This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.
- Behavioural Health
- Clinical Research
- Clinical Supply
- Vaccination


Pfizer hires Citi analyst Andrew Baum to manage portfolio strategy, business development
Fierce Pharma
MAY 6, 2024
In the role, he'll oversee the drugmaker's portfolio management and business development activities. .” | Citi analyst Andrew Baum, after covering Pfizer for more than a decade, will join the Big Pharma company as chief strategy and innovation officer.
Drug Excipient Business Development Reports now Available on Amazon
Drug Patent Watch
FEBRUARY 15, 2024
Elevate your excipient business strategies with the comprehensive Drug Excipient Business Development Reports from DrugPatentWatch.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Trending Sources
Bio Pharma Dive
Pharmaceutical Technology
- AuroBlog - Aurous Healthcare Clinical Trials blog
- Pharma Times

Teva CEO Francis poaches business development head Angus Grant from BeiGene
JULY 28, 2023
Teva’s CEO Richard Francis is adding to his constellation of new executives with a fresh business development hire from BeiGene. as its EVP of business development , the Israeli-American generics giant announced Friday. Teva has enlisted BeiGene's Angus Grant, Ph.D,

Key Business Development Skills Every Manager Should Have
JUNE 14, 2021
People working on business development are the ones responsible for dealing with the business side of the organization. Dealing with a lot of people, clients and a huge team is part of this job.

Divya Iyer, VP Strategy & Business Development at GoodRx, showcases digital health innovations at Digital Pharma East conference
NOVEMBER 10, 2022
Divya Iyer, VP Strategy & Business Development at GoodRx, showcases digital health innovations at Digital Pharma East conference. Thu, 11/10/2022 - 13:14.

Mainz Biomed Appoints Steve Quinn as VP Business Development
BioTech 365
JANUARY 27, 2022
Mainz Biomed Appoints Steve Quinn as VP Business Development Mainz Biomed Appoints Steve Quinn as VP Business Development Highly experienced diagnostics business development executive appointed to lead growth initiatives across international markets and drive international sales of ColoAlert at-home screening … Continue reading →

Customer Success: Law Firm Business Development
NOVEMBER 18, 2019
DrugPatentWatch has deep resources to support patent prosecution and patent litigation, but it is also used by law firms for business development . The post Customer Success: Law Firm Business Development appeared first on DrugPatentWatch - Make Better Decisions. Find weak claims One of the first tweaks….

PharmaSGP: Expected business development in Q1 2021
MAY 17, 2021
PharmaSGP: Expected business development in Q1 2021 Group revenues of € 12.4 DGAP-News: PharmaSGP Holding SE / Key word(s): Quarterly / Interim Statement 18.05.2021 / 07:30 The issuer is solely responsible for the content of this announcement. million and adjusted … Continue reading →

Customer Success: API and CDMO Business Development
DECEMBER 3, 2019
The post Customer Success: API and CDMO Business Development appeared first on DrugPatentWatch - Make Better Decisions. Changes in drug markets can create growth opportunities for API manufacturers and CDMOs alike. Whether change comes from generic entry, from new formulations of drugs, or from competition from novel….

Teva Appoints Angus Grant, PhD, as EVP of Business Development
Pharmaceutical Commerce
The pharma and biotech exec will begin his role on Aug.

I-MED Pharma USA Announces Michelle Schnabel as VP Business Development
NOVEMBER 9, 2021
I-MED Pharma USA Announces Michelle Schnabel as VP Business Development I-MED Pharma USA Announces Michelle Schnabel as VP Business Development MONTREAL, Nov. 09, 2021 (GLOBE NEWSWIRE) — With the recent expansion into the United States, I-MED Pharma USA is proud to announce … Continue reading →

eClinical Provider Crucial Data Solutions Appoints Brian Dufresne as SVP Business Development
Crucial Data Soutions
JUNE 27, 2022
Reno, NV, June 27, 2022 — Crucial Data Solutions (CDS), a provider of innovative low-code/no-code technologies to advance clinical research, announced today that industry veteran Brian Dufrense has joined the company as Senior Vice President, Business Development .

PharmaJet Announces Addition of Paul LaBarre as VP Global Business Development
JUNE 15, 2021
PharmaJet Announces Addition of Paul LaBarre as VP Global Business Development PharmaJet Announces Addition of Paul LaBarre as VP Global Business Development Brings extensive global health experience to leadership team GOLDEN, Colo.–(BUSINESS

Oxford Finance Appoints David Hickman to Managing Director of Business Development
APRIL 29, 2021
Oxford Finance Appoints David Hickman to Managing Director of Business Development Oxford Finance Appoints David Hickman to Managing Director of Business Development David has been appointed to Debbie Baker’s position upon her retirement announcement ALEXANDRIA, Va.–(BUSINESS

Business Development During COVID19 Quarantine For Clinical Research Sites and Job Seekers
Clinical Trial Gurus
SEPTEMBER 28, 2020
Business development is an ongoing process for each clinical research site. This video is based on a webinar that provides some strategies for improving the number of study leads that sites receive. Once a study lead is obtained, each site is responsible for pursuing each lead until the site gets activated.

Aperio Poised for Strategic Growth; Appoints Eric Ross as Vice President of Business Development
Aperio Poised for Strategic Growth; Appoints Eric Ross as Vice President of Business Development Aperio Poised for Strategic Growth; Appoints Eric Ross as Vice President of Business Development RESEARCH TRIANGLE PARK, N.C.–(BUSINESS

CAPAssurance, a Risk Purchasing Group, Names Mitch Temple as Vice President Business Development
JULY 19, 2021
CAPAssurance, a Risk Purchasing Group, Names Mitch Temple as Vice President Business Development CAPAssurance, a Risk Purchasing Group, Names Mitch Temple as Vice President Business Development Cooperative of American Physicians, Inc.

ERS Genomics Expands Team With Appointment of Jon Kratochvil as VP Business Development
MARCH 1, 2021
ERS Genomics Expands Team With Appointment of Jon Kratochvil as VP Business Development ERS Genomics Expands Team With Appointment of Jon Kratochvil as VP Business Development Jon Kratochvil joins as Vice President for Business Development & Licensing for North America … Continue reading →

Mainz Biomed Appoints Michele Pedrocchi, Former Head of Roche Diagnostics Business Development, to Strategic Advisory Board
JANUARY 12, 2022
Mainz Biomed Appoints Michele Pedrocchi, Former Head of Roche Diagnostics Business Development , to Strategic Advisory Board Mainz Biomed Appoints Michele Pedrocchi, Former Head of Roche Diagnostics Business Development , to Strategic Advisory Board BERKELEY, Calif. and MAINZ, Germany, Jan.

Juice Pharma Introduces New EVP of Business Development
JULY 20, 2023
Experienced exec joins the agency with over three decades of driving brand growth.
Immunovant data show potential for autoimmune disease drug
SEPTEMBER 26, 2023
Much anticipated clinical trial data for Immunovant’s experimental FcRn inhibitor could catalyze business development decisions for its parent, Roivant Sciences.

Bioventus Appoints Chris Yamamoto Senior Vice President of Business Development and Strategy
NOVEMBER 16, 2020
Bioventus Appoints Chris Yamamoto Senior Vice President of Business Development and Strategy Bioventus Appoints Chris Yamamoto Senior Vice President of Business Development and Strategy DURHAM, N.C.–(BUSINESS

Metrion Biosciences appoints Nick Foster as Chief Commercial Officer and Head of Global Business Development
JULY 26, 2021
Metrion Biosciences appoints Nick Foster as Chief Commercial Officer and Head of Global Business Development Metrion Biosciences appoints Nick Foster as Chief Commercial Officer and Head of Global Business Development Appointment supports international growth within ion channel contract research and … Continue reading →
Steven C. Tomlin Named SVP of Business Development for Corizon
OCTOBER 19, 2020
Tomlin has joined the company as Senior Vice President of Business Development , effective immediately. BRENTWOOD, Tenn., 19, 2020 (GLOBE NEWSWIRE) — Corizon Health announced today that longtime corrections treatment and healthcare executive Steven C. Tomlin, who most recently served … Continue reading →
Calyxt Appoints Sarah Reiter as Vice President of Business Development
OCTOBER 14, 2020
Expanded leadership team will drive new business opportunities to support the company’s go-to-market strategies ROSEVILLE, Minn.–(BUSINESS –( BUSINESS WIRE)–Calyxt, Inc. NASDAQ: CLXT), a plant-based technology company, announced today that it has appointed Sarah Reiter as Vice President, Business Development .

PharmaJet Announces Addition of Melissa Malhame as VP Business Development
SEPTEMBER 29, 2020
.–( BUSINESS WIRE)–PharmaJet®, the maker of innovative, needle-free injection technology, today announced that Melissa Malhame, has joined the leadership team as Vice President, Business Development . Malhame is … Continue reading →
MediClin AG: Business development to date shows an increasing capacity utilization in the clinics
NOVEMBER 2, 2021
Business development to date shows an increasing capacity utilization in the clinics In the first nine months of … Continue reading → DGAP-News: MediClin AG / Key word(s): Quarter Results 02.11.2021 / 13:15 The issuer is solely responsible for the content of this announcement.

Greenwich LifeSciences Hires Industry Expert Dr. Christine Fischette to Lead Business Development & Advise on Commercialization
APRIL 19, 2021
Greenwich LifeSciences Hires Industry Expert Dr. Christine Fischette to Lead Business Development & Advise on Commercialization Greenwich LifeSciences Hires Industry Expert Dr. Christine Fischette to Lead Business Development & Advise on Commercialization Previously led partnering transactions and strategic analyses for … Continue reading (..)

Applied DNA Appoints Clay Shorrock as Chief Legal Officer and Executive Director of Business Development
APRIL 1, 2021
Applied DNA Appoints Clay Shorrock as Chief Legal Officer and Executive Director of Business Development Applied DNA Appoints Clay Shorrock as Chief Legal Officer and Executive Director of Business Development – Shorrock Rejoins Company; Appointment Underscores Company’s Pursuit of Commercial … Continue reading →

Rapid Dose Therapeutics Signs Agreement with AB Strategic Ventures for Business Development Advisory Services with First Nations Enterprises
NOVEMBER 30, 2021
Rapid Dose Therapeutics Signs Agreement with AB Strategic Ventures for Business Development Advisory Services with First Nations Enterprises Rapid Dose Therapeutics Signs Agreement with AB Strategic Ventures for Business Development Advisory Services with First Nations Enterprises BURLINGTON, Ontario–( BUSINESS WIRE)–Rapid Dose … Continue (..)

InnoCare Appoints Dr. Manish Tandon as Vice President of Business Development
OCTOBER 11, 2020
BEIJING–( BUSINESS WIRE)–InnoCare Pharma (HKEX: 09969), a clinical stage biopharmaceutical company, announced today that the Company has appointed Dr. Manish Tandon as Vice President of Business Development , reporting to the Co-founder, Chairman and CEO, Dr. Jasmine Cui.
Ontario Bioscience Innovation Organization Secures Funding for Business Development Skills Program to Support Canadian Health Science Companies
MAY 25, 2021
Ontario Bioscience Innovation Organization Secures Funding for Business Development Skills Program to Support Canadian Health Science Companies Ontario Bioscience Innovation Organization Secures Funding for Business Development Skills Program to Support Canadian Health Science Companies Innovative program will provide critical capabilities … (..)

CytoDyn’s Chairman, CMO, and Head of Business Development Dr. Scott A. Kelly to Present at World Antiviral Congress 2021 Beginning Tomorrow
NOVEMBER 29, 2021
CytoDyn’s Chairman, CMO, and Head of Business Development Dr. Scott A. Kelly to Present at World Antiviral Congress 2021 Beginning Tomorrow CytoDyn’s Chairman, CMO, and Head of Business Development Dr. Scott A. Kelly to Present at World Antiviral Congress 2021 … Continue reading →
Biocontrol and Biostimulant Industry Leader Massimo Toni joins DunhamTrimmer as Vice President of New Business Development
NOVEMBER 13, 2020
Biocontrol and Biostimulant Industry Leader Massimo Toni joins DunhamTrimmer as Vice President of New Business Development Biocontrol and Biostimulant Industry Leader Massimo Toni joins DunhamTrimmer as Vice President of New Business Development Bio intelligence leader gains 20 years experience in … Continue reading →

PAOG Confirms CBD Nutraceuticals Business Development Update To Include ALKM, USMJ and PURA Highlights Scheduled This Wednesday, June 23rd
JUNE 21, 2021
PAOG Confirms CBD Nutraceuticals Business Development Update To Include ALKM, USMJ and PURA Highlights Scheduled This Wednesday, June 23rd PAOG Confirms CBD Nutraceuticals Business Development Update To Include ALKM, USMJ and PURA Highlights Scheduled This Wednesday, June 23rd Sandusky, Ohio, … Continue reading →

Seres Therapeutics Announces David Arkowitz to Join as Executive Vice President, Chief Financial Officer and Head of Business Development
MAY 20, 2021
Seres Therapeutics Announces David Arkowitz to Join as Executive Vice President, Chief Financial Officer and Head of Business Development Seres Therapeutics Announces David Arkowitz to Join as Executive Vice President, Chief Financial Officer and Head of Business Development CAMBRIDGE, Mass.–(BUSINESS

Medexus Appoints Michael Pine as Senior Vice President of Business Development and Strategy
SEPTEMBER 25, 2020
the “Company” or “Medexus”) (TSXV: MDP, OTCQX: MEDXF), today announced the appointment of Michael Pine as Senior Vice President of Business Development and Strategy, effective September 21, 2020. MONTREAL, Sept. 25, 2020 (GLOBE NEWSWIRE) — Medexus Pharmaceuticals Inc.

Women In Bio Elects Katie Williams, Associate Director of Business Development at Applied BioMath, as 2021 President-Elect
JANUARY 7, 2021
Women In Bio Elects Katie Williams, Associate Director of Business Development at Applied BioMath, as 2021 President-Elect Women In Bio Elects Katie Williams, Associate Director of Business Development at Applied BioMath, as 2021 President-Elect Sea Island, GA, Jan. 07, 2021 … Continue reading →

M1 Kliniken AG publishes figures for the 1st half of 2021: stable business development despite continued Corona burdens
AUGUST 25, 2021
M1 Kliniken AG publishes figures for the 1st half of 2021: stable business development despite continued … Continue reading → DGAP-News: M1 Kliniken AG / Key word(s): Half Year Report/Miscellaneous 25.08.2021 / 14:30 The issuer is solely responsible for the content of this announcement.
MediClin AG: The effects of the coronavirus pandemic are burdening the business development in the first quarter of 2021
MAY 3, 2021
The effects of the coronavirus pandemic are burdening the business development in the first quarter of … Continue reading → DGAP-News: MediClin AG / Key word(s): Quarterly / Interim Statement 03.05.2021 / 17:58 The issuer is solely responsible for the content of this announcement.

How Much Business Development Should Clinical Research Sites Be Doing
MARCH 19, 2021
Every site should have a strategy for finding new studies regardless of the current number of actively enrolling studies. Established sites tend to have a larger network that can help them find new studies based on the site’s reputation.

BMS reports 2% revenue loss in 2023 after Revlimid generics hurt sales
FEBRUARY 2, 2024
Bristol Myers Squibb has reported a 2% revenue decrease from 2022 as it focuses on business development to counteract patent losses.
Drägerwerk AG & Co. KGaA: Business development in the first half year significantly above prior year despite lower earnings in the second quarter
JULY 14, 2021
KGaA: Business development in the first half year significantly above prior year despite lower earnings in the second quarter 14-Jul-2021 / 19:29 CET/CEST Disclosure of … Continue reading → Drägerwerk AG & Co. KGaA / Key word(s): Half Year Results Drägerwerk AG & Co.

Business Development Is For Everyone In Clinical Research! Pharmacist Turned Entrepreneur Explains!
OCTOBER 5, 2021
Oftentimes as clinical research professionals we do not think of ourselves as sales people but expanding your opportunities in the life sciences industry is as important as anything else we will ever do for our careers.
Stay Connected
Join 32,000+ Insiders by signing up for our newsletter
- Participate in Clinical Research Informer
- Add a Source
- Add a Resource
- Thu. May 09
- Wed. May 08
- Tue. May 07
- Mon. May 06
- Apr 27 - May 03
- FDA Compliance
- Immune Response
- Laboratory Information Management System
- Clinical Trials
- More Topics
Input your email to sign up, or if you already have an account, log in here!
Enter your email address to reset your password. a temporary password will be e‑mailed to you., be in the know on.

Clinical Research Informer
Expert insights. Personalized for you.
We organize all of the trending information in your field so you don't have to. Join 32,000+ users and stay up to date on the latest articles your peers are reading.

Get the good stuff
Subscribe to the following Clinical Research Informer newsletters:
You must accept the Privacy Policy and Terms & Conditions to proceed.

You know about us, now we want to get to know you!
Check your mail, we've sent an email to . please verify that you have received the email..
We have resent the email to
Let's personalize your content
Use social media to find articles.
We can use your profile and the content you share to understand your interests and provide content that is just for you.
Turn this off at any time. Your social media activity always remains private.
Let's get even more personalized
Choose topics that interest you., so, what do you do.
Are you sure you want to cancel your subscriptions?
Cancel my subscriptions
Don't cancel my subscriptions
Changing Country?
Accept terms & conditions.
It looks like you are changing your country/region of residence. In order to receive our emails, you must expressly agree. You can unsubscribe at any time by clicking the unsubscribe link at the bottom of our emails.
You appear to have previously removed your acceptance of the Terms & Conditions.

We noticed that you changed your country/region of residence; congratulations! In order to make this change, you must accept the Aggregage Terms and Conditions and Privacy Policy. Once you've accepted, then you will be able to choose which emails to receive from each site .
You must choose one option
Please choose which emails to receive from each site .
- Update All Sites
- Update Each Site
Please verify your previous choices for all sites
Sites have been updated - click Submit All Changes below to save your changes.
We recognize your account from another site in our network , please click 'Send Email' below to continue with verifying your account and setting a password.
You must accept the Privacy Policy and Terms & Conditions to proceed.
This is not me
Sign in to your site’s geographic location:
The clinical trial business development lifecycle.

Webinar Date: February 17, 2020
Webinar Guests: Mark Ragusa, former Director of Research at Raleigh Neurology Associates
Don’t do clinical trial business development haphazardly. Mark Ragusa, former Director of Research at Raleigh Neurology, shares his framework for systematically targeting and nurturing your sponsor and CRO relationships to create a rich, site-appropriate study pipeline. Raleigh Neurology is one of the leading go-to sites in the space. Learn how Mark identifies sponsors, adjusts his site’s value proposition, and maintains a regular cadence of communication. This high-touch, disciplined process can be your key to growth.

21 CFR Part 11 Regulation Compliance Update
Compliance Update: The 21 CFR Part 11 Regulation is a cornerstone of conducting clinical trials in today’s world. The release of the regulation 1997 established guidelines for the use of electronic records and electronic signatures in FDA-regulated industries and had a significant impact on the pharmaceutical and medical device industries. The FDA began working on...

The Current State of Clinical Trials in Ukraine—A Conversation with Dr. Roman Fishchuk
Meet Dr. Roman Fishchuk, an esteemed otorhinolaryngologist (ENT) whose journey in the healthcare field has been marked by a commitment to providing medical care and innovative treatment options through clinical trials in his native Ukraine. Having graduated from Ivano-Frankivsk National Medical University, Dr. Fishchuk specialized in otolaryngology and completed a Master’s degree at the University...

Transitioning to CRIO? How to Get Your Data Ready for Migration
You have just signed on to use CRIO and you are excited to get started. Even if you are moving primarily from paper, you may have data that you want to migrate into CRIO rather than having to re-enter it. For many of our new clients, they have a wealth of information available in spreadsheets...
Get articles delivered to your inbox, every week

A Career with Clinical Research Organizations in Business Development (BD)
Business development team plays an essential role in all types of industries. In pharmaceutical and clinical research industries business development teams perform many important functions suchas understanding financial aspects of running clinical studies, setting objectives and goals for the successful completion of the projects, identify and approach new clients for new business opportunities and so on.
The Clinical Business Development team specialises in bidding for and obtaining new projects from new clientsacross various therapeutic areas of interest. The team supports in employing business tactics by building and applying strategic business plans to assist sales development and key account management techniques to make sure effective conductof studies. The Business Development team may be engaged in the recruiting and developing of new sites in order to achieveorganizational growth targets. The Business Development Executive (BDE) is responsibleto achieve business development objectives set bythe organization on a monthly and yearly basis. The BDE mayalso be supporting the Site Managers / Lead Coordinators in accomplishing their patient enrollment goals via the oversight of study marketing plans and educational materials.
Education Qualification & Training Requirements for Clinical Business Development (BD) Executive Position
Bachelor / Masters Degree in life science or health care related area; MBA will be advantage for people who want to begin their career in business development.
Experience / Training Required for Clinical Business Development Executive Position
Multinational CRO’s look for people who have 2-3 years relevant experience in business development or clinical research, but there are many CRO’s who hire freshers who have health care related degree and a PG Diploma in Clinical Research and Business Development .
Job Responsibilities of Clinical Business Development Team
- Recognize and prepare clinical trial research plans with CRO’s, pharmaceutical and biotech organizations.
- Explore various pharmaceutical organization’s pipelines to find out new and up coming clinical study possibilities with a focus on obtaining new therapeutic areas, new contacts, new pharmaceutical/biotech organizations and new CROs in the industry.
- Make calls to targeted companies, seek out new contacts, finish all surveys, make follow-up calls and evaluate web sites to make sure that all possible studies are being analyzed.
- Accountable for supporting with external advertising and provide support in enrollment of subjects for ongoing clinical research at study sites.
- Recognition of new possibilities for pipeline molecules / products
- Research of all new products/ideas coming to market and evaluate business viability
- Accomplish or exceed organizational goals that have been put in placeby the organization.
- Comply with all organization procedures, instructions and directives for the achievement of organization objectives and generate sales. Maintain an automated monitoring of all ongoing leads, sales and works in process.
- Be aware to market research reports, competitive strategies/studies and convey this information to management.
- Analyse new business models, methodologies, and markets that would maximize revenue share of the organization.
- Attend yearly industry conferences to enhance Clinical Research Benefits and to obtain further data on research that can help new business opportunities.
- Support with determining and recruiting new doctors to work as researchers at trial sites.
Annual Pay Scale for Clinical Business Development (BD) Executive
Related posts:

Profile cancel
Email Not published
How artificial intelligence can power clinical development
Clinical development and the randomized clinical trial (RCT) remain largely unaffected by the unprecedented wave of innovation in pharmaceutical R&D fueled by developments in artificial intelligence (AI), including generative AI (gen AI) and foundational models. However, they are under pressure from the rise of precision medicine and a more competitive development landscape. So far, the adoption of AI for clinical development has emphasized operational excellence and acceleration, but advances in scientific AI have made this the time to leverage modern analytical tools and novel sources of data to design more precise, efficient trials with greater success rates.
About the authors
This article is a collaborative effort by Chris Anagnostopoulos, David Champagne , Thomas Devenyns, Alex Devereson , and Heikki Tarkkila, representing views from McKinsey’s Life Sciences Practice.
The introduction of RCTs in the mid-20th century ushered in the modern era of evidence-based drug development. Their rigidity and simplicity were welcome defenses against an overreliance on anecdotes and case studies and have unarguably served patients well, supporting the introduction of countless safe, effective therapies. Yet RCTs are starting to be seen as bottlenecks that lengthen the time for therapies to gain approval and can increase costs. 1 Donald A Berry, “The brave new world of clinical cancer research: Adaptive biomarker-driven trials integrating clinical practice with clinical research,” Molecular Oncology , 2015, Volume 9, Issue 5; Michael Baumann et al., “How much does it cost to research and develop a new drug? A systematic review and assessment,” PharmacoEconomics , 2021, Volume 39, Issue 11. Meanwhile, as gen AI breaks milestone after milestone, patients eagerly await the translation of such unprecedented technological progress into pharmaceutical R&D that could deliver faster access to better treatments.
In addition to rising expectations about timelines, clinical development is also facing increasing demands to generate targeted and meaningful data. As precision medicine becomes mainstream, RCTs often must prove not only the general efficacy of a treatment but also whether it will benefit a specific segment of the patient population. The smaller that segment, the harder it can be to enroll enough patients in trials. In addition, the bar for what constitutes a clinically meaningful treatment effect is creeping higher to meet standards set by regulators and payers as well as competitive pressure. In most therapeutic areas today, there are, on average, 40 percent more assets per indication than in 2006, 2 McKinsey research on data from EvaluatePharma, March 2019. raising the need for greater differentiation. And clinicians and patients alike need more evidence to make decisions about the best available treatment at a given time.
All this comes as researchers accelerate drug discovery by making greater use of AI and gen AI, such as DeepMind’s AI-enabled platform AlphaFold, which can predict the 3-D structure of molecules 3 “ AI in biopharma research: A time to focus and scale ,” McKinsey, October 10, 2022. leading to a pipeline of more and better-designed preclinical assets with a better target validation. Companies are also striving to improve their operational processes with a view to speeding up the time it takes to apply for a first-in-human study. But if clinical development fails to keep pace, the benefits to patients of faster drug discovery will inevitably be delayed, and delivering on the promise of AI-enabled acceleration may flounder.
While the RCT will remain a central pillar of clinical development, the tide is changing. Regulators have recently issued guidance on appropriate use of real-world data (RWD). 4 See, for example, the US Food and Drug Administration’s real-world evidence activities responding to the 21st Century Cures Act. The healthcare data ecosystem is booming, leveraging privacy-respecting technology to make patient-level data safely available for research. And evidence generation from multiple data sources is now possible using causal machine learning, which aims to distinguish correlation from causation via a combination of biostatistics with machine learning (ML) and, increasingly, gen AI and foundational models.
Despite this level of promise, only a handful of established companies are deploying AI and data-driven approaches systematically in their clinical development. The focus so far has been on improving operational excellence and increasing acceleration rather than helping to inform trial design strategy. The remainder of this white paper dives deeper into the context, gives tangible example use cases and associated impact, and identifies challenges companies face when adopting AI for clinical development. Rather than staying at a high level, it aims to bring the topic to life through relevant details and case examples.

Creating value beyond the hype
Let’s deliver on the promise of technology from strategy to scale.
Expanding adoption of AI and RWD for clinical development
Today AI can draw upon growing volumes of RWD. Electronic health records and claims data from certain geographies are widely available, and novel data sources—including biobanks, data omics panels, population-wide genomic studies, patient registries, and imaging and digital pathology—are increasing in number and diversity. All these sources reflect a notable change in patients’ ability to share their data for the purpose of advancing research and treatments in privacy-respecting ways, including technology that enables the training of ML models in a manner that respects patients’ privacy.
In addition, new tools can systematically capture knowledge from unstructured data. Large language models (for example, BioGPT) are able to convert unstructured physician notes into high-quality structured data. Similarly, these models can search the vast corpus of published literature and identify connections between biological entities on a large scale, generating high-quality input for knowledge graphs that better represent the totality of available evidence on a given indication across domains, including genes, targets, proteins, pathways, and phenotypes.
Most pharma companies using AI and RWD in clinical development tend to do so only in isolated use cases, and they rarely deploy both in combination. For example, they might use machine learning to select trial sites or to predict patient enrollment rates. They might use RWD to measure disease prevalence—the size of an eligible population—to understand the natural course of a disease among untreated patients, or to construct an external control arm for regulatory purposes. While these use cases deliver value, a great deal more is now possible. Similarly, while the level of funding and investment going into AI-enabled drug discovery companies has tripled in the last five years, that same trend is not true for equivalent companies in the clinical development space.
Four use cases that demonstrate the potential of AI
Companies that are deploying AI combined with RWD are seeing impactful results. While RWD can be valuable supporting evidence in health authority submission packages, the most successful companies focus their use of AI and RWD to make better and more informed decisions to support the success of clinical development programs in every step, from asset and portfolio strategy to protocol and trial design:
- At the stage of defining an asset strategy, AI and RWD can reveal which indications are most promising indications to pursue for novel assets. Several leading biopharma companies identified multiple new indications for existing assets in this way, and an early-stage biotech used AI and RWD to assess whether to shift its indication selection strategy regarding a novel asset.
- AI and RWD can support decisions about the target patient population of a clinical trial, with subgroup discovery, refine trial eligibility criteria and help remove patients that are highly unlikely to benefit from the treatment, and shorten the length of trials. One early-stage biotech could better characterize “super-progressors” (that is, patients whose disease was likely to progress faster within the time frame of a clinical trial) and design a trial with similar expected benefits in a faster time frame.
- For decisions about portfolio strategy, AI and RWD can help companies identify the right combination of drugs for an indication or for the right patients. One biopharma company leveraged a prospective observational data set to generate evidence supportive of earlier positioning of its third-line treatment. Another was able to identify “super-responders” for several drugs in its portfolio—insights that helped the company position its assets optimally in a crowded indication.
- At the step of selecting and optimizing the end points of a clinical trial, AI and RWD can help a company identify patient attributes that closely track the primary end point over time. One biopharma company replaced a rare disease’s existing end point, which was an infrequent event, with end points that occurred with greater frequency or could be measured with blood tests. This cut the length of trials by 15 to 30 percent.
The following discussion looks in greater depth at four detailed use cases, illustrating the wealth of information that AI can unlock.
Indication selection for asset strategy
Selecting which indications to target with a specific molecule is one of the most important decisions a biopharma company makes. This decision is often informed by a combination of input from key opinion leaders, literature reviews, omics analysis (for example, genome-wide association studies), RCT data, and competitor decisions. Such decisions rarely are fully data driven, typically prove hard to integrate, and cover only part of the available evidence base, resulting in a subjective and suboptimal synthesis. In contrast, strategies informed by RWD and AI are objective and comprehensive in their use of multiple data sets and foundational models built on clinical data continually redefining the art of the possible.
For already-approved therapies, companies can use RWD to understand the therapies’ likely efficacy on alternative indications. One approach is examining the outcomes of patients who were prescribed the drug on a spontaneous basis by their physician; another is determining a drug’s average effect on patients exposed to the treatment because of incidental comorbidities. AI techniques can then extrapolate any findings to a set of patients whose characteristics may be distributed differently, by leveraging observed correlations between patient characteristics and outcomes.
Where companies are looking to expand the indication of an approved asset or identify for a novel asset, RWD and AI can estimate the biological proximity of one indication to another from a patient and clinical perspective—that is, whether the patient experiences similar symptoms, comorbidities, lab characteristics, and treatment journeys. Foundational models that treat medical events as words and patient medical histories as documents can uncover the semantic similarity of different events, including diagnoses. Each indication, from what is likely to be a sizable list of those with biological proximity, is scored according to its similarity to one or more anchor references—perhaps the indication for which the asset was originally approved or, in the case of a novel asset, one for which there is strong preclinical evidence of efficacy of the asset’s mechanism of action (MoA).
The scoring can also incorporate information from molecular knowledge graphs that show new connections—for example, between entities such as proteins or human biological pathways that have already been identified in literature or public data. Exhibit 1 shows a potential outcome of this approach: it can reveal the indications most strongly connected to each of the reference indications, serving as an anchor into the existing evidence base. The indications with the most connections to these references are further prioritized by unmet medical need, strategic fit, and technical feasibility, resulting in an evidence-based indication selection.
These analytical approaches can identify novel indications that can be rapidly validated via in vitro or animal models, can increase the confidence in selecting indications with a high probability of success, and can derisk resource allocation accordingly. The analysis provides a clearer and more holistic evidence base for investors, shareholders, and R&D leaders, and it can reduce the opportunity cost of blind alleys by helping new treatments reach patients faster.
Subgroup discovery for trial design
Once an asset has been matched with an indication, pharmaceutical companies pursue an all-comer clinical development and trial design strategy—that is, one that includes all patients except those deemed high-risk based on input from key opinion leaders and conventional wisdom. 5 The use of the all-comer strategy may reflect a low level of confidence in existing hypotheses that currently seek to explain response heterogeneity in patient subpopulations. As a result, patients unlikely to respond to a treatment may be included in the trial. A notable exception to this method is in precision oncology, where researchers use specific biomarkers discovered preclinically (such as genetic mutations) to stratify patients according to their probability of progression or to predict a patient’s response to different treatments. This approach is revolutionizing oncology treatments, but even it can identify only a fraction of possible patient subpopulations.
In contrast, AI and omics-rich RWD can examine thousands of genetic and/or phenotypic attributes to pinpoint the combinations most likely to influence prognostic or predictive scores, explain response heterogeneity, and improve a trial’s technical probability of success. Even in situations where the patient cohorts are modestly sized, foundational models trained on broad RWD can be used as a starting point for more bespoke modeling at the indication level.
One large biopharmaceutical company used RWD to remove likely nonresponders from a trial and to reduce its expected duration by between 5 and 10 percent without compromising its probability of success, which would allow the treatment to reach patients faster. Another company leveraged hospital episode RWD to identify super-responders to its comparator arm for an asset in Phase I, ensuring the trial was designed in a manner that focused on patients without access to effective treatments.
AI for subgroup discovery can also directly support the strategic trade-off between the size of the eligible population and the level of the treatment effect in a smaller population, in the form of an efficient frontier (Exhibit 2). The different subpopulations that are marked as blue on the exhibit are those for which it is impossible to simultaneously increase their breadth without reducing the drug’s expected effect. This approach widens the overlap between patients with access to this new treatment and patients who are most likely to benefit significantly from it.
Subgroup discovery and comparative efficacy for portfolio optimization
Not only can AI be used to discover subgroups to inform the design of a trial, it also can contribute to the wider portfolio strategy. The product pipelines and portfolios of many biopharmaceutical companies contain multiple assets aimed to treat a similar set of patients, often because companies lack sufficient evidence to inform the portfolio strategy differently, especially for new MoAs. However, the systematic analysis of all available data sources can predict the response of different patient subgroups (or disease endotypes), which could help companies hone their portfolio strategy—in some cases, resulting in a double-digit percentage improvement in net present value. This strategic stance can support targeted trials that produce the type of precision evidence that clinicians need to make the best possible treatment decisions for their patients amid a proliferation of novel assets.
In addition, several analytical approaches and data sources can be combined to determine the efficacy of novel MoAs in the portfolio relative to each other or to approved treatments of different patient subgroups. Enriching RWD with data from molecular knowledge graphs can enable foundational-model-powered representations of treatments that represent their associated biological pathways. From this, the company can estimate the efficacy of a novel treatment by observing the outcomes of approved treatments that share similar biological action mechanisms with the novel treatment. This exercise can be repeated across multiple disease endotypes or patient subgroups, identified through a combination of patient-level attributes (Exhibit 3).
End point optimization in clinical development
In many trials, there is no single, established end point. In others, it can be hard to know which of several would best measure the intended action of the drug. Some end points may take a long time to manifest, leading to extended development timelines. Others may detect progression that is more reliable for some types of patients than for others or that may be particularly invasive, increasing the trial’s burden on patients.
AI used on rich, patient-level longitudinal data sets can be deployed to identify patient attributes that closely track the primary end point over time. Such an application can control multiple confounding factors to ensure the association persists in future trials where the patient population may differ, thereby establishing potential novel end points.
Here, X-rays, CT and MRI scans, and other imaging techniques are often valuable sources of data for monitoring biomarkers in a noninvasive manner (as is the case with using MRIs to help detect Alzheimer’s disease 6 Ruth Stephen et al., “Change in CAIDE dementia risk score and neuroimaging biomarkers during a 2-year multidomain lifestyle randomized controlled trial: Results of a post-hoc subgroup analysis,” Journals of Gerontology: Series A , 2021, Volume 76, Issue 8. ). Foundational models using medical imaging are increasingly able to extract imaging biomarkers curated by human expertise from raw images at scale. They can even discover altogether novel, deeply hidden visual signatures of disease activity and severity with better predictive characteristics.
In all cases, the association between novel end points and desired patient outcomes such as survival or quality of life must persist even under treatment. The clinical data and the information present in RWD must be combined with a biological understanding of the mechanisms affected by the considered treatment.
Tackling the organizational challenges
Inevitably, supplementing a century-long, RCT-dependent approach to clinical development with a new, analytics-driven one using novel data poses organizational challenges. Here are some ways to tackle them.
Embed AI into clinical development
The governance processes and incentive structures in place in most biopharmaceutical companies encourage default solutions in clinical development at the asset team level and beyond. One consequence is the prevalence of all-comer trials, which target the broadest possible population without leveraging evidence about nonresponders and super-responders—a method that can increase trials’ duration and decrease their probability of success, resulting in longer waits for patients who would benefit from the treatment.
R&D leaders can help change that culture by encouraging the use of analytical methods to integrate all sources of available evidence when making key decisions. Individual incentives can help. A stronger step is to strengthen the development governance model to require that all key decisions be supported by AI.
Develop internal capabilities and talent
External providers can supply off-the-shelf AI solutions to support some use cases. But with so many new solutions emerging, no provider can yet support them all. Even if they could, companies would be unwise to become dependent upon them for critical strategic decisions. Providers with such rich intellectual property might eventually become competitors.
Biopharmaceutical companies could therefore consider building their own AI capabilities and products. However, significant investment is needed to acquire the relevant data sets; build up the necessary data science, data engineering, and business translator expertise; and establish agile product development processes that ensure use cases deliver business value in a timely and cost-efficient manner.
Companies will also have to work hard to attract and retain top data scientists and data engineers, as the biopharmaceutical industry is not typically their first choice. Measures that can help include offering competitive remuneration packages, building an innovative employer brand, and locating in key talent hubs. So, too, can a focus on the industry’s value proposition—offering employees the chance to help people lead longer and healthier lives.
Develop a targeted data strategy
With so much data becoming available, companies should consider formulating a detailed and iterative data strategy for each disease area. The purchase of certain commercial data sets, such as electronic health records, claims, and sometimes omics, can be relatively straightforward, even in therapeutic areas such as oncology, where biomarker-rich data are required. But other types of data sets—registries, biobanks, and clinical trials from other pharma companies—can involve complex negotiations and contracts, and companies will have to partner with a range of different data providers.
In addition, each data set is likely to be structured differently, with varying levels of quality and completeness. This means companies will likely need data curation and analytical capabilities for tasks such as combining different data sets to plug gaps in evidence and meeting regulatory expectations for the use of real-world data.
Build scalable products
AI’s power lies in its application across the clinical development portfolio, not restricted to isolated use cases. Achieving this kind of scale can be hard, due to limitations of the code base, data platforms, the company’s data engineering capabilities, and the analytical stack needed to collect, combine, and analyze data. As a result, pilots often remain just that—never reused or deployed against other assets in development.
To counter this, analytical use cases should be built as products, meaning solutions built in a manner that makes them easy to reuse and scale for different use cases, even at the proof-of-concept phase. The code base should be well annotated, for example, and deploy common, reusable analytical components that are either developed internally, open-source, or bought off the shelf. Shared ML development standards and coding frameworks can help by speeding up development work across different use cases because data scientists and engineers are familiar with them. Companies also need a mature and flexible ML operations stack—that is, the operational components needed to streamline the process of taking ML models to production and maintaining and monitoring them.
By harnessing novel data and the power of AI, biopharmaceutical companies can move beyond RCTs to vastly improve clinical development. The benefits accrue at each stage of the development process, from formulating asset strategy to designing the protocol and trial planning. For patients, the results can speed up access to new treatments, thanks to an accelerated development timeline, and make treatments more likely to elicit the strongest response.
High-impact solutions are already being implemented, and more will follow. Companies can benefit from beginning to build the capabilities they need and scaling them across the portfolio and their assets’ life cycles. The clinical development and trial design system is ripe for change, and the value to patients will be vast.
Chris Anagnostopoulos is an associate partner in McKinsey’s Athens office, David Champagne and Alex Devereson are partners in the London office, Thomas Devenyns is an associate partner in the Geneva office, and Heikki Tarkkila is an associate partner in the Helsinki office.
Explore a career with us
Related articles.

Generating real-world evidence at scale using advanced analytics

How AI could revolutionize drug discovery

AI in biopharma research: A time to focus and scale

- Clinical Research

Business Development in Clinical Research
News and blog.
Business Development in clinical research is in the process of a transition. The mounting costs of drug development and rationalizing of concerned departments is lashing many biotechnology and pharmaceutical companies to contract out clinical research programs . This is striking at the matching time as a tendency toward big pharma companies cementing chosen partnerships with a restricted number of CROs, successfully jamming out opposition.
Business development in clinical research is similar to drug development. It requires many trial opportunities to generate a few appropriate studies. How will companies ensure that their staff is equipped with the sales and business development expertise needed for the clinical settings, as well as required comprehension for a long-cherished relationship management? Furthermore, how companies evolve at business development strategy to facilitate long term growth?
The modalities involved in identifying and bagging clinical studies is called business development, also referred as sales. An efficient business development strategy boosts the likelihood of getting the studies required to keep an organization in business and ever growing. In some sectors, business development is carried out majorly by specialists, but in clinical research settings along with business development managers and marketing managers even the study personnel like principal investigators, study coordinators and site managers can all contribute. As the clinical research is scientifically intensive process, a professional with both technical and business development knowledge will surely contribute enormously to organization he is working for.
Biotechnology and pharmaceutical companies allocate a major percentage of their budgets to research than does any other U.S. industry. In 2010, the number of clinical studies in the U.S. is 25,992. This number is estimated to rise at a 5.7% CAGR (compound annual growth rate) to reach 32,318 in 2014. The bulk of the trials that are out sourced to developing countries are from US. Clinical research expenditure in 2010 is an approximated $25 billion and is projected to touch $28.5 billion by 2014.
The more the spending the more are the opportunities at the offering. Client-centric business strategies coupled with proper customer relationship management policies backed by skilled and well qualified professionals will help position any company at the helm of affairs. Business development in clinical research is always complex as the exact business that a company is looking for make it big or small.
What is Translational Research?
Careers in healthcare risk management, related posts.

Is Clinical Research Paving the Way in Africa?
Clinical research certification program: a step towards advancing your career, requirements for masters in clinical research, medical device development guidelines, challenges in clinical research, study anytime..

Get in touch

- Accreditations & Collaborations
- Management & Faculty
- CSR and The Foundation
- Quality Policy
- Image Gallery
- Bachelor of Public Health (BPH)
- BPH – Maternal & Child Health
- Master of Public Heath Mgmt (MPH)
- MPH – Disaster Management
- MPH – Epidemiology & Biostatistics
- MPH – Health Economics & Policy
- MPH – Maternal & Child Health
- MPH – Nutrition & Dietetics
- MPH – Tropical Medicine
- APGD in Global Health Mgmt & Policy
- APGD in Global Sexual & Reproductive Health
- APGD in Healthcare & Hospital Mgmt
- APGD in Maternal & Child Health
- APGD in Occupational Health & Safety Mgmt
- APGD in Public Health Research
- APGD in Tropical Medicine, Surv. & Immunization
- Advanced Certificate in Medical Law & Bioethics
- Certificate in Healthcare Risk Management
- Executive Certificate in Public Health Leadership & Research
- M.Sc in Clinical Research
- M.Sc in Pharmaceutical Medicine
- APGD in Clinical Trial Management
- APGD in Clinical Research & Pharmacovigilance
- APGD in Clinical Research & Medical Writing
- APGD in Clinical Research & Regulatory Affairs
- APGD in Pharmaceutical Medicine
- M.Sc in Agricultural Economics
- M.Sc in Banking & Finance
- M.Sc in Behavioural Economics
- M.Sc in Circular Economy & Sustainability
- M.Sc in Environmental Economics & Policy
- M.Sc in Financial Technology
- M.Sc in Health Economics
- B.Sc in Health Economics
- B.Sc in Behavioural Economics
- MBA in Clinical Research Management
- MBA in Health Economics
- Search All Courses
- Scholarships & Payment Plans
- Why Study At JLI?
- Academic Resources
- e-Learning & Study Methodology
- Student Profiles & Stories
- Student’s Speak
Insert/edit link
Enter the destination URL
Or link to existing content
Republish This Story
The Business of Clinical Trials Is Booming. Private Equity Has Taken Notice.
Disponible en Español

After finding success investing in the more obviously lucrative corners of American medicine — like surgery centers and dermatology practices — private equity firms have moved aggressively into the industry’s more hidden niches: They are pouring billions into the business of clinical drug trials.
To bring a new drug to market, the FDA requires pharmaceutical firms to perform extensive studies to demonstrate safety and efficacy, which are often expensive and time-consuming to conduct to the agency’s specifications. Getting a drug to market a few months sooner and for less expense than usual can translate into millions in profit for the manufacturer.
That is why a private equity-backed startup like Headlands Research saw an opportunity in creating a network of clinical sites and wringing greater efficiency out of businesses, to perform this critical scientific work faster. And why Moderna, Pfizer, Biogen, and other drug industry bigwigs have been willing to hire it — even though it’s a relatively new player in the field, formed in 2018 by investment giant KKR.
In July 2020, Headlands announced it won coveted contracts to run clinical trials of covid-19 vaccines, which would include shots for AstraZeneca, Johnson & Johnson, Moderna, and Pfizer.
In marketing its services, Headlands described its mission to “profoundly impact” clinical trials — including boosting participation among racial and ethnic minorities who have long been underrepresented in such research.
“We are excited,” CEO Mark Blumling said in a statement, to bring “COVID-19 studies to the ethnically diverse populations represented at our sites.” Blumling, a drug industry veteran with venture capital and private equity experience, told KHN that KKR backed him to start the company, which has grown by buying established trial sites and opening new ones.
Finding and enrolling patients is often the limiting and most costly part of trials, said Dr. Marcella Alsan, a public policy professor at Harvard Kennedy School and an expert on diverse representation in clinical trials, which have a median cost of $19 million for new drugs, according to Johns Hopkins University researchers .
Before covid hit, Headlands acquired research centers in McAllen, Texas; Houston; metro Atlanta; and Lake Charles, Louisiana, saying those locations would help it boost recruitment of diverse patients — an urgent priority during the pandemic in studying vaccines to ward off a disease disproportionately killing Black, Hispanic, and Native Americans.
Headlands’ sites also ran, among other things, clinical studies on treatments to combat Type 2 diabetes, postpartum depression, asthma, liver disease, migraines, and endometriosis, according to a review of website archives and the federal website ClinicalTrials.gov. But within two years, some of Headlands’ alluring promises would fall flat.
In September, Headlands shuttered locations in Houston — one of the nation’s largest metro areas and home to major medical centers and research universities — and Lake Charles, a move Blumling attributed to problems finding “experienced, highly qualified staff” to carry out the complex and highly specialized work of clinical research. The McAllen site is not taking on new research as Headlands shifts operations to another South Texas location it launched with Pfizer.
Email Sign-Up
Subscribe to KFF Health News' free Morning Briefing.
What impact did those sites have? Blumling declined to provide specifics on whether enrollment targets for covid vaccine trials, including by race and ethnicity, were met for those locations, citing confidentiality. He noted that for any given trial, data is aggregated across all sites and the drug company sponsoring it is the only entity that has seen the data for each site once the trial is completed.
A fragmented clinical trials industry has made it a prime target for private equity, which often consolidates markets by merging companies. But Headlands’ trajectory shows the potential risks of trying to combine independent sites and squeeze efficiency out of studies that will affect the health of millions.
Yashaswini Singh , a health economist at Johns Hopkins who has studied private equity acquisitions of physician practices, said consolidation has potential downsides. Singh and her colleagues published research in September analyzing acquisitions in dermatology, gastroenterology, and ophthalmology that found physician practices — a business with parallels to clinical trial companies — charged higher prices after acquisition.
“We’ve seen reduced market competition in a variety of settings to be associated with increases in prices, reduction in access and choice for patients, and so on,” Singh said. “So it’s a delicate balance.”
Dr. Aaron Kesselheim , a professor of medicine at Harvard Medical School, called private equity involvement in trials “concerning.”
“We need to make sure that patients” know enough to provide “adequate, informed consent,” he said, and ensure “protections about the privacy of the data.”
“We don’t want those kinds of things to be lost in the shuffle in the goals of making money,” he said.
Blumling said trial sites Headlands acquired are not charging higher prices than before. He said privacy “is one of our highest concerns. Headlands holds itself to the highest standard.”
Good or bad, clinical trials have become a big, profitable business in the private equity sphere, data shows.
Eleven of the 25 private equity firms identified by industry tracker PitchBook as the top investors in health care have bought stakes in clinical research companies, a KHN analysis found. Those companies have been involved in studies ranging from covid vaccines to treatments for ovarian cancer, Parkinson’s disease, and Alzheimer’s.
Contracted firms also analyze patient data and prepare materials to secure approval from regulatory agencies, in hopes of getting more drugs to market faster. And a big draw for investors: Clinical research companies make money whether or not a drug succeeds, making it less risky than investing in a drug company.
The number of clinical trials has exploded to more than 434,000 registered studies this year as of late November, more than triple the number a decade ago.
Still, most trial sites are physician practices that don’t consistently perform studies, according to a presentation by Boston-based investment firm Provident Healthcare Partners.
“Independent sites are being purchased by private equity, and they’re moving into larger site groups of 30, 40, and then their game plan is to roll that up into a business and then sell it again,” said Linda Moore Schipani, CEO of Clinical Research Associates, a Nashville-based company that worked on covid vaccine trials for AstraZeneca, Novavax, and Pfizer. “That’s kind of the endgame.”
Headlands is a prime example. It announced in November 2019 that it would acquire six centers in the U.S. and Canada, including three sites in Texas and Louisiana owned by Centex Studies that would help improve participation among Hispanics and African Americans.
It has made other acquisitions since then and opened new sites in areas with “extremely limited trial options,” something Blumling says distinguishes his company.
“I’m not an evangelist for private equity,” Blumling said. “The ability of KKR to be willing to invest in something that is a three- to five-year return versus a one- to two-year return is something that you won’t see out there.”
A research center in Brownsville, Texas — a stone’s throw from the U.S.-Mexico border and where 95% of the population is Hispanic or Latino — is one of several where it is partnering with Pfizer to boost patient diversity.
To recruit patients, Headlands “is really going beyond what a lot of sites do, which is social media,” Blumling said in an interview. “It’s going within churches, community fairs, really getting out into as much as possible the broader community.”
Headlands closed the Houston and Lake Charles sites because of staffing issues, Blumling said, and finished or moved their studies elsewhere. Blumling said the decision to close those locations “did not have anything to do with the speed of trials.”
Similarly, he said, Headlands is moving the McAllen site’s operations to Brownsville “because it had a larger population of trained personnel.”
“We want to continue to grow sites and do great work,” Blumling said. “If we can’t find the people in order to do that at the quality that we demand, which is at the highest level, then it doesn’t make sense to keep those sites.”
‘The Writing to Me Was on the Wall’
In 2006, Devora Torrence co-founded Centex Studies, which she described as “my little mom and pop business” in a 2021 podcast about female entrepreneurs in science. She said a flurry of interest from private equity came at the end of 2018. The appeal was evident: Drug companies were relying on bigger clinical trial networks.
“The thing is speed, getting it to market. With a bigger network, you get that speed,” Torrence said on the podcast. “The writing to me was on the wall that either I get some outside investment and scale up myself, or kind of listen to these guys and see if maybe now would be the right time to exit.”
Joining Headlands had its benefits during the pandemic because she could “lean on” its other sites with experience running vaccine trials. “Had we not gotten those … we may not still be here,” Torrence said.
Torrence, whose LinkedIn profile said she left the company in 2021, didn’t respond to messages from KHN.
Lyndon Fullen, a health care consultant and former Centex employee, said private equity provides funding that allows companies to add study sites.
“I completely support it,” he said. “If it’s about reaching that large patient population, it’s of course better to have larger groups with that funding.”
Opportunity in Long Covid
Contract research organization Parexel saw opportunity in the covid pandemic — millions of people were developing long covid after infection and there were few, if any, meaningful treatment options.
The company, which employs more than 19,000 people, was acquired in 2021 by EQT Private Equity and Goldman Sachs’ private equity arm for $8.5 billion , billions more than the $4.5 billion that private equity firm Pamplona Capital Management paid when it took Parexel private in 2017.
A growing body of research shows the debilitating effects of long covid, including a recent study of tens of thousands of patients in Scotland where nearly half had not fully recovered months later. But treatments addressing its root causes could be years away. “It’s a huge number of people,” said Dr. Nathalie Sohier, who leads Parexel’s infectious diseases and vaccines franchise. “There’s a lot of need.”
Long covid represents the promise and peril of the work to develop new drugs: Millions of patients create a potentially lucrative market for drug companies, and yet researchers and industry experts say they are reluctant to jump in. In part, that’s because “it’s not a well-defined disease, and that really makes it highly risky for companies to invest in research,” said Cecil Nick, a vice president for Parexel.
“How are we going to be able to tell the FDA that our drug works? We can’t count the number of people who died; we can’t count the number of people in the hospital,” said Dr. Steven Deeks , a University of California-San Francisco professor who is running an observational study on long covid patients.
As of August, among more than 4,400 covid studies, only 304 focused on long covid. A third of those were related to drug development, Sohier said.
Sohier said “there are few” companies in its long covid program. That hasn’t stopped Parexel from pitching itself as the ideal partner to shepherd new products, including by doing regulatory work and using remote technology to retain patients in trials. Parexel has worked on nearly 300 covid-related studies in more than 50 countries, spokesperson Danaka Williams said.
Michael Fenne, research and campaign coordinator with the Private Equity Stakeholder Project, which studies private equity investments, said Parexel and other contract research organizations are beefing up their data capacity. The aim? To have better information on patients.
“It kind of ties into access and control of patients,” Fenne said. “Technology makes accessing patients, and then also having more reliable information on them, easier.”
KHN senior correspondent Fred Schulte and Megan Kalata contributed to this report.
Related Topics
- Cost and Quality
- Health Care Costs
- Health Industry
- Pharmaceuticals
- Clinical Trials
- Investigation
- Patients for Profit
- Prescription Drugs
Copy And Paste To Republish This Story
By Rachana Pradhan December 2, 2022
After finding success investing in the more obviously lucrative corners of American medicine — like surgery centers and dermatology practices — private equity firms have moved aggressively into the industry’s more hidden niches: They are pouring billions into the business of clinical drug trials.
To bring a new drug to market, the FDA requires pharmaceutical firms to perform extensive studies to demonstrate safety and efficacy, which are often expensive and time-consuming to conduct to the agency’s specifications. Getting a drug to market a few months sooner and for less expense than usual can translate into millions in profit for the manufacturer.
That is why a private equity-backed startup like Headlands Research saw an opportunity in creating a network of clinical sites and wringing greater efficiency out of businesses, to perform this critical scientific work faster. And why Moderna, Pfizer, Biogen, and other drug industry bigwigs have been willing to hire it — even though it’s a relatively new player in the field, formed in 2018 by investment giant KKR.
In marketing its services, Headlands described its mission to “profoundly impact” clinical trials — including boosting participation among racial and ethnic minorities who have long been underrepresented in such research.
“We are excited,” CEO Mark Blumling said in a statement, to bring “COVID-19 studies to the ethnically diverse populations represented at our sites.” Blumling, a drug industry veteran with venture capital and private equity experience, told KHN that KKR backed him to start the company, which has grown by buying established trial sites and opening new ones.
Headlands’ sites also ran, among other things, clinical studies on treatments to combat Type 2 diabetes, postpartum depression, asthma, liver disease, migraines, and endometriosis, according to a review of website archives and the federal website ClinicalTrials.gov. But within two years, some of Headlands’ alluring promises would fall flat.
In September, Headlands shuttered locations in Houston — one of the nation’s largest metro areas and home to major medical centers and research universities — and Lake Charles, a move Blumling attributed to problems finding “experienced, highly qualified staff” to carry out the complex and highly specialized work of clinical research. The McAllen site is not taking on new research as Headlands shifts operations to another South Texas location it launched with Pfizer.
A fragmented clinical trials industry has made it a prime target for private equity, which often consolidates markets by merging companies. But Headlands’ trajectory shows the potential risks of trying to combine independent sites and squeeze efficiency out of studies that will affect the health of millions.
“We’ve seen reduced market competition in a variety of settings to be associated with increases in prices, reduction in access and choice for patients, and so on,” Singh said. “So it’s a delicate balance.”
Dr. Aaron Kesselheim , a professor of medicine at Harvard Medical School, called private equity involvement in trials “concerning.”
“We need to make sure that patients” know enough to provide “adequate, informed consent,” he said, and ensure “protections about the privacy of the data.”
“We don’t want those kinds of things to be lost in the shuffle in the goals of making money,” he said.
Blumling said trial sites Headlands acquired are not charging higher prices than before. He said privacy “is one of our highest concerns. Headlands holds itself to the highest standard.”
Eleven of the 25 private equity firms identified by industry tracker PitchBook as the top investors in health care have bought stakes in clinical research companies, a KHN analysis found. Those companies have been involved in studies ranging from covid vaccines to treatments for ovarian cancer, Parkinson’s disease, and Alzheimer’s.
Still, most trial sites are physician practices that don’t consistently perform studies, according to a presentation by Boston-based investment firm Provident Healthcare Partners.
“Independent sites are being purchased by private equity, and they’re moving into larger site groups of 30, 40, and then their game plan is to roll that up into a business and then sell it again,” said Linda Moore Schipani, CEO of Clinical Research Associates, a Nashville-based company that worked on covid vaccine trials for AstraZeneca, Novavax, and Pfizer. “That’s kind of the endgame.”
It has made other acquisitions since then and opened new sites in areas with “extremely limited trial options,” something Blumling says distinguishes his company.
“I’m not an evangelist for private equity,” Blumling said. “The ability of KKR to be willing to invest in something that is a three- to five-year return versus a one- to two-year return is something that you won’t see out there.”
A research center in Brownsville, Texas — a stone’s throw from the U.S.-Mexico border and where 95% of the population is Hispanic or Latino — is one of several where it is partnering with Pfizer to boost patient diversity.
To recruit patients, Headlands “is really going beyond what a lot of sites do, which is social media,” Blumling said in an interview. “It’s going within churches, community fairs, really getting out into as much as possible the broader community.”
Headlands closed the Houston and Lake Charles sites because of staffing issues, Blumling said, and finished or moved their studies elsewhere. Blumling said the decision to close those locations “did not have anything to do with the speed of trials.”
Similarly, he said, Headlands is moving the McAllen site’s operations to Brownsville “because it had a larger population of trained personnel.”
“We want to continue to grow sites and do great work,” Blumling said. “If we can’t find the people in order to do that at the quality that we demand, which is at the highest level, then it doesn’t make sense to keep those sites.”
‘The Writing to Me Was on the Wall’
In 2006, Devora Torrence co-founded Centex Studies, which she described as “my little mom and pop business” in a 2021 podcast about female entrepreneurs in science. She said a flurry of interest from private equity came at the end of 2018. The appeal was evident: Drug companies were relying on bigger clinical trial networks.
“The thing is speed, getting it to market. With a bigger network, you get that speed,” Torrence said on the podcast. “The writing to me was on the wall that either I get some outside investment and scale up myself, or kind of listen to these guys and see if maybe now would be the right time to exit.”
Joining Headlands had its benefits during the pandemic because she could “lean on” its other sites with experience running vaccine trials. “Had we not gotten those … we may not still be here,” Torrence said.
Torrence, whose LinkedIn profile said she left the company in 2021, didn’t respond to messages from KHN.
“I completely support it,” he said. “If it’s about reaching that large patient population, it’s of course better to have larger groups with that funding.”
The company, which employs more than 19,000 people, was acquired in 2021 by EQT Private Equity and Goldman Sachs’ private equity arm for $8.5 billion , billions more than the $4.5 billion that private equity firm Pamplona Capital Management paid when it took Parexel private in 2017.
A growing body of research shows the debilitating effects of long covid, including a recent study of tens of thousands of patients in Scotland where nearly half had not fully recovered months later. But treatments addressing its root causes could be years away. “It’s a huge number of people,” said Dr. Nathalie Sohier, who leads Parexel’s infectious diseases and vaccines franchise. “There’s a lot of need.”
Long covid represents the promise and peril of the work to develop new drugs: Millions of patients create a potentially lucrative market for drug companies, and yet researchers and industry experts say they are reluctant to jump in. In part, that’s because “it’s not a well-defined disease, and that really makes it highly risky for companies to invest in research,” said Cecil Nick, a vice president for Parexel.
“How are we going to be able to tell the FDA that our drug works? We can’t count the number of people who died; we can’t count the number of people in the hospital,” said Dr. Steven Deeks , a University of California-San Francisco professor who is running an observational study on long covid patients.
Sohier said “there are few” companies in its long covid program. That hasn’t stopped Parexel from pitching itself as the ideal partner to shepherd new products, including by doing regulatory work and using remote technology to retain patients in trials. Parexel has worked on nearly 300 covid-related studies in more than 50 countries, spokesperson Danaka Williams said.
“It kind of ties into access and control of patients,” Fenne said. “Technology makes accessing patients, and then also having more reliable information on them, easier.”
We encourage organizations to republish our content, free of charge. Here’s what we ask:
You must credit us as the original publisher, with a hyperlink to our kffhealthnews.org site. If possible, please include the original author(s) and KFF Health News” in the byline. Please preserve the hyperlinks in the story.
It’s important to note, not everything on kffhealthnews.org is available for republishing. If a story is labeled “All Rights Reserved,” we cannot grant permission to republish that item.
Have questions? Let us know at KHNHelp@kff.org
More From KFF Health News

Their First Baby Came With Medical Debt. These Illinois Parents Won’t Have Another.

Democrats Seek To Make GOP Pay for Threats to Reproductive Rights

Medical Residents Are Increasingly Avoiding States With Abortion Restrictions

KFF Health News' 'What the Health?': Newly Minted Doctors Are Avoiding Abortion Ban States
Thank you for your interest in supporting Kaiser Health News (KHN), the nation’s leading nonprofit newsroom focused on health and health policy. We distribute our journalism for free and without advertising through media partners of all sizes and in communities large and small. We appreciate all forms of engagement from our readers and listeners, and welcome your support.
KHN is an editorially independent program of KFF (Kaiser Family Foundation). You can support KHN by making a contribution to KFF, a non-profit charitable organization that is not associated with Kaiser Permanente.
Click the button below to go to KFF’s donation page which will provide more information and FAQs. Thank you!
- 919-625-7731

Matheus BD Connections
Providing experienced business development and sales services for clinical research industry service providers.
View Our Services...
Developing Relationships That Matter
Matheus BD Connections, LLC was founded by Chris Matheus to provide experienced business development and sales services for clinical research industry service providers. This approach allows small, new and/or niche providers the resources, experience and connections that has taken years to develop. Matheus BD has extensive experience in sales, marketing and business development in the pharmaceutical and clinical research industries. The company’s mission is connecting people for their mutual benefit in solving the issues that arise in clinical research.

JAMES LIND INSTITUTE
Careers in clinical trials, did you know.
People with training in clinical research business development highly sought after in the industry.
GAIN MORE CLIENTS & RETAIN THEM
Business development professionals who have excellent selling skills, assertive communication and a pleasing personality are a rare find. Even rarer are business development professionals who have a thorough understanding of the clinical trial processes as well as the business aspects.
Next Commencement Date
15 JUL
Clinical research business development career.
Clinical research offers multiple business development career options. Growth of the pharmaceutical industry has led to newer opportunities and avenues for clinical research business development professionals. Some positions available in the business development departments are:
– Business Development Executive – Business Development Manager – Business Analyst
BUSINESS DEVELOPMENT EXECUTIVE / MANAGER
Most pharmaceutical companies and contract research organizations now have global operations with clients spread across all major regions. Managing and interfacing with these clients is usually the responsibility of the Business Development Executive or Manager. A Clinical Research Business Development Career can be very stressful and demanding. Business development executives carry out negotiations for new business deals, draft agreements / contracts and bring new clinical trial related business to the company. They are also responsible for establishing initial interactions with potential clients. The most significant responsibility of a Business Development Executive/Manager is to bring as much as possible new business to the company and thus help generate more revenue. Of the several responsibilities of business development professionals, a few are listed below:
– Generating and maintaining important business reports and representing the organization at various conferences – Creating multiple types of marketing tools for advertising – Preparing proposals to meet client requirements – Generating new business leads and securing new business – Ongoing relationship building with potential clients
Competencies & Skills:
A number of competencies and skills are required to make a career as a Business Development Manager / Executive. Some of them are:
– Outstanding spoken and written communication skills – Knowledge of pharmaceutical industry and clinical research to understand client requirements better – Good negotiation skills – Good selling skills – High self-confidence – Knowledge of sales management techniques – Self-motivation and ability to work independently – Knowledge of basic word and data processing software’s like MS Word and Excel
– Bachelor’s or Master’s degree in life science, marketing, management or a business related field
RECOMMENDED PROGRAM
For a career as a Business Development Professional in the clinical research industry we suggest you consider the following online program(s):
– Advanced PG Diploma in Clinical Research & Regulatory Business Development
Participant Feedback
"I am very thankful for the prompt support offered by JLI every time. I appreciate that you went the extra mile for me when I was in need. Thanks again." Sushma D. Data Analyst Bangalore, India
- Discovery Platform
- Innovation Scouting
- Startup Scouting
- Technology Scouting
- Tech Supplier Scouting
- Startup Program
- Trend Intelligence
- Business Intelligence
- All Industries
- Industry 4.0
- Manufacturing
- Case Studies
- Research & Development
- Corporate Strategy
- Corporate Innovation
- Open Innovation
- New Business Development
- Product Development
- Agriculture
- Construction
- Sustainability
- All Startups
- Circularity
- All Innovation
- Business Trends
- Emerging Tech
- Innovation Intelligence
- New Companies
- Scouting Trends
- Startup Programs
- Supplier Scouting
- Tech Scouting
- Top AI Tools
- Trend Tracking
- All Reports [PDF]
- Circular Economy
- Engineering
- Oil & Gas

Share this:
- Click to share on Facebook (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
8 Clinical Trial Trends in 2023
Are you curious about which clinical trial trends & startups will soon impact your business? Explore our in-depth industry research on 1 449 clinical trial startups & scaleups and get data-driven insights into technology-based solutions in our Clinical Trial Innovation Map!
Clinical trials are dealing with complex procedures, slow patient recruitment, compliance issues, and high operation costs. Therefore, the medical and pharmaceutical industries are digitizing operations to resolve these problems. The COVID-19 pandemic also shifted research teams towards virtual solutions to avoid site selection. For example, startups and scaleups are using wearables and cloud computing to enable remote and patient-centric monitoring. This article provides an overview of the top 8 global clinical trial trends, including artificial intelligence (AI), blockchain, 5G, and decentralized clinical trials. Read more to discover the latest developments in clinical trials that impact your business.
Innovation Map outlines the Top 8 Clinical Trial Trends & 16 Promising Startups
For this in-depth research on the Top Clinical Trial Trends & Startups, we analyzed a sample of 1 449 global startups & scaleups. The result of this research is data-driven innovation intelligence that improves strategic decision-making by giving you an overview of emerging technologies & startups in the healthcare industry. These insights are derived by working with our Big Data & Artificial Intelligence-powered StartUs Insights Discovery Platform , covering 2 500 000+ startups & scaleups globally. As the world’s largest resource for data on emerging companies, the SaaS platform enables you to identify relevant startups, emerging technologies & future industry trends quickly & exhaustively.
In the Innovation Map below, you get an overview of the Top 8 Clinical Trial Trends & Innovations that impact 1 449 companies worldwide. Moreover, the Clinical Trial Innovation Map reveals 16 hand-picked startups, all working on emerging technologies that advance their field.
Top 8 Clinical Trial Trends
- Artificial Intelligence
- Data Management
- Decentralized Clinical Trials
- Cloud Computing
- Cybersecurity

Click to download
Tree Map reveals the Impact of the Top 8 Clinical Trial Trends
Based on the Clinical Trial Innovation Map, the Tree Map below illustrates the impact of the Top 8 Clinical Trial Trends in 2023. Startups and scaleups are leveraging AI to automate tasks and enable predictive measures to identify best-matching drug candidates. Data management has also been a keen area, where researchers are employing inclusive and digitized means to increase participation rates. Further, the introduction of wearable devices, 5G connectivity, and a decentralized approach enables precise data capture at home. Cloud-based platforms also reduce administrative workloads and enhance cross-collaboration. To protect sensitive patient data, startups utilize various cybersecurity measures together with blockchain, improving transparency and real-time visibility.
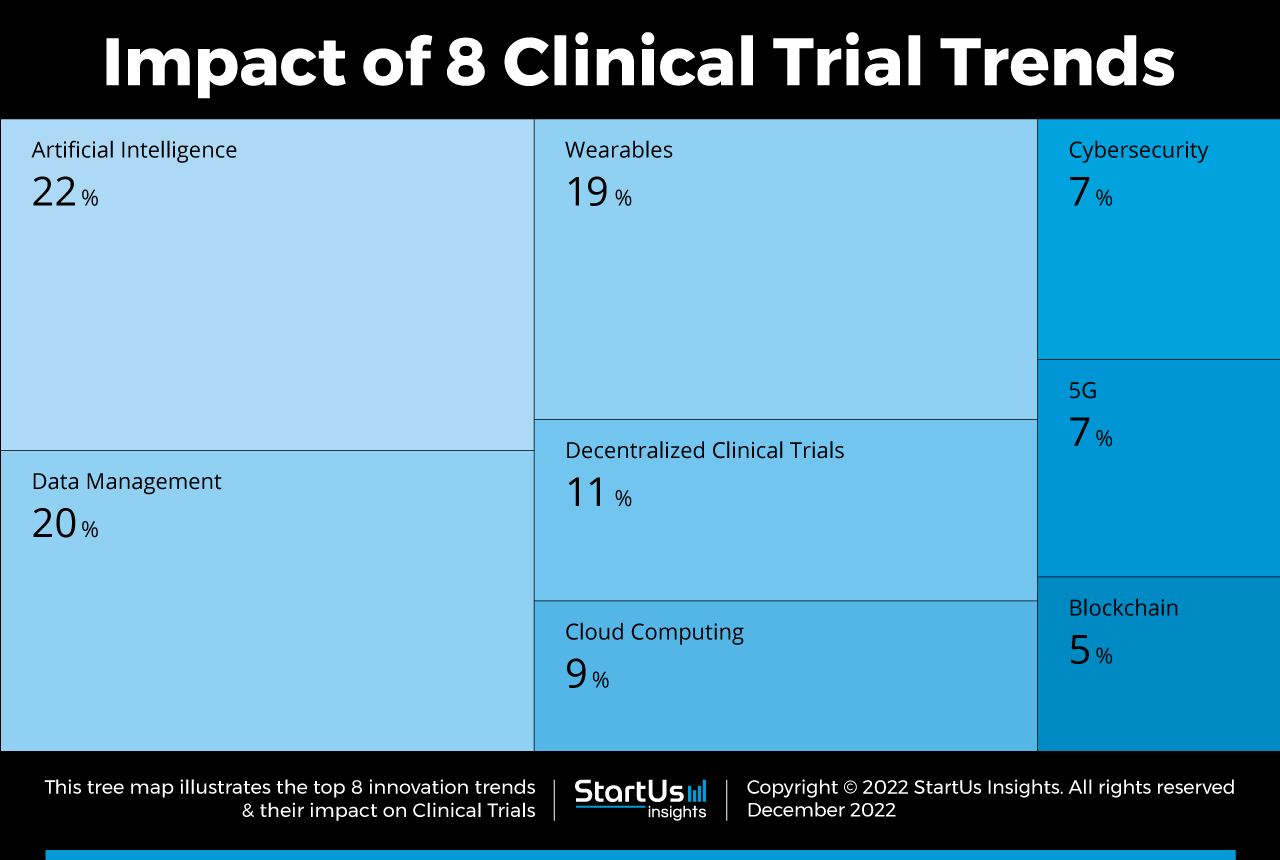
Schedule Demo
Global Startup Heat Map covers 1 449 Clinical Trial Startups & Scaleups
The Global Startup Heat Map below highlights the global distribution of the 1 449 exemplary startups & scaleups that we analyzed for this research. Created through the StartUs Insights Discovery Platform, the Heat Map reveals that the US sees the most startup activity.
Below, you get to meet 16 out of these 1 449 promising startups & scaleups as well as the solutions they develop. These clinical trial startups are hand-picked based on criteria such as founding year, location, funding raised, & more. Depending on your specific needs, your top picks might look entirely different.
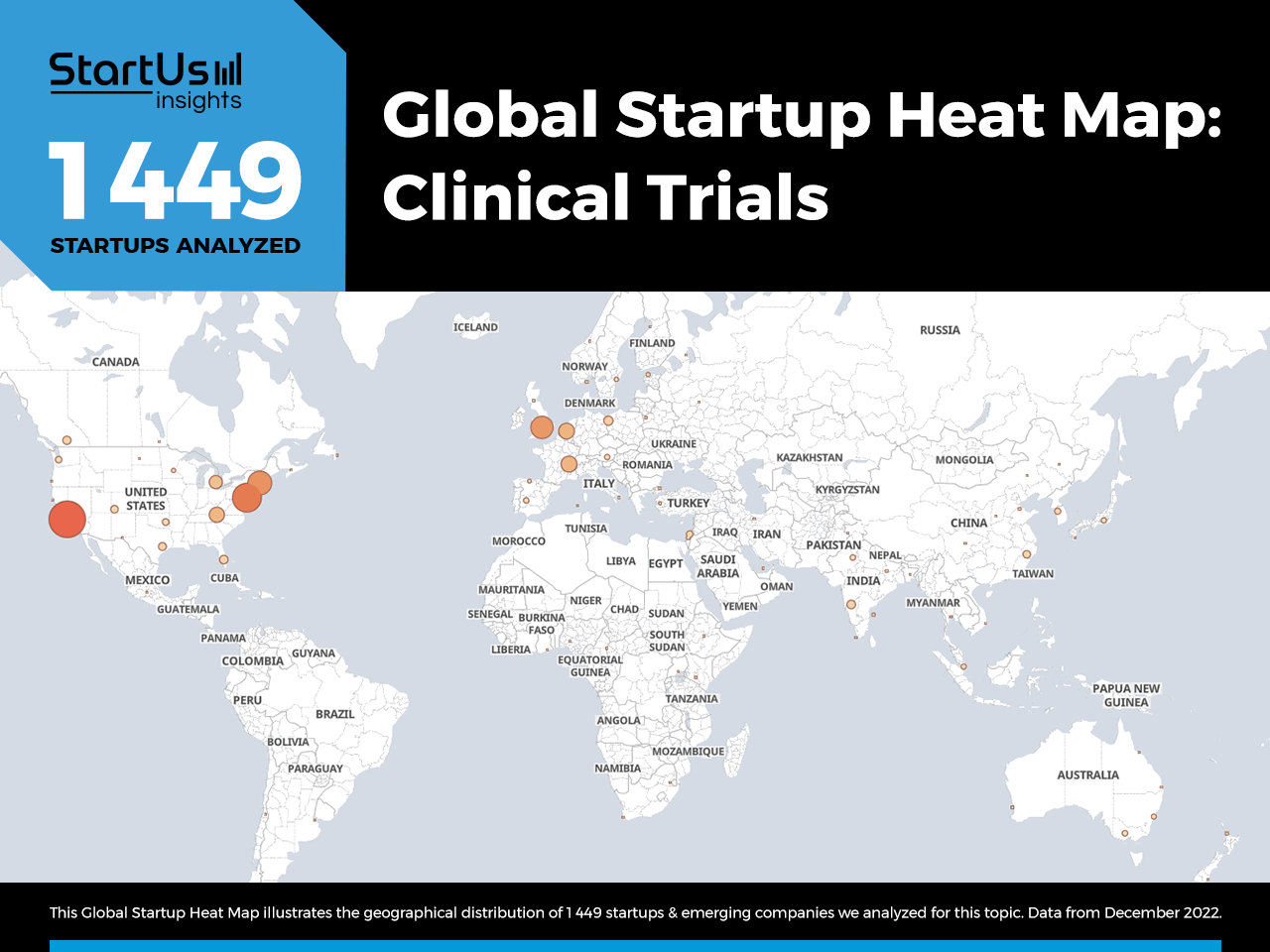
Top 8 Clinical Trial Trends in 2023
1. artificial intelligence.
Clinical research groups and pharma companies integrate AI to automate various processes during clinical trials, reducing staff workload and errors. Machine learning (ML) and computer vision mine enormous data to screen drug candidates quickly. Other tools, like natural language processing (NLP), deep learning (DL), and optical character recognition (OCR), accelerate data collection, analysis, and interpretation. Using ML-based predictive analytics, drug developers also forecast patient deterioration to avoid emergencies. Further, AI-driven innovations assist biopharma businesses in identifying target sites, priority candidates, and qualified investigators to ensure safety and cost-effectiveness.
RxE2 performs Behavior-based Interventions
US-based startup RxE2 leverages AI to perform behavior-based clinical trials. The startup’s platform, Habitu , adapts to patient habits and routines to optimize interventions. It also provides procedural, professional, and emotional support. This ranges from medication management and visit schedule to support groups onboarding and insights sharing. By translating patient behavior into data, Habitu assists clinical sites and biotech companies to prevent unwanted emergencies.
Altis Labs aids Imaging Data Acquisition
Canadian startup Altis Labs develops Nota , an AI-driven medical imaging platform for imaging data acquisition. It uses deep learning to analyze imaging features present in disease tissues and surrounding anatomy. Then, the platform develops radiographic imaging biomarkers that enable personalized treatment and stratification of early-stage cancers. Further, Nota enhances visibility into imaging protocol adherence and missing data as well as enables researchers to easily manage imaging data. This accelerates clinical trial operations and increases their transparency.
2. Data Management
Clinical data volumes are increasing at a fast pace and this leads to the growing interest in medical trial data management tools. Big data enables better predictive modeling of biological processes and drugs. Research organizations are also analyzing electronic health records (EHRs) to identify optimal patients for clinical trials. Product design teams apply data analytics to find patients from diverse populations, improving treatment efficacy for underrepresented groups. Other electronic tools such as electronic patient outcome (ePRO) and electronic case report form (eCRF)simplify participation processes.
Acclinate delivers Diverse Patient Engagement
US-based startup Acclinate makes e-DICT , a digital health analytics platform to increase the representation and diversity in genomic research and medical trials. It automates the identification, engagement, and retention of participants through predictive analytics. The platform also analyzes the trial characteristics against diverse engagement data to attract qualified participants. By making the data sources inclusive and trustworthy, Acclinate allows pharma and healthcare organizations to increase conversion rates and boost patient outcomes.
Mediaiplus creates a Clinical Trial Data Platform
South Korean startup Mediaiplus builds MediC , a platform to manage clinical trial participant data. It applies investigator-generated data, deep learning, and AI to learn medical and environmental factors affecting diseases. The platform also collects and integrates data from multiple sources to provide the best-fitted clinical trial information. It navigates optimized information to monitor similar cases’ status of progress. Further, the platform offers smart data matching that enables researchers to get the latest trends and customized reports. This allows research teams to make the decision-making process accurate without operational inefficiencies.
3. Wearables
The application of health-tracking wearables and devices in clinical research enables remote patient monitoring and, in turn, virtual trials. Wearable technology provides greater flexibility in collecting and analyzing participant data. Further, the integration of wearable medical devices with smartphones advances the growth of mobile health (mHealth) platforms. They reduce the logistical constraints of clinical trials and allow researchers to observe patients’ physiological and behavioral changes in real time. As a result, wearables are important for clinical trial sponsors to save money and time in achieving improved data quality.
ZEPHYRx provides Continuous Data Monitoring
US-based startup ZEPHYRx provides end-to-end remote respiratory monitoring (RRM) solutions. The startup’s FDA-cleared device, Spirobank Smart Spirometer , remotely performs pulmonary function tests (PFT). It measures the respiratory metrics of participants in real time and notifies them about health developments using a smart dashboard. The dashboard works in conjunction with a companion app, Breathe Easy , which offers video coaching, PFT instructions, and gamified breathing exercises. This way, ZEPHYRx empowers clinical teams, patients, and researchers with a continuous data monitoring system to improve trial accuracy and reduce dropouts at lower costs.
Preventric advances Remote Health Monitoring
US-based startup Preventric is the developer of smart devices designed to deliver hemodynamics measurements of health. The startup’s device, BPro , leverages sensors and AI-driven analytics to offer non-invasive and continuous measurement of ambulatory blood pressure. A 24-hour recording offers insights into hypertension diagnosis and management, which is more useful than traditional measurements. As a result, Preventric allows pharmaceutical companies to better target audiences and remotely gather vital information, leading to rapid and accurate results.
4. Decentralized Clinical Trials
Clinical trial organizers are continuously trying to adapt new technology to make clinical trials faster and more convenient for patients and physicians. This has resulted in the emergence of decentralized clinical trials. Advances in electronic communication tools, data transmission, digitization, biosensors, and virtual trials enable the decentralization of clinical trials. This allows researchers to reach a larger and more diverse pool of participants and reduces the workload of trial investigators. Moreover, this siteless approach aids drug developers to improve patient-centricity, recruitment, and retention at lower costs.
Trialize enables Hybrid Clinical Trials
French startup Trialize creates a hybrid decentralized clinical trial platform that streamlines clinical trial processes. It features a modular design and pre-configured options to reduce the need for customized programming, saving time and budget. Further, the startup’s other solution, Biometric TeleConsent , supports participant pre-screening, eConsent, and biometric facial scans. These tools promote smooth virtual experiences to simplify the consent process and clarify participant obligations. As a result, Trialize enables research teams to conduct clinical trials with increased transparency, minimized inclusion barriers, and low maintenance.
Curavit builds a Decentralized Clinical Trial Platform
US-based startup Curavit builds DCT , a decentralized clinical trial platform. It improves three areas – patient experience, data capture, and operations management. Simple virtual recruitment, telehealth, ePRO, and wearables devices leverage patient experience. Meanwhile, the platform delivers electronic data capture (EDC), patient health records (PHR), and other multimedia observations. Finally, it offers operation components including patient supply management, site investigator files, and quality management systems. This way, Curavit enhances patient safety, protocol adherence, and patient retention.

5. Cloud Computing
Cloud-enabled clinical trials allow easy storage and retrieval of large amounts of data without putting excessive load on the existing digital infrastructure. Using cloud-based electronic data capture and processing, research teams securely store medical data from multiple sources in centralized repositories. This is particularly useful for clinical trials with complex logistics. Cloud also offers a cost-effective means to rapidly scale trial operations to serve participants across a varied geographical area. With real-time data transfer, instant feedback, and rapid data cleaning, cloud computing allows contract research organizations (CROs) to make clinical trials fast, efficient, and cost-effective.
Jeeva Informatics Solutions accelerates Patient Recruitment
US-based startup Jeeva Informatics Solutions develops eClinical Cloud , a software-as-a-service SaaS platform to speed up remote patient recruitment. It works on any browser-enabled mobile device and reduces the time and logistical burden on study teams. The platform also easily integrates with health platforms to access patient information and match them accurately as per trial needs. This enables a cost-effective solution to set up and conduct clinical trials at various scales or duration. By solidifying patient enrollment, the startup’s cloud system aids hospital sites, academic centers, and biopharmaceutical sponsors to avoid risks.
Yaocheng enables Cloud-based Clinical Research Management
Chinese startup Yaocheng offers AuroraPrime , a SaaS clinical research management platform for life science studies. It leverages AI on the cloud that adapts to changing needs through an in-built engine. This accelerates patient data collection and study timelines through the quick configuration of trial parameters, data entries, and database review. The platform also offers real-time data insights throughout the trial to make accurate interventions. As a result, Yaocheng’s cloud infrastructure reduces costs and increases efficiency in medical trials.
6. Cybersecurity
The healthcare industry as a whole has been a key target of malicious cyber attacks due to sensitive patient information. To tackle such attacks, startups are innovating and developing security measures to detect and prevent cyber risks. This includes novel encryption models, secure authentication, and cryptography. By doing this, clinical research teams are able to build necessary trust with participants and protect businesses from the side costs due to data breaches.
Crescendo improves Research Health Record Security
US-based startup Crescendo secures health data collected during clinical research. The startup’s secure data management platform holds information in protected, cloud-based databases. It safeguards data using an AES 256-bit encryption key according to NIST standards. Further, the platform does not share patient information with third parties. This way, Crescendo enables researchers and medical professionals to conduct secure clinical trials and gain access to relevant patient data without hampering their privacy.
Triall provides Self-sovereign Identities for Clinical
Dutch startup Triall provides a digital clinical trial platform that leverages self-sovereign identity (SSI) technology to streamline and secure medical trials. It uses blockchain-based SSI to generate verifiable proof of identity. In addition to industry-standard encryption keys, the startup applies privacy-preserving hash codes as fingerprints to establish data authenticity. This offers medical professionals and participants more control and ownership over their data.
Patients are already using telehealth devices and wearables to collect, analyze, and exchange data. However, these sensors are not accurate or secure enough for the transmission of large files. That is why 5G technology is crucial for offering patients with medical-grade equipment for remote connectivity. It also enables decentralized clinical trials, making them more patient-centric. Moreover, the high speed of 5G encourages more users from remote and rural areas to participate. This way, 5G connectivity supports existing telemedicine infrastructure and pharmaceutical companies in reaching more effective medical research.
WKD.SMRT provides In-home Real-world Data (RWD)
US-startup WKD.SMRT utilizes wireless networking to collect real-world data at home. The startup’s platform combines vision and radar sensors, third-party inputs, and machine learning via 5G. This enables accurate and real-time RWD capture of patients’ phenotypical and cognitive metrics. For instance, the platform collects relevant biomarkers on the environment, daily living activities, and quality of life besides wearable measurements. This empowers drug developers with enriched real-world evidence for accurate and cost-effective trials at reduced risk.
Nexalta builds a Collaborative Computing Platform
Austrian startup Nexalta leverages 5G to develop Networks4Life , an open-source collaborative computing platform. It leverages AI, open data, blockchain, 5G, and the internet of things (IoT) to assist patients with chronic diseases. By deploying fast wireless broadband, wearable devices automatically collect, anonymize, and analyze big amounts of secured data. This way, 5G networks aid patients, caregivers, doctors, and researchers with a constant flow of data to speed up treatment tests.
8. Blockchain
Decentralization of data through blockchain improves data authenticity and credibility. Therefore, startups are using blockchain applications to improve patient data transparency and traceability. Due to its cryptographic nature, blockchain-powered clinical data records are immutably stored to ensure patient privacy along with quality. This also resolves intellectual property (IP) concerns for trial sponsors. Additionally, the use of a blockchain ledger enables clinical trial researchers to complete trials in an ethical and transparent manner.
PharmaTrail enhances Patient Data Transparency
Swiss startup PharmaTrail creates blockchain-driven medical software to capture and manage trial data. It ensures the security, reliability, and traceability of medical data by securing sensitive information on a decentralized ledger. This enables PharmaTrail to create an incorruptible audit trail of clinical trial data, which is traceable and visible to patients and medical professionals alike.
Aomics offers Blockchain-based Clinical Data Analysis
German startup Aomics leverages blockchain and deep learning to ensure data ownership in clinical trials. The startup’s platform uses a decentralized blockchain-based network to integrate data with pre-existing experimental data and patient records. The platform then predicts the outcomes of clinical trials. Meanwhile, the data stays secure over the blockchain network, enabling medical professionals to sustainably scale clinical trial operations.
Discover all Clinical Trial Trends, Technologies & Startups
Emerging technologies based on AI, cloud, and blockchain make clinical trials more secure, efficient, and cost-effective. They also automate data processing, predict patient outcomes, and contribute to data inclusivity. The adoption of cloud computing further assists clinical trial organizers in scaling up virtual studies. 5G, wearables, and enhanced cybersecurity measures will make clinical trials more decentralized, diversified, and fast.
The Clinical Trial Trends & Startups outlined in this report only scratch the surface of trends that we identified during our data-driven innovation & startup scouting process. Among others, blockchain, AI, and 5G will transform the sector as we know it today. Identifying new opportunities & emerging technologies to implement into your business goes a long way in gaining a competitive advantage. Get in touch to easily & exhaustively scout startups, technologies & trends that matter to you!
Your Name Business Email Company
Get our free newsletter on technology and startups.
Protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Discover our Free Healthcare Report 21 pages
Get in touch!

Healthcare 21 pages report
Pharma 22 pages report, biotech 22 pages report, manufacturing 22 pages report.
Leverage our unparalleled data advantage to quickly and easily find hidden gems among 4.7M+ startups, scaleups. Access the world's most comprehensive innovation intelligence and stay ahead with AI-powered precision.
Get in touch
Your Name Business Email Company How can we support you? (optional)
Business Email

Protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Working at a&r
- Business Development Manager Clinical Research (m/w/d)
analyze & realize GmbH (a&r) is a leading Contract Research Organization and consultancy located in Berlin, Germany, with a specific focus on natural health products. We provide highly specialized services to marketeers of pharmaceuticals, supplements, medical devices and cosmetics and ingredients.
Our key offerings in the Consumer Healthcare market are: Conduct of clinical studies (nationally and internationally) and strategic innovation consulting covering all aspects of regulatory requirements, science and product development.
Currently we are looking for a new Business Development Manager Clinical Research – preferred full-time (40h/week).
Main areas of responsibility:
- Oversight and management of all Clinical Research Business Development activities
- Offer management in close collaboration with Head of Clinical Operations and GM
- Growth of business with established and new clients
- Relationship management with key customer contacts
- Leading of clinical research communication and marketing activities
- Contributing to strategic development plan of the company
Education and experience:
- University degree in science, medicine, business administration or related areas
- Excellent understanding of innovation drivers in consumer healthcare space
- Exposure to and understanding of clinical trial processes
- Basic understanding of regulatory requirements in ingredients and Consumer Healthcare products
- Commitment, flexibility, initiative, ability to work under pressure and in a team
- Independent, problem-solving, and proactive working style
- Very good command of written and spoken English
- The opportunity to actively shape your area of responsibility
- Attractive salary according to your personal work experience
- The opportunity to actively shape your area of responsibility independently
- Flexible working time
- 30 vacation days with 40 hours per week
- Assumption of costs for BVG ticket (area AB)
- Capital-forming benefits
Your application documents:
- Meaningful cover letter explaining your motivation
- Curriculum vitae in table form
- Relevant certificates and references
- Possible starting date
- Salary expectations
We are looking forward to receiving your application!
Contact person: Sabine Schüler

Email: [email protected]
OTHER JOBS!
- Prüfarzt/Prüfärztin in Teilzeit auf Honorarbasis (m/w/d)
- Naturwissenschaftler/Lebensmittelchemiker als Berater (m/w/d)
- (Senior) Scientific Consultant (m/w/d)
- (Senior) Project Manager Clinical search (m/w/d)
Salutation Salutation Mrs Mr Dr Prof
First name *
Phone number
Subject* Subject* Supplements Herbal drugs Substance based medical devices Marketability of innovative ingredients Product specific claims Other*
How did you hear about us? How did you hear about us? Flyer Website Google LinkedIn Other*
Other Subject
Where did you here about us?
In case you do not wish to receive further information regarding new web and service offers by analyze & realize GmbH, you can object to this usage at any time. These e-mails are sent out in accordance with our data privacy statement: https://www.a-r.com/data-protection/. To object, please contact our data protection officer at [email protected]. Exercising your right to object incurs no further costs save the usual telecommunication costs. In case you do not wish to receive further information regarding new web and service offers by analyze & realize GmbH, you can object to this usage at any time. These e-mails are sent out in accordance with our data privacy statement: https://www.a-r.com/data-protection/. To object, please contact our data protection officer at [email protected]. Exercising your right to object incurs no further costs save the usual telecommunication costs. I've read and accept the conditions above.
- Open access
- Published: 10 May 2024
Assessing the impact of Medical Education's Innovation & Entrepreneurship Program in China
- Xiandi You 1 &
- Wenyi Wu 2 , 3 , 4
BMC Medical Education volume 24 , Article number: 519 ( 2024 ) Cite this article
Metrics details
A growing number of clinical undergraduates are chosen to enter institutions for higher education biotechnology and industry workforce, though most need more laboratory experience training and business practice. Innovation and Entrepreneurship Program (I&E Program) can benefit from biological experiment and commercialization training largely absent from standard clinical medical educational curricula. Our study investigates the impact and status of the I&E Program in enhancing medical students’ research and entrepreneurial abilities and provides recommendations for improving this program.
A cross-sectional study was applied by delivering a questionnaire to survey medical students from Central South University who participated in the I&E Program. The questionnaire consisted of three parts: basic information, the impact of the I&E Program on medical students’ research and entrepreneurial abilities, and attitudes and recommendations regarding the I&E Program.
Many students participating in the I&E Program have received competition awards and improved their academic experience, article writing, and application patents. Their research-related abilities have been enhanced, including in-lab techniques, theoretical research skills, data analysis knowledge, clinical research skills, experimental research skills, entrepreneurship, data analysis ability, teamwork, and communication. While 73.93% of students express satisfaction with the I&E Program, there are still several areas of improvement, including more robust practical components, increased support, and enhanced teamwork.
The scale of the I&E Program is rapidly expanding to address scientific research or business skills needed by college students in the new era. However, more programs still need to be discontinued during their further study. The I&E Program significantly enhances research abilities and fosters confidence in their study. This analysis emphasizes the importance of research-oriented and interdisciplinary education for students’ holistic development in medical schools compared with formal medical education.
Peer Review reports
Introduction
The National Innovation and Entrepreneurship Training Program for College Students (I&E Program) in China originated from the Ministry of Education’s policy recommendations in 2010. These recommendations aimed to promote innovation and entrepreneurship education in higher education, fostering students’ practical skills and innovative spirit. Implemented in 2011, the program received significant participation, with 16,300 projects submitted by Central South University under the ministries for approval [ 1 ]. More universities joined the program, which expanded nationwide with local government support and active university involvement, garnering widespread participation. In 2021, Hunan Province of China approved 11,394 I&E Program with a budget of 87.15 million yuan, involving 49,451 student participants. Central South University initiated 1,731 programs, including 432 in medical disciplines, comprising 391 innovative programs and 41 entrepreneurial programs [ 2 , 3 ]. American allopathic medical schools typically accept between 2 and 36% of their class size into I&E Program [ 4 ], whereas Chinese medical schools show broader participation. At least 2,100 undergraduate medical students are participating in the I&E Program in 2023 at Central South University, and this number is projected to grow. A survey at Yangtze University found 61.4% of clinical medicine majors involved in the Program [ 5 ], with similar expectations at Xiangya Medical School of Central South University.
The I&E Program provides a platform to develop their innovative ideas into lab experience or viable business. It aims to cultivate high-level innovative talents who can meet the needs of building an innovative-oriented nation [ 6 ]. It empowers students to conduct independent research, program design, implementation, and analysis under instructor guidance. They receive mentorship from seasoned scientists and industry experts. What’s more, the programs provide funding, access to cutting-edge facilities, and support for intellectual property protection. They encourage student collaboration and networking, fostering team formation and joint work. Universities annually solicit program proposals from students, securing financial backing at national, provincial, or university levels through application and evaluation processes. The programs fall into three categories: innovation training, entrepreneurship training, and entrepreneurship practice. They usually span a year, with universities conducting periodic inspections involving reports and poster presentations.
Interest in I&E education in healthcare is growing. However, formal educational programs in this field are relatively rare, and there needs to be more knowledge about the status and practices of I&E education in healthcare. To address these unmet needs, medical students inclined towards I&E can pursue these interests through graduate degrees, innovation scholarships, and other courses [ 4 , 7 ]. Also, it is necessary to continually report innovations in health education literature to identify successes and challenges in medical I&E education [ 8 ]. Existing research rarely includes nationwide I&E programs in Chinese healthcare [ 9 ]. The impetus for our study stemmed from a multifaceted understanding of clinical undergraduate education’s current landscape and evolving demands. Moreover, we want to assess current educational programs and recommend program improvement. Beyond evaluating the current status, there was a clear need to identify areas of improvement for the I&E Program to serve its participants better. Given the rapid expansion of such programs, it was imperative to provide evidence-based recommendations to enhance their effectiveness in meeting the new era’s demands on college students. We want to enhance the research and entrepreneurial abilities of medical students and improve their implementation and talent cultivation at medical schools.
In preparation for further survey, we first sought to identify the core I&E competencies we expect from all undergraduate participants and identify some concepts. The Entrepreneurship Competence Framework (EntreComp) generally defines entrepreneurship competency as the ability to turn ideas into actions [ 8 ]. Merriam-Webster dictionary defines “innovation” as a new idea, method, or device, which is core to bringing new approaches into a field. In business literature, innovation and entrepreneurship are widely regarded as closely intertwined concepts [ 10 ]. No medical I&E programs have publicly proposed a formal competency for the innovation [ 4 ]. However, EntreComp identified 15 competencies that would generate an “entrepreneurial mindset” for all citizens [ 8 ]. Another group validated all 15 competencies as curricular competencies in I&E for biomedical research trainees [ 11 ].
We also want to investigate what research competencies participants can gain from the I&E Program because research skills are fundamental for future clinician-researchers [ 12 ]. Some core competencies for clinical and translational scientists were also recognized [ 13 ]. Kansas City University defines six significant areas where students could demonstrate research competency [ 14 ]: project understanding, technical skills, attention to detail, analytical ability, communication with preceptor and research team members, and professionalism. These research competencies align with the Accreditation Council for Graduate Medical Education (ACGME) core competencies of Medical Knowledge. “Developing research skills in medical students: AMEE Guide No. 69,” published by The International Association for Health Professions Education (AMEE), defined several skills for medical students wanting to develop research skills. It isn’t easy to establish explicit connections between specific skills and innovation. The extensive skills identified in the literature as fostering innovation include fundamental abilities such as reading, academic skills, and technical expertise, as well as “soft” skills like problem-solving, teamwork ability, and leadership [ 12 ]. Competency is a cluster of related knowledge, attitudes, and skills that affect a significant part of one’s job [ 15 ]. Generally, the research competencies we hope to discuss include hard skills, most identified in AMEE Guide No. 69, and soft skills, aligned with I&E competencies.
Research design
This study adopts a cross-sectional survey design to understand the current levels of research competence among undergraduate medical students who have participated in the I&E Program. We employed a stratified random sampling method. This approach involved dividing the population into strata based on characteristics such as year of study, specialization, and prior exposure to research and entrepreneurial activities. The primary research questions include:
Does the I&E Program positively impact the I&E competencies and research competencies of medical students?
What challenges and obstacles do medical students face in the I&E Program?
Are medical students satisfied with the I&E Program, and how can it be improved?
Questionnaire design
To assess medical students’ research competency, a total of 66 closed-ended items based on the assessment form by Kansas City University [ 16 ], CRAI-12 [ 17 ], and AMEE Guide No. 69 [ 18 ] were employed to explore perceptions of their familiarity or mastery of knowledge related to the I&E Program and various their confidence in multiple competencies. These items were divided into the following sections:
General Student Information (10 items): This section covered demographics such as age, gender, and academic year.
Research Skills (3 items): This section included questions related to laboratory techniques, theoretical research skills, and knowledge of data analysis.
Research Competencies (by Different Domains):
Experimental Research Competencies (12 items)
Clinical Research Competencies (10 items)
Entrepreneurial Abilities (6 items)
Data Analysis Ability (4 items): This section assessed students' data processing and analysis proficiency.
Communication Skills (7 items): This section evaluated written and oral communication skills.
Satisfaction Levels and Advice (14 items): This section gauged students' satisfaction with the Innovation and Entrepreneurship program and collected their suggestions for improvement.
In the part of the study rooted in the “Self-Efficacy Theory [ 19 ]” and the “Social Cognitive Career Theory [ 20 ]”, we employed a Likert scale. This scale prompted students to rate their perspectives or experiences on a 5-point scale, ranging from “Strongly Disagree” to “Disagree,” “Neutral,” “Agree,” and “Strongly Agree.” An open-ended question was also included to elicit students’ feedback or opinions regarding the Innovation and Entrepreneurship program.
It is important to note that in the section concerning Research Competencies, each student was required to complete one subsection out of Clinical Research Skills, Experimental Research Skills, or Entrepreneurial Abilities based on the direction of their program participation.
These details in the questionnaire design will facilitate a comprehensive understanding of students’ research abilities and satisfaction levels across various dimensions and how their perspectives and experiences correlate with self-efficacy and social cognitive career theory.
Participants
After conducting a small-scale distribution, the questionnaire design was re-evaluated, and the time required for typical responses was estimated. Following the completion of questionnaire collection, researchers removed invalid questionnaires based on the time respondents took to answer questions and the completeness of questionnaire responses.
A total of 200 undergraduates were randomly selected. An online survey using an internet platform was conducted, and 188 valid responses were collected. These survey responses will be utilized to address research questions and conduct relevant statistical analyses. Participants were informed about data usage and confidentiality before participating in the survey, and their responses were anonymous.
The sample was sourced from the Xiangya School of Medicine (Central South University) students, encompassing individuals from various academic disciplines in medicine, year levels, and backgrounds. Participants were recruited by sharing the survey link on the university's online platforms. The recruitment process was extended over a specific duration to ensure diverse responses.
Program characteristics
Out of the 188 surveyed students, the predominant group comprises third-year clinical medicine students. They are in their third year of clinical medicine. Clinical medicine students have the highest number of students in most schools and are most interested in participating in scientific research during their third year. 61.70% of students have served as a leader at least once; among them, 67.53% have received funding at least at the provincial level (Table 1 ).
Research skills
Lab techniques.
Competence in research means possessing several skills and abilities to perform specific research. According to the survey in this study, many students became familiar with laboratory techniques. The data are shown in Fig. 1 A. Among the participants of the innovation program, the top three standard laboratory techniques are cell culture, protein separation and detection, and Nucleic acid extraction and analysis. Such results may be influenced by their versatility, applicability across different research areas, and ability to provide valuable insights into cellular and molecular processes.
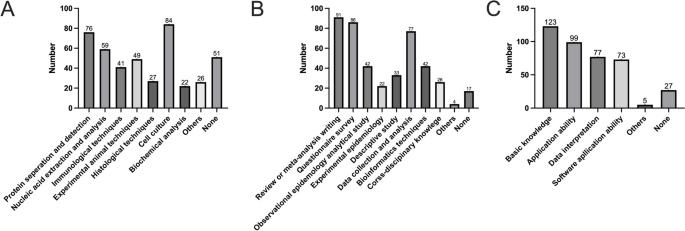
A Learning Outcomes in Lab Techniques from the I&E Program. B Learning Outcomes in Theoretic Research Skills from the I&E Program. C Learning Outcomes in Data Analysis Knowledge from the I&E Program
Although most students have mastered some laboratory techniques, there still needs to be a higher level of proficiency in other techniques and methods among students, such as immunological, experimental animal models, histological techniques, and biochemical analysis. However, 51 students (27.13%) claimed they didn’t learn any related methods.
Theoretic research skills
Theoretical research skills are vital for medical students, fostering critical thinking, research aptitude, and lifelong learning. Through the I&E Program, many students have become familiar with or mastered research methods and techniques. The data are shown in Fig. 1 B. Systematic review and meta-analysis writing (48.40%) and questionnaire surveys (45.74%) are the most common research methods. These methods play an essential role in scientific research by collecting and organizing research data and understanding people’s perspectives and opinions.
Data collection and analysis are also essential skills that many students have acquired (40.95%). Statistical methods are crucial in research, helping students collect and analyze data to draw scientific conclusions.
Various epidemiological methods are also techniques that some students have mastered. These methods are commonly used in epidemiological research to understand and analyze the spread and impact of diseases.
22.34% of these students have also acquired skills in bioinformatics. Bioinformatics is an interdisciplinary field that combines computer science and biology, and it can be used to analyze and interpret biological data. Because experimental epidemiological methods often involve research processes and are closely integrated with clinical practice, they may not be suitable for undergraduate innovation programs.
Only 26 (13.82%) students have acquired skills in other disciplines, such as medical engineering and cross-disciplinary knowledge. These skills may be related to the specific programs or research directions they are involved in. It cannot be denied that only a few students have been exposed to cross-disciplinary knowledge in medicine. This is because there is a need for more professionals in related fields, and students need a greater understanding of this field.
Data analysis knowledge
Through the innovation program, students are familiar with or have mastered data analysis in life science. 65.42% of the students are familiar with or have mastered fundamental concepts of statistics (Fig. 1 C), commonly used statistical methods, and analysis steps. This is important for their ability to analyze and interpret results effectively.
Regarding application ability, over half of the students select appropriate statistical methods and analyze data based on the situation. This indicates they have some data analysis skills and can apply statistical methods to real-world problems. In data interpretation, 40.95% of the students can explain the meaning and impact of statistical results (Fig. 1 C). This is crucial for them to draw meaningful conclusions and inferences from the data. Surprisingly, 38.83% of the students can use statistical software such as SPSS, R, Python, etc. for data analysis. These software tools are widely used in modern scientific research, and students have a relatively high level of proficiency in using them.
Research competencies
According to the research in which students participate, they can be divided into experimental, epidemiological, and entrepreneurial programs, and students’ research capabilities can be investigated accordingly. The I&E Program type distribution is shown in Fig. 2 .
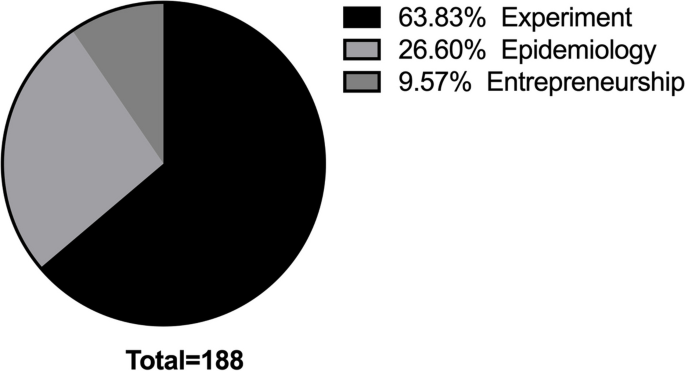
Distribution of Student Research Programs by Type
The curriculum and mentorship focus often influence the research programs medical students participate in. Considering schools and mentors prioritize experimental research, medical students are more likely to be involved in such programs. Meanwhile, Medical students’ backgrounds and interests may align more with experimental programs, making them more inclined to choose and participate in these types of research. Also, Chinese hospitals and research organizations often pay more attention to medical students’ laboratory experience and achievements when considering different candidates, leading them to focus more on research related to experimental programs to enhance related skills and competitiveness in the job market.
Epidemiological research (26.60%) and entrepreneurship programs (9.57%) may need to be adequately promoted and trained in medical education, resulting in medical students’ less knowledge and interest in these fields.
The situation may vary depending on the school, mentors, and individual student interests and backgrounds. For medical students, balancing participation in different research programs and improving epidemiological research and entrepreneurship skills can give them more opportunities and choices for comprehensive and future career development.
Clinical research competencies
For the students who took part in epidemiological research, this study adopted CRAI-12 [ 17 ], a shortened version of the Clinical Research Appraisal Inventory, to assess the confidence of students in performing clinical research based on self-efficacy theory [ 19 ] and social cognitive career theory [ 20 ]. The result is shown in Table 2 .
Given that most students are not experiment leaders or only partially involved in the core steps of the program, the Funding factor in CRAI-12 is not considered.
For most questions, students’ responses are between “Agree” and “Strong agree,” indicating that most students believe the program enhances their abilities in the relevant field.
Students often need more confidence regarding questions involving ethics and other related aspects. This may be because students, not being the leaders of the experiment, require a certain level of expertise and understanding in ethics. They must also gain experience managing human resources and working as a team.
Experimental research competencies
For the students who primarily participate in the experimental program, a questionnaire based on AMEE Guide No. 69 [ 18 ] and an assessment from Kansas City University [ 16 ] is used to assess their research competencies. The result is shown in Table 3 .
Several factors are considered in the questionnaire, including curiosity, professionalism, program understanding, experimental techniques, analytical ability, and interpersonal and communication skills.
Students’ responses are concentrated between “Agree” and “Strong agree,” indicating that most students believe the program enhances their abilities in the relevant field.
Students exhibit higher confidence in raising questions, sparking curiosity, enhancing self-learning, improving critical thinking, searching for professional literature, and using literature evidence, likely because these skills are closely aligned with their academic and career development and have been adequately taught and practiced. However, students need more confidence in understanding the importance of ethics and industry standards and assessing program strengths and limitations. This may be due to insufficient course content, less effective teaching methods, or individual disinterest. Enhancing ethics and industry standards education and providing more comprehensive program assessment training could help boost student confidence.
Entrepreneurship
Out of 188 students, only 18 students actively participated in the entrepreneurship program. Most believe participating in the Innovation and Entrepreneurship Program helps improve their entrepreneurial skills, but they need more confidence in leadership (Table 4 ).
Medicine is highly specialized, and medical students must dedicate significant time to acquiring medical knowledge and skills. This may limit their interest in entrepreneurship. They may be more inclined to focus on clinical practice and medical research, while medical schools must provide sufficient entrepreneurship education and support.
Data analysis ability
Data is crucial in most life science research, and data analysis skills are indispensable in enhancing the research abilities of medical students. Analytic skills enable medical students to design research studies that are more robust and scientifically rigorous. They can identify appropriate variables to measure, select suitable sample sizes, and determine the most influential research methods. It allows medical students to interpret and analyze research findings effectively, identify potential errors or biases in their research, and proceed with their study under the guidance of data analysis. Furthermore, a deep understanding of data means students can critically evaluate existing research, which helps them to understand the importance of evidence. These abilities are of great significance to students’ academic and career development. The assessment result is shown in Table 5 .
The innovation and entrepreneurship program positively impacts students’ data analysis and problem-solving abilities. Through the program, students can recognize the importance of data, enhance their ability to select appropriate statistical measures, accurately analyze and interpret program results, and proceed with subsequent processes under the guidance of preliminary results.
Teamwork and communication
Teamwork and communication skills are crucial for developing research abilities in medical students today [ 14 ]. The assessment result is shown in Table 6 .
Collaborating with professionals from different disciplines, such as doctors, researchers, and laboratory technicians, allows for sharing knowledge and experiences, complementing each other’s expertise, and ultimately improving the quality and efficiency of research programs. Effective communication is essential throughout the research process. Medical students must communicate effectively with team members, clarify research objectives, delegate tasks, and share progress and results, among other things. Strong communication skills facilitate collaboration, enhance team cohesion, and improve efficiency. Whether in future medical practice or medical research, medical students require effective teamwork and communication skills to learn and work efficiently. The Innovation and Entrepreneurship Training Program typically involves 3–5 students working under the guidance of 1–2 mentors, providing them with a small-scale team collaboration opportunity. These programs aim to create a supportive and inclusive environment where students can learn from each other, exchange ideas, and enhance their problem-solving abilities through practical experience.
Most students agree that participation in innovation programs develops teamwork and communication skills. They become better listeners, contributors, and leaders in team collaborations, and their communication skills with other team members and academic professionals are enhanced. However, it is worth noting that there are still disciplinary limitations in innovation programs, and there should be an emphasis on increasing the diversity of disciplines in future programs.
To investigate potential issues related to teamwork in the I&E Program, one item in the survey was designed to inquire whether the program leader took on the majority of tasks, thereby limiting the active participation of other team members. 62.5% agreed or strongly agreed that they have taken on most of the program work without assigning tasks for other members to be deeply involved in the program (Fig. 3 ). This suggests that the operation of most innovation programs may be satisfactory with teamwork.
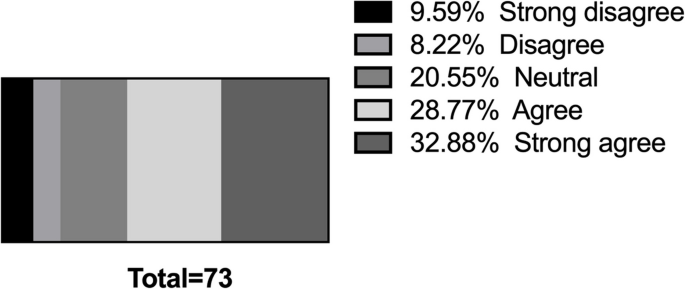
Agreement Levels among Program Leaders Regarding Task Distribution in the I&E Program
Satisfaction and improvement
On the positive side, 73.93% of students are satisfied with the innovation and entrepreneurship programs. 77.12% of the students agreed that the I&E Program positively impacts their research abilities (Table 7 ).
Although students have different opinions on whether the I&E Program affected their curriculum learning, 61.17% of students believe participating in the program helps their learning. Students with good time management and self-discipline can often effectively balance their research and course learning. Therefore, they tend to believe that the program did not affect their compulsory course learning and didn’t experience negative emotions related to the programs.
On the other hand, students who lack these skills may need more energy from the program, resulting in insufficient focus on their compulsory courses and thoughts of giving up. Other factors also influence this viewpoint. Some curriculums aim to develop students’ research abilities and even require them to participate in innovation and entrepreneurship programs. Students may invest unnecessary energy if the program is too complex, needs more smooth teamwork, or needs more guidance.
On the negative side, 53.19% of students believe their programs are vital in theory but weak in practical implementation. This is due to various reasons, such as insufficient funds, limited resources, lack of guidance, and insufficient long-term follow-up. Similarly, 54.25% of students agreed that their programs had yet to achieve the expected results due to a lack of support (Table 7 ).
To understand the purpose of medical students participating in innovation and entrepreneurship programs, a multiple-choice question was designed to inquire about the objectives of their involvement.
Most students participate in the program to enhance their abilities and improve personal competitiveness, as they recognize the importance of research skills in their medical careers during their undergraduate studies. Only 54.78% of students are driven by interest as they engage in the innovation and entrepreneurship program due to their curiosity about scientific questions. 35.64% of students passively participate in the program, either following the crowd or under the request of their teachers. 26.60% of students agree that they aim to engage in early career planning. The innovation and entrepreneurship program is crucial for the career planning of medical undergraduates, as most of them will pursue a master’s degree after graduation. This program helps them gain early exposure to research and determine whether they aspire to become scientists in the future (Fig. 4 ).
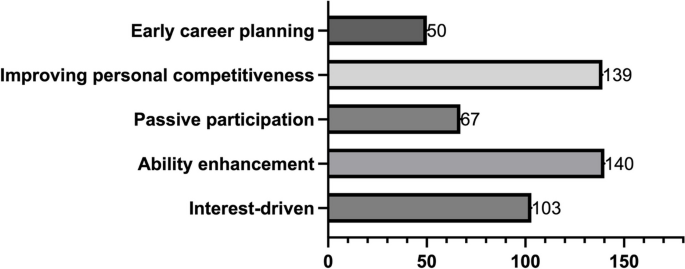
Student Objectives for Participating in the I&E Program
Since approximately half of the students believed that their program did not achieve the expected results, a multiple-choice question was designed to investigate the difficulties encountered by the students. The result is shown in Fig. 5 . Among them, 78.19% of students faced problems due to a lack of technical skills and experience, which is reasonable because most students were initially exposed to scientific research through innovation and entrepreneurship programs. 52.12%, 49.46%, and 44.69% of students, respectively, stated that they encountered difficulties due to resource limitations, tedious processes, and insufficient funding. Suggested improvement methods include providing more support regarding resources or funding and improving the standard operating procedure, such as enhancing the application procedure and fund reimbursement.
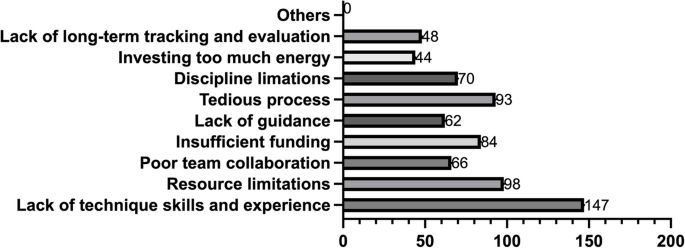
Challenges Faced by Students in the I&E Program
37.23% of students experienced difficulties due to disciplinary restrictions, while 32.97% and 35.10% encountered problems with their mentors and team members. Measures such as promoting the mentor’s attention to the program and improving the collaborative mode of innovation and entrepreneurship programs may be necessary. Some students (23.40%) felt overwhelmed due to excessive involvement. 25.53% of students needed long-term tracking and evaluation of their programs, which is crucial as it ensures the proper utilization of resources and funding and encourages students to execute their programs consistently.
Two multiple-choice questions are asked from the perspectives of subjective ability enhancement (Fig. 6 ) and objective awards and honors (Fig. 7 ) to inquire about the gains that students have obtained from the innovation and entrepreneurship program.
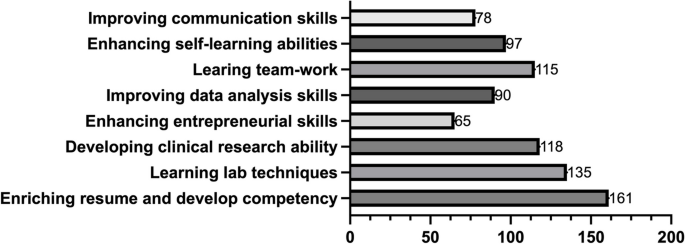
Skills Enhancement Perceived by Students in the I&E Program
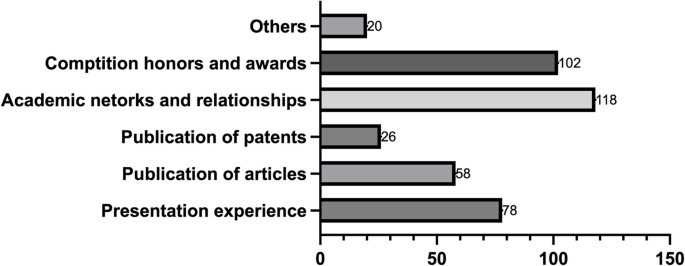
Achievements and Honors Attained by Students in the I&E Program
Most students agree that participating in innovation and entrepreneurship programs can enrich their resume and develop their career competency. This practical perspective is widely accepted among students. More than half of the students agree that medical students participate in innovation and entrepreneurship programs to develop laboratory techniques and clinical research abilities and learn teamwork and self-learning skills. Less than half of the students intend to improve their communication and data analysis abilities, with only 34.57% expressing a desire to enhance their entrepreneurial skills (Fig. 6 ).
The I&E Program has been highly beneficial for medical students. 54.26% of students gained competition awards and honors through their experience in the program. 62.77% of students acknowledged that their network was improved, which would benefit their future careers. 30.85% and 13.83% of students directly or indirectly obtained articles or patent publications due to the program. 41.49% of students have gained public speaking experience, which could be their first academic presentation, contributing to their development as capable and confident researchers (Fig. 7 ).
Historically, I&E education has focused on business [ 21 ], but it’s now expanding into medical education due to evolving healthcare demands [ 22 ]. We indicate the necessity of emphasizing the importance of the I&E program in China. Our survey targets students, but the primary obstacle to promoting this medicine program is the need for teachers to become familiar with I & E concepts, resulting in a shortage of new teaching methods [ 9 ]. Solutions to this issue include enhancing interdisciplinary collaboration between schools such as design, engineering, and architecture faculties and continuous faculty training to foster understanding of entrepreneurial/innovative concepts.
I&E education is key to boosting the employability of medical students [ 4 ]. Our study shows that students enhance teamwork, communication, and clinical research skills, aiming to increase competitiveness through these programs. They achieve awards, expand academic networks, and publish articles or patents. What’s more, the program helps educators link interests to future careers, enhancing students’ satisfaction with career planning.
According to surveys conducted in many countries, incorporating research as a part of undergraduate medical education is considered a potential approach to lay the foundation for future physician-scientists [ 23 , 24 ]. This may be the direction for developing the I&E Program in China. Students typically view their experiences in medical school research as favorable, often finding them stimulating in generating interest in research and cultivating scholarly research skills [ 25 ]. In our study, medical students who have participated in the I&E Program not only objectively acquire a variety of laboratory or theoretical research techniques and methods but also subjectively gain confidence in their potential to become future scientists, entrepreneurs, and collaborative team members based on the Self-Efficacy Theory [ 19 ] and Social Cognitive Career Theory [ 20 ]. The theory of self-efficacy states that believing in your ability to do something makes you more likely to succeed at it. Social cognitive career theory connects the anticipation of favorable results and achievements in specific behaviors to the inclination to pursue a career that demands these behaviors. Confidence doesn’t always mean ability, but being more confident and interested in clinical research shows that research training is helpful [ 26 ].
Undergraduate research is essential for progress in clinical medicine. Early research training linked to health practices helps tackle global health challenges effectively [ 27 ]. Surveys and our research indicate that undergraduate medical students generally hold a favorable view of scientific research [ 28 , 29 ]. However, the number of research students still needs to be higher [ 30 ]; they encounter several obstacles that hinder their engagement and contribution to the initiation and advancement of scientific projects. They need to balance their projects with their studies while investing more time and interacting with their tutors. It is worth noting that instruction from teachers is important in accomplishing the project [ 31 ]. On the other hand, students complain about inadequate funding. Approximately half of the students feel their programs need more support during implementation and fail to achieve the expected outcomes.
Contrary to what one might expect, most students do not believe the I&E Program will impact their mandatory coursework. More than half of the students believe that participating in the I&E Program contributes to their learning in required courses. This could be attributed to the fact that I&E education is woven into numerous undergraduate mandatory compulsory courses, and students recognize the significance of their current career planning. According to a previous study, there is a substantial overlap in crucial attributes from both professional and research standpoints [ 5 ], indicating a close integration between that research and the curriculum.
Most students expressed satisfaction with the programs. Students’ enjoyment contributes to enhancing their learning outcomes [ 32 ]. However, it’s noteworthy that many students have experienced negative emotions during the I&E Program and have even contemplated giving up. Further investigation is needed to uncover the specific reasons behind these experiences. What can be confirmed is that some students noted a disconnect between theoretical knowledge and practical implementation. Challenges included technical skill gaps, resource limitations, mentor issues, and team dynamics. Students highlighted the need for improved support, resources, and streamlined procedures.
According to the General Medical Council’s Tomorrow Doctor, teamwork is an important learning goal in medical curricula. Efficient health systems feature continuity, physician-patient partnerships, teamwork among healthcare providers, and inter-facility communication [ 33 ]. However, 35.10% of students reported teamwork difficulties, and 62.5% of program leaders acknowledge that they have taken on most of the work in the program, implying that other team members may have made minimal contributions and are likely to have yet to gain any skills. In such a situation, the data they provide may deviate from the actual impact of the I&E Program. To address these challenges, the I&E Program may benefit from implementing structured team-building exercises and fostering a culture of open communication and mutual respect among participants.
Based on teamwork, interdisciplinary collaboration is also essential for medical students as it improves their clinical skills and equips them with the ability to navigate the complexities of modern healthcare, foster innovation, and provide high-quality patient-centered care [ 34 ]. Considering students’ epistemic backgrounds, individual learning preferences, and the potential for synergy is crucial when designing interdisciplinary educational courses for a meaningful and effective learning experience [ 35 ]. As previously mentioned, multidisciplinary collaboration between medical schools and other schools also helps to improve I&E pedagogy.
When asking students for suggestions for improvement regarding the I&E Program, the most common complaints revolved around insufficient funding, often requiring them to self-finance or seek instructor assistance. In 2023, Central South University reduced its support for medical innovation projects. This reduction is evident as the number of projects receiving provincial or national-level support dropped to 173, a significant decline compared to 353 in 2022 and 291 in 2021. Additionally, there has been a notable increase in projects receiving less financial support, with most projects (56%) not receiving any financial backing [ 3 ]. Even the highest level of funding support is considered insufficient (about 10,000 ¥).
There needs to be more interest in research careers among medical students upon graduation in the US [ 36 ]. This is accompanied by a declining number of MDs seeking support from the NIH loan repayment program, a growing age at which physicians achieve their first R01 grant success, and fewer physicians considering research as their primary work activity [ 37 ]. In China, a similar situation of decreased or stagnant research funding is also occurring. The number of programs funded by the National Natural Science Foundation Of China (NSFC) in 2023 has reached 48,785, an increase of 468 compared to 2022. However, amidst a significant rise in the total number of program applications, the funding ratio continues to show a declining trend [ 38 ]. These trends raise concerns about the stability of the research career path. Furthermore, 2023 also witnessed the NSFC’s initiative to start providing research funding to undergraduate students. Considering these challenges, one recommendation for improvement is to enhance support for medical innovation programs, especially for undergraduate students, to encourage more aspiring physician-scientists.
The I&E Program could benefit from more innovation, as medical students often struggle to select research topics on their own. Improving the program may involve a budget increase and adopting active, interdisciplinary teaching methods to better engage students in research and provide more support. In conclusion, overcoming challenges with more support and mentorship prepares students for future careers through research and interdisciplinary education.
Our questionnaire survey aimed to understand the basic situation of innovative education in medical schools in our country. The cross-sectional design and lack of a control group are limitations of this study. The online questionnaire distribution may lead to subjective and recall biases. Due to inconsistencies in concepts, as well as limitations in the development of the questionnaire, we identified specific competencies to assess students’ acquisition of I&E competencies from the I&E Program. However, this may be partial and could be improved upon.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ministry of Education. Proposals on promoting education for innovation and entrepreneurship in higher education and on undergraduate independent entrepreneurship. http://www.moe.gov.cn/srcsite/A08/s5672/201005/t20100513_120174.html . Assessed 15 Mar 2024.
National undergraduate innovation and entrepreneurship. http://gjcxcy.bjtu.edu.cn/Index.aspx . Assessed 20 Oct 2023.
Central South University. https://www.csu.edu.cn/ . Assessed 20 Oct 2023.
Niccum BA, Sarker A, Wolf SJ, Trowbridge MJ. Innovation and entrepreneurship programs in US medical education: a landscape review and thematic analysis. Med Educ Online. 2017;22(1):1360722.
Article Google Scholar
Yu M, Liu W. The importance of College students’ innovative entrepreneurial training plan program in cultivation of medical undergraduates’ scientific research literacy. Open J Soc Sci. 2022;10(12):438–46.
Google Scholar
Grailer JG 3, Alhallak K, Antes AL, Kinch MS, Woods L, Toker E, Garbutt JM. A novel innovation and entrepreneurship (I&E) training program for biomedical research trainees. Acad Med. 2022;97(9):1335–40.
Brinton TJ, Kurihara CQ, Camarillo DB, Pietzsch JB, Gorodsky J, Zenios SA, et al. Outcomes from a postgraduate biomedical technology innovation training program: the first 12 years of Stanford biodesign. Ann Biomed Eng. 2013;41(9):1803–10.
Bacigalupo MKP, Punie Y, Van den Brande G. EntreComp: the entrepreneurship competence framework. Luxembourg: Publication Office of the European Union; 2016. Report No.: EUR 27939 EN.
Suryavanshi T, Lambert S, Lal S, Chin A, Chan TM. Entrepreneurship and innovation in health sciences education: a scoping review. Med Sci Educ. 2020;30(4):1797–809.
Audretsch DB, Falck O, Heblich S. Handbook of research on innovation and entrepreneurship. Edward Elgar Publishing; 2011.
Book Google Scholar
Garbutt J, Antes A, Mozersky J, Pearson J, Grailer J, Toker E, DuBois J. Validating curricular competencies in innovation and entrepreneurship for biomedical research trainees: a modified Delphi approach. J Clin Transl Sci. 2019;3(4):165–83.
Oecd. Skills for Innovation and research. Paris: OECD; 2011.
Valenta AL, Meagher EA, Tachinardi U, Starren J. Core informatics competencies for clinical and translational scientists: what do our customers and collaborators need to know? J Am Med Inform Assoc. 2016;23(4):835–9.
Chandrashekar A, Mohan J. Preparing for the National Health Service: the importance of teamwork training in the United Kingdom medical school curriculum. Adv Med Educ Pract. 2019;10:679–88.
Parry SB. Just what is a competency?(And why should you care?). Training. 1996;35(6):58.
Adkison LR, Glaros AG. Assessing research competency in a medical school environment. Med Sci Educ. 2012;22(S3):139–42.
Robinson GFWB, Switzer GE, Cohen ED, Primack BA, Kapoor WN, Seltzer DL, et al. A shortened version of the clinical research appraisal inventory. Acad Med. 2013;88(9):1340–5.
Laidlaw A, Aiton J, Struthers J, Guild S. Developing research skills in medical students: AMEE Guide No. 69. Med Teach. 2012;34(9):754–71.
Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215.
Lent RW, Brown SD, Hackett G. Toward a unifying social cognitive theory of career and academic interest, choice, and performance. J Vocat Behav. 1994;45(1):79–122.
Kuratko DF. The emergence of entrepreneurship education: development, trends, and challenges. Entrep Theory Pract. 2005;29(5):577–97.
Arshad S, Huda NU, Nadeem N, Ali S, Ahmad N, Anwar S, et al. Perceptions of medical students about research at undergraduate level. J Ayub Med Coll Abbottabad. 2021;33(1):129–33.
Laskowitz DT, Drucker RP, Parsonnet J, Cross PC, Gesundheit N. Engaging students in dedicated research and scholarship during medical school: the long-term experiences at Duke and Stanford. Acad Med. 2010;85(3):419–28.
Mahomed S, Ross A, Wyk J. Training and assessing undergraduate medical students’ research: learning, engagement and experiences of students and staff. Afr J Prim Health Care Fam Med. 2021;13(1):e1.
Chang Y, Ramnanan CJ. A review of literature on medical students and Scholarly Research. Acad Med. 2015;90(8):1162–73.
Mullikin EA, Bakken LL, Betz NE. Assessing research self-efficacy in physician-scientists: the clinical research APPraisal inventory. J Career Assess. 2007;15(3):367–87.
Schexnayder S, Starring H, Fury M, Mora A, Leonardi C, Dasa V. The formation of a medical student research committee and its impact on involvement in departmental research. Med Educ Online. 2018;23(1):1424449.
Hren D, Lukić IK, Marusić A, Vodopivec I, Vujaklija A, Hrabak M, Marusić M. Teaching research methodology in medical schools: students’ attitudes towards and knowledge about science. Med Educ. 2004;38(1):81–6.
Vujaklija A, Hren D, Sambunjak D, Vodopivec I, Ivaniš A, Marušić A, Marušić M. Can teaching research methodology influence students’ attitude toward science? Cohort study and nonrandomized trial in a single medical school. J Investig Med. 2010;58(2):282–6.
Muhandiramge J, Vu T, Wallace MJ, Segelov E. The experiences, attitudes and understanding of research amongst medical students at an Australian medical school. BMC Med Educ. 2021;21(1):267.
Murdoch-Eaton D, Drewery S, Elton S, Emmerson C, Marshall M, Smith JA, et al. What do medical students understand by research and research skills? Identifying research opportunities within undergraduate projects. Med Teach. 2010;32(3):e152-160.
Blunsdon B, Reed K, McNeil N, McEachern S. Experiential learning in social science theory: an investigation of the relationship between student enjoyment and learning. High Educ Res Dev. 2003;22(1):43–56.
Epstein RM. Defining and assessing professional competence. JAMA. 2002;287(2): 226.
Spelt EJH, Biemans HJA, Tobi H, Luning PA, Mulder M. Teaching and learning in interdisciplinary higher education: a systematic review. Educ Psychol Rev. 2009;21(4):365–78.
Oudenampsen J, van de Pol M, Blijlevens N, Das E. Interdisciplinary education affects student learning: a focus group study. BMC Med Educ. 2023;23(1):169.
Garrison HH, Ley TJ. Physician-scientists in the United States at 2020: trends and concerns. FASEB J. 2022;36(5):e22253.
Jain MK, Cheung VG, Utz PJ, Kobilka BK, Yamada T, Lefkowitz R. Saving the endangered physician-scientist — a plan for accelerating medical breakthroughs. N Engl J Med. 2019;381(5):399–402.
National Natural Science Foundation of China. https://www.nsfc.gov.cn/english/site_1/index.html . Assessed 20 Oct 2023.
Download references
Acknowledgements
The authors thank the students of the Central South University for their willingness.
This work was supported by a grant to W.W. from the National Natural Science Foundation of China (82271109), and Research on education and teaching reform in Central South University (2024jy168).
Author information
Authors and affiliations.
Xiangya Hospital, Xiangya School of Medicine, Central South University, Changsha, Hunan, China
Department of Ophthalmology, Xiangya Hospital, Central South University, Changsha, China
Hunan Key Laboratory of Ophthalmology, Changsha, China
National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
You can also search for this author in PubMed Google Scholar
Contributions
XY designed and distributed the questionnaire and wrote this manuscript. WW revised this manuscript.
Corresponding author
Correspondence to Wenyi Wu .
Ethics declarations
Ethics approval and consent to participate.
The study involving participants in the I&E Program has been submitted to the Medical Ethics Committee of Xiangya Hospital, Central South University. The questionnaire’s introduction outlines the research’s objectives, societal value, scope of information collection, potential privacy risks, and corresponding mitigation measures. Participants are required to read the questionnaire introduction and be informed that “submitting answers” is considered informed consent. Participants can exit at any time during the process, and informed consent was obtained from all subjects. Based on this process, the ethical risks of this study are extremely low, and it has received approval from the Medical Ethics Committee of Xiangya Hospital, Central South University, eliminating the need for further ethical review.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
You, X., Wu, W. Assessing the impact of Medical Education's Innovation & Entrepreneurship Program in China. BMC Med Educ 24 , 519 (2024). https://doi.org/10.1186/s12909-024-05467-2
Download citation
Received : 10 December 2023
Accepted : 24 April 2024
Published : 10 May 2024
DOI : https://doi.org/10.1186/s12909-024-05467-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- I&E program
- Medical education
- Questionnaire
- Research abilities
- Entrepreneurial abilities
BMC Medical Education
ISSN: 1472-6920
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- News & Analysis on Clinical Trial Services & Contract Research And Development
Phastar - recognizing the power of data in improving staff well-being
09-May-2024 - Last updated on 09-May-2024 at 13:12 GMT
- Email to a friend

As Mental Health Awareness Week 2024 approaches, employers must recognize the power of data in improving staff wellbeing.
Peter Taylor, senior director, and global head of human resources at Phastar, sheds light on this critical issue, emphasizing the need for proactive measures to support employees' mental health.
“The costs of low mental health, both personal and economic, are well-documented, with 42% of employees globally reporting a decline in mental health since 2020,” notes Taylor.
This statistic underscores the pressing need for employers to act and implement strategies to address mental health challenges in the workplace.
Despite the prevalence of mental health issues, Taylor observes a notable gap in applying data-driven approaches internally to support staff wellbeing. “In the clinical trials industry, we've seen the transformative impact of data analytics in supporting personalized medicines.
“Yet, we often neglect to apply the same data-driven approach internally to address mental health challenges facing our staff,” he explains.
As Mental Health Awareness Week approaches, Taylor highlights the event's theme, ‘Movement: Moving for our mental health’, which underscores the role of physical activity in reducing anxiety and depression. “Movement is key to promoting mental wellbeing, and employers can play a crucial role in encouraging exercise among their employees,” he emphasizes.
Furthermore, Taylor stresses that investing in employee mental health is not just a moral imperative but also a smart business decision.
“Good mental health is not just a human right but also a sound business investment,” he explains. The economic costs of depression and anxiety on productivity are staggering, with the World Health Organization estimating losses of $1 trillion annually.
Reflecting on Phastar's efforts to support employee mental health, Taylor shares promising results from their utilization of Govox wellness surveys.
“From October 2022 to July 2023, the overall happiness score increased from 76% to 79%, exceeding the average of other organizations using the Govox platform,” he reveals. These results underscore the effectiveness of data-driven interventions in improving employee well-being.
However, beyond Phastar, the broader life sciences industry also faces similar challenges in addressing mental health. Taylor's insights highlight the need for a concerted effort across all sectors to prioritize employee wellbeing and support mental health initiatives.
In the face of unprecedented challenges such as the global pandemic, the need for robust mental health support mechanisms has become even more critical. Taylor emphasizes the importance of proactive measures, including the provision of resources, training, and support networks, informed by data-driven insights.
As Taylor concludes, organizations must remain committed to fostering a healthy and dynamic working environment that prioritizes employee wellbeing. “Creating a healthy and dynamic working environment will allow us to increase productivity and attract the very best talent,” he says. By investing in employee mental health, organizations can ensure the continued success and sustainability of their operations, benefiting both employees and the broader society.
Related news

- Saama accelerates data review processes Saama | Download Infographic
- More Data, More Insights, More Progress Saama | Download Case Study
- Ultra Low Temperature Packaging solutions Almac Group | Download Case Study
On-demand webinars
- How to reduce crosslinking and ensure fast dissolution in soft caps
- Cell and Gene Therapy Manufacturing Webinar
- Innovation in Drug Delivery Webinar

Outsourcing-Pharma
- Advertise with us
- Why Register?
- Apply to reuse our content
- Press Releases – Guidelines
- Contact the Editor
- Report a technical problem
- Whitelist our newsletters
- Editorial Calendar
- Arcellx-stock
- News for Arcellx
Buy Rating Justified for Arcellx Inc. Amidst Progress in Anito-cel’s Clinical Development and Regulatory Milestones
Emily Bodnar , an analyst from H.C. Wainwright, maintained the Buy rating on Arcellx Inc ( ACLX – Research Report ). The associated price target was lowered to $80.00.
Emily Bodnar’s rating is based on the successful completion of the technology transfer of Arcellx’s key therapy candidate, anito-cel, to Kite’s manufacturing facility, which boasts significant cell therapy production capabilities. This development paves the way for manufacturing Phase 3 materials for the iMMagine-3 study, indicating progress in the company’s pipeline. Furthermore, the FDA’s clearance of the IND application for anito-cel suggests regulatory confidence in Arcellx’s work, while the anticipated release of preliminary data from the pivotal iMMagine-1 study in the second half of 2024 underscores the potential for significant advancements in the treatment of multiple myeloma. Additionally, the initiation of the iMMagine-3 study, which will compare anito-cel to standard of care in a population with a high unmet need, highlights Arcellx’s commitment to addressing key areas in multiple myeloma treatment. The study’s design, with a robust patient enrollment plan and key endpoints like PFS, complete response rate, and MRD negativity, indicates a thorough approach to evaluating anito-cel’s efficacy. Comparing these favorable factors to historical data from related studies, Bodnar’s confidence in anito-cel’s potential for superior efficacy is reinforced, justifying the Buy rating for Arcellx Inc.
Based on the recent corporate insider activity of 29 insiders, corporate insider sentiment is negative on the stock. This means that over the past quarter there has been an increase of insiders selling their shares of ACLX in relation to earlier this year.
TipRanks tracks over 100,000 company insiders, identifying the select few who excel in timing their transactions. By upgrading to TipRanks Premium, you will gain access to this exclusive data and discover crucial insights to guide your investment decisions. Begin your TipRanks Premium journey today.
Arcellx Inc (ACLX) Company Description:
Arcellx Inc is a clinical-stage biotechnology company reimagining cell therapy through the development of innovative immunotherapies for patients with cancer and other incurable diseases.
Read More on ACLX:
- 3 Best Stocks to Buy Now, 4/11/2024, According to Top Analysts
- Fly Insider: NextNav, Akamai among week’s notable insider trades
- Arcellx price target raised to $81 from $72 at Needham
Arcellx News MORE
Related stocks.

IMAGES
VIDEO
COMMENTS
Costs of clinical trials have been rising in the United States (DeVol et al., 2011). The workshop's third session, "Aligning Cultural and Financial Incentives," explored opportunities for developing a new business model for clinical trials based on the seamless integration of technological advances and research activities to realize increased efficiencies and reduced costs.
SEPTEMBER 28, 2020. Business development is an ongoing process for each clinical research site. This video is based on a webinar that provides some strategies for improving the number of study leads that sites receive. Once a study lead is obtained, each site is responsible for pursuing each lead until the site gets activated.
Don't do clinical trial business development haphazardly. Mark Ragusa, former Director of Research at Raleigh Neurology, shares his framework for systematically targeting and nurturing your sponsor and CRO relationships to create a rich, site-appropriate study pipeline. Raleigh Neurology is one of the leading go-to sites in the space.
Business Development - Patient Recruitment for Clinical Research Trials. Envision Trials LLC. Bonita Springs, FL 34134. $50,000 - $100,000 a year. Full-time + 2. Easily apply. You will play a vital role in the recruitment and enrolment of patients for our clinical trials through strategic business development efforts. Active 4 days ago ·.
Business development team plays an essential role in all types of industries. In pharmaceutical and clinical research industries business development teams perform many important functions such as understanding financial aspects of running clinical studies, setting objectives and goals for the successful completion of the projects, identify and approach new clients for new business ...
The Medical Affairs Leadership Academy, a 'field and forum' programme that aims to heighten the business and leadership capabilities of Medical Affairs divisions in our clients. The R&D Leadership Development Forum, which helps rising executives at pharmaceutical and biotech companies network and share their perspectives.
It seems to me that clinical business development is the process of determining the clinical applicability of a product or service, how it fits within the healt ... Research projects can be used ...
Subgroup discovery for trial design. Once an asset has been matched with an indication, pharmaceutical companies pursue an all-comer clinical development and trial design strategy—that is, one that includes all patients except those deemed high-risk based on input from key opinion leaders and conventional wisdom. 5 The use of the all-comer strategy may reflect a low level of confidence in ...
Business Development in clinical research is in the process of a transition. The mounting costs of drug development and rationalizing of concerned departments is lashing many biotechnology and pharmaceutical companies to contract out clinical research programs. This is striking at the matching time as a tendency toward big pharma companies cementing chosen partnerships with a restricted number ...
Finally, here is a recent webinar I have done with one of my business partners on exactly how to go about getting more business for your clinical research organization or site. Let me know what you think, and don't forget to sign up for my email list below.
Clinical research expenditure in 2010 is an approximated $25 billion and is projected to touch $28.5 billion by 2014. The more the spending the more are the opportunities at the offering. Client-centric business strategies coupled with proper customer relationship management policies backed by skilled and well qualified professionals will help ...
Newark, NJ $30 - $31. Be an early applicant. 1 day ago. Today's top 53,000+ Clinical Trials Business Development jobs in United States. Leverage your professional network, and get hired. New ...
he Associate Director, Business Development Clinical Research US will focus on building new and maintaining productive client relationships in the clinical research industry for the SGS Clinical Research Services. They will be able to achieve individual and team revenue and sales objectives through proactive communication and sales of SGS ...
Good or bad, clinical trials have become a big, profitable business in the private equity sphere, data shows. Eleven of the 25 private equity firms identified by industry tracker PitchBook as the top investors in health care have bought stakes in clinical research companies, a KHN analysis found. Those companies have been involved in studies ...
Today's top 17,000+ Business Development Clinical Research jobs in United States. Leverage your professional network, and get hired. New Business Development Clinical Research jobs added daily.
Matheus BD Connections, LLC was founded by Chris Matheus to provide experienced business development and sales services for clinical research industry service providers. This approach allows small, new and/or niche providers the resources, experience and connections that has taken years to develop. Matheus BD has extensive experience in sales ...
A Clinical Research Business Development Career can be very stressful and demanding. Business development executives carry out negotiations for new business deals, draft agreements / contracts and bring new clinical trial related business to the company. They are also responsible for establishing initial interactions with potential clients.
Senior Director, Clinical Business Development - Clinical Trials. Precision for Medicine. Remote in Frederick, MD 21701. $149,100 - $214,700 a year. Easily apply. Possesses a keen understanding of the CRO clinical development market and the role that Precision for Medicine plays in that environment. Posted 30+ days ago ·.
Top 8 Clinical Trial Trends in 2023. 1. Artificial Intelligence. Clinical research groups and pharma companies integrate AI to automate various processes during clinical trials, reducing staff workload and errors. Machine learning (ML) and computer vision mine enormous data to screen drug candidates quickly.
Currently we are looking for a new Business Development Manager Clinical Research - preferred full-time (40h/week). Main areas of responsibility: Oversight and management of all Clinical Research Business Development activities; Offer management in close collaboration with Head of Clinical Operations and GM
Clinical insights and drug data solutions can be used to inform trials, including evaluating eligibility criteria and improving target selection. Informing drug development and launch strategy. Clinical insights should be a core component of life science market research, in turn informing research and development.
A growing number of clinical undergraduates are chosen to enter institutions for higher education biotechnology and industry workforce, though most need more laboratory experience training and business practice. Innovation and Entrepreneurship Program (I&E Program) can benefit from biological experiment and commercialization training largely absent from standard clinical medical educational ...
Scientific Area/Specialty: Small business development (SBIR/STTR) research grants Job Type: Scientific Division of Behavioral and Social Research. Clinical Trials Compliance Coordinator (Health Specialist) ... GS 11/12/13 Scientific Area/Specialty: Clinical research, clinical trials, regulatory/policy compliance, research administration Job ...
The clinical trial process involves protocol development, designing a case record/report form (CRF), and functioning of institutional review boards (IRBs). It also includes data management and the monitoring of clinical trial site activities. The CRF is the most significant document in a clinical study.
Despite the prevalence of mental health issues, Taylor observes a notable gap in applying data-driven approaches internally to support staff wellbeing. "In the clinical trials industry, we've seen the transformative impact of data analytics in supporting personalized medicines.
The decreases were primarily due to the restructuring plans executed in 2023 to prioritize and focus our resources on clinical stage programs. Research and development expenses include non-cash ...
Emily Bodnar, an analyst from H.C. Wainwright, maintained the Buy rating on Arcellx Inc (ACLX - Research Report). The associated price target was lowered to $80.00. Emily Bodnar's rating is ...
Major accountabilities: Drive the implementation of data analytics reports and dashboards for optimal data review by working with the users to establish robust user specifications and with programmers to implement the optimal output -Translate business requirements into logical models and provide direction to the development team to translate business logic.Lead authoring of the user ...