
CHARLES KODNER, MD
Am Fam Physician. 2016;93(6):479-485
Patient information : See related handout on nephrotic syndrome , written by the author of this article.
Author disclosure: No relevant financial affiliations.
Nephrotic syndrome (NS) consists of peripheral edema, heavy proteinuria, and hypoalbuminemia, often with hyperlipidemia. Patients typically present with edema and fatigue, without evidence of heart failure or severe liver disease. The diagnosis of NS is based on typical clinical features with confirmation of heavy proteinuria and hypoalbuminemia. The patient history and selected diagnostic studies rule out important secondary causes, including diabetes mellitus, systemic lupus erythematosus, and medication adverse effects. Most cases of NS are considered idiopathic or primary; membranous nephropathy and focal segmental glomerulosclerosis are the most common histologic subtypes of primary NS in adults. Important complications of NS include venous thrombosis and hyperlipidemia; other potential complications include infection and acute kidney injury. Spontaneous acute kidney injury from NS is rare but can occur as a result of the underlying medical problem. Despite a lack of evidence-based guidelines, treatment consisting of sodium restriction, fluid restriction, loop diuretics, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy, and careful assessment for possible disease complications is appropriate for most patients. Renal biopsy is often recommended, although it may be most useful in patients with suspected underlying systemic lupus erythematosus or other renal disorders, in whom biopsy can guide management and prognosis. Immunosuppressive treatment, including corticosteroids, is often used for NS, although evidence is lacking. Routine prophylactic treatment to prevent infection or thrombosis is not recommended. A nephrologist should be consulted about use of anticoagulation and immunosuppressants, need for renal biopsy, and for other areas of uncertainty.
Nephrotic syndrome (NS) consists of peripheral edema, heavy proteinuria, and hypoalbuminemia, often with hyperlipidemia. Patients typically present with edema and fatigue, without heart failure or severe liver disease. Although there is limited evidence to guide management decisions, recent expert consensus guidelines and systematic reviews provide updated recommendations. This article focuses on diagnosis and management of NS in adults, which is different from that in children.

Epidemiology
The annual incidence of NS in adults is three per 100,000 persons. Approximately 80% to 90% of NS cases in adults are idiopathic. Membranous nephropathy is the most common cause in whites, and focal segmental glomerulosclerosis is most common in blacks; each of these disorders accounts for approximately 30% to 35% of NS cases in adults. Minimal change disease and immunoglobulin A nephropathy each account for approximately 15% of cases. The remaining 10% of cases are secondary to an underlying medical condition. 1
Assessing the cause of NS is important in guiding management decisions. Many underlying systemic conditions can cause NS, although type 2 diabetes mellitus and systemic lupus erythematosus are most common. NS may not present as a primary diagnosis, but instead as one of multiple disease manifestations, particularly in systemic lupus erythematosus. A published case report of a 29-year-old pregnant woman with lupus nephritis, preeclampsia, NS, and hemolytic anemia illustrates this scenario. 2 Secondary causes of NS are listed in Table 1 . 1 , 3
Pathophysiology
The mechanism of edema formation in NS is unclear. The primary defect seems to be increased glomerular permeability to albumin and other plasma proteins. Primary renal sodium retention and decreased oncotic pressure from hypoalbuminemia lead to increased extravasation of fluid from the intravascular space into the interstitial space, resulting in edema. 4
The pathophysiology of thrombogenesis in NS is also not completely understood but seems to be multifactorial, involving loss of coagulation regulatory proteins and a shift in the hemostatic balance toward a prothrombotic milieu. 5 Patients with NS and prothrombotic genetic mutations have a further increased risk of thrombosis.
Diagnostic Evaluation
New-onset edema, particularly in the lower extremities, is the most common presenting symptom of NS. Depending on disease severity, patients may have edema extending to the proximal lower extremities, lower abdomen, or genitalia. Ascites, periorbital edema, hypertension, and pleural effusion are also possible presenting features. Patients may report foamy urine, exertional dyspnea or fatigue, and significant fluid-associated weight gain. 1 , 3
The diagnostic criteria for NS are listed in Table 2 . 1 Confirmation of proteinuria via 24-hour urine collection is cumbersome for patients, and the specimen can be collected incorrectly. The protein-to-creatinine ratio from a single urine sample is commonly used to diagnose nephrotic-range proteinuria. Although this spot test has limited accuracy in patients who exercise heavily, are gaining or losing muscle mass, or have similar factors, in general, it is sufficient for diagnosing heavy proteinuria. 1
Further diagnostic assessment of patients with NS has three goals: to assess for complications, identify underlying disease, and potentially determine the histologic type of idiopathic NS. The role of renal biopsy in patients with NS is controversial, and there are no evidence-based guidelines regarding indications for biopsy. Whether biopsy is performed often depends on the preferences of consulting nephrologists. In patients with NS from a known secondary cause and who are responding to treatment appropriately, biopsy will likely add little to treatment. Biopsy may be more useful for treatment and prognosis in patients with idiopathic NS of an unknown histologic disease type or with suspected underlying systemic lupus erythematosus or other renal disorders.
Complications
Various systemic complications are commonly associated with NS. These are thought to result from overproduction of hepatic proteins and loss of low-molecular-weight proteins in the urine, although the specific mechanisms have not been fully described. 5 It is generally not necessary to screen otherwise asymptomatic patients for these complications. Figure 1 is an algorithm for the diagnosis and management of NS. 1
VENOUS THROMBOSIS
Venous thrombosis is one of the most important complications of NS, but the true incidence and risk are difficult to determine because of the heterogeneity of the clinical manifestations and causes of NS. The most common sites of venous thrombosis in adults are in the deep veins of the lower limbs, although thrombosis can also occur in the renal veins and can cause pulmonary embolism. Arterial thrombosis is rare in patients with NS. 1
In a historical case series of patients with NS, venous thrombosis of the lower limb occurred in 8% of patients, and renal venous thrombosis occurred in up to 25% of patients. However, more recent data suggest a much lower risk of venous thrombosis in patients with NS. 6 In a retrospective study, deep venous thrombosis occurred in 1.5% of adults with NS, and renal venous thrombosis occurred in 0.5% of adults with NS. 6 Venous thrombosis is much more common in adults than in children and is more common in adults with membranous nephropathy than other histologies, 5 with an incidence of up to 7%. 7 Unless the patient's history suggests a thromboembolic complication, screening otherwise asymptomatic patients for thromboembolic events is not indicated.
Bacterial infections, especially cellulitis, are a potential complication of NS. A Cochrane review found no relevant studies of infections in adults with NS. 8 There are no reliable data on the incidence of infection as a complication of NS and no current guidelines for the use of prophylactic antibiotics in adults with NS.
RENAL FAILURE
Acute kidney injury is considered a rare spontaneous complication of NS. It can coexist with NS when it is caused by the same factors that lead to edema and proteinuria, such as lupus nephritis and drug-induced interstitial nephritis. 1 , 9 Although acute kidney injury is uncommon in NS, tests for renal function, quantification of proteinuria, serum chemistry, and lipid profile are appropriate to assess renal function and determine the degree of hyperlipidemia. Table 3 shows the differential diagnosis of acute kidney injury in patients with NS. 3
HYPERLIPIDEMIA
Elevated lipid levels (potentially markedly elevated) are a common feature of NS. Any subtype of lipoprotein concentrations can be elevated. There are no recent epidemiologic data to indicate how common or severe this complication is, and no recent data regarding the impact of treatment for dyslipidemia associated with NS. However, resolving proteinuria and any underlying disease process is believed to improve or resolve the dyslipidemia. 1 , 10
Management of NS is limited by a lack of clear evidence-based guidelines, although recent expert consensus guidelines provide useful recommendations. 11 In addition to correction of treatable causes, management includes general measures to treat symptoms such as edema and, in some cases, immunosuppressant treatment of the renal pathology.
GENERAL TREATMENT MEASURES
Because of the possible pathophysiologic role of sodium retention, some experts recommend that routine treatment of patients with NS include restricting dietary sodium to less than 3 g per day and restricting fluid to less than 1,500 mL per day. 1
TREATING EDEMA
Patients with nephrosis are resistant to diuretics, even if the glomerular filtration rate is normal. Loop diuretics act in the renal tubule and must be protein-bound to be effective. Serum proteins are reduced in NS, limiting the effectiveness of loop diuretics, and patients may require higher-than-normal doses. 3 Other mechanisms for diuretic resistance are also possible. Oral loop diuretics with twice-daily administration are usually preferred because of the longer duration of action. However, with severe NS and edema, gastrointestinal absorption of the diuretic may be uncertain because of intestinal wall edema, and intravenous diuretics may be necessary. Diuresis should be relatively gradual and guided by daily weight assessment, with a target of 2 to 4 lb (1 to 2 kg) per day. 3
Furosemide (Lasix) at 40 mg orally twice daily or bumetanide at 1 mg twice daily is a reasonable starting dosage, with approximate doubling of the dose every one to three days if there is inadequate improvement in edema or other evidence of fluid overload. 3 An approximate upper limit for furosemide is 240 mg per dose or 600 mg total per day, 12 but there is no clear evidence or rationale for this limit. If there is still an inadequate clinical response, patients may be treated by changing to intravenous loop diuretics, adding oral thiazide diuretics, or giving an intravenous bolus of 20% human albumin prior to an intravenous diuretic bolus. 3
ANTICOAGULATION FOR VENOUS THROMBOSIS
Despite the known risk of venous thrombosis in patients with NS, there are no randomized controlled trials to guide whether prophylactic anticoagulation should be used and for how long. 1
Adult patients with NS should be assessed individually for underlying disease. Additional considerations are the severity of NS (i.e., serum albumin less than 2.0 to 2.5 g per dL [20 to 25 g per L] may be more likely to prompt anticoagulation prophylaxis 7 ), preexisting thrombophilic states, and the overall likelihood of serious bleeding events from the use of oral anticoagulation. The decision to treat with anticoagulants should be made individually. 13 Although the benefits of anticoagulation may outweigh the risks in selected patients at high risk of venous thrombosis (e.g., those with known prothrombotic tendency or a history of venous thrombosis), anticoagulation is not routinely used for primary prevention of thrombotic events in patients with NS. 6
TREATING AND PREVENTING INFECTION
Infection has been reported in up to 20% of adults with NS, although it is unclear if NS is causative or if the infection is a result of hospitalization, corticosteroid use, or other factors. 1 A Cochrane review found no strong evidence to recommend a specific intervention to prevent infection in adults with NS. 8
TREATING DYSLIPIDEMIA
A recent Cochrane review found insufficient evidence to determine if lipid-lowering agents are helpful in managing dyslipidemia in adults with NS and no other indications for treatment based on previously obtained lipid levels. 10
ANTIPROTEINURIC TREATMENT
Treatment with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers appears to reduce the risk of venous thrombosis, although this has not been confirmed. 14 Treatment with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers is often recommended for patients with NS because of their known antiproteinuric effects. However the degree of benefit for specific outcomes, such as renal failure or recovery, improvement in edema, or need for dialysis, is unproven, and the evidence supporting the routine use of these medications is conflicting.
IMMUNOSUPPRESSIVE THERAPY
Corticosteroids are often used in the treatment of NS despite an absence of supporting data. In recent years, corticosteroids and other immunosuppressive treatments have been investigated for use in NS ( Table 4 ) . 15 A Cochrane review showed that combining an alkylating agent with a corticosteroid has short- and long-term benefits for membranous nephropathy in adults with NS. 15 In general, immunosuppressive treatment has no proven benefit for most adults with idiopathic NS, and the potential risks may outweigh any benefits. The role of such treatment and specific treatment decisions, such as type and duration of therapy, depend on clinical factors and potentially on the histologic diagnosis identified on biopsy. If NS is steroid-resistant or does not improve, other immunosuppressive treatments should be considered in cooperation with a nephrologist. Immunosuppressive therapy for NS secondary to systemic lupus erythematosus is highly effective and supported by multiple studies, and may lead to partial or complete remission in patients with minimal change disease or primary focal segmental glomerulosclerosis.
The prognosis for NS is highly dependent on the underlying cause, the disease histology, and patient clinical factors. Although many patients improve with appropriate supportive care and do not require any specific therapy, others worsen despite aggressive, specific therapy and may require dialysis. In one study, routine treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, plus selective use of corticosteroids or other immunosuppressants, led to a remission rate of 76%, with 12% of patients requiring hemodialysis. 16
Idiopathic membranous nephropathy is one of the most common forms of primary NS in adults, and has a generally favorable prognosis. 15 The prognosis for this illness roughly follows a “rule of thirds”: about one-third of patients have a benign course with a high rate of remission; one-third have ongoing evidence of proteinuria or edema but maintain normal renal function; and somewhat less than one-third of patients progress toward end-stage renal disease within 10 years. 15
Adults with primary focal segmental glomerulosclerosis, however, tend to have a poorer prognosis, and the degree of proteinuria is a significant prognostic factor. Although about one-half of patients with nephrotic-range proteinuria progress to end-stage renal disease over five to 10 years, patients with very heavy proteinuria (10 to 14 g per day) will develop end-stage renal disease on average within two to three years. 17
Subspecialist Consultation
Consultation with nephrologists should guide decisions about use of anticoagulation and immunosuppressants, need for renal biopsy, and for other areas of uncertainty.
Data Sources : A Medline literature search was conducted using the key term nephrotic syndrome. The search was limited to English, human, core clinical journals ( Abridged Index Medicus ), and publication years between 2005 and 2015. Additional searches were conducted combining the baseline nephrotic syndrome search with other relevant key words, such as venous thrombosis, hyperlipidemia, infection, and acute kidney injury. Relevant original articles cited in reviews were used as the sources for cited data. Search dates: January 25, 2015, and December 10, 2015.
This review updates a previous article on this topic by the author. 18
Hull RP, Goldsmith DJ. Nephrotic syndrome in adults. BMJ. 2008;336(7654):1185-1189.
Williams WW, Ecker JL, Thadhani RI, Rahemtullah A. Case records of the Massachusetts General Hospital. Case 38-2005. A 29-year-old pregnant woman with the nephrotic syndrome and hypertension. N Engl J Med. 2005;353(24):2590-2600.
Floege J. Introduction to glomerular disease: clinical presentations. In: Johnson RJ, Feehally J, Floege J, eds. Comprehensive Clinical Nephrology . 5th ed. Philadelphia, Pa.: Elsevier Saunders; 2015.
Siddall EC, Radhakrishnan J. The pathophysiology of edema formation in the nephrotic syndrome. Kidney Int. 2012;82(6):635-642.
Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol. 2012;7(3):513-520.
Kayali F, Najjar R, Aswad F, Matta F, Stein PD. Venous thromboembolism in patients hospitalized with nephrotic syndrome. Am J Med. 2008;121(3):226-230.
Pincus KJ, Hynicka LM. Prophylaxis of thromboembolic events in patients with nephrotic syndrome. Ann Pharmacother. 2013;47(5):725-734.
Wu HM, Tang JL, Cao L, Sha ZH, Li Y. Interventions for preventing infection in nephrotic syndrome. Cochrane Database Syst Rev. 2012;4:CD003964.
Koomans HA. Pathophysiology of acute renal failure in idiopatic nephrotic syndrome. Nephrol Dial Transplant. 2001;16(2):221-224.
Kong X, Yuan H, Fan J, Li Z, Wu T, Jiang L. Lipid-lowering agents for nephrotic syndrome. Cochrane Database Syst Rev. 2013;12:CD005425.
Radhakrishnan J, Cattran DC. The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines—application to the individual patient. Kidney Int. 2012;82(8):840-856.
Furosemide dosage. Drugs.com. http://www.drugs.com/dosage/furosemide.html . Accessed January 13, 2016.
Glassock RJ. Prophylactic anticoagulation in nephrotic syndrome. J Am Soc Nephrol. 2007;18(8):2221-2225.
Mahmoodi BK, Mulder AB, Waanders F, et al. The impact of antiproteinuric therapy on the prothrombotic state in patients with overt proteinuria. J Thromb Haemost. 2011;9(12):2416-2423.
Chen Y, Schieppati A, Chen X, et al. Immunosuppressive treatment for idiopathic membranous nephropathy in adults with nephrotic syndrome. Cochrane Database Syst Rev. 2014;10:CD004293.
McQuarrie EP, Stirling CM, Geddes CC. Idiopathic membranous nephropathy and nephrotic syndrome. Nephrol Dial Transplant. 2012;27(1):235-242.
Korbet SM. Treatment of primary FSGS in adults. J Am Soc Nephrol. 2012;23(11):1769-1776.
Kodner C. Nephrotic syndrome in adults: diagnosis and management. Am Fam Physician. 2009;80(10):1129-1134.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2016 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Nephrotic syndrome
On this page, preparing for your appointment.
Tests and procedures used to diagnose nephrotic syndrome include:
- Urine tests. A urinalysis can reveal abnormalities in your urine, such as large amounts of protein. You might be asked to collect urine samples over 24 hours.
- Blood tests. A blood test can show low levels of the protein albumin and often decreased levels of blood protein overall. Loss of albumin is often associated with an increase in blood cholesterol and blood triglycerides. The creatinine and urea nitrogen levels in your blood also might be measured to assess your overall kidney function.
- Kidney biopsy. Your doctor might recommend removing a small sample of kidney tissue for testing. During a kidney biopsy, a needle is inserted through your skin and into your kidney. Kidney tissue is collected and sent to a lab for testing.
More Information
- Kidney biopsy
Treatment for nephrotic syndrome involves treating any medical condition that might be causing your nephrotic syndrome. Your doctor might also recommend medications and changes in your diet to help control your signs and symptoms or treat complications of nephrotic syndrome.
Medications might include:
Blood pressure medications. Drugs called angiotensin-converting enzyme (ACE) inhibitors reduce blood pressure and the amount of protein released in urine. Medications in this category include lisinopril (Prinivil, Qbrelis, Zestril), benazepril (Lotensin), captopril and enalapril (Vasotec).
Another group of drugs that works similarly is called angiotensin II receptor blockers (ARBs) and includes losartan (Cozaar) and valsartan (Diovan). Other medications, such as renin inhibitors, also might be used, though angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are generally used first.
- Water pills (diuretics). These help control swelling by increasing your kidneys' fluid output. Diuretic medications typically include furosemide (Lasix). Others include spironolactone (Aldactone, Carospir) and thiazides, such as hydrochlorothiazide or metolazone (Zaroxolyn).
Cholesterol-reducing medications. Statins can help lower cholesterol levels. However, it's not clear whether cholesterol-lowering medications can improve the outcomes for people with nephrotic syndrome, such as avoiding heart attacks or decreasing the risk of early death.
Statins include atorvastatin (Lipitor), fluvastatin (Lescol XL), lovastatin (Altoprev), pravastatin (Pravachol), rosuvastatin (Crestor, Ezallor) and simvastatin (Zocor).
- Blood thinners (anticoagulants). These might be prescribed to decrease your blood's ability to clot, especially if you've had a blood clot. Anticoagulants include heparin, warfarin (Coumadin, Jantoven), dabigatran (Pradaxa), apixaban (Eliquis) and rivaroxaban (Xarelto).
- Immune system-suppressing medications. Medications to control the immune system, such as corticosteroids, can decrease the inflammation that accompanies some of the conditions that can cause nephrotic syndrome. Medications include rituximab (Rituxan), cyclosporine and cyclophosphamide.
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Changes to your diet might help with nephrotic syndrome. Your doctor might refer you to a dietitian, who might recommend that you do the following:
- Choose lean sources of protein. Plant-based protein is helpful in kidney disease.
- Reduce the amount of fat and cholesterol in your diet to help control your blood cholesterol levels.
- Eat a low-salt diet to help control swelling.
- Reduce the amount of liquid in your diet.
Start by seeing your primary care doctor. If your doctor suspects you or your child has a kidney problem, such as nephrotic syndrome, you might be referred to a doctor who specializes in the kidneys (nephrologist).
Here's some information to help you get ready for your appointment.
What you can do
When you make the appointment, ask if there's anything you need to do in advance, such as restrict your diet. Take a family member or friend along, if possible, to help you remember the information you'll be given.
Make a list of:
- Your or your child's symptoms and when they began
- Key personal information, including major stresses or recent life changes
- All medications, vitamins or other supplements you or your child takes, including doses
- Questions to ask your doctor
For nephrotic syndrome, some questions to ask include:
- What's the most likely cause of my or my child's nephrotic syndrome?
- What tests do I or my child need?
- Is this condition likely temporary?
- What are the treatment options? And which do you recommend?
- Are there changes I can make to my or my child's diet? Could consulting a dietitian help?
- How can I best manage this condition with my or my child's other medical conditions?
- Are there brochures or other printed material that I can have? What websites do you recommend?
What to expect from your doctor
Your doctor is likely to ask you questions, such as:
- Do symptoms come and go, or do you have them all the time?
- How severe are the symptoms?
- Does anything seem to improve the symptoms?
- What, if anything, appears to worsen the symptoms?
Feb 23, 2022
- Ferri FF. Nephrotic syndrome. In: Ferri's Clinical Advisor 2020. Elsevier; 2020. https://www.clinicalkey.com. Accessed Nov. 22, 2019.
- Nephrotic syndrome in adults. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/kidney-disease/nephrotic-syndrome-adults. Accessed Nov. 22, 2019.
- Kelepouris E, et al. Overview of heavy proteinuria and the nephrotic syndrome. https://www.uptodate.com/contents/search. Accessed Nov. 24, 2019.
- A to Z health guide: Nephrotic syndrome. National Kidney Foundation. https://www.kidney.org/atoz/content/nephrotic. Accessed Nov. 22, 2019.
- Childhood nephrotic syndrome. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/kidney-disease/children/childhood-nephrotic-syndrome. Accessed Nov. 22, 2019.
- Symptoms & causes
- Doctors & departments
- Diseases & Conditions
- Nephrotic syndrome diagnosis & treatment
Associated Procedures
Products & services.
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Assortment of Compression Products at Mayo Clinic Store
- Newsletter: Mayo Clinic Health Letter — Digital Edition
CON-XXXXXXXX
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Kidney Disease
Nephrotic Syndrome in Adults
On this page:
What is nephrotic syndrome?
How common is nephrotic syndrome, who is more likely to develop nephrotic syndrome, what are the complications of having nephrotic syndrome, what are the symptoms of nephrotic syndrome, what causes nephrotic syndrome, how do health care professionals diagnose nephrotic syndrome, how do health care professionals treat nephrotic syndrome, how does eating, diet, and nutrition affect nephrotic syndrome, clinical trials for nephrotic syndrome.
Nephrotic syndrome is a group of symptoms that indicate your kidneys are not working properly. These symptoms include
- too much protein in your urine, called proteinuria
- low levels of a protein called albumin in your blood, called hypoalbuminemia
- swelling in parts of your body, called edema
- high levels of cholesterol and other lipids (fats) in your blood, called hyperlipidemia
Your kidneys are made up of about a million filtering units called nephrons. Each nephron includes a filter, called the glomerulus , and a tubule . The glomerulus filters your blood, and the tubule returns needed substances to your blood and removes wastes and extra water, which become urine . Nephrotic syndrome usually happens when the glomeruli are inflamed , allowing too much protein to leak from your blood into your urine.

Nephrotic syndrome is a combination of symptoms that can occur due to different causes. Among adults, the syndrome is most often caused by rare kidney diseases.
Nephrotic syndrome can affect children and adults of all ages. 1
Nephrotic syndrome can lead to serious complications, including 2
- blood clots that can lead to thrombosis
- higher risk of infection caused by the loss of immunoglobulins, proteins in your blood that help fight viruses and bacteria
- high blood pressure , also called hypertension
- brief or long-lasting kidney problems, including chronic kidney disease and kidney failure
Symptoms of nephrotic syndrome can include 3
- puffy eyelids and swelling in the legs, ankles, feet, lower abdomen, or other parts of your body
- foamy urine
- weight gain due to retaining too much fluid
- loss of appetite
Many disorders can cause nephrotic syndrome, including diseases that affect only the kidneys and diseases that affect many parts of the body, such as diabetes and lupus .
Kidney diseases
Diseases that affect only the kidneys and lead to nephrotic syndrome are called primary causes of nephrotic syndrome. The most common primary causes of nephrotic syndrome are 3
- Focal segmental glomerulosclerosis (FSGS). This disease affects the kidney’s glomeruli, causing some of these filters to become scarred. FSGS is the most common cause of nephrotic syndrome in Black adults.
- Membranous nephropathy . This disease causes protein to build up in a part of the kidney called the glomerular basement membrane. It is the most common cause of nephrotic syndrome in white adults.
- Minimal change disease . Also called nil disease, this disease is the main cause of nephrotic syndrome in children. Among adults, nephrotic syndrome is more common in older age.
Other causes
Other causes of nephrotic syndrome, also called secondary causes, include 3
- amyloidosis
- infections, such as HIV/AIDS , hepatitis B , and hepatitis C
- some allergic reactions
- some medicines, such as nonsteroidal anti-inflammatory drugs
- genetic disorders that affect the kidneys
Your health care professional can diagnose nephrotic syndrome through urine tests. The urine tests show if you are losing too much protein in your urine.
Tests for diagnosing nephrotic syndrome
Urine dipstick test. This simple test checks for albumin in your urine. Having albumin in the urine is called albuminuria . You collect the urine sample in a container during a visit to a health care professional’s office or lab. A health care professional places a strip of chemically treated paper, called a dipstick, into the urine for the test. The dipstick changes color if albumin is present in the urine.
To confirm the diagnosis of nephrotic syndrome, your health care professional may order one of these two urine tests
- 24-hour urine collection. For this test, you will need to collect urine samples over 24 hours. Your health care professional will then send the samples to a lab for analysis.
- Urine albumin-to-creatinine ratio (UACR). The UACR test uses a single urine sample to estimate the amount of albumin lost in 24 hours. The test measures both albumin and creatinine , a waste product of normal muscle breakdown.
Your health care professional may also order blood tests to check for low levels of protein in your blood and other problems linked to nephrotic syndrome.
Tests for identifying the cause
Once nephrotic syndrome has been diagnosed, your health care professional will use tests to identify what caused it and check your kidney function. Tests for finding the cause of nephrotic syndrome can include 3
- blood tests
- imaging tests, such as a kidney ultrasound
- kidney biopsy
Treating symptoms and complications
Treatment varies according to symptoms, causes, and the extent of kidney damage. Symptoms of nephrotic syndrome are most often treated with these medicines 3
- an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB) . ACEs and ARBs can help reduce protein loss and also lower blood pressure, which is often high in people with nephrotic syndrome.
- a diuretic (water pill), which reduces swelling by helping the kidneys remove fluid from the blood.
In some cases, your health care professional may also prescribe medicines that lower cholesterol, called statins . Blood thinners may also be used, but usually only if you develop a blood clot.
People with nephrotic syndrome should receive the pneumococcal vaccine , along with yearly flu shots, to prevent viral and bacterial infections.
Treating underlying causes
Other treatments vary, depending on underlying causes. In some cases, you may need to take medicines that suppress your immune system. For more on how health care professionals treat the underlying causes of nephrotic syndrome, see the NIDDK health topic Glomerular Diseases .
Once the cause has been treated, nephrotic syndrome may go away and kidney function returns to normal. Some patients may experience periods of remission followed by times when symptoms reappear. In some cases, nephrotic syndrome may lead to kidney failure .
Eating, diet, and nutrition have not been shown to play a role in causing or preventing nephrotic syndrome. However, if you have developed nephrotic syndrome, your health care professional may recommend that you
- limit intake of sodium (salt) and fluids to help control swelling
- reduce the amount of fat and cholesterol in your diet to help control your blood cholesterol levels
The NIDDK conducts and supports clinical trials in many diseases and conditions, including kidney diseases. The trials look to find new ways to prevent, detect, or treat disease and improve quality of life.
What are clinical trials for nephrotic syndrome?
Clinical trials—and other types of clinical studies —are part of medical research and involve people like you. When you volunteer to take part in a clinical study, you help doctors and researchers learn more about disease and improve health care for people in the future.
Researchers are studying many aspects of nephrotic syndrome, such as
- the kidney diseases that can lead to nephrotic syndrome, including genes that may cause these diseases
- quality of life among people with nephrotic syndrome
- how to best treat nephrotic syndrome
Find out if clinical studies are right for you .
Watch a video of NIDDK Director Dr. Griffin P. Rodgers explaining the importance of participating in clinical trials.
What clinical studies for nephrotic syndrome are looking for participants?
You can view a filtered list of clinical studies on nephrotic syndrome that are federally funded, open, and recruiting at www.ClinicalTrials.gov . You can expand or narrow the list to include clinical studies from industry, universities, and individuals; however, the National Institutes of Health does not review these studies and cannot ensure they are safe. Always talk with your health care provider before you participate in a clinical study.
This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public. Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts.
The NIDDK would like to thank: Andrew S. Bomback, M.D., Columbia University Irving Medical Center
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 30 September 2021
- Membranous nephropathy
- Pierre Ronco 1 , 2 ,
- Laurence Beck 3 ,
- Hanna Debiec 1 ,
- Fernando C. Fervenza ORCID: orcid.org/0000-0002-9952-209X 4 ,
- Fan Fan Hou ORCID: orcid.org/0000-0003-3117-7418 5 ,
- Vivekanand Jha ORCID: orcid.org/0000-0002-8015-9470 6 , 7 , 8 ,
- Sanjeev Sethi ORCID: orcid.org/0000-0002-4536-7709 9 ,
- Allison Tong 10 , 11 ,
- Marina Vivarelli 12 &
- Jack Wetzels ORCID: orcid.org/0000-0002-0650-6921 13
Nature Reviews Disease Primers volume 7 , Article number: 69 ( 2021 ) Cite this article
54k Accesses
161 Citations
134 Altmetric
Metrics details
- Autoimmunity
Membranous nephropathy (MN) is a glomerular disease that can occur at all ages. In adults, it is the most frequent cause of nephrotic syndrome. In ~80% of patients, there is no underlying cause of MN (primary MN) and the remaining cases are associated with medications or other diseases such as systemic lupus erythematosus, hepatitis virus infection or malignancies. MN is an autoimmune disease characterized by a thickening of the glomerular capillary walls due to immune complex deposition. Identification of the phospholipase A2 receptor (PLA2R) as the major antigen in adults in 2009 induced a paradigm shift in disease diagnosis and monitoring and several other antigens have since been characterized. Disease outcome is difficult to predict and around one-third of patients will undergo spontaneous remission. In those at high risk of progression, immunosuppressive therapy with cyclophosphamide plus corticosteroids has substantially reduced the need for kidney replacement therapy. Owing to carcinogenic risk, other treatments (calcineurin inhibitors and CD20-targeted B cell depletion therapy (rituximab)) have been developed. However, disease relapses are frequent when calcineurin inhibitors are stopped and the remission rate with rituximab is lower than with cyclophosphamide, particularly in patients with high PLA2R antibody titres. Other new drugs are already available and antigen-specific immunotherapies are being developed.
Similar content being viewed by others

IgA nephropathy
Eleni Stamellou, Claudia Seikrit, … Rafael Kramann

Membranous nephropathy: new pathogenic mechanisms and their clinical implications
Elion Hoxha, Linda Reinhard & Rolf A. K. Stahl
Lupus nephritis
Hans-Joachim Anders, Ramesh Saxena, … Chandra Mohan
Introduction
Membranous nephropathy (MN) is a pathologically defined disorder of the kidney glomerulus. The specific lesion is an apparent thickening of the glomerular capillary walls, which results from immune complex formation on the outer aspect of the basement membrane 1 . The immune deposits consist of immunoglobulin G (IgG), the relevant antigens and complement components, including the membrane attack complex (MAC). The consequence of the immunological conflict is the loss of large amounts of proteins in the urine (proteinuria), which is predominantly mediated by the pathophysiological disturbance of the podocyte structure caused by immune complex deposition and MAC formation. Patients experience a decrease in serum albumin levels and generalized oedema, which define a condition called nephrotic syndrome. Most patients report fatigue as an important symptom. MN accounts for ~30% of cases of nephrotic syndrome in adults and is only surpassed in prevalence among nephrotic non-diabetic glomerular diseases by podocytopathy presenting as focal and segmental glomerulosclerosis lesions in some populations (African and Hispanic American individuals). In ~80% of patients, there is no underlying cause of MN (primary MN (pMN)) and 20% are associated with medications, such as NSAIDs, or other diseases (secondary MN (sMN)) such as lupus (also referred to as systemic lupus erythematosus (SLE)), hepatitis B or hepatitis C, and malignancies 1 .
Patient outcomes are variable. For a long time, outcome was governed by the rule of thirds, with a third of the patients undergoing spontaneous remission, a third keeping variable levels of proteinuria and the remaining third progressing to advanced kidney failure. However, immunosuppressive therapy has substantially reduced the rate of kidney replacement therapy, which is ~10% with cyclophosphamide treatment but is not yet established for other newer agents such as rituximab 1 .
Substantial advances in the understanding of MN pathophysiology have occurred in the past two decades, starting with the identification of neutral endopeptidase (NEP), the first discovered human podocyte antigen, which is involved in a rare subset of patients with neonatal allo-immune MN 2 . This finding paved the way for the characterization of the M-type phospholipase A2 receptor (PLA2R) as another podocyte antigen in 2009. Antibodies to PLA2R are specific for MN and found in ~70% of adult patients with the disease 3 . This discovery demonstrated that pMN is an autoimmune disease in which the podocyte is both the target of circulating auto-antibodies and probably the main source of the auto-antigen. It induced a paradigm shift in thinking about establishing diagnosis (possibly without a kidney biopsy), predicting outcome and monitoring treatment in patients with pMN, including after transplantation. This discovery resulted in the rapid development of reliable assays for PLA2R antibodies and pMN can be considered as a model of organ-specific autoimmune disease and of personalized medicine. Subsequently, another podocyte antigen, thrombospondin type 1 domain-containing 7A (THSD7A) was identified 4 . THSD7A accounts for <5% of pMN 4 .
Owing to the technological advances in combining laser microdissection of glomeruli and mass spectrometry of solubilized digested proteins, several further antigens have been identified such as exostosin 1 (EXT1) and EXT2, protein kinase C-binding protein NELL1 (also known as neural epidermal growth factor like 1 protein), semaphorin 3B (SEMA3B), neural cell adhesion molecule 1 (NCAM1), protocadherin 7 (PCDH7) and serine protease HTRA1 (refs 5 , 6 ). These antigens, like PLA2R and THSD7A, can be associated with pMN or with a specific ‘cause’ of sMN, such as lupus, other autoimmune disease or cancer, recognizing that the link of causality is not established in many instances. The discovery of different antigens therefore challenges the classification that opposes pMN to sMN and leads to a discussion of an antigen-based classification in which each antigen-associated MN would have its specific immunological profile (IgG subclass), pattern of associated diseases and outcome. Future studies should evaluate whether management should be driven by the antigen specificity as is the case for PLA2R-associated MN, in which positive serology now replaces kidney biopsy and quantitative assessment of PLA2R antibody levels is helpful in predicting response to therapy. Within the coming few years, similar steps forward can be expected for the other antigens (although they are rarer and large patient cohorts will not be recruited). Thus, in this Primer, we adhere to the current nomenclature of pMN and sMN, while being mindful that it will need to be adapted based on evidence from future studies.
Despite these advances, treatment of MN remains controversial. For a long time, cyclophosphamide-based regimens were the standard of care as they have been shown to prevent the occurrence of advanced kidney failure; however, they expose patients to an increased risk of malignancy 1 , 7 . Treatment with calcineurin inhibitors (CNIs), such as cyclosporine or tacrolimus, induces a high rate of remission but with a high rate of relapse and renal toxic effects are a concern in prolonged treatment 1 , 7 . Therapy protocols with CD20-targeted agents, such as rituximab, are well tolerated but only 60–70% of patients reach persistent clinical remission compared with ~80% for cyclophosphamide and their efficacy in preventing kidney disease progression has not yet been proven 1 , 7 . Randomized controlled trials (RCTs) published in the past few years (GEMRITUX 8 , MENTOR 9 , STARMEN 10 and RI-CYCLO 11 ) have not clarified the dilemma between cyclophosphamide (more toxic) and CD20-targeted therapy (less efficient in the protocols used), which calls for new therapeutic approaches. The lack of RCTs with sufficient statistical power that directly compare these two treatments is a major reason for the enduring uncertainty. Furthermore, the dosage of rituximab is important to consider when effectivity is studied.
In this Primer, we summarize current knowledge of the epidemiology of MN and highlight the major advances that have led to a better understanding of MN pathophysiology, diagnosis and improved patient care. We also identify the major gaps in current knowledge and provide recommendations on how to improve long-term renal prognosis and quality of life.
Epidemiology
Incidence, prevalence and natural history.
MN occurs in all regions and all ethnicities. The data on the incidence of MN and MN subtypes remain quite limited as large population-based studies that are representative across diverse international populations are not available. The annual incidence rates of MN are estimated at 10–12 per million in North America and 2–17 per million in Europe 7 , 12 , 13 , 14 , 15 . The disease affects individuals of all ages with a mean age of diagnosis at 50–60 years 16 and a 2:1 male predominance for unknown reasons 17 . Whether incidence rates of MN vary between regions or ethnicities remains unclear as most data are from North America and Europe.
pMN is the most common cause of idiopathic nephrotic syndrome in non-diabetic adults worldwide, accounting for 20–37% in most kidney biopsy series and increasing to as high as 58% in adults >65 years of age 7 , 18 , 19 . MN is uncommon in children, accounting for <7% of biopsies 13 , 20 , 21 , 22 , 23 , 24 , and is often associated with other diseases such as hepatitis B 24 . The percentage of PLA2R-associated MN in adolescents is similar to that of adults.
No good data are available on the prevalence of the various aetiologies of sMN 1 , 25 . The main reason is that the epidemiology has varied with time, with infectious causes, such as hepatitis, strongly decreasing with vaccination, whereas others, such as lupus and drug exposure, are increasing.
In some regions, a temporal change in prevalence of MN has been reported 18 , 26 , 27 . A study in China analysed data from kidney biopsy samples of 71,151 patients in 938 hospitals in 282 cities taken over 11 years (2004–2014) encompassing all age groups 17 . The prevalence of MN increased by 13% annually, whereas the proportions of other major glomerulopathies remained stable. Areas with higher levels of fine particulate matter with diameters ≤2.5 µm (PM2.5) air pollution had the highest rates of MN. In areas with PM2.5 levels >70 μg/m 3 , each 10 μg/m 3 increase in PM2.5 concentration was associated with 14% higher odds for MN. This association was subsequently confirmed by another study in China 28 . However, whether the increase of MN is connected to a PLA2R-driven mechanism remains unclear as serum PLA2R antibody levels were not measured in most patients in these studies and whether air pollution is a causative risk of MN requires further confirmation.
The natural history of untreated MN has been reported with spontaneous complete remission rates of 20–30% and 10-year renal survival rates of 60–80% in most studies 29 , 30 , 31 . Despite its ‘benign’ presentation characteristics, MN has, for a long time, remained the second or third leading cause of kidney failure among the primary glomerulonephritis types in the USA and Europe 32 . In patients who continue to have nephrotic syndrome, kidney failure develops in 40–50% over a period of 10 years 33 . These patients are also at an increased risk of life-threatening thromboembolic and cardiovascular events 34 .
Genetic factors
MN is not a typical hereditary disease in Mendelian terms but there is growing evidence to support a strong genetic component. Like most autoimmune diseases, MN has a strong association with the class II antigens of the human leukocyte antigen (HLA) system that are encoded by the relevant alleles of the HLA-D locus on chromosome 6. HLA class II molecules are involved in the regulation of immune responses: their expression is mostly limited to antigen-presenting cells, such as B cells, monocytes, macrophages, dendritic cells and Langerhans cells of the skin, and their role is to present peptide antigens to the immune system. Thus, they are associated with autoimmune diseases. It has been known since 1979 and 1989 that MN is strongly associated with HLA-DR3 and HLA-DQA1, respectively, in Caucasian populations 35 , 36 . An initial genome-wide association study conducted in three European cohorts including 556 patients of white ancestry found that pMN in adults was strongly correlated with risk alleles in HLA-DQA1 (chromosome 6) and in PLA2R1 (chromosome 2) 37 . In individuals who were homozygous for the lead risk alleles, the risk of having MN was nearly 80-fold higher than in those who had neither risk allele. Interestingly, the risk allele that is most significantly associated with pMN is localized in an intron of the gene; thus, this allele is not expected to change PLA2R antigen auto-reactivity. Further studies did not detect mutations or rare variants in the sequence and the splice sites of PLA2R1 (ref. 38 ). Thus, it is unlikely that the immune reaction is triggered by a change in antigen sequence or conformation. It is more probable that post-translational modifications or increased expression of PLA2R antigen in podocytes or other cells have an important role.
A similar strong correlation between risk alleles of PLA2R1 and HLA-DQA1 and the risk of developing MN was found in a large Chinese cohort that included 1,112 patients with pMN and 1,020 healthy individuals 39 . Patients with both risk alleles had an >11-fold increased risk of developing MN. The presence of risk alleles was also correlated to PLA2R antibodies.
Two studies performed in Chinese cohorts revealed different HLA class II alleles associated with an increased risk of MN 40 , 41 and proposed that HLA associations may differ between ethnicities. This was corroborated in the largest international genetic study of MN performed to date. The study involved cohorts from East Asia (1,632 biopsy specimen-diagnosed cases and 3,209 controls) and of European ancestry (2,150 biopsy specimen-diagnosed cases and 5,829 controls) and found ethnic differences in HLA locus associations, defining DRB1*1501 as a major risk allele in East Asian individuals, DQA1*0501 in European individuals and DRB1*0301 in both ethnicities 42 . The PLA2R1 locus presented the most strongly associated non-HLA signals. In addition, two previously unreported loci, NFKB1 and IRF4 , which are both linked to inflammatory pathways, were identified. Genome-wide association study loci account for 32% and 25% of disease risk in East Asian and European individuals, respectively.
In addition to their likely role of presenting PLA2R antigen to the immune system, HLA-D alleles may also be modifiers of MN. Although DRB1*1501 is a major risk allele for the disease, DRB1*1502 , which differs from DRB1*1501 by a single amino acid, is not. However, DRB1*1502 has a strong predictive value when associated with HLA risk alleles, being associated with a lower estimated glomerular filtration rate (GFR) and a higher risk of kidney failure 43 . No data are available concerning genetic predisposition for the other antigens or sMN.
Mechanisms/pathophysiology
In the past 20 years, considerable advances have occurred in the understanding of the pathophysiology of MN with the description of the first podocyte antigens in neonatal 2 and adult 3 MN. These discoveries revealed the central role of the podocyte in MN pathogenesis. This section describes the classical antigens NEP, PLA2R and THSD7A, the newly reported potential antigens, the autoimmune reactions that lead to the development of antibodies, and the downstream events that lead to nephropathy.
In the 1970s, studies in the Heymann nephritis rat model of MN established the basis of MN pathophysiology. The studies showed that rats express megalin both in the proximal tubular brush-border and in podocytes and that actively or passively introduced megalin antibodies induced the aggregation of immune complexes at the basal surface of podocytes. This observation has led to the concept that a podocyte antigen, megalin (now called LRP2), could serve as a target of circulating antibodies leading to the in situ formation of immune complexes 44 , 45 , 46 . In humans, where megalin is not or only weakly present on podocytes 36 , 47 and cannot therefore be the target of circulating antibodies, NEP was identified in 2002 as the responsible antigen in a subset of patients with allo-immune antenatal MN 2 . In this rare condition, mothers carry a homozygous or compound heterozygous nonsense mutation in MME (encoding NEP), which results in the absence of the protein 48 (Fig. 1 ). During pregnancy, they become immunized to the paternally inherited NEP in the placenta and the resulting maternal IgG antibodies are then transferred to the fetus, which is not NEP-deficient and therefore develops active disease.
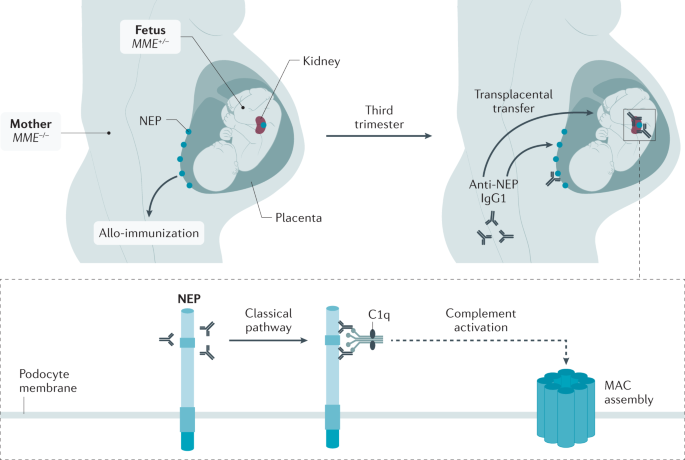
Antenatal membranous nephropathy is the result of a homozygous or compound heterozygous nonsense mutation in MME (encoding neutral endopeptidase (NEP)) in the pregnant mother, which results in the absence of NEP. The MME –/– pregnant mother develops allo-imunization against NEP that is expressed by the placenta (via paternal inheritance to the fetus) and recognized as a non-self protein by the mother’s immune system. In the third trimester of pregnancy, NEP antibodies are transferred to the MME +/– fetus and bind to the NEP antigen expressed on podocytes of the fetus, who develops active disease. NEP IgG1 antibodies bind to epitopes on NEP and activate the classical complement pathway through binding of complement component C1q to the NEP IgG1 antibodies, leading to the formation of C5b–9 (membrane attack complex of complement (MAC)). MAC insertion into the podocyte membrane induces cell damage. No podocyte damage occurs in fetuses from mothers producing only IgG4, which does not bind C1q and therefore fails to activate the complement cascade.
NEP is a membrane-bound zinc-dependent endopeptidase and is involved in the catabolism of regulatory peptides with vasoactive properties 49 , 50 . NEP is expressed in numerous tissues and its membrane form is naturally present on human podocytes at the sole of their foot processes 51 . Maternal production of complement-fixing NEP IgG1, which also inhibits NEP enzyme activity, is necessary for the disease to develop 52 . This suggests that some toxic effects of NEP antibodies may be associated with alterations of glomerular haemodynamics, endothelial permeability or tubular function. Analysis of these very rare cases strongly supports the idea that the circulating antibodies react with an intrinsic component of the podocyte membrane. Moreover, these observations suggest that similar truncating mutations of other podocyte antigens could lead to allo-immunization and renal disease in infants.
PLA2R is the most frequently targeted auto-antigen in MN (up to 80% of pMN cases) (Table 1 ). The antigen was initially identified by mass spectrometric analysis of an electrophoretic gel band that corresponded to a glycoprotein recognized by serum from patients with idiopathic (primary) MN but not by serum from patients with proteinuria or healthy controls 3 . PLA2R is a transmembrane glycoprotein abundantly expressed by the human podocyte 3 , present at the level of the foot process as well as on the apical surface, where it can be shed into the urine in vesicular structures during disease 53 . Its specific role in the glomerulus is not known. As some small mammals lack constitutive podocyte PLA2R expression, PLA2R may not be essential to podocyte function. Initially identified as a receptor for secreted phospholipase A2 enzymes 54 , PLA2R might bind and internalize these small and potentially toxic enzymes that pass through the glomerular basement membrane (GBM). The extracellular domain of PLA2R comprises 10 domains: an N-terminal cysteine-rich (CysR) domain, a fibronectin 2 domain and eight C-type lectin-like domains (CTLD) that harbour distinct humoral epitopes.
The auto-antibodies that form against PLA2R initially target the N-terminal immunodominant CysR region and a short amino acid sequence in this domain has been shown to stimulate the production of very high-affinity antibodies 55 . In most patients, epitope spreading and the production of antibodies to distal regions of the extracellular domain occurs, including CTLD1, CTLD7 and CTLD8 (refs 56 , 57 ). The detection of epitope spreading in a patient seems to confer an inferior prognosis compared with individuals who have CysR antibodies only 58 , although this notion has been challenged 57 . The predominant antibodies to PLA2R are of the IgG4 subclass but other subclasses, such as IgG1 and IgG3, are present in lower amounts 3 , 59 . Quantification of the overall titre of PLA2R antibodies has been a useful tool in monitoring the immune response to therapy in PLA2R-associated MN 13 and the decline and disappearance of PLA2R antibodies has been termed immunological remission and precedes and predicts clinical remission.
Thrombospondin type 1 domain-containing 7A (THSD7A) is a multidomain transmembrane glycoprotein expressed by the podocyte that serves as an auto-antigen in 2–3% of patients with MN 60 . Similar to PLA2R, it was identified by performing mass spectrometry on native, deglycosylated or proteolysed protein bands detectable by human auto-antibodies. Its presence in the glomerulus had not been demonstrated before its discovery as an MN target antigen, although it is now recognized as a conserved basal component of the podocyte, localizing directly between the slit diaphragm and the GBM 61 . No immunodominant epitope has been discovered and auto-antibodies seem to target multiple regions of the protein, including the N terminus 62 . Several cases of THSD7A-associated MN have been found in which a malignancy overexpresses THSD7A and may initiate the immune response that then causes MN in the kidney 63 , 64 . There are fewer data on using THSD7A antibodies than on PLA2R antibodies for monitoring of disease but THSD7A antibodies, similar to PLA2R antibodies, tend to decline with immunosuppressive treatment and their disappearance is associated with eventual clinical remission.
New and putative antigens
Laser microdissection of glomeruli followed by mass spectrometry has been used to identify novel antigens in MN 65 . The basic premise in the identification of an antigen is that the novel antigen has accumulated in the glomeruli (immune deposits) compared with other proteins and is unique to a subset of MN. Confirmation of the novel antigen is then provided by immunohistochemistry and/or immunofluorescence studies that show granular subepithelial staining of the novel antigen along the capillary walls, followed by confocal immunofluorescence studies that show co-localization of the novel antigen with IgG along the capillary walls, ideally elution of IgG from frozen biopsy material to show that the deposited IgG is specific for the novel antigen and, finally, serum western blot analysis to show that circulating antibodies to the novel antigen are present in the serum.
Six candidate antigens have been discovered using these techniques in the past few years (Table 1 ). Putative antigens EXT1 and EXT2 were discovered in 2019, NELL1 and SEMA3B in 2020, and PCDH7, HTRA1 and NCAM1 in 2021. EXT1 and EXT2 were the first novel proteins found in a subset of patients with MN. Granular EXT1 and EXT2 deposits were detected in MN secondary to autoimmune disease, mostly lupus 65 . Patients with MN associated with EXT1 and EXT2 deposits were young, predominantly female and their kidney biopsy samples showed features of MN usually associated with an autoimmune disease such as a full-house pattern of immunoglobulin on immunofluorescence studies, tubuloreticular inclusions in the endothelial cells and mesangial deposits. However, antibodies to EXT1 and EXT2 have yet to be found and these proteins are considered putative antigens. Positive staining for EXT1 and/or EXT2 is found in 30–40% of pure lupus MN (LMN; also referred to class V lupus nephropathy) and in around the same percentage of mixed class lupus nephropathy, that is, class III or class IV associated with class V. Patients with LMN and positive staining for EXT1 or EXT2 have fewer chronicity features, including glomerulosclerosis, tubular atrophy and interstitial fibrosis, and also have a better prognosis than those who are negative for EXT1 and EXT2 (refs 66 , 67 ).
NELL1 seems to be the second most common antigen following PLA2R. It is present in most patients with MN and no underlying disease association (pMN) but is also present in some patients with malignancy (sMN) 68 , 69 . One of the unique features of kidney biopsy samples is that staining for NELL1 can be segmental in some of the glomeruli 69 , 70 .
SEMA3B is another unique antigen in that it is primarily present in children (<2 years of age) and young adults. In children, kidney biopsy samples also show IgG staining along the tubular basement membrane. Interestingly, the tubular basement membrane deposits are negative for SEMA3B 71 . NCAM1 was identified as an antigen in both pMN and LMN 72 . Patients with NCAM1-associated LMN were younger, predominantly female and had a history of lupus, all of which are distinct features from those seen in the usual patients with MN. Similar to LMN associated with EXT1 and EXT2, NCAM1-associated LMN is not restricted to pure class V LMN but is also present in some patients with LMN who also have proliferative lupus nephritis.
PCDH7-associated MN is present in patients with a mean age of 63 years, close to that of the most common form of MN 73 . Most patients seem to not have an associated underlying disease but ~20% of patients with PCDH7-associated MN have a history of malignancy. Kidney biopsy samples lack complement deposits on immunofluorescence study. In addition, non-nephrotic-range proteinuria (proteinuria <3 g/day) is observed and remission follows conservative management in some patients with PCDH7-associated MN.
HTRA1-associated MN is present in patients with a mean age of 67 years and characterized by an IgG4-dominant subclass in the immune deposits without association to another disease except for anti-neutrophil cytoplasmic antibody-associated vasculitis in 1 of 14 patients 74 . Sera from two patients reacted by immunoblotting with glomerular extracts and with recombinant human HTRA1 under reducing and non-reducing conditions. Longitudinal sampling in these two patients suggests that HTRA1 antibody levels are correlated with clinical activity.
Whether these new antigens are true antigens or biomarkers is controversial as they have only been characterized in the past few years. As described, each antigen-associated MN has its own specific features, including demographic characteristics, immunopathological and sometimes morphological features, and associated diseases. The minimal definition of an antigen is the presence of the relevant antibodies in the blood and, ideally, in biopsy samples. Although co-localization of antigen and antibody by confocal microscopy is suggestive, definitive evidence of the reactivity of the deposited antibody against the potential antigen requires elution experiments that are hampered by the small size of the tissue specimen. Among the new potential antigens, such demonstration has only been achieved for PCDH7 (ref. 73 ). Further evidence of the pathogenic effect of the antibodies can be provided by correlation of antibody levels with clinical course, early recurrence in the transplanted kidney and development of experimental models. A few cases showing the parallel outcomes of immunological and clinical activity have been reported 69 , 71 , 73 , 74 . No observation of early recurrence after transplantation has been reported and animal models are not yet available.
Of note, more than a decade after the discovery of PLA2R, the only experimental model of PLA2R-associated MN is with the mouse antigen 75 and evidence of pathogenicity in humans mostly relies on early recurrence after transplantation 76 , 77 and time-course measurement of PLA2R antibody levels that precedes proteinuria 78 , 79 . Yet, the discovery of the role of the PLA2R antibody in MN has induced a paradigm shift in patient care, showing the way for future well-conducted clinical studies that aim to define the diagnostic and predictive values of potential antigens involved in MN. The pathophysiology of these new antigens is essentially unknown and will require further studies.
Exogenous antigens
Exogenous proteins, whether they are present or not in humans, can induce the production of allo-antibodies or xeno-antibodies , respectively. Important features of these proteins are their specific physicochemical properties that may lead to their trapping in the GBM. The first observation implicating a food antigen was reported in children with cationic bovine serum albumin (BSA)-related MN 80 . This observation was also inspired by an experimental model 81 , 82 . Some patients, mostly infants, with MN had high serum titres of anti-BSA antibodies reacting with one peptide region of BSA but not with human serum albumin. Only infants had circulating cationic BSA, which could interact with the negatively charged glomerular capillary wall. Why cationic BSA was formed is not known but differences in food processing or in the intestinal microbiota might be responsible for BSA modifications.
Other food antigens or non-dietary antigens from the environment might also be involved in MN. Extremely rare forms of allo-immune MN have been described in children affected with rare lysosomal storage diseases (mucopolysaccharidosis type VI and Pompe disease) receiving enzyme replacement therapy 83 , 84 . Because of the absence of enzymes, therapeutic proteins are potential allo-antigens that trigger immunization.
Autoimmune reactions
Regardless of the target antigen, the common denominator in MN is the accumulation of discrete deposits containing immunoglobulin and antigen that form and expand beneath the basal surface of the podocyte (Fig. 2 ). The mechanisms underlying the loss of tolerance to these self-antigens are not well understood but likely involve genetic factors, heightened expression of target antigens owing to polymorphisms in regulatory elements, upregulation by environmental factors, or even pathologic and dysregulated production of the antigen as might occur in malignancy. It is also not known why some patients have circulating antibodies well before the onset of nephrotic syndrome and even proteinuria 85 .
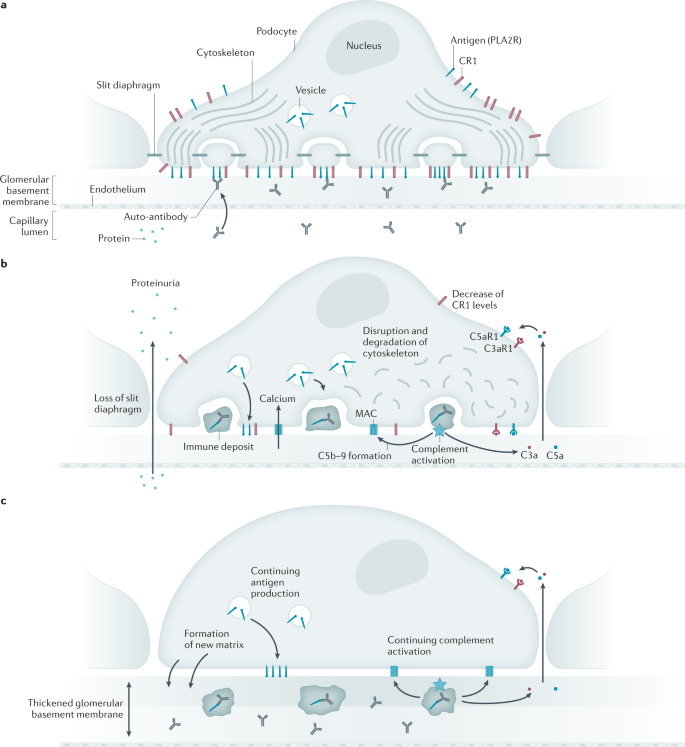
a | At the initiation of the humoral autoimmune response, the podocyte expresses phospholipase A2 receptor (PLA2R) and complement inhibitors such as complement receptor 1 (CR1) at the cell surface and has a cytoskeleton reflective of the differentiated podocyte state, with interdigitating foot processes bridged by slit diaphragms. Circulating auto-antibodies begin to target PLA2R on the basal aspect of the podocyte. Ongoing synthesis and delivery of PLA2R to the cell surface enables continued deposit formation and growth in the presence of circulating auto-antibodies. b | Immune deposits (stage 1) containing antigen and immunoglobulin form beneath the podocyte, where they begin to activate complement. The terminal complement components C5b–9 (membrane attack complex (MAC)) insert into the podocyte membrane and enable calcium influx, which initiates several maladaptive pathways. C3a and C5a generated by the complement cascade bind to and activate their cognate receptors C3aR1 and C5aR1, stimulating pathways that lead to the degradation of cytoskeletal elements. CR1 is downregulated, which enables complement-mediated cytotoxicity. The result is the simplification of the podocyte cyto-architecture, loss of slit diaphragms and increased flux of protein into the urinary space. PLA2R continues to be produced by the podocyte, leading to an increased mass of the immune deposits and ongoing complement activation. c | With ongoing injury, the podocyte secretes additional extracellular matrix components between (stage 2) and around (stage 3) the immune deposits, leading to the increased overall thickness of the glomerular basement membrane.
Several studies suggest an overall dysregulated immune phenotype in MN 86 , 87 characterized by a decreased proportion of regulatory T cells among all lymphocyte subpopulations in untreated patients with MN 88 . Numbers of plasma cells and regulatory B cells were statistically higher in patients with MN compared with healthy individuals or individuals with non-immune kidney disease and the amount of in vitro-expanded PLA2R-specific memory B cells could be correlated with circulating PLA2R antibody titres 89 . The specific site at which the immune response to PLA2R is initiated remains speculative 90 but intrarenal B cells in tertiary lymphoid follicles have been noted 91 , 92 . These tertiary follicles could represent the propagation of the immune response triggered by PLA2R shed from podocytes as exosomes into the tubular lumen 93 and later captured by intrarenal dendritic cells. The observation that the risk of recurrence after kidney transplantation is dependent on the donor’s gene variants of HLA-D and PLA2R1 can be seen as further evidence of a kidney-based source of antigen exposure 90 .
The paradigm of podocyte injury induced by the subepithelial immune deposits was elucidated in the experimental rat model of Heymann nephritis 94 . In this model, activation of the complement system and assembly of the terminal complement components C5b–9 were both necessary to induce podocyte injury and proteinuria. In the absence of complement-activating IgG or factors such as complement component C6, subepithelial deposits would form but no further injury would occur. Evidence for complement activation in human MN has been circumstantial and based on the consistent presence of specific complement factors within the subepithelial deposits. In routine immunofluorescence staining, IgG and C3 are present but C1q is usually weak or absent, suggesting that the classical complement pathway activation has a minor role in established disease. However, the fact that early deposits contain more IgG1 and IgG3 whereas later-stage deposits are enriched in IgG4 suggests that the classical complement pathway might initiate disease, which is then propagated by the alternative pathway or the lectin pathway 95 . The consistent presence of C4 (ref. 96 ) and of mannan-binding lectin (MBL) in many 97 but not all 98 patients with MN implicates the lectin pathway, but many components of the alternative pathway are also detected when assayed by mass spectrometric techniques 99 . The common final pathway is thought to involve the insertion of C5b–9 (also known as the MAC) channels into the podocyte membrane. This breach of cell integrity causes sublethal cell injury and the activation of maladaptive pathways causing cytoskeletal disassembly, loss of slit diaphragms and, over time, the production of normal and ectopic basement membrane elements 100 .
Mouse models using passive transfer of human or rabbit antibodies to PLA2R 75 or THSD7A 101 , 102 have been developed but they have not yet convincingly established a definite role for complement. Sophisticated co-culture models, such as the glomerulus-on-a-chip, hold promise for investigating the harmful effects of human anti-podocyte antibodies 103 . In a culture model of PLA2R-expressing podocytes, PLA2R IgG4 antibodies, especially those bearing glycan chains that lack terminal galactose residues, stimulated the lectin pathway through MBL and MBL-associated serine proteases to cause podocyte injury 104 . A novel finding from this work is the upregulation of receptors for C3a and C5a on the podocyte, also observed in human MN biopsy specimens, that may augment the cytotoxicity instigated by C5b–9 through cytoskeletal degradation in response to the anaphylatoxins generated by subepithelial activation of complement.
Research continues into non-complement-dependent mechanisms, for example, direct interference in normal pathways by the auto-antibodies. THSD7A antibodies seem likely to have direct effects on the slit diaphragm structure and function 102 , 103 given the close association of THSD7A with this structure. Intermolecular epitope spreading to intracellular antigens 105 or complement regulatory factors 106 may also augment the injury process.
Nephropathy
The downstream effects of injury caused by the subepithelial deposits are enormous, starting with the failure of the glomerular filtration barrier owing to the complement-mediated podocyte injury and the spilling of massive amounts of protein into more distal nephron segments. One consequence of delivery of these large amounts of protein, including specific proteases, to the distal nephron is the activation of the epithelial sodium channel ENaC, which is responsible for sodium reabsorption in the collecting duct, causing volume overload, weight gain, and localized oedema or generalized anasarca . The imbalance of promoters and inhibitors of the coagulation system in the setting of increased urinary losses and hepatic synthesis favours the thrombophilic state , which is more pronounced in MN than in other nephrotic disorders for as yet unknown reasons 107 , 108 . Deep vein thromboses, renal vein thrombosis and pulmonary embolism are all potential consequences of the nephrotic state in MN and are associated with the severity of hypoalbuminaemia 108 , 109 . Other consequences of the nephrotic state include mixed hyperlipidaemia, vitamin D deficiency and a general state of immunosuppression due to urinary loss of innate and humoral immune effectors such as complement factors and immunoglobulins. Similar to other glomerular diseases with sustained proteinuria over months to years, renal function can decline with progressive tubular atrophy and interstitial fibrosis 33 . Patients who develop end-stage MN and receive a kidney transplant are at risk of recurrence of the disease in the allograft if the circulating antibodies are still present or recur after transplantation.
Diagnosis, screening and prevention
Clinical presentation.
Nephrotic syndrome, defined as proteinuria >3.5 g per day and serum albumin <3.5 g/dl (when measured by bromocresol green) or <3.0 g/dl (when measured by bromocresol purple or immunonephelometric methods), respectively, is present in around two-thirds of patients with MN at presentation and can be severe 1 , 110 . The other third of patients present with asymptomatic proteinuria, usually ≤3.5 g per day. In most patients, GFR is normal and urinary sediment is unremarkable, although microscopic haematuria may be present in <25% of patients. Hypertension at presentation is uncommon (<20% of patients), which explains why most patients do not tolerate high-dose angiotensin II blockade. Thromboembolic events are reported in up to 8% of patients, with renal vein thrombosis accounting for 30% (Box 1 ). Hyperlipidaemia is common and characterized by an increase in total and LDL cholesterol and a decrease in HDLs, which is associated with a markedly increased risk of both myocardial infarction and coronary death compared with that of healthy individuals as well as with an increased risk for thromboembolism 111 . Rare cases of crescentic MN, usually presenting with proteinuria >3.5 g per day, haematuria and reduced GFR, in the absence of anti-neutrophil cytoplasmic antibodies or GBM antibodies, have been reported 112 .
Box 1 Complications of membranous nephropathy and its treatments
Nephrotic syndrome
Tiredness, oedema, dyspnoea, nausea, anorexia, ascites, venous thrombosis, arterial thrombosis, infections, kidney failure.
Hypokalaemia, hyponatraemia, hypomagnesaemia, alkalosis, gout, ototoxicity (with high-dose furosemide), muscle aches.
Prednisolone
Cushing face, obesity, skin bruising and striae, diabetes, osteoporosis, cataracts, infections, wound healing.
Cyclophosphamide
Anaemia, leukocytopenia, thrombocytopenia, infections, nausea, anorexia, bladder mucosal irritation with haematuria, liver function abnormalities, hair loss, infertility, malignancy, myelodysplasia.
Calcineurin inhibitor
Nephrotoxicity, diabetes, hair growth, gingiva hyperplasia, hypertension, liver dysfunction, neurotoxicity.
Infusion reaction, infections, hypogammaglobulinaemia, antibody formation.
See also Supplementary Table 1.
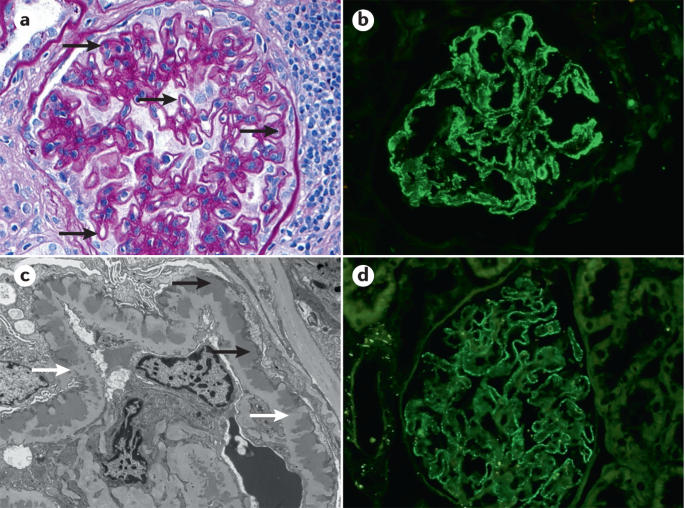
Histopathology
Kidney biopsy is the standard diagnostic approach for MN. On light microscopy, early stages of MN may show normal-appearing GBMs 113 . At later stages, basement membrane spikes and pinholes can be seen on silver methenamine and periodic acid-Schiff stains (Fig. 3 ).
a | Light microscopy image showing thickened glomerular basement membranes (arrows; periodic acid-Schiff stain, 40×). b | Immunofluorescence microscopy image showing granular staining for IgG along the capillary walls (40×). c | Electron microscopy image showing subepithelial electron-dense deposits (black arrows) and basement membrane material between the electron-dense deposits (white arrows; 4,800×). d | Immunofluorescence microscopy image showing staining for phospholipase A2 receptor (PLA2R) along the capillary walls (40×).
Proliferative features, such as mesangial and endocapillary proliferation, are typically absent 113 . In very rare cases, a concurrent crescentic pattern of injury is observed. In immunofluorescence microscopy, diffuse and granular staining for IgG, C3, and κ and λ light chains is seen along the capillary walls in pMN. In electron microscopy, numerous electron-dense deposits are seen in the basement membrane beneath the podocytes that show extensive foot process effacement even at the early stages. These subepithelial deposits are separated by expansions of basement membrane material or covered with extracellular matrix at later stages 114 .
Four stages are defined according to the location of the subepithelial deposits and matrix accumulation: stage 1, sparse small deposits without thickening of the GBM; stage 2, more extensive subepithelial deposits with the formation of basement membrane spikes between the deposits and thickening of the GBM; stage 3, a combination of stage 2 with deposits completely surrounded by basement membrane (intramembranous deposits); and stage 4, incorporation of deposits in the GBM, which are irregularly thickened (burned-out disease). In stage 4, the deposits are often fading, becoming less electron dense.
Features suggestive of sMN include mesangial or endocapillary proliferation; a full-house pattern of immunoglobulin staining, including staining for IgA and C1q on immunofluorescence microscopy; mesangial and/or subendothelial electron-dense deposits or deposits along the tubular basement membrane and vessel walls; substructures in the deposits; and endothelial tubuloreticular inclusions on electron microscopy 115 . The presence of scant superficially scattered subepithelial deposits on electron microscopy suggests drug-associated or malignancy-associated sMN. Staining for IgG subclasses may help in differentiating pMN from sMN. IgG1, IgG2 and IgG3 predominate in class V lupus nephritis, whereas IgG4 is the prevailing subclass associated with variable amounts of IgG1 in PLA2R-associated and THSD7A-associated pMN 69 , 71 , 116 . The prevailing subclass in MN associated with newly characterized antigens is IgG1 (ref. 117 ). In early reports in malignancy-associated sMN, IgG4 staining was usually absent 118 . However, one study found no differences in the IgG subclass distribution between patients with pMN and those with malignancy-associated sMN 119 . Furthermore, levels of antigen-specific IgG4 antibodies were not different between primary and malignancy-associated sMN and levels of all IgG subclasses did not differ between these groups.
Staining of kidney biopsy samples for PLA2R can also diagnose PLA2R-associated MN in patients who have negative PLA2R antibody serology 80 . This might occur if serum samples are collected when the patient is in immunological remission either spontaneously or following immunosuppressive therapy or PLA2R antibody serology may be falsely negative early in the disease course owing to the phenomenon of the kidney behaving like a ‘sink’ 120 . In this scenario, circulating PLA2R antibodies bind to the target antigens on the podocyte and are rapidly cleared from the blood. Only when the antibody production rate exceeds the buffering capacity of the kidney will seropositivity become apparent. Hence, serial assessment of PLA2R antibody levels should be performed in patients with positive glomerular PLA2R staining who are initially seronegative but have persistent proteinuria 12 . By contrast, positive PLA2R antibody serology and negative glomerular PLA2R staining are uncommon 121 , 122 and very likely reflect differences in staining techniques 123 .
Indications for kidney biopsy
Kidney biopsy is costly and can result in major complications 123 , 124 , 125 , 126 , 127 . Given the high specificity of PLA2R antibodies in patients with MN and the fact that PLA2R antibodies have not been detected in other glomerular diseases or healthy individuals, deferral of a kidney biopsy has been suggested in patients who have nephrotic syndrome and PLA2R antibodies 12 , 19 . This proposal has been supported by a study that showed that, in patients with preserved kidney function (eGFR >60 ml/min/1.73 m 2 ), no evidence of secondary cause (Box 2 ) and no diabetes mellitus, a positive serum PLA2R antibody titre equals a 100% probability of diagnosing MN and is therefore a reliable non-invasive method for the diagnosis of pMN 128 . In these patients, kidney biopsy did not provide any valuable information that altered treatment. This non-invasive diagnostic approach might be especially important for those at high risk of complications or in whom a kidney biopsy is contraindicated 129 . However, if kidney function is impaired or the patient has evidence of a potential secondary cause for MN, including diabetes mellitus, a kidney biopsy is needed to exclude concomitant kidney disease (for example, underlying diabetic nephropathy, acute interstitial nephritis or crescentic glomerulonephritis) and to estimate the extent of interstitial fibrosis and tubular atrophy, which may add useful information to guide management. Box 2 provides guidelines for the evaluation of associated conditions in patients with MN regardless of serology. The non-invasive diagnostic approach is supported by the new Kidney Disease: Improving Global Outcomes (KDIGO) guidelines on the management of glomerular disease 130 . In patients with a PLA2R − biopsy sample, staining for THSD7A, EXT1, EXT2, NELL1, NCAM1, SEMA3B, PCDH7 and HTRA1 antibodies should be performed depending on age, immunofluorescence pattern (segmental) and associated condition (autoimmunity).
Box 2 Evaluation for associated conditions (regardless of serology)
Phospholipase A2 receptor (PLA2R) and thrombospondin type 1 domain-containing 7A (THSD7A) antibody testing
Complete blood count
Extensive laboratory analysis with serum albumin
24-hour urine collection for protein quantification a and creatinine clearance
Antinuclear antibodies and anti-double-stranded DNA antibodies
Hepatitis B and C virus serology
Serum C3 and C4 complement levels
Monoclonal protein studies, including serum-free light chains and serum protein immunofixation
Age-appropriate cancer screening
Sarcoidosis
Autoimmune thyroiditis
Drug exposure
NSAIDs, penicillamine, elemental mercury, antitumour necrosis factor agents
Bone marrow transplant and graft-versus-host disease
IgG4-related disease
In patients with pancreatitis, tubulointerstitial nephritis or sialadenitis
Rare infectious causes
Schistosomiasis, malaria (specifically quartan fever), and congenital and secondary syphilis
The initial work-up for associated conditions may vary depending on local clinical practice. a A reasonable compromise is to collect an ‘intended’ 24-hour urine sample and measure a urinary protein to creatinine ratio in an aliquot of the collection.
pMN, sMN and a new antigen-based classification
In ~80% of patients, MN appears without an underlying cause and presents as a kidney-specific autoimmune disease. The target antigen in ~70% of patients is PLA2R, followed, in decreasing percentages, by NELL1, PCDH7, THSD7A, HTRA1 and SEMA3B (mainly in children and young adults), NCAM1, and unknown in the remaining 10–15% of patients with pMN. In ~20% of patients, MN occurs in association with other clinical conditions and is categorized as sMN 5 . Patients with pMN and those with sMN have similar clinical renal presentations.
However, this dichotomization of MN into primary and secondary has been challenged 5 , 131 . The complexity arises from the fact that clinical findings of the presence of antibodies do not accurately align with the definitions of pMN and sMN. Some patients with apparently sMN are also positive for PLA2R antibodies, most commonly those with hepatitis B virus (HBV) or hepatitis C virus (HCV) infection or sarcoidosis 132 , 133 , 134 , 135 . Whether these patients have true sMN or coincidental PLA2R-associated MN with a secondary disease is unclear. The facts that patients may enter spontaneous remission without treatment of the secondary cause or that treatment of the secondary cause does not result in remission of MN support the view that these antibodies occur coincidentally 136 . The same is true for patients with THSD7A-associated MN, for which an association with malignancy exists 64 , 137 . In these patients, remission has been observed with treatment of the malignancy alone 63 , 64 , 137 , 138 , but most patients with THSD7A-associated MN, with or without concomitant malignancy, respond to immunosuppression therapy 139 , 140 . In addition, most patients with NELL1-associated MN present without evidence of concomitant disease, but a concomitant malignancy is present in some of these patients 68 , 69 , 137 . Thus, a new molecular classification for MN based on target antigen has been proposed 5 , 131 . Future studies should enable the proposition of a better disease classification based on the presence of antibodies and the antigen specificity as well as the proven association (or not) with an underlying cause.
PLA2R antibody assays and identification of other target antigens
Various assays are available to detect PLA2R antibodies. The most commonly used assay is the ELISA commercialized by Euroimmun, which is 99.6% specific and enables the quantification of PLA2R antibody levels. The indirect immunofluorescence assay (IFA) from the same provider is a bit more sensitive than the ELISA and is 100% specific but does not enable quantitative assessment 141 . The currently recommended reference ranges for the ELISA assay are <14 relative units (RU)/ml (negative), 14–20 RU/ml (borderline) and >20 RU/ml (positive). Improved sensitivity without affecting specificity has been suggested by reducing the cut-off from 20 RU/ml to 2 RU/ml (refs 142 , 143 ) or by using a combination of IFA and ELISA 128 . In this study, all patients with ELISA values >2 RU/ml and <20 RU/ml and a positive IFA had MN confirmed on biopsy. An addressable laser bead immunoassay, showing similar performance to the IFA assay, has been developed but is not clinically available 144 . ELISA and IFA remain less sensitive than western blot analysis (not used in clinical practice) and antigen staining of the kidney biopsy sample 121 , 145 . Thus, these techniques may fail to detect PLA2R antibodies in patients with proven PLA2R-associated MN.
An indirect immunofluorescence assay for THSD7A antibodies is commercially available (Euroimmun) but tests for NELL1, NCAM1, SEMA3B, PCDH7 and HTRA1 antibodies have not yet been developed. However, antibodies specific for the antigens are commercially available and can be used for the detection of antigen after retrieval in paraffin-embedded kidney biopsy samples 117 .
MN and malignancies
The prevalence of malignancy in patients with MN is estimated at 6–22%, most commonly occurring in patients >60 years of age with most cancers discovered before or at the time of the diagnosis of MN 146 ; whether these are coincidental events or represent an aetiological association is unclear. Three criteria to ascertain an aetiological association have been proposed: remission occurs after complete removal of the tumour, renal relapse accompanies recurrence of the neoplasia, and the detection of tumour antigens and antitumor antibodies within subepithelial immune deposits 147 . However, clinical application of this paradigm can be difficult, in part because culprit antigens are generally unknown. Although fulfilment of these criteria provides strong support for an aetiological link, especially in patients with THSD7A-associated MN, their absence does not refute it.
The role of malignancy in patients with MN who are PLA2R antibody positive is debatable 148 . PLA2R antibody positivity has been reported in a minority of patients with MN associated with solid tumours but, in one study, no patient with PLA2R-associated MN went into remission with malignancy treatment alone, suggesting a coincidental process rather than a causal relation 131 . However, 10% of the patients tested negative for all seven known antigens associated with MN and 25% of these had a malignancy 131 . In a large series of 91 patients with NELL1-associated MN, 33% had an associated malignancy 68 . Patients with NELL1-associated MN were older than patients with PLA2R-associated MN (mean age 66.8 ± 10.8 years versus 56.4 ± 13.9 years, respectively) and patients with NELL1-associated MN with malignancy were significantly older than patients with NELL1-associated MN without malignancy (71.0 ± 8.6 years versus 65.0 ± 10.5 years; P = 0.01). By contrast, in a study from China that included 15 patients with NELL1-associated MN (median age 49 years, range 44–50 years), no association with malignancy was found 149 .
In practice, we recommend that patients who present with MN should undergo a thorough evaluation for possible occult malignancy, especially if they are negative for PLA2R antibodies or positive for THSD7A or NELL1 antibodies. The evaluation should consist of most age-appropriate screening tests, including colon cancer screening, mammography, kidney ultrasonography, a prostate-specific antigen assay in men and a chest radiograph (or a chest CT in patients at high risk) (Box 2 ; Supplementary Box 1 ) 134 . Ongoing vigilance is necessary because the diagnosis of cancer may not be immediately obvious.
MN rarely affects children (0.1 cases per 100,000 per year) 150 . However, its frequency may be underestimated in this age group as children <10–12 years of age with nephrotic-range proteinuria are usually treated empirically with oral glucocorticoids without performing a renal biopsy. As childhood progresses, MN as a cause of nephrotic syndrome becomes more frequent, increasing from 1–2% at ages 1–5 years to 18–22% at ages 18–20 years. A rare form of congenital MN that is limited to only a few families worldwide is caused by a maternal homozygous mutation in MME , which encodes NEP 52 , 151 . The SEM3B-associated form of pMN typically occurs in children (even <2 years of age) and in young adults 70 . BSA-related MN should also be considered in children <5 years of age 80 .
Similar to adults, particularly in regions with endemic HBV infection, young children presented with HBV-associated MN that is responsive to anti-viral treatment 152 . This form of MN has decreased steeply with widespread vaccination against HBV 152 . In adolescents, MN can occur in patients, typically female, with renal involvement secondary to SLE (class V lupus nephritis). At this age, pMN also occurs, especially but not exclusively associated with PLA2R 153 .
When MN is diagnosed in a child, the most likely causes must be identified, taking into account the child’s age 153 . Briefly, the congenital NEP form of MN must be suspected in neonates; the BSA-related form in infants; the SEMA3B form in children aged 1–3 years; the enzyme replacement therapy form in children with a metabolic disease receiving recombinant human arylsulfatase B or α-glucosidase; 154 the HBV-associated form in children living in areas with endemic HBV infection, particularly in those aged 5–7 years; and either SLE or primary forms of MN driven by one of the known antigens, particularly PLA2R, in children aged >10–12 years. In addition to SEMA3B-associated MN, the actual incidence of MN involving other known antigens in children still needs to be investigated; forms of MN associated with THSD7A have been described as well as a single patient each with NCAM1-associated and with EXT1 and EXT2-associated forms of MN.
The outcome of pMN in children is unpredictable and spontaneous remission occurs in ~30% of patients. Overall, prognosis in paediatric forms of MN seems better than in adults 153 . In a retrospective study of 217 children with pMN, after a median follow-up of 45 (23.5–74.0) months, the cumulative kidney survival rates at 5 years and 10 years after renal biopsy were 95.3% and 67.8%, respectively 155 .
Low-income regions
In low-income settings, patients with nephrotic syndrome due to MN often present with late-stage disease, sometimes with complications 156 . The presence of vascular thrombosis might prevent a kidney biopsy as patients need immediate therapeutic anticoagulation. Lack of specialist facilities limits the ability to fully evaluate these patients, including investigations to rule out secondary causes of MN, serological testing and kidney biopsy. Special attention needs to be given to rule out locally prevalent infections such as HBV or HCV. According to the Global Kidney Health Atlas, laboratory facilities for assessment of urinary protein levels or albumin creatinine ratios were available in <5% and <55% and renal pathology services were available in only 12% and 46% of low-income and lower-middle-income regions, respectively 157 . Even where pathology is available, immunofluorescence microscopy, PLA2R staining or electron microscopy are not routinely performed in many centres.
Infections with HBV, HCV or HIV are important preventable causes of MN. Of these, HBV infection can be prevented with a vaccine. According to the WHO, the prevalence of HBV infection is above the global median of 3.5% in sub-Saharan Africa, Southeast Asia, and Central Asia and exceeds 10% in several African regions. Universal HBV vaccination has effectively eliminated HBV-associated MN in high-income regions 25 . However, vaccination coverage remains dismal in low-income regions, with only 6% coverage in the WHO African region 158 .
Ingestion of heavy metals is associated with the development of MN. Heavy metals are often identified as contaminants in indigenous medications in India and China 159 . Better regulation of their use can help prevent MN. A study from China 18 identified a strong association between air pollution and the increasing prevalence of MN. These data identify important gaps in our knowledge of the broader environmental determinants of this condition. The findings highlight the need to develop sustainable approaches to address the preventable causes of MN through actions that regulate the use of indigenous medicines and address emerging environmental challenges 160 .
The initial non-immunosuppressive treatment of patients with pMN is guided by the complaints and (expected) complications at presentation and depends on the severity of proteinuria. Intensive monitoring enables the estimation of whether the patient is at risk of progressive kidney function deterioration. Immunosuppressive therapy, targeting the abnormal autoimmune response, should be considered in patients at risk. The introduction of a quantitative ELISA assay to measure antibody levels (only available for PLA2R antibodies) has enabled improved risk prediction and treatment guidance. For the time being, treatment approaches overall do not differ for the pMN forms associated with different antigens but most data are available for PLA2R-associated MN, which can facilitate more tailored management in patients with this form of MN. In patients with sMN, treatment of the underlying cause is warranted; however, patients with sMN who do not respond should receive therapy as proposed for pMN.
Supportive treatment of nephrotic syndrome
In nephrotic syndrome, oedema formation is dependent on water and sodium retention. Oedema contributes to immobility, fatigue, discomfort, skin blistering, infections and anxiety. Treatment is empirical and not evidence based 161 . Initial treatment includes a sodium-restricted diet and loop diuretics (furosemide or bumetanide). In patients with insufficient response, a second diuretic is added (thiazide, acetazolamide or a potassium-sparing diuretic). Theoretically, amiloride should be most advantageous in view of the evidence of increased activation of collecting duct ENaC 162 . In clinical practice, combinations of diuretics are often effective and the choice is guided by serum electrolyte levels and adverse effects of medications (Box 1 ). Patients with severe oedema often require hospital admission for treatment with intravenous diuretics, sometimes supplemented with albumin infusions 161 . In patients with hyponatraemia, water restriction is advised. After attaining sodium balance, blood pressure treatment should continue, targeting a blood pressure of 125–130/75–80 mmHg (refs 130 , 163 ). Angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers are preferred as they have antiproteinuric effects and evidence suggests that their use may increase spontaneous remission rates in patients with pMN 164 .
Patients with nephrotic syndrome often develop hypercholesterolaemia. Guidelines advise the use of statin therapy in patients with persistent proteinuria and hypercholesterolaemia, especially in patients >50 years of age, to provide cardiovascular protection 130 . The use of statins in MN is further supported by their suggested role in the prevention of venous thromboembolic events 165 .
Indeed, patients with MN are at high risk of venous and arterial thrombotic events and the incidence is highest within 6–12 months after disease onset 165 . Thus, already at the time of presentation and diagnosis, prophylactic anticoagulation must be considered, taking into account individual patient characteristics, disease severity, and patient and family history (Supplementary Box 2 ). Most guidelines suggest the use of early, prophylactic anticoagulation according to algorithms that consider all parameters that affect the risk of thrombotic events and bleeding 130 . Treatment with warfarin is often used as standard therapy; however, there are good arguments to start treatment with low-molecular-weight heparin (at low to moderate dose intensity) at the time of diagnosis and switch to warfarin after 3 months if nephrotic syndrome persists 166 . In patients who are not candidates for warfarin or are treated with low-intensity low-molecular-weight heparin and have other risk factors for atherosclerotic vascular events, the use of aspirin must be considered 167 . The use of the new class of direct-acting oral anticoagulant drugs for thrombosis prophylaxis in nephrotic syndrome cannot be recommended 168 . Direct-acting oral anticoagulant drugs have not been studied in nephrotic syndrome and some patients with nephrotic syndrome developed thrombosis while using them 169 . High protein binding, the contribution of renal clearance to drug elimination, and the role of CYP3A4 and p-glycoprotein in drug metabolism (interfering with the kinetics of CNIs) could all affect their use in nephrotic syndrome 168 .
Immunosuppressive therapy
Cyclophosphamide and corticosteroids.
Long before the identification of pathogenic antigens and specific auto-antibodies, pMN was recognized as an immune-mediated kidney disease, which fostered the use of prednisolone and other immunosuppressive drugs. RCTs showed that a combination of an alkylating agent (preferably cyclophosphamide) and prednisolone attenuated the progression to kidney failure in patients with MN 170 . However, the 2012 KDIGO guidelines did not recommend the unrestricted use of this therapy in patients with MN 163 . First, ~40% of untreated patients do not progress to kidney failure and many develop spontaneous proteinuria remission. Second, treatment with cyclophosphamide and corticosteroids is associated with considerable adverse effects (Box 1 ). Thus, it was advocated to restrict cyclophosphamide-based therapy to patients with MN and a high risk of kidney failure 171 . The guideline used persistent proteinuria of >4 g per day after 6 months of non-immunosuppressive therapy as the criterion of high risk. This criterion is not very accurate as the rate of spontaneous remission in these patients can be 45% 8 . The optimization of therapy (in terms of risk–efficacy balance) in patients with MN requires the early and accurate identification of patients who will progress to kidney failure (or will develop severe complications of nephrotic syndrome) and the introduction of effective, less toxic and alternative immunosuppressive therapies.
Risk prediction
Serum creatinine levels and proteinuria are the oldest biomarkers to estimate the risk of progressive disease associated with increased cardiovascular morbidity and mortality, and ultimately leading to kidney failure. Many other reported biomarkers lack sensitivity and/or specificity and have not been validated 172 (Table 2 ). Baseline serum creatinine levels (cut-off value 1.5 mg/dl) identify patients at high risk relatively late at a time when kidney function is already compromised. While patients with persistent non-nephrotic proteinuria do not develop renal insufficiency 173 , 174 , nephrotic syndrome by itself is not an accurate prognostic biomarker as 40–45% of patients with nephrotic syndrome do not progress. One study showed that no less than 22% of patients with baseline proteinuria >12 g/day develop spontaneous remission 164 . Monitoring the course of kidney function and proteinuria during follow-up improves risk prediction 164 , 175 , 176 . The Toronto risk score combines kidney function and proteinuria parameters. This score has been validated and showed good performance 177 but it can only be calculated after 12–24 months of follow-up. Measurement of low-molecular-weight proteins in urine at baseline provided similar accuracy as the Toronto risk score 178 . PLA2R antibody levels had no added value in a prognostic model that included proteinuria and serum creatinine levels but, in univariate analysis, high PLA2R antibody levels were specific (>80%) for predicting progression, with positive predictive values of 77% for 150–300 RU/ml and 87% for >300 RU/ml (ref. 179 ).
On the basis of biomarker assessment, the KDIGO 2021 guideline defines four risk categories: low, moderate, high and very high risk 130 (Table 3 ). Although the disease characteristics of most patients may not perfectly fit one category and risk classification is not very accurate and partly based on low-quality data, the classification provides guidance in patient management. Because patients are seen at various phases of the disease, some entering immunological remission while others having increasing immunological activity, one cannot rely on a snapshot measurement of PLA2R antibody levels and proteinuria to predict the risk of progression but rather on the trajectory of these variables 180 . Thus, risk evaluation is a dynamic process and it is important to re-evaluate risk prediction at 3 months and 6 months after diagnosis as the changes in PLA2R antibody levels and clinical parameters may affect treatment indications. For example, a severe nephrotic syndrome with persistently high PLA2R antibody levels at 3 months will prompt starting immunosuppressive treatment. Further studies are needed to define more specific biomarkers that would predict progression at baseline ideally from the urine via exosomes, although the varying time points of sampling in the natural history of the disease will probably make interpretation difficult.
CNIs and CD20-targeted therapy
The introduction of novel immunosuppressive agents offered hope for effective, less toxic therapy in patients with pMN. CNIs, such as cyclosporine and tacrolimus, indirectly affect B cell function and were proven effective in preventing immunological rejection after kidney transplantation. In addition, experimental studies suggested that CNIs directly target the podocyte, thereby reducing proteinuria 181 . The development of CD20 antibody treatments, such as rituximab, enabled effective depletion of B cells and thus the selective targeting of antibody production.
Both CNIs and rituximab, alone or in combination, are considered less toxic than cyclophosphamide and prednisolone. A review of clinical trials concluded that CNIs increased the likelihood of partial and complete proteinuria remission compared with no treatment (72–75% versus 22%) 170 . Additionally, remission rates at 12-month follow-up were comparable and numerically higher than those of cyclophosphamide treatment (CNIs 71–89%; cyclophosphamide 65–77%); however, the disease relapses after withdrawal of CNIs in most patients. In one RCT, relapses within 12 months after remission occurred in 40% of patients treated with tacrolimus and in 7% of patients treated with cyclophosphamide 182 . The efficacy of rituximab was also studied in RCTs. In the GEMRITUX trial, after 23 months of follow-up, the remission rate was 66% in patients treated with rituximab (total dose 750 mg/m 2 ) and 45% in those who received conservative treatment 8 . In the MENTOR trial, although rituximab (total dose 4 g) was non-inferior to cyclosporine in inducing remission at 12 months after treatment start, more patients who received rituximab maintained remission at 12 months after withdrawal of therapy (60% versus 20%) 9 . In the STARMEN trial, although sequential therapy with tacrolimus (for 6 months) and rituximab (single dose 375 mg/m 2 ) was inferior in inducing remission than the combination of cyclophosphamide and prednisolone (84% versus 58% at 24 months), the single dose of rituximab prevented relapse after withdrawal of tacrolimus 10 . The most recent RI-CYCLO trial compared rituximab (total dose 2 g) with cyclical cyclophosphamide and corticosteroids 11 . This study was underpowered and included mostly patients at moderate risk with low anti-PLA2R antibody levels. Although the authors concluded that the study provided “no signal of more benefit or less harm associated with rituximab”, the per-protocol analysis showed a significantly lower complete remission rate at 12 months in patients treated with rituximab than patients treated with cyclophosphamide and corticosteroids (13% versus 34%; OR 0.28, 95% CI 0.08–0.95). Subgroup analysis suggested superiority of cyclophosphamide treatment in men and in patients with increased proteinuria and decreased serum albumin levels.
Thus, there is evidence that CNIs and rituximab (alone or in combination) induce remission of proteinuria, which should translate into improved renal outcomes, although this has not yet been proven. However, overall failure rates with these agents are 30–35%, raising controversies on the ideal dosage and best protocol. Some data suggest that cyclophosphamide is more effective in patients at high risk 183 . The guidelines argue against the use of CNIs or rituximab in patients with deteriorating or decreased GFR; however, other data with rituximab are encouraging 184 .
Individualized treatment
Risk biomarkers, clinical characteristics, adverse effects, and patient and physician preferences can all be used to individualize therapy in patients with MN (Fig. 4 ). Patients at low risk do not need immunosuppressive therapy 130 . Patients at moderate risk can receive rituximab or a CNI — the controversial use of CNI in this indication is to decrease the duration of proteinuria in those with a high potential of spontaneous remission. In patients at high risk, single-agent rituximab treatment is now established in addition to combinations of cyclophosphamide and prednisolone. However, the long-term benefit on kidney function of CD20 antibody therapy remains unclear and its efficacy to induce immunological remission seems to be reduced in patients with very high antibody titres 183 , although this view has been challenged 185 . Patients at very high risk should receive a combination of cyclophosphamide and prednisolone.
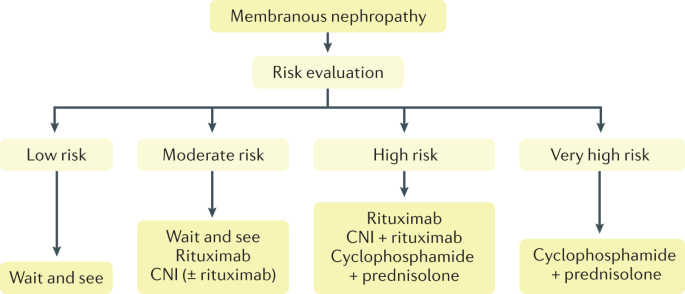
In patients with primary membranous nephropathy (MN), efforts must be made to estimate the risk of kidney function deterioration using clinical criteria (Table 2 ) and risk classification (Table 3 ). As the risk profile will change during the follow-up period, risk should be evaluated regularly. Immunosuppressive therapy should not be used in patients at low risk. By contrast, patients at very high risk should receive the most effective but also most toxic therapy with cyclophosphamide and prednisolone. Different treatment options can be considered in patients at moderate and high risk, guided by patient and physician preference, expected adverse effects, therapy reimbursement, and other factors. Although calcineurin inhibitor (CNI) monotherapy is probably less effective, it is an option in patients at moderate risk of progression. CNIs shorten the period of proteinuria and can be used in a regimen combined with rituximab. Adapted from ref. 130 , KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases.
In the individual patient, the ultimate choice of treatment requires a discussion of benefits and risks considering the expected burden of adverse effects (Supplementary Box 3 ; Supplementary Tables ). Future studies should define the best dosage and protocols adapted to the patient and integrate the new advances in the field of immunotherapy, including new CD20 antibodies, belimumab (a monoclonal antibody that inhibits the B cell-activating factor (BAFF)), anti-plasma cell therapy and anti-complement therapy. These new agents should be discussed in patients with refractory disease in expert centres.
Monitoring immunosuppressive therapy
Immunosuppressive therapy is usually given according to standard treatment protocols of therapy dosing and duration 130 . Patients should be monitored for adverse effects (Box 1 ) and therapy efficacy is ascertained by improvements in proteinuria, serum albumin levels and (if applicable) serum creatinine levels. Clinical response to cyclophosphamide or rituximab is notably slow and partial remission of proteinuria only develops after 6–18 months, often after withdrawal of therapy 10 , 78 . Thus, in patients with persistent nephrotic syndrome but stable eGFR after 6 months, no change in treatment is required.
In PLA2R-associated MN, measurement of PLA2R antibody levels aids treatment decisions (Fig. 5 ). Cyclophosphamide was more effective than rituximab in inducing immunological remission in patients with high PLA2R antibody levels 186 , which explains the low (30%) remission rate after one dose of rituximab in patients with high antibody levels 79 . Treatment should induce immunological remission. The disappearance of PLA2R antibodies (using the IFA; when using ELISA, the titre should fall below 2 RU/ml) predicted remission of proteinuria, whereas persistence of PLA2R antibodies after therapy was associated with progressive disease 78 , 187 .
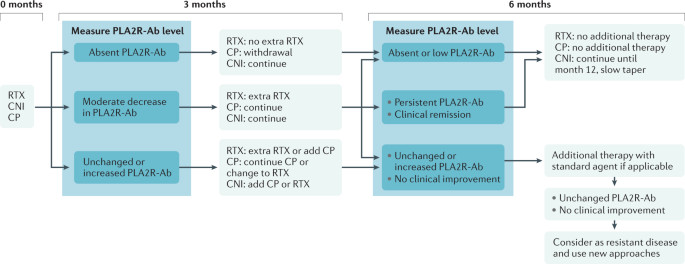
In patients with phospholipase A2 receptor (PLA2R)-associated primary membranous nephropathy, regular evaluation of the course of PLA2R antibody (PLA2R-Ab) levels after the start of immunosuppressive therapy enables the optimization of immunosuppressive therapy in the individual patient. Immunological remission is the goal of therapy. The disappearance of antibodies can be used to taper or withdraw therapy, whereas persistence or increasing antibody levels should lead to continuation of therapy or to switching to an alternative drug regimen. No validated cut-off values exist. A negative immunofluorescence assay result or PLA2R ELISA titre <2 RU/ml defines the absence of PLA2R-Ab. When measuring PLA2R-Ab at regular intervals (every 2–3 months), a large decrease of antibody levels (>50%) to values <50 RU/ml may be sufficient as an initial response criterion. CNI, calcineurin inhibitor; CP, cyclophosphamide; RTX, rituximab. Adapted from ref. 130 , KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases.
Given the rarity of paediatric MN, children with MN should be managed in expert centres and treatment should be targeted. Secondary forms of MN require management of the underlying condition (for example, HBV infection or SLE) 188 . Primary forms of MN, especially in adolescents, can be treated similarly to adults. Supportive therapy with inhibitors of the renin–angiotensin system and low-salt diet is essential. A key difference to the situation in adults is that many children, especially if <10–12 years old, are diagnosed with MN after already having received oral prednisolone for 4–8 weeks. Treatment with CNIs or rituximab follows the same dosing regimen employed for adults. When these therapies are available, cyclophosphamide is rarely used 189 .
In resource-poor settings, patients often do not present until the disease has progressed to a severe nephrotic state and/or a complication has occurred, for example, vascular thrombosis or infection such as pneumonia. This prevents the timely initiation of non-immunosuppressive antiproteinuric therapies with drugs that block the renin–angiotensin system. The lack of specialist nephrologists limits the application of personalized medicine principles such as individualizing evidence-based therapies according to the patient’s risk status 190 . Finally, the full range of treatments may not be available because a drug (for example, rituximab) is not marketed in the country or is too expensive. New treatments that are currently under investigation and monitoring approaches, such as PLA2R antibody assays, are likely to further increase treatment costs and therapeutic inequity. Treatment compliance can be a problem and contribute to suboptimal responses.
Potential solutions include a resource-sensitive adaptation of clinical practice guidelines by global organizations such as KDIGO and ISN 191 , using information technology tools to support the implementation of standard treatment pathways through the available workforce via linking with expert centres by international cooperations (for example, through ISN Sister Centers programmes or Project ECHO), greater use of generic compounds and clinical trials of therapies to optimize the risk–benefit balance (for example, eliminating methylprednisolone pulses in the Ponticelli regimen). Advocacy is needed for greater involvement of low-resource regions in clinical trials of novel therapies with the commitment to make these treatments available as has been done for HIV therapeutics and SARS-CoV-2 vaccination.
Patients with a kidney allograft
Patients with pMN who do not tolerate or do not respond to immunosuppressive therapy will develop kidney failure and need kidney replacement therapy. Kidney transplantation is considered the best treatment option provided there are no contraindications for this surgical procedure. Apart from the routine pretransplant evaluation, in patients with pMN, the risk for recurrent disease after kidney transplantation should be assessed and discussed. In these patients, the histological recurrence rate is 50–60% and the clinical recurrence rate is 30–40% 192 , 193 , 194 , 195 , 196 , 197 . Increased age, short waiting time to receiving the transplant, heavy proteinuria at pretransplant evaluation, and corticosteroid-free maintenance immunosuppression after transplantation are associated with an increased risk of recurrence 192 , 193 , 197 , 198 . Recurrence rates are approximately threefold higher after transplantation of an organ from a living related donor 195 , 196 , compatible with the observation that the risk of recurrence is dependent on donor HLA-D and PLA2R1 variants 90 .
Pretransplant and post-transplant measurement of PLA2R antibody levels aids patient management (Supplementary Box 4 ). The most critical step is to unequivocally establish that MN is associated with PLA2R. In PLA2R-associated MN, the pretransplantation presence of PLA2R antibodies, especially at high titres, is associated with a high risk of recurrence, whereas undetectable PLA2R antibody levels predict a low risk 195 , 197 , 199 . A gradual decrease and disappearance of PLA2R antibodies after transplantation are associated with a reduced risk of recurrence (25% versus 71%) 195 . Although PLA2R antibody level measurement is therefore helpful, there are pitfalls and exceptions to the rule (Supplementary Box 4 ).
Rituximab treatment is effective in patients with recurrent MN following kidney transplantation 193 , 197 . Most patients (>80%) respond with complete or partial remission of proteinuria, which is preceded by the disappearance of the antibodies in patients who are PLA2R antibody positive. In 20–30% of patients, recurrent disease is non-progressive 193 . Thus, rituximab should be withheld in patients with persistent proteinuria at <1 g/day. There is no evidence-based benefit on the post-transplant outcome of treatment with rituximab before transplantation.
Quality of life
According to the WHO, health-related quality of life (HRQOL) is defined as “an individual’s perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns” 200 . This broad concept encompasses multiple dimensions, including a person’s physical health, psychological status, level of independence, social relationships and personal beliefs 200 . Systematic reviews have shown that various measures for quality of life (for example, Short Form Health Survey (SF-36); EuroQoL 5 dimensions (EQ-5D); Kidney Disease Quality of Life Instrument (KDQOL)) have been used in patients with chronic kidney disease 201 , 202 . However, data on the quality of life in patients with MN are limited 203 .
Patients with MN have impaired quality of life and functioning compared with age-matched and gender-matched individuals in the general population 204 , 205 , which may be further exacerbated by symptoms, for example, fatigue and oedema, and treatment burden 206 . Some unique features of MN can also affect quality of life such as the increased risk of thrombotic events.
A study by the Cure Glomerulonephropathy Network (CureGN) involving 478 children and 1,115 adults with glomerular diseases, including MN, found that oedema, female sex, weight (obesity) and estimated GFR correlated with decrements in HRQOL domains 203 . The findings also indicated that HRQOL was similar across different types of primary glomerular disease 203 . Another study conducted in patients with primary glomerular disease found that HRQOL was lower compared with healthy controls and was associated with proteinuria 205 .
The need to include patient goals and preferences in the shared decision-making and assessment of interventions and care is increasingly recognized 207 . This is particularly relevant in MN because of the lack of well-defined tools for risk stratification to help with treatment decisions. Patient-reported outcomes, which reflect how patients feel and function, are assessed and reported directly by patients to provide a quantitative assessment of the effects of disease and treatment from the patient perspective 208 , 209 . However, patient-reported outcomes are infrequently reported in trials in patients with MN. HRQOL is an example of a patient-reported outcome; however, other outcomes are also important to patients, which may be implemented in research, practice and policy.
As part of the Standardized Outcomes in Nephrology – Glomerular Disease (SONG-GD) initiative, 134 patients with glomerular disease from Australia, the USA, the UK and Hong Kong identified and prioritized outcomes of importance and discussed the reasons for their choices. The highest-ranked patient-reported outcomes were life participation (defined as the ability to do meaningful activities), fatigue, anxiety, family impact and the ability to work 210 . The prioritization was driven by constraints on patients’ day-to-day existence, impaired agency and control over health, and threats to future health and their family 210 .
Further data on quality of life in patients with MN are needed, which may be gathered through routine measurement and reporting of HRQOL in research and clinical practice. Additionally, more evidence on interventions to improve quality of life and other patient-important outcomes, including life participation, fatigue, depression and anxiety, and the ability to work, is required. Ultimately, this may help to improve the care and outcomes of patients living with MN.
Despite the tremendous progress made over the past two decades, considerable gaps in our understanding of MN remain (Box 3 ). The application of new technologies and opportunities to adopt investigative strategies from other disciplines will enable a continued exponential growth in our understanding of MN. The use of mass spectrometric techniques, initially used to subtype the deposits of renal amyloidosis, has led to an unprecedented discovery of new antigens in MN and this progress should continue with the identification of additional target antigens in paediatric, adult idiopathic and secondary forms of MN in addition to a better understanding of the similarities and differences in complement and matrix proteins involved in the disease subtypes. Mass spectrometric imaging can surpass the resolution of more conventional glomerular laser capture approaches by limiting analysis to even more defined regions of the cell or GBM 211 , 212 .
Our ability to interrogate human and experimental biospecimens has become much more sophisticated. Advances in microscopy and cell labelling now include multiplexed immunofluorescence imaging technology for analysis of a large number of antigens or biomarkers (including complement components) and super-resolution imaging of the altered filtration barrier and molecules comprising the subepithelial deposits 213 . Single-cell RNA sequencing of human biopsy samples or experimental tissues can offer in-depth analysis of injury and repair pathways of all glomerular cell types as well as of downstream tubular and renal parenchymal and immune cells 214 . Similarly, single-cell RNA sequencing of circulating lymphocyte populations can characterize blood signatures of active disease, remission and progression, which could be used for patient monitoring and to define the antigen-specific B cell and T cell repertoire and their associated B cell and T cell receptors. These molecular data and computational analyses will further the efforts to develop novel antigen-specific or epitope-specific therapies, such as chimeric autoantibody receptor T cells that could target autoantibody-producing B cells 215 , or to develop the use of antigen-derived peptides coupled to MHC class II to study or target T cells 216 . Targeted multi-omics of the urine 217 should continue to be developed, with special emphasis on shed complement and matrix components to characterize signatures of active disease, remission and progression that enable better correlation of this ‘liquid biopsy’ with defined molecular features of kidney tissue.
High-resolution genetic data assembled from global registries of MN and control populations should be examined in the context of atlases of tissue-specific epigenetic regulatory elements and should be matched using machine learning approaches with records of phenotypic variation using information stored in electronic health records 218 . If this is possible at a large enough scale, previously unrecognized associations, susceptibilities and disease pathways in MN may come to light.
These knowledge gaps and new technologies should be prioritized in the next 5–10 years to focus on three major areas. First, understanding how genetic makeup, immune phenotype and exposures coalesce to cause disease initiation should be an overarching research goal, which in turn may lead to better prognostication, precision-guided therapeutics and monitoring, and avoidance of conditions that lead to relapse. Second, research should continue into the specific molecular mechanisms and pathways that cause glomerular injury and, in some individuals, rapid progression of parenchymal damage with ongoing proteinuria. Third, the field needs to move from broad immunosuppression of disease activity, which brings with it risks of infection and other substantial adverse effects, to more personalized and targeted therapeutics. Inhibition of the complement system seems to be the next therapeutic frontier in MN but further molecular understanding of the underlying pathological mechanisms could lead to even more novel ways to limit injury or stimulate reparative pathways. Our understanding of and ability to manipulate the immune system in MN is in its infancy but advances in immunology, oncology and infectious diseases (especially with the global emphasis on understanding the response to SARS-CoV-2) will drive our ability to rapidly interrogate the immune system in MN.
It is likely that substantial progress will be made in the therapeutic armamentarium in the near future, including new regimens to decrease the toxic effects of cyclophosphamide and corticosteroids, improving the use of CD20 antibodies with humanized, more potent molecules, such as obinutuzumab 219 , and new therapeutic approaches (anti-BAFF, anti-plasma cell and anti-complement therapy), which should be discussed in patients with resistant disease.
At present, the therapeutic armamentarium has already been enriched with new drugs. CD20-targeted humanized antibodies (ofatumumab, obinutuzumab and ocrelizumab) are now available. They induce prolonged B cell depletion with a very low risk of immunization 219 . These are currently mostly indicated in patients who have developed serum sickness with rituximab and in those with refractory or multi-relapsing MN 220 , 221 , 222 . One can predict a growing role for these more potent biotherapies as ~30% of patients treated with rituximab will not undergo clinical remission and relapses are frequent.
Belimumab is a human IgG1λ monoclonal antibody that inhibits BAFF, thereby affecting B cell proliferation and survival 223 . Because rituximab increases the circulating levels of BAFF, combination therapy with belimumab could be considered 224 . Belimumab was used successfully in patients with lupus. At the end of a 2-year RCT, the renal response was better in patients who received belimumab plus standard therapy than in those who received standard therapy alone 225 . Belimumab was used with some efficacy in patients with MN in a small open-label, prospective, single-arm study 226 but further studies are needed.
Biotherapies targeting plasma cells must be seriously considered. Indeed, the relatively low rate of sustained complete remission in patients treated with rituximab is usually attributed to the involvement of CD19 – CD20 − CD38 + CD138 + long-lived memory plasma cells that are niched naturally in the bone marrow and ectopically in the native and inflamed kidney. These non-proliferating plasma cells lacking CD19 and CD20 markers provide the basis for humoral memory and refractory autoimmune diseases 227 . These cells are targeted by anti-CD38 antibodies, which are used in ongoing trials (NCT04145440) 228 . Immunoadsorption and plasmapheresis have been used by several groups to enhance the depletion of circulating THSD7A antibodies and PLA2R antibodies in patients with severe MN 229 , 230 . Refractory disease is a current indication.
There is a strong interest in anti-complement therapies in glomerular diseases. MN seems to be among the most suitable for these treatments as complement is considered the main mediator of proteinuria, at least in experimental models. There are two windows of opportunity for anti-complement therapy: early before immunosuppressive drugs reach full efficacy and later in patients with partial or no remission. Several compounds targeting the lectin pathway and the alternative pathway are in early-phase trials 231 .
New drugs are urgently needed but preclinical models of the disease remain limited in their ability to assess the efficacy of novel therapeutics. The development of MN models using rodents with humanized components of the immune system may enable a more effective translation and relevance to human disease. The field has come to understand how human IgG4 PLA2R antibodies can activate the lectin pathway of complement activation using human podocytes grown in standard culture models 104 but such single-cell-type in vitro systems ignore the complex interplay of closely apposed cell types within the glomerulus. New model systems, such as the glomerulus-on-a-chip that combines podocytes with endothelium in a microfluidic flow chamber 103 or induced pluripotent stem cells (from healthy individuals or patients) in vascularized organoid cultures 232 , have the potential to enable a better understanding of the cell–cell interactions that drive disease and are amenable to high-throughput screening of compound libraries for drug discovery.
The future is bright for research into the pathogenesis and treatment of MN in all its forms and we encourage clinicians, scientists, patients, industry and regulatory agencies to come together with innovative and forward-reaching approaches. The involvement of patients both in defining the gaps and contributing to registries, biorepositories and research themes will be key to our common success.
Box 3 Overcoming the gaps in membranous nephropathy knowledge
Despite substantial progress, major gaps remain in our understanding of human membranous nephropathy (MN). The following points should be addressed as the field moves forwards.
Understand clinical variability and unpredictable disease outcomes with the goal of improving prognosis.
Assemble large databases of genetic, epigenetic and phenotypic information to fully define genetic control of disease susceptibility and expression.
Identify triggering factors for disease, such as air pollution, infection, tumour antigens and the target organ in which initial immunization occurs; explain why MN subtypes present at different patient ages.
Characterize the B cell and T cell populations involved at onset, remission, and relapse of disease and understand how they interact to modulate disease.
Identify precise B cell and T cell epitopes of phospholipase A2 receptor (PLA2R; and other antigens) at the molecular level, which is a prerequisite for targeted immunotherapy.
Establish the physiological roles of PLA2R, thrombospondin type 1 domain-containing 7A (THSD7A) and new antigens within the podocyte, glomerulus and/or extra-renal tissues.
Identify the as yet unknown antigens in uncharacterized forms of adult and paediatric MN as well as the composition of immune deposits in secondary MN.
Further analyse the role of complement, including the contribution of antibodies against complement regulatory proteins and functional polymorphisms in complement and complement regulatory proteins.
Fully establish the pathogenic mechanisms of antibodies, pathways of podocyte injury and glomerular basement membrane disorganization as well as repair mechanisms.
Use the emerging information to design new therapies such as anti-complement, anti-B cell-activating factor (BAFF) and targeted (epitope-specific) therapies.
Couser, W. G. Primary membranous nephropathy. Clin. J. Am. Soc. Nephrol. 12 , 983–997 (2017).
CAS PubMed PubMed Central Google Scholar
Debiec, H. et al. Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N. Engl. J. Med. 346 , 2053–2060 (2002). A landmark article that reports the first description of a podocyte antigen involved in a rare subset of neonatal MN.
PubMed Google Scholar
Beck, L. H. et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 361 , 11–21 (2009). A landmark article that first characterizes the major antigen in adult MN, leading to a paradigm shift in the diagnosis and monitoring of patients.
Tomas, N. M. et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N. Engl. J. Med. 371 , 2277–2287 (2014). The first report of an antigen found to be associated with cancer in a subset of patients.
PubMed PubMed Central Google Scholar
Ronco, P. & Debiec, H. Membranous nephropathy: current understanding of various causes in light of new target antigens. Curr. Opin. Nephrol. Hypertens. 30 , 287–293 (2021).
Sethi, S. New ‘antigens’ in membranous nephropathy. J. Am. Soc. Nephrol. 32 , 268–278 (2021).
CAS PubMed Google Scholar
Cattran, D. C. & Brenchley, P. E. Membranous nephropathy: integrating basic science into improved clinical management. Kidney Int. 91 , 566–574 (2017).
Dahan, K. et al. Rituximab for severe membranous nephropathy: a 6-month trial with extended follow-up. J. Am. Soc. Nephrol. 28 , 348–358 (2017).
Fervenza, F. C. et al. Rituximab or cyclosporine in the treatment of membranous nephropathy. N. Engl. J. Med. 381 , 36–46 (2019). This landmark randomized clinical trial provides the first comparison of rituximab and cyclosporine.
Fernández-Juárez, G. et al. The STARMEN trial indicates that alternating treatment with corticosteroids and cyclophosphamide is superior to sequential treatment with tacrolimus and rituximab in primary membranous nephropathy. Kidney Int. 99 , 986–998 (2021). This important randomized clinical trial provides the first comparison of cyclophosphamide (with corticosteroids) with a sequential combination of tacrolimus and rituximab.
Scolari, F. et al. Rituximab or cyclosphamide in the treatment of membranous nephropathy. J. Am. Soc. Nephrol. 32 , 972–982 (2021).
CAS PubMed Central Google Scholar
De Vriese, A. S., Glassock, R. J., Nath, K. A., Sethi, S. & Fervenza, F. C. A proposal for a serology-based approach to membranous nephropathy. J. Am. Soc. Nephrol. 28 , 421–430 (2017).
Kumar, V. et al. Antibodies to m-type phospholipase A2 receptor in children with idiopathic membranous nephropathy. Nephrology 20 , 572–575 (2015).
Debiec, H. & Ronco, P. Immunopathogenesis of membranous nephropathy: an update. Semin. Immunopathol. 36 , 381–397 (2014).
McGrogan, A., Franssen, C. F. & de Vries, C. S. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol. Dial. Transplant. 26 , 414–430 (2011).
Rychlik, I. et al. The Czech registry of renal biopsies. Occurrence of renal diseases in the years 1994-2000. Nephrol. Dial. Transplant. 19 , 3040–3049 (2004).
Hogan, S. L., Muller, K. E., Jennette, J. C. & Falk, R. J. A review of therapeutic studies of idiopathic membranous glomerulopathy. Am. J. Kidney Dis. 25 , 862–875 (1995).
Xu, X. et al. Long-term exposure to air pollution and increased risk of membranous nephropathy in China. J. Am. Soc. Nephrol. 27 , 3739–3746 (2016).
Ronco, P. & Debiec, H. Pathophysiological advances in membranous nephropathy: time for a shift in patient’s care. Lancet 385 , 1983–1992 (2015).
Arapovic, A. et al. Epidemiology of 10-year paediatric renal biopsies in the region of southern Croatia. BMC Nephrol. 21 , 65 (2020).
Chen, A. et al. Idiopathic membranous nephropathy in pediatric patients: presentation, response to therapy, and long-term outcome. BMC Nephrol. 8 , 11 (2007).
Eddy, A. A. & Symons, J. M. Nephrotic syndrome in childhood. Lancet 362 , 629–639 (2003).
Nie, S. et al. The spectrum of biopsy-proven glomerular diseases among children in China: a national, cross-sectional survey. Clin. J. Am. Soc. Nephrol. 13 , 1047–1054 (2018).
Johnson, R. J. & Couser, W. G. Hepatitis B infection and renal disease: clinical, immunopathogenetic and therapeutic considerations. Kidney Int. 37 , 663–676 (1990).
Moroni, G. & Ponticelli, C. Secondary membranous nephropathy. a narrative review. Front. Med. 7 , 611317 (2020).
Google Scholar
Xu, X., Nie, S., Ding, H. & Hou, F. F. Environmental pollution and kidney diseases. Nat. Rev. Nephrol. 14 , 313–324 (2018).
Narasimhan, B. et al. Characterization of kidney lesions in Indian adults: towards a renal biopsy registry. J. Nephrol. 19 , 205–210 (2006).
Li, J. et al. Primary glomerular nephropathy among hospitalized patients in a national database in China. Nephrol. Dial. Transpl. 33 , 2173–2181 (2018).
CAS Google Scholar
Schieppati, A. et al. Prognosis of untreated patients with idiopathic membranous nephropathy. N. Engl. J. Med. 329 , 85–89 (1993).
Ponticelli, C. et al. A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int. 48 , 1600–1604 (1995).
Jha, V. et al. A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 18 , 1899–1904 (2007).
Maisonneuve, P. et al. Distribution of primary renal diseases leading to end-stage renal failure in the United States, Europe, and Australia/New Zealand: results from an international comparative study. Am. J. Kidney Dis. 35 , 157–165 (2000).
Troyanov, S. et al. Idiopathic membranous nephropathy: definition and relevance of a partial remission. Kidney Int. 66 , 1199–1205 (2004).
Fervenza, F. C., Sethi, S. & Specks, U. Idiopathic membranous nephropathy: diagnosis and treatment. Clin. J. Am. Soc. Nephrol. 3 , 905–919 (2008).
Klouda, P. T. et al. Strong association between idiopathic membranous nephropathy and HLA-DRW3. Lancet 2 , 770–771 (1979).
Vaughan, R. W., Demaine, A. G. & Welsh, K. I. A DQA1 allele is strongly associated with idiopathic membranous nephropathy. Tissue Antigens 34 , 261–269 (1989).
Stanescu, H. C. et al. Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N. Engl. J. Med. 364 , 616–626 (2011).
Coenen, M. J. et al. Phospholipase A2 receptor (PLA2R1) sequence variants in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 24 , 677–683 (2013).
Lv, J. et al. Interaction between PLA2R1 and HLA-DQA1 variants associates with anti-PLA2R antibodies and membranous nephropathy. J. Am. Soc. Nephrol. 24 , 1323–1329 (2013).
Le, W. B. et al. HLA-DRB1*15:01 and HLA-DRB3*02:02 in PLA2R-Related membranous nephropathy. J. Am. Soc. Nephrol. 28 , 1642–1650 (2017).
Cui, Z. et al. MHC class II risk alleles and amino acid residues in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 28 , 1651–1664 (2017).
Xie, J. et al. The genetic architecture of membranous nephropathy and its potential to improve non-invasive diagnosis. Nat. Commun. 11 , 1600 (2020). The most extensive multi-ethnic genome-wide association study performed in membranous nephropathy revealing the complexity of HLA-D alleles associated with membranous nephropathy in different ethnic populations and signals in the NF-κ B and interferon pathways.
Wang, H. Y. et al. HLA class II alleles differing by a single amino acid associate with clinical phenotype and outcome in patients with primary membranous nephropathy. Kidney Int. 94 , 974–982 (2018).
Van Damme, B. J., Fleuren, G. J., Bakker, W. W., Vernier, R. L. & Hoedemaeker, P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. V. Fixed glomerular antigens in the pathogenesis of heterologous immune complex glomerulonephritis. Lab. Invest. 38 , 502–510 (1978).
Couser, W. G., Steinmuller, D. R., Stilmant, M. M., Salant, D. J. & Lowenstein, L. M. Experimental glomerulonephritis in the isolated perfused rat kidney. J. Clin. Invest. 62 , 1275–1287 (1978).
Kerjaschki, D. & Farquhar, M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J. Exp. Med. 157 , 667–686 (1983).
Prabakaran, T. et al. Receptor-mediated endocytosis of α-galactosidase A in human podocytes in Fabry disease. PLoS ONE 6 , e25065 (2011).
Debiec, H. et al. Role of truncating mutations in MME gene in feto-maternal allo-immunization and neonatal glomerulopathies. Lancet 364 , 1252–1259 (2004).
Turner, A. J., Isaac, R. E. & Coates, D. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays 23 , 261–269 (2001).
Lu, B. et al. The control of microvascular permeability and blood pressure by neutral endopeptidase. Nat. Med. 3 , 904–907 (1997).
Platt, J. L., Tucker, W. L. & Michael, A. F. Stages of renal ontogenesis identified by monoclonal antibodies reactive with lymphohematopoietic differentiation antigens. J. Exp. Med. 157 , 155–172 (1983).
Vivarelli, M. et al. Genetic homogeneity but IgG subclass-dependent clinical variability of alloimmune membranous nephropathy with anti-neutral endopeptidase antibodies. Kidney Int. 87 , 602–609 (2015).
Hogan, M. C. et al. Subfractionation, characterization, and in-depth proteomic analysis of glomerular membrane vesicles in human urine. Kidney Int. 85 , 1225–1237 (2014).
Ancian, P., Lambeau, G., Mattei, M. G. & Lazdunski, M. The human 180-kDa receptor for secretory phospholipases A2. Molecular cloning, identification of a secreted soluble form, expression, and chromosomal localization. J. Biol. Chem. 270 , 8963–8970 (1995).
Fresquet, M. et al. Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J. Am. Soc. Nephrol. 26 , 302–313 (2015).
Seitz-Polski, B. et al. Epitope spreading of autoantibody response to PLA2R associates with poor prognosis in membranous nephropathy. J. Am. Soc. Nephrol. 27 , 1517–1533 (2016).
Reinhard, L. et al. Clinical relevance of domain-specific phospholipase A2 receptor 1 antibody levels in patients with membranous nephropathy. J. Am. Soc. Nephrol. 31 , 197–207 (2020).
Seitz-Polski, B. et al. Phospholipase A2 receptor 1 epitope spreading at baseline predicts reduced likelihood of remission of membranous nephropathy. J. Am. Soc. Nephrol. 29 , 401–408 (2018).
Kanigicherla, D. et al. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 83 , 940–948 (2013).
Godel, M., Grahammer, F. & Huber, T. B. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N. Engl. J. Med. 372 , 1073 (2015).
Herwig, J. et al. Thrombospondin type 1 domain-containing 7A localizes to the slit diaphragm and stabilizes membrane dynamics of fully differentiated podocytes. J. Am. Soc. Nephrol. 30 , 824–839 (2019).
Seifert, L. et al. The most N-terminal region of THSD7A Is the predominant target for autoimmunity in THSD7A-associated membranous nephropathy. J. Am. Soc. Nephrol. 29 , 1536–1548 (2018).
Hoxha, E. et al. A mechanism for cancer-associated membranous nephropathy. N. Engl. J. Med. 374 , 1995–1996 (2016).
Hoxha, E. et al. An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7A-specific antibodies in membranous nephropathy. J. Am. Soc. Nephrol. 28 , 520–531 (2017).
Sethi, S. et al. Exostosin 1/exostosin 2-associated membranous nephropathy. J. Am. Soc. Nephrol. 30 , 1123–1136 (2019). This article reports the first use of a technological leap combining microdissection of glomeruli and mass spectrometry for the identification of the first biomarker of lupus membranous nephropathy.
Ravindran, A. et al. In patients with membranous lupus nephritis, exostosin-positivity and exostosin-negativity represent two different phenotypes. J. Am. Soc. Nephrol. 32 , 695–706 (2021).
Saidi, M. et al. The exostosin immunohistochemical status differentiates lupus membranous nephropathy subsets with different outcomes. Kidney Int. Rep. 6 , 1977–1980 (2021).
Caza, T. et al. NELL1 is a target antigen in malignancy-associated membranous nephropathy. Kidney Int. 99 , 967–976 (2021).
Sethi, S. et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 97 , 163–174 (2020). This article reports the identification of the second prevalent antigen (after PLA2R) in MN using glomerular microdissection followed by mass spectrometry.
Kudose, S. et al. The clinicopathologic spectrum of segmental membranous glomerulopathy. Kidney Int. 99 , 247–255 (2021).
Sethi, S. et al. Semaphorin 3B-associated membranous nephropathy is a distinct type of disease predominantly present in pediatric patients. Kidney Int. 98 , 1253–1264 (2020).
Caza, T. et al. Neural cell adhesion molecule 1 is a novel autoantigen in membranous lupus nephritis. Kidney Int. 100 , 171–181 (2021).
Sethi, S. et al. Protocadherin 7-associated Membranous Nephropathy. J. Am. Soc. Nephrol. 32 , 1249–1261 (2021).
Al-Rabadi, L. F. et al. Serine protease HTRA1 as a novel target antigen in primary membranous nephropathy. J. Am. Soc. Nephrol. https://doi.org/10.1681/ASN.2020101395 (2021).
Article PubMed Google Scholar
Meyer-Schwesinger, C. et al. A novel mouse model of phospholipase A2 receptor 1-associated membranous nephropathy mimics podocyte injury in patients. Kidney Int. 97 , 913–919 (2020).
Blosser, C. D., Ayalon, R., Nair, R., Thomas, C. & Beck, L. H. Jr. Very early recurrence of anti-Phospholipase A2 receptor-positive membranous nephropathy after transplantation. Am. J. Transplant. 12 , 1637–1642 (2012).
Stahl, R., Hoxha, E. & Fechner, K. PLA2R autoantibodies and recurrent membranous nephropathy after transplantation. N. Engl. J. Med. 363 , 496–498 (2010).
Beck, L. H. Jr et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J. Am. Soc. Nephrol. 22 , 1543–1550 (2011).
Ruggenenti, P. et al. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J. Am. Soc. Nephrol. 26 , 2545–2558 (2015).
Debiec, H. et al. Early-childhood membranous nephropathy due to cationic bovine serum albumin. N. Engl. J. Med. 364 , 2101–2110 (2011). A landmark article that identifies the first food antigen involved in rare cases of membranous nephropathy in children.
Border, W. A., Ward, H. J., Kamil, E. S. & Cohen, A. H. Induction of membranous nephropathy in rabbits by administration of an exogenous cationic antigen. J. Clin. Invest. 69 , 451–461 (1982).
Adler, S. G., Wang, H., Ward, H. J., Cohen, A. H. & Border, W. A. Electrical charge. Its role in the pathogenesis and prevention of experimental membranous nephropathy in the rabbit. J. Clin. Invest. 71 , 487–499 (1983).
Hunley, T. E. et al. Nephrotic syndrome complicating α-glucosidase replacement therapy for Pompe disease. Pediatrics 114 , e532–e535 (2004).
Debiec, H. et al. Allo-immune membranous nephropathy and recombinant aryl sulfatase replacement therapy: a need for tolerance induction therapy. J. Am. Soc. Nephrol. 25 , 675–680 (2013).
Burbelo, P. D. et al. Detection of PLA2R autoantibodies before the diagnosis of membranous nephropathy. J. Am. Soc. Nephrol. 31 , 208–217 (2020).
Cremoni, M. et al. Th17-immune response in patients with membranous nephropathy is associated with thrombosis and relapses. Front. Immunol. 11 , 574997 (2020).
Motavalli, R. et al. Altered Th17/Treg ratio as a possible mechanism in pathogenesis of idiopathic membranous nephropathy. Cytokine 141 , 155452 (2021).
Rosenzwajg, M. et al. B- and T-cell subpopulations in patients with severe idiopathic membranous nephropathy may predict an early response to rituximab. Kidney Int. 92 , 227–237 (2017).
Cantarelli, C. et al. A comprehensive phenotypic and functional immune analysis unravels circulating anti-phospholipase A2 receptor antibody secreting cells in membranous nephropathy patients. Kidney Int. Rep. 5 , 1764–1776 (2020).
Berchtold, L. et al. HLA-D and PLA2R1 risk alleles associate with recurrent primary membranous nephropathy in kidney transplant recipients. Kidney Int. 99 , 671–685 (2021).
Segerer, S. & Schlondorff, D. B cells and tertiary lymphoid organs in renal inflammation. Kidney Int. 73 , 533–537 (2008).
Kolovou, K. et al. B-cell oligoclonal expansions in renal tissue of patients with immune-mediated glomerular disease. Clin. Immunol. 217 , 108488 (2020).
Prunotto, M. et al. Proteomic analysis of podocyte exosome-enriched fraction from normal human urine. J. Proteom. 82 , 193–229 (2013).
Kerjaschki, D. & Neale, T. J. Molecular mechanisms of glomerular injury in rat experimental membranous nephropathy (Heymann nephritis). J. Am. Soc. Nephrol. 7 , 2518–2526 (1996).
Huang, C. C. et al. IgG subclass staining in renal biopsies with membranous glomerulonephritis indicates subclass switch during disease progression. Mod. Pathol. 26 , 799–805 (2013).
Val-Bernal, J. F., Garijo, M. F., Val, D., Rodrigo, E. & Arias, M. C4d immunohistochemical staining is a sensitive method to confirm immunoreactant deposition in formalin-fixed paraffin-embedded tissue in membranous glomerulonephritis. Histol. Histopathol. 26 , 1391–1397 (2011).
Hayashi, N. et al. Glomerular mannose-binding lectin deposition in intrinsic antigen-related membranous nephropathy. Nephrol. Dial. Transplant. 33 , 832–840 (2018).
Bally, S. et al. Phospholipase A2 receptor-related membranous nephropathy and mannan-binding lectin deficiency. J. Am. Soc. Nephrol. 27 , 3539–3544 (2016).
Ravindran, A. et al. Proteomic analysis of complement proteins in membranous nephropathy. Kidney Int. Rep. 5 , 618–626 (2020).
Cybulsky, A. V., Quigg, R. J. & Salant, D. J. Experimental membranous nephropathy redux. Am. J. Physiol. Ren. Physiol. 289 , F660–F671 (2005).
Tomas, N. M. et al. Autoantibodies against thrombospondin type 1 domain-containing 7A induce membranous nephropathy. J. Clin. Invest. 126 , 2519–2532 (2016).
Tomas, N. M. et al. A heterologous model of thrombospondin type 1 domain-containing 7A-associated membranous nephropathy. J. Am. Soc. Nephrol. 28 , 3262–3277 (2017).
Petrosyan, A. et al. A glomerulus-on-a-chip to recapitulate the human glomerular filtration barrier. Nat. Commun. 10 , 3656 (2019).
Haddad, G. et al. Altered glycosylation of IgG4 promotes lectin complement pathway activation in anti-PLA2R1 associated membranous nephropathy. J. Clin. Invest. 131 , e140453 (2021). This article provides the first demonstration of the pathogenic role of modified IgG4 specific for PLA2R in lectin pathway activation.
Ghiggeri, G. M. et al. Multi-autoantibody signature and clinical outcome in membranous nephropathy. Clin. J. Am. Soc. Nephrol. 15 , 1762–1776 (2020).
Seikrit, C., Ronco, P. & Debiec, H. Factor H autoantibodies and membranous nephropathy. N. Engl. J. Med. 379 , 2479–2481 (2018).
Barbour, S. J. et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int. 81 , 190–195 (2012).
Kerlin, B. A., Ayoob, R. & Smoyer, W. E. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin. J. Am. Soc. Nephrol. 7 , 513–520 (2012).
Lionaki, S. et al. Venous thromboembolism in patients with membranous nephropathy. Clin. J. Am. Soc. Nephrol. 7 , 43–51 (2012).
Cattran D. C. et al. (eds) National Kidney Foundation’s Primer on Kidney Diseases 7th Edn 188–197 (Elsevier, 2018).
Agrawal, S., Zaritsky, J. J., Fornoni, A. & Smoyer, W. E. Dyslipidaemia in nephritic syndrome: mechanisms and treatment. Nat. Rev. Nephrol. 14 , 57–70 (2018).
Rodriguez, E. F. et al. Membranous nephropathy with crescents: a series of 19 cases. Am. J. Kidney Dis. 64 , 66–73 (2014).
Fogo, A. B., Lusco, M. A., Najafian, B. & Alpers, C. E. Atlas of renal pathology: membranous nephropathy. Am. J. Kidney Dis. 66 , e43–e45 (2015).
Beck, L. H., Fervenza, F. C. In Molecular Mechanisms in the Pathogenesis of Idiopathic Nephrotic Syndrome (ed. Kaneko, K.) 181–205 (Springer Japan, 2016).
Markowitz, G. S. Membranous glomerulopathy: emphasis on secondary forms and disease variants. Adv. Anat. Pathol. 8 , 119–125 (2001).
Kuroki, A. et al. Glomerular and serum IgG subclasses in diffuse proliferative lupus nephritis, membranous lupus nephritis, and idiopathic membranous nephropathy. Intern. Med. 41 , 936–942 (2002).
Ronco, P., Plaisier, E. & Debiec, H. Advances in membranous nephropathy. J. Clin. Med. 10 , 607 (2021).
Qu, Z. et al. Absence of glomerular IgG4 deposition in patients with membranous nephropathy may indicate malignancy. Nephrol. Dial. Transplant. 27 , 1931–1937 (2012).
von Haxthausen, F. et al. Antigen-specific IgG subclasses in primary and malignancy-associated membranous nephropathy. Front. Immunol. 9 , 3035 (2018).
van de Logt, A. E., Hofstra, J. M. & Wetzels, J. F. Serum anti-PLA2R antibodies can be initially absent in idiopathic membranous nephropathy: seroconversion after prolonged follow-up. Kidney Int. 87 , 1263–1264 (2015).
Debiec, H. & Ronco, P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N. Engl. J. Med. 364 , 689–690 (2011).
Svobodova, B., Honsova, E., Ronco, P., Tesar, V. & Debiec, H. Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol. Dial. Transplant. 28 , 1839–1844 (2013).
Luo, J., Zhang, W., Su, C., Zhou, Z. & Wang, G. Seropositive PLA2R-associated membranous nephropathy but biopsy-negative PLA2R staining. Nephrol. Dial. Transplant. https://doi.org/10.1093/ndt/gfaa239 (2020).
Article PubMed PubMed Central Google Scholar
Poggio, E. D. et al. Systematic review and meta-analysis of native kidney biopsy complications. Clin. J. Am. Soc. Nephrol. 15 , 1595–1602 (2020).
Pombas, B. et al. Risk factors associated with major complications after ultrasound-guided percutaneous renal biopsy of native kidneys. Kidney Blood. Press. Res. 45 , 122–130 (2020).
Palsson, R. et al. Bleeding complications after percutaneous native kidney biopsy: results from the Boston Kidney Biopsy cohort. Kidney Int. Rep. 5 , 511–518 (2020).
Atwell, T. D. et al. The timing and presentation of major hemorrhage after 18,947 image-guided percutaneous biopsies. Am. J. Roentgenol. 205 , 190–195 (2015).
Bobart, S. A. et al. Noninvasive diagnosis of primary membranous nephropathy using phospholipase A2 receptor antibodies. Kidney Int. 95 , 429–438 (2019).
Bobart, S. A. et al. Kidney biopsy is required for nephrotic syndrome with PLA2R + and normal kidney function: the Con View. Kidney 360 , 890–893 (2020).
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 100 , S1–S276 (2021). A milestone for the management of glomerular diseases.
Bobart, S. A. et al. A target antigen-based approach to the classification of membranous nephropathy. Mayo Clin. Proc. 96 , 577–591 (2021).
Larsen, C. P. et al. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod. Pathol. 26 , 709–715 (2013).
Stehle, T. et al. Phospholipase A2 receptor and sarcoidosis-associated membranous nephropathy. Nephrol. Dial. Transplant. 30 , 1047–1050 (2015).
Xie, Q. et al. Renal phospholipase A2 receptor in hepatitis B virus-associated membranous nephropathy. Am. J. Nephrol. 41 , 345–353 (2015).
Berchtold, L. et al. Efficacy and safety of rituximab in hepatitis B virus-associated PLA2R-positive membranous nephropathy. Kidney Int. Rep. 3 , 486–491 (2018).
Qin, W. et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J. Am. Soc. Nephrol. 22 , 1137–1143 (2011).
Hanset, N. et al. Podocyte antigen staining to identify distinct phenotypes and outcomes in membranous nephropathy: a retrospective multicenter cohort study. Am. J. Kidney Dis. 76 , 624–635 (2020).
Zhang, Z. et al. Duodenal schwannoma as a rare association with membranous nephropathy: a case report. Am. J. Kidney Dis. 73 , 278–280 (2019).
Zaghrini, C. et al. Novel ELISA for thrombospondin type 1 domain-containing 7A autoantibodies in membranous nephropathy. Kidney Int. 95 , 666–679 (2019).
Sharma, S. G. & Larsen, C. P. Tissue staining for THSD7A in glomeruli correlates with serum antibodies in primary membranous nephropathy: a clinicopathological study. Mod. Pathol. 31 , 616–622 (2018).
Schlumberger, W. et al. Differential diagnosis of membranous nephropathy with autoantibodies to phospholipase A2 receptor 1. Autoimmun. Rev. 13 , 108–113 (2014).
Timmermans, S. A. et al. Evaluation of anti-PLA2R1 as measured by a novel ELISA in patients with idiopathic membranous nephropathy: a cohort study. Am. J. Clin. Pathol. 142 , 29–34 (2014).
Liu, Y. et al. Serum anti-PLA2R antibody as a diagnostic biomarker of idiopathic membranous nephropathy: the optimal cut-off value for Chinese patients. Clin. Chim. Acta 476 , 9–14 (2018).
Behnert, A. et al. Antiphospholipase A2 receptor autoantibodies: a comparison of three different immunoassays for the diagnosis of idiopathic membranous nephropathy. J. Immunol. Res. 2014 , 143274 (2014).
Pourcine, F. et al. Prognostic value of PLA2R autoimmunity detected by measurement of anti-PLA2R antibodies combined with detection of PLA2R antigen in membranous nephropathy. A single-centre study over 14 years. PLoS ONE 12 , e0173201 (2017).
Plaisier, E. & Ronco, P. Screening for cancer in patients with glomerular diseases. Clin. J. Am. Soc. Nephrol. 15 , 886–888 (2020).
Cambier, J. F. & Ronco, P. Onco-nephrology: glomerular diseases with cancer. Clin. J. Am. Soc. Nephrol. 7 , 1701–1712 (2012).
Timmermans, S. A. et al. Anti-phospholipase A2 receptor antibodies and malignancy in membranous nephropathy. Am. J. Kidney Dis. 62 , 1223–1225 (2013).
Wang, G. et al. Neural epidermal growth factor-Like 1 protein-positive membranous nephropathy in Chinese patients. Clin. J. Am. Soc. Nephrol. 8 , 727–735 (2021).
Ayalon, R. & Beck, L. H. Jr Membranous nephropathy: not just a disease for adults. Pediatr. Nephrol. 30 , 31–39 (2015).
Debiec, H., Ronco, P. & Vivarelli, M. Congenital membranous nephropathy due to fetomaternal anti-neutral endopeptidase alloimmunization. Orphanet https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=69063 (2020).
Bhimma, R. & Coovadia, H. M. Hepatitis B virus-associated nephropathy. Am. J. Nephrol. 24 , 198–211 (2004).
Safar-Boueri, L., Piya, A., Beck, L. H. Jr & Ayalon, R. Membranous nephropathy: diagnosis, treatment, and monitoring in the post-PLA2R era. Pediatr. Nephrol. 36 , 19–30 (2021).
Debiec, H. et al. Allo-immune membranous nephropathy and recombinat arylsulfatase replacement therapy: a need for tolerance induction therapy. J. Am. Soc. Nephrol. 25 , 675–680 (2014).
Wang, R. et al. Long-term renal survival and related risk factors for primary membranous nephropathy in Chinese children: a retrospective analysis of 217 cases. J. Nephrol. 34 , 589–596 (2021).
Ramachandran R. & Jha, V. in Glomerulonephritis (eds Trachtman H., Hogan J. J., Herlitz L. & Lerma E. V.) (Springer, 2020).
Htay, H. et al. Global access of patients with kidney disease to health technologies and medications: findings from the Global Kidney Health Atlas project. Kidney Int. Suppl. 8 , 64–73 (2018).
Hepatitis B Foundation. World Health Organization. Addressing Hepatitis B in Africa. Hepatitis B Foundation https://www.hepb.org/blog/addressing-hepatitis-b-africa/ (2020).
Kumar, M. N. et al. Membranous nephropathy associated with indigenous Indian medications containing heavy metals. Kidney Int. Rep. 5 , 1510–1514 (2020).
Landrigan, P. J. et al. The Lancet Commission on pollution and health. Lancet 391 , 462–512 (2018).
Gupta, S., Pepper, R. J., Ashman, N. & Walsh, S. B. Nephrotic syndrome: oedema formation and its treatment with diuretics. Front. Physiol. 9 , 1868 (2019).
Hinrichs, G. R., Jensen, B. L. & Svenningsen, P. Mechanisms of sodium retention in nephrotic syndrome. Curr. Opin. Nephrol. Hypertens. 29 , 207–212 (2020).
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int. Suppl. 2 , 186–197 (2012).
Polanco, N. et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 2 , 697–704 (2020).
Pincus, K. J. & Hynicka, L. M. Prophylaxis of thromboembolic events in patients with nephrotic syndrome. Ann. Pharmacother. 47 , 725–734 (2013).
Medjeral-Thomas, N. et al. Retrospective analysis of a novel regimen for the prevention of venous thromboembolism in nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 9 , 478–483 (2014).
Hofstra, J. M. & Wetzels, J. F. M. Should aspirin be used for primary prevention of thrombotic events in patients with membranous nephropathy? Kidney Int. 89 , 981–983 (2016).
Sexton, D. J. et al. Direct-acting oral anticoagulants as prophylaxis against thromboembolism in the nephrotic syndrome. Kidney Int. Rep. 3 , 784–793 (2018).
Reynolds, M. L., Nachman, P. H., Mooberry, M. J., Crona, D. J. & Derebail, V. K. Recurrent venous thromboembolism in primary membranous nephropathy despite direct Xa inhibitor therapy. J. Nephrol. 32 , 669–672 (2019).
van de Logt, A. E., Hofstra, J. M. & Wetzels, J. F. Pharmacological treatment of primary membranous nephropathy in 2016. Expert Rev. Clin. Pharmacol. 9 , 1463–1478 (2016).
van den Brand, J. A., van Dijk, P. R., Hofstra, J. M. & Wetzels, J. F. Long-term outcomes in idiopathic membranous nephropathy using a restrictive treatment strategy. J. Am. Soc. Nephrol. 25 , 150–158 (2014).
Reichert, L. J., Koene, R. A. & Wetzels, J. F. Prognostic factors in idiopathic membranous nephropathy. Am. J. Kidney Dis. 31 , 1–11 (1998).
Hladunewich, M. A., Troyanov, S., Calafati, J. & Cattran, D. C. Metropolitan Toronto Glomerulonephritis Registry. The natural history of the non-nephrotic membranous nephropathy patient. Clin. J. Am. Soc. Nephrol. 4 , 1417–1422 (2009).
Hoxha, E., Harendza, S., Pinnschmidt, H., Panzer, U. & Stahl, R. A. PLA2R antibody levels and clinical outcome in patients with membranous nephropathy and non-nephrotic range proteinuria under treatment with inhibitors of the renin-angiotensin system. PLoS ONE 9 , e110681 (2014).
Pei, Y., Cattran, D. & Greenwood, C. Predicting chronic renal insufficiency in idiopathic membranous glomerulonephritis. Kidney Int. 42 , 960–966 (1992).
Howman, A. et al. Immunosuppression for progressive membranous nephropathy: a UK randomised controlled trial. Lancet 381 , 744–751 (2013).
Cattran, D. C. et al. Validation of a predictive model of idiopathic membranous nephropathy: its clinical and research implications. Kidney Int. 51 , 901–907 (1997).
van den Brand, J. A., Hofstra, J. M. & Wetzels, J. F. Prognostic value of risk score and urinary markers in idiopathic membranous nephropathy. Clin. J. Am. Soc. Nephrol. 7 , 1242–1248 (2012).
van de Logt, A. E. et al. Anti-PLA2R1 antibodies as prognostic biomarker in membranous nephropathy. Kidney Int. Rep. 6 , 1677–1686 (2021).
Lerner, G. B., Virmani, S., Henderson, J. M., Francis, J. M. & Beck, L. H. Jr. A conceptual framework linking immunology, pathology, and clinical features in primary membranous nephropathy. Kidney Int. 100 , 289–300 (2021).
Faul, C. et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat. Med. 14 , 931–938 (2008).
Ramachandran, R. et al. Two-year follow-up study of membranous nephropathy treated with tacrolimus and corticosteroids versus cyclical corticosteroids and cyclophosphamide. Kidney Int. Rep. 2 , 610–616 (2017).
van de Logt, A. E. et al. Immunological remission in PLA2R-antibody-associated membranous nephropathy: cyclophosphamide versus rituximab. Kidney Int. 93 , 1016–1017 (2018).
Hanset, N. et al. Rituximab in patients with phospholipase A2 receptor-associated membranous nephropathy and severe CKD. Kidney Int. Rep. 5 , 331–338 (2019).
Dahan, K. et al. Retreatment with rituximab for membranous nephropathy with persistently elevated titers of anti-phospholipase A2 receptor antibody. Kidney Int. 95 , 233–234 (2019).
van de Logt, A. E. et al. The bias between different albumin assays may affect clinical decision-making. Kidney Int. 95 , 1514–1517 (2019).
Bech, A. P., Hofstra, J. M., Brenchley, P. E. & Wetzels, J. F. Association of anti-PLA 2 R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin. J. Am. Soc. Nephrol. 9 , 1386–1392 (2014).
Filler, G. et al. Another case of HBV associated membranous glomerulonephritis resolving on lamivudine. Arch. Dis. Child. 88 , F154–F156 (2003).
O’Shaughnessy, M. M. et al. Treatment patterns among adults and children with membranous nephropathy in the Cure Glomerulonephropathy Network (CureGN). Kidney Int. Rep. 4 , 1725–1734 (2019).
Riaz, P. et al. Workforce capacity for the care of patients with kidney failure across world countries and regions. BMJ Glob. Health 6 , e004014 (2021).
Jha, V. et al. Understanding kidney care needs and implementation strategies in low- and middle-income countries: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) controversies conference. Kidney Int. 90 , 1164–1174 (2016).
Cosio, F. G. & Cattran, D. C. Recent advances in our understanding of recurrent primary glomerulonephritis after kidney transplantation. Kidney Int. 91 , 304–314 (2017).
Grupper, A. et al. Recurrent membranous nephropathy after kidney transplantation: treatment and long-term implications. Transplantation 100 , 2710–2716 (2016).
Seitz-Polski, B. et al. Prediction of membranous nephropathy recurrence after transplantation by monitoring of anti-PLA2R1 (M-type phospholipase A2 receptor) autoantibodies: a case series of 15 patients. Nephrol. Dial. Transplant. 29 , 2334–2342 (2014).
Quintana, L. F. et al. Antiphospholipase A2 receptor antibody levels predict the risk of posttransplantation recurrence of membranous nephropathy. Transplantation 99 , 1709–1714 (2015).
Andrésdóttir, M. B. & Wetzels, J. F. Increased risk of recurrence of membranous nephropathy after related donor kidney transplantation. Am. J. Transplant. 12 , 265–266 (2012).
Gupta, G. et al. Pre-transplant phospholipase A2 receptor autoantibody concentration is associated with clinically significant recurrence of membranous nephropathy post-kidney transplantation. Clin. Transplant. 30 , 461–469 (2016).
Batal, I. et al. Association of HLA typing and alloimmunity with posttransplantation membranous nephropathy: a multicenter case series. Am. J. Kidney Dis. 76 , 374–383 (2020).
Kattah, A. et al. Anti-phospholipase A 2 receptor antibodies in recurrent membranous nephropathy. Am. J. Transplant. 15 , 1349–1359 (2015).
WHO. Programme on Mental Health. WHOQOL User Manual (World Health Organization, 1998).
Wyld, M., Morton, R. L., Hayen, A., Howard, K. & Webster, A. C. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med. 9 , e1001307 (2012).
Chuasuwan, A., Pooripussarakul, S., Thakkinstian, A., Ingsathit, A. & Pattanaprateep, O. Comparisons of quality of life between patients underwent peritoneal dialysis and hemodialysis: a systematic review and meta-analysis. Health Qual. Life. Outcomes 18 , 191 (2020).
Canetta, P. A. et al. Health-related quality of life in glomerular disease. Kidney Int. 95 , 1209–1224 (2019).
Cattran, D. Management of membranous nephropathy: when and what for treatment. J. Am. Soc. Nephrol. 16 , 1188–1194 (2005).
Libório, A. B. et al. Proteinuria is associated with quality of life and depression in adults with primary glomerulopathy and preserved renal function. PLoS ONE 7 , e37763 (2012).
Floege, J. et al. Management and treatment of glomerular diseases (part 1): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 95 , 268–280 (2019).
Murphy, S. L. et al. Longitudinal changes in health-related quality of life in primary glomerular disease: results from the CureGN study. Kidney Int. Rep. 5 , 1679–1689 (2020).
Center for Drug Evaluation and Research, Center for Devices and Radiological Health and Center for Biologics Evaluation and Research. Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development To Support Labeling Claims (FDA, 2009).
Bansal, D., Bhagat, A., Schifano, F. & Gudala, K. Role of patient-reported outcomes and other efficacy endpoints in the drug approval process in Europe (2008-2012). J. Epidemiol. Glob. Health 5 , 385–395 (2015).
Carter, S. A. et al. Identifying outcomes important to patients with glomerular disease and their caregivers. Clin. J. Am. Soc. Nephrol. 15 , 673–684 (2020).
Henkel, C. & Hoffmann, P. (Eds) MALDI Imaging. Biochim. Biophys. Acta 1865 , 725–978 (2017).
Neumann, E. K., Djambazova, K. V., Caprioli, R. M. & Spraggins, J. M. Multimodal imaging mass spectrometry: next generation molecular mapping in biology and medicine. J. Am. Soc. Mass. Spectrom. 31 , 2401–2415 (2020).
Unnersjö-Jess, D., Scott, L., Blom, H. & Brismar, H. Super-resolution stimulated emission depletion imaging of slit diaphragm proteins in optically cleared kidney tissue. Kidney Int. 89 , 243–247 (2016).
Park, J., Liu, C. L., Kim, J. & Susztak, K. Understanding the kidney one cell at a time. Kidney Int. 96 , 862–870 (2019).
Ellebrecht, C. T. et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 353 , 179–184 (2016).
Wraith, D. Autoimmunity: antigen-specific immunotherapy. Nature 530 , 422–423 (2016).
Abedini, A. et al. Urinary single-cell profiling captures the cellular diversity of the kidney. J. Am. Soc. Nephrol. 32 , 614–627 (2021).
Unlu, G. et al. GRIK5 genetically regulated expression associated with eye and vascular phenomes: discovery through iteration among biobanks, electronic health records, and Zebrafish. Am. J. Hum. Genet. 104 , 503–519 (2019).
Reddy, V. et al. Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology 56 , 1227–1237 (2017).
Klomjit, N., Fervenza, F. C. & Zand, L. Successful treatment of patients with refractory PLA 2 R-associated membranous nephropathy with obinutuzumab: a report of 3 cases. Am. J. Kidney Dis. 76 , 883–888 (2020).
Podestà, M. A., Ruggiero, B., Remuzzi, G. & Ruggenenti, P. Ofatumumab for multirelapsing membranous nephropathy complicated by rituximab-induced serum- sickness. BMJ Case. Rep. 13 , e232896 (2020).
Schmidt, T., Schulze, M., Harendza, S. & Hoxha, E. Successful treatment of PLA2R1-antibody positive membranous nephropathy with ocrelizumab. J. Nephrol. 34 , 603–606 (2021).
Samy, E., Wax, S., Huard, B., Hess, H. & Schneider, P. Targeting BAFF and APRIL in systemic lupus erythematosus and other antibody-associated diseases. Int. Rev. Immunol. 36 , 3–19 (2017).
Mahévas, M. et al. Efficacy, safety and immunological profile of combining rituximab with belimumab for adults with persistent or chronic immune thrombocytopenia: results from a prospective phase 2b trial. Haematologica https://doi.org/10.3324/haematol.2020.259481 (2020).
Article PubMed Central Google Scholar
Furie, R. et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N. Engl. J. Med. 383 , 1117–1128 (2020).
Barrett, C. et al. Effect of belimumab on proteinuria and anti-phospholipase A2 receptor autoantibody in primary membranous nephropathy. Nephrol. Dial. Transplant. 35 , 599–606 (2020).
Crickx, E., Weill, J. C., Reynaud, C. A. & Mahévas, M. Anti-CD20-mediated B-cell depletion in autoimmune diseases: successes, failures and future perspectives. Kidney Int. 97 , 885–893 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04145440 (2021).
Weinmann-Menke, J. et al. Treatment of membranous nephropathy in patients with THSD7A antibodies using immunoadsorption. Am. J. Kidney Dis. 74 , 849–852 (2019).
Podestà, M. A. et al. Accelerating the depletion of circulating anti-phospholipase A2 receptor antibodies in patients with severe membranous nephropathy: preliminary findings with double filtration plasmapheresis and ofatumumab. Nephron 144 , 30–35 (2020).
Zipfel, P. F. et al. Complement inhibitors in clinical trials for glomerular diseases. Front. Immunol. 10 , 2166 (2019).
Low, J. H. et al. Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network. Cell Stem. Cell 25 , 373–387 (2019).
Maas, R. J. et al. Kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as prognostic markers in idiopathic membranous nephropathy. Ann. Clin. Biochem. 53 , 51–57 (2016).
van den Brand, J. A., Hofstra, J. M. & Wetzels, J. F. Low-molecular-weight proteins as prognostic markers in idiopathic membranous nephropathy. Clin. J. Am. Soc. Nephrol. 6 , 2846–2853 (2011).
Download references
Acknowledgements
L.B. acknowledges institutional funding for the Glomerular Disease Center at Boston Medical Center in support of the preparation of this manuscript. J.W. acknowledges support from grants from the Dutch Kidney Foundation (grant nrNSN 17PhD12), funding from European Union Seventh Framework Programme FP7/2007-2013 grant 305608: European Consortium for High-Throughput Research in Rare Kidney Diseases. V.J. acknowledges research grants from Baxter Healthcare, GSK, and NephroPlus and honoraria/speaker fees from Baxter Healthcare and AstraZeneca (all monies paid to the employer). P.R. acknowledges support from grants from the National Research Agency: MNaims (ANR-17-CE17-0012-01) and SeroNegMN (ANR-20-CE17-0017-01). F.C.F. acknowledges unrestricted research grants from Genentech Inc., Roche and MorphoSys for research on Membranous Nephropathy (all funds paid to the institution). M.V., P.R. and J.W. acknowledge the European Rare Kidney Disease Network (ERKNet). A.T. is supported by a National Health and Medical Research Council Investigator Award (1197324). F.F.H. acknowledges support from the Clinical Innovation Research Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (2018GZR0201003).
Author information
Authors and affiliations.
Sorbonne Université, and Institut National de la Santé et de la Recherche Médicale, Unité Mixte de Recherche, S1155, Paris, France
Pierre Ronco & Hanna Debiec
Department of Nephrology, Centre Hospitalier du Mans, Le Mans, France
Pierre Ronco
Section of Nephrology, Department of Medicine, Boston Medical Center and Boston University School of Medicine, Boston, MA, USA
Laurence Beck
Division of Nephrology and Hypertension, Mayo Clinic, Rochester, MN, USA
Fernando C. Fervenza
National Clinical Research Center for Kidney Disease, Nanfang Hospital, State Key Laboratory of Organ Failure Research, Southern Medical University, Guangzhou, China
Fan Fan Hou
George Institute for Global Health, UNSW, New Delhi, India
Vivekanand Jha
School of Public Health, Imperial College, London, UK
Prasanna School of Higher Education, Manipal Academy of Higher Education, Manipal, India
Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, USA
Sanjeev Sethi
Sydney School of Public Health, University of Sydney, Sydney, NSW, Australia
Allison Tong
Centre for Kidney Research, The Children’s Hospital at Westmead, Sydney, NSW, Australia
Division of Nephrology and Dialysis - Bambino Gesù Pediatric Hospital IRCCS, Rome, Italy
Marina Vivarelli
Department of Nephrology, Radboud University Medical Center, Nijmegen, Netherlands
Jack Wetzels
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (P.R.); Epidemiology (P.R. and F.F.H.); Mechanisms/pathophysiology (L.B., H.D. and S.S.); Diagnosis/screening/prevention (F.C.F., V.J. and M.V.); Management (V.J., M.V. and J.W.); Quality of life (V.J. and A.T.); Outlooks (P.R. and L.B.); Overview of the Primer (P.R.).
Corresponding author
Correspondence to Pierre Ronco .
Ethics declarations
Competing interests.
L.B. is co-inventor on the patent “Diagnostics for Membranous Nephropathy” and has received consultancy fees from Alexion, Ionis, Genentech and Visterra for topics related to membranous nephropathy as well as research support from Sanofi Genzyme and Pfizer. P.R. has received consultancy fees from Alexion and MorphoSys for topics related to membranous nephropathy. F.C.F. has received consultancy fees from Alexion and MorphoSys for topics related to membranous nephropathy. F.F.H. has received consultancy fees from AbbVie and AstraZeneca. J.W. has received consultancy fees from Novartis and MorphoSys for topics related to membranous nephropathy. The other authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Disease Primers thanks L. Barisoni, S. Maruyama and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
A disease of the podocyte, the main glomerular cell controlling the filtration of proteins.
(GFR). The most reliable parameter to assess renal function and can be evaluated with formulas (eGFR) or measured (mGFR).
A model of membranous nephropathy developed by Walter Heymann in the rat in the 1950s that is still the most reliable experimental model for human membranous nephropathy.
Immunization against a non-self human antigen (protein or sugar) as occurs after blood transfusions or in case of Rhesus incompatibility during pregnancy, resulting in the production of allo-antibodies.
Diffusion of the immune response to additional epitopes on the same molecule or different molecules.
Immunofluorescence studies show staining for IgA, IgG, IgM, C1q, C3, κ and λ light chains.
Production of xeno-antibodies results from immunization against a non-self, non-human antigen (protein or sugar); a process termed xeno-immunization.
One of several proteolytic fragments generated by complement activation that promote acute inflammation and mediation of the immune response.
Severe and generalized form of oedema, with subcutaneous tissue swelling, that usually involves the cavities of the body.
A pathological condition that predisposes to thrombosis.

Rights and permissions
Reprints and permissions
About this article
Cite this article.
Ronco, P., Beck, L., Debiec, H. et al. Membranous nephropathy. Nat Rev Dis Primers 7 , 69 (2021). https://doi.org/10.1038/s41572-021-00303-z
Download citation
Accepted : 18 August 2021
Published : 30 September 2021
DOI : https://doi.org/10.1038/s41572-021-00303-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Pla2r-positive membranous nephropathy in igg4-related disease.
- Yusuke Ushio
- Taro Akihisa
- Junichi Hoshino
BMC Nephrology (2024)
Serum uric acid level is associated with glomerular ischemic lesions in patients with primary membranous nephropathy: an analytical, cross-sectional study
- Junjun Zhang
Scientific Reports (2024)
A guide to complement biology, pathology and therapeutic opportunity
- Dimitrios C. Mastellos
- George Hajishengallis
- John D. Lambris
Nature Reviews Immunology (2024)
Significance of the total renal chronicity score in predicting renal outcome in PLA2R-associated membranous nephropathy
Journal of Nephrology (2024)
Causal association between cardiovascular proteins and membranous nephropathy: a bidirectional Mendelian randomization
International Urology and Nephrology (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Case report
- Open access
- Published: 20 April 2024
Immunoglobulin G4-related disease presenting with nephrotic syndrome due to minimal change disease: a case report
- Amy Needleman 1 ,
- Michael Sheaff 2 ,
- Ruth J. Pepper 1 &
- Rhys D. R. Evans ORCID: orcid.org/0000-0001-7543-2041 1
Journal of Medical Case Reports volume 18 , Article number: 192 ( 2024 ) Cite this article
126 Accesses
1 Altmetric
Metrics details
Immunoglobulin G4-related disease is an inflammatory disease affecting multiple organs including the kidney. Immunoglobulin G4-related kidney disease most commonly manifests as a tubulointerstitial nephritis and is associated with glomerular disease in a proportion of cases. Membranous nephropathy is the most frequent glomerular lesion. Herein, we report the first documented case of immunoglobulin G4-related disease presenting with nephrotic syndrome owing to minimal change disease.
Case presentation
A 67-year-old South Asian male presented to our service with systemic upset and leg swelling. He had heavy proteinuria (urine protein:creatinine ratio 1042 mg/mmol) and was hypoalbuminemic (17 g/L) and hypercholersterolemic (9.3 mmol/L), consistent with the nephrotic syndrome. His serum creatinine was 140 μmol/L, and he was hypocomplementemic (C3 0.59 g/L, C4 < 0.02 g/L) with raised immunoglobulin G4 subclass levels (5.29 g/L). Kidney biopsy demonstrated minimal change disease alongside a plasma-cell-rich tubulointerstitial nephritis with strong positive staining for immunoglobulin G4. A diagnosis of minimal change disease in the setting of immunoglobulin G4-related disease was made. He was commenced on oral prednisolone at 60 mg daily but suffered infectious complications, including necrotizing fasciitis within 3 weeks of starting treatment, ultimately resulting in his death 52 days after initial presentation.
This case highlights the potential for immunoglobulin G4-related disease to be associated with a spectrum of glomerular pathologies including minimal change disease. It adds to the differential diagnosis of secondary causes of minimal change disease, and moreover, aids as an important reminder of the potential complications of high-dose steroids used in its treatment.
Peer Review reports
Immunoglobulin G4-related disease (IgG4-RD) is a multisystem disease associated with lymphoplasmacytic inflammation and fibrosis. It is characterized by IgG4 + plasma cell infiltration of affected tissues and elevated serum IgG4 in some patients. Kidney involvement in IgG4-RD (IgG4-related kidney disease; IgG4-RKD) can be the result of intrinsic kidney disease or be due to ureteric obstruction from retroperitoneal fibrosis [ 1 , 2 ]. Intrinsic kidney disease occurs in 12% of cases in the UK and usually consists of a tubulointerstitial nephritis (TIN) [ 3 ]. Kidney biopsy findings include lymphoplasmocytic infiltration, predominantly with IgG4+ plasma cells, with associated fibrosis that may be storiform in nature [ 4 ]. Tubular immune deposits are present in the majority of cases. TIN presents with a reduction in excretory kidney function or with mass lesions on imaging. It is associated with glomerular disease in around one-quarter of cases, which may present with edema especially if the nephrotic syndrome develops. The predominant glomerular lesion found in IgG4-RKD is membranous nephropathy (MN); IgA nephropathy and membranoproliferative glomerulonephritis have also been described [ 5 ]. Herein, we describe the first reported case of IgG4-RD presenting with minimal change disease (MCD). The case highlights the broad spectrum of glomerular pathologies associated with IgG4-RD and adds to the differential diagnosis of secondary causes of MCD.
A 67-year-old male of South Asian background with history of hypertension and type 2 diabetes presented with a 4-month history of systemic upset, 7 kg weight loss, lethargy, and migratory joint pains. He was referred to our service having developed leg swelling and shortness of breath with associated significant proteinuria (urine protein:creatinine ratio 1042 mg/mmol). On examination, there was pitting peripheral edema to the mid shins without rash, embolic phenomena, or cardiac murmur. There was reduced air entry in the lungs bi-basally, blood pressure was 145/89 and oxygen saturation was 98% on room air. His serum creatinine was 140 μmol/L, and he was hypoalbuminemic (17 g/L) and hypercholersterolemic (9.3 mmol/L), consistent with the nephrotic syndrome. Further investigation demonstrated hypocomplementemia (C3 0.59 g/L, C4 < 0.02 g/L) and raised IgG4 subclass levels (5.29 g/L) (Table 1 ). Ultrasound showed cortical irregularity of both kidneys and a positron emission tomography-computed tomography (PET-CT) demonstrated multiple avid lymph nodes in addition to uptake in the spleen, pancreas, and prostate (Fig. 1 a).
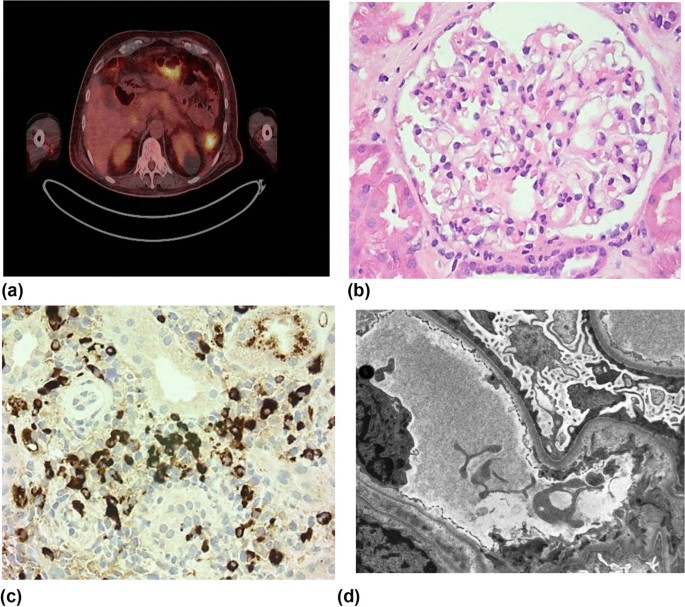
Imaging and histopathology demonstrating minimal change disease in the setting of immunoglobulin G4-related disease. a Positron emission tomography–computed tomography scan demonstrating splenic uptake and likely pancreatic uptake. b Light microscopy (hematoxylin and eosin stain; magnification × 400) demonstrating a histologically normal glomerulus. c Light microscopy (immunoglobulin G4 immunostain; magnification × 250) demonstrating multiple immunoglobulin G4 + plasma cells. d Electron microscopy demonstrating complete foot process effacement of podocytes with microvillus transformation and an absence of electron dense deposits (× 2500)
The differential diagnosis for nephrotic syndrome with low (consumed) complement includes immune-complex related glomerulonephritides [such as an infection-related glomerulonephritis (GN), membranoproliferative GN, lupus nephritis, and Sjögren’s syndrome-associated GNs], cholesterol embolization, and glomerular disease in the setting of IgG4-RD. The patient underwent diagnostic kidney biopsy to differentiate between these potential causes. Glomeruli were unremarkable on light microscopy with no glomerular immunoglobulin staining (Fig. 1 b). Electron microscopy demonstrated complete foot process effacement without the presence of electron dense deposits, consistent with MCD (Fig. 1 c). In addition, there was a plasma-cell-rich interstitial infiltrate with strong positive staining for IgG4 (52 IgG+ plasma cells/high power field and 60% IgG4/IgG ratio), fulfilling Raissan’s diagnostic criteria for IgG4-RKD [ 6 ] (Fig. 1 d). A unifying diagnosis of MCD in the setting of IgG4-RKD was made.
The patient was treated with oral prednisolone at 60 mg daily alongside conservative measures for the nephrotic syndrome (angiotensin converting enzyme inhibition, furosemide, atorvastatin, and heparin). There was no significant glomerular response, and on day 17 he presented to hospital with an enlarging erythematous, macular rash on his thigh, which was extremely tender to palpation. He was systemically unwell with fever and a clinical diagnosis of necrotizing fasciitis was made. Despite intravenous antibiotics and multiple surgical debridements, he became increasingly septic requiring ventilatory and renal support within intensive care. Immunosuppression was reduced, and intravenous immunoglobulin was given owing to new-onset hypogammaglobulinemia. Despite this, he developed bacteremia ( Enterobacter cloacae and Enterococcus faecium ) and fungemia ( Candida albicans ) with resultant mitral valve endocarditis. His disease course culminated in acute bowel ischemia and a decision was made at this point to provide palliative care. The patient died 52 days after initial presentation.
Glomerular diseases, including those that result in the nephrotic syndrome, may be primary processes or secondary to systemic disease and medications. MCD in both children and adults is most often primary, thought to be the result of T cell dysfunction and the production of a circulating permeability factor. Secondary causes, however, should be specifically excluded as their presence may impact management decisions, including targeting of the precipitating factor or disease process. Secondary causes of MCD include medications (such as nonsteroidal anti-inflammatory drugs), malignancies (most commonly lymphoproliferative diseases), infections, and allergy [ 7 ]. While MCD has previously been reported in Japan in a patient with known IgG4-RD [ 8 ], to the best of our knowledge, this is the first reported case of IgG4-RD presenting with MCD and the first case of IgG4-related MCD in a patient in Europe.
IgG4-RKD is associated with diverse renal manifestations. It was initially reported in cohorts predominantly from Asia and North America [ 9 , 10 , 11 , 12 ], but more recent studies are reported from Europe [ 3 , 13 ]. Intrinsic kidney disease occurs in 7–44% of patients and TIN is the most common histological lesion. This may be associated with glomerular disease in 9–39% of cases. We previously demonstrated that, in a UK-based cohort, 27% of TIN cases had glomerular disease; all glomerular disease in this cohort was MN [ 3 ]. This included one case of MN with no associated TIN, highlighting the potential for glomerular disease in IgG4-RD in isolation without tubulointerstitial inflammation. The pathogenesis of glomerular disease in IgG4-RD is unclear, irrespective of the pattern of glomerular injury. Patients with MN do not have detectable autoantibodies to the phospholipase A2 receptor, although antibodies to other podocyte antigens have been described [ 14 ]. The absence of immune deposits in MCD goes against this being an antibody mediated process; whether T cell dysfunction (as part of wider dysfunction of the immune system) or a circulating factor contributes to the pathogenesis of MCD in IgG4-RD are unexplored. There are no randomized controlled studies to guide the management of IgG4-RKD. TIN is often responsive to steroid therapy, but frequently relapses and steroid sparing agents are increasingly used. There are even less data to guide the management of IgG4-related glomerular disease. We approach management as we would for the respective primary glomerular lesion and aim to make management decisions within a dedicated IgG4-RD multidisciplinary team.
This case demonstrates both the need to consider IgG4-RD as a secondary cause of MCD (particularly in patients with hypocomplementemia which is not associated with primary MCD) and supports an increasing awareness of the broad range of glomerular pathologies associated with IgG4-RKD. It highlights the need for clinicians to be aware of IgG4-RKD as a potential cause in patients presenting with the nephrotic syndrome. Moreover, it reinforces the need for further research into the pathogenesis of glomerular disease in IgG4-RD, in addition to the need for more data to guide its management.
Availability of data and materials
The datasets during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Cornell LD. IgG4-related kidney disease. Curr Opin Nephrol Hypertens. 2012;21:279–88.
Article CAS PubMed Google Scholar
Saeki T, Kawano M. IgG4-related kidney disease. Kidney Int. 2014;85:251–7.
Evans RDR, et al . Clinical manifestations and long-term outcomes of IgG4-related kidney and retroperitoneal involvement in a United Kingdom IgG4-related disease cohort. Kidney Int Rep. 2019;4:48–58.
Article PubMed Google Scholar
Kawano M, et al . Proposal for diagnostic criteria for IgG4-related kidney disease. Clin Exp Nephrol. 2011;15:615–26.
Tian M, et al . Co-occurrence of IgA nephropathy and IgG4-Tubulointersitial nephritis effectively treated with tacrolimus: a case report. BMC Nephrol. 2021;22:279.
Article CAS PubMed PubMed Central Google Scholar
Raissian Y, et al . Diagnosis of IgG4-related tubulointerstitial nephritis. J Am Soc Nephrol JASN. 2011;22:1343–52.
Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal change disease. Clin J Am Soc Nephrol CJASN. 2017;12:332–45.
Yamada K, et al . A case developing minimal change disease during the course of IgG4-related disease. Mod Rheumatol. 2017;27:712–5.
Saeki T, et al . Clinicopathological characteristics of patients with IgG4-related tubulointerstitial nephritis. Kidney Int. 2010;78:1016–23.
Saeki T, et al . The clinical course of patients with IgG4-related kidney disease. Kidney Int. 2013;84:826–33.
Khosroshahi A, et al . Rethinking Ormond’s disease: ‘idiopathic’ retroperitoneal fibrosis in the era of IgG4-related disease. Medicine (Baltimore). 2013;92:82–91.
Kawano M, et al . Immunohistochemical characteristics of IgG4-related tubulointerstitial nephritis: detailed analysis of 20 Japanese cases. Int J Rheumatol. 2012;2012: 609795.
Article PubMed PubMed Central Google Scholar
Chaba A, et al . Clinical and prognostic factors in patients with IgG4-related kidney disease. Clin J Am Soc Nephrol CJASN. 2023;18:1031–40.
Buelli S, et al . Mitochondrial-dependent autoimmunity in membranous nephropathy of IgG4-related disease. EBioMedicine. 2015;2:456–66.
Download references
Acknowledgements
Not applicable.
Author information
Authors and affiliations.
UCL Centre for Kidney and Bladder Health, Royal Free Hospital, Pond Street, London, NW3 2QG, UK
Amy Needleman, Ruth J. Pepper & Rhys D. R. Evans
Department of Histopathology, Bart’s Health NHS Trust, London, UK
Michael Sheaff
You can also search for this author in PubMed Google Scholar
Contributions
AN, RP, and RE provided clinical care in the case. MS undertook histological examination of the kidney. AN and RE drafted the manuscript. All authors reviewed the manuscript and approved it for publication.
Corresponding author
Correspondence to Rhys D. R. Evans .
Ethics declarations
Ethics approval and consent to participate, consent for publication.
Written informed consent was obtained from the patient’s next-of-kin for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Needleman, A., Sheaff, M., Pepper, R.J. et al. Immunoglobulin G4-related disease presenting with nephrotic syndrome due to minimal change disease: a case report. J Med Case Reports 18 , 192 (2024). https://doi.org/10.1186/s13256-024-04494-3
Download citation
Received : 07 November 2023
Accepted : 06 March 2024
Published : 20 April 2024
DOI : https://doi.org/10.1186/s13256-024-04494-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- IgG4-related disease (IGG4-RD)
- IgG4-related kidney disease (IgG4-RKD)
- Minimal change disease (MCD)
- Tubulointerstitial nephritis (TIN)
- Necrotizing fasciitis
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.16(2); 2024 Feb
- PMC10924861

Nephrotic Syndrome: A Review
Priyanshu r verma.
1 Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research, Wardha, IND
Praful Patil
2 Microbiology, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research, Wardha, IND
Nephrotic syndrome (NS) is characterized by hypoalbuminemia, severe proteinuria, and peripheral edema, frequently in conjunction with hyperlipidemia. Individuals usually show symptoms of weariness and swelling, but no signs of serious liver damage or cardiac failure. With characteristic medical symptoms and evidence of hypoalbuminemia and severe proteinuria, NS can be diagnosed.
The majority of NS episodes are classified as unexplained or primary; the most prevalent histopathological subgroups of primary NS in people are focal segmental glomerulosclerosis and membraneous nephropathy. Thrombosis of the veins with high cholesterol levels is a significant NS risk. Acute renal damage and infection are further possible side effects.
The pathobiochemistry of NS involves alterations in genes that affect the selectivity of the kidneys and abnormalities in proteins related to podocytes. Understanding the molecular mechanisms that influence these processes is crucial to developing specific and targeted therapeutic approaches.
The need for invasive renal biopsies throughout the diagnosis process may be lessened by the development of non-invasive nephrotic syndrome biomarkers, such as microRNAs.
Corticosteroids are frequently used as the initial line of defense in NS treatment. However, some individuals need other treatments since a resistant type of NS also exists. The use of calcineurin inhibitors, mycophenolate mofetil, and rituximab is mentioned in the text, along with current research to identify safer and more efficient therapeutic choices.
The complicated kidney condition NS has several underlying causes and symptoms. For the diagnosis of this ailment as well as the creation of focused therapies, an understanding of the pathophysiology and the identification of possible biomarkers are essential.
Introduction and background
One of the causes of advanced kidney disease is nephrotic syndrome (NS). The primary symptom a physician should suspect in NS is a swollen body as the patient suffers from high blood pressure with hyperlipidemia. Membranous nephropathy, focal segmental glomerulosclerosis, and diabetic nephropathy are frequent causes in adults. Adults often have underlying diseases as the primary cause of many secondary causes. Through serologic testing and renal consulting, the cause of the NS should be identified. For patients whose condition has no recognized origin or classification, a renal biopsy is required. Symptoms, side effects, and the underlying reason are all addressed throughout treatment [ 1 ]. A living-donor transfusion might require weeks to months of waiting, instead of years. As increased dialysis duration predicts lower individual and donor mortality following transplantation; less time spent waiting is a significant benefit. Furthermore, as the donor and individual are usually in the same operating room, the organ suffers less ischemia injury. Generally, the kidney condition is better than in healthy donors, causing enhanced performance earlier on and roughly four years of transplant retention. Human cell antigen compatibility is also improved and indicates more accurate outcomes when an alive donor is linked to the individual [ 2 ].
According to area and ethnicity, the prevalence of idiopathic NS ranges from 1·15-16·9 per 100,000 children. Although the precise etiology of idiopathic NS is yet unclear, it is thought to be due to immunologic instability, chemicals circulating in our body, or inherited formational anomalies of the podocyte. The prognosis for long-term kidney outcomes is excellent for steroid-responsive illness [ 3 ].
Massive proteinuria, hypoalbuminemia, and edema are features of the problematic form of an NS known as congenital nephrotic syndrome (CNS). The major characteristic of CNS is significant plasma protein leakage. Patients may be diagnosed while still in the womb or the first few weeks after birth, usually before the age of three months. Either hereditary or nongenetic etiologies can be linked to CNS etiology. It has been suggested that this condition is caused by pathogenic mutations in NPHS1, NPHS2, LAMB2, WT1, and PLCE1 genes. The clinical course is made more difficult by substantial edema, infections, thrombosis, hypothyroidism, failure to thrive, and other factors. During their first month of life, the mainstays of therapy include gaining vascular access, routine IV albumin infusions, the use of diuretics, infection prevention, and nutritional support. Transplanting a kidney is the most effective treatment for these patients. Clinicians continue to face difficulties with CNS diagnosis and treatment. The pathogenesis, diagnosis, and treatment of CNS patients are now more well-known thanks to a review by AbuMaziad et al. [ 4 ].
The kidney illness known as NS, which is common in children, causes protein, fluid, and nutrition loss in the urine, and is characterized by changes in glomerular filtration. Peripheral, gravity-dependent edema affects the majority of patients; nevertheless, severe instances also show anasarca and ascites. Due to the underlying pathology and medications used to treat it, the condition has several long-term consequences, such as hyperlipidemia, metabolic bone disease, and deficits in certain micronutrients. The key to effective management is pharmacological and nutritional therapies. Edema is treated with corticosteroids, albumin, diuretics, sodium, and fluid restriction, and these medications are combined. Patients with recurrent episodes of illness or conditions that are resistant to steroid treatment may benefit from steroid-sparing treatments such as alkylating drugs, calcineurin inhibitors, and dietary changes that exclude dairy and gluten. To provide the best care possible for children with NS, nutrition experts should become familiar with the complexities of managing this condition [ 5 ].
Methodology
We undertook a systematic search through PubMed and PubMed Central in November 2020 using keywords such as "nephrotic syndrome" and "glomerulosclerosis" ((nephrotic syndrome [Title/ Abstract]) OR (NS [Title/ Abstract] OR ("nephrotic syndrome" [MeSH Terms]) AND ("glomerulosclerosis" [Title/ Abstract]) OR (GS [Title/ Abstract]) OR ("glomerulosclerosis" [MeSH Terms])).
We additionally searched for key references from bibliographies of the relevant studies. The search was updated in February 2022. One reviewer independently monitored the retrieved studies against the inclusion criteria, in the beginning, based on the title and abstract and then on full texts. Another reviewer also reviewed approximately 20% of these studies to validate the inclusion of studies (Figure (Figure1 1 ).

Pathogenesis and pathophysiology
Urinary protein loss (mostly albumin) is caused by damage to or dysfunction of the glomerulus's structural elements, such as the basement membrane, endothelium surface, or epithelial cells (podocytes). Only some proteins can be excreted in the urine due to restrictions on the size of the pore in the basement membrane and the charges of the barriers involved [ 1 ]. Edema, hypoalbuminemia, hyperlipidemia, and protein loss in the urine are all symptoms of NS. The glomerular podocytes are harmed by several disorders, which leads to NS. Together with the endothelial cells of the glomerular capillaries and the basal membrane, these specialized epithelial cells create a filter that traps plasma proteins in the bloodstream. Proteinuria results from a problem with this filter. Primary focal segmental glomerulosclerosis, membranous glomerulonephritis, and minimum change are the three most prevalent primary glomerular disorders. Although the well-known types are uncommon, our knowledge of podocyte function and the etiology of NS have considerably improved as a result of the discovery of important gene abnormalities [ 6 ]. There is no clear explanation for how edema develops in NS. Increased glomerular permeability to albumin and other plasma proteins appears to be the main problem. Edema is brought on by raised drainage of liquid in the space between blood vessels via the vascular region mainly related to kidney salt absorption with a lowering of flow due to a decrease of albumin in the bloodstream. Though not fully understood, the biological relation for thrombus formation by normal saline appears to be multifaceted and involves a raised drainage of liquid in the space between blood vessels via the vascular region. A patient's risk of thrombosis is substantially elevated if they also have prothrombotic genetic variants and NS [ 7 ].
Pathobiochemistry of NS
The increased permeability of the glomerular capillary wall for macromolecules is the underlying cause of NS. Persistent NS has a poor prognosis, a significant risk of developing end-stage renal failure, and a high risk of cardiovascular problems because of severe hyperlipidemia. It is still unclear what causes increased glomerular permeability in certain glomerular disorders. Recent research has provided fresh insight into the molecular processes of glomerular permselectivity by identifying the mutant genes for a few podocyte proteins in rare familial types of nephrotic disease. As time goes on, it becomes more and more clear that spontaneous mutations of podocyte proteins, such as podocin, may exist in some patients with acquired NS. Podocyte injury that causes either apoptosis or promotion of proliferation and some type of healing, including glomerular sclerosis, may cause the expression of additional podocyte proteins to vary over the course of experimental NS. Clear therapeutic implications could result from a deeper understanding of these systems. Although it is believed that glomerular permeability factors play a role in several non-inflammatory glomerular disorders, their molecular identification is difficult. This is likely due to the nonhomogeneous nature of the underlying diseases. For instance, spontaneous mutations in some podocyte protein genes, increased production of glomerular permeability factor (perhaps by thymus cells), or decreased levels of glomerular permeability factor inhibitors in nephrotic urine may all contribute to the development of localized segmental glomerulosclerosis.
There are distinct differences between the factors that contribute to glomerular sclerosis and those that increase glomerular permeability. Proteinuria does not just appear to result from glomerular injury; it may also damage tubules and start interstitial fibrosis, which would speed up the development of chronic renal failure in proteinuric renal disorders. The latest updates in the knowledge of the mechanisms behind tubular protein reabsorption may provide us with new methods for halting the growth of chronic kidney illness. In patients with severe proteinuria who are resistant to treatment, cubilin inhibitors may be able to reduce tubular and interstitial damage. All patients with prolonged NS should be treated for nephrotic hyperlipidemia, which increases the risk of cardiovascular problems. Prospective controlled trials are still needed to confirm the hypothesized beneficial effect of hypolipidemic medications (namely statins) on cardiovascular risk and chronic renal failure. Recent progress in understanding podocyte biology in uncommon hereditary glomerular disorders has opened up the possibility of understanding the molecular etiology of increased glomerular permeability in the far more commonly acquired forms of NS in the near future [ 8 ].
Refractory NS: a rare immune disease
Worldwide, both children and adults are affected by the rare but severe kidney illness known as NS. Its clinical manifestations frequently involve hyperlipidemia and include peripheral edema, severe proteinuria, and hypoalbuminemia. Two to seven per 100,000 children and three per 100,000 adults are the stated annual incidence rates. Even though it only occurs on occasion, it accounts for up to 20% of end-stage renal disease (ESRD) in children and roughly 12% of all ESRD causes. NS can have a variety of etiologies, including secondary illnesses brought on by medications, infections, and neoplasia as well as primary glomerulonephritis. Although there is a lot of evidence that indicates that immune-mediated disease that results in glomerular visceral epithelial cells destructs glomerular filters specifically affected by primary NS, its exact cause is still unknown [ 9 ].
Types of primary NS
Primary NS is divided into three main pathophysiological subtypes: focal segmental glomerulosclerosis (FSGS), minimal change disease (MCD), and idiopathic membranous nephropathy (IMN). They all have immunological damage to renal podocytes that are not very inflamed as part of their pathogenic pathways, despite differences in some features [ 9 ]. IMN, which is typically categorized as an autoimmune disease, acts as a catalyst in the sub-epithelial layers of the glomerulus and exhibits immune deposition, which causes the destruction of the walls of small blood vessels supplying the functional unit of the kidney [ 10 ]. The discovery that anti-podocyte-autoantigen antibodies are present in 5-10% of cases and that podocyte autoantigen detection is in 60-80% of cases was hailed as a revolutionary development in our understanding of the underlying pathomechanism of systemic nephropathy [ 11 , 12 ]. Conventionally, MCD and FSGS were regarded as separate entities. Nevertheless, given that FSGS represents an advanced stage of disease progression than MCD, there is now sufficient data to demonstrate different types of related chronic diseases [ 13 ]. It is believed that a general disturbance of T-cell activity is the immunological origin of these problems, bringing on the generation of pro-inflammatory factors or indirectly affecting the glomerulus and its function [ 14 ].
Among adults, IMN is the main cause of NS, despite being extremely uncommon in youngsters. Childhood NS is most frequently caused by MCD and FSGS. They account for 10-15% and 40%, respectively, of older instances of NS [ 9 , 15 ].
Massive proteinuria and low blood protein levels brought on by several disorders, such as minimal change NS (MCNS), FSGS, and membranous glomerulonephropathy, are clinical conditions known as NS. Invasive renal biopsies are generally required to distinguish between diagnoses. In rare instances, it can be challenging to distinguish between MCNS and FSGS even with a biopsy. There is currently no better choice for diagnosis than a kidney biopsy. Non-coding RNA molecules called microRNAs (miRNAs), which manage the removal of specific chromosomes during cell division steps, are found capable of removing the disease-causing, unwanted part from the gene and can be in length up to 20 nucleotides. MiRNAs have been demonstrated to function as non-invasive biological markers of a variety of illnesses, including kidney disorders, in serum and urine. In this paper, we provide a summary of the current understanding of miRNAs as potential NS indicators [ 16 ].
Corticosteroids are usually prescribed as the first line of treatment for NS. However, 20% of neonates having NS are resistant to steroids, even though the majority of neonates having the condition are steroid-sensitive. In addition, most pediatric children who react to steroids first get worse, and a lot of them do so frequently and require recurrent sessions of steroid therapy, leading to steroid dependence [ 17 ]. Therefore, the category of renal disease in sufferers who have become steroid-dependent or resistant with a high rate of recurrence is referred to as refractory NS (RNS) [ 18 ]. Children and adolescents with RNS make up approximately 25-40% of instances of NS, while adults with RNS are more likely to develop the condition in up to 70% of cases. For instance, 10-20% of mature MCD cases develop steroid rebellion, and the recurrence rate for MCD is around 50% [ 9 , 19 , 20 ]. The terms "NS" and "mature kidney disease" are often used interchangeably in journals and medical contexts due to the high occurrence of RNS in adult kidney disease.
Recent years have seen a significant increase in the use of second-line immunosuppressive treatments, such as cytotoxic drugs, calcineurin inhibitors, mycophenolate mofetil, and rituximab, in the treatment of RNS, with encouraging outcomes. However, prolonged utilization of analeptics and some of these immunosuppressants can have substantial negative consequences, including bone loss, lipid disorders, high blood sugar levels, renal toxicity, hypo-immunity, and an increased probability of getting infected. Additionally, these medicines have a poor risk/benefit profile. Severe unremitting edema, significant proteinuria, hypoalbuminemia, and occasionally impaired kidney function are still common in RNS patients. The ineffectiveness of the RNS treatments that are now not used has sparked ongoing research for safer and more efficient alternative therapies. There is a long history of using traditional Chinese medicine (TCM) to treat kidney disease symptoms like proteinuria and edema. TCM has collected a wealth of clinical expertise in treating this condition and has evolved unique views to explain NS through continuous acceptance, different theories, and also experimentation. It is logical that investigations of TCM be undertaken to treat RNS more effectively [ 9 ].
General Treatment Measures
Some experts advise that the liquid intake has to be below 1500 ml in 24 hours and dietary salt less than 3 g per day as part of the standard therapy of patients with NS due to the potential pathophysiologic involvement of sodium retention [ 21 ].
Treatment of Edema
Even when the glomerular filtration rate is normal, diuretics are ineffective for patients with nephrosis. Because serum proteins are decreased in NS, loop diuretics are less efficient, and patients may need higher dosages than usual. There may be additional diuretic resistance mechanisms. The prolonged duration of action of oral loop diuretics with twice-daily treatment is typically chosen. However, in cases with severe NS and edema, intestinal wall edema may make it difficult for the diuretic to be absorbed through the gastrointestinal tract, necessitating IV diuretics. One to two kilograms of water weight should be achieved in a 24-hour period, with a relatively progressive diuresis controlled by daily weight monitoring. Any acceptable starting dose of furosemide (Lasix) is 40 milligrams taken two times a day or for bumetanide is 1 milligram taken two times a day. If this shows any insufficient progression of dropsy or different signs of pitting swelling, the dose should be roughly doubled every one to three days. The maximum dosage of furosemide is roughly 240 milligrams one time else 600 milligrams once a day. However, this limit is not supported by any convincing evidence or logic. If the patient's clinical reaction is still inadequate, the patient may be treated by switching IV loop diuretics, giving chlorthalidone orally, or starting an IV meal containing 20 percent body protein before starting an IV diuretic meal in the stomach [ 7 ].
Hemodialysis (HD) has become more often used in newborns and young children with renal failure in recent years. Even in newborns with CNS nephrectomies, HD is possible. In sufferers having a live kidney transplant, those who reside nearby might receive HD treatment before the kidney transplant (KTx) for a few weeks. Even infants with peritoneal dialysis (PD) therapy issues (infections and technical issues) can benefit from HD [ 22 ]. It is the process through which CNS in children may be treated if renal failure develops or if a bilateral nephrectomy is performed. Infants were first given continuous PD in the 1980s [ 23 , 24 ]. Results later got more accurate, and it became clear that infants' non-renal comorbidity is the main risk factor for mortality [ 25 ].
Acute kidney injury aggravating the MCD type of NS
Of all cases of NS in children, MCD causes 70% to 90%. Adult individuals with the condition, notably those over 60, also develop NS. A foot-process fusion affects the purification of aqueous and dissolved substances, which causes a moderate alteration in renal function in 20% to 30% of individuals. When proteinuria is remitted, the glomerular filtration rate drops by 20% to 30% and returns to normal. Acute kidney injury has been documented in several publications over the past 50 years, roughly one-fifth to one-third of which occurred in older patients without preexisting or concurrent renal illness. Male preponderance, age > 50, significant protein in the urine, an acute drop of an abnormally small quantity of albumin in the blood, a history of hypertension, injury vessels on renal tissue extraction, and ischemic tubular necrosis are clinical indicators. Acute kidney damage can necessitate dialysis for a few weeks or months until the proteinuria goes away and the oliguria goes away. Renal function may occasionally not improve. A hypothesis has been made to explain tubular cell ischemia necrosis as a result of endothelin-1-induced vasoconstriction occurring at the outset of proteinuria. In individuals with minimally changed illnesses, diuretic-induced hypovolemia and nephrotoxic substances are the main causes of acute kidney injury. Acute renal injury is infrequent in children when there are no concurrent problems. The main risk factors include steroid resistance, nephrotoxic medicine, and infection. Supportive therapy's primary purpose in all patients is to purchase time until glucocorticoids can resolve proteinuria and reverse renal failure [ 26 ].
Glomerular filtration barrier
Substantial leaking of albumin present in the blood to urea is a key characteristic of CNS. Most of the time, this occurs due to the changes inside the chromosome, which produce albumin in the glomerular capillary wall and podocytes, which regulate renal cleaning [ 27 , 28 ]. Three layers make up this filter: the glomerular basement membrane (GBM), the fenestrated endothelium, and the epithelial cell layer (podocytes), which has interposed slit diaphragms (SD) and distal foot processes. Water and tiny plasma solutes are typically the only substances that can pass through the barrier because it functions as an efficient size- and charge-selective molecular sieve. Because the GBM and especially SD impede the flow of albumin and other bigger plasma proteins, only a small amount of protein is present in the ultrafiltrate that reaches the Bowman space. However, it is now understood that proteinuria can result from a fundamental abnormality in either the SD or GBM. The GBM's function in the renal ability of a membrane to discriminate between anions and cations has been challenged in recent times [ 29 ].
The GBM is a well-known protein network made up of negatively charged proteoglycans, type IV collagen, laminin, and nidogen. Elsewhere, SD's exact shape of the molecule could be a matter of debate. The foundation of SD is likely made up of recently discovered foot-like cells present in the kidney having proteins like FAT2, dendrin, FAT1, Neph2, nephrin, and Neph1 [ 29 , 30 ]. The adapter proteins podocin, cortactin-CD2-associated protein (CD2AP), zonula occludens-1 (ZO-1), calcium/calmodulin-dependent serine protein kinase (CASK), and membrane-associated guanylate kinase (MAGI-1), which are present inside foot-like cells present in the kidney, interact with these proteins and form extracellular associations with one another. These participate in cell signaling through the slits in the membrane region to foot processes by connecting to the actin cytoskeleton of the podocyte cells. The actin network and its connecting proteins, like actinin-4, are essential for maintaining the podocyte's intricate structure. It's interesting to note that effacement of the podocyte foot processes results from a disruption of that molecule group in the central nervous system with some illnesses having protein present in urine [ 29 ].
Infantile NS (INS), which develops during the early one to two years as typically has been earlier found, is distinguished from CNS, which starts showing symptoms within the initial three months [ 22 ]. CNS of the Finnish type (CNF) can be characterized by high levels of proteinuria, severe hypoproteinemia, edema, and subsequent symptoms brought on by high levels of proteinuria found soon after birth. There have been reports of familial and occasional CNF cases in newborns in Finland without Finnish heritage during the 1900s, which suggests that CNF had not been considered during those days [ 31 ]. The care of CNF is difficult even now even after the availability of advanced medical equipment. Since there is varying seriousness for the illness, patients having central nervous system complications have received treatment over the last two to three decades using a variety of protocols [ 22 , 32 ]. Due to the rarity of the central nervous system and its genetic makeup, there is insufficient evidence to establish a single therapy strategy for all CNS patients. The purpose of this study is to justify the timing and rationale of early aggressive treatment, which consists of improved nutrition, albumin inoculation, rapid bilateral nephrectomy, dialysis, and KTx at one to two years of age [ 22 ].
Steroid-resistant NS (SRNS)
After receiving daily corticosteroid therapy, more than 85% of children and adolescents with idiopathic NS (with the majority between one and 12 years old) exhibit complete remission of proteinuria. SRNS is a condition in which patients do not demonstrate remission following a four-week course of daily prednisolone therapy. The majority of patients' renal histology reveals the presence of FSGS, MCD, and (occasionally) mesangial proliferative glomerulonephritis. One of the essential podocyte genes has mutations in one-third of SRNS patients. The unknown moving component could generally be responsible for the rest of the cases of SRNS. It is advised that patients with hypertension receive further treatment with medications that block the renin-angiotensin axis to lower any lingering proteinuria. The last stage of kidney failure is a possibility for SRNS individuals who do not improve after being treated with immunosuppressive medications. These patients might also have deteriorating renal function. A third of the individuals who obtain a renal transplant have recurrent FSGS in the allograft, and rituximab, blood exchange, and increased immunosuppressive agents have been utilized in conjunction to treat this illness [ 33 ].
NS in pregnancy
It is quite uncommon for NS to manifest during pregnancy. According to reports, 0.028% of pregnancies result in primary renal disease-related nephrosis [ 34 ]. Because it can be challenging to distinguish from preeclampsia and because the two conditions may coexist, the incidence of NS secondary to primary glomerular disease is unknown without a histologic diagnosis. Elevated creatinine is a known risk factor for unfavorable pregnancy outcomes [ 35 , 36 ]. Additionally, the course of the mother's kidney illness may be sped up during pregnancy. The impact of NS on pregnancy outcomes in the absence of substantial renal impairment is less apparent. Women with NS alone who don't have substantial hypertension or renal insufficiency tend to do well. The literature contains scant information on this particular subset of patients. Knowing how these patients' clinical outcomes developed will help with NS counseling and direct the care of these high-risk pregnancies [ 34 , 37 , 38 ].
Table Table1 1 provides a summary of the articles included.
CNS: congenital nephrotic syndrome; NS: nephrotic syndrome; IMN: idiopathic membranous nephropathy; FSGS: focal segmental glomerulosclerosis; MCD: minimal change disease; RNS: refractory nephrotic syndrome; PD: peritoneal dialysis; GBM: glomerular basement membrane; SD: slit diaphragms; CNF: congenital nephrotic syndrome of the Finnish type
Conclusions
Proteinuria, or an excessive loss of protein in the urine, is a symptom of NS, which also involves various secondary alterations in the body's fluid, lipid, and coagulation balance. NS has a broad span of etiologies that can affect people of various ages, from congenital origins in childhood to acquired reasons in later adulthood. Relapses and/or steroid dependence, two challenging symptoms to treat, are more common in steroid-sensitive NS (SSNS) patients. Mycophenolic acid and calcineurin inhibitors (CNIs) have been found effective in lowering SSNS relapses. Rituximab is also necessary, although numerous questions regarding the first dosage, course repetitions, and long-term negative effects are still unresolved. SRNS may lead to chronic renal dysfunction, particularly if the condition is resistant to treatment.
The authors have declared that no competing interests exist.
Got any suggestions?
We want to hear from you! Send us a message and help improve Slidesgo
Top searches
Trending searches

suicide prevention
8 templates

computer network
75 templates

spring season
28 templates

cybersecurity
6 templates

46 templates

18 templates
Nephrotic Syndrome Management Case Report
Nephrotic syndrome management case report presentation, free google slides theme, powerpoint template, and canva presentation template.
Download the "Nephrotic Syndrome Management Case Report" presentation for PowerPoint or Google Slides. A clinical case is more than just a set of symptoms and a diagnosis. It is a unique story of a patient, their experiences, and their journey towards healing. Each case is an opportunity for healthcare professionals to exercise their expertise and empathy to help those in need. With this editable template for Google Slides or PowerPoint, you can describe in detail a clinical case, something that might be invaluable for medical students and fellow doctors.
Features of this template
- 100% editable and easy to modify
- Different slides to impress your audience
- Contains easy-to-edit graphics such as graphs, maps, tables, timelines and mockups
- Includes 500+ icons and Flaticon’s extension for customizing your slides
- Designed to be used in Google Slides, Canva, and Microsoft PowerPoint
- Includes information about fonts, colors, and credits of the resources used
How can I use the template?
Am I free to use the templates?
How to attribute?
Attribution required If you are a free user, you must attribute Slidesgo by keeping the slide where the credits appear. How to attribute?
Related posts on our blog.

How to Add, Duplicate, Move, Delete or Hide Slides in Google Slides

How to Change Layouts in PowerPoint

How to Change the Slide Size in Google Slides
Related presentations.

Premium template
Unlock this template and gain unlimited access

Register for free and start editing online
Clinical significance of rituximab-associated hypogammaglobulinemia in nephrotic syndrome: to treat or not to treat?
- Letter to the Editors
- Published: 19 April 2024
Cite this article
- Eugene Yu-hin Chan 1 , 2
54 Accesses
Explore all metrics
Avoid common mistakes on your manuscript.
To the editor,
I read with interest the manuscript by Zurowska et al. entitled “Rituximab-associated hypogammaglobulinemia in children with idiopathic nephrotic syndrome: results of an ESPN survey” [ 1 ]. This is an area of concern for paediatric nephrologists in the use of rituximab for glomerular diseases [ 2 , 3 ]. A total of 1328 children from 84 European centres were analysed in this huge survey. In concordance to previous reports, hypogammaglobulinemia was observed in up to 61% patients during rituximab.
In this study, 33 severe infections were reported in 2.5% of the cohort, of which 30 episodes had concurrent hypogammaglobulinemia. The total number of patients suffering from hypogammaglobulinemia is however unclear due to heterogeneous monitoring policy. The definite incidence of developing infection among patients with hypogammaglobulinemia therefore could not be ascertained. Serial values of immunoglobulin G and M, and concomitant use of immunosuppression would be of interest to inform the additional risk of developing infection.
The practice of prophylactic immunoglobulin substitution is varying since intravenous immunoglobulin is expensive and has been linked to acute kidney injury. It also has to be given monthly owing to short half-life and contains minimal IgM content. Given the risk of severe infection and mortality, it would be reasonable to at least replace immunoglobulin during active infection to prevent morbidity. Future trials are required to define the optimal threshold of immunoglobulin G below which treatment is indicated for asymptomatic, numerical hypogammaglobulinemia.
Zurowska A, Drozynska-Duklas M, Topaloglu R, Bouts A, Boyer O, Shenoy M, Vivarelli M (2023) Rituximab-associated hypogammaglobulinemia in children with idiopathic nephrotic syndrome: results of an ESPN survey. Pediatr Nephrol 38:3035–3042
Article PubMed PubMed Central Google Scholar
Chan EY-H, Wong S-W, Lai FF-Y, Ho T-W, Tong P-C, Lai W-M, Ma AL-T, Yap DY-H (2023) Long-term outcomes with rituximab as add-on therapy in severe childhood-onset lupus nephritis. Pediatr Nephrol 38:4001–4011. https://doi.org/10.1007/s00467-023-06025-6
Article PubMed Google Scholar
Chan EY-H, Ma AL-T, Tullus K (2022) Hypogammaglobulinaemia following rituximab therapy in childhood nephrotic syndrome. Pediatr Nephrol 37:927–931. https://doi.org/10.1007/s00467-021-05345-9
Download references
Author information
Authors and affiliations.
Paediatric Nephrology Centre, Hong Kong Children’s Hospital, Kowloon, Hong Kong SAR
Eugene Yu-hin Chan
Department of Paediatrics, Chinese University of Hong Kong, Shatin, Hong Kong SAR
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Eugene Yu-hin Chan .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Chan, E.Yh. Clinical significance of rituximab-associated hypogammaglobulinemia in nephrotic syndrome: to treat or not to treat?. Pediatr Nephrol (2024). https://doi.org/10.1007/s00467-024-06379-5
Download citation
Received : 06 April 2024
Revised : 06 April 2024
Accepted : 10 April 2024
Published : 19 April 2024
DOI : https://doi.org/10.1007/s00467-024-06379-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 22 April 2024
Mutations in the NUP93 , NUP107 and NUP160 genes cause steroid-resistant nephrotic syndrome in Chinese children
- Yanxinli Han 1 ,
- Hongyu Sha 2 ,
- Yuan Yang 1 ,
- Zhuowei Yu 1 ,
- Lanqi Zhou 1 ,
- Yi Wang 1 ,
- Fengjie Yang 1 ,
- Liru Qiu 1 ,
- Yu Zhang 1 &
- Jianhua Zhou 1
Italian Journal of Pediatrics volume 50 , Article number: 81 ( 2024 ) Cite this article
Metrics details
The variants of nucleoporins are extremely rare in hereditary steroid-resistant nephrotic syndrome (SRNS). Most of the patients carrying such variants progress to end stage kidney disease (ESKD) in their childhood. More clinical and genetic data from these patients are needed to characterize their genotype–phenotype relationships and elucidate the role of nucleoporins in SRNS.
Four patients of SRNS carrying biallelic variants in the NUP93 , NUP107 and NUP160 genes were presented. The clinical and molecular genetic characteristics of these patients were summarized, and relevant literature was reviewed.
All four patients in this study were female and initially presented with SRNS. The median age at the onset of the disease was 5.08 years, ranging from 1 to 10.5 years. Among the four patients, three progressed to ESKD at a median age of 7 years, ranging from 1.5 to 10.5 years, while one patient reached stage 3 chronic kidney disease (CKD3). Kidney biopsies revealed focal segmental glomerulosclerosis in three patients. Biallelic variants were detected in NUP93 in one patient, NUP107 in two patients, as well as NUP160 in one patient respectively. Among these variants, five yielded single amino acid substitutions, one led to nonsense mutation causing premature termination of NUP107 translation, one caused a single nucleotide deletion resulting in frameshift and truncation of NUP107. Furthermore, one splicing donor mutation was observed in NUP160 . None of these variants had been reported previously.
This report indicates that biallelic variants in NUP93 , NUP107 and NUP160 can cause severe early-onset SRNS, which rapidly progresses to ESKD. Moreover, these findings expand the spectrum of phenotypes and genotypes and highlight the importance of next-generation sequencing in elucidating the molecular basis of SRNS and allowing rational treatment for affected individuals.
Introduction
Nephrotic syndrome (NS) is common in children. Most of them respond well to glucocorticoid, while a minority are steroid-resistant [ 1 , 2 , 3 ]. Steroid-resistant nephrotic syndrome (SRNS) primarily presents as focal segmental glomerulosclerosis (FSGS), which is associated with an unfavorable renal prognosis [ 4 , 5 ]. Despite extensive research efforts, the etiology and pathogenesis of SRNS remain incompletely understood. Recent studies have identified an increasing number of genes associated with the development of SRNS, totally accounting for 29.5% of SRNS as reported by Hildebrandt [ 6 ]. About 66% of SRNS occurring within the first year of their life are caused by monogenic variants [ 7 ]. Thus far, more than 50 genes have been identified for SRNS worldwide [ 8 ]. Most of these gene products are located in slit diaphragm, cytoskeleton, mitochondria, lysosome and endocytic compartment of podocytes [ 8 , 9 ].
Currently, variants in several nucleoporins (NUPs) have been identified as the underlying causes of SRNS. The nuclear pore complex includes a variety of NUPs that are distributed across the nuclear envelope and play a critical role in macro-molecular transportation between nucleus and cytoplasm [ 10 , 11 ]. However, the reported cases of such variants are limited. Therefore, additional clinical and genetic data are required to characterize genotype–phenotype relationships and elucidate the role of NUPs in SRNS. This study aims to summarize the clinical and molecular genetic characteristics of four cases of SRNS caused by variants in NUP93 , NUP107 and NUP160 genes with the intention of providing new insights into this rare disease.
Patients and methods
Case presentation.
Case 1 was a 1-year-old female patient who initially presented with edema on the lower limbs and eyelids. She developed normally and was well-nourished. Upon physical examination, the patient presented with hypertension with a blood pressure of 115/68mmHg (≥95th percentile + 12 mmHg), while no other evident abnormalities were observed. The urine test showed proteinuria 3+ and hematuria 2+. The serum creatinine was 0.58mg/dl (eGFR 57ml/min/1.73m 2 ). Subsequent renal biopsy revealed FSGS. Despite receiving treatment with a combination of prednisone and cyclosporin A, the patient remained nephrotic after approximately six months of treatment and eventually progressed to end-stage kidney disease (ESKD). Whole-exome sequencing (WES) identified biallelic variants in the NUP93 gene.
Case 2 was a 5.3-year-old female patient who was hospitalized due to edema on lower limbs, with blood pressure of 141/108mmHg (≥95th percentile + 12 mmHg). Physical examination revealed no other significant abnormalities. Her growth and development were normal without any malformations. Urine examination showed hematuria 2+ and proteinuria 3+, while the serum creatinine level was 0.4mg/dl (eGFR 110ml/min/1.73m 2 ). Despite four weeks of treatment with prednisone, the patient showed no response. So renal biopsy and whole-exome sequencing (WES) were recommended. The renal biopsy revealed global sclerosis in 11 glomeruli and segmental glomerulosclerosis in 4 among total 30 glomeruli. WES identified biallelic variants in the NUP107 gene.
Case 3 involved a female child aged 10.5 years who presented with chest tightness and nausea. Physical examination showed hypertension with blood pressure ranging from 151/108 mmHg to 200/149 mmHg (≥95th percentile + 12 mmHg). There were no edema or growth and developmental delays. The plasma renin level was within the normal range, and the whole aortic computer tomography angiography (CTA) showed no remarkable findings. Echocardiography showed left ventricular hypertrophy with left ventricular ejection fractions (LVEF) at 38.7%. Ultrasound examination revealed no abnormalities in the kidneys, uterus, or ovaries. Urine analysis revealed a significant proteinuria level of 4+ without hematuria. The level of serum creatinine was 6.26 mg/dl (eGFR 9.6ml/min/1.73m 2 ). Subsequently, the patient underwent peritoneal dialysis, and whole-exome sequencing (WES) identified biallelic variants in the NUP107 gene.
Case 4 was a 3.5-year-old girl presenting with eyelid edema and hypertension. Urinalysis revealed proteinuria 4+ and hematuria 2+. The patient showed mild intellectual disability, as evidenced by a full-scale score of 48 on the Wechsler Preschool and Primary Scale of Intelligence (WPPSI), indicating an intellectual delay. A comprehensive neuropsychiatric assessment indicated delays in both gross and fine motor skills, adaptive abilities, language development and social behavior. Cognitive assessment demonstrated abnormal cognitive play and social communication behaviors. The Autism Behavior Checklist (ABC) confirmed the presence of autistic behaviors, with the Childhood Autism Rating Scale (CARS) indicating mild to moderate autism spectrum disorder. The ultrasound examination revealed the presence of a cord-like uterine, structure measuring 13 mm in length and 3 mm in anteroposterior diameter. The baseline level of serum creatinine was 0.34mg/dl (eGFR 114ml/min/1.73m 2 ). However, there was an increase level in serum creatinine to 0.98mg/dl (eGFR 42ml/min/1.73m 2 ) during the follow-up, suggesting the development of stage 3 chronic kidney disease (CKD3). Renal biopsy revealed FSGS. The patient was initially treated with prednisone and tacrolimus, which were discontinued due to no response. Thereafter WES identified biallelic variants in the NUP160 gene.
All of these cases had no family history of the disease. The specific family diagram is illustrated in Fig. 1 .
Family diagram of four patients. Black arrow indicates proband; WT: wild type
Detection and analysis of nucleoporin gene variants
After obtaining the informed consent of the patients’ parents, blood samples were collected from both the patients and their parents. DNA was extracted from peripheral white blood cells by using the MagPure Buffy Coat DNA Midi KF Kit according to manufacturer’s standard protocol. Genomic DNA was broken into 100–500 bp fragments by BGI’s enzyme kit (Segmentase, BGI), and 280–320 bp fragments were collected by magnetic bead. PCR amplification was performed using universal primers complementary to the adapter sequence to form a sequencing library. All amplified libraries were hybridized with exome capture probes (Agilent, USA) and sequenced. The clean reads derived from targeted sequencing and filtering were then aligned to the human genome reference (hg19) by using the BWA. Single-nucleotide variants (SNVs) and INDELs were detected with Sentieon (the same algorithm with GATK) analysis. The pathogenic variants were screened by ClinVar, OMIM, and HGMD databases. Functional prediction of missense mutations was conducted using PolyPhen-2, SIFT, and MutationTaster. All variants and potential pathogenic variants were validated via conventional Sanger sequencing methods.
Clinical and renal pathological features and long-term outcome
The clinical and laboratory data of four patients are shown in Table 1 . All patients initially presented with massive proteinuria with or without hematuria. Patient 2 and 4 had normal serum creatinine levels and eGFR at the onset, while patient 1 and 3 had elevated levels of serum creatinine and impaired renal function at beginning. Moreover, patient 3 presented with heart failure as an extra-renal manifestation, and patient 4 showed mild intellectual disability and uterine dysplasia. Renal biopsy revealed focal and segmental glomerular sclerosis (Fig. 2 ).
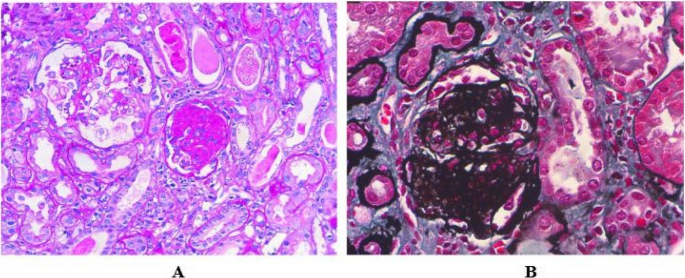
kidney biopsies reveal FSGS in the patients. A shows segmental sclerosis in a glomerulus and a global sclerosis in another glomerulus (HE, 100). B indicates global glomerulosclerosis (PASM, 100)
Patient 1 rapidly progressed to ESKD within six months of onset and regrettably passed away at 1.5 years of age. Patient 2 developed renal failure at 9 years old and subsequently underwent renal transplantation. Patient 3 was diagnosed with ESKD at the initial presentation and was on maintenance peritoneal dialysis. And patient 4 was diagnosed with CKD3 at 5 years old during the follow-up period (Table 1 ).
Identification of pathological variants in the NUP genes
Biallelic variants in the NUP genes were detected by WES in all patients. Specifically, patient 1 had biallelic missense mutations c.1235A>C (p.Tyr412Ser) and c.1286A>G (p.Tyr429Cys) in the NUP93 gene. Patient 2 showed biallelic variants c.1199G>A (p.Gly400Glu) and c.580C>T (p.Arg194*) in the NUP107 gene, and patient 3 also had biallelic variants c.2564delC (p.Pro855fsTer*23) and c.2753C>T (p.Pro918Leu) in NUP107 , resulting in amino acid substitutions and proteins truncation. Patient 4 was identified with a missense mutation c.3656T>G (p.Leu1219Trp) and a splicing donor mutation c.2241+1G>T in the NUP160 gene. Ployphen2, MutationTaster analysis indicated that all the missense mutations were harmful (Table 2 ).
Clinical and molecular genetic characterization of reported patients carrying biallelic variants of NUP genes
To date, a total of 60 cases of NUP -associated SRNS have been reported worldwide (Table 3 ). Among these reported cases, variants of NUP93 gene were observed in 24 individuals (40%), while NUP107 variants in 20 individuals (33.3%). Additionally, there were also eight cases (13.3%) with NUP133 mutations, four cases (6.7%) with NUP85 mutations, one case (1.7%) with NUP205 mutation and three cases (5%) with NUP160 mutations. The majority of these reported cases initially presented with SRNS and eventually progressed to ESKD. The detailed information of these variants were presented in Table 3 and Fig. 3 , involving 22 variants in NUP93 , 11 in NUP107 , 6 in NUP133 , 4 in NUP85 , 1 in NUP205 , and 3 in NUP160 . Missense mutations were the majority of all NUP mutations.
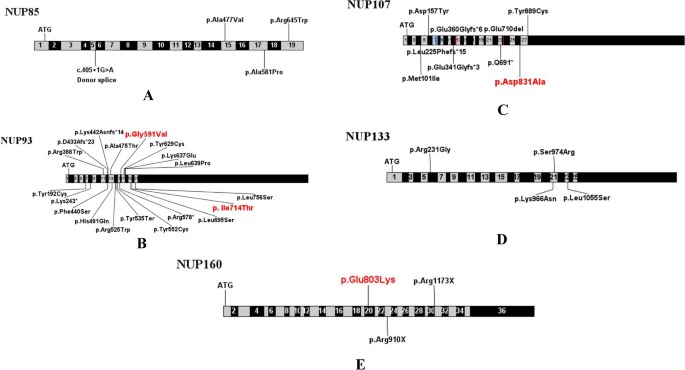
Schematic illustration of mutation sites in genes of NUP85 ( A ), NUP93 ( B ), NUP107 ( C ) , NUP133 ( D ) and NUP160 ( E ). Hot spot mutations are indicated in red
NUPs are situated in the nuclear membrane, and approximately 30 kinds of NUPs assemble to form the nuclear pore complex (NPC), serving as a special and unique transport channel across the nuclear membrane [ 29 ]. The NPC comprises a core scaffold, a nuclear basket, transmembrane nucleoporins and a central selective channel [ 11 ]. NUP93 is located in the inner ring of the core scaffold, while NUP107 and NUP160 are located in the outer ring also known as the “Y” complex [ 30 ]. NUP93, NUP107 and NUP160 interact with other NUPs and participate in the assembly of NPCs, which are crucial for trans-nuclear membrane transportation. Any alterations in the NUPs or defects in transport channels can hinder transmembrane transport, resulting in the abnormal accumulation of materials in nucleus or cytoplasm. Consequently, variants in NUPs are associated with a variety of diseases [ 31 ]. Previous studies have linked NUP93 , NUP107 and NUP160 to cancer, congenital heart disease, neurological diseases and gonadal dysgenesis [ 32 , 33 , 34 , 35 , 36 ]. Recently several SRNS cases caused by variants in NUPs have been reported and attracted the attention of pediatric nephrologists.
Previous studies had indicated that variants in NUP93 and NUP107 were the most frequent mutated NUP genes in individuals with SRNS. Patients with variants in these genes rapidly progressed to ESKD at a young age. Conversely, cases of SRNS with NUP160 variants presented relatively later and progressed to ESKD at an older age than those with NUP93 and NUP107 variants. Furthermore, patients with variants in NUP93 , NUP107 , and NUP133 were more likely to present with extra-renal manifestations.
Previously reported NUP variants included missense, nonsense, frameshift, small deletion and splicing mutations, among which missense mutations were found to be the most prevalent. Notably, variant hotspots were found in NUP93 and NUP107 genes. Specifically, the c.1772G>T (p.G591V) variant in NUP93 was identified as a pathogenic European founder variant and the c.1537+ 1G>A (deletion of exon 13) was found to be another pathogenic variant in Germans [ 13 ]. The c.2492A > C (D831A) variant in the NUP107 gene was considered to be unique to East Asians [ 23 ]. Furthermore, the variant c.2407G>A (p.Glu803Lys) in the NUP160 may be a hotspot variant in Asian according to previous reports.
The specific mechanisms underlying steroid-resistant nephrotic syndrome (SRNS) caused by NUPs remain unclear. A previous study demonstrated the presence of glomerular dysplasia and abnormal podocyte processes in zebrafish models with NUP107 knockdown [ 22 ]. Knockdown of NUP160 gene resulted in podocyte proliferation incapability, increased apoptosis, autophagy and cell migration, and altered expression and localization of nephrin, podocin, CD2AP and α-actinin-4 [ 37 ]. Furthermore, Braun [ 25 ] discovered upregulation of cdc42 in podocytes with knockout of NUP85 , NUP107 , and NUP133 genes. In addition, knockdown of NUP93 gene in podocytes disrupted BMP7-dependent SMAD signaling, potentially implicating it in the pathogenesis of SRNS [ 16 ].
Next-generation sequencing (NGS) is a powerful and high-throughput genetic test helping to identify the causative variants of genetic diseases. This technique offers valuable insights and evidence for the purposes of diagnosis, treatment, and genetic counseling [ 38 , 39 , 40 ]. Targeted panel sequencing is faster and cheaper than WES, making it a preferred choice for patients whose clinical data are highly consistent with a specific genetic defect or a known group of genes [ 41 ]. However, the limitation of targeted panel is that only genes within the panel are sequenced, which may result in missing genes located beyond the panel. As the cost of sequencing becomes cheaper and cheaper, WES has been widely accepted especially for patients with ambiguous phenotypes that make it difficult to apply targeted panel sequencing [ 42 ].
Cystic kidney diseases and renal tubulopathies subjected to WES result in relatively high rates of positive findings [ 43 ]. Generally speaking, an earlier onset of a disease is associated with a higher possibility of genetic etiology. Identifying the causes of sporadic non-syndromic SRNS is challenging. Overall, WES should be considered for SRNS patients with multisystem involvement as well as with unexplained clinical manifestations. In this study, all four patients lacked specific manifestation and two of them showed multisystemic involvement. Therefore, gene variant screening was the only way to identify genetic causes. Fortunately WES uncovered definitive pathogenic biallelic variants of NUP genes, thereby the ineffective treatment of prednisone and immunosuppressants was immediately discontinued. Our report demonstrates the necessity and diagnostic utility of genetic analysis in sporadic cases of SRNS and highlights the role of NGS in understanding the molecular mechanisms of SRNS and facilitating rational and individualized treatment for patients.
This paper is believed to be the first to report on a series of Chinese cases of SRNS associated with variants of NUP genes. Furthermore, all these variants in NUP93 , NUP107 and NUP160 have not been previously reported. Since this study is solely a case report, the limited number of cases prevented us from drawing conclusive phenotypic and genotypic correlations. In addition, how NUP variants cause SRNS remains unclear and requires further comprehensive molecular research.
In summary, this study reports four cases of sporadic SRNS caused by novel variants in the NUP93 , NUP107 , and NUP160 genes in Chinese children. The findings extend the spectrum of phenotypes and genotypes, and also highlight the importance of NGS in elucidating the molecular mechanisms of SRNS and allowing personalized treatment for affected individuals.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Steroid-resistant nephrotic syndrome
End stage kidney disease
Chronic kidney disease
Stage 3 chronic kidney disease
Nucleoporin
Whole-exome sequencing
Nuclear pore complex
Next-generation sequencing
Trautmann A, Vivarelli M, Samuel S, et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2020;35(8):1529–61.
Article PubMed PubMed Central Google Scholar
Downie M L, Gallibois C, Parekh R S, et al. Nephrotic syndrome in infants and children: pathophysiology and management[Z]. England: Taylor & Francis, 2017: 37, 248-258.
Tullus K, Webb H, Bagga A. Management of steroid-resistant nephrotic syndrome in children and adolescents. Lancet Child Adolesc Health. 2018;2(12):880–90.
Article PubMed Google Scholar
Lee JM, Kronbichler A, Shin JI, et al. Current understandings in treating children with steroid-resistant nephrotic syndrome. Pediatr Nephrol (Berlin, West). 2021;36(4):747–61.
Article Google Scholar
Shin JI, Kronbichler A, Oh J, et al. Nephrotic syndrome: genetics, mechanism, and therapies. Biomed Res Int. 2018;2018:6215942–6.
Sadowski CE, Lovric S, Ashraf S, et al. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2015;26(6):1279–89.
Article CAS PubMed Google Scholar
Hinkes BG, Mucha B, Vlangos CN, et al. Nephrotic syndrome in the first year of life: two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2). Pediatrics. 2007;119(4):e907–19.
Preston R, Stuart HM, Lennon R. Genetic testing in steroid-resistant nephrotic syndrome: why, who, when and how? Pediatr Nephrol (Berlin, West). 2019;34(2):195–210.
Trautmann A, Lipska-Ziętkiewicz BS, Schaefer F. Exploring the clinical and genetic spectrum of steroid resistant nephrotic syndrome: the podonet registry. Front Pediatr. 2018;6:200.
Lin DH, Hoelz A. The structure of the nuclear pore complex (an update). Ann Rev Biochem. 2019;88(1):725–83.
Hampoelz B, Andres-Pons A, Kastritis P, et al. Structure and assembly of the nuclear pore complex. Ann Rev Biophys. 2019;48(1):515–36.
Article CAS Google Scholar
Bierzynska A, Bull K, Miellet S, et al. Exploring the relevance of NUP93 variants in steroid-resistant nephrotic syndrome using next generation sequencing and a fly kidney model. Pediatr Nephrol (Berlin, West). 2022;37(11):2643–56.
Bezdíčka M, Štolbová Š, Seeman T, et al. Genetic diagnosis of steroid-resistant nephrotic syndrome in a longitudinal collection of Czech and Slovak patients: a high proportion of causative variants in NUP93. Pediatr Nephrol (Berlin, West). 2018;33(8):1347–63.
Rossanti R, Shono A, Miura K, et al. Molecular assay for an intronic variant in NUP93 that causes steroid resistant nephrotic syndrome. J Hum Genet. 2019;64(7):673–9.
Sandokji I, Marquez J, Ji W, et al. Identification of novel mutations and phenotype in the steroid resistant nephrotic syndrome gene NUP93: a case report. BMC Nephrol. 2019;20(1):271.
Braun DA, Sadowski CE, Kohl S, et al. Mutations in nuclear pore genes NUP93, NUP205 and XPO5 cause steroid-resistant nephrotic syndrome. Nat Genet. 2016;48(4):457–65.
Article CAS PubMed PubMed Central Google Scholar
Zhao B, Chen J, Liao Y, et al. Steroid-resistant nephrotic syndrome in infants caused by a novel compound heterozygous mutation of the NUP93: A CARE case report. Medicine (Baltimore). 2021;100(6):e24627.
Cason RK, Williams A, Chryst-Stangl M, et al. Collapsing focal segmental glomerulosclerosis in siblings with compound heterozygous variants in NUP93 expand the spectrum of kidney phenotypes associated with nucleoporin gene mutations. Front Pediatr. 2022;10:915174.
Acharya R, Upadhyay K. End-stage renal disease in a child with focal segmental glomerulosclerosis associated with a homozygous NUP93 variant. Clin Case Rep. 2021;9(11):e05111.
Hashimoto T, Harita Y, Takizawa K, et al. In Vivo expression of NUP93 and its alteration by NUP93 mutations causing focal segmental glomerulosclerosis. Kidney Int Rep. 2019;4(9):1312–22.
Al Riyami M S, Al Alawi I, Al Gaithi B, et al. Genetic analysis and outcomes of Omani children with steroid-resistant nephrotic syndrome. Mol Genet Genomic Med. 2023;11(9):e2201.
Miyake N, Tsukaguchi H, Koshimizu E, et al. Biallelic mutations in nuclear pore complex subunit NUP107 cause early-childhood-onset steroid-resistant nephrotic syndrome. Am J Hum Genet. 2015;97(4):555–66.
Park E, Ahn YH, Kang HG, et al. NUP107 mutations in children with steroid-resistant nephrotic syndrome. Nephrol Dial Transplan. 2017;32(6):1013–7.
CAS Google Scholar
Rosti RO, Sotak BN, Bielas SL, et al. Homozygous mutation in NUP107 leads to microcephaly with steroid-resistant nephrotic condition similar to Galloway-Mowat syndrome. J Med Genet. 2017;54(6):399–403.
Braun DA, Lovric S, Schapiro D, et al. Mutations in multiple components of the nuclear pore complex cause nephrotic syndrome. J Clin Investig. 2018;128(10):4313–28.
Zhao F, Zhu JY, Richman A, et al. Mutations in NUP160 are implicated in steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2019;30(5):840–53.
Fujita A, Tsukaguchi H, Koshimizu E, et al. Homozygous splicing mutation in NUP133 causes Galloway-Mowat syndrome. Ann Neurol. 2018;84(6):814–28.
Wang Q, Gu R, Li FW, et al. Steroid-resistant nephrotic syndrome caused by nuclear pore gene NUP133 variation. Clin Genet. 2023;104(2):272–4.
Schuller AP, Wojtynek M, Mankus D, et al. The cellular environment shapes the nuclear pore complex architecture. Nature. 2021;598(7882):667–71.
Beck M, Hurt E. The nuclear pore complex: understanding its function through structural insight. Nat Rev Mol Cell Biol. 2017;18(2):73–89.
Jamali T, Jamali Y, Mehrbod M, et al. Chapter six - Nuclear Pore Complex: Biochemistry and Biophysics of Nucleocytoplasmic Transport in Health and Disease[M]// Jeon K W . International Review of Cell and Molecular Biology. Academic Press, 2011:233-286.
Ouyang X, Hao X, Liu S, et al. Expression of Nup93 is associated with the proliferation, migration and invasion capacity of cervical cancer cells. Acta Biochimica Et Biophysica Sinica. 2019;51(12):1276–85.
Pan L, Song XW, Song JC, et al. Downregulation of NUP93 aggravates hypoxia-induced death of cardiomyocytes in vitro through abnormal regulation of gene transcription. Acta Pharmacol Sin. 2023;44(5):969–83.
Nong JS, Zhou X, Liu JQ, et al. Nucleoporin 107 is a prognostic biomarker in hepatocellular carcinoma associated with immune infiltration. Cancer Med. 2023;12(9):10990–1009.
Ren Y, Diao F, Katari S, et al. Functional study of a novel missense single-nucleotide variant of NUP107 in two daughters of Mexican origin with premature ovarian insufficiency. Mol Genet Genom Med. 2018;6(2):276–81.
Tarazon E, Rivera M, Rosello-Lleti E, et al. Heart failure induces significant changes in nuclear pore complex of human cardiomyocytes. Plos One. 2012;7(11):e48957.
Wang P, Zhao F, Nie X, et al. Knockdown of NUP160 inhibits cell proliferation, induces apoptosis, autophagy and cell migration, and alters the expression and localization of podocyte associated molecules in mouse podocytes. Gene. 2018;664:12–21.
Serra G, Antona V, D’Alessandro MM, Maggio MC, Verde V, Corsello G. Novel SCNN1A gene splicing-site mutation causing autosomal recessive pseudohypoaldosteronism type 1 (PHA1) in two Italian patients belonging to the same small town. Ital J Pediatr. 2021;47(1):138.
Piro E, Schierz IAM, Antona V, et al. Neonatal hyperinsulinemic hypoglycemia: case report of kabuki syndrome due to a novel KMT2D splicing-site mutation. Ital J Pediatr. 2020;46(1):136.
Serra G, Corsello G, Antona V, et al. Autosomal recessive polycystic kidney disease: case report of a newborn with rare PKHD1 mutation, rapid renal enlargement and early fatal outcome. Ital J Pediatr. 2020;46(1):154.
Platt CD, Zaman F, Bainter W, et al. Efficacy and economics of targeted panel versus whole-exome sequencing in 878 patients with suspected primary immunodeficiency. J Allergy Clin Immunol. 2021;147(2):723–6.
Lee JY, Oh SH, Keum C, Lee BL, Chung WY. Clinical application of prospective whole-exome sequencing in the diagnosis of genetic disease: Experience of a regional disease center in South Korea. Ann Hum Genet. Published online October 5, 2023.
Vilboux T, Doherty DA, Glass IA, et al. Molecular genetic findings and clinical correlations in 100 patients with Joubert syndrome and related disorders prospectively evaluated at a single center. Genet Med. 2017;19(8):875–82.
Download references
Acknowledgments
The authors thank all patients and their families for participation in this study.
The work for this study was supported by the National Key Scientific Research and Development Program of China (No.2022YFC2705193) and the Key Scientific Research and Development Program of Hubei Province (No.2022BCA047).
Author information
Authors and affiliations.
Department of Pediatrics, Tongji Hospital, Tongji Medical College, Huazhong University of Science & Technology, Wuhan, Hubei province, 430030, China
Yanxinli Han, Yuan Yang, Zhuowei Yu, Lanqi Zhou, Yi Wang, Fengjie Yang, Liru Qiu, Yu Zhang & Jianhua Zhou
Department of Pharmacy, Yantai Yuhuangding Hospital, Yantai, Shandong Province, 264000, China
You can also search for this author in PubMed Google Scholar
Contributions
HYXL and ZJH conceived the study. HYXL collected and analyzed the data and drafted the initial manuscript. SHY, YY, ZLQ, YZW, WY and YFJ helped to collect and supplement the data and participated in the care of these patients. QLR and ZY interpreted the results of WES and kidney biopsies. ZJH was the research grant recipient, revised the manuscript and supervised all the process. All authors had read and approved the manuscript.
Corresponding author
Correspondence to Jianhua Zhou .
Ethics declarations
Ethics approval and consent to participate.
Informed consent documents for this study were obtained from patients’ parents. The study was approved by the the Medical Ethics Committee of Tongji Medical College and was conducted in accordance with HIPAA regulations and with the tenets of the Declaration of Helsinki.
Consent for publication
Consent for publication were obtained from the institution and patients’ parents.
Competing interests
The authors declare no competing interests in this paper.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Han, Y., Sha, H., Yang, Y. et al. Mutations in the NUP93 , NUP107 and NUP160 genes cause steroid-resistant nephrotic syndrome in Chinese children. Ital J Pediatr 50 , 81 (2024). https://doi.org/10.1186/s13052-024-01656-3
Download citation
Received : 12 November 2023
Accepted : 07 April 2024
Published : 22 April 2024
DOI : https://doi.org/10.1186/s13052-024-01656-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- End-stage kidney disease
- Gene variants
Italian Journal of Pediatrics
ISSN: 1824-7288
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Cerebral Venous Sinus Thrombosis as an Initial Presentation of Nephrotic Syndrome: A Case Report
Affiliations.
- 1 Department of Internal Medicine, Somali-Sudanese Specialized hospital, Mogadishu, Somalia.
- 2 Department of Anesthesiology and Reanimation, Mogadishu Somalia Turkish Training and Research Hospital, Mogadishu, Somalia.
- 3 Department of Radiology, Mogadishu Somalia Turkish Training and Research Hospital, Mogadishu, Somalia.
- 4 Faculty of Medicine and Surgery, University of Somalia, Mogadishu, Somalia.
- 5 Department of Neurology, Mogadishu Somalia Turkish Training and Research Hospital, Mogadishu, Somalia.
- PMID: 38623542
- PMCID: PMC11018128
- DOI: 10.2147/VHRM.S458539
Cerebral sinovenous thrombosis (CSVT) encompasses a spectrum of disorders involving thrombosis of the cerebral venous system. As shown by previous epidemiological studies, the prevalence of cerebral sinovenous thrombosis is 4-7 cases per million people. Nephrotic syndrome was very rarely associated with thrombosis cerebral veins or sinuses. Hypercoagulability and thrombotic complications in nephrotic syndrome are most commonly seen in deep veins of the lower extremities and renal veins. Our case highlights a unique scenario in which cerebral sinovenous thrombosis was the initial presentation of nephrotic syndrome in a patient that was not an important past medical or surgical problem. The patient was brought to the emergency department with severe headache, vomiting, altered mental status, and generalized body swelling. Laboratory results showed proteinuria, hypoalbuminemia and hyperlipidemia. Non-contrast brain CT demonstrated hemorrhagic venous infarct associated with vasogenic edema. A subsequent brain MR venogram demonstrated occlusion of superior sagittal and right transverse sinuses. She was managed with low molecular weight heparin and intervenous corticosteroids then shifted to rivaroxaban and oral steroids, respectively, which resulted in massive clinical improvement and resolution of thrombus.
Keywords: MR venography; cerebral venous sinus thrombosis; nephrotic syndrome.
© 2024 Balla et al.
Publication types
- Case Reports
- Nephrotic Syndrome* / complications
- Nephrotic Syndrome* / diagnosis
- Nephrotic Syndrome* / drug therapy
- Sinus Thrombosis, Intracranial* / complications
- Sinus Thrombosis, Intracranial* / diagnostic imaging
- Sinus Thrombosis, Intracranial* / drug therapy
- Thrombosis*
Grants and funding

IMAGES
VIDEO
COMMENTS
Nephrotic syndrome (NS) is a clinical syndrome defined by massive proteinuria responsible for hypoalbuminemia, with resulting hyperlipidemia, edema, and various complications. It is caused by increased permeability through the damaged basement membrane in the renal glomerulus, especially infectious or thrombo-embolic. It results from an abnormality of glomerular permeability that may be ...
Nephrotic syndrome (NS) consists of peripheral edema, heavy proteinuria, and hypoalbuminemia, often with hyperlipidemia. ... Floege J. Introduction to glomerular disease: clinical presentations ...
Physical Examination. Edema is the salient feature of nephrotic syndrome and initially develops around the eyes and legs. With time, the edema becomes generalized and may be associated with an increase in weight, the development of ascites, or pleural effusions. Hematuria and hypertension manifest in a minority of patients.
Nephrotic syndrome is a kidney disorder that causes your body to pass too much protein in your urine. Nephrotic syndrome is usually caused by damage to the clusters of small blood vessels in your kidneys that filter waste and excess water from your blood. The condition causes swelling, particularly in your feet and ankles, and increases the ...
Nephrotic syndrome causes your kidneys to release too much protein in your urine. Causes include kidney diseases that affect the tiny filters inside your kidneys. Symptoms include swelling, high amounts of protein in your urine and low amounts of protein in your blood. Treatment includes medications that address its underlying causes.
Nephrotic syndrome has many causes, ... (See Presentation.) Classification. Nephrotic syndrome can be primary, being a disease specific to the kidneys, or it can be secondary, being a renal manifestation of a systemic general illness. In all cases, injury to glomeruli is an essential feature. Kidney diseases that affect tubules and interstitium ...
Nephrotic Syndrome what you should know • Depending on the disease and person's overall health, dietary changes and medicines are used to: - Lower excess salt and fluids in the body - Lower loss of protein in the urine - Lower cholesterol in the blood • Certain medicines that suppress or "calm" the immune system can be used.
The nephrotic syndrome is defined by the presence of heavy proteinuria (protein excretion greater than 3.0 g/24 hours), hypoalbuminemia (less than 3.0 g/dL), and peripheral edema. ... The etiology of glomerulonephritis can be classified by their clinical presentation (nephrotic, nephritic, rapidly progressive GN, chronic GN) or by ...
Tests and procedures used to diagnose nephrotic syndrome include: Urine tests. A urinalysis can reveal abnormalities in your urine, such as large amounts of protein. You might be asked to collect urine samples over 24 hours. Blood tests. A blood test can show low levels of the protein albumin and often decreased levels of blood protein overall.
The most common primary causes of nephrotic syndrome are 3. Focal segmental glomerulosclerosis. NIH external link. (FSGS). This disease affects the kidney's glomeruli, causing some of these filters to become scarred. FSGS is the most common cause of nephrotic syndrome in Black adults. Membranous nephropathy.
Nephrotic syndrome is one of the principal presentations of kidney disease, reflecting the pathophysiological effects of urinary losses of large quantities of protein. It is defined as urine total protein excretion of 3.5 g/d or greater, low serum albumin level, high serum cholesterol level, and peripheral edema.
Nephrotic syndrome (NS) is a condition characterized by increased permeability of the glomerular filtration barrier (GFB) leading to proteinuria with consequent hypoalbumine-mia, edema, and hyperlipidemia. Nephrotic-range protein-uria in children is de fined as a urine protein-to-creatinine ratio of 200 mg/mmol or greater ($2 mg/mg) or 24-hour ...
Nephrotic syndrome is a collection of symptoms due to kidney damage. This includes protein in the urine, low blood albumin levels, high blood lipids, and significant swelling.Other symptoms may include weight gain, feeling tired, and foamy urine. Complications may include blood clots, infections, and high blood pressure.. Causes include a number of kidney diseases such as focal segmental ...
Introduction. Nephrotic syndrome (NS) is the most common glomerular disease in children, but its occurrence in the first year of life is rare. It is defined as congenital nephrotic syndrome (CNS) if the disease onset is in the first 3 months of life, or infantile nephrotic syndrome (INS) if it occurs between 3 and 12 months of life.
Background and objectives: Nephrotic syndrome (NS) in the first year of life is called congenital (CNS) if diagnosed between 0-3 months, or infantile (INS) if diagnosed between 3-12 months of age. The aim of this study was to determine if there were clinically meaningful differences between CNS and INS patients, regarding clinical presentation, management and outcomes.
Clinical presentation. Nephrotic syndrome, defined as proteinuria >3.5 g per day and serum albumin <3.5 g/dl (when measured by bromocresol green) or <3.0 g/dl (when measured by bromocresol purple ...
2. Definition Nephrotic syndrome is a clinical complex characterized by a number of renal and extrarenal features, most prominent of which are Proteinuria (in practice > 3.0 to 3.5gm/24hrs), Hypoalbuminemia, Edema, Hypertension Hyperlipidemia, Lipiduria and Hypercoagulabilty. 3. 4. Classification Nephrotic syndrome can be Primary, being a ...
Citation 9 Our case report presents a unique scenario in which cerebral sinovenous thrombosis is an initial presentation of nephrotic syndrome, a rare condition that causes delays in diagnosis and treatment. Such cases are often missed due to the rarity and lack of awareness about the association between these two conditions, making it ...
Membranous nephropathy is the most frequent glomerular lesion. Herein, we report the first documented case of immunoglobulin G4-related disease presenting with nephrotic syndrome owing to minimal change disease. Case presentation. A 67-year-old South Asian male presented to our service with systemic upset and leg swelling.
A kidney illness known as nephrotic syndrome is characterized by high cholesterol levels, swelling (edema), low blood protein levels, and excess protein in the urine. Recognizing the signs of this ...
Hypercoagulability and thrombotic complications in nephrotic syndrome are most commonly seen in deep veins of the lower extremities and renal veins. Our case highlights a unique scenario in which cerebral sinovenous thrombosis was the initial presentation of nephrotic syndrome in a patient that was not an important past medical or surgical problem.
Download the "Nephrotic Syndrome" presentation for PowerPoint or Google Slides. Taking care of yourself and of those around you is key! By learning about various illnesses and how they are spread, people can get a better understanding of them and make informed decisions about eating, exercise, and seeking medical attention. This Google Slides ...
Nephrotic syndrome is treated with corticosteroids, but 20% of children are steroid-resistant, and 80-90% relapse, leading to recurrent therapy and steroid dependence. Saito et al. 2004: Therefore, the category of renal disease sufferers who have become steroid-dependent or resistant with a high rate of recurrence is referred to as RNS.
1. Nephrotic Syndrome Prepared By: Dr. Merwais Azizyar Supervisor: Assistant Clinical Professor Dr. Zabehullah Fazli 2019. 2. Introduction Definition of NS Classification of NS Pathophysiology of NS Clinical Manifestation of NS Complication of NS Differential Diagnosis (Edema) Laboratory Data Management.
Nephrotic Syndrome. 1. Presented by: Mr. Abhay Rajpoot. 2. Nephrotic syndrome is a primary glomerular disease characterized by proteinuria ,hypoalbuminemia , diffuse edema ,high serum cholesterol and hyperlipidemia. 3. Nephrotic syndrome is a collection of symptoms due to kidney damage. This includes protein in the urine, low blood albumin ...
Free Google Slides theme, PowerPoint template, and Canva presentation template. Download the "Nephrotic Syndrome Management Case Report" presentation for PowerPoint or Google Slides. A clinical case is more than just a set of symptoms and a diagnosis. It is a unique story of a patient, their experiences, and their journey towards healing.
Of note, only 8% patients in this study were reported to develop hypogammaglobulinemia. Indeed, hypogammaglobulinemia is a frequent complication following rituximab and can occur in up to 14-58% of children with nephrotic syndrome [2, 3].While rituximab is used in various paediatric glomerular diseases, the risk of hypogammaglobulinemia appears to be higher in nephrotic syndrome owing to ...
I read with interest the manuscript by Zurowska et al. entitled "Rituximab-associated hypogammaglobulinemia in children with idiopathic nephrotic syndrome: results of an ESPN survey" [].This is an area of concern for paediatric nephrologists in the use of rituximab for glomerular diseases [2, 3].A total of 1328 children from 84 European centres were analysed in this huge survey.
Nephrotic syndrome (NS) is common in children. Most of them respond well to glucocorticoid, while a minority are steroid-resistant [1,2,3].Steroid-resistant nephrotic syndrome (SRNS) primarily presents as focal segmental glomerulosclerosis (FSGS), which is associated with an unfavorable renal prognosis [4, 5].Despite extensive research efforts, the etiology and pathogenesis of SRNS remain ...
Hypercoagulability and thrombotic complications in nephrotic syndrome are most commonly seen in deep veins of the lower extremities and renal veins. Our case highlights a unique scenario in which cerebral sinovenous thrombosis was the initial presentation of nephrotic syndrome in a patient that was not an important past medical or surgical problem.