

Acute diabetic neuropathy following improved glycaemic control: a case series and review
- Get Citation Alerts
- Download PDF
We present three cases of acute diabetic neuropathy and highlight a potentially underappreciated link between tightening of glycaemic control and acute neuropathies in patients with diabetes. Case 1: A 56-year-old male with poorly controlled type 2 diabetes (T2DM) was commenced on basal-bolus insulin. He presented 6 weeks later with a diffuse painful sensory neuropathy and postural hypotension. He was diagnosed with treatment-induced neuropathy (TIN, insulin neuritis) and obtained symptomatic relief from pregabalin. Case 2: A 67-year-old male with T2DM and chronic hyperglycaemia presented with left lower limb pain, weakness and weight loss shortly after achieving target glycaemia with oral anti-hyperglycaemics. Neurological examination and neuro-electrophysiological studies suggested diabetic lumbosacral radiculo-plexus neuropathy (DLPRN, diabetic amyotrophy). Pain and weakness resolved over time. Case 3: A 58-year-old male was admitted with blurred vision diplopia and complete ptosis of the right eye, with intact pupillary reflexes, shortly after intensification of glucose-lowering treatment with an SGLT2 inhibitor as adjunct to metformin. He was diagnosed with a pupil-sparing third nerve palsy secondary to diabetic mononeuritis which improved over time. While all three acute neuropathies have been previously well described, all are rare and require a high index of clinical suspicion as they are essentially a diagnosis of exclusion. Interestingly, all three of our cases are linked by the development of acute neuropathy following a significant improvement in glycaemic control. This phenomenon is well described in TIN, but not previously highlighted in other acute neuropathies.
- Learning points:
A link between acute tightening of glycaemic control and acute neuropathies has not been well described in literature.
Clinicians caring for patients with diabetes who develop otherwise unexplained neurologic symptoms following a tightening of glycaemic control should consider the possibility of an acute diabetic neuropathy.
Early recognition of these neuropathies can obviate the need for detailed and expensive investigations and allow for early institution of appropriate pain-relieving medications.
Diabetic neuropathy is the most prevalent chronic complication of diabetes mellitus (DM). Diabetic neuropathy most commonly presents as a distal sensorimotor neuropathy, where reduced or absent protective sensation in the lower limbs leads to an increased risk of foot ulcers and peripheral neuropathic pain is common. Diabetic neuropathy can also present as acute neuropathies, however, in this article we present three of the less common acute presentations of diabetic neuropathy and discuss appropriate investigations, treatment and prognosis. We also highlight an important and potentially underappreciated link between tightening of glycaemic control and acute diabetic neuropathies.
Case presentation
A 56-year-old man presented to the Emergency Department complaining of an 8-month history of fatigue, polyuria, polydipsia, blurred vision and weight loss. Physical examination was normal, with a BMI of 21 kg/m 2 . Laboratory testing revealed a HbA1c of 145 mmol/mol (15.4%), confirming a diagnosis of DM. Basal-bolus insulin therapy was commenced. Six weeks later the patient returned to the hospital complaining of chest, shoulder, arm and leg pain with associated paraesthesia. The pain was described as severe, and he reported that light touching of his skin was extremely painful. He described further weight loss of approximately 10 kg since the first visit. Glucose readings had improved on insulin, with readings between 5 and 7 mmol/L. HbA1c had fallen to 55 mmol/mol.
Investigation case 1
Endoscopic examination of the stomach and duodenum did not show significant pathology, and other routine investigations were unremarkable.
Treatment case 1
Treatment-induced neuropathy (‘insulin neuritis’) was suspected, and pregabalin was prescribed.
Outcome and follow-up case 1
Weight increased and symptoms resolved on follow-up.
A 67-year-old man presented with a 1-year history of fatigue, polyuria, polydipsia, blurred vision and 7 kg weight loss. Physical examination revealed a BMI of 28 kg/m 2 and reduced peripheral vibration and monofilament sensation on both feet. Random blood glucose was elevated at 34 mmol/L and HbA1c was 140 mmol/mol (15.0%), confirming a diagnosis of DM. Basal-bolus insulin was instituted and resulted in resolution of hyperglycaemia and osmotic symptoms. Insulin was subsequently discontinued, and he was discharged on metformin 1000 mg twice a day and gliclazide modified release of 30 mg a day. Three months later, glucose readings were consistently below 6 mmol/L and without documented hypoglycaemia. The patient then complained of a new symptom of severe left-sided groin pain radiating down his left leg. There was associated left leg weakness, such that he walked with a stick. He had lost a further 3 kg in weight despite good glycaemic control. Examination revealed weakness of left hip flexion and left knee extension, an absent left patellar reflex and an atrophied left quadriceps. HbA1c checked 2 months later returned at 42 mmol/mol, indicating excellent glycaemic control.
Investigation case 2
Nerve conduction studies and electromyography (EMG) were performed. Results were suggestive of a left-sided lumbosacral plexopathy most severely affecting the quadriceps muscles, supporting the clinical diagnosis of DLRPN (diabetic amyotrophy).
Treatment case 2
Pregabalin was instituted, and physiotherapy was advised.
Outcome and follow-up case 2
Over time his pain resolved and he discontinued the pregabalin. One year later he continued to walk with a stick due to ongoing leg weakness, but at 2 years following initial presentation, he could walk unaided. Weight had increased by 10 kg since the initial presentation with DLRPN.
A 58-year-old man complained of double vision on a background of type 2 diabetes diagnosed 2 years prior to presentation. Three months prior to the current presentation, as a result of poorly controlled diabetes, his general practitioner had commenced dapagliflozin, and the patient had increased his exercise levels and adherence to dietary advice. Glucose readings in the weeks prior to his presentation had fallen into the normal range and HbA1c was 46 mmol/mol, indicating excellent glycaemic control. On examination, there was an almost complete ptosis of the right eyelid. Pupillary reflexes were intact. The right eye was fixed in the ‘down and out’ position indicating an oculomotor nerve palsy with intact cranial nerves IV and VI.
Investigation case 3
As investigations to rule out alternate pathology including MRI brain, anti-muscarinic and anti-acetylcholine receptor antibodies, anti-nuclear factor, anti-neutrophil cytoplasmic antibodies and erythrocyte sedimentation rate were normal, a diagnosis of a pupil-sparing third nerve palsy secondary to diabetic mononeuritis was made.
Treatment case 3
He was continued on the aforementioned medications and advised for close monitoring of blood sugars.
Outcome and follow-up case 3
Three months later the patient’s glycaemic control remained satisfactory, and his ophthalmoplegia had resolved.
- Discussion of 3 cases
The most common presentation of diabetic neuropathy, DSN, is a diffuse and nerve-length-dependent neuropathy. The pathophysiology of DSN is multifactorial but reasonably well understood, and the link between poor glycaemic control and DSN is well established. Optimisation of glycaemic control significantly reduces the relative risk of DSN in T1DM ( 1 ) and in T2DM ( 2 ). Symptoms of DSN are typically chronic and irreversible, but are frequently mild to the extent that some patients may present without symptoms – that is, the neuropathy is discovered on clinical examination.
The acute neuropathies experienced by the three patients we describe are an altogether different proposition: rare, acute, severe but reversible, not occurring as a consequence of chronic hyperglycaemia and poorly understood.
In this paper we present three cases of acute diabetic neuropathy – treatment-induced neuropathy (insulin neuritis), diabetic lumbosacral radiculoplexus neuropathy (diabetic amyotrophy) and a pupil sparing oculomotor nerve palsy. While all three acute neuropathies have been well described in the past, all are rare and hence require a high index of clinical suspicion, as diagnosis rests largely on recognizing the symptoms and signs in the appropriate context and excluding alternative pathology.
All three of our cases are linked by the acute development of neuropathy in patients who had achieved a significant improvement in their glycaemic control prior to the development of the acute neuropathy. In the first case this improved control was achieved with insulin, in the second case with insulin followed by metformin and a sulphonylurea and in the third case with an SGLT-2 inhibitor and lifestyle changes added to metformin.
An association between improved glycaemic control and acute worsening of diabetic retinopathy is well described ( 1 ), but a corresponding link between tight glycaemic control and acute neuropathies less well so. A link between acute lowering of blood glucose and acute neuropathies has been best described in cases of TIN and is reflected in the naming of the condition, but an association between tightening of glycaemic control and onset of DLPRN has not been previously highlighted. It is difficult to adequately scrutinize prior case series of DLRPN as the publications are typically from the era when assessment of glycaemic control rested on detection of glycosuria. When considering a potential link between acute lowering of blood glucose and DLRPN, it is interesting to review the three seminal cases described in detail by Casey et al. ( 3 ), however. In one patient the symptoms developed shortly after starting insulin treatment – a point not highlighted by the authors at the time as being relevant to the presentation. A second patient had insulin-treated diabetes and developed symptoms shortly after an admission to hospital with chest pain – again, in view of our contention that an acute tightening of glycaemic control might trigger an onset of DLRPN, it is tempting to speculate that the hospital admission resulted in an acute improvement in glycaemic control in this patient.
Why might an acute lowering of blood glucose cause an acute neuropathy? A small number of research papers have attempted to answer this question, largely prompted by the association between acute neuropathy and cases of TIN. Ohshima et al. , in a rat model, found reduced neural blood flow during 3 h of hypoglycaemia induced by the administration of insulin injections ( 4 , 5 ). Repeated induction of hypoglycaemia resulted in endothelial swelling of endoneural microvessels on histological examination 24 h following the hypoglycaemic events. Other authors have suggested that apoptosis of axons can occur following sudden glucose deprivation: using an in vitro model of dissociated rat dorsal root ganglions (DRG), Honma et al. found that neurons maintained in hypoglycaemic medium were less able to withstand exposure to acute hypoxia than neurons maintained in hyperglycaemic medium ( 6 ). Hypoxic conditions resulted in apoptosis of DRG neurons when maintained in hypoglycaemic medium.
None of the three patients we describe had hypoglycaemia per se , nor was documented hypoglycaemia present in any of the six patients in the largest case series of TIN ( 7 ). It could be argued, however, in a patient with prolonged severe hyperglycaemia, that a rapid drop to normal glucose levels could result in a relative hypoglycaemia – with osmotic shifts causing the same damage to the endoneural- microvessels as those described in the experimental conditions described in animal models.
The findings in the research papers referenced previously, the clear clinical correlation between improved glycaemic control and TIN in case reports and the clinical course in the patients we describe all lead us to postulate a link between a relative hypoglycemia – caused by rapid reduction in glucose levels in patients with chronic hyperglycaemia – and the development of acute diabetic neuropathies. These neuropathies could be considered, therefore, to be iatrogenic, and their onset may prompt consideration of a period of ‘permissive hyperglycaemia’ to improve symptomatology. In the first case of TIN described by Caravati in 1933 ( 8 ), the patient’s pain was refractory to analgesics and sedatives, but resolved within 3 days of stopping insulin – attempts to reintroduce insulin were met with similar levels of pain. Gibbons et al . propose limiting the fall in HbA1c to <2% over a 3-month period to prevent TIN ( 9 ), and we propose that this advice could apply equally to the other acute neuropathies we describe – but whether allowing permissive hyperglycaemia is the correct approach to adopt when patients develop symptoms remains a matter for debate.
Clinicians caring for patients with diabetes who develop otherwise unexplained neurologic symptoms following a tightening of glycaemic control should consider the possibility of an acute diabetic neuropathy. Early recognition of these neuropathies can obviate the need for detailed and expensive investigations, allow for early institution of appropriate pain relieving medications and could prompt consideration of a period of permissive hyperglycaemia.
- Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
- Patient consent
Written informed consent has been obtained from the patients for publication of the submitted article.
- Author contribution statement
N Siddique, R Durcan, S Smyth, T Kyaw Tun, S Sreenan and J H McDermott were involved in the diagnosis and management of all cases in this case series and they have contributed to writing and reviewing this manuscript.
Diabetes Control and Complications Trial Research Group , Nathan DM , Genuth S , Lachin J , Cleary P , Crofford O , Davis M , Rand L , Siebert C . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus . New England Journal of Medicine 1993 329 977 – 986 . ( https://doi.org/10.1056/NEJM199309303291401 )
- Search Google Scholar
- Export Citation
Ismail-Beigi F , Craven T , Banerji MA , Basile J , Calles J , Cohen RM , Cuddihy R , Cushman WC , Genuth S , Grimm RH , et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the accord randomised trial . Lancet 2010 376 419 – 430 . ( https://doi.org/10.1016/S0140-6736(10)60576-4 )
Casey EB , Harrison MJ . Diabetic amyotrophy: a follow up study . BMJ 1972 1 656 – 659 . ( https://doi.org/10.1136/bmj.1.5801.656 )
Ohshima J , Nukada H . Hypoglycaemic neuropathy: microvascular changes due to recurrent hypoglycaemic episodes in rat sciatic nerve . Brain Research 2002 947 84 – 89 . ( https://doi.org/10.1016/s0006-8993(02)02910-4 )
Ohshima J , Nukada H . Experimental hypoglycemic neuropathy in rat: nerve blood flow and nerve conduction study St. Marianna . Medical Journal 2006 34 85 91 .
Honma H , Podratz JL , Windebank AJ . Acute glucose deprivation leads to apoptosis in a cell model of acute diabetic neuropathy . Journal of the Peripheral Nervous System 2003 8 65 – 74 . ( https://doi.org/10.1046/j.1529-8027.2003.03009.x )
Dabby R , Sadeh M , Lampl Y , Gilad R , Watemberg N . Acute painful neuropathy induced by rapid correction of serum glucose levels in diabetic patients . Biomedicine and Pharmacotherapy 2009 63 707 – 709 . ( https://doi.org/10.1016/j.biopha.2008.08.011 )
Caravati C . Insulin neuritis: a case report . Virginia Medical Mon 1933 59 745 – 746 .
Gibbons CH , Freeman R . Treatment-induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes . Brain 2015 138 43 – 52 . ( https://doi.org/10.1093/brain/awu307 )
- Diabetes mellitus type 2
- Hyperglycaemia
- Hypotension
- Muscle atrophy
- Myasthaenia
- Ophthalmoplegia
- Paraesthesia
- T-reflex (absent)
- Vision - blurred
- Weight loss
- Electromyography
- Glucose (blood)
- Haemoglobin A1c
- Nerve conduction study
- Dapagliflozin
- SGLT2 inhibitors
- Sulphonylureas
- Error in diagnosis/pitfalls and caveats
Endocrinology, Diabetes and Metabolism Case Reports is committed to supporting researchers in demonstrating the impact of their articles published in the journal.
As an open-access title, EDMCR case reports are immediately available to read on publication, without restriction. The two types of article metrics we measure are (i) more traditional full-text views and pdf downloads, and (ii) Altmetric data, which shows the wider impact of articles in a range of non-traditional sources, such as social media.
More information is on the Reasons to publish page.
- Share on facebook Share on linkedin Share on twitter
Related Articles
Article information.
- PubMed Citation
- Article by N Siddique
- Article by R Durcan
- Article by S Smyth
- Article by T Kyaw Tun
- Article by S Sreenan
- Article by J H McDermott
- Similar articles in PubMed
Google Scholar
Online ISSN: 2052-0573
Author Information
Author Guidelines
Open Access Policy
Permissions
Librarian Information
General Information
Read and Publish Deal
Contact the journal
Advertising

Strengthening biomedical communities to advance science and health
Accessibility
Privacy and Cookies
Terms and Conditions
About Bioscientifica
All Bioscientifica Journals
Publishing Alliances
Bioscientifica Ltd | Starling House | 1600 Bristol Parkway North | Bristol BS34 8YU | UK
Bioscientifica Ltd | Registered in England no 3190519
© 2024 Bioscientifica Ltd
- [66.249.64.20|185.66.14.236]
- 185.66.14.236
Character limit 500 /500
CASE REPORT article
Case report: successful outcome for refractory diabetic peripheral neuropathy in patients with ultrasound-guided injection treatment.

- Department of Neurology, Affiliated Hospital of Guizhou Medical University, Guiyang, China
Diabetic peripheral neuropathy is the most prevalent chronic complication of diabetes and is based on sensory and autonomic nerve symptoms. Generally, intensive glucose control and nerve nourishment are the main treatments. However, it is difficult to improve the symptoms for some patients; such cases are defined as refractory diabetic peripheral neuropathy (RDPN). In this paper, we present five patients treated with saline and mecobalamin by ultrasound-guided injection. The Visual Analog Scale and Toronto Clinical Scoring System were used to evaluate the symptoms, and the neuro-ultrasound scoring system and electrophysiological severity scale were evaluated by ultrasound and electrophysiological examination. In brief, ultrasound-guided hydrodissection may be a safe way to treat RDPN.
Introduction
Hydrodissection was first proposed in 1997. It is used in treating entrapment neuropathy and inflammatory peripheral neuropathy and plays an important role in separating adjacent tissue and protecting nerves and blood vessels via the injection of saline or other agents to build a non-existent surgical plane ( 1 , 2 ). Previously, Smith has studied the treatment of carpal tunnel syndrome by hydrodissection ( 3 ).Without a unified standard, a visual needle is usually used to form a liquid clearance around nerves by ultrasound-guided injection with saline or another liquid (e.g., mecobalamin) to dissociate the nerves from their surroundings.
Currently, the treatment options for compressive neuropathy include: (1) medical treatment with oral drugs to provide nutrition to the nerves and blocking therapy ( 4 ) and (2) surgical treatment, after which symptoms may recur. Surgical procedures may damage the intraneural microcirculation, leading to pathological changes corresponding to chronic compressive neuropathy ( 5 ). However, hydrodissection minimizes the risk of nerve injury with its advantages of percutaneous punctures and blunt dissection. Ultrasound shows changes in the morphology, imaging, and echo of nerves, which could suddenly be attenuated at compressive sites, resulting in decreased echo intensity. In fact, honeycomb-like structures disappear on the axis, and the cross-sectional areas of proximal nerves increase due to edema. Therefore, ultrasound-guided neural hydrodissection represents a promising therapeutic strategy to treat various peripheral neuropathies.
Over the past decades, various investigations have been performed on neuropathies with mild to moderate, recurrent, and even severe nerve scars to explore the therapeutic effects of hydrodissection ( 6 – 9 ).
Hydrodissection has therapeutic effects on neural adhesion caused by inflammation, such as saphenous nerve ache. Fader has cured an athlete with compression of the right sural nerve on the right lateral foot and ankle using saline by hydrodissection to separate the sural nerve from the surrounding muscles; the athlete returned to participation in competitive sports without disability ( 10 ).
Based on 10 years of clinical experience, in this study, we demonstrate that ultrasound-guided neural hydrodissection has excellent therapeutic effects on compressive neuropathy and inflammatory adhesion and explore the therapeutic efficacy of ultrasound-guided injection in five patients with refractory peripheral neuropathy.
Patients and Methods
Five patients treated with intramuscular or oral mecobalamin were recruited. They were diagnosed with refractory diabetic peripheral neuropathy (RDPN) confirmed by electromyography (EMG). All patients, who had not experienced satisfactory effects after glycemic control and neurotrophic drug treatment, experienced symptoms of RDPN, such as pain, numbness, and sensory changes in the limb extremities. The exclusion criteria were cervical radiculopathy, history of trauma, immune dysfunction, severe cardiopulmonary insufficiency, and obvious myasthenia.
This study was approved by the institutional review board of the Affiliated Hospital of Guizhou Medical University (Guiyang, China), and written informed consent was obtained from all participants.
Case 1 : A 60-year-old female with weak dorsiflexion of the feet and numbness for one year, occasionally with tingling, type 2 diabetes for five years, a maximum fasting blood glucose level of 15 mmol/L, and glycosylated hemoglobin of 6.9%. The diagnosis of DPN was based on numbness and tingling, slow conduction velocity (40.0 m/s) in the tibial nerves, and a waveform that vanished in the bilateral peroneal nerves as assessed by EMG examination. In the past year, the patient had experienced paresthesia and dyskinesia of the lower limbs. After treatment with insulin, her symptoms were somewhat relieved; subsequently, her glycemia was controlled with oral hypoglycemic drugs. The extensor digitorum brevis (EDB) muscle had significantly atrophied by 0.32 cm 2 ( Figure 1A ), and the strength of the extensor of the thumb was grade 0/5 with steppage gait ( Table 1 ).
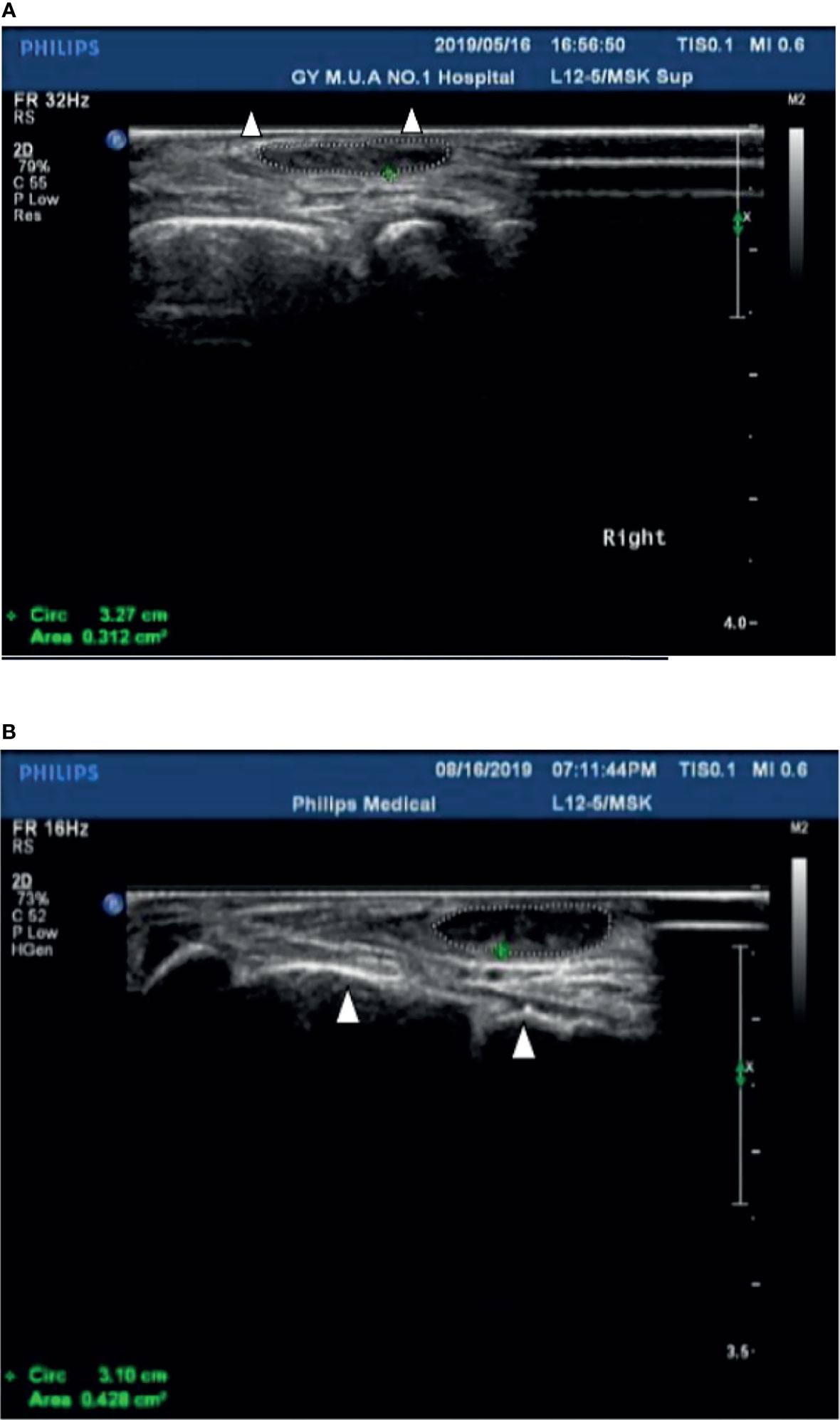
Figure 1 (A) The cross-sectional area of the extensor digitorum brevis of patient 1 before treatment was 0.312 cm 2 (Circled by a dotted line); The Triangle refers to the fourth and fifth metatarsal bones. (B) The cross-sectional area of the extensor digitorum brevis increased to 0.428 cm 2 (Circled by a dotted line) after two courses of treatment; The Triangle refers to the fourth and fifth metatarsal bones.
Table 1 Clinical symptoms and signs of the five patients.
After hospitalization, mecobalamin was started via ultrasound-guided injection of an appropriate dose (1 mg in 10 ml 0.9% NaCl). After therapy, the patient improved gradually. After one course of treatment, the foot strength returned to grade 2/5, with the calf circumference increasing from 25 to 28 cm (left) and 26 to 24 cm (right). The EMG results showed an insignificant change of 37.9 m/s in the bilateral peroneal nerve conduction velocity ( Table 2 ). The cross-sectional area of the EDB was 0.43 cm 2 after two courses of treatment ( Figure 1B ). The other changes are shown in Tables 3 and 4 .
Table 2 Electromyogram examination results in patients before and after therapy.
Table 3 Evaluation of symptoms, ultrasound and cross-sectional area in patients.
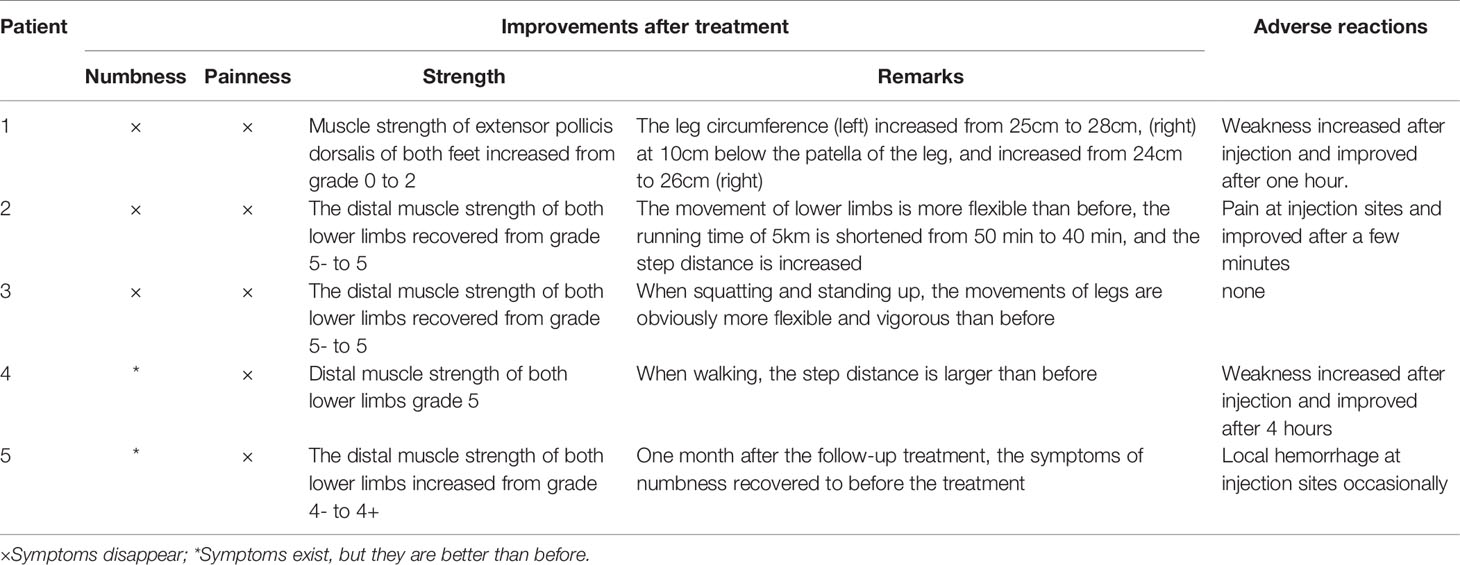
Table 4 Clinical manifestation changes after treatment.
Case 2 : A 56-year-old male with type 2 diabetes for six years accompanied by renal function impairment. The diagnosis of DPN was based on characteristic sock-like numbness, inflexible movement of the lower limbs, no waveform in the tibial nerves, neurogenic damage to the left anterior tibial muscle upon electromyographic examination, and exclusion of other peripheral neuropathies. He had experienced paresthesia of the distal extremities within the previous two years. After treatment with mecobalamin and epalrestat based on strict control of blood glucose, these symptoms gradually worsened, with frequent nocturnal tingling affecting sleep. The foot strength was grade –5/5 with hypalgesia ( Table 1 ).
After admission, mecobalamin was injected via ultrasound guidance at an appropriate dosage at the malleolus medialis of the posterior tibial nerves. Fortunately, the patient improved gradually after the therapy. After two courses, the foot strength returned to grade 5/5, the legs became more flexible, the walking distance increased, and there was no numbness or pain. An EMG examination showed no significant change in the bilateral peroneal nerves ( Table 2 ). The other changes are shown in Tables 3 and 4 .
Case 3 : A 52-year-old male with type 2 diabetes mellitus for 11 years and retinopathy stage III diagnosed with DPN due to slow conduction velocity of the tibial and peroneal nerves and no tibial nerve waveform found with EMG. Mecobalamin and vitamin B1 were taken orally for nearly one year, but areflexia, distal numbness of the limbs, and weakness had occurred when squatting within the last six months ( Table 1 ).
After two courses of treatment, the neurological examination showed that the muscle strength of the lower extremities was grade 5/5. Flexibility and numbness of the lower limbs improved, but no EMG results were obtained after treatment ( Table 2 ). Moreover, after ultrasound-guided injection of saline and mecobalamin, the symptom and ultrasound image scores were improved ( Table 3 ), and it was found that the EDB and muscles of the first interstitium (MIL) were larger than those before treatment ( Table 3 ).
Case 4 : A 76-year-old male with a history of type 2 diabetes for over 10 years diagnosed with DPN for 4 years. He had paresthesia and weakness in his lower limbs that was not relieved after treatment with mecobalamin, vitamin B1, and Maizhiling for more than two years. The EMG bilateral fibular motor nerve conduction velocity was 27.9 m/s (right, 52% ↓) and 36.2 m/s (left). The EMG showed that the conduction velocity of the bilateral tibial nerve waveforms had disappeared. The fibular sensory nerve values were 77.1 m/s (right) and 60.5 m/s (left) ( Table 2 ). After one course of therapy, the patient’s walking distance increased, though with persistent numbness. The muscle strength of the lower limbs was not ameliorated at grade –5/5. The other relevant change scores, muscle cross-sectional areas, and thickness values are shown in Table 3 .
Case 5 : A 44-year-old male with type 2 diabetes for 13 years, renal failure and retinal complications, persistent numbness of the distal limbs, paresthesia, and weakness of the lower limbs after walking 100 m for nearly 5 years as a result of poor glycemic control, and 11.9% glycosylated hemoglobin. The patient had been treated with painkillers during this period. The waveform of the bilateral peroneal motor nerves had disappeared, and the conduction velocity of the tibial motor nerves was 31.8 m/s (24% ↓). Moreover, the waveforms of the bilateral tibial and peroneal sensory nerves were not revealed by EMG examination ( Table 2 ).
After a course of treatment, the distal muscle strength of the lower limbs increased from grade –4/5 to +4/5, and the numbness was relieved gradually. Nevertheless, one month after follow-up, the symptoms appeared again. There was little change in the EMG results ( Table 2 ). The other improvement indicators are shown in Tables 3 and 4 .
Therapeutic Procedure
In our experience, the fibular head of the tibial nerves, the section at the start of the peroneal nerves, and the medial malleolus of the posterior tibial nerves should be chosen as treatment injection sites after ensuring there are no wounds on local skin sites. Herein, these injection sites were sterilized with povidone iodine, and the ultrasonic probe was covered with gloves after having been sterilized. Two syringes were prepared before injection (first syringe: 10 ml of 0.9% sodium chloride injection; second syringe: 0.5 mg of mecobalamin injection + 9 ml of 0.9% sodium chloride injection to prepare a 10-ml mixed solution with 1.5–2 ml for each injection site). The posterior tibial nerve was displayed with a clear neural boundary, an internal echo, and a honeycomb-like structure by high-resolution ultrasound ( Figure 2A ). The puncture needle was as perpendicular to the skin as possible, entering along the puncture point at a distance about 0.5 cm from the center of the probe. About 45°–60° was required between the needle and sonographic probe ( Figure 2B ). Real-time observation of the position of the puncture needle tip during injection was performed when the needle was connected to the first syringe, and 3–5 ml of saline was injected around the nerve to separate the nerve water from the surrounding tissue by ultrasound-guided injection. At the end of the hydrodissection, a hypoechoic ring could be observed on the transverse axis ( Figure 2C ). Then, the needle tip was kept still and substituted with the second syringe to inject 1.5–2 ml of solution into the target nerve at each injection point ( Figure 2D ). The surrounding liquid around the nerve was shown clearly on the longitudinal axis ( Figure 2E ), after which the needle was removed.
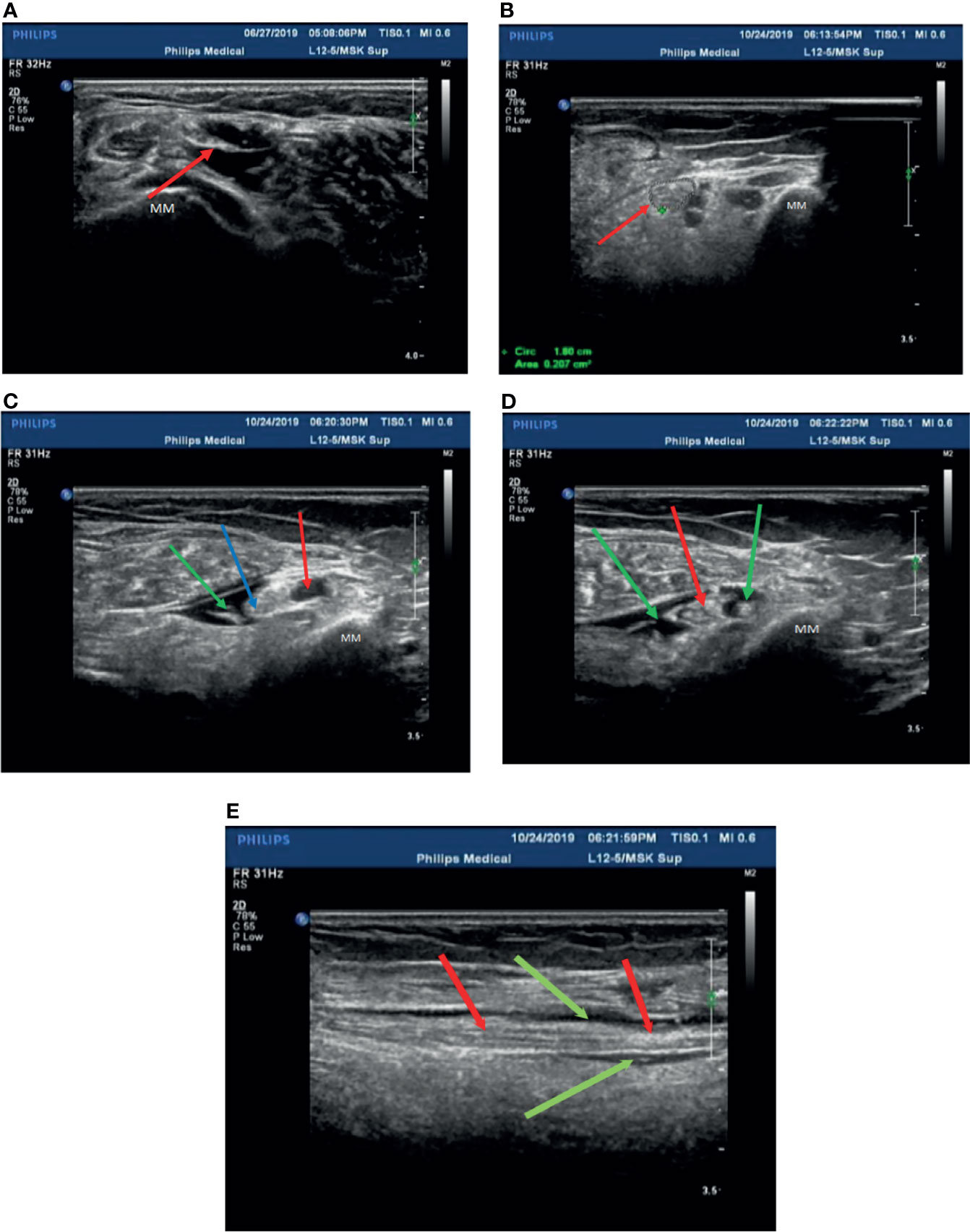
Figure 2 Patient 2 was treated with mecobalamin by ultrasound-guided hydrodissection injection at the malleolus medialis of the posterior tibial nerves (A–E) . (A) A normal posterior tibial nerve is shown as a clear neural boundary (cribriform network), normal internal echo (the posterior tibial nerve is below the red arrowhead). (B) The posterior tibial nerve of patient 2 was thickened with a fuzzy boundary in the medial malleolus (the red arrowhead indicates the posterior tibial nerve). (C) Injection with 3 ml saline at the medial malleolus of the posterior tibial nerve (the posterior tibial nerve at the red arrowhead, the point of a needle at the blue arrowhead, and the green arrowhead points to saline after injection). (D) The posterior tibial nerve was injected with mecobalamin at the medial malleolus after injection in the transverse section, and it can be seen that the solution is evenly wrapped around the posterior tibial nerve (red arrowhead pointed to the posterior tibial nerve, saline + mecobalamin at the green arrowhead). (E) The medial malleolus of the posterior tibial nerve after injection with mecobalamin in the longitudinal section, it can be seen that the fluid evenly covers the posterior tibial nerve (the red arrowhead points to the posterior tibial nerve, and saline + mecobalamin at the green arrowhead). MM; Medial malleolus.
It was essential to observe whether the patients had side effects in their limbs, such as numbness, weakness, and chills, during the injection process. Injection took place once every other day into the bilateral limbs, i.e., three times per week for four weeks per treatment course. Evaluation of the effect occurred after the treatment course. The injection operation was carried out by an experienced clinician and a sonographer.
Assessment of Symptoms
The symptoms were evaluated with objectively measurable scores, including the VAS and Toronto Clinical Scoring System (TCSS).
The VAS is a scale to evaluate pain before and after therapy. The TCSS is a grading system for assessing the severity of DPN with a score from 1 to 19 (mild to severe) ( 11 ).
Electrophysiological Examination
Each patient was diagnosed with DPN following an electrophysiological examination, wherein nerves were assessed with an electrophysiological severity scale comprising motor and sensory nerve conduction velocity detection. These tests were performed by a single physician using a Nicolet EDX EMG system (Madison, Wisconsin, U.S.A.). The measured nerves included the median nerve, ulnar nerve, tibial nerve, and common peroneal nerve. The sural nerve was not measured as it is easily affected by many factors. The motor conduction velocity of the median nerve and sensory conduction velocity of the ulnar and sural nerves, action potential amplitudes of all three nerves, and distal latency period of the median nerve in the wrist segment were determined.
The results were measured against the normal values obtained from the EMG room of Beijing Peking Union Medical College Hospital in China ( 12 ). Judging criteria: the examined nerve did not elicit potential (the statistical data were 0); the conduction velocity was over 20% lower than the normal low limit; the latency of the distal motor nerve exceeded 130% of the normal high limit; the amplitude of motor or sensory potential was decreased; the latency of the F wave exceeded 130% of the normal high limit; and the occurrence rate of the evoked wave was less than 50%.
Sensory nerve conduction measurement: The amplitude of the action potential of the sensory nerve decreases (particularly at the extremities of the lower limbs) although the conduction velocity is relatively normal, consistent with the characteristics of length-dependent axonal peripheral neuropathy. When there is compressive peripheral neuropathy, the sensory nerve conduction velocity at the embedded sites may be slowed. Among patients with autonomic nervous symptoms, sensory conduction can be normal. Thus, sensory nerve conduction measurement is helpful for the detection of subclinical lesions.
Measurement of motor nerve conduction: The latency and nerve conduction velocities of distal movement are usually normal in the early stage, and there is no partial conduction block of motor nerves or abnormal wave form dispersion. In later stages, the amplitude of the compound muscle action potential decreases, and the conduction velocity slows down slightly, similar to mononeuropathy or lumbosacral plexus disease. In patients with embedded peripheral neuropathy, the conduction velocity at the embedded sites can be significantly slowed.
Sonographic Examinations
All nerve image examinations were performed using ultrasound guidance and evaluated by measuring the size of the morphologic changes of the nerves using an ultrasound system (Philips Ulrasound iU22, Holland) with a 5- to 12-MHz linear array transducer. The subjects lay on the examination bed and kept their lower limbs relaxed in the correct position according to different nerves. The cross-sectional areas of the tibial nerves were measured at the start of the nerves, the fibula head, and the medial malleolus of the posterior tibial nerves. Three averages of the cross-sectional area and three measurements for each site were used. The neuro-ultrasound scoring system included four aspects of the target nerves with ultrasound regarding the neuroimaging clarity of the cross-sectional area, internal echo, and the presence or absence of nerve entrapment (DCEC), of which the abbreviated form of the DCEC scoring system was used for each patient. The detailed scoring rules are shown in Table 5 ( 13 ). Meanwhile, the extensor digitorum brevis (EDB) muscle and MIL were measured by ultrasound ( 14 ). The participants kept the ankle joint relaxed, and the cross-sectional area at the EDB midpoint and thickness of the MIL were determined.
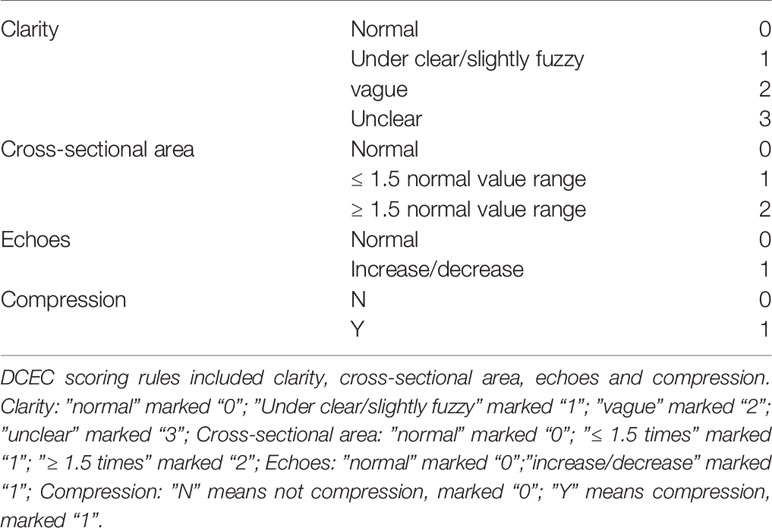
Table 5 DCEC scoring rules DCEC scoring rules.
All patients were injected using ultrasound guidance. Before injection, the patients were examined for temperature, pinprick sensation (small fiber function), and vibration sensation using a 128-Hz tuning fork (for large fiber function). They were assessed with respect to neurophysiological changes, neural morphology, leg circumference, the EDB and MIL, and muscle strength in the bilateral limbs at the time of each injection.
The patients were evaluated at the pre-injection timepoint as well as after one or two injection courses. The mean age in this study was 57.6 ± 11.87 years, and the mean duration of the participants with DPN was 9.0 ± 3.39 years at the pre-injection baseline ( Table 6 ). All five patients showed numbness and weakness in the lower limbs. A positive tendon reflex was found in three patients, and two patients had paroxysmal tingling pain in the lower limbs. However, only one patient had distal muscle atrophy of the lower extremities ( Table 1 ). There were some slight adverse reactions after the injection, such as short-term limb weakness, bleeding, and pain at the injection site. However, these did not have serious consequences, and the patients recovered within hours.
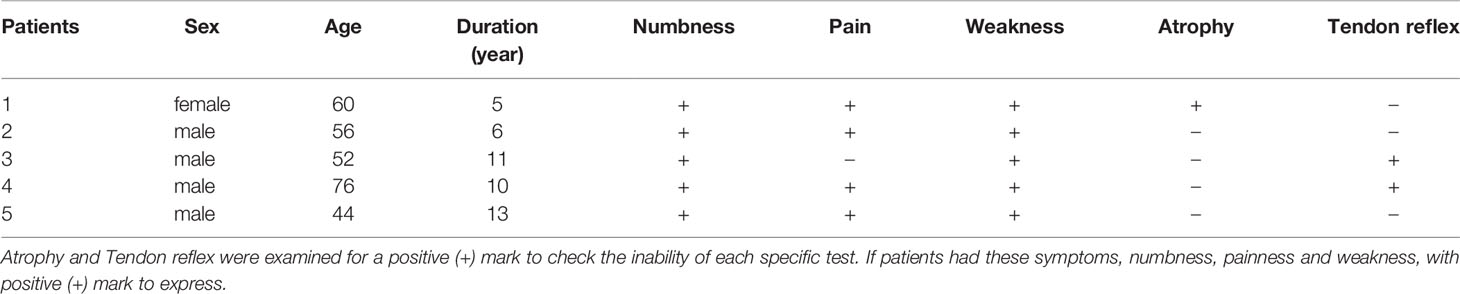
Table 6 General characteristics and clinical signs of the patients (n = 5).
Among the symptom scores, the VAS was decreased after the ultrasound-guided injection, with a mean of 3.4 points pre-injection and 0.6 points after treatment. The TCSS and DCEC scores did not significantly decrease after injection ( Table 3 and Figure 3 ).
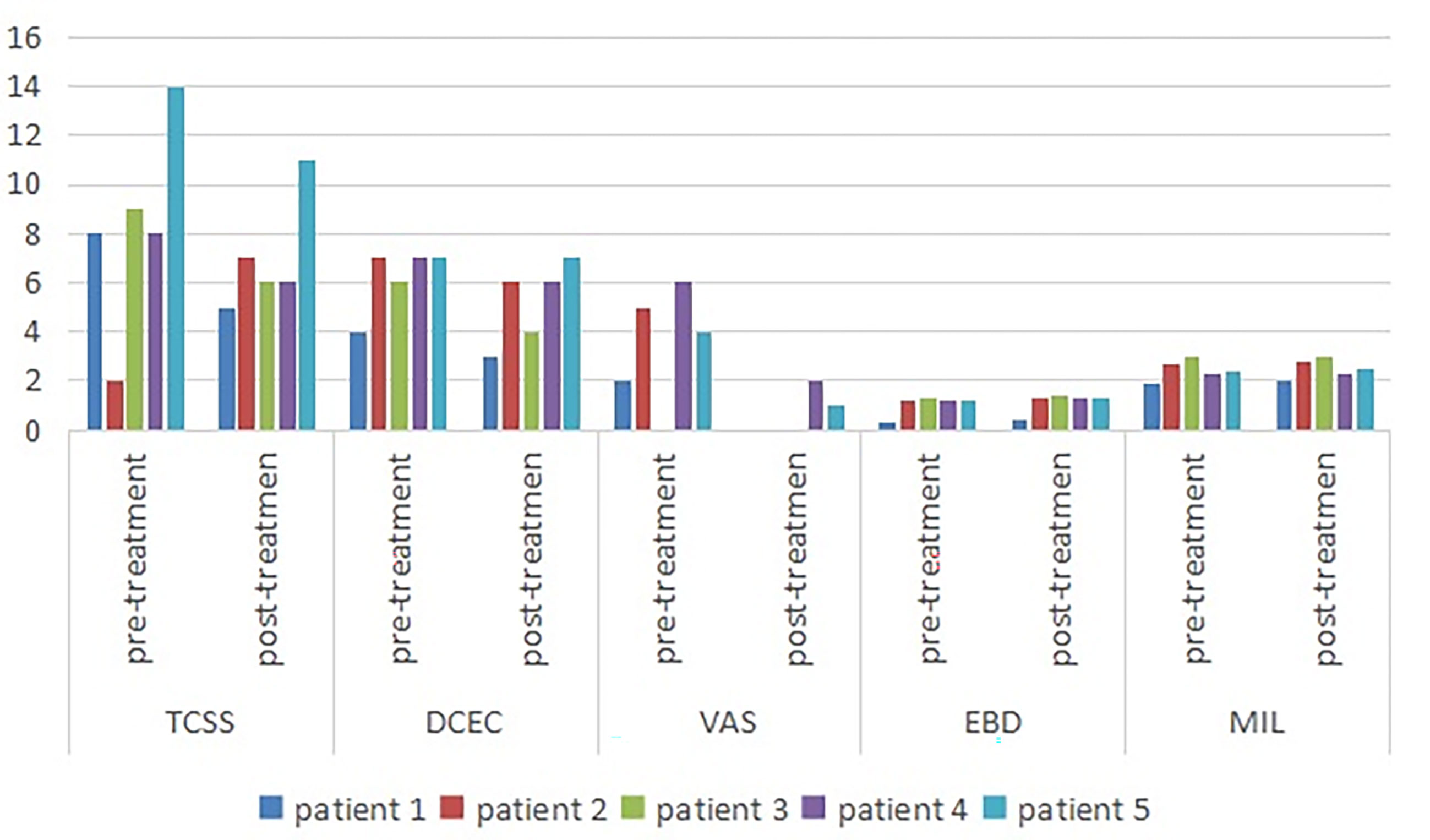
Figure 3 Symptom scores and morphologic changes were compared at baseline and post-injection. Among symptom scores, the VAS was significantly decreased, as compared to baseline. The DCEC score of nerves was measured by ultrasonography at limbs. Compared with baseline, the DCEC score and TCSS score showed a significant decrease post-injection. The EDB and MIL were increased after ultrasound-guided injection. DCEC, definition, cross sectional area, echogenicity and compression; TCSS, Toronto clinical scoring system; EDB, extensor digitorum brevis muscle; MIL, muscles of the first interstitium; VAS, visual analogy score.
The cross-sectional area of the EDB was measured by ultrasound at the midpoint of a straight line between the lateral malleolus and the fifth metatarsal tuberosity side of the dorsum pedis of the lower limbs, which determined the scanning plane. The cross-sectional areas of the EDB and MIL were decreased after injection ( Figure 1B ) compared with pre-injection, with a mean of 1.058 and 1.136 mm2, respectively ( Table 3 ).
We observed that the electrophysiology outcomes did not significantly change compared with those pre-injection, though the conduction velocity from the left peroneal nerve increased slightly in one patient.
The purpose of this investigation was to explore the clinical value of ultrasound-guided hydrodissection in the treatment of RDPN. The results showed that the TCSS and VAS of patients with RDPN treated with ultrasound-guided injection improved to some extent, with the latter improving the most. There was insignificant change in the DCEC scores and neuroelectrophysiological results. The improved neurotrophic effect was reflected in the increase of the leg circumference, EDB muscle, and MIL. It was obvious for Case 1 that the distal small muscle of the lower extremity atrophied but then grew after two courses of treatment. This was detected by ultrasonographic examination and measurement of the EDB and MIL from the innervation of the fibular nerve and tibial nerve, respectively. The degree of foot muscle atrophy was helpful in evaluating the neuropathology and the treatment effect on peripheral nerves.
The possible therapeutic mechanisms of ultrasound-guided hydrodissection are as follows: (1) Use the cutting force of fluid instead of needle to separate soft tissue. The primary objective of hydrodissection is to release the entrapment of the peripheral nerves by hydrodissecting the nerves ( 15 ). (2) By separating adhesion, reduce nerve compression, improve peripheral vein and lymph reflux, so as to promote nerve function ( 16 ). In addition, local injection of mecobalamin around nerves promotes axonal growth, which has significant effects on sensory symptoms and short courses ( 17 ). Mecobalamin injection after hydrodissection distributes evenly among the surrounding tissues, increases absorption, and plays a more efficient role.
DPN is a type of compressive disorder. In DPN treatment, especially in patients with pain, numbness, and other symptoms, in addition to controlling glycemia, we found that surgical decompression had an obvious therapeutic effect. As a result of nerve thickening, it could easily be compressed by the narrow pipe. The decompression operation relieved the nerve entrapment in the patients, improved the blood supply of axoplasmic transport in peripheral nerves, and repaired injured nerves, which significantly reduced the pain symptoms compared with the former ( 18 , 19 ). This research demonstrates the advantages of reducing nerve compression, improving the blood supply of nerves, increasing compliance, and reducing secondary nerve injury. The surgical decompression point was selected as the injection point of the hydrodissection in five patients. Mecobalamin is a type of endogenous co-enzyme B12 and can easily be transferred to the organelles of nerve cells in the process of methionine synthesis from homocysteine. It can promote the synthesis of nucleic acids and proteins, the transport and regeneration of axons, and the synthesis of phosphatidylcholine in myelination, whereby it has obvious effects on damaged nerve cells ( 20 ). Li Yuxin et al. carried out a study of axillary brachial plexus block compared with the traditional perivascular injection method and identified that expansion of the peripheral nerve space by ultrasound-guided saline reduced anesthetic doses ( 21 ). Consistent with our findings, earlier studies by Ide et al. also described that intrathecal injection with mecobalamin improved symptoms in patients with RDPN; however, the observation time was short, and nerve repair was not detected by the electrophysiological examination, which may explain why the EMG outcomes, including autonomic neural function, were insignificantly improved ( 22 ).
Limitations
In this study, only five patients were observed for a short time, and a standard could not be formed. In this study, patients with refractory diabetic peripheral neuropathy have been treated with different hypoglycemic and vegetative nerve regimens. The severity of the lesions may be different before using ultrasound-guided hydrodissection. In addition, the objective score of patients with symptoms is relatively low, and the response to treatment may not be comprehensive. However, as a small case report, this study is reasonable from the comparison before and after treatment and the selection of research objects. Otherwise, without treatment with hydrodissection and local mecobalamin injection, we cannot combine the results with the efficacy of hydraulic exfoliation, local injection of mecobalamin combined with efficient nutrition, or both. Thus, a large number of participants and researchers will not be able to distinguish the results from the efficacy of hydrodissection, local injection of mecobalamin, or a combination of both. In the future, we will recruit more participants to further study the treatment and prognosis, and we need to design a large sample size, controlled and multicenter study to determine whether ultrasound-guided hydrodissection has satisfactory efficacy in patients with RDPN compared with traditional methods.
In our patients, the nerves were thickened and obviously damaged at compression sites, and the recovery time was measured by electrophysiology improvements. Owing to the advantages of nutrition and safety in relieving nerve compression, the pain of the patients with RDPN was relieved. Therefore, we believe that the most effective method for the treatment of DPN is to reduce nerve injury by controlling blood sugar.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Guizhou Medical University. The patients/participants provided their written informed consent to participate in this study. All participants provided written informed consent for the publication of the case reports (including all data and images).
Author Contributions
HQH and SW conceived the idea and conceptualized the study.HLH, CT, ML and JCL completed the ultrasound for patients. JH performed nerve conduction tests. HQH completed the process of hydrodissection injection.HLH and HQH collected and analyzed the datas. Finally, HLH drafted and reviesd the manuscript,then YL and SW reviewed the manuscript.All authors contributed to the article and approved the submitted version.
Application and industrialization project of scientific and technological achievements in Guizhou Province, “The regional pilot of precision medical poverty alleviation powered by technology— Sub Project III, diagnosis and treatment of epilepsy, Parkinson’s disease and headache”, [Achievements of science and technology synthesis in Guizhou (2018) 4615-3]; National Key R&D Program of China (NO.2018YFC1312901); Guiyang Science and Technology Program, “the establishment and clinical application of the neuroultrasound scoring system”, [architecture contracts (2019) 9-1-7].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
1. Guru KA, Perlmutter AE, Butt ZM, Peabody JO. Hydrodissection for Preservation of Neurovascular Bundle During Robot-Assisted Radical Prostatectomy. Can J Urol (2008) 15:4000. doi: 10.17709/2409-2231-2016-3-4-5
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Bokey EL, Keating JP, Zelas P. Hydrodissection: An Easy Way to Dissect Anatomical Planes and Complex Adhesions. Aust New Z J Surg (1997) 67:643–4. doi: 10.1111/j.1445-2197.1997.tb04616.x
CrossRef Full Text | Google Scholar
3. Smith J, Wisniewski SJ, Finnoff JT, Payne JM. Sonographically Guided Carpal Tunnel Injection. J Ultrasound Med (2008) 27:1485–90. doi: 10.7863/jum.2008.27.10.1485
4. Jiang H, Yang W. Diagnosis and Treatment of Carpal Tunnel Syndrome. Chin J Integr Med Cardio Cerebrovasc Dis (2018) 16:65–7. doi: 10.12102/j.issn.1672-1349.2018.23.018
5. Rydevik B, Lundborg G, Nordborg C. Intraneural Tissue Reactions Induced by Internal Neurolysis. Scand J Plast Reconstr Surg Handb Surg (1976) 10:3–8. doi: 10.3109/02844317609169741
6. Delea SL, Chavez-Chiang NR, Poole JL, Norton HE, Sibbitt WL Jr, Bankhurst AD. Sonographically Guided Hydrodissection and Corticosteroid Injection for Scleroderma Hand. Clin Rheumatol (2011) 30:805–13. doi: 10.1007/s10067-010-1653-6
7. Wu YT, Chen SR, Li TY, Ho TY, Shen YP, Tsai CK, et al. Nerve Hydrodissection for Carpal Tunnel Syndrome: A Prospective, Randomized, Double-Blind, Controlled Trial. Muscle Nerve (2019) 59:174–80. doi: 10.1002/mus.26358
8. Fried SM, Nazarian LN. Ultrasound-Guided Hydroneurolysis of the Median Nerve for Recurrent Carpal Tunnel Syndrome. Hand (2019) 14:413–21. doi: 10.1177/1558944717731855
9. Lee JY, Park Y, Park KD, Lee JK, Lim OK. Effectiveness of Ultrasound-Guided Carpal Tunnel Injection Using in-Plane Ulnar Approach: A Prospective, Randomized, Single-Blinded Study. Med (Baltimore) (2014) 93:1–6. doi: 10.1097/MD.0000000000000350
10. Fader R, Mitchell J, Chadayammuri VP, Hill J, Wolcott ML. Percutaneous Ultrasound-Guided Hydrodissection of a Symptomatic Sural Neuroma. Orthopedics (2015) 38:e1046–50. doi: 10.3928/01477447-20151020-15
11. Chen MY, Cai HM, Chen JY, Zhang L, Wang R. Diagnostic Value of Michigan Diabetic Neuropathy Score and Toronto Clinical Scoring System in Diabetic Peripheral Neuropathy. Chin Gen Pract (2017) 20:427–31. doi: 10.3969/j.issn.1007-9572.2017.04.010
12. Cui L. Concise Electromyography Handbook . Beijing: Science Press. (2006) p. 184–200.
Google Scholar
13. Ou YQ, Wu XH, Lin YC, Tang C, Wu S. Ultrasonic Diagnostic Score For Diabetic Peripheral Neuropathy. Chin J Nerv Ment Dis (2019) 45:197–201. doi: 10.3969/j.issn.1002-0152.2019.04.002
14. Wang X, Chen L, Liu W, Su B, Zhang Y. Early Detection of Atrophy of Foot Muscles in Chinese Patients of Type 2 Diabetes Mellitus by High-Frequency Ultrasonography. J Diabetes Res (2014) 2014:1–6. doi: 10.1155/2014/927069
15. Cass SP. Ultrasound-Guided Nerve Hydrodissection: What is it? A Review of the Literature. Curr Sports Med Rep (2016) 15(1):20–2. doi: 10.1249/JSR.0000000000000226
16. Lam KHS, Hung CY, Chiang YP, Onishi K, Su DCJ, Clark TB, et al. Ultrasound-Guided Nerve Hydrodissection for Pain Management: Rationale, Methods, Current Literature, and Theoretical Mechanisms. J Pain Res (2020) 13:1957–68. doi: 10.2147/JPR.S247208
17. Suzuki K, Tanaka H, Ebara M, Uto K, Matsuoka H, Nishimoto S, et al. Electrospun Nanofiber Sheets Incorporating Methylcobalamin Promote Nerve Regeneration and Functional Recovery in a Rat Sciatic Nerve Crush Injury Model. Acta Biomaterialia (2017) 53:250–9. doi: 10.1016/j.actbio.2017.02.004
18. Liao CL, Yang M, Zhong WX, Zhang WC. Role of Pain Distribution in Surgical Decompression for Diabetic Peripheral Neuropathy. Chin J Minim Invasive Neurosurg (2015) 20:545–8. doi: 10.11850/j.issn.1009-122X.2015.12.006
19. Hu JD, Chen ZG. Surgical Treatment of Diabetic Peripheral Neuropathy. Fudan Univ J Med Sci (2016) 43:615–9. doi: 10.3969/j.issn.1672-8467.2016.05.018
20. Zhang YJ, Ding JQ, Li JH. Application of Mecobalamin in Nervous System Diseases. China Med Pharmac (2012) 2:19–21. doi: CNKI:SUN:GYKX.0.2012-14-011
21. Li YX, Xu Y, Hu Q, Cui D-R. Application of Perineural Space Expansion by Normal Saline in Axillary Brachial Plexus Block. J Shanghai Jiaotong Univ (Med Edition) (2018) 38:510–3. doi: CNKI:SUN:SHEY.0.2018-05-005
22. Ide H, Fujiya S, Asanuma Y, Tsuji M, Sakai H, Agishi Y. Clinical Usefulness of Intrathecal Injection of Methylcobalamin in Patients With Diabetic Neuropathy. Clin Ther (1987) 9:183–92. doi: 10.1097/00002826-198710000-00013
Keywords: hydrodissection, peripheral nerve compression, the scoring system of ultrasound, electrophysiological, refractory diabetic peripheral neuropathy
Citation: Hu HQ, Huang H, Huang J, Leng JC, Li M, Tang C, Li Y and Wu S (2021) Case Report: Successful Outcome for Refractory Diabetic Peripheral Neuropathy in Patients With Ultrasound-Guided Injection Treatment. Front. Endocrinol. 12:735132. doi: 10.3389/fendo.2021.735132
Received: 19 July 2021; Accepted: 21 September 2021; Published: 28 October 2021.
Reviewed by:
Copyright © 2021 Hu, Huang, Huang, Leng, Li, Tang, Li and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shan Wu, [email protected]
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Diabetic peripheral neuropathic pain: case studies
Affiliation.
- 1 Pain & Palliative Care Center, University of Minnesota Medical Center, Minneapolis, USA.
- PMID: 16608050
- DOI: 10.1016/s0025-6196(11)61476-6
Three case reports in this article illustrate the diagnostic methods used and the treatment course encountered for many patients with diabetic peripheral neuropathic pain (DPNP). Each case addresses an aspect of DPNP: pain that appears to be refractory to initial therapy, DPNP occurring with other medical conditions, and nondiabetlc neuropathy occurring in patients with diabetes mellitus. Together, these cases bring clarity to the confusing clinical experience for patients who have decreased sensation in combination with burning pain, and they apply the consensus guidelines for DPNP. Recently approved medications by the Food and Drug Administration for the treatment of DPNP offer hope for many patients whose pain was thought to be refractory to treatment.
Publication types
- Case Reports
- Comorbidity
- Diabetic Neuropathies / complications
- Diabetic Neuropathies / diagnosis*
- Diabetic Neuropathies / drug therapy*
- Diabetic Neuropathies / epidemiology
- Diagnosis, Differential
- Pain, Intractable / diagnosis
- Pain, Intractable / drug therapy
- Pain, Intractable / etiology*
- Parkinson Disease / epidemiology
- Peripheral Nervous System Diseases / diagnosis
- Polyneuropathies / diagnosis
- Practice Guidelines as Topic
Diabetic neuropathy
On this page, when to see a doctor, risk factors, complications.
Diabetic neuropathy is a type of nerve damage that can occur if you have diabetes. High blood sugar (glucose) can injure nerves throughout the body. Diabetic neuropathy most often damages nerves in the legs and feet.
Depending on the affected nerves, diabetic neuropathy symptoms include pain and numbness in the legs, feet and hands. It can also cause problems with the digestive system, urinary tract, blood vessels and heart. Some people have mild symptoms. But for others, diabetic neuropathy can be quite painful and disabling.
Diabetic neuropathy is a serious diabetes complication that may affect as many as 50% of people with diabetes. But you can often prevent diabetic neuropathy or slow its progress with consistent blood sugar management and a healthy lifestyle.
Products & Services
- A Book: The Essential Diabetes Book
There are four main types of diabetic neuropathy. You can have one type or more than one type of neuropathy.
Your symptoms depend on the type you have and which nerves are affected. Usually, symptoms develop gradually. You may not notice anything is wrong until considerable nerve damage has occurred.
Peripheral neuropathy
This type of neuropathy may also be called distal symmetric peripheral neuropathy. It's the most common type of diabetic neuropathy. It affects the feet and legs first, followed by the hands and arms. Signs and symptoms of peripheral neuropathy are often worse at night, and may include:
- Numbness or reduced ability to feel pain or temperature changes
- Tingling or burning feeling
- Sharp pains or cramps
- Muscle weakness
- Extreme sensitivity to touch — for some people, even a bedsheet's weight can be painful
- Serious foot problems, such as ulcers, infections, and bone and joint damage
Autonomic neuropathy
The autonomic nervous system controls blood pressure, heart rate, sweating, eyes, bladder, digestive system and sex organs. Diabetes can affect nerves in any of these areas, possibly causing signs and symptoms including:
- A lack of awareness that blood sugar levels are low (hypoglycemia unawareness)
- Drops in blood pressure when rising from sitting or lying down that may cause dizziness or fainting (orthostatic hypotension)
- Bladder or bowel problems
- Slow stomach emptying (gastroparesis), causing nausea, vomiting, sensation of fullness and loss of appetite
- Difficulty swallowing
- Changes in the way the eyes adjust from light to dark or far to near
- Increased or decreased sweating
- Problems with sexual response, such as vaginal dryness in women and erectile dysfunction in men

Proximal neuropathy (diabetic polyradiculopathy)
This type of neuropathy often affects nerves in the thighs, hips, buttocks or legs. It can also affect the abdominal and chest area. Symptoms are usually on one side of the body, but may spread to the other side. Proximal neuropathy may include:
- Severe pain in the buttock, hip or thigh
- Weak and shrinking thigh muscles
- Difficulty rising from a sitting position
- Chest or abdominal wall pain
Mononeuropathy (focal neuropathy)
Mononeuropathy refers to damage to a single, specific nerve. The nerve may be in the face, torso, arm or leg. Mononeuropathy may lead to:
- Difficulty focusing or double vision
- Paralysis on one side of the face
- Numbness or tingling in the hand or fingers
- Weakness in the hand that may result in dropping things
- Pain in the shin or foot
- Weakness causing difficulty lifting the front part of the foot (foot drop)
- Pain in the front of the thigh
More Information
- Types of diabetic neuropathy
Call your health care provider for an appointment if you have:
- A cut or sore on your foot that is infected or won't heal
- Burning, tingling, weakness or pain in your hands or feet that interferes with daily activities or sleep
- Changes in digestion, urination or sexual function
- Dizziness and fainting
The American Diabetes Association (ADA) recommends that screening for diabetic neuropathy begin immediately after someone is diagnosed with type 2 diabetes or five years after diagnosis with type 1 diabetes. After that, screening is recommended once a year.
From Mayo Clinic to your inbox
The exact cause of each type of neuropathy is unknown. Researchers think that over time, uncontrolled high blood sugar damages nerves and interferes with their ability to send signals, leading to diabetic neuropathy. High blood sugar also weakens the walls of the small blood vessels (capillaries) that supply the nerves with oxygen and nutrients.
Anyone who has diabetes can develop neuropathy. But these risk factors make nerve damage more likely:
- Poor blood sugar control. Uncontrolled blood sugar increases the risk of every diabetes complication, including nerve damage.
- Diabetes history. The risk of diabetic neuropathy increases the longer a person has diabetes, especially if blood sugar isn't well controlled.
- Kidney disease. Diabetes can damage the kidneys. Kidney damage sends toxins into the blood, which can lead to nerve damage.
- Being overweight. Having a body mass index (BMI) of 25 or more may increase the risk of diabetic neuropathy.
- Smoking. Smoking narrows and hardens the arteries, reducing blood flow to the legs and feet. This makes it more difficult for wounds to heal and damages the peripheral nerves.
Diabetic neuropathy can cause a number of serious complications, including:
- Hypoglycemia unawareness. Blood sugar levels below 70 milligrams per deciliter (mg/dL) — 3.9 millimoles per liter (mmol/L) — usually cause shakiness, sweating and a fast heartbeat. But people who have autonomic neuropathy may not experience these warning signs.
- Loss of a toe, foot or leg. Nerve damage can cause a loss of feeling in the feet, so even minor cuts can turn into sores or ulcers without being noticed. In severe cases, an infection can spread to the bone or lead to tissue death. Removal (amputation) of a toe, foot or even part of the leg may be necessary.
- Urinary tract infections and urinary incontinence. If the nerves that control the bladder are damaged, the bladder may not empty completely when urinating. Bacteria can build up in the bladder and kidneys, causing urinary tract infections. Nerve damage can also affect the ability to feel the need to urinate or to control the muscles that release urine, leading to leakage (incontinence).
- Sharp drops in blood pressure. Damage to the nerves that control blood flow can affect the body's ability to adjust blood pressure. This can cause a sharp drop in pressure when standing after sitting or lying down, which may lead to lightheadedness and fainting.
- Digestive problems . If nerve damage occurs in the digestive tract, constipation or diarrhea, or both are possible. Diabetes-related nerve damage can lead to gastroparesis, a condition in which the stomach empties too slowly or not at all. This can cause bloating and indigestion.
- Sexual dysfunction. Autonomic neuropathy often damages the nerves that affect the sex organs. Men may experience erectile dysfunction. Women may have difficulty with lubrication and arousal.
- Increased or decreased sweating. Nerve damage can disrupt how the sweat glands work and make it difficult for the body to control its temperature properly.
You can prevent or delay diabetic neuropathy and its complications by closely managing your blood sugar and taking good care of your feet.
Blood sugar management
The American Diabetes Association (ADA) recommends that people living with diabetes have a glycated hemoglobin (A1C) test at least twice a year. This test indicates your average blood sugar level for the past 2 to 3 months.
glycated hemoglobin (A1C) goals may need to be individualized, but for many adults, the ADA recommends an A1C of less than 7.0%. If your blood sugar levels are higher than your goal, you may need changes in your daily management, such as adding or adjusting your medications or changing your diet or physical activity.
Foot problems, including sores that don't heal, ulcers and even amputation, are common complications of diabetic neuropathy. But you can prevent many of these problems by having a thorough foot exam at least once a year. Also have your health care provider check your feet at each office visit and take good care of your feet at home.
Follow your health care provider's recommendations for good foot care. To protect the health of your feet:
- Check your feet every day. Look for blisters, cuts, bruises, cracked and peeling skin, redness, and swelling. Use a mirror or ask a friend or family member to help examine parts of your feet that are hard to see.
- Keep your feet clean and dry. Wash your feet every day with lukewarm water and mild soap. Don't soak your feet. Dry your feet and between your toes thoroughly.
- Moisturize your feet. This helps prevent cracking. But don't get lotion between your toes because it might encourage fungal growth.
- Trim your toenails carefully. Cut your toenails straight across. File the edges carefully so that you have smooth edges. If you can't do this yourself, a specialist in foot problems (podiatrist) can help.
- Wear clean, dry socks. Look for socks made of cotton or moisture-wicking fibers that don't have tight bands or thick seams.
- Wear cushioned shoes that fit well. Wear closed-toed shoes or slippers to protect your feet. Make sure your shoes fit properly and allow your toes to move. A foot specialist can teach you how to buy properly fitted shoes and to prevent problems such as corns and calluses. If you qualify for Medicare, your plan may cover the cost of at least one pair of shoes each year.
Apr 29, 2022
- Ferri FF. Diabetic polyneuropathy. In: Ferri's Clinical Advisor 2022. Elsevier; 2020. https://www.clinicalkey.com. Accessed Dec. 17, 2021.
- Diabetic neuropathy. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/nerve-damage-diabetic-neuropathies/all-content. Accessed Jan. 10, 2020.
- American Diabetes Association. Standards of medical care in diabetes — 2021. Diabetes Care. 2021. https://care.diabetesjournals.org/content/44/Supplement_1. Accessed Nov. 11, 2021.
- AskMayoExpert. Peripheral neuropathy (adult). Mayo Clinic; 2021.
- Feldman EL, et al. Management of diabetic neuropathy. https://www.uptodate.com/contents/search. Accessed Dec. 17, 2021.
- Diabetes and foot problems. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/foot-problems#healthyfeet. Accessed Jan. 10, 2020.
- Jankovic J, et al., eds. Disorders of peripheral nerves. In: Bradley and Daroff's Neurology in Clinical Practice. 8th ed. Elsevier; 2022. https://www.clinicalkey.com. Accessed Jan. 11, 2020.
- Baute V, et al. Complementary and alternative medicine for painful peripheral neuropathy. Current Treatment Options in Neurology. 2019; doi:10.1007/s11940-019-0584-z.
- Feldman EL, et al. Diabetic neuropathy. Nature Reviews — Disease Primers. 2019; doi:10.1038/s41572-019-0092-1.
- Cutsforth-Gregory (expert opinion). Mayo Clinic. Dec. 24, 2021.
- Castro MR (expert opinion). Mayo Clinic. Jan. 21, 2022.
- Diseases & Conditions
- Diabetic neuropathy symptoms & causes
- Diabetic neuropathy and dietary supplements
Associated Procedures
- Electromyography (EMG)
CON-XXXXXXXX
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.

- Previous Article
- Next Article
Case Presentation
Case study: a patient with uncontrolled type 2 diabetes and complex comorbidities whose diabetes care is managed by an advanced practice nurse.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Geralyn Spollett; Case Study: A Patient With Uncontrolled Type 2 Diabetes and Complex Comorbidities Whose Diabetes Care Is Managed by an Advanced Practice Nurse. Diabetes Spectr 1 January 2003; 16 (1): 32–36. https://doi.org/10.2337/diaspect.16.1.32
Download citation file:
- Ris (Zotero)
- Reference Manager
The specialized role of nursing in the care and education of people with diabetes has been in existence for more than 30 years. Diabetes education carried out by nurses has moved beyond the hospital bedside into a variety of health care settings. Among the disciplines involved in diabetes education, nursing has played a pivotal role in the diabetes team management concept. This was well illustrated in the Diabetes Control and Complications Trial (DCCT) by the effectiveness of nurse managers in coordinating and delivering diabetes self-management education. These nurse managers not only performed administrative tasks crucial to the outcomes of the DCCT, but also participated directly in patient care. 1
The emergence and subsequent growth of advanced practice in nursing during the past 20 years has expanded the direct care component, incorporating aspects of both nursing and medical care while maintaining the teaching and counseling roles. Both the clinical nurse specialist (CNS) and nurse practitioner (NP) models, when applied to chronic disease management, create enhanced patient-provider relationships in which self-care education and counseling is provided within the context of disease state management. Clement 2 commented in a review of diabetes self-management education issues that unless ongoing management is part of an education program, knowledge may increase but most clinical outcomes only minimally improve. Advanced practice nurses by the very nature of their scope of practice effectively combine both education and management into their delivery of care.
Operating beyond the role of educator, advanced practice nurses holistically assess patients’ needs with the understanding of patients’ primary role in the improvement and maintenance of their own health and wellness. In conducting assessments, advanced practice nurses carefully explore patients’ medical history and perform focused physical exams. At the completion of assessments, advanced practice nurses, in conjunction with patients, identify management goals and determine appropriate plans of care. A review of patients’ self-care management skills and application/adaptation to lifestyle is incorporated in initial histories, physical exams, and plans of care.
Many advanced practice nurses (NPs, CNSs, nurse midwives, and nurse anesthetists) may prescribe and adjust medication through prescriptive authority granted to them by their state nursing regulatory body. Currently, all 50 states have some form of prescriptive authority for advanced practice nurses. 3 The ability to prescribe and adjust medication is a valuable asset in caring for individuals with diabetes. It is a crucial component in the care of people with type 1 diabetes, and it becomes increasingly important in the care of patients with type 2 diabetes who have a constellation of comorbidities, all of which must be managed for successful disease outcomes.
Many studies have documented the effectiveness of advanced practice nurses in managing common primary care issues. 4 NP care has been associated with a high level of satisfaction among health services consumers. In diabetes, the role of advanced practice nurses has significantly contributed to improved outcomes in the management of type 2 diabetes, 5 in specialized diabetes foot care programs, 6 in the management of diabetes in pregnancy, 7 and in the care of pediatric type 1 diabetic patients and their parents. 8 , 9 Furthermore, NPs have also been effective providers of diabetes care among disadvantaged urban African-American patients. 10 Primary management of these patients by NPs led to improved metabolic control regardless of whether weight loss was achieved.
The following case study illustrates the clinical role of advanced practice nurses in the management of a patient with type 2 diabetes.
A.B. is a retired 69-year-old man with a 5-year history of type 2 diabetes. Although he was diagnosed in 1997, he had symptoms indicating hyperglycemia for 2 years before diagnosis. He had fasting blood glucose records indicating values of 118–127 mg/dl, which were described to him as indicative of “borderline diabetes.” He also remembered past episodes of nocturia associated with large pasta meals and Italian pastries. At the time of initial diagnosis, he was advised to lose weight (“at least 10 lb.”), but no further action was taken.
Referred by his family physician to the diabetes specialty clinic, A.B. presents with recent weight gain, suboptimal diabetes control, and foot pain. He has been trying to lose weight and increase his exercise for the past 6 months without success. He had been started on glyburide (Diabeta), 2.5 mg every morning, but had stopped taking it because of dizziness, often accompanied by sweating and a feeling of mild agitation, in the late afternoon.
A.B. also takes atorvastatin (Lipitor), 10 mg daily, for hypercholesterolemia (elevated LDL cholesterol, low HDL cholesterol, and elevated triglycerides). He has tolerated this medication and adheres to the daily schedule. During the past 6 months, he has also taken chromium picolinate, gymnema sylvestre, and a “pancreas elixir” in an attempt to improve his diabetes control. He stopped these supplements when he did not see any positive results.
He does not test his blood glucose levels at home and expresses doubt that this procedure would help him improve his diabetes control. “What would knowing the numbers do for me?,” he asks. “The doctor already knows the sugars are high.”
A.B. states that he has “never been sick a day in my life.” He recently sold his business and has become very active in a variety of volunteer organizations. He lives with his wife of 48 years and has two married children. Although both his mother and father had type 2 diabetes, A.B. has limited knowledge regarding diabetes self-care management and states that he does not understand why he has diabetes since he never eats sugar. In the past, his wife has encouraged him to treat his diabetes with herbal remedies and weight-loss supplements, and she frequently scans the Internet for the latest diabetes remedies.
During the past year, A.B. has gained 22 lb. Since retiring, he has been more physically active, playing golf once a week and gardening, but he has been unable to lose more than 2–3 lb. He has never seen a dietitian and has not been instructed in self-monitoring of blood glucose (SMBG).
A.B.’s diet history reveals excessive carbohydrate intake in the form of bread and pasta. His normal dinners consist of 2 cups of cooked pasta with homemade sauce and three to four slices of Italian bread. During the day, he often has “a slice or two” of bread with butter or olive oil. He also eats eight to ten pieces of fresh fruit per day at meals and as snacks. He prefers chicken and fish, but it is usually served with a tomato or cream sauce accompanied by pasta. His wife has offered to make him plain grilled meats, but he finds them “tasteless.” He drinks 8 oz. of red wine with dinner each evening. He stopped smoking more than 10 years ago, he reports, “when the cost of cigarettes topped a buck-fifty.”
The medical documents that A.B. brings to this appointment indicate that his hemoglobin A 1c (A1C) has never been <8%. His blood pressure has been measured at 150/70, 148/92, and 166/88 mmHg on separate occasions during the past year at the local senior center screening clinic. Although he was told that his blood pressure was “up a little,” he was not aware of the need to keep his blood pressure ≤130/80 mmHg for both cardiovascular and renal health. 11
A.B. has never had a foot exam as part of his primary care exams, nor has he been instructed in preventive foot care. However, his medical records also indicate that he has had no surgeries or hospitalizations, his immunizations are up to date, and, in general, he has been remarkably healthy for many years.
Physical Exam
A physical examination reveals the following:
Weight: 178 lb; height: 5′2″; body mass index (BMI): 32.6 kg/m 2
Fasting capillary glucose: 166 mg/dl
Blood pressure: lying, right arm 154/96 mmHg; sitting, right arm 140/90 mmHg
Pulse: 88 bpm; respirations 20 per minute
Eyes: corrective lenses, pupils equal and reactive to light and accommodation, Fundi-clear, no arteriolovenous nicking, no retinopathy
Thyroid: nonpalpable
Lungs: clear to auscultation
Heart: Rate and rhythm regular, no murmurs or gallops
Vascular assessment: no carotid bruits; femoral, popliteal, and dorsalis pedis pulses 2+ bilaterally
Neurological assessment: diminished vibratory sense to the forefoot, absent ankle reflexes, monofilament (5.07 Semmes-Weinstein) felt only above the ankle
Lab Results
Results of laboratory tests (drawn 5 days before the office visit) are as follows:
Glucose (fasting): 178 mg/dl (normal range: 65–109 mg/dl)
Creatinine: 1.0 mg/dl (normal range: 0.5–1.4 mg/dl)
Blood urea nitrogen: 18 mg/dl (normal range: 7–30 mg/dl)
Sodium: 141 mg/dl (normal range: 135–146 mg/dl)
Potassium: 4.3 mg/dl (normal range: 3.5–5.3 mg/dl)
Lipid panel
• Total cholesterol: 162 mg/dl (normal: <200 mg/dl)
• HDL cholesterol: 43 mg/dl (normal: ≥40 mg/dl)
• LDL cholesterol (calculated): 84 mg/dl (normal: <100 mg/dl)
• Triglycerides: 177 mg/dl (normal: <150 mg/dl)
• Cholesterol-to-HDL ratio: 3.8 (normal: <5.0)
AST: 14 IU/l (normal: 0–40 IU/l)
ALT: 19 IU/l (normal: 5–40 IU/l)
Alkaline phosphotase: 56 IU/l (normal: 35–125 IU/l)
A1C: 8.1% (normal: 4–6%)
Urine microalbumin: 45 mg (normal: <30 mg)
Based on A.B.’s medical history, records, physical exam, and lab results, he is assessed as follows:
Uncontrolled type 2 diabetes (A1C >7%)
Obesity (BMI 32.4 kg/m 2 )
Hyperlipidemia (controlled with atorvastatin)
Peripheral neuropathy (distal and symmetrical by exam)
Hypertension (by previous chart data and exam)
Elevated urine microalbumin level
Self-care management/lifestyle deficits
• Limited exercise
• High carbohydrate intake
• No SMBG program
Poor understanding of diabetes
A.B. presented with uncontrolled type 2 diabetes and a complex set of comorbidities, all of which needed treatment. The first task of the NP who provided his care was to select the most pressing health care issues and prioritize his medical care to address them. Although A.B. stated that his need to lose weight was his chief reason for seeking diabetes specialty care, his elevated glucose levels and his hypertension also needed to be addressed at the initial visit.
The patient and his wife agreed that a referral to a dietitian was their first priority. A.B. acknowledged that he had little dietary information to help him achieve weight loss and that his current weight was unhealthy and “embarrassing.” He recognized that his glucose control was affected by large portions of bread and pasta and agreed to start improving dietary control by reducing his portion size by one-third during the week before his dietary consultation. Weight loss would also be an important first step in reducing his blood pressure.
The NP contacted the registered dietitian (RD) by telephone and referred the patient for a medical nutrition therapy assessment with a focus on weight loss and improved diabetes control. A.B.’s appointment was scheduled for the following week. The RD requested that during the intervening week, the patient keep a food journal recording his food intake at meals and snacks. She asked that the patient also try to estimate portion sizes.
Although his physical activity had increased since his retirement, it was fairly sporadic and weather-dependent. After further discussion, he realized that a week or more would often pass without any significant form of exercise and that most of his exercise was seasonal. Whatever weight he had lost during the summer was regained in the winter, when he was again quite sedentary.
A.B.’s wife suggested that the two of them could walk each morning after breakfast. She also felt that a treadmill at home would be the best solution for getting sufficient exercise in inclement weather. After a short discussion about the positive effect exercise can have on glucose control, the patient and his wife agreed to walk 15–20 minutes each day between 9:00 and 10:00 a.m.
A first-line medication for this patient had to be targeted to improving glucose control without contributing to weight gain. Thiazolidinediones (i.e., rosiglitizone [Avandia] or pioglitizone [Actos]) effectively address insulin resistance but have been associated with weight gain. 12 A sulfonylurea or meglitinide (i.e., repaglinide [Prandin]) can reduce postprandial elevations caused by increased carbohydrate intake, but they are also associated with some weight gain. 12 When glyburide was previously prescribed, the patient exhibited signs and symptoms of hypoglycemia (unconfirmed by SMBG). α-Glucosidase inhibitors (i.e., acarbose [Precose]) can help with postprandial hyperglycemia rise by blunting the effect of the entry of carbohydrate-related glucose into the system. However, acarbose requires slow titration, has multiple gastrointestinal (GI) side effects, and reduces A1C by only 0.5–0.9%. 13 Acarbose may be considered as a second-line therapy for A.B. but would not fully address his elevated A1C results. Metformin (Glucophage), which reduces hepatic glucose production and improves insulin resistance, is not associated with hypoglycemia and can lower A1C results by 1%. Although GI side effects can occur, they are usually self-limiting and can be further reduced by slow titration to dose efficacy. 14
After reviewing these options and discussing the need for improved glycemic control, the NP prescribed metformin, 500 mg twice a day. Possible GI side effects and the need to avoid alcohol were of concern to A.B., but he agreed that medication was necessary and that metformin was his best option. The NP advised him to take the medication with food to reduce GI side effects.
The NP also discussed with the patient a titration schedule that increased the dosage to 1,000 mg twice a day over a 4-week period. She wrote out this plan, including a date and time for telephone contact and medication evaluation, and gave it to the patient.
During the visit, A.B. and his wife learned to use a glucose meter that features a simple two-step procedure. The patient agreed to use the meter twice a day, at breakfast and dinner, while the metformin dose was being titrated. He understood the need for glucose readings to guide the choice of medication and to evaluate the effects of his dietary changes, but he felt that it would not be “a forever thing.”
The NP reviewed glycemic goals with the patient and his wife and assisted them in deciding on initial short-term goals for weight loss, exercise, and medication. Glucose monitoring would serve as a guide and assist the patient in modifying his lifestyle.
A.B. drew the line at starting an antihypertensive medication—the angiotensin-converting enzyme (ACE) inhibitor enalapril (Vasotec), 5 mg daily. He stated that one new medication at a time was enough and that “too many medications would make a sick man out of me.” His perception of the state of his health as being represented by the number of medications prescribed for him gave the advanced practice nurse an important insight into the patient’s health belief system. The patient’s wife also believed that a “natural solution” was better than medication for treating blood pressure.
Although the use of an ACE inhibitor was indicated both by the level of hypertension and by the presence of microalbuminuria, the decision to wait until the next office visit to further evaluate the need for antihypertensive medication afforded the patient and his wife time to consider the importance of adding this pharmacotherapy. They were quite willing to read any materials that addressed the prevention of diabetes complications. However, both the patient and his wife voiced a strong desire to focus their energies on changes in food and physical activity. The NP expressed support for their decision. Because A.B. was obese, weight loss would be beneficial for many of his health issues.
Because he has a sedentary lifestyle, is >35 years old, has hypertension and peripheral neuropathy, and is being treated for hypercholestrolemia, the NP performed an electrocardiogram in the office and referred the patient for an exercise tolerance test. 11 In doing this, the NP acknowledged and respected the mutually set goals, but also provided appropriate pre-exercise screening for the patient’s protection and safety.
In her role as diabetes educator, the NP taught A.B. and his wife the importance of foot care, demonstrating to the patient his inability to feel the light touch of the monofilament. She explained that the loss of protective sensation from peripheral neuropathy means that he will need to be more vigilant in checking his feet for any skin lesions caused by poorly fitting footwear worn during exercise.
At the conclusion of the visit, the NP assured A.B. that she would share the plan of care they had developed with his primary care physician, collaborating with him and discussing the findings of any diagnostic tests and procedures. She would also work in partnership with the RD to reinforce medical nutrition therapies and improve his glucose control. In this way, the NP would facilitate the continuity of care and keep vital pathways of communication open.
Advanced practice nurses are ideally suited to play an integral role in the education and medical management of people with diabetes. 15 The combination of clinical skills and expertise in teaching and counseling enhances the delivery of care in a manner that is both cost-reducing and effective. Inherent in the role of advanced practice nurses is the understanding of shared responsibility for health care outcomes. This partnering of nurse with patient not only improves care but strengthens the patient’s role as self-manager.
Geralyn Spollett, MSN, C-ANP, CDE, is associate director and an adult nurse practitioner at the Yale Diabetes Center, Department of Endocrinology and Metabolism, at Yale University in New Haven, Conn. She is an associate editor of Diabetes Spectrum.
Note of disclosure: Ms. Spollett has received honoraria for speaking engagements from Novo Nordisk Pharmaceuticals, Inc., and Aventis and has been a paid consultant for Aventis. Both companies produce products and devices for the treatment of diabetes.
Email alerts
- Advanced Practice Care: Advanced Practice Care in Diabetes: Epilogue
- Advanced Practice Care: Advanced Practice Care in Diabetes: Preface
- Online ISSN 1944-7353
- Print ISSN 1040-9165
- Diabetes Care
- Clinical Diabetes
- Diabetes Spectrum
- Standards of Medical Care in Diabetes
- Scientific Sessions Abstracts
- BMJ Open Diabetes Research & Care
- ShopDiabetes.org
- ADA Professional Books
Clinical Compendia
- Clinical Compendia Home
- Latest News
- DiabetesPro SmartBrief
- Special Collections
- DiabetesPro®
- Diabetes Food Hub™
- Insulin Affordability
- Know Diabetes By Heart™
- About the ADA
- Journal Policies
- For Reviewers
- Advertising in ADA Journals
- Reprints and Permission for Reuse
- Copyright Notice/Public Access Policy
- ADA Professional Membership
- ADA Member Directory
- Diabetes.org
- X (Twitter)
- Cookie Policy
- Accessibility
- Terms & Conditions
- Get Adobe Acrobat Reader
- © Copyright American Diabetes Association
This Feature Is Available To Subscribers Only
Sign In or Create an Account
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Diabetic neuropathy: Clinical manifestations and current treatments
Brian c. callaghan.
(1) University of Michigan, Ann Arbor
Hsinlin Cheng
Catherine l. stables, andrea l. smith, eva l. feldman.
Diabetic peripheral neuropathy is a prevalent, disabling condition. The most common manifestation is a distal symmetric polyneuropathy (DSP), but many patterns of nerve injury can occur. Currently, the only effective treatments are glucose control and pain management. While glucose control dramatically decreases the development of neuropathy in those with type 1 diabetes, the effect is likely much smaller in those with type 2 diabetes. High levels of evidence support the use of certain anticonvulsants and antidepressants for pain management in diabetic peripheral neuropathy. However, the lack of disease modifying therapies for diabetic DSP makes the identification of new modifiable risk factors essential. Intriguingly, growing evidence supports an association between metabolic syndrome components, including pre-diabetes, and neuropathy. Future studies are needed to further explore this relationship with implications for new treatments for this common disease.
Introduction
Neuropathy, or damage to the nerves of the peripheral nervous system, is a debilitating yet surprisingly common and complex condition. Its prevalence is greater than 2% in the general population 1 , 2 and approximately 15% in those over the age of 40 3 . By far, the most common cause of neuropathy is diabetes 4 . In fact, the prevalence of neuropathy in patients with diabetes is approximately 30%, and up to 50% will eventually develop neuropathy during the course of their disease 5 . Diabetes can damage the peripheral nervous system in a variety of ways, but the most common presentation is a distal symmetric polyneuropathy (DSP). Other patterns of injury include small fiber predominant neuropathy, radiculoplexopathy, and autonomic neuropathy, amongst others. Since DSP is the most common neuropathy subtype and is the best studied, this will be the main focus of this review. Currently, the only treatments available to patients with diabetic DSP are improved glucose control and pain management. Both of these topics will be covered in depth.
Given the limitations in current clinical care, it is essential to identify new modifiable risk factors for the development of neuropathy. Top candidates include metabolic syndrome components such as hypertriglyceridemia, obesity, hyperglycemia, hypertension, and dyslipidemia. Establishing whether there is a causal relationship between these components, including pre-diabetes, has the potential to lead to new disease modifying therapies.
Diabetic neuropathy: clinical manifestations
Diabetes can impact the peripheral nervous system in a multitude of ways. DSP accounts for such a large proportion of all peripheral nerve manifestations attributed to diabetes that some use the terms diabetic DSP and diabetic neuropathy interchangeably. Patients with DSP typically have numbness, tingling, pain, and/or weakness that begin in the feet and spread proximally in a length-dependent fashion (stocking and glove distribution). The symptoms are symmetric with sensory symptoms more prominent than motor involvement. Many patients with neuropathy experience a sensation of their socks being bunched up or their shoes not fitting correctly. They even have the apparent paradox of numbness and exquisite sensitivity at the same time. Interestingly, which symptom predominates varies dramatically from patient to patient.
The constellation of symptoms from DSP creates many down-stream effects that can affect patients’ quality of life, both physical and mental 6 . DSP associated numbness often causes balance problems which can lead to falls. DSP is one of three main risk factors for falls in patients with diabetes, along with retinopathy and vestibular dysfunction 7 . In fact, patients with diabetic DSP are 2–3 times more likely to fall than those with diabetes and no neuropathy 7 . Additionally, patients with severe DSP are at risk for ulcerations and lower extremity amputations, with 15% developing an ulcer during the course of their disease 8 . Diabetes is the leading cause of lower extremity amputations, with a 15-fold increase in the likelihood of this life-changing complication 9 . Moreover, 80,000 lower extremity amputations are performed each year in patients with diabetes 9 . Overall, diabetic DSP can severely affect quality of life, particularly in those with pain 6 .
This common, disabling disease also profoundly impacts the health care system. Costs associated with diabetic neuropathy are estimated to be between 4.6 and 13.7 billion dollars, with most of the cost attributed to those with type 2 diabetes 8 . Therefore, neuropathy is associated with one fourth of the total costs of diabetes care in the United States.
Neuropathic pain is one of the major disabling symptoms of patients with DSP. It is a difficult condition to treat and therefore causes significant patient suffering and societal burden 10 . It is estimated that diabetic neuropathic pain (DNP) develops in 10% to 20% of the diabetic population overall, and can be found in 40% to 60% with documented neuropathy 11 – 13 . However, these numbers are likely to be underestimates, as one study showed that approximately 12% of patients with DNP had never mentioned this condition to their doctors 11 . Like other types of neuropathic pain, DNP is characterized by burning, electric, and stabbing sensations with or without numbness. Frequently, patients develop allodynia (painful sensations to innocuous stimuli) and hyperalgesia (increased sensitivity to painful stimuli). However, less than half are treated for pain, despite many available effective therapies 11 . Fortunately, there are multiple neuropathic pain screening instruments available to aid the clinician in identifying those who would benefit from treatment 14 .
Other types of peripheral nerve injury that can occur in patients with diabetes includes small fiber predominant neuropathy, autonomic neuropathy, radiculoplexopathy (diabetic amyotrophy), radiculopathy, mononeuritis multiplex, mononeuropathy, and treatment-induced neuropathy ( Figure 1 ). Small fiber-predominant neuropathy is an increasingly recognized pattern of involvement and typically is an early manifestation ( Figure 2 ). In fact, patients often progress from a small fiber-predominant neuropathy to a DSP. Small fiber-predominant neuropathy can be difficult to diagnose because the examination (decreased reflexes, impaired vibration, weakness) and electrodiagnostic testing can be normal. Autonomic neuropathy (a type of small fiber neuropathy) is also common in patients with diabetes. Symptoms include gastroparesis, constipation, urinary retention, erectile dysfunction, and cardiac arrhythmias. Importantly, patients with autonomic neuropathy are at a greater than 2-fold increased risk of death 15 . Diabetic radiculoplexopathy can involve either the lumbosacral (more common) or the cervical plexus. Patients present with pain and weight loss followed by weakness in the distribution of the involved plexus. Pathology reveals evidence of ischemic injury and a microvasculitis, but whether immunosuppressive medications are effective is unclear 16 , 17 . To date, only one randomized controlled trial has been completed for diabetic lumbar radiculoplexopathy and no significant effect was found on the primary outcome, although secondary outcome measures did show improvement with intravenous methylprednisolone 17 . Diabetes is also one of the few causes of non-compressive radiculopathy. Patients with diabetes can also present with mononeuritis multiplex without an underlying rheumatologic cause. Furthermore, patients are at increased risk of mononeuropathy which can be secondary to compressive or ischemic mechanisms. The most commonly involved nerves are the oculomotor and median nerves. Whether the mechanism of these four peripheral nerve manifestations (radiculoplexopathy, radiculopathy, mononeuritis multiplex, mononeuropathy) is the same is unclear. Though poor glucose control is associated with increased risk for neuropathy, the treatment of diabetes can also cause neuropathy. Treatment-induced neuropathy presents as acute pain and/or autonomic involvement, usually after the institution of insulin but can happen after any quick establishment of glucose control 18 . The pain and autonomic features can improve significantly with time, and this pattern of nerve injury underscores the fact that even quick improvements in glucose control can lead to neuropathy. Of note, there are also a substantial number of patients with diabetes that have asymptomatic neuropathy 19 . Thus, while DSP accounts for the vast majority of neuropathic manifestations in patients with diabetes, there are other important conditions for physicians to consider.

Many patterns of nerve injury are observed in patients with diabetes. By far the most common neuropathy subtype is distal symmetric polyneuropathy (DSP), which is the focus of this review. However, clinicians should be aware of all potential patterns as they have implications for the evaluation and treatment of these patients. For example, patients with diabetes can develop a radiculopathy without a disc herniation or degenerative changes in the spine. This knowledge could prevent a patient from spine surgery in the case where imaging results are equivocal. Furthermore, patients with diabetes can have more than one pattern of nerve injury, and the clinician needs to ask patients about specific symptoms such as autonomic involvement, which is often overlooked. The following patterns are shown in the figure: (A) DSP, small fiber predominant neuropathy, treatment induced neuropathy (B) radiculoplexopathy, radiculopathy (C) mononeuropathy, mononeuritis multiplex (D) autonomic neuropathy, treatment induced neuropathy. Note that small fiber predominant neuropathy has the same pattern as DSP but that the neurologic examination and electrodiagnostic studies are quite different, which has the potential to lead the clinician astray. Diabetic radiculoplexopathy may be responsive to immunotherapy and in contrast to most nerve injury in patients with diabetes, usually improves with time 16 , 17 . Treatment induced neuropathy is an under-recognized phenomenon 18 . Unlike the other peripheral manifestations of diabetes, this condition is caused by overaggressive control of glucose levels.

(A) Skin biopsy evaluating intra-epidermal nerve fiber density (stained with protein gene product 9.5, 50 micrometer sections) from a 41 year old male without neuropathy. Two nerves are seen crossing the dermal-epidermal junction. (B) Skin biopsy evaluating intra-epidermal nerve fiber density from a 50 year old male with diabetic neuropathy. No nerves are seen crossing the dermal-epidermal junction. (C) Sural nerve biopsy from a 44 year old male with diabetic neuropathy (40X magnification). Biopsy reveals axonal loss of small and large diameter nerves.
Glucose control in type 1 and type 2 diabetes
A body of research conducted over the past 20 years has added to our knowledge of glucose control as a modifiable risk factor for the development of neuropathy in patients with diabetes ( Table 1 ). Seventeen randomized, controlled clinical trials have studied the effects of enhanced glucose control over at least a 12 month period on neuropathy 20 – 37 . Seven of these studies focused on patients with type 1 diabetes, but only two of these reported on outcomes related to clinical impairment 20 , 23 , 26 , 29 , 31 , 32 , 34 . In 1993, the DCCT study group followed over 1400 subjects for five years and found a 60% reduction in the development of neuropathy in those receiving more frequent insulin dosing 20 . Similarly, Linn et al in 1996 followed 49 subjects for 5 years and reported an approximately 70% reduction in the development of neuropathy in those with enhanced glucose control 32 . Both of these groups revealed a large, statistically significant reduction in the development of neuropathy with tighter glucose control. Furthermore, only one of the seven studies did not show a statistically significant benefit of tighter glucose control.
Clinical trials investigating the role of enhanced glucose control on neuropathy
In contrast to the robust results seen in subjects with type 1 diabetes, the 8 randomized, controlled trials in type 2 diabetes have produced less definitive results 21 , 22 , 24 , 25 , 28 , 30 , 33 , 36 , 37 . Only four of these studies investigated the effects of glucose control on clinical impairment secondary to neuropathy. In 2010, the ACCORD study group compared the effectiveness of a lower hemoglobin A1C goal (less than 6 compared to 7–7.9) on the Michigan Neuropathy Screening instrument 28 . In the more than 5500 subjects followed for a median of 3.7 years, they discovered a 7% reduction in the development of neuropathy, which was not statistically significant. In 2009, Duckworth et al followed 1791 military veterans for a median of 5.6 years and found a non-significant 5% reduction in the development of neuropathy 24 . Studies by Azad et al and Tovi et al followed much smaller numbers of patients and their results produced relative risks (RR) with large confidence intervals (no statistically significant differences) 22 , 37 . However, three of the four studies that investigated nerve conduction studies and/or quantitative sensory testing revealed statistically significant results in favor of glucose control. One such study, performed by the UKPDS study group in 1998, is the second largest and longest randomized, controlled trial in patients with type 2 diabetes 21 . The main neuropathy outcome measure in this study was vibration threshold using a biothesiometer. This group followed 3867 subjects for 15 years and reported a RR of 0.95 (95%CI 0.76–1.18) at 3 years, 0.88 (95%CI 0.72–1.08) at 6 years, 0.84 (95%CI 0.68–1.04) at 9 years, 0.92 (95%CI 0.70–1.20) at 12 years, and 0.60 (95%CI 0.39–0.94) at 15 years (the only statistically significant result) in those receiving enhanced glucose control. Overall, these eight studies support only a modest reduction in the development of neuropathy in patients with type 2 diabetes receiving enhanced glucose control, which is in stark contrast to the substantial effect in those with type 1 diabetes. Possible explanations for this difference include the different outcome measures used, the different treatment regimens, the higher incidence of neuropathy in control subjects with type 2 diabetes, and the difference in baseline glucose control in these clinical trials. However, despite the similarities between type 1 and type 2 diabetes, these trials highlight the significant differences which exist in the disease mechanisms and complications of the two.
Pathophysiology of type 1 and type 2 diabetic neuropathy
Hyperglycemia is a major factor underlying diabetic neuropathy, but other changes also contribute. In type 2 diabetes, dyslipidemia is thought to play a major role 38 . Changes in insulin signaling are also key; in type 1 diabetes levels of both insulin and C-peptide are reduced, whereas in type 2 diabetes there is thought to be reduced neuronal insulin sensitivity 39 , 40 . Several recent reviews discuss the mechanisms of diabetic neuropathy in depth 38 , 41 – 43 . Therefore, we will briefly outline the major mechanisms ( Figure 3 ) and consider how the disease states in type 1 and type 2 diabetes are different, and why this may impact treatment efficacy.

Factors linked to type 1 diabetes (yellow), type 2 diabetes (blue) and both (green) cause DNA damage, ER stress, mitochondrial dysfunction, apoptosis and loss of neurotrophic signaling. This cell damage can occur in neurons, glial cells and vascular endothelial cells, as well as triggering macrophage activation, all of which can lead to nerve dysfunction and neuropathy. The relative importance of the pathways in this network will vary with cell type, disease profile and time. Abbreviations: AGE, advanced glycation end-products; LDL, low-density lipoprotein; HDL, high-density lipoprotein; FFA, free fatty acids; ROS, reactive oxygen species (red star); ER, endoplasmic reticulum; PI3K, phosphatidylinositol 3-kinase.
Hyperglycemia
Excess intracellular glucose is processed by increased flux through one or more glucose metabolism pathways, and prolonged hyperglycemia can lead to cellular damage in several ways. First, excess glycolysis may lead to overload of the mitochondrial electron transport chain and generation of reactive oxygen species (ROS) 43 . Second, increased flux through the polyol pathway can increase cellular osmolarity, reduce NADPH levels and lead to oxidative stress 44 . Finally, increased flux through the hexosamine pathway is associated with inflammatory injury 41 .
Another consequence of hyperglycemia is the generation of advanced glycation end products (AGEs) 45 , via attachment of reactive carbohydrate groups to proteins, lipids or nucleic acids. This tends to impair the biological function of protein AGEs, thus impacting cellular function 46 . Extracellular AGEs also bind to the receptor for AGE (RAGE), initiating inflammatory signaling cascades, activating NADPH oxidases and generating oxidative stress 47 . Long-term inflammatory responses are also triggered, including the upregulation of RAGE and activation of NFκB 48 .
Dyslipidemia
There is a high incidence of dyslipidemia in type 2 diabetic patients 49 . Dyslipidemia is linked to diabetic neuropathy 50 , and several underlying mechanisms have been identified. Free fatty acids (FFAs) have been shown to directly cause injury to Schwann cells in vitro 51 , but also have systemic effects such as promoting inflammatory cytokine release from adipocytes and macrophages 52 . Plasma lipoproteins, particularly low-density lipoproteins (LDLs), can be modified by oxidation (oxLDL) and/or glycation, and these modified LDLs can bind to extracellular receptors (including the oxLDL receptor LOX1 53 , Toll-like receptor 4 54 and RAGE 47 ), triggering signaling cascades that activate NADPH oxidase and subsequent oxidative stress 53 . Additionally, cholesterol may be oxidized to oxysterols, which have been shown to cause apoptosis in neurons 41 , 55 .
Impaired insulin signaling
While insulin is not involved in glucose uptake into neurons, it has been shown to have neurotrophic effects, promoting neuronal growth and survival 56 , 57 . Reduction of this neurotrophic signaling due to insulin deficiency (type 1 diabetes) or insulin resistance (IR; type 2 diabetes) is thought to contribute to the pathogenesis of diabetic neuropathy 39 . In neurons, IR occurs by inhibition of the PI3K/Akt signaling pathway, similarly to IR in muscle and adipose tissues 42 . Disruption of this pathway may also lead to mitochondrial dysfunction and oxidative stress, further promoting neuropathy 39 .
In type 1 diabetes, reduction in C-peptide may lead to nerve dysfunction in a number of ways, including reduction in Na/K ATPase activity, endothelial nitric oxide synthase (eNOS) activity, and endoneurial blood flow 40 . Treatment with C-peptide may slow progression of neuropathy in type 1 diabetic patients 58 .
The mechanisms outlined above lead to multiple cellular disturbances, including mitochondrial dysfunction, endoplasmic reticulum (ER) stress, DNA damage and apoptosis. Another layer of complexity is added when you consider that these processes of cell stress and/or damage occur in several different cell types within the nerves, including neurons (in axons and at nerve terminals), glial cells, and endothelial cells of the microvasculature. Furthermore, many of these changes will trigger activation and recruitment of macrophages 59 , feeding back into inflammatory mechanisms of cell stress and death. Ultimately, these different forms of cellular stress cause dysfunction and/or death of the nerve, which manifests as clinical neuropathy.
As discussed above, tight glucose control can reduce neuropathy in type 1 diabetic patients but is not as efficacious in type 2 patients 20 , 21 . This is likely to be related to differences in the underlying mechanisms: hyperglycemia and reduction in insulin signaling in type 1 diabetes, compared with a combination of hyperglycemia, dyslipidemia and IR in type 2 diabetes. Differences in the duration of pro-neuropathic changes prior to the onset/diagnosis of diabetes may also contribute to the differences in neuropathy progression between the two diseases. A patient does not typically develop type 2 diabetes rapidly; it occurs after many years of obesity and other aspects of the metabolic syndrome (see below). Tight glucose control will not necessarily reduce the dyslipidemia, systemic inflammation and IR, and following years of these insults it is not entirely surprising that neuropathy is difficult to halt/reverse. Although hyperglycemia contributes to the vicious cycles of oxidative stress, inflammation and cellular damage in type 2 diabetes, reducing hyperglycemia alone may not be enough to stop the cycle from continuing.
Pre-diabetes and neuropathy
Whereas the link between diabetes and neuropathy is well-established, there remains scientific uncertainty regarding the effects of pre-diabetes (impaired fasting glucose and/or impaired glucose tolerance (IGT)) on neuropathy. Two separate groups have shown that there is an increased prevalence of IGT in subjects with idiopathic neuropathy compared to literature-based controls 60 , 61 . A third group identified an increased prevalence of neuropathy in patients with IGT compared with controls 62 . In addition, Smith et al followed a cohort of subjects with IGT and neuropathy that underwent an extensive diet and exercise regimen. They found that these subjects had an increase in nerve fiber density (NFD) over time, which is in stark contrast to historical controls 63 . The implication of this study is that treatment of IGT may improve neuropathy outcomes, although this study lacked a control group for comparison. In contrast, Hughes et al did not find a statistically significant association of IGT with neuropathy in a small case-control study 64 . Similarly, Dyck et al found no difference in the prevalence of neuropathy in patients with IGT compared to matched controls in a population based study in Olmsted County (abstract only) 65 .
Since there are conflicting data linking pre-diabetes with neuropathy, there is a need for a comprehensive study investigating pre-diabetes to understand if it is one of the metabolic “drivers” underlying the onset and progression of neuropathy. The answer has direct implications for potential therapies for many patients with neuropathy. Currently, one third of adult Americans meet criteria for pre-diabetes 66 . Since less than 5% have received a formal diagnosis from their providers and only a small percentage are being treated for this condition, establishing a causal relationship between pre-diabetes and neuropathy would change the clinical management of a substantial number of patients 66 .
Pain management in DNP
While glucose control is the only disease modifying therapy for diabetic neuropathy, pain management is the other mainstay of treatment that can dramatically improve the quality of life of these patients. Over the last two decades, tremendous effort has been made to improve the treatment of DNP using randomized placebo controlled clinical trials. Data from these trials have provided support for the use of certain pharmacological treatments for DNP, as outlined below. Taking into consideration the efficacy of these interventions, several guidelines have been generated. The 2006 and 2010 guidelines from the European Federation of Neurological Societies task Force (EFNS) 67 , 68 and the 2011 guidelines from the American Academy of Neurology (AAN), the American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation 69 are the most thorough and recent guidelines on this topic. According to these guidelines, several classes of drugs are considered to be effective for the treatment of DNP ( Table 2 ). Here we discuss and compare the EFNS and AAN guidelines ( Table 3 ). The consensus from these guidelines provides information for the best evidence-based practice for the treatment of DNP.
Published class I and class II evidence of pharmacological treatment for DNP
DNP = Diabetic neuropathic pain
Comparison of the EFNS and AAN guidelines
EFNS = European Federation of Neurological Societies task Force, AAN = American Academy of Neurology
A=Established as effective, B=Probably effective, C=Possibly effective
Anticonvulsants
Pregabalin is classified as effective with Level A evidence by both the EFNS and AAN guidelines. This recommendation is based on three to four class I studies that all revealed superiority of pregabalin compared with placebo. Interestingly, the effect size was small in the highest quality studies. The recommended dosage for pregabalin is 300–600 mg/d.
Gabapentin is also classified as effective with Level A evidence by the EFNS, though the AAN (Level B) did not consider it to be a Level A drug based on the fact that only one class I study showed benefit and that one negative class II study has been published 70 . In contrast, the EFNS guidelines are based on a meta-analysis of 7 trials with class I evidence for a systematic review. The recommended dose is 900–3600 mg/d.
Lamotrigine is classified as ineffective or with discrepant results with Level A/B evidence by the EFNS because of one negative class I study and one class II study that showed comparable efficacy to amitryptiline 71 . Lamotrigine is also not recommended by the AAN based on two class I studies that failed to show benefit compared with placebo 72 . Similarly, both guidelines state that oxcarbazepine and lacosamide are not effective with Level A/B (EFNS) or Level B (AAN) evidence.
Sodium valproate is classified as ineffective or with discrepant results with Level A/B evidence by EFNS. In contrast, this medication is classified as effective with Level B evidence by the AAN for doses of 500–1200 mg/d. The EFNS justified its decision to classify it as ineffective or with discrepant results because both positive studies were published from the same group 73 , 74 and one negative study has been published 75 . The negative study was not discussed in the AAN guideline. Of note, the two positive studies did not report a significant placebo effect or significant side effects that are usually attributed to this medication. The two guidelines disagree on whether the current evidence supports or refutes the effectiveness of sodium valproate for the treatment of DNP.
Antidepressants
Tricyclic antidepressants (TCAs) are classified as effective with Level A evidence by the EFNS based on two class I meta-analyses 76 , 77 ; however the EFNS guidelines do not provide a recommendation of a specific drug within the TCA class. In contrast, the AAN states that amitriptyline (25–100 mg/d) is supported by Level B evidence based on one class I and two class II studies 78 , 79 , 80 . The AAN guidelines state that there is insufficient evidence in regards to other TCAs because only class III evidence is available for these drugs (Level U evidence).
Serotonin norepinephrine reuptake inhibitors (SNRIs) such as venlafaxine and duloxetine are supported by both the EFNS (Level A) and the AAN (Level B) guidelines. The reason for the discrepancy in the level of evidence is that the EFNS describes three class I studies for duloxetine whereas the AAN classifies only one of these studies as class I. Similarly, the AAN classifies only one of the two studies of venlafaxine as class I. The recommended dosages are 75–225 mg/d for venlafaxine and 60–120 mg/d for duloxetine.
Controlled release oxycodone is recommended by EFNS as effective with Level A evidence based on two class I studies. In contrast, the AAN recommends both controlled release oxycodone (mean 37 mg/d and up to 120 mg/day) based on three class II trials and morphine sulfate (up to 120 mg/d) based on one class II trial 81 as effective with Level B evidence.
Tramadol 200–400 mg/d and 37.5 mg + acetaminophen were listed by the EFNS as Level A effective treatments based on two class I studies. In contrast, only tramadol 210 mg/d was recommended by the AAN as effective with Level B evidence for DNP (two class II studies).
Other medications
Dextromethorphan (400 mg/d) is classified as effective with Level B evidence in both EFNS and AAN guidelines based on one class I and one class II study.
Topical capsaicin treatment (0.075% QID) is supported with Level B evidence in the AAN guidelines based on one class I 82 and one class II study 83 . However, EFNS classified capsaicin as Level A/B for inefficacy or discrepant results based on a systematic review of 5 class I–II studies.
Isosorbide dinitrate spray is backed by Level B evidence in the AAN guideline based on one class I trial 84 . Similarly, the EFNS cited the same study but also reported a study that used glyceryl trinitrate spray (class I) and determined treatment with nitrate derivatives is supported with Level A evidence based on these two studies 85 .
Nicotine derivative ABT-594 is listed by the EFNS as effective with Level A evidence based on one class I study 86 . This treatment is not discussed in the AAN guidelines.
Botulinum toxin and levodopa are classified as effective with Level B evidence by the EFNS based on one class II study for each medication; however, neither is discussed in the AAN guidelines.
The lidocaine patch is classified as effective with Level C evidence by the AAN based on two class III studies, but it was not discussed in the EFNS guidelines.
Overall, these two guidelines are in close agreement for the vast majority of medications evaluated. However, the data for sodium valproate and capsaicin cream is conflicting with one guideline providing evidence for and one revealing evidence against their use. The more subtle differences in Levels of evidence are likely due to the fact that the AAN guidelines required a completion rate of greater than 80%, which was not required by the EFNS. Therefore, many trials were downgraded from class I to class II because of this stringent criteria with a resulting effect that only pregabalin was shown to have Level A evidence in the AAN guidelines. Of note, with increasing knowledge of the proper conduct and reporting of clinical trials through the years, there is likely a bias in favor of newer medications. Furthermore, the levels of evidence do not take into account the number needed to treat or the number needed to harm. Rather, the levels of evidence are based on the number of high quality studies that show benefit. Unfortunately, few studies compare medications head to head or evaluate for effect on quality of life. Future studies are needed to further clarify the role of these medications in the treatment of DNP.
Treatment algorithm
Several review articles recommended treatment algorithms for DNP based on their efficacy and safety ( Figure 4 ). We reviewed algorithms from Jensen et al. 87 and EFNS 68 for treating DNP and Dworkin et al for treating neuropathic pain 88 , 89 . Importantly, there is no evidence to support one treatment algorithm versus another.

First and seconds line treatments for diabetic painful neuropathy.
1st line treatment
All three algorithms recommend gabapentin, pregabalin, TCAs, venlafaxine, and duloxetine as first line medications. Which agent to choose is largely determined by co-morbidities of the patient and side effect profiles of the medications. This is especially important in treating DNP because none of the drugs were designed specifically for neuropathic pain and therefore they each have other indications such as the treatment of seizures and depression. Dworkin et al also recommends topical lidocaine for those with localized neuropathic pain and in those with concern for central nervous system side effects. All three sources recommend titrating a first line medication to a maximum tolerated dose before switching to a second first line medication or combination therapy. Only once all these options fail is a second line agent recommended. All of these medications are supported by Level A evidence in the EFNS guidelines and by Level A or B evidence in the AAN guidelines.
All three algorithms also support opioid analgesics and tramadol as second line medications. While these medications are also backed by Level A evidence in the EFNS and Level B evidence in the AAN guidelines, concern exists over their long term use given their addiction potential, side effect profile, and waning effectiveness over time.
None of the recommendations incorporate cost into the decision, but this is also an important consideration for not only the patients, but also the health system. TCAs are the most affordable of the first line agents. Gabapentin and venlafaxine are cheaper than pregabalin and duloxetine, respectively.
Metabolic syndrome: implications for future treatments
Currently, glucose control and pain management are the backbones of treatment for diabetic neuropathy. However, glucose control is not the sole answer as patients with adequate glucose control continue to develop neuropathy or their neuropathy worsens over time. Furthermore, pain management is not a disease modifying therapy. Therefore, discovery of modifiable risk factors for neuropathy is essential, with metabolic syndrome (MetS) components representing one possibility. Over the last 10 years, there has been an increased interest in the possible role of MetS in the development of neuropathy. In 2001, Isomaa et al compared 85 subjects with MetS and type 2 diabetes to subjects without MetS controlled for age, gender, glycemic control, and duration of diabetes 90 . They found that subjects with MetS had a higher prevalence of neuropathy, but that in multiple logistic regression models, MetS and its components were not associated with neuropathy. Later, Costa et al and the Metascreen investigators used cross-sectional designs to independently show that MetS was associated with neuropathy in subjects with diabetes 91 , 92 . In 2007, Cull et al utilized the UKPDS cohort of 5102 subjects with type 2 diabetes followed for 10.3 years to assess for the association of MetS with neuropathy by using four different definitions of MetS 93 . They found that MetS was associated with a combined macrovascular endpoint, but not with a combined microvascular endpoint. Recently in 2008, Smith et al compared subjects with idiopathic neuropathy and normoglycemia to those with IGT and discovered that each group had the same prevalence of the separate components of the MetS 94 . This result suggests that the other components of the MetS besides IGT may have a role in the development of neuropathy. However, these studies have almost all been carried out on subjects with diabetes, have used cross-sectional designs, and have utilized inconsistent definitions of neuropathy.
Complementing the studies investigating the role of the MetS on neuropathy, many groups evaluated the effect of the individual MetS components on neuropathy. In 1994, Straub et al conducted a cross-sectional study of 91 subjects with type 2 diabetes, and stratified them based on Body Mass Index (BMI) 95 . Those subjects with a BMI > 26.5 had a worse clinical neuropathy score than those with a lower BMI. However, this study did not account for any confounding factors to this association. In 2005, Tesfaye et al followed 1172 patients with type 1 diabetes for a median of 7.3 years and found that BMI and smoking were independent risk factors for the development of neuropathy 96 . They also found associations with hypertension and LDL in minimally adjusted models. In the same year, De Block and colleagues performed a cross-sectional study in 592 subjects with type 1 diabetes 97 . Their study revealed no association between BMI, lipid abnormalities, triglycerides, or hypertension and neuropathy. More recently in 2009, Van Acker et al investigated 1111 subjects with diabetes in a cross-sectional design 6 . They discovered that obesity, HDL, and triglyceride levels were all independently associated with neuropathy. Moreover, Wiggins et al revealed that diabetic subjects with progressive neuropathy had higher triglyceride levels compared to non-progressors. 50 While most studies have shown an association between some MetS components and neuropathy, all of these studies have been performed in subjects with frank diabetes, most have used a cross-sectional design, and the definition of neuropathy has differed between studies. Given the conflicting results reported to date, further studies are needed to adequately define the role of MetS in the development and progression of neuropathy. There is also a need to better understand the underlying mechanisms by which MetS components cause neuropathy.
Pathophysiology of the Metabolic Syndrome on neuropathy
Our knowledge of how MetS components damage nerves is rapidly evolving. We have already outlined the involvement of dyslipidemia and IR and their contribution to neuropathy in patients with type 2 diabetes (see “Pathophysiology of type 1 and type 2 diabetic neuropathy”). Another central MetS component, visceral adiposity, may be particularly detrimental as it causes increased plasma FFAs and also induces a pro-inflammatory state by secretion of adipokines (also contributing to development of IR) 98 . Hypertension, another aspect of the MetS, may also be connected to neuropathy, though the link is less well-established. The renin-angiotensin system (RAS), which controls blood pressure, is upregulated in obesity, and may also contribute to development of type 2 diabetes (in part through promotion of IR and pro-inflammatory cytokine secretion from adipose tissue) 99 . Angiotensin-converting enzyme (ACE) inhibitors have been shown to improve diabetic neuropathy in animal studies 100 , 101 , but the mechanism is unclear. Microvascular dysfunction in the nerve and decreased endoneurial perfusion are also thought to contribute to neuropathy 102 . While this may be regulated by metabolic factors, upregulation of RAS might also contribute 102 .
These mechanisms are likely to be linked at multiple levels. Indeed, in terms of the mechanisms linking MetS and type 2 diabetes to neuropathy, it may be more accurate to describe these pathways as a network in which hyperglycemia, IR, dyslipidemia, systemic inflammation and RAS activation all feed into a self-perpetuating cycle of oxidative stress, inflammatory signals and disruption of normal cellular function. Thus, even in the absence of overt diabetes, other aspects of the MetS may be sufficient to cause neuropathy. One of the major challenges for research scientists is to determine which aspects of this network of mechanisms can be blocked at which times to effectively limit/prevent progression of the neuropathy.
Conclusions and future directions
Diabetes can injure peripheral nerves in a variety of distributions. The most common pattern is DSP, which is characterized by numbness, tingling, pain, and/or weakness that affect the nerves in a “stocking and glove” pattern beginning in the distal extremities. DSP leads to substantial pain, morbidity, and impaired quality of life. Societal, personal, and healthcare costs associated with diabetic neuropathy are high. Unfortunately, few interventions are currently available for the remediation of non-painful symptoms, and glucose control is the only proven disease-modifying intervention for these patients. While pain is a common feature, it is often under-reported and undertreated. However, many effective therapies exist for DNP including medicines designed to treat seizures and depression. Evidence-based consensus guidelines have been created to guide the use of these pain interventions.
There are many areas of research that are yet to be fully explored in regards to diabetic neuropathy, which could lead to improved prevention and treatment of the condition. The magnitude of the effect of glucose control on neuropathy is much smaller in patients with type 2 diabetes as compared to patients with type 1 diabetes. Given this small effect size and that many patients with type 2 diabetes continue to develop neuropathy despite adequate glucose control, discovery of modifiable risk factors for neuropathy is essential. MetS components, including pre-diabetes, are potential risk factors for neuropathy, and future studies are needed to define whether they are causally related to neuropathy with direct implications for new treatments.
Acknowledgments
Funding is provided by the A. Alfred Taubman Medical Research Institute, the Program for Neurology Research and Discovery, and the Kathy Rayner Program. B.C. is supported by an American Diabetes Association Junior Faculty Award and H.C. is supported by the NIH (KO8 NS061039-01A2A). C.S is supported by a JDRF fellowship. E.F. is supported by NIH 1 DP3 DK094292 and NIH 1 R24 DK082841. We thank J. Boldt and G. Walker for excellent secretarial support during the preparation of the manuscript. B.C. had full access to all the data and had final responsibility for the decision to submit for publication.
Contributors
Conflicts of Interest
There are no conflicts of interest to declare.

IMAGES
VIDEO
COMMENTS
It is estimated that 60-100% of the diabetic population has some form of neuropathy, ranging from barely detectable asymptomatic neuropathy to severe disabling painful disease to dense anesthesia. Few doubt that duration and degree of hyperglycemia are the major culprits, although the mechanisms involved are complex, not adequately or ...
Diabetic peripheral neuropathy is a symmetric distal sensorimotor neuropathy. Neuropathy is one of the most common complications of DM. The prevalence of neuropathy increases with duration of the disease and with degree of glycemic control with poorer control being associated with higher risks. Diabetic neuropathy can all types of neurons including
Three case reports in this article illustrate the diagnostic methods used and the treatment course encountered for many patients with diabetic peripheral neuropathic pain (DPNP). Each case addresses an aspect of DPNP: pain that appears to be refractory to initial therapy, DPNP occurring with other medical conditions, and nondiabetic neuropathy occurring in patients with diabetes mellitus.
Diabetic neuropathy. Diabetic neuropathy. Dr. Tushar Patil ... At the beginning of the presentation is a case study, a patient admitted and treated for chronic kidney disease. Other parts covered include relevant anatomy and physiology, aetiopathogenesis and pathophysiology of the condition, as well as management and prevention. ...
2. DEFINITION An internationally agreed simple definition of Diabetic neuropathy for clinical practice is "the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after the exclusion of other causes" Boulton AJ et al. Diabetic neuropathies: a statement by the American Diabetes Association.
Diabetic Neuropathy - Download as a PDF or view online for free. Submit Search. Upload. Diabetic Neuropathy ... Case Presentation: Hypertension (A case on refusal of Evidence Based Medicine) Case Presentation: ... case study on parkinson disease.
suggestive of overt diabetes. A nerve conduction study . performed four years prior was interpreted as bilateral sural neuropathy versus possible early peripheral neuropathy. The patient was referred to Neurology who noted that while the HbA1c did not suggest overt diabetes, the patient did have a single random glucose value of 200 mg/dL in the ...
Summary We present three cases of acute diabetic neuropathy and highlight a potentially underappreciated link between tightening of glycaemic control and acute neuropathies in patients with diabetes. Case 1: A 56-year-old male with poorly controlled type 2 diabetes (T2DM) was commenced on basal-bolus insulin. He presented 6 weeks later with a diffuse painful sensory neuropathy and postural ...
Several medications commonly used by people with diabetes may be associated with neuropathy. Evidence of a beneficial role for pyridoxine in the treatment of neuropathy is inconclusive. Dace L. Trence, MD, FACE, is associate director of the Diabetes Care Center and an assistant professor in the Division of Nutrition, Endocrinology, and ...
Three case reports in this article illustrate the diagnostic methods used and the treatment course encountered for many patients with diabetic peripheral neuropathic pain (DPNP). Each case addresses an aspect of DPNP: pain that appears to be refractory to initial therapy, DPNP occurring with other medical conditions, and nondiabetic neuropathy occurring in patients with diabetes mellitus.
Introduction. Hydrodissection was first proposed in 1997. It is used in treating entrapment neuropathy and inflammatory peripheral neuropathy and plays an important role in separating adjacent tissue and protecting nerves and blood vessels via the injection of saline or other agents to build a non-existent surgical plane (1, 2).Previously, Smith has studied the treatment of carpal tunnel ...
Painful diabetic peripheral neuropathy (DPN) is described as a superficial burning pain associated with other positive and/or negative sensory systems affecting the feet and lower extremities. It is one of the most commonly encountered neuropathic pain syndromes in clinical practice. Presentation may be complicated by multiple symptoms ...
Abstract. Three case reports in this article illustrate the diagnostic methods used and the treatment course encountered for many patients with diabetic peripheral neuropathic pain (DPNP). Each case addresses an aspect of DPNP: pain that appears to be refractory to initial therapy, DPNP occurring with other medical conditions, and nondiabetlc ...
View DIABETIC-NEUROPATHY-PPT.pptx from CON 102345 at Nueva Ecija University of Science and Technology. CASE OF DIABETIC NEUROPATHY Presented by: BSN III - Group A Jenny Juniora Ajoc George Palteng. ... CASE STUDY ASSIGNMENT: ASSESSING NEUROLOGICAL SYMPTOMS Case Study 2: Numbness and Tingling A 48 year old male with a history of diabetes ...
Current treatment options for DPN include mainly antidepressants and anticonvulsants, with other agents such as tramadol, dextromethorphan and memantine being employed or studied. This review article includes a case study of a patient with painful DPN to demonstrate the current management strategies for this neuropathic pain syndrome.
Case Studies In Painful Diabetic Neuropathy. Approximately 800,000 new cases of diabetes mellitus are diagnosed each year. The disease affects over 18 million people, approximately 6 percent of the population of the United States.1 Type 2 diabetes, which is typically not diagnosed in patients under age 45, is overwhelmingly the most prevalent ...
Online Exclusive. Case Study: Working Through The Differential Diagnosis Of Diabetic Neuropathy. Kathleen Satterfield, DPM. March 2009. Copied to clipboard. Given that neuropathy can have a complex range of manifestations, the diagnosis of the condition is not always as obvious as it initially seems. This author emphasizes the importance of a ...
After clinical evaluation and a nerve conduction study, diagnosis of diabetic cachectic neuropathy was made based on the rapid onset of severe neuropathic pain in the context of diabetic neuropathy, marked weight loss, and depressed mood. ... Case Presentation. M. M., a 46-year-old man, was admitted to the surgical department of Modena Hospital ...
Case Studies in Pain Management - October 2014. ... Chapter 8 - Diabetic neuropathy. from Section 1 - Neurological Disorders. Published online by Cambridge University Press: 05 October 2014 By. Gulshan Doulatram and. Tilak Raj. Edited by. Alan David Kaye and. Rinoo V. Shah. Show author details
Diabetic neuropathy is a type of nerve damage that can occur if you have diabetes. High blood sugar (glucose) can injure nerves throughout the body. Diabetic neuropathy most often damages nerves in the legs and feet. Depending on the affected nerves, diabetic neuropathy symptoms include pain and numbness in the legs, feet and hands.
The following case study illustrates the clinical role of advanced practice nurses in the management of a patient with type 2 diabetes. Case Presentation A.B. is a retired 69-year-old man with a 5-year history of type 2 diabetes.
Download now. Case study- Peripheral Neuropathy (Nerve Care forum) 1. DR SUDHIR KUMAR MD DM CONSULTANT NEUROLOGIST APOLLO HOSPITALS, HYDERABAD Case Study. 2. 2 Case Study Patient presentation A 49 year old man A Mechanical Engineer by profession, had weakness & wasting of Bilateral hands 6 months back. History: Initially noticed - inability ...
Diabetic peripheral neuropathy is a prevalent, disabling condition. The most common manifestation is a distal symmetric polyneuropathy (DSP), but many patterns of nerve injury can occur. Currently, the only effective treatments are glucose control and pain management. While glucose control dramatically decreases the development of neuropathy in ...
A 41-year-old Hispanic woman presents to her worksite clinic for an evaluation of acute onset polyuria and polydipsia. She was diagnosed with type 2 diabetes approximately 21 months earlier.
We report the case of a 27-year-old man with transthyretin amyloidosis secondary to the p.Val142Ile mutation with an atypical clinical presentation of predominantly lower limb polyneuropathy without cardiac involvement. p.Val142Ile is mainly associated with cardiopathy, whereas the neuropathic phenotype is mainly associated with p.Val50Met.