
Microbe Notes

Lock and Key Model- Mode of Action of Enzymes
Enzymes are biological catalysts. These are commonly proteins but also include RNA (ribozymes) molecules that catalyze chemical reactions by lowering the activation energy of a reaction. These are known to speed up the rate of a reaction millions of times faster than the reaction without enzymes. Nearly all biological reactions require enzymes to transform substrate into products. The substrate is the reactant molecule upon which enzymes act during a chemical reaction, and products are the substances formed as a result of a chemical reaction. A single reactant molecule can decompose to give multiple products. Similarly, two reactants can enter into a reaction to yield products. These are reusable even after the completion of the reaction. Chemical properties such as charge and pH are vital in enzymatic reactions.
Binding between enzymes and reactant molecules takes place in such a way that chemical bond-breaking and bond-forming processes occur more readily. Meanwhile, no change in ∆the G value of a reaction takes place, thereby not altering the energy-releasing or energy-absorbing process of the reaction. However, it lowers the energy of the transition state, the topmost unstable state where the activated complex is formed from reactants that later give products.
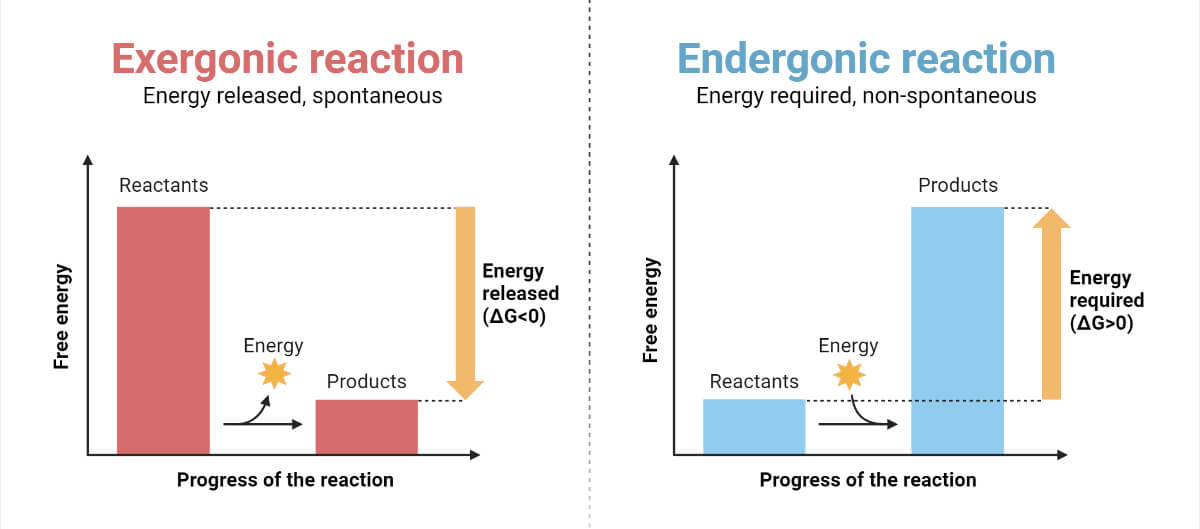
Table of Contents
Interesting Science Videos
Enzyme’s Active site and Substrate Specificity
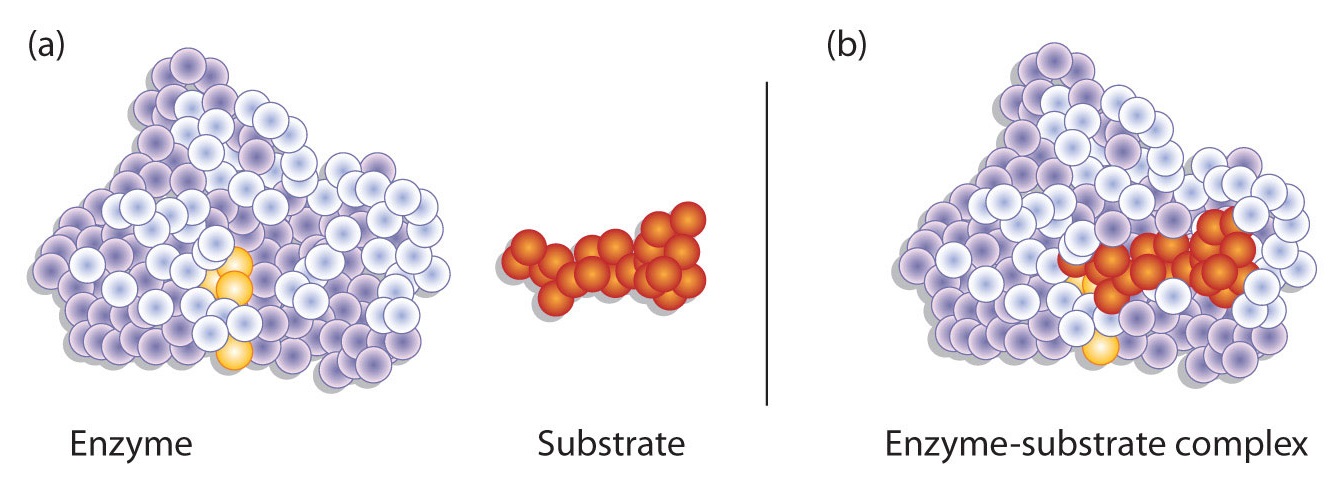
Enzymes are relatively larger than the substrates, whose only a small fraction is involved in catalysis by reducing chemical activation energy, also known as the catalytic site, and the other portion for binding with the substrate and orienting them also known as the binding site. The catalytic site and binding site altogether form the active site of an enzyme. Usually, there are two active sites in an enzyme.
- The active site of enzymes is a cleft portion, composed of a small number of a unique combination of amino acid residues, usually three to four in number, which make up only ~10-20% of the volume of an enzyme.
- The remaining amino acids are used to maintain tertiary structure by proper scaffold folding through non-covalent interactions.
- Non-covalent interaction between enzyme and substrate in correct orientation favors their reaction. These interactions include hydrogen bonds , hydrophobic bonds, ionic interactions, and Van der Waal’s interactions.
- However, transient covalent bonds between enzymes and substrates are also formed during the time of reaction.
- Side chains of amino acids play an important role in highly specific three-dimensional conformation at the level of the active site. These are large or small, hydrophilic or hydrophobic, acidic or basic.
- The specific shape, size, and chemical behavior of enzymes are determined by the nature of amino acids and their 3D space in the active site.
Specificity is a distinctive feature of enzymes where they have a unique ability to choose an exact substrate from a group of similar chemical molecules. Their specificity towards their substrate varies to a different extent. These are of different types, namely: Bond specificity, Group specificity. Substrate specificity, Stereospecificity, Geometrical specificity, and Co-factor specificity.
Substrate specificity is also k/a absolute specificity for the enzyme’s specificity towards one substrate and one reaction. For e.g., Lactase acts on the B-1-4 glycosidic linkage of lactose to yield galactose and glucose. The restrictive nature of enzymes towards the choice of substrate can be attributed to the enzymatic activity of two oxidoreductase enzymes. Alcohol dehydrogenase uses its substrate alcohol while lactic acid dehydrogenase act on lactic acid. Although these two enzymes function with the mechanism of oxidation and reduction reaction, their substrates can’t be used interchangeably. This is because the different structure of each substrate prevents their fitting into the active site of the alternative enzyme.
In most cases, cofactors, the non-protein molecules, are required to ensure an efficient enzyme-facilitated chemical reaction. These function to bind with enzymes via either ionic interaction or covalent interactions. Metal ions (such as minerals) and co-enzymes (vitamin derivatives) are cofactors.
Lock and Key Model
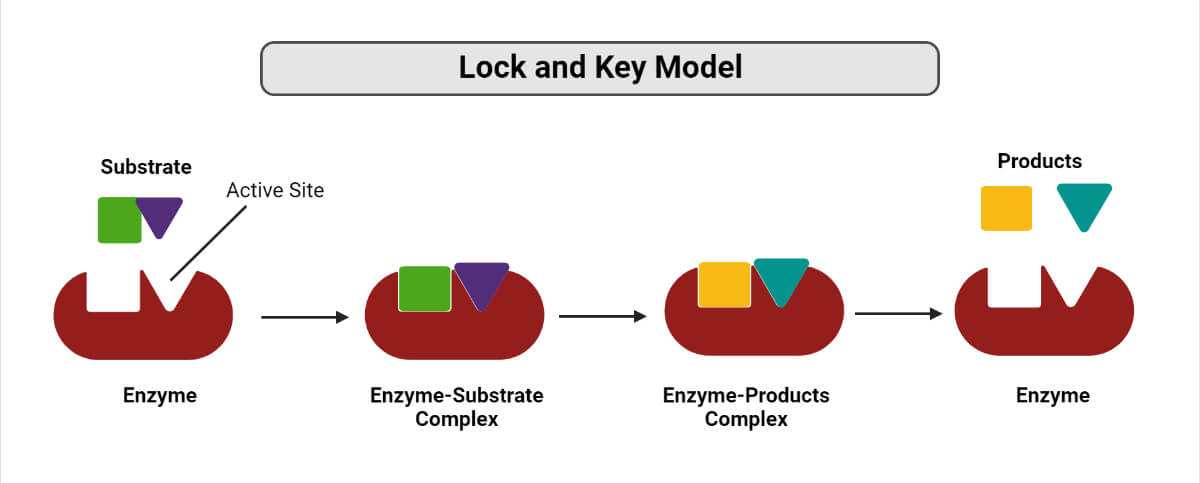
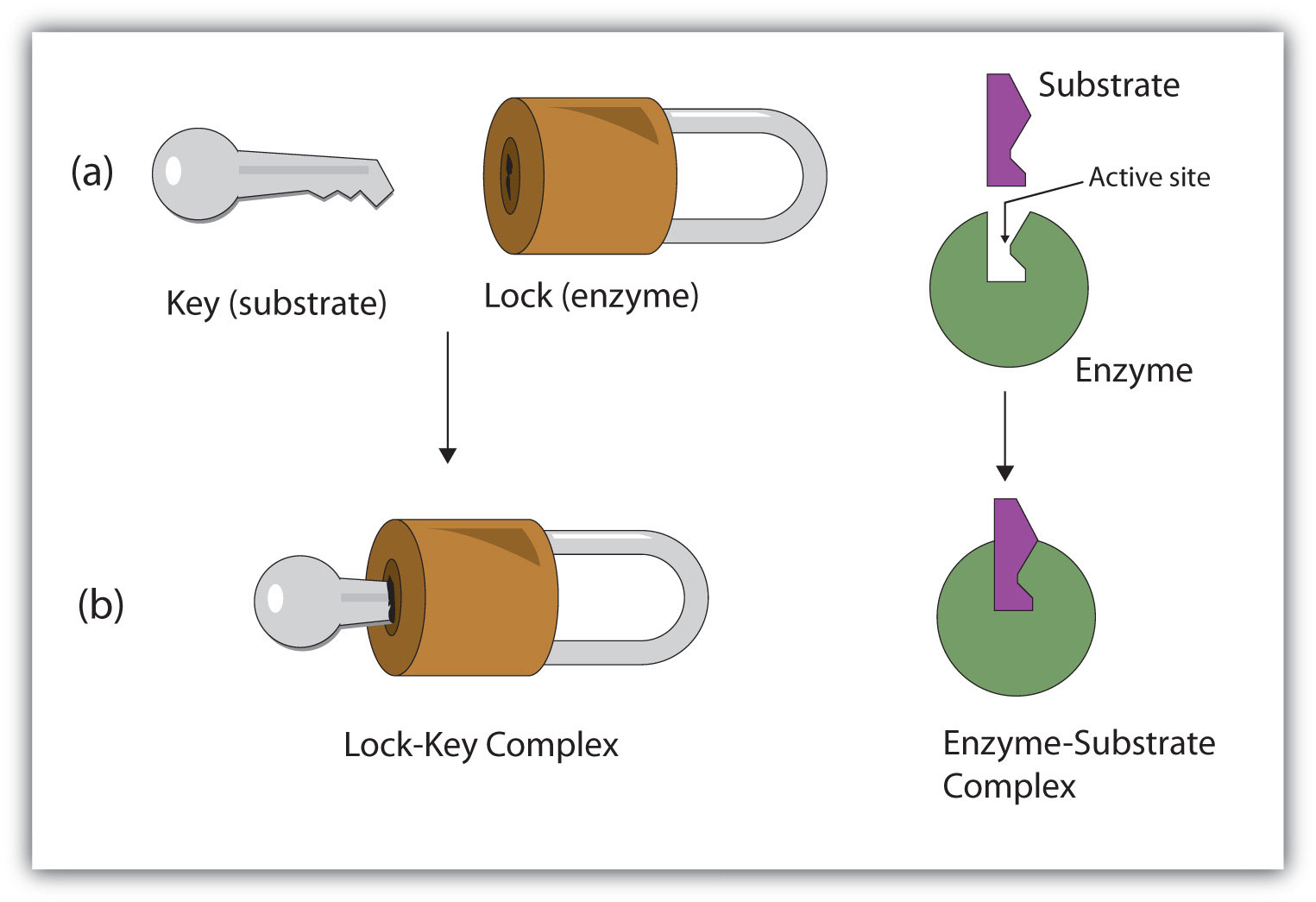
A German scientist, Emil Fischer postulated the lock and key model in 1894 to explain the enzyme’s mode of action. Fischer’s theory hypothesized that enzymes exhibit a high degree of specificity towards the substrate. This model assumes that the active site of the enzyme and the substrate fit perfectly into one another such that each possesses specific predetermined complementary geometric shapes and sizes. Thus, the shape of the enzyme and substrate do not influence each other. This specificity is analog to the lock and key model, where the lock is the enzyme, and the key is the substrate. In certain circumstances, if a second substrate similar in shape and size to the primary substrate is made to bind to the enzyme, this second substrate also fits in the active site too.
How does Lock and Key Model work?
- Binding of the substrate(s) to the enzyme at their active site takes place, thereby forming an enzyme-substrate complex.
- Enzymes catalyze the chemical reaction to take place, which can either be a synthesis reaction (favors bond formation) or a decomposition reaction (favors bond breakage).
- As a result, the formation of one or more products takes place, and the enzymes are released for their reuse in the next reaction.
Limitations of Lock and Key Model
- It doesn’t explain how the enzyme-substrate complex is stabilized in the transition state.
- This model supposes the enzyme is a rigid structure whose shape does not change upon binding with a suitable substrate. However, this is not supported by recent research, which states that there is a change in conformation of the active site of the enzyme upon binding of substrate.
- It does not describe the condition for binding multiple substrates to the enzyme.
Later, it was found that enzyme specificity toward one substrate is not always true. Although there are enzymes that specifically bind with only one substrate, there are also enzymes that exhibit broad specificity towards different but similarly structured substrates, such as lipase that can bind to different types of lipids. Similarly, proteases such as trypsin and chymotrypsin can degrade multiple types of proteins. Thus, the lock and key model is flawed, and the induced fit model was introduced to give a more refined view of the mode of enzymatic action.
- Blanco, A., & Blanco, G. (2017). Medical Biochemistry. Academic Press. https://www.khanacademy.org/science/ap-biology/cellular-energetics/enzyme-structure-and-catalysis/a/enzymes-and-the-active-site
- https://www.biologyonline.com/dictionary/substrate-specificity
- https://www.britannica.com/science/protein/The-mechanism-of-enzymatic-action
- https://www.biologyonline.com/dictionary/lock-and-key-model
- https://study.com/learn/lesson/lock-key-model-vs-induced-fit-model.html
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(McMurry)/26%3A_Biomolecules-_Amino_Acids_Peptides_and_Proteins/26.11%3A_Enzymes_and_Coenzymes
- https://en.wikibooks.org/wiki/Structural_Biochemistry/Protein_function/Lock_and_Key
- https://ib.bioninja.com.au/higher-level/topic-8-metabolism-cell/untitled-6/models-of-action.html
About Author
Prakriti Karki
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .

- My presentations
Auth with social network:
Download presentation
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you!
Presentation is loading. Please wait.
From lock and key model to induced fit model
Published by Modified over 5 years ago
Similar presentations
Presentation on theme: "From lock and key model to induced fit model"— Presentation transcript:
Enzymes … what do you know and will find out these next few lessons Lets find out millionaire styley remember the tips from “Mrs B how to answer MCQ”

WHAT IS THE NATURE OF SCIENCE?

Big Idea 2 : The Characteristics of Scientific Knowledge Description A: Scientific knowledge is based on empirical evidence, and is appropriate for understanding.

Chapter 13 Science and Hypothesis. Modern science has had a profound impact on our lives— mostly for the better. The laws and principles of science.

Scientific methodology is the heart of science. The full body of science includes more, as shown:

WHAT IS THE NATURE OF SCIENCE?. SCIENTIFIC WORLD VIEW 1.The Universe Is Understandable. 2.The Universe Is a Vast Single System In Which the Basic Rules.

What do we cover in section C?. Unit 4 research methods Explain the key features of scientific investigation and discuss whether psychology can be defined.

Enzymes Essential Questions: What is an enzyme? How do enzymes work? What are the properties of enzymes? How do they maintain homeostasis for the body?

The Sciences Natural and Human (Social) Sciences as Areas of Knowledge

WHAT IS THE NATURE OF SCIENCE?. THEORIES ARE THE SCIENTIFIC WORLD VIEW 1.The Universe Is Understandable. 2.The Universe Is a Vast Single System In Which.

False Assumptions 2012/03/25/false-assumptions-lesson/

Enzymes. What are they? Globular Proteins: This is important in explaining how heat can denature them – think tertiary structure Biological catalysts:

Enzymes and How They Work. Enzymes Enzymes are proteins. They are Biological catalysts (speed up the rate of reactions in living things without themselves.

Hypothesis-Based Science The Scientific Method. Science as Inquiry The process of investigation to answer questions about the natural world.
Hydrogen Peroxide Water + Oxygen

Virginia Standard of Learning BIO.1a-m

Section 1: Scientific Methods

Section 2: Scientific Methods

About project
© 2024 SlidePlayer.com Inc. All rights reserved.
Lock-and-key model
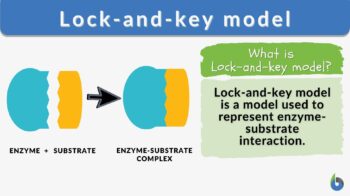
strong>Lock-and-key model n., [lɑk ænd ki ˈmɑdl̩] Definition: a model for enzyme-substrate interaction
Table of Contents
Lock-and-key model Definition
Lock-and-key model is a model for enzyme-substrate interaction suggesting that the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another. In this model, enzymes are depicted as highly specific. They must bind to specific substrates before they catalyze chemical reactions . The term is a pivotal concept in enzymology to elucidate the intricate interaction between enzymes and substrates at the molecular level. In the lock-and-key model, the enzyme-substrate interaction suggests that the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another. Like a key into a lock , only the correct size and shape of the substrate ( the key ) would fit into the active site ( the keyhole ) of the enzyme ( the lock ).
Compare: Induced fit model See also: enzyme , active site , substrate
Lock-and-key vs. Induced Fit Model
At present, two models attempt to explain enzyme-substrate specificity; one of which is the lock-and-key model , and the other is the Induced fit model . The lock and key model theory was first postulated by Emil Fischer in 1894. The lock-and-key enzyme action proposes the high specificity of enzymes. However, it does not explain the stabilization of the transition state that the enzymes achieve. The induced fit model (proposed by Daniel Koshland in 1958) suggests that the active site continues to change until the substrate is completely bound to the active site of the enzyme, at which point the final shape and charge are determined. Unlike the lock-and-key model, the induced fit model shows that enzymes are rather flexible structures. Nevertheless, Fischer’s Lock and Key theory laid an important foundation for subsequent research, such as during the refinement of the enzyme-substrate complex mechanism, as ascribed in the induced fit model. The lock-and-key hypothesis has opened ideas where enzyme action is not merely catalytic but incorporates a rather complex process in how they interact with the correct substrates with precision.
Key Components
Components of the lock and key model:
- Enzyme : the enzyme structure is a three-dimensional protein configuration, with an active site from where the substrate binds.
- Substrate : often an organic molecule, a substrate possesses a structural feature that complements the geometry of the enzyme’s active site.
In the lock and key model, both the enzymes and the substrates facilitate the formation of a complex that lowers the activation energy needed for a chemical transformation to occur. Such reduction in the activation energy allows the chemical reaction to proceed at a relatively faster rate, making enzymes crucial in various biological and molecular processes.
Lock-and-key Model Examples
Some of the common examples that are often discussed in the context of the Lock and Key Model are as follows:
- Enzyme lactate dehydrogenase with a specific active site for its substrates, pyruvate and lactate. The complex facilitates the interconversion of pyruvate and lactate during anaerobic respiration
- Enzyme carbonic anhydrase with a specific active site for the substrates carbon dioxide and water. The complex facilitates the hydration of carbon dioxide, forming bicarbonate
- Enzyme lysozyme binding with a bacterial cell wall peptidoglycan, which is a vital immune function
Choose the best answer.
Send Your Results (Optional)

- Aryal, S. and Karki, P. (2023). “Lock and Key Model- Mode of Action of Enzymes”. Microbenotes.com. https://microbenotes.com/lock-and-key-model-mode-of-action-of-enzymes/
- Farhana, A., & Lappin, S. L. (2023, May). Biochemistry, Lactate Dehydrogenase . Nih.gov; StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK557536/
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.
Last updated on January 11th, 2024
You will also like...
Cell Biology
The cell is defined as the fundamental, functional unit of life. Some organisms are comprised of only one cell whereas o..

Running Water Freshwater Communities
This tutorial introduces flowing water communities, which bring new and dithering factors into the equation for possible..

Plant Water Regulation
Plants need to regulate water in order to stay upright and structurally stable. Find out the different evolutionary adap..

Indicator Species and Endangered Species
Certain species are capable of expressing characteristics indicative of the state of the ecosystem they occupy. They are..

Seed Plants
Seed plants are vascular plants. They differ from the other vascular plants in producing seeds that germinate into a new..

Community Patterns
Learn about community patterns and the ecological factors influencing these patterns. Revisit some of the ecosystems you..
Related Articles...
No related articles found

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

4.7: Enzyme Action
- Last updated
- Save as PDF
- Page ID 178782
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
- To describe the interaction between an enzyme and its substrate.
Enzyme-catalyzed reactions occur in at least two steps. In the first step, an enzyme molecule (E) and the substrate molecule or molecules (S) collide and react to form an intermediate compound called the enzyme-substrate (E–S) complex . (This step is reversible because the complex can break apart into the original substrate or substrates and the free enzyme.) Once the E–S complex forms, the enzyme is able to catalyze the formation of product (P), which is then released from the enzyme surface:
\[S + E \rightarrow E–S \tag{\(\PageIndex{1}\)}\]
\[E–S \rightarrow P + E \tag{\(\PageIndex{2}\)}\]
Hydrogen bonding and other electrostatic interactions hold the enzyme and substrate together in the complex. The structural features or functional groups on the enzyme that participate in these interactions are located in a cleft or pocket on the enzyme surface. This pocket, where the enzyme combines with the substrate and transforms the substrate to product is called the active site of the enzyme (Figure \(\PageIndex{1}\)).
The active site of an enzyme possesses a unique conformation (including correctly positioned bonding groups) that is complementary to the structure of the substrate, so that the enzyme and substrate molecules fit together in much the same manner as a key fits into a tumbler lock. In fact, an early model describing the formation of the enzyme-substrate complex was called the lock-and-key model (Figure \(\PageIndex{2}\)). This model portrayed the enzyme as conformationally rigid and able to bond only to substrates that exactly fit the active site.
Working out the precise three-dimensional structures of numerous enzymes has enabled chemists to refine the original lock-and-key model of enzyme actions. They discovered that the binding of a substrate often leads to a large conformational change in the enzyme, as well as to changes in the structure of the substrate or substrates. The current theory, known as the induced-fit model , says that enzymes can undergo a change in conformation when they bind substrate molecules, and the active site has a shape complementary to that of the substrate only after the substrate is bound, as shown for hexokinase in Figure \(\PageIndex{3}\). After catalysis, the enzyme resumes its original structure.
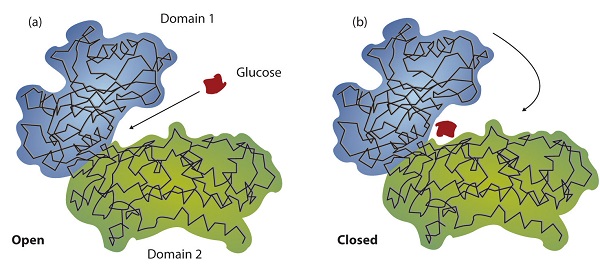
The structural changes that occur when an enzyme and a substrate join together bring specific parts of a substrate into alignment with specific parts of the enzyme’s active site. Amino acid side chains in or near the binding site can then act as acid or base catalysts, provide binding sites for the transfer of functional groups from one substrate to another or aid in the rearrangement of a substrate. The participating amino acids, which are usually widely separated in the primary sequence of the protein, are brought close together in the active site as a result of the folding and bending of the polypeptide chain or chains when the protein acquires its tertiary and quaternary structure. Binding to enzymes brings reactants close to each other and aligns them properly, which has the same effect as increasing the concentration of the reacting compounds.
Example \(\PageIndex{1}\)
- What type of interaction would occur between an OH group present on a substrate molecule and a functional group in the active site of an enzyme?
- Suggest an amino acid whose side chain might be in the active site of an enzyme and form the type of interaction you just identified.
- An OH group would most likely engage in hydrogen bonding with an appropriate functional group present in the active site of an enzyme.
- Several amino acid side chains would be able to engage in hydrogen bonding with an OH group. One example would be asparagine, which has an amide functional group.
Exercise \(\PageIndex{1}\)
- What type of interaction would occur between an COO − group present on a substrate molecule and a functional group in the active site of an enzyme?
One characteristic that distinguishes an enzyme from all other types of catalysts is its substrate specificity . An inorganic acid such as sulfuric acid can be used to increase the reaction rates of many different reactions, such as the hydrolysis of disaccharides, polysaccharides, lipids, and proteins, with complete impartiality. In contrast, enzymes are much more specific. Some enzymes act on a single substrate, while other enzymes act on any of a group of related molecules containing a similar functional group or chemical bond. Some enzymes even distinguish between D- and L-stereoisomers, binding one stereoisomer but not the other. Urease, for example, is an enzyme that catalyzes the hydrolysis of a single substrate—urea—but not the closely related compounds methyl urea, thiourea, or biuret. The enzyme carboxypeptidase, on the other hand, is far less specific. It catalyzes the removal of nearly any amino acid from the carboxyl end of any peptide or protein.
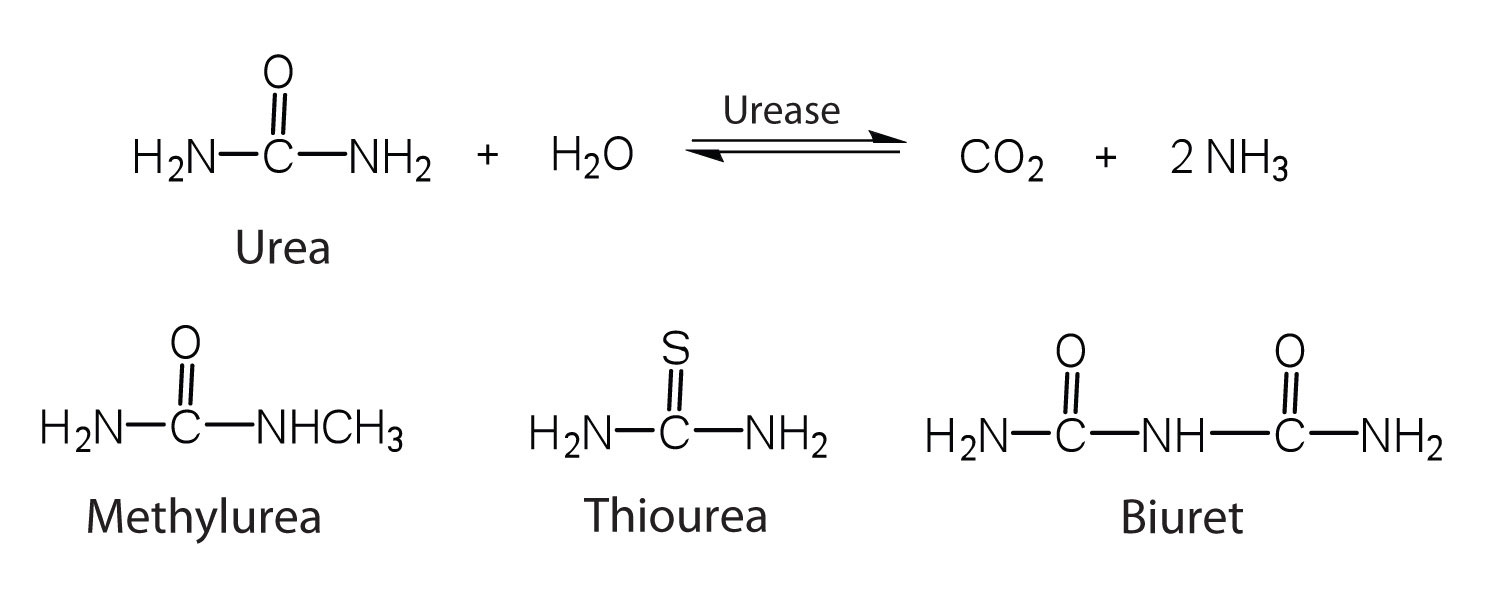
Enzyme specificity results from the uniqueness of the active site in each different enzyme because of the identity, charge, and spatial orientation of the functional groups located there. It regulates cell chemistry so that the proper reactions occur in the proper place at the proper time. Clearly, it is crucial to the proper functioning of the living cell.
A substrate binds to a specific region on an enzyme known as the active site, where the substrate can be converted to product. The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. The induced-fit model says that an enzyme can undergo a conformational change when binding a substrate. Enzymes exhibit varying degrees of substrate specificity.
Concept Review Exercises
- Distinguish between the lock-and-key model and induced-fit model of enzyme action.
- Which enzyme has greater specificity—urease or carboxypeptidase? Explain.
- The lock-and-key model portrays an enzyme as conformationally rigid and able to bond only to substrates that exactly fit the active site. The induced fit model portrays the enzyme structure as more flexible and is complementary to the substrate only after the substrate is bound.
- Urease has the greater specificity because it can bind only to a single substrate. Carboxypeptidase, on the other hand, can catalyze the removal of nearly any amino acid from the carboxyl end of a peptide or protein.
What type of interaction would occur between each group present on a substrate molecule and a functional group of the active site in an enzyme?
- CH(CH 3 ) 2
- COO −
For each functional group in Exercise 1, suggest an amino acid whose side chain might be in the active site of an enzyme and form the type of interaction you identified.
For each functional group in Exercise 2, suggest an amino acid whose side chain might be in the active site of an enzyme and form the type of interaction you identified.
- hydrogen bonding
- ionic bonding
- dispersion forces
- The amino acid has a polar side chain capable of engaging in hydrogen bonding; serine (answers will vary).
- The amino acid has a negatively charged side chain; aspartic acid (answers will vary).
- The amino acid has a polar side chain capable of engaging in hydrogen bonding; asparagine (answers will vary).
- The amino acid has a nonpolar side chain; isoleucine (answers will vary).
Skip to content

Get Revising
Join get revising, already a member.

Enzymes: Theory Of Lock and Key Powerpoint
A quick powerpoint that covers the basics of the Lock an Key Theory, for b4, OCR Twenty First Century Science. Good Luck! n_n
- Created by: Black Wolf
- Created on: 23-12-12 16:19
- Enzymes and digestion Enzymes and digestion
No comments have yet been made
Similar Biology resources:
B3- ENZYMES 0.0 / 5
Biology: Enzymes 0.0 / 5
LOCK AND KEY HYPOTHESIS 0.0 / 5
Chemical Properties of Enzymes 0.0 / 5
Enzymes in the human digestive system 0.0 / 5
Biology: Year 9 Term 3: Enzymes 0.0 / 5
Enzymes and Other Proteins 3.0 / 5 based on 1 rating
Enzymes 0.0 / 5
Enzymes Revision 0.0 / 5
GCSE Science Enzymes 2.0 / 5 based on 1 rating
Related discussions on The Student Room
- A level biology AQA »
- Media studies (a-level) »
- How do you revise A level Sociology? »
- How to take notes »
- What are effective note-taking methods for lectures? »
- CIE Biology A Level »
- Bio a level help - end product inhibition »
- How do you get A's in Biology A levels »
- enzymes examples urgent !! »
- OCR PAG help??? »
Lock and Key Model | Overview & Examples
Liz has taught high school science for over ten years. She has a BA in Biology from Lafayette College and a MA in Education from Gratz College. She is certified to teach biology in New Jersey and Pennsylvania. Relevant experience includes teaching high school biology and physics.
Chris has a master's degree in history and teaches at the University of Northern Colorado.
Table of Contents
Enzymes and the lock and key theory, lock and key model, induced fit model vs. lock and key, examples of the lock and key model, lesson summary, who proposed the lock and key model of enzyme action.
Emil Fischer proposed the Lock and Key model of enzyme action in 1899. The Lock and Key model explains that enzymes are specially shaped to fit one specific type of substrate.
Do enzymes work in a lock and key fashion?
Enzymes do generally work in a lock and key fashion in terms of the enzyme representing a "lock" and the substrate representing the "key." The substrate fits an enzyme's active site similar to how a key fits in a specific lock. However, a more accurate explanation is that an enzyme changes shape slightly to form a precise fit against any similarly shaped substrate.
What is the Lock & Key model and how does it relate to enzymes?
The lock and key model is a theory of enzyme action that explains how enzymes fit their substrate. The active site of an enzyme is structured to fit a specifically shaped substrate. Once the substrate binds to the active site, the enzyme will facilitate the reaction and release products of the reaction. A substrate is specific to one type of enzyme, similar to how a key is specific to one type of lock.
Why is it called the Lock and Key model?
The Lock and Key model of enzyme action is described as such because it explains that a substrate will fit a specific enzyme, similar to how a key fits a specific lock.
Enzymes are a type of protein that serve as catalysts to chemical reactions . Proteins are large molecules that serve multiple functions in organisms, such as composing important structures. A catalyst is any substance that speeds the rate of a chemical reaction. Enzymes do this by lowering the activation energy of a reaction. Activation energy is the energy needed to start a chemical reaction. When the activation energy is lowered, the reaction is able to start or activate sooner. Without enzymes, the chemical reactions in living things would take too long to occur naturally. There are several reasons why enzymes are able to speed up reactions:
- Enzymes are structured to bond with the substrate of a given reaction. A substrate is a substance that will attach to an enzyme and undergo a reaction.
- Enzymes can position molecules into the correct orientation to react together. It takes much longer for this to happen by random chance.
- Enzymes have chemical properties, such as charge and pH, that are ideal for the reaction to take place.
Each type of enzyme is uniquely structured to facilitate one specific reaction. This means that for every catalyzed reaction, there is one type of enzyme that facilitates it. The specifically shaped region that a substrate binds to is called the active site . Once the substrate binds to the active site, the chemical reaction will be initiated. The result of the reaction is called the product and will be a new substance or multiple substances if the substrate is broken down in the reaction. Enzymes are reusable after completing a reaction. This is known as the lock and key model of enzyme action and is discussed below. In some circumstances, if a second substrate is shaped similarly enough to the primary substrate that is supposed to bind to an enzyme, the second substrate may fit in the active site, too.
To unlock this lesson you must be a Study.com Member. Create your account

An error occurred trying to load this video.
Try refreshing the page, or contact customer support.
You must c C reate an account to continue watching
Register to view this lesson.
As a member, you'll also get unlimited access to over 88,000 lessons in math, English, science, history, and more. Plus, get practice tests, quizzes, and personalized coaching to help you succeed.
Get unlimited access to over 88,000 lessons.
Already registered? Log in here for access
Resources created by teachers for teachers.
I would definitely recommend Study.com to my colleagues. It’s like a teacher waved a magic wand and did the work for me. I feel like it’s a lifeline.
You're on a roll. Keep up the good work!
Just checking in. are you still watching.
- 0:01 Enzymes
- 1:20 How Enzymes Work
- 3:04 Lock and Key Hypothesis
- 3:44 Induced Fit Model
- 5:21 Lesson Summary
The Lock and Key model is a theory of enzyme action hypothesized by Emil Fischer in 1899. According to Fischer, enzymes exhibit a high degree of specificity to the substances they react with. He proposed that this is due to the shape of the enzyme fitting the shape of the substrate, similar to how a lock only fits a specific key. Using this analogy, the enzyme represents the lock and the substrate represents the key. The order in which the lock and key model works is as follows:
- The substrate(s) bind to the enzyme at the active site, forming an enzyme-substrate complex .
- The enzyme facilitates the chemical reaction. This reaction can be a synthesis reaction (building by forming bonds) or digestion (breaking bonds to form new substances).
- The enzyme releases the product(s) of the reaction. Once an enzyme completes a reaction, it can be reused.
It was later discovered that enzymes do not always show complete specificity to only one type of substrate. In this regard, the Lock and Key model is flawed. Even though many enzymes may react with only one substance, there are also many enzymes that are able to facilitate the same chemical reaction with different, although similarly structured, substrates. For example, there are several protein-digesting enzymes in the stomach that can break down multiple types of protein. These enzymes include pepsin and chymotrypsin . Synthetic substances that are similarly shaped like the natural substrate will also bind and react with enzymes.
A more accurate description of enzyme structure is the Induced Fit model of enzyme action . The Induced Fit model was proposed by Daniel Koshland in 1958. According to Koshland's hypothesis, the active site is shaped similarly enough and has specific chemical properties that attract a substrate to bind. Once the substrate binds, the active site is induced, or prompted, to change shape. This results in a more precise fit. The Induced Fit model better explains the phenomenon of more than one type of substrate binding and reacting to an enzyme when similarly structured.
The Induced Fit model is similar to the Lock and Key model. The order in which a reaction takes place is the same:
- A substrate binds to an enzyme, forming an enzyme-substrate complex.
- The enzyme facilitates the chemical reaction.
- The product(s) of the chemical reaction are released.
The main difference between the two models is how the active site reacts to the binding substrate. The Induced Fit model further details that the active site changes shape to better fit the substrate. This differs from the Lock and Key model, which proposes that the active site already matches the shape of a substrate before it binds.
Enzymes are able to speed up the rate of reactions by providing ideal conditions for the reaction to take place, such as pH, and by positioning molecules into the correct orientation to react. The molecules may react eventually in nature but providing the ideal conditions and removing random chance allows reactions to happen at a rate that can meet the needs of an organism.
The Lock and Key model was the first proposal on how enzymes work. Since enzymes seemed to conduct only one specific reaction, it was hypothesized that the Lock and Key mechanism was the most likely explanation. According to this theory, the active site of an enzyme is shaped to fit only one type of substrate, which it will then react with. This model did not explain how some enzymes are able to perform the same reaction with different, but similarly shaped, substrates. This phenomenon is explained by the induced fit model of enzyme action, in which the enzyme is able to change shape to better fit a substrate once it bonds to the active site.
For example, the active site of the enzyme amylase is specifically shaped to fit the large carbohydrate starch. Amylase is found in saliva, allowing the digestion of starch to begin in the mouth. The specific reaction conducted by amylase is the breakdown of starch into smaller sugar molecules called maltose. Maltose is composed of two glucose molecules bonded together; however, this is the smallest unit that amylase can break starch into. In order to further break down maltose, a different enzyme that is shaped to fit will conduct this separate reaction later during digestion.
Enzymes are molecules that catalyze , or speed up, the rate of chemical reactions in living things. Enzymes may break down molecules or bond molecules together depending on the type of chemical reaction. Chemical reactions happen faster when enzymes lower the activation energy , which is the amount of energy needed to start a reaction. Enzymes are able to accomplish this since they are structured to fit specific substrates , can position molecules into the correct orientation to react, and provide ideal conditions for a reaction to occur. Two different models of enzyme action have been proposed over time. Emil Fischer proposed the Lock and Key model in 1899, and Daniel Koshland proposed the Induced Fit model of enzyme action in 1958. Both models propose that enzymes are specific, yet while the Lock and Key model proposes that enzymes can only bond with one specific substrate, the Induced Fit model proposes that enzymes are able to change shape when bonded to a substrate. The Induced Fit model helps explain how many enzymes fit different substrates that are similarly shaped.
Video Transcript
See this piece of wood? If I place it here on the ground and leave it alone, eventually it will completely disintegrate until nothing but the basic carbon molecules are left. But, this could take a very, very long time, and I am rather impatient. So instead of waiting, I'll just set it on fire and achieve the same result in a much shorter time period. Apart from satisfying my pyromania, do you see what I did there? I used fire as a catalyst , which is just a substance to increase the rate of a chemical reaction. The wood would have disintegrated in time, but the fire sped up that process.
Well, as it turns out, nature is just as impatient as I am. These sorts of catalysts are all around us, and even within us. Nearly all cells contain large molecules that catalyze chemical reactions within the cell, called enzymes . Enzymes are important. The cell could wait for nutrients to dissolve into energy, but frankly the cell could die waiting for this to happen. Enzymes speed up the process. Sometimes it pays to be a little impatient.
How Enzymes Work
We know that enzymes are catalysts, but how exactly do they work? Well, this here is glucosidase, an enzyme that processes specific kinds of sugars. It starts when the cell ingests a complex sugar molecule, like this one here (see video). The complex molecule in this process is called the substrate . Over time, this complex sugar molecule will decompose into smaller molecules called products . In this case, the complex sugar becomes individual glucose molecules, which is a much simpler compound that the cell can use for energy. But, the cell can't just wait for the substrate to naturally dissolve into the products, so that's where the enzyme comes in. Glucosidase turns the substrate, the complex sugar, into two smaller products, simple glucose molecules.
See how that works? Now, it's important to note that this specific reaction can only be produced by glucosidase. Another enzyme cannot break complex sugars into glucose, and glucosidase cannot break down other substrates. Each chemical reaction can only be catalyzed by a specific kind of enzyme. Considering that there are roughly 5,000 chemical reactions that enzymes are known to catalyze, we've got a lot of enzymes with very specific focuses. So how do they know which molecules they can catalyze and which ones they can't? After all, real enzymes don't actually have hands and eyes like our cute little cartoons here. This is a major scientific debate, and there are two leading theories to explain this complex, but very important, process.
The Lock and Key Hypothesis
Scientists have long wondered exactly how enzymes know which substrates to process and which to ignore. In 1894, German chemist Emil Fischer proposed the lock and key theory , which states that enzymes have a specific shape that directly correlates to the shape of the substrate. Basically, substrates fit into an enzyme the way a key fits into a lock. If the substrate is not the correct shape, it won't fit into the enzyme, and no chemical reaction can occur. Only those substrates that exactly fit into the enzyme can be catalyzed.
The Induced Fit Model
As scientific technology improved, researchers began to notice a small problem with the lock and key theory. Enzymes don't actually maintain a rigid shape; they change slightly to accommodate their substrate. This means that enzymes aren't precisely shaped to be exact fits for their substrates, and in 1958, another scientist named Daniel Koshland proposed a modification to the lock and key theory. This new theory, called the induced fit model , states that enzymes are partially flexible and that the active site of the enzyme will reshape to fit the substrate. So if the active site can change shape, how does the enzyme know which substrates it can process? Well, the enzyme itself is still only partially flexible. The active site can change a bit to accommodate the correct substrate, but if it changes too much, then the enzyme can't function. The basic theory behind the lock and key model, the idea that substrates have to fit the enzyme, is still the same, but in the induced fit model the active site is simply less rigid. You can reshape it enough to accommodate variations in the individual substrate but not enough to fit an entirely new substrate. Why does the enzyme need to be more flexible? Maybe it was just too impatient to try and create a perfect fit every time. After all, the enzyme's job is to get it done quickly. Turns out, not even our cells like to wait.
While cells could ingest molecules and wait for them to naturally dissolve, this process could take way too long, so instead cells use a catalyst , a substance to increase the rate of chemical reaction. This catalyst is called an enzyme , a molecule that catalyzes chemical reactions within a cell. Each enzyme can only catalyze specific complex molecules, called substrates , so scientists have often wondered how enzymes recognize substrates. In 1894, Emil Fischer proposed the lock and key theory , which states that enzymes have a specific shape that directly correlates to the shape of the substrate. This model was accepted for a long time, until scientists noticed that enzymes were not actually so rigid. Then, in 1958, Daniel Koshland modified the lock and key theory with the induced fit model , claiming that enzymes are partially flexible and that the active site of the enzyme will reshape to fit the substrate. However, this model still acknowledged that only specific substrates could fit into the active site, which would reject substrates of dramatically different shapes. This theory explains how our cells speed up the process of chemical reactions. Turns out, our cells are just as impatient as we are.
Unlock Your Education
See for yourself why 30 million people use study.com, become a study.com member and start learning now..
Already a member? Log In
Recommended Lessons and Courses for You
Related lessons, related courses, recommended lessons for you.

Lock and Key Model | Overview & Examples Related Study Materials
- Related Topics
Browse by Courses
- Praxis Economics (5911) Prep
- FTCE School Psychologist PK-12 (036) Prep
- ILTS School Psychologist (237) Prep
- SAT Subject Test Chemistry: Practice and Study Guide
- SAT Subject Test US History: Practice and Study Guide
- ILTS English Language Arts (207): Test Practice and Study Guide
- Study.com SAT Reading Test Section: Review & Practice
- CSET Business Subtest III Prep
- GED Reasoning Through Language Arts
- NY Regents Exam - US History and Government: Tutoring Solution
- Study.com ACT® Science Test Section: Prep & Practice
- Study.com ACT® Writing Test Practice
- FTCE Marketing 6-12 (057) Prep
- GED Study Guide
- Study.com ACT® Math Test Section: Review & Practice
Browse by Lessons
- Enzymes Definition, Function & Uses
- Function of Enzymes | Overview, Diagram & Active Site
- Enzyme Substrate Complex | Definition, Product & Diagram
- Holoenzyme Overview, Functions & Examples
- Enzyme Action | Mechanisms, Models & Example
- The Function of Enzymes
- Enzymes Lesson for Kids
- Application of Chemistry & Enzymes in the Textile Industry
- Enzymes in Biological Reactions: Roles & Functions
- Structural & Conditional Factors that Impact Enzyme Activity
- Enzyme Function, Interactions & Regulation Activities for High School
- Emile Zola | Life, Books & Legacy
- John Webster: Biography & Plays
- Barbara Kingsolver | Novels & Books in Order
- John Dos Passos: Biography & Books
Create an account to start this course today Used by over 30 million students worldwide Create an account
Explore our library of over 88,000 lessons
- Foreign Language
- Social Science
- See All College Courses
- Common Core
- High School
- See All High School Courses
- College & Career Guidance Courses
- College Placement Exams
- Entrance Exams
- General Test Prep
- K-8 Courses
- Skills Courses
- Teacher Certification Exams
- See All Other Courses
- Create a Goal
- Create custom courses
- Get your questions answered
- International
- Education Jobs
- Schools directory
- Resources Education Jobs Schools directory News Search

GCSE Enzymes introduction including lock and key theory
Subject: Biology
Age range: 11-14
Resource type: Lesson (complete)
Last updated
9 July 2018
- Share through email
- Share through twitter
- Share through linkedin
- Share through facebook
- Share through pinterest

Tes paid licence How can I reuse this?
Your rating is required to reflect your happiness.
It's good to leave some feedback.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resource to let us know if it violates our terms and conditions. Our customer service team will review your report and will be in touch.
Not quite what you were looking for? Search by keyword to find the right resource:
- Corpus ID: 50084002
Russian Formalism | The Johns Hopkins Guide to Literary Theory and Criticism
- Uri Margolin
- Published 2011
- Linguistics
2 Citations
Body ≈ sounds : an emergent sonic practice, defamiliarization in innovation and usability, 14 references, russian formalist criticism : four essays, readings in russian poetics: formalist and structuralist views, supplement to a bibliography of russian formalism in english, language in literature, literary structure, evolution, and value: russian formalism and czech structuralism reconsidered, russian formalism : a collection of articles and texts in translation, related papers.
Showing 1 through 3 of 0 Related Papers

COMMENTS
Lock and key model & induced fit model. The document summarizes the lock-and-key hypothesis proposed by Emil Fischer in 1894 and the induced fit hypothesis proposed by Daniel Koshland in 1958 to describe enzyme-substrate binding. The lock-and-key hypothesis suggests that enzymes and substrates have complementary geometric shapes that fit exactly.
1 The Lock and Key Hypothesis. Fit between the substrate and the active site of the enzyme is exact Like a key fits into a lock very precisely The key is analogous to the enzyme and the substrate analogous to the lock. Temporary structure called the enzyme-substrate complex formed Products have a different shape from the substrate Once formed ...
Lock and Key Model. A German scientist, Emil Fischer postulated the lock and key model in 1894 to explain the enzyme's mode of action. Fischer's theory hypothesized that enzymes exhibit a high degree of specificity towards the substrate. This model assumes that the active site of the enzyme and the substrate fit perfectly into one another ...
Jun 17, 2014 • Download as PPTX, PDF •. The document defines key terms related to enzymes including substrate, active site, and cofactors. It describes the lock and key and induced fit models of how enzymes bind to substrates. Environmental factors like temperature, pH, and substrate concentration can affect the rate of enzymatic reactions.
Powerpoint enzymes models of action. Enzymes are proteins that act as biological catalysts, speeding up chemical reactions without being used up. They work via a "lock and key" mechanism, though the induced fit model suggests enzymes are flexible. Enzyme activity can be measured by looking at reaction rates under different temperatures, pH ...
The Key-Lock Theory and the Induced Fit Theory. Angew. Chem. Inl. Ed. Engl. 33, Koshland, D. E. (2004). Crazy, but correct. Nature, 432. Download ppt "From lock and key model to induced fit model" Similar presentations . Enzymes … what do you know and will find out these next few lessons Lets find out millionaire styley remember the tips from ...
Lock and key hypothesis This is the simplest model to represent how an enzyme works. The substrate simply fits into the active site to form a reaction intermediate. Induced fit hypothesis In this model the enzyme molecule changes shape as the substrate molecules gets close. The change in shape is 'induced' by the approaching substrate molecule.
Lock-and-key vs. Induced Fit Model. At present, two models attempt to explain enzyme-substrate specificity; one of which is the lock-and-key model, and the other is the Induced fit model.The lock and key model theory was first postulated by Emil Fischer in 1894.The lock-and-key enzyme action proposes the high specificity of enzymes.
Figure \(\PageIndex{2}\): The Lock-and-Key Model of Enzyme Action. (a) Because the substrate and the active site of the enzyme have complementary structures and bonding groups, they fit together as a key fits a lock. (b) The catalytic reaction occurs while the two are bonded together in the enzyme-substrate complex.
Therefore, each enzyme is substrate specific. Models of How Enzymes Work 1. Lock and Key model 2. Induced Fit model Lock and Key Model Substrate (key) fits to the active site (lock) which provides a microenvironment for the specific reaction. ... Enzyme Theory Last modified by: Elementary/Secondary Schools Created Date: 2/13/2006 12:27:42 PM ...
A quick powerpoint that covers the basics of the Lock an Key Theory, for b4, OCR Twenty First Century Science. Good Luck! n_n. 3.0 / 5 based on 3 ratings? Created by: Black Wolf; Created on: 23-12-12 16:19; Enzymes: Theory Of Lock and Key Powerpoint Powerpoint Presentation 211.7 Kb. Biology; Enzymes and digestion Enzymes and digestion; GCSE ...
And so, with the lock and key model, the shape of the active site on the enzyme is rigid and perfectly complementary to the substrates shape. And so what this means is that in the lock and key model, the shape of the active site is perfectly suited to the shape of the substrate to create a perfect puzzle piece like fit.
LOCK AND KEY HYPOTHESIS Enzymes are very specific and it was suggested by Fischer in 1890 that this was because the enzyme had a particular shape into which the substrate or substrates fit exactly.This is often referred as Lock and Key hypothesis. Lock and Key model: According to this model, shape of active site of enzyme is complementary to the shape of substrate molecules.
Lock and Key Model. The Lock and Key model is a theory of enzyme action hypothesized by Emil Fischer in 1899. According to Fischer, enzymes exhibit a high degree of specificity to the substances ...
The lock and key hypothesis models this. Enzymes are denatured at extremes of temperature and pH. Part of Combined Science Key concepts in biology. Save to My Bitesize Remove from My Bitesize.
Age range: 11-14. Resource type: Lesson (complete) File previews. pptx, 5.41 MB. This PowerPoint covers why enzymes are important, what they are, and lock and key theory. However, it is designed to introduce the concept of them being biological catalysts and the lock and key theory. This resource is designed for lower ability KS4 and KS3 students.
Herman J. Muller's single gene theory of the origin of life. 1.The first living being was a gene that appeared by chance in the primitive oceans; 2. The primordial gene was endowed with. a) autocatalysis (replication) b) heterocatalysis (metabolism) c) mutability (evolvability) Muller, 1926.
PPT abt co-enzymes and different co enzymes are explained. ... A theory called the "lock-key theory" of enzyme catalysts can be used to explain why inhibition occurs. Inhibitors 71. Inhibitors 72. • The lock and key theory utilizes the concept of an "active site." The concept holds that one particular portion of the enzyme surface has a ...
accompanied it, the Middle East has become a key testing ground for Russia's attempt to return to the global stage. To recap, Putin's general objective in the Middle East is to establish Russia's status and role as a major outside power in one of the world's most volatile regions. Other key objectives include:
Russian Formalism is the name for a group of literary scholars and linguists who between 1916 and 1929, while most were still in their twenties, developed a series of innovative theoretical concepts, claims, models, and methodological norms concerning various aspects of the literary system and its study. The group had two centers: the Moscow Linguistic Circle and OPOYAZ (the Society for the ...
1. 2. The Kremlin in the city of Moscow is known simply as the Kremlin. Triangular and surrounded by a crenellated wall, it occupies 90 acres (36.4 hectares) in the historic core of Moscow. It is bounded by the Moscow River (to the south), the Red Square (to the east), and the old Alexander Gardens (to the west).