An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Respir Med Case Rep

Case report: Three adult brothers with cystic fibrosis (delF508-delF508) maintain unusually preserved clinical profile in the absence of standard CF care
We present three cases in this report. Three adult brothers, homozygous for the delF508 cystic fibrosis mutation, have maintained an unusually preserved clinical condition even though they did not attend a CF Clinic during their childhood, do not attend a CF Clinic now, and do not follow standard CF care guidelines. The brothers use an alternative CF treatment regimen on which they have maintained normal lung function, height/weight, and bloodwork, and they utilize less than half the recommended dosage of pancreatic enzymes. The brothers culture only methicillin-sensitive Staphylococcus aureus, and have never cultured any other bacteria. Highly effective modulator therapies, such as elexacaftor/tezacaftor/ivacaftor, do not substantially reduce infection and inflammation in vivo in CF patients, and thus these three case reports are of special note in terms of suggesting adjunct therapeutic approaches. Finally, these three cases also raise important questions about standard CF care guidelines.
- • Three adult brothers, delF508 cystic fibrosis (CF) homozygotes, maintain unusually preserved clinical condition absent standard CF care.
- • An alternative CF treatment regimen has kept their lung function, weight/height, and lab parameters normal, with low pancreatic enzyme dose.
- • The brothers culture only methicillin-sensitive Staphylococcus aureus, and have never cultured any other bacteria.
- • Highly effective modulator therapies (HEMT) for CF do not substantially reduce infection and inflammation in vivo; these cases are thus of note.
- • These cases also raise important questions about standard CF care guidelines.
1. Introduction
Cystic fibrosis (CF) is a serious and life-shortening genetic disorder affecting approximately 70,000 persons worldwide [ 1 ]. Respiratory failure is the foremost cause of death in CF patients, and lung transplantation is often considered in end-stage CF disease. For those born with CF in the last five years, median predicted survival age is now 44, which is decades longer than survival rates in the recent past [ 2 ]. Indeed, new advances in CF modulator therapy and CF gene therapy may eventually provide a normal life expectancy for these individuals.
A key approach in fighting the ravages of CF while waiting for more advanced treatments to be developed has been to slow the inexorable decline in lung function. Typical rate of lung function decline in CF is approximately −1.2 to −1.6 FEV1% per year [ 3 ]. Rate of decline is strongly associated with type of CF mutation. The three most severe classes of CFTR, Classes I, II, and III, represent defects in protein production, protein processing, and protein regulation, respectively [ 4 ]. The most common CF-causing mutation is delF508, occurring in 70% of cases, which is a Class II mutation [ 5 ]. Being homozygous for the delF508 mutation confers a severe phenotype, including pancreatic insufficiency and a steeper rate of decline in lung function over time [ 6 ]. In the United States, it is estimated that approximately 50% of those with cystic fibrosis are homozygous for delF508 [ 7 ]. Standard clinical care for severe mutation cases is often aggressive, including but not limited to daily airway clearance, use of pancreatic enzymes at the level of 500-2,500 lipase units/kg/meal (and enteric feeding if adequate weight percentile cannot be maintained), common and repeated use of oral, inhaled and intravenous antibiotics, daily intake of water-miscible versions of fat-soluble vitamins, and quarterly CF Clinic visits where lung function parameters and cultures of lung bacteria and fungi are assessed [ 8 , 9 ]. Pulmonary exacerbations often result in hospitalization, which may occur one or more times per year, typically lasting 14–21 days and including intensive antibiotic treatment and chest physical therapy. Everyday treatment burden is high, with estimates of 2–3 hours per day, with adherence at an estimated 50% or less [ 10 ]. The mean annual cost of standard supportive CF care in the US in 2016 (in 2019 dollars), before CFTR modulator therapies, was estimated to average $77,143, with severe non-transplant cases experiencing multiple pulmonary exacerbations costing on average triple or quadruple that amount [ 11 ]. With the average cost of elexacaftor/tezacaftor/ivacaftor (Trikafta) treatment currently over $311,000 per year, average standard supportive CF care costs were expected to double in 2019 [ 12 ] and increase further over time, perhaps quadrupling, with wider adoption of that treatment by all eligible patients.
Here we report on three adult brothers who are delF508 homozygotes, and yet who have maintained an unusually preserved clinical profile in the absence of standard CF clinical care. At the time of this writing, Brother A is 23 years old, Brother B is 21 years old, and Brother C is 18 years old. They are full-blooded siblings.
2. Case reports
2.1. brother a.
Brother A, now aged 23, was born full-term weighing 10 lbs. 2 oz. to a carrier mother experiencing gestational diabetes who subsequently breastfed him. His weight percentile decreased significantly over time, and at 6 months, after a course of oral antibiotics for a suspected ear infection, he developed a severe Vitamin K deficiency manifesting in quarter-sized black bruises on his body, as well as Pseudo-Bartter Syndrome. He was hospitalized until IV fluids stabilized his condition and normalized his electrolytes. Vitamin K shots were also administered. At 9 months of age, he was diagnosed with cystic fibrosis, and the genetic mutation analysis identified him as a delF508 homozygote. Between the time of his hospitalization and his diagnosis, he suffered from malnutrition with accompanying protein edema and his weight percentile, which had been over 97th percentile when born, was under the 5th percentile adjusted for age and sex. Once started on pancreatic enzymes (CREON 5) after diagnosis, his weight percentile increased to approximately the 30th percentile.
Approximately one year after diagnosis, the parents of Brother A elected to depart from standard CF care, including an election to stop attending the CF Clinic, while continuing to be under the care of their family pediatrician. The treatment plan for the brothers is described in detail in a later section. The only prescription medicine taken during his childhood and continuing to this day remains CREON 5/6, with Brother A utilizing 4 CREON 5/6 per meal, less than half the lowest recommended dose for his weight. In the teen years, Brother A experienced three episodes of heat exhaustion requiring IV fluid stabilization in an emergency room, has had one endoscopic sinus cleaning for sinus pain at age 20, and also underwent an appendectomy for appendicitis at age 23, but otherwise has had no major clinical issues, though exhibiting digital clubbing. Brother A played ice hockey throughout his childhood and teen years. His height, weight, lung function, and lab results at age 23 are provided in Table 1 .
Clinical parameters, Brother A.
2.2. Brother B
Brother B, now aged 21, was born full-term, weighing 8 lbs. 8 oz., the mother supplementing with oral glutathione (GSH) during the pregnancy and subsequently breastfeeding him. Brother B has never attended a CF Clinic, was diagnosed at 2 weeks of age, and was under the care of the family's pediatrician only. Brother B's only prescription medication during his childhood was CREON 5/6, just as with Brother A, utilizing 4 capsules per meal. Brother B has never needed to be hospitalized or have surgery or antibiotics. While Brother B does not exhibit digital clubbing; when recovering from respiratory viruses, he does manifest a cough that lingers longer than it lingers for his brothers, though the cough ultimately resolves. Brother B played ice hockey in childhood and teen years, as well as participated in gymnastics, cross-country running, track and field, and weight-lifting. His height, weight, lung function, and lab results at age 21 are provided in Table 2 .
Clinical parameters, Brother B.
2.3. Brother C
Brother C, now aged 18, was born full-term weighing 9 lbs. 2 oz., the mother supplementing with oral glutathione (GSH) during the pregnancy and subsequently breastfeeding him. Brother C has never attended a CF Clinic, was diagnosed at 2 weeks of age, and was under the care of the family's pediatrician only. Brother C's only prescription medication during his childhood was CREON 5/6, just as with Brothers A and B, utilizing 4 capsules per meal. Brother C has never needed to be hospitalized, or have surgery or antibiotics. Brother C does not exhibit digital clubbing. Brother C played ice hockey in childhood and teen years, as well as participated in gymnastics. His height, weight, lung function, and lab results at age 18 are provided in Table 3 .
Clinical parameters, Brother C.
3. Description of treatment
Given the severity of the genotype involved and the almost complete non-adherence to standard CF guidelines (with the exception of a significantly lower-than-average dose of prescription pancreatic enzymes and a standard dose of water-miscible fat soluble vitamins), the preserved clinical profile of these three brothers is noteworthy. However, the family developed a regimen that went well beyond pancreatic enzymes and water-miscible vitamins. The treatment regimen is provided in Table 4 .
Description of Daily Regimen.

4. Discussion
There are several possibilities for the preserved clinical status of these three brothers in the absence of standard CF care:
- a) They avoided the CF Clinic setting. Recent research [ 13 ] has shown that Pseudomonas infections are more prevalent and lung function lower among CF patients in standard care versus CF patients in a telemedicine setting. It is possible these three brothers benefitted from not attending a standard CF Clinic, especially since during their childhood years at the turn of the century, Clinic infection control was not emphasized. For example, during Brother A's first few CF Clinic visits as an infant, families were expected to wait together in a communal area with communal toys, and health care professionals at the Clinic wore neither masks nor gloves as they moved from exam room to exam room.
- b) With the exception of Brother A, Brothers B and C have used no antibiotics at all. Brother A has only used antibiotics three times in his life; the first use in infancy precipitated Pseudo-Bartter Syndrome, leading to his diagnosis with cystic fibrosis. The other two uses were incident to endoscopic sinus scraping and an appendectomy. Recent research has shown the importance of the gut microbiome in maintenance of health (including respiratory function), digestion and immune signaling, and this is true in the case of cystic fibrosis as well [ [14] , [15] , [16] ]. As David Pride, Associate Director of Microbiology at UC San Diego, notes in an address to the 2019 North American Cystic Fibrosis Conference [ 17 ], “It is important to preserve our microbiomes because they play important roles in preventing pathogens from establishing infections, in the development of our immune systems to recognize and kill pathogens, and in metabolic processes such as the digestion of foods. Indiscriminate uses of antibiotics can have profound and long-lasting effects upon our microbiomes by killing many of the bacteria that make up our microbiome; thus, limiting their use may aid in keeping us healthy.”
- Prevalent, sometimes chronic, antibiotic use among CF patients results in a significant gut dysbiosis [ 18 ]. In addition, it has been noted that aggressive antibiotic use in CF, usually incident to the first manifestation of Staphylococcus aureus (SA), may allow Pseudomonas aeruginosa a greater foothold [ 19 ], and that aggressive treatment of Pseudomonas may, in turn, promote drug resistance and may allow additional bacteria, such as Stenotrophomonas maltophilia, an opportunity to proliferate [ 20 ]. Perhaps a preserved gut microbiome due to non-use of antibiotics may have played a role in the brothers' preserved clinical condition; this may also help account for the brothers’ significantly lower level of need for pancreatic enzymes. Perhaps also the decision not to aggressively treat their light to moderate growth of methicillin-sensitive SA may have precluded additional bacteria, including drug-resistant bacteria, from emerging.
- c) Other standard daily CF treatments were not employed, either, which might help account for their preserved clinical condition. For example, the brothers do not use bronchodilators; and beta-2 agonist bronchodilators, such as albuterol, have recently been shown to significantly reduce delF508 CFTR activation [ 21 ]. This reduction is even evident when CFTR modulators are used, with the finding of a more than 60% reduction of modulator-corrected CFTR activation in vitro, “sufficient to abrogate VX809/VX770 modulation of F508-del CFTR” [ 21 ]. In addition, the brothers do not use DNase, which has been associated with increased levels of neutrophil elastase in past research [ 22 ]. Last, after Brother A transitioned to his new treatment regimen at approximately 23 months of age, chest percussive therapy (CPT) was discontinued, and neither Brother B nor C underwent CPT at all. A Cochrane meta-review found that while CPT constituted the lion's share of treatment time burden in CF, the evidence that outcomes of CPT differed from no CPT was “very low quality” [ 23 ].
- d) Glutathione (GSH) is heavily emphasized in the brothers' daily regimen. Levels of GSH are strongly decreased in the extracellular milieu of CF patients, as its efflux from epithelial cells is compromised by CFTR mutation [ 24 ]. In the non-CF research literature, GSH in its ratio of reduced to oxidized forms (GSH:GSSG) has been shown to be the foundation of redox signaling in the body; GSH is also the body's primary water-soluble antioxidant and a potent mucolytic, and conserves NO through formation of GSNO. Given its pivotal roles, it is not surprising to find that GSH deficiency is noted in several other severe respiratory illnesses besides CF, including ARDS, COPD, IIP, IPF, IRDS, and DFA, and GSH deficiency is a key catalyst for (and GSH dosing a key treatment of) cachexia [ 24 ]. The use of GSH in the treatment of CF may reduce systemic inflammation, lessen the viscosity of mucus, and catalyze the efficacy of the immune system, including through GSNO. Indeed, a clinical study by Visca et al. found significantly increased BMI [ 25 ], significantly increased lung function [ 26 ], and even improved bacteriological results [ 27 ] from the daily use of oral glutathione in children with CF at a dose of 30 mg/lb body weight/day, spread out over 3–4 doses, over a time period of 6 months. In addition, the parents of these brothers noted a sudden increase in both saliva and appetite in Brother A after glutathione (GSH) was introduced when he was two years of age. Brothers B and C, on GSH from two weeks of birth (and with the mother supplementing with oral glutathione throughout pregnancy with these two brothers), never displayed low saliva or low appetite. The preserved clinical status of these three brothers may perhaps be related to this glutathione-heavy regimen.
- e) Other aspects of the brothers' regimen may offset their disease condition. The use of probiotics [ 28 ], the heavy emphasis on antioxidants in addition to glutathione (such as C, CoQ10, Alpha-lipoic acid, D, E, etc. [ 29 ]), amino acids (such as cysteine [ 30 ], carnitine [ 31 ], choline [ 32 , 33 ], taurine [ 34 ], and glycine [ 35 ]), curcumin [ 36 ], and additional digestive support beyond enzymes (lecithin, bile acid). It is possible that some or all of these supplementation efforts also helped to preserve the clinical status of the three brothers. In addition, exclusive breastfeeding of CF infants has been linked to significantly higher FEV1 at age 5 (difference significant at p ≤ 0.001 between breastfed and formula fed CF infants), perhaps contributing to the preservation of lung function beyond that time frame [ 37 ].
- f) Modifier alleles may be present. While no in-depth analysis of the brothers' genetic profile has been performed beyond the identification of their CF mutations, there are known modifier alleles that serve to lessen (or exacerbate) the severity of CF (see, for example [ 38 ]). It is possible all three brothers inherited some propitious set of modifier alleles.
5. Conclusion
In conclusion, while it is encouraging and heartening that new CF therapies, such as elexacaftor/tezacaftor/ivacaftor (Trikafta) and other HEMT (highly effective modulator therapies), now exist, it is instructive to consider how this family was able to preserve the clinical condition of three brothers, all delF508 homozygotes, in the absence of those therapies, and even in the absence of standard CF care. While HEMT certainly increase CFTR activity, there is substantially less effect on infection and inflammation in vivo [ 39 ]. As recently noted by Singh et al., “[I]f infection and inflammation become uncoupled from CFTR activity in established disease [due to HEMT use], drugs targeting CFTR may need to be initiated very early in life, or used in combination with agents that suppress infection and inflammation ” [ 39 ; emphasis ours]. These case reports may speak to that proposition.
Furthermore, each possible explanation for that preservation is an occasion for reflection on the current standard of CF care. We may feel to ask questions such as, “From the point of view of the patient's health, is the entire concept of the CF Clinic inherently flawed? Is the frequent, sometimes chronic, use of antibiotics and certain other medications in CF care a real double-edged sword for CF patients, with disadvantages possibly outweighing advantages in many cases? Are there measures we can take now, relatively inexpensive measures such as the use of glutathione (GSH) and other antioxidants and amino acids, that will help preserve the clinical status of CF patients, and that might synergize with cutting-edge treatments such as CFTR modulators to improve and safeguard health to an even greater degree, and which should be initiated as early in life as possible, possibly while the fetus is still in utero ?” The experience of these three brothers, so removed from standard CF care and yet so well preserved in their clinical status, highlights the need to consider such questions more urgently than we perhaps have heretofore considered them.
Funding sources
This work was supported by the Utah Valley Institute of Cystic Fibrosis, for publication costs only.
Acknowledgements
The author wishes to acknowledge Valerie M. Hudson, who assisted with the writing of this article.

Cystic Fibrosis: Overview and Practice Questions (2024)
by John Landry, BS, RRT | Updated: May 30, 2024
This inherited condition disrupts normal mucus production in the body, leading to severe respiratory and digestive complications.
25+ RRT Cheat Sheets and Quizzes
Get instant access to 25+ premium quizzes, mini-courses, and downloadable cheat sheets for FREE.
What is Cystic Fibrosis?
Cystic fibrosis is a genetic disorder that primarily affects the respiratory and digestive systems. It leads to the production of thick, sticky mucus that can clog airways and impair lung function, causing breathing difficulties and increasing the risk of lung infections.

Pathophysiology
Cystic fibrosis is characterized by a defect in the CFTR gene, which encodes for a protein that regulates the movement of chloride and sodium ions across epithelial membranes.
This defect impairs the function of the CFTR protein, leading to thick, sticky mucus production in various organs.
In the lungs, this mucus obstructs the airways, causing difficulty in breathing and making the lungs prone to bacterial infections and inflammation. This can lead to chronic respiratory diseases such as bronchiectasis.
In the digestive system, the thick mucus blocks pancreatic ducts, inhibiting the release of digestive enzymes. This results in malabsorption of nutrients and difficulties in digestion, often leading to malnutrition and poor growth.
Additionally, CF can affect the liver, leading to biliary cirrhosis due to blocked bile ducts, and can also impact the reproductive system. Sweat glands are also affected, producing sweat with an abnormally high salt content.
Summary: The pathology of cystic fibrosis involves multiple organ systems, primarily affecting the respiratory and digestive systems, and is characterized by chronic infections, inflammation, and complications related to mucus buildup.
Signs and Symptoms
The signs and symptoms of cystic fibrosis vary, often depending on the severity of the disease.
Key symptoms include:
- Respiratory Symptoms: Persistent cough, often producing thick mucus, frequent lung infections, leading to chronic bronchitis or pneumonia , and wheezing or shortness of breath.
- Digestive Symptoms: Poor weight gain and growth (failure to thrive) in infants and children, steatorrhea (fatty, smelly stools) due to poor absorption of nutrients. and intestinal blockage, particularly in newborns (meconium ileus).
- Salty-tasting Skin: Parents often notice that their child’s skin tastes salty when kissed.
- Additional Symptoms: Nasal polyps and sinusitis, clubbing (enlargement and rounding) of fingers and toes, fatigue, and reduced exercise tolerance.
- Male Reproductive System: Most males with CF are infertile due to obstructed or absent vas deferens.
- Other Complications: Diabetes, liver disease, and bone thinning (i.e., osteoporosis).
Note: Symptoms can vary in intensity, and individuals with CF might experience different sets of symptoms over time. Early diagnosis and management are crucial to improve quality of life and longevity.
Diagnosing cystic fibrosis involves several tests, often initiated due to the presence of symptoms or a positive newborn screening result.
Key diagnostic tests include:
- Sweat Chloride Test: The most definitive test for CF. It measures the amount of chloride in the sweat. High levels of chloride suggest CF.
- Genetic Testing: Used to identify mutations in the CFTR gene. This is particularly useful for confirming a diagnosis following a positive sweat test or as part of a newborn screening program.
- Newborn Screening: In many places, newborns are screened for CF using a blood test. This test looks for elevated levels of immunoreactive trypsinogen (IRT), an indicator of CF.
- Pulmonary Function Tests: These tests assess lung function and are often used to monitor the disease’s progression rather than for initial diagnosis.
- Chest X-rays or CT Scans: Imaging tests can identify lung infections and other abnormalities associated with CF.
- Stool Tests: These can be used to evaluate fat absorption, which can be impaired in CF due to pancreatic dysfunction.
- Sputum Tests: Analysis of sputum can detect lung infections common in people with CF.
Note: A combination of these tests, along with the evaluation of clinical symptoms, is typically used to diagnose cystic fibrosis. It’s important to start treatment early to manage symptoms and improve quality of life.
Treatment for cystic fibrosis is comprehensive and aims to manage symptoms, reduce complications, and improve quality of life.
Key aspects include:
- Airway Clearance Techniques : These help loosen and clear mucus from the lungs. Techniques include chest physiotherapy, positive expiratory pressure (PEP) therapy, and exercise.
- Inhaled Medications: Bronchodilators to open the airways, mucolytics to thin mucus, and antibiotics to treat and prevent lung infections.
- Pancreatic Enzyme Supplements: These aid in digestion and nutrient absorption for patients with pancreatic insufficiency.
- High-calorie, High-salt Diet: Essential to address malnutrition and maintain overall health. Dietitians often work with patients to create individualized meal plans.
- CFTR Modulators: These are a class of drugs that target the underlying genetic defect in CF, helping the CFTR protein function more effectively. Their availability and efficacy depend on the specific genetic mutations a person has.
- Regular Exercise: Encouraged to improve lung function and overall well-being.
- Management of Complications: Includes treatment for diabetes, liver disease, and other CF-related conditions.
- Psychosocial Support: Counseling and support groups help patients and families cope with the challenges of living with CF.
- Regular Monitoring: Regular follow-ups with healthcare providers to monitor lung function, nutritional status, and overall health.
- Lung Transplantation: In severe cases, a lung transplant may be considered when lung function significantly declines.
CF treatment is typically managed by a team of healthcare professionals, including doctors, nurses, dietitians, physiotherapists, and social workers, providing comprehensive care tailored to each individual’s needs.
Cystic Fibrosis Practice Questions
1. What is cystic fibrosis characterized by? Cystic fibrosis is a hereditary disease characterized by lung congestion and infection along with malabsorption of nutrients by the pancreas. It’s a multifaceted disease caused by genetically defective chloride ions in the apical surface of the epithelial lining resulting in impaired chloride flow across membranes through the cystic fibrosis conductance regulator (CFTR) channel.
2. What happens in cystic fibrosis? Mucus clogs the lungs leading to chronic respiratory infections, and mucus obstructs the ducts of the pancreas preventing digestive enzymes from reaching the intestines.
3. What system is cystic fibrosis a dysfunction of? Exocrine system
4. What causes thick secretions in cystic fibrosis? The disruption of the chloride transport causes thick secretions, and sodium is absorbed in the airways, pancreas, and bile ducts.
5. Which ethnicity is most likely to carry the CFTR gene? Caucasian/white
6. What does the x-ray typically look like in patients with cystic fibrosis? Translucencies, an enlarged heart, and a flattened diaphragm.
7. How is cystic fibrosis diagnosed? Sweat chloride test
8. What two infections are common in patients with cystic fibrosis? Staphylococcus aureus and pseudomonas
9. What common respiratory issues does a patient with cystic fibrosis have? Atelectasis, bronchial hyperreactivity, asthma, hemoptysis, pneumothorax, pulmonary hypertension, respiratory failure, sleep-related breathing problems, and symptomatic rhinosinusitis.
10. Aside from respiratory issues, what other clinical effects may come from cystic fibrosis? Anemia, appendicitis, and cystic fibrosis-related diabetes (CFRD) due to the scarring of the pancreas, gallbladder disease, gastroesophageal reflux disease (GERD) , malnutrition, growth failure, infertility, pancreatitis, and meconium ileus.
11. How many positive results should one have to be diagnosed with cystic fibrosis? Two positive results
12. What are the common signs and symptoms of cystic fibrosis? Salty sweat, residue on skin and/or clothes, abdominal distention and cramping, fatty stools, and malnutrition even with a ravenous appetite. Pulmonary indications include advanced chronic obstructive pulmonary disease (COPD), hypercapnia, and severe hypoxemia when the disease is advanced.
13. How can you manage patients with cystic fibrosis? Most patients suffering from cystic fibrosis are treated with pulmozyme, sympathomimetics, percussion, and postural drainage. In addition, positive expiratory pressure (PEP), flutter, vest therapy, hypertonic saline and xanthines must also be considered for managing this disease.
14. What other treatments are available for cystic fibrosis? Lung transplant, gene replacement, vitamins, food supplements, and oxygen therapy.
15. What is the prognosis for cystic fibrosis? The disease has no cure but can be managed. The life expectancy of a person suffering from this condition is 37.5 years.
16. What deficiencies does cystic fibrosis involve? Nutritional deficiencies
17. What is the typical arterial blood gas (ABG) for a patient with cystic fibrosis? Mixed respiratory acidosis and metabolic alkalosis
18. What kind of disease is cystic fibrosis? It is an inherited obstructive disease .
19. Where can you find the defective gene for cystic fibrosis? Chromosome 7
20. What pathological findings are associated with cystic fibrosis? Hyperinflation, thick tenacious mucus, and airway obstructions that lead to atelectasis .
21. What is a common sign of a person with cystic fibrosis? They present as malnourished with excessive secretions and may also suffer from meconium ileus (i.e., bowel obstruction).
22. What symptom of cystic fibrosis is common in men? Male infertility
23. What are the chances that a child will be a carrier of the cystic fibrosis gene if both the mother and father are carriers? There is a 50% chance that the child will be a carrier.
24. What may be seen on the fingers and toes of patients with cystic fibrosis? Digital clubbing
25. What is the cause of digital clubbing? Clubbing is mainly caused by chronically low levels of oxygen.
26. What is the median age of survival for people with cystic fibrosis? 37 years
27. What test must be elevated to diagnose cystic fibrosis? An elevated sweat chloride test is performed to diagnose cystic fibrosis.
28. What are the four commonly used therapies in the management of cystic fibrosis? Oral enzymes and vitamins, antibiotics, postural drainage, and expectorants.
29. A cystic fibrosis patient appears malnourished even on what kind of diet? A high-calorie diet.
30. Lung infections like pseudomonas aeruginosa are most often treated with what antibiotic? Tobramycin (TOBI)
31. A cystic fibrosis patient in generally good condition may be a candidate for what surgical procedure? Lung transplant
32. What percussion note if often heard in patients with cystic fibrosis? Hyperresonant percussion note
33. What are the typical breath sounds of a patient with cystic fibrosis? Crackles (i.e., rales)
34. What is a common non-respiratory clinical manifestation of cystic fibrosis? Sinusitis
35. What supplementary vitamins do cystic fibrosis patients need? A, E, D, and K
36. Cystic fibrosis patients can have chest percussion done manually, or they can use what? The theravest.
37. What medications are used to treat cystic fibrosis? Short-acting beta-agonists, hypertonic saline, pulmozyme, and tobramycin (TOBI).
38. What contributes to bone disease among individuals with cystic fibrosis? Malabsorption of vitamins D and K, failure to thrive, delayed puberty, physical inactivity, and the use of corticosteroids.
39. What protein is responsible for cystic fibrosis? Cystic fibrosis transmembrane conductance regulator protein.
40. What are the four reasons associated with poor appetite among children with cystic fibrosis? Depression, anxiety, fatigue and anorexia.
41. How are enzyme tablets administered to infants and young children? They can be mixed with soft acidic food.
42. What is the probability that child will have cystic fibrosis if both parents carry the cystic fibrosis gene? One in four children will inherit the disease.
43. What is the average life expectancy for people with cystic fibrosis? The average life expectancy for people with cystic fibrosis is 45 years.
44. Eighty-five percent of patients with cystic fibrosis has what condition? Pancreatic insufficiency
45. What are the two linked clinical manifestations of cystic fibrosis? Meconium Ileus and distal intestinal obstructive syndrome.
46. What lung and upper respiratory tract diseases are associated with cystic fibrosis? Persistent bacterial infection, inflammation, airway destruction, bronchiectasis, mucus plugged airways, goblet cell hyperplasia, and disrupted cilia function.
47. What is the most common amino acid that is deleted from the channel protein in cystic fibrosis? Phenylalanine changes the structure of the CFTR (cystic fibrosis transmembrane conductance regulator) protein (channel) in a way that it can no longer transport chloride.
48. What cellular mechanism takes place within the sweat glands of cystic fibrosis patients? There is an inhibited chloride reabsorption that results in inhibited sodium reabsorption, which makes sweat saltier than normal.
49. How would you explain the “low-volume” model as a result of cystic fibrosis? In the “low-volume” model, the chloride channel is involved in chloride outflow into the mucus that somewhat traps the sodium and water, retaining its fluidity. Normally, a significant amount of chloride remains in the mucus, causing sodium and water to remain in the mucus. In a cystic fibrosis patient, there are not enough chloride that outflows into the mucus. This results in less negative charge that is capable of keeping the sodium and water in the mucus, thus, causing the mucus to thicken and lose its fluidity.
50. What two organs are affected by the thickened mucus in cystic fibrosis patients? Lungs and pancreas
51. What happens in the lungs as a result of thickened mucus? Bronchiectasis is a result of thickened mucus that stays in the bronchioles and physically pushes them open, distending them and causing them to widen. The result is permanent and eventually leads to fibrosis of these bronchioles.
52. How does mucous affect cilia? It flattens the cilia
53. How is cystic fibrosis manifested in the lungs? Cystic fibrosis is manifested in lungs as acute exacerbations that include cough, dyspnea, decreased exercise tolerance, fatigue, and increased sputum production. This leads to accelerated permanent loss of lung function.
54. How are sinuses affected by cystic fibrosis? It may cause nasal and sinus polyps.
55. How is cystic fibrosis diagnosed? If a newborn screening is positive, a cystic fibrosis genetic panel is done followed by a sweat chloride test and complete CFTR sequencing.
56. What mucolytic agents are given for cystic fibrosis? Dornase alfa and hypertonic saline
57. What is the brand name for dornase alfa? Pulmozyme
58. How does Pulmozyme work? It breaks down extracellular DNA, decreased mucus viscosity, and increases the expectoration of mucus.
59. Which antibiotics are used to treat cystic fibrosis? Tobramycin (TOBI), aztreonam (Cayston) and colistemethate (Colistin).
60. How is tobramycin taken? 300 mg BID
61. What is the dose for tobramycin? 10 mg/kg IV every 24 hours
62. When are tobramycin levels checked? Two and ten hours after infusion.
63. What tests are given for cystic fibrosis? Sweat chloride test, pulmonary function testing (PFT), arterial blood gas (ABG) , and chest x-ray.
64. What results of the sweat chloride test indicate cystic fibrosis? Increased concentrations of NaCl (sodium chloride) and K (potassium) in the sweat.
65. What are the typical results of pulmonary function testing in patients with cystic fibrosis? Airflow obstruction
66. What can be observed in the chest x-ray of a person suffering from cystic fibrosis? Hyperinflation and increased pulmonary markings.
67. What do the ABG results show in patients with severe cystic fibrosis? Hypoxemia and hypercapnia
68. What methods are generally used to manage cystic fibrosis? Chest physiotherapy for secretion drainage, pancreatic enzyme replacement, administration of salbutamol and ipratropium bromide, inhalation of hypertonic saline or DNAse, and antibiotics for infectious exacerbations.
69. What is the gold standard treatment for cystic fibrosis? Lung transplant
70. How do you get a diagnosis of cystic fibrosis? Sweat chloride test, CFTR gene analysis, nasal electrical potential difference, and newborn screening
Final Thoughts
Cystic fibrosis is a complex genetic disorder with widespread effects, primarily impacting the respiratory and digestive systems.
Although there is no cure, advancements in diagnostic techniques and comprehensive treatment strategies have significantly improved the quality of life and life expectancy for those affected.
Ultimately, a multidisciplinary approach, combining medical, nutritional, and psychosocial support, is key to effectively managing cystic fibrosis and enhancing the lives of those living with this challenging condition.

Written by:
John Landry is a registered respiratory therapist from Memphis, TN, and has a bachelor's degree in kinesiology. He enjoys using evidence-based research to help others breathe easier and live a healthier life.
- Yu E, Sharma S. Cystic Fibrosis. [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023.
Recommended Reading
Sputum: definition, colors, causes, and conditions, best cystic fibrosis quotes and sayings for inspiration, bronchiectasis: overview and practice questions, chronic bronchitis: overview and practice questions, list of 97+ cardiopulmonary diseases you should know.
- Publications
- Conferences & Events
- Professional Learning
- Science Standards
- Awards & Competitions
- Instructional Materials
- Free Resources
- American Rescue Plan
- For Preservice Teachers
- NCCSTS Case Collection
- Science and STEM Education Jobs
- Interactive eBooks+
- Digital Catalog
- Regional Product Representatives
- e-Newsletters
- Bestselling Books
- Latest Books
- Popular Book Series
- Prospective Authors
- Web Seminars
- Exhibits & Sponsorship
- Conference Reviewers
- National Conference • Denver 24
- Leaders Institute 2024
- National Conference • New Orleans 24
- Submit a Proposal
- Latest Resources
- Professional Learning Units & Courses
- For Districts
- Online Course Providers
- Schools & Districts
- College Professors & Students
- The Standards
- Teachers and Admin
- eCYBERMISSION
- Toshiba/NSTA ExploraVision
- Junior Science & Humanities Symposium
- Teaching Awards
- Climate Change
- Earth & Space Science
- New Science Teachers
- Early Childhood
- Middle School
- High School
- Postsecondary
- Informal Education
- Journal Articles
- Lesson Plans
- e-newsletters
- Science & Children
- Science Scope
- The Science Teacher
- Journal of College Sci. Teaching
- Connected Science Learning
- NSTA Reports
- Next-Gen Navigator
- Science Update
- Teacher Tip Tuesday
- Trans. Sci. Learning
MyNSTA Community
- My Collections
Maggie’s Illness
Protein Structure and Function in Cystic Fibrosis
By Michaela Gazdik Stofer
Share Start a Discussion

This directed case study examines the molecular basis of cystic fibrosis to emphasize the relationship between the genetic code stored in a DNA sequence and the encoded protein’s structure and function. Cystic fibrosis is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) protein that functions to help maintain salt and water balance along the surface of the lung and gastrointestinal tract. This case introduces students to “Maggie,” who has just been diagnosed with cystic fibrosis. The students must identify the mutation causing Maggie’s disease by transcribing and translating a portion of the wildtype and mutated CFTR gene. Students then compare the three-dimensional structures of the resulting proteins to better understand the effect a single amino acid mutation can have on the overall shape of a protein. Students also review the concepts of tonicity and osmosis to examine how the defective CFTR protein leads to an increase in the viscosity of mucus in cystic fibrosis patients. This case was developed for use in an introductory college-level biology course but could also be adapted for use in an upper-level cell or molecular biology course.
Download Case
Date Posted
- Generate a protein sequence through transcription and translation of a given DNA gene sequence.
- Explain the chemistry of amino acid side chains and their importance in protein folding.
- Describe how a mutation in a protein sequence leads to changes in the overall tertiary structure of the protein.
- Examine various levels of protein structure using Cn3D to view three-dimensional protein structures from NCBI’s Entrez Structure database.
- Relate the loss of function of the CFTR protein to the physiological causes of cystic fibrosis.
Protein structure; transcription; translation; DNA mutation; cystic fibrosis; genetic disease; protein function; protein folding; protein; CFTR; Cn3D

Subject Headings
EDUCATIONAL LEVEL
Undergraduate lower division, Undergraduate upper division
TOPICAL AREAS
TYPE/METHODS
Teaching Notes & Answer Key
Teaching notes.
Case teaching notes are protected and access to them is limited to paid subscribed instructors. To become a paid subscriber, purchase a subscription here .
Teaching notes are intended to help teachers select and adopt a case. They typically include a summary of the case, teaching objectives, information about the intended audience, details about how the case may be taught, and a list of references and resources.
Download Notes
Answer Keys are protected and access to them is limited to paid subscribed instructors. To become a paid subscriber, purchase a subscription here .
Download Answer Key
Materials & Media
Supplemental materials.
The following two files should be viewed with the Cn3D software to view a single domain of the CFTR and ∆F508 CFTR proteins.
You may also like
Web Seminar
Join us on Tuesday, June 4, 2024, from 7:00 PM to 8:30 PM ET, to learn about the free lesson plans and storyline units designed for high school studen...
Join us on Thursday, October 24, 2024, from 7:00 PM to 8:00 PM ET, to learn about all NSTA Teacher Awards available and how to apply.Did you come up w...

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

Case Study: Cystic Fibrosis - CER
- Last updated
- Save as PDF
- Page ID 26446
This page is a draft and is under active development.
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Part I: A Case of Cystic Fibrosis
Dr. Weyland examined a six month old infant that had been admitted to University Hospital earlier in the day. The baby's parents had brought young Zoey to the emergency room because she had been suffering from a chronic cough. In addition, they said that Zoey sometimes would "wheeze" a lot more than they thought was normal for a child with a cold. Upon arriving at the emergency room, the attending pediatrician noted that salt crystals were present on Zoey's skin and called Dr. Weyland, a pediatric pulmonologist. Dr. Weyland suspects that baby Zoey may be suffering from cystic fibrosis.
CF affects more than 30,000 kids and young adults in the United States. It disrupts the normal function of epithelial cells — cells that make up the sweat glands in the skin and that also line passageways inside the lungs, pancreas, and digestive and reproductive systems.
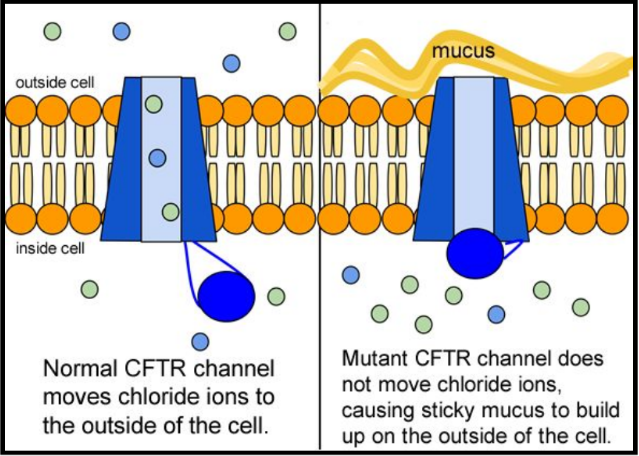
The inherited CF gene directs the body's epithelial cells to produce a defective form of a protein called CFTR (or cystic fibrosis transmembrane conductance regulator) found in cells that line the lungs, digestive tract, sweat glands, and genitourinary system.
When the CFTR protein is defective, epithelial cells can't regulate the way that chloride ions pass across cell membranes. This disrupts the balance of salt and water needed to maintain a normal thin coating of mucus inside the lungs and other passageways. The mucus becomes thick, sticky, and hard to move, and can result in infections from bacterial colonization.
- "Woe to that child which when kissed on the forehead tastes salty. He is bewitched and soon will die" This is an old saying from the eighteenth century and describes one of the symptoms of CF (salty skin). Why do you think babies in the modern age have a better chance of survival than babies in the 18th century?
- What symptoms lead Dr. Weyland to his initial diagnosis?
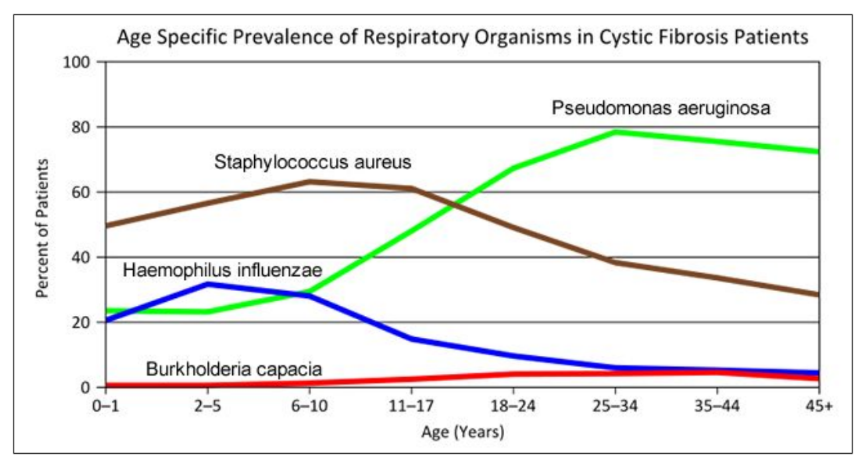
- Consider the graph of infections, which organism stays relatively constant in numbers over a lifetime. What organism is most likely affecting baby Zoey?
- What do you think is the most dangerous time period for a patient with CF? Justify your answer.
Part II: CF is a disorder of the cell membrane.
Imagine a door with key and combination locks on both sides, back and front. Now imagine trying to unlock that door blind-folded. This is the challenge faced by David Gadsby, Ph.D., who for years struggled to understand the highly intricate and unusual cystic fibrosis chloride channel – a cellular doorway for salt ions that is defective in people with cystic fibrosis.
His findings, reported in a series of three recent papers in the Journal of General Physiology, detail the type and order of molecular events required to open and close the gates of the cystic fibrosis chloride channel, or as scientists call it, the cystic fibrosis transmembrane conductance regulator (CFTR).
Ultimately, the research may have medical applications, though ironically not likely for most cystic fibrosis patients. Because two-thirds of cystic fibrosis patients fail to produce the cystic fibrosis channel altogether, a cure for most is expected to result from research focused on replacing the lost channel.
5. Suggest a molecular fix for a mutated CFTR channel. How would you correct it if you had the ability to tinker with it on a molecular level?
6. Why would treatment that targets the CFTR channel not be effective for 2⁄3 of those with cystic fibrosis?
7. Sweat glands cool the body by releasing perspiration (sweat) from the lower layers of the skin onto the surface. Sodium and chloride (salt) help carry water to the skin's surface and are then reabsorbed into the body. Why does a person with cystic fibrosis have salty tasting skin?
Part III: No cell is an island
Like people, cells need to communicate and interact with their environment to survive. One way they go about this is through pores in their outer membranes, called ion channels, which provide charged ions, such as chloride or potassium, with their own personalized cellular doorways. But, ion channels are not like open doors; instead, they are more like gateways with high-security locks that are opened and closed to carefully control the passage of their respective ions.
In the case of CFTR, chloride ions travel in and out of the cell through the channel’s guarded pore as a means to control the flow of water in and out of cells. In cystic fibrosis patients, this delicate salt/water balance is disturbed, most prominently in the lungs, resulting in thick coats of mucus that eventually spur life-threatening infections. Shown below are several mutations linked to CFTR:
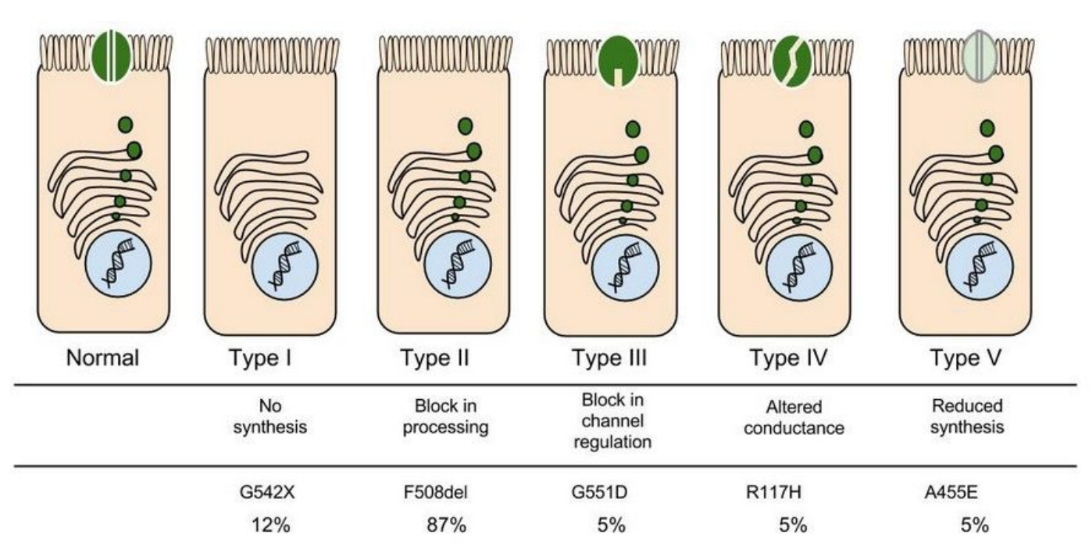
8. Which mutation do you think would be easiest to correct. Justify your answer. 9. Consider what you know about proteins, why does the “folding” of the protein matter?
Part IV: Open sesame
Among the numerous ion channels in cell membranes, there are two principal types: voltage-gated and ligand-gated. Voltage-gated channels are triggered to open and shut their doors by changes in the electric potential difference across the membrane. Ligand-gated channels, in contrast, require a special “key” to unlock their doors, which usually comes in the form of a small molecule.
CFTR is a ligand-gated channel, but it’s an unusual one. Its “key” is ATP, a small molecule that plays a critical role in the storage and release of energy within cells in the body. In addition to binding the ATP, the CFTR channel must snip a phosphate group – one of three “P’s” – off the ATP molecule to function. But when, where and how often this crucial event takes place has remains obscure.
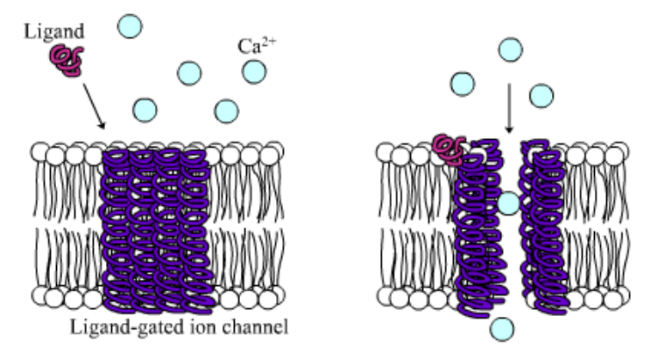
10. Compare the action of the ligand-gated channel to how an enzyme works.
11. Consider the model of the membrane channel, What could go wrong to prevent the channel from opening?
12. Where is ATP generated in the cell? How might ATP production affect the symptoms of cystic fibrosis?
13. Label the image below to show how the ligand-gated channel for CFTR works. Include a summary.
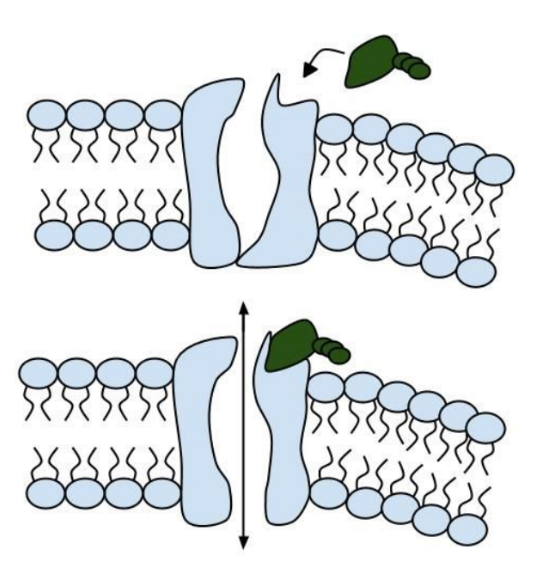
Part V: Can a Drug Treat Zoey’s Condition?
Dr. Weyland confirmed that Zoey does have cystic fibrosis and called the parents in to talk about potential treatments. “Good news, there are two experimental drugs that have shown promise in CF patients. These drugs can help Zoey clear the mucus from his lungs. Unfortunately, the drugs do not work in all cases.” The doctor gave the parents literature about the drugs and asked them to consider signing Zoey up for trials.
The Experimental Drugs
Ivacaftor TM is a potentiator that increases CFTR channel opening time. We know from the cell culture studies that this increases chloride transport by as much as 50% from baseline and restores it closer to what we would expect to observe in wild type CFTR. Basically, the drug increases CFTR activity by unlocking the gate that allows for the normal flow of salt and fluids.
In early trials, 144 patients all of whom were age over the age of 12 were treated with 150 mg of Ivacaftor twice daily. The total length of treatment was 48 weeks. Graph A shows changes in FEV (forced expiratory volume) with individuals using the drug versus a placebo. Graph B shows concentrations of chloride in patient’s sweat.
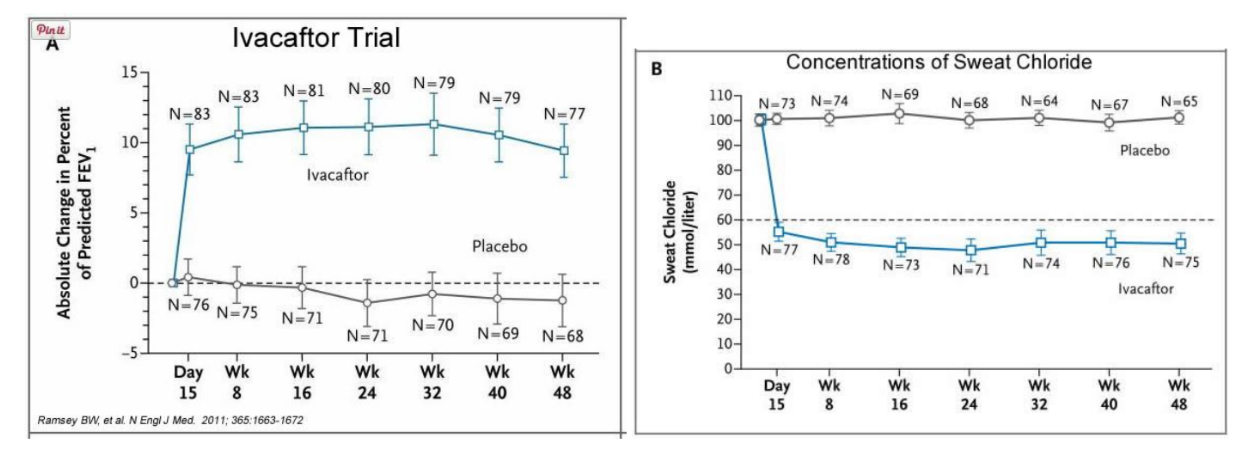
14. What is FEV? Describe a way that a doctor could take a measurement of FEV.
15. Why do you think it was important to have placebos in both of these studies?
16. Which graph do you think provides the most compelling evidence for the effectiveness of Ivacafor? Defend your choice.
17. Take a look at the mutations that can occur in the cell membrane proteins from Part III. For which mutation do you think Ivacaftor will be most effective? Justify your answer.
18. Would you sign Zoey up for clinical trials based on the evidence? What concerns would a parent have before considering an experimental drug?
Part VI: Zoey’s Mutation
Dr. Weyland calls a week later to inform the parents that genetic tests show that Zoey chromosomes show that she has two copies of the F508del mutation. This mutation, while the most common type of CF mutation, is also one that is difficult to treat with just Ivacaftor. There are still some options for treatment.
In people with the most common CF mutation, F508del, a series of problems prevents the CFTR protein from taking its correct shape and reaching its proper place on the cell surface. The cell recognizes the protein as not normal and targets it for degradation before it makes it to the cell surface. In order to treat this problem, we need to do two things: first, an agent to get the protein to the surface, and then ivacaftor (VX-770) to open up the channel and increase chloride transport. VX-809 has been identified as a way to help with the trafficking of the protein to the cell surface. When added VX-809 is added to ivacaftor (now called Lumacaftor,) the protein gets to the surface and also increases in chloride transport by increasing channel opening time.
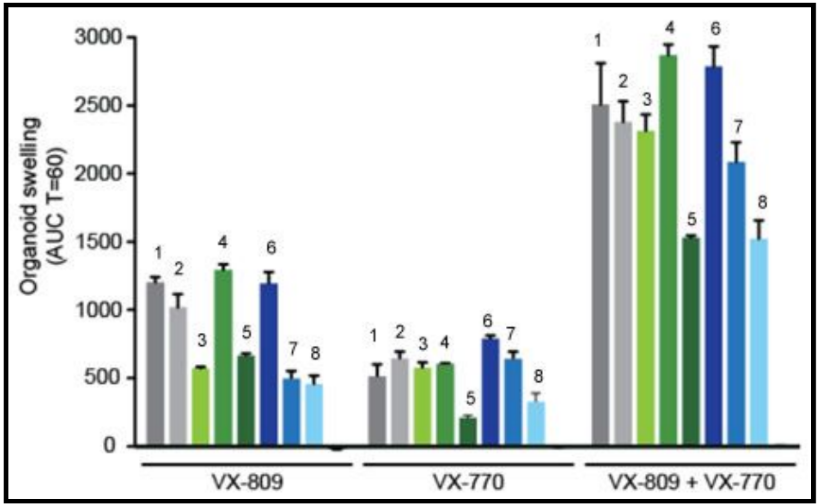
In early trials, experiments were done in-vitro, where studies were done on cell cultures to see if the drugs would affect the proteins made by the cell. General observations can be made from the cells, but drugs may not work on an individual’s phenotype. A new type of research uses ex-vivo experiments, where rectal organoids (mini-guts) were grown from rectal biopsies of the patient that would be treated with the drug. Ex-vivo experiments are personalized medicine, each person may have different correctors and potentiators evaluated using their own rectal organoids. The graph below shows how each drug works for 8 different patients (#1-#8)
19. Compare ex-vivo trials to in-vitro trials.
20. One the graph, label the group that represents Ivacaftor and Lumacaftor. What is the difference between these two drugs?
21. Complete a CER Chart. If the profile labeled #7 is Zoey, rank the possible drug treatments in order of their effectiveness for her mutation. This is your CLAIM. Provide EVIDENCE to support your claim. Provide REASONING that explains why this treatment would be more effective than other treatments and why what works for Zoey may not work for other patients. This is where you tie the graph above to everything you have learned in this case. Attach a page.

The Biology Corner
Biology Teaching Resources

Case Study: Cystic Fibrosis Mutations
This case study is a follow-up to the Cystic Fibrosis Case Study where students explore how changes in transport proteins affects the movement of ions, resulting in a build-up of chloride ions and the symptoms of the disease.
Students were introduced to the idea that different mutations can cause differences in the transport proteins, but in the first version, the origin of these mutations was not discussed.
Eventually, students get to the chapter on DNA, RNA, and protein synthesis, so it’s a good time to circle back to the CF case and explore how mutations in DNA can affect the protein made by the ribosomes.
Students should already have some background in the central dogma, but a review may be in order to remind students how to transcribe DNA to RNA and then use a codon chart to determine the sequence of amino acids. This practice worksheet on using codon charts is something they may have done in freshman biology.
CFTR Mutations

This case explore frameshift mutations, missense mutations, and nonsense mutations. Students are given a section of DNA to transcribe and compare it to mutant DNA. Students should see that changes in DNA can result in changes in the synthesized protein, though some changes are more profound than others.
The link below is a Google Doc designed for remote learning but will work for in-class lessons. An original in-class version is also available, where it doesn’t have the colored text boxes.
Shannan Muskopf
We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

Chapter 19: Case Study: Cystic Fibrosis
Julie M. Skrzat; Carole A. Tucker
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Introduction.
- Examination: Age 2 Months
- Evaluation, Diagnosis, and Prognosis
- Intervention
- Conclusion of Care
- Examination: Age 8 Years
- Examination: Age 16 Years
- Recommended Readings
- Full Chapter
- Supplementary Content
C ystic fibrosis (CF) is an autosomal recessive condition affecting approximately 30,000 Americans and 70,000 people worldwide. According to the Cystic Fibrosis Foundation ( Cystic Fibrosis Foundation, 2019a ), approximately 1,000 new cases are diagnosed yearly in the United States, with a known incidence of 1 per 3,900 live births. The disease prevalence varies greatly by ethnicity, with the highest prevalence occurring in Western European descendants and within the Ashkenazi Jewish population.
The CF gene, located on chromosome 7, was first identified in 1989. The disease process is caused by a mutation to the gene that encodes for the CF transmembrane conductance regulator (CFTR) protein. This mutation alters the production, structure, and function of cyclic adenosine monophosphate (cAMP), a dependent transmembrane chloride channel carrier protein found in the exocrine mucus glands throughout the body. The mutated carrier protein is unable to transport chloride across the cell membrane, resulting in an electrolyte and charge imbalance. Diffusion of water across the cell membrane is thus impaired, resulting in the development of a viscous layer of mucus. The thick mucus obstructs the cell membranes, traps nearby bacteria, and incites a local inflammatory response. Subsequent bacterial colonization occurs at an early age and ultimately this repetitive infectious process leads to progressive inflammatory damage to the organs involved in individuals with CF.
Pop-up div Successfully Displayed
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
- Gene Therapy
Gene Therapy Case Study: Cystic Fibrosis

IMAGES
VIDEO
COMMENTS
Finally, these three cases also raise important questions about standard CF care guidelines. Keywords: Cystic fibrosis ... digestion and immune signaling, and this is true in the case of cystic fibrosis as well ... including through GSNO. Indeed, a clinical study by Visca et al. found significantly increased BMI ...
In addition, many states have introduced newborn screening for CF, resulting in the detection of asymptomatic infants with CF. Case 12. Failure to Thrive: Workup Results in Diagnosis of Cystic Fibrosis. Mr. and Mrs. M, a white couple, have two children, a four-year-old son and a three-month-old daughter. The three-month-old has had considerable ...
suggest that they add extra salt to debbie's diet and watch her for dehydation. Study with Quizlet and memorize flashcards containing terms like Which statement by the mother supports the diagnosis of CF, which documentation further supports the diagnosis of CF, what information will the nurse include when teaching about the sweat test and more.
Cystic fibrosis is a life long, multisystem disorder. Other factors that can influence the quality of life with children with CF include: Study with Quizlet and memorize flashcards containing terms like Which assessment supports the diagnosis of CF?, The healthcare provider (HCP) reviews the client's medical chart.
Cystic fibrosis is characterized by a defect in the CFTR gene, which encodes for a protein that regulates the movement of chloride and sodium ions across epithelial membranes. This defect impairs the function of the CFTR protein, leading to thick, sticky mucus production in various organs. In the lungs, this mucus obstructs the airways, causing ...
This case introduces students to "Maggie," who has just been diagnosed with cystic fibrosis. The students must identify the mutation causing Maggie's disease by transcribing and translating a portion of the wildtype and mutated CFTR gene. Students then compare the three-dimensional structures of the resulting proteins to better understand ...
Case Study 15 Cystic Fibrosis, Disease Sum Questions 1-10. Course. Medical Surgical (VOCN300) 322 Documents. ... Have cystic fibrosis? - If both parents are carriers there is a 1 in 4 (25 percent) chance that both will pass on the non-functioning gene, which would result in a pregnancy affected with cystic fibrosis. ... Case Study 15 Cystic ...
Part I: A Case of Cystic Fibrosis. Dr. Weyland examined a six month old infant that had been admitted to University Hospital earlier in the day. The baby's parents had brought young Zoey to the emergency room because she had been suffering from a chronic cough. In addition, they said that Zoey sometimes would "wheeze" a lot more than they ...
This case study is a follow-up to the Cystic Fibrosis Case Study where students explore how changes in transport proteins affects the movement of ions, resulting in a build-up of chloride ions and the symptoms of the disease. Students were introduced to the idea that different mutations can cause differences in the transport proteins, but in ...
Cystic fibrosis (CF) is an autosomal recessive condition affecting approximately 30,000 Americans and 70,000 people worldwide.According to the Cystic Fibrosis Foundation (Cystic Fibrosis Foundation, 2019a), approximately 1,000 new cases are diagnosed yearly in the United States, with a known incidence of 1 per 3,900 live births.The disease prevalence varies greatly by ethnicity, with the ...
o The pancreatic enzymes would be increased with each meal and snack. · Pancreatic enzymes are adjusted to decrease the bulk of the stool. UMB NU373 NURS373 NU 373 NURS 373 Evidence-Concepts of Health and Illness II (UMB UMass Boston, Fall 2021) HESI Cystic Fibrosis - 26 questions.
As always, feel free to call our office with any questions. Our multidisciplinary team is here to provide education and support. Pediatric Program can be reached at 732-235-7899. Adult Program can be reached at 732-235-7840. Sincerely, Thomas F Scanlin, MD. Director, Cystic Fibrosis Center. Sabiha Hussain, MD.
Woe to That Child: A Case of Cystic Fibrosis. By Dayton J. Ford St Louis College of Pharmacy —Northern European folklore. Dr. Aldritch examined the four-month-old infant that had been admitted to Barnes-Jewish Hospital earlier in the day.
Genetic Science Learning Center. (2012, December 1) Gene Therapy Case Study: Cystic Fibrosis. Retrieved May 14, 2024, from https://learn.genetics.utah.edu/content ...
The clinical course of patients with cystic fibrosis (CF) is variable and probably determined by many interacting factors. We aimed to examine the influence of early social and clinical factors on long-term survival. A case-control study of adult CF patients was used to compare long-term survivors (aged ≥40 yrs) with patients who died before reaching 30 yrs of age. Each case (n = 78) was ...
Study with Quizlet and memorize flashcards containing terms like What treatment? Any future evaluation? Patient 1: 1 month old male + Newborn screen for CF IRT 213.5 1 mutation Sweat test: 89, 82 Poor growth Fecal elastase <6 Low protein, prealbumin, vitamin A, E, D, What Nutritional treatment? Patient 2: 2 year old with CF for routine visit Growth with recent plateau "Picky eater" Cough with ...
This case on cystic fibrosis was written as a joint effort by faculty members from the departments of physiology, cell biology, and biochemistry genetics as an integrated case for first-year medical students in a team-based learning format. The case provides basic background clinical information covering material that students were introduced ...
Cystic Fibrosis case study. unfolding case study. Course. Family Health Nursing (NUR 338) 10 Documents. Students shared 10 documents in this course. ... Nokia Questions; Debt and Insolvency Law - Handout - 2023-2024; Tran thien Grading Sheet for Unit One Spring 2024 Business Communication 310;
Study with Quizlet and memorize flashcards containing terms like Cystic Fibrosis is ..., Signs and Symptoms of Cystic Fibrosis, Most common test for Cystic Fibrosis and more. ... Mary Washington Pediatrics Case Study. 32 terms. Jerl101. Preview. Ch6 Wong tb . 19 terms. cassie_carranza. Preview. Mod d week 4 terminology word ... Pediatrics: Eye ...
Kisaury Rodriguez Cystic Fibrosis Case Study 09/21/2021 Questions: 1. After interpreting relevant clinical data, what is the primary problem? Pathophysiology of problem in own words. The patient is presenting with difficulty breathing, worsening respiratory status related to his new diagnosis of right lower pneumonia and COPD. He is also presenting with clubbing, cyanosis and an elevated HR.
Case Study: Genomics & Cystic Fibrosis (CF) Lisa is a healthy 19 year old woman who has come to the hospital to visit her 21 year old brother, Mark, her only sibling, who has cystic fibrosis (CF) and has been admitted with a lung infection. No one else in the family has CF.
Cystic fibrosis transmembrane conductance regulator (CFTR) is an ATP-gated anion channel with two remarkable distinctions. First, it is the only ATP-binding cassette (ABC) transporter that is known to be an ion channel—almost all others function as transport ATPases. Second, CFTR is the only ligand-gated channel that consumes its ligand (ATP ...
bronchiectasis: Bronchiectasis is a lung condition that causes coughing up mucus due to scarred tissue in the bronchi, or the passages that let air into the lungs. The condition is fairly common among people aged 75 years and older, but it can also happen to younger people. steatorrhoea: Steatorrhoea is the result of fat malabsorption, with diagnosis usually ascertained from patient history.
Mix the pancreatic enzymes with hot, starchy foods such as macaroni or pasta. Open the enzyme capsules and mix the beads in a protein food. Ensure that the child swallows the pancreatic enzyme capsule whole. Ensure that enzymes are administered within 30 min of consuming meals and snacks.