
About Systematic Reviews

The Difference Between Narrative Review and Systematic Review

Automate every stage of your literature review to produce evidence-based research faster and more accurately.
Reviews in scientific research are tools that help synthesize literature on a topic of interest and describe its current state. Different types of reviews are conducted depending on the research question and the scope of the review. A systematic review is one such review that is robust, reproducible, and transparent. It involves collating evidence by using all of the eligible and critically appraised literature available on a certain topic. To know more about how to do a systematic review , you can check out our article at the link. The primary aim of a systematic review is to recommend best practices and inform policy development. Hence, there is a need for high-quality, focused, and precise methods and reporting. For more exploratory research questions, methods such as a scoping review are employed. Be sure you understand the difference between a systematic review and a scoping review , if you don’t, check out the link to learn more.
When the word “review” alone is used to describe a research paper, the first thing that should come to mind is that it is a literature review. Almost every researcher starts off their career with literature reviews. To know the difference between a systematic review and a literature review , read on here. Traditional literature reviews are also sometimes referred to as narrative reviews since they use narrative analysis to synthesize data. In this article, we will explore the differences between a systematic review and a narrative review, in further detail.
Learn More About DistillerSR
(Article continues below)
Narrative Review vs Systematic Review
Both systematic and narrative reviews are classified as secondary research studies since they both use existing primary research studies e.g. case studies. Despite this similarity, there are key differences in their methodology and scope. The major differences between them lie in their objectives, methodology, and application areas.
Differences In Objective
The main objective of a systematic review is to formulate a well-defined research question and use qualitative and quantitative methods to analyze all the available evidence attempting to answer the question. In contrast, narrative reviews can address one or more questions with a much broader scope. The efficacy of narrative reviews is irreplaceable in tracking the development of a scientific principle, or a clinical concept. This ability to conduct a wider exploration could be lost in the restrictive framework of a systematic review.
Differences in Methodology
For systematic reviews, there are guidelines provided by the Cochrane Handbook, ROSES, and the PRISMA statement that can help determine the protocol, and methodology to be used. However, for narrative reviews, such standard guidelines do not exist. Although, there are recommendations available.
Systematic reviews comprise an explicit, transparent, and pre-specified methodology. The methodology followed in a systematic review is as follows,
- Formulating the clinical research question to answer (PICO approach)
- Developing a protocol (with strict inclusion and exclusion criteria for the selection of primary studies)
- Performing a detailed and broad literature search
- Critical appraisal of the selected studies
- Data extraction from the primary studies included in the review
- Data synthesis and analysis using qualitative or quantitative methods [3].
- Reporting and discussing results of data synthesis.
- Developing conclusions based on the findings.
A narrative review on the other hand does not have a strict protocol to be followed. The design of the review depends on its author and the objectives of the review. As yet, there is no consensus on the standard structure of a narrative review. The preferred approach is the IMRAD (Introduction, Methods, Results, and Discussion) [2]. Apart from the author’s preferences, a narrative review structure must respect the journal style and conventions followed in the respective field.
Differences in Application areas
Narrative reviews are aimed at identifying and summarizing what has previously been published. Their general applications include exploring existing debates, the appraisal of previous studies conducted on a certain topic, identifying knowledge gaps, and speculating on the latest interventions available. They are also used to track and report on changes that have occurred in an existing field of research. The main purpose is to deepen the understanding in a certain research area. The results of a systematic review provide the most valid evidence to guide clinical decision-making and inform policy development [1]. They have now become the gold standard in evidence-based medicine [1].
Although both types of reviews come with their own benefits and limitations, researchers should carefully consider the differences between them before making a decision on which review type to use.
- Aromataris E, Pearson A. The systematic review: an overview. AJN. Am J Nurs. 2014;114(3):53–8.
- Green BN, Johnson CD, Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropratic Medicine 2006;5:101–117.
- Linares-Espinós E, Hernández V, Domínguez-Escrig JL, Fernández-Pello S, Hevia V, Mayor J, et al. Metodología de una revisión sistemática. Actas Urol Esp. 2018;42:499–506.
3 Reasons to Connect

Systematic, Scoping and Narrative Reviews
- Open Access
- First Online: 24 October 2021
Cite this chapter
You have full access to this open access chapter

- Samiran Nundy 4 ,
- Atul Kakar 5 &
- Zulfiqar A. Bhutta 6
29k Accesses
2 Citations
1 Altmetric
A Systematic Review is an attempt to distill the essence of a large number of studies in medicine by first asking a research question and then first identifying and later synthesizing carefully chosen studies of a high quality which might provide the answers. A more precise definition is ‘a summary of the medical literature that uses explicit and reproducible methods to systematically search, critically appraise and synthesise the results of multiple primary studies related to each other by using strategies to reduce bias and random errors’ [1].
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others

Systematic Reviewing

Critical Appraisal of Systematic Reviews and Meta-Analyses

Systematic Reviews and Meta-Analysis: A Guide for Beginners
1 what is a systematic review.
A Systematic Review is an attempt to distill the essence of a large number of studies in medicine by first asking a research question and then first identifying and later synthesizing carefully chosen studies of a high quality which might provide the answers. A more precise definition is ‘a summary of the medical literature that uses explicit and reproducible methods to systematically search, critically appraise and synthesise the results of multiple primary studies related to each other by using strategies to reduce bias and random errors’ [ 1 ].
In 1979, Archibald Cochrane, a Scottish doctor, proposed: ‘It is surely a great criticism of our profession that we have not organised a critical summary, by specialty or subspecialty, adapted periodically, of all relevant randomised controlled trials’. Cochrane was one of the founding fathers of evidence-based medicine (Fig. 29.1 ). He highlighted and advocated the importance of critically summarizing the findings of research studies and designated the systematic review as a method of providing such a summary. This ultimately led to the development of the Cochrane Collaboration in 1993 [ 2 ]. The findings of systematic reviews are now widely used for clinical decision-making and have become integral towards the development of sound clinical practice guidelines and recommendations. In fact, they now occupy the summit of the pyramid for the quality of evidence.
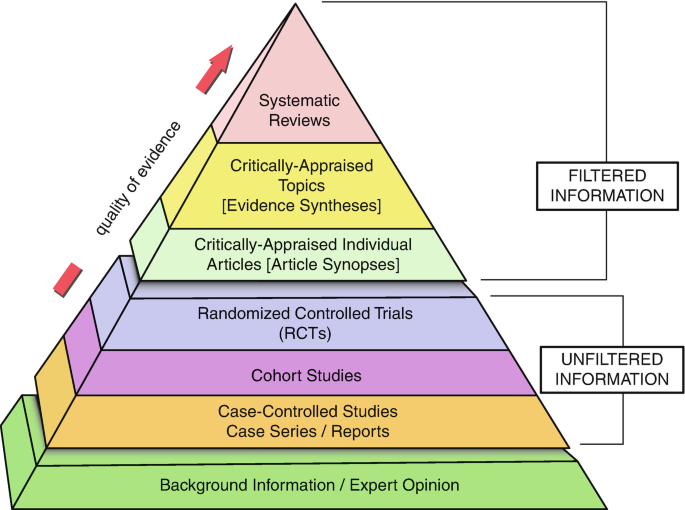
Shows the quality of evidence from various types of research papers
2 How Is a Systematic Review Done?
It is done using the following steps:
Step 1 —Defining the research question clearly and formulating criteria for which reports to include.
Searching for and selecting these studies and collecting their data. This will involve a review of all the available databases and citation indexes like the Web of Science, Embase, PubMed and others using different search technologies or even artificial intelligence-based tools. Each study should conform to the PRISMA (Preferred Reporting Items for Systemic Reviews and Meta-Analyses) guidelines or the standards of the Cochrane Collaboration [ 3 ].
The PRISMA guidelines (Fig. 29.2 ) are steps that depict the flow of information through the different phases of a systematic review. It maps out the number of records identified, included and excluded, and the reasons for exclusions.
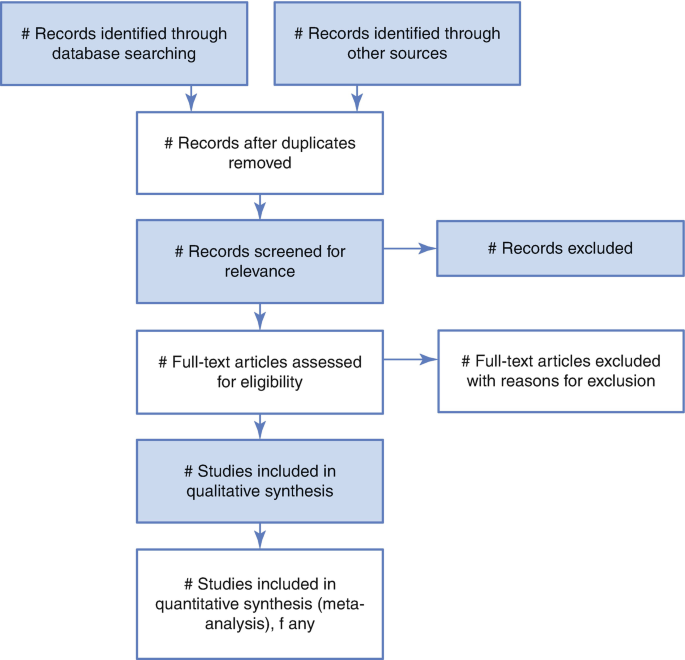
PRISMA guidelines
Step 2 —Assess their risk of bias. The review should use an objective and transparent approach for collection and synthesizing the data to minimize bias.
Step 3 —Analyse the data and undertake a meta-analysis. This may involve using complex statistical methods and the more data that is analyzed the more confident we can be of the result.
Step 4 —Write conclusions. Present the results and summarize the findings. Interpret the results, draw conclusions and suggest a message.
3 Why Is a Systematic Review Useful?
Many clinical decisions are guided by published studies but, unfortunately, there are now too many to choose from for a busy clinician. These studies often vary in their design, methodological quality, population involved and the intervention or condition considered. To take a rational clinical decision involves trying to reconcile the results of studies that provide different answers to the same question. Because it is often impractical for readers to track down and read all of the primary studies, systematic review articles are an important source of summarized evidence on a particular topic [ 4 , 5 ].
4 What Are Its Weaknesses?
Most systematic reviews focus on a single question when more than one may be relevant in a particular situation, e.g., the best treatment for variceal bleeding in a developed country may be endoscopic sclerotherapy but not in a person in a developing country who is poor, has good liver function and does not have access to sophisticated medical facilities. In him or her a portosystemic shunt operation and a one-time procedure may be the more appropriate.
Then search strategies are often not provided in detail, the selection of studies may be biased, and only the positive results may reach publication. It also takes about 6 months to complete a single systematic review [ 6 , 7 ].
5 What Is a Meta-Analysis? How Does It Differ from a Systematic Review?
A meta-analysis is a summary of data collected from multiple sources by collating it and helping to frame guidelines. While a systematic review includes the entire process of collecting, reviewing and presenting all available evidence, a meta-analysis only refers to the statistical technique of extracting and combining the data to produce a summary [ 7 , 8 ].
6 What Are Scoping and Narrative Reviews? How Do They Differ from a Systematic Review?
A Scoping review is a preliminary assessment of the potential size and scope of the available research literature. It aims to identify the nature and extent of research evidence (usually including ongoing research) and present an overview of a potentially large and diverse body of literature pertaining to a broad topic. In contrast, a systematic review attempts to collate empirical evidence from a relatively smaller number of studies pertaining to a focused research question.
A Narrative review is the type first-year college students often learn as a general approach. Its purpose is to identify a few studies that describe a problem of interest. Narrative reviews have no predetermined research question or specified search strategy, only a topic of interest. They are not systematic and follow no specified protocol. No standards or protocols guide the review. Although the reviewers will learn about the problem, they will not arrive at a comprehensive understanding of the state of the science related to the problem [ 9 , 10 ]. No strict rules are there for narrative review and can be done using the keywords.
7 Conclusions
A Systemic review gives a comprehensive and complete plan and search approach to study a topic of interest. This reduces the bias by recognizing, assessing and creating all relevant studies on a particular topic.
Systematic reviews can be ambiguous, not helpful, or even harmful when data are incorrectly handled.
A Meta-analysis involves using statistical methods to create the data from several studies into a single quantitative study.
Outcomes from a meta-analysis may help to estimate the effect of treatment or risk factors for disease, or other outcomes.
Uman LS. Systematic reviews and meta-analyses. J Can Acad Child Adolesc Psychiatry. 2011;20:57–9.
Article Google Scholar
Stavrou A, Challoumas D, Dimitrakakis G. Archibald Cochrane (1909–1988): the father of evidence-based medicine. Interact Cardiovasc Thorac Surg. 2014;18:121–4.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Last accessed on 3rd August 2020. Available on http://www.prisma-statement.org/ .
Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta-analysis: understanding the best evidence in primary healthcare. J Family Med Prim Care. 2013;2:9–14.
Article CAS Google Scholar
Why systematic reviews matter. A brief history, overview and practical guide for authors. Last accessed on 3rd August 2020. Available on https://www.elsevier.com/connect/authors-update/why-systematic-reviews-matter .
Yuan Y, Hunt RH. Systematic reviews: the good, the bad, and the ugly. Am J Gastroenterol. 2009;104:1086–92.
Ahn E, Kang H. Introduction to systematic review and meta-analysis. Korean J Anesthesiol. 2018;71(2):103–12.
Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14(Suppl 1):29–37.
CAS PubMed PubMed Central Google Scholar
Munn Z, Peters MDJ, Stern C, et al. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143.
Pham MT, Rajić A, Greig JD, Sargeant JM, Papadopoulos A, McEwen SA. A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Methods. 2014;5(4):371–85.
Download references
Author information
Authors and affiliations.
Department of Surgical Gastroenterology and Liver Transplantation, Sir Ganga Ram Hospital, New Delhi, India
Samiran Nundy
Department of Internal Medicine, Sir Ganga Ram Hospital, New Delhi, India
Institute for Global Health and Development, The Aga Khan University, South Central Asia, East Africa and United Kingdom, Karachi, Pakistan
Zulfiqar A. Bhutta
You can also search for this author in PubMed Google Scholar
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Reprints and permissions
Copyright information
© 2022 The Author(s)
About this chapter
Nundy, S., Kakar, A., Bhutta, Z.A. (2022). Systematic, Scoping and Narrative Reviews. In: How to Practice Academic Medicine and Publish from Developing Countries?. Springer, Singapore. https://doi.org/10.1007/978-981-16-5248-6_29
Download citation
DOI : https://doi.org/10.1007/978-981-16-5248-6_29
Published : 24 October 2021
Publisher Name : Springer, Singapore
Print ISBN : 978-981-16-5247-9
Online ISBN : 978-981-16-5248-6
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Subject Guides
Literature Review and Evidence Synthesis
- Reviews as Assignments
- Annotated Bibliography
What is a Narrative Literature Review
Literature review process.
- Integrative Review
- Scoping Review This link opens in a new window
- Systematic Review This link opens in a new window
- Other Review Types
- Subject Librarian Assistance with Reviews
- Grey Literature This link opens in a new window
Subject Librarians
Find your Subject Librarian Here

A narrative literature review is an integrated analysis of the existing literature used to summarize a body of literature, draw conclusions about a topic, and identify research gaps. By understanding the current state of the literature, you can show how new research fits into the larger research landscape.
A narrative literature review is NOT:
- Just a summary of sources
- A review of everything written on a particular topic
- A research paper arguing for a specific viewpoint - a lit review should avoid bias and highlight areas of disagreements
- A systematic review
Purposes of a narrative literature review:
- Explain the background of research on a topic
- Demonstrate the importance of a topic
- Suggest new areas of research
- Identify major themes, concepts, and researchers in a topic
- Identify critical gaps, points of disagreement, or flawed approaches for a research topic
1. Choose a topic & create a research question
- Use a narrow research question for more focused search results
- Use a question framework such as PICO to develop your research question
- Breakdown your research question into searchable concepts and keywords
- Research skills tutorials : How to choose a topic
- Ask a librarian for assistance
2. Select the sources for searching & develop a search strategy
- Identify databases to search for articles relevant to your topic
- Ask a librarian for recommended databases
- Develop a comprehensive search strategy using keywords, controlled vocabularies and Boolean operators
- Research skills tutorials: How to develop a search strategy
3. Conduct the search
- Use a consistent search strategy between databases
- Document the strategies employed to keep track of which are more successful
- Use a citation manager to organize your search results
- Ask a librarian for help or refer to the Research skills tutorials
4. Review the references
- Review the search results for relevant articles that answer your research question
- Review the bibliography of all relevant articles for additional sources
- Consider developing subfolders in the citation manager to organize sources by topic
- Use interlibrary loan for any articles without full text access
5. Summarize findings
- Synthesize the findings from the articles into a final paper
- The final paper should cover the themes identified in the research, explain any conflicts or disagreements, identify research gaps and potential future research areas, explain how this narrative review fits within the existing research and answer the research question .
For additional information :
Hempel. (2020). Conducting your literature review. American Psychological Association .
- Buchholz, & Dickins, K. A. (2023). Literature review and synthesis : a guide for nurses and other healthcare professionals . Springer Publishing Company, LLC.
- Coughlan, Michael, and Patricia Cronin. Doing a Literature Review in Nursing, Health and Social Care . 2nd edition., SAGE, 2017.
- Nundy, S., Kakar, A., Bhutta, Z.A. (2022). How to Do a Review of the Literature? . In: How to Practice Academic Medicine and Publish from Developing Countries?. Springer, Singapore. https://doi.org/10.1007/978-981-16-5248-6_18
- << Previous: Annotated Bibliography
- Next: Integrative Review >>
- Last Updated: Apr 24, 2024 10:53 AM
- URL: https://libraryguides.binghamton.edu/literaturereview
- share facebook
- share twitter
- share pinterest
- share linkedin
- share email
- Methodology
- Open access
- Published: 26 March 2019
SANRA—a scale for the quality assessment of narrative review articles
- Christopher Baethge ORCID: orcid.org/0000-0001-6246-3674 1 , 2 ,
- Sandra Goldbeck-Wood 1 , 3 &
- Stephan Mertens 1
Research Integrity and Peer Review volume 4 , Article number: 5 ( 2019 ) Cite this article
137k Accesses
626 Citations
38 Altmetric
Metrics details
Narrative reviews are the commonest type of articles in the medical literature. However, unlike systematic reviews and randomized controlled trials (RCT) articles, for which formal instruments exist to evaluate quality, there is currently no instrument available to assess the quality of narrative reviews. In response to this gap, we developed SANRA, the Scale for the Assessment of Narrative Review Articles.
A team of three experienced journal editors modified or deleted items in an earlier SANRA version based on face validity, item-total correlations, and reliability scores from previous tests. We deleted an item which addressed a manuscript’s writing and accessibility due to poor inter-rater reliability. The six items which form the revised scale are rated from 0 (low standard) to 2 (high standard) and cover the following topics: explanation of (1) the importance and (2) the aims of the review, (3) literature search and (4) referencing and presentation of (5) evidence level and (6) relevant endpoint data. For all items, we developed anchor definitions and examples to guide users in filling out the form. The revised scale was tested by the same editors (blinded to each other’s ratings) in a group of 30 consecutive non-systematic review manuscripts submitted to a general medical journal.
Raters confirmed that completing the scale is feasible in everyday editorial work. The mean sum score across all 30 manuscripts was 6.0 out of 12 possible points (SD 2.6, range 1–12). Corrected item-total correlations ranged from 0.33 (item 3) to 0.58 (item 6), and Cronbach’s alpha was 0.68 (internal consistency). The intra-class correlation coefficient (average measure) was 0.77 [95% CI 0.57, 0.88] (inter-rater reliability). Raters often disagreed on items 1 and 4.
Conclusions
SANRA’s feasibility, inter-rater reliability, homogeneity of items, and internal consistency are sufficient for a scale of six items. Further field testing, particularly of validity, is desirable. We recommend rater training based on the “explanations and instructions” document provided with SANRA. In editorial decision-making, SANRA may complement journal-specific evaluation of manuscripts—pertaining to, e.g., audience, originality or difficulty—and may contribute to improving the standard of non-systematic reviews.
Peer Review reports
Narrative review articles are common in the medical literature. Bastian et al. found that they constitute the largest share of all text types in medicine and they concluded that they “remain the staple of medical literature” [ 1 ]. Narrative reviews also appear popular among both authors and readers, and it is plausible to assume that they exercise an enormous influence among doctors in clinical practice and research. However, because their quality varies widely, they have frequently been compared in blanket, negative terms with systematic reviews.
We use the term narrative review to refer to an attempt to summarize the literature in a way which is not explicitly systematic, where the minimum requirement for the term systematic relates to the method of the literature search, but in a wider sense includes a specific research question and a comprehensive summary of all studies [ 2 ].
While systematic reviews are not per se superior articles and while certain systematic reviews have been criticized lately [ 3 ], non-systematic reviews or narrative reviews have been widely criticized as unreliable [ 1 , 4 ]. Hence, the hierarchy of evidence-based medicine places systematic reviews much higher than non-systematic ones. However, it is likely—and even desirable—that good quality narrative reviews will continue to play an important role in medicine: while systematic reviews are superior to narrative reviews in answering specific questions (for example, whether it is advisable to switch an antidepressant among antidepressant non-responders in patients with major depressive disorder [ 5 ]), narrative reviews are better suited to addressing a topic in wider ways (for example, outlining the general principles of diagnosing and treating depression [ 6 ]).
Critical appraisal tools have been developed for systematic reviews (e.g., AMSTAR 2 [A MeaSurement Tool to Assess Systematic Reviews] [ 7 ]) and papers on RCTs (e.g., the CASP [Critical Appraisal Skills Program] checklist for randomized trials [ 8 ]) and other types of medical studies. For narrative reviews, in contrast, no critical appraisal, or quality assessment tool is available. Such a tool, however, if simple and brief enough for day-to-day use, may support editors in choosing or improving manuscripts, help reviewers and readers in assessing the quality of a paper, and aid authors in preparing narrative reviews. It may improve the general quality of narrative reviews.
As a consequence, we have developed SANRA, the Scale for the Assessment of Narrative Review Articles, a brief critical appraisal tool for the assessment of non-systematic articles. Here, we present the revised scale and the results of a field test regarding its feasibility, item-total correlation, internal consistency, reliability, and criterion validity.
SANRA was developed between 2010 and 2017 by three experienced editors (CB, SGW, and SM) working at a general medical journal, Deutsches Ärzteblatt , the journal of the German Medical Association and the National Association of Statutory Health Insurance Physicians . It is intended to be a simple and brief quality assessment instrument not only to assist editors in their decisions about manuscripts, but also to help reviewers and readers in their assessment of papers and authors in writing narrative reviews.
Two earlier, seven-item versions of SANRA have been developed and tested by the authors, the first in 10 narrative reviews from the field of neurology as retrieved through a PubMed search, the second among 12 consecutive narrative reviews submitted to Deutsches Ärzteblatt —both showing satisfactory internal consistency and inter-rater reliability [ 9 ].
The current version of SANRA [ 10 ] has been revised by the authors in 2014 in order to simplify the scale and make it more robust. We simplified the wording of the items, and we deleted an item addressing a manuscript’s writing and accessibility because ratings of that item differed considerably. The six items that form the revised scale are rated in integers from 0 (low standard) to 2 (high standard), with 1 as an intermediate score. The maximal sum score is 12.
The sum score of the scale is intended to measure the construct “quality of a narrative review article” and covers the following topics: explanation of the review’s importance (item 1) and statement of the aims (item 2) of the review, description of the literature search (item 3), referencing (item 4), scientific reasoning (item 5), and presentation of relevant and appropriate endpoint data (item 6) (Fig. 1 ). For all items, we developed anchor definitions and examples to guide users in filling out the instrument, provided in the document “explanations and instructions,” accompanying the scale. This document was also edited to improve clarity (Fig. 2 ).

SANRA - Scale

SANRA—explanations and instructions document
In 2015, one rater (CB) screened all submissions to Deutsches Ärzteblatt in 2015, and the first 30 consecutive review manuscripts without systematic literature searches were selected for inclusion in the present study. All three raters (CB, SGW, and SM) are editors, with, in 2015, at least 10 years of experience each. They scored the manuscripts independently and blinded to each other’s ratings.

Statistical analysis
Descriptive data are shown as means or medians, as appropriate, and as ranges, standard deviations, or confidence intervals. This study aimed at testing SANRA’s internal consistency (Cronbach’s alpha) and the item-total correlation—indicating whether the items measure the same phenomenon, here different aspects of review paper quality—as well as SANRA’s inter-rater reliability with regard to its sum score. Inter-rater reliability, as a measure of the consistency among different raters, was expressed as the average measure intra-class correlation, ICC, using a two-way random effects model (consistency definition). As an approximation of SANRA’s criterion validity (Is the score predictive of other indicators of paper quality, e.g., acceptance and rejection or citations?), we analyzed post hoc whether average sum scores of SANRA were associated with the decision to accept or reject the 30 manuscripts under study (point biserial correlation for the association between a dichotomous and a continuous variable). All calculations were carried out using SPSS. Where possible, the presentation follows the recommendations of the Guidelines for Reporting Reliability and Agreement Studies (GRRAS) [ 11 ].
All 90 ratings (3 raters × 30 manuscripts) were used for statistical analysis. The mean sum score across all 30 manuscripts ( N = 90) was 6.0 out of 12 possible points (SD 2.6, range 1–12, median 6). Highest scores were rated for item 4 (mean 1.25; SD 0.70), item 2 (mean 1.14; SD 0.84), and item 1 (mean 1.1; SD 0.69) whereas items 6, 5, and 3 had the lowest scores (means of 0.81 (SD 0.65), 0.83 (SD 0.67), and 0.84 (SD 0.60), respectively) (all single-item medians: 1).
The scale’s internal consistency, measured as Cronbach’s alpha, was 0.68. Corrected item-total correlations ranged from 0.33 to 0.58 (Table 1 ). Tentative deletions of each item to assess the effect of these on consistency showed reduced internal consistency with every deleted item (0.58–0.67) (as shown by the alpha values in Table 1 ).
Across 180 single-item ratings (6 items × 30 manuscripts), the maximum difference among the 3 raters was 2 in 12.8% ( n = 23; most often in items 1, 2, and 4), in 56.7% ( n = 102), the raters differed by no more than 1 point, and in 30.6% ( n = 55), they entirely agreed (most often in items 2 and 3). The intra-class correlation coefficient (average measure) amounted to 0.77 [95% CI 0.57, 0.88; F 4.3; df 29, 58]. Disagreements most often occurred with regard to items 1 and 4.
Average SANRA sum scores of the 30 manuscripts were modestly associated with the editorial decision of acceptance (mean score 6.6, SD 1.9; n = 17) or rejection (mean score 5.1, SD 2.1; n = 13): point biserial correlation of 0.37 ( t = 2.09, df 28; two-sided p = 0.046).
All raters confirmed that completing the scale is feasible in everyday editorial work.
This study yielded three important findings: (1) SANRA can be applied to manuscripts in everyday editorial work. (2) SANRA’s internal consistency and item-total correlation are sufficient. (3) SANRA’s inter-rater reliability is satisfactory.
Feasibility
It is our experience with the current and earlier SANRA versions that editors, once accustomed to the scale, can integrate the scale into their everyday routine. It is important, however, to learn how to fill out SANRA. To this end, together with SANRA, we provide definitions and examples in the explanations and instructions document, and we recommend that new users train filling out SANRA using this resource. Editorial teams or teams of scientists and/or clinicians may prefer to learn using SANRA in group sessions.
Consistency and homogeneity
With Cronbach’s alpha of 0.68 and corrected item-total correlations between 0.33 and 0.58, we consider the scale’s consistency and item homogeneity sufficient for widespread application. It should be noted that because coefficient alpha increases with the number of items [ 12 ], simplifying a scale by reducing the number of items—as we did—may decrease internal consistency. However, this needs to be balanced against the practical need for brevity. In fact, the earlier seven-item versions of SANRA had higher values of alpha: 0.80 and 0.84, respectively [ 9 ]. Still, the number of items is not necessarily the only explanation for differences in alpha values. For example, the manuscripts included in the two earlier studies may have been easier to rate.
Inter-rater reliability
The scale’s intra-class correlation (0.77 after 0.76 in [ 9 ]) indicates that SANRA can be used reliably by different raters—an important property of a scale that may be applied for manuscript preparation and review, in editorial decision-making, or even in research on narrative reviews. Like internal consistency, reliability increases with the number of items [ 12 ], and there is a trade-off between simplicity (e.g., a small number of items) and reliability. While the ICC suggests sufficient reliability, however, the lower confidence limit (0.57) does not preclude a level of reliability normally deemed unacceptable in most applications of critical appraisal tools. This finding underscores the importance of rater training. Raters more often disagreed on items 1 and 4. After the study, we have therefore slightly edited these items, along with items 5 and 6 which we edited for clarity. In the same vein, we revised our explanations and instructions document.
It is important to bear in mind that testing of a scale always relates only to the setting of a given study. Thus, in the strict sense, the results presented here are not a general feature of SANRA but of SANRA filled out by certain raters with regard to a particular sample of manuscripts. However, from our experience, we trust that our setting is similar to that of many journals, and our sample of manuscripts represents an average group of papers. As a consequence, we are confident SANRA can be applied by other editors, reviewers, readers, and authors.
In a post hoc analysis, we found a modest, but statistically significant correlation of SANRA sum scores with manuscript acceptance. We interpret this as a sign of criterion validity, but emphasize that this is both a post hoc result and only a weak correlation. The latter, however, points to the fact that, at the level of submitted papers, other aspects than quality alone influence editorial decision-making: for example, whether the topic has been covered in the journal recently or whether editors believe that authors or topics of manuscripts have potential, even with low initial SANRA scores. SANRA will therefore often be used as one, and not the only, decision aid. Also, the decision to accept a paper has been made after the papers had been revised.
Moreover, additional results on criterion validity are needed, as are results on SANRA’s construct validity. On the other hand, SANRA’s content validity, defined as a scale’s ability to completely cover all aspects of a construct, will be restricted because we decided to limit the scale to six items, too few to encompass all facets of review article quality—SANRA is a critical appraisal tool and not a reporting guideline. For example, we deleted an item on the accessibility of the manuscript. Other possible domains that are not part of SANRA are, for example, originality of the manuscript or quality of tables and figures. These features are important, but we believe the six items forming SANRA are a core set that sufficiently indicates the quality of a review manuscript and, at the same time, is short enough to be applied without too much time and effort. SANRA’s brevity is also in contrast to other tools to assess articles, such as AMSTAR 2, for systematic reviews, or, to a lesser extent, CASP for RCTs, with its 16 and 11 items, respectively.
Throughout this paper we have referred to the current version of SANRA as the revision of earlier forms. This is technically true. However, because it is normal that scales go through different versions before publication and because this paper is first widespread publication of SANRA, we propose to call the present version simpy SANRA.
While medicine has achieved a great deal in the formalization and improvement of the presentation of randomized trials and systematic review articles, and also a number of other text types in medicine, much less work have been done with regard to the most frequent form of medical publications, the narrative review. There are exceptions: Gasparyan et al. [ 13 ], for example, have provided guidance for writing narrative reviews, and Byrne [ 14 ] as well as Pautasso [ 15 ] has written, from different angles, thoughtful editorials on improving narrative reviews and presented lists of key features of writing a good review—lists that naturally overlap with SANRA items (e.g., on referencing). These lists, however, are not tested scales and not intended for comparing different manuscripts. SANRA can be used in comparisons of manuscripts the way we used it in our editorial office, that is, in one setting. At the present time, however, it seems unwise to compare manuscripts across different settings because, so far, there are no established cut-offs for different grades of quality (e.g., poor-fair-moderate-good-very good). Still, in our experience, a score of 4 or below indicates very poor quality.
Limitations
The main limitation of this study is its sample size. While, in our experience, a group of 30 is not unusual in testing scales, it represents a compromise between the aims of representativeness for our journal and adequate power and feasibility; it took us about 6 months to sample 30 consecutive narrative reviews. Also, in this study, the authors of the scale were also the test-raters, and it is possible that inter-rater reliability is lower in groups less familiar with the scale. As for most scales, this underscores the importance of using the instructions that belong to the scale, in the present case the explanations and instructions document. It is also advisable to train using the scale before applying SANRA for manuscript rating. In addition, by design, this is not a study of test-retest reliability, another important feature of a scale. Finally, as previously acknowledged, although we believe in the representativeness of our setting for medical journals, the present results refer to the setting of this study, and consistency and reliability measures are study-specific.
We present SANRA, a brief scale for the quality assessment of narrative review articles, the most widespread form of article in the medical literature. We suggest SANRA can be integrated into the work of editors, reviewers, and authors. We encourage readers to consider using SANRA as an aid to critically appraising articles, and authors to consider its use on preparing narrative reviews, with a view to improving the quality of submitted and published manuscripts.
SANRA and its explanations and instructions document are available (open access) at: https://www.aerzteblatt.de/down.asp?id=22862 , https://www.aerzteblatt.de/down.asp?id=22861 .
Abbreviations
A MeaSurement Tool to Assess Systematic Reviews
Critical Appraisal Skills Program
Guidelines for Reporting Reliability and Agreement Studies
Intra-class correlation
Randomized controlled trial
Scale for the Assessment of Narrative Review Articles
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. https://doi.org/10.1371/journal.pmed.1000326 .
Article Google Scholar
Higgins JPT, Green S. (eds.). Cochrane Handbook for Systematic Reviews and Interventions. Version 5.1.0. The Cochrane Collaboration 2011, section 1.2.2., www.handbook.cochrane.org , retrieved on Oct 31, 2018.
Ioannidis JP. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q. 2016;94(3):485–514. https://doi.org/10.1111/1468-0009.12210 .
Mulrow CD. The medical review article: state of the science. Ann Intern Med. 1987;106:485–8.
Bschor T, Kern H, Henssler J, Baethge C. Switching the antidepressant after nonresponse in adults with major depression: a systematic literature search and meta-analysis. J Clin Psychiatry. 2018;79(1):16r10749. https://doi.org/10.4088/JCP.16r10749 .
Bschor T, Adli M. Treatment of depressive disorders. Dtsch Arztebl Int. 2008;105(45):782–92.
Google Scholar
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:J4008.
Critical Appraisal Skills Programme 2018. CASP Randomised controlled trial checklist available at: https://casp-uk.net/casp-tools-checklists/ . Accessed: 31 Oct 2018.
Baethge C, Mertens S, Goldbeck-Wood S. Development of a quality score for the assessment of non-systematic review articles (SANRA). In: Poster, Seventh International Congress on Peer Review and Biomedical Publication. USA: Chicago, Illinois; 2013.
Baethge C, Goldbeck-Wood S, Mertens S. A scale for the assessment of non-systematic review articles (SANRA). In: Poster, Eighth International Congress on Peer and Biomedical Publication. USA: Chicago, Illinois; 2017.
Kottner J, Audige L, Brorson S, Donner A, Gajewski BJ, Hrobjartsson A, Roberts C, Shoukri M, Streiner DL. Guidelines for reporting reliability and agreement studies (GRASS) were proposed. J Clin Epidemiol. 2011;64:96–106.
Streiner DL, Norman GR. Health measurment scales—a practical guide to their development and use. Fourth edition. New York: Oxford University Press; 2008.
Book Google Scholar
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int. 2011;31:1409–17.
Byrne JA. Improving the peer review of narrative literature reviews. Research Integrity and Peer Review. 2016;1(12). https://doi.org/10.1186/s41073-016-0019-2 .
Pautasso M. Ten simple rules for writing a literature review. PLoS Comput Biol. 2013;9(7):e1003149. https://doi.org/10.1371/journal.pcbi.1003149 .
Download references
Acknowledgements
This work has been presented at the Eighth International Congress on Peer Review and Scientific Publication in Chicago, Illinois, USA. (September 10-12, 2017) and at the 14th EASE Conference in Bucharest, Romania (June 8-10, 2018).
This work has not been externally funded.
Availability of data and materials
The dataset generated during the course of this study is available from the authors upon request.
Author information
Authors and affiliations.
Deutsches Ärzteblatt and Deutsches Ärzteblatt International, Dieselstraße 2, D-50859, Cologne, Germany
Christopher Baethge, Sandra Goldbeck-Wood & Stephan Mertens
Department of Psychiatry and Psychotherapy, University of Cologne Medical School, Cologne, Germany
Christopher Baethge
BMJ Sexual and Reproductive Health, London, UK
Sandra Goldbeck-Wood
You can also search for this author in PubMed Google Scholar
Contributions
All authors (CB, SM, and SGW) made substantial contributions to the conception of the study and to the acquisition and interpretation of data. CB analyzed the data and drafted the manuscript. SM and SGW revised the draft critically for important intellectual content. All authors sufficiently participated in this work to take public responsibility for its content, all finally approved the manuscript, and all are accountable for every aspect of this project.
Corresponding author
Correspondence to Christopher Baethge .
Ethics declarations
Ethics approval and consent to participate.
Not applicable
Consent for publication
Competing interests.
Non-financial competing interest: all authors (CB, SM, and SGW) had their part in the development of the scale under study. The authors declare that they have no financial competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Baethge, C., Goldbeck-Wood, S. & Mertens, S. SANRA—a scale for the quality assessment of narrative review articles. Res Integr Peer Rev 4 , 5 (2019). https://doi.org/10.1186/s41073-019-0064-8
Download citation
Received : 02 December 2018
Accepted : 26 February 2019
Published : 26 March 2019
DOI : https://doi.org/10.1186/s41073-019-0064-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Periodicals as topic
- Narrative review articles
- Non-systematic review articles
- Reliability
- Item-total correlation
- Internal consistency
- Cronbach’s alpha
- Intra-class correlation coefficient
Research Integrity and Peer Review
ISSN: 2058-8615
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Locations and Hours
- UCLA Library
- Research Guides
- Biomedical Library Guides
Systematic Reviews
- Types of Literature Reviews
What Makes a Systematic Review Different from Other Types of Reviews?
- Planning Your Systematic Review
- Database Searching
- Creating the Search
- Search Filters and Hedges
- Grey Literature
- Managing and Appraising Results
- Further Resources
Reproduced from Grant, M. J. and Booth, A. (2009), A typology of reviews: an analysis of 14 review types and associated methodologies. Health Information & Libraries Journal, 26: 91–108. doi:10.1111/j.1471-1842.2009.00848.x
- << Previous: Home
- Next: Planning Your Systematic Review >>
- Last Updated: Apr 17, 2024 2:02 PM
- URL: https://guides.library.ucla.edu/systematicreviews
- Search this site
- Systematic literature review
Narrative Review
- Scoping Review
- Systematic Review
- Rapid Review
- Research question
- Building your search string
- Optimal use of a literature database
- Fine-tuning your query
- Additional search methods
- Applications for literature review
- Support & contact
- Orientation
- Preparation
Key features of a narrative review
A narrative review is a general approach to literature review and is characterized by the lack of a rigid research methodology, such as PRISMA for a systematic review. As a result, it allows the researcher to choose their own approach and perspective for framing the research, i.e. the narrative. In a narrative review, literature on a topic is collected and summarized, e.g. by theme, perspective, or chronology. A narrative review does not require the researcher to determine inclusion and exclusion criteria prior to conducting the search, and so the researcher has the opportunity to include and discuss articles that are cross-disciplinary, and provide as examples to support the narrative.
Since a narrative review is largely shaped by the researcher's own views, it is generally considered less objective than a scoping review or a systematic review, because the potential for "bias" is more pronounced. With a method that is not transparent, the risk of bias is further exacerbated as it is unclear to the reader how thorough the review really is. Fortunately, there is a checklist available that tests the quality and objectivity of a narrative review, the SANRA . If you are planning to write a narrative review, it is advised to keep this checklist in mind - in addition to the other tips provided in this guide - to increase the quality of your review.
There are a number of situations in which a narrative review may be the best choice for your research. First, it is suitable for a chapter in a PhD thesis, as the researcher provides substantiation and evidence for the PhD research. Furthermore, it can serve as a framework for presenting research findings, to describe possible gaps, or reveal discussion points and ambiguities in the literature as a rationale for collecting the data. It may also point out gaps in the research area, using the researcher's view as a starting point. Finally, a narrative review can be a prelude to a more extensive literature review, such as a systematic review .
Before you start
Before you start writing a narrative review, always check whether a narrative review is the right review for your research. If you believe a complete overview and review is necessary on a topic in the field, a systematic review might be more fitting. Other considerations are:
- Has a narrative, rapid or systematic review already been conducted on the topic?
- Do you have sufficient knowledge about your topic to conduct a narrative review?
- Can you adequately substantiate and argue what gaps you find in the professional literature for this topic?
What do you need?
- Time: varies by topic (approximately 6-12 months);
- Access to a number of generic databases;
- Access to a leading domain-specific database;
- Reference manager (e.g. RefWorks );
- SANRA form;
- Log for your literature review ;
- Consultation with the information specialist (please fill out the intake form for support with your narrative review)
Determine your research question and associated keywords
You may already have a particular idea in mind for your narrative review that you want to substantiate. Another scenario is that you initially want to learn more about a topic and while reading the literature discover certain trends or gaps, which then lead to the narrative you want to present in your review. Either way, it is advisable to take the time to establish a research query and/or the relevant search terms and document them in a log, for example.
Tips for establishing and fine-tuning your research question and keywords for your literature review can be found in other sections of this guide.
Decide which databases you want to consult
Which databases you want to consult depends on your topic, the databases you have access to, and the available time for conducting a literature review. Hanze UAS has a number databases available that you can consult for a literature review . You may also have access to more and other relevant databases through the university where you are pursuing your PhD.
Again, take the time to determine which databases are available and useful to search and document your choices in your logbook.
Document the steps of your search strategy
Documenting the steps in your literature search is important for substantiating and arguing the aspects you want to address in the narrative review. A log has been developed for this purpose, which you can use to provide insight into your search process, but you are free to adopt your own approach. Another advantage of documenting your search strategy is that, with proper documentation, it is easier to pick up where you left off with your literature search if - unexpectedly - you are unable to work on your literature review for a while. This documentation is not a research protocol as is typically written for a scoping or systematic review, but rather an account of how you arrived at the results. A narrative review does not require protocol registration.
Conduct your search
As there is no set method for a narrative review, you can use a combination of free search terms and validated search terms (from a thesaurus or an ontology, for example). Elsewhere in this guide, you'll find helpful tips and tricks for setting up a search string and other search methods , among other things.
Other tips:
- keep a log (search terms and combinations thereof, databases consulted, filters, etc.)
- collect references in a reference manager, e.g. RefWorks
- create a data matrix (see table below), linking the aspects and results of your literature search
De-duplicate Duplicate results may occur when searching across databases. You can use RefWorks to deduplicate your search results; you can read more about this in the library guide on RefWorks .
Screening abstracts and papers The next step in the process is to screen the abstracts of the articles. Article abstracts follow an editor's requirements for writing an abstract. An abstract may contain the following elements:
- Objective/Background
- Conclusions
In most cases, search terms are also included with the abstract of the article or chapter; these can be found under ""keywords." After reading and screening the abstracts, you will become more familiar with the topic and the relevance of the articles, which will help you to substantiate and argue your view on the topic. Screening the papers as a whole allows you to make connections, identify gaps, discover trends in the scientific literature and check them against your own views on the topic.
A literature matrix and a reference manager can support you in organizing selection process. A literature matrix is an overview of the papers you screen. You create this overview by answering a number of key questions for each article so that you can easily compare sources.
Reporting your findings
After conducting the literature review and analyzing the results, writing a narrative review is the next phase. Write an outline of what you want to address in your narrative review. Your structure may be thematic, chronological, or hierarchical, and showcases the different angles and how they relate to the works cited. Some researchers like to first draw out the narrative schematically on a piece of paper, while others prefer to write the outline and then fill it in with the references that support the different parts.
A narrative review usually adheres to the following format:
- A summary of the article (article abstract);
- An introduction to the topic and its context;
- A motivation as to why a narrative review is needed;
- A description of the systematic search method, including search string, criteria, etc.;
- A main text divided into smaller sub-sections describing the similarities and differences of aspects in the literature;
- A conclusion (evidence and analysis) to highlight gaps and trends in the research field;
- A comprehensive reference list.
If you already have a (scientific) journal in mind where you would like to publish, then make sure to read the author's guide before you start writing. This way, you know in advance what the requirements are for a review publication in the relevant journal (e.g. maximum number of words, structure).
For your inspiration, we have added some systematic reviews published by Hanze researchers in the list of resources below. All publications are open access available.
SANRA method SANRA is an acronym for "Scale for the Assessment of Narrative Review Articles," and is a method for assessing the quality of a narrative review. SANRA provides transparency and evidence of the author's personal opinion. SANRA consists of 6 items, which are rated on a scale of 0-2, with 0 representing low level and 2 representing high level.
The following items are part of SANRA:
- Explanation of the importance of the topic;
- The goals of the narrative review;
- Report of the literature review conducted;
- References, related to the different aspects of the study;
- Presentation of the level of evidence for the author's views;
- Relevant data and references that support the research question.
To the SANRA review form.
Useful links and resources
- Useful links
- Examples of narrative review papers
More on SANRA:
- Baethge, C., Goldbeck-Wood, S. & Mertens, S. SANRA—a scale for the quality assessment of narrative review articles. Research Integrity Peer Review 4 , 5 (2019). https://doi.org/10.1186/s41073-019-0064-8
- Falcão, F., Costa, P. & Pêgo, J.M. (2022) Feasibility assurance: a review of automatic item generation in medical assessment. Advances in Health Sciences Education 27 , 405–425. DOI: 10.1007/s10459-022-10092-z
- Salman, L.A., Ahmed, G., Dakin, S.G. et al. (2023) Osteoarthritis: a narrative review of molecular approaches to disease management. Arthritis Research & Therapy, 25 , 27. DOI: 10.1186/s13075-023-03006-w
- Woodley, S.J., Hay-Smith, E.J.C. (2021). Narrative review of pelvic floor muscle training for childbearing women—why, when, what, and how. International Urogynecology Journal , 32 , 1977–1988. DOI: 10.1007/s00192-021-04804-z
- Cavada, M., & Rogers, C. D. F. (2020). Serious gaming as a means of facilitating truly smart cities: a narrative review. Behaviour & Information Technology , 39 (6), 695–710. DOI: 10.1080/0144929X.2019.1677775
- Chisholm, J. M., Zamani, R., Negm, A. M., Said, N., Abdel daiem, M. M., Dibaj, M., & Akrami, M. (2021). Sustainable waste management of medical waste in African developing countries: A narrative review. Waste Management & Researc h, 39 (9), 1149-1163. DOI: 10.1177/0734242X211029175
If you have questions, please contact the Information Specialist Research of your research center , or go to support & contact for more information and advice.
[anchornavigation]
Wearable Health Devices in Health Care: Narrative Systematic Review
Affiliation.
- 1 Department of Orthopaedic Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
- PMID: 33164904
- PMCID: PMC7683248
- DOI: 10.2196/18907
Background: With the rise of mobile medicine, the development of new technologies such as smart sensing, and the popularization of personalized health concepts, the field of smart wearable devices has developed rapidly in recent years. Among them, medical wearable devices have become one of the most promising fields. These intelligent devices not only assist people in pursuing a healthier lifestyle but also provide a constant stream of health care data for disease diagnosis and treatment by actively recording physiological parameters and tracking metabolic status. Therefore, wearable medical devices have the potential to become a mainstay of the future mobile medical market.
Objective: Although previous reviews have discussed consumer trends in wearable electronics and the application of wearable technology in recreational and sporting activities, data on broad clinical usefulness are lacking. We aimed to review the current application of wearable devices in health care while highlighting shortcomings for further research. In addition to daily health and safety monitoring, the focus of our work was mainly on the use of wearable devices in clinical practice.
Methods: We conducted a narrative review of the use of wearable devices in health care settings by searching papers in PubMed, EMBASE, Scopus, and the Cochrane Library published since October 2015. Potentially relevant papers were then compared to determine their relevance and reviewed independently for inclusion.
Results: A total of 82 relevant papers drawn from 960 papers on the subject of wearable devices in health care settings were qualitatively analyzed, and the information was synthesized. Our review shows that the wearable medical devices developed so far have been designed for use on all parts of the human body, including the head, limbs, and torso. These devices can be classified into 4 application areas: (1) health and safety monitoring, (2) chronic disease management, (3) disease diagnosis and treatment, and (4) rehabilitation. However, the wearable medical device industry currently faces several important limitations that prevent further use of wearable technology in medical practice, such as difficulties in achieving user-friendly solutions, security and privacy concerns, the lack of industry standards, and various technical bottlenecks.
Conclusions: We predict that with the development of science and technology and the popularization of personalized health concepts, wearable devices will play a greater role in the field of health care and become better integrated into people's daily lives. However, more research is needed to explore further applications of wearable devices in the medical field. We hope that this review can provide a useful reference for the development of wearable medical devices.
Keywords: chronic disease management; health monitoring; medical field; public health; rehabilitation.; wearable.
©Lin Lu, Jiayao Zhang, Yi Xie, Fei Gao, Song Xu, Xinghuo Wu, Zhewei Ye. Originally published in JMIR mHealth and uHealth (http://mhealth.jmir.org), 09.11.2020.
Publication types
- Research Support, Non-U.S. Gov't
- Systematic Review
- Delivery of Health Care
- Health Facilities
- Wearable Electronic Devices*
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Lau F, Kuziemsky C, editors. Handbook of eHealth Evaluation: An Evidence-based Approach [Internet]. Victoria (BC): University of Victoria; 2017 Feb 27.

Handbook of eHealth Evaluation: An Evidence-based Approach [Internet].
Chapter 9 methods for literature reviews.
Guy Paré and Spyros Kitsiou .
9.1. Introduction
Literature reviews play a critical role in scholarship because science remains, first and foremost, a cumulative endeavour ( vom Brocke et al., 2009 ). As in any academic discipline, rigorous knowledge syntheses are becoming indispensable in keeping up with an exponentially growing eHealth literature, assisting practitioners, academics, and graduate students in finding, evaluating, and synthesizing the contents of many empirical and conceptual papers. Among other methods, literature reviews are essential for: (a) identifying what has been written on a subject or topic; (b) determining the extent to which a specific research area reveals any interpretable trends or patterns; (c) aggregating empirical findings related to a narrow research question to support evidence-based practice; (d) generating new frameworks and theories; and (e) identifying topics or questions requiring more investigation ( Paré, Trudel, Jaana, & Kitsiou, 2015 ).
Literature reviews can take two major forms. The most prevalent one is the “literature review” or “background” section within a journal paper or a chapter in a graduate thesis. This section synthesizes the extant literature and usually identifies the gaps in knowledge that the empirical study addresses ( Sylvester, Tate, & Johnstone, 2013 ). It may also provide a theoretical foundation for the proposed study, substantiate the presence of the research problem, justify the research as one that contributes something new to the cumulated knowledge, or validate the methods and approaches for the proposed study ( Hart, 1998 ; Levy & Ellis, 2006 ).
The second form of literature review, which is the focus of this chapter, constitutes an original and valuable work of research in and of itself ( Paré et al., 2015 ). Rather than providing a base for a researcher’s own work, it creates a solid starting point for all members of the community interested in a particular area or topic ( Mulrow, 1987 ). The so-called “review article” is a journal-length paper which has an overarching purpose to synthesize the literature in a field, without collecting or analyzing any primary data ( Green, Johnson, & Adams, 2006 ).
When appropriately conducted, review articles represent powerful information sources for practitioners looking for state-of-the art evidence to guide their decision-making and work practices ( Paré et al., 2015 ). Further, high-quality reviews become frequently cited pieces of work which researchers seek out as a first clear outline of the literature when undertaking empirical studies ( Cooper, 1988 ; Rowe, 2014 ). Scholars who track and gauge the impact of articles have found that review papers are cited and downloaded more often than any other type of published article ( Cronin, Ryan, & Coughlan, 2008 ; Montori, Wilczynski, Morgan, Haynes, & Hedges, 2003 ; Patsopoulos, Analatos, & Ioannidis, 2005 ). The reason for their popularity may be the fact that reading the review enables one to have an overview, if not a detailed knowledge of the area in question, as well as references to the most useful primary sources ( Cronin et al., 2008 ). Although they are not easy to conduct, the commitment to complete a review article provides a tremendous service to one’s academic community ( Paré et al., 2015 ; Petticrew & Roberts, 2006 ). Most, if not all, peer-reviewed journals in the fields of medical informatics publish review articles of some type.
The main objectives of this chapter are fourfold: (a) to provide an overview of the major steps and activities involved in conducting a stand-alone literature review; (b) to describe and contrast the different types of review articles that can contribute to the eHealth knowledge base; (c) to illustrate each review type with one or two examples from the eHealth literature; and (d) to provide a series of recommendations for prospective authors of review articles in this domain.
9.2. Overview of the Literature Review Process and Steps
As explained in Templier and Paré (2015) , there are six generic steps involved in conducting a review article:
- formulating the research question(s) and objective(s),
- searching the extant literature,
- screening for inclusion,
- assessing the quality of primary studies,
- extracting data, and
- analyzing data.
Although these steps are presented here in sequential order, one must keep in mind that the review process can be iterative and that many activities can be initiated during the planning stage and later refined during subsequent phases ( Finfgeld-Connett & Johnson, 2013 ; Kitchenham & Charters, 2007 ).
Formulating the research question(s) and objective(s): As a first step, members of the review team must appropriately justify the need for the review itself ( Petticrew & Roberts, 2006 ), identify the review’s main objective(s) ( Okoli & Schabram, 2010 ), and define the concepts or variables at the heart of their synthesis ( Cooper & Hedges, 2009 ; Webster & Watson, 2002 ). Importantly, they also need to articulate the research question(s) they propose to investigate ( Kitchenham & Charters, 2007 ). In this regard, we concur with Jesson, Matheson, and Lacey (2011) that clearly articulated research questions are key ingredients that guide the entire review methodology; they underscore the type of information that is needed, inform the search for and selection of relevant literature, and guide or orient the subsequent analysis. Searching the extant literature: The next step consists of searching the literature and making decisions about the suitability of material to be considered in the review ( Cooper, 1988 ). There exist three main coverage strategies. First, exhaustive coverage means an effort is made to be as comprehensive as possible in order to ensure that all relevant studies, published and unpublished, are included in the review and, thus, conclusions are based on this all-inclusive knowledge base. The second type of coverage consists of presenting materials that are representative of most other works in a given field or area. Often authors who adopt this strategy will search for relevant articles in a small number of top-tier journals in a field ( Paré et al., 2015 ). In the third strategy, the review team concentrates on prior works that have been central or pivotal to a particular topic. This may include empirical studies or conceptual papers that initiated a line of investigation, changed how problems or questions were framed, introduced new methods or concepts, or engendered important debate ( Cooper, 1988 ). Screening for inclusion: The following step consists of evaluating the applicability of the material identified in the preceding step ( Levy & Ellis, 2006 ; vom Brocke et al., 2009 ). Once a group of potential studies has been identified, members of the review team must screen them to determine their relevance ( Petticrew & Roberts, 2006 ). A set of predetermined rules provides a basis for including or excluding certain studies. This exercise requires a significant investment on the part of researchers, who must ensure enhanced objectivity and avoid biases or mistakes. As discussed later in this chapter, for certain types of reviews there must be at least two independent reviewers involved in the screening process and a procedure to resolve disagreements must also be in place ( Liberati et al., 2009 ; Shea et al., 2009 ). Assessing the quality of primary studies: In addition to screening material for inclusion, members of the review team may need to assess the scientific quality of the selected studies, that is, appraise the rigour of the research design and methods. Such formal assessment, which is usually conducted independently by at least two coders, helps members of the review team refine which studies to include in the final sample, determine whether or not the differences in quality may affect their conclusions, or guide how they analyze the data and interpret the findings ( Petticrew & Roberts, 2006 ). Ascribing quality scores to each primary study or considering through domain-based evaluations which study components have or have not been designed and executed appropriately makes it possible to reflect on the extent to which the selected study addresses possible biases and maximizes validity ( Shea et al., 2009 ). Extracting data: The following step involves gathering or extracting applicable information from each primary study included in the sample and deciding what is relevant to the problem of interest ( Cooper & Hedges, 2009 ). Indeed, the type of data that should be recorded mainly depends on the initial research questions ( Okoli & Schabram, 2010 ). However, important information may also be gathered about how, when, where and by whom the primary study was conducted, the research design and methods, or qualitative/quantitative results ( Cooper & Hedges, 2009 ). Analyzing and synthesizing data : As a final step, members of the review team must collate, summarize, aggregate, organize, and compare the evidence extracted from the included studies. The extracted data must be presented in a meaningful way that suggests a new contribution to the extant literature ( Jesson et al., 2011 ). Webster and Watson (2002) warn researchers that literature reviews should be much more than lists of papers and should provide a coherent lens to make sense of extant knowledge on a given topic. There exist several methods and techniques for synthesizing quantitative (e.g., frequency analysis, meta-analysis) and qualitative (e.g., grounded theory, narrative analysis, meta-ethnography) evidence ( Dixon-Woods, Agarwal, Jones, Young, & Sutton, 2005 ; Thomas & Harden, 2008 ).
9.3. Types of Review Articles and Brief Illustrations
EHealth researchers have at their disposal a number of approaches and methods for making sense out of existing literature, all with the purpose of casting current research findings into historical contexts or explaining contradictions that might exist among a set of primary research studies conducted on a particular topic. Our classification scheme is largely inspired from Paré and colleagues’ (2015) typology. Below we present and illustrate those review types that we feel are central to the growth and development of the eHealth domain.
9.3.1. Narrative Reviews
The narrative review is the “traditional” way of reviewing the extant literature and is skewed towards a qualitative interpretation of prior knowledge ( Sylvester et al., 2013 ). Put simply, a narrative review attempts to summarize or synthesize what has been written on a particular topic but does not seek generalization or cumulative knowledge from what is reviewed ( Davies, 2000 ; Green et al., 2006 ). Instead, the review team often undertakes the task of accumulating and synthesizing the literature to demonstrate the value of a particular point of view ( Baumeister & Leary, 1997 ). As such, reviewers may selectively ignore or limit the attention paid to certain studies in order to make a point. In this rather unsystematic approach, the selection of information from primary articles is subjective, lacks explicit criteria for inclusion and can lead to biased interpretations or inferences ( Green et al., 2006 ). There are several narrative reviews in the particular eHealth domain, as in all fields, which follow such an unstructured approach ( Silva et al., 2015 ; Paul et al., 2015 ).
Despite these criticisms, this type of review can be very useful in gathering together a volume of literature in a specific subject area and synthesizing it. As mentioned above, its primary purpose is to provide the reader with a comprehensive background for understanding current knowledge and highlighting the significance of new research ( Cronin et al., 2008 ). Faculty like to use narrative reviews in the classroom because they are often more up to date than textbooks, provide a single source for students to reference, and expose students to peer-reviewed literature ( Green et al., 2006 ). For researchers, narrative reviews can inspire research ideas by identifying gaps or inconsistencies in a body of knowledge, thus helping researchers to determine research questions or formulate hypotheses. Importantly, narrative reviews can also be used as educational articles to bring practitioners up to date with certain topics of issues ( Green et al., 2006 ).
Recently, there have been several efforts to introduce more rigour in narrative reviews that will elucidate common pitfalls and bring changes into their publication standards. Information systems researchers, among others, have contributed to advancing knowledge on how to structure a “traditional” review. For instance, Levy and Ellis (2006) proposed a generic framework for conducting such reviews. Their model follows the systematic data processing approach comprised of three steps, namely: (a) literature search and screening; (b) data extraction and analysis; and (c) writing the literature review. They provide detailed and very helpful instructions on how to conduct each step of the review process. As another methodological contribution, vom Brocke et al. (2009) offered a series of guidelines for conducting literature reviews, with a particular focus on how to search and extract the relevant body of knowledge. Last, Bandara, Miskon, and Fielt (2011) proposed a structured, predefined and tool-supported method to identify primary studies within a feasible scope, extract relevant content from identified articles, synthesize and analyze the findings, and effectively write and present the results of the literature review. We highly recommend that prospective authors of narrative reviews consult these useful sources before embarking on their work.
Darlow and Wen (2015) provide a good example of a highly structured narrative review in the eHealth field. These authors synthesized published articles that describe the development process of mobile health ( m-health ) interventions for patients’ cancer care self-management. As in most narrative reviews, the scope of the research questions being investigated is broad: (a) how development of these systems are carried out; (b) which methods are used to investigate these systems; and (c) what conclusions can be drawn as a result of the development of these systems. To provide clear answers to these questions, a literature search was conducted on six electronic databases and Google Scholar . The search was performed using several terms and free text words, combining them in an appropriate manner. Four inclusion and three exclusion criteria were utilized during the screening process. Both authors independently reviewed each of the identified articles to determine eligibility and extract study information. A flow diagram shows the number of studies identified, screened, and included or excluded at each stage of study selection. In terms of contributions, this review provides a series of practical recommendations for m-health intervention development.
9.3.2. Descriptive or Mapping Reviews
The primary goal of a descriptive review is to determine the extent to which a body of knowledge in a particular research topic reveals any interpretable pattern or trend with respect to pre-existing propositions, theories, methodologies or findings ( King & He, 2005 ; Paré et al., 2015 ). In contrast with narrative reviews, descriptive reviews follow a systematic and transparent procedure, including searching, screening and classifying studies ( Petersen, Vakkalanka, & Kuzniarz, 2015 ). Indeed, structured search methods are used to form a representative sample of a larger group of published works ( Paré et al., 2015 ). Further, authors of descriptive reviews extract from each study certain characteristics of interest, such as publication year, research methods, data collection techniques, and direction or strength of research outcomes (e.g., positive, negative, or non-significant) in the form of frequency analysis to produce quantitative results ( Sylvester et al., 2013 ). In essence, each study included in a descriptive review is treated as the unit of analysis and the published literature as a whole provides a database from which the authors attempt to identify any interpretable trends or draw overall conclusions about the merits of existing conceptualizations, propositions, methods or findings ( Paré et al., 2015 ). In doing so, a descriptive review may claim that its findings represent the state of the art in a particular domain ( King & He, 2005 ).
In the fields of health sciences and medical informatics, reviews that focus on examining the range, nature and evolution of a topic area are described by Anderson, Allen, Peckham, and Goodwin (2008) as mapping reviews . Like descriptive reviews, the research questions are generic and usually relate to publication patterns and trends. There is no preconceived plan to systematically review all of the literature although this can be done. Instead, researchers often present studies that are representative of most works published in a particular area and they consider a specific time frame to be mapped.
An example of this approach in the eHealth domain is offered by DeShazo, Lavallie, and Wolf (2009). The purpose of this descriptive or mapping review was to characterize publication trends in the medical informatics literature over a 20-year period (1987 to 2006). To achieve this ambitious objective, the authors performed a bibliometric analysis of medical informatics citations indexed in medline using publication trends, journal frequencies, impact factors, Medical Subject Headings (MeSH) term frequencies, and characteristics of citations. Findings revealed that there were over 77,000 medical informatics articles published during the covered period in numerous journals and that the average annual growth rate was 12%. The MeSH term analysis also suggested a strong interdisciplinary trend. Finally, average impact scores increased over time with two notable growth periods. Overall, patterns in research outputs that seem to characterize the historic trends and current components of the field of medical informatics suggest it may be a maturing discipline (DeShazo et al., 2009).
9.3.3. Scoping Reviews
Scoping reviews attempt to provide an initial indication of the potential size and nature of the extant literature on an emergent topic (Arksey & O’Malley, 2005; Daudt, van Mossel, & Scott, 2013 ; Levac, Colquhoun, & O’Brien, 2010). A scoping review may be conducted to examine the extent, range and nature of research activities in a particular area, determine the value of undertaking a full systematic review (discussed next), or identify research gaps in the extant literature ( Paré et al., 2015 ). In line with their main objective, scoping reviews usually conclude with the presentation of a detailed research agenda for future works along with potential implications for both practice and research.
Unlike narrative and descriptive reviews, the whole point of scoping the field is to be as comprehensive as possible, including grey literature (Arksey & O’Malley, 2005). Inclusion and exclusion criteria must be established to help researchers eliminate studies that are not aligned with the research questions. It is also recommended that at least two independent coders review abstracts yielded from the search strategy and then the full articles for study selection ( Daudt et al., 2013 ). The synthesized evidence from content or thematic analysis is relatively easy to present in tabular form (Arksey & O’Malley, 2005; Thomas & Harden, 2008 ).
One of the most highly cited scoping reviews in the eHealth domain was published by Archer, Fevrier-Thomas, Lokker, McKibbon, and Straus (2011) . These authors reviewed the existing literature on personal health record ( phr ) systems including design, functionality, implementation, applications, outcomes, and benefits. Seven databases were searched from 1985 to March 2010. Several search terms relating to phr s were used during this process. Two authors independently screened titles and abstracts to determine inclusion status. A second screen of full-text articles, again by two independent members of the research team, ensured that the studies described phr s. All in all, 130 articles met the criteria and their data were extracted manually into a database. The authors concluded that although there is a large amount of survey, observational, cohort/panel, and anecdotal evidence of phr benefits and satisfaction for patients, more research is needed to evaluate the results of phr implementations. Their in-depth analysis of the literature signalled that there is little solid evidence from randomized controlled trials or other studies through the use of phr s. Hence, they suggested that more research is needed that addresses the current lack of understanding of optimal functionality and usability of these systems, and how they can play a beneficial role in supporting patient self-management ( Archer et al., 2011 ).
9.3.4. Forms of Aggregative Reviews
Healthcare providers, practitioners, and policy-makers are nowadays overwhelmed with large volumes of information, including research-based evidence from numerous clinical trials and evaluation studies, assessing the effectiveness of health information technologies and interventions ( Ammenwerth & de Keizer, 2004 ; Deshazo et al., 2009 ). It is unrealistic to expect that all these disparate actors will have the time, skills, and necessary resources to identify the available evidence in the area of their expertise and consider it when making decisions. Systematic reviews that involve the rigorous application of scientific strategies aimed at limiting subjectivity and bias (i.e., systematic and random errors) can respond to this challenge.
Systematic reviews attempt to aggregate, appraise, and synthesize in a single source all empirical evidence that meet a set of previously specified eligibility criteria in order to answer a clearly formulated and often narrow research question on a particular topic of interest to support evidence-based practice ( Liberati et al., 2009 ). They adhere closely to explicit scientific principles ( Liberati et al., 2009 ) and rigorous methodological guidelines (Higgins & Green, 2008) aimed at reducing random and systematic errors that can lead to deviations from the truth in results or inferences. The use of explicit methods allows systematic reviews to aggregate a large body of research evidence, assess whether effects or relationships are in the same direction and of the same general magnitude, explain possible inconsistencies between study results, and determine the strength of the overall evidence for every outcome of interest based on the quality of included studies and the general consistency among them ( Cook, Mulrow, & Haynes, 1997 ). The main procedures of a systematic review involve:
- Formulating a review question and developing a search strategy based on explicit inclusion criteria for the identification of eligible studies (usually described in the context of a detailed review protocol).
- Searching for eligible studies using multiple databases and information sources, including grey literature sources, without any language restrictions.
- Selecting studies, extracting data, and assessing risk of bias in a duplicate manner using two independent reviewers to avoid random or systematic errors in the process.
- Analyzing data using quantitative or qualitative methods.
- Presenting results in summary of findings tables.
- Interpreting results and drawing conclusions.
Many systematic reviews, but not all, use statistical methods to combine the results of independent studies into a single quantitative estimate or summary effect size. Known as meta-analyses , these reviews use specific data extraction and statistical techniques (e.g., network, frequentist, or Bayesian meta-analyses) to calculate from each study by outcome of interest an effect size along with a confidence interval that reflects the degree of uncertainty behind the point estimate of effect ( Borenstein, Hedges, Higgins, & Rothstein, 2009 ; Deeks, Higgins, & Altman, 2008 ). Subsequently, they use fixed or random-effects analysis models to combine the results of the included studies, assess statistical heterogeneity, and calculate a weighted average of the effect estimates from the different studies, taking into account their sample sizes. The summary effect size is a value that reflects the average magnitude of the intervention effect for a particular outcome of interest or, more generally, the strength of a relationship between two variables across all studies included in the systematic review. By statistically combining data from multiple studies, meta-analyses can create more precise and reliable estimates of intervention effects than those derived from individual studies alone, when these are examined independently as discrete sources of information.
The review by Gurol-Urganci, de Jongh, Vodopivec-Jamsek, Atun, and Car (2013) on the effects of mobile phone messaging reminders for attendance at healthcare appointments is an illustrative example of a high-quality systematic review with meta-analysis. Missed appointments are a major cause of inefficiency in healthcare delivery with substantial monetary costs to health systems. These authors sought to assess whether mobile phone-based appointment reminders delivered through Short Message Service ( sms ) or Multimedia Messaging Service ( mms ) are effective in improving rates of patient attendance and reducing overall costs. To this end, they conducted a comprehensive search on multiple databases using highly sensitive search strategies without language or publication-type restrictions to identify all rct s that are eligible for inclusion. In order to minimize the risk of omitting eligible studies not captured by the original search, they supplemented all electronic searches with manual screening of trial registers and references contained in the included studies. Study selection, data extraction, and risk of bias assessments were performed independently by two coders using standardized methods to ensure consistency and to eliminate potential errors. Findings from eight rct s involving 6,615 participants were pooled into meta-analyses to calculate the magnitude of effects that mobile text message reminders have on the rate of attendance at healthcare appointments compared to no reminders and phone call reminders.
Meta-analyses are regarded as powerful tools for deriving meaningful conclusions. However, there are situations in which it is neither reasonable nor appropriate to pool studies together using meta-analytic methods simply because there is extensive clinical heterogeneity between the included studies or variation in measurement tools, comparisons, or outcomes of interest. In these cases, systematic reviews can use qualitative synthesis methods such as vote counting, content analysis, classification schemes and tabulations, as an alternative approach to narratively synthesize the results of the independent studies included in the review. This form of review is known as qualitative systematic review.
A rigorous example of one such review in the eHealth domain is presented by Mickan, Atherton, Roberts, Heneghan, and Tilson (2014) on the use of handheld computers by healthcare professionals and their impact on access to information and clinical decision-making. In line with the methodological guidelines for systematic reviews, these authors: (a) developed and registered with prospero ( www.crd.york.ac.uk/ prospero / ) an a priori review protocol; (b) conducted comprehensive searches for eligible studies using multiple databases and other supplementary strategies (e.g., forward searches); and (c) subsequently carried out study selection, data extraction, and risk of bias assessments in a duplicate manner to eliminate potential errors in the review process. Heterogeneity between the included studies in terms of reported outcomes and measures precluded the use of meta-analytic methods. To this end, the authors resorted to using narrative analysis and synthesis to describe the effectiveness of handheld computers on accessing information for clinical knowledge, adherence to safety and clinical quality guidelines, and diagnostic decision-making.
In recent years, the number of systematic reviews in the field of health informatics has increased considerably. Systematic reviews with discordant findings can cause great confusion and make it difficult for decision-makers to interpret the review-level evidence ( Moher, 2013 ). Therefore, there is a growing need for appraisal and synthesis of prior systematic reviews to ensure that decision-making is constantly informed by the best available accumulated evidence. Umbrella reviews , also known as overviews of systematic reviews, are tertiary types of evidence synthesis that aim to accomplish this; that is, they aim to compare and contrast findings from multiple systematic reviews and meta-analyses ( Becker & Oxman, 2008 ). Umbrella reviews generally adhere to the same principles and rigorous methodological guidelines used in systematic reviews. However, the unit of analysis in umbrella reviews is the systematic review rather than the primary study ( Becker & Oxman, 2008 ). Unlike systematic reviews that have a narrow focus of inquiry, umbrella reviews focus on broader research topics for which there are several potential interventions ( Smith, Devane, Begley, & Clarke, 2011 ). A recent umbrella review on the effects of home telemonitoring interventions for patients with heart failure critically appraised, compared, and synthesized evidence from 15 systematic reviews to investigate which types of home telemonitoring technologies and forms of interventions are more effective in reducing mortality and hospital admissions ( Kitsiou, Paré, & Jaana, 2015 ).
9.3.5. Realist Reviews
Realist reviews are theory-driven interpretative reviews developed to inform, enhance, or supplement conventional systematic reviews by making sense of heterogeneous evidence about complex interventions applied in diverse contexts in a way that informs policy decision-making ( Greenhalgh, Wong, Westhorp, & Pawson, 2011 ). They originated from criticisms of positivist systematic reviews which centre on their “simplistic” underlying assumptions ( Oates, 2011 ). As explained above, systematic reviews seek to identify causation. Such logic is appropriate for fields like medicine and education where findings of randomized controlled trials can be aggregated to see whether a new treatment or intervention does improve outcomes. However, many argue that it is not possible to establish such direct causal links between interventions and outcomes in fields such as social policy, management, and information systems where for any intervention there is unlikely to be a regular or consistent outcome ( Oates, 2011 ; Pawson, 2006 ; Rousseau, Manning, & Denyer, 2008 ).
To circumvent these limitations, Pawson, Greenhalgh, Harvey, and Walshe (2005) have proposed a new approach for synthesizing knowledge that seeks to unpack the mechanism of how “complex interventions” work in particular contexts. The basic research question — what works? — which is usually associated with systematic reviews changes to: what is it about this intervention that works, for whom, in what circumstances, in what respects and why? Realist reviews have no particular preference for either quantitative or qualitative evidence. As a theory-building approach, a realist review usually starts by articulating likely underlying mechanisms and then scrutinizes available evidence to find out whether and where these mechanisms are applicable ( Shepperd et al., 2009 ). Primary studies found in the extant literature are viewed as case studies which can test and modify the initial theories ( Rousseau et al., 2008 ).
The main objective pursued in the realist review conducted by Otte-Trojel, de Bont, Rundall, and van de Klundert (2014) was to examine how patient portals contribute to health service delivery and patient outcomes. The specific goals were to investigate how outcomes are produced and, most importantly, how variations in outcomes can be explained. The research team started with an exploratory review of background documents and research studies to identify ways in which patient portals may contribute to health service delivery and patient outcomes. The authors identified six main ways which represent “educated guesses” to be tested against the data in the evaluation studies. These studies were identified through a formal and systematic search in four databases between 2003 and 2013. Two members of the research team selected the articles using a pre-established list of inclusion and exclusion criteria and following a two-step procedure. The authors then extracted data from the selected articles and created several tables, one for each outcome category. They organized information to bring forward those mechanisms where patient portals contribute to outcomes and the variation in outcomes across different contexts.
9.3.6. Critical Reviews
Lastly, critical reviews aim to provide a critical evaluation and interpretive analysis of existing literature on a particular topic of interest to reveal strengths, weaknesses, contradictions, controversies, inconsistencies, and/or other important issues with respect to theories, hypotheses, research methods or results ( Baumeister & Leary, 1997 ; Kirkevold, 1997 ). Unlike other review types, critical reviews attempt to take a reflective account of the research that has been done in a particular area of interest, and assess its credibility by using appraisal instruments or critical interpretive methods. In this way, critical reviews attempt to constructively inform other scholars about the weaknesses of prior research and strengthen knowledge development by giving focus and direction to studies for further improvement ( Kirkevold, 1997 ).
Kitsiou, Paré, and Jaana (2013) provide an example of a critical review that assessed the methodological quality of prior systematic reviews of home telemonitoring studies for chronic patients. The authors conducted a comprehensive search on multiple databases to identify eligible reviews and subsequently used a validated instrument to conduct an in-depth quality appraisal. Results indicate that the majority of systematic reviews in this particular area suffer from important methodological flaws and biases that impair their internal validity and limit their usefulness for clinical and decision-making purposes. To this end, they provide a number of recommendations to strengthen knowledge development towards improving the design and execution of future reviews on home telemonitoring.
9.4. Summary
Table 9.1 outlines the main types of literature reviews that were described in the previous sub-sections and summarizes the main characteristics that distinguish one review type from another. It also includes key references to methodological guidelines and useful sources that can be used by eHealth scholars and researchers for planning and developing reviews.
Typology of Literature Reviews (adapted from Paré et al., 2015).
As shown in Table 9.1 , each review type addresses different kinds of research questions or objectives, which subsequently define and dictate the methods and approaches that need to be used to achieve the overarching goal(s) of the review. For example, in the case of narrative reviews, there is greater flexibility in searching and synthesizing articles ( Green et al., 2006 ). Researchers are often relatively free to use a diversity of approaches to search, identify, and select relevant scientific articles, describe their operational characteristics, present how the individual studies fit together, and formulate conclusions. On the other hand, systematic reviews are characterized by their high level of systematicity, rigour, and use of explicit methods, based on an “a priori” review plan that aims to minimize bias in the analysis and synthesis process (Higgins & Green, 2008). Some reviews are exploratory in nature (e.g., scoping/mapping reviews), whereas others may be conducted to discover patterns (e.g., descriptive reviews) or involve a synthesis approach that may include the critical analysis of prior research ( Paré et al., 2015 ). Hence, in order to select the most appropriate type of review, it is critical to know before embarking on a review project, why the research synthesis is conducted and what type of methods are best aligned with the pursued goals.
9.5. Concluding Remarks
In light of the increased use of evidence-based practice and research generating stronger evidence ( Grady et al., 2011 ; Lyden et al., 2013 ), review articles have become essential tools for summarizing, synthesizing, integrating or critically appraising prior knowledge in the eHealth field. As mentioned earlier, when rigorously conducted review articles represent powerful information sources for eHealth scholars and practitioners looking for state-of-the-art evidence. The typology of literature reviews we used herein will allow eHealth researchers, graduate students and practitioners to gain a better understanding of the similarities and differences between review types.
We must stress that this classification scheme does not privilege any specific type of review as being of higher quality than another ( Paré et al., 2015 ). As explained above, each type of review has its own strengths and limitations. Having said that, we realize that the methodological rigour of any review — be it qualitative, quantitative or mixed — is a critical aspect that should be considered seriously by prospective authors. In the present context, the notion of rigour refers to the reliability and validity of the review process described in section 9.2. For one thing, reliability is related to the reproducibility of the review process and steps, which is facilitated by a comprehensive documentation of the literature search process, extraction, coding and analysis performed in the review. Whether the search is comprehensive or not, whether it involves a methodical approach for data extraction and synthesis or not, it is important that the review documents in an explicit and transparent manner the steps and approach that were used in the process of its development. Next, validity characterizes the degree to which the review process was conducted appropriately. It goes beyond documentation and reflects decisions related to the selection of the sources, the search terms used, the period of time covered, the articles selected in the search, and the application of backward and forward searches ( vom Brocke et al., 2009 ). In short, the rigour of any review article is reflected by the explicitness of its methods (i.e., transparency) and the soundness of the approach used. We refer those interested in the concepts of rigour and quality to the work of Templier and Paré (2015) which offers a detailed set of methodological guidelines for conducting and evaluating various types of review articles.
To conclude, our main objective in this chapter was to demystify the various types of literature reviews that are central to the continuous development of the eHealth field. It is our hope that our descriptive account will serve as a valuable source for those conducting, evaluating or using reviews in this important and growing domain.
- Ammenwerth E., de Keizer N. An inventory of evaluation studies of information technology in health care. Trends in evaluation research, 1982-2002. International Journal of Medical Informatics. 2004; 44 (1):44–56. [ PubMed : 15778794 ]
- Anderson S., Allen P., Peckham S., Goodwin N. Asking the right questions: scoping studies in the commissioning of research on the organisation and delivery of health services. Health Research Policy and Systems. 2008; 6 (7):1–12. [ PMC free article : PMC2500008 ] [ PubMed : 18613961 ] [ CrossRef ]
- Archer N., Fevrier-Thomas U., Lokker C., McKibbon K. A., Straus S.E. Personal health records: a scoping review. Journal of American Medical Informatics Association. 2011; 18 (4):515–522. [ PMC free article : PMC3128401 ] [ PubMed : 21672914 ]
- Arksey H., O’Malley L. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology. 2005; 8 (1):19–32.
- A systematic, tool-supported method for conducting literature reviews in information systems. Paper presented at the Proceedings of the 19th European Conference on Information Systems ( ecis 2011); June 9 to 11; Helsinki, Finland. 2011.
- Baumeister R. F., Leary M.R. Writing narrative literature reviews. Review of General Psychology. 1997; 1 (3):311–320.
- Becker L. A., Oxman A.D. In: Cochrane handbook for systematic reviews of interventions. Higgins J. P. T., Green S., editors. Hoboken, nj : John Wiley & Sons, Ltd; 2008. Overviews of reviews; pp. 607–631.
- Borenstein M., Hedges L., Higgins J., Rothstein H. Introduction to meta-analysis. Hoboken, nj : John Wiley & Sons Inc; 2009.
- Cook D. J., Mulrow C. D., Haynes B. Systematic reviews: Synthesis of best evidence for clinical decisions. Annals of Internal Medicine. 1997; 126 (5):376–380. [ PubMed : 9054282 ]
- Cooper H., Hedges L.V. In: The handbook of research synthesis and meta-analysis. 2nd ed. Cooper H., Hedges L. V., Valentine J. C., editors. New York: Russell Sage Foundation; 2009. Research synthesis as a scientific process; pp. 3–17.
- Cooper H. M. Organizing knowledge syntheses: A taxonomy of literature reviews. Knowledge in Society. 1988; 1 (1):104–126.
- Cronin P., Ryan F., Coughlan M. Undertaking a literature review: a step-by-step approach. British Journal of Nursing. 2008; 17 (1):38–43. [ PubMed : 18399395 ]
- Darlow S., Wen K.Y. Development testing of mobile health interventions for cancer patient self-management: A review. Health Informatics Journal. 2015 (online before print). [ PubMed : 25916831 ] [ CrossRef ]
- Daudt H. M., van Mossel C., Scott S.J. Enhancing the scoping study methodology: a large, inter-professional team’s experience with Arksey and O’Malley’s framework. bmc Medical Research Methodology. 2013; 13 :48. [ PMC free article : PMC3614526 ] [ PubMed : 23522333 ] [ CrossRef ]
- Davies P. The relevance of systematic reviews to educational policy and practice. Oxford Review of Education. 2000; 26 (3-4):365–378.
- Deeks J. J., Higgins J. P. T., Altman D.G. In: Cochrane handbook for systematic reviews of interventions. Higgins J. P. T., Green S., editors. Hoboken, nj : John Wiley & Sons, Ltd; 2008. Analysing data and undertaking meta-analyses; pp. 243–296.
- Deshazo J. P., Lavallie D. L., Wolf F.M. Publication trends in the medical informatics literature: 20 years of “Medical Informatics” in mesh . bmc Medical Informatics and Decision Making. 2009; 9 :7. [ PMC free article : PMC2652453 ] [ PubMed : 19159472 ] [ CrossRef ]
- Dixon-Woods M., Agarwal S., Jones D., Young B., Sutton A. Synthesising qualitative and quantitative evidence: a review of possible methods. Journal of Health Services Research and Policy. 2005; 10 (1):45–53. [ PubMed : 15667704 ]
- Finfgeld-Connett D., Johnson E.D. Literature search strategies for conducting knowledge-building and theory-generating qualitative systematic reviews. Journal of Advanced Nursing. 2013; 69 (1):194–204. [ PMC free article : PMC3424349 ] [ PubMed : 22591030 ]
- Grady B., Myers K. M., Nelson E. L., Belz N., Bennett L., Carnahan L. … Guidelines Working Group. Evidence-based practice for telemental health. Telemedicine Journal and E Health. 2011; 17 (2):131–148. [ PubMed : 21385026 ]
- Green B. N., Johnson C. D., Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. Journal of Chiropractic Medicine. 2006; 5 (3):101–117. [ PMC free article : PMC2647067 ] [ PubMed : 19674681 ]
- Greenhalgh T., Wong G., Westhorp G., Pawson R. Protocol–realist and meta-narrative evidence synthesis: evolving standards ( rameses ). bmc Medical Research Methodology. 2011; 11 :115. [ PMC free article : PMC3173389 ] [ PubMed : 21843376 ]
- Gurol-Urganci I., de Jongh T., Vodopivec-Jamsek V., Atun R., Car J. Mobile phone messaging reminders for attendance at healthcare appointments. Cochrane Database System Review. 2013; 12 cd 007458. [ PMC free article : PMC6485985 ] [ PubMed : 24310741 ] [ CrossRef ]
- Hart C. Doing a literature review: Releasing the social science research imagination. London: SAGE Publications; 1998.
- Higgins J. P. T., Green S., editors. Cochrane handbook for systematic reviews of interventions: Cochrane book series. Hoboken, nj : Wiley-Blackwell; 2008.
- Jesson J., Matheson L., Lacey F.M. Doing your literature review: traditional and systematic techniques. Los Angeles & London: SAGE Publications; 2011.
- King W. R., He J. Understanding the role and methods of meta-analysis in IS research. Communications of the Association for Information Systems. 2005; 16 :1.
- Kirkevold M. Integrative nursing research — an important strategy to further the development of nursing science and nursing practice. Journal of Advanced Nursing. 1997; 25 (5):977–984. [ PubMed : 9147203 ]
- Kitchenham B., Charters S. ebse Technical Report Version 2.3. Keele & Durham. uk : Keele University & University of Durham; 2007. Guidelines for performing systematic literature reviews in software engineering.
- Kitsiou S., Paré G., Jaana M. Systematic reviews and meta-analyses of home telemonitoring interventions for patients with chronic diseases: a critical assessment of their methodological quality. Journal of Medical Internet Research. 2013; 15 (7):e150. [ PMC free article : PMC3785977 ] [ PubMed : 23880072 ]
- Kitsiou S., Paré G., Jaana M. Effects of home telemonitoring interventions on patients with chronic heart failure: an overview of systematic reviews. Journal of Medical Internet Research. 2015; 17 (3):e63. [ PMC free article : PMC4376138 ] [ PubMed : 25768664 ]
- Levac D., Colquhoun H., O’Brien K. K. Scoping studies: advancing the methodology. Implementation Science. 2010; 5 (1):69. [ PMC free article : PMC2954944 ] [ PubMed : 20854677 ]
- Levy Y., Ellis T.J. A systems approach to conduct an effective literature review in support of information systems research. Informing Science. 2006; 9 :181–211.
- Liberati A., Altman D. G., Tetzlaff J., Mulrow C., Gøtzsche P. C., Ioannidis J. P. A. et al. Moher D. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Annals of Internal Medicine. 2009; 151 (4):W-65. [ PubMed : 19622512 ]
- Lyden J. R., Zickmund S. L., Bhargava T. D., Bryce C. L., Conroy M. B., Fischer G. S. et al. McTigue K. M. Implementing health information technology in a patient-centered manner: Patient experiences with an online evidence-based lifestyle intervention. Journal for Healthcare Quality. 2013; 35 (5):47–57. [ PubMed : 24004039 ]
- Mickan S., Atherton H., Roberts N. W., Heneghan C., Tilson J.K. Use of handheld computers in clinical practice: a systematic review. bmc Medical Informatics and Decision Making. 2014; 14 :56. [ PMC free article : PMC4099138 ] [ PubMed : 24998515 ]
- Moher D. The problem of duplicate systematic reviews. British Medical Journal. 2013; 347 (5040) [ PubMed : 23945367 ] [ CrossRef ]
- Montori V. M., Wilczynski N. L., Morgan D., Haynes R. B., Hedges T. Systematic reviews: a cross-sectional study of location and citation counts. bmc Medicine. 2003; 1 :2. [ PMC free article : PMC281591 ] [ PubMed : 14633274 ]
- Mulrow C. D. The medical review article: state of the science. Annals of Internal Medicine. 1987; 106 (3):485–488. [ PubMed : 3813259 ] [ CrossRef ]
- Evidence-based information systems: A decade later. Proceedings of the European Conference on Information Systems ; 2011. Retrieved from http://aisel .aisnet.org/cgi/viewcontent .cgi?article =1221&context =ecis2011 .
- Okoli C., Schabram K. A guide to conducting a systematic literature review of information systems research. ssrn Electronic Journal. 2010
- Otte-Trojel T., de Bont A., Rundall T. G., van de Klundert J. How outcomes are achieved through patient portals: a realist review. Journal of American Medical Informatics Association. 2014; 21 (4):751–757. [ PMC free article : PMC4078283 ] [ PubMed : 24503882 ]
- Paré G., Trudel M.-C., Jaana M., Kitsiou S. Synthesizing information systems knowledge: A typology of literature reviews. Information & Management. 2015; 52 (2):183–199.
- Patsopoulos N. A., Analatos A. A., Ioannidis J.P. A. Relative citation impact of various study designs in the health sciences. Journal of the American Medical Association. 2005; 293 (19):2362–2366. [ PubMed : 15900006 ]
- Paul M. M., Greene C. M., Newton-Dame R., Thorpe L. E., Perlman S. E., McVeigh K. H., Gourevitch M.N. The state of population health surveillance using electronic health records: A narrative review. Population Health Management. 2015; 18 (3):209–216. [ PubMed : 25608033 ]
- Pawson R. Evidence-based policy: a realist perspective. London: SAGE Publications; 2006.
- Pawson R., Greenhalgh T., Harvey G., Walshe K. Realist review—a new method of systematic review designed for complex policy interventions. Journal of Health Services Research & Policy. 2005; 10 (Suppl 1):21–34. [ PubMed : 16053581 ]
- Petersen K., Vakkalanka S., Kuzniarz L. Guidelines for conducting systematic mapping studies in software engineering: An update. Information and Software Technology. 2015; 64 :1–18.
- Petticrew M., Roberts H. Systematic reviews in the social sciences: A practical guide. Malden, ma : Blackwell Publishing Co; 2006.
- Rousseau D. M., Manning J., Denyer D. Evidence in management and organizational science: Assembling the field’s full weight of scientific knowledge through syntheses. The Academy of Management Annals. 2008; 2 (1):475–515.
- Rowe F. What literature review is not: diversity, boundaries and recommendations. European Journal of Information Systems. 2014; 23 (3):241–255.
- Shea B. J., Hamel C., Wells G. A., Bouter L. M., Kristjansson E., Grimshaw J. et al. Boers M. amstar is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. Journal of Clinical Epidemiology. 2009; 62 (10):1013–1020. [ PubMed : 19230606 ]
- Shepperd S., Lewin S., Straus S., Clarke M., Eccles M. P., Fitzpatrick R. et al. Sheikh A. Can we systematically review studies that evaluate complex interventions? PLoS Medicine. 2009; 6 (8):e1000086. [ PMC free article : PMC2717209 ] [ PubMed : 19668360 ]
- Silva B. M., Rodrigues J. J., de la Torre Díez I., López-Coronado M., Saleem K. Mobile-health: A review of current state in 2015. Journal of Biomedical Informatics. 2015; 56 :265–272. [ PubMed : 26071682 ]
- Smith V., Devane D., Begley C., Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. bmc Medical Research Methodology. 2011; 11 (1):15. [ PMC free article : PMC3039637 ] [ PubMed : 21291558 ]
- Sylvester A., Tate M., Johnstone D. Beyond synthesis: re-presenting heterogeneous research literature. Behaviour & Information Technology. 2013; 32 (12):1199–1215.
- Templier M., Paré G. A framework for guiding and evaluating literature reviews. Communications of the Association for Information Systems. 2015; 37 (6):112–137.
- Thomas J., Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. bmc Medical Research Methodology. 2008; 8 (1):45. [ PMC free article : PMC2478656 ] [ PubMed : 18616818 ]
- Reconstructing the giant: on the importance of rigour in documenting the literature search process. Paper presented at the Proceedings of the 17th European Conference on Information Systems ( ecis 2009); Verona, Italy. 2009.
- Webster J., Watson R.T. Analyzing the past to prepare for the future: Writing a literature review. Management Information Systems Quarterly. 2002; 26 (2):11.
- Whitlock E. P., Lin J. S., Chou R., Shekelle P., Robinson K.A. Using existing systematic reviews in complex systematic reviews. Annals of Internal Medicine. 2008; 148 (10):776–782. [ PubMed : 18490690 ]
This publication is licensed under a Creative Commons License, Attribution-Noncommercial 4.0 International License (CC BY-NC 4.0): see https://creativecommons.org/licenses/by-nc/4.0/
- Cite this Page Paré G, Kitsiou S. Chapter 9 Methods for Literature Reviews. In: Lau F, Kuziemsky C, editors. Handbook of eHealth Evaluation: An Evidence-based Approach [Internet]. Victoria (BC): University of Victoria; 2017 Feb 27.
- PDF version of this title (4.5M)
- Disable Glossary Links
In this Page
- Introduction
- Overview of the Literature Review Process and Steps
- Types of Review Articles and Brief Illustrations
- Concluding Remarks
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Recent Activity
- Chapter 9 Methods for Literature Reviews - Handbook of eHealth Evaluation: An Ev... Chapter 9 Methods for Literature Reviews - Handbook of eHealth Evaluation: An Evidence-based Approach
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

VIDEO
COMMENTS
Both systematic and narrative reviews are classified as secondary research studies since they both use existing primary research studies e.g. case studies. Despite this similarity, there are key differences in their methodology and scope. ... Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J ...
Introduction. Narrative reviews are a type of knowledge synthesis grounded in a distinct research tradition. They are often framed as non-systematic, which implies that there is a hierarchy of evidence placing narrative reviews below other review forms. 1 However, narrative reviews are highly useful to medical educators and researchers. While a systematic review often focuses on a narrow ...
Background. A systematic review, as its name suggests, is a systematic way of collecting, evaluating, integrating, and presenting findings from several studies on a specific question or topic.[] A systematic review is a research that, by identifying and combining evidence, is tailored to and answers the research question, based on an assessment of all relevant studies.[2,3] To identify assess ...
A narrative review is the "older" format of the two, presenting a (non-systematic) summation and analysis of available literature on a specific topic of interest. Interestingly, probably because the "approach" is non-systematic, there are no acknowledged formal guidelines for writing narrative reviews.
Our goal with this paper is to conduct a narrative review of the literature about systematic reviews and outline the essential elements of a systematic review along with the limitations of such a review. Keywords: systematic reviews, meta-analysis, narrative literature review, prisma checklist. A literature review provides an important insight ...
The labels Narrative Review and Literature Review are often describing the same type of review. For scientific purposes, the term Literature Review is the one used most often. ... The difference between a Systematic Review and a Narrative Review can be summarized as follows: Good Quality Systematic Reviews: Traditional Narrative Reviews:
Literature reviews establish the foundation of academic inquires. However, in the planning field, we lack rigorous systematic reviews. In this article, through a systematic search on the methodology of literature review, we categorize a typology of literature reviews, discuss steps in conducting a systematic literature review, and provide suggestions on how to enhance rigor in literature ...
The best reviews synthesize studies to draw broad theoretical conclusions about what a literature means, linking theory to evidence and evidence to theory. This guide describes how to plan, conduct, organize, and present a systematic review of quantitative (meta-analysis) or qualitative (narrative review, meta-synthesis) information.
Writing a narrative literature review requires careful planning. This chapter summarizes some key steps in reviewing the literature. First, a team needs to be formed. Second, a topic needs to be chosen. This needs to be relevant to the author's research/teaching interests and a well-defined issue. Third, a thorough search strategy using a ...
Most reviews fall into the following types: literature review, narrative review, integrative review, evidenced based review, meta-analysis and systematic review. This LibGuide will provide you a general overview of the specific review, offer starting points, and outline the reporting process.
A Narrative review is the type first-year college students often learn as a general approach. Its purpose is to identify a few studies that describe a problem of interest. Narrative reviews have no predetermined research question or specified search strategy, only a topic of interest. They are not systematic and follow no specified protocol.
This document aims to assist authors in planning their narrative analysis at protocol stage, and to highlight some issues for authors to consider at review stage. Narrative forms of synthesis are an area of emerging research, and so advice is likely to be adapted as methods develop. This document sits alongside the RevMan templates for ...
A narrative literature review is NOT: Just a summary of sources; A review of everything written on a particular topic; A research paper arguing for a specific viewpoint - a lit review should avoid bias and highlight areas of disagreements; A systematic review; Purposes of a narrative literature review: Explain the background of research on a topic
Narrative reviews are the commonest type of articles in the medical literature. However, unlike systematic reviews and randomized controlled trials (RCT) articles, for which formal instruments exist to evaluate quality, there is currently no instrument available to assess the quality of narrative reviews. In response to this gap, we developed SANRA, the Scale for the Assessment of Narrative ...
Health Sciences Literature Review Made Easy: The Matrix Method, Fifth Edition describes the practical and useful methods for reviewing scientific literature in the health sciences. Please note that an access code to supplemental content such as Appendix C: Data Visualization is not included with the eBook purchase.
The semi-systematic or narrative review approach is designed for topics that have been conceptualized differently and studied by various groups of researchers within diverse disciplines and that hinder a full systematic review process (Wong et al., 2013). That is, to review every single article that could be relevant to the topic is simply not ...
Qualitative, narrative synthesis. Thematic analysis, may include conceptual models. Rapid review. Assessment of what is already known about a policy or practice issue, by using systematic review methods to search and critically appraise existing research. Completeness of searching determined by time constraints.
Table 1 compares systematic and narrative reviews ( Table 1 ). Since the evidence-based medicine is the current trend and also mandatory for establishment of heath policy, the PI should also turn to encourage submission of systematic reviews rather than narrative reviews. Table 1. Comparison between narrative vs systematic review.
A narrative review is a general approach to literature review and is characterized by the lack of a rigid research methodology, such as PRISMA for a systematic review. As a result, it allows the researcher to choose their own approach and perspective for framing the research, i.e. the narrative.
Dec 2015. Rossella Ferrari. Reviews provide a synthesis of published literature on a topic and describe its current state-of-art. Reviews in clinical research are thus useful when designing ...
Results: A total of 82 relevant papers drawn from 960 papers on the subject of wearable devices in health care settings were qualitatively analyzed, and the information was synthesized. Our review shows that the wearable medical devices developed so far have been designed for use on all parts of the human body, including the head, limbs, and torso.
1. BACKGROUND. Cynthia Mulrow's important paper calling for literature reviews to be undertaken more systematically (and hence be more informative and reliable) is now 30 years old.1 A recent paper in BMC Medical Research Methodology compared the proportion of reviews that were systematic (as opposed to narrative) in five leading biomedical journals.2 The authors found significant diversity ...
Drawing on the critical literacy and affordances-in-practice frameworks, we explore the concept of critical social media literacy (CSML) through a systematic literature review to determine whether and how its components—users' goals, use context, inquiry, reflection, and action—have been addressed in the literature.
Parenteral, Non-Intravenous Analgesia in Acute Traumatic Pain—A Narrative Review Based on a Systematic Literature Search. Journal of Clinical Medicine. 2024; 13(9):2560 ... and Robert P. Weenink. 2024. "Parenteral, Non-Intravenous Analgesia in Acute Traumatic Pain—A Narrative Review Based on a Systematic Literature Search" Journal of ...
The narrative review is the "traditional" way of reviewing the extant literature and is skewed towards a qualitative interpretation of prior knowledge ... Keele University & University of Durham; 2007. Guidelines for performing systematic literature reviews in software engineering. Kitsiou S., Paré G., Jaana M. Systematic reviews and meta ...