Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- BMJ Journals More You are viewing from: Google Indexer

You are here
- Volume 14, Issue 5
- How to manage alcohol-related liver disease: A case-based review
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-1530-5328 James B Maurice 1 ,
- http://orcid.org/0000-0001-5140-517X Samuel Tribich 2 ,
- Ava Zamani 3 ,
- Jennifer Ryan 4
- 1 Department of Gastroenterology and Hepatology, Southmead Hospital , North Bristol NHS Trust , Bristol , UK
- 2 Department of Hepatology, Royal London Hospital , Barts Health NHS Trust , London , UK
- 3 Hammersmith Hospital , Imperial College Healthcare NHS Trust , London , UK
- 4 Department of Hepatology and Liver Transplantation, Royal Free Hospital , Royal Free London NHS Foundation Trust , London , UK
- Correspondence to Dr James B Maurice, Department of Gastroenterology and Hepatology, Southmead Hospital, North Bristol NHS Trust, Bristol BS10 5NB, UK; james.maurice{at}nbt.nhs.uk
https://doi.org/10.1136/flgastro-2022-102270
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
- alcoholic liver disease
- chronic liver disease
What is already known on this topic
Alcohol-related liver disease (ArLD) is a major cause of morbidity and mortality.
What this study adds
We present a typical case to illustrate current evidence-based investigation and management of a patient with ArLD.
This case-based review aims to concisely support the day-to-day decision making of clinicians looking after patients with ArLD, from risk stratification and fibrosis assessment in the community through to managing decompensated disease, escalation care to critical care and assessment for liver transplantation.
How this study might affect research, practice or policy
We summarise the evolving evidence for the benefit of liver transplantation in alcoholic hepatitis, and ongoing controversies shaping future research in this area.
ArLD is fundamentally a public health problem, and further efforts are required to implement effective policies to reduce consumption and prevent disease.
Introduction
Alcohol is the leading risk factor for premature death in young adults, of which alcohol-related liver disease (ArLD) is a major contributor. 1 The management of ArLD often requires complex decision-making, raising challenges for the clinician and wider multidisciplinary team. This case-based review follows the typical journey of a patient through the progressive stages of the disease process, from early diagnosis and risk stratification in the outpatient clinic through to alcoholic hepatitis and referral for liver transplantation. At each stage, we discuss a practical approach to clinical management and summarise the underlying evidence base.
Case part 1
A 47-year-old man is referred to the general hepatology clinic from his General Practitioner with abnormal liver function tests, ordered in the community following several episodes of non-specific abdominal pain which subsequently resolved. He is now asymptomatic. The referral states that he drinks one bottle of wine each weekday night and more at the weekends. He is on no regular medication, has no other significant medical history and works in construction. On clinical examination, there are a few spider naevi on the chest wall but no other stigmata of liver disease, and his body mass index is 26 kg/m 2 . The blood results show alanine aminotransferase (ALT) 65 IU/L, aspartate aminotransferase (AST) 92 IU/L, alkaline phosphatase (ALP) 100 IU/L, gamma-GT (GGT) 350 IU/L, bilirubin 15 µmol/L, albumin 45 g/L, platelets 256×10 9 /L, internation normalised ratio (INR) 1.0 and creatinine 50 µmol/L. Abdominal ultrasound reveals a mildly enlarged, hyperechoic liver but normal spleen and no ascites.
How can we risk-stratify patients with ArLD in the outpatient clinic?
Early diagnosis and risk stratification of patients enables appropriate selection of patients for follow-up in secondary care, while also providing an opportunity for preventative interventions in those with mild disease. Emergency admissions for hepatic decompensation, where up to 75% of patients present for the first time, represent a late stage of the disease process when 1-year mortality is very high. 2 It is therefore vital to make an early diagnosis of liver disease.
Hepatic fibrosis has been traditionally staged by liver biopsy; however, non-invasive methods of fibrosis staging have an emerging role in ArLD. Transient elastography (TE) has been validated against liver biopsy to accurately stage both advanced fibrosis and cirrhosis, 3–6 and current NICE guidance recommends TE for the diagnosis of cirrhosis in patients with ArLD.
Serological markers of fibrosis such as FIB-4 and AST-Platelet Ratio Index (APRI) have generally not performed well in ArLD, although the enhanced liver fibrosis (ELF) test, measuring direct markers of fibrosis in blood, has an Area Under the Receiver Operator Curve (AUROC) of 0.92 in diagnosing advanced fibrosis using a cut-off value of 10.5. 7
How can we screen for alcohol use disorder and ArLD?
The primary screening tools for alcohol use disorders are the Alcohol Use Disorders Identification Test (AUDIT) or abbreviated AUDIT-C questionnaires. 8 Although clear documentation of the amount of alcohol consumed is important, a diagnosis of alcohol use disorder is more nuanced than volume of alcohol alone, hence the improved sensitivity through use of validated questionnaires. Identifying increasing risk (AUDIT 8–15), higher risk (AUDIT 16–19) or possible dependence (AUDIT≥20) 8 provides an opportunity for targeted brief interventions in those who would most benefit and is a cost-effective method for reducing alcohol intake. 9 Typically only comprising a 5–20 min single interaction, brief interventions offer personalised advice using a motivational and empathetic style of interview ( Box 1 ). If delivered to all new patients registered in primary care, this could save 2500 alcohol-related deaths over 20 years. 10 Patients identified to have alcohol dependence through screening should be referred for specialist treatment.
Typical features of brief interventions
Feedback on the person’s alcohol use and any related harm.
Clarification as to what constitutes low-risk consumption.
Information on the harms associated with risky alcohol use.
Benefits of reducing intake.
Motivational enhancement to support change.
Analysis of high-risk situations for drinking.
Coping strategies and the development of a personal plan to reduce consumption.
Adapted from Public Health England Review: the public health burden of alcohol and the effectiveness and cost-effectiveness of alcohol control policies. An evidence review. 45
Although routine blood tests may be helpful in supporting a diagnosis of ArLD (eg, AST>ALT, increased GGT, macrocytosis), they are of limited value in determining the severity of liver disease before established cirrhosis has developed with impaired liver synthetic function (low albumin, high INR and bilirubin). Individuals drinking at harmful levels should be screened for liver fibrosis with TE. Hepatology referral should be considered in patients with TE 8–16 kPa, particularly in those who continue to drink at harmful levels. Patients with TE≥16 kPa are at high risk of developing complications of cirrhosis, therefore should be followed up in a specialist hepatology clinic and be screened for hepatocellular carcinoma and oesophageal varices. Screening endoscopy for varices should be offered when TE≥20 kPa or platelets≤150×10 9 /L. 11 In the primary care setting, fibrosis screening of individuals drinking at harmful levels may alternatively be done with the ELF test, although this is not uniformly available ( figure 1 ). 12
- Download figure
- Open in new tab
- Download powerpoint
Screening for cirrhosis in individuals drinking at hazardous and harmful levels. ARFI, Acoustic Radiation Force Impulse; AUDIT, Alcohol Use Disorder Identification Test; AUDIT-C, abbreviated Alcohol Use Disorder Identification Test; ELF, enhanced liver fibrosis; GGT, Gamma-GT; HCC, hepatocellular carcinoma; NICE, The National Institute of Health and Care Excellence. Reproduced with permission from Newsome et al . 46
A high-risk population that should be considered for ArLD screening are the patients admitted to hospital acutely with alcohol-related physical harm, such as acute alcohol withdrawal or alcohol-related trauma. These patients should all be referred to alcohol care teams, and in addition to their expertise in delivering brief interventions, tailored detoxification regimens and vital links to local alcohol support services in the community, some hospitals have trained to perform TE and screen for liver fibrosis. This has provided an opportunity to streamline at risk patients into the hepatology services.
Case part 2
The same patient presents on the acute medical take 1 year later with a 2-week history of jaundice and abdominal swelling. Unfortunately, he has continued drinking alcohol. On examination, he is jaundiced with moderate ascites, tender hepatomegaly and subtle asterixis. He is sarcopenic with arm muscle wasting. He has the following blood results: haemoglobin 100 g/L, mean cell volume 107 fL, white cell count 12×10 9 /L, platelets 135×10 9 /L, INR 2.3, sodium 132 mmol/L, potassium 3.0 mmol/L, creatinine 55 µmol/L, urea 2.0 µmol/L, bilirubin 250 µmol/L, ALT 25 IU/L, AST 60 IU/L, ALP 95 IU/L, GGT 200 IU/L, albumin 35 g/L, c-reative protein (CRP) 45 mg/L. A diagnostic paracentesis reveals an ascitic albumin 16 g/L, white cells 90/mm 3 (80% lymphocytes). An X-ray of the chest is normal. Ultrasound liver demonstrates hepatomegaly 17 cm, splenomegaly 15 cm, moderate ascites and a patent portal vein. A clinical diagnosis of alcoholic hepatitis (AH) is made.
What is the role of liver biopsy in the diagnosis of AH?
AH is a clinical syndrome characterised by jaundice and coagulopathy in the context of recent and prolonged heavy alcohol use. Rapid development of jaundice is accompanied by a systemic inflammatory response with constitutional symptoms and low-grade fever, with or without other features of decompensation.
The diagnosis of AH can be made using a standard consensus definition based on clinical and biochemical parameters ( table 1 ), originally established to allow inclusion in clinical trials without the need for a liver biopsy but now generalised to clinical practice. 13 Neither European Association for the Study of Liver Disease (EASL) nor American College of Gastroenterology (ACG) guidelines recommend liver biopsy in patients meeting the criteria for probable AH, 9 10 and these recommendations have been supported by more recent data, showing that liver biopsy rarely changes the diagnosis when clinical criteria are met for AH. 12 However, if diagnostic uncertainty remains, such as atypical biochemical markers, uncertain alcohol use or a suspected alternative cause of liver injury, a liver biopsy should be undertaken to confirm the diagnosis, particularly if planning to administer AH-directed medical therapies. 14
- View inline
Consensus definition for ‘probable’ alcohol hepatitis 13
In those patients in whom a biopsy is undertaken, specific histological features such as degree of neutrophil infiltration, fibrosis stage and presence of megamitochondria can be useful for prognostication using the Alcoholic Hepatitis Histologic Score, which is independently predictive of 90-day mortality. 15 However, the utility of this is significantly limited by interobserver variability between reporting pathologists. 16
Should this patient be treated with steroids?
Once a diagnosis of AH is established, patients should be risk stratified using a validated scoring system. The modified Maddrey’s discriminant function (mDF) is a commonly used score, which defines a cut-off of ≥32 as severe AH; however, this is very sensitive and risks over-treating patients with mild disease. 17 The Glasgow Alcoholic Hepatitis Score (GAHS) and Model for End-Stage Liver Disease (MELD) are better predictors of 28-day and 90-day mortality than mDF 18 19 and are also now included in EASL and ACG guidelines, with severe AH defined as GAHS≥9 or MELD≥21. 20 , 14
The STeroids Or Pentoxifylline for Alcoholic Hepatitis (STOPAH) study is the largest randomised controlled trial to investigate the efficacy of corticosteroids in the treatment of AH. It included 1103 participants with severe AH and the group that received prednisolone only had a small non-significant improvement in 28-day survival (OR 0.72, 95% CI 0.52 to 1.01, p=0.06), a benefit which was lost by 90 days and 1 year. In the multivariate analysis adjusting for baseline variables, prednisolone was associated with improved 28-day survival compared with placebo (OR 0.61, p=0.015), although not at 90 days or 1 year. 21 Further meta-analyses of pooled data have replicated these findings. 22
The EASL and ACG guidelines advise to take steroid treatment with prednisolone 40 mg per day in patients with severe AH, as defined by either the mDF, GAHS or MELD Score. Steroid responsiveness should be assessed using the Lille Score, typically on day 7, although there is data to support earlier application on day 4, 23 with steroids stopped in non-responders (Lille Score≥0.45); responders should complete a 28-day course. It may be possible to predict Lille response using a baseline neutrophil-to-lymphocyte ratio (NLR), with an NLR of 5–8 predictive of a significant reduction in 90-day mortality with corticosteroid treatment, compared with no reduction when NLR is less than 5 or more than 8. 24 Emerging data is further delineating which patients may derive the greatest benefit from corticosteroids 25 ; however, this remains an area of ongoing research.
Infection is a frequent complication of severe AH, contributing significantly to the high mortality rate and associated in particular with an increased 90-day mortality. 26 Corticosteroids are associated with an increased incidence of infection post-treatment compared with placebo (10% vs 6%), 21 and significantly worse 90-day mortality if patients develop infection within the first week of starting steroids. 26 Therefore, particular caution is required prior to starting prednisolone in patients with active sepsis, bearing in mind that patients with cirrhosis may not mount a classic immune response to infection. 26 Biomarkers to predict risk of incident infection on steroids are an area of research interest; baseline NLR of >8 is also associated with increased infection at day 7 of corticosteroid treatment (OR 2.60, p=0.006), but requires further validation. 24
In clinical practice, the commencement of steroids is delayed until infection is excluded, including negative cultures of blood, urine and ascitic fluid. This period also allows for the assessment of the bilirubin trend which, along with risk stratification scoring, helps to inform the decision to start corticosteroids. 27
What are the considerations in managing alcohol withdrawal in patients with advanced liver disease?
Alcohol withdrawal syndrome (AWS) should be assessed using the Clinical Institute Withdrawal Assessment for AlcoholScore, with a symptom-based regimen rather than fixed dosing in order to reduce drug accumulation. 28 Benzodiazepines reduce withdrawal symptoms and the risk of both seizures and delirium tremens and are considered the gold standard for treatment of AWS. Long-acting benzodiazepines such as chlordiazepoxide and diazepam should only be used with caution in patients with cirrhosis and impaired synthetic function due to their unpredictable half-life and significant accumulation in the presence of hepatic dysfunction, where the use of shorter-acting lorazepam or oxazepam may be preferable if available. In addition, benzodiazepines can both precipitate and worsen hepatic encephalopathy and so should be used with care.
Abstinence from alcohol remains the only independent predictor of long-term survival in patients presenting with severe AH 29 and early intervention from an alcohol liaison service during the hospital admission is of fundamental importance.
What is the role of nutrition in the management of AH?
Patients with both AH and cirrhosis are characterised by an almost universal state of malnutrition, sarcopenia and B vitamin deficiency, alongside increased resting energy expenditure and impaired metabolism of carbohydrates, lipids and proteins. 30 Early involvement of the dietetic team is vital to ensure patients with AH meet their nutritional requirements.
Increased caloric intake has been associated with a reduced incidence of infection, improved liver function and quicker resolution of hepatic encephalopathy in multiple randomised trials. 30 A recent large trial reported lower rates of infections and improved 1-month and 6-month mortality in patients with severe AH treated with corticosteroids who received a calorie intake of ≥21.5 kcal/kg/day compared with those who received<21.5 kcal/kg/day, regardless of Lille response or of the mode by which the calories were delivered. 31
EASL and European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines recommend an aim of 35–45 kcal/kg/day and a daily protein intake of 1.2–1.5 g/kg/day, with the oral route as first line and nasogastric feeding advised if oral intake is inadequate. 30 Intravenous thiamine replacement should be given to all patients with a history of alcohol use to reduce the risk of Wernicke’s encephalopathy. Several clinical trials have failed to demonstrate evidence for the use of various specialised dietary formulas and ESPEN recommend using standard nutritional supplements or feed with a high energy density, with a late evening supplement to reduce overnight starvation duration. 30
Case part 3
A full septic screen including blood cultures did not reveal any evidence of sepsis. The patient is managed with nutritional supplements, lactulose 20 mL three times a day and prednisolone 40 mg once daily. At day 7, his blood results are bilirubin 355, INR 3.0, PT 27, Cr 90, albumin 29. Lille Score is 0.60 (>0.45) indicating a poor prognosis, so prednisolone is stopped. Overall 90-day mortality in patients with AH is approximately 30%, increasing to around 45% in patients with a Lille Score>0.45 after 7 days of corticosteroids. 19
Is liver transplantation an option in severe AH?
In Europe, ArLD is the leading indication for liver transplant (LT), but the timing and selection of patients for liver transplantation with ArLD is controversial. A period of abstinence is vital to understand the extent of hepatic recompensation that can occur without the need to undergo LT and to ensure the patient is engaged with the process. However, although pretransplant abstinence is one important predictor of post-LT sobriety, it is not the only factor, and there is data that the risk of relapse is no higher in carefully selected patients transplanted with severe alcoholic hepatitis (SAH) compared with those with alcohol-related cirrhosis under standard selection criteria. 32
Challenging the traditional exclusion of patients with SAH from consideration for LT, a multicentre cohort study in France offered LT to patients with SAH who met specific stringent selection criteria, including non-response to steroid therapy, a first presentation of liver decompensation and a robust social support network. 33 This study showed significantly improved survival in the group offered LT at 2 years (71% vs 23%), a benefit almost entirely gained in the first 6 months. Long-term follow-up data was recently presented, showing overall survival at 1, 5 and 10 years of 83%, 70% and 56%, respectively. Severe alcohol relapse was evident in 10%, similar to other cohorts transplanted for ArLD using standard selection criteria. 34
The largest study in the USA on LT in SAH is a retrospective review of United Network for Organ Sharing data. In 147 patients transplanted with SAH between 2006 and 2017, with no previous decompensation and abstinence of less than 6 months, 1-year and 3-year survival was 94% and 84%, while return to sustained drinking occurred in 10% at 1 year and 17% at 3 years. 35 Interestingly, in a smaller retrospective case-controlled study comparing patients transplanted with SAH with<6 months abstinence (n=46) with a group transplanted for standard ArLD criteria and>6 months abstinence (n=34), the two groups had comparable 1-year survival (97% vs 100%, p=1) and return to harmful drinking after median follow-up of 532 days (17% vs 12%, p=0.5). 32
The only prospective trial of early liver transplantation in SAH was a non-randomised, non-inferiority, controlled trial recently published by the group in France. Over 2 years of follow-up, patients with SAH offered early liver transplant (n=68) had a small but non-significant increased risk of alcohol relapse compared with those transplanted for alcohol-related cirrhosis after ≥6 months of abstinence (n=93, relative risk 1.45, 95% CI 0.82 to 2.60), but also a greater risk of high levels of alcohol intake (RR 4.10, 95% CI 1.56 to 10.75). The 2-year post-transplantation survival was similar between these groups (89.7% and 88.2% respectively, HR 0.87, 95% CI 0.33 to 2.26), whereas the overall 2-year survival of patients with SAH who were not transplanted (n=47) was significantly reduced compared with those who were (28.3% vs 70.6%, HR 0.27, 95% CI 0.16 to 0.47). 36
The landscape of public and medical opinion on offering transplantation for SAH is changing in light of this data. However, concerns remain that predictive models are suboptimal and over 50% of patients with an unfavourable prognosis based on the Lille Score will survive without LT. 37 As such, the UK pilot on LT in SAH failed to recruit any patients over a 3-year period and was closed. 38 The current UK position recommends that if liver insufficiency persists after 3 months of documented alcohol abstinence in individuals with an index presentation of severe AH, consideration should be given to referral for liver transplantation if their psychosocial risk profile is favourable. 37
Case part 4
The patient’s clinical condition deteriorates over the following week. He becomes febrile, an ascitic tap confirms spontaneous bacterial peritonitis (white cells 700 cells/mL, neutrophils>90%) and he develops an oliguric acute kidney injury with haemodynamic instability requiring regular fluid boluses. His liver function remains poor (UK Model for End Stage Liver Disease (UKELD) 68). You call the intensive care unit (ITU) to review the patient, but questions are raised about his suitability for level 3 care.
Should this patient be escalated to critical care?
This patient now requires organ support with inotropes and likely renal replacement therapy. Historically, patients with ArLD have experienced barriers to timely escalation to ITU due to a perceived poor prognosis. Data in the National Confidential Enquiry into Patient Outcome and Death report in 2013 confirmed this practice, showing that 31% of patients deemed to require and be appropriate for escalation of care on independent review of the case notes did not receive such treatment. 39
In the same report, a review by the treating clinicians identified only 7% of cases who were appropriate for escalation but did not receive it. Subjective judgements detrimentally influenced these decisions and led the report to conclude that failure to escalate was due to clinicians having a prior view that it was not appropriate to escalate care in patients with ArLD.’ 39
Over the last 20 years, there has been a significant improvement in survival of patients admitted to ITU with organ failure complicating decompensated chronic liver disease, with mortality falling from 41.0% to 32.5% over this period, despite comparable scores for severity of illness at presentation. Although ArLD was associated with worse survival, improvements were also reported in this group over the same period (50.9% mortality to 41.9%, mean (Acute Physiology and Chronic Health Evaluation (APACHE) II Score 20 vs 19), such that the majority admitted to ITU will survive. 40
However, mortality rates for patients with ArLD and acute-on-chronic liver failure admitted to the ITU remain high, and escalation decisions require careful discussion and shared decision-making between the medical and critical care teams, alongside patients and their families as required. One of the first questions posed by the ITU team may be whether they are a transplant candidate. This is not a straightforward question to answer and may not be the most pertinent issue at this point in the patient’s care: in his case, the immediate answer would be ‘no’ in the UK, for reasons discussed above. But if he survives this admission, maintains abstinence but continues to have a qualifying UKELD Score he may be considered for a transplant assessment in 3 months.
In this instance, the patient’s age and the fact this is a first presentation with hepatic decompensation strongly support escalation at this stage, but the CLIF-ACLF prognostic scoring system can be helpful to add objective data to discussions between the managing team and ICU. While not including some increasingly recognised prognostic factors such as the presence of sarcopenia, the European Foundation for the Study of Chronic Liver Failure Acute-on-chronic Liver Failure (CLIF-C ACLF) Score has nevertheless been validated in a large dataset of patients with decompensated chronic liver disease and organ failure. 41 Days 3–7 on the ITU may be the optimal time to calculate this score, when an accurate assessment on the trajectory and likely outcome can be made. 42 Applying it in this way can support decision-making between the patient’s primary team and the ITU and provide timescales and goals with which to assess the benefits of level 3 care when there is disagreement or uncertainty.
However, even in the setting of treatment escalation it is important to remember that his overall prognosis is poor at this stage and, therefore, early involvement of the palliative care (PC) team should be considered. This can be done in parallel with full active medical care; it is important to bear in mind that PC is not synonymous with ‘end-of-life care’ and they are excellently equipped to optimise symptom control and begin to address wider holistic issues in the care of a patient at high risk of death. As such, early involvement of PC has been shown to improve symptom control and quality of life. 43
Case part 5
After 2 weeks in the ITU and a prolonged inpatient stay, the patient makes sufficient recovery to be discharged home. He continues to have moderate ascites managed with spironolactone and significant liver synthetic dysfunction (UKELD 60). He begins to attend his local alcohol support group and his partner has removed all alcohol from the house to support his abstinence.
When should you refer for transplant assessment?
Although he has made significant progress, this patient remains very unwell and at high risk of further deterioration. His ‘window of opportunity’ for transplant assessment is small. There is no fixed rule on this, but recent guidelines suggest that referral should be considered after 3 months of abstinence, or even sooner if the patient is actively engaged in alcohol cessation support, if there are issues that may complicate the workup assessment, or if the risk of death within 3 months is high. 44 In parallel to referral, every effort should be made to optimise his physical fitness, including dietician input and a graded exercise plan.
The patient maintains abstinence, is referred and successfully receives a life-saving liver transplant. A multidisciplinary team approach is required for patients admitted with decompensated ArLD, in which a dedicated alcohol care team is vital, but preventative measures on a population (eg, minimum unit pricing) and individual (eg, brief interventions and fibrosis stratification) level early in the disease course will have the greatest impact.
Ethics statements
Patient consent for publication.
Not applicable.
Ethics approval
- Griswold MG ,
- Fullman N ,
- Williams R ,
- Aspinall R ,
- Bellis M , et al
- Kettaneh A ,
- Tengher-Barna I , et al
- Pavlov CS ,
- Casazza G ,
- Nikolova D , et al
- Detlefsen S ,
- Sevelsted Møller L , et al
- Lackner C ,
- Bataller R ,
- Burt A , et al
- Madsen BS ,
- Hansen JF , et al
- National Institute for Health and Care Excellence
- Anderson P ,
- Chisholm D ,
- Gillespie D ,
- Ally A , et al
- de Franchis R , Baveno VI Faculty
- Forrest E ,
- Chalasani NP , et al
- Altamirano J ,
- Katoonizadeh A , et al
- Horvath B ,
- Allende D ,
- Xie H , et al
- Forrest EH ,
- Atkinson SR ,
- Richardson P , et al
- Singal AK ,
- Ahn J , et al
- Thursz MR ,
- Richardson P ,
- Allison M , et al
- Chandar AK , et al
- Garcia-Saenz-de-Sicilia M ,
- Altamirano J , et al
- Sinha R , et al
- Baeza N , et al
- Knapp S , et al
- Cabezas J ,
- Mirijello A ,
- D’Angelo C ,
- Ferrulli A , et al
- Labreuche J ,
- Artru F , et al
- Bischoff SC ,
- Dasarathy S , et al
- Deltenre P ,
- Senterre C , et al
- McCaul ME , et al
- Mathurin P ,
- Samuel D , et al
- Dharancy S ,
- Dumortier J , et al
- Platt L , et al
- Moreno C , et al
- Aldersley H ,
- Leithead JA , et al
- Juniper M ,
- Kelly K , et al
- McPhail MJW ,
- Parrott F ,
- Wendon JA , et al
- Pavesi M , et al
- Fernandez J ,
- Garcia E , et al
- Woodland H ,
- Forbes K , et al
- Millson C ,
- Considine A ,
- Cramp ME , et al
- Newsome PN ,
- Davison SM , et al
Twitter @jamesbmaurice
Contributors JBM conceptualised the original article and the case. JBM, ST and AZ drafted the initial version of the manuscript. JBM and ST contributed further editing of various sections. JR provided senior critical review and edited the manuscript. All authors agreed upon the final version.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Linked Articles
- Highlights from this issue UpFront R Mark Beattie Frontline Gastroenterology 2023; 14 357-358 Published Online First: 07 Aug 2023. doi: 10.1136/flgastro-2023-102519
Read the full text or download the PDF:
Cirrhosis Case Study (45 min)
Watch More! Unlock the full videos with a FREE trial
Included In This Lesson
Study tools.
Access More! View the full outline and transcript with a FREE trial
Mr. Garcia is a 43-year-old male who presented to the ED complaining of nausea and vomiting x 3 days. The nurse notes a large, distended abdomen and yellowing of the patient’s skin and eyes. The patient reports a history of alcoholic cirrhosis.
What initial nursing assessments should be performed?
- Full abdominal assessment, including assessing for ascites
- Heart and lung sounds
- Skin assessment – color, turgor, etc.
- Full set of vital signs
- Neurological assessment
What diagnostic testing do you anticipate for Mr. Garcia?
- LFT’s, CBC, BMP
Mr. Garcia’s vitals are stable, BP 100/58, bowel sounds are active but distant, and the nurse notes a positive fluid wave test on his abdomen. The patient denies itching but is constantly scratching at his chest. He is oriented to person only and his brother at the bedside reports he hasn’t been himself today. He keeps trying to get out of bed
Which finding is most concerning and needs to be reported to the provider? Why?
- Confusion, disorientation – this could indicate hepatic encephalopathy, which could lead to seizures and death if left untreated
What further diagnostic and lab tests should be ordered to determine Mr. Garcia’s priority problems?
- Abdominal X-ray and/or Abdominal ultrasound to visualize liver and whether the distended abdomen is related to ascites or other sources
- Ammonia level to determine if hepatic encephalopathy is the source of Mr. Garcia’s altered mental status.
The provider places orders for the following:
Keep SpO 2 > 92%
Keep HOB > 30 degrees
Insert 2 large bore PIV’s
500 mL NS IV bolus STAT
100 mL/hr NS IV continuous infusion
Hydrocodone/Acetaminophen 5-500 mg 1-2 tabs q4h PRN moderate to severe pain
Diphenhydramine 25 mg PO q8h PRN itching
Ondansetron 4 mg IV q6h PRN nausea
Lactulose 20 mg PO q6h
Mr. Garcia’s LFT’s and Ammonia level are elevated. He is extremely confused and agitated and appears somewhat short of breath. The patient’s current vital signs are as follows:
HR 82 RR 22
BP 94/56 SpO 2 93%
Temp 98.9°F
Which order should be implemented first? Why?
- Insert two large-bore IV’s. The patient requires IV fluids and has other IV meds ordered and will likely need labs drawn. This needs to be a priority.
- You could also say elevate the HOB to 30 degrees or higher, if there was indication that he was lying flat
- His SpO2 is >92%, so no intervention is required there.
- Lactulose should be the next priority intervention – to get the ammonia levels down – but it may take a bit for pharmacy to profile it, send it to the unit, etc.
Which order should be questioned? Why?
- Hydrocodone/Acetaminophen – Acetaminophen can be toxic for patients with liver disease. The way this order is written, this patient could receive anywhere from 3 g – 6 g of Acetaminophen in a 24-hour period. The max for a healthy person is 4 g, but for liver patients, it is 2 g max.
- Either the dose and frequency should be lowered significantly, or the medication should be changed altogether
The order is changed to Fentanyl 25 mcg IV q4h PRN moderate to severe pain. The provider notes somewhat shallow breathing and severe ascites and requests for you to set up for paracentesis. At this time, you express your concern that the patient is extremely confused and agitated and trying to get out of bed. You do not feel that he will be still enough for the procedure. The provider agrees and plans to postpone the paracentesis for now, but orders for you to report any signs of respiratory depression or hypoxia.
Why is Mr. Garcia so confused and agitated?
- His ammonia levels are elevated due to his liver failure – this causes hepatic encephalopathy – damage to the brain cells
- This causes altered mental status, agitation, confusion, and can eventually lead to seizures and death if left untreated
What is the rationale for performing a paracentesis for Mr. Garcia?
- The excess fluid in Mr. Garcia’s belly is compressing his thoracic cavity, causing him to feel short of breath and to only take shallow breaths. Draining this fluid will not only relieve some discomfort, but it can also help improve Mr. Garcia’s breathing
After 6 doses of lactulose, Mr. Garcia is much more calm and cooperative. He is oriented times 2-3 most times. The provider performs the paracentesis and is able to remove 1.5 L of fluid. The patient’s shortness of breath is relieved, and his breathing is less shallow. Ultrasound of the liver showed severe scarring on the liver. Mr. Garcia’s condition continues to improve, and the plan is to discharge him home tomorrow.
What discharge teaching should be included for Mr. Garcia, including nutrition?
- Mr. Garcia should not be eating a high protein diet as this can contribute to the increased ammonia levels and development of hepatic encephalopathy.
- Mr. Garcia should avoid drinking alcohol at all times
- Medication instructions for any new or changed medications
- Especially the importance of taking Lactulose regularly as ordered
- Signs to report to the provider of exacerbation or encephalopathy
View the FULL Outline
When you start a FREE trial you gain access to the full outline as well as:
- SIMCLEX (NCLEX Simulator)
- 6,500+ Practice NCLEX Questions
- 2,000+ HD Videos
- 300+ Nursing Cheatsheets
“Would suggest to all nursing students . . . Guaranteed to ease the stress!”
Nursing Case Studies

This nursing case study course is designed to help nursing students build critical thinking. Each case study was written by experienced nurses with first hand knowledge of the “real-world” disease process. To help you increase your nursing clinical judgement (critical thinking), each unfolding nursing case study includes answers laid out by Blooms Taxonomy to help you see that you are progressing to clinical analysis.We encourage you to read the case study and really through the “critical thinking checks” as this is where the real learning occurs. If you get tripped up by a specific question, no worries, just dig into an associated lesson on the topic and reinforce your understanding. In the end, that is what nursing case studies are all about – growing in your clinical judgement.
Nursing Case Studies Introduction
Cardiac nursing case studies.
- 6 Questions
- 7 Questions
- 5 Questions
- 4 Questions
GI/GU Nursing Case Studies
- 2 Questions
- 8 Questions
Obstetrics Nursing Case Studies
Respiratory nursing case studies.
- 10 Questions
Pediatrics Nursing Case Studies
- 3 Questions
- 12 Questions
Neuro Nursing Case Studies
Mental health nursing case studies.
- 9 Questions
Metabolic/Endocrine Nursing Case Studies
Other nursing case studies.

Liver Cirrhosis Case Study
56 year old Caucasian male with end-stage liver cirrhosis in hospice care, came in for stem cell therapy to help prolong his life. Patient had a history of chronic Hepatitis C, hypertension and diabetes, and did not qualify for liver transplant. Patient had declined steadily over the past year, and over the last month, he had an unintentional weight loss of 30 lbs. He had been struggling with fatigue, weakness, and occasional fever and chills, and also reported of shortness of breath, wheezing, and edema of lower extremities. At the time of treatment, patient presented with an distended abdomen (ascites), with very little energy to even speak.
Pin It on Pinterest

Citation, DOI, disclosures and case data
At the time the case was submitted for publication Abhijit Chikhlikar had no recorded disclosures.
Presentation
Swelling and fullness of the abdomen.
Patient Data
Ultrasound study shows a full spectrum of cirrhosis: small liver, nodular margins of the liver, dilated portal vein, ascites, splenomegaly and hepatofugal flow on portal vein. Incidental findings of a left ovarian cyst.
Case Discussion
Cirrhosis is the common endpoint of a wide variety of chronic disease processes which cause hepatocellular necrosis.
11 public playlists include this case
- LIVER US by Federico Giovanni Galbiati
- ecoografie by Popovici Alexandra
- R1 US by Bennett Battle
- abdomen by MD IRFAN ALAM
- Liver by Oleksii Burian
- UCD Medical Students 2018 by Danielle Byrne
- bizzarre by Federico Giovanni Galbiati
- QUIZ studenci by Adam
- liver by nour
- GK - Abdo - Liver by GLK
- GIT-Liver 2 by Mohamed shweel
Related Radiopaedia articles
- Hepatofugal
- Portal vein
- Splenomegaly
Promoted articles (advertising)
How to use cases.
You can use Radiopaedia cases in a variety of ways to help you learn and teach.
- Add cases to playlists
- Share cases with the diagnosis hidden
- Use images in presentations
- Use them in multiple choice question
Creating your own cases is easy.
- Case creation learning pathway
ADVERTISEMENT: Supporters see fewer/no ads
By Section:
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Feature Sponsor
- Expert advisers

- Introduction
- Conclusions
- Article Information
A, Cumulative recurrence ( P = .001; log-rank). B, Disease-specific survival ( P = .04; log-rank).
A, Number of recurrences in entire matched cohort (median [interquartile range]: solitary recurrence, 18.3 [14.7-64.4]; 2-3 recurrences, 9.6 [4.3-47.3]; and ≥4 recurrences, 5.8 [3.3-7.0]). B, Recurrences by site in entire matched cohort (median [interquartile range]: near, 12.8 [6.1-26.5]; distant, 19.8 [9.1-41.0]; and extrahepatic, 7.6 [4.7-12.6]). C, Number of recurrences in patients with normal liver (median [interquartile range]: solitary recurrence, 29.0 [15.4-56.0]; 2-3 recurrences, 4.7 [4.6-40.8]; and ≥4 recurrences, 5.8 [4.4-6.7]). D, Recurrences by site in patients with normal liver (median [interquartile range]: near, 8.9 [6.1-34.7]; distant, 22.8 [4.4-41.6]; and extrahepatic, 7.0 [4.4-14.0]). E, Number of recurrences in patients with liver cirrhosis (median [interquartile range]: solitary recurrence, 18.3 [9.4-36.4]; 2-3 recurrences, 9.7 [7.2-22.5]; and ≥4 recurrences, 8.1 [3.4-12.8]). F, Recurrences by site in patients with liver cirrhosis (median [interquartile range]: near, 13.5 [9.8-20.4]; distant, 19.8 [9.6-39.6]; and extrahepatic, 8.2 [6.5-8.7]).
A, Number of recurrences in entire matched cohort. B, Number of recurrences in patients with a normal liver. C, Number of recurrences in patients with liver cirrhosis. D, Recurrences by site in entire matched cohort. E, Recurrences by site in patients with a normal liver. F, Recurrences by site in patients with liver cirrhosis.
A, Estimated annual recurrence rate over time after hepatectomy. B, Cumulative hazard of recurrence.
eTable 1. Baseline Demographics and Clinical Characteristics
eTable 2. Baseline Demographics and Clinical Characteristics in Matched Cohort
- Hepatectomy for Early Hepatocellular Carcinoma JAMA Surgery Invited Commentary March 15, 2017 Roberto Hernandez-Alejandro, MD; Mark A. Levstik, MD; David C. Linehan, MD
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Sasaki K , Shindoh J , Margonis GA, et al. Effect of Background Liver Cirrhosis on Outcomes of Hepatectomy for Hepatocellular Carcinoma. JAMA Surg. 2017;152(3):e165059. doi:10.1001/jamasurg.2016.5059
Manage citations:
© 2024
- Permissions
Effect of Background Liver Cirrhosis on Outcomes of Hepatectomy for Hepatocellular Carcinoma
- 1 Division of Surgical Oncology, Department of Surgery, The Johns Hopkins Hospital, Baltimore, Maryland
- 2 Hepatobiliary Surgery Division, Department of Digestive Surgery, Toranomon Hospital, Tokyo, Japan
- 3 Okinaka Memorial Institute for Medical Research, Toranomon Hospital, Tokyo, Japan
- Invited Commentary Hepatectomy for Early Hepatocellular Carcinoma Roberto Hernandez-Alejandro, MD; Mark A. Levstik, MD; David C. Linehan, MD JAMA Surgery
Question What is the pattern and recurrence rate of de novo hepatocellular carcinoma among patients with liver cirrhosis and normal liver after resection of hepatocellular carcinoma?
Findings The median annual incidence of postoperative recurrence of hepatocellular carcinoma within 5 years after surgery was lower in the group with normal liver (5.9%) compared with the group with liver cirrhosis (12.7%). Multiple recurrences near the resection margin or at extrahepatic sites were more frequent in the group with normal liver (50.0% vs 15.4%), whereas solitary recurrence at a distant site was more common in the group with liver cirrhosis (53.8% vs 5.6%).
Meaning Comparison of the patterns and annual incidence of recurrence of hepatocellular carcinoma demonstrated that the poorer prognosis in the group with liver cirrhosis was likely owing to a higher hepatocarcinogenic potential among patients with cirrhosis.
Importance Background hepatocarcinogenesis is considered a major cause of postoperative recurrence of de novo hepatocellular carcinoma (HCC) in patients with liver cirrhosis (LC). The degree of underlying liver injury has reportedly correlated with surgical outcomes of HCC. However, the pattern and annual rate of recurrence of postoperative de novo HCC are still unclear.
Objective To clarify the pattern and rate of recurrence of de novo HCC in patients with LC.
Design, Setting, and Participants Data from 799 patients who underwent curative hepatectomy for HCC at Toranomon Hospital and The Johns Hopkins Hospital between January 1, 1995, and December 31, 2014, were retrospectively collected and analyzed. Of the patients who underwent curative hepatectomy for HCC, 424 met inclusion criteria: 73 with normal liver (NL) and 351 with LC. Sixty-four patients who had histologically proven NL parenchyma were matched with an equal number of patients who had established LC, and postoperative outcomes were compared.
Interventions Hepatectomy in patients with HCC.
Main Outcomes and Measures Patterns of recurrence of HCC and chronological changes in recurrence rates.
Results Among 128 matched patients in the study (mean [SD] age, 64.0 [12.7] years; 93 men and 35 women) 1-, 3-, and 5-year cumulative recurrence was 17.2%, 23.0%, and 37.5%, respectively, in the NL group vs 25.0%, 55.5%, and 72.1%, respectively, in the LC group ( P = .001). The 3- and 5-year disease-specific survival was 85.7% and 75.4%, respectively, in the NL group vs 74.9% and 59.1%, respectively, in the LC group ( P = .04). The median annual incidence of postoperative recurrence of HCC within 5 years after surgery was lower in the NL group (5.9%) compared with the LC group (12.7%) ( P = .003). Assessment of recurrence patterns revealed that multiple recurrences near the resection margin or at extrahepatic sites were more frequent in the NL group (9 [50.0%] vs 6 [15.4%]; P = .01), whereas solitary recurrence at a distant site was more common in the LC group (21 [53.8%] vs 1 [5.6%]; P < .001).
Conclusions and Relevance Comparison of the patterns and annual incidence of recurrence of HCC demonstrated that the poorer prognosis in the LC group was likely owing to a higher hepatocarcinogenic potential among patients with cirrhosis. Annual recurrence rates in the 2 groups indicate that de novo recurrence may continuously occur from the early postoperative period until the late period after resection of HCC.
Hepatocellular carcinoma (HCC) is the fifth most common cancer in men, and seventh among women, with more than half a million new cases diagnosed annually worldwide. In most cases, the etiologic factors of HCC are attributable to underlying liver injury due to chronic hepatitis or liver cirrhosis (LC). 1 , 2 Although hepatic resection is one of the most effective treatment options for patients with HCC and preserved hepatic functional reserve, the cumulative risk of recurrence during the first 5 years after surgery ranges from 70% to 100%. 3 - 5 As such, local tumor control remains a major issue in the clinical management of HCC. The high risk of recurrence after curative-intent hepatectomy is attributable to 2 unique patterns: recurrence derived from residual micrometastases and de novo recurrence owing to carcinogenic potential in the underlying liver. 6 , 7 Oncologic outcomes after resection of HCC have reportedly correlated with the degree of underlying liver injury, especially among patients with LC. 1 , 2 Given that the annual incidence of HCC arising from established LC has been reported to range between 2.5% and 6.6%, 8 - 10 it is estimated that the cumulative incidence of recurrence owing to de novo carcinogenesis would result in significant differences in long-term outcomes among patients with LC vs patients who have an undamaged liver.
Previous studies examining the influence of de novo recurrence of HCC attempted to distinguish the type of recurrence by using a time frame (early recurrence, occurring within 2 years after surgery, and late recurrence, occurring more than 2 years after surgery). 6 , 7 , 11 , 12 However, it is difficult to clearly distinguish the causes of recurrence of HCC, and the actual prognostic effect of de novo recurrence remains unclear. To address this issue, our study sought to determine the prognostic difference associated with the carcinogenic potential between patients with LC and those with a normal liver (NL) in a case-matched population adjusted for other oncologic factors.
A total of 799 adult patients without a history of previous HCC treatment who underwent curative-intent surgery for HCC between January 1, 1995, and December 31, 2014, at Toranomon Hospital in Tokyo, Japan, and at The Johns Hopkins Hospital in Baltimore, Maryland, were identified from a multi-institutional database. Only patients with HCC who had either histologically proven LC or NL were included in this study, which was approved by the Toranomon Hospital Human Ethics Review Committee and The Johns Hopkins University Institutional Review Board. No additional informed patient consent specific to this study was required given its retrospective nature.
Hepatitis B virus (HBV) infection was defined by seropositivity for HBV surface antigen and/or HBV DNA; hepatitis C virus (HCV) infection was defined as patients who were seropositive for HCV antibody and/or HCV-RNA. Alcohol abuse was defined as chronic liver injury resulting from alcohol consumption of 60 g/d or more without another clear etiologic factor and was confirmed by pathologic examination of the specimen. 13 The serum HBV-DNA level was investigated in patients with HCC without definitive etiologic factors to detect occult HBV infection. 14 Conditions such as autoimmune hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, Wilson disease, and hemochromatosis were included in the category of other etiologies. Types 1 and 2 diabetes were diagnosed based on the 2003 criteria of the American Diabetes Association. 15
Histopathologic variables were defined according to the pathologic classification system of the World Health Organization. 16 Specimens were fixed in 10% formalin and stained with hematoxylin-eosin, Masson trichrome, silver impregnation, and periodic acid–Schiff after diastase digestion. Only the area of noncancerous liver parenchyma that was distant from the tumor was evaluated to avoid influence from the tumor. Fibrosis and status of inflammation were evaluated using the METAVIR score. 17 Patients with no background liver damage, as evidenced by a liver that had had no fibrosis, moderate to severe inflammation, or severe steatosis (>30% of the total liver parenchyma) were assigned to the NL group. Patients with established cirrhosis (stage F4) were assigned to the LC group.
To control for the possibility of recurrence of HCC from residual micrometastases between the 2 groups, 1-to-1 individual paired case matching was performed for clinical factors other than underlying liver injury using R statistical software (R Foundation for Statistical Computing) with the optmatch package, which uses the optimal matching method with no caliper ( https://cran.r-project.org/web/packages/optmatch/optmatch.pdf ). Distance was calculated using Mahalanobis distance. These factors used for matching included sex, age, type of surgery, surgical margin status, tumor size, tumor differentiation, microvascular invasion, and preoperative serum α-fetoprotein level. After matching, the cumulative rate of recurrence, overall rate of survival, patterns of recurrence, and chronological changes in rates of recurrence were analyzed and compared.
Data were analyzed with SPSS software, version 19 (IBM SPSS), and EZR. 18 Continuous variables were expressed as median and range or interquartile range. Categorical variables were expressed as frequencies and percentages. The Mann-Whitney test was used to compare continuous variables between the 2 groups. The χ 2 test or Fisher exact test was used to compare categorical variables between the 2 groups as appropriate. Cumulative recurrence and disease-specific survival were determined using the Kaplan-Meier method. Differences between curves were assessed by the log-rank test. P < .05 (2-tailed) was considered statistically significant.
Among 799 adult patients who underwent primary curative-intent hepatectomy for HCC during the study period, 424 patients (285 men and 139 women) met the inclusion criteria. Of these patients, 73 had histologically proven NL parenchyma and 351 had LC. The baseline characteristics of the NL and LC groups are summarized in eTable 1 in the Supplement . Chronic hepatitis infection, including HBV and HCV, was the major cause of LC (HBV, 114 [32.5%] and HCV, 206 [58.7%]). The prevalence of diabetes in the NL group was higher than in the LC group, although the difference was marginal (NL, 17 [23.3%] and LC, 59 [16.8%]; P = .18). Data from baseline liver function tests, including aspartate aminotransferase and alanine aminotransferanse levels, total bilirubin level, platelet count, and Child-Pugh grade, were different between the NL and LC groups. The preoperative median serum α-fetoprotein level was also different between the 2 groups (NL, 5.0 ng/mL; LC, 20.7 ng/mL; P < .001) (to convert to micrograms per liter, multiply by 1.0).
With regard to treatment-associated factors, 36 patients (49.2%) in the NL group underwent anatomical liver resection, whereas 70 patients (19.9%) in the LC group underwent an anatomical resection ( P < .001). The proportion of resections with an R1 surgical margin was comparable between the groups (NL, 4 [5.5%] and LC, 32 [9.1%]; P = .49). With respect to tumor-associated characteristics, the proportion of multiple tumors was similar between the 2 groups (NL, 9 [15.5%] and LC, 41 [13.4%]; P = .84). The median maximum tumor diameter in the NL and LC groups was 40.5 mm (range, 20.0-250.0) and 20.0 mm (range, 2.0-150.0 mm), respectively ( P < .001). Tumors with poor histologic differentiation were more commonly found in the NL group (20 [27.4%]) than in the LC group (57 [16.2%]) ( P = .03). The presence of microvascular invasion tended to be more common in the NL group (20 [27.4%]) than in the LC group (69 [19.7%]) ( P = .16). Mild steatosis in the resected liver was noted in 30 patients (41.1%) in the NL group.
The clinicopathologic characteristics of the case-matched cohort are summarized in eTable 2 in the Supplement . Sixty-four patients who had histologically proven NL parenchyma were matched with an equal number of patients who had established LC. Matching variables included age, sex, operative factors, and tumor-associated factors. The proportion of patients with diabetes was similar between the 2 groups (NL, 18 [28.1%] and LC, 16 [25.0%]; P = .84). Despite matching, background liver function, including alanine aminotransferase level and platelet count, remained different between the 2 groups.
The median follow-up time of the matched cohort was 40.2 months (interquartile range, 21.5-62.6 months). The median follow-up period of the NL group was 39.4 months (interquartile range, 19.8-59. months), which was comparable with follow-up time for the LC group (median, 40.8 months; interquartile range, 24.2-67.5 months) ( P = .64). Recurrence was observed in 18 patients (28.1%) in the NL group and 39 patients (60.9%) in the LC group ( P < .001). Overall, 1-, 3-, and 5-year cumulative recurrence was 17.2%, 23.0%, and 37.5%, respectively, in the NL group vs 25.0%, 55.5%, and 72.1%, respectively, in the LC group ( P = .001; log-rank test) ( Figure 1 A). The 3- and 5-year disease-specific survival rate was 85.7% and 75.4%, respectively, in the NL group vs 74.9% and 59.1%, respectively, in the LC group ( P = .04; log-rank test) ( Figure 1 B). Of 64 patients in the NL group, no patients died of causes associated with liver disease other than recurrence of HCC; of 64 patients in the LC group, 3 patients died of liver failure and 1 patient died of spontaneous bacterial peritonitis.
When comparing patterns of recurrence in the matched cohort, both the number of recurring lesions (NL, 18 [28.1%] and LC, 39 [60.9%]; P < .001) and site of recurrence (NL: near resection site, 7 [38.9%], distant from resection site, 4 [22.2%], and extrahepatic, 7 [38.9%]; LC: near resection site, 13 [33.3%], distant from resection site, 23 [59.0%], and extrahepatic, 3 [7.7%]; P = .005) were significantly different between the 2 groups ( Table ). In the NL group, most patients (12 of 18 [66.7%]) experienced multiple recurrences, whereas most patients in the LC group (31 of 39 [79.5%]) experienced a solitary recurrence ( P = .001). Moreover, recurrence with 4 or more lesions was seen more frequently in the NL group than in the LC group (7 of 18 [38.9%] vs 4 of 39 [10.3%]; P = .03). In total, either multiple recurrences near the resection site and/or extrahepatic recurrence was the most frequent pattern of recurrence in the NL group than in the LC group (9 of 18 [50.0%] vs 6 of 39 [15.4%]; P = .01), whereas intrahepatic solitary recurrence distant from the resection site was the most frequent recurrence pattern in the LC group (NL, 1 of 18 [5.6%] and LC, 21 of 39 [53.8%]; P < .001)
The correlation between time to recurrence and patterns of recurrence is shown in Figure 2 and Figure 3 . In the matched cohort, the median time to recurrence among patients with a solitary recurrence was longer than that among patients who experienced multiple recurrences (18.3 vs 6.1 months; P = .001). The difference in median time to recurrence for patients with single vs multiple recurrences persisted in both the NL and LC groups (NL: 29.0 vs 5.7 months; P = .007; LC: 18.3 vs 9.7 months; P = .04). Recurrence at a site distant from the resection tended to develop later after resection compared with other sites of recurrence (median, 19.8 vs 10.0 months; P = .06), a finding that was similar in both NL and LC groups (NL: median, 22.8 vs 7.8 months; P = .92; and LC: median, 19.8 vs 11.2 months; P = .07). Recurrences distant from the resection site were consistently seen from the early period to the late period after resection. Differences in overall patterns of recurrence between the NL and LC groups persisted over time ( Figure 3 B).
The estimated annual rates of postoperative recurrence for both groups are shown in Figure 4 A. The median estimated annual rates of postoperative recurrence during the 5 years following resection were 5.9% in the NL group and 12.7% in the LC group ( P = .003). Although the risk of recurrence was bimodal for both the NL and LC groups, patients in the NL group tended to develop recurrences earlier than did patients in the LC group.
Our study aimed to characterize postoperative de novo recurrence of HCC among patients with LC using a case-matched analysis comparing patients with LC and those with NL who had oncologically equivalent tumors. Despite genetic analysis being the criterion standard to establish that recurrent disease is de novo HCC, few studies have used this approach. 19 - 21 Rather, most previous studies that have investigated postoperative recurrence of de novo HCC were based on the timing of recurrence. Previous investigators assumed that de novo recurrences developed exclusively in the late postoperative period and therefore classified such recurrences as de novo disease. 6 , 7 , 11 , 12 The carcinogenic potential in the liver remnant is, however, theoretically stable; as such, subsequent de novo recurrence can develop at any time, even in the early period after surgery. To mitigate the limitations associated with the assumption that postoperative recurrences of de novo HCC were based on the timing of recurrence, we conducted a case-matched analysis to define differences in postoperative recurrence of HCC among patients with NL and LC. Histologically proven NL parenchyma has a very low carcinogenic potential. Therefore, we hypothesize that the prognostic difference observed between the patients with a NL and those with LC could be attributed to the difference in carcinogenic potential between the 2 groups. We found that there was a marked difference in the patterns of recurrence between patients with NL and those with LC who underwent resection of an index HCC. Specifically, the main difference in the pattern of recurrence between the 2 groups was observed in the incidence of recurrent disease located distant from the resected portion of the liver. The recurrence rate among patients in the LC group remained consistently 6% to 15% higher than that in the NL group 1 to 4 years after resection.
In examining specific patterns of recurrence, the NL group more often had multiple recurrences near the resection site or at an extrahepatic site, mostly during the early period after resection. In contrast, the LC group tended to develop solitary recurrences located at a distance from the resected part of the liver; this pattern of recurrence among patients with LC was consistent from the early period after surgery to the late follow-up period. The differences in patterns of recurrence were also correlated with differences in postoperative recurrence. The reason for the different patterns of recurrence between the NL and LC groups was undoubtedly multifactorial. Theoretically, recurrence derived from residual micrometastases can develop in the relatively early period after surgery at or near the resection site (ie, intrahepatic micrometastases within a tumor-bearing portal territory) or at an extrahepatic site (ie, distant metastases through systemic circulation). 22 Moreover, time to recurrence was shorter for patients with multiple recurrences than for those with a single recurrence. Explanations of early multiple recurrences might be dissemination of the resected tumor or growth of multiple tumors at the initial surgery site, with microscopic nodules in the remaining liver being missed by imaging only to become apparent later. Conversely, an intrahepatic recurrence distant from the primary resection site may be considered a typical pattern of de novo recurrence. 23 , 24 In other words, the observed difference in patterns of recurrence are consistent with the hypothesis that postoperative recurrences in the NL group can be attributed largely to recurrence from residual cancer, whereas those of patients with LC are owing to both recurrence derived from micrometastases and de novo recurrence associated with the higher carcinogenic potential in the underlying liver.
Chronological changes in the annual incidence of recurrence demonstrated that recurrence in the LC group was about 6% to 15% higher per year than the risk of recurrence in the NL group ( Figure 4 A). Furthermore, after the first postoperative year, the cumulative hazards of recurrence continuously diverged over time ( Figure 4 B). These findings indicate that de novo recurrence may occur continuously from the early postoperative period until the late period. The annual incidence of primary HCC in patients with LC without a history of HCC is estimated to be 2.5% to 6.6%. 8 - 10 However, in patients with a history of HCC, a several-fold increase in the risk of developing a second primary HCC has been reported. 11 , 12 The difference in the annual rates of recurrence observed between the LC and NL groups is compatible with reported results in other patient populations. 7 , 10 , 11 , 25 , 26 The high annual incidence of de novo recurrence after resection persisted from the early to the late postoperative period.
The timing and bimodal nature of the rate of recurrence was roughly comparable between the NL and LC groups, although recurrence tended to be slightly earlier in the NL group ( Figure 4 ). The first peak of recurrence consisted of both recurrences owing to dissemination of resected tumor and small lesions that were not detected by imaging at the time of surgery. Considering that solitary recurrence at a distal site is much higher in the LC group, the first peak among the LC group would contain more undetected lesion at the time of surgery ( Figure 3 ). Imamura et al 11 reported a similar recurrence pattern in patients with LC and attributed the late peak to de novo recurrence of HCC owing to background LC. In our study, however, there was a late recurrence peak also seen in the NL group. Other investigators have similarly reported late recurrences arising among patients with NL. 25 - 28 These late recurrences might be attributed to unknown background liver carcinogenesis present in patients with NL and an index HCC. Given the low chance of carcinogenesis in histologically proven NL, late recurrences in a patient with NL may derive from slowly growing recurrences of the resected index tumor. To this end, a previous study that included 15 944 patients with nonviral hepatitis without a history of significant alcohol intake or fatty liver revealed that only 2 patients (0.01%) developed HCC during the 1-year follow-up period. 29 Although presence of fatty liver and diabetes are known risk factors of hepatocarcinogenesis, Kawamura et al 29 reported that the annual incidence of HCC among patients with nonalcoholic fatty liver disease was only 0.04%, while the reported hazard ratio of patients with diabetes compared with patients who do not have diabetes is about 2.2 to 3.5. 30 , 31 Even though patients with a history of HCC have a several-fold increase in hepatocarcinogenesis compared with patients without a previous HCC, the rate of carcinogenesis is still negligibly low compared with the risk among patients who have established LC. In the future, genetic investigations comparing primary and recurrent lesions in patients with NL are required to provide more compelling evidence regarding the origin of late recurrences.
Our study had some limitations, including the retrospective study design. Individual paired matching was performed using a large population of patients with LC to generate the matched pairs. Despite the attempt at matching, it is possible that some residual differences in the 2 groups persisted. 32 Although the biology of HCC may differ according to background etiologic factors, most traditional factors associated with aggressiveness of the disease were comparable between the 2 groups. However, residual differences of aggressiveness of the disease that arise in healthy vs cirrhotic livers may not have been fully accounted for. Furthermore, the different etiologic factors associated with the underlying LC were not taken into account. However, the incidence of HCC is fairly similar among patients who develop LC owing to different causes. 33 Although some differences may exist in the activity and modes of cancer promotion among patients with HBV infection, HCV infection, and alcohol abuse, the etiologic factors of background HCC were not associated with recurrence. Given the retrospective nature of the study, we also could not assess the presence or absence of a lead time bias or difference in screening protocol between the NL and LC groups.
Our study demonstrated different oncologic outcomes among patients with HCC and an underlying NL compared with patients who had LC. Specifically, patients with LC had a 6% to 15% higher annual risk of de novo recurrence compared with patients who had HCC resected from NL. Given the constant higher risk of de novo recurrence starting in the early postoperative period for patients with LC, preemptive treatment of viral hepatitis and close follow-up are important to decrease the risk of recurrence and detect early recurrent disease.
Accepted for Publication: October 28, 2016.
Corresponding Author: Timothy M. Pawlik, MD, MPH, PhD, Division of Surgical Oncology, Department of Surgery, The Johns Hopkins Hospital, 600 N Wolfe St, Blalock 688, Baltimore, MD 21287 ( [email protected] ).
Published Online: January 4, 2017. doi:10.1001/jamasurg.2016.5059
Author Contributions: Dr Pawlik had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Sasaki, Shindoh, Margonis, Nishioka, Andreatos, Sekine, Pawlik.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: Sasaki, Shindoh, Margonis, Nishioka, Andreatos, Sekine, Pawlik.
Statistical analysis: Sasaki, Shindoh, Nishioka, Andreatos, Sekine, Pawlik.
Administrative, technical, or material support: Hashimoto, Pawlik.
Study supervision: Shindoh, Margonis, Pawlik.
Conflict of Interest Disclosures: None reported.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

- Nutrition counsellor
- Food Allergy
- gastroenterology
- Heptologist In Delhi
- Liver transplant
- Alcoholic Liver Cirrhosis
Alcoholic liver cirrhosis is a late stage of fibrosis of the liver caused by many forms of liver diseases and conditions, such as chronic alcoholism. A person diagnosed with an alcoholic liver case may start from having fatty liver disease, then alcoholic hepatitis, and ultimately develop alcoholic cirrhosis. Hence, alcoholic liver cirrhosis stages in three levels. The diagnosed liver cirrhosis can be of two types :
- Compensated cirrhosis – when symptoms are not noticeable
- Uncompensated cirrhosis- when the symptoms can be noticed
The most common alcoholic liver causes are:
- Chronic alcohol consumption
- Chronic hepatitis
- Fatty liver disease
- Iron buildup in the body or hemochromatosis
- Copper accumulated in the liver (Wilson’s disease)
- Cystic fibrosis
- Biliary Atresia
- Inherited sugar metabolism or digestive disorders
- Infection like syphilis
Many other factors like the destruction of bile ducts(Primary Biliary Cirrhosis) or leaky gut also called, increased intestinal permeability are cofactors for the development of alcoholic liver cirrhosis.
Now, let us have a look at alcoholic liver cirrhosis symptoms:
- Food pipe problems
- Portal hypertension
- Swelling in legs (oedema) and abdomen (ascites)
- Bleeding in mouth
- Confusion, poor memory, loss of appetite
- Patchy red skin on palms (erythema)
Food pipe problem is also known as esophageal varices. Kidney failure and hypersplenism are other complications that happen due to this medical condition. If the symptoms are not taken seriously, then this deficient liver may arise a life-threatening situation. Hence, a person needs to keep a track of these indicators as if these signs are caught early and treated, it may slow down the progression of the disease.
How to treat alcoholic liver cirrhosis:
The first and foremost step in treatments is to help the patient to cease alcohol consumption. Medications like corticosteroids, calcium channel blockers, insulin can also be prescribed by the doctor as per the alcoholic liver care plan. Hepatologists may advise the patient to follow an alcoholic liver disease diet inclusive of fiber and protein. If the condition of the patient gets worsened, then the hepatologist may have to suggest a liver transplant surgery.
Dr. Nivedita Pandey is one of the best liver specialist doctors in Patna, Bihar. She is a well-renowned liver specialist doctor in Delhi, the best stomach doctor in Patna, the best gastroenterologist in Jammu, and a notable stomach doctor in Faridabad. Now, you can sway off all your gastroenterological worries online by booking an online gastroenterologist consultation with one of the best hepatologists in India. She is a liver specialist in Delhi NCR, one of the finest gastroenterologists in Jhansi and Jammu, and an acidity specialist doctor in Patna. Dr. Pandey’s gastroenterologist live chat has also helped people in several ways.
Case Review
This alcoholic liver case study presents a patient with liver cirrhosis. A 43-year-old man was brought into the hospital with a complaints of loss of appetite, abdominal distention, and arrhythmia. He also experienced itchy skin and blood in the stool. The patient’s family rushed him into the hospital, and he was in a half-conscious state. The patient was taken to the emergency room for evaluation. As told by the family, he had a past medical history and was a heavy alcohol consumer. This alcoholic liver case history consisted of various medical ailments like fatty liver, asthma, tuberculosis, malnutrition, hypertension, and hepatitis C. The patient had a heart attack three years back and stented for the same. Due to his health conditions, he was on several medications. In the emergency room, when the patient was under observation by a stomach specialist doctor in Patna and her team, they were able to diagnose from his symptoms that it was alcoholic liver cirrhosis. The patient went for a few scans including, a liver function test, liver ultrasound, and endoscopy along with CT, blood test, and urine tests.
Case Discussion
Though most of the liver cirrhosis causes remain unknown, with the help of her team, the best liver doctor and specialist in Patna was able to find out the reason for this one. The liver cirrhosis caused in this case was due to the medical history of the patient. His scans came out to be reasonably sound, and a liver biopsy was conducted to confirm the severity and type of liver disease. There were problems in his blood and urine culture, and they were taken care of by the team. His liver appeared swollen in the reports. There were certain other problems seen in his ultrasound and endoscopy. The crew decided to start with the treatment while keeping him under observation for the next 72 hours.
Clinical Symptoms
He was initially confused and was not able to respond or hear properly. According to the condition reported by his family i.e.- appetite loss, memory loss, and confusion were some other clinical symptoms of alcoholic liver cirrhosis. When the doctor talked to the family of the patient, she was able to get a clearer picture. The patient complained about acute abdominal pain. When Dr. Pandey, one of the best doctors in Patna for the stomach, observed the patient and talked to him, she noticed bleeding in his mouth. This further helped doctors to eliminate all doubts, and after looking at the lab results, they made out it was alcoholic liver cirrhosis.
The first and foremost management required when treating the alcoholic liver cirrhosis case is calming the patient down. The liver specialist with the help of her fellow doctors was able to counsel the patient and explain his medical condition to the family. After a complete diagnosis, the patient was taken to the ICU as he was under observation. The doctor prescribed him antioxidant drugs and insulin to control any future problems while treating the present one. The doctor is the best gastroenterologist in Faridabad, Delhi, and Patna, and she handled the situation well before any further complications. The patient got his discharge in due time and was sent back home in a healthy and sound condition.
1. Which Group of People are More Likely to get diagnosed with alcoholic liver cirrhosis?
A person who has drunk heavily for a long time is more prone to acquire this disease. Women are also at risk for this medical disease due to the absence of many enzymes which break down alcohol particles.
Consider consulting the best gastroenterologist in India , Dr. Nivedita Pandey who is also well known for her nutritional counselling services and teleconsultation services. She is also famous for her care from afar service. You can also find her as the best liver specialist doctor in Patna, Bihar or hepatologist in Patna or the best doctor for hepatitis b in Patna , a gastroenterologist in Faridabad , the best gastro doctor in Delhi, NCR , a gastroenterologist In Uttarakhand , a liver specialist in Jhansi , best gastroenterologist in Jammu take advantage of the online gastroenterology consultation to gastroenterologist live chat and receive the best treatment that your body deserves!
2. Is liver cirrhosis cancer?
No, liver cirrhosis is not a type of cancer. If a person has alcoholic liver cirrhosis, he/she has an increased risk of liver cancer.
3. Is liver cirrhosis a hereditary disease?
Negative, alcoholic liver cirrhosis is not a hereditary disease, rather it is a type of an acquired disease.
Privacy Preference Center
Privacy preferences, nutrition counselling.
Nutrition counseling is the assessment of an individual’s dietary intake after which, they are helped set achievable goals and taught various ways of maintaining these goals. The nutrition counselor provides information, educational materials, support and follow-up care to help an individual make and maintain the needed dietary changes for problems like obesity.
Obesity/ Food allergy
I assist people dealing with weight-related health problems by evaluating the health risks and help in obesity management. I also help patients manage various food allergies.
As a hepatologist, I specialize in the treatment of liver disorders, pancreas, gallbladder, hepatitis C, jaundice and the biliary tree. I also see patients suffering from pancreatitis, liver cancers alcoholic cirrhosis and drug induced liver disease(DILI), which has affected the liver.
Gastroenterology
As a gastroenterologist, my primary focus is the overall health of the digestive system. I treat everything from acid reflux to ulcers, IBS, IBD: Crohns disease and ulcerative colitis, and colon cancer.
Endoscopy is a nonsurgical procedure to examine a person’s digestive tract. It is carried out with an endoscope, a flexible tube with a light and camera attached to it so that the doctor can see pictures of the digestive tract on a color TV monitor.
Medical Gastroenterology
Gastroenterology is a specialty that evaluates the entire alimentary tract from the mouth to anus and involves studying the diseases of the pancreas.
Liver Transplant Services
A liver transplant is a surgical procedure that removes a patient’s non-functioning liver and replaces it with a healthy liver from a deceased donor or a portion of healthy liver from a living donor. It is reserved as a treatment option for people who have significant complications due to end-stage chronic liver disease or in case of sudden failure of a previously healthy liver.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 14, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
Study explores potential target to treat liver disease
by University of Birmingham
A clinical trial led by Birmingham researchers investigated targeting a molecule causing liver inflammation and fibrosis to treat patients with Primary sclerosing cholangitis (PSC)—a debilitating liver disease for which there is currently no treatment.
The findings, published in Hepatology Communications , highlight how the therapeutic agent timolumab was able to safely block the molecule of interest, a vascular adhesion protein called VAP-1, without causing significant adverse effects.
However, it did not produce significant change in the levels of alkaline phosphatase (ALP), an enzyme associated with liver disease, which meant the trial was stopped due to lack of treatment efficacy.
Nevertheless, researchers remain hopeful about the potential of VAP-1 as a target in inflammatory diseases —also thanks to the breadth of previous evidence gained in previous studies led by Professor David Adams at the University of Birmingham—and believe these findings may help guide future research focusing on this protein.
Dr. Chris Weston is Associate Professor at the University of Birmingham's Institute of Immunology and Immunotherapy, member of the NIHR Birmingham Biomedical Research Center's Inflammatory Liver Disease theme, and co-author of the study.
Weston said, "We believe that VAP-1 remains a viable target in inflammatory and fibrotic disease given the wealth of evidence in other preclinical models, and the use of small molecule inhibitors of VAP-1 that have been developed may provide an alternative means to target VAP-1.
"Our findings may well help guide other studies using monotherapy or combined therapy with VAP-1, both in the liver and in other sites of inflammation and fibrosis."
Lead Clinical Investigator for the trial Professor Gideon Hirschfield, Chair in Autoimmune Liver Disease Research at the Toronto Center for Liver Disease and Honorary Professor at the University of Birmingham, added, "Primary sclerosing cholangitis can be a very debilitating chronic disease, which most often affects people of working age.
"It is key that we continue to investigate this condition, its causes and potential avenues for treatment, to find effective ways to improve the lives of patients in the U.K. and across the globe."
Primary sclerosing cholangitis (PSC) is a progressive inflammatory liver disease where the body's immune attacks its own liver. It affects people of all ages, frequently in association with inflammatory bowel disease (IBD).
Explore further
Feedback to editors

New technique detects novel biomarkers for kidney diseases with nephrotic syndrome
May 25, 2024

In experiments, mice get ill from raw milk carrying bird flu virus
May 24, 2024
Understanding a broken heart—study finds link between stress and recurrent heart failure

Genetic cause of rare childhood immune disorders discovered

New surgical tool moves tiny bioparticles with robotics and acoustic energy
The link between defective autophagy and pancreatitis could point to new treatments

Possible association between tattoos and lymphoma revealed


Walkability in neighborhoods linked to health, study of siblings shows

Scientists uncover new treatment pathway for rare 'spider web' childhood brain tumors
How COVID-19 'breakthrough' infections alter your immune cells
Related stories.

Clinical trial identifies promising target for liver disease treatments
Nov 7, 2023
New inflammatory mechanisms unveiled in the setting of liver disease and liver cancer
Oct 30, 2023
Q&A: How to manage symptoms of liver disease
Jul 14, 2022

Clinical trial results suggest potential new treatment for primary biliary cholangitis
Nov 16, 2023
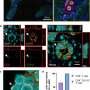
Research into autoimmune liver condition suggests unique cell movements may drive disease
Feb 2, 2024
NGM282—an engineered analogue of FGF19—shows promise in patients with primary sclerosing cholangitis
Apr 13, 2018
Recommended for you

Colon cancers are rising among the young: New study outlines the warning signs
How air pollution affects the digestive system
Study reveals right atrium changes in cardiovascular diseases
New global targets proposed to reduce AMR-linked deaths and improve access to essential antibiotics
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

CASE STUDY OF PATIENT WITH LIVER CIRRHOSIS

Livercirrhosisisadiseaseinwhichnormaltissueofliverreplacedwithscartissue,livercirrhosisisthe12thleadingcauseofdeathsbydiseaseintheworld.Livercirrhosisiscausedbyanyfactorthatcandamagelivertissues,mostlyfattyliverandchronicliverdiseasesarethemajorcauseoflivercirrhosis. WearepresentingherethecaseofanAsianmanwhowasthevictimoflivercirrhosisthatwascomplicatedbyuntreatedhepatitisc.Hewasexperiencinggeneralizedbodyweakness,brownishtintintheurine,andsuddenweightgainof8-kgswithinaperiodofthreeweeks.Bloodpressurecountwas100/60,pulseratefoundtobe76beats/min,temperature98F,andenlargedumbilical.Laboratorytestsincludingcompletebloodcount,liverfunctiontests,andureatestscameouttobesignificantlyabnormal,complicatingthecase.,ultrasoundreportrevealedthathisliverwasenlarged,urinarybladderpartiallyfilled,umbilicalherniagapereported,ascitespresent(retentionoffluidintheabdomen),Cirrhosisandsplenomegalywerereported.Hisliverwasdamagedandliverfunctioningtestswerenotreturningtonormal,hepatologistrecommendedlivertransplantationasthelastresortforhim. Thereistheneedofgoodclinicalevaluationbyaqualifiedtherapistanduseofappropriateinvestigativestudiestosecurepatientfromsuchacriticalhealthhazard. Keywords:chronicliverdisease,livertransplantation,
Related Papers
Journal of Pharmaceutical Research International
Jaya Khandar
Liver is the second largest organ in human body, more than 5,000 separate bodily functions .including helping blood to clot, cleansing the blood of toxins to converting food into nutrients to control hormone levels, fighting infections and illness, regenerating back after injury and metabolizing cholesterol, glucose, iron and controlling their levels. A 56- years old patient was admitted in AVBRH on date 9/12/2020 in ICU with the chief complaint of abdominal distension, breathlessness on exertion, pedal edema, fever since 8 days. After admitted in hospital all investigation was done including blood test, ECG, fluid cytology, peripheral smear, ultrasonography, etc. All investigation conducted and then final diagnosis confirmed as cirrhosis of liver. Patient was not having any history of communicable disease or any hereditary disease but he has history of hypertension and type II Diabetes mellitus for 12 years. Patient was COVID-19 negative and admitted in intensive care unit. Patient...
The Journal of medical research
Amrendra Yadav
The liver is the multi-functional and vital organ of the body. It is found in the upper abdomen region of the vertebrates. Due to long-term damage, liver stops functioning properly which may lead to cirrhosis. This long-term damage occurred when scar tissue replaces the normal tissue of the liver. This disease develops slowly and has no early symptoms, but when it develops and become worse, then it leads to tiredness, itchiness, weakness, yellow skin, swelling in the lower legs, spider-like blood vessels and an easy bruise on the skin with fluid in the abdomen. The severe complications like bleeding dilated veins in esophagus or stomach, hepatic encephalopathy leading to confusion and unconsciousness and liver cancer may occur in the body. This review article is focusing on the effect of liver damage in the
Asian Australasian Neuro and Health Science Journal (AANHS-J)
Hanun Mahyuddin
Liver cirrhosis is a chronic disease characterized by the presence of fibrosis and regeneration of nodules in the liver, the consequence of which is the development of portal hypertension and liver failure. Usually associated with infectious infectious diseases such as viral hepatitis, alcohol consumption, metabolic syndrome, autoimmune processes, storage diseases, toxic substances and drugs. Major complications include gastrointestinal variceal bleeding, ascites, spontaneous bacterial peritonitis infection, hepatorenal syndrome, hepatic encephalopathy, and hepatocellular carcinoma. A 23-year-old woman comes to the ER, dr. Soegiri Lamongan with complaints of vomiting blood. The patient also complained of black bloody stools. Referred patient from Intan Medika Hospital with the initial complaint of vomiting blood more than 5 times (± equivalent to one medium drinking bottle) four days ago. On examination also found anemic conjunctiva and found splenomegaly. On abdominal ultrasound ex...
Fortune Journals , Umair Akram
Cirrhosis results from chronic liver disease, and is characterized by advanced fibrosis, scarring, and formation of regenerative nodules leading to architectural distortion. The main objective of the study is to find the cirrhosis and its complication, other clinical complications except ACLF and critical illness. This descriptive study was conducted at DHQ hospital, Vehari, Pakistan during June 2022 to January 2023. A comprehensive literature search of the published data was performed in regard with the spectrum, diagnosis, and management of cirrhosis and its complications. Data was also collected from OPD of the hospital record. We include all patients suffering from liver cirrhosis and its complication. It is concluded that patients with cirrhosis have progressive disease and suffer from multiple complications like ascites, HE, variceal bleeding, hepatorenal syndrome, cirrhotic cardiomyopathy, pulmonary syndromes, sarcopenia, frailty, and HCC. The prevention, early diagnosis, treatment, and palliation of these complications are essential in comprehensive clinical care plans.
The Egyptian Journal of Hospital Medicine
ibrahim al rajeh
Clinical Chemistry
Corinne Fantz
Clinical Transplantation
Cyrille Feray
Asian J Pharm Clin Res
Dr Bibhu Prasad Behera
Objective: Efforts can be made to normalize the hematological parameters so that the morbidity and mortality in these patients could be effectively reduced. Methods: This observational study was carried out among 69 cirrhosis patients that fulfills the inclusion and exclusion criteria, attended the medicine outpatient department, and admitted in medicine ward Results: In our study, we had 59 male and 10 female patients with an average age of 49.8±13.19 years. About 92.75% of the patients were alcoholic. Abdominal distension (92.75%) and ascites (84.06%) were the most common presenting complaints. Pallor was present in 42 (60.87%) cases. Splenomegaly was present in 35 (50.72%) cirrhotic patients. Renal dysfunction was present in 23 (33.33%) cases. Sixty-six (95.65%) patients had anemia and 47 (68.12%) patients had thrombocytopenia. Conclusions: From this study, we can conclude that, in cirrhosis of liver patients, various hematological changes are very common which need to be identified and corrected early to reduce morbidity and mortality.
Faridpur Medical College Journal
Shahin Ul Islam
Liver cirrhosis is an important cause of death and disability globally. This cross-sectional study was carried out in Faridpur Medical College Hospital from November 2018 to April 2019 to see the patterns of clinical presentations and associated factors among admitted liver cirrhosis patients. A total of 89 patients were included. Data were collected by detailed history from patients or their relatives followed by thorough physical examination as well as diagnostic evaluation; then those were checked, verified for consistency and edited for result. Among total respondents, the majority were male (69.7%) with a male to female ratio of 1:0.44. Age of patients ranged between 22-106 years with mean age of 52.33 year. The patients predominantly presented with ascites (49.4%), gastrointestinal bleeding (27%), peripheral edema (24.7%), and encephalopathy (21.3%). The in-hospital case fatality rate was 11.2% and the patients presented with decreased urinary output, peripheral edema and ence...
RELATED PAPERS
Telecomunicação
Prof Vargas A Luiz Pinto
SSRN Electronic Journal
Rodrigo De-Losso
原版复制uc学位证书 查尔斯顿大学毕业证本科学位GRE成绩单原版一模一样
Maritime Technology and Research
Obed Ndikom
Den Obalanserade Hierarkin
Ann-Mari Sellerberg
Neuropsychologia
Simona Ghetti
Food Microbiology
Samantha Bolten
Journal of Aquatic Animal Health
Carole Engle
Jurnal Smart Keperawatan
evy kusgiarti
永久可查英国北安普顿大学毕业证 uom学位证书电子版学位证书留服认证原版一模一样
Steve Russell
Interciencia
Angeles Campos
International Journal of Scientific and Research Publications (IJSRP)
ghfkfg fdgdsf
多伦多大学毕业证书 购买加拿大学历Uof文凭学位证书
麦克马斯特大学毕业证书 加拿大学历MMU文凭学位证书成绩单
Sébastien Roy
Lutz Kirste
Systematic Parasitology
Sergey Mironov
The Indian Journal of Agricultural Sciences
Pratima Vaidya
Early Childhood Education Journal
Maureen Smith
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

Introduction
Research design and methods, conclusions, article information, metabolic syndrome traits increase the risk of major adverse liver outcomes in type 2 diabetes.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Ying Shang , Emilie Toresson Grip , Angelo Modica , Helena Skröder , Oskar Ström , Fady Ntanios , Soffia Gudbjörnsdottir , Hannes Hagström; Metabolic Syndrome Traits Increase the Risk of Major Adverse Liver Outcomes in Type 2 Diabetes. Diabetes Care 20 May 2024; 47 (6): 978–985. https://doi.org/10.2337/dc23-1937
Download citation file:
- Ris (Zotero)
- Reference Manager
Type 2 diabetes (T2D) increases the risk for major adverse liver outcomes (MALOs), including cirrhosis and its complications. Patients with T2D frequently have other traits of the metabolic syndrome (MetS). It remains uncertain whether there is a synergistic effect of accumulating MetS traits on future MALO risk.
Patients with T2D without a history of liver disease were identified from national registers in Sweden from 1998 to 2021. MetS traits included hypertension, low HDL level, hypertriglyceridemia, obesity, and albuminuria, in addition to T2D. MALO events were identified based on administrative coding from national registers until 31 October 2022. Data were analyzed using Cox regression models.
In total, 230,992 patients were identified (median age 64 years; 58% male), of whom 3,215 (1.39%) developed MALOs over a median follow-up of 9.9 years. Compared with patients with one MetS trait (only T2D) at baseline, those with more than one MetS trait had a higher rate of MALOs (adjusted hazard ratio [aHR] 2.33, 95% CI 1.53–3.54). The rate of MALOs increased progressively with increasing numbers of MetS traits at baseline (aHR 1.28 per added trait, 95% CI 1.23–1.33). During follow-up, patients who acquired additional MetS traits had a progressively higher rate of MALOs. The MetS trait with the largest association with incident MALOs was hypertension (aHR 2.06, 95% CI 1.57–2.71).
Having or acquiring additional traits of MetS increase the rate of progression to MALOs in patients with T2D. These results could be used to inform screening initiatives for liver disease.
Graphical Abstract

The metabolic syndrome (MetS) is a cluster of metabolic abnormalities that may affect the liver. Metabolic dysfunction–associated steatotic liver disease (MASLD) (recently renamed from nonalcoholic fatty liver disease) is considered the hepatic phenotype of the MetS, affecting 38% of the global adult population ( 1 , 2 ) and >55% of patients with type 2 diabetes (T2D) ( 3 ). Patients with T2D have more than twice as high a risk of developing cirrhosis or liver cancer compared with the general population ( 4 ). Recognizing this high risk for cirrhosis among patients with T2D, several international guidelines now actively recommend screening for MASLD-associated liver fibrosis in patients with T2D. Nevertheless, the implementation of such guidelines in clinical practice is modest at best ( 5 ). Identifying subgroups of patients with T2D who have a particularly high risk might help narrow down the target group intended for directed casefinding initiatives and improve the effectiveness of screening efforts.
A more severe metabolic state is associated with a higher rate of progression to cirrhosis, as evidenced by a study involving patients with a diagnosis of MASLD within a U.S. Veterans Affairs cohort ( 6 ). This study revealed a gradual increase in cirrhosis and liver cancer risk with the presence of additional metabolic traits, with T2D exhibiting the strongest association. However, for patients with T2D who already are at a high risk, it remains unclear whether a similar pattern is present. Additionally, it is unclear if acquiring additional metabolic traits beyond a T2D diagnosis further amplifies this risk and which specific traits hold the strongest association with liver disease. Such information is important as most patients with MASLD will never develop liver-related outcomes ( 7 ). Hence, a risk-based approach to screening or treatment has been advocated for ( 8 , 9 ). That is, instead of solely aiming to diagnose MASLD or fibrosis, one considers the risk a patient has for future liver-related outcomes and tailors appropriate actions accordingly.
Here, we used high-quality Swedish national registers to investigate the association between the number and subtypes of MetS traits and the risk of developing MALOs in Swedish patients with T2D. Our hypothesis was that increasing numbers of MetS traits at or after a diagnosis of T2D would be associated with an increase in the risk of incident liver cirrhosis or complications thereof.
Study Population
We used the Swedish National Diabetes Register (NDR) to identify patients with T2D. In NDR, T2D is defined using the following epidemiological definition: recorded T2D in clinical records, treatment with diet with or without oral antihyperglycemic agents, and treatment with oral antihyperglycemic agents with or without insulin ( 10 ). The NDR was initiated in 1996 and contains data for >90% of all patients aged >18 years with T2D in Sweden. This means that for patients diagnosed with T2D after 1996, their first registration in NDR is typically the time of their first diagnosis. The data are collected by trained nurses and physicians and include information on laboratory tests and medication use obtained from primary care and hospital outpatient clinics.
We included all patients recorded in the NDR between 1 January 1998 and 31 October 2021. The first entry date was defined as baseline. Patients were linked to several national registers using their personal identity number, which is assigned to all Swedish citizens ( 11 ). The National Patient Register (NPR) contains national coverage on hospital discharges since 1987 and contains data on outpatient visits in specialized care since 2001. It has a validity between 85 and 95%, depending on the diagnosis ( 12 ). Specifically, for hepatocellular carcinoma (HCC) and diagnoses related to cirrhosis, the validity rate ranges from 84 to 96% ( 13 ).
A total of 764,944 patients aged >18 years at the time of registration in NDR were eligible for inclusion (see flowchart in Supplementary Fig. 1 ). For the analysis on MetS traits to be comparable to each other, patients in the study sample needed to have information on the values for all MetS traits in this study. Therefore, 520,605 patients who had missing information on one or more of the MetS traits were excluded. We further excluded patients with preexisting MALOs and liver diseases other than MASLD based on ICD-10 codes and surgical procedure data from NPR. We also excluded patients with preexisting alcohol-related disease to minimize the impact of alcohol misuse. Since the use of ICD-10 codes began in 1997 in Sweden, we applied a look-back period of at least 1 year to identify preexisting diseases, with start of identification and follow-up from 1998. To reduce the potential impact of medications that may induce liver steatosis or fibrosis, we excluded patients who had ever used methotrexate or amiodarone prior to the index date from the Prescribed Drug Register. The Prescribed Drug Register was initiated in July 2005 and includes all drug dispensations at any Swedish pharmacy. Patients who emigrated before baseline were also excluded. Diagnostic, surgical, and Anatomical Therapeutic Chemical codes for all diagnoses and medications used in the study are listed in Supplementary Tables 1 – 3 .
MetS and Other Variables
We used World Health Organization (WHO) 1998 criteria to define the traits of the MetS ( 14 ), a definition that has been endorsed by the NDR for identifying the MetS among patients with T2D ( 15 ). The WHO definition may be better suited for populations with T2D because unlike other available definitions of the MetS, the WHO definition specifically mandates the presence of T2D as a diagnostic requirement ( 14 ), which is central to the pathophysiology of the MetS. In addition to T2D, the traits of MetS in this definition include hypertension, obesity, hypertriglyceridemia, a low level of HDL, and albuminuria. These were defined based on laboratory test data from the NDR, diagnoses from the NPR, or dispensed medications from the Prescribed Drug Register. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, a recorded diagnosis of hypertension, or any dispensed antihypertensive medication. Obesity was determined as BMI ≥30 kg/m 2 . A high level of triglycerides was identified as plasma triglyceride levels ≥1.7 mmol/L. A low level of HDL was defined as <0.9 mmol/L for men and <1.0 mmol/L for women. Finally, albuminuria was determined as a urinary albumin excretion rate ≥20 μg/min or an albumin-to-creatinine ratio ≥30 mg/mmol. We also calculated the number of MetS traits for each patient, which ranged from one to six.
The following information from the NDR recorded at each clinical visit was also included: time since diabetes diagnosis (years), glycated hemoglobin A 1c (HbA 1c ) (mmol/mol), weight (kg), height (m), total cholesterol (mmol/L), LDL (mmol/L), estimated glomerular filtration rate (eGFR) (mL/min/1.73 m 2 ), active smoking, and physical activity (walking or equivalent at least 30 min/day). Furthermore, the NDR contains information on glucose-lowering medications (defined as any oral medication or insulin), use of glucagon-like peptide 1 receptor agonists (GLP-1RAs), and lipid-lowering drugs but not on specific agents or doses. Besides this, clinical history of cardiovascular disease (CVD) (including myocardial infarct, hemorrhagic or ischemic stroke, and heart failure), chronic kidney disease (CKD), and other diseases included in the Charlson comorbidity index were obtained through linkage to the NPR ( 16 ). Hyperlipidemia was determined by recorded diagnosis in the NPR or any lipid-lowering drugs in the NDR or Prescribed Drug Register. Data on other medications, such as sodium–glucose cotransporter 2 (SGLT-2) inhibitors, were obtained from the Prescribed Drug Register.
We linked the NDR data set to the NPR, the Swedish Cancer Register (SCR), and the Causes of Death Register (CDR) to identify liver disease outcomes between 1 January 1998 and 31 October 2022. Malignancies are documented in the SCR, which contains ∼96% of all diagnosed cancers in Sweden ( 17 ). The CDR contains data on causes of death for all Swedish citizens. It is mandatory for Swedish physicians to report the cause of death and any diagnosis that might have contributed to death as recorded the CDR ( 18 ). We used both primary and contributing diagnoses to identify outcomes in the NPR and CDR.
MALOs, as a composite outcome, were defined as having any of the diagnoses defined by ICD-10 codes or surgical procedures in registers. These diagnoses include liver cirrhosis, decompensated cirrhosis, and associated complications (defined as coding for chronic or unspecified hepatic failure, esophageal varices with or without bleeding, portal hypertension, hepatorenal syndromes, ascites, or liver transplant), HCC, or death from any of these (definitions listed in Supplementary Table 1 ). In general, these diagnoses result in contact with health care or in death, leading to a high capture rate of the outcome in the relevant registries. Since the positive predictive value for hepatic ascites is generally low, we excluded outcomes with ascites because of other diseases, such as heart failure or extrahepatic cancer, defined as present at or before the ascites diagnosis.
Statistical Analyses
Baseline characteristics of the study population are expressed as median and interquartile range (IQR) or frequency and percentage, as appropriate. The follow-up time started at the baseline date, and patients were followed until the first event of MALOs or were censored at development of any other liver disease than MASLD, emigration, death, or the end of the study period (31 October 2022), whichever occurred first. Incidence rates (per 1,000 person-years) were calculated as the number of events divided by total person-time at risk, displayed overall by the number of metabolic traits and by each individual trait in the MetS as measured at baseline. A Cox proportional hazards model was used to estimate hazard ratios (HRs) and 95% CIs of the association between the risk of MALOs and prevalent MetS by numbers and by individual traits, where follow-up time was used as the timescale. We reported results from two models: model 1 was a univariate model with only each respective trait in the MetS as the dependent variable, and model 2 was adjusted for all MetS traits, age at baseline, sex, duration of T2D, HbA 1c , CKD, CVD, eGFR, LDL, smoking, hyperlipidemia, and glucose-lowering treatments, all recorded at baseline.
As the competing risk of non–liver-related mortality, e.g., cardiovascular death, is high in patients with T2D, we calculated cause-specific hazard rates of MALOs from a Cox regression model, considering non–liver mortality as a competing event. We did not use the Fine and Gray model since the subdistributional hazard derived from this does not have a straightforward interpretation and only gives the direction and not the strength of the association, which was our primary research question ( 19 , 20 ).
Statistical interaction between the main exposures (number and individual metabolic traits) and median age at baseline (age ≥65 or <65 years) and sex was tested separately. The associations between different combinations of baseline MetS traits and the risk of MALOs were estimated using HRs while adjusting for the same set of covariates at baseline. As patients with T2D may develop additional traits of the MetS during follow-up, we further examined the association between MetS and MALOs using time-varying Cox regression, considering both prevalent and incident metabolic traits. In brief, the time-varying Cox regression analyzed time to event with consideration of time-varying exposures by using all available data on MetS traits measured at multiple visits. We fitted two separate models, considering either the number or the different combinations of metabolic traits developed during follow-up as exposures, and reported corresponding HRs after adjusting for covariates at baseline. The cumulative incidence of MALOs by numbers of metabolic traits at baseline were calculated based on the Aalen-Johansen estimator, accounting for the competing risk of non–liver-related death ( 20 ).
A two-sided P < 0.05 was considered statistically significant, except in the case of interaction analysis, where P < 0.10 indicated the presence of a significant multiplicative interaction. All statistical analyses were performed using Stata MP 17.0 software (StataCorp).
Ethical Considerations
Ethics approval was granted from the ethical review board in Sweden (registration no. 2021-04422).
Data and Resource Availability
Requests of sharing deidentified data from this article will be considered on a case-by-case basis. A detailed proposal for how the data will be used is required, and a data access agreement must be signed upon request.
We identified 230,996 patents with T2D between 1998 and 2021. The median age of patients was 63.6 (IQR 56.0–72.0) years, and 58.2% were men. Among all patients, 19.6% had albuminuria, 92.4% had hypertension, 49.4% had hypertriglyceridemia, 16.2% had low HDL levels, and 46.5% had obesity. The median BMI was 29.6 (IQR 26.5–33.4) kg/m 2 , and the median Charlson comorbidity index was 1 (IQR 1–1). There were 22.2% patients with preexisting CVD and 1.2% with CKD. Baseline characteristics are presented in Table 1 .
Baseline characteristics of study population ( N = 230,996)
Number of MetS Traits and Rate of MALOs
During a median follow-up of 9.9 years (corresponding to 2,235,356 person-years), 3,215 (1.39%; incidence rate 1.44/1,000 person-years) patients developed MALOs ( Table 2 ). Within these outcomes, 1,344 cases of cirrhosis, 2,000 cases of decompensated cirrhosis and its complications, and 839 cases of HCC were identified. Patients with additional metabolic traits had a higher incidence of MALOs (1.46/1,000 person-years for two or more MetS traits vs. 0.56/1,000 person-years for those with only T2D). Furthermore, patients with a higher total number of metabolic traits at baseline had a higher incidence rate of MALOs, and the rate progressively increased as the number of metabolic traits increased, also after adjusting for other covariates (adjusted HR [aHR] 1.28, 95% CI 1.23–1.33, P trend < 0.001) (Table 2A ). Compared with patients with only T2D at baseline, having more than one MetS trait was associated with a 2.3-fold increased rate of MALOs (95% CI 1.53–3.54); for those with all MetS traits at baseline, the aHR was 4.09 (95% CI 2.50–6.68).
Incidence rate and HRs of MALOs associated with numbers and individual traits of the MetS present at baseline in patients with T2D
A : The model was adjusted for numbers of MetS traits (continuous), age, sex, smoking status, diabetes duration, HbA 1c level, CKD, CVD, eGFR, LDL level, hyperlipidemia, any diabetes treatment use, insulin, GLP-1RAs, and SGLT-2 inhibitors. B : The model was adjusted for albuminuria, hypertension, hypertriglyceridemia, low HDL levels, obesity, age, sex, smoking status, diabetes duration, HbA 1c level, CKD, CVD, eGFR, LDL level, hyperlipidemia, any diabetes treatment use, insulin, GLP-1RAs, and SGLT-2 inhibitors.
Individual MetS Traits and Rates of MALOs
Patients with individual MetS traits had a higher incidence rate of MALOs compared with those who did not display such traits (e.g., 1.50/1,000 person-years for patients with hypertension vs. 0.48/1,000 person-years for those without hypertension) (Table 2B ). The strongest association with incident MALOs was found for patients who had hypertension (aHR 2.06, 95% CI 1.57–2.71), followed by obesity (aHR 1.38, 95% CI 1.28–1.50), low HDL levels (aHR 1.37, 95% CI 1.23–1.51), albuminuria (aHR 1.23, 95% CI 1.13–1.35), and hypertriglyceridemia (aHR 1.11, 95% CI 1.02–1.20) (Table 2B ). We did not detect any statistical interaction between the number or individual traits of MetS and age or sex on the rate of MALOs ( P interaction > 0.1 for all). Among the other covariates, CKD (aHR 2.29, 95% CI 1.61–3.25), smoking (aHR 1.36, 95% CI 1.23–1.52), CVD (aHR 1.17, 95% CI 1.06–1.29), and eGFR (aHR 1.01, 95% CI 1.00–1.01) were associated with a higher rate of MALOs, while LDL (aHR 0.81, 95% CI 0.78–0.85) and hyperlipidemia (aHR 0.69, 95% CI 0.64–0.75) were related to a lower rate of MALOs in the fully adjusted models ( Supplementary Table 4 ).
Combinations of MetS Traits and Rates of MALOs
The associations of combinations of baseline MetS traits with MALOs are shown in Fig. 1 . For the different combinations, hypertension consistently had the strongest association with incident MALOs. For instance, patients with hypertension had a higher rate of MALOs (aHR 1.58, 95% CI 1.03–2.42) compared with those with only T2D. For patients with two additional MetS traits, those with comorbid hypertension and obesity had the highest rate of MALOs (aHR 2.77, 95% CI 1.80–4.26), followed by those with hypertension and low HDL (aHR 2.44, 95% CI 1.45–4.09). We consistently observed higher event rates in any combination of hypertension and other MetS traits with regard to all three and four combinations.

HRs and 95% CIs of baseline combinations of metabolic traits with MALOs in patients with T2D. The reference group comprises patients with T2D without any metabolic traits. The numbers of baseline metabolic traits are displayed as a shade of colored squares. NA, not applicable; TGR, triglycerides.
Cumulative incidences of MALOs by the total number of MetS traits over time are shown in Fig. 2 . Overall, patients with additional MetS traits had a higher probability of MALOs than those with only T2D. The highest probability was observed in patients with all traits of the MetS. For instance, the 10-year cumulative incidence of MALOs was 0.49, 0.87, 1.24, 1.23, 1.57, and 1.64% for patients with only T2D, two, three, four, five, and six MetS traits, respectively ( Supplementary Table 5 ). Regarding individual metabolic traits, there was a clear distinction in the probability of MALOs between patients with hypertension and those without. Similarly, there was a difference in the likelihood of outcome development among patients with obesity and without, and among patients with a low HDL level and without ( Supplementary Fig. 2 ). When considering incident traits of the MetS occurring after baseline using time-varying models, we observed slightly higher HRs with regard to both number and different combinations of MetS traits, providing additional validity to our findings ( Supplementary Fig. 3 ).

Cumulative incidence of MALOs by number of MetS traits at baseline.
Sensitivity Analysis
As the association might be driven by the development of HCC, we conducted a sensitivity analysis distinguishing between patients who developed HCC and those who did not. The results indicated that both the individual and the total number of metabolic traits are associated with HCC and non-HCC events; therefore, our estimates are robust ( Supplementary Table 6 ). We also found that each metabolic trait is associated with a higher risk of decompensated cirrhosis and complications and that hypertension, obesity, and low HDL level consistently demonstrate a higher risk across the spectrum of cirrhosis ( Supplementary Table 7 ).
In this population-based cohort study of >230,000 patients with T2D, we found that an increasing number of metabolic traits is associated with an increased risk of MALOs in patients with T2D. Each additional metabolic trait increased the risk for liver-related outcomes in a stepwise manner. We expand on previous knowledge by also showing that acquiring additional traits of the MetS after T2D diagnosis further increases the risk of liver-related outcomes, suggesting a strong association between poor metabolic health and liver disease risk.
Additionally, we investigated which individual parameters included in the MetS, as well as other traits of poor metabolic health that had differing associations with incident liver disease. The strongest association was seen for hypertension and CKD, whereas hyperlipidemia was negatively associated with incident liver disease. This may possibly be explained by a protective effect of statin treatment (which was included to define presence of hyperlipidemia), which has been suggested to have hepatoprotective effects and is associated with a reduced incidence of primarily HCC in patients with T2D ( 21 ). The other plausible explanation could be that patients with mutations in lipid-modifying genes, such as PNPLA3 , may have lower blood lipid levels but a higher risk for development of cirrhosis and HCC ( 22 , 23 ). An alternate explanation might involve hepatic synthetic failure during the early stages of cirrhosis, leading to a decrease in serum lipoprotein levels.
In the absence of primary care diagnoses, similarly to hyperlipidemia, hypertension was also defined by use of antihypertensive treatments in addition to having a recorded diagnosis in specialist care or an elevated blood pressure. As such, the risk increase associated with hypertension in this study may also warrant additional studies that can disentangle the effects associated with hypertension and the associated medical treatment.
In summary, our findings suggest that individualized risk prediction for incident MALOs is possible in patients with T2D. Future studies are needed to examine whether established noninvasive tests, such as the fibrosis 4 score or vibration-controlled transient elastography, can further increase prediction specifically in patients with T2D.
Comparison With Previous Studies
Our findings are in accordance with those of previous studies in this field in that each additional metabolic trait increased the risk of MALOs, regardless of alcohol intake ( 24–26 ). Indeed, the complex relationship between alcohol consumption and MetS complicates its individual impact of the MetS on liver outcomes. We investigated this by excluding all patients who had alcohol-related disease and found that even in the absence of known alcohol overconsumption with health care contacts, metabolic factors are independently related to liver-related outcomes.
The traits of the MetS seemingly have additive effects on the progression to cirrhosis, with hypertension demonstrating the strongest association among the individual traits. Indeed, any combination of hypertension with other MetS traits was associated with a higher risk of MALOs compared with only T2D, while such risk was not observed in the absence of hypertension. The presence of hypertension may indicate the occurrence of MASLD, as suggested by a meta-analysis that showed a bidirectional association between hypertension and MASLD independent of other cardiometabolic risk factors ( 27 ). As >90% of patients in this cohort had hypertension, we examined which combination of MetS traits, in addition to hypertension, conveyed the highest risk of MALOs in our cohort. We found that, in addition to hypertension, patients with obesity had the highest risk of MALOs, followed by those with low HDL, among all the two possible combinations of MetS traits. The risk increased substantially when patients had hypertension, low HDL, and comorbid albuminuria or obesity. This supports previous data from a large U.S. Veterans Affairs cohort of patients with a diagnosis of MASLD, where those with diabetes and coexisting hypertension and obesity or dyslipidemia had the highest risk for cirrhosis or HCC ( 6 ). That study also found a stepwise increase in risk for cirrhosis and HCC with each additional metabolic trait. Our findings extend this by including a less selected population of patients, all of whom have T2D. This is important since current guidelines promote screening for MASLD in all patients with T2D ( 28 , 29 ), aiming to identify patients on the path to cirrhosis. Our findings are important since they are more generalizable to the larger T2D population compared with previous data. A systematic review and meta-analysis found that in >22.8 million individuals with T2D, the overall risk of cirrhosis was 2.25-fold higher compared with people without diabetes ( 26 ). We extend this finding by showing that in patients with T2D, there are distinct risk profiles, possibly allowing for personalization of clinical management and follow-up.
Previous studies of the association between T2D and cirrhosis ( 30–33 ) often used mortality from cirrhosis as the outcome rather than incidence of nonfatal cirrhosis ( 31 , 33 ). Other studies reported an association between diabetes and more severe forms of liver disease, such as occurrence of hepatic failure ( 28 , 32 ). However, these studies did not differentiate between type 1 diabetes and T2D and were unable to study risk factors for development of liver-related outcomes.
Implications
Several important implications can be made based on these data. Although this study cannot show causality, our findings suggest the importance of adequately treating not only hyperglycemia in patients with T2D to reduce diabetes-related complications but also other comorbidities to reduce the risk for liver-related outcomes. Treating and preventing hypertension and obesity are particularly crucial to prevent the development of cirrhosis in this population. In addition to its impact on liver-related outcomes, a more severe metabolic profile in patients with T2D also increases the risk for cardiovascular events and overall mortality ( 34 ). By extension, this suggests that upcoming treatments for MASLD might be holistically more effective if they also improve the full metabolic profile of the patient. Data from randomized controlled trials are needed to corroborate this. In the absence of approved therapies to treat hepatic fibrosis due to MASLD, screening for liver fibrosis in all patients with T2D is currently controversial. The findings from this study may aid in identifying subgroups at high risk of developing future liver disease, which would be particularly efficient for targeted screening initiatives. To determine the cost-effectiveness of different interventions, such as screening for MASLD or advanced fibrosis, health economic evaluation is necessary. Our results suggest that patients with T2D have different metabolic risk factors, with a differing risk of progression to MALOs. Recognizing such distinct risk groups may be important in future clinical guidelines to tailor appropriate interventions and enhance patient outcomes.
Strengths and Limitations
The major strengths of this study are the use of high-quality registers with high coverage, minimal loss to follow-up, and an unprecedented sample size. Hence, estimates are more precise, accurate, and generalizable to the target population than that of previous studies. While large studies are often limited to register-based coding of metabolic traits, we used direct laboratory tests, in combination with medication use and register coding, to define metabolic traits. We further assessed the associations with repeated exposures to investigate whether newly developed metabolic traits also confer a higher risk of outcomes. We used hard outcomes that are validated and unlikely to be misclassified and that are important to patients ( 13 ). Several studies have shown that waist circumference may be a better predictor of central obesity than BMI and may also predict progression to MALO better than BMI; unfortunately, such data are not available in the used data sources ( 35 , 36 ). Additionally, to combine different MetS traits, we used categorized predictors. However, continuous versions of each MetS trait may provide an even more granular understanding. For instance, glycemic control is a better predictor for this purpose than diabetes status as a binary parameter ( 37 ). Combining continuous versions of MetS traits should be a focus for future studies. Another limitation is the lack of direct data on the presence and severity of MASLD, such as radiology or liver-related blood biochemistry, which are not available in the NDR. To address this limitation and further explore the severity of each MetS component, we plan to conduct future linkages to data sources that provide such detailed information. Furthermore, we excluded 486,377 patients because of missing data on at least one MetS trait. These patients tended to be older and more likely to have MetS, CVD, CKD, and severe diabetes as indicated by insulin use compared with those included in the study. Therefore, the estimated associations would have been stronger if we had included all eligible patients in the analysis.
In conclusion, in this large study of patients with T2D in Sweden, we found that an increasing number of metabolic comorbidities is associated with a higher rate of progression to MALOs, most likely attributable to MASLD. Hypertension and CKD were the parameters with the strongest association, whereas no positive association was seen for hyperlipidemia. This finding helps in narrowing down the specific group intended for directed casefinding among patients with T2D, potentially enhancing screening efficiency.
Acknowledgments. The authors thank the staff of the National Diabetes Register and all the study participants.
Funding. Y.S. was supported by Mag-Tarm Fonden, Swedish Gastroenterology Society, Karolinska Institutet research funding, and Region Stockholm. H.H. was supported by grants from Stockholm City County, The Swedish Cancer Society, and The Swedish Research Council. This study received financial support from Pfizer.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.S., E.T.G., A.M., H.S., O.S., F.N., S.G., and H.H. contributed to the data interpretation. Y.S., E.T.G., A.M., and H.H. contributed to the study concept and design. Y.S., E.T.G., H.S., and H.H. contributed to the statistical analysis. Y.S. and H.H. drafted the manuscript. E.T.G., O.S., and H.H. contributed to the data acquisition. All authors contributed to the critical revision of the manuscript. Y.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Handling Editors. The journal editors responsible for overseeing the review of the manuscript were Elizabeth Selvin and Amalia Gastaldelli.
This article contains supplementary material online at https://doi.org/10.2337/figshare.25280482 .
Email alerts
- Online ISSN 1935-5548
- Print ISSN 0149-5992
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Alcohol consumption and risk of liver cirrhosis: a systematic review and meta-analysis
Michael Roerecke, Afshin Vafaei, Omer SM Hasan, Bethany R Chrystoja, Jürgen Rehm
Centre for Addiction and Mental Health, Institute for Mental Health Policy Research, 33 Russell Street, Toronto, Ontario, Canada M5S 2S1
Marcus Cruz, Roy Lee, Manuela G Neuman
In Vitro Drug and Biotechnology, Banting Institute, lab. 217, 100 College St., Toronto, Ontario, M5G 1L5, Canada
Authors’ contributions
Associated Data
To systematically summarize the risk relationship between different levels of alcohol consumption and incidence of liver cirrhosis.
Medline and Embase were searched up to March 6 th , 2019 to identify case-control and cohort studies with sex-specific results and more than two categories of drinking in relation to incidence of liver cirrhosis. Study characteristics were extracted and random-effects meta-analyses and meta-regressions were conducted.
A total of seven cohort studies and two case-control studies met the inclusion criteria, providing data from 2,629,272 participants with 5,505 cases of liver cirrhosis. There was no increased risk for occasional drinkers. Consumption of 1 drink per day in comparison to long-term abstainers showed an increased risk for liver cirrhosis in women, but not in men. The risk for women was consistently higher compared to men. Drinking ≥5 drinks per day was associated with a substantially increased risk in both women (RR = 12.44, 95% CI: 6.65 – 23.27 for 5–6 drinks, and RR = 24.58, 95% CI: 14.77 – 40.90 for ≥7 drinks) and men (RR = 3.80, 95% CI: 0.85 – 17.02, and RR = 6.93, 95% CI: 1.07 – 44.99, respectively). Heterogeneity across studies indicated the additional impact of other risk factors.
Conclusions
Alcohol is a major risk factor for liver cirrhosis with risk increasing exponentially. Women may be at higher risk compared to men even with little alcohol consumption. More high-quality research is necessary to elucidate the role of other risk factors, such as genetic vulnerability, body weight, metabolic risk factors, and drinking patterns over the life course. High alcohol consumption should be avoided, and people drinking at high levels should receive interventions to reduce their intake.
INTRODUCTION
Alcohol is a major risk factor for liver disease in general, and for liver cirrhosis in particular.( 1 – 3 ) In fact, about half of the liver cirrhosis burden of morbidity and mortality would disappear in a world without alcohol.( 4 ) Mortality from liver cirrhosis has been on the rise in the US( 5 ) and Europe,( 6 ) more so in women than in men. Alcohol consumption is partly responsible, but liver disease is increasingly recognized as a multifactorial disease process.( 6 )
The importance of alcohol in the etiology of liver disease has led to establishing different codes for categories of liver diseases, which are considered to be primarily caused by alcohol. Thus, the International Classification of Diseases (ICD-10( 7 )) recognizes several forms of alcoholic liver disease (ICD-10, K70), sometimes considered stages( 8 ) that range from relatively mild and reversible alcoholic hepatic steatosis (fatty liver) (K70.0) and alcoholic hepatitis (K70.1), to alcoholic fibrosis and sclerosis of the liver (K70.2), and further to severe and irreversible stages such as alcoholic liver cirrhosis (K70.3) and alcoholic hepatic failure (K70.4). Alcohol consumption, in particular heavy use over time, has been found crucial in the etiology and progression of these diseases.( 1 , 9 , 10 ) However, liver diseases are multifactorial, and alcohol use may play a role in the progression of all types of cirrhosis,( 11 ) and even one drink per day may have an effect on the incidence of liver cirrhosis, ( 12 ). For scientific review of all liver cirrhosis, it is therefore crucial to include both alcoholic and non-alcoholic liver cirrhosis when examining the impact of alcohol use.
Most of the epidemiological literature to date has dealt with the level of drinking and incidence or mortality of liver cirrhosis.( 13 ) It followed the epidemiological tradition of the early studies of Lelbach and others,( 14 , 15 ) who, based on studies in people with alcohol use disorders, postulated a clear association between volume of alcohol use and liver cirrhosis.( 1 ) This association was corroborated in more rigorous studies.( 1 , 13 , 16 ) It remains to be determined, however, if a threshold for alcohol-related damage to the liver exists, or whether any amount of alcohol increases the risk for liver cirrhosis, which has been discussed recently.( 17 – 19 ) In fact, the last meta-analysis on the topic is now more than 10 years old and found some evidence for a protective association at low levels of alcohol intake in men.( 13 ) Furthermore, several large-scale studies have been published since then.( 20 – 22 )
The present review provides an overview of the current knowledge on the dose-response relationship between alcohol consumption and risk of liver cirrhosis in comparison to abstainers, with particular consideration given to the effects of study design and sex, and other subgroups where data were available. As noted above, our review was not restricted to alcoholic liver cirrhosis.
Search strategy and selection criteria
Following the MOOSE guidelines,( 23 ) we conducted a systematic electronic literature search using Medline and Embase from inception to March 6 th , 2019 for keywords and MeSH terms relating to alcohol consumption, liver cirrhosis, and observational studies ( Supplementary Table 1 ). Additionally, we searched reference lists of identified articles and published meta-analyses and reviews. Inclusion criteria were as follows:
- cohort and case-control studies examining the sex-specific association between average alcohol consumption and liver cirrhosis,
- analyses were adjusted for age at baseline,
- data for at least two quantitatively defined categories of average alcohol consumption in relation to non-drinkers, or data for former drinkers in relation to long-term abstainers were reported,
- more than 50 cases of liver cirrhosis occurred.
We did not apply language restrictions. At least two reviewers independently excluded articles based on title and abstract or full-text and abstracted the data. Any discrepancies were resolved in consultation with a third reviewer.
Data extraction
From all relevant articles we extracted authors’ names, year of publication, country, year(s) of baseline examination, follow-up period, setting of the study, study design, assessment of liver cirrhosis, age (range, mean or median) at baseline, sex, number of observed liver cirrhosis cases among participants by drinking group, number of total participants by drinking group, specific adjustment or stratification for potential confounders, and adjusted relative risks (RRs) and their confidence intervals (CIs) or standard errors. Risk estimates by sex were treated as independent samples. Where necessary, RRs within studies were re-calculated to contrast alcohol consumption categories against non-drinkers.( 24 )
Exposure and outcome assessment
Consolidating exposure measures across primary studies involved a two-step process. First, among drinkers, we converted reported alcohol intake categories in primary studies into an average of pure alcohol in grams per day (g/day) using the midpoints (mean or median) of reported drinking group categories. For open-ended categories, we added three quarters of the second highest category’s range to the lower limit of the open-ended category of alcohol intake if the mean was not reported. Standard drinks vary by country, with one standard drink containing approximately 8–14 g of pure alcohol.( 25 ) We used reported conversion factors when standard drinks were the unit of measurement to convert all measures to grams per day. Then, for reporting of our analyses, we considered categories with a mean of up to 12 grams pure ethanol as one standard drink for a global representation. Qualitative descriptions, such as ‘social’ or ‘frequent’ drinkers with no clear total alcohol intake in g/day were excluded. When current non-drinkers were the reference group (i.e., including both long-term abstainers and former drinkers), we adjusted risk estimates for the effect of former drinking compared to long-term abstention, based on the pooled risk for former drinking from two studies included in this review to avoid the sick-quitter effect. Long-term abstainers were defined as people who stated that they never consumed alcohol,( 20 ) people who stated that they never, or almost never, drank alcohol in the past,( 26 ) and when people who had greatly decreased their consumption in the last 10 years were excluded from non-drinkers.( 27 ) The logRR for former drinkers in comparison to long-term abstainers (RR former drinkers = 2.52) was multiplied by the mean fraction of former drinkers among current non-drinkers (0.23) and added to the respective logRRs of current drinking groups from primary studies used in our analysis when current non-drinkers was the reference group.
Liver cirrhosis due to known aetiology such as alcohol, and unspecified liver cirrhosis was defined as in the primary studies, which included ICD codes for liver cirrhosis (ICD-7: 581; ICD-8: 571; ICD-10: K70, K73, K74) and unspecified liver cirrhosis (ICD-8: 571.9, 456.0, 785.3; ICD-10: I85.0, I85.9, K74.6, R18.9). Because we aimed to estimate the relative risk in comparison to abstainers, we excluded several studies (e.g., ( 28 , 29 )) which focused only on alcoholic liver cirrhosis (or included alcoholism in addition to liver cirrhosis in the outcome),( 30 ) which, by definition, cannot occur in lifetime abstainers.
Quality assessment
Most quality scores are tailored for meta-analyses of randomized trials of interventions( 31 – 33 ) and many criteria do not apply to epidemiological studies examined in this study. Additionally, quality score use in meta-analyses remains controversial.( 34 – 36 ) As a result, study quality was enhanced by including quality components, such as study design, measurement of alcohol consumption and liver cirrhosis, adjustment for age in our inclusion and exclusion criteria, and further by investigating potential heterogeneity in several sensitivity analyses. We used the most adjusted RR reported and the most comprehensive data available for each analysis and gave priority to estimates where lifetime or long-term abstainers were used as the risk reference group.
In a formal risk of bias analysis, we used the Cochrane Risk of Bias Tool for Non-Randomized Studies (ROBINS-I( 37 )) to assess risk of bias in primary studies. We rated the evidence for the association between alcohol consumption and incidence of liver cirrhosis based on the Grades of Recommendation, Assessment, Development and Evaluation system (GRADE).( 38 )
Statistical analyses
In categorical analyses using standard drinks (12 grams pure alcohol) as the exposure measure, RRs were pooled with inverse-variance weighting using DerSimonian-Laird random-effect models to allow for between-study heterogeneity.( 39 ) Small-study bias was examined using Egger’s regression-based test.( 40 ) Variation in the effect size because of heterogeneity between studies was quantified using the I 2 statistic.( 41 )
Using studies that reported data for four or more alcohol intake groups, we conducted two-stage restricted cubic spline regression analysis in multivariate meta-regression models, taking into account the variance-covariance matrix for risk estimates derived from one reference group( 42 , 43 ) to test for non-linear dose-response relationships in relation to long-term abstainers. All meta-analytical analyses were conducted on the natural log scale in Stata Statistical Software, Version 14.2.
Role of funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
In total, out of 2,977 identified references, 385 articles were retrieved in full-text. Of these, seven cohort and two case-control studies fulfilled our inclusion criteria ( Figure 1 ). Four studies were conducted in the US,( 26 , 27 , 44 , 45 ), two in Italy,( 46 , 47 ) and one each in China,( 22 ) the UK,( 21 ) and Denmark.( 20 ) In total, data from 2,629,272 participants (579,592 men, 2,049,680 women) and 5,505 cases of liver cirrhosis (2,196 men, 3,309 women) were used in the analyses. All cohort studies included liver cirrhosis mortality as the outcome. The two case-control studies investigated first-time diagnosis of symptomatic liver cirrhosis in comparison to lifetime abstainers ( Table 1 ). The study by Liu et al contributed 2,078 liver cirrhosis cases from the National Health Service Million Women Study linked to death and morbidity registries.( 21 ) The proportion of non-drinkers varied widely, from 0.002% (lifetime abstainers) among men in the Danish study by Askgaard et al. ( 20 ) to 80% (current abstainers) in the study of women from the American Cancer Society I cohort by Garfinkel et al .( 44 ) All cohort studies used a one-time measurement of alcohol consumption as the baseline alcohol intake, while the three case-control studies from Italy assessed lifetime alcohol consumption retrospectively. All but one cohort study were rated to be of moderate quality mainly because of the one-time measurement of alcohol consumption at baseline (cohort studies), and the observational study design ( Supplementary Table 2 ). One cohort study( 44 ) had potential serious bias because the results were adjusted only for age.

Flowchart of study selection
Characteristics of 7 cohort and 2 case-control studies investigating risk of liver cirrhosis by alcohol intake, 1988–2017.
Abbreviations: BMI, body mass index; M, men; W, women; M, W, men and women stratified; M/W, men and women combined; SES, socioeconomic status; NHS, National Health System.
The pooled proportion of former drinkers among current abstainers( 20 , 26 ) was 23%, and the pooled RR for liver cirrhosis in comparison to long-term abstainers was 2.56 (95% CI: 0.93 – 6.79). Figure 2 displays the RRs for liver cirrhosis in cohort studies by alcohol intake in reference to long-term abstainers after current abstention at baseline was adjusted for the proportion and risk in former drinkers. Alcohol consumption beyond occasional drinking, which showed a similar risk compared to long-term abstainers, was associated with increasing risk for liver cirrhosis ( Figure 2 ) with a pooled RR of 10.70 (95% CI: 2.95–38.78) for consumption of 7 drinks or more per day. However, all drinking categories showed substantial heterogeneity across studies (I 2 between 70 and 98%, all P-values <0.001), resulting in large confidence intervals. We restricted analyses of small-study effects and influential studies to drinkers of 1 or 2 drinks per day for both sexes because of the small number of studies identified. We found no statistical evidence for small study bias for drinkers of 1 or 2 drinks per day (P = 0.94), the funnel plot showed similar results ( Supplementary Figure 1 ). None of the studies had an overly large impact on the pooled estimates ( Supplementary Figure 2 ).

Relative risk on the log scale. 1 standard drink = 12 grams pure ethanol per day. RR = relative risk.
Results for men and women are shown separately in Figures 3 and and4, 4 , respectively. Across all consumption levels, RRs in women were higher, reaching RR = 24.58 (95% CI: 14.77–40.90) for ≥7 drinks. While consumption of 1–2 drinks was associated with a substantially elevated risk for liver cirrhosis in women, this was not the case in men. However, these results need to be interpreted with caution because of the small number of studies available. Four cohort studies( 20 , 21 , 26 , 27 ) in women were adjusted for age, BMI or waist circumference, and smoking. The relationship was similar to the main analysis with an elevated and linearly increasing risk for consumption of 1 drink and beyond ( Supplementary Figure 3 ).

In both men and women, there was no evidence for a non-linear dose-response relationship on the log scale (P=0.24 and 0.27, respectively, Supplementary Figures 4 and 5 ). However, the number of studies available was low, resulting in little power to detect non-linearity.
The two case-control studies with liver cirrhosis morbidity as the outcome yielded smaller risks associated with alcohol consumption, with 1–4 drinks showing no risk increase compared to lifetime abstainers (pooled RR=1.19, 95% CI: 0.58–2.43, Figure 5 ). Risks for consumption of 5–8 and 9–13 drinks were associated with large heterogeneity with one study( 47 ) showing substantial risk increases for both men and women, while the other study( 46 ) showed no or marginally statistically significant risk increases for either men or women (see also Supplementary Figures 6 and 7 ).

Subgroup analyses
One cohort study( 21 ) showed that while the risk in smokers was higher than in never-smokers, the risk of liver cirrhosis by alcohol intake increased in never-smokers similar to current smokers, indicating that smoking is a confounder but not an effect modifier. In another report( 48 ) from the case-control studies by Corrao et al , it was shown that the relationship between alcohol and liver cirrhosis in all participants was similar to participants without serum HBsAg and/or positive anti-HCV status. One of the case-control studies( 46 ) included in our main analysis also showed that the risk for liver cirrhosis was greatest in drinkers who drank heavily for 10 or 20 years, but not for 30 years, indicating potentially a survivor bias. Similarly, the same study( 46 ) reported risk by age (≤60 years and > 60 years), showing that the risk increase was stronger in younger participants for both sexes. The cohort study by Askgaard et al. ( 20 ) reported results by frequency of drinking days adjusted for weekly alcohol intake. In men, there was an increased risk for daily drinking in comparison to drinking on 2–4 days per week (RR = 2.25, 95% CI: 1.68–3.00). In women, the RR was 1.28 (95% CI: 0.82–2.02). Results from the UK Million Women study( 49 ) showed that among drinkers daily drinking in comparison to non-daily drinking (RR = 1.61, 95% CI: 1.40–1.85) and drinking with meals in comparison to drinking outside of meals (RR = 0.69, 95% CI: 0.62–0.77) were associated with incidence of liver cirrhosis. These associations were similar in strata of BMI, smoking status, and type of alcoholic beverage. Women who both drank daily and outside of meals had a 2.47 (95% CI: 1.96–3.11) increased risk for liver cirrhosis with adjustment for amount and type of beverage.( 49 )
Given the observational nature of the studies included in this report, we rate the evidence for a causal effect of alcohol consumption and risk for liver cirrhosis as moderate. However, the dose-response relationship in addition to established biological pathways confirmed in randomized controlled trials( 50 ) give rise to high confidence in a causal dose-response relationship. There was no clear indication for a threshold effect, but we rate the quality of the evidence as low because of imprecision and the small number of studies reporting sex-specific RRs for low levels of drinking.
We conducted a systematic review and various meta-analyses on alcohol consumption and risk of liver cirrhosis. Contrary to prior analyses,( 13 ) we found overall no protective effects at any level of drinking when compared to long-term abstainers, and a steadily increasing dose-response relationship in women, and some evidence for a threshold effect in men. However, risks varied widely and the analysis of case-control studies showed no risk increase for consumption of 1–4 drinks per day. The high risk for heavy drinkers found in our meta-analysis of cohort studies is in line with prior research on risk for liver cirrhosis in people with alcohol use disorders.( 11 , 51 , 52 ) The pooled RRs from case-control studies were much smaller; however, the more recent case-control study( 47 ) corresponds with the risks found in people with alcohol use disorder. One of the cohort studies and one of the case-control studies reported very small RRs compared to the other studies. The reasons for this are unclear, although some outliers are to be expected in any statistical analysis.
Additionally, many studies were not well adjusted and of generally moderate methodological quality, mostly related to potential bias due to confounding and selection bias. While the increase in risk was stronger in women, confidence intervals were large and overlapped with those for men. Stronger effects in women are supported by studies in people with alcohol use disorder with or without liver cirrhosis( 53 , 54 ), and higher hepatotoxicity. While there is no doubt that heavy alcohol consumption is one of the main risk factors for liver cirrhosis, the large heterogeneity observed indicates that the multifactorial nature of development of liver cirrhosis has not been reflected in the epidemiological literature. Liver cirrhosis has a complex and not fully understood etiology, and the contributory role of other risk factors for liver cirrhosis, such as BMI, metabolic syndrome, diabetes, drinking frequency and outside of meals, and others, at any given level of alcohol intake over the life course, need more attention in both research and prevention efforts.
While several important confounders have been identified and should be adjusted for in epidemiological studies, it is likely that some of them are in fact effect modifiers that impact the risk associated with alcohol consumption. Most important here is genetic vulnerability. Twin studies have shown a three-fold higher disease concordance between monozygotic twins and dizygotic twins, but the genetic case-control studies have not yet led to conclusive results.( 55 ) Genetic vulnerability is seen as the major reason why only a minority of very heavy drinkers develop liver problems. With respect to other potential effect modifiers, it seems that the drinking frequency modifies the risk for liver cirrhosis associated with a given total weekly alcohol intake with fewer drinking days being associated with lower risk supporting the notion of a ‘liver holiday’.( 56 – 58 ) A report from the Singapore Chinese Health Study( 59 ) showed that among daily drinkers, consumption of even one drink a day was associated with a RR = 2.72 (95% CI: 0.98–7.50) for liver cirrhosis in comparison to non-drinkers. In more recent years, patterns of drinking, especially binge drinking, were introduced as potentially important for the etiology and progression of liver cirrhosis.( 60 , 61 ) However, evidence is limited and inconclusive at this point.( 62 , 63 ) Future research should include standard measures on patterns of drinking, such as measures of irregular heavy drinking in addition to average volume of drinking and drinking frequency, to test hypotheses about such patterns, and to determine whether there is a positive effect of abstinence days.( 64 ) The consumption of mostly wine, as opposed to beer or liquor, has been shown to modify the risk for alcoholic liver cirrhosis in some studies;( 29 , 58 ) however, as the UK Million Women study showed,( 49 ) this may be explained by the consumption of alcohol with meals, which is more common in wine drinkers than consumers of other types of alcohol.
An investigation of Midspan cohorts( 65 ) in Scotland indicated that BMI modifies the effect of alcohol consumption on liver disease, with obese participants being more susceptible to the harms from alcohol consumption than participants with lower BMI. An analysis of the Million Women Study( 66 ) confirmed that BMI and alcohol consumption interact in development of liver cirrhosis, in particular at alcohol intake of more than 150 g/week and BMI above 30. Potential interaction with drinking patterns seem possible.( 62 ) The effect of smoking in relation to alcohol consumption on liver cirrhosis is not clear. Several studies have reported an effect independent of alcohol consumption( 21 , 67 ), and no clear effect.( 68 , 69 ) Meta-analyses of the association of coffee consumption and risk for liver disease consistently show a decreased risk.( 70 , 71 ) Potential interaction with alcohol consumption should be explored. Liver cirrhosis severity may also play a role. Another analysis from the series of case-control studies from Italy showed the risk increase from alcohol consumption was characterized by a threshold effect at approximately 150 g/day, and a smaller risk at higher consumption for asymptomatic liver cirrhosis than for symptomatic liver cirrhosis. In other reports including the same participants,( 69 , 72 ) it was shown that HBV and HCV infection were risk factors independent from alcohol consumption. The role of nutrient intake is unclear. Several nutrients were investigated in reports from the Italian case-control studies. Possible interaction effects were observed for dietary intake of lipids,( 73 ) vitamin A,( 74 ) and iron.( 74 ) However, larger sample sizes are required to detect an effect with sufficient power. More and higher quality epidemiological studies are needed to reach firm conclusions about confounding and interaction effects of these risk and protective factors in men and women.( 51 )
Other limitations of this review are based on the underlying literature. First, the number of original articles was limited. This is surprising given the fact that the majority of liver cirrhosis cases would not exist in a counterfactual scenario without alcohol. Second, the quality of the contributions was limited. Because of the small number of studies published, we were unable to investigate in detail the role of many study design characteristics, such as adjustment for potential confounders, follow-up length, race/ethnicity, and others that may play a role in the development of liver cirrhosis. Low response rates and inclusion criteria in primary studies, such as participants in screening programs, may limit the generalizability of our findings. Although self-reported alcohol consumption is generally reliable,( 75 ) it may result in underestimation of the real consumption. No cohort study measured alcohol consumption more than once, thus opening the research to measurement and regression dilution bias, and underestimation of the real effect.( 76 ) While the two case-control studies from Italy were able to assess lifetime drinking retrospectively, these types of studies are prone to recall bias, and categories of alcohol consumption were large, and adjustments for other risk factors for liver cirrhosis were minimal. Again, even with similar methodology in the same country, the two studies observed large differences in risk for liver cirrhosis for a given total alcohol intake. One possibility for the difference in risk observed between cohort and case-control studies is because of the difference in outcome assessment (mortality vs morbidity).
In comparison to our earlier meta-analysis,( 13 ) the strengths of this meta-analysis lie in its clear definition of the outcome, and its methodological rigour. For example, we excluded studies with insufficient number of cases or adjustment,( 77 ) and provide an examination of age, drinking patterns, and type of beverage where data were available. This strength came at a cost - some of the most well-known studies in the field, which were limited to subcategories of liver cirrhosis, had to be excluded.( 28 , 29 ), which was crucial to quantify the risk of liver cirrhosis in comparison to abstainers, which by definition cannot develop alcoholic liver cirrhosis.
What are the clinical conclusions of this study? The exponential dose-response curve on the relative risk level indicates that the highest levels of average volume of alcohol consumption confer exponentially higher risks and should be avoided.( 78 ) For people at the high end of this trajectory, the risk for liver cirrhosis is very high,( 16 ) and reductions of the highest levels are associated with the highest health gains.( 79 ) This can be achieved on the individual level in two ways: first, the trajectory towards these levels should be interrupted early, and more than once. This should best be done at the general practitioner level with screening and brief interventions or treatment;( 80 ) however, screening for unhealthy alcohol use is still not conducted routinely.( 81 , 82 )` Second, ( 79 )to prevent liver cirrhosis and subsequent complications including death in people with continued high consumption, it is most important to reduce high levels, even if the new drinking level are still high, and even if the patients still qualify for alcohol use disorders. Of course, the larger the reduction from a given level, the larger the reduction of relative risk, but it should be taken into consideration that any reduction of high volume drinking will be beneficial.( 83 ) Finally, there are alcohol control policy measures. Measures like increase in price via taxation( 84 ) or restrictions in availability have historically shown to impact on liver cirrhosis deaths.( 85 ) Thus, the current high impact of alcohol consumption on liver cirrhosis is avoidable, and both individual interventions in the health care sector and alcohol control policies can contribute to reduce this impact.
Study Highlights
What is the current knowledge.
- Alcohol is involved in all types of liver disease, and high alcohol consumption is associated with high disease risk.
- Prior systematic evidence syntheses have included inconsistent definitions of alcohol exposure and liver cirrhosis.
What is new here
- The risk for incidence of liver cirrhosis for former drinkers in comparison to long-term abstainers was three-fold.
- With any alcohol consumption, the risk for liver cirrhosis increased exponentially among women; among men, the risk increased beyond consumption of 1 drink or more per day.
- Drinking daily and outside of meals increases the risk for liver cirrhosis at any given level of overall alcohol intake. Several other risk factors for liver cirrhosis may modify the association of alcohol with liver cirrhosis, such as genetics, age, BMI, metabolic risk factors, and others.
Supplementary Material
Supplementary file, financial support.
Research reported in this publication was supported by the National Institute On Alcohol Abuse And Alcoholism (NIAAA) of the National Institutes of Health under Award Number R21AA023521 to MR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The sponsor of the study (NIAAA) had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The authors collected the data, and had full access to all of the data in the study. The authors also had final responsibility for the decision to submit the study results for publication.
Declaration of interests
MR and JR report grants from National Institutes of Health (NIH), National Institute on Alcohol Abuse and Alcoholism (NIAAA), during the conduct of the study. JR reports grants and personal fees from Lundbeck and D&A Pharma outside of this work. AV, OSMH, BRC, MGN, RL, MC report no conflicts of interest.
¿En qué podemos ayudarle?
- Co n gresos
Study of the molecular mechanisms underlying liver diseases by mass spectrometry-based proteomics
- Autores: Laura Guerrero González
- Directores de la Tesis: Fernando J. Corrales ( dir. tes. ), Alberto Paradela Elizalde ( dir. tes. )
- Lectura: En la Universidad Autónoma de Madrid ( España ) en 2023
- Idioma: español
- Número de páginas: 213
- Estudio de los mecanismos moleculares asociados a enfermedad hepática mediante proteómica basada en espectrometría de masas
- Tesis en acceso abierto en: Biblos-e Archivo
Liver diseases cause approximately 2 million deaths per year worldwide and their incidence has increased over the last decade. Risk factors include excessive alcohol consumption, obesity, diabetes, ingestion of hepatotoxic substances such as aflatoxin, viral infections (viral hepatitis), and genetic determinants. Liver cancer is the sixth most prevalent and the third deadliest (second in men). The low survival rate (less than 20% after 5 years) is partly explained by late diagnosis, highlighting the need for new molecular biomarkers for early diagnosis. In this work, proteomic techniques based on mass spectrometry have been applied to the study of liver diseases such as cholestasis, liver cirrhosis, and hepatocellular carcinoma with the aim of understanding the underlying molecular mechanisms of these pathological processes, as well as discovering potential biomarkers for the diagnosis and prognosis of patients. Cholestasis is a disease characterized by the interruption of bile flow from hepatocytes to the small intestine. Its treatment, depending on the cause and severity of the disease, may include the administration of ursodeoxycholic acid, removal of gallstones obstructing the duct in cases of physical obstruction, or liver transplantation in severe cases. It is precisely in these latter cases where rapid and adequate intervention is essential for a favorable patient outcome. With the aim of developing tools for a more effective patient management, we conducted a study of the livers of patients with cholestasis of various etiologies, combining mass spectrometry and machine learning tools. In addition to identifying some of the cellular processes involved in the molecular pathogenesis of cholestasis, we have defined a panel of proteins using linear discriminant analysis (LDA) that allows for the stratification of patients according to the etiology of cholestasis with high precision (91%). This mathematical approach, based on the establishment of an importance criterion for the measured proteins in relation to their ability to discriminate the studied cases, represents an increasingly used analysis method to objectively define panels of biomolecules for patient stratification. Progressive familial intrahepatic cholestasis type 3 (PFIC3) is a serious and rare liver disease that affects between 1 in 50,000 and 1 in 100,000 children. It is caused by mutations in the phosphatidylcholine transporter ABCB4 (MDR3), leading to intrahepatic accumulation of free bile acids and resulting in chronic liver damage. PFIC3 typically manifests at an early age, progresses rapidly, and has a poor prognosis. Currently, besides the palliative use of ursodeoxycholic acid, the only available treatment for this disease is liver transplantation, which poses a challenge, especially in young patients. To uncover the molecular basis of PFIC3 progression and propose new strategies for its treatment, we conducted a proteomic and phosphoproteomic analysis comparing liver samples from PFIC3 patients and controls. The results were validated in an MDR2-deficient PFIC3 mouse model. The functional interpretation of the proteomic analysis results indicates that essential cellular processes, such as inflammation, cell proliferation, cytoskeletal organization, and significant reconfiguration of intermediary metabolism, particularly glucose metabolism and onecarbon metabolism (OCM), are affected in PFIC3. OCM is a highly relevant metabolic cycle that connects intermediary metabolism with epigenetic regulation. This cycle has a tissue-specific configuration, and in the liver, some of the participating enzymes show a specific expression pattern, which is essential for maintaining the differentiated and quiescent state of hepatocytes. Considering its critical role in hepatocyte biology, we have developed a targeted proteomic method to monitor specifically OCM enzymes. Our results indicate that systematic determination of the levels of these proteins reveals a reprogramming of OCM in chronic liver damage processes such as cholestasis, which is further accentuated in situations of severe liver damage, losing metabolic capacity in hepatocellular carcinoma (HCC) and adopting a nonspecific profile similar to that of other tissues. These results point to OCM as an indicator of liver function and differentiation that could help to monitor patients with chronic liver diseases, facilitating early prognoses that could enable more effective interventions. According to these results, we believe that the metabolic alteration of OCM occurring in liver damage processes is a relevant factor in disease progression, and the administration of metabolites to modulate this pathway could represent a potential therapeutic option. Therefore, we have studied the effect of methylthioadenosine (MTA), an intermediate metabolite of this cycle, on an established hepatocellular carcinoma cell line, evaluating the response through the analysis of the changes induced in the proteome. Our results show that treatment with MTA may have a hepatoprotective role by reversing several tumour phenotypic characteristics, such as proliferation and reconfiguration of OCM. In conclusion, the application of state-of-the-art proteomic techniques has allowed us to establish some of the mechanisms involved in the progression of chronic liver diseases, including cholestasis, cirrhosis, and HCC. Based on this knowledge, we have demonstrated the relevance of OCM and developed an analytical method to monitor OCM enzymes, enabling assessment of liver function and hepatocyte differentiation status. Therefore, it can be highly useful in monitoring patients with chronic liver diseases. Finally, we have developed an LDA method that allows the definition of models to stratify patients with similar pathologies, such as different etiologies of cholestasis. Our results suggest the great potential of proteomics in the development of precision medicine

Acceso de usuarios registrados
- Dialnet Plus

Opciones de compartir
Opciones de entorno.
- Sugerencia / Errata
© 2001-2024 Fundación Dialnet · Todos los derechos reservados
- Accesibilidad
- Aviso Legal
- R e gistrarse

Inflammatory myofibroblastic tumor of the liver after adrenal neuroblastoma surgery: a case report
- Case Report
- Open access
- Published: 18 May 2024
- Volume 15 , article number 174 , ( 2024 )
Cite this article
You have full access to this open access article

- Qiyang Shen 1 ,
- Xingyu Liu 2 ,
- Lijie Zhang 3 ,
- Tao Li 1 &
- Jianfeng Zhou 1
166 Accesses
Explore all metrics
A boy aged 55 months was diagnosed with stage IV Neuroblastoma (NB) of the right adrenal gland 2 years ago. Preoperative chemotherapy was given and he was then treated with retroperitoneal tumor resection and lymph node dissection. After surgery, the children were transferred to the Hemato-Oncology Department for chemotherapy according to the high-risk group NB, with outpatient follow-up every 6 months. In the second postoperative year, abdominal computed tomography (CT) scan revealed a rounded hypodense area in the upper part of the right posterior lobe of the liver, with marked inhomogeneous enhancement in the venous phase after enhancement, which was surgically resected, and postoperative pathology confirmed inflammatory myofibroblastic tumor (IMT) of liver. The patient was not given any special treatment after surgery. In this study, whole transcriptome sequencing was performed on the postoperative specimen of adrenal NB and the specimen of IMT of liver. This unusual case emphasizes the need for close monitoring of second tumor development in NB survivors even in the absence of known predisposing factors.
Similar content being viewed by others

Overview of the 2022 WHO Classification of Adrenal Cortical Tumors

Molecular Heterogeneity in Leiomyosarcoma and Implications for Personalised Medicine

Spleen Benign and Malignant Tumors and Tumor Conditions
Avoid common mistakes on your manuscript.
1 Background
With improved diagnostic and treatment modalities for neuroblastoma (NB), the prognosis for patients is getting better, but the risk of patients developing a second tumor remains [ 1 ]. A so-called second tumor is another tumor that develops near or away from the primary tumor [ 2 ]. The appearance of a mass in the organism after the complete cure of a malignant tumor does not necessarily mean the recurrence of the tumor or the development of another malignant tumor [ 3 ]. In addition, the development of a second tumor may or may not be related to the treatment of the prior tumor, as genetic risk factors or other external carcinogens may also be involved [ 4 ]. The development of a second malignant tumor is one of the most devastating events affecting the survival of these patients [ 5 , 6 ]. Studies suggest that the younger the cancer survivor, the more deadly the second primary malignancy [ 7 ]. Another study reported malignant gastrointestinal neuroectodermal tumour as the second primary malignant tumor after pediatric NB treatment [ 8 ]. IMT is a rare inert tumor that has been less frequently reported as a second tumor occurring after treatment of malignancy in pediatric patients, with cases of IMT occurring after treatment of pediatric nephroblastoma reported [ 9 ]. Characterized by spindle cell proliferation and inflammatory cell infiltration, IMT is an intermediate state tumor with malignant potential, and therefore should be given adequate attention when it appears as a second tumor [ 10 ]. This study reports a case of a child who developed liver inflammatory myofibroblastic tumor (IMT) after NB.
This study was reviewed by the ethics committee of the Children’s Hospital of Nanjing Medical University, batch number: NJCH2020137. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The legal guardians/next of kin of the participants provided written informed consent for participation in this study. Written informed consent for the publication of any potentially identifiable images or data contained herein was obtained from the legal guardian/next of kin of the minor.
2.1 Case presentation
A male child, aged 28 months, was admitted to the hospital for examination in 2018 when he was found to have a frontal parietal mass with fever for 6 days. Chest and whole abdomen CT scan showed soft tissue density mass shadow of the right adrenal gland, with multiple small nodular calcified foci seen internally; multiple nodular calcified foci seen in the retroperitoneum and pelvis, which was considered as NB with possible retroperitoneal pelvic multiple lymph node metastases (Fig. 1 A). Cranial CT scan revealed thickening of the cranial plate of the frontal parietal bone on both sides, radiolucent periosteal reaction was seen in the inner plate, and soft tissue mass formation was seen in the surrounding area, metastasis was considered (Fig. 1 B). The child underwent cranial tumor biopsy on 3/23/2018, and postoperative pathology showed a small round cell malignancy, and NB metastasis was considered in conjunction with the child’s clinical history and immunohistochemistry results. In this study, we used fluorescence in situ hybridization (FISH) to detect the amplification rate of the MYCN gene, and a MYCN (green signal)/CEP2 (orange signal) ratio > 4 was considered positive, i.e., MYCN amplification; a MYCN/CEP2 ratio ≥ 1 and < 4 was considered gain; and a MYCN/CEP2 ratio < 1 was considered negative. MYCN gene test result was gain (Fig. 2 ).

The patient’s first physical examination. A A whole-body CT shows a suspicious mass in the right adrenal gland. B CT scan of the head showed bony abnormalities of the frontal parietal bone with soft tissue masses on both sides, metastasis was considered

FISH assay to detect MYCN gene amplification, green signal for MYCN gene and orange gene for CEP2, × 1000. MYCN gene test result was gain. (Among the counted tumor cells, the MYCN/CEP2 ratio was 1.07, the mean MYCN signal was 2.45, and the mean CEP2 signal was 2.3)
The child was diagnosed with NB, stage IV, and was included in the ultra-high-risk group because of cranial, dural, and multiple vertebral metastases, and was subsequently transferred to the Hemato-Oncology Department for chemotherapy. Since 11/04/2018 patient was given 3 courses of chemotherapy in the ultra-high risk group regimen for NB (Course I: cyclophosphamide, topotecan; Course II: topotecan, cyclophosphamide; Course III: pedialyte glycosides, cisplatin). On June 29, 2018, the patient underwent retroperitoneal tumor resection and retroperitoneal lymph node dissection in the Department of General Surgery of Nanjing Children’s Hospital. Postoperative pathology revealed post-chemotherapy changes in NB (differentiated type) with lymph node metastasis (Fig. 3 ). Transferred to Hemato-Oncology for chemotherapy after surgery (Course 1: CTX + TOPO; Course 2: CTX + TOPO; Course 3: CDDP + VP16; Course 4: CTX + DOXO + VCR + MESNA; Course 5: CDDP + VP16; Course 6: CTX + DOXO + VCR + MESNA; Course 7: CTX + TOPO; Course 8: CDDP + VP16; Course 9: CTX + DOXO + VCR + MESNA; Course 10: CTX + TOPO) and the abdominal CT was reviewed regularly.

Hematoxylin and eosin stain of the mass. The patient had preoperative chemotherapy, and the postoperative NB pathological section results showed differentiated type ( A : 40 × ; B : 100 ×)
The child underwent a routine postoperative thoracic and abdominal CT review in June 2020, which revealed an abnormal occupancy in the upper right posterior lobe of the liver. Further examination of the whole abdomen using CT scan with contrast showed that the right lobe of the liver was visible as a classically rounded low-density area with an extent of about 22 × 24 mm, and enhancement reveals inhomogeneous enhancement, which is evident in the venous phase, within which enhanced nodules are also seen.; NB liver metastasis is considered (Fig. 4 ). Subsequently, hepatic anomalous occupancy resection was performed in our General Surgery Department, and the occupancy was anatomically resected using indocyanine green fluorescence navigated hepatic occupancy resection, and postoperative pathology showed IMT (Fig. 5 A). Ventana D5F3 immunohistochemical staining showed positive anaplastic lymphoma kinas (ALK), consistent with the diagnosis of IMT (Fig. 5 B). Whole transcriptome sequencing of the adrenal NB specimen and the IMT of liver revealed a large number of differentially expressed genes (Fig. 6 ). A VIT chemotherapy regimen (Vincristine, Irinotecan and Temozolomide) was used postoperatively with outpatient follow-up.
Abdominal CT showed that the liver was normal in shape and size, and the right lobe of the liver could be seen as a rounded hypodense area, with inhomogeneous enhancement seen in the venous phase, within which enhancement nodules could be seen

A Hematoxylin and eosin stain of the mass, postoperative pathology shows inflammatory myofibroblastic tumor (40 × ; 100 ×); B Immunohistochemistry for ALK shows strong positivity (100 × ; 200 ×)
Whole transcriptome sequencing of adrenal NB specimens and liver IMT specimens revealed a large number of differential genes
3 Discussion
Comprehensive treatment of NB is associated with multiple complications, including cognitive deficits, cardiotoxicity, and chronic kidney disease [ 1 , 11 , 12 ]. In addition, many individual cases have reported a second tumor after treatment [ 13 , 14 ]. IMT of the Liver is a tumor-like lesion characterized by fibrous tissue, capillary hyperplasia, and local tissue inflammatory cell infiltration following necrosis of liver tissue caused by various inflammatory factors. The condition as a second tumor is reported for the first time [ 15 ].
When two tumors appear in adjacent locations at different times, multiple etiologies may be involved [ 16 ]. The second tumor may represent an extension or metastasis of the primary tumor [ 17 ]. Irradiation and drug therapy used as adjuvant modalities during the preoperative and postoperative period may have contributed to the development of the new tumor [ 18 ]. In addition, a patient’s genetic susceptibility to cancer, other environmental factors, or unknown causes may also lead to the development of a second tumor [ 19 , 20 ]. Low-dose irradiation is known to induce a second tumor [ 21 ]. In general, the following criteria should be followed for the diagnosis of radiation tumors [ 22 ]: the new lesion appears in the radiated area; it is histologically distinct from the first tumor; it develops after a latent period that reasonably allows for the development of a secondary tumor, and there are no other conditions that predispose to tumor development. And the patient's medical records showed no other genetic or environmental factors predisposing him to cancer. Based on the fact that the patient received prior chemotherapy, the authors of this article do not exclude the possibility that chemotherapy may have acted as a causative factor in the development of hepatic IMT. However, it is not entirely clear whether this is the case.
In addition, in this study, whole transcriptome next-generation sequencing of adrenal NB specimens and IMT of liver specimens were performed. Whole transcriptome sequencing showed complete discordance in gene expression between adrenal NB specimens and liver IMT specimens, this transcriptomic level analysis again validated the non-homologous nature of the patient's two successive tumors. Genetic studies have similarly shown that rearrangement of the ALK gene may contribute to tumorigenesis, suggesting that IMT is likely to occur as a tumor entity and is not a reactive progression due to a particular disease [ 9 ]. Our immunohistochemistry results also showed strong positivity for ALK, which can be used to differentiate from NB. It is more likely that radiotherapy and medication used as adjuvant modalities during the preoperative and postoperative period contributed to the development of IMT of liver after adrenal NB surgery. Overall, the association between the two tumors awaits further study.
Data availability
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.
Castle JT, Levy BE, Rodeberg DA. Abdominal tumors: wilms, neuroblastoma, rhabdomyosarcoma, and hepatoblastoma [J]. Surg Clin North Am. 2022;102(5):715–37.
Article PubMed Google Scholar
Baird DC, Meyers GJ, Hu JS. Testicular cancer: diagnosis and treatment [J]. Am Fam Phys. 2018;97(4):261–8.
Google Scholar
Breslow NE, Lange JM, Friedman DL, et al. Secondary malignant neoplasms after Wilms tumor: an international collaborative study [J]. Int J Cancer. 2010;127(3):657–66.
Article CAS PubMed PubMed Central Google Scholar
Guan X, Wei R, Yang R, et al. Association of radiotherapy for rectal cancer and second gynecological malignant neoplasms [J]. JAMA Netw Open. 2021;4(1):e2031661.
Article PubMed PubMed Central Google Scholar
Attarbaschi A, Carraro E, Ronceray L, et al. Second malignant neoplasms after treatment of non-Hodgkin’s lymphoma-a retrospective multinational study of 189 children and adolescents [J]. Leukemia. 2021;35(2):534–49.
Article CAS PubMed Google Scholar
Fabius AWM, van Hoefen WM, van Leeuwen FE, et al. Subsequent malignant neoplasms in retinoblastoma survivors [J]. Cancers. 2021;13(6):1200.
Keegan THM, Bleyer A, Rosenberg AS, et al. Second primary malignant neoplasms and survival in adolescent and young adult cancer survivors [J]. JAMA Oncol. 2017;3(11):1554–7.
Ottaviano M, Maddalena C, D’Armiento M, et al. Systemic treatment of malignant gastrointestinal neuroectodermal tumour after childhood neuroblastoma: chemotherapy in malignant gastrointestinal neuroectodermal tumour [J]. Anticancer Drugs. 2019;30(9):959–63.
Li YP, Han WW, He LJ, et al. Inflammatory myofibroblastic tumor after treatment of wilms tumor in a 6-year-old boy: a case report and literature review [J]. Urology. 2021;149:e25–8.
Webb TR, Slavish J, George RE, et al. Anaplastic lymphoma kinase: role in cancer pathogenesis and small-molecule inhibitor development for therapy [J]. Expert Rev Anticancer Ther. 2009;9(3):331–56.
Cairo SB, Urias AR, Murphy JT. Pediatric abdominal malignancies and intravascular extension: contemporary single-center experience [J]. J Surg Res. 2022;280:396–403.
Uttinger KL, Riedmeier M, Reibetanz J, et al. Adrenalectomies in children and adolescents in Germany—a diagnose related groups based analysis from 2009–2017 [J]. Front Endocrinol. 2022;13:914449.
Article Google Scholar
Martin A, Schneiderman J, Helenowski IB, et al. Secondary malignant neoplasms after high-dose chemotherapy and autologous stem cell rescue for high-risk neuroblastoma [J]. Pediatr Blood Cancer. 2014;61(8):1350–6.
Applebaum MA, Vaksman Z, Lee SM, et al. Neuroblastoma survivors are at increased risk for second malignancies: a report from the international neuroblastoma risk group project [J]. Eur J Cancer. 2017;72:177–85.
Filips A, Maurer MH, Montani M, et al. Inflammatory myofibroblastic tumor of the liver: a case report and review of literature [J]. World J Hepatol. 2020;12(4):170–83.
Applebaum MA, Henderson TO, Lee SM, et al. Second malignancies in patients with neuroblastoma: the effects of risk-based therapy [J]. Pediatr Blood Cancer. 2015;62(1):128–33.
Westerveld ASR, van Dalen EC, Asogwa OA, et al. Neuroblastoma survivors at risk for developing subsequent neoplasms: a systematic review [J]. Cancer Treat Rev. 2022;104:102355.
Zhen H, Guan H, Ma J, et al. Risk of developing second malignant neoplasms in patients with neuroblastoma: a population study of the US SEER database [J]. Radiat Oncol. 2021;16(1):228.
Lee JS, Dubois SG, Coccia PF, et al. Increased risk of second malignant neoplasms in adolescents and young adults with cancer [J]. Cancer. 2016;122(1):116–23.
Yalcin B, Kremer LC, van Dalen EC. High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma [J]. Cochrane Database Syst Rev. 2010;12(5):CD006301.
Hawkins M, Bhatia S, Henderson TO, et al. Subsequent primary neoplasms: risks, risk factors, surveillance, and future research [J]. Pediatr Clin North Am. 2020;67(6):1135–54.
Park KJ, Kang SH, Lee HG, et al. Olfactory neuroblastoma following treatment for pituitary adenoma [J]. J Neurooncol. 2008;90(2):237–41.
Download references
Acknowledgements
We thank the patient and her parents for their participation in this research.
The study was based on the Pediatric Solid Tumor Cohort of Nanjing Children’s Hospital.
Author information
Authors and affiliations.
Department of Pediatric Surgery, Children’s Hospital of Nanjing Medical University, Nanjing, Jiangsu, China
Qiyang Shen, Tao Li & Jianfeng Zhou
Department of Pediatric Surgery, First Affiliated Hospital of Bengbu Medical College, Bengbu, Anhui, China
Xuzhou Medical University, Xuzhou, Jiangsu, China
Lijie Zhang
You can also search for this author in PubMed Google Scholar
Contributions
Qiyang Shen and Xingyu Liu conducted the research. Qiyang Shen, Tao Li and Jianfeng Zhou are responsible for tumor resection and pathology specimen collection. Xingyu Liu and Lijie Zhang analyzed data. Qiyang Shen and Xingyu Liu drafted the manuscript. Tao Li and Jianfeng Zhou reviewed the draft. All authors contribute to the article and approve the submitted version.
Corresponding authors
Correspondence to Tao Li or Jianfeng Zhou .
Ethics declarations
Ethics approval and consent to participate.
The study involving human participants was reviewed and approved by the Ethics Committee. The legal guardians/next of kin of the participants provided written informed consent for participation in this study. Written informed consent for the publication of any potentially identifiable images or data contained herein was obtained from the legal guardian/next of kin of the minor.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Shen, Q., Liu, X., Zhang, L. et al. Inflammatory myofibroblastic tumor of the liver after adrenal neuroblastoma surgery: a case report. Discov Onc 15 , 174 (2024). https://doi.org/10.1007/s12672-024-01039-4
Download citation
Received : 30 September 2023
Accepted : 15 May 2024
Published : 18 May 2024
DOI : https://doi.org/10.1007/s12672-024-01039-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Neuroblastoma
- Secondary tumor
- Inflammatory myofibroblastic tumor
- Whole transcriptome sequencing
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Diffuse liver disease includes etiologies such as the various viral and immune hepatitides, storage diseases, hepatic steatosis, fibrosis, and cirrhosis. 38 Ultrasonography findings are often non-specific in the case of diffuse liver disease, and biopsy is usually required to differentiate between the various etiologies. 38 In cases of acute ...
UNFOLDING Reasoning Case Study: STUDENT Cirrhosis History of Present Problem: ... Liver cirrhosis with hepatic encephalopathy and an AKI Admit today - recent admission 6 months ago Hepatic encephalopathy due to advanced liver cirrhosis Hep C +, ETOH use (x25 years), IVDU, liver cirrhosis ...
Recently published guidelines by European Association for Studies of Liver (EASL) 3 and European Society for Clinical Nutrition and Metabolism (ESPEN) 13 provide comprehensive overviews of nutrition in cirrhosis. Herein, we provide a case-based practical guide of the causes of malnutrition, assessments tools, daily requirements and dietary ...
Temp - 37.0 C. Weight 235 lbs. BMI- 33. Alert, oriented to person, place, year and month but not to the day. Sclerae were not icteric. Pulmonary and Cardiovascular exam normal. Abdomen distended with fluid wave, mild tenderness to palpation. No hepatosplenomegaly. 2+ edema to mid-calf / Pedal pulses barely palpable.
The first edition of the clinical practice guidelines for liver cirrhosis was published in 2010, and the second edition was published in 2015 by the Japanese Society of Gastroenterology (JSGE). The revised third edition was recently published in 2020. This version has become a joint guideline by the JSGE and the Japan Society of Hepatology (JSH).
Case Scenario 2 58-year-old male with BMI 34, diabetes and hypertension had his yearly labs that showed mildly elevated liver enzymes diagnosed with cirrhosis recently. (AST=40, ALT=55) Hb 14.5, WBC 4.8, platelet count 120. You saw him in clinic and ordered quality of care investigations. His lab work did not reveal any other etiology of liver ...
Alcohol-related liver disease (ArLD) is a major cause of morbidity and mortality. Effective management requires multi-disciplinary input at every stage of the disease trajectory. We present a typical case to illustrate current evidence-based investigation and management of a patient with ArLD. This case-based review aims to concisely support the day-to-day decision making of clinicians looking ...
No. 11. CASE STUDY IN CIRRHOSIS OF THE LIVER 1039. Albumin is an abnormal constituent but it was of the urine, sincere wish of both the and was most likely caused by medical renal conges- and nursing staff to try to. tion, which in turn was caused make by him the as liver comfortable and as con-.
Accepted 16 September 2021. Published 29 November 2021. ABSTRACT. Liver is the second largest organ in human body, more than 5,000 separate b odily functions. .including helping b lood to c lot ...
300+ Nursing Cheatsheets. Start Free Trial. "Would suggest to all nursing students . . . Guaranteed to ease the stress!". ~Jordan. Cirrhosis Case Study (45 min) is mentioned in these lessons. Nursing case study for patient with cirrhosis with unfolding questions and answers. Written by ICU nurses.
This case study examines the physiological nature of alcohol-induced cirrhosis and its pathogenesis, external and internal clinical presentations, and treatment options. Treatments for alcohol-induced cirrhosis include liver transplant for permanent correction as well as varied options to manage symptoms. This case study analyzes alcoholic ...
Vol 392 July 28, 2018. Professor of Pathology at Harvard Medical School, drew on new clinical and pathological work to redefine cirrhosis as a syndrome: "a chronic, progressive, destructive lesion of the liver combined with reparative activity and contraction on the part of the connective tissue". By the early 20th century, clinicians knew ...
Liver Cirrhosis Case Study. 56 year old Caucasian male with end-stage liver cirrhosis in hospice care, came in for stem cell therapy to help prolong his life. Patient had a history of chronic Hepatitis C, hypertension and diabetes, and did not qualify for liver transplant. Patient had declined steadily over the past year, and over the last ...
Case Report • 2014 • vol.3 •13-16 CASE STUDY OF PATIENT WITH LIVER CIRRHOSIS Muhammad Rashad, ZubairAhmad, Hassan Ali , shanulqadir , Muhammad umair Faculty of Pharmacy, University of Sargodha. [email protected] Abstract Liver cirrhosis is a disease in which normal tissue of liver replaced with scar tissue, liver cirrhosis is ...
ct. Liver cirrhotic changes seen as irregular borders and relatively prominent left lobe and caudate. This is complicated by portal hypertension seen as marked splenomegaly and dilated portal and splenic veins with multiple dilated venous collaterals, most prominent at the lower peri-oesophagal and oesophagal submucosal groups. Gynecomastia is ...
Age: 40 years. Gender: Female. ultrasound. Ultrasound study shows a full spectrum of cirrhosis: small liver, nodular margins of the liver, dilated portal vein, ascites, splenomegaly and hepatofugal flow on portal vein. Incidental findings of a left ovarian cyst.
current issue. current issue; browse recently published; browse full issue index; learning/cme
This case-matched population study examines the pattern and recurrence rate of de novo hepatocellular carcinoma in patients with liver cirrhosis. ... Occult hepatitis B virus infection increases hepatocellular carcinogenesis by eight times in patients with non-B, non-C liver cirrhosis: a cohort study.
Here, we report the case of a patient presenting with cirrhosis of the liver due to autoimmune hepatitis along with systemic sclerosis. Case presentation A 51-year-old non-obese female with no history of alcoholism was admitted with complaints of gradually progressive abdominal distension and swelling over both lower limbs for the last month.
This alcoholic liver case study presents a patient with liver cirrhosis. A 43-year-old man was brought into the hospital with a complaints of loss of appetite, abdominal distention, and arrhythmia. He also experienced itchy skin and blood in the stool. The patient's family rushed him into the hospital, and he was in a half-conscious state.
The most common causes are viral hepatitis and chronically high alcohol consumption. Chronic liver disease is the progressive deterioration (or weakening) of your liver function over many months ...
Short-term trials have shown a reduction in liver fat when saturated fatty acids (SFA) are substituted with polyunsaturated fatty acids (PUFA), or with low-glycemic carbohydrates. However, few cohort studies have been conducted to investigate the associations of replacing SFA and SFA-rich foods with different macronutrients and foods on more severe stages of liver disease; non-alcoholic fatty ...
Study explores potential target to treat liver disease. by University of Birmingham. Liver biochemistry pre-timolumab, during and post-timolumab treatment. (A) Median with IQRs of ALP (IU/L) in ...
Sixty-six (95.65%) patients had anemia and 47 (68.12%) patients had thrombocytopenia. Conclusions: From this study, we can conclude that, in cirrhosis of liver patients, various hematological changes are very common which need to be identified and corrected early to reduce morbidity and mortality. Download Free PDF.
A more severe metabolic state is associated with a higher rate of progression to cirrhosis, as evidenced by a study involving patients with a diagnosis of MASLD within a U.S. Veterans Affairs cohort ().This study revealed a gradual increase in cirrhosis and liver cancer risk with the presence of additional metabolic traits, with T2D exhibiting the strongest association.
Here we describe the case of a patient with severe thrombocytopenia at baseline due to liver cirrhosis who developed iTTP. The challenges of the case in terms of pursuing disease-directed treatment, defining laboratory parameters to guide treatment, and mitigating the risks of bleeding and disease exacerbation are discussed.
Clinical trial of lanifibranor in adult patients with nonalcoholic steatohepatitis without cirrhosis and stage 2/grade 3 liver fibrosis. THE AIM OF THIS STUDY IS TO SEE IF A DRUG NOT YET APPROVED BY ANY REGULATORY AUTHORITY FOR MARKETING, CALLED LANIFIBIBRANOR, WILL HELP IN THE TREATMENT OF NON-ALCOHOLIC STEATOHEPATITIS (NASH) WITHOUT CIRRHOSIS ...
The high risk for heavy drinkers found in our meta-analysis of cohort studies is in line with prior research on risk for liver cirrhosis in people with alcohol use disorders.(11, 51, 52) The pooled RRs from case-control studies were much smaller; however, the more recent case-control study corresponds with the risks found in people with alcohol ...
In this work, proteomic techniques based on mass spectrometry have been applied to the study of liver diseases such as cholestasis, liver cirrhosis, and hepatocellular carcinoma with the aim of understanding the underlying molecular mechanisms of these pathological processes, as well as discovering potential biomarkers for the diagnosis and ...
In this study, whole transcriptome sequencing was performed on the postoperative specimen of adrenal NB and the specimen of IMT of liver. This unusual case emphasizes the need for close monitoring of second tumor development in NB survivors even in the absence of known predisposing factors.