Cookies on Our Future Health
We use small data files to make the website work, known as essential cookies.
We'd like to use analytics cookies so we can improve our site's experience and measure the effectiveness of our marketing activities. We use Matomo and Mixpanel as they do not require us to share analytics data with any third parties.
You can read more in our privacy notice and cookie policy before you choose.

Our Future Health joins the UK Health Data Research Alliance
Convened by Health Data Research UK (HDR UK) , the Alliance includes members from across the healthcare and research sector, including the NHS, medical research charities, academia, health data research hubs and AI Centres of Excellence. Our Future Health is one of ten new organisations working together to develop and coordinate the adoption of standards, tools and technologies for the trustworthy use of data in health research.
The Alliance’s work is to develop a unified and standardised approach to the use of health data across the UK and increase the range and quality of data.
The new members of the Alliance include:
- Our Future Health
- Royal Devon and Exeter NHS Foundation Trust
- Oxford Health NHS Foundation
- Cambridge University Hospitals
- University Hospitals Bristol and Weston NHS Foundation Trust
- Lancashire Teaching Hospitals
- British Heart Foundation (BHF) Data Science Centre
Impact of the Alliance
The Alliance develops and coordinates the adoption of tools, techniques, conventions, technologies, and designs that enable the use of health data in a trustworthy and ethical way. By combining expertise and a shared commitment to work collaboratively, the Alliance is helping researchers to answer some of the most difficult questions and address the most important health challenges faced in the UK.
This year has seen the joint production of Green Papers on key issues for the sector, including the use of Trusted Research Environments (TREs) , Data Use Register Green Paper and Data Standards and Quality .
A shared vision of a unified health data platform
Alliance member organisations will also continue to make the datasets they host as custodians available to be listed on the Health Data Research Innovation Gateway , the UK’s unified platform to discover and request access to health data. This demonstrates their commitment to increasing safe access to health data for research that has the potential to deliver significant public benefit, as we have seen during the pandemic.
Each of these activities are important components in uniting the UK’s health datasets; thereby improving the ability for researchers to discover, access and analyse data to enable the insights that will ultimately improve healthcare for patients.
David Seymour, Alliance Executive Director, Health Data Research UK (HDR UK), said:
“The continued growth in members enriches the Alliance. It is testament to the value of working together across the sector. By co-ordinating our approach to standards, tools and technologies, we will continue to improve how we make health data available in an efficient, secure and trustworthy way for research and development that can change people’s lives. I look forward to working with our new and existing members on our programme of work in the coming months.”
Andrew Roddam, CEO of Our Future Health, said:
“Being part of the UK Health Data Research Alliance will help us deliver on our commitment to make the best use of data to help people live healthier lives for longer. Collaborations like this will ensure we are using data in the most trustworthy and ethical way, which is an essential part of creating a world-leading resource for health research.”
For more information, including a full list of member organisations, visit the Alliance website .
- Visit the Gateway
- Visit the Alliance
- Visit HDR UK Futures
Health Data Research UK Announces New Board Members
16 September 2022
Health Data Research UK is delighted to welcome five new trustees to the Non-Executive Board.

Share this page
Together with the existing eight board members , the new appointees will help ensure the effective governance and development of Health Data Research UK (HDR UK) and provide oversight on the delivery of the Institute’s strategy with the Director and the Senior Leadership Team.
Dr Graham Spittle , Chair of Health Data Research UK said:
“I am delighted to be welcoming Alison, Andrew, Ara, Claire and Mark to the Board of Health Data Research UK.
“They are joining us at a vital inflection point as we approach our second five years and implement our ambitious new strategy.
“Their wealth of knowledge and expertise will complement the strengths of our Senior Leadership Team and will be invaluable to HDR UK as it moves forward to delivering on the mission to unite the UK’s health data to make discoveries that improve people’s lives.”
Claire Bithell
Claire is a Science Communications Consultant with over 20 years’ experience communicating high-profile scientific research and policy using traditional, digital and social media.
During this time, she has held senior roles at a variety of organisations, including the Academy of Medical Sciences, the Human Tissue Authority, The Institute of Cancer Research and the Science Media Centre.
Claire has written widely about science in the media and has worked on numerous projects aimed at improving the communication of science, including contributing to the Science Media Centre’s ‘Changing Role of Science Press Officers’ report, supporting the development of a new labelling system for science press releases, and running a consultation on how mental health is portrayed in the media.
She holds a PhD in Biochemistry from the University of Manchester and was the winner of the Daily Telegraph ’s Young Science Writer of the Year Award in 2003.
Professor the Lord Darzi of Denham
Professor Darzi is Co-Director of the Institute of Global Health Innovation at Imperial College London and holds the Paul Hamlyn Chair of Surgery. He is a Consultant Surgeon at Imperial College NHS Trust and the Royal Marsden NHS Foundation Trust.
Professor Darzi also chairs the NHS Accelerated Access Collaborative, is Co-Director of the NHS Digital Academy and previously served for seven years as a Non-Executive Director of NHS England and NHS Improvement. He is also Chair for the Pre-emptive Medicine and Health Security Initiative at Flagship Pioneering UK plc.
In recognition of his academic achievements, Professor Darzi has been elected a Fellow of the Academy of Medical Sciences, a Fellow of the Royal Society, an Honorary Fellow of the Royal Academy of Engineering. He is also a past president of the British Science Association.
In 2002, Professor Darzi was knighted for his services to medicine and surgery, and in 2007 he was introduced as Lord Darzi of Denham to the UK’s House of Lords as the Parliamentary Under-Secretary of State for Health. He has been a member of Her Majesty’s Most Honourable Privy Council since 2009 and was awarded the Order of Merit in 2016.
Andrew Elder
Andrew Elder is Deputy Managing Partner at Albion Capital Group LLP, a leading independent venture capital investment company.
He initially practised as a surgeon, specialising in neurosurgery before joining the Boston Consulting Group (BCG) as a consultant in 2001.
Andrew focuses on health care and life science technologies, including digital health, and has served on the boards of companies ranging from diagnostics, imaging and software to pharmaceuticals and biomarkers.
He holds an MA and a Bachelor of Medicine and Surgery from Cambridge University and is a Fellow of the Royal College of Surgeons (England).
Sir Mark Walport
Mark Walport is Honorary Distinguished Professor of Medicine at Imperial College and chairs Imperial College Health Partners and the Imperial College Academic Health Sciences Centre.
In the 1990s and early 2000s, he was Professor of Medicine and Head of the Division of Medicine at Imperial College London, where he led a research team focussing on the immunology and genetics of rheumatic diseases.
From 2003 to 2013, Sir Mark took on the directorship of the Wellcome Trust before taking up the role of Government Chief Scientific Adviser (GCSA) and Head of the Government Office for Science.
Most recently, he was the first Chief Executive of United Kingdom Research and Innovation (UKRI).
Sir Mark received a knighthood in the 2009 New Year Honours List for services to medical research and was elected a Fellow of The Royal Society in 2011.
Alison Wilcox
After beginning her career in the NHS, Alison Wilcox moved on to a diverse range of roles spanning management consultancy, HR business partnering, strategy and leadership roles with international businesses Hay Group, Vodafone and BT plc.
Most recently, she was Group HR Director for BT plc, working to shape and drive a comprehensive enterprise-wide transformation agenda to modernise and simplify the business in parallel with creating new digital platforms, businesses and experiences to drive improvements for customers.
She stepped down from BT earlier this year.

We are an independent, registered charity supported by 10 funders, working across 31 locations within the UK, with our Central Team based in London.

Our Board Members
Our Board members bring exceptional and diverse skills and expertise to support us in the delivery of our mission to unite the UK’s health data to enable discoveries that improve people’s lives.

Our Community of Experts
Find out about the experts who are at the centre of our mission to unite the UK’s health data to make discoveries that improve lives. Use the filters below to search people by area of interest,...
- Visit the Gateway
- Visit the Alliance
- Visit HDR UK Futures
Health data research explained
Health data research is an exciting and developing area and can make positive changes in health and care for everyone. There are lots of examples of how health data science has improved our knowledge and helped to solve challenging health problems.
Health data research is a way of gathering, analysing and linking information about people and their health to improve healthcare for all.
Using health data for research helps us better understand diseases and health conditions , such as understanding their causes and symptoms or knowing how many people are affected. It provides new ways of identifying people most at risk of becoming ill, diagnosing diseases earlier, and providing better care and treatment . And it helps health services to run more efficiently and effectively, so everyone can get the care that they need.
Health data research has huge potential to transform healthcare, now and in the future. It has played a major role in the COVID-19 pandemic by helping scientists and doctors understand more about this new disease , and enabling the NHS and decision-makers to respond to the challenge.
Find out how health data research is making a difference
Join the HDR UK Voices Network to get involved with our work
Learn about health data research
What is health data.
Health data refers to data related to a person’s health ; their health conditions, information relating to maternity and children, causes of death, and quality of life. Health data includes, for example: patient health records, studies about the health of groups of people, data from blood or tissue samples, imaging data, and data from health and fitness devices.
Every day, large amounts of health-related data (known as datasets) are generated by the NHS and other health and care services, and from other sources such as academic studies and research . Some of these datasets come from general information about local or national populations, like the number of babies being born or how many people are admitted to hospital on any day. Other types of data are very personal, such as individual medical records or scans.
What is health data research?
Health data research is an exciting and developing area and can make positive changes in health and care for everyone. It combines maths, statistics, and technology to manage and analyse very large amounts of different health data sets across our health and care systems . It is a way of gathering, analysing and linking information about people and their health to enable us to make advances in healthcare and ultimately make improvement to healthcare for all.
There are lots of examples already of how it has improved our knowledge and helped to solve challenging health problems:
- How ethnicity has an impact on COVID-19 hospitalisation
- How the COVID-19 lockdown has impacted people with chronic obstructive pulmonary disease
- Risk factors for dementia development, frailty, and mortality in older adults with epilepsy
- Improvement recruitment for clinical trials through access to health data
- Using machine learning to predict and prevent patients having heart attacks
Combatting the COVID-19 pandemic has depended upon the ability to collect, link, access and use health data for research . It has allowed the NHS to identify and protect millions of people at high risk of COVID-19, to deliver and monitor the safety and effectiveness of the COVID-19 vaccinations programme, and to identify life-saving treatments for COVID-19, including dexamethasone.
These benefits must not stop with COVID-19. They must also extend to people living with other conditions such as mental illness, cancer, heart disease and diabetes . There is huge potential to make better use of information from people’s patient records. Data is vital for your individual care, and to improve health, care, and services across the NHS. If data from many different patients are linked up and pooled, researchers and doctors can look for patterns in the data, helping them develop new ways of predicting or diagnosing illness, and identify ways to improve clinical care .
Click the link to see a diagram from Understanding Patient Data which gives some examples of how health data can be used to make improvements to your individual care, in understanding and diagnosing diseases, improving treatments, evaluating healthcare policies, and for planning NHS services and ensuring patient safety.
Understanding Patient Diagram on data can be used to improve people’s lives.
The information from health data is really valuable to help understand more about disease, to develop new treatments, to monitor safety, to plan services and to evaluate NHS policy.
Data really does save lives. To view a series of animations about how better use of health data has made improvements for people with various health conditions, please go to Understanding Patient Data’s animations page .
Researchers can apply to access these health datasets for research and innovation . There are strict controls on how researchers can access health data information. The purpose must be approved before anyone can use the data, and they are only given access to the minimum amount of data necessary. The types of organisations that may use health data include:
- NHS providers and commissioners : use data to monitor trends and patterns in hospital activity, to assess how care in provided, and to support local service planning
- University researchers : use data to understand more about the causes of disease, to develop new ways of diagnosing illness or to identify ways to develop new treatments
- Charities : use data to evaluate services and identify ways to improve care
- Companies : use data if they are partnered with the NHS to provide care and research. The NHS can’t do all of the analysis on its own and companies may have the best expertise and technologies for making sense of large and complex data from hospitals, or for developing new treatments. If you have more questions about how and why companies access health data, please go to this webpage https://understandingpatientdata.org.uk/companies .
Who are health data scientists?
Health data scientists come from a range of backgrounds and include health researchers, innovators, technology specialists, mathematicians, and statisticians . At Health Data Research UK our community includes:
- doctors working in the NHS with an interest in using data for research
- academic scientists working in universities who use data to understand human health
- colleagues from industry who are using data to develop new tests and treatments
Everyone who uses health data for research and innovation has to work within legal frameworks . This includes the strict parameters of the Codes of Practice and the standards set out by the National Data Guardian and regulatory bodies including the Information Commissioner’s Office. Health data research is for everyone, so it’s important to us that we know what people think about it . We work with patients and the public to find out what matters to them and the kinds of research that should be done with health data.
The organisation which currently has your data (known as data custodians) – your GP practice, community health services, mental health or hospital trust, medical research charity, UK Biobank (a large-scale biomedical database and research resource, containing in-depth genetic and health information from half a million UK participants) or disease registry – is responsible for keeping your data safe.
Every member of staff who works for these organisations has a legal obligation to keep information about you confidential . For example, in the NHS, organisations maintain a duty of confidentiality by conducting annual training and awareness, ensuring access to personal data is limited to the appropriate staff and information is only shared with organisations and individuals that have a legitimate and legal basis for access.
Where systems have been set up to collect data from GP practices, NHS trusts, Health Boards and so on – for instance by NHS Digital in England, the Information Services Division of NHS Scotland, NHS Wales, and Health and Social Care Northern Ireland – the organisations collecting the data have responsibility for it. It is collected and, in most cases, de-identified before being released for research.
Data privacy is incredibly important in health data research . In almost all cases, except where it is directly relevant for personal healthcare, any identifying information (such as people’s names) is removed from datasets used in research. You can find out more about this process in this guide from Understanding Patient Data.
The datasets used by health data scientists come from lots of different sources including:
- Patient data from the NHS and social care, including hospital and GP data such as dates and times of appointments, through to information about treatment, medical and diagnostic tests
- Studies about the health of groups of people , which may be based on a particular health condition, such as cancer, or issues that affect the people’s health, like smoking
- Data from blood or tissue samples such as information about genetic makeup or biological markers of disease
- Data from images such as X-rays, MRI and CT scans that contain a huge amount of information
- Data from clinical trials investigating tests and treatments for health conditions
- Health and fitness devices , which provide data on things like heart rate, activity and calories
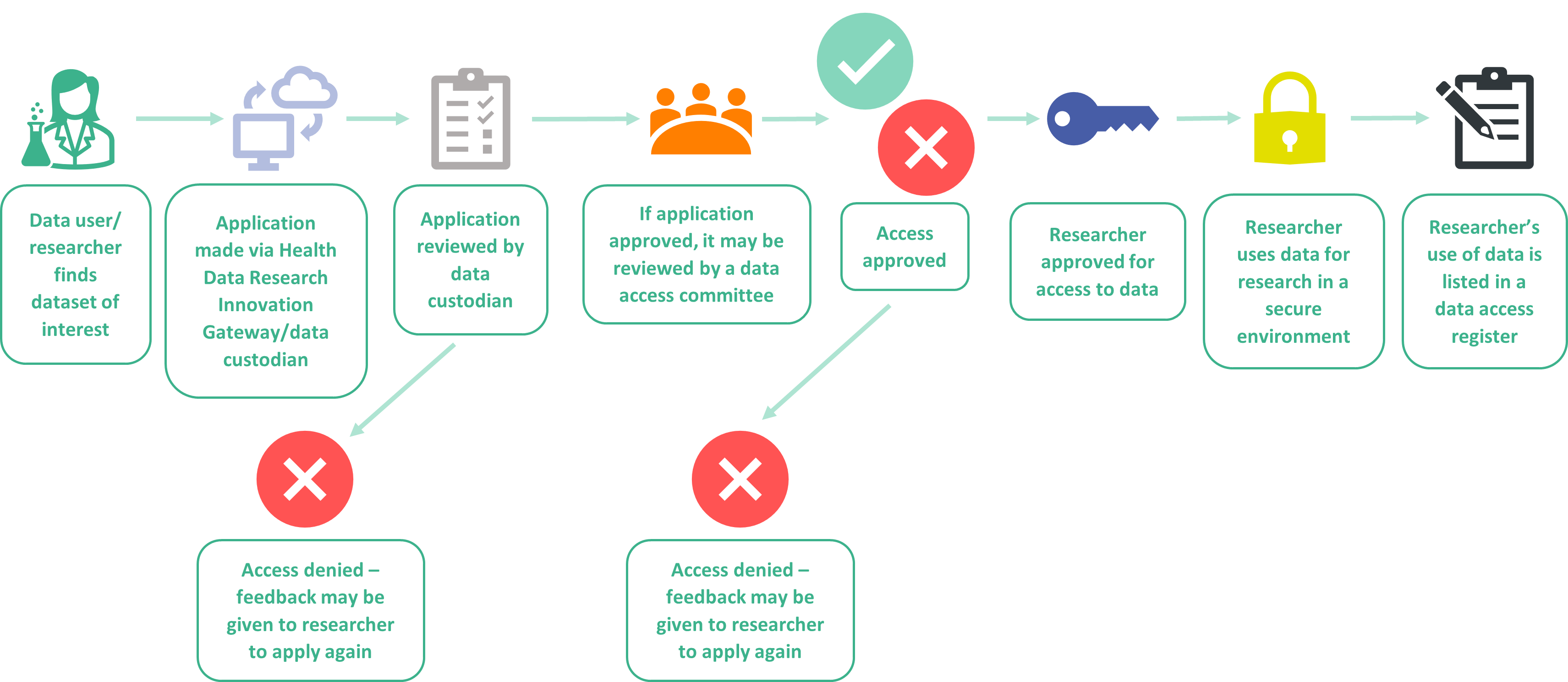
Diagram designed by HDR UK.
When a researcher or innovator (someone who can use health data to make discoveries that lead to patient benefit) wants to have access to a dataset, they have to apply for access by submitting a data access request . This is a form and while these vary slightly between data custodians, they all serve the purpose to establish the reason a researcher is asking for the data.
The form will ask for details on why the user needs the dataset for their research and how, by gaining access, they will use the data to improve health and care services and generate public benefit.
The data access request is submitted to the data custodian (the organisation that holds the data) . If a dataset is listed on the Innovation Gateway, researchers can submit their data access request through the Gateway, and this will be passed to the data custodian.
How is a data access request approved?
The data custodian will make the ultimate decision on whether access is granted to the researcher, and there will be a process in which they review the data access requests . For example, many data custodians have a Data Access Committee; this is a committee or an equivalent body such as a project steering committee, that is involved in access requests and overseeing the management and administration of data access.
They will meet and discuss the request for access and decide whether it should be approved or not, and they may make recommendations or ask questions of the researchers to clarify or improve their request. They may use set criteria to make their decision, and lay members may be members of these committees.
The Five Safes is a framework to help make decisions that will enable effective use of data which is confidential or sensitive . The Five Safes may be used when data custodians and these data access committees consider whether to approve or deny a data access request from a researcher. They will consider why the data is needed, who is accessing the data, and how the data will be protected.
It breaks down decisions surrounding data access and use into five separated dimensions, and these are likely to be used by data custodians through their data access approval processes.
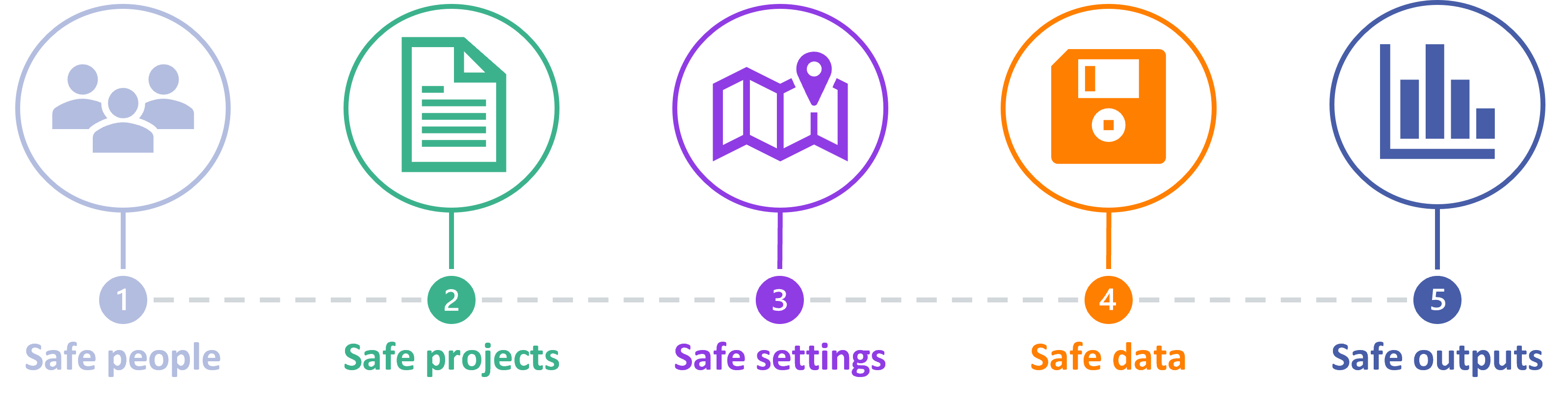
Diagram designed by HDR UK from information on the UK Data Service webpage ( link ).
- Safe People : can the data user be trusted to use the data in an appropriate manner? Do the researchers have the knowledge and skills to act in accordance with the required standards of behaviour?
- Safe Projects : is the use of this data appropriate, lawful, ethical, and sensible? Is the project expected to deliver public benefit?
- Safe settings : does the tool that the researcher is using to access the data prevent unauthorised use or mistakes? Are there controls on the way the data is accessed, both from a technology perspective and considering the physical environment?
- Safe Data : is there a disclosure risk in the data itself? Has the data been treated appropriately to minimise the potential for identification of individuals or organisations?
- Safe outputs : do the results of the research using the data prevent someone from identifying individuals from the data? What can be done to minimise risk when releasing the findings of the project?
There are various ways that health data is protected : by removing identifying information, using an independent review process, ensuring strict legal contracts are in place before data is transferred, and implementing robust data security standards. When researchers are granted access to a dataset, their use of it will be monitored carefully by the data custodian to ensure they are using it appropriately.
A legal contract must be signed before data can be transferred or accessed. This sets out strict rules about what an organisation can do with the data and has clear restrictions on what is not allowed. This data sharing contract may include:
- what data will be provided, and how
- the purpose for which the data can be used
- when and how data must be destroyed after use
- the data security requirements that must be followed
- data cannot be used in any way to re-identify an individual
- data cannot be linked with any other data, unless explicitly approved in the application
- data cannot be passed to any third parties, unless explicitly approved in the application
Data must also be stored securely, with controlled access and robust IT systems to keep data safe and the organisation can be audited to check data is being used appropriately . Technology can be used to protect data, for example by restricting access (using passwords or swipe cards to control access to data) or using encryption so the data can only be read with a code.
IT systems must be kept up to date to protect against viruses and hacking. Anyone accessing data must have appropriate training and be approved by the organisation . Finally, there must be an audit trail that records every time that personally identifiable data is viewed or used.
In many cases it is safer when the data is not transferred between the data custodian and the researcher. This can be done inside a Trusted Research Environment (TRE), also known as ‘Data Safe Havens’.
A TRE is a Trusted Research Environment. Also known as ‘Data Safe Havens’, TREs are highly secure computing environments that provide remote access to health data for approved researchers to use in research that can save and improve lives.
Click here to find out more about Trusted Research Environments
Hopefully the above information has helped your understanding of health data research, but if you would still like further information you may find the following links useful.
- HDR UK’s frequently asked questions
- The Health Data Research Innovation Gateway frequently asked questions
- Health Data Research Innovation Gateway explainer video
- Patient and public page on the Innovation Gateway
- Resources provided by Understanding Patient Data
- NHS Data Saves Lives and Improves Care information page
- Information provided by use MY data
- Data for public benefit report – understanding patient data
If you know of a resource that you think would be useful to include here, please get in touch with the Patient and Public Involvement and Engagement team at [email protected] .
We have created this glossary to help you understand key terms in health data research. We endeavour to update this regularly, but if you notice any key words or definitions that you think are missing, please get in touch with the Patient and Public Involvement and Engagement team at [email protected] .
Access Request/Data Access Request
Where a researcher submits a form to ask a data custodian for access to dataset.
Artificial Intelligence
Artificial Intelligence (AI) is the theory and development of computer systems that are able to perform tasks that normally require human intelligence, such as visual perception, speech recognition, decision-making, and translation between languages.
AI systems or machines can mimic human intelligence to perform tasks and can ‘learn’ and improve themselves based on the information that they collect.
The Caldicott Principles
The Caldicott Principles are a set of eight principles to ensure people’s information is help confidential and used appropriately. These principles apply to the use of confidential information within health and social care organisations and when such information is shared with other organisations and between individuals, both for individual care and for other purposes.
- Principle 1: Justify the purpose(s) for using confidential information
- Principle 2: Use confidential information only when it is necessary
- Principle 3: Use the minimum necessary confidential information
- Principle 4: Access to confidential information should be a strict need-to-know basis
- Principle 5: Everyone with access to confidential information should be aware of their responsibilities
- Principle 6: Comply with the law
- Principle 7: The duty to share information for individual care is as important as the duty to protect patient confidentiality
- Principle 8: Inform patients and service users about how their confidential information is used
To find out more, please go to this webpage The Caldicott Principles – GOV.UK (www.gov.uk) .
A dataset is a collection of data.
Data and Connectivity
A Health Data Research UK (HDR UK) National Core Study making data from all studies available and accessible to inform decision makers and catalyse COVID-19 research.
Data Access
Refers to the availability of data and the process of obtaining data for research. Not all data access is the same and researchers may need more or less types of data for specific projects. The conditions under which access to data is granted often vary by project, researcher, and data controller.
Data Custodian/Data Controller
A term used to describe an individual or organisation who controls the purposes for why and how any health data is accessed and used for research. It is the responsibility of the Controller to ensure that any processing of personally identifiable data is safe and lawful.
Data linkage
Data linkage is a method of bringing information from different sources together about the same person or population to create a new, richer dataset. For example, linking a dataset with information about outcomes from COVID-19 in Yorkshire with a dataset with information about outcomes from COVID-19 in London together, or linking information about health to educational information.
The linkage of information from different information sources enables us to understand how different factors may influence health outcomes, and is valuable in helping to inform policy and research into health and wellbeing.
Data Sharing
The disclosure of data from one or more organisations to another organisation or organisations, or the sending of data between different parts of a single organisation. This can take the form of routine data sharing, where the same data sets are shared between the same organisations for an on-going established purpose; and exceptional, one-off decisions to share data for a specific purpose.
Data Use Register
A data use register is a register or list of data that has been allowed access to for research by a data custodian. It is a public record of how data is being used, by whom and for what purpose.
Data Utility
A term used to refer to the usefulness of a dataset for a given purpose.
De-identified Data
De-identified data refers to data from which all personally identifiable information has been removed to protect individual identities and privacy. This includes items such as your NHS number, name, address, and date of birth (except year).
Disclosive Data
Any information from which you can deduce information about someone or identify individuals.
Electronic Health Record
An Electronic Health Record (EHR) or Electronic Medical Record (EMR) is a digital version of a patient’s medical history. EHRs are real-time, patient-centred records that make information available instantly and securely to authorized users.
FAIR Principles
The FAIR principles contain guidelines for good data management practice that aim to make data FAIR: Findable, Accessible, Interoperable, and Reusable.
- Findable : this means that data can be discovered by both humans and machines, for instance by exposing meaningful machine-actionable metadata and keywords to search engines and research data catalogues.
- Accessible : this means that data are archived in long-term storage and can be made available using standard technical procedures. This does not mean that the data have to be opening available for everyone, but information on how the data could be retrieved (or not) has to be available.
- Interoperable : this means that the data can be exchanged and used across different applications and systems – also in the future, for example, by using different file formats. It also means that the data can be integrated with other data from the same research field or data from other research fields.
- Reusable : the means that the data are well documented and curated and provide rich information about the context of data creation. The data should conform to community standards and include clear terms and conditions on how the data may be accessed and reused. Find out more here .
In data, the term federated means that datasets are held separately is different locations.
Federated Analytics
In data, federated analytics is where various distributed datasets are analysed, and research with those datasets can be carried out as though they are a single data source. The datasets are never combined together, and always stay separated, but researchers can analyse them as though they were a combined dataset.
Health Data
Refers to data related to health conditions, reproductive outcomes, causes of death, and quality of life. Health data includes, for example: patient data, studies about the health of groups of people, data from blood or tissue samples, imaging data, and data from health and fitness devices.
Health data research
A growing area of work and combines maths, statistics and technology to manage and analyse very large amounts of different datasets across our health and care systems. The information we get from health data research will enable us to make advances in healthcare.
Health Data Research Innovation Gateway
The Health Data Research Innovation Gateway (‘Gateway’) provides a common entry point for researchers and innovators (anyone who can use health data to make discoveries that lead to patient benefit i.e., researchers, clinicians, health data scientists, industry researchers) to discover and request access to health data held within UK health datasets. It does not hold or store any health data. It allows researchers to see descriptions (metadata) of health datasets available in the UK so that they can request access to them for research.
Health Data Research Hubs
HDR UK centres of excellence with expertise, tools, knowledge and ways of working to maximise the insights and innovations developed from the health data.
Information Commissioner’s Office
The UK’s independent body set up to uphold information rights https://ico.org.uk/about-the-ico/who-we-are/ .
Anyone who can use health data to make discoveries that lead to patient benefit i.e., researchers, clinicians, health data scientists, industry researchers.
Longitudinal Population Study (including cohort and panel studies)
Longitudinal Population Studies (LPS) track the health of large groups of people over time. These include cohorts, panel surveys and biobanks. They are valuable resources for the scientific community as there is no other way of understanding how biological, social and environmental factors interact over time in a population to produce health outcomes. LPS become more valuable with time, and often develop in unexpected ways, yielding outcomes that could not have been predicted at the outset.
Machine Learning
Machine learning is a type of artificial intelligence that provides computer programs with the ability to automatically learn and improve from experience, without being explicitly programmed. It focuses on the development of computer algorithms that can access data and use it to learn for themselves.
Descriptions and information about data, for example how many records, quality of certain details, or where further information can be found. Each dataset listed on the Gateway has metadata associated with it which can help users decide whether it would be of use to their work and whether they would be eligible to access the dataset.
National Core Studies
The National Core Studies (NCS) programme is enabling the UK to use health data and research to inform both our near and long-term responses to COVID-19, as well as accelerating progress to establish a world-leading health data and research infrastructure for the future.
Epidemiology and Surveillance – collecting data to inform safe levels of restrictions and protection against imminent outbreaks (led by Ian Diamond, UK National Statistician, Office for National Statistics).
Clinical Trials Infrastructure – building on established NIHR infrastructure (and equivalent in the devolved administrations) to accelerate delivery of large scale COVID trials for drugs and vaccines (led by Patrick Chinnery, Clinical Director, MRC and Divya Chadha Manek, Head of Business Development, Vaccines Task Force).
Transmission and Environment (also known as PROTECT) – improving our understanding of how the virus is transmitted in different settings and environments, including in workplaces, on transport and in other public places (led by Andrew Curran, Chief Scientific Adviser. Health and Safety Executive).
Immunity – understanding immunity again COVID to inform back-to-work policies (led by Paul Moss, Professor of Haematology, University of Birmingham).
Longitudinal Health and Wellbeing – using data from longitudinal studies to address the impact of COVID-19 and other associated viral suppression measure on health and wealth to inform mitigating strategies (led by Nishi Chaturvedi, professor of clinical epidemiology, University College London).
Data and Connectivity – making data from all of the above studies (and wider) available and accessible to inform decision makers and catalyse COVID-19 research (led by Andrew Morris, Director, HDR UK).
National Data Guardian
The National Data Guardian (NDG) advises and challenges the health and care system to help ensure that citizens’ confidential information is safeguarded securely and used properly.
Office for National Statistics Framework
The Office for National Statistics (ONS) is committed to providing access to de-identified survey and administrative data for statistical research that delivers a public benefit for the UK. Access is granted to researchers in a safe and consistent way, in line with legislation and ONS policies. We believe that better statistics lead to better decisions and that the research community is pivotal to achieving our national goals. Their research helps the UK understand and make informed decisions regarding the economy, society and people’s quality of life.
This framework provides certainty and clarity on the governance arrangements adopted by the ONS for access to data for research purposes, including the conditions under which access to these data is granted and the framework for accrediting researchers, research projects and data processors. This will help to ensure a consistent and transparent approach for access to data for research purposes so that the benefits are more easily realised, and the confidentiality of the data is protected.
Patient Data
Data that is collected about a patient whenever they go to a doctor or receive social care. It may include details about the individual’s physical or mental health, such as height and weight or detail of any allergies, and their social care needs and services received. It may also include next of kin information.
Pseudonymisation
Pseudonymisation means the processing of personal data in such a manner that the personal data can no longer be attributed to a specific data subject or individual without the use of additional information. This process de-identifies data and replaces personally identifiable information fields within the data record by artificial identifiers. For example, a dataset will remove NHS numbers of individuals who data is represented, and may replace exact date of birth and postcode of home address with the year of birth and a larger postal code.
Public Contributor (Lay Member)
The term public contributor (or lay member) is widely used to refer to a member of the public or a patient who is involved in research or activities with an organisation, for example by being part of a board or group, to provide their personal experiences and views and be a ‘critical friend’ for the organisation. Lay members will have an expert understanding of what matters most for people and help to bring that perspective.
Research Priorities – Better Care
The HDR UK Better Care programme aims to improve people’s lives by equipping clinicians and patients in the UK with the best possible data-based information to make decision about their care. Our vision is that by 2030, patients across the UK will benefit from healthcare decisions informed by large scale data and advanced analytics to identify what will work best for them.
Research Priorities – Clinical Trials
Getting enough people for a clinical trial is critical, as there is a level below which a trial’s results are just not robust enough. These days, a large portion of NHS records are electronic, so we are able to search the database for all patients that could benefit from a particular trial, and source participants from the entire population. The HDR UK Clinical Trials programme uses health data to ensure that every individual across the UK has access to the latest treatments and technologies through access to clinical trials.
Clinical trials track long-term health of participants by having consented access to participants’ NHS medical records. This allows for more efficient and longer-term follow-up processes. We want to provide a system that all clinical triallists can use to identify large numbers of the right trial participants. We also work on new approach to analyse trial results.
Research Priorities – Improving Public Health
The causes of poor health can be unexpected, long or short term, as well as local or global. We don’t always have the right data to know what effect a behaviour or intervention might have on public health. In many cases, the data we need already exists in GP surgeries and hospitals; the challenge is to create the infrastructure to link it all together. The HDR UK Improving Public Health programme aims to develop the capability to identify the things that cause ill-health in people, as well as the factors that result in improved health – no matter where they live or what their socioeconomic background – across the whole population of the UK.
We will enable data science to transform public health research through linking to data beyond health care, for example, to other government sectors, organisations, and data on environments that influence our health.
Research Priorities – Understanding the Causes of Diseases
The HDR UK Understanding the Causes of Disease programme aims to help advance understanding of disease prediction, causation, and progression through the integration of molecular data and other intermediate phenotypes with routine clinical data. The overarching hypothesis is that insights into biology and disease aetiology (the causes of a disease of condition) can be revealed by integration of information, at scale, on genomics, other biomolecular traits, and high-resolution electronic health records (EHRs).
The ultimate vision is to create new informatics infrastructures and data science methods that help achieve a deep integration of biology, biomedicine, and population health science.
Trusted Research Environment (Data Safe Haven)
Trusted Research Environments, also known as ‘Data Safe Havens’, are highly secure spaces for researchers to access sensitive data. They are based on the idea that researchers should access and use data within a single secure environment. In other words: users go to the data, the data doesn’t travel to them. Trusted Research Environments have multiple layers of security and safeguards in place, designed to minimise the risk of anyone’s data being misused.
UK Health Data Research Alliance
HDR UK’s Alliance of leading health, care and research organisations united to establish best practice around the ethical use of UK health data for research and innovation at scale. Alliance members have listed their datasets on the Gateway.
Involving and engaging patients and the public
We ensure our work is worthy of public trust and confidence by meeting people's needs. Visit our pages to see how you can get involved as a public member. If you're a researcher, take a look at our training and support.
The ICODA team led the delivery of the International COVID-19 Data Alliance on behalf of the Executive Leadership Team. The team combined skills from diverse backgrounds to achieve its goals.
Andrew Morris

Trudie Lang

Neil Postlethwaite

Anne Wozencraft

Sarah Fullegar

Matthew Retford

Sally Boylan

Edel McNamara

Jonas Häggström

Professor Andrew Morris has been the inaugural Director of Health Data Research UK since August 2017. HDR UK is the national Institute for health data science, with its mission is to unite the UK’s health data to enable discoveries that improve people’s lives. He is seconded from his position as Professor of Medicine, and Vice Principal of Data Science at the University of Edinburgh.
Andrew was Chief Scientist at the Scottish Government Health Directorate (2012-2017). Andrew currently chairs the Scottish COVID 19 Advisory Group which supports and provide analysis to the Scottish Government and senior clinical advisors.
He has published over 330 original papers, attracted over £100 million in grant funding and was the principal investigator of several programme grants including the Wellcome Trust United Kingdom Case Control Collection for Type 2 Diabetes, Generation Scotland, and the Wellcome Trust funded Scottish Health Informatics Programme (SHIP).

Global Research Director at Health Data Research (HDR) Global and Professor of Global Health Research at the University of Oxford and Head of The Global Health Network
Trudie Lang is Professor of Global Health Research; Head of The Global Health Network and Senior Research Scientist in Tropical Medicine, Nuffield Department of Medicine. She has over 20 years’ experience in running clinical trials, including trials in the developing world, for the pharmaceutical industry, the World Health Organisation and in academia.
Trudie focuses on combating diseases of poverty through the generation of high-quality evidence. She has worked in industry, academia and UN organisations. With her team and partners, she works to drive better health outcomes in vulnerable communities by enabling local leadership and ground-up implementation of high-quality health research studies.
Within the University of Oxford, she devised and leads The Global Health Network which is a major international collaborative enterprise that sets out to improve health by improving research.

Neil Postlethwaite is Technology Director for the International COVID-19 Data Alliance and joined HDR UK in September of 2020. He is responsible for ensuring successful product deliveries from all our technical alliance partners, including the Workbench, a trusted research environment that allows researchers to discover, access and analyse global datasets whilst respecting confidentiality and privacy.
Prior to joining HDR UK, Neil worked for 23 years at IBM where he held a variety of technical, management and executive positions, working across IBM’s Messaging and Integration middleware portfolio. He has been responsible for deliveries of both IBM MQ and IBM Integration products across a myriad of platforms and release levels. He was Director responsible for IBM’s acclaimed Watson Internet of Things Platform and worked extensively across the IoT ecosystem building strategic partnerships with silicon and device manufacturers and driving Open Standards.
Neil holds a degree in Electronic Engineering & Physics from Loughborough University.

Anne is Head of Partnerships at ICODA and is responsible for engagement with, and support for, the Alliance’s global partners. Prior to joining ICODA, she led the International Partnerships team at Cancer Research UK, where she developed the charity’s international strategy and expanded its partnerships in global research, policy and health. Previously, Anne held a series of executive board roles at the British Council, the UK’s international cultural relations organisation, where she led global programmes in science, research and higher education, and was then acting Head of International Relations at Imperial College London.
Anne began her career as a research scientist and lecturer at King’s College London, specialising in the immunology of infectious diseases. She completed her PhD at the London School of Hygiene and Tropical Medicine and held a post-doctoral fellowship at Scripps Clinic, La Jolla.

Sarah Fullegar is the Communications Manager at ICODA.
Sarah joined HDR UK as an Executive Assistant in October 2020 and has over 15 years of experience working in the charity and not for profit sector.
Sarah holds a BA in Media with Cultural Studies from Southampton Solent University.

Matthew Retford is Technical Project Manager and Agile Scrum Master for the International COVID-19 Data Alliance (ICODA). He joined HDR UK in May of 2021 and is responsible for the technical delivery and oversight of ICODA programmes and work-streams.
Matthew brings 5+ years of experience in the Health-Tech sectors in the UK and Australia. Prior to joining HDR UK Matthew was Lead Product Manager at Cera, the largest home care provider in the UK. His work at Cera focussed on the collection and analysis of health and operational data.
Matthew holds a degree in Computer Science from The University of Newcastle, Australia.

Sally is Project Manager and Grants and Agreements Coordinator within the ICODA team and supports 5 of the 10 Grand Challenges ICODA pilot projects on a day to day basis.
Previously, Sally worked at the Association of Charitable Foundations as Programme Manager for their Stronger Foundations initiative, a programme of inquiry into excellent foundation practice which took place over an 18 month period and which produced 5 thematic reports on Strategy and Governance, Transparency and Engagement, Funding Practices, Diversity, Equity and Inclusion and Learning and Evaluation. Prior to joining ACF, Sally worked as a freelance funding consultant for a number of years. Earlier in her career Sally led the institutional fundraising team at ActionAid and spent 11 years working for what is now the National Lottery Community Fund, managing a portfolio of funding programmes, and latterly leading a business change programme.

Data Protection and Information Governance Lead – Global

Jonas is an experienced biostatistician with 20+ years of experience in the life science field. Jonas is the Chair of ICODA Statistical Expert Group and VP of Global Health at Cytel. In addition, he also serves as an expert statistical consultant to the Bill & Melinda Gates Foundation, and as an expert evaluator to the European Commissio.
Prior to joining Cytel Jonas has had positions in industry, academia, and non-profit organizations, and his previous appointments includes; Food and Agriculture Organization (UN organization), Genentech Inc, and AstraZenec. At AstraZeneca Jonas was the Neuroscience Therapeutic Area Statistical Expert. In that role Jonas lead and managed the department, provided input to drug development strategy and design across all phases, and was responsible for the statistical methodology.
In addition to our ICODA core team members, we were supported by and worked very closely with all of our extended team within Health Data Research (HDR) UK , our convenor.
Health Data Research UK is a registered charity no. 1194431 funded by UK Research and Innovation, the Department of Health and Social Care in England and equivalents in Northern Ireland, Wales and Scotland, and leading medical research charities.
Executive Leadership Team
The Executive Leadership Team (ELT) developed the strategic direction for ICODA and was accountable for the delivery of our core work.
The ELT was overseen by, and accountable to HDR UK and our funders. Its work was also guided by advice from our Advisory Councils on Science and Strategy and on Ethics, and an expert group on Statistics.
The ELT brought together key stakeholders as well as members of our ICODA executive team:
- Andrew Morris (Chair) Director, Health Data Research UK
- Trudie Lang Global Research Director at Health Data Research UK, Professor of Global Health Research at the University of Oxford and Head of The Global Health Network
- Steve Kern Deputy Director, Quantitative Sciences; Global Health – Integrated Development, Bill and Melinda Gates Foundation
- David Sibbald CEO Aridhia Informatics
- N é vine Zariffa Principle and Founder, NMD Group LLC
- Aziz Sheikh Professor of Primary Care Research & Development and Director of the Usher Institute, University of Edinburgh
- Neil Postlethwaite Technical Director, ICODA
- Anne Wozencraft Director of Partnerships and Strategic operations, ICODA

David Sibbald

N é vine Zariffa

Aziz Sheikh

Steven E. Kern, PhD is Deputy Director of Quantitative Sciences at the Bill and Melinda Gates Foundation. He leads a team of scientists focused on gaining insight from data coming from Gates Foundation investments to inform program strategies in global health.
The Quantitative Sciences group is focused on quantitative data analysis to support program strategies for therapeutic projects that the foundation funds. This effort extends across all therapeutic areas in which the foundation is involved including maternal & child health, family planning, malaria, tuberculosis, neglected tropical diseases, HIV, and pandemic preparedness. He and his team are strong advocates of making research data “always FAIR and sometimes OPEN” to improve the impact data can have towards the problem it was collected to address, and beyond.
Prior to this, he was Global Head of Pharmacology Modeling at Novartis Pharma AG based in Basel Switzerland where he led a team focused on providing model-based drug development support to therapeutics in many disease conditions across all stages of drug development. He joined Novartis in 2010 from the University of Utah in Salt Lake City, Utah where he was Associate Professor of Pharmaceutics, Anesthesiology, and Bioengineering, and served as co-investigator for their NIH funded Pediatric Pharmacology Research Unit.
He has designed, conducted, and served as a principal investigator for clinical pharmacology studies that spanned the population from preterm infants to elderly adults

David is the CEO of Aridhia, which he founded with Professor Andrew Morris in 2008. He is also the Co-Founder and Board member of Sumerian, an IT analytics company he founded in 2002 and was previously the Co-Founder, Chairman and CEO of Atlantech Technologies, a communications software company which he founded in 1992 and sold to Cisco Systems in March 2000.
David is the Founder and Trustee of the Kate MacAskill Foundation, a charitable organisation providing education, care and micro-enterprise funding for children and young adults in the developing world and is Chairman of the Johari Foundation. He has been awarded honorary doctorates from Glasgow University, Glasgow Caledonian University, the University of Paisley and the University of Strathclyde for services to Scottish science and philanthropy. He is a Fellow of the Royal Society of Edinburgh, Scotland’s National Academy of Science and Letters. In the 2010 New Year Honours List, David was awarded an OBE for charitable services in Scotland and overseas.

Névine Zariffa, MMath is a highly accomplished thought leader in the fields of biostatistics and data science with extensive experience across all phases of drug development. Névine had a 25-year career in senior roles at GlaxoSmithKline and AstraZeneca where she also led the Enterprise Data & Analytics initiative. She has been a key contributor to development strategies for over 200 drug projects across oncology, cardiovascular, metabolic, respiratory, inflammation, and renal diseases. She served as a Board member of CDISC for 6 years, has been a reviewer for The Lancet and has over 30 peer reviewed publications to her name. She is currently a strategic consultant to select healthcare clients, several scientific data consortia ( ICODA and ctMoniTR ) and to the FDA’s Office of the Commissioner on the application of real-world evidence to COVID-19.

Aziz is a member of the Executive Leadership Team of ICODA and leads Driver Project 2.
Aziz Sheikh is Professor of Primary Care Research & Development, Director of the Usher Institute and Dean of Data at the University of Edinburgh. He is a clinical epidemiologist with substantive interests in health policy, health informatics and the quality and safety of health care. Aziz is Editor-in-Chief of the International Journal of Quality in Health Care. He is Director of the NIHR Global Respiratory Health Unit (RESPIRE), Director of the Health Data Research UK BREATHE Hub and co-Director of the NHS Digital Academy.
Aziz has been honoured with an OBE, fellowships from 9 learned societies and is the recipient of numerous national and international awards.
Scientific & Strategic Advisory Council
The Scientific & Strategic Advisory Council provided high-level advice to help us identify opportunities to tackle the COVID-19 pandemic, to deliver scientific impact and overcome the challenges of data sharing.
Aligned with ICODA’s commitment to transparency, the Scientific and Strategic Advisory Council’s Terms of Reference and meeting summaries are publicly available here .
"We are very fortunate to have the opportunity to design a platform for the global community of health researchers to generate better health outcomes for the world."
Agnes Binagwaho, MD, M(Ped), PhD
Co-Chair of Scientific Advisory Council;
Vice Chancellor, University of Global Health Equity;
Former Minister of Health of Rwanda;
Senior Lecturer on Global Health and Social Medicine, Harvard Medical School
"The Covid-19 pandemic presents our world a powerful opportunity … and responsibility … to share knowledge in real-time. Time must not be wasted because lives and livelihoods hang in the balance. ICODA has been created and dedicated to facilitating Partnership … Progress … that will derive Hope. We are stronger together. Our shared data will supercharge discovery that will save lives."
Martin J. Murphy, DMedSc, PhD, FASCO
Founding Chief Executive Officer, CEO Roundtable on Cancer
Founding CEO of Project Data Sphere, LLC, a non-profit enterprise devoted to cancer clinical trial data-transparency, data-sharing and data-analysis

Prof Agnes Binagwaho

Dr Martin J Murphy

Prof Frank Rockhold

Prof John Danesh

Prof Nicky Mulder

Prof Steve Webb
Prof Trudie Lang

Prof Wang Yu

Gail Stephens

Prof Liang Wannian

Professor Agnes Binagwaho co-chairs the Scientific & Strategic Advisory Council of the International COVID-19 Data Alliance (ICODA).
Professor Agnes Binagwaho, MD, M(Ped), PhD is a Rwandan paediatrician who returned to Rwanda in July of 1996, two years after the 1994 Genocide Against the Tutsi. Since then, she has provided clinical care in the public sector, served the Rwandan Health Sector (2001-2016) in high-level government positions, first as the Executive Secretary of Rwanda’s National AIDS Control Commission, then as Permanent Secretary of the Ministry of Health, and 5 years as Minister of Health. She co-founded the University of Global Health Equity (UGHE), an initiative of Partners In Health, which focuses on changing how health care is delivered around the world by training global health professionals who strive to deliver more equitable, quality health services for all.
Professor Binagwaho currently resides in Rwanda and is the Vice-Chancellor of the University of Global Health Equity. She is specialised in emergency paediatrics, neonatology, and the treatment of HIV/AIDS. She completed her MD at the Université Libre de Bruxelles and her MA in Paediatrics at the Université de Bretagne Occidentale. She was also awarded an Honorary Doctor of Science from Dartmouth College and earned a Doctor of Philosophy from the University of Rwanda College of Medicine.
Professor Binagwaho currently serves as a Senior Advisor to the Director General of the World Health Organisation, and as a member of multiple Advisory Board and Board of Directors including the Rockefeller Foundation Board. She is a member of a number of international working groups and task forces in global health for the United Nations and independent organizations and also sits on the Editorial Board of several scientific journals and serves on multiple scientific commissions.
Previously, she co-chaired the Millennium Development Goal Project Task Force on HIV/AIDS and Access to Essential Medicines for the Secretary-General of the United Nations under the leadership of Professor Jeffrey Sachs (MGGs). Professor Binagwaho also co-chaired the Joint Learning Initiative on Children and HIV/AIDS (JLICA) (2006–09) and founded the Rwandan Paediatric Society, chairing it until 2019. Since 2016, she has been a member of the American National Academy of Medicine and since 2017 a Fellow of the African Academy of Sciences. In 2015, Professor Binagwaho received the annual Roux Prize and Ronald McDonald House Charities Award of Excellence.
She is currently a senior Lecturer in the Department of Global Health and Social Medicine at Harvard Medical School, a Professor of Paediatrics at UGHE, as well as an Adjunct Clinical Professor of Paediatrics at Dartmouth’s Geisel School of Medicine. Professor Binagwaho’s academic engagements include research in implementation sciences, research on human rights to health, health services delivery systems strengthening, HIV/AIDS, and paediatric care. She has published over 190 peer- reviewed articles.

Martin J Murphy, DMedSc, PhD, FASCO co-chairs the Scientific & Strategic Advisory Council of the International COVID-19 Data Alliance (ICODA).
Martin J Murphy , was Founding CEO and Board Director of the 20-year-old non-profit CEO Roundtable on Cancer at the request of the 41 st President George H.W. Bush. Marty was also Founding CEO of Project Data Sphere ®, a non-profit initiative of the CEO Roundtable devoted to cancer clinical trial data-sharing and analysis. He is Founding Chief Executive Officer of the non-profit Shanghai TuoXin Health Promotion Center that has expanded its purview from cancer prevention and improving cancer patient outcomes to include international Covid-19 interventions and global pandemic preparedness.
Founding Executive Editor of the 26-year-old peer-reviewed journal, The Oncologist , Marty is also Founding Editor of Stem Cells , the 39-year-old flagship journal of stem cell biology, and Co-Founder of its 10-year-old sister journal, Stem Cells Translational Medicine reporting on stem cells in clinical trials. In 2021 he became Director Emeritus of the CEO Roundtable on Cancer .
An NIH principal investigator, Marty has authored more than 180 primary peer-accepted papers and 7 books, is co-founder of Society for Translational Oncology , member of six Scientific Advisory Boards, two corporate boards of directors, Chairman Emeritus of Conquer Cancer—ASCO Foundation , and Steering Member of Chinese Society of Clinical Oncology . He is an Emeritus Member of National Cancer Policy Forum of National Academy of Medicine ( US National Academy of Sciences), board member of Center for Public Health Advisory Board of the Milken Institute, Director Emeritus of Foundation for NIH , Fellow of American Society of Clinical Oncology , and former vice chairman of C-Change . Marty is married to Ann Murphy, PhD, president and founder of AlphaMed Press and co-founder and executive director of Society for Translational Oncology . They have five adult children and ten grandchildren.

Professor Frank Rockhold is a Professor of Biostatistics and Bioinformatics at Duke University Medical Centre, Affiliate Professor of Biostatistics at Virginia Commonwealth University, and Managing Partner of HunterRockhold, Inc. He has diverse research interests and consulting experience including trial design, data monitoring, benefit/risk, and pharmacovigilance and has been a leader in the scientific community in promoting data disclosure and transparency in clinical research. His career includes senior management positions in industry, most recently as Chief Safety Officer for GSK. Dr Rockhold served as Chairman of CDISC and president of the Society for Clinical Trials. He also served on the initial PCORI Advisory Board and is currently on the boards of the Frontier Science Foundation and an advisor to EMA.
Frank holds a BA in Statistics from The University of Connecticut, an ScM in Biostatistics from The Johns Hopkins University, and a PhD in Biostatistics from the Medical College of Virginia at Virginia Commonwealth University. He is an Elected Fellow of both the American Statistical Association, the Society for Clinical Trials, and the Royal Statistical Society. He is an Accredited Professional Statistician, PStat®, and a Chartered Statistician, CStat.

Professor John Danesh is Professor of Epidemiology and Medicine and Head of the Department of Public Health and Primary Care at the University of Cambridge. Alongside his role as Research Director, he is also Director of the British Heart Foundation’s Cardiovascular Epidemiology Unit, Associate Faculty Member at the Wellcome Trust Sanger Institute, and Honorary Consultant at the Cambridge University Hospital NHS Foundation Trust.
Before receiving his MSc from the London School of Health and Tropical Medicine, he trained in medicine at the University of Otago in New Zealand and at the Royal Melbourne Hospital in Australia. His main research interests are discovery genomics and cardiovascular genetics, therapeutic target prioritisation, international vascular health, screening and risk prediction, systems genomics and blood donor health and biology.

Professor Nicky Mulder heads the Computational Biology Division at the University of Cape Town (UCT), and is a full member of the Institute of Infectious Disease and Molecular Medicine. She leads H3ABioNet, a large Pan African Bioinformatics Network of 28 institutions in 17 countries, which aims to develop bioinformatics capacity to enable genomic data analysis on the continent. H3ABioNet has developed an extensive training program for African researchers. She also co-leads a Sickle Cell Disease Data Coordinating Centre and a Wellcome Trust Centre Data Integration Platform at UCT.
She received her PhD in Medical Microbiology from the University of Cape Town and then worked for 8.5 years at the European Bioinformatics Institute in Cambridge, as a Team Leader.
At UCT her research focuses on genetic determinants of susceptibility to disease, African genome variation, and microbial genomics and infectious diseases from both the host and pathogen perspectives. Her group provides bioinformatics services and training and develops new algorithms and resources for the analysis of complex African genetic data. Prof Mulder is actively involved in capacity development, including training, education and curriculum development in Bioinformatics. She also sits on a number of international scientific advisory boards.

Professor Steve Webb MBBS, MPH, PhD, FRACP, FCICM, FAAHMS
Dr Steve Webb is an ICU specialist at Royal Perth Hospital, a Professor of Critical Care Research at Monash University, and Deputy Chair of the Australian Clinical Trials Alliance. He designs and conducts clinical trials that generate evidence to improve patient care. He is named as an investigator on more than $115 million in research funding, has been an investigator on trials with an accumulated sample size of more than 50,000 patients, and has published more than 180 manuscripts including in the NEJM (7), JAMA (5), and The Lancet (2). He has particular experience with Bayesian adaptive platform trials and other innovative designs such as cluster cross-over trials.

Wang Yu, MD, PhD, is the distinguished Professor of Wanke School of Public Health, Tsinghua University. He served as Director General of the Chinese Center for Disease Control and Prevention for 13 years (2004-2017). He is the president of the Chinese Foundation for Hepatitis Prevention and Control.
In the academic field, Dr Wang Yu was dedicated to research on hepatitis viruses and hepatology. He did research and clinical practice in People’s Hospital, and became the director of the Institute of Hepatology, Peking Medical University. Later he was appointed vice president of Peking Medical University. He then transferred to the Ministry of Science and Technology as the deputy director of China National Center for Biotechnology Development (CNCBD).
Wang Yu participated in the response to the SARS outbreak, and led the national response to emerging infectious diseases and public health emergencies, such as the H1N1 Flu pandemic and the Wenchuan post-earthquake disease prevention. He led and promoted a national disease surveillance system initiative for investigation and intervention in areas with higher cancer prevalence. Internationally, he led and participated in the response to the Ebola outbreak in West Africa and was actively involved in the initiative of the public health system strengthening in Africa. Dr Wang Yu was invited to join the CEO Roundtable on Cancer in 2016, and currently serves as the president of Shanghai TuoXin Health Promotion Center, which is a Shanghai based non-profit focused on chronic disease prevention and education in workplace.
Dr Wang is on the Executive Board of the Chinese Medical Association. Internationally, he serves as the member of the WHO Regional Commission for the Certification of Poliomyelitis Eradication in the Western Pacific.

Gail Stephens is the executive champion of Health Care and Life Sciences at SAS, with a passion for helping customers harness data’s hidden intelligence to make the world a better place. She heads Research and Development (R&D), product management/strategy, industry consulting as well as philanthropic efforts in this industry. She consults with customers on data-driven initiatives, and evangelizes how SAS software delivers insights that transform organizations.
A principal responsibility is Project Data Sphere, the groundbreaking collaboration between researchers and SAS that makes historical clinical trial data and analytics broadly accessible to those seeking to advance cancer treatment research. Using a first-of-its-kind platform, the project integrates SAS Health and Life Sciences, machine learning and AI as well as Business Intelligence (BI) software.
Leading collaborative teams that deliver quality software has been Stephens’s mission for 35 years at SAS. In previous roles, she developed graphical user interfaces (GUIs) and managed teams that developed some of the earliest GUIs at SAS. She also spent many years guiding the overall BI R&D efforts at SAS, an experience that provided unique insights into streamlining BI software within the Health Care and Life Sciences projects she oversees today.
A North Carolina native, Stephens graduated from North Carolina State University with a bachelor’s degree in computer science. She also served on the Strategic Advisory Board for the computer science department. In addition, she has served on the executive board of North Carolina Healthcare Information and Communications Alliance.

Professor Liang Wannian, PhD, is the incumbent vice dean of the Vanke School of Public Health, Tsinghua University, and the leader of the National Health Commission’s COVID-19 Response & Reaction Expert Group.
From the dean of Teaching & Research Section of Sociological Medicine & Health Management to the vice president of the Capital Medical University (1997–2003), and from the deputy chief of the Beijing Municipal Health Bureau to the Minister of the Institution Reform Department of the National Health Commission (2003–2020), Liang held increasingly important positions both in the university and municipal/state functions. Liang played a central role in the national fight against both SARS and COVID-19, and gained rich practical experience in dealing with the pandemic situation.
Liang was awarded Outstanding Mid-Career Expert by the Ministry of Human Resources & Social Security of the People’s Republic of China in 1992, Municipal Outstanding Individual in Prevention of SARS by the United Group for SARS Prevention of Beijing in 2003, Honorary Title of the National Outstanding Science & Technology Worker by the China Association for Science & Technology, and another seven primary awards, along with ten technology awards.
Liang completed his MD at the Anhui Medical University and his PhD at Peking Union Medical College. He specialized in the fields of epidemiology, health statistics, health emergency management and general medicine. He has published 24 English papers in SCI and 480 Chinese papers and 46 textbooks and academic monographs as editor-in-chief.
Ethics Advisory Council
The Ethics Advisory Council provided high-level advice on ethics and data governance, helping us to build and demonstrate trustworthiness across all our activities.
- Effy Vayena (Chair) Chair of Bioethics, at the Swiss Institute of Technology (ETHZ)
- Pamela Andana Wits University, South Africa
- Claudia Emerson McMaster University, Canada, Director, Institute on Ethics & Policy for Innovation
- Sharon Kaur University of Malaysia
- Katherine Littler Co-Lead, Global Health Ethics & Governance Unit, World Health Organization
- Graeme Laurie , Professor of Medical Jurisprudence, University of Edinburgh
- Elettra Ronchi Senior Policy Analyst, OECD
Aligned with ICODA’s commitment to transparency, the Ethics Advisory Council’s Terms of Reference and meeting summaries are publicly available.
The Ethics Advisory Council has provided advice on the development of our Ethics and Governance Framework , and acted as an independent guardian of the Framework.

Effy Vayena

Professor Effy Vayena is Chair of Bioethics, at the Swiss Institute of Technology (ETHZ) and renowned expert at the intersection of medicine, data, and ethics. Her work focuses on important societal issues of data and technology as they relate to scientific progress and how it is or should be applied to public and personal health.
A keen interest in health policy has led her to work with the World Health Organization on ethical questions around reproductive medicine and research. Upon her return to academia, Vayena helped establish and coordinated the PhD program in Biomedical Ethics and Law at University of Zurich and was subsequently awarded a professorship by the Swiss National Science Foundation.Effy Vayena believes in the value of interdisciplinarity and the application of practical knowledge to investigate ethical questions of personalised health. Her widely recognised research has allowed her to collaborate and build strong relationships with a variety of scholars in Switzerland and from renowned institutions abroad.
Vayena is a leading expert in the dynamic and diverse field of health data and ethics, successfully leveraging her international network to promote a fruitful debate about the ethics of health in the digital age. She has previously worked with the Wellcome Trust, OECD, Commonwealth Fund, Chatham House, and academic institutions and governments around the world. She was born in Greece and studied medicine history, public health and bioethics at universities in Athens, London, Minnesota and Harvard. From 2000-2007 she worked as a technical manager for the WHO in the field of reproductive medicine and ethics. Since 2007 she has been a research associate and coordinator of the PhD program in biomedical ethics and law at the University of Zurich. She is researching, among others, the ethics of personalized medicine, the moral question of genetic testing, and the ethical challenges of using Big Data within health research and clinical practice.
Statistical Expert Group
The Statistical Expert Group (SEG) promoted and facilitated responsible sharing of data, use of analytical methods and tools, and high quality results to support dissemination of knowledge worldwide.
- Jonas Häggström (chair) VP Global Health, Cytel
- Frank Rockhold Professor of Biostatistics and Bioinformatics, Duke Clinical Research Institute
- Amy Racine Distinguish Scientist, Novartis
- Joan Buenconsejo Senior Director, Biostatistics, AstraZeneca
- Maia Leskovsky Associate Professor, University of Cape Town
- Ling Wang Executive Director Biostatistics, Pfizer
- John Norrie Chair of Medical Statistics and Trial Methodology, Director, Edinburgh Clinical Trials Unit (ECTU)
- Peter Mesenbrink Executive Director Biostatistics, Novartis
- Estelle Russek-Cohen Senior Advisor Office of Biostatistics, CDER
- Robin Mogg Clinical Biostatistics Leader – Bill & Melinda Gates Medical Research Institute
“There is this underlying tenet that everything we do is based on equality, collaboration, and open access to data and information. So, it was obvious for the Minderoo Foundation to understand the context of this initiative. We were impressed by the partners involved on the funder side and with HDR UK as the convener, so it seemed appropriate to support this Alliance.”
Steve Burnell
Minderoo Foundation
ICODA was convened by Health Data Research UK – the national institute for health data in the UK. It was supported by a grant from the COVID-19 Therapeutics Accelerator , a large-scale initiative initiated by the Bill & Melinda Gates Foundation , Wellcome , Mastercard with additional support from Minderoo Foundation , and other donors to speed the development of and access to therapies for COVID-19. Other funders to the International COVID-19 Alliance included Microsoft, through its AI for Health program:

Bill and Melinda Gates Foundation

Chan Zuckerberg Initiative

Microsoft AI for Health
ICODA provided the opportunity to make a real difference to harness the power of health data to help tackle the COVID-19 pandemic. ICODA was a rapidly-convened initiative and an ambitious and dynamic global programme.
We were a diverse group of experts working together, based on a set of united principles and a “One Institute” approach, a collaborative culture inherited from our convenor, Health Data Research UK.
ICODA is completing its activities in October 2022. Further job opportunities in HDR Global as they arise will be posted on HDR UK’s website in the jobs section .
Alternatively, please contact us .
Find more information on our Board of Directors/Trustees and Advisors to the Board, including individual bios.
Board of Directors/Trustees
Our Board is responsible for the effective governance and development of Research Data Scotland, supports the directors in overseeing the delivery of our strategy, monitors key risks, and ensures resources are managed effectively.
Our Directors/Trustees and Advisors bring exceptional and diverse skills and expertise to support us in the delivery of our mission to improve the economic, social and environmental wellbeing in Scotland by enabling access to and linkage of data about people, places and businesses for research in the public good.
- Board members
Advisors to the Board

Roger Halliday
Chief Executive Officer

Professor Paul Boyle
Chair of the Board


Professor Jill Pell
Board Member

Professor Mark Parsons

Scott Heald

Professor Julie Fitzpatrick, OBE

Martin Sinclair

Professor Andrew Morris
Advisor to the Board

Dr Emma Gordon

Dr Janet Egdell
Board of trustees, professor roger halliday.
Roger Halliday is Chief Executive of Research Data Scotland. He was previously the Scottish Government’s Chief Statistician and Co-Director of ADR Scotland. Before that, he worked in the Department of Health in England as a policy analyst managing evidence for decision-making across NHS issues. He qualified with a degree in statistics in 1993 from St. Andrews University. He has worked for various UK Government Departments and at the Scottish Government in several statistical and policymaking roles. His areas of expertise are around transforming services with data, and he has experience working in the fields of health, children, learning, skills and the economy.
Professor Paul Boyle, Vice-Chancellor
Professor Paul Boyle was appointed Vice-Chancellor of Swansea University in 2019 following his role as President and Vice-Chancellor of the University of Leicester. Previously, Paul was Chief Executive of the Economic and Social Research Council (ESRC), the UK’s largest funding agency for social science research; the International Champion of Research Councils UK, with responsibility for international strategy on behalf of all seven UK research councils; and President of Science Europe, representing over 50 European funding agencies. Paul is a Fellow of the British Academy and the Academy of Social Sciences, Chair of Universities Wales’ Research and Innovation Network and a Board Member of Universities UK, who provide leadership and support to executive heads of 133 UK university institutions.
Jill Pell is Henry Mechan Professor of Public Health and Director of the Institute of Health and Wellbeing at the University of Glasgow. She is a Fellow of the Academy of Medical Sciences and the Royal Society of Edinburgh. Jill is currently a member of Council at the Medical Research Council and a non-executive director and medical trustee of the British Health Foundation. She was awarded CBE in 2017 for services to public health research and has more than 30 years' experience of using routine data and record linkage for health research.
Professor Parsons is the Director of EPCC, the supercomputing centre at the University of Edinburgh. EPCC operates many of the UK’s national research computing services including the new ARCHER2 National Supercomputer. As Dean of Research Computing, he provides expert advice and guidance to the College of Science and Engineering and the University on all aspects of modern research infrastructures with a particular focus on supercomputing, AI and data infrastructure. Mark also works part-time for EPSRC at UKRI as the Director of Research Computing. He joined EPCC in 1994 as a software developer working on several industrial contracts following a PhD in Particle Physics undertaken on the LEP accelerator at CERN in Geneva.
Scott Heald is Public Health Scotland’s lead Director for data, digital and statistics. Before that, he held a variety of statistical leadership roles in the Information Services Division (ISD) of NHS National Services Scotland. He qualified in 1994 with a degree in mathematics and statistics from the University of Edinburgh. After initially working within the field of education statistics, Scott has spent most of his career in the health and care statistics arena and has a particular passion for the use of data and statistics to drive transformative change which improves the outcomes for individuals. Scott is a fellow of the Royal Statistical Society.
Julie Fitzpatrick was appointed Chief Scientific Adviser (CSA) for Scotland in June 2021. Alongside this part-time position within the Scottish Government, Julie also remains Scientific Director of Moredun Research Institute and CEO of The Moredun Foundation. She also holds a Chair in Food Security at the University of Glasgow’s College of Medicine, Veterinary Medicine and Life Sciences. As CSA Scotland, Julie champions the use of science to inform policy development. She works closely with the Scottish Science Advisory Council, of which she is an ex-officio member, to help ensure that the Scottish Government has access to the best scientific advice to inform its work across all policy areas.
Martin Sinclair is a qualified accountant and auditor with more than 30 years’ experience as an external auditor of central government working on behalf of the UK Parliament. For 15 years, prior to his retirement in 2015, he was a Management Board Member of the National Audit Office (NAO). During that time, he led the NAO’s financial audit practice for 10 years and subsequently was Executive leader for National Affairs, a client portfolio embracing many of the major offices of State. Since then, he has worked as a consultant in public financial management and corporate governance in a number of EU candidate countries and undertaken a various trustee and non- executive roles. These have included nine years as a trustee and Chair of the Finance and Audit Committee of Asthma UK and as a Non-Executive of two major NHS Trusts in the south of England. He was also for a time Treasurer of the Chartered Institute of Public Finance and Accountancy.
Professor Andrew Morris became the inaugural Director of Health Data Research UK in August 2017, the UK’s national Institute for health data science. Its mission is to unite the UK’s health data to improve people’s lives and is supported by 12 funders. He also convenes the International COVID 19 Data Alliance (ICODA) supported by the Bill and Melinda Gates Foundation and Minderoo Foundation. He is seconded from his position as Professor of Medicine, and Vice Principal of Data Science at the University of Edinburgh, having taken up position in August 2014. Prior to this Andrew was Dean of Medicine at the University of Dundee. Andrew was Chief Scientist at the Scottish Government Health Directorate (2012-2017) and has served and chaired numerous national and international grant committees and Governmental bodies. He is a Fellow of the Royal Society of Edinburgh and the Academy of Medical Sciences.
Dr Emma Gordon is the Director of the Administrative Data Research UK (ADR UK) programme at the Economic & Social Research Council (ESRC), which is opening up secure access to government administrative data across the UK, to support research and inform policy decisions. Emma joined ADR UK from HM Treasury, managing the Government Economic Service and Government Social Research profession, and prior to this was Head of Health Analysis at the Office for National Statistics (ONS). At the start of her career, Emma was a post-doctoral researcher on the Avon Longitudinal Study of Parents and Children (ALSPAC). Emma sits on the Executive Committee of the International Population Data Linkage Network (IPDLN), and is the Interim Deputy Editor in Chief of the International Journal of Population Data Science (IJPDS).
Dr Janet Egdell joined the National Records of Scotland (NRS) in February 2023 as Interim Chief Executive. In this capacity she also holds the offices of Registrar General for Scotland, Keeper of the Records of Scotland and Keeper of the Scottish Register of Tartans. Before joining NRS, Janet was Accountable Officer at Registers of Scotland from 2014. She started her working life as an economist, with the Ministry of Agriculture, Fisheries and Food, the Royal Society for the Protection of Birds and the University of Aberdeen. She joined the Scottish Government in 1999 working in a range of public sector policy areas, including water, fuel poverty, transport and infrastructure investment.
Governance documents
Browse and download key governance documents from RDS including board minutes, registers of interests and terms of reference.
Was this information helpful?
Thank you for your feedback..
You have characters remaining
Smart Data Research UK
Let’s do good things with data.
We’re the UK’s national programme for smart data research. Our mission is to unlock the power of data to improve lives.

What is smart data?
Smart data is made whenever we engage with the digital world. When we buy something, ‘like’ something, or get directions.
If used for scientific research, smart data can change our world. It can help us to understand important social challenges, from disease prevention to climate change.

Working with companies
We bring together companies, researchers and the public to unlock the power of smart data for research.

Supporting researchers
We provide researchers with safe access to smart data.

We prioritise public involvement, transparency, ethics and responsibility.
Research themes

Productivity and prosperity for all
Smart data can help us understand what drives growth and innovation. It helps us plan infrastructure for different communities across the UK, and tackle inequalities.

Health and wellbeing
Smart data can provide insights to improve population health and tackle inequalities, helping us live longer, healthier lives.

Digital society
Smart data can provide insights about online safety, misinformation, the digital divide and the future of work.

Sustainability
Smart data can help tackle issues such as decarbonisation, green growth and the transition to a sustainable future.
Latest news and funding
All news and funding articles ordered by publication date.

£1.8m for new smart data research
Smart Data Research UK is awarding funding to seven university teams under its new accelerator scheme.

Getting smarter about smart data
Elena Musi blogs about Smart Data Research UK's digital data dive at the University of Liverpool.

Financial data a key to social challenges
For the second in our new blog series, we welcome Frank Gauld, CEO of Smart Data Foundry.

Five more tips from our strategy
Dip into more tips and insights from Smart Data Research UK's stakeholder engagement work.
Sign up to receive our latest news updates
Appointments at Mayo Clinic
- Consumer health
What are the benefits of CBD — and is it safe to use?
A prescription cannabidiol (CBD) oil is considered an effective anti-seizure medication. However, further research is needed to determine CBD 's other benefits and safety.
CBD is a chemical found in marijuana. CBD doesn't contain tetrahydrocannabinol (THC), the psychoactive ingredient found in marijuana that produces a high. The usual CBD formulation is oil, but CBD is also sold as an extract, a vaporized liquid and an oil-based capsule. Food, drinks and beauty products are among the many CBD -infused products available online.
Currently, the only CBD product approved by the Food and Drug Administration is a prescription oil called Epidiolex. It's approved to treat two types of epilepsy. Aside from Epidiolex, state laws on the use of CBD vary. While CBD is being studied as a treatment for a wide range of conditions, including Parkinson's disease, schizophrenia, diabetes, multiple sclerosis and anxiety, research supporting the drug's benefits is still limited.
CBD use also carries some risks. Though it's often well-tolerated, CBD can cause side effects, such as dry mouth, diarrhea, reduced appetite, drowsiness and fatigue. CBD can also interact with other medications you're taking, such as blood thinners.
Another cause for concern is the unreliability of the purity and dosage of CBD in products. A recent study of 84 CBD products bought online showed that more than a quarter of the products contained less CBD than labeled. In addition, THC was found in 18 products.
If you plan to use products containing CBD , talk to your doctor.
Brent A. Bauer, M.D.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
- Candida cleanse diet
- Colloidal silver supplements
- Miller B. Labeling accuracy of cannabidiol extracts sold online. JAMA. 2017;318:1708.
- FDA approves first drug compromised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. U.S. Food and Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm611046.htm. Accessed Nov. 20, 2018.
- State medical marijuana laws. National Conference of State Legislatures. http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx#2. Accessed Nov. 27, 2018.
- Devinsky O, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut Syndrome. The New England Journal of Medicine. 2018;378:1888.
- Cannabidiol. Natural Medicines. https://naturalmedicines.therapeuticresearch.com. Accessed Nov. 5, 2018.
- Cannabidiol. Facts & Comparisons eAnswers. http://www.wolterskluwercdi.com/facts-comparisons-online/. Accessed Nov. 5, 2018.
- Portenoy RK, et al. Cancer pain management: Adjuvant analgesics (coanalgesics). https://www.uptodate.com/contents/search. Accessed Nov. 14, 2018.
Products and Services
- The Mayo Clinic Diet Online
- A Book: The Mayo Clinic Diet Bundle
- A Book: Mayo Clinic Guide to Pain Relief
- A very happy brain
- Alternative cancer treatments: 11 options to consider
- Colon cleansing
- Detox foot pads
- Diabetes treatment: Can cinnamon lower blood sugar?
- Do infrared saunas have any health benefits?
- Prickly pear cactus
- Herbal supplements and heart drugs
- Kombucha tea
- Kratom: Unsafe and ineffective
- Kratom for opioid withdrawal
- Learn to reduce stress through mindful living
- Medical marijuana
- Meditation 2.0: A new way to meditate
- Mindfulness exercises
- Natural remedies for depression: Are they effective?
- Tai Chi and Cardiac Rehab
- Valerian: A safe and effective herbal sleep aid?
- Alternative psoriasis treatments
- Do zinc supplements shorten colds?
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
- Healthy Lifestyle
- Expert Answers
- CBD Safe and effective
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.

Jun 4, 2024 | Jeff Comstock - Corporate Vice President, Dynamics 365 Customer Service
Announcing Dynamics 365 Contact Center – a Copilot-first cloud contact center to transform service experiences

Jun 3, 2024 | Kathleen Hall - Chief Brand Officer
Celebrating Pride and ‘Radical Joy’

Jun 2, 2024 | Noelle Walsh - Corporate Vice President, Cloud Operations and Innovation
Microsoft’s Datacenter Community Pledge: To build and operate digital infrastructure that addresses societal challenges and creates benefits for communities

May 21, 2024 | Frank X. Shaw - Chief Communications Officer, Microsoft
What’s next: Microsoft Build continues the evolution and expansion of AI tools for developers

May 20, 2024 | Yusuf Mehdi - Executive Vice President, Consumer Chief Marketing Officer
Introducing Copilot+ PCs
May 8, 2024 | Jared Spataro - CVP, AI at Work
Microsoft and LinkedIn release the 2024 Work Trend Index on the state of AI at work
May 3, 2024 | Microsoft Corporate Blogs
Prioritizing security above all else

Apr 24, 2024 | Judson Althoff - Executive Vice President and Chief Commercial Officer
Leading in the era of AI: How Microsoft’s platform differentiation and Copilot empowerment are driving AI Transformation

Apr 17, 2024 | Kathleen Mitford, CVP, Global Industry
Manufacturing for tomorrow: Microsoft announces new industrial AI innovations from the cloud to the factory floor

Apr 15, 2024 | Judson Althoff - Executive Vice President and Chief Commercial Officer
Microsoft and G42 partner to accelerate AI innovation in UAE and beyond

Apr 7, 2024 | Mustafa Suleyman, EVP and CEO of Microsoft AI
Announcing new Microsoft AI Hub in London
Apr 3, 2024 | Jason Zander - EVP, Strategic Missions and Technologies
Advancing science: Microsoft and Quantinuum demonstrate the most reliable logical qubits on record with an error rate 800x better than physical qubits

Apr 2, 2024 | Melanie Nakagawa - Chief Sustainability Officer
Sustainable by design: Advancing the sustainability of AI

Mar 20, 2024 | Nicole Dezen, Chief Partner Officer and CVP of Global Partner Solutions
From vision to reality: Microsoft’s partners embrace AI to deliver customer value
Press tools.
- Check us out on RSS
- English | en
- Spanish | ES
- French | FR
- German | DE
- Portuguese | PT
- Chinese | ZH
- Japanese | JA
Salary Benchmarking and Compensation Data
Salary Benchmarking and Compensation Data Now Available
Benchmark your organization against others, design a competitive compensation strategy, retain your best talent with pay data.
Get pay right with our comprehensive insights. Gain the data-driven knowledge you need to confidently design a competitive compensation and talent retention strategy.
Benefits from Korn Ferry’s Pay data:
- Evaluate and adjust salaries based on regional cost-of-living variations.
- Ensure fairness in your salary benchmarking approach.
- Discover ideal salary ranges tailored to your industry and experience levels.
- Fine-tune your compensation strategy with industry-specific data.
- Benchmark your organization against 150+ markets.
- Access information on salary increases, economic indicators, and more.
Why use Korn Ferry salary benchmarking data?
- Up-to-date Data: Our meticulously collected data ensures accuracy for your benchmarking needs.
- Reliable Sources: We gather data from over 26,000 companies in 150+ countries, offering the world's most reliable compensation and benefits data.
- Data Visualization: Explore data trends with customizable visualization tools. Access, analyze, and gain deeper insights into compensation surveys.
- Market-Leading Insights: Our cutting-edge analytics empower rewards experts to create competitive pay packages that attract and retain top talent.
Want to enhance your organization’s compensation strategy? Download the report now.
- Capabilities
- Business Transformation
- Organization Strategy
- Total Rewards
- Assessment & Succession
- Talent Acquisition
- Leadership & Professional Development
- Intelligence Cloud
- Consumer Markets
- Financial Services
- Healthcare & Life Sciences
- Specialties
- Board & CEO Services
- Corporate Affairs
- Cybersecurity
- FInancial Services
- Human Resources
- Information Technology
- Risk Management
- Supply Chain
- Sustainability
- Korn Ferry Architect
- Korn Ferry Assess
- Korn Ferry Listen
- Korn Ferry Pay
- Korn Ferry Sell
- Jobs with our clients
- Advance your career
- Join Korn Ferry
- Find a consultant
- Find an office
- Business impact
- Investor relations
- Press releases
© Korn Ferry. All rights reserved.
Terms of Use
Cookie Settings
Do Not Sell My Info

The Bartlett School of Planning
- Resources for Current Students
Publications

Health Citizenship
The Health Citizenship cluster is pursuing a new sphere of research around urban health and democratic governance.

1 April 2024
This cluster investigates wellbeing burdens/benefits and inequalities in their distribution; public understanding of the healthy life and how it can be sustained; & participatory approaches to health within urban planning and urban policy. Our work centres on the politics of producing health within urban environments. Globally there are ‘urban penalties’ on the health of individuals, and the impacts of urban living are very varied. Health issues are often addressed by diverse and siloed urban policies. We are exploring issues of health that are important to planning, such as air quality and food insecurity. We are providing insights that are immediately useful to communities involved in health initiatives and urban governance, and of wider value in work on future democracy and equality. In a world that is refocused on holistic well-being and planetary environmental disaster, there are new questions about urban civics where agency matters and citizens ostensibly seek to secure health through urban actions. Therefore Health Citizenship research has two areas of focus: 1) community-led actions; and 2) governance responses. We are interested in Community-led Action, where people are reshaping social processes and uses of the city, as well as amenities and materials for ‘the healthy life’. We see this as people claiming their rights to a healthy life, and the performance of citizenship. The resulting actions also offer routes to new and insurgent forms of planning and place-making. The attention given to such activities by governance actors, including commercial and corporate interests, and statutory institutions is critical. Animated by deepening inequalities, some cluster members have examined iconic COVID-19 cases for clean air and food security around the neighbourhood of UCL. These are hotbeds of local protest, concern, and conflict, and high-profile points of public communication on the nature of well-being, urban determinants of health, and trends in governance. We continue to monitor Health Citizenship in London, and are building bridges into work in other international cities.
Dr Lucy Natarajan, The Bartlett School of Planning Send Lucy an email View Lucy's profile
- Livingstone N, Natarajan L. Charity & Capitalism In The Politics of Food Insecurity and Food Poverty in Canada and the United Kingdom. Editors: Mendly-Zambo, Zsofia, Raphael, Dennis. Policy Press 01 Feb 2025 (Chapter) [ https://rps.ucl.ac.uk/viewobject.html?id=2248314&cid=1]
- Natarajan L, Cho H, Yanful B, Woodward A. Food resilient urbanism: reconstructing hunger with NGOs In Pandemic Recovery?. 124-138. Edward Elgar Publishing 12 Jan 2024 (Chapter) [ https://rps.ucl.ac.uk/viewobject.html?id=2140717&cid=1]
- Natarajan L, Grimble G, Cho H, Armstrong M, Woodward A. (2022) Food Security & Civil Society: Research Findings Report, 2022. [ https://rps.ucl.ac.uk/viewobject.html?id=2046878&cid=1]
Project records
- Grand Challenges Grant https://www.ucl.ac.uk/sustainable/news/2020/feb/grand-challenges-grant
- Podcast with GOSH CEO https://www.ucl.ac.uk/sustainable/case-studies/2020/oct/podcast-gosh-ceo
- Co-designing Sustainable Places series, from The Glass-House Community Led Design https://www.ucl.ac.uk/sustainable/news/2021/mar/students-co-design-air-q...
Follow The Bartlett School of Planning on X (formerly Twitter) Follow The Bartlett School of Planning on LinkedIn Follow Dr Lucy Natarajan on X (formerly Twitter) Follow the UCL Clean Air Community on X (formerly Twitter)
Go back to view all research clusters
Photo by Bruno Nascimento on Unsplash
- Career Center
- Member Login
Reality Meets Research: ARM 2024 Exploration of Disability and Complex Conditions
ARM 2024 Theme Leader Christine Von Raesfeld highlights sessions to attend in her theme Individuals Living with Disability or Other Complex Conditions.
As both a patient and a health care professional, I am eagerly anticipating the AcademyHealth Annual Research Meeting (ARM 2024). This is a pivotal meeting for the research community, and this year I am grateful for the opportunity to co-chair one of the many themes " Individuals Living with Disability or Other Complex Conditions " with Dr. Shriram Parashuram from NORC at the University of Chicago. Our planning discussions were intense, covering diverse perspectives, but with a shared, unwavering commitment to the community. We hope to expand on those conversations and to foster a meaningful dialogue that merges personal experiences with scientific research.
The Centers for Disease Control and Prevention (CDC) reports that an estimated 61 million adults in the U.S. live with a disability. Furthermore, around 129 million individuals have at least one major chronic illness, such as heart disease, cancer, diabetes, obesity, or hypertension. These figures represent about 51.8 percent of the civilian, noninstitutionalized adult population, with 24.6 percent having one chronic condition and 27.2 percent having multiple conditions, underscoring the significance of our topic.
My personal journey, marked by multiple disabilities and chronic conditions, has profoundly influenced my perspective on health care and strengthened my resolve in advocacy.
Selecting from a plethora of insightful submissions was challenging for Dr. Parashuram and me. Each submission reflected a researcher's commitment to enhancing lives and offered hope for patients like myself. Through collaboration with a dedicated team, we have crafted a program that spotlights innovative research and the multifaceted challenges faced by individuals with complex health conditions. These submissions are more than academic studies; they are narratives that emphasize the need for empathy and advocacy in health care.
Care for Individuals with Complex Conditions
Monday, July 1, 2024 8:00 - 9:15 AM EDT
Led by Dr. Parashuram, this session will explore the lifelong challenges faced by individuals with complex conditions. Highlights include the SEER-CAHPS study by Kim Danforth's team, examining the care experiences of those newly diagnosed with cancer, especially the complexities of managing concurrent health conditions. Seyeon Jang's analysis will shed light on the financial impact of prescription drug costs for patients with Alzheimer's Disease and Related Dementias (ADRD), informing policy decisions with data spanning 2003-2021.
Care for Individuals with Disabilities
Sunday, June 30, 2024, 1:45 - 3:00 PM EDT
This session, which I have the honor of chairing, will focus on the obstacles to accessible care. Discussions will include the challenges faced by Medicare beneficiaries with disabilities, as presented by Emma Achola's team. Additionally, Megan Morris's comparative effectiveness trial seeks to improve communication between health care providers and patients with communication disabilities, a vital component of patient-centered care.
Implementation of the NIH Disability Inclusion Recommendations 2024 Progress Report Monday, July 1, 2024, 11:00 - 12:15 PM EDT
This session will present the NIH Disability Inclusion Recommendations 2024 Progress Report by Dr. Jae Kennedy, Dr. Bonnielin Swenor, and Dr. Alison Cernich. It will examine the impact of these recommendations on health research and policy, aiming to connect empirical data with the realities of lived experiences.
Poster Session: Individuals Living with Disability or Other Complex Conditions
Sunday, June 30, 2024, 8:00 - 9:15 AM EDT
The poster session will complement the main events, presenting a tapestry of research that reflects the field's diversity. It offers a chance to engage with cutting-edge findings and network with peers.
The theme of our meeting is not merely theoretical; it mirrors the daily challenges of those living with chronic illnesses and disabilities.
Our goal is to dissect and discuss the barriers to care for individuals with disabilities. We will not only spotlight the hurdles but also present innovative solutions that envision a more inclusive future, where health care fully embraces the individual and the nuances of living with a disability or other complex conditions
To find the agenda and a more in-depth summary of our theme (including location for those attending) check out https://academyhealth.org/page/2024-arm-agenda
The Federal Register
The daily journal of the united states government, request access.
Due to aggressive automated scraping of FederalRegister.gov and eCFR.gov, programmatic access to these sites is limited to access to our extensive developer APIs.
If you are human user receiving this message, we can add your IP address to a set of IPs that can access FederalRegister.gov & eCFR.gov; complete the CAPTCHA (bot test) below and click "Request Access". This process will be necessary for each IP address you wish to access the site from, requests are valid for approximately one quarter (three months) after which the process may need to be repeated.
An official website of the United States government.
If you want to request a wider IP range, first request access for your current IP, and then use the "Site Feedback" button found in the lower left-hand side to make the request.

IMAGES
VIDEO
COMMENTS
Share this page. Professor Andrew Morris became the inaugural Director of Health Data Research UK in August 2017. He is seconded from his position as Professor of Medicine, and Vice Principal of Data Science at the University of Edinburgh, having taken up position in August 2014. Andrew has also been elected as the next President of the Academy ...
Board Announcement - CEO Departure. 15 December 2021. The Board of Trustees of Health Data Research UK (HDR UK) announce that, after more than three years as Chief Executive Officer, Caroline Cake is leaving HDR UK on 26 January 2022 to start the next chapter of her career in a new role at Oxford Science Enterprises.
Former CEO of University Hospitals Birmingham will oversee Health Data Research UK's work as Chair of the Board of Trustees . 11 July 2023 . Dame Julie Moore is to be the new Chair of Health Data Research UK (HDR UK) following an extensive recruitment process and the unanimous endorsement of the Board of Trustees.
The Senior Leadership Team. Director, HDR UK. Chief Scientist, Deputy Director of HDR UK and Director of the BHF Data Science Centre. Director of Infrastructure and Services. Director of Strategy, HDR UK. Head of Legal, Trust and Ethics. Chief Technology Officer at HDR UK, Interim Director at DARE UK and Chair of Health Data Science.
David Seymour joined Health Data Research UK (HDR UK) in June 2019 as Partnership Director to lead the development of the UK Health Data Research Alliance (the 'Alliance') as part of the Innovate UK funded Industrial Strategy Challenge Fund Digital Innovation Hub Programme. He subsequently became Alliance Executive Director, and, during the COVID-19 pandemic, David led HDR UK's work to ...
Health Data Research UK (HDR UK) has developed significantly since starting in 2018, in terms of the scale of communities involved, programmes of work and impact on the health of patients and public through research and innovation. ... Caroline Cake will take on the role of Chief Executive Officer (CEO) at HDR UK. A consolidated strategic ...
The UK Health Data Research Alliance is marking five remarkable years of progress and commitment to maximising the benefits of health data research for all. Find out more. CVD-COVID-UK / COVID-IMPACT. CVD-COVID-UK aims to understand the relationship between COVID-19 and cardiovascular diseases such as heart attack, heart failure, stroke, and ...
Configuring the UK health data ecosystem to facilitate research, whilst safeguarding patient confidentiality, is a goal shared by the whole research community, including ABPI members". Dr Janet Valentine, Executive Director of Innovation and Research Policy at ABPI.
12 January 2021. HDR UK, the national institute for health data science, today announces the appointment of Charles Gibbons as its new Chief Technology Officer and member of its Executive Committee. Charles was previously Chief IT Architect and Head of Software engineering at Francis Crick Institute. Prior to this, Charles spent 15 years ...
The UK Health Data Research Alliance was established in 2019 as an independent alliance of health data providers, custodians and curators dedicated to improving human health by maximising the potential of data to accelerate progress in biomedicine, health and care. ... The Executive Committee will shape the strategy and development of the ...
Health Data Research UK (HDR UK) Research Services London, England 16,798 followers We are the United Kingdom's national institute for health data science.
Former CEO of University Hospitals Birmingham will oversee Health Data Research UK's work as Chair of the Board of Trustees 11 July 2023 Dame Julie Moore is to be the new Chair of Health Data Research UK (HDR UK) following an extensive recruitment process and the unanimous endorsement of the Board of Trustees.
About. Welcome to Health Data Research UK (HDR UK) - we are the UK's national institute for health data science. We are uniting the UK's health data to enable discoveries that improve people's lives. Our vision is that every health and care interaction and research endeavour will be enhanced by access to large scale data and advanced analytics.
David Seymour, Alliance Executive Director, Health Data Research UK (HDR UK), said: "The continued growth in members enriches the Alliance. It is testament to the value of working together across the sector. By co-ordinating our approach to standards, tools and technologies, we will continue to improve how we make health data available in an ...
Dr Graham Spittle, Chair of Health Data Research UK said: "I am delighted to be welcoming Alison, Andrew, Ara, Claire and Mark to the Board of Health Data Research UK. "They are joining us at a vital inflection point as we approach our second five years and implement our ambitious new strategy. "Their wealth of knowledge and expertise ...
Amanda White, Executive Director of Engagement and Insights, Health Data Research UK (HDR UK), said: ... Following her cancer diagnosis in 2021, she stepped down from her role as Chief Executive of the Association of Medical Research Charities. Aisling is a passionate supporter of the responsible use of data for research and care.
Health data research explained. Health data research is an exciting and developing area and can make positive changes in health and care for everyone. There are lots of examples of how health data science has improved our knowledge and helped to solve challenging health problems. Health data research is a way of gathering, analysing and linking ...
Health Data Research UK is a registered charity no. 1194431 funded by UK Research and Innovation, the Department of Health and Social Care in England and equivalents in Northern Ireland, Wales and Scotland, and leading medical research charities. ... Founding CEO of Project Data Sphere, LLC, a non-profit enterprise devoted to cancer clinical ...
UK Health Data Research Alliance Gibbs Building, 215 Euston Road, London NW1 2BE [email protected] | @HDR_UK | ukhealthdata.org Health Data Research UK is a limited company registered in England and Wales No: 10887014 UK Health Data Research Alliance Board - Actions and Meeting Notes Thursday 26th January 2023 (13:00 - 15:00)
Health Data Research UK (HDR UK) has an important role in leading the development of best practice. The intention is to provide position papers and recommendations, developed conjunction with the health data community, that would encourage behaviours to improve data quality and usability. HDR UK can mandate adoption through its funded ...
Roger Halliday is Chief Executive of Research Data Scotland. He was previously the Scottish Government's Chief Statistician and Co-Director of ADR Scotland. ... Its mission is to unite the UK's health data to improve people's lives and is supported by 12 funders. He also convenes the International COVID 19 Data Alliance (ICODA) supported ...
Explore {Health Data Research UK's key management people. Discover current leadership team members including founders, CEO, other executives and board directors. Health Data Research UK CEO, Founder, Key Executive Team, Board of Directors & Employees
Health and wellbeing. Smart data can provide insights to improve population health and tackle inequalities, helping us live longer, healthier lives. ... Elena Musi talks about Smart Data Research UK's digital data dive at the University of Liverpool. ... 11 April 2024 . For the second in our new blog series, we welcome Frank Gauld, CEO of Smart ...
A prescription cannabidiol (CBD) oil is considered an effective anti-seizure medication. However, further research is needed to determine CBD's other benefits and safety.. CBD is a chemical found in marijuana.CBD doesn't contain tetrahydrocannabinol (THC), the psychoactive ingredient found in marijuana that produces a high. The usual CBD formulation is oil, but CBD is also sold as an extract ...
Apr 7, 2024 | Mustafa Suleyman, EVP and CEO of Microsoft AI Announcing new Microsoft AI Hub in London Apr 3, 2024 | Jason Zander - EVP, Strategic Missions and Technologies
UCL's Grand Challenge of Mental Health & Wellbeing (GC MHW) welcomes applications for "pump-priming" funding of up to £25,000 per project to support research that crosses disciplinary boundaries and accelerates intervention discovery and development.. The projects funded should represent impactful preliminary work that can lead to the development of a larger, externally funded project.
Evaluate and adjust salaries based on regional cost-of-living variations. Ensure fairness in your salary benchmarking approach. Discover ideal salary ranges tailored to your industry and experience levels. Fine-tune your compensation strategy with industry-specific data. Benchmark your organization against 150+ markets.
Therefore Health Citizenship research has two areas of focus: 1) community-led actions; and 2) governance responses. We are interested in Community-led Action, where people are reshaping social processes and uses of the city, as well as amenities and materials for 'the healthy life'. We see this as people claiming their rights to a healthy ...
As both a patient and a health care professional, I am eagerly anticipating the AcademyHealth Annual Research Meeting (ARM 2024). This is a pivotal meeting for the research community, and this year I am grateful for the opportunity to co-chair one of the many themes "Individuals Living with Disability or Other Complex Conditions" with Dr. Shriram Parashuram from NORC at the University of Chicago.
To obtain a copy of the data collection plans and instruments, submit comments in writing, or request more information on the proposed project, Start Printed Page 47971 contact: Scott Keasey, 428 Miller Drive, Suite 59 9742, Rockville, MD 20880 or call non-toll-free number 301-846-1115 or Email your request, including your address to: scott ...