- Search Menu
- Sign in through your institution
- Cytogenetics
- Cytotechnology
- Histotechnology
- Management/Administration
- Microbiology
- Molecular Biology
- Molecular Pathology
- Transfusion Medicine
- Advance articles
- COVID-19 articles
- Current Virtual Issue
- Cover Archive
- Author Guidelines
- Open Access
- Submission Site
- Why publish?
- Advertising and Corporate Services
- Advertising
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- About Laboratory Medicine
- About the American Society for Clinical Pathology
- Editorial Board
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
- < Previous
Laboratory Management: Quality in Laboratory Diagnosis
- Article contents
- Figures & tables
- Supplementary Data
Anthony Kurec, Laboratory Management: Quality in Laboratory Diagnosis, Laboratory Medicine , Volume 46, Issue 1, Winter 2015, Page e1, https://doi.org/10.1309/LMH0BBLXMPLLR8NQ
- Permissions Icon Permissions
Laboratory Management: Quality in Laboratory Diagnosis is one of a series of useful books that covers key areas of management in today’s complex clinical laboratory. Laboratory Management is a concise review of certain responsibilities of laboratory leaders and how those leaders can best manage some of the more common problems that they face.

For managers, supervisors, lead technologists, and aspiring laboratory leaders, this book serves as guide to focusing on tasks that are critical to proper laboratory management. The 12 chapters touch on the 4 cornerstones of laboratory management: financial management, operational management, human resource management, and marketing management. Specific topics include regulatory mandates; patient and laboratory safety; quality issues of preanalytic, analytic, and postanalytic problems; financial concerns related to billing, equipment purchases, and reference laboratory opportunities; personnel selection, compensation, and workload adjustments; and development of a strong marketing program by addressing phlebotomy centers, laboratory information system (LIS) needs, and test utilization issues.
Each chapter contains an overview of examples of real life issues encountered in the daily operations of today’s clinical laboratory. Each example provides a case study of an actual laboratory problem (Case with Error or Case with Error Averted) that influenced patient care and quality of services. The issue is then followed by an Explanation and Consequences section that details how that issue was resolved or could have been prevented. Each chapter concludes with a set of Standards of Performance that neatly summarizes the key points of the particular chapter.
This book is a quick read and is ideal for busy laboratory managers and supervisors; it contains a relatively complete index and additional reading sources for more-detailed management discussions. It is a particularly useful guide for individuals in Pathology residency training who need to know various aspects of laboratory management but may not have had much training or experience in this area. Laboratory Management provides the opportunity to learn from the mistakes of other individuals to stimulate readers to reflect on their own laboratory practices and to be proactive in establishing policies and procedures that promote quality laboratory services.
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 1943-7730
- Print ISSN 0007-5027
- Copyright © 2024 American Society for Clinical Pathology
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Reference Manager
- Simple TEXT file
People also looked at
Review article, automation in the life science research laboratory.

- Deanery of Biomedical Science and Synthsys Centre for Synthetic and Systems Biology, University of Edinburgh, Edinburgh, United Kingdom
Protocols in the academic life science laboratory are heavily reliant on the manual manipulation of tools, reagents and instruments by a host of research staff and students. In contrast to industrial and clinical laboratory environments, the usage of automation to augment or replace manual tasks is limited. Causes of this ‘automation gap’ are unique to academic research, with rigid short-term funding structures, high levels of protocol variability and a benevolent culture of investment in people over equipment. Automation, however, can bestow multiple benefits through improvements in reproducibility, researcher efficiency, clinical translation, and safety. Less immediately obvious are the accompanying limitations, including obsolescence and an inhibitory effect on the freedom to innovate. Growing the range of automation options suitable for research laboratories will require more flexible, modular and cheaper designs. Academic and commercial developers of automation will increasingly need to design with an environmental awareness and an understanding that large high-tech robotic solutions may not be appropriate for laboratories with constrained financial and spatial resources. To fully exploit the potential of laboratory automation, future generations of scientists will require both engineering and biology skills. Automation in the research laboratory is likely to be an increasingly critical component of future research programs and will continue the trend of combining engineering and science expertise together to answer novel research questions.
Introduction
The progressive integration of automation into work environments has enhanced the production rates, efficiency and quality of an enormous array of industrial processes ( Hitomi, 1994 ; Autor, 2015 ). From generation to generation, mechanised tooling has replaced swathes of manual tasks. More recent advances in robotics and information technology have further automated processes that were once the sole domain of human brawn or brain ( Hasegawa, 2009 ). Life science research conducted within academic institutions has also welcomed the ingress of mechanised equipment designed to automate a range of tasks. However, it is noticeable that a typical university research laboratory, often led by a single principal investigator, maintains a high level of manual manipulation in the form of undergraduate, postgraduate, post-doctoral and technical staff. Many experimental procedures remain heavily reliant upon the individual researcher manually carrying out protocols at the research bench.
This is in contrast to industrial environments, where widespread investment in automation has allowed companies to maximise their outputs and increase profits ( Ravazzi and Villa, 2009 ). Laboratories in a clinical setting have also experienced the benefits of adopting automation ( Hawker et al., 2018 ), increasing the speed and reliability of patient-specific data for use by clinicians ( Sarkozi et al., 2003 ; Lou et al., 2016 ). In this review, written from the perspective of an automation engineer now working in synthetic biology research and a Principal Investigator managing a research laboratory, we classify the current levels of automation in laboratories and highlight the benefits and limitations of its usage in research. We further attempt to summarise why automation has had such a limited impact in our workplace ( Jessop-Fabre and Sonnenschein, 2019 ) and ask whether the solution to including more automation into everyday laboratory tasks may reside in greater communication between scientists and engineers. Further, we suggest that it could be accelerated by beginning with a more low-tech approach rather than striving too soon for fully autonomous systems.
Current Laboratory Automation
Well-meaning predictions of the cybernetic laboratory ( Beugelsdijk, 1991 ) and a robotic revolution ( Boyd, 2002 ) have, at the time of writing, yet to materialise in the majority of life science research laboratories. Evidence from the proportional use of the terms ‘automation’ or ‘automated’ in the titles of PubMed listed articles does, however, exhibit a steady increase over the previous 4 decades. The terms ‘robot’ or ‘robotic’, which are often used interchangeably with automation, received negligible use until the mid 90’s and then showed a more marked elevation ( Figure 1 ). It should be noted however that, ‘robot’ or ‘robotic’ can also be used as an adjective for biological systems or medical devices and the increase in their prevalence may represent changes in language usage rather than an indication of greater automation usage. A more thorough text mining exercise than ours attempted to measure the extent of manual protocols that could potentially be automated through analysis of methods sections in published life science articles. The study concluded that 89% of articles featured a manual protocol that has an automated alternative ( Groth and Cox, 2017 ). Whilst there is a scale of automation, from the simple to the complex, that could be applied to these protocols, such data provides evidence that there remains a large potential for automation in most biology research laboratories. There are also clear claims in the literature that researchers working in academic institutions have been slow to embrace automation ( Sadowski et al., 2016 ; De Almeida and Ferreira, 2017 ; Jessop-Fabre and Sonnenschein, 2019 ).
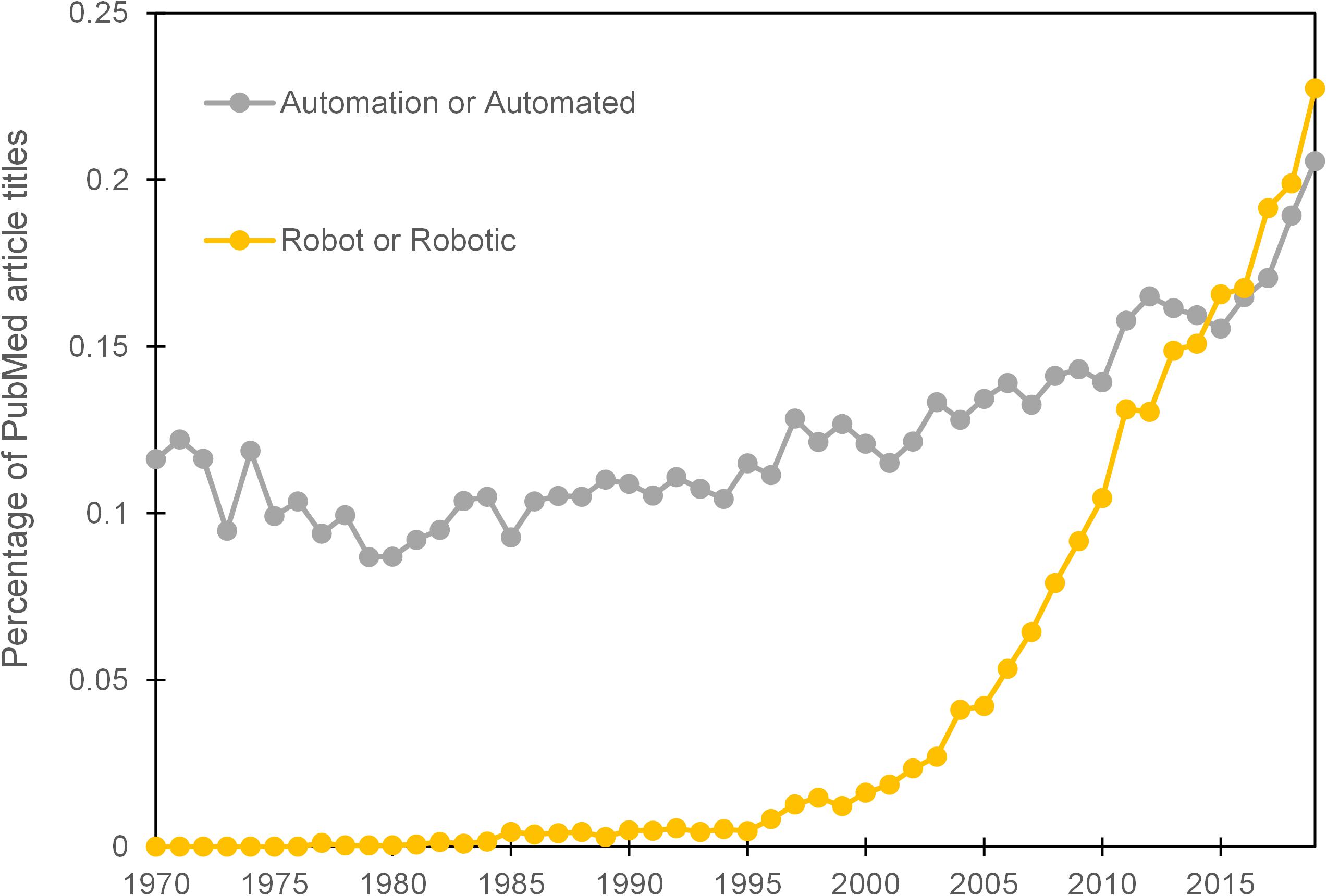
Figure 1. Prevalence of terms ‘automation’ or ‘automated’ and ‘robot’ or ‘robotic’ within the titles of PubMed articles per year over the period 1970–2019.
In this review we focus on automation where it describes equipment that physically manipulates items and we do not consider solely software-based technologies, such as image analysis and data mining tools. Within our scope there resides a diverse range of equipment that is found in research laboratories, from simple hand tools to entirely autonomous systems. A classification system for laboratory automation equipment has, to our knowledge, yet to be published, although a number of equivalent methods have been developed for classifying industrial automation. Frohm et al. (2008) reviewed these systems before proposing their own 7 levels of automation. These levels and descriptions are displayed in Table 1 , alongside examples typically seen in an academic research laboratory, and an indicative cost.
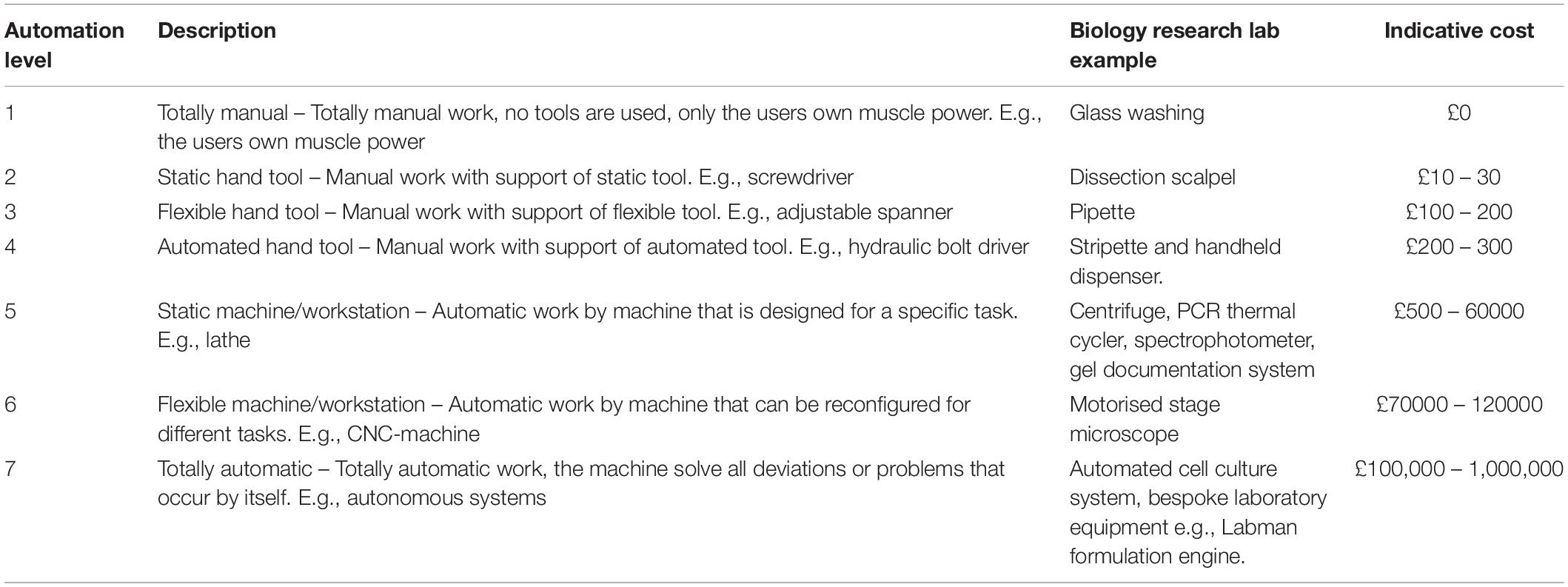
Table 1. Automation levels ( Frohm et al., 2008 ) with example laboratory automation equipment and an indicative cost range.
It is noticeable from Table 1 that the majority of equipment items that researchers would consider as the most expensive in their laboratory are categorised at level 5. Higher grade 6 and 7 items are a rarity in a biological research laboratory. Whilst mid-range level 5 automation items undoubtably increase the efficiency of laboratory research, they are designed for specific subtasks in a range of protocols. These items also generally require a large amount of manual manipulation both before and after machine usage. Within the research laboratory this category of equipment is commonplace and dominates equipment budgets. A further observation can be made in that the majority of research equipment in this category performs tasks that human operators would otherwise be incapable of carrying out themselves ( McClymont and Freemont, 2017 ). The rotation of samples at high speeds and observing microscale environments are examples of tasks that would be impossible without the use of centrifugation and microscopy equipment. Automation equipment which replaces manual handling tasks is rarer, and it the prevalence of these items where academic bioresearch facilities differ to industrial environments and clinical laboratories.
Access to high level 7 automated equipment can usually only be obtained through a pooled resource shared between across the parent organisation or wider research community; these are often referred to as biofoundries ( Chambers et al., 2016 ; Chao et al., 2017 ; Kitney et al., 2019 ). A new automation variant of the commercial contract research organisation has also arisen recently, the cloud lab. These provide researchers with remote access to heavily automated protocols available as a pay-per-experiment service ( Hayden, 2014 ). Cloud lab executives have made grand predictions regarding the impact these facilities will have on the future of biological research ( Miles and Lee, 2018 ; Segal, 2019 ), although doubts remain regarding experimental flexibility and the resulting inhibitory effect on experimental innovation ( Hayden, 2014 ).
Benefits of Laboratory Automation
Reproducibility.
There are multiple advantages and limitations in including automation into scientific processes and these are summarised in Figure 2 . Most pertinent is its use in improving the reproducibility of laboratory research ( Kitney et al., 2019 ). Reproducibility is a major concern for the research community both now ( Begley and Ioannidis, 2015 ; Baker, 2016 ) and historically (reviewed by Fanelli, 2018 ), with associated economic implications ( Freedman et al., 2015 ) and an undermining of public trust in science ( Saltelli and Funtowicz, 2017 ). Debate continues regarding the definition and scope of the reproducibility issue ( Casadevall and Fang, 2010 ; Goodman et al., 2018 ), alongside proposed improvements in scientific practices ( Peng, 2015 ; Munafò et al., 2017 ) and remedial technologies ( Benchoufi and Ravaud, 2017 ). Increasing the use of automation throughout research laboratories is one such proposition ( Jessop-Fabre and Sonnenschein, 2019 ; Kitney et al., 2019 ). An improvement in reproducibility is cited as a beneficial effect of automation implementation within clinical laboratories ( Hawker et al., 2018 ; Genzen et al., 2018 ).
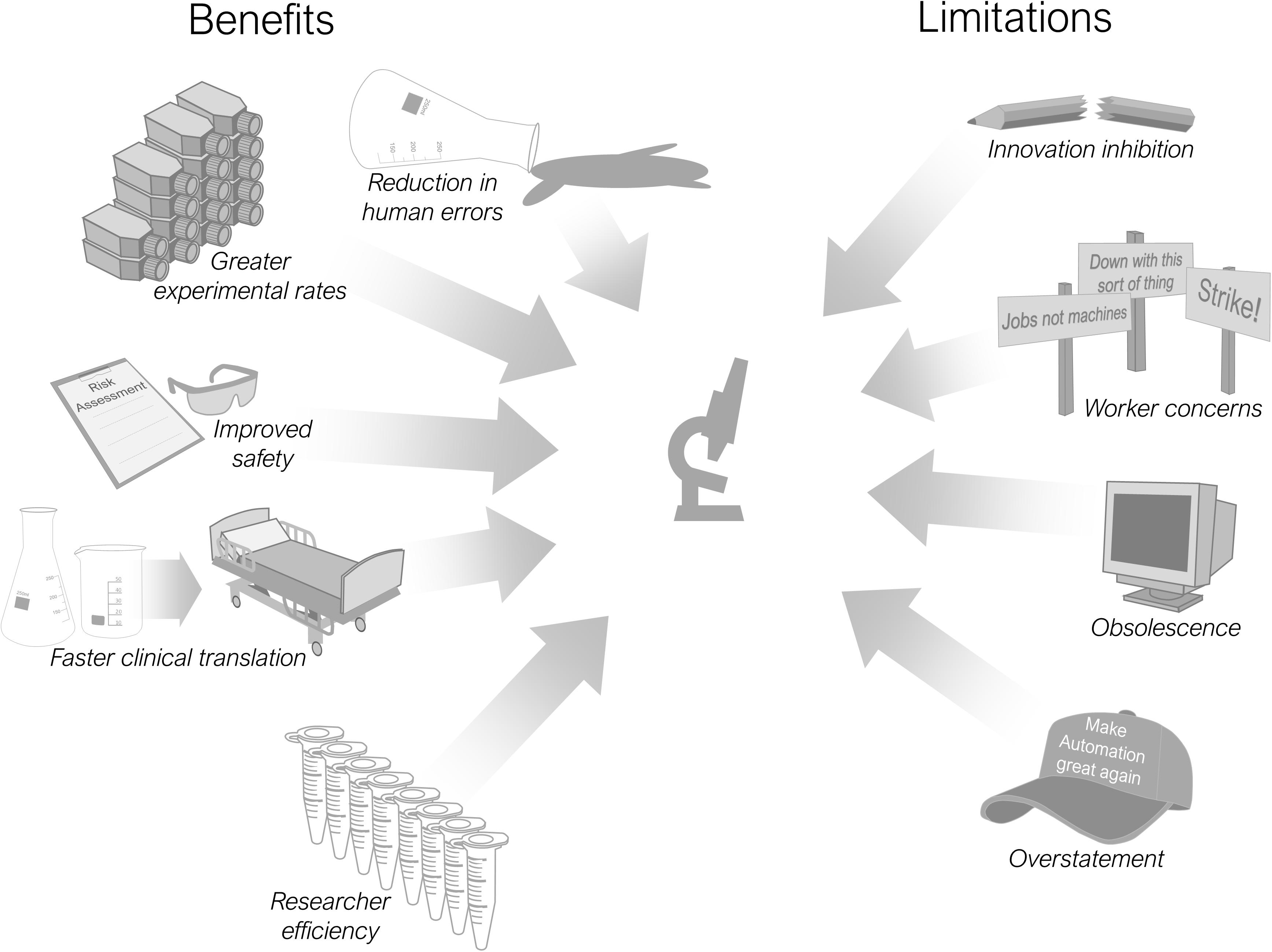
Figure 2. Benefits and limitations of research laboratory automation.
Automation can assist in improving reproducibility in three ways: a reduction in human-induced variability, an increase in the rate of data generation, and a decrease in contamination. The contribution each of these factors has on increasing reproducibility depends on the individual protocol. Firstly, experimental variability caused by humans is an omnipresent day-to-day reality in research laboratories ( Plebani, 2010 ; Price et al., 2015 ). Variation in protocols can arise from the same person unknowingly performing a task differently each time or between different individuals attempting to carry out the same procedure. Variability that is noticed at the time can be corrected for with repeated protocols or experimental redesign, although with an associated time penalty. However, variation that goes unnoticed will manifest itself in final datasets and published results. Automation can replace many, but not all, of these human-based sources of variability. Mechanised componentry is more suited to repetitive tasks ( Moutsatsou et al., 2019 ) in comparison to humans who are vulnerable to progressive mental fatigue ( Xu et al., 2018 ), physical weariness ( Björklund et al., 2000 ; Iridiastadi and Nussbaum, 2006 ) and also distracting influences ( Varao-Sousa et al., 2018 ). Laboratory protocols where manual operations have been automated demonstrate greater consistency in their results, improving experimental reproducibility ( Klevebring et al., 2009 ; Price et al., 2015 ). Secondly, a greater rate of experimental data capture, with an increased volume of results, can be achieved with automation alongside a wider range of experimental variables tested, including controls. Ultimately this increases the likelihood that others will be able to reproduce and build on their findings ( Maleki et al., 2019 ). Finally, there are those laboratory protocols that are susceptible to contamination that can arise from either from the researchers themselves ( Salter et al., 2014 ) or through increased exposure to environmental contaminants due to ponderous manual handling operations ( Greub et al., 2016 ). Automation can remove contact with human operators ( Wilke et al., 1995 ) or reduce potential contaminant exposure by lowering the required number of manual handling steps ( Mifflin et al., 2000 ; Moutsatsou et al., 2019 ).
Laboratory Efficiency
Efficiency is considered of paramount importance within manufacturing and can be defined as the rate of production, divided by the resources such as labour, input materials needed to accomplish this rate. By investing in automation, a company can increase the rate of production and also reduce the resources needed to achieve this rate. With a market available this can translate to a corresponding increase in profits ( Ceroni, 2009 ). A research laboratory investing in automation can improve the efficiency of its researchers ( Hawker and Schlank, 2000 ; Schneider, 2018 ) with machinery able to achieve a greater rate of experimental output than a manual based alternative ( Tacker et al., 2014 ; Price et al., 2015 ; Choi et al., 2018 ). It should be noted that an automated protocol need not take less time from start-to-finish to result in higher output than the manual alternative, as long as it demands less human intervention ( Reed et al., 2018 ). This is due the to the reward for academia differing from industry, with efficiency considered more as a time input to experimental output ratio. The key benefit derived from laboratory automation driven processes is therefore in the time saved by the researchers; time that can be spent on other parallel experiments. Automation in most cases will induce a transition from manual to cognitive labour ( Kaber et al., 2009 ). Allowing an operator to set a protocol in operation and walk away to think and focus on other tasks is a valuable function for any automation equipment. Researchers frequently have multiple projects, and experimental protocols operating in parallel as well as an array of responsibilities beyond the laboratory. With a greater rate of automation-driven experimental output researchers can also identify which aspects of their experiments don’t work and adjust more quickly ( Baranczak et al., 2017 ). Within industrial pharmaceutical development this methodology is known as fail fast, fail often ( Clark and Pickett, 2000 ; Khanna et al., 2016 ; Besteman and Bont, 2019 ). Efficiency gains can also extend to the use of expensive reagents and materials. Automation can provide a higher level of precision in reagent dispensing, reducing the amount needed per experiment.
Faster Translation
Automation has an important role in those laboratories engaged in applied research who are seeking to develop novel therapeutic interventions such as cell-based therapies, pharmaceutical developments or tissue-engineered constructs for implantation. Transition of these technologies from a purely research domain to final usage in a clinical setting is frequently difficult ( Ochs et al., 2017 ; Hua et al., 2018 ), often referred to as translation from the bench to the bedside ( Goldblatt and Lee, 2010 ). By considering and including automation at an early stage in the research process, crucial elements of the process can be mechanised, increasing product quality and production rates in the laboratory before the jump to manufacturing. The technological leap from laboratory-scale production to higher-volume manufacturing is therefore shortened. Researchers who include automation technologies at an early stage are subsequently better placed to upscale their processes allowing faster commercialisation rates and deployment to the clinic ( Kotin, 2011 ; Heathman et al., 2015 ; Rafiq and Thomas, 2016 ).
A number of protocols carried out in the research laboratory require the handling of dangerous reagents and occasionally of hazardous tooling. The manual manipulation of hazardous items places a burden on laboratories, particularly when contending with a continual turnover of short-term contract staff and students who require safety training and supervision. By assigning dangerous handling tasks to automated machinery, the exposure of humans to hazardous substances can be reduced ( Movsisyan et al., 2016 ; Caragher et al., 2017 ).
Examples of Automation Benefits
Evidence of automation benefits can be observed in recent success stories. In projects where high-throughput, reproducible results are demanded over short time frames automation has significant advantages over manual procedures. Recently a highly automated biofoundary, normally with a focus on research applications, was repurposed towards the development of SARS-CoV-2 assays for clinical diagnostics ( Crone et al., 2020 ). Automated liquid handling equipment was able to perform an extensive array of experimental procedures at a rate in excess of those that a manual based laboratory could carry out. Furthermore, in these time-pressured experiments, automation has an advantage over manual operators who are prone to fatigue and errors, with an associated negative effect on accuracy and reproducibility. Such work also clearly demonstrates the positive impact automation can have on public health challenges. It also an example of considerate design leading to systems that are flexible enough to be rapidly adapted to meet new experimental needs. This design feature is appropriately termed ‘facility agility.’
The use of automation to improve research efficiency is also demonstrated with a system comprising a mobile robotic platform that can autonomously navigate a laboratory performing reagent-dispensing and handling operations at a range of experimental benchtop stations ( Burger et al., 2020 ). In combination with an artificial intelligence search algorithm, the system was able to use initial data to decide on reagent combinations most likely to include an optimal reaction mix. The capacity of the robotic equipment to operate at all hours, with pausing only to charge batteries, allowed it to test five experimental hypotheses in a fraction of the time a manual research team would have required. Although it was used to answer a research question within a chemistry context the concept would be readily applicable to life science experimental laboratories. The system shares similar liquid and solid reagent handling operations to a life science laboratory as well as the common challenge of there being too many variables for researchers to explore manually in a reasonable time. A further crucial advantage of this arrangement resides in the possibility, with appropriate safety controls, of operating as a hybrid manual-automated laboratory. A staffed day shift performing high-skilled tasks requiring on-the-spot decisions could be followed by a robotic night shift carrying out the repetitive aspects of procedures.
Researchers aiming to translate stem cell-derived therapies towards clinical applications have considered automation for a range of projects. Such therapies will ultimately require the expansion of stem cells on a scale that is uneconomical for manual based laboratories, with large numbers also needed for research and clinical trials phases. The need for reliable methods of high-volume, quality-assured cells has led to the development of automated systems such as the StemCellFactory ( Doulgkeroglou et al., 2020 ), StemCellDiscovery ( Jung et al., 2018 ) and AUTOSTEM ( Ochs et al., 2017 ). The objective of these systems is to automate the normally manual stages of stem cell seeding, growth, colony selection, passaging, quality assessment, harvesting and potentially in later applications differentiation. In a similar fashion to the previous mobile robotic platform example, complex control algorithms are also being applied to these systems with the aim of improving cell yields and quality ( Egri et al., 2020 ). These projects are an important link between the domains of basic life science research, clinical application, and commercial cell product manufacturing. By developing these systems researchers have been able to generate high quantities of cells for research and testing purposes, hastening the route to clinical usage.
Limitations of Automation
Incorrect application.
Despite the range of benefits that laboratory automation can bring, there remains a number of limitations. Integrating automation into a research laboratory is not in itself a guarantee of success and, where applied incorrectly can even result in even less efficiency ( Zielinski et al., 2014 ). The nature of automated tasks also allows for rapid propagation of errors. An example would be a machine incorrectly dispensing a reagent repetitively which can then, if undetected, be distributed across many thousands of samples. In addition, the incorrect application and operation of automation may not improve the reproducibility of research between laboratories. Automation machinery carrying out the same experimental protocol in different laboratories may still produce different results. This can be due to variations in input materials, different equipment models or set-up and calibration errors. Even where automation has been carefully integrated into a laboratory and has demonstrated an improvement in reproducibility an inherent machine to machine variability can remain. What is more, this variability can be more hidden than more easily observed manual procedures. Careful maintenance, calibration and quality control measures are therefore essential in implementing any laboratory automation system ( Hawker and Schlank, 2000 ; Xie et al., 2004 ).
Obsolescence
Obsolescence is an inevitability for any technology and even, it can be argued, for scientists themselves. Many facilities will feature a dusty machine in the corner that is unused, because components and materials are no-longer available, the protocol itself has been supplanted or simply newer more effective equipment has taken over ( Croxatto et al., 2016 ). Predicting how and when a machine will become obsolete is an inherently difficult task in rapidly evolving research fields and can be specific to individual laboratories. Some researchers will find equipment is no-longer useful after a few years of operation whilst others may continue to happily use the same machine for decades. It is not only advances in hardware and software design that can render laboratory equipment obsolete. Scientific progress in reagent properties and resulting modifications to protocols can also be responsible. The advent of new thermostable polymerases obsoleted a whole generation of Polymerase Chain Reaction machinery designed upon a more repetitive protocol ( Hawker et al., 2018 ). Despite these difficulties, with considerate design allowing for reconfiguration and modification premature obsolescence can be delayed ( Harrison et al., 2007 ; Crombie et al., 2017 ), referred to in some industries as future-proofing. Understanding and planning for obsolescence is therefore an important part of any automation strategy.
Innovation Inhibition
There is a danger that automation can inhibit creativity in the experimental design process by limiting the opportunities for changing or tinkering with a protocol. A researcher may be less inclined to alter a protocol to optimise it for a new situation where a large number of steps are automated. This can be based upon the assumption that process steps carried out by machinery are already optimised and require no further improvement. They may also feel less able to begin changing things because they lack the confidence or maybe even the authorisation to open the box and begin modifying what is probably an expensive machine. Sharing of the machine with other users for whose purposes it is already optimised is also a brake to experimentation with parameters. Innovation inhibition is also a concern where protocols are outsourced to third party automated laboratories ( Hayden, 2014 ).
Workforce Impact
When integrating new automation into any workplace environment, the impact on workers and how they view new machinery must be carefully considered. Beginning in the rural English midlands with the machine breaking Luddite movement ( Roberts, 2017 ), societal resistance to automated machinery replacing manual labour and the threat it poses to livelihoods understandably continues into the present day ( Jones, 2013 ; Autor, 2015 ). Both positive and negative reactions to the introduction of automation have been observed amongst long-term workers in clinical laboratory settings ( Thomson and McElvania, 2019 ) and it is reasonable to anticipate that similar reactions may arise in research laboratories. The outright replacement of researchers by automation is unlikely as they are currently categorised as being amongst the lowest risk of being replaced ( White et al., 2019 ), due to their breadth of skills, including planning and creativity ( Reeves et al., 2019 ). However, researchers solely employed to perform repetitive manual tasks are more at risk and thus more likely to view automation as a threat. Those researchers with a multitude of other protocols and tasks beyond the laboratory are more likely to view automation assistance in their day to day roles in a positive manner. The short-term contracts that predominate in research will also lessen any hostility to automation. Employees who understand that they will be moving on to another position, will see a machine as more likely to be a replacement for their replacement rather than a replacement for themselves. Although the levels of militancy advocated by the early Luddites may not be repeated, laboratory managers who introduce automation will still, like their industrial and clinical counterparts, need to be sensitive to workforce reactions, particularly the impact on any long-term employees.
Automation Hyperbole
Both vendors of automation equipment and researchers must also be wary of overstating the benefits of automation and elevating expectations regarding the impact its introduction will have on future work practices. Automation hyperbole and the accompanying benefits is however part of a wider trend that is not only restricted to research ( Wajcman, 2017 ). Whilst automation can improve protocol reproducibility and efficiency the individual researcher will, in the majority of cases, still be responsible for correctly operating the equipment, with maintenance, quality of input materials, and calibration. These are tasks than can require a high level of personal discipline and tenacity. With notable exceptions ( King et al., 2009 ; Williams et al., 2015 ), automation will also be unable to undertake the overall experimental design and analysis. Journal publications have a responsibility too, to ensure that articles advocating laboratory automation equipment also highlight the limitations of their technologies, as well as identifying author conflicts of interests ( Miles and Lee, 2018 ). Greater awareness of limitations will allow more effective matching of automation solutions with laboratory problems and increase the trust between commercial vendors and academic institutions.
Laboratory Automation Obstacles
Automation is expensive and difficult to justify.
The most significant hurdle for PIs wishing to integrate automation systems into their laboratories is, unsurprisingly, cost. Commercially available automation equipment is expensive, whilst bespoke equipment for individual protocols costlier still. Cell culture is an example of a common, labor-intensive protocol familiar to generations of researchers. Equipment to automate cell culture is available and can save many hours of researcher effort from the process, but is tantalisingly out of reach for most laboratories. The cost of these items can be in excess of $1 M for a complete process system ( Storrs, 2013 ) placing them far beyond the reach of the majority of academic laboratories. Despite being commercially available for over 18 years ( Kempner and Felder, 2002 ) they remain a rare sight in research environments but are used in high volume cell-banking organisations ( Wrigley et al., 2014 ; Archibald et al., 2016 ; Daniszewski et al., 2018 ).
The development of automation equipment can be a time-consuming and expensive process. Initial rounds of iterative conceptual and prototype design and testing are followed by final design, build, and commissioning phases. Coordination is needed from a variety of disciplines including mechanical, electrical and software engineers alongside close collaboration with the end user. Most important for all automation projects however, is a source of capital investment. Industrial investment in automation is matched to business cases in which increasing confidence in the product and the associated income from projected sales is used to justify upfront capital expenditure. However, an academic principal investigator seeking to invest in automation for their laboratory is confronted by a different set of challenges. When compared to industrial and commercial organisations, a research laboratory’s output or success rate cannot be measured in using the same readily quantifiable metric of profit. Indeed, academic research output has long been a difficult entity to define both for individual researchers ( Klaus and del Alamo, 2018 ) and laboratories ( Kreiman and Maunsell, 2011 ; Abramo and D’Angelo, 2014 ). It is therefore more difficult to construct a ‘business’ case when seeking funding for laboratory automation equipment. A factory manager is able to justify a new item of automation based upon the argument that whilst it may initially cost X units of currency it will increase profits by X + Y units, measured in the same currency ( Ceroni, 2009 ). A clinical laboratory manager can present a similar case based upon both cost ( Archetti et al., 2017 ; Sarkozi et al., 2003 ) and the quantifiable output of turnaround time ( Hawkins, 2007 ; Archetti et al., 2017 ). A research laboratory manager however, in the same position applying for funding, will have greater difficulty in arguing that although the proposed equipment will cost X units of currency it will increase their laboratory’s research output by Y vaguely defined research outputs. The ambiguity of research success hinders laboratories seeking to invest in automation.
Research Funding Structures
The allocation of scientific funding to academic institutions further limits investment in automation. Research programs are most frequently funded through externally sourced grants that are applied for in a competitive environment, with pre-applied constraints on the amounts available and where these funds may be spent. Understandably the majority of funding calls open to scientific laboratories are seeking answers to novel scientific questions and not looking to develop items of equipment that are essentially engineering challenges. Should an applicant wish to include standard or bespoke automation when applying for grants, capital expenditure on large equipment, if even permitted, must be explicitly accounted for before the project starts. Unfortunately, the nature of research means that the details of protocols needed for the project are not always available during the early proposal phase. Estimating the both the timescales and cost of automation at such an early stage is a difficult task for supervisors of biological research laboratories who will have limited experience of budgeting for automation hardware. The time duration of funding grants also limits the development of automation, usually with the maximum being 5 years ( European Commission, 2016 ; Vaesen and Katzav, 2017 ). Automation strategies for industry are generally greater in duration and aligned to the anticipated lifecycle of the product, frequently extending into decades. In the case of commercialising a novel pharmaceutical product or medical device the automation strategy can be aligned to the 20-year exclusivity patent window. Automation expertise acquired over this time can then be exploited to maintain a competitive advantage when the window expires. Academic projects of a comparable length are rare. The Human Genome Project is one exception, and consequently was able to invest and substantially benefit from automation ( Meldrum, 2000 ). However, long-term, project specific funding stability is rarely available to most academic principal investigators, limiting automation investment.
Short-term research funding also places a limit on the individual researcher’s ability to develop automation. Hands-on researchers are best placed to determine which elements of their protocols would benefit from automation. However, these individuals are typically Ph.D. students or early career researchers with a time-limited contract or project. Such temporal limitation leaves little room for developing an idea for protocol automation into a functional system, particularly with specific scientific targets attached to the grant scheme funding their project. Short duration research positions reduce not only the time available to develop novel automated laboratory equipment but also the motivation for doing so. On completion, a researcher is likely to move on to a new laboratory contract or a career beyond academia ( van der Weijden et al., 2016 ). Researchers are therefore unlikely to experience any of the long-term benefits from planning automation. The cumulative effect of short-term, competitive grant allocations and transient researchers creates an environment unsuited to the long-term financial investment required for laboratory automation development.
A limited number of large grant funded projects have been successful in devising automation strategies and equipment, although often with a focus on industrial scale systems for clinical translation rather than research laboratories. One area that seen recent attention is the aforementioned development of high-volume manufacturing solutions for the production of Mesenchymal and Induced Pluripotent Stem Cells to meet anticipated future clinical demand ( Marx et al., 2013 ; Panchalingam et al., 2015 ; Rafiq et al., 2016 ; Ochs et al., 2017 ; Jossen et al., 2018 ). It is hoped that technology developed in these programs will, in the future, trickle down into more affordable systems that can be exploited by smaller research laboratories.
Stifled Commercial Development of New Laboratory Automation
Financial challenges also hinder those commercial organisations seeking to develop laboratory automation equipment. Industrial automation design and development is often a bespoke, collaborative arrangement for a particular challenge. A manufacturer will approach one or more automation developers to design a manufacturing system for their product. In this scenario the manufacturer is usually a much larger organisation with abundant reserves of capital and will also carry the majority of the risk should the product not sell as well as expected. To aid in mitigating this risk they are able to utilise their marketing, sales and distribution expertise within their particular market sector. For development of automated laboratory equipment, the scenario is often different. An automation developer may wish to partner with an academic research laboratory. However, as previously detailed, in such an arrangement the laboratory will be unable to operate as a cash-rich development partner unless a substantial funding grant can be obtained. The automation developer must therefore carry the risk that the equipment will not be commercially successful and assume the role of marketing and selling the product to the wider research community. Biological laboratories are best placed to identify where certain processes would benefit from automation, but don’t have the financial resources or expertise to develop these systems themselves. Automation companies, whilst having the capable expertise to develop automation equipment will be reluctant to pursue such a business strategy requiring up-front investment to develop a product for customers widely acknowledged to have little disposable capital.
Small-to medium-sized automation companies have often been most successful at innovative development of laboratory equipment, funded through grant schemes in cooperation with an academic institution or external venture capital funding. Examples include benchtop pipetting systems from Andrew Alliance and OpenTrons and Labman automation’s formulation engine. Access to joint research grants and funding schemes can encourage the development of novel automation solutions by increasing industrial and academic collaboration whilst also reducing the risk the commercial risk that developers are exposed to.
Laboratory Space
Alongside the financial investment required for automation researchers must also find physical laboratory space for new equipment, incurring a footprint cost ( Wong et al., 2018 ; Moutsatsou et al., 2019 ). The size and mass of many automation items means that it is not always practical or safe to tidy the item away and store it when it is not required. Laboratory space is often at a premium in many research institutions with territorial researchers often coming into conflict over the allocation of it ( Adams, 2004 ). A bench occupied by equipment is also an area that could be otherwise be utilised by productive researchers. The requirement for some laboratories to operate as a dual research and teaching environment further constrains the available space. It may also not be possible for automation to totally replace more manual based equipment and space in laboratories, with room required for both. The need to maintain cell culture hoods for teaching is one example. Developers of laboratory of automation have attempted to minimise the footprint of their machinery through innovative reworkings of traditional laboratory procedures. The use of hollow fibre arrays ( Russell et al., 2018 ) and multi-axis liquid and labware manipulation ( Kato et al., 2010 ) are examples of compact automated adherent cell culture systems. Spatial constraints may push future bench-based laboratory automation towards an architectural style resembling inner city skyscrapers.
Protocol Variation and Usage
The very nature of bioresearch involves the design and implementation of protocols aimed at the determining answers to novel research questions. In pursuit of these targets, researchers will devise new protocols or substantially modify existing ones to suit their needs. Recurring cycles of method generation and evolution within the research laboratory create a high-level of protocol variation that is not always easily automated. Matching commercially available automation equipment to these requirements is often not a feasible option with fixed componentry and locked-in software frequently being the limiting factors. Automated cell culture is an example where the available systems can be insufficiently flexible to accommodate the specific cell culture requirements of an individual laboratory ( Crombie et al., 2017 ), with some requiring a broad range of cell culture types and others having more focussed needs. A high level of experimental process variation is therefore more likely to require a bespoke automation system, the development of which will have an associated time and financial cost. Clinical laboratories, by comparison, have a greater level of consistency across protocols both within individual laboratories and across institutions, contributing to the widespread implementation of automated systems. High process variability is also cited as one of the major challenges for integrating automation into existing industrial environments ( Frohm et al., 2006 ) and is necessary when adapting to changing market conditions ( Froschauer et al., 2008 ). Across laboratory protocols there are process steps that are common, and it these where commercially available systems are more likely to be of assistance to the individual researcher. Liquid handling, through the manipulation of pipettes and receptacles is a one example ubiquitous to a range of molecular biology protocols, with a growing number of competing vendors offering more affordable and adaptable automation options ( Barthels et al., 2020 ).
How frequently a protocol is likely to be used over time is also a key factor when considering automation. A protocol developed for a specific project may only be used in a single laboratory for a short period, negating the long-term benefits that automation could provide. On occasion a researcher may find that their new protocol becomes widely adopted for an extended period in their own laboratory, and possibly throughout other laboratories too. In this scenario automation becomes a more attractive option and is not always driven by the original founding laboratory. Sequencing, is one example where the initial manual protocol developed by Sanger and colleagues ( Sanger et al., 1977 ) was eventually automated by researchers at different institutions ( García-Sancho, 2007 ).
Labware and Consumables
Automation equipment operates most effectively when input materials or consumables are standardised. In the case of standard shaped labware this allows non-adaptive, rigid automation components such as grippers to gain full custody of the device, allowing greater accuracy of placement and potentially faster actuations. Currently there remains a large amount of variation in labware not only between research laboratories but also within the same laboratory. The variant a researcher uses can change frequently based upon cost, availability or personal preference. Disposable plastics are an example where different manufacturers produce products that are, from an experimental, viewpoint functionally identical but with variations in the products dimensions and materials. The justification for these variants maybe a small improvement in handling, or simply to circumvent intellectual property assigned to a competing product. These present a significant challenge to automated handling equipment where even small variations, that are unnoticeable when handled manually, can render an automated system using non-adaptive handling elements useless. Clinical laboratories negate this issue by utilising standardised plastics for sample collections that can then be more readily processed autonomously. The recent advent of soft robotics may provide solutions to these challenges where rigid handling systems are replaced with pliable, adaptive designs sometimes based upon biomimetic examples ( Noel and Hu, 2018 ).
A counterstrategy to labware variation has emerged from commercial developers of automation. Unfortunately, the solution is often combined with a sales strategy aimed at securing a continuous revenue stream following the sale of the initial capital equipment. Commercially available systems are frequently designed in a fashion such that automation systems can only operate with specific consumables, available for purchase from themselves or a licensed distributor ( Huggett et al., 2009 ; Moutsatsou et al., 2019 ). Examples include the pipette tips for the Opentrons and Tecan EVO liquid dispensing systems, array tape for Douglas Scientific’s IntelliQube PCR system, purification cards for Invitrogens benchpro and spin kits for Qiagens Qiacube system. A laboratory binding themselves to a single consumable supplier has little or no guarantee of future price stability or even long-term supply should the commercial vendor cease to exist. Committing to a long-term, single vendor, supply chain is considered a very unwise strategy in a commercial context but is a worryingly frequent arrangement for automation equipment available to research laboratories.
There are two competing forces for labware standardisation; top-down and bottom up pressure, outlined in Figure 3 . Top-down pressure, as described above, is where commercial automation organisations seek to dominate a section of the market by forcing users to purchase specific labware through the sale of inflexible hardware. Bottom-up pressure acts in the opposite direction, when manufacturers of labware and laboratories slowly gravitate towards one standard form that automation developers are then forced to adopt. An example where bottom-up pressure has succeeded is in the largely standardised external dimensions of well plates, the ANSI/SLAS standard ( Society for Laboratory Automation and Screening, 2011 ), that has enabled automation of microscopy and plate reading procedures ( McClymont and Freemont, 2017 ). The range of automation equipment available for standard well plates is correspondingly larger, increasing competition, reducing running costs and making automation more affordable. There is likely to be a reciprocal benefit for labware manufacturers too, with an associated increase in demand for consumables. More instances of labware standardisation would allow a wider range of protocols to be automated.
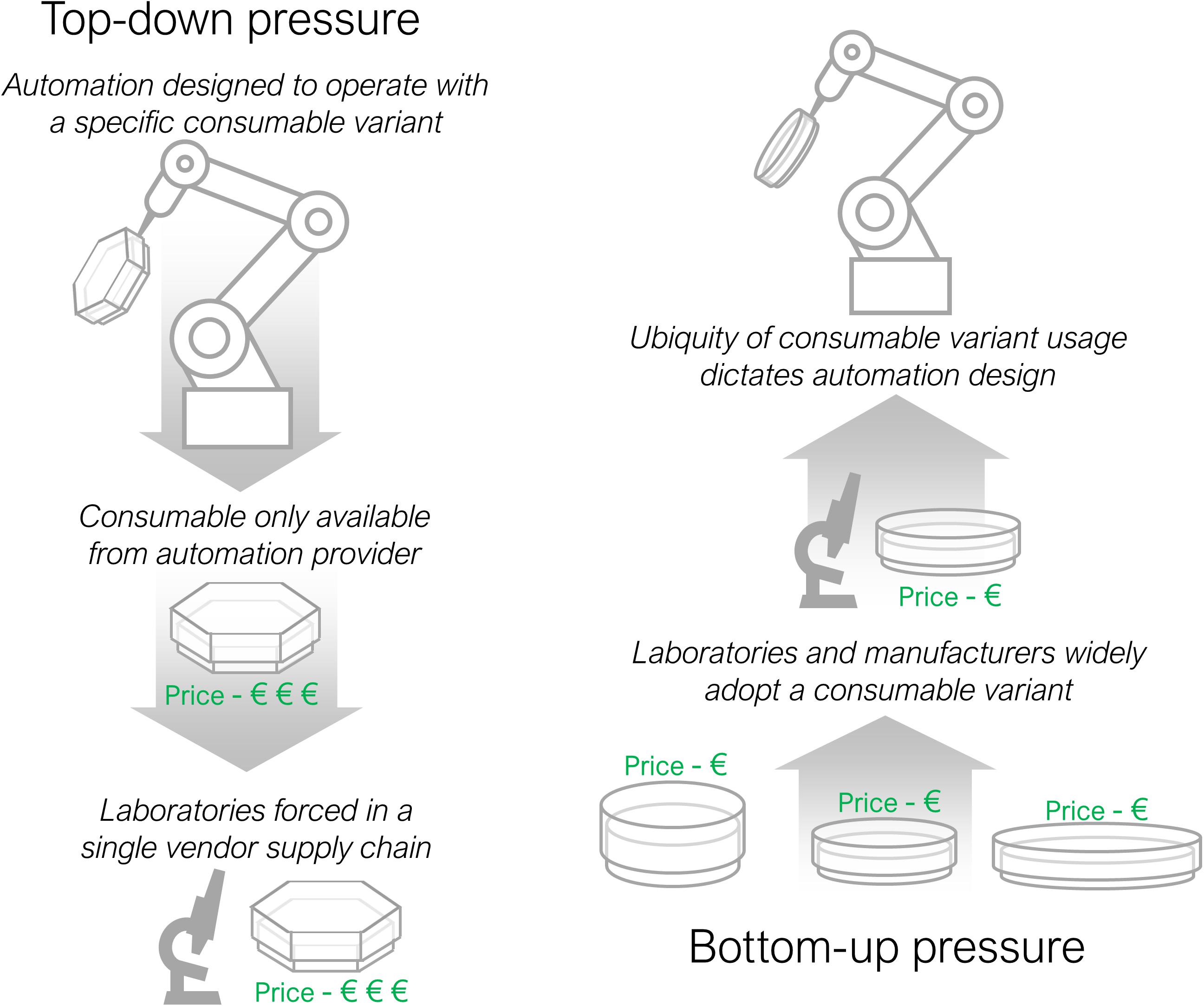
Figure 3. Top-down and bottom-up consumable adoption pressures. Top-down pressure occurs when an automation developer imposes a consumable on laboratories through tooling specific design. Bottom-up pressure acts in the reverse direction with laboratories and automation suppliers coalescing behind one consumable variant that then determines the design of automation equipment.
Environment Impact
The environmental impact that an item of equipment can have throughout its entire lifespan, from manufacture, to usage, to end-of-life disposal and recycling is an important consideration for many research institutions. A particular concern for laboratories is the rate at which automation consumes disposable plastics. Research institutions produce a large amount of plastic waste, estimated at 5.5 million tonnes annually ( Urbina et al., 2015 ), primarily to avoid contamination between samples. Commitments to minimising their use are part of a growing trend where laboratories aim to switch to recyclable or reusable alternatives ( Bistulfi, 2013 ; Krause et al., 2020 ). Automation designed around the same single-use plastic principle can generate even greater volumes of waste than human operators, due to higher experimental throughputs ( Howes, 2019 ). These designs are incompatible with research organisations who are committed to minimising their environmental impact. The consideration given to environmental concerns is currently very low or non-existent in many commercially available laboratory automation systems. An exception is Grenova’s pipette washing systems ( Safavi and Anderson, 2019 ) that can be integrated into existing automated liquid dispensing units. It is hoped that this type of equipment represents an emerging category of environmentally focused automation that will become ever more important to laboratories in the future.
There exists a fundamental culture difference between an academic research laboratory and the industrial workplace environment, that can inhibit investment in automation. It is hoped that the majority of principal investigators view their laboratory as a platform for staff and students to increase their skills and experience before they move onwards in their careers. This is a crucial ‘people’ output that accompanies the research output of a laboratory usually measured in scientific discoveries and publications. Although many companies also place a high-value on workforce upskilling their focus is primarily on profit and not on being a training institution to allow employee progression elsewhere. Consequently, many will favour investment in equipment over staff if a business case can be made ( Rampell, 2011 ). An academic principal investigator however, is likely to preferentially invest in additional people rather than equipment, with funding schemes frequently weighted this way too. Money spent on a large item of automation equipment could, for example, pay for several post-doctoral researchers or fund multiple Ph.D. projects. In the context of automation this culture could be described as a form of benevolent Luddism.
The availability and culture of undergraduate labour may also be inhibiting investment in laboratory automation. Undergraduates working in laboratories contribute by performing experiments that can generate preliminary data for grant applications or for publications. The benefits to the student reside in the acquisition of experience and skills that can enhance their employability prospects upon completion of their studies ( Seeling and Choudhary, 2016 ). This reciprocal arrangement and the high availability of undergraduates provides a means for carrying out labour intensive laboratory tasks. Not all principal investigators will view this relationship in such a cold manner, and will considerately assign duties that can generate useful data whilst simultaneously teaching students both the basics and realities of research. Unfortunately, there is evidence that some less altruistic supervisors do assign undergraduates to tasks that require a high degree of repetition ( Hayward et al., 2017 ). These are likely to be precisely the type of tasks where automation can be effectively applied.
The Laboratory Automation Interim Technology Gap
It is interesting to compare the relatively recent development of manual labour-saving laboratory automation equipment with other older, more mature automation processes. Here we refer to equipment that replaces manual human manipulation rather than machinery that performs operations operators are physically incapable of executing, such as centrifuging. Taking the millennia-old example of sewing, with just a needle, thread and cloth it is possible, given time, for a skilled human operator to create a garment. Equally the same items can be completely mechanised with expensive, high-level automation equipment and the garment produced with no human input necessary beyond the need to turn the machine on. Comparing with the laboratory process of cell culture which requires, media, pipettes, labware and some starting cells a skilled operator can also, given time, passage cells and create a sub-culture for experimentation. Again, the same output can also be produced using an entirely automated, costly, high-level system, with minimal operator input. However, in the case of needlework there exists a range of lower cost interim labour-saving automation options between these two extremes, such as motor driven stitching machinery, or manually powered mechanisms, exemplified in the Singer sewing machine ( McLoughlin and Mitchell, 2013 ). This is not currently the case for cell culture, there are no examples of commercially available low-cost machinery ( Figure 4 ).
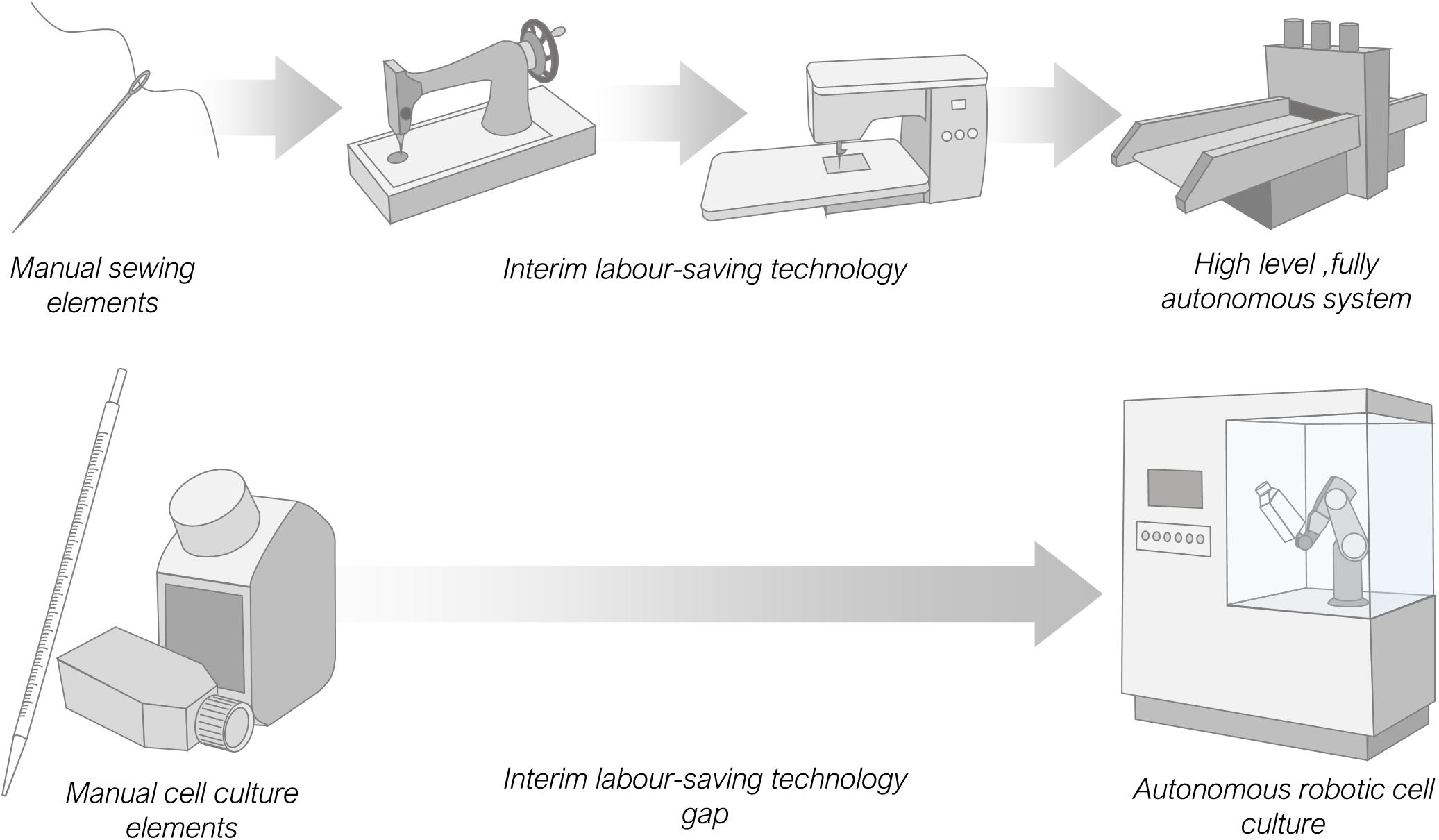
Figure 4. Comparison of available labour-saving automation options for the manual intensive processes of sewing and cell culture. Sewing has a range of interim automation options up to fully autonomous systems. Cell culture by contrast has only high-level automation equipment and no interim low-cost analogues to replace or augment manual labour.
Interim automation can arise in several scenarios. More commonly it occurs incrementally over time, as technological advances permit a shift from simple to complex machinery. Alternatively, on occasion a high-end complex automation system may be simplified due to new demands, such as an economic demand for cheaper equipment. For many laboratory automation processes there has been a rapid leap from simple to complex with, as yet, little or no development of lower cost automation technology. We believe this is due in part to the reasonable desire for academic laboratories and companies to be seen to be developing equipment at the forefront of technology. In simple terms, low-cost interim automation that removes some but not all of the manual labour from a protocol is not fashionable enough. It is unlikely to lead to a prestigious journal publication and, for commercial organisations, will not lead to financial rewards, with likely low sales volumes and low profit margins. There are therefore few incentives for academic and commercial automation developers to design such equipment.
In-House Laboratory Automation
Despite the hurdles facing researchers wishing to automate elements of their experimental procedures, there are many examples where laboratory automation development is carried out ‘in-house,’ without the assistance of a commercial partner or a large automation dedicated funding grant. Research teams are recognising that their protocols could be made more efficient by including automation but find themselves restricted financially and functionally by commercially available options ( Pilizota and Yang, 2018 ). A range of ingenious methods have been developed to build low-cost automation solutions, including the integration of Lego into microscopy automation ( Almada et al., 2019 ), microfluidics for DNA assembly ( Shih et al., 2015 ) and rapid synthesis and testing of small molecule libraries ( Baranczak et al., 2017 ). Laboratories with novel protocols that are nearly but not quite suited to existing automation equipment have been able to successfully upgrade commercially available systems for their specific needs ( McGraw et al., 2014 ; Richter et al., 2015 ; Zhang et al., 2016 ; Crombie et al., 2017 ; Konczal and Gray, 2017 ). Repurposing existing equipment in this fashion either through software or hardware modification is a cost-and time-efficient method of obtaining higher levels of protocol automation without the arduous task of designing and building an entirely novel system. The number of automation development tools, components and virtual training options available to research laboratories continues to broaden, increasing their capability to develop low-cost solutions to labour intensive processes. The advent of affordable 3D printing modalities ( Jones et al., 2011 ; Zluhan et al., 2016 ; Capel et al., 2018 ), off the shelf actuators and readily programable microcontrollers ( Mabbott, 2014 ; Kim et al., 2015 ; Wong et al., 2018 ) has given research laboratories the ability to produce componentry that can then be assembled, controlled and automated all for a relatively low cost ( Courtemanche et al., 2018 ; Needs et al., 2019 ; Barthels et al., 2020 ). Open source designs and software have an important enabling effect for researchers who may not have engineering or programming expertise. Researchers are also able to exploit the growing market for second hand laboratory automation equipment ( Zluhan et al., 2016 ), a case of one lab’s trash is another labs treasure. Developing automation internally, whilst often cheaper, and potentially a more rewarding and enjoyable process ( Pilizota and Yang, 2018 ) can however require a substantial investment in time ( May, 2019 ). That laboratories are frequently forced into developing their own systems is an indication of the paucity of commercially available options. Existing automation developers see an insufficient market for providing their services and expertise to develop bespoke items for individual laboratories and will be justifiably reluctant to provide open source solutions that may compromise their intellectual property.
Increasing the quantity and quality of laboratory automation within the research laboratory will require a concerted effort from funders, research institutions, automation developers and researchers themselves. The desire to automate elements of laboratory protocols exists. Researchers and their governmental funders ( Reeves et al., 2019 ) collectively recognise that mechanisation can improve reproducibility and efficiency. When attempting to develop laboratory automation three interrelated components are needed for success. Connecting researchers with automation needs to automation engineers, financing the resulting collaboration, and ensuring the resulting design meets the needs.
Collaboration
Encouraging academic researchers to engage and collaborate with industrial organisations has been a long-standing objective for their host institutions. Such joint enterprises are hindered by the significant differences in culture and attitudes to one another ( Berman, 2008 ) which are in part due to each partner having different timescales and expectations from projects. Academics build projects slowly through the funding stages and ultimately desire experimental data that can be packaged into publications. Industry often likes to move more quickly and would like intellectual property that can be reconstituted into a commercial opportunity ( Lynch, 2016 ). Contrary to widespread belief these viewpoints are, however, not always the most prominent motivations for collaboration, with altruistic aims also prevalent in both parties ( Berman, 2008 ).
Automation engineers and life science researchers operate in markedly different disciplines and in different work environments, rarely occupying the same space to share problems and ideas. Events where these disparate groups can be brought together would allow new ideas and projects to develop, in a similar fashion to academic conferences encouraging collaboration between different laboratories. Automation engagement events that feature all levels of employees from both sides of the divide would have the greatest effect. Interaction between industrial managers and academic supervisors as well as researchers who are researching and engineers who are engineering could allow the development of solutions to everyday automation challenges in the laboratory.
Collaboration can also be an internal academic arrangement. Life science laboratories often have a source of automation engineering expertise within their own institution in the form of engineering faculties. Both disciplines could benefit from increased interaction and discussion around laboratory automation, with examples of collaborating biomedicine and engineering departments producing innovative automated equipment ( Kato et al., 2010 ; Kane et al., 2019 ). Collaboration at an educational level can be beneficial too. Allowing undergraduate engineering students to undertake projects based upon automating a protocol within a laboratory would provide the host laboratory with designs and automation aids. Interdepartmental, interdisciplinary collaborations can bring benefits for students too, providing real world problems to develop their skills and the opportunity to apply theoretical knowledge ( Wilson and Zamberlan, 2012 ).
More varied career paths that allow employees with experience of industry-based automation to work in research environments can also develop new ideas that lead to mechanised laboratory equipment. Academic and industrial career paths diverge at early career stage and rarely reconnect. The majority of professional individuals progress from an academic institution into an industrial or commercial organisation. Researchers typically remain within a university environment accruing the required qualifications and experience as their career progresses. Reverse flow of employees, where an individual moves from industry to academia is less common ( Bonner, 2006 ). Encouraging a greater level of employees with experience of automation to work within life science laboratories will promote an exchange of ideas that can lead to experimental mechanisation. Such employee exchanges need not be permanent and can be sabbatical-style placements targeted at a specific project. The Knowledge Transfer Partnership is one successful long-running academic-industry exchange scheme in the authors host country that allows an employee to concurrently work on a project at both an academic and industrial organisation ( Howlett(ed.), 2010 ). These types of employee arrangements have a further benefit in deepening the relationships between Universities and industrial organisations. Academic institutions that can successfully foster relationships with industrial partners can reap substantial rewards not only in the form of publications and possible financial licencing agreements but greater reproducibility too ( Edwards, 2016 ). In a notable success story, automated sequencing technology, now the mainstay of genetic research, was successfully developed at Caltech, a research organisation with strong links to industry ( García-Sancho, 2007 ). Ultimately though any collaboration, regardless of the method of inception, is unlikely to succeed or even be embarked upon unless both partners are confident that they have the financial resources to proceed.
Greater implementation of automation can bestow benefits to funding organisations. Devoting financial resources towards automation engineering may seem paradoxical where the long-term objectives are targeted towards developing therapeutic interventions for biological diseases. However, the reproducibility of published research is essential for research financed by these organisations. Automation is a critical component in driving upwards the reproducibility of disseminated research ( Winder, 2019 ). In addition, as research confidence increases in a particular therapy consideration will eventually need to shift towards how the technology can be produced in sufficient quantities and at an affordable price so that it is available to the greatest range of patients. As previously discussed, including automation at earlier stage in the development process can help in attaining these goals, easing the transition from the experimentation phase to clinical usage. Competitive schemes, where funds are specifically are made available for developing laboratory automation would be beneficial in bridging the distance between the lab bench and the bedside.
Automation can provide benefits too for governments funding academic institutions. Increasing the level of automation across workplaces is acknowledged as strategy for economic progress ( Velásquez et al., 2009 ; Reeves et al., 2019 ) with research laboratories being no exception. Access to higher levels of automation increases the output of research laboratories that exist in publicly funded institutions. Any associated automation dividend will also require appropriately skilled technical staff to maintain, operate and enhance laboratory equipment. A greater range of dedicated grant schemes specifically targeted at developing laboratory automation will, in the long-term, increase the effectiveness of all research funding.
Laboratory Automation Design
Improvements can be made in automation design, how it is implemented in laboratories and the range of available automations options. A large amount of laboratory automation is based upon an anthropomorphic design framework that mimics human movement. Expensive laboratory equipment frequently features an over reliance on robotics to manipulate tooling, reagents and labware in a similar manner to how researchers would themselves. These types of designs can present as being visually high-tech and impressive and there is indeed an advantage to machinery that presents as more human-like in that it is more likely to be trusted by human operators ( de Visser et al., 2016 ). Unfortunately for many applications these designs are not always the most efficient means for automating a laboratory protocol. Robotic actuators featuring multiple axes and large operating envelopes also require even larger guarding enclosures and correspondingly complex control systems ( Yachie and Natsume, 2017 ). These design attributes render such equipment spatially and economically unsuitable for the majority of research laboratories. McClymont and Freemont provide an example where an assay requiring liquid handling can be more effectively processed and multiplexed with tooling that is not based upon an anthropomorphic design ( McClymont and Freemont, 2017 ). Hollow fibre cell culture systems are further examples of automation systems that have successfully eschewed more traditional anthropomorphic designs ( Eghbali et al., 2016 ).
Designing for flexibility is also an important factor for laboratories where there is a high level of protocol variation. Laboratory automations systems designs that anticipate future scientific developments and allow for subsequent adaptation will be less likely to become prematurely obsolete and thus more valuable to research laboratories. Machinery based upon modular based design is one approach to a flexible system. Modular automation systems can allow selective matching of automation to the protocol requirements, minimising the purchase of redundant features, and also providing the option for future upgrades should it be needed. There are indications that laboratory automation developers are becoming more aware of the need for flexibility. The ongoing development of technology such as Formulatrix’s rover system is one example where microwell plates are autonomously transferred between processing modules in a novel reworking of the robotic warehouse concept ( Wikholm and Lindblom, 2019 ).
The capability for an automation system to be modified without specialist engineering knowledge is desirable too. Allowing researchers to automate a wider range of process steps without the need for time consuming and expensive tooling redesign or extensive software reprogramming. An interesting extension of the modular design approach is to unify existing automation equipment so that it capable of performing the desired protocol in one continuous process stream. The recent development of software by the company Synthace that is capable of communicating and linking robotics from different manufacturers is one promising system for laboratories requiring highly flexible systems ( Sadowski et al., 2016 ; Jessop-Fabre and Sonnenschein, 2019 ).
To reduce the manual labour burden on laboratory research staff and students there is a need for a broader range of automation equipment. These designs should target the identified gap in labour saving automation with a focus on reducing price and footprint. In this regard employing multi axis robotics may not be the most optimal design solution and developers should be prepared to explore more cost-effective, low-tech routes to protocol automation, even if seems like a less fashionable option.
The Future of Laboratory Automation
It is with a certain degree of trepidation that we follow in the footsteps of others and attempt to predict the future of laboratory automation. The life science research laboratory of the future will undoubtably feature more automation equipment. How quickly automation is adopted will in all probability be slower than many would like and haphazard, with some fields being more suitable than others. Many of the obstacles to laboratory automation ingress we have described are long-standing and hardwired into the working practices of academic research. In particular financial hurdles faced by individual principal investigators are unlikely to be resolved and overcome in the immediate future. Bespoke, high-level automation solutions will remain beyond the reach of all but the most monied laboratories for a considerable time. Greater progress can be anticipated in the design and price of lower-level automation equipment. It is reasonable to assume that like other technologies laboratory automation will continue to mature with falling prices and more user centred designs. Hopefully incorporating more flexibility in response to consumer demand. In part this progression is already underway, with promising releases of low-cost liquid handling platforms and ongoing development of modular systems. The demand from research laboratories for automation that seeks to limit its impact on the environment will grow considerably and it is hoped that developers will create and adapt their designs to meet this need. Life science researchers will also continue to develop their own homemade laboratory automation and repurpose existing equipment, encouraging other laboratories to also take the leap into engineering. We predict that the second hand market will become an important resource for those choosing this route to automation.
Access to pooled resource, high-level, automation in the form of academic biofoundries is increasing and will continue to do so with expansion of existing facilities and the foundation of new ones. The outsourcing of protocols to commercial cloud laboratories has been predicted to become commonplace for a huge range of life science laboratories. From the perspective of the lab bench we are more circumspect in regards to the impact these organisations will have on day to day experimental research, with experimental range and flexibility key issues. Ultimately, the marketplace laws of supply and demand will dictate the success rate of these enterprises.
An appreciation of the limitations of automation both generally and for items of specific equipment is needed from academic, commercial and funding organisations and individuals. Of all the limitations discussed in this review we wish to particularly highlight the danger of innovation inhibition. Innovation in the laboratory is essential and the freedom to tinker and create new protocols needs to be retained if research is to retain a high degree of novelty. Ensuring that automation remains compatible with the curiously minded researcher will be a significant challenge for our field in the future.
In response to automation ingress the skills of life science researchers will need to adapt. The presence of more automation equipment will require more engineering type-skills to ensure correct equipment operation and implementation of protocols, along with a working knowledge of the biology under experimentation. Researchers will therefore need both biology ‘wet’ skills and ‘dry’ automation skills; such people have been imaginatively titled amphibious researchers by Mellingwood (2018) . It is therefore likely that automation will spawn a new generation of researchers with a range of interdisciplinary skills.
In summary, automation in life science laboratories lags behind its industrial and clinical counterparts due to an array of inhibiting factors, including financial, spatial and cultural challenges. Those who are able to surmount these barriers and integrate automation into their everyday protocols can reap significant reproducibility and efficiency benefits. It is essential that future laboratory automation systems are designed for flexibility to permit adaptation for changing laboratory needs and prevent the stifling of protocol innovation. A wider range of affordable bench top and remote automation options will steadily increase the ubiquity of mechanisation in life science research. Such progressive adoption of automation will emphasise the already growing interdisciplinary nature of research further blurring the boundary between science and engineering.
Author Contributions
IH conceived the study and wrote the manuscript with support from JD who critically reviewed it and also contributed content. Both authors contributed to the article and approved the submitted version.
The authors would like to acknowledge support from the Biotechnology and Biological Sciences Research Council (BBSRC: grant code BB/M018040/1).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
JD is a member of the Free Software Foundation, which campaigns for free (open-source) software in place of proprietary.
Acknowledgments
We would like to thank Sara Gómez Arnaiz and Fokion Glykofrydis for their feedback and discussion of the manuscript.
Abramo, G., and D’Angelo, C. (2014). How do you define and measure research productivity? Scientometrics 101, 1129–1144. doi: 10.1007/s11192-014-1269-8
CrossRef Full Text | Google Scholar
Adams, J. U. (2004). How to negotiate for academic lab space. Scientist 18, 42–43.
Google Scholar
Almada, P., Pereira, P. M., Culley, S., Caillol, G., Boroni-Rueda, F., Dix, C. L., et al. (2019). Automating multimodal microscopy with NanoJ-Fluidics. Nat. Commun. 10:1223. doi: 10.1038/s41467-019-09231-9
PubMed Abstract | CrossRef Full Text | Google Scholar
Archetti, C., Montanelli, A., Finazzi, D., Caimi, L., and Garrafa, E. (2017). Clinical laboratory automation: a case study. J. Public Health Res. 6:881. doi: 10.4081/jphr.2017.881
Archibald, P. R. T., Chandra, A., Thomas, D., Chose, O., Massouridès, E., Laâbi, Y., et al. (2016). Comparability of automated human induced pluripotent stem cell culture: a pilot study. Bioprocess Biosyst. Eng. 39, 1847–1858. doi: 10.1007/s00449-016-1659-9
Autor, D. H. (2015). Why are there still so many jobs? The history and future of workplace automation. J. Econ. Perspect. 29, 3–30. doi: 10.1257/jep.29.3.3
Baker, M. (2016). 1,500 scientists lift the lid on reproducibility. Nature 533, 452–454. doi: 10.1038/533452a
Baranczak, A., Tu, N. P., Marjanovic, J., Searle, P. A., Vasudevan, A., and Djuric, S. W. (2017). Integrated Platform for Expedited Synthesis-Purification-Testing of Small Molecule Libraries. ACS Med. Chem. Lett. 8, 461–465. doi: 10.1021/acsmedchemlett.7b00054
Barthels, F., Barthels, U., Schwickert, M., and Schirmeister, T. (2020). FINDUS: an open-source 3d printable liquid-handling workstation for laboratory automation in life sciences. SLAS Technol. 25, 190–199. doi: 10.1177/2472630319877374
Begley, C. G., and Ioannidis, J. P. A. (2015). Reproducibility in science: improving the standard for basic and preclinical research. Circ. Res. 116, 116–126. doi: 10.1161/CIRCRESAHA.114.303819
Benchoufi, M., and Ravaud, P. (2017). Blockchain technology for improving clinical research quality. Trials 18:335. doi: 10.1186/s13063-017-2035-z
Berman, J. (2008). Connecting with industry: bridging the divide. J. High. Educ. Policy Manage. 30, 165–174. doi: 10.1080/13600800801938762
Besteman, S. B., and Bont, L. J. (2019). Fail-fast in respiratory syncytial virus vaccine development. Am. J. Respir. Crit. Care Med. 200, 410–412. doi: 10.1164/rccm.201901-0233ED
Beugelsdijk, T. J. (1991). The future of laboratory automation. Genet. Anal. Biomol. Eng. 8, 217–220. doi: 10.1016/1050-3862(91)90016-K
Bistulfi, G. (2013). Reduce, reuse and recycle lab waste. Nature 502, 170–170. doi: 10.1038/502170a
Björklund, M., Crenshaw, A. G., Djupsjöbacka, M., and Johansson, H. (2000). Position sense acuity is diminished following repetitive low-intensity work to fatigue in a simulated occupational setting. Eur. J. Appl. Physiol. 81, 361–367. doi: 10.1007/s004210050055
Bonner, J. (2006). Back to Academia: A Mid-Life Crisis? New Scientist, 2006. Available online at: https://www.newscientist.com/article/mg19125711-900-back-to-academia-a-mid-life-crisis/ (accessed May 26, 2020).
Boyd, J. (2002). Robotic laboratory automation. Science 295, 517–518. doi: 10.1126/science.295.5554.517
Burger, B., Maffettone, P., Gusev, V., Aitchison, C., Bai, Y., Xiaoyan, W., et al. (2020). A Mobile Robotic chemist. Nature 583, 237–241. doi: 10.1038/s41586-020-2442-2
Capel, A. J., Rimington, R. P., Lewis, M. P., and Christie, S. D. R. (2018). 3D printing for chemical, pharmaceutical and biological applications. Nat. Rev. Chem. 2, 422–436. doi: 10.1038/s41570-018-0058-y
Caragher, T. E., Lifshitz, M. S., and DeCresce, R. (2017). “Analysis: clinical laboratory automation,” in Henry’s Clinical Diagnosis and Management by Laboratory Methods , eds R. A. McPherson and M. Pincus (Amsterdam: Elsevier), 60–65.
Casadevall, A., and Fang, F. C. (2010). Reproducible science. Infect. Immun. 78, 4972–4975. doi: 10.1128/IAI.00908-10
Ceroni, J. A. (2009). “Economic rationalization of automation projects,” in Springer Handbook of Automation , ed. S. Y. Nof (Berlin: Springer), 699–713. doi: 10.1007/978-3-540-78831-7_40
Chambers, S., Kitney, R., and Freemont, P. (2016). The foundry: the DNA synthesis and construction foundry at imperial college. Biochem. Soc. Trans. 44, 687–688. doi: 10.1042/BST20160007
Chao, R., Mishra, S., Si, T., and Zhao, H. (2017). Engineering biological systems using automated biofoundries. Metab. Eng. 42, 98–108. doi: 10.1016/j.ymben.2017.06.003
Choi, Q., Kim, H. J., Kim, J. W., Kwon, G. C., and Koo, S. H. (2018). Manual versus automated streaking system in clinical microbiology laboratory: performance evaluation of Previ Isola for Blood culture and body fluid samples. J. Clin. Lab. Anal. 32:e22673. doi: 10.1002/jcla.22373
Clark, D. E., and Pickett, S. D. (2000). Computational methods for the prediction of ‘drug-likeness. Drug Discov. Today 5, 49–58. doi: 10.1016/S1359-6446(99)01451-8
Courtemanche, J., King, S., and Bouck, D. (2018). Engineering novel lab devices using 3D printing and microcontrollers. SLAS Technol. 23, 448–455. doi: 10.1177/2472630318766858
Crombie, D. E., Daniszewski, M., Liang, H. H., Kulkarni, T., Li, F., Lidgerwood, G. E., et al. (2017). Development of a modular automated system for maintenance and differentiation of adherent human pluripotent stem cells. SLAS Discov. 22, 1016–1025. doi: 10.1177/2472555217696797
Crone, M. A., Priestman, M., Ciechonska, M., Jensen, K., Sharp, D. J., Anand, A., et al. (2020). A role for biofoundries in rapid development and validation of automated sars-cov-2 clinical diagnostics. Nat. Commun. 11:4464. doi: 10.1038/s41467-020-18130-3
Croxatto, A., Prod’hom, G., Faverjon, F., Rochais, Y., and Greub, G. (2016). Laboratory automation in clinical bacteriology: What system to choose? Clin. Microbiol. Infect. 22, 217–235. doi: 10.1016/j.cmi.2015.09.030
Daniszewski, M., Crombie, D. E., Henderson, R., Liang, H. H., Wong, R. C. B., Hewitt, A. W., et al. (2018). Automated cell culture systems and their applications to human pluripotent stem cell studies. SLAS Technol. 23, 315–325. doi: 10.1177/2472630317712220
De Almeida, M., and Ferreira, R. (2017). Taking Biotech to the Next Level with Laboratory Automation. Labiotech, 2017. Available online at: https://www.labiotech.eu/features/biotech-laboratory-automation/ (accessed April 1, 2020).
de Visser, E. J., Monfort, S. S., McKendrick, R., Smith, M. A. B., McKnight, P. E., Krueger, F., et al. (2016). Almost human: anthropomorphism increases trust resilience in cognitive agents. J. Exp. Psychol. Appl. 22, 331–349. doi: 10.1037/xap0000092
Doulgkeroglou, M. N., Nubila, A., Niessing, B., König, N., Schmitt, R. H., Damen, J., et al. (2020). Automation, monitoring, and standardization of cell product manufacturing. Front. Bioeng. Biotechnol. 8:811. doi: 10.3389/fbioe.2020.00811
Edwards, A. (2016). Reproducibility: team up with industry. Nature 531, 299–301. doi: 10.1038/531299a
Eghbali, H., Nava, M. M., Mohebbi-Kalhori, D., and Raimondi, M. T. (2016). Hollow fiber bioreactor technology for tissue engineering applications. Int. J. Artif. Organs 39, 1–15. doi: 10.5301/ijao.5000466
Egri, P., Csáji, B. C., Kis, K. B., Monostori, L., Váncza, J., Ochs, J., et al. (2020). Bio-inspired control of automated stem cell production. Procedia CIRP 88, 600–605. doi: 10.1016/j.procir.2020.05.105
European Commission (2016). H2020 Programme Fact Sheets Grants 2 (December). Available online at: http://ec.europa.eu/research/participants/data/ref/h2020/other/gm/h2020-grant-factsheet_en.pdf (accessed May 8, 2020).
Fanelli, D. (2018). Opinion: Is science really facing a reproducibility crisis, and do we need it to? Proc. Natl. Acad. Sci. U.S.A. 115, 2628–2631. doi: 10.1073/pnas.1708272114
Freedman, L. P., Cockburn, I. M., and Simcoe, T. S. (2015). The economics of reproducibility in preclinical research. PLoS Biol. 13:e1002165. doi: 10.1371/journal.pbio.1002165
Frohm, J., Lindström, V., Winroth, M., and Stahre, J. (2006). The Industry’s view on automation in manufacturing. IFAC Proc. Vol. 39, 453–458. doi: 10.3182/20060522-3-FR-2904.00073
Frohm, J., Lindström, V., Winroth, M., and Stahre, J. (2008). Levels of Automation in Manufacturing. Int. J. Ergon. Hum. Fact. 30, 71–74.
Froschauer, R., Dhungana, D., and Gruenbacher, P. (2008). “Managing the Life-Cycle of Industrial Automation Systems with Product Line Variability Models,” in Proceedings of the 2008 34th Euromicro Conference Software Engineering and Advanced Applications , Parma, 35–42. doi: 10.1109/SEAA.2008.21
García-Sancho, M. (2007). Sequencing As a Way of Work: A History of Its Emergence and Mechanisation – From Proteins To Dna, 1945-2000. Ph.D. thesis, Imperial College London, London.
Genzen, J. R., Burnham, C. A. D., Felder, R. A., Hawker, C. D., Lippi, G., and Peck Palmer, O. M. (2018). Challenges and opportunities in implementing total laboratory automation. Clin. Chem. 64, 259–264. doi: 10.1373/clinchem.2017.274068
Goldblatt, E. M., and Lee, W. H. (2010). From bench to bedside: the growing use of translational research in cancer medicine. Am. J. Transl. Res. 2, 1–18. doi: 10.1111/j.1468-3083.2010.03829.x
Goodman, S. N., Fanelli, D., and Ioannidis, J. P. A. (2018). What does research reproducibility mean? Sci. Transl. Med. 8:341ps12. doi: 10.1126/scitranslmed.aaf5027
Greub, G., Sahli, R., Brouillet, R., and Jaton, K. (2016). Ten years of R&D and full automation in molecular diagnosis. Future Microbiol. 11, 403–425. doi: 10.2217/fmb.15.152
Groth, P., and Cox, J. (2017). Indicators for the use of robotic labs in basic biomedical research: a literature analysis. PeerJ 5:e3997. doi: 10.7717/peerj.3997
Harrison, R., Colombo, A. W., West, A. A., and Lee, S. M. (2007). Reconfigurable modular automation systems for automotive power-train manufacture. Int. J. Flex. Manuf. Syst. 18, 175–190. doi: 10.1007/s10696-006-9008-y
Hasegawa, Y. (2009). “Advances in robotics and automation: historical perspectives,” in Springer Handbook of Automation , ed. Y. Shimon (Heidelberg: Springer), 3–4. doi: 10.1007/978-3-540-78831-7_1
Hawker, C. D., Genzen, J. R., and Wittwer, C. T. (2018). “Automation in the Clinical Laboratory,” in Tietz Textbook of Clinical Chemistry and Molecular Diagnostics , 6th Edn, eds N. Rifai, A. R. Horvath, and C. T. Wittwer (Philadelphia, PA: Elsevier Inc), 370.e1–370.e24. doi: 10.1016/B978-0-323-35921-4.00026-0
Hawker, C. D., and Schlank, M. R. (2000). Development of standards for laboratory automation. Clin. Chem. 46, 746–750. doi: 10.1093/clinchem/46.5.746
Hawkins, R. C. (2007). Laboratory turnaround time. Clin. Biochem. Rev. 28, 179–194. doi: 10.1093/ajcp/105.6.676
Hayden, E. C. (2014). The automated lab. Nature 516, 131–132. doi: 10.1038/516131a
Hayward, C. N., Laursen, S. L., and Thiry, H. (2017). Why work with undergraduate researchers? Differences in research advisors’ motivations and outcomes by career stage. CBE Life Sci. Educ. 16:ar13. doi: 10.1187/cbe.16-07-0229
Heathman, T. R. J., Nienow, A. W., McCall, M. J., Coopman, K., Kara, B., and Hewitt, C. J. (2015). The translation of cell-based therapies: clinical landscape and manufacturing challenges. Regen. Med. 10, 49–64. doi: 10.2217/rme.14.73
Hitomi, K. (1994). Automation — Its concept and a short history. Technovation 14, 121–128. doi: 10.1016/0166-4972(94)90101-5
Howes, L. (2019). Can laboratories move away from single-use plastic? ACS Cent. Sci. 5, 1904–1906. doi: 10.1021/acscentsci.9b01249
Howlett, R. J. (ed.) (2010). “Knowledge Transfer between UK Universities and Business,” in Innovation through Knowledge Transfer. Smart Innovation, Systems and Technologies , Vol. 5, (Berlin: Springer), 1–14. doi: 10.1007/978-3-642-14594-0_1
Hua, S., de Matos, M. B. C., Metselaar, J. M., and Storm, G. (2018). Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front. Pharmacol. 9:790. doi: 10.3389/fphar.2018.00790
Huggett, J., Green, C., and Zumla, A. (2009). Nucleic acid detection and quantification in the developing world. Biochem. Soc. Trans. 37, 419–423. doi: 10.1042/BST0370419
Iridiastadi, H., and Nussbaum, M. A. (2006). Muscular fatigue and endurance during intermittent static efforts: effects of contraction level, duty cycle, and cycle time. Hum. Factors 48, 710–720. doi: 10.1518/001872006779166389
Jessop-Fabre, M. M., and Sonnenschein, N. (2019). Improving reproducibility in synthetic biology. Front. Bioeng. Biotechnol. 7:18. doi: 10.3389/fbioe.2019.00018
Jones, R., Haufe, P., Sells, E., Iravani, P., Olliver, V., Palmer, C., et al. (2011). Reprap - the replicating rapid prototyper. Robotica 29, 177–191. doi: 10.1017/S026357471000069X
Jones, S. E. (2013). Against Technology: From the Luddites to Neo-Luddism. New York, NY: Routledge.
Jossen, V., van den Bos, C., Eibl, R., and Eibl, D. (2018). Manufacturing human mesenchymal stem cells at clinical scale: process and regulatory challenges. Appl. Microbiol. Biotechnol. 102, 3981–3994. doi: 10.1007/s00253-018-8912-x
Jung, S., Ochs, J., Kulik, M., König, N., and Schmitt, R. H. (2018). Highly modular and generic control software for adaptive cell processing on automated production platforms. Procedia CIRP 72, 1245–1250. doi: 10.1016/j.procir.2018.03.189
Kaber, D. B., Stoll, N., Thurow, K., Green, R. S., Kim, S. H., and Mosaly, P. (2009). Human-automation interaction strategies and models for life science applications. Hum. Fact. Ergon. Manuf. 19, 601–621. doi: 10.1002/hfm.20156
Kane, K. I. W., Moreno, E. L., Hachi, S., Walter, M., Jarazo, J., Oliveira, M. A. P., et al. (2019). Automated microfluidic cell culture of stem cell derived dopaminergic neurons. Sci. Rep. 9:1796. doi: 10.1038/s41598-018-34828-3
Kato, R., Iejima, D., Agata, H., Asahina, I., Okada, K., Ueda, M., et al. (2010). A compact, automated cell culture system for clinical scale cell expansion from primary tissues. Tissue Eng. Part C Methods 16, 947–956. doi: 10.1089/ten.tec.2009.0305
Kempner, M. E., and Felder, R. A. (2002). A review of cell culture automation. JALA 7, 56–62. doi: 10.1016/S1535-5535(04)00183-2
Khanna, R., Guler, I., and Nerkar, A. (2016). Fail often, fail big, and fail fast? Learning from small failures and R&D performance in the pharmaceutical industry. Acad. Manage. J. 59, 436–459. doi: 10.5465/amj.2013.1109
Kim, K. W., Lee, M. S., Ryu, M. H., and Kim, J. W. (2015). Arduino-Based Automation of a DNA Extraction System. Edited by Wen-Hsiang Hsieh. Technol. Health Care 24, S105–S112. doi: 10.3233/THC-151048
King, R. D., Rowland, J., Oliver, S. G., Young, M., Aubrey, W., Byrne, E., et al. (2009). The automation of science. Science 324, 85–89. doi: 10.1126/science.1165620
Kitney, R., Adeogun, M., Fujishima, Y., Goñi-Moreno, Á, Johnson, R., Maxon, M., et al. (2019). Enabling the advanced bioeconomy through public policy supporting biofoundries and engineering biology. Trends Biotechnol. 37, 917–920. doi: 10.1016/j.tibtech.2019.03.017
Klaus, B., and del Alamo, D. (2018). Talent identification at the limits of peer review: an analysis of the EMBO postdoctoral fellowships selection process. bioRxiv [Preprint]. doi: 10.1101/481655
Klevebring, D., Gry, M., Lindberg, J., Eidefors, A., and Lundeberg, J. (2009). Automation of CDNA synthesis and labelling improves reproducibility. J. Biomed. Biotechnol. 2009:396808. doi: 10.1155/2009/396808
Konczal, J., and Gray, C. H. (2017). Streamlining workflow and automation to accelerate laboratory scale protein production. Protein Expr. Purif. 133, 160–169. doi: 10.1016/j.pep.2017.03.016
Kotin, R. M. (2011). Large-scale recombinant adeno-associated virus production. Hum. Mol. Genet. 20, R2–R6. doi: 10.1093/hmg/ddr141
Krause, M., Gautam, K., Małgorzata, A., and Niraula, A. G. (2020). Reducing Plastic Waste in the Lab. Chemistry World, 2020. Available online at: https://www.chemistryworld.com/opinion/reducing-plastic-waste-in-the-lab/4011550 (accessed June 2, 2020).
Kreiman, G., and Maunsell, J. H. R. (2011). Nine criteria for a measure of scientific output. Front. Comput. Neurosci. 5:48. doi: 10.3389/fncom.2011.00048
Lou, A. H., Elnenaei, M. O., Sadek, I., Thompson, S., Crocker, B. D., and Nassar, B. (2016). Evaluation of the impact of a total automation system in a large core laboratory on turnaround time. Clin. Biochem. 49, 1254–1258. doi: 10.1016/j.clinbiochem.2016.08.018
Lynch, K. L. (2016). Collaboration at the heart of innovation. Clin. Chem. 62:1284. doi: 10.1373/clinchem.2016.260687
Mabbott, G. A. (2014). Teaching electronics and laboratory automation using microcontroller boards. J. Chem. Educ. 91, 1458–1463. doi: 10.1021/ed4006216
Maleki, F., Ovens, K., McQuillan, I., and Kusalik, A. J. (2019). Size matters: how sample size affects the reproducibility and specificity of gene set analysis. Hum. Genomics 13(Suppl. 1):42. doi: 10.1186/s40246-019-0226-2
Marx, U., Schenk, F., Behrens, J., Meyr, U., Wanek, P., Zang, W., et al. (2013). Automatic production of induced pluripotent stem cells. Procedia CIRP 5, 2–6. doi: 10.1016/j.procir.2013.01.001
May, M. (2019). A DIY approach to automating your lab. Nature 569, 587–588. doi: 10.1038/d41586-019-01590-z
McClymont, D. W., and Freemont, P. S. (2017). With all due respect to Maholo, lab automation isn’t anthropomorphic. Nat. Biotechnol. 35, 312–314. doi: 10.1038/nbt.3795
McGraw, J., Tatipelli, V. K., Feyijinmi, O., Traore, M. C., Eangoor, P., Lane, S., et al. (2014). A semi-automated method for purification of milligram quantities of proteins on the QIAcube. Protein Expr. Purif. 96, 48–53. doi: 10.1016/j.pep.2014.01.014
McLoughlin, J., and Mitchell, A. (2013). “Mechanisms of sewing machines,” in Joining Textiles , eds I. Jones, and G. K. Stylios (Amsterdam: Elsevier). doi: 10.1533/9780857093967.1.123
Meldrum, D. (2000). Automation for genomics, part one: preparation for sequencing. Genome Res. 10, 1081–1092. doi: 10.1101/gr.101400
Mellingwood, C. (2018). Amphibious Researchers: Working with Laboratory Automation in Synthetic Biology. Ph.D. thesis, University of Edinburgh, Edinburgh.
Mifflin, T. E., Estey, C. A., and Felder, R. A. (2000). Robotic automation performs a nested RT-PCR analysis for HCV without introducing sample contamination. Clin. Chim. Acta 290, 199–211. doi: 10.1016/S0009-8981(99)00192-8
Miles, B., and Lee, P. L. (2018). Achieving reproducibility and closed-loop automation in biological experimentation with an IoT-enabled lab of the future. SLAS Technol. 23, 432–439. doi: 10.1177/2472630318784506
Moutsatsou, P., Ochs, J., Schmitt, R. H., Hewitt, C. J., and Hanga, M. P. (2019). Automation in cell and gene therapy manufacturing: from past to future. Biotechnol. Lett. 41, 1245–1253. doi: 10.1007/s10529-019-02732-z
Movsisyan, M., Delbeke, E. I. P., Berton, J. K. E. T., Battilocchio, C., Ley, S. V., and Stevens, C. V. (2016). Taming hazardous chemistry by continuous flow technology. Chem. Soc. Rev. 45, 4892–4928. doi: 10.1039/c5cs00902b
Munafò, M. R., Nosek, B. A., Bishop, D. V. M., Button, K. S., Chambers, C. D., Du Sert, N. P., et al. (2017). A manifesto for reproducible science. Nat. Hum. Behav. 1:0021. doi: 10.1038/s41562-016-0021
Needs, S. H., Diep, T. T., Bull, S. P., Lindley-Decaire, A., Ray, P., and Edwards, A. D. (2019). Exploiting open source 3D printer architecture for laboratory robotics to automate high-throughput time-lapse imaging for analytical microbiology. PLoS One 14:e0224878. doi: 10.1371/journal.pone.0224878
Noel, A. C., and Hu, D. L. (2018). The Tongue as a Gripper. J. Exp. Biol. 221:jeb176289. doi: 10.1242/jeb.176289
Ochs, J., Barry, F., Schmitt, R., and Murphy, J. M. (2017). Advances in automation for the production of clinical-grade mesenchymal stromal cells: the AUTOSTEM Robotic Platform. Cell Gene Ther. Insights 3, 739–748. doi: 10.18609/cgti.2017.073
Panchalingam, K. M., Jung, S., Rosenberg, L., and Behie, L. A. (2015). Bioprocessing strategies for the large-scale production of human mesenchymal stem cells: a review. Stem Cell Res. Ther. 6:225. doi: 10.1186/s13287-015-0228-5
Peng, R. (2015). The reproducibility crisis in science: a statistical counterattack. Significance 12, 30–32. doi: 10.1111/j.1740-9713.2015.00827.x
Pilizota, T., and Yang, Y. T. (2018). ‘Do it yourself’ microbial cultivation techniques for synthetic and systems biology: cheap, fun, and flexible. Front. Microbiol. 9:1666. doi: 10.3389/fmicb.2018.01666
Plebani, M. (2010). The detection and prevention of errors in laboratory medicine. Ann. Clin. Biochem. 47, 101–110. doi: 10.1258/acb.2009.009222
Price, A. P., Godin, L. M., Domek, A., Cotter, T., D’Cunha, J., Taylor, D. A., et al. (2015). Automated decellularization of intact, human-sized lungs for tissue engineering. Tissue Eng. Part C Methods 21, 94–103. doi: 10.1089/ten.tec.2013.0756
Rafiq, Q. A., and Thomas, R. J. (2016). The evolving role of automation in process development & manufacture of cell & gene-based therapies. Cell Gene Ther. Insights 2, 473–479. doi: 10.18609/cgti.2016.058
Rafiq, Q. A., Twomey, K., Kulik, M., Leschke, C., O’Dea, J., Callens, S., et al. (2016). Developing an automated robotic factory for novel stem cell therapy production. Regen. Med. 11, 351–354. doi: 10.2217/rme-2016-0040
Rampell, C. (2011). Companies Spend on Equipment, Not Workers. New York Times. Available online at: https://www.nytimes.com/2011/06/10/business/10capital.html (accessed June, 2011).
Ravazzi, P., and Villa, A. (2009). “Economic aspects of automation,” in Springer Handbook of Automation , ed. S. Y. Nof (Berlin: Springer), 93–116. doi: 10.1007/978-3-540-78831-7_6
Reed, C. E., Fournier, J., Vamvoukas, N., and Koza, S. M. (2018). Automated preparation of MS-sensitive fluorescently labeled N-Glycans with a commercial pipetting robot. SLAS Technol. 23, 550–559. doi: 10.1177/2472630318762384
Reeves, R., Coaker, V., Hendry, D., Kerr, S., Kyle, P., Liddell-Grainger, I., et al. (2019). Automation and the Future of Work House of Commons Business, Energy and Industrial Strategy Committee.
Richter, F., Scheib, U. S., Mehlhorn, J., Schubert, R., Wietek, J., and Gernetzki, O. (2015). Upgrading a microplate reader for photobiology and all-optical experiments. Photochem. Photobiol. Sci. 14, 270–279. doi: 10.1039/C4PP00361F
Roberts, M. (2017). Rural luddism and the makeshift economy of the nottinghamshire framework knitters. Soc. Hist. 42, 365–398. doi: 10.1080/03071022.2017.1327644
Russell, A. L., Lefavor, R. C., and Zubair, A. C. (2018). Characterization and cost–benefit analysis of automated bioreactor-expanded mesenchymal stem cells for clinical applications. Transfusion 58, 2374–2382. doi: 10.1111/trf.14805
Sadowski, M. I., Grant, C., and Fell, T. S. (2016). Harnessing QbD, programming languages, and automation for reproducible biology. Trends Biotechnol. 34, 214–227. doi: 10.1016/j.tibtech.2015.11.006
Safavi, A., and Anderson, T. (2019). Pipette tip Washing Device. U.S. Patent No. US20190216290A1. Washington, DC: U.S. Patent and Trademark Office.
Saltelli, A., and Funtowicz, S. (2017). What is science’s crisis really about? Futures 91, 5–11. doi: 10.1016/j.futures.2017.05.010
Salter, S. J., Cox, M. J., Turek, E. M., Calus, S. T., Cookson, W. O., Moffatt, M. F., et al. (2014). Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 12:87. doi: 10.1186/s12915-014-0087-z
Sanger, F., Nicklen, S., and Coulson, A. R. (1977). DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. U.S.A. 74, 5463–5467. doi: 10.1073/pnas.74.12.5463
Sarkozi, L., Simson, E., and Ramanathan, L. (2003). The effects of total laboratory automation on the management of a clinical chemistry laboratory. Retrospective analysis of 36 years. Clin. Chim. Acta 329, 89–94. doi: 10.1016/S0009-8981(03)00020-2
Schneider, G. (2018). Automating Drug Discovery. Nat. Rev. Drug Discov. 17, 97–113. doi: 10.1038/nrd.2017.232
Seeling, J. M., and Choudhary, M. (2016). Professional practices in undergraduate research programs. J. Microbiol. Biol. Educ. 17, 246–251. doi: 10.1128/jmbe.v17i2.982
Segal, M. (2019). An operating system for the biology lab. Nature 573, S112–S113. doi: 10.1038/d41586-019-02875-z
Shih, S. C. C., Goyal, G., Kim, P. W., Koutsoubelis, N., Keasling, J. D., Adams, P. D., et al. (2015). A versatile microfluidic device for automating synthetic biology. ACS Synth. Biol. 4, 1151–1164. doi: 10.1021/acssynbio.5b00062
Society for Laboratory Automation and Screening (2011). ANSI SLAS 1-2004 (R2012) Footprint Dimensions. Chicago, IL: Society for Laboratory Automation and Screening.
Storrs, C. (2013). Set It and Forget It - A Tour of Three Systems for Automating Cell Culture. The Scientist, 2013. Available online at: https://www.the-scientist.com/lab-tools/set-it-and-forget-it-39696 (accessed March 27, 2020).
Tacker, D. H., Topardo, J., Mahaffey, C., and Perrotta, P. L. (2014). Workflow analysis comparing manual and automated specimen processing for mass spectrometry–based vitamin D testing. Lab. Med. 45, 361–367. doi: 10.1309/lmzl47en6kdodmxj
Thomson, R. B., and McElvania, E. (2019). Total laboratory automation: What is gained, what is lost, and who can afford it? Clin. Lab. Med. 39, 371–389. doi: 10.1016/j.cll.2019.05.002
Urbina, M. A., Watts, A. J. R., and Reardon, E. E. (2015). Labs should cut plastic waste too. Nature 528, 479–479. doi: 10.1038/528479c
Vaesen, K., and Katzav, J. (2017). How much would each researcher receive if competitive government research funding were distributed equally among researchers? PLoS One 12:e0183967. doi: 10.1371/journal.pone.0183967
van der Weijden, I., Teelken, C., de Boer, M., and Drost, M. (2016). Career satisfaction of postdoctoral researchers in relation to their expectations for the future. High. Educ. 72, 25–40. doi: 10.1007/s10734-015-9936-0
Varao-Sousa, T. L., Smilek, D., and Kingstone, A. (2018). In the lab and in the wild: how distraction and mind wandering affect attention and memory. Cogn. Res. Princ. Implic. 3:42. doi: 10.1186/s41235-018-0137-0
Velásquez, J. D., Chen, X. W., Yoon, S. W., and Ko, H. S. (2009). “Automation Statistics,” in Springer Handbook of Automation , ed. S. Y. Nof (Heidelberg: Springer), 1673–1701. doi: 10.1007/978-3-540-78831-7_94
Wajcman, J. (2017). Automation: Is it really different this time? Br. J. Sociol. 68, 119–127. doi: 10.1111/1468-4446.12239
White, S., Lacey, A., and Ardanaz-Badia, A. (2019). The Probability of Automation in England - Office for National Statistics. Office for National Statistics. Available online at: https://www.ons.gov.uk/employmentandlabourmarket/peopleinwork/employmentandemployeetypes/articles/theprobabilityofautomationinengland/2011and2017 (accessed April 24, 2020).
Wikholm, D., and Lindblom, R. (2019). Rover-based integrated laboratory system including autonomous mobile robots. U.S. Patent No. WO2019/139930. Washington, DC: U.S. Patent and Trademark Office.
Wilke, W. W., Jones, R. N., and Sutton, L. D. (1995). Automation of polymerase chain reaction tests. reduction of human errors leading to contamination. Diagn. Microbiol. Infect. Dis. 21, 181–185. doi: 10.1016/0732-8893(95)00041-8
Williams, K., Bilsland, E., Sparkes, A., Aubrey, W., Young, M., Soldatova, L. N., et al. (2015). Cheaper faster drug development validated by the repositioning of drugs against neglected tropical diseases. J. R. Soc. Interface 12:20141289. doi: 10.1098/rsif.2014.1289
Wilson, S., and Zamberlan, L. (2012). Show me yours: developing a faculty-wide interdisciplinary initiative in built environment higher education. Contemp. Issues Educ. Res. 5, 331–342. doi: 10.19030/cier.v5i4.7430
Winder, A. (2019). How Lab Automation Is Helping Drug Research. European Pharmaceutical Manufacturer, 2019. Available online at: https://www.epmmagazine.com/opinion/how-lab-automation-is-helping-drug-research/ (accessed May 19, 2020).
Wong, B. G., Mancuso, C. P., Kiriakov, S., Bashor, C. J., and Khalil, A. S. (2018). Precise, automated control of conditions for high-throughput growth of yeast and bacteria with EVOLVER. Nat. Biotechnol. 36, 614–623. doi: 10.1038/nbt.4151
Wrigley, J. D., McCall, E. J., Bannaghan, C. L., Liggins, L., Kendrick, C., Griffen, A., et al. (2014). Cell banking for pharmaceutical research. Drug Discov. Today 19, 1518–1529. doi: 10.1016/j.drudis.2014.05.006
Xie, I. H., Wang, M. H., Carpenter, R., and Wu, H. Y. (2004). Automated calibration of TECAN genesis liquid handling workstation utilizing an online balance and density meter. Assay Drug Dev. Technol. 2, 71–80. doi: 10.1089/154065804322966333
Xu, R., Zhang, C., He, F., Zhao, X., Qi, H., Zhou, P., et al. (2018). How physical activities affect mental fatigue based on EEG energy, connectivity, and complexity. Front. Neurol. 9:915. doi: 10.3389/fneur.2018.00915
Yachie, N., and Natsume, T. (2017). Robotic crowd biology with Maholo labdroids. Nat. Biotechnol. 35, 310–312. doi: 10.1038/nbt.3758
Zhang, C., Long, A. M., Swalm, B., Charest, K., Wang, Y., Hu, J., et al. (2016). Development of an automated mid-scale parallel protein purification system for antibody purification and affinity chromatography. Protein Expr. Purif. 128, 29–35. doi: 10.1016/j.pep.2016.08.005
Zielinski, D., Gordon, A., Zaks, B. L., and Erlich, Y. (2014). IPipet: sample handling using a tablet. Nat. Methods 11, 784–785. doi: 10.1038/nmeth.3028
Zluhan, E., Kelly, K., LeClair, N., Wortel, D., and Moody, K. (2016). Automating HESC differentiation with 3D printing and legacy liquid handling solutions. MethodsX 3, 569–576. doi: 10.1016/j.mex.2016.10.005
Keywords : laboratory automation, life science research, automation design, research efficiency, reproducibility, innovation inhibition, environmental design
Citation: Holland I and Davies JA (2020) Automation in the Life Science Research Laboratory. Front. Bioeng. Biotechnol. 8:571777. doi: 10.3389/fbioe.2020.571777
Received: 11 June 2020; Accepted: 26 October 2020; Published: 13 November 2020.
Reviewed by:
Copyright © 2020 Holland and Davies. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ian Holland, [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 09 July 2019
Management at the service of research: ReOmicS, a quality management system for omics sciences
- Antonella Lanati 1 na1 ,
- Marinella Marzano 2 na1 ,
- Caterina Manzari 2 ,
- Bruno Fosso 2 ,
- Graziano Pesole 2 , 3 &
- Francesca De Leo ORCID: orcid.org/0000-0003-0421-7699 2
Palgrave Communications volume 5 , Article number: 75 ( 2019 ) Cite this article
7613 Accesses
2 Citations
3 Altmetric
Metrics details
- Business and management
- Science, technology and society
Management and research represent a binomial almost unknown, whose potentialities and requirements have not yet been fully exploited even if, recently, the scientific and social communities have felt the burden of producing results and data requiring at the same time reproducibility, reliability, safety and efficacy of the discoveries, as well as a profitable use of resources. A Quality Management System (QMS) could represent a valid tool for these purposes, improving the quality of the research. The research community could ask whether and how it is possible to apply this approach in a research laboratory without hindering their creativity, and what the possible benefits might be. On the other hand, an international standard for a quality management system appropriate for a research laboratory is yet to come. The choice, the design and the application of a QMS, inspired by the Good Laboratory Practices, in a research laboratory specialized on “omics” sciences, is fully described in this paper. Its application has already shown good outcomes as testified by specific metric of efficiency and effectiveness. The approach is innovative as there is no obvious requirement for research laboratories to develop and define quality objectives. The paper highlights how the QMS approach enhances the relationship with public and private sectors by increasing customer confidence and loyalty, as well as improving the overall performance of the laboratory in terms of throughput and value of research. These results encourage proposing it as a QMS model providing a new and scalable operational strategy to be applied in a research environment with the same target and even in a generic research laboratory.
Similar content being viewed by others

Considerations for implementing electronic laboratory notebooks in an academic research environment
Reproducible, scalable, and shareable analysis pipelines with bioinformatics workflow managers
SciSciNet: A large-scale open data lake for the science of science research
Introduction.
Next Generation Sequencing (NGS) technologies have dramatically changed the field of genomics and are routinely applied to a variety of functional genomics investigations including, but not restricted to, whole genome sequencing, global identification of genomic rearrangements, epigenetic modifications, single nucleotide polymorphism (SNP) discovery, transcriptome profiling and metagenomics. In recent years, using these technologies thousands of genomes assembled from short DNA sequence readings of humans, plants, animals and microbes have been collected and explored, enabling scientists to develop a deeper understanding and gaining new insights into the molecular mechanisms related to different diseases, including many types of cancer, allergies, or other disorders (Wiese et al., 2018 ). Furthermore, the genomics has profoundly influenced the pharmaceutical industry and reshaped the processes allowing to discover, investigate, and develop new drugs. No less important is the research carried out using these technologies in the environmental field for industrial and biotechnological purposes (Tiwari et al., 2018 ). Indeed, NGS is a complex process that, on the one hand, requires the preparation of sequencing libraries that respond to the specific standard requirements of the platforms, and on the other hand generates unprecedented volumes of data to be analyzed (Stephens et al., 2015 ). Moreover, an NGS analysis usually involves collaboration between several departments, laboratories and data analysis groups, characterized by different scientific backgrounds and, above all, applying different experimental approaches. With the growing need of managing information, it has become challenging to keep track of data, processes and outcomes of research over long periods of time and across the collaborating units. Nowadays Big Data generation and its management create extraordinary challenges for storage, transfer, analysis, interpretation and last but not least security of information. Regarding this last aspect, the scientific community has recently expressed the necessity to manage NGS data according to the principles of Findability, Accessibility, Interoperability, and Re-usability of digital assets (FAIR data) (Wilkinson et al., 2016 ; Corpas et al., 2018 ). The expectation is to produce digital resources with more rigorous management and stewardship that can be used by the entire scientific community. Good data management is not a goal in itself, but rather it is the key conduit leading to discovery and innovation, through data integration and reuse by the scientific community after the publication process. Good data management, ensuring reliability and usability, needs a holistic approach tracking the process of data and metadata generation and all the different organizational aspect that, on the one hand may affect it, and on the other hand can keep it under control. This can be achieved by means of a management system focused on the quality of the results.
Furthermore, in recent years, in the context of the scientific research, we are witnessing a new phenomenon, defined as “reproducibility crisis” by Baker (Baker, 2016 ) and Dirnagl et al. (Dirnagl et al., 2018 ) characterized by the reduction of the reliability, reproducibility, traceability and predictability of research results. These problems can not only compromise the robustness and rigor of research (Dirnagl et al., 2018 ) but have also a significant impact from an economic point of view, reducing the profitability of research funds (Lanati, 2018 ). The standardization and simplification of experimental workflows, such as those applied for “omics” applications, is becoming a need both for academic and private research laboratories. As described by Endrullat et al. ( 2016 ), standards act as basic guidelines to ensure comparability and exchange of experimental data conducive to the acceleration of the innovation process, aiding improvement of transferability, transparency and reproducibility of results. Furthermore, the advantages deriving from the standardization of processes could reduce costs and increase services (Endrullat et al., 2016 ; Cargill, 2011 ). A Quality Management System (QMS) can support the correct management of the NGS research environment, providing directions for data and operations management. A suitable quality system ensures safety, reliability and reproducibility of the non-clinical tests on chemicals intended for use on humans, animals and the environment. A QMS supports the generation of high quality scientific data and associated services, it is also helpful in improving the economic and social impacts of research. Quality research management reinforces scientific communities and improves the attractiveness and effectiveness of the service. In a QMS activities are properly planned and documented, operations are regulated by means of standard operating procedures, and the correct behavior, compliant with internal and external standards, is guaranteed by regular inspections. A QMS can help in giving proper attention to sensitive data and in correctly managing them, setting internal standard, organizing rules and forms and maintaining due control. Good Research Practices, as a quality management standard dedicated to the research environment, are at present not yet organized in an international reference text and consist of different prescriptive documents that are drafted and/or personalized by each research institution interested in aspects of quality management. Researchers can only refer to the WHO Handbook of Quality in Biomedical Research (WHO, 2006 ) as a guiding text to comply with generic quality principles. However, several references for designing a QMS can be found among international standards suitable for the management of a research laboratory: ISO 9001:2015, the most general quality management standard; ISO 17025, derived from the ISO9001 and dedicated to test and calibration laboratories; and the Good Laboratory Practice, mandatory international reference for development and testing of drugs and other substances intended for human and animal use (Lanati, 2018 ).
In this paper, we describe the choice, the design and the application of a QMS, called ReOmicS (Research Environment management system for Omics Sciences), at the Molecular Biodiversity Laboratory (MoBiLab), a NGS research infrastructure located in Bari (Italy) at the Institute of Biomembranes, Bioenergetics and Molecular Biotechnologies of CNR (CNR-IBIOM). MoBiLab is a research environment, fully equipped with operative platforms based on the most innovative NGS technologies and powerful resources for data storage and computational analysis, whose mission is to contribute to innovation with original studies. Moreover, CNR-IBIOM is involved in the construction of the national nodes of ELIXIR and LifeWatch Research Infrastructures (included in the ESFRI Roadmap), as well as in a substantial empowering of its infrastructural components for omics data production and analysis consistently scaling up the available instruments and facilities. The described experience could represent a scalable model to be applied to MoBiLab and to other research laboratories in order to ensure the highest levels of reliability, reproducibility and traceability of the results, a process that is also expected to foster their potential exploitation.
Material and methods
Considering the managerial aspects of the study, methods described in this section are tools used in quality and organizational management, occasionally modified to be adopted for the specific use of a research laboratory, such as MoBiLab.
Decision grid
The decision grid (or matrix) is a tool that supports a decision among many options. Once the aim of the decision is clearly defined, the criteria used to characterize each solution must be identified. Each criterion is given a weight (1 = lowest to 5 = highest) based on its importance in the final decision. The selected options among which the choice has to be made is then assessed with respect to their suitability to each criterion (1 = lowest to 5 = highest). The sum of the weighted assessments gives the final score for each proposal. Referring to Table 1 , the head of the table defines the aim of the decision. The options are listed in the columns. Criteria are listed in the rows and weighted in importance in column W. Each option is given a specific assessment (column A) with respect to the relative criterion, and an overall score, which is the product of the importance of the criterion and the given assessment. The final score for the proposal is given by the sum of all scores for each criterion.
SWOT analysis
The SWOT analysis represents, within a rationale, the influence exerted by some key factors on a goal in order to identify actions that reinforce the positive factors and counteract the influence of negative factors. The key factors in the analysis, whose initials give the name to the technique, are:
Strength: a resource that can be used to best achieve the goal;
Weakness: an obstacle to achieving the goal;
Opportunity: a favorable situation in the external context that favors the achievement of the objective;
Threat: an external, unfavorable situation in the external context that potentially hinders the achievement of the objective.
The analysis combines internal factors (strengths and weaknesses) and external factors (threats and opportunities), as well as positive aspects (strengths, opportunities) and negative aspects (weaknesses, threats). In this way SWOT Analysis allows defining strategies aimed at capitalizing on strengths, eliminating weaknesses, exploiting opportunities and mitigating threats.
Risk assessment
A risk assessment was performed on the main analytical process, according to the requirements of an ISO High Level Structure (HLS) and the ISO 9001:2015 standard. For each experimental step (Fig. 1 , first column), some pitfalls were identified (second column); each pitfall was assessed with respect to its Severity S (from 1 = low to 3 = high) i.e., how serious would be the consequence of an error on the final result, and Probability P (from 1 = low to 3 = high), i.e., how frequently a specific mistake has recently occurred (column 3 and 4, respectively). The Risk R in column 5 was then calculated as S × P for each pitfall identified. Risk values range from 9 (greatest) to 1 (lowest). Operations with risk R greater or equal to 4 are judged worthy of specific interventions to prevent errors, as recorded in column 6 “Solution”. Interventions are prioritized according to the level of risk. Referring to the legend of Fig. 1 , colors indicate the need for improvement actions: red for urgent, orange for medium and yellow for minor need, while green indicates no need for action.

Risk assessment of the primary process at MoBiLab before the introduction of the ReOmicS. Pitfalls are placed in descending order according to R. Colors indicate the need for improvement actions: red for urgent, orange for medium and yellow for slight need, while green indicates no need for action
The SIPOC diagram, first outlined by Juran (Defeo and Juran, 2010 ) calling it TRIPOL, was then employed in the Six Sigma approach for analyzing a process. It is named SIPOC from the acronym of Supplier, Input, Process, Output, and Customer: the key elements of a process. A flowchart of the process is usually inserted in the third column “process” and for each step: input supplier, input needed by the operation, output of the operation and recipient of the output (customer) are listed.
The SIPOC-like flowchart is structured on the following categories:
source: the origin of the input
input: raw data, metadata, materials or samples needed by the study activities
process: steps of analysis and controls, logically linked
supervisor/person in charge: the supervisor and/or the person in charge to carry out each task of the previous column
output: the result/product of each task
procedure: the SOP describing the specific task
Due to the limited availability of data for the period preceding the introduction of the QMS, the metrics system has been necessarily simplified to two indicator of efficiency and three indicators of effectiveness:
Efficiency indicator: analysis throughput. The values of four parameters (total number of processed samples, the average number of samples, the number of sequencing runs and the sequencing platform output), related both to all the MoBiLab applications ( a ) and only to Metagenomics ( b ), referring to the 3-year periods (2013–15 and 2016–2018), are shown
efficiency:
outcome of the updated risk assessment, compared with the initial one performed in designing the QMS. With respect to the initial Risk Assessment, three new columns have been added: “New-Probability” with the updated probability of the pitfall(s) considered, “New risk Assessment” with the updated value for the parameter R = SxP, and “Audit” recording reasons and considerations regarding the improvement. A paired one-sided Wilcoxon test was performed, to verify whether the risk estimation prior to the QMS adoption was significantly higher than the next.
evaluation of the analysis throughput in terms of the total sequencing run number, the total number of processed samples, the average number of samples per sequencing run and the run output (Gb). For each parameter, the values related to the two three-year periods were collected. The analyses were carried out on both the data derived from all the MoBiLab applications (Genomics, Transcriptomics and Metagenomics) (Fig. 2a ) and those produced only from Metagenomics (Fig. 2b ).
effectiveness:
number of publications and the related impact factor (base: 3-year period): we considered the peer-reviewed publications of three researchers 100% involved in MoBiLab research projects (Source: JCR-ISI Web of Knowledge; https://login.webofknowledge.com ). In case of co-authorship the journal was counted once, and the number of publications within the period has been calculated considering the average number of the papers per year. The scientific areas of the journals are Biochemistry, Genetics and Molecular Biology Medicine Agricultural and Biological Sciences, Immunology and Microbiology, Multidisciplinary Environmental Science, Neuroscience, Computer science, Mathematics.
scientific attractiveness , i.e., number of active external collaborations in MoBiLab publications (base: 3-year period) (Source: PubMed-National Library of Medicine; https://www.nlm.nih.gov/bsd/pubmed.html ): author’s affiliation to the papers published in the first 3-year period 2013–15 (before the introduction of the QMS) are compared with author’s affiliation referring to the 3-year period 2016–2018, after the progressive introduction of the QMS.
satisfaction survey: two separate surveys were prepared using the online tool SurveyMonkey ( https://it.surveymonkey.com ). The first (B9M5SNL), dedicated to all customers/collaborators about perceived quality, was sent by mail to 54 MoBiLab collaborators. The second (BDDF65F) was sent, in addition to the first, only to those customers/collaborators (25/54) who worked with MoBiLab in both three-year periods before and after the introduction of the QMS. A two week deadline was given. The analysis results were provided by the tool and further analyzed and elaborated by the team (Supplementary Material (SM) 1 and 2 ).
The indicators of efficiency measure the ability of the MoBiLab to increase productivity and reduce costs, while the effectiveness indicators show the quality and importance of the analysis of results.
Results and discussion
Choosing the qms standard.
To choose the best reference standard for the characteristics of MoBiLab, we compared three international standards: ISO 9001:2015, ISO 17025, and GLPs by means of a decision grid. The criteria for this choice were identified as:
compatible with regulation environment
suitable for customer’s requirements
oriented to R&D
focused on analytical process
low management costs
easy to fit in Laboratory activities
not linked to external third parties
suitable for expansion
The results are illustrated in Table 1 . Evaluating the criteria for choice, we considered that the “customers” of the MoBiLab research services are laboratories already working under the principles of GLPs and they could benefit from a rigorous and standardized work environment for the production of their data, as well as from a common management language and references. All criteria are listed in the first column of Table 1 . The GLP obtained the best assessment weighted on the importance of each criterion, mainly for their suitability for customer’s requirements, the lower cost, the independence from third party evaluations and the opportunity for development.
GLPs are mandatory in OECD countries for preclinical tests, but should be also considered as a reference for laboratory management systems, that can be referred to as “GLP-like” quality systems, although outside GLPs main scope. As textually described by Kauffmann et al. ( 2017 ) the application of GLPs principles to “omics” studies based on NGS, in a regulatory context, would serve the following goals (i) to promote the consistent quality and validity of data used for determining the safety of chemical products—a primary objective of the GLP principles (OECD, 1998 ); (ii) to promote transparent process descriptions and thus support the traceability of study results; and (iii) to facilitate the exchange of information and enhance the regulatory impact of ‘ omics data, when successfully used for hazard and risk assessment purposes. The use of GLP standard system helps the management of the human genomic data sharing respecting the privacy of the data, the reuse of the data under the current legislation and any further integration.
Design of ReOmicS
A director, three researchers, a technician and a quality consultant have been the working group that has met fortnightly via videoconference. The team assessed the choice of the GLPs as main reference for ReOmicS by means of a SWOT analysis, whose results are illustrated in Fig. 3 . Strengths and opportunities validated the choice of following GLPs as main reference against minor weaknesses and threats. In any case, as illustrated in what follows, some actions have been planned and taken in order to tackle some weaknesses and threats in a near future.
SWOT analysis diagram. Strength, weaknesses, opportunities and threats have been evaluated for the choice of Good Laboratories Practices (GLPs) as QMS reference
The team then started the design of ReOmicS from the risk analysis of the main NGS process, based on a standard Risk Assessment. With this analysis, the team aimed at identifying the laboratory’s weaknesses in order to address them with specific interventions when developing the QMS.
Errors and problems experienced during the last 3 years were collected and attributed to the relevant process steps. Each problem was then assessed with respect to severity S and probability P. The risk R = S × P associated with each pitfall determined the need and the priority of a specific operating procedure. Figure 1 summarizes these results.
Major problems to be addressed when defining Standard Operating Procedures (SOPs) were identified in the fields of communication with the customer; traceability of samples; warehousing, archive, metadata and database management; planning; organizational structure; and controls and checks. No needs for specific instructions were envisaged for problems with R less than 4. Major needs and related priority were taken into account in the drafting phase of the SOPs.
As a first step, in order to define the internal context, the MoBiLab organizational structure was designed according to the GLP requirement taking into account the dimension and the constraints of the research institution: the major roles, such as Director, Study Director, Laboratory Manager, Principal Investigator, Archivist et al, were identified and assigned to laboratory staff (Fig. 4 ). Once defined the roles and responsibilities, the team analyzed the laboratory internal processes, identifying primary and support processes (see Table 2 ). The team outlined the main (primary) process by means of a Supplier-Input-Process-Output-Customer (SIPOC)-like flowchart, which includes the person in charge of the activity and the related documented information (SOPs, and records) (see Fig. 5 ).
Organizational Chart. Under the supervision of the MoBiLab director, resources are divided into two main groups: (i) managerial and technical support and (ii) research and experimentation
SIPOC-like flowchart of the Study management. Columns collect inputs and related providers, main flow-chart with tasks, person in charge/supervisor for every task, output and prescriptive document (SOP) for each task. The flowchart is divided into two main sections: Execution/experimentation and Analysis/results
This SIPOC-like chart acted as the backbone of the SOP of the management of the Study: moreover, most steps of the study process, together with the results of the risk assessment, led to the identification of technical/scientific procedures , these were then accompanied by attachments describing technical details as required. After having identified the SOPs required for the operational processes , researchers were provided with a template and with the instructions to draft them. In parallel, management procedures were defined by the whole team, drafted, and supported by flowcharts and other quality tools whenever needed (e.g., SIPOC). SOPs were ranked by priority, driven by the risk assessment results; few SOPs required by GLPs were not considered since were not needed for our specific research activities (e.g., management of test systems). The management support processes were described by SOPs and were, as far as possible, compliant with GLP requirements.
Following the results of the SWOT analysis (where this aspect was judged as a weakness) and in order to ensure the highest level of reliability, reproducibility and traceability of the results, the team also planned to develop and optimize a LIMS (Laboratory Information Management System) platform for managing all the laboratory activities through a suite of integrated modules, in collaboration with an Italian ICT company. The platform will be structured starting from the SIPOC-like flowchart for the management of the study and the modules will be developed and customized in agreement with the SOPs.
The structure of the SOP list (Table 2 ) is directly related to the allocation of responsibilities in the laboratory and conforms to the organization of the management of the studies (Fig. 5 ): the primary and the scientific SOPs are the responsibility of the researchers and technicians operating in the MoBiLab under the supervision of a Principal Investigator, nominated by the director of the study. At the same time, management and technical support SOPs govern staff indirectly involved in the project, caring for an environment suitable for the studies. The split into two different areas, research and support, is clearly represented in the organization chart (Fig. 4 ).
Indeed, the MoBiLab belongs to a public research institution whose mission is to achieve scientific outputs in national and international funded project. Staff organization is related to the skills required and tasks assigned in the study program. For this reason, in the primary SOP the study corresponds to the project and the director of the study refers to the scientist responsible of the project. Only in a few cases the MoBiLab is working as a service provider, producing genomic and data analysis directly commissioned by external customers. For all these reasons, the scientific SOPs can evolve by integrating new requirements highlighted by customers or scientific partners.
Following all these considerations, the ReOmicS was structured as illustrated in Table 3 . Each SOP is structured according to a general template with the following sections:
-definitions, terms and acronyms
-references
-activities and responsibilities
-materials and equipment
-safety rules
-history of revisions (change register)
Results of the application of ReOmicS
The system has been progressively introduced, starting in 2016 from the scientific SOPs. At present the application of ReOmicS is underway with few limitations, due to the fact that processes and their management are challenging to pursue without IT support. This is also the reason that a LIMS is being developed. For example, audits have been conducted in limited form, mainly focusing on the suitability and effectiveness of the quality management system. Despite these limitations, the MoBiLab, in the 3-years following the introduction of common rules and references, is experiencing a concrete improvement, as testified by the positive trend of the five metrics chosen: risk assessment, analysis throughput, number/quality of publications, external collaborations and satisfaction survey.
The first metric chosen for the assessment of efficiency is the comparison between the outcomes of the risk evaluation before and after the introduction of ReOmicS (Fig. 6 ). The results show a statistically significant improvement ( p = 0.0005 ) with respect to the initial assessment for the application of technical SOPs, while that of management SOPs is still limited. A better compliance to the QMS is expected in the future, mainly in the areas of study-planning, assignment of tasks and storage control.
Efficiency indicator. Efficiency is evaluated by means of the update of the risk assessment performed in the initial phase of the project, before the application of ReOmicS
A second metric has been chosen to evaluate the efficiency, i. e. the overall performance of MoBiLab, in terms of total number of processed samples, number of samples/sequencing runs, number of sequencing runs and sequencing platform output (GigaBase). The data shown in Fig. 2 compare the 3-year periods, 2013–2015 and 2016–2018. Considering all the MoBiLab applications (Genomic, Transcriptomics and Metagenomics), the analyzed parameters, except the number of sequencing run, show an improvement after the introduction of ReOmicS (Fig. 2a ). At the same time, it is important to underline that, despite the reduction in the number of sequencing runs due to a forced six months interruption of MoBiLab activities for logistical issues, the total number of samples processed and the average number of samples increased together with the platform output. In an NGS analysis, we can speculate that maintaining a high throughput whilst at the same time increasing the number of samples, represents an important laboratory challenge. These results can be ascribed to operator’s competences and training, but also to the improvement of the management of the process and the control of the analysis provided by the QMS. The efficiency of the laboratory was therefore assessed by taking into consideration the amount of data produced, referring mainly to Metagenomics analysis, the most requested application at MoBiLab (approximately 52% of the total amount of analysis performed) (Fig. 2a ). Overall, a positive increase was shown (Fig. 2b ) by all the parameters during the 3-year period 2016–2018.
To assess the influence of the QMS on the effectiveness of the MoBiLaB, three metrics have been chosen. The first one is the number of publications and the related Impact Factor (IF). In Table 4 , data referring to the first 3-year period 2013–15 (before the introduction of ReOmicS) are compared with data from the 3-year period after the progressive introduction of the QMS, 2016–2018. Table 4 also shows the average IF values obtained for each period. The lowest and the highest values of journal IF were excluded from the analysis. The QMS improved also downstream processes as demonstrated by the increased number of papers published in peer review journals. The number of published papers has grew from 13 to 23 in the last 3 years. Indeed, the number of published papers doubled even if the IF increase is not significant (data not shown). The second metric chosen for the assessment of effectiveness of ReOmicS, i.e., number of active external collaborations in MoBiLab publications (based on a 3-year period), shows the attitude of the laboratory to be a national and world leading scientific NGS laboratory and to be an enabling facility in the support of science. Table 4 shows the average number of external authors in the two 3-year periods. The total number of authors for each paper did not significantly change in the two periods considered, nor did the number of authors with an Italian affiliation. On the other hand, the number of general affiliations increased by a third and the number of international collaborations almost tripled.
The third effectiveness metric measures the satisfaction of customers and collaborators who had the opportunity to take advantage of the analysis service of MoBiLab, by means of two separate surveys: the first one dedicated to all customers/collaborators about perceived quality and the second to customers/collaborators who worked with MoBiLab in both three-year periods before and after the introduction of the QMS. The first survey was sent to 54 collaborators and 25 answers were collected. The second survey was sent to 25 collaborators, obtaining 12 answers. Of these last 12, 6 were discarded for inconsistency in answers to single questions, for this reason only a qualitative evaluation can be made. Results of both surveys are illustrated in Fig. 7 and show a good level of satisfaction from customers and collaborators, together with a demonstrable improvement of perceived quality after the introduction of ReOmicS.
Customer satisfaction. A qualitative measure of the customer satisfaction was evaluated analyzing the results obtained from the two surveys: the first one dedicated to all customers/collaborators about perceived quality ( a ) and the second to customers/collaborators who worked with MoBiLab in both three-year periods before and after the introduction of the QMS ( b )
As a final consideration on metrics, it was difficult to gather complete and detailed data regarding the projects developed in the years 2013–2015 for comparison with those pertaining to the years 2016–2018 because, before the ReOmicS introduction, a lot of the information was scattered among different research environments within and outside the MoBiLab. Since this data unavailability was judged unacceptable in maintaining due control on the work of the laboratory and on the improvement process, a more complete set of metrics has been studied which will be integrated in the planned LIMS (Table 5 ).
Main deviations from GLPs
Not all the requirements of GLP can be accomplished in the development of ‘omics’ studies, as clearly shown by Kauffmann et al. ( 2017 ). The limitations involve technical aspects, but in our case have had an impact also on the organizational requirements.
The first requirement that cannot be met is the management of test systems, because in the NGS procedures they are not used. External databases are used as reference and these are validated by the well-known mechanism of peer review. This is in partial disagreement with the GLP direction about data management and validation, but is common practice in genomics.
As far as data storage is concerned, MoBiLab depends on the servers made available by the INFN. The commercial agreement with INFN is stipulated by the IBIOM Institute: so far MoBiLab is not in the position to insert specific GLP requirements. With the planned development of the LIMS, new conditions and agreements more suitable for GLP compliance about data management will be implemented.
As an example of GLP procedure requirements that needed a new definition, the compliance statement required by the GLP is intended not towards the GLP, but to ReOmicS QMS itself.
As an example of organizational GLP requirements that could not be met, the dimension of the research unit and specifically of the laboratory is an issue when trying to identify an independent quality assurance structure. However, within the laboratory, a person has been appointed for the quality assurance tasks described in the dedicated SOP with support from an external quality consultant for methodological matters or concerns. Furthermore, the title of Principal Investigator (PI), which in the GLP is an individual who, for a multi-site study, acts on behalf of the Study Director, is known here as “research project manager”, as in the most common meaning for research laboratories working on funded projects.
Moreover, the role of archivist has not yet been allocated, since the planned introduction of a LIMS will ease the task of archiving and will allow a clearer allocation of responsibilities.
Conclusions and future perspectives
We can demonstrate that the application of a QMS, giving precise references for research management, introducing controls—thus increasing both result reliability and reducing opportunities for error—and promoting the efficiency in planning, conducting, analyzing and reporting on the processes, represents a valid tool for overcoming these difficulties and, at the same time, an opportunity for significant improvement for a research laboratory.
In the experience illustrated, GLPs—among different quality management standards—was judged most suitable for the purpose of the MoBiLab QMS, when implemented in the aspects appropriate to the characteristics of the laboratory. The development of the QMS was performed making use of quality techniques and methods to ensure a lean and rigorous process.
Among the positive outcomes that can be ascribed to the adoption of the QMS, we can stress an evident increase in the efficiency of the laboratory, evaluated by the decrease of the risks and of the errors occurring during analysis workflow. Moreover, staff was more motivated thanks to a better organization of the team and an acknowledgment of their competences. The effectiveness was also improved, increasing the number of collaborators, the customer confidence and the availability of a databases organized for future investigations.
Indeed, the experience of QMS in MoBiLab demonstrates that the performances of the analysis and number of the primary “products” of academic research—publications—increases after QMS introduction, together with the appeal of the laboratory as witnessed by more active international collaborations. Number of publications in high-impact factor journals, number of citations, and number of opportunities of excellent scientific collaborations indicate how the laboratory aims to be a national and world scientific leading infrastructure and an enabling facility supporting science.
Experimental and service data (throughput analysis) prove that the laboratory, thanks to the ReOmicS introduction, is increasing its ability to provide high quality scientific data and associated services.
The results of the surveys give evidence of a positive user satisfaction regarding support and collaboration, not only in reference to the facility, but also to the staff employed in the MoBiLab. Good opinions were also expressed comparing MoBiLab with other similar laboratories. We have planned to run such surveys periodically with different audiences to assess satisfaction, achievements, collaborations and expectations.
As future perspectives, scientific, economic and technological impact could be assessed reporting, for instance, the training of skilled researchers, the development of new methodologies and software for NGS, the growth of network and social interactions or the creation of a new firm (e.g., a spin-off).
As proposed by the OECD ( 2019 ) for the assessment of Research Infrastructures, the metrics chosen to evaluate efficiency and effectiveness of MoBiLab before and after the introduction of the QMS can be presented as possible indicators to demonstrate the impact of an NGS research and analysis laboratory.
The quantifiable impacts captured through quantitative metrics (number of publications, citations…), as well as the non-quantifiable metrics obtained by dedicated surveys, are also helpful when approaching an economy and policy impact analysis of the genomic and bioinformatic research.
Although the ReOmicS QMS did not incorporate all requirements of the GLPs, and its application is still to be completed with respect to a small number of controls and the complete traceability of results, the positive outcomes already obtained are also due to the gradual increase in confidence by laboratory staff with the quality approach and to the early adoption of the standard protocols described by the technical SOPs.
This experience and the results obtained prove that a NGS laboratory, and therefore any other research laboratory, can benefit from the introduction of a quality framework, if properly translated from the generic standards and adapted to the specific requirements of a research environment. The metrics and the indicators showed in this study will be followed up in the implementation of the MoBiLab thanks to the grant received by the Italian Research Minister to empower facilities and equipment of this laboratory.
The standards of the ISO 900 family are often declined in sector-specific versions (e.g., the already mentioned ISO 17025 for testing and calibration laboratories, ISO/TS 16949 for automotive, ISO 13485 for medical devices), adding requirements unique to a particular application. The ReOmicS has this precise purpose: to detail the requirements for the management and control of a generic research laboratory. To this end, as a first choice, we took as a reference the GLPs, whose adequacy for a research laboratory in ‘omics—despite some specific exclusions—is attested by Kauffman et al. ( 2017 ). The directions of the GLP are about management and control of the study and experimentations (the primary process), and of the tools and materials used, as well as the rules for reporting and archiving data, samples and documents (which are only a few of the support processes envisaged by the ISO9001 standard). In a general sense, these aspects are common to all research laboratories, regardless of the field of application. Starting from the structure of a GLP-like QMS, the ReOmicS can be completed with the specific requirements of ISO 9001 on the parts not governed by the GLP, such as context and risk assessment, supplier management and improvement process. In this light, we strongly believe that the ReOmicS can be taken as a reference for any type of research laboratory.
Indeed, the ReOmicS GLP-like quality system is expected to evolve into a complete quality system according to ISO9001 to achieve the specific certification. For this reason, a risk assessment—both strategic and operational has already been performed, and several SOPs have already been arranged to comply with main requirements of ISO 9001.
In the future, quality management would be streamlined by the introduction of the IT tool (LIMS). This development is expected to foster the potential exploitation of the NGS activities of the MoBiLab. The evolution of the ReOmicS is then expected to follow the Lean Production (a.k.a. Toyota Production System), which is a wide-ranging methodology developed in manufacturing to reduce waste and improve product quality (Womack et al., 1991 ) and recently used also in the research environment (Barnhart, 2013 ). The Lean approach will be strictly connected with the LIMS system, leveraging its features to ensure the best control. The GLP-like system and the Lean approach will allow the MoBiLab to improve its efficiency, limiting wastage of time and materials, and reducing opportunities for error, at the same time enhancing the effectiveness traceability and reproducibility of results.
Data availability
All data generated or analyzed during this study are included in this published article.
Baker M (2016) 1500 scientists lift the lid on reproducibility. Nature 533:452–454. https://doi.org/10.1038/533452a
Article ADS CAS Google Scholar
Barnhart T (2013) Creating a lean R&D system. CRC Press, Boca Raton
Google Scholar
Cargill CF (2011) Why standardization efforts fail. J Electron Publ. https://doi.org/10.3998/3336451.0014.103
Corpas M, Kovalevskaya NV, McMurray A, Nielsen FGG (2018) A FAIR guide for data providers to maximise sharing of human genomic data. PLOS Comput Biol 14(3):e1005873. https://doi.org/10.1371/journal.pcbi.1005873
Article ADS CAS PubMed PubMed Central Google Scholar
Defeo J, Juran JM (2010) Juran’s quality handbook: the complete guide to performance excellence 6/e McGraw-Hill Professional, US
Dirnagl U, Kurreck C, Castaños-Vélez E, Bernard R (2018) Quality management for academic laboratories: burden or boon? Professional quality management could be very beneficial for academic research but needs to overcome specific caveats. EMBO Rep. https://doi.org/10.15252/embr.201847143
Article Google Scholar
Endrullat C, Glökler J, Franke P, Frohme M (2016) Standardization and quality management in next-generation sequencing. Appl Transl Genomics. https://doi.org/10.1016/j.atg.2016.06.001
Kauffmann HM, Kamp H, Fuchs R, Chorley BN, Deferme L et al. (2017) Framework for the quality assurance of’omics technologies considering GLP requirements. Regul Toxicol Pharmacol. https://doi.org/10.1016/j.yrtph.2017.10.007
Lanati A (2018) Quality management in scientific research-challenging irreproducibility of scientific results. Springer, Cham, Switzerland
Book Google Scholar
Stephens ZD, Lee SY, Faghri F, Campbell RH, Zhai C et al. (2015) Big data: astronomical or genomical? PLoS Biol. https://doi.org/10.1371/journal.pbio.1002195
Tiwari R, Nain L, Labrou NE, Shukla P (2018) Bioprospecting of functional cellulases from metagenome for second generation biofuel production: a review. Crit Rev Microbiol. https://doi.org/10.1080/1040841X.2017.1337713
UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases & Scientific Working Group on Quality Practices inBasic Biomedical Research (2006) Handbook: quality practices in basic biomedical research/prepared for TDR by the Scientific Working Group on Quality Practices in Basic Biomedical Research. World Health Organization. https://apps.who.int/iris/handle/10665/43512
Wiese L, Schmitt AO, Gultas M (2018) Big data technologies for DNA sequencing. Springer, Basel
Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M et al. (2016) The FAIR Guiding Principles for scientific data management and stewardship. Sci Data. https://doi.org/10.1038/sdata.2016.18
Womack JP, Jones DT, Roos D (1991) The machine that changed the world. Harper Perennial, New York
OECD (1998) Organisation for economic co-operation and development principles on good laboratory practice (As Revised in 1997). OECD principles of good laboratory practice and compliance monitoring. No. 1. ENV/MC/CHEM(98)17
OECD (2019) Reference framework for assessing the scientific and socio-economic impact of research infrastructures. OECD Science, Technology and Industry Policy Papers, No. 65
Download references
Acknowledgements
This work was supported by the projects “Lifewatch” Roadmap ESFRI and “OMICS4FOOD”, cod. 1JLZKDPOR, Avviso “Innonetwork” A.D. n.124 del 16/10/2017, Puglia FESR-FSE 2014–2020 Azione 1.6. The authors thank Gabrielle Nasca Quadraccia for critical reading and English revision of the paper.
Author information
These authors contributed equally: Antonella Lanati, Marinella Marzano
Authors and Affiliations
Valore Qualità, 27100, Pavia, Italy
Antonella Lanati
Istituto di Biomembrane, Bioenergetica e Biotecnologie Molecolari (IBIOM), CNR, 70126, Bari, Italy
Marinella Marzano, Caterina Manzari, Bruno Fosso, Graziano Pesole & Francesca De Leo
Dipartimento di Bioscienze, Biotecnologie e Biofarmaceutica, Università degli Studi di Bari “Aldo Moro”, 70126, Bari, Italy
Graziano Pesole
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Antonella Lanati or Francesca De Leo .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Lanati, A., Marzano, M., Manzari, C. et al. Management at the service of research: ReOmicS, a quality management system for omics sciences. Palgrave Commun 5 , 75 (2019). https://doi.org/10.1057/s41599-019-0283-0
Download citation
Received : 28 February 2019
Accepted : 12 June 2019
Published : 09 July 2019
DOI : https://doi.org/10.1057/s41599-019-0283-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Accessibility Links
- Skip to content
- Skip to search IOPscience
- Skip to Journals list
- Accessibility help
- Accessibility Help
Click here to close this panel.
Purpose-led Publishing is a coalition of three not-for-profit publishers in the field of physical sciences: AIP Publishing, the American Physical Society and IOP Publishing.
Together, as publishers that will always put purpose above profit, we have defined a set of industry standards that underpin high-quality, ethical scholarly communications.
We are proudly declaring that science is our only shareholder.
Research on the Problems and Countermeasures of Laboratory Management in Colleges and Universities
Jiahua Zhang 1 and Hang Li 2
Published under licence by IOP Publishing Ltd Journal of Physics: Conference Series , Volume 1798 , 2020 International Conference on Applied Mechanics and Mechanical Engineering (ICAMME 2020) 25-27 September 2020, Hulun Buir, China Citation Jiahua Zhang and Hang Li 2021 J. Phys.: Conf. Ser. 1798 012006 DOI 10.1088/1742-6596/1798/1/012006

Article metrics
1152 Total downloads
Share this article
Author e-mails.
Author affiliations
1 School Enterprise Cooperation Division, University of Chifeng, Chifeng, 024000, Inner Mongolia, China
2 Departments of College of Physical and Intelligent Manufacturing Engineering, University of Chifeng, Chifeng, 024000, Inner Mongolia, China
Buy this article in print
University laboratories are responsible for providing professional venues for practical teaching and scientific research, and the quality of their management can directly affect the quality of research and research work in universities. At present, there are some problems in the laboratory management of colleges and universities in China, such as imperfect laboratory management system, imperfect laboratory personnel allocation and management, incomplete laboratory infrastructure construction, and incomplete laboratory safety management. In view of these problems, this paper puts forward five aspects of university laboratory management countermeasures.
Export citation and abstract BibTeX RIS
Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence . Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Research and Practice of Laboratory Safety Management Mode
- Conference paper
- First Online: 04 February 2020
- pp 1123–1128
- Cite this conference paper

- Bo Hu 17 &
- Baoxin Tai 17
Part of the book series: Advances in Intelligent Systems and Computing ((AISC,volume 1088))
110 Accesses
1 Citations
In the process of laboratory management, many problems will inevitably be encountered. In view of the problems in the laboratories of contemporary colleges and universities, a “three-level interaction” management model with modern characteristics has been developed by combining with the requirements of modern education for laboratory management. In this management mode, the student-centered management and teaching work are carried out from the aspects of practical teaching ideas, design of experimental content, experimental teaching methods and experimental teaching management, we can find from the study of the results of practice that the management model can greatly improve students’ practical ability, innovation ability, cooperation ability and management ability. In addition, the laboratory equipment has been well maintained to a certain extent, ensuring the normal operation and application of the laboratory.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Wei, Zheng. 2009. Construction of a “Firewall” for the safety management of university laboratories. Science and Education Literature Collection (first issue), (11).
Google Scholar
Qingmin, Liao. 2010. Reflections on the safety management of university laboratories. Laboratory Research and Exploration (1).
Yongqi, Wen, and Qin Huajun, Ouyangjin. 2010. Construction of a people-oriented laboratory security system. China Modern Educational Equipment (3).
Lihua, Zhou. 2010. Research on the construction of safety culture in university laboratories. Science and Education Literature Collection (late issue) (2).
Guo, Zhang. 2010. Discussion on the safety management of university laboratories. China Modern Education Equipment (11).
Shuangshuang, Liu. 2010. Reflections on strengthening the safety construction of university laboratories. China Higher Medical Education (8).
Zhenqi, He. 2005. To strengthen safety awareness and do a good job in laboratory safety management. China Modern Education Equipment (9).
Xiuping, Liao, Chen Jixin, and Xu Yehe. 2010. Change the concept, develop and innovate, and actively promote the construction of laboratory safety management in universities. Laboratory Research and Exploration (8).
Buyang, Cai. 1995. Discussion on the standardized management of laboratory safety. Experimental Technology and Management (2).
Ping, Yang, Chen Yanling, and Huang Jianping. 2008. Establishing laboratory safety assurance system to ensure safe operation of laboratories. Experimental Technology and Management (9).
Download references
Author information
Authors and affiliations.
Wuhan Donghu University, Wuhan, 430212, Hubei, China
Bo Hu & Baoxin Tai
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Bo Hu .
Editor information
Editors and affiliations.
Huazhong University of Science and Technology, Wuhan, China
Chuanchao Huang
Computer Science and Information Management, Providence University, Taichung, Taiwan
Yu-Wei Chan
University of Aizu, Fukushima, Japan
Rights and permissions
Reprints and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper.
Hu, B., Tai, B. (2020). Research and Practice of Laboratory Safety Management Mode. In: Huang, C., Chan, YW., Yen, N. (eds) Data Processing Techniques and Applications for Cyber-Physical Systems (DPTA 2019). Advances in Intelligent Systems and Computing, vol 1088. Springer, Singapore. https://doi.org/10.1007/978-981-15-1468-5_131
Download citation
DOI : https://doi.org/10.1007/978-981-15-1468-5_131
Published : 04 February 2020
Publisher Name : Springer, Singapore
Print ISBN : 978-981-15-1467-8
Online ISBN : 978-981-15-1468-5
eBook Packages : Intelligent Technologies and Robotics Intelligent Technologies and Robotics (R0)
Share this paper
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- BMC Public Health

Challenges and solutions for instituting an efficient maintenance program for laboratory equipment in Central Asian, and developing world, countries
Riza ikranbegiin.
1 World Health Organization, Regional Office for Europe, Copenhagen, Denmark
George Schmid
2 Centers for Disease Control and Prevention, Atlanta, GA USA
3 ICAP Global Health Action, Columbia University, Mailman School of Public Health, New York, NY USA
Andrew Young
4 Laboratory of Grady Health System, Grady Memorial Hospital, Atlanta, GA USA
Phyllis Della-Latta
5 Clinical Microbiology Service, Columbia University Medical Center NewYork-Presbyterian Hospital, New York, NY USA
Paul Spearman
6 Department of Pediatrics, Emory University, Children’s Healthcare of Atlanta, Atlanta, GA USA
Artur Ramos
Bereket alemayehu, begaiym achmetova.
7 ICAP Global Health Action, Columbia University, Mailman School of Public Health, New York, USA
9 ICAP Global Health Action, Columbia University, Mailman School of Public Health, Bishkek, Kyrgyzstan
Gulzhan Nauryzova
8 Clinical Diagnostic Laboratory of the Almaty AIDS Center, 2 Basenov Str., Almaty, Kazakhstan
Adilya Albetkova
Associated data.
Not applicable
We review the current state of quality assurance in laboratories of the five Central Asia Republics (CARs), focusing on laboratory equipment, and compare quality assurance approaches with CLSI standards. The laboratories of the CARs faced exceptional challenges including highly-structured laboratory systems that retain centralized and outmoded Soviet-era approaches to quality assurance, considerably jeopardizing the validity of laboratory tests. The relative isolation of the CARs, based on geography and almost exclusive use of the Russian language, further hamper change. CARs must make high-level government decisions to widely implement quality assurance programs within their laboratory systems, within which approaches to the management of laboratory equipment will be a prominent part.
At the crossroads between Europe and Asia, the Central Asia Republics (CARs) have received increased (but insufficient) attention from the international community due to the political and economic significance of the region. These countries – Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan and Uzbekistan – have faced enormous challenges in establishing and stabilizing their institutions since attaining independence in 1991, following the dissolution of the Soviet Union. Challenges involve governance - as authority has transitioned from highly standardized Soviet bureaucracies to more independent political structures at regional, national, provincial and local levels – and demographic. Although overall population density is low, many men have left - to work in Russia or elsewhere, leaving villages without men; poverty by national standards is widespread in all countries [ 1 ], particularly in rural areas; health care systems are poor [ 2 – 6 ]; and a growing percentage of the population is moving to cities to escape poverty and adopting Western lifestyles and diets, creating new health challenges.
Health care systems are changing but the traditional laboratory structures have operated from a centralized, national level, with oversight of service quality generally conducted by national ministries of health. In these dynamic societies, diagnostic services for patients will increasingly need to occur in local, non-specialized laboratories based in hospitals or clinics. The delivery of laboratory services to neglected rural populations requires additional decentralization. Based on these trends, quality management of clinical laboratories – including the management of equipment – will require attention at the local level, which is the model used for laboratories in developed countries and one espoused for health care systems by the World Health Organization European Office as it assists countries in the former Soviet Union [ 7 ].
The appropriate diagnosis and then management of illness requires valid test results. Valid laboratory results are also necessary for rapid detection and control of outbreaks of infectious diseases and other public health threats at their source and therefore essential for global health security but valid test results can be elusive. It is often thought that ex-Soviet health care systems are near-equivalents of Western systems…they are not. It is common in the CARs, for example, for people in cities with financial means to “laboratory shop” by visiting multiple laboratories, comparing conflicting results in an attempt to decipher what might be correct results. Poor people do not have even this poor option. Testing exists, but quality is uncertain.
Little is written about the quality of laboratory services in Central Asia (a search using “Central Asia health laboratory quality” with Google Scholar yielded no relevant article in the first 100 articles identified). Yet, our experience and that of others shows the need for Quality Management System (QMS) implementation in our region is big [ 8 – 10 ] and our observations important. The combined population of the five CARs is 61 million people in a region that retains an ex-Soviet heritage and which is relatively isolated from world literature by near-exclusive use of the Russian language. The challenges found in these countries are likely to be present in other countries with a similar heritage and similar systems [ 11 ].
In this paper, we provide a comprehensive review of QMS and its application within laboratories in the CARs, and then follow this with our observations regarding how widely used international standards are within the CARs and the need for their use there.
Quality management of clinical laboratories
International organizations have long supported disease-specific, “siloed” laboratory systems and attendant QMS. Consistent with an increased focus on integrated services, international organizations should support an approach that focuses on strengthening key cross-cutting elements, including a practical approach to QMS [ 12 ]. Accordingly, the concept of a total-laboratory QMS has been developed through the guidelines and standards of international and national organizations with recognized expertise, such as the International Organization of Standardization (ISO), the World Health Organization (WHO), the Clinical and Laboratory Standards Institute (CLSI) and the Centers for Disease Control and Prevention (CDC) (Table 1 ).
Reference documents for laboratory quality management systems
As defined by the ISO and CLSI, a QMS represents “coordinated activities to direct and control an organization with regard to quality.” With ISO developing standards and CLSI and WHO providing and coordinating with one another guidance on implementation of procedures to meet those standards, these QMS guidelines are consistent with one another, although they vary in degree of specificity. For example, CLSI documents often contain step-by-step suggestions, which are useful in developing countries that are establishing quality management processes for the first time.
In 2005, ISO [ 13 ] released requirements for both quality management and technical operations of testing and calibration in laboratories, followed by standards specific for the medical laboratory [ 14 ]. From 2006 to 2008, WHO, CDC and CLSI collaborated in a laboratory QMS initiative leading to publication of a CLSI guideline [ 15 ]. This guideline (GP26-A4, 2011, Fourth Edition) provides a structured approach for organizing, creating, and maintaining the management infrastructure for a quality laboratory system. Based on CLSI guidelines and the ISO 15189 standard, WHO published a handbook “Laboratory Quality Management System” [ 16 ] and tool kit “Laboratory Quality Management Systems Training Toolkit” [ 17 ], to guide and monitor laboratory QMS implementation.
The CLSI guidelines are based in part on the Clinical Laboratory Improvement Amendments (CLIA), which are American Federal laws implemented in 1988 to govern clinical laboratories in the USA. However, they are designed to be applicable to international laboratories.
Recently, step-wise international approaches to quality improvement have been developed which provide for progressive and incremental quality improvement. Such tools include the WHO Laboratory Quality Stepwise Implementation (LQSI) tool [ 18 ], which translates the ISO 15189 quality standard for medical laboratories into a stepwise process to implement a QMS, and, the WHO-Afro Strengthening Laboratory Management Towards Accreditation (SLMTA) program [ 19 ], which provides a laboratory management framework and curriculum to define and teach specific, measurable job tasks for laboratory personnel on how to manage quality in a laboratory. Progress is monitored with the Stepwise Laboratory Improvement Process Towards Accreditation (SLIPTA) checklist [ 19 ] and stepwise laboratory quality improvement is recognized based on the quantitative checklist score. The ultimate goal of these stepwise programs is to prepare laboratories in developing countries to establish sustainable quality management systems that meet international accreditation standards [ 20 ]. These tools are designed to complement one another and work in harmony to build capacities from the bench level and up in a national laboratory system and from the highest levels of decision making down through the system.
The WHO quality management model organizes all laboratory activities into 12 quality system essentials (QSEs) that serve as building blocks for coordinated and interrelated activities. Each QSE must be addressed for the overall goal of laboratory quality improvement to be achieved [ 16 ]. Failure in even one of the QSEs can result in inappropriate technical procedures.
Fundamental challenges to the quality of laboratory tests in less-resourced countries are: 1) the lack of a laboratory management infrastructure and quality management training curriculum that develops the competences of laboratory managers and quality coordinators; 2) lack of access to or knowledge of current international standards; and 3) an absence of national standard operating procedures that are based on these standards. As a consequence, there are significant quality gaps in laboratories of resource-limited countries relative to international standards. For example, these laboratories often find it very difficult to hire qualified medical technologists who are trained to follow established testing algoritms and quality control protocols, specific guidelines, workplace health regulations and instrument maintenance controls. In addition, there are few resources to conduct periodic competency testing and continuing education to assure that technologists retain core knowledge of authorized procedures and remain abreast of international and national standards.
To close these quality gaps, it will be important for developing countries and donor organizations to focus on implementing tools that assist laboratories to adopt QMS models that begin to address each of the QSEs.
Equipment management in resource-limited countries
Against this backdrop of need in countries with limited resources, the QSE that deals with equipment management and maintenance deserves special attention. Much of the laboratory equipment in developing countries, including the CARs, is donated by international aid organizations, or purchased with their funds. However, it is rare that funds are included to maintain equipment in a state necessary to produce reliable test results. In addition, there are few standardized indicators with which donor organizations can assess developing countries on how well they address equipment management and maintenance. Based upon the ISO, CLSI, and WHO guidelines [ 21 ], equipment management systems should be characterized by formal policies, processes and procedures for selection, qualification, validation, maintenance, calibration, troubleshooting, decommission and record keeping [ 15 , 21 ]. These systems ensure that a laboratory selects equipment that meets its needs; maintains it in a state that produces reliable test results; and documents its processes sufficiently for internal and external oversight. This approach has become common and has been implemented successfully in highly-resourced countries during the last 20 years. In contrast, few developing countries, including the CARs, have developed these quality management systems for effective laboratory equipment management.
Management of laboratory equipment
The following sections describes the management of laboratory equipment in the four Central Asian countries of Kazakhstan, Uzbekistan, Tajikistan and Kyrgyzstan (while differences exist in laboratory services between the individual CARs, there are enough similarities among their QMSs that they can be compared as a group) and compares them to CLSI standards [ 21 ] for: selecting appropriate equipment; performing installation qualification; and using, calibrating, and maintaining equipment according to established schedules and processes based on the international, national, and accreditation requirements . The CLSI guideline developed in line with ISO 15189 provides very specific guidance on equipment management.
Quality management systems in Central Asian countries
The overall laboratory QMS, which had been centralized from Moscow during the existence of the USSR, was not maintained during the period immediately after independence. After independence, the Ministry of Health (MOH) of each CAR gained oversight of laboratory services. But, the Constitution of each CAR left the authority for coordination of equipment management, which is one of the main part of QMS, to National Institute for Standards and Metrology (NISM). It is the responsibility of Metrology (NISM) to verify the required measurement accuracy and the functioning of the measuring system at defined intervals according to manufacturer’s instructions, also ISO requirements and to certify annually if the requirement was met. However, despite these intended levels of oversight, laboratory services experienced many funding shortfalls and the loss of highly experienced laboratory staff during the formation of the independent CARs. Currently, funding sources and levels for various laboratory networks differ, with research labs funded with competitive grants from governmental and non-governmental agencies, veterinary and “public health” laboratories supported by government funding, and clinical laboratories, while principally government-funded, are in some countries finding public-private partnerships or becoming exclusively privately funded. International donor programs have helped to build government capacity, increasingly according to “Western standards,” to improve access and delivery of clinical and veterinary laboratory services. Important developmental gaps remain, and a large gap is in including the lack of equipment management in laboratory services and the regulation of laboratories in the private sector.
Laboratory equipment management (EM) in CARs
CLSI document QMS13-A [ 21 ] provides recommendations for criteria and methods used in all operations that occur during the typical lifecycle of laboratory equipment, including selection, identification, validation, reverification, testing, and decommission. The guideline describes each of these operations and includes many sample forms and templates for use in documenting all aspects of the equipment life-cycle.
In the CLSI QMS13-A [ 21 ] guideline, laboratory equipment can be classified into two major categories: general laboratory equipment and laboratory instrumentation. General laboratory equipment is that which can be used in various laboratory settings or methods, while laboratory instrumentation is used to produce measurements in a specific examination/analytical system or method (Table 2 ). The distinction is useful. General laboratory equipment is often used for many purposes, and does not need frequent calibration or careful quality control. Laboratory instrumentation is used for more intricate and dedicated tasks, and does need frequent calibration and careful quality control. As equipment management includes both categories, in the following we use the term “Equipment” to refer to both.
Examples of general laboratory equipment and laboratory instrumentation
Below, the framework of QMS13-A [ 21 ] is used to compare equipment management systems in CAR laboratories with those in the United States (Table 3 ).
Policies and implementation of equipment management in laboratory services in the United States and the Central Asian Republics
*Ministries of Health in CAR
**National Institutes of Standards and Metrology in CAR
Planning for acquisition and implementation of new equipment
Clinical and research laboratories in CAR are authorized to provide plans for the need for new equipment (Table (Table3). 3 ). These plans, which include technical descriptions and estimates of the cost of requested equipment, must be signed by directors of the government organizations that oversee the laboratories, and then must be sent to the MOH, where the purchase of equipment is centralized. The purchasing of equipment by the MOH can be time consuming and is often dependent on funds provided by donors and development partners, which tend to reflect their particular interests. In some countries of Central Asia, equipment to be purchased must be included in the State System Register. In contrast, the acquisition of new equipment in the USA is entrusted to the individual laboratory or healthcare organization and is carried out through management of the organization’s yearly laboratory capital budget. A common flexible practice in the United States, but not in the CARs, is to enter into leasing agreements with manufacturers rather than purchase equipment (such as chemistry, hematology, blood culture or antimicrobial susceptibility systems). In these agreements, equipment is supplied by manufacturers as long as reagents for these instruments are purchased from the manufacturer. This system offers laboratories flexibility. They do not invest large sums of money in a purchase that “locks them into” a single piece of equipment, and the agreements — which will be for a specified period — typically can include wording that the manufacturer will upgrade or perform periodic calibration of equipment when desired or needed. The absence of this option in the CARs invites the purchase of equipment that will not be maintained and also that will become obsolete.
Equipment validation plan
A validation plan is essential to ensure that equipment functions as intended in daily work [ 21 ]. In the US, CLIA regulations require that validation be performed by the laboratory using the equipment. Initial validation must include an assessment of each test method performed on the equipment for the following parameters: precision (within- and between-run reproducibility); accuracy (bias versus a gold standard measurement); reportable range (the linear range for quantification); and local reference range. If the assay testing procedure requires the use of equipment, any laboratory modification of that equipment must establish the analytical sensitivity and specificity of the modified procedure. The CLSI guidelines include detailed recommendations for these validation studies. In many cases, equipment manufacturers provide technical personnel and procedures to assist laboratories with initial validation, and the laboratories conduct subsequent periodic assessments throughout the life cycle of the equipment according to regulatory requirements and manufacturer specifications. All validation, quality control or maintenance activities must be documented electronically or in manual logbooks. These must be signed by the personnel performing the activity and reviewed by a supervisor or director.
In the CARs, however, an initial validation plan is not used. The laboratory never provides an assessment of accuracy, reportable range and local reference range due to lack of requirements to adhere to international standards such as ISO 15189 and developed regulatory documents. This gap is a remnant of policies implemented during the time of the Soviet Union, where validation of equipment was centralized under the auspices of the Institute of Standardization and Validation because all laboratory equipment was manufactured in USSR. After the collapse of the USSR, laboratories started to use equipment from other countries. Equipment from other countries has different validation requirements and the current NISM does not have certified specialists who can validate and calibrate laboratory equipment manufactured outside of the former USSR. As a consequence, the accuracy of test results in the CARs is not assured as validation plans are uncommon.
Calibration and maintenance of equipment
Calibration verification, as per ISO, WHO and CLSI guidelines, should be performed according to manufacturer’s recommendations [ 22 ]. CLIA regulations require that calibration verification should be performed at a minimum defined frequency (e.g., every 6 months), whenever a complete change of reagents for a procedure is introduced, or when there is major preventive maintenance or replacement of parts of the instrument that may influence test performance [ 23 ].
In contrast, calibration verification in CAR laboratories is provided only yearly by National Institute of Standardization and Metrology (NISM) for general laboratory equipment. NISM has concluded that hoods, biologic safety cabinets (BSC) and polymerase chain reaction (PCR) machines are not subject to calibration verification. Laboratory instrumentation is tested annually (without calibration verification) solely to verify that instruments are operational, resulting in receipt of an NISM certificate. In general, the NISM do not have engineers with knowledge for calibration verification for recently purchased equipment such as PCR machines, readers, and cell counters. An NISM certificate is the sole requirement for continued operation in a laboratory, and laboratory directors have determined that calibration verification by distributors of the manufacturer is not necessary. Local distributors for the manufacturer provide free maintenance service only for a limited period, after which service contracts with laboratories need to be renewed. In practice, these contracts are rarely renewed. Technical specifications for some equipment are not available in the Russian language, and thus cannot be understood by laboratory staff. As a consequence, general equipment and laboratory instruments such as hematology analyzers, blood chemistry analyzers, blood typing equipment, flow cytometers, microbial identification instruments, thermal cyclers and BSC are never calibrated after the initial distributor’s service. For example, BSC began to be installed in the CAR clinical laboratories in the 1990’s, but have yet to be tested or certified by NISM [ 23 ]. Even if calibration of laboratory instrumentation occurs, records are not kept to document daily, monthly, and quarterly preventative maintenance of equipment in accordance with the manufacturer’s instructions.
In addition, the guidance provided in equipment maintenance documents provided by manufacturers is not part of laboratory practice in Central Asian countries (or most other developing countries). Preventive maintenance is intended to minimize unexpected failure of equipment or instruments so they continue to function as desired. The laboratory needs schedules for preventive maintenance; the manufacturer recommends these schedules. The laboratory needs to follow these schedules [ 21 ].
Manufacturers provide instructions for cleaning, adjusting, and replacing disposables on instruments and equipment. At a minimum, preventive maintenance records need to include the following:
- Instrument or equipment identification
- Date and time maintenance is performed
- Maintenance activities performed
- Identity of the person performing maintenance
- Any necessary follow-up actions taken
- Review and approval [ 21 ]
Preventive maintenance schedules recommended by manufacturer are in our experience never conducted in CAR laboratories.
Quality control of examination (analytical) equipment
International laboratory quality standards require a quality control (QC) policy for each instrument or component of the equipment to provide ongoing assurance that performance continues to meet specifications. A documented QC plan is needed for each examination system, which includes installation and maintenance of the equipment, quality of reagents, and skills of the operator to use the equipment. When examination systems have the ability to assay multiple analytes, a QC plan should be established for each analyte or set of analytes. When developing the plan, the laboratory should consider the stability of the equipment, its susceptibility to malfunction or error, and the risk associated with an undetected measurement error or other out-of-specification occurrence. The laboratory should follow the established schedule for frequency and timing of QC, as well as what ranges and types of QC materials should be used. The laboratory should establish limits of acceptability for QC results, and confirm that QC is within the acceptable range before releasing laboratory test results [ 21 ]. Laboratories operating according to international standards follow regulatory (CLIA [ 24 ]) or accreditation requirements (ISO15189: 2012 [ 14 ]) that define the number and frequency of control samples for quantitative assays (often two or three samples above and below the reference range, run daily or on every shift of operation). However, in some CAR laboratories, control materials are used only weekly, which may not be sufficient to ensure consistent, reliable results. Further, and for example, most AIDS Center Laboratories in one CAR country use equipment by one manufacturer, equipment which is supplied with QC program software. However, this software has been switched off by the vendors (personal observation). In addition, most CAR laboratories have not established limits of acceptability for results from their quality control (QC) materials, and have not developed statistical methods to monitor QC performance within individual test runs, or over a series of runs (such as graphical tools like Levey-Jennings plots). Furthermore, many CAR laboratories have not established specific procedures to take corrective action when QC results do not meet criteria for acceptability.
Troubleshooting, service, and repair
Due to the complexity of modern laboratory equipment, and the sensitivity of test results to subtle changes in equipment performance, laboratories must establish processes to detect and correct instrument malfunction. In the US, most laboratories are required to define their procedures for periodic maintenance, troubleshooting service and repair for all instruments throughout the entire span of their active use. These processes must be performed by qualified personnel (often through contracts with the manufacturers of their equipment). In CARs, troubleshooting, service, and repair are provided by local distributors of the manufacturer’s equipment without charge in the first year after purchase, as a component of the initial purchase price. Some laboratories have long-term contracts with local vendors for maintenance, but solely for broken equipment or replacement of critical parts. These contracts do not include scheduled periodic maintenance and calibration verification of equipment due to the lack of guidelines for equipment management. When the first year of free service ends, a continuing service agreement is generally not purchased due to budgetary constraints. As a consequence, damaged equipment in the CARs commonly remains unrepaired for lengthy periods of time.
Decommissioning of equipment
According to QMS13-A [ 21 ] decommissioning equipment involves a process to ensure the equipment meets the health and safety requirements for the equipment’s next use, such as reassignment to another facility or final disposition to an approved recycling/disposal center. Decommissioning requirements vary according to equipment type and the nature of substances used in operating the equipment. On many occasions, the manufacturer takes responsibility for decommissioning the equipment.
There are no specific requirements for decommissioning equipment in CAR. Every government-sector entity with a laboratory service (for example, AIDS Centers, hospitals and research institutes) has its own team for decommissioning equipment, which is approved by the director of the government entity. The decommissioning team may include a vice director, accountant, head of the laboratory and logistical experts from organizations and laboratories. Depending on equipment status or expected lifespan and typically according to requirements of the Rules for the Decommissioning and Utilization of the Material Values of the State Material Reserve (a Government decree), the team prepares a list of equipment to be decommissioned, which is signed by a director and sent to the MOH for a final decision. The MOH has its own group or department and authority for sign off on the list. Because of these multiple steps, actual decommissioning of problematic equipment is a lengthy process.
Managing equipment records
Equipment documents and records are an essential part of the quality system. The policies and procedures for installation and then maintenance should be defined in appropriate documents, and keeping good equipment records will allow for thorough evaluation of any problems that arise, as well as necessary inspections by regulatory or accreditation organizations. In the US, laboratories equipment documents and records for both types of equipment (general equipment and laboratory instrumentation) are maintained in equipment master files. In CAR, full equipment documents are ensured for some general equipment such as autoclaves. There are no ‘managing equipment records’ for laboratory instrumentation, aside from the manufacturer’s instructions, for installation and repair and receipt of the annual certificate from the NISM. Thus, accurate auditing of the physical status and performance of equipment in the laboratory is impossible.
International standards and training to reach those standards have been developed. In CAR laboratories, the approach to quality-assured testing--and in particular the management of laboratory equipment--is not in line with international standards. In the US, management of laboratory equipment depends on direct local administration, compliance with ISO and CLIA regulations, and compliance with federal, state, and local laws. In contrast, management of laboratory equipment is centralized in the CARs, with a number of organizations involved. While the NISMs play an essential role in management of equipment, it is not identified who—NISMs or others —is responsible for the entire range of services needed for standardization and quality management in laboratory services and annual validation of laboratory equipment. An NISM certificate of laboratory instrumentation, both initial and annual, is considered the sole requirement; however, NISMs do not have engineers with the requisite knowledge for calibration verification of laboratory instrumentation. An alternative approach, calibration verification by distributors of the manufacturer, has not been performed consistently. The responsibilities of vendors are not properly defined, and responsible organizations such as the national Ministries of Health are unaware of the current problems in equipment management. Therefore, laboratories often do not ensure essential elements of equipment management such as scheduled calibration, verification and QC. In addition, the recommendations issued by international organizations such as ISO have not been adapted for local conditions, and CLSI and WHO guidelines have not been implemented. These challenges threaten the validity of test results from laboratory services in CAR and serve as a barrier for rapid detection and control of outbreaks of infectious diseases and other public health threats at their source, and therefore represent a threat to global health security.
Recommendations
The recommendations below provide an approach Ministries of Health may adopt to help ensure accurate test results within CAR laboratories. The recommendations are not exhaustive and focus on equipment. While well-maintained and quality-controlled equipment is only one part of an overall approach to achieving comprehensive quality management in CAR laboratories, it is an essential part.
- A decision can be made within Ministries of Health that quality assurance of laboratory services must be improved and a national or international quality standard adopted.
- A Laboratory Quality Unit can be established within the MOH with the mandate to implement, monitor and certify laboratory quality management systems operations according to the national or international standard.
- Resources to develop a national strategy for the improvement of quality assurance of laboratory services can be provided along with resources to implement it.
- Resources and training for implementing quality assurance, including quality installation and maintenance of laboratory equipment, can be provided to directors and managers of laboratory services and laboratories.
- ISO, CLSI and WHO guidelines can be adapted for the conditions of CAR.
- Regulatory documents can be prepared that clearly describe the responsibilities of each organization with a role in equipment management.
- Laboratory accreditation programs can be established. These programs can be based on periodic laboratory inspections which ensure that laboratories are completing specific, measurable activities in quality management, similar to laboratory accreditation inspections in developed countries.
- CARs can define responsibilities of vendors of laboratory equipment. For example, NISMs could establish minimal standards for scheduled maintenance, which can be met for vendors to offer their equipment to CAR laboratories.
Acknowledgements
The authors would like to acknowledge with thanks all those who contributed to the development and review of this article, more specifically: Staff of IIE (International Institute of Education), Dr. Phil Brachman (Emory University; deceased), and Mary Helen O’Conner (Emory University).
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This article was developed through support of Hubert H. Humphrey Fellowship program at Emory University in Atlanta, USA. Publication costs are funded by the U.S. Centers for Disease Control and Prevention.
Availability of data and materials
About this supplement.
This article has been published as part of BMC Public Health Volume 19 Supplement 3, 2019: 10th anniversary of the Centers for Disease Control and Prevention - Global Disease Detection program. The full contents of the supplement are available online at https://bmcpublichealth.biomedcentral.com/articles/supplements/volume-19-supplement-3 .
Abbreviations
Authors’ contributions.
RI: manuscript concept; first draft, review and approval of the manuscript. GS: manuscript concept; first draft, revisions and approval of the manuscript. DH: manuscript first draft, revisions and approval of the manuscript. AY: review and approval of the manuscript. PD: review and approval of the manuscript. PS: review and approval of the manuscript. AR: review and approval of the manuscript. BA: review and approval of the manuscript. BAC: review and approval of the manuscript. GN: review and approval of the manuscript. AA: concept, development, review and approval of the manuscript. All authors read and approved the final version to be published.
Ethics approval and consent to participate
Consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Riza Ikranbegiin, Email: moc.oohay@niigebnarki .
George Schmid, Email: vog.cdc@dimhcSG .
David Hoos, Email: moc.liamg@013soohdivad .
Andrew Young, Email: ude.hmg@gnuoyn .
Phyllis Della-Latta, Email: ude.aibmuloc@32dp .
Paul Spearman, Email: [email protected] .
Artur Ramos, Email: vog.cdc@somaRA .
Bereket Alemayehu, Email: ude.aibmuloc.cmuc@7622hb .
Begaiym Achmetova, Email: [email protected] .
Gulzhan Nauryzova, Email: ur.liam@zyruan_nahzlug .
Adilya Albetkova, Email: vog.cdc@avokteblAA .

Research Clusters
- Geographic Clusters
Sectoral Clusters
Research support.
- Funding Opportunities + Grants
- Working Papers
- Career Opportunities
- Undergraduate Study Abroad Programs
- Request a Brochure
- Find a Program

Research Methods Cluster
Empirical research requires good data to complement statistical rigor in providing answers to global poverty questions. Poor data quality can lead to biases in causal inference, lower the probability of detecting the true effect of a program, and limit the generalizability of findings to other contexts. Without good quality data, policy decisions based on such evidence may be misguided, yet economists have little methodological research to inform the data generating process.
Evidence to Policy
“For 20 years I thought my job was as a basic scientist. Publish papers and throw them over the wall for someone else to apply. I now realize that there's no one on the other side of the wall. Just a huge pile of papers that we've all thrown over.” Duncan Watts in a speech at IC2S2 2019
Contact us about the Global Poverty Research Lab
847.467.3714

2024 Environmental Performance Index: A Surprise Top Ranking, Global Biodiversity Commitment Tested
The Baltic nation of Estonia is No. 1 in the 2024 rankings, while Denmark, one of the top ranked countries in the 2022 EPI dropped to 10 th place, highlighting the challenges of reducing emissions in hard-to-decarbonize industries. Meanwhile, “paper parks” are proving a global challenge to international biodiversity commitments.
Listen to Article
In 2022, at the UN Biodiversity Conference, COP 15, in Montreal over 190 countries made what has been called “the biggest conservation commitment the world has ever seen.” The Kunming-Montreal Global Biodiversity Framework called for the effective protection and management of 30% of the world’s terrestrial, inland water, and coastal and marine areas by the year 2030 — commonly known as the 30x30 target. While there has been progress toward reaching this ambitious goal of protecting 30% of land and seas on paper, just ahead of World Environment Day, the 2024 Environmental Performance Index (EPI) , an analysis by Yale researchers that provides a data-driven summary of the state of sustainability around the world, shows that in many cases such protections have failed to halt ecosystem loss or curtail environmentally destructive practices.
A new metric that assesses how well countries are protecting important ecosystems indicated that while nations have made progress in protecting land and seas, many of these areas are “paper parks” where commercial activities such as mining and trawling continue to occur — sometimes at a higher rate than in non-protected areas. The EPI analyses show that in 23 countries, more than 10% of the land protected is covered by croplands and buildings, and in 35 countries there is more fishing activity inside marine protected areas than outside.
“Protected areas are failing to achieve their goals in different ways,” said Sebastián Block Munguía, a postdoctoral associate with the Yale Center for Environmental Law and Policy (YCELP) and the lead author of the report. “In Europe, destructive fishing is allowed inside marine protected areas, and a large fraction of the area protected in land is covered by croplands, not natural ecosystems. In many developing countries, even when destructive activities are not allowed in protected areas, shortages of funding and personnel make it difficult to enforce rules.”
The 2024 EPI, published by the Yale Center for Environmental Law and Policy and Columbia University’s Center for International Earth Science Information Network ranks 180 countries based on 58 performance indicators to track progress on mitigating climate change, promoting environmental health, and safeguarding ecosystem vitality. The data evaluates efforts by the nations to reach U.N. sustainability goals, the 2015 Paris Climate Change Agreement, as well as the Kunming-Montreal Global Biodiversity Framework. The data for the index underlying different indicators come from a variety of academic institutions and international organizations and cover different periods. Protected area coverage indicators are based on data from March 2024, while greenhouse emissions data are from 2022.
Estonia has decreased its GHG emissions by 59% compared to 1990. The energy sector will be the biggest contributor in reducing emissions in the coming years as we have an aim to produce 100% of our electricity consumption from renewables by 2030.”
The index found that many countries that were leading in sustainability goals have fallen behind or stalled, illustrating the challenges of reducing emissions in hard-to-decarbonize industries and resistant sectors such as agriculture. In several countries, recent drops in agricultural greenhouse gas emissions (GHG) have been the result of external circumstances, not policy. For example, in Albania, supply chain disruptions led to more expensive animal feed that resulted in a sharp reduction in cows and, consequentially, nitrous oxide and methane emissions.
Estonia leads this year’s rankings with a 40% drop in GHG emissions over the last decade, largely attributed to replacing dirty oil shale power plants with cleaner energy sources. The country is drafting a proposal to achieve by 2040 a CO2 neutral energy sector and a CO2 neutral public transport network in bigger cities.
“Estonia has decreased its GHG emissions by 59% compared to 1990. The energy sector will be the biggest contributor in reducing emissions in the coming years as we have an aim to produce 100% of our electricity consumption from renewables by 2030,” said Kristi Klaas, Estonia’s vice-minister for Green Transition. Klaas discussed some of the policies that led to the country's success as well as ongoing challenges, such as reducing emissions in the agriculture sector, at a webinar hosted by YCELP on June 3. Dr. Abdullah Ali Abdullah Al-Amri, chairman of the Environment Authority of Oman, also joined the webinar to discuss efforts aimed at protecting the county's multiple ecosystems with rare biodiversity and endangered species, such as the Arabian oryx, and subspecies, such as the Arabian leopard.

Subscribe to “YSE 3”
Biweekly, we highlight three news and research stories about the work we’re doing at Yale School of the Environment.
Denmark, the top ranked country in the 2022 EPI dropped to 10th place, as its pace of decarbonization slowed, highlighting that those early gains from implementing “low-hanging-fruit policies, such as switching to electricity generation from coal to natural gas and expanding renewable power generation are themselves insufficient,” the index notes. Emissions in the world’s largest economies such as the U.S. (which is ranked 34th) are falling too slowly or still rising — such as in China, Russia, and India, which is ranked 176th.
Over the last decade only five countries — Estonia, Finland, Greece, Timor-Leste, and the United Kingdom — have cut their GHG emissions over the last decade at the rate needed to reach net zero by 2050. Vietnam and other developing countries in Southeast and Southern Asia — such as Pakistan, Laos, Myanmar, and Bangladesh — are ranked the lowest, indicating the urgency of international cooperation to help provide a path for struggling nations to achieve sustainability.
“The 2024 Environmental Performance Index highlights a range of critical sustainability challenges from climate change to biodiversity loss and beyond — and reveals trends suggesting that countries across the world need to redouble their efforts to protect critical ecosystems and the vitality of our planet,” said Daniel Esty, Hillhouse Professor of Environmental Law and Policy and director of YCELP.
- Daniel C. Esty
- Sebastián Block Munguía
- Ecosystem Management and Conservation
- Environmental Policy Analysis
Media Contact

Fran Silverman
Associate Director of Communications
Research in the News

Climate Change Threatens Resilience of Sri Lankan Rainforests

An Inside Look at Beech Leaf Disease

Achieving Sustainable Urban Growth on a Global Scale
Connect with us.
- Request Information
- Register for Events

File(s) under embargo
until file(s) become available
Development of Innovative Hardwood Products
In response to the growing significance of wood as a sustainable resource and the challenges within the wood products industry, there is a pressing need for innovation and collaboration across sectors. This study underscores the importance of mapping the wood products industry to gain a comprehensive understanding of material flows, which is essential for educational and research endeavors. The findings aim to uncover new economic opportunities and advocate for sustainable resource management. To address the complexities of the wood products industry, we developed a Generic Map, including a version tailored for the U.S. hardwood sector. Moreover, Dive-in Chain Maps were introduced to elaborate on the main production chains: Sawmill (I), Veneer Mill (II), Reconstituted Wood Manufacturing (III), and Pulp and Paper Mill (IV).
The study suggests four strategies to augment the value of hardwood through production, design, material modification, and by-products management. We showcased some strategies through two case studies.
The first focuses on Cross-laminated Timber (CLT), demonstrating value addition to hardwood. We conducted a literature review on the availability of raw materials in the US region and evaluated their performance across various stages of laboratory testing. This was followed by evaluating the feasibility and environmental effects of utilizing yellow poplar for CLT production. Additionally, we compared the Life Cycle Analysis (LCA) outcomes of yellow poplar CLT with those of traditional softwood CLT. This comparison aims to provide further insights for developing future by-product management or end-of-life strategies.
The second case study examines thermal modification, proposing an innovative method for efficient thermal treatment and employing an Artificial Neural Network (ANN) model to analyze the correlation between temperature, duration, and color change. We also compared the physical and mechanical properties of surface thermally treated samples to those of traditionally treated ones, discussing how different thermal treatments affect material properties.
Our findings illuminate the path for effective material flow and utilization, unveiling avenues for innovation and the creation of high-value products. Furthermore, the study provides strategies for waste minimization and informed end-of-life decision-making, thereby enhancing circularity and sustainability in the wood products industry.
Degree Type
- Doctor of Philosophy
- Forestry and Natural Resources
Campus location
- West Lafayette
Advisor/Supervisor/Committee Chair
Additional committee member 2, additional committee member 3, additional committee member 4, usage metrics.
- Forestry sciences not elsewhere classified


IMAGES
VIDEO
COMMENTS
Explore the latest full-text research PDFs, articles, conference papers, preprints and more on LABORATORY MANAGEMENT. Find methods information, sources, references or conduct a literature review ...
Competent laboratory management and leadership are vital for delivering quality laboratory services and laboratories need leaders who can utilise their resources effectively in a variable healthcare environment. 2 These leadership ... Approval to conduct the study was received from the Human Research Ethics Committee, University of New England ...
QMS software helps to address quality assurance aspects of laboratory activities. such as control of documented information, control of externally provided products. and services, tracking of non ...
The major theme among these papers was the improvement of clinical quality standards lab practice and training in the laboratory (n = 8), followed by the improvement of problems in the reception area (n = 5), the improvement of TTP (n = 4), the management of preanalytical errors (n = 4), and the evaluation and evolution of quality indicators (n ...
Money is the basic requirement for starting and running any laboratory. The required capital for a laboratory can be acquired in the form of equity (self/private/public) or in the form of debt. There are various advantages and disadvantages of either of these sources. Readers will get a better understanding of these concepts in Chap. 10.
Six tools group leaders love. • AnyDesk is free software for accessing and controlling computers remotely. • Google's Apps Script automates actions across the Google application suite ...
Laboratory medicine has undergone a profound evolution in organizational, methodological, and cultural terms in recent decades [].From the organizational point of view, we are living in the era of consolidation, i.e., the formation of networks of consolidated laboratories with marked automation and integration of the various branches of laboratory medicine [].
pages. . $40.00. ISBN: 978-1936287451. Laboratory Management: Quality in Laboratory Diagnosis is one of a series of useful books that covers key areas of management in today's complex clinical laboratory. Laboratory Management is a concise review of certain responsibilities of laboratory leaders and how those leaders can best manage some of ...
There is a broad consensus on the importance and advisability of testing laboratories adopting a Quality Management System (QMS) to support their work, no matter they are industrial or research oriented. However, laboratories involved in R&D have specific difficulties to implement a QMS due to the peculiar nature of their activity. This paper analyzes the main challenges and difficulties found ...
Abstract. Laboratory information management systems belong to the class of application software intended for storage and management of information obtained in the course of the work of the ...
The quality plan was significantly associated with the effective provision of improved laboratory service with good implementation of a laboratory quality management system. Laboratories that revealed evidence of available quality plans and utilization of quality indicators were 4.69 and 4.72 times, more likely to contribute to the reliability ...
1. laboratory information intelligent management system settings. 3.5. Allocate and audit laboratory equipment First of all, the school should arrange special funds to purchase the daily wear and tear of laboratory equipment to meet the teaching needs of students and teachers.
Protocols in the academic life science laboratory are heavily reliant on the manual manipulation of tools, reagents and instruments by a host of research staff and students. In contrast to industrial and clinical laboratory environments, the usage of automation to augment or replace manual tasks is limited. Causes of this 'automation gap' are unique to academic research, with rigid short ...
In this paper, we describe the choice, the design and the application of a QMS, called ReOmicS (Research Environment management system for Omics Sciences), at the Molecular Biodiversity Laboratory ...
With the rapid development of computer technology, computer-aided technology is widely used in the management and maintenance of university laboratories. This paper first analyzes the current situation of university laboratory management and maintenance, and points out the importance of computer-aided technology to manage and maintain ...
Paper • The following article is Open access. Research on the Problems and Countermeasures of Laboratory Management in Colleges and Universities . Jiahua ... University laboratories are responsible for providing professional venues for practical teaching and scientific research, and the quality of their management can directly affect the ...
With the development of modern education, the country pays more attention to the discipline training of experimental practice in science education, and high school laboratories become a high ground for students' science education and comprehensive quality education, while the current domestic laboratory management basically remains in the traditional manual management mode, with problems such ...
Laboratory. Safety management mode. Research and practice. Laboratory is an important basis and material condition for running a university nowadays. With the continuous improvement of education level, laboratory has become an important part of the modern education system. The application of laboratory in education can effectively help students ...
Leadership and team management. Laboratory managers must lead and supervise a team of scientists, technicians and support staff. As a result, they must possess leadership skills to guide, motivate and support the team members. This includes assigning tasks, monitoring progress and fostering collaboration while resolving conflicts.
A common and long-established practice of leading management journals is that they require that authors make a theoretical contribution (Boer et al., 2015).Rabetino et al. (2020) note that such contributions are based on diverse ontological, epistemological, and methodological assumptions; embrace disparate conceptual approaches (behavioral, institutional, evolutionary, etc.); and seek to ...
Fundamental challenges to the quality of laboratory tests in less-resourced countries are: 1) the lack of a laboratory management infrastructure and quality management training curriculum that develops the competences of laboratory managers and quality coordinators; 2) lack of access to or knowledge of current international standards; and 3) an ...
Full paper . Quantifying Device Flexibility With Shapley Values in Demand Side Management . Ivo A. M. Varenhorst (University of Twente), Johann Hurink (University of Twente), Marco E. T. Gerards (University of Twente) Full Paper . PhyGICS - A Physics-informed Graph Neural Network-based Intelligent HVAC Controller for Open-plan Spaces
Our projects produce working papers and datasets and use a cluster framework. Clusters can either be built around sectors or geographic areas. ... Global Poverty Research Lab 601 University Place | Scott Hall | Evanston, IL 60208 Kellogg School of Management Northwestern University 2211 Campus Drive Evanston, IL 60208 847.491.3300 ...
Structured Ethics Appendix. Social science researchers engaged in primary data collection often consider a range of ethical issues during the planning of their research, but such considerations are rarely articulated upfront or in subsequent articles generated from the research. We believe that building explicit steps for this can lead to ...
The Global Poverty Research Lab hosts several events each year. Our signature events include: Development Rookiefest. Rookiefest features promising doctoral students in development economics presenting their job talk paper to an audience of faculty and scholars from the Chicagoland and greater Midwest area. This event is invite only.
Undergraduate Research Assistants . The Global Poverty Research Lab (GPRL) is hiring several undergraduate Research Assistants to help conduct a series of literature reviews at the intersection of entrepreneurship, gender, and/or economic development. Tasks will include one or more of the following: searching for literature, coding articles and extracting statistics, and data management and ...
Research Methods Cluster. Empirical research requires good data to complement statistical rigor in providing answers to global poverty questions. Poor data quality can lead to biases in causal inference, lower the probability of detecting the true effect of a program, and limit the generalizability of findings to other contexts. Without good ...
Abstract. The aim of this paper is to show the advantages of implementing a Quality Management System. (QMS) in a research laboratory in order to improve the management of risks specific to ...
Fellows. [email protected]. 203-436-4842. The newly released 2024 Environmental Performance Index (EPI), an analysis by Yale researchers that provides a data-driven summary of the state of sustainability around the world, finds that many countries that were leading in sustainability goals have fallen behind or stalled, illustrating the ...
In response to the growing significance of wood as a sustainable resource and the challenges within the wood products industry, there is a pressing need for innovation and collaboration across sectors. This study underscores the importance of mapping the wood products industry to gain a comprehensive understanding of material flows, which is essential for educational and research endeavors ...