9.9 An Introduction to Organic Synthesis
9.9 • An Introduction to Organic Synthesis
As mentioned in the introduction, one of the purposes of this chapter is to use alkyne chemistry as a vehicle to begin looking at some of the general strategies used in organic synthesis—the construction of complex molecules in the laboratory. There are many reasons for carrying out the laboratory synthesis of an organic compound. In the pharmaceutical industry, new molecules are designed and synthesized in the hope that some might be useful new drugs. In the chemical industry, syntheses are done to devise more economical routes to known compounds. In academic laboratories, the synthesis of extremely complex molecules is sometimes done just for the intellectual challenge involved in mastering so difficult a subject. The successful synthesis route is a highly creative work that is sometimes described by such subjective terms as elegant or beautiful .
In this book, too, we will often devise syntheses of molecules from simpler precursors, but the purpose here is to learn. The ability to plan a successful multistep synthetic sequence requires a working knowledge of the uses and limitations of many different organic reactions. Furthermore, it requires the practical ability to piece together the steps in a sequence such that each reaction does only what is desired without causing changes elsewhere in the molecule. Planning a synthesis makes you approach a chemical problem in a logical way, draw on your knowledge of chemical reactions, and organize that knowledge into a workable plan—it helps you learn organic chemistry.
There’s no secret to planning an organic synthesis: all it takes is a knowledge of the different reactions and some practice. The only real trick is to work backward in what is often called a retrosynthetic direction. Don’t look at a potential starting material and ask yourself what reactions it might undergo. Instead, look at the final product and ask, “What was the immediate precursor of that product?” For example, if the final product is an alkyl halide, the immediate precursor might be an alkene, to which you could add HX. If the final product is a cis alkene, the immediate precursor might be an alkyne, which you could hydrogenate using the Lindlar catalyst. Having found an immediate precursor, work backward again, one step at a time, until you get back to the starting material. You have to keep the starting material in mind, of course, so that you can work back to it, but you don’t want that starting material to be your main focus.
Let’s work several examples of increasing complexity.

Worked Example 9.1
Devising a synthesis route.
How would you synthesize cis -2-hexene from 1-pentyne and an alkyl halide? More than one step is needed.
The product in this case is a cis-disubstituted alkene, so the first question is, “What is an immediate precursor of a cis-disubstituted alkene?” We know that an alkene can be prepared from an alkyne by reduction and that the right choice of experimental conditions will allow us to prepare either a trans-disubstituted alkene (using lithium in liquid ammonia) or a cis-disubstituted alkene (using catalytic hydrogenation over the Lindlar catalyst). Thus, reduction of 2-hexyne by catalytic hydrogenation using the Lindlar catalyst should yield cis -2-hexene.
Next ask, “What is an immediate precursor of 2-hexyne?” We’ve seen that an internal alkyne can be prepared by alkylation of a terminal alkyne anion. In the present instance, we’re told to start with 1-pentyne and an alkyl halide. Thus, alkylation of the anion of 1-pentyne with iodomethane should yield 2-hexyne.
Worked Example 9.2
How would you synthesize 2-bromopentane from acetylene and an alkyl halide? More than one step is needed.
What is an immediate precursor of an alkene? Perhaps an alkyne, which could be reduced.
What is an immediate precursor of a terminal alkyne? Perhaps sodium acetylide and an alkyl halide.
The desired product can be synthesized in four steps from acetylene and 1-bromopropane.
Worked Example 9.3
How would you synthesize 5-methyl-1-hexanol (5-methyl-1-hydroxyhexane) from acetylene and an alkyl halide?
What is an immediate precursor of a terminal alkene? Perhaps a terminal alkyne, which could be reduced.
What is an immediate precursor of 5-methyl-1-hexyne? Perhaps acetylene and 1-bromo-3-methylbutane.
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution-NonCommercial-ShareAlike License and you must attribute OpenStax.
Access for free at https://openstax.org/books/organic-chemistry/pages/1-why-this-chapter
- Authors: John McMurry, Professor Emeritus
- Publisher/website: OpenStax
- Book title: Organic Chemistry
- Publication date: Sep 20, 2023
- Location: Houston, Texas
- Book URL: https://openstax.org/books/organic-chemistry/pages/1-why-this-chapter
- Section URL: https://openstax.org/books/organic-chemistry/pages/9-9-an-introduction-to-organic-synthesis
© Jan 9, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.

Synthesis is the production of chemical compounds by reaction from simpler materials. The construction of complex and defined new molecules is a challenging and complicated undertaking, and one that requires the constant development of new reactions, catalysts and techniques.
Synthesis projects underpin developments in a very wide range of areas. This makes chemical synthesis a unique and enabling science; it means that the design of new molecules can be put into practice so that the target compounds can be made and tested for interesting properties or activity.

Areas in which synthesis is essential
Catalysts Catalysis is critical to a very wide range of industrial processes, encompassing both bulk and fine chemical manufacture. The rational design, synthesis and optimization of catalyst systems is therefore crucial to the development of more efficient, selective and environmentally tolerant processes. Research in this area is focussed on both metal-containing and metal-free systems, and targets not only better catalysts for existing processes but also entirely new catalytic transformations.
Medicine and drug discovery The development of new pharmaceutical products is an extremely important aspect of organic synthesis. This undertaking enables the discovery and optimisation of complex molecules with potent and selective biological activity. An understanding of synthetic chemistry allows balancing of chemical properties so that the molecules behave as desired in cells and patients. New reaction development is another essential facet of this work, because it opens up previously inaccessible routes to new compounds.
New materials The preparation of functional materials with custom-designed properties (e.g. electronic, optical, magnetic) is fundamental to breakthroughs in areas such as batteries, solar cell development, superconductors, smart materials etc., which hold much promise for future technologies. Oxford has a long-established track record in this area, with the fundamental synthetic work underpinning lithium ion battery technology having been carried out in the Department.
Chemical biology The synthesis of molecules that are designed to interact with and probe biological systems is very useful for investigating and understanding the processes involved in living systems. Such compounds allow us to understand fundamental biological processes more clearly, and to aid drug discovery through effective target validation.

Natural products The history of medicines, flavourings and agrochemicals illustrates the central importance of natural products. Synthetic chemistry is very useful in mimicking Nature and allowing us to prepare complex molecules that are produced naturally but without disrupting the source itself. Such natural products, and analogues thereof, have myriad uses as drugs, flavourings and agrochemicals.
Imaging Synthetic dyes and probes have been extremely important in recent developments in imaging, which means that more powerful and less intrusive techniques can be used in the search for diseased or damaged tissue.
Theme co-ordinators
News and articles related to this theme, researchers associated with this theme.
Jump to navigation

Cochrane Training
Chapter 9: summarizing study characteristics and preparing for synthesis.
Joanne E McKenzie, Sue E Brennan, Rebecca E Ryan, Hilary J Thomson, Renea V Johnston
Key Points:
- Synthesis is a process of bringing together data from a set of included studies with the aim of drawing conclusions about a body of evidence. This will include synthesis of study characteristics and, potentially, statistical synthesis of study findings.
- A general framework for synthesis can be used to guide the process of planning the comparisons, preparing for synthesis, undertaking the synthesis, and interpreting and describing the results.
- Tabulation of study characteristics aids the examination and comparison of PICO elements across studies, facilitates synthesis of these characteristics and grouping of studies for statistical synthesis.
- Tabulation of extracted data from studies allows assessment of the number of studies contributing to a particular meta-analysis, and helps determine what other statistical synthesis methods might be used if meta-analysis is not possible.
Cite this chapter as: McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV. Chapter 9: Summarizing study characteristics and preparing for synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook .
9.1 Introduction
Synthesis is a process of bringing together data from a set of included studies with the aim of drawing conclusions about a body of evidence. Most Cochrane Reviews on the effects of interventions will include some type of statistical synthesis. Most commonly this is the statistical combination of results from two or more separate studies (henceforth referred to as meta-analysis) of effect estimates.
An examination of the included studies always precedes statistical synthesis in Cochrane Reviews. For example, examination of the interventions studied is often needed to itemize their content so as to determine which studies can be grouped in a single synthesis. More broadly, synthesis of the PICO (Population, Intervention, Comparator and Outcome) elements of the included studies underpins interpretation of review findings and is an important output of the review in its own right. This synthesis should encompass the characteristics of the interventions and comparators in included studies, the populations and settings in which the interventions were evaluated, the outcomes assessed, and the strengths and weaknesses of the body of evidence.
Chapter 2 defined three types of PICO criteria that may be helpful in understanding decisions that need to be made at different stages in the review:
- The review PICO (planned at the protocol stage) is the PICO on which eligibility of studies is based (what will be included and what excluded from the review).
- The PICO for each synthesis (also planned at the protocol stage) defines the question that the specific synthesis aims to answer, determining how the synthesis will be structured, specifying planned comparisons (including intervention and comparator groups, any grouping of outcome and population subgroups).
- The PICO of the included studies (determined at the review stage) is what was actually investigated in the included studies.
In this chapter, we focus on the PICO for each synthesis and the PICO of the included studies , as the basis for determining which studies can be grouped for statistical synthesis and for synthesizing study characteristics. We describe the preliminary steps undertaken before performing the statistical synthesis. Methods for the statistical synthesis are described in Chapter 10 , Chapter 11 and Chapter 12 .
9.2 A general framework for synthesis
Box 9.2.a A general framework for synthesis that can be applied irrespective of the methods used to synthesize results
Box 9.2.a provides a general framework for synthesis that can be applied irrespective of the methods used to synthesize results. Planning for the synthesis should start at protocol-writing stage, and Chapter 2 and Chapter 3 describe the steps involved in planning the review questions and comparisons between intervention groups. These steps included specifying which characteristics of the interventions, populations, outcomes and study design would be grouped together for synthesis (the PICO for each synthesis: stage 1 in Box 9.2.a ).
This chapter primarily concerns stage 2 of the general framework in Box 9.2.a . After deciding which studies will be included in the review and extracting data, review authors can start implementing their plan, working through steps 2.1 to 2.5 of the framework. This process begins with a detailed examination of the characteristics of each study (step 2.1), and then comparison of characteristics across studies in order to determine which studies are similar enough to be grouped for synthesis (step 2.2). Examination of the type of data available for synthesis follows (step 2.3). These three steps inform decisions about whether any modification to the planned comparisons or outcomes is necessary, or new comparisons are needed (step 2.4). The last step of the framework covered in this chapter involves synthesis of the characteristics of studies contributing to each comparison (step 2.5). The chapter concludes with practical tips for checking data before synthesis (Section 9.4 ).
Steps 2.1, 2.2 and 2.5 involve analysis and synthesis of mainly qualitative information about study characteristics. The process used to undertake these steps is rarely described in reviews, yet can require many subjective decisions about the nature and similarity of the PICO elements of the included studies. The examples described in this section illustrate approaches for making this process more transparent.
9.3 Preliminary steps of a synthesis
9.3.1 summarize the characteristics of each study (step 2.1).
A starting point for synthesis is to summarize the PICO characteristics of each study (i.e. the PICO of the included studies, see Chapter 3 ) and categorize these PICO elements in the groups (or domains) pre-specified in the protocol (i.e. the PICO for each synthesis). The resulting descriptions are reported in the ‘Characteristics of included studies’ table, and are used in step 2.2 to determine which studies can be grouped for synthesis.
In some reviews, the labels and terminology used in each study are retained when describing the PICO elements of the included studies. This may be sufficient in areas with consistent and widely understood terminology that matches the PICO for each synthesis. However, in most areas, terminology is variable, making it difficult to compare the PICO of each included study to the PICO for each synthesis, or to compare PICO elements across studies. Standardizing the description of PICO elements across studies facilitates these comparisons. This standardization includes applying the labels and terminology used to articulate the PICO for each synthesis ( Chapter 3 ), and structuring the description of PICO elements. The description of interventions can be structured using the Template for Intervention Description and Replication (TIDIeR) checklist, for example (see Chapter 3 and Table 9.3.a ).
Table 9.3.a illustrates the use of pre-specified groups to categorize and label interventions in a review of psychosocial interventions for smoking cessation in pregnancy (Chamberlain et al 2017). The main intervention strategy in each study was categorized into one of six groups: counselling, health education, feedback, incentive-based interventions, social support, and exercise. This categorization determined which studies were eligible for each comparison (e.g. counselling versus usual care; single or multi-component strategy). The extract from the ‘Characteristics of included studies’ table shows the diverse descriptions of interventions in three of the 54 studies for which the main intervention was categorized as ‘counselling’. Other intervention characteristics, such as duration and frequency, were coded in pre-specified categories to standardize description of the intervention intensity and facilitate meta-regression (not shown here).
Table 9.3.a Example of categorizing interventions into pre-defined groups
* The definition also specified eligible modes of delivery, intervention duration and personnel.
While this example focuses on categorizing and describing interventions according to groups pre-specified in the PICO for each synthesis, the same approach applies to other PICO elements.
9.3.2 Determine which studies are similar enough to be grouped within each comparison (step 2.2)
Once the PICO of included studies have been coded using labels and descriptions specified in the PICO for each synthesis, it will be possible to compare PICO elements across studies and determine which studies are similar enough to be grouped within each comparison.
Tabulating study characteristics can help to explore and compare PICO elements across studies, and is particularly important for reviews that are broad in scope, have diversity across one or more PICO elements, or include large numbers of studies. Data about study characteristics can be ordered in many different ways (e.g. by comparison or by specific PICO elements), and tables may include information about one or more PICO elements. Deciding on the best approach will depend on the purpose of the table and the stage of the review. A close examination of study characteristics will require detailed tables; for example, to identify differences in characteristics that were pre-specified as potentially important modifiers of the intervention effects. As the review progresses, this detail may be replaced by standardized description of PICO characteristics (e.g. the coding of counselling interventions presented in Table 9.3.a ).
Table 9.3.b illustrates one approach to tabulating study characteristics to enable comparison and analysis across studies. This table presents a high-level summary of the characteristics that are most important for determining which comparisons can be made. The table was adapted from tables presented in a review of self-management education programmes for osteoarthritis (Kroon et al 2014). The authors presented a structured summary of intervention and comparator groups for each study, and then categorized intervention components thought to be important for enabling patients to manage their own condition. Table 9.3.b shows selected intervention components, the comparator, and outcomes measured in a subset of studies (some details are fictitious). Outcomes have been grouped by the outcome domains ‘Pain’ and ‘Function’ (column ‘Outcome measure’ Table 9.3.b ). These pre-specified outcome domains are the chosen level for the synthesis as specified in the PICO for each synthesis. Authors will need to assess whether the measurement methods or tools used within each study provide an appropriate assessment of the domains ( Chapter 3, Section 3.2.4 ). A next step is to group each measure into the pre-specified time points. In this example, outcomes are grouped into short-term (<6 weeks) and long-term follow-up (≥6 weeks to 12 months) (column ‘Time points (time frame)’ Table 9.3.b ).
Variations on the format shown in Table 9.3.b can be presented within a review to summarize the characteristics of studies contributing to each synthesis, which is important for interpreting findings (step 2.5).
Table 9.3.b Table of study characteristics illustrating similarity of PICO elements across studies
BEH = health-directed behaviour; CON = constructive attitudes and approaches; EMO = emotional well-being; ENG = positive and active engagement in life; MON = self-monitoring and insight; NAV = health service navigation; SKL = skill and technique acquisition. ANCOVA = Analysis of covariance; CI = confidence interval; IQR = interquartile range; MD = mean difference; SD = standard deviation; SE = standard error, NS = non-significant. Pain and function measures: Dutch AIMS-SF = Dutch short form of the Arthritis Impact Measurement Scales; HAQ = Health Assessment Questionnaire; VAS = visual analogue scale; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index. 1 Ordered by type of comparator; 2 Short-term (denoted ‘immediate’ in the review Kroon et al (2014)) follow-up is defined as <6 weeks, long-term follow-up (denoted ‘intermediate’ in the review) is ≥6 weeks to 12 months; 3 For simplicity, in this example the available data are assumed to be the same for all outcomes within an outcome domain within a study. In practice, this is unlikely and the available data would likely vary by outcome; 4 Indicates that an effect estimate and its standard error may be computed through imputation of missing statistics, methods to convert between statistics (e.g. medians to means) or contact with study authors. *Indicates the selected outcome when there was multiplicity in the outcome domain and time frame.
9.3.3 Determine what data are available for synthesis (step 2.3)
Once the studies that are similar enough to be grouped together within each comparison have been determined, a next step is to examine what data are available for synthesis. Tabulating the measurement tools and time frames as shown in Table 9.3.b allows assessment of the potential for multiplicity (i.e. when multiple outcomes within a study and outcome domain are available for inclusion ( Chapter 3, Section 3.2.4.3 )). In this example, multiplicity arises in two ways. First, from multiple measurement instruments used to measure the same outcome domain within the same time frame (e.g. ‘Short-term Pain’ is measured using the ‘Pain VAS’ and ‘Pain on walking VAS’ scales in study 3). Second, from multiple time points measured within the same time frame (e.g. ‘Short-term Pain’ is measured using ‘Pain VAS’ at both 2 weeks and 1 month in study 6). Pre-specified methods to deal with the multiplicity can then be implemented (see Table 9.3.c for examples of approaches for dealing with multiplicity). In this review, the authors pre-specified a set of decision rules for selecting specific outcomes within the outcome domains. For example, for the outcome domain ‘Pain’, the selected outcome was the highest on the following list: global pain, pain on walking, WOMAC pain subscore, composite pain scores other than WOMAC, pain on activities other than walking, rest pain or pain during the night. The authors further specified that if there were multiple time points at which the outcome was measured within a time frame, they would select the longest time point. The selected outcomes from applying these rules to studies 3 and 6 are indicated by an asterisk in Table 9.3.b .
Table 9.3.b also illustrates an approach to tabulating the extracted data. The available statistics are tabulated in the column labelled ‘Data’, from which an assessment can be made as to whether the study contributes the required data for a meta-analysis (column ‘Effect & SE’) ( Chapter 10 ). For example, of the seven studies comparing health-directed behaviour (BEH) with usual care, six measured ‘Short-term Pain’, four of which contribute required data for meta-analysis. Reordering the table by comparison, outcome and time frame, will more readily show the number of studies that will contribute to a particular meta-analysis, and help determine what other synthesis methods might be used if the data available for meta-analysis are limited.
Table 9.3.c Examples of approaches for selecting one outcome (effect estimate) for inclusion in a synthesis.* Adapted from López-López et al (2018)
9.3.4 Determine if modification to the planned comparisons or outcomes is necessary, or new comparisons are needed (step 2.4)
The previous steps may reveal the need to modify the planned comparisons. Important variations in the intervention may be identified leading to different or modified intervention groups. Few studies or sparse data, or both, may lead to different groupings of interventions, populations or outcomes. Planning contingencies for anticipated scenarios is likely to lead to less post-hoc decision making ( Chapter 2 and Chapter 3 ); however, it is difficult to plan for all scenarios. In the latter circumstance, the rationale for any post-hoc changes should be reported. This approach was adopted in a review examining the effects of portion, package or tableware size for changing selection and consumption of food, alcohol and tobacco (Hollands et al 2015). After preliminary examination of the outcome data, the review authors changed their planned intervention groups. They judged that intervention groups based on ‘size’ and those based on ‘shape’ of the products were not conceptually comparable, and therefore should form separate comparisons. The authors provided a rationale for the change and noted that it was a post-hoc decision.
9.3.5 Synthesize the characteristics of the studies contributing to each comparison (step 2.5)
A final step, and one that is essential for interpreting combined effects, is to synthesize the characteristics of studies contributing to each comparison. This description should integrate information about key PICO characteristics across studies, and identify any potentially important differences in characteristics that were pre-specified as possible effect modifiers. The synthesis of study characteristics is also needed for GRADE assessments, informing judgements about whether the evidence applies directly to the review question (indirectness) and analyses conducted to examine possible explanations for heterogeneity (inconsistency) (see Chapter 14 ).
Tabulating study characteristics is generally preferable to lengthy description in the text, since the structure imposed by a table can make it easier and faster for readers to scan and identify patterns in the information presented. Table 9.3.b illustrates one such approach. Tabulating characteristics of studies that contribute to each comparison can also help to improve the transparency of decisions made around grouping of studies, while also ensuring that studies that do not contribute to the combined effect are accounted for.
9.4 Checking data before synthesis
Before embarking on a synthesis, it is important to be confident that the findings from the individual studies have been collated correctly. Therefore, review authors must compare the magnitude and direction of effects reported by studies with how they are to be presented in the review. This is a reasonably straightforward way for authors to check a number of potential problems, including typographical errors in studies’ reports, accuracy of data collection and manipulation, and data entry into RevMan. For example, the direction of a standardized mean difference may accidentally be wrong in the review. A basic check is to ensure the same qualitative findings (e.g. direction of effect and statistical significance) between the data as presented in the review and the data as available from the original study.
Results in forest plots should agree with data in the original report (point estimate and confidence interval) if the same effect measure and statistical model is used. There are legitimate reasons for differences, however, including: using a different measure of intervention effect; making different choices between change-from-baseline measures, post-intervention measures alone or post-intervention measures adjusted for baseline values; grouping similar intervention groups; or making adjustments for unit-of-analysis errors in the reports of the primary studies.
9.5 Types of synthesis
The focus of this chapter has been describing the steps involved in implementing the planned comparisons between intervention groups (stage 2 of the general framework for synthesis ( Box 9.2.a )). The next step (stage 3) is often performing a statistical synthesis. Meta-analysis of effect estimates, and its extensions have many advantages. There are circumstances under which a meta-analysis is not possible, however, and other statistical synthesis methods might be considered, so as to make best use of the available data. Available summary and synthesis methods, along with the questions they address and examples of associated plots, are described in Table 9.5.a . Chapter 10 and Chapter 11 discuss meta-analysis (of effect estimate) methods, while Chapter 12 focuses on the other statistical synthesis methods, along with approaches to tabulating, visually displaying and providing a structured presentation of the findings. An important part of planning the analysis strategy is building in contingencies to use alternative methods when the desired method cannot be used.
Table 9.5.a Overview of available methods for summary and synthesis
9.6 Chapter information
Authors: Joanne E McKenzie, Sue E Brennan, Rebecca E Ryan, Hilary J Thomson, Renea V Johnston
Acknowledgements: Sections of this chapter build on Chapter 9 of version 5.1 of the Handbook , with editors Jonathan Deeks, Julian Higgins and Douglas Altman. We are grateful to Julian Higgins, James Thomas and Tianjing Li for commenting helpfully on earlier drafts.
Funding: JM is supported by an NHMRC Career Development Fellowship (1143429). SB and RR’s positions are supported by the NHMRC Cochrane Collaboration Funding Program. HT is funded by the UK Medical Research Council (MC_UU_12017-13 and MC_UU_12017-15) and Scottish Government Chief Scientist Office (SPHSU13 and SPHSU15). RJ’s position is supported by the NHMRC Cochrane Collaboration Funding Program and Cabrini Institute.
9.7 References
Chamberlain C, O’Mara-Eves A, Porter J, Coleman T, Perlen SM, Thomas J, McKenzie JE. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2017; 2 : CD001055.
Hollands GJ, Shemilt I, Marteau TM, Jebb SA, Lewis HB, Wei Y, Higgins JPT, Ogilvie D. Portion, package or tableware size for changing selection and consumption of food, alcohol and tobacco. Cochrane Database of Systematic Reviews 2015; 9 : CD011045.
Kroon FPB, van der Burg LRA, Buchbinder R, Osborne RH, Johnston RV, Pitt V. Self-management education programmes for osteoarthritis. Cochrane Database of Systematic Reviews 2014; 1 : CD008963.
López-López JA, Page MJ, Lipsey MW, Higgins JPT. Dealing with effect size multiplicity in systematic reviews and meta-analyses. Research Synthesis Methods 2018; 9 : 336–351.
For permission to re-use material from the Handbook (either academic or commercial), please see here for full details.
Browse Course Material
Course info.
- Prof. Donald Sadoway
Departments
- Materials Science and Engineering
As Taught In
- Chemical Engineering
Learning Resource Types
Introduction to solid state chemistry, 29. polymers: synthesis, properties & applications.
« Previous | Next »
Session Overview
Prerequisites.
Before starting this session, you should be familiar with:
- Session 28: Polymers: Structure & Composition
Looking Ahead
The next segment on biochemistry (Sessions 30 through 32) builds upon these introductory polymers sessions.
Learning Objectives
After completing this session, you should be able to:
- Define, compare and contrast the two forms of polymer synthesis.
- Summarize the key properties of polymers that determine their suitability for various applications.
- Explain the relationship between polymer structure and material properties , for instance the effect of crystallization zones on strength and transparency.
- Describe some factors that affect the recyclability of polymers.
Lecture Video
Lecture 29: Polymers: Synthesis, Properties & Applications
Lecture Slides (PDF - 3.0MB)
Lecture Summary
This session focuses on polymer synthesis, the relationships between polymer structure and properties, and the culture implications of polymers.
The two forms of polymer synthesis ( addition and condensation ) are described in terms of processes, resulting chemical structures and properties, and example materials. Factors affecting recyclability are described, along with defining thermoplastic and thermoset characteristics. Crystallization zones are presented as a means for controlling a polymer’s mechanical performance.
Prof. Sadoway summarizes the properties of polymers as follows:
- Electrically insulating
- Transparent to visible light (amorphous material) vs. opaque
- Chemically inert
- Strong covalent bonds (thus good for packaging)
- Low density
- Solid at room temperature
Polymers have had significant impact on society . The economic and performance improvements introduced by polymer-based substitute materials have transformed many aspects of modern daily life, and led to entirely new products. The class discussion ranges from early 20th century inventions (e.g. nylon , Bakelite ), to the late 1960s fascination with plastics , to present-day concerns about recycling and human health impacts.
Problems (PDF)
Solutions (PDF)
Textbook Problems
[JS] Chapter 13, Sample Problems 13.1 and 13.4
For Further Study
Supplemental readings.
Perree, R. Bakelite: The Material of a Thousand Uses. Amsterdam, NL: Cadre, 1996. ISBN: 9789053492338.
Meikle, J. American Plastic: A Culture History. New Brunswick, NJ: Rutgers University Press, 1995. ISBN: 9780813522357.
Carothers, W.H. Collected Papers of Wallace Hume Carothers on High Polymeric Substances . New York, NY: Interscience Publishers, 1940. ISBN: 9781406759259. [Download or view complete work from Internet Archive ]
Furukawa, Y. Inventing Polymer Science: Staudinger, Carothers, and the Emergence of Macromolecular Science . Philadelphia, PA: University of Pennsylvania Press, 1998. ISBN: 9780812233360.
Wallace Carothers
“Mr. Cellophane.” Chicago . DVD. Miramax, 2003.
The Graduate . Directed by M. Nichols. DVD. MGM, 1967.
Other OCW and OER Content

You are leaving MIT OpenCourseWare

- Walden University
- Faculty Portal
Using Evidence: Synthesis
Synthesis video playlist.
Note that these videos were created while APA 6 was the style guide edition in use. There may be some examples of writing that have not been updated to APA 7 guidelines.

Basics of Synthesis
As you incorporate published writing into your own writing, you should aim for synthesis of the material.
Synthesizing requires critical reading and thinking in order to compare different material, highlighting similarities, differences, and connections. When writers synthesize successfully, they present new ideas based on interpretations of other evidence or arguments. You can also think of synthesis as an extension of—or a more complicated form of—analysis. One main difference is that synthesis involves multiple sources, while analysis often focuses on one source.
Conceptually, it can be helpful to think about synthesis existing at both the local (or paragraph) level and the global (or paper) level.
Local Synthesis
Local synthesis occurs at the paragraph level when writers connect individual pieces of evidence from multiple sources to support a paragraph’s main idea and advance a paper’s thesis statement. A common example in academic writing is a scholarly paragraph that includes a main idea, evidence from multiple sources, and analysis of those multiple sources together.
Global Synthesis
Global synthesis occurs at the paper (or, sometimes, section) level when writers connect ideas across paragraphs or sections to create a new narrative whole. A literature review , which can either stand alone or be a section/chapter within a capstone, is a common example of a place where global synthesis is necessary. However, in almost all academic writing, global synthesis is created by and sometimes referred to as good cohesion and flow.
Synthesis in Literature Reviews
While any types of scholarly writing can include synthesis, it is most often discussed in the context of literature reviews. Visit our literature review pages for more information about synthesis in literature reviews.
Related Webinars
Didn't find what you need? Email us at [email protected] .
- Previous Page: Analysis
- Next Page: Citing Sources Properly
- Office of Student Disability Services
Walden Resources
Departments.
- Academic Residencies
- Academic Skills
- Career Planning and Development
- Customer Care Team
- Field Experience
- Military Services
- Student Success Advising
- Writing Skills
Centers and Offices
- Center for Social Change
- Office of Academic Support and Instructional Services
- Office of Degree Acceleration
- Office of Research and Doctoral Services
- Office of Student Affairs
Student Resources
- Doctoral Writing Assessment
- Form & Style Review
- Quick Answers
- ScholarWorks
- SKIL Courses and Workshops
- Walden Bookstore
- Walden Catalog & Student Handbook
- Student Safety/Title IX
- Legal & Consumer Information
- Website Terms and Conditions
- Cookie Policy
- Accessibility
- Accreditation
- State Authorization
- Net Price Calculator
- Contact Walden
Walden University is a member of Adtalem Global Education, Inc. www.adtalem.com Walden University is certified to operate by SCHEV © 2024 Walden University LLC. All rights reserved.
- More from M-W
- To save this word, you'll need to log in. Log In
Definition of synthesis
- amalgamation
- combination
- intermixture
Examples of synthesis in a Sentence
These examples are programmatically compiled from various online sources to illustrate current usage of the word 'synthesis.' Any opinions expressed in the examples do not represent those of Merriam-Webster or its editors. Send us feedback about these examples.
Word History
Greek, from syntithenai to put together, from syn- + tithenai to put, place — more at do
1589, in the meaning defined at sense 1a
Phrases Containing synthesis
synthesis gas
Dictionary Entries Near synthesis
Cite this entry.
“Synthesis.” Merriam-Webster.com Dictionary , Merriam-Webster, https://www.merriam-webster.com/dictionary/synthesis. Accessed 26 May. 2024.
Kids Definition
Kids definition of synthesis, medical definition, medical definition of synthesis, more from merriam-webster on synthesis.
Nglish: Translation of synthesis for Spanish Speakers
Britannica English: Translation of synthesis for Arabic Speakers
Britannica.com: Encyclopedia article about synthesis
Subscribe to America's largest dictionary and get thousands more definitions and advanced search—ad free!

Can you solve 4 words at once?
Word of the day.
See Definitions and Examples »
Get Word of the Day daily email!
Popular in Grammar & Usage
More commonly misspelled words, your vs. you're: how to use them correctly, every letter is silent, sometimes: a-z list of examples, more commonly mispronounced words, how to use em dashes (—), en dashes (–) , and hyphens (-), popular in wordplay, the words of the week - may 24, flower etymologies for your spring garden, birds say the darndest things, a great big list of bread words, 10 scrabble words without any vowels, games & quizzes.

Synthesis Reaction Definition and Examples
Overview of a Synthesis or Direct Combination Reaction
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction.
In a synthesis reaction, two or more chemical species combine to form a more complex product: A + B → AB.
In this form, a synthesis reaction is easy to recognize because you have more reactants than products. Two or more reactants combine to make one larger compound.
One way to think of synthesis reactions is that they are the reverse of a decomposition reaction .
Synthesis Reaction Examples
In the simplest synthesis reactions, two elements combine to form a binary compound (a compound made of two elements). The combination of iron and sulfur to form iron (II) sulfide is an example of a synthesis reaction :
8 Fe + S 8 → 8 FeS
Another example of a synthesis reaction is the formation of potassium chloride from potassium and chlorine gas :
2K (s) + Cl 2(g) → 2KCl (s)
As in these reactions, it's common for a metal to react with a nonmetal. One typical nonmetal is oxygen, as in the everyday synthesis reaction of rust formation :
4 Fe (s) + 3 O 2 (g) → 2 Fe 2 O 3 (s)
Direct combination reactions aren't always just simple elements reacting to form compounds: Another everyday synthesis reaction, for example, is the reaction that forms hydrogen sulfate, a component of acid rain. Here, the sulfur oxide compound reacts with water to form a single product:
SO 3 (g) + H 2 O (l) → H 2 SO 4 (aq)
Multiple Products
So far, the reactions you have seen have only one product molecule on the right-hand side of the chemical equation. Let's take a look at more complex reactions with multiple products. For example, the overall equation for photosynthesis:
CO 2 + H 2 O → C 6 H 12 O 6 + O 2
The glucose molecule is more complex than either carbon dioxide or water.
Remember, the key to identifying a synthesis or direct combination reaction is to recognize two or more reactants form a more complex product molecule.
Predictable Products
Certain synthesis reactions form predictable products. For example:
- Combining two pure elements will form a binary compound.
- A metallic oxide and carbon dioxide will form a carbonate.
- Binary salts combined with oxygen form a chlorate.
- Examples of Chemical Reactions in Everyday Life
- Synthesis Reaction Description Plus Examples
- Simple Chemical Reactions
- Chemical Reaction Definition and Examples
- Types of Chemical Reactions
- How Many Types of Chemical Reactions Are There?
- Reaction Definition in Chemistry
- Combination Reaction Definition
- 4 Types of Inorganic Chemical Reactions
- Decomposition Reaction Definition
- What Is a Chemical Reaction?
- Single-Displacement Reaction Definition and Examples
- Chemical Reaction Classification Practice Test
- Anabolism and Catabolism Definition and Examples
- Chemistry Vocabulary Terms You Should Know
- Name Reactions in Organic Chemistry
Methods of synthesis, characteristics, and environmental applications of Mxene: A comprehensive review
- Related Documents
The hetero-Friedel-Crafts-Bradsher Cyclizations with Formation of Ring Carbon-Heteroatom (P, S) Bonds, Leading to Organic Functional Materials
The interest in functional materials possessing improved properties led to development of new methods of their synthesis, which allowed to obtain new molecular arrangements with carbon and heteroatom motifs. Two of the classical reactions of versatile use are the Friedel-Crafts and the Bradsher reactions, which in the new heteroatomic versions allow to replace ring carbon atoms by heteroatoms. In the present work, we review methods of synthesis of C–S and C–P bonds utilizing thia- and phospha-Friedel-Crafts-Bradsher cyclizations. Single examples of C–As and lack of C–Se bond formation, involving two of the closest neighbors of P and S in the periodic table, have also been noted. Applications of the obtained π-conjugated molecules, mainly as semiconducting materials, flame retardants, and resins hardeners, designed on the basis of five- and six-membered cyclic molecules containing ring phosphorus and sulfur atoms, are also included. This comprehensive review covers literature up to August 2020.
Organotin(IV) complexes of carboxylic acid derivatives
AbstractA comprehensive review, >100 references, on organotin(IV) complexes of the carboxylic acid derivatives are presented with special reference to their methods of synthesis, spectroscopic and structural studies and their biological activities. The structures of these complexes are discussed on the basis of IR, multinuclear (1H-, 13C- and 119Sn-) NMR.
Recent developments in sensing methods for eutrophying nutrients with a focus on automation for environmental applications
A comprehensive review focusing on eutrophying nutrient monitoring using autonomous sensors, including novel analysis methods, standard analysis methods and state-of-the-art sensor technology.
Prospects of Microalgae for Biomaterial Production and Environmental Applications at Biorefineries
Microalgae are increasingly viewed as renewable biological resources for a wide range of chemical compounds that can be used as or transformed into biomaterials through biorefining to foster the bioeconomy of the future. Besides the well-established biofuel potential of microalgae, key microalgal bioactive compounds, such as lipids, proteins, polysaccharides, pigments, vitamins, and polyphenols, possess a wide range of biomedical and nutritional attributes. Hence, microalgae can find value-added applications in the nutraceutical, pharmaceutical, cosmetics, personal care, animal food, and agricultural industries. Microalgal biomass can be processed into biomaterials for use in dyes, paints, bioplastics, biopolymers, and nanoparticles, or as hydrochar and biochar in solid fuel cells and soil amendments. Equally important is the use of microalgae in environmental applications, where they can serve in heavy metal bioremediation, wastewater treatment, and carbon sequestration thanks to their nutrient uptake and adsorptive properties. The present article provides a comprehensive review of microalgae specifically focused on biomaterial production and environmental applications in an effort to assess their current status and spur further deployment into the commercial arena.
A comprehensive review of engineered biochar: Production, characteristics, and environmental applications
Raman and fluorescence spectra observed in laser microprobe measurements of several compositions in the ln-ba-cu-o system.
We are investigating by Raman microprobe measurements the superconducting and related phases in the LnBa2Cu3O7-x (for x=0 to 1) system where yttrium has been replaced by several of the lanthanide (Ln = Nd,Sm,Eu,Ho,Er) elements. The aim is to relate the observed optical spectra (Raman and fluorescence) to the compositional and structural properties of these solids as part of comprehensive materials characterization. The results are correlated with the methods of synthesis, the processing techniques of these materials, and their superconducting properties. Of relevance is the substitutional chemistry of these isostructural systems, the differences in the spectra, and their microanalytical usefulness for the detection of impurity phases, and the assessment of compositional homogeneity. The Raman spectra of most of these compounds are well understood from accounts in the literature.The materials examined here are mostly ceramic powders prepared by conventional solid state reaction techniques. The bulk samples are of nominally single-phase composition as determined by x-ray diffraction.
Tailoring microstructures of materials through biomimetics
Biomimetics involves investigation of structure, function, and methods of synthesis of biological composite materials. The goal is to apply this information to the design and synthesis of materials for engineering applications.Properties of engineering materials are structure sensitive through the whole spectrum of dimensions from nanometer to macro scale. The goal in designing and processing of technological materials, therefore, is to control microstructural evolution at each of these dimensions so as to achieve predictable physical and chemical properties. Control at each successive level of dimension, however, is a major challenge as is the retention of integrity between successive levels. Engineering materials are rarely fabricated to achieve more than a few of the desired properties and the synthesis techniques usually involve high temperature or low pressure conditions that are energy inefficient and environmentally damaging.In contrast to human-made materials, organisms synthesize composites whose intricate structures are more controlled at each scale and hierarchical order.
Road traffic injuries in developing countries: a comprehensive review of epidemiological studies
Environmental applications of chemometrics, environmental applications of nanoscale and microscale reactive metal particles, export citation format, share document.
Characteristics of Synthesis
In chemistry, synthesis refers to the process of combining different chemical compounds to create a new substance. The resulting substance can have different properties, structures, and uses than its individual components.
Synthesis is an essential part of chemistry and plays a critical role in the development of new drugs, materials, and technologies.
Characteristics
- Complexity: Synthesis often involves the creation of complex molecules from simpler building blocks. This complexity requires careful planning and precise control over reaction conditions to achieve the desired outcome.
- Precision: Synthesis requires precise measurements of the reactants and reaction conditions to ensure the creation of the desired product.
- Yield: The amount of product produced is a critical factor in synthesis. High yields are desirable to maximize the efficiency of the process and reduce costs.
- Selectivity: The ability to control the selectivity of the reaction is important in synthesis. This allows for the creation of specific products and minimizes the formation of unwanted by-products.
- Stereospecificity: Stereochemistry plays a critical role in the properties of many molecules. Synthesis can allow for the creation of specific stereoisomers of a molecule, which can have different properties and uses.
- Reaction Conditions: The choice of reaction conditions, including temperature, pressure, and solvent, can significantly impact the outcome of a synthesis reaction.
- Catalysts: The use of catalysts can improve the efficiency of a synthesis reaction by reducing the energy required for the reaction to occur.
- Purification: The purification of the final product is essential to ensure that it is free from impurities that could affect its properties or uses.
- Scale-up: Synthesis reactions must be scalable to allow for the production of larger quantities of a compound.
- Safety: Synthesis reactions can be hazardous, and safety protocols must be followed to prevent accidents and minimize risks.
- Drug Discovery: Synthesis is critical in the development of new drugs. It allows for the creation of molecules with specific properties that can target specific diseases.
- Materials Science: Synthesis is used to create new materials with specific properties, such as strength, flexibility, or conductivity.
- Polymer Chemistry: Synthesis is essential in the production of polymers, which are used in a wide range of products, from plastics to textiles.
- Energy Storage: Synthesis is used to create materials for energy storage devices, such as batteries and supercapacitors.
- Agriculture: Synthesis is used to create fertilizers, pesticides, and herbicides, which are essential in modern agriculture.
In conclusion, synthesis is an essential process in chemistry that allows for the creation of new substances with specific properties and uses.
Understanding the characteristics of synthesis is critical in designing and optimizing synthesis reactions, while knowledge of the uses of synthesis can guide its application in various fields, from medicine to agriculture.

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
What Is a Synthesis Reaction? Definition and Examples

A synthesis reaction is one of the four main types of chemical reactions , along with decomposition, single replacement , and double replacement reactions. Here is the synthesis reaction definition, examples of the reaction using elements and compounds, a look at how many reactants are involved, and how to recognize a synthesis reaction.
Synthesis Reaction Definition
A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product . A + B → AB This type of reaction is also called a direct combination reaction or simply a combination reaction. It’s the type of reaction that forms compounds from their elements. Synthesis reactions also make large molecules from smaller ones. A synthesis reaction is the opposite of a decomposition reaction , which breaks complex molecules into simpler ones.
Synthesis Reaction Examples
There are many examples of synthesis reactions. Some involve elements. In others, an element reacts with a compound. In still other cases, compounds react with other compounds to form larger molecules.
Synthesis Reactions Between Elements
- Iron and sulfur react to form iron sulfide. 8 Fe + S 8 → 8 FeS
- Potassium and chlorine react to form potassium chloride. 2K (s) + Cl 2(g) → 2KCl (s)
- Iron and oxygen react to form rust. 4 Fe (s) + 3 O 2 (g) → 2 Fe 2 O 3 (s)
- Hydrogen reacts with oxygen to form water. 2 H 2 (g) + O 2 (g) → 2 H 2 O(g)
Synthesis Reactions Between an Element and a Compound
- Carbon monoxide reacts with oxygen to form carbon dioxide. 2 CO(g) + O 2 (g) → 2CO 2 (g)
- Nitric oxide reacts with oxygen to form nitrogen dioxide. 2NO + O 2 → 2NO 2
- CH 2 CH 2 (g) + Br 2 (ℓ) → CH 2 BrCH 2 Br
Synthesis Reactions Between Compounds
- Sulfur oxide reacts with water to form sulfuric acid. SO 3 (g) + H 2 O (l) → H 2 SO 4 (aq)
- Calcium oxide reacts with water to form calcium hydroxide. 2CaO (s) + 2H 2 O (l) → 2Ca(OH) 2 (aq)
- Iron oxide and sulfur oxide react to form iron sulfate. Fe 2 O 3 + 3SO 3 → Fe 2 (SO 4 ) 3
How Many Reactants Are There?
Usually, there are two reactants in a synthesis reaction. They could be two elements, an element and a compound, or two compounds. However, sometimes more reactants combine to form a product. Here are examples of synthesis reactions involving three reactants:
- Sodium carbonate reacts with water and carbon dioxide to form sodium bicarbonate. Na 2 CO 3 + H 2 O + CO 2 → 2NaHCO 3
- Nitrogen reacts with water and oxygen to form ammonium nitrate. 2N 2 (g) + 4H 2 O(g) + O 2 (g) → 2NH 4 NO 3 (s)
How to Recognize a Synthesis Reaction
The easiest way to recognize a synthesis reaction is to look for a reaction where multiple reactants produce a single product. However, sometimes a synthesis reaction equation includes multiple products and reactants. A good example is the overall reaction for photosynthesis, in which carbon dioxide and water combine to form glucose and oxygen. CO 2 + H 2 O → C 6 H 12 O 6 + O 2 But, even in this case, two simpler molecules react to form a more complex one. So, this is the key in synthesis reaction identification.
Some synthesis reactions form predictable products. If you recognize them, it’s easy to recognize the reaction type:
- Reacting two elements forms a binary compound. For example, hydrogen and oxygen react to form water.
- When two nonmetals react, more than one product is possible. For example, sulfur and oxygen react to form sulfur dioxide or sulfur trioxide.
- Alkali metals react with nonmetals to form ionic compounds. For example, sodium and chlorine form sodium chloride.
- Transition metals react with nonmetals to form more than one possible product. To predict the product, you need to know the oxidation state (charge) or the metallic cation.
- Nonmetal oxides react with water to form acids. For example sulfur dioxide reacts with water to make sulfurous acid.
- Metallic oxides react with water to form bases.
- Nonmetal oxides react with one another to form salts.
Related Posts
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 22 May 2024
General synthesis of ionic-electronic coupled two-dimensional materials
- Xiang Xu 1 na1 ,
- Yunxin Chen 1 na1 ,
- Pengbin Liu 1 ,
- Hao Luo 2 ,
- Zexin Li 1 ,
- Dongyan Li 1 ,
- Haoyun Wang 1 ,
- Xingyu Song 1 ,
- Jinsong Wu ORCID: orcid.org/0000-0002-7305-7927 2 ,
- Xing Zhou ORCID: orcid.org/0000-0001-9031-0130 1 &
- Tianyou Zhai ORCID: orcid.org/0000-0003-0985-4806 1 , 3
Nature Communications volume 15 , Article number: 4368 ( 2024 ) Cite this article
1625 Accesses
2 Altmetric
Metrics details
- Nanoscale materials
- Two-dimensional materials
Two-dimensional (2D) AMX 2 compounds are a family of mixed ionic and electronic conductors (where A is a monovalent metal ion, M is a trivalent metal, and X is a chalcogen) that offer a fascinating platform to explore intrinsic coupled ionic-electronic properties. However, the synthesis of 2D AMX 2 compounds remains challenging due to their multielement characteristics and various by-products. Here, we report a separated-precursor-supply chemical vapor deposition strategy to manipulate the chemical reactions and evaporation of precursors, facilitating the successful fabrication of 20 types of 2D AMX 2 flakes. Notably, a 10.4 nm-thick AgCrS 2 flake shows superionic behavior at room temperature, with an ionic conductivity of 192.8 mS/cm. Room temperature ferroelectricity and reconfigurable positive/negative photovoltaic currents have been observed in CuScS 2 flakes. This study not only provides an effective approach for the synthesis of multielement 2D materials with unique properties, but also lays the foundation for the exploration of 2D AMX 2 compounds in electronic, optoelectronic, and neuromorphic devices.
Similar content being viewed by others
Engineering crystalline quasi-two-dimensional polyaniline thin film with enhanced electrical and chemiresistive sensing performances
Semiconductor physics of organic–inorganic 2D halide perovskites
Emerging nanoscience with discotic liquid crystals
Introduction.
The coupled ionic-electronic effects in two-dimensional (2D) materials have attracted tremendous interest in recent years as they endow the materials with diverse responses to external stimuli, further facilitating the development of next-generation electronic, optoelectronic, and neuromorphic devices 1 , 2 , 3 , 4 , 5 . For example, the hybrid of the ionic gate with 2D materials enables the modulation of the phase transition 6 , 7 , 8 and band structures 9 , 10 , 11 in 2D materials due to the strong gate control ability of the ionic gate. Furthermore, the extrinsic ionic states can be introduced into 2D materials through the pre-treatment, such as the intercalation of external ions 4 , 12 and plasma treatment 5 , 13 . Subsequently, employing an electric field to control the migration of ions allows for emulating the function of biological neurons and synapses, showing the vast potential in the field of neuromorphic computing 4 , 5 , 13 . It should be noted that these additional modification techniques require complex processes and result in interface states 4 , 11 , thereby impeding the exploration of novel physical and chemical properties, as well as hindering the development of high-density integrated devices. The intrinsic ionic-electronic coupled 2D materials are expected to solve the above problems.
AMX 2 is a family of mixed ionic-electronic conductors (where A is a monovalent metal ion, M is a trivalent transition or main group metal, and X is a chalcogen). The monovalent metal ions Cu + and Ag + have 3d-orbital electrons that exhibit second-order Jahn-Teller effect 14 , and normally possess a low ion migration barrier 15 . Thus, introducing the superionic conductivity 16 , 17 , 18 , multiferroics 19 , 20 , and magnetism 21 properties within the AMX 2 . Meanwhile, the multielement characteristic and various atomic structures give the AMX 2 rich band structures 19 , 22 , 23 , making the AMX 2 compounds excellent systems for studying intrinsic coupled ionic-electronic properties. While a few demonstrations of the synthesis of 2D AMX 2 have been reported 16 , 24 , the fabrication of most of these compounds remains elusive, hindering their exploration and application. Chemical vapor deposition (CVD) has been widely used in the synthesis of 2D materials 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 . For the synthesis of multielement compounds like AMX 2 , three kinds of precursors are required. In the common CVD process, the metal and chalcogen precursors transport along the same path, leading to uncontrollable pre-reactions and an unstable supply of precursors, thereby hindering the controllable synthesis of 2D AMX 2 .
In this work, we demonstrate a separated-precursor-supply strategy in which the suppressed by-reactions and controllable supply of precursors ensure the general synthesis of 20 distinct 2D AMX 2 , 18 types of which have never been reported. Interestingly, the as-grown AMX 2 flakes exhibit unique electronic and ionic properties. A 10.4 nm AgCrS 2 flake shows superionic conductor characteristics at room temperature with an ionic conductivity of up to 192.8 mS/cm. The as-grown CuScS 2 flakes exhibit semiconductor ferroelectric properties, and show a Curie temperature reaching ~370 K. Notably, the reconfigurable positive/negative photovoltaic current can be observed in CuScS 2 devices due to the adjustable ion migration drived by the external electric field. This work not only provides an effective strategy for synthesizing multielement 2D materials but also opens up opportunities for studying the properties and potential applications of a wide variety of 2D AMX 2 .
Results and discussion
General growth and characterization of amx 2.
We first discuss the difficulties of controllable synthesis 2D AMX 2 . For multielement compounds like AMX 2 , during the CVD process, there are many possible reactions between metal and chalcogen precursors (Supplementary Fig. 1 ). And the formation energy of most binary products is less than that of AMX 2 (Fig. 1a ) 35 . Even if the AMX 2 is more favorable thermodynamically, it is hard to control the reactions of precursors during transportation due to the premixing of the vaporized precursors in common CVD, which promotes the non-uniform distribution of precursors and results in undesired products (Fig. 1b ). Taking the synthesis of CuCrSe 2 as an example since other AMX 2 compounds have similar troubles. In the common CVD process, the Se vapor will pass by the Cu and Cr precursors before reaching the substrate, resulting in uncontrollable pre-reactions (Supplementary Fig. 2 ). Due to the consistent exposure of the metal source to the Se vapor, the metal precursor powders undergo excessive selenization (Supplementary Fig. 3a ), which will suppress the vaporization and destabilize the precursor supply, then giving rise to a large number of by-products such as Cu x Se on the substrate (Supplementary Fig. 3b , c ), hindering the controlled synthesis of AMX 2 .
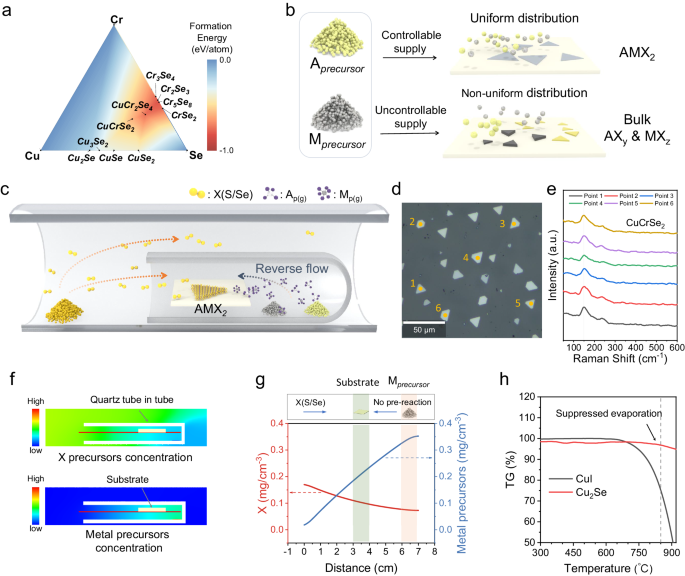
a The formation energy of CuCrSe 2 phase diagram 35 . b The kinetic growth process is influenced by the supply of metal precursors. A and M present the two kinds of metal elements of AMX 2 compounds, and X represents the chalcogen element. The x and y in the AX y and MX z demonstrate the possible stoichiometric ratio of the binary by-products in the ( a ) (such as CuSe, Cu 2 Se, etc.). c Schematic image of the CVD setup. The orange and blue dash arrows represent the transportation paths of the vapored chalcogen precursor and metal precursors (A p(g) and M p(g) ), respectively. d The large area optical image of the as-synthesized CuCrSe 2 nanosheets. e The Raman spectra of the flakes in the ( d ). The vertical dash line located at 150 cm −1 demonstrates the consistent Raman peaks of the as-synthesized CuCrSe 2 nanosheets. f The computational fluid dynamics (CFD) simulated distribution of X (S/Se) and metal precursors concentration. g The CFD simulated variation curve of precursor concentration along the red line in the ( f ). The green and pink shaded areas schematically represent the position of the substrate and metal precursors, respectively. h Thermogravimetric analysis (TGA) of CuI and Cu 2 Se powders. The black and red curves correspond to the weight-loss curves of CuI and Cu 2 Se, respectively. The vertical dash line located at 850°C demonstrates that the evaporation of excessively selenated metal precursors will be significantly suppressed.
To achieve the controllable synthesis of 2D AMX 2 compounds, it is imperative to suppress undesired by-reactions. We have approached this challenge from a kinetic perspective. Specifically, we report a separated-precursor-supply strategy to suppress the by-reactions during mass transportation. The schematic representation of the CVD setup can be found in Fig. 1c and Supplementary Fig. 4a . First, stable source feeding of chalcogen is important. Here, we placed the resolidified chalcogen source, which is believed to realize stable source feeding and further reduce chalcogen vacancy forming in the CVD process 36 , at the upstream. More importantly, we should ensure the temporal and spatial uniform supply of two metal precursors to support the synthesis of 2D AMX 2 , which is much more difficult than the synthesis of binary compounds. We placed two kinds of metal precursors at the bottom of a one-side-sealed quartz tube, then the small quartz tube was placed downstream of the furnace tube (see Methods for more details). In this system, the transport process of metal precursors is protected by the small quartz tube and is separated from the transport process of chalcogen vapor (Supplementary Fig. 4b ). Based on this method, we realized the uniform synthesis of the 2D AMX 2 materials (Fig. 1 d, e ).
Computational fluid dynamics (CFD) simulations predict that the gas flow inside the small quartz tube is primarily directed towards the open side, opposing the flow direction of carrier gas, and exhibits significantly lower velocity compared to the external gas flow outside the small tube (Supplementary Fig. 6 ). This results in a reverse mass flow opposite to the Se precursor’s transport direction (Supplementary Figs. 6 and 7 ). As a consequence, the concentration of metal precursor vapors is lower at the tube’s open side and higher at the tube’s sealed side, and the Se vapors’ concentration distribution is opposite to that of the metal precursor vapor (Fig. 1 f, g ). The relatively high concentration of the metal precursor vapor in the small tube prevents excessive selenization of the metal precursor powder, ensuring the stable vaporization of the metal precursor during the whole CVD process. In contrast, without the confinement of the small quartz tube, the vapor of the metal precursor and Se precursor meet before reaching the substrate (Supplementary Fig. 8 ) which will facilitate the occurrence of by-reactions. And the concentration of chalcogen precursor is much higher than the metal precursors. This will result in excessive selenization or sulfurization, making the supply of metal precursor unstable (Fig. 1h ). However, when the metal precursor is confined within the small quartz tube, its concentration is one order of magnitude higher compared to the situation without spatial confinement (Supplementary Fig. 9 ). The ample metal precursor supply, which matches the supply of chalcogen precursor, greatly suppress phase separation.
Taking the synthesis of 2D CuCrSe 2 as an example again. Different from the common CVD, the separated-precursor-supply strategy protected the metal precursors from excessive selenization and suppressed the pre-reactions between the Cu/Cr precursors and Se precursors (Supplementary Fig. 10 ), thereby ensuring a stable supply of metal precursors and suppressing undesired by-products. Through this approach, 2D CuCrSe 2 with consistent phase and uniform morphology can be obtained (Fig. 1 d, e ). Figure 2 shows a summary of optical images of the 20 kinds of 2D AMX 2 materials prepared using this method. The corresponding synthesis conditions are described in the Methods, more details are summarized in Supplementary Table 1 and Supplementary Methods. To our best knowledge, 18 of them have not been previously synthesized using CVD or mechanical exfoliation methods (Supplementary Table 2 ). The synthesized AMX 2 compounds are mainly selenides and sulfides and contain 9 metal elements including two monovalent metal ions (Cu + , Ag + ); three transition metals (Sc, Cr, Fe); and four main group metals (Ga, In, Sb, Bi). To show our uniform growth, the larger area optical images with more flakes are shown in Supplementary Fig. 11 . Most of the synthesized 2D AMX 2 compounds exhibit triangular or hexagonal shapes, and a small fraction shows rhombic or nanoribbon morphologies. The thickness of most samples can be reduced to below 10 nm, and some can even reach few unit-cell thickness, such as CuCrS 2 (2.56 nm), AgCrSe 2 (1.86 nm), CuFeSe 2 (1.9 nm), and CuSbS 2 (0.79 nm) (Supplementary Fig. 12 ), which demonstrates the effectivity and generality of our growth methods.
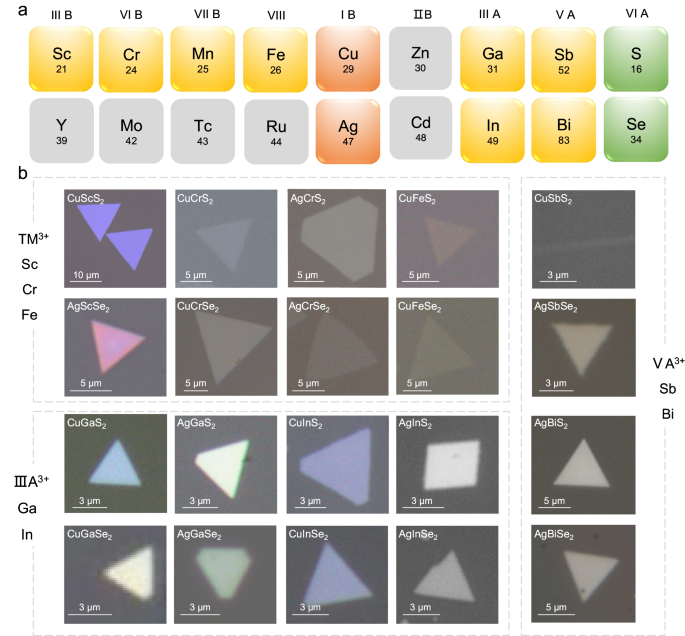
a Summary of 2D AMX 2 compounds that can be synthesized using this method. Orange, element A; yellow, element M; green, element X. b Optical images of the as-synthesized 2D AMX 2 nanosheets.
To elucidate the structural features of AMX 2 compounds, we conducted high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) characterization on three representative materials, namely AgCrS 2 with R 3 m space group, AgBiSe 2 with R \(\bar{3}\) m space group, and CuInS 2 with I \(\bar{4}\) 2 d space group. For the as-grown AgCrS 2 . The High-resolution transmission electron microscopy (HRTEM) image along the [001] crystal direction reveals its hexagonal atomic arrangement, with a measured lattice spacing of d(2 \(\bar{1}\) 0) = 1.71 Å (Fig. 3a ). The cross-sectional HAADF-STEM image along the [100] crystal direction is shown in Fig. 3b , with a measured lattice spacing of d(003) = 6.83 Å. Based on the layered characteristics, the structure can be understood as alternating stacking of CrS 2 layers and Ag + ion layers along the c-axis. The brightness variation of the dashed line in Fig. 3b is depicted in Fig. 3c , where Ag exhibits the highest brightness, followed by Cr with intermediate brightness, and S appears the darkest. The 3d orbital electrons of Cr 3+ in the material hybridize with the p orbital electrons of S, forming [CrS 6 ] octahedral coordination structure (shown at the blue quadrilateral position in Fig. 3b ). The [CrS 6 ] octahedra are edge-connected to form the CrS 2 layer (Supplementary Fig. 13 ). Meanwhile, Ag + ions orderly occupy the tetrahedral sites between the CrS 2 layers (shown at the red triangular position in Fig. 3b ). This ordered tetrahedral occupancy results in the breaking of inversion symmetry, leading the material to exhibit a pronounced optical second harmonic generation (SHG) response (Supplementary Fig. 14g ). Similarly, AgBiSe 2 also exhibits typical layered structure characteristics, with Ag + confined between the BiSe 2 layers. The HRTEM image along the [001] crystal direction also demonstrates the characteristic hexagonal atomic arrangement, with a measured lattice spacing of d(2 \(\bar{1}\) 0) = 2.07 Å (Fig. 3d ). However, different with the AgCrS 2 , the Ag + ions in the AgBiSe 2 occupy the octahedral sites between the BiSe 2 layers (shown at the red quadrilateral in Fig. 3e ), with a measured lattice spacing of d(003) = 6.77 Å. The structure of CuInS 2 is composed of [CuS 4 ] and [InS 4 ] tetrahedra. The exposed surface of the sample has a hexagonal atomic arrangement, namely the (112) plane (Fig. 3g ). From HRTEM images and selected area electron diffraction (SAED) patterns, the annotated lattice spacings are d(20 \(\bar{4}\) ) = 1.96 Å and d(112) = 3.26 Å, respectively. The measured crystal plane spacings for these three materials are consistent with previous reports. Additionally, we provided Raman spectra, photoluminescence spectra, and optical SHG response for each sample (Supplementary Figs. 14 – 18 ), and we conducted HRTEM and energy-dispersive spectroscopy (EDS) to characterize the 20 kinds of as-grown AMX 2 compounds (Supplementary Figs. 19 – 38 ). The synthesized AMX 2 compounds exhibit good agreement with the expected phases and show high crystalline quality.
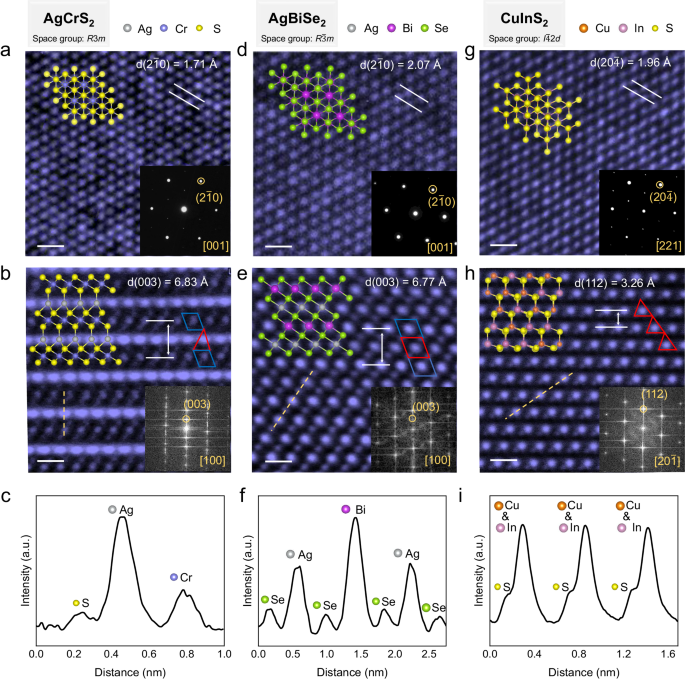
a , d , g High-resolution transmission electron microscopy (HRTEM) images of AgCrS 2 , AgBiSe 2 , and CuInS 2 along the direction out of the plane of the as-grown nanosheets, scale bar: 0.5 nm. The insets display the top view of atomic structure models and selected area electron diffraction (SAED) patterns. b , e , h Cross-sectional high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images of AgCrS 2 , AgBiSe 2 , and CuInS 2 , scale bar: 0.5 nm. The insets display the side view of the atomic structure models and the fast Fourier transform (FFT) patterns. The blue and red polygons represent the octahedral and tetrahedron sites. c , f , i Intensity profiles of the orange dash lines in ( b ), ( e ), and ( h ).
In summary, the as-grown AMX 2 compounds typically possess a quasi-2D layered structure, where monovalent metal ions Cu + and Ag + , known for their strong migration characteristics 17 , 18 , are confined within the interlayer space of MX 2 (Supplementary Fig. 13 ). This means that most AMX 2 materials possess inherent migratable ions, and have natural 2D ion migration pathways. As a result, AMX 2 materials exhibit intrinsic ion characteristics 18 , 23 , 37 . Additionally, the 3d orbital electrons of the transition metal in the MX 2 layers may introduce ferromagnetic or antiferromagnetic characteristics to the materials 19 , 20 , 38 (Supplementary Table 3 ). In addition to their layered structure characteristics, 14 kinds of the as-grown 2D AMX 2 possess features with broken inversion symmetry (Supplementary Table 4 ), endowing them with optical SHG properties, as well as piezoelectric and ferroelectric properties 24 , 39 .
The ionic and electronic properties of AMX 2 compounds
Building upon the structural attributes of AMX 2 , Cu + /Ag + ions can undergo hopping between the tetrahedral or octahedral sites within the MX 2 interlayer space when the temperature is higher than a certain point 18 , 37 . Simultaneously, under the influence of an external electric field, ions can exhibit directed migration, thereby manifesting superionic conductor features 16 , 40 (Fig. 4a ). We commence our investigation by delving into the ionic migration properties of AMX 2 , using AgCrS 2 as an illustrative example, we fabricated two-terminal electrode devices and employed Au as a blocking electrode for testing ion conductivity. The impedance spectra of samples with different thicknesses (Fig. 4b ) can all be fitted with two semicircles. These curves exhibit characteristic mixed ion-electron conductivity features 16 , 23 . According to the equivalent circuit of the mixed ionic-electronic conductor model (illustrated in the inset of Fig. 4b ), ionic conductivity can be obtained by fitting the electrochemical impedance curves, and the detail of the fitting process is described in Methods and Supplementary Fig. 39 . In a 10.4 nm AgCrS 2 nanosheet, we measured an ionic conductivity of 192.8 mS/cm. Interestingly, we observed that the ionic conductivity increases as the sample thickness decreases (Supplementary Fig. 40 ). This trend is consistent with the previous report on AgCrS 2 samples obtained via electrochemical exfoliation 16 . However, our samples exhibit a higher ionic conductivity, which could be attributed to the superior crystalline quality resulting from our synthesis method. Compared to other ion conductors, the ionic conductivity of the AgCrS 2 nanosheets synthesized by us remains at a relatively high level (Fig. 4c ) 16 , 40 , 41 , 42 , 43 , 44 , 45 , 46 .
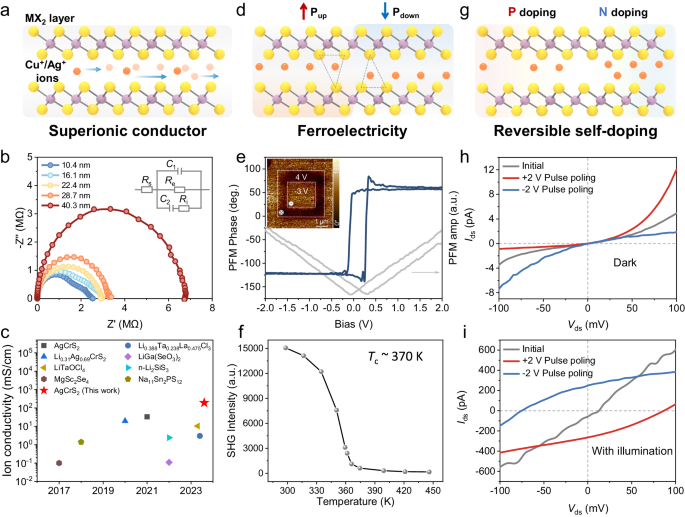
a , d , g Schematic diagrams of the superionic conductor ( a ), ferroelectricity ( d ), and reversible self-doping ( g ) properties that arise from the ionic-electronic coupling effects. The blue arrows in ( a ) present the directed long-distance migration of the Cu + /Ag + ions within the interlayer. The dashed triangles in ( d ) show the two enantiomeric tetrahedral sites of Cu + /Ag + ions, corresponding to the up and down ferroelectric polarization states of the materials. b The impedance spectroscopic measurement for AgCrS 2 nanosheets with different thicknesses. The inset shows the equivalent circuit, where R s , R e, and R i correspond to the contact resistance, electronic resistance, and ionic resistance, respectively, and C 1 and C 2 are the constant phase elements. The detailed calculation process of ionic conductivity is shown in Supplementary Fig. 39 . c The comparison of ionic conductivity with other reported superionic conductors 16 , 40 , 41 , 42 , 43 , 44 , 45 , 46 . e Piezoresponse force microscopy (PFM) phase and amplitude hysteresis loop of CuScS 2 nanosheet, the inset is the ferroelectric domains (white dashed square area) after forward and reverse DC bias polarization. The out-of-plane arrow symbols represent the P up and P down states of the nanosheet after polarization. f Temperature-dependent optical second harmonic generation (SHG) measurement of a CuScS 2 nanosheet, demonstrating a ferroelectric Curie temperature ( T c ) of ~ 370 K. h , i Memristor behavior ( h ) and switchable photovoltaic behavior under illumination (λ = 532 nm, 256.6 mW/cm 2 ) ( i ) after positive/negative pulse polarization of the CuScS 2 -based device.
Cu + /Ag + ions can not only undergo long-distance migration but also experience local displacements, giving rise to ferroelectricity, specifically ion displacement-induced ferroelectricity 47 . By applying a vertical electric field, we can drive ion displacements between the two enantiomeric tetrahedral sites within the MX 2 interlayer space, resulting in spontaneous polarization reversal and exhibiting ferroelectric properties (Fig. 4d ). Taking CuScS 2 as an example. The SHG mapping shows the uniform non-centrosymmetric crystal structure and the single domain characteristics of the as-synthesized CuScS 2 flake (Supplementary Fig. 41 ). Then, we employed piezoresponse force microscopy (PFM) testing methods to investigate the material’s room-temperature ferroelectric properties. The as-grown CuScS 2 flakes exhibited distinct ferroelectric phase hysteresis loops and typical amplitude butterfly curves (Fig. 4e ). Simultaneously, we performed domain read-write operations on the flakes. The PFM phase image shows two stable opposite polarization domain regions (the inset of Fig. 4e ), validating the out-of-plane ferroelectric properties of CuScS 2 at room temperature. We also tried to reveal the in-plane (IP) polarization of the CuScS 2 flakes. However, there is no typical IP PFM phase and amplitude hysteresis loop (as Supplementary Fig. 42 shows), suggesting no IP polarization in the as-synthesized CuScS 2 flakes. Furthermore, to reveal the temperature stability of the ionic displacement-type ferroelectricity in CuScS 2 , which is critical to the applications, we employed high-temperature SHG measurements to characterize its ferroelectric Curie temperature ( T C ). As the temperature increases to above 370 K, the SHG signal of CuScS 2 nearly quenches (Fig. 4f and Supplementary Fig. 43a ), indicating the disruption of the ordered occupancy of Cu + ions in the interlayer tetrahedral positions (Supplementary Fig. 43b ). This implies that the T C of CuScS 2 is approximately 370 K, demonstrating significant potential for extensive applications in the field of ferroelectricity.
The movement of ions within the material often results in concentration gradients, leading to different doping effects 48 . By employing electrical pulses to control the migration of ions within the material, reversible self-doping characteristics can be achieved (Fig. 4g ), consequently leading to interesting electrical and optoelectronic properties, which is exemplified by 2D CuScS 2 . First-principles calculations predict that the migration barrier for a Cu + ion to move through the tetrahedral-octahedral-tetrahedral path within the CuScS 2 is 0.24 eV (Supplementary Fig. 44 ). Such a low migration energy barrier is comparable to that for intercalated Li + ions in transition metal dichalcogenides (TMDs) 49 , indicating that a relatively small external electric field is sufficient to drive the migration of Cu + ions. Applying electric pulses for polarization is an effective approach for achieving controlled migration of interlayer ions as we can modulate the pulse width and amplitude precisely 50 . Firstly, we investigated the ionic-electronic coupled properties of CuScS 2 -based devices under dark conditions using triangular electric pulses (Supplementary Fig. 45a ). As shown in Fig. 4h , the initial state I - V curve of the device is symmetric in the positive and negative voltage ranges. After poling the device with a forward bias pulse, the I - V curve exhibits characteristic diode-like behavior, indicating the generation of a potential barrier within the device under the influence of the electric pulse. Furthermore, upon applying a reverse bias pulse, the rectification direction of the device is reversed, indicating that the direction of internal potential barriers within the material can be modulated by the electric pulse. This reconfigurable potential barrier allows for continuous modulation of the device resistance through electric pulses, enabling memristive functionality (Supplementary Fig. 45b–d ). Under illumination, compared to the initial state, the device exhibits a noticeable photovoltaic response after poling by 2 V/0.5 s electric pulse. The device demonstrates a photovoltaic short-circuit current ( I sc ) of ~270 pA when the source-drain bias voltage ( V ds ) is zero and shows a distinct open-circuit voltage ( V oc ) of ~85 mV. Similar to the dark situation, the photovoltaic response direction also reverses after the device undergoes reverse poling electric pulses (Fig. 4i ). This reconfigurable photovoltaic response exhibits great stability during I - t testing (Supplementary Fig. 46 ). To reveal the mechanism of this reconfigurable photovoltaic response, we firstly analyzed the possible bulk photovoltaic effect (BPVE) in the ferroelectric CuScS 2 . Based on the single-domain characteristic of as-synthesized CuScS 2 (Supplementary Fig. 41 ), the BPVE would induce obvious I sc at the initial-state device. However, the I sc of the initial-state device is ignorable (Fig. 4i ), demonstrating that the BPVE is negligible here. Moreover, the undetectable IP ferroelectricity of the as-synthesized CuScS 2 also proves this point. Then, we consider that the long-distance migration of the Cu + ions could induce reversible self-doping, which also can give rise to the reconfigurable photovoltaic response. Thus, we conducted cross-sectional EDS scanning tests on the polarized devices and observed that the distribution of Sc and S elements in the material remains uniformly distributed. However, the content of Cu elements beneath the electrode is significantly higher than in the channel region (Supplementary Fig. 47 ). This confirms the migration of Cu + ions after poling by the electric pulse. Moreover, the first-principles calculations indicate that the local absence or accumulation of Cu + ions resulting from ion migration will introduce significant p-type or n-type doping in the material (Supplementary Fig. 48 ), which is similar to the doping effect of point defects 5 . This coupling effect between ion migration and charge doping induces the emergence of the modulated potential barrier within 2D CuScS 2 and finally gives rise to the memristive behavior and reconfigurable photovoltaic response, which is crucial to the logic and neuromorphic devices 5 , 51 .
In summary, we have demonstrated a separated-precursor-supply CVD method to control the reactions and vaporization of precursors. 20 kinds of 2D AMX 2 compounds have been synthesized successfully showing the practicality of our approach. Detailed structural analysis and comprehensive characterization have revealed the high crystalline quality of the prepared AMX 2 materials. Notably, the as-grown 2D AMX 2 flakes show intriguing ionic and electronic properties. A high ionic conductivity of 192.8 mS/cm can be observed in a 10.4 nm AgCrS 2 flake at room temperature. The synthesized 2D CuScS 2 flakes show ion displacement-induced ferroelectricity at room temperature. Meanwhile, the reconfigurable photovoltaic response based on the coupling of ions migration and charge doping also can be observed in CuScS 2 . The achievement of generally synthesizing 2D AMX 2 compounds offers new insights into the vapor-phase synthesis of multielement 2D materials and provides an excellent material choice for exploration in electronics, optoelectronics, and neuromorphics.
2D AMX 2 compounds were synthesized by separated-precursor-supply CVD. Ultrahigh purity Ar (purity 99.999%) and H 2 (purity 99.999%) were used as the carrier gases. All the raw precursors were bought from Alfa Aesar with a purity higher than 99%. Freshly exfoliated mica was chosen as the growth substrate. A single temperature zone tube furnace (diameter, one inch) was used as the reaction instrument with a heating zone length of ~30 cm (Supplementary Fig. 5 ). A porcelain boat containing chalcogen precursor was placed upstream and heated to 150°C for sulfur (300°C for selenium). Two metal precursors were placed at the bottom of a one-side sealed small quartz tube with a length of 7 cm and a diameter of 10 mm. The growth substrate was placed in the small quartz tube too, 0.5-4 cm away from the metal precursors. Then, the small quartz tube was put downstream of the tube furnace and followed by the heating process. The heating rate is 50°C/min or 30°C/min for different AMX 2 , and the reactions were carried out at 1 atm pressure. The schematic image of the CVD setup can be seen in Fig. 1c and Supplementary Fig 4a . The detailed growth parameters and descriptions are shown in Supplementary Table 1 and Supplementary Methods. The detailed characterizations of the morphology, phase, and atomic structures of as-synthesized ultrathin 2D AMX 2 are shown in Supplementary Figs. 11 , 12 , 14 – 38 .
CFD simulations
To reveal the mass flow during the experiment, we did the numerical finite element simulation. During the modeling, we followed the real experiment setup of the tube furnace, the details can be seen in Supplementary Fig. 5 . The transient model is used to simulate the real CVD process. The gravity and convection heat transfer were considered. Argon and air are considered ideal gases. The shear stress transfer (SST) model is used here. It is a low Reynolds number and comprehensive turbulence model, which can give more accurate near-wall results 52 . Firstly, we conduct a steady analysis of the system. Similar to the real condition of the heating process of the furnace, we assume the system is stable when the temperature of the monitor point reaches the goal point and does not change again. Based on this result, the transient analysis was conducted to reveal the mass transportation process of two precursors.
TG-DSC testing was conducted using a DIAMOND TG/DTA thermal analyzer. Approximately 5 mg of the sample was added to an alumina crucible and heated from 20 °C to 900 °C at a rate of 50 K per minute in a pure argon atmosphere.
Characterizations
The morphologies of the as-grown AMX 2 nanosheets were characterized by an optical microscope (BX51, OLYMPUS) and atomic force microscope (Dimension icon, Bruker). Raman, photoluminescence, and SHG spectra were obtained by a confocal Raman system (Alpha 300 R, WITec) equipped with a 532 nm CW laser and a high-temperature test chamber (TS1000EV, Linkam). Femtosecond laser (Verdi, Coherent) was applied as the excitation source of SHG measurement. For the cross-sectional HAADF-STEM and EDS measurements, the samples were prepared by focus ion beam (Helios NanoLab G3 UC, FEI). The atomic resolution HAADF-STEM images were obtained by a double CS-corrected transmission electron microscopy (Titan Themis G2 60-300, FEI). For the TEM measurements, the samples were prepared with a poly (methyl methacrylate) (PMMA) assisted transfer method. TEM, SAED, and EDS were performed on an FEI Tecnai G2 F30 instrument.
Impedance spectra measurements
AgCrS 2 nanosheets were transferred to the silica substrate. Then, the Au/AgCrS 2 /Au devices were fabricated by electron-beam lithography (EBL, FEI Quanta 650 SEM & Raith Elphy Plus) and thermal evaporation coating (Angstrom Engineering, Nexdep). The ionic conductivity of AgCrS 2 nanosheets is obtained by fitting the electrochemical impedance spectra (Autolab PGSTAT 302 N) of the Au/AgCrS 2 /Ag devices at room temperature. The testing frequency range is 1 Hz to 1 MHz. To avoid electromagnetic interference the whole process was operated in a Faraday cage.
PFM measurements
The PFM measurements were conducted on the AFM platform (Dimension icon, Bruker). CuScS 2 nanosheets were transferred to the silica substrate that was covered with gold. A DC voltage was applied to the conductive tip coated with Pt/Ir to reverse the ferroelectric domain of the sample.
Device fabricating and electrical measurements
All the devices were transferred on the silicon substrate with a 300-nm-thick oxide film using a PMMA-assisted method. The Bi/Au was chosen to be the contact electrode. The electrical measurements were carried out with a semiconductor characterization system (4200SCS, Keithley) and a cryogenic probe station (CRX-6.5, Lake Shore).
Theory calculations
The present first principle DFT calculations are performed by Vienna Ab initio Simulation Package (VASP) 53 with the projector augmented wave (PAW) method 54 . The exchange-functional is treated using the generalized gradient approximation (GGA) of Perdew-Burke-Ernzerhof (PBE) 55 functional. The energy cutoff for the plane wave basis expansion was set to 450 eV and the force on each atom less than 0.02 eV/Å was set for the convergence criterion of geometry relaxation. The Brillouin zone integration is performed using 5 × 5 × 5 and 9 × 9 × 9 k-point sampling for structure optimization and electronic structure calculation, respectively. The self-consistent calculations apply a convergence energy threshold of 10 −5 eV. Transition state searching was performed using the climbing-image nudged elastic band (CI-NEB) method 56 .
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding authors upon request.
Sangwan, V. K. & Hersam, M. C. Neuromorphic nanoelectronic materials. Nat. Nanotechnol. 15 , 517–528 (2020).
Article ADS CAS PubMed Google Scholar
Fuller, E. J. et al. Parallel programming of an ionic floating-gate memory array for scalable neuromorphic computing. Science 364 , 6 (2019).
Article Google Scholar
Liu, C. et al. Two-dimensional materials for next-generation computing technologies. Nat. Nanotechnol. 15 , 545–557 (2020).
Zhu, X., Li, D., Liang, X. & Lu, W. D. Ionic modulation and ionic coupling effects in MoS 2 devices for neuromorphic computing. Nat. Mater. 18 , 141–148 (2019).
Article CAS PubMed Google Scholar
Li, T. et al. Reconfigurable, non-volatile neuromorphic photovoltaics. Nat. Nanotechnol. 18 , 1303–1310 (2023).
Wu, Y., Li, D., Wu, C.-L., Hwang, H. Y. & Cui, Y. Electrostatic gating and intercalation in 2D materials. Nat. Rev. Mater. 8 , 41–53 (2022).
Article ADS CAS Google Scholar
Li, L. J. et al. Controlling many-body states by the electric-field effect in a two-dimensional material. Nature 529 , 185–189 (2016).
Wu, C.-L. et al. Gate-induced metal–insulator transition in MoS 2 by solid superionic conductor LaF 3 . Nano Lett. 18 , 2387–2392 (2018).
Alam, M. H. et al. Lithium-ion electrolytic substrates for sub-1V high-performance transition metal dichalcogenide transistors and amplifiers. Nat. Commun. 11 , 3203 (2020).
Article ADS CAS PubMed PubMed Central Google Scholar
Domaretskiy, D. et al. Quenching the bandgap of two-dimensional semiconductors with a perpendicular electric field. Nat. Nanotechnol. 17 , 1078–1083 (2022).
Lee, S.-J. et al. Programmable devices based on reversible solid-state doping of two-dimensional semiconductors with superionic silver iodide. Nat. Electron. 3 , 630–637 (2020).
Article CAS Google Scholar
Edwards, B. et al. Giant valley-Zeeman coupling in the surface layer of an intercalated transition metal dichalcogenide. Nat. Mater. 22 , 459–465 (2023).
Zhang, H.-T. et al. Reconfigurable perovskite nickelate electronics for artificial intelligence. Science 375 , 533–539 (2022).
Burdett, J. K. & Eisenstein, O. From three- to four-coordination in copper(I) and silver(I). Inorg. Chem. 31 , 1758–1762 (1992).
Ma, J. & Wei, S.-H. Origin of novel diffusions of Cu and Ag in semiconductors: the case of CdTe. Phys. Rev. Lett. 110 , 235901 (2013).
Article ADS PubMed Google Scholar
Peng, J. et al. Stoichiometric two-dimensional non-van der Waals AgCrS 2 with superionic behaviour at room temperature. Nat. Chem. 13 , 1235–1240 (2021).
Rettie, A. J. E. et al. A two-dimensional type I superionic conductor. Nat. Mater. 20 , 1683–1688 (2021).
Niedziela, J. L. et al. Selective breakdown of phonon quasiparticles across superionic transition in CuCrSe 2 . Nat. Phys. 15 , 73–78 (2019).
Zhong, T., Li, X., Wu, M. & Liu, J.-M. Room-temperature multiferroicity and diversified magnetoelectric couplings in 2D materials. Natl Sci. Rev. 7 , 373–380 (2020).
Damay, F. et al. Magnetoelastic coupling and unconventional magnetic ordering in the multiferroic triangular lattice AgCrS 2 . Phys. Rev. B 83 , 184413 (2011).
Article ADS Google Scholar
Peng, J. et al. Even–odd‐layer‐dependent ferromagnetism in 2D non‐van‐der‐Waals CrCuSe 2 . Adv. Mater. 35 , 2209365 (2023).
Pang, C. et al. High-performance inorganically connected CuInSe 2 nanocrystal thin-film transistors and integrated circuits based on the solution process of colloidal synthesis, ligand exchange, and surface treatment. Chem. Mater. 33 , 8775–8785 (2021).
Harikesh, P. C. et al. Cubic NaSbS 2 as an ionic–electronic coupled semiconductor for switchable photovoltaic and neuromorphic device applications. Adv. Mater. 32 , 1906976 (2020).
Xu, X. et al. High- T C two-dimensional ferroelectric CuCrS 2 grown via chemical vapor deposition. ACS Nano 16 , 8141–8149 (2022).
Sun, L. et al. Chemical vapour deposition. Nat. Rev. Methods Prim. 1 , 5 (2021).
Zhu, J. et al. Low-thermal-budget synthesis of monolayer molybdenum disulfide for silicon back-end-of-line integration on a 200 mm platform. Nat. Nanotechnol. 18 , 456–463 (2023).
Zhou, J. et al. Composition and phase engineering of metal chalcogenides and phosphorous chalcogenides. Nat. Mater. 22 , 450–458 (2022).
Zhang, P. et al. Flux-assisted growth of atomically thin materials. Nat. Synth. 1 , 864–872 (2022).
Zhou, Z. et al. Stack growth of wafer-scale van der Waals superconductor heterostructures. Nature 621 , 499–505 (2023).
Li, J. et al. General synthesis of two-dimensional van der Waals heterostructure arrays. Nature 579 , 368–374 (2020).
Liu, L. et al. Uniform nucleation and epitaxy of bilayer molybdenum disulfide on sapphire. Nature 605 , 69–75 (2022).
Fu, J.-H. et al. Oriented lateral growth of two-dimensional materials on c-plane sapphire. Nat. Nanotechnol. 18 , 1289–1294 (2023).
Wang, J. et al. Dual-coupling-guided epitaxial growth of wafer-scale single-crystal WS 2 monolayer on vicinal a-plane sapphire. Nat. Nanotechnol. 17 , 33–38 (2022).
Xia, Y. et al. 12-inch growth of uniform MoS 2 monolayer for integrated circuit manufacture. Nat. Mater. 22 , 1324–1331 (2023).
Jain, A. et al. Commentary: The materials project: a materials genome approach to accelerating materials innovation. APL Mater. 1 , 011002 (2013).
Wu, Q. et al. Resolidified chalcogen precursors for high‐quality 2D semiconductor growth. Angew. Chem. Int. Ed. 62 , e202301501 (2023).
Li, B. et al. Liquid-like thermal conduction in intercalated layered crystalline solids. Nat. Mater. 17 , 226–230 (2018).
Takahashi, H. et al. Spin-orbit-derived giant magnetoresistance in a layered magnetic semiconductor AgCrSe 2 . Phys. Rev. Mater. 6 , 054602 (2022).
Shiomi, Y., Akiba, T., Takahashi, H. & Ishiwata, S. Giant piezoelectric response in superionic polar semiconductor. Adv. Electron. Mater. 4 , 1800174 (2018).
Peng, J. et al. Fast lithium ion conductivity in layered (li-Ag)CrS 2 . J. Am. Chem. Soc. 142 , 18645–18651 (2020).
Yin, Y.-C. et al. A LaCl 3 -based lithium superionic conductor compatible with lithium metal. Nature 616 , 77–83 (2023).
Jun, K. et al. Lithium superionic conductors with corner-sharing frameworks. Nat. Mater. 21 , 924–931 (2022).
Tanaka, Y. et al. New oxyhalide solid electrolytes with high lithium ionic conductivity > 10 mS cm −1 for all‐solid‐state batteries. Angew. Chem. Int. Ed. 62 , e202217581 (2023).
Huang, W. et al. Anomalously high ionic conductivity of Li 2 SiS 3 -type conductors. J. Am. Chem. Soc. 144 , 4989–4994 (2022).
Canepa, P. et al. High magnesium mobility in ternary spinel chalcogenides. Nat. Commun. 8 , 1759 (2017).
Article ADS PubMed PubMed Central Google Scholar
Zhang, Z. et al. Na 11 Sn 2 PS 12 : a new solid state sodium superionic conductor. Energy Environ. Sci. 11 , 87–93 (2018).
Brehm, J. A. et al. Tunable quadruple-well ferroelectric van der Waals crystals. Nat. Mater. 19 , 43–48 (2020).
Gong, Y. et al. Spatially controlled doping of two-dimensional SnS 2 through intercalation for electronics. Nat. Nanotechnol. 13 , 294–299 (2018).
Yang, Y. et al. 2D layered materials for fast‐charging lithium‐ion battery anodes. Small 19 , 2301574 (2023).
Yu, J. et al. Simultaneously ultrafast and robust two-dimensional flash memory devices based on phase-engineered edge contacts. Nat. Commun. 14 , 5662 (2023).
Pi, L. et al. Broadband convolutional processing using band-alignment-tunable heterostructures. Nat. Electron. 5 , 248–254 (2022).
Menter, F. R., Kuntz, M. & Langtry, R. Ten years of industrial experience with the SST turbulence model. Turbul. Heat. Mass Transf. 4 , 625–632 (2003).
Google Scholar
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6 , 15–50 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50 , 17953–17979 (1994).
Perdew, J. P. et al. Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 46 , 6671–6687 (1992).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113 , 9901–9904 (2000).
Download references
Acknowledgements
This work was supported by the National Natural Science Foundation of China (52322210, 22375069, 52172144, and U21A2069), the National Key Research and Development Program of China (2021YFA1200500), and the Innovation Project of Optics Valley Laboratory (OVL2023PY007). Here, the authors also thank the technical support from the Analytical and Testing Center at Huazhong University of Science and Technology.
Author information
These authors contributed equally: Xiang Xu, Yunxin Chen.
Authors and Affiliations
State Key Laboratory of Materials Processing and Die & Mould Technology, School of Materials Science and Engineering, Huazhong University of Science and Technology, Wuhan, 430074, P. R. China
Xiang Xu, Yunxin Chen, Pengbin Liu, Zexin Li, Dongyan Li, Haoyun Wang, Xingyu Song, Xing Zhou & Tianyou Zhai
Nanostructure Research Center, State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan, 430070, P. R. China
Hao Luo & Jinsong Wu
Optics Valley Laboratory, Hubei, 430074, P. R. China
Tianyou Zhai
You can also search for this author in PubMed Google Scholar
Contributions
X.Z., T.Z., and X.X. conceived and designed the experiments. X.X. and Y.C. synthesized the materials. X.X., P.L., Y.C., and D.L. performed the AFM, Raman, and SHG characterizations of the samples. X.X. and H.L. performed the HRTEM and HAADF-STEM characterizations and worked on the analysis of the results. X.X. performed the electrochemical impedance measurements. X.X., P.L., Y.C., H.W., and X.S. performed device fabrication and measurement. X.X. and Z.L. performed the PFM measurements. X.X. and Y.C. wrote the paper with inputs from T.Z., X.Z., and J.W. All authors participated in discussions and approved the manuscript.
Corresponding authors
Correspondence to Xing Zhou or Tianyou Zhai .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Xidong Duan, Wei-jin Hu and Jing Peng for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Xu, X., Chen, Y., Liu, P. et al. General synthesis of ionic-electronic coupled two-dimensional materials. Nat Commun 15 , 4368 (2024). https://doi.org/10.1038/s41467-024-48690-7
Download citation
Received : 03 February 2024
Accepted : 10 May 2024
Published : 22 May 2024
DOI : https://doi.org/10.1038/s41467-024-48690-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Cyclic polymers: synthesis, characteristics, and emerging applications
Affiliations.
- 1 Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany. [email protected].
- 2 Department of Chemistry and International Institute for Nanotechnology, Northwestern University, Evanston, Illinois 60208, USA.
- PMID: 35938292
- DOI: 10.1039/d2nh00242f
Cyclic polymers with a ring-like topology and no chain ends are a unique class of macromolecules. In the past several decades, significant advances have been made to prepare these fascinating polymers, which allow for the exploration of their topological effects and potential applications in various fields. In this Review, we first describe representative synthetic strategies for making cyclic polymers and their derivative topological polymers with more complex structures. Second, the unique physical properties and self-assembly behavior of cyclic polymers are discussed by comparing them with their linear analogues. Special attention is paid to highlight how polymeric rings can assemble into hierarchical macromolecular architectures. Subsequently, representative applications of cyclic polymers in different fields such as drug and gene delivery and surface functionalization are presented. Last, we envision the following key challenges and opportunities for cyclic polymers that may attract future attention: large-scale synthesis, efficient purification, programmable folding and assembly, and expansion of applications.
Publication types
- Research Support, Non-U.S. Gov't
- Gene Transfer Techniques*
- Macromolecular Substances / chemistry
- Polymers* / chemistry
- Macromolecular Substances
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Microbiol
- PMC10168541
A review on nanoparticles: characteristics, synthesis, applications, and challenges
The significance of nanoparticles (NPs) in technological advancements is due to their adaptable characteristics and enhanced performance over their parent material. They are frequently synthesized by reducing metal ions into uncharged nanoparticles using hazardous reducing agents. However, there have been several initiatives in recent years to create green technology that uses natural resources instead of dangerous chemicals to produce nanoparticles. In green synthesis, biological methods are used for the synthesis of NPs because biological methods are eco-friendly, clean, safe, cost-effective, uncomplicated, and highly productive. Numerous biological organisms, such as bacteria, actinomycetes, fungi, algae, yeast, and plants, are used for the green synthesis of NPs. Additionally, this paper will discuss nanoparticles, including their types, traits, synthesis methods, applications, and prospects.
1. Introduction
Nanotechnology evolved as the achievement of science in the 21st century. The synthesis, management, and application of those materials with a size smaller than 100 nm fall under the interdisciplinary umbrella of this field. Nanoparticles have significant applications in different sectors such as the environment, agriculture, food, biotechnology, biomedical, medicines, etc. like; for treatment of waste water ( Zahra et al., 2020 ), environment monitoring ( Rassaei et al., 2011 ), as a functional food additives ( Chen et al., 2023 ), and as a antimicrobial agents ( Islam et al., 2022 ). Cutting-edge properties of NPs such as; nature, biocompatibility, anti-inflammatory and antibacterial activity, effective drug delivery, bioactivity, bioavailability, tumor targeting, and bio-absorption have led to a growth in the biotechnological, and applied microbiological applications of NPs.
A particle of matter with a diameter of one to one hundred nanometers (nm) is commonly referred to as a nanoparticle or ultrafine particle. Nanoparticles frequently exhibit distinctive size-dependent features, mostly due to their tiny size and colossal surface area. The periodic boundary conditions of the crystalline particle are destroyed when the size of a particle approaches the nano-scale with the characteristic length scale close to or smaller than the de Broglie wavelength or the wavelength of light ( Guo et al., 2013 ). Because of this, many of the physical characteristics of nanoparticles differ significantly from those of bulk materials, leading to a wide range of their novel uses ( Hasan, 2015 ).
2. Emergence of nanotechnology
Nanotechnology emerged in the 1980s due to the convergence of experimental advances such as the invention of the scanning tunneling microscope in 1981 and the discovery of fullerenes in 1985 ( Bayda et al., 2019 ), with the elucidation. The popularization of a conceptual framework for nanotechnology goals began with the publication of the book Engines of Creation in 1986 ( Bayda et al., 2019 ).
2.1. Early stage of NPs
Carbon nanotubes have been discovered in pottery from Keeladi, India, dating from around 600–300 BC ( Bayda et al., 2019 ; Kokarneswaran et al., 2020 ). Cementite nanowires have been discovered in Damascus steel, a material that dates back to around 900 AD; nevertheless, its origin and creation method are unclear ( Kokarneswaran et al., 2020 ). However, it is unknown how they developed or whether the material containing them was used on purpose.
2.2. Discovery of C, Ag, Zn, Cu, and Au nanoparticles
Carbon NPs were found in 1991, and Iijima and Ichihashi announced the single-wall carbon nanotube synthesis with a diameter of 1 nanometer in 1993 ( Chen et al., 2021 ). Carbon nanotubes (CNTs), also known as Bucky tubes, are a kind of nanomaterial made up of a two-dimensional hexagonal lattice of carbon atoms. They are bent one way and joined to produce a hollow cylindrical cylinder. Carbon nanotubes are carbon allotropes that fall between Fullerene (0 dimensional) and Grapheme (2 dimensional) ( Chen et al., 2021 ).
In addition, M. C. Lea reported that the synthesis of citrate-stabilized silver colloid almost 120 years ago ( Nowack et al., 2011 ). This process produces particles with an average diameter of 7 to 9 nm. Nanoscale size and citrate stabilization are analogous to recent findings on nanosilver production employing silver nitrate and citrate ( Majeed Khan et al., 2011 ). The use of proteins to stabilize nanosilver has also been documented as early as 1902 ( Nowack et al., 2011 ; Beyene et al., 2017 ). Since 1897, a nanosilver known as “Collargol” has been made commercially and used for medicinal purposes ( Nowack et al., 2011 ). Collargol, a type of silver nanoparticle, has a particle size of about 10 nanometers (nm). This was determined as early as 1907, and it was found that the diameter of Collargol falls within the nanoscale range. In 1953, Moudry developed a different type of silver nanoparticle called gelatin-stabilized silver nanoparticles, with a diameter ranging from 2–20 nm. These nanoparticles were produced using another method than Collargol. The necessity of nanoscale silver was recognized by the creators of nanosilver formulations decades ago, as seen by the following remark from a patent: “for optimal efficiency, the silver must be disseminated as particles of colloidal size less than 25 nm in crystallite size”( Nowack et al., 2011 ).
Gold NPs (AuNPs) have a long history in chemistry, going back to the Roman era when they were used to decorate glassware by staining them. With the work of Michael Faraday, who may have been the first to notice that colloidal gold solutions have characteristics different from bulk gold, the contemporary age of AuNP synthesis began more than 170 years ago. Michael Faraday investigated the making and factors of colloidal suspensions of “Ruby” gold in 1857. They are among the magnetic nanoparticles due to their distinctive optical and electrical characteristics. Under specific illumination circumstances, Faraday showed how gold nanoparticles might create solutions of various colors ( Bayda et al., 2019 ; Giljohann et al., 2020 ).
3. Classification of NPs
Nanoparticles (NPs) are categorized into the following classes based on their shape, size, and chemical characteristics;
3.1. Carbon-based NPs
Fullerenes and carbon nanotubes (CNTs) are the two essential sub-categories of carbon-based NPs. NPs of globular hollow cages, like allotropic forms of carbon, are found in fullerenes. Due to their electrical conductivity, high strength, structure, electron affinity, and adaptability, they have sparked significant economic interest. These materials have organized pentagonal and hexagonal carbon units, each of which is sp2 hybridized. While CNTs are elongated and form 1–2 nm diameter tubular structures. These fundamentally resemble graphite sheets rolling on top of one another. Accordingly, they are referred to as single-walled (SWNTs), double-walled (DWNTs), or multi-walled carbon nanotubes (MWNTs) depending on how many walls are present in the rolled sheets ( Elliott et al., 2013 ; Astefanei et al., 2015 ).
3.2. Metal NPs
Metal NPs are purely made of metals. These NPs have distinctive electrical properties due to well-known localized surface Plasmon resonance (LSPR) features. Cu, Ag, and Au nanoparticles exhibit a broad absorption band in the visible region of the solar electromagnetic spectrum. Metal NPs are used in several scientific fields because of their enhanced features like facet, size, and shape-controlled synthesis of metal NPs ( Khan et al., 2019 ).
3.3. Ceramics NPs
Ceramic NPs are tiny particles made up of inorganic, non-metallic materials that are heat-treated and cooled in a specific way to give particular properties. They can come in various shapes, including amorphous, polycrystalline, dense, porous, and hollow, and they are known for heat resistance and durable properties. Ceramic NPs are used in various applications, including coating, catalysts, and batteries ( Sigmund et al., 2006 ).
3.4. Lipid-based NPs
These NPs are helpful in several biological applications because they include lipid moieties. Lipid NPs typically have a diameter of 10–1,000 nm and are spherical. Lipid NPs, i.e., polymeric NPs, have a solid lipid core and a matrix consisting of soluble lipophilic molecules ( Khan et al., 2019 ).
3.5. Semiconductor NPs
Semiconductor NPs have qualities similar to metals and non-metals. That is why Semiconductor NPs have unique physical and chemical properties that make them useful for various applications. For example, semiconductor NPs can absorb and emit light and can be used to make more efficient solar cells or brighter light-emitting diodes (LEDs). They can make smaller and faster electronic devices, such as transistors, and can be used in bio imaging and cancer therapy ( Biju et al., 2008 ).
3.6. Polymeric NPs
Polymeric NPs with a size between 1 and 1,000 nm can have active substances surface-adsorbed onto the polymeric core or entrapped inside the polymeric body. These NPs are often organic, and the term polymer nanoparticle (PNP) is commonly used in the literature to refer to them. They resemble Nano spheres or Nano capsules for the most part ( Khan et al., 2019 ; Zielińska et al., 2020 ).
4. Types of different metal-based NPs
Metal NPs are purely made of metal precursors. Due to well-known localized surface plasmon resonance (LSPR) characteristics, these NPs possess unique optoelectrical properties. NPs of the alkali and noble metals, i.e., Cu, Ag, and Au, have a broad absorption band in the visible zone of the solar electromagnetic spectrum. The facet, size, and shape-controlled synthesis of metal NPs are essential in present-day cutting-edge materials ( Dreaden et al., 2012 ; Khan et al., 2019 ).
4.1. Silver nanoparticles (AgNPs)
AgNPs are particles with a size range of 1–100 nanometers made of silver. They have unique physical and chemical properties due to their small size, high surface area-to-volume ratio, and ability to absorb and scatter light in the visible and near-infrared range. Because of their relatively small size and high surface-to-volume ratios, which cause chemical and physical differences in their properties compared to their bulk counterparts, silver nanoparticles may exhibit additional antimicrobial capabilities not exerted by ionic silver ( Shenashen et al., 2014 ).
Besides, AgNPs can be created in various sizes and forms depending on the manufacturing process, the most common of which is chemical reduction. The AgNPs were created by chemically reducing a 12 mM AgNO3 aqueous solution. The reaction was carried out in an argon environment using 70 mL of this solution containing PVP (keeping the molar ratio of the repeating unit of PVP and Ag equal to 34) and 21 mL of Aloe Vera. The mixture was agitated in ultrasonic for 45 min at ambient temperature, then heated 2°C/min to 80°C and left for 2 h to generate a transparent solution with tiny suspended particles that must be removed by simple filtering ( Shenashen et al., 2014 ; Gloria et al., 2017 ).

4.2. Zinc nanoparticles (ZnONPs)
Zinc nanoparticles (ZnONPs) are particles with a size range of 1–100 nm made of zinc. Zinc oxide (ZnO) NPs are a wide band gap semiconductor with a room temperature energy gap of 3.37 eV. Its catalytic, electrical, optoelectronic, and photochemical capabilities have made it widely worthwhile ( Kumar S.S. et al., 2013 ). ZnO nanostructures are ideal for catalytic reaction processes ( Chen and Tang, 2007 ). Laser ablation, hydrothermal methods, electrochemical depositions, sol-gel method, chemical vapor deposition, thermal decomposition, combustion methods, ultrasound, microwave-assisted combustion method, two-step mechanochemical-thermal synthesis, anodization, co-precipitation, electrophoretic deposition, and precipitation processes are some methods for producing ZnO nanoparticles ( Madathil et al., 2007 ; Moghaddam et al., 2009 ; Ghorbani et al., 2015 ).
4.3. Copper nanoparticles (CuNPs)
Copper nanoparticles (CuNPs) comprise a size range of 1–100 nm of copper-based particles ( Khan et al., 2019 ). Cu and Au metal fluorescence have long been known to exist. For excitation at 488 nm, a fluorescence peak centering on the metals’ interband absorption edge has been noted. Additionally, it was noted that the fluorescence peaked at the same energy at two distinct excitation wavelengths (457.9–514.5 and 300–400 nm), and the high-energy tail somewhat grows with increased photon energy pumping. A unique, physical, top-down EEW approach has been used to create Cu nanoparticles. The EEW method involves sending a current of *1,010 A/m2 (1,010 A/m2) across a thin Cu wire, which explodes on a Cu plate for a duration of 10–6 s ( Siwach and Sen, 2008 ).
4.4. Gold nanoparticles (AuNPs)
Gold nanoparticles(AuNPs) are nanometers made of gold. They have unique physical and chemical properties and can absorb and scatter light in the visible and near-infrared range ( Rad et al., 2011 ; Compostella et al., 2017 ).
Scientists around the turn of the 20th century discovered anisotropic AuNPs. Zsigmond ( Li et al., 2014 ) said that gold particles “are not always spherical when their size is 40 nm or lower” in his book, released in 1909. Additionally, he found anisotropic gold particles of various colors. Zsigmondy won the Nobel Prize in 1925 for “his demonstration of the heterogeneous character of colloidal solutions and the methods he utilized” and for developing the ultramicroscope, which allowed him to see the forms of Au particles. He noticed that gold frequently crystallized into a six-sided leaf shape ( Li et al., 2014 ).
AuNPs are the topic of extensive investigation due to their optical, electrical, and molecular-recognition capabilities, with numerous prospective or promised uses in a wide range of fields, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine ( Rad et al., 2011 ).
4.5. Aluminum nanoparticles (AlNPs)
Aluminum nanoparticles (AlNPs) are nanoparticles made of aluminum. Aluminum nanoparticles’ strong reactivity makes them promising for application in high-energy compositions, hydrogen generation in water processes, and the synthesis of alumina 2D and 3D structures ( Lerner et al., 2016 ).
4.6. Iron nanoparticles (FeNPs)
Iron nanoparticles(FeNPs) are particles with a size range of 1−100 nanometers ( Khan et al., 2019 ) made of iron. FeNPs have several potential applications, including their use as catalysts, drug delivery systems, sensors, and energy storage and conversion. They have also been investigated for use in photovoltaic and solar cells and water purification and environmental remediation. FeNPs can also be used in magnetic resonance imaging (MRI) as contrast agents to improve the visibility of tissues and organs. They can also be used in magnetic recording media, such as hard disk drives ( Zhuang and Gentry, 2011 ; Jamkhande et al., 2019 ).
As with any NPs, there are potential health and safety concerns associated with using FeNPs, e.g., FeNPs are used to deliver drugs to specific locations within the body, such as cancer cells and used in MRI, and used to remove contaminants from water ( Farrell et al., 2003 ; Zhuang and Gentry, 2011 ). Tables 1 , ,2 2 show the characteristics of metal-based nanoparticles and the techniques to study their characteristics, respectively.
Characteristics of metal based nanoparticles.
Different analytical techniques and their purposes in studying nanoparticles.
5. Approaches for the synthesis of metal NPs
There are mainly three types of approaches for the synthesis of NPs: the physical, chemical, and biological approaches. The physical approach is also called the top-down approach, while chemical and biological approaches are collectively called the bottom-up approach. The biological approach is also named green systems of NPs. All these approaches are further sub-categorized into various types based upon their method adopted. Figure 1 illustrates each approach’s reported methods for synthesizing NPs.

Approaches of NPs synthesis.
5.1. Top down/physical approach
Bulk materials are fragmented in top-down methods to create nano-structured materials ( Figure 2 ). They are additionally known as physical approaches ( Baig et al., 2021 ). The following techniques can achieve a top-down approach;

Difference between top-down and bottom-up approaches.
5.1.1. Mechanical milling
The mechanical milling process uses balls inside containers and may be carried out in various mills, typically planetary and shaker mills, which is an impact process with high energy ( Gorrasi and Sorrentino, 2015 ). Mechanical milling is a practical approach for creating materials at the nanoscale from bulk materials. Aluminum alloys that have been strengthened by oxide and carbide, spray coatings that are resistant to wear, nanoalloys based on aluminum, nickel, magnesium, and copper, and a variety of other nanocomposite materials may all be created mechanically. A unique class of nanoparticles known as ball-milled carbon nanomaterials has the potential to meet the needs for energy storage, energy conversion, and environmental remediation ( Yadav et al., 2012 ; Lyu et al., 2017 ).
5.1.2. Electrospinning
Typically, it is used to create nanofibers from various materials, most often polymers ( Ostermann et al., 2011 ). A technique for creating fibers called electrospinning draws charged threads from polymer melts or solutions up to fiber sizes of a few hundred nanometers ( Chronakis, 2010 ). Coaxial electrospinning was a significant advancement in the field of electrospinning. The spinneret in coaxial electrospinning is made up of two coaxial capillaries. Core-shell nanoarchitectures may be created in these capillaries using two viscous liquids, a viscous liquid as the shell and a non-viscous liquid as the core ( Du et al., 2012 ). Core-shell and hollow polymer, inorganic, organic, and hybrid materials have all been developed using this technique ( Kumar R. et al., 2013 ).
5.1.3. Laser ablation
A microfeature can be made by employing a laser beam to vaporize a single material ( Tran and Wen, 2014 ). Laser ablation synthesis produces nanoparticles by striking the target material with an intense laser beam. Due to the high intensity of the laser irradiation used in the laser ablation process, the source material or precursor vaporizes, causing the production of nanoparticles ( Amendola and Meneghetti, 2009 ). Laser ablation is an environmentally friendly for producing noble metal nanoparticles ( Baig et al., 2021 ). This method may be used to create a wide variety of nanomaterials, including metal nanoparticles, carbon nanomaterials, oxide composites, and ceramics ( Su and Chang, 2018 ; Baig et al., 2021 ).
5.1.4. Sputtering
Microparticles of a solid material are expelled off its surface during the phenomenon known as sputtering, which occurs when the solid substance is assaulted by intense plasma or gas particles ( Behrisch, 1981 ). According to the incident gaseous ion energy, energetic gaseous ions used in the sputtering deposition process physically expel tiny atom clusters off the target surface ( Muñoz-García et al., 2009 ). The sputtering method is intriguing because it is more affordable than electron-beam lithography, and the composition of the sputtered nanomaterials is similar to the target material with fewer contaminants ( Baig et al., 2021 ).
5.1.5. Electron explosion
In this technique, a thin metal wire is subjected to a high current pulse that causes an explosion, evaporation, and ionization. The metal becomes vaporized and ionized, expands, and cools by reacting with the nearby gas or liquid medium. The condensed vapor finally forms the nanoparticles ( Joh et al., 2013 ). Electron explosion method because it produces plasma from the electrical explosion of a metallic wire, which may produce nanoparticles from a Pt solution without using a reducing agent ( Joh et al., 2013 ).
5.1.6. Sonication
The most crucial step in the creation of nanofluids is sonication. After the mixture has been magnetically stirred in a magnetic stirrer, sonication is performed in an ultrasonication path, ultrasonic vibrator, and mechanical homogenizer. Sonicators have become the industry standard for Probe sonication and are noticeably more powerful and effective when compared to ultrasonic cleaner baths for nanoparticle applications. Probe sonication is highly effective for processing nanomaterials (carbon nanotubes, graphene, inks, metal oxides, etc.) ( Zheng et al., 2010 ).
5.1.7. Pulsed wire discharge method
This is the most used method for creating metal nanoparticles. A pulsating current causes a metal wire to evaporate, producing a vapor that is subsequently cooled by an ambient gas to form nanoparticles. This plan may quickly produce large amounts of energy ( Patil et al., 2021 ).
5.1.8. Arc discharge method
Two graphite rods are adjusted in a chamber with a constant helium pressure during the Arc Discharge procedure. It is crucial to fill the chamber with helium because oxygen or moisture prevents the synthesis of fullerenes. Arc discharge between the ends of the graphite rods drives the vaporization of carbon rods. Achieving new types of nanoparticles depends significantly on the circumstances in which arc discharge occurs. The creation of several nanostructured materials may be accomplished with this technique ( Berkmans et al., 2014 ). It is well-recognized for creating carbon-based materials such as fullerenes, carbon nanohorns (CNHs), carbon nanotubes ( Shi et al., 2000 ), few-layer graphene, and amorphous spherical carbon nanoparticles ( Kumar R. et al., 2013 ).
5.1.9. Lithography
Lithography typically uses a concentrated beam of light or electrons to create nanoparticles, a helpful technique ( Pimpin and Srituravanich, 2012 ). Masked and maskless lithography are the two primary categories of lithography. Without a mask, arbitrary nano-pattern printing is accomplished in maskless lithography. Additionally, it is affordable and easy to apply ( Brady et al., 2019 ).
5.2. Bottom-up approach
Tiny atoms and molecules are combined in bottom-up methods to create nano-structured particles ( Figure 2 ; Baig et al., 2021 ). These include chemical and biological approaches:
5.2.1. Chemical vapor deposition (CVD)
Through a chemical process involving vapor-phase precursors, a thin coating is created on the substrate surface during CVD ( Dikusar et al., 2009 ). Precursors are deemed appropriate for CVD if they exhibit sufficient volatility, high chemical purity, strong evaporation stability, cheap cost, a non-hazardous nature, and long shelf life. Additionally, its breakdown should not leave behind any contaminants. Vapor phase epitaxy, metal-organic CVD, atomic layer epitaxy, and plasma-enhanced CVD are only a few CVD variations. This method’s benefits include producing very pure nanoparticles that are stiff, homogeneous, and strong ( Ago, 2015 ). CVD is an excellent approach to creating high-quality nanomaterials ( Machac et al., 2020 ). It is also well-known for creating two-dimensional nanoparticles ( Baig et al., 2021 ).
5.2.2. Sol-gel process
A wet-chemical approach, called the sol-gel method, is widely utilized to create nanomaterials ( Das and Srivasatava, 2016 ; Baig et al., 2021 ). Metal alkoxides or metal precursors in solution are condensed, hydrolyzed, and thermally decomposed. The result is a stable solution or sol. The gel gains greater viscosity as a result of hydrolysis or condensation. The particle size may be seen by adjusting the precursor concentration, temperature, and pH levels. It may take a few days for the solvent to be removed, for Ostwald ripening to occur, and for the phase to change during the mature stage, which is necessary to enable the growth of solid mass. To create nanoparticles, the unstable chemical ingredients are separated. The generated material is environmentally friendly and has many additional benefits thanks to the sol-gel technique ( Patil et al., 2021 ). The uniform quality of the material generated, the low processing temperature, and the method’s ease in producing composites and complicated nanostructures are just a few of the sol-gel technique’s many advantages ( Parashar et al., 2020 ).
5.2.3. Co-precipitation
It is a solvent displacement technique and is a wet chemical procedure. Ethanol, acetone, hexane, and non-solvent polymers are examples of solvents. Polymer phases can be either synthetic or natural. By mixing the polymer solution, fast diffusion of the polymer-solvent into the non-solvent phase of the polymer results. Interfacial stress at two phases results in the formation of nanoparticles ( Das and Srivasatava, 2016 ). This method’s natural ability to produce high quantities of water-soluble nanoparticles through a straightforward process is one of its key benefits. This process is used to create many commercial iron oxide NP-based MRI contrast agents, including Feridex, Reservist, and Combidex ( Baig et al., 2021 ; Patil et al., 2021 ).
5.2.4. Inert gas condensation/molecular condensation
Metal NPs are produced using this method in large quantities. Making fine NPs using the inactive gas compression approach has been widespread, which creates NPs by causing a metallic source to disappear in an inert gas. At an attainable temperature, metals evaporate at a tolerable pace. Copper metal nanoparticles are created by vaporizing copper metal inside a container containing argon, helium, or neon. The atom quickly loses its energy by cooling the vaporized atom with an inert gas after it boils out. Liquid nitrogen is used to cool the gases, forming nanoparticles in the range of 2–100 nm ( Pérez-Tijerina et al., 2008 ; Patil et al., 2021 ).
5.2.5. Hydrothermal
In this method, for the production of nanoparticles, hydrothermal synthesis uses a wide temperature range from ambient temperature to extremely high temperatures. Comparing this strategy to physical and biological ones offers several benefits. At higher temperature ranges, the nanomaterials produced by hydrothermal synthesis could become unstable ( Banerjee et al., 2008 ; Patil et al., 2021 ).
5.2.6. Green/biological synthesis
The synthesis of diverse metal nanoparticles utilizing bioactive agents, including plant materials, microbes, and various biowastes like vegetable waste, fruit peel waste, eggshell, agricultural waste, algae, and so on, is known as “green” or “biological” nanoparticle synthesis ( Kumari et al., 2021 ). Developing dependable, sustainable green synthesis technologies is necessary to prevent the formation of undesirable or dangerous byproducts ( Figure 3 ). The green synthesis of nanoparticles also has several advantages, including being straightforward, affordable, producing NPs with high stability, requiring little time, producing non-toxic byproducts, and being readily scaled up for large-scale synthesis ( Malhotra and Alghuthaymi, 2022 ).

Schematic diagram for biosynthesis of NPs.
5.2.6.1. Biological synthesis using microorganisms
Microbes use metal capture, enzymatic reduction, and capping to create nanoparticles. Before being converted to nanoparticles by enzymes, metal ions are initially trapped on the surface or interior of microbial cells ( Ghosh et al., 2021 ). Use of microorganisms (especially marine microbes) for synthesis of metalic NPs is environmental friendly, fast and economical ( Patil and Kim, 2018 ). Several microorganisms are used in the synthesis of metal NPs, including:
Biosynthesis of NPs by bacteria: A possible biofactory for producing gold, silver, and cadmium sulfide nanoparticles is thought to be bacterial cells. It is known that bacteria may produce inorganic compounds either inside or outside of their cells ( Hulkoti and Taranath, 2014 ). Desulforibrio caledoiensis ( Qi et al., 2013 ), Enterococcu s sp. ( Rajeshkumar et al., 2014 ), Escherichia coli VM1 ( Maharani et al., 2016 ), and Ochrobactrum anhtropi ( Thomas et al., 2014 ) based metal NPs are reported previously for their potential photocatalytic properties ( Qi et al., 2013 ), antimicrobial activity ( Rajeshkumar et al., 2014 ), and anticancer activity ( Maharani et al., 2016 ).
Extracellular synthesis of NPs by bacteria: The microorganisms’ extracellular reductase enzymes shrink the silver ions to the nanoscale range. According to protein analysis of microorganisms, the NADH-dependent reductase enzyme carries out the bio-reduction of silver ions to AgNPs. The electrons for the reductase enzyme come from NADH, which is subsequently converted to NAD+. The enzyme is also oxidized simultaneously when silver ions are reduced to nanosilver. It has been noted that bio-reduction can occasionally be caused by nitrate-dependent reductase. The decline occurs within a few minutes in the quick extracellular creation of nanoparticles ( Mathew et al., 2010 ). At pH 7, the bacterium R. capsulata produced gold nanoparticles with sizes ranging from 10−20 nm. Numerous nanoplates and spherical gold nanoparticles were produced when the pH was changed to four ( Sriram et al., 2012 ). By adjusting the pH, the gold nanoparticles’ form may be changed. Gold nanoparticle shape was controlled by regulating the proton content at various pH levels. The bacteria R. capsulata ’s release cofactor NADH and NADH-dependent enzymes may cause the bioreduction of Au (3+) to Au (0) and the generation of gold nanoparticles. By using NADH-dependent reductase as an electron carrier, it is possible to start the reduction of gold ions ( Sriram et al., 2012 ).
Intracellular synthesis of NPs by bacteria: Three processes are involved in the intracellular creation of NPs: trapping, bioreduction, and capping. The cell walls of microorganisms and ions charge contribute significantly to creating NPs in the intracellular route. This entails specific ion transit in the presence of enzymes, coenzymes, and other molecules in the microbial cell. Microbes have a range of polysaccharides and proteins in their cell walls, which function as active sites for the binding of metal ions ( Slavin et al., 2017 ). Not all bacteria can produce metal and metal oxide nanoparticles. The only ions that pose a significant hazard to microorganisms are heavy metal ions, which, in response to a threat, cause the germs to react by grabbing or trapping the ions on the cell wall via electrostatic interactions. This occurs because a metal ion is drawn to the cell wall’s carboxylate groups, including cysteine and polypeptides, and certain enzymes with a negative charge ( Zhang et al., 2011 ).
Additionally, the electron transfers from NADH via NADH-dependent educates, which serves as an electron carrier and is located inside the plasma membrane, causing the trapped ions to be reduced into the elemental atom. The nuclei eventually develop into NPs and build up in the cytoplasm or the pre-plasmic space. On the other hand, the stability of NPs is provided by proteins, peptides, and amino acids found inside cells, including cysteine, tyrosine, and tryptophan ( Mohd Yusof et al., 2019 ).
Biosynthesis of NPs by fungi: Because monodisperse nanoparticles with distinct dimensions, various chemical compositions, and sizes may be produced, the biosynthesis of nanoparticles utilizing fungus is frequently employed. Due to the existence of several enzymes in their cells and the ease of handling, fungi are thought to be great candidates for producing metal and metal sulfide nanoparticles ( Mohanpuria et al., 2008 ).
The nanoparticles were created on the surface of the mycelia. After analyzing the results and noting the solution, it was determined that the Ag + ions are initially trapped on the surface of the fungal cells by an electrostatic interaction between gold ions and negatively charged carboxylate groups, which is facilitated by enzymes that are present in the mycelia’s cell wall. Later, the enzymes in the cell wall reduce the silver ions, causing the development of silver nuclei. These nuclei then increase as more Ag ions are reduced and accumulate on them.
The TEM data demonstrate the presence of some silver nanoparticles both on and inside the cytoplasmic membrane. The findings concluded that the Ag ions that permeate through the cell wall were decreased by enzymes found inside the cytoplasm and on the cytoplasmic membrane. Also possible is the diffusion of some silver nanoparticles over the cell wall and eventual cytoplasmic entrapment ( Mukherjee et al., 2001 ; Hulkoti and Taranath, 2014 ).
It was observed that the culture’s age does not affect the shape of the synthesized gold nanoparticles. However, the number of particles decreased when older cells were used. The different pH levels produce a variety of shapes of gold nanoparticles, indicating that pH plays a vital role in determining the shape. The incubation temperature also played an essential role in the accumulation of the gold nanoparticles. It was observed that the particle growth rate was faster at increased temperature levels ( Mukherjee et al., 2001 ; Ahmad et al., 2003 ). The form of the produced gold nanoparticles was shown to be unaffected by the age of the culture. However, when older cells were utilized, the particle count fell. The fact that gold nanoparticles take on various forms at different pH levels suggests that the pH is crucial in determining the shape. The incubation temperature significantly influenced the accumulation of the gold nanoparticles. It was found that higher temperatures caused the particle development rate to accelerate ( Mukherjee et al., 2001 ; Ahmad et al., 2003 ). Verticillium luteoalbum is reported to synthesize gold nanoparticles of 20–40 nm in size ( Erasmus et al., 2014 ). Aspergillus terreus and Penicillium brevicompactum KCCM 60390 based metal NPs are reported for their antimicrobial ( Li G. et al., 2011 ) and cytotoxic activities ( Mishra et al., 2011 ), respectively.
Biosynthesis of NPs using actinomycetes: Actinomycetes have been categorized as prokaryotes since they share significant traits with fungi. They are sometimes referred to as ray fungi ( Mathew et al., 2010 ). Making NPs from actinomycetes is the same as that of fungi ( Sowani et al., 2016 ). Thermomonospora sp., a new species of extremophilic actinomycete, was discovered to produce extracellular, monodispersed, spherical gold nanoparticles with an average size of 8 nm ( Narayanan and Sakthivel, 2010 ). Metal NPs synthesized by Rhodococcus sp. ( Ahmad et al., 2003 ) and Streptomyces sp. Al-Dhabi-87 ( Al-Dhabi et al., 2018 ) are reported for their antimicrobial activities.
Biosynthesis of NPs using algae: Algae have a high concentration of polymeric molecules, and by reducing them, they may hyper-accumulate heavy metal ions and transform them into malleable forms. Algal extracts typically contain pigments, carbohydrates, proteins, minerals, polyunsaturated fatty acids, and other bioactive compounds like antioxidants that are used as stabilizing/capping and reducing agents ( Khanna et al., 2019 ). NPs also have a faster rate of photosynthesis than their biosynthetic counterparts. Live or dead algae are used as model organisms for the environmentally friendly manufacturing process of bio-nanomaterials, such as metallic NPs ( Hasan, 2015 ). Ag and Au are the most extensively researched noble metals to synthesized NPs by algae either intracellularly or extracellularly ( Dahoumane et al., 2017 ). Chlorella vulgaris ( Luangpipat et al., 2011 ), Chlorella pyrenoidosa ( Eroglu et al., 2013 ), Nanochloropsis oculata ( Xia et al., 2013 ), Scenedesmus sp. IMMTCC-25 ( Jena et al., 2014 ) based metal NPs are reported for their potential catalytic ( Luangpipat et al., 2011 ; Eroglu et al., 2013 ) and, antimicrobial ( Eroglu et al., 2013 ; Jena et al., 2014 ) activities along with their use in Li-Ion batteries ( Xia et al., 2013 ).
Intracellular synthesis of NPs using algae: In order to create intracellular NPs, algal biomass must first be gathered and thoroughly cleaned with distilled water. After that, the biomass (living algae) is treated with metallic solutions like AgNO3. The combination is then incubated at a specified pH and a specific temperature for a predetermined time. Finally, it is centrifuged and sonicated to produce the extracted stable NPs ( Uzair et al., 2020 ).
Extracellular synthesis of NPs using algae: Algal biomass is first collected and cleaned with distilled water before being used to synthesize NPs extracellularly ( Uzair et al., 2020 ). The following three techniques are frequently utilized for the subsequent procedure:
(i) A particular amount of time is spent drying the algal biomass (dead algae), after which the dried powder is treated with distilled water and filtered.
(ii) The algal biomass is sonicated with distilled water to get a cell-free extract.
(iii) The resultant product is filtered after the algal biomass has been rinsed with distilled water and incubated for a few hours (8–16 h).
5.2.6.2. Biological synthesis using plant extracts
The substance or active ingredient of the desired quality extracted from plant tissue by treatment for a particular purpose is a plant extract ( Jadoun et al., 2021 ). Plant extracts are combined with a metal salt solution at room temperature to create nanoparticles. Within minutes, the response is finished. This method has been used to create nanoparticles of silver, gold, and many other metals ( Li X. et al., 2011 ). Nanoparticles are biosynthesized using a variety of plants. It is known that the kind of plant extract, its concentration, the concentration of the metal salt, the pH, temperature, and the length of contact time all have an impact on how quickly nanoparticles are produced as well as their number and other properties ( Mittal and Chisti, 2013 ). A leaf extract from Polyalthia longifolia was used to create silver nanoparticles, the average particle size was around 58 nm ( Kumar and Yadav, 2009 ; Kumar et al., 2016 ).
Acacia auriculiformis ( Saini et al., 2016 ), Anisomeles indica ( Govindarajan et al., 2016 ), Azadirachta indica ( Velusamy et al., 2015 ), Bergenia ciliate ( Phull et al., 2016 ), Clitoria ternatea , Solanum nigrum ( Krithiga et al., 2013 ), Coffea arabica ( Dhand et al., 2016 ), Coleus forskohlii ( Naraginti et al., 2016 ), Curculigo orchioides ( Kayalvizhi et al., 2016 ), Digitaria radicosa ( Kalaiyarasu et al., 2016 ), Dioscorea alata ( Pugazhendhi et al., 2016 ), Diospyros paniculata ( Rao et al., 2016 ), Elephantopus scaber ( Kharat and Mendhulkar, 2016 ), Emblica officinalis ( Ramesh et al., 2015 ), Euphorbia antiquorum L. ( Rajkuberan et al., 2017 ), Ficus benghalensis ( Nayak et al., 2016 ), Lantana camara ( Ajitha et al., 2015 ), Cinnamomum zeylanicum ( Soni and Sonam, 2014 ), and Parkia roxburghii ( Paul et al., 2016 ) are the few examples of plants which are reported for the green synthesis of metal NPs (i.e., AgNPs). These were evaluated for their antifilaria activity ( Saini et al., 2016 ), mosquitocidal activity ( Govindarajan et al., 2016 ), antibacterial activity ( Velusamy et al., 2015 ), catalytic activity ( Edison et al., 2016 ), antioxidant activity ( Phull et al., 2016 ), and Cytotoxicity ( Patil et al., 2017 ).
5.2.6.3. Biological synthesis using biomimetic
“Biomimetic synthesis” typically refers to chemical processes that resemble biological synthesis carried out by living things ( Dahoumane et al., 2017 ). In the biomimetic approach, proteins, enzymes, cells, viruses, pollen, and waste biomass are used to synthesize NPs. Two categories are used to classify biomimetic synthesis:
Functional biomimetic synthesis uses various materials and approaches to emulate particular characteristics of natural materials, structures, and systems ( Zan and Wu, 2016 ).
Process biomimetic synthesis is a technique that aims to create different desirable nanomaterials/structures by imitating the synthesis pathways, processes, or procedures of natural chemicals and materials/structures. For instance, several distinctive nano-superstructures (such as satellite structures, dendrimer-like structures, pyramids, cubes, 2D nanoparticle arrays, 3D AuNP tubes, etc.) have been put together in vitro by simulating the protein manufacturing process ( Zan and Wu, 2016 ).
6. Applications of NPs
6.1. applications of nps in environment industry.
Due to their tiny size and distinctive physical and chemical characteristics, NPs appeal to various environmental applications. The properties of nanoparticals and their advantages are illustrated in Figure 4 . The following are some possible NP uses in the environment.

Properties of nanoparticals and their advantages.
6.1.1. Bioremediation
Nanoparticles (NPs) can remove environmental pollutants, such as heavy metals from water or organic contaminants from soil ( Zhuang and Gentry, 2011 ). For example, silver nanoparticles (AgNPs) effectively degrade certain pollutants, such as organic dyes and compounds found in wastewater. Several nanomaterials have been considered for remediation purposes, such as nanoscale zeolites, metal oxides, and carbon nanotubes and fibers ( Zhuang and Gentry, 2011 ). Nanoscale particles used in remediation can access areas that larger particles cannot. They can be coated to facilitate transport and prevent reaction with surrounding soil matrices before reacting with contaminants. One widely used nanomaterial for remediation is Nanoscale zerovalent iron (nZVI). It has been used at several hazardous waste sites to clean up chlorinated solvents that have contaminated groundwater ( Elliott et al., 2013 ). Removing heavy metals such as mercury, lead, thallium, cadmium, and arsenic from natural water has attracted considerable attention because of their adverse effects on environmental and human health. Superparamagnetic iron oxide NPs are an effective sorbent material for this toxic soft material. So, no measurements of engineered NPs in the environment have been available due to the absence of analytical methods able to quantify the trace concentration of NPs ( Elliott et al., 2013 ).
6.1.2. Sensors in environment
Nanotechnology/NPs are already being used to improve water quality and assist in environmental clean-up activities ( Pradeep, 2009 ). Their potential use as environmental sensors to monitor pollutants is also becoming viable NPs can be used as sensors to detect the presence of certain compounds in the environment, such as heavy metals or pollutants. The nano-sensors small size and wide detection range provide great flexibility in practical applications. It has been reported that nanoscale sensors can be used to detect microbial pathogens and biological compounds, such as toxins, in aqueous environments ( Yadav et al., 2010 ). NPS can be designed to selectively bind to specific types of pollutants, allowing them to be detected at low concentrations. For example, gold nanoparticles (AuNPs) have been used as sensors for the detection of mercury in water ( Theron et al., 2010 ).
6.1.3. Catalysts in environment
Nanoparticles (NPs) are used as catalysts in chemical reactions, such as in the production of biofuels or environmental remediation processes, and to catalyze biomass conversion into fuels, such as ethanol or biodiesel. For example, platinum nanoparticles (PtNPs) have been explored for use in the production of biofuels due to their ability to catalyze the conversion of biomass into fuels ( Lam and Luong, 2014 ). PtNPs also showed promising sensing properties; for example, Using Pt NPs, the Hg ions were quantified in the range of 50–500 nM in MilliQ, tap, and groundwater samples, and the limit of quantifications for Hg ions were 16.9, 26, and 47.3 nM. The biogenic PtNPs-based probe proved to be applicable for detecting and quantifying Hg ions ( Kora and Rastogi, 2018 ).
Overall, NPs have significant potential for use in the environment and are being actively researched for a variety of applications.
6.2. Applications of NPs in medicine industry
Nanoparticles (NPs) have unique physical and chemical properties due to their small size, making them attractive for use in various applications, including the medicine industry. Some potential applications of NPs in medicine include:
6.2.1. Drug delivery
Technological interest has been given to AuNPs due to their unique optical properties, ease of synthesis, and chemical stability. The particles can be used in biomedical applications such as cancer treatment ( Sun et al., 2014 ), biological imaging ( Abdulle and Chow, 2019 ), chemical sensing, and drug delivery. Sun et al. (2014) mentioned in detail about two different methods of controlled release of drugs associated with NPs, which were (1) sustained (i.e., diffusion-controlled and erosion-controlled) and (2) stimuli-responsive (i.e., pH-sensitive, enzyme-sensitive, thermoresponsive, and photosensitive). Figure 5 illustrates that how NPs acts as targeted delivery of medicines to treat cancer cells ( Figure 5A ) and therapeutic gene delivery to synthesis proteins of interests in targeted cells ( Figure 5B ). NPs can deliver drugs to specific body areas, allowing for more targeted and effective treatment ( Siddique and Chow, 2020 ). For example AgNPs have been explored for use in drug delivery due to their stability and ability to accumulate in certain types of cancerous tumors ( Siddique and Chow, 2020 ). ZnONPs have also been explored for drug delivery due to their ability to selectively target cancer cells ( Anjum et al., 2021 ). CuNPs have been shown to have antimicrobial properties and are being explored for drug delivery to treat bacterial infections ( Yuan et al., 2018 ). AuNPs have unique optical, electrical, and catalytic properties and are being explored for drug delivery due to their ability to accumulate in certain cancerous tumors. Silver NPs (AgNPs) have been incorporated into wound dressings, bone cement, and implants ( Schröfel et al., 2014 ).

Application of nanoparticles as; targated drug delivery (A) , and therapeutic protein generation in targated cells (B) .
6.2.2. Diagnostics
Nanoparticles (NPs) can be used as imaging agents to help visualize specific body areas. For example, iron oxide nanoparticles (Fe 3 O 4 NPs) have been used as magnetic resonance imaging (MRI) contrast agents to help visualize tissues and organs ( Nguyen et al., 2013 ). AuNPs have unique optical, electrical, and catalytic properties and are being explored for diagnostics due to their ability to accumulate in certain cancerous tumors ( Siddique and Chow, 2020 ).
6.2.3. Tissue engineering
Nanoparticles (NPs) can help stimulate the growth and repair of tissues and organs. For example, titanium dioxide nanoparticles (TiO2 NPs) have been explored for tissue engineering due to their ability to stimulate the growth of bone cells ( Kim et al., 2014 ).
6.2.4. Antimicrobials
Some NPs, such as silver nanoparticles (AgNPs) and copper nanoparticles (CuNPs), have strong antimicrobial properties and are being explored for use in a variety of medical products, such as wound dressings and medical devices ( Hoseinzadeh et al., 2017 ).
Overall, NPs have significant potential for use in the medical industry and are being actively researched for various applications. However, it is essential to carefully consider the potential risks and benefits of using NPs in medicine and ensure their safe and responsible use.
6.3. Applications of NPs in agriculture industry
There are several ways in which nanoparticles (NPs) have the potential to alter the agricultural sector. NPs may be used in agriculture for a variety of reasons, including:
6.3.1. Pesticides and herbicides
Nanoparticles (NPs) can be used to deliver pesticides and herbicides in a targeted manner, reducing the number of chemicals needed and minimizing the potential for environmental contamination ( Khan et al., 2019 ). AgNPs and CuNPs have antimicrobial properties, making them potentially useful for controlling pests and diseases in crops. They can also be used as delivery systems for active ingredients, allowing for more targeted application and reducing the potential for environmental contamination ( Hoseinzadeh et al., 2017 ; Dangi and Verma, 2021 ).
It is important to note that using metal NPs in pesticides and herbicides is still in the early stages of development. More research is needed to understand their potential impacts on human health and the environment ( Dangi and Verma, 2021 ).
6.3.2. Fertilizers and plant growth
Nano fertilizers offer an opportunity for efficiently improving plant mineral nutrition. Some studies have shown that nanomaterials can be more effective than conventional fertilizers, with a controlled release of nutrients increasing the efficiency of plant uptake and potentially reducing adverse environmental outcomes associated with the loss of nutrients in the broader environment. However, other studies have found that nanomaterial has the same or even less effective effectiveness than conventional fertilizers. NPs used to deliver fertilizers to plants more efficiently, reducing the amount of fertilizer needed, and reducing the risk of nutrient runoff ( Kopittke et al., 2019 ).
Ag ( Jaskulski et al., 2022 ), Zn ( Song and Kim, 2020 ), Cu, Au, Al, and Fe ( Kopittke et al., 2019 ) based NPs have been shown to have fertilizing properties and plant growth-promoting properties, and may help provide essential nutrients to plants and improve plant growth and yield. It is important to note that the use of NPs in fertilizers is still in the early stages of development. More research is needed to understand their potential impacts on human health and the environment.
6.3.3. Food safety
Nanoparticles (NPs) can detect and eliminate pathogens in food products, improving food safety, and reducing the risk of foodborne illness ( Zhuang and Gentry, 2011 ).
6.3.4. Water purification
Nanoparticles (NPs) can purify irrigation water, reducing the risk of crop contamination and improving crop yield ( Zhuang and Gentry, 2011 ). Using NPs in agriculture can improve crop yields, reduce agriculture’s environmental impact, and improve food products’ safety and quality.
6.4. Applications of NPs in food industry
Numerous applications for nanoparticles (NPs) in the food sector are possible, including:
6.4.1. Food processing and food preservation/food packaging
Nanoparticles (NPs) can be used to improve the efficiency and performance of food processing operations, such as grinding, mixing, and drying, e.g., AgNPs have been used as a natural antimicrobial agent in food processing operations, helping to prevent the growth of bacteria and other microorganisms ( Dangi and Verma, 2021 ) and also NPs are used to enhance the performance of materials used in food packaging, making them more resistant to pollutants like moisture and gases.
6.4.2. Food fortification
Nanoparticles (NPs) can deliver essential nutrients to food products, such as vitamins and minerals, more efficiently and effectively. e.g., Fe 2 O 3 , and CuNPs have been used to fortify food products with iron, and Cu is an essential nutrient necessary for the metabolism of iron and other nutrients. Iron is an essential nutrient often lacking in many people’s diets, particularly in developing countries ( Kopittke et al., 2019 ).
6.4.3. Sensors
Nanoparticles (NPs) used to improve the sensitivity and specificity of food sensors, allowing them to detect a broader range of substances or signals ( Yadav et al., 2010 ).
Overall, using NPs in the food industry can improve the performance, safety, and nutritional value of a wide range of food products and processes.
6.5. Applications of NPs in electronics industry and automotive industry
In many aspects, nanoparticles (NPs) can transform the electronics sector. NPs may be used in a variety of electrical applications, such as:
6.5.1. Display technologies/storage devices
Nanoparticles (NPs) can be used to improve the performance of displays ( Park and Choi, 2019 ; Bahadur et al., 2021 ; Triana et al., 2022 ), such as LCD and OLED displays, by enhancing the brightness, color, and contrast of the image, such as silver NPs and gold NPs, have been explored for use in LCD and OLED displays as a means of improving the conductivity of the display ( Gwynne, 2020 ). NPs improve the performance and durability of energy storage devices, such as batteries and supercapacitors, by increasing energy density and charging speed. Zinc oxide nanoparticles (ZnO NPs) have the potential to be used in energy storage devices, such as batteries and supercapacitors, due to their ability to store and release energy ( Singh et al., 2011 ).
6.5.2. Data storage
Nanoparticles (NPs) can improve the capacity and speed of data storage devices, such as hard drives and flash drives. Magnetic NPs, such as iron oxide NPs, have been explored for use in data storage devices, such as hard drives, due to their ability to store, and retrieve data using magnetism. These NPs are often composed of a magnetic metal, such as iron, cobalt, or nickel. They can be magnetized and demagnetized, allowing them to store and retrieve data ( Ahmad et al., 2021 ).
Overall, the use of NPs in electronics has the potential to improve the performance and efficiency of a wide range of electronic devices and systems.
Applications of NPs in chemical industry: The chemical industry might be entirely transformed by nanoparticles (NPs) in various ways. The following are potential uses for NPs in the chemical industry ( Salem and Fouda, 2021 ).
6.5.3. Chemical processing/catalysis
Nanoparticles (NPs) can be used as catalysts in chemical reactions, allowing them to be carried out more efficiently and at lower temperatures. Some examples of metal NPs that have been used as catalysts in the chemical industry include: PtNPs have been used as catalysts in a variety of chemical reactions, including fuel cell reactions ( Bhavani et al., 2021 ), hydrogenation reactions, and oxidation reactions ( Lara and Philippot, 2014 ), PdNPs have been used as catalysts in a variety of chemical reactions, including hydrogenation reactions and cross-coupling reactions ( Pérez-Lorenzo, 2012 ), FeNPs have been used as catalysts in a variety of chemical reactions, including hydrolysis reactions ( Jiang and Xu, 2011 ), and oxygen reduction reactions, NiNPs have been used as catalysts in a variety of chemical reactions, including hydrogenation reactions, and hydrolysis reactions ( Salem and Fouda, 2021 ).
6.5.4. Separation and purification
NPs are used to separate and purify chemicals and other substances, such as gases and liquids, by exploiting their size-based properties ( Hollamby et al., 2010 ). Several types of metal nanoparticles (NPs) have been explored for use in separation and purification processes in the chemical industry, including Fe 2 O 3 NPs have been used to separate and purify gases, liquids, and chemicals. They have also been used to remove contaminants from water ( Pradeep, 2009 ; Siddique and Chow, 2020 ). AgNPs have been used to purify water and remove contaminants ( Pradeep, 2009 ), such as bacteria and viruses. They have also been used to remove heavy metals from water and other substances ( Zhuang and Gentry, 2011 ). AuNPs have been used to purify water and remove contaminants, such as bacteria and viruses ( Siddique and Chow, 2020 ). They have also been used to separate and purify gases and liquids ( Zhuang and Gentry, 2011 ). AlNPs have been used to remove contaminants from water and other substances, such as oils and fuels. They have also been used to purify gases ( Zhuang and Gentry, 2011 ).
6.6. Applications of NPs in defense industry
Nanoparticles (NPs) can be used to improve the efficiency and performance of chemical processing operations, such as refining and synthesizing chemicals ( Schröfel et al., 2014 ). Nanoparticles (NPs) have the potential to be used in the defense industry in several ways, including:
6.6.1. Sensors
Nanoparticles (NPs) can improve the sensitivity and specificity of sensors used in defense systems, such as sensors for detecting chemical, biological, or radiological threats ( Zheng et al., 2010 ).
6.6.2. Protective coatings
Nanoparticles (NPs) can improve the performance and durability of protective coatings applied to defense equipment, such as coatings resistant to chemical or biological agents. For example, metal NPs can improve the mechanical properties and durability of the coating, making it more resistant to wear and corrosion. For example, adding Al or Zn based NPs to a polymer coating can improve its corrosion resistance. In contrast, adding Ni or Cr-based NPs can improve their wear resistance ( Rangel-Olivares et al., 2021 ).
6.6.3. Weapons
Nanoparticles (NPs) are used as weapons against viruses, bacteria, etc, ( Ye et al., 2020 ) and as well as in the development of armor and protective materials. There have been some reports of the potential use of NPs in military and defense applications, such as in the development of armor and protective materials. For example, adding nanoparticles, such as ceramic or metal NPs, to polymers or other materials can improve their mechanical properties and make them more resistant to damage. In addition, there have been reports of the use of NPs in developing sensors and detection systems for defense purposes.
6.6.4. Manufacturing
Nanoparticles (NPs) can improve the performance and durability of materials used in defense equipment, such as armor or structural materials. Metal NPs can be used in materials by adding them as a filler or reinforcement in polymers. For example, the addition of metal NPs such as aluminum (Al), copper (Cu), or nickel (Ni) to polymers can improve the mechanical properties, thermal stability, and electrical conductivity of the resulting composite material ( Khan et al., 2019 ).
Metal NPs can also make functional materials, such as catalysts and sensors. For example, metal NPs, such as gold (Au), and platinum (Pt), can be used as catalysts in various chemical reactions due to their high surface area and ability to adsorb reactants ( Zheng et al., 2010 ).
6.6.5. Energy storage
Nanoparticles (NPs) can improve the performance and efficiency of energy storage systems used in defense systems, such as batteries or fuel cells ( Morsi et al., 2022 ). In batteries, nanoparticles can be used as a cathode material to increase the battery’s energy density, rate capability, and cycling stability. For example, lithium cobalt oxide (LiCoO 2 ) nanoparticles have been used as cathode materials in lithium-ion batteries due to their high capacity and good rate performance. In addition, nanoparticles of transition metal oxides, such as iron oxide (Fe 2 O 3 ), and manganese oxide (MnO 2 ), have been used as cathode materials in rechargeable lithium batteries due to their high capacity and good rate performance. In supercapacitors, nanoparticles can be used as the active material in the electrodes to increase the specific surface area, leading to an increase in the device’s capacitance ( Morsi et al., 2022 ). Using NPs in the defense industry can improve defense systems’ performance, efficiency, and safety.
7. Future perspectives
Metal nanoparticles (NPs) have many potential applications in various fields, including electronics, energy storage, catalysis, and medicine. However, there are also several challenges and potential future directions for developing and using metal NPs.
One major challenge is synthesizing and processing metal NPs with precise size and shape control. Many methods for synthesizing metal NPs involve high temperatures and harsh chemical conditions, which can be challenging to scale up for large-scale production. In addition, the size and shape of metal NPs can significantly impact their properties and potential applications, so it is essential to synthesize NPs with precise size and shape control.
Another challenge is the environmental impact of metal NPs. Some metal NPs, such as silver NPs, can be toxic to aquatic life and may have other environmental impacts. There is a need for more research on the environmental effects of metal NPs and the development of more environmentally friendly (Green) synthesis and processing methods.
In terms of future directions, one promising area is the use of metal NPs for energy storage, conversion, and protection of the environment. For example, metal NPs could be used to improve batteries’ performance or develop more efficient solar cells. In addition, metal NPs could be used in catalysis to improve the efficiency of chemical reactions. There is also ongoing research on metal NPs in medicine, including drug delivery and cancer therapy.
Author contributions
KAA: conceptualization, methodology, validation, formal analysis, investigation, writing – original draft, writing – review and editing, and visualization.
Acknowledgments
The author thanks Prof. Dr. Mona M. Sobhy, Department of Reproductive Diseases, Animal Reproduction Research Institute, ARC, Giza, Egypt, and Dr. Omar Hewedy, University of Guelph, Canada, for the critical reading of the manuscript.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
- Abdulle A., Chow J. C. (2019). Contrast enhancement for portal imaging in nanoparticle-enhanced radiotherapy: A Monte Carlo phantom evaluation using flattening-filter-free photon beams. Nanomaterials 9 : 920 . 10.3390/nano9070920 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ago H. (2015). “ CVD growth of high-quality single-layer graphene ,” in Frontiers of Graphene and Carbon Nanotubes , Ed. Matsumoto K. (Berlin: Springer; ), 3–20. 10.1007/978-4-431-55372-4_1 [ CrossRef ] [ Google Scholar ]
- Ahmad A., Alsaad A., Al-Bataineh Q. M., Al-Akhras M.-A. H., Albataineh Z., Alizzy K. A., et al. (2021). Synthesis and characterization of ZnO NPs-doped PMMA-BDK-MR polymer-coated thin films with UV curing for optical data storage applications. Polymer Bull. 78 1189–1211. 10.1007/s00289-020-03155-x [ CrossRef ] [ Google Scholar ]
- Ahmad A., Senapati S., Khan M. I., Kumar R., Ramani R., Srinivas V., et al. (2003). Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete. Rhodococcus species. Nanotechnology 14 : 824 . 10.1088/0957-4484/14/7/323 [ CrossRef ] [ Google Scholar ]
- Ahmad T., Wani I. A., Ahmed J., Al-Hartomy O. A. (2014). Effect of gold ion concentration on size and properties of gold nanoparticles in TritonX-100 based inverse microemulsions. Appl. Nanosci. 4 491–498. 10.1007/s13204-013-0224-y [ CrossRef ] [ Google Scholar ]
- Ajitha B., Reddy Y. A. K., Reddy P. S. (2015). Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Mater. Sci. Eng. C 49 373–381. 10.1016/j.msec.2015.01.035 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Al-Dhabi N. A., Mohammed Ghilan A.-K., Arasu M. V. (2018). Characterization of silver nanomaterials derived from marine Streptomyces sp. al-dhabi-87 and its in vitro application against multidrug resistant and extended-spectrum beta-lactamase clinical pathogens. Nanomaterials 8 : 279 . 10.3390/nano8050279 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Amendola V., Meneghetti M. (2009). Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 11 3805–3821. 10.1039/b900654k [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Anjum S., Hashim M., Malik S. A., Khan M., Lorenzo J. M., Abbasi B. H., et al. (2021). Recent advances in zinc oxide nanoparticles (Zno nps) for cancer diagnosis, target drug delivery, and treatment. Cancers 13 : 4570 . 10.3390/cancers13184570 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Astefanei A., Núñez O., Galceran M. T. (2015). Characterisation and determination of fullerenes: a critical review. Anal. Chim. Acta 882 1–21. [ PubMed ] [ Google Scholar ]
- Bahadur P. S., Jaiswal S., Srivastava R., Kumar A. (2021). “ Advanced application of nanotechnology in engineering ,” in Proceedings of the 2021 International Conference on Technological Advancements and Innovations (ICTAI) , (Piscataway, NJ: IEEE; ), 92–95. [ Google Scholar ]
- Baig N., Kammakakam I., Falath W. (2021). Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2 1821–1871. [ Google Scholar ]
- Banerjee A., Krishna R., Das B. (2008). Size controlled deposition of Cu and Si nano-clusters by an ultra-high vacuum sputtering gas aggregation technique. Appl. Phys. A 90 299–303. [ Google Scholar ]
- Bayda S., Adeel M., Tuccinardi T., Cordani M., Rizzolio F. (2019). The history of nanoscience and nanotechnology: from chemical–physical applications to nanomedicine. Molecules 25 : 112 . 10.3390/molecules25010112 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Behrisch R. (1981). Sputtering by Particle Bombardment Springer Verlag. Berlin-Heidelberg: Springer. [ Google Scholar ]
- Berkmans A. J., Jagannatham M., Priyanka S., Haridoss P. (2014). Synthesis of branched, nano channeled, ultrafine and nano carbon tubes from PET wastes using the arc discharge method. Waste Manag. 34 2139–2145. 10.1016/j.wasman.2014.07.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Beyene H. D., Werkneh A. A., Bezabh H. K., Ambaye T. G. (2017). Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. Sustain. Mater. Technol. 13 18–23. [ Google Scholar ]
- Bhattacharjee S. (2016). DLS and zeta potential–what they are and what they are not? J. Control. Release 235 337–351. [ PubMed ] [ Google Scholar ]
- Bhavani K. S., Anusha T., Brahman P. K. (2021). Platinum nanoparticles decorated on graphitic carbon nitride-ZIF-67 composite support: An electrocatalyst for the oxidation of butanol in fuel cell applications. Int. J. Hydr. Energy 46 9199–9214. [ Google Scholar ]
- Biju V., Itoh T., Anas A., Sujith A., Ishikawa M. (2008). Semiconductor quantum dots and metal nanoparticles: syntheses, optical properties, and biological applications. Anal. Bioanal. Chem. 391 2469–2495. [ PubMed ] [ Google Scholar ]
- Brady B., Wang P. H., Steenhoff V., Brolo A. G. (2019). “ Nanostructuring solar cells using metallic nanoparticles ,” in Metal Nanostructures for Photonics , eds Kassab L. R. P., De Araujo C. B. (Amsterdam: Elsevier; ), 197–221. [ Google Scholar ]
- Cadene A., Durand-Vidal S., Turq P., Brendle J. (2005). Study of individual Na-montmorillonite particles size, morphology, and apparent charge. J. Colloid Interf. Sci. 285 719–730. 10.1016/j.jcis.2004.12.016 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chen J., Zhu X. (2016). Magnetic solid phase extraction using ionic liquid-coated core–shell magnetic nanoparticles followed by high-performance liquid chromatography for determination of Rhodamine B in food samples. Food Chem. 200 10–15. 10.1016/j.foodchem.2016.01.002 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chen J., Guo Y., Zhang X., Liu J., Gong P., Su Z., et al. (2023). Emerging nanoparticles in food: sources, application, and safety. J. Agricult. Food Chem. 71 3564–3582. [ PubMed ] [ Google Scholar ]
- Chen J., Wei S., Xie H. (2021). “ A brief introduction of carbon nanotubes: history, synthesis, and properties ,” in Proceedings of the Journal of Physics: Conference Series , (United Kingdom: IOP Publishing; ), 012184. 10.1088/1742-6596/1948/1/012184 [ CrossRef ] [ Google Scholar ]
- Chen J.-C., Tang C.-T. (2007). Preparation and application of granular ZnO/Al2O3 catalyst for the removal of hazardous trichloroethylene. J. Hazardous Mater. 142 88–96. 10.1016/j.jhazmat.2006.07.061 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chronakis I. S. (2010). Micro-/nano-fibers by electrospinning technology: processing, properties and applications. Micromanufact. Eng. Technol. 2010 264–286. 10.1016/B978-0-8155-1545-6.00016-8 [ CrossRef ] [ Google Scholar ]
- Compostella F., Pitirollo O., Silvestri A., Polito L. (2017). Glyco-gold nanoparticles: synthesis and applications. Beilstein J. Org. Chem. 13 1008–1021. 10.3762/bjoc.13.100 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dahoumane S. A., Mechouet M., Wijesekera K., Filipe C. D., Sicard C., Bazylinski D. A., et al. (2017). Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology–a review. Green Chem. 19 552–587. 10.1039/C6GC02346K [ CrossRef ] [ Google Scholar ]
- Dangi K., Verma A. K. (2021). Efficient & eco-friendly smart nano-pesticides: Emerging prospects for agriculture. Mater. Today Proc. 45 3819–3824. [ Google Scholar ]
- Das S., Srivasatava V. C. (2016). Synthesis and characterization of ZnO–MgO nanocomposite by co-precipitation method. Smart Sci. 4 190–195. [ Google Scholar ]
- De La Calle I., Menta M., Klein M., Séby F. (2018). Study of the presence of micro-and nanoparticles in drinks and foods by multiple analytical techniques. Food Chem. 266 133–145. 10.1016/j.foodchem.2018.05.107 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Delvallée A., Feltin N., Ducourtieux S., Trabelsi M., Hochepied J. (2015). Direct comparison of AFM and SEM measurements on the same set of nanoparticles. Measur. Sci. Technol. 26 : 085601 . [ Google Scholar ]
- Dhand V., Soumya L., Bharadwaj S., Chakra S., Bhatt D., Sreedhar B. (2016). Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater. Sci. Eng. C 58 36–43. 10.1016/j.msec.2015.08.018 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dikusar A., Globa P., Belevskii S., Sidel’nikova S. (2009). On limiting rate of dimensional electrodeposition at meso-and nanomaterial manufacturing by template synthesis. Surf. Eng. Appl. Electrochem. 45 171–179. [ Google Scholar ]
- Dragovic R. A., Gardiner C., Brooks A. S., Tannetta D. S., Ferguson D. J., Hole P., et al. (2011). Sizing and phenotyping of cellular vesicles using nanoparticle tracking analysis. Nanomedicine 7 780–788. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Dreaden E. C., Alkilany A. M., Huang X., Murphy C. J., El-Sayed M. A. (2012). The golden age: gold nanoparticles for biomedicine. Chem. Soc. Rev. 41 2740–2779. 10.1039/c1cs15237h [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Du P., Song L., Xiong J., Li N., Xi Z., Wang L., et al. (2012). Coaxial electrospun TiO2/ZnO core–sheath nanofibers film: Novel structure for photoanode of dye-sensitized solar cells. Electrochim. Acta 78 392–397. [ Google Scholar ]
- Edison T. N. J. I., Lee Y. R., Sethuraman M. G. (2016). Green synthesis of silver nanoparticles using Terminalia cuneata and its catalytic action in reduction of direct yellow-12 dye. Pectrochimica Acta Part A 161 122–129. 10.1016/j.saa.2016.02.044 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Elliott J. A., Shibuta Y., Amara H., Bichara C., Neyts E. C. (2013). Atomistic modelling of CVD synthesis of carbon nanotubes and graphene. Nanoscale 5 6662–6676. 10.1039/c3nr01925j [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Erasmus M., Cason E. D., Van Marwijk J., Botes E., Gericke M., Van Heerden E. (2014). Gold nanoparticle synthesis using the thermophilic bacterium Thermus scotoductus SA-01 and the purification and characterization of its unusual gold reducing protein. Gold Bull. 47 245–253. [ Google Scholar ]
- Eroglu E., Chen X., Bradshaw M., Agarwal V., Zou J., Stewart S. G., et al. (2013). Biogenic production of palladium nanocrystals using microalgae and their immobilization on chitosan nanofibers for catalytic applications. RSC Adv. 3 1009–1012. [ Google Scholar ]
- Essajai R., Benhouria Y., Rachadi A., Qjani M., Mzerd A., Hassanain N. (2019). Shape-dependent structural and magnetic properties of Fe nanoparticles studied through simulation methods. RSC Adv. 9 22057–22063. 10.1039/c9ra03047f [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Falke S., Betzel C. (2019). “ Dynamic light scattering (DLS) ,” in Radiation in Bioanalysis , eds Pereira A. S., Tavares P., Limão-Vieira P. (Berlin: Springer; ), 173–193. [ Google Scholar ]
- Farrell D., Majetich S. A., Wilcoxon J. P. (2003). Preparation and characterization of monodisperse Fe nanoparticles. J. Phys. Chem. B 107 11022–11030. [ Google Scholar ]
- Feng L., Xuan Z., Ma J., Chen J., Cui D., Su C., et al. (2015). Preparation of gold nanorods with different aspect ratio and the optical response to solution refractive index. J. Exp. Nanosci. 10 258–267. [ Google Scholar ]
- Ghorbani H. R., Mehr F. P., Pazoki H., Rahmani B. M. (2015). Synthesis of ZnO nanoparticles by precipitation method. Orient. J. Chem. 31 1219–1221. [ Google Scholar ]
- Ghosh S., Ahmad R., Zeyaullah M., Khare S. K. (2021). Microbial nano-factories: synthesis and biomedical applications. Front. Chem. 9 : 194 . 10.3389/fchem.2021.626834 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Giljohann D. A., Seferos D. S., Daniel W. L., Massich M. D., Patel P. C., Mirkin C. A. (2020). Gold nanoparticles for biology and medicine. Spherical Nucleic Acids 49 3280–3294. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Giurlani W., Innocenti M., Lavacchi A. (2018). X-ray microanalysis of precious metal thin films: thickness and composition determination. Coatings 8 : 84 . [ Google Scholar ]
- Gloria E. C., Ederley V., Gladis M., César H., Jaime O., Oscar A., et al. (2017). “ Synthesis of silver nanoparticles (AgNPs) with antibacterial activity ,” in Proceedings of the Journal of Physics: Conference Series , (United Kingdom: IOP Publishing; ), 012023. [ Google Scholar ]
- Gorrasi G., Sorrentino A. (2015). Mechanical milling as a technology to produce structural and functional bio-nanocomposites. Green Chem. 17 2610–2625. [ Google Scholar ]
- Govindarajan M., Rajeswary M., Veerakumar K., Muthukumaran U., Hoti S., Benelli G. (2016). Green synthesis and characterization of silver nanoparticles fabricated using Anisomeles indica: mosquitocidal potential against malaria, dengue and Japanese encephalitis vectors. Exp. Parasitol. 161 40–47. 10.1016/j.exppara.2015.12.011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Graf C., Vossen D. L., Imhof A., Van Blaaderen A. (2003). A general method to coat colloidal particles with silica. Langmuir 19 6693–6700. [ PubMed ] [ Google Scholar ]
- Greczynski G., Hultman L. (2020). X-ray photoelectron spectroscopy: towards reliable binding energy referencing. Progr. Mater. Sci. 107 : 100591 . [ Google Scholar ]
- Guo D., Xie G., Luo J. (2013). Mechanical properties of nanoparticles: basics and applications. J. Phys. D 47 : 013001 . [ Google Scholar ]
- Guo W., Pleixats R., Shafir A. (2015). Water-soluble gold nanoparticles: from catalytic selective Nitroarene reduction in water to refractive index sensing. Chem. An Asian J. 10 2437–2443. 10.1002/asia.201500290 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gwynne K. (2020). Enhancement of the Photostability of Blue Phosphorescence Using Plasmonic Surfaces. New Brunswick, NJ: Rutgers University-School of Graduate Studies. [ Google Scholar ]
- Haasch R. T. (2014). “ X-ray photoelectron spectroscopy (XPS) and auger electron spectroscopy (AES) ,” in Practical Materials Characterization , Ed. Sardela M. (Berlin: Springer; ), 93–132. [ Google Scholar ]
- Hasan S. (2015). A review on nanoparticles: their synthesis and types. Res. J. Recent Sci. 2277 : 2502 . [ Google Scholar ]
- Holder C. F., Schaak R. E. (2019). Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. Washington, DC: ACS Publications. [ PubMed ] [ Google Scholar ]
- Hollamby M. J., Eastoe J., Chemelli A., Glatter O., Rogers S., Heenan R. K., et al. (2010). Separation and purification of nanoparticles in a single step. Langmuir 26 6989–6994. 10.1021/la904225k [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoo C. M., Starostin N., West P., Mecartney M. L. (2008). A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J. Nanopart. Res. 10 89–96. [ Google Scholar ]
- Hortin J., Anderson A., Britt D., Jacobson A., Mclean J. (2020). Copper oxide nanoparticle dissolution at alkaline pH is controlled by dissolved organic matter: influence of soil-derived organic matter, wheat, bacteria, and nanoparticle coating. Environ. Sci. 7 2618–2631. [ Google Scholar ]
- Hoseinzadeh E., Makhdoumi P., Taha P., Hossini H., Stelling J., Amjad Kamal M. (2017). A review on nano-antimicrobials: metal nanoparticles, methods and mechanisms. Curr. Drug Metab. 18 120–128. [ PubMed ] [ Google Scholar ]
- Hulkoti N. I., Taranath T. (2014). Biosynthesis of nanoparticles using microbes—a review. Colloids Surf. B Biointerf. 121 474–483. 10.1016/j.colsurfb.2014.05.027 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Islam F., Shohag S., Uddin M. J., Islam M. R., Nafady M. H., Akter A., et al. (2022). Exploring the journey of zinc oxide nanoparticles (ZnO-NPs) toward biomedical applications. Materials 15 : 2160 . 10.3390/ma15062160 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jadoun S., Arif R., Jangid N. K., Meena R. K. (2021). Green synthesis of nanoparticles using plant extracts: A review. Environ. Chem. Lett. 19 355–374. [ Google Scholar ]
- Jamkhande P. G., Ghule N. W., Bamer A. H., Kalaskar M. G. (2019). Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 53 101174 . [ Google Scholar ]
- Jana N. R., Earhart C., Ying J. Y. (2007). Synthesis of water-soluble and functionalized nanoparticles by silica coating. Chem. Mater. 19 5074–5082. [ Google Scholar ]
- Jaskulski D., Jaskulska I., Majewska J., Radziemska M., Bilgin A., Brtnicky M. (2022). Silver Nanoparticles (AgNPs) in urea solution in laboratory tests and field experiments with crops and vegetables. Materials 15 : 870 . 10.3390/ma15030870 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jayaraman V., Ghosh S., Sengupta A., Srivastava S., Sonawat H., Narayan P. K. (2014). Identification of biochemical differences between different forms of male infertility by nuclear magnetic resonance (NMR) spectroscopy. J. Assist. Reproduct. Genet. 31 1195–1204. 10.1007/s10815-014-0282-4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jena J., Pradhan N., Nayak R. R., Dash B. P., Sukla L. B., Panda P. K., et al. (2014). Microalga Scenedesmus sp.: a potential low-cost green machine for silver nanoparticle synthesis. J. Microbiol. Biotechnol. Adv. 24 522–533. 10.4014/jmb.1306.06014 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jiang H.-L., Xu Q. (2011). Catalytic hydrolysis of ammonia borane for chemical hydrogen storage. Catal. Today 170 56–63. [ Google Scholar ]
- Joh D.-W., Jung T.-K., Lee H.-S., Kim D.-H. (2013). Synthesis of nanoparticles using electrical explosion of Ni wire in Pt solution. J. Nanosci. Nanotechnol. 13 6092–6094. 10.1166/jnn.2013.7677 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kahle M., Kleber M., Jahn R. (2002). Review of XRD-based quantitative analyses of clay minerals in soils: the suitability of mineral intensity factors. Geoderma 109 191–205. [ Google Scholar ]
- Kalaiyarasu T., Karthi N., Sharmila G. V., Manju V. (2016). In vitro assessment of antioxidant and antibacterial activity of green synthesized silver nanoparticles from Digitaria radicosa leaves. Asian J. Pharm. Clin. Res. 9 297–302. [ Google Scholar ]
- Kayalvizhi T., Ravikumar S., Venkatachalam P. (2016). Green synthesis of metallic silver nanoparticles using Curculigo orchioides rhizome extracts and evaluation of its antibacterial, larvicidal, and anticancer activity. J. Environ. Eng. 142 : C4016002 . [ Google Scholar ]
- Khan A., Rashid R., Murtaza G., Zahra A. (2014). Gold nanoparticles: synthesis and applications in drug delivery. Trop. J. Pharm. Res. 13 1169–1177. [ Google Scholar ]
- Khan I., Saeed K., Khan I. (2019). Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 12 908–931. [ Google Scholar ]
- Khanna P., Kaur A., Goyal D. (2019). Algae-based metallic nanoparticles: Synthesis, characterization and applications. J. Microbiol Methods 163 : 105656 . 10.1016/j.mimet.2019.105656 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kharat S. N., Mendhulkar V. D. (2016). Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater. Sci. Eng. C 62 719–724. 10.1016/j.msec.2016.02.024 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kim J.-H., Sheikh F. A., Ju H. W., Park H. J., Moon B. M., Lee O. J., et al. (2014). 3D silk fibroin scaffold incorporating titanium dioxide (TiO2) nanoparticle (NPs) for tissue engineering. Int. J. Biol. Macromol. 68 158–168. 10.1016/j.ijbiomac.2014.04.045 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kohl H., Reimer L. (2008). Transmission Electron Microscopy. Berlin: Springer Series in Optical Sciences, 36. [ Google Scholar ]
- Kokarneswaran M., Selvaraj P., Ashokan T., Perumal S., Sellappan P., Murugan K. D., et al. (2020). Discovery of carbon nanotubes in sixth century BC potteries from Keeladi, India. Sci. Rep. 10 1–6. 10.1038/s41598-020-76720-z [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kopittke P. M., Lombi E., Wang P., Schjoerring J. K., Husted S. (2019). Nanomaterials as fertilizers for improving plant mineral nutrition and environmental outcomes. Environ. Sci. 6 3513–3524. 10.3390/biology10111123 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kora A. J., Rastogi L. (2018). Peroxidase activity of biogenic platinum nanoparticles: A colorimetric probe towards selective detection of mercuric ions in water samples. Sens. Actuators B Chem. 254 690–700. [ Google Scholar ]
- Kreizer M., Ratner D., Liberzon A. (2010). Real-time image processing for particle tracking velocimetry. Exp. Fluids 48 105–110. [ Google Scholar ]
- Krithiga N., Jayachitra A., Rajalakshmi A. (2013). Synthesis, characterization and analysis of the effect of copper oxide nanoparticles in biological systems. Ind. J. Ns 1 6–15. [ Google Scholar ]
- Kumar R., Singh R. K., Dubey P. K., Kumar P., Tiwari R. S., Oh I.-K. (2013). Pressure-dependent synthesis of high-quality few-layer graphene by plasma-enhanced arc discharge and their thermal stability. J. Nanopart. Res. 15 1–10. [ Google Scholar ]
- Kumar S. S., Venkateswarlu P., Rao V. R., Rao G. N. (2013). Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. Nano Lett. 3 1–6. [ Google Scholar ]
- Kumar V., Yadav S. K. (2009). Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 84 151–157. [ Google Scholar ]
- Kumar V., Bano D., Mohan S., Singh D. K., Hasan S. H. (2016). Sunlight-induced green synthesis of silver nanoparticles using aqueous leaf extract of Polyalthia longifolia and its antioxidant activity. Mater. Lett. 181 371–377. [ Google Scholar ]
- Kumari S. C., Dhand V., Padma P. N. (2021). Green synthesis of metallic nanoparticles: a review. Nanomaterials 2021 259–281. [ Google Scholar ]
- Lam E., Luong J. H. (2014). Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 4 3393–3410. [ Google Scholar ]
- Lara P., Philippot K. (2014). The hydrogenation of nitroarenes mediated by platinum nanoparticles: an overview. Catal. Sci. Technol. 4 2445–2465. [ Google Scholar ]
- Lerner M. I., Glazkova E. A., Lozhkomoev A. S., Svarovskaya N. V., Bakina O. V., Pervikov A. V., et al. (2016). Synthesis of Al nanoparticles and Al/AlN composite nanoparticles by electrical explosion of aluminum wires in argon and nitrogen. Powder Technol. 295 307–314. [ Google Scholar ]
- Lewczuk B., Szyryńska N. (2021). Field-emission scanning electron microscope as a tool for large-area and large-volume ultrastructural studies. Animals 11 : 3390 . 10.3390/ani11123390 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li G., He D., Qian Y., Guan B., Gao S., Cui Y., et al. (2011). Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int. J. Mol. Sci. 13 466–476. 10.3390/ijms13010466 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li N., Zhao P., Astruc D. (2014). Anisotropic gold nanoparticles: synthesis, properties, applications, and toxicity. Angewand. Chem. Int. Edn. 53 1756–1789. [ PubMed ] [ Google Scholar ]
- Li T., Senesi A. J., Lee B. (2016). Small angle X-ray scattering for nanoparticle research. Chem. Rev. 116 11128–11180. 10.1021/acs.chemrev.5b00690 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Li X., Xu H., Chen Z.-S., Chen G. (2011). Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011 : 270974 . [ Google Scholar ]
- Luangpipat T., Beattie I. R., Chisti Y., Haverkamp R. G. (2011). Gold nanoparticles produced in a microalga. J. Nanopart. Res. 13 6439–6445. [ Google Scholar ]
- Lyon L. A., Keating C. D., Fox A. P., Baker B. E., He L., Nicewarner S. R., et al. (1998). Raman spectroscopy. Anal. Chem. 70 341–362. [ PubMed ] [ Google Scholar ]
- Lyu H., Gao B., He F., Ding C., Tang J., Crittenden J. C. (2017). Ball-milled carbon nanomaterials for energy and environmental applications. ACS Sust. Chem. Eng. 5 9568–9585. 10.1016/j.biortech.2020.123613 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Machac P., Cichon S., Lapcak L., Fekete L. (2020). Graphene prepared by chemical vapour deposition process. Graph. Technol. 5 9–17. [ Google Scholar ]
- Madathil A. N. P., Vanaja K., Jayaraj M. (2007). “ Synthesis of ZnO nanoparticles by hydrothermal method ,” in Nanophotonic materials IV , eds Gaburro Z., Cabrini S. (Bellingham, WA: SPIE; ), 47–55. [ Google Scholar ]
- Maharani V., Sundaramanickam A., Balasubramanian T. J. E. (2016). In vitro anticancer activity of silver nanoparticle synthesized by Escherichia coli VM1 isolated from marine sediments of Ennore southeast coast of India. Enzyme Microb. Technol. 95 146–154. 10.1016/j.enzmictec.2016.09.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Majeed Khan M. A., Kumar S., Ahamed M., Alrokayan S. A., Alsalhi M. S. (2011). Structural and thermal studies of silver nanoparticles and electrical transport study of their thin films. Nanosc. Res. Lett. 6 1–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Malhotra S. P. K., Alghuthaymi M. A. (2022). Biomolecule-assisted biogenic synthesis of metallic nanoparticles. Agri-Waste Microb. Product. Sust. Nanomater. 2022 139–163. [ Google Scholar ]
- Mathew L., Chandrasekaran N., Mukherjee A. (2010). “ Biomimetic synthesis of nanoparticles: science, technology & applicability ,” Biomimetics learning from nature , Ed. Mukherjee A. (Norderstedt: Books on Demand; ). [ Google Scholar ]
- Mishra A., Tripathy S. K., Wahab R., Jeong S.-H., Hwang I., Yang Y.-B., et al. (2011). Microbial synthesis of gold nanoparticles using the fungus Penicillium brevicompactum and their cytotoxic effects against mouse mayo blast cancer C 2 C 12 cells. Appl. Microbiol. Biotechnol. Adv. 92 617–630. 10.1007/s00253-011-3556-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mittal A., Chisti Y. (2013). Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 31 346–356. [ PubMed ] [ Google Scholar ]
- Moghaddam A. B., Nazari T., Badraghi J., Kazemzad M. (2009). Synthesis of ZnO nanoparticles and electrodeposition of polypyrrole/ZnO nanocomposite film. Int. J. Electrochem. Sci. 4 247–257. [ Google Scholar ]
- Mohanpuria P., Rana N. K., Yadav S. K. (2008). Biosynthesis of nanoparticles: technological concepts and future applications. J. Nanopart. Res. 10 507–517. [ Google Scholar ]
- Mohd Yusof H., Mohamad R., Zaidan U. H., Rahman A. (2019). Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. J. Anim. Sci. Biotechnol. 10 1–22. 10.1186/s40104-019-0368-z [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Morsi M., Abdelrazek E., Ramadan R., Elashmawi I., Rajeh A. (2022). Structural, optical, mechanical, and dielectric properties studies of carboxymethyl cellulose/polyacrylamide/lithium titanate nanocomposites films as an application in energy storage devices. Polymer Test. 114 107705 . [ Google Scholar ]
- Mott D., Galkowski J., Wang L., Luo J., Zhong C.-J. (2007). Synthesis of size-controlled and shaped copper nanoparticles. Langmuir 23 5740–5745. [ PubMed ] [ Google Scholar ]
- Mukherjee P., Ahmad A., Mandal D., Senapati S., Sainkar S. R., Khan M. I., et al. (2001). Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett. 1 515–519. [ Google Scholar ]
- Muñoz-García J., Vázquez L., Cuerno R., Sánchez-García J. A., Castro M., Gago R. (2009). “ Self-organized surface nanopatterning by ion beam sputtering ,” in Toward Functional Nanomaterials , Ed. Wang Z. M. (Berlin: Springer; ), 323–398. [ Google Scholar ]
- Naraginti S., Kumari P. L., Das R. K., Sivakumar A., Patil S. H., Andhalkar V. V. (2016). Amelioration of excision wounds by topical application of green synthesized, formulated silver and gold nanoparticles in albino Wistar rats. Mater. Sci. Eng. C 62 293–300. 10.1016/j.msec.2016.01.069 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Narayanan K. B., Sakthivel N. (2010). Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interf. Sci. 156 1–13. [ PubMed ] [ Google Scholar ]
- Nayak D., Ashe S., Rauta P. R., Kumari M., Nayak B. (2016). Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng. C 58 44–52. 10.1016/j.msec.2015.08.022 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ndolomingo M. J., Meijboom R. (2016). Determination of the surface area and sizes of supported copper nanoparticles through organothiol adsorption—Chemisorption. Appl. Surf. Sci. 390 224–235. [ Google Scholar ]
- Newbury D. E., Ritchie N. W. (2013). Is scanning electron microscopy/energy dispersive X-ray spectrometry (SEM/EDS) quantitative? Scanning 35 141–168. 10.1002/sca.21041 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nguyen K. T., Menon J. U., Jadeja P. V., Tambe P. P., Vu K., Yuan B. (2013). Nanomaterials for photo-based diagnostic and therapeutic applications. Theranostics 3 152–166. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nowack B., Krug H. F., Height M. (2011). 120 Years of Nanosilver History: Implications for Policy Makers. Washington, DC: ACS Publications. [ PubMed ] [ Google Scholar ]
- Önal E. S., Yatkın T., Aslanov T., Ergüt M., Özer A. (2019). Biosynthesis and characterization of iron nanoparticles for effective adsorption of Cr (VI). Int. J. Chem. Eng. 2019 : 2716423 . [ Google Scholar ]
- Ostermann R., Cravillon J., Weidmann C., Wiebcke M., Smarsly B. M. (2011). Metal–organic framework nanofibers via electrospinning. Chem. Commun. 47 442–444. [ PubMed ] [ Google Scholar ]
- Parashar M., Shukla V. K., Singh R. (2020). Metal oxides nanoparticles via sol–gel method: a review on synthesis, characterization and applications. J. Mater. Sci. 31 3729–3749. [ Google Scholar ]
- Park C. Y., Choi B. (2019). Enhanced light extraction from bottom emission oleds by high refractive index nanoparticle scattering layer. Nanomaterials 9 : 1241 . 10.3390/nano9091241 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Patil M. P., Kim G.-D. (2018). Marine microorganisms for synthesis of metallic nanoparticles and their biomedical applications. Colloids Surf. B Biointerf. 172 487–495. [ PubMed ] [ Google Scholar ]
- Patil M. P., Ngabire D., Thi H. H. P., Kim M.-D., Kim G.-D. (2017). Eco-friendly synthesis of gold nanoparticles and evaluation of their cytotoxic activity on cancer cells. J. Clust. Sci. 28 119–132. [ Google Scholar ]
- Patil N., Bhaskar R., Vyavhare V., Dhadge R., Khaire V., Patil Y. (2021). Overview on methods of synthesis of nanoparticles. Int. J. Curr. Pharm. Res. 13 11–16. [ Google Scholar ]
- Patois E., Capelle M., Palais C., Gurny R., Arvinte T. (2012). Evaluation of nanoparticle tracking analysis (NTA) in the characterization of therapeutic antibodies and seasonal influenza vaccines: pros and cons. J. Drug Deliv. Sci. Technol. 22 427–433. [ Google Scholar ]
- Paul B., Bhuyan B., Purkayastha D. D., Dhar S. S. (2016). Photocatalytic and antibacterial activities of gold and silver nanoparticles synthesized using biomass of Parkia roxburghii leaf. J. Photochem. Photobiol. B Biol. 154 1–7. 10.1016/j.jphotobiol.2015.11.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pérez-Lorenzo M. (2012). Palladium nanoparticles as efficient catalysts for Suzuki cross-coupling reactions. J. Phys. Chem. Lett. 3 167–174. [ Google Scholar ]
- Pérez-Tijerina E., Pinilla M. G., Mejía-Rosales S., Ortiz-Méndez U., Torres A., José-Yacamán M. (2008). Highly size-controlled synthesis of Au/Pd nanoparticles by inert-gas condensation. Faraday Discuss. 138 353–362. 10.1039/b705913m [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Phull A.-R., Abbas Q., Ali A., Raza H., Zia M., Haq I.-U. (2016). Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliata. Fut. J. Pharm. Sci. 2 31–36. [ Google Scholar ]
- Pimpin A., Srituravanich W. (2012). Review on micro-and nanolithography techniques and their applications. Eng. J. 16 37–56. [ Google Scholar ]
- Pradeep T. (2009). Noble metal nanoparticles for water purification: a critical review. Thin Solid Films 517 6441–6478. [ Google Scholar ]
- Praseptiangga D., Zahara H. L., Widjanarko P. I., Joni I. M., Panatarani C. (2020). Preparation and FTIR spectroscopic studies of SiO2-ZnO nanoparticles suspension for the development of carrageenan-based bio-nanocomposite film. 100005 . [ Google Scholar ]
- Pugazhendhi S., Sathya P., Palanisamy P., Gopalakrishnan R. (2016). Synthesis of silver nanoparticles through green approach using Dioscorea alata and their characterization on antibacterial activities and optical limiting behavior. J. Photochem. Photobiol. B Biol. 159 155–160. 10.1016/j.jphotobiol.2016.03.043 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Qi P., Zhang D., Wan Y. (2013). Sulfate-reducing bacteria detection based on the photocatalytic property of microbial synthesized ZnS nanoparticles. Anal. Chim. Acta 800 65–70. 10.1016/j.aca.2013.09.015 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rad A. G., Abbasi H., Afzali M. H. (2011). Gold nanoparticles: synthesising, characterizing and reviewing novel application in recent years. Phys. Proc. 22 203–208. [ Google Scholar ]
- Rahmati-Abkenar M., Manteghian M. (2020). Effect of silver nanoparticles on the solubility of methane and ethane in water. J. Nat. Gas Sci. Eng. 82 : 103505 . [ Google Scholar ]
- Rajeshkumar S., Ponnanikajamideen M., Malarkodi C., Malini M., Annadurai G. (2014). Microbe-mediated synthesis of antimicrobial semiconductor nanoparticles by marine bacteria. J. Nanostruct. Chem. 4 1–7. [ Google Scholar ]
- Rajkuberan C., Prabukumar S., Sathishkumar G., Wilson A., Ravindran K., Sivaramakrishnan S. (2017). Facile synthesis of silver nanoparticles using Euphorbia antiquorum L. latex extract and evaluation of their biomedical perspectives as anticancer agents. J. Saudi Chem. Soc. 21 911–919. [ Google Scholar ]
- Ramesh P., Kokila T., Geetha D. (2015). Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochim. Acta Part A 142 339–343. 10.1016/j.saa.2015.01.062 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rangel-Olivares F. R., Arce-Estrada E. M., Cabrera-Sierra R. (2021). Synthesis and characterization of polyaniline-based polymer nanocomposites as anti-corrosion coatings. Coatings 11 : 653 . [ Google Scholar ]
- Rao N. H., Lakshmidevi N., Pammi S., Kollu P., Ganapaty S., Lakshmi P. (2016). Green synthesis of silver nanoparticles using methanolic root extracts of Diospyros paniculata and their antimicrobial activities. Mater. Sci. Eng. C 62 553–557. 10.1016/j.msec.2016.01.072 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rassaei L., Marken F., Sillanpää M., Amiri M., Cirtiu C. M., Sillanpää M. (2011). Nanoparticles in electrochemical sensors for environmental monitoring. TrAC Trends Anal. Chem. 30 1704–1715. [ Google Scholar ]
- Rocha F. S., Gomes A. J., Lunardi C. N., Kaliaguine S., Patience G. S. (2018). Experimental methods in chemical engineering: Ultraviolet visible spectroscopy—UV-Vis. Can. J. Chem. Eng. 96 2512–2517. [ Google Scholar ]
- Saini P., Saha S. K., Roy P., Chowdhury P., Babu S. P. S. (2016). Evidence of reactive oxygen species (ROS) mediated apoptosis in Setaria cervi induced by green silver nanoparticles from Acacia auriculiformis at a very low dose. Exp. Parasitol. 160 39–48. 10.1016/j.exppara.2015.11.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Saldarriaga J. F., Aguado R., Pablos A., Amutio M., Olazar M., Bilbao J. (2015). Fast characterization of biomass fuels by thermogravimetric analysis (TGA). Fuel 140 744–751. [ Google Scholar ]
- Salem S. S., Fouda A. (2021). Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol. Trace Element Res. 199 344–370. 10.1007/s12011-020-02138-3 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Salopek B., Krasic D., Filipovic S. (1992). Measurement and application of zeta-potential. Rudarsko-Geolosko-Naftni Zbornik 4 : 147 . [ Google Scholar ]
- Saw M. J., Ghosh B., Nguyen M. T., Jirasattayaporn K., Kheawhom S., Shirahata N., et al. (2019). High aspect ratio and post-processing free silver nanowires as top electrodes for inverted-structured photodiodes. ACS Omega 4 13303–13308. 10.1021/acsomega.9b01479 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schröfel A., Kratošová G., Šafa r ̄ ík I., Šafa r ̄ íková M., Raška I., Shor L. M. (2014). Applications of biosynthesized metallic nanoparticles–a review. Acta Biomater. 10 4023–4042. [ PubMed ] [ Google Scholar ]
- Shenashen M. A., El-Safty S. A., Elshehy E. A. (2014). Synthesis, morphological control, and properties of silver nanoparticles in potential applications. Part. Part. Syst. Char. 31 293–316. [ Google Scholar ]
- Shi Z., Lian Y., Liao F. H., Zhou X., Gu Z., Zhang Y., et al. (2000). Large scale synthesis of single-wall carbon nanotubes by arc-discharge method. J. Phys. Chem. Solids 61 1031–1036. 10.1166/jnn.2001.012 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Siddiqi K. S., Husen A. (2016). Green synthesis, characterization and uses of palladium/platinum nanoparticles. Nanosc. Res. Lett. 11 1–13. 10.1186/s11671-016-1695-z [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Siddique S., Chow J. C. (2020). Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 10 : 3824 . [ Google Scholar ]
- Sigmund W., Yuh J., Park H., Maneeratana V., Pyrgiotakis G., Daga A. (2006). Processing and structure relationships in electrospinning of ceramic fiber systems. J. Am. Ceramic Soc. 89 395–407. [ Google Scholar ]
- Singh R. P., Shukla V. K., Yadav R. S., Sharma P. K., Singh P. K., Pandey A. C. (2011). Biological approach of zinc oxide nanoparticles formation and its characterization. Adv. Mater. Lett. 2 313–317. [ Google Scholar ]
- Siwach O. P., Sen P. (2008). Synthesis and study of fluorescence properties of Cu nanoparticles. J. Nanopart. Res. 10 107–114. [ Google Scholar ]
- Slavin Y. N., Asnis J., Häfeli U. O., Bach H. (2017). Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 15 1–20. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Song U., Kim J. (2020). Zinc oxide nanoparticles: a potential micronutrient fertilizer for horticultural crops with little toxicity. Horticult. Environ. Biotechnol. 61 625–631. [ Google Scholar ]
- Soni N., Sonam P. (2014). Green nanoparticles for mosquito control. Sci. World J. 2014 1–6. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sowani H., Mohite P., Munot H., Shouche Y., Bapat T., Kumar A. R., et al. (2016). Green synthesis of gold and silver nanoparticles by an actinomycete Gordonia amicalis HS-11: mechanistic aspects and biological application. Process Biochem. 51 374–383. [ Google Scholar ]
- Sriram M. I., Kalishwaralal K., Barathmanikanth S., Gurunathani S. (2012). Size-based cytotoxicity of silver nanoparticles in bovine retinal endothelial cells. Nanosci. Methods 1 56–77. [ Google Scholar ]
- Stepanov A. L., Nuzhdin V. I., Valeev V. F., Kreibig U. (2011). “ Optical properties of metal nanoparticles ,” in Proceedings of the ICONO 2010: International Conference on Coherent and Nonlinear Optics , (Bellingham, WA: SPIE; ), 543–552. [ Google Scholar ]
- Su S. S., Chang I. (2018). “ Review of production routes of nanomaterials ,” in Commercialization of nanotechnologies–a case study approach , eds Brabazon D., Pellicer E., Zivic F., Sort J., Baró M. D., Grujovic N., Choy K.-L. (Berlin: Springer; ), 15–29. [ Google Scholar ]
- Sugihartono I., Dianisya D., Isnaeni I. (2018). “ Crystal structure analyses of ZnO nanoparticles growth by simple wet chemical method ,” in Proceedings of the IOP Conference Series: Materials Science and Engineering , (Bristol: IOP Publishing; ), 012077. [ Google Scholar ]
- Sun T., Zhang Y. S., Pang B., Hyun D. C., Yang M., Xia Y. J. A. C. I. E. (2014). Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. Engl. 53 12320–12364. [ PubMed ] [ Google Scholar ]
- Tavakoli A. H., Maram P. S., Widgeon S. J., Rufner J., Van Benthem K., Ushakov S., et al. (2013). Amorphous alumina nanoparticles: structure, surface energy, and thermodynamic phase stability. J. Phys. Chem. C 117 17123–17130. 10.1021/jp405820g [ CrossRef ] [ Google Scholar ]
- Theron J., Eugene Cloete T., De Kwaadsteniet M. (2010). Current molecular and emerging nanobiotechnology approaches for the detection of microbial pathogens. Crit. Rev. Microbiol. 36 318–339. 10.3109/1040841X.2010.489892 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Thomas R., Janardhanan A., Varghese R. T., Soniya E., Mathew J., Radhakrishnan E. (2014). Antibacterial properties of silver nanoparticles synthesized by marine Ochrobactrum sp. Braz. J. Microbiol. 45 1221–1227. 10.1590/s1517-83822014000400012 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Titus D., Samuel E. J. J., Roopan S. M. (2019). “ Nanoparticle characterization techniques ,” in Green synthesis, characterization and applications of nanoparticles , eds Shukla A., Iravani S. (Amsterdam: Elsevier; ), 303–319. 10.1016/B978-0-08-102579-6.00012-5 [ CrossRef ] [ Google Scholar ]
- Tran V., Wen X. (2014). “ Rapid prototyping technologies for tissue regeneration ,” in Rapid prototyping of biomaterials , Ed. Narayan R. (Sawston: Woodhead Publishing; ), 97–155. 10.1533/9780857097217.97 [ CrossRef ] [ Google Scholar ]
- Triana M. A., Hsiang E.-L., Zhang C., Dong Y., Wu S.-T. (2022). Luminescent nanomaterials for energy-efficient display and healthcare. ACS Energy Lett. 7 1001–1020. [ Google Scholar ]
- Uzair B., Liaqat A., Iqbal H., Menaa B., Razzaq A., Thiripuranathar G., et al. (2020). Green and cost-effective synthesis of metallic nanoparticles by algae: Safe methods for translational medicine. Bioengineering 7 : 129 . 10.3390/bioengineering7040129 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Van Thai P., Abe S., Kosugi K., Saito N., Takahashi K., Sasaki T., et al. (2019). Size/shape control of gold nanoparticles synthesized by alternating current glow discharge over liquid: The role of pH. Mater. Res. Expr. 6 : 095074 . [ Google Scholar ]
- Velusamy P., Das J., Pachaiappan R., Vaseeharan B., Pandian K. (2015). Greener approach for synthesis of antibacterial silver nanoparticles using aqueous solution of neem gum ( Azadirachta indica L.). Indus. Crops Products 66 103–109. [ Google Scholar ]
- Wang P., Menzies N. W., Lombi E., Sekine R., Blamey F. P. C., Hernandez-Soriano M. C., et al. (2015). Silver sulfide nanoparticles (Ag2S-NPs) are taken up by plants and are phytotoxic. Nanotoxicology 9 1041–1049. 10.3109/17435390.2014.999139 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wang Z., Li H., Tang F., Ma J., Zhou X. (2018). A facile approach for the preparation of nano-size zinc oxide in water/glycerol with extremely concentrated zinc sources. Nanosc. Res. Lett. 13 1–9. 10.1186/s11671-018-2616-0 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wiesendanger R., Güntherodt H.-J. (2013). Scanning tunneling microscopy III: theory of STM and related scanning probe methods. Berlin: Springer Science & Business Media. [ Google Scholar ]
- Xia Y., Xiao Z., Dou X., Huang H., Lu X., Yan R., et al. (2013). Green and facile fabrication of hollow porous MnO/C microspheres from microalgaes for lithium-ion batteries. ACS Nano 7 7083–7092. 10.1021/nn4023894 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yadav R., Dwivedi S., Kumar S., Chaudhury A. (2010). Trends and perspectives of biosensors for food and environmental virology. Food Environ. Virol. 2 53–63. [ Google Scholar ]
- Yadav T. P., Yadav R. M., Singh D. P. (2012). Mechanical milling: a top down approach for the synthesis of nanomaterials and nanocomposites. Nanosci. Nanotechnol. 2 22–48. [ Google Scholar ]
- Yang W., Wang L., Mettenbrink E. M., Deangelis P. L., Wilhelm S. (2021). Nanoparticle toxicology. Annu. Rev. Pharmacol. Toxicol. 61 269–289. [ PubMed ] [ Google Scholar ]
- Ye Q., Chen W., Huang H., Tang Y., Wang W., Meng F., et al. (2020). Iron and zinc ions, potent weapons against multidrug-resistant bacteria. Appl. Microbiol. Biotechnol. 104 5213–5227. 10.1007/s00253-020-10600-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yuan P., Ding X., Yang Y. Y., Xu Q. H. (2018). Metal nanoparticles for diagnosis and therapy of bacterial infection. Adv. Healthc. Mater. 7 : 1701392 . [ PubMed ] [ Google Scholar ]
- Zahra Z., Habib Z., Chung S., Badshah M. A. (2020). Exposure route of TiO2 NPs from industrial applications to wastewater treatment and their impacts on the agro-environment. Nanomaterials 10 : 1469 . 10.3390/nano10081469 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zan G., Wu Q. (2016). Biomimetic and bioinspired synthesis of nanomaterials/nanostructures. Adv. Mater. 28 2099–2147. [ PubMed ] [ Google Scholar ]
- Zhang X., Yan S., Tyagi R., Surampalli R. (2011). Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere 82 489–494. [ PubMed ] [ Google Scholar ]
- Zheng Z., Zhang X., Carbo D., Clark C., Nathan C.-A., Lvov Y. (2010). Sonication-assisted synthesis of polyelectrolyte-coated curcumin nanoparticles. Langmuir 26 7679–7681. 10.1021/la101246a [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhou C., Wang Y., Du L., Yao H., Wang J., Luo G. (2016). Precipitation preparation of high surface area and porous nanosized ZnO by continuous gas-based impinging streams in unconfined space. Indus. Eng. Chem. Res. 55 11943–11949. [ Google Scholar ]
- Zhou M., Wei Z., Qiao H., Zhu L., Yang H., Xia T. (2009). Particle size and pore structure characterization of silver nanoparticles prepared by confined arc plasma. J. Nanomater. 2009 : 968058 . [ Google Scholar ]
- Zhuang J., Gentry R. W. (2011). “ Environmental application and risks of nanotechnology: a balanced view ,” in Biotechnology and Nanotechnology Risk Assessment: Minding and Managing the Potential Threats around Us , eds Ripp S., Henry T. (Washington, DC: ACS Publications; ), 41–67. 10.3390/ijerph16234848 [ CrossRef ] [ Google Scholar ]
- Zielińska A., Carreiró F., Oliveira A. M., Neves A., Pires B., Venkatesh D. N., et al. (2020). Polymeric nanoparticles: production, characterization, toxicology and ecotoxicology. Molecules 25 : 3731 . 10.3390/molecules25163731 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
Introduction to Click Chemistry
A "click" away from discovery.
The traditional process of drug discovery based on natural secondary metabolites has often been slow, costly, and labor-intensive. Even with the advent of combinatorial chemistry and high-throughput screening in past decades, the generation of leads is dependent on the reliability of the individual reactions to construct the new molecular framework.
Click Chemistry Mechanism
Click chemistry is a newer approach to the synthesis of drug-like molecules that can accelerate the drug discovery process by utilizing a few practical and reliable reactions. Sharpless and coworkers defined what makes a click reaction as one that is wide in scope and easy to perform, uses only readily available reagents, and is insensitive to oxygen and water. In fact, in several instances, water is the ideal reaction solvent, providing the best yields and highest rates. Reaction work-up and purification uses benign solvents and avoids chromatography. 1
Click Chemistry Reaction Processes
- Simple to perform
- Wide in scope
- High yielding
- Stereospecific
- Adheres to the 12 Principles of Green Chemistry by generating harmless byproducts, removable by nonchromatographic methods
Click Chemistry Reaction Characteristics 1
- Simple reaction conditions
- Readily and easily available starting materials and reagents
- Use of no solvent, a benign solvent (such as water), or one that is easily removed
- Simple product isolation
- Product should be stable under physiological conditions
Click chemistry involves the use of a modular approach and has important applications in the fields of drug discovery, combinatorial chemistry, target-templated in situ chemistry, and DNA research. 1
Continue learning about click chemistry in drug discovery .
Network error: Failed to fetch
- Triazolylphenylglyoxal Reagents: Arginine-Directed Bioconjugation
- SAFC Biologic APIs and Conjugation Experts
- Click Chemistry in Drug Discovery
- Copper-Free Click Chemistry
- GlycoProfile™ Azido Sugars
- Guanylation of Amines by Bis(tert-butoxycarbonyl)thiopseudourea, Polymer-Bound
- Polybrene® Technical Bulletin
- Safe Manufacture of Antibody Drug Conjugates
To continue reading please sign in or create an account.
Research. Development. Production.
We are a leading supplier to the global Life Science industry with solutions and services for research, biotechnology development and production, and pharmaceutical drug therapy development and production.
© 2024 Merck KGaA, Darmstadt, Germany and/or its affiliates. All Rights Reserved.
Reproduction of any materials from the site is strictly forbidden without permission.
- English - EN
- Español - ES
Green Chemistry
Electrosynthesis of n/s-heterocycles.
The electrochemical generation of highly active and variable free radicals for the synthesis of organic compounds has emerged as a favored approach due to its mild and environmentally friendly characteristics, in contrast to traditional chemical redox reagents. As such, electrochemistry has been extensively applied in a wide range of synthetic transformations for the construction and functionalization of N/S-heterocycles in recent years. In this review, we present a comprehensive overview of our recent advancements in the methodology of electrochemically-assisted radical generation and its subsequent application in the synthesis and functionalization of diverse N/S-heterocyclic compounds. In practice, we have developed various methodologies for the functionalization of N/S-heterocyclic compounds, including thiocyanation, alkylation, sulfonation, hydrogenation, pyridination, and the synthesis of complex N/S-containing heterocyclic compounds from readily available starting materials under undivided cell. The methodology and mechanism of electrochemically-mediated synthesis and functionalization of N/S-heterocycles will be discussed in terms of direct anodic oxidation, indirect oxidation, cathodic reduction, and paired electrolysis.
- This article is part of the themed collection: 2024 Green Chemistry Reviews
Article information
Download citation, permissions.
J. Wen, Y. Yin, K. Yan, B. Li, M. Zhang and J. Yang, Green Chem. , 2024, Accepted Manuscript , DOI: 10.1039/D4GC01595A
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements
MgAl-Layered Double Hydroxides: Synthesis, Characteristics, and Their Effects on Flame Retardant and Mechanical Properties of Casting Polyurethane
- Published: 23 May 2024
- Volume 15 , pages 224–229, ( 2024 )
Cite this article

- T. V. A. Nguyen 1 ,
- B. P. Tolochko 1 , 2 ,
- F. K. Gorbunov 2 , 3 &
- A. A. Fadina 2
Explore all metrics
MgAl–layered double hydroxides with different molar ratios of cations were synthesized. The compound with a ratio of 2 : 1 was shown to exhibit better characteristics of thermal stability. The modification of polyurethane by using these hydroxides led to the improvement in the properties of composites: decrease by 47% in flame retardancy, and increase by 24.8 and 54.1% in tensile strength and Young’s modulus, respectively.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Chapanova, I.V., Subcheva, E.N., Sertsova, A.A., and Yurtov, E.V., The fire-resistant composite materials based on polymethyl methacrylate with the addition of nanoparticles of layered double hydroxides, Usp. Khim. Khim. Tekhnol. , 2017, vol. 31, no. 1, pp. 99–101.
Google Scholar
Jin, L., Zeng, H.-Y., Du, J.-Z., and Xu, S., Intercalation of organic and inorganic anions into layered double hydroxides for polymer flame retardancy, Appl. Clay Sci. , 2020, vol. 187, p. 105481. https://doi.org/10.1016/j.clay.2020.105481
Article CAS Google Scholar
Kopkova, E.K., Maiorov, D.V., and Kondratenko, T.V., Production and investigation of the structural, surface, and sorption properties of layered double hydroxides of magnesium and aluminium modified with polyethylene glycol, Sorbtsionnye Khromatogr. Protsessy , 2021, vol. 21, no. 6, pp. 894–904. https://doi.org/10.17308/sorpchrom.2021.21/3836
Nestroinaya, O.V., Ryl’tsova, I.G., Tarasenko, E.A., Yapryntsev, M.N., Solov’eva, A.A., and Lebedeva, O.E., Magnetic materials based on layered double hydroxides, Pet. Chem. , 2021, vol. 61, pp. 388–393. https://doi.org/10.1134/S096554412103004X
Santamaría, L., López-Aizpún, M., García-Padial, M., Vicente, M.A., Korili, S.A., and Gil, A., Zn–Ti–Al layered double hydroxides synthesized from aluminum saline slag wastes as efficient drug adsorbents, Appl. Clay Sci. , 2020, vol. 187, p. 105486. https://doi.org/10.1016/j.clay.2020.105486
Titov, E.N., Smal’chenko, D.E., and Lebedeva, O.E., Synthesis of Fe(II) containing layered double hydroxides for application as D-Limonene oxidation catalysts, Usp. Khim. Khim. Tekhnol. , 2021, vol. 35, no. 13, pp. 76–78.
Bhuvaneswari, K., Palanisamy, G., Pazhanivel, T., and Maiyalagan, T., r-GO supported g-C 3 N 4 /NiMgAl layered triple hydroxide hybrid as a Visible Light photocatalyst for organic dye removal, Colloids Surf., A , 2020, vol. 602, p. 125078. https://doi.org/10.1016/j.colsurfa.2020.125078
Nugmanova, A.G. and Kalinina, M.A., Supramolecular self-assembly of hybrid colloidal systems, Colloid J., 2022, vol. 84, no. 5, pp. 642–662. https://doi.org/10.1134/S1061933X22700107
Szycher, M., Szycher’s Handbook of Polyurethanes , CRC Press, 2013, 2nd ed.
Starukh, G., Budzinska, V., and Brychka, S.Ya., Structural characterization, thermal and mechanical properties of polyurethane–MgAl–layered double hydroxide nanocomposites prepared via physical dispersion, Appl. Nanosci. , 2019, vol. 9, pp. 987–996.
Chen, X., Jiang, Y., Liu, J., Jiao, C., Qian, Y., and Li, S., Smoke suppression properties of fumed silica on flame-retardant thermoplastic polyurethane based on ammonium polyphosphate, J. Therm. Anal. Calorim. , 2015, vol. 120, no. 3, pp. 1493–1501.
Li, L., Jiang, K., Qian, Y., Han, H., Qiao, P., and Zhang, H., Effect of organically intercalation modified layered double hydroxides-graphene oxide hybrids on flame retardancy of thermoplastic polyurethane nanocomposites, J. Therm. Anal. Calorim. , 2020, vol. 142, pp. 723–733.
Bourbigot, S., Turf, T., Bellayer, S., and Duquesne, S., Polyhedral oligomeric silsesquioxane as flame retardant for thermoplastic polyurethane, Polym. Degrad. Stab. , 2009, vol. 94, no. 8, pp. 1230–1237.
Cavani, F., Trifiro, F., and Vaccary, A., Hydrotalcite-type anionic clays: Preparation, properties and applications, Catal. Today, 1991, vol. 11, pp. 173–301.
Gorbunov, F.K., Poluboyarov, V.A., Baikina, L.K., and Voloskova, E.V., Influence of nanodispersed corundum on strength characteristics of hot-curing injection molded polyurethanes, Perspekt. Mater. , 2013, no. 3, pp. 71–76.
GOST (State Standard) 27484-87: Fire Hazard Testing. Test Methods. Needle-Flame Test .
Matrenin, S.V., Opredelenie plotnosti materialov: Metodicheskie ukazaniya po vypolneniyu laboratornykh rabot po kursu “Mekhanicheskie i fizicheskie svoistva materialov” (Determination of Material Density: Methodical Instructions for Laboratory Works on the Course “Mechanical and Physical Properties of Materials”), Tomsk: Tomsk Polytech. Univ., 2006.
GOST (State Standard) 15139-69: Plastics. Methods for the Determination of Density (Mass Density) .
GOST (State Standard) 11721-78: Cellular Rubber. Method for Determination of Elastic and Tensile Stress-Strain Properties .
Elmoubarki, R., Mahjoubi, F.Z., Elhalil, A., Tounsadi, H., Abdennouri, M., Sadiq, M., Qourzal, S., Zouhri, A., and Barka, N., Ni/Fe and Mg/Fe layered double hydroxides and their calcined derivatives: Preparation, characterization and application on textile dyes removal, J. Mater. Res. Technol. , 2017, vol. 6, no. 3, pp. 271–283.
Lin, C.-H., Chu, H.-L., Hwang, W.-S., Wang, M.-C., and Ko, H.H., Synthesis and optical properties of Mg‒Al layered double hydroxides precursor powders, AIP Adv., 2017, vol. 7, p. 125005. https://doi.org/10.1063/1.4990832
Naseem, S., Gevers, B., Boldt, R., Labuschagné, F.J.W.J., and Leuteritz, A., Comparison of transition metal (Fe, Co, Ni, Cu, and Zn) containing trimetal layered double hydroxides (LDHs) prepared by urea hydrolysis, RSC Adv. , 2019, vol. 9, pp. 3030–3040. https://doi.org/10.1039/c8ra10165e
Article CAS PubMed PubMed Central Google Scholar
Tamm, M.E. and Tret’yakov, Yu.D., Neorganicheskaya khimiya—Ionnye radiusy (po Shennonu i Pryuittu), Tom 1: Fiziko-khimicheskie osnovy neorganicheskoi khimii: Uchebnik dlya vysshikh uchebnykh zavedenii (Inorganic Chemistry – Ion Radii (by Shannon and Pruitt), vol. 1: Physico-Chemical Bases of Inorganic Chemistry: Manual for Higher Education Institutions), Tret’yakov, Yu.D., Ed., Moscow: Akademiya, 2004.
Bansal, R.K., A Textbook of Strength of Materials , New Delhi: Laxmi, 2009, 4th ed.
Gorbunov, F.K., Poluboyarov, V.A., Voloskova, E.V., and Kadimova, A.V., Influence of grain sizes on strength characteristics of polyurethane foams, Izv. Vyssh. Uchebn. Zaved., Tekhnol. Legk. Prom-st. , 2017, no. 1, pp. 109–113.
Download references
The work was carried out with financial support from the Ministry of Science and Education of the Russian Federation within the State Assignment for the Institute of Solid State Chemistry and Mechanochemistry SB RAS no. FWUS-2021-0004 and the Federal Targeted Program according to the Agreement no. 075-15-2021-1359 of 13.10.2021 (internal no. 15.SIN.21.0015).
Author information
Authors and affiliations.
Novosibirsk State University, 630090, Novosibirsk, Russia
T. V. A. Nguyen & B. P. Tolochko
Institute of Solid State Chemistry and Mechanochemistry, Siberian Branch, Russian Academy of Sciences, 630090, Novosibirsk, Russia
B. P. Tolochko, F. K. Gorbunov & A. A. Fadina
Novosibirsk State Technical University, 630087, Novosibirsk, Russia
F. K. Gorbunov
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to F. K. Gorbunov .
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s note..
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Nguyen, T.V., Tolochko, B.P., Gorbunov, F.K. et al. MgAl-Layered Double Hydroxides: Synthesis, Characteristics, and Their Effects on Flame Retardant and Mechanical Properties of Casting Polyurethane. Inorg. Mater. Appl. Res. 15 , 224–229 (2024). https://doi.org/10.1134/S2075113324010209
Download citation
Received : 07 November 2022
Revised : 26 January 2023
Accepted : 21 March 2023
Published : 23 May 2024
Issue Date : February 2024
DOI : https://doi.org/10.1134/S2075113324010209
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- MgAl-layered double hydroxides
- casting polyurethane
- flame retardant
- mechanical properties
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 25 May 2024
Single-atom nanozymes shines diagnostics of gastrointestinal diseases
- Sijia Hua 1 na1 ,
- Xiulin Dong 2 , 3 na1 ,
- Qiuxia Peng 3 ,
- Kun Zhang 3 ,
- Xiaofeng Zhang 2 &
- Jianfeng Yang 2
Journal of Nanobiotechnology volume 22 , Article number: 286 ( 2024 ) Cite this article
Metrics details
Various clinical symptoms of digestive system, such as infectious, inflammatory, and malignant disorders, have a profound impact on the quality of life and overall health of patients. Therefore, the chase for more potent medicines is both highly significant and urgent. Nanozymes, a novel class of nanomaterials, amalgamate the biological properties of nanomaterials with the catalytic activity of enzymes, and have been engineered for various biomedical applications, including complex gastrointestinal diseases (GI). Particularly, because of their distinctive metal coordination structure and ability to maximize atom use efficiency, single-atom nanozymes (SAzymes) with atomically scattered metal centers are becoming a more viable substitute for natural enzymes. Traditional nanozyme design strategies are no longer able to meet the current requirements for efficient and diverse SAzymes design due to the diversification and complexity of preparation processes. As a result, this review emphasizes the design concept and the synthesis strategy of SAzymes, and corresponding bioenzyme-like activities, such as superoxide dismutase (SOD), peroxidase (POD), oxidase (OXD), catalase (CAT), and glutathione peroxidase (GPx). Then the various application of SAzymes in GI illnesses are summarized, which should encourage further research into nanozymes to achieve better application characteristics.
Graphical abstract
Introduction
Hospitalizations for digestive system disorders have risen absolutely as a proportion of all hospitalizations over the past two decades, with the largest rises in disorders including, intestinal infections and pancreatitis, posing significant implications for the quality of life and overall health of patients [ 1 , 2 ]. Tumors of the digestive system (e.g., liver cancer [ 3 ], pancreatic [ 4 ], and colorectal cancer [ 5 ]) also account for a large percentage of solid tumors. Despite the constant updating of therapeutic approaches, the incidence and mortality of certain diseases, such as gastric, esophageal, and colorectal cancers, are increasing annually [ 6 ]. Although significant progress has been made in the detection and treatment of digestive illnesses since the advent of endoscopic techniques [ 7 ], novel therapeutic approaches are still required to address the complexity and diversity of disease types. Catalytic nanomaterials known as nanozymes have features similar to those of enzymes and come in a variety of sizes, shapes, and surface arrangements. These artificial enzymes exhibit both great catalytic stability and high catalytic efficiency. It has been demonstrated that nanozymes possess the biocatalytic activity of naturally occurring enzymes, such as SOD [ 8 , 9 , 10 ], OXD [ 11 , 12 ], POD [ 13 ], CAT [ 9 , 14 ] and so on. The focus of nanozyme research is currently on single-atom nanozymes (SAzymes), a novel class of nanozymes with the benefits of high atom utilization, high catalytic activity, cheap production cost, and great selectivity. SAzymes are also gradually being developed for the treatment of digestive diseases, such as liver cancer and cirrhosis [ 15 ], pancreatitis [ 16 ], and the control of intestinal inflammation [ 17 ].
Herein, an emphasis on their unprecedent design concepts to optimize dispersion, stability, and enzyme activity has been made. Based on it, we give a brief summary of the function of the biocatalytic-like activity of SAzymes and the corresponding application innovation of theme in the field of gastrointestinal disorders. Finally, in response to the problems and controversies of SAzymes in terms of bioactivity and biological applications, we point out the future opportunities and challenges of SAzymes and propose potential solutions and future directions to accelerate the development of SAzymes in biomedical fields (Fig. 1 ). Finally, an overview of the biological uses of SAzymes, along with issues and disagreements surrounding them, is provided. The outlook and challenges confronting SAzymes in the forthcoming period are also highlighted along with potential solutions and future pathways to expedite their development within the biomedical industry.
Schematic overview. Recent developments in nanozymes in terms of material preparation, enzyme-like catalytic activity and biological applications of nanozymes in Gastrointestinal (GI) diseases
Design principles and synthesis strategy of SAzymes
The preparation of frameworks, such as metal–organic frameworks (MOFs), covalent organic frameworks (COFs), hydrogen-bonded organic frameworks (HOFs) frameworks with various active centers, has actually been linked to a wide range of multi-enzyme activities. Because of this, scientists have experimented with a number of design approaches in an effort to maximize the dispersion, stability, especially catalytic activity in recent years. This section focuses on the remarkable advancements made recently in engineering techniques for activity tuning, such as modifications to the SAzymes framework and optimizations for SAzymes active centers. Based on this, the particular synthesis processes and modalities of SAzymes are described in order to further analyze their synthesis strategy (Fig. 2 ).
Optimization strategies for single-atom nanozymes at both framework structure and active center levels
Optimization of framework structure
It is essential to use an appropriate supporting substrate while synthesizing SAzymes. This is because, as a result of aggregating during catalysis, instability in the framework may cause the core metal atom to become inactive. The atoms that comprise the framework have the ability to interact with the atom of the core metal, so influencing the metal’s activity and, consequently, the total catalytic activity of nanozymes. Consequently, in this section, we investigate how the activity of nanozymes is affected by the optimization of various kinds of framework materials. MOFs, COFs, and HOFs are examples of crystalline porous materials that typically have excellent porosity and structural composition designability. They are also thought to have controllable morphologies, modifiable backbones, flexible active site structures, tunable charge transfer pathways, and designable porosities [ 18 , 19 ].
MOFs are unique because of their ability to fine-tune pore structure and their variety in design. They are created by coordinating metal ions with organic ligands [ 20 ]. They are typically made using a one-pot self-assembly method that creates metal-containing nodes on the spot. Ma et al. [ 21 ] introduced Zr-MOF nanozymatic coatings into natural bacterial cellulose (BC) nanofibers, and this design enabled the nanozymes to have multilayered macro–micro pores, which led to the full exposure of catalytic active sites and exhibited excellent enzyme-mimicking catalytic activity (Fig. 3 A). The same is true for CMZM, which has multi-enzymatic activity and can be utilized to reverse immunosuppressive TME. Additionally, the enzyme-like activities of CMZM improve the effectiveness of multimodal imaging-guided CDT and PDT treatments [ 14 ] (Fig. 3 B). The bimetallic MOF pathway has been applied to the optimization of MOFs. The Cu, Mn bimetallic nanozymes (Cu-TCPP-Mn) prepared from MOFs have combined high catalytic properties for SOD and CAT, and can synergistically scavenge ROS (Fig. 3 C) [ 22 ]. Another crystalline porous materials, COF, constructed through covalently linked organic units, show excellent properties for synthesizing SAzymes [ 23 , 24 , 25 ]. The one-dimensional iron porphyrin covalent organic skeleton (COF-CNT) coated on carbon nanotubes nanozymes were able to produce reactive oxygen species (ROS). Furthermore, the peroxidase-like activity of COF-CNT was much enhanced in the presence of an electric field, suggesting that the COF framework had a discernible impact on the enzymatic activity of the nanozymes [ 26 ]. When loaded with enzymes, pore congestion or partial clogging prevents COF from being used. HOFs are less stable than MOFs and COFs because they rely on hydrogen bonding interactions to stabilize their structure. However, because hydrogen bonding is typically weaker than ionic or covalent bonding, HOFs have an additional deformability that can be used to create flexible porous HOF materials [ 27 ].
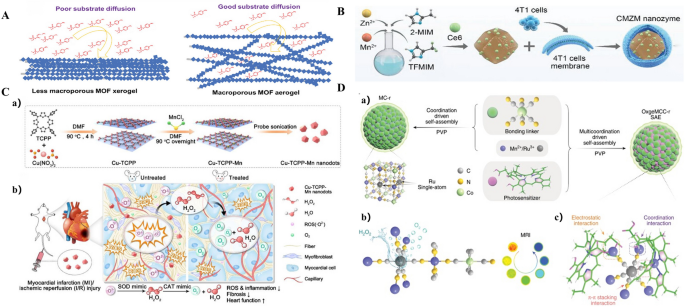
Copyright 2023. Wiley–VCH. B Illustration of CMZM preparation process. Reproduced with permission [ 14 ]. Copyright 2023. Wiley–VCH. C Schematic illustration of the design and synthesis of Cu-TCPP-Mn nanozyme for myocardial injury treatment. a) The bimetallic Cu-TCPP-Mn nanozyme was fabricated by embedding manganese and copper into the porphyrin via solvothermal method, followed by sonication into small MOF nanodots. b) Cu-TCPP-Mn nanozyme retained cascade activity that has been shown to scavenge ROS, inhibit inflammation, reduce myocardium fibrosis and promote constructive remodeling and vascularization in MI and I/R injury animal models. Reproduced with permission [ 22 ]. Copyright 2023. Ivyspring International Publisher. D Schematic illustration of OxgeMCC-r. a) Schematic illustration of OxgeMCC-r. OxgeMCC-r consists of catalytically active single-atom Ru site anchored in MCC with outer PVP protection layer. b) Partial molecular structure of OxgeMCC-r with active single-atom Ru site serving as catalase-like nanozyme for oxygen generation. c) Multicomponent coordination interactions within the OxgeMCC-r SAE. Reproduced with permission [ 28 ]. Copyright 2020. Springer Nature
Optimization scheme for MOF structures. A Illustration of the differences in diffusion between the less macroporous MOF nanozyme xerogel (left) and the microporous MOF nanozyme aerogel (right). Reproduced with permission [ 21 ].
Increasing the catalytic activity of SAzymes through optimization of these frameworks has become a major area of research interest. An essential technique for creating N-doped carbon materials for stabilizing and spreading substrates supported by enzymes is the pyrolysis of MOFs. The nanozymes prepared either by doping Ru into the framework [ 28 ] or by encapsulating ferritin in ZIF-8 [ 29 ] have excellent stability and catalytic activity (Fig. 3 D). It is also possible to tune the Fe–N coordination by optimizing the precursor type and pyrolysis temperature. Triple peroxidase-like activity and triple catalytic sites were added by Li et al. [ 30 ] to complement the synthesis of a unique core–shell nanocomposite, Prussian blue@Fe-covalent organic framework@Au (PB@Fe-COF@Au) (Fig. 4 A). On the other hand, the strategy of synthesizing porous N-doped carbon nano-enzymes using COF as a precursor increased the exposure of the catalytic site and exhibited stronger peroxidase-like activity [ 31 ]. Innovatively grown platinum nanoparticles and ultramicro rhodium nanoparticles on the surface of COF NPs, Gao et al. [ 32 ] and Zhang et al. [ 33 ], respectively, served as catalase mimics in situ. The optimized nanozymes demonstrated high affinity for the catalytic substrates and excellent peroxidase-mimicking activity (Fig. 4 B). In addition, the functional groups in the COF can act as Lewis acid–base sites within the porous skeleton to mimic the functions of amino acid residues and tailor the pore microenvironment around the active center, thus enhancing the catalytic activity of MOF-based nanozymes [ 34 ]. Constructing ionic HOFs is one of the strategies for HOF framework optimization. PFC-33 [ 35 ], the first anionic HOF synthesized, exhibits synergistic photodynamic and chemical antimicrobial efficiencies (Fig. 4 C). Based on this, Tong et al. [ 36 ] created three light-responsive HOFs nanomaterials with various pore structures. Next, they mimicked light-responsive oxidative enzymes using structurally well-defined hydrogen-bonded organic frameworks (HOFs), and by creating isostructured HOFs, they were able to demonstrate the importance of pore environments in mediating the activity of oxidative enzyme-like enzymes. This demonstrated that, in addition to active centers, pore environments have a significant impact on the activity of the nanozymes (Fig. 4 D). They suggested that the difference in pore channels could regulate the activity of isotope-like oxidase in structured HOFs. Ionic HOFs with anionic or cationic backbones were designed and synthesized to attract noncovalently bonded counterions in the pore channels. Currently, another optimization strategy for HOF frameworks is to add modifying substances to HOFs to improve the porosity and photodynamic efficiency. Yin et al. [ 37 ] performed surface modification by adding rabies virus glycoprotein (RVG)29. Not coincidentally, the final shell nanostructures were created by Liu et al. [ 38 ] utilizing a progressive ligand grafting technique (Fig. 4 E). It is evident that altering the morphology or spatial arrangement of HOFs affects the catalytic activity of the nanocomplexes built using them as frameworks; this may be due to the catalytic sites being completely exposed through physical or chemical mechanisms. Similar to the dual action of COF-MOFs, the combination of HOFs with MOFs also showed superior biological and chemical activities to those of MOFs alone [ 39 ].
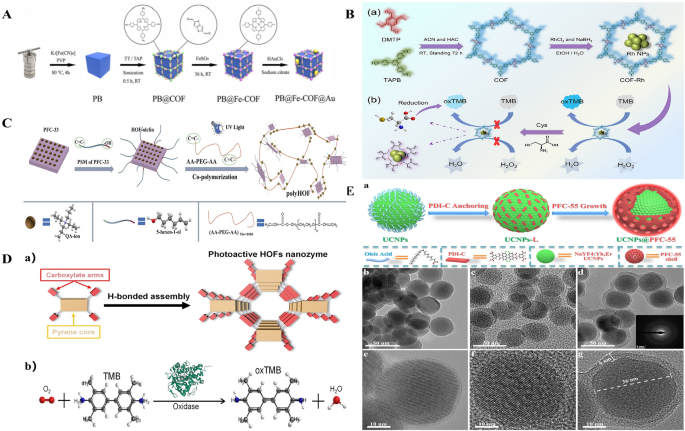
Copyright 2023. American Chemical Society. B Schematic illustration showing a) the synthesis of COF and COF-Rh, and b the strategy for Cys detection based on regulating the POD-like activity of COF-Rh. Reproduced with permission [ 33 ]. Copyright 2023. Elsevier. C Preparation of polyHOF by PSM followed by polymerization. Reproduced with permission [ 35 ]. Copyright 2020. Wiley–VCH. D a) Schematic of hydrogen bonding assembly of HOFs nanozymes. b The oxidase-like catalysis using TMB as the substrate. The colors used are: red for Oatom; blue for Natom; gray for Catom; white for Hatom. Reproduced with permission [ 36 ]. Copyright 2023. Wiley–VCH. E Fabrication of core–shell UCNPs@PFC-55. a) Fabrication of core–shell UCNPs@PFC-55. b, e TEM images of as-synthesized oleate-stabilized β-NaYF4:Yb,Er UCNPs; c, f PDI-C anchoring UCNPs-L; d, g the final products UCNPs@PFC-55 with inorganic UCNPs “cores” and organic PFC-55 “shells”. Reproduced with permission [ 38 ]. Copyright 2021. Wiley–VCH
Optimization scheme for COF and HOF structures. A Procedure for the Synthesis of PB@Fe-COF@Au. Reproduced with permission [ 30 ].
Carbon dots, a new type of carbon nanomaterial with luminescent properties, possess a rich source of raw materials, facile surface modification, low toxicit [ 40 ] and high biocompatibility [ 41 ]. Doping or loading individual metal atoms can boost the catalytic activity of carbon dots, including peroxidase activity, oxidase mimetic activity, and catalytic activity [ 10 , 42 ]. To enhance the biosafety, controllability, and catalytic activity of single-atom nanozymes, carbon dots were introduced during the synthesis of SAzymes [ 43 , 44 ]. By using a solvothermal technique, Han et al. [ 45 ] created carbon dots (CDs)-loaded single-atom iron nanozymes (ph-CDs-Fe SAzymes), which showed high POD activity (Fig. 5 A). Yu et al. [ 46 ] used polyethyleneimine (PEI) to modify carbon dots, receiving better optical characteristics (Fig. 5 B). Furthermore, by varying the reaction temperature, it was possible to optimize the various coordination structures of CD, thereby altering the bioactivity of the composites with SAzymes. For example, CD-loaded copper single-atom nanozymes with various coordination structures and peroxidase-like characteristics could be synthesized at various temperatures (Fig. 5 C) [ 47 ]. The aforementioned research has demonstrated a connection between the carbon dot form and the catalytic site variation, which in turn influences the biological activity of the composites.
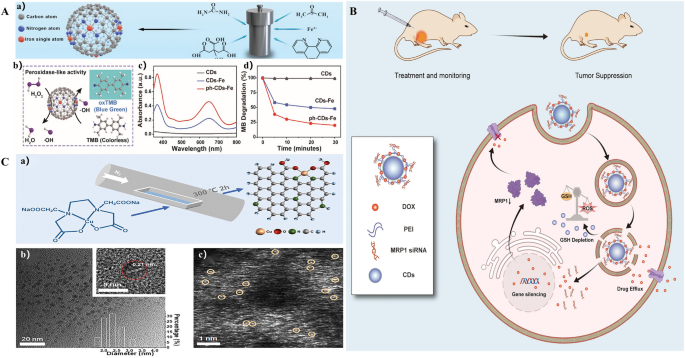
Copyright 2023. Wiley–VCH. B The schematic diagram delineates how CD-PEI-DOX-siMRP1 delivers doxorubicin to tumors and antagonizes chemoresistance by hindering drug efflux through knocking down MRP1 expression. Conversion of GSH to GSSG and subsequent ROS increase by CD-PEI oxidase and peroxidase activity further impairs MRP1 function. Collectively, CD-PEI-DOX-siMRP1 was capable of delivering drugs efficiently to tumor entities and retaining them in cells by hindering outflux of MRP1 through synergistic delivery of siRNA and perturbation of GSH-ROS balance. Reproduced with permission [ 46 ]. Copyright 2023. Dove Medical Press Ltd. C Synthesis and morphologies of Cu-CDs-300. a) Scheme of the calcining procedure for Cu-CDs-300 as an example. b) TEM and HRTEM images of Cu-CDs-300. c) Typical HAADF-STEM image of distributed single Cu atoms (orange circles) in Cu-CDs-300. Reproduced with permission [ 47 ]. Copyright 2023. Wiley–VCH
Optimization scheme for carbon dots structures. A Peroxidase-like activity of ph-CDs-Fe SAzyme. a) Schematic illustration of POD-like activity of CDs, Fe-CDs SAzyme, and ph-CDs-Fe SAzyme. b) POD-like activity of CDs, CDs-Fe, and ph-CDs-Fe SAzyme. c) Degradation of methylene blue (MB, 12.5 µg mL −1 , pH 5.0) by CDs, Fe-CDs SAzyme, and ph-CDs-Fe SAzyme in the presence of H 2 O 2 (5 mM). Reproduced with permission [ 45 ].
Because of their atomic characteristics and spatial arrangement, we found that materials that consist of crystalline frameworks, such as MOFs, COFs, and HOFs, are crucial for optimizing single-atom nanozyme complexes. MOFs structures possess a tendency to collapse in high-temperature environments, which is not conducive to a high degree of dispersion, and lead to changes in the oxidation state, phase purity and atomic level characterization of the metal, leaving the stability of the resulting nanozymes uncontrolled. Experimental data on COFs have collectively shown that COFs as a framework or carrier may cause complete or partial pore blockage on their surface, which significantly impacts the exposure of the active sites of the nanozymes. The inherent instability and structural recalcitrance are the primary obstacles to HOF development [ 48 ]. Previous research has conclusively demonstrated that the incorporation of functional groups typically shapes into new hydrogen bonds and alters the topology, which may have an effect on the function of SAzymes.
Optimization of active center
The experimental findings reveal an unbreakable link between particular atomic metal centers and catalytic activity. Ou et al. [ 49 ] suspected that material properties are affected by the spatial location of monatomic atoms. To examine their photocatalytic antibacterial activity, they created Cu single-atom-site nanozymes both within and outside of polyheptazinimide nano-(PHI) platforms. The results show that the interlayer-localized copper material (CuL/PHI) has a broader antimicrobial effect on a wide range of bacterial strains compared to other spatially arranged materials, and can achieve the same antimicrobial effect as antibiotics. During the catalytic process, Rh(V)N 4 and the preferentially formed Rh(V)-O-N 4 structure can act as active centers, enabling the SAzyme to exhibit excellent POD-like activity. This structure improves catalyst utilization and significantly reduces reaction energy through the “two-sided oxygen chain” catalytic reaction pathway [ 50 ]. According to Wang et al. [ 51 ], metal-H 2 O 2 interactions may have an impact on the catalytic activity of CAT. It was demonstrated that Ir-N 4 had the strongest interaction with H 2 O 2 , the lowest H 2 O 2 decomposition barrier, and the highest CAT-like activity out of the five active centers based on platinum group metals (M-N 4 , M = Ir, Ru, Rh, Pt, and Pd). In a manner similar to this, Cheng et al. [ 5 ] synthesized various active sites of metal-N to produce artificial metalloenzymes and a library of metalloenzyme imitators. Among the artificial metal nanozymes, they found that the Fe-like metal-centered nanozymes had the highest OXD-like activity, metal-centered nanozymes with a copper-like center demonstrated the highest level of POD-like activity.
Precious metals have strong catalytic activity and noble metal-precious metal bimetallic nanocatalysts have been reported. Fan et al. [ 53 ] wrapped a thin layer of palladium around gold nanorods (AuNRs) as the core to form Au@PdNRs, which showed notable oxidase-like activities under dark environment and plasma resonance excitation, and the enhanced catalytic activity was mainly due to the full exposure of the Pd surface used for catalysis. Both non-precious and precious characteristics of metals are displayed by nanozymes made of both types of metals. An ultrasmall single-atom Pt/CeO 2 with continuous catalytic activity was created by Yan et al. [ 54 ]. The highly ordered arrangement of metal atoms in the resultant nanocage allowed the SAzymes to have POD- and GSH peroxidase like (GSH-Px-like) catalytic activity and efficient ROS production under ultrasonic irradiation. The single-atom Pt generated a selective distribution on the crystalline surface of CeO 2 . Similarly, Zhong et al. [ 55 ] demonstrated that PtCu 3 nanocages can also be used as copper-based nanomaterials as novel acoustic sensitizers to generate ROS under irradiation.
It can be inferred from several experiments that the synergistic action of metals in multimetallic catalysts may result in greater selectivity and catalytic efficiency than in monometallic catalysts. A Cu/Zn bimetallic atom nanoconjugate enzyme (Cu/PMCS) was created by Liu et al. [ 56 ]. The findings indicated that doping Cu atoms increased the catalytic activity and GSH depletion of this nanoconjugate enzyme, which improved its anti-tumor capacity. Additionally, according to Lv et al. [ 57 ], the peroxidase catalytic activity of nanorods containing Au and Pt bimetallic atoms was significantly higher than that of nanozymes containing single metal atoms. Therefore, it is clear that adding more metal sites will increase the catalytic activity of nanozymes. The catalytic activity of single-atom nano-enzymes is connected with the ratio of metal atoms in addition to the influence of the quantity of metal atoms. Cai et al. [ 58 ] discovered that the shape, structure, and content of the products are influenced by the Au–Pd synthesis atomic ratio. In model oxidation reactions, 0D/2D Au–Pd nanocomposites demonstrated significantly increased peroxidase-mimetic catalysis. This was theorized to be directly related to changes in the electronic structure of Au–Pd and an increase in its specific surface area ratio.
Overall, a number of experiments have been carried out recently with the aim of enhancing the enzyme-like catalytic activity of single-atom nanozymes. These experiments range from the different types of single atoms and their spatial positions corresponding to different kinds of enzyme-like activities to the interactions between polyatoms and the changes in the ratio of polymetallic atoms significantly affecting the catalytic activity, which have produced encouraging results, and we anticipate the emergence of more effective and straightforward optimization based on the results.
Synthesis strategy of SAzymes
Synthesis process.
SAzymes have been synthesized in various ways, such as by constructing defect sites on metal hydroxide/oxide substrates, by creating spatially restricted domain effects on the substrate material, etc., which basically improve the interaction of metal atoms with the coordinating heteroatoms. For SAzymes, the most classical synthesis strategy is wet chemistry, including coprecipitation, impregnation, and ion exchange [ 59 ]. Using zeolite Y as a carrier, Cheng et al. [ 60 ] developed a straightforward wet impregnation technique to produce a stable nanocomposite (CeO 2 /Y). Tang et al. [ 61 ] developed a novel one-step wet chemical process after optimizing a number of investigations, and they used BSA to guide the formation of two-dimensional nanosheets (Fig. 6 A). To further improve the stability, biocompatibility, and surface functioning of the resulting BSA-NOTA-modified MnO 2 nanosheets (M-NS), they also presented an acoustic chemical synthesis method.

Copyright 2019. Wiley–VCH. B Schematic of the synthetic process for the Ni SAs@S/N-FCS. Reproduced with permission [ 65 ]. Copyright 2022. Wiley–VCH. C Schematic diagram of the synthesis process of ZnSA-HCNT. Reproduced with permission [ 66 ]. Copyright 2022. Wiley–VCH. D Illustration of the preparation process of PtTS-SAzyme. Reproduced with permission [ 67 ]. Copyright 2021. American Chemical Society. E Schematic of the Preparation Strategy for Fe–NO/NC. Reproduced with permission [ 68 ]. Copyright 2020. American Chemical Society. F Schematic showing the preparation of the SAFe-SWCNT film. Reproduced with permission [ 69 ]. Copyright 2021. Elsevier
Strategies for the synthesis of single-atom nanozymes. A Schematic illustration of two-step synthesis of M-NS. First, a novel “wet-chemical method” was applied to form 2D nanosheets under the direction of BSA. Second, a unique “sono-chemical method” was introduced to further enhance the stability, biocompatibility, and surface functionality of the M-NS. Reproduced with permission [ 61 ].
SAzymes can be prepared by direct pyrolysis for those precursor metal blocks or metal materials composed of thermodynamically unstable metals. Most types of SAzymes in the current study, especially those based on nitrogen-doped carbon materials, are mainly prepared by pyrolysis [ 62 ]. In the direct pyrolysis pathway, it is of utmost importance to break the metal–metal bonds of the precursor carriers and to minimize the escape of metal atoms at high temperatures [ 63 ]. Huang et al. [ 64 ] prepared single Fe atom nano-enzymes with Fe-N 5 active sites by pyrolyzing MOFs at 900 °C under N 2 atmosphere. Zhao et al. [ 65 ] pyrolyzed at 950 °C to generate Ni single-atom catalysts (Fig. 6 B). Similar to this, Li et al. [ 66 ] created zinc monoatomic nanozymes through the pyrolysis and high-temperature adsorption reactions of zinc with imidazole (Fig. 6 C). Coincidentally, Chen et al. [ 67 ] reversed the thermal sintering process and directly atomized platinum nanoparticles (Pt NPs) into single atoms to produce high-performance nanozymes (Fig. 6 D). However, the direct pyrolysis of MOFs has its drawbacks: during the pyrolysis process, the structure of MOFs tends to collapse due to high temperatures, leading to volume contraction, which is unfavorable for the high dispersion of monatomic metals.
In order to encourage the in-situ formation of volatile metal species in metal nanoparticles and induce their anchoring to the carrier, the gas migration technique entails the introduction of corrosive or reducing gases, such as ammonia, phosphine, and hydrogen chloride. Using a combination of NC, Fe NPs, and NH 4 Cl as precursors for pyrolysis, Li et al. [ 68 ] found that the resulting FeCl 2 could be readily vapor-diffused into the carriers at 500 °C, forming Fe monoatoms (Fig. 6 E). In a similar manner, Yu et al. [ 69 ] produced SAFe-SWCNT thin films by loading Fe NPs into single-walled carbon nanotubes (SWCNT) and then annealing the SWCNT during the process of XeF 2 introduction ( Fig. 6 F ) .
Methods for dispersing metal atoms
Single-atom metalloids can be obtained directly from precursors, while achieving a uniform dispersion of individual atoms and stopping them from migrating and aggregating to form NPs are the main goals of the synthesis of SAzymes [ 70 ]. UP to date, approaches based on enhanced metal-carrier interactions to capture isolated metal atoms on carriers mainly include spatial confinement, defect anchoring strategy and coordination stabilization. In addition, the research frequently uses chemical etching, gas migration, direct pyrolysis, and the electrochemical deposition approach.
Spatial confinement
The essence of spatial confinement lies in the encapsulation and separation of the metal precursor to immobilize the active center of the metal atom. Individual Pt atoms were carefully encapsulated in the six-membered ring of a Sodalite (SOD) cage within a Y molecular sieve by Chen et al. [ 71 ] using a template-guided approach. (Fig. 7 A). In order to create single-atom Mo SAzymes with varying coordination numbers, Wang et al. [ 72 ] enclosed MoO 2 (acac) 2 molecules in ZIF-8 pores. Following pyrolysis, the Mn 2+ absorbed by the hollow structure was transformed into single-atom Mn sites in the SAzymes. The molybdenum single-atom nanosize enzyme (MoSA-Nx-C) was established and verified for its peroxidase-like specificity (Fig. 7 B). By coordinating monoatomic manganese and nitrogen atoms in a hollow zeolite imidazolite skeleton, Yang et al. [ 73 ] created a polyethylene glycolated manganese-based SAE (Mn/PSAE). Mn/PSAE demonstrated notable therapeutic effects by producing a range of ROS and photothermal activities through stimulation of the tumor microenvironment (Fig. 7 C).
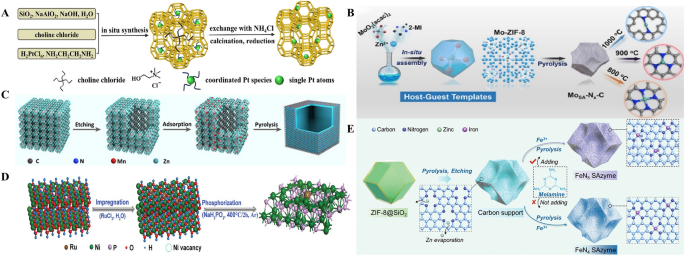
Copyright 2022. Wiley–VCH. B Schematic illustration for the fabrication strategy of MoSA-Nx-C catalysts. Reproduced with permission [ 72 ]. Copyright 2021. Elsevier. C Coordination of monoatomic manganese and nitrogen atoms in a hollow zeolitic imidazolite skeleton to construct Mn/PSAE. Reproduced with permission [ 73 ]. Copyright 2021. Wiley–VCH. D Schematic diagram for the synthesis of Ni5P 4 -Ru. Reproduced with permission [ 74 ]. Copyright 2020. Wiley–VCH. E Schematic illustration of synthesis process for FeN 5 SAzyme. Reproduced with permission [ 75 ]. Copyright 2022. Wiley–VCH
Methods for dispersing metal atoms: spatial confinement, defect anchoring strategy and coordination stabilization. A Illustration of the synthesis strategy to selectively encage single Pt atoms into the six-membered rings of SOD cages within Y zeolite. Reproduced with permission [ 71 ].
Defect anchoring strategy
Immobilizing metal atoms can also be accomplished effectively by carrier defects. By using crystal surface defects of CeO 2 clusters to trap Pt atoms, Yan et al. [ 54 ] successfully synthesized single-atom Pt/CeO 2 clusters for the treatment of traumatic brain damage. He et al. [ 74 ] constructed Ni(OH) 2 -rich nickel vacancies with RuCl 3 and obtained the target Ni 5 P 4 -Ru SAC by subsequent phosphorylation treatment, where strong interactions between the nickel vacancy defects and Ru cations enhanced the Ru doping rate (Fig. 7 D). Defects on the carbon carriers that served as anchor sites for the insertion of Fe atoms were created by Xu and associates [ 75 ]. The Fe atoms inserted into the flaws were immobilized by the N species in the NC substrate, and the FeN 5 SAzymes exhibited superior peroxidase-like activity in the tumor microenvironment. ZIF-8 precursors covered with SiO 2 that were pyrolyzed to cause Zn volatilization (Fig. 7 E).
Coordination stabilization
With regard to the ligand stabilization doctrine, it has been shown that metal-nonmetal bonds can significantly enhance metal-carrier interactions [ 70 ]. In order to improve the metal-carrier interactions and the charge redistribution of the surrounding atoms, Au atoms were added to NiFe layered double hydroxides by Zhang et al. [ 76 ], where Au atoms on O atoms produced the hydroxides. As a result, catalytic performance was enhanced. This led to improved catalytic performance. Ag atoms were uniformly distributed on TiO 2 carriers by Wang et al. [ 77 ], who then created Ag SAzymes with potent antiviral properties by means of strong Ag–O metal-carrier interactions. In order to facilitate the effective oxidative breakdown of propranolol in water, single Fe atoms were trapped in two-dimensional MoS nanosheets (Fe MoS) by Huang et al. [ 78 ] as extremely reactive catalysts for the non-homogeneous activation of sulfites.
The spatial confinement approach encapsulates fleeing metal atoms primarily through metal-carrier interactions. The stability of SAzymes is mostly caused by the interaction between the metal and the carrier [ 79 ]. However, the matrix framework in the wet-chemical synthesis method hides some of the metal active centers, resulting in a reduced usage of monoatomic metals. Similar to how the gas migration strategy chooses certain gases with corrosive or reducing properties to make the metal nanoparticles more volatile and increase their anchoring efficiency, the direct pyrolysis strategy uses high temperature to forcibly break strong metal–metal (M-M) bonds in the metal nanoparticles in the metal nanozymes.
Bioenzymatic-like catalytic activity of SAzymes
Artificial enzymes of different types and structures have different catalytic mechanisms, which lead to different final catalytic results (Fig. 8 ). This section will discuss several oxidoreductases mimicked by SAzymes that have been identified in recent studies, including POD, OXD, CAT, SOD, and GSH-Px (Table 1 ).
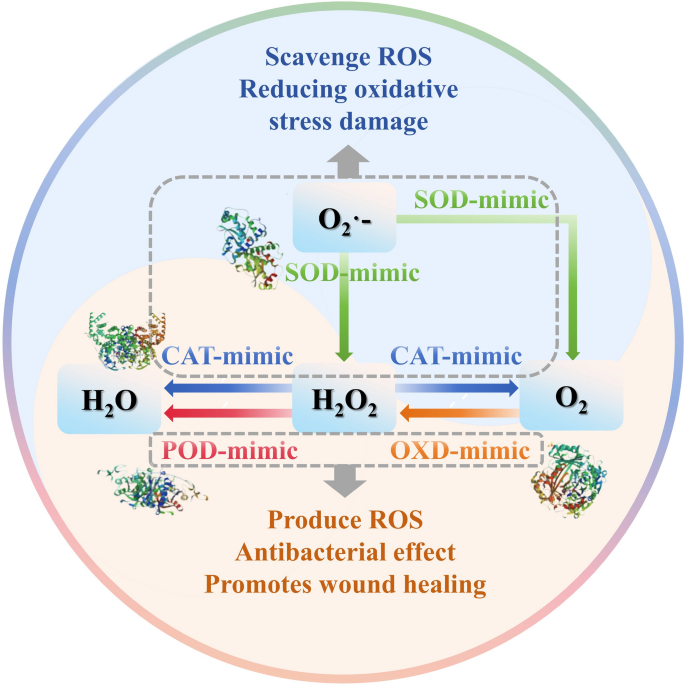
Schematic diagrams of the main enzyme-like catalytic activities of various nanozymes. POD-like activity of the nanozymes catalyzes peroxidation reaction using H 2 O 2 as substrate. OXD-like activity nanozymes catalyze the generation of H 2 O 2 in the presence of O 2 . POD-like activity and OXD-like activity nanozymes usually promote the production of ROS in organisms, resulting in a bactericidal effect. CAT-like activity helps to catalyze the decomposition of H 2 O 2 into O 2 and H 2 O. SOD-like activity mainly catalyzes the disproportionation of superoxide anion radicals (O2·-) into H 2 O2 and O 2 .POD-like activity and OXD-like activity nanozymes mainly scavenge excess ROS in the body and mitigate oxidative stress damage. OXD oxidase, POD peroxidase, CAT catalase, SOD superoxide dismutase, ROS reactive oxygen species
POD-mimetic activity
With H 2 O 2 serving as the electron acceptor, the POD-like activity of SAzymes catalyzes the synthesis of -OH, which severely damages cancer cells through oxidative stress by producing copious amounts of reactive oxygen species. POD-like SAzymes are widely distributed, and their nanomaterials contain a wide variety of transition metals, primarily Fe, Cu, Au, Pt, Co, and Ce [ 80 ]. Research has demonstrated that Fe-N-C single-atom nano-enzymes (Fe-N-C SAzymes), which are prepared by high-temperature calcination, have peroxidase-like activity [ 81 ]. More precisely, their enzymatic activity is less than that of peroxidase-like activity and is more akin to that of natural metalloproteinases. Fe-N-C SAzymes can detect H 2 O 2 colorimetrically and have a better catalytic efficiency, which makes them more useful in biology. For example, Fe-N-C single atom nanozymes have remarkable peroxidase mimic activity. Butyrylcholinesterase (BChE) activity can be highly sensibly biosensitized with these SAzymes. POD activity is also shown by other SAzymes that include Fe single atoms as their active centers [ 82 ]. New SAzymes based on carbon nanotubes loaded with Fe single atoms (CNT/FeNC) were used to detect H 2 O 2 , ascorbic acid, and glucose. These enzymes also showed good peroxidase-like activity [ 83 ]. According to the previously cited Wu et al. [ 84 ], Cu-N-C was produced on carbon nanosheets with a high concentration of Cu sites by replacing Fe in SAzymes with Cu, and it was shown that the peroxidase activity remained present. Furthermore, due of the dense dispersion of active copper atoms (≈5.1 wt%), the Cu-N-C SAzymes showed remarkable activity in mimicking natural peroxidase. Excellent POD activity was also shown by a series of single-atom nano-enzymatic liquids with different metal active centers. Furthermore, research on molybdenum single-atom nanozymes (MoSA-Nx-C) showed that the quantity of ligands at a particular molybdenum site controlled the specificity of the enzyme similar to peroxidase, offering a useful approach for the logical construction of the targeted nanozymes [ 72 ]. Carbon nanomaterials derived from zinc-based zeolite imidazolate frameworks (ZIF-8), which have zinc atoms dispersed atomically, can be used to make efficient single-atom peroxidase mimics [ 85 ].
OXD-mimetic activity
Using O 2 as the reaction substrate, OXD-like activities are a class of redox reactions that can imitate amino acid oxidase (AAO), uric acid oxidase (UOX), and glucose oxidase (GOX), depending on the kind of hydrogen donor [ 80 ]. Whereas SAzymes with limited reducing activity convert O 2 to H 2 O, those with significant reducing characteristics convert O 2 straight to H 2 O 2 . To examine the structure–activity link, Xu et al. [ 86 ] looked at Fe-N-C single-atom catalysts with two distinct coordination structures (NG-Heme and G-Heme). In particular, the contact between the active site and the intermediate can eventually boost its intrinsic oxidase activity. It was discovered that NG-Heme exhibits stronger oxidase activity than G-Heme. It has a high sensitivity for colorimetric carcinoembryonic antigen detection in clinical settings. Other research had looked into the performance of Fe-N-C SAzymes as oxidase-like SAzymes. These enzymes have atomically dispersed metal active sites that can be activated to produce reactive oxygen species [ 87 ]. Ru single atom loading based on carbon dots has produced SAzymes with strong stability, outstanding biocompatibility, and great activity [ 88 ]. Ru SAzymes can function as surrogates for glutathione oxidases, peroxidases, and other oxidative enzymes. ROS formation and glutathione depletion can be catalyzed simultaneously by them, which can exacerbate ROS-induced cell damage and ultimately lead to cancer cell death—a crucial stage in tumor treatment. Zhu et al. [ 73 ] reported that a manganese-based PEGylated SAN (Mn/PSAE) could efficiently produce large amounts of harmful ROS and mimic the different catalytic capabilities of OXD, POD, and CAT enzymes in addition to having better photothermal properties. FeN 5 , when contained within a carbon nanoframework (FeN 5 SA/CNF), possesses a well-defined active part and important synergistic effects among its single-atom nano-enzymatic active centers, which confer outstanding oxidase-like activity and multifunctional antimicrobial activities [ 64 ]. This implies that the axial N coordination in FeN 5 SA/CNF has an oxidase-like driving effect, increasing its catalytic activity relative to other SAzymes.
CAT-mimetic activity
An important antioxidant enzyme in the metabolism of ROS and H 2 O 2 is CAT. Cancer cells suffer significant oxidative damage when there is an abundance of ROS. By breaking down endogenous H 2 O 2 into O2, the CAT-like activity of SAzymes helps to improve the hypoxic environment around tumors in cancer cells. CAT-like nanozymes mimic natural CAT by generating H 2 O and O 2 from H 2 O 2 as substrates, providing electrons from the catalytic activity center to align and disproportionate H 2 O 2 . With atomically dispersed active centers, Cao et al. [ 89 ] constructed Co/PMCS as an enzyme-mimicking single-atom catalyst. It atomically dispersed active centers, which can eliminate H 2 O 2 by mimicking superoxide dismutase, catalase, and glutathione peroxidase, and its elimination efficiency is significantly higher than that of other nanozymes. With single-atom ruthenium serving as an active catalytic site anchored in the metal–organic framework Mn 3 [Co(CN) 6 ] 2 , the self-assembled photodynamic therapy nanoreagent OxgeMCC-r SAzymes produces OxgeMCC-r with a high loading capacity and CAT-like activity. Endogenous H 2 O 2 reacts with single-atom Ru in OxgeMCC-r to generate O 2 in situ, reducing hypoxic conditions in the tumor microenvironment [ 28 ]. The dual catalytic activity of single-atom iron-dispersed N-doped mesoporous carbon nanospheres (SAFe-NMCNs) nanozymes imitate both oxidase and peroxidase [ 90 ]. Experiments conducted in vitro and in vivo shown that the SAFe-NMCNs nanozymes could effectively limit the proliferation of tumor cells and had a synergistic therapeutic effect when combined with photothermal-enhanced nanocatalytic therapy. It is possible to mimic the actions of catalase (CAT) and SOD by anchoring atomically dispersed Fe-N 4 sites on N-doped porous carbon materials (Fe-SAs/NC) [ 91 ]. Because of this, Fe-SAs/NC can work as a dual-purpose single-atom-based enzyme (SAzyme) that scavenges reactive oxygen species (ROS) and gets rid of extra ROS that are created when cells are under oxidative stress.
SOD-mimetic activity
The transition metal elements Cu, Zn, Mn, and Fe are the key components of antioxidant metalloenzymes, which are found in organisms and regulate ROS levels in cells. These enzymes have SOD-like activity. Consequently, Cu, Mn, and Au are also the primary components of SOD-like activity in nanozymes including transition metal elements [ 80 ]. Because of their special qualities, carbon-based SOD mimetic nanozymes have lately been employed as promising antioxidant nanotherapeutics. The majority of reactive oxygen species (ROS) in the body are produced by superoxide anions, which can be scavenged by SAzymes having SOD-like activity. Yan et al. [ 54 ] developed a single-atom Pt/CeO with long-lasting catalytic activity, and the single-atom Pt greatly increased the endogenous CAT activity, resulting in greatly increased scavenging activity and enzyme-like activity of RONS of the final SAzymes compared to other materials. Co/PMCS was found to have atomically dispersed ligand-unsaturated active centers and hence also possesses SOD-like, CAT- and glutathione peroxidase activities [ 89 ]. In the end, this lowers pro-inflammatory cytokine levels and shields organs from harm. Due to its wealth of electronic energy levels and surplus of transition metal electronic states, Au offers a strong foundation for the development of atomic-level enzymes. It was demonstrated that the activities of Au 25 , Au 24 Cu 1 , and Au 24 Cd 1 are similar to those of GSH-Px, CAT, and SOD, respectively [ 92 ]. Au 24 Cu 1 reduces peroxides in the damaging brain, whereas Au 24 Cd 1 prioritizes the use of superoxides and significantly reduces inflammatory factors. In order to enhance the suppression of tumor growth, Lu et al. [ 93 ] created an N-doped carbon sphere doped with monoatomic copper, called Cu-HNCS, whose SAzymes may directly catalyze the breakdown of oxygen and hydrogen peroxide into ROS. And more research revealed that monoatomic copper was the primary source of the strong catalytic activity of Cu-HNCS.
GSH-Px-mimetic activity
The primary mechanism of catalysis in single-atom nanozymes exhibiting glutathione peroxidase activity involves the depletion of intracellular glutathione superoxide (GSH), resulting in the elimination of microbes and cancerous cells. Cu single-atom sites/N-doped porous carbon (Cu SASs/NPC) can function as GSH-Px-like nanozymes, which can deplete GSH in bacteria and hence greatly enhance the bactericidal effect. Cu SASs/NPC have a greater glutathione (GSH) depletion capacity than non-Cu-doped NPC [ 94 ]. In addition, copper SAzymes based on a bionic single-atom nano-enzyme system have been prepared in other studies and validated to have excellent POD-like activity [ 95 ]. It demonstrated effective tumor targeting both in vivo and in vitro, preventing the ability of cells to synthesize GSH from the source. An adaptive iron mutation platform developed by Cao et al. [ 96 ] builds upon single-atom nanozymes (SAzymes). By enabling SAzymes to deplete GSH in tumor cells on demand, the platform speeds up safe and selective iron apoptosis. Studies on breast and colon cancers have shown evidence of this adaptive anti-tumor response.
Biological applications of SAzymes in gastrointestinal (GI) diseases
While nanozymes have catalytic activity similar to that of genuine enzymes, they suffer from a number of serious problems, such as insufficient substrate selectivity, confusing architectures, and imprecise catalytic processes [ 97 ]. SAzymes have the advantages of facile separation, homogenous active sites, customizable coordination environments, and high atom utilization because they allow for the rational design of the coordination environment and the selection of metal atoms and their valence states. In particular, nitrogen-doped carbon-loaded SAzymes have structurally similar metal-Nx(M-Nx) sites to those of natural enzymes, and thus are considered to have a wide range of prospects for mimicking natural enzyme activities [ 85 ]. This section summarizes the biological application areas of nanozymes, primarily based on recent basic research, as illustrated in the image. These fields include bioassays, antibacterial, anticancer therapy, and anti-inflammatory effects (Fig. 9 ).
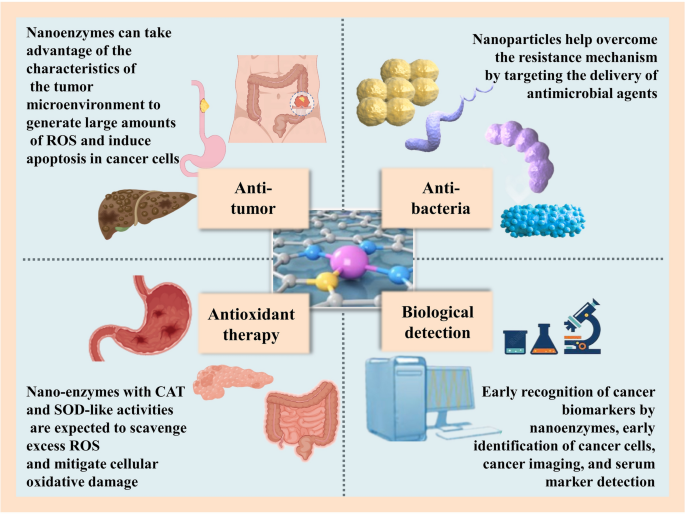
Various biological applications of nanozymes in gastrointestinal diseases
Antibacterial application
ROS and reactive oxygen species (RNS) are the most common free radicals, and excessive accumulation of free radicals can help nanoparticles to fight against microorganisms or to reduce microbial resistance [ 98 ]. SAzymes can undergo a Fenton-like reaction, which is utilized to kill bacteria by the ROS produced by the reaction, and have demonstrated excellent bactericidal effects in GI diseases (Table 2 ).
Due to their broad-spectrum and drug-free antibacterial capabilities, single-atom nanozymes with antimicrobial activity have been increasingly significant in antimicrobial therapy in recent years [ 99 ]. Escherichia coli , Staphylococcus aureus , and some multidrug-resistant bacteria (MDR) are also common pathogens in GI disorders. The zinc-based ZIF-8 with atomically dispersed zinc atoms was reported by Xu et al. [ 100 ] and its derived carbon nanomaterials can be used as effective single-atom peroxidase mimics. Lastly, it was confirmed that SAzyme with unsaturated Zn-N 4 sites is a powerful antimicrobial agent for wound treatment. It has strong antibacterial action against Pseudomonas aeruginosa as well as outstanding peroxidase-like activity. They conducted an analysis based on the particular mechanism by which Ag single atoms with high electrical conductivity could stimulate the MnO 2 oxygen vacancies, hence facilitating the entry of reactive compounds that are photocatalytic. In addition, the photothermal conversion efficiency is enhanced by the catalysis of single-atom Ag, leading to the enhancement of the redox properties of the crystalline materials. Additionally, it has been investigated that Cu single-atom site/N-doped porous carbon (Cu SASs/NPC) was discovered to exhibit POD-like activity after being effectively synthesized using a sequence of pyrolysis-adsorption procedures [ 56 ]. The POD activity was greatly increased by doping the material with a single atom of Cu. Additionally, photo-thermal characteristics of the materials were specifically and simultaneously optimized to speed up the consumption of GSH. This synergistic effect enabled Cu SASs/NPC to exhibit excellent antimicrobial properties against Escherichia coli. and methicillin-resistant S. aureus (MRSA). The effective in vitro antibacterial and in vivo anti-infective qualities of single iron atom nanocatalysts have also been emphasized in a number of research conducted in recent years. Huo et al. [ 101 ] created nitrogen-doped amorphous carbon (SAF NCs) nanocatalysts attached with single iron atoms to cause peroxidase-like activity in the presence of H 2 O 2 , which efficiently eliminated Staphylococcus aureus and Escherichia coli . In order to eradicate intracellular MRSA, Liu et al. [ 102 ] created the highly sought-after missile-like nanotherapeutic medication [email protected]. and produced extremely toxic ROS through enzymatic activity at the center of FeSAs. A multifunctional Cu single-atom nanozyme (l-Arg@Cu-SAzymes) loaded with l-arginine was created by Qiu et al. [ 103 ]. This strategy was thought to be a promising way to treat MDR infection because it synergized with ROS and RNS to give the therapeutic system a strong antimicrobial efficacy and an enhanced tissue remodeling ability. In addition, antimicrobial activities against Escherichia coli [ 64 ] . and MRSA [ 89 ] have been demonstrated in ongoing experiments.
Despite the fact that ROS typically cause great harm to bacterial cells by damaging their cell membranes, as demonstrated by the aforementioned research, single-atom nanozymes are crucial for antibacterial applications primarily because of their biomimetic properties (particularly their POD-like activity).
Antitumor therapy
One of the primary mechanisms for inducing apoptosis in tumor cells is the generation of ROS. In the biological arena, particularly in the area of anti-tumor therapy, they have achieved remarkable strides due to the multiple mimetic enzyme activities of SAzymes and the simultaneous creation of multiple ROS [ 104 ].
Wang et al. [ 105 ] designed a protein-supported single-atom copper nanozyme (BSA-Cu SAzymes), which possesses ROS-generating and GSH-depleting effects, to effectively restore the elevated autophagy level of F. nucleatum and the ROS resistance of the tumor cells in situ to synergistically killing colorectal cancer (CRC) cells (Fig. 10 ). Furthermore, BSA-Cu SAzymes have a good biosafety profile, making them a promising new treatment for colorectal cancer as they can be processed by the kidneys. Also relevant to the tumor microenvironment, probiotics may enable cancer biotherapy by secreting antitumor or immunomodulatory drugs in the tumor microenvironment. However, the efficacy and accuracy of probiotics in cancer treatment is limited, and the addition of nanozymes can ameliorate this shortcoming. Furthermore, studies on colon cancer both in vivo and in vitro have shown that the composite nanozyme system can efficiently suppress tumor growth via photothermally enhanced nano-catalytic synergistic therapies, offering a novel way to boost the effectiveness of chemodynamic therapy (CDT) [ 106 , 107 ].
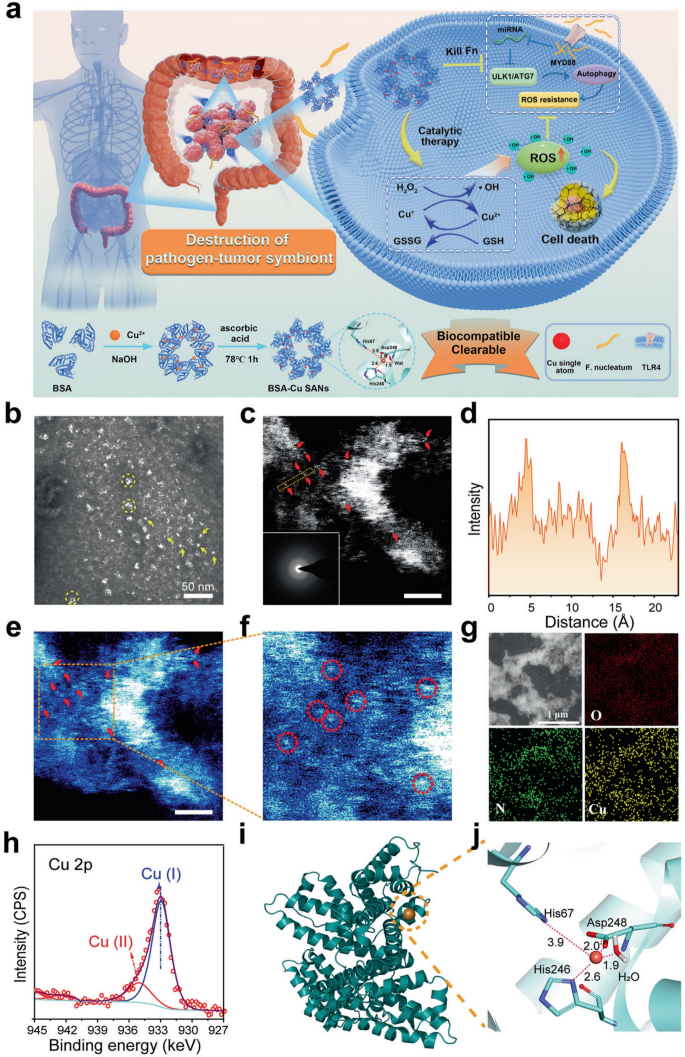
Formation and characterization of BSA-Cu SAN. a Schematic illustration of the synthesis of BSA-Cu SAN and its function of destroying pathogen-tumor symbionts for antitumor therapy, b Negative-stain electron microscopy image of BSA-Cu SAN. White bots represent BSA-Cu SAN, some of which are marked by yellow arrows. c Atomic HAADF image of BSA-Cu SAN with SAED pattern inset in the bottom left corner and partial single Cu atoms highlighted in yellow dash circles. d The intensity spectrum image along the distance in the yellow dash tangle in ( c ). e Pseudo-color image of corresponding intensity of ( c ). f The enlarged image of ( e ). g Elemental mapping of BSA-Cu SAN. h Cu 2p XPS spectra of BSA-Cu SAN. i Binding site of Cu + on BSA based on the highest docking score. j Protein-ligand interaction diagram for Cu + . Reproduced with permission [ 105 ]. Copyright 2023, Springer Nature
Liu et al.’s study [ 108 ] produced novel and effective Ir-N 5 single-atom nanozymes for the treatment of liver tumors. These SAzymes mimic the enzyme cascade and disrupt the redox and metabolic homeostasis of the tumor region, producing an anticancer effect at the tumor site and amplifying oxidative stress by increasing ROS, which kills tumor cells with less impact on normal cells, resulting in an effective cancer therapy. One of the worst tumors is pancreatic cancer (PDAC), and using nanomaterials offers a fresh strategy to treat drug-resistant pancreatic. Currently, more experiments in the field of pancreatic cancer are carried out through the synergistic action of drugs or organic molecules with nanozymes to target delivery to the tumor site and reduce the drug resistance of cancer cells. Zhao et al. [ 109 ] synergized a metal-based SAzymes coupling (Fe@Fe 3 O 4 ) with a naturally occurring biologically active organic molecule (ginseng saponin RG3) to construct a novel nanomedicine, which releases Fe 3+ in the tumor TME and Fe 2+ in the tumor TME and efficiently generates ROS thereby promoting cancer cell apoptosis. This also provides a new strategy for metal–organic nanocomposites to play a role in anticancer therapy.
Based on the cytochrome P450 structure, Sun et al. [ 110 ] created sandwich-like Sazymes and discovered that IrN 4 -S-TMN 4 (TM = Co, Rh, Pd) showed selective CO 2 reduction to CO with a special artificial CO dehydrogenase (CODH). They hypothesize that the unique enzyme-like activity and selectivity of SANs result from the precise control of sulfur atoms over the electron density, and that this control is contingent upon the nature and characteristics of the transition metals present in the surface layer. Feng et al. [ 111 ] immobilized ultramicro gold nanozymes in a metal–organic skeleton to establish a gold-iron SAzyme, which showed promising anti-esophageal cancer effects in vivo via the ferroptosis pathway. The same application of the ferroptosis pathway was also validated among the palladium nanozymes constructed by Zhang et al. [ 112 ]. Furthermore, an adaptive SAzymes-based iron death platform was constructed. This platform not only specifically enhances ROS generation activity, but also empowers SAzymes to consume GSH on demand in tumor cells, thereby accelerating selective and safe ferroptosis. The above anti-tumor responses have been demonstrated in colon cancers [ 96 ].
The limitations of traditional tumor treatment techniques have spawned the development of novel nanotechnology for the treatment of tumors. Currently, the application of nanozymes in gastrointestinal tumors is mainly limited to colorectal, liver and pancreatic cancers, and more studies are needed to explore their biological applications in other solid tumors (Table 2 ).
Antioxidative therapy
Through chemical redox processes, antioxidant nanostructures can neutralize ROS. Numerous studies have suggested that by scavenging ROS and lowering the release of inflammatory factors, nanozymes can display superior anti-inflammatory and antioxidant benefits in the treatment of ulcerative colitis and inflammatory bowel disease (IBD) alone [ 9 , 113 ]. Nevertheless, the contribution of biological SAzymes structures, which resemble natural enzymes in their activity, to the elimination of ROS remains incompletely understood.
ROS are scavenged by atomically dispersed Fe-N 4 sites anchored on N-doped porous carbon materials (Fe-SAs/NC), which act as a bifunctional SAzyme for the removal of excess ROS produced during cellular oxidative stress. These sites mimic two antioxidant enzymes, CAT-like and SOD-like [ 91 ]. Comparable Fe-N/C monoatomic catalysts also exhibit glutathione-like, catalase-like, oxidase-like, and peroxidase-like activity. Intracellular H 2 O 2 levels were successfully regulated by Fe-N/C SAzymes [ 114 ]. The findings of the two studies mentioned above demonstrated that the synthesized Fe-N/C SACs and Fe-SAs/NC were both more effective at scavenging the excess ROS that the oxidatively stressed cells produced. This suggests that the SAzymes could effectively shield the cells from the harm caused by cellular oxidative stress by scavenging the intracellular ROS, opening the door to the treatment of diseases linked to ROS. Not coincidentally, single-atom Pt/CeO 2 treats brain injury by scavenging RONS by a mechanism that exhibits POD-like, CAT-like, and OXD-like multienzymatic catalytic activities [ 54 ]. Similar phenomena were found in an in vitro E. coli mouse model, which demonstrated the antioxidant activity of Co-SAzymes [ 89 ]. The CAT-like, SOD-like, and GSH-Px-like activities of Co-SAzymes reduced ROS and RNS in sepsis by 60–80%, and pro-inflammatory cytokine levels were reduced to 10% after 2 weeks of treatment. Because of its unique activity and kinetic features that are naturally similar to those of APX, graphitized carbon nitride (Cu SAs/CN) anchored with isolated single copper atoms can be employed to successfully protect H 2 O 2 -treated cells from oxidative damage in vitro [ 115 ]. Liu et al. [ 116 ] synthesized Pt@PCN222-Mn on the basis of manganese (III) porphyrin, which was doped with Mn-MOF to mimic superoxide dismutase and convert oxygen radicals into hydrogen peroxide. The experiments also mimicked the catalytic activity of catalase by doping platinum nanoparticles. Both in vitro and in vivo experimental measurements demonstrated the synergistic ROS scavenging ability of this integrated cascade of nanozymes.
Recent research has demonstrated that single-atom nanozymes, with their huge specific surface area, unusual electron packing, and superior electrical conductivity, possess intrinsic antioxidant characteristics. The application of nanozymes is a viable strategy to remove the excess intracellular ROS and to maintain the homeostasis of the cellular redox system because, despite the presence of several antioxidant natural enzymes such as CAT, SOD, and GSH-Px in the cellular system, the overexpression of ROS induced by pathological conditions inhibits the activity of the natural enzymes [ 117 , 118 ] (Table 2 ).
Biological detection
Excess GSH is a cancer biomarker that is crucial for preserving intracellular redox balance in tumor cells. Hemoglobin (Hb) as a Fe source embedded in zeolite imidazolate framework (ZIF-8) was utilized by Chen et al. [ 119 ] to create porous single-atom Fe nanozymes (pFe SAzymes) in a convenient and large-scale method, and then developed a pFe SAzymes-GSH assay, which can accurately detect GSH levels in tumor cells transplanted from liver in situ. The pFe SAzymes-GSH assay can detect GSH levels in liver in situ transplanted tumor cells with millimolar accuracy and avoids the need for complex manipulation, making it a simple, fast and accurate visualization method for identifying tumor boundaries. Catalytic nanomaterials called iron SAzymes (Fe-N-C SAzymes) were also made, and it was discovered that adenine and thymine exhibited a greater adsorption affinity on Fe-N-C SAzymes. Sun et al. [ 120 ] designed Apt/Fe-N-C SAzymes for the colorimetric assay of cancer cells based on the observation that one DNA sequence (adenine) in duplex DNA binds to Fe-N-C SAzymes and the other DNA sequence (i.e., aptamer) binds to cancer cells. This discovery offers a novel application of SAzymes in biomedicine. Nanozymes have also been used to distinguish between normal and cancerous cells for early identification of cancer. SAzymes has been utilized in investigations for the detection of various blood indicators in addition to tumor-related diagnoses (Table 2 ). In order to enable practical uric acid (UA) monitoring in serum samples, Hu et al. [ 121 ] investigated an A-Co-NG single-atom catalyst for electrochemical UA detection for the first time. They did this by attaching high-density and isolated cobalt atoms on an N-doped graphene substrate. Fe-SAzyme, which was produced by Zhou et al. [ 122 ], has a built-in colorimetric assay for galactose measurement and can be utilized as a substitute approach for diagnosing galactosemia. A flow-injection chemiluminescence immunoassay was created for the quick and accurate detection of serum 5-fluorouracil (5-Fu) in serum [ 123 ] based on the validation of the Fenton-like activity of Co-SAzymes.
Discussion and perspectives
Among the deadliest cancers, gastrointestinal cancers cause around one-third of cancer-related deaths globally [ 124 ]. A number of gastrointestinal and liver conditions, such as inflammatory bowel disease (IBD) [ 125 ], colorectal cancer (CRC) [ 126 ], and alcohol-associated liver disease [ 127 ], have been related to alterations in the human gut microbiota. When inflammation reaches advanced stages, it can result in multiple organ dysfunction syndrome (MODS), infectious necrosis, and systemic inflammatory response syndrome (SIRS). One such condition is pancreatitis, which has a high morbidity and mortality rate and can be fatal [ 128 ]. Even though the digestive system is now developing quickly, new technologies are still required to solve current issues due to the diversity and complexity of disorders of the digestive tract, which places more demands on early diagnosis and accurate treatment.
Environmental protection, antibacterial, anticancer, and sensing are just a few of the many applications for SAzymes. Rethinking the connection between the structure of SAzymes and their active roles has proven difficult in light of the substantial changes that have occurred recently in the content, structure, and morphology of these molecules. Enzymes possessing atomically distributed metal active sites, as well as the ability to bind any ligand on appropriate carriers, exhibit significant promise for medicinal uses. This work establishes the groundwork for a later discussion of the function of SAzymes in gastrointestinal disorders by reviewing the most recent design principles and the preparation procedure of SAzymes, as well as by classifying and elaborating the biomimetic activities.
In addition, the complex internal environment and immune microenvironment in the body may affect the efficacy of SAzymes. The future research of SAzymes strategy is suggested by the significant number of vacancies in their study of inflammatory digestive system disorders such pancreatitis and cholecystitis. Currently, clinical investigations of SAzymes are not met, mostly due to the uncertainty over their biosafety. The ability of single-atom nanozymes to withstand degradation is one of the more significant variables. Despite numerous animal investigations have demonstrated that many SAzymes do not have harmful effects on other organs, the risks associated with a continuous presence of these enzymes in the bloodstream remain unclear, particularly with regard to critical organs like the heart and brain [ 129 ]. Furthermore, since different SAzymes have different active centers and framework structures, toxicity occurs when their structural composition, elemental loading composition, internal environment, and mode of administration are changed. Future research should focus on improving the in vivo biostability of single-atom nanozymes and clarifying the scope of their uses in microbial bioassay, targeted drug delivery, immune regulation and anti-inflammatory therapy [ 130 , 131 ]. A thorough safety evaluation that takes into account of pharmacokinetics, body organ distribution, and in vivo metabolism is still necessary for the clinical use of SAzymes.
Availability of data and materials
Not applicable.
Meyers WC. A study of gastric mucosa in various diseases affecting the upper part of the gastrointestinal tract. Gastroenterology. 1948;10:923–38.
CAS PubMed Google Scholar
Hanauer SB. The burdens of digestive diseases. Nat Rev Gastroenterol Hepatol. 2009;6:377.
Article PubMed Google Scholar
Islami F, Miller KD, Siegel RL, Fedewa SA, Ward EM, Jemal A. Disparities in liver cancer occurrence in the United States by race/ethnicity and state. CA Cancer J Clin. 2017;67:273–89.
Ap K. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021. https://doi.org/10.1038/s41575-021-00457-x .
Article Google Scholar
Dekker E, Tanis P, Vleugels J, Kasi P, Wallace M. Colorectal cancer. Lancet. 2019. https://doi.org/10.1016/S0140-6736(19)32319-0 .
Hindson J. Digestive disease week 2023. Nat Rev Gastroenterol Hepatol. 2023;20:483.
Wadhwa V, Patel P, Grover D, Ali F, Thosani N. Interventional gastroenterology in oncology. CA: A Cancer J Clin. 2023. https://doi.org/10.3322/caac.21766 .
Zhong J, Yang X, Gao S, Luo J, Xiang J, Li G, Liang Y, Tang L, Qian C, Zhou J, Zheng L, Zhang K, Zhao J. Geometric and electronic structure-matched superoxide dismutase-like and catalase-like sequential single-atom nanozymes for osteoarthritis recession. Adv Funct Mater. 2023;33:2209399.
Article CAS Google Scholar
Cao Y, Cheng K, Yang M, Deng Z, Ma Y, Yan X, Zhang Y, Jia Z, Wang J, Tu K, Liang J, Zhang M. Orally administration of cerium oxide nanozyme for computed tomography imaging and anti-inflammatory/anti-fibrotic therapy of inflammatory bowel disease. J Nanobiotechnol. 2023;21:21.
Geng H, Chen J, Tu K, Tuo H, Wu Q, Guo J, Zhu Q, Zhang Z, Zhang Y, Huang D, Zhang M, Xu Q. Carbon dot nanozymes as free radicals scavengers for the management of hepatic ischemia-reperfusion injury by regulating the liver inflammatory network and inhibiting apoptosis. J Nanobiotechnol. 2023;21:500.
Zhe Y, Wang J, Zhao Z, Ren G, Du J, Li K, Lin Y. Ascorbate oxidase-like nanozyme with high specificity for inhibition of cancer cell proliferation and online electrochemical DOPAC monitoring. Biosens Bioelectron. 2023;220:114893.
Article CAS PubMed Google Scholar
Li M, Chen J, Wu W, Fang Y, Dong S. Oxidase-like MOF-818 nanozyme with high specificity for catalysis of catechol oxidation. J Am Chem Soc. 2020;142:15569–74.
Cao C, Zhang T, Yang N, Niu X, Zhou Z, Wang J, Yang D, Chen P, Zhong L, Dong X, Zhao Y. POD nanozyme optimized by charge separation engineering for light/pH activated bacteria catalytic/photodynamic therapy. Signal Transduct Target Ther. 2022;7:86.
Article CAS PubMed PubMed Central Google Scholar
Wang R, Qiu M, Zhang L, Sui M, Xiao L, Yu Q, Ye C, Chen S, Zhou X. Augmenting immunotherapy via bioinspired MOF-based ROS homeostasis disruptor with nanozyme-cascade reaction. Adv Mater. 2023;35:e2306748.
Jing H, Ren Y, Zhou Y, Xu M, Krizkova S, Heger Z, Lu Q, Wang S, Liang X, Adam V, Li N. Remodeling of the liver fibrosis microenvironment based on nilotinib-loaded multicatalytic nanozymes with boosted antifibrogenic activity. Acta Pharm Sin B. 2023;13:5030–47.
Liu L, Zhang Y, Li X, Deng J. Microenvironment of pancreatic inflammation: calling for nanotechnology for diagnosis and treatment. J Nanobiotechnology. 2023;21:443.
Min DK, Kim YE, Kim MK, Choi SW, Park N, Kim J. Orally administrated inflamed colon-targeted nanotherapeutics for inflammatory bowel disease treatment by oxidative stress level modulation in colitis. ACS Nano. 2023;17:24404–16.
Wang C, Lv Z, Yang W, Feng X, Wang B. A rational design of functional porous frameworks for electrocatalytic CO2 reduction reaction. Chem Soc Rev. 2023;52:1382–427.
Ding G, Zhao J, Zhou K, Zheng Q, Han S-T, Peng X, Zhou Y. Porous crystalline materials for memories and neuromorphic computing systems. Chem Soc Rev. 2023;52:7071–136.
Xu K, Zhang S, Zhuang X, Zhang G, Tang Y, Pang H. Recent progress of MOF-functionalized nanocomposites: from structure to properties. Adv Colloid Interface Sci. 2023;323:103050.
Ma K, Cheung YH, Kirlikovali KO, Xie H, Idrees KB, Wang X, Islamoglu T, Xin JH, Farha OK. Fibrous Zr-MOF nanozyme aerogels with macro-nanoporous structure for enhanced catalytic hydrolysis of organophosphate toxins. Adv Mater. 2023. https://doi.org/10.1002/adma.202300951 .
Article PubMed PubMed Central Google Scholar
Xiang K, Wu H, Liu Y, Wang S, Li X, Yang B, Zhang Y, Ma L, Lu G, He L, Ni Q, Zhang L. MOF-derived bimetallic nanozyme to catalyze ROS scavenging for protection of myocardial injury. Theranostics. 2023;13:2721–33.
Liang R-R, Ru-Han A, Shun-Qi X, Qi Q-Y, Zhao X. Fabricating organic nanotubes through selective disassembly of two-dimensional covalent organic frameworks. J Am Chem Soc. 2020;142:70–4.
Sun Q, Fu CW, Aguila B, Perman J, Wang S, Huang H-Y, Xiao F-S, Ma S. Pore environment control and enhanced performance of enzymes infiltrated in covalent organic frameworks. J Am Chem Soc. 2018;140:984–92.
An S, Xu Q, Ni Z, Hu J, Peng C, Zhai L, Guo Y, Liu H. Construction of covalent organic frameworks with crown ether struts. Angew Chem Int Ed Engl. 2021;60:9959–63.
Yao S, Zhao X, Wang X, Huang T, Ding Y, Zhang J, Zhang Z, Wang ZL, Li L. Bioinspired electron polarization of nanozymes with a human self-generated electric field for cancer catalytic therapy. Adv Mater. 2022;34:e2109568.
Zhang Z, Ye Y, Xiang S, Chen B. Exploring multifunctional hydrogen-bonded organic framework materials. Acc Chem Res. 2022;55:3752–66.
Wang D, Wu H, Phua SZF, Yang G, Qi Lim W, Gu L, Qian C, Wang H, Guo Z, Chen H, Zhao Y. Self-assembled single-atom nanozyme for enhanced photodynamic therapy treatment of tumor. Nat Commun. 2020;11:357.
Liang J, Johannessen B, Wu Z, Webster RF, Yong J, Zulkifli MYB, Harbort JS, Cheok YR, Wen H, Ao Z, Kong B, Chang SLY, Scott J, Liang K. Regulating the coordination environment of mesopore-confined single atoms from metalloprotein-MOFs for highly efficient biocatalysis. Adv Mater. 2022;34:e2205674.
Li J, Gao M, Xia X, Cen Y, Wei F, Yang J, Wang L, Hu Q, Xu G. Spherical hydrogel sensor based on PB@Fe-COF@Au nanoparticles with triplet peroxidase-like activity and multiple capture sites for effective detection of organophosphorus pesticides. ACS Appl Mater Interfaces. 2023;15:6473–85.
Gao P, Wang K, Wei R, Shen X, Pan W, Li N, Tang B. A covalent organic framework-derived M1 macrophage mimic nanozyme for precise tumor-targeted imaging and NIR-II photothermal catalytic chemotherapy. Biomater Sci. 2023;11:7616–22.
Gao P, Wei R, Chen Y, Li X, Pan W, Li N, Tang B. Pt nanozyme-bridged covalent organic framework-aptamer nanoplatform for tumor targeted self-strengthening photocatalytic therapy. Biomaterials. 2023;297:122109.
Zhang L, Zhang W, Nie Y, Wang Y, Zhang P. Covalent organic framework-supported ultrasmall Rh nanoparticles as peroxidase mimics for colorimetric sensing of cysteine. J Colloid Interface Sci. 2023;636:568–76.
Zhang L, Liu Z, Deng Q, Sang Y, Dong K, Ren J, Qu X. Nature-inspired construction of MOF@COF nanozyme with active sites in tailored microenvironment and pseudopodia-like surface for enhanced bacterial inhibition. Angew Chem Int Ed Engl. 2021;60:3469–74.
Liu B, Pan X, Nie D, Hu X, Liu E, Liu T. Ionic hydrogen-bonded organic frameworks for ion-responsive antimicrobial membranes. Adv Mater. 2020;32:e2005912.
Tong L, Lin Y, Kou X, Shen Y, Shen Y, Huang S, Zhu F, Chen G, Ouyang G. Pore-environment-dependent photoresponsive oxidase-like activity in hydrogen-bonded organic frameworks. Angew Chem Int Ed Engl. 2023;62:e202218661.
Yin N, Wang Y, Liu Y, Niu R, Zhang S, Cao Y, Lv Z, Song S, Liu X, Zhang H. A cholesterol metabolic regulated hydrogen-bonded organic framework (HOF)-based biotuner for antibody non-dependent immunotherapy tailored for glioblastoma. Adv Mater. 2023;35:e2303567.
Liu B, Pan X, Zhang D, Wang R, Chen J, Fang H, Liu T. Construction of function-oriented core-shell nanostructures in hydrogen-bonded organic frameworks for near-infrared-responsive bacterial inhibition. Angew Chem Int Ed. 2021;60:25701–7.
Cai Z, Xia Y, Ito Y, Ohtani M, Sakamoto H, Ito A, Bai Y, Wang Z, Yamauchi Y, Fujita T. General synthesis of MOF nanotubes via hydrogen-bonded organic frameworks toward efficient hydrogen evolution electrocatalysts. ACS Nano. 2022;16:20851–64.
Wang C, Pan C, Wei X, Yang F, Wu W, Mao L. Emissive carbon dots derived from natural liquid fuels and its biological sensing for copper ions. Talanta. 2020;208:120375.
Kang L, Hu Y, Liu L, Wu J, Zhang S, Zhao Q, Ding F, Li Q, Zhang J. Growth of close-packed semiconducting single-walled carbon nanotube arrays using oxygen-deficient TiO2 nanoparticles as catalysts. Nano Lett. 2015;15:403–9.
Molaei MJ. Carbon quantum dots and their biomedical and therapeutic applications: a review. RSC Adv. 2019;9:6460–81.
Li X, Ding S, Lyu Z, Tieu P, Wang M, Feng Z, Pan X, Zhou Y, Niu X, Du D, Zhu W, Lin Y. Single-atomic iron doped carbon dots with both photoluminescence and oxidase-like activity. Small. 2022;18:e2203001.
Zhang L, Dong Q, Hao Y, Wang Z, Dong W, Liu Y, Dong Y, Wu H, Shuang S, Dong C, Chen Z, Gong X. Drug-primed self-assembly of platinum-single-atom nanozyme to regulate cellular redox homeostasis against cancer. Adv Sci. 2023;10:e2302703.
Han Y, Ge K, Zhao Y, Bottini M, Fan D, Wu W, Li L, Liu F, Gao S, Liang X, Zhang J. Modulating the coordination environment of carbon-dot-supported Fe single-atom nanozymes for enhanced tumor therapy. Small. 2023. https://doi.org/10.1002/smll.202306656 .
Yu H, Tang K, Cai Z, Lin X, Huang Y, Yu T, Zhang Q, Wang Q, Wu L, Yang L, Shan H, Luo H. Carbon dots-based nanozyme for drug-resistant lung cancer therapy by encapsulated doxorubicin/siRNA cocktail. Int J Nanomed. 2023;18:933–48.
Ma Y, Zhang M, Wu J, Zhao Y, Du X, Huang H, Zhou Y, Liu Y, Kang Z. The key effect of carboxyl group and CuN2 O2 coordinate structure for Cu, N Co-doped carbon dots with peroxidase-like property. Small. 2023;19:2300883.
Yu D, Zhang H, Ren J, Qu X. Hydrogen-bonded organic frameworks: new horizons in biomedical applications. Chem Soc Rev. 2023;52:7504–23.
Ou H, Qian Y, Yuan L, Li H, Zhang L, Chen S, Zhou M, Yang G, Wang D, Wang Y. Spatial position regulation of cu single atom site realizes efficient nanozyme photocatalytic bactericidal activity. Adv Mater. 2023;35:2305077.
Ji S, Jiang B, Hao H, Chen Y, Dong J, Mao Y, Zhang Z, Gao R, Chen W, Zhang R, Liang Q, Li H, Liu S, Wang Y, Zhang Q, Gu L, Duan D, Liang M, Wang D, Yan X, Li Y. Matching the kinetics of natural enzymes with a single-atom iron nanozyme. Nat Catal. 2021;4:407–17.
Wang Z, Wang W, Wang J, Wang D, Liu M, Wu Q, Hu H. Single-atom catalysts with ultrahigh catalase-like activity through electron filling and orbital energy regulation. Adv Funct Mater. 2023;33:2209560.
Cao S, Zhao Z, Zheng Y, Wu Z, Ma T, Zhu B, Yang C, Xiang X, Ma L, Han X, Wang Y, Guo Q, Qiu L, Cheng C. A library of ROS-catalytic metalloenzyme mimics with atomic metal centers. Adv Mater. 2022;34:e2200255.
Fan H, Li Y, Liu J, Cai R, Gao X, Zhang H, Ji Y, Nie G, Wu X. Plasmon-enhanced oxidase-like activity and cellular effect of Pd-coated gold nanorods. ACS Appl Mater Interfaces. 2019;11:45416–26.
Yan R, Sun S, Yang J, Long W, Wang J, Mu X, Li Q, Hao W, Zhang S, Liu H, Gao Y, Ouyang L, Chen J, Liu S, Zhang X-D, Ming D. Nanozyme-based bandage with single-atom catalysis for brain trauma. ACS Nano. 2019;13:11552–60.
Zhong X, Wang X, Cheng L, Tang Y, Zhan G, Gong F, Zhang R, Hu J, Liu Z, Yang X. GSH-depleted PtCu3 nanocages for chemodynamic- enhanced sonodynamic cancer therapy. Adv Funct Mater. 2020;30:1907954.
Liu L, Zhang H, Xing S, Zhang Y, Shangguan L, Wei C, Peng F, Liu X. Copper-zinc bimetallic single-atom catalysts with localized surface plasmon resonance-enhanced photothermal effect and catalytic activity for melanoma treatment and wound-healing. Adv Sci. 2023;10:2207342.
Lv F, Gong Y, Cao Y, Deng Y, Liang S, Tian X, Gu H, Yin J-J. A convenient detection system consisting of efficient Au@PtRu nanozymes and alcohol oxidase for highly sensitive alcohol biosensing. Nanoscale Adv. 2020;2:1583–9.
Cai S, Fu Z, Xiao W, Xiong Y, Wang C, Yang R. Zero-dimensional/two-dimensional AuxPd100- x nanocomposites with enhanced nanozyme catalysis for sensitive glucose detection. ACS Appl Mater Interfaces. 2020;12:11616–24.
Fan Y, Liu S, Yi Y, Rong H, Zhang J. Catalytic nanomaterials toward atomic levels for biomedical applications: from metal clusters to single-atom catalysts. ACS Nano. 2021;15:2005–37.
Cheng X, Huang L, Yang X, Elzatahry AA, Alghamdi A, Deng Y. Rational design of a stable peroxidase mimic for colorimetric detection of H2O2 and glucose: a synergistic CeO2/Zeolite Y nanocomposite. J Colloid Interface Sci. 2019;535:425–35.
Tang W, Fan W, Zhang W, Yang Z, Li L, Wang Z, Chiang Y-L, Liu Y, Deng L, He L, Shen Z, Jacobson O, Aronova MA, Jin A, Xie J, Chen X. Wet/sono-chemical synthesis of enzymatic two-dimensional MnO2 nanosheets for synergistic catalysis-enhanced phototheranostics. Adv Mater. 2019;31:1900401.
Sun T, Xu L, Wang D, Li Y. Metal organic frameworks derived single atom catalysts for electrocatalytic energy conversion. Nano Res. 2019;12:2067–80.
Umer M, Umer S, Anand R, Mun J, Zafari M, Lee G, Kim KS. Transition metal single atom embedded GaN monolayer surface for efficient and selective CO2 electroreduction. J Mater Chem A. 2022;10:24280–9.
Huang L, Chen J, Gan L, Wang J, Dong S. Single-atom nanozymes. Sci Adv. 2019;5:5490.
Zhao Y, Lu XF, Fan G, Luan D, Gu X, Lou XWD. Surface-exposed single-Ni atoms with potential-driven dynamic behaviors for highly efficient electrocatalytic oxygen evolution. Angew Chem Int Ed Engl. 2022;61:e202212542.
Li X, Ye W, Xu P, Huang H, Fan J, Yuan R, Zheng M-S, Wang M-S, Dong Q. An encapsulation-based sodium storage via Zn-single-atom implanted carbon nanotubes. Adv Mater. 2022;34:e2202898.
Chen Y, Wang P, Hao H, Hong J, Li H, Ji S, Li A, Gao R, Dong J, Han X, Liang M, Wang D, Li Y. Thermal atomization of platinum nanoparticles into single atoms: an effective strategy for engineering high-performance nanozymes. J Am Chem Soc. 2021;143:18643–51.
Li Y, Wang S, Wang X-S, He Y, Wang Q, Li Y, Li M, Yang G, Yi J, Lin H, Huang D, Li L, Chen H, Ye J. Facile top-down strategy for direct metal atomization and coordination achieving a high turnover number in CO2 photoreduction. J Am Chem Soc. 2020;142:19259–67.
Meng Y, Li J, Zhao S, Shi C, Li X, Zhang L, Huo P, Liu C, Cheng H. Fluorination-assisted preparation of self-supporting single-atom Fe-N-doped single-wall carbon nanotube film as bifunctional oxygen electrode for rechargeable Zn-Air batteries. Appl Catal B. 2021;294:120239.
Peng C, Pang R, Li J, Wang E. Current advances on the single-atom nanozyme and its bioapplications. Adv Mater. 2023. https://doi.org/10.1002/adma.202211724 .
Chen Q, Peng P, Yang G, Li Y, Han M, Tan Y, Zhang C, Chen J, Jiang K, Liu L, Ye C, Xing E. Template-guided regioselective encaging of platinum single atoms into Y zeolite: enhanced selectivity in semihydrogenation and resistance to poisoning. Angew Chem Int Ed Engl. 2022;61:e202205978.
Wang Y, Jia G, Cui X, Zhao X, Zhang Q, Gu L, Zheng L, Li LH, Wu Q, Singh DJ, Matsumura D, Tsuji T, Cui Y, Zhao J, Zheng W. Coordination number regulation of molybdenum single-atom nanozyme peroxidase-like specificity. Chem. 2021;7:436–49.
Zhu Y, Wang W, Cheng J, Qu Y, Dai Y, Liu M, Yu J, Wang C, Wang H, Wang S, Zhao C, Wu Y, Liu Y. Stimuli-responsive manganese single-atom nanozyme for tumor therapy via integrated cascade reactions. Angew Chem Int Ed Engl. 2021;60:9480–8.
He Q, Tian D, Jiang H, Cao D, Wei S, Liu D, Song P, Lin Y, Song L. Achieving efficient alkaline hydrogen evolution reaction over a Ni5 P4 catalyst incorporating single-atomic Ru sites. Adv Mater. 2020;32:e1906972.
Xu B, Li S, Zheng L, Liu Y, Han A, Zhang J, Huang Z, Xie H, Fan K, Gao L, Liu H. A bioinspired five-coordinated single-atom iron nanozyme for tumor catalytic therapy. Adv Mater. 2022;34:e2107088.
Zhang J, Liu J, Xi L, Yu Y, Chen N, Sun S, Wang W, Lange KM, Zhang B. Single-atom Au/NiFe layered double hydroxide electrocatalyst: probing the origin of activity for oxygen evolution reaction. J Am Chem Soc. 2018;140:3876–9.
Wang D, Zhang B, Ding H, Liu D, Xiang J, Gao XJ, Chen X, Li Z, Yang L, Duan H, Zheng J, Liu Z, Jiang B, Liu Y, Xie N, Zhang H, Yan X, Fan K, Nie G. TiO2 supported single Ag atoms nanozyme for elimination of SARS-CoV2. Nano Today. 2021;40:101243.
Huang L, Wei X, Gao E, Zhang C, Hu X, Chen Y, Liu Z, Finck N, Lützenkirchen J, Dionysiou DD. Single Fe atoms confined in two-dimensional MoS2 for sulfite activation: a biomimetic approach towards efficient radical generation. Appl Catal B. 2020;268:118459.
Akri M, Zhao S, Li X, Zang K, Lee AF, Isaacs MA, Xi W, Gangarajula Y, Luo J, Ren Y, Cui Y-T, Li L, Su Y, Pan X, Wen W, Pan Y, Wilson K, Li L, Qiao B, Ishii H, Liao Y-F, Wang A, Wang X, Zhang T. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat Commun. 2019;10:5181.
Chen Z, Yu Y, Gao Y, Zhu Z. Rational design strategies for nanozymes. ACS Nano. 2023;17:13062–80.
Jiao L, Xu W, Yan H, Wu Y, Liu C, Du D, Lin Y, Zhu C. Fe-N-C single-atom nanozymes for the intracellular hydrogen peroxide detection. Anal Chem. 2019;91:11994–9.
Niu X, Shi Q, Zhu W, Liu D, Tian H, Fu S, Cheng N, Li S, Smith JN, Du D, Lin Y. Unprecedented peroxidase-mimicking activity of single-atom nanozyme with atomically dispersed Fe–Nx moieties hosted by MOF derived porous carbon. Biosens Bioelectron. 2019;142:111495.
Cheng N, Li J, Liu D, Lin Y, Du D. Single-atom nanozyme based on nanoengineered Fe–N–C catalyst with superior peroxidase-like activity for ultrasensitive bioassays. Small. 2019;15:1901485.
Wu Y, Wu J, Jiao L, Xu W, Wang H, Wei X, Gu W, Ren G, Zhang N, Zhang Q, Huang L, Gu L, Zhu C. Cascade reaction system integrating single-atom nanozymes with abundant Cu sites for enhanced biosensing. Anal Chem. 2020;92:3373–9.
Xu B, Wang H, Wang W, Gao L, Li S, Pan X, Wang H, Yang H, Meng X, Wu Q, Zheng L, Chen S, Shi X, Fan K, Yan X, Liu H. A single-atom nanozyme for wound disinfection applications. Angew Chem Int Ed Engl. 2019;58:4911–6.
Xu W, Song W, Kang Y, Jiao L, Wu Y, Chen Y, Cai X, Zheng L, Gu W, Zhu C. Axial ligand-engineered single-atom catalysts with boosted enzyme-like activity for sensitive immunoassay. Anal Chem. 2021;93:12758–66.
Wu Y, Jiao L, Luo X, Xu W, Wei X, Wang H, Yan H, Gu W, Xu BZ, Du D, Lin Y, Zhu C. Oxidase-like Fe-N-C single-atom nanozymes for the detection of acetylcholinesterase activity. Small. 2019;15:1903108.
Wang W, Zhu Y, Zhu X, Zhao Y, Xue Z, Xiong C, Wang Z, Qu Y, Cheng J, Chen M, Liu M, Zhou F, Zhang H, Jiang Z, Hu Y, Zhou H, Wang H, Li Y, Liu Y, Wu Y. Biocompatible ruthenium single-atom catalyst for cascade enzyme-mimicking therapy. ACS Appl Mater Interfaces. 2021;13:45269–78.
Cao F, Zhang L, You Y, Zheng L, Ren J, Qu X. An enzyme-mimicking single-atom catalyst as an efficient multiple reactive oxygen and nitrogen species scavenger for sepsis management. Angew Chem Int Ed Engl. 2020;59:5108–15.
Su Y, Wu F, Song Q, Wu M, Mohammadniaei M, Zhang T, Liu B, Wu S, Zhang M, Li A, Shen J. Dual enzyme-mimic nanozyme based on single-atom construction strategy for photothermal-augmented nanocatalytic therapy in the second near-infrared biowindow. Biomaterials. 2022;281:121325.
Ma W, Mao J, Yang X, Pan C, Chen W, Wang M, Yu P, Mao L, Li Y. A single-atom Fe-N4 catalytic site mimicking bifunctional antioxidative enzymes for oxidative stress cytoprotection. Chem Commun. 2018;55:159–62.
Liu H, Li Y, Sun S, Xin Q, Liu S, Mu X, Yuan X, Chen K, Wang H, Varga K, Mi W, Yang J, Zhang X-D. Catalytically potent and selective clusterzymes for modulation of neuroinflammation through single-atom substitutions. Nat Commun. 2021;12:114.
Lu X, Gao S, Lin H, Yu L, Han Y, Zhu P, Bao W, Yao H, Chen Y, Shi J. Bioinspired copper single-atom catalysts for tumor parallel catalytic therapy. Adv Mater. 2020;32:e2002246.
Wang X, Shi Q, Zha Z, Zhu D, Zheng L, Shi L, Wei X, Lian L, Wu K, Cheng L. Copper single-atom catalysts with photothermal performance and enhanced nanozyme activity for bacteria-infected wound therapy. Bioact Mater. 2021;6:4389–401.
CAS PubMed PubMed Central Google Scholar
Zhu D, Ling R, Chen H, Lyu M, Qian H, Wu K, Li G, Wang X. Biomimetic copper single-atom nanozyme system for self-enhanced nanocatalytic tumor therapy. Nano Res. 2022;15:7320–8.
Cao F, Sang Y, Liu C, Bai F, Zheng L, Ren J, Qu X. Self-adaptive single-atom catalyst boosting selective ferroptosis in tumor cells. ACS Nano. 2022;16:855–68.
Wu J, Wang X, Wang Q, Lou Z, Li S, Zhu Y, Qin L, Wei H. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem Soc Rev. 2019;48:1004–76.
Zhang Q, Song L, Zhang K. Breakthroughs in nanozyme-inspired application diversity. Mat Chem Front. 2023;7:44–64.
Yang D, Chen Z, Gao Z, Tammina SK, Yang Y. Nanozymes used for antimicrobials and their applications. Colloids Surf B Biointerfaces. 2020;195:111252.
Xia D, Liu H, Xu B, Wang Y, Liao Y, Huang Y, Ye L, He C, Wong PK, Qiu R. Single Ag atom engineered 3D-MnO2 porous hollow microspheres for rapid photothermocatalytic inactivation of E. coli under solar light. Appl Catal B-Environ. 2019;245:177–89.
Huo M, Wang L, Zhang H, Zhang L, Chen Y, Shi J. Construction of single-iron-atom nanocatalysts for highly efficient catalytic antibiotics. Small. 2019;15:1901834.
Liu H, Liu X, Wang H, Ren J, Qu X. A homing missile-like nanotherapeutic with single-atom catalytic sites for in situ elimination of intracellular bacterial pathogens. Small. 2023;19:2207510.
Qiu X, Zhuang L, Yuan J, Wang H, Dong X, He S, Guan S, Chang Z, Bao P. Constructing multifunctional Cu single-atom nanozyme for synergistic nanocatalytic therapy-mediated multidrug-resistant bacteria infected wound healing. J Colloid Interface Sci. 2023;652:1712–25.
Chan M, Chen B-G, Huang W, Su T, Hsiao M, Liu R. Tunable single-atom nanozyme catalytic system for biological applications of therapy and diagnosis. Mater Today Adv. 2023;17:100342.
Wang X, Chen Q, Zhu Y, Wang K, Chang Y, Wu X, Bao W, Cao T, Chen H, Zhang Y, Qin H. Destroying pathogen-tumor symbionts synergizing with catalytic therapy of colorectal cancer by biomimetic protein-supported single-atom nanozyme. Signal Transduct Target Ther. 2023;8:277.
Lv Q, Chi K, Shi X, Liu M, Li X, Zhou C, Shi L, Fan H, Liu H, Liu J, Zhang Y, Wang S, Wang L, Wang Z. Nanozyme-like single-atom catalyst combined with artesunate achieves photothermal-enhanced nanocatalytic therapy in the near-infrared biowindow. Acta Biomater. 2023;158:686–97.
Madhuvilakku R, Hong Y, Nila IS, Villagra Moran VM, Subramanian P, Khan ZA, Jeong S, You SG. Quantification of neuronal cell-released hydrogen peroxide using 3D mesoporous copper-enriched prussian blue microcubes nanozymes: a colorimetric approach in real time and anticancer effect. ACS Appl Mater Interfaces. 2023;15:55466–85.
Liu Y, Wang B, Zhu J, Xu X, Zhou B, Yang Y. Single-atom nanozyme with asymmetric electron distribution for tumor catalytic therapy by disrupting tumor redox and energy metabolism homeostasis. Adv Mater. 2023;35:2208512.
Zhao X, Wu J, Zhang K, Guo D, Hong L, Chen X, Wang B, Song Y. The synthesis of a nanodrug using metal-based nanozymes conjugated with ginsenoside Rg3 for pancreatic cancer therapy. Nanoscale Adv. 2021;4:190–9.
Sun H, Liu J. A feasible strategy for designing cytochrome P450-mimic sandwich-like single-atom nanozymes toward electrochemical CO2 conversion. J Colloid Interface Sci. 2024;661:482–92.
Feng N, Li Q, Bai Q, Xu S, Shi J, Liu B, Guo J. Development of an Au-anchored Fe single-atom nanozyme for biocatalysis and enhanced tumor photothermal therapy. J Colloid Interface Sci. 2022;618:68–77.
Chang M, Hou Z, Wang M, Yang C, Wang R, Li F, Liu D, Peng T, Li C, Lin J. Single-atom Pd nanozyme for ferroptosis-boosted mild-temperature photothermal therapy. Angew Chem Int Ed Engl. 2021;60:12971–9.
Deng Z, Ma W, Ding C, Wei C, Gao B, Zhu Y, Zhang Y, Wu F, Zhang M, Li R, Zhang M. Metal polyphenol network/cerium oxide artificial enzymes therapeutic nanoplatform for MRI/CT-aided intestinal inflammation management. Nano Today. 2023;53:102044.
Lu M, Wang C, Ding Y, Peng M, Zhang W, Li K, Wei W, Lin Y. Fe-N/C single-atom catalysts exhibiting multienzyme activity and ROS scavenging ability in cells. Chem Commun. 2019;55:14534–7.
Chen Y, Zou H, Yan B, Wu X, Cao W, Qian Y, Zheng L, Yang G. Atomically dispersed Cu nanozyme with intensive ascorbate peroxidase mimic activity capable of alleviating ROS-mediated oxidation damage. Adv Sci. 2022;9:2103977.
Liu Y, Cheng Y, Zhang H, Zhou M, Yu Y, Lin S, Jiang B, Zhao X, Miao L, Wei C-W, Liu Q, Lin Y-W, Du Y, Butch CJ, Wei H. Integrated cascade nanozyme catalyzes in vivo ROS scavenging for anti-inflammatory therapy. Sci Adv. 2020;6:2695.
Jo S-M, Zhang KAI, Wurm FR, Landfester K. Mimic of the cellular antioxidant defense system for a sustainable regeneration of nicotinamide adenine dinucleotide (NAD). ACS Appl Mater Interfaces. 2020;12:25625–32.
Morry J, Ngamcherdtrakul W, Yantasee W. Oxidative stress in cancer and fibrosis: opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles. Redox Biol. 2017. https://doi.org/10.1016/j.redox.2016.12.011 .
Chen D, Xia Z, Guo Z, Gou W, Zhao J, Zhou X, Tan X, Li W, Zhao S, Tian Z, Qu Y. Bioinspired porous three-coordinated single-atom Fe nanozyme with oxidase-like activity for tumor visual identification via glutathione. Nat Commun. 2023;14:7127.
Sun L, Li C, Yan Y, Yu Y, Zhao H, Zhou Z, Wang F, Feng Y. Engineering DNA/Fe-N-C single-atom nanozymes interface for colorimetric biosensing of cancer cells. Anal Chim Acta. 2021;1180:338856.
Hu FX, Hu T, Chen S, Wang D, Rao Q, Liu Y, Dai F, Guo C, Yang HB, Li CM. Single-atom cobalt-based electrochemical biomimetic uric acid sensor with wide linear range and ultralow detection limit. Nanomicro Lett. 2020;13:7.
PubMed PubMed Central Google Scholar
Zhou X, Wang M, Chen J, Xie X, Su X. Peroxidase-like activity of Fe-N-C single-atom nanozyme based colorimetric detection of galactose. Anal Chim Acta. 2020;1128:72–9.
Li J, Li Y, Wu K, Deng A, Li J. Ultra-sensitive detection of 5-fluorouracil by flow injection chemiluminescence immunoassay based on Fenton-like effect of single atom Co nanozyme. Talanta. 2023;265:124870.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
Ni J, Wu G, Albenberg A, Tomov V. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. 2017;14:573–84.
Wong SH, Jun Y. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019;16:690–704.
Lang S, Duan Y, Liu J, Torralba M, Kuelbs K, Ventura-Cots M, Abraldes J, Abraldes F, Verna E, Brown R, Brown V, Altamirano J, Caballería J, Shawcross D, Lucey M, Louvet A, Mathurin P, Garcia-Tsao G-T, Ho S, Tu X, Bataller R, Stärkel P, Fouts F, Schnabl B. Intestinal fungal dysbiosis and systemic immune response to fungi in patients with alcoholic hepatitis. Hepatology. 2020;71:522–38.
Tenner S, Baillie J, DeWitt J, Vege S. American college of gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–15.
Zhu Y, Liao Y, Zou J, Cheng J, Pan Y, Lin L, Chen X. Engineering single-atom nanozymes for catalytic biomedical applications. Small. 2023;19:2300750.
Zhang Y, Wang T, Dong X, Zhu C, Peng Q, Liu C, Zhang Y, Chen F, Zhang K. Salivary amylase-responsive buccal tablets wipe out chemotherapy-rooted refractory oral mucositis. Adv Sci. 2024;11:2308439.
Jiao R, Lin X, Zhang Q, Zhang Y, Qin W, Yang Q, Xu C, Chen F, Zhang K. Anti-tumor immune potentiation targets-engineered nanobiotechnologies: design principles and applications. Prog Mater Sci. 2024;142:101230.
Download references
Zhejiang Province's 2024 Key R&D Plan Project (Grant No. 2024C03048); Zhejiang Provincial Traditional Chinese Medicine Science and Technology Project (Grant No. GZY-ZJ-KJ-24093); Hangzhou Science and Technology Commission (202004A14); and the Construction Fund of Medical Key Disciplines of Hangzhou (OO20190001); Research Fund of Sichuan Academy of Medical Sciences and Sichuan Provincial People's Hospital.
Author information
Sijia Hua and Xiulin Dong have contributed equally to this work.
Authors and Affiliations
Zhejiang University of Chinese Medicine, No. 548 Binwen Road, Binjiang District, Hangzhou, 310053, Zhejiang, China
Department of Gastroenterology, School of Medicine, Affiliated Hangzhou First People’s Hospital, Westlake University, No. 261 Huansha Road, Hangzhou, 310006, Zhejiang, China
Xiulin Dong, Xiaofeng Zhang & Jianfeng Yang
Department of Pharmacy and Central Laboratory, School of Medicine, Sichuan Academy of Medical Sciences, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, No. 32, West Second Section, First Ring Road, Chengdu, 610072, Sichuan, People’s Republic of China
Xiulin Dong, Qiuxia Peng & Kun Zhang
You can also search for this author in PubMed Google Scholar
Contributions
# Hua S.J., Dong X.L. and Peng Q.X. contributed equally to this work. Conceptualization: Hua S.J.; Writing-original draft preparation: Hua S.J and Peng Q.X.; Writing-review and editing: Dong X.L.; Supervision: Zhang X.F., Zhang K.; Funding acquisition: Yang J.F.. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Correspondence to Kun Zhang , Xiaofeng Zhang or Jianfeng Yang .
Ethics declarations
Informed consent, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hua, S., Dong, X., Peng, Q. et al. Single-atom nanozymes shines diagnostics of gastrointestinal diseases. J Nanobiotechnol 22 , 286 (2024). https://doi.org/10.1186/s12951-024-02569-3
Download citation
Received : 16 March 2024
Accepted : 20 May 2024
Published : 25 May 2024
DOI : https://doi.org/10.1186/s12951-024-02569-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Single-atom nanozymes
- Catalytic regulation
- Gastrointestinal diseases
- Cancer therapy
- Antibacterial
Journal of Nanobiotechnology
ISSN: 1477-3155
- Submission enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
chemical synthesis, the construction of complex chemical compounds from simpler ones. It is the process by which many substances important to daily life are obtained. It is applied to all types of chemical compounds, but most syntheses are of organic molecules.. Chemists synthesize chemical compounds that occur in nature in order to gain a better understanding of their structures.
Planning a synthesis makes you approach a chemical problem in a logical way, draw on your knowledge of chemical reactions, and organize that knowledge into a workable plan—it helps you learn organic chemistry. There's no secret to planning an organic synthesis: all it takes is a knowledge of the different reactions and some practice.
Synthesis. Synthesis is the production of chemical compounds by reaction from simpler materials. The construction of complex and defined new molecules is a challenging and complicated undertaking, and one that requires the constant development of new reactions, catalysts and techniques. Synthesis projects underpin developments in a very wide ...
Box 9.2.a provides a general framework for synthesis that can be applied irrespective of the methods used to synthesize results. Planning for the synthesis should start at protocol-writing stage, and Chapter 2 and Chapter 3 describe the steps involved in planning the review questions and comparisons between intervention groups. These steps included specifying which characteristics of the ...
In this paper a critical overview of nanomaterials, their varieties, characteristics, synthesis techniques, and applications in various fields is offered. 1 INTRODUCTION. The meaning of the word 'nano' is nanos, which indicates a person of very low height or a very small object that is a dwarf. Consider that in an international system of ...
The following section provides an overview of cyclic biomacromolecules, highlighting some of their unique behaviour and characteristics. The first discovery of cyclic DNA
The two forms of polymer synthesis (addition and condensation) are described in terms of processes, resulting chemical structures and properties, and example materials. Factors affecting recyclability are described, along with defining thermoplastic and thermoset characteristics.
This chapter presents an overview of the synthesis methods of nanomaterials, including top-down and bottom-up approaches, and discusses the unique properties that arise from their small size and high surface area-to-volume ratio. ... Synthesis, Characteristics, and Applications of Nanomaterials. In: Khan, T., Jawaid, M., Ahmad, K.A., Singh, B ...
The primary characteristics of NPs are dictated by the synthesis conditions. These characteristics include but not limited to size and size distribution, crystallinity, shape, directional properties, mutual alignment. The main category of NPs synthesis methods are bottom up approaches and top down approaches.
1. Introduction. The examination of the characteristics of matter at the nanoscale is known as nanoscience. Distinctive, solid state materials' size-dependent characteristics are the primary focus of this field of study [1].Nanotechnology is specifically concerned with the creation, advancement, and use of substances known as nanomaterials, which have sizes between 1 and 100 nm [2].
These versatile characteristics acknowledge Mxene as a well-proven active component for building batteries as well as supercapacitors. With new developments, MXene finds enormous applications by its favorable performance characteristics, such as use in conductive polymeric substances as polypyrrole and polyaniline.
Local synthesis occurs at the paragraph level when writers connect individual pieces of evidence from multiple sources to support a paragraph's main idea and advance a paper's thesis statement. A common example in academic writing is a scholarly paragraph that includes a main idea, evidence from multiple sources, and analysis of those ...
synthesis: [noun] the composition or combination of parts or elements so as to form a whole. the production of a substance by the union of chemical elements, groups, or simpler compounds or by the degradation of a complex compound.
Synthesis Reaction Examples. In the simplest synthesis reactions, two elements combine to form a binary compound (a compound made of two elements). The combination of iron and sulfur to form iron (II) sulfide is an example of a synthesis reaction : 8 Fe + S 8 → 8 FeS. Another example of a synthesis reaction is the formation of potassium ...
Biomimetics involves investigation of structure, function, and methods of synthesis of biological composite materials. The goal is to apply this information to the design and synthesis of materials for engineering applications.Properties of engineering materials are structure sensitive through the whole spectrum of dimensions from nanometer to macro scale.
Characteristics of Synthesis. In chemistry, synthesis refers to the process of combining different chemical compounds to create a new substance. The resulting substance can have different properties, structures, and uses than its individual components. Synthesis is an essential part of chemistry and plays a critical role in the development of ...
Synthesis Reaction Definition. A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product. A + B → AB. This type of reaction is also called a direct combination reaction or simply a combination reaction. It's the type of reaction that forms compounds from their elements.
Fig. 1: Synthesis mechanism of chemical vapor deposition (CVD) growth AMX 2. Fig. 2: Optical microscopy images of the 20 kinds of as-synthesized 2D AMX 2 compounds. Fig. 4: The ionic and ...
Subsequently, representative applications of cyclic polymers in different fields such as drug and gene delivery and surface functionalization are presented. Last, we envision the following key challenges and opportunities for cyclic polymers that may attract future attention: large-scale synthesis, efficient purification, programmable folding ...
2.2. Discovery of C, Ag, Zn, Cu, and Au nanoparticles. Carbon NPs were found in 1991, and Iijima and Ichihashi announced the single-wall carbon nanotube synthesis with a diameter of 1 nanometer in 1993 (Chen et al., 2021).Carbon nanotubes (CNTs), also known as Bucky tubes, are a kind of nanomaterial made up of a two-dimensional hexagonal lattice of carbon atoms.
The physical, chemical, and interfacial characteristics of the resulting SNPs, as well as the performance of practical applications, from different starch sources must be evaluated. Focus on tailoring SNPs that exhibit a physical (e.g., surface area) and chemical structure (e.g., available functional groups) able to induce several ...
Click chemistry is a newer approach to the synthesis of drug-like molecules that can accelerate the drug discovery process by utilizing a few practical and reliable reactions. ... Click Chemistry Reaction Characteristics 1. Simple reaction conditions; Readily and easily available starting materials and reagents; Use of no solvent, a benign ...
The electrochemical generation of highly active and variable free radicals for the synthesis of organic compounds has emerged as a favored approach due to its mild and environmentally friendly characteristics, in contrast to traditional chemical redox reagents. As such, electrochemistry has been extensively 2024 Green Chemistry Reviews
Abstract MgAl-layered double hydroxides with different molar ratios of cations were synthesized. The compound with a ratio of 2 : 1 was shown to exhibit better characteristics of thermal stability. The modification of polyurethane by using these hydroxides led to the improvement in the properties of composites: decrease by 47% in flame retardancy, and increase by 24.8 and 54.1% in tensile ...
This review is an attempt to quantify the intriguing characteristics of MXene nanocomposites by embracing design tools like the 'iceberg model'. To further evaluate the performance of MXenes fabricated using green strategies (such as eutectic etching) we have made an attempt to critically compare them with conventional MXenes by examining ...
Various clinical symptoms of digestive system, such as infectious, inflammatory, and malignant disorders, have a profound impact on the quality of life and overall health of patients. Therefore, the chase for more potent medicines is both highly significant and urgent. Nanozymes, a novel class of nanomaterials, amalgamate the biological properties of nanomaterials with the catalytic activity ...
These versatile characteristics acknowledge Mxene as a well-proven active component for building batteries as well as supercapacitors. With new developments, MXene finds enormous applications by its favorable performance characteristics, such as use in conductive polymeric substances as polypyrrole and polyaniline.
In general, MXenes are represented as M n+1 X n T x where n ranges between 1 and 3 and T implies the functional groups (-O, -F, and/or -OH) resulting from the interaction with acids during the etching step. As the n values in the MAX phases range from 1 to 3, the resulting MXene exists in three possible lattice structures: M 2 X, M 3 X 2 ...
Salinity reduces crop yields and quality, causing global economic losses. Zinc oxide nanoparticles (ZnO-NPs) improve plant physiological and metabolic processes and abiotic stress resistance. This study examined the effects of foliar ZnO-NPs at 75 and 150 mg/L on tomato Kecskeméti 549 plants to alleviate salt stress caused by 150 mM NaCl. The precipitation procedure produced ZnO-NPs that were ...
Activated carbon's outstanding characteristics, such as its distinctive electrical and thermal stability, high resistance to corrosion, ... (AC), enhancing its hydrogen storage capacity. Synthesis occurred under hydrothermal conditions, with in situ AC incorporation at varying proportions. The AC@MIL-101/A composite, containing 0.63 wt% AC, ...