Research articles
Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first line treatment for advanced gastric or gastro-oesophageal junction adenocarcinoma, epidural analgesia during labour and severe maternal morbidity, exposure to antibiotics during pregnancy or early infancy and risk of neurodevelopmental disorders, clinical and healthcare use outcomes after cessation of long term opioid treatment due to prescriber workforce exit, effect of the hpv vaccination programme on incidence of cervical cancer by socioeconomic deprivation in england, long acting progestogens vs combined oral contraceptive pill for preventing recurrence of endometriosis related pain, ultra-processed food consumption and all cause and cause specific mortality, comparative effectiveness of second line oral antidiabetic treatments among people with type 2 diabetes mellitus, efficacy of psilocybin for treating symptoms of depression, reverse total shoulder replacement versus anatomical total shoulder replacement for osteoarthritis, effect of combination treatment with glp-1 receptor agonists and sglt-2 inhibitors on incidence of cardiovascular and serious renal events, prenatal opioid exposure and risk of neuropsychiatric disorders in children, temporal trends in lifetime risks of atrial fibrillation and its complications, antipsychotic use in people with dementia, predicting the risks of kidney failure and death in adults with moderate to severe chronic kidney disease, impact of large scale, multicomponent intervention to reduce proton pump inhibitor overuse, esketamine after childbirth for mothers with prenatal depression, glucagon-like peptide 1 receptor agonist use and risk of thyroid cancer, use of progestogens and the risk of intracranial meningioma, delirium and incident dementia in hospital patients, derivation and external validation of a simple risk score for predicting severe acute kidney injury after intravenous cisplatin, quality and safety of artificial intelligence generated health information, large language models and the generation of health disinformation, 25 year trends in cancer incidence and mortality among adults in the uk, cervical pessary versus vaginal progesterone in women with a singleton pregnancy, comparison of prior authorization across insurers, diagnostic accuracy of magnetically guided capsule endoscopy with a detachable string for detecting oesophagogastric varices in adults with cirrhosis, ultra-processed food exposure and adverse health outcomes, added benefit and revenues of oncology drugs approved by the ema, exposure to air pollution and hospital admission for cardiovascular diseases, short term exposure to low level ambient fine particulate matter and natural cause, cardiovascular, and respiratory morbidity, optimal timing of influenza vaccination in young children, effect of exercise for depression, association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes, duration of cpr and outcomes for adults with in-hospital cardiac arrest, clinical effectiveness of an online physical and mental health rehabilitation programme for post-covid-19 condition, atypia detected during breast screening and subsequent development of cancer, publishers’ and journals’ instructions to authors on use of generative ai in academic and scientific publishing, effectiveness of glp-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes, neurological development in children born moderately or late preterm, invasive breast cancer and breast cancer death after non-screen detected ductal carcinoma in situ, all cause and cause specific mortality in obsessive-compulsive disorder, acute rehabilitation following traumatic anterior shoulder dislocation, perinatal depression and risk of mortality, undisclosed financial conflicts of interest in dsm-5-tr, effect of risk mitigation guidance opioid and stimulant dispensations on mortality and acute care visits, update to living systematic review on sars-cov-2 positivity in offspring and timing of mother-to-child transmission, perinatal depression and its health impact, christmas 2023: common healthcare related instruments subjected to magnetic attraction study, using autoregressive integrated moving average models for time series analysis of observational data, demand for morning after pill following new year holiday, christmas 2023: christmas recipes from the great british bake off, effect of a doctor working during the festive period on population health: experiment using doctor who episodes, christmas 2023: analysis of barbie medical and science career dolls, christmas 2023: effect of chair placement on physicians’ behavior and patients’ satisfaction, management of chronic pain secondary to temporomandibular disorders, christmas 2023: projecting complete redaction of clinical trial protocols, christmas 2023: a drug target for erectile dysfunction to help improve fertility, sexual activity, and wellbeing, christmas 2023: efficacy of cola ingestion for oesophageal food bolus impaction, conservative management versus laparoscopic cholecystectomy in adults with gallstone disease, social media use and health risk behaviours in young people, untreated cervical intraepithelial neoplasia grade 2 and cervical cancer, air pollution deaths attributable to fossil fuels, implementation of a high sensitivity cardiac troponin i assay and risk of myocardial infarction or death at five years, covid-19 vaccine effectiveness against post-covid-19 condition, association between patient-surgeon gender concordance and mortality after surgery, intravascular imaging guided versus coronary angiography guided percutaneous coronary intervention, treatment of lower urinary tract symptoms in men in primary care using a conservative intervention, autism intervention meta-analysis of early childhood studies, effectiveness of the live zoster vaccine during the 10 years following vaccination, effects of a multimodal intervention in primary care to reduce second line antibiotic prescriptions for urinary tract infections in women, pyrotinib versus placebo in combination with trastuzumab and docetaxel in patients with her2 positive metastatic breast cancer, association of dcis size and margin status with risk of developing breast cancer post-treatment, racial differences in low value care among older patients in the us, pharmaceutical industry payments and delivery of low value cancer drugs, rosuvastatin versus atorvastatin in adults with coronary artery disease, clinical effectiveness of septoplasty versus medical management for nasal airways obstruction, ultrasound guided lavage with corticosteroid injection versus sham lavage with and without corticosteroid injection for calcific tendinopathy of shoulder, early versus delayed antihypertensive treatment in patients with acute ischaemic stroke, mortality risks associated with floods in 761 communities worldwide, interactive effects of ambient fine particulate matter and ozone on daily mortality in 372 cities, association between changes in carbohydrate intake and long term weight changes, future-case control crossover analysis for adjusting bias in case crossover studies, association between recently raised anticholinergic burden and risk of acute cardiovascular events, suboptimal gestational weight gain and neonatal outcomes in low and middle income countries: individual participant data meta-analysis, efficacy and safety of an inactivated virus-particle vaccine for sars-cov-2, effect of invitation letter in language of origin on screening attendance: randomised controlled trial in breastscreen norway, visits by nurse practitioners and physician assistants in the usa, non-erosive gastro-oesophageal reflux disease and oesophageal adenocarcinoma, venous thromboembolism with use of hormonal contraception and nsaids, food additive emulsifiers and risk of cardiovascular disease, balancing risks and benefits of cannabis use, promoting activity, independence, and stability in early dementia and mild cognitive impairment, effect of home cook interventions for salt reduction in china, cancer mortality after low dose exposure to ionising radiation, effect of a smartphone intervention among university students with unhealthy alcohol use, long term risk of death and readmission after hospital admission with covid-19 among older adults, mortality rates among patients successfully treated for hepatitis c, association between antenatal corticosteroids and risk of serious infection in children, follow us on, content links.
- Collections
- Health in South Asia
- Women’s, children’s & adolescents’ health
- News and views
- BMJ Opinion
- Rapid responses
- Editorial staff
- BMJ in the USA
- BMJ in South Asia
- Submit your paper
- BMA members
- Subscribers
- Advertisers and sponsors

Explore BMJ
- Our company
- BMJ Careers
- BMJ Learning
- BMJ Masterclasses
- BMJ Journals
- BMJ Student
- Academic edition of The BMJ
- BMJ Best Practice
- The BMJ Awards
- Email alerts
- Activate subscription
Information
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Transforming Clinical Research to Meet Health Challenges
- 1 Office of the Director, National Institutes of Health, Bethesda, Maryland
The COVID-19 pandemic made “clinical trials” a household phrase, highlighting the critical value of clinical research in creating vaccines and treatments and demonstrating the need for large-scale, well-designed, and rapidly deployed clinical trials to address the public health emergency. As the largest public funder of clinical trials, the National Institutes of Health (NIH) launched a high-level effort to absorb the lessons of the pandemic and to assess and build on ongoing initiatives to improve efficiency, accountability, and transparency in clinical research.
Read More About
Wolinetz CD , Tabak LA. Transforming Clinical Research to Meet Health Challenges. JAMA. 2023;329(20):1740–1741. doi:10.1001/jama.2023.3964
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

Research shows GLP-1 receptor agonist drugs are effective but come with complex concerns

Drugs like Ozempic, Wegovy and Mounjaro have been around for years, but they’ve recently been making headlines due to a rise in popularity as weight loss agents. They all belong to a class of drugs known as glucagon-like peptide-1 receptor agonists (GLP-1RAs), which mimic a hormone (GLP-1) in the body that helps control insulin and blood glucose levels and promotes feelings of satiety.
These drugs are extremely effective for blood glucose control and weight management, which, combined with their relatively limited side effect profile, makes them very appealing for diabetes treatment — the purpose for which they originally received FDA approval.
However, off-label use fueled by celebrities and social media is a growing concern. And even when physicians are prescribing GLP-1RAs for their intended uses, it’s not a magic formula — there are complex considerations such as dosages, costs, side effects and comparisons between specific drugs.
“The current fervor for GLP-1RAs in the capital markets as well as in the general public, especially in terms of weight reduction, is probably going to result in overuse,” said Chun-Su Yuan, MD, PhD , the Cyrus Tang Professor of Anesthesia and Critical Care at the University of Chicago. “This should raise a red flag.”
Living up to the hype
While experts caution against overusing GLP-1RAs or viewing them as a universal cure-all for obesity, physicians and researchers agree that the drugs are highly effective for weight management and Type 2 diabetes treatment.
“Some other treatments for Type 2 diabetes can actually cause weight gain, whereas GLP-1RA drugs effectively control blood glucose levels while also reducing body weight,” Yuan said.
Yuan and a group of other researchers recently published a paper comparing the effectiveness of different GLP-1RAs. Different drugs performed better in different areas, but all 15 GLP-1RAs they analyzed were very successful in lowering blood glucose and achieving weight loss. They also identified some secondary benefits, such as lowering cholesterol.
Similarly, Eric Polley, PhD , a UChicago data science and public health expert, recently led a study published in Nature Cardiovascular Research that used statistical modeling to simulate a clinical trial comparing the effects of four different classes of diabetes medication in patients with moderate cardiovascular risk. GLP-1RA drugs came out on top, not only controlling blood glucose and weight but also reducing the risk of major heart-related events and the risk of death overall.
Not a silver bullet: making nuanced decisions for each patient
However, GLP-1RAs are not universally effective for all patients, and Yuan said that even after deciding to prescribe this drug class, physicians should consider multiple factors when selecting a specific drug and dosage. For example, co-morbid conditions like hyperlipidemia could tip the scale and make one drug more suitable for a specific patient.
Polley pointed out that even patients with similar clinical profiles might prioritize different aspects of their health or quality of life.
“If cardiovascular health is what you think is important for deciding between these drug classes, I think our most recent study provides some strong evidence. But if there are other outcomes that your patient is concerned about, then you have to consider the effect size for those other outcomes,” Polley said. He and other experts are working on subsequent research examining the effects of different diabetes treatments on other health outcomes and concerns, including a patient’s risk of cancer, blindness or amputation.
Another key consideration is side effects, which can vary significantly from patient to patient. While Yuan’s recent study confirmed the efficacy of GLP-1RAs, the researchers also found that some patients did experience adverse side effects, especially related to gastrointestinal issues like nausea and vomiting. They highlighted the need to consider potential tradeoffs between efficacy and side effects, finding that higher doses can have stronger efficacy but also induce more severe side effects.
“It’s also important to note that the long-term side effects of these drugs are not yet well-studied,” Yuan said. “If large swathes of the general public start taking them off-label for weight loss and then we find out years later that there are bad side effects, it could be a real issue.”
Rethinking long-term weight management strategies to overcome cost barriers
Yet another dimension affecting the use of GLP-1RAs is cost. The drugs are expensive, and experts say the recent spike in popularity has already led to shortages and increased hesitancy among insurance providers to cover these drugs.
“We know these drugs represent a massive breakthrough in our long fight against obesity-related clinical conditions, but their high cost has been the subject of substantial debate,” said David Kim, PhD , a UChicago health economist. “It presents a key barrier to equitable access to this great innovation.”
In pursuit of more equitable and cost-effective approaches to leveraging GLP-1RAs, Kim and a group of other researchers analyzed the potential impact of alternative weight loss programs. Specifically, they proposed an approach in which GLP-1RAs could be prescribed for an initial period of weight loss before patients transitioned to cheaper alternative interventions for weight maintenance such as lower-cost medications, behavioral health programs and support from nutritionists.
“We wanted to challenge the assumption that once you’re on a GLP-1RA drug, you have to keep taking it forever,” Kim said. “That’s where some of the affordability concerns are coming from: large populations are potentially eligible to take these drugs, and we can’t pay for a lifetime supply for everyone.”
The researchers’ model suggested that even though the alternative weight-maintenance programs might be slightly less effective than long-term, full-dosage GLP-1RA use, the clinical benefits would only decrease slightly, while lifetime healthcare spending would decrease substantially.
“We argue that this alternative framework is a viable solution that provides greater flexibility for managing a limited drug supply and giving healthcare payers financial headroom to support more patients accessing effective weight management treatment,” Kim said.
“ Comparative effectiveness of GLP-1 receptor agonists on glycaemic control, body weight, and lipid profile for type 2 diabetes: systematic review and network meta-analysis ” was published in The BMJ in January 2024. Authors included Haiqiang Yao, Anqi Zhang, Delong Li, Yuqi Wu, Chong-Zhi Wang, Jin-Yi Wan and Chun-Su Yuan.
“ Effectiveness of glucose-lowering medications on cardiovascular outcomes in patients with type 2 diabetes at moderate cardiovascular risk ” was published in Nature Cardiovascular Research in April 2024. Authors included Rozalina G. McCoy, Jeph Herrin, Kavya Sindhu Swarna, Yihong Deng, David M. Kent, Joseph S. Ross, Guillermo E. Umpierrez, Rodolfo J. Galindo, William H. Crown, Bijan J. Borah, Victor M. Montori, Juan P. Brito, Joshua J. Neumiller, Mindy M. Mickelson and Eric C. Polley.
“ Balancing Innovation and Affordability in Anti-Obesity Medications: The Role of Alternative Weight Maintenance Program ” was published in Health Affairs Scholar in May 2024. Authors included David D. Kim, Jennifer H. Hwang and A. Mark Fendrick.
Leading Change in Cancer Clinical Research, Because Our Patients Can’t Wait
May 31, 2024 , by W. Kimryn Rathmell, M.D., Ph.D., and Shaalan Beg, M.D.

Greater use of technologies that can increase participation in cancer clinical trials is just one of the innovations that can help overcome some of the bottlenecks holding up progress in clinical research.
Thanks to advances in technology, data science, and infrastructure, the pace of discovery and innovation in cancer research has accelerated, producing an impressive range of potential new treatments and other interventions that are being tested in clinical studies . The extent of the innovative ideas that might help people live longer, improve our ability to detect cancer early, or otherwise transform care is staggering.
Our understanding of tumor biology is also evolving, and those gains in knowledge are being translated into the continued discovery of targets for potential interventions and the development of novel types of treatments. Some of these therapies are producing unprecedented clinical responses in studies, including in traditionally difficult-to-treat cancers.
These advances have contributed to a record number of Food and Drug Administration (FDA) approvals in recent years with, arguably, the most notable approvals being those for drugs that can be used for any cancer, regardless of where it is in the body .
In some instances, the activity of new agents has been so profound that clinical investigators are having to rethink their criteria for implementation in patient care and their definitions of treatment response.
For example, although HER2 has been a known therapeutic target in breast cancer for many decades, the new antibody-drug conjugates (ADCs) that target HER2 have proven to be vastly more effective than the original HER2-targeted therapies. This has forced researchers to rethink fundamental questions about how these ADCs are used in patient care: Can they be effective in people whose tumors have lower expression of HER2 than we previously thought was needed ? And, if so, do we need to redefine how we classify HER2-positive cancer?
As more innovative therapies like ADCs hit the clinic at a far more rapid cadence than ever before, the research community is being inundated with such fundamentally important questions.
However, the remarkable progress we're experiencing with novel new therapies is tempered by a critical bottleneck: the clinical research infrastructure can’t be expected to keep pace in this new landscape.
Currently, many studies struggle to enroll enough participants. At the same time, there are patients who don’t have ready access to studies from which they might benefit. Furthermore, ideas researchers have today for studies of innovative new interventions might not come to fruition for 2 or 3 years, or even longer—years that people with cancer don’t have.
The key to overcoming this bottleneck is to invite innovation to help reshape our clinical trials infrastructure. And here’s how we plan to accomplish that.
Testing Innovation in Cancer Clinical Trials
A transformation in cancer clinical research is already underway. That transformation has been led in part by the success of novel precision oncology approaches, such as those tested in the NCI-MATCH trial .
This innovative study ushered in novel ways of recruiting participants and involving oncologists at centers big and small. And NCI-MATCH has spawned several successor studies that are incorporating and building on its innovations and achievements.
An innovation that emerged from the COVID pandemic was the increase of remote work, even in the clinical trials domain. Indeed, staffing shortages have caused participation in NCI-funded trials to decline. In response, NCI is piloting a Virtual Clinical Trials Office to offer remote support staff to participating study sites. This support staff includes research nurses, clinical research associates, and data specialists, all of whom will help NCI-Designated Cancer Centers and community practices engaged in clinical research activities.
Such technology-enabled services can allow us to reimagine how clinical trials are designed and run. This includes developing technologies and processes for remotely identifying clinical trial participants, shipping medications to participants at home, having imaging performed in the health care settings where our patients live, and empowering local physicians to participate in clinical trials.
We also need mechanisms to test and implement innovations in designing and conducting clinical studies.
For example, NCI recently established the Clinical Trials Innovation Unit (CTIU) to pressure test a variety of innovations. One of the first trials to emerge from the CTIU’s initial efforts was the Pragmatica-Lung Cancer Treatment Trial , a phase 3 study designed to be easy to launch, enroll, and interpret its results.
The CTIU, which includes leadership from FDA and NCI’s National Clinical Trials Network , is already working on future innovations, including those that will streamline data collection and apply innovative approaches for other cancers, all with the goal of making cancer clinical studies less burdensome to run and easier for patients to participate.
Data-Driven Solutions
The era of data-driven health care is here, providing still more opportunities to transform cancer clinical research.
The emergence of artificial intelligence (AI) solutions, large language models, and informatics brings real potential for wholesale changes in how we match patients to clinical studies, assess side effects, and monitor events like disease progression.
Recognizing this potential, NCI is offering funding opportunities and other resources that will fuel the development of AI tools for clinical research, allow us to carefully test their usefulness, and ultimately deploy them across the oncology community.
Creating Partnerships and Expanding Health Equity
To be sure, none of this will be, or can be, done by NCI alone. All these innovations require partnerships. We will increase our engagement with partners in the public- and private-sectors, including other government agencies and nonprofits.
That includes high-level engagement with the Office of the National Coordinator for Health Information Technology (ONC), with input from FDA, Centers for Medicare & Medicaid Services, and Centers for Disease Control and Prevention.

Dr. W. Kimryn Rathmell, M.D., Ph.D.
NCI Director
One example of such a partnership is the USCDI+ Cancer program . Conducted under the auspices of the ONC, this program will further the aims of the White House's reignited Cancer Moonshot SM by encouraging the adoption and utilization of interoperable cancer health IT standards, providing resources to support cancer-specific use cases, and promoting alignment between federal partners.
And just as importantly, the new partnerships we create must include those with patients, advocates, and communities in ways we have never considered before.
A central feature of this community engagement must involve intentional efforts to expand health equity, to create study designs that are inclusive and culturally appropriate. Far too many marginalized communities and populations today are further harmed by studies that fail to provide findings that apply to their unique situations and needs.
Very importantly, the future will require educating our next generation of clinical investigators and empowering them with the tools that enable new ways of managing clinical studies. By supporting initiatives spearheaded by FDA and professional groups like the American Society of Clinical Oncology, NCI is making it easier for community oncologists to participate in clinical trials and helping clarify previously misunderstood regulatory requirements.
These efforts must also ensure that we have a clinical research workforce that is representative of the people it is intended to serve. Far too many structural barriers have prevented this from taking place in the past, and it’s time for that to change.
Expanding our capacity doesn’t mean doing more of the same, it means challenging ourselves to work differently. This will let us move forward to a new state, one in which clinical research is integrated in everyday practice. It is only with more strategic partnerships and increased inclusivity that we can open the doors to seeing clinical investigation in new ways, with new standards for success.
A Collaborative Effort

Shaalan Beg, M.D.
Senior Advisor for Clinical Research
To make the kind of progress we all desire, we have to recognize that our clinical studies system needs to evolve.
There was a time when taking years to design, launch, and complete a clinical trial was acceptable. It isn’t acceptable anymore. We are in an era where we have the tools and the research talent to make far more rapid progress than we have in the past.
And we can do that by engaging with many different communities and stakeholders in unique and dynamic ways—making them partners in our effort to end cancer as we know it.
Together, our task is to capitalize on this work so we can move faster and enable cutting-edge research that benefits as many people as possible.
We also know that there are more good ideas in this space, and part of this transformation includes grass roots efforts to drive systemic change. So, we encourage you to share your ideas on how we can transform clinical research. Because achieving this goal can’t be done by any one group alone. We are all in this together.
Featured Posts
March 27, 2024, by Edward Winstead
March 21, 2024, by Elia Ben-Ari
March 5, 2024, by Carmen Phillips
- Biology of Cancer
- Cancer Risk
- Childhood Cancer
- Clinical Trial Results
- Disparities
- FDA Approvals
- Global Health
- Leadership & Expert Views
- Screening & Early Detection
- Survivorship & Supportive Care
- February (6)
- January (6)
- December (7)
- November (6)
- October (7)
- September (7)
- February (7)
- November (7)
- October (5)
- September (6)
- November (4)
- September (9)
- February (5)
- October (8)
- January (7)
- December (6)
- September (8)
- February (9)
- December (9)
- November (9)
- October (9)
- September (11)
- February (11)
- January (10)
Loading metrics
Open Access
Peer-reviewed
Research Article
Factors influencing the participation of pregnant and lactating women in clinical trials: A mixed-methods systematic review
Contributed equally to this work with: Mridula Shankar, Alya Hazfiarini
Roles Data curation, Formal analysis, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Gender and Women’s Health Unit, Nossal Institute for Global Health, School of Population and Global Health, University of Melbourne, Carlton, Victoria, Australia
Roles Formal analysis, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing
Roles Formal analysis, Methodology, Writing – review & editing
Roles Methodology, Writing – review & editing
Affiliation Maternal, Child and Adolescent Health Program, Burnet Institute, Melbourne, Victoria, Australia
Roles Data curation, Methodology, Writing – review & editing
Affiliation University Library, University of Melbourne, Carlton, Victoria, Australia
Affiliation Women’s and Children’s Health Research Unit, KLE Academy of Higher Education and Research, Jawaharlal Nehru Medical College, Belagavi, Karnataka, India
Affiliation Concept Foundation, Geneva, Switzerland/Bangkok, Thailand
Roles Conceptualization, Funding acquisition, Methodology, Writing – review & editing
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing
- Mridula Shankar,
- Alya Hazfiarini,
- Rana Islamiah Zahroh,
- Joshua P. Vogel,
- Annie R. A. McDougall,
- Patrick Condron,
- Shivaprasad S. Goudar,
- Yeshita V. Pujar,
- Manjunath S. Somannavar,

- Published: May 30, 2024
- https://doi.org/10.1371/journal.pmed.1004405
- Peer Review
- Reader Comments
Poor representation of pregnant and lactating women and people in clinical trials has marginalised their health concerns and denied the maternal–fetal/infant dyad benefits of innovation in therapeutic research and development. This mixed-methods systematic review synthesised factors affecting the participation of pregnant and lactating women in clinical trials, across all levels of the research ecosystem.
Methods and findings
We searched 8 databases from inception to 14 February 2024 to identify qualitative, quantitative, and mixed-methods studies that described factors affecting participation of pregnant and lactating women in vaccine and therapeutic clinical trials in any setting. We used thematic synthesis to analyse the qualitative literature and assessed confidence in each qualitative review finding using the GRADE-CERQual approach. We compared quantitative data against the thematic synthesis findings to assess areas of convergence or divergence. We mapped review findings to the Theoretical Domains Framework (TDF) and Capability, Opportunity, and Motivation Model of Behaviour (COM-B) to inform future development of behaviour change strategies.
We included 60 papers from 27 countries. We grouped 24 review findings under 5 overarching themes: (a) interplay between perceived risks and benefits of participation in women’s decision-making; (b) engagement between women and the medical and research ecosystems; (c) gender norms and decision-making autonomy; (d) factors affecting clinical trial recruitment; and (e) upstream factors in the research ecosystem. Women’s willingness to participate in trials was affected by: perceived risk of the health condition weighed against an intervention’s risks and benefits, therapeutic optimism, intervention acceptability, expectations of receiving higher quality care in a trial, altruistic motivations, intimate relationship dynamics, and power and trust in medicine and research. Health workers supported women’s participation in trials when they perceived clinical equipoise, had hope for novel therapeutic applications, and were convinced an intervention was safe. For research staff, developing reciprocal relationships with health workers, having access to resources for trial implementation, ensuring the trial was visible to potential participants and health workers, implementing a woman-centred approach when communicating with potential participants, and emotional orientations towards the trial were factors perceived to affect recruitment. For study investigators and ethics committees, the complexities and subjectivities in risk assessments and trial design, and limited funding of such trials contributed to their reluctance in leading and approving such trials. All included studies focused on factors affecting participation of cisgender pregnant women in clinical trials; future research should consider other pregnancy-capable populations, including transgender and nonbinary people.
Conclusions
This systematic review highlights diverse factors across multiple levels and stakeholders affecting the participation of pregnant and lactating women in clinical trials. By linking identified factors to frameworks of behaviour change, we have developed theoretically informed strategies that can help optimise pregnant and lactating women’s engagement, participation, and trust in such trials.
Author summary
Why was this study done.
- Pregnant and lactating women and people are routinely excluded from participating in drug and vaccine clinical trials, resulting in limited options for prevention and treatment of medical conditions.
- Challenges to including pregnant and lactating women and people in clinical research have been identified at multiple levels of the research and health systems, but the full range of barriers and facilitators to participation are not well known.
What did the researchers do and find?
- We conducted a mixed-methods systematic review and identified 60 research articles from 27 countries on the views and experiences of pregnant and lactating women’s participation in clinical research, from the perspectives of cisgender women, family and community members, health workers, and people involved in the conduct of clinical research.
- Using a thematic synthesis approach, we identified barriers affecting participation including women having a limited appetite for risk during pregnancy and lactation, concerns about women’s bodily autonomy during pregnancy, and challenges in obtaining ethical approval for clinical research with pregnant women.
- We also identified facilitators of participation including the potential for personal health benefits, expectations of higher quality care, trust in the medical and research systems, and strong teamwork between researchers and health workers.
What do these findings mean?
- Our findings demonstrate the need for multipronged strategies to address barriers and reinforce facilitators across the various levels of the research and health systems.
- The actions that are needed to overcome these barriers and reinforce facilitators must be discussed, prioritised, and adapted to specific contexts.
- All included studies focused on factors affecting participation of cisgender pregnant women in clinical trials; future research should consider other pregnancy-capable populations, including transgender and nonbinary people.
Citation: Shankar M, Hazfiarini A, Zahroh RI, Vogel JP, McDougall ARA, Condron P, et al. (2024) Factors influencing the participation of pregnant and lactating women in clinical trials: A mixed-methods systematic review. PLoS Med 21(5): e1004405. https://doi.org/10.1371/journal.pmed.1004405
Received: December 20, 2023; Accepted: April 19, 2024; Published: May 30, 2024
Copyright: © 2024 Shankar et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting Information files.
Funding: The research in this publication was supported by funding from MSD (grant MFM-22-159697 to Concept Foundation), through its MSD for Mothers initiative ( https://www.msdformothers.com/ ) and is the sole responsibility of the authors. MSD for Mothers is an initiative of Merck & Co., Inc., Rahway, NJ, U.S.A. MAB’s time is supported by an Australian Research Council Discovery Early Career Researcher Award (DE200100264) and a Dame Kate Campbell Fellowship (University of Melbourne Faculty of Medicine, Dentistry and Health Sciences). JPV is supported by an Australian National Health and Medical Research Council (NHMRC) Investigator grant (GNT1194248). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations: BCW, Behaviour Change Wheel; COM-B, Capability, Opportunity, and Motivation Model of Behaviour; MMAT, Mixed Methods Appraisal Tool; TDF, Theoretical Domains Framework
Introduction
Clinical trials are the foundation for knowledge on the efficacy and safety of biomedical interventions to protect health and treat illness. The fundamental questions of who participates and whose data contributes to trials have implications for understanding the risks and benefits of interventions, and the societal value of such interventions to specific populations. Pregnant and lactating women and people have long been underrepresented or excluded entirely from participating in therapeutic and vaccine clinical trials [ 1 ]. Notwithstanding valid concerns regarding fetal and infant safety, an outright exclusionary response to this complex issue has denied the maternal–fetal/infant dyad the health benefits of biomedical innovation, despite demonstrated public health need [ 2 , 3 ]. As a recent example, during the COVID-19 pandemic, pregnant women and people were excluded from early therapeutic and vaccine trials despite greater severity of infection-related illness [ 4 – 9 ].
Including pregnant and lactating women and people as research participants is vital: pregnancy is a unique physiological state where the body undergoes adaptations that can lead to pregnancy-specific disorders or worsen preexisting conditions [ 10 ]. These changes can influence how effective a drug is, whether and how the body responds to the drug, and the dosages at which the drug is optimally effective and minimally harmful. Most pregnant women take at least 1 medication during pregnancy [ 11 ], yet many of these medications are provided with limited information on efficacy, appropriate dosing, and safety in these populations [ 1 ]. Pregnant and lactating women with preexisting illnesses may also be advised to discontinue medications to minimise potential harms, without full appreciation of the possible consequences of unmedicated disease progression [ 12 ].
The current state of maternal health and the limited therapeutic options available for pregnant and lactating populations illustrates the consequences of these evidence gaps. Each year, complications of pregnancy and childbirth result in approximately 287,000 maternal deaths [ 13 ], 1.9 million stillbirths [ 14 ], and 2.3 million neonatal deaths [ 15 ]. Most of these deaths occur from preventable or treatable obstetric causes (e.g., postpartum haemorrhage, preeclampsia/eclampsia, sepsis) that are generally treated using repurposed medications that were originally developed and approved for use in other non-obstetric conditions [ 16 ]. Over the past 3 decades, only 2 drugs have been registered to specifically treat pregnancy-related complications: Atosiban—a tocolytic to prevent preterm birth, and Carbetocin—an oxytocin analogue for managing postpartum haemorrhage [ 17 ]. Pregnancy-specific medicines rarely progress through the research and development pipeline due to a multitude of factors, including the absence of public stewardship, chronic underinvestment, and regulatory and market barriers [ 18 , 19 ]. Maternal mortality rates have largely remained static in the Sustainable Development Goal era: progress has halted or reversed in 150 countries [ 13 ]. Without significant investments in pharmaceutical development, the 2030 target of a global maternal mortality ratio less than 70 maternal deaths per 100,000 live births [ 20 ] is unlikely to be achieved.
Poor representation of pregnant and lactating women and people in clinical research, and the absence of a pregnancy-focused research and development agenda violates fundamental ethical principles of justice and equity [ 12 , 21 ]. Challenges to equitable inclusion operate across all research stages: “upstream” barriers include a lack of appropriate animal models, pharmaceutical industry risk aversion, and clinical trials and liability insurance challenges [ 12 , 18 , 22 , 23 ]. “Downstream” barriers include perceptions that pregnant and lactating women do not want to take part in clinical trials, or that their inclusion makes research activities too risky or onerous [ 23 ]. Overall, there is a lack of a comprehensive understanding of the full range of these factors from the perspectives of key stakeholder groups. This mixed-methods systematic review seeks to address this gap by synthesising current research evidence on factors (i.e., barriers and facilitators) affecting the participation of pregnant and lactating women in vaccine and therapeutic clinical trials. We use behavioural [ 24 , 25 ] frameworks to provide a theory-informed basis for the development and implementation of appropriate behaviour change intervention strategies to promote their meaningful inclusion.
This review is reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines ( S1 Appendix ), Enhancing Transparency in Reporting the Synthesis of Qualitative Research (ENTREQ) statement ( S2 Appendix ), and based on guidance from the Cochrane Effective Practice and Organisation of Care group [ 26 ]. The protocol has been registered (PROSPERO: CRD42023462449).
Types of studies
We included primary qualitative, quantitative, and mixed-methods studies. There were no limitations on publication date, language, or country.
We excluded publications that were not primary research, including conceptual scholarship on the ethics of inclusion/exclusion, case reports, reviews, commentaries, short communications, editorials, news articles, letters to the editor, conference abstracts, workshop summaries, theses or dissertations, book chapters, book reviews, and regulatory or committee guidance or decisions.
Topic of interest
This review focuses on systematically identifying the factors, including barriers and facilitators, influencing the participation of pregnant and lactating women in drug or vaccine trials (i.e., therapeutic or prophylactic trials). We recognise that people who are capable of pregnancy have diverse gender identities. We use the terminology “pregnant and lactating women,” acknowledging that empirical literature on this topic has been focused on the experiences of cisgender women. Extrapolating these data to apply to people with other gender identities may lead to inaccurate or incomplete conclusions.
We included studies that described the attitudes, perspectives, and experiences of multiple stakeholders: women who participated and declined participation in clinical trials during pregnancy and lactation, partners or husbands, family members, community leaders, health workers, research staff, study investigators, ethics committee members, regulators, funders, pharmaceutical representatives, policy makers, and other relevant stakeholders.
We excluded the following types of interventions from this review: (a) lifestyle or behavioural interventions; (b) trials of diagnostics or medical devices; (b) workforce interventions to improve clinical care outcomes; (c) alternative or complementary medicine; (d) trials evaluating health policies or clinical protocols; (e) fetal tissue research, bio-banking, and genetic testing; (f) facilitators and barriers to engaging pregnant women in observational research; (g) supports to clinicians or pregnant or lactating women regarding decision-making on medication; and (h) research solely focused on substance use prevention and treatment, due to the particularly distinct barriers and facilitators given overlapping vulnerabilities among substance-using pregnant women, and unique considerations in relation to fetal health such as in utero exposure to alcohol and other substances. We also excluded clinical trial protocols and publications of randomised controlled trials that did not contain data related to facilitators or barriers to trial participation.
Search methods for identification of relevant studies
We searched 8 databases from inception to 14 February 2024: MEDLINE (Ovid), CINAHL Complete, Family & Society Studies Worldwide, SocINDEX, Scopus, Web of Science Core Collection, Embase (Ovid), and Global Health (Ovid). PC, an Information Specialist developed the final search strategy ( S3 Appendix ), using a combination of terms relevant to pregnant and lactating women, and perspectives and experiences of stakeholders regarding their inclusion/exclusion and participation in drug or vaccine clinical trials. No restrictions were placed on publication year, language, or geographical setting.
Selection of studies
We imported the search results into Covidence [ 27 ] and removed duplicates. Five review authors (MS, AH, MAB, AM, and AA) independently screened titles and abstracts. Titles and abstracts of non-English publications were screened with the assistance of Google Translate. Three reviewers (MS, AH, and AM) independently reviewed full texts. One French publication that met the inclusion criteria was translated to English using ChatGPT [ 28 ], and translation accuracy was subsequently verified with a native French speaker in our research network. At each screening stage, differences in decisions regarding record inclusion were resolved through discussion and final decisions were made through consensus with a third review author (MAB).
Data extraction and assessing methodological limitations
Two review authors (MS and AH) extracted relevant data, including study aims, methodological characteristics, geographical settings, population of interest (pregnant women, lactating women, or both), intervention type (therapy or vaccine), specific areas of research, and study findings (author-generated themes, supporting explanations, participant quotes, survey results, and relevant tables and figures). We developed a data extraction form and refined it by extracting data from a subset of 6 studies. All extracted data was cross-checked for accuracy and completeness, and differences resolved via consensus.
Two reviewers (MS and AH) independently assessed the methodological limitations of each study using an adapted Mixed Methods Appraisal Tool (MMAT) [ 29 ]. For qualitative studies, evaluative criteria included alignment of methodology and data collection with research aims, rigour in data analysis and reporting of study findings, ethical considerations, and researcher reflexivity. We assessed quantitative studies based on the suitability of sampling strategy, reporting on sample representativeness, use of appropriate measures, level of nonresponse bias, ethical considerations, and relevance of statistical analyses conducted. In addition to the aforementioned criteria, we assessed mixed-methods studies to determine whether authors demonstrated sufficient rationale for the use of a mixed-methods approach, effectiveness of integration of study components and outputs, and discussion of data triangulation. All differences in assessments between the 2 review authors were resolved through discussion. The assessment of methodological limitations did not affect the inclusion or exclusion of studies but rather served as a mechanism for determining confidence in the evidence.
Data analysis and synthesis
We used a thematic synthesis approach to analyse qualitative data [ 30 ]. After selecting 6 data-rich studies, 2 reviewers (MS and AH) independently applied line-by-line coding to the textual data to create summative codes. Codes were discussed for consistency in meaning and refined if necessary. The remaining studies were each coded by one of the 2 reviewers, and new codes were added as necessary. Through discussion, we subsumed codes of similar meaning under broader categories, gradually developing “summary layers” in a hierarchical grouping structure. We applied the gender domains of the gender analysis matrix [ 31 ] as a lens to our findings to understand how our data on factors influencing participation were shaped by aspects such as distribution of labour and roles, gender norms and beliefs, access to resources, decision-making power, and institutional policies. We consolidated our results into a set of 5 overarching themes and 24 review findings through an iterative process of identifying, comparing, and discussing conceptual boundaries between and among thematic data outputs.
Two review authors (MS and AH) used the GRADE-CERQual (Confidence in the Evidence from Reviews of Qualitative research) approach [ 32 , 33 ] to assess our confidence in each of the 24 qualitative review findings. GRADE-CERQual assesses confidence in the evidence, based on the following 4 key components [ 26 ]:
- methodological limitations of included studies [ 34 ];
- coherence of the review finding [ 35 ];
- adequacy of the data contributing to the review finding [ 36 ]; and
- relevance of the included studies to the review question [ 37 ].
After assessing each component, we made a judgement via consensus about the overall confidence—rated as high, moderate, low, or very low—in the evidence supporting the review finding [ 32 ]. Detailed descriptions of the GRADE-CERQual assessments are in S4 Appendix .
We then mapped data from the quantitative studies onto the findings of the qualitative evidence synthesis, and determined areas of convergence or divergence, and whether any additional factors arose that had previously not been discussed. We regarded the quantitative data as (a) “supporting” of a qualitative evidence synthesis finding if the information synthesised from the contributory quantitative studies were similar to the finding; (b) “extending” if the data offered additional details in line with a review finding; and (c) “contradictory” if the data conflicted with a review finding. Summaries of the quantitative findings are presented in S5 Appendix .
Finally, we mapped our review findings to the Theoretical Domains Framework (TDF) [ 24 ] and the Capability, Opportunity, and Motivation (COM-B) [ 25 ] models of behavioural determinants and the Behaviour Change Wheel (BCW) to identify and provide a rational basis for the development and implementation of appropriate behaviour change strategies.
Review team and reflexivity
The review author team has diverse personal backgrounds, including gender, personal experiences of pregnancy, countries of origin and residence, and linguistic traditions. Our professional and academic backgrounds and experiences are varied, and include the social, behavioural, and biomedical sciences, medicine, clinical epidemiology, and public health. Some review authors have led and implemented trials in maternal and perinatal health. As an interdisciplinary team with diverse social and professional backgrounds, we maintained a reflexive stance through all stages of the review process by engaging in multiple reflective dialogues to interrogate and interpret the data and findings. Through this process, we named and critiqued assumptions that underpinned the analysis and challenged disciplinary biases. In doing so, we aimed to develop review findings that were inclusive of different disciplinary lenses.
Sixty papers from 53 studies met the inclusion criteria [ 38 – 97 ]. Fig 1 presents the PRISMA flowchart. Table 1 reports the summary characteristics of included papers and S6 Appendix includes more detailed individual characteristics of the included papers.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pmed.1004405.g001
https://doi.org/10.1371/journal.pmed.1004405.t001
Description of papers
Thirty-nine papers used qualitative methodologies [ 39 , 40 , 42 – 48 , 53 , 54 , 56 – 66 , 69 , 70 , 72 – 74 , 78 , 81 , 82 , 84 – 87 , 89 – 92 , 96 ], 18 papers used quantitative methodologies [ 38 , 41 , 50 – 52 , 67 , 68 , 71 , 75 – 77 , 79 , 80 , 88 , 93 – 95 , 97 ], and 3 papers used mixed-methods study designs [ 49 , 55 , 83 ].
The 60 papers present data from 27 countries and 4 geographic regions: 13 countries in Africa [ 44 – 47 , 65 , 73 , 78 , 84 , 85 ], 8 countries in Europe [ 38 , 39 , 41 , 48 – 50 , 53 – 56 , 58 , 59 , 61 , 62 , 64 , 67 – 69 , 72 , 74 , 80 – 83 , 86 , 89 , 90 , 92 , 94 , 96 ], 3 countries in the Americas [ 42 , 43 , 51 , 52 , 57 , 60 , 63 , 66 , 70 , 71 , 75 , 77 , 79 , 85 , 88 , 91 , 93 , 95 ], and 3 countries in the Western Pacific [ 40 , 76 , 87 , 97 ].
Fifty-one papers focused on pregnant women only [ 38 – 41 , 44 , 47 – 50 , 52 , 53 , 55 – 70 , 72 – 94 , 97 ], 2 papers focused on lactating women only [ 46 , 96 ], and 7 papers focused on pregnant and lactating women [ 42 , 43 , 45 , 51 , 54 , 71 , 95 ]. Thirty-seven papers addressed a therapeutic drug-related intervention [ 38 , 40 , 41 , 44 – 49 , 53 , 56 , 59 – 62 , 66 , 69 , 70 , 72 , 73 , 77 , 79 – 90 , 92 , 93 , 96 , 97 ], 11 papers focused on a vaccine-related intervention [ 50 , 51 , 55 , 57 , 58 , 63 , 64 , 67 , 68 , 78 , 94 ], and 12 papers were about pregnant and/or lactating women’s participation in interventional clinical trials generally [ 39 , 42 , 43 , 52 , 54 , 65 , 71 , 74 – 76 , 91 , 95 ].
Twenty-five papers included perspectives of pregnant women [ 38 , 45 , 47 , 48 , 51 , 57 , 58 , 60 , 61 , 64 , 65 , 67 , 71 – 75 , 77 , 85 , 89 – 91 , 94 , 95 , 97 ], 28 papers included perspectives of postpartum women [ 39 – 41 , 44 – 46 , 49 , 51 , 56 , 57 , 59 , 62 , 63 , 69 – 71 , 74 , 79 – 87 , 92 , 95 ], and 14 papers included health workers’ perspectives [ 44 , 47 , 50 , 52 – 54 , 61 , 64 , 65 , 67 , 87 , 88 , 91 , 94 ]. For other stakeholder groups, please refer to Table 1 .
Methodological limitations of included studies
Assessments of methodological limitations of the included studies are available in S7 Appendix . Across qualitative studies, the most common methodological limitations concerned recruitment approaches and strategies, descriptions of analytical methods, ethical considerations, specifically steps or precautions taken to protect from loss of privacy and confidentiality, data security and integrity, and most studies did not include a reflexivity statement. Across quantitative studies, authors rarely reported on indicators of sample representativeness of the target population, most did not report on or were judged at high risk of nonresponse bias, and ethical considerations pertaining to data security and integrity were frequently missing. For the 3 mixed-methods studies, limitations were identified at the level of integrating methodological approaches at the methods, interpretation, and reporting levels.
Themes and findings from the qualitative and quantitative evidence synthesis
We developed 5 overarching themes and 24 review findings in the qualitative evidence synthesis ( Table 2 ):
- interplay between perceived risks and benefits of participation in women’s decision-making (9 review findings);
- engagement between women and the medical and research ecosystems (2 review findings);
- gender norms and decision-making autonomy (3 review findings);
- factors affecting clinical trial recruitment (7 review findings); and
- upstream factors in the research ecosystem (3 review findings).
https://doi.org/10.1371/journal.pmed.1004405.t002
We graded 6 review findings as high confidence, 11 as moderate confidence, and 7 as low confidence. An explanation for each GRADE-CERQual assessment is presented in the evidence profile ( S4 Appendix ).
Interplay between perceived risks and benefits of participation in women’s decision-making
Findings 1 to 9 are categorised under this theme with 48 studies exploring women’s perspectives on clinical trial participation and factors influencing their decision-making. These factors include balancing risks and benefits, experiences and expectations of high quality care, understanding of study design features, acceptability and stigma associated with the intervention, altruistic motivations and financial incentives.
Finding 1 : Women have a limited appetite and higher perception of risk during pregnancy or lactation . Perception of risks influenced pregnant and lactating women’s willingness to participate in trials, which varied based on their individual levels of risk tolerance, previous trial experiences, observations of others’ experiences, stage of pregnancy or lactation, existing health conditions, and a sense of responsibility for their health and that of the fetus/infant. Women were more likely to decline participation if the experimental intervention was previously untested and were more confident to participate when convinced of no harm (high confidence) [ 39 , 40 , 47 , 48 , 57 , 58 , 60 , 63 – 65 , 69 , 72 , 74 , 83 , 84 , 87 , 89 , 91 , 92 , 96 ].
The most salient factors affecting perceptions of risk were concerns of potential harm to the fetus or baby, including in the longer term, and fears of side-effects [ 39 , 48 , 57 , 58 , 60 , 63 , 69 , 72 , 74 , 83 , 84 , 87 , 89 , 91 , 92 , 96 ]. The uncertainty of these negative outcomes contributed to women’s reluctance to take medications [ 48 , 64 , 69 , 72 ] or participate in experimental interventions, with some likening the experience to being treated as “guinea pigs” [ 39 , 56 , 58 , 69 , 90 ]. Women willing to consider participation wanted proof of safety from previous research evidence [ 57 , 58 , 84 ], online resources [ 96 ], discussions with research staff and health workers [ 96 ], and knowing the experiences of others who had taken the intervention [ 47 , 96 ].
Quantitative evidence supported the qualitative findings that women were apprehensive about taking an experimental product during pregnancy or lactation [ 79 ] primarily due to concerns of fetal or infant harm [ 38 , 51 , 67 , 71 , 75 , 83 , 94 , 95 ], side-effects [ 77 , 80 ], and the possibility of unknown longer-term negative sequelae [ 67 , 75 , 77 ]. Prior knowledge of the health condition [ 68 ], information about drug safety in pregnant and nonpregnant populations [ 51 ], and information that large numbers of pregnant women had already enrolled in the trial [ 67 ] were factors that increased willingness to participate.
Finding 2 : Making trade-offs between risk and severity of the condition and risk-benefit ratio of intervention . Before participating, women weighed the risk of their medical condition and its impact, especially on the baby, against the risks of an intervention and its potential benefits. Women were less likely to participate if they felt healthy or perceived themselves at low risk of experiencing or being negatively affected by the condition, believed they had nothing to gain from participating, or felt concerned that the intervention risks were too high (moderate confidence) [ 39 , 48 , 57 – 60 , 63 , 64 , 69 , 72 , 74 , 87 , 91 , 96 ].
Women were more willing to participate when they had concerns about their risk factors [ 70 ], had previously experienced the condition [ 48 , 70 ], or personally knew someone who had [ 48 ], were anxious about the baby suffering health problems [ 57 – 60 ], or perceived the intervention to be helpful based on past use [ 87 ], or the only course of action to avoid (further) ill-health [ 57 – 59 , 63 , 91 ]. For some women with preconceived notions that research entailed significant risks, their perceptions did not change in the presence of information, including about intervention safety [ 48 ].
Quantitative evidence supported the qualitative findings that, when coupled with risks that were considered minimal or manageable [ 83 ], women with greater knowledge about [ 83 ] or direct exposure to the condition [ 94 ] were more likely to participate in a vaccine or therapeutic trial. However, prior exposure to the medical condition did not consistently lead to higher participation in trials [ 51 ].
Finding 3 : Benefits to health arising from participation . A key motivating factor for pregnant and lactating women to participate in trials was the expectation of personal health benefits, such as improved knowledge about how the condition affected them, protecting their fetus or infant from harm, and reducing mother-to-child disease transmission. When women saw the potential for these benefits, deciding not to participate was viewed as potentially putting the baby’s life at risk (high confidence) [ 40 , 47 , 55 , 60 , 61 , 63 , 64 , 70 , 73 , 83 , 84 , 87 , 90 – 92 , 96 ].
Quantitative evidence supported this finding that women were more willing to participate in a trial when they were convinced about the potential short and longer-term benefits of the intervention for the health of the fetus [ 38 , 51 , 75 , 77 , 80 ], and their own health [ 38 , 41 , 51 , 75 , 80 , 95 ] and education [ 41 , 95 ].
Finding 4 : Experiences and expectations of high-quality care motivate participation . Pregnant and lactating women were motivated to participate as a token of appreciation to health workers who provided good quality care. Additionally, women were more likely to participate when they perceived that it would result in higher quality clinical care or access to vaccines or therapeutic products that had previously been denied or were otherwise not accessible outside the context of a trial (high confidence) [ 39 , 48 , 49 , 60 , 63 , 70 , 72 , 83 , 84 , 86 , 87 , 92 , 96 ].
In addition to free medications and vaccines, women’s perceptions of higher quality care were linked to greater frequency of diagnostic and monitoring tests [ 72 , 83 , 84 , 92 ], detailed information regarding care provided [ 63 ], and closer and continuous clinical observation [ 49 , 63 , 70 , 92 ]. Occasionally, women perceived care associated with a trial as lower quality due to the “experimental” nature of the intervention [ 39 ].
Quantitative evidence supported the qualitative finding that women expected trial participation to engender more and better quality care through enhanced monitoring [ 38 , 41 , 67 , 68 , 80 ], more tests [ 67 ], better therapeutic treatment [ 38 , 49 ], and the general feeling of being provided a high standard of medical care [ 51 , 75 , 80 ].
Finding 5 : Knowledge of the rationale for study design features . The rationale behind certain trial design features such as randomisation, blinding or inclusion of a placebo arm could be a source of confusion, concern, or reassurance for potential participants, impacting their decisions to participate. These features could be viewed as preferential treatment of one group over another, adding burden with little opportunity for personal benefit, a mechanism to reduce bias or conversely for researchers to avoid accountability for an adverse outcome (moderate confidence) [ 39 , 40 , 45 , 59 , 62 , 63 , 69 , 72 , 74 , 87 , 91 , 92 ].
Quantitative evidence extended understanding of women’s views about participation in placebo-controlled trials. Some women expressed reluctance to participate due to the possibility of being assigned to the control or placebo group [ 67 , 77 , 79 , 83 ]. However, others expressed that the uncertainty of assignment would not affect their decision, and for a minority, the possibility of assignment to the control condition motivated their participation as it could minimise risk but still provide ancillary benefits [ 67 ]. Women were keen to be unblinded regarding the arm to which they were assigned, once the trial was complete [ 80 ].
Finding 6 : Acceptability of the intervention is key to pregnant and lactating women’s willingness to participate in a trial and for research staff to recruit for a trial . Interventions that were most acceptable to women and research staff were those that simplified intervention delivery, were less onerous or painful than usual care, had negligible risk, were noninvasive, placed limited demands on time, did not involve invasive procedures, and where prior knowledge about the condition intersected with positive attitudes towards the therapeutic product (high confidence) [ 40 , 45 , 48 , 53 , 54 , 61 , 64 , 65 , 72 , 73 , 81 , 83 , 86 , 87 , 90 – 92 , 96 ].
For health workers involved in recruitment and trial operations, acceptability of the intervention was closely linked to their perceptions of the safety of the experimental therapy, derived from previous positive experiences administering the drug in a different clinical setting [ 53 ].
Quantitative evidence supported this qualitative finding that some women might be more willing to participate in a trial when they were less likely to be inconvenienced by or experience discomfort from trial procedures, additional and lengthy study visits [ 38 , 41 , 80 ]. Decliners cited blood tests, additional scans, and availability of suitable noninvasive alternatives as reasons for nonparticipation [ 51 , 83 ]. In the case of vaccine trials, quantitative data extended this qualitative finding by suggesting that women indicated greater acceptability of inactivated virus vaccines compared to live-attenuated virus vaccines [ 51 ].
Finding 7 : Fears around data sharing and use . Some women feared that trial participation, including provision of blood samples, could expose them to stigmatisation and judgement due to unwanted diagnoses and disclosure of disease status, data sharing regarding sensitive behaviours, and the threat of their data being used in ways that would compromise confidentiality and safety (low confidence) [ 65 , 85 , 86 ]. In the context of HIV trials, some women discussed concerns that an HIV diagnosis would lead to abandonment by their husbands [ 85 ].
No quantitative evidence was identified in this domain.
Finding 8 : Altruistic motivations . Pregnant women expressed willingness to participate in trials for the purpose of contributing to societal benefits of research, including the potential to improve health and healthcare for pregnant women in the future. Altruistic motivations could act as a stand-alone stimulus, secondary to or alongside beliefs around personal benefit, or conditional on no additional risk for participation (moderate confidence) [ 39 , 40 , 47 , 48 , 55 – 61 , 63 , 64 , 70 , 72 – 74 , 83 , 86 , 87 , 89 , 91 , 92 ].
In addition to helping other women, altruistic sentiments were linked to perceptions that the research effort was worthy [ 48 , 59 , 61 ], well-intentioned [ 61 ], filled an important scientific gap [ 58 , 70 , 72 ], and addressed a pressing need [ 48 , 63 , 73 , 91 ].
Quantitative evidence supported the qualitative finding that altruistic motivations influenced willingness to participate in trials, alongside personal benefits [ 38 , 41 , 49 , 51 , 67 , 77 , 80 , 95 ]. Women expressed having a sense of fulfilment that participation would have a positive impact on women’s health in the future.
Finding 9 : Financial incentives . Pregnant and lactating women had mixed attitudes to financial incentives for research participation. Some viewed financial incentives as acceptable, with higher remuneration as an appropriate strategy to encourage participation, whereas others viewed financial incentives as potentially coercive, especially in the context of poverty. Some women felt that financial reimbursements did not play a substantial role in women’s decision-making (low confidence) [ 39 , 55 , 65 , 83 , 96 ].
Negative views on renumeration arose from concerns that financial incentives would entice women to enrol multiple times [ 65 ], or make it challenging for them to withdraw from the study [ 39 ].
Quantitative evidence extended this qualitative finding by suggesting that attitudes to financial compensation differed based on levels of education attainment [ 97 ]. In one study, less than 1 in 10 women discussed that financial incentives would increase their likelihood of participation in medication or vaccine-based research [ 75 ], whereas in another, 4 in 10 women agreed that they volunteered to participate due to financial compensation [ 41 ].
Engagement between women and the medical and research ecosystems
Findings 10 and 11 are categorised under this theme, with 34 contributing studies examining factors operating at the intersection of women and the medical and research ecosystems. The factors include women’s reliance on health workers’ clinical opinions to assist decision-making, and the role of therapeutic hope and optimism in women’s decisions to participate and health worker and research staffs’ motivations to administer trials.
Finding 10 : Roles of trust and power in the medical and research ecosystem . Pregnant and lactating women’s willingness to participate in trials was driven by trust, confidence, and faith in medicine and research, and women relied on the opinions of the health workers that they consulted with regarding the efficacy and safety of the intervention. Simultaneously, power imbalances between women and health workers, coupled with women’s therapeutic misconceptions, could lead to coercion in participation. This ethical dilemma was recognised by study investigators, ethics committee members, and women, especially in the context of the dual roles of clinician-researchers; however, power and credibility when combined with good rapport and clear communication generated trust to participate or comfort to decline. While rare, some women had larger concerns about the vested interests of pharmaceutical companies (high confidence) [ 39 , 40 , 42 – 45 , 47 – 49 , 56 – 61 , 65 , 69 , 70 , 72 – 74 , 81 , 82 , 86 , 87 , 89 , 91 , 92 ].
Quantitative data supported the qualitative finding that trust (or lack thereof) in health workers, research teams, and pharmaceutical companies affected participation [ 38 , 51 , 75 , 95 ]. Some women felt pressured to participate by health workers and were disappointed by the lack of an individualised approach to recruitment [ 80 ]. Among decliners of a vaccine trial, some noted that recommendations from a health worker could motivate a change of mind [ 51 ].
Finding 11 : The role of therapeutic hope and optimism . Therapeutic hope and optimism played a critical role for health workers and research staff to administer trials, and for pregnant and lactating women to participate in trials. Prior knowledge about and experience with using the intervention, observation of potential beneficial effects, and trust in health workers shaped feelings of therapeutic hope and optimism. However, for some women, a lack of understanding of the differences between research and clinical care when combined with therapeutic hope led to therapeutic misconceptions and unmet expectations about the personal benefits arising from trial participation (moderate confidence) [ 42 , 45 , 47 , 53 , 65 , 70 , 74 , 81 , 82 , 87 ].
Health workers expressed the importance of women and themselves comprehending the differences between research and clinical care to minimise participation arising from therapeutic misconceptions [ 47 ].
Gender norms and decision-making autonomy
Findings 12 to 14 are categorised under this theme with 24 contributing studies discussing women’s roles as mothers and caregivers, mixed perceptions of women’s autonomous decision-making, and intimate male partner involvement in decision-making.
Finding 12 : Expectations of women’s roles as mothers and caregivers . Pregnant and lactating women’s decisions to participate in clinical trials were often influenced by their strong sense of responsibility towards the health and care of their fetus or infant, themselves, and their families. This sense of responsibility was endorsed and reinforced by familial and societal expectations of what it means to be a good mother (low confidence) [ 60 , 61 , 64 , 91 , 96 ].
For some women, this responsibility to protect their baby translated to not engaging in any actions that might risk jeopardising the baby’s health [ 91 ].
Finding 13 : Role of bodily autonomy in decision-making . Some women, health workers, ethics committee members, and regulators perceived that pregnant women might not be able to make decisions by themselves about trial participation due to fetal involvement, inability to make rational choices during pregnancy, hormones, the stressful context of hospitalisation and financial inducements. However, research staff and some women believed in the right to bodily autonomy to make decisions by themselves despite having discussions with partners, family members, support persons, or health workers. Women viewed other people making decisions regarding their participation as a violation of this right, though some women declined participation due to pressure from family members (moderate confidence) [ 39 , 40 , 43 , 47 , 54 , 56 , 72 , 74 , 81 , 82 , 85 , 87 , 90 , 92 ].
Women also believed that research could be an avenue through which women demanded their rights in the healthcare [ 65 ].
Quantitative evidence supported qualitative findings that women believed in their capability to make decisions regarding trial participation, with some doing so autonomously and others receiving support from family members [ 38 , 83 ].
Finding 14 : Relationship dynamics , gender roles , and norms are key to women’s attitudes to partner involvement and paternal consent . Pregnant women often discussed the benefits and risks of trial participation with their partners—especially in the context of fetal involvement—and their final decision may or may not have been influenced by their partners’ own attitudes. In some settings, pregnant women’s trial participation was contingent on partners’ buy-in, and the formality justified in the context of gender norms and roles. These could be the partner being the household head, to allay men’s suspicions about women’s whereabouts and interactions, and to minimise any misunderstanding related to positive tests or disease status that might cast doubt on women’s fidelity to their husbands (moderate confidence) [ 39 , 40 , 42 , 43 , 47 , 60 , 64 , 65 , 69 , 72 , 74 , 81 , 83 , 85 , 87 , 90 , 91 ].
Partner involvement was not preferred when that partner was abusive or uninvolved, or when a woman was unmarried, or the pregnancy had occurred in the context of rape [ 85 ]. Furthermore, imposing a paternal consent rule in these circumstances was a serious barrier to participation [ 85 ]. When research participation violated gender roles and norms, it sometimes resulted in partner violence, marital breakdown, or rejection of the baby [ 85 ].
Factors affecting clinical trial recruitment
Findings 15 through 21 are categorised under this theme with 41 contributing studies exploring the importance of cultural acceptability and safety of intervention procedures, development of reciprocal relationships between research staff and health workers, the importance of resource availability, trial visibility and emotional orientations, and woman-centred approach to recruitment.
Finding 15 : Developing trusting and reciprocal relationships with the community as part of the research process . Designing and embedding research within communities required engaging with community norms, beliefs, and practices. Some community members expressed how they viewed research negatively in the context of historical and ongoing oppressions that people experience due to colonisation, corruption, extractive practices, and civil and political conflict. Central to the acceptability and cultural safety of the research were investments in developing trusting relationships with community representatives and leaders (moderate confidence) [ 44 , 45 , 60 , 65 , 66 , 74 , 78 , 83 , 90 , 92 ].
This was achieved through dialogue and engagement starting at research conceptualisation, collaborating with community representatives and previous research participants to develop communication and mobilisation strategies, providing accurate information about study procedures, and ensuring alignment of these procedures with community norms, beliefs, and practices.
Finding 16 : Increasing visibility and awareness of the trial . Increasing visibility and awareness of the trial to potential participants, health workers, and community representatives influenced trial recruitment. Recommended strategies included paper and electronic promotional materials, regular physical presence of research staff in the areas where recruitment was taking place, and reminders to health workers about recruitment pathways and trial protocols through trainings (low confidence) [ 54 , 62 , 65 , 74 , 87 ].
Quantitative evidence extended the qualitative finding that women preferred to have information about trials through their health workers [ 67 ].
Finding 17 : Inadequate resources . Inadequate physical infrastructure, time, finances, and insufficient quantity and quality of human resources were barriers for research staff to recruit women for clinical trials. For health workers specifically, heavy workloads made it challenging to incorporate trial recruitment into clinical workflows, and the added burden and sometimes insufficient compensation, contributed to poor morale (low confidence) [ 44 , 54 , 55 , 62 , 87 , 89 ].
In terms of competency of human resources, research staff shared that their recruiting capability was built through practice and working alongside more experienced colleagues [ 54 ]. A key limiting factor in the recruitment of women from non-English speaking backgrounds was the unavailability of interpreters [ 87 ].
Quantitative evidence similarly reported that lack of infrastructure and limited time due to heavy workloads for health workers were barriers to including pregnant women in trials [ 50 , 67 , 88 ].
Finding 18 : Engaging health workers in trials . Research staff perceived the importance of building reciprocal and collaborative relationships with health workers because some acted as gatekeepers. Some health workers, however, were reluctant to engage women in clinical trials due to a lack of knowledge about trial design and the research value, varying levels of acceptability of risk, perceived obligation to protect women, and a lack of trust in the research team. Health workers supported inclusion when trial protocols included close monitoring of risks and when there was clinical equipoise alongside therapeutic hope in the trial intervention. These factors were informed by their clinical knowledge, previous clinical experiences using the intervention, and observed outcomes in the current trial (high confidence) [ 47 , 53 – 55 , 60 , 62 , 64 , 65 , 87 , 89 – 91 ].
Quantitative evidence supported qualitative findings that knowledge of the relevance, feasibility, and ethical obligations to include pregnant and lactating women in trials, perceptions that pregnant women were a vulnerable population, lack of interest in trials, and preferences for noninvasive treatment were factors influencing whether health workers encouraged pregnant women’s clinical trial participation [ 50 , 52 , 67 , 88 , 94 , 95 ].
Finding 19 : Research staff’s emotional orientations towards clinical trials . Having a sense of trial ownership, supportive teamwork, a shared sense of team achievement and motivation to achieve recruitment targets could support successful trial recruitment. However, feeling pressured by the recruitment process, seeing it as a procedural activity and needing to implement complex study designs impacted research staffs’ ability to recruit women, leading to frustration and lower enthusiasm (low confidence) [ 53 , 54 , 62 ].
Finding 20 : Women-centred approach encourages participation . Women valued an individualised, humanised, and transparent approach to communication, and adequate time during trial recruitment to discuss details and concerns related to the trial. These helped ensure they had sufficient capacity and opportunity to make informed decisions. Similarly, research staff found that approaching potential participants at the “right time” and in an appropriate manner by considering their physical and mental state, providing adequate information and engaging in discussions increased recruitment success (moderate confidence) [ 39 , 40 , 54 , 56 , 62 , 66 , 69 , 70 , 72 , 74 , 86 , 87 , 92 ].
To support an individualised recruitment approach, research staff reviewed obstetric information from women’s charts [ 54 , 86 ] and had discussions with health workers [ 86 ] to tailor the recruitment information to women’s personal situations. They also discussed using intuition to determine when and whom to approach for trial participation [ 54 ], considering the extent to which women looked sick or unwell at the time of recruitment [ 86 ].
Quantitative data supported this qualitative finding of women noting the significance of having detailed and well-explained trial information, including about risks and benefits, and adequate time to make decisions regarding participation [ 80 , 95 ]. Some women expressed disappointment when they felt they had been ill-informed about study procedures by research staff [ 80 ].
Finding 21 : Recruitment for intrapartum research . Pain, intensity, and duration of labour motivated pregnant women to participate in intrapartum clinical trials. However, women, their partners, and research staff recognised the challenges in ensure women make informed decisions during this sensitive time, as decisions had to be made quickly, and partners were reluctant to make decisions on women’s behalf, even during emergencies, due to fears of negative outcomes. To optimise women making informed decisions, research staff provided information clearly and succinctly during the intrapartum period and tried to offer adequate time for decision-making. Most women recommended having trial information provided in the antenatal period, and revisiting trial details, including having a de-briefing about one’s own experience, prior to discharge (moderate confidence) [ 43 , 49 , 56 , 59 , 61 , 62 , 81 , 82 , 86 , 91 ].
Quantitative data extended this qualitative finding with most ethics committee members considering consent in-labour as ethical. Factors that ethics committee members considered when approving labour trials, included the level of risk involved and women’s ability to provide informed consent [ 76 ]. Most ethics committee members also supported the involvement of partners in the consent process [ 76 ]. Aligned with the qualitative data, women expressed a preference to be approached for a labour trial earlier to have adequate time for discussion and an informed decision [ 79 , 80 ].
Upstream factors affecting the research ecosystem
Findings 22 to 24 are categorised under this theme with 13 studies discussing factors operating at the level of study investigators, ethics committees, and funders. The factors include study investigators’ personal and professional motivations to pursue research with pregnant women, complexities in obtaining ethical approval, and limited interest of funders to support clinical trials with pregnant and lactating women.
Finding 22 : Factors affecting motivation of study investigators . The underlying factors that motivated many study investigators to conduct research with pregnant women were ethical responsibility, passion towards equity, and dedication to improving women’s health status and care, and filling scientific gaps. Additionally, lived experience of being pregnant, having mentors in this area in early careers, and previous research experiences with pregnant women contributed to study investigators’ motivations. However, concerns about risks of teratogenicity demotivated some investigators (moderate confidence) [ 42 , 43 , 66 , 78 , 89 , 91 ].
Finding 23 : Challenges in gaining ethical approvals for trials with pregnant women . While some regulators, ethics committee members, and study investigators strongly support inclusion of pregnant women in clinical trials, most stakeholders start from a presumption of minimal risk to the fetus. This results in women’s exclusion, especially in the context of poor public stewardship, ambiguous guidelines, insufficient data on intervention safety, complexities and subjectivities in risk assessment, poor agreement on appropriate trial design, time-consuming ethical processes, and concerns about reputation (moderate confidence) [ 42 , 43 , 66 , 78 , 82 , 89 – 91 ].
Study investigators and ethics committee members reported that these challenges could be overcome through shared institutional commitment to pregnant women’s inclusion, close collaboration between investigators and ethics committee members from protocol inception, mutual understanding about each other roles, responsibilities, and intentions, development and implementation of practical guidance for consistency in regulatory interpretation and risk assessment, safety monitoring during implementation, and safeguards for injury compensation [ 42 , 66 , 78 , 89 , 91 ].
Quantitative evidence supported qualitative findings that obtaining regulatory approval for clinical trials that include pregnant women was challenging [ 88 ] due to ethics committees’ preference for observational studies over trials [ 93 ], and varied opinions on the inclusion of pregnant women and what constituted minimal risk [ 76 , 93 ]. Most ethics committee members were also aware that they did not have adequate policy or guidance to inform their decisions to ensure equitable subject selection [ 76 , 93 ].
Finding 24 : Role of funders . Limited interest of public and private funders and pharmaceutical companies to financially invest in trials due to the ethical complexities, potential for adverse events, liability, and possibility of political fallout was a barrier to conduct trials with pregnant and lactating women. When funding was available, funders’ requests might facilitate the inclusion of pregnant women or create ethical challenges in conducting trials (low confidence) [ 54 , 62 , 66 , 78 ].
Mapping review findings to TDF, COM-B, and potential implementation strategies
Table 3 presents the mapping of review findings to the applicable TDF [ 24 ] and COM-B model domains [ 25 ], and the BCW intervention types to inform proposed strategies that address these factors. The strategies that we have identified are designed to provide a theoretically informed guide to the types of actions that can be taken to address barriers at various levels associated with different stakeholder groups. Which actions are appropriate for a given context should therefore be discussed, prioritised, and adapted to a particular setting.
https://doi.org/10.1371/journal.pmed.1004405.t003
Some of these strategies may already be in place as part of ethical conduct for trial recruitment, for example, sharing information transparently with potential participants about safety, risks, benefits, and side effects of the trial intervention (BCW intervention type: education). Given pregnant and lactating women’s concerns around risks of the intervention, such strategies can be enhanced through personalised discussions about how the intervention relates to women’s personal and clinical circumstances, for example, using a decision-aid tool (BCW intervention type: enablement). Developing clear and context-specific ways to explain study design features in plain language, and involvement of trusted sources (such as health workers), to communicate trial information can aid the decision-making process. Engaging with patient advocates and women’s groups and conducting formative research with potential participants to receive feedback on acceptability of trial components can streamline trial procedures and enhance acceptability and contextual alignment. Considerations should include how societal and gender norms, and gender roles impact various aspects of participation.
Given potential concerns among health workers regarding safety of interventions during pregnancy, providing access to credible resources on the risks, benefits and potential side-effects of the product being trialled, and elaborating on the trial rationale, potential benefits, and where the trial fits into existing evidence can help address fear and uncertainty regarding intervention safety (BCW intervention type: education, training).
At the health systems-level, strategies include creating a research-friendly environment within health facilities. In addition to promoting buy-in from hospital leadership, this would include infrastructural enhancements such as creating research spaces within health facilities (e.g., offices, meeting rooms, labs, data storage, research information systems), and hiring and training research support staff (e.g., research midwives), among other aspects.
Strategies to promote alignment between study investigators and ethics committee members include: educating ethics committee members about the health consequences of excluding pregnant women from research, and useful approaches for monitoring and managing risks associated with trial inclusion (BCW intervention type: education); developing a shared institutional commitment to inclusion of pregnant women research as the standard, and developing a common understanding of regulatory guidelines and associated documentation such as standard operating procedures, worksheets, and checklists to facilitate consistency in guideline application by institutional ethics committees and researchers.
This review provides a comprehensive overview of the range of factors affecting the participation of pregnant and lactating women in clinical trials across the research ecosystem. At the upstream levels, we identified barriers arising from limited interest of funders to invest in clinical trials with pregnant and lactating women, and reluctance of ethics committees to approve protocols due to potential for risks, particularly to fetal health. Factors at the interface between health systems and communities included developing trusting and reciprocal relationships among community members, research staff, and healthcare workers, and taking a woman-centred approach to recruitment. For women, determining the risk-benefit ratio of participation, trust (or lack thereof) in medicine and research, the potential to access high-quality care through trial participation, and altruistic motivations were key factors. Incorporating a gender lens to the data, we found that participation was impacted by gender relations of power sustained by gender norms, gender role expectations of women as mothers and caregivers, and mixed opinions regarding bodily and decisional autonomy during pregnancy.
Our findings on factors influencing pregnant women’s decisions regarding participation are aligned with those identified by Van der Zande and colleagues [ 98 ] who found that the potential for personal benefits alongside altruistic motivations were crucial drivers, while participation burdens, risks, and mistrust in research were key barriers to participation. Some of these findings, such as the role of altruism and potential for personal benefit, concerns about randomisation and other study design features, burdensome trial procedures, fears associated with taking an experimental therapy, and health worker attitudes towards trials are also consistent with the broader literature on factors associated with trial participation [ 99 – 101 ]. Across the findings, women and research staff emphasised the importance of a woman-centred approach to trial recruitment, with careful consideration of women’s individual clinical and personal circumstances, transparency in information, and support for informed and unhurried decision-making. These aspects were found to be challenging to navigate in intrapartum trials, given the timing of recruitment coinciding with birth, often in the context of impending or ongoing complications. For example, a recent analysis of uterotonic trials for prevention of postpartum haemorrhage found considerable variability between trials in the timing of informed consent—most obtained consent during labour, with a minority in the antenatal period [ 102 ]. Our findings suggest that women prefer consent in the antenatal period to optimise informed and unhurried decision-making. However, there are ethical concerns about seeking antenatal consent as it may exclude participation of women who do not regularly access antenatal care [ 102 ]. Indeed, the informed consent process in intrapartum trials is an issue of current debate and ethical interest [ 103 ], and more empirical work is needed to understand women’s preferences and needs to optimise informed decision-making.
We found that healthcare workers’ engagement was crucial in recruiting women as they play a vital role in bridging communication between potential participants and research staff. Many studies reported that women relied on health workers advice in making decisions about participation. Health workers in turn encouraged or discouraged participation based on their own attitudes towards clinical research in pregnancy and knowledge about or personal experience using the therapy under investigation. Given the roles of trust and power in women’s decision-making processes, it is important to promote transparent and open communication between women and health workers regarding trials, and their associated risks and benefits [ 104 , 105 ]. It is also important to clarify differences between clinical trial and regular clinical care to minimise the potential for therapeutic misconceptions, the consequences of which could lead to the eroding of trust in the medical system, affecting future health-seeking behaviour.
The complicated issue of autonomy in decision-making during pregnancy was raised by multiple stakeholders. Many women discussed trial participation with their partners and other family members but considered the final decision to be their own. In some settings, usually in the context of rigid gender norms, women required partners’ permission to participate; if violated, this could result in the threat of violence or marital discord. Separately, the imposition of a paternal consent requirement was viewed as a significant barrier for women who were in unstable relationships, unmarried, or wanted to exercise fully autonomous decision-making. Widmer and colleagues [ 102 ] argue that it is the role of research staff to guarantee and protect women’s autonomy. We found that women’s decisional autonomy was impacted by intimate partner relationship dynamics, and wider sociocultural and gender norms that required nuanced understandings of the context and multistakeholder engagement to create an enabling environment for women to exercise choice.
We also identified barriers experienced by researchers, ethics committees, and funders of clinical trials. Study investigators had trouble obtaining ethical approval as ethics committees have mixed perspectives on the inclusion of pregnant and lactating women in trials, particularly in the absence of clear guidelines. In line with previously reported upstream barriers [ 16 , 23 ], limited interest in funding clinical trials with pregnant and lactating women due to potential risks, high liability, and reputational consequences also inhibits the implementation of trials. These findings demonstrate a need to develop holistic strategies addressing barriers experienced by stakeholders operating at the upstream levels of clinical research.
The TDF and COM-B mapping in our review (Table 3) can be used by study investigators, research staff, health workers, ethics committees, and funders to inform the development of implementation strategies to address barriers to pregnant and lactating women’s participation in clinical trials. Formative research to identify specific barriers and facilitators in specific settings and contexts is a recommended starting point before developing appropriate strategies.
A limitation is that we did not include grey literature, which may have expanded the types of evidence and/or contexts of the review. However, our search strategies yielded high coverage of published literature. The studies included in the review had good coverage of countries from the African region, but sparse representation of countries from Latin America, and no representation of countries in the Eastern Mediterranean or South-East Asian regions. A growing number of trials addressing maternal and perinatal health are being implemented in these settings [ 106 ], calling for significantly greater focus in formative and process evaluation research with pregnant and lactating women and people, family members, health workers, local researchers, and ethics committee members to understand context-specific motivations for and concerns regarding conduct of and participation in research during pregnancy and lactation. The AIM-Gender project [ 107 ] aims to address this limitation through qualitative research on the topic in India and Nigeria—2 countries that together account for 37% of global maternal deaths [ 13 ]. Future work must also consider inclusion of pregnancy-capable transgender and nonbinary people, as knowledge gaps regarding factors affecting their participation in pregnancy and lactation clinical research are particularly pronounced. We also draw attention to 2 relevant reviews on factors affecting participation of racial and ethnically marginalised populations in pregnancy and lactation research, a related topic that was beyond the scope of this review [ 108 , 109 ].
Our review builds on previous work [ 98 ] by examining the full range of factors and perspectives of multiple stakeholders operating at the upstream and downstream levels of the research ecosystem. We optimised the available data by including qualitative, quantitative, and mixed-methods primary research. We applied the GRADE-CERQual approach to assess confidence in each finding, i.e., the extent to which the finding adequately represented the phenomenon of interest [ 32 , 33 ]. These assessments have important practical implications for increasing the applicability and usability of these findings by stakeholders seeking to enhance research and development in maternal health. This review additionally integrates the use of behavioural frameworks [ 24 , 25 ] to propose a theory-informed set of behaviour change interventions to address factors affecting clinical trial participation among pregnant and lactating women.
Supporting information
S1 appendix. preferred reporting items for systematic reviews and meta-analyses (prisma) reporting checklist..
https://doi.org/10.1371/journal.pmed.1004405.s001
S2 Appendix. Enhancing transparency in reporting the synthesis of qualitative research (ENTREQ) reporting checklist.
https://doi.org/10.1371/journal.pmed.1004405.s002
S3 Appendix. Search strategies.
https://doi.org/10.1371/journal.pmed.1004405.s003
S4 Appendix. GRADE-CERQual evidence profile.
https://doi.org/10.1371/journal.pmed.1004405.s004
S5 Appendix. Summaries of quantitative findings.
https://doi.org/10.1371/journal.pmed.1004405.s005
S6 Appendix. Characteristics of included papers.
https://doi.org/10.1371/journal.pmed.1004405.s006
S7 Appendix. Critical appraisal.
https://doi.org/10.1371/journal.pmed.1004405.s007
Acknowledgments
We are grateful to Alessandra Fleurent at Concept Foundation for her assistance with verifying the accuracy of the translated French paper included in this review.
- View Article
- PubMed/NCBI
- Google Scholar
- 12. Task Force on Research Specific to Pregnant Women and Lactating Women. Report to Secretary, Health and Human Services, Congress. 2018. Available from: https://www.nichd.nih.gov/sites/default/files/2018-09/PRGLAC_Report.pdf .
- 13. World Health Organization. Trends in maternal mortality 2000 to 2020: Estimates by WHO, UNICEF, UNFPA, World Bank Group and UNDESA/Population Division. Geneva: World Health Organization; 2023. 9240069259.
- 14. UN Inter-agency Group for Child Mortality Estimation. Never forgotten–the situation of stillbirth around the globe. New York: United Nations Children’s Fund; 2023.
- 15. UN Inter-agency Group for Child Mortality Estimation. Levels and trends in child mortality: Report 2022 estimates developed by the United Nations Inter-agency Group for Child Mortality Estimation. New York: United Nations Children’s Fund; 2023.
- 17. Bhattacharya S. Safe and effective medicines for use in pregnancy: A call to action. Birmingham: University of Birmingham, 2021. Available from: https://www.birmingham.ac.uk/documents/college-mds/centres/bctu/21560-policy-commission-maternal-health-report.pdf .
- 18. Concept Foundation. Market challenges and potential solutions for the development and introduction of medicines for pregnancy specific conditions. Geneva Concept Foundation; 2021. Available from: https://www.conceptfoundation.org/wp-content/uploads/2023/04/Maternal-health-medicines-market.pdf .
- 20. U. N. General Assembly. Transforming our world: The 2030 Agenda for Sustainable Development. New York: United Nations; 2015.
- 27. Veritas Health Innovation. Covidence systematic review software [internet]. 2023.
- 28. OpenAI. Chatgpt. 2023.
- 29. Hong QN, Pluye P, Fàbregues S, Bartlett G, Boardman F, Cargo M, et al. Mixed Methods Appraisal Tool (MMAT), version 2018. Registration of copyright (#1148552), Canadian Intellectual Property Office, Industry Canada. 2018.
- 107. Concept Foundation. Accelerating innovation for mothers—AIM Gender. 2023 [cited 2023 Nov 10]. Available from: https://www.conceptfoundation.org/accelerating-innovation-for-mothers/aim-gender/ .
National Cancer Institute - Cancer.gov
Clinical trial researching combination immunotherapy for colorectal cancer

Image credit: Canva
Colorectal cancer that has returned or progressed after treatment and spread to other organs is known as metastatic colorectal cancer (mCRC). Most people with mCRC survive only about two years. A trial led by Jason M. Redman, M.D. , Assistant Research Physician in the Center for Immuno-Oncology , is studying combination immunotherapy, including a tumor-targeted vaccine, for the disease. The trial includes two mandatory biopsies.
The trial will take place at the NIH Clinical Center in Bethesda, Maryland, and there is no cost for participation.
For more information, please contact the NCI Medical Oncology Referral Office at (888) 624-1937 or [email protected] .
Clinicaltrials.gov identifier: NCT06149481
NCI Protocol ID: IRB001563
Official Title: Phase I/II Study of the Combination Immunotherapy Regimen: SX-682, TriAdeno Vaccine, Retifanlimab and IL-15 Agonist N-803 (STAR15) for Metastatic Colorectal Cancer (mCRC)
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow, treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here , and subscribe to have the latest CCR clinical trials sent directly to your inbox.

Related News

Clinical trial researching combination therapy for solid tumors
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.25(4); 2020 Jun

Clinical research nursing and factors influencing success: a qualitative study describing the interplay between individual and organisational leadership influences and their impact on the delivery of clinical research in healthcare
Linda tinkler.
Trust Lead, Nursing, Midwifery and AHP Research, Newcastle upon Tyne Hospitals NHS Foundation Trust, Freeman Hospital, Newcastle upon Tyne, UK
Lisa Robinson
Consultant Allied Health Professional, Major Trauma Rehabillitation Department, The Newcastle upon Tyne Hospitals NHS Foundation Trust, Royal Victoria Infirmary, Newcastle upon Tyne, UK
Clinical research delivery is a term increasingly used to describe the work undertaken to implement studies which explore and test prevention, diagnosis and treatment in healthcare. Such studies range from multi-site clinical trials to single site observational projects. Whilst widely acknowledged as fundamental to effective healthcare, clinical research is complex to deliver and is met with challenges in the busy clinical environment. The perceptions and experiences of Clinical Research Nurses, whose work is central to this agenda, have emerged as important, yet frequently overlooked insights in relation to the potential success of research delivery. Increased understanding of these insights is essential to address the barriers and maximise facilitators to success in clinical research.
To better understand how the perceptions and experiences of Clinical Research Nurses relate to potential for success in research delivery.
Seven focus groups and two one-to-one interviews (27 participants) were conducted in a large tertiary North East England National Health Service Foundation Trust between March and June 2017.
Clinical Research Nurses’ described perceptions and experiences of working to co-ordinate and deliver a range of research as individuals, within their teams, throughout the wider organisation, and beyond. Two key elements situated within an overarching theme of leadership emerged as likely to impact on how successfully they were able to practise:
• the individual (self-leadership).
• the organisation (leadership culture).
Conclusions
The framework emerging from this study illustrates a complex interplay between personal attributes and organisational culture, mediated by national strategies and targets associated with the clinical research agenda. When situated within the concept of leadership, it broadens the potential for understanding the underlying issues and increases the range of possible support mechanisms to improve experiences for Clinical Research Nurses. Framing the challenges in this way contributes new knowledge to the dialogue surrounding clinical research delivery.
Introduction
The successful delivery of clinical research is fundamental to enabling continuous improvements in health ( Cooksey, 2006 ; Department of Health, 2017 ). Research-active organisations report better patient outcomes and research activity and engagement at all organisational levels is fundamental to high-quality care provision. The Care Quality Commission (CQC) now enables NHS organisations to showcase research engagement as part of its inspection programme ( CQC, 2017 ; Ozdemir, 2015 ).
Trial recruitment challenges are relevant to the entire healthcare community and increasingly cited in the literature ( Adams et al., 2015 ; Donovan et al., 2014a , b , 2016 ; Elliot et al., 2017 ). The Clinical Research Nurse (CRNurse) workforce is a key element of successful research delivery and an integral part of the research journey for patients. The perceptions and experiences of their role and the content and context of their work are emerging as an important marker in understanding the complexities of trial delivery ( Adams et al., 2015 ; Gibbs and Lowton, 2012 ; Hamer, 2017 ; Hill, 2018; Jones, 2017 ; Kunhunny and Salmon, 2017 ; MacArthur et al., 2014; Tinkler et al., 2018 ; Whitehouse and Smith, 2018 ).
Previous research from our team explored the role and the ethical perceptions of Clinical Research Nurses (CRNurses) specifically in relation to their roles, undertaking study recruitment and the informed consent process ( Tinkler et al., 2018 ). The findings emerging from that study identified numerous factors that could adversely impact on successful research delivery. Factors included a range of intrinsic and extrinsic issues, such as role transition and the conflict between core clinical values and perceived pressure to recruit. Altered relationships with multidisciplinary colleagues and perceptions of how the CRNurses were viewed by the wider organisation led to reduced morale and contributed to staff turnover. Kunhunny and Salmon (2017) reported similar findings in relation to issues undermining the professional identity of CRNurses and role-related challenges. The work of Whitehouse and Smith (2018 ) has highlighted the need for clearer CRNurse strategies and structure, and further research into professional identity, understanding of the role by multi-professional team members and work to increase the evidence base regarding the role and impact.
The recent National Institute for Health Research (NIHR) CRNurse strategy has further highlighted the need for visible leaders in clinical research nursing along with the imperative to demonstrate the impact of the CRNurse in the patient journey. The International Association of Clinical Research Nurses (IACRN) embodies similar aims through their Scope and Standards of Practice ( American Nurses Association and International Association of Clinical Research Nursing, 2016 ). As such clinical research nursing is recognised as specialty nursing practice by the American Nurses Association, though this status is not the case in the UK.
With increasing interest and expertise emerging in this area, there is an opportunity to influence policy and practice in relation to the CRNurse role, whilst contributing to the dialogue on trial recruitment challenges through a focus on self-leadership and organisational culture ( Jones, 2017 ; Whitehouse and Smith, 2018 ).
Methodology
A qualitative study design used focus groups as the primary method of data collection. Focus groups maximise the benefit of group interaction and generate data that highlight underlying factors influencing the feelings, attitudes and behaviour of participants ( Kitzinger, 2006 ; Krueger and Casey, 2015 ). Focus groups were also chosen due to their time effectiveness for busy clinical staff. One-to-one interviews were added approximately one month into data collection in response to requests from potential participants. The lead author (LT) adopted a constructivist approach, facilitating participants to share their experiences, focusing on enablers and barriers to their practice as CRNurses, in a supportive, confidential environment ( Mills and Birks, 2014 ).
Sample/participants
The host organisation for this study is one of the largest NHS foundation trusts in the UK, with a sustained successful track record in clinical research delivery; 108 CRNurses were employed by the organisation at the time of study recruitment. These individuals were invited to participate, via email of the participant information sheet (PIS) from team leaders. The email invited potential participants to opt in by returning an expression of interest form via email to LT. The email stated that involvement was voluntary and that a decision not to take part would not affect subsequent interactions or relationships with the researcher or the organisation. Participants were given up to four weeks to decide on participation and were free to ask questions prior to making a decision. LT attended organisational meetings to present the study and its background, however participants were not known to LT prior to study commencement.
Data collection
Data were collected during the working week between March and June 2017. Focus groups were conducted at one hospital site, utilising two dedicated meeting rooms situated away from the clinical area to reduce interruptions. One-to-one interviews were conducted at a venue of the participants’ choice in the workplace. Each focus group comprised of between two and seven participants and lasted approximately two hours. Interviews lasted approximately one hour and 30 minutes. All interviews and focus groups were digitally audio-recorded with participants’ consent and transcribed verbatim by an independent transcriber approved by the study sponsor. LT, an adult nurse qualified to Masters’ Level, with 14 years’ clinical experience, (nine of these in designated research roles) moderated all focus groups and interviews. Open-ended questioning was supported by a topic guide ( Table 1 ).
Topic guide.
Data analysis
Data were analysed systematically by LT using an inductive approach which incorporated thematic analysis ( Braun and Clarke, 2006 ) supported by NVivo 11 qualitative data analysis software. Thematic analysis enabled an iterative process of engagement with the data through familiarisation by repeatedly listening to recordings and comparing with transcripts, generating initial codes and organizing data excerpts within NVivo, searching for and identifying themes, reviewing, defining and naming themes, before producing the final report.
Although they did not emerge as a useful strategy for increasing participation, the semi-structured interviews confirmed the themes generated by the focus groups and proved to be a valuable source of data triangulation ( Carter et al., 2014 ).
A high level of consensus was reached by the third focus group. Although data saturation may have occurred, data collection continued until everyone who had expressed an interest in participating had been given the opportunity to share their views. No new themes emerged in the subsequent data collection.
LT’s experience as a CRNurse and her developing knowledge and expertise in this area has contributed to the development of concepts, subsequently informing the study design and conduct. These experiences have stimulated a strong commitment to objectively exploring the subject and have subsequently fostered a level of trust and credibility with participants, meaning they were comfortable with LT’s motivations as a researcher. Data verification was sought from LR, via regular discussions throughout the data collection and analysis process.
A post-data-collection feedback workshop was undertaken to present results and provide opportunities for staff to discuss their implications and plan actions. This was well attended by original participants, other CRNurses who were unable to contribute to the original data collection and allied health professionals with an interest in the work. Outputs from the workshop confirmed the themes emerging were an accurate and relevant reflection of the content of sessions, and it emerged that they were also reflective of experiences of those who were not present at data collection, adding further validity to the themes.
Of the 108 staff invited, 47 responded to the initial email invitation: 27 went on to participate, through seven focus groups and two one-to-one interviews. Non-participation resulted from work pressures, sickness, annual leave, leaving post or conflicting patient visits. Focus groups and interviews comprised of CRNurses working in a variety of practice settings across the organisation, from ward-like clinical research units to independent specialty teams operating out of offices.
The framework presented in Figure 1 illustrates the complex interplay between personal attributes, organisational culture, national priorities and strategic direction in clinical research delivery. Preliminary data analysis generated over 80 initial ‘nodes’ within NVivo. These were refined, combined and eventually translated into two overarching themes and seven sub-themes:
- 1. The individual:
- 1.1. prior experience and personal attributes;
- 1.2. the patient;
- 1.3. the role.
- 2. The organisation
- 2.1. communication;
- 2.2. feeding the machine;
- 2.3. structure and teams;
- 2.4. the reality.

Theoretical framework.
These themes and subthemes form the basis of our presentation of the qualitative findings. We will demonstrate how a focus on leadership at an individual and organisational level has the potential to enhance our understanding of the experiences of the CRNurse, shifting the emphasis towards the development of support strategies to overcome the challenges associated with clinical research delivery.
The individual
Experiences of the CRNurse role are impacted by the individual in the post. Intrinsic individual factors may explain why some CRNurses react differently to others with the challenges and complexity of the role.
I think for some people, their personality is better to deal with that [negativity from other staff] in research … other staff might not be as resilient to put up with things like that. It’s another stress in the day isn’t it I suppose … (IP2)
Prior experience and personal attributes
The importance of ‘know-how’ was debated. Narratives varied depending on whether participants had prior experiences of delivering research or knowledge of the CRNurse role. Prior knowledge of the specialty, or familiarity with staff in that area made a difference.
it probably makes the job a little bit easier initially if you know a little of the terminology about the specialty. Maybe know some of the doctors, or some of the team already. (P6FG1)
It was agreed that experience of delivering research is hard to come by and this is often the main element of know-how in the role that needs to be developed for the new CRNurse. However, if the CRNurse is coming into post as a research novice and also picking up an unfamiliar clinical specialty, this is particularly challenging. One participant (P3FG7) described this as ‘coming in with two hands [tied] behind your back’.
There was a lack of uniformity in the approach to inductions across the teams. As this became apparent it led to various debates with mixed views about what was important and how it should be developed. This suggests role transition and induction is critically important.
I have been in this post a year and have only just got to feel like I’m starting to get a handle on it. There’s still lots I don’t know … (P1FG7)
Despite the challenges described, participants recognised the value of their role and were keen to express the positive elements that they feel are little known.
And actually, it’s a privilege isn’t it, and I didn’t realise, the privilege, and actually, these patients want to be here … I enjoy coming to work so much more … I didn’t think that would have been as big a thing to me. I just thought it would be a job, I feel if people knew this, more people would want to work in research. (P7 FG1)
The patient
The nurse–patient relationship was a prominent and positive element of individual narratives, providing them with familiarity with previous nursing roles, as well as a close holistic nurse-patient relationship.
You feel you are doing something good towards improving nursing and treatments for patients which is why a lot of patients seem to want to be on studies and I think that it feels good to be able to contribute to that. (IP2)
CRNurses consistently described themselves as advocates for patients. Although this sometimes led to conflict with medical PIs, they expressed a clear vision of their role and positive contribution to the patient pathway. They described knowing instinctively when it was or was not appropriate to approach patients. Experiences of receiving informed consent and conducting research in areas where there were little or no other treatment options left were discussed. Whilst posing challenges around timing, appropriateness of approach and patient priorities during difficult times, CRNs felt offering hope through research participation was important.
Maybe it’s like a, you know that gut feeling isn’t it. It’s hard to describe … We’ve had patients that have signed up for an early phase study and yes, they understood the information and signed the consent form but talking to them, I was like, this patient doesn’t want to do this study. And then I had to go back to the consultant and say, this is just not sitting right with me … (P1FG5)
Some CRNurses described a reduction in patient contact resulting from moving into a research post, however this was not the case in every area.
I personally don’t have as much contact with patients and people have found that quite challenging and they still want to keep their hand in and in some studies you do, you have a lot of contact but some you don’t, and I think that is probably one of the biggest things you have to find out straightaway that you are not going to be over bothered by the amount of contact that you have with patients. (P3FG5)
CRNurses described the positives of having time to spend with patients. They sympathised with their non-research counterparts expressing how limited quality time with patients can be in areas outside of research.
I think that is the main thing that I get from the job, especially the older population, I love just going and chatting to them. Having the time, whereas normally when I’m on the ward you can’t because there’s either a buzzer or someone wants tablets, there is always something going on. (P4FG5)
The range of ways CRNurses deal with the role as individuals depends on numerous factors. This impacts on experiences, thoughts, mind-set, job satisfaction and intention to remain in post. The consensus was that the CRNurse should feel positive about the specialty they are practising in and the job itself should be intrinsically motivating. If this is not the case it can impact on morale and mind-set, ultimately affecting study delivery.
Factors such as the type of study, its design, sponsor and complexity were important and there was a mixture of opinions about the value of commercial versus academic research. Some participants preferred commercial studies:
I love commercial studies … I just feel it is a lot more professional, I feel they are run better, I feel like the protocols are more in depth, more detailed, I feel like academic protocols are rushed to a degree. I’m not saying that academic you know, there’s a place for it. But as a nurse, when I get an academic study, I’m like oh, nightmare. (P7FG1)
Others preferred academic work, and expressed frustration at aspects of commercial trial delivery:
I think commercially … when they’re writing them, they have no idea what happens in clinical practice … I think generally they don’t consider that there’s clinical things going on, everything just has to be done, they’re very narrow-thinkers. (P4FG1)
Individual capacity and capability were debated, along with the challenges of measuring workload, intensity, and when to decline studies. There was agreement that what one person may find challenging and exciting, another may find stressful and overwhelming. The support received in day-to-day practice varied, and a feeling of responsibility and lack of ability to hand over caused tension for some. Some individuals valued the autonomy and associated ownership of their studies, including a sense of achievement in delivering it successfully.
That’s what I found really difficult coming to the role. Knowing that there wasn’t another shift coming after me … knowing that there is not another team coming on after, you know, and having to get everything organised weeks in advance, like really forward planning for your patients. I found that really difficult. And managing my own time is, difficult. (P1FG5)
There was a passion for attracting others into CRNurse roles, and participants debated how to inspire others to consider trying research nursing. There was an appetite to raise awareness of the role with other nurses to help them understand the role. Offering student placements was particularly positive for those who had the opportunity to do so, recognising the value of the opportunity to inspire the next generation.
it’s about being quite outgoing and upfront about what you are doing and talking to people and just getting integrated … I think, you know, they’re introducing students into research I think that might help as well. (P4FG1)
The organisation
There was an understanding that healthcare is complex, with numerous priorities and strategies that CRNurse teams and individuals contribute to. In research these can sometimes feel more complex because of the increased number of external stakeholders, and the link to other national priorities.
Communication
Communication discussions focused on dialogue between the CRNurse and the Medical PI.
I think some of them do it because they maybe feel they should do be doing it, rather than actually being enthusiastic about it. The consultant we are talking about, I don’t know why they chose the two studies because they’re not interested in them really. It is almost like a pain. You are a nuisance, to ask them to sign this, or see the patient. (P1FG4)
Communication was discussed in relation to the individual teams, external study teams and the wider organisation.
I tried to go to one of those specialist nurse groups and I felt absolutely out of place. And I thought, well I shouldn’t, because we should be here but I did feel I didn’t belong there really … I don’t know I think I was probably the only CRNurse. (P1FG4)
Internal meetings and the tone of communication was raised by the majority of participants.
It’s about perspective, we can come out of a meeting and feel that we’ve been absolutely flogged because we are not meeting targets, but then you look and we are at the top … It’s not reflected in the culture, it’s just ‘duf duf duf’ [gestures towards pressure]. (P1FG6)
Feeding the machine
One message that was clearly identified throughout the study was around funding and targets. ‘Feeding the machine’ was a poignant description to make sense of the challenges the CRNurse workforce experiences, with messages of reduced funding and the pressure to deliver every study successfully to time and target.
It is, it’s all about targets isn’t it – meeting targets and if you don’t then heaven help you. (P2FG6)
Concerns around job security were shared. These were identified as a new feeling in comparison to working on a ward, as discussions about funding repeatedly linked patient recruitment to funding for posts.
But you also get told well numbers are jobs. So, we need to keep our accruals up or we lose funding and then that will be somebody leaving. You know, you do get told that. (P1FG4)
Despite such observations, some teams admitted to enjoying, even thriving on a little healthy competition.
I must admit, I think I am a little bit target driven. I quite enjoy it to an extent actually … they are not such a bad thing because there is something to aim for … (P1FG6)
Structure and teams
Structure and team working impacted on experiences. Working across specialties was described as less positive than a focused approach. Participants acknowledged that this was necessary to enable effective delivery of the wide range of studies across different clinical areas in the context of a limited research delivery workforce. They also recognised the resulting opportunities to enhance skills development and the potential to support colleagues in other teams, though this approach to working introduced competing priorities.
You were just trying to spread yourself too thinly … you were working with different PI’s who wanted to see patients on the same day at the same time and you couldn’t … (P1FG3)
Those working in areas with limited contact with their clinical counterparts felt isolated, describing the challenges of motivating teams to support their roles.
So I think we are a product of our own success in that we have managed to successfully separate research and deliver research in parallel to the clinical service, but it’s not embedded in the clinical service because you’ve got a whole separate research department and so the clinical staff can now think oh no that’s for the research department … (P1FG6)
Those who were more embedded clinically either through where they were based geographically or through their specific role, reported more positive experiences.
I prefer and I would suggest the reason I get so much out of my job is because I feel part of the directorate, the clinical directorate … I like being part of that … (IP)
The reality
The reality of operating within the organisation varied across the teams and individuals. Visibility and awareness of the role outside of research was linked with communication, structures and teams, and it also linked back to individual attributes.
I think a lot of people don’t know what we do and don’t understand that all the current drives that research should be an option for all patients and that it’s actually good for patients and it can benefit them … I think they don’t realise that being nicer to us and allowing us to get access to the patients would actually be them supporting their patients … (IP)
Access to space and facilities varied across the teams.
I mean one of the pressures we have at the minute is that we do not have a designated area to see our patients so we go down to out-patients and you are not stressing about whether the patient’s going to go in [the study] or not you are stressing about whether you are going to have a room. (P1FG3)
Others described a feeling of isolation in terms of where they were based geographically, and a struggle to be accepted into the clinical areas they needed to access to be able to undertake their patient visits.
We are the last in the pecking order of who needs to see. When I am waiting to see somebody in clinic … the dietician is free now so they will just pinch them. So I will wait, and they will come out of the dietician and the psychologist is waiting … and then before you know it you’ve been waiting there an hour to see somebody, and they want to go then because they’ve been there for that long [laughs]. Because there was nowhere for you to go and you always felt the clinical need to go and see somebody was greater than yours. (P3FG5)
There was talk of emotional labour, making an extra effort to be welcomed into the area or allowed space to practise.
I’ve gone back to being a second class citizen in the Trust, so I feel that quite a lot, I feel like I have to justify what I do. (P6FG1)
There were excellent examples of work going on to raise awareness and visibility of the CRNurse contribution to the patient pathway. Posters, information boards, staff presentations, research champions and linking in with multi-disciplinary meetings were described. These activities and the impact of them weren’t shared widely, and those attending the focus groups seemed to learn from the experience, taking practical strategies away with them to try.
On each ward there’s somebody allocated, and they will come to a meeting so many times a year where we tell them a little bit about research, about things we have to do and a bit about the studies that again is relevant to their ward … There’s posters on every ward, again it’s a directorate thing, they want very much to integrate research and the directorate stuff … (P4FG1)
The findings illustrated above indicate a complex blend of factors with the potential to impact on successful research delivery. These factors arise from a combination of interplay between the personal attributes of CRNurses, their experiences and behaviours and how they are impacted by and can equally impact on organisational culture and national priorities. This combination of factors and a focus on self-leadership and organisational culture has the potential to contribute to the dialogue on challenges in clinical research delivery and should be considered by policy makers and organisational leads.
The King’s Fund (2011) acknowledges high levels of complexity and debate related to the many definitions of leadership and its impact.
Historically, leadership has been described and interpreted in a positive manner:
Leadership is interpersonal influence, exercised in a situation, and directed, through the communication process, toward the attainment of a specified goal or goals ( Tannenbaum et al., 1961 ).
In its most basic form, however, leadership is derived from individual approaches to influencing, in order to modify the behaviours of others towards a specific goal, agenda or outcome. Zalenik (1992) captures the essence of leadership as follows:
Leadership requires using power to influence the thoughts and actions of other people.
An increased focus on leadership in recent years, has led to improved awareness of how individual behaviours can impact on performance and experiences, ultimately translating into the quality of outcomes, whether for individual consumers or businesses as a whole ( Blanchard, 2010 ; Goleman, 2013 ; Kouzes and Posner, 2012 ; Sinek, 2014 ; Yukl, 2013 ).
The findings from the present study describe intrinsic feelings, perceptions and motivators that have the potential to impact on actions and reactions to events, pressures and expectations placed on individuals to varying degrees. These intrinsic factors are often described through psychological trait theory, which is reported to influence how we act in addition to situational factors such as learned behaviours, lived experiences and social interactions. This results in what is described as self-leadership ( Goleman, 2013 ; Northouse, 2013 ).
Trait theory historically indicates that the majority of intrinsic characteristics are relatively fixed, with limited scope for development; however, behavioural research has increasingly identified that individuals have the capacity for development and change through increased self-awareness ( Northouse, 2013 ). Increased self-awareness, understanding the relevance of prior experiences and knowledge, in addition to greater awareness of the CRNurse role prior to being appointed, could assist in recruitment and selection for posts, improve experiences of role transition and enhance performance within the role.
The suggestion that some nurses are more suited to research delivery posts than others and the individual nature of how one person’s ability to ‘cope’ with the complexities of the role differ to another, relate to the concept of resilience. This is the subject of increasing debate in healthcare at present. Whilst implementing strategies to enhance personal resilience may assist individuals in dealing with the challenges they face, the very concept of resilience is argued to foster a submissive mindset, placing responsibility for coping with adversity in the hands of the individual rather than enabling action to explore the core of the issues and identify what support is required ( Traynor, 2017 , 2018 ).
Intrinsic issues are also associated with professional identity; a broad term emerging from our previous study, described as a continuing process of development affected by numerous social interactions and environmental factors ( Franco and Tavares, 2013 ; Workman and Pickard, 2008 ). A positive professional identity is fundamental to performance, impacting on patient care, job satisfaction and decisions to remain in a particular post ( Cowin et al., 2008 ; Kunhunny and Salmon, 2017 ). Professional identity in clinical research nursing is under-researched, and the results of this study suggest a positive, embedded organisational research culture, is a key mediator of a positive professional identity.
The experiences described by participants in relation to organisational challenges, indicate an increasing level of discomfort with the current target-based culture in research delivery, and whilst CRNurses were able to articulate the relevance of targets, they were negatively impacted by the focus on funding linked to those targets. This left them feeling there was limited focus on improved patient outcomes and the value and benefit of research participation for patients.
The Nuffield Trust (2015 ) identified funding crises, NHS management and culture, and the effectiveness of performance targets as priorities for the Government to address. Re-connecting with the core NHS workforce to improve engagement and empowerment, and implementing successful workforce development and planning, were also key priorities, however, growing workloads and staff shortages underline the current pressure on the NHS workforce, impacting staff morale ( The King’s Fund, 2017 ).
Whilst the research arena has historically been less affected by funding crises, the issues described above are increasingly evident, and equally important, and the majority of participants acknowledged this. Difficulties meeting trial recruitment targets, combined with the threat of funding reductions based on reduced activity, may have affected the morale of CRNurses ( Tinkler et al., 2018 ). Reduced morale across the NHS may also have potentially contributed to disconnection between CRNurse teams and their wider organisational colleagues.
Organisational and team-focused research predominates the healthcare leadership literature, with a focus on collective and multilevel models to define the most effective approaches to leadership (Yukl, 2013; MacPhee et al., 2013 ). Increased performance and safer care are synonymous with satisfied and contented teams and a recent shift in leadership culture within the NHS has identified increasingly that a top-down approach results in disengaged staff, ultimately leading to poorer standards of care ( The King’s Fund, 2011 , 2012 ; Turnbull James, 2011 ; West, 2012 ). Turnbull James (2011) also identified that it is vital to engage and empower staff to recognise the importance of their unique contribution in the provision of high-quality care. This is as important in research delivery as any other sphere of healthcare delivery.
Healthcare priorities are driven by various national and government strategies influencing and directing every element of practice. The NIHR was established in the UK through one such government strategy ( Department of Health, 2006 ). The seven high-level objectives (HLOs) from the NIHR influence practice and performance in research delivery in the UK. The general tone of these HLOs is about increasing numbers of patients into studies and reducing the time taken to achieve this. The core foundations of these high-level objectives are with patients in mind, in being able to prevent ill health, improve diagnosis and offer better treatments more readily ( NHS England, 2014 , 2016 ). Funding is also a key foundation of the HLOs aimed at improving the UK’s reputation as a setting for research ( Department of Health, 2017 ; NHS England, 2013 ). As identified by the participants in this study, the tone of the message from national strategies and how those messages are translated into practice have the potential to affect organisational culture, which mediates individual experiences as professionals operating at the frontline.
‘The NHS Long Term Plan’, launched in January of 2019 articulates the clear necessity for change in the health service, including a commitment to release pressure at the frontline whilst capitalising on the medical advances we currently benefit from. The plan commits to increased funding to support staff whilst continuing to sustain and further develop a vibrant life sciences sector ( NHS England, 2019a ).
The subsequent launch of ‘The NHS Interim People Plan’ built on the commitments of this through dedicating a strategy to improving the leadership culture, tackling the nursing workforce challenges we currently see and making the NHS the best place to work ( NHS England, 2019b ). By harnessing the recommendations in this plan there is scope to further influence the Clinical Research Nursing agenda through improving awareness and attitudes towards research, whilst working to address some of the unhelpful behaviours that have impacted on relationships, experiences and productivity in the research area.
The framework and results presented in this paper provide a valuable contribution to the evidence base in this under-represented area, with the potential to influence policy and practice in relation to clinical research delivery. It highlights the numerous factors that have the potential to impact on the practice of CRNurses and introduces how they may subsequently affect how well research is delivered. Themes emerging from this piece of work build on previous work and confirm that there is transferability between areas of practice and types of organisation.
A focus on self-leadership and organisational culture, whilst positioning the discussion within the context of the wider health service and national priorities provides a context for the issues described and shifts dialogue towards increased focus on approaches to communication, awareness, visibility and support mechanisms to inform policy and practice and overcome challenges associated with clinical research delivery.
The challenges described also suggest there is further engagement work to do to improve understanding of the importance of the CRNurse role in the patient pathway. Within its recently launched CRNurse strategy (2017–20), clear recognition of CRNurses, and their unique role in the delivery of quality clinical research care was identified as key to achieving the NIHR mission. Increasing the understanding of the experiences and perceptions of the CRNurse is essential to address the barriers to research delivery and increase awareness and understanding of the role of the CRNurse and associated challenges.
The following key points have been generated from this study:
- This study contributes to the increasing literature indicating that work to deliver research in the clinical environment is complex and that CRNursing roles are often poorly understood outside of research delivery teams
- Exploring the experiences and perceptions of CRNurses is increasingly highlighting important and inherently complex factors related to successful research delivery. This approach should be further developed to fully understand the issues and influence policy in relation to organisation of the workforce
- This study adds new knowledge in relation to the finding that research delivery relies on a complex, multi-faceted range of factors originating from individual clinical research nurses and the organisations in which they operate. This is mediated by the context of national drivers and strategies influencing their work and this should be considered in relation to the wider NHS workforce
- The concept of leadership is a useful frame in which to situate the challenges associated with clinical research delivery. This has the potential to facilitate a broader dialogue on the subject and increases the potential for developing appropriate support mechanisms to address the barriers, whilst maximising the facilitators to clinical research delivery
Limitations
Although data were collected in a single organisation the findings were consistent with previous and other emerging research.
It became apparent during the recruitment process that the agreed approach to sampling staff had not been followed by all team leads. Some expressed discomfort with the email approach and therefore did not send emails to their teams. Instead they made Participant Information Leaflets available to staff through placing them at the nurses’ station. At this time, it is not possible to say with certainty whether all CRNurses were given the opportunity to participate, or whether they felt able to do so confidentially, due to this unforeseen change to the sampling process.
Key points for policy, practice and/or research
- Consideration should be given to the relevance of strategies to increase personal resilience and consider where these may assist in addressing the challenges identified.
- Work should be undertaken to improve the induction process and more broadly improve the organisational support offered to CRNurses in the delivery of their roles.
- The utility of the target-based culture in the NHS should be explored further, more specifically in relation to where this may be impacting on clinical research delivery roles.
- Further exploration of the utility of the NIHR HLOs should be considered, including how they may be adapted to reduce the focus on recruitment to time and target and include metrics measuring the impact of research, dissemination and knowledge transfer and real impact on health inequalities and unwarranted variations in care.
- The interface between the CRNurse role and their multiprofessional colleagues from the perspective of those colleagues could be investigated to enable enhanced understanding of their needs in relation to supporting the delivery of research and the opportunities for patients in their care to participate in research.
Acknowledgements
The lead author would like to thank Aileen Burn, Matron for Clinical Research Delivery at Newcastle upon Tyne Hospitals NHS Foundation Trust.
Linda Tinkler is the Trust Lead for Nursing, Midwifery and Allied Health Professionals Research at Newcastle upon Tyne Hospitals NHS Foundation Trust. Linda's role involves leading and implementing research specific strategies, policies and initiatives on behalf of the Executive Chief Nurse team. This includes building research capacity and capability to enable staff in the NMAHP disciplines to develop along the research continuum from research awareness to research leadership. Linda's own research interests include leadership culture in the NHS and the interface between Clinical Research Delivery and Clinical Practice. She is currently undertaking a PhD through a Royal College of Nursing Strategic Research Alliance with the University of Sheffield, is a Florence Nightingale Leadership Scholar and an NIHR 70@70 Senior Nurse Research Leader.
Lisa Robinson is currently employed as Consultant Allied Health Professional – Major Trauma Rehabilitation. Her clinical and research interests are informed by four key areas: improving healthcare organization and delivery; improving patient experience and outcomes; advancing complex interventions research methods in a rehabilitation setting; and workforce development and professional learning in the nursing, midwifery and allied health professions, with a particular emphasis on building research capacity and capability.
Contributor Information
Linda Tinkler, Trust Lead, Nursing, Midwifery and AHP Research, Newcastle upon Tyne Hospitals NHS Foundation Trust, Freeman Hospital, Newcastle upon Tyne, UK.
Lisa Robinson, Consultant Allied Health Professional, Major Trauma Rehabillitation Department, The Newcastle upon Tyne Hospitals NHS Foundation Trust, Royal Victoria Infirmary, Newcastle upon Tyne, UK.
Declaration of conflicting interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The study was assessed and approved through the UK Health Research Authority (HRA: 17/HRA/0125), and capacity and capability to participate was confirmed by the sponsor/host organisation. The study did not require full Research Ethics Committee approval due to the involvement of NHS staff members only. Participants provided written informed consent prior to data collection.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Study funding was awarded to the lead author through a competitive process by Newcastle upon Tyne Hospitals NHS Foundation Trust, utilising NIHR Research Capability Funding (Ref. No. 1617-039).
Linda Tinkler https://orcid.org/0000-0003-3052-0640
- Adams M, Caffrey L, McKevitt C. (2015) Barriers and opportunities for enhancing patient recruitment and retention in clinical research: Findings from an interview study in an NHS academic health science centre . Health Research Policy and Systems 12 ( 13 ): . [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Blanchard K. (2010) Leading at a Higher Level: Blanchard on How to Be a High Performing Leader , Edinburgh: Pearson Education. [ Google Scholar ]
- Braun V, Clarke V. (2006) Using thematic analysis in psychology . Qualitative Research in Psychology 3 ( 2 ): 77–101. [ Google Scholar ]
- Carter N, Bryant-Lukosius D, DiCenso A, et al. (2014) The use of triangulation in qualitative research . Oncology Nursing Forum 41 ( 5 ): 545–547. [ PubMed ] [ Google Scholar ]
- Cooksey D (2006) A review of UK health research funding Crown Copyright HMSO. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/228984/0118404881.pdf (accessed 15 December 2018).
- Cowin LS, Johnson M, Craven RG, et al. (2008) Causal modelling of self-concept, job satisfaction and retention of nurses . International Journal of Nursing Studie 45 : 1449–1459. [ PubMed ] [ Google Scholar ]
- CQC (2017) Driving improvement. Case studies from eight NHS trusts. Available at: https://www.cqc.org.uk/sites/default/files/20170614_drivingimprovement.pdf (accessed 3 January 2019).
- Department of Health (2006) Best Research for Best Health A New National Health Research Strategy. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/136579/dh_4127153.pdf (accessed 3 January 2019).
- Department of Health (2017) Life Sciences Industrial Strategy: A report to the Government from the life sciences sector. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/650447/LifeSciencesIndustrialStrategy_acc2.pdf (accessed 3 January 2019).
- Donovan JL, Paramasivan S, deSalis I, et al. (2014. a) Clear obstacles and hidden challenges: Understanding recruiter perspectives in six pragmatic randomised controlled trials . Trials 15 ( 5 ): . [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Donovan JL, de Salis I, Toerien MG, et al. (2014. b) The intellectual challenges and emotional consequences of equipoise contributed to the fragility of recruitment in six randomized controlled trials . Journal of Clinical Epidemiology 67 ( 8 ): 912–920. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Donovan JL, Rooshenas L, Jepson M, et al. (2016) Optimising recruitment and informed consent in randomised controlled trials: The development and implementation of the Quintet Recruitment Intervention (QRI) . Trials 17 ( 283 ): . [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Elliot D, Husbands S, Hamdy F, et al. (2017) Understanding and improving recruitment to randomised controlled trials: Qualitative research approaches . European Urology 72 ( 5 ): 799–800. [ PubMed ] [ Google Scholar ]
- Franco M, Tavares P. (2013) The influence of professional identity on the process of nurses’ training: An empirical study . Leadership in Health Services 26 ( 2 ): 118–134. [ Google Scholar ]
- Gibbs C, Lowton K. (2012) The role of the clinical research nurse . Nursing Standard 26 ( 27 ): 37–40. [ PubMed ] [ Google Scholar ]
- Goleman D. (2013) What Makes A Leader: Why Emotional Intelligence Matters , 1st edn. Florence, MA: More than Sound. [ Google Scholar ]
- Hamer S (2017) NIHR Clinical Research Network Developing our Clinical Research Nursing Strategy 2017–2020. Available at: https://www.nihr.ac.uk/our-research-community/clinical-research-staff/clinical-research-nurses/Clinical%20Research%20Nurse%20Strategy%202017_2020FINAL.pdf (accessed 15 December 2018).
- Hill G (2018) E xploring Clinical Research Nurses’ Experiences of Working with Clinical Nurses. PhD thesis, Queen Margaret University. Available at: https://eresearch.qmu.ac.uk/bitstream/handle/20.500.12289/9707/9707.pdf?sequence=1&isAllowed=y (accessed 31 August 2019).
- American Nurses Association and International Association of Clinical Research Nursing (2016) Clinical Research Nursing: Scope and Standards of Practice , Silver Spring, MD: ANA & IACRN. [ Google Scholar ]
- Jones H (2017) Exploring the experience of Clinical Research Nurses working within acute NHS trusts and determining the most effective way to structure the workforce: A mixed methods study . PhD thesis, Kings College London, UK. Available at: https://kclpure.kcl.ac.uk/portal/files/80806720/2017_Jones_Helen_0853556_ethesis.pdf (accessed 15 December 2018).
- Kitzinger J. (2006) Focus groups . In: Pope C, Mays N. (eds) Qualitative Research in Health Care , 3rd edn. Oxford: Blackwell Publishing Ltd, pp. 21–31. [ Google Scholar ]
- Kouzes J, Posner B. (2012) The Leadership Challenge , 5th edn. Hoboken, NJ: John Wiley & Sons. [ Google Scholar ]
- Krueger RA, Casey MA. (2015) Focus Groups: A Practical Guide for Applied Research , 5th edn. London: SAGE. [ Google Scholar ]
- Kunhunny S, Salmon D. (2017) The evolving professional identity of the clinical research nurse: A qualitative exploration . Journal of Clinical Nursing 26 ( 23–24 ): 5121–5132. [ PubMed ] [ Google Scholar ]
- MacArthur J, Hill G, Callister C. (2014) Professional issues associated with the clinical research nurse role . Nursing Standard 29 ( 14 ): 37–43. [ PubMed ] [ Google Scholar ]
- MacPhee M, Chang L, Lee D, et al. (2013) Global health care leadership development: Trends to consider . Journal of Healthcare Leadership 5 : 21–29. [ Google Scholar ]
- Mills J and Birks M (eds) (2014 ) Qualitative Methodology. A Practical Guide. London: SAGE . .
- Northouse PG. (2013) Leadership Theory and Practice , 6th edn. London: SAGE. [ Google Scholar ]
- NHS England (2019a) The NHS long term plan. Available at: https://www.longtermplan.nhs.uk/ (accessed 31 August 2019).
- NHS England (2019b) The Interim People Plan. Available at: https://www.longtermplan.nhs.uk/wp-content/uploads/2019/05/Interim-NHS-People-Plan_June2019.pdf (accessed 31 August 2019).
- NHS England (2013) Research and Development Strategy 2013–2018. Research is everybody’s business. Available at: http://www.invo.org.uk/wp-content/uploads/2014/02/NHS-England-Research-Strategy-Consultation.pdf (accessed 15 December 2018).
- NHS England (2014) Five Year Forward View. Available at: https://www.england.nhs.uk/wp-content/uploads/2014/10/5yfv-web.pdf (accessed 3 January 2019).
- NHS England (2016) Leading Change Adding Value: A framework for nursing, midwifery and care staff. Available at: https://www.england.nhs.uk/wp-content/uploads/2016/05/nursing-framework.pdf (accessed 15 December 2018).
- Ozdemir BA, Karthikesalingam A, Sinha S, et al. (2015) Research activity and the association with mortality . PLoS ONE 10 ( 2 ): 10.e0118253. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sinek S. (2014) Leaders Eat Last: Why Some Teams Pull Together and Others Don't , London: Penguin Books Ltd. [ Google Scholar ]
- The King’s Fund (2011) The Future of Leadership and Management in the NHS No More Heroes , London: The King’s Fund. [ Google Scholar ]
- The King’s Fund (2012) Leadership and Engagement for Improvement in the NHS Together we can , London: The King’s Fund. [ Google Scholar ]
- The King’s Fund (2017) What are the Priorities for Health and Social Care? , London: The King’s Fund. [ Google Scholar ]
- Tannenbaum RJ, Weschler IR, Massarik F. (1961) Leadership and Organization: A Behavioural Science Approach , New York: McGraw-Hill. [ Google Scholar ]
- Tinkler L, Smith V, Yiannakou Y, et al. (2018) Professional identity and the Clinical Research Nurse: A qualitative study exploring issues impacting on participant recruitment in research . Journal of Advanced Nursing 74 ( 2 ): 318–328. [ PubMed ] [ Google Scholar ]
- The Nuffield Trust (2015) Health and social care priorities for the Government: 2015–2020 Policy Briefing. Available at: https://www.nuffieldtrust.org.uk/resource/health-and-social-care-priorities-for-the-government-2015-2020 (accessed 20 July 2018).
- Traynor M. (2017) Critical Resilience for Nurses , London: Routledge. [ Google Scholar ]
- Traynor M. (2018) Guest editorial: What’s wrong with resilience . Journal of Research in Nursing 23 ( 1 ): 5–8. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Turnbull James K. (2011) Leadership in Context: Lessons from new Leadership Theory and Current Leadership Development Practice , London: Kings Fund. [ Google Scholar ]
- West M. (2012) Effective Teamwork , 3rd edn. Chichester: John Wiley & Sons Ltd. [ Google Scholar ]
- Whitehouse CL and Smith HA (2018) The Whitehouse Report: Review of research nursing and midwifery structures, strategies and sharing of learning across the UK and Ireland in 2017. The Florence Nightingale Foundation. Available at: https://florence-nightingale-foundation.org.uk/wp-content/uploads/2018/06/FNF-Whitehouse-report-CWhitehouse-final-revisions-6June2018.pdf (accessed 15 December 2018).
- Workman A, Pickard J. (2008) Professional identity in multi-disciplinary teams: The staff speak . Journal of Integrated Care 16 ( 3 ): 29–37. [ Google Scholar ]
- Yukl G. (2013) Leadership in Organisations , 8th edn. London: Pearson Education. [ Google Scholar ]
- Zaleznik A. (1992) Managers and leaders: Are they different? Harvard Business Review 70 ( 2 ): 126–135. [ PubMed ] [ Google Scholar ]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Clinical Research Article
Eltrombopag combined with immunosuppressive therapy for pediatric severe aplastic anemia
- Lingling Fu

The Ladder of Inference as a tool to reduce implicit bias in pediatric clinical practice
- Beatrice E. Lechner
- Stephanie K. Kukora
- Autumn Fiester
Differences in autophagy marker levels at birth in preterm vs. term infants
- Noëmi Künstle
- Olga Gorlanova
- Florian Wyler
Artificial intelligence model system for bone age assessment of preschool children
- Chengcheng Gao
- Chunfeng Hu
- Zhongxiang Ding
Serum eosinophil-derived neurotoxin: a new promising biomarker for cow’s milk allergy diagnosis
- Wael A. Bahbah
- Ahmed S. Abo Hola
- Heba M. S. El Zefzaf
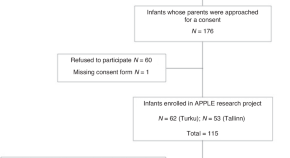
Exposure to the parents’ speech is positively associated with preterm infant’s face preference
- Anette Aija
- Jukka Leppänen
- Liisa Lehtonen
Cardiovascular and cerebrovascular effects of caffeine maintenance in preterm infants during the transitional period
- Roberta Parladori
- Topun Austin
- Silvia Martini
Faecal lipid profile as a new marker of fat maldigestion, malabsorption and microbiota
- Andrea Asensio-Grau
- Miguel Ferriz-Jordán
- Joaquim Calvo-Lerma
Quantification of testicular fat content: the value of evaluating testicular function after cryptorchidism surgery
- Qingling Li
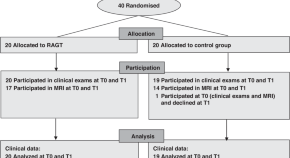
Robot-assisted gait training improves walking and cerebral connectivity in children with unilateral cerebral palsy
- Laura Julien
- Guillemette Moreau-Pernet
- Catherine Sarret
Nutritional intake and growth until two years of age in moderate and late preterms
- Anne H. Lafeber
- Roxanne C. de Jong
- Femke de Groof
Family caregivers of children transitioning hospital to home receiving nasogastric feeding: descriptive qualitative study
- Samantha Mekhuri
- Anam Shahil-Feroz
- Reshma Amin
Quantitative EEG features during the first day correlate to clinical outcome in perinatal asphyxia
- Anna Tuiskula
- Alexey S. Pospelov
- Sampsa Vanhatalo
High frequency of MEFV disease-causing variants in children with very-early-onset inflammatory bowel disease
- Aasem Abu Shtaya
- Naama Orenstein
- Lina Basel-Salmon
Neonatal outcomes of preterm infants with pulmonary hypertension: clustering based on prenatal risk factors
- Seong Phil Bae
- Sung Shin Kim
- Suyeon Park
Clinical and metagenomic characteristics of lymphadenopathy related to fever of unknown origin in children
- Yajuan Zhou
Blood pressure and cerebral oxygenation with physiologically-based cord clamping: sub-study of the BabyDUCC trial
- Shiraz Badurdeen
- Douglas A. Blank
- Peter G. Davis
Young hearts, early risks: novel cardiovascular biomarkers in former very preterm infants at kindergarten age
- Wolfgang Mitterer
- Irena Odri Komazec
- Ursula Kiechl-Kohlendorfer
Body composition and motor function in children born large for gestational age at term
- Tadashi Ito
- Nobuhiko Ochi
Sleep and internalizing problems in primary school children with attention-deficit hyperactivity disorder
- Han-Yu Dong
- Chun-Yue Miao
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
Images in Clinical Medicine. May 29, 2024. Perspective. May 25, 2024. The New England Journal of Medicine (NEJM) is a weekly general medical journal that publishes new medical research and review ...
Tirzepatide to Treat Obesity in ChinaMay 31, 2024Original Investigation Tirzepatide for Weight Reduction in Chinese Adults With Obesity: The SURMOUNT-CN Randomized Clinical Trial Lin Zhao, MD; Zhifeng Cheng, MD; Yibing Lu, MD; et al. Editorial Efficacy of Tirzepatide for Weight Loss in China:Implications for the Global Obesity Epidemic Frank ...
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management. Moreover, any research that evaluates the aspects of a ...
Association between antenatal corticosteroids and risk of serious infection in children. August 2, 2023. Can't find what you're looking for? Continue to all research articles. Original research studies that can improve decision making in clinical medicine, public health, health care policy, medical education, or biomedical research.
Clinical trials articles from across Nature Portfolio. A clinical trial involves the study of the safety, efficacy and/or dosage regimen of a therapeutic intervention (such as a drug) in humans ...
L.D. Fiore and P.W. LavoriN Engl J Med 2016;374:2152-2158. Clinical trials of interventions in common practice can be built into the workflow of an electronic medical record. The authors review ...
Recognizing Historical Injustices in Medicine and the Journal : Malicious Midwives, Fruitful Vines, and Bearded Women — Sex, Gender, and Medical Expertise in the Journal. B. Maldonado and Others ...
Despite questions remain about the impact of virus variants and the duration of the immune response, messenger RNA (mRNA)-based and adenoviral vectored vaccines have demonstrated an overall efficacy from 70 to 95% in both phase III trials and real life. In addition, all these vaccines also reduce the severe forms of the disease and might ...
Anna-Maria Kellen Clinical Accelerator, Cancer Research Institute, New York, NY, USA. Since the first CAR-T cell product was approved by the FDA in 2017, five additional CAR-T cell products have ...
The COVID-19 pandemic made "clinical trials" a household phrase, highlighting the critical value of clinical research in creating vaccines and treatments and demonstrating the need for large-scale, well-designed, and rapidly deployed clinical trials to address the public health emergency. As the largest public funder of clinical trials, the ...
A peer-reviewed journal of the Royal College of Physicians, Clinical Medicine (ClinMed) publishes advances in general and specialist medicine and authoritative reviews on thought-provoking topics.It aims to directly improve readers' clinical practice by providing evidence-supported and relevant learning. This will be achieved by publishing original research, guideline summaries and clinically ...
Clinical Trials is dedicated to advancing knowledge on the design and conduct of clinical trials related research methodologies. Covering the design, conduct, analysis, synthesis and evaluation of key methodologies, the journal remains on the cusp of the latest topics, including ethics, regulation and policy impact.
Abstract. The goal of this review was to present the essential steps in the entire process of clinical research. Research should begin with an educated idea arising from a clinical practice issue. A research topic rooted in a clinical problem provides the motivation for the completion of the research and relevancy for affecting medical practice ...
Jennifer Litton is the vice president of clinical research and a professor in the Breast Medical Oncology department, ... Cite this article. Arnold, C. 11 clinical trials that will shape medicine ...
Temporal trends and disparities of population attributable fractions of modifiable risk factors for dementia in China: a time-series study of the China health and retirement longitudinal study (2011-2018) The Lancet Regional Health - Western Pacific. Vol. 47101106Published: May 29, 2024.
A two-dose regimen of BNT162b2 (30 μg per dose, given 21 days apart) was found to be safe and 95% effective against Covid-19. The vaccine met both primary efficacy end points, with more than a 99 ...
Clinical Nursing Research (CNR) is a leading international nursing journal, published eight times a year.CNR aims to publish the best available evidence from multidisciplinary teams, with the goal of reporting clinically applicable nursing science and phenomena of interest to nursing. Part of CNR's mission is to bring to light clinically applicable solutions to some of the most complex ...
Research shows GLP-1 receptor agonist drugs are effective but come with complex concerns. May 30, 2024. Drugs like Ozempic, Wegovy and Mounjaro have been around for years, but they've recently been making headlines due to a rise in popularity as weight loss agents. They all belong to a class of drugs known as glucagon-like peptide-1 receptor ...
Clinical research is the comprehensive study of the safety and effectiveness of the most promising advances in patient care. Clinical research is different than laboratory research. It involves people who volunteer to help us better understand medicine and health. Lab research generally does not involve people — although it helps us learn ...
Introduction. In clinical research, our aim is to design a study, which would be able to derive a valid and meaningful scientific conclusion using appropriate statistical methods that can be translated to the "real world" setting. 1 Before choosing a study design, one must establish aims and objectives of the study, and choose an appropriate target population that is most representative of ...
Read the latest Research articles in Clinical trials from Scientific Reports. ... Clinical trials articles within Scientific Reports. Featured. Article 15 May 2024 | Open Access.
Testing Innovation in Cancer Clinical Trials. A transformation in cancer clinical research is already underway. That transformation has been led in part by the success of novel precision oncology approaches, such as those tested in the NCI-MATCH trial. This innovative study ushered in novel ways of recruiting participants and involving ...
What did the researchers do and find? We conducted a mixed-methods systematic review and identified 60 research articles from 27 countries on the views and experiences of pregnant and lactating women's participation in clinical research, from the perspectives of cisgender women, family and community members, health workers, and people involved in the conduct of clinical research.
While the biologic benefits of biospecimen research have been well established, the benefit of translational research on the Veteran care experience has yet to be defined. In this study, we evaluated: 1. Whether Veteran enrollment in a biospecimen-based prostate cancer research study improved their VA patient care experience and satisfaction and 2.
A trial led by Jason M. Redman, M.D., Assistant Research Physician in the Center for Immuno-Oncology, is studying combination immunotherapy, including a tumor-targeted vaccine, for the disease. The trial includes two mandatory biopsies. The trial will take place at the NIH Clinical Center in Bethesda, Maryland, and there is no cost for ...
Clinical research delivery is a term increasingly used to describe the work undertaken to implement studies which explore and test prevention, diagnosis and treatment in healthcare. Such studies range from multi-site clinical trials to single site observational projects. Whilst widely acknowledged as fundamental to effective healthcare ...
Even so, enrolment in clinical trials run by the SWOG Cancer Research Network, a large clinical-trials programme funded by the NCI, dropped by about half between March and May 2020. Small, early ...
Clinical research is the lifeblood of the biopharmaceutical industry. In January 2024, there were more than 480,000 registered studies on ClinicalTrials.gov, a significant increase from three years ago, when there were 365,000. This uptick is driving demand for researchers and clinical trial participants—and the staggering amount of money and ...
Blood pressure and cerebral oxygenation with physiologically-based cord clamping: sub-study of the BabyDUCC trial. Shiraz Badurdeen. Douglas A. Blank. Peter G. Davis. Clinical Research Article ...