- Search by keyword
- Search by citation
Page 1 of 164

Forest cover percentage drives the peak biting time of Nyssorhynchus darlingi (Diptera: Culicidae) in the Brazilian Amazon
Deforestation is an important driver of malaria dynamics, with a relevant impact on mosquito ecology, including larval habitat availability, blood-feeding behaviour, and peak biting time. The latter is one of ...
- View Full Text
Insecticide susceptibility status of Anopheles albimanus populations in historical malaria foci in Quintana Roo, Mexico
Mexico has experienced a significant reduction in malaria cases over the past two decades. Certification of localities as malaria-free areas (MFAs) has been proposed as a steppingstone before elimination is ac...
Identifying suitable methods for evaluating the sterilizing effects of pyriproxyfen on adult malaria vectors: a comparison of the oviposition and ovary dissection methods
Nets containing pyriproxyfen, an insect growth regulator that sterilizes adult mosquitoes, have become available for malaria control. Suitable methods for investigating vector susceptibility to pyriproxyfen an...
Evaluation of naturally acquired immune responses against novel pre-erythrocytic Plasmodium vivax proteins in a low endemic malaria population located in the Peruvian Amazon Basin
Plasmodium vivax represents the most geographically widespread human malaria parasite affecting civilian and military populations in endemic areas. Targeting the pre-erythrocytic (PE) stage of the parasite life c...
Towards malaria elimination: a reflection about digital notification modules to improve malaria cases notification speed and follow-up in the Brazilian Amazon region
Health information systems (HIS) are a pivotal element in epidemiological surveillance. In Brazil, malaria persists as a public health challenge, with 99% of its occurrences concentrated in the Amazon region, ...
The behaviour of adult Anopheles gambiae , sub-Saharan Africa’s principal malaria vector, and its relevance to malaria control: a review
Mosquitoes of the Anopheles gambiae complex are one of the major vectors of malaria in sub-Saharan Africa. Their ability to transmit this disease of major public health importance is dependent on their abundance,...
Can neonicotinoid and pyrrole insecticides manage malaria vector resistance in high pyrethroid resistance areas in Côte d'Ivoire?
Anopheles mosquito resistance to insecticide remains a serious threat to malaria vector control affecting several sub-Sahara African countries, including Côte d’Ivoire, where high pyrethroid, carbamate and organo...
Considerations for first field trials of low-threshold gene drive for malaria vector control
Sustainable reductions in African malaria transmission require innovative tools for mosquito control. One proposal involves the use of low-threshold gene drive in Anopheles vector species, where a ‘causal pathway...
Population pharmacokinetics of primaquine and its metabolites in African males
Primaquine (PQ) is the prototype 8-aminoquinoline drug, a class which targets gametocytes and hypnozoites. The World Health Organization (WHO) recommends adding a single low dose of primaquine to the standard ...
Assessment of environmental and spatial factors influencing the establishment of Anopheles gambiae larval habitats in the malaria endemic province of Woleu-Ntem, northern Gabon
This study aimed to assess the spatial distribution of Anopheles mosquito larval habitats and the environmental factors associated with them, as a prerequisite for the implementation of larviciding.
Field testing of user-friendly perennial malaria chemoprevention packaging in Benin, Côte d’Ivoire and Mozambique
Perennial malaria chemoprevention (PMC) aims to protect children at risk from severe malaria by the administration of anti-malarial drugs to children of defined ages throughout the year. Sulfadoxine-pyrimetham...
Exploring existing malaria services and the feasibility of implementing community engagement approaches amongst conflict-affected communities in Cameroon: a qualitative study
Cameroon is one of the countries with the highest burden of malaria. Since 2018, there has been an ongoing conflict in the country, which has reduced access to healthcare for populations in affected regions, a...
IgG and IgM responses to the Plasmodium falciparum asexual stage antigens reflect respectively protection against malaria during pregnancy and infanthood
Plasmodium falciparum malaria is a public health issue mostly seen in tropical countries. Until now, there is no effective malaria vaccine against antigens specific to the blood-stage of P. falciparum infection. ...
Characteristics of the Western Province, Zambia, trial site for evaluation of attractive targeted sugar baits for malaria vector control
The attractive targeted sugar bait (ATSB) is a novel malaria vector control tool designed to attract and kill mosquitoes using a sugar-based bait, laced with oral toxicant. Western Province, Zambia, was one of...
Ten-year trend analysis of malaria prevalence in Gindabarat district, West Shawa Zone, Oromia Regional State, Western Ethiopia
Malaria is a major public health concern in Ethiopia, where more than half of the population lives in malaria risk areas. While several studies have been conducted in different eco-epidemiological settings in ...
A screen for Plasmodium falciparum sporozoite surface protein binding to human hepatocyte surface receptors identifies novel host–pathogen interactions
Sporozoite invasion of hepatocytes is an essential step in the Plasmodium life-cycle and has similarities, at the cellular level, to merozoite invasion of erythrocytes. In the case of the Plasmodium blood-stage, ...
Expansion of artemisinin partial resistance mutations and lack of histidine rich protein-2 and -3 deletions in Plasmodium falciparum infections from Rukara, Rwanda
Emerging artemisinin partial resistance and diagnostic resistance are a threat to malaria control in Africa. Plasmodium falciparum kelch13 ( k13 ) propeller-domain mutations that confer artemisinin partial resistan...
Evidence of Plasmodium vivax circulation in western and eastern regions of Senegal: implications for malaria control
Malaria elimination in Senegal requires accurate diagnosis of all Plasmodium species. Plasmodium falciparum is the most prevalent species in Senegal, although Plasmodium malariae , Plasmodium ovale, and recently P...
Efficacy of Pirikool® 300 CS used for indoor residual spraying on three different substrates in semi-field experimental conditions
Vector control using insecticides is a key prevention strategy against malaria. Unfortunately, insecticide resistance in mosquitoes threatens all progress in malaria control. In the perspective of managing thi...
Cost-effectiveness of village health worker-led integrated community case management (iCCM) versus health facility based management for childhood illnesses in rural southwestern Uganda
In Uganda, village health workers (VHWs) manage childhood illness under the integrated community case management (iCCM) strategy. Care is provided for malaria, pneumonia, and diarrhoea in a community setting. ...
Mixed-method evaluation study of a targeted mass drug administration of long-acting anti-malarials among children aged 3 months to 15 years in the Bossangoa sub-prefecture, Ouham, Central African Republic, during the COVID-19 pandemic
In 2020, during the COVID-19 pandemic, Médecins Sans Frontières (MSF) initiated three cycles of dihydroartemisin-piperaquine (DHA-PQ) mass drug administration (MDA) for children aged three months to 15 years w...
Optimal balance of benefit versus risk for tafenoquine in the treatment of Plasmodium vivax malaria
A single 300 mg dose of tafenoquine (an 8-aminoquinoline), in combination with a standard 3-day course of chloroquine, is approved in several countries for the radical cure (prevention of relapse) of Plasmodium v...
Therapeutic efficacy and tolerability of artemether–lumefantrine for uncomplicated Plasmodium falciparum malaria in Niger, 2020
Monitoring therapeutic efficacy is important to ensure the efficacy of artemisinin-based combination therapy (ACT) for malaria. The current first-line treatment for uncomplicated malaria recommended by the Nat...
Prevalence of malaria and associated risk factors among household members in South Ethiopia: a multi-site cross-sectional study
Despite continuous prevention and control strategies in place, malaria remains a major public health problem in sub-Saharan Africa including Ethiopia. Moreover, prevalence of malaria differs in different geogr...
Hesitancy towards R21/Matrix-M malaria vaccine among Ghanaian parents and attitudes towards immunizing non-eligible children: a cross-sectional survey
The newly developed malaria vaccine called “R21/Matrix-M malaria vaccine” showed a high safety and efficacy level, and Ghana is the first country to approve this new vaccine. The present study aimed to evaluat...
Phytochemical evaluation of Ziziphus mucronata and Xysmalobium undulutum towards the discovery and development of anti-malarial drugs
The development of resistance by Plasmodium falciparum is a burdening hazard that continues to undermine the strides made to alleviate malaria. As such, there is an increasing need to find new alternative strateg...
Kinetics of glucose-6-phosphate dehydrogenase (G6PD) activity during Plasmodium vivax infection: implications for early radical malaria treatment
Plasmodium vivax relapses due to dormant liver hypnozoites can be prevented with primaquine. However, the dose must be adjusted in individuals with glucose-6-phosphate-dehydrogenase (G6PD) deficiency. In French G...
Genetic polymorphism and evidence of signatures of selection in the Plasmodium falciparum circumsporozoite protein gene in Tanzanian regions with different malaria endemicity
In 2021 and 2023, the World Health Organization approved RTS,S/AS01 and R21/Matrix M malaria vaccines, respectively, for routine immunization of children in African countries with moderate to high transmission...
Molecular markers of artemisinin resistance during falciparum malaria elimination in Eastern Myanmar
Artemisinin resistance in Plasmodium falciparum threatens global malaria elimination efforts. To contain and then eliminate artemisinin resistance in Eastern Myanmar a network of community-based malaria posts was...
A qualitative look at bed net access and use in Burkina Faso, Mozambique, Nigeria, and Rwanda following piloted distributions of dual-active ingredient insecticide-treated nets
Universal coverage with insecticide-treated nets (ITNs) is important for malaria control and elimination. The emergence and intensification of insecticide resistance threatens progress made through the deploym...
Leveraging malaria vaccines and mRNA technology to tackle the global inequity in pharmaceutical research and production towards disease elimination
Malaria vaccine introduction in endemic countries is a game-changing milestone in the fight against the disease. This article examines the inequity in the global pharmaceutical research, development, manufactu...
A simple, field-applicable method to increase the infectivity of wild isolates of Plasmodium falciparum to mosquito vectors
The direct membrane feeding assay (DMFA), whereby gametocyte-infected blood is collected from human donors and from which mosquitoes feed through a membrane, is proving essential for assessing parameters influ...
A new long-read mitochondrial-genome protocol (PacBio HiFi) for haemosporidian parasites: a tool for population and biodiversity studies
Studies on haemosporidian diversity, including origin of human malaria parasites, malaria's zoonotic dynamic, and regional biodiversity patterns, have used target gene approaches. However, current methods have...
Statistical design and analysis of controlled human malaria infection trials
Malaria is a potentially life-threatening disease caused by Plasmodium protozoa transmitted by infected Anopheles mosquitoes. Controlled human malaria infection (CHMI) trials are used to assess the efficacy of in...
Repurposing of anti-malarial drugs for the treatment of tuberculosis: realistic strategy or fanciful dead end?
Drug repurposing offers a strategic alternative to the development of novel compounds, leveraging the known safety and pharmacokinetic profiles of medications, such as linezolid and levofloxacin for tuberculos...
Predictors of accessing seasonal malaria chemoprevention medicines through non-door-to-door distribution in Nigeria
In Nigeria, seasonal malaria chemoprevention (SMC) is typically administered door-to-door to children under five by community medicine distributors during high transmission seasons. While door-to-door distribu...
Plasmodium falciparum alters the trophoblastic barrier and stroma villi organization of human placental villi explants
The sequestration of Plasmodium falciparum infected erythrocytes in the placenta, and the resulting inflammatory response affects maternal and child health. Despite existing information, little is known about the...
Exploring the role of spending on malaria incidence in Uganda using the auto-regressive distributed lag approach
Malaria has remained a persistent global health problem. Despite multiple government and donor initiatives to eradicate malaria and its detrimental effects on Uganda's health outcomes, the incidence of malaria...
Access to quality-assured artemisinin-based combination therapy and associated factors among clients of selected private drug outlets in Uganda
Malaria treatment in sub-Saharan Africa is faced with challenges including unreliable supply of efficacious agents, substandard medicines coupled with high price of artemisinin-based combinations. This affects...
Field evaluation of the residual efficacy of new generation insecticides for potential use in indoor residual spray programmes in South Africa
The decreasing residual efficacy of insecticides is an important factor when making decisions on insecticide choice for national malaria control programmes. The major challenge to using chemicals for vector co...
Blood count changes in malaria patients according to blood groups (ABO/Rh) and sickle cell trait
Introduction: Malaria continues to be the leading cause of hospitalization and death in Angola, a country in sub- Saharan Africa. In 2023, in the first quarter, 2,744,682 cases were registered, and of these 2,...
Therapeutic efficacy of generic artemether–lumefantrine in the treatment of uncomplicated malaria in Ghana: assessing anti-malarial efficacy amidst pharmacogenetic variations
Despite efforts made to reduce morbidity and mortality associated with malaria, especially in sub-Saharan Africa, malaria continues to be a public health concern that requires innovative efforts to reach the W...
Factors influencing fever care-seeking for children under five years of age in The Gambia: a secondary analysis of 2019–20 DHS data
Malaria contributes to excess child mortality in The Gambia. Children under five are at risk of severe malaria and death if not treated promptly and appropriately. It is crucial that a child with fever receive...
Fidelity of implementation of national guidelines on malaria diagnosis for children under-five years in Rivers State, Nigeria
Malaria is still a disease of global public health importance and children under-five years of age are the most vulnerable to the disease. Nigeria adopted the “test and treat” strategy in the national malaria ...

The Anopheles coluzzii range extends into Kenya: detection, insecticide resistance profiles and population genetic structure in relation to conspecific populations in West and Central Africa
Anopheles coluzzii is a primary vector of malaria found in West and Central Africa, but its presence has hitherto never been documented in Kenya. A thorough understanding of vector bionomics is important as it en...
Identifying and characterizing high-risk populations in pilot malaria elimination districts in Madagascar: a mixed-methods study
In Madagascar, the districts of Antsirabe II, Faratsiho and Antsiranana I have relatively low malaria incidence rates and have been selected by the National Malaria Control Programme for pilot elimination stra...
A qualitative study on determinants of the use of malaria rapid diagnostic test and anti-malarial drug prescription practices by primary healthcare workers in Ebonyi state, Nigeria
The increased availability and use of malaria rapid diagnostic test (RDT) by primary healthcare (PHC) workers has made universal diagnostic testing before malaria treatment more feasible. However, to meaningfu...
Publisher Correction: Progress towards malaria elimination in the Greater Mekong Subregion: perspectives from the World Health Organization
The original article was published in Malaria Journal 2024 23 :64
Willingness to accept malaria vaccines amongst women presenting at outpatient and immunization clinics in Enugu state, Southeast Nigeria
There are giant steps taken in the introduction of the novel malaria vaccine poised towards reducing mortality and morbidity associated with malaria.
Prospective study of malaria in pregnancy, placental and congenital malaria in Northwest Colombia
Pregnancy Associated Malaria (PAM) include malaria in pregnancy (MiP), placental malaria (PM), and congenital malaria (CM). The evidence available in Colombia on PAM focuses on one of the presentations (MiP, P...

- Editorial Board
- Manuscript editing services
- SNAPP Editor link
- Instructions for Editors
- Contact Support for Editors
- Sign up for article alerts and news from this journal
- Follow us on Twitter
Annual Journal Metrics
2022 Citation Impact 3.0 - 2-year Impact Factor 3.2 - 5-year Impact Factor 1.148 - SNIP (Source Normalized Impact per Paper) 1.237 - SJR (SCImago Journal Rank)
2023 Speed 7 days submission to first editorial decision for all manuscripts (Median) 131 days submission to accept (Median)
2023 Usage 4,093,320 downloads 5,053 Altmetric mentions
- More about our metrics
Malaria Journal
ISSN: 1475-2875
- Submission enquiries: [email protected]
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Emily Buck ; Nancy A. Finnigan .
Affiliations
Last Update: July 31, 2023 .
- Continuing Education Activity
Malaria is a parasitic infection transmitted by the Anopheles mosquito that leads to acute life-threatening disease and poses a significant global health threat. Two billion people risk contracting malaria annually, including those in 90 endemic countries and 125 million travelers. The Plasmodium parasite has a multistage lifecycle, which leads to characteristic cyclical fevers. With timely treatment, most people experience rapid resolution of symptoms; however, significant complications may occur, including cerebral malaria, severe malarial anemia, coma, or death. This activity reviews the epidemiology, presentation, and complications of Plasmodium malaria and the role of the interprofessional team in evaluating and managing patients with this life-threatening infection.
- Review the epidemiology of malaria infection.
- Describe the pathophysiology of malaria infection.
- Summarize the pharmacologic treatment strategies for malaria infection.
- Outline the importance of collaboration amongst an interprofessional team to improve outcomes for patients receiving malaria treatment.
- Introduction
Malaria is a parasitic infection transmitted by the Anopheles mosquito that leads to acute life-threatening disease and poses a significant global health threat. Two billion people risk contracting malaria annually, including those in 90 endemic countries and 125 million travelers, and 1.5 to 2.7 million people die in a year. [1] The Plasmodium parasite has a multistage lifecycle, which leads to characteristic cyclical fevers. With timely treatment, most people experience rapid resolution of symptoms; however, significant complications may occur, including cerebral malaria, severe malarial anemia, coma, or death. Preferred antimalarial therapeutic and chemoprophylactic regimens get dictated by species, geography, susceptibility, and patient demographics. Latent or reactivating infections may be reported years following exposure.
The incubation period, and therefore time to symptom development, varies by species: 8 to 11 days for P. falciparum , 8 to 17 days for P. vivax , 10 to 17 days for P. ovale , 18 to 40 days for P. malariae (though possibly up to several years), and 9 to 12 days for P. knowlesi . [1] The periodicity of the Plasmodium lifecycle creates the classic "malarial paroxysm" of rigors, followed by several hours of fever, followed by diaphoresis, and a drop to normal body temperature ( P. vivax infection establishes a 48-hour cycle), though this is less commonly seen today due to rapid identification and treatment. [1]
- Epidemiology
Forty percent of the global population resides in or visits malaria-endemic regions annually. [1] P. falciparum is present in Western and sub-Saharan Africa and displays the highest morbidity and mortality of the Plasmodia species. [2] P. vivax is present in South Asia, the Western Pacific, and Central America. [2] P. ovale and P. malariae are present in Sub-Saharan Africa. [2] P. knowlesi is present in Southeast Asia. [2] As many as 500 million malaria cases occur annually, with 1.5 to 2.7 million deaths. [1] Ninety percent of fatalities occur in Africa. [1] Those at highest risk include children under age 5, pregnant women, and disease naïve populations, including refugees in Central and Eastern Africa, nonimmune civilian and military travelers, and immigrants returning to their place of origin. [2]
Of the 125 million travelers who visit endemic locations each year, 10000 to 30000 develop malaria, and 1% of these will die from complications of their disease. [2] [3] Rising average global temperatures and changes in weather patterns are projected to expand the burden of malaria; a rise of 3 degrees Celsius is postulated to increase malaria incidence by 50 to 80 million. [1]
- Pathophysiology
Five Plasmodium species possess the ability to infect humans: P. falciparum, P. ovale, P. vivax, P. malariae , and P. knowlesi . [2] The female Anopheles mosquito ingests gametes during a blood meal, which form sporozoites that replicate in the gut. [1] During subsequent bloodmeals, saliva containing sporozoites gets released into a human host's bloodstream. [1] Within 60 minutes, sporozoites reach the liver, invade hepatocytes, and then rapidly divide, forming merozoites. In an active infection, organisms reenter the bloodstream and invade erythrocytes. [1] [4] Within erythrocytes, Plasmodia consume hemoglobin and develop from immature trophozoites (ring stage) to either mature trophozoites or gametocytes (CDC Malaria 2019). Mature trophozoites replicate, forming schizonts, disrupting erythrocyte cell membrane integrity, and leading to capillary endothelial adherence and cell lysis. [1]
Free heme is released into the peripheral blood, which stimulates endothelial activation. [5] [6] Untreated malaria lasts 2 to 24 months. [1] P. vivax and P. ovale infections may display "dormant schizogony," where inactive intrahepatic parasites (hypnozoites) remain until reactivation months to years in the future. [1] Although hypnozoite parasites do not routinely develop in the liver in the setting of P. falciparum and P. malariae infection, there are few reports of resurgent P. falciparum infection years after initial exposure. [7]
Pathogenesis stems from toxin-induced IFN-gamma and TNF-alpha secretion. [8] The innate immune response is dominated by monocyte and macrophage phagocytosis within the splenic red pulp. Adaptive immunity develops by IFN-gamma and TNF-alpha-induced class switching of CD4-positive lymphocytes. [4] TNF also suppresses hematopoiesis, which contributes to anemia. The liver and spleen enlarge, causing massive splenomegaly. [8]
Low arginine, low nitric oxide, and elevated arginase activity have been observed in severe malaria in peripheral blood. [9] Studies have shown that the parasite's arginase enzyme may contribute to low arginine in severely ill patients, thus reducing nitric oxide production. Low nitric may lead to subsequent pulmonary hypertension and myocardial wall stress in children. Therefore, peripheral arginine or inhaled nitric oxide are possible treatment options. [10]
Parasitemia dictates symptom onset and severity: symptoms typically develop with 0.002% parasitemia in naïve patients and 0.2% parasitemia in previously exposed patients. [1] Severe infection usually exhibits parasitemia of 5%. [1] [4]
- Histopathology
Intracellular digestion of hemoglobin by parasites forms hemozoin and makes the membrane less deformable, which results in hemolysis or splenic clearance.
- History and Physical
In taking a history, it is essential to inquire about the location of residence, recent travel and use of chemoprophylaxis, exposures (including sick contacts, fresh water, caves, farm/wild animals, insects/arthropods), HIV status, history of current or recent pregnancy, history of G6PD deficiency, history of sickle cell disease, history of anemia, history of blood or other cancers, and history of prior malarial infections (including successful or failed treatments).
Fever is the dominant symptom of malaria—fever, especially for seven or more days, in a patient residing in or with recent travel to an endemic region is highly suspicious and should prompt evaluation. [3] Adults may exhibit headaches, malaise, weakness, gastrointestinal distress, upper respiratory symptoms, and muscle aches; severe cases may include jaundice, confusion, seizures, and dark urine. [2] [1] Children are more likely to present with non-specific or gastrointestinal symptoms such as fever, lethargy, malaise, nausea, vomiting, abdominal cramps, and somnolence. [2] They are more likely to develop hepatomegaly, splenomegaly, and severe anemia without major organ dysfunction than adults. In the case of severe malaria, they present with more frequent seizures (60 to 80%), hypoglycemia, and concomitant sepsis but are less likely to develop pulmonary edema and renal failure than adults. [11] [2]
Pregnant Women
The clinical features of infection in pregnancy vary from asymptomatic to severe, depending on the degree of (incomplete) immunity that a woman had acquired by the time she got pregnant. In semi-immune pregnant women, only a few infections result in fever or other symptoms. [12] Malaria in pregnancy has a devastating effect on maternal health and has been associated with increased infant mortality due to low birth weight caused by either intrauterine growth restriction or preterm labor, or both. [12] P. falciparum infections are associated with complications such as maternal anemia, low birth weight, miscarriage, stillbirths, and congenital malaria. [13] [12] It is more likely for a pregnant woman in the second or third trimester to develop severe malaria with complications such as hypoglycemia and pulmonary edema compared to non-pregnant adults. [14]
Initial evaluation of undifferentiated fever in stable patients with possible malaria exposure includes a complete blood count, comprehensive metabolic panel, coagulation panel, blood culture, urinalysis, chest radiograph, and thick and thin blood smears. In patients with altered mental status, when cerebral malaria is suspected, a lactate level, arterial blood gas, and lumbar puncture may also be indicated. [2]
In patients with malaria, complete blood count reveals thrombocytopenia in 60-70% of all cases and varying degrees of anemia in 29% of adults and 78% of children. [2] Anemia is more severe in P. falciparum due to invasion of all aged erythrocytes and capillary and splenic erythrocyte sequestration secondary to decreased flexibility and cytoadherence. [1] Anemia is typically moderate with P. vivax and P. malariae due to preferential invasion of reticulocytes and older erythrocytes, respectively. [1] A comprehensive metabolic panel may reveal hepatocellular injury secondary to parasitic invasion, indirect hyperbilirubinemia due to hemolysis, electrolyte abnormalities secondary to the release of intracellular contents, concomitant dehydration, and kidney injury secondary to glomerular damage. [2] The coagulation panel may reveal coagulopathy concerning bleeding risk in patients with severe thrombocytopenia or liver dysfunction. Urinalysis may show proteinuria indicative of nephrotic syndrome. [1]
The gold standard for malaria diagnosis is a microscopic evaluation of Giemsa-stained thick and thin smears of a free-flowing venipuncture blood specimen. [2] [1] Examination with oil immersion must be completed at 100-times and 1000-times magnification to avoid missing low-level parasitemia or "delicate ring forms." [1] The extent of parasitemia is estimated by the number of organisms per high-powered field. [1] Varying microscopic appearance of infected erythrocytes guides speciation:
- The ring stage in P. falciparum appears as a "purple spot with a thin ring;" in P. vivax as a "purple spot with a deformed body;" in P. ovale as a "ring with a large purple spot;" in P. malariae as a "purple spot with a thick body;" and in P. knowlesi as a "purple spot (or spots) with an amorphous thick ring." [15]
- The trophozoite stage in P. falciparum appears as "a bigger spot [growing] around a smaller spot;" in P. vivax as "a misshapen circle which contains an extended spot;" in P. ovale as "an oval circle (sometimes with small corners) which contains a purple spot with undefined shapes;" in P. malariae as "basket or band-shaped [without a] spot;" and in P. knowlesi as a "purple branched spot." [15]
- The schizont stage in P. falciparum is not established; in P. vivax, it appears as "not defined purple spots inside a circle;" in P. ovale as "more than one spot inside an oval circle (sometimes with small corners);" in P. malariae as "diffuse purple spots around a darker spot;" and in P. knowlesi as "defined purple spots [that are] easy to count." [15]
- The gametocyte stage in P. falciparum appears as "banana [or] sausage-shaped;" in P. vivax as an "extended, big spot;" in P. ovale as a "row of accumulated spots;" in P. malariae as a "big stained spot which almost fills[s] the circle;" and in P. knowlesi as a "big spot which contains small spots." [15]
An initial negative smear does not rule out malaria, as infected erythrocytes may become intravascularly sequestered; if clinical suspicion of malaria is high, smears require repetition in 12 and 24 hours. [2] The malarial pigment in monocytes and neutrophils may also manifest on the blood smear, particularly in patients with cerebral malaria. [1]
Other diagnostic modalities include rapid diagnostic testing (RDT), microhematocrit centrifugation, and polymerase chain reaction (PCR). RDTs detecting parasitic antigens histidine-rich-protein-2, lactate dehydrogenase, and aldolase are increasingly being utilized to diagnose P. falciparum infection. [2] [16] Sensitivities approach 100%, though microscopy is still a recommendation at the time of presentation and 12 and 24 hours. Limitations of RDTs include the detection of P. falciparum species only, the inability to quantify parasitic burden, and false-positive results occurring weeks after infection due to persistent blood antigens. [2] Microhematocrit centrifugation isolates infected erythrocytes, then binds to acridine in the collection tube, causing the fluorescence of parasites. [1] PCR is useful in low-level parasitemia detection and speciation.
- Treatment / Management
Treatment for patients diagnosed with malaria includes schizonticidal medications, supportive care, and hospitalization for high-risk patients. Naïve adult and pediatric patients receiving active antimalarial treatment should remain inpatient for at least 24 hours to ensure adequate and correctly timed medication dosing and to trend parasitemia to evaluate treatment response. Higher initial parasitemia and poor downtrend are associated with fluid imbalance, renal dysfunction, and respiratory distress syndrome. [2] Unstable patients, particularly those with cerebral malaria or significant respiratory sequelae, require intensive care. [2]
Treatment involves combination therapy targeting both the hepatic and erythrocytic forms. [17] The chief antimalarials are chloroquine, hydroxychloroquine, primaquine, artemisinin-based combination therapy (ACT), and atovaquone-proguanil. Chloroquine and hydroxychloroquine are synthetic forms of quinine. [18] [19] They disrupt the erythrocytic stage by interfering with parasitic hemoglobin metabolism and increasing intracellular pH. [18] [19] They generally require two days of treatment, allowing for better tolerance and shorter admissions. [2] However, chloroquine may enhance gametogenesis, contributing to resistance, which is a concern, particularly in South Asia. [17] Primaquine is a hypnozointocidal agent added for P. vivax or P. ovale infection for the eradication of liver parasites and the prevention of dormancy and relapse. [2] [20]
Primaquine is contraindicated in pregnant and G6PD deficient patients due to fetal teratogenicity and hemolytic reaction (will see bite cells and Heinz bodies on blood smear), respectively. [3] Artemisinins are active against all parasite lifecycle stages. [2] Atovaquone targets the cellular electron transport chain inhibiting ATP production; proguanil enhances atovaquone’s effect by sensitizing parasitic mitochondria. [21] Atovaquone-proguanil is active against the erythrocytic and extraerythrocytic forms. [17] [21]
Per the 2019 CDC Guidelines below, appropriate treatment depends on the Plasmodium species, clinical stability, age of the patient, and regional antimalarial susceptibility:
- Uncomplicated P. falciparum, P. malariae or P. knowlesi infections in chloroquine-sensitive regions are treated with a chloroquine phosphate 600 mg (pediatric: 10 mg/kg) loading dose, followed by 300 mg (pediatric: 5 mg/kg) at 6, 24, 48 hours; or a hydroxychloroquine 620 mg (pediatric: 10 mg/kg) loading dose, followed by 310 mg (pediatric: 5 mg/kg) at 6, 24, and 48 hours.
- Uncomplicated P. falciparum infections in chloroquine-resistant or unknown regions are treated with atovaquone-proguanil 250 mg/100 mg 4 tabs (pediatric: varied weight-based dosing, 6.5 mg/25 mg tabs) daily for 4 days; or artemether-lumefantrine 20 mg/120 mg 4 tabs (pediatric: varied weight-based tabs) at initial dose, then 8 hours later, then twice daily for 2 days; or quinine sulfate 542 mg (pediatric: 8.3 mg/kg) three times daily for 3 days (7 days if in Southeast Asia) plus either doxycycline 100 mg daily for 7 days (pediatrics 2.2 mg/kg every 12 hours), or tetracycline 250 mg daily for 7 days (pediatric: 25 mg/kg/day divided four times daily for 7 days), or clindamycin 20 mg/kg/day divided three times daily for 7 days (pediatric: same); or mefloquine 684 mg (pediatric: 13.7 mg/kg) loading dose followed by 456 mg (pediatric: 9.1 mg/kg) every 6 to 12 hours for total of 1250 mg (pediatric total: 25 mg/kg).
- Uncomplicated P. vivax or P. ovale infections in chloroquine-sensitive regions receive treatment with chloroquine phosphate or hydroxychloroquine as per above, plus either primaquine phosphate 30 mg (pediatric: 0.5 mg/kg) daily for 14 days or tafenoquine 300 mg once (same in children older than 16 years).
- Uncomplicated P. vivax infections in chloroquine-resistant regions (Indonesia, Papua New Guinea) get treated with quinine sulfate as per above plus either doxycycline, primaquine, or tafenoquine as per above; or atovaquone-proguanil as per above plus either primaquine or tafenoquine; or mefloquine as per above plus either primaquine or tafenoquine as per above.
- Uncomplicated infections with any species in pregnant women in chloroquine-sensitive regions require treatment with chloroquine or hydroxychloroquine as per above.
- Uncomplicated infections with any species in pregnant women in chloroquine-resistant regions are treated with quinine sulfate as per above plus either clindamycin or mefloquine as per above in the first, second, or third trimesters; or artemether-lumefantrine as per above in only the second and third trimesters.
- Severe malaria infection in unstable, non-pregnant patients in all regions includes IV artesunate 2.4 mg/kg (pediatric: children greater than 20 kg receive 2.4 mg/kg, children less than 20 kg receive 3.0 mg/kg) at 0, 12, 24, and 48 hours and either artemether-lumefantrine, atovaquone-proguanil, doxycycline, or mefloquine as per above.
- Differential Diagnosis
The differential for undifferentiated fever is extremely broad and varies based on geographic location and age. In a 2017 review of fever in returning travelers, 77% had protozoal malaria, 18% had a bacterial enteric fever ( Salmonella enterica, typhi, or paratyphi ), and 5% had another infection. In patients presenting with fever and significant somnolence or seizures, viral or bacterial meningitis or meningoencephalitis must remain on the differential and prompt consideration of lumbar puncture. [2] [22] Viral etiologies include avian influenza, Middle East respiratory syndrome coronavirus, hemorrhagic fever (Ebola virus, Lassa fever, Marburg hemorrhagic fever, Crimean-Congo hemorrhagic fever), yellow fever, dengue, Japanese encephalitis, Rift Valley fever, hepatitis virus (A or B), viral gastroenteritis, and rabies. [22] Bacterial etiologies include anthrax, epidemic typhus, ehrlichiosis, leptospirosis, melioidosis, murine (endemic) typhus, spotted fever group rickettsioses, Q fever, and Yersinia pestis. [22] [2]
The differential in children varies by region, with the most likely etiology being a viral or bacterial infection. In a 2014 study of febrile children in a tropical region, 10.5% were diagnosed with malaria, 62% were diagnosed with a respiratory infection, 13.3% with a systemic bacterial infection (usually staphylococcus or streptococcus bacteremia), and 10.3% with gastroenteritis (viral or bacterial). [23] Urinary tract infection and typhoid may also be considerations. Meningitis must be ruled out in somnolent children. [23]
- Treatment Planning
Table 1. Artemisinin combination therapy (ACT) regimens for treatment of uncomplicated Plasmodium falciparum malaria in nonpregnant adults and children
Artemether 20 mg/ lumefantrine 120 mg Artemether 40 mg/ lumefantrine 240 mg
Table 2. Oral regimens for treatment of Plasmodium falciparum malaria in pregnant women regions with chloroquine-resistant P. falciparum infection.
Quinine: 542 mg base (= 650 mg salt) three times daily for a weekClindamycin: 20 mg base/kg/day (up to 1.8 grams) divided three times daily for a week OR Artemisinin combination therapy can be used as an alternate therapy in the first trimester if the above (more...)
Table 3. Oral regimens for treatment of Plasmodium falciparum malaria in pregnant women in regions with chloroquine-sensitive P. falciparum infection
00 hours: 600 mg base (= 1000 mg salt) 06 hours: 300 mg base (= 500 mg salt)
The duration of untreated infection and time to relapse vary by location and species. P. falciparum and P. ovale infections last 2 to 3 weeks and may relapse 6 to 18 months later, usually from a new primary infection. [1] P. vivax infection lasts 3 to 8 weeks and may relapse months to up to 5 years later. [1] P. malariae infection lasts 3 to 24 weeks and may relapse up to 20 years later. [1]
Relapse is a case of recurrent symptoms months to years after the resolution of erythrocytic organisms due to reinfection or hypnozoite activation. [2] [1] Recrudescence is defined as recurrent symptoms within days to weeks of acute illness due to remaining parasitemia after ineffective or incomplete treatment or failed host immune response, more commonly in P. falciparum . [2] [1] Appropriate, complete treatment usually results in a full resolution of symptoms.
The two main determinants reflecting the outcome for both adults and children were the level of consciousness assessed by coma scales and the degree of metabolic acidosis, assessed clinically by breathing pattern or, more precisely, with measurement of bicarbonate, base deficit, and plasma lactate. [32] While the general mortality of treated severe malaria is between 10 to 20%, the mortality in pregnant women reaches approximately 50%. [14]
- Complications
The significant complications of malaria are cerebral malaria, severe malarial anemia, and nephrotic syndrome (NS).
Cerebral malaria accounts for 80% of fatal malaria cases, most often occurring with P. falciparum infection. [1] It presents as slow-onset altered mental status, violent behavior, headache, and extremely high fever (up to 42 degrees C), followed by coma, metabolic acidosis, hypoglycemia, and possibly seizures and death. [1] [4] It most commonly affects children under age 5, with a case fatality rate of 18%. [33] Pathogenesis involves malarial rosettes (one infected erythrocyte surrounded by three uninfected erythrocytes), causing cerebral sequestration and vasodilation, as well as excessive oxygen free radicals, IFN-gamma, and TNF-alpha leading to an extreme inflammatory response. [1] [4] [33] This leads to congestion, decreased perfusion, endothelial activation, impairment of the blood-brain barrier, and cerebral edema, which increases brain volume. [33]
Increased brain volume is the major contributor to mortality in cerebral malaria. In a 2015 study of Malawian children with cerebral malaria, 84% of those who died had severely increased brain volume on MRI; children who survived showed lower initial brain volume or a downtrend over time. [33]
Severe malarial anemia stems from TNF-alpha-mediated mechanisms involving both increased destruction and decreased production of erythrocytes, including cell lysis as parasites replicate and exit erythrocytes, splenic removal and autoimmune lysis of immune-marked erythrocytes, poor iron incorporation into new heme molecules, and bone marrow suppression during severe infection leading to decreased production. [1] [4] Blackwater fever is severe anemia with hemoglobinuria and renal failure in the context of "massive intravascular hemolysis" in the setting of repeat P. falciparum infections treated with chronic quinine; it is rare and thought to be associated with G6PD deficiency. [34]
Nephrotic syndrome occurs secondary to glomerular antigen-antibody complex deposition and presents similarly to membranoproliferative glomerulonephritis with proteinuria and decreased renal function, which may lead to renal failure. Nephrotic syndrome is common in P. malariae and P. knowlesi , possible in P. vivax , and rare in P. falciparum and P. ovale infections. [1]
Additional complications include:
- Bilious remittent fever presents with abdominal pain and persistent vomiting that may lead to severe dehydration, jaundice, and dark urine.
- Algid malaria is an adrenal insufficiency due to parasitic congestion and subsequent necrosis of the adrenal glands.
- Acute respiratory distress syndrome, circulatory collapse, disseminated intravascular coagulation, pulmonary edema, coma, and death. [1]
Malaria infection during pregnancy may result in low birth weight or fetal demise. [1]
- Consultations
Recommended consultations for non-infectious disease experts in the management or prevention of malaria include infectious disease and preventive or travel medicine.
- Deterrence and Patient Education
The recommendation is that patients schedule a pre-travel appointment with a preventive medicine or infectious disease physician for education regarding malaria deterrence. Malaria prevention centers around vector control and chemoprophylaxis while exposed to mosquito-ridden environments.
Vector control is the prevention of mosquito bites by way of insecticide-impregnated bed nets, permethrin treatment of clothing, and DEET application to the skin. [3] The three main prophylactic agents for Plasmodium falciparum are atovaquone-proguanil, doxycycline, and mefloquine. Atovaquone-proguanil is taken once daily during and one week after travel to an endemic region; it suppresses the hepatic stage and does not have approval for pregnancy. [2] Doxycycline is taken once daily during and one month after travel; it suppresses the blood stage. [2] Doxycycline has the added benefit of prophylaxis against Rickettsial disease, Q fever, leptospirosis, and travelers’ diarrhea; however, it may cause gastrointestinal distress, photosensitivity, and increased risk of candida infection. Mefloquine is taken once weekly during and one month after travel; it suppresses the blood stage. [2] It has the benefit of safety in the second and third trimester of pregnancy; however, it has a far higher risk of neuropsychiatric side effects. [2] The US military primarily utilizes doxycycline if susceptibilities are equal. [2] For pregnant women in the first trimester or breastfeeding women, chloroquine or mefloquine prophylaxis are preferable; data regarding the safety of atovaquone-proguanil prophylaxis in pregnancy is limited. [35]
- Enhancing Healthcare Team Outcomes
The timely care of patients diagnosed with malaria and clinically relevant research regarding advancing diagnostic techniques and treatment requires interprofessional teamwork and communication between clinicians, infectious disease experts, pharmacists, nurses, and global health professionals.
Any clinician treating malaria will initiate treatment as outlined above. Still, it is good policy to include an infectious disease specialist and involve an infectious disease board-certified pharmacist, who can also examine the regimen and agents chosen, as well as verify dosing and drug interactions. A nurse with infectious disease specialty training can also help by answering patient questions, serving as a bridge to the treating clinician, and monitoring treatment progress and potential adverse drug reactions. All team members must keep accurate and updated records, so everyone involved in treatment has the same information on the patient's case. If there are any concerns, each team member must be free to communicate with other team members so that appropriate interventions can be started or therapeutic modifications can be implemented. This collaborative interprofessional approach can optimize outcomes for malaria patients. [Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Life Cycle of the Malaria Parasite Contributed by Wikimedia Commons, National Institutes of Health (NIH) (Public Domain)
Aedes species mosquito Image courtesy of S Bhimji MD
Blood smear malaria Image courtesy S Bhimji MD
Table 1 - Diagnostic criteria for severe P.falciparum malaria. Contributed by Lara Zekar, MD
Disclosure: Emily Buck declares no relevant financial relationships with ineligible companies.
Disclosure: Nancy Finnigan declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Buck E, Finnigan NA. Malaria. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. [Cochrane Database Syst Rev. 2022] Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Malaria Surveillance - United States, 2016. [MMWR Surveill Summ. 2019] Malaria Surveillance - United States, 2016. Mace KE, Arguin PM, Lucchi NW, Tan KR. MMWR Surveill Summ. 2019 May 17; 68(5):1-35. Epub 2019 May 17.
- Malaria surveillance--United States, 2010. [MMWR Surveill Summ. 2012] Malaria surveillance--United States, 2010. Mali S, Kachur SP, Arguin PM, Division of Parasitic Diseases and Malaria, Center for Global Health, Centers for Disease Control and Prevention (CDC). MMWR Surveill Summ. 2012 Mar 2; 61(2):1-17.
- Review [The reemergence of malaria in Israel?]. [Harefuah. 2004] Review [The reemergence of malaria in Israel?]. Anis E, Pener H, Goldmann D, Leventhal A. Harefuah. 2004 Nov; 143(11):815-9, 838, 837.
- Review Diagnosis and Treatment of the Febrile Child. [Reproductive, Maternal, Newbor...] Review Diagnosis and Treatment of the Febrile Child. Herlihy JM, D’Acremont V, Hay Burgess DC, Hamer DH. Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities, Third Edition (Volume 2). 2016 Apr 5
Recent Activity
- Malaria - StatPearls Malaria - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 14 May 2024
Severe outcomes of malaria in children under time-varying exposure
- Pablo M. De Salazar ORCID: orcid.org/0000-0002-8096-2001 1 , 2 ,
- Alice Kamau 3 ,
- Aurelien Cavelan 1 , 2 ,
- Samuel Akech 3 ,
- Arthur Mpimbaza 4 ,
- Robert W. Snow ORCID: orcid.org/0000-0003-3725-6088 3 , 5 na1 &
- Melissa A. Penny 1 , 2 , 6 , 7 na1
Nature Communications volume 15 , Article number: 4069 ( 2024 ) Cite this article
Metrics details
- Epidemiology
- Policy and public health in microbiology
In malaria epidemiology, interpolation frameworks based on available observations are critical for policy decisions and interpreting disease burden. Updating our understanding of the empirical evidence across different populations, settings, and timeframes is crucial to improving inference for supporting public health. Here, via individual-based modeling, we evaluate a large, multicountry, contemporary Plasmodium falciparum severe malaria dataset to better understand the relationship between prevalence and incidence of malaria pediatric hospitalizations - a proxy of malaria severe outcomes- in East-Africa. We find that life-long exposure dynamics, and subsequent protection patterns in children, substantially determine the likelihood of malaria hospitalizations relative to ongoing prevalence at the population level. Unsteady transmission patterns over a lifetime in children -increasing or decreasing- lead to an exponential relationship of hospitalization rates versus prevalence rather than the asymptotic pattern observed under steady transmission. Addressing this increase in the complexity of malaria epidemiology is crucial to update burden assessments via inference models that guide current and future policy decisions.
Similar content being viewed by others

A meta-analysis on global change drivers and the risk of infectious disease

A guide to vaccinology: from basic principles to new developments

Dynamics of measles immunity from birth and following vaccination
Introduction.
Assessing the burden of malaria life-threatening outcomes in populations at risk is a critically important step in evaluating and improving control efforts. Malaria mortality is challenging to measure accurately in the community 1 but remains a fundamental component of statistical-based interpolation from prevalence estimates, resulting in high uncertainty 2 . Inference of disease burden has been approached with different grades of sophistication, ranging from purely data-driven fits to multi-level mechanistic microsimulations 3 , 4 , 5 , 6 . Independent of the complexity of the approach, the ability of a model to generate accurate, robust, and valid malaria disease outcomes using exposure predictors, such as prevalence, requires (1) high-quality data as input from real-world observations, and (2) a comprehensive understanding and identification of the key factors determining the relationship between exposure and clinical outcomes.
Severe, life-threatening malaria syndromes presenting to hospitals are a valuable proxy for malaria-related death among communities. High exposure rates in children at a very young age are known to offset the risk of severe clinical outcomes at older ages 7 . This leads to a characteristic asymptotic pattern between exposure and disease risk at the population level, consistent with consensual malaria theory and historical observations 4 , 7 , 8 , 9 . Recent work has assessed the empirical relationship between community prevalence and the risk of severe malaria syndromes, namely severe anemia, cerebral malaria, and respiratory distress among children in East Africa, based on the largest standardized Plasmodium falciparum malaria pediatric dataset available to date 10 . Findings show that the occurrence of these severe malaria outcomes in the population may relate differently to increasing community prevalence. Particularly, an asymptotic relationship was observed when predicting severe anemia over community prevalence, while an exponential relationship was favored when predicting a combined outcome comprising the three syndromes. Evaluating new sources of standardized data, such as this contemporary dataset, contextualized with historical sources of data 9 , 11 improves our understanding of the accuracy, robustness, and validity of the inference frameworks.
Here, we use a previously validated multi-level individual-based malaria model 12 , OpenMalaria ( https://github.com/SwissTPH/openmalaria/wiki ), to systematically investigate clinical and epidemiological factors influencing the relationship between potentially life-threatening hospital malaria admissions among children upon a given observed community prevalence. We use community-based malaria hospitalization incidence rates in small catchment populations and adjusted for case under-ascertainment as an empirical proxy of the incidence of severe malaria outcomes. Our analysis framework interrogates standardized malaria data obtained in Sub-Saharan African time-sites within the 1990s throughout 2020 9 , 10 , 11 , 13 , aiming to improve and update our understanding of the dynamics from P. falciparum malaria infection to the occurrence of severe outcomes in children.
Malaria admissions were assembled from individual records of 21 hospitals representing 35 time-sites in Kenya, Uganda, and Tanzania, among children resident in specific catchment areas -within a defined distance radius from the hospital where surveillance took place- excluding urban settings where it was possible to estimate single-year censused population estimates. We assume that the hospitalization rates per time-site represent a lower limit of the hospitalization incidence and can be reasonably comparable when further adjusting for case ascertainment. Cases were included if malaria was the primary cause of hospitalization, and those with underlying conditions were excluded 10 , 13 . We further used data on malaria hospitalization incidence obtained with similar approaches between 1992 and 1997 at seven hospital time-site locations in Kenya, The Gambia, and Malawi 9 , 11 . This allows us to compare and interpret our findings with a dataset that has been classically used for informing malaria inference models 4 , 8 , 14 aiming to estimate severe outcomes of malaria across populations.
For each of the time-sites in the above datasets, the average number of hospitalizations due to malaria among children three months to 9 years old per 1000 children per year were paired with age-diagnostic method standardized community prevalence estimates, as empirical P. falciparum Parasite Rates among children 2–10 years old ( ePf PR 2–10 ). The data was obtained from community and school surveys undertaken during the period of hospital surveillance within the same catchment areas 9 , 10 , 11 . Further, we assessed the past exposure dynamics using catchment site-specific time-series of modeled age- and test-standardized parasite rates estimates, herein referred to as mPf PR 2–10 . For each time-site of the contemporary dataset, annual mPf PR 2–10 estimates were obtained using a Bayesian hierarchical geospatial model detailed elsewhere 13 , 15 . In those time sites where modeled estimates were available for at least 7 past years ( n = 27), and up to a maximum of 9 years, we computed the median mPf PR 2–10 of the time series. We assume that the median prevalence across the past 7–9 years roughly represents the cumulative past transmission to which the population of children up to 10 years of age have been long-life, which can then be compared to the empirical prevalence at the time of the survey to evaluate the gap between past and present-day transmission
Visual inspection of the empirical relationship between prevalence and hospitalization rates for the 35 time-sites included in the contemporary dataset does not suggest an asymptotic relationship of malaria hospitalization incidence across the ePf PR 2–10 range within the full dataset (Fig. 1a .). For illustration, we highlight four representative time-sites in Fig. 1a and alongside the mPf PR 2–10 time-series of these time-sites for the years prior to the collection of the empirical data (Fig. 1b ). Three major patterns of time-varying transmission are depicted, showing (1) a substantial increase in the mPf PR 2–10 (Apac A), (2) relative constant mPf PR 2–10 (e.g, Busia) (3) steady increase (Mubende B) and 4) substantial decrease in the Pf PR 2–10 (e.g., Jinja B). As depicted in Fig. 1c , there is substantial change between the ePf PR 2–10 and the median value of the mPf PR 2–10 over the previous years for each of the time-sites comparing at least seven years and up to ten years of modeled past exposure estimates. The difference between the ePf PR 2–10 and the median value of past mPf PR 2–10 can be interpreted as the gap in past exposure relative to ongoing exposure. For those time-sites at the higher end of the current ePf PR 2–10 range (i.e., higher than the median empirical prevalence, 20%), exposure had primarily substantially increased or remained relatively stable (12 out of 14 sites). For those time sites at the lower end of the range (lower than 20%), exposure had decreased or remained relatively stable (13 out of 13 sites). All available mPf PR 2–10 time series for the 35-time sites are shown in Fig. S1 13 , 15 .
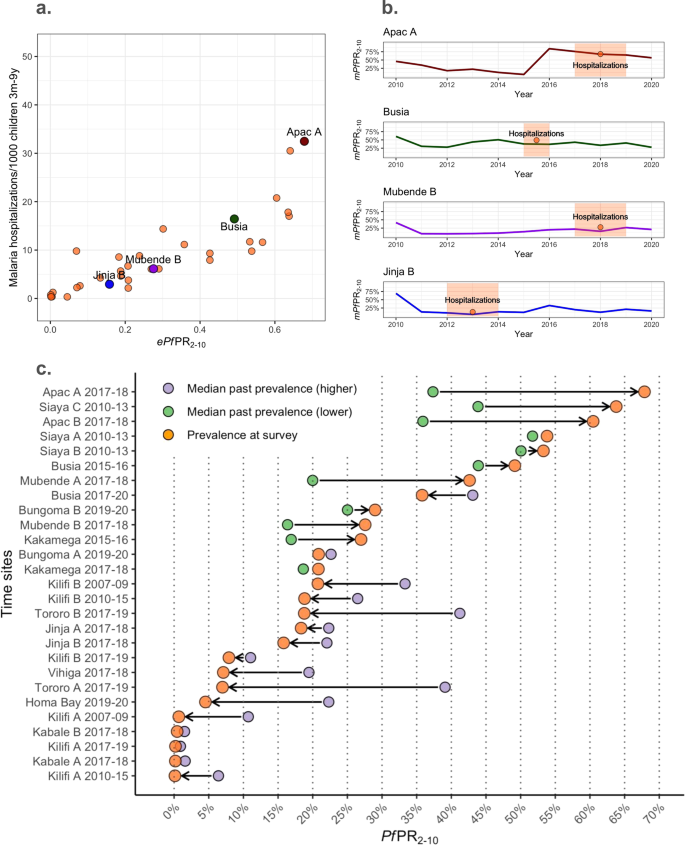
a The ePf PR 2–10 -severe malaria incidence empirical relationships highlighting four representative time-sites (red, green, purple, and blue colored dots) within all time-sites (orange dots). b Present day mPf PR 2–10 over time in four representative time-sites (red, green, purple, and blue colored lines) and time-site ePf PR 2–10 (orange) with highlighted time periods for which hospitalization incidence estimates were available for the empirical relations in ( a ). c Summarizing the prevalence trends over time estimated for each time-site as an increasing trend -when the estimated median mPf PR 2–10 in the past 7 to 9 years is lower than the ePf PR 2–10 at the time of assessment of severe outcomes incidence- or decreasing trend the estimated median mPf PR 2–10 in the past 7–9 years is lower than the ePf PR 2–10 at the time of assessment of severe outcomes incidence. The median past mPf PR 2–10 per time-site is plotted as yellow (for those with increasing trends) or red (for those time-sites with reducing trends), and ePf PR 2–10 per time-site is plotted in orange. Estimates have been computed among the 27-time sites with at least seven years of available past mPf PR 2–10 estimates.
Understanding the complex relationship between malaria exposure, immunity, and clinical outcomes across populations and time requires causal analytical frameworks that (a) can combine empirical observations with theory (b) can address multiple interacting causal effects, threshold dynamics, and interference (c) have generally accepted principles to build the models, populate and calibrate their parameters and test their predictions for avoiding misspecification. Individual-based models are amongst the few modeling tools that fulfill these requirements 1 , 2 .
Open-Malaria ( https://github.com/SwissTPH/openmalaria/wiki ) is an multi-level individual-based model that includes several key features that allow to generate counterfactuals of the effect of malaria exposure on clinical disease under different scenarios of population structure, changing transmission, health-access, diagnostic thresholds, drug-efficacy, and other major malaria control and prevention interventions 3 . Random effects can be incorporated into the modeled processes and allow the inclusion of uncertainty and heterogeneity in the simulations. Relevant to our analyses, the framework encompasses submodels specifically parameterized to empirical data including (1) population structure 4 , 5 ; (2) within-host dynamics of parasite burden and addressing the effect on single individuals of repeated infections in developing immunity and subsequent infections 6 , 7 , 8 ; (3) disease progression 6 , 7 and health-seeking behavior including rates of individuals accessing health services, as well as time to diagnostic and treatment 4 ; (4) efficacy of case-management including diagnostic sensitivity and specificity, first- and second-line treatment effectiveness and efficacy of hospitalization 4 ; and (5) the effect of age-structured comorbidities on severe malaria outcomes upon infection 5 . Further details are provided in the Supplementary Note 2 .
We iteratively interrogated the data under different sets of plausible parameterizations of our individual-based model, hereafter referred to as scenario analysis. The scenario analysis explores hypotheses of the impact of well-known determinants on disease risk and changes in contemporary disease risk compared to historical observations, including the deployment and availability of artemisinin-derivatives in primary- and hospital-care, improved treatment adherence, the reduction in the occurrence and progression of malaria-associated comorbidities. In addition to addressing the detailed major key changes between the historical and contemporary datasets, we further address the life-long exposure dynamics evidenced by the modeled community prevalence estimates (Fig. 1b and Fig. S1 ). The risk of malaria disease outcomes, including those severe, depends on immunity, which in turn depends on previous exposure dynamics. We hypothesize that the year-to-year variability of PfPR could strongly influence the risk of hospitalization. Thus, we further assessed the impact of this variability on malaria exposure on the ongoing hospital admission risk estimated from our individual-based model scenarios. Here, we define steady exposure as the exposure of a specific population that does not change substantially over the years, and we define unsteady exposure as an exposure that shows substantial increasing or decreasing dynamics over time. We performed simulations that included generic step-up and step-down exposure dynamics with differences between pre- and intra-survey prevalence within the range of those observed empirically. Further details are found in the Supplementary Note 3 . For each scenario analysis, we performed over 1000 individual-based simulations. We tested the model outputs— Pf PR 2–10 and incidence rate of malaria admissions—against three regression models, namely an intercept-only, a log-logistic, and a log-linear model, which was previously used to evaluate the prevalence-hospitalization relationship 10 .
The historical dataset shows higher levels of hospitalization rates at similar prevalence, consistent with the expected reduction of comorbidities and improved management effectiveness in the contemporary dataset (Fig. 2 and Supplementary Note 5 ). Figure 2a, b shows (1) the historical (Fig. 2a ) and contemporary (Fig. 2b ) empirical estimates, (2) their respective time-frame-specific simulations under the assumption of steady exposure, and (3) the regression-based predicted relationship, shown as the median and 50% and 95% prediction intervals. For both the historical and the contemporary predictions, the log-logistic regression model provides a better fit to the model outputs, with the asymptote reaching around 60% and 40% Pf PR 2–10 , respectively. However, while the prediction model based on steady transmission is consistent with the historical empirical data, it fails to recover the contemporary dataset relationship, with a substantial number of time-sites outlying from the predictions range, particularly for the highest ePf PR 2–10 values (i.e., over 60%).
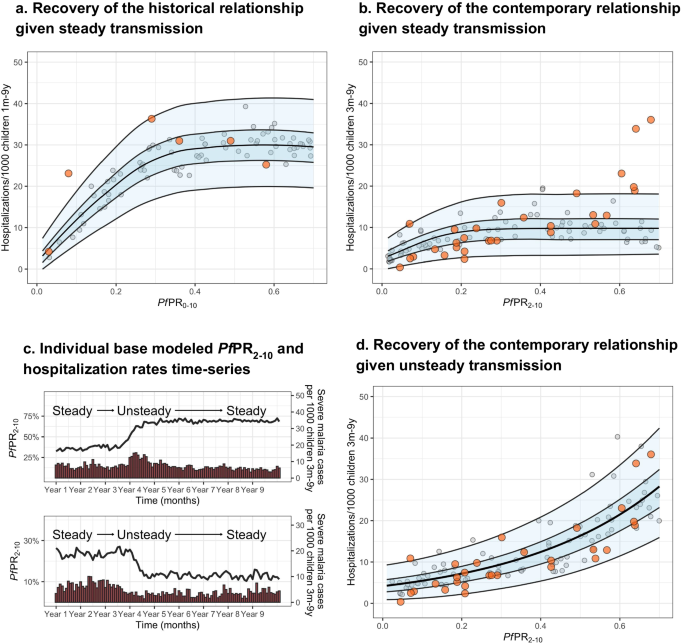
Showing the empirical Pf PR 2–10 -hospitalization rates relationship obtained from a the historical dataset (orange dots), and b the contemporary dataset (orange dots) overlapping respective Pf PR-hospitalization model-based estimates obtained through simulations consistent with steady transmission (gray dots, n = 100) and respective levels of health care access, treatment and comorbidities ( b ), and the best-fit model-based log-logistic regressions (median black line, blue ribbons 50% and 95% prediction intervals). c Representative simulation patterns of Pf PR 2–10 (black lines, increasing at the top and decreasing at the bottom) and subsequent malaria hospitalization incidence over time (red columns). d The empirical Pf PR 2–10 -hospitalization rates relationship obtained from the contemporary dataset (orange dots) overlapping Pf PR 2–10 -severity model-based estimates obtained through simulations scenarios consistent with time-varying exposure (gray dots, n = 100) and levels in health care access, treatment, and comorbidities, and the best-fit model-based log-linear regression (median black line, blue ribbons 50% and 95% prediction intervals).
Given that model predictions based on steady exposure do not capture the pattern of the empirical contemporary data, we further evaluated scenarios with unsteady past malaria transmission (either increasing or decreasing dynamics), and how these different trends affect the Pf PR 2–10 -hospitalization rate incidence relationship. Specifically, we performed simulations that included step-up and step-down exposure dynamics with differences between pre- and intra-survey prevalence within the range of those observed empirically (see Methods). Simulations captured representative patterns of time-varying exposure, with decreasing, steady or increasing transmission before computing severe disease incidence (Fig. 2c ) over the range of Pf PR 2–10 values. The best fit to a regression model is then obtained using the log-linear model (Fig. 2d ). Based on performance metrics 16 , simulations under the assumption of unsteady patterns of past exposure predict the relationship of the contemporary dataset more accurately than those based on steady exposure. Further, modeled predictions under unsteady exposure show hospitalization rates in different age groups increase towards higher ePf PR 2–10 in a similar way as observed in the empirical estimates (Fig. S2 ). However, this is opposite to the pattern in historical observations under steady state exposure; severe disease incidence among youngest children is typically higher in high transmission settings than in low transmission settings, whereas the opposite occurs among older children 4 , 7 , 8 , 9 . Consistent with these results, our model recovers more accurately the observed hospitalization age structure (Fig. S3 , left column for representative time-sites) under the unsteady transmission assumption (Fig. S3 , middle column) than under the steady-state assumption (Fig. S3 , right column). Further, when assessing the model estimates of hospitalization risk later in time (i.e., allowing the scenarios to maintain a steady-state level transmission over 5 years), the prevalence-hospitalization rates relationship transitions to the asymptotic pattern expected for steady transmission (Fig. S4 ).
Via scenario analysis, we have systematically evaluated the major clinical and epidemiological determinants influencing the occurrence of malaria hospitalization upon infection at the population level and thus proved a contemporary characterization of relationship trends and changes between malaria community prevalence and life-threatening disease risk in children. We found that the asymptotic relationship between prevalence and hospitalization disease risk, expected under a relatively steady transmission, is lost when children have been exposed to unstable, time-varying past malaria transmission. Overall, our analyses support the assumption that substantial fluctuations in malaria transmission over the years have led to a particular prevalence-hospitalization relationship observed among the East-African settings 10 , where increasing prevalence does not necessarily lead to saturating disease risk but increases toward the highest rates in an exponential manner. However, our analyses show that if transmission is further maintained at a steady-state level over sufficient time, disease risk would also eventually re-equilibrate back to the asymptotic relationship relative to the parasite rates (fig. S4 ). To date, inference frameworks aiming to estimate or predict severe outcomes of malaria used for policy decisions and public health action do not explicitly include past exposure as an independent variable 5 , 17 . Our analysis framework is capable of reconciling historical and contemporary observations encompassing three decades in sub-Saharan Africa and underscores the importance of taking the variability of past malaria exposure among children into account when predicting severe disease risk.
Our analyses have several limitations that need to be acknowledged. First, we use community-based hospitalization in non-urban settings as an empirical proxy of the incidence of severe outcomes of malaria. While these estimates could under- or over-estimate the true number of severe outcomes, the data was obtained aiming to standardized the under-ascertainment of cases, estimates would be affected by site specific treatment-seeking behaviors and therefore represent a lower limit for hospitalization rate at each time site. Also, the empirical contemporary dataset does not necessarily represent urban settings, where the referral pathways from infection to hospitalization might be more complex to understand or subject to other potential biases. Further, the curated data does not include cases where malaria was not the major syndrome for hospitalization. Our modeling approach allows access to health (i.e., access to diagnosis, treatment, and/or hospitalization) to randomly vary within the range of the rates estimated for the three countries, with sensitivity analysis showing that deviations from this assumption do not influence the overall relationship between prevalence and hospitalization. Nevertheless, if the data represents stronger deviations from these assumptions regarding case identification but remains relatively similar across time sites, the overall prevalence-severe disease trend will still hold. Second, the empirical Pf PR estimates obtained through community and school surveys might not necessarily reflect the underlying prevalence dynamics for the full catchment population, given how heterogeneous malaria exposure can be at a very granular spatial level. However, we obtain a similar prevalence-hospitalization relationship using estimates computed using the geospatial model (see Section “Discussion”, Fig. S10 ), thus supporting the assumption of the empirical values being a representative summarizing value for the catchment populations. Also, the time series of Pf PR values used to compute exposure steadiness can bear high uncertainty on the precise estimates. Nevertheless, given that we did not aim to replicate the prevalence changes over time but addressed this matter focusing on the relative change, our framework will remain well informed if the estimates approximate true trends. Third, our model OpenMalaria simulations are based on spatially homogeneous malaria transmission because the catchment populations in the empirical data are small. Assuming a heterogeneous transmission structure can affect the magnitude of the clinical outcomes, central estimates will not change 18 . Fourth, while parameterization of the hospitalization rates, efficacy of treatment, and comorbidities are consistent with the literature, for simplicity, we assumed similar ranges of values across time-sites with stochastic variation. In Supplementary Note 4 , we provide an uncertainty analysis of these mechanistic parameters, showing that our results hold under no major deviations from tenable assumptions. Last, we have applied parametric regression models to simulation data, which likely misspecifies the mechanical interpretation of the model. Still, we believe this is justified given this approach was originally used to determine the empirical relationship 10 and we aimed to replicate the trends under potential plausible mechanistic scenarios.
We have provided evidence that variation in malaria transmission and subsequent disease protection after life-long exposure can strongly influence severe disease risk estimates under otherwise equivalent ongoing force of transmission. Notably, frequent implementation and withdrawal of infection prevention strategies can strongly contribute to unsteady malaria exposure patterns and thus increase severe disease risk. Other potential sources of variability in the exposure include substantial movement of individuals from areas with different community prevalence (i.e., via increasing or reducing the overall population-level susceptibility to severe disease) or strong environmental changes influencing entomological inoculation rates such as urbanization or climatic drivers (i.e., prolonged drought or excessive rainfall).
Under constant, similar malaria exposure and health access rates, a population of children with higher immunity will substantially reduce their risk of severe malaria and, therefore, the number of severe cases. However, if malaria transmission has marked changes, either sudden reductions or increases, the resulting severe disease will be significantly lower or higher, respectively, than if the population had remained under constant transmission. This understanding is critical to evaluate the effects of interventions, and such mechanistic processes need to be included in future analytical approaches providing predictions of malaria disease burden. The processes that must be incorporated into disease burden estimates are best defined through differences in the age–structure patterns of the risk conditional to past exposure. For example, it is expected that following a strong reduction in malaria transmission, severe cases among age groups of children at a certain prevalence will be reduced earlier in time but will likely increase in later periods if malaria prevalence reaches steady levels. Similarly, the withdrawal of effective prevention and control strategies will lead to a higher number of severe cases than those expected when malaria prevalence has remained unchanged over time. In short, if past exposure and the dynamics described here are not accounted for in burden estimates, it will lead to long-term overestimation of severe malaria risk in places with recent effective interventions. And conversely, it will lead to long-term underestimation of severe malaria in places with deterioration of interventions. Finally, our findings underpin the need to build back rigorous clinical surveillance of severe malaria under the changing landscape of parasite exposure in Africa. It is striking that only two longitudinal clinical series exist since the launch of the Roll Back Malaria initiative in 2000 19 , 20 .
Overall, our findings provide evidence that inference in malaria epidemiology, such as the generation of counterfactual scenarios with predictions on clinical outcomes for policy decisions, should account from now on past exposure and subsequent protection to avoid substantial bias in such risk predictions and highlight the increase in the complexity of malaria epidemiology arising from unsteady transmission dynamics.
Contemporary malaria hospitalization incidence data and paired community prevalence estimates
The contemporary P. falciparum malaria hospitalization incidence data has been obtained from 35 time-sites in Kenya ( n = 18), Uganda ( n = 14), and Tanzania ( n = 3) between 2006 and 2020 and has been previously described elsewhere 10 . The data was collated from individual records of 21 hospitals, including hospitalized malaria cases in children resident in specific catchment areas within a 30 km range and excluding urban settings. Given potential differences in treatment-seeking behaviors at the different sites, the computed estimates represent the lower limit for hospitalization incidence, thus requiring further assumptions for adjusting to under ascertainment (see model parameterization in the Supplementary Text). for In the present analysis, cases were included if malaria was deemed the primary cause of hospitalization. Per each time-site, the number of hospitalizations among children 3 months to 9 years old per 1000 children per year was computed as a proxy of life-threatening disease incidence from the catchment population, obtained from census data and census data projections. Hospitalization data was paired with community surveys performed at the same periods of the hospital surveys for each time-site, adjusting for diagnostic accuracy (i.e., microscopy vs. rapid test) and standardized by computing the Pf PR 2–10 as described elsewhere 21 , 22 . Further details on hospitalization data, community prevalence, and estimates of hospital catchment population can be found in Supplementary Note 1 .
Modeled community prevalence time-series
We assessed the past exposure dynamics using catchment site-specific time series of modeled age- and test-standardized parasite rates estimates, referred to as mPf PR 2–10 13 , 15 . Modeled mPf PR 2–10 estimates were explicitly obtained for each time-site catchment population using a geospatial model detailed elsewhere 13 , 15 , a Bayesian hierarchical geostatistical framework based on more than 180000 geo-coded empirical prevalence survey data points from East Africa, interpolated in time to 1 × 1 km resolutions using climatic and ecological covariates. For a set of sites ( n = 11), annual mPf PR 2–10 estimates are available since 2000, while for the rest ( n = 15) available data begin in 2010. Time series of all the available mPf PR 2–10 per site are shown in Fig. S1 . Thus, for 27 out of the 35 present-day time sites, mPf PR 2–10 time series included at least seven-time points of past annual estimates. Further, to estimate the gap between past and present transmission, we first computed the median value of the annual mPf PR 2–10 time-series for each of the time-sites, under the assumption that it roughly represents the life-long transmission at which the surveyed population of children have been exposed in the previous years previous. This median mPf PR 2–10 can then be compared to the empirical Pf PR 2–10 that is estimated at the time of surveying the malaria hospitalization incidence.
Historical malaria hospitalization incidence data and paired community prevalence estimates
As an alternative source of data, we analyzed a historical dataset obtained between 1992 and 1997, encompassing the relation between severe disease incidence measured as malaria hospitalization rates and the Pf PR 2–10 up to 70% and published elsewhere 9 . Similar inclusion and exclusion criteria had been used to obtain both datasets explicitly to allow comparison. Thus, comparable approaches to those used in the contemporary dataset were used to estimate malaria hospitalization incidence at six hospital time-site locations in The Gambia ( n = 3), Kenya ( n = 2), and Malawi ( n = 1) and paired community prevalence estimates 7 , 9 , 11 . Further details can be found in Supplementary Note 1 .
Agent-based model of malaria transmission, immunity, and disease dynamics
To recover the relationship between hospitalization rates and Pf PR 2–10 , we used an individual-based model of P. falciparum malaria transmission and disease dynamics, OpenMalaria, previously described and calibrated elsewhere 12 . Briefly, OpenMalaria features within-host parasite dynamics, the progression of clinical disease, development of immunity, individual care-seeking behavior, vector exposure, and pharmaceutical and non-pharmaceutical antimalarial interventions at vector and human level ( https://github.com/SwissTPH/openmalaria/wiki ) 14 , 23 , 24 . The full model is calibrated to fit 23 parameters to 11 objectives representing different epidemiological outcomes, including age-specific prevalence and incidence patterns, age-specific mortality rates, and hospitalization rates, using a Bayesian optimization approach 12 . A detailed description of the model and references for the key technical details are provided in Supplementary Note 2 .
Model parameterization on disease management, comorbidities, and health access
In order to compare model predictions to empirical data, we first parameterized several key inputs of the individual-based model to be consistent with both historical and contemporary datasets and the corresponding existing clinical and epidemiological knowledge, including different sets of variables addressing (a) the individual probability of malaria exposure per time-step to cover a range of Pf PR 2–10 up to 70%, (b) the effectiveness in malaria case management, including the rate of access of uncomplicated and severe malaria cases to health-care (i.e., rate of accurate diagnostic of true occurrence and subsequent timely treatment) and the efficacy of the available malaria drugs at each period (e.g., the efficacy of artemisinin derivatives combination therapies in clearing malaria), and (c) the co-occurrence of other diseases with influence on malaria that affect the severe progression of malaria across age-groups.
To evaluate the contemporary dataset, model parameterization for the main analysis was as follows: (1) Pf PR 2–10 estimates ranged up to 70%; (2) drug efficacy ranged between 85 and 100% 25 , 26 ; (3) the rates of individuals suffering from malaria that required hospitalization who received diagnostic and treatment ranged from 60 to 90% while the rate of individuals with uncomplicated malaria accessing diagnostic and treatment ranged 20–60% 27 ; and (4) co-occurrence of diseases contributing to malaria hospitalization was substantially reduced by 60–80% since the 1990s 28 , 29 , 30 . For (2–4), we parameterized the model by randomly sampling values from a uniform distribution defined by the respective ranges. Sensitivity analysis for these assumptions can be found in the Supplementary Note 3 . To evaluate the historical dataset, we include the following: (1) Pf PR 2–10 estimates ranged up to 70% (2) first-line drug efficacy for uncomplicated malaria was negligible 31 ; (3) access rates of malaria requiring hospitalization ranged from 40–60% 8 , 32 , and (4) co-occurrence of diseases contributing to malaria hospitalization approximated a hyperbolic decay distribution over age-groups 8 . Details on key model parameters and submodels are provided in Supplementary Note 3 .
Time-varying malaria exposure on severity estimates
To evaluate how time-varying malaria exposure rates influence the ongoing Pf PR 2–10 -hospitalization rates relationship, we produced a set of plausible malaria-transmission scenarios consistent with time-varying exposure as for those obtained from a Bayesian hierarchical geostatistical framework 13 , 15 across all our time-sites. Particularly, we evaluated how a rapid reduction or increase of the Pf PR 2–10 level over 6 months (e.g., Pf PR 2–10 increasing from 10% to 70%) could affect the severe malaria incidence relative to a steady-state transmission assumption described before. This was performed by setting (1) the initial exposure rate (i.e., the individual probability of exposure to infectious bites pre-survey as a single rate over simulation time-steps), independent from (2) the final exposure rate (i.e., the probability of exposure during the survey period, also as a single rate over time steps). For simplicity, we set constant initial and final exposure rates. The relationship between the initial and final exposure rate was parameterized to reflect archetypical mPf PR 2–10 patterns,—depicted in Fig. 1b —showing (1) a substantial increase in the mPf PR 2–10 (e.g., Apac A), (2) relative constant mPf PR 2–10 (e.g., Busia), and (3) substantial decrease in the Pf PR 2–10 (e.g., Jinja B). Thus, our definition of time-varying (unsteady) exposure comprises both substantial increasing and decreasing dynamics. For each time site with retrospective data encompassing at least 7 years, we computed the average mPf PR 2–10 available up to 9 years prior to the date when the hospitalization data was available, used it as a proxy of the initial exposure rate, and computed the fold-change Pf PR 2–10 . We then performed a local polynomial regression model to obtain predictions of the relationship between the final exposure rate and the corresponding relative change per time site. See fig. S5 in Supplementary depicting the relationship between the contemporary community prevalence as ePf PR 2–10 , and estimated relative change at survey computed from the median value of mPf PR 2–10 of the past 7–9 years for each time-site. We then used these inputs of exposure to produce simulations using a combination of initial and final exposure rates to map a range of simulation-based Pf PR 2–10 -hospitalization rates.
Scenario analysis to recover the empirical relationship
To evaluate under which scenarios OpenMalaria can recover the empirical prevalence-hospitalization, we implemented an iterative 4-step procedure to explore hypotheses of the impact of the determinants on disease risk and changes in disease risk—see sections above for details. The procedure is represented schematically in the Supplementary (Fig. S6 ). For each scenario analysis, we performed four iterative steps as follows:
Using a high-performance computing framework ( http://scicore.unibas.ch/ ) we performed 1000 population-level individual-based model simulations of malaria transmission at a steady-state (i.e., same entomological inoculation rate for each simulation) over long periods of time (i.e., over 90 years which ensures that lifelong malaria exposure has occurred all the generations evaluated prospectively); computing hospitalization rates among children 3 months- 9 years per 1000 persons-year across values of Pf PR 2–10 within the input range. In each of the simulations, we parameterized the model, mapping the range of values set up for the major epidemiological determinants described in the previous section, namely disease management (which includes rates and efficacy of diagnostic and treatment of severe and uncomplicated malaria) and co-occurrence of comorbidities. We set the parameters according to the empirical dataset we were evaluating (i.e., historical or contemporary). At the same time, we evaluated the model outcomes simulating steady and unsteady transmission.
In order to assess how accurately the model simulations represent the empirical data, we evaluated the performance of two regression models to recover the prevalence-hospitalization relationship obtained via scenario analysis, namely a log-linear model and a log-logistic model. The approach was chosen to be consistent with the previous analysis framework of the contemporary dataset 10 , which analyses severe malaria syndrome-specific cases against prevalence. See Supplementary Note 3 for details on the regression models, model selection criteria, and computation of uncertainty estimates.
We used the best-fit regression model among the above to evaluate how the modeling framework predicted the empirical ePf PR 2–10 -hospitalization incidence relationship. Specifically, we evaluated the accuracy of the model predictions using prediction performance metrics 16 (see Supplementary Note 3 and Table S2 ).
We updated model assumptions based on the evaluation above and reparametrized the scenario(/s) accordingly.
Evaluation of key assumptions for scenario modeling
To evaluate the robustness of the simulation-based predictions of the Pf PR 2–10 -hospitalization relationship under time-varying exposure, we performed three major sensitivity analyses: (a) how increased or reduced rates of both uncomplicated case management and hospitalizations at the population level influence the severe disease incidence and prevalence relationship; (b) how the relationship changes over higher or lower drug efficacy estimates; (c) how changes on the incidence of comorbidities influence the severe disease risk. Details of the evaluation are provided in Supplementary Note 4 and Figs. S7 and S8 .
The age structure of the severity estimates and the effect of time-varying malaria
Last, to assess if the model framework recovers hospitalization risk over different age groups, we compared both empirical and simulation-based estimates disaggregated by age. We performed the 4-steps iterative analysis described above for the contemporary dataset under the steady and time-varying transmission assumptions and evaluated the Pf PR 2–10 -hospitalization relationship by age. More specifically, we computed hospitalization incidence over age groups at values of Pf PR2–10 equal to those estimated in the for four representative time sites Apac A, Busia, Mubende B, and Jinja B. Further details on the age-structured analysis are provided in Supplementary Note 5 .
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Empirical contemporary data used in this analysis have been curated and uploaded to the Harvard Dataverse: https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/XGDB3K . Correspondence and requests for materials should be addressed to the KEMRI Wellcome Data Governance Committee ([email protected]). These data are available through a formal requesting process to the KEMRI Institutional Data Access/Ethics Committee. Guideline details can be found on the KEMRI Wellcome website: https://kemri-wellcome.org/about-us/#ChildVerticalTab_15 .
Code availability
Model code, plotting code, and simulation data are available at https://github.com/PDeSalazarSwissTPH/SevereMalaria .
Snow, R. W. Sixty years trying to define the malaria burden in Africa: have we made any progress? BMC Med. 12 , 227 (2014).
Article PubMed PubMed Central Google Scholar
White, N. J., Day, N. P. J., Ashley, E. A., Smithuis, F. M. & Nosten, F. H. Have we really failed to roll back malaria? Lancet 399 , 799–800 (2022).
Camponovo, F., Bever, C. A., Galactionova, K., Smith, T. & Penny, M. A. Incidence and admission rates for severe malaria and their impact on mortality in Africa. Malar. J. 16 , 1 (2017).
Griffin, J. T. et al. Gradual acquisition of immunity to severe malaria with increasing exposure. Proc. Biol. Sci. 282 , 20142657 (2015).
PubMed PubMed Central Google Scholar
Weiss, D. J. et al. Mapping the global prevalence, incidence, and mortality of Plasmodium falciparum , 2000-17: a spatial and temporal modelling study. Lancet 394 , 322–331 (2019).
Bhatt, S. et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526 , 207–211 (2015).
Article ADS CAS PubMed PubMed Central Google Scholar
Snow, R. & Marsh, K. The consequences of reducing transmission of Plasmodium falciparum in Africa. Adv. Parasitol. https://doi.org/10.1016/s0065-308x(02)52013-3 (2002).
Ross, A., Maire, N., Molineaux, L. & Smith, T. An epidemiologic model of severe morbidity and mortality caused by Plasmodium falciparum . Am. J. Trop. Med. Hyg. 75 , 63–73 (2006).
Article PubMed Google Scholar
Marsh, K. & Snow, R. W. Malaria transmission and morbidity. Parassitologia 41 , 241–246 (1999).
Paton, R. S. et al. Malaria infection and severe disease risks in Africa. Science 373 , 926–931 (2021).
Snow, R. W. et al. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. The Lancet https://doi.org/10.1016/s0140-6736(97)02038-2 (1997).
Reiker, T. et al. Emulator-based Bayesian optimization for efficient multi-objective calibration of an individual-based model of malaria. Nat. Commun. 12 , 7212 (2021).
Kamau, A. et al. Malaria hospitalisation in East Africa: age, phenotype and transmission intensity. BMC Med. 20 , 28 (2022).
Smith, T. et al. Mathematical modeling of the impact of malaria vaccines on the clinical epidemiology and natural history of Plasmodium falciparum malaria: overview. Am. J. Trop. Med. Hyg. 75 , 1–10 (2006).
Alegana, V. A. et al. Plasmodium falciparum parasite prevalence in East Africa: Updating data for malaria stratification. PLOS Global Public Health https://doi.org/10.1371/journal.pgph.0000014 (2021).
Dziak, J. J., Coffman, D. L., Lanza, S. T., Li, R. & Jermiin, L. S. Sensitivity and specificity of information criteria. Brief. Bioinform. 21 , 553–565 (2020).
World malaria report. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023 (2023).
Penny, M. A. et al. Public health impact and cost-effectiveness of the RTS,S/AS01 malaria vaccine: a systematic comparison of predictions from four mathematical models. Lancet 387 , 367–375 (2016).
Njuguna, P. et al. Observational study: 27 years of severe malaria surveillance in Kilifi, Kenya. BMC Med. 17 , 124 (2019).
Guinovart, C. et al. The epidemiology of severe malaria at Manhiça District Hospital, Mozambique: a retrospective analysis of 20 years of malaria admissions surveillance data. Lancet Glob. Health 10 , e873–e881 (2022).
Article CAS PubMed Google Scholar
Smith, D. L., Guerra, C. A., Snow, R. W. & Hay, S. I. Standardizing estimates of the Plasmodium falciparum parasite rate. Malar. J. 6 , 1–10 (2007).
Article Google Scholar
Mappin, B. et al. Standardizing Plasmodium falciparum infection prevalence measured via microscopy versus rapid diagnostic test. Malar. J. 14 , 460 (2015).
Smith, T. et al. Towards a comprehensive simulation model of malaria epidemiology and control. Parasitology 135 , 1507–1516 (2008).
Smith, T. et al. Ensemble modeling of the likely public health impact of a pre-erythrocytic malaria vaccine. PLoS Med. 9 , e1001157 (2012).
World Health Organization. Guidelines for the Treatment of Malaria . (World Health Organization, 2010).
World Health Organization. Guidelines for the Treatment of Malaria . Third Edition. (World Health Organization, 2015).
The DHS Program. https://dhsprogram.com/methodology/survey-types/mis.cfm .
GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390 , 1151–1210 (2017).
Reiner, R. C. et al. Identifying residual hotspots and mapping lower respiratory infection morbidity and mortality in African children from 2000 to 2017. Nat. Microbiol. 4 , 2310–2318 (2019).
Reiner, R. C. Jr et al. Variation in childhood diarrheal morbidity and mortality in Africa, 2000-2015. N. Engl. J. Med. 379 , 1128–1138 (2018).
Amin, A. A. et al. The challenges of changing national malaria drug policy to artemisinin-based combinations in Kenya. Malar. J. 6 , 72 (2007).
Goodman, C. A., Coleman, P. G. & Mills, A. Economic analysis of malaria control in sub-Saharan Africa. (Global Forum for Health Research, Geneva, 2000).
Download references
Acknowledgements
Calculations were performed at sciCORE ( http://scicore.unibas.ch/ ) scientific computing center at the University of Basel. Funding : This study was partly funded by Bill & Melinda Gates Foundation (INV- 025569 to MAP) supporting M.A.P. and P.M.S. M.A.P. also received support via a Swiss National Science Foundation Professorship (PP00P3_203450 to M.A.P.); R.W.S. is supported under a Wellcome Trust Principal Research Fellowship (212176/Z/18/Z), which also provided support for A.K.; R.W.S., A.K. and S.A. are grateful for the support from the Wellcome Trust to the Core Award for the East Africa Major Overseas Program (203077/Z/16/Z). S.A. was supported by the CDC Foundation through funding from the World Health Organization for the RTS,S evaluation (project requisition number 2018/854999).
Author information
These authors contributed equally: Robert W. Snow, Melissa A. Penny.
Authors and Affiliations
Department of Epidemiology and Public Health, Swiss Tropical and Public Health Institute, Allschwil, Switzerland
Pablo M. De Salazar, Aurelien Cavelan & Melissa A. Penny
University of Basel, Basel, Switzerland
Kenya Medical Research Institute (KEMRI)-Wellcome Trust Research Programme, Nairobi, Kenya
Alice Kamau, Samuel Akech & Robert W. Snow
Child Health and Development Centre, College of Health Sciences, Makerere University, Kampala, Uganda
Arthur Mpimbaza
Centre for Tropical Medicine and Global Health, Nuffield Department of Clinical Medicine, University of Oxford, Oxford, UK
Robert W. Snow
Telethon Kids Institute, Nedlands, WA, Australia
Melissa A. Penny
Centre for Child Health Research, University of Western Australia, Crawley, WA, Australia
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: P.M.S., M.A.P., A.K., R.W.S.; methodology: P.M.S., M.A.P., R.W.S.; investigation: P.M.S., S.A., A.M., A.K., A.C., R.W.S., M.A.P.; visualization: P.M.S., M.A.P.; funding acquisition: M.A.P., R.W.S.; writing—original draft: P.M.S., M.A.P., R.W.S.; writing—review & editing: P.M.S., M.A.P., R.W.S., A.K., A.C., S.A., A.M.
Corresponding authors
Correspondence to Pablo M. De Salazar or Melissa A. Penny .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethics declaration
Kenya hospital surveillance: Kenya Medical Research Institute/ Scientific and Ethics Review Unit (KEMRI SERU) IRB numbers 1433, 3057, 3149; 1801, 2558, 2465, 3459, and 3771; Uganda hospital surveillance approved by the CDC as public health surveillance/non-research (NCEZID 031416), Tanzania hospital surveillance approved by the ethics committees of the National Institute for Medical Research, Tanzania, and the London School of Hygiene and Tropical Medicine. Kenya community parasite surveys KEMRI SERU IRB numbers 3149, 3592, 1801, 2558, 2675, 2801, and 3822; Uganda community prevalence surveys Vector Control Division Research Ethics Committee, MoH, Kampala: VCDREC/117.
Peer review
Peer review information.
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
De Salazar, P.M., Kamau, A., Cavelan, A. et al. Severe outcomes of malaria in children under time-varying exposure. Nat Commun 15 , 4069 (2024). https://doi.org/10.1038/s41467-024-48191-7
Download citation
Received : 31 August 2023
Accepted : 22 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1038/s41467-024-48191-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.

IMAGES
VIDEO
COMMENTS
5.6eported malaria cases in R HBHI countries since 2018 and comparisons with estimated cases 48 6.vestments in malaria programmes and research In 52 6.1unding trends for malaria control and elimination 52F 6.2vestments in malaria-related R&D 56In 7.istribution and coverage of malaria prevention, diagnosis and treatment D 58
PDF | INTRODUCTION Malaria is a mosquito-borne infectious disease of humans and other animals caused by protists (a type of microorganism) of the genus... | Find, read and cite all the research ...
6. Investments in malaria programmes and research 44 6.1 Funding trends for malaria control and elimination 45 6.2 Global trends in real GDP growth and out-of-pocket health expenditure 54 6.3 Investments in malaria-related R&D 58 7. Distribution and coverage of malaria prevention, diagnosis and treatment 60 7.1 Distribution and coverage of ITNs 60
However, there continues to be a significant funding gap for basic research and product development. There is an urgent need to ramp up investment in new malaria tools, to address emerging threats such as urban malaria and the spread of antimalarial drug resistance. We face many challenges, but there are many reasons for hope.
The Technical Strategy was developed in close alignment with the Roll Back Malaria Partnership's Action and Investment to defeat Malaria 2016-2030 (AIM) to ensure shared goals and complementarity. Many thanks to the AIM Task force and Vanessa Racloz for the strong coordination and collaboration.
researchers and academics to identify priority research areas for malaria. » In 1998, WHO, the World Bank, the United Nations Development Programme and the United Nations Children's Fund created the Roll Back Malaria initiative with the goal of halving the global burden of malaria by 2010. Two years later, leaders of malaria-endemic countries
The 2023 WHO World malaria report. The estimatednumber ofglobalmalaria cases in 2022 exceeded pre-COVID-19 pandemic levels in 2019, according to WHO's 2023 World malaria report. Several threats to the malaria global response are highlighted in the report, including climate change. The report, an annual assessment of global trends in malaria ...
only ITNs for the prevention and control of malaria in children and adults living in areas with ongoing malaria transmission where the principal malaria vector(s) exhibit pyrethroid resistance that is: a) confirmed, b) of intermediate level, and c) conferred (at least in part) by a monooxygenase-based resistance mechanism, as
Malaria case incidence (i.e. cases per 1000 population at risk) reduced from 80 in 2000 to 58 in 2015 and 57 in 2019 (Table 3.1, Fig. 3.2). Between 2000 and 2015, malaria case incidence declined by 27% and then by less than 2% in the period 2015-2019, indicating a slowing of the rate of decline since 2015 (Fig. 3.2).
The World malaria report 2018 estimates that there were 219 million cases of malaria in 2017. The 10 highest burden African countries saw an estimated 3.5 million more malaria cases in 2017 compared with the previous year. Malaria continues to claim the lives of more than 435 000 people each year, largely in Africa.
PDF | Malaria is a global public health burden with an estimated 229 million cases reported worldwide in 2019. About 94% of the reported cases were... | Find, read and cite all the research you ...
Suitable methods for investigating vector susceptibility to pyriproxyfen an... Alesha Myers, Josias Fagbohoun, Georgine Houetohossou, Boris Ndombidje, Renaud Govoetchan, Damien Todjinou and Corine Ngufor. Malaria Journal 2024 23 :164. Research Published on: 24 May 2024. Full Text.
1. Introduction. Malaria affected an estimated 219 million people causing 435,000 deaths in 2017 globally. This burden of morbidity and mortality is a result of more than a century of global effort and research aimed at improving the prevention, diagnosis, and treatment of malaria [].Malaria is the most common disease in Africa and some countries in Asia with the highest number of indigenous ...
METHODS IN MALARIA RESEARCH 6th edition . Welcome to this new edition of Methods in Malaria Research which contains protocols provided by 122 scientists from the global malaria community. The manual is considered a "working document" that, with the help of our readers and users, will continuously grow and evolve as new and improved methods are
Global Malaria Epidemiology. The number of total malaria cases globally increased in 2021 (from 245 million in 2020 to 247 million in 2021), with most of the increase occurring in Africa. However, case incidence remained stable from 2020 to 2021 (59 cases per 1000 population at risk) following an increase from 2019 (57 cases/1000 population ...
control of malaria in children and adults living in areas with ongoing malaria transmission. Remark: • WHO recommends ITNs that have been prequalified by WHO for deployment in protecting populations at risk of malaria. • ITNs are most effective where the principal malaria vector(s) bite predominantly at night after people have retired under
Malaria is a parasitic infection transmitted by the Anopheles mosquito that leads to acute life-threatening disease and poses a significant global health threat. Two billion people risk contracting malaria annually, including those in 90 endemic countries and 125 million travelers, and 1.5 to 2.7 million people die in a year.[1] The Plasmodium parasite has a multistage lifecycle, which leads ...
Severe pediatric malaria remains a concern in many countries. Here, the authors use an individual-based modeling approach to evaluate the relationship between malaria prevalence and incidence of ...
MALARIA DIAGNOSIS, TREATMENT AND PREVENTION: BRIEF GUIDELINE FOR UN MEDICAL STAFF (APRIL 2019) UNITED NATIONS | DEPARTMENT OF OPERATIONAL SUPPORT . 1 . MALARIA DIAGNOSIS, TREATMENT & PREVENTION: BRIEF GUIDELINE FOR UN MEDICAL STAFF . BACKGROUND . Malaria is a common and life-threatening disease in many tropical and subtropical areas where UN ...
Malaria research. Each year there are more than 200 million new cases of malaria, a preventable and treatable disease. According to the World Health Organization's (WHO) World malaria report 2019, there were no global gains in reducing new infections between 2014 and 2018, and nearly as many people died from malaria in 2018 as in the previous ...
Overview. Malaria is a life-threatening disease spread to humans by some types of mosquitoes. It is mostly found in tropical countries. It is preventable and curable. The infection is caused by a parasite and does not spread from person to person. Symptoms can be mild or life-threatening. Mild symptoms are fever, chills and headache.
Haematological and biochemical findings in severe malaria. Anaemia is normocytic and may be 'severe' (haemoglobin < 5g/dl or erythrocyte volume fraction (haematocrit) < 15%. Thrombocytopenia (< 100 000 platelets/μl) is usual in malaria, and in some cases the platelet count may be extremely low (< 20 000/μl).
Kinshasa, urban and semirural, where malaria transmission is endemic and perennial. 13. Ethical aspects . The research adhered to the ethical principles outlined in the Declaration of Helsinki for medical studies involving human subjects. All participants (or their parents or guardians in the case of minors)
This review ofprogress in malaria research over theperiods 1951-1970and1970-1973 indicates the results sofar achieved in research on theparasite, on the immune response ofthe host, andon the vector; refers to the means ofcontrolling or eradicating malaria that have been developed in recent years; andoutlines thepresent status ofthe malaria
malaria-free world. Medicines for Malaria. Venture. 28 May. 07:30-09:00 CET. Scaling up integrated models of care to address maternal and child. mortality. The African Union. Commission, the United. Republic of Tanzania, EGPAF. and Save the Children. 28 May. 07:15-08:45 CET. Empowering tomorrow: Sexual and reproductive health and rights
Overview . In response to ever increasing financial constraints, the WHO Global Malaria Programme and Regional Offices, in consultation with selected national malaria programme managers and technical partners, have developed guiding principles for prioritizing interventions in resource-constrained country contexts to achieve maximum impact for national malaria control programmes.
Research to assess the safety, effi cacy and programme feasibility of other antimalarials in intermittent preventive treatment is under way. 3 information are provided in Annexes 1-5, and the household survey ... malaria indicator survey, multiple indicator cluster surveys, demographic