
- KI Open Archive Home
- Avhandlingar / Theses
- Doktorsavhandlingar / Doctoral Theses

Experiences of signs and symptoms among men with advanced prostate cancer
Collections.
- Doktorsavhandlingar / Doctoral Theses - K8 (CNS)
- Doktorsavhandlingar / Doctoral Theses - SwePub
Total Visits
Total visits per month, file visits, top country views, top cities views, general information.
- Skip to main content
- Accessibility information

- Enlighten Enlighten
Enlighten Theses
- Latest Additions
- Browse by Year
- Browse by Subject
- Browse by College/School
- Browse by Author
- Browse by Funder
- Login (Library staff only)
In this section
Analysis of the incidence and patient survival for prostate cancer in the West of Scotland
Shafique, Kashif (2012) Analysis of the incidence and patient survival for prostate cancer in the West of Scotland. PhD thesis, University of Glasgow.
Prostate cancer has emerged as the most frequently diagnosed cancer, except for non-melanoma skin cancer, among men in many Western countries in the last decade. In the United Kingdom (UK), prostate cancer accounts for nearly a quarter of all new male cancer diagnoses. Increasing age and some genetic and ethnic risk factors have been identified but few modifiable risk factors are known. The introduction of Prostate Specific Antigen (PSA) testing has increased the detection of previously undiagnosed disease but its contribution to the observed increases in prostate cancer incidence is not clear. Considerable variations in the incidence of prostate cancer have been observed in different geographic regions and socio-economic groups across the UK but it is not known whether, or to what extent, these may be attributed to differential uptakes of PSA testing. Prostate cancer is the third most common cause of cancer death in men but many cases do not progress. There is therefore an important clinical need for better prognostic markers so that the increasing numbers of men with prostate cancer can be appropriately managed. This thesis begins with a descriptive epidemiological study using cancer registry incidence data from the West of Scotland from 1991 to 2007. The aim was to determine whether the incidence of prostate cancer was continuing to rise and to describe any demographic or socio-economic patterns that might suggest particular at-risk groups. To understand whether any socio-economic differentials in incidence might be due to PSA testing, I examined Gleason grade-specific prostate cancer incidence by socio-economic groups over time. Socio-economic circumstances were measured using census-derived Carstairs scores. Overall (age adjusted) prostate cancer incidence increased by 70% from 44 per 100,000 in 1991 to 75 per 100,000 in 2007, an average annual growth of 3.59%. This pattern was driven by significant increases in both low and high grade cancers with no convincing change in their proportions over time. Incidence was inversely associated with deprivation with the highest rates among the most affluent groups. To explore the role of potentially modifiable risk factors on prostate cancer incidence, the Midspan and Collaborative prospective cohort studies were analysed. An analysis of the relationship between cholesterol and prostate cancer incidence was conducted on the Midspan cohort, which comprises 12,926 men who were enrolled between 1970 and 1976 and followed up to 31st December 2007. Cox Proportional Hazards Models were used to evaluate the association between baseline plasma cholesterol and Gleason grade-specific prostate cancer incidence. Following up to 37 years’ follow-up, 650 men developed prostate cancer. Their baseline plasma cholesterol level was positively associated with hazard of high grade (Gleason score ≥8) prostate cancer incidence (n=119). The association was greatest among men in the 4th highest quintile for cholesterol, 6.1 to <6.69 mmol/l, Hazard Ratio 2.28, 95% CI 1.27 to 4.10, compared with the baseline of <5.05 mmol/l. This association remained significant after adjustment for age, body mass index, smoking and socio-economic status. Evidence on the possible role of tea and coffee consumption in the development of prostate cancer remains limited to a small number of studies with short follow-up and small numbers of cases. Therefore to understand the relationship of tea and coffee consumption with overall as well as grade-specific prostate cancer, a prospective cohort study of 6016 men was carried out, who were enrolled in the Collaborative cohort study between 1970 and 1973 and followed up to 31st December 2007. Three hundred and eighteen men developed prostate cancer in up to 37 years’ follow-up. I found a positive association between consumption of tea and overall risk of prostate cancer incidence (p=0.02). The association was greatest among men who drank ≥7 cups of tea per day (HR 1.50, 95% CI 1.06 to 2.12) compared with the baseline of 0-3 cups per day. However, I did not find any significant association between tea intake and low (Gleason < 7) or high grade (Gleason 8-10) prostate cancer incidence. Higher coffee consumption was inversely associated with risk of high grade disease (HR 0.46, 95% CI 0.21-0.99) but not with overall risk of prostate cancer. These associations remained significant after adjustment for age, Body Mass Index, smoking, social class, cholesterol level, systolic blood pressure and alcohol consumption. Although survival of prostate cancer patients has improved over time, little is known about the major prognostic factors. To understand the socio-economic differences and major determinants of survival, an investigation was carried out using cancer registry incidence data from the West of Scotland from 1991 to 2007, linked with General Registrar Office (Scotland) death records up to 31st December 2008. Socio-economic circumstances were measured using the Scottish Index for Multiple Deprivation (SIMD). Age, sex and deprivation specific mortality rates were obtained from General Registrar Office for Scotland (GRO(S)). One, three and five year relative survival was estimated using the complete approach. Survival gradients across deprivation quintiles were estimated using linear regression, weighted by the variance of the relative survival estimate, using STATA software (StataCorp, version 11). Five year relative survival increased from 58.2% to 78.6% in men over the same period (an average deprivation adjusted increase of 10.2% between six years periods). Despite substantial improvements in survival of prostate cancer patients, there was a deprivation gap (that is, better survival for the least deprived compared with the most deprived) between the three time periods. The deprivation gap in five year relative survival widened from -4.76 in 1991-1996 to -10.08 in 2003-2007. Age, Gleason grade and socio-economic status appeared as significant determinants of survival. There is some evidence that systemic inflammation may be associated with survival in patients with prostate cancer although its relationship to tumour grade and socio-economic circumstances has not been previously studied. I therefore investigated the association between inflammation-based prognostic scores and survival, using the modified Glasgow Prognostic Score (mGPS) and Neutrophil Lymphocyte Ratio (NLR) as well as Gleason grade. The patient cohort within the Glasgow Inflammation Outcome Study who had a diagnosis of prostate cancer was included in this study. The mGPS is a categorical score constructed by combining serum C-reactive protein and albumin levels, while the NLR is obtained by calculating the ratio of neutrophils to lymphocytes. The relationship between mGPS and NLR and five-year relative survival was explored after adjusting for age, socio-economic circumstances and Gleason grade. Of the 897 prostate cancer patients in the Glasgow Inflammation Outcome Study, 422 (47%) died during a maximum follow-up of 6.2 years. Systemic inflammation had a significant prognostic value. The mGPS predicted poorer 5-year overall and relative survival independent of age, socio-economic circumstances, disease grade and NLR. Raised mGPS also had a significant association with excess risk of death (mGPS 2: Relative Excess Risk = 2.08, 95% CI 1.13-3.81) among aggressive, clinically significant prostate cancer (Gleason score 8-10). Prostate cancer patients with a raised mGPS had significantly higher risks of death overall as well as for high grade disease. Inflammation-based prognostic scores can potentially predict patient outcome and a further prospective study is warranted to assess their clinical value. Although the study of the epidemiology of prostate cancer is complicated by changing diagnostic sensitivity and disease grade definitions, the increasing number of men diagnosed with the disease demands continuing research into understanding risk factors, prognostic factors and more effective treatment. However, it seems unlikely that a simple, modifiable risk factor exists for prostate cancer and that PSA testing and an aging population will continue to drive increasing incidence.
Actions (login required)
Downloads per month over past year
View more statistics

The University of Glasgow is a registered Scottish charity: Registration Number SC004401
- Open access
- Published: 06 May 2021
Knowledge of prostate cancer presentation, etiology, and screening practices among women: a mixed-methods systematic review
- Ebenezer Wiafe ORCID: orcid.org/0000-0002-0496-5737 1 , 2 ,
- Kofi Boamah Mensah 1 , 3 ,
- Adwoa Bemah Boamah Mensah 3 ,
- Varsha Bangalee 1 &
- Frasia Oosthuizen 1
Systematic Reviews volume 10 , Article number: 138 ( 2021 ) Cite this article
5 Citations
2 Altmetric
Metrics details
With the burden of prostate cancer, it has become imperative to exploit cost-effective ways to tackle this menace. Women have demonstrated their ability to recognize early cancer signs, and it is, therefore, relevant to include women in strategies to improve the early detection of prostate cancer. This systematic review seeks to gather evidence from studies that investigated women’s knowledge about (1) the signs and symptoms, (2) causes and risk factors, and (3) the screening modalities of prostate cancer. Findings from the review will better position women in the fight against the late detection of prostate cancer.
The convergent segregated approach to the conduct of mixed-methods systematic reviews was employed. Five databases, namely, MEDLINE (EBSCOhost), CINAHL (EBSCOhost), PsycINFO (EBSCOhost), Web of Science, and EMBASE (Ovid), were searched from January 1999 to December 2019 for studies conducted with a focus on the knowledge of women on the signs and symptoms, the causes and risk factors, and the screening modalities of prostate cancer.
Of 2201 titles and abstracts screened, 22 full-text papers were retrieved and reviewed, and 7 were included: 3 quantitative, 1 qualitative, and 3 mixed-methods studies. Both quantitative and qualitative findings indicate that women have moderate knowledge of the signs and symptoms and the causes and risk factors of prostate cancer. However, women recorded poor knowledge about prostate cancer screening modalities or tools.
Conclusions
Moderate knowledge of women on the signs and symptoms and the causes and risk factors of prostate cancer was associated with education. These findings provide vital information for the prevention and control of prostate cancer and encourage policy-makers to incorporate health promotion and awareness campaigns in health policies to improve knowledge and awareness of prostate cancer globally.
Systematic review registration
Open Science Framework (OSF) registration DOI: https://doi.org/10.17605/OSF.IO/BR456
Peer Review reports
Prostate cancer (PCa) is the most common non-skin cancer occurring in men and is accountable for 3.8% of all mortality caused by cancer in men [ 1 , 2 ]. According to the GLOBOCAN, 2018 database, it is estimated that it is the fifth primary cause of cancer death in men globally. It further reported that the highest mortality rate is found in the Caribbean and Southern African men worldwide [ 1 , 3 ]. A recent study by Yeboah-Asiamah et al. reported that PCa was the second most common cancer in areas such as Australia, the USA, and New Zealand [ 4 ]. Though fewer than 30% of all incidence of PCa are from developing countries, these countries have previously been estimated to have the highest mortality from PCa due to late diagnosis [ 5 , 6 ]. Although sub-Saharan Africa (SSA) has a low rate of the disease, the incidence is projected to increase if screening is encouraged [ 7 ]. Hence, PCa remains a vital public health concern in both developed and developing countries.
The Centers for Disease Control and Prevention (CDC) in North America organized a workshop with the motive to explore strategies to control and prevent the disease based on the increasing incidence and mortality rate of PCa [ 8 ]. To address mortality rates related to the disease, participants recommended strategies to improve PCa awareness [ 8 ]. Also, as documented by many studies, PCa incidence is a direct reflection of the rate at which high-risk groups screen for the disease [ 4 , 9 ]. In Europe, early screening was attributed to a 20% reduction in PCa mortality rate [ 10 ]. Although there is evidence suggesting a reduction in PCa mortality due to early screening, a United States (US) study did not highlight a reduction in mortality [ 11 ]. The prostate-specific antigen (PSA) test and the digital rectal examination (DRE) are useful screening tools, although initial controversies were surrounding the use of these tools [ 12 ]. Because of overlap in PSA levels in men with prostatitis, benign prostatic hyperplasia, and PCa, it was assumed that PCa cannot be screened using the PSA test [ 13 ]. Catalona et al. demonstrated that PSA could be utilized as a screening tool for PCa, and it has widely been adopted [ 14 ]. DRE is the only procedure whereby physicians can examine part of the prostate gland [ 15 ]. The findings are only based on the physician impression, hence poor inter-rater reliability and also a limitation to the palpable region of the prostate gland [ 15 ]. However, DRE sometimes detects PCa in men with PSA, 4.0 ng/mL [ 16 ]. Regardless of the controversial nature of screening and the potential for early screening to reduce mortality, studies support the need to encourage screening [ 4 , 12 ].
Women have essential characteristics that make them better managers of family health as compared to men. Therefore, it is not surprising that there is evidence positioning women as individuals who make adequate observations about the health of their partners [ 9 , 17 ]. In promoting the early detection of PCa, women have been documented to observe the slightest symptoms presented by their partners and push them to seek medical attention [ 9 , 18 ]. In a study conducted by Blanchard et al., it was recommended that efforts must be made to actively involve women in improving the timely detection of PCa through the closure of knowledge gaps [ 19 ].
Also, men admit seeking out their wives’ opinions as sources of health information [ 20 ]. In the context of the early detection of PCa, women can play various roles such as information seekers, advocates, health advisors, and support persons [ 21 ]. Therefore, there is the need to gather current evidence about women’s knowledge of PCa as the findings will be vital in equipping women to contribute towards the early detection of the disease.
In light of the availability of limited evidence addressing the awareness of women on prostate cancer, this review will seek to combine quantitative and qualitative data to increase the validity of findings through data triangulation as recommended by Caruth and supported by Lizarondo et al. [ 22 , 23 ]. Thus, this review seeks to map out current evidence regarding women’s awareness of PCa under the scopes of (1) signs and symptoms, (2) risk factors and causes, and (3) screening guidelines.
Review question
Do women have adequate knowledge about prostate cancer?
Methodology
The Joanna Briggs Institute (JBI) reviewer’s manual for the conduct of mixed-methods critical appraisal and synthesis formed the backbone of the study [ 23 ]. With guidance from the JBI manual, a protocol was developed to guide the review process according to the convergent segregated approach [ 23 ]. The respective DOIs of the review protocol and review, registered with the Open Science Framework (OSF), are https://doi.org/10.17605/OSF.IO/EYHF2 and https://doi.org/10.17605/OSF.IO/BR456 . The review protocol is readily available to the scientific community [ 24 ].
Inclusion criteria
The following were grounds for the inclusion of studies:
Studies that were conducted among women aged 18 years and above.
Studies that were conducted among women of all racial backgrounds.
Studies published from January 1999 to December 2019.
Studies that were conducted among women of all geographical locations.
Studies of all research designs.
Studies that were conducted to investigate the knowledge of women on the signs and symptoms of prostate cancer as highlighted in the review protocol.
Studies that were conducted to investigate the knowledge of women on the causes and risk factors of prostate cancer as highlighted in the review protocol.
Studies that were conducted to investigate the knowledge of women on the screening recommendations of prostate cancer as highlighted in the review protocol.
Studies that were published in the English language.
Studies with abstract and full text available.
Exclusion criteria
The following were grounds for the exclusion of studies:
Studies that were published before January 1999 or after December 2019.
Studies that were not published in the English language.
Studies that include women below the age of 18 years.
Studies in which the age of included women cannot be established.
Studies that did not indicate the number/percentage of included women.
Studies that exclusively included men without any women component (18 years and above).
Studies conducted among women who were previously given education on prostate cancer.
Studies that exclusively involved lesbian, gay, bisexual, transsexual/transgender, and queer/questioning (LGBTQ) participants.
Studies that exclusively included healthcare professionals.
Studies that exclusively involved healthcare and college/university students.
Studies that do not include the outcome of interest.
Book chapters.
Reviews and overviews.
Abstracts and conference papers.
Dissertations and thesis.
Commentaries and letters to editors.
Studies published without abstracts.
Information sources and search strategy
An initial explorative search in PubMed founded search terms in preparation for comprehensive electronic search. The selected search terms, applied as MeSH terms, were combined with Boolean operators for a comprehensive electronic search in MEDLINE (EBSCOhost), CINAHL (EBSCOhost), PsycINFO (EBSCOhost), Web of Science, and EMBASE (Ovid) as “(prostate cancer ) AND (awareness OR knowledge) AND (signs OR symptoms) AND (risk factors OR causes) AND (screening) AND (women)”. The search strategy (Additional file 1 ), so developed, was utilized by the first (EW) and second (KBM) reviewers to independently conduct a literature search as outlined in the review protocol e24].
Selection of studies
The first and second reviewers, being guided by the developed review protocol, singularly screened and compared the titles and abstracts of the literature search outcomes to a developed standard (the inclusion and exclusion criteria). Studies that successfully passed the initial stage of screening were subjected to the independent full-text reading by EW and KBM before consideration for data extraction. Lastly, hand-searching and snowballing on references of selected articles were done to find eligible studies in the grey area. There were no disagreements between EW and KBM. Hence, the third reviewer (ABBM) assessed the studies before data extraction was conducted by the lead author according to the JBI data extraction tools outlined in the review protocol [ 24 ]. The characteristic of studies that successfully went through the data extraction, the key findings that were extracted, and a summary of the study selection process are detailed respectively (Tables 1 and 2 and Fig. 1 ).
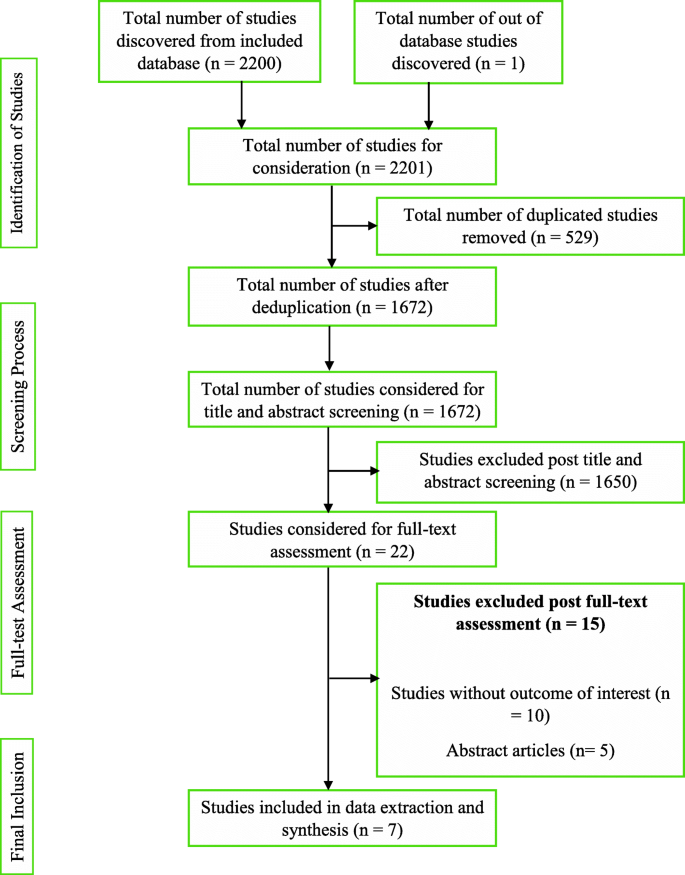
Summary of study selection process
Quality assessment
As described in the review protocol [ 24 ], the methodological quality assessment tool (Additional file 2 ) was adopted and modified for this review due to the similarities this review shares with the study conducted by Mensah et al. [ 30 ]. The tool appraised the studies’ quality based on the study sample representativeness, response rate, reliability, and validity of the data collection tool. The tool was modified to suit the results from the included studies. A score was calculated, and the quality of the studies was classified as weak (0 to 33.9%), moderate (34 to 66.9%), or strong (67 to 100%). Eligible records were subjected to independent quality assessment by EW and KBM. Methodological quality outcomes were not grounds for exclusion.
Synthesis and integration of findings
The review findings were subjected to the convergent segregated approach to synthesis and integration according to the developed review protocol [ 24 ]. A narrative synthesis was separately performed for qualitative and quantitative findings. The heterogeneous nature of the review findings did not support the conduct of a meta-analysis. The results were finally integrated.
Conducting the review, according to the developed protocol, yielded 2200 study results. A detailed citation screening led to an additional study, which increased the total number of studies to 2201. Regarding the summary of the study selection process (Fig. 1 ), 1672 studies were obtained after 529 duplicates were removed from the pool of data. Post-titles and abstract review excluded 1650 studies leaving 22 studies. The 22 studies were further reduced to 7 after a full-text reading resulted in the exclusion of 15 studies.
Characteristics of included studies
The data extracted from the seven (7) studies are detailed (Table 1 ). The publication years ranged from 2003 to 2018 with 5 studies having been conducted in the USA. One of the studies was a multicenter study that involved multinationals [ 28 ]. The study with the highest female participants (4040 women) was conducted in Spain [ 26 ]. Webb et al. recruited the lowest sample size, 14 women [ 29 ]. A total of 5634 women were involved in the 7 studies. Two studies were solely conducted in women, three included other diseases, and two did not disclose study duration.
Quality of included studies
According to the scoring scheme of the quality assessment tool (Additional file 2 ), two studies [ 26 , 29 ] were evaluated as moderate quality whilst five studies were evaluated as strong quality. None of the studies was excluded based on methodological quality assessment outcomes. There was no disagreement between EW and KBM.
Review findings
Study findings, presented in Table 2 , were heterogeneous. Quantitative studies indicate that women knew about the existence of PCa. In exploring qualitative evidence, women exhibited knowledge of PCa. Therefore, both arms of the review are supportive of each other.
Women had moderate knowledge about the signs and symptoms of PCa drawing from quantitative findings. Women knew about the asymptomatic nature of the early stages of PCa. They also moderately knew urinary symptoms such as urinary frequency, difficulty in urinating, and dysuria. Qualitative studies indicate that women were aware prostate cancer patients, usually in advanced stages, could present with signs and symptoms such as urinary frequency, difficulty in urinating, glandular enlargement of the prostate, and erectile dysfunction. Hence, quantitative and qualitative findings revealed that women moderately knew the urinary symptoms of PCa.
Quantitative studies indicate an average score of women on knowledge of risk factors of PCa. Risk factors women knew were increasing age, presence of a first-degree relative, being genetically linked to Africa, and excessive truncal obesity. Qualitative evidence recognized all risk factors documented by the quantitative findings except truncal obesity. Also, identified risk factors included poor diet, inadequate exercise, stressful lifestyle, poor screening habits, cigarette smoking, and poor access to quality healthcare. Women wrongly reported sexual orientation and frequent sexual activity as risk factors. Therefore, qualitative findings confirm the quantitative claim that women have shared knowledge about the risk factors of PCa.
Quantitative studies indicate that women had poor knowledge about PCa screening guidelines, appropriate screening samples, and tools. Although it was reported that women knew about PSA and DRE, the proportions of women who had correct responses to screening knowledge items were not appreciable. Women poorly recognized urine as a screening sample, PSA as an exclusive diagnostic tool (where only 17.5% answered correctly), and failed to identify more than one screening tool (between 41 and 71% of women failed). Qualitative studies respectively reported PSA and blood as a screening tool and sample. Colonoscopy was wrongly reported as a PCa screening tool. Conclusively, both arms of the review reported women knew about PSA and had poor knowledge about PCa screening.
The heterogeneity of the study findings warranted the synthesis as a narrative [ 23 , 31 ]. The convergent segregated approach was employed according to the recommendation of the JBI reviewer’s manual [ 23 ].
Generally, from the quantitative evidence, women knew about prostate cancer [ 19 , 25 , 27 , 28 ]. The knowledge of women was found to have increased with educational and financial status [ 19 ], and disease familiarity [ 19 , 25 ]. The awareness of women about the existence of PCa increased when the disease was mentioned compared to an initial request for women to list cancers [ 28 ]. Qualitative evidence showed that women were aware of PCa [ 18 , 27 ]. They appreciated and specifically requested for PCa education partly because they could not tell the location of the prostate gland [ 18 ]. Thus, quantitative and qualitative evidence indicates that women know about PCa. Women’s awareness could be due to their role in family health management and the possible health-seeking behavior of educated and financially strong women. As persons are faced with the experiences of a health condition, they will seek to make sense of this illness by acquiring knowledge [ 32 ], experiences, and beliefs; hence, this theory might explain the improved awareness of women who were familiar with the disease.
Most of the quantitative studies indicate that women are aware of the asymptomatic nature of early-stage PCa [ 19 , 25 , 27 ]. Symptoms that women had a fair knowledge about included urinary frequency, difficulty in urinating, and dysuria [ 25 ]. Findings from one of the qualitative studies indicate that women fairly recognized urinary frequency, difficulty in urinating, glandular enlargement of the prostate, and erectile dysfunction as signs and symptoms of PCa [ 18 ]. Being familiar with the disease may explain the awareness of women of the urinary symptoms associated with PCa.
According to Okoro and colleagues’ quantitative study, although knowledge of PCa was not adequate, women knew associated risk factors such as being a first-degree relative, being a man of African descent, and excessive truncal obesity [ 27 ]. Blanchard et al. also documented women’s recognition of increasing age as a PCa risk factor [ 19 ]. One of the qualitative studies indicates women knew increasing age could increase a man’s chance for PCa development [ 18 , 29 ]. Other causes and risk factors women identified included poor diet, inadequate exercise, stressful lifestyle, family history of the disease, being of African descent, poor screening habits, cigarette smoking, and poor access to quality healthcare [ 18 ]. Erroneously, one study reported that women perceived sexual orientation and frequent sexual activity as risk factors [ 18 ]. Both quantitative and qualitative findings documented women knew increasing age, family history, and African descent as PCa risk factors.
Quantitatively, women’s responses to queries about PCa screening were poor [ 25 , 28 ]. Some women were unable to recognize at least a PCa screening tool whilst others mistakenly recognized urine as a suitable sample for PCa screening [ 28 ]. According to Okoro et al., the majority of women exclusively tagged PSA elevation as a basis for PCa diagnosis [ 27 ]. This, therefore, calls for extensive education because benign prostatic hyperplasia, prostatitis, and PCa usually present with elevated PSA [ 13 ]. Evidence from qualitative findings indicated women knew physical examination must augment blood analysis [ 29 ]. Also, women mentioned PSA and colonoscopy as screening tools [ 18 ]. The results from included qualitative studies confirmed that women had poor knowledge about PCa screening. The mention of colonoscopy as a screening tool further supports a lack of adequate knowledge about PCa screening.
This critical appraisal and synthesis revealed over the 20 years of study search, only four studies out of the seven included studies investigated all the outcomes of interest. Two studies did not investigate women’s awareness of the signs and symptoms [ 26 , 29 ] and the causes and risk factors [ 25 , 26 ] of PCa. Therefore, although quantitative and qualitative findings were supportive of each other, studies investigating the causes and risk factors, as well as the signs and symptoms of PCa, were lacking.
Recommendations for practice
From the review findings, it is recommended that PCa control programs should also focus on educating women. Clinicians and public health practitioners should include women in prostate cancer health promotion. Women should be encouraged to attend PCa clinics with their male significant others suffering from the disease, and the effect of this strategy in reducing PCa mortality rate must be investigated.
Recommendations for research
Further studies are recommended to investigate the knowledge of women living in low- and middle-income countries (LMIC) about PCa. Such studies should focus extensively on the knowledge of women on PCa screening. Also, it is recommended for research to develop and pilot a PCa educational intervention model, applicable to women to reduce the burden of the disease. This tool should be culturally specific for easy acceptance and recognition. Also, current evidence on the willingness of women to offer social support to men with PCa should be investigated.
Study limitations
The various restrictions that were imposed on the literature search included a search range from January 1999 to December 2019, a search into only 5 databases, and the outright exclusion of non-English publications. These constitute selection bias. Therefore, some important studies could have been left out of the review.
Although five (5) out of the seven (7) included studies explicitly indicated recruiting participants of African backgrounds, none of the studies were conducted in Africa. Hence, the global generalizability of the review findings, to most importantly cover low and middle-income countries, cannot be documented.
The exclusion of studies conducted in women who received education on prostate cancer, healthcare professionals, healthcare students, and college/university students, and further exclusion of studies that involved (LGBTQ) participants further constitute selection bias.
It is imperative to note that the various limitations, in connection to the included studies, documented in Table 2 have an effect on this review and, as such, could be considered as potential limitations.
Availability of data and materials
Data and other pieces of information are available at https://doi.org/10.17605/OSF.IO/BR456
Abbreviations
Centers for disease control and prevention
Digital rectal examination
Global cancer incidence, mortality and prevalence
Joanna briggs institute
Lesbian, gay, bisexual, transsexual/transgender, and queer/questioning
Open science framework
Low- and middle-income countries
- Prostate cancer
Prostate-specific antigen
Sub-Saharan Africa
United States
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492 .
Article PubMed Google Scholar
James LJ, Wong G, Craig JC, Hanson CS, Ju A, Howard K, et al. Men’s perspectives of prostate cancer screening: a systematic review of qualitative studies. PloS One. 2017;12(11):e0188258. https://doi.org/10.1371/journal.pone.0188258 .
Article CAS PubMed PubMed Central Google Scholar
GLOBOCAN. GLOBOCAN 2008: Ghana Fact Sheets. 2018. Available from: file:///C:/Users/user-pc/Downloads/288-ghana-fact-sheets.pdf.
Yeboah-Asiamah B, Yirenya-Tawiah D, Baafi D, Ackumey M. Perceptions and knowledge about prostate cancer and attitudes towards prostate cancer screening among male teachers in the Sunyani Municipality, Ghana. Afr J Urol. 2017;23(4):184-91.
Rebbeck TR, Devesa SS, Chang B-L, Bunker CH, Cheng I, Cooney K, et al. Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of African descent. Prostate Cancer. 2013;2013:1–12. https://doi.org/10.1155/2013/560857 .
Article Google Scholar
Mofolo N, Betshu O, Kenna O, Koroma S, Lebeko T, Claassen FM, et al. Knowledge of prostate cancer among males attending a urology clinic, a South African study. SpringerPlus. 2015;4(1):67. https://doi.org/10.1186/s40064-015-0824-y .
Article PubMed PubMed Central Google Scholar
Jemal A, Bray F, Forman D, O'Brien M, Ferlay J, Center M, et al. Cancer burden in Africa and opportunities for prevention. Cancer. 2012;118(18):4372–84. https://doi.org/10.1002/cncr.27410 .
Dimah KP, Dimah A. Prostate cancer among African American men: a review of empirical literature. Journal of African American Studies. 2003;7(1):27–46. https://doi.org/10.1007/s12111-003-1001-x .
Rashid P, Denham J, Madjar I. Do women have a role in early detection of prostate cancer? Lessons from a qualitative study. Aust Fam Phys. 2007;36(5):375.
Google Scholar
Schröder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–8. https://doi.org/10.1056/NEJMoa0810084 .
Andriole GL, Crawford ED, Grubb RL III, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–9. https://doi.org/10.1056/NEJMoa0810696 .
Nakandi H, Kirabo M, Semugabo C, Kittengo A, Kitayimbwa P, Kalungi S, et al. Knowledge, attitudes and practices of Ugandan men regarding prostate cancer. Afr J Urol. 2013;19(4):165–70. https://doi.org/10.1016/j.afju.2013.08.001 .
Catalona WJ. Prostate cancer screening. Med Clin. 2018;102(2):199–214. https://doi.org/10.1016/j.mcna.2017.11.001 .
Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324(17):1156–61. https://doi.org/10.1056/NEJM199104253241702 .
Article CAS PubMed Google Scholar
Wilbur J. Prostate cancer screening: the continuing controversy. Am Fam Phys. 2008;78(12):1377–84.
Federman DG, Pitkin P, Carbone V, Concato J, Kravetz JD. Screening for prostate cancer: are digital rectal examinations being performed? Hosp Pract. 2014;42(2):103–7. https://doi.org/10.3810/hp.2014.04.1108 .
Karim R, Lindberg L, Wamala S, Emmelin M. Men’s perceptions of women’s participation in development initiatives in rural Bangladesh. Am J Mens Health. 2018;12(2):398–410. https://doi.org/10.1177/1557988317735394 .
Owens OL, Friedman DB, Hebert J. Commentary: Building an evidence base for promoting informed prostate cancer screening decisions: an overview of a cancer prevention and control program. Ethn Dis. 2017;27(1):55–62. https://doi.org/10.18865/ed.27.1.55 .
Blanchard K, Proverbs-Singh T, Katner A, Lifsey D, Pollard S, Rayford W. Knowledge, attitudes and beliefs of women about the importance of prostate cancer screening. J Natl Med Assoc. 2005;97(10):1378–85.
PubMed PubMed Central Google Scholar
Taylor KL, Turner RO, Davis JL III, Johnson L, Schwartz MD, Kerner J, et al. Improving knowledge of the prostate cancer screening dilemma among African American men: an academic-community partnership in Washington, DC. Public Health Rep. 2016;116(6):590–8.
Miller SM, Roussi P, Scarpato J, Wen KY, Zhu F, Roy G. Randomized trial of print messaging: the role of the partner and monitoring style in promoting provider discussions about prostate cancer screening among African American men. Psychooncol. 2014;23(4):404–11. https://doi.org/10.1002/pon.3437 .
Caruth GD. Demystifying mixed methods research design: a review of the literature. Mevlana Int J Educ. 2013;3(2):112–22. https://doi.org/10.13054/mije.13.35.3.2 .
Lizarondo L, Stern C, Carrier J, Godfrey C, Rieger K, Salmond S, et al. Chapter 8: Mixed methods systematic reviews.: The Joanna Briggs Institute; 2017 [Available from: https://wiki.joannabriggs.org/display/MANUAL/Chapter+8%3A+Mixed+methods+systematic+reviews .
Wiafe E, Mensah KB, Mensah ABB, Bangalee V, Oosthuizen F. The awareness of women on prostate cancer: a mixed-methods systematic review protocol. Syst Rev. 2020;9(1):1–6.
Brown N, Naman P, Homel P, Fraser-White M, Clare R, Browne R. Assessment of preventive health knowledge and behaviors of African-American and Afro-Caribbean women in urban settings. J Natl Med Assoc. 2006;98(10):1644–51.
Carrasco-Garrido P, Hernandez-Barrera V, Lopez de Andres A, Jimenez-Trujillo I, Gallardo Pino C, Jimenez-Garcıa R. Awareness and uptake of colorectal, breast, cervical and prostate cancer screening tests in Spain. Eur J Public Health. 2014;24(2):264–70. https://doi.org/10.1093/eurpub/ckt089 .
Okoro ON, Rutherford CA, Witherspoon SF. Leveraging the family influence of women in prostate cancer efforts targeting African American Men. J Racial Ethnic Health Disparities. 2018;5(4):820–30. https://doi.org/10.1007/s40615-017-0427-0 .
Article CAS Google Scholar
Schulman CC, Kirby R, Fitzpatrick JM. Awareness of prostate cancer among the general public: findings of an independent international survey. European Urology. 2003;44(3):294–302. https://doi.org/10.1016/S0302-2838(03)00200-8 .
Webb CR, Kronheim L, Williams JE, Hartman TJ. An evaluation of the knowledge, attitudes, and beliefs of African-American men and their female significant others regarding prostate cancer screening. Ethn Dis. 2006;16(1):234–8.
PubMed Google Scholar
Mensah KB, Oosthuizen F, Bonsu AB. Cancer awareness among community pharmacist: a systematic review. BMC Cancer. 2018;18(1):299. https://doi.org/10.1186/s12885-018-4195-y .
Lockwood C, Porritt K, Munn Z, Rittenmeyer L, Salmond S, Bjerrum M, et al. Chapter 2: Systematic reviews of qualitative evidence: the Joanna Briggs Institute; 2017 [Available from: https://wiki.joannabriggs.org/display/MANUAL/Chapter+2%3A+Systematic+reviews+of+qualitative+evidence .
Petrie K, Weinman J. Why illness perceptions matter. Clin Med. 2006;6(6):536–9. https://doi.org/10.7861/clinmedicine.6-6-536 .
Download references
Acknowledgements
The review team gives recognition to Dr. Richard Ofori-Asenso.
Author information
Authors and affiliations.
University of KwaZulu-Natal, Durban, South Africa
Ebenezer Wiafe, Kofi Boamah Mensah, Varsha Bangalee & Frasia Oosthuizen
Ho Teaching Hospital, Ho, Ghana
Ebenezer Wiafe
Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
Kofi Boamah Mensah & Adwoa Bemah Boamah Mensah
You can also search for this author in PubMed Google Scholar
Contributions
EW is credited with the conception of the review, the coordination of the systematic review, the development of the search strategy, the search and selection of studies to be included in the review, the extraction and management of quantitative and qualitative data, the assessment of methodological quality, the filtering of all reference materials, the integration and interpretation of the data, and the drafting of the manuscript, and is the principal reviewer. KBM is credited with the conception of the review, the review of the search strategy, the search and selection of studies to be included in the review, the extraction and management of quantitative and qualitative data, the assessment of methodological quality, the integration and interpretation of the data, and the review of the manuscript. ABBM is credited with the review of the search strategy, the assessment of the studies before data extraction, and the review of the manuscript. VB is credited with the review of the manuscript, the coordination of the systematic review, and the as co-supervisor of the review. FO is credited with the conception of the review, the review of the manuscript, and the overall supervision of the review. All authors have reviewed and accepted the final manuscript of the review for publication. The authors read and approved the final manuscript.
Corresponding author
Correspondence to Ebenezer Wiafe .
Ethics declarations
Ethics approval and consent to participate.
Ethical permission was not required since the study did not involve the enrollment of humans or animals as study subjects.
Consent for publication
Competing interests, additional information, publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:..
Proposed search strategy using Medline via EBSCOhost
Additional file 2:.
Assessment of methodological quality of included studies
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wiafe, E., Mensah, K.B., Mensah, A.B.B. et al. Knowledge of prostate cancer presentation, etiology, and screening practices among women: a mixed-methods systematic review. Syst Rev 10 , 138 (2021). https://doi.org/10.1186/s13643-021-01695-5
Download citation
Received : 01 December 2020
Accepted : 29 April 2021
Published : 06 May 2021
DOI : https://doi.org/10.1186/s13643-021-01695-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Signs and symptoms
- Causes and risk factors
- Screening recommendations
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Quantitative Prostate Diffusion MRI and Multi-Dimensional Diffusion-Relaxation Correlation MRI for Characterization of Prostate Cancer
- Zhang, Zhaohuan
- Advisor(s): Wu, Holden H. HHW
Prostate Cancer (PCa) remains the second most common cause of cancer-related death in men in the U.S. Multi-parametric (mp) MRI is playing an increasingly important role for the localization, detection, and risk stratification of PCa. However, prostate mp-MRI still misses PCa in up to 45% of men and faces challenges in distinguishing clinically significant PCa from indolent PCa. Therefore, MRI technology must be improved to enhance diagnostic performance for PCa. This thesis aimed to improve prostate MRI by addressing two challenges. First, the diffusion-weighted imaging (DWI) component of mp-MRI often suffers from artifacts such as distortion and low signal-to-noise ratio (SNR), which can lead to low diagnostic image quality. Second, prostate microstructure features are key determinants for histopathological assessment of cancer aggressiveness; however, current MRI techniques have limitations in capturing this information. To address the first challenge, in Aim 1, we translated and evaluated an eddy current-nulled convex optimized diffusion encoding (ENCODE) based prostate DWI technique that achieves short echo time (TE) to maintain SNR while reducing prostate geometric distortion from eddy currents and susceptibility effects. Further, in Aim 2, we developed a combined TE-minimized ENCODE diffusion encoding acquisition with a random matrix theory-based denoising reconstruction technique to improve the SNR and robustness of high-resolution (in-plane: 1.0x1.0 mm2) prostate DWI and apparent diffusion coefficient mapping. To address the second challenge, in Aim 3, we performed a first proof-of-concept ex vivo evaluation and validation of the diffusion-relaxation correlation spectrum imaging (DR-CSI) technique at 3T for quantifying microscopic tissue compartments (epithelium, stroma, and lumen) in PCa using whole-mount digital histopathology as the reference standard. Further, in Aim 4, we explored and evaluated sequential backward selection analysis for the acceleration of DR-CSI through subsampling of the diffusion-relaxation contrast encoding space while maintaining the accuracy of prostate microstructure mapping in PCa.
Enter the password to open this PDF file:
- Original Research
- Open access
- Published: 07 November 2020
Assessment of knowledge, practice and attitude towards prostate cancer screening among male patients aged 40 years and above at Kitwe Teaching Hospital, Zambia
- Sakala Gift ORCID: orcid.org/0000-0003-0438-6804 1 ,
- Kasongo Nancy 2 &
- Mwanakasale Victor 1
African Journal of Urology volume 26 , Article number: 70 ( 2020 ) Cite this article
6 Citations
Metrics details
Prostate cancer is a leading cause of cancer death in men. Evaluating knowledge, practice and attitudes towards the condition is important to identify key areas where interventions can be instituted.
This was a hospital-based descriptive cross-sectional study aimed at assessing knowledge, practice and attitude towards prostate cancer screening among male patients aged 40 years and above at Kitwe Teaching Hospital, Zambia.
A total of 200 men took part in the study (response rate = 100%). Of the 200 respondents, 67 (33.5%) had heard about prostate cancer and 58 (29%) expressed knowledge of prostate cancer out of which 37 (63.8%) had low knowledge. Twenty-six participants (13%) were screened for prostate cancer in the last 2 years. 98.5% of the participants had a positive attitude towards prostate cancer screening. Binary logistic regression results showed that advanced age ( p = 0.017), having secondary or tertiary education ( p = 0.041), increased knowledge ( p = 0.023) and family history of cancer ( p = 0.003) increased prostate cancer screening practice. After multivariate analysis, participants with increased knowledge ( p = 0.001) and family history of cancer ( p = 0.002) were more likely to practice prostate cancer screening.
The study revealed low knowledge of prostate cancer, low prostate cancer screening practice and positive attitude of men towards prostate cancer screening. These findings indicate a need for increased public sensitization campaigns on prostate cancer and its screening tests to improve public understanding about the disease with the aim of early detection.
1 Background
Prostate cancer, or adenocarcinoma of the prostate as it is called in some settings, can be described as cancer of the prostate gland.
The prostate is a small fibromuscular accessory gland of male reproductive system weighing about 20 g. It is located posterior to the pubic symphysis, superior to the perineal membrane, inferior to the bladder and anterior to the rectum. It produces and secretes proteolytic enzymes into semen, to facilitate fertilization [ 1 , 2 ].
Prostate cancer is characterized by both physical and psychological symptoms [ 3 ]. Early-stage prostate cancer is usually asymptomatic [ 4 ]. More advanced disease has similar symptoms with benign prostate conditions such as weak or interrupted urine flow, hesitancy, frequency, nocturia, hematuria or dysuria. Late-stage prostate cancer commonly spreads to bones and cause pain in the hips, spine or ribs [ 4 ]. The 2 commonly used screening methods for prostate cancer are digital rectal examination (DRE) and prostate-specific antigen (PSA) test.
Prostate cancer is one of the leading causes of cancer-related deaths among males globally [ 4 ]. The 2018 Global Cancer Project (GLOBOCAN) report estimated 1 276 106 new cases in 2018, representing 7.1% of all cancers worldwide [ 5 ]. The report further estimated the number of deaths due to prostate cancer at 358 989, representing 3.8% of all cancers globally. It was thus ranked the second most common cancer and the fifth leading cause of cancer death in men. The American Cancer Society 2019 report showed that an estimated 174,650 new cases of prostate cancer would be diagnosed in the USA during 2019 [ 4 ]. The report further stated that an estimated 31,620 deaths from prostate cancer would occur in 2019. It further put the incidence of prostate cancer to about 60% higher in blacks than in whites suggesting a genetic predilection to the cancer.
Africa is no exception to this global trend of high incidence and mortality of prostate cancer with age-standardized incidence and mortality rates of 26.6 and 14.6 per 100 000 men, respectively [ 5 ]. This placed prostate cancer as the third most common cancer among both sexes and the fourth leading cause of all cancer deaths among both sexes in the region. Current statistics on Zambia indicate that Zambia has one of the world’s highest estimated mortality rates from prostate cancer [ 6 , 7 ]. The age standardized incidence and mortality rates from prostate cancer are at 45.6 and 28.4 per 100,000 men, respectively [ 5 ].
Although the causes of prostate cancer are not yet fully understood, it is thought that advanced age (above 50 years), positive family history of prostate cancer and an African-American ethnic background are risk factors [ 4 , 8 ].
In mitigating the effects of diseases like prostate cancer, evaluating knowledge, practice and attitudes towards the condition is important to identify key areas where interventions can be instituted. For instance, studies in other countries that accessed these factors were able to identify the role that health workers and political will could play in increasing knowledge and screening for prostate cancer [ 8 , 9 , 10 ]. Furthermore, low level of awareness about prostate cancer or the complete lack of it has been identified as the cause of late presentation and poor prognosis [ 11 , 12 ].
Despite Zambia having one of the world’s highest estimated mortality rates from prostate cancer [ 6 , 7 ] coupled with an increased suggested genetic predilection to the cancer [ 4 ], since the majority of the male population are black, no studies assessing knowledge, practice and attitude towards prostate cancer screening have been done. This study therefore sought to address the gap.
The aims of the study were to determine the knowledge, practice and attitude towards prostate cancer screening at Kitwe Teaching Hospital (KTH). In addition, the study also aimed to determine the association between demographics of participants and knowledge, knowledge of participants and attitude towards prostate cancer screening, knowledge of participants and prostate cancer screening practice as well as attitude of participants towards prostate cancer screening and prostate cancer screening practice.
2.2 Study site and design
The study was done at KTH in the Copperbelt province of Zambia. It is a tertiary referral hospital in the region whose catchment area includes Copperbelt, Luapula and North-western provinces. It has a bed capacity of 630 [ 13 ].
This was a descriptive cross-sectional study of knowledge, practice and attitude towards prostate cancer screening among male patients aged 40 years and above at KTH. The study design was chosen because it is simple to use, cost-effective and time economic.
2.3 Study participants
The sample size was ascertained using the ‘Stalcalc’ function of Epi Info Version 7.1.5. In a month, nearly 419 male patients presented to the target areas for this study at KTH, namely Out-Patient Department (OPD), medical and surgical admission wards. Since data for this study were collected in 1 month, 419 was used as the total population size. A confidence level was 95% and a confidence limit was 5% (at 95% confidence level) and the expected frequency was 50%. Therefore, a sample size of 200 was calculated for this study. Study participants were randomly selected from target areas. All consenting male patients aged 40 years and above in the target areas for this study at KTH were enrolled until the targeted sample size was reached. Male patients aged less than 40 years and participants who did not give consent were excluded from the study.
2.4 Study duration
The study was done in a period of 6 months from April to September 2019.
2.5 Data collection and analysis
The study objectives were explained to participants, and written and informed consent was obtained. Participants were enrolled utilizing a well-structured questionnaire as shown in “Appendix”. The questionnaire collected demographic information including age, marital status, education and occupation. It also collected data on family history of cancer as well as knowledge, practice and attitudes towards prostate cancer screening. Translations to the questionnaire were done from English to a suitable local language according to the participant’s preference. The responses were recorded as given by the participants.
Data collected during the study were checked for completeness and double-entered into the Epi Info version 7 software. Frequency tables and graphs were generated for relevant variables. The data were analysed using Statistical Package for Social Sciences (SPSS) version 23. For comparing associations between variables, Pearson Chi-square test was performed. A p value of equal or less than 0.05 was considered significant. Binary logistic regression, as well as multivariate analysis, was done. Low knowledge was defined as scoring 1–3 correct responses in the knowledge section, moderate knowledge as scoring 4–6 correct responses and high knowledge as scoring 7–9. Positive attitude was defined as scoring 2 or more correct responses in the section assessing attitudes, while negative was defined as scoring less than 2 correct responses. Practice was assessed with a closed-ended question in the practice section.
A total of 200 participants were enrolled.
3.1 Background characteristics
As illustrated in Table 1 , more than half, 149 (74.5%), of the participants in the study were in the 40–60 years age range. All participants were Christians, 161 (80.5%) had no formal education or had primary education, and 198 (99%) were in informal employment or unemployed.
3.2 Knowledge of prostate cancer
Of the 200 participants enrolled, 67 (33.5%) had heard about prostate cancer, while 133 (66.5%) had never heard about it. Majority, 55.3%, of the participants who had had heard about prostate cancer pointed to a doctor or nurse as a source of information as shown in Table 2 .
Of the 200 participants enrolled in the study, 58 (29%) expressed knowledge on prostate cancer. Among participants who had knowledge, majority of them, 37 (63.8%) had low knowledge as shown in Fig. 1 .
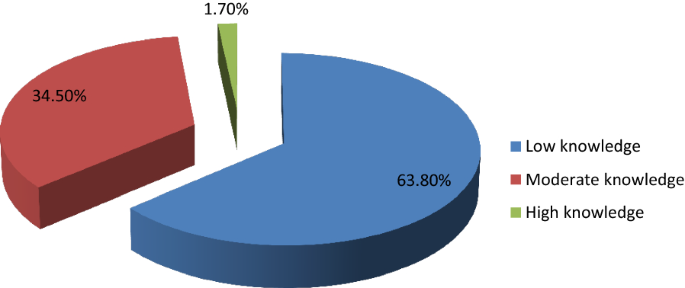
Levels of knowledge
Participants who had secondary school or tertiary education were more knowledgeable about prostate cancer than those who did not have ( p < 0.001). Participants who had heard about prostate cancer were more knowledgeable than those who had not ( p < 0.001). Participants who had heard about prostate cancer had high levels of knowledge compared to those who had not ( p = 0.009). Participants older than 60 years had more knowledge on prostate cancer compared with those below 60 years ( p = 0.026).
3.3 Practice of prostate cancer screening
Of the 200 participants, only 26 (13%) had been screened in the last 2 years. Among participants who had screened, 20 (76.9%) pointed out DRE as the method used, while 3 (11.5%) pointed out PSA, 2 (7.69%) reported both DRE and PSA, and 1 (3.85%) did not know which screening method was used. Among the 26 participants that had screened in the last 2 years, 18 (69.2%) had a positive prostate cancer outcome, while 8 (30.8%) had a negative prostate cancer outcome. 199 (99.5%) of the participants expressed intentions to screen in future.
Age above 60 years was associated with a positive prostate cancer outcome ( p = 0.002). The study also found that participants who were knowledgeable about prostate cancer were more likely to undergo prostate cancer screening ( p < 0.001) and that high level of knowledge was associated with prostate cancer screening practice ( p = 0.024). Increasing age of participants (over 60) was also associated with prostate cancer screening practice in the last 2 years ( p < 0.001).
3.4 Attitude towards prostate cancer screening
Among 200 participants enrolled in the study, 197 (98.5%) had a positive attitude towards prostate cancer screening, while 3 (1.5%) had a negative attitude. There were no statistically significant associations between age and attitude towards prostate cancer screening ( p = 0.099), knowledge and attitude towards prostate cancer screening ( p = 0.868) as well as between practice in the last 2 years and attitude towards prostate cancer screening ( p = 0.291).
3.5 Factors affecting prostate cancer screening practice
Binary logistic regression analysis was performed to identify factors that affect prostate cancer screening. As shown in Table 3 , prostate cancer screening practice was associated with age ( p = 0.017), education ( p = 0.041), knowledge ( p = 0.023) and family history of cancer ( p = 0.003).
All factors that were significant in binary logistic analysis (with p < 0.05) were analysed using multivariate logistic regression. A backward step-by-step elimination method was employed to manually eliminate factors with insignificant p values. As illustrated in Table 4 , only two factors remained statistically significant, namely knowledge and family history of cancer. Participants who had knowledge about prostate cancer were nearly 11 times more likely to practice prostate cancer screening than those who did not have ( p = 0.001). Participants who had a family history of cancer were 26 times more likely to practice prostate cancer screening than those who had a negative family history of cancer ( p = 0.002).
4 Discussion
The study targeted male patients aged 40 years and above due to available literature which indicates that prostate cancer screening should start at 40 years [ 4 , 14 ]. Literature indicates that the average age of a man to be diagnosed with prostate cancer is about 66 years and above [ 4 , 15 ]. Since the majority of the participants in the study, 149 (74.5%), were in the 40–60 years age group, as also observed by Mofolo and colleagues in their study [ 16 ], there was an over representation of participants at the lowest risk of prostate cancer.
The study found low levels of awareness and knowledge. This is similar to findings of other studies done in other countries [ 10 , 17 ]. This implies that there is little sensitization being done to the public and expresses the need for more public sensitization campaigns utilizing both electronic and print media with the aim of early detection and treatment to improve the prognosis [ 7 , 8 , 11 ]. However, other studies found high levels of awareness [ 9 , 18 , 19 , 20 ]. Of the studies that found high levels of knowledge, one of them was done on a group of public servants who were educated, had good access to health information and this was not a reflection of the general population who are mostly uneducated [ 9 ]. The study also demonstrated that majority of the participants who were knowledgeable about prostate cancer had low level of knowledge which was consistent with findings by other studies [ 10 , 18 , 19 , 21 , 22 , 23 ]. This indicates a need for comprehensive knowledge on prostate cancer to promote early detection.
Participants with higher level of education were more knowledgeable about prostate cancer than those who had lower level of education or no formal education at all consistent with findings by similar studies done in other countries [ 16 , 17 , 21 , 24 ]. However, some studies done in Nigeria and Kenya in 2018 did not find such an association [ 10 , 19 ]. In one of the studies that did not find an association between higher education and knowledge, the sample was drawn from a rural part of the country with more than 60% having no formal education or had primary education [ 19 ]. This could have resulted in the finding.
Participants older than 60 years had more knowledge on prostate cancer than those below 60 years as also demonstrated by Adibe et al. [ 24 ]. This highlights a possible bias that might be present in the provision of information on prostate cancer where individuals who are at an advanced age are educated about it because of their increased risk. It could also indicate the natural history of how older patients are more likely to have information about prostate cancer as they visit healthcare centres for urologic problems like benign prostate hyperplasia which are quiet frequent [ 4 , 15 ].
In addition to health workers contributing to the increase in knowledge of prostate cancer, utilizing media platforms that are widely accessible such as radio presents a great opportunity to achieve this. A 2018 study by Kinyao and Kishoyian which assessed attitudes, perceived risk and intention in a rural county found that over 60% of the participants learnt about prostate cancer from the radio [ 19 ]. This shows how much more applicable this media platform can be in developing regions like Africa.
The low level of prostate cancer screening practice demonstrated in the study is consistent with findings from similar studies [ 10 , 11 , 19 , 21 , 25 ] though majority of participants in this study were willing to be screened after discussing about the condition with them consistent with a study done in Kenya [ 21 ]. However, it is inconclusive whether increased knowledge would increase screening as other factors apart from knowledge on prostate cancer appear to influence this practice. A similar study by Kinyao and Kishoyian in 2018 found that many participants had strong fatalistic attitudes towards screening such as “if I am meant to get prostate cancer, I will get it” and these appeared to influence screening [ 19 ]. Thus in sharing information on prostate cancer, cultural beliefs and fatalistic attitudes must also be addressed.
DRE was the most commonly used method of prostate cancer screening contrary to findings by similar studies done in Nigeria that found PSA to be the most commonly used method [ 24 , 25 ]. This suggests a possible cost barrier to utilization of the PSA screening method in our sample.
The finding demonstrated in the study that participants were more likely to screen for prostate cancer if they were older than 60 years is consistent with findings of similar studies done in Uganda and Nigeria [ 11 , 25 ]. This implies that there is a risk of late presentation and consequently poor prognosis. As such, intensified public sensitization campaigns are needed to attain early detection and treatment as well as good prognosis. Participants who were more educated were more likely to undergo prostate cancer screening consistent with findings from a Nigerian study [ 25 ]. The statistically significant association between knowledge on prostate cancer and prostate cancer screening practice is consistent with other similar studies done [ 10 , 11 , 21 ]. This is another indication of the need to intensify prostate cancer sensitization campaigns.
The high positive attitude level demonstrated in the study was similar to findings from other studies done [ 9 , 23 , 24 ]. However, a Ugandan study found a negative attitude towards prostate cancer screening [ 11 ]. This could be because the study explored other factors under attitude that our study did not. The lack of any statistically significant association between age of participants and attitude towards prostate cancer screening concurs with findings from a study done in Uganda [ 11 ]. However, a 2017 study done in Nigeria found an association between age and attitude [ 24 ].
5 Limitations of Study
Generalizability of findings of this study must be done with caution since this was a hospital-based study. There is thus a need for more studies to be done in other institutions such as universities and colleges, urban and rural communities, district, general, central and other teaching hospitals to have comprehensive knowledge. In addition, certain aspects of knowledge were not assessed, for example, that prostate cancer can present without symptoms. As such, the study findings were limited to comparisons with studies that also did not assess the asymptomatic presentation of prostate cancer.
6 Conclusion
The study revealed low knowledge of prostate cancer, low prostate cancer screening practice and positive attitude of men towards prostate cancer screening. Practice of prostate cancer screening was associated with age, education level, knowledge and family history of cancer.
Being the first study to assess knowledge, practice and attitude towards prostate cancer screening in Zambia, it has bridged the knowledge gap and has also provided valuable information for healthcare intervention.
Availability of data and material
Data used in the study is available in additional file 1 captioned ‘Dataset for AFJU-D-19-00041R2 manuscript’ and authors agree to share it.
Abbreviations
- Digital rectal examination
- Prostate-specific antigen
Out-Patient Department
Tropical Diseases Research Centre
National Health Research Authority
Kitwe Teaching Hospital
Global Cancer Project
Blandy J, Kaisary A (2009) Lecture notes urology, 6th edn. Wiley, Hoboken
Google Scholar
Shenoy KR, Shenoy A (2019) Manipal manual of surgery, 4th edn. CBS Publishers Pvt Ltd., Shenoy Nagar
Desousa A, Sonavane S, Mehta J (2012) Psychological aspects of prostate cancer. Prostate Cancer and Prostatic Dis 15(2):120–127
Article CAS Google Scholar
American Cancer Society (2019) Cancer facts and figures, 2019. American Cancer Society, Atlanta
GloboCan (2018). http://globocan.iarc.fr
GloboCan (2012). http://globocan.iarc.fr
National Cancer Control Strategic Plan 2016–2021. Ministry of Health Zambia
So WK, Choi KC, Tang WP, Lee PC, Shiu AT, Ho SS et al (2014) Uptake of prostate cancer screening and associated factors among Chinese men aged 50 or more: a population-based survey. Cancer Biol Med 11(1):56–63
PubMed PubMed Central Google Scholar
Oranusi CK, Mbieri UT, Oranusi IO, Nwofor AME (2012) Prostate cancer awareness and screening among male public servants in Anambra State Nigeria. Afr J Urol 18(2):72–74
Article Google Scholar
Awosan KJ, Yunusa EU, Agwu NP, Taofiq S (2018) Knowledge of prostate cancer and screening practices among men in Sokoto, Nigeria. Asian J Med Sci 9(6):51–56
Nakandi H, Kirabo M, Semugabo C, Kittengo A, Kitayimbwa P, Kalungi S, Maena J (2013) Knowledge, attitudes and practices of Ugandan men regarding prostate cancer. Afr J Urol 19(4):165–170
Ito K (2014) Prostate cancer in Asian men. Nat Rev Urol 11:197
Sichula M, Kabelenga E, Mwanakasale V (2018) Factors influencing malnutrition among under five children at Kitwe Teaching Hospital, Zambia. Int J Curr Innov in Adv Res 1(7):9–18
Canadian National Institute of Health (2013)
Cancer Diseases Hospital (2013) Annual report
Mofolo N, Betshu O, Kenna O, Koroma S, Lebeko T, Claassen FM, Joubert Gina (2015) Knowledge of prostate cancer among males attending a Urology clinic, a South African study. SpringerPlus 4:67
Kabore FA, Kambou T, Zango B, Ouédraogo A (2014) Knowledge and awareness of prostate cancer among the general public in Burkina Faso. J Cancer Educ 29:69–73. https://doi.org/10.1007/s13187-013-0545-2
Article PubMed Google Scholar
Muhammad FHMS, Soon LK, Azlina Y (2016) Knowledge, awareness and perception towards prostate cancer among male public staffs in Kelantan. Int J of Public Health Clin Sci 3(6):105–115
Kinyao M, Kishoyina G (2018) Attitude, perceived risk and intention to screen for prostate cancer by adult men in Kasikeu sub location, Makueni County, Kenya. Ann Med Health Sci Res 8(3):125–132
Agbugui JO, Obarisiagbon EO, Nwajei CO, Osaigbovo EO, Okolo JC, Akinyele AO (2013) Awareness and knowledge of prostate cancer among men in Benin City, Nigeria. J Med Biomed Res 12(2):42–47
Wanyagah P (2013) Prostate cancer awareness, knowledge, perception on self-vulnerability and uptake of screening among men in Nairobi. Kenyatta University, Kenya
Arafa MA, Rabah DM, Wahdan IH (2012) Awareness of general public towards cancer prostate and screening practice in Arabic communities: a comparative multi-center study. Asian Pac J Cancer Prev 13:4321–4326
Makado E, Makado RK, Rusere MT (2015) An assessment of knowledge of and attitudes towards prostate cancer screening among men aged 40 to 60 years at Chitungwiza Central Hospital in Zimbabwe. Int J Humanit Soc Stud 3(4):45–55
Adibe MO, Oyine DA, Abdulmuminu I, Chibueze A (2017) Knowledge, attitudes and perceptions of prostate cancer among male staff of the University of Nigeria. Asian Pac J Cancer Prev 18(7):1961–1966
Ebuechi OM, Otumu IU (2011) Prostate screening practices among male staff of the University of Lagos, Lagos, Nigeria. Afr J Urol 17(4):122–134
Download references
Acknowledgements
First and foremost, I express my sincere, heartfelt and profound gratitude to the Almighty God for guiding, protecting and seeing me through the entire process of conducting this study. I am also grateful to my supervisor Prof. Victor Mwanasakale for his zeal and tireless efforts in seeing to it that this work becomes a reality. Let me also express my gratitude to Prof. Seter Siziya and the entire public health team for all the advice, encouragement. I would also like to thank the entire management at the Copperbelt University Michael Chilufya Sata School of Medicine for a friendly atmosphere. I would also like to thank my family back home for all the support and trust vested in me as well as my friends, roommates and class mates for the moral support.
A thesis submitted in partial fulfilment for the award of the Bachelors degree in medicine and surgery (MBChB).
This work was funded by the Government of the Republic of Zambia through the Ministry of Higher Education through its Higher Education Loans and Scholarships Board. As part of policy, the Ministry of Higher Education through its Higher Education Loans and Scholarships Board finances students in Higher Education institutions whose training demands the carrying out of research. Funding given covers for such expenses incurred during research such as printing and photocopying of data collection tools, transport charges for the researcher during the whole process and ethical clearance charges.
Author information
Authors and affiliations.
Michael Chilufya Sata School of Medicine, The Copperbelt University, P.O. Box 71191, Ndola, Zambia
Sakala Gift & Mwanakasale Victor
Pan - African Organization for Health, Education and Research (POHER), Lusaka, Zambia
Kasongo Nancy
You can also search for this author in PubMed Google Scholar
Contributions
SG is the corresponding author, and KN and MV are the contributing authors. SG constructed the manuscript, collected data, analysed and interpreted data and also edited the manuscript. KN constructed the manuscript, analysed and interpreted data and extensively edited the manuscript. MV constructed the manuscript, extensively edited the manuscript and supervised this thesis study. All authors read and approved the final manuscript.
Author’s information
The corresponding author (S.G) is currently pursuing his Bachelors degree in Medicine and Surgery (MBChB) at the Copperbelt University Michael Chilufya Sata School of Medicine in Zambia. K.N is a Pan African Organization for Health, Education and Research (POHER) scholar with a rich research background, who has presented at so many conferences. She was a recipient of the international research elective which took place at University of Missouri School of Medicine, USA, for 2–3 months. She holds a Bachelors Degree of Medicine and Surgery (MBChB) and graduated as the Best student. M.V was also the supervisor of this work. He holds BSc Human Biology, MBChB, MSc and a PhD in Parasitology and is an Associate Professor of Parasitology at the Copperbelt University Michael Chilufya Sata School of Medicine, Zambia.
Corresponding author
Correspondence to Sakala Gift .
Ethics declarations
Ethics approval and consent to participate.
A request to conduct the study was sought from the Tropical Diseases Research Centre (TDRC) research ethics committee (IRB Registration Number: 00002911, FWA Number: 00003729) as well as the National Health Research Authority (NHRA). Management at Kitwe Teaching Hospital was assured that confidentiality would not be breached and that the data obtained in the study would not be used for any other purpose besides that specified in the study protocol. Informed and written consent was obtained from participants. During data collection, no identifying images or other personal or clinical details of participants were collected. They were treated with at-most respect and dignity and their rights to privacy and confidentiality were not violated at any point.
Consent for publication
In this study, no data that could compromise the anonymity of participants such as images or other personal or clinical details were collected. As such, it was not applicable.
Competing interest
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1:.
Dataset for AFJU-D-19-00041R2 manuscript.
Appendix: Questionnaire
Topic: assessment of knowledge, practice and attitude towards prostate cancer screening among male patients aged 40 years and above at Kitwe Teaching Hospital.
NAME OF INTERVIEWER:…………………………………………………………
SERIAL NUMBER OF PARTICIPANT:…………………………………………….
DATE:…………………………………………………………………………………
1.1 Section A: demographic characteristics
Instruction: Please, tick as appropriate.
1. Age:…………years
2. Marital status: Single [] Married [] Divorced [] Separated []
3. Religion: Christian [] Muslim [] Traditional []
4. Educational level: Primary [] Secondary [] Tertiary [] No formal education []
5. Occupation: Trader [] Civil servant [] Taxi driver [] Businessman [] Electrician [] Mechanic [] Barber [] Other (please specify)
1.2 Section B: family history of cancer
6. Does anyone in your family have cancer? Yes [] No []
i) What type of cancer………………………………………………………
ii) What is their relation to you………………………………………………
7. Has anyone in your family died of Cancer Yes [] No []
i) What type of cancer……………………………………………………..
ii) What is their relation to you……………………………………………
1.3 Section C: knowledge
8. Have you heard of prostate cancer before: Yes [] No []
i) Where did you hear it from Friends [] Read about it [] TV [] Radio [] Doctor [] Nurse [] Relative []
ii) Which gender does prostate cancer affect Men only [] Women only [] Both men and women [] I do not know []
iii) Which of the following factors could make a person more likely to develop prostate cancer. Please tick as many as possible
a) Family history of the disease [] b) Drinking alcohol [] c) Age [] d)Exercise [] e) Diet [] f) Smoking []
9. Do you know symptoms of prostate cancer Yes [] No []
If Yes, what are they? Tick as many as possible. a) Excessive urination at night [] b)Headache [] c) blood in urine [] d) High temperature [] e) Bone pain [] f) Painful sex [] g) Loss of sex drive [] h) Infertility [] i) cough []
10. Is prostate cancer preventable Yes [] No [] I do not know []
a) How can it be prevented? Genital hygiene [] regular screening [] condom use [] use of right diet [] avoiding many sexual partners []
11. Is prostate cancer curable Yes [] No [] I don’t know []
1.4 Section D: practice
12. Have you been screened for prostate cancer within the last two years? Yes [] No []
a) Which method was used Prostate Specific Antigen (PSA) [] Digital Rectal Examination (DRE) [] I do not know []
b) What was the outcome of the screening? Positive [] Negative []
13. Do you have any intention of getting screened in the nearest future? Yes [] No []
1.5 Section E: attitude towards prostate cancer screening
14. Prostate cancer screening is good Yes [] No []
15. Going for prostate cancer screening is a waste of time Yes [] No []
16. Prostate cancer screening has side effects that can cause harmful effects to the body Yes [] No []
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Gift, S., Nancy, K. & Victor, M. Assessment of knowledge, practice and attitude towards prostate cancer screening among male patients aged 40 years and above at Kitwe Teaching Hospital, Zambia. Afr J Urol 26 , 70 (2020). https://doi.org/10.1186/s12301-020-00067-0
Download citation
Received : 01 November 2019
Accepted : 16 September 2020
Published : 07 November 2020
DOI : https://doi.org/10.1186/s12301-020-00067-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Prostate cancer screening
- Help & FAQ
Identifying potential new stem cell biomarkers for prostate cancer
- Dhafer Alghezi
- Department of Life Sciences
Student thesis : Doctoral Thesis › PhD
- prostate cancer, Biomarkers, Stem cells, IHC, Rnascope
File : application/pdf, 7.98 MB
Type : Thesis
Embargo End Date : 5 Jun 2020
Advertisement
Prostate cancer biomarkers: from early diagnosis to precision treatment
- REVIEW ARTICLE
- Published: 14 May 2024
Cite this article

- Versha Dahiya 1 ,
- Sanjana Hans 1 na1 ,
- Ruchi Kumari 1 na1 &
- Gargi Bagchi ORCID: orcid.org/0000-0001-6416-2584 1
Prostate cancer (PCa) is the second most prevalent cancer in men. In 2020, approximately 1,414,259 new cases were reported that accounted for 3,75,324 deaths (Sung et al. in CA 71:209–249, 2021). PCa is often asymptomatic at early stages; hence, routine screening and monitoring based on reliable biomarkers is crucial for early detection and assessment of cancer progression. Early diagnosis of disease is key step in reducing PCa-induced mortality. Biomarkers such as PSA have played vital role in reducing recent PCa deaths. Recent research has identified many other biomarkers and also refined PSA-based tests for non-invasive diagnosis of PCa in patients. Despite progress in screening methods, an important issue that influences treatment is heterogeneity of the cancer in different individuals, necessitating personalized treatment. Currently, focus is to identify biomarkers that can accurately diagnose PCa at early stage, indicate the stage of the disease, metastatic nature and chances of survival based on individual patient profile (Fig. 1 ).
Graphical abstract
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others


Biomarkers in prostate cancer: new era and prospective

Role of Molecular Diagnostics in Prostate Cancer

Prostate Cancer
Data availability.
Not applicable.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2021;71(3):209–49.
PubMed Google Scholar
Rice SM, Oliffe JL, Kelly MT, Cormie P, Chambers S, Ogrodniczuk JS, et al. Depression and prostate cancer: examining comorbidity and male-specific symptoms. Am J Mens Health. 2018;12(6):1864–72.
Article PubMed PubMed Central Google Scholar
Madu CO, Lu Y. Novel diagnostic biomarkers for prostate cancer. J Cancer. 2010;1:150.
Gutman AB, Gutman EB. An “acid” phosphatase occurring in the serum of patients with metastasizing carcinoma of the prostate gland. J Clin Investig. 1938;17(4):473–8.
Article CAS PubMed PubMed Central Google Scholar
Wang MC, Valenzuela LA, Murphy GP, Chu TM. Purification of a human prostate specific antigen. Invest Urol. 1979;17(2):159–63.
CAS PubMed Google Scholar
Stamey TA, Yang N, Hay AR, McNeal JE, Freiha FS, Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317(15):909–16.
Article CAS PubMed Google Scholar
Mettlin C, Lee F, Drago J, Murphy GP. The American cancer society national prostate cancer detection project. Findings on the detection of early prostate cancer in 2425 men. Cancer. 1991;67(12):2949–58.
Basler JW, Thompson IM. Lest we abandon digital rectal examination as a screening test for prostate cancer. J Natl Cancer Inst. 1998;90(23):1761–3.
Mahon SM. Screening for prostate cancer: informing men about their options. Clin J Oncol Nurs. 2005;9(5):625.
Article PubMed Google Scholar
Hara M. Some physiochemical characteristics of gamma-semino-protein: An antigenic component specific for human plasma. Jpn J Legal Med. 1971;25:322–4.
CAS Google Scholar
Rao AR, Motiwala HG, Karim OMA. The discovery of prostate-specific antigen. BJU Int. 2008;101(1):5–10.
Klotz L. Active surveillance for prostate cancer: overview and update. Curr Treat Options Oncol. 2013;14:97–108.
Panzone J, Byler T, Bratslavsky G, Goldberg H. Applications of focused ultrasound in the treatment of genitourinary cancers. Cancers. 2022;14(6):1536.
Crawford ED, Scholz MC, Kar AJ, Fegan JE, Haregewoin A, Kaldate RR, et al. Cell cycle progression score and treatment decisions in prostate cancer: results from an ongoing registry. Curr Med Res Opin. 2014;30(6):1025–31.
Welti J, Rodrigues DN, Sharp A, Sun S, Lorente D, Riisnaes R, et al. Analytical validation and clinical qualification of a new immunohistochemical assay for androgen receptor splice variant-7 protein expression in metastatic castration-resistant prostate cancer. Eur Urol. 2016;70(4):599–608.
Van der Kwast TH. Prognostic prostate tissue biomarkers of potential clinical use. Virchows Arch. 2014;464(3):293–300.
Bazzichetto C, Conciatori F, Pallocca M, Falcone I, Fanciulli M, et al. PTEN as a prognostic/predictive biomarker in cancer: an unfulfilled promise? Cancers. 2019;11(4):435.
Coradduzza D, Solinas T, Balzano F, Culeddu N, Rossi N, Cruciani S, et al. miRNAs as molecular biomarkers for prostate cancer. J Mol Diagn. 2022;24(11):1171–80.
Rana S, Valbuena GN, Curry E, Bevan CL, Keun HC. MicroRNAs as biomarkers for prostate cancer prognosis: a systematic review and a systematic reanalysis of public data. Br J Cancer. 2022;126(3):502–13.
Wang W, Wang M, Wang L, Adams TS, Tian Y, Xu J. Diagnostic ability of% p2PSA and prostate health index for aggressive prostate cancer: a meta-analysis. Sci Rep. 2014;4(1):5012.
De La Calle C, Patil D, Wei JT, Scherr DS, Sokoll L, Chan DW, et al. Multicenter evaluation of the prostate health index to detect aggressive prostate cancer in biopsy naive men. J Urol. 2015;194(1):65–72.
Stephan C, Ralla B, Jung K. Prostate-specific antigen and other serum and urine markers in prostate cancer. Biochem Biophys Acta. 2014;1846(1):99–112.
Filella X, Gimenez N. Evaluation of [− 2] proPSA and Prostate Health Index (phi) for the detection of prostate cancer: a systematic review and meta-analysis. Clin Chem Lab Med. 2013;51(4):729–39.
Hernández J, Thompson IM. Prostate-specific antigen: a review of the validation of the most commonly used cancer biomarker. Cancer. 2004;101(5):894–904.
Punnen S, Freedland SJ, Polascik TJ, Loeb S, Risk MC, Savage S, et al. A multi-institutional prospective trial confirms noninvasive blood test maintains predictive value in African American men. J Urol. 2018;199(6):1459–63.
Hayes VM, Bornman MR. Prostate cancer in Southern Africa: does Africa hold untapped potential to add value to the current understanding of a common disease? J Global Oncol. 2018;4:1–7.
Google Scholar
Voigt JD, Dong Y, Linder V, Zappala S. Use of the 4Kscore test to predict the risk of aggressive prostate cancer prior to prostate biopsy: Overall cost savings and improved quality of care to the us healthcare system. Rev Urol. 2017;19(1):1.
PubMed PubMed Central Google Scholar
Zappala SM, Scardino PT, Okrongly D, Linder V, Dong Y. Clinical performance of the 4Kscore Test to predict high-grade prostate cancer at biopsy: A meta-analysis of us and European clinical validation study results. Rev urol. 2017;19(3):149.
Lin DW, Newcomb LF, Brown MD, Sjoberg DD, Dong Y, Brooks JD, et al. Evaluating the four kallikrein panel of the 4Kscore for prediction of high-grade prostate cancer in men in the Canary Prostate Active Surveillance Study. Eur Urol. 2017;72(3):448–54.
Stattin P, Vickers AJ, Sjoberg DD, Johansson R, Granfors T, Johansson M, et al. Improving the specificity of screening for lethal prostate cancer using prostate-specific antigen and a panel of kallikrein markers: a nested case–control study. Eur Urol. 2015;68(2):207–13.
Catalona WJ, Partin AW, Sanda MG, Wei JT, Klee GG, Bangma CH, et al. A multicenter study of [-2] pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J Urol. 2011;185(5):1650–5.
Porzycki P, Ciszkowicz E. Modern biomarkers in prostate cancer diagnosis. Central Eur J Urol. 2020;73(3):300.
Filella X, Foj L, Augé JM, Molina R, Alcover J. Clinical utility of% p2PSA and prostate health index in the detection of prostate cancer. Clin Chem Lab Med. 2014;52(9):1347–55.
Loeb S, Sanda MG, Broyles DL, Shin SS, Bangma CH, Wei JT, et al. The prostate health index selectively identifies clinically significant prostate cancer. J Urol. 2015;193(4):1163–9.
Hussein AA, Baban R, Hussein A. Prostate-specific antigen and free prostate-specific antigen/prostate-specific antigen ratio in patients with benign prostatic hyperplasia and prostate cancer. Baghdad J Biochem Appl Biol Sci. 2020;1(01):18–26.
Article Google Scholar
Eyrich NW, Morgan TM, Tosoian JJ. Biomarkers for detection of clinically significant prostate cancer: contemporary clinical data and future directions. Transl Androl Urol. 2021;10(7):3091.
Cui Y, Cao W, Li Q, Shen H, Liu C, Deng J, et al. Evaluation of prostate cancer antigen 3 for detecting prostate cancer: a systematic review and meta-analysis. Sci Rep. 2016;6(1):25776.
Kornberg Z, Cooperberg MR, Spratt DE, Feng FY. Genomic biomarkers in prostate cancer. Transl Androl Urol. 2018;7(3):459.
Tutrone R, Donovan MJ, Torkler P, Tadigotla V, McLain T, Noerholm M, et al. Clinical utility of the exosome based ExoDx Prostate (IntelliScore) EPI test in men presenting for initial Biopsy with a PSA 2–10 ng/mL. Prostate Cancer Prostatic Dis. 2020;23(4):607–14.
Marrugo-Ramírez J, Mir M, Samitier J. Blood-based cancer biomarkers in liquid biopsy: a promising non-invasive alternative to tissue biopsy. Int J Mol Sci. 2018;19(10):2877.
Jamaspishvili T, Kral M, Khomeriki I, Student V, Kolar Z, Bouchal J. Urine markers in monitoring for prostate cancer. Prostate Cancer Prostatic Dis. 2010;13(1):12–9.
Tomlins SA, Bjartell A, Chinnaiyan AM, Jenster G, Nam RK, Rubin MA, et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur Urol. 2009;56(2):275–86.
Hessels D, Schalken JA. The use of PCA3 in the diagnosis of prostate cancer. Nat Rev Urol. 2009;6(5):255–61.
Nicholson A, Mahon J, Boland A, Beale S, Dwan K, Fleeman N, et al. The clinical effectiveness and cost-effectiveness of the PROGENSA® prostate cancer antigen 3 assay and the Prostate Health Index in the diagnosis of prostate cancer: a systematic review and economic evaluation. Health Technol Assess. 2015;19(87):1–31.
Munroz Rodríguez SVM, García-Perdomo HA. Diagnostic accuracy of prostate cancer antigen 3 (PCA3) prior to first prostate biopsy: A systematic review and meta-analysis. Can Urol Assoc J. 2020;14(5):E214.
Kristiansen G. Diagnostic and prognostic molecular biomarkers for prostate cancer. Histopathology. 2012;60(1):125–41.
Kretschmer A, Tilki D. Biomarkers in prostate cancer–current clinical utility and future perspectives. Crit Rev Oncol Hematol. 2017;120:180–93.
Alford AV, Brito JM, Yadav KK, Yadav SS, Tewari AK, Renzulli J. The use of biomarkers in prostate cancer screening and treatment. Rev Urol. 2017;19(4):221.
Kohaar I, Petrovics G, Srivastava S. A rich array of prostate cancer molecular biomarkers: opportunities and challenges. Int J Mol Sci. 2019;20(8):1813.
Fujita K, Nonomura N. Urinary biomarkers of prostate cancer. Int J Urol. 2018;25(9):770–9.
Demichelis F, Fall K, Perner S, Andrén O, Schmidt F, Setlur SR, et al. TMPRSS2: ERG gene fusion associated with lethal prostate cancer in a watchful waiting cohort. Oncogene. 2007;26(31):4596–9.
McKiernan J, Donovan MJ, O’Neill V, Bentink S, Noerholm M, Belzer S, et al. A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncol. 2016;2(7):882–9.
Humphrey PA. Histopathology of prostate cancer. Cold Spring Harb Perspect Med. 2017;7(10):a030411.
Basourakos SP, Tzeng M, Lewicki PJ, Patel K, Awamlh BAHA, Venkat S, et al. Tissue-based biomarkers for the risk stratification of men with clinically localized prostate cancer. Front Oncol. 2021;11:676716.
Lokeshwar SD, Klaassen Z, Saad F. Treatment and trials in non-metastatic castration-resistant prostate cancer. Nat Rev Urol. 2021;18(7):433–42.
Moschini M, Spahn M, Mattei A, Cheville J, Karnes RJ. Incorporation of tissue-based genomic biomarkers into localized prostate cancer clinics. BMC Med. 2016;14(1):1–7.
Khoo A, Liu LY, Nyalwidhe JO, Semmes OJ, Vesprini D, Downes MR, et al. Proteomic discovery of non-invasive biomarkers of localized prostate cancer using mass spectrometry. Nat Rev Urol. 2021;18(12):707–24.
Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71(4):618–29.
Ahmed HU, Bosaily AES, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–22.
Erho N, Crisan A, Vergara IA, Mitra AP, Ghadessi M, Buerki C, et al. Discovery and validation of a prostate cancer genomic classifier that predicts early metastasis following radical prostatectomy. PLoS ONE. 2013;8(6):e66855.
Spratt DE, Dai DL, Den RB, Troncoso P, Yousefi K, Ross AE, et al. Performance of a prostate cancer genomic classifier in predicting metastasis in men with prostate-specific antigen persistence postprostatectomy. Eur Urol. 2018;74(1):107–14.
Behm-Ansmant I, Rehwinkel J, Izaurralde E. MiRNAs silence gene expression by repressing protein expression and/or by promoting mRNA decay. Quant Biol. 2006;71:523–30.
Article CAS Google Scholar
Pedroza-Torres A, Romero-Córdoba SL, Justo-Garrido M, Salido-Guadarrama I, Rodríguez-Bautista R, Montaño S, et al. MicroRNAs in tumor cell metabolism: roles and therapeutic opportunities. Front Oncol. 2019;9:1404.
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci. 2008;105(30):10513–8.
Meiri E, Mueller WC, Rosenwald S, Zepeniuk M, Klinke E, Edmonston TB, et al. A second-generation microRNA-based assay for diagnosing tumor tissue origin. Oncologist. 2012;17(6):801–12.
Li D, Hao X, Song Y. Identification of the key MicroRNAs and the miRNA-mRNA regulatory pathways in prostate cancer by bioinformatics methods. Biomed Res Int. 2018. https://doi.org/10.1155/2018/6204128 .
Guo H, Qi RQ, Sheng J, Liu C, Ma H, Wang HX, et al. MiR-155, a potential serum marker of extramammary Paget’s disease. BMC Cancer. 2018;18:1–8.
Wani S, Kaul D, Mavuduru RS, Kakkar N, Bhatia A. Urinary-exosomal miR-2909: a novel pathognomonic trait of prostate cancer severity. J Biotechnol. 2017;259:135–9.
Matin F, Jeet V, Moya L, Selth LA, Chambers S, Yeadon APCB, et al. A plasma biomarker panel of four microRNAs for the diagnosis of prostate cancer. Sci Rep. 2018;8(1):6653.
Leite KR, Morais DR, Reis ST, Viana N, Moura C, Florez MG, et al. MicroRNA 100: a context dependent miRNA in prostate cancer. Clinics. 2013;68:797–802.
Ghamlouche F, Yehya A, Zeid Y, Fakhereddine H, Fawaz J, Liu YN, Abou-Kheir W. MicroRNAs as clinical tools for diagnosis, prognosis, and therapy in prostate cancer. Transl Oncol. 2023;28:101613.
Barceló M, Castells M, Bassas L, Vigués F, Larriba S. Semen miRNAs contained in exosomes as non-invasive biomarkers for prostate cancer diagnosis. Sci Rep. 2019;9(1):13772.
Cuzick J, Swanson GP, Fisher G, Brothman AR, Berney DM, Reid JE, et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 2011;12(3):245–55.
Huang X, Yuan T, Liang M, Du M, Xia S, Dittmar R, et al. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur Urol. 2015;67(1):33–41.
Penney KL, Sinnott JA, Fall K, Pawitan Y, Hoshida Y, Kraft P, et al. mRNA expression signature of Gleason grade predicts lethal prostate cancer. J Clin Oncol. 2011;29(17):2391.
Prensner JR, Iyer MK, Sahu A, Asangani IA, Cao Q, Patel L, et al. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat Genet. 2013;45(11):1392–8.
Xiong T, Li J, Chen F, Zhang F. PCAT-1: a novel oncogenic long non-coding RNA in human cancers. Int J Biol Sci. 2019;15(4):847.
Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47(3):199–208.
Camacho CV, Choudhari R, Gadad SS. Long noncoding RNAs and cancer, an overview. Steroids. 2018;133:93–5.
Qiu M, Xu Y, Wang J, Zhang E, Sun M, Zheng Y, et al. A novel lncRNA, LUADT1, promotes lung adenocarcinoma proliferation via the epigenetic suppression of p27. Cell Death Dis. 2015;6(8):e1858–e1858.
Lemos AEG, Ferreira LB, Batoreu NM, de Freitas PP, Bonamino MH, Gimba ERP. PCA3 long noncoding RNA modulates the expression of key cancer-related genes in LNCaP prostate cancer cells. Tumor Biol. 2016;37:11339–48.
Sanda MG, Feng Z, Howard DH, Tomlins SA, Sokoll LJ, Chan D, et al. Association between combined TMPRSS2:ERG and PCA3 RNA urinary testing and detection of aggressive prostate cancer. JAMA Oncol. 2017;3(8):1085–93. https://doi.org/10.1001/jamaoncol.2017.0177 .
Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1(5):391–407.
Prensner JR, Sahu A, Iyer MK, Malik R, Chandler B, Asangani IA, et al. The lncRNAs PCGEM1 and PRNCR1 are not implicated in castration resistant prostate cancer. Oncotarget. 2014;5(6):1434.
Mehra R, Shi Y, Udager AM, Prensner JR, Sahu A, Iyer MK, et al. A novel RNA in situ hybridization assay for the long noncoding RNA SChLAP1 predicts poor clinical outcome after radical prostatectomy in clinically localized prostate cancer. Neoplasia. 2014;16(12):1121–7.
Cozar JM, Robles-Fernandez I, Rodriguez-Martinez A, Puche-Sanz I, Vazquez-Alonso F, Lorente JA, et al. The role of miRNAs as biomarkers in PCa. Mutat Res Rev Mutat Res. 2019;781:165–74.
Allemailem KS, Almatroudi A, Alrumaihi F, Makki Almansour N, Aldakheel FM, Rather RA, et al. Single nucleotide polymorphisms (SNPs) in prostate cancer: its implications in diagnostics and therapeutics. Am J Transl Res. 2021;13(4):3868–89.
CAS PubMed PubMed Central Google Scholar
Chang HH, Lee CH, Chen YT, Huang CY, Yu CC, Lin VC, et al. Genetic analysis reveals the prognostic significance of the DNA mismatch repair gene MSH2 in advanced prostate cancer. Cancers. 2022;14(1):223.
Lee CH, Pao JB, Lu TL, Lee HZ, Lee YC, Liu CC, et al. Prognostic value of prostaglandin-endoperoxide synthase 2 polymorphisms in prostate cancer recurrence after radical prostatectomy. Int J Med Sci. 2016;13(9):696–700. https://doi.org/10.7150/ijms.16259 .
Mangolini A, Rocca C, Bassi C, Ippolito C, Negrini M, Dell’Atti L, et al. Detection of disease-causing mutations in prostate cancer by NGS sequencing. Cell Biol Int. 2022;46(7):1047–61.
Goel S, Bhatia V, Kundu S, Biswas T, Carskadon S, Gupta N, et al. Transcriptional network involving ERG and AR orchestrates Distal-less homeobox-1 mediated prostate cancer progression. Nat Commun. 2021;12(1):5325.
Singh JP, Dagar M, Dagar G, Kumar S, Rawal S, Bagchi G, et al. Activation of GPR56, a novel adhesion GPCR, is necessary for nuclear androgen receptor signaling in prostate cells. PLoS ONE. 2020;15(9):e0226056.
Sánchez Iglesias Á, Morillo Macías V, Picó Peris A, Fuster-Matanzo A, Nogué Infante A, Muelas Soria R, et al. Prostate region-wise imaging biomarker profiles for risk stratification and biochemical recurrence prediction. Cancers. 2023;15(16):4163.
Padhani AR, Schoots IG. Prostate cancer screening—stepping forward with MRI. Eur Radiol. 2023;33(10):6670–6.
Eklund M, Jäderling F, Discacciati A, Bergman M, Annerstedt M, Aly M, et al. MRI-targeted or standard biopsy in prostate cancer screening. N Engl J Med. 2021;385(10):908–20.
Nordström T, Discacciati A, Bergman M, Clements M, Aly M, Annerstedt M, et al. Prostate cancer screening using a combination of risk-prediction, MRI, and targeted prostate biopsies (STHLM3-MRI): a prospective, population-based, randomised, open-label, non-inferiority trial. Lancet Oncol. 2021;22(9):1240–9.
Yaman Agaoglu F, Kovancilar M, Dizdar Y, Darendeliler E, Holdenrieder S, Dalay N, et al. Investigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancer. Tumor Biol. 2011;32:583–8.
Download references
Acknowledgements
We are thankful to Science & Engineering Research Board (SERB) grant CRG/2019/002583.
Science and Engineering Research Board, CRG/2019/002583, Gargi Bagchi.
Author information
Sanjana Hans and Ruchi Kumari have contributed equally to this work.
Authors and Affiliations
Amity Institute of Biotechnology, Amity University Haryana, Gurgaon, India, 122413
Versha Dahiya, Sanjana Hans, Ruchi Kumari & Gargi Bagchi
You can also search for this author in PubMed Google Scholar
Contributions
Gargi Bagchi: conceptualization, supervision, review and editing. Versha Dahiya: manuscript writing, preparing figures and tables. Sanjana Hans and Ruchi Kumari: manuscript writing.
Corresponding author
Correspondence to Gargi Bagchi .
Ethics declarations
Conflict of interests.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical statement
“No ethical approval was required as this study did not involve human participants or laboratory animals.”
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Dahiya, V., Hans, S., Kumari, R. et al. Prostate cancer biomarkers: from early diagnosis to precision treatment. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03508-2
Download citation
Received : 26 February 2024
Accepted : 26 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1007/s12094-024-03508-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Prostate cancer
- New generation biomarkers
- Find a journal
- Publish with us
- Track your research
Prostate Cancer Research Results and Study Updates
See Advances in Prostate Cancer Research for an overview of recent findings and progress, plus ongoing projects supported by NCI.
Under a new FDA approval, enzalutamide (Xtandi) can now be used alone, or in combination with leuprolide, to treat people with nonmetastatic prostate cancer that is at high risk of returning after surgery or radiation.
FDA approved enzalutamide (Xtandi) combined with talazoparib (Talzenna) for metastatic castration-resistant prostate cancer with alterations in any of 12 DNA repair genes. The drug combination, which blocks both DNA repair activities and hormones that fuel cancer growth, was more effective than the standard treatment in a large clinical trial.
The Decipher genomic test found high-risk prostate cancer even when conventional tests said the tumors were lower risk. This discrepancy appeared to happen more frequently for African-American men.
Men diagnosed with low-risk prostate cancer are increasingly opting against immediate treatment and choosing active surveillance instead, a new study finds. In fact, rates of active surveillance more than doubled between 2014 and 2021.
Adding darolutamide (Nubeqa) to ADT and docetaxel (Taxotere) can improve how long men with hormone-sensitive metastatic prostate cancer live without causing more side effects, results from the ARASENS trial show.
Many with prostate cancer can safely receive shorter, higher-dose radiation therapy after surgery, a new study has found. The approach, called HYPORT, didn’t harm patients’ quality of life compared with the standard radiation approach, trial finds.
A drug called Lu177-PSMA-617 may be a new option for treating advanced prostate cancer. In a large clinical trial, adding the drug—a type of radiopharmaceutical—to standard treatments improved how long participants lived.
For some men with prostate cancer, a genetic biomarker test called Decipher may help predict if their cancer will spread elsewhere in the body. The test could help determine whether hormone therapy, which can cause distressing side effects, is needed.
FDA’s recent approval of relugolix (Orgovyx) is expected to affect the treatment of men with advanced prostate cancer. A large clinical trial showed that relugolix was more effective at reducing testosterone levels than another common treatment.
FDA has approved olaparib (Lynparza) and rucaparib (Rubraca) to treat some men with metastatic prostate cancer. The PARP inhibitors are approved for men whose cancers have stopped responding to hormone treatment and have specific genetic alterations.
For some men with prostate cancer at high risk of spreading, a large clinical trial shows an imaging method called PSMA PET-CT is more likely to detect metastatic tumors than the standard imaging approach used in many countries.
Testing for prostate cancer with a combined biopsy method led to more accurate diagnosis and prediction of the course of the disease in an NCI study. The method is poised to reduce the risk of prostate cancer overtreatment and undertreatment.
In the Veterans Affairs health care system—where all patients have equal access to care—African American men did not appear to have more-aggressive prostate cancer when diagnosed or a higher death rate from the disease than non-Hispanic white men.
In two large clinical trials, the drugs enzalutamide (Xtandi) and apalutamide (Erleada), respectively, combined with the androgen deprivation therapy, improved the survival of men with metastatic prostate cancer that still responds to hormone-suppressing therapies.
The Prostate Cancer Prevention Trial showed that finasteride can reduce the risk of prostate cancer, but might increase the risk of aggressive disease. NCI’s Howard Parnes talks about subsequent findings and what they mean for men aged 55 and older.
The investigational drug darolutamide can help delay the spread of prostate cancer in some men with the disease, a recent clinical trial shows. In addition, the drug caused fewer side effects than similar prostate cancer drugs.
For African American men, the risk of dying from low-grade prostate cancer is double that of men of other races, a new study has found. But, despite the increase, the risk is still small.
Researchers have found that men with advanced prostate cancer may be more likely than previously thought to develop a more aggressive form of the disease. The subtype, called t-SCNC, was linked with shorter survival than other subtypes.
RESPOND is the largest coordinated study on biological and non-biological factors associated with aggressive prostate cancer in African-American men. The study is an effort to learn why these men disproportionally experience aggressive disease.
In a small clinical trial, researchers compared the efficacy of a much lower dose of the cancer drug abiraterone (Zytiga) taken with a low-fat breakfast with a full dose taken on an empty stomach, as directed on the drug’s label.
In the trial that led to the approval, apalutamide (Erleada) delayed cancer metastasis for men with prostate cancer that is resistant to androgen deprivation therapy.
A new study in mice has revealed a molecular link between a high-fat diet and the growth and spread of prostate cancer. The findings, the study leaders believe, raise the possibility that changes in diet could potentially improve treatment outcomes in some men.
The Food and Drug Administration (FDA) has expanded the approval of abiraterone (Zytiga®) for men with prostate cancer. The agency approved abiraterone, in combination with the steroid prednisone, for men with metastatic prostate cancer that is responsive to hormone-blocking treatments (also known as castration-sensitive) and is at high risk of progressing.
Researchers have identified an emerging subtype of metastatic prostate cancer that is resistant to therapies that block hormones that fuel the disease.
In two large clinical trials, adding the hormone-blocking drug abiraterone to androgen-deprivation therapy (ADT) allowed men with metastatic hormone-sensitive prostate cancer to live longer than men who were treated with ADT alone.
Findings from a new study show testing for two biomarkers in urine may help some men avoid an unnecessary biopsy to detect a suspected prostate cancer.
Long-term results from an NCI-sponsored clinical trial suggest that adding androgen deprivation therapy to radiation therapy can improve survival for some men with recurrent prostate cancer.
Researchers estimate that nearly 12% of men with advanced prostate cancer have inherited mutations in genes that play a role in repairing damaged DNA.
Researchers have identified a potential alternative approach to blocking a key molecular driver of an advanced form of prostate cancer, called androgen-independent or castration-resistant prostate cancer.
MSc Thesis: Non-invasive and accurate prediction of prostate cancer aggressiveness
With estimates of 1 600 000 cases and more than 350 000 deaths annually worldwide, prostate cancer is among the most common cancers in men [1]. Diagnosis of prostate cancer is typically done by using ultrasound-guided needle biopsies. An approach that not only involves risks, due to the invasive nature of the method, but also generally underestimates the aggressiveness of the tumor [2]. Standard positron emission tomography (PET) imaging with prostate cancer specific tracers and specifically PSMA-PET have shown to be advantageous for delineating suspicious lesions during biopsy [3, 4].
We want to use the information provided by these PET and multi-parametric MRI images to directly predict Gleason scores that accurately report the aggressiveness of the prostate cancers without the risks associated with an invasive biopsy. Recent research at our institution has produced promising results predicting Gleason scores from handcrafted radiomic features [5]. This work aims at building on top of this research and to develop an end-to-end deep learning pipeline for predicting tumor aggressiveness directly from different imaging modalities. We have exclusive access to a dataset which includes PSMA-11 PET/MRI scans, segmentation maps and the pathological information of the tumors. Additional public data is available through the PI-CAI Challenge . The focus of this work is to build a complete pipeline for analysing multi-modal medical imaging datasets. This includes research on understanding the diverse data available, exploring different approaches for dealing with the limited dataset size (data augmentation, pre-training etc.) and finding a suitable model and evaluation metric for this use case.
Your qualifications:
We are looking for a highly motivated Master’s student in Computer Science, Physics, Engineering or Mathematics. You will establish a comprehensive medical imaging pipeline in PyTorch to extract information from these images. You will be working together with me and under the supervision of Prof. Daniel Rückert. Importantly, we aim to publish the results of this work, with you, in a follow up study at a high-impact medical imaging conference or in an academic journal.
- Strong motivation and interest in machine learning and medical imaging.
- Advanced programming skills in Python and a common DL framework (PyTorch, Tensorflow).
- Strong interest in teamwork and interdisciplinary research.
What we offer:
- An exciting research project with many possibilities to bring in your own ideas.
- Close supervision and access to state-of-the-art computer hardware.
- The chance to work in a team of highly qualified experts in image processing, computer vision and deep learning.
[1] 1. Global Burden of Disease Cancer Collaboration (2017). Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524. [2] Cohen et al. (2008). Comparing the Gleason prostate biopsy and Gleason prostatectomy grading system: the Lahey Clinic Medical Center experience and an international meta-analysis. EurUrol. 2008;54(2):371-81. [3] Fendler et al. (2017). Joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44(6):1014–24. [4] Maurer et al. (2016). Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13(4):226–35. [5] Solari et al. (2021). The added value of PSMA PET/MR radiomics for prostate cancer staging. Eur J Nucl Med Mol Imaging. 2022;49:527–538.
Florian A. Hölzl
Phd student.
My research interests include privacy-preserving deep learning in general and in medical imaging specifically.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-.

PDQ Cancer Information Summaries [Internet].
Prostate cancer prevention (pdq®).
PDQ Screening and Prevention Editorial Board .
Published online: March 7, 2024.
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about prostate cancer prevention. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
This summary is reviewed regularly and updated as necessary by the PDQ Screening and Prevention Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Note: The Overview section summarizes the published evidence on this topic. The rest of the summary describes the evidence in more detail.
Other PDQ summaries on Prostate Cancer Screening ; Prostate Cancer Treatment ; and Levels of Evidence for Cancer Screening and Prevention Studies are also available.
Benefits From Finasteride and Dutasteride Chemoprevention
Chemoprevention with finasteride and dutasteride reduces the incidence of prostate cancer, but the evidence is inadequate to determine whether chemoprevention with finasteride or dutasteride reduces mortality from prostate cancer.
Magnitude of Effect : In the Prostate Cancer Prevention Trial (PCPT), absolute reduction in incidence for more than 7 years with finasteride as compared with placebo was 6% (18.4% with finasteride and 24.4% with placebo); relative risk reduction (RRR) for incidence was 24.8% (95% confidence interval [CI], 18.6%–30.6%). With long-term follow-up (median, 18.4 years), prostate cancer mortality was not statistically different between men in the placebo and finasteride groups of PCPT (hazard ratio [HR], finasteride vs. placebo, 0.75; 95% CI, 0.50–1.12). Long-term follow-up (median, 16 years) of PCPT participants found that with 7 years of finasteride therapy, there was a 21.1% relative reduction in risk of prostate cancer.[ 1 ]
In the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) randomized trial of dutasteride versus placebo, using the restricted crude rate, the absolute risk reduction was 5.1% at 4 years, and the RRR was 22.8% (95% CI, 15.2%–29.8%; P < .001). There was no difference in prostate cancer or overall mortality, although the number of deaths was small and none were due to prostate cancer. The reduction in prostate cancer incidence occurred primarily in Gleason score 5 to 6 cancers.[ 2 ] That the reduction in incidence was primarily in less aggressive cancers (i.e., Gleason score 5–6) and not in more aggressive cancers (i.e., Gleason score 7–10) raises the question of whether this reduction in incidence would lead to any reduction in mortality. This question is presently unanswered.
- Study Design : Two randomized controlled trials; one for finasteride and one for dutasteride.
- Internal Validity : Good for the outcome of incidence, poor for the outcome of mortality.
- Consistency : Good.
- External Validity : The studies focused on different populations. The finasteride trial enrolled men with a prostate-specific antigen (PSA) of less than 3 ng/mL, constituting the majority of U.S. men, but those with a lower risk of cancer. In the dutasteride trial, men were at somewhat higher risk, with a PSA of 2.5 to 10.0 ng/mL and a prior negative biopsy. As such, results are generalizable primarily to these respective populations.
Harms From Finasteride and Dutasteride Chemoprevention
Finasteride.
Men in the finasteride group had statistically significantly more erectile dysfunction, loss of libido, and gynecomastia than men in the placebo group. Men in the finasteride group had a statistically significant higher incidence of high-grade (Gleason score 7–10) cancers during the study than did men in the placebo group (relative risk, 1.27; 95% CI, 1.07–1.50).[ 3 ] Subsequent studies showed that diagnostic tests (PSA, prostate digital rectal exam, and prostate biopsy) had improved performance for detection of cancer and of high-grade cancer in men who received finasteride.[ 4 - 6 ] Long-term follow-up in the finasteride trial (PCPT) found no increased risk of prostate cancer mortality (HR, finasteride vs. placebo, 0.75; 95% CI, 0.50–1.12).
Magnitude of Effect : Statistically significant increases in the following outcomes were observed in the finasteride group (a greater fraction of men in the finasteride group [36.8%] temporarily discontinued treatment at some time during the study for reasons other than death or a diagnosis of prostate cancer than in the placebo group [28.9%]):
- Reduced volume of ejaculate (60.4% vs. 47.3%).
- Erectile dysfunction (67.4% vs. 61.5%).
- Loss of libido (65.4% vs. 59.6%).
- Gynecomastia (4.5% vs. 2.8%).
- Internal Validity : Good: The finasteride trial used two subject-completed sexual functioning instruments administered at enrollment, randomization, 6 months, and annually over the 7-year study. The dutasteride trial administered a sexual functioning instrument after completion of placebo run-in and annually thereafter.
- Consistency : Good (evidence other than the randomized controlled trial supports these effects).
- External Validity : As above, the studies evaluated two different populations: PSA less than or equal to 3 ng/mL in the finasteride trial and PSA of 2.5 to 10.0 ng/mL with a prior negative biopsy in the REDUCE trial. The results are most generalizable to these two populations.
Dutasteride
Overall, 4.3% of men in the dutasteride group compared with 2% of men in the placebo group discontinued the trial because of drug-related adverse events ( P < .001). Men in the dutasteride group had a higher incidence of decreased libido, loss of libido, decreased semen volume, erectile dysfunction, and gynecomastia than men in the placebo group.[ 2 ]
Magnitude of Effect : Increases in the following outcomes were observed in the dutasteride group:
- Decreased libido (3.3% vs. 1.6%).
- Loss of libido (1.9% vs. 1.3%).
- Decreased semen volume (1.4% vs. 0.2%).
- Erectile dysfunction (9.0% vs. 5.7%).
- Gynecomastia (1.9% vs. 1.0%).
U.S. Food and Drug Administration (FDA) Review of Finasteride and Dutasteride
The Oncology Drugs Advisory Committee of the FDA examined both finasteride and dutasteride in 2010. Neither agent was recommended for use for chemoprevention of prostate cancer.
Other Prevention Interventions
The Selenium and Vitamin E Cancer Prevention Trial (SELECT [NCT00006392]) was a large randomized placebo-controlled trial of vitamin E and selenium. It showed no reduction in prostate cancer period prevalence, but an increased risk of prostate cancer with vitamin E alone.[ 7 ]
Magnitude of Effect : Compared with the placebo group in which 529 men developed prostate cancer, there was a statistically significant increase in prostate cancer in the vitamin E group (620 cases) but not in the selenium plus vitamin E group (555 cases) or in the selenium group (575 cases). The magnitude of increase in prostate cancer risk with vitamin E alone was 17%.
- Study Design for Vitamin E and Selenium : Randomized, placebo-controlled trial of selenium (200 µg/d from L-selenomethionine), vitamin E (400 IU/d of all-rac-[alpha]-tocopheryl acetate), or both.
- Internal Validity : Good.
- External Validity : Good.
- Unger JM, Hershman DL, Till C, et al.: Using Medicare Claims to Examine Long-term Prostate Cancer Risk of Finasteride in the Prostate Cancer Prevention Trial. J Natl Cancer Inst 110 (11): 1208-1215, 2018. [ PMC free article : PMC6235685 ] [ PubMed : 29534197 ]
- Andriole GL, Bostwick DG, Brawley OW, et al.: Effect of dutasteride on the risk of prostate cancer. N Engl J Med 362 (13): 1192-202, 2010. [ PubMed : 20357281 ]
- Thompson IM, Goodman PJ, Tangen CM, et al.: The influence of finasteride on the development of prostate cancer. N Engl J Med 349 (3): 215-24, 2003. [ PubMed : 12824459 ]
- Thompson IM, Chi C, Ankerst DP, et al.: Effect of finasteride on the sensitivity of PSA for detecting prostate cancer. J Natl Cancer Inst 98 (16): 1128-33, 2006. [ PubMed : 16912265 ]
- Thompson IM, Tangen CM, Goodman PJ, et al.: Finasteride improves the sensitivity of digital rectal examination for prostate cancer detection. J Urol 177 (5): 1749-52, 2007. [ PubMed : 17437804 ]
- Lucia MS, Epstein JI, Goodman PJ, et al.: Finasteride and high-grade prostate cancer in the Prostate Cancer Prevention Trial. J Natl Cancer Inst 99 (18): 1375-83, 2007. [ PubMed : 17848673 ]
- Klein EA, Thompson IM, Tangen CM, et al.: Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 306 (14): 1549-56, 2011. [ PMC free article : PMC4169010 ] [ PubMed : 21990298 ]
- Incidence and Mortality of Prostate Cancer
Carcinoma of the prostate is the most common tumor in men in the United States (other than skin cancer), with an estimated 299,010 new cases and 35,250 deaths expected in 2024.[ 1 ] A wide range of estimates of the impact of the disease are notable. The disease is histologically evident in as many as 34% of men in their fifth decade and in up to 70% of men aged 80 years and older.[ 2 , 3 ] The lifetime risk of being diagnosed with prostate cancer for U.S. men is 12.9%, while the lifetime risk of dying from prostate cancer is 2.3%.[ 4 ] The estimated reduction in life expectancy of men who die of prostate cancer is approximately 9 years.[ 5 ]
The extraordinarily high rate of clinically occult prostate cancer in the general population compared with the 20-fold lower likelihood of death from the disease indicates that many of these cancers have low biological risk. Concordant with this observation are the many series of patients with lower-risk (i.e., Gleason grade 6 and some low-volume Gleason grade 7 tumors) prostate cancer managed by surveillance alone with high survival rates at 5 and 10 years of follow-up.[ 6 ] Data demonstrate, however, that with longer follow-up, higher-grade cancers are associated with a greater risk of prostate cancer death.[ 7 , 8 ]
Because of marked variability in tumor differentiation from one microscopic field to another, many pathologists will report the range of differentiation among the malignant cells that are present in a biopsy using the Gleason grading system. This grading system includes five histological patterns distinguished by the glandular architecture of the cancer. The architectural patterns are identified and assigned a grade from 1 to 5 with 1 being the most differentiated and 5 being the least differentiated. The sum of the grades of the predominant and next most prevalent will range from 2 (well-differentiated tumors) to 10 (undifferentiated tumors).[ 9 , 10 ] Systematic changes to the histological interpretation of biopsy specimens by anatomical pathologists have occurred during the prostate-specific antigen (PSA) screening era (i.e., since about 1985) in the United States.[ 11 ] This phenomenon, sometimes called grade inflation, is the apparent increase in the distribution of high-grade tumors in the population for a period of time but in the absence of a true biological or clinical change. It is possibly the result of an increasing tendency for pathologists to read tumor grade as more aggressive, resulting in a higher preponderance to treat these cancers aggressively.[ 12 ] In general, these changes in interpretation have resulted in almost all prostate cancers being graded with Gleason grades of 3, 4, or 5; Gleason grades of 1 or 2 are highly unusual.
Treatment options available for prostate cancer include radical prostatectomy, external-beam radiation therapy, brachytherapy, cryotherapy, focal ablation, androgen deprivation with luteinizing hormone-releasing hormone analogs and/or antiandrogens, intermittent androgen deprivation, cytotoxic agents, and watchful waiting. Of all the means of management, only radical prostatectomy has been tested in a randomized clinical trial to assess survival benefit. In this study, prostatectomy was found to be superior to surveillance in men with localized prostate cancer, diagnosed in an era before widespread PSA screening. There were reduced rates of prostate cancer mortality (relative risk [RR], 0.56; 95% confidence interval [CI], 0.41–0.77) and overall mortality (RR, 0.71; 95% CI, 0.59–0.86).[ 13 ] Only 12% of the men had nonpalpable T1x tumors, suggesting that a minority of tumors were detected by PSA screening, whereas the majority were clinically detected. The relative efficacy of radical prostatectomy compared with other forms of treatment has not been adequately addressed.[ 14 ] Previous studies that compared radical prostatectomy with radiation therapy and brachytherapy closed because of poor patient accrual. Confounding issues in the treatment of prostate cancer include side effects of treatment, inability to predict the natural history of a given cancer, patient comorbidity that may affect an individual’s likelihood of surviving long enough to be at risk of disease morbidity and mortality, and an increasing body of evidence suggesting that, with careful PSA monitoring following treatment, a substantial fraction of patients may suffer disease recurrence.[ 15 ]
Because of considerable uncertainty regarding the efficacy of treatment and the difficulty with selecting patients for whom there is a known risk of disease progression, opinion in the medical community is divided regarding screening for carcinoma of the prostate. While both digital rectal examination and PSA screening have demonstrated reasonable performance characteristics (sensitivity, specificity, and positive predictive value) for the early detection of prostate cancer, conflicting outcomes of randomized trials examining the impact of screening on mortality has led some organizations to recommend for and others to recommend against screening.[ 16 ]
The tremendous impact of prostate cancer on the U.S. population and the financial burden of the disease for both patients and society have led to an increased interest in primary disease prevention.
The main treatment modalities for prostate cancer are surgery, radiation, hormonal, and active surveillance. For a detailed discussion, see Prostate Cancer Treatment . The goal of prostate cancer prevention interventions is to reduce the occurrence of prostate cancer, thereby obviating the need for treatment. As the effectiveness of prevention interventions improves, it is expected that the need for treatment will diminish.
- American Cancer Society: Cancer Facts and Figures 2024. American Cancer Society, 2024. Available online . Last accessed January 17, 2024.
- Sakr WA, Haas GP, Cassin BF, et al.: The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J Urol 150 (2 Pt 1): 379-85, 1993. [ PubMed : 8326560 ]
- Hølund B: Latent prostatic cancer in a consecutive autopsy series. Scand J Urol Nephrol 14 (1): 29-35, 1980. [ PubMed : 6154966 ]
- Surveillance Research Program, National Cancer Institute: SEER*Explorer: An interactive website for SEER cancer statistics. Bethesda, MD: National Cancer Institute. Available online . Last accessed March 6, 2024.
- Horm JW, Sondik EJ: Person-years of life lost due to cancer in the United States, 1970 and 1984. Am J Public Health 79 (11): 1490-3, 1989. [ PMC free article : PMC1349798 ] [ PubMed : 2817158 ]
- Cooperberg MR, Carroll PR, Klotz L: Active surveillance for prostate cancer: progress and promise. J Clin Oncol 29 (27): 3669-76, 2011. [ PubMed : 21825257 ]
- Lu-Yao GL, Albertsen PC, Moore DF, et al.: Outcomes of localized prostate cancer following conservative management. JAMA 302 (11): 1202-9, 2009. [ PMC free article : PMC2822438 ] [ PubMed : 19755699 ]
- Jones CU, Hunt D, McGowan DG, et al.: Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med 365 (2): 107-18, 2011. [ PubMed : 21751904 ]
- Gleason DF, Mellinger GT: Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 111 (1): 58-64, 1974. [ PubMed : 4813554 ]
- Gleason DF: Histologic grading and clinical staging of prostatic carcinoma. In: Tannenbaum M: Urologic Pathology: The Prostate. Lea and Febiger, 1977, pp 171-197.
- Albertsen PC, Hanley JA, Barrows GH, et al.: Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst 97 (17): 1248-53, 2005. [ PubMed : 16145045 ]
- Thompson IM, Canby-Hagino E, Lucia MS: Stage migration and grade inflation in prostate cancer: Will Rogers meets Garrison Keillor. J Natl Cancer Inst 97 (17): 1236-7, 2005. [ PubMed : 16145036 ]
- Bill-Axelson A, Holmberg L, Garmo H, et al.: Radical Prostatectomy or Watchful Waiting in Prostate Cancer - 29-Year Follow-up. N Engl J Med 379 (24): 2319-2329, 2018. [ PubMed : 30575473 ]
- Middleton RG, Thompson IM, Austenfeld MS, et al.: Prostate Cancer Clinical Guidelines Panel Summary report on the management of clinically localized prostate cancer. The American Urological Association. J Urol 154 (6): 2144-8, 1995. [ PubMed : 7500479 ]
- Moul JW: Prostate specific antigen only progression of prostate cancer. J Urol 163 (6): 1632-42, 2000. [ PubMed : 10799151 ]
- Carter HB, Albertsen PC: Re: Relative value of race, family history and prostate specific antigen as indications for early initiation of prostate cancer screening. J Urol 193 (3): 1063-4; discussion 1064, 2015. [ PubMed : 25476113 ]
- Risk Factors for Prostate Cancer Development
Prostate cancer incidence escalates with increasing age. Although it is an unusual disease in men younger than 50 years, incidence rates increase substantially thereafter. Data from the Surveillance, Epidemiology and End Results (SEER) program for 2016 to 2020 showed that incidence rates were 111.7 per 100,000 for men aged 50 to 54 years, 253.9 per 100,000 for men aged 55 to 59 years, 438.1 per 100,000 for men aged 60 to 64 years, and 679.7 per 100,000 for men aged 65 to 69 years. After age 70 years, incidence rates stabilized or decreased modestly. Mortality rates showed a greater increasing trend with age than did incidence, increasing from 3.0 per 100,000 for men aged 50 to 54 years to 39.5 per 100,000 for men aged 65 to 69 years to 210.9 per 100,000 for men aged 80 to 84 years.[ 1 ]
Family History
Approximately 15% of men with a diagnosis of prostate cancer will be found to have a first-degree relative (e.g., brother, father) with prostate cancer, compared with approximately 8% of the U.S. population.[ 2 ] Approximately 9% of all prostate cancers may result from heritable susceptibility genes.[ 3 ] Several authors have completed segregation analyses, and though a single, rare autosomal gene has been suggested to cause cancer in some of these families, the burden of evidence suggests that the inheritance is considerably more complex.[ 4 - 6 ] Evidence from the Prostate Cancer Prevention Trial (PCPT) and Selenium and Vitamin E Cancer Prevention Trial suggest that physician and patient bias lead to a greater likelihood of prostate biopsy, which contributes significantly to the increased risk of prostate cancer diagnosis in men with a family history of the disease.[ 7 ]
The development of the prostate is dependent upon the secretion of dihydrotestosterone (DHT) by the fetal testis. Testosterone causes normal virilization of the Wolffian duct structures and internal genitalia and is acted upon by the enzyme 5-alpha-reductase (5AR) to form DHT. DHT has a 4-fold to 50-fold greater affinity for the androgen receptor than testosterone, and it is DHT that leads to normal prostatic development. Children born with abnormal 5AR (due to a change in a single base pair in exon 5 of the normal type II 5AR gene), are born with ambiguous genitalia (variously described as hypospadias with a blind-ending vagina to a small phallus) but masculinize at puberty because of the surge of testosterone production at that time. Clinical, imaging, and histological studies of kindreds born with 5AR deficiency have demonstrated a small, pancake-appearing prostate with an undetectable prostate-specific antigen (PSA) level and no evidence of prostatic epithelium.[ 8 ] Long-term follow-up demonstrates that neither benign prostatic hyperplasia (BPH) nor prostate cancer develop.
Other evidence suggesting that the degree of cumulative exposure of the prostate to androgens is related to an increased risk of prostate cancer includes the following:
- Neither BPH nor prostate cancer have been reported in men castrated prior to puberty.[ 9 ]
- Androgen deprivation in almost all forms leads to involution of the prostate, a fall in PSA levels, apoptosis of prostate cancer and epithelial cells, and a clinical response in prostate cancer patients.[ 10 , 11 ]
- The results of two large-scale chemoprevention trials using 5AR inhibitors (finasteride and dutasteride) demonstrate that intraprostatic androgens modulate prostate cancer risk. In both studies, reductions in overall prostate cancer risk were identified although with increased risk of high-grade disease.[ 12 , 13 ]
Ecological studies have found a correlation between serum levels of testosterone, especially DHT, and overall risk of prostate cancer among African American, White, and Japanese males.[ 14 - 16 ] However, evidence from prospective studies of the association between serum concentrations of sex hormones, including androgens and estrogens, does not support a direct link.[ 17 ] A collaborative analysis of 18 prospective studies, pooling prediagnostic measures on 3,886 men with incident prostate cancer and 6,438 control subjects, found no association between the risk of prostate cancer and serum concentrations of testosterone, calculated-free testosterone, DHT sulfate, androstenedione, androstanediol glucuronide, estradiol, or calculated-free estradiol.[ 17 ] A caution for interpreting the data is the unknown degree of correlation between serum levels and prostate tissue level. Androstanediol glucuronide may most closely reflect intraprostatic androgen activity, and this measure was not associated with the risk of prostate cancer. This lack of association affirms that risk stratification cannot be made on serum hormone concentrations.
The risk of developing and dying of prostate cancer is higher among Black men (Hispanic and non-Hispanic), is of intermediate levels among White men (Hispanic and non-Hispanic), and is lowest among native Japanese men.[ 1 , 18 ] Conflicting data have been published regarding the etiology of these outcomes, but some evidence is available that access to health care may play a role in disease outcomes.[ 19 ] According to the Surveillance, Epidemiology, and End Results (SEER) Program, incidence of prostate cancer in African American men exceeds those of White men at all ages.[ 20 ]
Dietary Fat
An interesting observation is that although the incidence of latent (occult, histologically evident) prostate cancer is similar throughout the world, clinical prostate cancer varies from country to country by as much as 20-fold.[ 21 ] Previous ecological studies have demonstrated a direct relationship between a country’s prostate cancer-specific mortality rate and average total calories from fat consumed by the country’s population.[ 22 , 23 ] Studies of immigrants from Japan have demonstrated that native Japanese have the lowest risk of clinical prostate cancer, first-generation Japanese American men have an intermediate risk, and subsequent generations have a risk comparable to the U.S. population.[ 24 , 25 ] Animal models of explanted human prostate cancer have demonstrated decreased tumor growth rates in animals who are fed a low-fat diet.[ 26 , 27 ] Evidence from many case-control studies has shown an association between dietary fat and prostate cancer risk,[ 28 - 30 ] although studies have not uniformly reached this conclusion.[ 31 - 33 ] In a review of published studies of the relationship between dietary fat and prostate cancer risk, among descriptive studies, approximately half found an increased risk with increased dietary fat and half found no association.[ 34 ] Among case-control studies, about half of the studies found an increased risk with increasing dietary fat, animal fat, and saturated and monounsaturated fat intake while approximately half found no association. Only in studies of polyunsaturated fat intake were three studies reported of a significant negative association between prostate cancer and fat intake. Fat of animal origin seems to be associated with the highest risk.[ 19 , 35 ] In a series of 384 patients with prostate cancer, the risk of cancer progression to an advanced stage was greater in men with a high fat intake.[ 36 ] The announcement in 1996 that cancer mortality rates had fallen in the United States prompted the suggestion that this may be caused by decreases in dietary fat intake during the same time period.[ 37 , 38 ]
Two studies were conducted within the PCPT in which prospective nutritional information was collected and all participants were recommended to undergo biopsy. Findings included that among 9,559 participants, there was no association between any supplement or nutrient (including fat) and risk of prostate cancer overall, but the risk of high-grade cancer was associated with high intake of polyunsaturated fats. In a subset of 1,658 cases and 1,803 controls, specific fatty acids were examined, and docosahexaenoic acid was associated with risk of high-grade disease while trans -fatty acids (TFA) 18:1 and TFA 18:2 were inversely associated with risk of high-grade disease. These large-scale studies suggest a complex relationship between nutrients such as fat and risk of prostate cancer.[ 39 , 40 ]
The explanation for this possible association between prostate cancer and dietary fat is unknown. Several hypotheses have been advanced, including the following:
- Dietary fat may increase serum androgen levels, thereby increasing prostate cancer risk. This hypothesis is supported by observations from South Africa and the United States that changes in dietary fat intake change urinary and serum levels of androgens.[ 41 , 42 ]
- Certain types of fatty acids or their metabolites may initiate or promote prostate carcinoma development. The evidence for this hypothesis is conflicting, but one study suggests that linoleic acid (omega-6 polyunsaturated fatty acid) may stimulate prostate cancer cells, while omega-3 fatty acids inhibit cell growth.[ 43 ]
- An observation made in an animal model is that male offspring of pregnant rats who are fed a high-fat diet will develop prostate cancer at a higher rate than animals who are fed a low-fat diet.[ 44 ] This observation may explain some of the variations in prostate cancer incidence and mortality among ethnic groups; an observation has been made that first trimester androgen levels in pregnant Black men are higher than those in White men.[ 45 ]
Dairy and Calcium Intake
A meta-analysis of ten cohort studies (eight from the United States and two from Europe) concluded that men with the highest intake of dairy products (relative risk [RR], 1.11; 95% confidence interval [CI], 1.00–1.22; P = .04) and calcium (RR, 1.39; 95% CI, 1.09–1.77; P = .18) were more likely to develop prostate cancer than men with the lowest intake. The pooled RRs of advanced prostate cancer were 1.33 (95% CI, 1.00–1.78; P = .055) for the highest versus lowest intake categories of dairy products and 1.46 (95% CI, 0.65–3.25; P > .2) for the highest versus lowest intake categories of calcium. High intake of dairy products and calcium may be associated with an increased risk of prostate cancer, although the increase may be small.[ 46 ]
Multivitamin Use
Regular multivitamin use has not been associated with the risk of early or localized prostate cancer. However, in this large (295,344 men) study, there was a statistically significantly increased risk of advanced and fatal prostate cancer among men with excessive use of multivitamins.[ 47 ]
The Aspirin/Folate Polyp Prevention Study, a placebo-controlled randomized trial of aspirin and folic acid supplementation for the chemoprevention of colorectal adenomas, was conducted between July 6, 1994, and December 31, 2006. In a secondary analysis, the authors addressed the effect of folic acid supplementation on the risk of prostate cancer. Participants were followed for up to 10.8 (median, 7.0; interquartile range, 6.0–7.8) years and asked periodically to report all illnesses and hospitalizations.[ 48 ] Supplementation with 1 mg of folic acid was associated with an increased risk of prostate cancer. However, dietary and plasma levels among nonmultivitamin users were inversely associated with risk. These findings highlight the potentially complex role of folate in prostate carcinogenesis.[ 48 , 49 ]
Cadmium Exposure
Cadmium exposure is occupationally associated with nickel-cadmium batteries and cadmium recovery plant smelters and is associated with cigarette smoke.[ 50 ] The earliest studies of this agent documented an apparent association with prostate cancer, but better-designed studies have failed to note an association.[ 51 , 52 ]
Dioxin Exposure
Dioxin (2,3,7,8 tetrachlorodibenzo-p-dioxin or TCDD) is a contaminant of an herbicide used in Vietnam. This agent is similar to many components of herbicides used in farming. A review of the linkage between dioxin and prostate cancer risk, by the National Academy of Sciences Institute of Medicine Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides, found only two articles on prostate cancer with sufficient numbers of cases and follow-up to allow analysis.[ 53 , 54 ] The analysis of all available data suggests that the association between dioxin exposure and prostate cancer is not conclusive.[ 55 ]
Prostatitis
Several case-control and cohort studies, as well as two meta-analyses, suggested a significant but modest increase in the risk of prostate cancer in men with prostatitis (RR, 1.6) and in those with a history of syphilis or gonorrhea (RR, 1.4).[ 56 , 57 ] However, PSA values can be elevated with prostatitis, leading to more prostate biopsies and a greater likelihood of making the diagnosis of cancer. This is an example of ascertainment bias, and this bias can be significant in prostate cancer. Any factor associated with an elevation in serum PSA would be expected to lead to more biopsies being performed, and consequently an artifactual elevation in prostate cancer diagnoses. Despite a significant body of work relating inflammation to cancer, a cause and effect relationship has not been established between prostatitis and prostate cancer.[ 56 , 57 ]
- Steinberg GD, Carter BS, Beaty TH, et al.: Family history and the risk of prostate cancer. Prostate 17 (4): 337-47, 1990. [ PubMed : 2251225 ]
- Grönberg H, Isaacs SD, Smith JR, et al.: Characteristics of prostate cancer in families potentially linked to the hereditary prostate cancer 1 (HPC1) locus. JAMA 278 (15): 1251-5, 1997. [ PubMed : 9333266 ]
- Carter BS, Steinberg GD, Beaty TH, et al.: Familial risk factors for prostate cancer. Cancer Surv 11: 5-13, 1991. [ PubMed : 1841757 ]
- Schaid DJ, McDonnell SK, Blute ML, et al.: Evidence for autosomal dominant inheritance of prostate cancer. Am J Hum Genet 62 (6): 1425-38, 1998. [ PMC free article : PMC1377141 ] [ PubMed : 9585590 ]
- Bauer JJ, Srivastava S, Connelly RR, et al.: Significance of familial history of prostate cancer to traditional prognostic variables, genetic biomarkers, and recurrence after radical prostatectomy. Urology 51 (6): 970-6, 1998. [ PubMed : 9609635 ]
- Tangen CM, Goodman PJ, Till C, et al.: Biases in Recommendations for and Acceptance of Prostate Biopsy Significantly Affect Assessment of Prostate Cancer Risk Factors: Results From Two Large Randomized Clinical Trials. J Clin Oncol 34 (36): 4338-4344, 2016. [ PMC free article : PMC5455311 ] [ PubMed : 27998216 ]
- Imperato-McGinley J, Gautier T, Zirinsky K, et al.: Prostate visualization studies in males homozygous and heterozygous for 5 alpha-reductase deficiency. J Clin Endocrinol Metab 75 (4): 1022-6, 1992. [ PubMed : 1400866 ]
- Isaacs JT: Hormonal balance and the risk of prostatic cancer. J Cell Biochem Suppl 16H: 107-8, 1992. [ PubMed : 1289665 ]
- Peters CA, Walsh PC: The effect of nafarelin acetate, a luteinizing-hormone-releasing hormone agonist, on benign prostatic hyperplasia. N Engl J Med 317 (10): 599-604, 1987. [ PubMed : 2441256 ]
- Kyprianou N, Isaacs JT: Expression of transforming growth factor-beta in the rat ventral prostate during castration-induced programmed cell death. Mol Endocrinol 3 (10): 1515-22, 1989. [ PubMed : 2608047 ]
- Ellis L, Nyborg H: Racial/ethnic variations in male testosterone levels: a probable contributor to group differences in health. Steroids 57 (2): 72-5, 1992. [ PubMed : 1621259 ]
- Ross RK, Bernstein L, Lobo RA, et al.: 5-alpha-reductase activity and risk of prostate cancer among Japanese and US white and black males. Lancet 339 (8798): 887-9, 1992. [ PubMed : 1348296 ]
- Wu AH, Whittemore AS, Kolonel LN, et al.: Serum androgens and sex hormone-binding globulins in relation to lifestyle factors in older African-American, white, and Asian men in the United States and Canada. Cancer Epidemiol Biomarkers Prev 4 (7): 735-41, 1995 Oct-Nov. [ PubMed : 8672990 ]
- Roddam AW, Allen NE, Appleby P, et al.: Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst 100 (3): 170-83, 2008. [ PMC free article : PMC6126902 ] [ PubMed : 18230794 ]
- Bunker CH, Patrick AL, Konety BR, et al.: High prevalence of screening-detected prostate cancer among Afro-Caribbeans: the Tobago Prostate Cancer Survey. Cancer Epidemiol Biomarkers Prev 11 (8): 726-9, 2002. [ PubMed : 12163325 ]
- Optenberg SA, Thompson IM, Friedrichs P, et al.: Race, treatment, and long-term survival from prostate cancer in an equal-access medical care delivery system. JAMA 274 (20): 1599-605, 1995 Nov 22-29. [ PubMed : 7474244 ]
- Cancer incidence in the United States (SEER) age-specific rates. In: Harras A, Edwards BK, Blot WJ, eds.: Cancer Rates and Risks. 4th ed. National Cancer Institute, 1996, pp 22.
- Wynder EL, Mabuchi K, Whitmore WF: Epidemiology of cancer of the prostate. Cancer 28 (2): 344-60, 1971. [ PubMed : 5109447 ]
- Armstrong B, Doll R: Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer 15 (4): 617-31, 1975. [ PubMed : 1140864 ]
- Rose DP, Connolly JM: Dietary fat, fatty acids and prostate cancer. Lipids 27 (10): 798-803, 1992. [ PubMed : 1435098 ]
- Haenszel W, Kurihara M: Studies of Japanese migrants. I. Mortality from cancer and other diseases among Japanese in the United States. J Natl Cancer Inst 40 (1): 43-68, 1968. [ PubMed : 5635018 ]
- Shimizu H, Ross RK, Bernstein L, et al.: Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer 63 (6): 963-6, 1991. [ PMC free article : PMC1972548 ] [ PubMed : 2069852 ]
- Wang Y, Corr JG, Thaler HT, et al.: Decreased growth of established human prostate LNCaP tumors in nude mice fed a low-fat diet. J Natl Cancer Inst 87 (19): 1456-62, 1995. [ PubMed : 7545759 ]
- Connolly JM, Coleman M, Rose DP: Effects of dietary fatty acids on DU145 human prostate cancer cell growth in athymic nude mice. Nutr Cancer 29 (2): 114-9, 1997. [ PubMed : 9427973 ]
- Ross RK, Shimizu H, Paganini-Hill A, et al.: Case-control studies of prostate cancer in blacks and whites in southern California. J Natl Cancer Inst 78 (5): 869-74, 1987. [ PubMed : 3471995 ]
- Kolonel LN, Yoshizawa CN, Hankin JH: Diet and prostatic cancer: a case-control study in Hawaii. Am J Epidemiol 127 (5): 999-1012, 1988. [ PubMed : 3358418 ]
- Whittemore AS, Kolonel LN, Wu AH, et al.: Prostate cancer in relation to diet, physical activity, and body size in blacks, whites, and Asians in the United States and Canada. J Natl Cancer Inst 87 (9): 652-61, 1995. [ PubMed : 7752270 ]
- Giovannucci E: Epidemiologic characteristics of prostate cancer. Cancer 75 (Suppl 7): 1766-77, 1995.
- Mettlin C, Selenskas S, Natarajan N, et al.: Beta-carotene and animal fats and their relationship to prostate cancer risk. A case-control study. Cancer 64 (3): 605-12, 1989. [ PubMed : 2743255 ]
- Severson RK, Nomura AM, Grove JS, et al.: A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res 49 (7): 1857-60, 1989. [ PubMed : 2924323 ]
- Zhou JR, Blackburn GL: Bridging animal and human studies: what are the missing segments in dietary fat and prostate cancer? Am J Clin Nutr 66 (6 Suppl): 1572S-1580S, 1997. [ PubMed : 9394717 ]
- Rose DP, Boyar AP, Wynder EL: International comparisons of mortality rates for cancer of the breast, ovary, prostate, and colon, and per capita food consumption. Cancer 58 (11): 2363-71, 1986. [ PubMed : 3768832 ]
- Bairati I, Meyer F, Fradet Y, et al.: Dietary fat and advanced prostate cancer. J Urol 159 (4): 1271-5, 1998. [ PubMed : 9507851 ]
- Cole P, Rodu B: Declining cancer mortality in the United States. Cancer 78 (10): 2045-8, 1996. [ PubMed : 8918396 ]
- Wynder EL, Cohen LA: Correlating nutrition to recent cancer mortality statistics. J Natl Cancer Inst 89 (4): 324, 1997. [ PubMed : 9048839 ]
- Kristal AR, Till C, Platz EA, et al.: Serum lycopene concentration and prostate cancer risk: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev 20 (4): 638-46, 2011. [ PMC free article : PMC3070045 ] [ PubMed : 21335507 ]
- Kristal AR, Arnold KB, Neuhouser ML, et al.: Diet, supplement use, and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol 172 (5): 566-77, 2010. [ PMC free article : PMC2950820 ] [ PubMed : 20693267 ]
- Hill P, Wynder EL, Garbaczewski L, et al.: Diet and urinary steroids in black and white North American men and black South African men. Cancer Res 39 (12): 5101-5, 1979. [ PubMed : 498137 ]
- Hämäläinen E, Adlercreutz H, Puska P, et al.: Diet and serum sex hormones in healthy men. J Steroid Biochem 20 (1): 459-64, 1984. [ PubMed : 6538617 ]
- Rose DP, Connolly JM: Effects of fatty acids and eicosanoid synthesis inhibitors on the growth of two human prostate cancer cell lines. Prostate 18 (3): 243-54, 1991. [ PubMed : 2020620 ]
- Kondo Y, Homma Y, Aso Y, et al.: Promotional effect of two-generation exposure to a high-fat diet on prostate carcinogenesis in ACI/Seg rats. Cancer Res 54 (23): 6129-32, 1994. [ PubMed : 7525054 ]
- Henderson BE, Bernstein L, Ross RK, et al.: The early in utero oestrogen and testosterone environment of blacks and whites: potential effects on male offspring. Br J Cancer 57 (2): 216-8, 1988. [ PMC free article : PMC2246431 ] [ PubMed : 3358915 ]
- Gao X, LaValley MP, Tucker KL: Prospective studies of dairy product and calcium intakes and prostate cancer risk: a meta-analysis. J Natl Cancer Inst 97 (23): 1768-77, 2005. [ PubMed : 16333032 ]
- Lawson KA, Wright ME, Subar A, et al.: Multivitamin use and risk of prostate cancer in the National Institutes of Health-AARP Diet and Health Study. J Natl Cancer Inst 99 (10): 754-64, 2007. [ PubMed : 17505071 ]
- Figueiredo JC, Grau MV, Haile RW, et al.: Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst 101 (6): 432-5, 2009. [ PMC free article : PMC2657096 ] [ PubMed : 19276452 ]
- Kristal AR, Lippman SM: Nutritional prevention of cancer: new directions for an increasingly complex challenge. J Natl Cancer Inst 101 (6): 363-5, 2009. [ PubMed : 19276449 ]
- Pienta KJ: Epidemiology and etiology of prostate cancer. In: Raghavan D, Scher HI, Leibel SA, eds.: Principles and Practice of Genitourinary Oncology. Lippincott-Raven Publishers, 1997, pp 379-385.
- García Sánchez A, Antona JF, Urrutia M: Geochemical prospection of cadmium in a high incidence area of prostate cancer, Sierra de Gata, Salamanca, Spain. Sci Total Environ 116 (3): 243-51, 1992. [ PubMed : 1615308 ]
- Boffetta P: Methodological aspects of the epidemiological association between cadmium and cancer in humans. In: Nordberg GF, Herber RF, Alessio L, eds.: Cadmium in the Human Environment: Toxicity and Carcinogenicity. Lyon, France: International Agency for Research on Cancer, 1992, pp 425-434. [ PubMed : 1303970 ]
- Fingerhut MA, Halperin WE, Marlow DA, et al.: Cancer mortality in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. N Engl J Med 324 (4): 212-8, 1991. [ PubMed : 1985242 ]
- Bertazzi PA, Zocchetti C, Pesatori AC, et al.: Ten-year mortality study of the population involved in the Seveso incident in 1976. Am J Epidemiol 129 (6): 1187-200, 1989. [ PubMed : 2729256 ]
- Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides: Veterans and Agent Orange: Update 1996. In: Washington DC, National Academy Press, 1996.
- Dennis LK, Dawson DV: Meta-analysis of measures of sexual activity and prostate cancer. Epidemiology 13 (1): 72-9, 2002. [ PubMed : 11805589 ]
- Dennis LK, Lynch CF, Torner JC: Epidemiologic association between prostatitis and prostate cancer. Urology 60 (1): 78-83, 2002. [ PubMed : 12100928 ]
- Opportunities for Prevention
Hormonal Prevention
The Prostate Cancer Prevention Trial (PCPT), a large randomized placebo-controlled trial of finasteride (an inhibitor of alpha-reductase), was performed in 18,882 men aged 55 years or older. At 7 years, the incidence of prostate cancer was 18.4% in the finasteride group versus 24.4% in the placebo group, a relative risk reduction (RRR) of 24.8% (95% confidence interval [CI], 18.6%–30.6%; P < .001). The finasteride group had more patients with Gleason grade 7 to 10, but the clinical significance of Gleason scoring is uncertain in conditions of androgen deprivation.[ 1 ] High-grade cancers (Gleason score 7–10) were noted in 6.4% of finasteride patients, compared with 5.1% of men who received placebo, yielding a relative risk (RR) of 1.27 (95% CI, 1.07–1.50). The increase in high-grade tumors was seen within 1 year of finasteride exposure and did not increase during this time period.[ 2 ]
Finasteride decreases the risk of prostate cancer but may also alter the detection of disease through effects on prostate-specific antigen (PSA), prostate digital rectal examination (DRE), and decreased prostate volume (24%), creating a detection bias.[ 3 ] Adjustment of PSA in men taking finasteride preserves the performance characteristics for cancer detection.[ 4 ]
Examination of the outcomes of the PCPT found that finasteride significantly reduced the risk of high-grade prostatic intraepithelial neoplasia (HGPIN); HGPIN alone was reduced by 15% (RR, 0.85; 95% CI, 0.73–0.99) and HGPIN with prostate cancer was reduced by 31% (RR, 0.69; 95% CI, 0.56–0.85).[ 3 , 5 ] The concern that finasteride may increase the risk of high-grade cancer prompted an examination of the rate of cancer development in the PCPT. While a gradual and progressive increase in the number of high-grade tumors would have been expected over the study duration of 7 years, when compared with placebo, this was not the case. The increase in high-grade tumors was seen within 1 year of finasteride exposure and did not increase during this time period.[ 2 ] An analysis of the PCPT data adjusted for the sources of detection bias found that finasteride reduced the incidence of Gleason score 5 to 7 and Gleason score 3 to 4 prostate cancer, but not Gleason score 2 to 3 or Gleason score 8 to 10. The reduction in the incidence of Gleason score 7 (22%) was less than the reduction in the incidence of Gleason 5 score (58%) and Gleason score 6 (52%).[ 6 ] An analysis using different methodologies found an overall reduction of both low-grade (Gleason score <6) and high-grade (Gleason score >7) cancers.[ 7 ]
A follow-up analysis of the PCPT of finasteride mapped study participants with the National Death Index, allowing for an analysis of prostate cancer-specific mortality. With 296,842 person-years of follow-up and a median follow-up of 18.4 years, of the 9,423 men randomly assigned to the finasteride group, there were 3,048 deaths of which 42 were caused by prostate cancer; of the 9,457 men randomly assigned to the placebo group, there were 2,979 deaths of which 56 were caused by prostate cancer. The 25% reduction in risk of prostate cancer death with finasteride was not statistically significant (hazard ratio, finasteride vs. placebo, 0.75; 95% CI, 0.50–1.12). It was concluded that the early concern for an increased risk of high-grade prostate cancer with finasteride was not borne out. In this study, it was notable that, of the 61 prostate cancer deaths for which original Gleason grading was available, 23 (38%) of the prostate cancer deaths were seen in men whose original biopsy Gleason grade was less than or equal to 6.[ 8 ]
A retrospective, population-based, cohort study from the U.S. Department of Veterans Affairs health care system examined the impact of 5-alpha reductase inhibitor (5-ARI) use before prostate cancer diagnosis on prostate cancer-specific mortality.[ 9 ] The authors found that prediagnostic use of 5-ARIs was associated with a delayed diagnosis (median time from first elevated PSA was 3.6 years for men who received 5-ARIs versus 1.4 years for non–5-ARI users) and worsened cancer-specific outcomes (e.g., higher grade, higher clinical stage, more with positive nodes, and higher rates of metastatic disease) in men with prostate cancer. A subsequent letter to the editor pointed out the following challenges with the analysis:
- A 39% improvement in prostate cancer mortality with a 2-year earlier diagnosis and with only 5.9 years of follow-up is implausible, given that the very best reduction in prostate cancer mortality in a randomized clinical trial was 20%.
- Because the study could not assess 5-ARI medication adherence, PSA misadjustment was a serious concern.
- Because men treated with 5-ARIs are very different than those not treated (i.e., more urinary symptoms, older, larger prostates, etc.), major differences in baseline characteristics, as reported in the study, prevented adequate adjustment in outcomes.
- As demonstrated by the PCPT, because finasteride (a 5-ARI) prevents a substantial proportion of low-grade tumors, a greater proportion of high-grade tumors would be expected.
- Because national treatment guidelines recommend 5-ARIs for men with larger prostates, which have higher PSA values, and as prostate cancers are more commonly missed in larger prostates (and may be identified at a subsequent biopsy, often with a magnetic resonance imaging-directed biopsy), a later diagnosis would be common in this patient population.
- The authors' analysis did not adjust for survival bias; men not receiving a 5-ARI had an earlier diagnosis, and therefore, an inherent longer survival.
When taken together, these biases call into question the conclusions, which appear to be at odds with the prostate cancer–specific mortality outcomes of the randomized PCPT.
The Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial randomly assigned 8,231 men aged 50 to 75 years at higher risk of prostate cancer (i.e., PSA 2.5–10.0 ng/mL) with one recent negative prostate biopsy to dutasteride at 0.5 mg daily or to placebo. The primary end point was prostate cancer diagnosed by prostate biopsy at 2 years and 4 years after randomization. After 4 years, among the 6,729 men (82% of initial population) who had at least one prostate biopsy, 25.1% of the placebo group and 19.9% of the dutasteride group had been diagnosed with prostate cancer, a statistically significant difference (absolute risk reduction, 5.1% and RRR, 22.8% [95% CI, 15.2%–29.8%]). The RRR in years 3 to 4 was similar to the RRR in years 1 to 2. The difference between the groups was entirely due to a reduction in prostate cancers with Gleason score 5 to 7. For years 3 to 4 there was a statistically significant increase in the dutasteride group compared with the placebo group in prostate cancers with Gleason score 8 to 10 (12 cancers in the dutasteride group vs. 1 cancer in the placebo group).[ 10 ]
Overall, there was no statistically significant difference in high-grade tumors for Gleason score 8 to 10 cancers in years 1 to 4 (29 tumors in the dutasteride group vs. 19 tumors in the placebo group, 0.9% vs. 0.6%; P = .15). However, in a retrospective analysis there was a statistically significant difference between years 3 to 4. Because this is a small retrospective subgroup, the finding of an increase in Gleason score 8 to 10 cancers is of uncertain validity. However, the finding of no reduction in these cancers is more significant.[ 10 ]
While long-term data are unavailable for dutasteride as a cancer prevention agent, evidence is now available that finasteride does not have a significant effect on overall survival or prostate cancer–specific survival. Its effect is primarily in preventing the diagnosis of prostate cancer and the subsequent events (staging, treatment, follow-up, and management of treatment-related side effects) after diagnosis.
Agents that are used for hormonal therapy of existing prostate cancers would be unsuitable for prostate cancer chemoprevention because of the cost and wide variety of side effects including sexual dysfunction, osteoporosis, and vasomotor symptoms (hot flushes).[ 11 ] Newer antiandrogens may play a role as preventive agents in the future.[ 12 ]
A Cochrane systematic review of all published studies of clinical outcome investigations of the prostate preventive effects of 5-ARIs through 2010 that were at least 1 year in duration concluded that finasteride and dutasteride reduce the risk of being diagnosed with prostate cancer among men who are screened regularly for prostate cancer. The review also concluded that mortality effects could not be assessed from these studies and that persistent use of these agents increased sexual and erectile dysfunction. The review was based on MEDLINE and Cochrane Collaboration Library computerized searches through June 2010 using the Medical Subject Headings terms and text words finasteride , dutasteride , neoplasms , azasteroids , reductase inhibitors , and enzyme inhibitors to identify randomized trials. Eight studies met the inclusion criteria. Only the PCPT and the REDUCE study were designed to assess the impact of 5-ARIs on prostate cancer period prevalence. Reviews of all eight studies concluded that compared with placebo, 5-ARIs resulted in 25% RR reduction in prostate cancers detected for cause (RR, 0.75; 95% CI, 0.67–0.83 and 1.4% absolute risk reduction [3.5% vs. 4.9%]). Six trials of 5-ARIs versus placebo assessed prostate cancers detected overall. Among these there was a 26% RR reduction favoring 5-ARIs (RR, 0.74; 95% CI, 0.55–1.00 and 2.9% absolute risk reduction [6.3% vs. 9.2%]). There were reductions across age, race, and family history. One placebo-controlled trial of men considered at greater risk for prostate cancer based on age, elevated PSA, and previous suspicion of prostate cancer leading to a prostate biopsy reported that dutasteride did not reduce prostate cancers detected for cause based on needle biopsy but did reduce risk of overall incident prostate cancer detected by biopsy by 23% (RR, 0.77; 95% CI, 0.7–0.85 and absolute risk reduction, 16.1% vs. 20.8%). There were reductions across age, family history of prostate cancer, PSA level, and prostate volume subgroups. The Cochrane review defined for cause cancers as follows:
- Suspected clinically from symptoms, abnormal DRE, or PSA and confirmed on biopsy.
- Study protocol recommended biopsy, but it was not done and the end-of-study biopsy showed prostate cancer.
- The end-of-study biopsy with PSA less than 4 ng/mL and/or suspicious DRE showed prostate cancer.[ 13 ]
Dietary Prevention With Fruit, Vegetables, and a Low-fat Diet
Results from studies of the association between dietary intake of fruits and vegetables and risk of prostate cancer are not consistent. A study evaluated 1,619 prostate cancer cases and 1,618 controls in a multicenter, multiethnic population. The study found that intake of legumes and yellow-orange and cruciferous vegetables was associated with a lower risk of prostate cancer.
The European Prospective Investigation into Cancer and Nutrition examined the association between fruit and vegetable intake and subsequent prostate cancer. After an average follow-up of 4.8 years, 1,104 men developed prostate cancer among the 130,544 male participants. No statistically significant associations were observed for fruit intake, vegetable intake, cruciferous vegetable intake, or the intake of fruits and vegetables combined.[ 14 ]
One study of dietary intervention over a 4-year period with reduced fat and increased consumption of fruit, vegetables, and fiber had no impact on serum PSA levels.[ 15 ] It is unknown whether dietary modification through the use of a low-fat, plant-based diet will reduce prostate cancer risk. While this outcome is unknown, multiple additional benefits may be observed in patients following such a diet, including a lower risk of hyperlipidemia, better control of blood pressure, and a lower risk of cardiovascular disease—all of which may merit adoption of such a diet.
Chemoprevention
While several agents, including alpha-tocopherol, selenium, lycopene, difluoromethylornithine,[ 16 - 20 ] vitamin D,[ 21 - 23 ] and isoflavonoids,[ 24 , 25 ] have shown potential in either clinical or laboratory studies for chemoprevention of prostate cancer. However, the correlations of cancer prevention with these agents are increasingly of concern given the statistically significant increased risk of prostate cancer with alpha-tocopherol in the Selenium and Vitamin E Cancer Prevention Trial (SELECT) and the lack of preventive effect (actually, a nonsignificant increase in prostate cancer risk) with selenium.
Chemoprevention with selenium and vitamin E
The SELECT ( NCT00006392 ) was a large randomized placebo-controlled trial of vitamin E and selenium. It showed no reduction in prostate cancer period prevalence, but an increased risk of prostate cancer with vitamin E alone.[ 26 ]
Compared with the placebo group in which 529 men developed prostate cancer, there was a statistically significant increase in prostate cancer in the vitamin E group (620 cases), but not in the selenium plus vitamin E group (555 cases) or in the selenium group (575 cases). The magnitude of increase in prostate cancer risk with vitamin E alone was 17%. Of interest, the statistically increased risk of prostate cancer among men receiving vitamin E was seen after study supplements had been discontinued suggesting a longer-term effect of this agent.[ 26 ]
Chemoprevention with lycopene
Evidence exists that a diet with a high intake of fruits and vegetables is associated with a lower risk of cancer. Which, if any, micronutrients may account for this reduction is unknown. One group of nutrients often postulated as having chemoprevention properties is the carotenoids. Lycopene is the predominant circulating carotenoid in Americans and has a number of potential activities, including an antioxidant effect.[ 27 ] It is encountered in a number of vegetables, most notably tomatoes, and is best absorbed if these products are cooked and in the presence of dietary fats or oils.
The earliest studies of the association of lycopene and prostate cancer risk were generally negative before 1995 with only one study of 180 case-control patients showing a reduced risk.[ 28 - 31 ] In 1995, an analysis of the Physicians’ Health Study found a one-third reduction in prostate cancer risk in the group of men with the highest consumption of tomato products when compared with the group with the lowest level of consumption, which was attributed to the lycopene content of these vegetables.[ 32 ] This large analysis prompted several subsequent studies, the results of which were mixed.[ 33 , 34 ] A review of the published data concluded that the evidence is weak that lycopene is associated with a reduced risk because previous studies were not controlled for total vegetable intake (i.e., separating the effect of tomatoes from vegetables), dietary intake instruments are poorly able to quantify lycopene intake, and other potential biases.[ 35 ] Specific dietary supplementation with lycopene remains to be demonstrated to reduce prostate cancer risk. In the largest prospective study to date, the PCPT, lycopene was not associated with any reduction in risk of prostate cancer among 9,559 men studied. Similarly, there was no relationship between lycopene serum concentrations and risk of prostate cancer.[ 36 , 37 ]
- Thompson IM, Klein EA, Lippman SM, et al.: Prevention of prostate cancer with finasteride: US/European perspective. Eur Urol 44 (6): 650-5, 2003. [ PubMed : 14644115 ]
- Andriole G, Bostwick D, Civantos F, et al.: The effects of 5alpha-reductase inhibitors on the natural history, detection and grading of prostate cancer: current state of knowledge. J Urol 174 (6): 2098-104, 2005. [ PubMed : 16280736 ]
- Etzioni RD, Howlader N, Shaw PA, et al.: Long-term effects of finasteride on prostate specific antigen levels: results from the prostate cancer prevention trial. J Urol 174 (3): 877-81, 2005. [ PubMed : 16093979 ]
- Thompson IM, Lucia MS, Redman MW, et al.: Finasteride decreases the risk of prostatic intraepithelial neoplasia. J Urol 178 (1): 107-9; discussion 110, 2007. [ PubMed : 17499284 ]
- Kaplan SA, Roehrborn CG, Meehan AG, et al.: PCPT: Evidence that finasteride reduces risk of most frequently detected intermediate- and high-grade (Gleason score 6 and 7) cancer. Urology 73 (5): 935-9, 2009. [ PubMed : 19328538 ]
- Redman MW, Tangen CM, Goodman PJ, et al.: Finasteride does not increase the risk of high-grade prostate cancer: a bias-adjusted modeling approach. Cancer Prev Res (Phila Pa) 1 (3): 174-81, 2008. [ PMC free article : PMC2844801 ] [ PubMed : 19138953 ]
- Goodman PJ, Tangen CM, Darke AK, et al.: Long-Term Effects of Finasteride on Prostate Cancer Mortality. N Engl J Med 380 (4): 393-394, 2019. [ PubMed : 30673548 ]
- Sarkar RR, Parsons JK, Bryant AK, et al.: Association of Treatment With 5α-Reductase Inhibitors With Time to Diagnosis and Mortality in Prostate Cancer. JAMA Intern Med 179 (6): 812-819, 2019. [ PMC free article : PMC6503564 ] [ PubMed : 31058923 ]
- Thompson I, Feigl P, Coltman C: Chemoprevention of prostate cancer with finasteride. Important Adv Oncol : 57-76, 1995. [ PubMed : 7672814 ]
- Nelson PS, Gleason TP, Brawer MK: Chemoprevention for prostatic intraepithelial neoplasia. Eur Urol 30 (2): 269-78, 1996. [ PubMed : 8875211 ]
- Wilt TJ, Macdonald R, Hagerty K, et al.: 5-α-Reductase inhibitors for prostate cancer chemoprevention: an updated Cochrane systematic review. BJU Int 106 (10): 1444-51, 2010. [ PubMed : 20977593 ]
- Key TJ, Allen N, Appleby P, et al.: Fruits and vegetables and prostate cancer: no association among 1104 cases in a prospective study of 130544 men in the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer 109 (1): 119-24, 2004 Mar10. [ PubMed : 14735477 ]
- Shike M, Latkany L, Riedel E, et al.: Lack of effect of a low-fat, high-fruit, -vegetable, and -fiber diet on serum prostate-specific antigen of men without prostate cancer: results from a randomized trial. J Clin Oncol 20 (17): 3592-8, 2002. [ PubMed : 12202659 ]
- Heby O: Role of polyamines in the control of cell proliferation and differentiation. Differentiation 19 (1): 1-20, 1981. [ PubMed : 6173280 ]
- Danzin C, Jung MJ, Grove J, et al.: Effect of alpha-difluoromethylornithine, an enzyme-activated irreversible inhibitor of ornithine decarboxylase, on polyamine levels in rat tissues. Life Sci 24 (6): 519-24, 1979. [ PubMed : 431333 ]
- Metcalf BW, Bey P, Danzin C, et al.: Catalytic irreversible inhibition of mammalian ornithine decarboxylase (E.C. 4.1.1.17) by substrate and product analogues. J Am Chem Soc 100(8): 2551-2553, 1978.
- Heston WD, Kadmon D, Lazan DW, et al.: Copenhagen rat prostatic tumor ornithine decarboxylase activity (ODC) and the effect of the ODC inhibitor alpha-difluoromethylornithine. Prostate 3 (4): 383-9, 1982. [ PubMed : 6812032 ]
- Abeloff MD, Slavik M, Luk GD, et al.: Phase I trial and pharmacokinetic studies of alpha-difluoromethylornithine--an inhibitor of polyamine biosynthesis. J Clin Oncol 2 (2): 124-30, 1984. [ PubMed : 6422008 ]
- Schwartz GG, Hulka BS: Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis). Anticancer Res 10 (5A): 1307-11, 1990 Sep-Oct. [ PubMed : 2241107 ]
- Eisman JA, Barkla DH, Tutton PJ: Suppression of in vivo growth of human cancer solid tumor xenografts by 1,25-dihydroxyvitamin D3. Cancer Res 47 (1): 21-5, 1987. [ PubMed : 3024816 ]
- Chida K, Hashiba H, Fukushima M, et al.: Inhibition of tumor promotion in mouse skin by 1 alpha,25-dihydroxyvitamin D3. Cancer Res 45 (11 Pt 1): 5426-30, 1985. [ PubMed : 3840412 ]
- Adlercreutz H, Markkanen H, Watanabe S: Plasma concentrations of phyto-oestrogens in Japanese men. Lancet 342 (8881): 1209-10, 1993. [ PubMed : 7901532 ]
- Peterson G, Barnes S: Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate 22 (4): 335-45, 1993. [ PubMed : 8497428 ]
- Gerster H: The potential role of lycopene for human health. J Am Coll Nutr 16 (2): 109-26, 1997. [ PubMed : 9100211 ]
- Hsing AW, Comstock GW, Abbey H, et al.: Serologic precursors of cancer. Retinol, carotenoids, and tocopherol and risk of prostate cancer. J Natl Cancer Inst 82 (11): 941-6, 1990. [ PubMed : 2342127 ]
- Mills PK, Beeson WL, Phillips RL, et al.: Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer 64 (3): 598-604, 1989. [ PubMed : 2743254 ]
- Schuman LM, Mandel JS, Radke A, et al.: Some selected features of the epidemiology of prostatic cancer: Minneapolis-St. Paul, Minnesota case-control study, 1976-1979. [Abstract] Trends in Cancer Incidence: Causes and Practical Implications (Proceedings of a Symposium Held in Oslo, Norway, Aug. 6-7, 1980) pp 345-354.
- Le Marchand L, Hankin JH, Kolonel LN, et al.: Vegetable and fruit consumption in relation to prostate cancer risk in Hawaii: a reevaluation of the effect of dietary beta-carotene. Am J Epidemiol 133 (3): 215-9, 1991. [ PubMed : 2000838 ]
- Giovannucci E, Ascherio A, Rimm EB, et al.: Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst 87 (23): 1767-76, 1995. [ PubMed : 7473833 ]
- Jain MG, Hislop GT, Howe GR, et al.: Plant foods, antioxidants, and prostate cancer risk: findings from case-control studies in Canada. Nutr Cancer 34 (2): 173-84, 1999. [ PubMed : 10578485 ]
- Key TJ, Silcocks PB, Davey GK, et al.: A case-control study of diet and prostate cancer. Br J Cancer 76 (5): 678-87, 1997. [ PMC free article : PMC2228001 ] [ PubMed : 9303371 ]
- Kristal AR, Cohen JH: Invited commentary: tomatoes, lycopene, and prostate cancer. How strong is the evidence? Am J Epidemiol 151 (2): 124-7; discussion 128-30, 2000. [ PubMed : 10645814 ]
- Latest Updates to This Summary (03/07/2024)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Updated statistics with estimated new cases and deaths for 2024 (cited American Cancer Society as reference 1).
This summary is written and maintained by the PDQ Screening and Prevention Editorial Board , which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
- About This PDQ Summary
Purpose of This Summary
Reviewers and updates.
This summary is reviewed regularly and updated as necessary by the PDQ Screening and Prevention Editorial Board , which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us . Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Screening and Prevention Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Screening and Prevention Editorial Board. PDQ Prostate Cancer Prevention. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/prostate/hp/prostate-prevention-pdq . Accessed <MM/DD/YYYY>. [PMID: 26389405]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online , a collection of over 2,000 scientific images.
The information in these summaries should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us .
- Cite this Page PDQ Screening and Prevention Editorial Board. Prostate Cancer Prevention (PDQ®): Health Professional Version. 2024 Mar 7. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-.
Version History
- NBK65968.19 March 7, 2024 (Displayed Version)
- NBK65968.18 October 26, 2023
- NBK65968.17 May 22, 2023
- NBK65968.16 March 2, 2022
- NBK65968.15 August 24, 2021
- NBK65968.14 March 18, 2021
- NBK65968.13 March 27, 2020
- NBK65968.12 February 12, 2020
- NBK65968.11 December 13, 2019
- NBK65968.10 June 19, 2019
- NBK65968.9 April 26, 2019
- NBK65968.8 March 1, 2019
- NBK65968.7 September 28, 2018
- NBK65968.6 March 2, 2018
- NBK65968.5 March 17, 2017
- NBK65968.4 April 8, 2016
- NBK65968.3 March 9, 2016
- NBK65968.2 February 4, 2016
- NBK65968.1 February 6, 2015
In this Page
Related publications.
- Patient Version
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Prostate Cancer Screening (PDQ®): Health Professional Version. [PDQ Cancer Information Summari...] Review Prostate Cancer Screening (PDQ®): Health Professional Version. PDQ Screening and Prevention Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Prostate Cancer Treatment (PDQ®): Health Professional Version. [PDQ Cancer Information Summari...] Review Prostate Cancer Treatment (PDQ®): Health Professional Version. PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Genetics of Prostate Cancer (PDQ®): Health Professional Version. [PDQ Cancer Information Summari...] Review Genetics of Prostate Cancer (PDQ®): Health Professional Version. PDQ Cancer Genetics Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Cancer Prevention Overview (PDQ®): Health Professional Version. [PDQ Cancer Information Summari...] Review Cancer Prevention Overview (PDQ®): Health Professional Version. PDQ Screening and Prevention Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Anal Cancer Prevention (PDQ®): Health Professional Version. [PDQ Cancer Information Summari...] Review Anal Cancer Prevention (PDQ®): Health Professional Version. PDQ Screening and Prevention Editorial Board. PDQ Cancer Information Summaries. 2002
Recent Activity
- Prostate Cancer Prevention (PDQ®) - PDQ Cancer Information Summaries Prostate Cancer Prevention (PDQ®) - PDQ Cancer Information Summaries
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Open access
- Published: 24 May 2024
Disrupting Na + ion homeostasis and Na + /K + ATPase activity in breast cancer cells directly modulates glycolysis in vitro and in vivo
- Aidan M. Michaels 1 ,
- Anna Zoccarato 2 ,
- Zoe Hoare 2 ,
- George Firth 1 ,
- Yu Jin Chung 2 ,
- Philip W. Kuchel 3 ,
- Ajay M. Shah 2 ,
- Michael J. Shattock 2 ,
- Richard Southworth 1 &
- Thomas R. Eykyn 1
Cancer & Metabolism volume 12 , Article number: 15 ( 2024 ) Cite this article
5 Altmetric
Metrics details
Glycolytic flux is regulated by the energy demands of the cell. Upregulated glycolysis in cancer cells may therefore result from increased demand for adenosine triphosphate (ATP), however it is unknown what this extra ATP turnover is used for. We hypothesise that an important contribution to the increased glycolytic flux in cancer cells results from the ATP demand of Na + /K + -ATPase (NKA) due to altered sodium ion homeostasis in cancer cells.
Live whole-cell measurements of intracellular sodium [Na + ] i were performed in three human breast cancer cells (MDA-MB-231, HCC1954, MCF-7), in murine breast cancer cells (4T1), and control human epithelial cells MCF-10A using triple quantum filtered 23 Na nuclear magnetic resonance (NMR) spectroscopy. Glycolytic flux was measured by 2 H NMR to monitor conversion of [6,6- 2 H 2 ] d -glucose to [ 2 H]-labelled l -lactate at baseline and in response to NKA inhibition with ouabain. Intracellular [Na + ] i was titrated using isotonic buffers with varying [Na + ] and [K + ] and introducing an artificial Na + plasma membrane leak using the ionophore gramicidin-A. Experiments were carried out in parallel with cell viability assays, 1 H NMR metabolomics of intracellular and extracellular metabolites, extracellular flux analyses and in vivo measurements in a MDA-MB-231 human-xenograft mouse model using 2-deoxy-2-[ 18 F]fluoroglucose ( 18 F-FDG) positron emission tomography (PET).
Intracellular [Na + ] i was elevated in human and murine breast cancer cells compared to control MCF-10A cells. Acute inhibition of NKA by ouabain resulted in elevated [Na + ] i and inhibition of glycolytic flux in all three human cancer cells which are ouabain sensitive, but not in the murine cells which are ouabain resistant. Permeabilization of cell membranes with gramicidin-A led to a titratable increase of [Na + ] i in MDA-MB-231 and 4T1 cells and a Na + -dependent increase in glycolytic flux. This was attenuated with ouabain in the human cells but not in the murine cells. 18 FDG PET imaging in an MDA-MB-231 human-xenograft mouse model recorded lower 18 FDG tumour uptake when treated with ouabain while murine tissue uptake was unaffected.
Conclusions
Glycolytic flux correlates with Na + -driven NKA activity in breast cancer cells, providing evidence for the ‘centrality of the [Na + ] i -NKA nexus’ in the mechanistic basis of the Warburg effect.
Introduction
Glycolysis is an evolutionarily conserved metabolic pathway that oxidises one molecule of glucose to form two molecules of pyruvate, typically producing a net of two molecules of ATP. The product pyruvate can either enter the tricarboxylic acid (TCA) cycle in the mitochondria, where it is further oxidized, phosphorylating ~ 32 more molecules of adenosine diphosphate (ADP); or it is reduced to lactate with conversion of NADH to NAD + (nicotinamide adenine dinucleotide) that is recycled as a co-substrate of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), thus enabling glycolysis to continue; or it is transaminated to form alanine. In normal cells, increased glycolytic flux is typically observed under anaerobic conditions where oxygen supply is not able to meet demand and oxidative phosphorylation is inhibited in the mitochondria, the build-up of lactate and H + is prevented by efflux via plasma membrane monocarboxylate transporter protein(s). The Warburg effect [ 1 , 2 , 3 ] is a phenomenon in which many cancer cell types (although not all) preferentially use glycolysis, even in the presence of plentiful oxygen. The premise of a ‘glycolytic switch’ as the basis of this effect can be misleading, as it has been extensively reported that cancer cells have fully functional mitochondria [ 4 , 5 , 6 , 7 ], contrary to Warburg’s initial thesis. However, cancer cells are reported to have slower TCA cycle flux than healthy cells [ 8 ]. Therefore, a fundamental question remains: Why do cancer cells have increased glycolytic metabolism and what is the extra ATP used for? This paradoxical observation, in the sense that uncontrolled growth of cells would require the most efficient extraction of energy from metabolic fuels like glucose, would imply up-regulation of oxidative phosphorylation, not the reverse [ 9 ]. It has often been argued that the switch in metabolism in cancer cells favours the accumulation of biomass since increased flux through glycolysis supports metabolic shunts such as the pentose phosphate pathway (PPP) and various branch pathways leading to the synthesis of amino acids required for protein synthesis [ 10 ]. However, recent suggestions are that glycolysis supplies the ATP required to satisfy the fluctuating anabolic demands of the cell [ 11 ], rather than being used to maintain a steady state metabolite concentration, and that glycolysis directly provides the ATP for other energy-demanding processes like the maintenance of the trans-plasma-membrane ionic gradient [ 12 ].
The enzyme-catalysed reactions of glycolysis transduce covalent-bond energy from glucose to form ATP (from ADP and inorganic phosphate); and it is known that flux through the pathway is ‘ATP-demand regulated’ [ 13 ]. For the cell to remain in a metabolic steady-state the number of moles of ATP generated must match the number of moles of ATP consumed in any time interval, by processes separate from the glycolytic reactions [ 11 ]. Hence, an increase in the rate of ATP hydrolysis will release more ADP and increase the [ADP]/[ATP] ratio; this results in the allosteric stimulation of glycolysis via phosphofructokinase-1 (PFK-1), producing more ATP to match demand [ 14 ].
The major cytosolic sinks for ATP are Na + /K + -ATPase (NKA), Ca 2+ -ATPases, H + /K + -ATPase, and H + -ATPases which belong to the superfamily of ion pumps known as P-type ATPases [ 15 ]. Of these NKA is reported to be responsible for ~ 30% of the total cellular ATP turnover with some estimates as much as 50% of total ATP turnover in the brain [ 16 ]. In the human erythrocyte, which lacks mitochondria, ATP generation is entirely glycolytic, and it has previously been shown through inhibition of the NKA with ouabain, or stimulation of Na + influx with the ionophore monensin, that the stoichiometry of Na + efflux via NKA is close to the theoretical 6 ions of Na + per 1 molecule of glucose consumed [ 17 ]. This highlights the intimate connection between the activity of the pump and glycolytic flux in these cells. A tight coupling between glycolytic metabolism and NKA activity has been reported in renal MDCK cells [ 18 ], permeabilized rat cardiomyocytes [ 19 ], and Ehrlich ascites tumour cells [ 14 ]. Thus, increased activity of NKA would increase ATP hydrolysis which in turn stimulates glycolytic production of ATP that matches demand. However, it is not known to what extent this process also drives the high glycolytic rates typically seen in cancer cells.
Na + ion homeostasis is widely reported to be dysregulated in cancer [ 20 ], being elevated inside the cell [ 21 ] and tumours [ 21 , 22 , 23 ]. Increased intracellular Na + could either result from decreased activity of the NKA, the main extruder of Na + from the cell, or an increase in Na + influx [ 24 , 25 ]. Observations that Na + -channels are overexpressed in cancer cells [ 25 , 26 ] suggests that increased influx may be the major driver of elevated [Na + ] i , which is consistent with the overexpression of sodium channel protein type 5 subunit alpha (Na V 1.5) [ 27 ] and other voltage gated Na + -channels [ 28 ] being associated with more rapid disease progression and worse patient outcomes [ 29 ]. Other Na + influx pathways include sodium hydrogen exchange (NHE) and sodium bicarbonate co-transport (NBC), which are linked to cytosolic pH through the action of carbonic anhydrases (CAs) [ 30 ], as well as sodium glucose linked transport (SGLT) where Na + transport is coupled to glucose uptake. Independent of the mechanism of Na + influx, a consequence of increased intracellular [Na + ] would be to push NKA up its activation curve (i.e., above its K m for Na + ), thereby increasing ATP turnover by NKA. A further consequence of elevated [Na + ] i will be an elevation of cytosolic Ca 2+ through the action of the plasma membrane sodium-calcium exchange (NCX) and a decrease of mitochondrial Ca 2+ through the action of the mitochondrial sodium-calcium exchange (NCLX) which lead to wide ranging effects of altered Na + ion homeostasis in cancer cells.
We focussed on the contribution of NKA to glycolytic metabolism and hypothesised that the elevated rates of glycolysis that underpins the Warburg effect in cancer is causally linked to the flux of Na + through the NKA. We tested this hypothesis in a panel of three human breast cancer cells MDA-MB-231, HCC1954, MCF-7, a murine breast cancer cell line 4T1, and a control human epithelial cell line MCF-10A. Nuclear magnetic resonance (NMR) techniques were applied to living cells, to probe [Na + ] i non-invasively with triple quantum filtered (TQF) [ 23 ] 23 Na-NMR spectroscopy [ 31 ] and glycolytic flux measurements with 2 H-NMR to measure conversion of [6,6- 2 H 2 ] d -glucose to [ 2 H]-labeled l -lactate, in real time, and in response to NKA inhibition with ouabain [ 32 ]. Intracellular [Na + ] i was titrated using modified isotonic extracellular buffers with varying [Na + ] and [K + ] and introducing an artificial Na + plasma membrane leak using the ionophore gramicidin-A, to create a controlled and constant permeabilization to Group 1 cations, which equilibrates [Na + ] across the cell membrane. Experiments were carried out in parallel with cell viability assays, 1 H NMR metabolomics of intracellular and extracellular metabolites, extracellular flux analyses using the commercial Seahorse assay method to measure extracellular acidification rate (ECAR) and oxygen consumption rate (OCR), and in vivo measurements in an MDA-MB-231 human-xenograft mouse model using 2-deoxy-2-[ 18 F]fluoroglucose ( 18 F-FDG) positron emission tomography (PET) [ 33 ].
Materials and Methods
Cell culture and treatment protocols.
Human cell lines MDA-MB-231 (CRM-HTB-26); MCF-7 (HTB-22); HCC1954 (CRL-2338) and 4T1 (CRL-2539) mouse cell line were obtained from American Type Culture Collection (ATCC). MCF-10A (CRL-10317) were a gift from Dr Christopher Switzer. All cells were used at early passage and tested regularly for mycoplasma. Cells were cultured as monolayers under standard conditions in the supplier’s recommended media: RPMI-1640 (#R0883-500ML, Sigma, USA), DMEM (#D5671-500ML, Sigma, USA); DMEM/F-12 (#11,330–032, Gibco). RPMI-1640 and DMEM were supplemented with 10% (v/v) fetal bovine serum (FBS) (#F7524-500ML, Sigma-Aldrich), 4 mM L-glutamine (#G7513-100ML, Sigma-Aldrich), 170 µM penicillin and 170 µM streptomycin (#P4333-100ML, Sigma-Aldrich). DMEM/F-12 (Gibco) was supplemented with 5% (v/v) horse serum (26,050,088, ThermoFisher Scientific) 10 µg mL −1 human insulin (A11382II, Gibco), 20 ng mL −1 human epidermal growth factor (hEGF) (#CC-4107, Lonza), 100 ng mL −1 cholera toxin (#C8052, Sigma-Aldrich), 0.5 µg mL −1 hydrocortisone (H0888, Sigma-Aldrich).
For investigations using ouabain, normal medium was replaced 1 h prior to the start of the experiment with complete DMEM supplemented with 1 µM ouabain (#PHR1945, Sigma-Aldrich). For control MCF-10A cells, complete medium was replaced 16 h prior to experimentation by DMEM/F-12 solely supplemented with 5% (v/v) horse serum to avoid artificial growth stimulation.
Cell volume measurement
Cell volumes were measured using a Scepter 2.0 Automatic cell counter (#PHCC20040, Merck Millipore, USA) with Scepter™ 60 µm sensors attached (#PHCC60050, Merck Millipore, USA). Samples were taken as part of routine passaging during experiments and assessed in serum-free DMEM supplemented with 4 mM L-glutamine, 170 µM penicillin and 170 µM streptomycin. The mean cell volume (pL) was used to calculate the average cell size, which was used for normalization throughout (data in Supplementary Fig. S1).
MTT cytotoxicity assays
Cells were seeded at 1 × 10 5 cells mL −1 into a 96-well plate. Following overnight adherence, the cells were cultured with complete medium ± ouabain (titrated 1 × 10 –12 – 1 × 10 –3 M) for 24 h. The medium was then aspirated and replaced with 20 μL solution of reconstituted powder 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; #M6494, ThermoFisher Scientific) in each well and mixed. After 3 h, supernatants were removed and 50 μL dimethyl sulfoxide (DMSO) was added to each well to dissolve the formazan crystal precipitate. Light absorbance was measured at a wavelength of 570 nm using a Spectrostar Nano plate reader (BMG LabTech, Germany). Data were fitted using a nonlinear regression model in GraphPad Prism version 9.4.1 for Windows (GraphPad Software, USA) to estimate EC 50 following 24 h ouabain treatment. Normalised absorbance is presented as a % of maximum.
Trypan blue exclusion
Cells were seeded at densities of 5 × 10 5 per well in 2 mL of complete medium and left to adhere overnight. The following day, the medium was aspirated and replaced with DMEM ± 1 µM ouabain for 1 h. Medium was aspirated, and the cells were washed three times with 1 mL Dulbecco’s phosphate buffered saline (DPBS; #D8537, Sigma Life Science), 0.05% Trypsin–EDTA solution (#15,400–054, Gibco) was added to each well until the cells detached. The cells were resuspended in serum-free DMEM and centrifuged at 350 × g for 5 min. Cell pellets were resuspended in serum-free DMEM and diluted to give concentrations of 5 × 10 4 cells mL −1 and vortexed to ensure a homogeneous suspension. Fractions of each cell suspension were mixed 1:1 with 0.4% Trypan Blue solution (#T8154, Sigma Life Sciences) and pipetted onto a haemocytometer. Studies were performed within 5 min of mixing with Trypan Blue. Cell viability was calculated by \(\text{Cell viability}=(\text{Viable cells}/\text{Total cells})\times 100\%\) . Unpaired, two-tailed t-tests were performed in GraphPad Prism version 9.4.1 for Windows (GraphPad).
1 H NMR metabolomics
Cells were harvested by scraping the surface and then subjecting to methanol / water / chloroform dual phase extraction. Cells were vortexed in 2 mL each of ice-cold methanol, chloroform, and Millipore water and then centrifuged for 1 h at 2500 × g at 4 °C to separate aqueous, protein, and lipid layers. The upper aqueous phase was separated; 20–30 mg Chelex-100 was added to chelate paramagnetic ions, vortexed and centrifuged at 2500 × g for 1 min at 4 °C. The supernatant was transferred to a fresh Falcon tube containing 10 μL of universal pH indicator solution followed by freeze drying. Dual-phase-extracted metabolites were reconstituted in 600 μL of deuterium oxide [containing 8 g L −1 NaCl, 0.2 g L −1 KCl, 1.15 g L −1 Na 2 HPO 4 , 0.2 g L −1 KH 2 PO 4 and 0.0075% w/v 3-(trimethylsilyl)-2,2,3,3-d 4 -tetradeuteropropionic acid (TSP)] and adjusted to pH ~ 7 by titrating with 100 mM hydrochloric acid. Cell culture medium samples (500 µL) were collected from the culture flask after 24 h. Fresh medium samples were also collected. 100 µL of D 2 O buffer were added to each sample prior to NMR spectral acquisition.
1 H nuclear magnetic resonance spectra were acquired using a vertical-bore, ultra-shielded Bruker (Bruker, Karlsruhe, Germany) 14.1 T (600 MHz) spectrometer equipped with a prodigy probe, at 298 K using the Bruker noesygppr1d pulse sequence for residual water suppression. Acquisition parameters were: 64 transients; 4 dummy transients; 20.8 ppm spectral width; acquisition time, 2.6 s; pre-scan delay, 4 s; 90° flip angle; and experiment duration of 7.5 min per sample. TopSpin (version 4.0.5) software was used for data acquisition and for metabolite quantification. Free induction decays (FIDs) were multiplied by a line broadening factor of 0.3 Hz and Fourier transformed, phase, and automatic baseline corrected. Chemical shifts were normalised by setting the TSP signal to 0.0 ppm. Metabolite peaks of interest were initially integrated automatically using a pre-written integration-region text file and then manually adjusted as required. Assignment of peaks to their respective metabolites was based on previously obtained in-house data, confirmed by chemical shift, and using Chenomx NMR Profiler Version 8.1 (Chenomx, Canada). Metabolite concentrations were normalised to the number of cells (measured in a parallel flask) and cell volume, expressed in mM.
23 Na triple quantum filtered NMR
Cells were seeded 48 h prior to experiments to reach 70–80% confluence at the time of harvesting. For ouabain treatment groups the medium was replaced 1 h prior to harvesting. Monolayer cultures were harvested by trypsinisation, resuspended in DMEM without 10% FBS, counted, and their viability assessed by the trypan blue exclusion method. Cell suspensions were centrifuged at 350 × g for 5 min at room temperature. The supernatant was aspirated, and the cell pellet was rapidly resuspended in 250 µL DMEM without 10% FBS. The cell suspension was transferred to a 5 mm OD Shigemi NMR tube, 8 mm bottom glass slug, (D 2 O matched #BMS-005B, Fluorchem) containing a 20-mm long, 1-mm OD capillary tube that contained 2% w/v agarose (#17,850, ThermoFisher Scientific), 5 mM 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetramethylenephosphonate (Tm[DOTP] 5− ; #M-155, Macrocyclics, Plano TX, USA) without using a deuterium-solvent lock. The NMR tube was then placed in a Bruker Avance III 400 MHz wide-bore spectrometer with a BBO probe maintained at 310 K (37 °C). Cells were transferred from their culture medium and studied by NMR within 10 min. Magnetic field homogeneity was ensured by shimming on the 1D 23 Na spectrum prior to each experiment. Triple quantum filtered 23 Na NMR experiments were performed as previously described [ 31 , 34 ], with acquisition of 384 transients, 6 dummy transients, a spectral width of 50 ppm, pre-scan delay of 150 ms, and an acquisition time of 2 min 16 s. TQF transients were repeated three times in succession to assess reproducibility.
2 H NMR of glycolytic flux
Cell samples were cultured, harvested, and prepared as described above. Cell pellets were resuspended in 250 µL glucose-free DMEM (#11,966,025, ThermoFisher Scientific) and transferred to a 5 mm OD Shigemi NMR tube. A 40 µL bolus of [6,6- 2 H 2 ] d -glucose (#282,650, Sigma-Aldrich) was added to give a final concentration of 10 mM D-glucose within the sample. The cell suspension was then placed in the Bruker Avance III 400 MHz wide-bore spectrometer, with a BBO probe (Bruker) maintained at 37 °C for the duration of the experiment. To improve sensitivity in the detection of 2 H, the X transmitter amplifier was routed via the 2 H preamplifier, and onto the X-channel of the 5-mm BBO probe. Time series of spectra were acquired in a pseudo-2-dimensional fashion using inverse gated 1 H decoupling (waltz16), with 128 transients, a spectral width of 20 ppm, a pre-scan delay of 10 ms, and an acquisition time of 200 ms. Each spectrum was averaged over 28.4 s with 64 spectra acquired in a time course of ~ 30 min. Spectra were processed and analysed using TopSpin 4.2.0 (Bruker). Peak integrals were measured for [ 2 H]HOD, [6,6- 2 H 2 ] d -glucose and [3,3- 2 H 2 ] l -lactate as a function of time. The dynamic intensity versus time data were analysed using nonlinear least squares fitting to a two-compartment model in MATLAB R2022b (Mathworks, USA) to the estimate rate constant for the conversion of glucose to lactate. Briefly, a phenomenological model given by the following set of differential equations was employed; it assumed a single rate constant k gl for glucose to lactate conversion and an additional rate constant k loss describing flux of glucose via other pathways,
Experimental data were fitted in a least squares fashion using an ordinary differential equation (ODE) solver. Glycolytic rate constants ( k gl ) were multiplied by the number of moles of glucose added, and normalised to cell number, and cell volume (Supplementary Fig. S1) to give glycolytic flux in units of mol (total cell volume) −1 s −1 .
Extracellular Flux
Extracellular acidification rate (ECAR) was measured with an XFe Extracellular Flux Analyzer (Agilent, USA) that was run according to the manufacturer’s instructions. Cells were seeded at 5 ✕ 10 4 cells per well, plated on Seahorse XFe24 culture plates (Agilent, #100,777–004) in the preferred medium (RPMI-1640 or DMEM) supplemented with 10% (v/v) FBS, 4 mM L-glutamine, 170 µM penicillin, and 170 µM streptomycin, and incubated overnight.
Two hours prior to the start of the experiment, the culture medium was replaced with DMEM ± 1 µM ouabain for 1 h and then placed into standard culture conditions. The medium was aspirated, and replaced with unbuffered DMEM (#D5030, SigmaAldrich) supplemented with 4 mM L-glutamine, pH 7.4, and incubated for 1 h at 37 °C in a CO 2 -free incubator. The glycolysis stress-test was then performed according to Seahorse commercial protocols with final concentrations of 10 mM glucose, 1 μM oligomycin, and 100 mM 2-deoxy- d -glucose. The ECAR reading was normalised to DNA content in each well using DRAQ5 nuclear stain (Thermo Scientific, #62,251) at the end of the experiment. Full traces of the glycolytic stress test were presented, including all repeats ± SD.
Cells were prepared in 175 cm 2 culture flasks and seeded 36 h prior to each experiment (80–90% confluence). Cell culture medium was aspirated and replaced with complete DMEM ± 1 µM ouabain for 1 h. The medium was then aspirated and the cells were washed with DPBS. Trypsin–EDTA was used to detach the cells, and the medium was neutralised by adding modified Krebs–Henseleit buffer (Supplementary Table S1) not containing Na + . Cell suspensions were centrifuged at 350 ✕ g for 5 min. The supernatant was aspirated and the cells were resuspended in 250 µL of the respective buffers in (mM) 10, 20, 30, 50 or 70 [Na + ] containing 10 µM Gramicidin (#G5002, Sigma-Aldrich) dissolved in sterile dimethyl sulfoxide (#12,611, Cell Signalling Technology) and transferred to a 5-mM OD Shigemi NMR-tube. To this tube either the Tm-DOTP reference tube was added for 23 Na- TQF NMR experiments or 10 mM [6,6- 2 H 2 ] d -glucose was added for 2 H NMR experiments. The sample was then subject to the same NMR experiments for acquisition of either 23 Na TQF spectra to measure [Na + ] i or 2 H time series to measure glycolytic flux.
Animal models
All animal experiments were conducted in the UK and were performed in accordance with the United Kingdom Home Office Animal (Scientific Procedures) Act, 1986. Experiments were performed unblinded. BALB/c Nude (CAnN.Cg-Foxn1nu/Crl) were purchased from Charles River Laboratories. Mice arrived at age 6–8 weeks and were acclimatized for 7 d before undergoing experimental procedures. Animals were able to access drinking water and standard diet ad libitum.
18 F-FDG PET preclinical imaging
Tumours were established by injecting 100 µL of suspension containing 1 × 10 6 MDA-MB-231 cells in a 1:1 PBS/Matrigel (#AC-M082706, Acro Biosystems) solution. Tumour growth was monitored daily by electronic calliper measurement with volume calculated using the following equation: volume (mm 3 ) = ((π/6) × h × w × l), where h, w and l represent height, weight, and length, respectively. When tumour volumes reached > 100 mm 3 , animals underwent PET imaging protocols. At t = 0, sterile saline ± 0.04 mg kg −1 ouabain was injected via tail vein cannulation as a 50 µL bolus to anaesthetised mice. The injected concentration of ouabain was 270 µM to yield a final blood concentration of ~ 6.75 µM; this was above the EC 50 measured for human MDA-MB-231 cells but below the EC 50 for mouse cells. Mice were anaesthetised with isoflurane (2–2.5% in O 2 ) by nose cone at 37 °C until transferred to the nanoScan® microPET/CT scanner (Mediso). At t = 60 min, mice were injected with 7.15 ± 1.33 MBq 18 F-FDG in saline (n = 12), and a dynamic PET acquisition was performed for 1 h post injection. After completion of scanning protocols, mice were euthanized by cervical dislocation. For quantification of radiotracer retention in tissue by PET/CT, tumour volumes were estimated from 2D regions drawn manually using the CT image as reference using VivoQuant™ (Invicro, Needham, MA, USA). Data were expressed as %ID g −1 , assuming a tissue density of d = 1 g mL −1 .
All data are presented as mean ± standard deviation (SD). Data acquired during 24 h ouabain exposure MTT (Fig. 1 g) were transformed to % of untreated control viability and then an empirical nonlinear fit was applied to determine the EC 50 for each cell line. Two-tailed unpaired t-tests (assuming equal SD) were used for vehicle versus ouabain-treated samples where p < 0.05 was taken to be significant and * denotes p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Statistical tests were performed using GraphPad Prism. Unclassified principal components analysis (PCA) was performed in MATLAB. Metabolite peak integrals were first normalised to total spectrum area and was then auto-scaled prior to PCA calculation using Venetian blinds cross validation and five cross validation groups. Hierarchical cluster analysis was performed in MATLAB using the function clustergram.
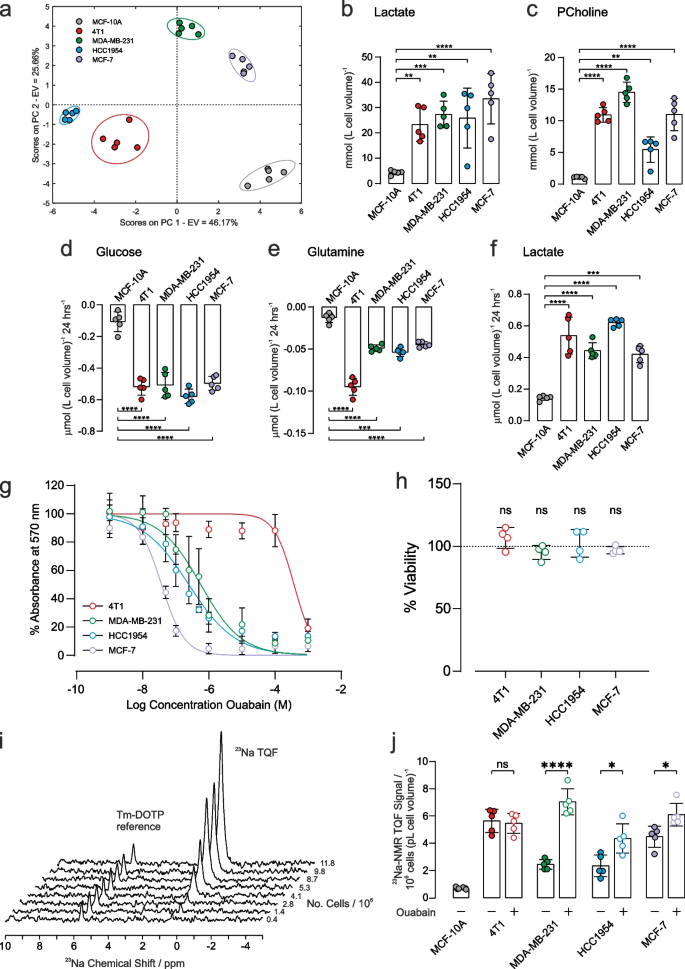
Metabolic characterization and response to ouabain treatment on [Na + ] i . a Principal component analysis of intracellular metabolites (mean values given in Supplementary Table S2). Intracellular concentrations of b lactate (left panel) and c phosphocholine (right panel) were significantly higher in all cancer cells with respect to control epithelial cells ( n = 5). Extracellular metabolite concentrations of d glucose, e glutamine and f lactate after 24 h cell culture. Respective metabolite concentration from fresh media were subtracted such that negative concentrations refer to metabolite consumption while positive concentrations refer to production ( n = 5). g MTT cytotoxicity assay dose response curves following 24 h treatment with ouabain. Measured EC 50 values were: 4T1: 40 µM, MDA-MB-231: 0.4 µM, HCC1954: 0.2 µM, MCF-7: 0.04 µM. h Cell viability in response to 1 µM ouabain for 1 h measured by trypan blue exclusion assay measured no change in cell viability. i Representative TQF 23 Na NMR spectra showing proportionality with cell number. The Tm-DOTP reference peak is from the internal standard. j Quantification of TQF 23 Na NMR relative to cell number and cell volume. Baseline [Na + ] i was higher in all cancer cells with respect to control epithelial cells ( n = 5). Treatment with 1 µM ouabain for 1 h led to a significant increase in [Na + ] i in all human cancer cell lines compared to vehicle control ( n = 5). [Na + ] i was unchanged in the murine 4T1 cell line following 1 h treatment with 1 µM ouabain, ( p = 0.7, n = 5). Significance was assessed using a two-tailed unpaired t-test, ns p > 0.05, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Data plotted as mean ± SD
Metabolic profiles
Metabolic profiles were first characterized for the different cells using NMR-based metabolomics. Intracellular metabolite concentrations were measured in the three human cancer cell lines (MDA-MB-231, HCC1954, and MCF-7), in the mouse cancer cells (4T1) and in the control human epithelial cells (MCF-10A). A full table of data is given in Supplementary Table S2, and representative annotated 1 H spectra are shown in Fig. S2. Principle component analysis, Fig. 1 a, and hierarchical cluster analysis, Supplementary Fig. S3, showed a good separation of intracellular metabolites in the different cell lines, highlighting distinct metabolic profiles. Intracellular lactate, Fig. 1 b, was significantly higher in all cancer cells with respect to control epithelial cell line, (left panel, mmol (L cell volume) −1 , n = 5): MCF-10A: 4.4 ± 0.4, 4T1: 23.3 ± 2.6 ( p < 0.0001), MDA-MB-231: 27.3 ± 2.1 ( p < 0.001), HCC1954: 25.9 ± 4.7 ( p < 0.01), MCF-7: 34 ± 4 ( p < 0.001). Intracellular phosphocholine (a marker of phospholipid membrane turnover in proliferating cells), Fig. 1 c, was higher in all cancer cells with respect to control epithelial cell line, (right panel, mmol (L cell volume) −1 , n = 5), MCF-10A: 1.05 ± 0.06, 4T1: 10.9 ± 0.5 ( p < 0.0001), MDA-MB-231: 14.5 ± 0.6 ( p < 0.0001), HCC1954: 5.4 ± 0.8 ( p < 0.001), MCF-7: 11.0 ± 1.0 ( p < 0.0001).
The concentrations of extracellular metabolites in the culture medium of each cell line were measured after 24 h at 37 °C; the 1 H NMR spectra of respective control media were subtracted to measure metabolite utilisation or excretion. A full table of data is given in Supplementary Table S3, and representative annotated subtracted 1 H spectra are shown in Fig. S4. Glucose consumption, Fig. 1 d, was significantly higher in all cancer cells, compared with the control epithelial cell line, (µmol 10 –6 cells, n = 5): MCF-10A: 0.11 ± 0.03, 4T1: 0.52 ± 0.02, MDA-MB-231: 0.51 ± 0.03, HCC1954: 0.57 ± 0.02, MCF-7: 0.50 ± 0.02. Glutamine utilisation, Fig. 1 e, was significantly higher in all cancer cells, compared with the control epithelial cell line, (µmol 10 –6 cells, n = 5): MCF-10A: 0.013 ± 0.002, 4T1: 0.095 ± 0.004, MDA-MB-231: 0.049 ± 0.002, HCC1954: 0.054 ± 0.002, MCF-7: 0.045 ± 0.001. Lactate excretion, Fig. 1 f, was significantly higher in all cancer cells, compared with the control epithelial cell line, (µmol 10 –6 cells, n = 5): MCF-10A: 0.15 ± 0.01, 4T1: 0.54 ± 0.05, MDA-MB-231: 0.44 ± 0.02, HCC1954: 0.62 ± 0.01, MCF-7: 0.42 ± 0.02 µmol 10 –6 cells.
Taken together, these data are indicative of all four breast cancer cell lines displaying significantly elevated aerobic glycolysis compared to the control epithelial cell line.
Ouabain cytotoxicity – establishing EC 50
Ouabain is an inhibitor of NKA, with reported effects on cell proliferation and survival [ 35 , 36 ]. The methylthiazolyl tetrazolium (MTT) cell-cytotoxicity assay was used to determine the concentration of ouabain that led to a defined level of cell death over 24 h, Fig. 1 g. Human breast cancer cells showed differing sensitivity to ouabain, with EC 50 values in the sub micromolar range of 0.5 µM (MDA-MB-231), 0.2 µM (HCC1954), and 0.04 µM (MCF-7). For the murine 4T1 cell line, EC 50 = 40 µM, thus showing that it was 80–1000-fold less sensitive to ouabain, as reported for cells derived from rodents [ 37 , 38 , 39 ]. Given the EC 50 estimates, exposure to 1 µM ouabain for 1 h was chosen for subsequent experiments, which is above the 24 h EC 50 for the human cells and below the EC 50 for the 4T1 cells. Since the MTT cytotoxicity assay reports on metabolism as a surrogate measure of viability, the trypan blue exclusion assay was used to measure membrane integrity to assess viability more directly in the absence of acute effects on metabolism. Figure 1 h shows that 1-h acute exposure to ouabain did not reveal any cytotoxicity with 100% viability as assessed by trypan blue exclusion.
23 Na NMR spectroscopy of [Na + ] i
Triple quantum filtered (TQF) 23 Na-NMR is an established method [ 34 ] for non-invasively measuring [Na + ] i in red blood cells [ 40 ], the perfused heart [ 31 , 41 ] and other tissues [ 42 ]. We adapted the method for cancer cell suspensions using 5-mm OD Shigemi NMR tubes, which retain the cells within the active volume of the radio-frequency (RF) coils of the NMR probe. The method detects Na + that is in exchange-binding to macromolecules, while filtering out the signal from unbound extracellular [Na + ] e , yielding a spectral peak with an intensity that is proportional to [Na + ] i . A reference capillary (inserted into the cell suspension) containing a set agarose gel, with the shift reagent Tm-DOTP, together with a known concentration of NaCl (140 mM), yielded a TQF peak that was shifted away from the intracellular one, Fig. 1 i, thus enabling normalization of the relative peak areas. The peak area of the 23 Na-TQF signal was linear with cell number across all cancer cell lines, Fig. 1 i and Supplementary Fig. S5. The 23 Na-TQF signal was normalised to the internal standard, cell number (10 6 ), and cell volume (pL) enabling the measurement of relative changes in [Na + ] i . All cancer cells displayed elevated [Na + ] i compared with the breast epithelial cell line MCF-10A, Fig. 1 j (filled symbols).
Control cell suspensions maintained a stable [Na + ] i over a 1 h time course, whereas ouabain treatment led to the steady accumulation of [Na + ] i within the same period, Supplementary Fig. S6. Treatment with 1 µM ouabain for 1 h led to a significant increase in [Na + ] i in all human cancer cells compared to vehicle control, Fig. 1 j (open symbols, n = 5), MDA-MB-231 by 188 ± 23% ( p < 0.0001); HCC1954 by 90 ± 40% ( p = 0.0101); MCF-7 by 36 ± 12% ( p = 0.0127). Ouabain elicited no effect on [Na + ] i in the murine 4T1 cells compared to vehicle control, implying that the increase in the human cells was due to NKA inhibition.
Treatment effect of ouabain on extracellular acidification rate
Seahorse XFe24 (ECAR) flux-analysis used a commercial multi-well instrument and prescribed protocol to study glycolytic flux through to lactic acid (and hence a change in pH; a proxy for glycolytic rate) and oxygen consumption (OCR) in an adherent monolayer of cells. It was applied to the panel of cancer cells that had been pre-treated with 1 µM ouabain for 1 h. Figures 2 a and 2b show the ECAR and OCR time courses plotted for the MDA-MB-231 cells, with ECAR vs OCR shown in Fig. 2 c, demonstrating significant decreases in ECAR, with no change in OCR during the addition of 10 mM glucose, in the presence of ouabain. Time courses for the other cells are given in Supplementary Fig. S9. Figures 2 d-2g show the quantified ECAR values and OCR after the addition of 10 mM glucose. Ouabain elicited a reduction in glycolytic rate in the MDA-MB-231 and MCF-7 cells, while rate-change in HCC1954 cells did not reach significance. Ouabain elicited no effect on ECAR in the murine 4T1 cells showing that decreased glycolytic rate was a consequence of NKA inhibition by ouabain. We recorded no difference in OCR in the four cancer cell lines, indicating that there was no change in oxidative metabolism (Figs. 2 d-2g).
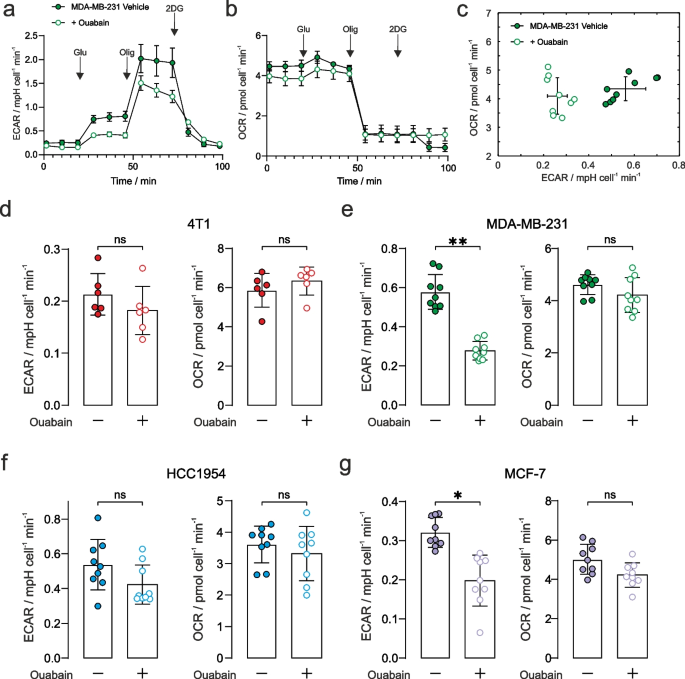
Extracellular acidification rate with NKA inhibition . a Seahorse XFe24 glycolytic stress test of extracellular change in pH for the MDA-MB-231 cancer cells (± SEM representing biological reproducibility of n = 3 biological repeats where each n = 3 technical repeats were first averaged). Complete time courses for the other cell lines are given in Supplementary Fig. S8. The stress test comprised 10 mM glucose, 1 μM oligomycin, and 100 mM 2-deoxy-D-glucose indicated by arrows. b Oxygen consumption rate (OCR) measured simultaneously with the ECAR data in panel a . c Plot of the measured ECAR glycolytic rate vs OCR during the 10 mM glucose time window defined in Supplementary Fig. S6 ( n = 3 biological repeats each with n = 3 technical repeats) showing a reduction in glycolytic rate and no change in OCR. Quantified extracellular acidification rate corresponding to glycolytic rates during the 10 mM glucose time window (left panels) and their corresponding OCR (right panels), in control and ouabain treated cells: d 4T1: ECAR ( p = 0.24), OCR ( p = 0.39); e MDA-MB-231: ECAR decreased by 52% ( p = 0.006), OCR ( p = 0.40); f HCC1954: ECAR decreased by 21% ( p = 0.08), OCR ( p = 0.66); g MCF-7: ECAR decreased by 38% ( p = 0.015), OCR ( p = 0.16). n = 3 biological repeats each with n = 3 technical repeats, significance was assessed using a nested unpaired t-test. ns p > 0.05, * p < 0.05, ** p < 0.01
The above glycolytic flux experiments reported on the terminal H + production measured by extracellular acidification rate (ECAR) as a surrogate for actual glycolysis. De Feyter et al. (2018) have shown that metabolism of [6,6- 2 H 2 ] d -glucose via glycolysis to [3,3- 2 H 2 ] l -lactate (or [3- 2 H]L-lactate) can be followed non-invasively in cells, and in vivo in real time, yielding quantitative estimates of the rate of glucose consumption and lactate production. Accordingly, cancer cells were resuspended in glucose-free Dulbecco’s modified Eagle’s medium (DMEM) that was supplemented with 10 mM [6,6- 2 H] d -glucose then immediately placed in the NMR spectrometer. 2 H NMR spectra were acquired every 30 s for ~ 30 min to quantify the rate of glucose consumption, and lactate production (Fig. 3 a). Importantly, there was no significant loss of 2 H label to water in the cell suspension; hence, deuterated water (HOD) conveniently served as an internal chemical shift and peak-intensity reference for these studies, [ 2 H]HOD has a natural abundance in water of 16.7 mM. Figure 3 b shows time courses of the peak integrals of [6,6- 2 H 2 ] d -glucose and [3,3- 2 H 2 ] l -lactate for 4T1 cells and Fig. 3 c for MDA-MB-231 cells; vehicle treated (filled symbols) and 1µM ouabain treated (open symbols). Decreased glycolytic flux can be seen in MDA-MB-231 cells following ouabain treatment, while no change in rate is seen in the 4T1 cells.
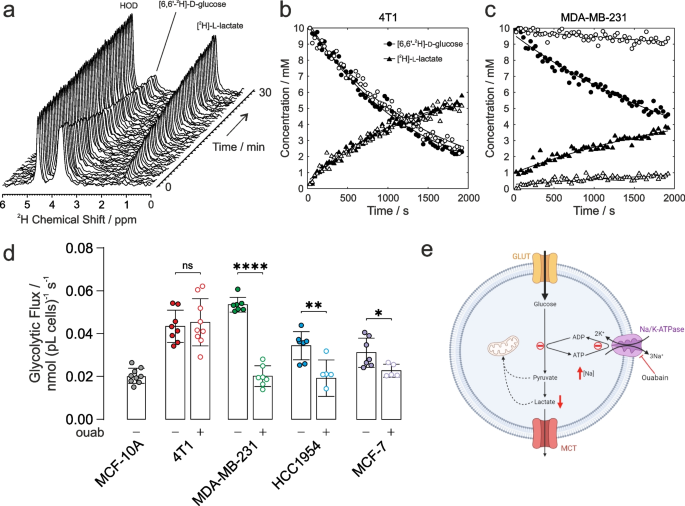
Glycolytic flux measured with 2 H-NMR. a Time-series of 2 H-NMR spectra showing metabolism of [6,6- 2 H 2 ] d -glucose to [3,3- 2 H 2 ] l -lactate by 4T1 cells in suspension. No 2 H label was lost to HOD, serving as an internal chemical shift and intensity standard at 16.7 mM. Time course and empirical fits performed in Matlab of the normalized peak integrals of the [6,6- 2 H 2 ] d -glucose and [3,3- 2 H 2 ] l -lactate spectral peaks: b 4T1 cells and c MDA-MB-231 cells for vehicle control (filled symbols) and ouabain treated (open symbols). d Quantified glycolytic flux in MCF-10A cells was 0.020 ± 0.003 nmol (pL cells) −1 s −1 ( n = 9). 4T1 cells had a higher baseline glycolytic rate of 0.043 ± 0.007 nmol (pL cells) −1 s −1 ( n = 8) and unchanged rate after ouabain treatment, 0.045 ± 0.010 nmol (pL cells) −1 s −1 ( n = 9, p = 0.6949). Human breast cancer cells all showed higher baseline glycolytic rates than control epithelial cells, MDA-MB-231: 0.054 ± 0.003 nmol (pL cells) −1 s −1 ( n = 7); HCC1954: 0.034 ± 0.006 nmol (pL cells) −1 s −1 ( n = 7); MCF-7: 0.031 ± 0.006 nmol (pL cells) −1 s −1 ( n = 7). Human cells showed a decreased glycolytic rate following ouabain-treatment vs vehicle control, MDA-MB-231: 0.020 ± 0.004 nmol (pL cells) −1 s −1 ( n = 7; p < 0.0001); HCC1954: 0.019 ± 0.008 nmol (pL cells) −1 s −1 ( n = 6; p = 0.004); MCF-7: 0.023 ± 0.003 nmol (pL cells) −1 s −1 ( n = 5; p = 0.029). e Schematic of the proposed mechanism of the effect of ouabain inhibition of NKA on glycolytic flux (Figure created using BioRender.com). ns p > 0.05, * p < 0.05, ** p < 0.01, **** p < 0.0001. Data plotted as mean ± SD
Figure 3 d shows the calibrated rates of glycolytic flux derived from fitting the data in MATLAB, normalised to cell number (10 6 ) and cell volume (pL). All cancer cells showed significantly higher baseline glycolytic rates than the healthy epithelial cell line, giving further evidence of the Warburg phenotype in the chosen panel of cancer cells. Glycolytic rates measured in the human breast cancer cells were all significantly lower following ouabain treatment vs vehicle controls, while the glycolytic rate measured in the murine 4T1 cells was unchanged following ouabain treatment.
Membrane permeabilization with Gramicidin-A
To investigate whether there was a graded relationship between [Na + ] i , NKA activity, and glycolytic rate, we used gramicidin-A to permeabilise the plasma membranes to monovalent cations to allow the intracellular [Na + ] i concentration to be matched to a series of extracellular concentrations. Extracellular solutions were designed to be isotonic with Na + replaced with K + to give a range of NKA-activating Na + concentrations, while [Ca 2+ ] and pH were held constant (see Supplementary Table S1). NKA is maximally activated at all K + concentrations above 10 mM and hence extracellular [K + ] did not limit the rate under these conditions.
Upon permeabilization with gramicidin-A, and resuspension of the cells in buffers with [Na + ] e ranging from 10 – 70 mM, intracellular [Na + ] i (measured by 23 Na TQF NMR) showed a linear dependence on [Na + ], Fig. 4 b and 4c, in both the murine 4T1 and the human MDA-MB-231 breast cancer cells. Under the same titrated conditions of [Na + ] ranging from 10 – 70 mM, the rates of glycolytic flux measured by 2 H-NMR correlated directly with increasing [Na + ] i in both cell lines, Fig. 4 d. The K m for Na + from the plots in Fig. 4 d were 14 mM for 4T1 cells and 25 mM for MDA-MB-231 cells with a similar V max (plateau) at high [Na + ]. NKA pump current (activity) has been previously measured as a function of [Na + ] using patch clamping and an analytical expression has been reported (Fig. 3 in Silverman et al. [ 43 ]). Using this expression and converting [Na + ] into NKA pump current (as a % of maximum), glycolytic flux was found to be directly proportional to pump current (NKA activity), Fig. 4 e. Finally, Fig. 4 f shows the glycolytic fluxes measured at the highest [Na + ] i = 70 mM (closed symbols) and when treated with ouabain (open symbols), which was unchanged in 4T1 cells whereas there was nearly a 60% decrease in glycolytic flux in ouabain treated MDA-MB-231 cells.
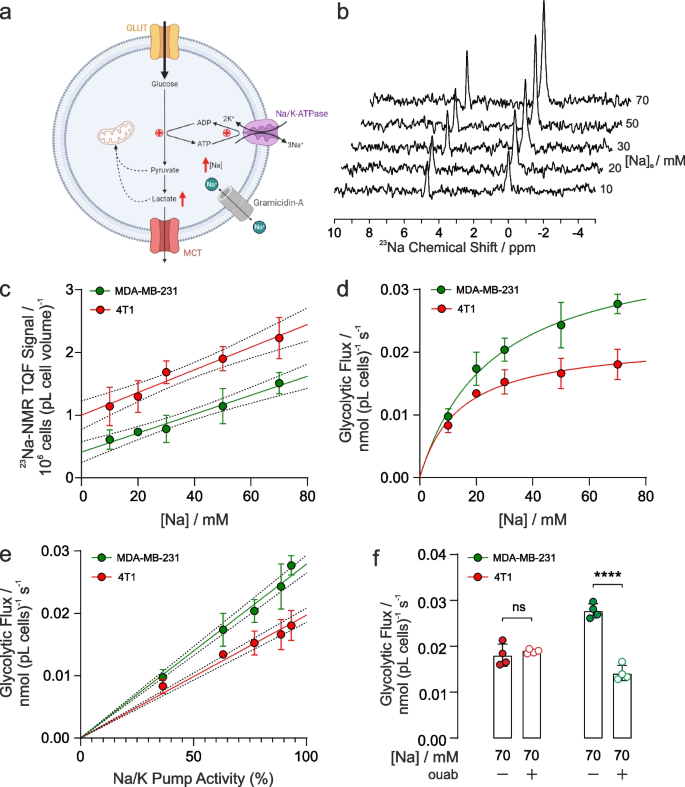
Glycolytic flux affected by intracellular [Na] i and NKA function. a Schematic of the proposed mechanism of the effect of gramicidin-A on [Na + ] i and glycolysis. Gramicidin introduces an artificial Na + leak, increasing [Na + ] i and glycolytic metabolism (Figure created using BioRender.com). b 23 Na TQF spectra showing the intracellular [Na + ] i peak relative to a reference capillary in MDA-MB-231 cells following membrane permeabilization with gramicidin and varying concentrations of titrated extracellular [Na + ] e . c Quantification of TQF 23 Na NMR spectra (proportional to [Na + ] i ) following exposure to isotonic solutions of titrated [Na + ] e ( n = 4), for 4T1 cells and MDA-MB-231 cells. d Quantification of glycolytic fluxes measured by the rate of [6,6- 2 H 2 ] d -glucose to [3,3- 2 H 2 ] l -lactate conversion at different concentrations of titrated [Na + ] i in murine 4T1 and human MDA-MB-231 breast cancer cells ( n = 4). e Glycolytic fluxes in panel d replotted as a function of pump current derived from the analytical expression given by Silverman et al. [ 43 ] f Glycolytic flux measured at the highest concentration of 70 mM [Na + ] e following treatment with 1 µM ouabain treatment was not significantly altered in murine 4T1 cells but was significantly decreased in human MDA-MB-231 cells. ns p > 0.05, **** p < 0.0001. Data plotted as mean ± SD
In vivo PET imaging of tumour metabolism
To investigate tumour metabolism in vivo, we used 18 F-FDG, an analogue of glucose that is used in clinical oncology to assess tumour burden and treatment response. Immunodeficient mice bearing subcutaneous human MDA-MB-231 xenograft tumours were injected intravenously with vehicle ± ouabain 1 h prior to injection with 18 F-FDG. At 20 min post injection (p.i.) of 18 F-FDG, mice were scanned for 1 h and PET data were reconstructed dynamically to evaluate pharmacokinetics and biodistribution. Representative images demonstrated decreased tumour uptake of 18 F-FDG in ouabain-treated compared to vehicle controls (Figs. 5 a and 5b). The difference in uptake was significant from 40–60 min scan time (Figs. 5 c) in-line with clinical protocols, which add a 1 h delay between injection and PET scanning [ 44 ]. The area under the curve of 40–60 min scan time revealed a significant decrease in 18 F-FDG uptake of -24.5% in ouabain-treated versus vehicle treated controls, Fig. 5 d. The biodistribution (major organ uptake) of 18 F-FDG was comparable between groups in all other murine tissues, demonstrating the insensitivity of murine cells to cardiotonic steroids compared to human cells (Supplementary Fig. S11).
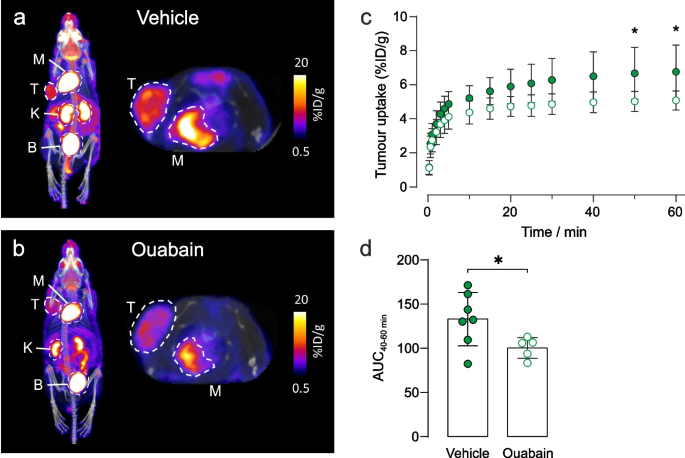
Ouabain decreases 18 F-FDG uptake in MDA-MB-231 tumour xenografts. Representative images of the biodistribution of 18 F-FDG in a vehicle and b ouabain treated tumours acquired at 60 min scan time. The tumour and organs of interest are denoted by dotted circles, where T = tumour; K = kidney, M = myocardium, B = bladder. c The pharmacokinetics of 18 F-FDG uptake were determined via the time-activity curves. A decreased avidity for 18 F-FDG was observed in tumours following treatment with ouabain (6.75 µM final blood concentration). At 50 min post 18 F-FDG injection, uptake was significantly lower in ouabain treated tumours 5.0 ± 0.6%ID/g ( n = 5) compared to control tumours 6.7 ± 1.6%ID/g ( n = 7), * p < 0.05, two-way ANOVA with Šidák’s multiple comparisons. d The area under the curve (AUC) derived from the time-activity curves at 40–60 min post injection of 18 F-FDG revealed a significant decrease in the uptake of 18 F-FDG in ouabain treated, AUC 40-60 min = 100 ± 12 ( n = 5) versus vehicle controls, AUC 40-60 min = 130 ± 40 ( n = 7), * p < 0.05, two-tailed unpaired t-test. Data plotted as mean ± SD
Intracellular [Na + ] i —correlation with glycolytic flux
Our experiments demonstrated a direct, functional link between intracellular [Na + ] i , NKA activity, Na + membrane permeability, and glycolytic flux in the breast cancer cells. Acute inhibition of NKA with 1 µM ouabain in the human cells MDA-MB-231, HCC1954 and MCF-7 resulted in significantly elevated [Na + ] i and a concomitant decrease in glycolytic flux. Treatment of murine 4T1 cells with 1 µM ouabain did not alter [Na + ] i nor glycolytic flux, due the lack of inhibition, and therefore acted as a negative control. Murine cells are typically less sensitive to ouabain than human cells due to two amino acid substitutions in the K + binding site of the α1 subunit of NKA (Q111R, N122D) causing ~ 1000-fold decreased ouabain sensitivity [ 37 , 38 , 39 ]. These experiments demonstrated the direct association between NKA activity and the ‘energy demand regulation’ of glycolytic flux. A schematic of the proposed mechanism is shown in Fig. 3 e.
The influence of membrane permeability of Na + on glycolytic flux was demonstrated in human MDA-MB-231 cells, and in murine 4T1 cells by using gramicidin-A. Gramicidin inserts into the plasma membrane (and has also been reported to insert into the mitochondrial membrane) creating a mono-valent cation-permeable pore. Under these conditions, the Na + (and K + and H + ) concentrations equilibrate (become equal) across the membrane(s) removing any trans-membrane Na + gradient and dissipating the membrane potential(s). Dissipating the mitochondrial H + gradient will inhibit mitochondrial ATP synthesis and therefore metabolism is expected to be dominated by glycolysis. Using modified extracellular buffers where [Na + ] i was tightly controlled, ranging from 10 – 70 mM, the 23 Na TQF experiments showed a linear dependence on [Na + ]. Our 23 Na TQF experiment preferentially report on intracellular Na + , however, due to the mechanism of triple quantum filtering, which selects for Na + that is slowly tumbling due to its association with larger macromolecules, there is a contribution to the signal both from intracellular Na + bound to protein and a contribution from extracellular Na + bound to the surfaces of membranes. This gives an offset to the TQF signal. Thus, while the TQF signal is proportional to [Na + ] i it is not directly so, ie. a calibration line of signal intensity versus [Na + ] does not pass through the origin.
Enhanced glycolytic flux correlated directly with increasing [Na + ] i in both cell lines, which could subsequently be inhibited with 1 µM ouabain in MDA-MB-231 cells, but not in the 4T1 cells. The K m values found from the fits in Fig. 4 d are close to the reported K m value of 15.5 mM for NKA pump current (activity) and [Na + ] [ 43 ]. The Na + dependence of glycolysis is therefore dependent on the kinetics of NKA. Glycolytic fluxes, Fig. 4 d, showed a plateau (V max ) at high [Na + ] which could reflect the saturation of NKA by Na + or the saturation of glycolysis and glucose transport. When [Na + ] is converted to pump activity using the expression of Silverman et al. [ 43 ] glycolytic fluxes show a linear dependence on activity. Hence, the plateaux in the plots of glycolytic flux in Fig. 4 d are due to saturation of NKA by Na + at its V max and not due to saturation of glycolytic flux or glucose transport. This suggests that over the range of [Na + ] that we used, glucose supply and glycolysis were not rate determining for the NKA. Thus, what limits glycolytic flux at high [Na + ] concentration in Fig. 4 d is the saturation of NKA by Na + and not [ATP] supply. A further interesting point in the curve fits of glycolytic activity vs [Na + ], Fig. 4 d, is that they pass through the origin when it is extrapolated to [Na + ] = 0 mM. This finding suggests that in the presence of gramicidin, when the [Na + ], [K + ] and [H + ] gradients across the membrane are dissipated (and when [Ca 2+ ] is low there will very little residual Ca-ATPase activity), glycolytic activity would be very low (or zero). Of relevance to this interpretation, the magnitudes of glycolytic flux measured with gramicidin-A present were lower than those measured in unpermeabilised cells. It is possible that treatment with gramicidin-A led to a reduction in other related ATPase activities, notably H + -ATPases due to dissipation of the H + gradient. Given these observations/ deductions it is notable that ouabain did not completely inhibit glycolytic flux in the MDA-MB-231 cells; this probably resulted from using a submaximal concentration of ouabain. These experiments demonstrated that [Na + ] i directly contributes to the enhanced glycolytic flux observed, and that this was dependent on NKA activity. In other words, the [Na + ] i dependent increase in NKA activity caused increased energy demand (ATP turnover) of the pump that led to stimulation of ATP supply by glycolysis, thus matching this demand. A schematic of the proposed mechanism is shown in Fig. 4 a.
Elevated basal [Na + ] i in cancer cells is predicted to push NKA up its activation curve leading to an enhancement of glycolytic flux. Taken together, our data can be understood in the light of the known negative feedback controls of ATP/ADP cycling and glycolysis in which accumulation of ATP allosterically inhibits hexokinase, and MgATP of PFK-1 [ 45 ]; these reactions that have high flux control coefficients in glycolysis [ 46 ]. Therefore, inhibition of NKA leads to a significant reduction in overall ATP turnover by the cell, causing almost immediate reductions in glycolytic flux. Elevated [Na + ] i (whether due to increased influx or decreased efflux) also has the potential to modulate intracellular pH through the action of NHE and CA-NBC. The H + gradient across the plasma membrane is known to be reversed in cancer cells with acidification of the extracellular environment and alkalinisation of the cytosol. This also drives accelerated glycolysis, while acidification of the intracellular environment is known to inhibit glycolysis [ 47 ]. Since the H + gradient is abolished by gramicidin-A in our experiments then intracellular acidification would not contribute to the reduced rates of glycolysis observed in MDA-MB-231 cells on ouabain treatment.
The Warburg effect
In the ~ 100 years since the discovery by Otto Warburg of the upregulation of glycolysis in cancer cells [ 3 ], there have been multiple hypotheses that “explain” the basis of this phenomenon. Most recent hypotheses posit genome, transcriptome, and proteomic-level alterations in cancer cells that underlie the uncontrolled cell proliferation, which is the hallmark of neoplasia [ 48 ]. There is strong evidence that ‘metabolic reprogramming’ of cancer cells leads to an advantageous phenotype with greater anabolic capacity of branch pathways; this occurs via upregulation of glycolysis, the PPP, decreased TCA cycle activity, as well as modifications of the extracellular environment (e.g., lower pH), leading to modification of the extracellular matrix, immune cell evasion, and genetic reprogramming thus favouring natural selection via survival of the fittest cells [ 49 ].
Previous studies have reported a link between the NKA and glycolysis in several cell types: erythrocytes [ 17 ], neuronal [ 16 ], skeletal [ 50 , 51 ], and cardiomyocytes [ 19 ]. Our other recent work further demonstrates the importance of the NKA and elevated intracellular [Na + ] i as a modulator of cardiac metabolism [ 52 ] with a switch in metabolism away from fatty acid oxidation towards glucose utilisation in ouabain-treated hearts, with similar observations in hypertrophic hearts and transgenic phospholemman (PLM 3SA ) mice, that have chronic cardiac myocyte [Na + ] i elevation [ 52 ]. Paradoxically, the metabolic response that we observed to ouabain in cancer cells is opposite to what we observed in the beating heart in which ouabain is known to increase cardiac contractility and substrate consumption [ 52 ].
Numerous studies have reported upregulated expression of voltage gated sodium channels (VGSCs) and other Na + -ion transporters in several cancers [ 53 , 54 , 55 , 56 , 57 , 58 , 59 ]. Further work supports the anti-cancer properties of Na + -channel ‘blockers’ [ 60 ] as well as NKA inhibitors [ 61 ]. There is mounting evidence as to how these changes in ion channel expression or kinetics provide a selective advantage for cancer cell survival (and perhaps morphogenesis) [ 25 , 62 ]. The data presented here, indicate that alterations to Na + ion homeostasis across the plasma membrane can directly affect glycolytic rate in breast cancer cells. Since glycolysis and ATPase activity both generate H + , elevated proton efflux facilitates extracellular acidosis which provides tumours with one aspect of their ‘naturally selective’ microenvironment. However, intracellular pH will also play an important role in this mechanism since H + efflux via NHEs would further amplify the Na + leak into the cell.
Our hypothesis is of relevance in cancer imaging, underpinning the practice of clinical 18 F-FDG PET. Specifically, we demonstrated that ouabain treatment of a murine human MDA-MB-231 xenograft model led to decreased tumour uptake of 18 F-FDG, while murine tissues were unaffected thus further evidencing NKA-dependency of glucose demand in tumours. Indications from clinical 23 Na-MRI also support the finding that tumours have elevated [Na + ] i compared with surrounding healthy tissue [ 22 , 23 ], while 2 H-MRI and 82 Rb-PET imaging are emerging techniques in clinical oncology [ 63 , 64 , 65 , 66 ].
A further consequence of altered Na + influx in cells is the impact that this has on the maintenance of a depolarized membrane [ 67 ]. Cone et al. demonstrated that mitotic cell division and proliferation rates are controlled by membrane depolarization [ 68 , 69 , 70 , 71 , 72 ]. Thus, an increased Na + permeability could both increase NKA-driven glycolysis, and hence metabolite availability, while also promoting rapid cell proliferation. Although the current study has not identified the structural proteins and enzymes that are most likely to lead to the functional juxtaposition of glycolysis with the NKA, we have identified a major sink for the glycolytic ATP production in a manner that is similar to what occurs in the human erythrocyte [ 17 ]. It is possible that this functional proximity is indeed a structural one where key glycolytic enzymes are associated with the plasma membrane [ 11 ], namely a ‘metabolon’. The exact identity of the Na + influx pathways is complex, but it includes the voltage gated Na + -channels as well as Na + /H + exchangers, NBC, Na + K + 2Cl − cotransporters and others.
The underlying causes of the Warburg effect observed in cancer cells has been a long-standing conundrum to which the answer is multifaceted. The functional ‘redirection’ of metabolism yields metabolic intermediates that enhance cell growth, and via efflux of lactic acid in particular, a reduction in extracellular pH leading to a modification of the extracellular environment, thus altering the ability to selectively invade surrounding tissue. We demonstrated, further to previous hypotheses, that glycolysis is dependent on ionic homeostasis across the plasma membrane, specifically an Na + -dependent consumption of ATP via the NKA. We hypothesise that this is caused by increased Na + -influx (which we invoked here with the ionophore gramicidin-A) resulting in increased NKA activity that re-established a steady state of [Na + ] i that was elevated.
Availability of data and materials
Data are provided within the manuscript or supplementary information files.
Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519–30.
Article CAS PubMed PubMed Central Google Scholar
Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14.
Article CAS PubMed Google Scholar
Warburg O. Über den Stoffwechsel der Carcinomzelle. Naturwissenschaften. 1924;12(50):1131–7.
Article CAS Google Scholar
Vyas S, Zaganjor E, Haigis MC. Mitochondria and cancer. Cell. 2016;166(3):555–66.
Schell JC, Olson KA, Jiang L, Hawkins AJ, Van Vranken JG, Xie J, et al. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol Cell. 2014;56(3):400–13.
Vacanti NM, Divakaruni AS, Green CR, Parker SJ, Henry RR, Ciaraldi TP, et al. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Mol Cell. 2014;56(3):425–35.
Yang C, Ko B, Hensley CT, Jiang L, Wasti AT, Kim J, et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell. 2014;56(3):414–24.
Bartman CR, Weilandt DR, Shen Y, Lee WD, Han Y, TeSlaa T, et al. Slow TCA flux and ATP production in primary solid tumours but not metastases. Nature. 2023;614(7947):349–57.
Locasale JW, Cantley LC. Metabolic flux and the regulation of mammalian cell growth. Cell Metab. 2011;14(4):443–51.
Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–64.
Epstein T, Xu L, Gillies RJ, Gatenby RA. Separation of metabolic supply and demand: aerobic glycolysis as a normal physiological response to fluctuating energetic demands in the membrane. Cancer & Metabolism. 2014;2:7.
Article Google Scholar
Iorio J, Petroni G, Duranti C, Lastraioli E. Potassium and sodium channels and the Warburg effect: Biophysical regulation of cancer metabolism. Bioelectricity. 2019;1(3):188–200.
Article PubMed PubMed Central Google Scholar
Kuchel PW, Shishmarev D. Accelerating metabolism and transmembrane cation flux by distorting red blood cells. Science Advances. 2017;3(10):eaao1016.
Scholnick P, Lang D, Racker E. Regulatory mechanisms in carbohydrate metabolism. IX. Stimulation of aerobic glycolysis by energy-linked ion transport and inhibition by dextran sulfate. J Biol Chem. 1973;248(14):5175–82.
Kuhlbrandt W. Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Biol. 2004;5(4):282–95.
Article PubMed Google Scholar
Meyer DJ, Diaz-Garcia CM, Nathwani N, Rahman M, Yellen G. The Na + /K + pump dominates control of glycolysis in hippocampal dentate granule cells. eLife. 2022;11:e81645.
Puckeridge M, Chapman BE, Conigrave AD, Grieve SM, Figtree GA, Kuchel PW. Stoichiometric relationship between Na + ions transported and glucose consumed in human erythrocytes: Bayesian analysis of 23 Na and 13 C NMR time course data. Biophys J. 2013;104(8):1676–84.
Lynch RM, Balaban RS. Coupling of aerobic glycolysis and Na + -K + -ATPase in renal cell line MDCK. Am J Physiol. 1987;253(2 Pt 1):C269–276.
Sepp M, Sokolova N, Jugai S, Mandel M, Peterson P, Vendelin M. Tight coupling of Na + /K + -ATPase with glycolysis demonstrated in permeabilized rat cardiomyocytes. PLoS ONE. 2014;9(6):e99413.
Leslie TK, James AD, Zaccagna F, Grist JT, Deen S, Kennerley A, et al. Sodium homeostasis in the tumour microenvironment. Biochim Biophys Acta Rev Cancer. 2019;1872(2):188304.
James AD, Leslie TK, Kaggie JD, Wiggins L, Patten L, Murphy O’Duinn J, et al. Sodium accumulation in breast cancer predicts malignancy and treatment response. Br J Cancer. 2022;127(2):337–49.
Bhatia A, Lee VK, Qian Y, Paldino MJ, Ceschin R, Hect J, et al. Quantitative sodium ( 23 Na) MRI in pediatric gliomas: Initial experience. Diagnostics (Basel). 2022;12(5):1150.
Google Scholar
Poku LO, Phil M, Cheng Y, Wang K, Sun X. 23 Na-MRI as a noninvasive biomarker for cancer diagnosis and prognosis. J Magn Reson Imaging. 2021;53(4):995–1014.
Djamgoz MBA, Fraser SP, Brackenbury WJ. In vivo evidence for voltage-gated sodium channel expression in carcinomas and potentiation of metastasis. Cancers (Basel). 2019;11(11):1675.
Prevarskaya N, Skryma R, Shuba Y. Ion channels and the hallmarks of cancer. Trends Mol Med. 2010;16(3):107–21.
Kunzelmann K. Ion channels and cancer. J Membr Biol. 2005;205(3):159–73.
Brackenbury WJ. Voltage-gated sodium channels and metastatic disease. Channels (Austin). 2012;6(5):352–61.
Fiske JL, Fomin VP, Brown ML, Duncan RL, Sikes RA. Voltage-sensitive ion channels and cancer. Cancer Metastasis Rev. 2006;25(3):493–500.
Joshi AD, Parsons DW, Velculescu VE, Riggins GJ. Sodium ion channel mutations in glioblastoma patients correlate with shorter survival. Mol Cancer. 2011;10:17.
Swietach P, Boedtkjer E, Pedersen SF. How protons pave the way to aggressive cancers. Nat Rev Cancer. 2023;23(12):825–41.
Eykyn TR, Aksentijevic D, Aughton KL, Southworth R, Fuller W, Shattock MJ. Multiple quantum filtered 23 Na NMR in the Langendorff perfused mouse heart: Ratio of triple/double quantum filtered signals correlates with [Na] i . J Mol Cell Cardiol. 2015;86:95–101.
De Feyter HM, Behar KL, Corbin ZA, Fulbright RK, Brown PB, McIntyre S, et al. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci Adv. 2018;4(8):eaat7314.
Almuhaideb A, Papathanasiou N, Bomanji J. 18F-FDG PET/CT imaging in oncology. Ann Saudi Med. 2011;31(1):3–13.
Elliott SJ, Eykyn TR, Kuchel PW. Multiple quantum filtered nuclear magnetic resonance of 23 Na + in uniformly stretched and compressed hydrogels. J Chem Phys. 2023;159(3):034903.
Pongrakhananon V, Chunhacha P, Chanvorachote P. Ouabain suppresses the migratory behavior of lung cancer cells. PLoS ONE. 2013;8(7):e68623.
Liu N, Li Y, Su S, Wang N, Wang H, Li J. Inhibition of cell migration by ouabain in the A549 human lung cancer cell line. Oncol Lett. 2013;6(2):475–9.
Abeywardena MY, McMurchie EJ, Russell GR, Charnock JS. Species variation in the ouabain sensitivity of cardiac Na+/K+-ATPase. A possible role for membrane lipids. Biochem Pharmacol. 1984;33(22):3649–54.
Herzig S, Mohr K. Action of ouabain on rat heart: comparison with its effect on guinea-pig heart. Br J Pharmacol. 1984;82(1):135–42.
Price EM, Lingrel JB. Structure-function relationships in the Na, K-ATPase alpha subunit: site-directed mutagenesis of glutamine-111 to arginine and asparagine-122 to aspartic acid generates a ouabain-resistant enzyme. Biochemistry. 1988;27(22):8400–8.
Knubovets T, Shinar H, Navon G. Quantification of the contribution of extracellular sodium to 23 Na multiple-quantum-filtered NMR spectra of suspensions of human red blood cells. J Magn Reson. 1998;131(1):92–6.
Navon G, Werrmann JG, Maron R, Cohen SM. 31 P NMR and Triple Quantum Filtered 23 Na NMR-studies of the effects of inhibition of Na + /H + exchange on intracellular sodium and pH in working and ischemic hearts. Magn Reson Med. 1994;32(5):556–64.
Eliav U, Navon G. Analysis of double-quantum-filtered NMR-spectra of Na-23 in biological tissues. J Magn Reson, Ser B. 1994;103(1):19–29.
Silverman BDZ, Warley A, Miller JIA, James AF, Shattock MJ. Is there a transient rise in sub-sarcolemmal Na and activation of Na/K pump current following activation of I Na in ventricular myocardium? Cardiovasc Res. 2003;57(4):1025–34.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–54.
Berg JM, Stryer L, Tymoczko JL, Gatto GJ. Biochemistry. W.H. Freeman & Co Ltd; 9th ed. 2019.
Mulquiney PJ, Bubb WA, Kuchel PW. Model of 2,3-bisphosphoglycerate metabolism in the human erythrocyte based on detailed enzyme kinetic equations: in vivo kinetic characterization of 2,3-bisphosphoglycerate synthase/phosphatase using 13 C and 31 P NMR. Biochem J. 1999;342:567–80.
Russell S, Xu LP, Kam Y, Abrahams D, Ordway B, Lopez AS, et al. Proton export upregulates aerobic glycolysis. BMC Biol. 2022;20(1):163.
Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491(7424):364–73.
Liberti MV, Locasale JW. The Warburg effect: How does it benefit cancer cells? Trends Biochem Sci. 2016;41(3):211–8.
Okamoto K, Wang W, Rounds J, Chambers EA, Jacobs DO. ATP from glycolysis is required for normal sodium homeostasis in resting fast-twitch rodent skeletal muscle. Am J Physiol Endocrinol Metab. 2001;281(3):E479–88.
James JH, Wagner KR, King JK, Leffler RE, Upputuri RK, Balasubramaniam A, et al. Stimulation of both aerobic glycolysis and Na + -K + -ATPase activity in skeletal muscle by epinephrine or amylin. Am J Physiol Endocrinol Metab. 1999;277(1):E176–86.
Aksentijevic D, Karlstaedt A, Basalay MV, O’Brien BA, Sanchez-Tatay D, Eminaga S, et al. Intracellular sodium elevation reprograms cardiac metabolism. Nat Commun. 2020;11(1):4337.
Fulgenzi G, Graciotti L, Faronato M, Soldovieri MV, Miceli F, Amoroso S, et al. Human neoplastic mesothelial cells express voltage-gated sodium channels involved in cell motility. Int J Biochem Cell Biol. 2006;38(7):1146–59.
Roger S, Rollin J, Barascu A, Besson P, Raynal PI, Iochmann S, et al. Voltage-gated sodium channels potentiate the invasive capacities of human non-small-cell lung cancer cell lines. Int J Biochem Cell Biol. 2007;39(4):774–86.
Diaz D, Delgadillo DM, Hernandez-Gallegos E, Ramirez-Dominguez ME, Hinojosa LM, Ortiz CS, et al. Functional expression of voltage-gated sodium channels in primary cultures of human cervical cancer. J Cell Physiol. 2007;210(2):469–78.
Gao R, Shen Y, Cai J, Lei M, Wang Z. Expression of voltage-gated sodium channel alpha subunit in human ovarian cancer. Oncol Rep. 2010;23(5):1293–9.
CAS PubMed Google Scholar
House CD, Vaske CJ, Schwartz AM, Obias V, Frank B, Luu T, et al. Voltage-gated Na + channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 2010;70(17):6957–67.
Pancrazio JJ, Viglione MP, Tabbara IA, Kim YI. Voltage-dependent ion channels in small-cell lung cancer cells. Cancer Res. 1989;49(21):5901–6.
Schrey M, Codina C, Kraft R, Beetz C, Kalff R, Wolfl S, et al. Molecular characterization of voltage-gated sodium channels in human gliomas. NeuroReport. 2002;13(18):2493–8.
Fairhurst C, Watt I, Martin F, Bland M, Brackenbury WJ. Sodium channel-inhibiting drugs and survival of breast, colon and prostate cancer: a population-based study. Sci Rep. 2015;5:16758.
Stenkvist B, Bengtsson E, Dahlqvist B, Eriksson O, Jarkrans T, Nordin B. Cardiac glycosides and breast cancer, revisited. N Engl J Med. 1982;306(8):484.
Brackenbury WJ. Voltage-gated sodium channels and metastatic disease. Channels. 2012;6(5):352–61.
Jochumsen MR, Bouchelouche K, Nielsen KB, Frokiær J, Borre M, Sörensen J, et al. Repeatability of tumor blood flow quantification with Rubidium PET/CT in prostate cancer-a test-retest study. EJNMMI Res. 2019;9:58.
Jochumsen MR, Sörensen J, Pedersen BG, Nyengaard JR, Krag SRP, Frokiær J, et al. Tumour blood flow for prediction of human prostate cancer aggressiveness: a study with Rubidium-82 PET, MRI and Na/K-ATPase-density. Eur J Nucl Med Mol Imaging. 2021;48(2):532–42.
Nielsen MO, Schledermann H, Jensen LT. Primary breast cancer diagnosed by 82-Rubidium myocardial perfusion PET-scan. J Nucl Cardiol. 2023;30(5):2252–3.
Oldan JD, Femi-Abodunde AD, Muhleman MA, Khandani AH. Rubidium Uptake in Chest Tumors on PET/CT. World J Nucl Med. 2022;21(01):18–27.
Yang M, Brackenbury WJ. Membrane potential and cancer progression. Front Physiol. 2013;4:185.
Cone CD Jr. Variation of the transmembrane potential level as a basic mechanism of mitosis control. Oncology. 1970;24(6):438–70.
Cone CD Jr. Unified theory on the basic mechanism of normal mitotic control and oncogenesis. J Theor Biol. 1971;30(1):151–81.
Cone CD Jr, Tongier M Jr. Control of somatic cell mitosis by simulated changes in the transmembrane potential level. Oncology. 1971;25(2):168–82.
Cone CD Jr, Cone CM. Induction of mitosis in mature neurons in central nervous system by sustained depolarization. Science. 1976;192(4235):155–8.
Cone CD Jr, Tongier M Jr. Contact inhibition of division: involvement of the electrical transmembrane potential. J Cell Physiol. 1973;82(3):373–86.
Download references
Acknowledgements
We are grateful to Declan Bolster for informative discussions on the [6,6- 2 H 2 ] d -glucose experiments. The authors would like to thank the Centre for Biomolecular Spectroscopy for access to the 600 MHz NMR spectrometer for metabolomics data acquisition, funded by the Wellcome Trust and British Heart Foundation (ref. 202767/Z/16/Z and IG/16/2/32273)
This work was supported by the EPSRC Centre for Doctoral Training in Smart Medical Imaging (EP/S022104/1), by the Centre of Excellence in Medical Engineering funded by the Wellcome Trust and EPSRC (WT 203148/Z/16/Z), the BHF Centre of Research Excellence (RE/18/2/34213), a British Heart Foundation Programme Grant RG/12/4/29426 (to MJS) and the NIHR Biomedical Research Centre at Guy's and St Thomas' NHS Foundation Trust and KCL. PWK’s work was funded by an Australian Research Council Discovery Project Grant DP190100510. AMS is supported by the British Heart Foundation (CH/1999001/11735; RG/20/3/34823).
Author information
Authors and affiliations.
School of Biomedical Engineering and Imaging Sciences, King’s College London, St Thomas’ Hospital, London, SE1 7EH, UK
Aidan M. Michaels, George Firth, Richard Southworth & Thomas R. Eykyn
School of Cardiovascular and Metabolic Medicine and Sciences, King’s College London, London, UK
Anna Zoccarato, Zoe Hoare, Yu Jin Chung, Ajay M. Shah & Michael J. Shattock
School of Life and Environmental Sciences, University of Sydney, Sydney, NSW, 2006, Australia
Philip W. Kuchel
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: AMM, MJS, RS, TRE. Methodology: AMM, AZ, ZH, YJC, PWK, MJS, TRE. Investigation: AMM, AZ, ZH, YJC, MJS, RS, TRE. Funding acquisition: TRE, RS, AMS, MJS, Project administration and Supervision: TRE, RS. Writing – original draft: AMM, TRE. All authors reviewed the manuscript.
Corresponding author
Correspondence to Thomas R. Eykyn .
Ethics declarations
Ethics approval and consent to participate.
All animal studies were performed in accordance with the United Kingdom Home Office Animal (Scientific Procedures) Act, 1986, under home office licence PP 998/2297.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Michaels, A.M., Zoccarato, A., Hoare, Z. et al. Disrupting Na + ion homeostasis and Na + /K + ATPase activity in breast cancer cells directly modulates glycolysis in vitro and in vivo. Cancer Metab 12 , 15 (2024). https://doi.org/10.1186/s40170-024-00343-5
Download citation
Received : 22 February 2024
Accepted : 16 May 2024
Published : 24 May 2024
DOI : https://doi.org/10.1186/s40170-024-00343-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Intracellular sodium
- Warburg effect
Cancer & Metabolism
ISSN: 2049-3002
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Abstract. The current diagnostic and stratification pathway for prostate cancer has led to over-diagnosis and over- treatment. This thesis aims to improve the prostate cancer diagnosis pathway by developing a minimally invasive blood test to inform diagnosis alongside mpMRI and to understand the true Gleason 4 burden which will help better stratify disease and guide clinicians in treatment ...
The prevalence of prostate cancer is high in Sweden with 70 459 men living with prostate cancer (31/12 2008)2. Figure 1: The trends in prostate cancer incidence and mortality in Sweden 1970-2007. Adapted from the National board of health and welfare. In 2007, 2 470 men died from prostate cancer constituting 21.5 % of all cancer deaths among men3.
prostate cancer therapies. Overall these results suggest that androgen receptor phosphorylated at serine 81 and serine 578 are associated with poor outcomes in prostate cancer and are potential targets for new drug therapies. Additional studies are required to validate these results in a larger multi-centre cohort of prostate cancer patients
Prostate cancer diagnosis after prostate biopsies, and the subsequent treatment decision making, affect millions of men worldwide yearly [1, 2, 3]. ... This thesis is also emphasized by the fact that IHC was performed in a high proportion of patients (>47%) in order to rule out prostate cancer diagnoses (negative biopsy), which is also very ...
Front-line Test for Prostate Cancer Screening Citation Al-Faouri, Ra'ad. 2022. The Effect of TNF-α Inhibitors Therapy on the Growth of the Prostate Gland & Cost-Effectiveness of Biparametric Magnetic Resonance Imaging as a Front-line Test for Prostate Cancer Screening. Master's thesis, Harvard Medical School. Permanent link
Screening for prostate cancer often includes a blood test for the prostate specific antigen (PSA) and/or a digital rectal exam (DRE; CDC 2010). Disagreement regarding guidelines for prostate cancer screening intensified recently (Bazell 2012). Issues of disagreement include the age at which men should begin screening for prostate cancer and the ...
The thesis is based on data from two different overall projects: a survey sent to all members of the Swedish Prostate Cancer Federation (SPCF) and the PROstate Cancer-Experiences and Expectations During treatment (PROCEED) project. ... Results: Men with advanced prostate cancer experience an uncertain illness situation when living with a life ...
Prostate cancer has emerged as the most frequently diagnosed cancer, except for non-melanoma skin cancer, among men in many Western countries in the last decade. ... This thesis begins with a descriptive epidemiological study using cancer registry incidence data from the West of Scotland from 1991 to 2007. The aim was to determine whether the ...
Prostate cancer (PCa) is the second most frequently diagnosed cancer in men world-wide and the fifth leading cause of cancer death in men, with an estimated 1.4 ... thesis, effort can be allocated to the creation of a unified platform composed of the CAD systems presented in this thesis, and extensions thereof; active learning should ...
isomiRs in prostate cancer Sharmila Rana November 2020 A thesis submitted in accordance with the requirements for the degree of ... I dedicate this thesis to you both. 6. Contents Declaration 2 Abstract 3 Acknowledgements 5 List of Figures 9 List of Tables 11 List of Acronyms 12 1 Introduction 16
masks and lesion scores for 157 men with prostate cancer. The dataset is further explored in Chapter 3. The two main problems addressed in this thesis are the classification of lesions, including PIRADS and clinical significance, and the detection and segmentation of the prostate cancer lesions. For these problems different experiments were ...
Genetic medicine has been introduced for the diagnosis and treatment of prostate cancer. In this Special Issue, experts in this field reviewed the recent progress in important topics associated with prostate cancer. We present two systematic reviews and eight original articles on this topic. In this editorial, we summarize the main findings of ...
Results. A total of 1 414 259 new cases of prostate cancer and 375 304 related deaths were reported in 2020 globally. HDI was positively correlated with ASIRs (P < 0.001) and negatively correlated with ASMRs (P < 0.001).In the past two decades, ASIRs have been increasing in 65 countries, stable in 15 countries and decreasing in 9 countries, and ASMRs have been increasing in 19 countries ...
Background With the burden of prostate cancer, it has become imperative to exploit cost-effective ways to tackle this menace. Women have demonstrated their ability to recognize early cancer signs, and it is, therefore, relevant to include women in strategies to improve the early detection of prostate cancer. This systematic review seeks to gather evidence from studies that investigated women ...
Author(s): Zhang, Zhaohuan | Advisor(s): Wu, Holden H. HHW | Abstract: Prostate Cancer (PCa) remains the second most common cause of cancer-related death in men in the U.S. Multi-parametric (mp) MRI is playing an increasingly important role for the localization, detection, and risk stratification of PCa. However, prostate mp-MRI still misses PCa in up to 45% of men and faces challenges in ...
Prostate cancer is a leading cause of cancer death in men. Evaluating knowledge, practice and attitudes towards the condition is important to identify key areas where interventions can be instituted. This was a hospital-based descriptive cross-sectional study aimed at assessing knowledge, practice and attitude towards prostate cancer screening among male patients aged 40 years and above ...
UBIRA ETheses - University of Birmingham eData Repository
Student thesis: Doctoral Thesis › PhD. Abstract Few biomarkers have been identified for prostate cancer diagnosis/prognosis and there are clinical difficulties in distinguishing between relapsing and non-relapsing tumours. Therefore, identifying new biomarkers for prostate cancer has become a priority. Recently, potential stem cells found ...
Prostate cancer (PCa) is the second most prevalent cancer in men. In 2020, approximately 1,414,259 new cases were reported that accounted for 3,75,324 deaths (Sung et al. in CA 71:209-249, 2021). PCa is often asymptomatic at early stages; hence, routine screening and monitoring based on reliable biomarkers is crucial for early detection and assessment of cancer progression. Early diagnosis ...
Prostate cancer has been a disease of older men but age at diagnosis is falling in Sweden. ... The overall purpose of this thesis was to identify and describe fatigue and its influence on men's lives when undergoing examinations for suspected prostate cancer and diagnosed with prostate cancer. Further, the purpose was to understand if ...
lesions of the prostate is a subject of great interest analyzed in numerous publications. Despite many studies conducted over this area each year, there were still important questions remain about the cause and prevention of prostate cancer. Inspite of significant advances in the early detection of prostatic
The Prostate Cancer Prevention Trial showed that finasteride can reduce the risk of prostate cancer, but might increase the risk of aggressive disease. NCI's Howard Parnes talks about subsequent findings and what they mean for men aged 55 and older. Darolutamide Delays the Spread of Some Prostate Cancers.
MSc Thesis: Non-invasive and accurate prediction of prostate cancer aggressiveness. Florian A. Hölzl. Jul 28, 2022. With estimates of 1 600 000 cases and more than 350 000 deaths annually worldwide, prostate cancer is among the most common cancers in men [1]. Diagnosis of prostate cancer is typically done by using ultrasound-guided needle ...
Background and objectives: Microbiota of the urinary tract may be associated with urinary tract malignancy, including prostate cancer. Materials and Methods: We retrospectively collected patients with newly diagnosed prostate cancer and subjects without prostate cancer from the National Health Insurance Research Database (NHIRD) in Taiwan between 1 January 2000 and 31 December 2016. A total of ...
Memorial Sloan Kettering Cancer Center (MSK) researchers are testing Illuminare-1 in a phase 1 clinical trial in patients undergoing prostate cancer surgery. At the 2024 Annual Meeting of the American Urological Association held May 3-6, results from the first 28 patients to receive the agent demonstrated it to be safe and effective, with ...
Published online: March 7, 2024. This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about prostate cancer prevention. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for ...
Glycolytic flux is regulated by the energy demands of the cell. Upregulated glycolysis in cancer cells may therefore result from increased demand for adenosine triphosphate (ATP), however it is unknown what this extra ATP turnover is used for. We hypothesise that an important contribution to the increased glycolytic flux in cancer cells results from the ATP demand of Na+/K+-ATPase (NKA) due to ...