Cohort Studies: Design, Analysis, and Reporting
Affiliations.
- 1 Department of Quantitative Health Sciences, Lerner Research Institute, Cleveland Clinic, Cleveland, OH. Electronic address: [email protected].
- 2 Department of Quantitative Health Sciences, Lerner Research Institute, Cleveland Clinic, Cleveland, OH.
- PMID: 32658655
- DOI: 10.1016/j.chest.2020.03.014
Cohort studies are types of observational studies in which a cohort, or a group of individuals sharing some characteristic, are followed up over time, and outcomes are measured at one or more time points. Cohort studies can be classified as prospective or retrospective studies, and they have several advantages and disadvantages. This article reviews the essential characteristics of cohort studies and includes recommendations on the design, statistical analysis, and reporting of cohort studies in respiratory and critical care medicine. Tools are provided for researchers and reviewers.
Keywords: bias; cohort studies; confounding; prospective; retrospective.
Copyright © 2020 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.

Publication types
- Cohort Studies*
- Data Interpretation, Statistical
- Guidelines as Topic
- Research Design / statistics & numerical data*
Quantitative study designs: Cohort Studies
Quantitative study designs.
- Introduction
- Cohort Studies
- Randomised Controlled Trial
- Case Control
- Cross-Sectional Studies
- Study Designs Home
Cohort Study
Did you know that the majority of people will develop a diagnosable mental illness whilst only a minority will experience enduring mental health? Or that groups of people at risk of having high blood pressure and other related health issues by the age of 38 can be identified in childhood? Or that a poor credit rating can be indicative of a person’s health status?
These findings (and more) have come out of a large cohort study started in 1972 by researchers at the University of Otago in New Zealand. This study is known as The Dunedin Study and it has followed the lives of 1037 babies born between 1 April 1972 and 31 March 1973 since their birth. The study is now in its fifth decade and has produced over 1200 publications and reports, many of which have helped inform policy makers in New Zealand and overseas.
In Introduction to Study Designs, we learnt that there are many different study design types and that these are divided into two categories: Experimental and Observational. Cohort Studies are a type of observational study.
What is a Cohort Study design?
- Cohort studies are longitudinal, observational studies, which investigate predictive risk factors and health outcomes.
- They differ from clinical trials, in that no intervention, treatment, or exposure is administered to the participants. The factors of interest to researchers already exist in the study group under investigation.
- Study participants are observed over a period of time. The incidence of disease in the exposed group is compared with the incidence of disease in the unexposed group.
- Because of the observational nature of cohort studies they can only find correlation between a risk factor and disease rather than the cause.
Cohort studies are useful if:
- There is a persuasive hypothesis linking an exposure to an outcome.
- The time between exposure and outcome is not too long (adding to the study costs and increasing the risk of participant attrition).
- The outcome is not too rare.
The stages of a Cohort Study
- A cohort study starts with the selection of a group of participants (known as a ‘cohort’) sourced from the same population, who must be free of the outcome under investigation but have the potential to develop that outcome.
- The participants must be identical, having common characteristics except for their exposure status.
- The participants are divided into two groups – the first group is the ‘exposure’ group, the second group is free of the exposure.
Types of Cohort Studies
There are two types of cohort studies: Prospective and Retrospective .
How Cohort Studies are carried out
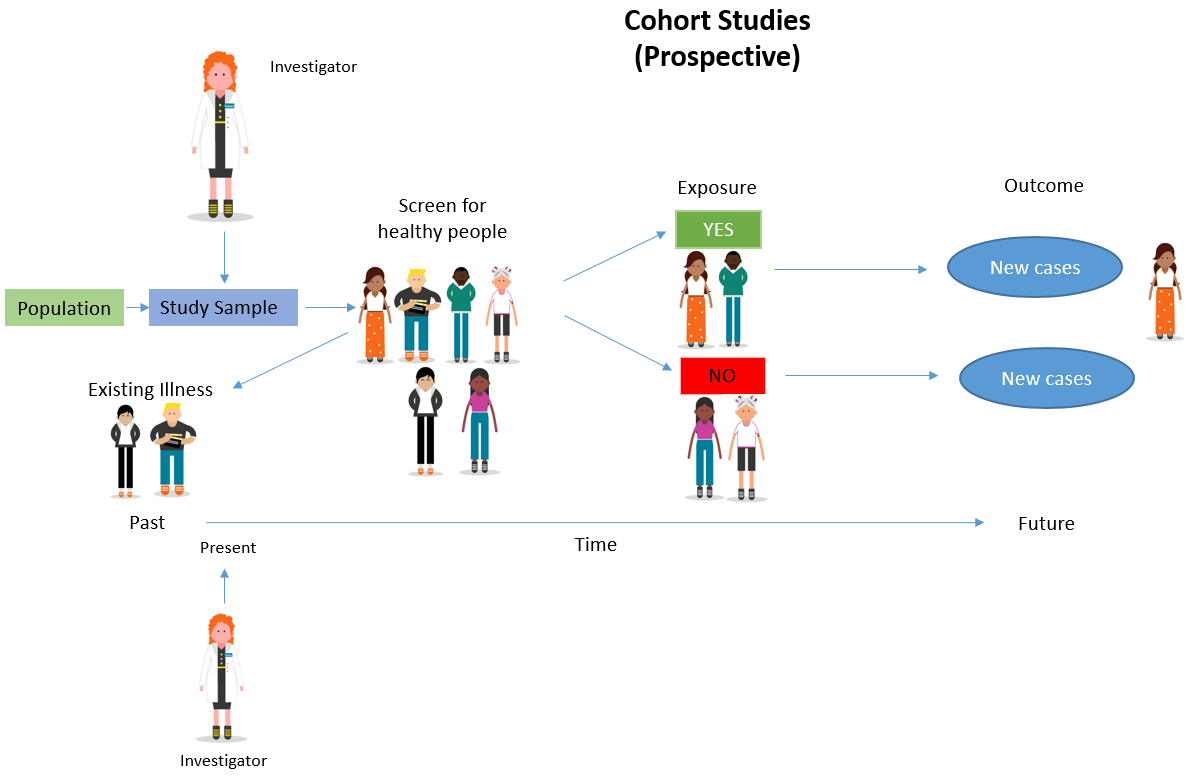
Adapted from: Cohort Studies: A brief overview by Terry Shaneyfelt [video] https://www.youtube.com/watch?v=FRasHsoORj0)
Which clinical questions does this study design best answer?
What are the advantages and disadvantages to consider when using a cohort study, what does a strong cohort study look like.
- The aim of the study is clearly stated.
- It is clear how the sample population was sourced, including inclusion and exclusion criteria, with justification provided for the sample size. The sample group accurately reflects the population from which it is drawn.
- Loss of participants to follow up are stated and explanations provided.
- The control group is clearly described, including the selection methodology, whether they were from the same sample population, whether randomised or matched to minimise bias and confounding.
- It is clearly stated whether the study was blinded or not, i.e. whether the investigators were aware of how the subject and control groups were allocated.
- The methodology was rigorously adhered to.
- Involves the use of valid measurements (recognised by peers) as well as appropriate statistical tests.
- The conclusions are logically drawn from the results – the study demonstrates what it says it has demonstrated.
- Includes a clear description of the data, including accessibility and availability.
What are the pitfalls to look for?
- Confounding factors within the sample groups may be difficult to identify and control for, thus influencing the results.
- Participants may move between exposure/non-exposure categories or not properly comply with methodology requirements.
- Being in the study may influence participants’ behaviour.
- Too many participants may drop out, thus rendering the results invalid.
Critical appraisal tools
To assist with the critical appraisal of a cohort study here are some useful tools that can be applied.
Critical appraisal checklist for cohort studies (JBI)
CASP appraisal checklist for cohort studies
Real World Examples
Bell, A.F., Rubin, L.H., Davis, J.M., Golding, J., Adejumo, O.A. & Carter, C.S. (2018). The birth experience and subsequent maternal caregiving attitudes and behavior: A birth cohort study . Archives of Women’s Mental Health .
Dykxhoorn, J., Hatcher, S., Roy-Gagnon, M.H., & Colman, I. (2017). Early life predictors of adolescent suicidal thoughts and adverse outcomes in two population-based cohort studies . PLoS ONE , 12(8).
Feeley, N., Hayton, B., Gold, I. & Zelkowitz, P. (2017). A comparative prospective cohort study of women following childbirth: Mothers of low birthweight infants at risk for elevated PTSD symptoms . Journal of Psychosomatic Research , 101, 24–30.
Forman, J.P., Stampfer, M.J. & Curhan, G.C. (2009). Diet and lifestyle risk factors associated with incident hypertension in women . JAMA: Journal of the American Medical Association , 302(4), 401–411.
Suarez, E. (2002). Prognosis and outcome of first-episode psychoses in Hawai’i: Results of the 15-year follow-up of the Honolulu cohort of the WHO international study of schizophrenia . ProQuest Information & Learning, Dissertation Abstracts International: Section B: The Sciences and Engineering , 63(3-B), 1577.
Young, J.T., Heffernan, E., Borschmann, R., Ogloff, J.R.P., Spittal, M.J., Kouyoumdjian, F.G., Preen, D.B., Butler, A., Brophy, L., Crilly, J. & Kinner, S.A. (2018). Dual diagnosis of mental illness and substance use disorder and injury in adults recently released from prison: a prospective cohort study . The Lancet. Public Health , 3(5), e237–e248.
References and Further Reading
Greenhalgh, T. (2014). How to Read a Paper : The Basics of Evidence-Based Medicine , John Wiley & Sons, Incorporated, Somerset, United Kingdom.
Hoffmann, T. a., Bennett, S. P., & Mar, C. D. (2017). Evidence-Based Practice Across the Health Professions (Third edition. ed.): Elsevier.
Song, J.W. & Chung, K.C. (2010). Observational studies: cohort and case-control studies . Plastic and Reconstructive Surgery , 126(6), 2234-42.
Mann, C.J. (2003). Observational research methods. Research design II: cohort, cross sectional, and case-control studies . Emergency Medicine Journal , 20(1), 54-60.
- << Previous: Introduction
- Next: Randomised Controlled Trial >>
- Last Updated: May 15, 2024 11:37 AM
- URL: https://deakin.libguides.com/quantitative-study-designs
Prospective Cohort Study Design: Definition & Examples
Julia Simkus
Editor at Simply Psychology
BA (Hons) Psychology, Princeton University
Julia Simkus is a graduate of Princeton University with a Bachelor of Arts in Psychology. She is currently studying for a Master's Degree in Counseling for Mental Health and Wellness in September 2023. Julia's research has been published in peer reviewed journals.
Learn about our Editorial Process
Saul Mcleod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul Mcleod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
On This Page:
A prospective study, sometimes called a prospective cohort study, is a type of longitudinal study where researchers will follow and observe a group of subjects over a period of time to gather information and record the development of outcomes.

The participants in a prospective study are selected based on specific criteria and are often free from the outcome of interest at the beginning of the study. Data on exposures and potential confounding factors are collected at regular intervals throughout the study period.
By following the participants prospectively, researchers can establish a temporal relationship between exposures and outcomes, providing valuable insights into the causality of the observed associations.
This study design allows for the examination of multiple outcomes and the investigation of various exposure levels, contributing to a comprehensive understanding of the factors influencing health and disease.
How it Works
Participants are enrolled in the study before they develop the outcome or disease in question and then are observed as it evolves to see who develops the outcome and who does not.
Cohort studies are observational, so researchers will follow the subjects without manipulating any variables or interfering with their environment.
Similar to retrospective studies , prospective studies are beneficial for medical researchers, specifically in the field of epidemiology, as scientists can watch the development of a disease and compare the risk factors among subjects.
Before any appearance of the disease is investigated, medical professionals will identify a cohort, observe the target participants over time, and collect data at regular intervals.
Weeks, months, or years later, depending on the duration of the study design, the researchers will examine any factors that differed between the individuals who developed the condition and those who did not.
They can then determine if an association exists between an exposure and an outcome and even identify disease progression and relative risk.
Determine cause-and-effect relationships
Because researchers study groups of people before they develop an illness, they can discover potential cause-and-effect relationships between certain behaviors and the development of a disease.
Multiple diseases and conditions can be studied at the same time
Prospective cohort studies enable researchers to study causes of disease and identify multiple risk factors associated with a single exposure. These studies can also reveal links between diseases and risk factors.
Can measure a continuously changing relationship between exposure and outcome
Because prospective cohort studies are longitudinal, researchers can study changes in levels of exposure over time and any changes in outcome, providing a deeper understanding of the dynamic relationship between exposure and outcome.
Limitations
Time consuming and expensive.
Prospective studies usually require multiple months or years before researchers can identify a disease’s causes or discover significant results.
Because of this, they are often more expensive than other types of studies. Recruiting and enrolling participants is another added cost and time commitment.
Requires large subject pool
Prospective cohort studies require large sample sizes in order for any relationships or patterns to be meaningful. Researchers are unable to generate results if there is not enough data.
- Framingham Heart Study: Studied the effects of diet, exercise, and medications on the development of hypertensive or arteriosclerotic cardiovascular disease in residents of the city of Framingham, Massachusetts.
- Caerphilly Heart Disease Study: Examined relationships between a wide range of social, lifestyle, dietary, and other factors with incident vascular disease.
- The Million Women Study: Analyzed data from more than one million women aged 50 and over to understand the effects of hormone replacement therapy use on women’s health.
- Nurses’ Health Study: Studied the effects of diet, exercise, and medications on the development of hypertensive or arteriosclerotic cardiovascular disease.
- Sleep-Disordered Breathing and Mortality: Determined whether sleep-disordered breathing and its sequelae of intermittent hypoxemia and recurrent arousals are associated with mortality in a community sample of adults aged 40 years or older (Punjabi et al., 2009)
Frequently Asked Questions
1. what does it mean when an observational study is prospective.
A prospective observational study is a type of research where investigators select a group of subjects and observe them over a certain period.
The researchers collect data on the subjects’ exposure to certain risk factors or interventions and then track the outcomes. This type of study is often used to study the effects of suspected risk factors that cannot be controlled experimentally.
2. What is the primary difference between a randomized clinical trial and a prospective cohort study?
In a retrospective study, the subjects have already experienced the outcome of interest or developed the disease before the start of the study.
The researchers then look back in time to identify a cohort of subjects before they had developed the disease and use existing data, such as medical records, to discover any patterns.
In a prospective study, on the other hand, the investigators will design the study, recruit subjects, and collect baseline data on all subjects before any of them have developed the outcomes of interest.
The subjects are followed and observed over a period of time to gather information and record the development of outcomes.
3. What is the primary difference between a randomized clinical trial and a prospective cohort study?
In randomized clinical trials , the researchers control the experiment, whereas prospective cohort studies are purely observational, so researchers will observe subjects without manipulating any variables or interfering with their environment.
Researchers in randomized clinical trials will randomly divide participants into groups, either an experimental group or a control group.
However, in prospective cohort studies, researchers will identify a cohort and observe the target participants as a whole to examine any factors that differ between the individuals who develop the condition and those who do not.
Euser, A. M., Zoccali, C., Jager, K. J., & Dekker, F. W. (2009). Cohort studies: prospective versus retrospective. Nephron. Clinical practice, 113(3), c214–c217. https://doi.org/10.1159/000235241
Hariton, E., & Locascio, J. J. (2018). Randomised controlled trials – the gold standard for effectiveness research: Study design: randomised controlled trials. BJOG : an international journal of obstetrics and gynaecology, 125(13), 1716. https://doi.org/10.1111/1471-0528.15199
Netherlands Cooperative Study on the Adequacy of Dialysis-2 Study Group de Mutsert Renée r. de_mutsert@ lumc. nl Grootendorst Diana C Boeschoten Elisabeth W Brandts Hans van Manen Jeannette G Krediet Raymond T Dekker Friedo W. (2009). Subjective global assessment of nutritional status is strongly associated with mortality in chronic dialysis patients. The American journal of clinical nutrition, 89(3), 787-793.
Punjabi, N. M., Caffo, B. S., Goodwin, J. L., Gottlieb, D. J., Newman, A. B., O”Connor, G. T., Rapoport, D. M., Redline, S., Resnick, H. E., Robbins, J. A., Shahar, E., Unruh, M. L., & Samet, J. M. (2009). Sleep-disordered breathing and mortality: a prospective cohort study. PLoS medicine, 6(8), e1000132. https://doi.org/10.1371/journal.pmed.1000132
Ranganathan, P., & Aggarwal, R. (2018). Study designs: Part 1 – An overview and classification. Perspectives in clinical research, 9(4), 184–186.
Song, J. W., & Chung, K. C. (2010). Observational studies: cohort and case-control studies. Plastic and reconstructive surgery, 126(6), 2234–2242. https://doi.org/10.1097/PRS.0b013e3181f44abc.
Further Information
- Euser, A. M., Zoccali, C., Jager, K. J., & Dekker, F. W. (2009). Cohort studies: prospective versus retrospective. Nephron Clinical Practice, 113(3), c214-c217.
- Design of Prospective Studies
- Hammoudeh, S., Gadelhaq, W., & Janahi, I. (2018). Prospective cohort studies in medical research (pp. 11-28). IntechOpen.
- Nabi, H., Kivimaki, M., De Vogli, R., Marmot, M. G., & Singh-Manoux, A. (2008). Positive and negative affect and risk of coronary heart disease: Whitehall II prospective cohort study. Bmj, 337.
- Bramsen, I., Dirkzwager, A. J., & Van der Ploeg, H. M. (2000). Predeployment personality traits and exposure to trauma as predictors of posttraumatic stress symptoms: A prospective study of former peacekeepers. American Journal of Psychiatry, 157(7), 1115-1119.
Related Articles
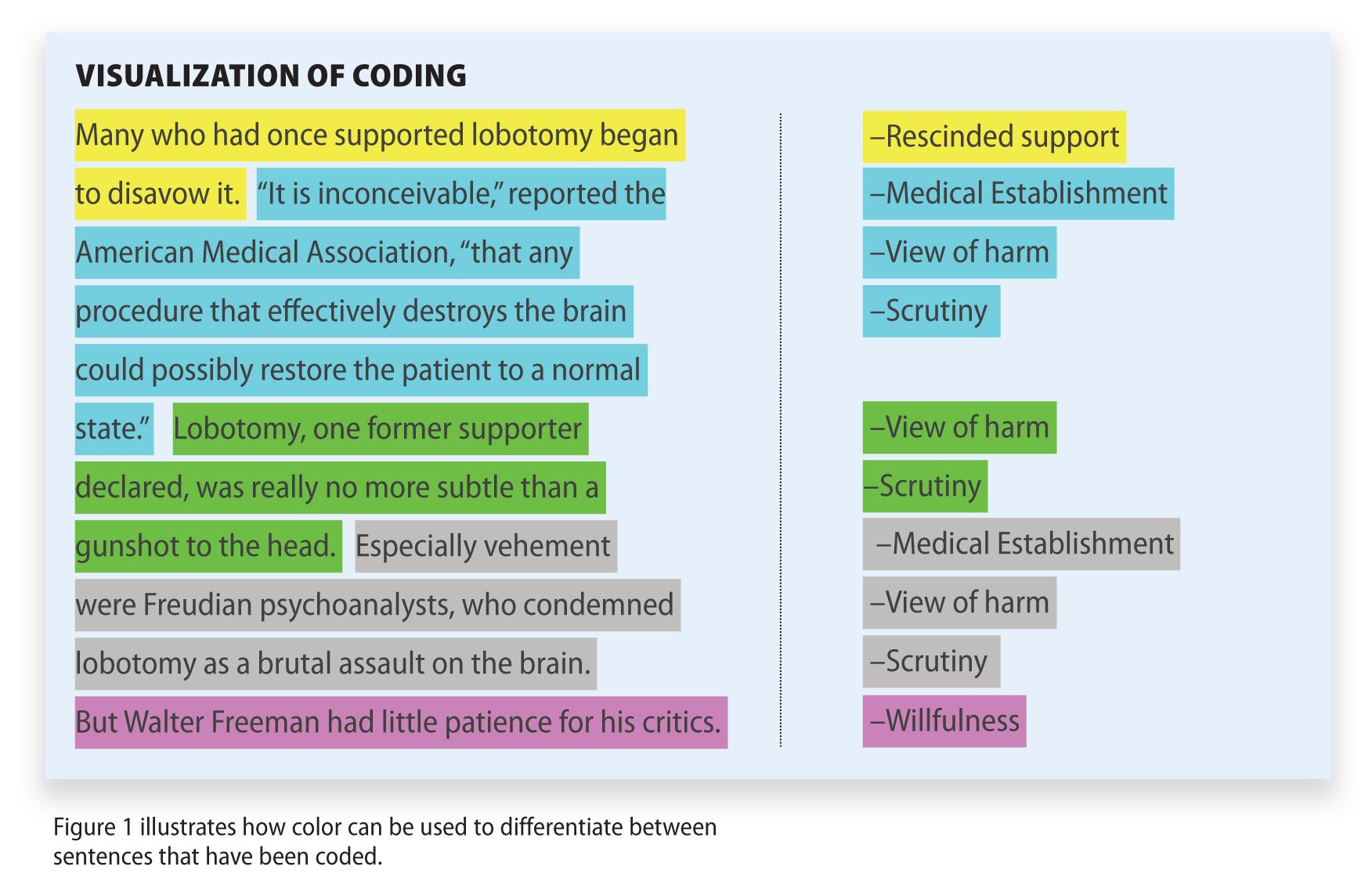
Research Methodology
Qualitative Data Coding

What Is a Focus Group?
Cross-Cultural Research Methodology In Psychology

What Is Internal Validity In Research?

Research Methodology , Statistics
What Is Face Validity In Research? Importance & How To Measure

Criterion Validity: Definition & Examples
Study Design 101: Cohort Study
- Case Report
- Case Control Study
- Cohort Study
- Randomized Controlled Trial
- Practice Guideline
- Systematic Review
- Meta-Analysis
- Helpful Formulas
- Finding Specific Study Types
A study design where one or more samples (called cohorts) are followed prospectively and subsequent status evaluations with respect to a disease or outcome are conducted to determine which initial participants exposure characteristics (risk factors) are associated with it. As the study is conducted, outcome from participants in each cohort is measured and relationships with specific characteristics determined
- Subjects in cohorts can be matched, which limits the influence of confounding variables
- Standardization of criteria/outcome is possible
- Easier and cheaper than a randomized controlled trial (RCT)
Disadvantages
- Cohorts can be difficult to identify due to confounding variables
- No randomization, which means that imbalances in patient characteristics could exist
- Blinding/masking is difficult
- Outcome of interest could take time to occur
Design pitfalls to look out for
The cohorts need to be chosen from separate, but similar, populations.
How many differences are there between the control cohort and the experiment cohort? Will those differences cloud the study outcomes?
Fictitious Example
A cohort study was designed to assess the impact of sun exposure on skin damage in beach volleyball players. During a weekend tournament, players from one team wore waterproof, SPF 35 sunscreen, while players from the other team did not wear any sunscreen. At the end of the volleyball tournament players' skin from both teams was analyzed for texture, sun damage, and burns. Comparisons of skin damage were then made based on the use of sunscreen. The analysis showed a significant difference between the cohorts in terms of the skin damage.
Real-life Examples
Hoepner, L., Whyatt, R., Widen, E., Hassoun, A., Oberfield, S., Mueller, N., ... Rundle, A. (2016). Bisphenol A and Adiposity in an Inner-City Birth Cohort. Environmental Health Perspectives, 124 (10), 1644-1650. https://doi.org/10.1289/EHP205
This longitudinal cohort study looked at whether exposure to bisphenol A (BPA) early in life affects obesity levels in children later in life. Positive associations were found between prenatal BPA concentrations in urine and increased fat mass index, percent body fat, and waist circumference at age seven.
Lao, X., Liu, X., Deng, H., Chan, T., Ho, K., Wang, F., ... Yeoh, E. (2018). Sleep Quality, Sleep Duration, and the Risk of Coronary Heart Disease: A Prospective Cohort Study With 60,586 Adults. Journal Of Clinical Sleep Medicine, 14 (1), 109-117. https://doi.org/10.5664/jcsm.6894
This prospective cohort study explored "the joint effects of sleep quality and sleep duration on the development of coronary heart disease." The study included 60,586 participants and an association was shown between increased risk of coronary heart disease and individuals who experienced short sleep duration and poor sleep quality. Long sleep duration did not demonstrate a significant association.
Related Formulas
- Relative Risk
Related Terms
A group that shares the same characteristics among its members (population).
Confounding Variables
Variables that cause/prevent an outcome from occurring outside of or along with the variable being studied. These variables render it difficult or impossible to distinguish the relationship between the variable and outcome being studied).
Population Bias/Volunteer Bias
A sample may be skewed by those who are selected or self-selected into a study. If only certain portions of a population are considered in the selection process, the results of a study may have poor validity.
Prospective Study
A study that moves forward in time, or that the outcomes are being observed as they occur, as opposed to a retrospective study, which looks back on outcomes that have already taken place.
Now test yourself!
1. In a cohort study, an exposure is assessed and then participants are followed prospectively to observe whether they develop the outcome.
a) True b) False
2. Cohort Studies generally look at which of the following?
a) Determining the sensitivity and specificity of diagnostic methods b) Identifying patient characteristics or risk factors associated with a disease or outcome c) Variations among the clinical manifestations of patients with a disease d) The impact of blinding or masking a study population
Evidence Pyramid - Navigation
- Meta- Analysis
- Case Reports
- << Previous: Case Control Study
- Next: Randomized Controlled Trial >>

- Last Updated: Sep 25, 2023 10:59 AM
- URL: https://guides.himmelfarb.gwu.edu/studydesign101

- Himmelfarb Intranet
- Privacy Notice
- Terms of Use
- GW is committed to digital accessibility. If you experience a barrier that affects your ability to access content on this page, let us know via the Accessibility Feedback Form .
- Himmelfarb Health Sciences Library
- 2300 Eye St., NW, Washington, DC 20037
- Phone: (202) 994-2850
- [email protected]
- https://himmelfarb.gwu.edu

Exploring the Relationship Between Early Life Exposures and the Comorbidity of Obesity and Hypertension: Findings from the 1970 The British Cohort Study (BCS70)
- Find this author on Google Scholar
- Find this author on PubMed
- Search for this author on this site
- ORCID record for S Stannard
- For correspondence: [email protected]
- ORCID record for R Owen
- ORCID record for A Berrington
- ORCID record for N Ziauddeen
- ORCID record for SDS Fraser
- ORCID record for S Paranjothy
- ORCID record for RB Hoyle
- ORCID record for N A Alwan
- Info/History
- Supplementary material
- Preview PDF
Background Epidemiological research commonly investigates single exposure-outcome relationships, while children’s experiences across a variety of early lifecourse domains are intersecting. To design realistic interventions, epidemiological research should incorporate information from multiple risk exposure domains to assess effect on health outcomes. In this paper we identify exposures across five pre-hypothesised childhood domains and explored their association to the odds of combined obesity and hypertension in adulthood.
Methods We used data from 17,196 participants in the 1970 British Cohort Study. The outcome was obesity (BMI of ≥30) and hypertension (blood pressure>140/90mm Hg or self-reported doctor’s diagnosis) comorbidity at age 46. Early life domains included: ‘prenatal, antenatal, neonatal and birth’, ‘developmental attributes and behaviour’, ‘child education and academic ability’, ‘socioeconomic factors’ and ‘parental and family environment’. Stepwise backward elimination selected variables for inclusion for each domain. Predicted risk scores of combined obesity and hypertension for each cohort member within each domain were calculated. Logistic regression investigated the association between domain-specific risk scores and odds of obesity-hypertension, controlling for demographic factors and other domains.
Results Adjusting for demographic confounders, all domains were associated with odds of obesity-hypertension. Including all domains in the same model, higher predicted risk values across the five domains remained associated with increased odds of obesity-hypertension comorbidity, with the strongest associations to the parental and family environment domain (OR1.11 95%CI 1.05-1.18) and the socioeconomic factors domain (OR1.11 95%CI 1.05-1.17).
Conclusions Targeted prevention interventions aimed at population groups with shared early-life characteristics could have an impact on obesity-hypertension prevalence which are known risk factors for further morbidity including cardiovascular disease.
Competing Interest Statement
R.O. is a member of the National Institute for Health and Care Excellence (NICE) Technology Appraisal Committee, member of the NICE Decision Support Unit (DSU), and associate member of the NICE Technical Support Unit (TSU). She has served as a paid consultant to the pharmaceutical industry and international reimbursement agencies, providing unrelated methodological advice. She reports teaching fees from the Association of British Pharmaceutical Industry (ABPI). R.H. is a member of the Scientific Board of the Smith Institute for Industrial Mathematics and System Engineering.
Funding Statement
This work is part of the multidisciplinary ecosystem to study lifecourse determinants and prevention of early-onset burdensome multimorbidity (MELD-B) project which is supported by the National Institute for Health Research (NIHR203988). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Author Declarations
I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained.
The details of the IRB/oversight body that provided approval or exemption for the research described are given below:
Ethics approval for this work has been obtained from the University of Southampton Faculty of Medicine Ethics committee (ERGO II Reference 66810).
I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals.
I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as ClinicalTrials.gov. I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance).
I have followed all appropriate research reporting guidelines, such as any relevant EQUATOR Network research reporting checklist(s) and other pertinent material, if applicable.
Data Availability Statement
The BCS70 datasets generated and analysed in the current study are available from the UK Data Archive repository (available here: http://www.cls.ioe.ac.uk/page.aspx?&sitesectionid=795 ).
View the discussion thread.
Supplementary Material
Thank you for your interest in spreading the word about medRxiv.
NOTE: Your email address is requested solely to identify you as the sender of this article.

Citation Manager Formats
- EndNote (tagged)
- EndNote 8 (xml)
- RefWorks Tagged
- Ref Manager
- Tweet Widget
- Facebook Like
- Google Plus One
Subject Area
- Epidemiology
- Addiction Medicine (324)
- Allergy and Immunology (628)
- Anesthesia (165)
- Cardiovascular Medicine (2379)
- Dentistry and Oral Medicine (289)
- Dermatology (207)
- Emergency Medicine (379)
- Endocrinology (including Diabetes Mellitus and Metabolic Disease) (838)
- Epidemiology (11775)
- Forensic Medicine (10)
- Gastroenterology (703)
- Genetic and Genomic Medicine (3748)
- Geriatric Medicine (350)
- Health Economics (634)
- Health Informatics (2400)
- Health Policy (933)
- Health Systems and Quality Improvement (898)
- Hematology (341)
- HIV/AIDS (782)
- Infectious Diseases (except HIV/AIDS) (13319)
- Intensive Care and Critical Care Medicine (768)
- Medical Education (366)
- Medical Ethics (105)
- Nephrology (398)
- Neurology (3508)
- Nursing (198)
- Nutrition (528)
- Obstetrics and Gynecology (675)
- Occupational and Environmental Health (665)
- Oncology (1825)
- Ophthalmology (538)
- Orthopedics (219)
- Otolaryngology (287)
- Pain Medicine (233)
- Palliative Medicine (66)
- Pathology (446)
- Pediatrics (1035)
- Pharmacology and Therapeutics (426)
- Primary Care Research (420)
- Psychiatry and Clinical Psychology (3179)
- Public and Global Health (6145)
- Radiology and Imaging (1280)
- Rehabilitation Medicine and Physical Therapy (748)
- Respiratory Medicine (828)
- Rheumatology (379)
- Sexual and Reproductive Health (372)
- Sports Medicine (323)
- Surgery (402)
- Toxicology (50)
- Transplantation (172)
- Urology (146)
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Association of ultra...
Association of ultra-processed food consumption with all cause and cause specific mortality: population based cohort study
Linked editorial.
Ultra-processed foods linked to higher mortality
- Related content
- Peer review
- Zhe Fang , doctoral student 1 ,
- Sinara Laurini Rossato , adjunct professor 2 3 ,
- Dong Hang , associate professor 3 4 ,
- Neha Khandpur , assistant professor 3 5 6 ,
- Kai Wang , research associate 1 ,
- Chun-Han Lo , resident physician 7 ,
- Walter C Willett , professor 1 3 8 ,
- Edward L Giovannucci , professor 1 3 ,
- Mingyang Song , associate professor 1 3 9
- 1 Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA
- 2 Laboratory of Research and Extension in Epidemiology (Lapex-Epi), Institute of Geography, Universidade Federal de Uberlândia, Uberlândia, MG, Brazil
- 3 Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA
- 4 Department of Epidemiology, Jiangsu Key Lab of Cancer Biomarkers, Prevention and Treatment, Collaborative Innovation Center for Cancer Personalized Medicine, School of Public Health, Gusu School, Nanjing Medical University, Nanjing, China
- 5 Division of Human Nutrition and Health, Wageningen University, Wageningen, Netherlands
- 6 Department of Nutrition, School of Public Health, University of São Paulo, São Paulo, Brazil
- 7 Department of Internal Medicine, Kirk Kerkorian School of Medicine, University of Nevada, Las Vegas, NV, USA
- 8 Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
- 9 Clinical and Translational Epidemiology Unit and Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
- Correspondence to: M Song msong{at}hsph.harvard.edu (or @MingyangSong3 on X/Twitter)
- Accepted 13 March 2024
Objective To examine the association of ultra-processed food consumption with all cause mortality and cause specific mortality.
Design Population based cohort study.
Setting Female registered nurses from 11 US states in the Nurses’ Health Study (1984-2018) and male health professionals from all 50 US states in the Health Professionals Follow-up Study (1986-2018).
Participants 74 563 women and 39 501 men with no history of cancer, cardiovascular diseases, or diabetes at baseline.
Main outcome measures Multivariable Cox proportional hazard models were used to estimate hazard ratios and 95% confidence intervals for the association of ultra-processed food intake measured by semiquantitative food frequency questionnaire every four years with all cause mortality and cause specific mortality due to cancer, cardiovascular, and other causes (including respiratory and neurodegenerative causes).
Results 30 188 deaths of women and 18 005 deaths of men were documented during a median of 34 and 31 years of follow-up, respectively. Compared with those in the lowest quarter of ultra-processed food consumption, participants in the highest quarter had a 4% higher all cause mortality (hazard ratio 1.04, 95% confidence interval 1.01 to 1.07) and 9% higher mortality from causes other than cancer or cardiovascular diseases (1.09, 1.05 to 1.13). The all cause mortality rate among participants in the lowest and highest quarter was 1472 and 1536 per 100 000 person years, respectively. No associations were found for cancer or cardiovascular mortality. Meat/poultry/seafood based ready-to-eat products (for example, processed meat) consistently showed strong associations with mortality outcomes (hazard ratios ranged from 1.06 to 1.43). Sugar sweetened and artificially sweetened beverages (1.09, 1.07 to 1.12), dairy based desserts (1.07, 1.04 to 1.10), and ultra-processed breakfast food (1.04, 1.02 to 1.07) were also associated with higher all cause mortality. No consistent associations between ultra-processed foods and mortality were observed within each quarter of dietary quality assessed by the Alternative Healthy Eating Index-2010 score, whereas better dietary quality showed an inverse association with mortality within each quarter of ultra-processed foods.
Conclusions This study found that a higher intake of ultra-processed foods was associated with slightly higher all cause mortality, driven by causes other than cancer and cardiovascular diseases. The associations varied across subgroups of ultra-processed foods, with meat/poultry/seafood based ready-to-eat products showing particularly strong associations with mortality.
Introduction
Ultra-processed foods are ready-to-eat/heat industrial formulations made mostly or entirely from substances derived from foods, including flavors, colors, texturizers, and other additives, with little if any intact whole food. 1 Ultra-processed foods, which are typically of low nutritional quality and high energy density, have been dominating the food supply of high income countries, and their consumption is markedly increasing in middle income countries. 2 Ultra-processed food consumption accounts for 57% of daily energy intake among adults and 67% among youths in the US according to the National Health and Nutrition Examination Survey (NHANES). 3 4
Ultra-processed foods usually disproportionately contribute added sugars, sodium, saturated fats and trans fats, and refined carbohydrates to the diet together with low fiber. 5 6 As well as having low nutritional quality, ultra-processed foods may contain harmful substances, such as additives and contaminants formed during the processing. 7 8 9 10 Growing evidence from large prospective cohorts show that ultra-processed food is associated with adverse health outcomes, such as overweight/obesity, cardiovascular diseases, type 2 diabetes, and colorectal cancer. 11 12 13 14 A systematic review showed that high ultra-processed food consumption was associated with increased risk of all cause mortality, cardiovascular diseases, metabolic syndrome, depression, and postmenopausal breast cancer. 15 However, few prospective cohort studies with a follow-up longer than 20 years have examined the association for all cause mortality or cause specific mortality, especially mortality due to cancer. High quality evidence from cohorts with a long follow-up is critical to inform dietary recommendations and food policies.
Leveraging the rich data obtained through repeated assessments for more than 30 years in two large US prospective cohorts, we examined the associations of total ultra-processed food and subgroups of ultra-processed food with mortality from all causes and major individual causes.
Study population
We used data from two large prospective cohorts in the US: the Nurses’ Health Study (NHS) began in 1976 and included 121 700 female registered nurses aged 30-55 years from 11 states; the Health Professionals Follow-up Study (HPFS) began in 1986 and enrolled 51 529 male health professionals aged 40-75 years from all 50 states. Every two years participants completed a mailed questionnaire enquiring about medical and lifestyle information. The baseline of this study was set to 1984 for the NHS and 1986 for the HPFS when the ultra-processed food data were first available. We excluded participants at baseline if they had reported a history of cancer, cardiovascular diseases, or diabetes; left more than 70 food items blank in the food frequency questionnaire or had implausible caloric intakes (<800 or >4200 kcal/d for men; <600 or >3500 kcal/d for women); or had missing data on ultra-processed food intakes. After exclusions, we included 74 563 women from the NHS and 39 501 men from the HPFS (supplementary figure A).
Assessment of ultra-processed food intake
Diet was assessed using a validated semiquantitative food frequency questionnaire administered every four years. 16 We grouped all foods into four categories of the Nova classification: unprocessed or minimally processed foods, processed culinary ingredients, processed foods, and ultra-processed foods, which has been described in detail elsewhere. 17 we further categorized ultra-processed foods into nine mutually exclusive subgroups (supplementary table B; supplementary figure B): ultra-processed breads and breakfast foods; fats, condiments, and sauces; packaged sweet snacks and desserts; sugar sweetened and artificially sweetened beverages; ready-to-eat/heat mixed dishes; meat/poultry/seafood based ready-to-eat products (for example, processed meat); packaged savory snacks; dairy based desserts; and other. Because alcohol is a well studied risk factor for premature death and a distinct factor in diet, we did not consider alcohol in ultra-processed foods in the primary analysis. Moreover, as wholegrain foods have established benefit for lowering all cause mortality, 18 we removed whole grains from ultra-processed foods in the primary analysis. We measured ultra-processed food intake as servings per day and adjusted it for total energy intake by using the residual method. 19
Ascertainment of outcomes
Death of a cohort member was notified by the next of kin via the post office when questionnaires or newsletters were returned or was identified through searches of the vital records of states and of the National Death Index. Study investigators blinded to the exposure status reviewed death certificates and extracted information from medical records to confirm the cause of death according to ICD-8 (international classification of diseases, 8th revision). The primary outcome of this study was all cause mortality. The secondary outcomes included deaths from cancer (ICD-8 codes 140-207), cardiovascular diseases (ICD-8 codes 390-459), and other causes (including respiratory diseases (ICD-8 codes 460-519) and neurodegenerative diseases (ICD-8 codes 290, 332, 340, 342, and 348)).
Assessment of covariates
Biennial follow-up questionnaires were used to collect self-reported information on body weight, marital status, smoking status and pack years, physical activity, family history of cancer/cardiovascular diseases/diabetes, and physical examination for screening purposes, as well as menopausal status and postmenopausal hormone use for women. We calculated body mass index as weight in kilograms divided by height squared in meters. Physical activity was assessed with a validated questionnaire and converted into metabolic equivalent task hours. 20 Alcohol drinking was measured by food frequency questionnaires as the number of drinks per week (considering one drink as one glass, bottle, or can of beer; one 4 ounce glass of wine; or one shot of liquor) and then converted into grams per day. We assessed overall dietary quality by using the Alternative Healthy Eating Index-2010 (AHEI) score. 21
Statistical analysis
Follow-up time accrued from the date of return of the baseline questionnaire to the date of death or the end of follow-up (30 June 2018 for NHS; 31 January 2018 for HPFS), whichever came first. To better represent long term dietary habits and to minimize within person variation, we calculated cumulative averages of ultra-processed food consumption as the primary exposure. We did primary analyses in pooled cohorts and a secondary analysis in each cohort separately. We used time varying Cox proportional hazards models stratified by age (months), questionnaire cycle (two year interval), and cohort (in pooled analyses) with the counting process data structure to estimate the hazard ratios and 95% confidence intervals according to quarters of ultra-processed food consumption. We calculated P for trend on the basis of the Wald test by assigning the median intake to each quarter and modeling it as a continuous variable. In the multivariable model, we adjusted for race/ethnicity, marital status, physical activity, body mass index, smoking status and pack years, alcohol consumption, physical examination performed for screening purposes, family history of diabetes mellitus, myocardial infarction, or cancer, and menopausal status and hormone use (women only). We carried forward non-missing values from the previous survey cycle to replace missing data. If the value remained missing, we created missing indicators. The percentage of missing data is shown in supplementary table A. We also tested for the dose-response relation by using the restricted cubic spline regression. 22
In secondary analyses, we further categorized ultra-processed foods into mutually exclusive subgroups (supplementary tables B and C) to investigate whether the associations were driven by specific food groups. 13 Furthermore, to assess the independent and combined association of ultra-processed food consumption and overall dietary quality with mortality, we categorized individuals jointly according to quarters of AHEI score and quarters of ultra-processed food intake and estimated the hazard ratios by using participants with the highest quarter of AHEI score and lowest quarter of ultra-processed food intake as the reference.
We did several sensitivity analyses to test the robustness of the results. Firstly, given that people are likely to change their dietary habits after the diagnosis of certain chronic diseases, we stopped updating ultra-processed food consumption after the diagnosis of cardiovascular diseases, cancer, or diabetes during follow-up. Secondly, because of the uncertainty of the etiological time window, we introduced an eight to 12 year lag period between assessment of ultra-processed food intake and each follow-up period (for example, we used ultra-processed food intake from the 1986 questionnaire to assess the mortality risk in the period of 1994 to 1998). Thirdly, we added back to total ultra-processed food whole grains and distilled alcohol individually and in combination (that is, using the standard Nova definition) and repeated the analysis. Finally, we removed from the multivariable model pack years of smoking, which was not adjusted for in most previous studies, and further adjusted for AHEI score, to assess the confounding by smoking and dietary quality, respectively. We also removed from the multivariable model body mass index, which might be a mediator. Furthermore, we did the stratified analysis by major risk factors and repeated the primary analysis with ultra-processed food intake measured by percentage of energy.
We used SAS statistical package (version 9.4) for all the statistical analyses. We considered a P value <0.05 (two sided) to be statistically significant unless otherwise specified.
Patient and public involvement
The public was concerned about the health effects of ultra-processed foods, and their concerns informed our research question. Although participants were not involved in the study design, they played a central role in the conduct of the study by completing the biennial questionnaires in our cohorts, and we appreciate their contributions. We could not directly involve members of the public in this study, as no funding was available or set aside for patient and public involvement and our study team was not trained to work directly with the public.
During a median of 34 years of follow-up, we documented 48 193 deaths (30 188 deaths of women and 18 005 deaths of men), including 13 557 deaths due to cancer, 11 416 deaths due to cardiovascular diseases, 3926 deaths due to respiratory diseases, and 6343 deaths due to neurodegenerative diseases. Table 1 shows the characteristics of participants according to quarters of energy adjusted ultra-processed food consumption throughout follow-up. Participants with higher ultra-processed food consumption were younger, more physically inactive, and more likely to smoke and had higher body mass index, lower consumption of alcohol, whole fruits and vegetables, and whole grains, and lower AHEI score.
Age standardized characteristics of study participants according to quarters of ultra-processed food (UPF) consumption across entire follow-up period. Values are number (percentage) of person years unless stated otherwise
- View inline
Table 2 shows the hazard ratios of mortality according to quarters of ultra-processed food consumption. In the age, sex, and total calorie adjusted analysis, we observed strong positive associations between ultra-processed food and mortality outcomes. The associations became substantially attenuated in the multivariable analysis ( table 2 ; supplementary figure C). Compared with participants in the lowest quarter (median 3.0 servings/day), those in the highest quarter (median 7.4 servings/day) had a 4% higher risk of total deaths (multivariable adjusted hazard ratio 1.04, 95% confidence interval 1.01 to 1.07; P for trend=0.005) and a 9% higher risk of other deaths (1.09, 1.05 to 1.13; P for trend<0.001), including an 8% higher risk of neurodegenerative deaths (1.08, 1.01 to 1.17; P for trend=0.1). We found no associations for deaths due to cardiovascular diseases, cancer, or respiratory diseases. The all cause mortality rate among participants in the lowest and highest quarter of ultra-processed food consumption was 1472 and 1536 per 100 000 person years, respectively.
Hazard ratios and 95% confidence intervals for mortality according to quarters of ultra-processed food (UPF) consumption
Table 3 shows the associations for nine subgroups of ultra-processed foods. Meat/poultry/seafood based ready-to-eat products (for example, processed meat) showed the strongest association with higher all cause mortality (hazard ratio 1.13 (1.10 to 1.16) comparing highest versus lowest quarter) and mortality due to individual causes other than cardiovascular diseases and neurodegenerative diseases (hazard ratios ranged from 1.06 to 1.43). Other subgroups also showed an association with higher all cause mortality, including sugar sweetened and artificially sweetened beverages (1.09, 1.07 to 1.12), other ultra-processed foods (mainly composed of artificial sweeteners) (1.08, 1.05 to 1.11), dairy based desserts (1.07, 1.04 to 1.10), and ultra-processed breakfast foods excluding whole grains (1.04, 1.02 to 1.07). When further separating sugar sweetened and artificially sweetened beverages, we found a generally stronger association for sugar sweetened than artificially sweetened beverages; we present these results and those for other selected individual ultra-processed food categories in supplementary table D.
Multivariable hazard ratios and 95% confidence intervals for mortality according to quarters of subgroups of ultra-processed food consumption *
When we examined ultra-processed food intake and AHEI score together ( fig 1 ), we did not observe a consistent association of ultra-processed foods with mortality within each quarter of the AHEI score, whereas AHEI score generally showed an inverse association with mortality within each of the quarters of ultra-processed food consumption.

Joint analysis for mortality according to quarters of ultra-processed food (UPF) consumption and quarters of Alternative Healthy Eating Index-2010 (AHEI) score. Alcohol was removed from calculation of AHEI score. Each participant was categorized according to their quarter of UPF intake and their quarter of AHEI score, resulting in 16 distinct groups. Using this combined variable as exposure, its association with mortality outcomes was assessed, with reference group being participants in highest quarter of AHEI score (Q4) and lowest quarter of UPF intake (Q1). Results were from multivariable Cox proportional hazards model stratified by age (months), questionnaire cycle (two year interval), and cohort and adjusted for total energy intake, race, marital status, physical activity, body mass index, smoking status and pack years, alcohol consumption, physical examination performed for screening purposes, and family history of diabetes mellitus, myocardial infarction, or cancer; for women, also menopausal status and hormone use. Markers denote point estimates of hazard ratios and error bars indicate 95% confidence intervals
- Download figure
- Open in new tab
- Download powerpoint
We found similar results in men and women (supplementary table E). The results of sensitivity analyses are summarized in supplementary table F. The lagged analysis showed similar results to the primary analysis. The associations were attenuated when we stopped updating the information on ultra-processed food intake at a diagnosis of chronic disease, likely owing to the increased intake of ultra-processed foods over time (supplementary figures D and E). Unsurprisingly, including wholegrain products in ultra-processed foods weakened the associations, whereas including distilled alcohol strengthened the associations. Removing pack years of smoking from the multivariable model led to a much stronger positive association, whereas adjusting for the AHEI score attenuated the association toward null.
In the stratified analysis by major risk factors, the associations between ultra-processed food intake and all cause mortality seemed to be stronger in participants consuming less alcohol (P for interaction=0.005) and not currently smoking (P for interaction<0.001), but we found no interaction by body mass index or physical activity (supplementary table G). We repeated the primary analysis using percentage of energy to measure ultra-processed food intake and observed similar results (supplementary table H).
In two large prospective cohorts with up to 34 years of follow-up, we found that higher consumption of ultra-processed foods was associated with modestly higher all cause mortality. We found no associations for mortality due to cancer or cardiovascular diseases. The associations varied across subgroups of ultra-processed foods, with meat/poultry/seafood based ready-to-eat products consistently showing associations with higher all cause mortality and cause specific mortality. The associations between ultra-processed food consumption and mortality were attenuated after we accounted for overall dietary quality.
Comparison with other studies and possible explanations
Existing evidence suggests a relation between ultra-processed food consumption and mortality. A meta-analysis of prospective cohorts reported that the highest ultra-processed food consumption was associated with higher all cause mortality compared with the lowest consumption (hazard ratio 1.21, 1.13 to 1.30). 23 Two studies were conducted in the US, 24 25 whereas the other six were conducted in Spain, 26 27 28 France, 29 Italy, 30 and the UK. 31 Unlike our study, which excluded alcohol from ultra-processed foods and carefully controlled for smoking status and pack years, all the above studies included alcohol in ultra-processed foods and adjusted for smoking status (never, former, and current) only. As noted in our sensitivity analysis, pack years of smoking strongly confounded the association—additionally adjusting for smoking pack years remarkably attenuated the hazard ratios toward the null. That may partly explain why the associations found in our study were weaker than those in previous studies. Another possible reason could be tighter control for socioeconomic status because our participants were all health professionals and had similar levels of education.
The evidence on mortality due to cancer is relatively sparse. Consistently, the Moli-sani Study did not observe a statistically significant association but reported a positive association with other mortality. 30 An analysis of three cohorts including the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO), NHANES (1999-2018), and UK Biobank reported null findings for mortality due to cancer in the PLCO and NHANES (1999-2018). 32 By contrast, the UK Biobank study found that every 10% increment in ultra-processed food consumption was associated with a 6% higher cancer mortality. 33 Diet was assessed in the UK Biobank through multiple 24 hour recalls between 2009 and 2012, and 40% of the participants had only one 24 hour recall, thus limiting the ability to capture long term dietary intake.
In agreement with our study, the Prospective Urban and Rural Epidemiology study from 25 high income, middle income, and low income countries in America, Europe, Africa, and Asia observed a null association with mortality due to cardiovascular diseases but a positive association with non-cardiovascular disease mortality. 34 Our findings on the relation between ultra-processed foods and mortality due to cardiovascular diseases are inconsistent with previous evidence from Europe but consistent with the null finding in the US NHANES III (1988-94). 24 25 30 Moreover, a much stronger positive association was reported in the UK Biobank (1.28, 1.13 to 1.45) compared with the two US cohorts (1.12, 1.05 to 1.09; 1.11, 0.92 to 1.34). 32 In addition to the methodological differences mentioned above, different study populations, ultra-processed food compositions, and eating patterns may also contribute. Ultra-processed food intake in our two US cohorts is mainly contributed by “sauces, spreads, and condiments” and “sweet snacks and desserts,” which together accounted for nearly 50% (supplementary figure B), but neither of the two subgroups was associated with increased mortality due to cardiovascular diseases. On the other hand, compelling evidence shows that nuts and (dark) chocolate, common constituents of “sweet snacks and desserts,” are inversely associated with cardiovascular diseases. 35 36 We observed that dark chocolate in the subgroup “packaged sweet snacks and desserts” was associated with decreased mortality (supplementary table D). Therefore, the diverse array of constituents contained in ultra-processed foods with heterogeneous health effects may have contributed to the discrepant findings. Our findings suggest that meat/poultry/seafood based ready-to-eat products and sugar sweetened and artificially sweetened beverages are major factors contributing to the harmful influence of ultra-processed foods on mortality, which is in accordance with previous studies. 13 37 38 39
Few studies have investigated the relation with cause specific mortality other than that due to cancer and cardiovascular diseases. We found that ultra-processed food intake was associated with higher neurodegenerative mortality. Increasing evidence suggests that ultra-processed food is linked to higher risk of central nervous system demyelination (a precursor of multiple sclerosis), 40 lower cognitive function, 41 and dementia. 42 Studies have shown that a diet rich in ultra-processed foods may drive neuroinflammation and impairment of the blood-brain barrier, leading to neurodegeneration. 43 44 Of note, among ultra-processed food subgroups, diary based desserts showed the strongest association with neurodegenerative mortality. Earlier finding from the HPFS and NHS cohorts showed that intake of sherbet/frozen yogurt was associated with an increased risk of Parkinson’s disease. 45 Furthermore, we found a positive association between ultra-processed food intake measured by percentage of energy and respiratory mortality. Emerging evidence suggests that higher ultra-processed food intake is associated with increased risk of respiratory multimorbidity. 46 The increased respiratory mortality associated with processed red meat may be partly due to heme iron and nitrate/nitrite. 47
An important question not answered by previous studies is whether and how food processing level and nutritional quality jointly influence health. We observed that in the joint analysis, the AHEI score but not ultra-processed food intake showed a consistent association with mortality and that further adjustment for the AHEI score attenuated the association of ultra-processed food intake with mortality. Although including AHEI in the multivariable model for ultra-processed food may represent an overadjustment because common foods are included in both the AHEI and ultra-processed food, our data together suggest that dietary quality has a predominant influence on long term health, whereas the additional effect of food processing is likely to be limited. Furthermore, foods may have dual attributes according to their processing level and nutritional quality, and these two features may have quantitatively and even qualitatively different effects on health. Another added value of our study is the exclusion of wholegrain products that fall in the ultra-processed foods from the primary exposure, based on the well established health benefits associated with whole grains. By taking this approach, we aim to rectify the potential misperception that all ultra-processed food products should be universally restricted and to avoid oversimplification when formulating dietary recommendations.
Besides neglecting overall nutritional quality, the ultra-processed food classification system has other limitations. The Nova classification is based on broad categories that do not capture the full complexity of food processing, 48 leading to potential misclassification. Further work is needed to improve the assessment and categorization of ultra-processed foods. On the other hand, dietary guidelines should provide clear and sound food selections that are available, actionable, attainable, and affordable for the largest proportion of the population. Thus, careful deliberation is necessary when considering incorporation of ultra-processed foods into dietary guidelines. 49 50 Again, on the basis of our data, limiting total ultra-processed food consumption may not have a substantial influence on premature death, whereas reducing consumption of certain ultra-processed food subgroups (for example, processed meat) can be beneficial.
We note that mortality is a more complicated endpoint than disease incidence and is also influenced by several factors including early detection, treatment, and individuals’ overall health status. The findings for mortality should not be regarded as synonymous with those pertaining to disease incidence but rather considered as more comprehensive assessment of the health impact of risk factors.
Strengths and limitations of study
The strengths of the study include the prospective study design, large sample size, long follow-up, and detailed, validated, and repeated measurements. In addition, we rigorously controlled for confounding, did thorough sensitivity analyses, explored major specific causes of mortality, and examined individual ultra-processed food subgroups. Several limitations should also be noted. Firstly, we cannot rule out unmeasured and residual confounding due to the nature of the observational study. Secondly, our participants are health professionals and predominantly non-Hispanic white, limiting the generalizability of our findings. Thirdly, as the food frequency questionnaires collected intake of only a limited number of pre-defined items representing the primary source of energy and nutrients in the US population and were not designed to classify foods by processing level, they may not capture the full spectrum of ultra-processed foods. Although the food frequency questionnaires used in our cohorts have been validated for foods and nutrients, they were not specifically validated for ultra-processed foods. Moreover, we classified ultra-processed foods by using the same algorithm throughout follow-up that did not account for changes in the grade of food processing over time. These factors may have introduced non-differential misclassification, likely biasing our results toward the null.
Conclusions
Higher ultra-processed food intake was associated with slightly increased all cause mortality. The mortality associations for ultra-processed food consumption were more modest than those for dietary quality and varied across ultra-processed food subgroups, with meat/poultry/seafood based ready-to-eat products generally showing the strongest and most consistent associations with mortality. The findings provide support for limiting consumption of certain types of ultra-processed food for long term health. Future studies are warranted to improve the classification of ultra-processed foods and confirm our findings in other populations.
What is already known on this topic
Ultra-processed foods have been suggested to have adverse health effects
Evidence is limited on the influence of ultra-processed food consumption on mortality outcomes in large cohorts with long term follow-up and repeated dietary assessment
What this study adds
A higher intake of ultra-processed foods was associated with slightly higher all cause mortality, driven by causes other than cancer and cardiovascular diseases
The positive associations were mainly driven by meat/poultry/seafood based ready-to-eat products, sugar and artificially sweetened beverages, dairy based desserts, and ultra-processed breakfast foods
Dietary quality was observed to have a more predominant influence on mortality outcomes than ultra-processed food consumption
Ethics statements
Ethical approval.
The Nurses’ Health Study I and the Health Professionals Follow-up Study were approved by the Institutional Review Board at the Brigham and Women’s Hospital, the Harvard T.H. Chan School of Public Health (IRB protocol number: 1999-P-011114 and 10162). The completion of the self-administered questionnaire was considered to imply informed consent.
Data availability statement
Data can be shared through mechanisms detailed at https://www.nurseshealthstudy.org and https://www.hsph.harvard.edu/hpfs/ .

Acknowledgments
We thank the participants of the Nurses’ Health Study and the Health Professionals Follow-up Study and the staff of the Channing Division of Network Medicine for their valuable contributions. We acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. Central registries may also be supported by state agencies, universities, and cancer centers. Participating central cancer registries include the following: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Indiana, Iowa, Kentucky, Louisiana, Massachusetts, Maine, Maryland, Michigan, Mississippi, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, Seattle SEER Registry, South Carolina, Tennessee, Texas, Utah, Virginia, West Virginia, and Wyoming. The authors assume full responsibility for analyses and interpretation of these data.
Contributors: ZF did the statistical analysis and drafted the manuscript. SLR and NK made a substantial contribution to the concept of the article. DH, WK, CHL, WCW, and ELG were involved in the acquisition and interpretation of data. MS was responsible for the study design. All authors critically assessed, edited, and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. MS is the guarantor.
Funding: This work was supported by the US National Institutes of Health grants (UM1 CA186107; P01 CA87969; U01 CA167552; U01 CA261961; R01 CA263776; and K99 CA283146). The funders had no role in considering the study design or in the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from the National Institutes of Health for the submitted work; NK received a consulting fee from the Pan American Health Organization for three months on the topic of nutrition disclosure initiatives and nutrient profiling models; no other relationships or activities that could appear to have influenced the submitted work.
Transparency: The manuscript’s guarantor affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The research findings are disseminated to participants through periodic newsletters and study websites at https://www.nurseshealthstudy.org and https://www.hsph.harvard.edu/hpfs/ . The manuscript will be disseminated to the general public through press releases.
Provenance and peer review: Not commissioned; externally peer reviewed.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
- Monteiro CA ,
- Moubarac JC ,
- Martinez-Steele E ,
- Martínez Steele E ,
- Mendez MA ,
- Louzada MLC ,
- Buckley JP ,
- Monteiro CA
- Kesse-Guyot E ,
- Khandpur N ,
- Desjardins C ,
- Giovannucci EL ,
- Stampfer MJ ,
- Colditz GA ,
- Rossato S ,
- Drouin-Chartier JP ,
- Giovannucci E ,
- Chasan-Taber S ,
- McCullough ML ,
- Gauthier J ,
- Suksatan W ,
- Rico-Campà A ,
- Martínez-González MA ,
- Alvarez-Alvarez I ,
- Blanco-Rojo R ,
- Sandoval-Insausti H ,
- López-Garcia E ,
- Romero Ferreiro C ,
- Martín-Arriscado Arroba C ,
- Cancelas Navia P ,
- Lora Pablos D ,
- Gómez de la Cámara A
- Schnabel L ,
- Bonaccio M ,
- Di Castelnuovo A ,
- Costanzo S ,
- Gunter MJ ,
- Dehghan M ,
- Rangarajan S ,
- Prospective Urban Rural Epidemiology (PURE) study investigators
- Guasch-Ferré M ,
- Schwedhelm C ,
- Canhada SL ,
- Cordova R ,
- Viallon V ,
- Fontvieille E ,
- Mannino A ,
- Ausimmune Investigator Group
- R Cardoso B ,
- Machado P ,
- Więckowska-Gacek A ,
- Mietelska-Porowska A ,
- Wydrych M ,
- Martínez Leo EE ,
- Segura Campos MR
- Hughes KC ,
- Etemadi A ,
- Petrus RR ,
- do Amaral Sobral PJ ,
- Tadini CC ,
- Vadiveloo MK ,
- Comeau ME ,
- Casperson S ,
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 14 May 2024
Identification and validation of microbial biomarkers from cross-cohort datasets using xMarkerFinder
- Wenxing Gao ORCID: orcid.org/0000-0002-1740-8227 1 na1 ,
- Weili Lin 1 na1 ,
- Qiang Li 2 na1 ,
- Wanning Chen 1 ,
- Wenjing Yin 1 ,
- Xinyue Zhu ORCID: orcid.org/0009-0009-4168-6196 1 ,
- Sheng Gao 1 ,
- Lei Liu 1 ,
- Wenjie Li 3 ,
- Dingfeng Wu 4 ,
- Guoqing Zhang ORCID: orcid.org/0000-0001-8827-7546 2 ,
- Ruixin Zhu ORCID: orcid.org/0000-0002-5070-6453 1 &
- Na Jiao ORCID: orcid.org/0000-0003-3976-6313 4 , 5
Nature Protocols ( 2024 ) Cite this article
157 Accesses
1 Altmetric
Metrics details
- Bioinformatics
- Computational biology and bioinformatics
- Microbiology
Microbial signatures have emerged as promising biomarkers for disease diagnostics and prognostics, yet their variability across different studies calls for a standardized approach to biomarker research. Therefore, we introduce xMarkerFinder, a four-stage computational framework for microbial biomarker identification with comprehensive validations from cross-cohort datasets, including differential signature identification, model construction, model validation and biomarker interpretation. xMarkerFinder enables the identification and validation of reproducible biomarkers for cross-cohort studies, along with the establishment of classification models and potential microbiome-induced mechanisms. Originally developed for gut microbiome research, xMarkerFinder’s adaptable design makes it applicable to various microbial habitats and data types. Distinct from existing biomarker research tools that typically concentrate on a singular aspect, xMarkerFinder uniquely incorporates a sophisticated feature selection process, specifically designed to address the heterogeneity between different cohorts, extensive internal and external validations, and detailed specificity assessments. Execution time varies depending on the sample size, selected algorithm and computational resource. Accessible via GitHub ( https://github.com/tjcadd2020/xMarkerFinder ), xMarkerFinder supports users with diverse expertise levels through different execution options, including step-to-step scripts with detailed tutorials and frequently asked questions, a single-command execution script, a ready-to-use Docker image and a user-friendly web server ( https://www.biosino.org/xmarkerfinder ).
The authors describe xMarkerFinder, a four-stage computational framework for microbial biomarker identification with comprehensive validations from cross-cohort datasets.
xMarkerFinder is the first computational framework aggregating meta-analyses and machine learning models for the establishment and validation of universally robust microbial biomarkers across multiple cohorts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
251,40 € per year
only 20,95 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Identifying proteomic risk factors for cancer using prospective and exome analyses of 1463 circulating proteins and risk of 19 cancers in the UK Biobank

Genome-wide association studies

Microbiota in health and diseases
Data availability.
Example input/output files, codes, frequently asked questions and a user message board for xMarkerFinder are provided in our GitHub repository ( https://github.com/tjcadd2020/xMarkerFinder ). Source data used for generating Figs. 3 and 9 can be accessed as Supplementary information.
Code availability
The single-command execution option and the step-to-step scripts for xMarkerFinder can be obtained at https://github.com/tjcadd2020/xmarkerfinder . The ready-to-use Docker image can be pulled from Docker Hub ( https://hub.docker.com/r/tjcadd2022/xmarkerfinder ) and the dockerfile used for creating this Docker image is also provided. The user-friendly web server for xMarkerFinder is available at https://www.biosino.org/xmarkerfinder/ . The code in this protocol has been peer reviewed.
Cullin, N., Azevedo Antunes, C., Straussman, R., Stein-Thoeringer, C. K. & Elinav, E. Microbiome and cancer. Cancer Cell 39 , 1317–1341 (2021).
Article CAS PubMed Google Scholar
LaCourse, K. D., Johnston, C. D. & Bullman, S. The relationship between gastrointestinal cancers and the microbiota. Lancet Gastroenterol. Hepatol. 6 , 498–509 (2021).
Article PubMed PubMed Central Google Scholar
Fan, Y. & Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19 , 55–71 (2021).
Britton, G. J. et al. Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORγt+ regulatory T cells and exacerbate colitis in mice. Immunity 50 , 212–224. e214 (2019).
Article CAS PubMed PubMed Central Google Scholar
Mima, K. et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 65 , 1973–1980 (2016).
McQuade, J. L., Daniel, C. R., Helmink, B. A. & Wargo, J. A. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 20 , e77–e91 (2019).
Article PubMed Google Scholar
Wu, Y. et al. Identification of microbial markers across populations in early detection of colorectal cancer. Nat. Commun. 12 , 3063 (2021).
Liu, N.-N. et al. Multi-kingdom microbiota analyses identify bacterial–fungal interactions and biomarkers of colorectal cancer across cohorts. Nat. Microbiol. 7 , 238–250 (2022).
Ma, S. et al. Population structure discovery in meta-analyzed microbial communities and inflammatory bowel disease using MMUPHin. Genome Biol. 23 , 208 (2022).
Gao, W. et al. Multimodal metagenomic analysis reveals microbial single nucleotide variants as superior biomarkers for early detection of colorectal cancer. Gut Microbes 15 , 2245562 (2023).
Zhu, X. et al. Multi-kingdom microbial signatures in excess body weight colorectal cancer based on global metagenomic analysis. Commun. Biol. 7 , 24 (2024).
Gao, S. et al. Microbial genes outperform species and SNVs as diagnostic markers for Crohn’s disease on multicohort fecal metagenomes empowered by artificial intelligence. Gut Microbes 15 , 2221428 (2023).
Relman, D. A. The human microbiome and the future practice of medicine. JAMA 314 , 1127–1128 (2015).
Gao, L. et al. Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell 9 , 488–500 (2018).
Byrd, A. L., Belkaid, Y. & Segre, J. A. The human skin microbiome. Nat. Rev. Microbiol. 16 , 143–155 (2018).
Wang, Z. et al. Inflammatory endotype-associated airway microbiome in chronic obstructive pulmonary disease clinical stability and exacerbations: a multicohort longitudinal analysis. Am. J. Respir. Crit. Care Med. 203 , 1488–1502 (2021).
Zhou, J. et al. Signatures of mucosal microbiome in oral squamous cell carcinoma identified using a random forest model. Cancer Manag. Res. 12 , 5353–5363 (2020).
Zhang, L., Liu, Y., Zheng, H. J. & Zhang, C. P. The oral microbiota may have influence on oral cancer. Front. Cell Infect. Microbiol. 9 , 476 (2019).
Zhao, H. et al. Variations in oral microbiota associated with oral cancer. Sci. Rep. 7 , 11773 (2017).
Sunagawa, S. et al. Structure and function of the global ocean microbiome. Science 348 , 1261359 (2015).
Salazar, G. et al. Gene expression changes and community turnover differentially shape the global ocean metatranscriptome. Cell 179 , 1068–1083 e1021 (2019).
Govaere, O. et al. Transcriptomic profiling across the nonalcoholic fatty liver disease spectrum reveals gene signatures for steatohepatitis and fibrosis. Sci. Transl. Med. 12 , eaba4448 (2020).
Pantano, L. et al. Molecular characterization and cell type composition deconvolution of fibrosis in NAFLD. Sci. Rep. 11 , 18045 (2021).
Gerhard, G. S. et al. Transcriptomic profiling of obesity-related nonalcoholic steatohepatitis reveals a core set of fibrosis-specific genes. J. Endocr. Soc. 2 , 710–726 (2018).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12 , R60 (2011).
Xiao, L., Zhang, F. & Zhao, F. Large-scale microbiome data integration enables robust biomarker identification. Nat. Comput. Sci. 2 , 307–316 (2022).
Wirbel, J. et al. Microbiome meta-analysis and cross-disease comparison enabled by the SIAMCAT machine learning toolbox. Genome Biol. 22 , 93 (2021).
Jiang, J. MIIDL: a Python package for microbial biomarkers identification powered by interpretable deep learning. Preprint at https://arxiv.org/abs/2109.12204 (2021).
Wang, Y. & LeCao, K. A. Managing batch effects in microbiome data. Brief. Bioinform. 21 , 1954–1970 (2020).
Ling, W. et al. Batch effects removal for microbiome data via conditional quantile regression. Nat. Commun. 13 , 5418 (2022).
Gibbons, S. M., Duvallet, C. & Alm, E. J. Correcting for batch effects in case-control microbiome studies. PLoS Comput. Biol. 14 , e1006102 (2018).
Dai, Z., Wong, S. H., Yu, J. & Wei, Y. Batch effects correction for microbiome data with Dirichlet-multinomial regression. Bioinformatics 35 , 807–814 (2018).
Article Google Scholar
Knight, R. et al. Best practices for analysing microbiomes. Nat. Rev. Microbiol. 16 , 410–422 (2018).
Coelho, L. P. et al. NG-meta-profiler: fast processing of metagenomes using NGLess, a domain-specific language. Microbiome 7 , 84 (2019).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G. Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39 , 175–191 (2007).
Faul, F., Erdfelder, E., Buchner, A. & Lang, A.-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods 41 , 1149–1160 (2009).
Kelly, B. J. et al. Power and sample-size estimation for microbiome studies using pairwise distances and PERMANOVA. Bioinformatics 31 , 2461–2468 (2015).
Casals-Pascual, C. et al. Microbial diversity in clinical microbiome studies: sample size and statistical power considerations. Gastroenterology 158 , 1524–1528 (2020).
Pasolli, E. et al. Accessible, curated metagenomic data through ExperimentHub. Nat. Methods 14 , 1023–1024 (2017).
Dai, D. et al. GMrepo v2: a curated human gut microbiome database with special focus on disease markers and cross-dataset comparison. Nucleic Acids Res. 50 , D777–D784 (2022).
Janssens, Y. et al. Disbiome database: linking the microbiome to disease. BMC Microbiol. 18 , 50 (2018).
Shoaie, S. et al. Global and temporal state of the human gut microbiome in health and disease. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-339282/v1 (2021).
Weiss, S. et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 5 , 27 (2017).
Molder, F. et al. Sustainable data analysis with Snakemake. F1000Res 10 , 33 (2021).
Download references
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers 92251307 to R.Z. and G.Z., 82170542 to R.Z., 82000536 to N.J.), and the National Key Research and Development Program of China (grant number 2021YFF0703702 to R.Z.). We thank X. Huang, K. Chen and Y. Huang for testing xMarkerFinder and providing their constructive feedback.
Author information
These authors contributed equally: Wenxing Gao, Weili Lin, Qiang Li.
Authors and Affiliations
The Shanghai Tenth People’s Hospital, School of Life Sciences and Technology, Tongji University, Shanghai, P. R. China
Wenxing Gao, Weili Lin, Wanning Chen, Wenjing Yin, Xinyue Zhu, Sheng Gao, Lei Liu & Ruixin Zhu
National Genomics Data Center & Bio-Med Big Data Center, Chinese Academy of Sciences Key Laboratory of Computational Biology, Shanghai Institute of Nutrition and Health, University of the Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, P. R. China
Qiang Li & Guoqing Zhang
Shanghai Southgene Technology Co., Ltd., Shanghai, P. R. China
National Clinical Research Center for Child Health, the Children’s Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, P. R. China
Dingfeng Wu & Na Jiao
State Key Laboratory of Genetic Engineering, Fudan Microbiome Center, School of Life Sciences, Fudan University, Shanghai, P. R. China
You can also search for this author in PubMed Google Scholar
Contributions
N.J., R.Z. and G.Z. conceived and designed the study. W.G. and W.Lin wrote the codes and step-to-step protocol. Q.L. and W.Li designed the user interface and wrote related codes. W.G., W.Lin and N.J. drafted the manuscript. W.C., W.Y., X.Z. and S.G. tested the protocol. L.L., D.W., G.Z., R.Z. and N.J. reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Guoqing Zhang , Ruixin Zhu or Na Jiao .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Protocols thanks Kang Ning, Jiachao Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Wu, Y. et al. Nat. Commun . 12 , 3063 (2021): https://doi.org/10.1038/s41467-021-23265-y
Liu, N.-N. et al. Nat. Microbiol . 7 , 238–250 (2022): https://doi.org/10.1038/s41564-021-01030-7
Gao, S. et al. Gut Microbes 15 , 2221428 (2023): https://doi.org/10.1080/19490976.2023.2221428
Gao, W et al. Gut Microbes 15 , 2245562 (2023): https://doi.org/10.1080/19490976.2023.2245562
Zhu, X et al. Commun. Biol . 7 , 24 (2024): https://doi.org/10.1038/s42003-023-05714-0
Key data used in this protocol
Extended data
Supplementary information, supplementary information.
Supplementary Figs. 1 and 2 and Table 1.
Reporting Summary
Supplementary data 1.
Example data for the execution of xMarkerFinder.
Source data
Source data fig. 3.
Statistical source data.
Source Data Fig. 9
Rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Gao, W., Lin, W., Li, Q. et al. Identification and validation of microbial biomarkers from cross-cohort datasets using xMarkerFinder. Nat Protoc (2024). https://doi.org/10.1038/s41596-024-00999-9
Download citation
Received : 24 January 2023
Accepted : 05 March 2024
Published : 14 May 2024
DOI : https://doi.org/10.1038/s41596-024-00999-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Ethics and practice of Trials within Cohorts: An emerging pragmatic trial design
Scott yh kim.
1 Department of Bioethics, Clinical Center, National Institutes of Health, Bethesda, MD, USA
2 Center for Bioethics and Social Sciences in Medicine, University of Michigan, Ann Arbor, MI, USA
James Flory
3 Memorial Sloan Kettering Cancer Center, New York, NY, USA
Clare Relton
4 University of Sheffield, Sheffield, UK
With increasing emphasis on pragmatic trials, new randomized clinical trial designs are being proposed to enhance the “real world” nature of the data generated. We describe one such design, appropriate for unmasked pragmatic clinical trials in which the control arm receives usual care, called “Trials within Cohorts” that is increasingly used in various countries because of its efficiency in recruitment, advantages in reducing subject burden, and ability to better mimic real-world consent processes.
Descriptive, ethical, and US regulatory analysis of the Trials within Cohorts design.
Trials within Cohorts design involves, after recruitment into a cohort, randomization of eligible subjects, followed by an asymmetric treatment of the two arms: those selected for the experimental arm provide informed consent for the intervention trial, while the data from the control arm are used based on prior broad permission. Thus, unlike the traditional Zelen post-randomization consent design, the cohort participants are informed about future research within the cohort; however, the extent of this disclosure currently varies among studies. Thus, ethical analysis is provided for two types of situations: when the pre-randomization disclosure and consent regarding the embedded trials are fairly explicit and detailed versus when they consist of only general statements about future data use. These differing ethical situations could have implications for how ethics review committees apply US research rules regarding waivers and alterations of informed consent.
Trials within Cohorts is a promising new pragmatic randomized controlled trial design that is being increasingly used in various countries. Although the asymmetric consent procedures for the experimental versus control arm subjects can initially raise ethical concerns, it is ethically superior to previous post-randomization consent designs and can have important advantages over traditional trial designs.
There is increasing recognition of the value of pragmatic clinical trials, especially as it relates to the vision of a learning healthcare system that aims to closely integrate the delivery of medical services with clinical research. In such a system, the generation of knowledge would be “embedded into the core of the practice of medicine” leading to “continual improvement in care.” 1 The advent of a modern electronic health record system makes it feasible and relatively inexpensive to conduct studies in the context of routine clinical practice. 2 Such a vision provides an opportunity to think creatively about novel trial designs that can fulfill this pragmatic imperative.
In this article, we describe an emerging pragmatic trial paradigm called “Trials within Cohorts” (TwiCs) which involves longitudinal cohort studies that provide a platform for randomized clinical trials. To date, studies using the design have obtained research ethics committee approval in 10 countries (Australia, Canada, Finland, France, Germany, Mexico, the Netherlands, Spain, United Kingdom and the United States) with the most growth in the United Kingdom, 3 – 7 Canada, 8 – 11 and the Netherlands. 12 The rare disease SPIN (Scleroderma Patient-centered Intervention Network) cohort has obtained institutional review board (IRB) approval to recruit to its cohort and conduct four intervention trials using the design in the United States, Canada, Mexico, France, and Spain. 10
We first describe the features of TwiCs, and their strengths and limitations as a pragmatic randomized controlled trial (RCT) design. Because the TwiCs design is novel and unfamiliar to most research ethics committees/IRBs—and also because it involves an element of post-randomization consent which has a history of controversy 13 —we largely focus on the ethical issues in conducting TwiCs. We place TwiCs within a brief history of RCTs that obtain informed consent after randomization and then provide an ethical analysis of TwiCs, including a discussion of how it might be regulated by US IRBs.
Trials within Cohorts
RCTs remain the gold standard to prove effectiveness of interventions and this is no less true when the goal is to show the real-world effectiveness of the intervention in learning healthcare systems. However, the standard approach to RCTs is often complicated by slow recruitment rates, limited generalisability, limited long-term follow up, and high costs. The “Trials within Cohorts” design (formerly referred to as the “cohort multiple RCT design” 14 , 15 ) was created to address these problems when unmasked studies are used to compare an intervention of interest with a usual care control arm.
In the TwiCs design, a cohort of participants with the condition(s) of interest is recruited for a longitudinal cohort study. At the time of recruitment into the cohort, the participants are given information about the process for their potential involvement in future intervention studies (i.e. TwiCs) and consent is obtained for potential future use of their data. A critical point, and one which varies from TwiCs to TwiCs, is whether this discussion also includes an explicit consent to be randomly assigned to control or intervention in unspecified future trials. Some implementations of TwiCs have given no specific information about future clinical trials (only general information about future use of their data in other studies) to the cohort participants, 4 , 16 while others obtain consent (at initial recruitment into the cohort) regarding future randomization prior to TwiCs and use of data in future TwiCs 12 , 17 as described further below.
After randomization to any given trial within the cohort, additional consent to receive the intervention is obtained from participants who have been randomly assigned to the new intervention. Those assigned to the treatment as usual control arm do not provide any additional consent after randomization.
The TwiCs design has several advantages over standard RCT design. First, difficulty with recruitment is a common concern in RCTs. The TwiCs design takes advantage of the fact that recruitment into observational cohort studies is often easier and less selective. Once a cohort is established, controls for multiple future clinical trials are available without further recruitment efforts. There is now preliminary evidence that recruitment for RCTs within such established cohorts can be highly efficient when compared to recruitment without such cohorts. 18
Second, disclosure of information and informed consent can be tailored to the needs of the participants (e.g. those not offered the new intervention are not burdened with information about the risks and potential benefits of trial intervention). Thus, the informed consent process is “patient centered” and “real world” in its goals—replicating, as much as is ethically feasible, the real-world routine healthcare where clinicians provide patients with the information they need, at the time they need it. This may in fact increase the autonomous decision-making by patient-subjects by reducing some of the widely discussed challenges in the consent process, such as decisional burden, confusion, and information overload. 19
Third, the design reduces some problems related to patient preferences in standard RCT designs. For instance, when a condition does not have highly effective interventions, the prospect of trying a new, if unproven, intervention is often an incentive for patients to enrol. In standard RCT designs, this often results in those randomized to the “treatment as usual” arm dropping out or experiencing disappointment. But this does not occur in the TwiCs design.
Another advantage of embedding RCTs within an established cohort is that periodic research data collection that is part of the longitudinal study can provide outcome data in addition to data from medical records. 10
The TwiCs design does have limitations. It is only applicable to unmasked studies and requires (at least) one “usual care” control arm; however, it is not unusual for pragmatic studies emulating “real world” conditions to have unmasked, usual care control designs. TwiCs will also share the limitations of unmasked studies in general regarding potentially biased outcome reporting.
Another limitation of TwiCs design is the potential bias introduced by non-compliance in the intervention arm (i.e. patients who decline to enroll as well as those who enrol but do not adhere). This involves two issues. First, in the traditional approach to informed consent only those willing to try either arm are recruited. This approach will usually result in fewer dropouts in the intervention arm than in the TwiCs approach. However, this is because traditional designs will have excluded the “unwilling to enroll” at an earlier stage, and the actual number of persons complying with the intervention may be similar in TwiCs. Also, the traditional design is less pragmatic (less generalizable) because only those willing to enter the RCT are enrolled in either arm. Furthermore, in the TwiCs design, added information on the acceptability and adherence rates of new treatments in the real world is provided by the behaviour of those in the intervention arm.
Second, to reduce bias due to non-compliance in the intervention arm, TwiCs studies are typically analyzed as intention-to-treat. But if the dropouts in the intervention arm in TwiCs design are greater than in a traditional design RCT, there could be relative disadvantage in terms of loss of power. One mitigating factor is that because the dropouts in the control arm will be very rare in a TwiCs design, a TwiCs design has “room for more non-compliance” in the intervention arm in comparison with an RCT where non-compliance is expected in both arms. 20 This relative power advantage in TwiCs may not apply, however, if the non-compliance in the intervention arm is very high. 20
A brief background on RCT designs with consent following randomization
The TwiCs design is a descendent of a family of older proposals variously known as “Zelen design,” “randomized consent,” or “pre-randomization” designs. 21 A brief history of these proposals and their implementation illustrates some of their strengths and weaknesses and also helps to clarify how TwiCs is different from these earlier proposals.
The original proposal for post-randomization con-sent, called a “Zelen single-consent design,” (named after the biostatistician who proposed it) was the simplest: patients were, without prior consent or knowledge, randomized between “best standard” care (usual care) and an intervention. 21 Subjects assigned to the intervention were then asked for consent, while the others served as control subjects without their knowledge (thus the label “single-consent”). Several advantages were proposed for the single-consent design. 21 It reduces the need for investigators to present, and patients to confront, stressful aspects of research participation, such as knowing that their treatment is going to be randomly chosen and being denied access to an experimental treatment. Furthermore, single-consent designs might increase the efficiency of accrual, in part because patients (assigned to the intervention arm) might be more inclined to enroll knowing that they were guaranteed to receive the intervention.
Zelen 22 seems to have interpreted the US Federal research regulations to say that as long as research subjects received only “established and accepted methods necessary to meet [their] needs,” informed consent was not necessary. However, the Office of Protection from Research Risks (OPRR) eventually disagreed and reprimanded the investigators of a study of neonates which used a Zelen single-consent design for failing to obtain consent from parents of the control group neonates. 23
Criticism of the single-consent procedure led to greater interest in the “double-consent Zelen design,” in which both the usual care and intervention arms are approached for consent. In double consent, in contrast to single consent, all participants are at least informed that they are participating in research. However, in addition to the obvious difference from a traditional RCT in obtaining consent after treatment assignment, Zelen double consent may include little or no information about the other arm of the trial, or indeed about the fact of randomization. 13
Trials using Zelen double- and single-consent designs have remained relatively uncommon—as of 2006, two reviews suggest that approximately 83 unique studies employing Zelen designs had been conducted. 24 , 25 This relative unpopularity has no definitive explanation, but the experiences of investigators who have used post-randomization consent designs reveal both ethical and logistical problems.
First, Zelen designs have attracted considerable ethical criticism. 26 Even though patients assigned to the control group undergo no harm, and might actually be spared burdens related to a traditional consent process, they might still reasonably expect to know that a new intervention is being tested for their condition and that they have been randomly assigned to a group whose data are used for comparison. The perception that information is being withheld has been described as causing an “outcry” of concern about the ethics of the earliest Zelen proposals, and subsequent modifications have not fully allayed these concerns. 27 As we note below, however, despite the 1990 reprimand by the OPRR, pragmatic RCTs are beginning to be conducted in the United States with post-randomization single-consent procedures with the apparent knowledge of the Office of Human Research Protections (the successor to the OPRR). 28
Another problem with post-randomization consent is that it has not always proven to be as efficient as had been hoped. Analysis of a post-randomization study has to be done as intention-to-treat, including patients who declined the intervention, which reduces study power. 27 Post-randomization designs must improve accrual and withdrawal rates sufficiently to make up for this loss of power; these improvements are difficult to predict and are not guaranteed. 22 , 29 , 30
Ethics of informed consent for TwiCs and regulatory implications
There are variations in practice when it comes to the content of the initial consent procedures regarding future embedded RCTs within the cohort. We first describe a consent procedure 31 which involves the greatest amount of disclosure regarding the elements of potential trials within a cohort. We then discuss other variations.
TwiCs with pre-randomization broad consent about TwiCs elements
In some jurisdictions, investigators implementing TwiCs have run into regulatory obstacles; this has led to the development of a consent model that includes explicit consent for some elements of future embedded trials. 31 At the time of recruitment into the cohort, subjects provide specific consent for the cohort study and also provide broad consent—”broad” since the consent covers a range of unspecified future studies—that specifically includes information about randomizations for future TwiCs, for future contact if randomized to the intervention arm of TwiCs, and for use of their data in future TwiCs if randomized to the control arm (see Figure 1 ).

Comparison of a more detailed (left column) versus a general (right column) pre-randomization broad consent for Trials within Cohorts.
For those whose data will serve as the control arm data, their participation in an embedded trial is exhausted by two elements: (a) being randomly selected as a control and (b) use of their data in the embedded trial (usually collected from clinical medical records, or in some cases, from measurements that are part of the longitudinal cohort study 10 ). Despite these elements, the entirety of their clinical experience will be decided by what their physicians consider to be the best care for them. When someone is randomized to the usual care arm, they have been randomized, not to a specific treatment, but to the ordinary interactions and decision-making processes with his or her doctor; thus, how his or her care is determined and provided is not disturbed at all. Furthermore, no additional research measures are needed; thus, no additional interactions or interventions of research are involved when these persons’ data are used in TwiCs. In sum, persons in the control arm receive care that is decided by usual clinical considerations and will have given consent to every element of their research participation.
The lack of specificity in broad consent (i.e. broad permission for future use without specific consent for each use) has led to some prominent controversies in other domains of research, such as the much publicized Havasupai case in which the controversy centered around researchers’ use of samples and data that went beyond the disease domains of initial focus of the research. 32 The difference in the TwiCs context is that unlike in most biobank-based research, the cohorts are disease-based (or at risk of it) as are the trials within them; thus, given the specificity of the domain of research, there is little risk of violating any subject’s non-welfare interests such as their cultural, religious, and moral commitments. 33 For instance, for a person in a diabetes cohort who provides broad consent for use of their electronic health record and other data for evaluation of future diabetes treatments, there is little danger of patients’ non-welfare interests (regarding the type of uses to which their data are put) being compromised. (However, it should be noted that if a cohort of interest were a very general one—for example, one encompassing all patients in an integrated health delivery system—conducting TwiCs in such a cohort would require further ethical analysis regarding the content of the initial broad consent.)
Those randomized to the intervention arms of TwiCs will have given consent to be approached for enrolment in such trials. After being told that they have been randomly selected to an embedded clinical trial, they will then provide informed consent for the trial intervention. They would not be enrolled in an embedded trial unless they explicitly give consent after they are provided all the usually required elements of informed consent for an RCT. Thus, everyone who enrolls in the intervention arm of the TwiCs will also have given informed consent to every aspect of their research participation in the TwiCs.
TwiCs with only general pre-randomization discussion of future research
Some TwiCs do not obtain explicit pre-randomization consent covering the possibility of future randomization and future contact for intervention studies. Consent is still obtained, but for unspecified future uses of their data (as part of the initial consent for enrolling in the cohort). 4 , 10 , 16 The rationale is as follows. For the inter-vention arm group of a future embedded trial, when they are randomized into the intervention arm and then subsequently contacted to be asked if they wish to enroll in the trial, it is not that different from someone in a clinic being approached to participate in a traditional RCT. There is no “cold contact” involved; the subjects are aware that the clinic is a locus of clinical research, and they should not be surprised that they are being asked to consider participation in an RCT.
For the control arm, it might be argued that by enrolling in the cohort study (on, say, diabetes), their permission to the researchers to use their medical records and other data includes a variety of future research uses, and this is sufficient to permit their use for comparison purposes in a TwiC testing an intervention to treat diabetes.
There are two potential objections to not employing pre-randomization consent that explicitly includes relevant elements about future embedded trials. First, some may argue that randomization is a research procedure that always requires consent prior to the act of randomization. A contrary point of view would be that, if intervention recipients are being selected to be approached at random from a pool of patients receiving usual care, those not selected do not need to give consent for that random selection any more than individuals who are not selected in a random-digit-dial telephone survey need to give prior consent for randomization. Of course, research studies where the randomization leads to any potential alterations in the way the subjects are treated (e.g. using an experimental intervention) always require consent before such alterations are implemented. But in the control arm of the TwiCs study in question, there are no deviations from the usual way the subjects are treated and in the intervention arm informed consent is obtained before any deviations from the usual are implemented.
Second, it is plausible that some persons, enrolled in a cohort, who later find out that there are embedded randomized trials in that cohort may feel that the researchers could easily have made their plans for embedding trials in the cohort clearer from the beginning. Some of these participants may feel that the researchers were not as transparent as they could have been, even while recognizing that the lack of transparency has no impact on their welfare (benefits and harms/burdens).
Different people will have different moral intuitions about whether pre-randomization broad consent that specifically mentions elements about future embedded trials is ethically necessary. On one side of the argument, there is a potential for mistrust due to the lack of transparency such that it may be not only ethically right but prudent to obtain an explicit consent to future embedded trials and randomization, especially if the burden of obtaining it is low. On the other side is the view that consent is not only unnecessary but could cause confusion (since the idea of broad consent to future randomization with asymmetric consequences for the participants could be a challenging set of concepts to digest), then it may be better to avoid it. We suspect that a part of the answer will rest on the particular features of the TwiCs—the nature of the cohort, the interventions involved, and the setting in which the study is done and the reasonable expectations that researchers might anticipate in the participants.
Implications for US regulations
Although the use of TwiCs is gaining momentum, most of the activity has been in countries outside the United States. Given the potential advantages of the TwiCs design, it may prove useful for US researchers as well. However, the regulations do differ among jurisdictions, especially regarding the issue of when it is permissible to deviate from the traditional informed consent procedures.
How might IRBs apply the US research regulations to TwiCs? The task for the IRBs will be different depending on whether cohort studies employ pre-randomization broad consent for future embedded randomized trials in that cohort. We begin with the assumption that pre-randomization broad consent including explicit discussion of randomization is used.
First, unlike recent debates in the United States, in which the focus has been on whether traditional informed consent is necessary for pragmatic trials in learning health systems, 34 , 35 TwiCs does not need to rely on waivers or alterations of informed consent. As noted above, the intervention arm participants, before consenting to the intervention in the TwiC, would have received all of the information that persons enrolling in traditional RCTs would receive. The only difference is that the information is given (and consent for the intervention obtained) after randomization while consent for the randomization would have been given separately at the time of enrolment into the cohort.
The control arm participants’ consent would not be waived or altered either. They would have provided informed consent for the cohort study, and also given broad consent for randomization and for the use of their data for TwiCs. Since those in the control group will have given consent to every element of their research participation, there is no need to invoke the criteria for waiver or alteration of consent in the Common Rule.
What about TwiCs that are proposed without a substantive pre-randomization broad consent? The regulatory situation could involve the IRBs requiring the investigator to show that the waiver or alteration criteria in the US regulations are met. As we saw above, the intuition concerning the need for pre-randomization broad consent that specifies the elements of future embedded trials varies, and will likely vary among IRBs. It is possible that some IRBs will see the lack of transparency regarding randomization and future TwiCs as implying at least an alteration of informed consent, and therefore will require that such a proposal meet the several regulatory criteria for waiver or alteration of informed consent in 45CFR46.116: (a) the research must be minimal risk, (b) the research would be impracticable to conduct without the waiver or alteration, (c) the participants’ rights or welfare would not be adversely affected by the waiver or alteration, and (d) whenever appropriate, participants are provided with additional pertinent information after participation.
How might these criteria apply to studies that forgo substantive pre-randomization broad consent? Although some TwiCs will be minimal risk, many will not be minimal risk; whether an IRB would or should analyze the risk-benefit issue separately for the intervention and the control arms is not clear. In terms of the practicability of research criterion, it would be difficult to argue that the trial is impracticable without an alteration or waiver since there are examples of TwiCs that are being successfully conducted with substantive pre-randomization broad consent. And we have already noted that some people may see the lack of transparency about randomization into TwiCs as something that goes against their legitimate expectations—this could be interpreted by some as at odds with the condition that waiver or alteration not adversely affect subjects’ rights and welfare. 36 Finally, an IRB would need to determine whether debriefing after the embedded trial would be necessary for those assigned to the control arm. Thus, some IRBs could require the use of substantive pre-randomization broad consent for TwiCs.
It is, however, difficult to predict how this issue would finally be decided by the regulators. Of particular interest is a pragmatic clinical trial in the United States involving approximately 20,000 subjects comparing care management, skills training, and treatment as usual for the prevention of suicide attempts among out-patients who endorse suicidal thoughts on a routine clinical measure. 28 According to the investigators, this study uses a modified Zelen design (control arm patients are unaware of the RCT; subjects in the intervention arms provide clinical consent to the interventions) that has been approved by IRBs of multiple institutions, and the investigators report having held “extensive discussions” with the Office of Human Research Protections. Thus, it appears that the study is deemed to pose no more than minimal incremental risk and also that it would have been impracticable to conduct without the waiver and alteration of consent, despite what amounts to a single-consent Zelen design. However, the authors do not provide further details about how their IRBs made these determinations.
For conditions in which longitudinal cohort studies can be valuable (which likely includes most chronic conditions), recruiting and conducting multiple randomized trials within such cohorts provide significant scientific and ethical advantages over both traditional and stand-alone Zelen designs. With the increasing emphasis on pragmatic trials, 15 , 37 investigators from many countries are now using this design. One of the main obstacles to its use is the concern over the ethics of obtaining informed consent for the embedded trials after randomizing the subjects and only from the intervention arm. Pre-randomization consent to cohort participation as well as, in some cases, to more explicit broad consent to elements of future TwiCs (including for randomization, and use of data specifically for TwiCs) mitigates this ethical concern. However, regulatory policies vary among jurisdictions and interpretations of those policies vary among research ethics committees. Investigators who hope to benefit from the scientific and practical advantages of the TwiCs design will need to clearly articulate its ethical and scientific strengths and limitations.
Acknowledgments
The authors thank Frank Miller for helpful comments on an earlier draft. The opinions expressed in this article are the authors’ and do not represent the views or policies of the National Institutes of Health, Department of Health and Human Services, or the US government.
This research was supported in part by the Intramural Research Program at the National Institutes of Health (S.Y.H.K.). C.R. was funded and supported by the Wellcome Trust and the NIHR Collaboration for Leadership in Applied Health Research and Care Yorkshire and Humber (NIHR CLAHRC YH; www.clahrc-yh.nihr.ac.uk ) and her views expressed in this paper are not necessarily those of the NHS, the NIHR, or the Department of Health.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
- Systematic Review
- Open access
- Published: 13 May 2024
Sarcopenia and sarcopenic obesity among older adults in the nordic countries: a scoping review
- Fereshteh Baygi 1 na1 ,
- Sussi Friis Buhl 1 na1 ,
- Trine Thilsing 1 ,
- Jens Søndergaard 1 &
- Jesper Bo Nielsen 1
BMC Geriatrics volume 24 , Article number: 421 ( 2024 ) Cite this article
325 Accesses
Metrics details
Sarcopenia and sarcopenic obesity (SO) are age-related syndromes that may compromise physical and mental health among older adults. The Nordic countries differ from other regions on prevalence of disease, life-style behavior, and life expectancy, which may impact prevalence of sarcopenia and SO. Therefore, the aim of this study is to review the available evidence and gaps within this field in the Nordic countries.
PubMed, Embase, and Web of science (WOS) were searched up to February 2023. In addition, grey literature and reference lists of included studies were searched. Two independent researcher assessed papers and extracted data.
Thirty-three studies out of 6,363 searched studies were included in this scoping review. Overall prevalence of sarcopenia varied from 0.9 to 58.5%. A wide prevalence range was still present for community-dwelling older adults when definition criteria and setting were considered. The prevalence of SO ranged from 4 to 11%, according to the only study on this field. Based on the included studies, potential risk factors for sarcopenia include malnutrition, low physical activity, specific diseases (e.g., diabetes), inflammation, polypharmacy, and aging, whereas increased levels of physical activity and improved dietary intake may reduce the risk of sarcopenia. The few available interventions for sarcopenia were mainly focused on resistance training with/without nutritional supplements (e.g., protein, vitamin D).
The findings of our study revealed inadequate research on SO but an increasing trend in the number of studies on sarcopenia. However, most of the included studies had descriptive cross-sectional design, small sample size, and applied different diagnostic criteria. Therefore, larger well-designed cohort studies that adhere to uniform recent guidelines are required to capture a full picture of these two age-related medical conditions in Nordic countries, and plan for prevention/treatment accordingly.
Peer Review reports
The number of older adults with age-related disorders is expected to increase worldwide [ 1 , 2 ]. Sarcopenia and sarcopenic obesity (SO) are both age-related syndromes that may compromise the physical and mental health of older adults and increase their need for health care services in old age [ 3 , 4 ], and this may challenge the sustainability of health care systems economically and by shortage of health care personnel [ 5 ].
Sarcopenia is characterized by low muscle mass in combination with low muscle strength [ 4 ]. SO is characterized by the co-existence of obesity (excessive adipose tissue) and sarcopenia [ 3 ]. Sarcopenia and SO are both associated with physical disability, risk of falls, morbidity, reduced quality of life and early mortality [ 4 , 6 , 7 , 8 , 9 ]. In SO the consequences of sarcopenia and obesity are combined and maximized [ 4 , 6 , 7 , 8 ].
Etiology of sarcopenia and SO is multifactorial and closely linked to multimorbidity [ 3 , 7 , 8 , 9 , 10 ]. Nevertheless, lifestyle and behavioral components particularly diet and physical activity, are important interrelated factors that potentially can be modified. Physical inactivity and sedentary behavior may accelerate age-related loss of muscle mass, reduce energy expenditure, and increase risk of obesity [ 3 , 11 ]. In addition, weight cycling (the fluctuations in weight following dieting and regain) and an unbalanced diet (particularly inadequate protein intake) may accelerate loss of muscle mass and increase severity of sarcopenia and SO in older adults [ 3 , 12 ]. International guideline for the treatment of sarcopenia emphasizes the importance of resistance training potentially in combination with nutritional supplementation to improve muscle mass and physical function [ 13 ]. Similar therapeutic approach is suggested for treatment of SO [ 14 ]. However, more research is needed to confirm optimal treatment of SO [ 14 ].
According to a recently published meta-analysis the global prevalence of sarcopenia ranged from 10 to 27% in populations of older adults ≥ 60 years [ 15 ]. Further the global prevalence of SO among older adults was 11% [ 8 ]. So, sarcopenia and SO are prevalent conditions, with multiple negative health outcomes and should be given special attention [ 16 ]. Despite the large burden on patients and health care systems, the awareness of the importance of skeletal muscle maintenance in obesity is low among clinicians and scientists [ 3 , 16 ].
A recent meta-analysis on publication trends revealed that despite an increase in global research on sarcopenia, the Nordic countries were only limitedly represented [ 6 ]. Nordic countries may differ from other regions on aspects associated with the prevalence and trajectory of sarcopenia and SO and challenge the representativeness of research findings from other parts of the world. These include a different prevalence pattern of noncommunicable diseases [ 17 ], different life-style behavior and life-style associated risk factors [ 15 , 18 ], and higher life expectancy [ 18 ].
The Nordic countries including Sweden, Finland, Iceland, Norway, Denmark, and three autonomous areas (Åland Islands, Greenland and Faroe Islands) share common elements of social and economic policies such as a comprehensive publicly financed health care system [ 18 , 19 ]. Additionally, these countries have a strong tradition of collaboration including a common vision of a socially sustainable region by promoting equal health and inclusive participation in society for older adults [ 20 ]. Therefore, more insight into the etiology, prevalence, and risk factors for sarcopenia and SO among older adults is a prerequisite for the development and implementation of effective strategies to prevent and treat these complex geriatric conditions in this geographic region. So, the aim of this study is to conduct a scoping review to systematically identify and map the available evidence while also addressing knowledge gaps and exploring the following research questions: (1) What are the prevalence of sarcopenia and SO in older adults living in the Nordic countries? (2) Which risk factors or contributing conditions are involved in the development of sarcopenia and SO in the Nordic Countries? (3) Which interventions to prevent or counteract negative health outcomes of sarcopenia and SO have been tested or implemented among older adults living in the Nordic countries?
Identification of relevant studies
The development and reporting of this review were done by following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [ 21 ].
The literature search was developed to target three main areas: Sarcopenia, sarcopenic obesity, and aging (See Appendix 1 for full search strategy). All studies published before the end of February 2023 were included in this scoping review. The optimal sensitivity of search was obtained by simultaneous search of the following databases: PubMed, Embase, and Web of science (WOS). Additionally, a detailed search for grey literature was performed in relevant databases (e.g., Research Portal Denmark, Libris, Oria, Research.fi). Besides, reference lists of the included studies were reviewed to identify eligible studies. Duplicates and non-peer reviewed evidence (e.g., PhD thesis) were excluded but if the latter contained published articles of relevance, these were included. If more than one publication on similar outcomes (e.g., prevalence) were based on a single study, just one publication was included. Data were extracted from large studies with combined data from several countries only when findings were presented separately for the Nordic countries.
Inclusion and exclusion criteria
The inclusion criteria were as follow : Broad selection criteria were used to be comprehensive: (1) studies with any outcome (e.g., prevalence, risk factors, etc.) to address our research questions on sarcopenia and SO, (2) studies on subjects with age ≥ 60 years in any type of settings (e.g., community, nursing homes, general practice, hospital, outpatients, homecare, etc.), (3) studies using any definition of sarcopenia and SO without restriction for criteria and cutoff values, (4) all type of study designs (e.g., randomized control trials, cohort studies, cross-sectional, etc.), (5) studies should be conducted in the Nordic countries The exclusion criteria are as follow : (1) studies without relevant outcome to sarcopenia or SO, (2) studies without sufficient information to determine eligibility.
Study selection and data extraction
Two independent researchers screened literature and conducted data extraction. Any discrepancies between them were resolved through discussion.
First, duplicates were removed by using EndNote 20.6 software, then titles and abstracts were screened to narrow down the list of potentially eligible studies. Finally, the full text review was done to examine in detail the studies that were not excluded in first step. For more clarification, the reasons for the exclusion were recorded (Fig. 1 ).
PRISMA diagram for searching resources
The following information was extracted: (1) study characteristics (e.g., first author’s name, country, year of publication), (2) characteristics of the target population (e.g., age, sex), (3) study design, setting, intervention duration and follow-up time (if applicable), measurements, tools, criteria, and results.
Study selection
A combined total of 6,358 studies were identified through the initial electronic database and grey literature searches. An additional five articles were identified through other sources (citation searching). After removing duplication, 3,464 articles remained. A total of 3107 articles were excluded based on screening titles and abstracts. Out of the remaining 357 studies, 324 were excluded after the full-text review. Finally, 33 studies met our inclusion criteria and were included in this current scoping review [ 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 ] (Fig. 1 ).
Study characteristics
Table 1 summarized characteristics of the included studies.
The number of documents showed an increasing trend between 2020 and 2021. A peak in the number of publications was observed in 2021 (24.2% of all documents). All the studies were conducted across four (Denmark, Norway, Sweden, and Finland) out of the five Nordic countries and three autonomous areas. The highest contribution in this field was made by Sweden ( n = 12).
Most studies were conducted in community-dwelling settings [ 22 , 23 , 24 , 28 , 30 , 31 , 35 , 36 , 38 , 39 , 40 , 42 , 45 , 46 , 47 , 48 , 49 , 54 ]. Seven studies included patients with acute diseases (hospital-setting) [ 26 , 27 , 33 , 37 , 50 , 51 , 52 ], while four studies included patients with chronic conditions (out-patient setting) [ 25 , 32 , 41 , 44 ], and one study including nursing-home residents [ 34 ]. In terms of study design, most of the studies were observation studies with a cross-sectional or longitudinal design ( 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 33 , 34 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 ), while three studies [ 32 , 35 , 46 ] applied interventions. It appears, however, that one study [ 32 ] out of the above three interventions is sub-project conducted within the framework of larger intervention program. Sample size ranged from 49 in a cross-sectional case control study [ 52 ] to 3334 in a cohort study [ 30 ].
Five studies were among males only [ 22 , 24 , 36 , 45 , 53 ] and three studies included females only [ 38 , 47 , 54 ]. The rest of the studies had a mixed sample. Top subject area was sarcopenia (31 out of the 33 included studies), and on this subject, publications were categorized into the following research areas (with some studies addressing more areas): prevalence [ 22 , 23 , 24 , 25 , 26 , 27 , 29 , 30 , 33 , 35 , 36 , 37 , 40 , 42 , 44 , 45 , 47 , 49 , 50 , 51 , 52 , 53 , 54 ], risk factors [ 24 , 27 , 28 , 30 , 31 , 34 , 38 , 40 , 42 , 44 , 47 , 49 , 50 , 51 ], and effectiveness of interventions on sarcopenia or indicator of sarcopenia [ 32 , 35 , 46 ].
In most studies sarcopenia was defined according to the criteria set by the European Working Group on Sarcopenia in Older People in the updated version from 2019 (EWGSOP2) ( n = 15) or the original version from 2010 (EWGSOP) ( n = 14). However, in some studies multiple criteria such as EWGSOP, EWGSOP2, and National Institutes of Health Sarcopenia Project definition (FNIH) were applied [ 27 , 39 , 43 ], and in other studies alternative criteria were used [ 26 , 33 , 35 , 45 , 57 ].
Different assessment methods of muscle mass including Dual energy X-ray absorptiometry (DXA) [ 22 , 24 , 25 , 27 , 29 , 30 , 32 , 33 , 38 , 39 , 40 , 41 , 45 , 46 , 47 , 52 , 53 , 54 ], Bioelectrical Impedance Analysis (BIA) [ 28 , 31 , 34 , 44 , 48 , 49 ], Bioimpedance Spectroscopy (BIS) [ 35 , 42 , 43 ], Computed Tomography (CT) [ 33 ], and Computed Tomography Angiogram (CTA) [ 26 ] were used in the included studies.
SO were defined by the co-existence of sarcopenia with obesity. Studies on SO used the EWGSOP2 criteria [ 39 ], or the EWGSOP2 criteria for hand grip strength only (probable sarcopenia) [ 23 ] in combination with obesity estimated from BMI cut points [ 23 , 39 ], waist circumference [ 23 , 39 ], and fat mass percentage [ 39 ]. Lastly, one study used measures of body composition measures that reflect adiposity as estimates of SO [ 48 ].
Four studies reported the prevalence of “probable sarcopenia” [ 23 , 30 , 36 , 45 ], while two studies reported the prevalence of sarcopenia and comorbidities (e.g., osteopenia, pre-frailty, malnutrition) [ 33 , 40 ].
Narrative synthesis
Due to the heterogeneity of the studies in definition of sarcopenia, settings, and sample size, the overall reported prevalence was variable and ranged from 0.9% [ 54 ] to 58.5% [ 26 ]. However, according to the most commonly used criteria (EWGSOP2) the highest (46%) and lowest (1%) prevalence of sarcopenia was reported in Sweden among inpatients in geriatric care [ 27 ], and community-dwelling older adults [ 30 ], respectively.
Prevalence of sarcopenia according to population and definition criteria is illustrated in Table 2 . Higher prevalence rates of sarcopenia were found in females compared to males among community-dwelling older adults [ 49 ] and in older adults acutely admitted to hospital [ 51 ]. Further, acutely admitted female patients also presented with more severe sarcopenia compared to male patients [ 51 ].
Frequency of sarcopenia was higher (9.1–40.0%) in patients with diabetes (with and without complications of charcot osteoarthropathy), compared to age-matched healthy adults [ 52 ].
The prevalence of “probable sarcopenia” ranged between 20.4% (reduced muscle strength only) and 38.1% (fulfilling one of the following criteria: reduced muscle strength, reduced muscle mass, or low physical function) in Finnish community-dwelling adults [ 23 , 36 ], while longitudinal studies on Swedish community-dwelling old (70 years) and very old adults (≥ 85 years) the prevalence of “probable sarcopenia” (reduced muscle strength only) ranged from 1.8 to 73%, respectively [ 30 , 45 ]. Lastly, in a Swedish study among nursing home residents the prevalence of probable sarcopenia was 44% (evaluated by an impaired chair stand test) [ 34 ].
Prevalence of Osteosarcopenia (sarcopenia and osteoporosis) was 1.5% [ 36 ], and the prevalence of co-occurrence of all three following conditions: pre-frail, malnutrition, and sarcopenia was 7% [ 34 ].
We only identified two studies with prevalence of SO [ 39 ] and probable SO [ 23 ]. The prevalence of SO in a Swedish population was 4% and 11% in females and males, respectively, while the prevalence of probable SO among Finnish community-dwelling ranged between 5.8% and 12.6%, depending on the criteria to define the obesity (e.g., BMI, waist circumference, etc.) [ 23 ].
Several studies investigated aspects of etiology and risk factors for sarcopenia [ 24 , 27 , 28 , 30 , 31 , 34 , 36 , 38 , 40 , 42 , 43 , 44 , 47 , 49 , 50 , 51 ] and one study focused on SO [ 49 ]. Higher physical activity was associated with a decreased likelihood of sarcopenia [ 30 ]. In addition, adhering to world health organization (WHO) guidlines for physical activity and the Nordic nutritional recommendations for protein intake was positively associated with greater physical function and lower fat mass in older female community-dwellers [ 38 ]. In older adults who are physically active, eating a healthy diet (based on the frequency of intake of favorable food like fish, fruits, vegetables, and whole grains versus unfavorable foods like red/processed meats, desserts/sweets/sugar-sweetened beverages, and fried potatoes) was associated with lower risk of sarcopenia [ 28 ]. Further, among older adults who already meet the physical activity guidelines, additional engagement in muscle-strengthening activities was associated with a lower sarcopenia risk score and improved muscle mass and chair rise time [ 31 ].
Associations between sarcopenia, risk of sarcopenia and malnutrition or nutritional status was identified in geriatric patients [ 27 , 51 ], older patients with hip fracture [ 50 ], nursing home residents [ 34 ] and in community-dwelling older adults [ 49 ]. Moreover, the importance of nutritional intake was investigated in the following studies [ 24 , 36 , 47 ]. A study among community-dwelling men revealed an inverse association between total energy intake, protein intake (total, plant, and fish protein), intake of dietary fibers, fat (total and unsaturated), and vitamin D with sarcopenia status [ 36 ]. In a cohort of 71-year-old men a dietary pattern characterized by high consumption of fruit, vegetables, poultry, rice and pasta was associated with lower prevalence of sarcopenia after 16 years [ 24 ]. A longitudinal Finnish study on sarcopenia indices among postmenopausal older women, showed that lower adherence to the Mediterranean (focuses on high consumption of olive oil) or Baltic Sea (focuses on the dietary fat quality and low-fat milk intake) diets resulted in higher loss of lean mass over a 3-year period [ 47 ]. Further, a higher adherence to the Baltic Sea diet was associated with greater lean mass and better physical function, and higher adherence to the Mediterranean diet was associated with greater muscle quality [ 47 ].
In a study of patients with hip fracture age, polypharmacy, and low albumin levels was associated with sarcopenia [ 50 ]. Exocrine pancreatic insufficiency was an independent risk factor for sarcopenia [ 44 ]. This study also revealed that sarcopenia was associated with reduced quality of life, physical function, and increased risk of hospitalization [ 44 ]. In a longitudinal study of community-dwelling adults (+ 75 years) at risk of sarcopenia, high physical function, muscle strength, muscle mass and low BMI predicted better physical function and reduced need for care after four years [ 42 ]. Furthermore, in community-dwelling adults with sarcopenia, muscle mass, muscle strength and physical function are independent predictors of all-cause mortality. As a result, they have been proposed by researchers as targets for the prevention of sarcopenia-related over-mortality [ 43 ]. Lastly, community-dwelling older adults with sarcopenia had lower bone mineral density compared to those without sarcopenia and they were more likely to develop osteoporosis (Osteosarcopenia) [ 40 ].
Regarding SO risk factors, a longitudinal study among community-dwelling older adults in Finland found that SO (operationalized by measures of adiposity) were associated with poorer physical function after ten years [ 48 ].
Our literature search identified three randomized controlled trials investigating the effectiveness of interventions to prevent or counteract sarcopenia in older adults of Norway, Finland, and Sweden, respectively [ 32 , 35 , 46 ]. The Norwegian study [ 32 ] was a double-blinded randomized controlled trial (RCT). The study included those who were at risk of developing sarcopenia, including patients with chronic obstructive pulmonary disease (COPD) or individuals who showed diagnostic indications of sarcopenia. Participants received either vitamin D 3 or placebo supplementation for 28 weeks. Additionally, resistance training sessions were provided to all participants from weeks 14 to 27. Vitamin D supplementation did not significantly affect response to resistance training in older adults at risk of sarcopenia with or without COPD [ 32 ].
Furthermore, a RCT among pre-sarcopenic Swedish older adults investigated the effectiveness of three weekly sessions of instructor-led progressive resistance training in combination with a non-mandatory daily nutritional supplement (175 kcal, 19 g protein) compared to control group. The 10 weeks intervention resulted in significant between group improvements of physical function and a significant improvement in body composition in the intervention group [ 46 ].
Another intervention study revealed that a 12-month intervention with two daily nutritional supplements (each containing 20 g whey protein) did not attenuate the deterioration of physical function and muscle mass in sarcopenic older community-dwelling adults compared to isocaloric placebo supplements or no supplementation. All participants were given instructions on home-based exercises, importance of dietary protein and vitamin D supplementation [ 35 ].
Based on our broad literature search 33 studies were identified that concerned sarcopenia and SO and met the inclusion criteria. However, research on SO was very limited with only three studies identified. Narrative synthesis of the included studies revealed that the most reported classification tool for sarcopenia in Nordic countries was the EWGSOP2. Moreover, some studies estimated sarcopenia using EWGSOP. The overall prevalence of sarcopenia in Nordic countries according to EWGSOP2 ranged between 1% and 46% [ 25 , 28 ]. The prevalence of SO, however, was reported only in one study in Sweden (4–11%) [ 39 ]. Even though the previous systematic reviews and meta-analysis have reported the prevalence of sarcopenia and SO in different regions and settings (e.g., community-dwelling, nursing home, etc.) [ 8 , 15 , 55 , 56 ], this current scoping review is to the best of our knowledge the first study that provides an overview of research on sarcopenia and SO in the Nordic countries.
Based on our findings from 24 studies, there were large variability in prevalence of sarcopenia in studies conducted in the Nordic countries. We think that the wide variation in estimated prevalence of sarcopenia in our scoping review might be due to a different definition/diagnostic criterion (e.g., EWGSOP, EWGSOP2, FNIH), methodology to measure muscle mass (DXA, BIA, CT), and heterogeneity in characteristics of the study population (e.g., setting, age, medical conditions, co-occurrence of multiple risk factors). A previous study on prevalence of sarcopenia in Swedish older people showed significant differences between prevalence of sarcopenia based on EWGSOP2 and EWGSOP1 [ 29 ]. Therefore, researchers stressed that prevalence is more dependent on cut-offs than on the operational definition [ 29 , 57 ]. Further, we know that various international sarcopenia working groups have issued expert consensus and such diagnostic criteria are being updated [ 4 , 58 ]. Since the revision of criteria focuses primarily on the adjustment of cut-off values, the main reason for differences in prevalence even when using an updated version of one diagnosis criteria is modification in cut-off values. For instance, if the cut-off value for gait speed was increased by 0.2 m/s, the prevalence of sarcopenia may increase by 8.5% [ 57 ]. Meaning that even a small change in cut-off value can have a big impact on how sarcopenia is diagnosed. Besides when we take definition criteria into account (Table 2 ), the prevalence of sarcopenia is still variable in the population of community-dwelling adults for instance. We believe it is basically because studies have applied different assessment tools and tests to identify older adults with low muscle mass and muscle strength, although using the same definition criteria (Table 1 ). Previous studies have illustrated that choice of methodology to assess muscle strength (e.g., hand grip strength, chair rise) [ 59 ] and muscle mass (e.g., DXA, BIA, anthropometry) [ 60 , 61 , 62 ] in older adults may impact findings and this variability may explain some of the variability in our findings. So, adherence to the latest uniform diagnostic criteria for future studies is recommended to simplify the comparison of findings within the same country, across countries, and regions. Moreover, we suggest that medical community particularly GPs to come to an agreement on assessment methods for muscle mass and muscle strength and the use of one set of definition criteria for sarcopenia.
In previous meta-analyses [ 15 ], sub-group analyses based on region and classification tool, revealed that the prevalence of sarcopenia was higher in European studies using EWGSOP (12%) compared to rest of the studies using Asian Working Group for Sarcopenia (AWGS), FNIH, and EWGSOP (3%) [ 15 ]. In our scoping review, we also found a high prevalence of sarcopenia in Nordic countries. Longevity and life expectancy is higher in the Nordic countries compared to estimates for rest of the world [ 18 ], which means that in this region many people reach old age, and consequently they are more likely to be diagnosed with sarcopenia as an age-related disorder. Therefore, the authors of this current scoping review emphasis the importance of preventive strategies targeted major risk factors and effective interventions to limit the consequences of sarcopenia in the Nordic populations. Besides, we think that the health care system in the Nordic countries should be better equipped with the necessary healthcare resources for both a timely diagnosis and dealing with this major age-related issue in the years to come. However, due to the limitations regarding the timely diagnosis, we highly recommend a comprehensive approach including establishment of support services, implement educational programs, offer training for health care professionals, and engage the community.
Many countries have conducted research on SO [ 7 , 39 , 63 , 64 , 65 ]. Based on our findings, however, among the Nordic countries only Sweden and Finland have investigated the prevalence of probable SO and SO [ 23 , 29 ]. Besides, we only found one study investigating the association between body adiposity and physical function over time [ 54 ]. We did not find any literature on risk factors or interventions among older adults with SO in this region. Therefore, we call on medical and research community in Nordic countries to attach importance to screening of SO in elderly people to capture a full picture of this public health risk to aging society and allocate healthcare resources accordingly.
In terms of risk factors for sarcopenia, our study revealed that malnutrition, low levels of physical activity, specific diseases (e.g., diabetes, osteoporosis), inflammation, polypharmacy (multiple medicines), BMI, and ageing are potential risk factor for sarcopenia in populations of the Nordic region. However, evidence on risk factors derived mainly from cross-sectional associations [ 27 , 28 , 30 , 31 , 34 , 40 , 44 , 49 , 50 , 51 ], and only to a limited extend from longitudinal studies [ 24 , 38 , 43 , 47 ]. Therefore, the associations between risk factors and sarcopenia should be interpreted with caution due to the possibility of reverse causality and confounding affecting the results. Moreover, our findings on risk factors mainly came from community-dwelling older adults, and only to a limited extend hospital and nursing home settings. We think that risk factors may vary depending on population characteristics (e.g., age, sex, health condition) and setting (e.g., hospital, nursing home, community). Therefore, we encourage researchers of the Nordic countries to perform well-designed prospective cohort studies in different settings to enhance the possibility to establish causal inference as well as understanding degree and direction of changes over time.
A recently published meta-analyses revealed a higher risk of having polypharmacy in Europe among individuals with sarcopenia compared to people without this condition [ 66 ]. A nationwide register-based study in Swedish population also showed that the prevalence of polypharmacy has increased in Sweden over the last decade [ 67 ]. Sarcopenia itself is associated with morbidity (identified by specific disease or inflammatory markers) and different health-related outcomes (e.g., disability) [ 7 ]; therefore, future research should investigate whether polypharmacy is a major factor to sarcopenia development [ 66 ]. Although we lack information on polypharmacy in Nordic countries other than Sweden, we encourage researchers in this region to examine the above research gap in their future studies.
According to previous studies physiological changes in ageing include systemic low-grade inflammation which results in insulin resistance, affect protein metabolism and leads to increased muscle wasting [ 68 ]. Acute and chronic disease may increase the inflammatory response and accelerate age-related loss of muscle mass and increase risk of sarcopenia [ 68 , 69 ]. Hence, we think that special attention should be made by health care professionals particularly GPs to older adults with acute or chronic conditions to limit the risk of sarcopenia.
Literature from the Nordic countries also indicated that higher levels of physical activity and different dietary patterns (e.g., higher protein intake, fruit, vegetables, fibers) were associated with reduced risk of sarcopenia or improvement in indicators of sarcopenia. There was a large heterogeneity in the studied aspect which makes direct comparison of studies difficult. Nevertheless, according to findings from a recent systematic review of meta-analyses on sarcopenia the identified risk factors are in alignment with previously identified risk factors globally [ 70 ]. Other potential lifestyle-related risk factors suggested from the above meta-analysis included smoking and extreme sleep duration. However, we did not identify studies investigating these health behaviors in the Nordic populations. Therefore, high-quality cohort studies are needed to deeply understand such associations with the risk of sarcopenia.
In this current review, we only found three intervention studies in Nordic countries. However, two of them were sub-projects of big intervention programs, meaning that such studies were not designed explicitly for the prevention/treatment of sarcopenia. Therefore, explicit intervention studies on sarcopenia in this region is recommended.
We believe that on a global level, research on sarcopenia will carry on with nutrition, exercise, and understanding of molecular mechanisms. Furthermore, examining the link between sarcopenia and other medical conditions/diseases would be the next step [ 6 ]. In the Nordic countries, however, already performed studies have a basic and descriptive design, so that, well-designed research and advanced analyses are lacking. Hence, we recommend conducting large well-designed and adequately powered studies to (a) explore the scale of this age-related health issue on country and regional level, (b) investigate the patterns of physical activity and sedentary behavior to understand if this should be a target in older adults with SO and sarcopenia, (c) determine whether elderly populations are suffering from nutritional deficiency or are at risk of malnutrition. The latest can support further studies to assess the impact of combined physical activity and dietary intake, which are still lacking globally [ 6 ].
A previous systematic review on therapeutic strategies for SO revealed that exercise-based interventions (e.g., resistance training) reduced total adiposity and consequently improved body composition. However, evidence of other therapeutic strategies (e.g., nutritional supplementation) was limited due to scarcity of data and lack of unique definition for SO [ 69 ]. Therefore, authors suggested that more research should be done to clarify optimal treatment options for various age-groups and not only for older adults [ 14 ].
In our scoping review, the included studies, did not provide a status of either SO or the prevention/treatment methods in this region. We believe that SO is practically neglected in clinical practice and research as well, and this is mainly because it is difficult to separate it from general obesity. The consequence of lacking knowledge in this research area is that when older adults with SO are recommended weight loss- a frequently used strategy for management of general obesity- this may accelerate the loss of muscle mass and increase the severity of the sarcopenia [ 3 ]. Consequently, we think that this issue may have adverse effects both on patients (e.g., decreasing quality of their life) and on the health care system (e.g., increasing the health care demands) of this region. Therefore, we encourage researchers to perform cohort studies to understand the epidemiology and etiological basis of SO, which are poorly understood even on a global scale [ 8 ]. We think that the consensus definition on SO from the European Society for Clinical Nutrition and Metabolism (ESPEN) and European Association for the Study of Obesity (EASO) which was published in 2022 [ 3 ], can positively affect the ability to define studies on prevalence and prevention of SO. Besides, we recommend conducting further research to find the optimal treatment for SO and reduce its adverse consequences both at individual and society levels. Additionally, we think that the concepts of sarcopenia and SO might be somehow unfamiliar to health care personnel. Therefore, it is highly recommended that more information be provided to bring their attention to the significance of prevention, timely diagnosis, and treatment of these two aging disorders.
Strengths and limitations of the study
This is the first study providing an overview of available evidence on sarcopenia and SO among older adults in the Nordic countries. These countries have important similarities in welfare sectors and on a population level and we believe that our findings will be a significant benefit for researchers and health care providers to understand the knowledge gaps and plan for future studies in this geographical region. However, the current scoping review has limitations. This review was limited to studies among individuals more than 60 years old which may limit the overview of available research in this field, as well as understanding risk factors, confounders for prevention, and the potential for early detection of these two diseases in younger age population. The included cross-sectional studies in our review cannot provide information on causality of the associations.
Sarcopenia and SO are generally prevalent syndromes among older adults in Nordic countries, even though the prevalence of them varies according to the criteria for definition, population, and setting. Research among older adults with SO was very limited in this region. Besides, studies on risk factors were primarily cross-sectional and only few intervention studies were identified. Therefore, we encourage researchers performing well-designed studies (e.g., prospective cohorts) to understand the epidemiology and etiological basis of these two age-related disorders. For the next step, implementation of interventions targeting risk factors (e.g., combined physical activity and dietary intake) and evaluating of their impact on prevention or treatment of sarcopenia and SO is recommended. Furthermore, for the comprehensive advancement of muscle health in older adults, we recommend implementing interventions directed at health care personnel and encouraging more collaboration among clinicians, professional societies, researchers, and policy makers to ensure comprehensive and effective approach to health care initiatives.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
sarcopenic obesity
Web of science
Preferred Reporting Items for Systematic Reviews and Meta-analyses
European Working Group on Sarcopenia in Older People in the updated version from 2019
National Institutes of Health Sarcopenia Project definition
Dual energy X-ray absorptiometry
Bioelectrical Impedance Analysis
Bioimpedance Spectroscopy
Computed Tomography
Computed Tomography Angiogram
World Health Organization
General Practitioner
Randomized Controlled Trial
Chronic Obstructive Pulmonary Disease
European Association for the Study of Obesity
United, Nations, Department of Economic and Social Affairs., Population Division (2019). World Population Prospects 2019: Highlights (ST/ESA/SER.A/423).
United, Nations, Department of Economic and Social Affairs., Population Division (2019). World Population Ageing 2019: Highlights (ST/ESA/SER.A/430).
Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar MD, Batsis JA, Bauer JM, Boirie Y, Cruz-Jentoft AJ, Dicker D, Frara S, Frühbeck G, Genton L, Gepner Y, Giustina A, Gonzalez MC, Han HS, Heymsfield SB, Higashiguchi T, Laviano A, Lenzi A, Nyulasi I, Parrinello E, Poggiogalle E, Prado CM, Salvador J, Rolland Y, Santini F, Serlie MJ, Shi H, Sieber CC, Siervo M, Vettor R, Villareal DT, Volkert D, Yu J, Zamboni M, Barazzoni R. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO Consensus Statement. Obes Facts. 2022;15(3):321–35. doi: 10.1159/000521241. Epub 2022 Feb 23. PMID: 35196654; PMCID: PMC9210010.
Article PubMed PubMed Central Google Scholar
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. https://doi.org/10.1093/ageing/afy169 . Erratum in: Age Ageing. 2019;48(4):601. PMID: 30312372; PMCID: PMC6322506.
Article PubMed Google Scholar
Cylus J, Figueras J, Normand C. Will population ageing spell the end of the welfare state? A review of evidence and policy options [Internet]. Sagan A, Richardson E, North J, White C, editors. Copenhagen (Denmark): European Observatory on Health Systems and Policies; 2019. PMID: 31820887.
Yuan D, Jin H, Liu Q, Zhang J, Ma B, Xiao W, Li Y. Publication trends for Sarcopenia in the World: a 20-Year bibliometric analysis. Front Med (Lausanne). 2022;9:802651. https://doi.org/10.3389/fmed.2022.802651 . PMID: 35223902; PMCID: PMC8873525.
Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, Meinow B, Fratiglioni L. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430–9. https://doi.org/10.1016/j.arr.2011.03.003 . Epub 2011 Mar 23. PMID: 21402176.
Gao Q, Mei F, Shang Y, Hu K, Chen F, Zhao L, Ma B. Global prevalence of sarcopenic obesity in older adults: a systematic review and meta-analysis. Clin Nutr. 2021;40(7):4633–41. https://doi.org/10.1016/j.clnu.2021.06.009 . Epub 2021 Jun 21. PMID: 34229269.
Molino S, Dossena M, Buonocore D, Verri M. Sarcopenic obesity: an appraisal of the current status of knowledge and management in elderly people. J Nutr Health Aging. 2016;20(7):780-8. https://doi.org/10.1007/s12603-015-0631-8 . PMID: 27499312.
Khadra D, Itani L, Tannir H, Kreidieh D, El Masri D, El Ghoch M. Association between sarcopenic obesity and higher risk of type 2 diabetes in adults: a systematic review and meta-analysis. World J Diabetes. 2019;10(5):311–23. https://doi.org/10.4239/wjd.v10.i5.311 . PMID: 31139318; PMCID: PMC6522758.
Aggio DA, Sartini C, Papacosta O, Lennon LT, Ash S, Whincup PH, Wannamethee SG, Jefferis BJ. Cross-sectional associations of objectively measured physical activity and sedentary time with Sarcopenia and sarcopenic obesity in older men. Prev Med. 2016;91:264–72. Epub 2016 Aug 26. PMID: 27575317; PMCID: PMC5061552.
Rossi AP, Rubele S, Calugi S, Caliari C, Pedelini F, Soave F, Chignola E, Vittoria Bazzani P, Mazzali G, Dalle Grave R, Zamboni M. Weight cycling as a risk factor for low muscle mass and strength in a population of males and females with obesity. Obesity (Silver Spring). 2019;27(7):1068–1075. https://doi.org/10.1002/oby.22493 . PMID: 31231958.
Dent E, Morley JE, Cruz-Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, Bauer JM, Pahor M, Clark BC, Cesari M, Ruiz J, Sieber CC, Aubertin-Leheudre M, Waters DL, Visvanathan R, Landi F, Villareal DT, Fielding R, Won CW, Theou O, Martin FC, Dong B, Woo J, Flicker L, Ferrucci L, Merchant RA, Cao L, Cederholm T, Ribeiro SML, Rodríguez-Mañas L, Anker SD, Lundy J, Gutiérrez Robledo LM, Bautmans I, Aprahamian I, Schols JMGA, Izquierdo M, Vellas B. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148–1161. https://doi.org/10.1007/s12603-018-1139-9 . PMID: 30498820.
Poggiogalle E, Parrinello E, Barazzoni R, Busetto L, Donini LM. Therapeutic strategies for sarcopenic obesity: a systematic review. Curr Opin Clin Nutr Metab Care. 2021;24(1):33–41. https://doi.org/10.1097/MCO.0000000000000714 . PMID: 33323715.
Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, Celis-Morales C. Global prevalence of Sarcopenia and severe Sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13(1):86–99. https://doi.org/10.1002/jcsm.12783 . Epub 2021 Nov 23. PMID: 34816624; PMCID: PMC8818604.
Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr. 2012;31(5):583–601. https://doi.org/10.1016/j.clnu.2012.06.010 . Epub 2012 Jul 17. PMID: 22809635.
Article CAS PubMed Google Scholar
Balaj M, Huijts T, McNamara CL, Stornes P, Bambra C, Eikemo TA. Non-communicable diseases and the social determinants of health in the nordic countries: findings from the European Social Survey (2014) special module on the social determinants of health. Scand J Public Health. 2017;45(2):90–102. Epub 2017 Jan 27. PMID: 28128015.
Nordic Burden of Disease Collaborators. Life expectancy and disease burden in the Nordic countries: results from the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet Public Health. 2019;4(12): e658-e669. doi: 10.1016/S2468-2667(19)30224-5. Epub 2019 Nov 20. PMID: 31759894; PMCID: PMC7098475.
Stockmarr A, Hejgaard T, Matthiessen J. Obesity prevention in the Nordic Countries. Curr Obes Rep. 2016;5(2):156 – 65. https://doi.org/10.1007/s13679-016-0206-y . PMID: 27033877.
Cuadrado A, Stjernberg M, Huynh D. Active and healthy ageing: heterogenous perspectives and nordic indicators. Nordens välfärdscenter/Nordic Welfare Centre; 2022.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336 – 41. https://doi.org/10.1016/j.ijsu.2010.02.007 . Epub 2010 Feb 18. Erratum in: Int J Surg. 2010;8(8):658. PMID: 20171303.
Sallfeldt ES, Mallmin H, Karlsson MK, Mellström D, Hailer NP, Ribom EL. Sarcopenia prevalence and incidence in older men - a MrOs Sweden study. Geriatr Nurs. 2023 Mar-Apr;50:102–8. https://doi.org/10.1016/j.gerinurse.2023.01.003 . Epub 2023 Feb 10. PMID: 36774676.
Sääksjärvi K, Härkänen T, Stenholm S, Schaap L, Lundqvist A, Koskinen S, Borodulin K, Visser M. Probable Sarcopenia, obesity, and risk of all-cause mortality: a pooled analysis of 4,612 participants. Gerontology. 2023;69(6):706–15. Epub 2023 Jan 30. PMID: 36716714.
Karlsson M, Becker W, Cederholm TE, Byberg L. A posteriori dietary patterns in 71-year-old Swedish men and the prevalence of Sarcopenia 16 years later. Br J Nutr Camb Univ Press. 2022;128(5):909–20. https://doi.org/10.1017/S0007114521003901 .
Article CAS Google Scholar
Dolin TG, Mikkelsen MK, Jakobsen HL, Vinther A, Zerahn B, Nielsen DL, Johansen JS, Lund CM, Suetta C. The prevalence of Sarcopenia and cachexia in older patients with localized colorectal cancer. J Geriatr Oncol. 2023;14(1):101402. Epub 2022 Nov 21. PMID: 36424269.
Paajanen P, Lindström I, Oksala N, Väärämäki S, Saari P, Mäkinen K, Kärkkäinen JM. Radiographically quantified Sarcopenia and traditional cardiovascular risk assessment in predicting long-term mortality after endovascular aortic repair. J Vasc Surg. 2022;76(4):908–e9152. Epub 2022 Mar 31. PMID: 35367563.
Sobestiansky S, Åberg AC, Cederholm T. Sarcopenia and malnutrition in relation to mortality in hospitalised patients in geriatric care - predictive validity of updated diagnoses. Clin Nutr ESPEN. 2021;45:442–8. Epub 2021 Jul 16. PMID: 34620352.
Papaioannou KG, Nilsson A, Nilsson LM, Kadi F. Healthy eating is Associated with Sarcopenia Risk in physically active older adults. Nutrients. 2021;13(8):2813. https://doi.org/10.3390/nu13082813 . PMID: 34444973; PMCID: PMC8401667.
Article CAS PubMed PubMed Central Google Scholar
Wallengren O, Bosaeus I, Frändin K, Lissner L, Falk Erhag H, Wetterberg H, Rydberg Sterner T, Rydén L, Rothenberg E, Skoog I. Comparison of the 2010 and 2019 diagnostic criteria for Sarcopenia by the European Working Group on Sarcopenia in Older people (EWGSOP) in two cohorts of Swedish older adults. BMC Geriatr. 2021;21(1):600. https://doi.org/10.1186/s12877-021-02533-y . PMID: 34702174; PMCID: PMC8547086.
Scott D, Johansson J, Gandham A, Ebeling PR, Nordstrom P, Nordstrom A. Associations of accelerometer-determined physical activity and sedentary behavior with Sarcopenia and incident falls over 12 months in community-dwelling Swedish older adults. J Sport Health Sci. 2021;10(5):577–84. Epub 2020 Feb 5. PMID: 34088651; PMCID: PMC8500807.
Veen J, Montiel-Rojas D, Nilsson A, Kadi F. Engagement in muscle-strengthening activities lowers Sarcopenia Risk in older adults already adhering to the Aerobic Physical Activity guidelines. Int J Environ Res Public Health. 2021;18(3):989. https://doi.org/10.3390/ijerph18030989 . PMID: 33499423; PMCID: PMC7908493.
Mølmen KS, Hammarström D, Pedersen K, Lian Lie AC, Steile RB, Nygaard H, Khan Y, Hamarsland H, Koll L, Hanestadhaugen M, Eriksen AL, Grindaker E, Whist JE, Buck D, Ahmad R, Strand TA, Rønnestad BR, Ellefsen S. Vitamin D3 supplementation does not enhance the effects of resistance training in older adults. J Cachexia Sarcopenia Muscle. 2021;12(3):599–628. https://doi.org/10.1002/jcsm.12688 . Epub 2021 Mar 31. PMID: 33788419; PMCID: PMC8200443.
Simonsen C, Kristensen TS, Sundberg A, Wielsøe S, Christensen J, Hansen CP, Burgdorf SK, Suetta C, de Heer P, Svendsen LB, Achiam MP, Christensen JF. Assessment of Sarcopenia in patients with upper gastrointestinal tumors: prevalence and agreement between computed tomography and dual-energy x-ray absorptiometry. Clin Nutr. 2021;40(5):2809–16. Epub 2021 Mar 26. PMID: 33933747.
Faxén-Irving G, Luiking Y, Grönstedt H, Franzén E, Seiger Å, Vikström S, Wimo A, Boström AM, Cederholm T. Do malnutrition, sarcopenia and frailty overlap in nursing-home residents? J Frailty Aging. 2021;10(1):17–21. https://doi.org/10.14283/jfa.2020.45 . PMID: 33331617.
Björkman MP, Suominen MH, Kautiainen H, Jyväkorpi SK, Finne-Soveri HU, Strandberg TE, Pitkälä KH, Tilvis RS. Effect of protein supplementation on physical performance in older people with sarcopenia-a randomized controlled trial. J Am Med Dir Assoc. 2020;21(2):226–e2321. Epub 2019 Nov 14. PMID: 31734121.
Jyväkorpi SK, Urtamo A, Kivimäki M, Strandberg TE. Macronutrient composition and sarcopenia in the oldest-old men: the Helsinki businessmen study (HBS). Clin Nutr. 2020;39(12):3839–41. https://doi.org/10.1016/j.clnu.2020.04.024 . Epub 2020 Apr 24. PMID: 32376097.
Probert N, Lööw A, Akner G, Wretenberg P, Andersson ÅG. A comparison of patients with hip fracture, ten years apart: morbidity, malnutrition and sarcopenia. J Nutr Health Aging. 2020;24(8):870–877. https://doi.org/10.1007/s12603-020-1408-2 . PMID: 33009538.
Sjöblom S, Sirola J, Rikkonen T, Erkkilä AT, Kröger H, Qazi SL, Isanejad M. Interaction of recommended levels of physical activity and protein intake is associated with greater physical function and lower fat mass in older women: Kuopio osteoporosis risk Factor- (OSTPRE) and fracture-Prevention Study. Br J Nutr. 2020;123(7):826–39. Epub 2020 Jan 8. PMID: 31910914; PMCID: PMC7054249.
von Berens Å, Obling SR, Nydahl M, Koochek A, Lissner L, Skoog I, Frändin K, Skoglund E, Rothenberg E, Cederholm T. Sarcopenic obesity and associations with mortality in older women and men - a prospective observational study. BMC Geriatr. 2020;20(1):199. https://doi.org/10.1186/s12877-020-01578-9 . PMID: 32517653; PMCID: PMC7285448.
Nielsen BR, Andersen HE, Haddock B, Hovind P, Schwarz P, Suetta C. Prevalence of muscle dysfunction concomitant with osteoporosis in a home-dwelling Danish population aged 65–93 years -the Copenhagen Sarcopenia Study. Exp Gerontol. 2020;138:110974. https://doi.org/10.1016/j.exger.2020.110974 . Epub 2020 May 25. PMID: 32464171.
Van Ancum JM, Alcazar J, Meskers CGM, Nielsen BR, Suetta C, Maier AB. Impact of using the updated EWGSOP2 definition in diagnosing Sarcopenia: a clinical perspective. Arch Gerontol Geriatr 2020 Sep-Oct;90:104125. https://doi.org/10.1016/j.archger.2020.104125 . Epub 2020 May 23. PMID: 32534364.
Björkman M, Jyväkorpi SK, Strandberg TE, Pitkälä KH, Tilvis RS. Sarcopenia indicators as predictors of functional decline and need for care among older people. J Nutr Health Aging. 2019;23(10):916–922. https://doi.org/10.1007/s12603-019-1280-0 . PMID: 31781719.
Björkman MP, Pitkala KH, Jyväkorpi S, Strandberg TE, Tilvis RS. Bioimpedance analysis and physical functioning as mortality indicators among older sarcopenic people. Exp Gerontol. 2019;122:42–6. https://doi.org/10.1016/j.exger.2019.04.012 . Epub 2019 Apr 24. PMID: 31026498.
Olesen SS, Büyükuslu A, Køhler M, Rasmussen HH, Drewes AM. Sarcopenia associates with increased hospitalization rates and reduced survival in patients with chronic pancreatitis. Pancreatology. 2019;19(2):245–51. https://doi.org/10.1016/j.pan.2019.01.006 . Epub 2019 Jan 14. PMID: 30665702.
Sobestiansky S, Michaelsson K, Cederholm T. Sarcopenia prevalence and associations with mortality and hospitalisation by various sarcopenia definitions in 85–89 year old community-dwelling men: a report from the ULSAM study. BMC Geriatr. 2019;19(1):318. https://doi.org/10.1186/s12877-019-1338-1 . PMID: 31747923; PMCID: PMC6864927.
Vikberg S, Sörlén N, Brandén L, Johansson J, Nordström A, Hult A, Nordström P. Effects of resistance training on functional strength and muscle mass in 70-Year-old individuals with pre-sarcopenia: a randomized controlled trial. J Am Med Dir Assoc. 2019;20(1):28–34. Epub 2018 Nov 7. PMID: 30414822.
Isanejad M, Sirola J, Mursu J, Rikkonen T, Kröger H, Tuppurainen M, Erkkilä AT. Association of the baltic sea and mediterranean diets with indices of sarcopenia in elderly women, OSPTRE-FPS study. Eur J Nutr. 2018;57(4):1435–48. https://doi.org/10.1007/s00394-017-1422-2 . Epub 2017 Mar 16. PMID: 28303397.
Mikkola TM, von Bonsdorff MB, Salonen MK, Simonen M, Pohjolainen P, Osmond C, Perälä MM, Rantanen T, Kajantie E, Eriksson JG. Body composition as a predictor of physical performance in older age: a ten-year follow-up of the Helsinki Birth Cohort Study. Arch Gerontol Geriatr. 2018 Jul-Aug;77:163–8. doi: 10.1016/j.archger.2018.05.009. Epub 2018 May 14. PMID: 29783137; PMCID: PMC5994345.
Ottestad I, Ulven SM, Øyri LKL, Sandvei KS, Gjevestad GO, Bye A, Sheikh NA, Biong AS, Andersen LF, Holven KB. Reduced plasma concentration of branched-chain amino acids in sarcopenic older subjects: a cross-sectional study. Br J Nutr. 2018;120(4):445–53. Epub 2018 Jun 18. PMID: 29909813.
Steihaug OM, Gjesdal CG, Bogen B, Kristoffersen MH, Lien G, Ranhoff AH. Sarcopenia in patients with hip fracture: a multicenter cross-sectional study. PLoS ONE. 2017;12(9):e0184780. https://doi.org/10.1371/journal.pone.0184780 . PMID: 28902873; PMCID: PMC5597226.
Jacobsen EL, Brovold T, Bergland A, Bye A. Prevalence of factors associated with malnutrition among acute geriatric patients in Norway: a cross-sectional study. BMJ Open. 2016;6(9):e011512. https://doi.org/10.1136/bmjopen-2016-011512 . PMID: 27601491; PMCID: PMC5020767.
Jansen RB, Christensen TM, Bülow J, Rørdam L, Holstein PE, Svendsen OL. Sarcopenia and body composition in diabetic Charcot osteoarthropathy. J Diabetes Complications. 2015 Sep-Oct;29(7):937–42. https://doi.org/10.1016/j.jdiacomp.2015.05.020 . Epub 2015 Jun 5. PMID: 26139557.
Frost M, Nielsen TL, Brixen K, Andersen M. Peak muscle mass in young men and Sarcopenia in the ageing male. Osteoporos Int. 2015;26(2):749–56. https://doi.org/10.1007/s00198-014-2960-6 . Epub 2014 Nov 22. PMID: 25416073.
Patil R, Uusi-Rasi K, Pasanen M, Kannus P, Karinkanta S, Sievänen H. Sarcopenia and osteopenia among 70-80-year-old home-dwelling finnish women: prevalence and association with functional performance. Osteoporos Int. 2013;24(3):787–96. https://doi.org/10.1007/s00198-012-2046-2 . Epub 2012 Jun 12. PMID: 22688541.
Papadopoulou SK, Tsintavis P, Potsaki P, Papandreou D. Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. a systematic review and meta-analysis. J Nutr Health Aging. 2020;24(1):83–90. https://doi.org/10.1007/s12603-019-1267-x . PMID: 31886813.
Mayhew AJ, Amog K, Phillips S, Parise G, McNicholas PD, de Souza RJ, Thabane L, Raina P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing. 2019;48(1):48–56. https://doi.org/10.1093/ageing/afy106 . PMID: 30052707.
Cao M, Lian J, Lin X, Liu J, Chen C, Xu S, Ma S, Wang F, Zhang N, Qi X, Xu G, Peng N. Prevalence of Sarcopenia under different diagnostic criteria and the changes in muscle mass, muscle strength, and physical function with age in Chinese old adults. BMC Geriatr. 2022;22(1):889. https://doi.org/10.1186/s12877-022-03601-7 . PMID: 36418979; PMCID: PMC9682713.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European working group on sarcopenia in older people. sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–23. https://doi.org/10.1093/ageing/afq034 . Epub 2010 Apr 13. PMID: 20392703; PMCID: PMC2886201.
Verstraeten LMG, de Haan NJ, Verbeet E, van Wijngaarden JP, Meskers CGM, Maier AB. Handgrip strength rather than chair stand test should be used to diagnose s in geriatric rehabilitation inpatients: restoring health of acutely unwell adulTs (RESORT). Age Ageing. 2022;51(11):afac242. https://doi.org/10.1093/ageing/afac242 . PMID: 36413590; PMCID: PMC9681126.
Cheng KY, Chow SK, Hung VW, Wong CH, Wong RM, Tsang CS, Kwok T, Cheung WH. Diagnosis of sarcopenia by evaluating skeletal muscle mass by adjusted bioimpedance analysis validated with dual-energy X-ray absorptiometry. J Cachexia Sarcopenia Muscle. 2021;12(6):2163–73. Epub 2021 Oct 4. PMID: 34609065; PMCID: PMC8718029.
Sousa-Santos AR, Barros D, Montanha TL, Carvalho J, Amaral TF. Which is the best alternative to estimate muscle mass for sarcopenia diagnosis when DXA is unavailable? Arch Gerontol Geriatr. 2021 Nov-Dec;97:104517. https://doi.org/10.1016/j.archger.2021.104517 . Epub 2021 Sep 3. PMID: 34547538.
González Correa CH, Marulanda Mejía F, Castaño González PA, Vidarte Claros JA, Castiblanco Arroyabe HD. Bioelectrical impedance analysis and dual x-ray absorptiometry agreement for skeletal muscle mass index evaluation in sarcopenia diagnosis. Physiol Meas. 2020;41(6):064005. https://doi.org/10.1088/1361-6579/ab8e5f . PMID: 32348971.
Hwang B, Lim JY, Lee J, Choi NK, Ahn YO, Park BJ. Prevalence rate and associated factors of sarcopenic obesity in Korean elderly population. J Korean Med Sci. 2012;27(7):748–55. https://doi.org/10.3346/jkms.2012.27.7.748 . Epub 2012 Jun 29. PMID: 22787369; PMCID: PMC3390722.
Kera T, Kawai H, Hirano H, Kojima M, Fujiwara Y, Ihara K, Obuchi S. Differences in body composition and physical function related to pure Sarcopenia and sarcopenic obesity: a study of community-dwelling older adults in Japan. Geriatr Gerontol Int. 2017;17(12):2602–9. https://doi.org/10.1111/ggi.13119 . Epub 2017 Jun 28. PMID: 28657168.
Aibar-Almazán A, Martínez-Amat A, Cruz-Díaz D, Jiménez-García JD, Achalandabaso A, Sánchez-Montesinos I, de la Torre-Cruz M, Hita-Contreras F. Sarcopenia and sarcopenic obesity in Spanish community-dwelling middle-aged and older women: Association with balance confidence, fear of falling and fall risk. Maturitas. 2018;107:26–32. Epub 2017 Oct 7. PMID: 29169576.
Prokopidis K, Giannos P, Reginster JY, Bruyere O, Petrovic M, Cherubini A, Triantafyllidis KK, Kechagias KS, Dionyssiotis Y, Cesari M, Ibrahim K, Scott D, Barbagallo M, Veronese N, the Task Force on Pharmaceutical Strategy of the European Geriatric Medicine Society (EuGMS). Special interest group in Systematic Reviews and Meta-analyses and sarcopenia is associated with a greater risk of polypharmacy and number of medications: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14(2):671–683. https://doi.org/10.1002/jcsm.13190 . Epub 2023 Feb 13. PMID: 36781175; PMCID: PMC10067503.
Zhang N, Sundquist J, Sundquist K, Ji J. An increasing Trend in the prevalence of polypharmacy in Sweden: a nationwide register-based study. Front Pharmacol. 2020;11:326. https://doi.org/10.3389/fphar.2020.00326 . PMID: 32265705; PMCID: PMC7103636.
Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol. 2017;8:1045. https://doi.org/10.3389/fphys.2017.01045 . PMID: 29311975; PMCID: PMC5733049.
Riuzzi F, Sorci G, Arcuri C, Giambanco I, Bellezza I, Minelli A, Donato R. Cellular and molecular mechanisms of sarcopenia: the S100B perspective. J Cachexia Sarcopenia Muscle. 2018;9(7):1255–68. https://doi.org/10.1002/jcsm.12363 . Epub 2018 Nov 30. PMID: 30499235; PMCID: PMC6351675.
Yuan S, Larsson SC. Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism. 2023;144:155533. https://doi.org/10.1016/j.metabol.2023.155533 . Epub 2023 Mar 11. PMID: 36907247.
Download references
Acknowledgements
Not applicable.
Open access funding provided by University of Southern Denmark
This work was done without any fund.
Author information
Fereshteh Baygi, Sussi Friis Buhl contributed equally to this work.
Authors and Affiliations
Research Unit of General Practice, Department of Public Health, University of Southern Denmark, Odense, Denmark
Fereshteh Baygi, Sussi Friis Buhl, Trine Thilsing, Jens Søndergaard & Jesper Bo Nielsen
You can also search for this author in PubMed Google Scholar
Contributions
FB conceived and designed the review, participated in literature review, data extraction, interpretation of the results and wrote the manuscript. SFB designed the review, participated in literature review, data extraction, and revised the manuscript. TT, JBN and JS contributed to the conception of the study and revised the manuscript critically. All the authors approved the final manuscript.
Corresponding authors
Correspondence to Fereshteh Baygi or Sussi Friis Buhl .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Baygi, F., Buhl, S.F., Thilsing, T. et al. Sarcopenia and sarcopenic obesity among older adults in the nordic countries: a scoping review. BMC Geriatr 24 , 421 (2024). https://doi.org/10.1186/s12877-024-04970-x
Download citation
Received : 12 November 2023
Accepted : 12 April 2024
Published : 13 May 2024
DOI : https://doi.org/10.1186/s12877-024-04970-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Sarcopenic obesity
- Nordic countries
BMC Geriatrics
ISSN: 1471-2318
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Examples of cohort studies. Cohort studies are common in fields like medicine, epidemiology, and healthcare. Example: Prospective cohort study. You are examining the relationship between exposure to pesticides and the incidence of a diagnosis of Parkinson's disease.. You recruit a group of healthy participants, all of whom were free of Parkinson's disease at the beginning of your study.
One famous example of a cohort study is the Nurses' Health Study. This was a large, long-running analysis of female health that began in 1976. ... Observational research methods. Research design ...
A prospective cohort study is a type of longitudinal research where a group of individuals sharing a common characteristic (cohort) is followed over time to observe and measure outcomes, often to investigate the effect of suspected risk factors. In a prospective study, the investigators will design the study, recruit subjects, and collect ...
This paper continues the series on the observational study designs, focusing on the cohort design. The word 'cohort' was adopted from the Roman term of 300 to 600 fighting soldiers who march together ( Hood, 2009; Hulley, 2013 ). The epidemiology community-initiated using 'cohort' during the 1930s to mean a "designated group which are ...
Examined from the perspective of research design, cohort studies are empirical because they collect and examine data. They are sample-based because a group of individuals is studied. ... As an example of a prospective cohort study, pregnant women can be recruited across the course of two years; relevant participant and gestational data can be ...
It is a type of nonexperimental or observational study design. The term "cohort" refers to a group of people who have been included in a study by an event that is based on the definition decided by the researcher. For example, a cohort of people born in Mumbai in the year 1980. This will be called a "birth cohort.".
A study design where one or more samples (called cohorts) are followed prospectively and subsequent status evaluations with respect to a disease or outcome are conducted to determine which initial participants exposure characteristics (risk factors) are associated with it. As the study is conducted, outcome from participants in each cohort is ...
Abstract. Cohort studies are types of observational studies in which a cohort, or a group of individuals sharing some characteristic, are followed up over time, and outcomes are measured at one or more time points. Cohort studies can be classified as prospective or retrospective studies, and they have several advantages and disadvantages.
Examined from the perspective of research design, cohort studies are empirical because they collect and examine data. They are sample-based because a group of individuals is studied. ... As an example of a prospective cohort study, pregnant women can be recruited across the course of two years; relevant participant and gestational data can be ...
This design determines whether exposure to a risk factor affects an outcome. Cohort studies are a type of longitudinal study because they track the same set of subjects over time. For example, if researchers hypothesize that exposure to a chemical increases skin cancer, they can form a cohort based on exposure to that chemical.
research design, cohort studies are empir-ical because they collect and examine data. They are sample-based because a group of individuals is studied. They are always longitudinal because there is a follow-up, but can be prospectively HOW TO CITE THIS ARTICLE: Andrade C. Research Design: Cohort Studies. Indian J Psychol Med. 2022;44(2):189-191.
Cohort studies can be either prospective or retrospective. The type of cohort study is determined by the outcome status. If the outcome has not occurred at the start of the study, then it is a prospective study; if the outcome has already occurred, then it is a retrospective study. 4 Figure 1 presents a graphical representation of the designs of prospective and retrospective cohort studies.
Example: In a retrospective cohort study researchers used previously collected data to investigate whether there was an association between birth experience and subsequent maternal care-giving attitudes and behaviour over a 12-month period ... Observational research methods. Research design II: cohort, cross sectional, and case-control studies ...
Prospective cohort studies require large sample sizes in order for any relationships or patterns to be meaningful. Researchers are unable to generate results if there is not enough data. ... E., & Locascio, J. J. (2018). Randomised controlled trials - the gold standard for effectiveness research: Study design: randomised controlled trials ...
Design, Analysis, and Reporting. Cohort studies are types of observational studies in which a cohort, or a group of individuals sharing some characteristic, are followed up over time, and outcomes are measured at one or more time points. Cohort studies can be classified as prospective or retrospective studies, and they have several advantages ...
A cohort study is a particular form of longitudinal study that samples a cohort (a group of people who share a defining characteristic, typically those who experienced a common event in a selected period, such as birth or graduation), performing a cross-section at intervals through time. It is a type of panel study where the individuals in the panel share a common characteristic.
Research study design is a framework, or the set of methods and procedures used to collect and analyze data on variables specified in a particular research problem. Research study designs are of many types, each with its advantages and limitations. ... This design is known as a cohort study. For example, a researcher can follow a group of ...
A study design where one or more samples (called cohorts) are followed prospectively and subsequent status evaluations with respect to a disease or outcome are conducted to determine which initial participants exposure characteristics (risk factors) are associated with it. As the study is conducted, outcome from participants in each cohort is ...
Abstract Background: Epidemiological research commonly investigates single exposure-outcome relationships, while childrens experiences across a variety of early lifecourse domains are intersecting. To design realistic interventions, epidemiological research should incorporate information from multiple risk exposure domains to assess effect on health outcomes. In this paper we identify ...
Objective To examine the association of ultra-processed food consumption with all cause mortality and cause specific mortality. Design Population based cohort study. Setting Female registered nurses from 11 US states in the Nurses' Health Study (1984-2018) and male health professionals from all 50 US states in the Health Professionals Follow-up Study (1986-2018). Participants 74 563 women ...
A research design is a strategy for answering your research question using empirical data. Creating a research design means making decisions about: Your overall research objectives and approach. Whether you'll rely on primary research or secondary research. Your sampling methods or criteria for selecting subjects. Your data collection methods.
Originally developed for gut microbiome research, xMarkerFinder's adaptable design makes it applicable to various microbial habitats and data types. ... -b the column name of cohort (in example ...
In this article, we describe an emerging pragmatic trial paradigm called "Trials within Cohorts" (TwiCs) which involves longitudinal cohort studies that provide a platform for randomized clinical trials. To date, studies using the design have obtained research ethics committee approval in 10 countries (Australia, Canada, Finland, France ...
Many governments or research centers carry out longitudinal studies and make the data freely available to the general public. For example, anyone can access data from the 1970 British Cohort Study, which has followed the lives of 17,000 Brits since their births in a single week in 1970, through the UK Data Service website.
However, most of the included studies had descriptive cross-sectional design, small sample size, and applied different diagnostic criteria. Therefore, larger well-designed cohort studies that adhere to uniform recent guidelines are required to capture a full picture of these two age-related medical conditions in Nordic countries, and plan for ...