
MARY JO GROVES, MD
Am Fam Physician. 2016;93(11):928-934
Patient information : See related handout on genital herpes , written by the author of this article.
Author disclosure: No relevant financial affiliations.
Genital herpes is a common sexually transmitted disease, affecting more than 400 million persons worldwide. It is caused by herpes simplex virus (HSV) and characterized by lifelong infection and periodic reactivation. A visible outbreak consists of single or clustered vesicles on the genitalia, perineum, buttocks, upper thighs, or perianal areas that ulcerate before resolving. Symptoms of primary infection may include malaise, fever, or localized adenopathy. Subsequent outbreaks, caused by reactivation of latent virus, are usually milder. Asymptomatic shedding of transmissible virus is common. Although HSV-1 and HSV-2 are indistinguishable visually, they exhibit differences in behavior that may affect management. Patients with HSV-2 have a higher risk of acquiring human immunodeficiency virus (HIV) infection. Polymerase chain reaction assay is the preferred method of confirming HSV infection in patients with active lesions. Treatment of primary and subsequent outbreaks with nucleoside analogues is well tolerated and reduces duration, severity, and frequency of recurrences. In patients with HSV who are HIV-negative, treatment reduces transmission of HSV to uninfected partners. During pregnancy, antiviral prophylaxis with acyclovir is recommended from 36 weeks of gestation until delivery in women with a history of genital herpes. Elective cesarean delivery should be performed in laboring patients with active lesions to reduce the risk of neonatal herpes.
Genital herpes is a common sexually transmitted disease caused by herpes simplex virus (HSV) and characterized by lifelong infection and periodic reactivation. HSV, a DNA virus, is named from its protein coat as HSV-1 or HSV-2. HSV-1 is the chief cause of orolabial herpes. Until recently, genital herpes was more likely to be caused by HSV-2. However, the incidence of primary genital infection with HSV-1 is now as common or more common than HSV-2 in the United States. 1

Epidemiology
Worldwide, more than 400 million persons have genital herpes caused by HSV-2. 2 In the United States, nearly one in five adults (approximately 50 million persons) has HSV-2 infection, with 1 million new infections occurring each year. 3 – 5 Overall seroprevalence of HSV-1 is decreasing because of less childhood exposure to orolabial herpes; however, genital acquisition rates have increased simultaneously, with HSV-1 now representing at least one-half of new cases. 1 , 6 – 8 This increase has been partly attributed to changing adolescent sexual practices involving more oral-genital contact. 8 , 9
The risk of genital herpes varies by race, sex, and ethnicity. Risk factors, including the number of lifetime sex partners, are listed in Table 1 . 3 , 10
Pathophysiology
Primary HSV infection results from a previously unexposed person having close contact with someone who is actively shedding the virus from skin or secretions. There may be a prodrome of hours to days consisting of pain, tingling, itching, or burning at the site of exposure. Epithelial damage at the portal of entry leads to eruption of vesicles that open, ulcerate, and reepithelialize during an outbreak that lasts about two weeks. During initial infection, viral DNA travels by axon to the spinal cord sensory ganglion where it persists for life. 8 Reactivation of HSV causes migration back through the axon, its branches, or contralateral axons to the skin and mucosa. 11
Clinical Manifestations
A visible outbreak consists of single or clustered vesicles on the genitalia ( Figures 1 and 2 12 ) , perineum, buttocks, upper thighs, or perianal areas that ulcerate before resolving. Primary infections may cause malaise, fever, or localized adenopathy. Subsequent outbreaks are usually milder and are caused by reactivation of latent virus. 1 , 3 The classic presentation of HSV, whether primary infection or secondary outbreak, is absent most of the time, with many patients reporting minimal or no symptoms. 8 Studies consistently report 65% to 90% of patients with genital HSV infection are unaware of its presence. 1 , 3 , 13
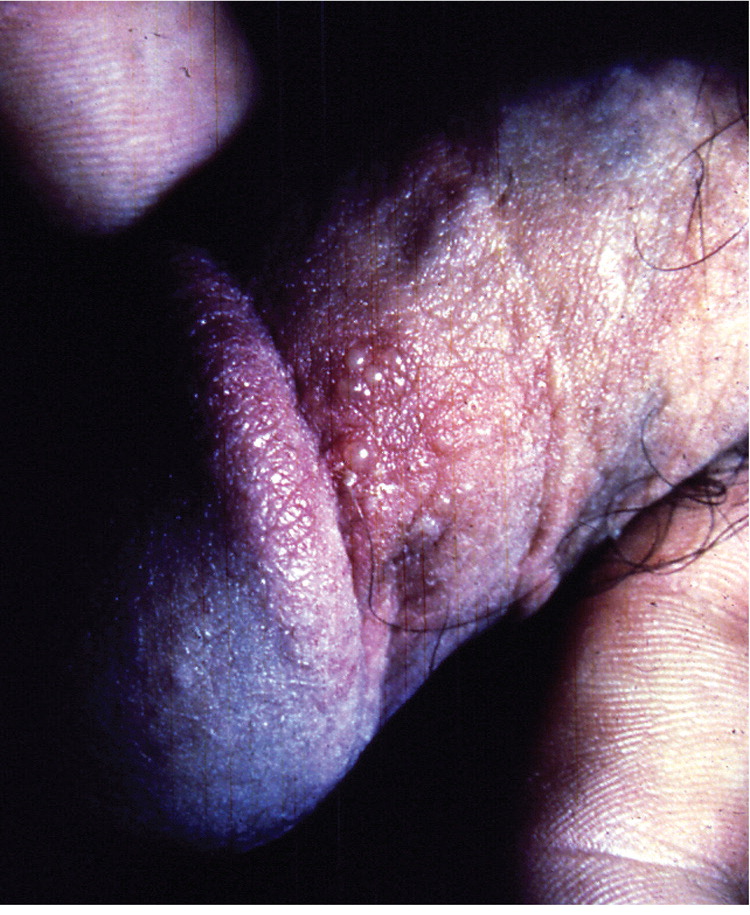
Clinically apparent secondary outbreaks may have a prodrome anywhere along the involved axon, are milder, and usually heal within six to 12 days. Primary and secondary genital HSV-1 infections tend to be milder than HSV-2 infections. Patients with HSV-1 infection average zero to one recurrence per year, whereas HSV-2 recurs four to five times annually, with both types decreasing in recurrence over time. The wide variability in clinical expression may have greater significance to the individual's symptomatic course than the viral type. 8
Asymptomatic viral shedding is common, occurring on 10% to 20% of all days and more often during the first year of infection, but continuing over the extended years studied. 14 – 16 The frequency of subclinical infection and asymptomatic shedding facilitates HSV transmission. The first recognizable outbreak may not occur until well after the primary infection. 1 , 17
Complications
Persons with HSV-2 infection have a threefold increase in the risk of acquiring human immunodeficiency virus (HIV) infection. 18 This may be related to open ulcers, or lymphocytes at the site of eruptions, facilitating HIV invasion during sexual contact. 19
Concurrent infection with HSV-2 and HIV increases the severity of HSV episodes and the likelihood of atypical presentations. 20 The relationship between genital HSV-1 and HIV infections has not been well studied. 21
The presence of either HSV-1 or HSV-2 antibody may provide a small degree of protection against developing an infection with the other HSV type at the same or a new site; however, patients should still be counseled on safe sex practices to prevent genital infections. 7 , 8
Complications of genital herpes are listed in Table 2 . 8 However, the predominant morbidity may be the psychological burden attributable to lifelong dilemmas of conduct, and disclosure to sex partners. 8 , 22 , 23
Although herpes is the most common ulcerative genital disease in the United States, the coexistence of multiple etiologies must be considered. Table 3 lists the differential diagnosis. 24 , 25 Because HSV-1 and HSV-2 infections are indistinguishable visually, suspected infection should be confirmed by type-specific testing to guide management. 1 , 24 In the presence of active lesions, polymerase chain reaction assay is the preferred test, with sensitivity and specificity greater than 95%. 24 , 26 , 27
Serologic testing may be useful if there is a history of suggestive symptoms but no lesions are present or polymerase chain reaction assay results are negative, or when the patient's partner is infected. 24 Western blot is the diagnostic standard, although glycoprotein G tests are comparable ( Table 4 ) . 28 Older immunoglobulin G and M antibody tests are not reliable and should not be used. 28
There is a window of two weeks to six months after HSV exposure to formation of detectable antibody, so repeat serologic testing may be needed to confirm recent acquisition. When the likelihood of infection is low, false-positive test results are more common. It is unclear how to counsel patients with a positive serologic test result but no history of genital herpes symptoms. 29 Therefore, the U.S. Preventive Services Task Force and the Centers for Disease Control and Prevention (CDC) recommend against serologic screening for genital herpes. 24 , 30 Type-specific serologic testing should be offered to partners of patients with HSV infection to determine the risk of acquisition 15 , 24 , 29 ( Table 5 31 ) .
Interpretation of serology results is critical to accurately counsel patients. Serology confirms infection and the viral type. Because HSV-2 is almost always genitally acquired, the presence of HSV-2 antibody implies anogenital disease, even if there is no history of symptoms. 24 However, the site of HSV-1 infection cannot be identified by positive HSV-1 serology results alone. 5 , 24 HSV-1 antibody is present in 54% of the U.S. population, primarily acquired orolabially in childhood, 5 and it is often asymptomatic whether orolabial or genital. Considering the increased incidence of new genital HSV-1 infections and changing sexual practices, clinicians should counsel patients who test positive for HSV-1 antibody that they may be able to transmit HSV to uninfected partners through oral or genital sex, and that they remain at risk of acquiring HSV-2 infection. 8 , 24
Treatment and Prevention
Episodic and suppressive treatment of herpes is aimed at reducing the severity, duration, and recurrence of symptoms, and at preventing transmission to uninfected partners. 24 , 32 Suppressive treatment may be intermittent or continuous. Three nucleoside analogues, which work by inhibiting viral DNA, are approved and well tolerated: acyclovir, famciclovir (Famvir), and valacyclovir (Valtrex). Regimens are identical for HSV-1 and HSV-2.
Nucleoside analogues are equally effective in treating first and subsequent episodes of HSV infection, in reducing the frequency and severity of recurrence, and in decreasing viral shedding. 24 , 33 – 35 However, shedding is not completely eliminated with any treatment regimen, nor does treatment eliminate latent virus or affect transmission risk or symptoms once the medication is discontinued. 24 Oral regimens 32 recommended in the 2015 CDC guidelines are provided in Tables 6 , 7 , and 8 . 24
In patients who have both HSV-2 and HIV infections, anti-HSV suppressive therapy does not reduce the risk of HSV transmission to uninfected partners. 36 Also, suppressive therapy in patients with HSV-2 infection does not reduce the risk of acquiring HIV infection. 37
Treatment should be based on the patient's disease profile, sexual practices, and psychosocial needs. Persons with infrequent or mild recurrences may opt for episodic treatment. Patient-initiated episodic treatment in those with known genital herpes is safe and effective, and avoids delay in initiating treatment. 38 Chronic suppression is valuable for patients with frequent recurrences and for protecting at-risk partners. Drug resistance with long-term nucleoside analogue use is rare in immunocompetent persons. 39 Topical acyclovir has minimal benefit for local symptom reduction, and does not improve episode duration, recurrence, or transmission rates. 24 , 40
There are no approved vaccines for the treatment or prevention of genital herpes. Key points for counseling patients with genital herpes are listed in Table 9 . 24
Considerations in Pregnancy
One in five pregnant women is seropositive for HSV-2, and more than 60% of pregnant women are positive for HSV-1. 41 Yet, neonatal herpes is uncommon, occurring once in 3,200 live births. However, maternal primary infection with HSV-1 or HSV-2 at the time of delivery carries a 60% risk of neonatal herpes.
Antiviral prophylaxis with acyclovir is recommended from 36 weeks' gestation until delivery in women with a history of genital herpes to minimize active recurrence at the time of delivery. 42 All three nucleoside analogues are U.S. Food and Drug Administration category B for breastfeeding and pregnancy. Elective cesarean delivery should be performed in laboring patients with active lesions to decrease the risk of HSV transmission. 42
Data Sources : PubMed was searched using the key terms genital herpes, diagnosis, testing, prognosis, treatment, clinical features, pregnancy, neonatal herpes, and vaccines. Also searched were the Cochrane database, Essential Evidence Plus, Clinical Key, Clinical Evidence, the Centers for Disease Control and Prevention website and selected issues of Morbidity and Mortality Weekly Report for 2015 STD treatment guidelines and epidemiologic data. Search dates: June 2015 through March 2016.
note: This review updates a previous article on this topic by Beauman . 12
Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis. 2013;56(3):344-351.
Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012 [published correction appears in PLoS One . 2015;10(5):e0128615]. PLoS One. 2015;10(1):e114989.
Centers for Disease Control and Prevention (CDC). Seroprevalence of herpes simplex virus type 2 among persons aged 14–49 years—United States, 2005–2008. MMWR Morb Mortal Wkly Rep. 2010;59(15):456-459.
Satterwhite CL, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187-193.
Bradley H, et al. Seroprevalence of herpes simplex virus types 1 and 2—United States, 1999–2010. J Infect Dis. 2014;209(3):325-333.
Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296(8):964-973.
Gupta R, Warren T, Wald A. Genital herpes. Lancet. 2007;370(9605):2127-2137.
Corey L, Wald A. Genital herpes. In: Holmes KK, Sparling PF, Stamm WE, et al. Sexually Transmitted Diseases . 4th ed. New York, NY: McGraw Hill Medical; 2008:399–438.
Halpern-Felsher BL, Cornell JL, Kropp RY, Tschann JM. Oral versus vaginal sex among adolescents: perceptions, attitudes, and behavior. Pediatrics. 2005;115(4):845-851.
Cherpes TL, et al. Genital tract shedding of herpes simplex virus type 2 in women: effects of hormonal contraception, bacterial vaginosis, and vaginal group B Streptococcus colonization. Clin Infect Dis. 2005;40(10):1422-1428.
Tata S, Johnston C, Huang ML, et al. Overlapping reactivations of herpes simplex virus type 2 in the genital and perianal mucosa. J Infect Dis. 2010;201(4):499-504.
Beauman JG. Genital herpes: a review. Am Fam Physician. 2005;72(8):1527-1534.
Schillinger JA, McKinney CM, Garg R, et al. Seroprevalence of herpes simplex virus type 2 and characteristics associated with undiagnosed infection: New York City, 2004. Sex Transm Dis. 2008;35(6):599-606.
Tronstein E, et al. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA. 2011;305(14):1441-1449.
Engelberg R, Carrell D, Krantz E, Corey L, Wald A. Natural history of genital herpes simplex virus type 1 infection. Sex Transm Dis. 2003;30(2):174-177.
Phipps W, Saracino M, Magaret A, et al. Persistent genital herpes simplex virus-2 shedding years following the first clinical episode. J Infect Dis. 2011;203(2):180-187.
Diamond C, et al. Clinical course of patients with serologic evidence of recurrent genital herpes presenting with signs and symptoms of first episode disease. Sex Transm Dis. 1999;26(4):221-225.
Freeman EE, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73-83.
Corey L. Synergistic copathogens—HIV-1 and HSV-2 [published correction appears in N Engl J Med . 2007; 356(14):1487]. N Engl J Med. 2007;356(8):854-856.
Leeyaphan C, et al. Clinical characteristics of hypertrophic herpes simplex genitalis and treatment outcomes of imiquimod: a retrospective observational study. Int J Infect Dis. 2015;33:165-170.
Tan DH, Kaul R, Walsmley S. Left out but not forgotten: should closer attention be paid to coinfection with herpes simplex virus type 1 and HIV?. Can J Infect Dis Med Microbiol. 2009;20(1):e1-e7.
Dunphy K. Herpes genitalis and the philosopher's stance. J Med Ethics. 2014;40(12):793-797.
Gilbert LK, Omisore F. Common questions about herpes: analysis of chat-room transcripts. Herpes. 2009;15(3):57-61.
Centers for Disease Control and Prevention. 2015 sexually transmitted diseases treatment guidelines: genital HSV infections. http://www.cdc.gov/std/tg2015/herpes.htm . Accessed March 1, 2016.
Johnston C, Morrow RA, Moreland A, Wald A. Genital herpes. In: Morse SA, Ballard RC, Holmes KK, Moreland AA, eds. Atlas of Sexually Transmitted Diseases and AIDS . 4th ed. London, UK: Saunders Elsevier; 2010:169–185.
Scoular A, Gillespie G, Carman WF. Polymerase chain reaction for diagnosis of genital herpes in a genitourinary medicine clinic. Sex Transm Infect. 2002;78(1):21-25.
Wald A, et al. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188(9):1345-1351.
Wald A, Ashley-Morrow R. Serological testing for herpes simplex virus (HSV)-1 and HSV-2 infection. Clin Infect Dis. 2002;35(suppl 2):S173-S182.
Scoular A. Using the evidence base on genital herpes: optimising the use of diagnostic tests and information provision. Sex Transm Infect. 2002;78(3):160-165.
U.S. Preventive Services Task Force. Final update summary: Genital herpes: Screening. March 2005. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/genital-herpes-screening . Accessed October 7, 2015.
Centers for Disease Control and Prevention. Genital herpes—CDC fact sheet (detailed). http://www.cdc.gov/std/herpes/stdfact-herpes-detailed.htm . Accessed March 1, 2016.
Hollier LM, Eppes C. Genital herpes: Oral antiviral treatments. BMJ Clin Evid. 2015;2015:1603.
Corey L, et al. An update on short-course episodic and prevention therapies for herpes genitalis. Herpes. 2007;14(suppl 1):5A-11A.
Lebrun-Vignes B, et al. A meta-analysis to assess the efficacy of oral antiviral treatment to prevent genital herpes outbreaks. J Am Acad Dermatol. 2007;57(2):238-246.
Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Intern Med. 2008;168(11):1137-1144.
Mujugira A, Magaret AS, Celum C, et al.; Partners in Prevention HSV/HIV Transmission Study Team. Daily acyclovir to decrease herpes simplex virus type 2 (HSV-2) transmission from HSV-2/HIV-1 coinfected persons: a randomized controlled trial. J Infect Dis. 2013;208(9):1366-1374.
Celum C, Wald A, Hughes J, et al.; HPTN 039 Protocol Team. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371(9630):2109-2119.
Abudalu M, Tyring S, Koltun W, Bodsworth N, Hamed K. Single-day, patient-initiated famciclovir therapy versus 3-day valacyclovir regimen for recurrent genital herpes: a randomized, double-blind, comparative trial. Clin Infect Dis. 2008;47(5):651-658.
Reyes M, Shaik NS, Graber JM, et al.; Task Force on Herpes Simplex Virus Resistance. Acyclovir-resistant genital herpes among persons attending sexually transmitted disease and human immunodeficiency virus clinics. Arch Intern Med. 2003;163(1):76-80.
Kinghorn GR, Turner EB, Barton IG, Potter CW, Burke CA, Fiddian AP. Efficacy of topical acyclovir cream in first and recurrent episodes of genital herpes. Antiviral Res. 1983;3(5–6):291-301.
Xu F, Markowitz LE, Gottlieb SL, Berman SM. Seroprevalence of herpes simplex virus types 1 and 2 in pregnant women in the united states. Am J Obstet Gynecol. 2007;196(1):43.e1-e6.
Stephenson-Famy A, Gardella C. Herpes simplex virus infection during pregnancy. Obstet Gynecol Clin North Am. 2014;41(4):601-614.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2016 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.

Type your tag names separated by a space and hit enter
Herpes Simplex Virus
Microbiology.
- Herpes simplex virus 1 and 2 (HSV-1, HSV-2): members of the Herpes DNA virus family, Herpesviridae , aka Human Herpes Virus 1 and 2 (HHV-1 and HHV-2).
- After primary infection, the virus establishes latency in neurons, with potential for reactivation--usually near the site of initial acquisition.
- Tissue culture using Vero cell culture line or similar with tube or shell vial technique. Growth is often quick within 1-2d, with a cytopathic effect—confirmation of virus by immunologic staining.
- Blood viral culture: use whole blood in a heparinized tube to obtain a buffy coat, an insensitive method.
- HSV-1: 50-80% of adults seropositive (U.S. data)
- HSV-2: 20-40% of adults seropositive
- HSV-1: herpes labialis is the most common form of recurrent HSV-1, but 30% of genital HSV is nowadays HSV-1.
- Asymptomatic in two-thirds of both HSV-1 and 2. Most HSV is acquired from an infected but asymptomatic person.
- Primary gingivostomatitis: fever, sore throat, cervical lymphadenopathy , oral cavity vesicular enanthem that may involve the lips, tongue and mucosal surfaces [ Fig 1] .
- Mononucleosis syndrome: pharyngitis , fever, cervical lymphadenopathy ; not uncommon primary infection of adolescents.
- Genital infection: see the section below.
- Neonatal infection: risk ~40% if primary genital HSV infection occurs in the mother during the third trimester.
- Diagnostics: note that serotype-specific serology helps confirm seroconversion in primary infection; the role in non-primary infection diagnosis is poorly defined.
- Women: infection is often asymptomatic, but when clinically active, may include sores or vesicles around the vaginal region, including the vulva and cervix, perianal, buttocks or thighs [ Fig 2 ]. Dysuria or difficulty voiding may accompany.
- Men: often asymptomatic, less commonly with ulcers or vesicles on the penis, including glans and shaft [ Fig 3 ], perianal or on buttocks/thighs.
- Primary infection may be associated with constitutional symptoms, often with urinary retention (in women), with or without aseptic meningitis (30% women; 10% men) and takes longer to resolve than recurrent disease.
- HSV-2 accounts for 70-80% of cases; HSV-1 for 20-30% of cases.
- HSV-2 is more likely to have clinical recurrences.
- Genital ulcer disease, including that caused by genital HSV, increases the risk of acquiring and transmitting HIV infection.
- Obtain scraping of cells or fluid by unroofing intact blister with a sterile needle.
- HSV DNA PCR from lesions 98-100% sensitive.
- Viral shedding is intermittent, so negative PCR does not exclude the diagnosis. Specificity is 97%.
- 1) Recurrent genital or atypical symptoms with negative HSV PCR or culture.
- 2) Clinical diagnosis of genital herpes without laboratory confirmation.
- 3) A patient whose partner has genital herpes.
- See the Genital Ulcer Disease module for additional details.
- The psychological impact of genital HSV cannot be overstated; 60% report being "devastated" when first told of their dx.
- Recurrent genital herpes (>9 episodes/yr) in non-immunosuppressed may be due to persistently lower levels of IgG1, IgG3, and complement compared with infected persons without recurrent disease [15] .
- Condom use and valacyclovir reduce transmission of genital herpes in serodiscordant couples [22] (this observation was not replicated in HIV/HSV-2 discordant couples [10] ).
- Shedding may occur without clinically evident lesions.
- CSF viral culture is insensitive.
- Rarely CSF PCR negative, yet virus identified on brain biopsy/autopsy.
- The prognosis is poor if the brain MRI shows edema with a shift.
- Mortality is 70% without therapy.
- Definition: >2 recurrences of fever and meningismus lasting 2-5 d with spontaneous recovery.
- If presentation follows genital HSV recurrence, onset typically 5-7 days later.
- Other viruses ( EBV , VZV , echoviruses) have been implicated.
- M:F is 1:2 with mean age = 35 years.
- Recurrences are usually less frequent over time.
- Syndrome: fever, headache (can be severe), photophobia, meningismus; symptoms reach maximum intensity in a few hours.
- 50% have transient neurologic signs/symptoms, including cranial nerve palsies, diplopia, hallucinations, seizures, and altered consciousness. Thus, BRLM must be a dx of exclusion.
- Hallmark is large granular plasma cells (Mollaret cells) seen by Papanicolaou stain.
- Patients are completely well between episodes with CSF normalizing and spontaneous clinical resolution.
- A study showed no benefit in risk reduction of meningitis recurrence at 2 years with suppressive valacyclovir (500mg twice daily ) [12] .
- Approximately 50,000 new and recurrent ocular HSV/yr in the U.S., a leading cause of corneal opacification and infection-related visual loss.
- Atopy increases the risk of ocular herpes [9] .
- The recurrence rate is 20% by two years, 40% by five years, and 67% by seven years.
- Herpetic Eye Disease Study Group has shown that oral acyclovir suppression following initial ocular herpes decreases recurrence by 45% in the first year; the most significant suppressive effect may be seen in those with concomitant history of atopy. This approach, however, may increase the risk of refractory disease due to the emergence of acyclovir resistance [11] .
- Acute follicular conjunctivitis and kerato- conjunctivitis : foreign body sensation, lacrimation, photophobia, conjunctival hyperemia followed by vesicular blepharitis , ulceration, blurring of vision secondary to keratitis, and ultimate healing without scarring.
- It begins with foreign body sensation, lacrimation, photophobia and decreased vision that is slow to heal; repeated recurrences can lead to scarring. It can also be sight-threatening.
- Caused by viral reactivation, previously dormant in trigeminal ganglia.
- Patients with atopy may have unusually severe keratitis due to impaired cell-mediated immunity.
- Response to topical antivirals poor; use oral agents.
- Herpes retinitis: rarer than VZV-related retinal necrosis, can lead to acute retinal necrosis secondary to occlusive vasculitis, sight-threatening.
- HIV-infected persons: 60-70% in the U.S. are infected with HSV-2; disseminated infection with visceral involvement can be seen when CD4 count < 200 cells/mL and is potentially life-threatening.
- Acyclovir is the most studied and is the preferred drug to use.
- HSV outbreak in pregnancy, recommend treating for 7-10 days.
- Pregnant women w/ HSV or a history of genital HSV are recommended to take oral antiviral suppression at 36 weeks.
- Acute immunosuppression: may reactivate HSV within 2 wks of immunosuppression onset.
- HSV esophagitis: seen in immunocompromised patients and must be differentiated from other causes of esophagitis, including CMV and Candida .
- Usually seen in those with significant anti-HSV medication exposure and immunosuppression.
- Acyclovir resistance is primarily due to TK-deficient strains.
- Severe, recurrent ano- genital herpes : commonly seen in patients with AIDS with low CD4 counts (< 200 cells/mL) and high viral loads. HIV-infected, especially w/ AIDS, need more prolonged treatment and/or higher doses for episodic cutaneous HSV.
- HSV tracheobronchitis: rare but most commonly seen in immunosuppressed or elderly intubated patients.
- Herpes dermatitis: seen in athletes (herpes gladiatorum), health care workers (herpetic whitlow) and patients with eczema who become superinfected with HSV (Kaposi’s varicelliform eruption).
- Usually due to HSV-1, but HSV-2 has increasingly been identified.
- It may reactivate after exposure to sunlight, wind, cold, emotional stressors or the late stage of the menstrual cycle.
- Erythema multiforme: a condition in ~5% following recurrent or symptomatic HSV.
- Mean duration of recurrence (vesicles to the healing of lesions): 7-8 d.
- Mean duration of viral shedding: approximately 60h (measured by PCR) with a peak viral load during the vesicle/ulcer stage.
- Data suggest that lesion size, progression to ulcers and duration are improved with topical acyclovir 5%/hydrocortisone 1% combination compared to topical acyclovir 5% alone.
- Occurs in 1:3,000-20,000 live births, 50% of cases are due to HSV type 1 and 50% to type 2.
- The vertical transmission risk to the neonate is 40-80% if primary transmission occurs at the delivery time.
- Congenital herpes occurs by the transfer of infection in utero and is extremely rare.
- Cesarean section if active disease at the time of labor.
- Cutaneous/ocular/oral: lesions may be seen.
- CNS: encephalitis may occur without mucocutaneous findings.
- Disseminated infection: multiple organs involved, including the above and hepatitis, pneumonitis, and DIC.
- Recurrent genital herpes is associated with a very low risk of neonatal herpes (0-3% for vaginal delivery).
- Most neonates with CNS infection will not have skin/eye or mouth manifestations.
- Neonates with CNS herpes may have better neurological development if continued on suppressive acyclovir after initial treatment [13] .
- Elective Cesarean section and suppressive therapy with acyclovir (400mg TID) at or beyond 36 weeks of gestation are recommended for women with first-episode genital lesions during the third trimester.
- Vaginal delivery and suppressive acyclovir therapy are recommended for recurrent genital lesions during the third trimester.
- Note that ~15% of women with "primary" genital herpes present with recurrent infections.
- Re-testing is not needed for prior confirmed history.
- Active lesions: obtain a specimen for culture, antigen or molecular testing.
- History of ulcers, no active lesions: obtain type-specific serology.
- Insufficient data to recommend routine screening of all pregnant women for HSV.
- Oral antiviral suppression is recommended at 36 weeks for those with genital HSV history.
SITES OF INFECTION
- Oro-facial: primary gingivostomatitis; recurrent stomatitis; herpes labialis
- Genital: genital ulcer disease
- Eye: follicular conjunctivitis , keratitis , acute retinal necrosis syndrome, endophthalmitis
- Other skin areas: eczema herpeticum, herpetic whitlow, herpes gladiatorum
- Central nervous system: sporadic encephalitis , meningoencephalitis, aseptic meningitis ; sacral radiculopathy, benign recurrent lymphocytic meningitis (Mollaret’s meningitis)
- Esophagus: esophagitis
- Respiratory system: pneumonia , tracheobronchitis
- Liver: hepatitis
- Rectum: proctitis
- Multiple organs: disseminated infection
Mucocutaneous Infections
- Acyclovir 400 mg PO three times daily
- Acyclovir 200 mg PO five times daily
- Famciclovir 250 mg PO three times daily
- Valacyclovir 1g PO twice daily
- Acyclovir 400 mg PO three times daily x 2d
- Acyclovir 800 mg PO twice daily x 5d
- Acyclovir 800 mg PO three times daily x 5d
- Valacyclovir 500mg PO twice daily x 3d
- Famciclovir 125 mg PO three times daily x 5d
- Famciclovir 1000mg PO twice daily x 1 day alternative short-course regimen
- Acyclovir 400 mg PO three times daily x 5-10d
- Famciclovir 500 mg PO twice daily x 5-10d
- Valacyclovir 1g PO twice daily x 5-10d
- Acyclovir 400 mg PO twice daily
- Famciclovir 250 mg PO twice daily
- Valacyclovir 500 mg PO daily
- Valacyclovir 1g PO daily (recommended for persons with >10 recurrences/year)
- 400 mg three times daily used in pregnancy due to enhanced renal clearance rather than twice daily dosing
- Not suggested in pregnancy
- Above dose in pregnancy.
- Use oral acyclovir per the above regimen with initial HSV infection or if highly symptomatic recurrent HSV.
- Parenteral acyclovir is needed for life-threatening infection.
- Acyclovir 400 mg PO three times a day x 7-10d
- Acyclovir 200 mg PO five times a day x 7-10d
- Famciclovir 250 mg PO q8h X 7-10d
- Valacyclovir 1g PO twice daily x 7-10d
- Valacyclovir 250 mg PO twice daily
- Valacyclovir 500 mg PO once daily
- Valacyclovir 1000 mg PO once daily
- Acyclovir 400-800 mg PO five times daily
- Famciclovir 500mg PO twice or thrice daily
- Acyclovir 5 mg/kg IV q8h
Central Nervous System Infection
- Duration of treatment x 21d advocated by some to minimize relapse.
- Neonatal CNS infection is usually maintained on acyclovir suppression.
- HSV meningitis often with hemorrhagic features in CSF fluid.
- Recent data suggests that 11% have sequelae six months after infection [6] .
- Antiviral treatment is not necessary to use as the condition is usually self-limiting.
- A study showed no benefit of suppressive valacyclovir in preventing the recurrence of meningitis [12] .
Severe, non-CNS infections
- May convert to an oral regimen upon sufficient clinical response.
Ocular Infection
- Requires ophthalmological consultation. Suggestions adapted from [3] .
- Use a therapeutic oral or topical agent. • Acyclovir 400 mg 3–5 times daily for 7–10 days • Valacyclovir 500 mg twice daily for 7–10 days • Famciclovir 250 mg twice daily for 7–10 days • Trifluridine 1% 1 drop 9 times daily for 7 days (not to exceed 21 days) • Acyclovir 3% ointment 5x daily for 7 days • Ganciclovir ointment 0.15% 1 drop 5 times daily for 7 days
- Epithelial debridement
- Taper slowly to the lowest dose required to control inflammation
- PLUS prophylactic oral antiviral • Acyclovir 400 mg 2 times daily • Valacyclovir 500 mg daily • Famciclovir 250 mg daily
- Use Prednisolone acetate 1% 1 to 4 times daily
- PLUS • Acyclovir 800 mg 5 times daily for 7–10 days • Valacyclovir 1 gm 3 times daily for 7–10 days • Famciclovir 500 mg 2–3 times daily for 7–10 days
- Acyclovir 800 mg 5 times daily
- Valacyclovir 1 gm 3 times daily
- Famciclovir 500 mg 2 times daily
- PLUS prednisolone acetate 1% 4 times daily
Prophylaxis for Immunosuppressed Patients
- Initiation: acyclovir 5 mg/kg IV q8h X 7d.
- Maintenance: acyclovir 200-400 mg PO 3-5x daily x 1-3 mos.
- Acyclovir 400-800 mg PO twice or thrice daily
- Valacyclovir 500 mg daily
- Famciclovir 500 mg twice daily
- Burn pts: acyclovir 5 mg/kg IV q8h x 7d, then 200 mg PO 5x/d x 7-14d.
Acyclovir-Resistant Strains
Acyclovir resistance should be suspected in unresponsive cases.
- Foscarnet 40-80mg/kg IV q8h until clinical resolution.
- Topical cidofovir gel 1% for genital or perirectal lesions daily X 5 d may be tried (local pharmacy must compound).
- Imiquimod 5% q8H 3 times/week applied to the lesion until clinical resolution is an alternative therapy for HSV-resistant genital infections.
- Parenteral cidofovir : rarely used option due to toxicities, but may be considered for resistant systemic HSV infection at 5mg/kg once weekly.
Selected Drug Comments
- Resistance testing is not routinely recommended unless there is an apparent clinical failure.
- Suppression therapy does not obviate recurrences but reduces frequency and severity. Relapse if on suppressive therapy is not solved by escalating the dose.
- Moderate 40%
- Mild or normal 30%
- < 30 years of age
- < 4 days of symptoms
- Glasgow coma scale (GCS) score > 6
- If GCS < 6 and > 30 years, only 35% with normal or mild impairments if they survive.
- Neonatal HSV-2 infection is worse than HSV-1.
OTHER INFORMATION
- HSV suppressive therapy does NOT decrease the risk of HIV acquisition among HSV-infected, HIV-uninfected women.
- Breastfeeding is not contraindicated in women with active herpes simplex virus unless there is a lesion in the breast. Given that postnatally-acquired herpes can be as lethal as that acquired during delivery special consideration of handwashing should be taken by mothers and family members with active lesions in any part of the body.
- HSV serologic testing should be considered for persons presenting for an STD evaluation (especially for those persons with multiple sex partners), persons with HIV infection, and MSM at increased risk for HIV acquisition. Screening for HSV-1 and HSV-2 in the general population is not indicated.
- Abstinence during the presence of symptoms or signs is not an effective strategy to reduce the risk of transmission since asymptomatic shedding plays a significant role in HSV transmission.
- Transmission can be reduced with either suppressive therapy or condom use.
Basis for recommendation
Comment: Updated CDC STI Treatment Guidelines used in this module.
Comment: Recommendations for diagnosis and management in pregnancy are used in this module.
Comment: The spectrum of disease and dosing recommendations, also photos.
Comment: 2014 update of the 2007 guidelines on the management of anogenital herpes.
Comment: Clinically useful overview focusing on neurological disease ranging from pregnancy/neonatal to adults.d
Comment: Database analysis from Denmark looked at 205 patients and found less favorable outcomes in 31%, evaluated at discharge. More concerning was 11% after 6 months, unrelated to any clinical or lab-based factors. Almost all received antiviral therapy (96%, majority oral ), so unclear whether it helped.
Comment: Negative trial to see if patients with prolonged ventilation and (+) HSV secretions benefited from antiviral suppression.
Comment: Review of 1028 infants with HSV PCR performed in blood and CSF specimens. Of the 21 who had positive CSF PCR, 76% also had positive HSV PCR in their blood. The important conclusion is that a blood PCR in this population cannot be used to exclude CNS HSV infection.
Comment: This was a retrospective, population-based case-control study of 114 patients with HSV ocular disease and 137 with herpes-zoster ocular disease (HZO) in Hawaii. Authors found that patients with atopy had a 2.6-fold (95% CI, 1.6-4.2) higher odds of having HSVocular disease and 1.8-fold (95% CI, 1.2-2.8) increased odds of having HZO compared to patients without atopy. Patients with 2 or more atopic conditions had an 8.9-fold (95% CI, 3.5-22.6) higher odds of having HSVocular disease and a 2.9-fold (95% CI, 1.1-7.7) higher odds of having HZO. Rating: Important
Comment: This randomized controlled trial evaluated the impact of acyclovir 400mg twice daily on the prevention of transmission of HSV-2 genital herpes in HIV-1/HSV-2 discordant couples in Africa. Key findings : Treatment of HSV-2/HIV-1-infected persons with daily suppressive acyclovir did not decrease the risk of HSV-2 transmission to susceptible partners. Rating: Important
Comment: This retrospective study analyzed 169 corneal swabs from 78 immunocompetent patients with recurrent herpetic keratitis for acyclovir resistance. Key findings : 1) 26% of the isolates were acyclovir-resistant, 2) acyclovir prophylaxis x ≥12 m and recurrence duration of ≥45 days were associated with acyclovir resistance and acyclovir refractory disease, 3) acyclovir-resistant isolates were a risk factor for acyclovir refractory disease (OR 2.28; 95% CI, 1.06–4.89). Rating: Important
Comment: This Sweedish randomized, double-blind, placebo-controlled multicenter trial investigated the effect of valacyclovir on the prevention of recurrence of HSV meningitis. Patients received valacyclovir 500 mg twice daily (n=50) or placebo (n=51) for 1 year after primary or recurrent, confirmed or probable, HSV meningitis. Patients were followed for 2 years. Key finding : no difference between the 2 groups during the first year however, during the second year, the risk of recurrence was higher among patients exposed to valacyclovir (HR, 3.29 [95% confidence interval, 10.06–10.21]). Rating: Important
Comment: A study of 74 infants with neonatal HSV: 45 with CNS involvement were enrolled in a study; 29 with skin, eye and mouth involvement (enrolled in a different study). All 45 neonates with CNS involvement received 14-21 d of parenteral acyclovir and were randomly assigned to receive acyclovir suppression TID x 6 mo vs. placebo. The Mental Development Index of the Bayley Scales of Infant Development (in which scores range from 50 to 150, with a mean of 100 and with higher scores indicating better neurodevelopmental outcomes) was assessed in 28 of the 45 infants with CNS involvement (62%) at 12 months of age. Infants surviving neonatal HSV disease with CNS involvement had significantly improved neurodevelopmental outcomes after receiving suppressive therapy with oral acyclovir for 6 months. Rating: Important
Comment: This randomized, double-blind, placebo-controlled multicenter, multinational phase III clinical trial among HIV-uninfected, HSV-2 seropositive heterosexual women (n=1358) and men who have sex with men (MSM; n=1814) examined the primary outcome of new HIV-1 acquisition and the secondary outcome of the incidence of genital ulcers amongst those receiving twice daily acyclovir (400 mg) and placebo. Amongst participants from all countries, no reduction in HIV-1 incidence was noted between the treatment and control groups. HSV-2 positive ulcers were reduced by 63% in the treatment group compared with the control group (Relative risk = 0.37, Confidence Interval 0.31-0.45). No serious drug effects were noted in the study. Rating: Important
Comment: This prospective case-control study examined immunogenetic risk factors for recurrent genital herpes. the study population included 52 consecutive eligible patients, without immunodeficiency, with culture-confirmed HSV-2 from an active lesion >12 months before enrollment and >9 recurrences per year and 80 HSV seropositive and 70 HSV seronegative controls. Anti-HSV-2 antibodies did not correlate with protection from recurrence. Risk factors for recurrence included lower IgG1 antibody -Confidence Interval (CI), 2.0-12.5; p< 0.001 and IgG3 antibody - CI 1.7-7,8, p< .001. Complement levels were lower in patients with recurrent symptomatic infections. Rating: Important
Comment: This Australian community-based cohort study of 1,427 HIV-negative gay men examined risk factors for herpes simplex virus type 1 (HSV-1) and HSV type 2 (HSV-2) over a median follow-up period of 2 years. At enrolment, the prevalence of HSV-1 was 75%, and HSV-2 was 23%, and both infections had a lower prevalence in those < 25 years. The incidence of HSV-1 infection was 5.58/100 person-years (PY) and 1.45/100 PY for HSV-2. Using multivariate analysis, significant independent risk factors for HSV-1 infection were insertive oral intercourse with casual sex partners (hazards ratio = 3.91; 95% confidence interval [CI] =1.23-12.44) and younger age (p< 0.03). A significant risk factor for HSV-2 acquisition was anal sex with casual partners. Rating: Important
Comment: This randomized, controlled Phase IIb trial of a 10-session behavioral intervention vs. brief counseling session (control group) to reduce HIV acquisition among 4295 high-risk HIV-uninfected men who have sex with men (MSM). Sera and behavioral data collected during this trial were subsequently examined to determine risk factors for herpes simplex virus type 2 (HSV-2, ) evaluate the role of prevalent and incident HSV-2 infection in HIV infection acquisition, and determine the impact of the behavioral intervention on HSV acquisition (already shown not to have a role in HIV acquisition). 91% of subjects had evaluable data; 20.3% were HSV-2 positive (by serology) at enrolment; 4.3% acquired infection over the 24-month study period, and 75.4% remained uninfected with HSV-2. Risk factors for seroconversion included unprotected anal receptive intercourse in the prior 6 months, having at least 1 HIV-infected partner in the past 6 months, and having >5 male sex partners in the last 6 months. HIV risk was increased among MSM with recent HSV-2 infection identified compared with HSV-2 uninfected MSM. The intense behavioral intervention did not increase the risk of HSV-2 infection. Rating: Important
Comment: This is the report of a 27-site multicenter randomized, double-blinded parallel placebo control trial examining the efficacy of 1-gram valacyclovir (VAC) in reducing HSV-2 viral shedding in both clinical and asymptomatic infections among immunocompetent persons. 152 persons were randomized--43 placebo (40 completed) and 109 VAC (94 completed. Over 60 days, each participant reported daily on the presence or absence of genital lesions and collected daily genital and anorectal samples. VAC significantly decreased total days of viral shedding amongst clinical and subclinical cases and a viral load when shedding compared with the controls. In the intent-to-treat group, a 71% reduction in total shedding was noted (p< 0.001), a 58% reduction in subclinical shedding (p< 0.001), and a 64% reduction in clinical shedding (p< 0.01) was seen in the VAC group. There were no major adverse effects noted with VAC over the 60-day study period. Rating: Important
Comment: Excellent review on RBLM.
Comment: The authors report the largest case series of 11 patients/12 eyes with HSV-2 acute retinal necrosis (ARN) and review the world’s literature. Although other infections are associated with ARN, the authors identify some HSV-2-specific features, including young age at DX, hx of HSV at birth, and a preexisting chorioretinal scar in the ARN eye, triggering events such as trauma or steroids. Also, the clinical syndrome described with HSV-2 is more aggressive and rapid. This is a sight-threatening condition and requires prompt consultative referral to an ophthalmologist. Rating: Important
Comment: The authors report the findings of a study among 89 pts with HSV-like lesions--81 with genital and 8 with cutaneous lesions. Specimens were collected for quantitative duplex PCR and culture; 64% were PCR positive, 51% were cx positive. PCR detected 30 of 34 primary and 24 of 29 recurrent infections. 2 HSV-1 samples were positive on cx only despite repeated PCR attempts. Symptomatic pts had significantly higher copy numbers on PCR. In this study, duplex qPCR for HSV-1 and HSV-2 was more sensitive than the gold standard cx for mucocutaneous HSV. Rating: Important
Comment: The authors conducted a study among 528 mutually-monogamous heterosexual couples discordant for HSV-2 infection. Although the antiviral was the intervention under observation, data were also collected re: condom use. When condoms were used more than 70% of the time by the discordant pairs with a positive man and a negative woman, transmission risk was reduced by 60%, even in the absence of antiviral suppression. Acquisition of infection by the seronegative partner and recurrence and shedding by the positive partner were significantly reduced when valacyclovir was used. Rating: Important
Comment: Report of a chart review of 170 patients seen on referral to a dermatology clinic found to have culture-confirmed HSV. This specialty practice was likely to see "outliers" in presentation as only 49% had "typical" cluster genital lesions. Single ulcers, erosion, crusts, fissures, edema, and erythema were seen. Women were more likely to have extragenital lesions than men. Rating: Important
Comment: The author reviewed 29 published clinical trials. Notable that ACV ointment did cause superficial punctate keratitis in 9.8% of 998 pts and 4% noted burning of the eye with the application of the agent. Found to compare favorably with other topical antiherpetics available. Rating: Important
Primary Herpes Simplex virus infection
Primary Herpes Simplex virus infection involving lips and tongue.
Source: CDC
Genital HSV female
Ulcers and vesicle from genital HSV are seen around vaginal introitus due to HSV-2.
Genital HSV male
Vesicle seen on penile shaft due to HSV-2.
Source: CDC/Suan Lindsley
1. Download the Johns Hopkins Guides app by Unbound Medicine
2. Select Try/Buy and follow instructions to begin your free 30-day trial
Want to regain access to Johns Hopkins Guides?
Renew my subscription
Not now - I'd like more time to decide
Log in to Johns Hopkins Guides
Forgot your password, forgot your username, contact support.
- unboundmedicine.com/support
- [email protected]
- 610-627-9090 (Monday - Friday, 9 AM - 5 PM EST.)
- Patient Care & Health Information
- Diseases & Conditions
- Genital herpes
Genital herpes is a common sexually transmitted infection (STI). The herpes simplex virus (HSV) causes genital herpes. Genital herpes can often be spread by skin-to-skin contact during sexual activity.
Some people infected with the virus may have very mild symptoms or no symptoms. They can still able to spread the virus. Other people have pain, itching and sores around the genitals, anus or mouth.
There is no cure for genital herpes. Symptoms often show up again after the first outbreak. Medicine can ease symptoms. It also lowers the risk of infecting others. Condoms can help prevent the spread of a genital herpes infection.
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
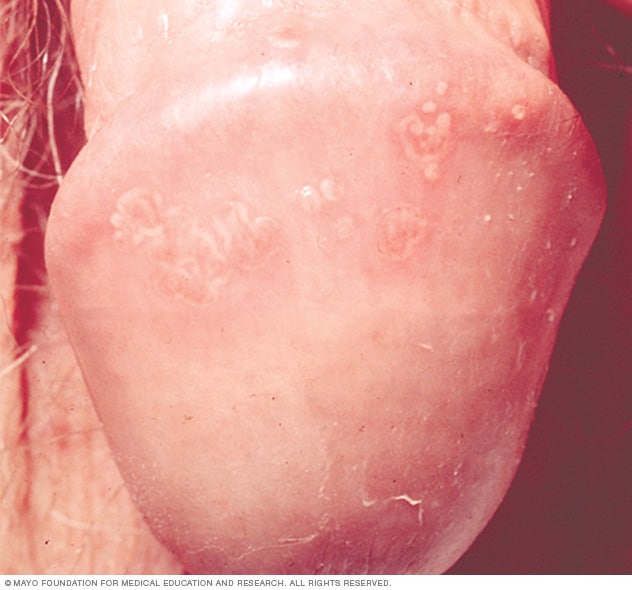
Sores associated with genital herpes can be small bumps, blisters or open sores. Scabs eventually form and the sores heal, but they tend to recur.
Most people infected with HSV don't know they have it. They may have no symptoms or have very mild symptoms.
Symptoms start about 2 to 12 days after exposure to the virus. They may include:
- Pain or itching around the genitals
- Small bumps or blisters around the genitals, anus or mouth
- Painful ulcers that form when blisters rupture and ooze or bleed
- Scabs that form as the ulcers heal
- Painful urination
- Discharge from the urethra, the tube that releases urine from the body
- Discharge from the vagina
During the first outbreak, you may commonly have flu-like symptoms such as:
- Swollen lymph nodes in the groin
Differences in symptom location
Sores appear where the infection enters the body. You can spread the infection by touching a sore and then rubbing or scratching another area of your body. That includes your fingers or eyes.
Sore can develop on or in the:
Repeat outbreaks
After the first outbreak of genital herpes, symptoms often appear again. These are called recurrent outbreaks or recurrent episodes.
How often recurrent outbreaks happen varies widely. You'll usually have the most outbreaks the first year after infection. They may appear less often over time. Your symptoms during recurrent outbreaks usually don't last as long and aren't as severe as the first.
You may have warning signs a few hours or days before a new outbreak starts. These are called prodromal symptoms. They include:
- Genital pain
- Tingling or shooting pain in the legs, hips or buttocks
When to see a doctor
If you suspect you have genital herpes, or any other STI , see your health care provider.
There is a problem with information submitted for this request. Review/update the information highlighted below and resubmit the form.
From Mayo Clinic to your inbox
Sign up for free and stay up to date on research advancements, health tips, current health topics, and expertise on managing health. Click here for an email preview.
Error Email field is required
Error Include a valid email address
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Thank you for subscribing!
You'll soon start receiving the latest Mayo Clinic health information you requested in your inbox.
Sorry something went wrong with your subscription
Please, try again in a couple of minutes
Genital herpes is caused by two types of herpes simplex virus. These types include herpes simplex virus type 2 (HSV-2) and herpes simplex virus type 1 (HSV-1). People with HSV infections can pass along the virus even when they have no visible symptoms.
HSV-2 is the most common cause of genital herpes. The virus can be present:
- On blisters and ulcers or the fluid from ulcers
- The moist lining or fluids of the mouth
- The moist lining or fluids of the vagina or rectum
The virus moves from one person to another during sexual activity.
HSV-1 is a version of the virus that causes cold sores or fever blisters. People may be exposed to HSV-1 as children due to close skin-to-skin contact with someone infected.
A person with HSV-1 in tissues of the mouth can pass the virus to the genitals of a sexual partner during oral sex. The newly caught infection is a genital herpes infection.
Recurrent outbreaks of genital herpes caused by HSV-1 are often less frequent than outbreaks caused by HSV-2 .
Neither HSV-1 nor HSV-2 survives well at room temperature. So the virus is not likely to spread through surfaces, such as a faucet handle or a towel. But kissing or sharing a drinking glass or silverware might spread the virus.
More Information
- Genital herpes: Can you get it from a toilet seat?
Risk factors
A higher risk of getting genital herpes is linked to:
- Contact with genitals through oral, vaginal or anal sex. Having sexual contact without using a barrier increases your risk of genital herpes. Barriers include condoms and condom-like protectors called dental dams used during oral sex. Women are at higher risk of getting genital herpes. The virus can spread more easily from men to women than from women to men.
- Having sex with multiple partners. The number of people you have sex with is a strong risk factor. Contact with genitals through sex or sexual activity puts you at higher risk. Most people with genital herpes do not know they have it.
- Having a partner who has the disease but is not taking medicine to treat it. There is no cure for genital herpes, but medicine can help limit outbreaks.
- Certain groups within the population. Women, people with a history of sexually transmitted diseases, older people, Black people in in the United States and men who have sex with men diagnosed with genital herpes at a higher than average rate. People in groups at higher risk may choose to talk to a health care provider about their personal risk.
Complications
Complications associated with genital herpes may include:
- Other sexually transmitted infections. Having genital sores raises your risk of giving or getting other STI s, including HIV / AIDS .
- Newborn infection. A baby can be infected with HSV during delivery. Less often, the virus is passed during pregnancy or by close contact after delivery. Newborns with HSV often have infections of internal organs or the nervous system. Even with treatment, these newborns have a high risk of developmental or physical problems and a risk of death.
- Internal inflammatory disease. HSV infection can cause swelling and inflammation within the organs associated with sexual activity and urination. These include the ureter, rectum, vagina, cervix and uterus.
- Finger infection. An HSV infection can spread to a finger through a break in the skin causing discoloration, swelling and sores. The infections are called herpetic whitlow.
- Eye infection. HSV infection of the eye can cause pain, sores, blurred vision and blindness.
- Swelling of the brain. Rarely, HSV infection leads to inflammation and swelling of the brain, also called encephalitis.
- Infection of internal organs. Rarely, HSV in the bloodstream can cause infections of internal organs.
Prevention of genital herpes is the same as preventing other sexually transmitted infections.
- Have one long-term sexual partner who has been tested for STI s and isn't infected.
- Use a condom or dental dam during sexual activity. These reduce the risk of disease, but they don't prevent all skin-to-skin contact during sex.
- Don't have sex when a partner with genital herpes has symptoms.
Pregnancy precautions
If you are pregnant and know you have genital herpes, tell your health care provider. If you think you might have genital herpes, ask your provider if you can be tested for it.
Your provider may recommend that you take herpes antiviral medicines late in pregnancy. This is to try to prevent an outbreak around the time of delivery. If you have an outbreak when you go into labor, your provider may suggest a cesarean section. That is a surgery to remove the baby from your uterus. It lowers the risk of passing the virus to your baby.
- Genital herpes: CDC detailed fact sheet. U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/std/herpes/stdfact-herpes-detailed.htm. Accessed Sept. 28, 2022.
- Genital herpes. Sexually Transmitted Infections Treatment Guidelines, 2021. U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/std/treatment-guidelines/herpes.htm. Accessed Sept. 28, 2022.
- AskMayoExpert. Simplex herpes virus (SHV) (adult). Mayo Clinic; 2022.
- Loscalzo J, et al., eds. Herpes simplex virus infections. In: Harrison's Principles of Internal Medicine. 21st ed. McGraw Hill; 2022. https://accessmedicine.mhmedical.com. Accessed Sept. 28, 2022.
- Schiffer JT et al. Herpes simplex virus. In: Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 9th ed. Elsevier; 2020. https://www.clinicalkey.com. Accessed Sept. 28, 2022.
- FAQs: Genital herpes. American College of Obstetricians and Gynecologists. https://www.acog.org/Patients/FAQs/Genital-Herpes. Accessed Sept. 28, 2022.
- Dinulos JGH. Sexually transmitted viral infections. In: Habif's Clinical Dermatology. 7th ed. Elsevier; 2021. https://www.clinicalkey.com. Accessed Oct. 31, 2022.
- Symptoms & causes
- Diagnosis & treatment
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women
Affiliation.
- 1 Division of Infectious Diseases, Cincinnati Children's Hospital Medical Center, University of Cincinnati, Cincinnati, OH 45229, USA. [email protected]
- PMID: 23087395
- PMCID: PMC3540038
- DOI: 10.1093/cid/cis891
Background: Herpes simplex virus infections type 1 (HSV-1) and type 2 (HSV-2) are common, but the epidemiology of HSV disease is changing.
Methods: HSV-seronegative women, aged 18-30 years, who were in the control arm of the HERPEVAC Trial for Women were followed for 20 months for primary HSV infections.
Results: Of the 3438 evaluable participants, 183 became infected with HSV: 127 (3.7%) with HSV-1 and 56 (1.6%) with HSV-2. The rate of infection for HSV-1 (2.5 per 100 person-years) was more than twice that for HSV-2 (1.1 per 100 person-years). Most infections (74% of HSV-1 and 63% of HSV-2) occurred without recognized signs or symptoms of herpes disease. The HSV-2 infection rate was 2.6 times higher in non-Hispanic black participants than in Hispanics and 5.5 times higher than in non-Hispanic whites (P < .001), while the HSV-1 infection rate was 1.7 times higher in non-Hispanic whites than non-Hispanic blacks. Younger participants (18-22 years) were more likely to acquire HSV-1 infections and less likely to develop recognized disease than older participants. Overall, 84% of recognized disease cases were genital. No differences were noted in the clinical manifestations of genital HSV-1 vs genital HSV-2 disease. The clinicians' assessment that cases were caused by HSV was good when they assessed cases as clinically confirmed or unlikely (validated in 83% and 100% of cases, respectively).
Conclusions: HSV-1 is now more common than HSV-2 as a cause of oral and genital mucosal infections in young women, but there are important age and race differences.
Publication types
- Research Support, N.I.H., Extramural
- Research Support, Non-U.S. Gov't
- Antibodies, Viral / blood*
- Antibody Formation / immunology
- Herpes Genitalis / epidemiology*
- Herpes Genitalis / immunology
- Herpes Simplex / epidemiology*
- Herpes Simplex / immunology
- Herpesvirus 1, Human / immunology*
- Herpesvirus 2, Human / immunology*
- Prospective Studies
- Young Adult
- Antibodies, Viral
Grants and funding
- N01AI45250/AI/NIAID NIH HHS/United States
- P01 AI030731/AI/NIAID NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
- N01-AI-45250/AI/NIAID NIH HHS/United States
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
INTRODUCTION
The pathogenesis, clinical manifestations, diagnosis, and treatment of HSV-1 encephalitis will be reviewed here. Neonatal encephalitis and other manifestations of HSV-1 infection are discussed separately. (See "Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection" .)
EPIDEMIOLOGY
In up to 50 percent of HSV-1 encephalitis cases, the strains detected in the central nervous system and the skin differ in the same patients [ 10 ]. Thus, in patients with a prior history of herpes labialis, primary infection with a different strain may cause HSV-1 encephalitis.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Sexually Transmitted Infections (STIs)
Genital Herpes
- Health Topics A-Z
Screening for Genital Herpes
- Genital herpes is a common sexually transmitted infection (STI) can be challenging to diagnose.
- CDC recommends herpes testing for people with genital symptoms.
- These frequently asked questions provide answers about herpes tests and CDC's genital herpes testing recommendations.
Who should get tested
Genital herpes is common. shouldn’t cdc recommend testing for everyone.
CDC recommends herpes testing for people who have genital symptoms to confirm if they have it. Testing allows a healthcare provider to talk with patients about what to expect in the future. This includes talking about medications that help with symptoms. Providers can also tell patients how to lower the risk of transmitting herpes to sex partner(s).
CDC does not recommend herpes testing for people without symptoms in most situations. This is because of the limits of a herpes blood test and the possibility of a wrong test result. The chances of wrong test results are higher for people who are at low risk of infection.
Blood tests might be useful if:
- You have genital symptoms that could be related to herpes
- You have (or have had) a sex partner with genital herpes
- Your provider found signs of herpes, but you still need a test to confirm it
If you are sexually active, talk openly and honestly with your healthcare provider about what tests are right for you. These tips can help.
Does my healthcare provider include a blood test for genital herpes when they test me for “everything” (all STIs)?
Herpes blood tests may or may not be part of the tests your healthcare provider gives you. They may choose tests based on several factors (e.g., number of sex partners, if you had an STI before, etc.).
They will also evaluate you for signs or symptoms of herpes to choose which tests to use. This is why it's important to talk openly and honestly with your provider during your visit. Ask them which infections they are and are not testing you for and why.
Additional Resources
- Sample questions you might ask your provider
- A list of questions your provider might ask you
- Conversation tips
I found out my partner has herpes. When should I get tested?
Talk with your healthcare provider if your partner has herpes. They can recommend specific tests and help guide when you should get tested. After exposure, it can take up to 16 weeks or more for current tests to detect infection.
Quick facts
Diagnosing genital herpes, is it true that genital herpes is hard to diagnose.
Diagnosing genital herpes can be challenging. This is for two main reasons:
Many people with herpes have no symptoms
A healthcare provider may diagnose herpes by looking at any blisters or sores. They can also take a sample or swab from a blister or sore that is not already crusted over or healing. In fact, the tests that use these samples work best. However, most people with genital herpes do not have symptoms or can mistake them for other skin conditions like a pimple or ingrown hair.
There are limits to the current tests
If a patient has no blisters or sores, providers may use a blood test to see if they have herpes. These tests have limits. For example, if a person gets a blood test too soon after an infection, the result could be wrong. A wrong result is also possible when the person has a low risk of infection.
If you're sexually active, talk openly and honestly with your provider about testing for herpes and other STIs. They can help you decide what is best for you based on your sexual and medical history. These tips can help.
Why are false positive tests an argument against routine testing for genital herpes, but not for other STIs, which can also have false positives?
False positive test results show that a person has an infection or condition when they do not. This can happen with many kinds of diagnostic tests. However, the chance of a false positive herpes test result is much higher than when testing for STIs like chlamydia or gonorrhea. This is because current herpes tests are not as exact as tests for chlamydia and gonorrhea.
Can testing and treating genital herpes decrease the risk for HIV infection?
No. Studies show that HIV risk is not lowered by genital herpes testing or treatment. Learn more about the link between genital herpes and HIV in this fact sheet .
Finding support
I have genital herpes. where can i find the latest information about ongoing genital herpes research, including clinical trials.
The U.S. National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID) supports research to develop prevention methods and treatments for genital herpes. Details about current research efforts can be found on the NIAID website . NIH also maintains a database with information about clinical trials around the world . This database includes information on all genital herpes studies that are actively recruiting volunteers.
Genital herpes is a common sexually transmitted infection (STI) that can be treated.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 13 May 2024
Gene editing for latent herpes simplex virus infection reduces viral load and shedding in vivo
- Martine Aubert ORCID: orcid.org/0000-0003-1125-6856 1 ,
- Anoria K. Haick 1 ,
- Daniel E. Strongin 2 ,
- Lindsay M. Klouser ORCID: orcid.org/0000-0002-9644-361X 1 , 2 ,
- Michelle A. Loprieno ORCID: orcid.org/0000-0002-2905-485X 1 ,
- Laurence Stensland 2 ,
- Tracy K. Santo 2 ,
- Meei-Li Huang 2 ,
- Ollivier Hyrien 1 ,
- Daniel Stone ORCID: orcid.org/0000-0003-1619-7541 1 &
- Keith R. Jerome ORCID: orcid.org/0000-0002-8212-3789 1 , 2
Nature Communications volume 15 , Article number: 4018 ( 2024 ) Cite this article
3115 Accesses
174 Altmetric
Metrics details
- Herpes virus
- Molecular medicine
- Viral infection
Anti-HSV therapies are only suppressive because they do not eliminate latent HSV present in ganglionic neurons, the source of recurrent disease. We have developed a potentially curative approach against HSV infection, based on gene editing using HSV-specific meganucleases delivered by adeno-associated virus (AAV) vectors. Gene editing performed with two anti-HSV-1 meganucleases delivered by a combination of AAV9, AAV-Dj/8, and AAV-Rh10 can eliminate 90% or more of latent HSV DNA in mouse models of orofacial infection, and up to 97% of latent HSV DNA in mouse models of genital infection. Using a pharmacological approach to reactivate latent HSV-1, we demonstrate that ganglionic viral load reduction leads to a significant decrease of viral shedding in treated female mice. While therapy is well tolerated, in some instances, we observe hepatotoxicity at high doses and subtle histological evidence of neuronal injury without observable neurological signs or deficits. Simplification of the regimen through use of a single serotype (AAV9) delivering single meganuclease targeting a duplicated region of the HSV genome, dose reduction, and use of a neuron-specific promoter each results in improved tolerability while retaining efficacy. These results reinforce the curative potential of gene editing for HSV disease.

Similar content being viewed by others

Gene editing and elimination of latent herpes simplex virus in vivo
Targeting herpes simplex virus with CRISPR–Cas9 cures herpetic stromal keratitis in mice

Initial TK-deficient HSV-1 infection in the lip alters contralateral lip challenge immune dynamics
Introduction.
HSV infections can cause recurrent orofacial, corneal, anogenital, or other lesions, and infections of newborns can lead to disseminated disease and devastating neurological sequelae. Genital infection with HSV-2 increases the risk of acquisition of HIV, and is a major driver of the global HIV pandemic 1 . Current antiviral therapy can treat acute episodes and suppress outbreaks, but does not cure established infection 2 , 3 , 4 , 5 , 6 . Recurrent outbreaks result from the ability of HSV to establish latent infection within ganglionic neurons innervating the affected sites. Latent HSV in ganglia is unaffected by traditional antivirals, explaining the inability of antivirals to cure, and reactivations typically commence again once therapy is stopped.
A promising potentially curative strategy involves gene editing directed at latent HSV itself 7 . In a recent study, AAV-delivered meganucleases eliminated over 90% of HSV-1 genomes from the superior cervical ganglia of latently infected mice 8 . Despite this impressive reduction in ganglionic HSV loads after gene editing, the relevance that such reduction would have for human HSV infection is uncertain. Infected persons are typically not concerned with ganglionic viral loads per se, but instead about symptomatic disease and/or viral shedding, and the associated risk of transmission to others 9 . Existing mouse models are limited in their ability to address these issues, since latently infected mice rarely spontaneously reactivate HSV or show viral shedding at peripheral tissues.
Here, we introduce a model of small-molecule induction of HSV-1 reactivation and peripheral shedding in latently infected mice, and demonstrate that gene editing mediates a dramatic reduction not only in ganglionic viral loads, but also in induced viral shedding. Optimization of the therapeutic approach through regimen simplification, dose reduction, and tissue restriction of meganuclease expression results in almost complete elimination of undesired effects on liver and ganglia, supporting the continued clinical development of this strategy.
Meganuclease therapy reduces ganglionic viral load after ocular or genital HSV infection
We previously evaluated several AAV serotypes for delivery of meganucleases to latently infected mice, and found the best results with AAV-Rh10, followed by AAV8 and AAV1 8 . To further improve efficacy, we tested additional neurotropic AAV serotypes, including AAV7, AAV9, AAV-DJ, and AAV-DJ/8 10 , 11 , for delivery of the anti-HSV1 meganuclease, m5, at a dose of 10 12 AAV genomes (vg) per mouse (Fig. S1a ), using our model of orofacial HSV disease. Both AAV9 and AAV-Dj/8 were superior to 10 12 vg AAV-Rh10, the best of our previously used serotypes 8 , showing HSV reductions in superior cervical ganglia (SCG) of 95% ( p = 10 −5 ) and 90% ( p = 0.018), respectively, relative to untreated controls (Fig. S1b ), comparing favorably with the 65% reduction previously obtained with m5 alone delivered with AAV-Rh10 8 . Similarly, AAV9 and AAV-Dj/8 showed better activity than AAV-Rh10 in trigeminal ganglia (TG), with HSV load reductions of 48% ( p = 0.07) and 41% ( p = 0.5), respectively (Fig. S1b ), compared with our prior observation of no detectable reduction using AAV-Rh10 delivering m5 8 . The route of AAV administration (retro-orbital vein vs. intradermally into the whisker pad) did not have any detectable impact on either AAV transduction or gene editing efficiencies (Fig. S1e–g ).
We previously demonstrated that gene editing of HSV could be increased by using combinations of AAV serotypes for meganuclease delivery, rather than a single AAV serotype, a finding we ascribed to the heterogeneity of neuronal subsets within HSV-infected ganglia 8 . We therefore evaluated gene editing with the anti-HSV1 meganuclease m5, which cleaves a sequence in the UL19 gene coding for the major capsid protein VP5 12 , when delivered using single AAV serotypes vs. combinations of AAV9, AAV-Dj/8, and AAV-Rh10 (Fig. S2a ) administered as a total dose of 10 12 vg per mouse. In agreement with our previous results, combinations of AAV serotypes led to robust HSV gene editing, with the triple combination of AAV9, AAV-Dj/8, and AAV-Rh10 showing especially strong reductions in HSV loads and mutagenesis of residual HSV across both SCG and TG (Fig. S2b–g ).
While orofacial infections with HSV are extremely common, genital infections, which lead to latent infection of dorsal root ganglia (DRG), also represent a major cause of morbidity. We therefore established latent genital infections in mice by intravaginal inoculation with HSV-1 after treatment with Depo Provera, which synchronizes the estrus cycle and increases HSV infection 13 . Infected mice were treated with a total dose of 3 × 10 12 vg of the AAV9, AAV-Dj/8, and AAV-Rh10 combination delivering two HSV1-specific meganucleases simultaneously (m5 along with m8, which targets a sequence in the UL30 gene coding for the catalytic subunit of the viral DNA polymerase 12 . In parallel, we tested the same AAV combination against latent orofacial HSV infection as described above (Fig. 1a, b ). Remarkably, efficacy in the vaginal model of infection was the highest we have observed to date, with a 97.7% reduction in HSV viral load in DRG (Fig. 1c ). This compared favorably with the orofacial infection group treated in parallel, in which (in agreement with our previous studies) we observed robust gene editing with significant reductions of ganglionic HSV loads of 89% in SCG and 61% in TG (Fig. 1d, e ).
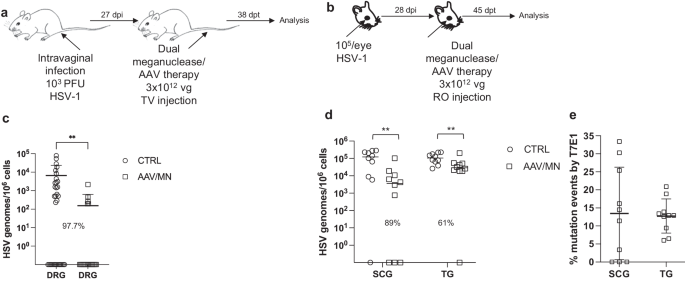
a , b Experimental timeline of ( a ) vaginal or ( b ) ocular infection and meganuclease therapy. RO, retroorbital; TV, tail vein. c HSV loads in DRGs from control ( n = 7) and dual meganuclease-treated ( n = 4) mice vaginally infected with HSV-1; p = 0.001. d . HSV loads in SCGs and TGs from control ( n = 10) and dual meganuclease-treated ( n = 10) mice ocularly infected with HSV-1; p = 0.0046 and 0.0034 for SCG and TG, respectively. e Gene editing at the m5 target site of residual virus quantified by T7E1 assay in SCG and TG from dual meganuclease-treated mice ( n = 10). Each graph shows individual and mean values with standard deviation, percent decrease of HSV loads in treated mice compared to control mice and statistical analysis (unpaired one-tailed Mann-Whitney test with ** p < 0.01). AAV loads are shown in Supplemental Fig. 9a, b . Source data are provided as a Source Data file.
Induction of HSV shedding using the BET bromodomain inhibitor JQ1
Mice generally show little if any spontaneous HSV reactivation, with minimal to no viral shedding at peripheral sites, limiting their utility in cure studies. The BET (Bromo and Extra-Terminal domain) bromodomain inhibitor JQ1 was reported to reactivate latent HSV in vitro in primary neuronal cultures, and HSV could be detected in the eyes of about one-third of latently infected mice treated with JQ1 14 . To evaluate the utility of JQ1 for our cure work, we extended these studies to determine the quantitative kinetics of HSV shedding after JQ1 therapy.
A single intraperitoneal (IP) injection of JQ1 (50 mg/kg) given to latently infected mice (Fig. S3a ) led to detectable shedding from the eyes of 56% (5/9) of animals, compared with 0/9 animals treated with vehicle alone (Fig. S3b, c ). Viral shedding was transient, peaking at 2 days post JQ1, with maximal viral loads ranging from about 10 2 to almost 10 6 copies/swab (Fig. S3c ). A direct comparison suggested that JQ1 may be a more powerful reactivation stimulus for HSV than hyperthermic stress (HS) 15 , which in our hands led to detectable virus shedding in less than 20% of animals (2/12 HS vs 4/10 JQ1), with peak shedding viral loads two logs lower than after JQ1 treatment (Fig. S3d–f ). Sequential treatment with JQ1 at one-week intervals led to repeated shedding episodes with similar kinetics as observed above (Fig. S4a–e ). Over the course of three sequential JQ1 reactivations (Fig. S4e ), shedding from individual mice was stochastic; 10/12 (83%) mice shed detectable virus at least once, but only 1/12 (8%) shed after all three treatments, while 4/12 (33%) and 5/12 (42%) shed only after two or one of the three treatments, respectively (Fig. S4e and Table S1 ). Shedding was typically unilateral (only detected in one eye), despite the initial inoculation being to both eyes (unilateral shedding was observed in 33/37 (89%) of events). The side of shedding in one episode was not predictive of the side of future shedding events (Table S1 ). Importantly for cure studies, repeated weekly reactivation of virus with JQ1 up to 7 weekly injections did not change ganglionic viral loads compared with control animals (Fig. S4g, h ).
Reduction of ganglionic HSV load is associated with reduced peripheral shedding
The ability to reproducibly induce HSV reactivation and shedding with JQ1 allowed us to investigate the relationship between ganglionic viral load reduction using meganucleases and subsequent viral shedding at peripheral sites. Latently infected mice were treated as above using the AAV9, AAV-Dj/8, and AAV-Rh10 combination delivering HSV1-specific meganucleases m5 and m8 at a total dose of 3 × 10 12 vg, or left untreated as controls. One month later, mice were administered JQ1, and eye swabs were collected daily for 4 days (Fig. 2a ). Consistent with our previous results, ganglionic tissues from treated mice showed a 98% and 42% reduction in mean viral loads in SCG and TG, respectively, when compared to control untreated animals (Fig. 2b, c ) and gene editing in the remaining viral genomes (Fig. 2d ). After JQ1 administration, only 3/10 (30%) of the dual meganuclease-treated mice had detectable virus in eye swabs, compared with 5/10 (50%) of control untreated animals (Fig. 2e, f ). The mean titer of HSV in positive eye swabs was 3 × 10 4 copies/ml in the meganuclease-treated animals, compared with 1.2 × 10 5 copies/ml in the control animals. Area under the curve analysis (AUC) demonstrated a 95% reduction ( p = 0.15) in total viral shedding in treated vs. control animals (Fig. 2g ). In a separate experiment performed similarly (Fig. 3a ), ganglionic tissues from treated mice showed 97% reduction in mean latent HSV genomes in both SCG and TG when compared to control untreated animals (Fig. 3b, c ) and gene editing in the remaining genomes (Fig. 3d ). While 3/8 mice from the control group shed virus with a mean viral titer of 8.2 × 10 5 copies/ml, 0/8 meganuclease-treated mice had detectable shedding, representing a 100% decrease in total virus shed ( p = 0.10, Fig. 3e–g ).
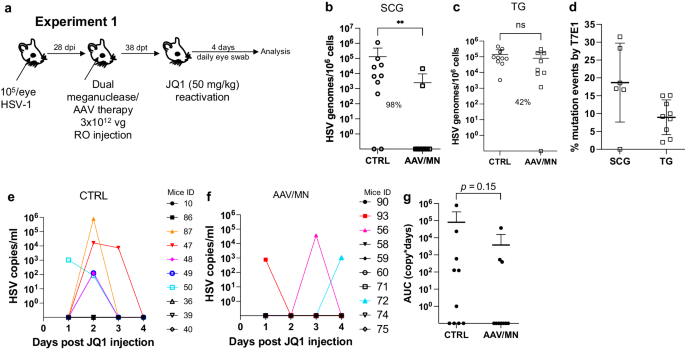
Experimental timeline of ocular infection, meganuclease treatment and viral reactivations with JQ1. a Experiment 1 ( n = 10 per group). HSV loads in SCGs ( b : p = 0.0057) and TGs ( c ). Percent decrease of HSV loads in treated mice compared to control mice and statistical analysis (unpaired one-tailed Mann–Whitney test with ns: not significant, ** p < 0.01) are indicated. Gene editing at the m5 target site of residual virus quantified by T7E1 assay in SCG and TG from dual meganuclease-treated mice ( d ). HSV titers in eye swabs collected daily from day 1 to 4 post JQ1 reactivation from control ( e ) and dual meganuclease-treated ( f ) infected mice. Panels 2i-k show data for both SCG and both TG from each mouse. Area under the curve (AUC) analysis ( g ) with p value (un p aired one-tailed Mann–Whitney test). AAV loads are shown in Supplemental Fig. 9c, d . Each graph shows individual and mean values with standard deviation. Source data are provided as a Source Data file.
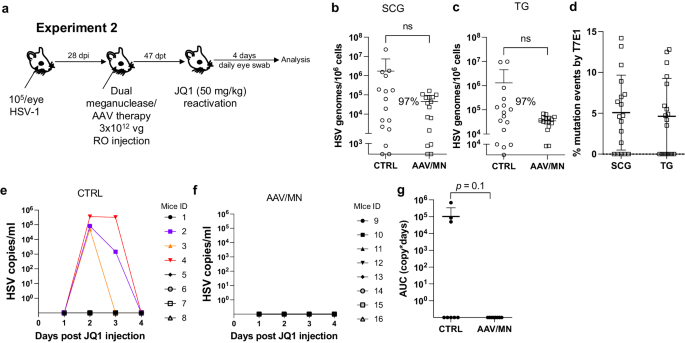
Experimental timeline of ocular infection, meganuclease treatment and viral reactivations with JQ1. a Experiment 2 ( n = 8 per group). HSV loads in SCGs ( b ) and TGs ( c ). Percent decrease of HSV loads in treated mice compared to control mice and statistical analysis (unpaired one-tailed Mann-Whitney test with ns: not significant, ** p < 0.01) are indicated. Gene editing at the m5 target site of residual virus quantified by T7E1 assay in SCG and TG from dual meganuclease-treated mice ( d ). HSV titers in eye swabs collected daily from day 1 to 4 post JQ1 reactivation from control ( e ) and dual meganuclease-treated ( f ) infected mice. Panels 2 b - d show data for both SCG and both TG from each mouse. Area under the curve (AUC) analysis ( g ) with p value (un p aired one-tailed Mann–Whitney test). AAV loads are shown in Supplemental Fig. 9e, f . Each graph shows individual and mean values with standard deviation. Source data are provided as a Source Data file.
Safety of AAV/meganuclease therapy
AAV-vectored therapies are generally considered safe. Nevertheless, dose-limiting liver toxicity has been observed after AAV administration in humans, non-human primates, and mice, typically at doses of 2 × 10 14 vg/kg or greater. The 3 × 10 12 vg/animal dose (~1 × 10 14 vg/kg) used in the experiments described in Figs. 1 – 3 approached the level associated with liver toxicity in previous studies. Across multiple studies we observed that 7/70 (10%) animals treated with the 3 × 10 12 vg/animal dose exhibited clinical signs consistent with hepatotoxicity, including weight change, bloating, and general health decline. Hepatotoxicity was confirmed in these animals by subsequent histopathological evaluation (Fig. S5 and Table S2 ). We therefore evaluated lower total doses of triple AAV serotype/dual meganuclease therapy (0.6, 1.2, or 1.8 × 10 12 vg/animal or 1.8, 3.6, or 5.4 × 10 13 vg/kg) for their tolerability and effects on viral load and JQ1-induced HSV shedding (Fig. 4a ). These doses showed substantially improved tolerability, both clinically and upon histopathological examination and quantification of the number of inflammatory cell foci (ICF) in livers (Fig. S6a ). Dose-dependent reductions in ganglionic HSV loads were observed across the three treatment groups compared to controls, ranging from 69% and 47% in SCG and TG, respectively, at the 0.6 × 10 12 dose to 94% and 73% at the 1.8 × 10 12 dose (Fig. 4b, c ). To evaluate the effect of these reduced doses on HSV shedding, treated mice were subjected to three weekly rounds of JQ1 administration, followed by eye swabbing as described above. While the percentage of dual meganuclease-treated animals shedding virus after the first JQ1 reactivation was not reduced compared with the control mice, it was substantially lower than controls at all doses by the third JQ1 reactivation (0% (0/12), 8% (1/12) and 0% (0/12) for 0.6, 1.2, and 1.8 × 10 12 vg/mouse groups, respectively, versus 18% (2/11) in the control group) (Fig. 4f–i ). This finding that may relate to the two additional weeks available for meganuclease expression and gene editing activity by the time of the third JQ1 reactivation. Consistent with this interpretation, the reduction in total viral shedding, as determined by AUC analysis, appeared to become more complete over time, with up to a 97–100% reduction in all three groups by the final JQ1 reactivation (Fig. 4j–l ). The efficacy of reduced-dose dual meganuclease therapy (1.8 × 10 12 vg) was confirmed in a separate experiment (Fig. 5a ), showing a significant decrease in ganglionic viral loads in both SCG and TG (Fig. 5b, c ). In this experiment, 7 of 12 control animals showed detectable viral shedding after JQ1 reactivation, compared with only 1 of 12 animals treated with AAV-meganuclease therapy, (Fig. 5d, e ) and reduction in total viral shedding, as determined by AUC analysis (Fig. 5f, g ). While none of the treated mice exhibited any clinical signs of hepatotoxicity, we did observe higher numbers of ICF in liver of treated animals receiving the 1.8 × 10 12 vg dose compared to control mice (Fig. S6b ). Histologic analysis of H&E stained TG sections from both control and treated animals revealed subtle evidence of neuronal injury, manifesting as neuronal degeneration, necrosis, and axonopathy. The scores grading prevalence and severity of the microscopic changes were higher in treated animals compared to control mice (Fig. S7 and Table S3 ). However, no mice in either the control or experimental group showed detectable signs of neuropathy.
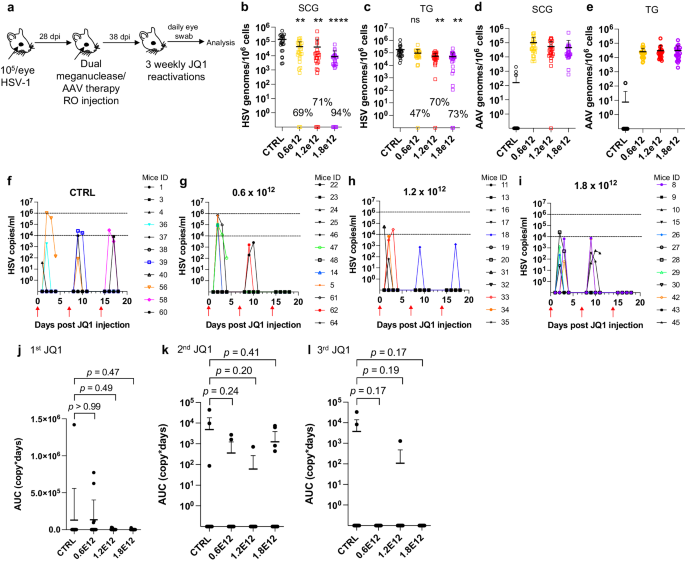
a Experimental timeline of ocular infection, meganuclease treatment and viral reactivations with JQ1. b , c HSV loads and d , e , AAV loads in SCGs ( b ; p = 0.0016, 0.0012, and <0.0001 for 0.6, 1.2, and 1.8 × 10 12 , respectively, d ) and TGs ( c ; p = 0.068, 0.0025 and 0.0016 for 0.6, 1.2, and 1.8 × 10 12 , respectively, e ) in control infected mice ( n = 11) and infected mice treated with dual therapy delivered with 0.6 ( n = 12), 1.2 ( n = 12), or 1.8 ( n = 12) × 10 12 total vg AAV dose. Percent decrease of HSV loads in treated mice compared to control mice and statistical analysis (ordinary one-way Anova, multiple comparisons with ns: not significant, ** p < 0.01, **** p < 0.0001) are indicated. f – i Virus titers in eye swabs collected daily from day 1 to 4 after each weekly JQ1 reactivation from control infected mice ( f ) and infected mice treated with dual therapy delivered with 0.6 ( g ), 1.2 ( h ), or 1.8 ( i ) ×10 12 total vg AAV dose. j – l Area under the curve (AUC) ana l ysis of virus shedding after first ( j ), second ( k ), and third ( l ) JQ1 reactivation from control infected mice ( n = 11) and infected mice treated with dual therapy delivered with 0.6 ( n = 12), 1.2 ( n = 12) or 1.8 ( n = 12) × 10 12 total vg AAV dose. p values (unpaired, ordinary one-way Anova, with multiple comparisons) compared virus shedding between treatment groups and the control group. Each graph shows individual and mean values with standard deviation. Source data are provided as a Source Data file.
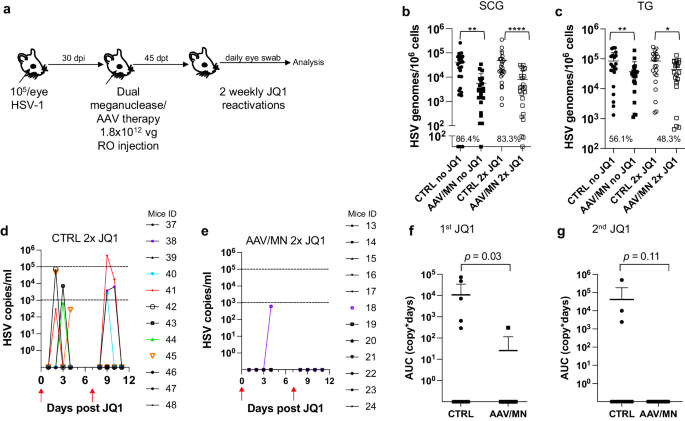
a Experimental timeline of ocular infection, meganuclease treatment and viral reactivations with JQ1. b HSV loads in SCGs ( b ; p = 0.0012, and <0.0001 for CTRL vs AAV/MN no JQ1 and for CTRL vs AAV/MN 2x JQ1, respectively), and TGs ( c ; p = 0.0089, and 0.0293 for CTRL vs AAV/MN no JQ1 and for CTRL vs AAV/MN 2x JQ1, respectively) of control infected mice either unreactivated (CTRL no JQ1) or reactivated (CTRL 2x JQ1) and infected mice treated with dual therapy delivered with 1.8 × 10 12 total AAV dose either unreactivated (AAV/MN no JQ1) or reactivated (AAV/MN 2x JQ1), with n = 12 per group. Percent decrease of HSV loads in treated mice compared to control mice and statistical analysis (unpaired one-tailed Mann-Whitney test with * p < 0.05; ** p < 0.01, **** p < 0.0001) are indicated. d , e Virus titers in eye swabs collected daily from day 1 to 4 after two weekly JQ1 reactivations (red arrows) from control infected mice ( d ) and infected mice treated with dual therapy delivered with 1.8 × 10 12 total AAV dose ( e ). f , g Area under the curve (AUC) analysis after the first ( f ), and the second ( g ) JQ1 reactivation from control infected mice either unreactivated (CTRL no JQ1) or reactivated (CTRL 2x JQ1) and infected mice treated with dual therapy delivered with 1.8 × 10 12 total AAV dose either unreactivated (AAV/MN no JQ1) or reactivated (AAV/MN 2x JQ1), with n = 12 per group. p values (unpaired one-tailed Mann–Whitney test) are indicated. Each graph shows individual and mean values with standard deviation. AAV loads are shown in Supplemental Fig. 9g–h . Source data are provided as a Source Data file.
Reduced dose of AAV/meganuclease treatment of genitally infected animals decreases DRG latent viral load and may reduce genital HSV shedding
As noted above, genital HSV infection is a major cause of morbidity in humans. We therefore evaluated the reduced-dose dual meganuclease therapy (total dose of 1.8 × 10 12 vg/animal) in vaginally infected mice (Fig. 6a ). In agreement with our previous results, the reduced-dose therapy led to a 78.8% ( p = 0.02) to 95.6% ( p = 0.006) reduction in latent virus genomes in DRGs (Fig. 6b ).
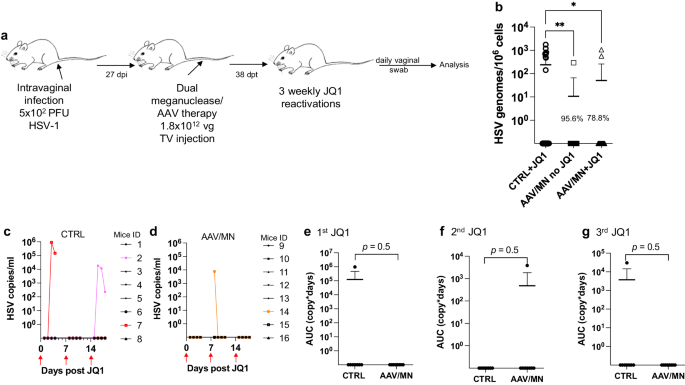
a Experimental timeline of intravaginal HSV-1 infection, meganuclease treatment and viral reactivations with JQ1. b HSV loads in DRGs from control infected mice reactivated with 3 weekly JQ1 injections and infected mice treated with dual therapy unreactivated, or reactivated with 3 weekly JQ1 injections with n = 8 per group; p = 0.0055, and 0.0198 (ordinary one-way Anova, multiple comparisons) for CTRL + JQ1 vs AAV/MN no JQ1 and for CTRL + JQ1 vs AAV/MN + JQ1, respectively. c , d HSV titers in vaginal swabs collected daily from day 1 to 4 post JQ1 injections (red arrows) from control ( c ) and dual meganuclease-treated ( d ) infected mice. Area under the curve (AUC) analysis after the first ( e ), second ( f ), and third ( g ) JQ1 reactivation from control infected mice ( n = 8) and infected mice treated with dual therapy, both reactivated with 3 weekly JQ1 injections ( n = 8). p values (unpaired one-tailed Mann–Whitney test) are indicated. Each graph shows individual and mean values with standard deviation. The AAV viral loads are shown in Supplemental Fig. 9i . Source data are provided as a Source Data file.
We then sought to evaluate whether JQ1 could induce HSV shedding in the genital infection model, as we previously observed in the ocular infection model. Over 3 sequential JQ1 reactivations, only 2 of 8 control animals (and 1 of 8 AAV/meganuclease-treated animals) shed detectable virus, a rate lower than the 40–50% reactivation we typically observe after ocular infection (Fig. 6c, d ). The apparently lower rate of reactivation seen in the vaginal model compared to the ocular model may be due to lower levels of ganglionic HSV loads in the DRG (10 2 –10 3 vg/10 6 cells in DRG, Fig. 6b vs 10 4 –10 5 vg/10 6 cells in SCG or TG, Fig. 5b, c ). While this lower reactivation rate prohibited meaningful statistical analysis, the observation that 2 out of the 8 control mice shed virus over 2 to 3 sequential days, while only 1 of the 8 AAV-treated mice shed virus, on a single day and at a substantially lower level, is qualitatively in agreement with our observations after ocular infection (Fig. 6c–g ).
Meta-analysis of the effect of AAV/meganuclease therapy on HSV shedding
The stochastic nature of clinical HSV reactivation 16 , recapitulated when induced by JQ1 in mice (Fig. S4 and Table S1 ), makes evaluation of viral shedding extremely resource-intensive. Practical constraints, including the number of animals that can be housed and studied at the same time, along with the extended duration of each study ( ~ 3 months), limited the statistical power of our individual experiments. We therefore performed a meta-analysis of data from all experiments presented above (Figs. 1 – 6 ), combining evidence from infection sites (orofacial or genital), thus comparing 174 swabs from AAV/meganuclease-treated animals to 99 swabs from experimentally-matched controls. The primary endpoint was viral shedding, expressed either as a binary variable (equal to 1 for samples in which HSV was detected and 0 otherwise) or the log10-transformed AUC for quantitative viral shedding. The experiments depicted in Figs. 1 – 6 represent all of the shedding studies with the dual meganuclease/triple AAV therapy we have performed as of this writing, and each suggests a strong and consistent trend toward a substantial reduction in viral shedding after AAV/meganuclease therapy. Across all studies, the proportion of swabs with detectable HSV was 48% lower among AAV/meganuclease-treated animals compared to controls. The meta-analysis confirmed that animals receiving AAV/meganuclease therapy had a statistically significant reduction in viral shedding (OR = 0.41, p = 0.010, by generalized linear mixed models, GLMM).
We then asked whether dose or duration of meganuclease therapy was associated with the probability of viral shedding (expressed as a binary variable) or the quantity of viral shedding (expressed as the log10-transformed AUC). Overall, the probability of viral shedding significantly decreased with the dose of AAV/meganuclease (OR = 0.66; p = 0.023, GLMM), and also with the duration of meganuclease therapy (OR = 0.42; p < 0.001, GLMM) in treated animals compared to controls. The data further indicate that overall, the quantity of virus shed (AUC) significantly decreased with the AAV/meganuclease dose at a rate of -0.36 log 10 copy-days per 10 12 increase in dose (LMM; p = 0.028), and also with the duration of meganuclease therapy, at a rate of −0.48 log 10 copy-days per additional week after treatment (LMM; p = 0.017). No significant association was detected between the log10-transformed AUC and the interaction between time and dose (LMM; p = 0.59).
Simplification of the AAV-meganuclease regimen
The studies described above were performed using a triple AAV serotype/dual meganuclease approach, resulting in each animal receiving a total of 6 unique vectors (3 serotypes × 2 meganucleases). Clinical translation of such a complex regimen could raise manufacturing and quality control issues. We therefore sought to simplify AAV/meganuclease therapy to reduce the complexity of our therapeutic regimen. We took advantage of the dual cutting meganuclease m4, which recognizes a sequence in the duplicated gene ICP0 in the HSV-1 genome and was previously shown to induce significant decrease of latent viral loads in ganglia of latently infected mice 8 . Latently infected mice were administered a total dose of 5 × 10 11 vg of either the combination of AAV9, AAV-Dj/8, and AAV-Rh10, or each single AAV serotype delivering the HSV1-specific meganuclease m4 (Fig. 7a ). Consistent with the results using the lower dose of 6 x 10 11 of the triple AAV-dual MN therapy (Fig. 4 ), ganglionic tissues from treated mice with the triple AAV combination delivering m4 showed a 73.9% ( p < 0.0001) and 43.7% ( p = 0.014) reduction in mean viral loads in SCG and TG, respectively, when compared to control untreated animals. When m4 was delivered using single AAV serotypes, the data confirmed that AAV9 on its own could recapitulate the viral load decrease seen with the triple AAV serotype combination, with 77.8% ( p < 0.0001) and 49% ( p = 0.0046) reduction in mean viral loads in SCG and TG, respectively (Fig. 7b, c ). Furthermore, mice having received AAV9 alone showed the lowest levels of liver inflammation of any of the groups, similar to those in the control liver (Fig. 7d ). At this reduced dose, regardless of the AAV serotype combination used, no detectable neurotoxicity was observed compared to the control animals (Fig. 7e, f ).
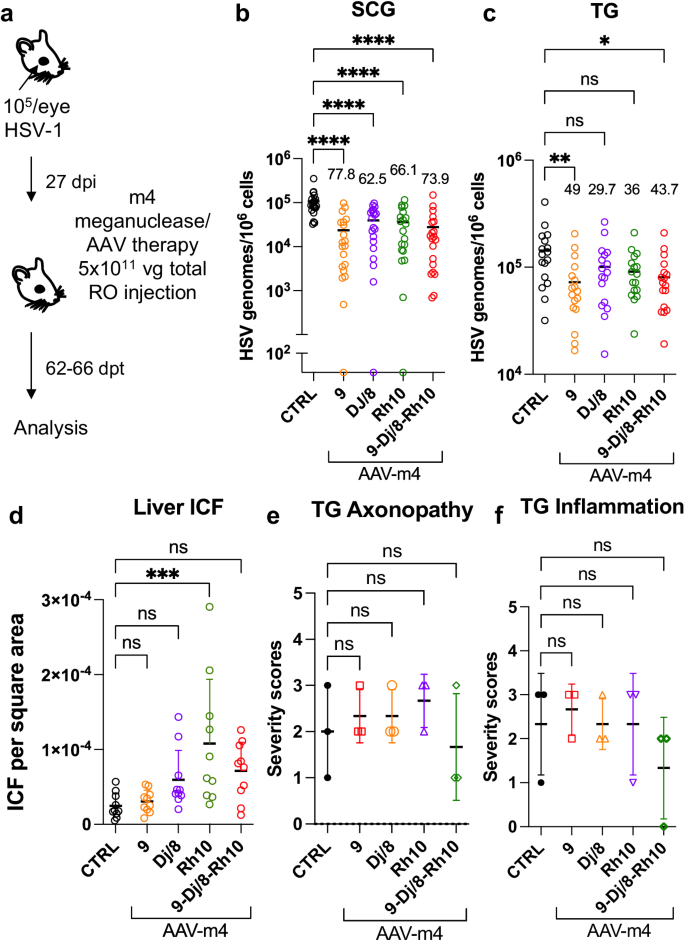
a Experimental timeline of ocular infection and meganuclease therapy. b , c HSV loads in SCGs ( b ; p < 0.0001) and TGs ( c ; p = 0.0046 for AAV9 and 0.0142 for 9-Dj/8-Rh10) from infected control and infected mice treated with m4 delivered by retro-orbital (RO)) injections of 5 × 10 11 vg total of the single or triple combinations of AAV9, -Dj/8 and -Rh10. Percent decrease of HSV loads in treated mice ( n = 10 per group) compared to control mice ( n = 10) and statistical analysis (Ordinary one-way Anova, multiple comparisons with * p < 0.05; ** p < 0.01, **** p < 0.0001; ns: not significant). d Inflammatory cell foci (ICF) in liver sections from either HSV-infected control mice ( n = 10), or mice treated with m4 delivered using AAV single or triple combinations of AAV9, -Dj/8, and -Rh10 ( n = 10 per group); p = 0.0009 for Rh10. e , f Severity scores of axonopathy ( e ) and inflammation ( f ) in TG from infected control mice ( n = 3 TG) and infected mice treated with m4 delivered using single or triple combinations of AAV9, -Dj/8 and -Rh10 ( n = 3 TG per group) and statistical analysis (Ordinary one-way Anova, multiple comparisons with ns: not significant; *** p < 0.001). Each graph shows individual and mean values with standard deviation. The AAV viral loads are shown in Supplemental Fig. 9j–l . Source data are provided as a Source Data file.
To confirm that a simplified regimen composed of AAV9-m4 was also able to reduce peripheral virus shedding, latently infected mice were treated as above using AAV9 delivering either HSV1-specific meganuclease m4 or a catalytically inactive version (m4i) at a dose of 1 × 10 12 vg. One month later, mice were subjected to two weekly rounds of JQ1 administration, followed by daily eye swabbing for 3 days (Fig. 8a, b ). A decrease of ganglionic viral loads of 89.6% ( p < 0.0001) and 69% ( p = 0.03) in SCG and TG respectively, was observed in m4-treated mice but not in mice treated with the inactive form of the meganuclease m4i (Fig. 8c, d ). Furthermore, 6 out of 9 control mice and 6 out of 10 m4i-treated mice shed virus after JQ1 reactivations, while only 3 out of 10 m4-treated mice had detectable virus shedding after reactivation (Fig. 8e–i ). These data demonstrate that our simplified regimen can substantially reduce ganglionic viral loads, with an associated decrease in virus shedding after reactivation, and that these effects are dependent on an active enzyme and not on AAV itself. In this experiment, mice treated with m4 had slightly higher levels of liver ICF and TG axonopathy, but not more TG inflammation, compared to control mice (Fig. 8j–l ).
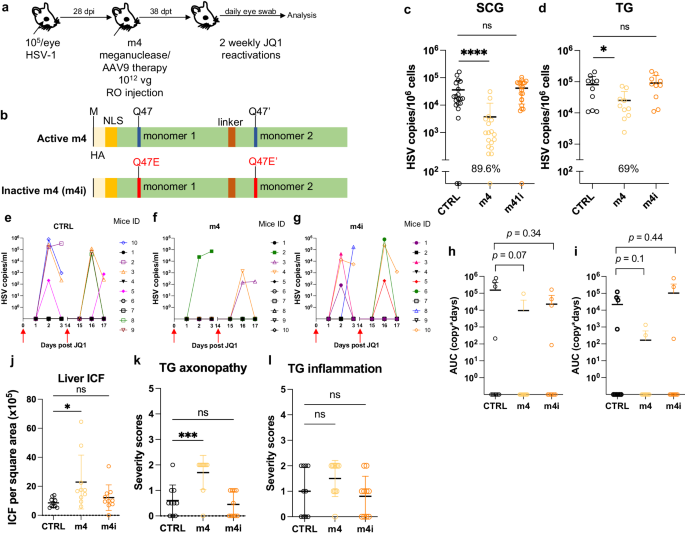
a Experimental timeline of ocular HSV-1 infection, meganuclease treatment and viral reactivations with JQ1. b Schematic of active m4 and inactive m4i meganuclease. c - d , HSV loads in both SCGs ( c ; p < 0.0001 for m4) and both TGs ( d ; p = 0.003 for m4) from control infected mice and infected mice treated the active m4 or inactive m4i ( n = 10 per group). Percent decrease of HSV loads in treated mice compared to control mice and statistical analysis (unpaired one-tailed Mann-Whitney test with * p < 0.05; **** p < 0.0001; ns: not significant) are indicated. e – g Virus titers in eye swabs collected at day 1 to 3 after each JQ1 reactivation from control infected mice ( e ) and infected mice treated with active m4 ( f ) or inactive m4i ( g ). h , i Area under the curve (AUC) analysis of virus shedding after first ( h ), and second ( i ) JQ1 reactivation from control i nfected mice and infected mice treated with active m4 or inactive m4i ( n = 10 per group). p values (unpaired one-tailed Mann–Whitney test) are indicated. j Inflammatory cell foci (ICF) in liver sections from either HSV-infected control mice, mice treated with active m4 or inactive m4i ( n = 10 per group); p = 0.0234 for m4. k , l Severity scores of axonopathy ( k ; p = 0.0007 for m4) and inflammation ( l ) in TG from HSV-infected contro l mice, mice treated with active m4 or inactive m4i ( n = 10 per group) with statistical analysis (Ordinary one-way Anova, multiple comparisons with ns: not significant; * p < 0.05; *** p < 0.001). Each graph shows individual and mean values with standard deviation. AAV viral loads are shown in Supplemental Fig. 9m–o . Source data are provided as a Source Data file.
Tissue restriction of meganuclease expression improves tolerability
Across our studies, we observed that ~10% of the animals treated with a high dose of AAV/meganuclease (2–3 × 10 12 vg/animal, or approximately 6–9 × 10 13 vg/kg) exhibited clinical signs consistent with hepatotoxicity, including weight change, bloating, and general health decline. When lower doses were evaluated, we observed substantially improved tolerability, both clinically and upon histopathological examination and ICF quantification. To further reduce hepatoxicity, we evaluated the use of neuron-specific promoters (Calmodulin Kinase II (CamKII) and human Synapsin (hSyn)) combined with the CMV enhancer 17 , to test the hypothesis that limiting enzyme expression to neuronal tissues would decrease or perhaps prevent liver toxicity (Fig. 9a ). We found that latently infected mice treated with a high dose (2 × 10 12 vg) of AAV9-E/CamKII-m4 or AAV9-E/hSyn-m4 did not show any clinical signs of hepatotoxicity (weight change, general health decline, or ICF), in contrast to mice treated with 2 × 10 12 vg AAV9-CBh-m4 (Fig. 9b, c ). Moreover, while liver inflammation increased over time in AAV9-CBh-m4-treated mice, it remained low in AAV9-E/CamKII-m4 (Fig. S11d ). Intriguingly, histopathologic signs of neurotoxicity in TG from AAV9-E/CamKII-m4 or AAV9-E/hSyn-m4 treated mice were also similar to those in control mice, while they were significantly higher in TG from AAV9-CBh-m4 treated mice (Fig. 9d, e ). A decrease of ganglionic viral loads of 67.9% ( p = 0.07) and 70.4% ( p = 0.05) in SCG and TG respectively, was observed in AAV9-E/CamKII-m4-treated mice but not in mice treated with the AAV9-E/hSyn-m4 (Fig. 9f, g ). Assessment of m4 expression in neuronal tissues at different times post administration of either AAV9-CBh-m4 or AAV9-E/CamKII-m4 showed that the m4 expression increased over time but was in general slightly lower in tissues from AAV9-E/CamKII-m4-treated mice compared with AAV9-CBh-m4-treated mice (Fig. S11a, b ). This may explain the slightly lesser degree of viral load reduction in AAV9- E/CamKII-m4-treated mice compared with AAV9- CBh-m4-treated mice (Fig. 9f, g ). We conclude that the AAV9-E/CamKII-m4 regimen retains efficacy and shows improved tolerability compared to AAV9-CBh-m4.
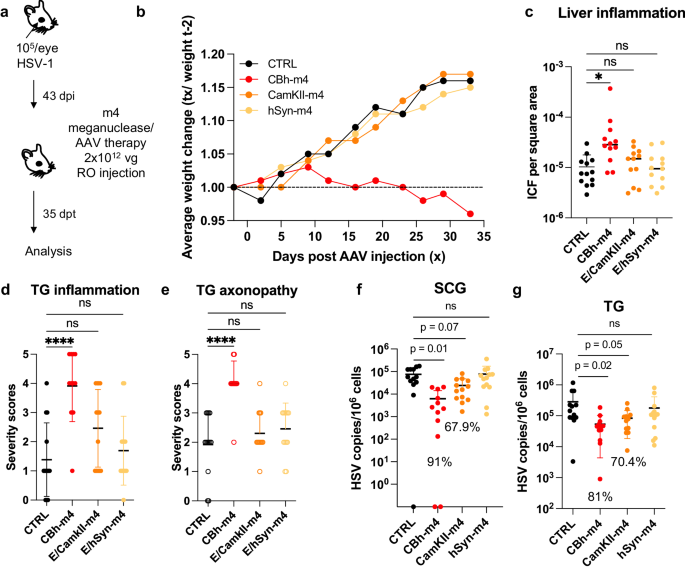
a Experimental timeline of ocular infection and meganuclease therapy. b Average weight change of infected control mice ( n = 13) or HSV-infected mice treated with m4 expressed from either the ubiquitous CBh promoter, or the neuronal promoters E/CamKII or E/hSyn ( n = 12 per group). c Inflammatory cell foci (ICF) in liver sections from either HSV-infected control mice ( n = 13), or mice treated with m4 expressed from either the CBh, E/CamKII or E/hSyn promoter ( n = 12 per group); p = 0.00455 for CBh-m4. d , e Severity scores of inflammation ( d ; p = <0.0001 for CBh-m4) and axonopathy ( e ; p = <0.0001 for CBh-m4) in TG from infected control mice ( n = 10) and infected mice treated with m4 expressed from either the CBh, E/CamKII or E/hSyn promoter ( n = 12 per group) with statistical analysis (Ordinary one-way Anova, multiple comparisons with ns: not significant; * p < 0.05; **** p < 0.0001). f , g HSV loads in SCGs ( f ) and TGs ( g ) from infected control ( n = 10) and infected mice treated with m4 expressed from either the CBh, E/CamKII or E/hSyn promoter ( n = 12 per group). Percent decrease of HSV loads in treated mice compared to control mice and statistical analysis (Ordinary one-way Anova, multiple comparisons with p values; ns: not significant). Each graph shows individual and mean values with standard deviation. The AAV viral loads are shown in Supplemental Figure 10a–c . Source data are provided as a Source Data file.
Human infection with HSV is lifelong, and while current antiviral approaches can reduce symptoms and transmission, they do not cure. As such, there is a strong unmet desire for new and potentially curative approaches for HSV 9 . Here we extend our previous study of gene editing as a potential curative therapy for HSV in three important ways. First, we established a model of HSV reactivation in mice using a small molecule, to show that a reduction in ganglionic HSV loads via gene editing results in a significant reduction in viral shedding from mice with established orofacial infection. Second, we demonstrated high efficacy of gene editing of latent HSV in DRG after genital HSV infection. Third, we reduced or even eliminated the hepato- and neurotoxicity detected in some animals by decreasing the AAV dose, simplifying the therapy regimen, and using a cell type-specific promoter. Together, these findings address several of the major drivers of interest in HSV cure 9 , and support further development of gene editing for HSV infection.
Mice are easily infected with HSV, and have been critical in defining many aspects of HSV infection, latency, and immune control 18 . A major drawback, however, has been the fact that latent HSV infection in mice exhibits minimal to no spontaneous reactivation or peripheral virus shedding 19 . Thus, mice have been of limited utility in studying HSV therapeutics or vaccines that are directed at control of latent infection and reactivation. HSV reactivation in mice can be induced by various stimuli such as immunosuppression 20 , hyperthermic stress 21 , or ultraviolet B irradiation 22 , but these approaches induce minimal shedding, can be cumbersome, and may be applicable to only certain specific mouse strains or HSV isolates. Here we evaluated the reactivation of HSV by intraperitoneal injection of JQ1, a bromodomain inhibitor that has been proposed as a latency-reversing agent for human immunodeficiency virus (HIV) 23 . Previously, JQ1 was reported to reactivate HSV in cell culture models of latency 14 and induce shedding from the eyes of latently infected mice, although the amount and timing of shedding was not fully defined 14 . Here we demonstrate that JQ1 reproducibly induces detectable viral shedding at the periphery from a substantial subset of latently infected mice, at quantitative levels (10 2 to 10 6 copies/mL) that are similar to those observed in human observational studies 24 . Additional studies in C57BL/6 mice latently infected with HSV-1 showed that virus shedding could be also induced with JQ1 administration (Supplemental Table 4 ), suggesting that JQ1-induced virus shedding is not limited to the Swiss Webster strain. Thus, the JQ1 reactivation model should prove useful for future studies regarding the mechanisms and determinants of HSV reactivation and peripheral shedding, and vaccines or therapeutics aiming to reduce such shedding.
Among people infected with HSV, a major concern and driver of the desire for cure is the risk of transmission of the virus to others 9 . While our previous work demonstrated an up to 90% reduction of latent HSV within ganglia after gene editing 8 , it remained unclear what effect such reduction would have on viral shedding at the periphery. Using the JQ1 reactivation model, we found that reduction of ganglionic load via gene editing has a profound effect on viral shedding, both in terms of the fraction of samples with detectable virus, and also in the amount of virus shed. In humans, the relationship between HSV shedding quantity and the likelihood of viral transmission remains incompletely understood, but previous mathematical modeling suggests that reduction of shedding to levels below 10 4 viral copies (as observed in most of our treated animals that exhibited residual shedding) would be expected to greatly reduce, if not fully eliminate, the risk of viral transmission 25 .
One challenge with the JQ1 reactivation model in mice is the stochastic nature of the induced viral reactivation and shedding. Within a given cohort, only a subset of JQ1-treated mice shed detectable HSV, and shedding in one episode was not predictive of subsequent shedding after repeated JQ1 reactivations. Similarly, individual mice often shed unilaterally from a single eye; again, this was not predictive of the laterality of subsequent shedding episodes. These findings are similar to observations in humans, in whom shedding is episodic and stochastic, and can occur at distinct anatomical locations during different shedding episodes 26 . However, they constituted a challenge in adequately powering experiments designed to detect a reduction in viral shedding. While each of our individual experiments demonstrated a similar trend toward reduction of HSV shedding after AAV/meganuclease therapy, many individual experiments did not achieve statistical significance. This was particularly apparent after genital HSV infection, where the lower latent viral load in DRG appeared to result in lower reactivation rates after JQ1 administration. Optimization of the genital infection model to increase latent DRG loads may facilitate further evaluation of the effect of gene editing on viral shedding, although in our experience efforts to increase latent viral loads must be carefully balanced against increased animal mortality during the acute phase of infection. In any event, meta-analysis of the combined data from all our experiments confirmed a highly significant reduction in HSV shedding from AAV/meganuclease-treated animals compared with controls. This reduction in shedding proved to be both dose- and duration-of-therapy-dependent. The latter observation is particularly encouraging in regard to the potential clinical translation of the work. Here, mice were evaluated for ganglionic load and shedding approximately one month after AAV/meganuclease administration, but our data suggest that HSV gene editing efficacy likely continues past this point, which may lead to more complete reduction or elimination of viral shedding at later time points. Experiments are currently underway to evaluate HSV gene editing in mice over periods of a year or more, and to determine whether overall efficacy ultimately plateaus.
A second important finding in our studies is that AAV-delivered meganucleases can readily enter neurons and edit HSV within DRG, the site of HSV latency in genital disease. We have consistently observed superior HSV viral load reduction in SCG (which are autonomic ganglia; typically ~90% reduction) compared to TG (sensory ganglia; typically ~50-60% reduction). This raised the possibility that DRG (also sensory ganglia) might also show similar modest rates of genome reduction. However, gene editing of latent HSV genomes proved to be highly efficient within DRG (97% reduction), suggesting that the differing efficiencies are not intrinsic to the type of ganglion (sensory vs . autonomic). We currently favor the hypothesis that the relative efficiencies of AAV transduction in the various ganglia are driven mainly by the relative permeability of the blood/ganglionic barrier for each ganglionic type, and have a series of experiments underway to address this issue.
One limitation of our work is that the HSV-1 target sequences of the meganucleases used in this study are not well conserved in HSV-2. We therefore performed the vaginal infection experiments using HSV-1, to make the point that there appears to be no barrier to treating genital vs . orofacial disease; that is, the latently infected neurons in both sites are readily accessible to AAV vectors as well as meganuclease-mediated editing of latent HSV genomes. We would point out that successful treatment of genital HSV-1 infection is not a trivial result – over half of new cases of genital herpes in the US are now due to HSV-1 27 . Nevertheless, we fully recognize the importance of HSV-2 as a target, and current work in our laboratory focuses on development of anti-HSV-2 meganucleases and their testing in mice and other models of infection. We are especially interested in targeting duplicated regions of HSV-2, which our results here suggest will allow effective single-nuclease therapy, and we would suggest that duplicated or repeated sites should be considered for gene editing efforts targeting other viruses.
AAV vectors generally have been considered safe, particularly in comparison with other gene therapy vectors 28 . However, at high doses AAV vectors can induce liver toxicity, manifesting initially as transaminase elevation. At AAV doses higher than those used in the experiments presented here, liver toxicity can be severe, and has led to liver failure in several animal models 29 , 30 . We were therefore encouraged that we observed strong anti-HSV activity at AAV doses that were well tolerated in our mouse model. More recently, histological evidence of neuronal injury after AAV administration has been described in mice, rats, piglets, dogs, and non-human primates 29 , 31 , 32 , 33 , and in at least two human trial participants at autopsy 34 . The causative mechanism of such injury remains unclear; among the current leading hypotheses are saturation of neural protein-folding capacity 35 and TLR9-mediated recognition of vector or transgene RNA 36 . Despite histological evidence of neuronal injury, clinical signs in experimental animals have been rare, consisting mainly of mild gait or balance disturbance 29 , 37 , 38 . Such signs have only been reported in a single patient among several thousand human participants in trials of AAV-delivered gene therapies 39 . Consistent with these other studies, we observed subtle evidence of neuronal injury in experimental mice, manifesting as neuronal degeneration, necrosis, and axonopathy, but our mice have not shown any associated behavioral alterations. Our results obtained using a neuronal-specific promoter to drive the meganuclease expression were somewhat surprising, in that they show an absence of the neuronal toxicity readily detected with use of an ubiquitous promoter. While not definitive in themselves, these results support consideration of an alternative hypothesis, that ganglionic neurotoxicity is mediated indirectly through AAV effects on non-neuronal cells, rather than on the neurons themselves. In any event, our data with regimen simplification, dose reduction, and tissue restriction of transgene expression is reassuring that mitigation avenues can be designed to eliminate both hepato- and neurotoxicity. Additional studies in alternative animal models of HSV infection, such as guinea pigs or even non-human primates, are therefore important, and if such studies confirm anti-HSV efficacy with an acceptable safety profile, human trials may be warranted. This is supported by the recent report of the use of a gene editing strategy to remove HSV from humans in an investigator-initiated, open-label, single-arm, non-randomized interventional trial in 3 patients with severe refractory herpetic stromal keratitis (HSK). In this study, no off-target cleavages or systemic adverse events were detected in the 18 months follow-up while preventing viral relapse 40 .
In all studies, 5- to 8-week old female Swiss Webster mice were purchased from either Taconic or Charles River, and housed in accordance with the Fred Hutch Cancer Center and NIH guidelines on the care and use of animals in research. Experimental procedures performed and approved by the Institutional Animal Care and Use Committee (IACUC) of the Fred Hutch Cancer Center. Standard housing, diet, bedding, enrichment, and light/dark cycles were implemented under animal biosafety level 2 (ABSL2) containment.
A limited set of preliminary experiments was performed in C57BL/6 mice (Charles River), and we observed similar rates of viral load reduction and gene editing by T7 assay, suggesting that our results were not strain-specific.
Ocular HSV infection
Mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (12 mg/kg). Mice were infected in both eyes by dispensing 10 5 PFU of HSV1 syn17+ contained in 4 ul following corneal scarification using a 28-gauge needle.
Vaginal HSV infection
Mice were treated with 2 mg of Depo-Provera injected subcutaneously. Five to seven days later, they were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (12 mg/kg) and intravaginally infected with either 5 × 10 2 or 10 3 PFU of HSV1 syn17+ contained in 4 ul using a pipette after clearing the vaginal lumen with a Calginate swab.
AAV inoculation
Mice anesthetized with isoflurane were administered the indicated AAV vector dose by either unilateral intradermal whisker pad (WP), or intravenous injection using unilateral retro-orbital (RO; ocular HSV-infected mice) or tail vein (TV; ocular and vaginal HSV-infected mice) injection. Tissues were collected at the indicated time.
Study approval
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Fred Hutchinson Cancer Center. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (“The Guide”).
HSV reactivation
JQ1 reactivation was performed by intraperitoneal (IP) injection of (+)-JQ-1 (JQ1, MedChemExpress) at a dose of 50 mg/kg at the indicated time. JQ1 was prepared from a stock solution (50 mg/ml in DMSO, Sigma) by 1:10 dilution in a vehicle solution 10% w/v 2-hydroxypropyl-β-cyclodextrin in PBS (Sigma). Alternatively, JQ1 reactivation was performed with 2 IP injections separated by 12 h, which resulted in the detection of virus shedding in the eyes of 6 out of 9 (66.6%) mice (Supplemental Fig. 5 ). A limited set of experiments was performed in C57BL/6 mice (Charles River), and we observed similar rates and kinetics of peripheral viral load shedding, suggesting that the results of JQ1 reactivation were not mouse strain specific. Hyperthermic stress (HS) reactivation was performed as previously described 15 .
Cells, herpes simplex viruses, and AAV stocks
HEK293 41 and Vero cell lines (ATCC # CCL-81) were propagated in Dubelcco’s modified Eagle medium supplemented with 10% fetal bovine serum. HSV-1 strain syn 17 + (kindly provided by Dr. Sawtell) was propagated and titered on Vero cells.
AAV production and titering. AAV vector plasmids pscAAV-CBh-m5, pscAAV-CBh-m8, pscAAV-CBh-m4, pscAAV-CBh -m4i and pscAAV-E/CamKII-m4 were used to generate the AAV stocks in this study (Fig. S12 ). All AAV stocks were generated from transfected HEK293 cells and culture media produced by the Viral Vector Core of the Wellstone Muscular Dystrophy Specialized Research Center (Seattle). AAV stocks were generated by PEG-precipitation of virus from cell lysates and culture media, followed by iodixanol gradient separation 42 , 43 and concentration into PBS using an Amicon Ultra-15 column (EMD Millipore). AAV stocks were aliquoted and stored at −80 o C. All AAV vector stocks were quantified by qPCR using primers/probe against the AAV ITR, with linearized plasmid DNA as a standard, according to the method of Aurnhammer et al. 44 . AAV stocks were treated with DNase I and Proteinase K prior to quantification.
Quantification of viral loads in tissues
Total genomic DNA was isolated from ganglionic tissues using the DNeasy Blood and tissues kit (Qiagen, Germantown, MD) per the manufacturer’s protocol. Viral genomes were quantified by ddPCR in tissue DNA samples using an AAV ITR primer/probe set for AAV, and a gB primer/probe set for HSV as described previously 7 . Cell numbers in tissues were quantified by ddPCR using mouse-specific RPP30 primer/probe set: Forward 5′-GGCGTTCGCAGATTTGGA, Reverse 5′- TCCCAGGTGAGCAGCAGTCT, probe 5′-ACCTGAAGGCTCTGCGCGGACTC. In some control ganglia, sporadic samples showed positivity for AAV genomes, although the levels were typically >2-3 logs lower than in ganglia from treated mice having received AAV. We attribute this to low-level contamination of occasional tissue samples. The ganglionic AAV loads for experiments presented in Figs. 1 – 3 and 5 – 8 are shown in Supplemental Fig. 9 and those for the experiment presented in Fig. 9 are shown in Supplemental fig. 10 . The statistical analysis was performed using GraphPad Prism version 9.4.1. The test used for each data set is indicated in the figure legends.
Quantification of HSV in eye swabs
Swab samples were collected into vials containing 1 ml of digestion buffer (KCL, Tris HCl pH8.0, EDTA, Igepal CA-630). DNA was extracted from 200 ml of digestion buffer using QiaAmp 96 DNA Blood Kits (Qiagen, Germantown, MD) and eluted into 100 ml AE buffer (Qiagen). Then, 10 ml of DNA was used to setup 30 ml real-time Taqman quantitative PCR reactions. The primers and probes were as described previously 45 . QuantiTect multiplex PCR mix (Qiagen) was used for PCR assays. The PCR cycling conditions were as follows: 1 cycle at 50 °C for 2 min, 1 cycle at 95 °C for 15 min, and 45 cycles of 94 °C for 1 min and 60 °C for 1 min. Exo internal control was spiked into each PCR reaction to monitor inhibition. A negative result was accepted only if the internal control was positive with a cycle threshold (CT) within 3 cycles of the Exo CT of no template controls.
Western blot detection
Tissue lysates were obtained from 1 TG per mouse collected in 200 μl RIPA buffer (PIERCE, Thermo Scientific) with protease inhibitor cocktail (Roche) and disrupted by sonication on ice. Thirteen microliters of tissue lysates were loaded onto 4-12% NuPAGE gel, transfer onto nitrocellulose membrane and probe for m4 expression using rabbit anti-HA antibody (1:1000 mAb clone C29F, Cell signaling) and β-actin (1: 1000 mAb clone13E5, cell signaling) for protein loading. Membrane hybridization and detection were performed using PIERCE Fast Western blotting kit Super signal, West pico Rabbit (Thermo Scientific) per manufacturer protocol and imaged using ChemiDoc Imaging system (BIO-RAD).
Inflammatory cell foci quantification
Liver tissues were paraffin-embedded, sectioned and H&E stained by the Experimental histopathology shared resources of the Fred Hutchinson Cancer Center. ICF were counted by a blinded observer and expressed as the number of ICF per surface area which was determined using Fiji 46 .
Grading of neuronal changes within trigeminal ganglia
Trigeminal ganglia were paraffin-embedded, sectioned, and H&E stained by the Experimental histopathology shared resources of the Fred Hutchinson Cancer Center. Microscopic changes were graded as to severity by a veterinary pathologist using a standard grading system whereby 0 = no significant change, 1 = minimal, 2 = mild, 3 = moderate, and 4 = severe.
Statistical analysis
Statistical analyses for each individual experiment were performed using GraphPad Prism version 9.4.1 and R. Tests were two-sided and p-values smaller than 0.05 considered significant. The specific test used for each analysis is indicated in the corresponding figure legend.
Meta-analyses were performed on combined data from all experiments (Figs. 1 – 6 ). To assess whether AAV/MN-treated mice shed virus less often than control animals and whether the frequency of viral shedding decreased with the duration of meganuclease therapy, we used generalized linear mixed models (GLMM) describing the probability of viral shedding (with viral shedding defined as an AUC > 0) as a function of therapy duration and dose treated as continuous variables, while adjusting for experiment. Animal-specific random intercepts were included in the model to capture intra-mice dependencies between observations. We also considered including an interaction term between dose and duration to evaluate whether change in the probability of viral shedding over time was affected by dose (in particular, whether it decreased faster with dose). Association between viral shedding and covariates (dose, therapy duration) are reported as odds ratios (OR). The significance of the association between the probability of viral shedding and dose, therapy duration, and their interactions was evaluated using two-sided Wald tests.
To study the relationship between the quantity of virus shedding and therapy dose and duration, we used linear mixed models (LMM) describing the log10-transformed AUC as a function of therapy dose and duration, treated as continuous variables, while adjusting for experiment. The model included an interaction between dose and therapy duration to assess whether change in AUC over time was impacted by dose (e.g., whether the log10-transformed AUC decreased faster with dose). Animal-specific random intercepts were also included to capture intra-mice dependencies between observations. The standard errors of regression coefficients were estimated using a robust, sandwich-type estimator. Association between viral shedding and covariates (dose, therapy duration) are reported as odds ratios (OR). The significance of the association between the log10-transformed AUC and dose, therapy duration, and their interactions was evaluated using Wald tests. Both sets of analyses also evaluated models that included dose squared and square root of dose to perform sensitivity analysis and assess whether the relationship between the probability of viral shedding (or the log10-transformed AUC) and dose were nonlinear. Likewise, models were considered that included squared therapy duration or square root of therapy duration to perform sensitivity analysis and assess whether relationships between the probability of shedding (or the log10-transformed AUC) and therapy duration were nonlinear.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated are provided in the main text, the supplementary materials or the Source Data file. Meganuclease sequences are Cellectis proprietary information and material. Source data is provided with this paper. Source data are provided with this paper.
Schiffer, J. T. & Corey, L. New concepts in understanding genital herpes. Curr. Infect. Dis. Rep. 11 , 457–464 (2009).
Article PubMed PubMed Central Google Scholar
Corey, L. et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N. Engl. J. Med. 350 , 11–20 (2004).
Article CAS PubMed Google Scholar
Gupta, R. et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J. Infect. Dis. 190 , 1374–1381 (2004).
Fife, K. H. et al. Effect of valacyclovir on viral shedding in immunocompetent patients with recurrent herpes simplex virus 2 genital herpes: a US-based randomized, double-blind, placebo-controlled clinical trial. Mayo Clin Proc. 81 , 1321–1327 (2006).
Mertz, G. J. et al. Long-term acyclovir suppression of frequently recurring genital herpes simplex virus infection. A multicenter double-blind trial. JAMA 260 , 201–206 (1988).
Wald, A., Zeh, J., Barnum, G., Davis, L. G. & Corey, L. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann. Intern. Med. 124 , 8–15, (1996).
Aubert, M. et al. In vivo disruption of latent HSV by designer endonuclease therapy. JCI Insight https://doi.org/10.1172/jci.insight.88468 (2016).
Aubert, M. et al. Gene editing and elimination of latent herpes simplex virus in vivo. Nat. Commun. 11 , 4148 (2020).
Article ADS CAS PubMed PubMed Central Google Scholar
Oseso, L., Magaret, A. S., Jerome, K. R., Fox, J. & Wald, A. Attitudes and willingness to assume risk of experimental therapy to eradicate genital herpes simplex virus infection. Sex Transm. Dis. 43 , 566–571 (2016).
Jacques, S. J. et al. AAV8(gfp) preferentially targets large diameter dorsal root ganglion neurones after both intra-dorsal root ganglion and intrathecal injection. Mol Cell Neurosci. 49 , 464–474 (2012).
Samaranch, L. et al. Strong cortical and spinal cord transduction after AAV7 and AAV9 delivery into the cerebrospinal fluid of nonhuman primates. Hum. Gene Ther. 24 , 526–532 (2013).
Article CAS PubMed PubMed Central Google Scholar
Grosse, S. et al. Meganuclease-mediated inhibition of HSV1 infection in cultured cells. Mol. Ther. 19 , 694–702 (2011).
Parr, M. B. et al. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 70 , 369–380 (1994).
CAS PubMed Google Scholar
Alfonso-Dunn, R. et al. Transcriptional elongation of HSV immediate early genes by the super elongation complex drives lytic infection and reactivation from latency. Cell Host Microbe 21 , 507–517.e505 (2017).
Sawtell, N. M. Detection and quantification of the rare latently infected cell undergoing herpes simplex virus transcriptional activation in the nervous system in vivo. Methods Mol. Biol. 292 , 57–72 (2005).
Schiffer, J. T. et al. Frequent release of low amounts of herpes simplex virus from neurons: results of a mathematical model. Sci. Transl. Med. 1 , 7ra16 (2009).
Article ADS PubMed PubMed Central Google Scholar
Hioki, H. et al. Efficient gene transduction of neurons by lentivirus with enhanced neuron-specific promoters. Gene Ther. 14 , 872–882 (2007).
Kollias, C. M., Huneke, R. B., Wigdahl, B. & Jennings, S. R. Animal models of herpes simplex virus immunity and pathogenesis. J. Neurovirol. 21 , 8–23 (2015).
Hill, T. J. & Shimeld, C. Models of recurrent infection with HSV in the skin and eye of the mouse. Methods Mol. Med. 10 , 273–289, (1998).
Cook, S. D. et al. Ocular herpes simplex virus reactivation in mice latently infected with latency-associated transcript mutants. Invest. Ophthalmol. Vis. Sci. 32 , 1558–1561 (1991).
Sawtell, N. M. & Thompson, R. L. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J. Virol. 66 , 2150–2156 (1992).
Laycock, K. A., Lee, S. F., Brady, R. H. & Pepose, J. S. Characterization of a murine model of recurrent herpes simplex viral keratitis induced by ultraviolet B radiation. Invest. Ophthalmol. Vis. Sci. 32 , 2741–2746 (1991).
Alamer, E., Zhong, C., Hajnik, R., Soong, L. & Hu, H. Modulation of BRD4 in HIV epigenetic regulation: implications for finding an HIV cure. Retrovirology 18 , 3 (2021).
Ramchandani, M. et al. Herpes simplex virus type 1 shedding in tears and nasal and oral mucosa of healthy adults. Sex Transm. Dis. 43 , 756–760 (2016).
Schiffer, J. T., Mayer, B. T., Fong, Y., Swan, D. A. & Wald, A. Herpes simplex virus-2 transmission probability estimates based on quantity of viral shedding. J. R. Soc. Interface 11 , 20140160 (2014).
Schiffer, J. T. et al. Rapid localized spread and immunologic containment define Herpes simplex virus-2 reactivation in the human genital tract. Elife 2 , e00288 (2013).
Ryder, N., Jin, F., McNulty, A. M., Grulich, A. E. & Donovan, B. Increasing role of herpes simplex virus type 1 in first-episode anogenital herpes in heterosexual women and younger men who have sex with men, 1992-2006. Sex Transm. Infect. 85 , 416–419 (2009).
Bulcha, J. T., Wang, Y., Ma, H., Tai, P. W. L. & Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 6 , 53 (2021).
Hinderer, C. et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum. Gene Ther. 29 , 285–298 (2018).
Morales, L., Gambhir, Y., Bennett, J. & Stedman, H. H. Broader implications of progressive liver dysfunction and lethal sepsis in two boys following systemic high-dose AAV. Mol. Ther. 28 , 1753–1755 (2020).
Palazzi, X. et al. Biodistribution and tolerability of AAV-PHP.B-CBh-SMN1 in wistar han rats and cynomolgus macaques reveal different toxicologic profiles. Hum Gene Ther. 33 , 175–187 (2022).
Schuster, D. J. et al. Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Front. Neuroanat. 8 , 42 (2014).
Hinderer, C. et al. Neonatal tolerance induction enables accurate evaluation of gene therapy for MPS I in a canine model. Mol. Genet. Metab. 119 , 124–130 (2016).
Mullard, A. Gene therapy community grapples with toxicity issues, as pipeline matures. Nat. Rev. Drug Discov. 20 , 804–805 (2021).
Hordeaux, J. et al. MicroRNA-mediated inhibition of transgene expression reduces dorsal root ganglion toxicity by AAV vectors in primates. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aba9188 (2020).
Hamilton, B. A. & Wright, J. F. Challenges posed by immune responses to AAV vectors: addressing root causes. Front. Immunol. 12 , 675897 (2021).
Hordeaux, J. et al. Adeno-associated virus-induced dorsal root ganglion pathology. Hum. Gene Ther. 31 , 808–818 (2020).
Keiser, M. S. et al. Toxicity after AAV delivery of RNAi expression constructs into nonhuman primate brain. Nat. Med. 27 , 1982–1989 (2021).
Kuzmin, D. A. et al. The clinical landscape for AAV gene therapies. Nat. Rev. Drug Discov. 20 , 173–174 (2021).
Wei, A. et al. In vivo CRISPR gene editing in patients with herpetic stromal keratitis. Mol. Ther. 31 , 3163–3175 (2023).
Graham, F. L., Smiley, J., Russell, W. C. & Nairn, R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36 , 59–74, (1977).
Choi, V. W., Asokan, A., Haberman, R. A. & Samulski, R. J. Production of recombinant adeno-associated viral vectors for in vitro and in vivo use. Curr. Protoc. Mol. Biol. https://doi.org/10.1002/0471142727.mb1625s78 (2007).
Zolotukhin, S. et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 6 , 973–985 (1999).
Aurnhammer, C. et al. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum. Gene Ther. Methods 23 , 18–28 (2012).
Jerome, K. R., Huang, M. L., Wald, A., Selke, S. & Corey, L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J. Clin. Microbiol. 40 , 2609–2611 (2002).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9 , 676–682 (2012).
Download references
Acknowledgements
We are grateful to Cellectis (Paris, France) for the original development of meganucleases m4, m5, and m8, as well as Philippe Duchateau and Roman Galetto (Cellectis) for helpful discussions. This work was supported by National Institutes of Health grant R01AI132599 (KRJ), Caladan Foundation (KRJ), National Institutes of Health grant 5P50AR065139 (Viral Vector Core of the Wellstone Muscular Dystrophy Specialized Research Center, Seattle), National Institutes of Health / National Cancer Institutes, Cancer Center Support Grant P30 CA015704 (Fred Hutchinson Cancer Center Shared Resources Division), donations from the Kenneth Hill Foundation, Krieger Family Trust, Tiny Foundation, and over 2000 individual donors.
Author information
Authors and affiliations.
Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, WA, 98109, USA
Martine Aubert, Anoria K. Haick, Lindsay M. Klouser, Michelle A. Loprieno, Ollivier Hyrien, Daniel Stone & Keith R. Jerome
Department of Laboratory Medicine and Pathology, University of Washington, Seattle, WA, 98133, USA
Daniel E. Strongin, Lindsay M. Klouser, Laurence Stensland, Tracy K. Santo, Meei-Li Huang & Keith R. Jerome
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: K.R.J., M.A., D.S., D.E.S. and O.H. Investigation: M.A., A.K.H., D.E.S., L.M.K., M.A.L., L.S., T.K.S. and O.H. Funding acquisition: K.J.R., M.A. and D.S. Supervision: K.R.J., M.A., D.S. and M.L.H. Writing – original draft: M.A., K.R.J. and O.H. Writing – review & editing: M.A., K.R.J., D.S. and O.H.
Corresponding author
Correspondence to Keith R. Jerome .
Ethics declarations
Competing interests.
K.R.J. is a founder and holds equity in Caladan Therapeutics, is a paid advisor and holds equity in Excision Biosciences, participates in sponsored research agreements with Excision Biosciences and Emendo Biotherapeutics, and is co-inventor of International Patent Application No. PCT/US2022/013757 and U.S. Provisional Application No. 63/503,541 held by Fred Hutch for the treatment of HSV-1 and HSV-2 using meganucleases. There are no restrictions on publication of data. M.A. and D.S. have sponsored research agreements with Excision Biosciences. The remaining authors declare no conflict of interest.
Peer review
Peer review information.
Nature Communications thanks Joseph Glorioso, Paul Kinchington and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Aubert, M., Haick, A.K., Strongin, D.E. et al. Gene editing for latent herpes simplex virus infection reduces viral load and shedding in vivo. Nat Commun 15 , 4018 (2024). https://doi.org/10.1038/s41467-024-47940-y
Download citation
Received : 22 January 2023
Accepted : 15 April 2024
Published : 13 May 2024
DOI : https://doi.org/10.1038/s41467-024-47940-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Candel Therapeutics-stock
- News for Candel Therapeutics
Candel Therapeutics to Host Non-Small Cell Lung Cancer (NSCLC) R&D Breakfast Panel During 2024 ASCO Annual Meeting
NEEDHAM, Mass., May 20, 2024 (GLOBE NEWSWIRE) -- Candel Therapeutics, Inc. (Candel or the Company) (Nasdaq: CADL), a clinical stage biopharmaceutical company focused on developing multimodal biological immunotherapies to help patients fight cancer, today announced it will be hosting a webcasted R&D breakfast panel featuring prominent scientific and medical thought leaders to discuss topline overall survival data from its phase 2 clinical trial of CAN-2409, its multimodal biological immunotherapy candidate, in Non-Small Cell Lung Cancer (NSCLC).
The event will be held on Monday, June 3, 2024, at 7:00 AM Central Time, during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago.
Paul Peter Tak, MD, PhD, FMEDSci, President and Chief Executive Officer of Candel , will be hosting the event and moderating the guest panel, which includes:
- Charu Aggarwal, MD, MPH, FASCO Leslie M. Heisler Associate Professor for Lung Cancer Excellence, Perelman School of Medicine, University of Pennsylvania
- Roy S. Herbst, MD, PhD Chief of Medical Oncology Yale School of Medicine Candel Research Advisory Board
- Daniel H. Sterman, MD, FCCP, ATSF, DAABIP Professor and Director, Pulmonary, Critical Care and Sleep Medicine NYU Langone Health
A live webcast will be available by selecting Events and Presentations, under the News & Events tab, in the Investors section on Candeltx.com. A replay of the webcast will be archived for up to 90 days following the session date.
EDITOR’S NOTE: Media representatives interested in attending the event should please contact Kyle Evans at [email protected].
About CAN-2409
CAN-2409, Candel’s most advanced multimodal biological immunotherapy candidate, is an investigational, off-the-shelf, replication-defective adenovirus designed to deliver the herpes simplex virus thymidine kinase (HSV-tk) gene to a patient’s specific tumor and induce an individualized, systemic anti-tumor immune response. HSV-tk is an enzyme that locally converts orally administered valacyclovir into a toxic metabolite that kills nearby cancer cells. Together, this regimen is designed to induce an individualized and specific CD8+ T cell mediated response against the injected tumor and uninjected distant metastases for broad anti-tumor activity, based on in situ vaccination against a variety of tumor antigens. Because of its versatility, CAN-2409 has the potential to treat a broad range of solid tumors. Encouraging monotherapy activity as well as combination activity with standard of care radiotherapy, surgery, chemotherapy, and immune checkpoint inhibitors have previously been shown in several preclinical and clinical settings. Furthermore, more than 1,000 patients have been dosed with CAN-2409 with a favorable tolerability profile to date, supporting the potential for combination with other therapeutic strategies without inordinate concern of overlapping adverse events.
Currently, Candel is evaluating the effects of treatment with CAN-2409 in NSCLC, borderline resectable pancreatic ductal adenocarcinoma (PDAC), and localized, non-metastatic prostate cancer in ongoing clinical trials. CAN-2409 plus prodrug (valacyclovir) has been granted Fast Track Designation by the U.S. Food and Drug Administration (FDA) for treatment of stage III/IV NSCLC in patients who are resistant to first line PD-(L)1 inhibitor therapy and who do not have activating molecular driver mutations or have progressed on directed molecular therapy, treatment of borderline resectable PDAC, and treatment of localized prostate cancer. The FDA has also granted Orphan Drug Designation to CAN-2409 for the treatment of PDAC. The Company’s pivotal phase 3 clinical trial in prostate cancer is being conducted under a Special Protocol Assessment by the FDA.
About Candel Therapeutics
Candel is a clinical stage biopharmaceutical company focused on developing off-the-shelf multimodal biological immunotherapies that elicit an individualized, systemic anti-tumor immune response to help patients fight cancer. Candel has established two clinical stage multimodal biological immunotherapy platforms based on novel, genetically modified adenovirus and herpes simplex virus (HSV) gene constructs, respectively. CAN-2409 is the lead product candidate from the adenovirus platform and is currently in ongoing clinical trials in NSCLC (phase 2), borderline resectable PDAC (phase 2), and localized, non-metastatic prostate cancer (phase 2 and phase 3). CAN-3110 is the lead product candidate from the HSV platform and is currently in an ongoing phase 1b clinical trial in recurrent high-grade glioma (rHGG). In October 2023, the Company announced that Nature published results from this ongoing clinical trial. Finally, Candel’s enLIGHTEN™ Discovery Platform is a systematic, iterative HSV-based discovery platform leveraging human biology and advanced analytics to create new viral immunotherapies for solid tumors. The Company presented preclinical data at the AACR Annual Meeting in April 2024, unveiling the second candidate from this platform, a first-in-class multimodal immunotherapy candidate to induce tertiary lymphoid structures (TLS), being developed as a novel therapeutic for solid tumors.
For more information about Candel, visit: www.candeltx.com.
Forward-Looking Statements
This press release includes certain disclosures that contain “forward-looking statements,” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, express or implied statements regarding the timing and advancement of the Company’s development programs, including key data readout presentations; expectations regarding the therapeutic benefit of the Company’s programs; and the ability of CAN-2409 to improve the median overall survival of patients with NSCLC. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements in this press release are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward-looking statements contained in this press release, including, without limitation, those risks and uncertainties related to the timing and advancement of development programs; the Company’s ability to continue as a going concern; expectations regarding the therapeutic benefit of the Company’s programs; that final data from the Company’s pre-clinical studies and completed clinical trials may differ materially from reported interim data from ongoing studies and trials; the Company’s ability to efficiently discover and develop product candidates; the Company’s ability to obtain and maintain regulatory approval of product candidates; the Company’s ability to maintain its intellectual property; the implementation of the Company’s business model, including strategic plans for the Company’s business and product candidates; and other risks identified in the Company’s filings with the U.S. Securities and Exchange Commission (SEC) including the Company’s most recent Quarterly Report on Form 10-Q filed with the SEC and subsequent filings with the SEC. The Company cautions you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. The Company disclaims any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions, or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. Any forward-looking statements contained in this press release represent the Company’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date.
Investor Contact: Theodore Jenkins VP, Investor Relations and Business Development Candel Therapeutics, Inc. [email protected]
Media Contact: Kyle Evans ICR Westwicke [email protected]

Candel Therapeutics News MORE
Related stocks.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Clin Infect Dis

Editor's choice
Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women, david i. bernstein.
1 Division of Infectious Diseases, Cincinnati Children's Hospital Medical Center,, University of Cincinnati, Ohio
Abbie R. Bellamy
2 EMMES Corporation, Rockville, Maryland
Edward W. Hook, III
3 Departments of Medicine, Microbiology, and Epidemiology, University of Alabama at Birmingham
Myron J. Levin
4 Section of Pediatric Infectious Diseases, Anschutz Medical Campus, University of Colorado Denver,, Aurora
5 Department of Medicine, Epidemiology, and Laboratory Medicine, University of Washington
6 Vaccine and Infectious Diseases Division, Fred Hutchinson Cancer Research Center,, Seattle, Washington
Marian G. Ewell
Peter a. wolff.
7 National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland
Carolyn D. Deal
Thomas c. heineman.
8 GlaxoSmithKline, King of Prussia, Pennsylvania
Robert B. Belshe
9 Division of Infectious Diseases and Immunology, Saint Louis University, Missouri
In this large prospective study of primary herpes simplex virus (HSV) infections in young, HSVseronegative women, we found that the rate of HSV-1 infections was more than twice that for HSV-2, but there were important age and race differences.
Background. Herpes simplex virus infections type 1 (HSV-1) and type 2 (HSV-2) are common, but the epidemiology of HSV disease is changing.
Methods. HSV-seronegative women, aged 18–30 years, who were in the control arm of the HERPEVAC Trial for Women were followed for 20 months for primary HSV infections.
Results. Of the 3438 evaluable participants, 183 became infected with HSV: 127 (3.7%) with HSV-1 and 56 (1.6%) with HSV-2. The rate of infection for HSV-1 (2.5 per 100 person-years) was more than twice that for HSV-2 (1.1 per 100 person-years). Most infections (74% of HSV-1 and 63% of HSV-2) occurred without recognized signs or symptoms of herpes disease. The HSV-2 infection rate was 2.6 times higher in non-Hispanic black participants than in Hispanics and 5.5 times higher than in non-Hispanic whites ( P < .001), while the HSV-1 infection rate was 1.7 times higher in non-Hispanic whites than non-Hispanic blacks. Younger participants (18–22 years) were more likely to acquire HSV-1 infections and less likely to develop recognized disease than older participants. Overall, 84% of recognized disease cases were genital. No differences were noted in the clinical manifestations of genital HSV-1 vs genital HSV-2 disease. The clinicians’ assessment that cases were caused by HSV was good when they assessed cases as clinically confirmed or unlikely (validated in 83% and 100% of cases, respectively).
Conclusions. HSV-1 is now more common than HSV-2 as a cause of oral and genital mucosal infections in young women, but there are important age and race differences.
(See the Editorial Commentary by Whitley on pages 352–3.)
Herpes simplex virus (HSV) is a common cause of both genital and oral disease. HSV type 2 (HSV-2), a sexually transmitted pathogen, infects >500 million people worldwide and causes an estimated 23 million new infections each year [ 1 ]. HSV type 1 (HSV-1) is even more common, with an estimated seroprevalence of >90% in many nations [ 2 ]. HSV-1 is frequently acquired during early childhood, primarily through oral secretions [ 3 ]. However, the epidemiology of HSV-1 is changing, such that the frequency of sexual transmission of HSV-1 has increased in many countries, including the United States [ 4 , 5 ].
The control arm of an investigational HSV-2 vaccine study, the HERPEVAC Trial for Women [ 6 ], provided the opportunity to prospectively follow a large cohort of HSV-seronegative women in order to characterize the epidemiology, clinical manifestations, and antibody response to primary HSV infections. Thus, all infections identified in these women were primary infections in which the etiology (HSV-1 or HSV-2) could be confirmed by Western blot analysis and the identification of symptomatic vs unrecognized primary infections categorized with a high degree of certainty. This obviated limitations present in past studies where primary and recurrent infections might be misclassified, and where the ability to serologically distinguish HSV-1 from HSV-2, was suboptimal.
Study Population
A subset of participants enrolled in the HERPEVAC Trial for Women, a phase 3 herpes vaccine study [ 6 ] at 50 sites in the United States and Canada from 2003 to 2007, were included in this analysis. Women aged 18–30 years who were seronegative for HSV-1 and HSV-2, absent of significant health problems, not pregnant, and willing to use effective methods of birth control, were enrolled. The analysis presented here includes the 3438 women who only received the control hepatitis A vaccine (Havrix) in this trial, and had at least 1 follow-up visit. Race and ethnicity were self-described by participants and classified as non-Hispanic white, non-Hispanic black, or Hispanic (any race) for analysis. Women with missing data or not fitting into one of these categories were classified as “other.”
Study Design
Participants were followed for 20 months after study entry. They were educated regarding the signs and symptoms of genital and nongenital herpes disease and requested to attend clinic within 48 hours, or as soon as possible thereafter, if they noted possible signs or symptoms. Active surveillance for suspected HSV disease was conducted monthly by telephone call, e-mail, text message, or social media communication. Serum for evaluation of infection (seroconversion) was obtained prior to vaccination (month 0) and at months 2, 6, 7, 12, 16, and 20. At clinic visits for suspected genital or nongenital herpes disease, participants were examined, viral cultures were obtained, and treatment was initiated at the discretion of the local investigator. The protocol was approved by the institutional review boards/ethics committees at all sites, and participants gave written informed consent.
An HSV infection was defined as unrecognized if a woman seroconverted to HSV-1 or HSV-2 and did not present with any signs or symptoms of disease within the previous 6 months. HSV disease was defined by the presence of compatible signs and/or symptoms with confirmation by virus culture and/or seroconversion within 6 months after onset of symptoms. Cases were determined centrally by an independent blinded endpoint review committee.
Laboratory Methods
Serum samples were evaluated for antibodies to HSV-2 with HerpeSelect-2 (Focus Technologies, Cypress, CA) after each blood draw. Samples testing positive, using the manufacturer's criteria, were then tested for HSV-1 and HSV-2 antibody using Western blot (WB) at the University of Washington [ 7 ]. For each participant, the first and last serum samples were also tested by WB. Seroconversion was defined as a positive HSV-1 and/or HSV-2 WB in a participant with a previously negative result for the corresponding HSV type. If seroconversion was detected using the first and last sample, all samples were tested to determine the time of seroconversion.
Statistical Analysis
Categorical variables were compared by Fisher exact test, and continuous variables were compared by Student t test. Cox proportional hazards models were used to compare rates of HSV-1 and HSV-2 infection by age group and race/ethnicity.
The association between genital HSV disease and participants’ reported signs and symptoms was assessed by fitting separate univariate logistic regression models for each sign or symptom (lesion location [genital or perianal/buttocks], papules, vesicles, ulcers, crusts, fissures, pain, painful urination, redness, vaginal discharge, swelling, headache, malaise, muscle ache, fever). Then, any sign or symptom found to be significant ( P < .05) in the univariate models was considered for inclusion in a multivariate logistic regression model. Signs and symptoms were chosen for inclusion in the multivariate model using a forward selection algorithm; where at each step the variable that most reduced Akaike information criterion was selected. The variables selected by the model were used to define classification rules for genital HSV disease, and the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of each rule compared with the participant's true HSV genital disease status was calculated. The logistic regression modeling analyses included 165 participants who presented with at least 1 suspected genital herpes episode and provided complete diary card data; 17 participants without completed diary cards were excluded.
Of the 3438 subjects included in the natural history analysis, 98 (3%) did not have any samples tested by WB, 2 (<1%) had only baseline WB results, and 3338 (97%) had first and last samples tested by WB. Of these 3438 participants, 183 became infected with HSV: 127 (3.7%) with HSV-1 and 56 (1.6%) with HSV-2. Of the 242 women who presented for evaluation of signs and/or symptoms that the participant felt were compatible with HSV infection, as they had been instructed, 54 (22%) had confirmed HSV disease (Figure (Figure1). 1 ). Irrespective of virus type, most (84%) recognized disease was genital. Of the 54 participants with symptomatic HSV, 33 had HSV-1 disease (5 oral, 24 genital, 4 both genital and oral), and 21 had HSV-2 disease (all genital). Two additional participants from this group who were not infected at the time of their clinical evaluation later developed unrecognized HSV-1 infections, while 6 developed unrecognized HSV-2 infections (Figure (Figure1). 1 ). An additional 92 participants who did not present with suspected disease became infected with HSV-1, while 29 who did not present with suspected disease became infected with HSV-2. Thus, most infections in this study (74% of HSV-1 and 63% of HSV-2) occurred without recognized signs or symptoms of herpes disease despite frequent reminders to return to the clinics with any sign or symptom compatible with HSV disease. The rate of infection for HSV-1 was 2.5 cases per 100 person-years (py) and for HSV-2, 1.1 cases per 100 py (Table (Table1). 1 ). Among non-Hispanic black participants, 74% (20 of 27) of those who acquired HSV were found to have HSV-2 (rate of infection, 4.4 per 100 py). In comparison, HSV-2 accounted for 23% (31 of 135) of HSV infections in non-Hispanic white participants (rate of infection, 0.8 per 100 py; P < .0001 vs non-Hispanic black participants). In Hispanic participants with HSV infections, 40% (4 of 10) were HSV-2 (attack rate, 1.7 per 100 py), while 9% (1 of 11) of HSV infections in participants of other race/ethnicity were HSV-2. In contrast, the rate of infection for HSV-1 was 1.5 per 100 py in non-Hispanic blacks, and 2.6 per 100 py in both non-Hispanic whites and in Hispanics. Participants 18–22 years of age were significantly more likely than older participants to acquire HSV-1 infections and significantly more likely to develop asymptomatic or unrecognized disease. This trend was similar for HSV-2 infections, although the differences were not significant.
Rate and Frequency of Herpes Simplex Virus Infection in Healthy Young Women
Abbreviations: HSV, herpes simplex virus; py, person-years.
a P = .001 for comparison with 18–22-year-olds.
b P = .03 for comparison with 18–22-year-olds.
c P = .002 for comparison with 18–22-year-olds.
d P ≤ .0001 for comparison with non-Hispanic white participants.

Natural history of herpes simplex virus (HSV) infections in herpes simplex virus (HSV)–seronegative women aged 18–30 years. The analysis presented here includes the 3438 women who only received the control hepatitis A vaccine (Havrix) in the HERPEVAC trial, were not infected with HSV at baseline, and had at least 1 follow-up visit. Abbreviation: HSV, herpes simplex virus.
Genital HSV Disease
We compared the clinical manifestations of genital disease caused by HSV-1 (n = 28) to those caused by HSV-2 (n = 21) in the 49 women who developed genital symptoms and lesions (Table (Table2). 2 ). Lesions were reported by 93% of participants with HSV-1 and 81% of participants with HSV-2 genital disease ( P = .38). Similarly, lesions were documented by the clinician in 71% of participants with either HSV-1 or HSV-2 genital disease. The most frequent lesion types reported by participants were similar for HSV-1 and HSV-2: ulcers (HSV-1, 75%; HSV-2, 52%), vesicles (HSV-1, 64%; HSV-2, 48%), and papules (HSV-1, 61%; HSV-2, 57%). Approximately 50% of women with genital HSV disease had systemic symptoms, most commonly malaise, regardless of the HSV type (Table (Table2). 2 ). Fever was noted in 18% of those infected with HSV-1 and 33% of those infected with HSV-2 ( P = .32). Muscle aches were reported by 36% of participants with HSV-1 and 38% of participants with HSV-2 ( P = 1.0). Additionally, local symptoms of genital HSV-1 or HSV-2 disease were similar, with about 90% reporting pain, burning, or itching, and about 50% reporting a vaginal discharge.
Local Manifestations of Primary Symptomatic Genital and Oral Herpes Disease
Abbreviation: HSV, herpex simplex virus.
a P = .0003 for comparison between HSV-1 and HSV-2 for symptoms collected on subject diary card.
b A subject may have had >1 location of lesions recorded.
c “Other” locations specified by clinician for 3 subjects included (1) internal genital, (2) cervix and mons pubis, (3) cervicovaginal.
d Local and systemic symptoms were only recorded on the subjects’ diary cards.
Oral HSV Disease
All oral HSV disease (n = 9) was caused by HSV-1. Systemic symptoms were reported by 56% of the participants. Local symptoms included pain/burning/itching (100%), redness (44%), and swelling (22%).
Accuracy of Clinical Assessment for Genital HSV Disease
When clinicians assessed the participants presenting for possible genital herpes as clinically confirmed herpes, their assessments were validated by HSV culture and/or serological test results 83% of the time (Table (Table3). 3 ). When their assessment was that genital herpes was possible, 30% were validated. No cases that the investigator felt were not genital herpes were confirmed as genital herpes by laboratory testing.
Accuracy of Clinician Assessment
Thirteen subjects with missing investigator assessment or clinical exam date were excluded.
Univariate logistic regression analysis of participants reporting signs and symptoms of genital herpes demonstrated significant associations between the diagnosis of genital HSV disease and the presence of vesicles, ulcers, pain, painful urination, redness, swelling, malaise, and muscle aches. These 8 variables were considered for inclusion in a multivariate logistic regression model. A forward selection algorithm resulted in a model with 4 predictors (in order of selection): ulcers, vesicles, painful urination, and pain, which were used to define 8 classification rules: classify with HSV disease if woman has (1) ulcers; (2) ulcers or vesicles; (3) ulcers or vesicles or painful urination; (4) ulcers or vesicles or painful urination or pain; (5) at least 1 of the 4 signs/symptoms; (6) at least 2 of the 4 signs/symptoms; (7) at least 3 of the 4 signs/symptoms; (8) all 4 of the 4 signs/symptoms. The predictive ability of each classification rule is summarized in Table Table4. 4 . Specificity, positive predictive value, and accuracy for the diagnosis of genital herpes were all highest when ulcers were present.
Signs and Symptoms Predictive of Laboratory-Confirmed Genital Herpes Disease
Abbreviations: NPV, negative predictive value; PPV, positive predictive value.
HSV Antibody Response
When the 28 participants with HSV-1 genital disease (23 culture confirmed) and the 21 participants with HSV-2 genital disease (16 culture confirmed) were evaluated by WB, all but 3 women with HSV-1 infection seroconverted after developing genital herpes. Of these, 1 did not return for follow-up and 2 had not developed antibodies at 8 and 13 months, after culture-confirmed HSV-1 genital infection. Of the 5 participants who developed oral disease without genital disease, 3 had disease confirmed by culture and all seroconverted to HSV-1. Of particular interest, 6 participants (1 genital HSV-1, 4 genital HSV-2, and 1 oral HSV-1) seroconverted prior to developing recognized symptoms of herpes disease (data not shown). Their first recognized symptoms occurred from 176 to 319 days after they were first infected.
During the study, 32 participants developed an indeterminate WB (14 for HSV-1, 13 for HSV-2, and 5 for both HSV-1 and HSV-2). A designation of indeterminate is assigned when the test does not meet the definition for a positive but has some evidence indicating the presence of HSV antibody. Of these participants, 25 later developed a positive WB response (average, 184 days after the first indeterminate result [range, 30–434 days]). Seven participants with an indeterminate WB did not develop a positive WB during study follow-up, 3 did not have any further samples tested after the indeterminate result was obtained, and 4 remained indeterminate throughout study follow-up (range, 23–421 days).
This is the largest prospective study of HSV acquisition in HSV-seronegative women ever performed. It confirms and extends several observations of primary HSV infections. The rate of infection for HSV-1 (2.5 per 100 py) was more than twice that for HSV-2 (1.1 per 100 py) in young women. This is quite different from the rate of 1.0 case per 100 py for HSV-1 and 6.8 per 100 py for HSV-2 in women in another prospective study evaluating participants in an HSV vaccine study conducted from 1993 to 1995 [ 8 ]. However, that study was enriched for women with a high risk of exposure to HSV-2 (HSV-discordant couples and sexually transmitted infection [STI] clinic attendees). Further, women with and without previous HSV-1 infections were included and only women with ≥4 sexual partners in the prior year prior were enrolled. In a previous prospective study of women recruited from STI clinics from 1992 to 1995, the HSV-2 infection rate was also considerably higher, 20.5 per 100 py [ 9 ], whereas in a study of young adolescents conducted in early 2000, the rate was 4.4 and 3.2 per 100 py for HSV-2 and HSV-1, respectively [ 10 ]. The higher HSV-2 rate of infection in this study may reflect the predominance of non-Hispanic black participants in this trial.
In the young adult women studied here, clinically recognized HSV-1 infections presented 3 times more commonly as genital than oral disease. Thus, while HSV-1 acquired in childhood occurs as oral infections, in young adults the majority may be acquired as genital infection. This finding must be tempered by the fact that while participants were instructed to report any oral or genital HSV disease, the emphasis for the study was genital disease. Furthermore, the majority (74%) of HSV-1 infections did not produce recognizable disease and thus the site of infection is unknown.
Infection rates for HSV-1 and HSV-2 differed markedly between racial groups. HSV-2 infections accounted for 74% of HSV infections in non-Hispanic blacks (the rate of infection was 2.6 times higher than in Hispanics and 5.5 times higher than in non-Hispanic whites). In contrast, the rate of HSV-1 infection was 1.7 times higher in non-Hispanic whites than in non-Hispanic blacks, and it was identical in Hispanics and non-Hispanic whites. In a previous study, [ 8 ] the HSV-2 acquisition rate was similarly nearly double for non-white women (11.2 per 100 py) compared with white women (5.8 per 100 py). This suggests that there are differences in the prevalence of HSV types in the source partners of the study participants as documented in large sero-surveys [ 11 , 12 ]. Alternatively, the variability in type-specific HSV rates of infection may be in part attributable to race-based differences in sexual practices or other behaviors.
The rates of infection and the development of recognized HSV disease also differed by age. Younger participants were more likely to acquire HSV-1 infections compared with older participants and less likely to develop recognized disease. Differences in HSV type may reflect differences in sexual practices by age, with younger participants more likely to engage in oral than vaginal sex [ 13 ]. Differences in the development of recognized signs and symptoms of HSV disease may reflect a maturing ability to recognize changes in sexual health or a real difference in the development of the signs and symptoms.
As noted in previous studies, [ 8 , 13 – 15 ] most primary infections with HSV do not produce recognized signs or symptoms. In the study reported here, 74% of HSV-1 and 63% of HSV-2 infections did not produce participant-recognized disease (or at least did not bring them to the study clinics). Thus, despite educational efforts, most participants were unaware of their infections and would therefore not undertake efforts to prevent transmission .
The manifestations of HSV-1 were compared to HSV-2 and, as previously noted [ 16 ], the infections produced indistinguishable disease.
As reported previously [ 17 , 18 ], the first clinical presentation of genital HSV can occur long after the primary infection. In the study reported here, 6 participants were infected and developed HSV antibody responses 176–319 days before developing the first oral (n = 1) or genital (n = 5) symptoms of HSV. In a previous study, 8% of participants developed their first clinical episode of genital HSV-2 after developing HSV-2 antibodies [ 8 ].
Western blots are often obtained to determine if a patient has been infected with HSV. Physicians may receive a report of indeterminate results either because the infection is recent or for other unexplained reasons. Of the 32 WBs reported as indeterminate, 25, became positive and 7 remained indeterminate Thus, the majority were infected with HSV.
Finally, it is difficult to distinguish genital HSV infections from other diseases that cause similar signs and symptoms by clinical exam, and thus culture (or polymerase chain reaction) and/or serology is recommended as an aid to the clinician. [ 14 , 15 , 19 ]. We developed a multivariate logistic regression model using a forward selection algorithm of 8 signs or symptoms that were associated with genital HSV by univariate analysis. This resulted in a model with 4 predictors, which were used to define rules for identifying genital HSV infections. Use of only 1 of these signs/symptoms to identify genital HSV infections had the highest sensitivity (98%), whereas the rule that required all 4 to be present had the best specificity (100%) and accuracy (83%).
There are several factors that might influence our findings and their generalizability. The population evaluated was a diverse group of women, 18–30 years of age. However, they represent a self-selected sample that volunteered to be in a study of a herpes vaccine and therefore could conceivable be more at risk for acquiring an HSV infection. Thus, it is possible our estimates of rates of infection could be somewhat higher than the general population of young women.
In summary, this is the largest prospective study of documented primary HSV infections in which the identification of symptomatic vs unrecognized primary infections could be categorized with a high degree of certainty. We found that, overall, the rate of infection for HSV-1 was higher than for HSV-2, but that there were significant age and racial differences. Infections by either type were most often not recognized by the participants, despite the efforts to educate them regarding possible signs and symptoms of HSV disease. These findings have important implications for the design and implementation of treatment and prevention strategies.
Author contributions. D. I. B. and R. B. B. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases ( N01-AI-45250 ) and GlaxoSmithKline.
Potential conflicts of interest. D. I. B. has received lecture fees and royalties from GlaxoSmithKline. R. B. B. has served as a board member of Vivaldi Biosciences, has received consulting fees from GlaxoSmithKline, has received consulting fees and lecture fees from MedImmune, and has received lecture fees from Merck. G. D. is an employee of and has received stock and travel, accommodations, and meeting expenses from GlaxoSmithKline and royalties from Pfizer. T. C. H. is an employee of GlaxoSmithKline and has received travel, accommodations, meeting expenses, and stock equity from GlaxoSmithKline. M. J. L. has received consulting fees and grants from GlaxoSmithKline. A. W. has received consulting fees from AiCuris, Agenus, and ViruLite, and grant support from Genocea, Gilead, GlaxoSmithKline, and the Washington Vaccine Alliance. E. W. H. has received consulting fees from Cempra, grants from Becton Dickinson and GlaxoSmithKline, honoraria from Becton Dickinson, and royalties from McGraw-Hill. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.

COMMENTS
Clinical features: The incubation period of primary genital herpes is 3-7 days (range, 1 day to 3 weeks). Constitutional symptoms include fever, headache, malaise, and myalgia (prominent in the first 3-4 days). Local symptoms include pain, itching, dysuria, vaginal and urethral discharge, and tender lymphadenopathy.
- Herpes simplex virus infection orofacial with crust - Herpetic whitlow thumb - Herpetic whitlow vesicles - Erythema multiforme palm - Eczema herpeticum on face ... This topic will review the epidemiology, clinical manifestations, and diagnosis of HSV-1 infection. Other topic reviews related to herpes simplex type 1 include:
Herpes simplex virus type 1 (HSV-1) is a member of the Alphaherpesviridae subfamily. Its structure is composed of linear dsDNA, an icosahedral capsid that is 100 to 110 nm in diameter, with a spikey envelope. ... When symptoms do occur, there is a wide range of clinical presentations including orolabial herpes, herpetic sycosis (HSV ...
Herpes genitalis can be caused by the herpes simplex virus type 1 or 2, manifesting as a primary or recurrent infection.[1] Most commonly, viral replication occurs in epithelial tissue and establishes dormancy in sensory neurons, reactivating periodically as localized recurrent lesions.[2] It remains one of the most common sexually transmitted infections (STI) but continues to be ...
Genital herpes simplex virus (HSV) infections are a major global public health problem: A dramatic upsurge in genital HSV infections has been documented from seroprevalence studies. There is a wide diversity of the clinical spectrum of genital HSV disease.
The clinical presentation of symptomatic initial genital herpes does not differ between HSV-1 and HSV-2 infection. 8 After an incubation period of 4 to 7 days, multiple lesions appear on the ...
Bernstein DI, Bellamy AR, Hook EW, et al. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect ...
Genital herpes. Presentation: classically presents as a small number of painful, clustered vesicles with an erythematous base; increased pain noted upon rupture, leaving shallow ulcers that heal spontaneously without treatment over 4-10 days. ... Usually due to HSV-2, but the history of clinical genital herpes is not common. If presentation ...
CLINICAL PRESENTATION. HSV-2 infection is the most common cause of recurrent genital ulcer disease (GUD) worldwide. Symptomatic genital HSV is a lifelong condition that can be characterized by frequent symptomatic recurrences. Most initial infections are asymptomatic or atypical, therefore the majority of people with HSV-2 infection have not ...
Clinical presentation 9 Laboratory diagnosis 10 1.2 Rationale for new recommendations 10 1.3 Objectives 10 1.4 Target audience 10 1.5 Structure of the guidelines 11 ... Recommendations for treatment of genital herpes simplex virus 17 4.1 First clinical episode of genital HSV infection 17 Recommendation 1 17
INTRODUCTION — Herpes simplex virus type 1 (HSV-1) is a cause of recurrent vesiculoulcerative lesions of the oral or genital mucosa. It can also cause infection in the eye, skin, central nervous system, and/or visceral organs. This topic will review treatment and prevention of primary and recurrent HSV-1 infections in immunocompetent adolescents and adults.
Only 10-30% of orolabial infections are symptomatic. The most common clinical presentation of first-episode, primary herpes simplex virus infection in children (usually aged 6 mo to 5 y) is acute herpetic gingivostomatitis, as is shown in the image below. Primary herpes simplex virus (HSV) gingivostomatitis in an infant is shown.
Genital herpes is a common sexually transmitted infection (STI). The herpes simplex virus (HSV) causes genital herpes. Genital herpes can often be spread by skin-to-skin contact during sexual activity. Some people infected with the virus may have very mild symptoms or no symptoms. They can still able to spread the virus.
Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women Clin Infect Dis. 2013 Feb;56 ... No differences were noted in the clinical manifestations of genital HSV-1 vs genital HSV-2 disease. The clinicians' assessment that cases were caused by HSV was good when ...
Clinical herpes simplex virus (HSV) infections appear as clustered vesicles on an erythematous base. They often progress to pustular or ulcerated lesions with a scalloped border, and they eventually form a crust. ... Turiansky GW, Redfield R, James WD. Viral folliculitis. Atypical presentations of herpes simplex, herpes zoster, and molluscum ...
HSV-1 and HSV-2 are enveloped dsDNA viruses known for their short replicative cycles and ability to infect a variety of host tissues resulting in a wide range of clinical presentations. HSV-2 usually results in genital infections, whereas HSV-1 can cause genital infections as well as other mucocutaneous infections such as orolabial herpes ...
Genital Herpes Presentations •Neonatal HSV is a rare complication of genital HSV infection, in which virus is transmitted when genital shedding occurs during birth process, highest risk when HSV is acquired in pregnancy ... -Clinical diagnosis of genital herpes without laboratory confirmation-Partner with genital herpes
Herpes genitalis is caused by the herpes simplex virus type 1 or type 2 and can manifest as primary or recurrent infection. It is one of the most common sexually transmitted infections and due to associated physical and psychological morbidity it constitutes a considerable, often underestimated medical problem. ... Clinical presentation HSV ...
INTRODUCTION. Herpes simplex virus type 1 (HSV-1) encephalitis is the most common cause of sporadic fatal encephalitis worldwide. The clinical syndrome is often characterized by the rapid onset of fever, headache, seizures, focal neurologic signs, and impaired consciousness [].HSV-1 encephalitis is a devastating disease with significant morbidity and mortality, despite available antiviral therapy.
I have genital herpes. Where can I find the latest information about ongoing genital herpes research, including clinical trials? The U.S. National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID) supports research to develop prevention methods and treatments for genital herpes.
Fife, K. H. et al. Effect of valacyclovir on viral shedding in immunocompetent patients with recurrent herpes simplex virus 2 genital herpes: a US-based randomized, double-blind, placebo ...
Unusual presentations occur. Both herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2) may produce a more subacute encephalitis, apparent psychiatric syndromes, and benign recurrent meningitis. Less commonly, HSV-1 may produce a brain stem encephalitis, and HSV-2 may produce a myelitis.
Herpes simplex virus (HSV) is the leading cause of genital ulcers worldwide. In rare cases, mostly among immunocompromised hosts, HSV infections can present as hypertrophic pseudotumoral forms simulating malignancies or often mistaken as other viral infections, usually resistant to conventional antiviral therapy and often requiring alternative therapeutic approaches.
Herpes simplex encephalitis is caused by herpes simplex virus type 1 (HSV-1) or type 2 (HSV-2). HSV-1 causes encephalitis in children (beyond the neonatal period) and adults, and it is the most common etiology for sporadic encephalitis worldwide. ... The clinical presentation of herpes encephalitis can be acute or subacute. A prodromal phase of ...
Clinical presentation and imaging are similar to that of acute pyogenic meningitis but milder. ... where the most common causing viruses are herpes simplex virus (HSV), West Nile virus (WNV), Enterovirus, varicella zoster virus (VZV), cytomegalovirus (CMV) and human herpesvirus type 6 (HHV-6).
Candel has established two clinical stage multimodal biological immunotherapy platforms based on novel, genetically modified adenovirus and herpes simplex virus (HSV) gene constructs, respectively.
Patients with herpes simplex virus (HSV) keratitis may report the following: Pain. Photophobia. Blurred vision. Tearing. Redness. A history of prior episodes in patients with recurrent disease may exist. Patients with ocular HSV who have previous stromal involvement have a significantly higher risk for subsequent stromal keratitis; in contrast ...
Poster presentation details are as follows: Title: Murine Toxicology Study of Repeat-Dose Inhaled KB408, an HSV-1 ... The Company is rapidly advancing a robust preclinical and clinical pipeline of ...
As reported previously [17, 18], the first clinical presentation of genital HSV can occur long after the primary infection. In the study reported here, 6 participants were infected and developed HSV antibody responses 176-319 days before developing the first oral (n = 1) or genital (n = 5) symptoms of HSV.