Clinical Presentation of COVID19 in Dementia Patients
Affiliation.
- 1 Angelo Bianchetti, MD. Department Medicine and Rehabilitation, Istituto Clinico S.Anna Hospital, via del Franzone 31, 25122 Brescia, Italy; e-mail: [email protected], https://orcid.org/0000-0002-2914-0627, phone: +390303197409 - fax: +390303198687.
- PMID: 32510106
- PMCID: PMC7227170
- DOI: 10.1007/s12603-020-1389-1
Objective: No studies analyzing the role of dementia as a risk factor for mortality in patients affected by COVID-19. We assessed the prevalence, clinical presentation and outcomes of dementia among subjects hospitalized for COVID19 infection.
Design: Retrospective study.
Setting: COVID wards in Acute Hospital in Brescia province, Northern Italy.
Participants: We used data from 627 subjects admitted to Acute Medical wards with COVID 19 pneumonia.
Measurements: Clinical records of each patients admitted to the hospital with a diagnosis of COVID19 infection were retrospectively analyzed. Diagnosis of dementia, modalities of onset of the COVID-19 infection, symptoms of presentation at the hospital and outcomes were recorded.
Results: Dementia was diagnosed in 82 patients (13.1%). The mortality rate was 62.2% (51/82) among patients affected by dementia compared to 26.2% (143/545) in subjects without dementia (p<0.001, Chi-Squared test). In a logistic regression model age, and the diagnosis of dementia resulted independently associated with a higher mortality, and patients diagnosed with dementia presented an OR of 1.84 (95% CI: 1.09-3.13, p<0.05). Among patients diagnosed with dementia the most frequent symptoms of onset were delirium, especially in the hypoactive form, and worsening of the functional status.
Conclusion: The diagnosis of dementia, especially in the most advanced stages, represents an important risk factor for mortality in COVID-19 patients. The clinical presentation of COVID-19 in subjects with dementia is atypical, reducing early recognition of symptoms and hospitalization.
Keywords: COVID19 infection; dementia; mortality risk.
- Aged, 80 and over
- Betacoronavirus*
- Coronavirus Infections / complications*
- Dementia / complications*
- Dementia / epidemiology
- Italy / epidemiology
- Logistic Models
- Middle Aged
- Pneumonia, Viral / complications*
- Retrospective Studies
- Risk Factors
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Year in Review
- Published: 06 January 2021
NEUROLOGY AND COVID-19 IN 2020

The effects of the COVID-19 pandemic on people with dementia
- Katya Numbers 1 &
- Henry Brodaty ORCID: orcid.org/0000-0001-9487-6617 1 , 2
Nature Reviews Neurology volume 17 , pages 69–70 ( 2021 ) Cite this article
26k Accesses
124 Citations
122 Altmetric
Metrics details
- Alzheimer's disease
The COVID-19 pandemic has posed unique risks to people with Alzheimer disease and dementia. Research from 2020 has shown that these people have a relatively high risk of contracting severe COVID-19, and are also at risk of neuropsychiatric disturbances as a result of lockdown measures and social isolation.
Key advances
People with dementia are at high risk of SARS-CoV-2 infection because cognitive symptoms cause difficulty with following safeguarding procedures and living arrangements in care homes facilitate viral spread 1 .
Once infected, older adults with dementia are more likely to experience severe virus-related outcomes, including death, than are people without dementia 4 .
A homozygous APOE ε4 genotype is associated with an increased risk of hospitalization for COVID-19 (ref. 8 ), possibly owing to exacerbated inflammation and cytokine production that leads to a cytokine storm.
Older adults with dementia, especially those in care homes, are at high risk of worsening psychiatric symptoms and severe behavioural disturbances as a result of social isolation during the pandemic 3 .
The COVID-19 pandemic has had a unique impact on people with Alzheimer disease (AD) and other dementias. As research into this impact has accumulated throughout 2020, a clear picture has emerged that this population is particularly susceptible not just to SARS-CoV-2 infection and its effects, but also to the negative effects of the measures taken worldwide to control the spread of the virus.
Large-scale clinical data suggest that, even when old age and medical comorbidities such as hypertension and diabetes are taken into account, people with dementia are more likely to contract COVID-19 than people without dementia. Several reasons underlie the increased risk of SARS-CoV-2 infection in people with dementia, which are described in an important overview published in August 1 . First, cognitive impairment and neuropsychiatric symptoms make it challenging for individuals with dementia to understand and comply with safeguarding procedures, such as wearing masks and maintaining appropriate physical distancing 1 . Ignoring or forgetting warnings and an inability to follow self-quarantine measures increase the risk of infection.
people with dementia are more likely to contract COVID-19 than people without dementia
In addition, most people who live in institutional settings (nursing or care homes), where rates of infection are disproportionately high worldwide 2 , have dementia. Such living arrangements facilitate rapid transmission of the virus as residents and staff congregate and live within close proximity. Physical distancing is not feasible for residents who are dependent on staff to assist with basic activities of daily living (for example, toileting, bathing and eating). Furthermore, dementia-associated neuropsychiatric symptoms, such as agitation, intrusiveness or wandering, can also undermine safety protocols and increase the risk of infection among staff and other residents 1 . Accordingly, nursing and care homes have implemented increasingly severe lockdown measures, which further exacerbate pre-existing neuropsychiatric symptoms among residents with dementia 3 .
As well as being at increased risk of contracting COVID-19, older adults with dementia are also more likely to have more severe disease consequences than those without dementia 4 , 5 , 6 , 7 . A large community cohort study conducted in the UK has shown that the risk of serious COVID-19 (defined as a requirement for hospitalization) was threefold higher for individuals with a diagnosis of dementia than for those without dementia 4 . The risk factors for dementia — age, obesity, cardiovascular disease, hypertension and diabetes mellitus — are also risk factors for SARS-CoV-2 infection 6 and for severe COVID-19. However, some evidence suggests that more specific mechanistic aspects of dementia and pre-existing brain pathology can increase the risk of neurological complications from COVID-19 (ref. 8 ). In particular, a study of the UK Biobank cohort showed that the risk of COVID-19-related hospitalization was more than twofold higher among individuals who were homozygous for APOE ε4 than among individuals with the most common APOE ε3/ε3 genotype 8 .
One possible mechanistic explanation for this association is that increased blood–brain barrier permeability associated with APOE ε4 leads to more extensive CNS inflammation in response to SARS-CoV-2 infection — in line with this hypothesis, APOE ε4 is known to exacerbate microglia-mediated neuroinflammation and subsequent neurodegeneration 9 . In addition, APOE ε4 is associated with increased cytokine production in response to inflammatory stimuli, which could intensify the already aggressive inflammatory response associated with COVID-19, resulting in a so-called cytokine storm 10 . The cytokine storm has been directly associated with lung injury, multi-organ failure and severe COVID-19 outcomes, including death 10 .
The restrictions that have been implemented in many countries to control the pandemic have also had important neuropsychiatric consequences for patients with dementia. In the population as a whole, forced social isolation has led to an increase in reported psychiatric symptoms (for example, stress, anxiety and depression) for all individuals; this relationship seems to be moderated by the loneliness associated with prolonged periods of lockdown 1 , 3 , 9 . In nursing and care homes, older adults are likely to experience additional distress owing to the absence of relatives who would normally visit them, as well as strict limitations on social activities and interactions with fellow residents. Data collected during the first half of 2020 show that such social isolation during the pandemic is associated with manifestation and/or exacerbation of neuropsychiatric symptoms even in cognitively healthy older adults 1 , 9 .
Several studies — summarized in a review published in October 3 — have shown that, in older adults with dementia, psychiatric symptoms caused by social isolation are linked to more severe neuropsychiatric and behavioural disturbances 3 . Social isolation combined with confusion in care home residents with dementia might result in even greater agitation, boredom and loneliness than in residents without dementia, thereby leading to more severe neuropsychiatric symptoms. These neuropsychiatric symptoms seem to arise directly from social restrictions, as longer lockdown periods result in more severe neuropsychiatric symptoms 3 . Furthermore, some experts have suggested that behavioural complications that result from prolonged periods of lockdown in older adults with dementia could become chronic 3 . Some consequences of neuropsychiatric disturbances, such as increased aggression and agitation, can be particularly challenging for carers and care home staff to manage.
longer lockdown periods result in more severe neuropsychiatric symptoms
In summary, the evidence to date indicates that older adults with dementia have a high risk of contracting COVID-19 and, once infected, have a high risk of disease-related morbidity and mortality. This population is often the first to go into, and the last to come out of, strict and prolonged periods of isolation to prevent SARS-CoV-2 infection, yet is at extremely high risk of worsening neuropsychiatric symptoms and severe behavioural disturbance as a direct result. Therefore, during and after the pandemic, implementation of caregiver support and the presence of skilled nursing home staff are essential to maintain social interaction and to provide extra support to older adults with dementia.
Mok, V. C. et al. Tackling challenges in care of Alzheimer’s disease and other dementias amid the COVID-19 pandemic, now and in the future. Alzheimers Dement. 16 , 1571–1581 (2020).
Article Google Scholar
Comas-Herrera, A. et al. Mortality associated with COVID-19 outbreaks in care homes: early international evidence. LTCCOVID https://ltccovid.org/2020/04/12/mortality-associated-with-covid-19-outbreaks-in-care-homes-early-international-evidence/ (2020).
Manca, R., De Marco, M. & Venneri, A. The impact of COVID-19 infection and enforced prolonged social isolation on neuropsychiatric symptoms in older adults with and without dementia: a review. Front. Psychiatry 11 , 1086 (2020).
Atkins, J. L. et al. Preexisting comorbidities predicting severe COVID-19 in older adults in the UK Biobank community cohort. J. Gerontol. A Biol. Sci. Med. Sci. 75 , 2224–2230 (2020).
Onder, G., Rezza, G. & Brusaferro, S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 323 , 1775–1776 (2020).
CAS Google Scholar
Williamson, E. J. et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 584 , 430–436 (2020).
Article CAS Google Scholar
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395 , 1054–1062 (2020).
Kuo, C.-L. et al. APOE e4 genotype predicts severe COVID-19 in the UK Biobank community cohort. J. Gerontol. A Biol. Sci. Med. Sci. 75 , 2231–2232 (2020).
Brown, E. E., Kumar, S., Rajji, T. K., Pollock, B. G. & Mulsant, B. H. Anticipating and Mitigating the Impact of COVID-19 Pandemic on Alzheimer’s Disease and Related Dementias. Am. J. Geriatr. Psychiatry 28 , 712–721 (2020).
Ragab, D., Salah Eldin, H., Taeimah, M., Khattab, R. & Salem, R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 11 , 1446 (2020).
Download references
Author information
Authors and affiliations.
CHeBA (Centre for Healthy Brain Ageing), School of Psychiatry, University of New South Wales, Sydney, New South Wales, Australia
Katya Numbers & Henry Brodaty
Dementia Centre for Research Collaboration, School of Psychiatry, University of New South Wales, Sydney, New South Wales, Australia
Henry Brodaty
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Henry Brodaty .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Numbers, K., Brodaty, H. The effects of the COVID-19 pandemic on people with dementia. Nat Rev Neurol 17 , 69–70 (2021). https://doi.org/10.1038/s41582-020-00450-z
Download citation
Published : 06 January 2021
Issue Date : February 2021
DOI : https://doi.org/10.1038/s41582-020-00450-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Impact of the covid-19 pandemic on mortality and loss to follow-up among patients with dementia receiving anti-dementia medications.
- Hyuk Sung Kwon
- Wonjae Sung
Scientific Reports (2024)
The Impact of the Pandemic on Health and Quality of Life of Informal Caregivers of Older People: Results from a Cross-National European Survey in an Age-Related Perspective
- Marco Socci
- Mirko Di Rosa
- Sara Santini
Applied Research in Quality of Life (2024)
Impact of COVID-19 on the Residential Aged Care Workforce, and Workers From Culturally and Linguistically Diverse Backgrounds: A Rapid Literature Review
- Samantha Battams
- Angelita Martini
Ageing International (2024)
Factors influencing mobility in community-dwelling older adults during the early COVID-19 pandemic: a cross-sectional study
BMC Public Health (2023)
Emotions, action strategies and expectations of health professionals and people with dementia regarding COVID-19 in different care settings in Switzerland: a mixed methods study
- Steffen Heinrich
- Inga Weissenfels
- Adelheid Zeller
BMC Geriatrics (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Clinical Presentation of COVID19 in Dementia Patients
- Published: 15 May 2020
- Volume 24 , pages 560–562, ( 2020 )
Cite this article

- Angelo Bianchetti 1 , 4 na1 ,
- R. Rozzini 2 , 4 na1 ,
- F. Guerini 1 , 4 na1 ,
- S. Boffelli 2 , 4 na1 ,
- P. Ranieri 1 , 4 na1 ,
- G. Minelli 1 , 4 na1 ,
- L. Bianchetti 3 , 4 na1 &
- M. Trabucchi 4 na1
15k Accesses
214 Citations
149 Altmetric
Explore all metrics
No studies analyzing the role of dementia as a risk factor for mortality in patients affected by COVID-19. We assessed the prevalence, clinical presentation and outcomes of dementia among subjects hospitalized for COVID19 infection.
Retrospective study.
COVID wards in Acute Hospital in Brescia province, Northern Italy.
Participants
We used data from 627 subjects admitted to Acute Medical wards with COVID 19 pneumonia.
Measurements
Clinical records of each patients admitted to the hospital with a diagnosis of COVID19 infection were retrospectively analyzed. Diagnosis of dementia, modalities of onset of the COVID-19 infection, symptoms of presentation at the hospital and outcomes were recorded.
Dementia was diagnosed in 82 patients (13.1%). The mortality rate was 62.2% (51/82) among patients affected by dementia compared to 26.2% (143/545) in subjects without dementia (p<0.001, Chi-Squared test). In a logistic regression model age, and the diagnosis of dementia resulted independently associated with a higher mortality, and patients diagnosed with dementia presented an OR of 1.84 (95% CI: 1.09–3.13, p<0.05). Among patients diagnosed with dementia the most frequent symptoms of onset were delirium, especially in the hypoactive form, and worsening of the functional status.
The diagnosis of dementia, especially in the most advanced stages, represents an important risk factor for mortality in COVID-19 patients. The clinical presentation of COVID-19 in subjects with dementia is atypical, reducing early recognition of symptoms and hospitalization.
Similar content being viewed by others

COVID-19 in adults with dementia: clinical features and risk factors of mortality—a clinical cohort study on 125 patients
Delirium in covid-19: epidemiology and clinical correlations in a large group of patients admitted to an academic hospital.
COVID-19 mortality risk factors in older people in a long-term care center
Avoid common mistakes on your manuscript.
Introduction
In Italy, SARS-CoV-2 outbreak was catastrophic with 135,586 confirmed cases and 17,127 deaths at April, 8th ( 1 ). In clinical series of patients who died of COVID-19 comorbidities (especially hypertension, cardiac ischemic disease, diabetes and obesity) were identified as significant risk factors for mortality, while dementia was described as a comorbid condition in only 6.8% of COVID-19 patients ( 2 ).
Although dementia is known to be an important mortality risk factor among older people, so far there are no studies analyzing the role of dementia as a risk factor for mortality in patients affected by COVID-19 ( 3 , 4 ).
In the Province of Brescia, an administrative district in eastern Lombardy home to 1.2 million people, between February 22nd and April 8th, 9,900 cases of Covid-19 have been diagnosed and 1,800 deaths have been reported. About 53% (2265 out of 4200) of hospital beds have been dedicated to treat patients affected by Covid-19 pneumonia. Specific units were created to cater to these patients: acute medical units, named COVID Wards, and intensive care units, with the last accounting fort the 8.5% of all the beds dedicated to COVID-19 patients.
Methods and study population
During this period, 627 patients diagnosed with COVID-19 pneumonia were admitted to our hospitals. All patients admitted to COVID Wards were positive to RT-PCR for SARS-Cov-2 conducted on a nasopharyngeal specimen and presented respiratory failure. Each patient underwent a thorough medical evaluation and, if over 65, a geriatric multi-dimensional assessment, comprehensive of evaluation of cognitive and functional status and presence of delirium.
Dementia was diagosed according to clinical history and results of the cognitive assessment. The modalities of onset of the COVID-19 infection, the symptoms of presentation at the hospital emergency department and the outcomes were recorded.
Dementia was diagnosed in 82 patients (13.1%). The mean age of patients diagnosed with dementia was 82.6 (SD 5.3; IQR 80–86), versus 68.9 (SD 12.7; IQR 60–68) in patients not affected by dementia (p<0.001; Student’s t test). Females were 47 (57.3%) among patients with dementia and 288 (52.8%) among patients not diagnosed with dementia, respectively.
The mortality rate was 62.2% (51/82) among patients affected by dementia compared to 26.2% (143/545) in subjects without dementia (p<0.001, Chi-Squared test). (Table 1 )
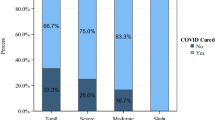
The Clinical Dementia Rating Scale (CDR) ( 5 ) was used to determine the severity of dementia: 36 patients (43.4%) were classified in stage 1, 15 (18.3%) in stage 2 and 31 (37.8%) in stage 3. The Mortality rates were, respectively, 41.7%, 66.7%, and 83.9% (p<0.001, one-way ANOVA). (table 2 )
To assess if the diagnosis of dementia was associated with a worse outcome regardless of age and sex, we built a logistic regression model. According to this model age, and the diagnosis of dementia resulted independently associated with a higher mortality. For every increased year of age, the Odds Ratio (OR) for mortality was 1.09 (95% CI: 1.07–1.12, p<0.001), and patients diagnosed with dementia presented an OR of 1.84 (95% CI: 1.09–3.13, p<0.05). According to this model sex was not associated with a change in mortality risk. (Table 3 )
As shown in table 4 , among patients diagnosed with dementia the most frequent symptoms of onset were delirium (67%, especially in the hypoactive form, 50%) and worsening of the functional status. The classic symptoms of COVID-19 infection were less frequent: only 47% of patients had fever, 44% dyspnea and 14% cough.
Conclusions
Caring for patients with dementia during the current pandemic is a complex task, involving the management of patients in different settings. Some patients need to be treated at home, often with caregivers burdened by isolation due to lockdown measures and by limitation of home services. Other patients are cared in nursing homes, which often lack adequate and trained staffs and access to personal protective equipment. Hospital patient’s management has been difficult due to the scarce collaboration offered by the patient and difficulties in communication, immobility, and limited availability of trained staff members ( 6 ). There are also ethical concerns regarding hospitalization of patients with dementia due to resource constraints during the current pandemic ( 7 ).
To our knowledge, the proportion of subjects with dementia among patients admitted to an acute hospital for COVID-19 has never been evaluated. The prevalence of demented patient found in the present study (13.1%) is lower than the previous estimates of the prevalence of dementia in hospital, which vary from 15% to 42% ( 7 ). According to our data, the diagnosis of dementia, especially in the most advanced stages, represents an important risk factor for mortality in COVID-19 patients. The clinical presentation of COVID-19 in subjects with dementia is atypical, reducing early recognition of symptoms and hospitalization. We suggest that the onset of hypoactive delirium and worsening functional status in people with dementia may be considered a sign of possible COVID-19 infection during this epidemic. Early recognition of COVID-19 in demented people can help provide timely treatment and adequate isolation. Hospitals should develop integrated care models, create Special Care Geriatric COVID units and promote guidelines to ensure the better possible treatment for frail older persons.
Word Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report - 79. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200408-sitrep-79-covid-19.pdf?sfvrsn=4796bl43_4
Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. Published online March 23, 2020. doi: https://doi.org/10.1001/jama.2020.4683
Morandi A, Di Santo SG, Zambón A et al , Italian Study Group on Delirium (ISGoD). Delirium, Dementia, and In-Hospital Mortality: The Results From the Italian Delirium Day 2016, A National Multicenter Study. J Gerontol A Biol Sci Med Sci;2019;74:910–916.
Article Google Scholar
D’Adamo H, Yoshikawa T, Ouslander JG. Coronavirus Disease 2019 in Geriatrics and Long-term Care: The ABCDs of COVID-19. JAGS 2020;doi: https://doi.org/10.1111/jgs.16445
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. The British Journal of Psychiatry; 1982;140, 566–572.
Article CAS Google Scholar
Wang H, Li T, Barbarino P, Gauthier S, Brodaty H, Molinuevo JL, Xie H, Sun Y, Yu E, Tang Y, Weidner W, Yu X. Dementia care during COVID-19. Lancet. 2020;395(10231):1190–1191. doi: https://doi.org/10.1016/S0140-6736(20)30755-8 . Epub 2020 Mar 30.
Aprahamian I, Cesari M. Geriatric Syndromes and SARS-COV-2: More than Just Being Old [published online ahead of print,]. J Frailty Aging. 2020;1–3. doi: https://doi.org/10.14283/jfa.2020.17
Jackson TA, Gladman JR, Harwood RH et al. Challenges and opportunities in understanding dementia and delirium in the acute hospital. PLoS Med:e 2017;1002247.
Download references
Acknowledgement
Anita Chizzoli, MD; Marzia Cristo, MD; Silvia Comini, MD; Assunta Di Stasio, MD and Antonella Ricci, MD for the support in clinical evaluation of patients.
Funding: No funding.
Author information
All the authors contributed equally to the drafting of this manuscript.
Authors and Affiliations
Department Medicine and Rehabilitation, Istituto Clinico S.Anna Hospital, via del Franzone 31, 25122, Brescia, Italy
Angelo Bianchetti, F. Guerini, P. Ranieri & G. Minelli
Geriatric Department, Fondazione Poliambulanza Istituto Ospedaliero Hospital, Brescia, Italy
R. Rozzini & S. Boffelli
Geriatric Reahabilitation Unit, Anni Azzurri, Rezzato, Brescia, Italy
L. Bianchetti
Italian Association of Psychogeriatrics, Rome, Italy
Angelo Bianchetti, R. Rozzini, F. Guerini, S. Boffelli, P. Ranieri, G. Minelli, L. Bianchetti & M. Trabucchi
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Angelo Bianchetti .
Ethics declarations
Conflicts of interest/Competing interests: The authors declare that they have no conflict of interest.
Ethics approval: This is a review study; the protocol was approved by the institutional committee.
Rights and permissions
Reprints and permissions
About this article
Bianchetti, A., Rozzini, R., Guerini, F. et al. Clinical Presentation of COVID19 in Dementia Patients. J Nutr Health Aging 24 , 560–562 (2020). https://doi.org/10.1007/s12603-020-1389-1
Download citation
Received : 25 April 2020
Accepted : 11 May 2020
Published : 15 May 2020
Issue Date : June 2020
DOI : https://doi.org/10.1007/s12603-020-1389-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- COVID19 infection
- mortality risk
- Find a journal
- Publish with us
- Track your research
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- Covid-19 and lack of...
Impact of COVID-19 on people living with dementia: emerging international evidence
Rapid response to:
Covid-19 and lack of linked datasets for care homes
Read our latest coverage of the coronavirus pandemic.
- Related content
- Article metrics
- Rapid responses
Rapid Response:
Dear Editor,
High rates of COVID-19 related deaths in people living with dementia have been reported since the pandemic started. This is likely associated with the fact that many COVID-19 deaths correspond to care home residents, most of whom have dementia. People with dementia may be at increased risk of developing more severe COVID-19 infection (1) and die from it (2) according to studies conducted on hospital cohorts. Furthermore, carriers of ApoEε4/ε4 genotype, the strongest genetic risk factor for Alzheimer’s disease, are more likely to develop complications from COVID-19 (3). Although it remains unclear whether dementia is directly associated with the severity of COVID-19 or influenced by other factors (e.g. older age and associated comorbidity), growing evidence indicates that this population is extremely vulnerable to the effects of the virus. Not only that, but up to 5.7% of patients with severe presentation of COVID-19 have stroke, which can precipitate cognitive decline in people already living with progressive cognitive difficulties (4). To prevent infection, people with dementia have gone through confinement and isolation, both in the community and in care homes. These measures, also involving the removal of essential sources of support, care and meaningful contact with family members (including spouses and main partners in care), may have long-lasting deleterious effects. A survey conducted among patients attending an Italian memory clinic showed that up to 31% of people with dementia had experienced significant cognitive deterioration during the first month of lockdown and 54% a worsening of agitation, apathy and depression (5).
Mitigating the impact of COVID-19 on people with dementia should be a public health priority. The measures required include ensuring access to enough PPE and training on infection prevention and control for care workers, comprehensive testing policies, access to quarantine and step-down facilities, and implementation of guidance on compassionate isolation and person-centred care to lessen the psychological and cognitive detrimental effect of confinement. In many parts of the world, the rates of infection are beginning to decrease. We now have the opportunity to learn from these first experiences with COVID-19 and be better prepared so that, in future waves, people living with dementia are not left behind.
1. Atkins JL, Masoli JAH, Delgado J, et al. Preexisting comorbidities predicting severe COVID-19 in older adults in the UK Biobank community cohort. https://doi.org/10.1101/2020.05.06.20092700 2. Bianchetti A, Rozzini R, Guerini F., et al. Clinical presentation of COVID-19 in dementia patients. J Nutr Health Aging 24, 560-562 doi.org/10.1007/s12603-020-1389-1 3. Kuo CL, Pilling LC, Atkin JL, et al. APOE e4 Genotype predicts severe COVID-19 in the UK Biobank community cohort. The journals of Gerontology: series A, glaa131: https://doi.org/10.1093/gerona/glaa131 4. Mao L, Huijuna J, Wang M et al., Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690. doi:10.1001/jamaneurol.2020.1127 5. Canevelli m, Valleta M, Toccaceli M., et al. Facing dementia during the covid-19 outbreak. J Am Geriatr Soc. 2020 Jun 9. doi: 10.1111/jgs.16644.
Funding: ASG is supported by the ESRC/NIHR Dementia Research Initiative (ES/S010467/1). ACH is supported by the UK Research and Innovation’s Global Challenges Research Fund (ES/P010938/1).
Competing interests: GL and ACH report no competing interests. ASG reports fees from MedAvante Pro-Phase. All reported financial activities are unrelated to this correspondence.
Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.
Search life-sciences literature (44,074,510 articles, preprints and more)
- Free full text
- Citations & impact
- Similar Articles
Clinical Presentation of COVID19 in Dementia Patients.
Author information, affiliations.
- Bianchetti A 1
ORCIDs linked to this article
- BIANCHETTI A | 0000-0002-2914-0627
The Journal of Nutrition, Health & Aging , 01 Jan 2020 , 24(6): 560-562 https://doi.org/10.1007/s12603-020-1389-1 PMID: 32510106 PMCID: PMC7227170
Abstract
Participants, measurements, free full text .

Clinical Presentation of COVID19 in Dementia Patients
Angelo bianchetti.
1 Department Medicine and Rehabilitation, Istituto Clinico S.Anna Hospital, via del Franzone 31, 25122 Brescia, Italy
4 Italian Association of Psychogeriatrics, Rome, Italy
2 Geriatric Department, Fondazione Poliambulanza Istituto Ospedaliero Hospital, Brescia, Italy
S. Boffelli
L. bianchetti.
3 Geriatric Reahabilitation Unit, Anni Azzurri, Rezzato, Brescia, Italy
M. Trabucchi
No studies analyzing the role of dementia as a risk factor for mortality in patients affected by COVID-19. We assessed the prevalence, clinical presentation and outcomes of dementia among subjects hospitalized for COVID19 infection.
Retrospective study.
COVID wards in Acute Hospital in Brescia province, Northern Italy.
We used data from 627 subjects admitted to Acute Medical wards with COVID 19 pneumonia.
Clinical records of each patients admitted to the hospital with a diagnosis of COVID19 infection were retrospectively analyzed. Diagnosis of dementia, modalities of onset of the COVID-19 infection, symptoms of presentation at the hospital and outcomes were recorded.
Dementia was diagnosed in 82 patients (13.1%). The mortality rate was 62.2% (51/82) among patients affected by dementia compared to 26.2% (143/545) in subjects without dementia (p<0.001, Chi-Squared test). In a logistic regression model age, and the diagnosis of dementia resulted independently associated with a higher mortality, and patients diagnosed with dementia presented an OR of 1.84 (95% CI: 1.09–3.13, p<0.05). Among patients diagnosed with dementia the most frequent symptoms of onset were delirium, especially in the hypoactive form, and worsening of the functional status.
The diagnosis of dementia, especially in the most advanced stages, represents an important risk factor for mortality in COVID-19 patients. The clinical presentation of COVID-19 in subjects with dementia is atypical, reducing early recognition of symptoms and hospitalization.

Introduction
In Italy, SARS-CoV-2 outbreak was catastrophic with 135,586 confirmed cases and 17,127 deaths at April, 8th ( 1 ). In clinical series of patients who died of COVID-19 comorbidities (especially hypertension, cardiac ischemic disease, diabetes and obesity) were identified as significant risk factors for mortality, while dementia was described as a comorbid condition in only 6.8% of COVID-19 patients ( 2 ).
Although dementia is known to be an important mortality risk factor among older people, so far there are no studies analyzing the role of dementia as a risk factor for mortality in patients affected by COVID-19 ( 3 , 4 ).
In the Province of Brescia, an administrative district in eastern Lombardy home to 1.2 million people, between February 22nd and April 8th, 9,900 cases of Covid-19 have been diagnosed and 1,800 deaths have been reported. About 53% (2265 out of 4200) of hospital beds have been dedicated to treat patients affected by Covid-19 pneumonia. Specific units were created to cater to these patients: acute medical units, named COVID Wards, and intensive care units, with the last accounting fort the 8.5% of all the beds dedicated to COVID-19 patients.
Methods and study population
During this period, 627 patients diagnosed with COVID-19 pneumonia were admitted to our hospitals. All patients admitted to COVID Wards were positive to RT-PCR for SARS-Cov-2 conducted on a nasopharyngeal specimen and presented respiratory failure. Each patient underwent a thorough medical evaluation and, if over 65, a geriatric multi-dimensional assessment, comprehensive of evaluation of cognitive and functional status and presence of delirium.
Dementia was diagosed according to clinical history and results of the cognitive assessment. The modalities of onset of the COVID-19 infection, the symptoms of presentation at the hospital emergency department and the outcomes were recorded.
Dementia was diagnosed in 82 patients (13.1%). The mean age of patients diagnosed with dementia was 82.6 (SD 5.3; IQR 80–86), versus 68.9 (SD 12.7; IQR 60–68) in patients not affected by dementia (p<0.001; Student’s t test). Females were 47 (57.3%) among patients with dementia and 288 (52.8%) among patients not diagnosed with dementia, respectively.
The mortality rate was 62.2% (51/82) among patients affected by dementia compared to 26.2% (143/545) in subjects without dementia (p<0.001, Chi-Squared test). (Table (Table1 1 )
Characteristics of 627 patients consecutively hospitalized for COVID19 pneumonia in two Italian hospitals according to the diagnosis of dementia
* Pearson’s chi-squared test; ** Student’s t-test
The Clinical Dementia Rating Scale (CDR) ( 5 ) was used to determine the severity of dementia: 36 patients (43.4%) were classified in stage 1, 15 (18.3%) in stage 2 and 31 (37.8%) in stage 3. The Mortality rates were, respectively, 41.7%, 66.7%, and 83.9% (p<0.001, one-way ANOVA). (table (table2 2 )
Characteristics of 627 patients consecutively hospitalized for COVID19 pneumonia in two Italian hospitals according to CDR classification
* one-way ANOVA
To assess if the diagnosis of dementia was associated with a worse outcome regardless of age and sex, we built a logistic regression model. According to this model age, and the diagnosis of dementia resulted independently associated with a higher mortality. For every increased year of age, the Odds Ratio (OR) for mortality was 1.09 (95% CI: 1.07–1.12, p<0.001), and patients diagnosed with dementia presented an OR of 1.84 (95% CI: 1.09–3.13, p<0.05). According to this model sex was not associated with a change in mortality risk. (Table (Table3 3 )
Binary Logistic Regression Model for mortality by Age, Sex and Dementia
* Wald Test for Analysis of Variance
As shown in table table4, 4 , among patients diagnosed with dementia the most frequent symptoms of onset were delirium (67%, especially in the hypoactive form, 50%) and worsening of the functional status. The classic symptoms of COVID-19 infection were less frequent: only 47% of patients had fever, 44% dyspnea and 14% cough.
Symptoms at ER admission among 82 dementia patients consecutively hospitalized for COVID19 pneumonia in two Italian hospitals
Conclusions
Caring for patients with dementia during the current pandemic is a complex task, involving the management of patients in different settings. Some patients need to be treated at home, often with caregivers burdened by isolation due to lockdown measures and by limitation of home services. Other patients are cared in nursing homes, which often lack adequate and trained staffs and access to personal protective equipment. Hospital patient’s management has been difficult due to the scarce collaboration offered by the patient and difficulties in communication, immobility, and limited availability of trained staff members ( 6 ). There are also ethical concerns regarding hospitalization of patients with dementia due to resource constraints during the current pandemic ( 7 ).
To our knowledge, the proportion of subjects with dementia among patients admitted to an acute hospital for COVID-19 has never been evaluated. The prevalence of demented patient found in the present study (13.1%) is lower than the previous estimates of the prevalence of dementia in hospital, which vary from 15% to 42% ( 7 ). According to our data, the diagnosis of dementia, especially in the most advanced stages, represents an important risk factor for mortality in COVID-19 patients. The clinical presentation of COVID-19 in subjects with dementia is atypical, reducing early recognition of symptoms and hospitalization. We suggest that the onset of hypoactive delirium and worsening functional status in people with dementia may be considered a sign of possible COVID-19 infection during this epidemic. Early recognition of COVID-19 in demented people can help provide timely treatment and adequate isolation. Hospitals should develop integrated care models, create Special Care Geriatric COVID units and promote guidelines to ensure the better possible treatment for frail older persons.
Acknowledgement
Anita Chizzoli, MD; Marzia Cristo, MD; Silvia Comini, MD; Assunta Di Stasio, MD and Antonella Ricci, MD for the support in clinical evaluation of patients.
Funding: No funding.
Conflicts of interest/Competing interests: The authors declare that they have no conflict of interest.
Ethics approval: This is a review study; the protocol was approved by the institutional committee.
All the authors contributed equally to the drafting of this manuscript.
Full text links
Read article at publisher's site: https://doi.org/10.1007/s12603-020-1389-1
Citations & impact
Impact metrics, citations of article over time, smart citations by scite.ai smart citations by scite.ai include citation statements extracted from the full text of the citing article. the number of the statements may be higher than the number of citations provided by europepmc if one paper cites another multiple times or lower if scite has not yet processed some of the citing articles. explore citation contexts and check if this article has been supported or disputed. https://scite.ai/reports/10.1007/s12603-020-1389-1, article citations, a longitudinal cohort study on the use of health and care services by older adults living at home with/without dementia before and during the covid-19 pandemic: the hunt study..
Ibsen TL , Strand BH , Bergh S , Livingston G , Lurås H , Mamelund SE , Voshaar RO , Rokstad AMM , Thingstad P , Gerritsen D , Selbæk G
BMC Health Serv Res , 24(1):485, 19 Apr 2024
Cited by: 0 articles | PMID: 38641570 | PMCID: PMC11027287
COVID-19 and Comorbidities: What Has Been Unveiled by Metabolomics?
Camelo ALM , Zamora Obando HR , Rocha I , Dias AC , Mesquita AS , Simionato AVC
Metabolites , 14(4):195, 30 Mar 2024
Cited by: 0 articles | PMID: 38668323 | PMCID: PMC11051775
COVID-19 and Alzheimer's disease: Impact of lockdown and other restrictive measures during the COVID-19 pandemic.
Nawaz AD , Haider MZ , Akhtar S
Biomol Biomed , 24(2):219-229, 11 Mar 2024
Cited by: 0 articles | PMID: 38078809 | PMCID: PMC10950341
New score to predict COVID-19 progression in vaccine and early treatment era: the COVID-19 Sardinian Progression Score (CSPS).
De Vito A , Saderi L , Colpani A , Puci MV , Zauli B , Fiore V , Fois M , Meloni MC , Bitti A , Moi G , Maida I , Babudieri S , Sotgiu G , Madeddu G
Eur J Med Res , 29(1):123, 15 Feb 2024
Cited by: 0 articles | PMID: 38360688 | PMCID: PMC10868088
Investigating the Potential Shared Molecular Mechanisms between COVID-19 and Alzheimer's Disease via Transcriptomic Analysis.
Fan Y , Liu X , Guan F , Hang X , He X , Jin J
Viruses , 16(1):100, 09 Jan 2024
Cited by: 0 articles | PMID: 38257800 | PMCID: PMC10821526
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
- http://www.ebi.ac.uk/biostudies/studies/S-EPMC7227170?xr=true
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Delirium in COVID-19: epidemiology and clinical correlations in a large group of patients admitted to an academic hospital.
Ticinesi A , Cerundolo N , Parise A , Nouvenne A , Prati B , Guerra A , Lauretani F , Maggio M , Meschi T
Aging Clin Exp Res , 32(10):2159-2166, 18 Sep 2020
Cited by: 56 articles | PMID: 32946031 | PMCID: PMC7498987
Effectiveness of Streptococcus Pneumoniae Urinary Antigen Testing in Decreasing Mortality of COVID-19 Co-Infected Patients: A Clinical Investigation.
Desai A , Santonocito OG , Caltagirone G , Kogan M , Ghetti F , Donadoni I , Porro F , Savevski V , Poretti D , Ciccarelli M , Martinelli Boneschi F , Voza A
Medicina (Kaunas) , 56(11):E572, 29 Oct 2020
Cited by: 6 articles | PMID: 33138045 | PMCID: PMC7693839
Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy.
Inciardi RM , Adamo M , Lupi L , Cani DS , Di Pasquale M , Tomasoni D , Italia L , Zaccone G , Tedino C , Fabbricatore D , Curnis A , Faggiano P , Gorga E , Lombardi CM , Milesi G , Vizzardi E , Volpini M , Nodari S , Specchia C , [...] Metra M
Eur Heart J , 41(19):1821-1829, 01 May 2020
Cited by: 300 articles | PMID: 32383763 | PMCID: PMC7239204
Delirium Assessment in Critically Ill Older Adults: Considerations During the COVID-19 Pandemic.
Duggan MC , Van J , Ely EW
Crit Care Clin , 37(1):175-190, 14 Aug 2020
Cited by: 12 articles | PMID: 33190768 | PMCID: PMC7427547
Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: A single center study of 100 patients in Brescia, Italy.
Toniati P , Piva S , Cattalini M , Garrafa E , Regola F , Castelli F , Franceschini F , Airò P , Bazzani C , Beindorf EA , Berlendis M , Bezzi M , Bossini N , Castellano M , Cattaneo S , Cavazzana I , Contessi GB , Crippa M , Delbarba A , [...] Latronico N
Autoimmun Rev , 19(7):102568, 03 May 2020
Cited by: 476 articles | PMID: 32376398 | PMCID: PMC7252115
Europe PMC is part of the ELIXIR infrastructure
Hospital Medicine Virtual Journal Club
Clinical Presentation of COVID19 in Dementia Patients.
Link to article at PubMed
J Nutr Health Aging. 2020 May 15;:1-3
Authors: Bianchetti A, Rozzini R, Guerini F, Boffelli S, Ranieri P, Minelli G, Bianchetti L, Trabucchi M
Abstract Objective: No studies analyzing the role of dementia as a risk factor for mortality in patients affected by COVID-19. We assessed the prevalence, clinical presentation and outcomes of dementia among subjects hospitalized for COVID19 infection. Design: Retrospective study. Setting: COVID wards in Acute Hospital in Brescia province, Northern Italy. Participants: We used data from 627 subjects admitted to Acute Medical wards with COVID 19 pneumonia. Measurements: Clinical records of each patients admitted to the hospital with a diagnosis of COVID19 infection were retrospectively analyzed. Diagnosis of dementia, modalities of onset of the COVID-19 infection, symptoms of presentation at the hospital and outcomes were recorded. Results: Dementia was diagnosed in 82 patients (13.1%). The mortality rate was 62.2% (51/82) among patients affected by dementia compared to 26.2% (143/545) in subjects without dementia (p<0.001, Chi-Squared test). In a logistic regression model age, and the diagnosis of dementia resulted independently associated with a higher mortality, and patients diagnosed with dementia presented an OR of 1.84 (95% CI: 1.09-3.13, p<0.05). Among patients diagnosed with dementia the most frequent symptoms of onset were delirium, especially in the hypoactive form, and worsening of the functional status. Conclusion: The diagnosis of dementia, especially in the most advanced stages, represents an important risk factor for mortality in COVID-19 patients. The clinical presentation of COVID-19 in subjects with dementia is atypical, reducing early recognition of symptoms and hospitalization.
PMID: 32425646 [PubMed - as supplied by publisher]
Share this:
- Click to share on Facebook (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Click to share on Mastodon (Opens in new window)
- Click to share on Reddit (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
- Click to email a link to a friend (Opens in new window)
- Click to print (Opens in new window)
Leave a Reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Notify me of new posts by email.
ORIGINAL RESEARCH article
Delirium: clinical presentation and outcomes in older covid-19 patients.

- 1 Geriatric Department, Fondazione Poliambulanza Istituto Ospedaliero, Brescia, Italy
- 2 Associazione Italiana di Psicogeriatria, Brescia, Italy
- 3 Medicine and Rehabilitation Department, Istituto Clinico S. Anna Hospital, Brescia, Italy
- 4 Geriatric Rehabilitation Unit, Anni Azzurri, Brescia, Italy
The aim of the study is to describe the clinical characteristics and outcomes of a series of older patients consecutively admitted into a non-ICU ward due to SARS-CoV-2 infection (14, males 11), developing delirium. Hypokinetic delirium with lethargy and confusion was observed in 43% of cases (6/14 patients). A total of eight patients exhibited hyperkinetic delirium and 50% of these patients (4/8) died. The overall mortality rate was 71% (10/14 patients). Among the four survivors we observed two different clinical patterns: two patients exhibited dementia and no ARDS (acute respiratory distress syndrome), while the remaining two patients exhibited ARDS and no dementia. The observed different clinical patterns of delirium (hypokinetic delirium; hyperkinetic delirium with or without dementia; hyperkinetic delirium with or without ARDS) identified patients with different prognosis: we believe these observations may have an impact on the management of older subjects with delirium due to COVID-19.
Introduction
Although the most frequent and life-threatening complications of coronavirus disease 19 (COVID-19) are respiratory, there are increasing reports of neurological and psychiatric involvement ( 1 ).
It is known that delirium can be the symptom of the presentation of many diseases, particularly in frail and older patients, and is recognized as an independent risk factor for mortality ( 2 ). The overall prevalence of delirium in the hospital setting is about 14–24%; its prevalence is higher, about 30%, in emergency, surgical, or medical wards ( 3 , 4 ). To date, the clinical presentation of delirium in older patients with COVID-19 infection have rarely been described; in fact, although some studies focus on epidemiological data and outcome, few studies analyze the clinical aspects of delirium in COVID-19 ( 5 – 8 ). The aim of this study is to describe clinical characteristics and outcomes of a series of elderly patients presenting delirium as the main symptom of COVID-19.
Materials and Methods
The study was carried out in the COVID ward in an Acute Care Hospital located in Brescia, one of the hardest hit cities by SARS-CoV-2 infection in northern Italy ( 9 ). We collected the characteristics of 14 older patients (age range 70–90, mean age 78.2; 11 males) consecutively admitted developing prevalent or incident delirium (respectively 10 and 4 cases). All the patients were admitted with a diagnosis of COVID-19, confirmed by a real-time reverse-transcriptase-polymerase chain reaction (rRT-PCR); three patients came from nursing homes, the remainder from home.
Medical information collected were age, sex, PaO 2 /FiO 2 , chest x-ray or CT, comorbidities [ischemic heart disease, chronic obstructive pulmonary disease (COPD), hypertension, diabetes, malignancies, neurodegenerative diseases], blood tests [hemoglobin, platelets count, neutrophils, lymphocytes, C-reactive protein (CRP), urea, and creatinine], and oxygen therapy (i.e., from nasal cannula to high flow cannula oxygen therapy to non-invasive ventilation). To assess the severity of COVID-19 pneumonia the SIAARTI criteria were followed, i.e., mild ARDS (acute respiratory distress syndrome): PaO 2 /FiO ratio 201–300; moderate ARDS : 101–200, and s evere ARDS : ≤ 100 ( 10 ). The diagnosis of dementia was made on the basis of the data collected from clinical records, while the severity of dementia was assessed by CDR ( 11 ) and functional status by the Barthel Index ( 12 ). CDR was estimated based on information collected from family members and the records of patients. Delirium was detected through 4At (assessment test for delirium and cognitive impairment) ( 13 ).
Clinical criteria were used to characterize delirium subtypes: hypoactive or hyperactive. The presence of a disturbance of consciousness was retrospectively defined by altered arousal.
Hyperactive delirium with aggression and agitation was observed in eight patients, while the remaining six patients exhibited hypoactive delirium with lethargy and confusion.
Moreover, dementia was diagnosed in six out of 14 patients; among these, four developed hypokinetic delirium, while the remaining two developed hyperkinetic delirium. Patients without dementia were younger, with a mean age of 74.1 years (see Table 1 ).
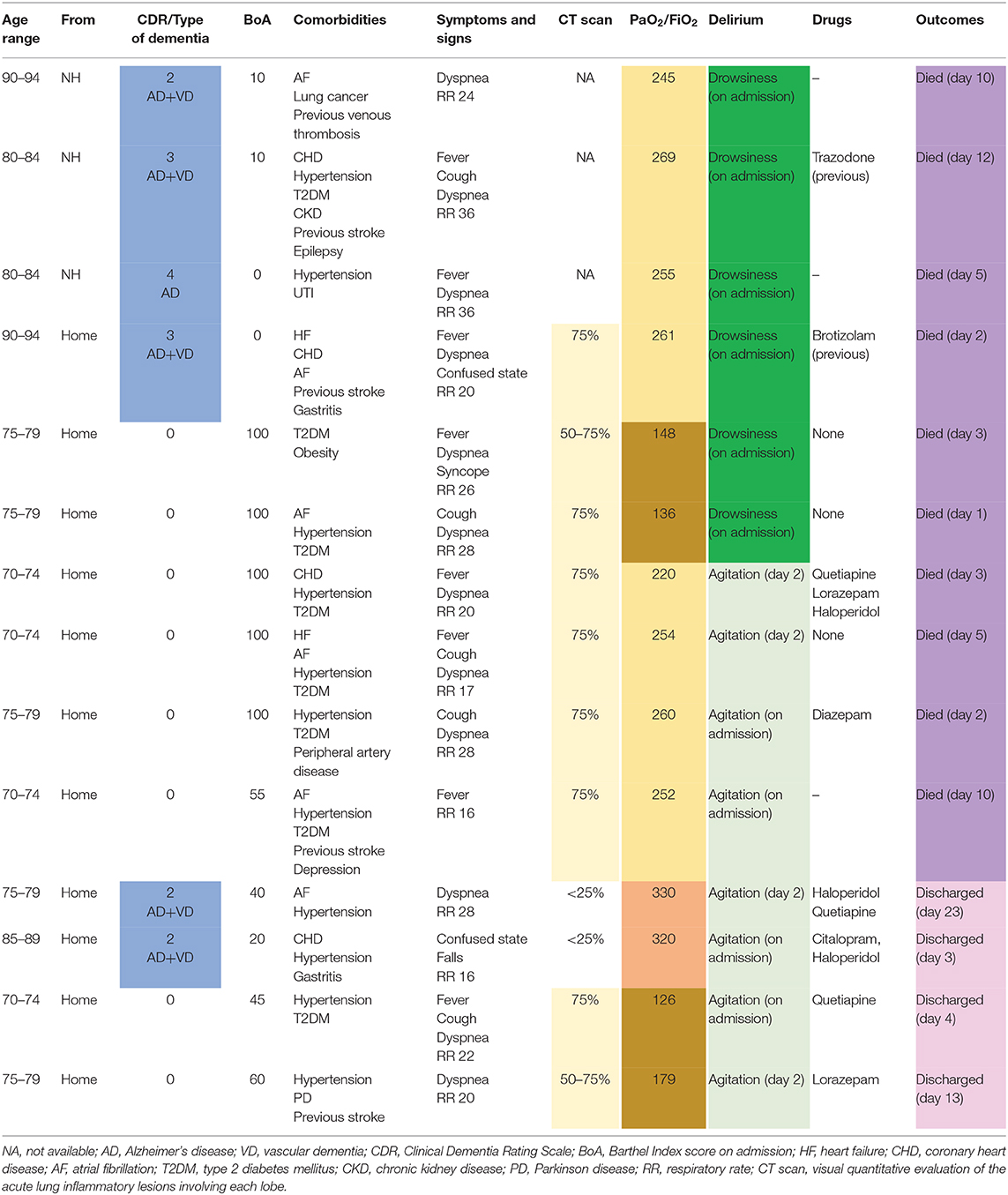
Table 1 . Characteristics and outcomes of 14 older patients with confirmed diagnosis of COVID-19 and delirium.
The drugs used to treat patients with hyperkinetic delirium were: lorazepam (2 cases), diazepam (1 cases), quetiapine (3 cases), and haloperidol (3 cases). Two patients with hypokinetic prevalent delirium were treated before hospitalization with brotizolam (1 case) and trazodone (1 case).
Two of the patients were hospitalized for stage III pneumonia (PaO 2 /FiO 2 ratio >300), eight patients were hospitalized for stage IV pneumonia-mild ARDS (200<PaO 2 /FiO 2 <3008), and four patients were hospitalized with stage IV pneumonia-moderate ARDS (100<PaO 2 /FiO 2 <200).
Upon admission, the patients presented the following symptoms: fever (7 cases), dyspnea (12 cases), cough (4 cases), fall and syncope (one case).
Almost all the patients (12/14) had a respiratory rate greater than 19.
The overall mortality rate was 71% (10/14 patients). All 6 of the patients exhibiting hypokinetic delirium and the 50% of patients (4/8) with hyperkinetic delirium died. Patients with hypokinetic delirium exhibited dementia and mild ARDS in four cases and no dementia and moderate ARDS in two cases.
Among the four survivors we observed two different clinical patterns: two patients exhibited dementia and no ARDS, while the remaining two patients exhibited ARDS and no dementia.
All patients living in a nursing home developed hypokinetic delirium and died.
A chest CT scan was taken for 11 of the patients: in two cases the lung involvement was less than 25%, in two cases it was from 50 to 75%, and in seven cases it was greater than 75%. The two cases with lower lung involvement survived; one patient with intermediate (50–75%) and one with greater involvement (>75%) also survived.
Each patient showed a high number of comorbidities: nine patients were affected by cardiovascular diseases (mainly coronary heart disease, atrial fibrillation, and heart failure), 12 by hypertension, and eight by diabetes. In particular, only four patients had no more than two comorbid conditions. In detail: survivors with hyperkinetic delirium had two or three comorbidities; deceased patients with hyperkinetic delirium had three or more comorbidities; deceased patients with hypokinetic delirium had two comorbidities in two cases and three or more in four cases.
With increasing frequency, delirium is reported as a symptom of the presentation of COVID-19 in older patients, although clinical aspects are rarely characterized ( 14 ). In a French series of elderly patients with COVID-19, delirium was present in 26.7% of patients, in two thirds of the cases in the hypokinetic form ( 15 ). In a series of hospitalized older patients with COVID-19 in the UK, delirium was observed in 25.2% of the sample ( 16 ). In older patients with dementia, delirium was a clinical manifestation of COVID-19 in 67% of cases, in 75% of these cases in the hypokinetic form ( 17 ). Mortality rates in these case series related to COVID-19 disease are still inconclusive and so comparison with other literature is uncertain.
In our patients with delirium, mortality was higher (71%) than previously reported for cases of hospitalized older people with delirium (ranging from 9 to 25%) ( 4 ). All subjects who developed hypokinetic delirium died. According to the literature, this form of delirium is associated with worse outcomes, particularly among patients affected by dementia ( 18 ). Multimorbidity is a condition associated with higher mortality, especially among patients who developed hypokinetic delirium: thus, hypokinetic delirium needs to be considered a marker of poor prognosis even in previously fit patients ( 3 ).
The onset of delirium is due to a complex interaction between the baseline vulnerability of the patient or predisposing factors and noxious insults or precipitating factors; recent observations lead us to believe that frailty and immunosenescence constitute factors that explain the excess mortality in elderly subjects with COVID-19 ( 19 ).
In our study, hyperkinetic delirium in cognitively unimpaired patients with mild ARDS had a better prognostic value than hypokinetic delirium in those with the same lung impairment. Hyperkinetic delirium in patients with dementia was observed in non-ARDS pneumonia (PaO 2 /FiO 2 > 300). Patients with hyperkinetic delirium who died had a higher noxious insult (i.e., 200 < PaO 2 /FiO 2 < 300) or dementia, and high level of comorbidities.
The high mortality rate of subjects developing delirium as an onset symptom of COVID-19, particularly in its hypokinetic form, could suggest brain involvement rather than the worsening effect of a pre-existing condition of frailty. Taking cognizance of the emergency due to the outbreak of COVID-19 and the consequent necessity of brief and easy-to-use tools and the involvement of non-expert doctors and nurses in COVID wards, to diagnose delirium we decided to use the 4AT test, a reliable tool designed for delirium detection in clinical practice ( 13 ).
Based on our observations, we hypothesize that delirium subtypes may be markers of biological severity of precipitating disease in COVID-19 patients. Specifically, patients suffering from a higher involvement of brain function and thus manifesting hypokinetic delirium, have a worse prognosis, while those who develop hyperkinetic delirium with a lower degree of dysregulation induced by the disease have a better chance of survival. Data on the ARDS stage confirm this interpretation since deceased patients with hypokinetic delirium and dementia were the most biologically compromised (with the most severe form of ARDS).
These different clinical patterns (hypokinetic delirium; hyperkinetic delirium with or without dementia; hyperkinetic delirium with or without ARDS) identify patients with different prognosis. Although the data were collected in a relatively limited number of cases, these observations may have an impact on the management of older subjects with delirium due to COVID-19.
In conclusion, our study indicates that delirium, particularly in the hypokinetic form, is related to a high risk of mortality in patients with COVID-19, especially in the presence of dementia. Therefore, a systematic recognition of this syndrome in COVID-19 patients is crucial for establishing a reliable prognosis.
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Comitato Etico di Brescia. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
RR, FM, and GC contribute to evaluation of cases, data management and discussion. AB, LB, and MT reviewed and discussed the manuscript. AB and RR wrote the first draft. All authors carefully reviewed, discussed and contributed to various draft of the manuscript. All authors approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Leonardi M, Padovani A, McArthur JC. Neurological manifestations associated with COVID-19: a review and a call for action. J Neurol. (2020) 267:1573–6. doi: 10.1007/s00415-020-09896-z
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Persico I, Cesari M, Morandi A, Haas J, Mazzola P, Zambon A, et al. Frailty and delirium in older adults: a systematic review and meta-analysis of the literature. J Am Geriatr Soc. (2018) 66:2022–30. doi: 10.1111/jgs.15503
3. Bellelli G, Morandi A, Di Santo SG, Mazzone A, Cherubini A, Mossello E, et al. “Delirium Day”: a nationwide point prevalence study of delirium in older hospitalized patients using an easy standardized diagnostic tool. BMC Med. (2016) 14:106. doi: 10.1186/s12916-016-0649-8
4. Morandi A, Di Santo SG, Zambon A, Mazzone A, Cherubini A, Mossello E, et al. Delirium, dementia, and in-hospital mortality: the results from the italian delirium day 2016, A National Multicenter Study. J Gerontol Ser A Biol Sci Med Sci. (2019) 74:910–6. doi: 10.1093/gerona/gly154
5. Tay HS, Harwood R. Atypical presentation of COVID-19 in a frail older person. Age Ageing. (2020) 49:523–4. doi: 10.1093/ageing/afaa068
6. Alkeridy WA, Almaghlouth I, Alrashed R, Alayed K, Binkhamis K, Alsharidi A, et al. A Unique presentation of delirium in a patient with otherwise asymptomatic COVID-19. J Am Geriatr Soc. (2020) 68:1382–4. doi: 10.1111/jgs.16536
7. Kotfis K, Williams Roberson S, Wilson JE, Dabrowski W, Pun BT, Ely EW. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care. (2020) 24:176. doi: 10.1186/s13054-020-02882-x
8. Marengoni A, Zucchelli A, Grande G, Fratiglioni L, Rizzuto D. The impact of delirium on outcomes for older adults hospitalised with COVID-19. Age Ageing . (2020) 49:923–6. doi: 10.1093/ageing/afaa189
9. Rozzini R, Bianchetti A. COVID Towers: low- and medium-intensity care for patients not in the ICU. CMAJ . (2020) 192:E463–4. doi: 10.1503/cmaj.75334
10. SIAARTI. Percorso Assistenziale per il Paziente Affetto da COVID-19 . Sezione 1 - Procedure area critica - versione 02 (2020).
Google Scholar
11. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. (1982) 140:566–72.
PubMed Abstract | Google Scholar
12. Mahoney F, Barthel D. Functional evaluation: the Barthel Index. Md Med J . (1995) 14:61–5.
13. Bellelli G, Morandi A, Davis DH, Mazzola P, Turco R, Gentile S, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing. (2014) 43:496–502. doi: 10.1093/ageing/afu021
14. Bianchetti A, Rozzini R, Guerini F, Boffelli S, Ranieri P, Minelli G, et al. Clinical presentation of COVID19 in dementia patients. J Nutr. Health Aging. (2020) 24:560–2. doi: 10.1007/s12603-020-1389-1
15. Annweiler C, Sacco G, Salles N, Aquino JP, Gautier J, Berrut G, et al. National French survey of COVID-19 symptoms in people aged 70 and over. Clin Infect Dis. (2020) ciaa792. doi: 10.1093/cid/ciaa792
16. Zazzara MB, Penfold RS, Roberts AL, Lee K, Dooley H, Sudre CH, et al. Delirium is a presenting symptom of COVID-19 in frail, older adults: a cohort study of 322 hospitalised and 535 community-based older adults. medRxiv . (2020) 2020.06.15.20131722. doi: 10.1101/2020.06.15.20131722
17. Bianchetti A, Bellelli G, Guerini F, Marengoni A, Padovani A, Rozzini R, et al. Improving the care of older patients during the COVID-19 pandemic. Aging Clin Exp Res. (2020) 32:1883–8. doi: 10.1007/s40520-020-01641-w
18. Rosgen BK, Krewulak KD, Stelfox HT, Ely EW, Davidson JE, Fiest KM. The association of delirium severity with patient and health system outcomes in hospitalised patients: a systematic review. Age Ageing. (2020) 49:549–57. doi: 10.1093/ageing/afaa053
19. Knopp P, Miles A, Webb TE, Mcloughlin BC, Mannan I, Raja N, et al. Presenting features of COVID-19 in older people: relationships with frailty, inflammation and mortality. medRxiv . (2020) 2020.06.07.20120527. doi: 10.1101/2020.06.07.20120527
Keywords: COVID 19, delirium, elderly, frailty, mortality
Citation: Rozzini R, Bianchetti A, Mazzeo F, Cesaroni G, Bianchetti L and Trabucchi M (2020) Delirium: Clinical Presentation and Outcomes in Older COVID-19 Patients. Front. Psychiatry 11:586686. doi: 10.3389/fpsyt.2020.586686
Received: 23 July 2020; Accepted: 22 September 2020; Published: 12 November 2020.
Reviewed by:
Copyright © 2020 Rozzini, Bianchetti, Mazzeo, Cesaroni, Bianchetti and Trabucchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renzo Rozzini, renzo.rozzini@poliambulanza.it
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Introduction
- Conclusions
- Article Information
A and B, Histologic findings in an adult man with severe cardiac magnetic resonance imaging abnormalities 67 days after COVID-19 diagnosis. High-sensitivity troponin T level on the day of cardiac magnetic resonance imaging was 16.7 pg/mL. The patient recovered at home from COVID-19 illness with minimal symptoms, which included loss of smell and taste and only mildly increased temperature lasting 2 days. There were no known previous conditions or regular medication use. Histology revealed intracellular edema as enlarged cardiomyocytes with no evidence of interstitial or replacement fibrosis. Panels A and B show immunohistochemical staining, which revealed acute lymphocytic infiltration (lymphocyte function–associated antigen 1 and activated lymphocyte T antigen CD45R0) as well as activated intercellular adhesion molecule 1. C to F, Representative cardiac magnetic resonance images of an adult woman with COVID-19–related perimyocarditis. Panels C and D show significantly raised native T1 and native T2 in myocardial mapping acquisitions. Panels E and F show pericardial effusion and enhancement (yellow arrowheads) and epicardial and intramyocardial enhancement (white arrowheads) in late gadolinium enhancement (LGE) acquisition.
There was a small but significant difference between patients who recovered at home vs in the hospital for native T1 (median [interquartile range], 1119 [1092-1150] ms vs 1141 [1121-1175] ms; P = .008) and high-sensitivity troponin T (4.2 [3.0-5.9] pg/dL vs 6.3 [3.4-7.9] pg/dL; P = .002) but not for native T2 or N-terminal pro–b-type natriuretic peptide. For the coronavirus disease 2019 (COVID-19) home recovery group, dark circles indicate symptomatic illness and light circles indicate asymptomatic illness. Boxes indicate overlays of box-whisker plots, midlines indicate medians, and whiskers indicate the farthest data point not regarded as an outlier (ie, within 1.5-fold the interquartile range).
There was no significant correlation with duration between the positive test for COVID-19 and the measures (native T1: r = 0.07; P = .47; native T2: r = 0.14; P = .15; high-sensitivity troponin T: r = −0.07; P = .50). The trend line indicates the linear regression trend, and the shaded area indicates 95% CIs of the mean.
eFigure. STROBE diagram
- Younger Adults Cautioned to Take COVID-19 Seriously as Demographics Shift JAMA Medical News & Perspectives December 1, 2020 This Medical News article discusses the health implications of severe acute respiratory syndrome coronavirus 2 infection among young and middle-aged adults. Jennifer Abbasi
- JAMA Network Articles of the Year 2020 JAMA Medical News & Perspectives December 15, 2020 This Medical News article discusses the top-viewed research articles published across the JAMA Network in the past year. Jennifer Abbasi
- Researchers Investigate What COVID-19 Does to the Heart JAMA Medical News & Perspectives March 2, 2021 This Medical News article discusses reports of myocardial injury and myocarditis among patients with COVID-19. Jennifer Abbasi
- Coronavirus Disease 2019 (COVID-19) and the Heart JAMA Cardiology Editorial November 1, 2020 Clyde W. Yancy, MD, MSc; Gregg C. Fonarow, MD
- Errors in Statistical Numbers and Data in Study of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From COVID-19 JAMA Cardiology Comment & Response November 1, 2020 Eike Nagel, MD; Valentina O. Puntmann, MD, PhD
- Errors in Statistical Numbers and Data JAMA Cardiology Correction November 1, 2020
- Cardiac Involvement After Recovering From COVID-19 JAMA Cardiology Comment & Response February 1, 2021 Łukasz A. Małek, MD, PhD
- Cardiac Involvement After Recovering From COVID-19 JAMA Cardiology Comment & Response February 1, 2021 Laura Filippetti, MD; Nathalie Pace, MD; Pierre-Yves Marie, MD, PhD
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Puntmann VO , Carerj ML , Wieters I, et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265–1273. doi:10.1001/jamacardio.2020.3557
Manage citations:
© 2024
- Permissions
Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19)
- 1 Institute for Experimental and Translational Cardiovascular Imaging, DZHK Centre for Cardiovascular Imaging, University Hospital Frankfurt, Frankfurt am Main, Germany
- 2 Department of Biomedical Sciences and Morphological and Functional Imaging, University of Messina, Messina, Italy
- 3 Department of Infectious Diseases, University Hospital Frankfurt, Frankfurt am Main, Germany
- 4 Department of Diagnostic and Interventional Radiology, University Hospital Frankfurt, Frankfurt am Main, Germany
- 5 Department of Cardiology, Goethe University Hospital Frankfurt, Frankfurt am Main, Germany
- 6 Department of Hospital Therapy No. 1, I.M. Sechenov First Moscow State Medical University, Moscow, Russia
- 7 Institute for Cardiac Diagnostic and Therapy, Berlin, Germany
- Editorial Coronavirus Disease 2019 (COVID-19) and the Heart Clyde W. Yancy, MD, MSc; Gregg C. Fonarow, MD JAMA Cardiology
- Medical News & Perspectives Younger Adults Cautioned to Take COVID-19 Seriously as Demographics Shift Jennifer Abbasi JAMA
- Medical News & Perspectives JAMA Network Articles of the Year 2020 Jennifer Abbasi JAMA
- Medical News & Perspectives Researchers Investigate What COVID-19 Does to the Heart Jennifer Abbasi JAMA
- Comment & Response Errors in Statistical Numbers and Data in Study of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From COVID-19 Eike Nagel, MD; Valentina O. Puntmann, MD, PhD JAMA Cardiology
- Correction Errors in Statistical Numbers and Data JAMA Cardiology
- Comment & Response Cardiac Involvement After Recovering From COVID-19 Łukasz A. Małek, MD, PhD JAMA Cardiology
- Comment & Response Cardiac Involvement After Recovering From COVID-19 Laura Filippetti, MD; Nathalie Pace, MD; Pierre-Yves Marie, MD, PhD JAMA Cardiology
Question What are the cardiovascular effects in unselected patients with recent coronavirus disease 2019 (COVID-19)?
Findings In this cohort study including 100 patients recently recovered from COVID-19 identified from a COVID-19 test center, cardiac magnetic resonance imaging revealed cardiac involvement in 78 patients (78%) and ongoing myocardial inflammation in 60 patients (60%), which was independent of preexisting conditions, severity and overall course of the acute illness, and the time from the original diagnosis.
Meaning These findings indicate the need for ongoing investigation of the long-term cardiovascular consequences of COVID-19.
Importance Coronavirus disease 2019 (COVID-19) continues to cause considerable morbidity and mortality worldwide. Case reports of hospitalized patients suggest that COVID-19 prominently affects the cardiovascular system, but the overall impact remains unknown.
Objective To evaluate the presence of myocardial injury in unselected patients recently recovered from COVID-19 illness.
Design, Setting, and Participants In this prospective observational cohort study, 100 patients recently recovered from COVID-19 illness were identified from the University Hospital Frankfurt COVID-19 Registry between April and June 2020.
Exposure Recent recovery from severe acute respiratory syndrome coronavirus 2 infection, as determined by reverse transcription–polymerase chain reaction on swab test of the upper respiratory tract.
Main Outcomes and Measures Demographic characteristics, cardiac blood markers, and cardiovascular magnetic resonance (CMR) imaging were obtained. Comparisons were made with age-matched and sex-matched control groups of healthy volunteers (n = 50) and risk factor–matched patients (n = 57).
Results Of the 100 included patients, 53 (53%) were male, and the mean (SD) age was 49 (14) years. The median (IQR) time interval between COVID-19 diagnosis and CMR was 71 (64-92) days. Of the 100 patients recently recovered from COVID-19, 67 (67%) recovered at home, while 33 (33%) required hospitalization. At the time of CMR, high-sensitivity troponin T (hsTnT) was detectable (greater than 3 pg/mL) in 71 patients recently recovered from COVID-19 (71%) and significantly elevated (greater than 13.9 pg/mL) in 5 patients (5%). Compared with healthy controls and risk factor–matched controls, patients recently recovered from COVID-19 had lower left ventricular ejection fraction, higher left ventricle volumes, and raised native T1 and T2. A total of 78 patients recently recovered from COVID-19 (78%) had abnormal CMR findings, including raised myocardial native T1 (n = 73), raised myocardial native T2 (n = 60), myocardial late gadolinium enhancement (n = 32), or pericardial enhancement (n = 22). There was a small but significant difference between patients who recovered at home vs in the hospital for native T1 mapping (median [IQR], 1119 [1092-1150] ms vs 1141 [1121-1175] ms; P = .008) and hsTnT (4.2 [3.0-5.9] pg/dL vs 6.3 [3.4-7.9] pg/dL; P = .002) but not for native T2 mapping. None of these measures were correlated with time from COVID-19 diagnosis (native T1: r = 0.07; P = .47; native T2: r = 0.14; P = .15; hsTnT: r = −0.07; P = .50). High-sensitivity troponin T was significantly correlated with native T1 mapping ( r = 0.33; P < .001) and native T2 mapping ( r = 0.18; P = .01). Endomyocardial biopsy in patients with severe findings revealed active lymphocytic inflammation. Native T1 and T2 were the measures with the best discriminatory ability to detect COVID-19–related myocardial pathology.
Conclusions and Relevance In this study of a cohort of German patients recently recovered from COVID-19 infection, CMR revealed cardiac involvement in 78 patients (78%) and ongoing myocardial inflammation in 60 patients (60%), independent of preexisting conditions, severity and overall course of the acute illness, and time from the original diagnosis. These findings indicate the need for ongoing investigation of the long-term cardiovascular consequences of COVID-19.
The global pandemic of coronavirus disease 2019 (COVID-19) continues to cause considerable morbidity and mortality worldwide. 1 Thus far, the main emphasis of the research communication has been on acute respiratory complications, especially in critically ill patients. A number of case reports and small series suggested that COVID-19 prominently affects the cardiovascular system by exacerbating heart failure in patients with preexisting cardiac conditions 1 - 3 and troponin elevation in critically ill patients. 4 Fulminant myocarditis was suspected in 7% of patients with lethal outcome. 5 Quiz Ref ID The proposed pathophysiological mechanisms of cardiac injury include inflammatory plaque rupture, stent thrombosis, cardiac stress due to high cardiac output, and infection via the angiotensin-converting enzyme 2 receptors causing systemic endothelitis. 6 , 7 A small number of autopsy cases suggest infiltration by interstitial mononuclear inflammatory cells, 8 suggesting myocardial inflammation as the underlying mechanism, and some severe cases of myocarditis have been reported. 3 , 9 In a small study of recovered patients with ongoing cardiac symptoms, cardiovascular magnetic resonance (CMR) imaging revealed cardiac involvement in 58% of patients consisting of myocardial edema and scar by late gadolinium enhancement (LGE). 10 There remains poor insight into the cardiovascular sequelae in unselected patients, including those with no preexisting conditions, who were not hospitalized, or had no or only mild symptoms. To better understand the prevalence, extent, and type of cardiovascular sequelae, we proactively examined patients with a documented recent COVID-19 infection using serological markers of cardiac injury and highly standardized in-depth imaging with CMR.
This is a prospective observational cohort study of 100 patients diagnosed with severe acute respiratory syndrome coronavirus 2 by reverse transcription–polymerase chain reaction on swab test of the upper respiratory tract who fulfilled inclusion criteria for this CMR investigation. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guideline (eFigure in the Supplement ). Participants were identified from the University Hospital Frankfurt COVID-19 Registry via the Department of Infectious Diseases and the Institute for Experimental and Translational Cardiovascular Imaging, Hesse, Germany, and were recruited between April and June 2020. All participants were considered eligible after a minimum of 2 weeks from the original diagnosis if they had resolution of respiratory symptoms and negative results on a swab test at the end of the isolation period. Patients recently recovered from COVID-19 referred for a clinical CMR due to active cardiac symptoms were not included in this analysis. Exclusion criteria were unwillingness to participate or provide informed consent or absolute contraindications for a contrast-enhanced magnetic resonance study. The study protocol was approved by the institutional ethics committee of the University Hospital Frankfurt (Improving Cardiovascular Risk Stratification Using T1 Mapping in General Population study 11 ). Comparisons were made with age-matched and sex-matched control groups of normotensive adults who were taking no cardiac medications, had normal cardiac volumes and function, and had no evidence of scar (healthy controls; n = 50). Comparisons were also made with risk factor–matched patients (n = 57) for age, sex, hypertension, diabetes, smoking, known coronary artery disease, or comorbidities, sourced from the International T1 Multicenter Outcome Study. 12 All procedures were performed in concordance with the Declaration of Helsinki and International Conference on Harmonization of Good Clinical Practice. All patients provided written informed consent.
Clinical demographic characteristics, medications, blood test results, endomyocardial biopsy results, and imaging measurements on the day of CMR examination were recorded using REDCap electronic data capture tools. 13 All participants underwent venous blood sampling immediately prior to the CMR study. Blood samples were processed using standardized commercially available test kits for analysis of high-sensitivity troponin T (hsTnT) and N-terminal pro–b-type natriuretic peptide (Elecsys 2010; Roche). The local laboratory cutoff value for detectable hsTnT was greater than 3 pg/mL, whereas values above the 99th percentile (13.9 pg/mL) counted as a significant increase. 14
Cardiac magnetic resonance imaging was performed on clinical 3-T scanners (Magnetom Skyra; Siemens Healthineers), using standardized and unified imaging protocols (Goethe CVI Approaches). Conventional sequences were used for acquisition of cardiac function, volumes, mass, and scar imaging. Myocardial T1 and T2 mapping were acquired in a single midventricular short-axis slice using a validated variant of a modified Look-Locker Imaging sequence (Goethe CVI MOLLI), whereas for T2 mapping, a validated sequence for measurement of myocardial edema was used (T2-FLASH). 15 - 17 Due to the proven sensitivity of Goethe CVI MOLLI for abnormal myocardium and evidence of superior diagnostic and prognostic performance, 18 postcontrast T1 mapping was not part of the standardized protocol. Late gadolinium enhancement imaging was performed approximately 10 minutes after administration of 0.1 mmol/kg of body weight of gadobutrol (Gadovist; Bayer).
Cardiac volumes, function, and mass were measured using an artificial intelligence–based automated contour detection with manual correction if required (SuiteHeart; Neosoft). Myocardial T1 and T2 relaxation times were measured conservatively within the septal myocardium of the midventricular SAX slice using motion-corrected images, as per internal standardized operating procedures 19 and with quality control by the core laboratory staff, blinded to the underlying clinical information using pseudonymized data sets. Areas of LGE were excluded from the measurements to avoid confounding diffuse fibrosis with replacement scar. Interpretation of LGE images followed standardized postprocessing recommendations; myocardial LGE was visually defined by 2 observers based on the presence and predominant pattern as ischemic or nonischemic. 20 Pericardial LGE was considered present when enhancement involved both pericardial layers, irrespective of the presence of pericardial effusion. The distinction from the pericardial fat was ascertained using T1 mapping images.
Normality of distributions were tested using Shapiro-Wilk test. Categorical data are presented as counts (percentages) and continuous variables as means (standard deviation) and medians (interquartile ranges [IQRs]). Comparisons between patients’ groups were conducted using one-way analysis of variance for normally distributed parameters and Kruskal-Wallis for nonnormally distributed data with post hoc tests for significance between groups. Fischer exact and χ 2 tests were used for proportions. Receiver operating characteristic curve analyses were used to examine discrimination (expressed as area under the receiver operating characteristic curve) between patients recently recovered from COVID-19 and control groups. Associations were explored using Pearson or Spearman correlation analyses, as appropriate for the type of data. Abnormal native T1 and T2 values were defined as greater than 1105 ms and greater than 37.4 ms, respectively, based on previously derived sequence-specific cutoffs of 2 SDs above the respective means in a healthy population. 18 , 21 , 22 Significant abnormalities were defined as greater than 1136 ms for T1 and greater than 40 ms for T2, using 4 SDs above those means. Classification into abnormal and significantly abnormal served to distinguish the patients with a potential high risk of adverse events. 23 , 24 All tests were 2-tailed, and P values less than .05 were considered statistically significant. Analysis was performed using SPSS software version 25.0 (IBM) and RStudio version 1.2.5001 (RStudio).
An unselected cohort of 100 patients who recently recovered from COVID-19 infection were included, of which 53 (53%) were male, and the mean (SD) age was 49 (14) years. Baseline characteristics are provided in Table 1 . Most patients recovered at home (n = 67), with severity of the acute COVID-19 illness ranging from asymptomatic (n = 18) to minor to moderate symptoms (n = 49). A total of 33 severely unwell patients (33%) required hospitalization. In this group, 2 patients (2%) underwent mechanical ventilation, and 17 (17%) underwent noninvasive ventilation with positive airway pressure. Oxygen supplementation was required in 28 patients. In addition to respiratory support, patients received antiviral (n = 1), antibiotic (n = 15), and steroid (n = 8) therapy. Treatment with hydrochloroquine was initiated in a single patient but discontinued within days due to severe leukopenia. During hospitalization, a significant rise (greater than 13.9 pg/mL) in hsTnT values was documented in 15 patients (15%). Preexisting cardiovascular conditions included hypertension, diabetes, and known coronary artery disease but no previously known heart failure or cardiomyopathy. Other significant conditions included asthma (n = 10) and chronic obstructive pulmonary disease (n = 11). All preexisting conditions were similarly distributed between patients who recovered at home vs hospitalized.
Patient characteristics and the results of the imaging parameters and blood markers on the day of CMR are shown in Table 1 . Body mass index, hypertension, diabetes, hypercholesterolemia, known coronary artery disease, and chronic obstructive pulmonary disease or asthma were associated with COVID-19 diagnosis compared with the healthy controls, but there were no differences between those with COVID-19 and the risk factor–matched patients. The median (IQR) duration between the positive COVID-19 testing and the CMR examination was 71 (64-92) days. On the day of CMR examination, direct questioning about symptoms revealed atypical chest pain (n = 17) and palpitations (n = 20). Compared with pre–COVID-19 status, 36 patients (36%) reported ongoing shortness of breath and general exhaustion, of whom 25 noted symptoms during less-than-ordinary daily activities, such as a household chore. Only 4 of these 25 patients (16%) were previously hospitalized. No patient reported typical angina symptoms or a recent syncope. High-sensitivity troponin T values were detectable (greater than 3 pg/mL) in 71 patients recently recovered from COVID-19 (71%) and significantly elevated (greater than 13.9 pg/mL) in 5 (5%). Compared with healthy controls and risk factor–matched controls, patients recently recovered from COVID-19 had lower left ventricular and right ventricular ejection fraction, higher left ventricular volume, and raised native T1 and T2 measures. A total of 78 patients recently recovered from COVID-19 had abnormal CMR findings, including at least one of the following: raised myocardial native T1 (n = 73), 21 raised myocardial native T2 (n = 60), 22 myocardial LGE (n = 32), or pericardial enhancement (n = 22) ( Figure 1 ). A total of 12 patients recently recovered from COVID-19 had an ischemic-type pattern of myocardial LGE. Three patients with severe abnormalities (significantly higher hsTnT, native T1, and native T2 measures, LGE, and left ventricular ejection fraction less than 50%) were referred to endomyocardial biopsy, revealing active lymphocytic inflammation with no evidence of any viral genome. Figure 2 and Figure 3 show the findings for native T1 and T2 mapping and hsTnT values based on the COVID-19 illness presentation (home-based recovery vs hospitalization) and in relation to the time from the original COVID-19 diagnosis. There was a significant difference between patients who recovered at home vs in the hospital for native T1 measures (median [IQR], 1119 [1092-1150] ms vs 1141 [1121-1175] ms; P = .008) and hsTnT (4.2 [3.0-5.9] pg/dL vs 6.3 [3.4-7.9] pg/dL; P = .002) but not for native T2 or N-terminal pro–b-type natriuretic peptide. There was no significant correlation with duration between the positive test for COVID-19 and the measures (native T1: r = 0.07; P = .47; native T2: r = 0.14; P = .15; hsTnT: r = −0.07; P = .50) ( Figure 3 ). High-sensitivity troponin T was significantly correlated with native T1 ( r = 0.33; P < .001), native T2 ( r = 0.18; P = .01), and left ventricle mass ( r = 0.25; P = .01). There was also a cross-correlation between native T1 and T2 ( r = 0.40; P < .001). The associations of hsTnT with mapping measures remained significant despite controlling for the presence of comorbidities (overall or separately) or treatment received during the COVID-19 illness.
Table 2 shows the results of the receiver operating characteristic curve analyses for discrimination between the control groups and patients recently recovered from COVID-19 using imaging measures and blood biomarkers. Native T1 and T2 were the measures with the best discriminatory ability to detect COVID-19–related myocardial pathology.
A total of 78 patients who recovered from COVID-19 infection (78%) had cardiovascular involvement as detected by standardized CMR, irrespective of preexisting conditions, the severity and overall course of the COVID-19 presentation, the time from the original diagnosis, or the presence of cardiac symptoms. Quiz Ref ID The most prevalent abnormality was myocardial inflammation (defined as abnormal native T1 and T2 measures), detected in 60 patients recently recovered from COVID-19 (60%), followed by regional scar and pericardial enhancement. Findings on classic parameters, such as volumes and ejection fractions, were mildly abnormal. Myocardial measures, native T1 measures, and native T2 measures provided the best discriminatory value against healthy controls and risk factor–matched controls for exclusion of any myocardial disease or confirmation of COVID-19–related involvement, respectively.
To our knowledge, this is the first prospective report on a cohort of unselected patients with a recent COVID-19 infection identified from a local testing center who voluntarily underwent evaluation for cardiac involvement with CMR. The results of our study provide important insights into the prevalence of cardiovascular involvement in the early convalescent stage. Our findings demonstrate that participants with a relative paucity of preexisting cardiovascular condition and with mostly home-based recovery had frequent cardiac inflammatory involvement, which was similar to the hospitalized subgroup with regards to severity and extent. Our observations are concordant with early case reports in hospitalized patients showing a frequent presence of LGE, 3 , 25 diffuse inflammatory involvement, 10 , 26 and significant rise of troponin T levels. 4 Unlike these previous studies, our findings reveal that significant cardiac involvement occurs independently of the severity of original presentation and persists beyond the period of acute presentation, with no significant trend toward reduction of imaging or serological findings during the recovery period. Our findings may provide an indication of potentially considerable burden of inflammatory disease in large and growing parts of the population and urgently require confirmation in a larger cohort. Although the long-term health effects of these findings cannot yet be determined, several of the abnormalities described have been previously related to worse outcome in inflammatory cardiomyopathies. 27 - 29 Quiz Ref ID Most imaging findings point toward ongoing perimyocarditis after COVID-19 infection. This is further confirmed by the cross-correlation between the T1 and T2 measures and hsTnT as well as histological verification of inflammatory changes in more severe cases.
Each of the abnormal imaging parameters can be linked to an underlying pathophysiological process and worse outcome. The peri-epicardial LGE in the areas with increased contrast agent uptake represents regional damage due to myocardial inflammation. Especially in combination with pericardial effusion, these observations can be attributed to fibrosis and/or edema due to an ongoing active pericarditis. Nonischemic patterns of myocardial LGE are mainly observed in patients with acute or healed myocarditis and have been strongly linked to reduced outcome. 23 , 24 , 30 , 31 Quiz Ref ID Increased native T1 measures represent diffuse myocardial fibrosis and/or edema, whereas native T2 is specific for edema. 18 Thus, patients with increased native T1 and T2 measures have an active inflammatory process, while those with increased native T1 and normal native T2 measures have healed with some residual diffuse myocardial damage (although native T1 measures can be increased in a variety of pathophysiology, as many different pathways lead to diffuse fibrosis, including hypertension or genetic cardiomyopathies). Yet the combination with histological findings as well as the increase relative to age-matched, sex-matched, and risk factor–matched controls makes a COVID-19–related inflammatory process as the underlying pathophysiology highly likely. Increased native T1 measures have been strongly related to worse outcome in patients with ischemic heart disease and nonischemic cardiomyopathies. 23 , 24 , 30 , 31 Increased troponin T and C-reactive protein levels similarly indicate inflammatory and partially ongoing myocardial damage and have been related to worse outcome, even if only minimally increased. 32 Quiz Ref ID While left and right ventricular ejection fraction were significantly reduced, there was a large overlap between patients recently recovered from COVID-19 and both control groups, demonstrating that volumes and function are inferior markers of disease detection compared with direct tissue characterization with mapping measures. Importantly, volumes and function have consistently been demonstrated to be less relevant for predicting outcome than LGE and mapping, highlighting the relevance of the more sensitive markers of early cardiac damage. 23 , 24 , 30 , 31
Our study has limitations. The findings are not validated for the use in pediatric patients 18 years and younger. They also do not represent patients during acute COVID-19 infection or those who are completely asymptomatic with COVID-19. Several patients within our cohort had new or persistent symptoms, thus increasing the likelihood of positive CMR findings. Outcome data remain outstanding. The imaging sequences used in this study have been well validated, standardized, and locked for the use in multicenter settings. The use of other imaging protocols, sequence parameters, or postprocessing approaches may yield different results.
Taken together, we demonstrate cardiac involvement in 78 patients (78%) and ongoing myocardial inflammation in 60 patients (60%) with recent COVID-19 illness, independent of preexisting conditions, severity and overall course of the acute illness, and the time from the original diagnosis. These findings indicate the need for ongoing investigation of the long-term cardiovascular consequences of COVID-19.
Accepted for Publication: July 6, 2020.
Published Online: July 27, 2020. doi:10.1001/jamacardio.2020.3557
Correction: This article was corrected on August 25, 2020, to fix pervasive errors in statistical numbers and data in the Abstract, Methods and Results sections, Tables, and Figures 1 and 2.
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2020 Puntmann VO et al. JAMA Cardiology .
Corresponding Author: Eike Nagel, MD, Institute for Experimental and Translational Cardiovascular Imaging, DZHK Centre for Cardiovascular Imaging, University Hospital Frankfurt, Theodor-Stern-Kai 7, Frankfurt am Main 60590, Germany ( [email protected] ).
Author Contributions: Drs Puntmann and Nagel had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design : Puntmann, Shchendrygina, Vasa-Nicotera, Zeiher, Nagel.
Acquisition, analysis, or interpretation of data : Puntmann, Carerj, Wieters, Fahim, Arendt, Hoffmann, Escher, Vehreschild, Nagel.
Drafting of the manuscript : Puntmann, Shchendrygina, Nagel.
Critical revision of the manuscript for important intellectual content : Puntmann, Carerj, Wieters, Fahim, Arendt, Hoffmann, Escher, Vasa-Nicotera, Zeiher, Vehreschild, Nagel.
Statistical analysis : Puntmann, Nagel.
Obtained funding : Puntmann, Zeiher, Nagel.
Administrative, technical, or material support : Puntmann, Carerj, Wieters, Fahim, Arendt, Escher, Vehreschild, Nagel.
Study supervision : Puntmann, Wieters, Arendt, Vasa-Nicotera, Vehreschild, Nagel.
Conflict of Interest Disclosures: Dr Escher has received personal fees from Institut Kardiale Diagnostik und Therapie outside the submitted work. Dr Zeiher has received grants from the German Centre for Cardiovascular Research during the conduct of the study and personal fees from Sanofi, Amgen, Boehringer Ingelheim, and Novo Nordisk outside the submitted work. Dr Vehreschild has received grants from BioNTech and Takeda outside the submitted work. Dr Nagel has received grants from Bayer, the German Ministry for Education and Research, Deutsche Herzstiftung e.V., Neosoft Technologies, and Cardio-Pulmonary Institute and personal fees from Bayer. No other disclosures were reported.
Funding/Support: Drs Puntmann, Arendt, Escher, Vasa-Nicotera, Zeiher, and Nagel were supported by grants from the German Ministry of Education and Research via the German Centre for Cardiovascular Research (DZHK) partner site RheinMain, Deutsche Herzstiftung e.V., Bayer, and Cardio-Pulmonary Institute
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We acknowledge the dedicated support of clinical research support staff of the Institute of Experimental and Translational Cardiovascular Imaging, including Tammy Wolf, Thourier Azdad, Franziska Weis, Deniz Desik, BA, and Layla Laghchioua, MSc, as well as of the Department of Infectious Diseases, University Hospital Frankfurt. We are very grateful to our colleagues of the Department of Anaesthesiology, Intensive Care Medicine and Pain Therapy, University Hospital Frankfurt, Frankfurt am Main, Germany, for treating all critically ill patients with COVID-19. Contributors were not compensated for their work.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Aging Neurosci
Dementia as Risk Factor for Severe Coronavirus Disease 2019: A Case-Control Study
Mariantonietta pisaturo.
1 Infectious Diseases Unit, Department of Mental Health and Public Medicine, University of Campania Luigi Vanvitelli, Naples, Italy
Federica Calò
Antonio russo, clarissa camaioni, agnese giaccone.
2 Infectious Diseases Unit, Federico II University, Naples, Italy
Biagio Pinchera
Ivan gentile, filomena simeone.
3 Infectious Disease Unit, AORN Caserta, Caserta, Italy
Angelo Iodice
Paolo maggi, nicola coppola, associated data.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The aim of the present study was to investigate the outcome of patients with SARS-CoV-2 infection and dementia.
Patients and Methods
In a multicenter, observational, 1:2 matched case-control study all 23 patients with a history of dementia, hospitalized with a diagnosis of SARS-CoV-2 infection from February 28th 2020 to January 31st 2021 were enrolled. For each Case, 2 patients without dementia observed in the same period study, pair matched for gender, age (±5 years), PaO 2 /FiO 2 (P/F) ratio at admission (<200, or >200), number of comorbidities (±1; excluding dementia) were chosen (Control group).
The majority of patients were males (60.9% of Cases and Controls) and very elderly [median age 82 years (IQR: 75.5–85) in the Cases and 80 (IQR: 75.5–83.75) in the Controls]. The prevalence of co-pathologies was very high: all the Cases and 43 (93.5%) Controls showed a Charlson comorbidity index of at least 2. During hospitalization the patients in the Case group less frequently had a moderate disease of COVID-19 (35 vs. 67.4%, p = 0.02), more frequently a severe disease (48 vs. 22%, p = 0.03) and more frequently died (48 vs. 22%, p = 0.03). Moreover, during coronavirus disease 2019 (COVID-19), 14 (60.8%) patients in the Case group and 1 (2.1%; p < 0.000) in the Control group showed signs and symptoms of delirium.
Patients with dementia are vulnerable and have an increased risk of a severe disease and death when infected with COVID-19.
Introduction
The novel coronavirus SARS-CoV-2, first identified in China on 31 December 2019, has rapidly spread around the world causing a global pandemic with over two million deaths.
The clinical presentation of the coronavirus disease 2019 (COVID-19) is variable, ranging from an asymptomatic infection to mild and more severe progressive respiratory failure ( Macera et al., 2020 ; Cascella et al., 2021 ). Several risk factors for poor outcomes and mortality have been identified, such as age, hypertension, obesity, diabetes, and cancer ( Gautret et al., 2020 ; Marfella et al., 2020 ; Onder et al., 2020 ; Zhou et al., 2020 ; Fedeli et al., 2021 ; Monari et al., 2021 ).
Older adults are particularly susceptible to COVID-19 infection due to the presence of multiple comorbidities and chronic diseases ( Wynants et al., 2020 ). Moreover, the cognitive decline due to dementia, such as Alzheimer’s disease, exposes elderly subjects to a greater risk of becoming infected with COVID-19 ( Korczyn, 2020 ); in fact, the poor adherence to infection control measures (e.g., hand washing, social distancing, and wearing masks) and their close physical contact with caregivers are risk factors for SARS-CoV-2 infection ( Canevelli et al., 2020a ). Furthermore, they often show an atypical clinical presentation ( Bianchetti et al., 2020 ; Isaia et al., 2020 ; Ward et al., 2020 ) that may delay diagnosis and appropriate treatment and consequently impact their prognosis and survival ( Alonso-Lana et al., 2020 ). Moreover, in the case of respiratory failure, the compliance with oxygen (O 2 ) treatment with non-invasive or invasive ventilation is very low, with a possible poor prognosis.
Few data have been published on the impact of SARS-CoV-2 infection in patients with dementia ( Canevelli et al., 2020b ; Caratozzolo et al., 2020 ; Burns et al., 2021 ; Tsapanou et al., 2021 ; Wang et al., 2021 ; West et al., 2021 ). Although results are controversial, a worse outcome has been described among these patients ( Hariyanto et al., 2020 ; Liu et al., 2020 ; McMichael et al., 2020 ). However, being older the patients with dementia had multiple comorbidities, so the nature of the association between dementia and poor prognosis of COVID-19 without the evaluation of age and co-pathologies associated has not yet been clearly evaluated.
The aim of the present pair-matched case-control study was to investigate the outcome of patients with SARS-CoV-2 infection and dementia, compared with patients without dementia but of the same age, presence of co-morbidities and clinical presentation at hospitalization, in order to assess its impact on the mortality and severity of the disease.
Study Design and Setting
We performed a multicenter, observational, 1:2 matched case-control study involving three COVID-19 Units in two cities in the Campania region in southern Italy, Naples and Caserta.
The patients enrolled were adults (≥18 years), hospitalized with a diagnosis of SARS-CoV-2 infection confirmed by a positive reverse transcriptase-polymerase chain reaction (RT-PCR) on a naso-oropharyngeal swab. Viral RNA was extracted by naso-oropharyngeal swab with QIAamp Viral RNA Kits (Qiagen GmbH, Hilden, Germany); the detection of SARS-CoV-2 was performed by RT-PCR test using Bosphore ® Novel Coronavirus (Anatolia Diagnostics and Biotechnology Products Inc., İstanbul, Turkey) Detection Kit V3, by primers designed on three viral regions: E, ORF1ab, and N regions.
The study period was from February 28th 2020 to January 31st 2021. All the patients with a diagnosis of dementia observed in the study period in one of the three centers participating were enrolled as Cases (Case group). For each Case, two patients without dementia observed by the same centers in the same study period, pair matched for gender, age (±5 years), PaO 2/ FiO 2 (P/F) ratio at admission (<200, or >200), number of comorbidities (±1; excluding dementia) were chosen (Control group).
All demographic, clinical and laboratory data of both Cases and Controls were collected in a database. From this database we extrapolated the data.
The study was approved by the Ethics Committee of the University of Campania L. Vanvitelli, Naples ( n °10877/2020). All procedures performed in this study were in accordance with the ethics standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethics standards. Informed consent was obtained from all participants included in the study.
Variables and Definitions
The microbiological diagnosis of SARS-CoV-2 infection was defined as a positive RT-PCR test on a naso-oropharyngeal swab.
Dementia was diagnosed according to the clinical history of the patients.
The P/F ratio was considered as the arterial partial pressure of oxygen (aPP O 2 ) investigated through hemogas analysis divided by the fraction of inspired oxygen concentration (FiO 2 ) at the time of hospital admission.
The presence of underlying chronic diseases were defined according to the Charlson age-comorbidity index ( Charlson et al., 1987 ), while medical conditions at risk of clinical deterioration were defined through a modified Early Warning Score (MEWS) ( Subbe et al., 2001 ).
We defined patients with a mild, moderate or severe disease according to the clinical presentation of COVID-19. Precisely, patients with a mild infection did not need O 2 therapy and/or had a MEWS score below 3 points. Patients with a moderate infection required low flow O 2 therapy or non-invasive O 2 therapy and/or had a MEWS score equal to or above 3 points (≥3). Lastly, patients with a severe infection needed management in an intensive care unit (ICU) and/or mechanical ventilation; in this definition we also included patients who died because of respiratory failure, multi-organ failure or septic shock. Patients were followed until SARS-CoV-2-RNA negativity at naso-oropharyngeal swab or discharge from hospital.
Statistical Analysis
For the descriptive analysis, categorical variables were presented as absolute numbers and their relative frequencies. Continuous variables were summarized as mean and standard deviation if normally distributed or as median and interquartile range (IQR) if not normally distributed. We performed a comparison of patients with dementia and without dementia using Pearson chi-square or Fisher exact test for categorical variables and Student’s t - or Mann-Whitney tests for continuous variables.
A p -value below 0.05 was considered statistically significant. Analyses were performed by STATA.
During the study period, overall 672 patients with SARS-CoV-2 infection were observed in the three centers participating in the study. Of these 672 patients enrolled, 23 had a pre-existing diagnosis of dementia before the development of COVID-19 and were included in the Case group. Among the 649 patients observed without dementia, 46 were chosen as the Control group.
Of the 23 patients in the Case group, nine had a history of senile dementia, six of vascular dementia, five of Alzheimer’s disease, one of frontotemporal dementia, one of Parkinson dementia, and one of human immunodeficiency virus (HIV)-related dementia. Twelve patients in the Case group were in chronic treatment ( Table 1 ): memantine in two, dopaminergic drug in two, benzodiazepine in one, selective serotonin reuptake inhibitors in one, antiepileptic drug in one, antipsychotic drug and benzodiazepine in one, antipsychotic drug and NMDA receptor antagonist in one, antipsychotic drug and gabapentin in one, antipsychotic drug and lithium in one; antipsychotic drug and acetylcholinesterase inhibitors in one. Eight patients had a history of delirium before COVID-19 that required pharmacological treatment.
The therapies of the patients in case group.
Table 2 shows the epidemiological and clinical characteristics of the Cases and Controls. There were no statistically significant differences in age, gender, and co-morbidities among COVID-19 patients with and without dementia. The majority of patients were males (60.9% of Cases and Controls) and very elderly [median age 82 years (IQR: 75.5–85) in the Cases and 80 (IQR: 75.5–83.75) in the Controls] ( Table 2 ). The prevalence of co-pathologies was very high: all the Cases and 43 (93.5%) Controls showed a Charlson comorbidity index of at least 2; moreover, the median Charlson comorbidity index was similar in the two groups of patients [median 6 (IQR: 5–7) in Case group vs. 6 (IQR: 4–6) in the Control group] ( Table 2 ). However, the patients in the Control group more frequently showed as underlying chronic diseases arterial hypertension (86.9 vs. 56.8%, p = 0.004).
Demographic and clinical characteristics of the patients according to the presence or absence of dementia.
Table 3 shows the data on the clinical presentation of COVID-19 in the Cases and Controls. No statistically significant differences were found at admission between P0 2 [median 69.5 mmHg (IQR: 56.3–79) in the Cases and 65 (IQR: 59.5–74. 5) in the Controls] and P/F [median 244.5 (IQR: 169.7–320.5) in the Cases and 245 (IQR: 205–290) in the Controls] ( Table 3 ).
Clinical presentation of coronavirus disease 2019 (COVID-19) in case and control groups.
As regards the most serious respiratory support needed during hospitalization, a similar prevalence was found in high flow nasal cannulas (HFNC) [9 (39.1%) vs. 9 (19.6%; p = 0.08] in continuous positive airway pressure (CPAP) [1 (4.3%) vs. 8 (17.4%); p = 0.25] in non-invasive ventilation (NIV) [4 (17.4%) vs. 6 (13%); p = 0.72]. In the Case group, the prevalence of patients needing invasive ventilation was higher [10 (43.5) vs. 10 (21.7); p = 0.06], but with a difference not significant to the statistical analysis ( Table 2 ). However, during hospitalization, with respect to the patients in the Control group, those in the Case group less frequently had a moderate disease of COVID-19 (35 vs. 67.4%, p = 0.02), more frequently a severe disease (48 vs. 22%, p = 0.03) and more frequently died (48 vs. 22%, p = 0.03) ( Table 2 ). Moreover, although no difference between the two group of patients was observed in time from admission to discharge, the patients with dementia had a shorter period between admission and death [median and IQR of 12 (9–21) days vs. 19 (12.5–30) days], a difference without statistical significance ( Table 3 ).
During COVID-19, 14 (60.8%) patients in the Case group and 1 (2.1%; p < 0.000) in the Control group showed signs and symptoms of delirium and required the addition of drugs to control these ( Table 1 ): antipsychotic drug in three, benzodiazepine in seven and both in three patients in the Case group; the only patient in the Control group showing signs of delirium required the addition of an antipsychotic drug and benzodiazepine.
In the Case group, no difference in mortality was observed between the 14 patients with signs and symptoms of delirium during COVID-19 and the nine without (35.7 vs. 66.6%, p = 0.21).
In the present 1:2 case-control study performed in three COVID-19 Units in southern Italy we found that the patients with pre-existing dementia showed a worse prognosis of COVID-19. They more frequently showed a severe clinical outcome and more frequently died than those without dementia, but showed a similar age, number of pre-existing co-pathologies and respiratory failure at admission.
We know that globally more than 50 million people have dementia that has emerged as a pandemic in an aging society ( Fox and Petersen, 2013 ; Alzheimer’s Disease International, 2019 ). Thus, the double hit of dementia and COVID-19 pandemics has raised great concern.
A meta-analysis on 24 studies with 46,391 dementia patients showed that dementia was associated with severe COVID-19 [RR 2.63 (95% CI 1.41–4.90), p = 0.002] and mortality from COVID-19 infection [RR 2.62 (95% CI 2.04–3.36), p < 0.00001] ( Hariyanto et al., 2020 ). However, the data available in the literature on this topic cannot be considered conclusive. In fact, since the patients with dementia were very elderly, they had a lot of co-pathologies. Thus, the impact of age and the presence of co-pathologies in the clinical presentation of patients with dementia have not been clearly analyzed. For example, Bianchetti et al. (2020) showed that the mortality rate was higher (62.2%) among 82 patients suffering from dementia than that (26.2%) observed in 545 without. Instead, the 82 patients with dementia were older (mean age of 82.6 ± standard deviation 5.3) than the 545 without (68.9 ± 12.7), with no analysis of the presence of co-pathologies.
Interestingly, the majority (69.5%) of patients with dementia in the present study during COVID-19 showed symptoms that required the addition of antipsychotic or benzodiazepine drugs. Thus, as already suggested by other authors ( Kales et al., 2019 ), the SARS-CoV-2 patients with dementia who need hospital care represent a challenge for COVID-19 units and an increase in stress to manage non-compliant patients and with behavioral problems. In fact, delirium caused by hypoxia, a prominent clinical feature of COVID-19, can complicate the presentation of dementia ( Marcantonio, 2017 ) and increase the suffering of people with dementia hospitalized for COVID-19, as well as the cost of medical care and the need for dementia support.
Although without a difference in the statistical significance probably due to the small number of patients enrolled, it seems interesting that the patients with dementia had a shorter period between admission and death compared with those without. These data are in agreement with the observation of the Italian Institute of Health: considering the data on the 2,621 deaths due to COVID-19 in Italy, the patients with dementia showed a more rapid clinical worsening compared with individuals with intact cognition ( Canevelli et al., 2020a ).
The factors involved in the association between dementia and worse prognosis of COVID-19 could be many. Of course, the patient’s lack of cooperation in performing the main therapy for SARS-CoV-2 pneumonia could be one of the reasons for the negative outcome of the disease in these patients. Then, the neurotropism of the virus and the presence of angiotensin-converting enzyme 2 (ACE-2) receptor, the cellular receptor for the SARS-CoV-2, on the brain and glial tissue makes the central nervous system a potential target for the virus ( Yan et al., 2020 ; Barillari et al., 2021 ). The virus could infect the brain also through a disrupted blood-brain barrier that was often compromised in the aging brain and in neurodegenerative diseases, such as Alzheimer’s disease ( Hascup and Hascup, 2020 ). In view of this, it is likely that neurological manifestations caused by the virus worsen the already damaged neurological function of patients with dementia, making the prognosis worse. Furthermore, some studies had shown how the ACE receptor polymorphisms could influence the prognosis of patients with COVID 19 and are associated with Alzheimer’s disease ( Cao et al., 2020 ; Delanghe et al., 2020 ; Gómez et al., 2020 ).
Finally, severe COVID-19 outcomes are often associated with a “cytokine storm” ( Castelli et al., 2020 ); so elderly individuals affected by dementia could be at a higher risk due to a higher baseline of inflammation that steadily increases with age ( Rea et al., 2018 ; Naughton et al., 2020 ).
Our study shows several limits; first, the retrospective nature of the study; second, we evaluated only in-hospital mortality; third, the number of patients enrolled with dementia was low. The strengths of the study are the multicenter and case-control nature of the design, which makes it possible to look at multiple risk factors at the same time, especially age and the presence of co-pathologies.
In conclusion, patients with dementia are vulnerable and have an increased risk of serious morbidity, admission to ICUs, and death when infected with COVID-19. Thus, it is necessary to carry out an early diagnosis of SARS-CoV-2 infection in this population and to implement all measures to ensure proper management of the disease at home, with the use of telemedicine and digital technological devices, such as smart phones, which can be very useful in remote monitoring and care. Ideally, the use of monoclonal antibodies can be considered in these patients in an early phase to reduce the need of hospitalization and progression of the disease. In addition, it is necessary to establish a multidisciplinary team with an infectious disease specialist, a psychiatrist, a psychologist, social workers, nurses and volunteers to manage this difficult-to-treat-population. Finally, implementing the anti-COVID-19 vaccination in these patients is a priority.
Data Availability Statement
Ethics statement.
The studies involving human participants were reviewed and approved by AOU Vanvitelli. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MP, FC, and NC were involved in study concept and design, and drafting of the manuscript. PM and IG were involved in critical revision of the manuscript for important intellectual content. CC, AR, AG, and BP were involved in acquisition of data, analysis and interpretation of data, and in critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. POR Campania FESR 2014-2020-Avviso per l’acquisizione di manifestazioni di interesse per la realizzazione di servizi di ricerca e sviluppo per la lotta contro il Covid-19 (DGR n. 140 del 17 marzo 2020), Project: “Identificazione dei Fattori Demografici, Clinici, Virologici, Genetici, Immunologici e Sierologici Associati ad Outcome Sfavorevole Nei Soggetti Con COVID-19”, Regione Campania, Italy.
- Alonso-Lana S., Marquié M., Ruiz A., Boada M. (2020). Cognitive and neuropsychiatric manifestations of COVID-19 and effects on elderly individuals with dementia. Front. Aging Neurosci. 12 : 588872 . 10.3389/fnagi.2020.588872 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Alzheimer’s Disease International. (2019). World Alzheimer’s Report 2019: Attitudes to Dementia. Available online at: https://www.alz.co.uk/research/WorldAlzheimerReport2019.pdf (accessed March 15, 2020). [ Google Scholar ]
- Barillari M. R., Bastiani L., Lechien J. R., Mannelli G., Molteni G., Cantarella G., et al. (2021). A structural equation model to examine the clinical features of mild-to-moderate COVID-19: a multicenter Italian study. J. Med. Virol. 93 983–994. 10.1002/jmv.26354 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bianchetti A., Rozzini R., Guerini F., Boffelli S., Ranieri P., Minelli G., et al. (2020). Clinical presentation of COVID19 in dementia patients. J. Nutr. Health Aging. 24 560–562. 10.1007/s12603-020-1389-1 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Burns A., Lobo A., Olde Rikkert M., Robert P., Sartorius N., Semrau M., et al. (2021). COVID-19 and dementia: experience from six European countries. Int. J. Geriatr. Psychiatry. 36 943–949. 10.1002/gps.5497 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Canevelli M., Palmieri L., Raparelli V., Lo Noce C., Colaizzo E., Tiple D., et al. (2020a). Prevalence and clinical correlates of dementia among COVID-19-related deaths in Italy. Alzheimers Dement (Amst). 12 : e12114 . 10.1002/dad2.12114 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Canevelli M., Valletta M., Toccaceli Blasi M., Remoli G., Sarti G., Nuti F., et al. (2020b). Facing dementia during the COVID-19 outbreak. J. Am. Geriatr. Soc. 68 1673–1676. 10.1111/jgs.16644 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X., et al. (2020). Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 6 : 11 . 10.1038/s41421-020-0147-1 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Caratozzolo S., Zucchelli A., Turla M., Cotelli M. S., Fascendini S., Zanni M., et al. (2020). The impact of COVID-19 on health status of home-dwelling elderly patients with dementia in East Lombardy, Italy: results from COVIDEM network. Aging Clin. Exp. Res. 32 2133–2140. 10.1007/s40520-020-01676-z [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cascella M., Rajnik M., Aleem A., Dulebohn S. C., Di Napoli R. (2021). “ Features, evaluation, and treatment of coronavirus (COVID-19) ,” in StatPearls [Internet]. (Treasure Island: StatPearls Publishing; ). [ PubMed ] [ Google Scholar ]
- Castelli V., Cimini A., Ferri C. (2020). Cytokine storm in COVID-19: “When you come out of the storm, you won’t be the same person who walked in”. Front. Immunol. 11 : 2132 . 10.3389/fimmu.2020.02132 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Charlson M. E., Pompei P., Ales K. L., MacKenzie C. R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40 373–383. 10.1016/0021-9681(87)90171-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Delanghe J. R., Speeckaert M. M., De Buyzere M. L. (2020). COVID-19 infections are also affected by human ACE1 D/I polymorphism. Clin. Chem. Laboratory Med. (CCLM) 58 1125–1126. 10.1515/cclm-2020-0425 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fedeli U., Schievano E., Avossa F., Pitter G., Barbiellini Amidei C., Grande E., et al. (2021). Different approaches to the analysis of causes of death during the COVID-19 epidemic. Eur. Rev. Med. Pharmacol. Sci. 25 3610–3613. 10.26355/eurrev_202105_25844 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fox N. C., Petersen R. C. (2013). The G8 dementia research summit–a starter for eight? Lancet 382 1968–1969. 10.1016/S0140-6736(13)62426-5 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gautret P., Million M., Jarrot P. A., Camoin-Jau L., Colson P., Fenollar F., et al. (2020). Natural history of COVID-19 and therapeutic options. Expert Rev. Clin. Immunol. 16 1159–1184. 10.1080/1744666X.2021.1847640 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gómez J., Albaiceta G. M., García-Clemente M., López-Larrea C., Amado-Rodríguez L., Lopez-Alonso I., et al. (2020). Angiotensin-converting enzymes (ACE, ACE2) gene variants and COVID-19 outcome. Gene 762 : 145102 . 10.1016/j.gene.2020.145102 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hariyanto T. I., Putri C., Arisa J., Situmeang R. F. V., Kurniawan A. (2020). Dementia and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review and meta-analysis. Arch. Gerontol Geriatr. 93 : 104299 . 10.1016/j.archger.2020.104299 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hascup E. R., Hascup K. N. (2020). Does SARS-CoV-2 infection cause chronic neurological complications? GeroScience 42 1083–1087. 10.1007/s11357-020-00207-y [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Isaia G., Marinello R., Tibaldi V., Tamone C., Bo M. (2020). Atypical presentation of Covid-19 in an older adult with severe Alzheimer disease. Am. J. Geriatr. Psychiatry 28 790–791. 10.1016/j.jagp.2020.04.018 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kales H. C., Lyketsos C. G., Miller E. M., Ballard C. (2019). Management of behavioral and psychological symptoms in people with Alzheimer’s disease: an international Delphi consensus. Int. Psychogeriatr. 31 83–90. 10.1017/S1041610218000534 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Korczyn A. D. (2020). Dementia in the COVID-19 period. J. Alzheimers Dis . 75 1071–1072. 10.3233/JAD-200609 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liu N., Sun J., Wang X., Zhao M., Huang Q., Li H. (2020). The impact of dementia on the clinical outcome of COVID-19: a systematic review and meta-analysis. J. Alzheimers Dis. 78 1775–1782. 10.3233/JAD-201016 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Macera M., De Angelis G., Sagnelli C., Coppola N. Vanvitelli Covid-Group. (2020). Clinical presentation of COVID-19: case series and review of the literature. Int. J. Environ. Res. Public Health 17 : 5062 . 10.3390/ijerph17145062 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marcantonio E. R. (2017). Delirium in hospitalized older adults. N. Engl. J. Med. 377 1456–1466. 10.1056/NEJMcp1605501 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marfella R., Paolisso P., Sardu C., Bergamaschi L., D’Angelo E. C., Barbieri M., et al. (2020). Negative impact of hyperglycaemia on tocilizumab therapy in Covid-19 patients. Diabetes Metab. 46 403–405. 10.1016/j.diabet.2020.05.005 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McMichael T. M., Currie D. W., Clark S., Pogosjans S., Kay M., Schwartz N. G., et al. (2020). Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N. Engl. J. Med. 382 2005–2011. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Monari M., Sagnelli C., Maggi P., Sangiovanni V., Numis F. G., Gentile I., et al. (2021). More severe covid-19 in patients with active cancer: the results of a multicenter cohort study. Front. Oncol. 11 : 662746 . 10.3389/fonc.2021.662746 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Naughton S. X., Raval U., Pasinetti G. M. (2020). Potential novel role of COVID-19 in Alzheimer’s disease and preventative mitigation strategies. J. Alzheimers Dis. 76 21–25. 10.3233/JAD-200537 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Onder G., Rezza G., Brusaferro S. (2020). Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 323 1775–1776. 10.1001/jama.2020.4683 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rea I. M., Gibson D. S., McGilligan V., McNerlan S. E., Alexander H. D., Ross O. A., et al. (2018). Age and age-related diseases: role of inflammation triggers and cytokines. Front. Immunol. 9 : 586 . 10.3389/fimmu.2018.00586 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Subbe C. P., Kruger M., Rutherford P., Gemmel L. (2001). Validation of a modified early warning score in medical admissions. QJM 94 521–526. 10.1093/qjmed/94.10.521 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tsapanou A., Papatriantafyllou J. D., Yiannopoulou K., Sali D., Kalligerou F., Ntanasi E., et al. (2021). The impact of COVID-19 pandemic on people with mild cognitive impairment/dementia and on their caregivers. Int. J. Geriatr. Psychiatry. 36 583–587. 10.1002/gps.5457 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang Q., Davis P. B., Gurney M. E., Xu R. (2021). COVID-19 and dementia: analyses of risk, disparity, and outcomes from electronic health records in the US. Alzheimers Dement 10.1002/alz.12296 [Epub ahead of print]. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ward C. F., Figiel G. S., Mcdonald W. M. (2020). Altered mental status as a novel initial clinical presentation for COVID-19 infection in the elderly. Am. J. Geriatr. Psychiatry 28 808–811. 10.1016/j.jagp.2020.05.013 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- West E., Nair P., Barrado-Martin Y., Walters K. R., Kupeli N., Sampson E. L., et al. (2021). Exploration of the impact of the COVID-19 pandemic on people with dementia and carers from black and minority ethnic groups. BMJ Open 11 : e050066 . 10.1136/bmjopen-2021-050066 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wynants L., Van Calster B., Collins G. S., Riley R. D., Heinze G., Schuit E., et al. (2020). Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ 369 : m1328 . 10.1101/2020.03.24.20041020 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. (2020). Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367 1444–1448. 10.1126/science.abb2762 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395 1054–1062. 10.1016/S0140-6736(20)30566-3 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]

IMAGES
VIDEO
COMMENTS
Measurements: Clinical records of each patients admitted to the hospital with a diagnosis of COVID19 infection were retrospectively analyzed. Diagnosis of dementia, modalities of onset of the COVID-19 infection, symptoms of presentation at the hospital and outcomes were recorded. Results: Dementia was diagnosed in 82 patients (13.1%). The ...
BMC Geriatrics (2023) The COVID-19 pandemic has posed unique risks to people with Alzheimer disease and dementia. Research from 2020 has shown that these people have a relatively high risk of ...
Clinical characteristics of dementia patients who died from COVID between Feb‐Apr 2020. Dementia patients were less likely to present with cough, to have faster clinical deterioration, have reduced access to intensive care, antivirals, hydroxychloroquine and chloroquine. ... Eight studies reported that presentation of COVID‐19 in dementia ...
Objective No studies analyzing the role of dementia as a risk factor for mortality in patients affected by COVID-19. We assessed the prevalence, clinical presentation and outcomes of dementia among subjects hospitalized for COVID19 infection. Design Retrospective study. Setting COVID wards in Acute Hospital in Brescia province, Northern Italy. Participants We used data from 627 subjects ...
Delirium caused by hypoxia, a prominent clinical feature of COVID-19, could complicate the presentation of dementia, increasing the suffering of the people living with dementia, the cost of medical care, and the need for dementia support. During the COVID-19 outbreak in China, five organisations, including the Chinese Society of Geriatric ...
virus Disease 2019 (COVID-19) pandemic could cause more deleterious eects on people living with dementia [2]. ese patients are at a higher risk of experiencing severe COVID-19 due to factors such as older age, frailty, inammation, and the presence of comorbidities, espe-cially cardiovascular diseases [3].
The diagnosis of dementia, especially in the most advanced stages, represents an important risk factor for mortality in COVID-19 patients, and the clinical presentation in subjects with dementia is atypical, reducing early recognition of symptoms and hospitalization. Objective No studies analyzing the role of dementia as a risk factor for mortality in patients affected by COVID-19.
Not only that, but up to 5.7% of patients with severe presentation of COVID-19 have stroke, which can precipitate cognitive decline in people already living with progressive cognitive difficulties (4). ... Bianchetti A, Rozzini R, Guerini F., et al. Clinical presentation of COVID-19 in dementia patients. J Nutr Health Aging 24, 560-562 doi.org ...
Europe PMC is an archive of life sciences journal literature. Search life-sciences literature (41,605,652 (41,605,652
A year after contracting COVID-19, all of the patients with dementia had experienced a significant increase in fatigue and depression, as well as worsening attention, memory, speech, visuospatial capabilities, and executive functions. All the patients also had cerebral atrophy, which is the loss of neurons and connections between neurons, and ...
The medical community was quick to recognize that dementia and other comorbidities of older age left older individuals prone to severe illness and death from COVID-19. 1,2 Yet the impact of the COVID-19 pandemic has had far broader consequences to population health than can be attributed to the virus itself. The indirect effects of COVID-19, including increased adoption of telehealth ...
Measurements: Clinical records of each patients admitted to the hospital with a diagnosis of COVID19 infection were retrospectively analyzed. Diagnosis of dementia, modalities of onset of the COVID-19 infection, symptoms of presentation at the hospital and outcomes were recorded. Results: Dementia was diagnosed in 82 patients (13.1%).
Of the 30 laboratory-diagnosed COVID-19 elderly patients with dementia, only three (10⋅0%) were detected with delirium ... Indeed, such a clinical presentation in elderly patients with dementia might represent a prodromal phase of SARS-CoV-2 infection. As also suggested by other authors, (10. Bianchetti A. Rozzini R. Guerini F. Boffelli S.
Approximately 40% - 60% of people with dementia in residential care facilities experience behavioral and psychological symptoms (BPSD), such as agitation, psychosis, or apathy [1]. During the COVID-19, older adults with dementia were likely to develop behavioral changes [2]. Among multiple factors contributing to the behavioral disturbances in unprecedented times, delirium was not well ...
Results Of 8916 patients with ADRD and dysphagia included in the propensity score matched analysis, the mean (SD) age was 85.7 (8.0) years and 4829 were female (54.2%). A total of 4458 patients receiving a thick liquid diet were matched with 4458 patients receiving a thin liquid diet.
The aim of the study is to describe the clinical characteristics and outcomes of a series of older patients consecutively admitted into a non-ICU ward due to SARS-CoV-2 infection (14, males 11), developing delirium. Hypokinetic delirium with lethargy and confusion was observed in 43% of cases (6/14 patients). A total of eight patients exhibited ...
Key Points. Question What are the cardiovascular effects in unselected patients with recent coronavirus disease 2019 (COVID-19)?. Findings In this cohort study including 100 patients recently recovered from COVID-19 identified from a COVID-19 test center, cardiac magnetic resonance imaging revealed cardiac involvement in 78 patients (78%) and ongoing myocardial inflammation in 60 patients (60% ...
Before the COVID-19 outbreak, Annette Adams-Brown's 87-year-old mother was an avid follower of TV news. Now Adams-Brown has to channel-surf for a less stressful pastime. Her mother, Bertha, has dementia, and each time she hears the news about a terrible disease spreading through the country, it's like she is hearing it for the first time.
Background/Objectives: The newly emergent COVID-19 pandemic involved primarily the respiratory system and had also major cardiovascular system (CVS) implications, revealed by acute myocardial infarction (AMI), arrhythmias, myocardial injury, and thromboembolism. CVS involvement is done through main mechanisms—direct and indirect heart muscle injury, with high mortality rates, worse short ...
There were no statistically significant differences in age, gender, and co-morbidities among COVID-19 patients with and without dementia. The majority of patients were males (60.9% of Cases and Controls) and very elderly [median age 82 years (IQR: ... Clinical presentation of COVID-19: case series and review of the literature. Int. J. Environ.
SANTA ANA, Calif., May 16, 2024 (GLOBE NEWSWIRE) -- NKGen Biotech, Inc. (Nasdaq: NKGN) ("NKGen" or the "Company"), a clinical-stage biotechnology company focused on the development and commercialization of innovative autologous, allogeneic and CAR-NK natural killer ("NK") cell therapeutics, today announced that Paul Y. Song, MD, Chairman and Chief Executive Officer of NKGen, will ...