An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springer Nature - PMC COVID-19 Collection


Determinants of healthy ageing: a systematic review of contemporary literature
1 School of Medicine, Medical Sciences and Nutrition, University of Aberdeen, Aberdeen, AB25 2ZD UK
Georgios Kounidas
Kathryn r. martin.
2 Academic Primary Care, Institute of Applied Health Sciences, Aberdeen Centre for Arthritis and Musculoskeletal Health, University of Aberdeen, Aberdeen, AB25 2ZD UK
Martin Werth
3 Autumn Project Limited, London, SE27 0BY UK
4 School of Health Sciences, Robert Gordon University, Aberdeen, AB10 7QG UK
Phyo Kyaw Myint
Associated data.
All data generated or analysed during this study are included in this article and its supplementary material files. Further enquiries can be directed to the corresponding author.
Healthy ageing frameworks have been highly explored. Our objective was to assess existing frameworks for healthy ageing and to identify commonly described factors that can potentially act as determinants of healthy ageing.
We carried out a systematic review by searching five electronic databases (EMBASE, MEDLINE, Cochrane, PsychINFO, and CINAHL) from January 2010 to November 2020 to capture contemporary evidence. Eligible studies needed to report a clear framework of healthy ageing in humans, within one or more of three domains (physical, mental/cognitive, social), in English. No restriction was placed on geographical location. Retrospective studies, studies that did not report a framework of healthy ageing, and studies with a focus on diagnostic measures were excluded.
Of 3329 identified records, nine studies met our eligibility criteria and were included. Most of the studies were qualitative or cross sectional, and a majority were carried out in Asia, followed by North America, Australia, and Africa. The ten determinants identified for healthy ageing include physical activity, diet, self-awareness, outlook/attitude, life-long learning, faith, social support, financial security, community engagement, and independence.
Conclusions
We identified ten determinants of healthy ageing proposed by the contemporary evidence base. There appears to be increasing acknowledgement of the instrumental role of social and mental/cognitive well-being as determinants of healthy ageing. The extent to which each determinant contributes to healthy ageing requires further evaluation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40520-021-02049-w.
Introduction
Worldwide, the population aged over 65 is increasing at a faster pace than all other age groups [ 1 ]. As a result of this demographic shift, it is important to look at ways to improve the quality of life of older adults and support independent living. The COVID-19 pandemic has disproportionately affected people over 65 years of age, who had previously been in good health [ 2 ]. Given the global impact of COVID-19, it is more crucial than ever to identify determinants of healthy ageing that can be applicable across different communities and countries to build their path to better health.
Ageing as a concept has been vastly explored, a particularly important aspect being how to define what it means to age well. Key leaders in the field of ageing, such as Rowe and Kahn, defined successful ageing as the absence of physical impairment and chronic diseases, as well as optimal social participation and mental well-being [ 3 ]. Rowe and Kahn brought the field forward with their inclusion of mental and social well-being. The idea that to age healthily one must be free of disease or impairment is something that has been carried throughout the years, but in more contemporary times this has been disputed and modified.
Previous reviews in this field have provided valuable information on internal and external factors that promote healthy ageing in older age, as well as better engagement in healthier and active lifestyles [ 4 , 5 ]. In 2013 Lara et al. developed five fundamental domains of healthy ageing: physiological and metabolic health; physical capability; cognitive function; social well-being, and psychological well-being [ 6 ]. Comparatively in 2017 Hornby-Turner et al. categorised four domains: personal, social, economic, and environmental [ 4 ]. This shows the lack of consensus of what ageing well entails due to the variability between studies.
Lu et al., a review comparing methods used to assess healthy ageing, evaluated the common terms used in ageing studies (e.g. successful ageing, active ageing), and established that the term healthy ageing was most appropriate for their study [ 7 ]. The main reason as to why healthy was preferred was because of the World Health Organization’s (WHO) definition. The WHO defines health as “a state of complete physical, mental/cognitive, and social well-being, rather than merely the absence of disease or infirmity” [ 8 ]. The WHO established their definition of health in their constitution in 1948 and still stand by the initial definition. It highlights that being healthy is not solely determined by the absence of disease, even though may be a contributor. The WHO’s definition also highlights the three main domains of health: physical, mental, and social well-being [ 8 ]. Separating healthy ageing into these three domains can facilitate the development of a framework to assess and guide an individual towards healthy ageing.
The aim of this systematic review was to synthesise the evidence on healthy ageing frameworks by critically evaluating existing frameworks, identifying the methods used in frameworks to evaluate health ageing, and if appropriate to propose a revised, contemporary framework for healthy ageing. In doing so also to identify factors that can act as determinants of healthy ageing within the domains of physical, mental/cognitive, and social well-being in line with the WHO definition of health [ 8 ].
We carried out a systematic literature review by searching five databases [EMBASE (Ovid), MEDLINE (Ovid), Cochrane Central Register of Controlled Trials (Ovid), PsychINFO (Ovid), CINAHL (EBSCO)] in November 2020, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [ 9 ]. The PRISMA checklist was included in the supplementary material, as in Table Table1. 1 . A PRISMA protocol was not registered.
Characteristics of included studies
Search strategy
The following search terms were used in OVID (EMBASE, MEDLINE, Cochrane, PsychINFO): healthy ageing.mh. or (healthy ageing or healthy ageing).tx,tw,ab,hw,kw.) and (measurement tool or scale or instrument or questionnaire).mp. and EBSCOhost (CINAHL): MH(healthy ageing) OR TX(healthy ageing OR healthy ageing) AND (measurement tool OR scale OR instrument OR questionnaire).
Eligibility
To be eligible for this systematic review, studies were required to meet the following criteria: (1) Studies published in English, (2) Articles published between January 2010 and November 2020 (to capture contemporary evidence), and (3) Studies that were conducted in humans. There were no restrictions for inclusion based on geographical location. The following exclusion criteria were applied: (1) Retrospective studies, (2) Studies that did not report a framework of healthy ageing, and (3) Studies with a focus on clinical diagnostic measures (e.g. Magnetic Resonance Imaging (MRI)).
Study identification
All identified studies were transferred to Covidence (Melbourne, Australia) systematic review software where they were deduplicated [ 10 ]. The titles and abstracts were screened by two independent reviewers (GK, TA) with conflicts resolved by discussion or a third reviewer (PKM). Following that, full-text screening was conducted on all retrieved studies by two independent reviewers, with conflicts similarly resolved by discussion or a third reviewer (PKM). Reasons for exclusion at full-text screening stage are reported in the PRISMA flow chart (Fig. 1 ).

PRISMA 2009 Flow Diagram
Outcomes and data extraction
The main outcome was a framework for successful healthy ageing. For this systematic review, outcomes also included identification of determinants that fall within the three domains of physical, mental/cognitive, and social well-being. Data were independently extracted from included studies by two reviewers (TA, GK). Disagreement was resolved by discussion and/or by a senior author (PKM). The following data were extracted: country, study design, age, number of participants, gender, specific population studied, main framework, and healthy ageing domains.
Derived frameworks and categorisation into domains
Following full-text screening and data extraction, due to the nature of studies, meta-analysis was not feasible; therefore, we conducted a narrative synthesis. A framework for healthy ageing was identified as a primary outcome in all included studies (Supplementary material).
Quality assessment
Included studies were critically appraised independently by two researchers (TA, GK), using the Critical Appraisal Skills Programme (CASP) Checklist for qualitative studies and the Newcastle–Ottawa Quality Assessment Scale (NOS) adapted for cross-sectional studies [ 11 , 12 ].
Study selection
Of 3329 studies initially identified, after removing duplicates, 2970 studies underwent title/abstract screening during which 2818 studies were excluded for the following reasons: did not focus on healthy ageing and/or had a focus on diagnostic measures (e.g. MRI). Thus, a total of 152 studies were retrieved in full and screened against the inclusion and exclusion criteria by two reviewers independently (GK, TA) to determine their eligibility; 143 studies were excluded, as they did not report a framework for healthy ageing. Nine studies that reported frameworks of healthy ageing were included in the review (Fig. 1 ) [ 13 – 21 ].
All studies were found to be of high quality according to the CASP Checklist for qualitative studies and the NOS for cross-sectional studies (Supplementary Table 2, Supplementary Table 3). Five qualitative studies did not adequately report the relationship between the researcher and the participants [ 14 – 21 ]. Two cross-sectional studies did not report the comparability between respondents and non-respondents [ 13 , 18 ].
Study characteristics
The total number of participants in this review was of 2407, ranging from 11 to 683 participants in individual studies (Table (Table1). 1 ). Most studies had a sample size greater than 100, and were predominantly conducted in Asia [ 13 – 16 ]. Eight studies were carried out on both genders and one was solely on females. A majority of participants were above sixty years of age: study mean ages ranged from 64 to 85.2. Most of the studies were qualitative in nature and employed either semi-structured interviews or focus groups. Three studies used cross-sectional design (e.g. surveys) [ 13 , 17 , 18 ]. There were four studies that were conducted in people with specific conditions or circumstances. Two focused on Multiple Sclerosis (MS) patients [ 17 , 19 ], one on incarcerated women [ 15 ], and one on immigrants [ 20 ].
Determinants of healthy ageing
Six out of the nine studies included determinants of successful ageing within the three healthy ageing domains of physical, mental/cognitive, and social well-being (Table (Table2, 2 , Fig. 2 ) [ 14 – 17 , 20 , 21 ]. Three studies only addressed the mental/cognitive and social domains. Of the nine studies, there were five that had determinants that covered more than a single domain, meaning the determinant could not be solely classified into one domain [ 14 , 15 , 17 , 18 , 20 ]. Ten overall determinants were identified, with independence being present in all three domains. Figure 2 shows the combination of determinants found in each study by the overlapping of the shapes, each of which represents a study.

Pictorial representation of determinants of healthy ageing. 0: no shared studies, 1: one shared study, 2: two shared studies. There are ten shapes, each representing a determinant. The border of the label of each shape is colour-coded according to the domain they correspond to. The numbers within each shape overlap represents how many studies included that combination of determinants. Venn diagram created using Bioinformatics and Evolutionary Genomics ( http://bioinformatics.psb.ugent.be/cgi-bin/liste/Venn/calculate_venn.htpl )
Physical well-being
Seven studies included determinants within the physical domain [ 14 – 18 , 20 , 21 ]. These studies emphasised the need to maintain a good level of physical capability to enhance successful healthy ageing. Wallack et al. focused on MS participants; therefore, physical activity was addressed as a subtype of “lifestyle choices and habits” specifically in the body category [ 27 ]. This included exercise but also alternative therapies and medication management due to their potential effects on the body. Conversely, the other studies focused more on the aspect of exercise and keeping active as physical activity. Three studies used diet as a determinant for physical health, yet the specifics of the kind of diet or nutritional elements were not reported [ 14 , 15 , 17 ]. Lucas et al. included diet as part of the sustaining phase of healthy ageing due to its role in maintaining and supporting physical health [ 15 ].
Mental/cognitive well-being
All studies included mental/cognitive determinants of successful healthy ageing. Four main determinants emerged in relation to the mental/cognitive well-being domain, namely, self-awareness, outlook/attitude, life-long learning, and faith.
The determinant of self-awareness included self-esteem, self-achievement [ 13 ], resilience [ 19 ], body awareness, and sense of purpose [ 17 ]. Ploughman et al. defined resilience as “the participants ability to adapt to changes” specifically being conscious of the new circumstances they are presented with and choosing to modify their choices to support the new conditions [ 19 ]. This definition of resilience closely relates to Wallack et al. definition of body awareness, specifically relating to one’s lifestyle choices [ 17 ]. Additionally, body awareness differs in the Wallack et al. study due to the specific circumstance of MS being studied [ 17 ].
The determinant of outlook/attitude, found in seven studies, ties into self-awareness [ 15 – 21 ]. Amosun et al. divided their findings into two overarching themes, one focused on participants found to have future-oriented behaviour and the second for participants without a future-oriented behaviour [ 21 ]. The final themes for successful ageing were specified within those who had a future-oriented behaviour, which included the theme of preparing for the afterlife. It was noted that having a good outlook and attitude towards the future impacted ageing in a positive way, rather than “awaiting death” [ 21 ].
Life-long learning (e.g. reading, taking up a new hobby, or learning a new language), found in three studies, is intricately connected with outlook/attitude [ 14 , 18 , 20 ]. Thanakwang et al. specifies that “engaging in active learning” is very important in successful healthy ageing particularly in the field of technology [ 14 ]. Additionally, continuous learning has a good cognitive impact aiding in maintaining one’s cognitive function as they age.
Lastly, faith was found in five studies, which included the aspects of beliefs, religion, and spirituality [ 14 , 15 , 17 , 18 , 21 ]. Lucas et al. focused on incarcerated women as participants and created a framework that had the five stages of successful ageing [ 15 ]. Within the third phase (“reforming phase”) and the fifth phase (“sustaining phase”), faith was significant [ 15 ]. Being in isolation has a large impact on mental health and immersing in faith was shown to support stability as well as increase motivation. Both of which support a good outlook towards life as the participants age and began to develop illnesses. Additionally, Robleda et al. found that participants reported that as you age it becomes more difficult to look forward to the future and immersing oneself in faith gave their life a higher sense of purpose [ 18 ].
Social well-being
All studies included social determinants of successful healthy ageing [ 13 – 21 ]. Three main determinants (Social Support, Financial Security, Community Engagement) were identified for the social domain.
Social support was reported across seven out of the nine studies [ 13 – 15 , 17 – 20 ]. Social support was defined as establishing relationships and building rapport not only with family members but also with acquaintances. Additionally, Wallack et al. focused on MS patients, and brought up the factor of effective and accessible healthcare, which was classified as social support because participants’ relationships with their care providers were valued [ 17 ].
Community engagement (identified in seven studies) ranged from volunteering to religious gatherings, such as going to church, and feeling acquainted with the community [ 14 – 18 , 20 , 21 ]. According to Amosun et al. engaging in community activities gave the participants a sense of purpose [ 21 ]. This was particularly explored by Hui Chian Teh et al. who focused on Chinese immigrants living in Australia [ 20 ].
The last determinant, which was identified across seven studies, was financial security [ 14 , 16 – 21 ]. Robleda et al. defined financial security as being able to maintain a good quality of life [ 18 ], whereas Hui Chian Teh et al. focused on the aspect of not having to be a financial burden to family [ 20 ]. What both studies have in common was the emphasis on being able to maintain a good lifestyle; Hui Chian Teh et al. specified that having access and the ability to afford proper care as you age was highly important [ 20 ], which Wallack et al. agreed with for their MS participants [ 17 ]. The key aspect found across all studies that included financial security was the ability to continue to live a comfortable life and for many it included not having to rely on others.
Independence as an overlap determinant
Independence as a determinant was explored in six studies and it is present across all three domains [ 13 , 14 , 17 – 20 ]. It includes aspects, such as one’s physical or mental/cognitive ability to live without support as well as being financially independent from family or friends. It was clearly shown in different studies that how independence is perceived changes according to the individual’s circumstances. For Ploughman et al. and Wallack et al. both of whom focused on participants with MS, physical independence played a significant role in terms of how far their physical capability spanned [ 17 , 19 ]. The studies that did not research participants with MS also found independence to affect the physical domain as well as the social and mental/cognitive well-being domains. Due to the lack of a chronic disease, when independence was mentioned in these studies, it was not solely focused on the individual’s physical independence. For Thanakwang et al. being self-reliant was a very important factor in the active ageing scale used [ 14 ].
On 14th December 2020, the United Nations General Assembly declared 2021–2030 as the Decade of Healthy Ageing [ 22 ]. Healthy ageing replaced the WHO previous focus on active ageing. Although the concept of active healthy ageing has been widely researched and discussed in academic, political, and popular media arenas, systematic reviews that assess existing healthy ageing frameworks are lacking. To the best of our knowledge, this review illustrates the first attempt to systematically identify key determinants related to healthy ageing. The novelty of this research lies in the comparison of contemporary healthy ageing frameworks that have already been proposed. We identified ten determinants for healthy ageing, namely, physical activity, diet, self-awareness, outlook/attitude, life-long learning, faith, social support, financial security, community engagement, and independence.
The determinants of healthy ageing can vary depending on many factors, including culture, age, and gender. Therefore, it is important to consider that the studies were from varied geographical locations. This may have a large effect on what is considered important for achieving healthy ageing due to the difference in culture/customs [ 23 ]. Additionally, including a study with the premise of being an immigrant made it clear how integral community immersion and engagement is for an immigrant as they age, further emphasizing cultural differences. However, the geographical diversity arguably provided more depth and spread to this review, because it enabled the identification of commonalities, such as social support, independence, and financial security. This in turn will increase opportunities for local and global initiatives to optimise healthy ageing across different communities and countries.
Often, studies investigating healthy ageing focus on the biological factors (e.g. genetics and illnesses) that play a role in ageing [ 24 ]. We sought to identify modifiable factors to provide a better insight into healthy ageing. By doing this, non-biological factors, such as social, mental/cognitive, and physical well-being, were shown to play a substantial role [ 24 ]. For example, Wallack et al. who studied MS patients focused on the participants’ acceptance and awareness of their body and its capability and how that largely impacted their mental health [ 17 ].
Our results illustrated that many of the determinants of physical, mental/cognitive, and social well-being are interrelated. For example, in the physical domain both determinants, physical activity and diet can affect the mental/cognitive determinant of attitude/outlook. Increasing physical activity and eating a balanced diet has been shown to boost the mood and energy levels of individuals which consequently improves their attitude/outlook towards life [ 25 , 26 ]. There was a contrast in terms of physical activity depending on the targeted group of participants, e.g. those with MS differed from those without. The inter-relation of determinants establishes the idea that healthy ageing cannot be segmented into isolated factors but is an inter-dependent measure. An example is how faith is linked to outlook/attitude, as it can be part of goal setting and gives individuals something to work on and improve as they age. Additionally, often, having a strong sense of faith aids an individual to find a greater sense of purpose. These inter-relations could be because different people place a higher value on different determinants, depending on their subjective views or life experiences [ 27 ]. Additionally, the inter-dependence between determinants supports the idea that healthy ageing is not a single stable measure, but that it is a balance that is constantly adjusted between all the determinants [ 28 , 29 ]. Therefore, to successfully evaluate healthy ageing there is a need to assess all the identified determinants and understand the value and hierarchy the individual ascribes to each determinant at the individual level. Independence could not be classified in only one domain since it has been found to be “highly significant for life satisfaction” and its loss to be a highly feared occurrence in ageing [ 30 ]. Thus, it was more appropriate to categorise it into an overlapping determinant included across all three domains.
This review gains its strengths from the combination of rigorous search and extraction methods and the underlying theoretical framework which guided the synthesis. Another strength of our work is that one of the exclusion criteria was studies that used clinical measurements for their results. This makes our proposed determinants more widely applicable to groups that do not have access to clinical diagnostic measures (e.g. blood tests, MRI). Additionally, by limiting the years of inclusion from 2010 to 2020, it was possible to focus on the most contemporary research available which builds on early established research in healthy ageing [ 28 ].
One of the limitations stems from the point of the original studies’ definitions and categorisation. Most studies included in this review defined determinants differently, which made direct cross-cultural comparisons challenging. Only studies written in the English language were included, which might affect the ability to generalise results to non-English-speaking countries and may have resulted in us excluding relevant studies. Moreover, the studies included were cross sectional in nature, and therefore did not allow for investigation of causality between determinants and reports of healthy ageing. There was a larger proportion of female participants in the included studies, which might under-represent what males consider to be healthy ageing. The concept of healthy ageing is likely to be a dynamic process meaning important determinants may even vary within an individual depending on their age, further evaluation of relative contribution these determinants is warranted, albeit this is beyond the scope of the current study.
The application of the results from this review to pre-existing longitudinal cohort data could provide direct comparison of these determinants in their contribution to healthy ageing at population level. Through our review we have created a more specialised understanding of healthy ageing by finding commonalities and differences among the nine identified frameworks. Future research would be to conduct a sense-checking exercise via focus group work with older adults to propose the new framework and whether this framework fits with their concept of healthy ageing. This is particularly important to evaluate whether all determinants have the same weighting towards defining healthy ageing and how it may vary with age, gender, race/ethnicity, and socioeconomic factors. Another alternative would be to cross reference this framework with large self-reported health studies to see how reliable and applicable these data are. Moreover, future studies should have an agreed terminology on how to better define determinants, which will be crucial for cross-cultural comparisons. Our results support the use of the term healthy ageing rather than successful or active ageing, in accordance with Lu et al. as it more holistically encompasses the domains of health as defined by the WHO [ 7 , 8 ]. Additionally, going forward we suggest using the terms determinants rather than factors as it encompasses the direct effect that the determinants have on healthy ageing.
In summary, we have systematically reviewed the contemporary literature on frameworks of healthy ageing and identified ten determinants of successful healthy ageing. These are as follows: physical activity, diet, self-awareness, outlook/attitude, life-long learning, faith, social support, financial security, community engagement, and independence. Healthy ageing appears to be the result of all these determinants being optimised. By creating a clear framework of the factors that influence healthy ageing at an individual level, public service providers and policy makers can be guided to identify and give incentives to work towards improvement in health focusing on specific determinants that are relevant to an individual’s circumstances.
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Dr Stavroula Lila Kastora for constructive criticism of the manuscript.
Author contributions
TA and GK are joint first authors. KRM, KC, MW, and PKM contributed to conceptualisation, funding acquisition, and supervision. TA and GK were involved in data curation, formal analysis, investigation, methodology, visualisation, and writing––original draft. All the authors contributed to writing––review and editing.
TA and GK are recipients of The Autumn Project Research Scholarships (Ageing Clinical & Experimental Research) funded by the Development Trust, University of Aberdeen. The authors acknowledge the donor of these scholarships, the Autumn Project Limited.
Data availability
Declarations.
The authors declare no competing interests to declare.
An ethics statement was not required for this study type, no human or animal subjects or materials were used.
Not applicable.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Thais Abud and Georgios Kounidas are joint first authors.
New methodologies in ageing research
Affiliations.
- 1 Center for Healthy Aging, Department of Cellular and Molecular Medicine, University of Copenhagen, Copenhagen, Denmark.
- 2 Center for Healthy Aging, Department of Cellular and Molecular Medicine, University of Copenhagen, Copenhagen, Denmark. Electronic address: [email protected].
- PMID: 32512174
- DOI: 10.1016/j.arr.2020.101094
Ageing is arguably the most complex phenotype that occurs in humans. To understand and treat ageing as well as associated diseases, highly specialised technologies are emerging that reveal critical insight into the underlying mechanisms and provide new hope for previously untreated diseases. Herein, we describe the latest developments in cutting edge technologies applied across the field of ageing research. We cover emerging model organisms, high-throughput methodologies and machine-driven approaches. In all, this review will give you a glimpse of what will be pushing the field onwards and upwards.
Keywords: Ageing; High-throughput methods; Machine learning; Model organisms.
Copyright © 2020 The Author(s). Published by Elsevier B.V. All rights reserved.
Publication types
- Research Support, Non-U.S. Gov't
Ageing Research Reviews

Subject Area and Category
- Biochemistry
- Biotechnology
- Molecular Biology
Elsevier Ireland Ltd
Publication type
15681637, 18729649
Information
How to publish in this journal
The set of journals have been ranked according to their SJR and divided into four equal groups, four quartiles. Q1 (green) comprises the quarter of the journals with the highest values, Q2 (yellow) the second highest values, Q3 (orange) the third highest values and Q4 (red) the lowest values.
The SJR is a size-independent prestige indicator that ranks journals by their 'average prestige per article'. It is based on the idea that 'all citations are not created equal'. SJR is a measure of scientific influence of journals that accounts for both the number of citations received by a journal and the importance or prestige of the journals where such citations come from It measures the scientific influence of the average article in a journal, it expresses how central to the global scientific discussion an average article of the journal is.
Evolution of the number of published documents. All types of documents are considered, including citable and non citable documents.
This indicator counts the number of citations received by documents from a journal and divides them by the total number of documents published in that journal. The chart shows the evolution of the average number of times documents published in a journal in the past two, three and four years have been cited in the current year. The two years line is equivalent to journal impact factor ™ (Thomson Reuters) metric.
Evolution of the total number of citations and journal's self-citations received by a journal's published documents during the three previous years. Journal Self-citation is defined as the number of citation from a journal citing article to articles published by the same journal.
Evolution of the number of total citation per document and external citation per document (i.e. journal self-citations removed) received by a journal's published documents during the three previous years. External citations are calculated by subtracting the number of self-citations from the total number of citations received by the journal’s documents.
International Collaboration accounts for the articles that have been produced by researchers from several countries. The chart shows the ratio of a journal's documents signed by researchers from more than one country; that is including more than one country address.
Not every article in a journal is considered primary research and therefore "citable", this chart shows the ratio of a journal's articles including substantial research (research articles, conference papers and reviews) in three year windows vs. those documents other than research articles, reviews and conference papers.
Ratio of a journal's items, grouped in three years windows, that have been cited at least once vs. those not cited during the following year.
Evolution of the percentage of female authors.
Evolution of the number of documents cited by public policy documents according to Overton database.
Evoution of the number of documents related to Sustainable Development Goals defined by United Nations. Available from 2018 onwards.
Leave a comment
Name * Required
Email (will not be published) * Required
* Required Cancel
The users of Scimago Journal & Country Rank have the possibility to dialogue through comments linked to a specific journal. The purpose is to have a forum in which general doubts about the processes of publication in the journal, experiences and other issues derived from the publication of papers are resolved. For topics on particular articles, maintain the dialogue through the usual channels with your editor.

Follow us on @ScimagoJR Scimago Lab , Copyright 2007-2024. Data Source: Scopus®

Cookie settings
Cookie Policy
Legal Notice
Privacy Policy
Harvard Science Review
The undergraduate science publications of Harvard University
The New Age of Aging Research
By: Eric Sun
Aging. To some, this word symbolizes equality, wisdom, and progress; to others, this word represents weakness, disease, and death. To me, aging has taken on a mixed meaning. When I was a small child, I remember lying awake in bed and counting my heartbeats as if the thumping in my chest was also the ticking of my biological clock. I imagined that each person was given a certain number of heartbeats in a lifetime. Aging, to my young mind, was simply the slow and eventual countdown of these limited heartbeats. Although not many people will admit it, the fear of aging and death is extremely common (1). Throughout history, our fascination with mortality has contributed to the rise and spread of religions and legends. Since then, scientific research has begun to shed light on one of life’s greatest mysteries.
Aging Research in History
During the late Middle Ages, alchemy was a booming practice. The holy grail of alchemy was procurement of the fabled philosopher’s stone—a stone that had the ability to extend the life of its wielder indefinitely (1). Despite numerous efforts, there are no records of any successful attempts at synthesizing the object. The philosopher’s stone was not the only fabled anti-aging object. After the discovery of the Americas, there was growing interest in the possibility of uncovering the fountain of youth in this uncharted territory. In 1513, the conquistador Juan Ponce de León set out on a quest to find the fabled fountain, which ultimately ended in failure (1).
In the following decades, the interest in anti-aging ‘research’ faded along with its associated myths. In fact, aging research was under the public radar from the Renaissance until the 1940s when James Birren produced a theory involving what he called the “tertiary, secondary, and primary processes of aging” (1). Birren developed the field of gerontology, which is the study of aging, and expanded it firstly socially and secondly scientifically. At this time, aging research was widely considered a pseudoscience—a label that was not helped by the unscientific blood and serum transfusions championed by charlatans as anti-aging treatments. Ironically, a recent study reported that old mice that received plasma transfusions from younger mice were physiologically healthier, although this has yet to be validated in humans and although it was quite clear that these results were unknown in the early 1900s (2). Under Birren’s lead, the scientific stigma surrounding aging began to dissipate as more scientists were attracted to this young and growing field of research.
Notable Advances
In the following years, aging research underwent a series of profound, exciting breakthroughs. Perhaps the most famous discovery was that of the telomere. Telomeres are the repeating DNA sequences at the end of each of the chromosomes. Through the DNA replication mechanism, the telomeres deteriorate after each cycle of replication and the chromosomes become shorter. Although telomeres themselves do not appear to have any significant function outside of protecting other DNA sequences from degradation, when a cell exhausts its telomeres, each successive division results in deterioration of essential genes and deleterious effects that often result in cellular death (3).
The first indication of telomeres came in 1962 through Leonard Hayflick’s discovery of the limit on somatic cell replication. Hayflick, considered by many to be the father of modern aging research, carried out a groundbreaking experiment that indicated that somatic cells could only divide a finite number of times (1). At the time, it was widely accepted that cell lineages were immortal and that each body cell was capable of an indefinite number of divisions. It was only until several other scientists replicated Hayflick’s result that the socalled Hayflick limit became largely accepted by the scientific community. This limit to cellular division was typically 50-54 divisions for human somatic cells (1). In the 1970s, Jack Szostak discovered the existence of telomeres at the end of chromosomes, which explained the Hayflick limit phenomenon (3). If cell replication was restricted by the length of the telomere, and the telomere was of a finite length, then surely cell lineages are finite. Szostak garnered a Nobel Prize for his work. Soon after the discovery of telomeres, the enzyme that extends telomeres on chromosomes, telomerase, was discovered. In recent years, overexpression of telomerase has been linked to the vicious proliferation and immortality of cancer cells (3). Telomeres serve as the switch for immortality—at least on a cellular level.
An often overlooked, but perhaps even greater breakthrough was the development of several notable theories of aging. Imagine an organism as a car. Cars, no matter how well kept or maintained, begin to lose function with time. At first, there may be a few scratches to the windshield, buildup in the exhaust pipe, and worn-out tires. These are minor issues that can be amended relatively easily. Then, the engine starts to malfunction, the wires begin to rust, and the car becomes unsalvageable. Like a car, the organism has many parts that are being used daily. Similarly, an organism can break down through continuous wear and tear. This seemingly obvious idea has been revolutionary in the field of aging research. Contrary to other theories that proposed that humans were genetically programmed to age, the cumulative damage theory presented aging as a random process (1). As such, it may be reasonable to conclude that aging is the byproduct of environmental effects. Surely, this would mean that after centuries of medical advancement, which included vaccines, antibiotics, and surgery among its ranks, humans have been able to increase their life spans considerably. Yet, despite significant increases in life expectancy, meaning more humans are realizing the full extent of their maximum lifespans, the actual human lifespan has stayed relatively the same (3). A more recent theory proposed that the maximum lifespan is determined genetically and that environmental factors can only contribute to expedited biological aging. Given the saturation of human population survival curves, this theory is especially convincing (3).
As a corollary to the cumulative damage theory of aging, aging is regarded as a holistic process—a process that is affected by a multitude of genes and environmental factors. One suspected contributor to the aging process is free radical damage.1 Free radicals are molecules that harbor a single, unpaired valence electron and induce oxidative damage in cellular machinery. Free radicals are byproducts of cellular respiration and can damage DNA. In particular, mtDNA (mitochondrial DNA) is at risk of oxidative damage due to both its proximity to free radical formation, as cellular respiration occurs in mitochondria, and significantly lower levels of DNA repair. The free radical theory of aging has become especially popular in the health industry where antioxidants, compounds that neutralize free radicals, have become synonymous with anti-aging treatments.3 The effectiveness of antioxidant consumption in retarding aging has not been validated. Other notable candidates for contributing to aging include protein aggregation, cross linkage, and induced apoptosis (1).
In order to discern other contributing factors, several longitudinal studies on aging have been implemented. Longitudinal studies offer one major advantage over the cross-sectional studies traditionally employed in medical research in that they allow scientists to track an individual’s health as they grow older. The Baltimore Longitudinal Study of Aging (BLSA) is the most prominent of these studies and was started in 1958 by Nathan Shock, a pioneer in the field of aging research, along with over 1,000 participants (4). Since then, several other studies have taken root including The SardiNIA Project executed by the National Institute on Aging that includes 6,100 participants from the island of Sardinia off the coast of Italy (3). Armed with the powerful tools of bioinformatics, these studies have become potential windows from which to understand the intricacies of human aging.
Aging Research Today
Aging research has gained steady momentum in recent years. In fact, one of the most famous aging experiments was conducted in 1993 by Cynthia Kenyon, a professor at UCSF and now vice president at Calico. Kenyon discovered that mutations in the daf-2 and daf- 16 genes doubled the lifespan of C. elegans, a model organism. Her future work saw increasingly lengthened lifespans from modulating these two gene (5). The search for homologous counterparts in humans is ongoing. A recent subset of aging research has focused on life extension treatments in more complex model organisms such as D. melanogaster, lab mice, and Rhesus monkeys (1).
Recently, other molecular mechanisms have been implicated with aging. These include reservatrol, sirtuins, and rapamycin. Reservatrol, a compound commonly found in red wines, activates sirtuin deacetylases, which extend the lifespan of lower organisms and may also be involved in human aging (6). Reservatrol has also been related to cardioprotective benefits. Discovery of these mechanisms and possible relations to aging have been led by pioneers such as David Sinclair of Harvard Medical School. Treatments involving rapamycin, an immunosuppressant, have increased the longevity of mice (7). The search for contributing molecular factors of aging is an active and promising facet of aging research.
In the past decade, the advent of computational tools for large-scale data analysis has revealed fascinating insights into aging. Computational biology and bioinformatics have expedited the search for biomarkers of aging. Traditionally, pulse wave velocity and telomere length served as the gold standards of biological age measurement, but only explained a fraction of individual variance in aging (3). Recent research has implicated a litany of cardiovascular traits, physical and mental characteristics, and genetic mutations as potential biomarkers. In 2014, Steve Horvath, a professor at UCLA, developed a method for deriving an estimate of biological age (DNAm) from DNA methylation patterns, which was highly correlated with chronological age and seemed to explain several tendencies in both aging and disease (8). There is ongoing research in detection of a central aging signal that explains most physiological causes of aging.
Aging research has garnered considerable public spotlight in the past several years. Aubrey de Grey, a computer scientist turned biologist and founder of the SENS foundation, gave an extremely well-received TED talk on a strategy that he has proposed to tackle the obstacle of aging. The strategy involves partitioning the aging process into several major factors: aggregates, cellular senescence and growth, cross linkage, and mutations. By targeting medical advancements in each field separately, the problem becomes more manageable and the human lifespan could potentially be elongated in small increments over a long period of breakthroughs (9). Other social movements such as transhumanism have highlighted the potential of anti-aging treatments in the near future. Transhumanism embraces emerging technologies and their potential in bettering the human body or quality of life—including extension of the healthy lifespan (9).
Controversies
Since the age of alchemy, aging research has been a field brewing with controversy. Today, there are two major concerns with developments in aging research and rejuvenation technology. First, critics of anti-aging research are concerned with the very real possibility of overpopulation. The current age distribution of ages in the United States is a micro-example of what an ageless population might entail. There are already concerns that the aging Baby Boomers generation may overburden the healthcare and Social Security systems. Imagine this same effect but with continuous, cumulative addition to the old end of the age spectrum. Critics espousing this belief, however, do not take into consideration what current aging research implies about future anti-aging therapies. Nearly all current testing in model organisms has indicated that anti-aging treatments tend to promote extended, healthy aging. That is, the relative age of individuals would simply be stretched across a longer temporal span. Individuals under treatment who are chronologically 70 years old may instead be 50 years old biologically. As such, fears of skewing towards an elderly population are largely unfounded in a relative world. Additionally, longer healthy life spans would entail greater productivity from an individual over their lifetime (9).
Other opponents of aging research cite religious and ethical concerns (10). After all, if we are extending our lifespans beyond their natural limit, are we not playing God? There is no simple solution to address these concerns. There will always be advocates and critics of aging research and scientists should be attentive to these ethical concerns as they continue to pursue this line of research. In the end, if an anti-aging treatment is procured, it is only an additional opportunity that has been extended and would be by no means obligatory.
The Path Ahead
Aging research is an exciting and growing field. Developments in our understanding of the fundamental aging process are likely to proffer increased insight in related research areas such as cancer, diabetes, and Alzheimer’s research. Aging is still a relatively underpopulated field of research and looks to benefit from the recent explosion of biotechnology and big data-aided research (11). In the coming decades, one can expect to see greater innovation and progress in aging research. Perhaps one day, even the fabled philosopher’s stone or fountain of youth may manifest as a product of this push for greater understanding.
Eric Sun ‘20 is a freshman in Hollis Hall.
WORKS CITED
[1] Hayflick, L. How and Why We Age; Ballantine Books: New York, 1996.
[2] Scudellari, M. Nature 2015, 517, 426-429.
[3] Austad, S. Why We Age, 1st ed.; Wiley: Hoboken, NJ, 1999.
[4] Shock, N. et al. Normal Human Aging: The Baltimore Longitudinal Study of Aging; NIH-84-2450; NIH: Washington, D.C., 1984.
[5] Kenyon, C., et al. Nature 1993, 366, 461- 464.
[6] Baur, J. A.; Sinclair, D. A. Nat. Rev. Drug Discov. 2006, 5, 493-506.
[7] Wilkinson, J.E., et al. Aging Cell 2012, 4, 675-682.
[8] Horvath, S. Genome Biology 2013, 14, R115.
[9] de Grey, A.; Rae, M. Ending Aging: The Rejuvenation Breakthroughs That Could Reverse Human Aging, 1st ed.; St. Martin’s Press: New York, 2007.
[10] Green, B. Radical Life Extension: An Ethical Analysis. Santa Clara University [Online], February 27, 2017. http://www.scu.edu/ethics/all-about-ethics/radical-life-extension/ (accessed Mar. 26, 2017).
[11] Arking, R. Biology of Aging: Observations and Principles, 2nd ed.; Oxford University Press: Oxford, U.K., 2006.
- Open access
- Published: 24 May 2024
Molecular mechanisms of aging and anti-aging strategies
- Yumeng Li 1 ,
- Xutong Tian 1 ,
- Juyue Luo 1 ,
- Tongtong Bao 1 ,
- Shujin Wang 2 &
Cell Communication and Signaling volume 22 , Article number: 285 ( 2024 ) Cite this article
737 Accesses
10 Altmetric
Metrics details
Aging is a complex and multifaceted process involving a variety of interrelated molecular mechanisms and cellular systems. Phenotypically, the biological aging process is accompanied by a gradual loss of cellular function and the systemic deterioration of multiple tissues, resulting in susceptibility to aging-related diseases. Emerging evidence suggests that aging is closely associated with telomere attrition, DNA damage, mitochondrial dysfunction, loss of nicotinamide adenine dinucleotide levels, impaired macro-autophagy, stem cell exhaustion, inflammation, loss of protein balance, deregulated nutrient sensing, altered intercellular communication, and dysbiosis. These age-related changes may be alleviated by intervention strategies, such as calorie restriction, improved sleep quality, enhanced physical activity, and targeted longevity genes. In this review, we summarise the key historical progress in the exploration of important causes of aging and anti-aging strategies in recent decades, which provides a basis for further understanding of the reversibility of aging phenotypes, the application prospect of synthetic biotechnology in anti-aging therapy is also prospected.
Aging will be a major social problems worldwide in the coming decades [ 1 , 2 , 3 ]. During the aging process, the body tissues and organs of the older people undergo functional decline or deterioration, thus increasing their susceptibility to age-related diseases and shortening their healthy life span, which has brought enormous financial pressure to countries worldwide in terms of pension, medical expenses, and health care [ 4 , 5 , 6 ]. Therefore, exploring the biological nature of aging, searching for safe and effective intervention strategies to positively regulate health status, and prolonging the healthy lifespan of the aging population are important for reducing the global pension burden and promoting healthy aging.
Aging is a progressive degenerative state that can be physiological and pathological [ 7 , 8 , 9 ] (Fig. 1 ). Physiological aging is observed in across many species, and is a degenerative process that occurs after maturation, including telomere attrition [ 10 , 11 ], DNA damage [ 12 , 13 ], mitochondrial dysfunction [ 14 , 15 ], loss of nicotinamide adenine dinucleotide (NAD + ) levels [ 16 , 17 ], impaired macro-autophagy [ 18 , 19 ], stem cell exhaustion, inflammation [ 20 , 21 ], loss of protein balance [ 22 ], deregulated nutrient-sensing [ 23 ], altered intercellular communication [ 24 , 25 , 26 ] and dysbiosis [ 27 , 28 ], thereby leading to systemic functional decline. Importantly, these changes are decentralised and interactive, not independent of each other. Pathological aging includes the senile pathological aging changes, which are caused by various external factors, such as cardiovascular disease [ 29 ], cerebrovascular disease [ 30 ], degenerative joint disease [ 31 , 32 ], diabetes [ 33 ], Parkinson’s disease [ 34 , 35 ], Alzheimer’s disease [ 36 ], cancer [ 37 , 38 , 39 ], and degeneration of multiple organ functions. These aging-induced cellular physiological and pathological changes can reflect the underlying nutrient sensing, intercellular communication, protein stabilisation, epigenetics, and molecular abnormalities in DNA damage repair, leading to genomic instability and damage. Further understanding of the different molecular mechanisms involved in the aging process is of great importance for preventing aging and prolonging the lifespan.
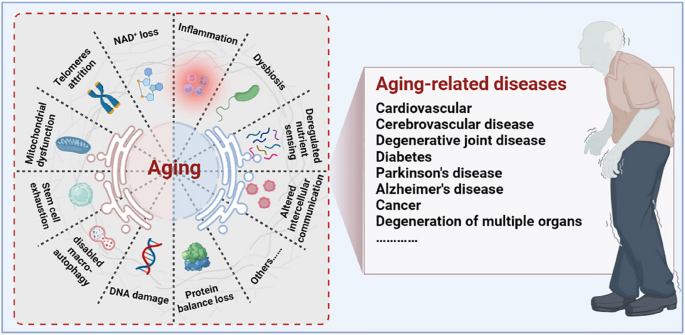
Aging drivers and age-related diseases. Major physiological features of aging include NAD + loss, telomeres attrition, mitochondrial dysfunction, stem cell exhaustion, disabled macro-autophagy, DNA damage, protein balance loss, inflammation, dysbiosis, deregulated nutrient sensing, and altered cellular communication. These physiological characteristics of aging are primitive, antagonistic, and integrated, and their interaction promotes aging. When aging reaches a certain threshold, organ and tissue function continues to deteriorate, which increases the incidence and mortality of aging-related diseases, including cardiovascular, cerebrovascular, degenerative joint disease, diabetes, Parkinson’s disease, Alzheimer’s disease, and cancer
In recent years, a large number of animal and clinical experiments have been conducted to study factors that induce aging, such as morphological and pathological changes and functional decline of the aging organism. Indeed, some differences between biological and chronological age reflect the validity of age-accelerated or deceleration procedures, which are well-known biomarkers of the aging process. Researchers have gradually expanded from traditional methods of measuring aging (including maximal energy expenditure at the respiratory, sensory, psychomotor, and cognitive levels) to modern biotechnological methods, such as genomics, epigenomics, transcriptomics, proteomics, and metabolomics. These techniques may have implications for assessing the spatiotemporal patterns of health degradation and effectiveness of anti-aging strategies.
Briefly, aging is a complex process, and its characteristics are interdependent. Each of these factors should be considered as an entry point for future exploration of the aging process and the development of novel life extensions. Here, we review the history and current state of aging research and summarise the characteristics of aging and the mechanisms promoting aging. In addition, we review different types of aging mechanisms and their corresponding anti-aging strategies. This knowledge can guide the design of preventive and therapeutic strategies to delay aging and age-related diseases and extend human health and longevity.
Potential triggers and molecular mechanisms of aging
Aging is a complex result of many biological processes, and many key factors trigger aging, such as DNA damage, telomere dysregulation, mitochondrial dysregulation, NAD + loss, autophagy disorders, and stem cell exhaustion. Here, we summarise the main causes and underlying molecular mechanisms contributing to the aging process.
Aging and DNA damage
DNA damage is a major internal factor that leads to genomic instability, epigenetic changes, protein stress, impaired mitochondrial function, and telomere dysfunction [ 12 ]. The continuous accumulation of DNA-damaged cells triggers cell death and senescence, ultimately leading to chronic inflammation, loss of function, atrophy, and disease in cells and tissues [ 40 ].
Molecular mechanisms of DNA damage
Genomic instability manifests as permanent and transmissible changes in DNA sequence [ 13 , 41 ]. DNA damage caused by an inherently unstable genome includes spontaneous deamination, hydrolysis, and many other chemical changes such as different types of breaks, changes in base positions, gaps, DNA-protein cross-links, and other subtle chemical modifications [ 12 , 42 ]. Abnormal DNA structures (e.g. G-quadruplexes, R-loops, and persistent single-stranded regions), as well as abnormal intermediates in DNA transactions (e.g. stalled transcription, replication, and recombination complexes), are considered phenotypes of DNA damage [ 13 ]. Genomic mutations caused by DNA instability adversely affect cellular functions and are major causes of cancer and genetic diseases. However, DNA instability is also the most important substrate in the evolution of species [ 43 , 44 ]. DNA integrity is maintained by the continuous repair of highly complex DNA repair and DNA damage response (DDR) systems that counteract the time-dependent erosion and destruction of genetic DNA information. Progressive telomere shortening is another major contributor to DNA damage that accelerates the aging process [ 45 ].
DNA damage is the major driver of age-related epigenetic changes. The epigenome, which includes DNA methylation and histone modifications, is unstable throughout the life cycle of somatic cells [ 46 ]. DNA damage leads to persistent chromatin changes that enrich aging-enhancing DNA fragments (DNA-scars) in senescent cells [ 47 ]. Persistent DNA damage and repair-related cellular physiological effects may leave epigenetic marks, resulting in epigenetic heterogeneity among cells. Transcription appears to change considerably more in senescent cells than in young cells. Thus, DDR may be a major cause of epigenetic changes that impair gene expression control, leading to somatic heterogeneity and a time-dependent decline in overall function.
Relationship between DNA damage and aging
During aging, numerous exogenous and endogenous genotoxins, photoaging, and mechanical stress in tissues continuously induce DNA damage (Fig. 2 ). Approximately 10 5 DNA damage events occur in mammalian cells every day, although most of the DNA damage is effectively excised or repaired. Notably, a small portion escapes the DNA damage detection and repair system, subsequently resulting in failure to repair or repair errors [ 48 ]. Many studies using mammalian models have confirmed an inextricable link between DNA damage and aging [ 49 , 50 , 51 , 52 ]. As aging progresses, the DNA repair capacity gradually declines, and the increased molecular phenotype of genomic instability becomes the main marker of aging. Markers of DNA damage are found in patients with age-related diseases such as cardiovascular disease [ 53 ], Alzheimer’s disease [ 54 ], and cancer [ 55 ], suggesting that DNA damage is directly related to the incidence of these diseases. Patients with genetic or acquired defects in DNA repair proteins also exhibit features of premature aging and that differences in the location of the defect in the DNA repair system can lead to premature aging in different organs [ 56 ]. Specifically, RecQ helicase plays an important role in DNA recombination, replication, repair, and telomere maintenance, and its mutation may increase the incidence of Werner, Bloom, and Rothmund-Thomson syndromes [ 57 ]. Global genome nucleotide excision repair deficiency leads to a thousand-fold increase in skin cancer susceptibility and may accelerate neurodegeneration [ 58 ]. Impaired transcription-coupled repair mechanisms can cause typical age-related pathologies, such as neurodegeneration, osteoporosis, and atherosclerosis [ 59 ]. Hutchinson-Gilford progeria is associated with nuclear genome instability, defects in DNA double-strand break repair leading to telangiectasia and Nijmegen break syndrome, and defects in DNA cross-linking repair leading to anaemia [ 60 ]. In addition, DNA damage caused by mitochondrial defects is another underlying factor in a class of progressive diseases that affect multiple organs.
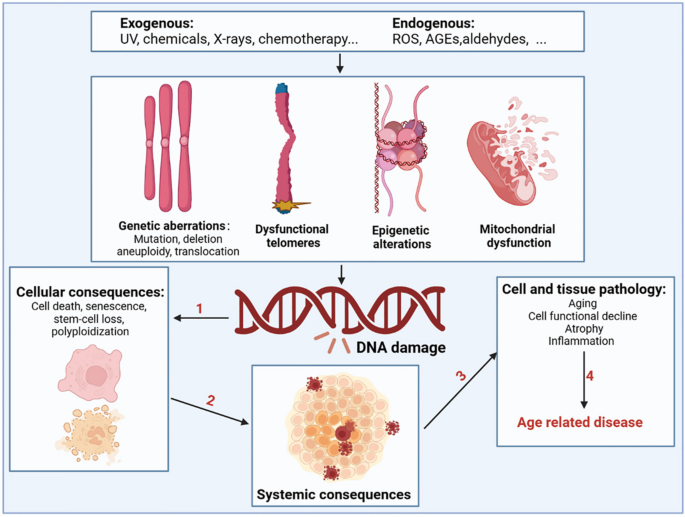
Drivers of DNA damage and the resulting systemic consequences. The nuclear and mitochondrial genomes are constantly exposed to exogenous substances (such as ultraviolet and X-rays, chemicals in food, water, and air), endogenous substances such as ROS, advanced glycation end products (AGEs), and aldehydes; this results in genetic abnormalities, including mutation, deletion, aneuploidy, translocation, dysfunctional telomeres, epigenetic alterations, and mitochondrial dysfunction. DNA damage and DNA damage response caused by the above factors can shock molecular processes and alter cell fate, such as cell death, senescence, and systemic breakdown of repair functions, eventually leading to the loss of cell and organ function and promoting the occurrence and development of age-related diseases
Overall, defects in the DNA damage repair system directly leads to the continued accumulation of genomic mutations, which underlie many segmental forms of premature aging in humans, suggesting a close link between genome integrity and aging. Although considerable progress has been achieved in the study of the mechanistic connection between DNA damage and aging, there are still many issues to be further explore the specific molecular mechanisms by which DNA damage affects diseases in older people. Therefore, fundamentally addressing the aging process and combating age-related diseases are important for exploring the relationship between DNA damage and anti-aging effects.
Aging and telomere attrition
Telomeres are small stretches of DNA-protein complexes present at the ends of linear chromosomes in eukaryotic cells, which maintain chromosomal integrity, control the cell division cycle, and are essential for an organism’s healthy life span and reproduction [ 61 ]. As early as the 1960s, a scientist named Leonardo Hayflick discovered that cultured human fibroblasts had limited and reproducible replication capacity and were governed by cell-autonomous mechanisms [ 62 , 63 ]. Even if the cold stops the cell division, once the temperature rises again, the cells will continue to divide before freezing, until 50 times after the cessation of division. Heverick realized that cells have a deep-seated internal mechanism that controls the number of times they divide [ 64 ]. In the 1970s, Olovnikov [ 65 ] and Watson [ 66 ] discovered the “end duplication problem” by looking at asymmetries in linear DNA replication and predicting that each cell division results in chromosomal DNA at the ends of the lagging strands loss, eventually leading to the gradual shortening of chromosomes. Limited telomere length reserve is an obstacle to cell proliferation and viability, and the loss of telomere function is closely associated with age-related functional decline and increased incidence of disease [ 67 ] (Fig. 3 A).
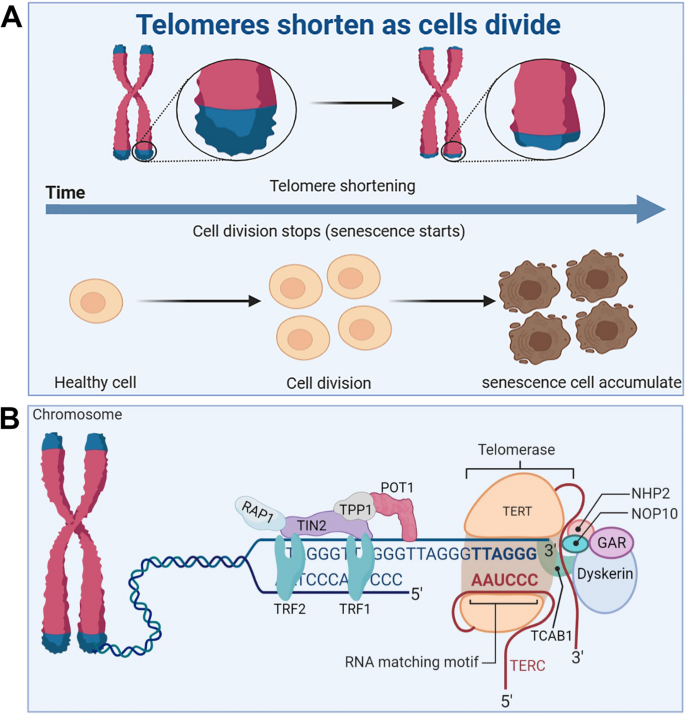
Telomere and telomerase structure, and their relationship with cell senescence. A Telomeres shorten during cell division, leading to accumulation of senescent cells. B The structure of the telomere-telomerase complex. TERT, telomerase reverse transcriptase; TERC, telomerase RNA component; NOP10, nucleolar protein family A, member 3; NHP2, nucleolar protein family A, member 2; GAR, nucleolar protein family A; TIN2, TERF1-interacting nuclear factor 2; TPP1, telomere protection protein 1; TRF1, telomeric repeat binding factor 1; TRF2, telomeric repeat binding factor 2; POT1, protection of telomeres 1; RAP1, TERF2-interacting protein. The telomere diagram is derived from “biorender”
Telomere and telomerase structure
Telomere end protection is evolutionarily highly conserved from lower to higher multicellular organisms [ 68 ]. Structurally, telomeres consist of repeating nucleotide sequences 3’-[TTAGGG]-5’ in tandem, ranging from a few to tens of bases, terminated at the 3’ end by a single strand of guanine-rich nucleotides of 75 to 300 nt, forming a “cap structure” (Fig. 3 B). Telomeres are covered by a special protein called the shelterin complex, which is a multimer of six protein subunits (TRF1, TRF2, TPP1, POT1, TIN2, and RAP1) that work together to protect the chromosomes and regulate telomere length [ 68 ]. Telomeres and shelterin complexes form a sophisticated higher-order structure that protects DNA repair programmes from fusing ends by mediating non-homologous end-joining of telomeric DNA through double-stranded DNA break detection, ultimately involved in the capping, protection, and regulation of telomeres [ 69 ]. Correspondingly, mutations in these six protein components can disrupt the shelterin-telomere complex, resulting in terminal fusion and premature senescence. Specifically, telomere maintenance is inseparable from normal expression of TRF1 [ 70 , 71 ]. TRF1 deletion induces telomeric DNA to form a fragile site phenotype, whereas TRF1 overexpression impairs telomerase binding to telomere ends, eventually resulting in telomere shortening [ 72 , 73 ]. TRF2 folds telomeric DNA into T-loops, inhibits the ataxia telangiectasia mutated-dependent DDR at chromosome ends, and suppresses end-to-end chromosome fusion and canonical homologous end joining [ 74 ]. In addition, TIN2 plays a connecting role in the shelterin complex and forms bridges between different shelterin proteins [ 75 ]. TIN2 mutations do not interfere with the spatial structure of other shelterin components on telomeres; however, the TIN2-R282H mutation activates telomeric DNA damage signalling, which results in telomere instability associated with telomerase activity, eventually leading to a premature cellular senescence phenotype [ 76 ]. Uncontrolled POT1 impairs telomerase binding to telomere ends, resulting in shortened telomeres [ 77 ]. TPP1 interacts with telomerase reverse transcriptase (TERT) to recruit telomerase and its loss elicits a robust telomeric DNA damage response [ 78 ]. Rap1 is a key telomere-capping protein that prevents non-homologous end joining and telomere fusion, and its overexpression causes histone loss and accelerates cellular senescence [ 79 , 80 ]. Overall, the biological functional integrity of telomeres depends on the interaction of telomeres and the shelterin complex, which together regulate telomere length and the cell life cycle. It should be noted that normally shortened telomeres alone do not drive senescence (biology) if telomeres become so short that they are perceived as double-stranded DNA breaks, then these telomeres will recruit the DDR and induce the cells into a normal apoptotic or senescence program.
Telomerase is a riboprotease composed of two basic subunits: TERT and telomerase RNA component (TERC) [ 81 ]. The H/ACA domain of Cajal body protein 1 in TERC binds to telomerase to form telomerase Cajal body protein 1, which catalyzes telomerase activity and transports telomerase to the ends of telomeres [ 82 ]. In addition, multiple core protein components, including dyskerin, NHP2, NOP10, and GAR1, are essential for the normal catalytic function of telomerase [ 83 ]. Normally, telomerase is abundantly expressed in undifferentiated stem [ 67 ] and progenitor cells of germ cells [ 84 ], the skin, intestine [ 85 ], haematopoietic system [ 82 ], hair bulge [ 86 ], and testis [ 7 ]. Nevertheless, it is extremely low or undetectable in differentiated adult cells, such as neuroblasts [ 87 ], fibroblasts [ 88 ], cardiomyocytes [ 89 ], and sperm cells [ 90 ]. In the germ line and in some stem cells, telomerase can compensate for this loss of telomere duplication, which decreases with cell division [ 91 ]. Telomerase is silent during the early development of most somatic cells, limiting the number of cell divisions until the telomeres become very short [ 92 ]. The pathogenicity of telomere shortening during aging is a characteristic antagonistic pleiotropic effect. On the one hand, cells with telomere dysfunction are prone to genome instability and may become cancer cells. On the other hand, the normal replicative shortening of telomeres can restrict unrestricted cell proliferation and induce cell apoptosis or senescence, thus preventing the formation of tumors. Robinson et al. found a way to help telomeres maintain their length, a technique known as alternative lengthening of telomeres (ALT) [ 93 ]. In osteosarcoma and bread cancer cell lines, the potential relationship between telomere lengthening and inhibition of tumor growth is cleverly orchestrated in cell lines that maintain telomere length by the ALT [ 94 ]. It helps that tumors can be suppressed even when telomeres are lengthened.
Maintenance of adequate telomere length in normal cells requires intact telomere structure and highly sophisticated regulation of telomerase [ 95 ]. However, each associated protein in the telomere and telomerase complexes is susceptible to uncontrollable factors in the tissue microenvironment [ 96 ]. However, there is still some scientific debate regarding how the telomerase complex is sensed, expressed, and recruited to telomere ends for functional regulation to determine the role of telomeres and telomerases in the pathogenesis of systemic aging and degenerative diseases. Recently, telomere dysfunction has been described as a molecular feature of senescent cells, and the loss of telomere function is closely associated with genomic instability [ 97 ], DDR [ 98 ], and age-related decline in fitness [ 99 ]. Most importantly, telomere dysfunction during aging can amplify and drive other aging mechanisms and the progeria syndrome.
Relationship between telomere and telomerase dysfunction and aging
Organismal cellular telomere reserves are limited, and the loss of telomere function is closely associated with age-related adaptive decline [ 99 , 100 , 101 ] (Fig. 4 ). Excellent telomere and telomerase structures are essential for ensuring the normal physiological function of mothers and offspring, and their integrity has a certain genetic intergenerational effect [ 102 , 103 ]. Mice with knockout of TERT that are crossbred in successive generations, the telomeres of the offspring gradually shorten, finally developing telomere dysfunction in the third generation [ 104 ]. Additionally, low telomerase levels and continued tissue turnover lead to decades of progressive telomere attrition in the progenitor cells of highly proliferative tissues, including the haematopoietic system, gastrointestinal tract, and skin [ 10 , 11 ]. Excessive telomere attrition ultimately triggers DDR such as cell cycle arrest [ 105 ], apoptosis [ 106 , 107 ], differentiation disorders [ 108 ] and senescence [ 109 ]. Notably, as the aging process progresses, hypoproliferative tissues, including the heart, brain, and liver, may suffer from the effects of reactive oxygen species (ROS), which further induce telomere sequence damage, telomere attrition, and uncapping [ 86 , 110 ]. Thus, the aforementioned telomere properties make them a focal point in the biology of aging.
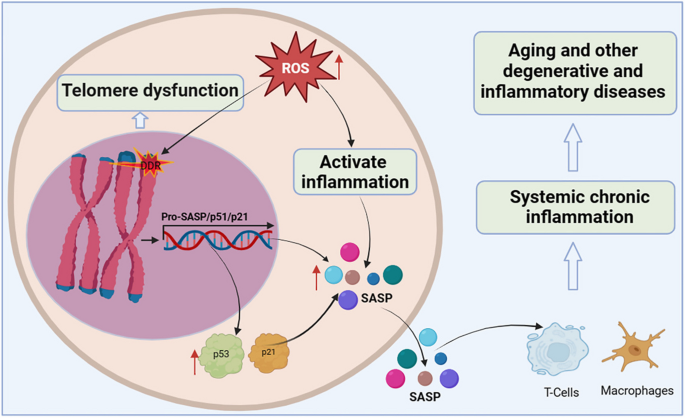
Telomere dysfunction activates DDR to drive cellular senescence. ROS induce telomere sequence damage, leading to telomere shortening and decapitation, triggering DDR, inducing the overexpression of cell cycle inhibition markers p53 and p21, and accelerating cell senescence. Senescent cells secrete SASP, which alter extracellular matrix composition, recruit and enhance T cells and macrophages, which can spread the aging phenotype to surrounding cells, thus promoting systemic chronic inflammation and inflammation-related diseases
Shortening of telomeres to a critical length leads to replicative cellular senescence [ 86 , 111 , 112 , 113 ]. Chromosomal telomeres gradually shorten as DNA replicates. When telomeres reach a critical length, they cannot bind enough telomere-covering proteins and are perceived as exposed DNA ends [ 114 ]. One or a few very short telomeres are sufficient to trigger the DNA damage response and induce overexpression of the cell cycle inhibitory markers p53 and p21, thereby forcibly inhibiting cell proliferation [ 115 ]. Accumulated senescent cells secrete a complex set of pro-inflammatory cytokines, termed the senescence-associated secretory phenotype (SASP), including interleukins, interleukin chemokines, proteases, and growth factors. The SASP alters the composition of the extracellular matrix and propagates the senescent phenotype to surrounding cells, leading to systemic chronic inflammation [ 116 ]. Interestingly, persistent telomere cohesion protected aged cells from premature senescence [ 117 ]. Therefore, telomere dysfunction-associated DNA damage response signalling events are key determinants of cell fate and organismal aging.
In summary, telomeres and telomerase play important roles in the core mechanisms that drive aging and many major human diseases. However, many knowledge gaps remain, such as the elucidation of the mechanisms regulating telomerase expression and activity, the non-canonical function of TERT, and the interactions between telomere dysfunction, inflammation, fibrosis, and degenerative disease. Therefore, there is an urgent need to develop telomerase activators for the treatment of aging and age-related diseases to prevent and treat fatal diseases caused by telomere shortening by rescuing telomeres and telomerase damage.
Aging and mitochondrial dysfunction
Mitochondria are the only organelles that retain their own genome and transcriptional and translational machinery, and are important cellular organelles for cellular energy conversion and signalling. The functional integrity of mitochondria is affected by intramitochondrial protein folding, mitochondrial membrane dynamics, mitosis, and intracellular environmental stress products. One of the classic features of aging is a progressive decline in mitochondrial activity and stress resilience. Mitochondrial dysfunction is closely associated with aging and age-related metabolic diseases.
Mitochondrial dysregulation by pleiotropic stress pathways
A healthy mitochondrial network generates adenosine triphosphate (ATP) through the tricarboxylic acid cycle (TCA cycle) and oxidative phosphorylation, which maintain the basic energy conversion and information exchange within the cell and are essential for life [ 118 ]. Studies have shown that in normal cells, the nuclear gene-encoded transcription factor PCG1NRF1 induces the expression of mitochondrial-encoding genes, which further regulate mitochondrial biogenesis or increase mitochondrial activity to regulate cellular energy metabolism [ 119 ]. Conversely, metabolic perturbations of mitochondrial physiology, such as intramitochondrial protein stabilisation stress, energy deficit, and increased ROS production, trigger transcriptional reprogramming of nuclear genes for metabolic adaptation [ 120 ]. Notably, nuclear genes encode most of the mitochondrial proteome, whereas only a few protein-coding genes are encoded by the circular mtDNA. Therefore, to ensure protein balance and functional stability of the mitochondria, it is necessary to maintain excellent mitochondrial-nuclear genome-encoded communication channels [ 121 ].
In addition, mitochondria are the main cellular organelles that regulate energy homeostasis in cellular metabolism, and the dynamic balance of small molecules (including adenosine 5’-monophosphate (AMP), nicotinamide adenine dinucleotide (NAD + ), oxygen, ROS, and TCA cycle components) produced by mitochondria affect the information of mitochondria, nucleus, and other cellular organelles [ 14 ]. Specifically, ATP is a sensitive signal of mitochondrial health, and a continuous decrease in intracellular ATP levels increases the relative AMP content and activates the AMP-protein kinase signalling pathway [ 122 ]. The activated 5’-AMP-activated protein kinase (AMPK) signalling pathway further regulates key enzymes in other metabolic pathways (including fat and glucose metabolism, mitochondrial dynamics, autophagy, and protein synthesis) through phosphorylation and indirectly restores the energy balance in the mitochondria [ 123 , 124 ]. Disruption of this mechanism results in various mitochondria-related diseases. Similarly, NAD + is a cofactor for many metabolic reactions and a key factor in sensing the mitochondrial metabolic state and communicating it to other cellular organelles. We will elaborate on the important role of NAD + in the aging process in Sect. 2.4 . Oxygen is another small molecule that affects mitochondrial function; low intracellular oxygen levels reduce the ability of mitochondria to generate ATP [ 125 ]. Under normal conditions, cells can stabilise the structure of the proline hydroxylase domain of hypoxia-inducible factor-1/2a, limiting the potential impairment of mitochondrial function caused by low oxygen supply. In addition, toxic byproducts, such as ROS generated in mitochondria, can act on mitochondrial permeability pores together with excess Ca 2+ in mitochondria, resulting in oxidative damage and swelling of the mitochondria, thereby triggering inflammation and affecting mitochondrial function [ 126 ]. Small molecules in the TCA cycle, such as acetyl-CoA, α-ketoglutarate, succinic acid, and fumaric acid, are all signalling molecules that characterise the physiological state of mitochondria.
Relationship between mitochondrial dysfunction and aging
Mitochondrial dysfunction has pleiotropic effects (Fig. 5 ). Maintaining healthy and excellent mitochondrial metabolic function is a key factor in ensuring long-term health during the aging process, and the genetic stability of mtDNA and nuclear DNA determines the energy supply capacity of an organism’s tissues throughout life [ 121 ]. Unlike mitosis of the nuclear genome, mtDNA can replicate continuously, independently of the cell cycle. Owing to the low repair efficiency of the mtDNA repair system, mutated mtDNA copies accumulate in the cells over time. When the life cycle of an organism enters the later stages of life, heterogeneous mutations generated in both nuclear DNA and mtDNA exceed a certain threshold. These harmful physiological consequences promote the process of aging and age-related diseases, including disturbed glycolipid metabolism, reduced recognition knowledge, and shortened lifespan. Studies have reported that mtDNA mutant mice are more likely to develop signs of premature aging, such as a shortened lifespan, reduced fertility, anaemia, osteoporosis and hearing loss [ 127 ]. Notably, perturbation of the mtDNA epigenome has also been implicated in human progeria and disease [ 119 , 128 ]. The methylation of mtDNA is an important epigenetic modification. During the life cycle, mtDNA methylation is susceptible to environmental interference, endogenous metabolites, and other factors. Studies have found that individuals with reduced methylation in the D-loop region of mtDNA are more likely to develop amyotrophic lateral sclerosis and Parkinson’s disease, whereas those with reduced methylation of Mt-ND1 are more likely to develop Alzheimer’s disease [ 129 ] owing to the effect of mitochondrial dysfunction on normal cells. Conversely, senescent cells display changes in mitochondrial morphology, physiology, dynamics, and function. Studies have reported decreased mitochondrial membrane potential, increased proton leakage, and ROS production in senescent cells, further reducing cellular fatty acid oxidation and disrupting mitochondrial metabolism.
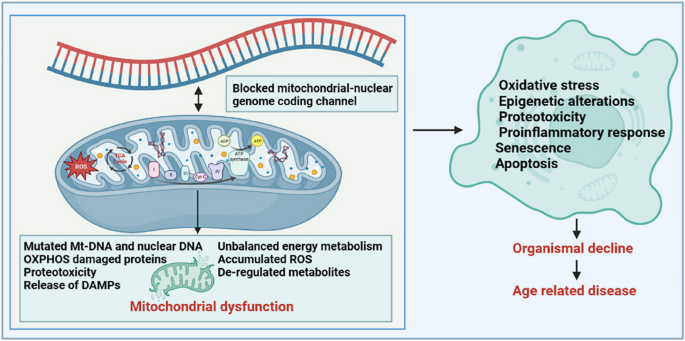
Mitochondrial dysfunction has pleiotropic effects in aging. Inducers such as the accumulation of mtDNA mutations, release of damaged toxic mitochondrial material, the production of mtROS, proteotoxicity, and deregulated metabolites (TCA intermediates, NAD + ) all contribute to mitochondrial dysfunction. Alterations in mitochondrial function have widespread adverse effects on intracellular homeostasis and lead to systemic organ decline and the development of several age-related diseases through complex signalling mechanisms (involving mitogens, metabolites, etc.)
In summary, many factors impair mitochondrial function during the life cycle, among which excessively reduced ATP, NAD + and oxygen levels, excessively accumulated ROS levels, and disrupted TCA cycle small molecules are the major contributors. Correspondingly, mitochondrial dysfunction is mainly reflected in transcriptional and epigenetic regulation caused by mitochondrial stress responses, such as mtDNA mutation, and the induction of other cellular organelle disorders, such as lysosomal storage disorders, impaired mitochondrial removal disorders, endoplasmic reticulum response, and changes in the cytoplasmic microenvironment. Based on the sensitivity of mitochondria to their microenvironment, mitochondrial dysfunction has been identified as an important trigger for aging and aging-related metabolic diseases. However, more research is needed to elucidate the interrelationships between mitochondrial dysfunction, aging, and aging-related diseases, as well as the underlying mechanisms of action, to discover new targets for anti-aging interventions.
Aging and NAD + loss
Nicotinamide adenine dinucleotide (NAD + ) is an important cofactor in the nucleus, cytoplasm, and mitochondria [ 130 ]. NAD + is involved in the regulation of cell redox reactions and energy metabolism, and its abnormal metabolism can affect cell metabolism, DNA repair, organelle function, immune cell viability, and cell aging [ 131 ]. However, aging is accompanied by a gradual decline in NAD + levels in tissues and cells, which accelerates the aging process and increases the prevalence of age-related diseases. Therefore, maintaining NAD + levels in tissue cells is important to alleviate the loss of tissue cell function, stabilise metabolic homeostasis, and promote healthy aging.
NAD + regulatory network and its role in cellular processes
NAD + is an important coenzyme in cellular redox reactions and is at the centre of energy metabolism [ 132 ]. It is involved in regulating the activity of dehydrogenase in metabolic pathways such as cellular glycolysis, fatty acid oxidation, and L-glutamine metabolism [ 133 ]. In these reactions, NAD + receives hydrogen ions, forms its reduced form NADH, transfers the accepted electrons to the electron transport chain, and generates ATP to supply energy to the cell. Conversely, NAD + is phosphorylated to form NADP + , which then receives hydrogen ions to form NADPH, a process that protects the reducing anabolic pathways from oxidative stress. Notably, NAD + is also a cofactor and substrate for hundreds of cellular enzymes and is one of the major contributors to maintaining cellular processes and ensuring cellular physiological functions [ 134 ]. In the early and middle stages of life, NAD + synthesis, metabolism, and consumption are in a balanced state. Specifically, NAD + is continuously utilised in cells by NAD + -consuming enzymes, including NAD + glycohydrolases, NADases (CD38, CD157, and Sarm1), and the protein deacetylase family of Sirtuins and poly ADP-ribose polymerases (PARPs), which participate in a variety of important cellular functions and generate the byproduct nicotinamide (NAM). To maintain intracellular NAD + levels, in the NAM recycling pathway, NAM is converted to NMN by nicotinamide phosphoribose transferase (NAMPT) and further converted to NAD + by nicotinamide mononucleotide adenosyltransferases NMNat1 (nucleus), NMNat2 (cytosolic face of the Golgi apparatus), and NMNat3 (mitochondria) [ 132 ]. In addition, NAD + can be synthesised from tryptophan via the kenuridine pathway and from vitamin precursors such as nicotinic acid via the Preiss-Handler pathway. Most tryptophan is metabolised to NAM in the liver and converted to NAD + via the NAM rescue pathway [ 135 ]. Thus, the NAM rescue pathway appears to be a major contributor to system-wide NAD + levels.
Under normal circumstances, NAD + is continuously decomposed, synthesised, and recycled to maintain the balance and stability of intracellular NAD + levels [ 136 ]. However, studies have found that the balance between NAD + catabolic and anabolic processes is altered during aging, with NAD + degradation rates exceeding the capacity for intracellular NAD + synthesis, or excess NAM being broken down by alternative intracellular metabolic pathways, effectively shifting it away from the NAM rescue pathway and further affecting NAD + levels [ 137 ]. Studies have demonstrated that when rodents or humans reach middle age, the level of NAD + in the body is reduced to half that at a young age, which severely impairs cellular energy metabolism and various biological pathways, accelerates the aging process, and increases the incidence of age-related diseases [ 138 ].
Relationship between NAD + loss and aging
NAD + levels are strongly associated with health and aging in both rodents and humans (Fig. 6 ). In 1937, scientists discovered that low levels of NAD + can lead to symptoms such as dermatitis, diarrhoea, and dementia. NAD + levels gradually decrease during aging, but the mechanism of this reduction is not fully understood. Recent studies have found that aging itself causes inflammation and oxidative stress, which affect the activity of NAMPT, the rate-limiting enzyme of NAD + synthesis, and further affect the activity of downstream NAD + -dependent enzymes (including Sirtuins, PARPs, CD38, and CD157) [ 135 ]. Notably, Sirtuins, PARPs, and CD38 are the main enzymes that consume NAD + , and their content and activity strongly affect intracellular NAD + level [ 139 ]. Sirtuins contain seven proteins (Sirtuin1–7) which are a class of NAD + -dependent deacetylases. They regulate the activity of various proteins and gene expression by consuming NAD + , and has been shown to be an important mechanism for regulating the life span [ 140 ]. PARPs activity is an important factor in intracellular NAD + catabolism. PARPs levels increase with age, possibly because DNA damage caused by aging requires PARPs enzymes to participate in repair; however, excessive activation of PARPs promotes the reduction of NAD + levels [ 141 ]. In addition, some studies have found that inflammation and SASP accumulation during aging promote the expression and activity of CD38 protein, leading to a partial reduction in NAD + levels and mitochondrial function through the regulation of SIRT3 [ 142 ].
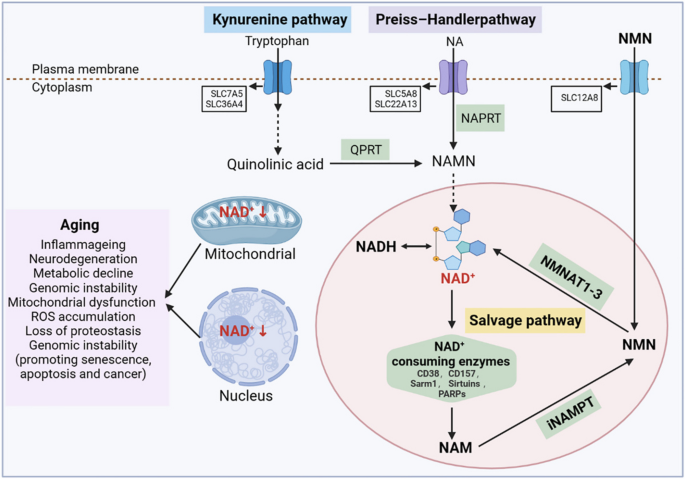
NAD + metabolism and its relationship with aging. NAD + levels are maintained by three independent biosynthetic pathways. The kynurenine pathway uses the dietary amino acid tryptophan to produce NAD + . Tryptophan enters cells through the transporters SLC7A5 and SLC36A4. In the cell, tryptophan undergoes a series of reactions to form quinolinic acid, which is then converted by the quinolinic acid phosphoribosyl glycosyltransferase (QPRT) into nicotinamide mononucleotide (NAMN), where it converges with the Preiss-Handler pathway. In the Preiss-Handler pathway, niacin (NA) enters cells via SLC5A8 or SLC22A13 transporters, and is catalysed by the nicotinic acid phosphoribosyltransferase (NAPRT) to produce NAMN, which is then converted into NAD + by a series of reactions. The NAD + salvage pathway recycles the nicotinamide (NAM) generated as a by- product of the enzymatic activities of NAD + -consuming enzymes (sirtuins, poly (ADP- ribose) polymerases (PARPs) and the NAD + glycohydrolase and cyclic ADP- ribose synthases CD38, CD157 and Sarm1). Intracellular nicotinamide phosphoribotransferase (INAMPT) circulates NAM to nicotinamide mononucleotide (NMN), a portion of which enters the cell via SLC12A8 transporter and is then converted to NAD + by different NMNATs. Decreased levels of NAD + in cells during senescence give rise to a range of problems, including inflammageing, neurodegeneration, genomic instability (promoting senescence, apoptosis, and cancer), mitochondrial dysfunction, ROS accumulation, and loss of proteostasis
At present, many measures are used to inhibit NAD + consumption caused by aging or disease, including supplementation with various NAD + precursors such as nicotinamide mononucleotide (NMN) [ 143 , 144 , 145 ] and nicotinamide riboside (NR) [ 146 ], activation of NAMPT activity [ 147 ], and inhibition of CD38 activity [ 148 ]. Notably, Sirtuins, PARPs, and CD38 play active physiological roles in healthy cells. Thus, not every NAD + promotion strategy has a purely beneficial effect on the organism. Increasing NAD + levels by inhibiting PARPs activity reduces the ability of cells to repair DNA damage. Activation of Sirtuins enzyme expression objectively depletes NAD + , but also prolongs the lifespan of mice. In conclusion, to gain a more in-depth and comprehensive understanding of the effects of various NAD + promotion strategies, more clinical studies are needed to promote them for practical applications more safely, effectively, and scientifically.
Aging and disabled macro-autophagy
Autophagy is an indispensable part of cell metabolism that mediates the degradation and elimination of defective cellular components, including damaged nucleic acids, misfolded protein aggregates, abnormal lipids, and organelles, to promote homeostasis, differentiation, development, and survival through lysosomes [ 149 ]. Among the molecular phenotypic changes that occur during cellular aging, autophagy disorder has become an important physiological feature and has a causal relationship with aging-related diseases. Therefore, maintenance of an excellent autophagy process is essential for long-term health.
Cellular processes involved in autophagy
Autophagy is a highly conserved cell clearance pathway that targets macromolecules and organelles, and the integrity of its biological processes is related to the maintenance of cellular tissue homeostasis. Autophagy can be classified into three main types: macro-autophagy, micro-autophagy, and molecular chaperone-mediated autophagy. Specific target substances of autophagy can be divided into glycophagy and lipidophagy, mitochondrial autophagy, endoplasmic reticulum autophagy, nuclear autophagy, heterologous autophagy, and lysosomal autophagy [ 150 ]. These autophagy processes can be summarised as follows: expanded membrane structures (phagocytes) wrap some of the target material, such as defective organelles and misfolded protein aggregates, forming double-membrane sequestering vesicles (autophagosomes). Autophagosomes fuse with lysosomes and release their contents into the lysosomal lumen. The inner membrane of the autophagosome is degraded along with the encapsulated contents, and the resulting macromolecules are released into the cytoplasm for recycling via lysosomal membrane permeases [ 151 ]. Autophagy is a tightly regulated pathway that plays an important role in the regulation of basic metabolic functions, enabling cells to remove damaged or harmful components through catabolism and recycling and maintain the dynamic balance of nutrients and energy. Autophagy is also a major protective mechanism that allows cells to survive multiple stress conditions such as nutrient or growth factor deprivation, hypoxia, ROS, DNA damage, or intracellular pathogens [ 152 ]. In addition, autophagy is involved in many aging-related pathophysiological processes, such as tumours, metabolic and neurodegenerative diseases, and cardiovascular and pulmonary diseases [ 153 ].
Relationship between autophagy and aging
The stability or disturbance of autophagy has a causal relationship with health, aging and disease [ 154 ]. Increasing evidence indicates that intracellular lysosomal proteolytic function is impaired with aging in various model organisms, which impairs autophagic flux, exacerbates cell damage, and promotes the occurrence of aging-related diseases [ 155 ]. In both human clinical studies and rodent models, the expression of autophagy priming-related proteins ATG5-ATG12 and Becn1 decreased with increasing age, whereas the expression of mTOR increased [ 152 ]. The fusion rate of neuronal autophagosomes and lysosomes is decreased in aged mice, and neuronal autophagy is reduced, which further leads to the appearance of misfolded, mislocalized, and aggregated proteins in the nervous system and increases the probability of neurodegeneration [ 156 , 157 ]. These findings suggest a causal relationship between impaired autophagy and aging [ 158 , 159 ]. This conclusion was confirmed in animal models by manipulating key genes regulating autophagy. Thus, an increase in autophagy caused by heredity, gene mutation, or pharmacological intervention can prolong the life of animals. Studies in C. elegans have found that daf-2 inactivation mutations are dependent on autophagy genes, such as bec-1 , lgg-1 , atg-7 , and atg-12 , and that this mutation significantly extends the lifespan of C. elegans [ 160 ]. Enhanced autophagy in aging mice can also activate mitochondrial SIRT3, inhibit oxidative stress and maintain immune memory [ 161 ]. Accordingly, researchers have found more damaged autophagy sites in aging model animals, which manifest as reduced autophagosomes and impaired lysosome fusion or degradation ability, accompanied by the accumulation of abnormal organelles or biological macromolecules in the cell, leading to cell dysfunction and even death. This eventually increases the incidence of age-related diseases, such as neurodegenerative, heart, and metabolic diseases [ 162 ]. Although the important role of autophagy in inhibiting aging and prolonging lifespan has been widely confirmed, excessive upregulation of autophagy under certain physiological conditions may also cause cell metabolic disorders. For example, the overexpression of Rubicon, a negative regulator of autophagy in aged mice, disrupts adipose metabolism in tissue cells, and the lack of serum/glucocorticoid-regulated kinase-1 (sgk-1) leads to increased mitochondrial permeability and enhanced autophagy, which further leads to reduced environmental adaptability in C. elegans and mice [ 163 , 164 ].
In conclusion, aging is accompanied by a decline in autophagy. Enhancing the autophagic ability in aging model animals is essential for maintaining homeostasis of cell metabolic function, prolonging life span, and improving pathological aging and diseases. Concurrently, autophagy is also one of the important regulators in the aging process. Enhancing or restoring autophagy function to a certain extent is beneficial to the health and longevity of various animal models, whereas dysregulation of autophagy in any direction, whether too low or too high, leads to cell defects and a decline in body function.
Aging and stem cell exhaustion
Physiologically, the decline in stem cell regenerative ability is closely related to the degree of senescence, which is manifested in the accumulation of global harmful cell metabolites caused by aging, and also impairs the regenerative ability of stem cells. Conversely, stem cell decline is an important cellular driver of a variety of tissue senescence-related pathophysiologies.
Main causes of stem cell exhaustion
Stem cells are progenitor cells with the potential for self-replication and multidirectional differentiation. Through self-renewal and differentiation, they can produce mature effector cells, replenish and repair damaged organs, and maintain the health and vitality of the human body; thus, they promote a steady state of continuous organisation throughout the life course [ 165 ]. Numerous studies have demonstrated that stem cells play an irreplaceable role in different stages of life. During the growth and development stages, stem cells continue to differentiate into a variety of new cells for growth and development [ 166 ]. During adulthood, stem cells replace senescent or damaged cells to maintain normal physiological metabolism of the organism [ 167 ]. Notably, throughout the life cycle, stem cells can recognise the signals released by aging damaged cells in the body, localise to the place that needs repair and regeneration, and differentiate into cells at that location to achieve an overall improvement of body functions. However, during the aging process, the proportion of stem cells to total cells gradually decreases. The proportion of mesenchymal stem cell cells in the bone marrow during aging is reportedly 200 times lower than that at birth [ 168 ]. Numerous studies have shown that during aging, a series of changes occurs in the tissues and cells of an organism, including increased DNA damage, replication stress, loss of polarity, mitochondrial dysfunction, altered autophagy, and epigenetic disorders, all of which contribute to stem cell aging and exhaustion [ 20 , 21 , 169 , 170 ]. In addition, the stem cell microenvironment (also called “Niche”) plays a crucial role in maintaining and regulating stem cell function and tissue homeostasis [ 171 ]. During aging, stem cells also accumulate in large quantities as the niche changes, and functional differences between “young and old” stem cells can be more dependent on mechanical differences in the stem cell niche, rather than cell-autonomous age-related changes [ 172 ]. This is also risk for stem-cell injection treatments, as the niche itself may need rejuvenation prior to fresh stem cells.
Although stem cells are not affected by replicative senescence, they are still susceptible to damage and accumulate in large quantities during the aging process. Based on the importance of stem cells on the basis of cell lineages, their dysfunction may have a greater impact than that of other cell types [ 173 ]. As aging progresses, stem cells tend to accumulate DNA damage, which reduces their ability to regenerate cell lineages, exhibiting age-related loss of organ function and homeostasis, and increasing the incidence of age-related diseases [ 174 ]. However, little is known about the cause of this damage or the mechanism by which it leads to a decline in senescent stem cell function. In some cases, DNA damage can lead to stem cell apoptosis, aging, and differentiation, thereby reducing stem cell numbers. Studies have also shown that increased ROS levels in aging mesenchymal stem cells and increased ROS expression in haematopoietic and neural stem cells in mice lead to abnormal cell proliferation, tumour-like changes, and decreased self-replication of stem cells [ 175 ]. Similarly, dysregulation of autophagy during aging leads to defects in protein homeostasis, impaired protein folding, and the accumulation of toxic proteins, resulting in cell damage and tissue dysfunction, and stem cells can also be damaged or depleted [ 176 , 177 ]. Increased mitochondrial DNA point mutations and deletions, along with a shortened lifespan and premature aging, result in decreased nutrient uptake by stem cells. Conversely, enhanced mitochondrial function is accompanied by enhanced stem cell function and tissue regeneration [ 178 ]. Epigenetic regulation also plays an important role in the regulation of stem cell function and changes in the epigenome during aging affect the aging process of stem cells [ 179 , 180 ]. DNA methyltransferases balance self-renewal and differentiation in multiple adult stem cell compartments [ 181 ]. Conditional knockout of DNA methyltransferases results in reduced proliferation, abnormal differentiation, and impaired self-renewal of stem cells [ 182 ]. In addition, proper histone modification is necessary for stem cell self-renewal, and the activity of histone acetyltransferases is important for maintaining the homeostasis and function of neural stem and progenitor cells [ 182 , 183 ]. In summary, stem cells are the source cells of organism renewal, and their function is affected by many microenvironmental factors in senescent cells; therefore, stem cell senescence is closely related to the drivers of aging, health, and longevity.
Relationship between stem cell exhaustion and aging
During the aging process, a decrease in stem cell number and function is closely related to a decline in tissue function and repair ability. In recent years, with the development of new molecular techniques, such as single-cell transcriptomics, lineage tracing, and clonal analysis, scientists have discovered the commonality and heterogeneity of stem cell senescence across tissues. Particularly, the ability of stem cells to produce offspring is impaired during aging. The number of activated neural stem cells and mature nerve cells (offspring) decreases with age, with older haematopoietic stem cells producing fewer lymphoid cells that are dynamically activated and differentiate more slowly [ 184 ]. The fate and behaviour of stem cells in senescent tissues are abnormal, and they may be in a senescent, over-activated, or abnormal differentiation state [ 185 ]. In addition, somatic cells in the stem cell pool are more susceptible to mutations and clonal competition during aging, while the heterogeneity of the resting stem cell pool increases, and the ability to produce established offspring decreases [ 186 ]. Specific age-related transcriptomic and proteomic markers accumulate in senescent stem cells and induce the infiltration of different types of immune cells into the stem cell microenvironment. Researchers have also found that clonal expansion of T-and B-cell infiltrates occurs in the aging brain tissue of mice and humans, and this infiltrate is more pronounced in age-related diseases [ 187 , 188 ].
In summary, stem cells, as primitive and undifferentiated cells, possess a strong regenerative capacity and are essential for environmental homeostasis and organ regeneration in mammalian tissues. However, the number of stem cells continues to decrease with age, and their ability to self-renew and differentiate decreases, leading to impaired tissue or organ regeneration. Therefore, stem cell senescence is closely associated with aging. Because of the important role of stem cells in maintaining functional homeostasis during aging, they have attracted considerable attention in the fields of disease therapy, regenerative medicine, and new drug development.
Aging and cellular senescence
Senescent cells in tissues and organs are thought to be essential not only for the aging process but also for the onset of chronic diseases [ 189 ]. During aging, cells exposed to metabolic, genotoxic, or oncogene-induced stress undergo a basically irreversible cell cycle arrest called cellular senescence [ 190 ]. A major phenotype of senescent cells and how they are thought to promote disease is an increase in inflammatory mediators, mainly cytokines and chemokines, known as the SASP, it causes dynamic equilibrium damage by interfering with stem cell regeneration, tissue and wound repair, and inflammation [ 191 ]. As the number of senescent cells increases with age, cell senescence has been associated with several age-related diseases, the elimination of senescent cells with drugs for aging may be an effective treatment for several previously untreatable diseases [ 192 ].
Main causes of cellular senescence
Cell senescence is a kind of cell state caused by stress injury and some physiological processes, which is characterized by irreversible cell cycle arrest, accompanied by secretory features, macromolecular damage and metabolic changes, these functions can depend on each other to jointly drive the aging process [ 193 ]. Cell senescence may be an alarmist response to deleterious stimuli or aberrant proliferation, including cell cycle exit quiescence and terminal differentiation. Quiescence is a state of temporary arrest in which proliferation can be restored with appropriate stimulation; terminal differentiation is the acquisition of specific cellular functions, accompanied by persistent cell cycle arrest mediated by pathways distinct from cellular senescence [ 194 ]. In senescent cells, the cyclin-dependent kinase (CDK2) inhibitor P21 WAF1/CIP1 (CDKN1A) and the CDK4/6 inhibitor p16 INK4A (CDKN2A) accumulate, this accumulation leads to sustained activation of retinoblastoma (RB) family proteins, inhibition of E2F transactivation, and subsequent cell cycle arrest [ 195 ]. ARF (an alternative reading frame protein at the P16 INK4A gene locus that activates p53) has an important role in regulating cell cycle arrest [ 196 ]. In addition, cell cycle arrest is also characterized by defects in ribosome biogenesis and retrotransposon [ 197 ]. Senescent cells secrete a number of cytokines, including proinflammatory cytokine and chemokines, growth regulators, angiogenic factors and matrix metalloproteinase, collectively known as the SASP or the senescence information secretory group. SASP has been recognized as a marker of senescent cells and mediates many pathophysiological effects [ 198 ]. In addition, DNA damage, telomere depletion, epigenetic changes, protein damage, lipid damage, dysfunction of mitochondria and lysosomes, ROS and inflammation are all important inducers of cell senescence [ 199 ].
Relationship between cellular senescence and aging
Aging is a complex biological process, which is closely related to cell function [ 200 ]. Over the past few decades, a growing body of research has found that cellular senescence is a key driver of many age-related diseases. Excessive accumulation of senescent cells may lead to many chronic diseases and accelerate organ aging. This process not only affects health, but also promotes mutual cellular and physical aging.
Senescent cells exhibit abnormally high levels of damage accumulation, including DNA damage, telomere dysfunction, mitochondrial dysfunction and ROS accumulation [ 201 ]. Elimination of senescent cells reduces the number of cells with the highest degree of damage [ 191 , 198 ]. Therefore, treatment methods that improve or delay cellular senescence characteristics are important strategies for delaying aging. Studies found that after clearance of senescent cells, lower levels of telomere-associated foci were detected in aortic epithelial and hepatocytes of aging mice [ 202 , 203 ]. Similarly, after genetic or pharmacological clearance of senescent cells in mice with chemotherapy-or whole-body irradiation-induced senescence, a reduction in cells bearing persistent DNA damage was observed [ 204 ]. Notably, after clearance of senescent cells, the above evidence relates only to DNA damage, and no data currently provide insights into protein or lipid damage or other senescence-associated phenotypes after clearance of senescent cells.
A new class of drugs, “Senolytics,” eliminate senescent cells by inhibiting a targeted pathway that ultimately damages cell apoptosis [ 205 ]. The senolytic approach aims to selectively eliminate senescent cells, with a pioneering study showing that about 30% of senescent cells are cleared and that heart, kidney and adipose tissue function is improved [ 206 , 207 ]. Subsequent research has focused on specific age-related diseases such as frailty, Idiopathic pulmonary fibrosis, arteriosclerosis, osteoporosis, liver steatosis, and osteoarthritis, in these cases senescent cell clearance has been shown to be beneficial [ 208 ]. Notably, elimination of senescent cells has been shown to alleviate age-related diseases, but not necessarily successfully delay aging. Aging is generally considered a negative phenomenon, but in the context of disease-free aging, senescent cells can retain at least part of their pre-senescent phenotype and function [ 209 ]. The elimination of senescent cells will also result in the filling of the empty space by new cells, which requires the proliferation of stem cells or other resident cells, which may lead to the depletion of their regenerative potential and replication of senescence [ 198 ]. Cellular senescence appears to be a trade-off between tissue function and the risk associated with injury accumulation. Therefore, the therapeutic strategy of intermittent short-term clearance of senescent cells provides a new perspective to solve this problem. In summary, the rate at which cellular damage accumulates determines the rate at which cells senescence, and implies the rate at which the aging process occurs in a disease/healthy state. It is reasonable to understand that proper elimination of senescent cells may be an effective strategy to control aging-related diseases and delay aging.
Other possible causes of aging
Aging is a complex biological process characterised by age-related adaptive decline and a decline in organic physiological systems and metabolic pathways. In addition to the major contributors to aging that we have reviewed, factors such as inflammation, loss of epigenetic information, resurrection of endogenous retroviruses, loss of protein balance, deregulated nutrient sensing, altered intercellular communication, and tissue dysbiosis are also important drivers of accelerated aging.
Many studies have demonstrated that chronic inflammatory response activates the nuclear factor (NF)-κB signalling pathway, a key intracellular signalling pathway for inflammation, which then influences the fate of tissue cells towards senescence by regulating the downstream mechanistic target of rapamycin (mTOR) pathway, insulin and insulin-like growth factor pathways, the AMPK, Sirtuin, forkhead box O families, and p53-related pathways [ 12 , 210 , 211 , 212 , 213 ]. The loss of epigenetic information is also an important cause of aging in mammals. In yeast, epigenetic information is lost over time due to the relocalization of chromatin-modifying proteins to DNA breaks, causing cells to lose their identity, a hallmark of yeast aging [ 214 ]. It was reported the act of faithful DNA repair advances aging at physiological, cognitive, and molecular levels, including erosion of the epigenetic landscape, cellular exdifferentiation, senescence, and advancement of the DNA methylation clock, which can be reversed by OSK-mediated rejuvenation [ 215 ]. The latest research has found that the resurrection of endogenous retroviruses is a hallmark and driving force of cellular senescence and tissue aging. Activation of endogenous retroviruses has been observed in organs of elderly primates and mice, as well as in human tissues and serum of elderly individuals. Their inhibition alleviates cellular aging and tissue degradation, and to some extent, alleviates the aging of the body [ 216 ].
In addition, a decline in the activity of the protein-folding chaperone network and loss of intracellular protein balance are important markers of aging [ 217 ]. Decreased Sirtuin1 and HSP70 levels in senescent cells impair protein homeostasis and heat shock response [ 22 , 218 ]. A growing body of literature has also shown that the ubiquitin-proteasome system is the main non-lysosomal pathway by which cells control protein degradation, either by promoting central lifespan regulators or by aberrant folding and degradation of damaged proteins, and it plays a potential role in regulating the aging process [ 219 , 220 ]. Aging-induced telomere shortening, mitochondrial dysfunction, and DNA damage affect cellular nutrient perception [ 221 , 222 , 223 ]. Aging is also associated with progressive changes in cell-to-cell communication, including factors in the blood-borne system that promote aging or prolong life, interaction of different communication systems between cells, and interference of two-way extracellular matrix communication during aging [ 223 ]. Among the blood-derived factors with pro-aging effects, chemokines CCL11, eosinophil granulocyte, and inflammation-related protein b2-microglobulin can reduce neurogenesis, IL-6 and transforming growth factor β can inhibit the haematopoietic system, and complement factor C1q can affect muscle repair [ 25 , 26 ]. Notably, these blood-derived factors are secreted in the context of SASP, and may contribute to the “infectious” aging phenomenon. Moreover, it has been demonstrated that the anti-aging blood transmissible factors in the blood of young mice can effectively restore the renewal and repair ability of old mice, and reduce the expression of age-related genes [ 224 ]. Cell-to-cell communication also involves short-term从 n extracellular molecules, including ROS, nitric oxide, nucleic acids, prostaglandins, and other lipophilic molecules [ 25 ]. The interactions between soluble factors released from different tissues may also play a role in pro- or anti-aging effects during the aging process. In essence, all of the above-mentioned causes of senescence can lead to dynamic equilibrium disorder within the cell, thus providing a stable proliferative arrest response to various stressors -- cellular senescence. Although senescence promotes programming during development and wound healing, it also limits tumor initiation. However, dynamic equilibrium disorders within senescent cells and their production of large amounts of SASP will induce an inflammatory state that triggers local and systemic inflammation and tissue damage. The pathological accumulation of senescent cells is also associated with a range of diseases and age-related diseases across the organ system. In preclinical and clinical models of aging and chronic diseases, therapeutic approaches that induce apoptosis of senescent cells or inhibit the senescence-associated secretory phenotype have been shown to be pharmacological targets for delaying systemic aging in the body.
Changes in the gut microflora during aging have also attracted great interest from scientists. The gut microbiota is involved in many physiological processes, such as digestion and absorption of nutrients, protection from pathogens, and production of essential metabolites, including vitamins, amino acid derivatives, secondary bile acids, and short-chain fatty acids [ 225 ]. The gut microbiota also signals to peripheral and central nervous system organs and other distant organs and has a strong impact on the overall maintenance of host health [ 226 ]. The interruption of the two-way communication between bacteria and the host leads to biological disorders and causes various pathological conditions, such as obesity, type 2 diabetes, ulcerative colitis, neurological disease, cardiovascular disease, and cancer [ 28 , 227 ]. In addition, vitamin D and magnesium deficiency is associated with aging-related diseases [ 228 ]. Vitamin D aids in magnesium absorption, and magnesium helps in the synthesis and activation of vitamin D in the body [ 229 ]. This interaction reduces the formation of age-related insoluble proteins, and a deficiency of either vitamin D or magnesium can affect muscle, bone, nerve and heart health.
In summary, many studies have linked biological processes such as telomere dynamics, DNA damage response, mitochondrial dysfunction, NAD + loss, autophagy dysregulation, stem cell exhaustion, inflammation, loss of protein balance, dysregulation of nutrient sensing, changes in cell-to-cell communication, and dysbiosis to the triggers behind the characteristics of aging. Aberrant perturbations in these biological processes create feedback loops that amplify the aging phenotype, accelerate the aging process, and increase the incidence of age-related diseases. However, in the early stage of development, the above-mentioned physiological characteristics of aging to a certain extent also promote the healthy development of juvenile factors. For example, the activation of nutrient-sensing signals during early development contributes to organ development in adolescents; and the activation of nutrient-sensing signals after aging has a largely pro-aging effect [ 230 , 231 ]; Low-dose mitochondrial dysfunction can stimulate cells to engage in a beneficial antagonistic response through mitosis [ 232 ]; appropriate levels of cellular senescence contribute to inhibition of tumour generation and promote wound healing [ 233 ]. Therefore, it is an important prerequisite to understand the physiological characteristics of aging and its molecular mechanism in specific life stages and physiological states.
- Anti-aging strategies
The interaction and mutual promotion between aging triggers, aging phenotypic characteristics, and inherited or acquired age-related diseases have become key hotspots of interest for scientists to explore anti-aging strategies. To date, there have been a wide range of interventions aimed at mitigating aging or aging-related morbidity, including dietary regulation and caloric restriction, improved sleep quality, enhanced physical activity, altered microbiota, and exogenous active molecular interventions targeting specific senescence-promoting molecular targets.
The role of diet and calorie-restriction in delaying aging
Diet is strongly correlated with longevity and disease development. In rodents and humans, the excessive intake of high-calorie foods increases body fat production, fat storage, and obesity. People with obesity are more likely to develop symptoms such as elevated insulin, blood sugar, cholesterol, and triglyceride levels during aging, and a combination of these factors can activate the aging pathway and accelerate the aging process, leading to disease and death [ 234 ]. Studies based on diet regulation and calorie restriction have shown that moderate calorie restriction in mice modestly extends the lifespan and improves metabolic, cerebrovascular, and cognitive function indices [ 235 , 236 , 237 ]. Dietary restriction improves cognition and reduces plaque burden in mice with Alzheimer’s disease through a mechanism related to mitochondrial function in hippocampal neurons [ 238 ]. Researchers recently found that controlling the intake of nutrients, including total calories and macronutrient balance, had a greater effect on aging and metabolic health than the three commonly used life-extension drugs (metformin, sirolimus, and resveratrol) [ 239 , 240 ]. A recent study also showed that moderate caloric restriction can reduce the production of acidic and cysteine-rich (SPARC) proteins, which are associated with aging, and extend the healthy lifespan of older people, further confirming that improving the diet is an important way to live a long and healthy life [ 241 ].
The role of sleep quality in delaying aging
Sleep is an important factor in the recovery and improvement of physiological systems, including metabolism, endocrine function, immune response, and brain metabolism. Poor sleep accelerates aging and increases the incidence of age-related diseases, such as cognitive decline, Alzheimer’s disease, haematopoietic stem cell dysfunction, and coronary heart disease. Numerous studies have reported that improving the quantity and quality of sleep can be considered as an anti-aging treatment that can prevent, slow, or even reverse the physical decline and degeneration associated with the aging process [ 242 ]. One clinical trial reported a significant association between better quality and quantity of sleep and increased plasma levels of S-Klotho, a gene family known as a senescence suppressor that is overexpressed to prolong life [ 243 ]. In addition, researchers followed 411 volunteers for eight years and found that poor sleep quality may be a new modifiable risk factor for coronary heart disease in older adults, independent of traditional cardiovascular risk factors [ 244 ]. In human trials, adequate sleep has been found to regulate the epigenome of haematopoietic stem and progenitor cells, inhibit inflammatory output, and maintain clonal diversity to slow the decline in the hematopoietic system [ 245 ]. Sleep and health undergo age-related changes throughout life, and the impact of sleep deprivation in older people is particularly important [ 246 , 247 , 248 ]. However, to improve sleep quality in the older people and reduce age-related sleep issues, further research is needed to investigate the relevant mechanisms.
The role of exercise in delaying aging
Physical activity causes a series of integrated physiological responses in many tissues in the entire animal kingdom and has been widely accepted to improve the health of physiological tissues. Exercise is strongly associated with changes in plasma microsecretory factors, such as immunomodulatory cytokines, regulatory T cells in lymphoid organs, and inflammatory monocytes. Increased physical activity in aged mice significantly slowed cognitive aging and neurodegeneration, and these improvements were associated with the reduced expression of neuroinflammatory genes in the hippocampus. Proteomics of plasma from exercising mice has revealed a significant increase in the complement cascade inhibitor rapamycin, which binds to brain endothelial cells and reduces the expression of neuroinflammatory genes in mice with acute encephalitis and Alzheimer’s disease [ 249 ]. In addition, exercise can induce hippocampal precursor cell proliferation in aged mice by activating platelets, that is, increasing the systemic levels of the platelet-derived exerkine CXCL4/platelet factor 4 (PF4) ameliorates age-related regenerative and cognitive impairments in a hippocampal neurogenesis-dependent manner [ 250 ]. Although the benefits of exercise have been demonstrated in several studies, older people are less likely to exercise if they are physically weak or have poor health status. Based on this, researchers were able to ameliorate age-related neurogenesis and cognitive impairment in the hippocampus in the older people by systematically administering plasma from exercising mice, and the key molecular target of this regulation was identified; exercise increased the concentration of liver-derived glycosylphosphatidylinositol-specific phospholipase D1 in the plasma of aged mice [ 251 ]. Similarly, many studies have found that exercise can enhance myocardial contractility [ 252 ], improve heart pumping function and heart ejection fraction [ 253 ], improve blood supply [ 254 ] and oxygen supply [ 255 ] in all organ systems of the body, and speed up metabolism [ 256 ], prolonging the lifespan of cells, thereby slowing the aging process of organs and skin.
The role of exogenous active molecules in delaying aging
Lifestyle changes, including calorie restriction, sleep regulation, and exercise, are insufficient to extend the healthy lifespan of older people or prevent age-related diseases. Therefore, many studies have focused on the mechanisms underlying the aging process and explored ways to target the hallmark features of aging. Currently, the most promising mechanisms for preventing senescence include inhibition of the mTORC1 signalling pathway [ 257 ], clearance of senescent cells [ 258 ], and the use of natural metabolites to rejuvenate stem cells [ 221 ]. Therefore, the development of synthetic or natural small-molecule compounds that inhibit these signature features is a promising anti-aging strategy.
To date, many synthetic or natural small-molecule compounds have been reported to have the potential to genetically protect or regulate senescence in one or more model species (Table 1 ). Among them, studies on synthetic compounds including metformin, klotho, PF4, hyaluronan acid, taurine, acarbose, rapamycin, spermidine, NAD + enhancers, nonsteroidal anti-inflammatory drugs, lithium, reverse transcriptase inhibitors, systemic circulating factors, glucosamine, glycine, and 17α-oestradiol, focused on telomere attrition, DNA damage, mitochondrial dysfunction, NAD + loss, disabled macro-autophagy, stem cell exhaustion, regulation of tissue cell perception of nutrients, cell-to-cell communication, and improved stem cell function. By improving the interactions among the hallmarks of aging, these synthetic compounds can eventually alleviate or even reverse the decline in age-related bodily functions.
Senescent cells cannot continue to divide or die in tissues, and secrete a range of pro-inflammatory factors that may recruit inflammatory cells to reshape the extracellular environment, induce abnormal cell death and fibrosis, and inhibit stem cell function [ 258 ]. Senescent cells are also closely related to the pathogenesis of osteoporosis, atherosclerosis, hepatic steatosis, pulmonary fibrosis, and osteoarthritis. Therefore, anti-aging methods targeting senescent cells are also very important anti-aging strategies and can be divided into two categories: senescent cell lytic agents (senolytics), whose role is to clear senescent cells, and compounds that combat the effects of various cytokines secreted by senescent cells. Currently, senolytics class targeting senescent cells and SASP include natural polyphenol extract, kinase inhibitors, BCL-2 family inhibitors, heat shock protein inhibitors, BET family protein degraders, P53 stabilizers, cardiac steroids, PPRα agonists and antibiotics.
Other anti-aging strategies
Heterochronic blood exchange models have shown that the blood of aging mice accelerates the aging of tissues and cells in young mice [ 304 , 305 ]. Based on this, the researchers showed that systemic exposure of aged male mice to a fraction of blood plasma from young mice containing platelets decreased neuroinflammation in the hippocampus at the transcriptional and cellular level and ameliorated hippocampal-dependent cognitive impairments [ 263 ]. Despite the growing number of reports about heterochronic blood exchange research, it still faces great challenges and risks in the field of anti-aging, both at the technical level and in the application of safety. In addition, mesenchymal stem cell therapy has been shown to improve frailty and facial skin aging [ 306 ]. Intravenous mesenchymal stem cell may be an effective treatment for frailty in the elderly. However, the safety and efficacy of stem cell therapy remain controversial, and more studies are needed to verify it. Studies have found that deliberate cold exposure can enhance the nervous system, as well as injury and speed recovery [ 307 ]. Saunas are effective at activating cell longevity and anti-cancer factors through heat, this may be a useful tool for people who are too old to engage in physical activity [ 308 ], but its physiological metabolic mechanisms and safety thresholds remain to be further confirmed.
Conclusions and perspectives
The physiological characteristics of aging summarised in this article gradually accumulate over time and contribute to the aging process. Notably, antagonism of an organism’s response to the characteristics of aging also plays a subtle role in the aging process. When the cumulative damage caused by primary and antagonistic markers is no longer compensated for by the complex markers of aging, it means that the rate of aging is accelerated. Furthermore, senescence also relies on the integration of cell-autonomous and non-cell-autonomous mechanisms, and mechanisms that promote senescence can be transmitted between different types of organs and cells. In a metachronous experiment linking the vasculature of young and old mice, extensively characterised by single-cell transcription levels, a spatiotemporal map of the ability of the young system to rejuvenate the senescent system was confirmed, and vice versa; the factors that promote aging have the ability to accelerate the aging of young cells. This may explain why programmed aging usually affects multiple organs in a nearly synchronous manner, causes a comprehensive systemic decline in physiological function, and is an important factor that induces pathological aging.
In conclusion, aging is a gradual and complex process of decline in physiological function, and experiments in animal models have shown that certain interventions may not only extend lifespan, but also increase healthy longevity. However, in vitro models, tissue culture studies, and in vivo animal models, which are ultimately translated into human studies, are complex and diverse, and only a few models can be used to investigate these differences. There are also significant differences between physiological and pathological aging, and the scientific problem of slowing down aging and extending the healthy lifespan of humans involves a number of challenges, including inadequate regulation, barriers to clinical validation, failure to identify more biomarkers of human aging, and the unknown challenges of introducing new interventions to the market. It is gratifying that years of basic research in the anti-aging field have laid the foundation for explosive biotechnology and industrial applications. In a recent report, researchers used a “visual genetic circuit” to control two pathways of yeast aging, alternative approaches including the lysine deacetylase Sir2-related pathway and the haeme-activating protein (HAP)-related pathway, successfully extending yeast lifespan by 82% [ 309 ]. Hence, using modern biological techniques, including genetic manipulation or cell-based therapies with broad implementation prospects, to focus on the discovery of physiological mechanisms and interventions underlying the aging process will greatly advance anti-aging research, delay human aging to the maximum extent, maintain human physiological functions in later years, and mitigate the surge in age-related chronic diseases.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
Nicotinamide adenine dinucleotide
DNA damage response
Telomerase reverse transcriptase
Telomerase RNA component
Reactive oxygen species
Senescence-associated secretory phenotype
Adenosine triphosphate
Adenosine 5’-monophosphate
Tricarboxylic acid
Nicotinamide
Nicotinamide phosphoribose transferase
Poly ADP-ribose polymerases
5'-AMP-activated protein kinase
Platelet factor 4
Colchero F, et al. The long lives of primates and the “invariant rate of ageing” hypothesis. Nat Commun. 2021;12:3666.
Giaimo S, Traulsen A. The selection force weakens with age because ageing evolves and not vice versa. Nat Commun. 2022;13:686.
Gokbilen SO, Becer E, Vatansever HS. Senescence-mediated anticancer effects of quercetin. Nutr Res. 2022;104:82–90.
Article Google Scholar
Zhang KX, et al. The promotion of active aging through older adult education in the context of population aging. Front Public Health. 2022;10:998710.
Lehr U. Social- and behavioral-scientific gerontology; age and ageing as a social problem and individual theme. Z Gerontol Geriatr. 2005;38(3):218–218.
Google Scholar
Shabashova NI, et al. Demographic, social and medical problems of the aging population. Vopr Onkol. 2001;47(5):523–35.
PubMed Google Scholar
Aguilar-Hernandez L, et al. Cellular mechanisms in brain aging: Focus on physiological and pathological aging. J Chem Neuroanat. 2023;128:102210.
Sacco A, Belloni L, Latella L. From Development to Aging: The Path to Cellular Senescence. Antioxid Redox Signal. 2021;34(4):294–307.
Article CAS PubMed PubMed Central Google Scholar
Aunan JR, et al. Molecular and biological hallmarks of ageing. Br J Surg. 2016;103(2):e29-46.
Article CAS PubMed Google Scholar
Hao LY, et al. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123(6):1121–31.
Ding Z, et al. Telomerase reactivation following telomere dysfunction yields murine prostate tumors with bone metastases. Cell. 2012;148(5):896–907.
Zhao Y, et al. DNA damage and repair in age-related inflammation. Nat Rev Immunol. 2023;23(2):75–89.
Stead ER, Bjedov I. Balancing DNA repair to prevent ageing and cancer. Exp Cell Res. 2021;405(2):112679.
Yang J, et al. Progress in Understanding Oxidative Stress, Aging, and Aging-Related Diseases. Antioxidants. 2024;13(4):394.
Zhao Q, et al. Targeting Mitochondria-located circRNA SCAR alleviates NASH via reducing mROS output. Cell. 2020;183(1):76-e9322.
Igarashi M, et al. NAD(+) supplementation rejuvenates aged gut adult stem cells. Aging Cell. 2019;18(3):e12935.
Article PubMed PubMed Central Google Scholar
Xie N, et al. NAD(+) metabolism: pathophysiologic mechanisms and therapeutic potential. Signal Transduct Target Ther. 2020;5(1):227.
Kaushik S, et al. Autophagy and the hallmarks of aging. Ageing Res Rev. 2021;72:101468.
Cassidy LD, Narita M. Autophagy at the intersection of aging, senescence, and cancer. Mol Oncol. 2022;16(18):3259–75.
Kalamakis G, et al. Quiescence modulates stem cell maintenance and regenerative capacity in the aging brain. Cell. 2019;176(6):1407-e141914.
Schneider JL, et al. The aging lung: physiology, disease, and immunity. Cell. 2021;184(8):1990–2019.
Holwerda AM, et al. Dose-Dependent increases in whole-body net protein balance and dietary protein-derived amino acid incorporation into myofibrillar protein during recovery from Resistance Exercise in older men. J Nutr. 2019;149(2):221–30.
Slack C, et al. The ras-Erk-ETS-Signaling pathway is a drug target for longevity. Cell. 2015;162(1):72–83.
Villeda SA, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90-U157.
Yang BA, et al. Engineered Tools to Study Intercellular Communication. Adv Sci. 2021;8(3):2002825.
Fafian-Labora JA, O’Loghlen A. Classical and Nonclassical Intercellular Communication in Senescence and Ageing. Trends Cell Biol. 2020;30(8):628–39.
Article PubMed Google Scholar
DeJong EN, Surette MG, Bowdish DME. The gut microbiota and unhealthy aging: disentangling cause from Consequence. Cell Host Microbe. 2020;28(2):180–9.
Alsegiani AS, Shah ZA. The influence of gut microbiota alteration on age-related neuroinflammation and cognitive decline. Neural Regeneration Res. 2022;17(11):2407–12.
Zhou MG, et al. Aging and Cardiovascular Disease: Current Status and Challenges. Rev Cardiovasc Med. 2022;23(4):135.
Juttukonda MR, Donahue MJ. Neuroimaging of vascular reserve in patients with cerebrovascular diseases. NeuroImage. 2019;187:192–208.
O’Brien MS, McDougall JJ. Age and frailty as risk factors for the development of osteoarthritis. Mech Ageing Dev. 2019;180:21–8.
Rezus E, et al. The Link Between Inflammaging and Degenerative Joint Diseases. Int J Mol Sci. 2019;20(3):614.
Wang XR, Hu JJ, Wu DP. Risk factors for frailty in older adults. Medicine. 2022;101(34):e30169.
Berardelli I, et al. Suicide in Parkinson’s Disease: a systematic review. Cns Neurol Disorders-Drug Targets. 2019;18(6):466–77.
Article CAS Google Scholar
Bloem BR, Okun MS, Klein C. Parkinson’s Disease Lancet. 2021;397(10291):2284–303.
CAS PubMed Google Scholar
Heavener KS, Bradshaw EM. The aging immune system in Alzheimer’s and Parkinson’s diseases. Semin Immunopathol. 2022;44(5):649–57.
Wang S, et al. Cancer Treatment-Induced Accelerated Aging in Cancer Survivors: Biology and Assessment. Cancers. 2021;13(3):427.
Chatsirisupachai K, Lagger C, Magalhaes JPD. Age-associated differences in the cancer molecular landscape. Trends Cancer. 2022;8(11):962–71.
Bhatia R, et al. Do Cancer and Cancer treatments accelerate aging? Curr Oncol Rep. 2022;24(11):1401–12.
Gorbunova V, et al. The role of retrotransposable elements in ageing and age-associated diseases. Nature. 2021;596(7870):43–53.
Peters A, Nawrot TS, Baccarelli AA. Hallm Environ Insults Cell. 2021;184(6):1455–68.
CAS Google Scholar
Petr MA, et al. Protecting the aging genome. Trends Cell Biol. 2020;30(2):117–32.
Carloni V, et al. The Adaptability of Chromosomal Instability in Cancer Therapy and Resistance. Int J Mol Sci. 2023;24(1):245.
Balzano E, Giunta S. Centromeres under Pressure: Evolutionary Innovation in Conflict with Conserved Function. Genes. 2020;11(8):912.
Coluzzi E, Leone S, Sgura A. Oxidative Stress Induces Telomere Dysfunction and Senescence by Replication Fork Arrest. Cells. 2019;8(1):19.
Tiwari M, Parvez S, Agrawala PK. Role of some epigenetic factors in DNA damage response pathway. AIMS Genet. 2017;4(1):69–83.
Zheng GQ, Fu Y, He C. Nucleic acid oxidation in DNA damage repair and epigenetics. Chem Rev. 2014;114(8):4602–20.
Ping Y, Zhifang LI, Wen C. Epigenetic mechanisms of DNA damage and repair. J Med Mol Biology. 2009;6(5):459–62.
Fischer KE, Riddle NC. Sex differences in aging: genomic instability. Journals Gerontol Ser a-Biological Sci Med Sci. 2018;73(2):166–74.
Li WT, et al. Epigenetic Regulation of Nucleotide Excision Repair. Front Cell Dev Biology. 2022;10:847051.
Zhang JW. Brothers in arms: emerging roles of RNA epigenetics in DNA damage repair. Cell Bioscience. 2017;7(1):24.
Kaufmann W. Epigenetics and the DNA damage response. Environ Mol Mutagen. 2012;53:S27-27.
Shukla PC, et al. DNA damage repair and cardiovascular diseases. Can J Cardiol. 2010;26:A13-6.
Lin XZ, et al. Contributions of DNA Damage to Alzheimer’s Disease. Int J Mol Sci. 2020;21(5):1666.
Alhmoud JF, et al. DNA Damage/Repair Manage Cancers. Advances in Medical Biochemistry, Genomics, Physiology, and Pathology. 2021:309–39.
Lu T, Xu K. The multifunction of mismatch repair protein. Chem Life. 2008;28(6):696–700.
Monnat RJ. Human RECQ helicases: roles in DNA metabolism, mutagenesis and cancer biology. Sem Cancer Biol. 2010;20(5):329–39.
Diderich K, Alanazi M, Hoeijmakers JHJ. Premature aging and cancer in nucleotide excision repair-disorders. DNA Repair. 2011;10(7):772–80.
Sepe S, et al. Nucleotide excision repair in chronic neurodegenerative diseases. DNA Repair. 2013;12(8):568–77.
Xu SW, Jin ZG. Hutchinson-Gilford Progeria Syndrome: Cardiovascular pathologies and potential therapies. Trends Biochem Sci. 2019;44(7):561–4.
David L, et al. Telomeres and genomic instability during early development. European journal of medical genetics. 2020;63(2):103638.
Hayflick L. HUMAN CELLS AND AGING. Sci Am. 1968;218(3):32.
Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1(1):72–6.
Hayflick L. LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965;37(3):614.
Watson JD. Origin of Concatemeric T7DNA. Nat New Biology. 1972;239(94):197–201.
Olovnikov AM. A theory of marginotomy: the incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973;41(1):181–90.
Bernadotte A, Mikhelson VM, Spivak IM. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging. 2016;8(1):3–11.
Roake CM, Artandi SE. Regulation of human telomerase in homeostasis and disease. Nat Rev Mol Cell Biol. 2020;21(7):384–97.
Benarroch-Popivker D, et al. TRF2-Mediated control of Telomere DNA Topology as a mechanism for chromosome-end Protection. Mol Cell. 2016;61(2):274–86.
Hohensinner PJ, et al. Age intrinsic loss of telomere protection via TRF1 reduction in endothelial cells. Biochim Biophys Acta. 2016;1863(2):360–7.
Yang Z, et al. Break-induced replication promotes fragile telomere formation. Genes Dev. 2020;34(19-20):1392–405.
Diotti R, Loayza D. Shelterin complex and associated factors at human telomeres. Nucleus. 2011;2(2):119–35.
Van SB, De LT. Control of telomere length by the human telomeric protein TRF1. Nature. 1997;385(6618):740–3.
Dimitrova N, et al. 53BP1 promotes non-homologous end joining of telomeres by increasing chromatin mobility. Nature. 2008;456(7221):524–8.
Frank AK, et al. The shelterin TIN2 subunit mediates recruitment of telomerase to telomeres. PLoS Genet. 2015;11(7):e1005410.
Bhanot M, Smith S. TIN2 Stability is regulated by the E3 ligase Siah2. Mol Cell Biol. 2012;32(2):376–84.
Zeng L, et al. Construction of the POT1 promoter report gene vector, and the effect and underlying mechanism of the POT1 promoter in regulating telomerase and telomere length. Oncol Lett. 2017;14(6):7232–40.
Kibe T, et al. Telomere Protection by TPP1 is mediated by POT1a and POT1b. Mol Cell Biology. 2010;30(4):1059–66.
Lototska L, et al. Human RAP1 specifically protects telomeres of senescent cells from DNA damage. EMBO Rep. 2020;21(4):e49076.
Platt JM, et al. Rap1 relocalization contributes to the chromatin-mediated gene expression profile and pace of cell senescence. Genes Dev. 2013;27(12):1406–20.
Wu RA, et al. Telomerase mechanism of Telomere Synthesis. Annu Rev Biochem. 2017;86(1):439.
Jiang J, et al. The architecture of Tetrahymena telomerase holoenzyme. Nature. 2013;496(7444):187–92.
Liu BC, et al. Structure of active human telomerase with telomere shelterin protein TPP1. Nature. 2022;604(7906):578.
Maillard, et al. The shelterin complex and hematopoiesis. J Clin Invest. 2016;126(5):1621–9.
Schepers AG, et al. Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J. 2011;30(6):1104–9.
Ko E, Seo HW, Jung G. Telomere length and reactive oxygen species levels are positively associated with a high risk of mortality and recurrence in hepatocellular carcinoma. Hepatology. 2018;67(4):1378–91.
Caporaso GL, et al. Telomerase activity in the subventricular zone of adult mice. Mol Cell Neurosci. 2003;23(4):693–702.
Drayton S, et al. The significance of p16(INK4a) in cell defenses against transformation. Cell Cycle. 2004;3(5):611–5.
Parkinson EK, Newbold RF, Keith WN. The genetic basis of human keratinocyte immortalisation in squamous cell carcinoma development: the role of telomerase reactivation. Eur J Cancer. 1997;33(5):727–34.
Di Donna S, et al. Regenerative capacity of human satellite cells: the mitotic clock in cell transplantation. Neurol Sci. 2000;21(5):S943-51.
Albanell J, et al. Telomerase activity in germ cell cancers and mature teratomas. J Natl Cancer Inst. 1999;91(15):1321–6.
He X, et al. Research progress of telomerase and somatic cell reprogramming. J South Argiculture. 2019;50(5):1133–40.
Henson JD, et al. A robust assay for alternative lengthening of telomeres in tumors shows the significance of alternative lengthening of telomeres in sarcomas and astrocytomas. Clin Cancer Res. 2005;11(1):217–25.
Robinson NJ, Schiemann WP. Means to the ends: the role of telomeres and telomere processing machinery in metastasis. Biochim Et Biophys Acta-Reviews Cancer. 2016;1866(2):320–9.
Heaphy CM, et al. Altered telomeres in tumors with ATRX and DAXX mutations. Science. 2011;333(6041):425.
Marzec P, et al. Nuclear-receptor-mediated telomere insertion leads to genome instability in ALT cancers. Cell. 2015;160(5):913–27.
Yegorov YE, et al. Role of Telomeres Shortening in Atherogenesis: An Overview. Cells. 2021;10(2):395.
Opresko PL, Shay JW. Telomere-associated aging disorders. Ageing Res Rev. 2017;33:52–66.
Freitas-Simoes TM, Ros E, Sala-Vila A. Telomere length as a biomarker of accelerated aging: is it influenced by dietary intake? Curr Opin Clin Nutr Metab Care. 2018;21(6):430–6.
Hong J, Yun CO. Telomere Gene Therapy: Polarizing Therapeutic Goals for Treatment of Various Diseases. Cells. 2019;8(5):392.
Pousa PA, et al. Telomere Shortening and Psychiatric Disorders: A Systematic Review. Cells. 2021;10(6):1423.
Martínez P, et al. Telomere-driven diseases and telomere-targeting therapies. The Journal of cell biology. 2017;216(4):875.
Shay JW, Wright WE. Telomeres and telomerase: three decades of progress. Nat Rev Genet. 2019;20(5):299–309.
Blasco MA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91(1):25–34.
Saretzki G, et al. Telomere shortening triggers a p53-dependent cell cycle arrest via accumulation of G-rich single stranded DNA fragments. Oncogene. 1999;18(37):5148–58.
Karlseder J, et al. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science. 1999;283(5406):1321–5.
Hemann MT, et al. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107(1):67–77.
Wang HT, et al. Impairment of osteoblast differentiation due to proliferation-independent telomere dysfunction in mouse models of accelerated aging. Aging Cell. 2012;11(4):704–13.
Berman AJ, et al. SnapShot: Telomeres and Telomerase. Cell. 2012;151(5):1138.
Berby B, et al. Oxidative Stress Is Associated with Telomere Interaction Impairment and Chromatin Condensation Defects in Spermatozoa of Infertile Males. Antioxidants. 2021;10(4):593.
Erusalimsky JD. Oxidative stress, telomeres and cellular senescence: what non-drug interventions might break the link? Free Radic Biol Med. 2020;150:87–95.
Nalobin D, et al. Telomeres and Telomerase in Heart Ontogenesis, Aging and Regeneration. Cells. 2020;9(2):503.
Razgonova MP, et al. Telomerase and telomeres in aging theory and chronographic aging theory. Mol Med Rep. 2020;22(3):1679–94.
Berman AJ, Cech TR. SnapShot: telomeres and telomerase. Cell. 2012;151(5):1138-e11381.
Victorelli S, Passos JF. Telomeres: beacons of autocrine and paracrine DNA damage during skin aging. Cell Cycle. 2020;19(5):532–40.
Zhu Y, et al. Telomere and its role in the aging pathways: telomere shortening, cell senescence and mitochondria dysfunction. Biogerontology. 2019;20(1):1–16.
Azarm K, et al. Persistent telomere cohesion protects aged cells from premature senescence. Nat Commun. 2020;11(1):3321.
Hebert SL, Lanza IR, Nair KS. Mitochondrial DNA alterations and reduced mitochondrial function in aging. Mech Ageing Dev. 2010;131(7–8):451–62.
Amorim JA, et al. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat Rev Endocrinol. 2022;18(4):243–58.
Weindel CG, et al. Mitochondrial ROS promotes susceptibility to infection via gasdermin D-mediated necroptosis. Cell. 2022;185(17):3214-e323123.
Mottis A, Herzig S, Auwerx J. Mitocellular communication: shaping health and disease. Science. 2019;366(6467):827–32.
Traba J, Satrustegui J, del Arco A. Transport of adenine nucleotides in the mitochondria of Saccharomyces cerevisiae: interactions between the ADP/ATP carriers and the ATP-Mg/Pi carrier. Mitochondrion. 2009;9(2):79–85.
Mikhail AI, et al. AMPK is mitochondrial medicine for neuromuscular disorders. Trends Mol Med. 2023;29(7):512–29.
Wu SN, Zou MH. AMPK, Mitochondrial Function, and Cardiovascular Disease. Int J Mol Sci. 2020;21(14):4987.
Schottlender N, Gottfried I, Ashery U. Hyperbaric Oxygen Treatment: Effects on Mitochondrial Function and Oxidative Stress. Biomolecules. 2021;11(12):1827.
Alfatni A, et al. Peripheral Blood Mononuclear Cells and Platelets Mitochondrial Dysfunction, Oxidative Stress, and Circulating mtDNA in Cardiovascular Diseases. J Clin Med. 2020;9(2):311.
Lima T, et al. Pleiotropic effects of mitochondria in aging. Nat Aging. 2022;2(3):199–213.
Lee J. Mitochondrial drug targets in neurodegenerative diseases. Bioorg Med Chem Lett. 2016;26(3):714–20.
Jin H, et al. Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: preclinical and clinical outcomes. Biochim Et Biophys Acta-Molecular Basis Disease. 2014;1842(8):1282–94.
Covarrubias AJ, et al. NAD(+) metabolism and its roles in cellular processes during ageing. Nat Rev Mol Cell Biol. 2021;22(2):119–41.
Gerner RR, et al. NAD metabolism fuels human and mouse intestinal inflammation. Gut. 2018;67(10):1813–23.
Katsyuba E, et al. NAD(+) homeostasis in health and disease. Nat Metab. 2020;2(1):9–31.
Nakagawa T, Guarente L. SnapShot: sirtuins, NAD, and aging. Cell Metab. 2014;20(1):192-e1921.
Yoshino J, Baur JA, Imai SI. NAD(+) intermediates: the Biology and therapeutic potential of NMN and NR. Cell Metab. 2018;27(3):513–28.
Rajman L, Chwalek K, Sinclair DA. Therapeutic potential of NAD-Boosting molecules: the in vivo evidence. Cell Metab. 2018;27(3):529–47.
Belenky P, Bogan KL, Brenner C. NAD + metabolism in health and disease. Trends Biochem Sci. 2007;32(1):12–9.
Bertoldo MJ, et al. NAD(+) repletion rescues female fertility during Reproductive Aging. Cell Rep. 2020;30(6):1670-e16817.
Schumacher B, et al. The central role of DNA damage in the ageing process. Nature. 2021;592(7856):695–703.
Chini CCS, et al. Evolving concepts in NAD(+) metabolism. Cell Metab. 2021;33(6):1076–87.
Canto C, Menzies KJ, Auwerx J. NAD(+) metabolism and the Control of Energy Homeostasis: a Balancing Act between Mitochondria and the Nucleus. Cell Metabol. 2015;22(1):31–53.
Bai P, Canto C. The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metabol. 2012;16(3):290–5.
Salmina AB, et al. NAD+-converting enzymes in neuronal and glial cells: CD38 as a novel target for neuroprotection. Vestn Ross Akad Med Nauk. 2012;67(10):29–37.
Miao Y, et al. Nicotinamide Mononucleotide supplementation reverses the declining quality of maternally aged oocytes. Cell Rep. 2020;32(5):107987.
Li Y, et al. Alleviation of hepatic insulin resistance and steatosis with NMN via improving endoplasmic reticulum–Mitochondria miscommunication in the liver of HFD mice. Biomedicine & Pharmacotherapy. 2024;175:116682.
Ru M, et al. Nicotinamide mononucleotide supplementation protects the intestinal function in aging mice and D-galactose induced senescent cells. Food & Function. 2022;13(14):7507–19.
Chi YL, Sauve AA. Nicotinamide Riboside, a trace nutrient in foods, is a vitamin B3 with effects on energy metabolism and neuroprotection. Curr Opin Clin Nutr Metab Care. 2013;16(6):657–61.
Yao H, et al. Discovery of small-molecule activators of nicotinamide phosphoribosyltransferase (NAMPT) and their preclinical neuroprotective activity. Cell Res. 2022;32(6):570–84.
Chini EN, et al. The pharmacology of CD38/NADase: an emerging target in Cancer and diseases of Aging. Trends Pharmacol Sci. 2018;39(4):424–36.
Aman Y, et al. Autophagy in healthy aging and disease. Nat Aging. 2021;1(8):634–50.
Ktistakis NT, Tooze SA. Digesting the Expanding mechanisms of Autophagy. Trends Cell Biol. 2016;26(8):624–35.
Yim WW-Y, Mizushima N. Lysosome biology in autophagy. Cell Discovery. 2020;6:6.
Honda S, et al. Association between Atg5-independent alternative autophagy and neurodegenerative diseases. J Mol Biol. 2020;432(8):2622–32.
Ichimiya T, et al. Autophagy and Autophagy-Related Diseases: A Review. Int J Mol Sci. 2020;21(23):8974.
Liu B, Wen X, Cheng Y. Survival or death: disequilibrating the oncogenic and tumor suppressive autophagy in cancer. Cell Death Dis. 2013;4(10):e892.
Kang C, Elledge SJ. How autophagy both activates and inhibits cellular senescence. Autophagy. 2016;12(5):898–9.
Ou-Yang P, Cai ZY, Zhang ZH. Molecular Regulation Mechanism of Microglial Autophagy in the Pathology of Alzheimer’s Disease. Aging and Disease. 2023;14(4):1166.
Menzies FM, Fleming A, Rubinsztein DC. Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci. 2015;16(6):345–57.
Rubinsztein DC, Marino G, Kroemer G. Autophagy Aging Cell. 2011;146(5):682–95.
Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42.
Sun YN, et al. Lysosome activity is modulated by multiple longevity pathways and is important for lifespan extension in C. elegans. Elife. 2020;9:e55745.
Fernandez AF, et al. Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature. 2018;558(7708):136.
Zhong ZY, Sanchez-Lopez E, Karin M. Autophagy, inflammation, and immunity: a troika governing Cancer and its treatment. Cell. 2016;166(2):288–98.
Kumsta C, et al. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. Elegans. Nat Commun. 2017;8:14337.
Kumsta C, Hansen M. Hormetic heat shock and HSF-1 overexpression improve C. Elegans survival and proteostasis by inducing autophagy. Autophagy. 2017;13(6):1076–7.
Ono N, Balani DH, Kronenberg HM. S tem and progenitor cells in skeletal development, in Vertebrate Skeletal Development, B.R. Olsen, Editor. 2019. p. 1–24.
Leeanansaksiri W, Dechsukhum C. Regulation of stem cell fate in hematopoietic development. J Med Association Thail = Chotmaihet Thangphaet. 2006;89(10):1788–97.
Plasschaert RN, Bartolomei MS. Genomic imprinting in development, growth, behavior and stem cells. Development. 2014;141(9):1805–13.
Navarro Negredo P, Yeo RW, Brunet A. Aging and rejuvenation of neural stem cells and their niches. Cell Stem Cell. 2020;27(2):202–23.
Bigot A, et al. Age-Associated methylation suppresses SPRY1, leading to a failure of re-quiescence and loss of the Reserve Stem Cell Pool in Elderly muscle. Cell Rep. 2015;13(6):1172–82.
Schultz MB, Sinclair DA. When stem cells grow old: phenotypes and mechanisms of stem cell aging. Development. 2016;143(1):3–14.
Brunet A, Goodell MA, Rando TA. Ageing and rejuvenation of tissue stem cells and their niches. Nat Rev Mol Cell Biol. 2023;24(1):45–62.
Lukjanenko L, et al. Aging disrupts muscle stem cell function by impairing Matricellular WISP1 secretion from Fibro-adipogenic progenitors. Cell Stem Cell. 2019;24(3):433-e4467.
Pentinmikko N, et al. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature. 2019;571(7765):398–402.
Qin Z, Hubbard EJ. Non-autonomous DAF-16/FOXO activity antagonizes age-related loss of C. Elegans germline stem/progenitor cells. Nat Commun. 2015;6:7107.
Wang K, et al. Redox homeostasis: the linchpin in stem cell self-renewal and differentiation. Cell Death & Disease; 2013. p. 4.
Lee HJ, Gutierrez-Garcia R, Vilchez D. Embryonic stem cells: a novel paradigm to study proteostasis? FEBS J. 2017;284(3):391–8.
Tavasolian F, et al. Unfolded protein response-mediated modulation of mesenchymal stem cells. IUBMB Life. 2020;72(2):187–97.
Chakrabarty RP, Chandel NS. Mitochondria as Signaling Organelles Control mammalian stem cell fate. Cell Stem Cell. 2021;28(3):394–408.
Li HY, et al. Epigenetic regulation of Gene expression in epithelial stem cells fate. Curr Stem Cell Res Therapy. 2018;13(1):46–51.
Thompson C, et al. Possible roles of epigenetics in stem cell therapy for Parkinson’s disease. Epigenomics. 2020;12(7):647–56.
Lu JY, et al. Application of Epigenome-Modifying Small molecules in Induced Pluripotent Stem cells. Med Res Rev. 2013;33(4):790–822.
Horii T, Hatada I. Regulation of CpG methylation by Dnmt and Tet in pluripotent stem cells. J Reprod Dev. 2016;62(4):331–5.
Wang YC, Peterson SE, Loring JF. Protein post-translational modifications and regulation of pluripotency in human stem cells. Cell Res. 2014;24(2):143–60.
Rossi DJ, Jamieson CHM, Weissman IL. Stems Cells Pathways Aging cancer Cell. 2008;132(4):681–96.
Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132(4):598–611.
Alvarado AS, Yamanaka S. Rethinking differentiation: stem cells, regeneration, and plasticity. Cell. 2014;157(1):110–9.
Article PubMed Central Google Scholar
Naik S, et al. Two to Tango: Dialog between Immunity and Stem cells in Health and Disease. Cell. 2018;175(4):908–20.
Wu JY, et al. Stem cell-derived exosomes: a New Method for reversing skin aging. Tissue Eng Regenerative Med. 2022;19(5):961–8.
Ogrodnik M. Cellular aging beyond cellular senescence: markers of senescence prior to cell cycle arrest in vitro and in vivo. Aging Cell. 2021;20(4):e13338.
Mohamad Kamal NS, et al. Aging of the cells: insight into cellular senescence and detection methods. Eur J Cell Biol. 2020;99(6):151108.
Chiche A, Chen C, Li H. The crosstalk between cellular reprogramming and senescence in aging and regeneration. Exp Gerontol. 2020;138:111005.
Giacconi R, et al. Cellular Senescence and Inflammatory Burden as determinants of Mortality in Elderly people until the Extreme old age. EBioMedicine. 2015;2(10):1316–7.
Schmeer C, et al. Dissecting Aging and Senescence-Current Concepts and Open Lessons. Cells. 2019;8(11):1446.
Schwartz RE, Conboy IM. Non-Intrinsic, Systemic Mechanisms of Cellular Senescence. Cells. 2023;12(24):2769.
Degirmenci U, Lei S. Role of lncRNAs in Cellular Aging. Front Endocrinol (Lausanne). 2016;7:151.
Wei Z, et al. Editorial: Cellular Senescence and Cellular communications within tissue microenvironments during aging. Front Physiol. 2022;13:890577.
Nacarelli T, Liu P, Zhang R. Epigenetic Basis of Cellular Senescence and Its Implications in Aging. Genes (Basel). 2017;8(12):343.
Wan R, et al. Cellular Senescence: A Troy Horse in Pulmonary Fibrosis. Int J Mol Sci. 2023;24(22):16410.
Gasek NS, et al. Strategies for targeting senescent cells in Human Disease. Nat Aging. 2021;1(10):870–9.
Diwan B, Sharma R. Nutritional components as mitigators of cellular senescence in organismal aging: a comprehensive review. Food Sci Biotechnol. 2022;31(9):1089–109.
Sikora E, et al. Impact of cellular senescence signature on ageing research. Ageing Res Rev. 2011;10(1):146–52.
Roos CM, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15(5):973–7.
Ogrodnik M, et al. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8:15691.
Tuttle CSL, et al. Cellular senescence and chronological age in various human tissues: a systematic review and meta-analysis. Aging Cell. 2020;19(2):e13083.
Al-Naggar IMA, Kuchel GA, Xu M. Senolytics: targeting senescent cells for age-associated diseases. Curr Mol Biology Rep. 2020;6(4):161–72.
Jia M, et al. LEF1 isoforms regulate cellular senescence and aging. Aging Cell. 2023;22(12):e14024.
Bahar R, et al. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature. 2006;441(7096):1011–4.
Baar MP, et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to Chemotoxicity and Aging. Cell. 2017;169(1):132.
Yosef R, et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun. 2016;7(1):11190.
Guerville F, et al. Does Inflammation Contribute to Cancer Incidence and Mortality during Aging? A Conceptual Review. Cancers (Basel). 2022;14(7):1622.
Trott DW, Fadel PJ. Inflammation as a mediator of arterial ageing. Exp Physiol. 2019;104(10):1455–71.
He H, Wang J. Inflammation and hematopoietic stem cells aging. Blood Sci. 2021;3(1):1–5.
Chung HY, et al. Redefining chronic inflammation in aging and age-related diseases: proposal of the Senoinflammation Concept. Aging Dis. 2019;10(2):367–82.
Zhou Z, et al. Engineering longevity-design of a synthetic gene oscillator to slow cellular aging. Science. 2023;380(6643):376–81.
Yang JH, et al. Loss of epigenetic information as a cause of mammalian aging. Cell. 2023;186(2):305-e32627.
Blikstad V, et al. Evolution of human endogenous retroviral sequences: a conceptual account. Cell Mol Life Sci. 2008;65(21):3348–65.
Lopez-Otin C, et al. Hallmarks of aging: an expanding universe. Cell. 2023;186(2):243–78.
Andreeva NV, et al. Recombinant HSP70 and mild heat shock stimulate growth of aged mesenchymal stem cells. Cell Stress Chaperones. 2016;21(4):727–33.
Kaszubowska L, et al. Expression of cellular protective proteins SIRT1, HSP70 and SOD2 correlates with age and is significantly higher in NK cells of the oldest seniors. Immunity & Ageing; 2017. p. 14.
Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475(7356):324–32.
Guo LL, et al. PGRP-SC2 promotes gut Immune Homeostasis to Limit Commensal Dysbiosis and extend lifespan. Cell. 2014;156(1–2):109–22.
Heintz C, et al. Splicing factor 1 modulates dietary restriction and TORC1 pathway longevity in C. Elegans. Nature. 2017;541(7635):102.
Hughes CE, et al. Cysteine toxicity drives age-related mitochondrial decline by Altering Iron Homeostasis. Cell. 2020;180(2):296.
Jaskelioff M, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469(7328):102-U1700.
Mangiola F, et al. Gut microbiota and aging. Eur Rev Med Pharmacol Sci. 2018;22(21):7404–13.
Boehme M, et al. Microbiota from young mice counteracts selective age-associated behavioral deficits. Nat Aging. 2021;1(8):666–76.
Tibbs TN, Lopez LR, Arthur JC. The influence of the microbiota on immune development, chronic inflammation, and cancer in the context of aging. Microb Cell. 2019;6(8):324–34.
Li S, et al. Effect of vitamin D, vitamin E and magnesium on pork quality. J Northeast Agricultural Univ. 2010;41(7):84–8.
Ford J, et al. Hypovitaminosis d: a contributor to psychiatric disorders in elderly? Can Geriatr Journal: CGJ. 2012;15(3):80–4.
Carroll B, Korolchuk VI. Nutrient sensing, growth and senescence. FEBS J. 2018;285(11):1948–58.
Fernandes SA, Demetriades C. The multifaceted role of Nutrient Sensing and mTORC1 Signaling in Physiology and Aging. Front Aging. 2021;2:707372.
Gao JL, et al. Characterizations of mitochondrial uncoupling induced by chemical mitochondrial uncouplers in cardiomyocytes. Free Radic Biol Med. 2018;124:288–98.
Li D, de Glas NA, Hurria A. Cancer and Aging: General principles, Biology, and Geriatric Assessment. Clin Geriatr Med. 2016;32(1):1.
Mishra A, et al. Fasting-mimicking diet prevents high-fat diet effect on cardiometabolic risk and lifespan. Nat Metab. 2021;3(10):1342–56.
Hernandez AR, et al. A ketogenic Diet improves cognition and has biochemical effects in Prefrontal Cortex that Are Dissociable from Hippocampus. Front Aging Neurosci. 2018;10:391.
Newman JC, et al. Ketogenic Diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 2017;26(3):547-e5578.
Roberts MN, et al. A ketogenic Diet extends longevity and Healthspan in Adult mice. Cell Metab. 2018;27(5):1156.
Pawlosky RJ, et al. A Dietary Ketone Ester Normalizes Abnormal Behavior in a Mouse Model of Alzheimer’s Disease. Int J Mol Sci. 2020;21(3):1044.
David G. Does diet influence aging Evidence from animal studies. J Intern Med. 2024;295(4):400–15.
Le Couteur DG, et al. Nutritional reprogramming of mouse liver proteome is dampened by metformin, resveratrol, and rapamycin. Cell Metabol. 2021;33(12):2367-e23794.
Ryu S, et al. The matricellular protein SPARC induces inflammatory interferon-response in macrophages during aging. Immunity. 2022;55(9):1609.
Gao X, et al. Role of sleep quality in the acceleration of biological aging and its potential for preventive interaction on air pollution insults: Findings from the UK Biobank cohort. Aging Cell. 2022;21(5):e13610.
Mochón-Benguigui S, et al. Is Sleep Associated with the S-Klotho anti-aging protein in sedentary middle-aged adults? The FIT-AGEING study. Antioxidants. 2020;9(8):738.
Song CX, et al. Sleep quality and risk of coronary heart disease - a prospective cohort study from the English longitudinal study of ageing. Aging-Us. 2020;12(24):25005–19.
McAlpine CS, et al. Sleep exerts lasting effects on hematopoietic stem cell function and diversity. J Exp Med. 2022;219(11):e20220081.
Yuan RK, et al. Differential effect of sleep deprivation on place cell representations, sleep architecture, and memory in young and old mice. Cell Rep. 2021;35(11):109234.
Li SB, et al. Hyperexcitable arousal circuits drive sleep instability during aging. Science. 2022;375(6583):838.
Hafycz JM, Strus E, Naidoo N. Reducing ER stress with chaperone therapy reverses sleep fragmentation and cognitive decline in aged mice. Aging Cell. 2022;21(6):e13598.
De Miguel Z, et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature. 2021;600(7889):494–9.
Leiter O, et al. Platelet-derived exerkine CXCL4/platelet factor 4 rejuvenates hippocampal neurogenesis and restores cognitive function in aged mice. Nat Commun. 2023;14(1):4375.
Grevendonk L, et al. Impact of aging and exercise on skeletal muscle mitochondrial capacity, energy metabolism, and physical function. Nat Commun. 2021;12(1):4773.
Wen DT, et al. The activation of cardiac dSir2-related pathways mediates physical exercise resistance to heart aging in old Drosophila. Aging-Us. 2019;11(17):7274–93.
Koshy A, et al. Association between heart rate variability and haemodynamic response to exercise in chronic heart failure. Scandinavian Cardiovasc J. 2019;53(2):77–82.
Horowitz AM, et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science. 2020;369(6500):167.
Nemoto EM, et al. Skeletal Muscle Deoxygenation and Its Relationship to Aerobic Capacity During Early and Late Stages of Aging. In: Nemoto EM, editor., et al., Oxygen Transport to Tissue Xli. 2021. p. 77–82.
Chapter Google Scholar
Tzimou A, et al. Effects of lifelong exercise and aging on the blood metabolic fingerprint of rats. Biogerontology. 2020;21(5):577–91.
Ham DJ, et al. Distinct and additive effects of calorie restriction and rapamycin in aging skeletal muscle. Nat Commun. 2022;13(1):2025.
Partridge L, Fuentealba M, Kennedy BK. The quest to slow ageing through drug discovery. Nat Rev Drug Discov. 2020;19(8):513–32.
Hu D, et al. Metformin: a potential candidate for Targeting Aging mechanisms. Aging Dis. 2021;12(2):480–93.
Kulkarni AS, Gubbi S, Barzilai N. Benefits of Metformin in attenuating the hallmarks of Aging. Cell Metabol. 2020;32(1):15–30.
Dubal DB. Klotho, a longevity factor, improves cognitive function in aging nonhuman primates. Nat Aging. 2023;3(8):915–6.
Castner SA, et al. Longevity factor klotho enhances cognition in aged nonhuman primates. Nat Aging. 2023;3(8):931.
Schroer AB, et al. Platelet factors attenuate inflammation and rescue cognition in ageing. Nature. 2023;620(7976):1071–9.
Zhang ZH, et al. Increased hyaluronan by naked mole-rat Has2 improves healthspan in mice. Nature. 2023;621(7977):196–205.
McGaunn J, Baur JA. Taurine linked with healthy aging, vol. 380. New York, N.Y.): Science; 2023. p. 1010–1.
Singh P, et al. Taurine deficiency as a driver of aging, vol. 380. New York, N.Y.): Science; 2023. p. eabn9257.
Lardinois CK. Type 2 diabetes: glycemic targets and oral therapies for older patients. Geriatrics. 1998;53(11):22.
Brewer RA, Gibbs VK, Smith DL Jr. Targeting glucose metabolism for healthy aging. Nutr Healthy Aging. 2016;4(1):31–46.
Mannick JB, Lamming DW. Targeting the biology of aging with mTOR inhibitors. Nat aging. 2023;3(6):642–60.
Liang Y, et al. eIF5A hypusination, boosted by dietary spermidine, protects from premature brain aging and mitochondrial dysfunction. Cell Rep. 2021;35(2):108941.
Minois N. Molecular basis of the ‘Anti-Aging’ effect of Spermidine and other natural polyamines a Mini-review. Gerontology. 2014;60(4):319–26.
Hofer SJ, et al. Spermidine-induced hypusination preserves mitochondrial and cognitive function during aging. Autophagy. 2021;17(8):2037–9.
Kiss T, et al. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience. 2020;42(2):527–46.
Zaman FY, et al. Non-aspirin non-steroidal anti-inflammatory drugs in colorectal cancer: a review of clinical studies. Br J Cancer. 2022;127(10):1735–43.
Xie Y, et al. Dose-dependent roles of aspirin and other non-steroidal anti-inflammatory drugs in abnormal bone remodeling and skeletal regeneration. Cell Bioscience. 2019;9(1):1–11.
Feng ZL, et al. The drug likeness analysis of anti-inflammatory clerodane diterpenoids. Chin Med. 2020;15(1):1–13.
Salarda EM, et al. Mini-review: The anti-aging effects of lithium in bipolar disorder. Neurosci Lett. 2021;759:136051.
Bajaj S, et al. Targeting telomerase for its advent in cancer therapeutics. Med Res Rev. 2020;40(5):1871–919.
Pluvinage JV, Wyss-Coray T. Systemic factors as mediators of brain homeostasis, ageing and neurodegeneration. Nat Rev Neurosci. 2020;21(2):93–102.
Kassab A, Rizk N, Prakash S. The Role of Systemic Filtrating Organs in Aging and Their Potential in Rejuvenation Strategies. Int J Mol Sci. 2022;23(8):4338.
Derwich M, et al. Oral Glucosamine in the Treatment of Temporomandibular Joint Osteoarthritis: A Systematic Review. Int J Mol Sci. 2023;24(5):4925.
Liu B, Yang WX, Zhang K. Role of glucosamine and chondroitin in the Prevention of Cancer: a Meta-analysis. Nutr Cancer-an Int J. 2023;75(3):785–94.
Johnson AA, Cuellar TL. Glycine and aging: evidence and mechanisms. Ageing Res Rev. 2023;87:101922.
Scarano A, et al. Malar augmentation with hyaluronic acid enriched with glycine and proline: a clinical evaluation. J Biol Regul Homeost Agents. 2021;35(2):187–94.
Cano M, et al. Application of kinase inhibitors for anti-aging intervention. Curr Pharm Des. 2017;23(29):4351–68.
Li J, et al. Oxidized quercetin has stronger anti-amyloid activity and anti-aging effect than native form. Comparative Biochemistry and Physiology C-Toxicology & Pharmacology; 2023. p. 271.
Wang YY, et al. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging-Us. 2016;8(11):2915–26.
Mahoney SA, et al. Intermittent supplementation with fisetin improves arterial function in old mice by decreasing cellular senescence. Aging Cell. 2024;23(3):e14060.
Mahjoob M, Stochaj U. Curcumin nanoformulations to combat aging-related diseases. Ageing Res Rev. 2021;69:101364.
Xu QX, et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat Metabolism. 2021;3(12):1706.
Moaddel R, et al. Identification of gingerenone A as a novel senolytic compound. PLoS ONE. 2022;17(3):e0266135.
Nieto M, Könisgberg M, Silva-Palacios A. Quercetin and dasatinib, two powerful senolytics in age-related cardiovascular disease. Biogerontology. 2024;25(1):71–82.
Cho HJ, et al. Nintedanib induces senolytic effect via STAT3 inhibition. Cell Death Dis. 2022;13(9):760.
Takaya K, et al. Navitoclax (ABT-263) rejuvenates human skin by eliminating senescent dermal fibroblasts in a Mouse/Human chimeric model. Rejuven Res. 2023;26(1):9–20.
Bertram KL, et al. 17-DMAG regulates p21 expression to induce chondrogenesis in vitro and in vivo. Disease Models & Mechanisms. 2018;11(10):dmm033662.
Fuhrmann-Stroissnigg H, et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun. 2017;8.
Diczfalusy U, et al. On the formation and possible biological role of 25-hydroxycholesterol[J]. Biochimie, 2013;95(3):455–60.
Wakita M, et al. A selective BET family protein degrader provokes senolysis by targeting NHEJ and autophagy in senescent cells. Cancer Sci. 2021;112:554–554.
Mannarino M, et al. Senolytic Combination Treatment Is More Potent Than Single Drugs in Reducing Inflammatory and Senescence Burden in Cells from Painful Degenerating IVDs. Biomolecules. 2023;13(8):1257.
Chen C, et al. Synthesis and biological evaluation of thiazole derivatives as novel USP7 inhibitors. Bioorg Med Chem Lett. 2017;27(4):845–9.
Deryabin PI, Shatrova AN, Borodkina A. Apoptosis resistance of senescent cells is an intrinsic barrier for senolysis induced by cardiac glycosides`. Cell Mol Life Sci. 2021;78(23):7757–76.
Kowalski J, et al. E ffect of fluvastatin and fenofibrate on reactive oxygen species generation and lipid peroxidation in patients with dyslipidemia. Polski merkuriusz lekarski: organ Polskiego Towarzystwa Lekarskiego. 2003;14(82):279–84.
Sié A, et al. Azithromycin during Routine Well-Infant visits to prevent death. N Engl J Med. 2024;390(3):221–9.
Han Q, et al. Age-related changes in metabolites in young donor livers and old recipient sera after liver transplantation from young to old rats. Aging Cell. 2021;20(7):e13425.
Jeon OH, et al. Systemic induction of senescence in young mice after single heterochronic blood exchange. Nat Metab. 2022;4(8):995–1006.
Sarkar TJ, et al. Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells. Nat Commun. 2020;11(1):1545.
Shefer VI, Talan MI. Change in heat loss as a part of adaptation to repeated cold exposures in adult and aged male C57BL/6J mice. Exp Gerontol. 1997;32(3):325–32.
Strandberg TE, et al. Sauna bathing, health, and quality of life among octogenarian men: the Helsinki businessmen study. Aging Clin Exp Res. 2018;30(9):1053–7.
Zhou Z, et al. Engineering longevity-design of a synthetic gene oscillator to slow cellular aging, vol. 380. New York, N.Y.): Science; 2023. p. 376–81.
Download references
Acknowledgements
Not applicable.
This work was supported by Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (TSBICIP-CXRC-031).
Author information
Authors and affiliations.
Tianjin Institute of Industrial Biotechnology, Chinese Academy of Sciences; National Center of Technology Innovation for Synthetic Biology, Tianjin, China
Yumeng Li, Xutong Tian, Juyue Luo, Tongtong Bao & Xin Wu
Institute of Life Sciences, Chongqing Medical University, Chongqing, China
Shujin Wang
You can also search for this author in PubMed Google Scholar
Contributions
Yumeng Li wrote the manuscript. Xutong Tian, Juyue Luo and Tongtong Bao prepared the figures. Shujin Wang reviewed the manuscript. Xin Wu offered the idea of the manuscript. All authors reviewed the manuscript.
Corresponding author
Correspondence to Xin Wu .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Li, Y., Tian, X., Luo, J. et al. Molecular mechanisms of aging and anti-aging strategies. Cell Commun Signal 22 , 285 (2024). https://doi.org/10.1186/s12964-024-01663-1
Download citation
Received : 03 February 2024
Accepted : 15 May 2024
Published : 24 May 2024
DOI : https://doi.org/10.1186/s12964-024-01663-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Aging triggers
Cell Communication and Signaling
ISSN: 1478-811X
- General enquiries: [email protected]
- Open access
- Published: 27 May 2024
Spatiotemporal multi-omics: exploring molecular landscapes in aging and regenerative medicine
- Liu-Xi Chu 1 , 2 , 3 na1 ,
- Wen-Jia Wang 4 na1 ,
- Xin-Pei Gu 5 , 6 na1 ,
- Ping Wu 2 , 3 ,
- Chen Gao 4 ,
- Quan Zhang 7 ,
- Da-Wei Jiang 1 , 2 , 3 ,
- Jun-Qing Huang 2 , 3 , 9 ,
- Xin-Wang Ying 2 , 3 ,
- Jia-Men Shen 3 ,
- Yi Jiang 3 ,
- Li-Hua Luo 10 ,
- Jun-Peng Xu 1 , 2 , 3 ,
- Yi-Bo Ying 3 ,
- Hao-Man Chen 3 ,
- Ao Fang 2 , 3 ,
- Zun-Yong Feng 2 , 3 , 11 , 12 , 13 , 14 ,
- Shu-Hong An 6 ,
- Xiao-Kun Li 2 , 3 &
- Zhou-Guang Wang ORCID: orcid.org/0000-0001-9903-0362 1 , 2 , 3 , 9
Military Medical Research volume 11 , Article number: 31 ( 2024 ) Cite this article
150 Accesses
2 Altmetric
Metrics details
Aging and regeneration represent complex biological phenomena that have long captivated the scientific community. To fully comprehend these processes, it is essential to investigate molecular dynamics through a lens that encompasses both spatial and temporal dimensions. Conventional omics methodologies, such as genomics and transcriptomics, have been instrumental in identifying critical molecular facets of aging and regeneration. However, these methods are somewhat limited, constrained by their spatial resolution and their lack of capacity to dynamically represent tissue alterations. The advent of emerging spatiotemporal multi-omics approaches, encompassing transcriptomics, proteomics, metabolomics, and epigenomics, furnishes comprehensive insights into these intricate molecular dynamics. These sophisticated techniques facilitate accurate delineation of molecular patterns across an array of cells, tissues, and organs, thereby offering an in-depth understanding of the fundamental mechanisms at play. This review meticulously examines the significance of spatiotemporal multi-omics in the realms of aging and regeneration research. It underscores how these methodologies augment our comprehension of molecular dynamics, cellular interactions, and signaling pathways. Initially, the review delineates the foundational principles underpinning these methods, followed by an evaluation of their recent applications within the field. The review ultimately concludes by addressing the prevailing challenges and projecting future advancements in the field. Indubitably, spatiotemporal multi-omics are instrumental in deciphering the complexities inherent in aging and regeneration, thus charting a course toward potential therapeutic innovations.
Aging and regeneration, are fundamental biological processes, that have long captivated the scientific community. Aging, a multifaceted phenomenon, is characterized by a progressive decline in physiological functions coupled with an increased vulnerability to age-related diseases [ 1 , 2 , 3 , 4 , 5 ]. To elucidate this complex process, López-Otín et al. [ 3 ] introduced a comprehensive framework encompassing twelve hallmarks of aging. These hallmarks, which span a range of molecular, cellular, and organ processes, contribute significantly to aging. They include genomic instability, telomere depletion, epigenetic alterations, proteostasis impairment, macroautophagy dysfunction, dysregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell decline, altered intercellular communication, chronic inflammation, and dysbiosis (Fig. 1 a [ 3 ]). These hallmarks furnish pivotal insights into the mechanisms underlying aging and hole potential for informing interventions aimed at promoting healthier aging trajectories and mitigating age-related diseases.
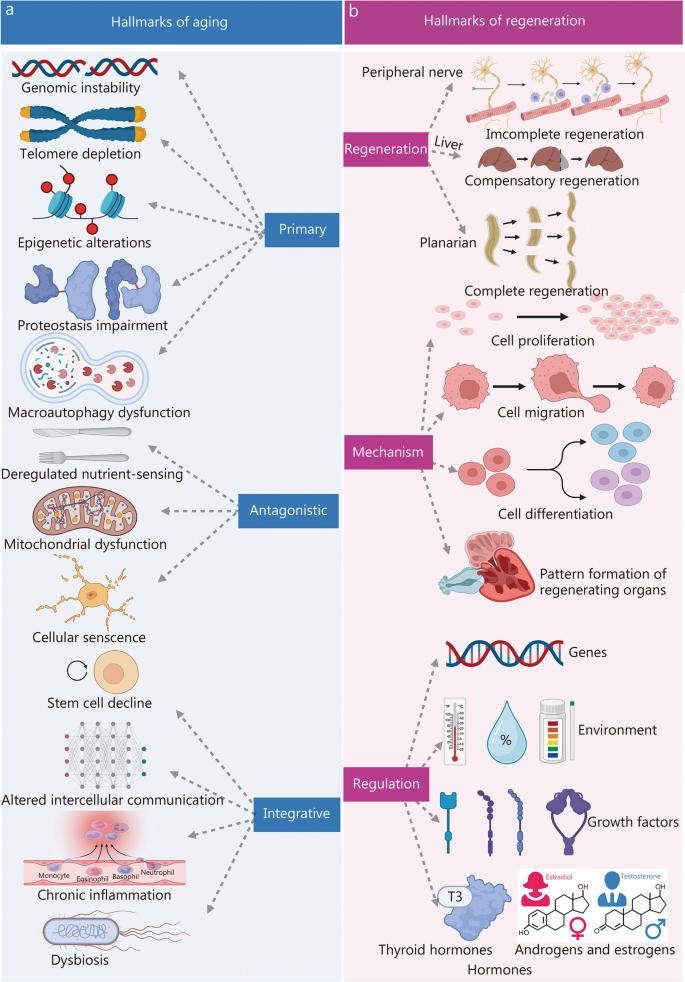
Key features of aging and regenerative processes. a The twelve hallmarks of aging included genomic instability, telomere depletion, epigenetic alterations, proteostasis impairment, macroautophagy dysfunction, dysregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell decline, altered intercellular communication, chronic inflammation, and dysbiosis. Reprinted with permission from [ 3 ]. Copyright © 2022 Elsevier Inc. b The hallmarks of regeneration can be classified into three main areas: the types of regeneration, the underlying mechanisms governing it, and the regulatory processes orchestrating regenerative events. Regeneration is divided into three types: complete, incomplete, or compensatory, depending on the extent of restoration achieved. Processes involve cell activities like proliferation, migration, differentiation, and pattern formation. Proliferation creates new tissues through cell division. Migration moves cells for correct structure. Differentiation changes cells into specialized types. Pattern formation arranges new tissues or organs. Genes, environment, hormones, and growth factors affect regeneration, T3 triiodothyronine
In contrast, regeneration signifies the extraordinary ability of certain organisms to repair and restore damaged or lost cells, tissues, or organs, ultimately achieving tissue homeostasis and functional recovery [ 6 , 7 , 8 , 9 , 10 ]. This process is intricately orchestrated, involving a coordinated cascade of molecular events. The phenomena of aging and regeneration are closely intertwined; notably, a decline in regenerative capacity is frequently associated with aging [ 11 , 12 , 13 ]. Deciphering the molecular underpinnings of aging and regeneration is crucial for the development of interventions and therapies that support healthy aging and enhance tissue repair capabilities.
A comprehensive understanding of aging and regeneration necessitates an analysis of molecular dynamics across both spatial and temporal dimensions. The functional state of senescent and those undergoing regenerative repair is intricately controlled by the spatiotemporal regulation of gene expression [ 14 , 15 , 16 ]. However, conventional transcriptomics methods fall short of capturing the simultaneous spatial and temporal dependencies of RNA profiles [ 17 , 18 , 19 ]. While existing transcriptomics techniques adeptly dissect gene expression in heterogeneous cell types within a histomorphological context, providing static snapshots, they are limited [ 20 , 21 , 22 ]. Cutting-edge single-cell sequencing technologies and metabolic RNA labeling methods allow for temporal analysis of nascent single-cell transcriptomes, yet they lack precise spatial resolution [ 23 , 24 , 25 ]. Although live cell imaging facilitates the tracking of intracellular RNA trajectories, visualizing multiple transcripts simultaneously remains a significant challenge [ 26 , 27 , 28 ]. Thus, the pursuit of sequencing methodologies that combine high multiplexing capabilities with simultaneous spatiotemporal resolution is imperative. Such methodologies would enable in situ monitoring of de novo messenger RNA (mRNA) at subcellular and single-cell resolution. Furthermore, in response to stress signals from the microenvironment, senescent and regenerative cells rapidly assemble functional protein complexes to propagate these signals [ 29 , 30 , 31 ]. These cellular signal processes, encompassing post-translational protein modifications, the assembly of functional protein complexes, and the intricate interplay of subcellular spatial migration, contribute significantly to the orchestration and regulation of dynamic protein complexes in a spatiotemporally dynamic manner.
To effectively address the challenges inherent in studying aging and regeneration, the deployment of spatiotemporal multi-omics technology is paramount. Enhanced by the addition of a temporal dimension, spatiotemporal multi-omics facilitates a thorough examination of the temporal variations exhibited by entities such as cells, genes, and proteins during the aging and regeneration processes. These methodologies are instrumental in identifying key regulatory moments and dynamic mechanisms, offering a more intricate portrayal of the temporal characteristics underpinning biological processes and the formation of precise biomolecular networks. Notably, spatiotemporal multi-omics reveal the temporal correlations and interactions that govern molecular dynamics within complex networks throughout the progression of aging and regeneration. Additionally, it unveils insights into the dynamic trajectory of diseases; comparisons of spatiotemporal multi-omics data from healthy and diseased states illuminate the dynamic changes associated with aging and regeneration, providing more accurate insights into the mechanisms driving these processes.
This review endeavors to provide a comprehensive exploration of the application of spatiotemporal multi-omics in the fields of aging and regeneration. It delves into the capabilities of these technologies to probe molecular dynamics, cellular interactions, and signaling pathways within a spatial and temporal framework, thereby shedding light on the multifaceted aspects of these processes. The review commences with an overview of the various spatiotemporal multi-omics methodologies, followed by a detailed examination of recent advancements in the field of aging and regeneration. Importantly, this analysis extends to identifying challenges and offering insights into the future trajectory of these methodologies. Consequently, the integration of spatiotemporal multi-omics approaches emerges as a transformative avenue for untangling the intricacies inherent to aging and regeneration. This, in turn, fosters a fertile ground for the cultivation of innovative therapeutic strategies poised to shape the landscape of future medical interventions.
Spatiotemporal multi-omics analysis
This section provides a comprehensive overview of the principles, procedures, and categorizations of spatiotemporal multi-omics, which includes spatiotemporal transcriptomics (STT), spatiotemporal proteomics (STP), spatiotemporal metabolomics (STM), and spatiotemporal epigenomics (STE). The introduction previously in this paper establishes a foundational understanding of complex organizational structures on a grander scale and across more elevated dimensions. Spatiotemporal multi-omics can be broadly classified into two principal categories. The first category involves pseudo-temporal analysis, which leverages spatial multi-omics technologies (Fig. 2 ). This approach entails the systematic collection of tissue samples across a series of temporal stages, followed by meticulous analysis at multiple, distinct time points. Such a method enables a nuanced comprehension of both temporal and spatial fluctuations within the tissue matrix. The second category involves the application of authentic spatiotemporal techniques, which require the labeling of specific intracellular molecules. Subsequent longitudinal tracking allows for the observation and characterization of these molecules' dynamic trajectories through time and space. These approaches collectively enhance our understanding of cellular behavior in complex biological systems. Crucially, these innovative methodologies hold immense potential in unraveling the intricate details underlying the processes of aging and regeneration, thereby driving forward our understanding and fostering the development of groundbreaking therapeutic strategies.
The multi-dimensional approach of spatial multi-omics. a Spatial transcriptomics. b Spatial proteomics. c Spatial metabolomics. d Spatial epigenomics. ISS in situ sequencing, ISH in situ hybridization, TSA tyramide signal amplification, MICS MACSima imaging cyclic staining, CODEX co-detection by IndEXing, IMC imaging mass cytometry, MIBI multiplex ionbeam imaging, DESI desorption electrospray ionization, SIMS secondary ion mass spectrometry, MALDI-MS matrix-assisted laser desorption/ionization mass spectrometry, NGS next-generation sequencing, MERFISH multiplexed error-robust fluorescence in situ hybridization, CUT&Tag cleavage under targets and tagmentation, ATAC-seq assay for transposase-accessible chromatin sequencing, mIHC multiplex immunohistochemistry, DEI diffraction enhanced imaging, MELC multi-epitope-ligand cartography, CyCIF cyclic immunofluorescence, MxIF multiplexed immunofluorescence, IBEX Iterative bleaching extends multiplexity, MSI mass spectrometry imaging. 4i iterative indirect immunofluorescence, mIHC multiplex immunohistochemistry, Immuno-SABER immuno-signal amplification by exchange reaction
Overview of STT
The widespread application of single-cell RNA sequencing (scRNA-seq) technology has yielded valuable insights into the diversity of cellular composition and gene expression status in tissues, enabling the resolution of temporal changes through sampling at distinct time points [ 23 , 24 , 25 , 32 , 33 , 34 , 35 , 36 ]. It is crucial to recognize that gene expression exhibits both temporal and spatial heterogeneity. The process of scRNA-seq often involves enzymatic digestion or mechanical separation of cells into suspensions before sequencing. This procedure results in the loss of original location information and disrupts the cellular communication network, thus hindering our understanding of cellular interactions with neighboring cells and extracellular matrix. To overcome these limitations, the advent of STT technology emerged as a promising solution. STT aims to provide gene expression profiles while preserving spatial context and concurrently offering data on the temporal dimension, enabling a more comprehensive exploration of cellular interactions within the tissue microenvironment [ 37 , 38 , 39 , 40 ].
STT approaches, developed in recent years, provide a more extensive exploration of the temporal and spatial dimensions of cellular interactions within tissues. These can be broadly divided into two categories. The primary methodology employs a pseudotemporal analysis based on spatial transcriptomics techniques. It involves the systematic collection of tissue sections across various temporal stages. By conducting detailed analyses of these sections at multiple discrete time points, the method elucidates spatiotemporal variations within the tissue matrix. Presently, this pseudotemporal approach is widely applied. Conversely, the secondary methodology employs an authentic STT technique. This approach necessitates the labeling of specific intracellular substances, followed by longitudinal tracking to discern the dynamic spatiotemporal shifts they undergo. A detailed exposition of these two methodologies is presented in the subsequent sections (Fig. 3 [ 41 , 42 , 43 ]).
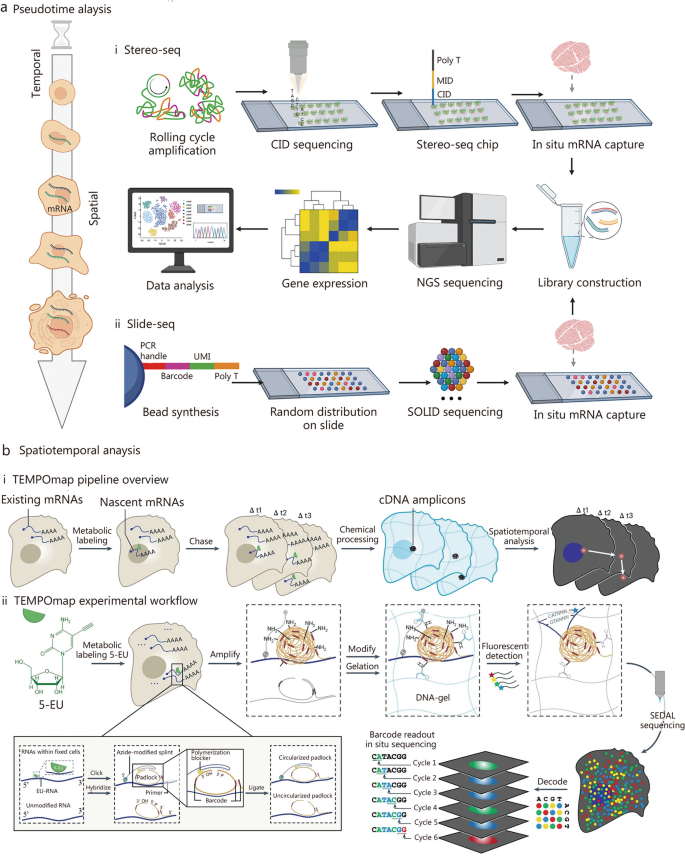
Integrating pseudotemporal analysis with spatiotemporal transcriptomics. a Spatial transcriptomic analyses at different time points. (i) Workflows of Sereo-seq. Reprinted with permission from [ 42 ]. Copyright © 2022. Published by Elsevier Inc. (ii) Workflows of Slide-seq. Reprinted with permission from [ 41 ]. Copyright © 2019, The American Association for the Advancement of Science. b Spatiotemporal transcriptomic analysis using 5-ethynyl uridine (5-EU) metabolic markers. (i) TEMPOmap pipeline overview: the process involves collecting and in situ sequencing nascent RNAs from various time points, followed by comprehensive spatiotemporal RNA analysis. (ii) TEMPOmap experimental workflow: the procedure starts with labeling cells using 5-EU. Three distinct probes - splint, primer, and padlock - are combined with cellular mRNAs, leading to enzymatic cDNA amplicon generation from each padlock sequence. These amplicons are integrated into a hydrogel network, secured by a specially designed acrylic component (depicted in blue). The resulting composite structure of DNA and hydrogel is visualized as blue undulating lines. Each amplicon, carrying a unique five-base barcode, undergoes sequential decoding through a six-stage process known as SEDAL fluorescence. This multiplexed RNA quantification approach precisely reveals gene expression patterns in nascent subcellular locations. Reprinted with permission from [ 43 ]. Copyright © 2023, Published by Springer Nature. CID coordinate encoding, MID molecularly encoded, NGS next-generation sequencing, PCR polymerase chain reaction, SOLID sequencing by oligonucleotide ligation and detection, SEDAL sequencing with error-reduction by dynamic annealing and ligation, cDNA complementary DNA, UMI unique molecular identifier
Pseudotemporal-based STT
We begin by presenting a brief overview of spatial transcriptomics (Fig. 2 a), given that pseudotemporal analyses are fundamentally reliant on these techniques. Spatial transcriptomics technologies can be categorized into two types based on sequencing throughput. The first category includes low-throughput spatial technologies such as microdissected gene expression technologies [ 44 , 45 , 46 , 47 ], in situ hybridization technologies [ 48 , 49 , 50 ], and in situ sequencing methods [ 51 , 52 , 53 ]. The second category encompasses high-throughput spatial technologies that utilize spatial barcodes. Examples include Nanostring [ 54 , 55 ], 10× Visium [ 56 , 57 ], Slide-seq [ 41 , 58 ], Slide-seq V2 [ 57 , 59 ], Stereo-seq [ 42 , 60 , 61 ], Seq-scope [ 62 , 63 ], and sci-Space [ 64 , 65 ]. The foundational principles and comprehensive categorization of these techniques have been extensively discussed in our recent publications [ 66 ] and elsewhere [ 44 , 47 , 67 , 68 , 69 , 70 , 71 ]. The primary principles and steps are succinctly summarized (Fig. 3 a [ 41 , 42 ] ) . This section focuses on providing an overview of high-throughput spatial technologies, with a particular emphasis on Stereo-seq [ 42 , 61 , 72 , 73 , 74 ]. Random barcode sequences are incorporated into DNA nanoballs (DNBs) which are then positioned on a modified chip using photolithographic etching techniques. Subsequently, the array undergoes micro-graphically treatment with primers and sequencing, revealing the sequence of each etched DNB. A data matrix containing the coordinate encoding (CID) for each etched DNB is obtained. Molecularly encoded (MID) and oligonucleotide-containing polyp sequences are then bound to each position through hybridization with the CID. Frozen sections of fresh tissue are placed onto the chip’s surface for fixation, permeabilization, reverse transcription, and amplification, aimed at capturing polyA-tailed RNA from the tissue. The amplified complementary DNAs (cDNAs) serve as templates for library preparation and co-sequenced with CID. In standard data analysis, the 1 cm × 1 cm chip incorporates 400 million DNBs, a result of amplifying [ 61 ]. The Stereo-seq technology overcomes the limitations of previous spatial transcriptomic methods by simultaneously enhancing resolution, gene capture efficiency, and field of view. By introducing a novel combination of DNB patterning and in situ, RNA capture, Stereo-seq establishes a new standard for generating high-resolution, comprehensive STT atlases, holding promise for application in the realms of aging and regeneration research. It is expected to uncover potential regulatory mechanisms underlying age-related changes and tissue regeneration, thus advancing our understanding of aging and regeneration. Moreover, this technology will offer a framework for targeted interventions in age-related diseases and regenerative medicine.
Spatiotemporal-based STT
The authentic STT involves the tagging of individual cells or distinct intracellular components to observe their spatiotemporal dynamics. Presently, metabolic labeling of mRNA is instrumental in distinguishing newly synthesized mRNA molecules from those synthesized earlier. Due to advancements in biochemical methodologies, a range of RNA metabolic labeling techniques have been integrated into scRNA-seq. These methods enable the discrimination between “old” and “new” RNAs [ 75 ]. Commonly, modified exogenous nucleotides, such as 4-thiouridine (s 4 U) [ 76 ] and 5-ethynyl uracil [ 77 ], are employed for labeling newly transcribed RNAs. Through oxidative nucleophilic substitution, these exogenous nucleotides can be converted into different nucleotides, introducing base mutations in cDNA. This process facilitates the differentiation of “old” and “new” RNAs based on mutation sites in subsequent sequencing. Integrating the RNA metabolic labeling with high-throughput scRNA-seq, Qiu et al. [ 78 ] developed metabolic labeling-based single-cell RNA tagging sequencing (scNT-seq) using droplet microfluidic technology. In this technique, cells labeled with s 4 U are encapsulated in droplets along with barcoded beads. Following cell lysis, the beads capture both pre-existing and s 4 U-labeled newly transcribed RNA. The s 4 U on the beads is then chemically converted to cytosine derivatives, allowing base-pair with guanine during transcription. This process enables the location and information of new transcripts to be inferred from sequencing reads showing T to C substitutions. Although scNT-seq provides high-throughput temporal information on cellular mRNAs, it does not offer detailed transcriptional dynamics on an hourly scale.
To enhance understanding of the temporal dynamics in gene expression, Rodriques et al. [ 79 ] developed an innovative RNA timestamping technique. This method is designed to ascertain the “age” of individual RNA molecules and monitor historical shifts in gene expression within single cells. Utilizing an adenine-rich RNA template with an MS2 binding site, this RNA timestamp serves as a substrate for the enzyme adenine deaminase ADAR. In this methodology, the catalytic domain of ADAR is fused with the MS2 capsid protein. This fusion allows ADAR to specifically target and edit adenine within the MS2 binding site, leading to adenine-to-inosine (A-to-I) mutations. As ADAR binds to the RNA timestamps over time, these A-to-I edits accumulate, providing a reliable measure of the age of RNA molecules on an hourly basis. Additionally, a molecular biology protocol integrating this timestamping technique with droplet-based RNA sequencing was developed. This combination demonstrates the compatibility of the timestamping system with advanced, high-throughput single-cell transcriptomic sequencing methods. Applying this methodology enables the discernment of transcriptional changes at specific time intervals, revealing cellular diversity and applying these insights to various biological systems. This approach not only enhances understanding of transcriptional processes but also holds potential for tracking stimulus-specific responses. However, current metabolic RNA labeling methods, while enabling temporal analysis of nascent single-cell transcriptomes, lack essential spatial resolution. Although live cell imaging can track intracellular RNA trajectories, visualizing multiple transcripts concurrently remains a formidable challenge [ 80 , 81 ]. There is a pressing need for highly multiplexed sequencing techniques that offer both spatial and temporal resolution, capable of effectively tracing nascent mRNA from its inception to its conclusion at subcellular and single-cell levels.
In response to this need, Ren et al. [ 43 ] developed the TEMPOmap method, designed for tracing the spatiotemporal progression of the nascent transcriptome at subcellular resolution. TEMPOmap employs the selective amplification of metabolic-labeled and pulse-labeled nascent transcriptomes, combined with advanced three-dimensional (3D) in situ RNA sequencing within hydrogel cellular scaffolds. The introduction of pulse-tracking markers enables simultaneous tracking of multiple genes throughout their RNA lifecycle, capturing vital kinetic parameters such as transcription, decay, nuclear export, and cytoplasmic translocation rates. Analysis of these spatiotemporal metrics reveals that different mRNAs undergo distinct regulation at various stages of the RNA life cycle and during cell cycle phases, influencing gene functionality. TEMPOmap represents a novel method for spatiotemporally resolved transcriptomics, utilizing metabolically labeled RNA along with a triad of probe sets: a splint DNA probe, a padlock probe, and a primer-probe. The splint DNA probe establishes a covalent bond with labeled mRNA via copper(I)-catalyzed azide-alkyne cycloaddition. Simultaneously, the padlock probe identifies the mRNA target and can loop when adjacent to the splint DNA probe on the same RNA molecule. In situ, primer probes amplify these circular padlocks through rolling circular amplification, leading to the formation of cDNA nanospheres or amplicons. This amplification occurs only for mRNAs that simultaneously bind all three probes, ensuring selective detection of labeled mRNA populations. To facilitate highly multiplexed transcriptome detection, in situ-generated cDNA amplicon libraries were embedded within hydrogel matrices. This was followed by several rounds of fluorescence imaging. Subsequently, the genes encoded by barcodes were deciphered using sequencing for error reduction by dynamic annealing and ligation (SEDAL). This approach was thoroughly tested on human cells, encompassing 991 genes, which displayed diverse spatial and temporal RNA expression patterns (Fig. 3 b [ 43 ]).
As a key exemplar in the realm of spatiotemporal transcriptome research, TEMPOmap stands out for its innovative approach. This advanced technology in single-cell transcriptomics enables the simultaneous analysis of RNA with remarkable subcellular and temporal precision. It proficiently demonstrates TEMPOmap’s capability to trace the subcellular distribution and cytoplasmic translocation of transcripts over time, thereby providing a comprehensive insight into RNA subcellular dynamics at the single-cell level. Moreover, the observed strong correlation between the dynamic patterns of RNA and the molecular function of genes suggests that a functionally driven regulation of the RNA lifecycle has likely evolved to manage spatiotemporal gene expression with both precision and efficiency [ 82 ]. The validation of TEMPOmap was conducted across various cell types, including human induced pluripotent stem cell-derived cell cultures and primary cell cultures. This process illuminated the cell type-specific regulation of RNA dynamics [ 43 ]. While the kinetics of RNA generally mirror the inherent characteristics of genes grouped by molecular function, the kinetic behavior of genes crucial to specific cellular functions is notably influenced by the cell’s state and type. It is important to recognize that TEMPOmap may display sequence bias and necessitates the use of uridine analogs for metabolic labeling and the formulation of DNA probes.
An insight into STP
Traditional proteomic analyses of bulk tissues often fail to the spatial distribution and cellular specificity due to tissue homogenization, resulting in merely an average representation of protein expression levels in the mass spectrometry signal [ 82 ]. Conversely, advanced spatial proteomics provides a precise delineation of protein expression profiles across varied cells and tissue regions [ 83 ]. The integration of spatial, cell-type, and proteomic data offers critical insights into tissue spatial microenvironments. The integration facilitates the identification of accurate biomarkers and elucidates novel functional pathways [ 84 ]. While spatial transcriptomics effectively captures spatial data through methods like multiplexed fluorescence in situ hybridization or sequencing, inferring protein expression from transcriptomic data is a challenging endeavor [ 17 ]. The relationship between mRNA and its resulting protein is intricate and non-linear, often leading to discrepancies between mRNA and protein expression levels [ 85 , 86 ]. Notably, transcript expression tends to exhibit more variability compared to protein expression, with proteins generally presenting lower variation coefficients relative to their corresponding mRNAs [ 87 ]. Direct spatial proteomic measurements thus provide a more authentic depiction of cellular functions and states.
STP unveils unparalleled perspectives on protein dynamics, localization, and interactions across both spatial and temporal dimensions. It offers deeper insights into cellular processes than what can be gleaned from spatial or bulk proteomics alone [ 88 , 89 ]. STP can be categorized into two primary methodologies, each characterized by its distinct approach to in vivo protein labeling for tracking intricate spatial and temporal dynamics (Fig. 4 [ 90 , 91 ]). The first approach utilizes pseudotemporal analysis, where spatial proteomics techniques are employed to meticulously examine tissue sections at defined temporal intervals. Fortified by algorithms specifically designed for pseudotemporal analysis, this method extrapolates protein behaviors across the temporal and spatial spectra. Conversely, the second methodology is rooted in genuine STP. This sophisticated approach involves the in vivo tagging of targeted proteins localized within specific subcellular organelles, utilizing biotin in a live cell environment. The overarching objective here is to astutely monitor and decipher the nuanced alterations in these proteins across the intertwined dimensions of space and time [ 90 ]. While the basics of pseudotemporal analysis are well-established in spatial proteomics literature, our primary focus in this discourse is on the latter, the authentic aspect of STP. An in-depth overview of spatial proteomic methodologies is essential before delving into STP, as STP is intrinsically built upon the foundational concepts and techniques of spatial proteomics.
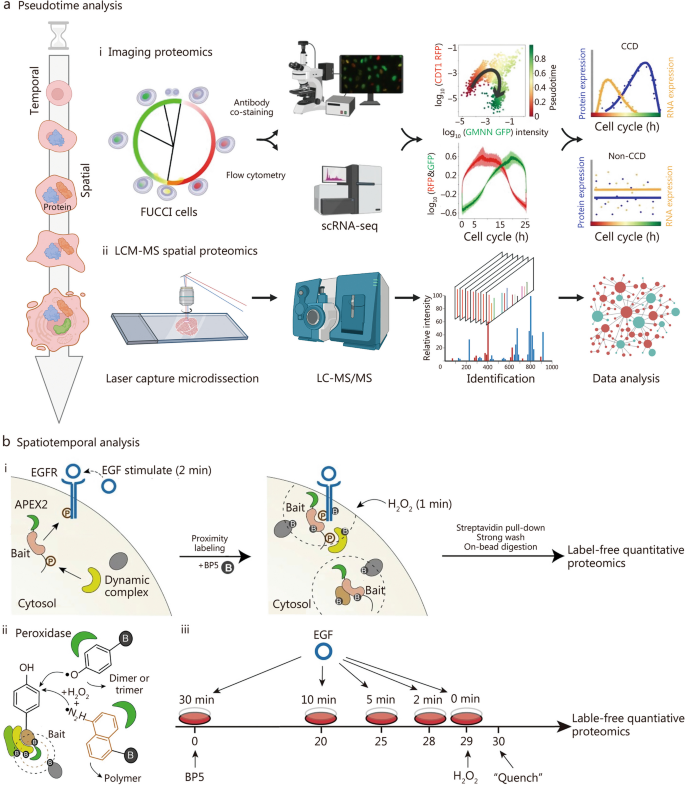
Temporal pseudotemporal analysis and spatiotemporal proteomic analysis. a Spatial proteomics analyses at different time points. (i) Spatial proteomic analysis uses U2OS FUCCI cells with dual fluorescent cell cycle indicators (CDT1 in G1 marked by red RFP and GMNN in S and G 2 highlighted in green GFP). This system provides insights into cell cycle behavior, particularly during the G 1 – S transition, where both markers are active, creating a distinct yellow hue. Using a polar model for RFP and GFP intensity, the cell cycle’s progression is streamlined into a linear format, facilitating the comparison of independent RNA and protein expression measurements aligned by individual cell pseudotime. Reprinted with permission from [ 91 ]. Copyright © 2021, Published by Springer Nature. (ii) LCM-MS spatial proteomics: utilizing laser capture microdissection followed by mass spectrometry (LCM-MS) for spatial proteomic analysis. b Spatiotemporal proteomics based on proximity labeling. (i) BP5 proximity proteomics: studies the adapter protein interactome response to epidermal growth factor (EGF) stimulation in living cells using BP5-based proximity labeling. (ii) Peroxidase-catalyzed proximity labeling: uses peroxidases for proximity labeling in both living cells and in vitro settings. (iii) Proximity proteomics workflow: involves a five-time-course EGF stimulation in HeLa cell lines stably expressing APEX2-FLAG-STS1. After EGF stimulation, cells are labeled with BP5 as indicated in the study design. Reprinted with permission from [ 90 ]). Copyright © 2021, Published by Springer Nature. CCD cell-cycle-dependent, scRNA-seq single-cell RNA sequencing, CDT1 chromatin licensing and DNA replication factor 1, RFP red fluorescent protein, GMNN geminin, GFP green fluorescent protein, EGFR epidermal growth factor receptor
Spatial proteomics
Spatial proteomics, an advanced field of study, utilizes a range of techniques, such as immunohistochemistry, immunofluorescence, mass spectrometry, and cytometry, to delineate protein distributions across scales ranging from whole tissues to subcellular entities [ 92 , 93 ]. Navigating these techniques involves careful consideration of trade-offs, including spatial resolution, analytical depth, molecular and cellular throughput, as well as data acquisition durations [ 94 , 95 ]. The techniques can be broadly categorized into two main groups: multiplexed antibody-based approaches and mass spectrometry-based methods, depending on the use of antibodies or other specific conjugates (Fig. 2 b and Table 1 [ 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 ]).
Multiplexed antibody-based techniques employ various antibody labeling mechanisms, including fluorophores, metal markers, and DNA barcodes [ 94 ]. Each labeling method has its advantages and disadvantages, necessitating the use of validated antibodies for accuracy. Notable fluorescent techniques include SWITCH [ 96 ], multiplex immunofluorescence [ 97 ], tissue-based circular immunofluorescence [ 98 , 99 ], and iterative bleach extended multiplexing [ 100 ], each distinguished by its approach to fluorescence signal removal. Indirect methods include the iterative indirect immunofluorescence [ 101 ] and the OPAL system [ 102 ]. Metal-based imaging methods, such as imaging cytometry [ 103 , 104 ] and multiplex ion beam imaging by time of flight [ 105 ], utilize metal-coupled antibodies and mass spectrometry for detection. DNA barcoded techniques like DNA exchange imaging [ 106 ], CODEX [ 107 , 108 ], and immuno-SABER [ 109 ], address the spectral limitations of fluorescence and bolster multiplexing capabilities. Despite the efficiency and versatility of DNA barcoding, meticulous antibody validation remains imperative.
The mass spectrometry-based method offers an alternative to antibody-based techniques. In the matrix-assisted laser desorption/ionization (MALDI) method, a pulsed laser ionizes biomolecules and peptides with near single-cell resolution (10 – 50 μm) [ 110 ]. Initially, surface proteins undergo in situ digestion to provide a peptide proteome representation. While MALDI offers high-resolution analysis of biological molecules and clarifies molecular pathways in aging and regeneration, it faces challenges such as sample degradation and matrix interferences [ 111 ]. Integrating antibody-based imaging with proteome characterization promises deeper tissue biology insights. The deep visual proteomics concept fuses AI-guided imaging with ultra-sensitive mass spectrometry-based proteomics, enhancing cell phenotype identification [ 112 , 113 ]. Additionally, expansion proteomics (ProteomEx) enables high-resolution proteome profiling, recently identifying proteins in Alzheimer’s disease (AD)-afflicted mouse brains [ 114 ], marking advancements in spatial proteomics.
Pseudotemporal-based STP
The initial pseudotemporal analysis presented herein focuses on instances of single-cell proteomics methodologies applied across various phases of the cell cycle. Cell division is intricately controlled by specific proteins, encompassing their presence, and activity. These proteins are meticulously regulated in both temporal and spatial dimensions through mechanisms such as transcriptional regulation, post-translational modifications, and protein degradation [ 115 , 116 ]. While traditional studies of the cell cycle have concentrated on cell populations, a deeper understanding of the intricate interplay between the cell cycle, senescence, and regeneration is of paramount importance [ 117 ]. With the progression of aging, there is an increasing dysregulation of the cell cycle, leading to reduced cellular senescence and regenerative capabilities [ 118 ]. This imbalance can precipitate age-related tissue dysfunction and the onset of diseases [ 119 ]. Comprehending and effectively manipulating the cell cycle within the contexts of senescence and regeneration are imperative for developing interventions that enhance tissue repair and delay the adverse effects of aging. Consequently, the cell cycle stands as a pivotal nexus linking the domains of aging and tissue regeneration. Previous studies have notably enhanced our comprehension of the cell cycle [ 115 , 116 ], but technical constraints have limited investigations into the variability of protein expression at the single-cell level. The advent of single-cell analyses has opened novel avenues for cell cycle research. Mahdessian et al. [ 91 ] employed single-cell proteomics, coupled with single-cell transcriptomics, to identify 1180 proteins expressed in U2OS cells out of a total of 2193 proteins characterized by cellular heterogeneity. This study involved customizing specific antibodies for these proteins. Through large-scale immunostaining and systematic antibody specificity validation, single-cell proteomic data were obtained, unveiling a total of 539 cell cycle-dependent proteins, with 301 proteins previously unassociated with the cell cycle, constituting 56% of the discoveries (Fig. 4 a [ 91 ]). This groundbreaking study established precise temporal expression profiles, tagged numerous proteins that play pivotal roles in proliferation, and marked the first temporal and spatial mapping of human proteome heterogeneity, systematically identifying cell cycle-associated proteins with heterogeneous expression at both mRNA and protein levels.
Spatiotemporal-based STP
The authentic STP involved the in vivo labeling of target proteins within distinct subcellular organelles using biotin in live cells, enabling the monitoring of their temporal and spatial dynamics. A recent study by Tian’s research team introduced two highly selective proximity labeling proteomics techniques [ 90 ]. These approaches revealed the spatiotemporal dynamics of interacting proteomes with remarkable temporal resolution at the subcellular level [ 90 ]. Specifically, a set of unique biotin analog probes was engineered to modulate the labeling efficiency of APEX2, thereby finely tuning the selectivity of protein complex labeling within live cells. This investigation involved the design of twelve APEX2 substrate probes, each incorporating varying electron-donating and electron-withdrawing groups strategically positioned around the phenolic structure of biotinol. These probes enable the capture of transient and weakly interacting protein complexes within living cells. A comprehensive series of in vitro and in vivo tests aimed at assessing labeling efficiency and selectivity culminated in the identification of two novel biotin analog probes, BP5 and BN2. Notably, both BP5 and BN2 probes demonstrated superior reactivity and selectivity for in vitro protein complex labeling within live cells compared to conventional biotinophenol probes (Fig. 4 b [ 90 ]). This enhanced specificity in protein complex labeling is primarily attributed to their reactivity and pronounced capacity to form dimers, trimers, and even multimers, thus enabling efficient labeling of intricate protein assemblages within a confined spatial domain while reducing self-quenching. This technological advancement not only promises enhanced accuracy in deciphering protein complexes within live cells but also furnishes robust tools and methodologies for proteomic inquiries, especially in exploring intricate biological mechanisms governed by spatiotemporal dynamics at a detailed level.
Genomics typically predicts potential outcomes, proteomics elucidates ongoing processes, and metabolomics reveals past events, thereby underscoring the profound capability of metabolomics to directly and precisely characterize the terminal state and phenotype of organisms [ 120 , 121 ]. STM, compared to spatial metabolomics and conventional bulk metabolomics, is particularly notable in the realms of aging and regeneration research [ 122 , 123 ]. STM offers precise tracking of temporal dynamics, enabling the pinpointing of transient regenerative events and distinguishing age-related metabolic changes within specific cellular compartments. This enhances our understanding of aging and regeneration with a finer level of detail. Notably, the biosynthesis, accumulation, and catabolism of metabolites in organisms exhibit a highly precise spatiotemporal distribution. The physiological functionalities of organisms are intricately intertwined with the spatial distribution of metabolites within tissues, extending even to the level of individual cells [ 24 , 124 ]. Therefore, unraveling metabolite heterogeneity in both temporal and spatial dimensions is pivotal in comprehending the intricate physiological and pathological alterations occurring in organisms. Consequently, the emergence of spatial metabolomics, integrated with advanced imaging techniques, has provided a preliminary means to visualize metabolites in biological specimens, addressing our quest for metabolite visualization since the onset of the current century [ 125 , 126 ]. Contemporary STM primarily relies on spatial proteomics methodologies, where tissue sections are analyzed at multiple time points, followed by the application of pseudo-spatiotemporal analysis algorithms to deduce the metabolite status across both temporal and spatial dimensions. In the following section, we will first introduce the spatial metabolomics approach, and then illustrate a representative case of STM through pseudotemporal analysis.
Spatial metabolomics
Spatial metabolomics, focusing on understanding the spatial distribution and organization of metabolites (small molecules involved in cellular processes) within biological systems such as organisms, tissues, or cells, employs mass spectrometry as a powerful method for the concurrent analysis of proteins, natural products, and metabolic derivatives [ 127 , 128 , 129 , 130 ]. Traditional mass spectroscopy techniques, lacking spatial information, have been supplemented by mass spectrometry-based imaging strategies using different ionization methods [ 131 ]. These methods include MALDI mass spectrometry [ 132 , 133 ], desorption electrospray ionization (DESI) [ 134 , 135 ], and secondary ion mass spectrometry (SIMS) [ 134 , 135 ]. These techniques enable label-free detection and mapping of a wide array of metabolites, including small molecules, lipids, peptides, organic compounds, and elemental ions, and mapping within cells and tissues (Table 2 [ 123 , 124 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 ]). For instance, atmospheric pressure MALDI achieves up to 2 μm resolution [ 138 ] , Space-MALDI allows metabolic profiling at single-cell scales [ 137 ], and transmission-mode MALDI-2 (t-MALDI-2) reaches a resolution of 1 – 2 μm [ 138 ] for detecting phospholipids and certain biomolecules. DESI, operational under ambient conditions [ 139 , 140 , 141 ], achieves 50 – 200 μm resolution, while its variations like nanoDESI reach 10 – 15 μm [ 142 ]. SIMS-based techniques like time-of-flight (TOF)-SIMS [ 143 ] and 3D OrbiSIMS [ 144 , 145 ] provide resolutions as low as 1 μm and 0.3 μm, respectively. Despite some challenges, these methods illuminate intricate molecular landscapes in tissues. Additionally, the spatial single nuclear metabolomics (SEAM) method has been introduced to tackle challenges related to segmentation and representation in SIMS data [ 124 ]. SEAM ensures the preservation of the sample’s native state through rapid and minimalistic processing, offering in situ metabolic fingerprints and individual nuclei clustering [ 124 ].
Pseudotemporal-based STM
Built upon spatial proteomics methodologies, the analyses conducted at these intervals enable a refined mapping of metabolite fluctuations, enhancing our understanding of their dynamics within biological systems. We delve into an in-depth discussion of this pseudo-STM approach, drawing from recent literature. Notably, Jin et al. [ 123 ] recently employed their novel air flow-assisted desorption electrospray ionization (AFADESI)-MSI technique, merging spatially resolved metabolomics with isotope tracer analysis. Their extensive study not only examined the action mechanism of the sedative-hypnotic drug YZG-331 but also performed a multi-target analysis using an established mass spectrometry imaging method. This investigation provided a systematical exploration of both the spatial and temporal distribution of endogenous metabolites within specific microregions of the rat brain following YZG-331 administration, showcasing the sensitive and comprehensive capabilities of AFADESI-MSI (Fig. 5 a [ 123 ]). The study identified functionally relevant metabolites associated with the drug action of YZG-331, localizing them within two metabolic pathways. Analysis of the “glutamine-glutamate-gamma aminobutyric acid (GABA)” metabolic pathway and isotopic glucose tracer analysis indicated a significant increase in the GABA/glutamate ratio in the hypothalamus, suggesting an enhanced glutamate decarboxylase activity post-administration of YZG-331. Furthermore, examining the “histidine-histamine-1-methylhistamine” metabolic pathway, coupled with isotopic histamine tracer studies, mainly attributed to increased peripheral histamine penetrating the pineal gland. Additionally, 1-methylhistamine levels significantly increased in the thalamic and hypothalamic regions post-administration.
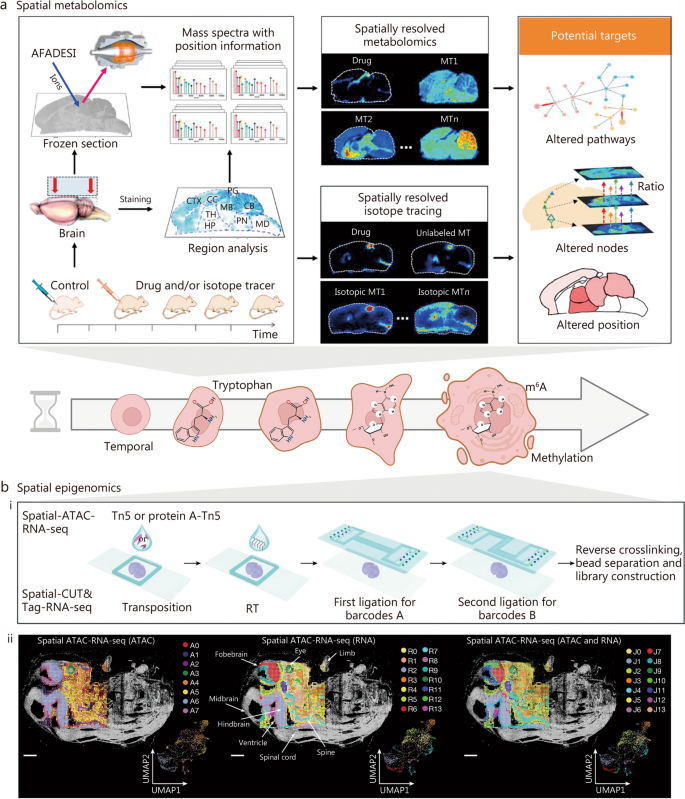
Temporal pseudotemporal analysis of spatial metabolomics and epigenomics. a Spatial metabolomics analyses at different time points: this section discusses an integrated approach using mass spectrometry imaging (MSI)-based spatiotemporally resolved metabolomics combined with isotope tracing to elucidate the multifaceted targets of central nervous system drugs. The analysis spans various brain regions, including the metabolite (MT), thalamus (TH), pineal gland (PG), hypothalamus (HP), midbrain (MB), cerebellum (CB), cortex (CTX), corpus callosum (CC), pons (PN), and medulla (MD). Reprinted with permission from [ 123 ]. Copyright © 2022, Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V. b Spatial epigenomic analyses at different time points. (i) Schematic workflow: this part delineates a structured workflow for spatial epigenomic analysis. (ii) Spatial distribution and uniform manifold approximation and projection (UMAP) analysis: the spatial distribution and UMAP of all clusters are presented for assay for transposase-accessible chromatin (ATAC), RNA, and ATAC and RNA data. The alignment of these clusters with tissue imaging demonstrates that the spatial clusters correspond precisely with anatomical regions. The pixel size for this analysis is set at 50 µm, with scale bars representing 1 mm. Reprinted with permission from [ 146 ]. Copyright © 2023, Published by Springer Nature. m6A mRNA modification N6-methyladenosine, Tn5 pA-Tn5 transposase 5 pre-adapter-transposase 5, RT reverse transcription, CUT&Tag cleavage under targets and tagmentation
This research contributes vital insights into the metabolic mechanisms underlying YZG-331’s effects, potentially informing our understanding of age-related changes and regeneration processes. Further explorations in these areas could unveil novel therapeutic interventions for aging and regenerative medicine. Moreover, this study highlights the efficacy of advanced mass spectrometry-based imaging techniques, such as AFADESI-MSI, in elucidating complex biological phenomena. The ability to map endogenous metabolites both spatially and temporally within specific brain microregions enhances our understanding of drug actions. Additionally, integrating isotope tracer analysis amplifies our capacity to unravel intricate metabolic pathways, showcasing the potential of these cutting-edge analytical techniques in aging and regeneration research.
Epigenetics, which refers to alterations in gene expression levels stemming from modifications in non-genetic sequences, is a subject of considerable focus [ 147 ]. Traditional epigenetic methods have predominantly utilized bulk sequencing, where cells from the acquired tissue are pooled before sequencing. These methods reflect an averaged state of all cells and may obscure the inherent heterogeneity among different cell types [ 148 , 149 , 150 ].
Technological advancements have facilitated single-cell-level epigenetic analysis, enabling researchers to isolate individual cells from the same tissue and assess their epigenetic status through sequencing [ 151 , 152 , 153 , 154 ]. However, this approach has its limitations, as tissue dissociation during single-cell suspension preparation may induce changes in intracellular gene expression and result in the loss of critical spatial information necessary for understanding cell function and corresponding biological mechanisms [ 153 , 155 ]. To address these challenges, spatial epigenomics has emerged, encompassing two primary analysis methodologies: one based on next-generation sequencing, exemplified by the spatial cleavage under targets and tagmentation (CUT&Tag) technology [ 156 , 157 ], which is the main focus of this paper; the other relying on high-resolution imaging techniques, such as multifluorescence in situ hybridization. Presently, spatial epigenomics primarily concentrates on transcriptome and proteome analysis [ 158 ], allowing for the simultaneous evaluation of spatial transcriptome and proteome profiles in the same tissue. The spatial CUT&Tag technology integrates microfluidic coding, a fundamental principle of the platform, with the CUT&Tag technology to investigate epigenetic modifications, enabling the precise localization of histone modifications. Understanding phenotypes relies on information from coupled genomic and transcriptomic analyses, but this does not tackle why identical DNA sequences exhibit diverse expression patterns in distinct cells. Integrating single-cell epigenomic analysis with transcriptomics can directly elucidate DNA epigenetic features, including DNA methylation, chromatin accessibility, and histone modifications associated with the originating transcriptomes. Current STE heavily relies on the spatial epigenomics approach, where tissue sections are examined at multiple time points. Pseudotemporal analysis algorithms are then applied to infer the status of gene epigenetic modifications in both temporal and spatial dimensions [ 159 ]. Therefore, we will begin by discussing the three frequently encountered forms of epigenetic modification: DNA methylation [ 160 , 161 , 162 , 163 ], chromatin accessibility [ 164 , 165 ], and histone modifications [ 156 , 166 , 167 , 168 , 169 , 170 , 171 , 172 ]. We will delineate the distinct characteristics of each modification and summarize the commonly employed methods for their investigation ( Table 3 [ 156 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 ] ) . Subsequently, a representative case study of STE research through pseudotemporal analysis will be illustrated.
These three epigenetic modifications intricately influence genomic function and exhibit cell type specificity. Simultaneously, there is a profound correlation between tissue structure and cellular function. As such, spatial epigenomics holds the potential to inaugurate a groundbreaking era within the field of advanced spatial genomics. In early 2022, Deng et al. [ 157 ] utilized the spatial CUT&Tag technology, an in-situ tissue encoding method, to pioneer a high spatial resolution analysis targeting specific histone modifications. This breakthrough facilitates a comprehensive examination of tissue development across spatial and genome-wide dimensions, elucidating epigenetic mechanisms underpinning both development and disease. Thereafter, Deng et al. [ 173 ] utilized spatial ATAC-seq technology to achieve in situ spatially resolved whole-genome sequencing of chromatin accessibility within tissues, marking a significant advancement. They further delved into epigenetic regulation by introducing combined spatial epigenome and spatial transcriptome sequencing approaches, embracing spatial multi-omics technologies [ 146 ]. Liu et al. [ 174 ] conducted spatial ATAC-RNA-seq combined with spatial CUT&Tag-RNA-seq analysis on mouse embryos, effectively distinguishing various organs within the embryos through the integration of epigenomic and transcriptomic data. This investigation focused on unraveling the differentiation trajectory from radial glia to postmitotic premature neurons, aiming to explore the intricate spatial and temporal correlations between chromatin accessibility and gene expression during embryonic development (Fig. 5 b [ 146 ]). These findings underscore the potential of spatial ATAC-RNA-seq technology as a powerful tool for investigating gene regulatory mechanisms and unraveling spatiotemporal dynamics in the context of tissue development. Integration with single-cell data demonstrated the technique’s ability to attain cellular or near single-cell resolution.
Spatiotemporal multi-omics techniques in aging research
In this section, we delve into the applications of spatiotemporal multi-omics in the realm of aging research, underscoring their pivotal role in unraveling the complexities of aging. This exploration includes how STT reveals aging signatures and the dynamics of cellular senescence, how STP sheds light on protein biomarkers and interaction networks relevant to aging, how STM uncovers metabolic shifts and their correlations with age-related phenotypes, and how STE decodes DNA methylation patterns and epigenetic clocks that gauge biological age. Through the integration of these multidimensional omics datasets, researchers are positioned to gain deeper insights into the molecular foundations of aging, identify novel therapeutic targets for age-related diseases, and ultimately pave the way for interventions that promote healthy aging. The convergence of spatiotemporal multi-omics with aging research signals an exciting epoch of discovery. It brings us closer to understanding the intricate tapestry of the aging process and fuels the pursuit of extending the quality and duration of human life.
Application of STT in characterizing age-related tissue changes
STT enables a more comprehensive investigation of cellular interactions within the aging tissue microenvironment, facilitating the discernment of dynamic changes in gene expression patterns over time and across various spatial regions within tissues. This approach provides insights into how cells communicate and adapt in the context of aging, revealing the complex molecular mechanisms driving age-related alterations in tissue structure and function [ 175 ]. STT in aging research primarily employs the pseudotemporal-based approach, which involves sequencing tissues organized at various time points using spatial transcriptomics techniques. For instance, Hahn et al. [ 176 ] employed spatial transcriptomics combined with single-cell sequencing to map the spatiotemporal transcriptome of the aging mouse brain comprehensively. Their detailed investigation revealed pronounced regional disparities in glial cell senescence, particularly within cerebral white matter glial cells, and identified specific cerebral regions responsive to regenerative interventions. Another study employed spatial multi-omics combined with single-cell sequencing across different age points to investigate the impact of apolipoprotein E (APOE) genotypes on aging, inflammatory responses, and amyloid reactions [ 177 ]. This study highlighted the role of the microglial subpopulation (Mi_6) in APOE4 carriers and senescent AD model groups, revealing how APOE4-associated microglia promote inflammation through regulatory pathways, leading to chronic neuroinflammation (Fig. 6 a-g). Second, spatial transcriptomics, revealed the prevalence of PIG high /OLIG low in APOE4 brains, suggesting complement activation, aberrant synaptic pruning, and disrupted axonal myelin sheath formation, which perpetuates neuroinflammation and hinders lipid metabolism (Fig. 6 h-j). Furthermore, spatial metabolomics techniques identified a region in APOE4 brains associated with lipid metabolism (Fig. 6 k), elucidating regulatory mechanisms making certain brain regions more susceptible to neurodegeneration in APOE4 carriers. Other studies, such as those by Stoeger et al. [ 178 ] have investigated the molecular aspects of aging by analyzing transcriptomic data from multiple studies, finding that changes in transcript length are associated with longevity. Spatial transcriptomics has been instrumental in uncovering the complex mechanisms underlying age-related changes in various tissues. Russ et al. [ 179 ] utilized this technique to examine transcriptomic changes in young and aged mouse ovaries, identifying cell-specific mechanisms that contribute to age-related fertility decline. Building on this approach, Ståhl et al. [ 19 ] mapped gene expression patterns in aged brain tissue at different times to reveal spatially distinct changes associated with aging, providing insights into the temporal dynamics of gene expression. Further demonstrating the utility of spatial transcriptomics, Asp et al. [ 44 ] characterized age-related heterogeneity within tissues, showing how different cells respond to aging processes. Besides, Kiss et al. [ 180 ] employed spatial transcriptomics to pinpoint regions in the aging mouse where senescent cells accumulate, leading to the development of inflammatory foci. This accumulation may impact age-related cognitive decline and dementia, linking cellular senescence to specific pathological outcomes in aging brains. Additionally, the utilization of spatial transcriptomic techniques and statistical methods has significantly advanced our understanding of spatial gene expression patterns, cellular senescence, and age-related processes. The introduction of Giotto by Dries et al. [ 181 ] marked a significant enhancement in analyzing and visualizing spatial transcriptomic data through a comprehensive, flexible, robust, and open-source pipeline. This development set the stage for further innovations, such as SPARK by Sun et al. [ 182 ], a statistical method specially designed to identify spatial expression patterns in spatially resolved transcriptomic, advancing our capability to interpret complex data landscapes. Building on these analytical advancements, Zhao et al. [ 183 ] introduced BayesSpace, a Bayesian method that not only enhances resolution in spatial transcriptomic data but also facilitates detailed clustering analysis, allowing for finer distinctions in tissue sample studies. Concurrently, Shang et al. [ 184 ] developed SpatialPCA, which extracts low-dimensional representations of spatial transcriptomics data while preserving the inherent biological signals and spatial correlations — essential for understanding cellular senescence and spatial gene expression patterns. Further complementing these methodological innovations, LaRocca et al. [ 185 ] discovered that noncoding repetitive element transcripts accumulate with age, serving as a reliable marker of biological age. Lastly, Kasemeier-Kulesa et al. [ 186 ] bridged single-cell and spatial transcriptomics through age- and location-matched scRNA-seq and 10× Genomics Visium analyses, providing a comprehensive view of gene expression and cellular behavior in aging tissues.
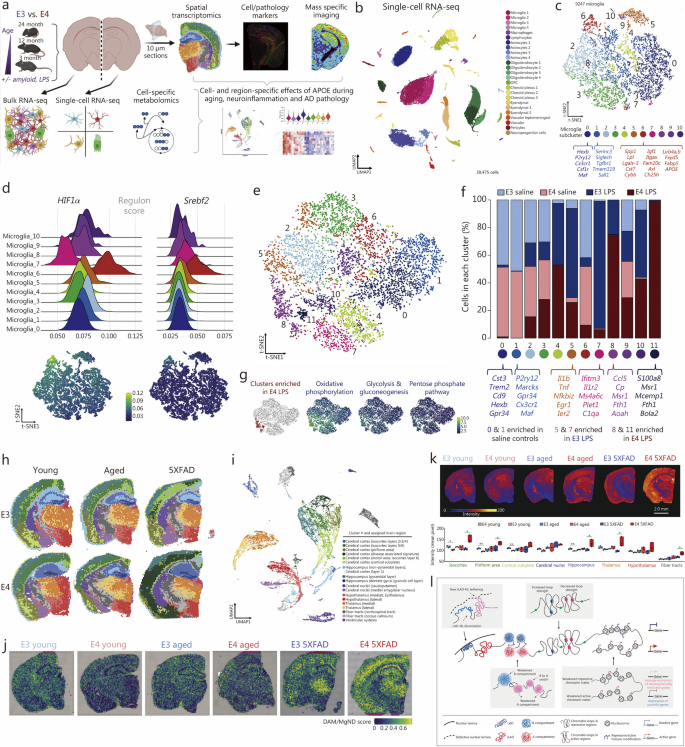
Implementing spatiotemporal multi-omics in aging research. a Brain analysis in APOE3 and APOE4 mice were systematically analyzed at different life stages (3, 12, and 24 months) and under specific conditions such as inflammatory challenge [lipopolysaccharide (LPS)] and Alzheimer’s disease (AD) pathology (characterized by amyloid overexpression). b Uniform manifold approximation and projection (UMAP) classification of gene expression clusters: a UMAP analysis identified 24 distinct clusters based on canonical gene expression markers. c Microglia sub-cluster analysis using t-distributed stochastic neighbor embedding (t-SNE): t-SNE revealed the microglial sub-clusters. Key biomarkers for “homeostatic” clusters (0 and 1) and disease-associated microglia (DAM)-like cluster 6 are annotated below the respective cluster labels. d Analysis of regulon activity scores: ridge plots (left) and t-SNE plots (right) illustrate the activity scores of HIF1a and Srebf2 regulons. e Microglial sub-cluster distribution in LPS- or saline-treated E3 and E4 mice: a t-SNE plot displays the distribution of 12 microglia sub-clusters in mice treated with LPS or saline, differentiated by colors. f The allocation of experimental groups across various microglia sub-clusters is depicted through a stacked bar chart. This visualization effectively illustrates the distribution of experimental groups among the different sub-clusters of microglia. g Within E4 LPS brains, t-SNE plots have uncovered a significant upregulation in crucial carbon pathways related to energy production. This increased activity is notably prevalent in specific subclusters found in brains with the E4 genotype that have undergone LPS treatment. h Spatial transcriptomic analysis is applied to brain sections from young, aged, and amyloid-overexpressing E3 and E4 mice, offering a detailed examination of genetic expression across different brain regions and conditions. i A thorough UMAP analysis is conducted for comprehensive data interpretation. j In spatial transcriptomic plots, DAM/neurodegenerative microglia (MgND) scores are displayed for each spot, calculated via AUCell. This analysis incorporates a brain from each experimental group for comparison. k The study also includes an analysis of a specific lipid, phosphatidylcholine (16:0/18:2). Top scans illustrate the lipid’s spatial distribution in coronal brain sections, while the bottom part quantifies the average pixel intensity of this phosphatidylcholine variant across different brain regions, offering a visual and quantitative insight into its distribution. Reprinted with permission from [ 177 ]. Copyright © 2023 The Author(s). Published by Elsevier Inc. l Schematic overview of spatial genome architectures in growing (center) and senescent (gray boxes) stem cells. Reprinted with permission from [ 187 ]. Copyright © 2022, Oxford University Press. APOE apolipoprotein E, LAD-NL lamina-associated domain-nuclear lamina, iLAD inter-LAD-NL
Collectively, these studies provide valuable insights into the spatial distribution of gene expression, the impact of cellular senescence on cognitive decline and neuroinflammation, and the potential mechanisms underlying regenerative and non-regenerative healing. The development of robust pipelines and statistical methods has enabled more effective analysis and visualization of spatial transcriptomic data, facilitating a deeper understanding of cellular processes and interactions within tissues. These findings have significant implications for future research in understanding the molecular changes underlying aging and developing novel therapeutic strategies for age-related disorders.
STP and STM approaches to elucidate molecular dynamics during aging
STP has revolutionized our understanding of aging by providing a dynamic perspective, enabling the identification of key specific proteins and pathways central to age-related changes [ 188 ]. This knowledge is invaluable for developing interventions targeting the molecular drivers of aging. A recent study employing STP systematically analyzed comprehensive proteomic profiles across various brain regions in non-human primates from fetuses to neonates [ 189 ]. This approach offers valuable insights into normal brain development and informs our understanding of mechanisms underlying dysfunctions and disorders in humans, with profound implications for neuroscience research. Hosp et al. [ 190 ] utilized STP to investigate proteomic changes during the progression of Huntington’s disease (HD) in the R6/2 mouse model. This study highlighted substantial reconfiguration of the soluble brain proteome, closely associated with the emergence of insoluble aggregates as the disease advanced. The accumulation of proteins in these aggregates was linked to their expression levels and specific sequence characteristics, underscoring the role of cellular protein dysfunction in HD toxicity. STP has identified proteins associated with aging, such as telomerase [ 191 , 192 ] and sirtuins [ 193 , 194 ]. The aberrant modulation of protein expression and functionality during aging is recognized as a key influence on the aging phenomenon [ 195 ]. Telomerase, typically quiescent in most adult cells but active in cancer cells and stem cells, presents a promising target for potentially decelerating or even reversing the aging process [ 191 ]. Huang et al. [ 196 ] utilized spatial proteomic analyses in 4 distinct brain regions, revealing differential proteins associated with resilient populations in AD. These studies underscore the significance of STP in elucidating key protein targets in aging and forming a basis for therapeutic interventions in age-related diseases.
Similarly, STM provides a comprehensive view of metabolic changes throughout the aging process. Walker et al. [ 197 ] at the University of California produced the first spatial and temporal metabolite map of the mouse brain across the aging spectrum, from adolescence to old age. This extensive dataset, encompassing 1547 molecules across 10 brain regions and 4 developmental stages [ 197 ], highlights significant variations in metabolites with no sex correlation. Notable findings include changes in sphingolipid patterns, indicative of myelin remodeling, and metabolic pathways during aging. The study revealed weakening metabolic correlations in the cerebrum from adolescence to adulthood and reduced cerebral segregation in old age [ 198 ]. These metabolic shifts, when correlated with gene and protein brain atlases, offer insights into brain metabolism’s spatial and temporal dynamics during aging. Additionally, spatial metabolomics techniques have been employed to investigate age-related metabolic changes, revealing spatially distinct metabolic signatures of aging [ 199 ]. Integrating spatial proteomic and metabolomic data has facilitated the identification of molecular interactions and pathways involved in aging [ 200 , 201 , 202 ]. These studies highlight the importance of proteomics and metabolomics in comprehensively elucidating the molecular mechanisms of aging in both spatial and spatial dimensions, leveraging advancements in STP and metabolomic techniques. Understanding molecular dynamics within tissues aids in deciphering age-related changes, identifying potential biomarkers, and developing targeted interventions for healthy aging.
STE profiling in a spatial context to understand age-related modifications
STE has arisen as a transformative methodology, enabling the elucidation of intricate interactions among epigenetic modifications, spatial arrangement, and temporal dynamics within the context of aging. This section delves into the pivotal function of STE in enhancing our comprehension of the molecular mechanisms underlying the aging process. Drawing upon perspectives from prominent researchers and recent empirical discoveries, this section insights into the diverse applications of STE in the field of aging research. STE provides a dynamic view of how our epigenome evolves with age. By understanding these changes within a spatial and temporal framework, we gain unprecedented insights into the epigenetic basis of aging, offering potential avenues for interventions to promote healthy aging.
The aging process is characterized by a multitude of epigenetic alterations, encompassing DNA methylation, chromatin accessibility, and histone modifications [ 2 , 3 , 203 ]. These tightly regulated and frequently reversible modifications exert influence over gene expression and various cellular processes, ultimately contributing to the onset and progression of numerous age-associated human disorders. A plethora of enzyme systems, including DNA methyltransferases, histone acetylases, deacetylases, methylases, and demethylases, in conjunction with chromatin remodeling factors, participate in establishing and maintaining epigenetic patterns [ 204 ].
The landscape of DNA methylation in humans has undergone cumulative alterations over time [ 204 ]. Initial investigations highlighted generalized age-related hypomethylation, yet subsequent analyses unveiled specific loci, including those associated with certain tumor suppressor genes and polycomb target genes, exhibiting heightened methylation levels during aging [ 203 ]. Additionally, cells derived from individuals and mice manifesting progeria-like syndromes demonstrate DNA methylation shifts that partially mirror those observed in typical aging processes [ 203 , 205 ]. However, the functional implications of these age-associated DNA methylation modifications remain elusive, primarily due to a lack of understanding regarding their temporal and spatial dynamics. STE, equipped to elucidate the distribution of epigenetic marks within cells and tissues across space and time, introduces a fresh vantage into the molecular dynamics underpinning aging. Rodriguez-Muela et al. [ 206 ] pioneered the use of spatial epigenomics to investigate how DNA methylation patterns shift with age, identifying specific epigenetic alterations that vary distinctly across different tissue regions. Building on this foundation, Zocher et al. [ 207 ] extended these analyses to brain tissue, where they mapped the spatial variability of DNA methylation changes, further detailing the epigenetic landscape of aging neural tissue. Smith et al. [ 208 ] underscored the importance of comprehending these spatiotemporal dynamics, emphasizing that a comprehensive grasp of epigenetic modifications throughout the aging process is crucial for developing targeted anti-aging therapies. Complementing these findings, Wu et al. [ 209 ] broadened the scope of the STE study by examining how the common mRNA modification N 6 -methyladenosine (m 6 A) affects primate tissue health and aging. They discovered tissue-specific m 6 A changes in the liver, heart, and skeletal muscle of both young and aged nonhuman primates, particularly emphasizing the susceptibility of skeletal muscle to m 6 A reduction during aging and highlighting the crucial role of the m 6 A methyltransferase-like 3 (METTL3) in maintaining muscle health. These findings shed light on the mechanisms underlying tissue aging and reveal a METTL3-m 6 A- nephronectin (NPNT) axis that helps mitigate muscle degeneration associated with aging.
Aging is strongly associated with both global histone loss and tissue-specific alterations in post-translational modifications of histones [ 3 , 210 ]. Enhanced histone expression has been linked to extended lifespan in Drosophila, while investigations in fibroblasts from older individuals and patients with progeria have revealed increased histone H4K16 acetylation or H3K4 trimethylation, alongside reduced levels of H3K9 or H3K27 trimethylation [ 211 ]. These modifications of histones have the potential to induce shifts in transcriptional activity, disrupt cellular homeostasis, and contribute to age-related metabolic decline. Notably, the diminishment of telomeric heterochromatic markers has been demonstrated to result in telomere elongation.
In addition to DNA and histone-modifying factors, various chromosomal proteins and chromatin remodeling factors, including heterochromatin protein 1a and polycomb histones, play a role in genome-stabilizing DNA repair and senescence regulation [ 212 ]. The lowest hierarchical level of chromosomal organization involves the folding of chromatin fibers, intimately associated with chromatin recycling [ 187 ]. This recycling process, characterized by myriad DNA and histone modifications, plays a pivotal role in transcriptional regulation, determining DNA accessibility to the transcriptional machinery (Fig. 6 l [ 187 ]). Modifications in these epigenetic elements result in significant shifts in chromatin configuration, encompassing widespread heterochromatin depletion and repositioning, prevalent occurrences in senescent cells. For instance, Zhang et al. [ 213 ] employed advanced epigenetic high-throughput methods, including chromatin accessibility sequencing, chromatin immunoprecipitation sequencing, limited enzyme digestion, and isotope-labeled quantitative proteomics, to unveil fundamental patterns of histone epigenetic modifications in senescent cells, elucidating the dynamics of chromatin spatial accessibility, and highlighting the significant contributions of epigenetic factors like lysine demethylase 4. The study underscores the potential targeting value of these molecules for manipulating senescence.
STE offers an innovative perspective for exploring the dynamic epigenetic alterations accompanying aging. By unveiling the spatiotemporal distribution of epigenetic marks, this paradigm enhances our understanding of the molecular mechanisms driving the aging process while delineating potential avenues for interventions aimed at fostering healthy aging.
Spatiotemporal multi-omics techniques in regeneration studies
Regeneration stands as a remarkable biological phenomenon observed across the animal kingdom, encompassing a broad spectrum of organisms, from invertebrates like planarians to vertebrates like salamanders. However, within mammals, including humans, regenerative potential remains comparatively limited, predominantly confined to select tissues, such as the liver and skin. In the realm of regenerative medicine, the integration of spatiotemporal multi-omics approaches holds great promise. Drawing upon insights gleaned from existing literature, the hallmarks of regeneration can be conveniently categorized into three principal domains: the classification of regeneration types, the underlying mechanisms governing regeneration, and the regulatory processes that orchestrate these events. Regeneration is typically classified into three types, complete, incomplete, or compensatory, depending on the extent of restoration achieved [ 214 , 215 , 216 , 217 ]. Complete regeneration entails the comprehensive reinstatement of a lost or damaged component, achieving both structural and functional recovery. This phenomenon is observed in organisms as diverse as planarians. In contrast, incomplete regeneration results in only partial restoration, potentially recovering some, but not all, aspects of structure or function, as seen in peripheral nerves. Compensatory regeneration, on the other hand, allows an organ or tissue to regrow to functional sufficiency without replicating the original form or achieving complete functionality. A prime example is live regeneration in various vertebrates, including humans, where hepatocytes proliferate to regain function, though not the original liver structure (Fig. 1 b).
The processes governing regeneration predominantly encompass cell proliferation, migration, differentiation, and the precise patterning of regenerating tissues and organs [ 218 , 219 ]. Cell proliferation serves as the foundational process, where new tissues or organs arise through cellular division and propagation [ 220 , 221 , 222 , 223 ]. Cell migration involves the movement of cells from one location to another, establishing the correct structural arrangement [ 221 , 224 , 225 ]. Cell differentiation marks the transformation of cells from an undifferentiated state into specialized cell types, such as muscle or nerve cells [ 220 , 221 ]. Pattern formation of regenerating organs refers to the capacity of newly generated tissues or organs to develop in a specific sequence and arrangement during the regeneration process [ 226 , 227 ]. Governing the regeneration process is an array of influential factors, encompassing genes (i.e, MSX , p53 , HOX ) [ 228 , 229 , 230 , 231 , 232 , 233 ], environmental elements (i.e . , temperature) [ 234 , 235 ], hormones (i.e., androgens and estrogens) [ 128 , 129 , 236 , 237 , 238 , 239 ], and growth factors (fibroblast growth factor and epidermal growth factor) [ 240 , 241 , 242 , 243 ]. Of particular significance, genes play a pivotal role in the regulation of cell proliferation, differentiation, and migration, intricately controlling the complex process of regeneration.
The integration of spatiotemporal multi-omics techniques into regenerative medicine holds the potential to offer invaluable insights into the intricate and dynamic molecular events governing tissue regeneration. This integration may pave the way for novel therapeutic approaches and enhance our understanding of the regenerative potential within mammals, including humans. In this review, we explore recent applications of spatiotemporal multi-omics approaches within the field of regenerative medicine, shedding light on their potential to revolutionize our understanding of regenerative processes and their therapeutic implications.
Utilizing STT in investigating tissue regeneration processes
STT, integrating high-throughput gene expression profiling with spatial and temporal data, enables detailed characterization of gene expression patterns in regenerating tissues. This technique captures the spatial organization of gene expression over time, allowing researchers to identify specific cell types, signaling pathways, and molecular signatures crucial to tissue regeneration [ 19 , 244 ]. It provides a comprehensive perspective on transcriptional changes during different stages of tissue repair, illuminating the dynamic cellular responses and regulatory networks involved in regeneration. For instance, Cui et al. [ 245 ] conducted a pioneering study on planarian regeneration. By merging spatial transcriptomics with single-cell sequencing, they created a 3D transcriptome atlas detailing characteristic cell distributions and gene expression patterns at six-time points, later expanded with two additional time points (Fig. 7 ai). This study revealed a totipotent stem cell cluster and identified multiple gene expression patterns linked to planarian regeneration (Fig. 7 aii). Using spatial module screening tools, the study pinpointed polar gene pairs crucial to wound-specific expression and to regions pre-/post-planarian. A key regulatory gene, plk1 , was identified as critical in planarian formation and regeneration (Fig. 7 aiii-iv), with functional analyses indicating its role as an early response element in blastocyst formation and subsequent regeneration events (Fig. 7 av-vi). These insights notably enhance our comprehension of the intricate regulatory mechanism’s planarian regeneration, relevant to both specific planarian studies and broader mammalian regeneration research.
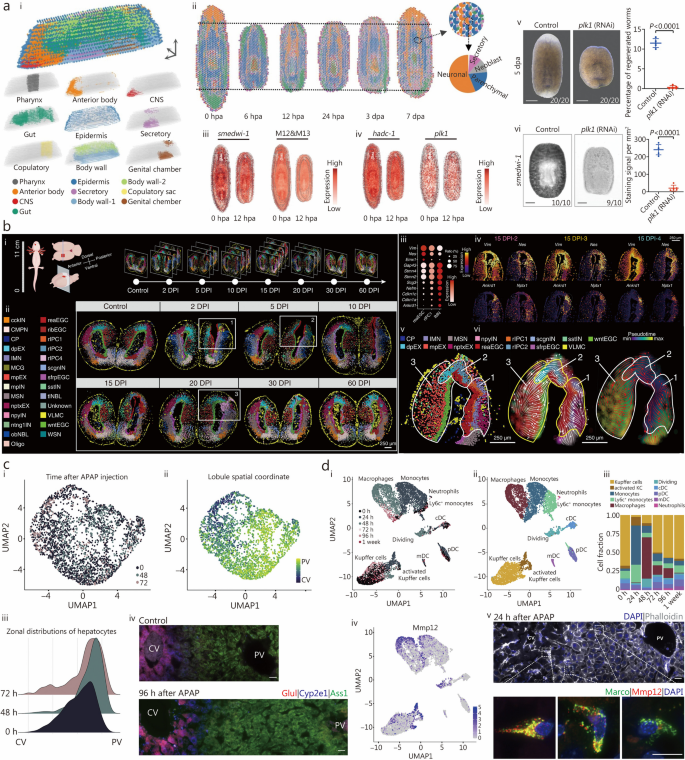
Leveraging spatiotemporal multi-omics in regenerative studies. a Planarian regeneration analysis: (i) spatial transcriptomics spots were grouped using the STAGATE method, labeling clusters based on their spatial expression patterns. (ii) The study visually tracks primary cell types’ spatial distribution during regeneration stages using pie charts in corresponding spatial transcriptomics spots. (iii) Spatial distribution mapping of smedwi-1 and neoblast-related genes provides insights into their localization. (iv) Spatial transcriptomics data at 0 and 12 hpa examines spatial expression patterns of regulatory genes hdac-1 and plk1 during early regeneration. (v) A plk1 knockdown effect in planarians at 5 dpa is illustrated in a bright-field image. (vi) WISH staining of smedwi-1 in control and plk1 knockdown planarians at 3 dpa. Reprinted with permission from [ 245 ]. Copyright © 2023 The Author(s). Published by Springer Nature. b Axolotl telencephalon regeneration research: (i) schematic illustrates sample collection and examined sections during homeostatic and regenerative phases. (ii) Stereo-seq maps cell types in axolotl telencephalon sections using various stages of regeneration. (iii) Bubble chart displays the fluctuating expression of marker genes defining cell types crucial for telencephalon regeneration. (iv) Heatmap shows spatial expression trends of essential markers within the injured area at 15 DPI-2, -3, and -4. (v) The spatial arrangement of cell types around the regeneration site is shown for the 15 DPI-4 section. (vi) RNA velocity plots illustrate predicted cellular lineage transitions in regenerating axolotl telencephalon area. Reprinted with permission from [ 60 ]. Copyright © 2022, The American Association for the Advancement of Science. c Hepatocyte study post-APAP injection: (i) UMAP visual representation of hepatocytes color-coded by time post-APAP injection. (ii) UMAP of hepatocytes color-coded by inferred spatial coordinates within the lobule. (iii) Distribution of spatial coordinates among hepatocytes for different time intervals post-injection. (iv) smFISH images of a liver lobule exhibit zonated genes at different time points post-APAP. Reprinted with permission from [ 246 ]. Copyright © 2022 Elsevier Inc. d Myeloid cell analysis post-APAP administration: (i) UMAP visualization of myeloid cell populations color-coded by time post-APAP administration. (ii) UMAP color-coded by myeloid cell types. (iii) Proportions of various myeloid cell subtypes at each examined time point. (iv) UMAP visualization of myeloid cells, colored by expression levels of Mmp12. (v) smFISH scan (top) and zoomed-in insets (bottom) of a liver lobule 24 h after APAP injection. Reprinted with permission from [ 246 ]. Copyright © 2022 Elsevier Inc. CNS central nervous system, hpa hours post-amputation, dpa days post-amputation, WISH whole-mount in situ hybridization, reaEGC reactive ependymoma cell, IMN immature neuron, rIPC1 regeneration intermediate progenitor cells 1, UMAP uniform manifold approximation and projection, APAP acetaminophen, CV central vein, PV portal vein, smFISH single-molecule fluorescence in situ hybridization, KC kupffer cells, pDC plasmacytoid dendritic cells, cDC conventional dendritic cells, mDC myeloid dendritic cells, Mmp12 matrix metallopeptidase 12, UMI unique molecular identifier
In the regeneration field, a multi-institute research team led by the Beijing Genomics Institute recently applied Stereo-seq to examine self-healing processes of brain injuries in axolotls [ 60 ]. They conducted Stereo-seq analysis on a cerebral cortex section from the salamander’s telencephalon during a 60-day post-recovery period (Fig. 7 bi). This approach identified 28 distinct cell types (Fig. 7 bii), revealing that morphological wound healing occurred around 30-d post-injury, with complete cell type restoration by 60-d post-injury. This study identified an additional cell type that exhibits patterns similar to those of reactive ependymoma cells (reaEGCs) and immature neurons (IMNs), and expressing markers from both categories (Fig. 7 biii-iv). This cell type, termed “regeneration intermediate progenitor cells 1” (rIPC1) suggests a sequential transition of cell types (reaEGCs-rIPC1-IMNs) during regeneration. RNA velocity analysis confirmed the interconnected sequence of these cell types (Fig. 7 bv-vi), providing compelling evidence for their interdependence in the regeneration process. The establishment of spatiotemporal cellular maps for salamander brain development and regeneration is crucial in understanding brain regeneration, the intricate structure of amphibian brains, and evolutionary brain structure trajectories. It also opens new avenues for clinical interventions in human tissue and organ self-repair and regeneration, while offering a valuable data repository for species evolution studies. Ben-Moshe et al . [ 246 ] conducted a comprehensive investigation into liver regeneration dynamics using spatial transcriptomics, single-cell sequencing, and bulk transcriptome sequencing. Their work elucidated the mechanisms underlying zonal hepatocyte regeneration and the supportive role of various region-specific non-parenchymal cells (Fig. 7 c). They noted the downregulation of major histocompatibility complex class I and class II molecules, inhibiting adaptive immune activation during liver regeneration (Fig. 7 d). This study severs as a comprehensive resource on the coordinated mechanisms of zonal liver regeneration, enriching our understanding of this complex process.
Collectively, these studies highlight the potential role of STT in regenerative medicine. Employing axolotls, planarians, and liver models, they provide invaluable insights into the mechanisms of tissue repair. By mapping gene expression changes within a spatial and temporal context, researchers can identify key genes, cell types, and pathways essential for successful regeneration. This methodology not only deepens our comprehension of regenerative phenomena but also offers valuable tools for advancing regenerative medicine research. STT is poised to unravel the complexities of tissue restoration and drive progress in regenerative therapies.
STP and STM in identifying key factors and pathways in regeneration
Recent advancements in STP and STM have yielded powerful tools for dissecting the intricate processes involved in regeneration. This section delves into the invaluable contributions of these cutting-edge technologies in identifying key factors and pathways crucial for the successful regeneration of tissue and organs. By elucidating protein expression patterns and metabolic changes, researchers can unravel the spatially restricted molecular dynamics that underlie the process of tissue repair [ 34 , 247 , 248 ]. This innovative approach facilitates the discovery of novel biomarkers and therapeutic targets, thereby enhancing the prospects of regeneration.
STP is geared towards elucidating the spatial distribution and temporal dynamics of proteins within regenerating tissues. This approach has played a pivotal role in unraveling the molecular mechanisms that drive regeneration across diverse model organisms. In a notable study, Mallah et al. [ 249 ] conducted a spatiotemporal microproteomic analysis to explore traumatic brain injury (TBI) over a 10-d post-injury period. Their findings showcased a restoration of the brain’s protein profile to its pre-injury state within this timeframe. Intriguingly, they also identified a pronounced upregulation of proteins associated with Parkinson’s disease (PD) in the brain’s substantia nigra as early as 3 d post-injury, hinting at a potential association between TBI and PD. This investigation, employing spatiotemporal microproteomics, provides invaluable insights for regenerative medicine. By identifying specific protein markers and tracing their trajectories immediately following injury, the study sheds light on the intrinsic regenerative capacities of the brain. The suggested connection between TBI and PD underscores the need to comprehend the broader implications of traumatic injuries, a perspective crucial for shaping regenerative and neurotherapeutic strategies. Furthermore, the employment of in vitro models to validate these findings amplifies the translational significance of the study. Huang et al. [ 196 ] examined 4 distinct brain regions such as the caudate nucleus, hippocampus, inferior temporal parietal lobule, and the middle gyrus of the superior temporal gyrus in their study involving 11 healthy controls, 12 individuals demonstrating resilience to Alzheimer’s disease (RAD), and 20 patients with AD. Their results identified 33 proteins differentially expressed in the resilient group. Notably, 5 of these proteins — platelet-activating factor acetylhydrolase Isoform 1B subunit 3 (PA1B3), testican-3 (TICN3), intercellular adhesion molecule 1 (ICAM1), immunity-related GTPase Q (IRGQ), and aldehyde dehydrogenase 1 family member L1 (AL1L1) — were uniquely associated with RAD, suggesting their potential significance in the pathogenesis of AD. This study, by highlighting reduced soluble Aβ levels and pinpointing specific proteins linked to RAD, provides crucial insights that may prove instrumental for regenerative medicine research and future therapeutic interventions for AD.
Spatiotemporal protein expression profiling also offers fresh insights into peripheral nerve regeneration mechanisms and potential therapeutic targets. Bryan et al. [ 250 ] employed a reverse phase protein microarray approach to investigate the dynamic protein expression patterns during the 28-d course of peripheral nerve regeneration in a rat sciatic nerve transection injury model. The research delves into the spatiotemporal profiles of various proteins involved in nerve regeneration, including growth factors, extracellular matrix proteins, and those related to adhesion and migration. The findings reveal distinct changes in protein expression across different segments of the regenerating nerve and suggest potential implications for future research in understanding the molecular mechanisms underpinning nerve regeneration and identifying therapeutic targets.
STM, on the other hand, centers on the study of the changes in metabolite levels and fluxes within tissues during regeneration, offering insights into the metabolic processes that underpin tissue repair. A study on liver regeneration after acute damage integrated spatial metabolomics with spatially resolved scRNA-seq to elucidate the dynamics of regeneration [ 246 ]. This integrated approach unveiled the coordinated roles of different cell types, including hepatocytes, endothelial cells, hepatic stellate cells, and macrophages, in liver tissue repair. STP and STM have emerged as indispensable tools for dissecting the complexities of tissue and organ regeneration. They provide a comprehensive view of the molecular events that transpire during the repair process. Ongoing research in this domain holds great promise for regenerative medicine, as it offers insights into potential targets for therapeutic interventions aimed at enhancing tissue regeneration.
Epigenetic regulation in spatial and temporal dimensions during tissue repair
Epigenetic modifications play a critical role in regulating gene expression and cellular identity during tissue regeneration [ 36 , 251 ]. Spatial epigenetic profiling allows for the investigation of epigenetic changes within the spatial and temporal context of tissue repair [ 206 ]. By examining DNA methylation patterns and chromatin states, researchers can gain insights into the spatially defined epigenetic regulation of regeneration-associated genes and the establishment of cell fate during tissue repair. This knowledge enhances our understanding of the epigenetic mechanisms governing regeneration.

Challenges and future prospects
In the fields of aging and regenerative medicine, a host of challenges beckon, including the complexity of data integration, the imperative for high-resolution techniques, the quest to decipher temporal dynamics, the enigma of interpreting multimodal data, and the quest for standardization. The horizon, however, gleams with promise in the realms of single-cell profiling, multi-omics integration, machine learning and AI, functional validation, and precision medicine. These avenues offer profound insights, interventions, and early detection mechanisms in the relentless pursuit of unraveling aging and tissue regeneration (Fig. 8 ).
Challenges and future directions in spatiotemporal multi-omics research. In the domains of aging and regenerative medicine, challenges encompass complexities in data integration, the imperative for high-resolution techniques, comprehension of temporal dynamics, interpretation of multimodal data, and the establishment of standardization. Looking ahead, there is great promise in single-cell profiling, multi-omics integration, machine learning and AI, functional validation, and precision medicine. These advancements hold the potential to provide profound insights, interventions, and early detection capabilities in the study of aging and tissue regeneration. m 6 A mRNA modification N 6 -methyladenosine, AI artificial intelligence
Data integration complexity
In the realm of aging and regeneration research, deciphering the intricate relationship of spatial and temporal factors stands as a linchpin for uncovering the core molecular mechanisms. Traditional multi-omics approaches, while offering significant molecular insights, frequently fall short in terms of spatial and temporal resolution [ 82 , 252 , 253 , 254 , 255 , 256 , 257 ]. This limitation threatens to obscure a comprehensive comprehension of processes like aging and tissue regeneration. Consequently, spatiotemporal multi-omics techniques that integrate both spatial and temporal dimensions have gained prominence. Such integration captures the dynamic intra-tissue changes over time and discerns molecular signatures specific to various aging or regeneration phases, enriching our understanding of spatial and temporal nuances [ 37 , 258 ].
However, the analysis of extensive spatiotemporal omics datasets presents distinct challenges, necessitating bespoke computational methods. These challenges include handling the data’s high dimensionality and intricate spatial structures [ 259 , 260 ], as well as discerning spatial patterns and cellular diversities [ 261 ]. In response, tools such as spatial clustering algorithms and spatially constrained dimensionality reduction have been developed. For instance, the utilization of spatially constrained non-negative matrix factorization aids in identifying unique cell types and their gene expression profiles [ 34 , 262 , 263 ]. Moreover, the integration of diverse spatiotemporal datasets, spanning transcriptomics to epigenomics, presents a formidable undertaking [ 264 , 265 , 266 ]. These datasets often derive from different platforms, leading to disparities in data types and scales. Overcoming these demands, the creation of robust analytical tools for data harmonization and the introduction of advanced techniques, including multi-omics integration algorithms, becomes imperative. These tools are instrumental in harmonizing and deciphering the varied and intricate datasets, thereby enabling researchers to distill invaluable insights from the integration of diverse omics layers. This integrative approach stands as a cornerstone for unraveling the multifaceted aspects of aging and regeneration, providing a more comprehensive understanding of their spatiotemporal intricacies. The achievement of proficient data integration thus becomes pivotal in our pursuit of comprehending the intricate spatiotemporal intricacies of aging and regeneration.
High-resolution techniques
Obtaining high-resolution data capable of capturing dynamic changes at the cellular and subcellular levels emerges as a categorical imperative. Existing techniques, often lacking the necessary spatial and temporal granularity to dissect intricate processes during aging and regeneration, necessitate a quantum leap. The development of cutting-edge imaging and omics technologies stands as the panacea to surmount this challenge. For instance, single-cell multi-omics approaches have the potential to unveil granular insights into individual cell behaviors over time [ 165 , 267 , 268 , 269 ]. Advanced imaging techniques, including super-resolution microscopy and live cell imaging, offer high-resolution spatial and temporal data [ 270 , 271 , 272 , 273 , 274 ]. Strategic investment in research and innovation to refine and expand these techniques will be critical for gaining a deeper into the spatiotemporal dynamics within these domains.
Temporal dynamics
Comprehending the temporal dynamics of biological processes takes center stage in aging and regeneration research. Current methods often provide snapshots of static states, thereby rendering the capture of dynamic changes a formidable task. In Part II, spatiotemporal multi-omics bifurcate into two main classes. The first entails pseudotemporal analysis through the utilization of spatial multi-omics, examining tissue sections from diverse time intervals. The second employs genuine spatiotemporal genomics techniques, labeling specific cellular components to investigate temporal changes. However, the pseudotemporal approach remains dominant in aging research due to its established methodology and ease of use [ 43 , 275 ]. Addressing this challenge requires the conception of longitudinal and time-series experimental designs. Researchers must employ technologies that facilitate real-time monitoring and sampling of biological systems over extended durations. Additionally, computational tools for modeling and analyzing temporal data necessitate enhancement to extract meaningful patterns and identify critical transition points during aging and regeneration.
Interpreting multimodal data
The integration of multiple omics modalities, encompassing genomics, proteomics, and metabolomics, beckons the need for comprehensive frameworks for interpreting data effectively. The intricate interactions across these layers in aging and regeneration require innovative approaches to unravel intricate networks and pathways [ 276 ]. Techniques for multimodal data integration, including network-based analyses and pathway enrichment methods, must be refined to account for dependencies and interactions between distinct omics components [ 277 , 278 , 279 , 280 ]. Furthermore, interdisciplinary collaboration spanning biology, bioinformatics, and systems biology, is indispensable for advancing our ability to interpret multimodal data proficiently. Contemporary biotechnology enables the simultaneous measurement of multiple high-dimensional modalities (e.g., RNA, DNA accessibility, and proteins) within a single tissue sample. To comprehensively elucidate the role of gene regulation in steering biodiversity and function, a combination of diverse analytical approaches becomes imperative. However, prevailing methods primarily cater to single-task analysis, offering only a partial insight into multimodal data. Recent breakthroughs in single-cell biotechnology empower the concurrent assessment of gene expression and other high-dimensional modalities within the same cell, yielding multimodal histological data [ 281 , 282 , 283 ]. This data vantage furnishes a comprehensive view of cellular transcriptional and functional processes. Nonetheless, traditional methodologies fall short of handling such multimodal data, necessitating the development of novel approaches to fully harness their potential [ 277 , 278 ]. While researchers have put forth various methods for multimodal analysis, tackling tasks such as joint group identification, cross-modal prediction, and cross-modal correlation discovery, these methodologies are typically tailored with a singular objective, posing challenges for their integration into a unified framework [ 284 , 285 , 286 , 287 ]. To bridge this divide, Tang et al. [ 288 ] introduced interpretable multi-task deep neural networks, demonstrating efficacy in navigating the intricacies of multimodal data analysis. The network utilizes an encoder-decoder-discriminator architecture, which is iteratively trained to perform tasks such as joint cluster identification and cross-modal prediction. Such a framework holds significant potential to accelerate the uncovering of cell-type-specific regulatory dynamics across transcriptomics and other modalities.
Standardization
The establishment of standardized protocols and data formats for spatiotemporal multi-omics data assumes paramount importance, ensuring data reproducibility and comparability across studies [ 289 , 290 ]. Presently, variations in experimental protocols, data preprocessing steps, and data reporting impede data harmonization. Collaborative endeavors among researchers are imperative to define and embrace common data standards and best practices. This includes the development of metadata standards encompassing experimental conditions, sample information, and data preprocessing steps. Moreover, the establishment of data repositories and platforms adhering to these standards becomes imperative to facilitate data sharing and reproducibility within the scientific community. Standardization efforts will enhance the reliability and utility of spatiotemporal multi-omics data in aging and regeneration research.
Future prospects
Integration of additional layers such as 3d configuration and single-cell resolution.
The horizon of spatiotemporal multi-omics research in the context of aging and regeneration radiates with great promise, especially within the realm of single-cell analysis. These advancements will furnish a granular perspective of individual cells as they evolve over time [ 291 , 292 , 293 ]. This approach will uncover previously veiled cellular heterogeneity and dynamics, enabling the discernment of rare cell populations, transitional states, and responses of cells to environmental cues. Single-cell spatiotemporal profiling is poised to be instrumental in unraveling the intricacies of tissue regeneration, elucidating the roles of distinct cell types, and meticulously tracking their behavior throughout the intricate process.
Moreover, the incorporation of additional layers of information, such as 3D configuration and single-cell resolution, constitutes a pivotal direction for advancing spatiotemporal omics within aging and regeneration research. 3D imaging techniques, including spatial transcriptomics in 3D and expansion microscopy, possess the capability to bestow spatial context with greater resolution, thereby facilitating the exploration of tissue architecture and cellular interactions [ 44 , 294 , 295 , 296 ] . Furthermore, the fusion of single-cell transcriptomics with spatial omics techniques is poised to unveil the landscape of cell heterogeneity and lineage trajectories during aging and regeneration, ultimately streamlining the identification of critical cell types and their functional roles [ 24 , 296 , 297 , 298 ]. Combining these multidimensional approaches will offer a more comprehensive understanding of the complex biological processes involved.
It is imperative to acknowledge that the spatiotemporal transcription approach employed by TEMPOmap may potentially introduce sequence bias, necessitating the incorporation of U analogs for metabolic labeling and thoughtful design of DNA probes [ 43 ] Subsequent investigations could synergize TEMPOmap with high-throughput single-cell functional genomics to pinpoint pivotal molecular factors shaping the kinetic landscape of the RNA life cycle. Furthermore, optimizing the conditions for metabolic labeling and integrating diverse molecular probing protocols hold the potential to extend the applicability of this method to isolated or in vivo tissue samples, thereby systematically unraveling dynamic events in tissue biology. The coordination of STT patterns may yield insights into the molecular mechanisms governing a range of biological phenomena, encompassing developmental processes, pattern formation, memory and learning, and biological rhythms, as well as the initiation and progression of disease.
Multi-omics integration to advance the understanding of biological systems
The future is set to witness an unwavering emphasis on the development of integrated multi-omics approaches, thereby ushering in a comprehensive and interconnected understanding of aging and regeneration processes. The fusion of genomics, proteomics, metabolomics, and other omics data will empower researchers to construct holistic models that capture the multidimensional facets of biological systems. Advanced computational tools will abet the integration of diverse omics layers, ultimately enabling the identification of key regulatory hubs and cross-talk between different molecular components. This integrated perspective is poised to pave the path toward more targeted and precise interventions and personalized therapies within the domain of aging-related diseases and regenerative medicine.
Addressing technical and computational challenges for broader adoption
While the realm of spatiotemporal omics teems with potential, it concurrently presents several technical and computational challenges. The formulation of standardized protocols for sample preparation, data acquisition, and analysis is essential to ensure reproducibility and comparability across studies [ 289 , 299 ]. Additionally, the scrutiny of large-scale spatiotemporal omics datasets requires advanced computational methods and tools for data integration, visualization, and interpretation [ 280 , 300 ]. The development of robust bioinformatic pipelines, machine learning algorithms, and statistical models assumes a crucial role in distilling meaningful insights from complex spatial omics data [ 288 , 301 , 302 , 303 , 304 ]. Furthermore, concerted efforts should be channeled toward augmenting data sharing and fostering collaboration among researchers, thereby nurturing the progression and adoption of spatiotemporal omics within the realms of aging and regeneration.
Overall, the future trajectory of spatiotemporal omics within aging and regeneration research holds great promise. The integration of emerging technologies, the incorporation of supplementary layers of information, and the resolution of technical and computational challenges are poised to further illuminate the molecular mechanisms underlying these processes. This enlightenment will, in turn, lay the foundation for pioneering therapeutic strategies.
Machine learning and artificial intelligence (AI) are set to occupy a pivotal role in unearthing meaningful insights from the expansive and intricate datasets engendered by spatiotemporal multi-omics studies. These technologies will serve as the vanguards of data-driven discoveries by uncovering patterns, correlations, and predictive models. AI-driven analyses will help uncover novel biomarkers, pathways, and regulatory networks associated with aging and regeneration. Additionally, machine learning algorithms will facilitate data interpretation, aiding researchers in understanding the functional implications of omics findings. The integration of AI into spatiotemporal multi-omics research will accelerate our ability to uncover hidden biological mechanisms.
Machine learning and statistical modeling techniques play a crucial role in extracting meaningful insights from spatiotemporal omics data within the field of aging and regeneration. These approaches facilitate the identification of key biomarkers, regulatory networks, and predictive models. Supervised machine learning algorithms, such as support vector machines (SVMs) and random forests, can be applied to classify spatial omics data based on predefined phenotypes or outcomes. Notably, SVM has been instrumental in classifying regenerating tissue regions based on gene expression profiles, ultimately leading to the identification of genes associated with successful regeneration [ 305 ].
Unsupervised machine learning methods, including clustering and dimensionality reduction techniques, furnish the capability to unearth novel cell populations and spatial patterns within complex tissue samples. Techniques such as t-distributed stochastic neighbor embedding and uniform manifold approximation and projection have been judiciously employed to unravel cellular heterogeneity and spatial organization within the context of aging and regeneration [ 306 , 307 ]. Statistical modeling approaches, inclusive of differential expression analysis and spatial enrichment analysis, aid in the identification of genes and pathways significantly associated with aging and regeneration processes. These methods open avenues for the discernment of spatially regulated genes, spatially enriched pathways, and the coordinated expression of genes in spatial domains [ 308 , 309 ]. Through the adept utilization of specialized computational methods, bioinformatic tools, and machine learning techniques, researchers stand poised to glean invaluable insights into the spatial and temporal dynamics of biological processes, thereby accelerating our comprehension of aging and regeneration.
Emerging technologies and techniques in spatiotemporal omics
The field of spatiotemporal omics is undergoing rapid evolution, marked by the emergence of cutting-edge technologies and techniques that are poised to shape its future significantly. Notably, advancements in imaging technologies, such as multiplexed imaging and super-resolution microscopy, are catalyzing higher resolution and multiplexed analyses of spatial omics data [ 310 , 311 , 312 ]. Additionally, the integration of spatial transcriptomics with other omics modalities, including spatial proteomics and metabolomics, holds the potential for a more comprehensive understanding of the intricacies underlying aging and regeneration processes [ 313 , 314 , 315 ]. These emerging technologies herald new frontiers for exploring the multifaceted molecular dynamics in spatial and temporal dimensions.
Additionally, it is imperative to recognize that the regulatory state and functionality of cells are intricately governed by the spatiotemporal orchestration of gene expression. One pivotal contributor to the emergence of RNA expression heterogeneity lies in the precise control of mRNA metabolism and transport. To fully grasp the landscape of transcriptional and post-transcriptional gene regulatory mechanisms in cells and tissues, a systematic exploration of RNA expression across both time and space becomes imperative. However, prevailing transcriptome analysis techniques fall short of capturing the concurrent temporal and spatial attributes of RNA molecules. On one hand, current spatial transcriptome analysis methods enable a thorough dissection of gene expression within heterogeneous cell populations while considering tissue morphology. Yet, they are confined to providing static snapshots of cells and tissues, thereby failing to illuminate the dynamic flux of gene expression. On the other hand, established metabolic labeling approaches facilitate temporal profiling of nascent RNA, but they lack spatial resolution. Meanwhile, live cell imaging allows for tracking RNA trajectories within cells, yet visualizing multiple transcripts poses a formidable challenge. Consequently, there is an urgent demand for transcriptome analysis methodologies capable of affording both temporal and spatial resolution, thereby enabling the monitoring of mRNA dynamics during the life process.
Functional validation
While spatiotemporal multi-omics technologies provide valuable insights, the significance of functional validation studies is set to ascend. These experiments bridge the gap between omics findings and the elucidation of biological mechanisms, substantiating the relevance of identified biomolecules and pathways in the context of aging and regeneration. Functional validation encompasses experimental techniques such as gene knockout studies, perturbation assays, and functional genomics approaches [ 316 , 317 , 318 ]. By establishing causality and shedding light on the roles of specific molecules or pathways, functional validation studies fortify the robustness and translatability of omics discoveries, thus paving the way for targeted interventions and therapeutic development.
Spatiotemporal multi-omics in precision medicine
Central to the concept of aging is the notion of regeneration, denoting the body’s capacity to restore and rejuvenate compromised tissues and organs. Regenerative efficacy diminishes with age, ushering in a decline in tissue functionality and the onset of age-related ailments. Spatiotemporal multi-omics methodologies provide an unparalleled lens through which to scrutinize tissue regeneration with exceptional precision [ 319 , 320 ]. By scrutinizing the spatial dispersion of cellular constituents and their molecular profiles, investigators can unveil the diversity of regenerative mechanisms across distinct tissues and life stages [ 321 , 322 , 323 ]. This insight proves invaluable in devising tactics to amplify tissue reparation and invigoration, potentially stalling the onset of age-linked maladies.
The assimilation of spatiotemporal insights into multi-omics analyses augments our comprehension of aging. Spatiotemporal multi-omics entails the delineation of molecular changes not solely across diverse omics strata, but also within the spatial milieu of tissues and organs. This paradigm furnishes a deeper grasp of how cellular interplays, local habitats, and cellular kinetics impact the aging trajectory [ 73 , 300 ]. Furthermore, it facilitates the detection of localized molecular imprints concomitant with precise aging-associated phenotypes, proffering promising biomarkers for early detection and intervention.
The confluence of aging research, inquiries into regeneration, and spatiotemporal multi-omics paints a trajectory for precision health applications vis-à-vis aging-linked disorders. Precision medicine aims to provide tailored interventions and therapeutics grounded in an individual’s idiosyncratic genetic makeup, lifestyle, and environmental encounters [ 319 ]. Leveraging the revelations derived from spatiotemporal multi-omics, investigators can pinpoint molecular targets for interventions that foster healthy aging and mitigate the toll of age-linked maladies [ 324 , 325 ]. These interventions span a spectrum from lifestyle adaptations to personalized therapies attuned to an individual’s specific molecular profile.
The interdisciplinary synergy among aging, regeneration, and spatiotemporal multi-omics promises to elevate our understanding of the aging trajectory and its associated disorders. Through untangling the intricate molecular networks governing aging, researchers are forging a path toward precision health interventions, which have the potential to extend health span and augment the quality of life for aging populations. As technology continues to advance, the quest to unlock the mysteries of aging and attain precision health becomes more auspicious than ever.
Conclusions
Spatiotemporal multi-omics methods have emerged as invaluable tools in unraveling the complexities of aging and regeneration research. This review has elucidated their pivotal roles in illuminating molecular dynamics across both spatial and temporal dimensions. The integration of STT, STP, STM, and STE has enabled a profound exploration of age-associated tissue alterations and the intricate processes of tissue regeneration. By harmonizing spatial and temporal data, these methodologies have yielded transformative insights into the molecular underpinnings of age-related ailments and regenerative therapies. Of particular significance is the ability of spatiotemporal multi-omics to unveil the spatial heterogeneity of molecular changes associated with aging, shedding light on the fundamental cellular processes governing the aging phenotype. Spatial transcriptomics, for instance, has uncovered intricate transcriptional variations and the evolving states of cellular populations in age-related tissues. Concurrently, spatial proteomics and metabolomics have cast a spotlight on critical components within the dynamic landscape of aging, offering promising targets for therapeutic interventions.
In the realm of tissue regeneration, spatiotemporal multi-omics methods have provided intricate blueprints for regenerating tissues. They have identified distinct cell types and traced their gene expression trajectories, while also capturing the molecular and metabolic shifts that underlie successful restoration. Furthermore, studies in spatiotemporal epigenetics have enriched our understanding of tissue repair mechanisms. Looking ahead, the promise of spatiotemporal omics looms large on the horizon. Continuous technological advancements will grant us deeper and more granular insights into these biological phenomena, propelling our understanding to new heights. Moreover, fostering a culture of data sharing and collaborative efforts among researchers will solidify the indispensable role of spatiotemporal omics in the field of biomedicine.
In summary, spatiotemporal omics techniques have been transformative, offering unprecedented clarity at the molecular level and paving the way for tailored therapies and advancements in aging and regenerative medicine. The future of this burgeoning field holds immense potential to unravel the intricate mechanisms underlying aging and regeneration, ultimately leading to improved health outcomes.
Availability of data and materials
Not applicable.
Abbreviations
Three-dimensional
Alzheimer’s disease
Artificial intelligence
Complementary DNA
Coordinate encoding
Desorption electrospray ionization
mRNA modification N 6 -methyladenosine
Matrix-assisted laser desorption/ionization
Messenger RNA
Parkinson’s disease
Resilience to Alzheimer’s disease
Single-cell RNA tagging sequencing
Single-cell RNA sequencing
Secondary ion mass spectrometry
Spatiotemporal metabolomics
Spatiotemporal proteomics
Spatiotemporal transcriptomics
Support vector machine
Traumatic brain injury
4-thiouridine
Khosla S, Farr JN, Tchkonia T, Kirkland JL. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol. 2020;16(5):263–75.
Article CAS PubMed Google Scholar
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217.
Article PubMed PubMed Central Google Scholar
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186(2):243–78.
Article PubMed Google Scholar
Guo J, Huang X, Dou L, Yan M, Shen T, Tang W, et al. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct Target Ther. 2022;7(1):391.
Article CAS PubMed PubMed Central Google Scholar
Li X, Li C, Zhang W, Wang Y, Qian P, Huang H. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct Target Ther. 2023;8(1):239.
Faroni A, Mobasseri SA, Kingham PJ, Reid AJ. Peripheral nerve regeneration: experimental strategies and future perspectives. Adv Drug Deliv Rev. 2015;82–83:160–7.
Gaharwar AK, Singh I, Khademhosseini A. Engineered biomaterials for in situ tissue regeneration. Nat Rev Mater. 2020;5(9):686–705.
Article CAS Google Scholar
Chen J, Zhou R, Feng Y, Cheng L. Molecular mechanisms of exercise contributing to tissue regeneration. Signal Transduct Target Ther. 2022;7(1):383.
Ji SF, Zhou LX, Sun ZF, Xiang JB, Cui SY, Li Y, et al. Small molecules facilitate single factor-mediated sweat gland cell reprogramming. Mil Med Res. 2022;9(1):13.
CAS PubMed PubMed Central Google Scholar
Xiong Y, Mi BB, Lin Z, Hu YQ, Yu L, Zha KK, et al. The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: from mechanism to therapeutic opportunity. Mil Med Res. 2022;9(1):65.
Sahinyan K, Lazure F, Blackburn DM, Soleimani VD. Decline of regenerative potential of old muscle stem cells: contribution to muscle aging. FEBS J. 2023;290(5):1267–89.
Palmer AK, Kirkland JL. Aging and adipose tissue: potential interventions for diabetes and regenerative medicine. Exp Gerontol. 2016;86:97–105.
Rhinn M, Ritschka B, Keyes WM. Cellular senescence in development, regeneration and disease. Development. 2019;146(20):dev151837.
Moses L, Pachter L. Museum of spatial transcriptomics. Nat Methods. 2022;19(5):534–46.
Chen WT, Lu A, Craessaerts K, Pavie B, Frigerio CS, Corthout N, et al. Spatial transcriptomics and in situ sequencing to study Alzheimer’s disease. Cell. 2020;182(4):976–91.e19.
Ito Y, Hoare M, Narita M. Spatial and temporal control of senescence. Trends Cell Biol. 2017;27(11):820–32.
Burgess DJ. Spatial transcriptomics coming of age. Nat Rev Genet. 2019;20(6):317.
Tian L, Chen F, Macosko EZ. The expanding vistas of spatial transcriptomics. Nat Biotechnol. 2023;41(6):773–82.
Ståhl PL, Salmén F, Vickovic S, Lundmark A, Navarro JF, Magnusson J, et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science. 2016;353(6294):78–82.
Costa V, Angelini C, De Feis I, Ciccodicola A. Uncovering the complexity of transcriptomes with RNA-Seq. J Biomed Biotechnol. 2010;2010:853916.
Velculescu VE, Madden SL, Zhang L, Lash AE, Yu J, Rago C, et al. Analysis of human transcriptomes. Nat Genet. 1999;23(4):387–8.
Zeng H, Huang J, Zhou H, Meilandt WJ, Dejanovic B, Zhou Y, et al. Integrative in situ mapping of single-cell transcriptional states and tissue histopathology in a mouse model of Alzheimer’s disease. Nat Neurosci. 2023;26(3):430–46.
CAS PubMed Google Scholar
Tang F, Lao K, Surani MA. Development and applications of single-cell transcriptome analysis. Nat Methods. 2011;8(4 Suppl):S6–11.
Armand EJ, Li J, Xie F, Luo C, Mukamel EA. Single-cell sequencing of brain cell transcriptomes and epigenomes. Neuron. 2021;109(1):11–26.
Wang Y, Navin NE. Advances and applications of single-cell sequencing technologies. Mol Cell. 2015;58(4):598–609.
Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biochem. 2010;111(2):488–96.
Lange S, Katayama Y, Schmid M, Burkacky O, Brauchle C, Lamb DC, et al. Simultaneous transport of different localized mRNA species revealed by live-cell imaging. Traffic. 2008;9(8):1256–67.
Held M, Schmitz MHA, Fischer B, Walter T, Neumann B, Olma MH, et al. Cell Cognition: time-resolved phenotype annotation in high-throughput live cell imaging. Nat Methods. 2010;7(9):747–54.
Galluzzi L, Yamazaki T, Kroemer G. Linking cellular stress responses to systemic homeostasis. Nat Rev Mol Cell Biol. 2018;19(11):731–45.
Yin Y, Chen H, Wang Y, Zhang L, Wang X. Roles of extracellular vesicles in the aging microenvironment and age-related diseases. J Extracell Vesicles. 2021;10(12):e12154.
Leontieva OV, Natarajan V, Demidenko ZN, Burdelya LG, Gudkov AV, Blagosklonny MV. Hypoxia suppresses conversion from proliferative arrest to cellular senescence. Proc Natl Acad Sci U S A. 2012;109(33):13314–8.
Hu X, Xu W, Ren Y, Wang Z, He X, Huang R, et al. Spinal cord injury: molecular mechanisms and therapeutic interventions. Signal Transduct Target Ther. 2023;8(1):245.
Lin D, Shen L, Luo M, Zhang K, Li J, Yang Q, et al. Circulating tumor cells: biology and clinical significance. Signal Transduct Target Ther. 2021;6(1):404.
Bai YM, Yang F, Luo P, Xie LL, Chen JH, Guan YD, et al. Single-cell transcriptomic dissection of the cellular and molecular events underlying the triclosan-induced liver fibrosis in mice. Mil Med Res. 2023;10(1):7.
Wang Y, Wang JY, Schnieke A, Fischer K. Advances in single-cell sequencing: insights from organ transplantation. Mil Med Res. 2021;8(1):45.
PubMed PubMed Central Google Scholar
Tang L, Huang ZP, Mei H, Hu Y. Insights gained from single-cell analysis of chimeric antigen receptor T-cell immunotherapy in cancer. Mil Med Res. 2023;10(1):52.
Cao J, Chen X, Huang S, Shi W, Fan Q, Gong Y, et al. Microfluidics-based single cell analysis: from transcriptomics to spatiotemporal multi-omics. TrAC-Trend Anal Chem. 2023;158:116868.
Lin S, Liu Y, Zhang M, Xu X, Chen Y, Zhang H, et al. Microfluidic single-cell transcriptomics: moving towards multimodal and spatiotemporal omics. Lab Chip. 2021;21(20):3829–49.
Magoulopoulou A, Salas SM, Tiklová K, Samuelsson ER, Hilscher MM, Nilsson M. Padlock probe-based targeted in situ sequencing: overview of methods and applications. Annu Rev Genomics Hum Genet. 2023;24:133–50.
Patkulkar PA, Subbalakshmi AR, Jolly MK, Sinharay S. Mapping spatiotemporal heterogeneity in tumor profiles by integrating high-throughput imaging and omics analysis. ACS Omega. 2023;8(7):6126–38.
Chelvanambi S, Hester JM, Sharma S, Lahm T, Frump AL. Slide-seq for spatially mapping gene expression. Metabolic syndrome exacerbates group 2 pulmonary hypertension, and NAD metabolism is influenced by tissue origin. Am J Respir Cell Mol Biol. 2020;62(1):112-4.
Xia K, Sun HX, Li J, Li J, Zhao Y, Chen L, et al. The single-cell Stereo-seq reveals region-specific cell subtypes and transcriptome profiling in Arabidopsis leaves. Dev Cell. 2022;57(10):1299–310.e4.
Ren J, Zhou H, Zeng H, Wang CK, Huang J, Qiu X, et al. Spatiotemporally resolved transcriptomics reveals the subcellular RNA kinetic landscape. Nat Methods. 2023;20(5):695–705.
Asp M, Bergenstrahle J, Lundeberg J. Spatially resolved transcriptomes-next generation tools for tissue exploration. Bioessays. 2020;42(10):e1900221.
Zhang X, Wang Z, Zhang C, Li Y, Lu S, Steffens S, et al. Laser capture microdissection-based mrna expression microarrays and single-cell rna sequencing in atherosclerosis research. Methods Mol Biol. 2022;2419:715–26.
Zhou Y, Jia E, Pan M, Zhao X, Ge Q. Encoding method of single-cell spatial transcriptomics sequencing. Int J Biol Sci. 2020;16(14):2663–74.
Baysoy A, Bai Z, Satija R, Fan R. The technological landscape and applications of single-cell multi-omics. Nat Rev Mol Cell Biol. 2023;24(10):695–713.
McGinnis LM, Ibarra-Lopez V, Rost S, Ziai J. Clinical and research applications of multiplexed immunohistochemistry and in situ hybridization. J Pathol. 2021;254(4):405–17.
Chen TY, You L, Hardillo JAU, Chien MP. Spatial transcriptomic technologies. Cells. 2023;12(16):2042.
Cassidy A, Jones J. Developments in in situ hybridisation. Methods. 2014;70(1):39–45.
Chen X, Sun YC, Church GM, Lee JH, Zador AM. Efficient in situ barcode sequencing using padlock probe-based BaristaSeq. Nucleic Acids Res. 2018;46(4):e22.
Bowes AL, Tarabichi M, Pillay N, Van Loo P. Leveraging single-cell sequencing to unravel intratumour heterogeneity and tumour evolution in human cancers. J Pathol. 2022;257(4):466–78.
Pervez MT, Hasnain MJU, Abbas SH, Moustafa MF, Aslam N, Shah SSM. A comprehensive review of performance of next-generation sequencing platforms. Biomed Res Int. 2022;2022:3457806.
Kulkarni MM. Digital multiplexed gene expression analysis using the NanoString nCounter system. Curr Protoc Mol Biol. 2011;Chapter 25:Unit25B.10.
Molania R, Gagnon-Bartsch JA, Dobrovic A, Speed TP. A new normalization for Nanostring nCounter gene expression data. Nucleic Acids Res. 2019;47(12):6073–83.
Li Z, Song T, Yong J, Kuang R. Imputation of spatially-resolved transcriptomes by graph-regularized tensor completion. PLoS Comput Biol. 2021;17(4):e1008218.
Li X, Wang CY. From bulk, single-cell to spatial RNA sequencing. Int J Oral Sci. 2021;13(1):36.
Rodriques SG, Stickels RR, Goeva A, Martin CA, Murray E, Vanderburg CR, et al. Slide-seq: a scalable technology for measuring genome-wide expression at high spatial resolution. Science. 2019;363(6434):1463–7.
Mantri M, Scuderi GJ, Abedini-Nassab R, Wang MFZ, McKellar D, Shi H, et al. Spatiotemporal single-cell RNA sequencing of developing chicken hearts identifies interplay between cellular differentiation and morphogenesis. Nat Commun. 2021;12(1):1771.
Wei X, Fu S, Li H, Liu Y, Wang S, Feng W, et al. Single-cell Stereo-seq reveals induced progenitor cells involved in axolotl brain regeneration. Science. 2022;377(6610):eabp9444.
Chen A, Liao S, Cheng M, Ma K, Wu L, Lai Y, et al. Spatiotemporal transcriptomic atlas of mouse organogenesis using DNA nanoball-patterned arrays. Cell. 2022;185(10):1777–92.e21.
Cho CS, Xi J, Si Y, Park SR, Hsu JE, Kim M, et al. Microscopic examination of spatial transcriptome using Seq-Scope. Cell. 2021;184(13):3559–72.e22.
Do TH, Ma F, Andrade PR, Teles R, de Andrade Silva BJ, Hu C, et al. TREM2 macrophages induced by human lipids drive inflammation in acne lesions. Sci Immunol. 2022;7(73):eabo2787.
Srivatsan SR, Regier MC, Barkan E, Franks JM, Packer JS, Grosjean P, et al. Embryo-scale, single-cell spatial transcriptomics. Science. 2021;373(6550):111–7.
Weber C. Single-cell spatial transcriptomics. Nat Cell Biol. 2021;23(11):1108.
PubMed Google Scholar
Wang WJ, Chu LX, He LY, Zhang MJ, Dang KT, Gao C, et al. Spatial transcriptomics: recent developments and insights in respiratory research. Mil Med Res. 2023;10(1):38.
Li PH, Kong XY, He YZ, Liu Y, Peng X, Li ZH, et al. Recent developments in application of single-cell RNA sequencing in the tumour immune microenvironment and cancer therapy. Mil Med Res. 2022;9(1):52.
Hsieh WC, Budiarto BR, Wang YF, Lin CY, Gwo MC, So DK, et al. Spatial multi-omics analyses of the tumor immune microenvironment. J Biomed Sci. 2022;29(1):96.
Mund A, Brunner AD, Mann M. Unbiased spatial proteomics with single-cell resolution in tissues. Mol Cell. 2022;82(12):2335–49.
Cheng M, Jiang Y, Xu J, Mentis AA, Wang S, Zheng H, et al. Spatially resolved transcriptomics: a comprehensive review of their technological advances, applications, and challenges. J Genet Genomics. 2023;50(9):625–40.
Moffitt JR, Lundberg E, Heyn H. The emerging landscape of spatial profiling technologies. Nat Rev Genet. 2022;23(12):741–59.
Liu C, Li R, Li Y, Lin X, Zhao K, Liu Q, et al. Spatiotemporal mapping of gene expression landscapes and developmental trajectories during zebrafish embryogenesis. Dev Cell. 2022;57(10):1284–98.e5.
Wang M, Hu Q, Lv T, Wang Y, Lan Q, Xiang R, et al. High-resolution 3D spatiotemporal transcriptomic maps of developing Drosophila embryos and larvae. Dev Cell. 2022;57(10):1271–83.e4.
Chen A, Liao S, Cheng M, Ma K, Wu L, Lai Y, et al. Large field of view-spatially resolved transcriptomics at nanoscale resolution. bioRxiv. 2021. https://doi.org/10.1101/2021.01.17.427004 .
Singha M, Spitalny L, Nguyen K, Vandewalle A, Spitale RC. Chemical methods for measuring RNA expression with metabolic labeling. Wiley Interdiscip Rev RNA. 2021;12(5):e1650.
Schofield JA, Duffy EE, Kiefer L, Sullivan MC, Simon MD. TimeLapse-seq: adding a temporal dimension to RNA sequencing through nucleoside recoding. Nat Methods. 2018;15(3):221–5.
Battich N, Beumer J, de Barbanson B, Krenning L, Baron CS, Tanenbaum ME, et al. Sequencing metabolically labeled transcripts in single cells reveals mRNA turnover strategies. Science. 2020;367(6482):1151–6.
Qiu Q, Hu P, Qiu X, Govek KW, Cámara PG, Wu H. Massively parallel and time-resolved RNA sequencing in single cells with scNT-seq. Nat Methods. 2020;17(10):991–1001.
Rodriques SG, Chen LM, Liu S, Zhong ED, Scherrer JR, Boyden ES, et al. RNA timestamps identify the age of single molecules in RNA sequencing. Nat Biotechnol. 2021;39(3):320–5.
Han KY, Leslie BJ, Fei J, Zhang J, Ha T. Understanding the photophysics of the spinach–DFHBI RNA aptamer–fluorogen complex to improve live-cell RNA imaging. J Am Chem Soc. 2013;135(50):19033–8.
Wang D, Shalamberidze A, Arguello AE, Purse BW, Kleiner RE. Live-cell RNA imaging with metabolically incorporated fluorescent nucleosides. J Am Chem Soc. 2022;144(32):14647–56.
Pade LR, Stepler KE, Portero EP, DeLaney K, Nemes P. Biological mass spectrometry enables spatiotemporal ’omics: From tissues to cells to organelles. Mass Spectrom Rev. 2023;43(1):106–38.
Brozova K, Hantusch B, Kenner L, Kratochwill K. Spatial proteomics for the molecular characterization of breast cancer. Proteomes. 2023;11(2):17.
Lohani V, A RA, Kundu S, Akhter MQ, Bag S. Single-cell proteomics with spatial attributes: tools and techniques. ACS Omega. 2023;8(20):17499-510.
de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. Global signatures of protein and mRNA expression levels. Mol Biosyst. 2009;5(12):1512–26.
Google Scholar
Bosia C, Sgrò F, Conti L, Baldassi C, Brusa D, Cavallo F, et al. RNAs competing for microRNAs mutually influence their fluctuations in a highly non-linear microRNA-dependent manner in single cells. Genome Biol. 2017;18(1):37.
Cai L, Friedman N, Xie XS. Stochastic protein expression in individual cells at the single molecule level. Nature. 2006;440(7082):358–62.
Selkrig J, Li N, Hausmann A, Mangan MSJ, Zietek M, Mateus A, et al. Spatiotemporal proteomics uncovers cathepsin-dependent macrophage cell death during Salmonella infection. Nat Microbiol. 2020;5(9):1119–33.
Marmor-Kollet H, Siany A, Kedersha N, Knafo N, Rivkin N, Danino YM, et al. Spatiotemporal proteomic analysis of stress granule disassembly using APEX reveals regulation by SUMOylation and links to ALS pathogenesis. Mol Cell. 2020;80(5):876–91.e6.
Ke M, Yuan X, He A, Yu P, Chen W, Shi Y, et al. Spatiotemporal profiling of cytosolic signaling complexes in living cells by selective proximity proteomics. Nat Commun. 2021;12(1):71.
Mahdessian D, Cesnik AJ, Gnann C, Danielsson F, Stenstrom L, Arif M, et al. Spatiotemporal dissection of the cell cycle with single-cell proteogenomics. Nature. 2021;590(7847):649–54.
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419.
Lundberg E, Borner GHH. Spatial proteomics: a powerful discovery tool for cell biology. Nat Rev Mol Cell Biol. 2019;20(5):285–302.
Hickey JW, Neumann EK, Radtke AJ, Camarillo JM, Beuschel RT, Albanese A, et al. Spatial mapping of protein composition and tissue organization: a primer for multiplexed antibody-based imaging. Nat Methods. 2022;19(3):284–95.
Lewis SM, Asselin-Labat ML, Nguyen Q, Berthelet J, Tan X, Wimmer VC, et al. Spatial omics and multiplexed imaging to explore cancer biology. Nat Methods. 2021;18(9):997–1012.
Murray E, Cho JH, Goodwin D, Ku T, Swaney J, Kim SY, et al. Simple, scalable proteomic imaging for high-dimensional profiling of intact systems. Cell. 2015;163(6):1500–14.
Gerdes MJ, Sevinsky CJ, Sood A, Adak S, Bello MO, Bordwell A, et al. Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proc Natl Acad Sci U S A. 2013;110(29):11982–7.
Lin JR, Fallahi-Sichani M, Sorger PK. Highly multiplexed imaging of single cells using a high-throughput cyclic immunofluorescence method. Nat Commun. 2015;6:8390.
Venkatesan M, Zhang N, Marteau B, Yajima Y, De Zarate Garcia NO, Fang Z, et al. Spatial subcellular organelle networks in single cells. Sci Rep. 2023;13(1):5374.
Radtke AJ, Kandov E, Lowekamp B, Speranza E, Chu CJ, Gola A, et al. IBEX: a versatile multiplex optical imaging approach for deep phenotyping and spatial analysis of cells in complex tissues. Proc Natl Acad Sci U S A. 2020;117(52):33455–65.
Gut G, Herrmann MD, Pelkmans L. Multiplexed protein maps link subcellular organization to cellular states. Science. 2018;361(6401):eaar7042.
Zhu C, Qiu J, Pongkitwitoon S, Thomopoulos S, Xia Y. Inverse opal scaffolds with gradations in mineral content for spatial control of osteogenesis. Adv Mater. 2018;30(29):e1706706.
Article Google Scholar
Giesen C, Wang HA, Schapiro D, Zivanovic N, Jacobs A, Hattendorf B, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods. 2014;11(4):417–22.
Angelo M, Bendall SC, Finck R, Hale MB, Hitzman C, Borowsky AD, et al. Multiplexed ion beam imaging of human breast tumors. Nat Med. 2014;20(4):436–42.
Liu CC, McCaffrey EF, Greenwald NF, Soon E, Risom T, Vijayaragavan K, et al. Multiplexed ion beam imaging: insights into pathobiology. 2022;17:403-23.
Wang Y, Woehrstein JB, Donoghue N, Dai M, Avendano MS, Schackmann RCJ, et al. Rapid sequential in situ multiplexing with DNA exchange imaging in neuronal cells and tissues. Nano Lett. 2017;17(10):6131–9.
Goltsev Y, Samusik N, Kennedy-Darling J, Bhate S, Hale M, Vazquez G, et al. Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell. 2018;174(4):968–81.e15.
Schürch CM, Bhate SS, Barlow GL, Phillips DJ, Noti L, Zlobec I, et al. Coordinated cellular neighborhoods orchestrate antitumoral immunity at the colorectal cancer invasive front. Cell. 2020;182(5):1341–59.e19.
Saka SK, Wang Y, Kishi JY, Zhu A, Zeng Y, Xie W, et al. Immuno-SABER enables highly multiplexed and amplified protein imaging in tissues. Nat Biotechnol. 2019;37(9):1080–90.
Spraggins JM, Djambazova KV, Rivera ES, Migas LG, Neumann EK, Fuetterer A, et al. High-performance molecular imaging with MALDI trapped ion-mobility time-of-flight (timsTOF) mass spectrometry. Anal Chem. 2019;91(22):14552–60.
Gekenidis MT, Studer P, Wuthrich S, Brunisholz R, Drissner D. Beyond the matrix-assisted laser desorption ionization (MALDI) biotyping workflow: in search of microorganism-specific tryptic peptides enabling discrimination of subspecies. Appl Environ Microbiol. 2014;80(14):4234–41.
Palla G, Fischer DS, Regev A, Theis FJ. Spatial components of molecular tissue biology. Nat Biotechnol. 2022;40(3):308–18.
Mund A, Coscia F, Kriston A, Hollandi R, Kovács F, Brunner AD, et al. Deep visual proteomics defines single-cell identity and heterogeneity. Nat Biotechnol. 2022;40(8):1231–40.
Li L, Sun C, Sun Y, Dong Z, Wu R, Sun X, et al. Spatially resolved proteomics via tissue expansion. Nat Commun. 2022;13(1):7242.
Scholey JM, Brust-Mascher I, Mogilner A. Cell division. Nature. 2003;422(6933):746–52.
Salazar-Roa M, Malumbres M. Fueling the cell division cycle. Trends Cell Biol. 2017;27(1):69–81.
Karlsson J, Kroneis T, Jonasson E, Larsson E, Stahlberg A. Transcriptomic characterization of the human cell cycle in individual unsynchronized cells. J Mol Biol. 2017;429(24):3909–24.
Macieira-Coelho A. Cell division and aging of the organism. Biogerontology. 2011;12(6):503–15.
Polymenis M, Kennedy BK. Unbalanced growth, senescence and aging. Adv Exp Med Biol. 2017;1002:189–208.
Hasin Y, Seldin M, Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18(1):83.
Subramanian I, Verma S, Kumar S, Jere A, Anamika K. Multi-omics data integration, interpretation, and its application. Bioinform Biol Insights. 2020;14:1177932219899051.
Irie M, Fujimura Y, Yamato M, Miura D, Wariishi H. Integrated MALDI-MS imaging and LC-MS techniques for visualizing spatiotemporal metabolomic dynamics in a rat stroke model. Metabolomics. 2014;10:473–83.
Jin B, Pang X, Zang Q, Ga M, Xu J, Luo Z, et al. Spatiotemporally resolved metabolomics and isotope tracing reveal CNS drug targets. Acta Pharm Sin B. 2023;13(4):1699–710.
Yuan Z, Zhou Q, Cai L, Pan L, Sun W, Qumu S, et al. SEAM is a spatial single nuclear metabolomics method for dissecting tissue microenvironment. Nat Methods. 2021;18(10):1223–32.
Fehr M, Frommer WB, Lalonde S. Visualization of maltose uptake in living yeast cells by fluorescent nanosensors. Proc Natl Acad Sci U S A. 2002;99(15):9846–51.
Han G, Wei P, He M, Teng H, Chu Y. Metabolomic profiling of the aqueous humor in patients with wet age-related macular degeneration using UHPLC-MS/MS. J Proteome Res. 2020;19(6):2358–66.
Spraker JE, Luu GT, Sanchez LM. Imaging mass spectrometry for natural products discovery: a review of ionization methods. Nat Prod Rep. 2020;37(2):150–62.
Chu L, Huang Y, Xu Y, Wang LK, Lu Q. An LC-APCI + -MS/MS-based method for determining the concentration of neurosteroids in the brain of male mice with different gut microbiota. J Neurosci Methods. 2021;360:109268.
Chu L, Li N, Deng J, Wu Y, Yang H, Wang W, et al. LC-APCI + -MS/MS method for the analysis of ten hormones and two endocannabinoids in plasma and hair from the mice with different gut microbiota. J Pharm Biomed Anal. 2020;185:113223.
Chu L, Shu X, Huang Y, Chu T, Ge M, Lu Q. Sex steroid hormones in urinary exosomes as biomarkers for the prediction of prostate cancer. Clinica Chimica Acta. 2022;531:389–98.
Murayama C, Kimura Y, Setou M. Imaging mass spectrometry: principle and application. Biophys Rev. 2009;1(3):131.
Stoeckli M, Chaurand P, Hallahan DE, Caprioli RM. Imaging mass spectrometry: a new technology for the analysis of protein expression in mammalian tissues. Nat Med. 2001;7(4):493–6.
Kaufmann R. Matrix-assisted laser desorption ionization (MALDI) mass spectrometry: a novel analytical tool in molecular biology and biotechnology. J Biotechnol. 1995;41(2–3):155–75.
Takats Z, Wiseman JM, Gologan B, Cooks RG. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306(5695):471–3.
Takats Z, Wiseman JM, Cooks RG. Ambient mass spectrometry using desorption electrospray ionization (DESI): instrumentation, mechanisms and applications in forensics, chemistry, and biology. J Mass Spectrom. 2005;40(10):1261–75.
Taylor MJ, Lukowski JK, Anderton CR. Spatially resolved mass spectrometry at the single cell: recent innovations in proteomics and metabolomics. J Am Soc Mass Spectrom. 2021;32(4):872–94.
Rappez L, Stadler M, Triana S, Gathungu RM, Ovchinnikova K, Phapale P, et al. SpaceM reveals metabolic states of single cells. Nat Methods. 2021;18(7):799–805.
Zavalin A, Todd EM, Rawhouser PD, Yang J, Norris JL, Caprioli RM. Direct imaging of single cells and tissue at sub-cellular spatial resolution using transmission geometry MALDI MS. J Mass Spectrom. 2012;47(11):i.
Kauppila TJ, Talaty N, Kuuranne T, Kotiaho T, Kostiainen R, Cooks RG. Rapid analysis of metabolites and drugs of abuse from urine samples by desorption electrospray ionization-mass spectrometry. Analyst. 2007;132(9):868–75.
Kauppila TJ, Wiseman JM, Ketola RA, Kotiaho T, Cooks RG, Kostiainen R. Desorption electrospray ionization mass spectrometry for the analysis of pharmaceuticals and metabolites. Rapid Commun Mass Spectrom. 2006;20(3):387–92.
Wiseman JM, Ifa DR, Venter A, Cooks RG. Ambient molecular imaging by desorption electrospray ionization mass spectrometry. Nat Protoc. 2008;3(3):517–24.
Roach PJ, Laskin J, Laskin A. Nanospray desorption electrospray ionization: an ambient method for liquid-extraction surface sampling in mass spectrometry. Analyst. 2010;135(9):2233–6.
Walther F, Koerver R, Fuchs T, Ohno S, Sann J, Rohnke M, et al. Visualization of the interfacial decomposition of composite cathodes in argyrodite-based all-solid-state batteries using time-of-flight secondary-ion mass spectrometry. Chem Mater. 2019;31(10):3745–55.
Kotowska AM, Trindade GF, Mendes PM, Williams PM, Aylott JW, Shard AG, et al. Protein identification by 3D OrbiSIMS to facilitate in situ imaging and depth profiling. Nat Commun. 2020;11(1):5832.
Passarelli MK, Pirkl A, Moellers R, Grinfeld D, Kollmer F, Havelund R, et al. The 3D OrbiSIMS-label-free metabolic imaging with subcellular lateral resolution and high mass-resolving power. Nat Methods. 2017;14(12):1175–83.
Zhang D, Deng Y, Kukanja P, Agirre E, Bartosovic M, Dong M, et al. Spatial epigenome–transcriptome co-profiling of mammalian tissues. Nature. 2023;616(7955):113–22.
Fitz-James MH, Cavalli G. Molecular mechanisms of transgenerational epigenetic inheritance. Nat Rev Genet. 2022;23(6):325–41.
Foley DL, Craig JM, Morley R, Olsson CA, Dwyer T, Smith K, et al. Prospects for epigenetic epidemiology. Am J Epidemiol. 2009;169(4):389–400.
Tollefsbol TO. Advances in epigenetic technology. Methods Mol Biol. 2011;791:1–10.
Wu Y, Terekhanova NV, Caravan W, Naser Al Deen N, Lal P, Chen S, et al. Epigenetic and transcriptomic characterization reveals progression markers and essential pathways in clear cell renal cell carcinoma. Nat Commun. 2023;14(1):1681.
Ramalingam N, Jeffrey SS. Future of liquid biopsies with growing technological and bioinformatics studies: opportunities and challenges in discovering tumor heterogeneity with single-cell level analysis. Cancer J. 2018;24(2):104–8.
Trapp A, Kerepesi C, Gladyshev VN. Profiling epigenetic age in single cells. Nat Aging. 2021;1(12):1189–201.
Bheda P, Schneider R. Epigenetics reloaded: the single-cell revolution. Trends Cell Biol. 2014;24(11):712–23.
Rudolph KL. DNA-methylation aging at single-cell level. Nature Aging. 2021;1(12):1086–7.
Casado-Pelaez M, Bueno-Costa A, Esteller M. Single cell cancer epigenetics. Trends Cancer. 2022;8(10):820–38.
Kaya-Okur HS, Wu SJ, Codomo CA, Pledger ES, Bryson TD, Henikoff JG, et al. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat Commun. 2019;10(1):1930.
Deng Y, Bartosovic M, Kukanja P, Zhang D, Liu Y, Su G, et al. Spatial-CUT&Tag: spatially resolved chromatin modification profiling at the cellular level. Science. 2022;375(6581):681–6.
Liu Y, Yang M, Deng Y, Su G, Enninful A, Guo CC, et al. High-spatial-resolution multi-omics sequencing via deterministic barcoding in tissue. Cell. 2020;183(6):1665–81.e18.
Yu P, Xiao S, Xin X, Song CX, Huang W, McDee D, et al. Spatiotemporal clustering of the epigenome reveals rules of dynamic gene regulation. Genome Res. 2013;23(2):352–64.
Smallwood SA, Lee HJ, Angermueller C, Krueger F, Saadeh H, Peat J, et al. Single-cell genome-wide bisulfite sequencing for assessing epigenetic heterogeneity. Nat Methods. 2014;11(8):817–20.
Ogbeide S, Giannese F, Mincarelli L, Macaulay IC. Into the multiverse: advances in single-cell multiomic profiling. Trends Genet. 2022;38(8):831–43.
Angermueller C, Clark SJ, Lee HJ, Macaulay IC, Teng MJ, Hu TX, et al. Parallel single-cell sequencing links transcriptional and epigenetic heterogeneity. Nat Methods. 2016;13(3):229–32.
Macaulay IC, Haerty W, Kumar P, Li YI, Hu TX, Teng MJ, et al. G&T-seq: parallel sequencing of single-cell genomes and transcriptomes. Nat Methods. 2015;12(6):519–22.
Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of native chromatin for fast and sensitive epigenomic profiling of open chromatin, DNA-binding proteins and nucleosome position. Nat Methods. 2013;10(12):1213–8.
Ma A, McDermaid A, Xu J, Chang Y, Ma Q. Integrative methods and practical challenges for single-cell multi-omics. Trends Biotechnol. 2020;38(9):1007–22.
Grosselin K, Durand A, Marsolier J, Poitou A, Marangoni E, Nemati F, et al. High-throughput single-cell ChIP-seq identifies heterogeneity of chromatin states in breast cancer. Nat Genet. 2019;51(6):1060–6.
Skene PJ, Henikoff S. An efficient targeted nuclease strategy for high-resolution mapping of DNA binding sites. Elife. 2017;6:e21856.
Bartosovic M, Kabbe M, Castelo-Branco G. Single-cell CUT&Tag profiles histone modifications and transcription factors in complex tissues. Nat Biotechnol. 2021;39(7):825–35.
Janssens DH, Otto DJ, Meers MP, Setty M, Ahmad K, Henikoff S. CUT&Tag2for1: a modified method for simultaneous profiling of the accessible and silenced regulome in single cells. Genome Biol. 2022;23(1):81.
Zhang B, Srivastava A, Mimitou E, Stuart T, Raimondi I, Hao Y, et al. Characterizing cellular heterogeneity in chromatin state with scCUT&Tag-pro. Nat Biotechnol. 2022;40(8):1220–30.
Zhu C, Zhang Y, Li YE, Lucero J, Behrens MM, Ren B. Joint profiling of histone modifications and transcriptome in single cells from mouse brain. Nat Methods. 2021;18(3):283–92.
Xiong H, Luo Y, Wang Q, Yu X, He A. Single-cell joint detection of chromatin occupancy and transcriptome enables higher-dimensional epigenomic reconstructions. Nat Methods. 2021;18(6):652–60.
Deng Y, Bartosovic M, Ma S, Zhang D, Kukanja P, Xiao Y, et al. Spatial profiling of chromatin accessibility in mouse and human tissues. Nature. 2022;609(7926):375–83.
Liu Y, DiStasio M, Su G, Asashima H, Enninful A, Qin X, et al. Spatial-CITE-seq: spatially resolved high-plex protein and whole transcriptome co-mapping. Res Sq [Preprint]. 2022.
Enge M, Arda HE, Mignardi M, Beausang J, Bottino R, Kim SK, et al. Single-cell analysis of human pancreas reveals transcriptional signatures of aging and somatic mutation patterns. Cell. 2017;171(2):321–30.e14.
Hahn O, Foltz AG, Atkins M, Kedir B, Moran-Losada P, Guldner IH, et al. Atlas of the aging mouse brain reveals white matter as vulnerable foci. Cell. 2023;186(19):4117–4133.e22.
Lee S, Devanney NA, Golden LR, Smith CT, Schwartz JL, Walsh AE, et al. APOE modulates microglial immunometabolism in response to age, amyloid pathology, and inflammatory challenge. Cell Rep. 2023;42(3):112196.
Stoeger T, Grant RA, McQuattie-Pimentel AC, Anekalla KR, Liu SS, Tejedor-Navarro H, et al. Aging is associated with a systemic length-associated transcriptome imbalance. Nat Aging. 2022;2(12):1191–206.
Russ JE, Haywood ME, Lane SL, Schoolcraft WB, Katz-Jaffe MG. Spatially resolved transcriptomic profiling of ovarian aging in mice. iScience. 2022;25(8):104819.
Kiss T, Nyul-Toth A, DelFavero J, Balasubramanian P, Tarantini S, Faakye J, et al. Spatial transcriptomic analysis reveals inflammatory foci defined by senescent cells in the white matter, hippocampi and cortical grey matter in the aged mouse brain. Geroscience. 2022;44(2):661–81.
Dries R, Zhu Q, Dong R, Eng CL, Li H, Liu K, et al. Giotto: a toolbox for integrative analysis and visualization of spatial expression data. Genome Biol. 2021;22(1):78.
Sun S, Zhu J, Zhou X. Statistical analysis of spatial expression patterns for spatially resolved transcriptomic studies. Nat Methods. 2020;17(2):193–200.
Zhao E, Stone MR, Ren X, Guenthoer J, Smythe KS, Pulliam T, et al. Spatial transcriptomics at subspot resolution with BayesSpace. Nat Biotechnol. 2021;39(11):1375–84.
Shang L, Zhou X. Spatially aware dimension reduction for spatial transcriptomics. Nat Commun. 2022;13(1):7203.
LaRocca TJ, Cavalier AN, Wahl D. Repetitive elements as a transcriptomic marker of aging: Evidence in multiple datasets and models. Aging Cell. 2020;19(7):e13167.
Kasemeier-Kulesa JC, Morrison JA, McKinney S, Li H, Gogol M, Hall K, et al. Cell-type profiling of the sympathetic nervous system using spatial transcriptomics and spatial mapping of mRNA. Dev Dyn. 2023;252(8):1130–42.
Zhao D, Chen S. Failures at every level: breakdown of the epigenetic machinery of aging. Life Medicine. 2022;1(2):81–3.
Djuric U, Rodrigues DC, Batruch I, Ellis J, Shannon P, Diamandis P. Spatiotemporal proteomic profiling of human cerebral development. Mol Cell Proteomics. 2017;16(9):1548–62.
Wei J, Dai S, Yan Y, Li S, Yang P, Zhu R, et al. Spatiotemporal proteomic atlas of multiple brain regions across early fetal to neonatal stages in cynomolgus monkey. Nat Commun. 2023;14(1):3917.
Hosp F, Gutiérrez-Ángel S, Schaefer MH, Cox J, Meissner F, Hipp MS, et al. Spatiotemporal proteomic profiling of Huntington’s disease inclusions reveals widespread loss of protein function. Cell Rep. 2017;21(8):2291–303.
Draskovic I, Arnoult N, Steiner V, Bacchetti S, Lomonte P, Londono-Vallejo A. Probing PML body function in ALT cells reveals spatiotemporal requirements for telomere recombination. Proc Natl Acad Sci U S A. 2009;106(37):15726–31.
Schey KL, Wang Z, Friedrich MG, Garland DL, Truscott RJW. Spatiotemporal changes in the human lens proteome: Critical insights into long-lived proteins. Prog Retin Eye Res. 2020;76:100802.
Budayeva HG, Cristea IM. Human sirtuin 2 localization, transient interactions, and impact on the proteome point to its role in intracellular trafficking. Mol Cell Proteomics. 2016;15(10):3107–25.
Zhang J, Qiu Z, Zhang Y, Wang G, Hao H. Intracellular spatiotemporal metabolism in connection to target engagement. Adv Drug Deliv Rev. 2023;200:115024.
Schulz C, Massberg S. Inflammaging aggravates stroke pathology. Nat Immunol. 2023;24(6):887–8.
Huang Z, Merrihew GE, Larson EB, Park J, Plubell D, Fox EJ, et al. Brain proteomic analysis implicates actin filament processes and injury response in resilience to Alzheimer’s disease. Nat Commun. 2023;14(1):2747.
Walker JM, Orr ME, Orr TC, Thorn EL, Christie TD, Yokoda RT, et al. Spatial proteomics of hippocampal subfield-specific pathology in Alzheimer’s disease and primary age-related tauopathy. Alzheimers Dement. 2024;20(2):783–97.
Thul PJ, Akesson L, Wiking M, Mahdessian D, Geladaki A, Ait Blal H, et al. A subcellular map of the human proteome. Science. 2017;356(6340):eaal3321.
Hogan KA, Zeidler JD, Beasley HK, Alsaadi AI, Alshaheeb AA, Chang YC, et al. Using mass spectrometry imaging to visualize age-related subcellular disruption. Front Mol Biosci. 2023;10:906606.
Simpson CE, Ambade AS, Harlan R, Roux A, Graham D, Klauer N, et al. Spatial and temporal resolution of metabolic dysregulation in the Sugen hypoxia model of pulmonary hypertension. Pulm Circ. 2023;13(3):e12260.
Zhao C, Dong J, Deng L, Tan Y, Jiang W, Cai Z. Molecular network strategy in multi-omics and mass spectrometry imaging. Curr Opin Chem Biol. 2022;70:102199.
Zheng P, Zhang N, Ren D, Yu C, Zhao B, Zhang Y. Integrated spatial transcriptome and metabolism study reveals metabolic heterogeneity in human injured brain. Cell Rep Med. 2023;4(6):101057.
Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115.
Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13(7):484–92.
Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19(6):371–84.
Rodriguez-Muela N, Litterman NK, Norabuena EM, Mull JL, Galazo MJ, Sun C, et al. Single-cell analysis of SMN reveals its broader role in neuromuscular disease. Cell Rep. 2017;18(6):1484–98.
Zocher S, Overall RW, Lesche M, Dahl A, Kempermann G. Environmental enrichment preserves a young DNA methylation landscape in the aged mouse hippocampus. Nat Commun. 2021;12(1):3892.
Smith ZD, Chan MM, Mikkelsen TS, Gu H, Gnirke A, Regev A, et al. A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature. 2012;484(7394):339–44.
Wu Z, Lu M, Liu D, Shi Y, Ren J, Wang S, et al. m 6 A epitranscriptomic regulation of tissue homeostasis during primate aging. Nat Aging. 2023;3(6):705–21.
Yi SJ, Kim K. New insights into the role of histone changes in aging. Int J Mol Sci. 2020;21(21):8241.
DiTacchio L, Le HD, Vollmers C, Hatori M, Witcher M, Secombe J, et al. Histone lysine demethylase JARID1a activates CLOCK-BMAL1 and influences the circadian clock. Science. 2011;333(6051):1881–5.
Swer PB, Sharma R. ATP-dependent chromatin remodelers in ageing and age-related disorders. Biogerontology. 2021;22(1):1–17.
Zhang B, Long Q, Wu S, Xu Q, Song S, Han L, et al. KDM4 orchestrates epigenomic remodeling of senescent cells and potentiates the senescence-associated secretory phenotype. Nat Aging. 2021;1(5):454–72.
Goss RJ. Principles of regeneration [M]. Elseriver, 2013.
Mescher AL. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration (Oxf). 2017;4(2):39–53.
Wang Z, Zhao H, Tang X, Meng T, Khutsishvili D, Xu B, et al. CNS organoid surpasses cell-laden microgel assembly to promote spinal cord injury repair. Research (Wash D C). 2022;2022:9832128.
You WJ, Xu ZY, Chen WT, Yang X, Liu SQ, Wang LY, et al. Cellular and transcriptional dynamics during brown adipose tissue regeneration under acute injury. Research. 2023;6:0268.
Brockes JP, Kumar A. Comparative aspects of animal regeneration. Annu Rev Cell Dev Biol. 2008;24:525–49.
Maddaluno L, Urwyler C, Werner S. Fibroblast growth factors: key players in regeneration and tissue repair. Development. 2017;144(22):4047–60.
Jiao L, Liu Y, Yu XY, Pan X, Zhang Y, Tu J, et al. Ribosome biogenesis in disease: new players and therapeutic targets. Signal Transduct Target Ther. 2023;8(1):15.
Fu M, Hu Y, Lan T, Guan KL, Luo T, Luo M. The Hippo signalling pathway and its implications in human health and diseases. Signal Transduct Target Ther. 2022;7(1):376.
Li G, Han Q, Lu P, Zhang L, Zhang Y, Chen S, et al. Construction of dual-biofunctionalized chitosan/collagen scaffolds for simultaneous neovascularization and nerve regeneration. Research (Wash D C). 2020;2020:2603048.
Wang F, Ye Y, Zhang Z, Teng W, Sun H, Chai X, et al. PDGFR in PDGF-BB/PDGFR signaling pathway does orchestrates osteogenesis in a temporal manner. Research (Wash D C). 2023;6:0086.
Chen Y, Ren B, Yang J, Wang H, Yang G, Xu R, et al. The role of histone methylation in the development of digestive cancers: a potential direction for cancer management. Signal Transduct Target Ther. 2020;5(1):143.
Pearson JRD, Regad T. Targeting cellular pathways in glioblastoma multiforme. Signal Transduct Target Ther. 2017;2:17040.
Di X, Gao X, Peng L, Ai J, Jin X, Qi S, et al. Cellular mechanotransduction in health and diseases: from molecular mechanism to therapeutic targets. Signal Transduct Target Ther. 2023;8(1):282.
Sun K, Li YY, Jin J. A double-edged sword of immuno-microenvironment in cardiac homeostasis and injury repair. Signal Transduct Target Ther. 2021;6(1):79.
Wang KC, Helms JA, Chang HY. Regeneration, repair and remembering identity: the three Rs of Hox gene expression. Trends Cell Biol. 2009;19(6):268–75.
Yokoyama H. Initiation of limb regeneration: the critical steps for regenerative capacity. Dev Growth Differ. 2008;50(1):13–22.
Yun MH, Gates PB, Brockes JP. Regulation of p53 is critical for vertebrate limb regeneration. Proc Natl Acad Sci U S A. 2013;110(43):17392–7.
Bayascas JR, Castillo E, Munoz-Marmol AM, Salo E. Planarian Hox genes: novel patterns of expression during regeneration. Development. 1997;124(1):141–8.
Call MK, Tsonis PA. Vertebrate limb regeneration. Adv Biochem Eng Biotechnol. 2005;93:67–81.
Charni M, Aloni-Grinstein R, Molchadsky A, Rotter V. p53 on the crossroad between regeneration and cancer. Cell Death Differ. 2017;24(1):8–14.
Carlson BM. Some principles of regeneration in mammalian systems. Anat Rec Part B. 2005;287(1):4–13.
Sabine AM, Smith TB, Williams DE, Brandt ME. Environmental conditions influence tissue regeneration rates in scleractinian corals. Mar Pollut Bull. 2015;95(1):253–64.
Chu L, Liu S, Wu Y, Yang J, Qiao S, Zhou Y, et al. Hair levels of steroid, endocannabinoid, and the ratio biomarkers predict viral suppression among people living with HIV/AIDS in China. Clin Chim Acta. 2022;535:143–52.
Chu L, Liu W, Deng J, Wu Y, Yang H, Wang W, et al. Age-related changes in endogenous glucocorticoids, gonadal steroids, endocannabinoids and their ratios in plasma and hair from the male C57BL/6 mice. Gen Comp Endocrinol. 2021;301:113651.
Qi D, Wang W, Chu L, Wu Y, Wang W, Zhu M, et al. Associations of schizophrenia with the activities of the HPA and HPG axes and their interactions characterized by hair-based biomarkers. Psychoneuroendocrinology. 2024;165:107049.
Feng Z, Zhang X, Zhou J, Li Q, Chu L, Di G, et al. An in vitro-transcribed circular RNA targets the mitochondrial inner membrane cardiolipin to ablate EIF4G2 + /PTBP1 + pan-adenocarcinoma. Nat Cancer. 2024;5(1):30–46.
Poss KD, Shen J, Nechiporuk A, McMahon G, Thisse B, Thisse C, et al. Roles for Fgf signaling during zebrafish fin regeneration. Dev Biol. 2000;222(2):347–58.
Ding JY, Chen MJ, Wu LF, Shu GF, Fang SJ, Li ZY, et al. Mesenchymal stem cell-derived extracellular vesicles in skin wound healing: roles, opportunities and challenges. Mil Med Res. 2023;10(1):36.
Wang Z, Huang Y, He Y, Khor S, Zhong X, Xiao J, et al. Myocardial protection by heparin-based coacervate of FGF10. Bioact Mater. 2021;6(7):1867–77.
Ying Y, Huang Z, Tu Y, Wu Q, Li Z, Zhang Y, et al. A shear-thinning, ROS-scavenging hydrogel combined with dental pulp stem cells promotes spinal cord repair by inhibiting ferroptosis. Bioact Mater. 2023;22:274–90.
Tower RJ, Busse E, Jaramillo J, Lacey M, Hoffseth K, Guntur AR, et al. Spatial transcriptomics reveals metabolic changes underly age-dependent declines in digit regeneration. Elife. 2022;11:e71542.
Cui G, Dong K, Zhou JY, Li S, Wu Y, Han Q, et al. Spatiotemporal transcriptomic atlas reveals the dynamic characteristics and key regulators of planarian regeneration. Nat Commun. 2023;14(1):3205.
Ben-Moshe S, Veg T, Manco R, Dan S, Papinutti D, Lifshitz A, et al. The spatiotemporal program of zonal liver regeneration following acute injury. Cell Stem Cell. 2022;29(6):973–89.e10.
Tu A, Said N, Muddiman DC. Spatially resolved metabolomic characterization of muscle invasive bladder cancer by mass spectrometry imaging. Metabolomics. 2021;17(8):70.
Clair G, Piehowski PD, Nicola T, Kitzmiller JA, Huang EL, Zink EM, et al. Spatially-resolved proteomics: rapid quantitative analysis of laser capture microdissected alveolar tissue samples. Sci Rep. 2016;6(1):39223.
Mallah K, Quanico J, Raffo-Romero A, Cardon T, Aboulouard S, Devos D, et al. Mapping spatiotemporal microproteomics landscape in experimental model of traumatic brain injury unveils a link to Parkinson’s disease. Mol Cell Proteomics. 2019;18(8):1669–82.
Bryan DJ, Litchfield CR, Manchio JV, Logvinenko T, Holway AH, Austin J, et al. Spatiotemporal expression profiling of proteins in rat sciatic nerve regeneration using reverse phase protein arrays. Proteome Sci. 2012;10(1):9.
Ji JJ, Fan J. Discovering myeloid cell heterogeneity in the lung by means of next generation sequencing. Mil Med Res. 2019;6(1):33.
Alchahin AM, Tsea I, Baryawno N. Recent advances in single-cell RNA-sequencing of primary and metastatic clear cell renal cell carcinoma. Cancers (Basel). 2023;15(19):4734.
Cassotta M, Pistollato F, Battino M. Rheumatoid arthritis research in the 21st century: Limitations of traditional models, new technologies, and opportunities for a human biology-based approach. ALTEX. 2020;37(2):223–42.
Fangma Y, Liu M, Liao J, Chen Z, Zheng Y. Dissecting the brain with spatially resolved multi-omics. J Pharm Anal. 2023;13(7):694–710.
Li Y, Ma L, Wu D, Chen G. Advances in bulk and single-cell multi-omics approaches for systems biology and precision medicine. Brief Bioinform. 2021;22(5):bbab024.
Swenson CS, Pillai KS, Carlos AJ, Moellering RE. Spatial chemoproteomics for mapping the active proteome. Isr J Chem. 2023;63(3–4):e202200104.
Zheng Y, Lin C, Chu Y, Gu S, Deng H, Shen Z. Spatial metabolomics in head and neck tumors: a review. Front Oncol. 2023;13:1213273.
Cao J, Packer JS, Ramani V, Cusanovich DA, Huynh C, Daza R, et al. Comprehensive single-cell transcriptional profiling of a multicellular organism. Science. 2017;357(6352):661–7.
Satija R, Farrell JA, Gennert D, Schier AF, Regev A. Spatial reconstruction of single-cell gene expression data. Nat Biotechnol. 2015;33(5):495–502.
Su M, Pan T, Chen QZ, Zhou WW, Gong Y, Xu G, et al. Data analysis guidelines for single-cell RNA-seq in biomedical studies and clinical applications. Mil Med Res. 2022;9(1):68.
Galeano Nino JL, Wu H, LaCourse KD, Kempchinsky AG, Baryiames A, Barber B, et al. Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer. Nature. 2022;611(7937):810–7.
Klaus A, Martins GJ, Paixao VB, Zhou P, Paninski L, Costa RMJN. The spatiotemporal organization of the striatum encodes action space. Neuron. 2017;95(5):1171–80.
Chen R, Blosser TR, Djekidel MN, Hao J, Bhattacherjee A, Chen W, et al. Decoding molecular and cellular heterogeneity of mouse nucleus accumbens. Nat Neurosci. 2021;24(12):1757–71.
Wood J, Dykes J, Slingsby A, Clarke K. Interactive visual exploration of a large spatio-temporal dataset: reflections on a geovisualization mashup. IEEE Trans Vis Comput Graph. 2007;13(6):1176–83.
Ansari MY, Ahmad A, Khan SS, Bhushan G, Mainuddin. Spatiotemporal clustering: a review. Artif Intell Rev. 2019;53(4):2381–423.
Li J, Li Y, He L, Chen J, Plaza A. Spatio-temporal fusion for remote sensing data: an overview and new benchmark. Sci China Inf Sci. 2020;63(4):3–19.
Haghverdi L, Ludwig LS. Single-cell multi-omics and lineage tracing to dissect cell fate decision-making. Stem Cell Rep. 2023;18(1):13–25.
Bai D, Peng J, Yi C. Advances in single-cell multi-omics profiling. RSC Chem Biol. 2021;2(2):441–9.
Deng Y, Bai Z, Fan R. Microtechnologies for single-cell and spatial multi-omics. Nat Rev Bioeng. 2023;1:769–84.
Godin AG, Lounis B, Cognet L. Super-resolution microscopy approaches for live cell imaging. Biophys J. 2014;107(8):1777–84.
Traenkle B, Rothbauer U. Under the microscope: single-domain antibodies for live-cell imaging and super-resolution microscopy. Front Immunol. 2017;8:1030.
Nozaki T, Imai R, Tanbo M, Nagashima R, Tamura S, Tani T, et al. Dynamic organization of chromatin domains revealed by super-resolution live-cell imaging. Mol Cell. 2017;67(2):282–93.e7.
Wang L, Frei MS, Salim A, Johnsson K. Small-molecule fluorescent probes for live-cell super-resolution microscopy. J Am Chem Soc. 2018;141(7):2770–81.
Lukinavičius G, Umezawa K, Olivier N, Honigmann A, Yang G, Plass T, et al. A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nat Chem. 2013;5(2):132–9.
Saul D, Kosinsky RL. Spatial transcriptomics herald a new era of transcriptome research. Clin Transl Med. 2023;13(5):e1264.
Wen ZQ, Lin J, Xie WQ, Shan YH, Zhen GH, Li YS. Insights into the underlying pathogenesis and therapeutic potential of endoplasmic reticulum stress in degenerative musculoskeletal diseases. Mil Med Res. 2023;10(1):54.
Cao Y, Fu L, Wu J, Peng Q, Nie Q, Zhang J, et al. Integrated analysis of multimodal single-cell data with structural similarity. Nucleic Acids Res. 2022;50(21):e121.
Hao Y, Hao S, Andersen-Nissen E, Mauck WM 3rd, Zheng S, Butler A, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184(13):3573–87.e29.
Argelaguet R, Arnol D, Bredikhin D, Deloro Y, Velten B, Marioni JC, et al. MOFA+: a statistical framework for comprehensive integration of multi-modal single-cell data. Genome Biol. 2020;21(1):111.
Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM 3rd, et al. Comprehensive integration of single-cell data. Cell. 2019;177(7):1888–902.e21.
Singh R, Hie BL, Narayan A, Berger B. Schema: metric learning enables interpretable synthesis of heterogeneous single-cell modalities. Genome Biol. 2021;22(1):131.
Chen W, Li Y, Easton J, Finkelstein D, Wu G, Chen X. UMI-count modeling and differential expression analysis for single-cell RNA sequencing. Genome Biol. 2018;19(1):70.
Eraslan G, Simon LM, Mircea M, Mueller NS, Theis FJ. Single-cell RNA-seq denoising using a deep count autoencoder. Nat Commun. 2019;10(1):390.
Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36(5):411–20.
Gala R, Budzillo A, Baftizadeh F, Miller J, Gouwens N, Arkhipov A, et al. Consistent cross-modal identification of cortical neurons with coupled autoencoders. Nat Comput Sci. 2021;1(2):120–7.
Wu KE, Yost KE, Chang HY, Zou J. BABEL enables cross-modality translation between multiomic profiles at single-cell resolution. Proc Natl Acad Sci U S A. 2021;118(15):e2023070118.
Yang KD, Belyaeva A, Venkatachalapathy S, Damodaran K, Katcoff A, Radhakrishnan A, et al. Multi-domain translation between single-cell imaging and sequencing data using autoencoders. Nat Commun. 2021;12(1):31.
Tang X, Zhang J, He Y, Zhang X, Lin Z, Partarrieu S, et al. Explainable multi-task learning for multi-modality biological data analysis. Nat Commun. 2023;14(1):2546.
Rutter L, Barker R, Bezdan D, Cope H, Costes SV, Degoricija L, et al. A new era for space life science: international standards for space omics processing. Patterns (N Y). 2020;1(9):100148.
Bressan D, Battistoni G, Hannon GJ. The dawn of spatial omics. Science. 2023;381(6657):eabq4964.
Schwartzman O, Tanay A. Single-cell epigenomics: techniques and emerging applications. Nat Rev Genet. 2015;16(12):716–26.
Brown RB, Audet J. Current techniques for single-cell lysis. J R Soc Interface. 2008;5 Suppl 2(Suppl 2):S131-8.
Kashima Y, Sakamoto Y, Kaneko K, Seki M, Suzuki Y, Suzuki A. Single-cell sequencing techniques from individual to multiomics analyses. Exp Mol Med. 2020;52(9):1419–27.
Liao J, Lu X, Shao X, Zhu L, Fan X. Uncovering an organ’s molecular architecture at single-cell resolution by spatially resolved transcriptomics. Trends Biotechnol. 2021;39(1):43–58.
Vickovic S, Schapiro D, Carlberg K, Lotstedt B, Larsson L, Hildebrandt F, et al. Three-dimensional spatial transcriptomics uncovers cell type localizations in the human rheumatoid arthritis synovium. Commun Biol. 2022;5(1):129.
Baccin C, Al-Sabah J, Velten L, Helbling PM, Grunschlager F, Hernandez-Malmierca P, et al. Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. Nat Cell Biol. 2020;22(1):38–48.
Li B, Zhang W, Guo C, Xu H, Li L, Fang M, et al. Benchmarking spatial and single-cell transcriptomics integration methods for transcript distribution prediction and cell type deconvolution. Nat Methods. 2022;19(6):662–70.
Long Y, Ang KS, Li M, Chong KLK, Sethi R, Zhong C, et al. Spatially informed clustering, integration, and deconvolution of spatial transcriptomics with GraphST. Nat Commun. 2023;14(1):1155.
Eriksson JO, Sanchez Brotons A, Rezeli M, Suits F, Marko-Varga G, Horvatovich P. MSIWarp: a general approach to mass alignment in mass spectrometry imaging. Anal Chem. 2020;92(24):16138–48.
Schlotter F, Halu A, Goto S, Blaser MC, Body SC, Lee LH, et al. Spatiotemporal multi-omics mapping generates a molecular atlas of the aortic valve and reveals networks driving disease. Circulation. 2018;138(4):377–93.
Mereu E, Lafzi A, Moutinho C, Ziegenhain C, McCarthy DJ, Alvarez-Varela A, et al. Benchmarking single-cell RNA-sequencing protocols for cell atlas projects. Nat Biotechnol. 2020;38(6):747–55.
Chen C, Wang J, Pan D, Wang X, Xu Y, Yan J, et al. Applications of multi-omics analysis in human diseases. MedComm (2020). 2023;4(4):e315.
Erfanian N, Heydari AA, Feriz AM, Iañez P, Derakhshani A, Ghasemigol M, et al. Deep learning applications in single-cell genomics and transcriptomics data analysis. Biomed Pharmacother. 2023;165:115077.
Gaddy MR, Unkelbach J, Papp D. Robust spatiotemporal fractionation schemes in the presence of patient setup uncertainty. Med Phys. 2019;46(7):2988–3000.
Saeed S, Abdullah A. Informatics. Recognition of brain cancer and cerebrospinal fluid due to the usage of different MRI image by utilizing support vector machine. Bulletin Electr Eng. 2020;9(2):619–25.
Seifert AW, Muneoka K. The blastema and epimorphic regeneration in mammals. Dev Biol. 2018;433(2):190–9.
Becht E, McInnes L, Healy J, Dutertre CA, Kwok IW, Ng LG, et al. Dimensionality reduction for visualizing single-cell data using UMAP. Nat Biotechnol. 2019;37(1):38–44.
Huang L, Ma F, Chapman A, Lu S, Xie XS. Single-cell whole-genome amplification and sequencing: methodology and applications. Annu Rev Genomics Hum Genet. 2015;16:79–102.
Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, et al. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature. 2018;563(7731):347–53.
Chen F, Tillberg PW, Boyden ES. Optical imaging. Expansion microscopy. Science. 2015;347(6221):543–8.
Bravo Gonzalez-Blas C, Quan XJ, Duran-Romana R, Taskiran II, Koldere D, Davie K, et al. Identification of genomic enhancers through spatial integration of single-cell transcriptomics and epigenomics. Mol Syst Biol. 2020;16(5):e9438.
Velten B, Braunger JM, Argelaguet R, Arnol D, Wirbel J, Bredikhin D, et al. Identifying temporal and spatial patterns of variation from multimodal data using MEFISTO. Nat Methods. 2022;19(2):179–86.
Akhoundova D, Rubin MA. Clinical application of advanced multi-omics tumor profiling: Shaping precision oncology of the future. Cancer Cell. 2022;40(9):920–38.
Fang S, Chen B, Zhang Y, Sun H, Liu L, Liu S, et al. Computational approaches and challenges in spatial transcriptomics. Genom Proteom Bioinform. 2023;21(1):24–47.
Singh KS, van der Hooft JJJ, van Wees SCM, Medema MH. Integrative omics approaches for biosynthetic pathway discovery in plants. Nat Prod Rep. 2022;39(9):1876–96.
Karczewski KJ, Snyder MP. Integrative omics for health and disease. Nat Rev Genet. 2018;19(5):299–310.
Wheelock CE, Goss VM, Balgoma D, Nicholas B, Brandsma J, Skipp PJ, et al. Application of’omics technologies to biomarker discovery in inflammatory lung diseases. Eur Respir J. 2013;42(3):802–25.
Bonaguro L, Schulte-Schrepping J, Ulas T, Aschenbrenner AC, Beyer M, Schultze JL. A guide to systems-level immunomics. Nat Immunol. 2022;23(10):1412–23.
Zhang J, Yin J, Heng Y, Xie K, Chen A, Amit I, et al. Spatiotemporal omics-refining the landscape of precision medicine. Life Med. 2022;1(2):84–102.
Almet AA, Cang Z, Jin S, Nie Q. The landscape of cell-cell communication through single-cell transcriptomics. Curr Opin Syst Biol. 2021;26:12–23.
Wang X. Clinical trans-omics: an integration of clinical phenomes with molecular multiomics. Cell Biol Toxicol. 2018;34(3):163–6.
Maniatis S, Aijo T, Vickovic S, Braine C, Kang K, Mollbrink A, et al. Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science. 2019;364(6435):89–93.
Wang Z, Wu W, Kim MS, Cai D. GnRH pulse frequency and irregularity play a role in male aging. Nat Aging. 2021;1(10):904–18.
Wang X, Fan J. Spatiotemporal molecular medicine: a new era of clinical and translational medicine. Clin Transl Med. 2021;11(1):e294.
Feng DC, Zhu WZ, Wang J, Li DX, Shi X, Xiong Q, et al. The implications of single-cell RNA-seq analysis in prostate cancer: unraveling tumor heterogeneity, therapeutic implications and pathways towards personalized therapy. Mil Med Res. 2024;11(1):21.
Download references
Acknowledgments
The images were created by Biorender.
This work was supported by the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (2023R01002), and the National Natural Science Foundation of China (82271629, 82301790).
Author information
Liu-Xi Chu, Wen-Jia Wang and Xin-Pei Gu contributed equally to this work.
Authors and Affiliations
Affiliated Cixi Hospital, Wenzhou Medical University, Ningbo, 315300, Zhejiang, China
Liu-Xi Chu, Da-Wei Jiang, Jun-Peng Xu & Zhou-Guang Wang
Oujiang Laboratory (Zhejiang Lab for Regenerative Medicine, Vision and Brain Health), School of Pharmaceutical Science, Wenzhou Medical University, Wenzhou, 325035, Zhejiang, China
Liu-Xi Chu, Ping Wu, Da-Wei Jiang, Jun-Qing Huang, Xin-Wang Ying, Jun-Peng Xu, Ao Fang, Zun-Yong Feng, Xiao-Kun Li & Zhou-Guang Wang
National Key Laboratory of Macromolecular Drug Development and Manufacturing, School of Pharmaceutical Science, Wenzhou Medical University, Wenzhou, 325035, Zhejiang, China
Liu-Xi Chu, Ping Wu, Da-Wei Jiang, Jun-Qing Huang, Xin-Wang Ying, Jia-Men Shen, Yi Jiang, Jun-Peng Xu, Yi-Bo Ying, Hao-Man Chen, Ao Fang, Zun-Yong Feng, Xiao-Kun Li & Zhou-Guang Wang
State Key Laboratory of Bioelectronics, School of Biological Science & Medical Engineering, Southeast University, Nanjing, 210096, China
Wen-Jia Wang & Chen Gao
School of Pharmaceutical Sciences, Guangdong Provincial Key Laboratory of New Drug Screening, Southern Medical University, Guangzhou, 510515, China
Department of Human Anatomy, Shandong First Medical University & Shandong Academy of Medical Sciences, Taian, 271000, Shandong, China
Xin-Pei Gu & Shu-Hong An
Integrative Muscle Biology Laboratory, Division of Regenerative and Rehabilitative Sciences, University of Tennessee Health Science Center, Memphis, TN, 38163, United States
Key Laboratory for Laboratory Medicine, Ministry of Education, Zhejiang Provincial Key Laboratory of Medical Genetics, School of Laboratory Medicine and Life Science, Wenzhou Medical University, Wenzhou, 325035, Zhejiang, China
Key Laboratory of Imaging Diagnosis and Minimally Invasive Intervention Research, Institute of Imaging Diagnosis and Minimally Invasive Intervention Research, the Fifth Affiliated Hospital of Wenzhou Medical University, Lishui Hospital of Zhejiang University, Lishui, 323000, Zhejiang, China
Jun-Qing Huang & Zhou-Guang Wang
School and Hospital of Stomatology, Wenzhou Medical University, Wenzhou, 324025, Zhejiang, China
Departments of Diagnostic Radiology, Surgery, Chemical and Biomolecular Engineering, and Biomedical Engineering, Yong Loo Lin School of Medicine and College of Design and Engineering, National University of Singapore, Singapore, 119074, Singapore
Zun-Yong Feng
Clinical Imaging Research Centre, Centre for Translational Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, 117599, Singapore
Nanomedicine Translational Research Program, NUS Center for Nanomedicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, 117597, Singapore
Institute of Molecular and Cell Biology, Agency for Science, Technology, and Research (A*STAR), Singapore, 138673, Singapore
You can also search for this author in PubMed Google Scholar
Contributions
LXC, WJW, and XPG collected the related papers and wrote the manuscript. PW, CG, QZ, JW, DWJ, JQH, XWY, JMS, YJ, LHL, JPX, YBY, HMC, and AF investigated and prepared the figures. ZGW, XKL, SHA, and ZYF helped to revise the manuscript. ZGW, XKL, SHA, and ZYF made important suggestions to the paper. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Zun-Yong Feng , Shu-Hong An , Xiao-Kun Li or Zhou-Guang Wang .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Chu, LX., Wang, WJ., Gu, XP. et al. Spatiotemporal multi-omics: exploring molecular landscapes in aging and regenerative medicine. Military Med Res 11 , 31 (2024). https://doi.org/10.1186/s40779-024-00537-4
Download citation
Received : 15 December 2023
Accepted : 07 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1186/s40779-024-00537-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Spatiotemporal multi-omics
- Aging and regeneration
- Cellular interactions
- Innovative therapeutic strategies
Military Medical Research
ISSN: 2054-9369
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

- Better Hearing Consumer
- Dizziness Depot
- FindHearing
- Hearing News Watch
- Hearing Economics
- Hear The Music
- Hear In Private Practice
- Hearing Technologies
- Hearing and Kids
- This Week in Hearing
- Hearing Technology Innovator Awards
- Submit Your Product

New Study: Hearing Loss Increases Risk of Cognitive Impairment, Dementia, and Alzheimer’s Disease

A new systematic review and meta-analysis published in Ageing Research Reviews examined the relationship between adult-onset hearing loss and cognitive impairment. Analyzing data from fifty cohort studies involving over 1.5 million participants, the study suggests that hearing loss significantly increases the risk of cognitive decline, dementia, and Alzheimer’s disease (AD).
Hearing loss is a major global health issue affecting an estimated 1.5 billion people worldwide, a number that is expected to rise with the aging population. The World Health Organization and the Lancet Commission on Dementia have identified hearing loss as a potentially modifiable risk factor for dementia, suggesting that treatments like hearing aids might prevent or delay cognitive decline.
“…this meta-analysis of cohort studies provided compelling evidence across diverse study settings and designs of adult-onset hearing loss being a robust and consistent independent risk factor for dementia.”
Hearing Loss and Dementia Risk
This comprehensive review aimed to summarize existing cohort evidence on adult-onset hearing loss as a risk factor for cognitive impairment and dementia. The study explored the dose-response relationship, risk for various dementia subtypes, and potential moderators that might influence this association.
The researchers included cohort studies with participants who had no dementia at baseline, assessed hearing at the beginning of the study, and followed them for at least two years. They used random-effect models and conducted subgroup and meta-regression analyses to examine the impact of different moderators.
Key Findings
The analysis included fifty studies with a total of 1,548,754 participants. The findings revealed that:
- Increased Dementia Risk : Hearing loss was associated with a 35% increased risk of incident dementia (HR=1.35), a 29% increased risk of mild cognitive impairment (MCI) (HR=1.29), and a 56% increased risk of Alzheimer’s disease dementia (HR=1.56). However, the association with vascular dementia was not statistically significant (HR=1.30).
- Dose-Response Relationship : Each 10-decibel worsening of hearing was linked to a 16% increase in dementia risk, indicating a dose-response relationship where greater hearing loss correlates with higher dementia risk.
- Consistency Across Subtypes : The impact of hearing loss on dementia risk did not vary significantly across different types of dementia or other moderators such as baseline age or cardiovascular health.

The research suggests several possible mechanisms for this link, including reduced social interaction and accelerated brain pathology due to hearing loss. However, the potential for residual confounding factors such as age and cardiovascular health cannot be entirely ruled out.
Implications
The study’s findings underscore the importance of early identification and treatment of hearing loss, potentially through the use of hearing aids , as a strategy to reduce the risk of cognitive decline and dementia. This emphasizes the need for regular hearing assessments, particularly in older adults, and integrating hearing care into broader dementia prevention strategies.
“…Adult-onset hearing loss is also potentially treatable, most often with hearing aids. Our findings suggest that this treatment may also reduce dementia risk.”
Despite the large participant pool and diverse settings, variations in hearing and cognitive assessment methods, follow-up durations, and analysis techniques introduced some residual heterogeneity, suggesting that other unexamined factors might also play a role. Nonetheless, the systematic review and meta-analysis provide strong evidence that adult-onset hearing loss is a significant and independent risk factor for dementia.
Addressing hearing loss through interventions like hearing aids could mitigate this risk, highlighting the need to incorporate hearing loss management into public health strategies to reduce the incidence of dementia and cognitive impairment.
- Yu, R.-C., Proctor, D., Soni, J., Pikett, L., Livingston, G., Lewis, G., Schilder, A., Bamiou, D., Mandavia, R., Omar, R., Pavlou, M., Lin, F., Goman, A. M., & Costafreda Gonzalez, S. (2024). Adult-onset hearing loss and incident cognitive impairment and dementia – A systematic review and meta-analysis of cohort studies. Ageing Research Reviews , 102346. ISSN 1568-1637. https://doi.org/10.1016/j.arr.2024.102346 .

Leave a Reply Cancel Reply
Save my name, email, and website in this browser for the next time I comment.
- Open access
- Published: 27 May 2024
The role of sarcopenia in fragility fractures of the pelvis – is sarcopenia an underestimated risk factor?
- Olivia Mair 1 ,
- Jan Neumann 2 ,
- Philipp Rittstieg 1 ,
- Michael Müller 1 ,
- Peter Biberthaler 1 &
- Marc Hanschen 1
BMC Geriatrics volume 24 , Article number: 461 ( 2024 ) Cite this article
63 Accesses
Metrics details
Fragility fractures of the pelvis (FFPs) represent a significant health burden, particularly for the elderly. The role of sarcopenia, an age-related loss of muscle mass and function, in the development and impact of these fractures is not well understood. This study aims to investigate the prevalence and impact of osteoporosis and sarcopenia in patients presenting with FFPs.
This retrospective study evaluated 140 elderly patients with FFPs. The diagnosis of sarcopenia was assessed by psoas muscle area (PMA) and the height-adjusted psoas muscle index (PMI) measured on computed tomography (CT) scans. Clinical data, radiological findings and functional outcomes were recorded and compared with the presence or absence of sarcopenia and osteoporosis.
Our study cohort comprised 119 female (85.0%) and 21 (15.0%) male patients. The mean age at the time of injury or onset of symptoms was 82.26 ± 8.50 years.
Sarcopenia was diagnosed in 68.6% ( n = 96) patients using PMA and 68.8% ( n = 88) using PMI. 73.6% ( n = 103) of our study population had osteoporosis and 20.0% ( n = 28) presented with osteopenia. Patients with sarcopenia and osteoporosis had longer hospital stays ( p < 0.04), a higher rate of complications ( p < 0.048) and functional recovery was significantly impaired, as evidenced by a greater need for assistance in daily living ( p < 0.03). However, they were less likely to undergo surgery ( p < 0.03) and the type of FFP differed significantly ( p < 0.04). There was no significant difference in mortality rate, pre-hospital health status, age or gender.
Our study highlights the important role of sarcopenia in FFPs in terms of the serious impact on health and quality of life in elderly patients especially when osteoporosis and sarcopenia occur together.
Identifying and targeting sarcopenia in older patients may be an important strategy to reduce pelvic fractures and improve recovery. Further research is needed to develop effective prevention and treatment approaches that target muscle health in the elderly.
Peer Review reports
The WHO classified fragility fractures as fractures, which result from minimal and low-energy trauma such as falls from standing heights with the underlying cause attributed to reduced compressive and/or torsional strength of the bone [ 1 ]. Fragility fractures of the pelvis (FFP) are one of the most common entities of fragility fractures. It has been reported in the literature that their incidence is continually rising due to the aging population, but also due to a more frequent detection caused by the ample use of computed tomography (CT) [ 2 , 3 , 4 , 5 , 6 ]. As their morphology differs significantly from that of high-energy pelvic fractures, Rommens and coworkers have introduced a comprehensive classification system for FFPs based on the stability of the fractures [ 7 ].
While osteoporosis is widely acknowledged as one of the leading causes for fragility fractures in the older population, sarcopenia moves increasingly into focus [ 8 ]. Sarcopenia is defined as “a progressive and generalized skeletal muscle disorder that is associated with increased likelihood of adverse outcomes including falls, fractures, physical disability and mortality” by the European Working Group on Sarcopenia in Older People (EWGSOP) [ 9 ].
Sarcopenia is associated with reduced muscle strength and muscle mass and can be quantified in different ways. While functional tests such as grip strength and gait speed are highly sensitive for measuring the severity of sarcopenia, sarcopenia can also be identified by assessing skeletal muscle mass and muscle quality radiographically. One of the validated methods for assessing muscle quantity is the measurement of the lumbar muscle cross-sectional area on CT or MRI scans [ 9 ]. Often, the cross-sectional area of specific muscles, such as the psoas muscle area (PMA) are analyzed. They are then adjusted by height to calculate, for example the psoas muscle index (PMI). This method has proven useful in several disciplines. For example, multiple authors showed that PMA and PMI were significant markers for postoperative complications after colorectal surgery, acute pancreatitis and also mechanically ventilated patients [ 10 , 11 , 12 , 13 , 14 , 15 ].
Osteoporosis is one of the leading risk factors in developing fragility fractures. In recent years measuring osteoporosis on routine CT scans of areas involving the lumbar spine by assessing bone mineral density in HU has increasingly been utilized in clinical practice with good success. Although it might not be as precise as dual energy X-ray absorptiometry (DXA), this technique offers an opportunity for opportunistic and cost-effective screening for osteoporosis without any additional radiation [ 16 , 17 , 18 , 19 , 20 ].
However, extensive research into the exact role and especially the interaction of sarcopenia and osteoporosis in FFPs is still missing.
Therefore, this study aims to investigate the prevalence of sarcopenia and osteoporosis in patients presenting with FFPs and the role the occurrence of both of these conditions together plays in the outcome after FFPs.
Data collection
This study was conducted at a university level 1 trauma center. Approval and Authorization by the local Institutional Review Board was obtained (Study Nr.: 2023–179-S-NP).
In this retrospective study all patients who sustained a FFP from 1st of January 2020 to 31 st of December 2022 were included. We excluded all patients with isolated acetabular fractures or patients who sustained their pelvic fracture due to high velocity trauma. Moreover, the inclusion criteria for CT scans were stringently limited to those encompassing, at a minimum, the L3, L4, and L5 vertebral segments.
Demographic data of patients and information about the time of trauma or the onset of symptoms, if applicable, data on surgical interventions and duration of stay were documented retrospectively. Complications during the hospital stay were also documented and their severity classified according to the Clavien-Dindo-Classification system [ 21 ].
Additional information on the health status before the surgery including ASA (American Society of Anesthesiologists)- status, preexisting conditions, the place of residence and the level of care needed prior to surgery and after discharge from hospital were collected.
Measuring PMI and MRA
To quantify sarcopenia, we used psoas muscle area (PMA), psoas muscle index (PMI) to analyze muscle quantity, and muscle radiation attenuation (MRA) for muscle quality. We chose to use the psoas muscle as the reference muscle because CT scans of the pelvis are routinely obtained in FFPs. One of the other available radiological measurement tools is the skeletal muscle index (SMI), which is usually also measured on the level of the third lumbar vertebra by tracing the whole cross sectional skeletal muscle area (SMA). While SMA is also valid to identify sarcopenia, it has been criticized, as tracing skeletal muscle mass can be difficult due to irregular muscle shapes and unclear boundaries of the muscles. Therefore, the margin of error is larger in SMI opposed to PMI and additionally is more time consuming due to the larger muscle area which needs to be traced. Furthermore, it has been critiqued as SMI lacks in quantifying muscle mass in women [ 22 ].
PMA was measured on an axial CT-scan on the baseplate level of the L3 vertebra by manually tracing the perimeter of the right and left psoas muscle. The sum divided by two is the PMA (in cm 2 ). PMI (cm 2 /m 2 ) was then calculated from the PMA divided by height squared (m 2 ) (Fig. 1 ).
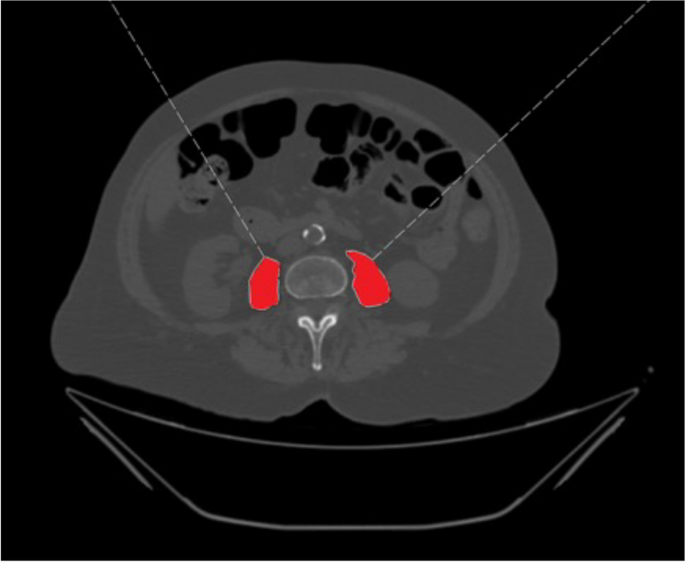
Exemplary measurement method of the psoas muscle area on an axial CT scan, the psoas muscle circumference is traced manually and the area within the tracing calculated in (cm. 2 )
All measurements were obtained utilizing the PACS Viewer (IDS7, Sectra Workstation, Version 24.2, Sectra AB, Linköping, Sweden). Axial, non-contrast-enhanced CT images utilizing bone window settings (with a slice thickness of 3 mm) were uniformly acquired within the hospital premises, employing a 256-row multidetector CT scanner (iCT 256; Philips Healthcare, Best, The Netherlands).
As mentioned before, there are no standardized cut-off values to define sarcopenia. We used the proposed cut-off value to determine between pathological and normal values for PMA and PMI proposed by Fu and coworkers [ 14 ]. Therefore, values below 11.50 cm 2 for PMA and 3.85 cm 2 /m 2 for PMI were considered pathological in men. Values below 8.22 cm 2 for PMA and 3.20 cm 2 /m 2 for PMI were considered pathological in women.
Measuring osteoporosis
The same CT scans used to quantify sarcopenia were utilized for measuring osteoporosis. Radiation attenuation was measured in Hounsfield Units (HU) using a round region of interest (ROI) area of 10mm 2 on a sagittal, coronal and axial reconstruction on the level of the L3 vertebra avoiding the cortex and posterior venous plexus. The mean value of these three measurements was then calculated. When the L3 vertebra could not be used due to fractures, hemangioma or other issues, the next lower possible vertebra was used for measurements. HU were than transformed to bone mineral density (BMD) by asynchronous calibration based on scanner specific equations [ 23 ]. We then used the cut-off values proposed by the American College of Radiology to define osteoporosis (BMD < 80.0 mg/cm 3 ) and osteopenia ≥ 80.0 to < 120.0 mg/cm 3 ) [ 24 ].
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS, version 27; IBM Inc., Chicago, IL, USA). Patients’ characteristics were described using mean and standard deviation (SD) for continuous variables. Absolute numbers and relative percentages were used for categorical variables. Significances were calculated using paired t-tests or Fisher´s-Exact Test where appropriate. P -values < 0.05 were considered statistically significant.
Demographic data of patients
Applying the relevant exclusion criteria, we were able to include 140 patients in this study. There were 119 female patients (85.0%) and 21 (15.0%) male patients. The mean age at the time of injury or onset of symptoms was 82.26 ± 8.50 years (Table 1 ).
There were no healthy patients (ASA 1) in our study population, but 68 (48.6%) of patients in the ASA 2 group, 71 (50.7%) in the ASA 3 group and 1 (0.7%) in the ASA 4 group. Cardiac impairments were the most common condition ( n = 126), followed by neurologic conditions such as dementia or Parkinson’s disease ( n = 90), pulmonary conditions ( n = 44), a history of oncological disease ( n = 38) and diabetes ( n = 28). Further, 90 patients had health conditions other than mentioned in the above categories. 53 patients (37.9%) had a prior history of osteoporosis and 39 patients (27.9%) were medically treated for osteoporosis.
To assess pre-injury health status further we also examined the walking distance, walking aids and living arrangements. 24 patients (17.1%) were mobile only within their homes, while 50 patients (35.7%) were able to walk distances of up to 1 km, 38 patients (27.1%) had unrestricted mobility and 2 patients (1.4%) were bedridden. This information could not be obtained in 26 patients.
35.7% of patients ( n = 50) were mobile without any walking aids, while 47.9% ( n = 67) were reliant on walkers/ walking sticks and 5.7% ( n = 8) on a wheelchair. Information was missing in 15 cases.
Additionally, most of our patient cohort was still living in their homes ( n = 118, 84.3%), with 62 (44.3%) even living on their own without any professional care. 21 patients (15.0%) lived in nursing homes. Information on one patient was missing.
Injury and treatment
90.7% ( n = 127) patients sustained a fall from standing height, while the remaining 9.3% ( n = 13) of patients experienced pain and were diagnosed with FFPs without any trauma.
FFPs were all classified according to the comprehensive classification introduced by Rommens and coworkers [ 5 ]. The distribution was as follows: Type Ia 27.9% ( n = 39), Type Ib none, Type IIa 2.9% ( n = 4), Type IIb 18.6% ( n = 26), Type IIc 28.6% ( n = 40), Type IIIa 1.4% ( n = 2), Type IIIb 2.1% ( n = 3), Type IIIc 4.3% ( n = 6), Type IVa none, Type IVb 4.3% ( n = 6) and Type IVc 10.0% ( n = 14).
Twenty-one patients (15.0%) underwent surgery, with only 4 of the cases primarily indicated for surgery. Generally, in the absence of neurological deficits, patients with FFPs receive conservative treatment, which includes oral pain management and mobilization under physiotherapeutic assistance, according to the standardized treatment protocol of FFPs in the treating hospital. If mobilization fails due to unmanageable pain or secondary dislocation of the injury, surgery will be considered.
During their hospital stay 66 patients (47.1%) suffered some sort of. According to the Clavien—Dindo—Classification there were 41 Grade I (minor) complications, 9 Grade II, 7 Grade III and no Grade IV complications. 9 patients (6.4%) died within the hospital stay after approximately 22.78 ± 17.75 days.
The average length of stay was 9.48 ± 6.72 days. 52 patients (37.1%) each were discharged home or discharged to nursing homes, while 22 patients (15.7%) were discharged to rehabilitation centers and 7 patients (5.0%) were transferred to peripheral hospitals.
In women mean values for PMA and PMI were 7.73 ± 1.86 cm 2 and 2.91 ± 0.67 cm 2 /m 2 respectively. In men PMA and PMI were 10.24 ± 3.19 cm 2 and 3.41 ± 1.01 cm 2 /m 2 respectively.
When applying the above- mentioned cut-off values for defining sarcopenia, 68.6% ( n = 96) of the patients had pathological values for PMA and 68.8% ( n = 88) for PMI. As height is needed to calculated PMI, the PMI of 12 patients could not be calculated as information about height was missing in these patients’ records.
There were no statistically significant differences for sarcopenia rates between men and women (PMA: p < 0.48, PMI: p < 0.88) or according to age (PMA: p < 0.57, PMI: p < 0.52 respectively).
Additionally, PMA and PMI did not differ between ASA groups, pre-injury mobility, living conditions, complication rate and mortality. Although independent from sarcopenia, men were significantly more likely to die than women ( p < 0.001).
Type of pelvic injury (by FFP classification) differed significantly in patients with sarcopenia (PMA p < 0.005, PMI p < 0.004), however the difference was not significant in MRA values ( p < 0.41). Furthermore, significantly more patients with sarcopenia underwent surgery (PMA p < 0.03, PMI p < 0.04). Patients with sarcopenia also had a lower outcome in terms of place of discharge as more patients with sarcopenia were discharged to nursing homes instead of lower care facilities (PMA p < 0.02, PMI p < 0.01).
- Osteoporosis
Mean BMD was 45.17 ± 27.33 mg/cm 3 with 73.6% ( n = 103) of our study population presenting with osteoporosis. Osteopenia was recognized in 20.0% ( n = 28) of our study cohort and only 9 patients (6.4%) had healthy bone density.
There was no statistically significant difference between pre-injury health and mobility status, rate of surgery, complication rate or place discharged to. Patients with osteoporosis had significantly longer hospital stays ( p < 0.027).
Only 39.7% of the patients diagnosed with osteoporosis based on our CT scans were previously aware of their condition, even though this was not statistically significant ( p < 0.84). Additionally, only 29.8% of the patients in our study cohort diagnosed with osteoporosis received medical treatment for their condition ( p < 0.43).
Sarcopenia and osteoporosis
Ninety-one patients (65.0%) suffered from both osteoporosis and sarcopenia. Those patients had significantly longer hospital stays ( p < 0.04) (Fig. 2 ), had higher complication rates ( p < 0.048) and were more likely to be discharged to high grade care facilities ( p < 0.03).
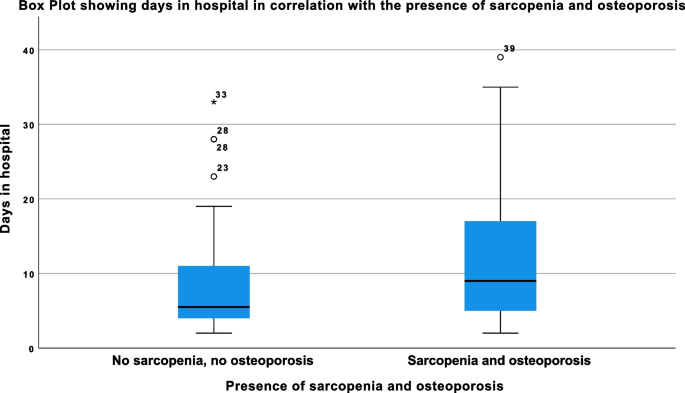
Boxplot showing the correlation of length of hospital stay and the presence of osteoporosis and sarcopenia; the outliers, depicted as circles or asterixis beyond the whiskers, represent patients that had significantly longer hospital stays, the values next to the circles/ asterixis show the days in hospital; these outliers are also partially responsible for the high standard deviation in the “days in hospital” (With sarcopenia and osteoporosis: 12.45 ± 9.88 days, without sarcopenia and osteoporosis: 7.45 ± 5.8 days), also see discussion section
Furthermore, types of FFPs differed significantly between the groups ( p < 0.04). Additionally, patients with sarcopenia and osteoporosis were significantly less likely to undergo surgery ( p < 0.03).
There was no significant difference in mortality, pre- injury health status, gender or age (Table 2 ).
In this study, we focused in particular on the influence that the combined presence of sarcopenia and osteoporosis has on the short-term outcome after FFPs. As mentioned before FFPs are some of the most common fragility fractures in elderly patients and their number is expected to rise to 200 Mio. until 2050 [ 8 ].
Our study population comprised 140 patients, predominantly women (85.0%) and were over 80 years old at the time of injury or onset of symptoms. These demographics closely resemble those reported in other studies investigating fragility fractures and sarcopenia [ 2 , 25 ]. It is widely recognized that women are significantly more prone to sustaining fragility fractures compared to men due to several factors. Firstly, women typically have lower bone density, a condition often exacerbated by hormonal changes during menopause. Furthermore, different pelvic anatomy, lower muscle strength as well as longer life expectancy further add to the higher prevalence of FFPs in women [ 6 , 26 ].
As stated in the consensus paper by the EWGSOP, there are multiple different methods for identifying and quantifying sarcopenia. These include measurement methods based on radiological data as well as methods based on clinical tests [ 9 ]. One of the measurement methods based on radiological data is the psoas muscle index (PMI), also called the psoas cross sectional area. Several reasons led to our decision to utilize PMI in this study. Due to the retrospective nature of the study, clinical tests, such as the grip strength test or the chair stand test to evaluate sarcopenia at the time of injury are impossible to obtain. Additionally, the lower lumbar spine is usually imaged on CT scans, which are routinely performed in patients with FFPs. Therefore, the region of interest for measuring PMI is available in the hospital records. The easy availability and low cost of CT scans compared to DXA or bioelectrical impedance analysis (BIA) is considered advantageous when using PMA [ 17 ]. In addition, since we also measured osteoporosis on the same CT scans in this study, the use of PMA to detect sarcopenia is even more valuable. However, we acknowledge that CT scans might not always be available, especially in smaller hospitals or during off-hours. Yet, we believe that in these cases, implementing clinical tests such as hand grip strength can also be utilized to identify sarcopenia and initiate appropriate treatment.
PMA and PMI have also been validated by multiple authors, and their predictive value for perioperative complications has been demonstrated in various patient cohorts, including colorectal cancer patients, liver cirrhosis patients and patients with acute pancreatitis [ 10 , 11 , 13 , 27 ].
However, clearly defined cut-off values to determine between pathological and normal values for PMA and PMI are missing. Several authors tried to establish these cut-offs to define sarcopenia using different methods [ 14 , 22 , 28 , 29 ]. As explained above we used cut-off values defined by Fu et al. as they presented values adjusted by gender and utilized the most detailed method for determining cut-off values [ 14 ]. We hypothesized that generating cut-off values based on our dataset, such as adopting thresholds lower than the 5th percentile, as observed in previous studies, might have led to an underestimation of sarcopenia. This potential underestimation can be attributed to the selection bias inherent in our study population only including elderly patients with existing fragility fractures.
68.7% of the patients in our study cohort had sarcopenia at the time of injury, with other authors presenting the prevalence of sarcopenia ranging between 17 – 70% [ 8 , 30 , 31 , 32 ]. The prevalence of sarcopenia in this study is relatively high. This can be attributed, on one hand, to our inclusion criteria, as this study focuses an old, fragile cohort with FFPs. On the other hand, this could also be due to the measurement method we used for sarcopenia, as PMA and PMI have been criticized for being unreliable, as they only measure one muscle [ 30 ]. Contrarily, multiple authors have demonstrated high predictive value for outcome and mortality in other diseases [ 10 , 12 , 13 , 33 ].
93.6% of our study population had osteopenia or osteoporosis. It is common knowledge that osteoporosis is the biggest risk factor for developing fragility fractures. Even though this is well known, in clinical practice osteoporosis might still be undertreated. This is also reflected in our study as only 39.7% of the patients with osteoporosis had prior knowledge of their osteoporosis and only 29.8% were actually treated for osteoporosis. The underdiagnosing and undertreating of patients with osteoporosis and fragility fractures is widely discussed in literature [ 34 ].
In this study, we focused in particular on the influence that the combined presence of sarcopenia and osteoporosis has on the short-term outcome after FFPs. We found that patients with sarcopenia and osteoporosis had more low-grade FFPs (Type I and II) compared to patients without sarcopenia and osteoporosis. Similarly, Honda et al. established that sarcopenia patients were at lower risk of secondary displacement than patients without sarcopenia, which they attested to larger muscle mass causing displacement [ 25 ].
While there was no significant difference when either osteoporosis or sarcopenia was present, the rate of complications increased significantly in patients with both conditions.
Additionally, patients with both conditions had significantly longer hospital stays, even though the rate of surgery was significantly lower. This could be due to the significantly higher complication rate in this study cohort, leading to longer hospital stays. While there was no difference in pre-hospital living conditions, patients were discharged much more often to high grade facilities such as specialized nursing homes. This can also lead to longer hospitals stays due to socioeconomic factors, as there is a high demand on nursing home placements, prolonging hospital stays due to the lack of available nursing home spots. Steihaug et al. also found similar results after osteoporotic hip fractures [ 35 ].
This study has several limitations. First of all, this is a retrospective study, which comes with the usual limitations such as missing or inaccurate data and the possibility of a selection bias due to chosen inclusion criteria. Furthermore, there is no follow-up to evaluate long-term outcome after FFPs, however this is also under consideration for future studies. Additionally, we used cut-off values based on a predominantly Asian population [ 14 ]. Yet similar studies defining cut-off values for PMA and PMI have found very similar values [ 28 , 29 ].
This study shows that sarcopenia and osteoporosis together are highly predictive of lower outcome after FFPs and are associated with significantly higher mortality, higher complication rates, prolonged length of stay and poorer functional recovery. More prospective clinical studies are needed in order to provide uniform guidelines to define sarcopenia as treatment of the geriatric population will become even more demanding in the future. Additionally, prevention of sarcopenia and osteoporosis is key to keep the socioeconomic burden low and to enhance specialized care for the older population.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Association of Anesthesiologists
Bioelectrical impedance analysis
Bone mineral density
Computed tomography
European Working Group on Sarcopenia in Older People
- Fragility fractures of the pelvis
Hounsefield units
- Psoas muscle area
- Psoas muscle index
Skeletal muscle area
Skeletal muscle index
Organization WH. Guidelines for preclinical evaluation and clinical trials in osteoporosis. World Health Organization; 1998. https://iris.who.int/handle/10665/42088 .
Andrich S, Haastert B, Neuhaus E, Neidert K, Arend W, Ohmann C, Grebe J, Vogt A, Jungbluth P, Rösler G, Windolf J, Icks A. Epidemiology of pelvic fractures in Germany: considerably high incidence rates among older people. PLoS One. 2015;10(9):e0139078. https://doi.org/10.1371/journal.pone.0139078 .
Article CAS PubMed PubMed Central Google Scholar
Kannus P, Parkkari J, Niemi S, Sievänen H. Low-Trauma Pelvic Fractures in Elderly Finns in 1970–2013. Calcif Tissue Int. 2015;97(6):577–80. https://doi.org/10.1007/s00223-015-0056-8 .
Article CAS PubMed Google Scholar
Nanninga GL, de Leur K, Panneman MJ, van der Elst M, Hartholt KA. Increasing rates of pelvic fractures among older adults: The Netherlands, 1986–2011. Age Ageing. 2014;43(5):648–53. https://doi.org/10.1093/ageing/aft212.
Article PubMed Google Scholar
Rommens PM, Hofmann A. Comprehensive classification of fragility fractures of the pelvic ring: recommendations for surgical treatment. Injury. 2013;44(12):1733–44. https://doi.org/10.1016/j.injury.2013.06.023 .
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–75. https://doi.org/10.1359/jbmr.061113 .
Rommens PM, Arand C, Hofmann A, Wagner D. When and how to operate fragility fractures of the pelvis? Indian J Orthop. 2019;53(1):128–37. https://doi.org/10.4103/ortho.IJOrtho_631_17 .
Article PubMed PubMed Central Google Scholar
Chen H, Ma J, Liu A, Cui Y, Ma X. The association between sarcopenia and fracture in middle-aged and elderly people: a systematic review and meta-analysis of cohort studies. Injury. 2020;51(4):804–11. https://doi.org/10.1016/j.injury.2020.02.072 .
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. https://doi.org/10.1093/ageing/afy169 .
Hanaoka M, Yasuno M, Ishiguro M, Yamauchi S, Kikuchi A, Tokura M, Ishikawa T, Nakatani E, Uetake H. Morphologic change of the psoas muscle as a surrogate marker of sarcopenia and predictor of complications after colorectal cancer surgery. Int J Colorectal Dis. 2017;32(6):847–56. https://doi.org/10.1007/s00384-017-2773-0 .
Uehara H, Yamazaki T, Iwaya A, Kameyama H, Utsumi S, Harada R, Komatsu M, Hirai M, Kubota A, Katada T, Kobayashi K, Sato D, Yokoyama N, Kuwabara S, Otani T. s radiological psoas muscle area measurement a predictor of postoperative complications after rectal resection for rectal cancer? A retrospective study. Surg Today. 2022;52(2):306–15. https://doi.org/10.1007/s00595-021-02346-x .
Herrod PJJ, Boyd-Carson H, Doleman B, Trotter J, Schlichtemeier S, Sathanapally G, Somerville J, Williams JP, Lund JN. Quick and simple; psoas density measurement is an independent predictor of anastomotic leak and other complications after colorectal resection. Tech Coloproctol. 2019;23(2):129–34. https://doi.org/10.1007/s10151-019-1928-0 .
Weijs PJ, Looijaard WG, Dekker IM, Stapel SN, Girbes AR, Oudemans-van Straaten HM, Beishuizen A. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care. 2014;18(2):R12. https://doi.org/10.1186/cc13189 .
Fu H, Li P, Xing Q, Jiang H, Sui H. Cutoff value of psoas muscle area as reduced muscle mass and its association with acute pancreatitis in China. Int J Gen Med. 2023;16:2733–51. https://doi.org/10.2147/ijgm.S413308 .
Yamada R, Todo Y, Kurosu H, Minowa K, Tsuruta T, Minobe S, Matsumiya H, Kato H, Mori Y, Osanai T. Validity of measuring psoas muscle mass index for assessing sarcopenia in patients with gynecological cancer. Jpn J Clin Oncol. 2021;51(3):393–9. https://doi.org/10.1093/jjco/hyaa218 .
van Dijk DP, Bakens MJ, Coolsen MM, Rensen SS, van Dam RM, Bours MJ, Weijenberg MP, Dejong CH, Olde Damink SW. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8(2):317–26. https://doi.org/10.1002/jcsm.12155 .
Yang G, Wang H, Wu Z, Shi Y, Zhao Y. Prediction of osteoporosis and osteopenia by routine computed tomography of the lumbar spine in different regions of interest. J Orthop Surg Res. 2022;17(1):454. https://doi.org/10.1186/s13018-022-03348-2 .
Vadera S, Osborne T, Shah V, Stephenson JA. Opportunistic screening for osteoporosis by abdominal CT in a British population. Insights Imaging. 2023;14(1):57. https://doi.org/10.1186/s13244-023-01400-1 .
Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158(8):588–95. https://doi.org/10.7326/0003-4819-158-8-201304160-00003 .
Kim KJ, Kim DH, Lee JI, Choi BK, Han IH, Nam KH. Hounsfield units on lumbar computed tomography for predicting regional bone mineral density. Open Med (Wars). 2019;14:545–51. https://doi.org/10.1515/med-2019-0061 .
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. https://doi.org/10.1097/01.sla.0000133083.54934.ae .
Kong M, Geng N, Zhou Y, Lin N, Song W, Xu M, Li S, Piao Y, Han Z, Guo R, Yang C, Luo N, Wang Z, Jiang M, Wang L, Qiu W, Li J, Shi D, Li R, Cheung EC, Chen Y, Duan Z. Defining reference values for low skeletal muscle index at the L3 vertebra level based on computed tomography in healthy adults: A multicentre study. Clin Nutr. 2022;41(2):396–404. https://doi.org/10.1016/j.clnu.2021.12.003 .
Dieckmeyer M, Löffler MT, El Husseini M, Sekuboyina A, Menze B, Sollmann N, Wostrack M, Zimmer C, Baum T, Kirschke JS. Level-specific volumetric BMD threshold values for the prediction of incident vertebral fractures using opportunistic QCT: a case-control study. Front Endocrinol (Lausanne). 2022;13:882163. https://doi.org/10.3389/fendo.2022.882163 .
Radiology ACo. ACR–SPR–SSR practice parameter for the performance of musculoskeletal quantitative computed tomography (QCT). American College of Radiology, Reston. 2018. Available via https://www.acr.org/-/media/ACR/Files/Practice-Parameters/QCT.pdf .
Honda S, Ota S, Yamashita S, Yasuda T. Inverse association between sarcopenia and displacement in the early phase of fragility fractures of the pelvis. Osteoporos Sarcopenia. 2022;8(1):24–9. https://doi.org/10.1016/j.afos.2022.03.002 .
Audretsch CK, Siegemund A, Ellmerer A, Herath SC. Sex Differences in Pelvic Fractures-a Retrospective Analysis of 16 359 Cases From the German Trauma Registry. Dtsch Arztebl Int. 2023;120(13):221–2. https://doi.org/10.3238/arztebl.m2022.0402 .
Aubrey J, Esfandiari N, Baracos VE, Buteau FA, Frenette J, Putman CT, Mazurak VC. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf). 2014;210(3):489–97. https://doi.org/10.1111/apha.12224 .
Derstine BA, Holcombe SA, Goulson RL, Ross BE, Wang NC, Sullivan JA, Su GL, Wang SC. Quantifying sarcopenia reference values using lumbar and thoracic muscle areas in a healthy population. J Nutr Health Aging. 2017;21(10):180–5. https://doi.org/10.1007/s12603-017-0983-3 .
Bahat G, Turkmen BO, Aliyev S, Catikkas NM, Bakir B, Karan MA. Cut-off values of skeletal muscle index and psoas muscle index at L3 vertebra level by computerized tomography to assess low muscle mass. Clin Nutr. 2021;40(6):4360–5. https://doi.org/10.1016/j.clnu.2021.01.010 .
Pigneur F, Di Palma M, Raynard B, Guibal A, Cohen F, Daidj N, Aziza R, El Hajjam M, Louis G, Goldwasser F, Deluche E. Psoas muscle index is not representative of skeletal muscle index for evaluating cancer sarcopenia. J Cachexia Sarcopenia Muscle. 2023;14(4):1613–20. https://doi.org/10.1002/jcsm.13230 .
Lim S-K, Beom J, Lee SY, Kim BR, Chun S-W, Lim J-Y, Shin Lee E. Association between sarcopenia and fall characteristics in older adults with fragility hip fracture. Injury. 2020;51(11):2640–7. https://doi.org/10.1016/j.injury.2020.08.031 .
Yoo J-I, Ha Y-C, Kwon H-B, Lee Y-K, Koo K-H, Yoo M-J. High Prevalence of Sarcopenia in Korean Patients after Hip Fracture: a Case-Control Study. JKMS 2016;31(9):1479–1484. https://doi.org/10.3346/jkms.2016.31.9.1479 .
Wagner D, DeMarco MM, Amini N, Buttner S, Segev D, Gani F, Pawlik TM. Role of frailty and sarcopenia in predicting outcomes among patients undergoing gastrointestinal surgery. World J Gastrointest Surg. 2016;8(1):27–40. https://doi.org/10.4240/wjgs.v8.i1.27 .
Miller PD. Underdiagnoses and Undertreatment of Osteoporosis: The Battle to Be Won. J Clin Endocrinol Metab. 2016;101(3):852–9. https://doi.org/10.1210/jc.2015-3156 .
Steihaug OM, Gjesdal CG, Bogen B, Kristoffersen MH, Lien G, Hufthammer KO, Ranhoff AH. Does sarcopenia predict change in mobility after hip fracture? a multicenter observational study with one-year follow-up. BMC Geriatr. 2018;18(1):65. https://doi.org/10.1186/s12877-018-0755-x .
Download references
Acknowledgements
Not applicable
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
School of Medicine and Health, Klinikum Rechts Der Isar, Department of Trauma Surgery, Technical University of Munich, Munich, Germany
Olivia Mair, Philipp Rittstieg, Michael Müller, Peter Biberthaler & Marc Hanschen
School of Medicine and Health, Klinikum Rechts Der Isar, Department of Radiology, Technical University of Munich, Munich, Germany
Jan Neumann
You can also search for this author in PubMed Google Scholar
Contributions
OM: Collection of data, wrote the main Manuscript, prepared figures. JN: Helped with conceptualizing of radiological aspects of this study, figure collection, revised the manuscript. MM: aided with data collection, revised the manuscript. PR: writing and revision of manuscript, helped with preparing figures. PB: revised the article. MH: conceptualized the study idea, has substantially revised the draft. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Olivia Mair .
Ethics declarations
Ethics approval and consent to participate.
Approval and Authorization by the "Ethikkommission der Technischen Universität München" (Ethics Committee of the Technical University of Munich, Germany) was obtained (Study Nr.: 2023–179-S-NP). The ethics committee further waived the need for informed consent as this was a retrospective data and no patient identifying data was included in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Mair, O., Neumann, J., Rittstieg, P. et al. The role of sarcopenia in fragility fractures of the pelvis – is sarcopenia an underestimated risk factor?. BMC Geriatr 24 , 461 (2024). https://doi.org/10.1186/s12877-024-05082-2
Download citation
Received : 12 March 2024
Accepted : 15 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1186/s12877-024-05082-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
BMC Geriatrics
ISSN: 1471-2318
- Submission enquiries: [email protected]
- General enquiries: [email protected]

- May 28, 2024 | Scientists May Have Discovered an Achilles Heel for Hepatitis B
- May 28, 2024 | Challenging Classical Physics: Surprising Properties of Elastic Turbulence Discovered
- May 28, 2024 | Johns Hopkins Scientists Solve 30-Year Biological Mystery of Night Blindness
- May 28, 2024 | Cutting-Edge Phage Research Promises New Solutions for Old Pathogens
- May 28, 2024 | Vanishing Without a Trace: Why Stars Mysteriously Disappear From the Night Sky
Unlocking Longevity – New Research Suggests That Aging Could Be Influenced by Random Changes
By University of Cologne May 20, 2024

Aging clocks, which measure biological age with precision, can deviate from chronological age due to environmental influences like smoking or diet. Researchers at the University of Cologne found that these clocks actually track increasing random cellular changes, suggesting that biological aging could be influenced by stochastic variations in processes like DNA methylation and gene activity.
Aging clocks can accurately determine a person’s biological age, which can differ from their chronological age—the age calculated from their date of birth—due to environmental influences like diet or smoking. The precision of these clocks indicates that the aging process follows a program.
Scientists David Meyer and Professor Dr. Björn Schumacher at CECAD, the Cluster of Excellence Cellular Stress Responses in Aging-Associated Diseases of the University of Cologne , have now discovered that aging clocks actually measure the increase in stochastic changes in cells. The study was recently published published in Nature Aging .
“Aging is triggered when the building blocks in our cells become damaged. Where this damage occurs is for the most part random. Our work combines the accuracy of aging clocks with the accumulation of entirely stochastic changes in our cells,” said Professor Schumacher.
Fewer Checks, More Noise
With increasing age, controlling the processes that occur in our cells becomes less effective, resulting in more stochastic results. This is particularly evident in the accumulation of stochastic changes in DNA methylation. Methylation refers to the chemical changes that affect DNA, the genome’s building blocks. These methylation processes are strictly regulated within the body. However, during the course of one’s life, random changes occur in the methylation patterns. The accumulation of variation is a highly accurate indicator of a person’s age.
The loss of control over the cells and the increase in stochastic variation are not restricted to DNA methylation. Meyer and Schumacher demonstrate that the increase in stochastic variations also in gene activity can be used as an aging clock. “In principle it would be feasible to take this even further, allowing the stochastic variations in any process in the cell to predict age,” Schumacher said. According to the authors, it is above all crucial to ascertain if such aging clocks can show the success of interventions that slow the aging process or harmful factors that accelerate aging.
Using the available datasets, the scientists showed that smoking increases the random changes in humans and that ‘anti-aging’ interventions such as lower calorie intake in mice reduce the variation in methylation patterns. They also showed that the stochastic noise is even reversible by means of reprogramming body cells to stem cells. The scientists compared human fibroblasts from the skin that were reprogrammed into stem cells and as a result of the reprogramming are rejuvenating. The high variation indicative of the age of the body cells was indeed reversed to the low stochastic noise of young stem cells.
Meyer and Schumacher hope that their findings on the loss of regulation and the accumulating stochastic variations will lead to new interventions that can tackle the root cause of aging and may even lead to cellular rejuvenation. A target for such interventions could be repairing stochastic changes in DNA or improved control of gene expression.
Reference: “Aging clocks based on accumulating stochastic variation” by David H. Meyer, and Björn Schumacher, 9 May 2024, Nature Aging . DOI: 10.1038/s43587-024-00619-x
More on SciTechDaily

Ocean’s Silent Plastic Invasion: Marine Mammals Now Carry Microplastics Within

Catch a Cold, Catch Dementia? The Surprising Connection Unveiled

Bird-Brained AI: Pigeons and Artificial Intelligence Share Surprising Learning Techniques

NASA Prepares for Future Artemis Missions Using Data From the First SLS Flight
Nasa state-of-the-art asteroid tracking system now capable of full sky search.
New COVID-19 Treatment for People With Diabetes Shows Early Promise

Turning Back the Clock: Genetic Engineers Rewire Cells for an 82% Increase in Lifespan

Camouflage Skin Developed That Provides On-Demand Cloaking in Both Daylight and Night
2 comments on "unlocking longevity – new research suggests that aging could be influenced by random changes".
From the article: “Using the available datasets, the scientists showed that smoking increases the random changes in humans and that ‘anti-aging’ interventions such as lower calorie intake in mice reduce the variation in methylation patterns.”
Higher education seems to blind researchers to the “…forest for the trees.” For a start, as a now eighty year old American male who’s been compelled by officially (FDA in the US) approved food poisoning to explore new areas of the ‘forest’ for forty-three years and counting with some successes, I can state with some confidence, again, that the “…available datasets…” have multiple fatal flaws. First, they don’t factor-in a kind of nearly subclinical non-IgE-mediated food (minimally; other) allergy reaction which can be acquired and/or inherited. More specifically, when it comes to ‘smoking,’ a person’s individual allergic reaction to tobacco or some substance in the smoke is probably what makes some smokers ill and others not. Next, they don’t know to factor-in the relationship between repetitious trauma and acquired allergies; a psychosomatic factor. Additionally, the ‘available datasets’ do not factor-in increasing numbers of officially approved toxic food additives, namely added soy since the late 1960s, MSG since 1980 and the increasingly pervasive cooking oil preservative TBHQ since 1972; others. Finally, for now, they don’t yet factor-in allergy related acidic blood and/or possible nutritional deficiencies like calcium (unreliable serum testing), especially in mothers due to the calcium demands of the fetus, and they certainly don’t factor-in how it is probably where the acidic flow of blood is the greatest that the greatest immediate physical harm and ‘DNA methylation’ occur.
Cell rejuvenation aside, as long as the primary goal of researchers is to find ‘new interventions’ instead of identifying and eliminating ‘root causes,’ the ‘forest’ is likely to remain invisibly hidden behind huge trees of deceit, medical errors, ignorance and fatally flawed so-called “evidence-based” datasets.
I think this is too generalizing in research , we have to taking in the baskets of geography Culture, local food production, genders and climate into consideration.
Leave a comment Cancel reply
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
MIT Technology Review
- Newsletters
That viral video showing a head transplant is a fake. But it might be real someday.
BrainBridge is best understood as the first public billboard for a hugely controversial scheme to defeat death.
- Antonio Regalado archive page
An animated video posted this week has a voice-over that sounds like a late-night TV ad, but the pitch is straight out of the far future. The arms of an octopus-like robotic surgeon swirl, swiftly removing the head of a dying man and placing it onto a young, healthy body.
This is BrainBridge, the animated video claims—“the world’s first revolutionary concept for a head transplant machine, which uses state-of-the-art robotics and artificial intelligence to conduct complete head and face transplantation.”
First posted on Tuesday, the video has millions of views, more than 24,000 comments on Facebook, and a content warning on TikTok for its grisly depictions of severed heads. A slick BrainBridge website has several job postings, including one for a “neuroscience team leader” and another for a “government relations adviser.” It is all convincing enough for the New York Post to announce that BrainBridge is “a biomedical engineering startup” and that “the company” plans a surgery within eight years.
We can report that BrainBridge is not a real company—it’s not incorporated anywhere. The video was made by Hashem Al-Ghaili, a Yemeni science communicator and film director who in 2022 made a viral video called “EctoLife,” about artificial wombs, that also left journalists scrambling to determine if it was real or not .
Yet BrainBridge is not merely a provocative work of art. This video is better understood as a public billboard for a hugely controversial scheme to defeat death that’s recently been gaining attention among some life-extension proponents and entrepreneurs.
“It’s about recruiting newcomers to join the project,” says Al-Ghaili.
This morning, Al-Ghaili, who lives in Dubai, was up at 5 a.m., tracking the video as its viewership ballooned around social media. “I am monitoring its progress,” he says, but he insists he didn’t make the film for clicks: “Being viral is not the goal. I can be viral anytime. It’s pushing boundaries and testing feasibility.”
The video project was bankrolled in part by Alex Zhavoronkov, the founder of Insilico Medicine, a large AI drug discovery company , who is also a prominent figure in anti-aging research. After Zhavoronkov posted the video on his LinkedIn account, commenters noticed that it is his face on the two bodies shown in the video.
“I can confirm I helped design and fund a few things,” Zhavoronkov told MIT Technology Review in a WhatsApp message, in which he also claimed that “some important and famous people are supporting [it] financially.”
Zhavoronkov declined to name these individuals. He also didn’t respond when asked if the job ads—whose cookie-cutter descriptions of qualifications and responsibilities appear to have been written by an AI—are real roles or make-believe positions.
Aging bypass
What is certain is that head transplantation—or body transplant, as some prefer to call it—is a subject of growing, if speculative, interest in longevity circles, the kind inhabited by biohackers, techno-anarchists, and others on the fringes of biotechnology and the startup scene and who form the most dedicated cadre of extreme life-extensionists.
Many proponents of longer life spans will admit things don’t look good. Anti-aging medicine so far hasn’t achieved any breakthroughs. In fact, as research advances into the molecular details, the problem of death only looks more and more complicated. As we age, our billions of cells gradually succumb to the irreversible effects of entropy. Fixing that may never be possible.
By comparison, putting your head on a young body looks comparatively easy—a way to bypass aging in a single stroke, at least as long as your brain holds out. The idea was strongly endorsed in a technical road map put forward this year by the Longevity Biotech Fellowship , a group espousing radical life extension, which rated “body replacement” as the cheapest, fastest pathway to “solve aging.”
Will head transplants work? In a crude way, they already have. In the early 1970s, the American neurosurgeon Robert White performed a “cephalic exchange,” cutting off the head of a monkey, placing it on the body of another, and sewing together their circulatory systems. Reports suggest the head remained conscious, and able to see, for a few days before it died.
Most likely, a human head transplant would also be fatal. But even if you lived, you’d be a mind atop a paralyzed body, since exchanging heads means severing the spinal cord.
Yet head-swapping proponents can point to plausible solutions for that, too—a number of which appear in the BrainBridge video. In Europe, for instance, some paralyzed people have walked again after doctors bridged their spinal injuries with electronics. Other scientists in China are studying growth factors to regrow nerves.
Joined at the neck
As shocking as the video is, BrainBridge is in some ways overly conventional in its thinking. If you want to keep your brain going, why must it be on a human body? You might instead keep the head alive on a heart-lung machine—with an Elon Musk neural implant to let it surf the internet, for as long as it lives. Or consider how doctors hoping to solve the organ shortage have started putting hearts and kidneys from genetically engineered pigs into patients. If you don’t mind having a tail and four legs, maybe your head could be placed onto a pig’s body.
Let’s take it a step further. Why does the body “donor” have to be dead at all? Anatomically, it’s possible to have two heads. There are conjoined twins who share one body. If your spouse were diagnosed with a fatal cancer, you would surely welcome his or her head next to yours, if it allowed their mind to live on. After all, the concept of a "living donor" is widely accepted in transplant medicine already, and married couples are often said to be joined at the hip. Why not at the neck, too?
If the video is an attempt to take the public’s temperature and gauge reactions, it’s been successful. Since it was posted, thousands of commenters have explored the moral dilemmas posed by the procedure. For instance, if someone is left brain dead—say, in a motorcycle accident—surgeons can use their heart, liver, and kidneys to save multiple other people. Would it be ethical to use a body to help only one person?
“The most common question is ‘Where do you get the bodies from?’” says Al-Ghaili. The BrainBridge website answers this question by stating it will source “ethically grown” unconscious bodies from EctoLife, the artificial womb company that is Al-Ghaili’s previous fiction. He also suggests that people undergoing euthanasia because of chronic pain, or even psychiatric problems, could provide an additional supply.
For the most part, the public seems to hate the idea. On Facebook, a pastor, Matthew. W. Tucker, called the concept “disgusting, immoral, unnecessary, pagan, demonic and outright idiotic,” adding that “they have no idea what they are doing.” A poster from the Middle East apologized for the video, joking that its creator “is one of our psychiatric patients who escaped last night.” “We urge the public to go about [their] business as everything is under control,” this person said.
Al-Ghaili is monitoring the feedback with interest and some concern. “The negativity is huge, to be honest,” he says. “But behind that are the ones who are sending emails. These are people who want to invest, or who are expressing their personal health challenges. These are the ones who matter.”
Biotechnology and health
Google helped make an exquisitely detailed map of a tiny piece of the human brain.
A small brain sample was sliced into 5,000 pieces, and machine learning helped stitch it back together.
- Cassandra Willyard archive page
The effort to make a breakthrough cancer therapy cheaper
CAR-T cells could revolutionize the treatment of a wide variety of diseases, if only we can make them cheaper.
Beyond Neuralink: Meet the other companies developing brain-computer interfaces
Companies like Synchron, Paradromics, and Precision Neuroscience are also racing to develop brain implants
Cancer vaccines are having a renaissance
After years of lackluster results, cancer vaccines seem poised for success. Finally.
Stay connected
Get the latest updates from mit technology review.
Discover special offers, top stories, upcoming events, and more.
Thank you for submitting your email!
It looks like something went wrong.
We’re having trouble saving your preferences. Try refreshing this page and updating them one more time. If you continue to get this message, reach out to us at [email protected] with a list of newsletters you’d like to receive.
Shannex donates $3.5M to N.S., N.B universities for healthy aging research
Dalhousie, university of new brunswick and mount saint vincent are the benefactors of the donation.

Social Sharing
A family-owned Nova Scotia business that operates seniors' facilities is donating $3.5 million to three universities in Nova Scotia and New Brunswick.
The money is going to Dalhousie University ($2 million) and Mount Saint Vincent University ($500,000) in Halifax, and the University of New Brunswick ($1 million).
"The population that we serve today will easily more than double in the next fifteen years," said Shannex president Jason Shannon. "This is why we are investing this money — to support research on healthy aging."
The aim is to use artificial intelligence and other technologies to help decision making and policy planning. Other goals are to improve food and nutrition for seniors and to find strategies to improve wellness and quality of work life for long-term care staff.
"We are grateful for the support of Shannex to help us find solutions for one of the most vital areas of health, improving the lives and well-being of older adults," said Kim Brooks, president and vice-chancellor of Dalhousie University.

The donation to Dalhousie will create the Shannex Research Chair in Artificial Intelligence and Healthy Aging.
The chair will be jointly appointed to the university's health and computer science faculties. They will develop models to enhance resident safety and use data to improve the well-being of seniors.
"This is probably one of the single most important projects that's going to happen in this province for people my age," said Shannex founder Joe Shannon, who purchased a nursing home in 1988 in his hometown of Sydney, N.S.
Shannex now operates more than 25 facilities in Nova Scotia, as well as facilities in New Brunswick and Ontario.
- Lobster prices fall as crates of crustaceans pile up in Cape Breton harbours
- Downward spiral for Atlantic cod continues in Gulf of St. Lawrence
- N.S. is pursuing more family doctors, but not all want to stick to primary care
- Wildfires destroyed their homes. They've had to rebuild a day at a time
- Halifax needs better emergency management strategy instead of 'ad hoc' policies: report
ABOUT THE AUTHOR

Paul Palmeter is an award-winning video journalist born and raised in the Annapolis Valley. He has covered news and sports stories across Nova Scotia for 30 years.
Related Stories
- Pro-Palestinian encampment set up at Dalhousie University in Halifax
- Suicide is a leading cause of death for young people, but most universities don't track it
- McGill University seeks injunction against pro-Palestinian encampment
- New N.S. tourism company that would highlight Black cultural sites faces pushback
- Volunteers pack healthy food for kids in need this summer
CBC Nova Scotia
Add some “good” to your morning and evening.
Get the latest top stories from across Nova Scotia in your inbox every weekday.
This site is protected by reCAPTCHA and the Google Privacy Policy and Google Terms of Service apply.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Ageing articles from across Nature Portfolio
Ageing is the process during which structural and functional changes accumulate in an organism as a result of the passage of time. The changes manifest as a decline from the organism’s peak fertility and physiological functions until death.
Quantifying stochasticity in the aging DNA methylome
Aging-related DNA methylation changes are numerous. Their precise measurement has opened new avenues to explore aging-related disease pathology, including the construction of chronological and biological age predictors (termed DNA methylation ‘clocks’). Three studies investigate the substantial stochastic contribution to these epigenetic changes and further our understanding of aging biology, as well as of these predictors.
- Christopher G. Bell
Latest Research and Reviews
Cell-type-specific consequences of mosaic structural variants in hematopoietic stem and progenitor cells
This study uses Strand-seq to explore the landscape of mosaic structural variants (mSVs) in human hematopoietic stem and progenitor cells from people of different ages. The analysis highlights patterns of enrichment for mSVs in specific cell types, with associated phenotypes, and suggests that clonal expansions due to mSVs are generally restricted to older individuals.
- Karen Grimes
- Hyobin Jeong
- Jan O. Korbel
Validation of human telomere length multi-ancestry meta-analysis association signals identifies POP5 and KBTBD6 as human telomere length regulation genes
Here the authors conduct a multi-ancestry meta-analysis of telomere length, used diverse approaches to identify genes underlying association signals, and experimentally validated POP5 and KBTBD6 as regulators of telomere length in human cells.
- Rebecca Keener
- Surya B. Chhetri
- Alexis Battle
Adipose tissue as a linchpin of organismal ageing
Nguyen and Corvera review distinct changes that occur in adipose tissue during ageing, discuss potential mechanisms by which these changes impact whole-body metabolism, immunity and longevity, and highlight therapeutic opportunities.
- Tammy T. Nguyen
- Silvia Corvera
An improved epigenetic counter to track mitotic age in normal and precancerous tissues
DNA methylation (DNAm) clocks can track mitotic age, but their potential use for cancer risk prediction remains less explored. Here, the authors develop a DNAm counter of total mitotic age (stemTOC) that shows an increase of mitotic age in normal tissues and precancerous lesions.
- Andrew E. Teschendorff
DDX5 inhibits hyaline cartilage fibrosis and degradation in osteoarthritis via alternative splicing and G-quadruplex unwinding
Liu et al. identify downregulation of DDX5, an RNA helicase, in the cartilage of patients and mouse model of osteoarthritis. Targeted upregulation of DDX5 in mouse chondrocytes inhibits hyaline cartilage fibrosis and degradation via pre-mRNA splicing and G4 unwinding, a potential therapeutic strategy against osteoarthritis.
- Qianqian Liu
- Mingrui Han
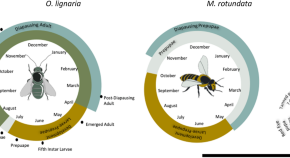
Telomere length is longer following diapause in two solitary bee species
- Courtney C. Grula
- Joshua D. Rinehart
- Julia H. Bowsher
News and Comment
Don’t leave out joints and bones in exercise studies.
- Francis Berenbaum
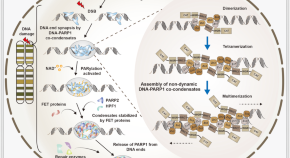
PARP1-DNA co-condensation: the driver of broken DNA repair
- Fangfang Zhou
How to kill the ‘zombie’ cells that make you age
Researchers are using new molecules, engineered immune cells and gene therapy to kill senescent cells and treat age-related diseases.
- Carissa Wong
Defining responsible use of AI chatbots in social care for older adults
Artificial intelligence (AI) chatbots offer the potential to enhance many aspects of social care for older adults, but also pose ethical risks. This Comment explores the responsible use of AI chatbots, which recognizes the distinct features of social care provision.
- Caroline Emmer De Albuquerque Green
Rebalancing the aged immune system
- Hannah Walters
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
Ageing Research Reviews is a peer-reviewed journal that publishes reviews on various aspects of ageing and age-related diseases. The journal covers topics such as genetics, biochemistry, behaviour, nutrition, exercise, and interventions for ageing and age-related conditions.
Browse the past and current volumes of Ageing Research Reviews, a peer-reviewed journal that covers all aspects of ageing research. Find articles on biology, medicine, psychology, sociology, and more related to ageing.
Ageing Research Reviews publishes critical reviews and viewpoints on mechanisms of ageing and age-related disease. Learn about the journal's scope, types of articles, submission guidelines, and ethical policies.
This Review summarizes current research on cellular senescence including its molecular basis and examines how drugs may be targeted against senescent cells to treat age-related multimorbidities ...
Most of the studies were qualitative or cross sectional, and a majority were carried out in Asia, followed by North America, Australia, and Africa. The ten determinants identified for healthy ageing include physical activity, diet, self-awareness, outlook/attitude, life-long learning, faith, social support, financial security, community ...
Fig. 1: Timeline of ageing research. Key discoveries in the ageing field are highlighted, starting with the discovery of the effect of calorie restriction on ageing in 1930. Full size image ...
Ageing Research Reviews. Published by Elsevier BV. Print ISSN: 1568-1637. Articles. Commentary to the recently published review "Drug pipeline in neurodegeneration based on transgenic mice ...
Severe infections as a gateway to dementia. A study in Nature Aging on electronic health records from 1.7 million people in New Zealand reveals that most patients with dementia have a history of ...
Affiliations 1 Laboratory of Epidemiology and Population Science, National Institute on Aging, National Institutes of Health, NIH Biomedical Research Center, 251 Bayview Boulevard, Baltimore, MD 21224, USA. Electronic address: [email protected]. 2 Translational Gerontology Branch, National Institute on Aging, National Institutes of Health, NIH Biomedical Research Center, 251 Bayview ...
Ageing Research Reviews (ARR) is a journal that covers the trends and advances in the multidisciplinary field of ageing research. It publishes critical reviews and viewpoints on mechanisms of ageing and age-related disease, as well as applications of basic ageing research to lifespan extension and disease prevention.
Research on Aging (ROA), peer-reviewed and published eight times a year, is an interdisciplinary journal designed to reflect the expanding role of research in the field of social gerontology. For over four decades, scholars, researchers and professionals like yourself have turned to ROA for the latest analyses on the critical issues facing today's elderly population.
Abstract. Ageing is arguably the most complex phenotype that occurs in humans. To understand and treat ageing as well as associated diseases, highly specialised technologies are emerging that reveal critical insight into the underlying mechanisms and provide new hope for previously untreated diseases. Herein, we describe the latest developments ...
Ageing Research Reviews is a peer-reviewed journal that publishes critical reviews and viewpoints on mechanisms of ageing and age-related disease. It covers topics such as telomerase, stem cells, caloric restriction, and lifespan extension, and ranks among the top journals in biochemistry, biotechnology, molecular biology, and neurology.
Prevalence and health outcomes of polypharmacy and hyperpolypharmacy in older adults with frailty: A systematic review and meta-analysis. Janice Jia Yun Toh, Hui Zhang, Yang Yue Soh, Zeyu Zhang, Xi Vivien Wu. Article 101811. View PDF.
Ageing Research Reviews is a peer-reviewed scientific journal publishing review articles covering research on ageing, aging-associated diseases, and human life expectancy. The editor-in-chief is Claudio Franceschi (University of Bologna). Abstracting and indexing. The journal is abstracted and indexed in: BIOSIS; Chemical Abstracts
Uncategorized. The New Age of Aging Research. By: Eric Sun. Aging. To some, this word symbolizes equality, wisdom, and progress; to others, this word represents weakness, disease, and death. To me, aging has taken on a mixed meaning. When I was a small child, I remember lying awake in bed and counting my heartbeats as if the thumping in my ...
Aging is a complex and multifaceted process involving a variety of interrelated molecular mechanisms and cellular systems. Phenotypically, the biological aging process is accompanied by a gradual loss of cellular function and the systemic deterioration of multiple tissues, resulting in susceptibility to aging-related diseases. Emerging evidence suggests that aging is closely associated with ...
Aging is a gradual and irreversible pathophysiological process. It presents with declines in tissue and cell functions and significant increases in the risks of various aging-related diseases ...
Aging is primarily due to accumulated chemical damage to molecules and cells. Research on rapamycin and cellular reprogramming offers potential for extending lifespan. The anti-aging industry is booming, but caution is needed due to potential conflicts of interest and exaggerated claims. Source: Harvard. Mayflies live for only a day.
Aging and regeneration represent complex biological phenomena that have long captivated the scientific community. To fully comprehend these processes, it is essential to investigate molecular dynamics through a lens that encompasses both spatial and temporal dimensions. Conventional omics methodologies, such as genomics and transcriptomics, have been instrumental in identifying critical ...
A new systematic review and meta-analysis published in Ageing Research Reviews examined the relationship between adult-onset hearing loss and cognitive impairment. Analyzing data from fifty cohort studies involving over 1.5 million participants, the study suggests that hearing loss significantly increases the risk of cognitive decline, dementia, and Alzheimer's disease (AD).
Cortical excitability and plasticity in Alzheimer's disease and mild cognitive impairment: A systematic review and meta-analysis of transcranial magnetic stimulation studies. Ying-hui Chou, Mark Sundman, Viet Ton That, Jacob Green, Chrisopher Trapani.
Aging Clinical and Experimental Research (ACER) is dedicated to advancing knowledge and understanding in the field of aging research, encompassing a broad spectrum of topics relevant to the health and well-being of older adults. In alignment with the journal's scope, the topic of "Climate Change and Health in Aging Populations" presents a crucial area of investigation at the intersection of ...
Scholars have studied the construction of smart home care services since the 1980s, and there are some related studies at this stage. According to the literature search results of WOS of "smart home" and "elderly care" related topics shown in Figure 1, it can be found that the main research directions are sensors, ambient-assisted living (AAL), and health care.
Fragility fractures of the pelvis (FFPs) represent a significant health burden, particularly for the elderly. The role of sarcopenia, an age-related loss of muscle mass and function, in the development and impact of these fractures is not well understood. This study aims to investigate the prevalence and impact of osteoporosis and sarcopenia in patients presenting with FFPs.
Aging clocks can accurately determine a person's biological age, which can differ from their chronological age—the age calculated from their date of birth—due to environmental influences like diet or smoking. The precision of these clocks indicates that the aging process follows a program. ... New Research Suggests That Aging Could Be ...
A recent review in the journal Neuroscience & Biobehavioral Reviews presents a multidimensional model of loneliness, exploring its impact on social interactions, the oxytocin system, and illness.
Anti-aging medicine so far hasn't achieved any breakthroughs. In fact, as research advances into the molecular details, the problem of death only looks more and more complicated.
The donation to Dalhousie will create the Shannex Research Chair in Artificial Intelligence and Healthy Aging. The chair will be jointly appointed to the university's health and computer science ...
Ageing is the process during which structural and functional changes accumulate in an organism as a result of the passage of time. The changes manifest as a decline from the organism's peak ...