Purdue Online Writing Lab Purdue OWL® College of Liberal Arts

Writing a Literature Review

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
A literature review is a document or section of a document that collects key sources on a topic and discusses those sources in conversation with each other (also called synthesis ). The lit review is an important genre in many disciplines, not just literature (i.e., the study of works of literature such as novels and plays). When we say “literature review” or refer to “the literature,” we are talking about the research ( scholarship ) in a given field. You will often see the terms “the research,” “the scholarship,” and “the literature” used mostly interchangeably.
Where, when, and why would I write a lit review?
There are a number of different situations where you might write a literature review, each with slightly different expectations; different disciplines, too, have field-specific expectations for what a literature review is and does. For instance, in the humanities, authors might include more overt argumentation and interpretation of source material in their literature reviews, whereas in the sciences, authors are more likely to report study designs and results in their literature reviews; these differences reflect these disciplines’ purposes and conventions in scholarship. You should always look at examples from your own discipline and talk to professors or mentors in your field to be sure you understand your discipline’s conventions, for literature reviews as well as for any other genre.
A literature review can be a part of a research paper or scholarly article, usually falling after the introduction and before the research methods sections. In these cases, the lit review just needs to cover scholarship that is important to the issue you are writing about; sometimes it will also cover key sources that informed your research methodology.
Lit reviews can also be standalone pieces, either as assignments in a class or as publications. In a class, a lit review may be assigned to help students familiarize themselves with a topic and with scholarship in their field, get an idea of the other researchers working on the topic they’re interested in, find gaps in existing research in order to propose new projects, and/or develop a theoretical framework and methodology for later research. As a publication, a lit review usually is meant to help make other scholars’ lives easier by collecting and summarizing, synthesizing, and analyzing existing research on a topic. This can be especially helpful for students or scholars getting into a new research area, or for directing an entire community of scholars toward questions that have not yet been answered.
What are the parts of a lit review?
Most lit reviews use a basic introduction-body-conclusion structure; if your lit review is part of a larger paper, the introduction and conclusion pieces may be just a few sentences while you focus most of your attention on the body. If your lit review is a standalone piece, the introduction and conclusion take up more space and give you a place to discuss your goals, research methods, and conclusions separately from where you discuss the literature itself.
Introduction:
- An introductory paragraph that explains what your working topic and thesis is
- A forecast of key topics or texts that will appear in the review
- Potentially, a description of how you found sources and how you analyzed them for inclusion and discussion in the review (more often found in published, standalone literature reviews than in lit review sections in an article or research paper)
- Summarize and synthesize: Give an overview of the main points of each source and combine them into a coherent whole
- Analyze and interpret: Don’t just paraphrase other researchers – add your own interpretations where possible, discussing the significance of findings in relation to the literature as a whole
- Critically Evaluate: Mention the strengths and weaknesses of your sources
- Write in well-structured paragraphs: Use transition words and topic sentence to draw connections, comparisons, and contrasts.
Conclusion:
- Summarize the key findings you have taken from the literature and emphasize their significance
- Connect it back to your primary research question
How should I organize my lit review?
Lit reviews can take many different organizational patterns depending on what you are trying to accomplish with the review. Here are some examples:
- Chronological : The simplest approach is to trace the development of the topic over time, which helps familiarize the audience with the topic (for instance if you are introducing something that is not commonly known in your field). If you choose this strategy, be careful to avoid simply listing and summarizing sources in order. Try to analyze the patterns, turning points, and key debates that have shaped the direction of the field. Give your interpretation of how and why certain developments occurred (as mentioned previously, this may not be appropriate in your discipline — check with a teacher or mentor if you’re unsure).
- Thematic : If you have found some recurring central themes that you will continue working with throughout your piece, you can organize your literature review into subsections that address different aspects of the topic. For example, if you are reviewing literature about women and religion, key themes can include the role of women in churches and the religious attitude towards women.
- Qualitative versus quantitative research
- Empirical versus theoretical scholarship
- Divide the research by sociological, historical, or cultural sources
- Theoretical : In many humanities articles, the literature review is the foundation for the theoretical framework. You can use it to discuss various theories, models, and definitions of key concepts. You can argue for the relevance of a specific theoretical approach or combine various theorical concepts to create a framework for your research.
What are some strategies or tips I can use while writing my lit review?
Any lit review is only as good as the research it discusses; make sure your sources are well-chosen and your research is thorough. Don’t be afraid to do more research if you discover a new thread as you’re writing. More info on the research process is available in our "Conducting Research" resources .
As you’re doing your research, create an annotated bibliography ( see our page on the this type of document ). Much of the information used in an annotated bibliography can be used also in a literature review, so you’ll be not only partially drafting your lit review as you research, but also developing your sense of the larger conversation going on among scholars, professionals, and any other stakeholders in your topic.
Usually you will need to synthesize research rather than just summarizing it. This means drawing connections between sources to create a picture of the scholarly conversation on a topic over time. Many student writers struggle to synthesize because they feel they don’t have anything to add to the scholars they are citing; here are some strategies to help you:
- It often helps to remember that the point of these kinds of syntheses is to show your readers how you understand your research, to help them read the rest of your paper.
- Writing teachers often say synthesis is like hosting a dinner party: imagine all your sources are together in a room, discussing your topic. What are they saying to each other?
- Look at the in-text citations in each paragraph. Are you citing just one source for each paragraph? This usually indicates summary only. When you have multiple sources cited in a paragraph, you are more likely to be synthesizing them (not always, but often
- Read more about synthesis here.
The most interesting literature reviews are often written as arguments (again, as mentioned at the beginning of the page, this is discipline-specific and doesn’t work for all situations). Often, the literature review is where you can establish your research as filling a particular gap or as relevant in a particular way. You have some chance to do this in your introduction in an article, but the literature review section gives a more extended opportunity to establish the conversation in the way you would like your readers to see it. You can choose the intellectual lineage you would like to be part of and whose definitions matter most to your thinking (mostly humanities-specific, but this goes for sciences as well). In addressing these points, you argue for your place in the conversation, which tends to make the lit review more compelling than a simple reporting of other sources.

- University of Texas Libraries
Literature Reviews
- What is a literature review?
- Steps in the Literature Review Process
- Define your research question
- Determine inclusion and exclusion criteria
- Choose databases and search
- Review Results
- Synthesize Results
- Analyze Results
- Librarian Support
What is a Literature Review?
A literature or narrative review is a comprehensive review and analysis of the published literature on a specific topic or research question. The literature that is reviewed contains: books, articles, academic articles, conference proceedings, association papers, and dissertations. It contains the most pertinent studies and points to important past and current research and practices. It provides background and context, and shows how your research will contribute to the field.
A literature review should:
- Provide a comprehensive and updated review of the literature;
- Explain why this review has taken place;
- Articulate a position or hypothesis;
- Acknowledge and account for conflicting and corroborating points of view
From S age Research Methods
Purpose of a Literature Review
A literature review can be written as an introduction to a study to:
- Demonstrate how a study fills a gap in research
- Compare a study with other research that's been done
Or it can be a separate work (a research article on its own) which:
- Organizes or describes a topic
- Describes variables within a particular issue/problem
Limitations of a Literature Review
Some of the limitations of a literature review are:
- It's a snapshot in time. Unlike other reviews, this one has beginning, a middle and an end. There may be future developments that could make your work less relevant.
- It may be too focused. Some niche studies may miss the bigger picture.
- It can be difficult to be comprehensive. There is no way to make sure all the literature on a topic was considered.
- It is easy to be biased if you stick to top tier journals. There may be other places where people are publishing exemplary research. Look to open access publications and conferences to reflect a more inclusive collection. Also, make sure to include opposing views (and not just supporting evidence).
Source: Grant, Maria J., and Andrew Booth. “A Typology of Reviews: An Analysis of 14 Review Types and Associated Methodologies.” Health Information & Libraries Journal, vol. 26, no. 2, June 2009, pp. 91–108. Wiley Online Library, doi:10.1111/j.1471-1842.2009.00848.x.
Meryl Brodsky : Communication and Information Studies
Hannah Chapman Tripp : Biology, Neuroscience
Carolyn Cunningham : Human Development & Family Sciences, Psychology, Sociology
Larayne Dallas : Engineering
Janelle Hedstrom : Special Education, Curriculum & Instruction, Ed Leadership & Policy
Susan Macicak : Linguistics
Imelda Vetter : Dell Medical School
For help in other subject areas, please see the guide to library specialists by subject .
Periodically, UT Libraries runs a workshop covering the basics and library support for literature reviews. While we try to offer these once per academic year, we find providing the recording to be helpful to community members who have missed the session. Following is the most recent recording of the workshop, Conducting a Literature Review. To view the recording, a UT login is required.
- October 26, 2022 recording
- Last Updated: Oct 26, 2022 2:49 PM
- URL: https://guides.lib.utexas.edu/literaturereviews

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- CAREER FEATURE
- 04 December 2020
- Correction 09 December 2020
How to write a superb literature review
Andy Tay is a freelance writer based in Singapore.
You can also search for this author in PubMed Google Scholar
Literature reviews are important resources for scientists. They provide historical context for a field while offering opinions on its future trajectory. Creating them can provide inspiration for one’s own research, as well as some practice in writing. But few scientists are trained in how to write a review — or in what constitutes an excellent one. Even picking the appropriate software to use can be an involved decision (see ‘Tools and techniques’). So Nature asked editors and working scientists with well-cited reviews for their tips.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
doi: https://doi.org/10.1038/d41586-020-03422-x
Interviews have been edited for length and clarity.
Updates & Corrections
Correction 09 December 2020 : An earlier version of the tables in this article included some incorrect details about the programs Zotero, Endnote and Manubot. These have now been corrected.
Hsing, I.-M., Xu, Y. & Zhao, W. Electroanalysis 19 , 755–768 (2007).
Article Google Scholar
Ledesma, H. A. et al. Nature Nanotechnol. 14 , 645–657 (2019).
Article PubMed Google Scholar
Brahlek, M., Koirala, N., Bansal, N. & Oh, S. Solid State Commun. 215–216 , 54–62 (2015).
Choi, Y. & Lee, S. Y. Nature Rev. Chem . https://doi.org/10.1038/s41570-020-00221-w (2020).
Download references
Related Articles

- Research management

Defying the stereotype of Black resilience
Career Q&A 30 MAY 24

How I overcame my stage fright in the lab
Career Column 30 MAY 24

I had my white colleagues walk in a Black student’s shoes for a day
Career Q&A 28 MAY 24

Researcher parents are paying a high price for conference travel — here’s how to fix it
Career Column 27 MAY 24

How researchers in remote regions handle the isolation
Career Feature 24 MAY 24

Guidelines for academics aim to lessen ethical pitfalls in generative-AI use
Nature Index 22 MAY 24
Who will make AlphaFold3 open source? Scientists race to crack AI model
News 23 MAY 24

Egypt is building a $1-billion mega-museum. Will it bring Egyptology home?
News Feature 22 MAY 24

Pay researchers to spot errors in published papers
World View 21 MAY 24
Postdoctoral Associate - Amyloid Strain Differences in Alzheimer's Disease
Houston, Texas (US)
Baylor College of Medicine (BCM)
Postdoctoral Associate- Bioinformatics of Alzheimer's disease
Postdoctoral associate- alzheimer's gene therapy, postdoctoral associate, kaust global postdoctoral fellowship.
The KAUST Global Fellowship Program is designed to attract emerging research leaders working across areas under the four research priorities of KAUST.
Saudi Arabia (SA)
King Abdullah University of Science and Technology (KAUST
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- UConn Library
- Literature Review: The What, Why and How-to Guide
- Introduction
Literature Review: The What, Why and How-to Guide — Introduction
- Getting Started
- How to Pick a Topic
- Strategies to Find Sources
- Evaluating Sources & Lit. Reviews
- Tips for Writing Literature Reviews
- Writing Literature Review: Useful Sites
- Citation Resources
- Other Academic Writings
What are Literature Reviews?
So, what is a literature review? "A literature review is an account of what has been published on a topic by accredited scholars and researchers. In writing the literature review, your purpose is to convey to your reader what knowledge and ideas have been established on a topic, and what their strengths and weaknesses are. As a piece of writing, the literature review must be defined by a guiding concept (e.g., your research objective, the problem or issue you are discussing, or your argumentative thesis). It is not just a descriptive list of the material available, or a set of summaries." Taylor, D. The literature review: A few tips on conducting it . University of Toronto Health Sciences Writing Centre.
Goals of Literature Reviews
What are the goals of creating a Literature Review? A literature could be written to accomplish different aims:
- To develop a theory or evaluate an existing theory
- To summarize the historical or existing state of a research topic
- Identify a problem in a field of research
Baumeister, R. F., & Leary, M. R. (1997). Writing narrative literature reviews . Review of General Psychology , 1 (3), 311-320.
What kinds of sources require a Literature Review?
- A research paper assigned in a course
- A thesis or dissertation
- A grant proposal
- An article intended for publication in a journal
All these instances require you to collect what has been written about your research topic so that you can demonstrate how your own research sheds new light on the topic.
Types of Literature Reviews
What kinds of literature reviews are written?
Narrative review: The purpose of this type of review is to describe the current state of the research on a specific topic/research and to offer a critical analysis of the literature reviewed. Studies are grouped by research/theoretical categories, and themes and trends, strengths and weakness, and gaps are identified. The review ends with a conclusion section which summarizes the findings regarding the state of the research of the specific study, the gaps identify and if applicable, explains how the author's research will address gaps identify in the review and expand the knowledge on the topic reviewed.
- Example : Predictors and Outcomes of U.S. Quality Maternity Leave: A Review and Conceptual Framework: 10.1177/08948453211037398
Systematic review : "The authors of a systematic review use a specific procedure to search the research literature, select the studies to include in their review, and critically evaluate the studies they find." (p. 139). Nelson, L. K. (2013). Research in Communication Sciences and Disorders . Plural Publishing.
- Example : The effect of leave policies on increasing fertility: a systematic review: 10.1057/s41599-022-01270-w
Meta-analysis : "Meta-analysis is a method of reviewing research findings in a quantitative fashion by transforming the data from individual studies into what is called an effect size and then pooling and analyzing this information. The basic goal in meta-analysis is to explain why different outcomes have occurred in different studies." (p. 197). Roberts, M. C., & Ilardi, S. S. (2003). Handbook of Research Methods in Clinical Psychology . Blackwell Publishing.
- Example : Employment Instability and Fertility in Europe: A Meta-Analysis: 10.1215/00703370-9164737
Meta-synthesis : "Qualitative meta-synthesis is a type of qualitative study that uses as data the findings from other qualitative studies linked by the same or related topic." (p.312). Zimmer, L. (2006). Qualitative meta-synthesis: A question of dialoguing with texts . Journal of Advanced Nursing , 53 (3), 311-318.
- Example : Women’s perspectives on career successes and barriers: A qualitative meta-synthesis: 10.1177/05390184221113735
Literature Reviews in the Health Sciences
- UConn Health subject guide on systematic reviews Explanation of the different review types used in health sciences literature as well as tools to help you find the right review type
- << Previous: Getting Started
- Next: How to Pick a Topic >>
- Last Updated: Sep 21, 2022 2:16 PM
- URL: https://guides.lib.uconn.edu/literaturereview
Literature Reviews
- Tools & Visualizations
- Literature Review Examples
- Videos, Books & Links
Business & Econ Librarian

Click to Chat with a Librarian
Text: (571) 248-7542
What is a literature review?
A literature review discusses published information in a particular subject area. Often part of the introduction to an essay, research report or thesis, the literature review is literally a "re" view or "look again" at what has already been written about the topic, wherein the author analyzes a segment of a published body of knowledge through summary, classification, and comparison of prior research studies, reviews of literature, and theoretical articles. Literature reviews provide the reader with a bibliographic history of the scholarly research in any given field of study. As such, as new information becomes available, literature reviews grow in length or become focused on one specific aspect of the topic.
A literature review can be just a simple summary of the sources, but usually contains an organizational pattern and combines both summary and synthesis. A summary is a recap of the important information of the source, whereas a synthesis is a re-organization, or a reshuffling, of that information. The literature review might give a new interpretation of old material or combine new with old interpretations. Or it might trace the intellectual progression of the field, including major debates. Depending on the situation, the literature review may evaluate the sources and advise the reader on the most pertinent or relevant.
A literature review is NOT:
- An annotated bibliography – a list of citations to books, articles and documents that includes a brief description and evaluation for each citation. The annotations inform the reader of the relevance, accuracy and quality of the sources cited.
- A literary review – a critical discussion of the merits and weaknesses of a literary work.
- A book review – a critical discussion of the merits and weaknesses of a particular book.
- Teaching Information Literacy Reframed: 50+ Framework-Based Exercises for Creating Information-Literate Learners
- The UNC Writing Center – Literature Reviews
- The UW-Madison Writing Center: The Writer’s Handbook – Academic and Professional Writing – Learn How to Write a Literature Review
What is the difference between a literature review and a research paper?
The focus of a literature review is to summarize and synthesize the arguments and ideas of others without adding new contributions, whereas academic research papers present and develop new arguments that build upon the previously available body of literature.
How do I write a literature review?
There are many resources that offer step-by-step guidance for writing a literature review, and you can find some of them under Other Resources in the menu to the left. Writing the Literature Review: A Practical Guide suggests these steps:
- Chose a review topic and develop a research question
- Locate and organize research sources
- Select, analyze and annotate sources
- Evaluate research articles and other documents
- Structure and organize the literature review
- Develop arguments and supporting claims
- Synthesize and interpret the literature
- Put it all together
What is the purpose of writing a literature review?
Literature reviews serve as a guide to a particular topic: professionals can use literature reviews to keep current on their field; scholars can determine credibility of the writer in his or her field by analyzing the literature review.
As a writer, you will use the literature review to:
- See what has, and what has not, been investigated about your topic
- Identify data sources that other researches have used
- Learn how others in the field have defined and measured key concepts
- Establish context, or background, for the argument explored in the rest of a paper
- Explain what the strengths and weaknesses of that knowledge and ideas might be
- Contribute to the field by moving research forward
- To keep the writer/reader up to date with current developments in a particular field of study
- Develop alternative research projects
- Put your work in perspective
- Demonstrate your understanding and your ability to critically evaluate research in the field
- Provide evidence that may support your own findings
- Next: Tools & Visualizations >>
- Last Updated: May 6, 2024 3:20 PM
- URL: https://subjectguides.library.american.edu/literaturereview
Want to publish a literature review? Think of it as an empirical paper
What to consider if you want to publish a literature review paper
Tatiana Andreeva - Fri 23 Apr 2021 07:50 (updated Fri 27 Oct 2023 17:26)

[Guest post by CYGNA member Tatiana Andreeva ]
When you’ve been reading a lot on a particular topic – for example, reviewing the literature for your research project or for your PhD – at some point it looks like you have enough material and reflections to publish this piece of work as a separate paper. Recognize this? If you ever tried it, you might know that publishing a literature review paper in an academic journal is a tricky task. The literature review publications come in so many forms, and there is no single cheat-sheet or established format like for empirical papers that you could follow to ensure success in publication.
Through my own journey of trial-and-error on this path, as well as through reviewing for journals and for PhD students in my course, I came up with an idea that will help you to increase the chances of publishing a literature review: think of a literature review as simply another empirical research project. Think of it as an empirical study, in which your data comes not from your usual fieldwork but from the articles that you review.
Many literature reviews can be thought of as a qualitative empirical study, in which the papers included in the review substitute interviews or field observations that you would usually collect and code. Some literature reviews, e.g., meta-analyses, are more like a quantitative empirical paper, in which various numbers you extract from the papers in your dataset substitute your survey data.
Seeing literature review in this way has three important implications for how we think about our literature review, and how we can design it to increase its chances of being interesting to others - that is, of being published.
Start with a relevant research problem and an interesting research question
We learn early in our academic career that any empirical paper should have a clear research problem and a clear research question. We frequently hear from journal editors and reviewers that just having a gap in the literature, or the fact that something has not been researched before, are not good enough to justify doing yet another empirical study. They say: you need to have a problem that your study can address, and you need to have a question that we currently don’t have an answer to. Only then your empirical study can add value to existing research.
When we think of a literature review as of an empirical study, just with the different type of data at hand, we realize that the very same rationale applies. From this perspective the arguments that I often see in literature reviews – that there is no literature review in this particular area or that the existing literature reviews are quite dated – are not sufficient in the journal’s eyes to justify the publication of a literature review on a topic. If you aim to publish your literature review, start by thinking – what is the problem I would like to address? What would be my research question about this problem, that other readers would find interesting?
Design a methodologically-sound data collection and analysis protocol
When we think of any empirical study, we know that if we want to have reliable findings that will be accepted by our peers as trustworthy, we need to follow a transparent and well-thought data collection protocol. We also need to carefully choose and correctly apply relevant data analysis method. This goes without saying, right?
The same applies to the literature review! If we want our readers to trust our conclusions from the literature review, we need to make sure that the data we collect speaks to our research question, is of good quality, representative of the field, etc. The growing attention in business and management field to the systematic approach to literature reviews (Denyer & Tranfield, 2009; Rojon et al., 2021) reflects the rising expectations of the quality of the data used in literature review papers. Indeed, this approach offers exactly that: a clear data collection protocol, transparently communicated, so that someone else could replicate your study. For example, do the very same thing in 10 years and see how thinking on the topic has changed.

In the literature on doing literature reviews you will read that systematic literature review is only one of the types of literature reviews. Yet all recommendations on doing different types of the literature reviews share the idea that the data that you base your conclusions on has to be collected in a rigorous and transparent way (e.g., Callahan, 2014). In this post you can find more references on how to ensure that your literature review “data collection” protocol meets the quality expectations.
So now you have all the papers you have carefully selected, how do you go about analysing them, so that peer academics would recognize your conclusions as reliable and robust? This is the trickiest part, and we have limited methodological advice published on this. In this post I’ve mentioned some papers that discuss specific methods of literature analysis. For example, I found that a sophisticated coding rubric leveraged our literature analysis to a different level (Sergeeva & Andreeva, 2016), but must acknowledge that developing this rubric was one of the most challenging tasks of this review paper. In O’Higgins et al. (forthcoming) we used a combination of qualitative content analysis with Pearson’s chi-squared (χ²) goodness of fit test in order to validate some of our conclusions. The trick is - as with any empirical study - your choice of the analytical method needs to fit with your research question. In sum, the message is: choose your method for analysis of the selected literature carefully, apply it rigorously, and explain it transparently.
Think of the theoretical contribution beyond description of the findings
When we think of our usual empirical work, be it qualitative or quantitative, we are well-aware that just the description of our data wouldn’t do. We know that we need to leverage what our data shows to explain how it informs the broader theory, how it compares to previous studies, what is new that we see from this data?
Again, the same logic applies to the literature reviews. In practice though, we often find it difficult to apply this advice to our literature review papers, because the description of the field in itself seems to be novel, especially if nobody did such a review before. In my experience, this argument does not persuade editors and reviewers of the journals, and often rightfully so.

For example, think of a typical quantitative empirical paper: a descriptive statistics table must be provided, but no one would claim a contribution based on it, right? Cropanzano (2009:1306-1307) offers a good exercise that explains why reviewers often don’t buy the description of the field as a novel contribution. He suggests: imagine somebody who read all the primary articles in your dataset, would they still learn anything from your literature review? And if the answer is “no”, then it’s likely that your review paper doesn’t have yet the level of contribution that is needed to turn it into a publication.
I think this exercise can also help to stimulate your thinking of what a theoretical contribution of your literature review could be. For example, think – what it is that I see in this literature that others are not likely to see? In this blogpost you can find some papers that offer insights on how to leverage your literature review to have a theoretical contribution.
Callahan, J.L. (2014). Writing literature reviews: A reprise and update. Human Resource Development Review , 13(3), 271–275. https://doi.org/10.1177/1534484314536705
Cropanzano, R. (2009). Writing nonempirical articles for Journal of Management: General thoughts and suggestions. Journal of Management , 35(6), 1304–1311. https://doi.org/10.1177/0149206309344118
Denyer, D., Tranfield, D. (2009). Producing a systematic review. In Buchanan, D., Bryman, A. (Eds.), The Sage handbook of organizational research methods (pp. 671–689). London, UK: Sage.
O’Higgins, C., Andreeva, T., Aramburu, N. (forthcoming). International management challenges of professional service firms: a synthesis of the literature. Review of International Business and Strategy.
Rojon, C., Okupe, A., McDowall, A. (2021). Utilization and development of systematic reviews in management research: What do we know and where do we go from here? International Journal of Management Reviews, 1– 33. https://doi.org/10.1111/ijmr.12245
Sergeeva, A., Andreeva, T. (2016). Knowledge sharing: bringing the context back in, Journal of Management Inquiry , 25, 240-261. https://doi.org/10.1177/1056492615618271
Related blogposts
- Resources on doing a literature review
- Do you really want to publish your literature review? Advice for PhD students
- How to keep up-to-date with the literature, but avoid information overload?
- Is a literature review publication a low-cost project?
- Using Publish or Perish to do a literature review
- How to conduct a longitudinal literature review?
- New: Publish or Perish now also exports abstracts
Find the resources on my website useful?
I cover all the expenses of operating my website privately. If you enjoyed this post and want to support me in maintaining my website, consider buying a copy of one of my books (see below) or supporting the Publish or Perish software .
A story about perseverance and publication failures
Copyright © 2023 Tatiana Andreeva . All rights reserved. Page last modified on Fri 27 Oct 2023 17:26

Tatiana Andreeva's profile and contact details >>
Conduct a literature review
What is a literature review.
A literature review is a summary of the published work in a field of study. This can be a section of a larger paper or article, or can be the focus of an entire paper. Literature reviews show that you have examined the breadth of knowledge and can justify your thesis or research questions. They are also valuable tools for other researchers who need to find a summary of that field of knowledge.
Unlike an annotated bibliography, which is a list of sources with short descriptions, a literature review synthesizes sources into a summary that has a thesis or statement of purpose—stated or implied—at its core.
How do I write a literature review?
Step 1: define your research scope.
- What is the specific research question that your literature review helps to define?
- Are there a maximum or minimum number of sources that your review should include?
Ask us if you have questions about refining your topic, search methods, writing tips, or citation management.
Step 2: Identify the literature
Start by searching broadly. Literature for your review will typically be acquired through scholarly books, journal articles, and/or dissertations. Develop an understanding of what is out there, what terms are accurate and helpful, etc., and keep track of all of it with citation management tools . If you need help figuring out key terms and where to search, ask us .
Use citation searching to track how scholars interact with, and build upon, previous research:
- Mine the references cited section of each relevant source for additional key sources
- Use Google Scholar or Scopus to find other sources that have cited a particular work
Step 3: Critically analyze the literature
Key to your literature review is a critical analysis of the literature collected around your topic. The analysis will explore relationships, major themes, and any critical gaps in the research expressed in the work. Read and summarize each source with an eye toward analyzing authority, currency, coverage, methodology, and relationship to other works. The University of Toronto's Writing Center provides a comprehensive list of questions you can use to analyze your sources.
Step 4: Categorize your resources
Divide the available resources that pertain to your research into categories reflecting their roles in addressing your research question. Possible ways to categorize resources include organization by:
- methodology
- theoretical/philosophical approach
Regardless of the division, each category should be accompanied by thorough discussions and explanations of strengths and weaknesses, value to the overall survey, and comparisons with similar sources. You may have enough resources when:
- You've used multiple databases and other resources (web portals, repositories, etc.) to get a variety of perspectives on the research topic.
- The same citations are showing up in a variety of databases.
Additional resources
Undergraduate student resources.
- Literature Review Handout (University of North Carolina at Chapel Hill)
- Learn how to write a review of literature (University of Wisconsin-Madison)
Graduate student and faculty resources
- Information Research Strategies (University of Arizona)
- Literature Reviews: An Overview for Graduate Students (NC State University)
- Oliver, P. (2012). Succeeding with Your Literature Review: A Handbook for Students [ebook]
- Machi, L. A. & McEvoy, B. T. (2016). The Literature Review: Six Steps to Success [ebook]
- Graustein, J. S. (2012). How to Write an Exceptional Thesis or Dissertation: A Step-by-Step Guide from Proposal to Successful Defense [ebook]
- Thomas, R. M. & Brubaker, D. L. (2008). Theses and Dissertations: A Guide to Planning, Research, and Writing
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Dissertation
- What is a Literature Review? | Guide, Template, & Examples
What is a Literature Review? | Guide, Template, & Examples
Published on 22 February 2022 by Shona McCombes . Revised on 7 June 2022.
What is a literature review? A literature review is a survey of scholarly sources on a specific topic. It provides an overview of current knowledge, allowing you to identify relevant theories, methods, and gaps in the existing research.
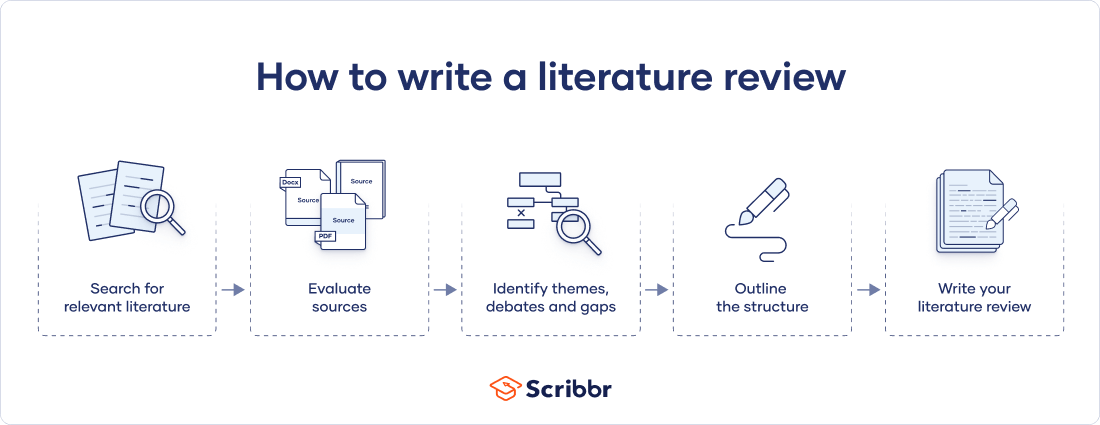
There are five key steps to writing a literature review:
- Search for relevant literature
- Evaluate sources
- Identify themes, debates and gaps
- Outline the structure
- Write your literature review
A good literature review doesn’t just summarise sources – it analyses, synthesises, and critically evaluates to give a clear picture of the state of knowledge on the subject.
Instantly correct all language mistakes in your text
Be assured that you'll submit flawless writing. Upload your document to correct all your mistakes.

Table of contents
Why write a literature review, examples of literature reviews, step 1: search for relevant literature, step 2: evaluate and select sources, step 3: identify themes, debates and gaps, step 4: outline your literature review’s structure, step 5: write your literature review, frequently asked questions about literature reviews, introduction.
- Quick Run-through
- Step 1 & 2
When you write a dissertation or thesis, you will have to conduct a literature review to situate your research within existing knowledge. The literature review gives you a chance to:
- Demonstrate your familiarity with the topic and scholarly context
- Develop a theoretical framework and methodology for your research
- Position yourself in relation to other researchers and theorists
- Show how your dissertation addresses a gap or contributes to a debate
You might also have to write a literature review as a stand-alone assignment. In this case, the purpose is to evaluate the current state of research and demonstrate your knowledge of scholarly debates around a topic.
The content will look slightly different in each case, but the process of conducting a literature review follows the same steps. We’ve written a step-by-step guide that you can follow below.
The only proofreading tool specialized in correcting academic writing
The academic proofreading tool has been trained on 1000s of academic texts and by native English editors. Making it the most accurate and reliable proofreading tool for students.
Correct my document today
Writing literature reviews can be quite challenging! A good starting point could be to look at some examples, depending on what kind of literature review you’d like to write.
- Example literature review #1: “Why Do People Migrate? A Review of the Theoretical Literature” ( Theoretical literature review about the development of economic migration theory from the 1950s to today.)
- Example literature review #2: “Literature review as a research methodology: An overview and guidelines” ( Methodological literature review about interdisciplinary knowledge acquisition and production.)
- Example literature review #3: “The Use of Technology in English Language Learning: A Literature Review” ( Thematic literature review about the effects of technology on language acquisition.)
- Example literature review #4: “Learners’ Listening Comprehension Difficulties in English Language Learning: A Literature Review” ( Chronological literature review about how the concept of listening skills has changed over time.)
You can also check out our templates with literature review examples and sample outlines at the links below.
Download Word doc Download Google doc
Before you begin searching for literature, you need a clearly defined topic .
If you are writing the literature review section of a dissertation or research paper, you will search for literature related to your research objectives and questions .
If you are writing a literature review as a stand-alone assignment, you will have to choose a focus and develop a central question to direct your search. Unlike a dissertation research question, this question has to be answerable without collecting original data. You should be able to answer it based only on a review of existing publications.
Make a list of keywords
Start by creating a list of keywords related to your research topic. Include each of the key concepts or variables you’re interested in, and list any synonyms and related terms. You can add to this list if you discover new keywords in the process of your literature search.
- Social media, Facebook, Instagram, Twitter, Snapchat, TikTok
- Body image, self-perception, self-esteem, mental health
- Generation Z, teenagers, adolescents, youth
Search for relevant sources
Use your keywords to begin searching for sources. Some databases to search for journals and articles include:
- Your university’s library catalogue
- Google Scholar
- Project Muse (humanities and social sciences)
- Medline (life sciences and biomedicine)
- EconLit (economics)
- Inspec (physics, engineering and computer science)
You can use boolean operators to help narrow down your search:
Read the abstract to find out whether an article is relevant to your question. When you find a useful book or article, you can check the bibliography to find other relevant sources.
To identify the most important publications on your topic, take note of recurring citations. If the same authors, books or articles keep appearing in your reading, make sure to seek them out.
You probably won’t be able to read absolutely everything that has been written on the topic – you’ll have to evaluate which sources are most relevant to your questions.
For each publication, ask yourself:
- What question or problem is the author addressing?
- What are the key concepts and how are they defined?
- What are the key theories, models and methods? Does the research use established frameworks or take an innovative approach?
- What are the results and conclusions of the study?
- How does the publication relate to other literature in the field? Does it confirm, add to, or challenge established knowledge?
- How does the publication contribute to your understanding of the topic? What are its key insights and arguments?
- What are the strengths and weaknesses of the research?
Make sure the sources you use are credible, and make sure you read any landmark studies and major theories in your field of research.
You can find out how many times an article has been cited on Google Scholar – a high citation count means the article has been influential in the field, and should certainly be included in your literature review.
The scope of your review will depend on your topic and discipline: in the sciences you usually only review recent literature, but in the humanities you might take a long historical perspective (for example, to trace how a concept has changed in meaning over time).
Remember that you can use our template to summarise and evaluate sources you’re thinking about using!
Take notes and cite your sources
As you read, you should also begin the writing process. Take notes that you can later incorporate into the text of your literature review.
It’s important to keep track of your sources with references to avoid plagiarism . It can be helpful to make an annotated bibliography, where you compile full reference information and write a paragraph of summary and analysis for each source. This helps you remember what you read and saves time later in the process.
You can use our free APA Reference Generator for quick, correct, consistent citations.
Prevent plagiarism, run a free check.
To begin organising your literature review’s argument and structure, you need to understand the connections and relationships between the sources you’ve read. Based on your reading and notes, you can look for:
- Trends and patterns (in theory, method or results): do certain approaches become more or less popular over time?
- Themes: what questions or concepts recur across the literature?
- Debates, conflicts and contradictions: where do sources disagree?
- Pivotal publications: are there any influential theories or studies that changed the direction of the field?
- Gaps: what is missing from the literature? Are there weaknesses that need to be addressed?
This step will help you work out the structure of your literature review and (if applicable) show how your own research will contribute to existing knowledge.
- Most research has focused on young women.
- There is an increasing interest in the visual aspects of social media.
- But there is still a lack of robust research on highly-visual platforms like Instagram and Snapchat – this is a gap that you could address in your own research.
There are various approaches to organising the body of a literature review. You should have a rough idea of your strategy before you start writing.
Depending on the length of your literature review, you can combine several of these strategies (for example, your overall structure might be thematic, but each theme is discussed chronologically).
Chronological
The simplest approach is to trace the development of the topic over time. However, if you choose this strategy, be careful to avoid simply listing and summarising sources in order.
Try to analyse patterns, turning points and key debates that have shaped the direction of the field. Give your interpretation of how and why certain developments occurred.
If you have found some recurring central themes, you can organise your literature review into subsections that address different aspects of the topic.
For example, if you are reviewing literature about inequalities in migrant health outcomes, key themes might include healthcare policy, language barriers, cultural attitudes, legal status, and economic access.
Methodological
If you draw your sources from different disciplines or fields that use a variety of research methods , you might want to compare the results and conclusions that emerge from different approaches. For example:
- Look at what results have emerged in qualitative versus quantitative research
- Discuss how the topic has been approached by empirical versus theoretical scholarship
- Divide the literature into sociological, historical, and cultural sources
Theoretical
A literature review is often the foundation for a theoretical framework . You can use it to discuss various theories, models, and definitions of key concepts.
You might argue for the relevance of a specific theoretical approach, or combine various theoretical concepts to create a framework for your research.
Like any other academic text, your literature review should have an introduction , a main body, and a conclusion . What you include in each depends on the objective of your literature review.
The introduction should clearly establish the focus and purpose of the literature review.
If you are writing the literature review as part of your dissertation or thesis, reiterate your central problem or research question and give a brief summary of the scholarly context. You can emphasise the timeliness of the topic (“many recent studies have focused on the problem of x”) or highlight a gap in the literature (“while there has been much research on x, few researchers have taken y into consideration”).
Depending on the length of your literature review, you might want to divide the body into subsections. You can use a subheading for each theme, time period, or methodological approach.
As you write, make sure to follow these tips:
- Summarise and synthesise: give an overview of the main points of each source and combine them into a coherent whole.
- Analyse and interpret: don’t just paraphrase other researchers – add your own interpretations, discussing the significance of findings in relation to the literature as a whole.
- Critically evaluate: mention the strengths and weaknesses of your sources.
- Write in well-structured paragraphs: use transitions and topic sentences to draw connections, comparisons and contrasts.
In the conclusion, you should summarise the key findings you have taken from the literature and emphasise their significance.
If the literature review is part of your dissertation or thesis, reiterate how your research addresses gaps and contributes new knowledge, or discuss how you have drawn on existing theories and methods to build a framework for your research. This can lead directly into your methodology section.
A literature review is a survey of scholarly sources (such as books, journal articles, and theses) related to a specific topic or research question .
It is often written as part of a dissertation , thesis, research paper , or proposal .
There are several reasons to conduct a literature review at the beginning of a research project:
- To familiarise yourself with the current state of knowledge on your topic
- To ensure that you’re not just repeating what others have already done
- To identify gaps in knowledge and unresolved problems that your research can address
- To develop your theoretical framework and methodology
- To provide an overview of the key findings and debates on the topic
Writing the literature review shows your reader how your work relates to existing research and what new insights it will contribute.
The literature review usually comes near the beginning of your dissertation . After the introduction , it grounds your research in a scholarly field and leads directly to your theoretical framework or methodology .
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator.
McCombes, S. (2022, June 07). What is a Literature Review? | Guide, Template, & Examples. Scribbr. Retrieved 27 May 2024, from https://www.scribbr.co.uk/thesis-dissertation/literature-review/
Is this article helpful?
Shona McCombes
Other students also liked, how to write a dissertation proposal | a step-by-step guide, what is a theoretical framework | a step-by-step guide, what is a research methodology | steps & tips.

Literature Reviews
What this handout is about.
This handout will explain what literature reviews are and offer insights into the form and construction of literature reviews in the humanities, social sciences, and sciences.
Introduction
OK. You’ve got to write a literature review. You dust off a novel and a book of poetry, settle down in your chair, and get ready to issue a “thumbs up” or “thumbs down” as you leaf through the pages. “Literature review” done. Right?
Wrong! The “literature” of a literature review refers to any collection of materials on a topic, not necessarily the great literary texts of the world. “Literature” could be anything from a set of government pamphlets on British colonial methods in Africa to scholarly articles on the treatment of a torn ACL. And a review does not necessarily mean that your reader wants you to give your personal opinion on whether or not you liked these sources.
What is a literature review, then?
A literature review discusses published information in a particular subject area, and sometimes information in a particular subject area within a certain time period.
A literature review can be just a simple summary of the sources, but it usually has an organizational pattern and combines both summary and synthesis. A summary is a recap of the important information of the source, but a synthesis is a re-organization, or a reshuffling, of that information. It might give a new interpretation of old material or combine new with old interpretations. Or it might trace the intellectual progression of the field, including major debates. And depending on the situation, the literature review may evaluate the sources and advise the reader on the most pertinent or relevant.
But how is a literature review different from an academic research paper?
The main focus of an academic research paper is to develop a new argument, and a research paper is likely to contain a literature review as one of its parts. In a research paper, you use the literature as a foundation and as support for a new insight that you contribute. The focus of a literature review, however, is to summarize and synthesize the arguments and ideas of others without adding new contributions.
Why do we write literature reviews?
Literature reviews provide you with a handy guide to a particular topic. If you have limited time to conduct research, literature reviews can give you an overview or act as a stepping stone. For professionals, they are useful reports that keep them up to date with what is current in the field. For scholars, the depth and breadth of the literature review emphasizes the credibility of the writer in his or her field. Literature reviews also provide a solid background for a research paper’s investigation. Comprehensive knowledge of the literature of the field is essential to most research papers.
Who writes these things, anyway?
Literature reviews are written occasionally in the humanities, but mostly in the sciences and social sciences; in experiment and lab reports, they constitute a section of the paper. Sometimes a literature review is written as a paper in itself.
Let’s get to it! What should I do before writing the literature review?
If your assignment is not very specific, seek clarification from your instructor:
- Roughly how many sources should you include?
- What types of sources (books, journal articles, websites)?
- Should you summarize, synthesize, or critique your sources by discussing a common theme or issue?
- Should you evaluate your sources?
- Should you provide subheadings and other background information, such as definitions and/or a history?
Find models
Look for other literature reviews in your area of interest or in the discipline and read them to get a sense of the types of themes you might want to look for in your own research or ways to organize your final review. You can simply put the word “review” in your search engine along with your other topic terms to find articles of this type on the Internet or in an electronic database. The bibliography or reference section of sources you’ve already read are also excellent entry points into your own research.
Narrow your topic
There are hundreds or even thousands of articles and books on most areas of study. The narrower your topic, the easier it will be to limit the number of sources you need to read in order to get a good survey of the material. Your instructor will probably not expect you to read everything that’s out there on the topic, but you’ll make your job easier if you first limit your scope.
Keep in mind that UNC Libraries have research guides and to databases relevant to many fields of study. You can reach out to the subject librarian for a consultation: https://library.unc.edu/support/consultations/ .
And don’t forget to tap into your professor’s (or other professors’) knowledge in the field. Ask your professor questions such as: “If you had to read only one book from the 90’s on topic X, what would it be?” Questions such as this help you to find and determine quickly the most seminal pieces in the field.
Consider whether your sources are current
Some disciplines require that you use information that is as current as possible. In the sciences, for instance, treatments for medical problems are constantly changing according to the latest studies. Information even two years old could be obsolete. However, if you are writing a review in the humanities, history, or social sciences, a survey of the history of the literature may be what is needed, because what is important is how perspectives have changed through the years or within a certain time period. Try sorting through some other current bibliographies or literature reviews in the field to get a sense of what your discipline expects. You can also use this method to consider what is currently of interest to scholars in this field and what is not.
Strategies for writing the literature review
Find a focus.
A literature review, like a term paper, is usually organized around ideas, not the sources themselves as an annotated bibliography would be organized. This means that you will not just simply list your sources and go into detail about each one of them, one at a time. No. As you read widely but selectively in your topic area, consider instead what themes or issues connect your sources together. Do they present one or different solutions? Is there an aspect of the field that is missing? How well do they present the material and do they portray it according to an appropriate theory? Do they reveal a trend in the field? A raging debate? Pick one of these themes to focus the organization of your review.
Convey it to your reader
A literature review may not have a traditional thesis statement (one that makes an argument), but you do need to tell readers what to expect. Try writing a simple statement that lets the reader know what is your main organizing principle. Here are a couple of examples:
The current trend in treatment for congestive heart failure combines surgery and medicine. More and more cultural studies scholars are accepting popular media as a subject worthy of academic consideration.
Consider organization
You’ve got a focus, and you’ve stated it clearly and directly. Now what is the most effective way of presenting the information? What are the most important topics, subtopics, etc., that your review needs to include? And in what order should you present them? Develop an organization for your review at both a global and local level:
First, cover the basic categories
Just like most academic papers, literature reviews also must contain at least three basic elements: an introduction or background information section; the body of the review containing the discussion of sources; and, finally, a conclusion and/or recommendations section to end the paper. The following provides a brief description of the content of each:
- Introduction: Gives a quick idea of the topic of the literature review, such as the central theme or organizational pattern.
- Body: Contains your discussion of sources and is organized either chronologically, thematically, or methodologically (see below for more information on each).
- Conclusions/Recommendations: Discuss what you have drawn from reviewing literature so far. Where might the discussion proceed?
Organizing the body
Once you have the basic categories in place, then you must consider how you will present the sources themselves within the body of your paper. Create an organizational method to focus this section even further.
To help you come up with an overall organizational framework for your review, consider the following scenario:
You’ve decided to focus your literature review on materials dealing with sperm whales. This is because you’ve just finished reading Moby Dick, and you wonder if that whale’s portrayal is really real. You start with some articles about the physiology of sperm whales in biology journals written in the 1980’s. But these articles refer to some British biological studies performed on whales in the early 18th century. So you check those out. Then you look up a book written in 1968 with information on how sperm whales have been portrayed in other forms of art, such as in Alaskan poetry, in French painting, or on whale bone, as the whale hunters in the late 19th century used to do. This makes you wonder about American whaling methods during the time portrayed in Moby Dick, so you find some academic articles published in the last five years on how accurately Herman Melville portrayed the whaling scene in his novel.
Now consider some typical ways of organizing the sources into a review:
- Chronological: If your review follows the chronological method, you could write about the materials above according to when they were published. For instance, first you would talk about the British biological studies of the 18th century, then about Moby Dick, published in 1851, then the book on sperm whales in other art (1968), and finally the biology articles (1980s) and the recent articles on American whaling of the 19th century. But there is relatively no continuity among subjects here. And notice that even though the sources on sperm whales in other art and on American whaling are written recently, they are about other subjects/objects that were created much earlier. Thus, the review loses its chronological focus.
- By publication: Order your sources by publication chronology, then, only if the order demonstrates a more important trend. For instance, you could order a review of literature on biological studies of sperm whales if the progression revealed a change in dissection practices of the researchers who wrote and/or conducted the studies.
- By trend: A better way to organize the above sources chronologically is to examine the sources under another trend, such as the history of whaling. Then your review would have subsections according to eras within this period. For instance, the review might examine whaling from pre-1600-1699, 1700-1799, and 1800-1899. Under this method, you would combine the recent studies on American whaling in the 19th century with Moby Dick itself in the 1800-1899 category, even though the authors wrote a century apart.
- Thematic: Thematic reviews of literature are organized around a topic or issue, rather than the progression of time. However, progression of time may still be an important factor in a thematic review. For instance, the sperm whale review could focus on the development of the harpoon for whale hunting. While the study focuses on one topic, harpoon technology, it will still be organized chronologically. The only difference here between a “chronological” and a “thematic” approach is what is emphasized the most: the development of the harpoon or the harpoon technology.But more authentic thematic reviews tend to break away from chronological order. For instance, a thematic review of material on sperm whales might examine how they are portrayed as “evil” in cultural documents. The subsections might include how they are personified, how their proportions are exaggerated, and their behaviors misunderstood. A review organized in this manner would shift between time periods within each section according to the point made.
- Methodological: A methodological approach differs from the two above in that the focusing factor usually does not have to do with the content of the material. Instead, it focuses on the “methods” of the researcher or writer. For the sperm whale project, one methodological approach would be to look at cultural differences between the portrayal of whales in American, British, and French art work. Or the review might focus on the economic impact of whaling on a community. A methodological scope will influence either the types of documents in the review or the way in which these documents are discussed. Once you’ve decided on the organizational method for the body of the review, the sections you need to include in the paper should be easy to figure out. They should arise out of your organizational strategy. In other words, a chronological review would have subsections for each vital time period. A thematic review would have subtopics based upon factors that relate to the theme or issue.
Sometimes, though, you might need to add additional sections that are necessary for your study, but do not fit in the organizational strategy of the body. What other sections you include in the body is up to you. Put in only what is necessary. Here are a few other sections you might want to consider:
- Current Situation: Information necessary to understand the topic or focus of the literature review.
- History: The chronological progression of the field, the literature, or an idea that is necessary to understand the literature review, if the body of the literature review is not already a chronology.
- Methods and/or Standards: The criteria you used to select the sources in your literature review or the way in which you present your information. For instance, you might explain that your review includes only peer-reviewed articles and journals.
Questions for Further Research: What questions about the field has the review sparked? How will you further your research as a result of the review?
Begin composing
Once you’ve settled on a general pattern of organization, you’re ready to write each section. There are a few guidelines you should follow during the writing stage as well. Here is a sample paragraph from a literature review about sexism and language to illuminate the following discussion:
However, other studies have shown that even gender-neutral antecedents are more likely to produce masculine images than feminine ones (Gastil, 1990). Hamilton (1988) asked students to complete sentences that required them to fill in pronouns that agreed with gender-neutral antecedents such as “writer,” “pedestrian,” and “persons.” The students were asked to describe any image they had when writing the sentence. Hamilton found that people imagined 3.3 men to each woman in the masculine “generic” condition and 1.5 men per woman in the unbiased condition. Thus, while ambient sexism accounted for some of the masculine bias, sexist language amplified the effect. (Source: Erika Falk and Jordan Mills, “Why Sexist Language Affects Persuasion: The Role of Homophily, Intended Audience, and Offense,” Women and Language19:2).
Use evidence
In the example above, the writers refer to several other sources when making their point. A literature review in this sense is just like any other academic research paper. Your interpretation of the available sources must be backed up with evidence to show that what you are saying is valid.
Be selective
Select only the most important points in each source to highlight in the review. The type of information you choose to mention should relate directly to the review’s focus, whether it is thematic, methodological, or chronological.
Use quotes sparingly
Falk and Mills do not use any direct quotes. That is because the survey nature of the literature review does not allow for in-depth discussion or detailed quotes from the text. Some short quotes here and there are okay, though, if you want to emphasize a point, or if what the author said just cannot be rewritten in your own words. Notice that Falk and Mills do quote certain terms that were coined by the author, not common knowledge, or taken directly from the study. But if you find yourself wanting to put in more quotes, check with your instructor.
Summarize and synthesize
Remember to summarize and synthesize your sources within each paragraph as well as throughout the review. The authors here recapitulate important features of Hamilton’s study, but then synthesize it by rephrasing the study’s significance and relating it to their own work.
Keep your own voice
While the literature review presents others’ ideas, your voice (the writer’s) should remain front and center. Notice that Falk and Mills weave references to other sources into their own text, but they still maintain their own voice by starting and ending the paragraph with their own ideas and their own words. The sources support what Falk and Mills are saying.
Use caution when paraphrasing
When paraphrasing a source that is not your own, be sure to represent the author’s information or opinions accurately and in your own words. In the preceding example, Falk and Mills either directly refer in the text to the author of their source, such as Hamilton, or they provide ample notation in the text when the ideas they are mentioning are not their own, for example, Gastil’s. For more information, please see our handout on plagiarism .
Revise, revise, revise
Draft in hand? Now you’re ready to revise. Spending a lot of time revising is a wise idea, because your main objective is to present the material, not the argument. So check over your review again to make sure it follows the assignment and/or your outline. Then, just as you would for most other academic forms of writing, rewrite or rework the language of your review so that you’ve presented your information in the most concise manner possible. Be sure to use terminology familiar to your audience; get rid of unnecessary jargon or slang. Finally, double check that you’ve documented your sources and formatted the review appropriately for your discipline. For tips on the revising and editing process, see our handout on revising drafts .
Works consulted
We consulted these works while writing this handout. This is not a comprehensive list of resources on the handout’s topic, and we encourage you to do your own research to find additional publications. Please do not use this list as a model for the format of your own reference list, as it may not match the citation style you are using. For guidance on formatting citations, please see the UNC Libraries citation tutorial . We revise these tips periodically and welcome feedback.
Anson, Chris M., and Robert A. Schwegler. 2010. The Longman Handbook for Writers and Readers , 6th ed. New York: Longman.
Jones, Robert, Patrick Bizzaro, and Cynthia Selfe. 1997. The Harcourt Brace Guide to Writing in the Disciplines . New York: Harcourt Brace.
Lamb, Sandra E. 1998. How to Write It: A Complete Guide to Everything You’ll Ever Write . Berkeley: Ten Speed Press.
Rosen, Leonard J., and Laurence Behrens. 2003. The Allyn & Bacon Handbook , 5th ed. New York: Longman.
Troyka, Lynn Quittman, and Doug Hesse. 2016. Simon and Schuster Handbook for Writers , 11th ed. London: Pearson.
You may reproduce it for non-commercial use if you use the entire handout and attribute the source: The Writing Center, University of North Carolina at Chapel Hill
Make a Gift

Get science-backed answers as you write with Paperpal's Research feature
What is a Literature Review? How to Write It (with Examples)

A literature review is a critical analysis and synthesis of existing research on a particular topic. It provides an overview of the current state of knowledge, identifies gaps, and highlights key findings in the literature. 1 The purpose of a literature review is to situate your own research within the context of existing scholarship, demonstrating your understanding of the topic and showing how your work contributes to the ongoing conversation in the field. Learning how to write a literature review is a critical tool for successful research. Your ability to summarize and synthesize prior research pertaining to a certain topic demonstrates your grasp on the topic of study, and assists in the learning process.
Table of Contents
- What is the purpose of literature review?
- a. Habitat Loss and Species Extinction:
- b. Range Shifts and Phenological Changes:
- c. Ocean Acidification and Coral Reefs:
- d. Adaptive Strategies and Conservation Efforts:
How to write a good literature review
- Choose a Topic and Define the Research Question:
- Decide on the Scope of Your Review:
- Select Databases for Searches:
- Conduct Searches and Keep Track:
- Review the Literature:
- Organize and Write Your Literature Review:
- How to write a literature review faster with Paperpal?
- Frequently asked questions
What is a literature review?
A well-conducted literature review demonstrates the researcher’s familiarity with the existing literature, establishes the context for their own research, and contributes to scholarly conversations on the topic. One of the purposes of a literature review is also to help researchers avoid duplicating previous work and ensure that their research is informed by and builds upon the existing body of knowledge.

What is the purpose of literature review?
A literature review serves several important purposes within academic and research contexts. Here are some key objectives and functions of a literature review: 2
1. Contextualizing the Research Problem: The literature review provides a background and context for the research problem under investigation. It helps to situate the study within the existing body of knowledge.
2. Identifying Gaps in Knowledge: By identifying gaps, contradictions, or areas requiring further research, the researcher can shape the research question and justify the significance of the study. This is crucial for ensuring that the new research contributes something novel to the field.
Find academic papers related to your research topic faster. Try Research on Paperpal
3. Understanding Theoretical and Conceptual Frameworks: Literature reviews help researchers gain an understanding of the theoretical and conceptual frameworks used in previous studies. This aids in the development of a theoretical framework for the current research.
4. Providing Methodological Insights: Another purpose of literature reviews is that it allows researchers to learn about the methodologies employed in previous studies. This can help in choosing appropriate research methods for the current study and avoiding pitfalls that others may have encountered.
5. Establishing Credibility: A well-conducted literature review demonstrates the researcher’s familiarity with existing scholarship, establishing their credibility and expertise in the field. It also helps in building a solid foundation for the new research.
6. Informing Hypotheses or Research Questions: The literature review guides the formulation of hypotheses or research questions by highlighting relevant findings and areas of uncertainty in existing literature.
Literature review example
Let’s delve deeper with a literature review example: Let’s say your literature review is about the impact of climate change on biodiversity. You might format your literature review into sections such as the effects of climate change on habitat loss and species extinction, phenological changes, and marine biodiversity. Each section would then summarize and analyze relevant studies in those areas, highlighting key findings and identifying gaps in the research. The review would conclude by emphasizing the need for further research on specific aspects of the relationship between climate change and biodiversity. The following literature review template provides a glimpse into the recommended literature review structure and content, demonstrating how research findings are organized around specific themes within a broader topic.
Literature Review on Climate Change Impacts on Biodiversity:
Climate change is a global phenomenon with far-reaching consequences, including significant impacts on biodiversity. This literature review synthesizes key findings from various studies:
a. Habitat Loss and Species Extinction:
Climate change-induced alterations in temperature and precipitation patterns contribute to habitat loss, affecting numerous species (Thomas et al., 2004). The review discusses how these changes increase the risk of extinction, particularly for species with specific habitat requirements.
b. Range Shifts and Phenological Changes:
Observations of range shifts and changes in the timing of biological events (phenology) are documented in response to changing climatic conditions (Parmesan & Yohe, 2003). These shifts affect ecosystems and may lead to mismatches between species and their resources.
c. Ocean Acidification and Coral Reefs:
The review explores the impact of climate change on marine biodiversity, emphasizing ocean acidification’s threat to coral reefs (Hoegh-Guldberg et al., 2007). Changes in pH levels negatively affect coral calcification, disrupting the delicate balance of marine ecosystems.
d. Adaptive Strategies and Conservation Efforts:
Recognizing the urgency of the situation, the literature review discusses various adaptive strategies adopted by species and conservation efforts aimed at mitigating the impacts of climate change on biodiversity (Hannah et al., 2007). It emphasizes the importance of interdisciplinary approaches for effective conservation planning.

Strengthen your literature review with factual insights. Try Research on Paperpal for free!
Writing a literature review involves summarizing and synthesizing existing research on a particular topic. A good literature review format should include the following elements.
Introduction: The introduction sets the stage for your literature review, providing context and introducing the main focus of your review.
- Opening Statement: Begin with a general statement about the broader topic and its significance in the field.
- Scope and Purpose: Clearly define the scope of your literature review. Explain the specific research question or objective you aim to address.
- Organizational Framework: Briefly outline the structure of your literature review, indicating how you will categorize and discuss the existing research.
- Significance of the Study: Highlight why your literature review is important and how it contributes to the understanding of the chosen topic.
- Thesis Statement: Conclude the introduction with a concise thesis statement that outlines the main argument or perspective you will develop in the body of the literature review.
Body: The body of the literature review is where you provide a comprehensive analysis of existing literature, grouping studies based on themes, methodologies, or other relevant criteria.
- Organize by Theme or Concept: Group studies that share common themes, concepts, or methodologies. Discuss each theme or concept in detail, summarizing key findings and identifying gaps or areas of disagreement.
- Critical Analysis: Evaluate the strengths and weaknesses of each study. Discuss the methodologies used, the quality of evidence, and the overall contribution of each work to the understanding of the topic.
- Synthesis of Findings: Synthesize the information from different studies to highlight trends, patterns, or areas of consensus in the literature.
- Identification of Gaps: Discuss any gaps or limitations in the existing research and explain how your review contributes to filling these gaps.
- Transition between Sections: Provide smooth transitions between different themes or concepts to maintain the flow of your literature review.
Write and Cite as you go with Paperpal Research. Start now for free.
Conclusion: The conclusion of your literature review should summarize the main findings, highlight the contributions of the review, and suggest avenues for future research.
- Summary of Key Findings: Recap the main findings from the literature and restate how they contribute to your research question or objective.
- Contributions to the Field: Discuss the overall contribution of your literature review to the existing knowledge in the field.
- Implications and Applications: Explore the practical implications of the findings and suggest how they might impact future research or practice.
- Recommendations for Future Research: Identify areas that require further investigation and propose potential directions for future research in the field.
- Final Thoughts: Conclude with a final reflection on the importance of your literature review and its relevance to the broader academic community.

Conducting a literature review
Conducting a literature review is an essential step in research that involves reviewing and analyzing existing literature on a specific topic. It’s important to know how to do a literature review effectively, so here are the steps to follow: 1
Choose a Topic and Define the Research Question:
- Select a topic that is relevant to your field of study.
- Clearly define your research question or objective. Determine what specific aspect of the topic do you want to explore?
Decide on the Scope of Your Review:
- Determine the timeframe for your literature review. Are you focusing on recent developments, or do you want a historical overview?
- Consider the geographical scope. Is your review global, or are you focusing on a specific region?
- Define the inclusion and exclusion criteria. What types of sources will you include? Are there specific types of studies or publications you will exclude?
Select Databases for Searches:
- Identify relevant databases for your field. Examples include PubMed, IEEE Xplore, Scopus, Web of Science, and Google Scholar.
- Consider searching in library catalogs, institutional repositories, and specialized databases related to your topic.
Conduct Searches and Keep Track:
- Develop a systematic search strategy using keywords, Boolean operators (AND, OR, NOT), and other search techniques.
- Record and document your search strategy for transparency and replicability.
- Keep track of the articles, including publication details, abstracts, and links. Use citation management tools like EndNote, Zotero, or Mendeley to organize your references.
Review the Literature:
- Evaluate the relevance and quality of each source. Consider the methodology, sample size, and results of studies.
- Organize the literature by themes or key concepts. Identify patterns, trends, and gaps in the existing research.
- Summarize key findings and arguments from each source. Compare and contrast different perspectives.
- Identify areas where there is a consensus in the literature and where there are conflicting opinions.
- Provide critical analysis and synthesis of the literature. What are the strengths and weaknesses of existing research?
Organize and Write Your Literature Review:
- Literature review outline should be based on themes, chronological order, or methodological approaches.
- Write a clear and coherent narrative that synthesizes the information gathered.
- Use proper citations for each source and ensure consistency in your citation style (APA, MLA, Chicago, etc.).
- Conclude your literature review by summarizing key findings, identifying gaps, and suggesting areas for future research.
Whether you’re exploring a new research field or finding new angles to develop an existing topic, sifting through hundreds of papers can take more time than you have to spare. But what if you could find science-backed insights with verified citations in seconds? That’s the power of Paperpal’s new Research feature!
How to write a literature review faster with Paperpal?
Paperpal, an AI writing assistant, integrates powerful academic search capabilities within its writing platform. With the Research feature, you get 100% factual insights, with citations backed by 250M+ verified research articles, directly within your writing interface with the option to save relevant references in your Citation Library. By eliminating the need to switch tabs to find answers to all your research questions, Paperpal saves time and helps you stay focused on your writing.
Here’s how to use the Research feature:
- Ask a question: Get started with a new document on paperpal.com. Click on the “Research” feature and type your question in plain English. Paperpal will scour over 250 million research articles, including conference papers and preprints, to provide you with accurate insights and citations.
- Review and Save: Paperpal summarizes the information, while citing sources and listing relevant reads. You can quickly scan the results to identify relevant references and save these directly to your built-in citations library for later access.
- Cite with Confidence: Paperpal makes it easy to incorporate relevant citations and references into your writing, ensuring your arguments are well-supported by credible sources. This translates to a polished, well-researched literature review.
The literature review sample and detailed advice on writing and conducting a review will help you produce a well-structured report. But remember that a good literature review is an ongoing process, and it may be necessary to revisit and update it as your research progresses. By combining effortless research with an easy citation process, Paperpal Research streamlines the literature review process and empowers you to write faster and with more confidence. Try Paperpal Research now and see for yourself.
Frequently asked questions
A literature review is a critical and comprehensive analysis of existing literature (published and unpublished works) on a specific topic or research question and provides a synthesis of the current state of knowledge in a particular field. A well-conducted literature review is crucial for researchers to build upon existing knowledge, avoid duplication of efforts, and contribute to the advancement of their field. It also helps researchers situate their work within a broader context and facilitates the development of a sound theoretical and conceptual framework for their studies.
Literature review is a crucial component of research writing, providing a solid background for a research paper’s investigation. The aim is to keep professionals up to date by providing an understanding of ongoing developments within a specific field, including research methods, and experimental techniques used in that field, and present that knowledge in the form of a written report. Also, the depth and breadth of the literature review emphasizes the credibility of the scholar in his or her field.
Before writing a literature review, it’s essential to undertake several preparatory steps to ensure that your review is well-researched, organized, and focused. This includes choosing a topic of general interest to you and doing exploratory research on that topic, writing an annotated bibliography, and noting major points, especially those that relate to the position you have taken on the topic.
Literature reviews and academic research papers are essential components of scholarly work but serve different purposes within the academic realm. 3 A literature review aims to provide a foundation for understanding the current state of research on a particular topic, identify gaps or controversies, and lay the groundwork for future research. Therefore, it draws heavily from existing academic sources, including books, journal articles, and other scholarly publications. In contrast, an academic research paper aims to present new knowledge, contribute to the academic discourse, and advance the understanding of a specific research question. Therefore, it involves a mix of existing literature (in the introduction and literature review sections) and original data or findings obtained through research methods.
Literature reviews are essential components of academic and research papers, and various strategies can be employed to conduct them effectively. If you want to know how to write a literature review for a research paper, here are four common approaches that are often used by researchers. Chronological Review: This strategy involves organizing the literature based on the chronological order of publication. It helps to trace the development of a topic over time, showing how ideas, theories, and research have evolved. Thematic Review: Thematic reviews focus on identifying and analyzing themes or topics that cut across different studies. Instead of organizing the literature chronologically, it is grouped by key themes or concepts, allowing for a comprehensive exploration of various aspects of the topic. Methodological Review: This strategy involves organizing the literature based on the research methods employed in different studies. It helps to highlight the strengths and weaknesses of various methodologies and allows the reader to evaluate the reliability and validity of the research findings. Theoretical Review: A theoretical review examines the literature based on the theoretical frameworks used in different studies. This approach helps to identify the key theories that have been applied to the topic and assess their contributions to the understanding of the subject. It’s important to note that these strategies are not mutually exclusive, and a literature review may combine elements of more than one approach. The choice of strategy depends on the research question, the nature of the literature available, and the goals of the review. Additionally, other strategies, such as integrative reviews or systematic reviews, may be employed depending on the specific requirements of the research.
The literature review format can vary depending on the specific publication guidelines. However, there are some common elements and structures that are often followed. Here is a general guideline for the format of a literature review: Introduction: Provide an overview of the topic. Define the scope and purpose of the literature review. State the research question or objective. Body: Organize the literature by themes, concepts, or chronology. Critically analyze and evaluate each source. Discuss the strengths and weaknesses of the studies. Highlight any methodological limitations or biases. Identify patterns, connections, or contradictions in the existing research. Conclusion: Summarize the key points discussed in the literature review. Highlight the research gap. Address the research question or objective stated in the introduction. Highlight the contributions of the review and suggest directions for future research.
Both annotated bibliographies and literature reviews involve the examination of scholarly sources. While annotated bibliographies focus on individual sources with brief annotations, literature reviews provide a more in-depth, integrated, and comprehensive analysis of existing literature on a specific topic. The key differences are as follows:
References
- Denney, A. S., & Tewksbury, R. (2013). How to write a literature review. Journal of criminal justice education , 24 (2), 218-234.
- Pan, M. L. (2016). Preparing literature reviews: Qualitative and quantitative approaches . Taylor & Francis.
- Cantero, C. (2019). How to write a literature review. San José State University Writing Center .
Paperpal is an AI writing assistant that help academics write better, faster with real-time suggestions for in-depth language and grammar correction. Trained on millions of research manuscripts enhanced by professional academic editors, Paperpal delivers human precision at machine speed.
Try it for free or upgrade to Paperpal Prime , which unlocks unlimited access to premium features like academic translation, paraphrasing, contextual synonyms, consistency checks and more. It’s like always having a professional academic editor by your side! Go beyond limitations and experience the future of academic writing. Get Paperpal Prime now at just US$19 a month!
Related Reads:
- Empirical Research: A Comprehensive Guide for Academics
- How to Write a Scientific Paper in 10 Steps
- How Long Should a Chapter Be?
- How to Use Paperpal to Generate Emails & Cover Letters?
6 Tips for Post-Doc Researchers to Take Their Career to the Next Level
Self-plagiarism in research: what it is and how to avoid it, you may also like, mla works cited page: format, template & examples, how to ace grant writing for research funding..., powerful academic phrases to improve your essay writing , how to write a high-quality conference paper, how paperpal’s research feature helps you develop and..., how paperpal is enhancing academic productivity and accelerating..., how to write a successful book chapter for..., academic editing: how to self-edit academic text with..., 4 ways paperpal encourages responsible writing with ai, what are scholarly sources and where can you....
Loading metrics
Open Access
Ten Simple Rules for Writing a Literature Review
* E-mail: [email protected]
Affiliations Centre for Functional and Evolutionary Ecology (CEFE), CNRS, Montpellier, France, Centre for Biodiversity Synthesis and Analysis (CESAB), FRB, Aix-en-Provence, France
- Marco Pautasso

Published: July 18, 2013
- https://doi.org/10.1371/journal.pcbi.1003149
- Reader Comments
Citation: Pautasso M (2013) Ten Simple Rules for Writing a Literature Review. PLoS Comput Biol 9(7): e1003149. https://doi.org/10.1371/journal.pcbi.1003149
Editor: Philip E. Bourne, University of California San Diego, United States of America
Copyright: © 2013 Marco Pautasso. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This work was funded by the French Foundation for Research on Biodiversity (FRB) through its Centre for Synthesis and Analysis of Biodiversity data (CESAB), as part of the NETSEED research project. The funders had no role in the preparation of the manuscript.
Competing interests: The author has declared that no competing interests exist.
Literature reviews are in great demand in most scientific fields. Their need stems from the ever-increasing output of scientific publications [1] . For example, compared to 1991, in 2008 three, eight, and forty times more papers were indexed in Web of Science on malaria, obesity, and biodiversity, respectively [2] . Given such mountains of papers, scientists cannot be expected to examine in detail every single new paper relevant to their interests [3] . Thus, it is both advantageous and necessary to rely on regular summaries of the recent literature. Although recognition for scientists mainly comes from primary research, timely literature reviews can lead to new synthetic insights and are often widely read [4] . For such summaries to be useful, however, they need to be compiled in a professional way [5] .
When starting from scratch, reviewing the literature can require a titanic amount of work. That is why researchers who have spent their career working on a certain research issue are in a perfect position to review that literature. Some graduate schools are now offering courses in reviewing the literature, given that most research students start their project by producing an overview of what has already been done on their research issue [6] . However, it is likely that most scientists have not thought in detail about how to approach and carry out a literature review.
Reviewing the literature requires the ability to juggle multiple tasks, from finding and evaluating relevant material to synthesising information from various sources, from critical thinking to paraphrasing, evaluating, and citation skills [7] . In this contribution, I share ten simple rules I learned working on about 25 literature reviews as a PhD and postdoctoral student. Ideas and insights also come from discussions with coauthors and colleagues, as well as feedback from reviewers and editors.
Rule 1: Define a Topic and Audience
How to choose which topic to review? There are so many issues in contemporary science that you could spend a lifetime of attending conferences and reading the literature just pondering what to review. On the one hand, if you take several years to choose, several other people may have had the same idea in the meantime. On the other hand, only a well-considered topic is likely to lead to a brilliant literature review [8] . The topic must at least be:
- interesting to you (ideally, you should have come across a series of recent papers related to your line of work that call for a critical summary),
- an important aspect of the field (so that many readers will be interested in the review and there will be enough material to write it), and
- a well-defined issue (otherwise you could potentially include thousands of publications, which would make the review unhelpful).
Ideas for potential reviews may come from papers providing lists of key research questions to be answered [9] , but also from serendipitous moments during desultory reading and discussions. In addition to choosing your topic, you should also select a target audience. In many cases, the topic (e.g., web services in computational biology) will automatically define an audience (e.g., computational biologists), but that same topic may also be of interest to neighbouring fields (e.g., computer science, biology, etc.).
Rule 2: Search and Re-search the Literature
After having chosen your topic and audience, start by checking the literature and downloading relevant papers. Five pieces of advice here:
- keep track of the search items you use (so that your search can be replicated [10] ),
- keep a list of papers whose pdfs you cannot access immediately (so as to retrieve them later with alternative strategies),
- use a paper management system (e.g., Mendeley, Papers, Qiqqa, Sente),
- define early in the process some criteria for exclusion of irrelevant papers (these criteria can then be described in the review to help define its scope), and
- do not just look for research papers in the area you wish to review, but also seek previous reviews.
The chances are high that someone will already have published a literature review ( Figure 1 ), if not exactly on the issue you are planning to tackle, at least on a related topic. If there are already a few or several reviews of the literature on your issue, my advice is not to give up, but to carry on with your own literature review,
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
The bottom-right situation (many literature reviews but few research papers) is not just a theoretical situation; it applies, for example, to the study of the impacts of climate change on plant diseases, where there appear to be more literature reviews than research studies [33] .
https://doi.org/10.1371/journal.pcbi.1003149.g001
- discussing in your review the approaches, limitations, and conclusions of past reviews,
- trying to find a new angle that has not been covered adequately in the previous reviews, and
- incorporating new material that has inevitably accumulated since their appearance.
When searching the literature for pertinent papers and reviews, the usual rules apply:
- be thorough,
- use different keywords and database sources (e.g., DBLP, Google Scholar, ISI Proceedings, JSTOR Search, Medline, Scopus, Web of Science), and
- look at who has cited past relevant papers and book chapters.
Rule 3: Take Notes While Reading
If you read the papers first, and only afterwards start writing the review, you will need a very good memory to remember who wrote what, and what your impressions and associations were while reading each single paper. My advice is, while reading, to start writing down interesting pieces of information, insights about how to organize the review, and thoughts on what to write. This way, by the time you have read the literature you selected, you will already have a rough draft of the review.
Of course, this draft will still need much rewriting, restructuring, and rethinking to obtain a text with a coherent argument [11] , but you will have avoided the danger posed by staring at a blank document. Be careful when taking notes to use quotation marks if you are provisionally copying verbatim from the literature. It is advisable then to reformulate such quotes with your own words in the final draft. It is important to be careful in noting the references already at this stage, so as to avoid misattributions. Using referencing software from the very beginning of your endeavour will save you time.
Rule 4: Choose the Type of Review You Wish to Write
After having taken notes while reading the literature, you will have a rough idea of the amount of material available for the review. This is probably a good time to decide whether to go for a mini- or a full review. Some journals are now favouring the publication of rather short reviews focusing on the last few years, with a limit on the number of words and citations. A mini-review is not necessarily a minor review: it may well attract more attention from busy readers, although it will inevitably simplify some issues and leave out some relevant material due to space limitations. A full review will have the advantage of more freedom to cover in detail the complexities of a particular scientific development, but may then be left in the pile of the very important papers “to be read” by readers with little time to spare for major monographs.
There is probably a continuum between mini- and full reviews. The same point applies to the dichotomy of descriptive vs. integrative reviews. While descriptive reviews focus on the methodology, findings, and interpretation of each reviewed study, integrative reviews attempt to find common ideas and concepts from the reviewed material [12] . A similar distinction exists between narrative and systematic reviews: while narrative reviews are qualitative, systematic reviews attempt to test a hypothesis based on the published evidence, which is gathered using a predefined protocol to reduce bias [13] , [14] . When systematic reviews analyse quantitative results in a quantitative way, they become meta-analyses. The choice between different review types will have to be made on a case-by-case basis, depending not just on the nature of the material found and the preferences of the target journal(s), but also on the time available to write the review and the number of coauthors [15] .
Rule 5: Keep the Review Focused, but Make It of Broad Interest
Whether your plan is to write a mini- or a full review, it is good advice to keep it focused 16 , 17 . Including material just for the sake of it can easily lead to reviews that are trying to do too many things at once. The need to keep a review focused can be problematic for interdisciplinary reviews, where the aim is to bridge the gap between fields [18] . If you are writing a review on, for example, how epidemiological approaches are used in modelling the spread of ideas, you may be inclined to include material from both parent fields, epidemiology and the study of cultural diffusion. This may be necessary to some extent, but in this case a focused review would only deal in detail with those studies at the interface between epidemiology and the spread of ideas.
While focus is an important feature of a successful review, this requirement has to be balanced with the need to make the review relevant to a broad audience. This square may be circled by discussing the wider implications of the reviewed topic for other disciplines.
Rule 6: Be Critical and Consistent
Reviewing the literature is not stamp collecting. A good review does not just summarize the literature, but discusses it critically, identifies methodological problems, and points out research gaps [19] . After having read a review of the literature, a reader should have a rough idea of:
- the major achievements in the reviewed field,
- the main areas of debate, and
- the outstanding research questions.
It is challenging to achieve a successful review on all these fronts. A solution can be to involve a set of complementary coauthors: some people are excellent at mapping what has been achieved, some others are very good at identifying dark clouds on the horizon, and some have instead a knack at predicting where solutions are going to come from. If your journal club has exactly this sort of team, then you should definitely write a review of the literature! In addition to critical thinking, a literature review needs consistency, for example in the choice of passive vs. active voice and present vs. past tense.
Rule 7: Find a Logical Structure
Like a well-baked cake, a good review has a number of telling features: it is worth the reader's time, timely, systematic, well written, focused, and critical. It also needs a good structure. With reviews, the usual subdivision of research papers into introduction, methods, results, and discussion does not work or is rarely used. However, a general introduction of the context and, toward the end, a recapitulation of the main points covered and take-home messages make sense also in the case of reviews. For systematic reviews, there is a trend towards including information about how the literature was searched (database, keywords, time limits) [20] .
How can you organize the flow of the main body of the review so that the reader will be drawn into and guided through it? It is generally helpful to draw a conceptual scheme of the review, e.g., with mind-mapping techniques. Such diagrams can help recognize a logical way to order and link the various sections of a review [21] . This is the case not just at the writing stage, but also for readers if the diagram is included in the review as a figure. A careful selection of diagrams and figures relevant to the reviewed topic can be very helpful to structure the text too [22] .

Rule 8: Make Use of Feedback
Reviews of the literature are normally peer-reviewed in the same way as research papers, and rightly so [23] . As a rule, incorporating feedback from reviewers greatly helps improve a review draft. Having read the review with a fresh mind, reviewers may spot inaccuracies, inconsistencies, and ambiguities that had not been noticed by the writers due to rereading the typescript too many times. It is however advisable to reread the draft one more time before submission, as a last-minute correction of typos, leaps, and muddled sentences may enable the reviewers to focus on providing advice on the content rather than the form.
Feedback is vital to writing a good review, and should be sought from a variety of colleagues, so as to obtain a diversity of views on the draft. This may lead in some cases to conflicting views on the merits of the paper, and on how to improve it, but such a situation is better than the absence of feedback. A diversity of feedback perspectives on a literature review can help identify where the consensus view stands in the landscape of the current scientific understanding of an issue [24] .
Rule 9: Include Your Own Relevant Research, but Be Objective
In many cases, reviewers of the literature will have published studies relevant to the review they are writing. This could create a conflict of interest: how can reviewers report objectively on their own work [25] ? Some scientists may be overly enthusiastic about what they have published, and thus risk giving too much importance to their own findings in the review. However, bias could also occur in the other direction: some scientists may be unduly dismissive of their own achievements, so that they will tend to downplay their contribution (if any) to a field when reviewing it.
In general, a review of the literature should neither be a public relations brochure nor an exercise in competitive self-denial. If a reviewer is up to the job of producing a well-organized and methodical review, which flows well and provides a service to the readership, then it should be possible to be objective in reviewing one's own relevant findings. In reviews written by multiple authors, this may be achieved by assigning the review of the results of a coauthor to different coauthors.
Rule 10: Be Up-to-Date, but Do Not Forget Older Studies
Given the progressive acceleration in the publication of scientific papers, today's reviews of the literature need awareness not just of the overall direction and achievements of a field of inquiry, but also of the latest studies, so as not to become out-of-date before they have been published. Ideally, a literature review should not identify as a major research gap an issue that has just been addressed in a series of papers in press (the same applies, of course, to older, overlooked studies (“sleeping beauties” [26] )). This implies that literature reviewers would do well to keep an eye on electronic lists of papers in press, given that it can take months before these appear in scientific databases. Some reviews declare that they have scanned the literature up to a certain point in time, but given that peer review can be a rather lengthy process, a full search for newly appeared literature at the revision stage may be worthwhile. Assessing the contribution of papers that have just appeared is particularly challenging, because there is little perspective with which to gauge their significance and impact on further research and society.
Inevitably, new papers on the reviewed topic (including independently written literature reviews) will appear from all quarters after the review has been published, so that there may soon be the need for an updated review. But this is the nature of science [27] – [32] . I wish everybody good luck with writing a review of the literature.
Acknowledgments
Many thanks to M. Barbosa, K. Dehnen-Schmutz, T. Döring, D. Fontaneto, M. Garbelotto, O. Holdenrieder, M. Jeger, D. Lonsdale, A. MacLeod, P. Mills, M. Moslonka-Lefebvre, G. Stancanelli, P. Weisberg, and X. Xu for insights and discussions, and to P. Bourne, T. Matoni, and D. Smith for helpful comments on a previous draft.
- 1. Rapple C (2011) The role of the critical review article in alleviating information overload. Annual Reviews White Paper. Available: http://www.annualreviews.org/userimages/ContentEditor/1300384004941/Annual_Reviews_WhitePaper_Web_2011.pdf . Accessed May 2013.
- View Article
- Google Scholar
- 7. Budgen D, Brereton P (2006) Performing systematic literature reviews in software engineering. Proc 28th Int Conf Software Engineering, ACM New York, NY, USA, pp. 1051–1052. doi: https://doi.org/10.1145/1134285.1134500 .
- 16. Eco U (1977) Come si fa una tesi di laurea. Milan: Bompiani.
- 17. Hart C (1998) Doing a literature review: releasing the social science research imagination. London: SAGE.
- 21. Ridley D (2008) The literature review: a step-by-step guide for students. London: SAGE.
- Have your assignments done by seasoned writers. 24/7
- Contact us:
- +1 (213) 221-0069
- [email protected]

Can Literature Reviews Be Published: Can I Publish on my Own

Publishing Literature Reviews
A literature review is a piece of academic writing that demonstrates the understanding and knowledge that exists of a given topic. It is an overview of works that have been previously published.
Literature reviews can either be part of a published scholarly work such as a book or a full article on its own. The length of a literature review usually depends on the type of work being done and the topic of study.

The more the topic is studied the longer the literature review will be. Research papers and dissertations also usually have longer literature reviews.
People Also Read: How to Use Personal Experience in Research Paper or Essay
Can Literature Reviews be Published?

Literature reviews can be published. When reviewing the literature on a topic and you gather enough material that can be published then you can publish the literature review if you want.
The main purpose of publishing literature reviews is to inform others mainly experts in a certain field about something beyond the existing literature.
Also, reviews can be used to inform the experts and general audience about future research agendas that have been untapped in that particular topic.
For the literature reviews to achieve they must be clear with a focus directed at specific research problems.
Can I Publish a Review Paper on my Own?
You can publish your literature review in journals yourself. However, you will need to have a regular affiliation. This can be either related to academic research or not. It gives you the guarantee to publish the research even without needing supervisors.
Your review, however, must be of high quality for it to be published in journals. The notion that writing publishable literature reviews is easy is a myth because it is usually not simple to produce the quality needed. Also, data is not unique in literature reviews because it has already been written and published and everyone can access it if they want.
Therefore, you have to find a problem that has not been solved and try to solve it.
How to Publish a Literature Review?
There is no one-fit-all approach to how literature reviews should be published. However, there are typical steps that you can go through if you want to publish one:

Choosing a Journal
Deciding the journal where you will publish your literature review is an important decision that you have to make.
The journal you choose determines the reach and the impact of your literature review.
Also, it helps shape your literature review to build on research that has already been published in that journal. This will help the editors see how it adds value to the conversation in their journal.
Additionally, by choosing a journal you improve the chances of your literature review getting accepted because you will write it based on its specifications, preferences, and audience.
Writing the Literature Review
How you write the review will depend on the journal chosen, the subject area, and the type of paper. While writing the target audience and keywords recommended by the journal should be kept in mind.
Also, all the editorial policies recommended should be followed to the latter when writing the paper. If anything is not clear, it is recommendable that you contact the editorial team so that you can deliver a manuscript that is in line with their terms.
Submitting the Manuscript
The process of submitting a literature review varies in different journals. All you need to do is make sure that you have followed the rules that each stipulates in that regard.
Some of the common rules in most journals include following the writer’s instructions, providing an effective cover letter, ensuring data is prepared as required, and appropriately navigating the journal submission system.
To ensure that you have included everything you can use the read-to-submit checklist to avoid any possible errors.
Navigating the Peer Review Process

Peer review is the assessment of the literature review by experts in a particular field. The common things that they judge include the significance, validity, and originality of the paper that you have submitted.
Peer reviewers ensure that the literature review is in line with the recommendations of the journal and if it is ready or not for publication.
They can also return feedback and ask you to improve your paper before it is submitted for publication.
This is a collaborative process where dialogue is recommended between the reviewers and the author to ensure that there is mutual agreement and support to advance the work presented.
The Production
If your literature review is accepted for publication, it will head into production. Before it is produced you may be needed to check and correct the proofs of your article.
Also, you may need to decide whether you want to produce a video abstract to accompany it or not. After this, the review is published and you can access it in the journal and promote it to make sure that it has an impact.
People Also Read: Is it Plagiarism if you Cite? Incorrect or Paraphrase yourself
Why Literature Review is Important for a Research
Literature reviews are important for research because of the following reasons:
Literature reviews help establish the context for research. They put research into perspective and show how it relates to what has been done before. Gaps in the existing knowledge are also identified and new research questions are formulated
Literature reviews help identify problems. Through literature reviews, researchers identify issues and controversies that exist in particular topics. These problems can be addressed in their work and broaden the scope of research by contributing new ideas
Literature reviews help identify theoretical frameworks. This helps provide conceptual underpinnings of research. They also help clarify the rationale of the study and show how research is linked to existing theories
Literature reviews help identify the experts in particular topics. As a researcher, you will be able to identify the best scholar and researchers on your topic through the literature reviews. As a result, you will have expert knowledge of the content that is available and unavailable under your research topic
Determination of methodologies. Through literature reviews, you can identify successful methods that other researchers used in conducting research which you can also adapt to yours.
People Also Read: What is a Good SAT Essay Score in 2022: Combined out of 24
Challenges Hindering Publishing of Literature Reviews
Quality of literature reviews.
Quality is one of the challenges hindering the publishing of literature reviews.

Most people are not able to provide a paper that meets the requirements that many journals require to publish reviews.
Most journals prefer publishing articles and full research papers instead of literature reviews. When writing a research one is required to include the literature review in them.
Therefore, publishing a literature review on its own is not their preference and many view it as repetition and not ideal.
Failing the Peer-Reviewing Process
One can have a quality literature review and have it not get published because of peer reviewing experts.
The article can be very good but fail to meet the originality, validity, and significance that peer reviewers require to allow publications.
Change of Demands
What is required when publishing literature reviews changes from time to time? Therefore, if one is not keen on the current demands then the literature review may not be published.
People Also Read: Best Research Paper Font and Size: Best Styles for an Essay
List of 10 Journals that Publish Literature Reviews
Not all journals accept literature reviews or theoretical work but the following do:
- American Journal of Play
- Creativity Research Journal
- Journal of Applied Design
- International Journal of Online Pedagogy and Course Design
- American Journal of Distance Education
- Current Issues in Education
- Applied Measurement in Education
- Journal of Computing in Higher Education
- International Journal of web-based learning and teaching technology
- Online Journal of Distance Learning Administration

With over 10 years in academia and academic assistance, Alicia Smart is the epitome of excellence in the writing industry. She is our chief editor and in charge of the writing department at Grade Bees.
Related posts

Is Doing a Dissertation worth It
Is Doing a Dissertation worth It: Benefits of writing it

Optimal Dissertation Length
Dissertation Length: Optimal Length in Words and Pages

Can your Dissertation be a Question
Can a Dissertation be a Question?: How to Choose Good Titles
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Mark Access Health Policy
- v.11(1); 2023
- PMC10392303

Rapid literature review: definition and methodology
Beata smela.
a Assignity, Cracow, Poland
Mondher Toumi
b Public Health Department, Aix-Marseille University, Marseille, France
Karolina Świerk
Clement francois, małgorzata biernikiewicz.
c Studio Slowa, Wroclaw, Poland
Emilie Clay
d Clever-Access, Paris, France
Laurent Boyer
Introduction: A rapid literature review (RLR) is an alternative to systematic literature review (SLR) that can speed up the analysis of newly published data. The objective was to identify and summarize available information regarding different approaches to defining RLR and the methodology applied to the conduct of such reviews.
Methods: The Medline and EMBASE databases, as well as the grey literature, were searched using the set of keywords and their combination related to the targeted and rapid review, as well as design, approach, and methodology. Of the 3,898 records retrieved, 12 articles were included.
Results: Specific definition of RLRs has only been developed in 2021. In terms of methodology, the RLR should be completed within shorter timeframes using simplified procedures in comparison to SLRs, while maintaining a similar level of transparency and minimizing bias. Inherent components of the RLR process should be a clear research question, search protocol, simplified process of study selection, data extraction, and quality assurance.
Conclusions: There is a lack of consensus on the formal definition of the RLR and the best approaches to perform it. The evidence-based supporting methods are evolving, and more work is needed to define the most robust approaches.
Introduction
A systematic literature review (SLR) summarizes the results of all available studies on a specific topic and provides a high level of evidence. Authors of the SLR have to follow an advanced plan that covers defining a priori information regarding the research question, sources they are going to search, inclusion criteria applied to choose studies answering the research question, and information regarding how they are going to summarize findings [ 1 ].
The rigor and transparency of SLRs make them the most reliable form of literature review [ 2 ], providing a comprehensive, objective summary of the evidence for a given topic [ 3 , 4 ]. On the other hand, the SLR process is usually very time-consuming and requires a lot of human resources. Taking into account a high increase of newly published data and a growing need to analyze information in the fastest possible way, rapid literature reviews (RLRs) often replace standard SLRs.
There are several guidelines on the methodology of RLRs [ 5–11 ]; however, only recently, one publication from 2021 attempted to construct a unified definition [ 11 ]. Generally, by RLRs, researchers understand evidence synthesis during which some of the components of the systematic approach are being used to facilitate answering a focused research question; however, scope restrictions and a narrower search strategy help to make the project manageable in a shorter time and to get the key conclusions faster [ 4 ].
The objective of this research was to collect and summarize available information on different approaches to the definition and methodology of RLRs. An RLR has been run to capture publications providing data that fit the project objective.
To find publications reporting information on the methodology of RLRs, searches were run in the Medline and EMBASE databases in November 2022. The following keywords were searched for in titles and abstracts: ‘targeted adj2 review’ OR ‘focused adj2 review’ OR ‘rapid adj2 review’, and ‘methodology’ OR ‘design’ OR ‘scheme’ OR ‘approach’. The grey literature was identified using Google Scholar with keywords including ‘targeted review methodology’ OR ‘focused review methodology’ OR ‘rapid review methodology’. Only publications in English were included, and the date of publication was restricted to year 2016 onward in order to identify the most up-to-date literature. The reference lists of each included article were searched manually to obtain the potentially eligible articles. Titles and abstracts of the retrieved records were first screened to exclude articles that were evidently irrelevant. The full texts of potentially relevant papers were further reviewed to examine their eligibility.
A pre-defined Excel grid was developed to extract the following information related to the methodology of RLR from guidelines:
- Definition,
- Research question and searches,
- Studies selection,
- Data extraction and quality assessment,
- Additional information.
There was no restriction on the study types to be analyzed; any study reporting on the methodology of RLRs could be included: reviews, practice guidelines, commentaries, and expert opinions on RLR relevant to healthcare policymakers or practitioners. The data extraction and evidence summary were conducted by one analyst and further examined by a senior analyst to ensure that relevant information was not omitted. Disagreements were resolved by discussion and consensus.
Studies selection
A total of 3,898 records (3,864 articles from a database search and 34 grey literature from Google Scholar) were retrieved. After removing duplicates, titles and abstracts of 3,813 articles were uploaded and screened. The full texts of 43 articles were analyzed resulting in 12 articles selected for this review, including 7 guidelines [ 5–11 ] on the methodology of RLRs, together with 2 papers summarizing the results of the Delphi consensus on the topic [ 12 , 13 ], and 3 publications analyzing and assessing different approaches to RLRs [ 4 , 14 , 15 ].
Overall, seven guidelines were identified: from the World Health Organization (WHO) [ 5 ], National Collaborating Centre for Methods and Tools (NCCMT) [ 7 ], the UK government [ 8 ], the Oxford Centre for Evidence Based Medicine [ 9 ], the Cochrane group [ 6 , 11 ], and one multi-national review [ 10 ]. Among the papers that did not describe the guidelines, Gordon et al. [ 4 ] proposed 12 tips for conducting a rapid review in the right settings and discussed why these reviews may be more beneficial in some circumstances. The objective of work conducted by Tricco et al. [ 13 ] and Pandor et al. [ 12 ] was to collect and compare perceptions of rapid reviews from stakeholders, including researchers, policymakers, industry, journal editors, and healthcare providers, and to reach a consensus outlining the domains to consider when deciding on approaches for RLRs. Haby et al. [ 14 ] run a rapid review of systematic reviews and primary studies to find out the best way to conduct an RLR in health policy and practice. In Tricco et al. (2022) [ 15 ], JBI position statement for RLRs is presented.
From all the seven identified guidelines information regarding definitions the authors used for RLRs, approach to the PICOS criteria and search strategy development, studies selection, data extractions, quality assessment, and reporting were extracted.
Cochrane Rapid Reviews Methods Group developed methods guidance based on scoping review of the underlying evidence, primary methods studies conducted, as well as surveys sent to Cochrane representative and discussion among those with expertise [ 11 ]. They analyzed over 300 RLRs or RLR method papers and based on the methodology of those studies, constructed a broad definition RLR, one that meets a minimum set of requirements identified in the thematic analysis: ‘ A rapid review is a form of knowledge synthesis that accelerates the process of conducting a traditional systematic review through streamlining or omitting a variety of methods to produce evidence in a resource-efficient manner .’ This interpretation aligns with more than 50% of RLRs identified in this study. The authors additionally provided several other definitions, depending on specific situations or requirements (e.g., when RLR is produced on stakeholder’s request). It was additionally underlined that RLRs should be driven by the need of timely evidence for decision-making purposes [ 11 ].
Rapid reviews vary in their objective, format, and methods used for evidence synthesis. This is a quite new area, and still no agreement on optimal methods can be found [ 5 ]. All of the definitions are highlighting that RLRs are completed within shorter timeframes than SLRs, and also lack of time is one of the main reasons they are conducted. It has been suggested that most rapid reviews are conducted within 12 weeks; however, some of the resources suggest time between a few weeks to no more than 6 months [ 5 , 6 ]. Some of the definitions are highlighting that RLRs follow the SLR process, but certain phases of the process are simplified or omitted to retrieve information in a time-saving way [ 6 , 7 ]. Different mechanisms are used to enhance the timeliness of reviews. They can be used independently or concurrently: increasing the intensity of work by intensifying the efforts of multiple analysts by parallelization of tasks, using review shortcuts whereby one or more systematic review steps may be reduced, automatizing review steps by using new technologies [ 5 ]. The UK government report [ 8 ] referred to two different RLRs: in the form of quick scoping reviews (QSR) or rapid evidence assessments (REA). While being less resource and time-consuming compared to standard SLRs, QSRs and REAs are designed to be similarly transparent and to minimize bias. QSRs can be applied to rather open-ended questions, e.g., ‘what do we know about something’ but both, QSRs and REAs, provide an understanding of the volume and characteristics of evidence on a specific topic, allowing answering questions by maximizing the use of existing data, and providing a clear picture of the adequacy of existing evidence [ 8 ].
Research questions and searches
The guidelines suggest creating a clear research question and search protocol at the beginning of the project. Additionally, to not duplicate RLRs, the Cochrane Rapid Reviews Methods Group encourages all people working on RLRs to consider registering their search protocol with PROSPERO, the international prospective register of reviews; however, so far they are not formally registered in most cases [ 5 , 6 ]. They also recommend involving key stakeholders (review users) to set and refine the review question, criteria, and outcomes, as well as consulting them through the entire process [ 11 ].
Regarding research questions, it is better to structure them in a neutral way rather than focus on a specific direction for the outcome. By doing so, the researcher is in a better position to identify all the relevant evidence [ 7 ]. Authors can add a second, supportive research question when needed [ 8 ]. It is encouraged to limit the number of interventions, comparators and outcomes, to focus on the ones that are most important for decision-making [ 11 ]. Useful could be also reviewing additional materials, e.g., SLRs on the topic, as well as conducting a quick literature search to better understand the topic before starting with RLRs [ 7 ]. In SLRs researchers usually do not need to care a lot about time spent on creating PICOS, they need to make sure that the scope is broad enough, and they cannot use many restrictions. When working on RLRs, a reviewer may spend more or less time defining each of the components of the study question, and the main step is making sure that PICOS addresses the needs of those who requested the rapid review, and at the same time, it is feasible within the required time frame [ 7 ]. Search protocol should contain an outline of how the following review steps are to be carried out, including selected search keywords and a full strategy, a list of data sources, precise inclusion and exclusion criteria, a strategy for data extraction and critical appraisal, and a plan of how the information will be synthesized [ 8 ].
In terms of searches running, in most cases, an exhaustive process will not be feasible. Researchers should make sure that the search is effective and efficient to produce results in a timely manner. Cochrane Rapid Reviews Methods Group recommends involving an information specialist and conducting peer review of at least one search strategy [ 11 ]. According to the rapid review guidebook by McMaster University [ 7 ], it is important that RLRs, especially those that support policy and program decisions, are being fed by the results of a body of literature, rather than single studies, when possible. It would result in more generalizable findings applied at the level of a population and serve more realistic findings for program decisions [ 7 ]. It is important to document the search strategy, together with a record of the date and any date limits of the search, so that it can easily be run again, modified, or updated. Furthermore, the information on the individual databases included in platform services should always be reported, as this depends on organizations’ subscriptions and must be included for transparency and repeatability [ 7 , 8 ]. Good solution for RLRs is narrowing the scope or searching a limited number of databases and other sources [ 7 ]. Often, the authors use the PubMed/MEDLINE, Cochrane Library, and Embase databases. In most reviews, two or more databases are searched, and common limits are language (usually restricted to English), date, study design, and geographical area. Some RLRs include searching of grey literature; however, contact with authors is rather uncommon [ 5 , 8 ]. According to the flexible framework for restricted systematic review published by the University of Oxford, the search should be run in at least one major scientific database such as PubMed, and one other source, e.g., Google Scholar [ 9 ]. Grey literature and unpublished evidence may be particularly needed and important for intervention questions. It is related to the fact that studies that do not report the effects of interventions are less likely to be published [ 8 ]. If there is any type of evidence that will not be considered by the RLRs, e.g., reviews or theoretical and conceptual studies, it should also be stated in the protocol together with justification [ 8 ]. Additionally, authors of a practical guide published by WHO suggest using a staged search to identify existing SLRs at the beginning, and then focusing on studies with other designs [ 5 ]. If a low number of citations have been retrieved, it is acceptable to expand searches, remove some of the limits, and add additional databases and sources [ 7 ].
Searching for RLRs is an iterative process, and revising the approach is usually needed [ 7 ]. Changes should be confirmed with stakeholders and should be tracked and reflected in the final report [ 5 ].
The next step in the rapid review is the selection of studies consisting of two phases: screening of titles and abstracts, and analysis of full texts. Prior to screening initiation, it is recommended to conduct a pilot exercise using the same 30–50 abstracts and 5–10 full-texts for the entire screening team in order to calibrate and test the review form [ 11 ]. In contrast to SLRs, it can be done by one reviewer with or without verification by a second one. If verification is performed, usually the second reviewer checks only a subset of records and compares them. Cochrane Group, in contrast, recommends a stricter approach: at least 20% of references should be double-screened at titles and abstracts stage, and while the rest of the references may be screened by one reviewer, the excluded items need to be re-examined by second reviewer; similar approach is used in full-text screening [ 11 ]. This helps to ensure that bias was reduced and that the PICOS criteria are applied in a relevant way [ 5 , 8 , 9 , 11 ]. During the analysis of titles and abstracts, there is no need to report reasons for exclusion; however, they should be tracked for all excluded full texts [ 7 ].
Data extraction and quality assessment
According to the WHO guide, the most common method for data extraction in RLRs is extraction done by a single reviewer with or without partial verification. The authors point out that a reasonable approach is to use a second reviewer to check a random sample of at least 10% of the extractions for accuracy. Dual performance is more necessary for the extraction of quantitative results than for descriptive study information. In contrast, Cochrane group recommends that second reviewer should check the correctness and completeness of all data [ 11 ]. When possible, extractions should be limited to key characteristics and outcomes of the study. The same approach to data extraction is also suggested for a quality assessment process within rapid reviews [ 5 , 9 , 11 ]. Authors of the guidebook from McMaster University highlight that data extraction should be done ideally by two reviewers independently and consensus on the discrepancies should always be reached [ 7 ]. The final decision on the approach to this important step of review should depend on the available time and should also reflect the complexity of the research question [ 9 ].
For screening, analysis of full texts, extractions, and quality assessments, researchers can use information technologies to support them by making these review steps more efficient [ 5 ].
Before data reporting, a reviewer should prepare a document with key message headings, executive summary, background related to the topic and status of the current knowledge, project question, synthesis of findings, conclusions, and recommendations. According to the McMaster University guidebook, a report should be structured in a 1:2:20 format, that is, one page for key messages, two pages for an executive summary, and a full report of up to 20 pages [ 7 ]. All the limitations of the RLRs should be analyzed, and conclusions should be drawn with caution [ 5 ]. The quality of the accumulated evidence and the strength of recommendations can be assessed using, e.g., the GRADE system [ 5 ]. When working on references quoting, researchers should remember to use a primary source, not secondary references [ 7 ]. It would be worth considering the support of some software tools to automate reporting steps. Additionally, any standardization of the process and the usage of templates can support report development and enhance the transparency of the review [ 5 ].
Ideally, all the review steps should be completed during RLRs; however, often some steps may need skipping or will not be completed as thoroughly as should because of time constraints. It is always crucial to decide which steps may be skipped, and which are the key ones, depending on the project [ 7 ]. Guidelines suggest that it may be helpful to invite researchers with experience in the operations of SLRs to participate in the rapid review development [ 5 , 9 ]. As some of the steps will be completed by one reviewer only, it is important to provide them with relevant training at the beginning of the process, as well as during the review, to minimize the risk of mistakes [ 5 ].
Additional information
Depending on the policy goal and available resources and deadlines, methodology of the RLRs may be modified. Wilson et al. [ 10 ] provided extensive guidelines for performing RLR within days (e.g., to inform urgent internal policy discussions and/or management decisions), weeks (e.g., to inform public debates), or months (e.g., to inform policy development cycles that have a longer timeline, but that cannot wait for a traditional full systematic review). These approaches vary in terms of data synthesis, types of considered evidence and project management considerations.
In shortest timeframes, focused questions and subquestions should be formulated, typically to conduct a policy analysis; the report should consist of tables along with a brief narrative summary. Evidence from SLRs is often considered, as well as key informant interviews may be conducted to identify additional literature and insights about the topic, while primary studies and other types of evidence are not typically feasible due to time restrictions. The review would be best conducted with 1–2 reviewers sharing the work, enabling rapid iterations of the review. As for RLRs with longer timeline (weeks), these may use a mix of policy, systems and political analysis. Structure of the review would be similar to shorter RLRs – tabular with short narrative summary, as the timeline does not allow for comprehensive synthesis of data. Besides SLRs, primary studies and other evidence may be feasible in this timeframe, if obtained using the targeted searches in the most relevant databases. The review team should be larger, and standardized procedures for reviewing of the results and data extraction should be applied. In contrast to previous timeframe, merit review process may be feasible. For both timeframes, brief consultations with small transdisciplinary team should be conducted at the beginning and in the final stage of the review to discuss important matters.
For RLRs spanning several months, more comprehensive methodology may be adapted in terms of data synthesis and types of evidence. However, authors advise that review may be best conducted with a small review team in order to allow for more in-depth interpretation and iteration.
Studies analyzing methodology
There have been two interesting publications summarizing the results of Delphi consensus on the RLR methodology identified and included in this review [ 12 , 13 ].
Tricco et al. [ 13 ] first conducted an international survey and scoping review to collect information on the possible approaches to the running of rapid reviews, based on which, they employed a modified Delphi method that included inputs from 113 stakeholders to explore the most optimized approach. Among the six most frequent rapid review approaches (not all detailed here) being evaluated, the approach that combines inclusion of published literature only, a search of more than one database and limitations by date and language, study selection by one analyst, data extraction, and quality assessment by one analyst and one verifier, was perceived as the most feasible approach (72%, 81/113 responses) with the potentially lowest risk of bias (12%, 12/103). The approach ranked as the first one when considering timelines assumes updating of the search from a previously published review, no additional limits on search, studies selection and data extraction done by one reviewer, and no quality assessment. Finally, based on the publication, the most comprehensive RLRs can be made by moving on with the following rules: searching more than one database and grey literature and using date restriction, and assigning one reviewer working on screening, data extraction, and risk of bias assessment ( Table 1 ). Pandor et al. [ 12 ] introduced a decision tool for SelecTing Approaches for Rapid Reviews (STARR) that were produced through the Delphi consensus of international experts through an iterative and rigorous process. Participants were asked to assess the importance of predefined items in four domains related to the rapid review process: interaction with commissioners, understanding the evidence base, data extraction and synthesis methods, and reporting of rapid review methods. All items assigned to four domains achieved > 70% of consensus, and in that way, the first consensus-driven tool has been created that supports authors of RLRs in planning and deciding on approaches.
Six most frequent approaches to RLRs (adapted from Tricco et al. [ 13 ]).
Haby et al. [ 14 ] run searches of 11 databases and two websites and developed a comprehensive overview of the methodology of RLRs. With five SLRs and one RCT being finally included, they identified the following approaches used in RLRs to make them faster than full SLRs: limiting the number and scope of questions, searching fewer databases, limited searching of grey literature, restrictions on language and date (e.g., English only, most recent publications), updating the existing SLRs, eliminating or limiting hand searches of reference lists, noniterative search strategies, eliminating consultation with experts, limiting dual study selection, data extraction and quality assessment, minimal data synthesis with short concise conclusions or recommendations. All the SLRs included in this review were consistent in stating that no agreed definition of rapid reviews is available, and there is still no final agreement on the best methodological rules to be followed.
Gordon et al. [ 4 ] explained the advantages of performing a focused review and provided 12 tips for its conduction. They define focused reviews as ‘a form of knowledge synthesis in which the components of the systematic process are applied to facilitate the analysis of a focused research question’. The first tip presented by the authors is related to deciding if a focused review is a right solution for the considered project. RLRs will suit emerging topics, approaches, or assessments where early synthesis can support doctors, policymakers, etc., but also can direct future research. The second, third, and fourth tips highlight the importance of running preliminary searches and considering narrowing the results by using reasonable constraints taking into account the local context, problems, efficiency perspectives, and available time. Further tips include creating a team of experienced reviewers working on the RLRs, thinking about the target journal from the beginning of work on the rapid review, registering the search protocol on the PROSPERO registry, and the need for contacting authors of papers when data available in publications are missing or incongruent. The last three tips are related to the choice of evidence synthesis method, using the visual presentation of data, and considering and describing all the limitations of the focused review.
Finally, a new publication by Tricco et al. from 2022, describing JBI position statement [ 15 ] underlined that for the time being, there is no specific tool for critical appraisal of the RLR’s methodological quality. Instead, reviewers may use available tools to assess the risk of bias or quality of SLRs, like ROBIS, the JBI critical appraisal tools, or the assessment of multiple systematic reviews (AMSTAR).
Inconsistency in the definitions and methodologies of RLR
Although RLR was broadly perceived as an approach to quicken the conduct of conventional SLR, there is a lack of consensus on the formal definition of the RLR, so as to the best approaches to perform it. Only in 2021, a study proposing unified definition was published; however, it is important to note that the most accurate definition was only matching slightly over 50% of papers analysed by the authors, which underlines the lack of homogeneity in the field [ 11 ]. The evidence-based supporting methods are evolving, and more evidence is needed to define the most robust approaches [ 5 ].
Diverse terms are used to describe the RLR, including ‘rapid review’, focused systematic review’, ‘quick scoping reviews’, and ‘rapid evidence assessments’. Although the general principles of conducting RLR are to accelerate the whole process, complexity was seen in the methodologies used for RLRs, as reflected in this study. Also, inconsistencies related to the scope of the questions, search strategies, inclusion criteria, study screening, full-text review, quality assessment, and evidence presentation were implied. All these factors may hamper decision-making about optimal methodologies for conducting rapid reviews, and as a result, the efficiency of RLR might be decreased. Additionally, researchers may tend to report the methodology of their reviews without a sufficient level of detail, making it difficult to appraise the quality and robustness of their work.
Advantages and weaknesses of RLR
Although RLR used simplified approaches for evidence synthesis compared with SLR, the methodologies for RLR should be replicable, rigorous, and transparent to the greatest extent [ 16 ]. When time and resources are limited, RLR could be a practical and efficient tool to provide the summary of evidence that is critical for making rapid clinical or policy-related decisions [ 5 ]. Focusing on specific questions that are of controversy or special interest could be powerful in reaffirming whether the existing recommendation statements are still appropriate [ 17 ].
The weakness of RLR should also be borne in mind, and the trade-off of using RLR should be carefully considered regarding the thoroughness of the search, breadth of a research question, and depth of analysis [ 18 ]. If allowed, SLR is preferred over RLR considering that some relevant studies might be omitted with narrowed search strategies and simplified screening process [ 14 ]. Additionally, omitting the quality assessment of included studies could result in an increased risk of bias, making the comprehensiveness of RLR compromised [ 13 ]. Furthermore, in situations that require high accuracy, for example, where a small relative difference in an intervention has great impacts, for the purpose of drafting clinical guidelines, or making licensing decisions, a comprehensive SLR may remain the priority [ 19 ]. Therefore, clear communications with policymakers are recommended to reach an agreement on whether an RLR is justified and whether the methodologies of RLR are acceptable to address the unanswered questions [ 18 ].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Utilities for Complications Associated with Type 2 Diabetes: A Review of the Literature
- Open access
- Published: 21 May 2024
Cite this article
You have full access to this open access article

- William J. Valentine ORCID: orcid.org/0000-0003-4844-6813 1 ,
- Kirsi Norrbacka 2 &
- Kristina S. Boye 3
225 Accesses
1 Altmetric
Explore all metrics
Introduction
Utility values are used in health economic modeling analyses of type 2 diabetes (T2D) to quantify the effect of acute and long-term complications on quality of life (QoL). For accurate modeling projections, it is important that the utility values used are up to date, accurate and representative of the simulated model cohort.
A literature review was performed to identify utility values for health states representing acute and chronic T2D-related complications including cardiovascular complications, stroke, renal disease, ophthalmic complications, neuropathy, diabetic foot, amputation and hypoglycemia. Searches were performed using the PubMed, Embase and Cochrane Library databases and limited to articles published since 2010. Supplementary searches were performed to identify data published at congresses in 2019–2023.
A total of 54 articles were identified that reported utility values for T2D-related complications. The most frequently used elicitation method/instrument was the EQ-5D ( n = 42 studies) followed by the Short Form-6 dimensions ( n = 6), time tradeoff ( n = 5), the Health Utilities Index Mark 2 or Mark 3 ( n = 2), 15D ( n = 1), visual analog scale ( n = 1) and standard gamble ( n = 1). Stroke and amputation were consistently associated with the largest decrements in QoL. There is a lack of published data that distinguishes between severity of several complications including renal disease, retinopathy and neuropathy.
Conclusions
Diabetes-related complications can have a profound impact on QoL; therefore, it is important that these are captured accurately and appropriately in health economic models. Recently published utility values for diabetes-related complications that can be used to inform health economic models are summarized here.
Similar content being viewed by others

Estimating Quality of Life Decrements Due to Diabetes Complications in the United States: The Health Utility Index (HUI) Diabetes Complication Equation
Evaluation of health utility values for diabetic complications, treatment regimens, glycemic control and other subjective symptoms in diabetic patients using the EQ-5D-5L
Does the choice of eq-5d tariff matter a comparison of the swedish eq-5d-3l index score with uk, us, germany and denmark among type 2 diabetes patients.
Avoid common mistakes on your manuscript.
Health state utility values provide a quantitative indication of the extent to which a particular disease, complication or treatment-related side effect influences the quality of life (QoL) of an individual. Utility values quantify QoL on a scale of 0–1, where 1 represents perfect health and 0 represents death. Some utility elicitation methods, such as the EuroQoL 5 Dimensions (EQ-5D), allow negative values, which correspond to states considered to be worse than death [ 1 , 2 ]. Utility values are key inputs in health economic models that are used to project the long-term clinical and economic outcomes associated with new treatments and upon which payer and reimbursement decisions are often based. It is therefore important that these values accurately represent the QoL decrement associated with specific health states.
Individuals with type 2 diabetes (T2D) are at an elevated risk for a number of acute and long-term complications including cardiovascular disease, stroke, ophthalmic complications, renal disease, diabetic foot, neuropathy and hypoglycemic events, many of which can have a considerable impact on QoL. Poor glycemic control exacerbates the risk for long-term complications, and pharmacologic treatments aimed at improving glycemic control can in turn reduce the risk for long-term complications. However, the duration of clinical trials is not sufficient to capture the effect of new treatments on the incidence of long-term complications. Consequently, long-term health economic modeling analyses represent an important component of the reimbursement decision-making process for payers and policy makers.
The International Society for Pharmacoeconomics and Outcomes Research (ISPOR) good practice recommendations state that the use of up-to-date QoL data is an important aspect of long-term health economic analyses but also note that, in practice, many economic models use outdated utility values that may not adequately capture recent advances in treatment [ 3 ]. A previous review published by Beaudet et al. collated utility values for T2D-related complications, published over the period 1995–2012 [ 4 ]. The aim of the current review was to update and expand the previously published review by Beaudet et al. to provide a synopsis of utility values that could be utilized in future health economic models of T2D. The scope of the review was expanded to capture utility values elicited by the EQ-5D and other methods including (but not limited to) the Short Form 6 Dimensions (SF-6D), 15D, Health Utilities Index-2 or -3 (HUI-2/3), time-trade-off (TTO) and standard gamble (SG) and also to include utility/disutility values related to treatment-related attributes such as dosing frequency and timing flexibility, injection device-related attributes and unpleasant treatment-related side effects such as nausea or weight gain. The time frame of the review was also updated such that the review was limited to studies published since 2010, thereby capturing contemporary clinical practice relating to the management of people with T2D. Only findings related to T2D-related complications are presented here; summary findings of utilities for treatment-related attributes are presented separately in Part 2 of this review.
The literature review was performed using the PubMed, Embase and Cochrane Library databases. Search strategies were designed in alignment with recommendations outlined in the UK-based National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) Technical Support Document 9 [ 5 ]. Search strategies utilized high level Medical Subject Heading (MeSH) terms supplemented with free-text terms, and search syntax was adjusted as required for use across the different databases (full details of the search strategies used are provided in Supplementary Material, Tables 1–3). Supplementary hand searches were also performed to identify pertinent studies presented at major congresses between late 2019 and 2023( specifically the virtual meeting of the American Diabetes Association [ADA], the ISPOR Annual Congress and the 55th annual meeting of the European Association for the Study of Diabetes [EASD] in 2019). Relevant abstracts presented at the 2019 ISPOR meeting have been published; therefore, relevant publications should have been captured within the literature database searches. Studies published only in abstract form prior to 2019 were excluded on the basis that study results were likely to have been subsequently published in full-text form.
The time horizon of the searches was limited to articles published since 2010, and all searches were performed in March 2020. For inclusion in the review, studies were required to be published in full-text form (except for recent abstracts as outlined above) in English and present utility or disutility values for health states related to acute or long-term T2D-related complications or treatment-related attributes or process characteristics. Complications captured in the review included cardiovascular disease (angina, myocardial infarction [MI], and congestive heart failure [CHF]), stroke, renal disease (albuminuria/proteinuria, end-stage renal disease [ESRD] and renal transplant), ophthalmic complications (retinopathy, macular edema, cataract and severe vision loss/blindness), neuropathy, diabetic foot, amputation, peripheral vascular disease, overweight/obesity, hypoglycemia and fear of hypoglycemia (FoH). Studies that were conducted in mixed populations of patients with type 1 and type 2 diabetes were excluded if results were not presented according to diabetes type. Secondary studies (i.e., studies listing previously published utility values), and discrete choice experiments were also excluded.
Here, reporting of results is limited to acute and long-term complications; utility values for treatment-related attributes and process characteristics are reported in a separate review [ 6 ].
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Literature Searches
Literature searches across the three databases yielded a total of 8566 hits, which included 1383 duplicates, therefore resulting in a total of 7183 unique hits. First-round screening of titles and abstracts was performed by one investigator and identified a total of 241 hits for full-text review (Fig. 1 ). During second-round screening, a further 176 articles were excluded, leaving a total of 65 articles detailing utilities/disutilities associated with either T2D-related complications or treatment-related attributes for inclusion. A further three articles were identified via bibliographies of included articles. Searches of meeting abstract databases identified one relevant abstract for inclusion. The final review therefore included a total of 69 studies. Of these, a total of 39 presented findings exclusively related to acute or long-term diabetes-related complications, 15 presented findings exclusively related to the influence of treatment-related attributes on QoL, and 15 captured findings on both complications and treatment attributes. In total, 54 articles presented utility/disutility values for T2D-related complications (Table 1 ); these included a total of 18 studies conducted in Asia [ 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 ], 13 conducted in Europe [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 ], 13 in North America [ 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 ], 2 in Latin America [ 51 , 52 ], one in the Middle East [ 53 ] and 1 in Africa [ 54 ], and 6 were multinational (or setting not stated) [ 55 , 56 , 57 , 58 , 59 , 60 ]. Nearly all identified studies were conducted in individuals with T2D, although two presented data gathered from general population samples [ 34 , 56 ].
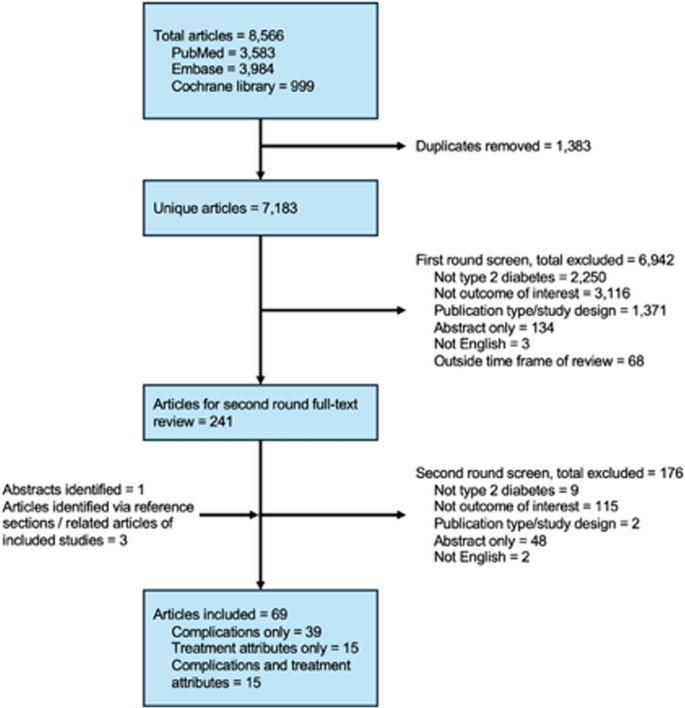
Summary of literature searches. T2D, type 2 diabetes. Publication/study type refers to articles that were reviews, editorials, letters, case reports, secondary sources of utility values or discrete choice experiments
T2D with No Complications
Eleven studies were identified that provided baseline utility values for individuals with T2D with no complications, all of which used the EQ-5D-3L or EQ-5D-5L (where studies did not state which version of the EQ-5D was used it was assumed that the EQ-5D-3L was used) (Table 2 ) [ 7 , 14 , 16 , 22 , 24 , 28 , 35 , 36 , 38 , 44 , 49 ] with one additionally using the SF-6D [ 38 ]. Five of the identified studies were conducted in Asia [ 7 , 14 , 16 , 22 , 24 ], three in North America [ 38 , 44 , 49 ] and three in European populations [ 28 , 35 , 36 ].
Using the EQ-5D, reported mean baseline values for individuals with T2D with no complications ranged from a low of 0.76 in a community-dwelling sample in Canada aged ≥ 40 years and with HbA1c ≥ 7% [ 44 ] to a high of 0.956 in a sample in China with mean (SD) age of 64.9 (9.1) years [ 14 ]. One study conducted in Vietnam reported a median baseline value of 1.0, which corresponds to perfect health [ 16 ], and is also slightly higher than that previously reported in a general population sample in Vietnam [ 61 ]. Differences in baseline characteristics such as age, BMI and duration of disease as well as the source of utility weights may have contributed towards the differences in baseline values between the different study populations.
For economic modeling analyses, the optimal baseline utility values should be as closely representative of the simulated patient population under investigation as possible. In particular, factors such as setting, baseline age and duration of diabetes should be taken into account and, if necessary, adjustments applied to the reference population. The description of the reference population of patients with no complications varied between studies; however, one US-based study provided a comprehensive description. Zhang et al. [ 49 ] reported a baseline utility value of 0.92 for no complications with this value referring specifically to individuals that were male, non-Hispanic white, with BMI < 30 kg/m 2 and with no risk factors for cardiovascular disease, with income > USD 40,000 per annum and not treated with insulin.
Cardiovascular and Cerebrovascular Complications
A total of ten studies that presented disutility values for MI [ 10 , 26 , 28 , 33 , 37 , 44 , 46 , 55 , 57 , 58 ], were identified (Table 3 ) (three studies presented utility values for MI; data not shown [ 11 , 36 , 46 ]). Two further studies presented disutilities for angina [ 10 , 46 ], and nine studies were identified that presented disutility values for CHF [ 10 , 27 , 28 , 37 , 46 , 49 , 55 , 57 , 58 ]. In studies that utilized the EQ-5D, the reported disutility associated with an MI ranged from − 0.073 in a sample of individuals with T2D attending outpatient clinics in South Korea [ 10 ] to − 0.0119 in a sample of patients in Sweden [ 28 ]. One study also reported a positive value of + 0.004 in a post hoc analysis of the multinational LEADER trial [ 58 ]. However, this value was not an event-related disutility per se but specifically referred to the change in utility value reported in patients who experienced an MI at any point during the 36-month follow-up period rather than a recent MI and may therefore potentially have captured improvements in QoL in patients recovering from an MI. Indeed, in a post hoc analysis of ACCORD trial data, Shao et al. noted that the timing of the event relative to the timing of measurement of QoL was an important determinant of QoL and although the effect of an MI waned over time a degree of long-term impairment persisted [ 46 ]. More specifically, using the HUI-3, Shao et al. reported that the mean (SE) disutility associated with MI was − 0.042 (0.016) in the year of the event but − 0.011 (0.006) in subsequent years after the event.
Two studies reported disutility values associated with angina (two studies also reported utility values for patients with angina; data not shown [ 36 , 46 ]). In a South Korean study that utilized the EQ-5D, Lee et al. reported a mean (SE) disutility of − 0.0266 (0.0114) [ 10 ], while for US-based patients, Shao et al., who utilized the HUI-3, reported a disutility of − 0.032 (0.006) in the year of event but − 0.010 (0.021) in subsequent years [ 46 ].
A total of nine studies reported disutility values for CHF [ 10 , 27 , 28 , 37 , 46 , 49 , 55 , 57 , 58 ], eight of which used the EQ-5D [ 10 , 27 , 28 , 37 , 49 , 55 , 57 , 58 ], with the remaining study using the HUI-3 [ 46 ] (four studies presented utility values for patients with CHF; data not shown [ 11 , 27 , 36 , 46 ]). In studies using the EQ-5D, the mean disutility for CHF ranged from − 0.0821 in Sweden [ 28 ] to − 0.037 in a study conducted in Greece [ 37 ]. Additionally, a US-based study that used the HUI-3 reported a disutility of − 0.089 in the year of diagnosis and − 0.041 in subsequent years [ 46 ].
The interpretation of the QoL impact of stroke was complicated by the heterogeneity in the definition of stroke used between different studies. In two studies, separate disutility values were presented for stroke and transient ischemic attack (TIA) [ 10 , 46 ] and one study further separated stroke into events with and without hemiplegia [ 46 ]. In comparison, other studies either did not state how stroke was defined or grouped TIA and stroke together. A total of 14 studies reported disutility values for stroke [ 10 , 24 , 26 , 28 , 33 , 35 , 37 , 44 , 46 , 49 , 54 , 55 , 57 , 58 ] (Table 4 ), and 8 studies presented utility values for stroke [ 11 , 18 , 24 , 29 , 36 , 38 , 46 , 54 ] (data not shown). Overall, stroke was typically associated with profound deficits in QoL relative to other complications. In studies that used the term stroke, disutility values ranged from − 0.59 to − 0.035 (Table 4 ). Notably, the two studies that reported the largest disutilities for stroke used the HUI-2 or HUI-3 [ 46 , 54 ], and another study reporting one of the smallest decrements of − 0.04 utilized the SF-6D [ 33 ]. When limited to studies that elicited utility values using the EQ-5D, the disutility associated with stroke ranged from − 0.135 [ 35 ] to − 0.035 [ 49 ]. Notably, the − 0.035 value reported in a US-based study by Zhang et al. did not include strokes resulting in hemiplegia; for events resulting in hemiplegia, the disutility was − 0.094 [ 49 ].
Renal Disease
Heterogeneity regarding definitions applied was also evident for studies presenting disutility values for renal complications. The literature review was initially designed to capture studies that reported utility/disutility values for the health states of microalbuminuria, macroalbuminuria, end-stage renal disease (ESRD), dialysis and renal transplant. However, few studies were identified that delineated renal disease according to this terminology, with many studies instead utilizing the overarching term of nephropathy. However, the definition of the nephropathy health state was in general poorly defined, and in instances where definitions were provided, these were inconsistent between studies. For example, Grandy et al. defined nephropathy as “self-reported diagnosis of chronic kidney disease, dialysis, ESRD, kidney transplant or proteinuria” [ 39 ]. In contrast, Luk et al. applied a more specific definition of nephropathy as “either proteinuria or chronic kidney disease (defined by the Renal Association as estimated glomerular filtration rate < 60 ml/min/1.73 m [ 2 ] on at least two occasions 90 days apart with or without markers of kidney damage, which include albuminuria, hematuria, electrolyte disorders due to tubular disorders, renal histologic abnormalities, structural abnormalities evident on imaging or a history of renal transplantation” [ 62 ]) [ 12 ]. The broad definition applied in analyses such as that presented by Grandy et al. means that the term nephropathy captures both patients with microalbuminuria and ESRD, despite these states representing very different severities of renal disease.
A total of seven studies presented disutility values for the broad health state of nephropathy [ 10 , 12 , 16 , 37 , 39 , 50 , 58 ] (all of which used the EQ-5D), and a further four studies presented values for ESRD [ 44 , 46 , 49 , 57 ], three of which used the EQ-5D [ 44 , 49 , 57 ] and one used the HUI-3 (Table 5 ) [ 46 ]. The mean disutility for nephropathy ranged from − 0.08 in a sample of patients with good glycemic control (median HbA1c 6.8%) and median duration of diabetes of 7 years based in Vietnam [ 16 ] to –0.0044 in patients with a mean age of 57 years based in South Korea [ 10 ]. For individuals with ESRD, the mean disutility value in EQ-5D studies ranged from –0.049 [ 57 ] to − 0.1018 [ 44 ], while the one study that utilized the HUI-3 reported a disutility of − 0.024 [ 46 ].
Ophthalmic Complications
The review was designed to capture the QoL impact of ophthalmic complications including retinopathy (background and proliferative), macular edema, cataract and severe vision loss/blindness. However, no studies were identified that examined the effect of macular edema on QoL, and only one study that presented a disutility value for cataract was identified (Table 6 ) [ 10 ]. Additionally, none of the retinopathy studies identified distinguished in severity between background and proliferative retinopathy; however, one Chinese study did distinguish between unilateral and bilateral retinopathy [ 15 ]. Here, using the EQ-5D-5L, the mean disutilities for unilateral and bilateral retinopathy were –0.013 and − 0.019, respectively [ 15 ]. A total of nine further studies reported disutility values for retinopathy elicited using the EQ-5D [ 10 , 14 , 16 , 24 , 26 , 28 , 37 , 39 , 58 ]. These included one US-based longitudinal study that captured the decline in QoL in individuals with retinopathy over a 5-year period. Using the EQ-5D, Grandy et al. reported a decline in EQ-5D of − 0.058 over 5 years, assuming a linear rate of decline over time corresponding to an annual decline of − 0.0116 per year [ 39 ]. Excluding the longitudinal study by Grandy et al., the mean disutility associated with retinopathy, elicited using the EQ-5D, ranged from − 0.0578 in a sample of people with T2D aged > 65 years (mean [SD] age 70.3 [9.3] years) based in Germany [ 26 ] to − 0.001 in a multinational study by Nauck et al. [ 58 ]. A further study utilized the 15D questionnaire and reported a mean disutility for retinopathy of − 0.036 [ 29 ]. The same authors also noted that in their analysis both the EQ-5D and SF-6D were relatively insensitive to retinopathy.
Three further studies presented disutility values for health states defined as “impaired vision,” “severe vision loss” and “blindness” (Table 6 ), findings that collectively suggested a deterioration in QoL with increasing severity of vision loss. In a Norwegian study, visual impairment (severity not defined) was associated with a mean (EQ-5D) disutility of − 0.012 [ 35 ]. In a US-based study, severe vision loss (defined as visual acuity of < 20/200 on a Snellen chart) was associated with a mean (HUI-3) disutility of − 0.057 [ 46 ], and in a multinational study blindness was associated with a mean (EQ-5D) disutility of − 0.083 [ 57 ].
Neuropathy, Foot Ulcer and Amputation
A total of 11 studies reported disutility values for people experiencing neuropathy (Table 7 ) [ 12 , 14 , 19 , 26 , 29 , 35 , 37 , 39 , 46 , 49 , 51 ]; however, only 1 disutility study distinguished between painful and non-painful neuropathy [ 49 ] (8 studies also reported utility values for the health state of neuropathy, 1 of which distinguished between painful and non-painful neuropathy; data not shown [ 9 , 12 , 13 , 14 , 18 , 24 , 29 , 46 ]). For US-based patients, using the EQ-5D, the mean disutility for patients with non-painful neuropathy was –0.039, but for painful neuropathy the mean decrement was as expected, notably more pronounced at –0.105 [ 49 ]. Another study identified in the review compared utility (rather than disutility values) and patient characteristics for those with painful versus non-painful neuropathy [ 9 ]. Patients with painful neuropathy were slightly older and had longer duration of disease than those with non-painful neuropathy, and the mean (SD) EQ-5D score was 0.8 (0.1) for those with non-painful neuropathy compared with 0.6 (0.3) for those with painful neuropathy [ 9 ]. Allied to this, a further US-based study captured the decline in QoL over time in patients with neuropathy, which may at least partially address the issue of progression/increased severity over time. Using the EQ-5D, Grandy et al. reported a decline in QoL of − 0.061 over a period of 5 years, which corresponds to an annual decline of − 0.0122 per year assuming a linear rate of decline [ 39 ].
The magnitude of the disutility associated with neuropathy was also influenced by the elicitation method used. For patients in Greece, Kontodimopoulos et al. report a disutility for neuropathy of − 0.117 using the SF-6D, but using the EQ-5D the corresponding value was − 0.247, suggesting different sensitivities to the impact of neuropathy between the two methods used [ 29 ].
Diabetic Foot and Amputation
The definitions and terminology used for diabetic foot/foot ulcer health states were heterogenous between studies and in some instances poorly defined, thereby making it challenging to differentiate between the impact of events such as the occurrence of superficial uninfected ulcers that healed without complications and infected/gangrenous ulcer. Additionally, two studies included amputation within the overarching terms of diabetic foot [ 50 , 58 ]. Reported mean disutility values for diabetic foot/foot ulcer ranged from − 0.016 in a Norwegian study by Solli et al. [ 35 ] to − 0.206 in a Greek study by Kontodimopoulos et al. [ 29 ], with both of these values elicited using the EQ-5D (Table 7 ). The broad range of disutility values may be partly attributable to the heterogeneity and differences in severity in terms of the terminology and categorizations used between different studies. Furthermore, as with neuropathy, reported disutility values were influenced by the elicitation method used. Using both the 15D and SF-6D Kontodimopoulos et al. reported a mean disutility of − 0.093, but when the EQ-5D-3L was used the mean utility decrement was notably greater at − 0.206 [ 29 ]. Additionally, for amputation specifically, reported disutility values (elicited using the EQ-5D) ranged from − 0.122 [ 57 ] to − 0.0631 [ 44 ] (Table 7 ).
Hypoglycemia
Literature searches identified a total of 24 studies that presented either utility or disutility values for patients experiencing hypoglycemia of various severities [ 11 , 12 , 17 , 20 , 21 , 24 , 25 , 30 , 31 , 32 , 34 , 37 , 40 , 41 , 42 , 43 , 45 , 47 , 48 , 55 , 56 , 58 , 59 , 60 ] as well as three studies that examined the effect of FoH on QoL [ 34 , 35 , 47 ]. As with other complications, the terminology and categorization of hypoglycemia varied widely between studies. Some studies categorized hypoglycemic events as either non-severe or severe, with non-severe events typically defined as events that could be resolved by the individual and severe events as those requiring third-party assistance. Other investigators used the categorization of mild, moderate, severe and very severe, with mild events typically defined as events causing no interruption of activities, moderate events as those resulting in some interruption of activities, severe events as requiring the assistance of others and very severe events as events that required medical assistance [ 41 , 60 ]. Some studies employed broader terminology, presenting utility/disutility values for any symptomatic event, and several focused on historical events describing utility values according to hypoglycemia experienced in the previous 1-, 3- or 6-month period. Additionally, a total of four studies distinguished between daytime and nocturnal hypoglycemic events [ 11 , 20 , 40 , 56 ]. As with long-term complications, the EQ-5D was the most frequently used utility elicitation method employed to determine the influence of hypoglycemic events on QoL; however, a total of five studies that specifically focused on hypoglycemic events used TTO methodology to determine disutility values [ 20 , 34 , 40 , 56 , 59 ].
Three studies reported disutility values for hypoglycemic events categorized as non-severe, all three of which included values elicited from both people with T2D and general population samples using TTO methodology [ 20 , 40 , 56 ]. For people with T2D, the disutility for a daytime non-severe event ranged from − 0.0028 in a Canadian study [ 40 ] to − 0.0283 in Malaysia (Table 8 ) [ 20 ]. Similarly, for general population samples, the corresponding range was from − 0.004 in a multinational study [ 56 ] to − 0.0354 in Malaysia [ 20 ]. For people with T2D, two of the three studies reported that a nocturnal non-severe event was associated with a greater decrement compared with an event occurring during the daytime. For example, in Canada, the decrement associated with a daytime non-severe event was − 0.0028, whereas a nocturnal non-severe event was associated with a utility decrement of − 0.0076 [ 40 ].
A further two studies (both of which used the EQ-5D) categorized events as either mild or moderate and reported disutility values of − 0.009 [ 41 ] and − 0.018 [ 31 ] for mild events and − 0.055 [ 41 ] and − 0.043 [ 31 ] for moderate events, respectively. A total of 13 studies examined the effect of severe hypoglycemic events on QoL [ 11 , 20 , 24 , 31 , 34 , 37 , 40 , 41 , 43 , 46 , 56 , 58 , 60 ], including 8 that reported disutility values for severe events [ 20 , 24 , 31 , 37 , 40 , 41 , 56 , 58 ]. In people with T2D, reported mean disutility values for a severe hypoglycemic event ranged from − 0.008 in China [ 24 ] (determined using the EQ-5D-3L) to − 0.1938 (determined using TTO methods) in a cross-sectional study conducted in Malaysia [ 20 ]. Three studies distinguished between severe events occurring during the day and nocturnal severe events [ 20 , 40 , 56 ], and in people with T2D nocturnal events were consistently associated with a greater disutility than daytime events.
Additionally, in a US-based study presenting disutility values elicited using the EQ-5D, Marrett et al. [ 41 ] distinguished between severe events requiring third-party assistance and very severe events requiring medical assistance; the mean disutilities associated with severe and very severe events were − 0.131 and − 0.208, respectively.
Three further studies quantified the influence of FoH on QoL; of these, two were conducted in people with T2D in Norway [ 35 ] and the USA [ 47 ], respectively, and the third was conducted in a UK-based general population sample [ 34 ]. Both studies conducted in people with T2D used the EQ-5D, and in the US, Shi et al. [ 47 ] reported that any FoH was associated with a disutility of − 0.003. In Norway, Solli et al. [ 35 ] reported that a large FoH was associated with a disutility of − 0.078 compared with a small FoH.
It is well established that diabetes-related complications can have a profound effect on QoL. Here, a synopsis of recently published disutility values that reflect current management/treatment practices is provided, which can be used in turn to inform future health economic models and analyses of novel interventions. New treatments for T2D generally provide incremental benefits in terms of glycemic control and/or adverse event rates, in particular hypoglycemia, relative to the standard of care. Glycemic control is a key determinant of the risk for long-term complications [ 63 ]. As such, for economic models to project valid outcomes such as quality-adjusted life expectancy for diabetes interventions, it is important that the most appropriate disutility values are used to best reflect the impact of individual complications on QoL.
When selecting utility/disutility values to inform economic models, there are a number of issues that warrant consideration by model developers. These include the elicitation method used and whether utility/disutility values selected are from a population that aligns with the simulated patient cohort under investigation. The choice of elicitation method is in some instances influenced by national guidelines. For example, for economic analyses performed in Sweden or Denmark, direct methods (e.g., TTO or SG) are preferred, whereas in other jurisdictions (e.g., England, Scotland) guidelines advocate the use of utility values elicited using the EQ-5D [ 64 ]. Indeed, in national guidelines where a specific generic multi-attribute utility instrument is recommended, this is most commonly the EQ-5D, although several countries do not stipulate which instrument should be used, instead providing examples of acceptable methods [ 65 ]. In terms of population, it may also be desirable to source disutility values that are generalizable to the simulated model cohort in terms of location, baseline demographics and disease characteristics. For example, values derived from individuals with newly diagnosed T2D based in Europe may not be appropriate when modeling new interventions in a population with long-standing disease in a country in Asia such as China or Singapore. The statistical approach used and the parameters adjusted for should also be considered when selecting utility values. In some instances, disutilities were calculated simply by subtracting the mean utility value of patients with a particular complication from the mean value for those without, while in other analyses disutilities were calculated using multivariate regression models and adjusted for baseline demographics and disease characteristics. The factors controlled for should be considered when selecting sources of disutility values. For example, women were consistently shown to have lower QoL relative to men [ 14 , 24 , 28 , 33 , 46 , 49 , 53 ], so it is important to consider whether values have been adjusted according to gender and other baseline demographics and disease characteristics.
Model developers may also need to consider the best way in which to address data gaps in terms of the need to utilize disutility values from multiple different sources. Research across different therapy areas has consistently shown that, for particular health states, utility values elicited using direct methods are consistently higher than values for the same state elicited using indirect methods [ 66 , 67 ], which may potentially lead to the introduction of bias in an analysis. Allied to this, for indirect methods consistency in terms of the source of preference weights used should also be considered owing to potential differences in cultural norms and healthcare provision between different settings [ 46 ]. Two studies identified in the current review showed that the source of preference weights can have a considerable influence on baseline utility values [ 28 , 57 ]. In particular, in a Swedish analysis, Kiadaliri et al. [ 28 ] noted that when using a Swedish tariff, the mean utility value for individuals with no complications was 0.89, but when using a UK tariff the corresponding value was considerably lower at 0.79. Similarly, when using the UK tariff, the disutilities associated with MI, heart failure and stroke were approximately two-fold greater than with the Swedish tariff [ 28 ].
A few studies identified in the review documented the phenomenon of diminishing marginal disutility specifically relating to cardiovascular events. Diminishing marginal disutility has previously been demonstrated with hypoglycemic events [ 68 ] and refers to instances where subsequent events are judged to have a lesser effect on QoL than first events. Briggs et al. observed diminishing marginal disutility with a composite endpoint of major cardiovascular event [ 55 ]. Here, a first major cardiovascular event was associated with a disutility of − 0.05, but the decrement associated with a subsequent event was less at − 0.038. Similar findings were reported by Kiadaliri et al. in terms of the effect of first and subsequent MIs [ 28 ]. Shao et al. also demonstrated that while the QoL impact of events such as MIs wanes over time, an MI within the previous year was associated with a disutility of − 0.042 but the disutility associated with a history of MI was − 0.011 [ 46 ]. However, with any retrospective analysis of events, it is possible that the interval between the event and utility elicitation may influence the QoL finding owing to response shift or recall bias, the potential impact of which should be considered when interpreting results [ 69 ].
Several diabetes-related complications such as retinopathy or renal disease may be classified according to severity. However, few studies identified in the review captured differing levels of severity for complications, although the notable exception to this was hypoglycemia, where events were frequently classified as non-severe and severe, or mild, moderate, severe and very severe. For retinopathy, no studies were identified that distinguished between background retinopathy and proliferative retinopathy. The decrement associated with vision loss/blindness, which may occur as a result of proliferative retinopathy, was however captured by several investigators. Additionally, only two studies distinguished between painful and non-painful neuropathy [ 9 , 49 ]. However, a longitudinal US-based study by Grandy et al. [ 39 ] captured the decline in EQ-5D index score over a time period of 5 years for patients with various complications including retinopathy and neuropathy. Annualizing this decrement may therefore represent an alternative method of modeling progression/increasing severity of complications over time.
For most complications, the EQ-5D was the most frequently used elicitation method, with more recently published studies tending to use the EQ-5D-5L rather than EQ-5D-3L. Two studies also directly compared values derived from the EQ-5D-3L and EQ-5D-5L in people with T2D, with both concluding that the 5L version showed greater sensitivity and discriminative ability as well as a reduced ceiling effect relative to the 3L [ 13 , 22 ]. Although the EQ-5D was frequently used for long-term complications, three studies that focused exclusively on hypoglycemic events used TTO methodology [ 20 , 40 , 56 ], with the rationale for this being the greater sensitivity of TTO methods relative to generic instruments such as the EQ-5D. This increased sensitivity may be particularly beneficial for complications such as hypoglycemia where events are distinguished by severity as well as the time of day/night at which events occur.
As with all literature reviews, there are limitations associated with the present review. No formal assessment of study quality or critical evaluation was performed; as a result, no ranking of the data is identified. This was a deliberate approach as different modeling and country-specific approaches may lead to differential priorities for the selection of utilities in a health economic analysis. Little was reported on the management of diabetes-related complications in the studies identified in this review. Different approaches to managing these conditions could lead to different utility scores being reported by patients across studies, likely contributing to between-country variation in outcomes reported. Demographic and socio-economic factors regarding utility scores were not reported in this review, which may also influence the selection of the most appropriate utility scores for health economic analysis. The application of inclusion and exclusion criteria was limited in the present review in an effort to align the work with that of Beaudet et al. and to minimize the risk of introducing bias through study selection [ 4 ]. However, this approach also has the potential to capture studies with smaller populations and/or limited quality and imply an equal weight to their results.
The ISPOR good practice recommendations note that economic modeling analyses should utilize up-to-date utility values as these reflect contemporary clinical practices and recent advances in treatment that may influence patients’ QoL [ 3 ]. The findings presented here provide a synopsis of recently published disutility values for major complications in T2D diabetes than can be used to inform future economic modeling analyses.
EuroQol 5 dimension questionnaire. Available at: https://euroqol.org/eq-5d-instruments/ . Last accessed September 14, 2020.
Brooks R, Boye KS, Slaap B. EQ-5D: a plea for accurate nomenclature. J Patient Rep Outcomes. 2020;4(1):52.
Article PubMed PubMed Central Google Scholar
Brazier J, Ara R, Azzabi I, Busschbach J, Chevrou-Séverac H, Crawford B, Cruz L, Karnon J, Lloyd A, Paisley S, Pickard AS. Identification, review, and use of health state utilities in cost-effectiveness models: an ISPOR good practices for outcomes research task force report. Value Health. 2019;22(3):267–75.
Article PubMed Google Scholar
Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health. 2014;17(4):462–70.
Papaioannou D, Brazier J, Paisley S. NICE DSU Technical Support Document 9: the identification, review and synthesis of health state utility values from the literature. October 201. http://www.nicedsu.org.uk .
Valentine W, Norrbacka K, Boye KS. Evaluating the impact of therapy on quality of life in type 2 diabetes: a literature review of utilities associated with treatment-related attributes. Patient Relat Outcome Meas. 2022;12(13):97–111.
Article Google Scholar
Butt M, Ali AM, Bakry MM. Health-related quality of life in poorly controlled type 2 diabetes patients- association of patients’ characteristics with EQ-5D domains, mean EQ-5D scores, and visaul analog scale score. Asian J Pharm Clin Res. 2018;11(1):93–8.
Ji L, Zou D, Liu L, Qian L, Kadziola Z, Babineaux S, Zhang HN, Wood R. Increasing body mass index identifies Chinese patients with type 2 diabetes mellitus at risk of poor outcomes. J Diabetes Complicat. 2015;29(4):488–96.
Kim SS, Won JC, Kwon HS, Kim CH, Lee JH, Park TS, Ko KS, Cha BY. Prevalence and clinical implications of painful diabetic peripheral neuropathy in type 2 diabetes: results from a nationwide hospital-based study of diabetic neuropathy in Korea. Diabetes Res Clin Pract. 2014;103(3):522–9.
Lee WJ, Song KH, Noh JH, Choi YJ, Jo MW. Health-related quality of life using the EuroQol 5D questionnaire in Korean patients with type 2 diabetes. J Korean Med Sci. 2012;27(3):255–60.
Lin YJ, Wang CY, Cheng SW, Ko Y. Patient preferences for diabetes-related complications in Taiwan. Curr Med Res Opin. 2019;35(1):7–13.
Article CAS PubMed Google Scholar
Luk AOY, Zhang Y, Ko GTC, Brown N, Ozaki R, et al. Health-related quality of life in chinese patients with type 2 diabetes: an analysis of the Joint Asia Diabetes Evaluation (JADE) program. J Diabetes Metab. 2014;5:333.
Google Scholar
Pan CW, Sun HP, Wang X, Ma Q, Xu Y, Luo N, Wang P. The EQ-5D-5L index score is more discriminative than the EQ-5D-3L index score in diabetes patients. Qual Life Res. 2015;24(7):1767–74.
Pan CW, Sun HP, Zhou HJ, Ma Q, Xu Y, Luo N, Wang P. Valuing health-related quality of life in type 2 diabetes patients in China. Med Decis Making. 2016;36(2):234–41.
Pan CW, Wang S, Wang P, Xu CL, Song E. Diabetic retinopathy and health-related quality of life among Chinese with known type 2 diabetes mellitus. Qual Life Res. 2018;27(8):2087–93.
Pham TB, Nguyen TT, Truong HT, Trinh CH, Du HNT, Ngo TT, Nguyen LH. Effects of diabetic complications on health-related quality of life impairment in Vietnamese patients with type 2 diabetes. J Diabetes Res. 2020;23(2020):4360804.
Pratipanawatr T, Satirapoj B, Ongphiphadhanakul B, Suwanwalaikorn S, Nitiyanant W. Impact of hypoglycemia on health-related quality of life among type 2 diabetes: a cross-sectional study in Thailand. J Diabetes Res. 2019;23(2019):5903820.
Quah JH, Luo N, Ng WY, How CH, Tay EG. Health-related quality of life is associated with diabetic complications, but not with short-term diabetic control in primary care. Ann Acad Med Singap. 2011;40(6):276–86.
Riandini T, Wee HL, Khoo EYH, Tai BC, Wang W, Koh GCH, Tai ES, Tavintharan S, Chandran K, Hwang SW, Venkataraman K. Functional status mediates the association between peripheral neuropathy and health-related quality of life in individuals with diabetes. Acta Diabetol. 2018;55(2):155–64.
Shafie AA, Ng CH, Thanimalai S, Haron N, Manocha AB. Estimating the utility value of hypoglycaemia according to severity and frequency using the visual analogue scale (VAS) and time trade-off (TTO) survey. J Diabetes Metab Disord. 2018;17(2):269–75.
Terauchi Y, Ozaki A, Zhao X, Teoh C, Jaffe D, Tajima Y, Shuto Y. Humanistic and economic burden of cardiovascular disease related comorbidities and hypoglycaemia among patients with type 2 diabetes in Japan. Diabetes Res Clin Pract. 2019;149:115–25.
Wang P, Luo N, Tai ES, Thumboo J. The EQ-5D-5L is more discriminative than the EQ-5D-3L in patients with diabetes in Singapore. Value Health Reg Issues. 2016;9:57–62.
Yan BP, Zhang Y, Kong AP, Luk AO, Ozaki R, Yeung R, Tong PC, Chan WB, Tsang CC, Lau KP, Cheung Y, Wolthers T, Lyubomirsky G, So WY, Ma RC, Chow FC, Chan JC, Hong Kong JADE Study Group. Borderline ankle-brachial index is associated with increased prevalence of micro- and macrovascular complications in type 2 diabetes: a cross-sectional analysis of 12,772 patients from the Joint Asia Diabetes Evaluation Program. Diab Vasc Dis Res. 2015;12(5):334–44.
Zhang Y, Wu J, Chen Y, Shi L. EQ-5D-3L decrements by diabetes complications and comorbidities in China. Diabetes Ther. 2020;11(4):939–50.
Cvetanović G, Stojiljković M. Miljković M Estimation of the influence of hypoglycemia and body mass index on health-related quality of life, in patients with type 2 diabetes mellitus. Vojnosanit Pregl. 2017;74(9):831–9.
Hunger M, Schunk M, Meisinger C, Peters A, Holle R. Estimation of the relationship between body mass index and EQ-5D health utilities in individuals with type 2 diabetes: evidence from the population-based KORA studies. J Diabetes Complicat. 2012;26(5):413–8.
Kamradt M, Krisam J, Kiel M, Qreini M, Besier W, Szecsenyi J, Ose D. Health-related quality of life in primary care: which aspects matter in multimorbid patients with type 2 diabetes mellitus in a community setting? PLoS ONE. 2017;12(1): e0170883.
Kiadaliri AA, Gerdtham UG, Eliasson B, Gudbjörnsdottir S, Svensson AM, Carlsson KS. Health utilities of type 2 diabetes-related complications: a cross-sectional study in Sweden. Int J Environ Res Public Health. 2014;11(5):4939–52.
Kontodimopoulos N, Pappa E, Chadjiapostolou Z, Arvanitaki E, Papadopoulos AA, Niakas D. Comparing the sensitivity of EQ-5D, SF-6D and 15D utilities to the specific effect of diabetic complications. Eur J Health Econ. 2012;13(1):111–2.
Mitchell BD, Vietri J, Zagar A, Curtis B, Reaney M. Hypoglycaemic events in patients with type 2 diabetes in the United Kingdom: associations with patient-reported outcomes and self-reported HbA1c. BMC Endocr Disord. 2013;19(13):59.
Pagkalos E, Thanopoulou A, Sampanis C, Bousboulas S, Melidonis A, Tentolouris N, Alexandrides T, Migdalis I, Karamousouli E, Papanas N. The real-life effectiveness and care patterns of type 2 diabetes management in Greece. Exp Clin Endocrinol Diabetes. 2018;126(1):53–60.
Pettersson B, Rosenqvist U, Deleskog A, Journath G, Wändell P. Self-reported experience of hypoglycemia among adults with type 2 diabetes mellitus (Exhype). Diabetes Res Clin Pract. 2011;92(1):19–25.
Schunk M, Reitmeir P, Schipf S, Völzke H, Meisinger C, Ladwig KH, Kluttig A, Greiser KH, Berger K, Müller G, Ellert U, Neuhauser H, Tamayo T, Rathmann W, Holle R. Health-related quality of life in women and men with type 2 diabetes: a comparison across treatment groups. J Diabetes Complicat. 2015;29(2):203–11.
Article CAS Google Scholar
Shingler S, Fordham B, Evans M, Schroeder M, Thompson G, Dewilde S, Lloyd AJ. Utilities for treatment-related adverse events in type 2 diabetes. J Med Econ. 2015;18(1):45–55.
Solli O, Stavem K, Kristiansen IS. Health-related quality of life in diabetes: the associations of complications with EQ-5D scores. Health Qual Life Outcomes. 2010;4(8):18.
Wermeling PR, Gorter KJ, van Stel HF, Rutten GE. Both cardiovascular and non-cardiovascular comorbidity are related to health status in well-controlled type 2 diabetes patients: a cross-sectional analysis. Cardiovasc Diabetol. 2012;5(11):121.
Yfantopoulos J, Chantzaras A. Health-related quality of life and health utilities in insulin-treated type 2 diabetes: the impact of related comorbidities/complications. Eur J Health Econ. 2020. https://doi.org/10.1007/s10198-020-01167-y . ( Epub ahead of print ).
Sayah FA, Qiu W, Xie F, Johnson JA. Comparative performance of the EQ-5D-5L and SF-6D index scores in adults with type 2 diabetes. Qual Life Res. 2017;26(8):2057–66.
Grandy S, Fox KM, SHIELD Study Group. Change in health status (EQ-5D) over 5 years among individuals with and without type 2 diabetes mellitus in the SHIELD longitudinal study. Health Qual Life Outcomes. 2012;10:99.
Harris S, Mamdani M, Galbo-Jørgensen CB, Bøgelund M, Gundgaard J, Groleau D. The effect of hypoglycemia on health-related quality of life: Canadian results from a multinational time trade-off survey. Can J Diabetes. 2014;38(1):45–52.
Marrett E, Radican L, Davies MJ, Zhang Q. Assessment of severity and frequency of self-reported hypoglycemia on quality of life in patients with type 2 diabetes treated with oral antihyperglycemic agents: a survey study. BMC Res Notes. 2011;21(4):251.
McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Self-report of hypoglycemia and health-related quality of life in patients with type 1 and type 2 diabetes. Endocr Pract. 2013;19(5):792–9.
Meneghini LF, Lee LK, Gupta S, Preblick R. Association of hypoglycaemia severity with clinical, patient-reported and economic outcomes in US patients with type 2 diabetes using basal insulin. Diabetes Obes Metab. 2018;20(5):1156–65.
Article CAS PubMed PubMed Central Google Scholar
O’Reilly DJ, Xie F, Pullenayegum E, Gerstein HC, Greb J, Blackhouse GK, Tarride JE, Bowen J, Goeree RA. Estimation of the impact of diabetes-related complications on health utilities for patients with type 2 diabetes in Ontario, Canada. Qual Life Res. 2011;20(6):939–43.
Pawaskar M, Iglay K, Witt EA, Engel SS, Rajpathak S. Impact of the severity of hypoglycemia on health—related quality of life, productivity, resource use, and costs among US patients with type 2 diabetes. J Diabetes Complicat. 2018;32(5):451–7.
Shao H, Yang S, Fonseca V, Stoecker C, Shi L. Estimating quality of life decrements due to diabetes complications in the United States: the Health Utility Index (HUI) diabetes complication equation. Pharmacoeconomics. 2019;37(7):921–9.
Shi L, Shao H, Zhao Y, Thomas NA. Is hypoglycemia fear independently associated with health-related quality of life? Health Qual Life Outcomes. 2014;30(12):167.
Williams SA, Pollack MF, Dibonaventura M. Effects of hypoglycemia on health-related quality of life, treatment satisfaction and healthcare resource utilization in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2011;91(3):363–70.
Zhang P, Brown MB, Bilik D, Ackermann RT, Li R, Herman WH. Health utility scores for people with type 2 diabetes in US managed care health plans: results from Translating Research Into Action for Diabetes (TRIAD). Diabetes Care. 2012;35(11):2250–6.
Zhao H, McClure NS, Johnson JA, Soprovich A, Al Sayah F, Eurich DT. A longitudinal study on the association between diabetic foot disease and health-related quality of life in adults with type 2 diabetes. Can J Diabetes. 2020;44(3):280-286.e1.
da Mata AR, Álvares J, Diniz LM, da Silva MR, Alvernaz dos Santos BR, Guerra Júnior AA, Cherchiglia ML, Andrade EI, Godman B, Acurcio Fde A. Quality of life of patients with diabetes mellitus types 1 and 2 from a referal health centre in Minas Gerais, Brazil. Expert Rev Clin Pharmacol. 2016;9(5):739–46.
Romero-Naranjo F, Espinosa-Uquillas C, Gordillo-Altamirano F, Barrera-Guarderas F. Which factors may reduce the health-related quality of life of ecuadorian patients with diabetes? P R Health Sci J. 2019;38(2):102–8.
PubMed Google Scholar
Javanbakht M, Abolhasani F, Mashayekhi A, Baradaran HR, Jahangiri Noudeh Y. Health related quality of life in patients with type 2 diabetes mellitus in Iran: a national survey. PLoS ONE. 2012;7(8):e44526.
Adibe MO, Aguwa CN. Sensitivity and responsiveness of health utility indices (HUI2 and HUI3) among type 2 diabetes patients. Trop J Pharm Res. 2013;12(5):835–42.
Briggs AH, Bhatt DL, Scirica BM, Raz I, Johnston KM, Szabo SM, Bergenheim K, Mukherjee J, Hirshberg B, Mosenzon O. Health-related quality-of-life implications of cardiovascular events in individuals with type 2 diabetes mellitus: a subanalysis from the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)-TIMI 53 trial. Diabetes Res Clin Pract. 2017;130:24–33.
Evans M, Khunti K, Mamdani M, Galbo-Jørgensen CB, Gundgaard J, Bøgelund M, Harris S. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes. 2013;3(11):90.
Hayes A, Arima H, Woodward M, Chalmers J, Poulter N, Hamet P, Clarke P. Changes in quality of life associated with complications of diabetes: results from the ADVANCE study. Value Health. 2016;19(1):36–41.
Nauck MA, Buse JB, Mann JFE, Pocock S, Bosch-Traberg H, Frimer-Larsen H, Ye Q, Gray A, LEADER Publication Committee for the LEADER Trial Investigators. Health-related quality of life in people with type 2 diabetes participating in the LEADER trial. Diabetes Obes Metab. 2019;21(3):525–32.
Polster M, Zanutto E, McDonald S, Conner C, Hammer M. A comparison of preferences for two GLP-1 products—liraglutide and exenatide—for the treatment of type 2 diabetes. J Med Econ. 2010;13(4):655–61.
Sheu WH, Ji LN, Nitiyanant W, Baik SH, Yin D, Mavros P, Chan SP. Hypoglycemia is associated with increased worry and lower quality of life among patients with type 2 diabetes treated with oral antihyperglycemic agents in the Asia-Pacific region. Diabetes Res Clin Pract. 2012.
Nguyen LH, Tran BX, Le Hoang QN, Tran TT, Latkin CA. Quality of life profile of general Vietnamese population using EQ-5D-5L. Health Qual Life Outcomes. 2017;15(1):199.
The Renal Association. Chronic kidney disease stages. Available at: https://renal.org/information-resources/the-uk-eckd-guide/ckd-stages/ . Last accessed July 29, 2020.
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53. ( Erratum in: Lancet 1999 Aug 14;354(9178):602 ).
European Network for Health Technology Assessment. 2015. Methods for health economic evaluations. Available at: https://www.eunethta.eu/wpcontent/uploads/2018/03/Methods_for_health_economic_evaluations.pdf . Last accessed September 23, 2020.
Kennedy-Martin M, Slaap B, Herdman M, van Reenen M, Kennedy-Martin T, Greiner W, Busschbach J, Boye KS. Which multi-attribute utility instruments are recommended for use in cost-utility analysis? A review of national health technology assessment (HTA) guidelines. Eur J Health Econ. 2020;21(8):1245–57.
Blieden Betts M, Gandra SR, Cheng LI, Szatkowski A, Toth PP. Differences in utility elicitation methods in cardiovascular disease: a systematic review. J Med Econ. 2018;21(1):74–84.
Arnold D, Girling A, Stevens A, Lilford R. Comparison of direct and indirect methods of estimating health state utilities for resource allocation: review and empirical analysis. BMJ. 2009;22(339): b2688.
Lauridsen JT, Lønborg J, Gundgaard J, Jensen HH. Diminishing marginal disutility of hypoglycaemic events: results from a time trade-off survey in five countries. Qual Life Res. 2014;23(9):2645–50.
McPhail S, Haines T. Response shift, recall bias and their effect on measuring change in health-related quality of life amongst older hospital patients. Health Qual Life Outcomes. 2010;10(8):65.
Download references
Medical Writing and Editorial Assistance.
The authors are grateful to Jayne Smith-Palmer for medical writing support (when Dr. Palmer was an employee of Ossian). Eli Lilly and Company provided financial support to Ossian Health Economics and Communications to perform the medical writing work.
Eli Lilly and Company provided financial support to Ossian Health Economics and Communications to perform the literature review described in this paper. Eli Lilly and Company also funded the journal’s Rapid Service Fee and the Open Access Fee.
Author information
Authors and affiliations.
Ossian Health Economics and Communications GmbH, Bäumleingasse 20, 4051, Basel, Switzerland
William J. Valentine
Eli Lilly Finland, Helsinki, Finland
Kirsi Norrbacka
Eli Lilly and Company, Indianapolis, IN, USA
Kristina S. Boye
You can also search for this author in PubMed Google Scholar
Contributions
William J Valentine, Kristina Secnik Boye and Kirsi Norrbacka were involved in the concept and design of the literature review, review of the data for inclusion in the publication and review and/or drafting of the manuscript for publication.
Corresponding author
Correspondence to William J. Valentine .
Ethics declarations
Conflict of interest.
William J Valentine is an employee of Ossian Health Economics and Communications. Krisi Norrbacka and Kristina Secnik Boye were both employees of Eli Lilly and Company at the time of the literature review.
Ethical Approval
Supplementary information.
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 213 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
Valentine, W.J., Norrbacka, K. & Boye, K.S. Utilities for Complications Associated with Type 2 Diabetes: A Review of the Literature. Adv Ther (2024). https://doi.org/10.1007/s12325-024-02878-x
Download citation
Received : 22 May 2023
Accepted : 17 April 2024
Published : 21 May 2024
DOI : https://doi.org/10.1007/s12325-024-02878-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Diabetes-related complications
- Health state utility
- Quality of life
- Type 2 diabetes
- Find a journal
- Publish with us
- Track your research
- Systematic Review
- Open access
- Published: 23 May 2024
Systematic literature review of real-world evidence for treatments in HR+/HER2- second-line LABC/mBC after first-line treatment with CDK4/6i
- Veronique Lambert ORCID: orcid.org/0000-0002-6984-0038 1 ,
- Sarah Kane ORCID: orcid.org/0009-0006-9341-4836 2 na1 ,
- Belal Howidi ORCID: orcid.org/0000-0002-1166-7631 2 na1 ,
- Bao-Ngoc Nguyen ORCID: orcid.org/0000-0001-6026-2270 2 na1 ,
- David Chandiwana ORCID: orcid.org/0009-0002-3499-2565 3 ,
- Yan Wu ORCID: orcid.org/0009-0008-3348-9232 1 ,
- Michelle Edwards ORCID: orcid.org/0009-0001-4292-3140 3 &
- Imtiaz A. Samjoo ORCID: orcid.org/0000-0003-1415-8055 2 na1
BMC Cancer volume 24 , Article number: 631 ( 2024 ) Cite this article
320 Accesses
1 Altmetric
Metrics details
Cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) combined with endocrine therapy (ET) are currently recommended by the National Comprehensive Cancer Network (NCCN) guidelines and the European Society for Medical Oncology (ESMO) guidelines as the first-line (1 L) treatment for patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative, locally advanced/metastatic breast cancer (HR+/HER2- LABC/mBC). Although there are many treatment options, there is no clear standard of care for patients following 1 L CDK4/6i. Understanding the real-world effectiveness of subsequent therapies may help to identify an unmet need in this patient population. This systematic literature review qualitatively synthesized effectiveness and safety outcomes for treatments received in the real-world setting after 1 L CDK4/6i therapy in patients with HR+/ HER2- LABC/mBC.
MEDLINE®, Embase, and Cochrane were searched using the Ovid® platform for real-world evidence studies published between 2015 and 2022. Grey literature was searched to identify relevant conference abstracts published from 2019 to 2022. The review was conducted in accordance with PRISMA guidelines (PROSPERO registration: CRD42023383914). Data were qualitatively synthesized and weighted average median real-world progression-free survival (rwPFS) was calculated for NCCN/ESMO-recommended post-1 L CDK4/6i treatment regimens.
Twenty records (9 full-text articles and 11 conference abstracts) encompassing 18 unique studies met the eligibility criteria and reported outcomes for second-line (2 L) treatments after 1 L CDK4/6i; no studies reported disaggregated outcomes in the third-line setting or beyond. Sixteen studies included NCCN/ESMO guideline-recommended treatments with the majority evaluating endocrine-based therapy; five studies on single-agent ET, six studies on mammalian target of rapamycin inhibitors (mTORi) ± ET, and three studies with a mix of ET and/or mTORi. Chemotherapy outcomes were reported in 11 studies. The most assessed outcome was median rwPFS; the weighted average median rwPFS was calculated as 3.9 months (3.3-6.0 months) for single-agent ET, 3.6 months (2.5–4.9 months) for mTORi ± ET, 3.7 months for a mix of ET and/or mTORi (3.0–4.0 months), and 6.1 months (3.7–9.7 months) for chemotherapy. Very few studies reported other effectiveness outcomes and only two studies reported safety outcomes. Most studies had heterogeneity in patient- and disease-related characteristics.
Conclusions
The real-world effectiveness of current 2 L treatments post-1 L CDK4/6i are suboptimal, highlighting an unmet need for this patient population.
Peer Review reports
Introduction
Breast cancer (BC) is the most diagnosed form of cancer in women with an estimated 2.3 million new cases diagnosed worldwide each year [ 1 ]. BC is the second leading cause of cancer death, accounting for 685,000 deaths worldwide per year [ 2 ]. By 2040, the global burden associated with BC is expected to surpass three million new cases and one million deaths annually (due to population growth and aging) [ 3 ]. Numerous factors contribute to global disparities in BC-related mortality rates, including delayed diagnosis, resulting in a high number of BC cases that have progressed to locally advanced BC (LABC) or metastatic BC (mBC) [ 4 , 5 , 6 ]. In the United States (US), the five-year survival rate for patients who progress to mBC is three times lower (31%) than the overall five-year survival rate for all stages (91%) [ 6 , 7 ].
Hormone receptor (HR) positive (i.e., estrogen receptor and/or progesterone receptor positive) coupled with negative human epidermal growth factor 2 (HER2) expression is the most common subtype of BC, accounting for ∼ 60–70% of all BC cases [ 8 , 9 ]. Historically, endocrine therapy (ET) through estrogen receptor modulation and/or estrogen deprivation has been the standard of care for first-line (1 L) treatment of HR-positive/HER2-negative (HR+/HER2-) mBC [ 10 ]. However, with the approval of the cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) palbociclib in combination with the aromatase inhibitor (AI) letrozole in 2015 by the US Food and Drug Administration (FDA), 1 L treatment practice patterns have evolved such that CDK4/6i (either in combination with AIs or with fulvestrant) are currently considered the standard of care [ 11 , 12 , 13 , 14 , 15 , 16 , 17 ]. Other CDK4/6i (ribociclib and abemaciclib) in combination with ET are approved for the treatment of HR+/HER2- LABC/mBC; 1 L use of ribociclib in combination with an AI was granted FDA approval in March 2017 for postmenopausal women (with expanded approval in July 2018 for pre/perimenopausal women and for use in 1 L with fulvestrant for patients with disease progression on ET as well as for postmenopausal women), and abemaciclib in combination with fulvestrant was granted FDA approval in September 2017 for patients with disease progression following ET and as monotherapy in cases where disease progression occurs following ET and prior chemotherapy in mBC (with expanded approval in February 2018 for use in 1 L in combination with an AI for postmenopausal women) [ 18 , 19 , 20 , 21 ].
Clinical trials investigating the addition of CDK4/6i to ET have demonstrated significant improvement in progression-free survival (PFS) and significant (ribociclib) or numerical (palbociclib and abemaciclib) improvement in overall survival (OS) compared to ET alone in patients with HR+/HER2- advanced or mBC, making this combination treatment the recommended option in the 1 L setting [ 22 , 23 , 24 , 25 , 26 , 27 ]. However, disease progression occurs in a significant portion of patients after 1 L CDK4/6i treatment [ 28 ] and the optimal treatment sequence after progression on CDK4/6i remains unclear [ 29 ]. At the time of this review (literature search conducted December 14, 2022), guidelines by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) recommend various options for the treatment of HR+/HER2- advanced BC in the second-line (2 L) setting, including fulvestrant monotherapy, mammalian target of rapamycin inhibitors (mTORi; e.g., everolimus) ± ET, alpelisib + fulvestrant (if phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha mutation positive [PIK3CA-m+]), poly-ADP ribose polymerase inhibitors (PARPi) including olaparib or talazoparib (if breast cancer gene/partner and localizer of BRCA2 positive [BRCA/PALB2m+]), and chemotherapy (in cases when a visceral crisis is present) [ 15 , 16 ]. CDK4/6i can also be used in 2 L [ 16 , 30 ]; however, limited data are available to support CDK4/6i rechallenge after its use in the 1 L setting [ 15 ]. Depending on treatments used in the 1 L and 2 L settings, treatment in the third-line setting is individualized based on the patient’s response to prior treatments, tumor load, duration of response, and patient preference [ 9 , 15 ]. Understanding subsequent treatments after 1 L CDK4/6i, and their associated effectiveness, is an important focus in BC research.
Treatment options for HR+/HER2- LABC/mBC continue to evolve, with ongoing research in both clinical trials and in the real-world setting. Real-world evidence (RWE) offers important insights into novel therapeutic regimens and the effectiveness of treatments for HR+/HER2- LABC/mBC. The effectiveness of the current treatment options following 1 L CDK4/6i therapy in the real-world setting highlights the unmet need in this patient population and may help to drive further research and drug development. In this study, we conducted a systematic literature review (SLR) to qualitatively summarize the effectiveness and safety of treatment regimens in the real-world setting after 1 L treatment with CDK4/6i in patients with HR+/HER2- LABC/mBC.
Literature search
An SLR was performed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions [ 31 ] and reported in alignment with the Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses (PRISMA) statement [ 32 ] to identify all RWE studies assessing the effectiveness and safety of treatments used for patients with HR+/HER2- LABC/mBC following 1 L CDK4/6i therapy and received subsequent treatment in 2 L and beyond (2 L+). The Ovid® platform was used to search MEDLINE® (including Epub Ahead of Print and In-Process, In-Data-Review & Other Non-Indexed Citations), Ovid MEDLINE® Daily, Embase, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews by an experienced medical information specialist. The MEDLINE® search strategy was peer-reviewed independently by a senior medical information specialist before execution using the Peer Review of Electronic Search Strategies (PRESS) checklist [ 33 ]. Searches were conducted on December 14, 2022. The review protocol was developed a priori and registered with the International Prospective Register of Systematic Review (PROSPERO; CRD42023383914) which outlined the population, intervention, comparator, outcome, and study design (PICOS) criteria and methodology used to conduct the review (Table 1 ).
Search strategies utilized a combination of controlled vocabulary (e.g., “HER2 Breast Cancer” or “HR Breast Cancer”) and keywords (e.g., “Retrospective studies”). Vocabulary and syntax were adjusted across databases. Published and validated filters were used to select for study design and were supplemented using additional medical subject headings (MeSH) terms and keywords to select for RWE and nonrandomized studies [ 34 ]. No language restrictions were included in the search strategy. Animal-only and opinion pieces were removed from the results. The search was limited to studies published between January 2015 and December 2022 to reflect the time at which FDA approval was granted for the first CDK4/6i agent (palbociclib) in combination with AI for the treatment of LABC/mBC [ 35 ]. Further search details are presented in Supplementary Material 1 .
Grey literature sources were also searched to identify relevant abstracts and posters published from January 2019 to December 2022 for prespecified relevant conferences including ESMO, San Antonio Breast Cancer Symposium (SABCS), American Society of Clinical Oncology (ASCO), the International Society for Pharmacoeconomics and Outcomes Research (ISPOR US), and the American Association for Cancer Research (AACR). A search of ClinicalTrials.gov was conducted to validate the findings from the database and grey literature searches.
Study selection, data extraction & weighted average calculation
Studies were screened for inclusion using DistillerSR Version 2.35 and 2.41 (DistillerSR Inc. 2021, Ottawa, Canada) by two independent reviewers based on the prespecified PICOS criteria (Table 1 ). A third reviewer was consulted to resolve any discrepancies during the screening process. Studies were included if they reported RWE on patients aged ≥ 18 years with HR+/HER2- LABC/mBC who received 1 L CDK4/6i treatment and received subsequent treatment in 2 L+. Studies were excluded if they reported the results of clinical trials (i.e., non-RWE), were published in any language other than English, and/or were published prior to 2015 (or prior to 2019 for conference abstracts and posters). For studies that met the eligibility criteria, data relating to study design and methodology, details of interventions, patient eligibility criteria and baseline characteristics, and outcome measures such as efficacy, safety, tolerability, and patient-reported outcomes (PROs), were extracted (as available) using a Microsoft Excel®-based data extraction form (Microsoft Corporation, WA, USA). Data extraction was performed by a single reviewer and was confirmed by a second reviewer. Multiple publications identified for the same RWE study, patient population, and setting that reported data for the same intervention were linked and extracted as a single publication. Weighted average median real-world progression-free survival (rwPFS) values were calculated by considering the contribution to the median rwPFS of each study proportional to its respective sample size. These weighted values were then used to compute the overall median rwPFS estimate.
Quality assessment
The Newcastle-Ottawa scale (NOS) for nonrandomized (cohort) studies was used to assess the risk of bias for published, full-text studies [ 36 ]. The NOS allocates a maximum of nine points for the least risk of bias across three domains: (1) Formation of study groups (four points), (2) Comparability between study groups (two points), (3) Outcome ascertainment (three points). NOS scores can be categorized in three groups: very high risk of bias (0 to 3 points), high risk of bias (4 to 6), and low risk of bias (7 to 9) [ 37 ]. Risk of bias assessment was performed by one reviewer and validated by a second independent reviewer to verify accuracy. Due to limited methodological data by which to assess study quality, risk of bias assessment was not performed on conference abstracts or posters. An amendment to the PROSPERO record (CRD42023383914) for this study was submitted in relation to the quality assessment method (specifying usage of the NOS).
The database search identified 3,377 records; after removal of duplicates, 2,759 were screened at the title and abstract stage of which 2,553 were excluded. Out of the 206 reports retrieved and assessed for eligibility, an additional 187 records were excluded after full-text review; most of these studies were excluded for having patients with mixed lines of CDK4/6i treatment (i.e., did not receive CDK4/6i exclusively in 1 L) (Fig. 1 and Table S1 ). The grey literature search identified 753 records which were assessed for eligibility; of which 752 were excluded mainly due to the population not meeting the eligibility criteria (Fig. 1 ). In total, the literature searches identified 20 records (9 published full-text articles and 11 conference abstracts/posters) representing 18 unique RWE studies that met the inclusion criteria. The NOS quality scores for the included full-text articles are provided in Table S2 . The scores ranged from four to six points (out of a total score of nine) and the median score was five, indicating that all the studies suffered from a high risk of bias [ 37 ].
Most studies were retrospective analyses of chart reviews or medical registries, and all studies were published between 2017 and 2022 (Table S3 ). Nearly half of the RWE studies (8 out of 18 studies) were conducted in the US [ 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ], while the remaining studies included sites in Canada, China, Germany, Italy, Japan, and the United Kingdom [ 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 ]. Sample sizes ranged from as few as 4 to as many as 839 patients across included studies, with patient age ranging from 26 to 86 years old.
Although treatment characteristics in the 1 L setting were not the focus of the present review, these details are captured in Table S3 . Briefly, several RWE studies reported 1 L CDK4/6i use in combination with ET (8 out of 18 studies) or as monotherapy (2 out of 18 studies) (Table S3 ). Treatments used in combination with 1 L CDK4/6i included letrozole, fulvestrant, exemestane, and anastrozole. Where reported (4 out of 18 studies), palbociclib was the most common 1 L CDK4/6i treatment. Many studies (8 out of 18 studies) did not report which specific CDK4/6i treatment(s) were used in 1 L or if its administration was in combination or monotherapy.
Characteristics of treatments after 1 L CDK4/6i therapy
Across all studies included in this review, effectiveness and safety data were only available for treatments administered in the 2 L setting after 1 L CDK4/6i treatment. No studies were identified that reported outcomes for patients treated in the third-line setting or beyond after 1 L CDK4/6i treatment. All 18 studies reported effectiveness outcomes in 2 L, with only two of these studies also describing 2 L safety outcomes. The distribution of outcomes reported in these studies is provided in Table S4 . Studies varied in their reporting of outcomes for 2 L treatments; some studies reported outcomes for a group of 2 L treatments while others described independent outcomes for specific 2 L treatments (i.e., everolimus, fulvestrant, or chemotherapy agents such as eribulin mesylate) [ 42 , 45 , 50 , 54 , 55 ]. Due to the heterogeneity in treatment classes reported in these studies, this data was categorized (as described below) to align with the guidelines provided by NCCN and ESMO [ 15 , 16 ]. The treatment class categorizations for the purpose of this review are: single-agent ET (patients who exclusively received a single-agent ET after 1 L CDK4/6i treatment), mTORi ± ET (patients who exclusively received an mTORi with or without ET after 1 L CDK4/6i treatment), mix of ET and/or mTORi (patients who may have received only ET, only mTORi, and/or both treatments but the studies in this group lacked sufficient information to categorize these patients in the “single-agent ET” or “mTOR ± ET” categories), and chemotherapy (patients who exclusively received chemotherapy after 1 L CDK4/6i treatment). Despite ESMO and NCCN guidelines indicating that limited evidence exists to support rechallenge with CDK4/6i after 1 L CDK4/6i treatment [ 15 , 16 ], two studies reported outcomes for this treatment approach. Data for such patients were categorized as “ CDK4/6i ± ET ” as it was unclear how many patients receiving CDK4/6i rechallenge received concurrent ET. All other patient groups that lacked sufficient information or did not report outcome/safety data independently (i.e., grouped patients with mixed treatments) to categorize as one of the treatment classes described above were grouped as “ other ”.
The majority of studies reported effectiveness outcomes for endocrine-based therapy after 1 L CDK4/6i treatment; five studies for single-agent ET, six studies for mTORi ± ET, and three studies for a mix of ET and/or mTORi (Fig. 2 ). Eleven studies reported effectiveness outcomes for chemotherapy after 1 L CDK4/6i treatment, and only two studies reported effectiveness outcomes for CDK4/6i rechallenge ± ET. Eight studies that described effectiveness outcomes were grouped into the “other” category. Safety data was only reported in two studies: one study evaluating the chemotherapy agent eribulin mesylate and one evaluating the mTORi everolimus.
Effectiveness outcomes
Real-world progression-free survival
Median rwPFS was described in 13 studies (Tables 2 and Table S5 ). Across the 13 studies, the median rwPFS ranged from 2.5 months [ 49 ] to 17.3 months [ 39 ]. Out of the 13 studies reporting median rwPFS, 10 studies reported median rwPFS for a 2 L treatment recommended by ESMO and NCCN guidelines, which ranged from 2.5 months [ 49 ] to 9.7 months [ 45 ].
Weighted average median rwPFS was calculated for 2 L treatments recommended by both ESMO and NCCN guidelines (Fig. 3 ). The weighted average median rwPFS for single-agent ET was 3.9 months ( n = 92 total patients) and was derived using data from two studies reporting median rwPFS values of 3.3 months ( n = 70) [ 38 ] and 6.0 months ( n = 22) [ 40 ]. For one study ( n = 7) that reported outcomes for single agent ET, median rwPFS was not reached during the follow-up period; as such, this study was excluded from the weighted average median rwPFS calculation [ 49 ].
The weighted average median rwPFS for mTORi ± ET was 3.6 months ( n = 128 total patients) and was derived based on data from 3 studies with median rwPFS ranging from 2.5 months ( n = 4) [ 49 ] to 4.9 months ( n = 25) [ 54 ] (Fig. 3 ). For patients who received a mix of ET and/or mTORi but could not be classified into the single-agent ET or mTORi ± ET treatment classes, the weighted average median rwPFS was calculated to be 3.7 months ( n = 17 total patients). This was calculated based on data from two studies reporting median rwPFS values of 3.0 months ( n = 5) [ 46 ] and 4.0 months ( n = 12) [ 49 ]. Notably, one study of patients receiving ET and/or everolimus reported a median rwPFS duration of 3.0 months; however, this study was excluded from the weighted average median rwPFS calculation for the ET and/or mTORi class as the sample size was not reported [ 53 ].
The weighted average median rwPFS for chemotherapy was 6.1 months ( n = 499 total patients), calculated using data from 7 studies reporting median rwPFS values ranging from 3.7 months ( n = 249) [ 38 ] to 9.7 months ( n = 121) [ 45 ] (Fig. 3 ). One study with a median rwPFS duration of 5.6 months was not included in the weighted average median rwPFS calculation as the study did not report the sample size [ 53 ]. A second study was excluded from the calculation since the reported median rwPFS was not reached during the study period ( n = 7) [ 41 ].
Although 2 L CDK4/6i ± ET rechallenge lacks sufficient information to support recommendation by ESMO and NCCN guidelines, the limited data currently available for this treatment have shown promising results. Briefly, two studies reported median rwPFS for CDK4/6i ± ET with values of 8.3 months ( n = 302) [ 38 ] and 17.3 months ( n = 165) (Table 2 ) [ 39 ]. The remaining median rwPFS studies reported data for patients classified as “Other” (Table S5 ). The “Other” category included median rwPFS outcomes from seven studies, and included a myriad of treatments (e.g., ET, mTOR + ET, chemotherapy, CDK4/6i + ET, alpelisib + fulvestrant, chidamide + ET) for which disaggregated median rwPFS values were not reported.
Overall survival
Median OS for 2 L treatment was reported in only three studies (Table 2 ) [ 38 , 42 , 43 ]. Across the three studies, the 2 L median OS ranged from 5.2 months ( n = 3) [ 43 ] to 35.7 months ( n = 302) [ 38 ]. Due to the lack of OS data in most of the studies, weighted averages could not be calculated. No median OS data was reported for the single-agent ET treatment class whereas two studies reported median OS for the mTORi ± ET treatment class, ranging from 5.2 months ( n = 3) [ 43 ] to 21.8 months ( n = 54) [ 42 ]. One study reported 2 L median OS of 24.8 months for a single patient treated with chemotherapy [ 43 ]. The median OS data in the CDK4/6i ± ET rechallenge group was 35.7 months ( n = 302) [ 38 ].
Patient mortality was reported in three studies [ 43 , 44 , 45 ]. No studies reported mortality for the single-agent ET treatment class and only one study reported this outcome for the mTORi ± ET treatment class, where 100% of patients died ( n = 3) as a result of rapid disease progression [ 43 ]. For the chemotherapy class, one study reported mortality for one patient receiving 2 L capecitabine [ 43 ]. An additional study reported eight deaths (21.7%) following 1 L CDK4/6i treatment; however, this study did not disclose the 2 L treatments administered to these patients [ 44 ].
Other clinical endpoints
The studies included limited information on additional clinical endpoints; two studies reported on time-to-discontinuation (TTD), two reported on duration of response (DOR), and one each on time-to-next-treatment (TTNT), time-to-progression (TTP), objective response rate (ORR), clinical benefit rate (CBR), and stable disease (Tables 2 and Table S5 ).
Safety, tolerability, and patient-reported outcomes
Safety and tolerability data were reported in two studies [ 40 , 45 ]. One study investigating 2 L administration of the chemotherapy agent eribulin mesylate reported 27 patients (22.3%) with neutropenia, 3 patients (2.5%) with febrile neutropenia, 10 patients (8.3%) with peripheral neuropathy, and 14 patients (11.6%) with diarrhea [ 45 ]. Of these, neutropenia of grade 3–4 severity occurred in 9 patients (33.3%) [ 45 ]. A total of 55 patients (45.5%) discontinued eribulin mesylate treatment; 1 patient (0.83%) discontinued treatment due to adverse events [ 45 ]. Another study reported that 5 out of the 22 patients receiving the mTORi everolimus combined with ET in 2 L (22.7%) discontinued treatment due to toxicity [ 40 ]. PROs were not reported in any of the studies included in the SLR.
The objective of this study was to summarize the existing RWE on the effectiveness and safety of therapies for patients with HR+/HER2- LABC/mBC after 1 L CDK4/6i treatment. We identified 18 unique studies reporting specifically on 2 L treatment regimens after 1 L CDK4/6i treatment. The weighted average median rwPFS for NCCN- and ESMO- guideline recommended 2 L treatments ranged from 3.6 to 3.9 months for ET-based treatments and was 6.1 months when including chemotherapy-based regimens. Treatment selection following 1 L CDK4/6i therapy remains challenging primarily due to the suboptimal effectiveness or significant toxicities (e.g., chemotherapy) associated with currently available options [ 56 ]. These results highlight that currently available 2 L treatments for patients with HR+/HER2- LABC/mBC who have received 1 L CDK4/6i are suboptimal, as evidenced by the brief median rwPFS duration associated with ET-based treatments, or notable side effects and toxicity linked to chemotherapy. This conclusion is aligned with a recent review highlighting the limited effectiveness of treatment options for HR+/HER2- LABC/mBC patients post-CDK4/6i treatment [ 56 , 57 ]. Registrational trials which have also shed light on the short median PFS of 2–3 months achieved by ET (i.e., fulvestrant) after 1 L CDK4/6i therapy emphasize the need to develop improved treatment strategies aimed at prolonging the duration of effective ET-based treatment [ 56 ].
The results of this review reveal a paucity of additional real-world effectiveness and safety evidence after 1 L CDK4/6i treatment in HR+/HER2- LABC/mBC. OS and DOR were only reported in two studies while other clinical endpoints (i.e., TTD, TTNT, TTP, ORR, CBR, and stable disease) were only reported in one study each. Similarly, safety and tolerability data were only reported in two studies each, and PROs were not reported in any study. This hindered our ability to provide a comprehensive assessment of real-world treatment effectiveness and safety following 1 L CDK4/6i treatment. The limited evidence may be due to the relatively short period of time that has elapsed since CDK4/6i first received US FDA approval for 1 L treatment of HR+/HER2- LABC/mBC (2015) [ 35 ]. As such, almost half of our evidence was informed by conference abstracts. Similarly, no real-world studies were identified in our review that reported outcomes for treatments in the third- or later-lines of therapy after 1 L CDK4/6i treatment. The lack of data in this patient population highlights a significant gap which limits our understanding of the effectiveness and safety for patients receiving later lines of therapy. As more patients receive CDK4/6i therapy in the 1 L setting, the number of patients requiring subsequent lines of therapy will continue to grow. Addressing this data gap over time will be critical to improve outcomes for patients with HR+/HER2- LABC/mBC following 1 L CDK4/6i therapy.
There are several strengths of this study, including adherence to the guidelines outlined in the Cochrane Handbook to ensure a standardized and reliable approach to the SLR [ 58 ] and reporting of the SLR following PRISMA guidelines to ensure transparency and reproducibility [ 59 ]. Furthermore, the inclusion of only RWE studies allowed us to assess the effectiveness of current standard of care treatments outside of a controlled environment and enabled us to identify an unmet need in this patient population.
This study had some notable limitations, including the lack of safety and additional effectiveness outcomes reported. In addition, the dearth of studies reporting PROs is a limitation, as PROs provide valuable insight into the patient experience and are an important aspect of assessing the impact of 2 L treatments on patients’ quality of life. The studies included in this review also lacked consistent reporting of clinical characteristics (e.g., menopausal status, sites of metastasis, prior surgery) making it challenging to draw comprehensive conclusions or comparisons based on these factors across the studies. Taken together, there exists an important gap in our understanding of the long-term management of patients with HR+/HER2- LABC/mBC. Additionally, the effectiveness results reported in our evidence base were informed by small sample sizes; many of the included studies reported median rwPFS based on less than 30 patients [ 39 , 40 , 41 , 46 , 49 , 51 , 60 ], with two studies not reporting the sample size at all [ 47 , 53 ]. This may impact the generalizability and robustness of the results. Relatedly, the SLR database search was conducted in December 2022; as such, novel agents (e.g., elacestrant and capivasertib + fulvestrant) that have since received FDA approval for the treatment of HR+/HER2- LABC/mBC may impact current 2 L rwPFS outcomes [ 61 , 62 ]. Finally, relative to the number of peer-reviewed full-text articles, this SLR identified eight abstracts and one poster presentation, comprising half (50%) of the included unique studies. As conference abstracts are inherently limited by how much content that can be described due to word limit constraints, this likely had implications on the present synthesis whereby we identified a dearth of real-world effectiveness outcomes in patients with HR+/HER2- LABC/mBC treated with 1 L CDK4/6i therapy.
Future research in this area should aim to address the limitations of the current literature and provide a more comprehensive understanding of optimal sequencing of effective and safe treatment for patients following 1 L CDK4/6i therapy. Specifically, future studies should strive to report robust data related to effectiveness, safety, and PROs for patients receiving 2 L treatment after 1 L CDK4/6i therapy. Future studies should also aim to understand the mechanism underlying CDK4/6i resistance. Addressing these gaps in knowledge may improve the long-term real-world management of patients with HR+/HER2- LABC/mBC. A future update of this synthesis may serve to capture a wider breadth of full-text, peer-reviewed articles to gain a more robust understanding of the safety, effectiveness, and real-world treatment patterns for patients with HR+/HER2- LABC/mBC. This SLR underscores the necessity for ongoing investigation and the development of innovative therapeutic approaches to address these gaps and improve patient outcomes.
This SLR qualitatively summarized the existing real-world effectiveness data for patients with HR+/HER2- LABC/mBC after 1 L CDK4/6i treatment. Results of this study highlight the limited available data and the suboptimal effectiveness of treatments employed in the 2 L setting and underscore the unmet need in this patient population. Additional studies reporting effectiveness and safety outcomes, in addition to PROs, for this patient population are necessary and should be the focus of future research.
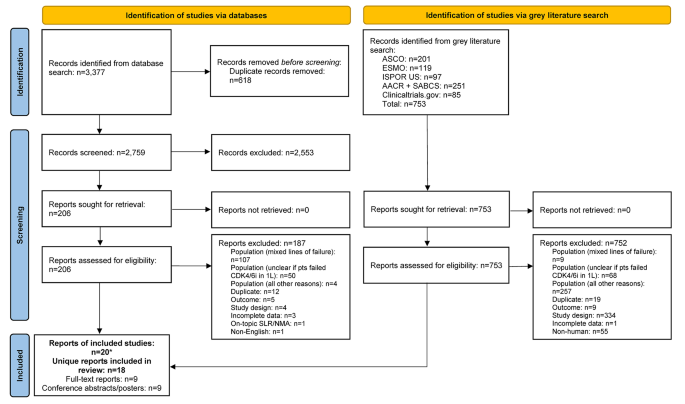
PRISMA flow diagram. *Two included conference abstracts reported the same information as already included full-text reports, hence both conference abstracts were not identified as unique. Abbreviations: 1 L = first-line; AACR = American Association of Cancer Research; ASCO = American Society of Clinical Oncology; CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; ESMO = European Society for Medical Oncology; ISPOR = Professional Society for Health Economics and Outcomes Research; n = number of studies; NMA = network meta-analysis; pts = participants; SABCS = San Antonio Breast Cancer Symposium; SLR = systematic literature review.
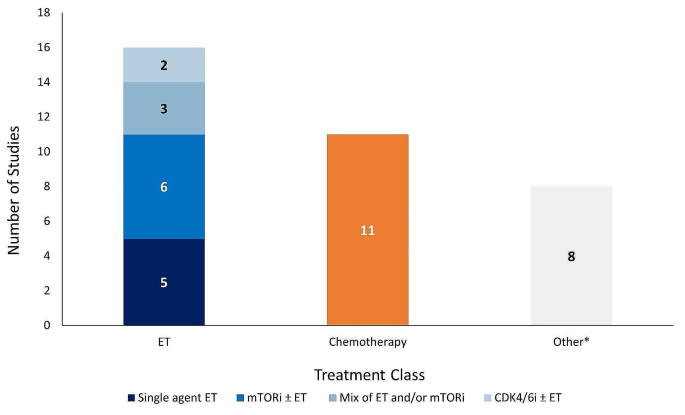
Number of studies reporting effectiveness outcomes exclusively for each treatment class. *Studies that lack sufficient information on effectiveness outcomes to classify based on the treatment classes outlined in the legend above. Abbreviations: CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; ET = endocrine therapy; mTORi = mammalian target of rapamycin inhibitor.
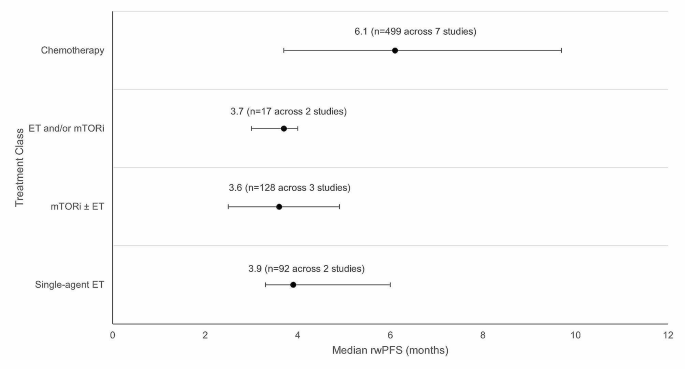
Weighted average median rwPFS for 2 L treatments (recommended in ESMO/NCCN guidelines) after 1 L CDK4/6i treatment. Circular dot represents weighted average median across studies. Horizontal bars represent the range of values reported in these studies. Abbreviations: CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; ESMO = European Society for Medical Oncology; ET = endocrine therapy, mTORi = mammalian target of rapamycin inhibitor; n = number of patients; NCCN = National Comprehensive Cancer Network; rwPFS = real-world progression-free survival.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files]. This study is registered with PROSPERO (CRD42023383914).
Abbreviations
Second-line
Second-line treatment setting and beyond
American Association of Cancer Research
Aromatase inhibitor
American Society of Clinical Oncology
- Breast cancer
breast cancer gene/partner and localizer of BRCA2 positive
Clinical benefit rate
Cyclin-dependent kinase 4/6 inhibitor
Complete response
Duration of response
European Society for Medical Oncology
Food and Drug Administration
Human epidermal growth factor receptor 2
Human epidermal growth factor receptor 2 negative
Hormone receptor
Hormone receptor positive
Professional Society for Health Economics and Outcomes Research
Locally advanced breast cancer
Metastatic breast cancer
Medical Literature Analysis and Retrieval System Online
Medical subject headings
Mammalian target of rapamycin inhibitor
National Comprehensive Cancer Network
Newcastle Ottawa Scale
Objective response rate
Poly-ADP ribose polymerase inhibitor
Progression-free survival
Population, Intervention, Comparator, Outcome, Study Design
Partial response
Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses
Patient-reported outcomes
- Real-world evidence
San Antonio Breast Cancer Symposium
- Systematic literature review
Time-to-discontinuation
Time-to-next-treatment
Time-to-progression
United States
Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A, Breast, Cancer—Epidemiology. Risk factors, classification, prognostic markers, and current treatment Strategies—An. Updated Rev Cancers. 2021;13(17):4287.
Google Scholar
World Health Organization (WHO). Breast Cancer Facts Sheet [updated July 12 2023. https://www.who.int/news-room/fact-sheets/detail/breast-cancer .
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022;66:15–23.
Article PubMed PubMed Central Google Scholar
Wilkinson L, Gathani T. Understanding breast cancer as a global health concern. Br J Radiol. 2022;95(1130):20211033.
Article PubMed Google Scholar
Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A et al. Breast Cancer Statistics, 2022. CA: A Cancer Journal for Clinicians. 2022;72(6):524– 41.
National Cancer Institute (NIH). Cancer Stat Facts: Female Breast Cancer [updated 2020. https://seer.cancer.gov/statfacts/html/breast.html .
American Cancer Society. Key Statistics for Breast Cancer [ https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html .
Zagami P, Carey LA. Triple negative breast cancer: pitfalls and progress. npj Breast Cancer. 2022;8(1):95.
Article CAS PubMed PubMed Central Google Scholar
Matutino A, Joy AA, Brezden-Masley C, Chia S, Verma S. Hormone receptor-positive, HER2-negative metastatic breast cancer: redrawing the lines. Curr Oncol. 2018;25(Suppl 1):S131–41.
Lloyd MR, Wander SA, Hamilton E, Razavi P, Bardia A. Next-generation selective estrogen receptor degraders and other novel endocrine therapies for management of metastatic hormone receptor-positive breast cancer: current and emerging role. Ther Adv Med Oncol. 2022;14:17588359221113694.
Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, et al. 4th ESO-ESMO International Consensus guidelines for advanced breast Cancer (ABC 4)†. Ann Oncol. 2018;29(8):1634–57.
Article CAS PubMed Google Scholar
US Food Drug Administration. Palbociclib (Ibrance) 2017 [updated March 31, 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/palbociclib-ibrance .
US Food Drug Administration. FDA expands ribociclib indication in HR-positive, HER2-negative advanced or metastatic breast cancer 2018 [updated July 18. 2018. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-expands-ribociclib-indication-hr-positive-her2-negative-advanced-or-metastatic-breast-cancer .
US Food Drug Administration. FDA approves abemaciclib for HR positive, HER2-negative breast cancer 2017 [updated Sept 28. 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-hr-positive-her2-negative-breast-cancer .
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast Cancer 2022 [ https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf .
Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–95.
Beaver JA, Amiri-Kordestani L, Charlab R, Chen W, Palmby T, Tilley A, et al. FDA approval: Palbociclib for the Treatment of Postmenopausal Patients with estrogen Receptor-Positive, HER2-Negative metastatic breast Cancer. Clin Cancer Res. 2015;21(21):4760–6.
US Food Drug Administration. Ribociclib (Kisqali) [ https://www.fda.gov/drugs/resources-information-approved-drugs/ribociclib-kisqali#:~:text=On%20March%2013%2C%202017%2C%20the,hormone%20receptor%20(HR)%2Dpositive%2C .
US Food Drug Administration. FDA approves new treatment for certain advanced or metastatic breast cancers [ https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-certain-advanced-or-metastatic-breast-cancers .
US Food Drug Administration. FDA expands ribociclib indication in HR-positive, HER2-negative advanced or metastatic breast cancer. 2018 [ https://www.fda.gov/drugs/resources-information-approved-drugs/fda-expands-ribociclib-indication-hr-positive-her2-negative-advanced-or-metastatic-breast-cancer .
US Food Drug Administration. FDA approves abemaciclib as initial therapy for HR-positive, HER2-negative metastatic breast cancer [ https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-initial-therapy-hr-positive-her2-negative-metastatic-breast-cancer .
Turner NC, Slamon DJ, Ro J, Bondarenko I, Im S-A, Masuda N, et al. Overall survival with Palbociclib and fulvestrant in advanced breast Cancer. N Engl J Med. 2018;379(20):1926–36.
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of Ribociclib and Fulvestrant in hormone Receptor-Positive, human epidermal growth factor receptor 2-Negative advanced breast Cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–72.
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast Cancer. J Clin Oncol. 2017;35(32):3638–46.
Gopalan PK, Villegas AG, Cao C, Pinder-Schenck M, Chiappori A, Hou W, et al. CDK4/6 inhibition stabilizes disease in patients with p16-null non-small cell lung cancer and is synergistic with mTOR inhibition. Oncotarget. 2018;9(100):37352–66.
Watt AC, Goel S. Cellular mechanisms underlying response and resistance to CDK4/6 inhibitors in the treatment of hormone receptor-positive breast cancer. Breast Cancer Res. 2022;24(1):17.
Goetz M. MONARCH 3: final overall survival results of abemaciclib plus a nonsteroidal aromatase inhibitor as first-line therapy for HR+, HER2- advanced breast cancer. SABCS; 2023.
Munzone E, Pagan E, Bagnardi V, Montagna E, Cancello G, Dellapasqua S, et al. Systematic review and meta-analysis of post-progression outcomes in ER+/HER2– metastatic breast cancer after CDK4/6 inhibitors within randomized clinical trials. ESMO Open. 2021;6(6):100332.
Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Annals of Oncology. 2021;32(12):1475-95.
European Society for Medical Oncology (ESMO). ESMO Metastatic Breast Cancer Living Guideline: ER-positive HER2-negative Breast Cancer [updated May 2023. https://www.esmo.org/living-guidelines/esmo-metastatic-breast-cancer-living-guideline/er-positive-her2-negative-breast-cancer .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Welch PM VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). www.training.cochrane.org/handbook : Cochrane; 2021.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40–6.
Fraser C, Murray A, Burr J. Identifying observational studies of surgical interventions in MEDLINE and EMBASE. BMC Med Res Methodol. 2006;6(1):41.
US Food Drug Administration. Palbociclib (Ibrance). Silver Spring, MD: US Food and Drug Administration; 2017.
Book Google Scholar
GA Wells BS, D O’Connell J, Peterson V, Welch M, Losos PT. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [ https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
Lo CK-L, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14(1):45.
Martin JM, Handorf EA, Montero AJ, Goldstein LJ. Systemic therapies following progression on first-line CDK4/6-inhibitor treatment: analysis of real-world data. Oncologist. 2022;27(6):441–6.
Kalinsky KM, Kruse M, Smyth EN, Guimaraes CM, Gautam S, Nisbett AR et al. Abstract P1-18-37: Treatment patterns and outcomes associated with sequential and non-sequential use of CDK4 and 6i for HR+, HER2- MBC in the real world. Cancer Research. 2022;82(4_Supplement):P1-18-37-P1-18-37.
Choong GM, Liddell S, Ferre RAL, O’Sullivan CC, Ruddy KJ, Haddad TC, et al. Clinical management of metastatic hormone receptor-positive, HER2-negative breast cancer (MBC) after CDK 4/6 inhibitors: a retrospective single-institution study. Breast Cancer Res Treat. 2022;196(1):229–37.
Xi J, Oza A, Thomas S, Ademuyiwa F, Weilbaecher K, Suresh R, et al. Retrospective Analysis of Treatment Patterns and effectiveness of Palbociclib and subsequent regimens in metastatic breast Cancer. J Natl Compr Canc Netw. 2019;17(2):141–7.
Rozenblit M, Mun S, Soulos P, Adelson K, Pusztai L, Mougalian S. Patterns of treatment with everolimus exemestane in hormone receptor-positive HER2-negative metastatic breast cancer in the era of targeted therapy. Breast Cancer Res. 2021;23(1):14.
Bashour SI, Doostan I, Keyomarsi K, Valero V, Ueno NT, Brown PH, et al. Rapid breast Cancer Disease Progression following cyclin dependent kinase 4 and 6 inhibitor discontinuation. J Cancer. 2017;8(11):2004–9.
Giridhar KV, Choong GM, Leon-Ferre R, O’Sullivan CC, Ruddy K, Haddad T, et al. Abstract P6-18-09: clinical management of metastatic breast cancer (MBC) after CDK 4/6 inhibitors: a retrospective single-institution study. Cancer Res. 2019;79:P6–18.
Article Google Scholar
Mougalian SS, Feinberg BA, Wang E, Alexis K, Chatterjee D, Knoth RL, et al. Observational study of clinical outcomes of eribulin mesylate in metastatic breast cancer after cyclin-dependent kinase 4/6 inhibitor therapy. Future Oncol. 2019;15(34):3935–44.
Moscetti LML, Riggi L, Sperduti I, Piacentini FOC, Toss A, Barbieri E, Cortesi L, Canino FMA, Zoppoli G, Frassoldati A, Schirone A, Dominici MECF. SEQUENCE OF TREATMENTS AFTER CDK4/6 THERAPY IN ADVANCED BREAST CANCER (ABC), A GOIRC MULTICENTER RETRO/ PROSPECTIVE STUDY. PRELIMINARY RESULTS IN THE RETROSPECTIVE SERIES OF 116 PATIENTS. Tumori. 2022;108(4S):80.
Menichetti AZE, Giorgi CA, Bottosso M, Leporati R, Giarratano T, Barbieri C, Ligorio F, Mioranza E, Miglietta F, Lobefaro R, Faggioni G, Falci C, Vernaci G, Di Liso E, Girardi F, Griguolo G, Vernieri C, Guarneri V, Dieci MV. CDK 4/6 INHIBITORS FOR METASTATIC BREAST CANCER: A MULTICENTER REALWORLD STUDY. Tumori. 2022;108(4S):70.
Marschner NW, Harbeck N, Thill M, Stickeler E, Zaiss M, Nusch A, et al. 232P Second-line therapies of patients with early progression under CDK4/6-inhibitor in first-line– data from the registry platform OPAL. Annals of Oncology. 2022;33:S643-S4
Gousis C, Lowe KMH, Kapiris M. V. Angelis. Beyond First Line CDK4/6 Inhibitors (CDK4/6i) and Aromatase Inhibitors (AI) in Patients with Oestrogen Receptor Positive Metastatic Breast Cancer (ERD MBC): The Guy’s Cancer Centre Experience. Clinical Oncology2022. p. e178.
Endo Y, Yoshimura A, Sawaki M, Hattori M, Kotani H, Kataoka A, et al. Time to chemotherapy for patients with estrogen receptor-positive breast Cancer and cyclin-dependent kinase 4 and 6 inhibitor use. J Breast Cancer. 2022;25(4):296–306.
Li Y, Li W, Gong C, Zheng Y, Ouyang Q, Xie N, et al. A multicenter analysis of treatment patterns and clinical outcomes of subsequent therapies after progression on palbociclib in HR+/HER2- metastatic breast cancer. Ther Adv Med Oncol. 2021;13:17588359211022890.
Amaro CP, Batra A, Lupichuk S. First-line treatment with a cyclin-dependent kinase 4/6 inhibitor plus an aromatase inhibitor for metastatic breast Cancer in Alberta. Curr Oncol. 2021;28(3):2270–80.
Crocetti SPM, Tassone L, Marcantognini G, Bastianelli L, Della Mora A, Merloni F, Cantini L, Scortichini L, Agostinelli V, Ballatore Z, Savini A, Maccaroni E. Berardi R. What is the best therapeutic sequence for ER-Positive/HER2- Negative metastatic breast cancer in the era of CDK4/6 inhibitors? A single center experience. Tumori. 2020;106(2S).
Nichetti F, Marra A, Giorgi CA, Randon G, Scagnoli S, De Angelis C, et al. 337P Efficacy of everolimus plus exemestane in CDK 4/6 inhibitors-pretreated or naïve HR-positive/HER2-negative breast cancer patients: A secondary analysis of the EVERMET study. Annals of Oncology. 2020;31:S382
Luhn P, O’Hear C, Ton T, Sanglier T, Hsieh A, Oliveri D, et al. Abstract P4-13-08: time to treatment discontinuation of second-line fulvestrant monotherapy for HR+/HER2– metastatic breast cancer in the real-world setting. Cancer Res. 2019;79(4Supplement):P4–13.
Mittal A, Molto Valiente C, Tamimi F, Schlam I, Sammons S, Tolaney SM et al. Filling the gap after CDK4/6 inhibitors: Novel Endocrine and Biologic Treatment options for metastatic hormone receptor positive breast Cancer. Cancers (Basel). 2023;15(7).
Ashai N, Swain SM. Post-CDK 4/6 inhibitor therapy: current agents and novel targets. Cancers (Basel). 2023;15(6).
Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). www.training.cochrane.org/handbook : Cochrane; 2022.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Serdar CC, Cihan M, Yücel D, Serdar MA. Sample size, power and effect size revisited: simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem Med (Zagreb). 2021;31(1):010502.
US Food Drug Administration. FDA approves elacestrant for ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer [updated January 27 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-elacestrant-er-positive-her2-negative-esr1-mutated-advanced-or-metastatic-breast-cancer .
US Food Drug Administration. FDA approves capivasertib with fulvestrant for breast cancer [updated November 16 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-capivasertib-fulvestrant-breast-cancer .
Download references
Acknowledgements
The authors would like to acknowledge Joanna Bielecki who developed, conducted, and documented the database searches.
This study was funded by Pfizer Inc. (New York, NY, USA) and Arvinas (New Haven, CT, USA).
Author information
Sarah Kane, Belal Howidi, Bao-Ngoc Nguyen and Imtiaz A. Samjoo contributed equally to this work.
Authors and Affiliations
Pfizer, 10017, New York, NY, USA
Veronique Lambert & Yan Wu
EVERSANA, Burlington, ON, Canada
Sarah Kane, Belal Howidi, Bao-Ngoc Nguyen & Imtiaz A. Samjoo
Arvinas, 06511, New Haven, CT, USA
David Chandiwana & Michelle Edwards
You can also search for this author in PubMed Google Scholar
Contributions
VL, IAS, SK, BH, BN, DC, YW, and ME participated in the conception and design of the study. IAS, SK, BH and BN contributed to the literature review, data collection, analysis, and interpretation of the data. VL, IAS, SK, BH, BN, DC, YW, and ME contributed to the interpretation of the data and critically reviewed for the importance of intellectual content for the work. VL, IAS, SK, BH, BN, DC, YW, and ME were responsible for drafting or reviewing the manuscript and for providing final approval. VL, IAS, SK, BH, BN, DC, YW, and ME meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Corresponding author
Correspondence to Imtiaz A. Samjoo .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors of this manuscript declare that the research presented was funded by Pfizer Inc. and Arvinas. While the support from Pfizer Inc. and Arvinas was instrumental in facilitating this research, the authors affirm that their interpretation of the data and the content of this manuscript were conducted independently and without bias to maintain the transparency and integrity of the research. IAS, SK, BH, and BN are employees of EVERSANA, Canada, which was a paid consultant to Pfizer in connection with the development of this manuscript.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lambert, V., Kane, S., Howidi, B. et al. Systematic literature review of real-world evidence for treatments in HR+/HER2- second-line LABC/mBC after first-line treatment with CDK4/6i. BMC Cancer 24 , 631 (2024). https://doi.org/10.1186/s12885-024-12269-8
Download citation
Received : 26 January 2024
Accepted : 16 April 2024
Published : 23 May 2024
DOI : https://doi.org/10.1186/s12885-024-12269-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- First-line CDK4/6i
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Open access
- Published: 24 May 2024
Cost-effectiveness of differentiated care models that incorporate economic strengthening for HIV antiretroviral therapy adherence: a systematic review
- Annie Liang 1 ,
- Marta Wilson-Barthes ORCID: orcid.org/0000-0002-9845-7142 2 &
- Omar Galárraga ORCID: orcid.org/0000-0002-9985-9266 3
Cost Effectiveness and Resource Allocation volume 22 , Article number: 46 ( 2024 ) Cite this article
105 Accesses
1 Altmetric
Metrics details
There is some evidence that differentiated service delivery (DSD) models, which use a client-centered approach to simplify and increase access to care, improve clinical outcomes among people living with HIV (PLHIV) in high HIV prevalence countries. Integrating economic strengthening tools (e.g., microcredit, cash transfers, food assistance) within DSD models can help address the poverty-related barriers to HIV antiretroviral therapy (ART). Yet there is minimal evidence of the cost-effectiveness of these types of multilevel care delivery models, which potentially prohibits their wider implementation.
Using a qualitative systematic review, this article synthesizes the literature surrounding the cost-effectiveness of differentiated service delivery models that employ economic strengthening initiatives to improve HIV treatment adherence in low- and middle-income countries. We searched three academic databases for randomized controlled trials and observational studies published from January 2000 through March 2024 in Sub-Saharan Africa. The quality of each study was scored using a validated appraisal system.
Eighty-nine full texts were reviewed and 3 met all eligibility criteria. Two of the three included articles were specific to adolescents living with HIV. Economic strengthening opportunities varied by care model, and included developmental savings accounts, microenterprise workshops, and cash and non-cash conditional incentives. The main drivers of programmatic and per-patient costs were ART medications, CD4 cell count testing, and economic strengthening activities.
All economic evaluations in this review found that including economic strengthening as part of comprehensive differentiated service delivery was cost-effective at a willingness to pay threshold of at least 2 times the national per capita gross domestic product. Two of the three studies in this review focused on adolescents, suggesting that these types of care models may be especially cost-effective for youth entering adulthood. All studies were from the provider perspective, indicating that additional evidence is needed to inform the potential cost-savings of DSD and economic strengthening interventions to patients and society. Randomized trials testing the effectiveness of DSD models that integrate economic strengthening should place greater emphasis on costing these types of programs to inform the potential for bringing these types of multilevel interventions to scale.
Introduction
Responding to the World Health Organization’s Treat All Policy, low- and middle-income countries (LMICs) are increasingly using differentiated service delivery (DSD) models as a way to rapidly scale up access to life-saving antiretroviral therapy for people living with HIV (PLHIV) [ 1 ]. According to the International AIDS Society, “differentiated service delivery (DSD), previously referred to as differentiated care, is a client-centred approach that simplifies and adapts HIV services across the cascade to reflect the preferences, expectations and needs of people living with and affected by HIV, while reducing unnecessary burdens on the health system” [ 2 ]. DSD models aim to make care “patient-centered” while reducing logistical and administrative burden(s) on traditional, resource-constrained care facilities [ 1 ]. These models have shown to be effective for increasing treatment adherence, but most do not address the persistent poverty-related barriers to HIV care engagement (e.g., long and costly distances to facilities, food insecurity, HIV stigma). A recent systematic review from 20 LMICs found that economic strengthening interventions such as conditional cash transfers, microcredit, and transportation assistance can improve medication adherence and care-seeking behaviors among persons living with HIV, with more moderate impacts on clinical outcomes [ 3 ]. Two other systematic reviews found that, on their own, differentiated HIV service delivery approaches in Sub-Saharan Africa (SSA) generally cost the same as or less than standard HIV care in terms of the cost per patient per year from a patient perspective [ 1 , 4 ]. For providers and health systems, the available economic evidence suggests that DSD models in SSA are not cost saving compared to more traditional facility-based care models [ 4 ]. A 2017 modeling study found that differentiated service delivery models aiming to increase access to ART in SSA could yield up to a 17.5% reduction in health system costs and health workforce requirements over 5 years [ 5 ]. It remains to be seen whether differentiated service delivery models that additionally aim to address poverty-related barriers to care (e.g., food insecurity, long and costing distances to facilities, restricted access to income-generating opportunities) are cost-effective for patients, providers, or society as a whole [ 6 , 7 ].
The purpose of this systematic review is to (i) summarize the current evidence surrounding the cost and cost-effectiveness of differentiated HIV service delivery models that include economic strengthening compared to differentiated service delivery without economic strengthening and to standard HIV care, and (ii) offer a conceptual framework that can help future researchers understand the key components influencing the incremental cost-effectiveness of these holistic models for patients and providers.
Eligibility criteria
Our review focused on studies of the cost-effectiveness of differentiated HIV care models that incorporated at least one economic strengthening component. Articles were excluded if they were not a randomized controlled trial or observational study, did not include both an economic strengthening and a differentiated care component for promoting ART adherence, or did not report a standard metric for assessing cost-effectiveness of an ART adherence intervention. Economic strengthening included any activity that aimed to generate individual- or household-level income or wealth, such as microfinance groups, social protection programs, savings accounts, or training in financial literacy or entrepreneurship. Articles that were not peer reviewed, published in English, or conducted in SSA were also excluded. There were no restrictions on the study population in terms of age, gender, or SSA region. During the abstract round of screening if the study fit all other criteria (differentiated service delivery in Sub-Saharan Africa with economic strengthening) but did not mention whether a cost-analysis was performed, the study was included for full text screening to account for ancillary costeffectiveness analyses.
Information sources & search strategy
We conducted a literature search of articles in PubMed (National Center for Biotechnology Information, Bethesda, Maryland) and EconLit (American Economic Association, Nashville, Tennessee), supplemented by an Internet search of Google Scholar. Prior reviews indicate that DSD interventions have been implemented since the 2000s. Thus, we searched articles published from January 1, 2000 through March 31, 2024 using the terms “HIV or AIDS”, “ antiretroviral therapy”, “economic strengthening”, “differentiated service delivery”, “Sub-Saharan Africa” “cost analysis”, “cost-effectiveness” and “cost-savings”. Literature searched in PubMed used MeSH (Medical Subject Headings) controlled vocabulary to select key search terms. The full search strategy implemented for each database is provided in Additional File 1 .
Selection process
Initial search results were reviewed by one reviewer (AL). Abstracts and main texts of articles that met all eligibility criteria were double reviewed (AL and MWB), with a third reviewer consulted when necessary (OG).
Data collection process
A data extraction tool was developed to capture the following indicators: study context (e.g., country and region of study), design, population, DSD component(s), economic strengthening activity, costing perspective, main drivers of intervention and per-patient costs, cost-effectiveness metric (e.g., incremental cost-effectiveness ratio), willingness-to-pay threshold (WTP), and a binary indicator of whether the intervention showed to be cost-effective (yes/no). Due to significant heterogeneity across studies in terms of effectiveness and cost-effectiveness outcomes, a meta-analysis was not performed. Search findings were reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [ 8 ].
Quality assessment
Full texts that were standard health economic evaluations were assessed using the validated Quality of Health Economic Studies (QHES) appraisal system developed by Chiou [ 9 , 10 ]. The quality of each full text article was assessed based on the sixteen weighted criteria listed in Additional File 2 . Weighted scores for each criterion were summed to generate an overall quality score ranging from 0 (extremely poor quality) to 100 (excellent quality). Four quality categories (0–25, 25.1–50, 50.1–75, and 75.1–100) were used with scores > 75 indicating high quality studies [ 10 ]. Systematic reviews, micro-costing studies, and qualitative analyses were not scored given our focus on randomized controlled trials (RCTs) and observational studies.
Conceptual framework
Drawing on the papers included in the review, we adapted an existing conceptual framework to synthesize the key components that could be understood to drive the incremental cost-effectiveness of HIV differentiated service delivery models for SSA health systems.
Identified articles
Figure 1 documents the flow of articles through the review and reasons for exclusion. Most of the 89 articles were peer-reviewed journal articles (93.2%), followed by preprints (2.2%), and scientific reports (2.2%). Of the 57 articles that included a DSD intervention, the most common differentiated service delivery model was community-based ART support and adherence counseling. Of the 40 articles that included an economic strengthening (ES) component, conditional economic (cash and non-cash) incentives and microfinance engagement were the most common ES activities. The most common reasons for exclusion were no economic strengthening component and no cost-effectiveness analysis. Eleven of the 89 reviewed articles were traditional cost-effectiveness analyses and thus were appraised for quality using the Chiou grading system; those that were not appraised using the grading system included costing, budget impact, or other types of non-cost-effectiveness evaluations. The 11 articles had an average quality score of 80.73 (out of 100), and all satisfied at least 11 of the 16 grading criteria (Additional File 2 ). Of the 89 full text articles that were assessed, three papers met all eligibility criteria and were included in this narrative review.
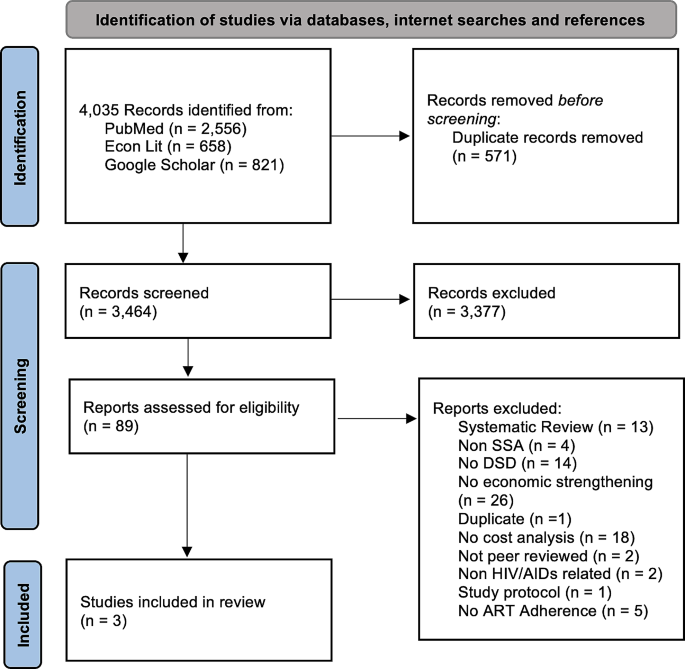
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram
Background and summary of included articles
All 3 studies scored above a 75 (out of 100) on the QHES appraisal system, indicating high quality studies [ 10 ]. Tozan et al. and Ekwunife et al. scored an 85 on the QHES, satisfying the same criteria. Stevens et al. scored 100 on the QHES, satisfying all criteria. Only Stevens et al. displayed a clear economic model, study methods and analysis, and components of numerator and denominator and justified choice of economic model, main assumptions, and limitations of the study. Although all three included studies were of high quality according to the QHES, each provided minimal rationale for their use of a given economic model which may hinder replicability.
Details of the three included studies are summarized in Table 1 . In brief, Tozan et al. [ 11 ] estimated the incremental costs of providing additional counseling sessions for HIV and ART adherence as well as an incentivized savings account and workshops on asset building to adolescents living with HIV in Uganda. Incremental intervention costs were compared to the cost of providing routine HIV care and social support alone. Ekwunife et al. [ 12 ] estimated the cost-effectiveness of a differentiated care model for young adults living with HIV in Nigeria that included motivational interview sessions and economic incentives based on viral load over 12 months. Stevens et al. [ 13 ] modelled the cost-effectiveness of scaling-up a combination care package in Swaziland, which included SMS reminders for ART adherence, counseling and health commodities for ART adherence (e.g., pillboxes and informational materials), and non-cash financial incentives for adults who newly tested positive for HIV. All included studies utilized a facility-based DSD model. For each study, the additional cost for a given intervention compared to the status quo was $970 [95% CI: $508 − 10,275] per additional patient virally suppressed [ 11 ], $1,419 per additional patient with undetected viral load [ 12 ], and $3,560 per additional quality-adjusted life year (QALY) gained [ 13 ].
Cost-effectiveness of differentiated care with economic strengthening
Table 2 presents the cost-effectiveness outcomes from each included study. All analyses used a provider perspective.
The threshold at which a given intervention was deemed cost-effective varied across studies. Tozan et al. did not report a pre-specified willingness to pay threshold [ 11 ]. Ekwunife et al. specified a willingness to pay threshold of $1,137 per additional QALY gained by the intervention [ 12 ]. Stevens et al. reported a threshold of $9,840 per additional QALY gained (3x Swaziland’s GDP per capita); the Link4Health combination package yielded an incremental cost effectiveness ratio (ICER) of $3,560 per additional QALY gained from the health sector perspective, which the authors deemed cost-effective at a willingness to pay threshold of 3 x Swaziland’s per capita GDP in 2018 [ 13 ]. The cost-effectiveness analysis by Ekwunife et al. [ 12 ] found that combing conditional economic incentives and motivational interviewing was not cost-effective compared to standard care at the authors’ pre-defined willingness to pay threshold of 0.51 times Nigeria’s per capita GDP; the intervention was cost-effective at 1 x Nigeria’s per capita GDP in 2021 ($2,027.80). Tozan et al. [ 11 ] did not report the cost-effectiveness of the combined adherence mentoring and incentivized financial savings account intervention in relation to a pre-defined cost-effectiveness threshold; however the intervention cost less than 2 x Uganda’s per capita GDP ($847.30 in 2021). The respective interventions analyzed by Ekwunife et al. [ 12 ] and Tozan et al. [ 11 ] were cost-effective (compared to standard care) assuming the World Health Organization’s willingness to pay thresholds of 2 to 3 times the national per capita GDP in the trial year. Across the three studies, the main drivers of programmatic and per-patient costs were ART treatment costs, CD4 cell count testing, and economic strengthening activities including the costs to provide non-financial incentives. In the Uganda cluster-randomized trial [ 12 ], the largest cost drivers for the intervention came from viral load tests, CD4 count testing, and patient transportation. Financial incentives and point of care CD4 testing were the main drivers of the observed cost differences in the analysis of the Link4Health cluster-RCT [ 13 ]. For Tozan et al. [ 11 ], intervention activities including health education sessions, microenterprise workshops, and savings accounts contributed the largest difference in costs between intervention and standard care. All interventions were more expensive than standard care in terms of total cost per patient.
Synthesizing framework
Based on the three papers in this review, we adapted an existing conceptual model originally developed by Kahn and colleagues [ 14 ] to illustrate – from a health system perspective – the key components that can be hypothesized to influence the cost-effectiveness of differentiated service delivery models that incorporate economic strengthening. (Fig. 2 ) Increasing patient access to antiretroviral therapy immediately following diagnosis and sustaining access over time (e.g., by offering community- or home-based care visits; accelerating ART initiation following point of care CD4 cell count testing) can be expected to add costs to the health system via an increased demand for higher drug quantities, follow-up tests, and personnel time. Similarly, providing economic strengthening opportunities that address known poverty-related barriers to ART adherence will almost always increase the incremental costs of these care delivery approaches if the initiatives are not self-sustaining. For example, providing economic incentives conditional on achieving a viral load below an assay’s lower detection limit will incur additional costs to health ministries who wish to offer this incentive scheme as part of a government social protection program. However, economic strengthening interventions have the potential to be cost-neutral to health systems if they can generate economic growth on their own, as in the case of saving and lending microfinance groups [ 15 , 16 ] or no fee savings accounts [ 11 ]. Averting new HIV infections and decreasing HIV-related morbidity by achieving an undetectable viral load via ART leads to substantial reductions in both disability-adjusted life years and treatment costs. However, as individuals live longer due to ART, they may develop other chronic diseases that incur additional costs to themselves and the health system [ 17 ]. Thus, differentiated service delivery models that integrate economic strengthening and treatment for co-occurring conditions have the potential to further reduce disease burden without substantially increasing treatment costs.
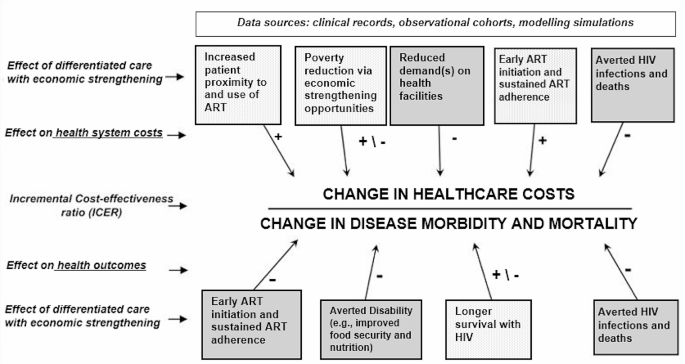
Conceptual Framework. The conceptual framework was adapted from an existing conceptual model developed by Kahn et al. [ 11 ] The framework illustrates the key components that can be hypothesized to influence the cost-effectiveness of differentiated HIV care approaches that incorporate economic strengthening activities, from a health system perspective
All elements of this synthesizing conceptual framework are drawn from the authors’ analyses of the supporting literature. Further research on the cost-effectiveness impact of these mechanisms is required to support their validity.
This systematic narrative review found one of three studies testing a differentiated service delivery model that includes economic strengthening to be cost-effective for providers at the authors’ pre-determined WTP threshold. All three included articles were cost-effective at the WHO willingness to pay threshold of at least 2 times a given country’s per capita GDP. Sensitivity analyses [ 11 , 12 ] and modeling projections [ 13 ] in these papers suggest that the cost-effectiveness of these types of multilevel interventions would increase as these care models are brought to scale. Ekwunife et al. [ 12 ] found that if CD4 + count tests were performed triannually rather than four times a year, the intervention would become cost-effective. Thus, only minimal adjustments to the differentiated service delivery and ES components could increase the interventions’ cost-effectiveness.
Two of three studies in this review were among adolescents living with HIV. This suggests that cultivating routine medication taking behaviors and establishing positive economic skills (e.g., having a savings account, managing microcredit) may be especially important for lower income adolescents living with HIV who can carry these practices into adulthood. Additionally, two recent feasibility studies did not meet inclusion criteria (i.e., being an RCT or observational study) but were initially screened in this review. Findings from these studies further support the potential of integrating DSD with economic strengthening for improving HIV treatment outcomes along the care continuum (testing, linkage to care, and ART adherence) [ 18 , 19 ].
The World Health Organization’s Treat-All guidance recommends CD4 testing before initiating antiretroviral therapy (ART) and recommends routine viral load monitoring (over CD4 cell count monitoring) for patients on ART [ 20 , 21 ]. Viral load monitoring remains the gold standard for monitoring ART adherence and viral suppression among persons living with diagnosed HIV, even in settings where health systems face financial and resource constraints [ 22 , 23 , 24 ]. Thus, given that the focus of our review is on cost-effectiveness of models for ART adherence among persons with diagnosed HIV, our findings can inform scale-up of DSD models that support the most widely used HIV treatment outcomes.
Recent protocol studies reveal that there remains space in the literature to continue to examine DSD with economic strengthening interventions as an effective and cost-effective method of enhancing ART adherence [ 25 ]. For future research and policymaking, these findings suggest there may be potential for implementing scaled-up DSD with economic strengthening interventions enhancing ART adherence among adolescents and young adults specifically.
Limitations of this systematic review stemmed from the large variability in population, context, and target outcomes across studies, which limited our ability to calculate an overall combined economic effect of these interventions. Additionally, all of the cost-effectiveness analyses in this review calculated cost according to the provider perspective, which limits our ability to quantify the potential economic impact of these combination differentiated care models on patients or society. We aimed to mitigate any potential reviewer bias in the inclusion/exclusion of a quality assessment by using a standardized data extraction tool.
Despite calls for novel cost-effectiveness data of holistic differentiated care models in low- and middle-income countries [ 1 , 6 , 26 , 27 , 28 ], the evidence base surrounding the scale-up potential of DSD interventions and economic strengthening remains sparse. To our knowledge, this is the first review to synthesize the available evidence of poverty-addressing DSD models from a health economics perspective. This evidence is critical for policymakers and health care advocates working to address the economic determinants of HIV treatment adherence with limited resources.
This brief systematic review demonstrated that including economic strengthening tools as part of differentiated service delivery models is effective and largely cost-effective at common thresholds compared to traditional HIV care. Modelling projections suggest that scaling these types of multilevel intervention may improve their cost-effectiveness in the short and medium term. Future research should consider the cost-effectiveness and cost-savings of these comprehensive HIV care models from a patient and societal perspective.
Data availability
Data sharing is not applicable to this article as no new datasets were generated or analyzed during the current study.
Abbreviations
Adolescents Living with HIV
Antiretroviral Therapy
Differentiated Service Delivery
Economic Strengthening
Gross Domestic Product
Incremental Cost-Effectiveness Ratio
Income-Generating Activity
Low- or Middle-Income Country
Motivational Interviewing
People Living with HIV
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Quality-Adjusted Life Year
Randomized Controlled Trial
- Sub-Saharan Africa
Willingness-to-Pay
Roy M, Bolton Moore C, Sikazwe I, Holmes CB. A review of Differentiated Service Delivery for HIV Treatment: effectiveness, mechanisms, Targeting, and Scale. Curr HIV/AIDS Rep. 2019;16(4):324–34. https://doi.org/10.1007/s11904-019-00454-5 .
Article PubMed Google Scholar
Differentiated Service Delivery. International AIDS Society. 2024. Accessed April 24, 2024. https://www.iasociety.org/ias-programme/differentiated-service-delivery .
Swann M. Economic strengthening for retention in HIV care and adherence to antiretroviral therapy: a review of the evidence. AIDS Care. 2018;30(3):99–125. https://doi.org/10.1080/09540121.2018.1479030 .
Article Google Scholar
Rosen S, Nichols B, Guthrie T, Benade M, Kuchukhidze S, Long L. Do differentiated service delivery models for HIV treatment in sub-saharan Africa save money? Synthesis of evidence from field studies conducted in sub-saharan Africa in 2017–2019. Gates Open Res. 2022;5:177. https://doi.org/10.12688/gatesopenres.13458.2 .
Article PubMed PubMed Central Google Scholar
Barker C, Dutta A, Klein K. Can differentiated care models solve the crisis in HIV treatment financing? Analysis of prospects for 38 countries in sub-saharan Africa. J Int AIDS Soc. 2017;20(Suppl 4):21648. https://doi.org/10.7448/IAS.20.5.21648 .
Nachega JB, Adetokunboh O, Uthman OA, Knowlton A, Altice FL, Schechter M, et al. Community-based interventions to improve and sustain antiretroviral therapy adherence, Retention in HIV Care and clinical outcomes in low- and Middle-Income Countries for achieving the UNAIDS 90-90-90 targets. Curr HIV/AIDS Rep. 2016;13:241–55. https://doi.org/10.1007/s11904-016-0325-9 .
Munyayi FK, van Wyk B, Mayman Y. Interventions to Improve Treatment outcomes among adolescents on antiretroviral therapy with unsuppressed viral loads: a systematic review. IJERPH. 2022;19(7):3940. https://doi.org/10.3390/ijerph19073940 .
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71 .
Chiou CF, Hay JW, Wallace JF, Bloom BS, Neumann PJ, Sullivan SD, et al. Development and validation of a grading system for the quality of cost-effectiveness studies. Med Care. 2003;41(1):32–44. https://doi.org/10.1097/00005650-200301000-00007 .
Spiegel BM, Targownik LE, Kanwal F, et al. The quality of published health economic analyses in digestive diseases: a systematic review and quantitative appraisal. Gastroenterology. 2004;127(2):403–11. https://doi.org/10.1053/j.gastro.2004.04.020 .
Tozan Y, Capasso A, Sun S, Neilands TB, Damulira C, Namuwonge F, et al. The efficacy and cost-effectiveness of a family-based economic empowerment intervention (suubi + adherence) on suppression of HIV viral loads among adolescents living with HIV: results from a Cluster Randomized Controlled Trial in southern Uganda. JIAS. 2021;24(6):e25752. https://doi.org/10.1002/jia2.25752 .
Ekwunife OI, Ofomata CJ, Okafor CE, Anetoh MU, Kalu SO, Ele PU, et al. Cost-effectiveness and feasibility of conditional economic incentives and motivational interviewing to improve HIV health outcomes of adolescents living with HIV in Anambra State, Nigeria. BMC Health Serv Res. 2021;21:685. https://doi.org/10.1186/s12913-021-06718-4 .
Stevens ER, Li L, Nucifora KA, Zhou Q, McNairy ML, Gachuhi A, et al. Cost-effectiveness of a combination strategy to enhance the HIV care continuum in Swaziland: Link4Health. PLoS ONE. 2018;13(9):e0204245. https://doi.org/10.1371/journal.pone.0204245 .
Article CAS PubMed PubMed Central Google Scholar
Kahn JG, Marseille EA, Bennett R, Williams BG, Granich R. Cost-effectiveness of antiretroviral therapy for prevention. Curr HIV Res. 2011;9(6):405–15. https://doi.org/10.2174/157016211798038542 .
Genberg BL, Wachira J, Steingrimsson JA, Pastakia S, Tran DNT, Said JA, et al. Integrated community-based HIV and non-communicable disease care within microfinance groups in Kenya: study protocol for the Harambee Cluster randomised trial. BMJ Open. 2021;11(5):e042662. https://doi.org/10.1136/bmjopen-2020-042662 .
Pastakia SD, Manyara SM, Vedanthan R, Kamano JH, Menya D, Andama B, et al. Impact of bridging Income Generation with Group Integrated Care (BIGPIC) on hypertension and diabetes in Rural Western Kenya. J Gen Intern Med. 2017;32(5):540–8. https://doi.org/10.1007/s11606-016-3918-5 .
Negin J, Bärnighausen T, Lundgren JD, Mills EJ. Aging with HIV in Africa: the challenges of living longer. AIDS. 2012;26:S1–5. https://doi.org/10.1097/QAD.0b013e3283560f54 .
Kim HY, Inghels M, Mathenjwa T, et al. The impact of a conditional financial incentive on linkage to HIV care: findings from the HITS cluster randomized clinical trial in rural South Africa. Preprint medRxiv. 2024. https://doi.org/10.1101/2024.03.15.24304278 . 2024.03.15.24304278. Published 2024 Mar 18.
Kibel M, Nyambura M, Embleton L, et al. Enabling adherence to treatment (EAT): a pilot study of a combination intervention to improve HIV treatment outcomes among street-connected individuals in western Kenya. BMC Health Serv Res. 2023;23(1):1331. https://doi.org/10.1186/s12913-023-10215-1 . Published 2023 Nov 30.
Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. who.int. Published September 1, 2015. Accessed April 24. 2024. https://www.who.int/publications/i/item/9789241509565 .
Brazier E, Tymejczyk O, Zaniewski E, et al. Effects of National Adoption of treat-all guidelines on Pre-antiretroviral Therapy (ART) CD4 testing and viral load monitoring after ART initiation: a regression discontinuity analysis. Clin Infect Dis. 2021;73(6):e1273–81. https://doi.org/10.1093/cid/ciab222 .
Pham P MD, Nguyen HV, Anderson D, Crowe S, Luchters S. Viral load monitoring for people living with HIV in the era of test and treat: progress made and challenges ahead - a systematic review. BMC Public Health. 2022;22(1):1203. https://doi.org/10.1186/s12889-022-13504-2 . Published 2022 Jun 16.
Okoboi S, Musaazi J, King R, et al. Adherence monitoring methods to measure virological failure in people living with HIV on long-term antiretroviral therapy in Uganda. PLOS Glob Public Health. 2022;2(12):e0000569. https://doi.org/10.1371/journal.pgph.0000569 . Published 2022 Dec 30.
World Health Organization. Updated recommendations on HIV prevention, infant diagnosis, antiretroviral initiation and monitoring. who.int. Published March 17, 2021. Accessed April 24. 2024. https://www.who.int/publications/i/item/9789240022232 .
van Heerden A, Szpiro A, Ntinga X, Celum C, van Rooyen H, Essack Z, Barnabas R. A sequential multiple assignment Randomized Trial of scalable interventions for ART delivery in South Africa: the SMART ART study. Trials. 2023;24(1):32. https://doi.org/10.1186/s13063-022-07025-x .
Decroo T, Rasschaert F, Telfer B, Remartinez D, Laga M, Ford N. Community-based antiretroviral Therapy Programs can overcome barriers to Retention of Patients and Decongest Health Services in Sub-saharan Africa: a systematic review. Int Health. 2013;5(3):169–79. https://doi.org/10.1093/inthealth/iht016 .
Chaiyachati KH, Ogbuoji O, Price M, Suthar AB, Negussie EK, Bärnighausen T. Interventions to improve adherence to antiretroviral therapy: a Rapid systematic review. AIDS. 2014;28:187–204. https://doi.org/10.1097/QAD.0000000000000252 .
Creese A, Floyd K, Alban A, Guinness L. Cost-effectiveness of HIV/AIDS interventions in Africa: a systematic review of the evidence. Lancet. 2002;359(9318):1635–42. https://doi.org/10.1016/S0140-6736(02)08595-1 .
Download references
Acknowledgements
We thank the authors of the original source papers, whose work we drew on considerably.
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under award number R01MH118075, and by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health through the Providence/Boston Center for Aids Research (CFAR) (award number P30AI042853). One hundred percent of this research was financed with Federal money. The design of the study and collection, analysis and interpretation of data and writing of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and affiliations.
Brown University School of Public Health, Providence, RI, USA
Annie Liang
Department of Epidemiology, Brown University School of Public Health, Providence, RI, USA
Marta Wilson-Barthes
Department of Health Services, Policy and Practice; and International Health Institute, Brown University School of Public Health, 121 South Main Street, Box G-S121-2, Providence, RI, USA
Omar Galárraga
You can also search for this author in PubMed Google Scholar
Contributions
AL and OG conceived and designed the work. AL led the analysis and interpretation of the data, and drafted the work. MWB contributed to the analysis and interpretation of data. MWB and OG substantively revised the work. All authors approved the submitted version of the manuscript, and agree to be personally accountable for their own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Correspondence to Omar Galárraga .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Additional file 1: Search syntax
Additional file 2: quality assessment of full text articles that were standard health economic evaluations, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liang, A., Wilson-Barthes, M. & Galárraga, O. Cost-effectiveness of differentiated care models that incorporate economic strengthening for HIV antiretroviral therapy adherence: a systematic review. Cost Eff Resour Alloc 22 , 46 (2024). https://doi.org/10.1186/s12962-024-00557-w
Download citation
Received : 13 April 2023
Accepted : 19 May 2024
Published : 24 May 2024
DOI : https://doi.org/10.1186/s12962-024-00557-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Differentiated care
- Differentiated service delivery
- Economic strengthening
- Microfinance
- Conditional cash transfer
- Cost-effectiveness
- Antiretroviral therapy
Cost Effectiveness and Resource Allocation
ISSN: 1478-7547
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
A diversity of feedback perspectives on a literature review can help identify where the consensus view stands in the landscape of the ... but also of the latest studies, so as not to become out-of-date before they have been published. Ideally, a literature review should not identify as a major research gap an issue that has just been ...
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
A literature review is a document or section of a document that collects key sources on a topic and discusses those sources in conversation with each other (also called synthesis ). The lit review is an important genre in many disciplines, not just literature (i.e., the study of works of literature such as novels and plays).
A literature review can broadly be described as a more or less systematic way of collecting and synthesizing previous research (Baumeister & Leary, 1997; Tranfield, Denyer, & Smart, 2003). ... How to get your literature review published. While there are many arguments in favor for conducting a literature review, publishing it can be challenging
Writing a literature review requires a range of skills to gather, sort, evaluate and summarise peer-reviewed published data into a relevant and informative unbiased narrative. Digital access to research papers, academic texts, review articles, reference databases and public data sets are all sources of information that are available to enrich ...
A literature or narrative review is a comprehensive review and analysis of the published literature on a specific topic or research question. The literature that is reviewed contains: books, articles, academic articles, conference proceedings, association papers, and dissertations. It contains the most pertinent studies and points to important ...
The best proposals are timely and clearly explain why readers should pay attention to the proposed topic. It is not enough for a review to be a summary of the latest growth in the literature: the ...
(analytical evaluation) then your literature review is concentrating on the nature of the problem, its cause and effect as a basis for action to solve it. FORMATIVE When a literature review emphasizes explanation of what you believe the knowledge stemming from previous literature means (formative evaluation) it compares and contrasts the ...
"A literature review is an account of what has been published on a topic by accredited scholars and researchers. In writing the literature review, your purpose is to convey to your reader what knowledge and ideas have been established on a topic, and what their strengths and weaknesses are.
The stand-alone literature review can be categorized by the purpose for the review, which needs to be determined before any work is done. Building from Paré et al. (2015) and Templier and Paré (2015), we group literature reviews into four categories based on the review's purpose: describe, test, extend, and critique. This section provides a ...
A literature review discusses published information in a particular subject area. Often part of the introduction to an essay, research report or thesis, the literature review is literally a "re" view or "look again" at what has already been written about the topic, wherein the author analyzes a segment of a published body of knowledge through summary, classification, and comparison of prior ...
Many literature reviews can be thought of as a qualitative empirical study, in which the papers included in the review substitute interviews or field observations that you would usually collect and code. Some literature reviews, e.g., meta-analyses, are more like a quantitative empirical paper, in which various numbers you extract from the ...
1. Outline and identify the purpose of a literature review. As a first step on how to write a literature review, you must know what the research question or topic is and what shape you want your literature review to take. Ensure you understand the research topic inside out, or else seek clarifications.
The most recently published documents should be considered, but relevant texts published before a predefined cutoff year can be included if they are classic documents in that field. Extra care should be employed when translating documents. ... (how to develop the literature review section). The LR can cover the literature from one or more ...
A literature review is a summary of the published work in a field of study. This can be a section of a larger paper or article, or can be the focus of an entire paper. Literature reviews show that you have examined the breadth of knowledge and can justify your thesis or research questions.
The purpose of looking at a prospective manuscript using this lens is the opposite of publishing it: if is the literature review is not published, what effect it would have on scholarly knowledge and knowledge of practitioners. ... Furthermore, a literature review can have different purposes, which should be articulated. For example, Rocco and ...
A literature review is a survey of scholarly sources on a specific topic. It provides an overview of current knowledge, allowing you to identify relevant theories, methods, and gaps in the existing research. There are five key steps to writing a literature review: Search for relevant literature. Evaluate sources. Identify themes, debates and gaps.
A literature review discusses published information in a particular subject area, and sometimes information in a particular subject area within a certain time period. A literature review can be just a simple summary of the sources, but it usually has an organizational pattern and combines both summary and synthesis. A summary is a recap of the ...
Writing a literature review requires a range of skills to gather, sort, evaluate and summarise peer-reviewed published data into a relevant and informative unbiased narrative. Digital access to research papers, academic texts, review articles, reference databases and public data sets are all sources of information that are available to enrich ...
A literature review is a critical analysis and synthesis of existing research on a particular topic. It provides an overview of the current state of knowledge, identifies gaps, and highlights key findings in the literature. 1 The purpose of a literature review is to situate your own research within the context of existing scholarship ...
Literature reviews are in great demand in most scientific fields. Their need stems from the ever-increasing output of scientific publications .For example, compared to 1991, in 2008 three, eight, and forty times more papers were indexed in Web of Science on malaria, obesity, and biodiversity, respectively .Given such mountains of papers, scientists cannot be expected to examine in detail every ...
A literature review is an overview of the previously published works on a topic. The term can refer to a full scholarly paper or a section of a scholarly work such as a book, or an article. Either way, a literature review is supposed to provide the researcher/author and the audiences with a general image of the existing knowledge on the topic under question.
It is an overview of works that have been previously published. Literature reviews can either be part of a published scholarly work such as a book or a full article on its own. The length of a literature review usually depends on the type of work being done and the topic of study. The more the topic is studied the longer the literature review ...
Discipline norms: a literature review for one subject (e.g., history) would be different than another (e.g., medicine). Organization: a review can be organized the following ways: Topical or narrative: by subject or theme of documents included in the review. Chronological: by when the included documents were published. Geographical: by regions ...
Introduction: A rapid literature review (RLR) is an alternative to systematic literature review (SLR) that can speed up the analysis of newly published data. The objective was to identify and summarize available information regarding different approaches to defining RLR and the methodology applied to the conduct of such reviews.
The literature review was performed using the PubMed, Embase and Cochrane Library databases. Search strategies were designed in alignment with recommendations outlined in the UK-based National Institute for Health and Care Excellence (NICE) Decision Support Unit (DSU) Technical Support Document 9 [].Search strategies utilized high level Medical Subject Heading (MeSH) terms supplemented with ...
This systematic literature review qualitatively synthesized effectiveness and safety outcomes for treatments received in the real-world setting after 1 L CDK4/6i therapy in patients with HR+/ HER2- LABC/mBC. MEDLINE®, Embase, and Cochrane were searched using the Ovid® platform for real-world evidence studies published between 2015 and 2022.
Background: This abstract presents a review of published research literature to determine research investigators' rate of adoption of the International Cognition and Cancer Task Force (ICCTF) recommended criteria for defining cancer-related cognitive impairment (CRCI). Published in 2011, the ICCTF recommendations were intended to identify psychometric standards that would facilitate ...
Using a qualitative systematic review, this article synthesizes the literature surrounding the cost-effectiveness of differentiated service delivery models that employ economic strengthening initiatives to improve HIV treatment adherence in low- and middle-income countries. ... We searched three academic databases for randomized controlled ...
The need for a new review on masks was highlighted by a widely publicized polarization in scientific opinion. The masks section of a 2023 Cochrane review of non-pharmaceutical interventions was—controversially—limited to randomized controlled trials (RCTs).It was interpreted by the press and by some but not all of its own authors to mean that "masks don't work" and "mask mandates ...