

Handbook of 200 Medicinal Plants
A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications
- © 2020
- Shahid Akbar 0
Stockton, USA
You can also search for this author in PubMed Google Scholar
- Provides comprehensive review of 200 medicinal plants
- Includes all clinical trials conducted
- PresentsToxicity Studies and potential interactions
- Thorough spectrum of references included
34k Accesses
170 Citations
22 Altmetric
This is a preview of subscription content, log in via an institution to check access.
Access this book
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
Table of contents (201 chapters)
Front matter, introduction.
Shahid Akbar
Abelmoschus moschatus Medik (Malvaceae)
Abrus precatorius l. (fabaceae/leguminosae), abutilon indicum (link) sweet (malvaceae), acacia catechu oliver (fabaceae/leguminosae), acacia nilotica (l.) delile. (fabaceae/leguminosae), achillea millefolium l. (asteraceae/compositae), achyranthes aspera l. (amaranthaceae), aconitum napellus l. (ranunculaceae), acorus calamus l. (acoraceae), adiantum venustum g. don./ adiantun capillus - veneris l. (pteridaceae), aegle marmelos (l.) corrêa (rutaceae), agrimonia eupatoria l. (rosaceae), alhagi maurorum medik. (fabaceae/leguminosae), alkanna tinctoria (l.) tausch (boraginaceae), allium cepa l. (amaryllidaceae), allium sativum l. (amaryllidaceae), aloe vera (l.) burm.f. (asphodelaceae/xanthorrhoeaceae), alpinia galanga (l.) willd. (zingiberaceae).
- Indian Medicinal plants
- medicinal natural products
- vernacular names
- phytoconstituents
- pharmacology of medicinal plants
- medicinal plants clinical trials
- medicinal plants toxicity
- traditional medical plants
About this book
Authors and affiliations, about the author.
Dr. Akbar holds an M.D. in Pharmacology of natural products from Aligarh Muslim University, Aligarh, India, and obtained his Ph.D. in Pharmacology from the University of the Pacific, Stockton, California. He served as a Professor of Pharmacology, and has over thirty years of experience in pharmacological research and teaching to medical and pharmacy students. He also served as Director of Pharmacy College Research Center, Qassim University, Saudi Arabia. He has many research papers published in international journals, book chapters on beta-blockers, and books related to natural products and general health. His earlier books were intended to raise awareness of healthcare issues and the use of health (herbal) supplements. The current book is an effort to provide detailed reviews of selected plants, especially for their uses in traditional medicines around the world, their updated phytochemical, pharmacological and clinical (if any) research, point out the reasons for variations and discrepancies, and highlight other nuances involved in research on medicinal plants and the obtained results.
Bibliographic Information
Book Title : Handbook of 200 Medicinal Plants
Book Subtitle : A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications
Authors : Shahid Akbar
DOI : https://doi.org/10.1007/978-3-030-16807-0
Publisher : Springer Cham
eBook Packages : Biomedical and Life Sciences , Biomedical and Life Sciences (R0)
Copyright Information : The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG 2020
Hardcover ISBN : 978-3-030-16806-3 Published: 23 April 2020
eBook ISBN : 978-3-030-16807-0 Published: 21 April 2020
Edition Number : 1
Number of Pages : XXX, 2156
Number of Illustrations : 1 b/w illustrations, 198 illustrations in colour
Topics : Pharmacology/Toxicology , Pharmacy , Medicinal Chemistry
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 11 May 2021
Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources
- Manyou Yu 1 ,
- Irene Gouvinhas 1 ,
- João Rocha 2 &
- Ana I. R. N. A. Barros 1 , 3
Scientific Reports volume 11 , Article number: 10041 ( 2021 ) Cite this article
27k Accesses
124 Citations
1 Altmetric
Metrics details
- Biochemistry
Plants with medicinal properties play an increasingly important role in food and pharmaceutical industries for their functions on disease prevention and treatment. This study characterizes the phenolic composition and antioxidant activity of seven medicinal and food plants, including the leaves of Salvia officinalis L., Rosmarinus officinalis L., Olea europaea L., and Punica granatum L., as well as the leaves and young stems of Ruta graveolens L., Mentha piperita L., and Petroselinum crispum , Mill., by using colorimetric, chromatographic, and spectrophotometric assays. Results revealed that the hydro-methanolic leaf extracts of P. granatum (pomegranate) displayed the highest content of total phenols (199.26 mg gallic acid per gram of plant dry weight), ortho -diphenols (391.76 mg gallic acid per gram of plant dry weight), and tannins (99.20 mg epicatechin per gram of plant dry weight), besides a higher content of flavonoids (24 mg catechin per gram of plant dry weight). The highest antioxidant capacity measured by ABTS, DPPH, and FRAP (2.14, 2.27, and 2.33 mM Trolox per gram of plant dry weight, respectively) methods was also obtained in pomegranate leaf extracts, being 4–200 times higher than the other species. Such potent antioxidant activity of pomegranate leaves can be ascribed to the presence of different types of phenolic compounds and the high content in tannins, whilst phenolic acids and flavonoids were found to be the dominant phenolic classes of the other six plants. Consequently, despite the well-known antioxidant properties of these plant species, our study suggests pomegranate leaf can stand out as a relatively more valuable plant source of natural bioactive molecules for developing novel functional food-pharma ingredients, with potential for not only promoting human health but also improving bio-valorization and environment.
Similar content being viewed by others

Screening of 20 species from Lamiaceae family based on phytochemical analysis, antioxidant activity and HPLC profiling
Phytochemical composition and in vitro antioxidant and antimicrobial activities of Bersama abyssinica F. seed extracts

New insights of phenolic compounds from optimized fruit extract of Ficus auriculata
Introduction.
The recent development of functional foods and pharmaceutical products based on medicinal and food (namely fruits and vegetables) plants has brought improvements to all aspects of life, including the alleviation of physical disorders, the reduction in the use of synthetic antibiotics, and the increase in life expectancy 1 , 2 . Indeed, these plants have long been used as safe, effective and sustainable sources of natural antioxidants or free radical scavengers, particularly phenolic compounds, such as phenolic acids, flavonoids, tannins, stilbenes, and anthocyanins 2 . Those phenolics are mostly regarded to confer upon the antioxidant activity of medicinal and food plants, making a marked contribution in the fight against many pathological conditions such as cancer, diabetes, aging, cardiovascular, and other degenerative diseases 2 , 3 , 4 , 5 .
Salvia officinalis L., Rosmarinus officinalis L., and Mentha piperita L. commonly named as sage, rosemary, and peppermint, respectively, belongs to the family of Lamiaceae. They are well-known herbs and spices used in foods for flavors and aromas. Infusions, leaves or essential oils of its each species are reported to possess therapeutics in anti-cancer, anti-microbial, anti-diabetes, and gastrointestinal diseases, etc. 3 , 6 , 7 , 8 . Several bioactivities of sage like antinociceptive, hypolipidemic, and memory-enhancing effects have been demonstrated with clinical trials 7 . Rosmarinic acid is abundant both in sage and rosemary, contributing to their anti-inflammatory properties 3 , 6 , 7 . Flavonoids, phenolic lignans and stilbenes, and essential oils are expected to be responsible for the aroma effects of peppermint 8 .
Rue ( Ruta graveolens L.) has been one of the key plants of the European pharmacopoeia since ancient times for the use in tremors, paralysis, nervine disorders, and joint pain 9 . And nowadays, it becomes medicine in Mediterranean region, due to its prominent biological activities, especially neuroprotection 9 , 10 . Rutin, psoralen, limonene, and pinene are reported as main constituents in this plant extracts or rue oils 9 , 10 .
Olive ( Olea europaea L.) oil is one of the major components of the Mediterranean diets. Recently, phenolics present in olive leaves, especially the oleuropein, are reviewed to be potential economic and renewable source of natural by-products, attributed to its antioxidant, antihypertensive, hypoglycemic, hypocholesterolemic and cardioprotective activity 11 , 12 .
Parsley ( Petroselinum crispum Mill.), used as culinary and medicinal herb, is originated from Mediterranean region. Phytochemicals particularly apigenin, coumarins, myristicin, and apiol are active compounds rich in parsley leaves, exhibiting diverse pharmacological properties, such as cyto-, gastro-, brain-, nephron-protective effects, and so on 13 , 14 , 15 .
Pomegranate ( Punica granutum L.) a deciduous shrub in the family of Lythraceae, is one of the oldest known plants. Both the edible (namely fruit juice) and non-edible parts (including seeds, peels, leaves, roots and bark) of this plant have been evidenced to have a wide range of health benefits, largely resulting from its abundant phenolic acid, flavonoids, tannins, amino acids, and alkaloids 16 , 17 . However, the importance of pomegranate leaves, as agricultural and industrial waste, is of great interest and value to be emphasized by means of describing its beneficial effects and studies performed on this field.
Within the frame, materials from the seven medicinal and food plants aforementioned, that is, leaves and young stems (easy for picking) of rue, peppermint, and parsley, as well as the leaves of sage, rosemary, olive, and pomegranate are outstanding for their higher levels of phenolic contents and antioxidant capacities, along with relatively lower (dose-dependent) or inexistent toxicity 6 , 7 , 8 , 9 , 11 , 13 , 15 , 17 . Therefore, in an attempt to explore plant-based alternative solutions in promoting health, as well as paving the way towards our future pre-clinical and clinical studies, we aimed to analyze the phenolic classes (total phenols, ortho -diphenols, flavonoids, and tannins) and antioxidant activities of different plant species under the same evaluation condition. Furthermore, the principal phenolic constituents were chromatographically characterized to investigate the relationship between the phenolic content and antioxidant activity.
Results and discussion
Phenolic content of tested medicinal and food plants.
Results of colorimetric and spectrophotometric analysis of seven medicinal and food plants were showed in Table 1 . In general, the total phenolic content of the selected plant species was found to be at the highest level in pomegranate leaf extracts at 199.26 mg of gallic acid equivalents per gram of plant dry weight (mg GAE g −1 DW), followed by three Lamiaceae species, including peppermint (70.06 mg GAE g −1 DW), sage (50.89 mg GAE g −1 DW) and rosemary (48.48 mg GAE g −1 DW). On the contrary, parsley displayed the lowest value of total phenols (6.94 mg GAE g −1 DW). The same trend was observed concerning the content of ortho -diphenols and tannins of all investigated samples, reporting the following sequence: pomegranate > peppermint > sage > rosemary > rue > olive > parsley. The ortho -diphenol and tannin content of the methanolic extracts ranged from 26.40 to 391.76 mg GAE g −1 DW, and from 1.33 to 99.20 mg of epicatechin equivalents per gram of plant dry weight (mg ECE g −1 DW), respectively. Moreover, results on total flavonoids content showed a different pattern compared to other phenolic classes, with peppermint showing maximum values at 70.14 mg of catechin equivalents per gram of plant dry weight (mg CATE g −1 DW), following with rosemary (49.14 mg CATE g −1 ), sage (43.92 mg CATE g −1 ), and pomegranate (24.34 mg CATE g −1 ). Furthermore, the flavonoid content of olive leaf was higher than that of rue, in contrast to the trend of the other phenolic classes. Rosemary and sage had comparatively high levels of flavonoids, while the minimum values were reported for parsley.
Different phenolic contents of different plant samples have been reported in the literature 12 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 . For instance, the total phenol content of sage and peppermint was 27.94 and 45.25 mg GAE 100 g −1 DW, meanwhile the flavonoid content of them was 27.54 and 25.17 mg catechin per 100 g, which were much lower than that of our results 19 . Parsley extracts had 1.583 GAE mL −1 of total phenols, 0.091 mg catechin mL −1 of flavonoids, and 1.167 mg catechin mL −1 of condensed tannins 26 . Salama et al. 12 described significant differences in the amounts of total phenolics, flavonoids, and tannins of olive leaves, under different extraction solvents, ranging from 42.02 to 85.50 mg GAE g −1 , 31.22 to 105.19 mg quercetin g −1 , and 30.92 to 51.03 mg tannic acid g −1 , respectively. The contents of phenolic and flavonoid compounds in rue were 14.1 GAE g −1 and 15.8 mg rutin g −1 of dry extracts 20 . Some studies 27 , 28 , 29 have evidenced considerably high level of phenolics in pomegranate leaf extracts, up to 328 mg GAE g −1 DW. Interestingly, pomegranate leaves are characterized by carbohydrates, reducing sugars, sterols, saponins, flavonoids, ellagitannins, piperidine alkaloids, flavones, glycosidic compounds, which are the richest source of phytochemicals when considering the non-edible parts of this species, some food products (red wine, green tea, etc.), and another 109 medicinal plants 30 , 31 , 32 . Our results disclosed that tannins were the main phenolic compounds of pomegranate leaf extract, which has also been corroborated by other studies 33 .
As shown in data (Table 1 ), significant differences ( p < 0.001) around 29, 15, 92 and 75 times were observable respectively for total phenols, ortho -diphenols, flavonoids and tannins in the seven plant extracts, indicating that each phenolic classes exhibited considerably different content among the studied plants. This result was in agreement with other authors 34 , who found that depending on the plant species and botanical family, strong differences were found among 10 medicinal herbs and 11 spices. Meanwhile, the same authors 34 observed a wide variance of phenolics in different samples of the same species, such as the total phenolic content of nine independent samples of peppermint was from 18.3 to 284.3 mg GAE g −1 . Moreover, contents of total phenolics, flavonoids, and condensed tannins of 13 different provenances of rosemary, collected in different seasons ranged from 22.46 to 44.57 mg GAE g −1 DW, from 1.49 to 5.01 mg quercetin g −1 , and from 0.81 to 1.71 mg CATE g −1 DW, respectively 18 . Our results showed inconsistency with this observation, probably attributed to the varieties, or geographical differences, as well as to the collection time, agroclimatic conditions and other relevant factors 24 , 25 . However, to some extent, pomegranate leaf was supposed to have a relatively higher phenolic content than many other medicinal plants. Therefore, it can be inferred that pomegranate leaf could be an important valuable source of bioactive compounds for medicinal purposes and health care.
In addition, in the current study, the colorimetric analysis of flavonoids varied between pomegranate leaf (orange-yellowish) with other plants (pink) and the standard (catechin, pink) under the same conditions (as below described in the methods). This visual observation may be related to the fact that leaves from pomegranate have different predominant sub-classes of flavonoids, different from that existing in the other studied plants 32 . So, the methodology, especially to normalize the use of standards such as quercetin or rutin 35 should be modified to accurately quantify the amount of flavonoids.
In vitro antioxidant activity
The in vitro antioxidant activity assays were carried out to assess the capacity of plant extracts to scavenge free radicals including 2,2′‐azino‐bis(3‐ethylbenzothiazoline‐6‐sulfonic acid radical cation (ABTS +· ) and 2,2‐di(4‐tert‐octylphenyl)‐1‐picrylhydrazyl radical (DPPH·), as well as the ability to reduce ferric (III) iron to ferrous (II) iron. Overall, Table 1 revealed that all the species displayed high antioxidant capacities, although significant differences were observed ( p < 0.001), ranging from 0.01 to 2.14 mM Trolox per gram of plant dry weight (mM Trolox g −1 ) for ABTS, from 0.01 to 2.27 mM Trolox g −1 for DPPH, and from 0.01 to 2.33 mM Trolox g −1 for FRAP (ferric reducing antioxidant power), with large variation over 210-fold. It was found that pomegranate always exhibited the highest antioxidant properties (2.14–2.33 mM Trolox g −1 ) throughout the three measurements, followed by peppermint (0.35–0.50 mM Trolox g −1 ), sage (0.27–0.40 mM Trolox g −1 ), rosemary (0.27–0.42 mM Trolox g −1 ), rue (0.10–0.16 mM Trolox g −1 ), and olive leaf (0.11–0.15 mM Trolox g −1 ). No significant difference was observed between sage and rosemary, and between rue and olive leaf. However, parsley extracts reported the lowest antioxidant potential (0.01 mM Trolox g −1 ).
Previous data regarding the antioxidant capacities of sage, rosemary, rue, olive leaf, peppermint, parsley, and pomegranate leaf have been reported by several authors 12 , 14 , 18 , 22 , 26 , 31 , 36 . The IC 50 values of ABTS and DPPH radical scavenging activity, as well as the EC 50 values of reducing powder regarding olive leaves ranged from 20.13 to 190.95 µg mL −1 , from 17.97 to 41.64 µg mL −1 , and from 90 to 216 µg mL −1 , arising from diverse extraction solvents 12 . Rosemary leaves displayed 75.04 and 9.08 µg mL −1 of IC 50 by ABTS and DPPH assay, along with 4.12 µM by FRAP method 18 . Farnad et al. 22 reported the methanol-ethanol (1:1) extract of peppermint had the best DPPH radical scavenging ability (10.05 mg mL −1 of IC 50 ) and ferric reducing power (184.22 µmol per 100 g powder). The ethanolic extract of parsley displayed 0.34 mg AAE mL −1 (milligrams of ascorbic acid equivalents per milliliter) of DPPH and 0.942 mg AAE mL −1 of FRAP, which was correlated with the anti-glycation activity of this extract 26 . The best antioxidant capacities conducted by DPPH (17.09% of IC 50 ) and FRAP (458.26 mmol Fe II L −1 ) were determined for sage leaves which were collected in May 36 . Cefali et al. 14 stated the rue extracts exhibited antioxidant potential against DPPH (281.02 µg mL −1 of IC 50 ) and ABTS (587.98 µg mL −1 of IC 50 ) radicals, indicating the premature aging protective effect.
Importantly, several studies in vitro and in vivo have recorded the superior antioxidant capacity of pomegranate leaves by contrast with its non-edible parts, of which leaves are as effective as peels in the anti-bacterial, analgesic, acute and chronic anti-inflammatory effects 37 , 38 , while more potent than flowers, stems, and seeds 31 , 39 , 40 , 41 , 42 . Authors proved the potency of pomegranate leaf was higher than that of flower in the prevention of ethylene glycol-induced nephrolithiasis, in the inhibition of DPPH and hydroxyl radicals, and in the reduction of ferric iron 39 , 40 . Data 41 highlighted leaves worked more effectively than stems and led to the most loss of MMP (mitochondrial membrane permeability) potential, consequently suggested as an anti-cancer and anti-proliferative agent. Elfalleh et al. 31 illustrated the highest reducing power (348.68 µg mL −1 of EC 50 ) occurring in the aqueous extract of pomegranate leaf. Furthermore, a higher antioxidant and enzyme inhibitory activity was exposed in two extracts (methanolic and water) of pomegranate leaves among different fruit tree leaves 28 . The ethanolic extracts of pomegranate leaf also exhibited remarkable antioxidant and anti-glycation ability of twenty edible and medicinal plants 29 . The level of anti-radical and ferric reducing properties of pomegranate leaves in our results was similar to some authors 42 . However, comprehensively comparative research involving in the phytochemical and antioxidant properties between pomegranate leaves and other numerous medicinal plants is still scarce; Widely practical application of pomegranate leaf hasn’t come into being, although different biological activities of this material extracts are studied increasingly. Many authors have deeply reviewed for sage, rosemary, peppermint, rue, parsley, and olive leaf. Thus it is worth stressing on the brilliant phenolics and antioxidant property of pomegranate leaves, and developing high added-value products from these materials in the food, pharmaceutical, or even nutraceutical and cosmeceutical industries.
Chromatographic analysis of phenolic compounds
With the development of chromatographic techniques, the phenolic chemistry of many plants has been explored and analyzed to a certain degree, providing us important reference data. To obtain a more complete picture of the quality and quantity of phenolic constituents in the selected plants, 64 phenolic compounds were identified (Table 2 ), of which 59 were quantified with authentic standards relying on RP-HPLC-DAD, as well as by comparison with the literature (retention time, UV/Visible λ max , and spectra). Concentrations of identified phenolics were expressed as milligram per gram dry weight of plant (mg g −1 ).
As shown in Table 2 and Fig. S1 , phenolic profile of diverse plants was significantly different. Leaf extracts of both sage and rosemary were characterized by a high proportion of rosmarinic acid (4.61 mg g −1 or 4.31 mg g −1 , respectively). Rue presented the highest content of rutin (26.10 mg g −1 ), followed by epicatechin gallate (7.82 mg g −1 ). The major phenolic components in olive leaves were oleuropein and its derivatives. Flavanones, especially eriodictyol glycosides, following rutin were found as predominant in the leaf and stem extracts of peppermint. Parsley was described in high amount of apigenin-7- O -apiosylglucoside also called apiin (4.04 mg g −1 ) and epicatechin (3.72 mg g −1 ) in its leaf and stem extracts. The principal phenolic constituents in pomegranate leaves were hydrolyzable tannins, particularly ellagitannin I (56.06 mg g −1 ) and ellagitannin II (45.16 mg g −1 ), ranking the highest concentrations among all identified compounds.
On the other hand, results from Table 2 and Fig. S1 also showed that the most abundant phenolic classes in the tested samples were phenolic acids, flavonoids, tannins, and phenylethanoids. A considerable variation of phenolics was found, ranging, for instance, from 0.03 mg g −1 of 2,3-hydrocybenzoic acid to 56.06 mg g −1 of ellagitannin I. For each identified compound, significant differences were observed ( p < 0.05), such as gallocatechin. The most widespread phenolic acids present in the studied samples included hydroxybenzoic acids (gallic acid and its derivative, vanillic acid), hydroxycinnamic acids (caffeic acid, chlorogenic acid, and neochlorogenic acid), and their ester derivatives (e.g. rosmarinic acid). Significantly high contents of chlorogenic acid (1.54 mg g −1 ) and neochlorogenic acid (1.96 mg g −1 ), and the presence of coumaric acid were perceptible in rue extracts. Ellagic acid and its derivatives were abundant in pomegranate leaves. Except in sage and rosemary, rosmarinic acid was also found in peppermint (0.23 mg g −1 ), but its concentration was lower than that in literature 43 . The special existence of rosmarinic acid, rosmanol, epirosmanol, carnosol, and carnosic acid in sage and rosemary was consistent with other authors 23 , 25 , 44 , 45 .
Besides flavanols including gallocatechin, catechin and epicatechin gallate, then various flavones (luteolin and apigenin) and flavonols (quercetin and diosmetin), mainly in the forms of their derivatives were widely distributed in the most of the studied species. Among them, the highest content of gallocatechin (2.10 mg g −1 ), catechin (3.61 mg g −1 ) and epicatechin gallate (7.82 mg g −1 ) was detected in parsley, rosemary, and rue, respectively. Epicatechin was only found in parsley with a good quantity (3.72 mg g −1 ). Furthermore, the main flavonoids from our data present in sage, rosemary, rue, peppermint, and parsley were apigenin glycosides, luteolin glycosides, quercetin glycosides, flavanone glycosides, and apigenin glycosides, respectively. In addition, peppermint also had comparative amounts of luteolin and quercetin glycosides. Likewise, pomegranate leaves possessed several apigenin and luteolin glycosides. Particularly, rutin presented the highest proportion (26.10 mg g −1 ) in rue, followed by peppermint (9.90 mg g −1 ), while the lowest (0.86 mg g −1 ) in olive leaf.
An important observation is that pomegranate leaf extracts held the greatest number of hydrolyzable tannins, especially ellagitannins. Nevertheless, no ellagitannins were detected by the HPLC method in the other six plants, while condensed tannins were present by the spectrophotometric approach. This was possibly caused by the lack of authentic standards involving different tannins, which need to be performed in the chromatographic analysis. In practice, certain studies have reported the tannins present in sage, rosemary, peppermint, rue, parsley, and olive leaves 12 , 18 , 19 , 26 , 46 , 47 , 48 , mainly in the form of condensed tannins.
In some cases, phenylethanoids, which are phenethyl alcohol-structured phenolic antioxidants, were abundantly found in olive leaves, including oleuropein and its derivatives, followed by tyrosol and verbascoside. These molecules may conduct to its high antioxidant properties 11 , 49 . Tyrosol existed in highest concentration in rosemary (4.56 mg g −1 ), but in small quantity in olive leaf (0.75 mg g −1 ) and rue (0.19 mg g −1 ).
Many studies have described the domination of rosmarinic acid in sage and rosemary, detected in varied amounts depending on phenophase, genotypes, extraction methods, and geographical conditions 23 , 24 , 25 , 36 , 44 , 45 , 50 . A concentration ranging from 0.27 to 2.49% of rosmarinic acid was determined in rosemary leaf extract, according to regions 44 . Khaleel et al. 45 reported 4.5 µg mL −1 of rosmarinic acid in aqueous extract of rosemary, whereas 17.3 µg mL −1 was measured in our methanolic extract of this plant. Exceptionally high content of rosmarinic acid was found in May extract (19.375 mg L −1 ) of sage leaves described by Generalić et al. 36 , very close to our data (18.653 mg L −1 ). Roby et al. 23 declared that the predominant phenolic compounds in sage methanolic extract were ferulic acid (18%), rosmarinic acid (17%) and apigenin (14%) of the total extracted phenols, while in our results, rosmarinic acid and apigenin glycoside III were primary and accounted for 9% and 13% of the total phenolics of sage. Among more than one-hundred active ingredients of rue, rutin, as one of its major compounds, has been a topic of interest for researchers 9 , 20 . Asgharian et al. 20 detected a high level of rutin (40.15 mg g −1 ) by extraction with 70% ethanol, which was higher than that of our study. Melnyk et al. 46 identified rutin as the highest content of phenolics in the rue methanolic extract, consistent with the present work. Several studies 48 , 49 have reported oleuropein and its derivatives as the dominant phenolics in the olive leaf, according with our results. As shown in data (Table 2 and Fig. S1 ), up to 20 phenolic compounds were identified in methanolic extract of olive leaf, more than those identified in other six plants, evidencing it as a rich source of bioactive compounds. However, the composition of olive leaf shows a remarkable variability due to location, climatic-seasonal factors, and cultivation practices, suggesting a trend to understand the factors that control the composition of olive leaves. This can be worthy for the harvesting and production of suitable extracts to be applied in human health. Kapp et al. 43 demonstrated eriocitrin, as a powerful bioactive compound, was the most abundant phenolics in peppermint, in accord with our records, composed of 38% of its aqueous extract, or reaching from 19.9 to 68.1% in 26 peppermint tea samples, respectively. However, the same authors 43 reported that rosmarinic acid accounted for a highest proportion (54.2%) of phenolics in one peppermint tea sample which was originated from Estonia. Additionally, other authors 22 , 47 , 51 also pointed out different dominant phenolics in peppermint, such as epicatechin, naringenin, caffeic acid, chlorogenic acid, 4-hydroxybenzoic acid, which can be attributed to diverse varieties, growing environment, and extraction conditions. The main finding of the present work performed on parsley corresponded to several studies 15 , 52 that apiin extractability was maximum when the solvent was ethanol, methanol or acetone. Yet Hozayen et al. 53 and Aissani et al. 21 conducted rosmarinic acid and quinic acid as the most abounded constituent in aqueous and methanol extracts of parsley, respectively. Fourteen phenolic constituents (Figure S1 ) of pomegranate leaf extracts were preliminarily identified and quantified by reference to chromatographic parameters and the literature. These results are agreeable to other researchers 33 , 54 , 55 , 56 , highlighting that ellagic acid and its derivatives, ellagitannins (punicalin, granatin A and B, etc.), flavone (apigenin, luteolin) and its glycosides, and flavonol (kaempferol) and its glycosides, are the principal phenolics in pomegranate leaves. In addition, many ellagitannins (such as punicalagins, punicafolin, castalagin, corilagin, strictinin, tercatain, brevifolin), and their galloyl and/or hexahydroxydiphenoyl (HHDP) substitutions, have been isolated from the leaf 57 . Other flavonoid derivatives like kaempferol, gossypin, quercetin, and rutin were also detected as major constituents in hydro-methanolic or hydro-ethanolic leaf extracts of pomegranate leaves 33 , 57 . However, the detailed structures of tannins and flavonoids of pomegranate leaf will require further identification by mass spectrometry and nuclear magnetic resonance spectroscopy.
Correlation analysis
In order to better understand the relationship between the antioxidant activity (by ABTS, DPPH, FRAP assays) and the phenolic composition (total phenols, ortho -diphenols, flavonoids, tannins) of the studied plants, correlation coefficients ( r ) were determined (Fig. 1 ). Strong relationships were characterized between antioxidant capacities with total phenols and ortho -diphenols (Fig. 1 a,b), indicating that phenolic compounds contribute to the inhibition of oxidative processes. The content of tannins was well correlated with antioxidant potential (Fig. 1 d). No correlation of antioxidant activities was found with flavonoid content (Fig. 1 c). However, a better relationship of flavonoids (Fig. 1 e) or tannins (Fig. 1 f) can be obtained with the antioxidant activity if excluding pomegranate or peppermint from the data, respectively. The above analysis demonstrated that the antioxidant potential from different plants was dependent on both the concentrations and the structures of phenolic compounds, in line with Cai et al. 30 . Compared to radical scavenging assays (ABTS and DPPH), the stronger correlation between reducing power and phenolic contents confirmed that FRAP was more closely related to total phenols, ortho -diphenols and tannins, which was also mentioned by Li et al. 1 .
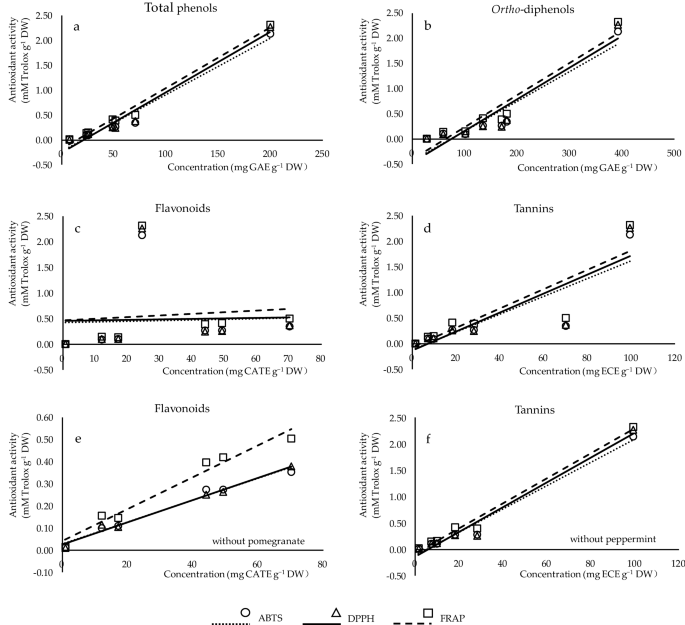
Correlation analysis between the contents of phenolic classes (x-axis) and antioxidant capacities (y-axis) measured by ABTS (circles), DPPH (triangles), and FRAP (squares). ( a – d ) The correlation of total phenols ( r ABTS , DPPH , FRAP = 0.985***, 0.984***, 0.993***), ortho -diphenols ( r ABTS , DPPH , FRAP = 0.859*, 0.861*, 0.878**), flavonoids ( r ABTS , DPPH , FRAP = 0.038, 0.031, 0.098), and tannins ( r ABTS , DPPH , FRAP = 0.859*, 0.861*, 0.878**) of the studied plants with their antioxidant activity, respectively. ( e ) The correlation of flavonoids ( r ABTS , DPPH , FRAP = 0.989***, 0.992***, 0.983***) of studied plants excluding pomegranate with their antioxidant activity. ( f ) The correlation of tannins of studied plants excluding peppermint ( r ABTS , DPPH , FRAP = 0.989***, 0.987***, 0.993***) with their antioxidant activity.
There is a highly correlation between the phenolic composition and antioxidant properties of plants. High anti-radical activity of rosemary leaf in summer was strongly related to high amounts of total phenols, total flavonoids, condensed tannins, and carnosic acid 18 . It is suggested that intraperitoneal of hydroalcoholic extract of rue increased serum and brain antioxidant capacity, due to their potent antioxidant activities of total phenolic and flavonoids content, especially rutin, caffeic acid, and apigenin 20 . Parsley methanolic extract inhibited human glioblastima cancer and oxidative stress owing to its antioxidant properties primarily related to phenolic content 21 . Peppermint extracted by various alcoholic solvents are found to have different levels of antioxidant potential, attributed to the presence of vast flavonoids, anthocyanins, and total phenols 22 . The strong reducing power, free radical scavenging capacity, and the inhibition of hydro-peroxide radicals activity of sage leaves can be linked to the high quantity of phenolic acids, especially rosmarinic acid, and certain flavonoids like catechins and flavanols 36 . Makowska-Wąs et al. 49 revealed considerable antioxidant and cytotoxic properties of olive leaf against several human cancers, largely concerned in the existence of phenolic acids, flavonoids, oleuropein, fatty acids, and volatile oils. The high concentration of phenolic components in pomegranate leaf extracts such as tannins, flavonoids, phyto-steroids, terpenoids, and saponins can be responsible for its high antioxidant activity in vitro and in vivo 27 , 28 , 29 , 32 , 58 .
To date, amount of studies have reported the close relationship not only between the phenolic contents but also between the phenolic structures and the antioxidant capacities 28 , 30 , 59 . The level of antioxidant potential of plants mainly depends on the presence and hydroxyl groups of (poly)phenolic compounds. Specifically, the antioxidant ability of phenolic acids is firstly related to the number and position of phenolic hydroxyls, and secondly to the methoxy and carboxylic acid groups 59 . Rosmarinic acid which was mainly detected in sage, rosemary, and peppermint in our work, is an ester of caffeic acid and 3,4-dihydroxyphenyl lactic acid, comprising two catechol moieties, thus having two pairs of ortho hydroxyl groups grafted on two phenolic rings 18 . Gallic and chlorogenic acid are well-known antioxidant agents, due to three and two active hydroxyl groups on the aromatic ring, respectively 59 . Moreover, the catechol structure in the B-ring, the 2,3-double bond conjugated to a 4-oxo functionality, and the available of both 3- and 5-hydroxyl groups of flavonoids are essential for assessing their antioxidant properties 28 . Rutin is a rutinoside of quercetin with one of the four hydroxyl groups at position C-3 substituted with glucose and rhamnose sugar groups 20 . Apiin or eriocitrin is a apigenin or eriodictyol glycoside, on which the different glycoside moiety is located at position C-7 via a glycosidic linkage along with two or three residual hydroxyl groups on the phenolic rings 15 , 43 . Furthermore, phenylethanoids are characterized by a phenethyl alcohol (C6–C2) moiety attached to a β-glucopyranose/β-allopyranose via a glycosidic bond. Studies indicated the ortho -dihydroxyphenyl groups were the most significant, and the steric hindrance, the number and the position of phenolic hydroxyls were also thought to play an important role 60 . Oleuropein with two hydroxyl groups is an ester of elenolic acid and hydroxytyrosol, and has a oleosidic skeleton that is common to the secoiridoid glucosides of Oleaceae 49 . The strong correlation of antioxidant property with well-identified phenolic acids, flavonoids, and oleuropein present in sage, rosemary, peppermint, rue, parsley, and olive leaves has been individually demonstrated to explain their diverse biological functions 6 , 7 , 8 , 9 , 11 , 13 . In addition, ellagic acid and tannins, defined as polyphenols, are complex chemical substances, possessing plentiful hydroxyl groups, especially ortho -dihydroxyl or galloyl groups 61 . Bigger tannin molecules appear more galloyl and ortho -dihydroxyl groups, consequently, their activities are stronger 61 . Ellagitannins, ellagic acid, and their metabolites have been reported to exhibit numerous beneficial effects on human health including antioxidant, anti-inflammatory, anti-cancer, prebiotic, and cardio-protective properties 61 . Thus they deserve to be part of a healthy diet as functional foods.
The researches on the structure–activity relationship between phenolics and their antioxidant activities have focused on phenolic acids and flavonoids, as well as oleuropein and its derivatives owing to their partially acknowledged health-promoting effects 2 , 30 . However, the benefits of medicinal and food plants may arise from the action of some less well-studied antioxidant molecules or from a synergy of certain antioxidants 30 . Cai et al. 30 found some anticancer-related medicinal plants contained higher quantities and more sorts of tannins, quinones, phenolic terpenoids and special phenolic glycosides than that of phenolic acids and flavonoids. Regarding pomegranate leaves, some authors detected kaempferol 54 or kaempferol 3- O -glycoside 33 as the main compound in ethanolic extracts, while others found as ellagic acid 55 . The principal ellagitannins of pomegranate leaves also differed from one another, considered as granatin B 56 , or castalagin derivative 33 , or undefined galloy-HHDP derivatives 55 . This difference may be induced by varieties, phenology, and growing conditions. In our study, the potent antioxidant capacity of pomegranate leaves was highly correlated with the content of tannins, which can be considered as the key antioxidant contributors of this plant material. However, the chemical structures of the tentatively identified ellagitannins were not determined, and studies on these constituents are also incomplete. Therefore, it is important to note although this is a preliminary study to provide a baseline of data for future investigations, a major limitation is that identified phyto-constituents were neither isolated, nor separately analyzed for their bioactivities. Moreover, the association between these compounds and antioxidant effect of pomegranate leaf is yet to be well understood. In this regard, it is necessary to further characterize the structure of these less-exploited phenolics (tannins) and their associated biological properties within pomegranate leaf. Hence, the results presented in our study confirm pomegranate leaf as a promising natural alternative in the development of antioxidant products, thereby assisting in the prevention and treatment of some diseases.
Conclusions
The level of different phenolic classes, antioxidant capacities and the phenolic profiles of seven medicinal and food plants were evaluated and correlated, including the leaves of sage, rosemary, olive, and pomegranate, as well as the leaves and young stems of rue, peppermint, and parsley. This study compared and demonstrated these plant extracts as valuable sources of bioactive compounds, likely for preparing novel functional products in various industries. High correlations of phenolic composition with antioxidant potential were investigated in our analysis. Different kinds of phenolic acids and flavonoids along with their derivatives were found widespread in the studied plant materials. Phenylethanoids especially oleuropein and its derivatives were characterized as the most abundant constituents of olive leaf extracts, probably contributing to its beneficial biological properties. While tannins particularly ellagitannins were supposed to be the main contributor to the features of pomegranate leaf. Interestingly, our results highlighted that the hydro-methanolic extracts of Punica granatum L. (pomegranate) leaves displayed the greatest levels of free radical scavenging capacity and ferric reducing antioxidant power, as well as the highest contents of total phenols, ortho -diphenols and tannins; a relatively high content of flavonoids was also found. Studies have increasingly evidenced the close association of tannins and less-studied compounds with antioxidant activity in medicinal and food plants 12 , 18 , 19 , 26 , 48 . Thus it is expected that richer phenolic types, namely tannins and phenolic glycosides, and their higher concentrations, are maintained in pomegranate leaves, making it possible to explore active ingredients and bioavailable products in the food-pharm, nutraceutical or cosmeceutical industries.
Moreover, only a limited number of researches have pointed out the comparison of biological activities and phenolic components of the tested plant organs, which belong to tree plants or shrub plants with large or small leaves. Many authors have stated the importance of vegetables, fruits, medicinal and aromatic plants in the current dietary patterns 2 , 3 , 4 , 5 , 29 , 30 , 50 . However, it doesn’t mean the agricultural and industrial waste like the tree leaves are useless for application. Extracts of olive leaves have attracted more attention recently, being reviewed as promising cheap, renewable and plenty source of bio-phenols for by-products. Some articles proved pomegranate leaf as a safe substrate due to its lower or inexistent toxicity 17 , 35 . In addition, ellagitannins as effective ingredients in teas are considered to be more abundant in the large-leaf tree than those from the small-leaf tree 61 , 62 . Therefore, as per olive leaf, research into finding new uses for by-products of pomegranate leaf may be proved as a strong argument for not only promoting human health but also improving bio-valorization and environment. However, samples of pomegranate leaves were not collected from different varieties or different seasons. Hence, studies on these issues would be of much interest in the future, in order to select the most promising matrix of the wasted bio-phenol materials.
Materials and methods
Chemicals and standards.
Compounds: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS ·+ ), (±)-6-hydroxy-2,5,7,8-tetramethylchromone-2-carboxylic acid (Trolox), 2,2-diphenyl-1-picrylhidrazyl radical (DPPH · ), 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), sodium carbonate, sodium molybdate, potassium persulfate, and hydrochloric acid, all extra pure (> 99%) were obtained from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Reagents: ferric chloride, methanol, aluminum chloride, sodium nitrite, all extra pure (> 99%), and methyl cellulose (1500 centipoises viscosity at 2%) were acquired from Merck (Merck, Darmstadt, Germany). Sodium hydroxide, ammonium sulfate, Folin-Ciocalteu’s reagent and acetic acid, all extra pure (> 99%) were purchased from Panreac (Panreac Química S.L.U., Barcelona, Spain). Authentic standards of phenolic compounds used in the chromatographic analysis, including that protocatechuic acid (> 97%), p -hydroxybenzoic acid (> 99%), benzoic acid (> 99.5%) were obtained from Fluka (Fluka Chemika, Neu-Ulm, Switzerland), and caffeic acid (> 98%) was from Panreac (Panreac Química S.L.U., Barcelona, Spain). Standards: neochlorogenic acid (> 95%), chlorogenic acid (> 99%), vanillic acid (> 97%), syringic acid (≥ 99%), myricitin-3- O -glucoside (≥ 99%), p -coumaric acid (> 99%), rutin (quercetin-3-rutinoside) (≥ 94%), ellagic acid (≥ 95%), ferulic acid (> 99%), apigenin-7- O -glucoside (≥ 95%), rosmarinic acid (≥ 98%), luteolin (≥ 98%), quercetin (> 95%), trans -cinnamic acid (> 95%), and kaempferol (> 90%) were purchased from Chem-Lab (Chem-Lab N.V., Zedelgem, Belgium). Gallic acid (> 97.5%), tyrosol (> 98%), caftaric acid (≥ 97%), catechin (≥ 98%), gentisic acid (≥ 98%), epicatechin (≥ 98%), 4-hydrocinnamic acid (> 95%), luteolin-7- O -glucoside (≥ 98%), isorhamnetina-3- O -glucoside (> 95%), oleuropein (> 98%), resveratrol (≥ 99%), and trans -stilben (> 96%) were acquired from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA). Chromatography solvents were of RP-HPLC-DAD grade according to the analysis performed. Ultrapure water was obtained using a Water Purification System (Arioso Power, Human Corporation, Seoul, Korea).
Plant materials
From about one-hundred common medicinal and food plants reported in literature references, we have selected seven medicinal and food plants (Table S1 ) in this study according to following criteria: (1) higher phenolic content and antioxidant capacity, (2) lower or inexistent toxicity. Plant species were botanically authenticated by Prof. António Crespí (Department of Biology and Environment, University of Trás-os-Montes e Alto Douro, UTAD, Portugal) and Dr. João Rocha (Chemistry Centre-Vila Real, UTAD, Portugal). Samples of each species were hand-picked randomly from a pool of individual specimens (n > 10) that are naturally growing in the Botanical Garden of UTAD (Vila Real, Portugal), which belongs to the international network of botanical gardens. Sage, rosemary, rue, peppermint, and parsley are present in the Aromatic and Medicinal Plants collection; olive is present in the Mediterranean Calcareous collection; pomegranate is present in the Garden Fruits collection (more detailed information of each plant species can be checked at http://jb.utad.pt/ ). Thus, a mixture sample for each species was obtained and used for the subsequent analysis. The collected samples were immediately dried at 40 ℃ (Drying Cabinet, LEEC, Nottingham, UK) for 72 h, before being ground into a fine powder with a blender (MB 800, KINEMATICA AG, Malters, Switzerland), and hermetically stored in the dark, at room temperature (RT) until analysis. Experimental research and field studies on plants (either cultivated or wild), including the collection of plant material have complied with relevant institutional, national, and international guidelines and legislation.
Preparation of plant phenolic extracts
The sample powder of each species was weighed and extracted in triplicate with 40 mg of dry weight (DW). The extraction was performed by agitating (30 min, 200 rpm, RT) the mixture of the powder and 1.5 mL of a hydro-methanolic solution (methanol:H 2 O, 70:30, v/v) in an orbital shaker (GFL 3005, GEMINI, Apeldoorn, Netherlands). Afterwards, the suspensions were centrifuged (10,000 rpm, 4 ℃) for 15 min (Sigma 2-16KL Refrigerated Centrifuges, Sigma Laborzentrifugen, Berlin, Germany). The supernatants were collected in a 5 mL volumetric flask, and the solid residues were then extracted twice via the same procedure. All the three supernatants from successive extractions were kept together and the final volume came to 5 mL with the above-mentioned extraction solvent.
Content of different phenolic classes
The content of total phenols, ortho -diphenols, and flavonoids was determined by colorimetric and spectrophotometric approaches according to the literature 63 . The content of tannins was evaluated by the methyl cellulose (MC) methodology previously reported by Dambergs et al. 64 .
For the determination of total phenol content, 20 μL of diluted sample, 100 μL of diluted Folin-Ciocalteu reagent (90%, v/v), and 80 μL aqueous sodium carbonate (7.5%, w/v) were mixed in sequence. The mixture was incubated for 30 min at 42 ℃ in the dark and measured at 750 nm, using gallic acid as standard. Results were expressed in milligrams of gallic acid equivalents per gram of plant dry weight (mg GAE g −1 DW).
For the assessment of ortho -diphenols content, 40 μL of sodium molybdate solution (5%, w/v) prepared with hydro-methanol (50%, v/v) was added to 160 μL of diluted extract. The mixture was stood for 15 min at RT, protected from light, before the absorbance at 375 nm was read. The content was quantified using gallic acid as standard. Results were defined in mg GAE g −1 DW.
For the quantification of total flavonoids content, 24 μL of diluted extract and 28 μL of sodium nitrite (5%, w/v) were mixed. After 5 min at RT, 28 μL of a 10% (w/v) aluminum chloride solution was added in the mixture and reacted for 6 min. Afterwards, 120 μL of sodium hydroxide (1 M) was added and the final mixture was read at 520 nm after agitation for 30 s in a microplate reader. The results were expressed in milligrams of catechin equivalents per gram of plant dry weight (mg CATE g −1 DW).
The above-mentioned assays were undertaken with a microplate reader (Multiskan FC Microplate Photometer, Thermo Fisher Scientific, Vantaa, Finland) in 96-well microplates (PrimeSurface MS-9096MZ, Frilabo, Maia, Portugal) with a final volume of 200 µL.
The content of tannins was evaluated both in treatment and control groups simultaneously, by adding 600 μL of methyl cellulose (MC) solution (treatment) or water (control) to 200 μL of sample in a 2 mL Eppendorf. The mixture was stirred manually for 2–3 min at RT. Four hundred μL of saturated ammonium sulfate and 800 μL of water were added successively both in the treatment and control groups until 2 mL of total volume was reached. The final mixture was vortexed and kept for 10 min. After centrifugation (10,000 rpm, 16 ℃, 5 min), the absorbance was read at 280 nm, by using a conventional spectrophotometer (Helios Gamma UV Spectrophotometer, Thermo Electron Corporation, Warwickshire, UK). The absorbance of tannins was obtained by subtracting the treatment absorbance from the value registered from the control, using epicatechin as standard. The results were described in milligrams of epicatechin equivalents per gram of plant dry weight (mg ECE g −1 DW).
Evaluation of in vitro antioxidant activity
The antioxidant activity of sample extracts was determined by ABTS, DPPH and FRAP (ferric reducing antioxidant power) spectrophotometric methods, reported by Mena et al. 65 , with some modifications.
The ABTS + radicals were produced by mixing 5 mL of ABTS stock solution (7.0 mM) with 88 μL of potassium persulfate (148 mM), and diluted to a working solution with sodium acetate buffer (20 mM, pH 4.5), showing an absorbance of 0.70 ± 0.02 at 734 nm. Subsequently, 188 μL of ABTS working solution and 12 μL of sample dilutions (water used as blank) were mixed and reacted for 30 min at RT, and then the absorbance was read at 734 nm.
The DPPH radicals (8.87 mM) were formed with methanol (99.9%) and diluted in a working solution with hydro-methanol (70%, v/v), achieving an absorbance of 1000 at 520 nm. A mixture of 190 μL of DPPH working solution and 10 μL of sample dilutions (70% hydro-methanol used as blank) was incubated for 15 min at RT, reading the absorbance at 520 nm.
The FRAP working solution was prepared by mixing 10-volume acetate buffer (300 mM, pH 3.6), 1-volume TPTZ (10 mM dissolved in hydrochloric acid), and 1-volume ferric chloride (20 mM in water). The mixture was maintained at 37 ℃ for 10 min before use. The reaction of FRAP working solution (180 μL) and sample dilutions (20 μL) was kept at 37 ℃ for 30 min and the absorbance read at 593 nm.
The three antioxidant assays were adapted to microscale using 96-well microplates (PrimeSurface MS-9096MZ, Frilabo, Maia, Portugal) and microplate readers (Multiskan GO Microplate Photometer, Thermo Fisher Scientific, Vantaa, Finland), using Trolox as standard. All the results were expressed in millimoles of Trolox per gram of plant dry weight (mM Trolox g −1 DW).
Reverse phase-high performance liquid chromatography-diode array detector (RP-HPLC-DAD) system (Thermo Finnigan, San Diego, CA, USA) was carried out to determine the (poly)phenolic profile of each plant extract, as previously described 63 . The analysis equipment is composed of three parts, including LC pump (Surveyor), autosampler (Surveyor), and PDA detector (Surveyor). Sample extracts, in triplicate, and 31 pure standard compounds (all in HPLC grade), including 17 phenolic acids, 10 flavonoids, 2 phenylethanoids and 2 stilbenoids, were prepared and filtered through 0.45 μm PVDF filters (Millex-HV Syringe Filter Unit, Merck Millipore, Bedford, MA, USA) and injected into a C18 column (250 × 4.6 mm, 5 μm particle size; ACE, Aberdeen, Scotland), using a mobile phase composed of water/formic acid (99.9:0.1, v/v) (solvent A) and acetonitrile/formic acid (99.9:0.1, v/v) (solvent B). The linear gradient program (t in min and %B) was: t = 0–0%; t = 5–0%; t = 20–20%; t = 35–50%; t = 40–100%; t = 45–0%; and t = 65–0%. The injection volume was 20 μL and the flow rate was kept at 1.0 mL min −1 . UV/Vis detection was recorded from 200 to 600 nm range. Peaks were monitored at 280 and 330 nm, and identified by congruent retention time compared with standards. Data acquisition, peak integration and analysis were performed using Chromeleon software (Version 7.1; Thermo Scientific, Dionex, USA). The three extracts of each medicinal plant were chromatographed and results were expressed in milligram per liter of sample extracts (mg L −1 ).
Data and statistical analysis
All the measurements of phenolic phytochemicals and antioxidant activity of the plant extracts were conducted in triplicate. The results of phenolic content and antioxidant activity are presented as mean ± standard deviation (SD). Concentrations of individual identified phenolic compounds are presented as mean (n = 3) with the determination of the Least Significant Difference (LSD) for a p value < 0.05. The obtained data were subjected to analysis of variance (ANOVA) and a multiple range test (Tukey’s test) with IBM SPSS statistics 21.0 software (SPSS Inc., Chicago, USA). Pearson ( r ) analysis was carried out to establish correlations between phenolic chemical classes and antioxidant activity.
Li, Q. et al. Cholinesterase, β -amyloid aggregation inhibitory and antioxidant capacities of Chinese medicinal plants. Ind. Crops Prod. 108 , 512–519. https://doi.org/10.1016/j.indcrop.2017.07.001 (2017).
Article CAS Google Scholar
Nollet, L. M. & Gutierrez-Uribe, J. A. Phenolic Compounds in Food: Characterization and Analysis 1st edn, 167 (CRC Press, 2018).
Book Google Scholar
Uritu, C. M. et al. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018 , 7801543. https://doi.org/10.1155/2018/7801543 (2018).
Article PubMed PubMed Central Google Scholar
Etkin, N. L. Plants and Indigenous Medicine and Diet: Biobehavioral Approaches eBook, 336 (Taylor & Francis, 2019).
Watson, R. R. & Preedy, V. R. Fruits, Vegetables, and Herbs: Bioactive Foods in Health Promotion 1st edn. (Elsevier Academic Press, 2016).
Google Scholar
Alavi, M. S., Fanoudi, S., Ghasemzadeh Rahbardar, M., Mehri, S. & Hosseinzadeh, H. An updated review of protective effects of rosemary and its active constituents against natural and chemical toxicities. Phytother. Res. https://doi.org/10.1002/ptr.6894 (2020).
Article PubMed Google Scholar
Ghorbani, A. & Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 7 , 433–440. https://doi.org/10.1016/j.jtcme.2016.12.014 (2017).
Mahendran, G. & Rahman, L.-U. Ethnomedicinal, phytochemical and pharmacological updates on Peppermint ( Mentha × piperita L.)—a review. Phytother. Res. 34 , 2088–2139. https://doi.org/10.1002/ptr.6664 (2020).
Shamal Badhusha, P. A. et al. Traditional uses, phytochemistry and ethanopharmacology of Ruta graveolens Linn: A review. Int. J. Pharm. Drug Anal. 20 , 1–4 (2020).
Colucci-D’Amato, L. & Cimaglia, G. Ruta graveolens as a potential source of neuroactive compounds to promote and restore neural functions. J. Tradit. Complement. Med. 10 , 309–314. https://doi.org/10.1016/j.jtcme.2020.05.002 (2020).
Şahin, S. & Bilgin, M. Olive tree ( Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 98 , 1271–1279. https://doi.org/10.1002/jsfa.8619 (2018).
Article CAS PubMed Google Scholar
Salama, Z. A. et al. In-vitro antioxidant, antimicrobial and anticancer activities of banana leaves ( Musa acuminata ) and olive leaves ( Olea europaea L.) as by-products. Res. J. Pharm. Technol. 13 , 687–696. https://doi.org/10.5958/0974-360X.2020.00132.8 (2020).
Article Google Scholar
Farzaei, M. H., Abbasabadi, Z., Ardekani, M. R. S., Rahimi, R. & Farzaei, F. Parsley: A review of ethnopharmacology, phytochemistry and biological activities. J. Tradit. Chin. Med. 33 , 815–826. https://doi.org/10.1016/S0254-6272(14)60018-2 (2013).
Cefali, L. C. et al. Evaluation of in vitro solar protection factor (SPF), antioxidant activity, and cell viability of mixed vegetable extracts from Dirmophandra mollis Benth, Ginkgo biloba L., Ruta graveolens L., and Vitis vinífera L.. Plants 8 , 453. https://doi.org/10.3390/plants8110453 (2019).
Article CAS PubMed Central Google Scholar
Mara de Menezes Epifanio, N. et al. Chemical characterization and in vivo antioxidant activity of parsley ( Petroselinum crispum ) aqueous extract. Food Funct. 11 , 5346–5356. https://doi.org/10.1039/D0FO00484G (2020).
Vučić, V., Grabež, M., Trchounian, A. & Arsić, A. Composition and potential health benefits of pomegranate: A review. Curr. Pharm. Des. 25 , 1817–1827. https://doi.org/10.2174/1381612825666190708183941 (2019).
Viswanatha, G. L., Venkataranganna, M. V., Prasad, N. B. L. & Ashok, G. Evaluation of anti-epileptic activity of leaf extracts of Punica granatum on experimental models of epilepsy in mice. J. Intercult. Ethnopharmacol. 5 , 415–421. https://doi.org/10.5455/jice.20160904102857 (2016).
Article CAS PubMed PubMed Central Google Scholar
Yeddes, W., Chalghoum, A., Aidi-Wannes, W., Ksouri, R. & Saidani Tounsi, M. Effect of bioclimatic area and season on phenolics and antioxidant activities of rosemary ( Rosmarinus officinalis L.) leaves. J. Essential Oil Res. 31 , 432–443. https://doi.org/10.1080/10412905.2019.1577305 (2019).
Christova-Bagdassarian, V. L., Bagdassarian, K. S., Atanassova, M. S. & Ahmad, M. A. Comparative analysis of total phenolic and total flavonoid contents, rutin, tannins and antioxidant capacity in Apiaceae and Lamiaceae families. Indian Hortic. J. 4 , 131–140 (2014). https://www.academia.edu/15503276/Comparative_Analysis_of_Total_Phenolic_and_Total_Flavonoid_Contents_Rutin_Tannins_and_Antioxidant_Capacity_in_Apiaceae_and_Lamiaceae_families?source=swp_share
Asgharian, S. et al. Ruta graveolens and rutin, as its major compound: Investigating their effect on spatial memory and passive avoidance memory in rats. Pharm. Biol. 58 , 447–453. https://doi.org/10.1080/13880209.2020.1762669 (2020).
Aissani, N., Albouchi, F. & Sebai, H. Anticancer effect in human glioblastoma and antioxidant activity of Petroselinum crispum L. methanol extract. Nutr. Cancer https://doi.org/10.1080/01635581.2020.1842894 (2020).
Farnad, N., Heidari, R. & Aslanipour, B. Phenolic composition and comparison of antioxidant activity of alcoholic extracts of Peppermint ( Mentha piperita ). J. Food Meas. Charact. 8 , 113–121. https://doi.org/10.1007/s11694-014-9171-x (2014).
Roby, M. H. H., Sarhan, M. A., Selim, K.A.-H. & Khalel, K. I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme ( Thymus vulgaris L.), sage ( Salvia officinalis L.), and marjoram ( Origanum majorana L.) extracts. Ind. Crops Prod. 43 , 827–831. https://doi.org/10.1016/j.indcrop.2012.08.029 (2013).
Dent, M., Dragović-Uzelac, V., Penić, M., Bosiljkov, T. & Levaj, B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in Dalmatian wild sage ( Salvia officinalis L.) extracts. Food Technol. Biotech. 51 , 84–91 (2013).
CAS Google Scholar
Mulinacci, N. et al. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: An HPLC/DAD/MS study. Talanta 85 , 167–176. https://doi.org/10.1016/j.talanta.2011.03.050 (2011).
Ramkissoon, J. S., Mahomoodally, M. F., Ahmed, N. & Subratty, A. H. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac. J. Trop. Med. 6 , 561–569. https://doi.org/10.1016/S1995-7645(13)60097-8 (2013).
Mestry, S. N., Dhodi, J. B., Kumbhar, S. B. & Juvekar, A. R. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J. Tradit. Complement. Med. 7 , 273–280. https://doi.org/10.1016/j.jtcme.2016.06.008 (2017).
Uysal, S., Zengin, G., Aktumsek, A. & Karatas, S. Chemical and biological approaches on nine fruit tree leaves collected from the Mediterranean region of Turkey. J. Funct. Foods 22 , 518–532. https://doi.org/10.1016/j.jff.2016.02.006 (2016).
Kaewnarin, K., Niamsup, H., Shank, L. & Rakariyatham, N. Antioxidant and antiglycation activities of some edible and medicinal plants. Chiang Mai J. Sci. 41 , 105–116 (2014).
Cai, Y., Luo, Q., Sun, M. & Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 74 , 2157–2184. https://doi.org/10.1016/j.lfs.2003.09.047 (2004).
Elfalleh, W. et al. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med. Plants Res. 6 , 4724–4730 (2012).
Fellah, B. et al. Untargeted metabolomics reveals changes in phenolic profile following in vitro large intestine fermentation of non-edible parts of Punica granatum L.. Food Res. Int. 128 , 108807. https://doi.org/10.1016/j.foodres.2019.108807 (2020).
Pinheiro, A. J. M. C. R. et al. Punica granatum L. leaf extract attenuates lung inflammation in mice with acute lung injury. J. Immunol. Res. 2018 , 1–11. https://doi.org/10.1155/2018/6879183 (2018).
Ulewicz-Magulska, B. & Wesolowski, M. Total phenolic contents and antioxidant potential of herbs used for medical and culinary purposes. Plant Food Hum. Nutr. 74 , 61–67. https://doi.org/10.1007/s11130-018-0699-5 (2019).
Ankita, P., Deepti, B. & Nilam, M. Flavonoid rich fraction of Punica granatum improves early diabetic nephropathy by ameliorating proteinuria and disturbed glucose homeostasis in experimental animals. Pharm. Biol. 53 , 61–71. https://doi.org/10.3109/13880209.2014.910533 (2015).
Generalić, I. et al. Influence of the phenophase on the phenolic profile and antioxidant properties of Dalmatian sage. Food Chem. 127 , 427–433. https://doi.org/10.1016/j.foodchem.2011.01.013 (2011).
Salwe, K. J. & Sachdev, D. Evaluation of antinociceptive and anti-inflammatory effect of the hydroalcoholic extracts of leaves and fruit peel of P. granatum in experimental animals. Asian J. Pharm. Clin. Res. 7 , 137–141 (2014).
AlFadel, F., Al Laham, S. & Alkhatib, R. The anti-bacterial activity of various parts of Punica granatum on antibiotics resistance Escherichia coli . Seeds 21 , 76 (2014).
Gheith, I. & El-Mahmoudy, A. Potent anti-oxidant and anti-inflammatory potentials of Punica granatum leaf and flower hydromethanolic extracts in vitro. Biosci. J. https://doi.org/10.14393/BJ-v33n2-33736 (2017).
Pararin, S., Rouhi, L. & Ghasemi Pirbalouti, A. The beneficial effect of hydro-alcoholic extract of Punica granatum L. leaves and flower on ethylene glycol-induced kidney calculi in RATS. J. Herb. Drugs (Int. J. Med. Herbs) 7 , 59–64 (2016).
Kiraz, Y., Neergheen-Bhujun, V. S., Rummun, N. & Baran, Y. Apoptotic effects of non-edible parts of Punica granatum on human multiple myeloma cells. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 37 , 1803–1815. https://doi.org/10.1007/s13277-015-3962-5 (2016).
Rummun, N., Somanah, J., Ramsaha, S., Bahorun, T. & Neergheen-Bhujun, V. S. Bioactivity of nonedible parts of Punica granatum L.: A potential source of functional ingredients. Int. J. Food Sci. 2013 , 602312. https://doi.org/10.1155/2013/602312 (2013).
Kapp, K. et al. Commercial peppermint ( Mentha × piperita L) teas: Antichlamydial effect and polyphenolic composition. Food Res. Int. 53 , 758–766. https://doi.org/10.1016/j.foodres.2013.02.015 (2013).
Meziane-Assami, D., Tomao, V., Ruiz, K., Meklati, B. Y. & Chemat, F. Geographical differentiation of rosemary based on GC/MS and fast HPLC analyses. Food Anal. Method 6 , 282–288. https://doi.org/10.1007/s12161-012-9430-6 (2013).
Khaleel, I. R., Kadhim, M. I. & Subhi, J. H. Effect of some biotic and abiotic elicitors on phenolic acids and diterpenes production from rosemary ( Rosmarinus officinalis L.) leaf and callus analyzed by high performance liquid chromatography (Hplc). Al-Nahrain J. Sci. 14 , 20 (2018).
Melnyk, M., Vodoslavskyi, V. & Obodianskyi, M. Research of phenolic compounds of ruta graveolens l and stellaria media (l.) vill. Asian J. Pharm. Clin. Res. 11 , 152–156 (2018).
Figueroa-Pérez, M. G. et al. Diabetic nephropathy is ameliorated with peppermint ( Mentha piperita ) infusions prepared from salicylic acid-elicited plants. J. Funct. Foods 43 , 55–61. https://doi.org/10.1016/j.jff.2018.01.029 (2018).
Dekanski, D. et al. Phytochemical analysis and gastroprotective activity of an olive leaf extract. J. Serb. Chem. Soc. 74 , 367–377. https://doi.org/10.2298/JSC0904367D (2009).
Makowska-Wąs, J. et al. Identification of predominant phytochemical compounds and cytotoxic activity of wild olive leaves ( Olea europaea L. ssp. sylvestris) harvested in South Portugal. Chem. Biodiv. 14 , e1600331. https://doi.org/10.1002/cbdv.201600331 (2017).
Skotti, E. et al. Biological activity of selected Greek medicinal and aromatic plants extracts on Alternaria alternata. Emirates J. Food Agric. 28 , 796–804. https://doi.org/10.9755/ejfa.2016-06-618 (2016).
Arruda, M. O. et al. The hydroalcoholic extract obtained from Mentha piperita L. leaves attenuates oxidative stress and improves survival in lipopolysaccharide-treated macrophages. J. Immunol. Res. 2017 , 2078794. https://doi.org/10.1155/2017/2078794 (2017).
Luthria, D. L., Mukhopadhyay, S. & Kwansa, A. L. A systematic approach for extraction of phenolic compounds using parsley ( Petroselinum crispum ) flakes as a model substrate. J. Sci. Food Agric. 86 , 1350–1358. https://doi.org/10.1002/jsfa.2521 (2006).
Hozayen, W. G., El-Desouky, M. A., Soliman, H. A., Ahmed, R. R. & Khaliefa, A. K. Antiosteoporotic effect of Petroselinum crispum , Ocimum basilicum and Cichorium intybus L. in glucocorticoid-induced osteoporosis in rats. Bmc. Complement. Altern. Med. 16 , 165. https://doi.org/10.1186/s12906-016-1140-y (2016).
Marques, L. C. et al. Anti-inflammatory effects of a pomegranate leaf extract in LPS-induced peritonitis. Planta Med. 82 , 1463–1467. https://doi.org/10.1055/s-0042-108856 (2016).
Swilam, N. & Nematallah, K. A. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci. Rep. 10 , 14851. https://doi.org/10.1038/s41598-020-71847-5 (2020).
Article ADS CAS PubMed PubMed Central Google Scholar
Akkawi, M., Abu-Lafi, S. & Abu-Remeleh, Q. Phytochemical screening of Pomegranate juice, peels, leaves and membranes water extracts and their effect on β-hematin formation, a comparative study. Pharm. Pharmacol. Int. J. 7 , 193–200 (2019).
Pinheiro, A. C. et al. Galloyl-hexahydroxydiphenoyl (HHDP)-glucose isolated from Punica granatum L. leaves protects against lipopolysaccharide (LPS)-induced acute lung injury in BALB/c mice. Front. Immunol. 10 , 1978. https://doi.org/10.3389/fimmu.2019.01978 (2019).
Lakshminarayanashastry Viswanatha, G., Venkatanarasappa Venkataranganna, M. & Lingeswara Prasad, N. B. Methanolic leaf extract of Punica granatum attenuates ischemia-reperfusion brain injury in Wistar rats: Potential antioxidant and anti-inflammatory mechanisms. Iran. J. Basic Med. Sci. 22 , 187–196. https://doi.org/10.22038/ijbms.2018.30660.7389 (2019).
Chen, J. et al. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 10 , 2611. https://doi.org/10.1038/s41598-020-59451-z (2020).
Xue, Z. & Yang, B. Phenylethanoid glycosides: Research advances in their phytochemistry, pharmacological activity and pharmacokinetics. Molecules (Basel, Switzerland) 21 , 991. https://doi.org/10.3390/molecules21080991 (2016).
Fraga-Corral, M. et al. Technological application of tannin-based extracts. Molecules 25 , 614. https://doi.org/10.3390/molecules25030614 (2020).
Yang, X. & Tomás-Barberán, F. A. Tea is a significant dietary source of ellagitannins and ellagic acid. J. Agric. Food Chem. 67 , 5394–5404. https://doi.org/10.1021/acs.jafc.8b05010 (2019).
Gouvinhas, I. et al. Monitoring the antioxidant and antimicrobial power of grape ( Vitis vinifera L.) stems phenolics over long-term storage. Ind. Crops Prod. 126 , 83–91. https://doi.org/10.1016/j.indcrop.2018.10.006 (2018).
Dambergs, R. G., Mercurio, M. D., Kassara, S., Cozzolino, D. & Smith, P. A. Rapid measurement of methyl cellulose precipitable tannins using ultraviolet spectroscopy with chemometrics: Application to red wine and inter-laboratory calibration transfer. Appl. Spectrosc. 66 , 656–664. https://doi.org/10.1366/11-06516 (2012).
Article ADS CAS PubMed Google Scholar
Mena, P. et al. Phytochemical characterisation for industrial use of pomegranate ( Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 91 , 1893–1906. https://doi.org/10.1002/jsfa.4411 (2011).
Download references
Acknowledgements
The experiments were approved by the FCT-Portuguese Foundation for Science and Technology (PD/BD/135333/2017), under the Doctoral Programme “Agricultural Production Chains-from fork to farm” (PD/00122/2012).
This research was funded by the FCT (Portuguese Foundation for Science and Technology) Grant number UIDB/04033/2020.
Author information
Authors and affiliations.
Centre for the Research and Technology of Agro-Environmental and Biological Sciences, CITAB, University de Trás-os-Montes e Alto Douro, UTAD, 5000-801, Vila Real, Portugal
Manyou Yu, Irene Gouvinhas & Ana I. R. N. A. Barros
Chemistry Centre-Vila Real, CQ-VR, UTAD, 5000-801, Vila Real, Portugal
Department of Chemistry, School of Life Sciences and Environment, UTAD, Quinta de Prados, 5001-801, Vila Real, Portugal
Ana I. R. N. A. Barros
You can also search for this author in PubMed Google Scholar
Contributions
M.Y. carried out data analysis, wrote the manuscript, and participated in all experimental measurements. I.G. developed and performed the chromatographic analysis. J.R. supervised botanical identification and sample collection. A.I.R.N.A.B. conceived all experiments, performed theoretical calculations, and supervised data analysis and interpretation. All authors reviewed the manuscript and participated in editing the manuscript.
Corresponding authors
Correspondence to Manyou Yu or Ana I. R. N. A. Barros .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information 1., supplementary information 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Yu, M., Gouvinhas, I., Rocha, J. et al. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci Rep 11 , 10041 (2021). https://doi.org/10.1038/s41598-021-89437-4
Download citation
Received : 04 January 2021
Accepted : 27 April 2021
Published : 11 May 2021
DOI : https://doi.org/10.1038/s41598-021-89437-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Insight into antioxidant-like activity and computational exploration of identified bioactive compounds in talinum triangulare (jacq.) aqueous extract as potential cholinesterase inhibitors.
- Olakunle Bamikole Afolabi
- Oluwaseun Ruth Olasehinde
- Oghenerobor Benjamin Akpor
BMC Complementary Medicine and Therapies (2024)
Integrated metabolomic and transcriptomic dynamic profiles of endopleura coloration during fruit maturation in three walnut cultivars
- Hengzhao Liu
- Huijuan Zhou
BMC Plant Biology (2024)
Chemoprofiling and medicinal potential of underutilized leaves of Cyperus scariosus
- Yashika Gandhi
- Vijay Kumar
- Thomas J. Webster
Scientific Reports (2024)
Rheological characteristics of wheat flour fortified with Musaceae flours and physicochemical properties of layered flat-bread (Parotta)
- K. Ashwath Kumar
- Crassina Kasar
- P. Prabhasankar
Journal of Food Measurement and Characterization (2024)
Physicochemical, antioxidant and antimicrobial characteristics of two types of mumies(shilajit)
- Sahar Elahi
- Ali Mohamadi Sani
- Mahboobe Sarabi-Jamab
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Open access
- Published: 01 April 2022
Phytochemical analysis of some selected traditional medicinal plants in Ethiopia
- Misganaw Gedlu Agidew 1
Bulletin of the National Research Centre volume 46 , Article number: 87 ( 2022 ) Cite this article
42k Accesses
45 Citations
Metrics details
This review of relevant medicinal plants is based on the fundamental knowledge accumulated by indigenous people of Ethiopia and to identify which types of selected medicinal plants for phytochemical analysis were analyzed and which one is not analyzed at Ethiopian levels. In this review, the most traditional medicinal plant species found and used in Ethiopia are chosen.
The qualitative phytochemical analysis, some of which are the most important phytochemicals such as phenolic, tannins, alkaloids, saponins, cardiac glycosides, steroids, terpenoids, flavonoids, phlobatannins, anthraquinones, and reducing sugars are studied by the researcher. Most studies have revealed that some phytochemicals are present in some medicinal plants while some are absent. The phytochemical properties of some species were studied like Artemisia afra (Ariti), Aloe Vera (Erret), Yzygium guineense (Dokuma), Ruta chalepensis (Tenadam), Ocimum grattissimum (Damakese), Nigella sativa (Tikur Azmud), Lepidium sativum (Feto), Hagenia abyssinica (Kosso), Croton macrostachyus (Bisana), and Rhamnus prinoides (Gesho).
Conclusions
This review has shown that traditional medicinal plants whose phytochemical properties are not studied have various medicinal purposes like treating mastitis, preventing boils, hemorrhoids, congestion, headache, hepatitis, liver, vertigo, stomatitis, kidneys, liver, and vision for treating anemia, hemorrhoid coughs, fluxes, and stomatitis in most animals and human beings. So that identifying the plants based on the investigation and analysis of phytochemical properties of such plant species are more important than Ethiopian levels.
Medicinal plants still play important roles in the daily lives of people living in developing countries of Asia and Africa, including Ethiopia. Medicinal plants not only serve as complements or substitutes for modern medical treatments, which are often inadequately available but also enhance the health and security of local people. Thus, these plants play indispensable roles in daily life and are deeply connected to diverse social, cultural, and economic events associated with life, aging, illness, and death (JAFICOAF 2008). Medicinal plants are used to treat and diagnose diseases and infections. From ancient times, plants have been rich sources of effective and safe medicines (Russell-Smith et al. 2006 ).
The world health organization (WHO) defined traditional medicine as the total combination of knowledge and practices that can be formally explained or used in the prevention and elimination of physical, mental, or social imbalance and relying exclusively on practical experience and observation handed down from generation to generation, whether verbally or in writing. About 75–90% of the rural population in the world (excluding western countries) relies on traditional medicines as their only health care system. This is not only because of poverty where people cannot afford to buy expensive modern drugs, but traditional systems are also more culturally acceptable and meet the psychological needs in a way modern medicine does not (Fassil Kibebe 2001 ).
Ethnomedicinal practices are believed to be one of the potential bases for the development of safe and effective treatments. Ethiopia has a long history of a traditional health care system, but studies on traditional medicinal plants (TMP) have been limited in comparison to the country’s multiethnic, cultural, and flora diversity (Fentahun et al. 2017 ), Also, the use of medicinal plants to treat infections is an old practice in large parts of Ethiopia to solve health problems for livestock and humans (Redda et al. 2014 ; Giday et al. 2009 ; Regassa 2013 ; Abera 2014 ; Tamene 2020 ; Mulatu 2020 ).
Increasing traditional medicines and natural plant products
The main phytochemical components, present in medicinal plants are tannins, alkaloids, saponins, cardiac glycosides, steroids, terpenoids, flavonoids, phlobatannins, anthraquinones, and reducing sugars. As proposed by WHO, the primary health care of most population of developing countries depend on traditional medicines and mostly natural plant products (Vines 2004 ). Like the worldwide countries, populations of Ethiopia use traditional medicines in both rural and urban areas. Traditional practice and activities have a long history in many areas in the Ethiopia and it will continue to give useful and applicable tools for treating disease (Helen et al. 2019 ).
Different traditional medicinal plant species are studied by different researchers in the world and in the Ethiopian. Ethiopia comprises people with many languages, cultures, and beliefs. This makes for a rich and diverse knowledge and practice of traditional medicine, including herbal remedies (Helen et al. 2019 ). There are different literature reviews that investigated and studied the Ethnobotanical and Ethnopharmacological evidence of some Ethiopian medicinal plants traditionally used for the Treatment of Cancer, skin problems, leprosy, and external parasites, Evil eye, and wound treatment in the Ethiopia. However, there is no report that could show phytochemical composition and its expanded pharmacological application in the folk medicine of some traditional medicinal plants in the country of Ethiopia. Moreover, this knowledge of identifications of studied and unstudied phytochemical composition of medicinal plants in Ethiopia can serve as the baseline data for researchers and analyzers for the further study of traditional medicinal plants in Ethiopia (Helen et al. 2019 ).
The medicinal power of traditional plants species lies in phytochemical components that cause definite pharmacological action on the human body (Naseem 2014 ). Based on their metabolism activity in the plant, phytochemicals components are generally can be mainly divided into two groups, which are primary which has mainly sugars, amino acids, chlorophyll and proteins, and secondary constituents while secondary constituents consist of alkaloids, flavonoids, saponins, tannins, phenolic compounds and many more (Krishnaiah 2007 ).
The most important components of the medicinal plant were isolated by the extraction methods by using the right solvent. Each researcher in the published articles in this review, different methods of extraction such as ethanol, methanol, chloroform, acetone, hexane, petroleum ether, ethyl acetate, and aqueous (water) were used to the phytochemical composition of plant species. The objective of this review was to collect and summarize the information about the medicinal plant and to classify the plants based on the studies of their phytochemical composition as well as this provides information for the research community to conduct further scientific investigations in Ethiopia’s medicinal plants.
Materials and methods
In this review, the data and information on the traditional medicinal plants in Ethiopia were collected from the published papers, which are available online in different forms such as books, published articles, and research reports. Different online sources such as Google Scholar and gray literature were the source of published articles by browsing the different words or terms like medicinal plants and Ethiopian traditional plants. For this review, scientific name, family name, local name, and important, obtained from the published articles that were obtained online, and the data are shown in Table 23 .
There are various traditional medicinal plants used to treat different illnesses and diseases in Ethiopia which did not describe plant species by scientific names; and review articles, are excluded. For this review paper, a total of 53 plant species that are recognized and grown in Ethiopia are documented. From those plant species, the phytochemical composition of some plant species is studied by a researcher and some are not studied. The most important components of the medicinal plant were isolated by the extraction methods by using different solvents. In all reported literature, different solvent such as ethanol, methanol, chloroform, acetone, hexane, petroleum ether, ethyl acetate, and water was used as solvent.
The main aim of this review is to collect and summarize the information about the medicinal plant and to classify the plants based on the studies of their phytochemical composition as well as to provide information for the research community to conduct further scientific investigations on the Ethiopia medicinal plants.
Results and discussions
Phytochemical analysis.
Traditional medicine plays a significant role in the healthcare of the people in developing countries, including Ethiopia, and medicinal plants provide a valuable contribution to this practice (Tesfahuneygn and Gebreegziabher 2019 ). In this review, around 33 medicinal plants species were identified from published articles. The different parts of the plant such as root, leaves, and fruit, in which these different parts have many traditional values, pharmacological uses, and phytochemical constituents were mentioned. From few medication values of plant parts, to treat rheumatism, madness, snakebite, chest pain, jaundice chest pain, malaria, headache, cough, etc. All the medicinal plants are shown in the table form with the scientific name, families, local name, and importance. Most plants were reported and investigated in Ethiopia. As reported by many authors, some medicinal plants with their scientific name, family, local name and their importance are shown in Table 23 , and these plant species listed in this review were often used by the people in Ethiopia.
Phytochemicals
Analysis of the phytochemical properties of the medicinal plants used to show and isolate the drug, lead compounds and components from the parts of the plant. The unique biological activity of the plants can be identified by their phytochemicals properties. Most parts of the plants used for the analysis of the phytochemical properties were leaves, roots, stem barks, and fruits. In this review, medicinal plants were investigated for phytochemical constituents of ethanol, methanol, chloroform, acetone, hexane, petroleum ether, ethyl acetate, and aqueous (water) extraction of different phytochemicals.
In this review, the most published articles recognized the presence of phytochemical components in the plants was indicated by the positive sign (+) and the absence of phytochemical components in the plants, by the negative sign (−) as shown in table.
Alkaloids are one of the main and largest components produced by plants, and they are metabolic byproducts that are derived from the amino acids (Naseem 2014 ). Based on the published articles in these reviews, alkaloids were extracted from the different parts of the plants using different solvents such as ethanol, methanol, chloroform, acetone, hexane, petroleum ether, ethyl acetate, and aqueous (water). These types of solvents extract phytochemical components from medicinal plants like leaves, roots, stem bark, and fruits.
Flavonoids consist of a large group of polyphenol compounds having a benzoyl-γ-pyrone structure and are ubiquitously present in plants. They are synthesized by the phenylpropanoid pathway. Available reports tend to show that secondary metabolites of a phenolic nature including flavonoids, are responsible for the variety of pharmacological activities (Mahomoodally et al. 2005 ; Pandey 2007 ). Flavonoids are hydroxylated phenolic substances and are known to be synthesized by plants in response to microbial infection (Dixon et al. 1983 ). In this review, flavonoids were detected in most plant species but in some medicinal plants were not present the same plant but different solvents like eucalyptus and Agenda Abyssinia leaves.
The term tannin is widely applied to a complex large biomolecule of polyphenol nature having sufficient hydroxyls and other suitable groups such as carboxyl to form strong complexes with various macromolecules (Navarrete 2013 ). In this present review, tannins were detected in most plant species like peel and juice of Citrus medica, mango ( Mangifera indica L .) leaves, Avocado fruit ( Persea Americana ), Dioscorea alata leaf , of Leucas aspera L . leaf and root, Ocimum gratissimum Linn leaf, Rhamnus prinoides root, extract of Rhizomes , Zingiber officinale and Curcuma longa and also for different solvent give different response for the same plant species like Bersama abyssinica leaf, F lax seeds , Nigella sativa , Ruta chalepensis leaves, and Syzygium guineense and not totally detected in part of plants like Lepidium sativum seeds and love Gilbetii root. Tannins are generally used in the tanning process and used as healing agents in inflammation, burn, piles, and gonorrhea (Boroushaki et al. 2016 ).
Saponins are an important group of plant secondary metabolites that are widespread throughout the plant kingdom. Saponins are basically phytochemicals that are found in most vegetables, beans, and herbs (Francis et al. 2002 ; Haralampidis et al. 2002 ). In this review, saponins were detected in most medicinal plants like citrus fruit juice , of Mango ( Mangifera indica L .) leaves, Avocado fruit ( Persea americana ), Leucas aspera L . leaf, and root, Rhamnus prinoides root, Bitter ( Vernonia amygdalina ) leaf and Stem bark of Vernonia amygdalina in common plant species and some plants were shown different results, that depends on solvent and also not totally detected in part of the plant such as Bersama abyssinica leaf, Dioscorea alata leaf, love Gilbertii root, and Flax seeds .
The word steroid is derived from sterol, which is a natural or synthetic chemically active hormone-like element. A steroid is one of a large group of chemical substances classified by a specific carbon structure. Steroids include drugs used to relieve swelling and inflammation, such as prednisone and cortisone; vitamin D; and some sex hormones, such as testosterone and estradiol (Hill et al. 2007 ). For this review, Steroids were detected in most plant species like citrus fruit juice , peel and juice of citrus Medica , Flaxseeds , Nigella sativa , Ocimum gratissimum Linn leaf, Syzygium guineans root, and Root and Stem bark of Vernonia amygdalina in common plant species while in some plant species were shown variable result that depends on the given solvents and not totally detected in the part of the plant like Rhamnus prinoides root.
Terpenoids are small molecular products synthesized by plants and are probably the most widespread group of natural products. Terpenoids show significant pharmacological activities, such as antiviral, antibacterial, antimalarial, anti-inflammatory, inhibition of cholesterol synthesis, and anti-cancer activities (Boroushaki et al. 2016 ). As mentioned earlier, Terpenoids were detected in most analysis plant species such as citrus fruit juice , Hagenia abyssinica leaves, Leucas aspera L . leaf and root, Flax seeds , Ocimum gratissimum linn leaf, Ruta chalepensis leaves, and Syzygium guineans root while in some plants its result depends on the types of solvents.
Phenolic compounds are secondary metabolites, which are produced in the shikimic acid of plants and pentose phosphate through phenylpropanoid metabolization (Derong Lin et al. 2016 ). In this review, phenolic was detected in most the medicinal plants like citrus fruit juice, peel and juice of citrus medica, mango ( Mangifera indica L .) leaves and Avocado fruit ( Persea Americana ), eucalyptus leaves, Flax seeds , Rhamnus prinoides root, of Rhizomes , Zingiber officinale, and Curcuma longa but some medicinal plant is given different response and depend on the solvents.
Even though there are so many medicinal plants in Ethiopia, this review of the phytochemical analysis shows that some medicinal plants were studied by the investigator in different areas of Ethiopia, while some traditional plants are not studied. According to the data of published articles, the extraction techniques of the medicinal plants were mainly digestion and aqueous-alcohol extraction. From Tables show that phytochemical investigation results are available in the Ethiopia area levels.
Above the Table 1 , phytochemical screening of alkaloids, tannins, saponins, flavonoids, phenols and phytosterols were the secondary metabolites found in the crude extract of Echinops amplexicaulis , Ruta chalepensis , and Salix subserrata . The methanol extracts of Echinops amplexicaulis and Salix subserrata contain most of the secondary metabolites.
In terms of the qualitative phytochemical investigation of the medicinal plants, the medicinal plants extract had different phytochemicals constituents such as saponins, tannins, alkaloids, terpenoids, anthraquinones, phenolic compounds, cardiac glycosides, and flavonoids (Table 2 ).
Phytochemical investigations from these medicinal plants have shown a large number of organic complex and biologically active compounds.
The results of the qualitative phytochemicals analysis showed that the leaf extracts of Lippia adonis var. koseret also indicated the presence of tannins, flavonoids, polyphenols, alkaloids and saponins, while in the case of ethyl acetate alkaloids were not detected and tannins were absent in petroleum ether extract (Table 3 ). Amino acids and carbohydrates were absent in all three extracts.
In this review, phytochemical screening of Bersama abyssinica leaf in Table 4 shown that the most published articles recognized the presences of specific phytochemical components in the plants was indicated by the positive sign (+) and the absence of phytochemical components in the plants, by the negative sign (−). These phytochemical constituents in Bersama abyssinica leaf were shown variable results that depend on the given solvents and are not totally detected in Bersama abyssinica leaf.
The results in Table 5 show that there are phytochemical components in Citrus fruit juice concentrates. These phytochemical constituents all are found in citrus fruit juice concentrates except cardiac glycosides were not detected in lemon and they indicated highly medicinal values. It can be suggested that the presence of phenols, alkaloids, flavonoids, saponins, steroids, and reducing sugar in Citrus fruit juice indicates are highly medicinal value.
From Table 6 , flavonoids, phenols, tannins, steroids, coumarin and cardioactive glycosides: have shown positive tests of ethyl acetate, and methanol extracts of peel and juice of citrus medica, while some phytochemical positive test and totally not detected like (anthraquinones, alkaloids, and terpenoids). These secondary metabolites are known to be biologically active and play significant roles in the bioactivity of medicinal plants because the medicinal values of the medicinal plant lie in these phytochemical compounds which produce a definite and specific action on the human body.
Based on the given data from Table 7 , phytochemical screening of ethanol extract of mango ( Mangifera indica L .) leaves and Avocado ( Persea americana ) fruits almost all are were detected but terpenoids were not detected in Mango ( Mangifera indica L .). The phytochemical are naturally occurring chemicals in plants which serve as medicinal for the protection of human disease; the phytochemicals are nonnutritive plants chemical that have protection or disease preventive properties.
In this review, the phytochemical analysis revealed the presence of flavonoids, phenols, and tannins while the terpenoids positive test of methanol extract and the remaining phytochemical components are were not detected. These results show that phytochemical depend on solvents (Table 8 ).
Table 9 , the presence of flavonoid, tannin, and phenol in methanol extract. The acetone extract obtained from the eucalyptus leaves was screened for phytochemicals. Qualitative phytochemical screening of acetone extract of eucalyptus leaves demonstrated the presence of saponins, carbohydrate, tannin, and phenol, while quinone, fat, protein, and flavonoid were absent.
In this review, the methanol, ethanol, n-hexane, and petroleum ether extract obtained from the Hagenia abyssinica leaves were screened for various phytochemicals from Table 10 . Qualitative phytochemical screening of methanol extract of Hagenia abyssinica leaves demonstrated the presence of saponins, flavonoids, phenols, terpenoids, steroids, and glycosides, while tannins, anthraquinones, and alkaloids were absent. Phytochemical analysis of ethanol extract of Hagenia abyssinica leaves demonstrated the presence of saponins, tannins, phenols, terpenoids, and alkaloids, while steroids, glycosides and phlobatannins were absent. A similarity that phytochemical screening of n -hexane extract of Hagenia abyssinica leaves demonstrated the presence of flavonoids, anthraquinones and terpenoids but saponins, tannins, alkaloids, steroids, glycosides, and phlobatannins are not detected and Hagenia abyssinica leaves extracted by petroleum ether were obtained presence of phytochemical only saponins and terpenoids, while other phytochemicals are not detected.
Phytochemicals screening in the plant extracts revealed the presence of flavonoid, stereol and polyterpenes, and saponified present in both methanol and ethyl acetate extract of Lepidium sativum s eeds and also flavonoids were present in petroleum ether extract of Lepidium sativum seeds while other phytochemical components were not detected (Table 11 ).
In this review, phytochemical screening of the aqueous, methanol, and hexane extracts of Leucas aspera L . leaf and root revealed the presence of various medically active constituents from Table 12 . Almost all phytochemical compounds present in the aqueous, methanol, and hexane extracts of Leucas aspera L . leaf and root were identified except cholesterol and steroids in the parts of leaf and root by aqueous. These plants indicate highly medicinal values.
Phytochemical screening of the love Gilbertii root suggests the presence of major phytochemicals in the root extracts (Table 13 ). Dichloromethane: methanol of roots showed the presence of alkaloids, anthraquinones, and flavonoids whereas; tannins, saponins, and terpenoids were not presented.
As result in Table 14 , screening for phytochemicals in the plant extracts almost all presents in both acetone and methanol extracts of Flax seeds, while some phytochemical is not detected like tannins, saponins in acetone extract of Flax seeds and also saponins were presented by methanol extract of flaxseeds. In addition to this phytochemicals screening of ethanol and water extract of flaxseeds almost phytochemical components presents and some phytochemicals not totally detected. These secondary metabolites are known to be biologically active and play significant roles in the bioactivity of medicinal plants because the medicinal values of the medicinal plant lie in these phytochemical compounds which produce a definite and specific action on the human body.
This review was shown in the (Table 15 ) phytochemical analysis of petroleum ether and ethyl acetate seed extract of Nigella sativa contains tannins, steroids, terpenoids and alkaloids, flavonoids, phenol, glycosides and steroids were found in the extract and are potent methanol soluble while some phytochemicals were not presented since it depends on the solvents.
In the present review, phytochemical screening of methanol and aqueous extracts of Ocimum gratissimum Linn leaf showed that the presence of tannins, phlorotannins, steroids, terpenoids, flavonoids and cardiac glycosides with steroidal ring whereas, saponins and sugar were not present in methanol solvent and also alkaloids were not absent in Table 16 . These detected phytochemical compounds are known to have beneficial importance in medicinal as well as physiological activities. In this manner, isolating and identifying these bioactive compounds, new drugs can be formulated to treat various diseases and disorders.
Table 17 shows the phytochemicals detected in Rhamnus prinoides root extract. Tests for triterpenes, saponins, tannins, phenols, glycosides, cardiac glycosides, and resins were positive in both aqueous and methanol/water extracts. Alkaloids were detected only in the methanol/water extract while steroids, flavonoids, flavones, and anthraquinones were not detected in both aqueous and methanol/water extracts. These phytochemicals may be responsible for the medicinal value of Rhamnus prinoides .
Phytochemical screening of ethanol/water (1:1) extract of Rhizomes, Zingiber officinale, and Curcuma longa showed the presence of phenolic, flavonoids, glycosides, and tannins whereas alkaloids were not present (Table 18 ).
The phytochemical analysis of Ruta chalepensis leaves extract in methanol showed that phytochemical components include; alkaloids, flavonoids, terpenoids, cardiac glycosides, phenols, saponins, tannins and anthraquinones and steroids were not present. Steroids, terpenoids and saponins were additionally present in both ethyl acetate and acetone extract, and also flavonoids, terpenoids, and anthraquinones were detected in the n-hexane extract, while others were not totally found in Table 19 .
In Table 20 , the presence of steroids, terpenoids, saponins, flavonoids, flavonoids, tannins, alkaloids, phenol, and glycosides were present in both dichloromethane/methanol and methanol extracts and steroids and terpenoids also were present in n-hexane extract whereas other phytochemicals components were not detected.
From Table 21 , it can be seen that the sample extracts showed positive tests for the presence of alkaloids, saponin, tannins, phlorotannin, glycosides, and flavonoids except for anthraquinones. Therefore, Bitter ( Vernonia amygdalina ) is the most frequently used for medicinal purposes.
In this review, the results revealed the presence of alkaloids, steroids, glycosides, saponin, and phlorotannin methanol extracts from the root and stem bark of Vernonia amygdalina whereas only tannins and phenols were not detected (Table 22 ). Therefore, the phytochemical screening results reveals that the presence of these phytochemical constituents supports the use of the Vernonia amygdalina plant in folklore medications and it is probable that these phytochemicals are responsible for the healing properties.
A total of 53 traditional medicinal plants were identified in this review. All of the reviewed plants have direct traditional uses for treating either ailment with cancer-like symptoms (determined by the traditional practitioner) or for laboratory-confirmed cancer cases. Medicinal plants have continued to be the most affordable and easily accessible source for the treatment of several human and livestock ailments in Ethiopia. Besides treating cancer, the plants selected in this review are also cited for their various traditional uses, including for the treatment of eczema, leprosy, rheumatism, gout, ringworm, diabetes, respiratory complaints, warts, hemorrhoids, syphilis, and skin diseases (Table 23 ). The output calls for the need for further phytochemical and pharmacological investigation giving priority to those plants which have been cited most for their use to treat cancer.
In Ethiopia, there are increasing demands for many most popular, more available, and effective plant species by the people. As stated by the different authors in the above Tables, different phytochemicals were investigated in different plant species with different solvent concentrations. Even though different phytochemicals were analyzed for different plant species, their concentration varied from one plant species to another plant species for different parts of the plant. Based on the above information from the Table, one type of phytochemical cannot be detected in all plant species and the concentration of one phytochemical content varies from one part of the plant to another part which mean the concentration of one phytochemical content in leaves can vary from the concentrations of phytochemical contents in root and fruits. Generally, even though there are various medicinal plants in Ethiopia, there are no studies that show enough information about qualitative and quantitative phytochemical contents for most plant species in the country. This may be due to the lack of enough laboratory facilities and modern technology available in the country for improving the synthesis and extraction of phytochemical components for developing the new drug product and drug leading compounds from the different parts of the medicinal plants by the government and private company.
In conclusion, this study showed the wide use of medicinal plants in Ethiopia. Even though there is a wealth of indigenous knowledge transfer is declining from generation to generation as a result of oral transmission. Human beings around the world have spent their lives for a long time to discovering a new drug to diagnose, prevent and treat various diseases. To save their lives from dangerous diseases, a new and powerful drug must be discovered and developed from the different parts of the plant. In order to future promote for development of new drug synthesis and extraction of bioactive components from the parts of the plant, availability, and value of information is very important. From tables, phytochemicals analysis of different medicinal plants revealed the presence of various bioactive compounds such as polyphenols, flavonoids, phenolic compounds alkaloids, saponins, tannins, phlobatannins, glycosides, anthraquinones, steroids, terpenoids, and triterpene. Based on the above data available in the review, most phytochemical components of traditional medicinal plants in Ethiopia are not analyzed. This leads to more traditional plants in Ethiopia are not being recognized by the international scientific organization, not how to use medicinal plants for disease treatment and they do not have scientific names. This review recommended finding further most common medicinal plants to investigate in scientific research and to governing them in the scientific naming system and as well as further studies should focus on green synthesis of heavy metals on different types of medicinal plants in Ethiopia. Based on this review, the studied phytochemical characteristics of medicinal plants in Ethiopia are few, so further study could be needed for examining, and characterizing the properties of unrecognized plant species in Ethiopia.
Availability of data and materials
The datasets used during the current study are available online in different forms such as books, various published journals and google scholar.
Abbreviations
Cirsium Englerianum
Cucumis Pustulatus
Discopodium Penninervium
Euphorbia Depauperata
Lippia Adoensis
Polysphaeria Aethiopica
Rumex Abyssinica
World Health Organization
Abdurohaman Mengesha Yessuf (2015) Phytochemical extraction and screening of bio active compounds from black cumin (Nigella Sativa) seeds extract. Am J Life Sci 3(5):358–364
Google Scholar
Abera B (2014) Medicinal plants used in traditional medicine by Oromo people, Ghimbi District, Southwest Ethiopia. J Ethnobiol Ethnomed 10(1):1–15. https://doi.org/10.1186/1746-4269-10-4
Article Google Scholar
Abhishek D, Dipankar C, Nikhil BG, Anupam C, Nripendranath M (2014) phytochemical analysis, antioxidant and anticancer potential of leaf extracts from edible greater yam, dioscorea alata l., from north-east India. Int J Phytopharmacol 5(2):109–119
Akinmoladun AC, Ibukun EO, Afor E, Obuotor EM, Farombi EO (2007) Phytochemical constituent and antioxidant activity of extract from the leaves of Ocimum gratissimum . Sci Res Essay 2(5):163–166
Alemayehu M, Welday D (2021) Comparative study of the antioxidant and antibacterial activities of Rumex abyssinicus with commercially available Zingiber ofcinale and Curcuma longa in Bahir Dar city, Ethiopia. Chem Biol Technol Agricn 8:2
Alves RRN, Rosa IML (2007) Biodiversity, traditional medicine and public health: where do they meet? J Ethnobiol Ethnomed 3:1–9
Amare F, Getachew G (2019) An ethnobotanical study of medicinal plants in chiro district, West Hararghe, Ethiopia. Afr J Plant Sci 13:309–323. https://doi.org/10.5897/AJPS2019.1911
Amde A, Masresha G, Hansha H, Asafa O (2020) Ethnobotanical study of traditional medicinal plants in Debark District, North Gondar, Ethiopia. Int J Sci Res Multidiscip Stud 6(11):16–23
Aragaw TJ, Afework DT, Getahun KA (2020) Assessment of knowledge, attitude, and utilization of traditional medicine among the communities of Debre Tabor Town, Amhara Regional State, North Central Ethiopia: a cross-sectional study. Evid Based Complement Altern Med e6565131. Available from: https://www.hindawi.com/journals/ecam/6565131/
Arias BA, Ramón-Laca L (2005) Pharmacological properties of citrus and their ancient and medieval uses in the Mediterranean region. J Ethnopharmacol 97:89–95
Article PubMed Google Scholar
Asgarpanah J, Khoshkam R (2012) Phytochemistry and pharmacological properties of Ruta graveolens L. J Med Plants Res 6:3942–3949
CAS Google Scholar
Atanu FO, Ebiloma UG, Ajayi EI (2010) A review of the pharmacological aspects of Solanum nigrum Linn. Biotechnol Mol Biol Rev 6:01–07
Aung EE, Kristanti AN, Aminah NS, Takaya Y, Ramadhan R (2020) Plant description, phytochemical constituents and bioactivities of Syzygium genus : a review. Open Chem 18:1256–1281
Article CAS Google Scholar
Aveen NA (2015) Phytochemical analysis and evaluation antibacterial activity of Citrus medica peel and juice growing in Kurdistan/Iraq. J Appl Pharmaceut Sci 5(10):136–141
Badreldin HA et al (2008) Some phytochemical, pharmacological and toxicological properties of ginger ( Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol 48:409–420
Bosch CH (2008) Bersama abyssinica Fresen. In: Schmelzer GH, Gurib-Fakim A (eds) Medicinal plants/Plantes médicinales. PROTA, Wageningen, Netherlands
BNT, Kim Y (2022) Ethnobotanical study of Medicinal plants used as Antimalarial and Repellent by Sidama people of Hawassa Zuria district, Southern Ethiopia. https://www.jocmr.com/?mno=15231 . Accessed 19 Feb. https://doi.org/10.5455/jcmr.20181102063241
Boroushaki MT, Mollazadeh H, Afshari AR (2016) Pomegranate seed oil: a comprehensive review on its therapeutic effects. Int J Pharm Sci Res 7(2):430
Chatoui K, Talbaoui A, Aneb M, Bakri Y, Harhar H, Tabyaoui M (2016) Phytochemical screening, antioxidant and antibacterial activity of Lepidium sativum seeds from Morocco. J Mater Environ Sci 7(8):2938–2946
De Castro Moreira ME, Pereira RGFA, Dias DF, Gontijo VS, Vilela FC, de Moraes GDOI, Giusti-Paiva A, dos Santos MH (2013) Anti-inflammatory effect of aqueous extracts of roasted and green Coffea Arabica L. J Funct Food 5(1):466–474
Derong L, Xiao M, Zhao J, Li Z, Xing B, Li X, Kong M, Li L, Zhang Q, Liu Y, Chen H, Qin W, Wu H, Chen S (2016) An overview of plant phenolic compounds and their importance in human nutrition and management of type diabetes. Molecules 21:1374
Dixon RA, Dey PM, Lamb CJ (1983) Phytoalexins: enzymology and molecular biology. Adv Enzymol Relat Areas Mol Biol 55:1–136
CAS PubMed Google Scholar
Djoukeng JD et al (2005) Antibacterial triterpenes from Syzygium guineense (Myrtaceae). J Ethnopharmacol 1:283–286
Fentahun S, Makonnen E, Awas T, Giday M (2017) In vivo antimalarial activity of crude extracts and solvent fractions of leaves of Strychnos mitis in Plasmodium berghei infected mice. BMC Complement Altern Med 17(1):1–12
Lalisa WD, Daniel M (2017) Phytochemical screening and antioxidant activity of selected mango ( Mangifera indica L.) and avocado ( Persea americana ) fruits in illu Ababor zone, Oromia regional state, Ethiopia indo. Am J Pharmaceut Res
Ehigbai IO, Omoregie ES, Oviasogie FE, Oriakhi K (2016) Phytochemical, antimicrobial, and antioxidant activities of different citrus juice concentrates. Food Sci Nutr 4(1):103–109
Hussein AEM et al (2013) Antioxidant activity of Vicia faba L. Vicine and its
Milkyas E, Berhanu E, Israel A, Belayhun K, Fikre M (2016) Phytochemical analysis of roots of Aloe gilbertii and Millettia ferruginea . J Sci Develop 4(1)
Farombi EO, Owoeye O (2011) Antioxidative and chemopreventive properties of Vernonia amygdalina and Garcinia bi flavonoid. Int J Environ Res Public Health 8:2533–2555
Article PubMed PubMed Central Google Scholar
Francis G, Zohar K, Harinder PS, Klaus B (2002) The biological action of saponins in animal systems. Br J Nutr 88:587–605
Article CAS PubMed Google Scholar
Giday M, Asfaw Z, Woldu Z (2009) Medicinal plants of the Meinit ethnic group of Ethiopia: an ethnobotanical study. J Ethnopharmacol 124(3):513–521. https://doi.org/10.1016/j.jep
Hanaa MH, Ismail HA, Mahmoud ME, Ibrahim HM (2017) Antioxidant activity and phytochemical analysis of flaxseeds ( Linum usitatisimum L.). Minia J Agric Res Develop 37(1):129–140
Haralampidis K, Trojanowska M, Osbourn AE (2002) Biosynthesis of triterpenoid saponins in plants. Adv Biochem Eng Biotechnol 75:31–49
Helen B, Haftom G, Kald BT, Mariamawit Y (2019) Ethiopian medicinal plants traditionally used for wound treatment: a systematic review. J Health Dev 33:2
Hill JA, Suker JR, Sachs K, Brigham C (2007) The athletic po1ydrug abuse phenomenon. Am J Sports Med 8(4):269–271
Audu I, Shuaibu BS, Alona CL, Murtala MM, Jiya JA (2018) Phytochemical analysis and antimicrobial activity of bitter leaf ( Vernonia amygdalina ) collected from Lapai, Niger state, Nigeria on some selected pathogenic microorganisms. Sci World J 13
Israili ZH, Lyoussi B (2009) Ethnopharmacology of the plants of genus Ajuga. Pak J Pharm Sci 22:425–462
Japan Association for International Collaboration of Agriculture and Forestry (2008) Medicinal crops” in Ethiopia Current status and future potentials. Japan. Available at: http://www.jaicaf.or.jp/publications/ethiopia_en.pdf
Kebede T, Gadisa E, Tufa A (2021a) Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: a possible alternative in the treatment of multidrug-resistant microbes. https://doi.org/10.1371/journal.pone.0249253 .
Taye K, Eshetu G, Abreham T (2021b) Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: a possible alternative in the treatment of multidrug-resistant microbes
Ketema AD, Engidaw SM (2019) Phytochemical analysis, antibacterial and antioxidant activity of the leave extracts of Ruta chalepensis . Chem Mater Res11
Fassil K (2001) The status and availability of oral and written knowledge on traditional health care in Ethiopia. In: Conservation and sustainable use of medicinal plants in Ethiopia, pp 107–119
Krishnaiah D, Sarbatly R, Bono A (2007) Phytochemical antioxidants for health and medicine a move towards nature. Biotechnol Mol Biol Rev 2(4):97–104
Kuiate JR et al (2006) Chemical composition and antidermatophytic properties of volatile fractions of hexanic extract from leaves of Cupressus lusitanic a Mill. From Cameroon. J Ethnopharmacol 2:160–165
Kumar HD, Laxmidhar S (2011) A review on phytochemical and pharmacological of eucalyptus globulus: a multipurpose tree. Int J Res Ayurveda Pharm 2:1527–1530
Londhe VP, Gavasane AT, Nipate SS, Bandawane DD, Chaudhari PD (2011) Role of garlic ( Allium sativum ) in various diseases: an overview. J Pharmaceut Res Opin 4:129–134
Machocho AK et al (2003) Pentacyclic triterpenoids from Embelia schimperi . Phytochemistry 4:573–577
Mahomoodally MF, Gurib-Fakim A, Subratty AH (2005) Antimicrobial activities and phytochemical profiles of endemic medicinal plants of Mauritius. Pharmaceut Biol 43:237–242
Lencho MM, Getachew MD, Dagmawit AB, Edilu JS, Askale G, Wakuma MB, Morka DB, Petros A, Abraham M, Miressa T, Kebede A, Dejene B (2021) Phytochemical screening and in-vitro evaluation of antibacterial activities of Echinops amplexicaulis , Ruta chalepensis and Salix subserrata against selected pathogenic bacterial strains in West Shewa Zone, Ethiopia. J Exp Pharmacol
Marzouk MS et al (2006) Deglycosylation product divicine. Int J Pharma Sci 3:200–205
Mathewos A, Feleke W, Solomon L, Fikre M, Milkyas E (2015) Phytochemical screening and antibacterial activity of leaves extract of Bersama abyssinica . J Adv Bot Zool 3:2
Mbwambo ZH et al (2012) Phytochemical and pharmacological investigations of Garcinia volkensii Engl. J Complement Med Drug Discov 2:1–7
Melkamu FF, Padmanabhan R, Gurmessa GT (2016) Phytochemical investigation and in vitro antibacterial evaluation on root extracts of Rumex abyssinicus . Nat Prod Chem Res
Mengistu M, Kebede D, Oncho D, Abebe A, Alemnie D (2019) Status and utilization of medicinal and aromatic plants in Eastern Hararghe, Ethiopia. Cogent Food Agric. https://doi.org/10.1080/23311932.2019.1701349201
Mulatu G (2020) Antibacterial activities of Calpurnia aurea against selected animal pathogenic bacterial strains. Adv Pharmacol Pharm Sci. https://doi.org/10.1155/2020/8840468
Musarra-Pizzo M, Ginestra G, Smeriglio A, Pennisi R, Sciortino MT, Mandalari G (2019) Antimicrobial and antiviral activity of polyphenols from almond ( Prunus dulcis L.) skin. Nutrients 11(10):2355
Article CAS PubMed Central Google Scholar
Mworia JK, Ngeranwa J, Ngugi M (2020) Analgesic potential of dichloromethane leaf extracts of Eucalyptus globulus (Labill) and Senna didymobotrya (Fresenius) in mice models. J Herbmed Pharmacol. https://doi.org/10.34172/jhp.49
Naseem U, Muhammad Z, Farhat Ali K, Shazeb K (2014) A review on general introduction to medicinal plants, its phytochemicals and role of heavy metal and inorganic constituents. Life Sci 11(7s):520–527
Navarrete P, Pizzi A, Pasch H, Rode K (2013) Delmotte characterization of two maritime pine tannins as wood adhesives. J Adhes Sci Technol 27(22):2462–2479
Nayak BS, Vinutha B, Sudha B (2005) Experimental evaluation of Pentas lanceolata flowers for wound healing activity in rats. Fitoterapia 7:671–675
Ngo Bum E et al (2012) Decoctions of Bridelia micrantha and Croton macrostachyus may have anticonvulsant and sedative effects. Epilepsy Behav 3:319–323
Oloyede O et al (2011) Antioxidative properties of ethyl acetate fraction of unripe pulp of carica papaya in mice. J Microbiol 1:409–425
Pandey AK (2007) Anti-staphylococcal activity of a pan-tropical aggressive and obnoxious weed Parihenium histerophorus: an in vitro study. Natl Acad Sci Lett 30:383–386
Prabhu KS et al (2009) Ocimum gratissimum : a review of its chemical, pharmacological and ethnomedicinal properties. Open Complement Med J 1:1–15
Prabhu KS, Lobo R, Shirwaikar AA, Shirwaikar A (2009) Ocimum gratissimum : a review of its chemical, pharmacological and ethnomedicinal properties. Open Complement Med J 1:1–15
Raimi CO, Oyelade AR, Adesola OR (2020) phytochemical screening and in-vitro antioxidant activity on Vernonia amygdalina (ewuro- bitter leaf). Eur J Agric for Res 8(2):12–17
Rather MA et al (2014) Foeniculum vulgare : a comprehensive review of its traditional use, phytochemistry, pharmacology, and safety . Arab J Chem
Redda YT, Kebede E, Cruz C, Gugsa G, Awol N, Mengeste B (2014) Potential antibacterial activity of crude extracts from Aloe vera, Zingiber officinale and Vinca major medicinal plants. Intl J 5(3):202–207
Regassa R (2013) Assessment of indigenous knowledge of medicinal plant practice and mode of service delivery in Hawassa city, southern Ethiopia. J Med Plants Res 7(9):517–535
Reyes-Escogido MDL, Gonzalez-Mondragon EG, Vazquez-Tzompantzi E (2011) Chemical and pharmacological aspects of capsaicin: a review. Molecules 16:1253–1270
Russell-Smith J, Karunaratne NS, Mahindapala R (2006) Rapid inventory of wild medicinal plant populations in Sri Lanka. Biol Cons 132(1):22–32
Sharma NK et al (2009) Medicinal and pharmacological potential of Nigella sativa: a review. Ethnobotanical Rev 13:946–955
Shubhreet K, Saurabh G, Priyae BG (2019) Phytochemical analysis of Eucalyptus leaves extract. J Pharmacogn Phytochem 8(1):2442–2446
Stapf, Croton macrostachyus Del. Against Anopheles arabiensis patton, a potent malaria vector. Eur Rev Med Pharmacol Sci 14:57–62
Sumanthy V et al (2011) In vitro bioactivity and phytochemical screening of musa Acuminata flower. Pharmacology 2:118–127
Tamene S (2020) Ethnobotanical study of indigenous knowledge on medicinal plant uses and threatening factors around the Malga District, Southern Ethiopia. Int J Biodivers Conserv 12(3):215–226. https://doi.org/10.5897/IJBC2020.1416
Tefera B, Kim Y (2019) Ethnobotanical study of medicinal plants in the fDistrict, Sidama zone, Southern Ethiopia. J Ethnobiol Ethnomed. https://doi.org/10.1186/s13002-019-0302-7
Teka A, Asfaw Z, Demissew S, Van Damme P (2020) Traditional medicinal plant use of indigenous communities in Gurage Zone. Ethiopia Ethnobotany Res Appl. https://doi.org/10.32859/era.19.41.1-31
Teklit Gebregiorgis Amabye (2015) Evaluation of phytochemical, chemical composition, antioxidant and antimicrobial screening parameters of Rhamnus prinoides (Gesho). Available in the Market of Mekelle, Tigray, Ethiopia. Natural Products Chemistry and Research
Tesfahuneygn G, Gebreegziabher G (2019) Medicinal plants used in traditional medicine by ethiopians: a review article. J Respirat Med Lung Dis 4(1):1–3
Thomsen H et al (2012) Characterization of constituents and anthelmintic properties of Hagenia abyssinica . Sci Pharm 80:433–446
Article CAS PubMed PubMed Central Google Scholar
Tsegay B, Mazengia E, Beyene T (2019a) Short communication: diversity of medicinal plants used to treat human ailments in rural Bahir Dar, Ethiopia. Asian J Forest. Available from: https://smujo.id/ajf/article/view/4163
Tsegay B, Mazengia E (2019b) Diversity of medicinal plants used to treat human ailments in rural Bahir Dar, Ethiopia. A review on significant of traditional medicinal plants for human use in case of Ethiopia. Plant Pathol Microbiol 10:484
Vines G (2004) Herbal harvests with a future: towards sustainable sources for medicinal plants. Plant Life Int
Wolde T, Bizuayehu B, Hailemariam T, Tiruha K (2016) Phytochemical analysis and antimicrobial activity of Hagenia abyssinica . Indian J Pharm Pharmacol 3(3):127–134
Worku A (2019) A review on significant of traditional medicinal plants for human use in case of Ethiopia, Plant Pathol Microbiol 10:484
Yasudha K, Archana D, Mutyalamma B, Kishori B (2019) Phytochemical screening, antimicrobial, and antioxidant activities of root and leaf extracts of Leucas aspera . Asian J Pharm Clin Res 12:141–147
Yordanos G, Tegenu M (2021) Identification of bioactive compound, phytochemical analysis and antimicrobial activity of Lippia adoensis var. koseret from Ethiopia. Sch Int J Tradit Complement Med 4(8):139–153
Zanwar AA, Hedge MV, Bodhankar SL (2010) In vitro antioxidant activity of ethanolic extract of Linum usitatissimum . Pharmacologyonline 1:683–696
Download references
Acknowledgements
First and foremost, I would like to praise the Almighty God, and his wife Elsa Aweke for bestowing upon my health, strength, patience and protection throughout my study period.
Not applicable.
Author information
Authors and affiliations.
Chemistry Department, College of Natural and Computational Sciences, Bonga University, P.O. Box 334, Bonga, Ethiopia
Misganaw Gedlu Agidew
You can also search for this author in PubMed Google Scholar
Contributions
MGA designed and reviewed and approved the final version of the manuscript for publication.
Corresponding author
Correspondence to Misganaw Gedlu Agidew .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The author declares that I have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Agidew, M.G. Phytochemical analysis of some selected traditional medicinal plants in Ethiopia. Bull Natl Res Cent 46 , 87 (2022). https://doi.org/10.1186/s42269-022-00770-8
Download citation
Received : 29 January 2022
Accepted : 17 March 2022
Published : 01 April 2022
DOI : https://doi.org/10.1186/s42269-022-00770-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Medicinal plants
- Phytochemicals analysis
- Bioactive compounds
- Traditional medicines
- Ethiopian levels
- Open access
- Published: 24 March 2022
Ethnomedicinal study of medicinal plants used by Mizo tribes in Champhai district of Mizoram, India
- T. B. C. Laldingliani 1 ,
- Nurpen Meitei Thangjam 1 ,
- R. Zomuanawma 2 ,
- Laldingngheti Bawitlung 1 ,
- Anirban Pal 3 &
- Awadhesh Kumar ORCID: orcid.org/0000-0003-4751-1142 1
Journal of Ethnobiology and Ethnomedicine volume 18 , Article number: 22 ( 2022 ) Cite this article
10k Accesses
11 Citations
8 Altmetric
Metrics details
Medicinal plants have been used countless times for curing diseases mainly in developing countries. They are easily available with little to no side effects when compared to modern medicine. This manuscript encompasses information on ethnomedicinal plants in Champhai district, located in the North East Region (NER) of India. The region lies within Indo-Burma biodiversity hotspot. This study will be the first quantitative report on the ethnomedicinal plants used by the local tribes of this region. Knowledge of medicinal plants is mostly acquired by word of mouth, and the knowledge is dying among the local youths with the prevalence of modern medicine. Hence, there is urgency in deciphering and recording such information.
Information was gathered through interviews with 200 informants across 15 villages of the Champhai district. From the data obtained, we evaluate indices such as used report (UR), frequency of citation (FC), informant consensus factor ( F ic ), cultural values (CVs) and relative importance (RI) for all the plant species. Secondary data were obtained from scientific databases such as Pubmed, Sci Finder and Science Direct. The scientific name of the plants was matched and arranged in consultation with the working list of all plant species ( http://www.theplantlist.org ).
Totally, 93 plant species from 53 families and 85 genera were recorded. The most common families are Euphorbiaceae and Asteraceae with six and five species representatives, respectively. Leaves were the most frequently used part of a plant and were usually used in the form of decoction. Curcuma longa has the most cultural value (27.28 CVs) with the highest used report (136 FC), and the highest RI value was Phyllanthus emblica . The main illness categories as per Frequency of citation were muscle/bone problem (0.962 F ic ), gastro-intestinal disease (0.956 F ic ) and skin care (0.953 F ic ).
The people of Mizoram living in the Champhai district have an immense knowledge of ethnomedicinal plants. There were no side effects recorded for consuming ethnomedicinal plants. We observed that there is a scope of scientific validation of 10 plant species for their pharmacological activity and 13 species for the phytochemical characterisation or isolation of the phytochemicals. This might pave the path for developing a scientifically validated botanical or lead to semisyntheic derivatives intended for modern medicine.
Plants have been known to be a major source of diverse chemical compounds possessing both medicinal properties and commercial value. There have been several reports on medicinal plants as a source for drug discovery. However, new diseases will likely continue to emerge along with drug-resistant pathogens. This dynamic nature of pathogens has constantly challenged researchers to look for alternatives. The past few decades have witnessed the surge in ethnomedicinal plant research [ 1 ], one of the reasons being that the natural products have played an important role in the development of drugs, contributing more than 50% of clinical drugs in the pharmaceutical industry [ 2 ]. Further, the rapid growth in human population has raised the demand which in turn has increased the quest for novel plant resources, triggering a threat to natural resources [ 3 ].
Traditional knowledge and practices of herbal remedies have been passed on to new generations over the centuries and will continue to do so, with some variations taking place every generation. Plants have been the essential source for therapeutic regimes since ages, and traditional practices are proved to have little known side effects besides their low cost and easy availability. India has been well known worldwide for its indigenous traditional pieces of knowledge and practices from ancient times, through different systems of medicine such as Ayurveda, Siddha and Unani [ 4 ]. Although more than 427 tribal communities are having vast diversity of ancient traditions, still there has been criticism of ethnomedicines due to regional variation, political and socio-economic challenges [ 5 ]. Reports are stating that several plants have been increasingly utilised by the indigenous people of India [ 1 ]. Generally, in India, it was estimated that 6,000 species are used in traditional and herbal medicine which represent about 75% of the needs of the third world, and meanwhile, 3,000 plants were officially acknowledged due to their medicinal values [ 6 ].
The healthcare system of India witnesses a wide variation encompassing urban and rural populations which rely on both modern and traditional systems of medicine. The recently implemented Ayushman Bharat Pradhan Mantri Jan Arogya Yojana from the Commonwealth Fund enables cashless secondary and tertiary care at private facilities [ 7 ]. Besides, health insurance schemes also exist for institutions and factories. Catering to the huge population has its limitations, and thus, many of the ailments are treated either by traditional healers or through traditional knowledge and practices, especially in remote areas. One such state in the North-Eastern part of India is Mizoram.
Although some researchers [ 8 , 9 , 10 , 11 , 12 , 13 ] have documented and identified several ethnomedicinal plants of Mizoram mentioning their mode of preparation, usage, distribution and habitat, they mostly reported from the core areas of the cities. Their studies highlighted the qualitative data. However, there are no in-depth ethnobotanical studies recorded in Champhai district. Therefore, the present study aims to carry out a quantitative study using different cultural importance indices to assess the most valued plants and document the ethnomedicinal practices involving medicinal plants of the Champhai district of Mizoram, India. Their practical knowledge has been established based on more than a century of credence and observation.
Description of the study area
Mizoram lies within the Indo-Burma biodiversity hotspot region and shares two international borders with Bangladesh in the west and Myanmar in the east. According to Champion and Seth (1968), Mizoram forests are classified into Tropical semi-evergreen forests, tropical wet evergreen forests and mountain sub-tropical pine forests [ 14 ]. The study area, i.e. the Champhai district, is classified as a rural area where healthcare facilities are relatively poor which drives the people to rely on traditional medicines. The traditional healers using medicinal plant-based formulations for various ailments indicate that traditional medicines are still one of the mainstays in their contemporary health care. It is felt that prospection and research on the medicinal plants that play such an important role in the health care of Mizo tribes need a more intensified effort.
Champhai is one of the 8 districts in Mizoram, amidst the North-East Region of India. It is located in the eastern part of Mizoram, internationally bordered by Myanmar and therefore becoming the main gate of trading for India and Myanmar. It lies between 23.456° N latitude and 93.328°E longitude. The average annual rainfall is approximately 1814 mm, and the temperature remains around 18.6 °C which is slightly colder than the rest of the state during winter. The total land area is 3185.83 sq kilometres at an elevation around 1678 m above sea level, population density is 10 per sq kilometres (32,734). According to an official Census (2011), Champhai reported a population of 1,26,000, of which male and female were 62,357 and 63,388, respectively [ 15 ]. The study area was divided into 15 village council areas (Vengthlang, Vengthlang North, Venglai, Vengsang, Electric veng, Kanan, Kahrawt, Bethel, New Champhai, Zotlang, Hmunhmeltha, Tualcheng, Ngopa, Khawzawl and East Lungdar) for extensive data collection (Fig. 1 ). The majority of people living in this area are Mizo tribe and use the Mizo dialect in common.
Location of the present study area: Champhai district, Mizoram, India
Investigative method
In the field study, formal questionnaires were distributed to each participant while having face to face interviews at their residence. At least 16 people were interviewed in each village council area. Only those people who have knowledge in the art of preparing medicines either for their families or their neighbourhood were considered for the interaction. The interactions primarily focussed on their experience, type of dosage form, duration of usage, any adverse effects observed and the source of their knowledge about the plant and their parts used. This information was then correlated with the scientific data curated from related databases (Pubmed, SciFinder and Science Direct). In most of the cases, the voucher specimens were deposited (Herbarium, Mizoram University, Aizawl, Mizoram, India) for their authentication and archiving.
Characteristics of demographic data
This demonstrated the socio-economic information of the informant including qualities like age, sex, education level and occupation. Using random sampling method, 200 people (12–14 individuals from each village) in the ages group of 18–71 years were interviewed, of which 112 and 82 were males and females, respectively. Respondents belonged to various professions while some were students. Most of the informants do not engage in full-time ethnomedicinal practice or as a profession. The feature of demographical characteristics obtained in the study is tabulated below (Table 1 ).
Quantitative analysis
Frequency of citation
Frequency of citation was used to further examine the primary data by finding the sum of total citations/usage reports for a particular species. The usage report is the quotation of one plant by an informant [ 16 ].
Use value or UV is used to express the correlative importance of each particular plant species locally known and was calculated by the following equation [ 17 ].
where ‘ U i ’ represents the number of citations of each species by the informants and ‘ n ’ represents the total number of informants in the study area. The larger the number of citations, the greater is the use-value.
Informant consensus factor
F ic or ICF is used to represent the consistency of the information among the informants, indicating whether there were shared knowledge and concurrence in the use of plants for treating the ailment category among the plant’s users in the study area. It was calculated by the following equation [ 18 ].
where ‘ N ur ’ refers to the number of users reports in each illness category and ‘ N t ’ refers to the number of plant species used for a particular illness category by all the informants.
Further, F ic value with 1 or either close to 1 indicates that a large number of informants had agreed on using few plants for curing an illness category while low F ic value signified that there was an argument on using medicinal plants to treat illness amidst the category.
Relative importance
When calculating RI, both the informants who mentioned the useful plant species and their various kinds of uses are considered. So, it was calculated by the following equation [ 19 ].
where ‘NUC’ refers to the number of illnesses use category of each species divided by the total number of most use categories among the species and ‘NT’ refers to the number of illness types of uses of each species divided by the total number of most types of uses among the species.
Cultural values
In this index, the use category is taken into account and it was calculated by using the following equation [ 20 ].
where ‘UCs’ is the number of the used reports for each species divided by the total number of use categories of that species. ‘ICs’ is the number of informants who mention each plant as effective divided by the total number of informants, and ∑IUCs is the number of informants who report the use of each species divided by the total number of informants.
Demographic characteristics
All the 200 respondents were randomly selected from 15 village council areas interviewing at least 16 persons in each area with no equal separation of male–female ratio. Amongst them, the elderly in their seventies and above occupied 6.5% only, while people between 31 and 50 years old occupied 34.5%. The average age among the informants was 54 years. Mizoram is the second most literate state in the country (2011 census), and all the informants were literate having at least primary school level education. Out of the total informants, 32.5% were engaged in government jobs like teachers, officers, while 35% were self-employed like farmers, carpenters, skilled workers, small businesses and the rest 32.5% of the informants were unemployed including students and housewives (Table 1 ).

Taxonomy identification
In the present study, 93 medicinal plant species belonging to 53 families and 85 genera have been reported for treating various kinds of ailments. The most prominent families were Euphorbiaceae with 6 plant species followed by Asteraceae with 5 plant species and 4 species each among Cucurbitaceae and Zingiberaceae. Liliaceae, Fabaceae, Verbenaceae, Solanaceae, Rutaceae, Anacardiaceae are with 3 species each while Orchidaceae, Combrethaceae Theaceae, Arecaceae, Apocynaceae, Musaceae, Rubiaceae, Scrophulariaceae, Lamiaceace, Mimosaceae, Smilacaceae are with 2 species each and other 34 families with one species each as shown in Table 2 . The high usage report of this large family like Euphorbiaceae (6 species), Asteraceae (5 species) and Zingiberaceae (4 species) occupied 10.8%, 9.2% and 8.35% of the total used report, respectively, indicating that most people in the study area are inclined to use plants that are easily available and abundant around them (Table 2 ).
Frequency of usage of parts of plants
The most commonly used medicinal plants fell under herbs (35.5%) followed by trees (33.3%), shurbs (18.3%) and creepers (12.9%) as shown in (Fig. 2 ). Among the parts, leaves, fruits and barks were mainly utilised by the informants (Fig. 3 ). A detailed analysis concluded that leaves (47%) followed by fruits (14%), barks (11%), seeds (10%), rhizomes (6%), stems (4%), young shoot (2%), oil (1%) and in some cases the whole plant (3%) were used for ethnomedicinal purposes.
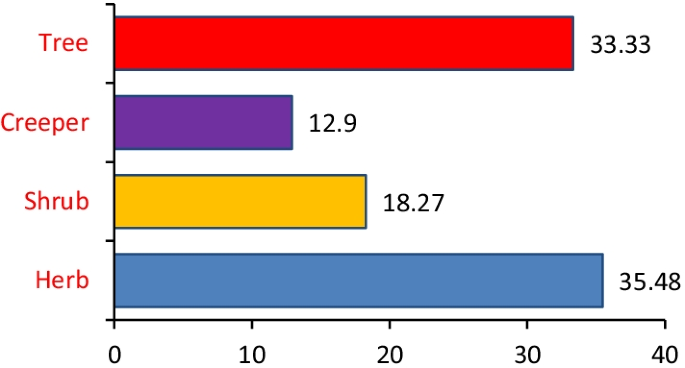
Percentage of plants habit

Percentage of parts used
Mode of preparation and administration
The mode of formulation preparation or administration was observed to be in the form of decoction (44.2%) followed by paste (23%), raw (19.5%), juice (9.73%), powder (1.77%) and others like maceration and oil (1.77%) (Fig. 4 .).
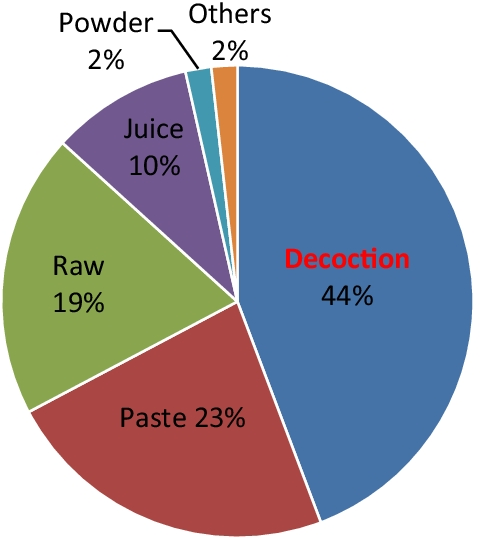
Distribution of formulation usage
Usage analysis based on the treatment of ailments
The total number of user reports documented in this study was 2717, in which all different illnesses were categorised into 16 groups using International Classification of Primary Care (ICPC) with a slight modification. Among the illness category, the gastro-intestinal disease has the highest usage report (940) followed by skincare (259) cardiovascular (222), kidney disease (196), hyperglycaemia (175), ENT (159), genito-urinary disease (139) and so on as shown in Table 3 .
Data analysis
Among the total number of user reports (UR) cited, Curcuma longa L. (136 FC), Flueggea virosa (Roxb. ex Willd.) Royle (126 FC), Psidium guajava L. (98 FC), Chromolaena odorata (L.) R.M. King & H. Rob. (87 FC), Mikania micrantha Kunth. (82 FC), Citrus limon (L.) Osbeck (68 FC), Carica papaya L. (53 FC), Ananas comosus (L.) Merr. (49 FC), Sarcococca pruniformis Lindl. (49 FC), Phyllanthus emblica L. (48 FC), Rhus chinensis Mill. (45 FC), Clerodendrum glandulosum Lindl. (44 FC), Senecio scandens Buch- Ham. ex D. Don (43 FC) were those species having the highest FC (Table 4 ).
Plant use value
From the UV value evaluation, Curcuma longa L. (0.68), Flueggea virosa (Roxb. ex Willd.) Royle (0.63), Psidium guajava L. (0.49), Chromolaena odorata (L.) R.M. King & H. Rob. (0.43), Mikania micrantha Kunth. (0.41), Citrus limon (L.) Osbeck (0.34), Carica papaya L. (0.26), Ananas comosus (L.) Merr. (0.24), Sarcococca pruniformis Lindl. (0.24), Phyllanthus emblica L. (0.24), Clerodendrum glandulosum Lindl. (0.22), Rhus chinensis Mill. (0.22), Senecio scandens Buch- Ham. ex D. Don (0.21) were reported to have the highest use value (UV).
We calculated the informant consensus factor by categorising the reported illness into 16 ailment groups along with the number of users report and taxa (Table 5 ). In our study, F ic values ranged from 0.866 to 0.962 which were all close to 1.
Relative importance and cultural value
Results of top-ranking species in terms of both indices of relative importance and cultural value are given in Table 6 . This study elucidates the highest cultural valued species and relative importance species utilised by the inhabitants of the study area. In general, the evaluated values were quite high in case of CVs and an average value of RI (0.607 ± 0.38) clarified that the versatile species, i.e. Phyllanthus emblica (RI = 2) was 3.3 times more relevant than the rest of the listed species.
Correlation and validation studies
An attempt was made to compare the use of all the medicinal plants reported by the informants with the previous papers published for their biological activity or ethnomedicines (Table 4 ). According to the studies conducted by Cakilcioglu et al., 2011, it was stated that if a medicinal plant has been reported for similar use in other parts of the world, its pharmacological effect could be more easily known [ 209 ].
The use of crude juice of Allium cepa L. showed a significantly higher hair growth rate than tap water when applied twice a day for two months which corroborated the present report of hair regrowth [ 22 ]. Metallothionein, an antioxidant protein present in Aloe vera (L.) gel, has been reported to have a protective effect against UV and gamma radiation damage to the skin. It scavenges free radicals by preventing the suppression of glutathione peroxidase and superoxide dismutase in the skin [ 30 ]. So, this validated the used of A. vera for skin care and burning by the Mizo tribes. In the present study, Betula alnoides Buch- Ham. ex D. Don has been used as toothpaste for whitening teeth while it was proved that 80% methanolic bark extract had the potential α-glucosidase inhibitory effect that prevent the (98.4%) at 40 µg/mL concentration [ 2 ]. Cajanus cajan (L.) Millsp is used effectively in Champhai district to treat jaundice and intestinal worms. To certify this, the methanolic extracts showed hepatoprotective activity in Swiss albino mice by inducing carbon tetrachloride (CCl 4 ) that cause liver damage. It lowers the serum levels of glutamate pyruvate transaminase (SGPT), or alanine aminotransferase (ALT) aspartate aminotransferase (AST) or serum glutamate oxaloacetate transaminase (SGOT) significantly [ 58 ].
When the aqueous extracts of Carica papaya L. and Ananas comosus L. were given to Spraque Dawley rats orally at doses of 5 and 10 mg/kg, both possessed mild to strong diuretic activity. Careful measure should be taken when using these plants as increase in the level of urinary K + , serum BUN and creatinine were mentioned [ 71 ]. This validated the used of C. papaya and A. comosus in kidney disease and urinary infection. The contemporary reports showed that Drymaria cordata (L.) was used as an instant pain killer for rheumatism; meanwhile, the scientific study also demonstrated that the aqueous whole plant extract exhibited analgesic and antipyretic properties at doses of 100, 200, and 400 mg/kg p.o mediated through peripheral and central mechanisms [ 210 ]. The latex water-soluble fraction of Euphorbia royleana Boiss. showed dose-dependent anti-arthritic and anti-inflammatory effects in rats and mice administered through gavage at doses of 50–200 mg/kg having more than 1500 mg/kg oral LD 50 in both [ 135 ]. Dose-dependent and significant decline in the number of abdominal constrictions induced by intraperitoneal administration of acetic acid was observed in methanol extract of Lablab purpureus (L.) Sweet. at a dose of 200 mg and 400 mg exhibited far better analgesic activity than 200 mg aspirin per kg of body weight [ 211 ].
Colocasia esculenta (L.) Schott and Elaeagnus caudata Schltdl. ex Momiy. were declared to use to discharge placenta after birth and to treat vaginal discharge (Lochia) for women in present study. Besides this record, in Cachar hills district of Assam, India, 5 ml of Elaeagnus caudata fresh root extract diluted in 10 ml of fresh water was also administered orally once a week to prevent miscarriage during pregnancy although there is no scientific study to backup this claim [ 118 ]. Apart from present report in Jamaica, Mikania micrantha Kunth. was most popularly used too for wound healing and its extract showed anti-inflammatory and antimicrobial activity against common pathogens, namely Escherichia coli , Staphylococcus aureus and Streptococcus sp. [ 212 ]. The decoction of Psidium guajava leaf was effectively used for diarrhoea which already proved to have antidiarrhoeal and protein conservative effects in diarrhoeal rats at a dose of 50 and 100 mg/kg of body weight. It increased the kidney weight and concentration of sodium, potassium and chloride significantly [ 213 ]. In the animal study of anti-urolithialic activity of Solanum nigrum , the fruit hydroalcoholic extract elicited potent activity against calcium oxalate urolithiasis effected by ethylene glycol through tumour necrosis factor adiponectin stimulation and alpha inhibition, also maintained the balance between stone promoter and inhibitor such as calcium and magnesium, respectively [ 214 ]. Thus, this authenticated the used of S. nigrum for removing kidney stone by the Mizo tribes in India.
Anoectochilus brevilabris Lindl. , Begonia inflata C.B. Clark , Dysoxylum excelsum Blume , Embelia vestita Roxb , Ensete glaucum (Roxb.) Cheesman , Gomphogyne cissiformis Griff. , Helicia robusta (Roxb.) R. Br. ex Blume , Laurocerasus undulata (Buch- Ham. ex D. Don) M. Roem. and Lobelia angulata G. Forst., Sarcococca pruniformis Lindl. were the plants that did not have biological activity reported previously which means that there is no scientific validation to support their application. Therefore, these plants were especially recommended in carrying out further investigation.
In addition, we compiled the secondary metabolite isolated chemical constituents done by several researchers for all the documented plants in the present study. Further investigation revealed that secondary metabolites from 13 plant species that have neither less nor none chemical compound isolated or identified— Anoectochilus brevilabris Lindl., Begonia inflata C. B. Clark., Castanopsis tribuloides (Sm.) A. DC., Combretum wallichii DC, Elaeagnus caudata Schltdl. ex Momiy., Embelia vestita Roxb., Ensete glaucum (Roxb.) Cheesman, Gomphogyne cissiformis Griff., Helicia robusta (Roxb.) R. Br. ex Blume, Laurocerasus undulata (Buch- Ham. ex D. Don) M. Roem., Lobelia angulata G. Forst., Pandanus odorifer (Forssk.) Kuntze, Sarcococca pruniformis Lindl. (Table 4 ) which will surely have great potent on ethnopharmacological study.
According to our findings, women practitioners (44%) were less than men (56%) which may be explained partly by the low sex ratio of the district; however, it can be assumed that women play lesser role in ethnomedicinal practices [ 215 , 216 ]. Among self-employed, farmers account for 58.5%, business persons 34.2% and carpenters were 21.4%. Farmers represented the highest percentage as they often lack access to modern healthcare facilities due to various issues ranging from financial, transportation and higher education. These issues forced them to rely on traditional medicines, cultivating and utilising them more regularly than others and somehow playing a big role in conservation too. Through this study, we observed that young informants like students around 18 to 25 years old have little expertise in practicing ethnomedicine and utilised them rarely as compared to elder informants. This may be due to change in mentality brought by education to rely only on prescribed medicines. Further, the results of the usage of plants dominated by the families were followed and confirmed the work done by some researchers stating that greater the plants grew in the study area the more it will be favourably and commonly used [ 217 ]. This supports the non-random plant selection hypothesis by Moerman 1979 [ 218 ]. Large families such as Asteraceae and Euphorbiaceae were most utilised while Orchidaceae and Poaceae were underutilised (low used report). However, due to non-random selection, small families like Cucurbitaceae and Zingiberaceae became over-represented (high used report). Thus, this implies that medicinal plants are not selected randomly by the inhabitants of Champhai district but are utilised based on their cultural and traditional knowledge [ 219 ]. In the present study, we laid out the only accepted botanical names by ‘The Plant List’ and their family, local name, habit, mode of preparation and ailments as illustrated in Table 4 .
Out of 93 species, 40 were cultivated species, whereas 53 were found in the wild. There were also 6 invasive alien species most notably Chromolaena odorata and Mikania micrantha which were commonly used to treat wounds topically. This is because wounds are the most common form of injury and these two species can be found almost everywhere [ 219 ]. The frequent use of herbaceous plants as medicines among the informants was due to their richness, abundance as well as their ability to grow easily in nature. Meanwhile, many parts of the world have been commonly using herbs as their medicinal ingredients due to their wide range of medicinal properties [ 220 ]. Leaves are the most utilised part of the plants due to their ease off collection as compared to their underground part. It is also the active site of photosynthesis accompanied by the production of metabolites [ 1 , 221 ]. In addition, leaves can be easily prepared and stored. It can be dried quickly under the sun in lesser amount of time than other parts like stem, bark and rhizome.
Similarly, it is also reported that decoction was the most common preparation method for herbal medicine while in some other tribal community [ 3 ], preparation of paste was the most common method applied [ 1 , 216 , 222 ]. For decoction the plant part was washed thoroughly and boiled with water administering the juice orally, whereas for paste the materials were crushed or rubbed within palms and applied topically. To make fine powder plant parts were shade dried and ground. Intake of oral administration and external topical formulation were the main mode of administration used in traditional herbal medicines which has also been previously reported [ 215 , 223 ]. Regarding the duration of consumption of herbal medicine, it depends on the illness whether it was short term or long term. For instance, short-term illness like cold, flu, stomach upset and skin problem, the consumption period did not last more than 1 week. On the contrary, the long-term illness like diabetes, kidney failure and heart diseases, the consumption period of plants (e.g., Flueggea virosa ) was much longer and last more than a month and so on.
The inhabitants of the study area extensively exploited medicinal plants to treat various illnesses and other needs which have not been previously reported. For instance, Anoetochilus brevilabris was used for pile treatments, Betula alnoides as toothpaste, Capsicum annuum to soothe and prevent scars from skin burns. Colocasia esculenta to expel lochia, Euphorbia milii as antidiarrhoea, Lablab purpureus as a pain reliever, Mussaenda macrophylla to stop internal bleeding and Parkia timoriana for treating baby umbilical cord. From this study it was clear that among the informants, stomach problems like ulcer, indigestion, diabetes, hypertension and kidney problems were common illness resulting in high user rate of consuming herbal medicines and similar record was reported by Mahwasane et al., [ 224 ]. Further, skin problem like dermatitis which was the second highest usage report was the highest ailment in most other tribal communities like Malda district in West Bengal reported by Saha et al. [ 225 ].
Generally, majority of the informants did not consume the medicines prescribed by the Doctor’s prescribed medicines along with their herbal medicine and claimed that many plants like Sarcococca pruniformis (tonsil), Psidium guajava (diarrhoea), Mikania micrantha (cut/wound), Flueggea virosa (chicken pox), Elaeagnus caudata (veginal discharge) were really effective and most importantly, none of them reported any adverse effect such as vomiting, headache, nausea, allergic reactions and/or skin rashes. Moreover, regarding the expenditure on buying medicines, 38% of the informants usually purchased their herbal medicines either in raw form ( Allium sativum, Allium cepa, Beta vulgaris ) or in processed form like juice ( Citrus limon, Phyllanthus emblica, Citrus aurantiifolia ), fruits ( Punica granatum, Phyllanthus emblica, Cucumis sativus ), and powder ( Curcuma longa ). Concerned about the source of their knowledge, all the informants reported that they have heard and learned some of their information from their elders, family and/or acquaintances. Besides these, 30% of the informants have also gathered additional information through social media and 10% through books, magazines and newspapers. This documentation clearly showed that knowledge and cultural practices of herbal medicines had been shared through the indigenous community through word of mouth.
Frequency of citation showed the sociocultural importance of medicinal plants to identify their therapeutic value [ 16 ]. The FC value is directly proportional to the use value (UV), the more FC value will increase the used value significantly.
Curcuma longa L. is one of the main commercially grown as seasoning plants in India. In Southeast Asia including India and China, turmeric powder has been used extensively for spice and colouring food material. It had a wide range of medicinal value that curcumin was the main bioactive chemical constituents [ 226 ]. C. longa was a mandatory spice that each and every household kept it that’s why the reason used report (UR) for medicinal value and cultural value (CVs) were high among the informants. In case of CVs, the high value was due to a greater number of the used report with lesser-used categories. The informants in present study reported that Flueggea virosa have a prominent effectiveness against diabetes (59 UR) and chickenpox (50 UR). The Mizo tribes extensively used F. virosa and Embelia vestita Roxb. plant to treat chickenpox and measles by bathing once a day with the decoction of leaf mixed with water. Apart from the degree of the used report, this index also attributed to the effectiveness of their use and importance.
Higher in the UV value indicates the more rate of agreeing and sharing their knowledges and practices of the medicinal plants among the informants [ 216 ]. Among the Terai forest of western Nepal Curcuma longa L. was also reported as the highest used value [ 227 ] similar to this result.
The plants with low UV value were Colocasia esculenta (L.) Schott, Eulophia nuda Lindl. and Ocimum americanum L., Maesa indica (Roxb.) A. DC, Morus macroura Miq, Tectona grandis L.f., Hibiscus sinensis Mill, Elaeis guineensis Jacq, Smilax perfoliata Lour with less than 0.05 UV as shown in Table 4 . Tectona grandis L.f. was also described with very low UV value by Ayyanar and Ignacimuthu as relevant to this result [ 1 ]. According to Chaudhary et al. 2006, the plants with low used value were in at risk of misrecollecting and passing on to the young generation which might be gradually disappearing [ 228 ]. On the other hand, the relevance of knowing the plant used value was for the convenience of pharmacological study and their used reliability [ 229 ].
However, Rajakumar and Shivanna had mentioned that the value of F ic depends on the accessibility of the taxa for the treatment of various diseases in the study area. Muscle/Bone problem with 81 UR have the highest F ic value of 0.962 followed by gastro-intestinal disease (GID) with 940 UR and skin care (SC) with 259 UR (Table 5 ). The lowest F ic value in the present study was the General Health (GH) category (Cold, fever, immunity boost) with 0.887 which was still more than the previous maximum F ic value report in Shimoga district, Karnataka, India i.e. 0.77 in Liver complaints [ 230 ]. Most RI value ( Phyllanthus emblica ) is considered to be versatile on its uses which would also increase the importance of the plant when it is used to treat more illnesses. The high RI values of some species may be attributed to their abundance and availability in the study area [ 19 ].
Overall, the quantitative analysis revealed that Curcuma longa was the most relevant species with the highest used value, frequency of citation and cultural value except in relative importance. This is due to the fact that the RI value is independent of the number of informants used report. On the conflict of these report, our study indicated that there was high consistency of the indigenous informant knowledge in the practices of ethnomedicines and utilised the same plants to treat it.
Conclusions
The present study concluded that the native people in the study area have their unique way of utilising medicinal plants to treat different kinds of ailments. We documented 93 valuable medicinal plants belonging to 55 families and 85 genera in which Euphorbiaceae and Asteraceae family were the most widely used in the area. This study supported the non-random selection of medicinal plants hypothesis. Among the plants part, leaves were the most commonly used. No new medicinal taxa were reported, but this study is a first quantitative report of ethnomedicine in this region and no informant had reported an adverse effect of herbal medicines. Their traditional pieces of knowledge had been passed on from their elders mostly through word of mouth. This study also revealed that younger generations between the ages of 18 and 30 have little to no knowledge of preparation of herbal medicines and their use as compared to the older age groups. This is mostly due to the availability of modern clinical drugs in the villages. Therefore, the traditional knowledge and practices of medicinal plants in the study area are somehow at risk of dying. This is why it is important to document the valuable knowledge as well as for conservation of the taxa.
The use of quantitative indices was essential in the field of ethnobotany to determine the most valuable plants along with their role played in a particular culture and to develop conservation initiatives. The plants which have high usage report and frequency of citation were known to possess numerous phytochemical compounds. The calculated informant consensus factor was extremely high, which means that the acquired data can be used as reference and reliable for ethnopharmacological study in the future. Even though the remedial value of many high cited plants has already been verified, there are still some plants that need to be validated. Hence, they are strongly recommended for further studies to develop alternative drugs.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
Used reports
Whole plants
Young shoot
Ayyanara M, Ignacimuthub S. Ethnobotanical survey of medicinal plants commonly used by Kani tribals in Tirunelveli hills of Western Ghats, India. J Ethnopharmacol. 2011;134:851–64. https://doi.org/10.1016/j.jep.2011.01.029 .
Article Google Scholar
Ghimire BK, Tamang JP, Yu CY, Jung SJ, Chung IM. Antioxidant, antimicrobial activity and inhibition of α-glucosidase activity by Betula alnoides Buch. bark extract and their relationship with polyphenolic compounds concentration. Immunopharmacol Immunotoxicol. 2012;34:824–31. https://doi.org/10.3109/08923973.2012.661739 .
Article PubMed Google Scholar
Simbo DJ. An ethnobotanical survey of medicinal plants in Babungo, Northwest Region, Cameroon. J Ethnobiol Ethnomed. 2010;6:8. https://doi.org/10.1186/1746-4269-6-8 .
Article PubMed PubMed Central Google Scholar
Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109:69–75. https://doi.org/10.1289/ehp.01109s169 .
Article CAS PubMed PubMed Central Google Scholar
Kadhirvel K, Ramya S, Sudha TS, Ravi AV, Rajasekaran C, Selvi RV, Jayakumararaj R. Ethnomedicinal survey on plants used by tribals in Chitteri Hills. Environ We Int J Sci Tech. 2010;5:35–46.
Google Scholar
Rai PK, Lalramnghinglova H. Ethnomedicinal plant resources of Mizoram, India: Implication of traditional knowledge in health care system. Ethnobot Leafl. 2010;3:6.
Tikkanen R, Osborn R, Mossialos E, Djordjevic A, Wharton GA. International Health Care System Profiles, India. The Commonwealth Fund. 2020 https://www.commonwealthfund.org/international-health-policy-enter/countries/india. Accessed 21 June 2021.
Lalramnghinglova H, Jha LK. Ethnomedicine from Mizoram—North East India. Ethnobotany. 1997;9:105–11.
Lalnundanga, Sahoo UK, Jha LK. Ethnobotanical flora in the humid sub-tropical semi-evergreen forest of Mizoram. In: Proceedings national conference on healthcare and developments of herbal medicines, Raipur. 1997.
Sharma HK, Chhangte L, Dolui AK. Traditional medicinal plants in Mizoram, India. Fitoterapia. 2001;72(2):146–61. https://doi.org/10.1016/S0367-326X(00)00278-1 .
Article CAS PubMed Google Scholar
Lalramnghinglova H. Ethno-medicinal plants of mizoram. Bishen Singh Mahendra, Pal Singh, Dehradun, India; 2003.
Singh NP, Singh KP, Singh DK. Flora of Mizoram. Botanical Survey of India. Ministry of Environment and Forest. Government of India, Kolkata; 2002.
Sawmliana M. The Book of mizoram plants. Zakhuma P, Aizawl, Mizoram; 2003.
Champion GH, Seth SK. A revised survey of the forest types of India. Dehradun: Natraj Publishers; 1968.
Champhai district. Government of Mizoram, India. 2021. https://champhai.nic.in/about-district/ . Accessed 03 Jan 2021.
Khajuria AK, Manhas RK, Kumar H, Bisht NS. Ethnobotanical study of traditionally used medicinal plants of Pauri District of Uttarakhand, India. J Ethnopharmacol. 2021;276: 114204. https://doi.org/10.1016/j.jep.2021.114204 .
Gazzaneo LRS, Lucena RFP, Albuquerque UP. Knowledge and use of medicinal plants by local specialists in a region of Atlantic Forest in the state of Pernambuco. J Ethnobiol Ethnomed. 2005;1:9. https://doi.org/10.1186/1746-4269-1-9 .
Heinrich M, Ankli A, Frei B, Weimann C, Sticher O. Medicinal plants in Mexico: healers’ consensus and cultural importance. Soc Sci Med. 1998;47:1859–71. https://doi.org/10.1016/S0277-9536(98)00181-6 .
Albuquerque UP, Lucena RF, Monteiro JM, Florentino AT, Cecília de Fátima CB. Evaluating two quantitative ethnobotanical techniques. Ethnobot. Res. Appl. 2006;4:051–60. https://ethnobotanyjournal.org/index.php/era/article/view/101.
Sujarwo W, Caneva G. Using quantitative indices to evaluate the cultural importance of food and nutraceutical plants: comparative data from the Island of Bali (Indonesia). J Cult Herit. 2016;18:342–8. https://doi.org/10.1016/j.culher.2015.06.006 .
Kundan SB, Anupam S. Phytoconstituents and therapeutic potential of Allium cepa Linn—a review. Phcog Rev. 2009;3(5):170–80.
Sharquie KE, Al-Obaidi HK. Onion juice ( Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol. 2002;29:343–6. https://doi.org/10.1111/j.1346-8138.2002.tb00277.x .
Teshika JD, Zakariyyah AM, Zaynab T, Zengin G, Rengasamy KR, Pandian SK, Fawzi MM. Traditional and modern uses of onion bulb ( Allium cepa L.): a systematic review. Crit Rev Food Sci Nutr. 2019;59(1):39–70. https://doi.org/10.1080/10408398.2018.1499074 .
Article CAS Google Scholar
Batiha GES, Beshbishy AM, Elewa Y, Taha A. Chemical constituents and pharmacological activities of garlic ( Allium sativum L.): a review. Nutrients. 2020;12:872. https://doi.org/10.3390/nu12030872 .
Miron T, Rabinkov A, Mirelman D, Wilchek M, Weiner L. The mode of action of allicin: its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim Biophys Acta. 2000;1463:20–30. https://doi.org/10.1016/S0005-2736(99)00174-1 .
Harris JC, Cottrell SL, Plummer S, Lloyd D. Antimicrobial properties of Allium sativum (garlic). Appl Microbial Biotechnol. 2001;57:282–6. https://doi.org/10.1007/s002530100722 .
Ishtiaq M, Hanif W, Khan MA, Ashraf M, Butt AM. An ethnomedicinal survey and documentation of important medicinal folklore food phytonims of Flora of Samahni Valley, (Azad Kashmir) Pakistan. Pakistan J Biol Sci. 2007;10:2241–56. https://doi.org/10.3923/pjbs.2007.2241.2256 .
Singh VK, Singh DK. Pharmacological effects of garlic ( Allium sativum L.). Annu Rev Biomed Sci. 2008;10:6–26.
Arunkumar S, Muthuselvam M. Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens. World J Agric Sci. 2009;5:572–6.
CAS Google Scholar
Christaki E, Florou-Paneri PC. Aloe vera: a plant for many uses. J Food Agric Environ. 2010;8:245–9.
Sahu PK, Giri DD, Singh R, Pandey P, Gupta S, Shrivastava AK, Kumar A, Pandey KD. Therapeutic and medicinal uses of Aloe vera : a review. J Pharm Pharmacol. 2013;4:599–610. https://doi.org/10.4236/pp.2013.48086 .
Kalaria P, Gheewala P, Chakraborty M, Kamath J. A phytopharmacological review of Alstonia scholaris : a panoramic herbal medicine. Int J Res Ayurveda Pharm. 2012;3:367–71.
Arulmozhi S, Mazumder PM, Ashok P, Narayanan LS. Pharmacological activities of Alstonia scholaris Linn. (Apocynaceae)—a review. Pharmacogn Rev. 2007;1:163–70.
Baliga MS. Review of the phytochemical, pharmacological and toxicological properties of Alstonia scholaris Linn. R. Br (Saptaparna). Chin J Integr Med. 2012. https://doi.org/10.1007/s11655-011-0947-0 .
Huang XJ, Chen WH, Ji MH, Guo FY, Shu HM, Zheng CJ. Chemical constituents from leaves of Ananas comosus and their biological activities. Zhong Cao Yao. 2015;46:949–54. https://doi.org/10.7501/j.issn.0253-2670.2015.07.002 .
Pavan R, Jain S, Kumar A. Properties and therapeutic application of bromelain: a review. Biotechnol Res Int. 2012;6: 976203. https://doi.org/10.1155/2012/976203 .
Rathnavelu V, Alitheen NB, Sohila S, Kanagesan S, Ramesh R. Potential role of bromelain in clinical and therapeutic applications. Biomed Rep. 2016;3:283–8. https://doi.org/10.3892/br.2016.720 .
Lin TC, Tanaka T, Nonaka G, Nishioka I, Young TJ. Isolation and characterization of novel complex tannins (Flavano-ellagitannins). Anogeissinin and Anogeissusin-B, from Anogeissus acuminata (Roxb ex Dc) Guill et Perr var Lanccolata Wall ex Clarke. Chem Pharm Bull. 1991;39:1144–7. https://doi.org/10.1248/cpb.39.1144 .
Monali PM, Padhy R. Antibacterial activity of green silver nanoparticles synthesized from Anogeissus acuminata against multidrug resistant urinary tract infecting bacteria in vitro and host-toxicity testing. J Appl Biomed. 2018;16:120–5. https://doi.org/10.1016/j.jab.2017.11.003 .
Panda SK, Laxmipriya P, Pieter L, Maoxuan L, Johan N, Walter L. Antimicrobial anthelmintic, and antiviral activity of plants traditionally used for treating infectious disease in the Similipal Biosphere Reserve, Odisha, India. Front Pharmacol. 2017;8:658. https://doi.org/10.3389/fphar.2017.00658 .
Panda SS, Girgis AS, Prakash A, Khanna L, Khanna P, Shalaby EM, Fawzy NG, Jain SC. Protective effects of Aporosa octandra bark extract against d -galactose induced cognitive impairment and oxidative stress in mice. Heliyon. 2018. https://doi.org/10.1016/j.heliyon.2018.e00951 .
Sahu BP, Gouda P, Patnaik C. Aporosa octandra , a Less studied plant species with potential drug activities-I: identification of a new compound from aqueous ethanolic extract of its stem bark. Int J Adv Res Sci Eng Tech. 2016;3:2219–24.
Vabeiryureilai M, Lalrinzuali K, Rosangkima G, Jagetia GC. Qualitative phytochemical analysis and antioxidant activity of Aporosa octandra (Buch-Ham. ex D. Don) extracts. Int J Pharmacol Res. 2014;6:68–73.
Siddiqui BS, Ali ST, Rasheed M, Kardar MN. Chemical constituents of the flowers of Azadirachta indica . Helv Chim Acta. 2003;86:2787–96. https://doi.org/10.1002/hlca.200390229 .
Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. Biological activities and medicinal properties of neem ( Azadirachta indica ). Curr Sci. 2002;82:1336–45.
Doshi GM, Nalawade VV, Mukadam AS, Chaskar PK, Zine SP, Somani RR, Une HD. Structural elucidation of chemical constituents from Benincasa hispida seeds and Carissa congesta roots by gas chromatography: mass spectroscopy. Pharmacogn Res. 2015;7(3):282–93.
Al-Snafi AE. The pharmacological importance of Benincasa hispida . A review. Int J Pharm Sci Res. 2013;4:165–70.
Qadrie ZL, Hawisa NT, Khan MA, Samuel M, Anandan R. Antinociceptive and anti-pyretic activity of Benincasa hispida (Thunb.) cogn. in wistar albino rats. Pak J Pharm Sci. 2009;22:287–90.
PubMed Google Scholar
El-Hawary SS, Hammouda FM, Tawfik WA, Kassem HA, Abdelshafeek KA, El-Shamy SS. Investigation of some chemical constituents, cytotoxicity and antioxidant activities of Beta vulgaris var. altissima cultivated in Egypt. Rasayan J Chem. 2017;10:1391–401. https://doi.org/10.7324/RJC.2017.1041936 .
El-Beltagi HS, Mohamed HI, Megahed BHM, Gamal M, Safwat G. Evaluation of some chemical constituents, antioxidant, antibacterial and anticancer activities of Beta vulgaris L. root. Fresenius Environ Bull. 2018;27:6369–78.
Ninfali P, Angelino D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia. 2013;89:188–99. https://doi.org/10.1016/j.fitote.2013.06.004 .
Dũng NX, Mõi LD, Leclercq PA. Constituents of the bark oil of Betula alnoides Ham ex. D. Don from Vietnam. J Essent Oil Res. 1995;7:565–6. https://doi.org/10.1080/10412905.1995.9698589 .
Thu NB, Trung TN, Ha DT, Khoi NM, Hung TV, Hien TT, Yim NH, Bae KH. Screening of Vietnamese medicinal plants for cytotoxic activity. Nat Prod Sci. 2010;16:43–9.
Yang DS, Yang YP, Yong-hong Y, Xiao-li L. Chemical constituents of Bischofia javanica . Nat Prod Res Dev. 2013;25:1056–9. https://doi.org/10.3969/j.issn.1001-6880.2013.08.009 .
Khan MR, Kihara M, Domoloso A. Anti-microbial activity of Bidens pilosa , Bischofia javanica, Elmerillia papuana and Sigesbekia orientalis . Fitoterapia. 2001;72:662–5. https://doi.org/10.1016/S0367-326X(01)00261-1 .
Lingadurai S, Roy S, Joseph RV, Nath LK. Antileukemic activity of the leaf extract of Bischofia javanica blume on human leukemic cell lines. Indian J Pharmacol. 2011;43:143–9.
Anadebe VC, Okafor NA, Ezeugo JO, Amanjide IJ, Ogide BC. GC-MS analysis of phytochemical compounds in Cajanus cajan leaf. J Chem Pharm Res. 2017;9:360–3.
Pal D, Mishra P, Sachan N, Ghosh AK. Biological activities and medicinal properties of Cajanus cajan (L) Millsp. J Adv Pharm Technol Res. 2011;2:207–14.
Tu Y, Sun L, Guo M, Chen W. The medicinal uses of Callicarpa L. in traditional Chinese medicine: an ethnopharmacological, phytochemical and pharmacological review. J Ethnopharmacol. 2013;146:465–81. https://doi.org/10.1016/j.jep.2012.12.051 .
Zhu HB, Li BM, Liu C, Chen RY. Chemical constituents of Camellia sinensis var. assamica. Zhongguo Zhong yao za. 2013;38:1386–9. https://doi.org/10.4268/cjcmm20130925 .
Sarangi AB. Medicinal and therapeutic potentialities of tea ( Camellia sinensis L.)—a review. Food Res Int. 2009;42:529–35. https://doi.org/10.1016/j.foodres.2009.01.007 .
Hamilton-Miller JM. Antimicrobial properties of tea ( Camellia sinensis L.) antimicrobe. Agents Chemother. 1995;39:2375–7.
Grutenherman F, Russo E. Cannabis and cannabinoids, pharmacology, toxicology and therapeutic potential. New York: The Howarth Integrative Healing Press; 2002.
Nuutinen T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus . Eur J Med Chem. 2018;157:198–228. https://doi.org/10.1016/j.ejmech.2018.07.076 .
Siddiqui BS, Aslam H, Ali ST, Khan S, Begum S. Chemical constituents of Centella asiatica . J Asian Nat Prod Res. 2007;9:407–14. https://doi.org/10.1080/10286020600782454 .
Kalshetty P, Aswar U, Bodhankar S, Sinnathambi A, Mohan V, Thakurdesai P. Antidepressant effects of standardized extract of Centella asiatica L. in olfactory bulbectomy model. Biomed Aging Pathol. 2012;2:48–53. https://doi.org/10.1016/j.biomag.2012.03.005 .
Punturee K, Wild CP, Kasinrerk W, Vinitketkumnuen U. Immunomodulatory activities of Centella asiatica and Rhinacanthus nasutus extracts. Asian Pac J Cancer Prev. 2005;6:396–400.
Marin A, Ferreres F, Tomás-Barberán FA, Gil MI. Characterization and quantitation of antioxidant constituents of sweet pepper ( Capsicum annuum L.). J Agric Food Chem. 2004;52:3861–9. https://doi.org/10.1021/jf0497915 .
Khan FA, Mahmood T, Ali M, Saeed A, Maalik A. Pharmacological importance of an ethnobotanical plant: Capsicum annuum L. Nat Prod Res. 2014;28:1267–74. https://doi.org/10.1080/14786419.2014.895723 .
Yogiraj V, Goyal PK, Chauhan CS, Goyal A, Vyas B. Carica papaya Linn: an overview. Int J Herbal Med. 2014;2:1–8.
Adam Y, Nasaruddin AA, Zuraini A, Arifah AK, Zakaria MOFZ, Somchit MN. Diuretic activity of roots from Carica papaya L. and Ananas comosus L. Int J Pharm Sci Rev Res. 2013;23:163–7.
Krishna KL, Paridhavi M, Patel KL. Review on nutritional, medicinal and pharmacological properties of Papaya ( Carica papaya Linn.). Nat Prod Radiance. 2008;7:364–73. http://nopr.niscair.res.in/handle/123456789/5695
Prakash D, Upadhyay G, Gupta C, Pushpangadan P, Singh KK. Antioxidant and free radical scavenging activities of some promising wild edible fruits. Int Food Res J. 2012;19:1109–16.
Ferreres F, Pereira DM, Valentão P, Andrade PB, Seabra RM, Sottomayor M. New Phenolic compounds and antioxidant potential of Catharanthus roseus . J Agric Food Chem. 2008;56:9967–74.
Aslam J, Khan SH, Siddiqui ZH, Fatima Z, Maqsood M, Bhat MA, Nasim SA, Ilah A, Ahmad IZ, Khan SA, Mujib A. Catharanthus roseus (L.) G. Don. An important drug: it’s applications and production. Pharmacie Globale. 2010;4:1–16.
Gajalakshmi S, Vijayalakshmi SD, Rajeswari V. Pharmacological activities of Catharanthus roseus : a perspective review. Int J Pharm Bio Sci. 2013;4:431–9.
Hassan HM, Eldesoky AM, Al-Rashdi A, Ahmed HM. Green corrosion electrochemistry: Cheilocostus speciosus extract (CSE) investigated electro-analytically as a potential green corrosion inhibitor for copper within acidic solution. Int J Emerg Trends Eng Develop. 2016;7:72–98.
Ayam VS, Gogoi P. Evaluation of antioxidant activity of the selected frequently used traditional wild medicinal plants from Lakhimpur, Assam (India). Pleione. 2018;12:187–92. https://doi.org/10.26679/Pleione.12.2.2018 .
Benelli G, Govindarajan M, Rajeswary M, Vaseeharan B, Alyahya SA, Alharbi NS, Kadaikunnan S, Khaled JM, Maggi F. Insecticidal activity of camphene, zerumbone and α-humulene from Cheilocostus speciosus rhizome essential oil against the Old-World bollworm Helicoverpa armigera . Ecotoxicol Environ Saf. 2018;148:781–6. https://doi.org/10.1016/j.ecoenv.2017.11.044 .
Owolabi MS, Ogundajo A, Yusuf KO, Lajide L, Villanueva HE, Tuten JA, Setzer WN. Chemical composition and bioactivity of the essential oil of Chromolaena odorata from Nigeria. Rec Nat Prod. 2010;4:72–8.
Taiwo OB, Olajide OA, Soyannwo OO, Makinde JM. Anti-inflammatory, antipyretic and antispasmodic properties of Chromolaena Odorata . Pharm Biol. 2000;38:367–70. https://doi.org/10.1076/phbi.38.5.367.5970 .
Vaisakh MN, Pandey A. The invasive weed with healing properties: a review on Chromolaena odorata . Int J Pharm Sci Res. 2012;3:80–3.
Abyaneh M, Shams-Ghahfarokhi M, Rezaee MB, Jaimand K, Alinezhad S, Saberi R, Yoshinari T. Chemical composition and antiaflatoxigenic activity of Carum carvi L., Thymus vulgaris and Citrus aurantifolia essential oils. Food Control. 2009;20:1018–24. https://doi.org/10.1016/j.foodcont.2008.12.007 .
Pathan RK, Gali PR, Pathan P, Gowtham T, Pasupuleti S. In vitro antimicrobial activity of Citrus aurantifolia and its phytochemical screening. Asian Pac J Trop Dis. 2012;2:328–31. https://doi.org/10.1016/S2222-1808(12)60176-5 .
Taur DJ, Kulkarni VB, Patil RY, Patil RN. Anthelmintic activity of Ocimum sanctum and Citrus aurantifolia oils. Pharmacologyonline. 2009;3:495–9.
Xu YR, Zhang KF, Xie QJ, Lin JX, Huan KX, Liao Y. Chemical constituents from young fruits of Citrus maxima cv. Shatian. Zhong Yao Cai. 2015;38:1879–81.
CAS PubMed Google Scholar
Abirami A, Nagarani G, Siddhuraju P. Antimicrobial activity of crude extract of Citrus hystrix and Citrus maxima . Int J Pharm Sci. 2013;4:1–5.
Shivananda A, Rao DM, Jayaveera KN. Analgesic and anti-inflammatory activities of Citrus maxima (J. Burm) Merr in animal models. Res J Pharm Biol Chem Sci. 2013;4:1800–10. http://rjpbcs.com/pdf/2013_4(2 )/
Kefford JF. The chemical constituents of citrus fruits. Adv Food Res. 1960;2016(9):285–372. https://doi.org/10.1016/S0065-2628(08)60278-5 .
Szczykutowicz MK, Szopa A, Ekiert H. Citrus limon (Lemon) phenomenon—a review of the chemistry, pharmacological properties, applications in the modern pharmaceutical, food, and cosmetics industries, and biotechnological studies. Plants. 2020;9:119. https://doi.org/10.3390/plants9010119 .
Jadeja RN, Thounaojam MC, Singh TB, Devkar RV, Ramachandran AV. Traditional uses, phytochemistry and pharmacology of Clerodendron glandulosum Coleb—a review. Asian Pac J Trop Med. 2012;5:1–6. https://doi.org/10.1016/S1995-7645(11)60236-8 .
Prajapati R, Kalariya M, Umbarkar R, Parmar S, Sheth N. Colocasia esculenta : a potent indigenous plant. Int J Nutr Pharmacol Neurol Dis. 2011;1:90–6. http://www.ijnpnd.com/text.asp?2011/1/2/90/84188
Agyare C, Boakye YD, Apenteng JA, Dapaah SO, Appiah T, Adow A. Antimicrobial and anti-inflammatory properties of Anchomanes difformis (Bl.) Engl. and Colocasia esculenta (L.) Schott. Biochem Pharmacol. 2016;5:1. https://doi.org/10.4172/2167-0501.1000201.
Kubde MS, Khadabadi SS, Farooqui IA, Deore SL. In-vitro anthelmintic activity of Colocasia esculenta . Der Pharma Lett. 2010;2:82–5.
Patil BR, Ageely HM. Antihepatotoxic activity of Colocasia esculenta leaf juice. Int J Adv Biotech Res. 2011;2:296–304.
Koné WM, Vargas M, Keiser J. Anthelmintic activity of medicinal plants used in Côte d’Ivoire for treating parasitic diseases. Parasitol Res. 2012;110:2351–62. https://doi.org/10.1007/s00436-011-2771-z .
Joshi RK. Study on essential oil composition of the roots of Crassocephalum crepidioides (Benth.) S. Moore. J Chil Chem Soc. 2014;59:2363–5. https://doi.org/10.4067/S0717-97072014000100025 .
Tomimori K, Nakama S, Kimura R, Tamaki K, Ishikawa C, Mori N. Antitumor activity and macrophage nitric oxide producing action of medicinal herb Crassocephalum crepidioides . BMC Complement Altern Med. 2012;12:1–11. https://doi.org/10.1186/1472-6882-12-78 .
Bahar E, Akter KM, Lee GH, Lee HY, Rashid HO, Choi MK, Bhattarai KR, Hossain MMM, Ara J, Mazumder K, Raihan O. β-Cell protection and antidiabetic activities of Crassocephalum crepidioides (Asteraceae) Benth. S. Moore extract against alloxan-induced oxidative stress via regulation of apoptosis and reactive oxygen species (ROS). BMC Complement Altern Med. 2017;17:179. https://doi.org/10.1186/s12906-017-1697-0 .
Rezig L, Chouaibi M, Msaada K, Hamdi H. Chemical composition and profile characterisation of pumpkin ( Cucurbita maxima ) seed oil. Ind Crops Prod. 2012;37:82–7. https://doi.org/10.1016/j.indcrop.2011.12.004 .
Md PM, Md TK. Overview on Cucurbita maxima seed. J Dent Med Sci. 2017;16:29–33. https://doi.org/10.9790/0853-1603132933 .
Borah A. Chemical composition, antioxidant, anti-inflammatory, anti-microbial and in-vitro cytotoxic efficacy of essential oil of Curcuma caesia Roxb. leaves: an endangered medicinal plant of North East India. Ind Crops Prod. 2019;129:448–54. https://doi.org/10.1016/j.indcrop.2018.12.035 .
Baghel SS, Baghel RS, Sharma K, Sikarwar I. Pharmacological activities of Curcuma caesia . Int J Green Pharm. 2013;7:1–5. https://doi.org/10.22377/ijgp.v7i1.287 .
Lateef EA. Bioactive chemical constituents of Curcuma longa L. rhizomes extract inhibit the growth of human hepatoma cell line (HepG2). Acta Pharm. 2016;66:387–98. https://doi.org/10.1515/acph-2016-0028 .
Krup V, Prakash LH, Harini A. Pharmacological activities of turmeric ( Curcuma longa Linn): a review. J Homeop Ayurv Med. 2013;2:133. https://doi.org/10.4172/2167-1206.1000133 .
Wu XY, Chao ZM, Wang C, Tan ZG, Sun W. Chemical constituents contained in fatty oil from seeds of Cucumis sativus . Zhongguo Zhong Yao Za Zhi. 2012;37:3252–5.
Saeedi R, Sultana A, Rahman K. Ethnomedicinal uses and pharmacological activities of different parts of Cucumis sativus Linn: an update. Int J Pharm Sci Res. 2020;11:1549–56. https://doi.org/10.13040/IJPSR.0975-8232.11,4,1549-56 .
Zhu SH, Zhang QJ, Chen Q, Zhou T, Yao RJ. Study on the chemical constituents of Dichrocephala integrifolia . Zhong Yao Cai. 2010;33:53–5.
Emégam NK, Nguepi MSD, Lambou AF, Okomolo FCM, Sotoing GT, Bougolla DP, Pale S, Kameni JSN, Bum EN. Antioxidant properties of Dichrocephala integrifolia (Asteraceae) in a mouse model of monosodium glutamate-induced neurotoxicity. Afr J Tradit Complement Altern Med. 2017;14:147–55.
Kouémou NE, Taiwe GS, Moto FC, Pale S, Ngoupaye GT, Njapdounke JS, Nkantchoua GC, Pahaye DB, Bum EN. Nootropic and neuroprotective effects of Dichrocephala integrifolia on scopolamine mouse model of Alheimer’s disease. Front Pharmacol. 2017. https://doi.org/10.3389/fphar.2017.00847 .
Lee CL, Yen MH, Hwang TL, Yang JC, Peng CY, Chen CJ, Chang WY, Wu YC. Anti-inflammatory and cytotoxic component from Dichrocephala integrifolia . Phytochem Lett. 2015;12:237–42. https://doi.org/10.1016/j.phytol.2015.04.012 .
Gandhi D, Mehta P. Dillenia indica Linn. and Dillenia pentagyna Roxb.: pharmacognostic, phytochemical and therapeutic aspects. J App Pharm Sci. 2013;3:134–42. https://doi.org/10.7324/JAPS.2013.31124 .
Cheng WY, Kuo YH, Huang CJ. Isolation and identification of novel estrogenic compounds in Yam Tuber ( Dioscorea alata Cv. Tainung No. 2). J Agric Food Chem. 2007;55:7350–8. https://doi.org/10.1021/jf0711690 .
Das A, Chaudhuri D, Chatterjee A. Study of antioxidant and reactive oxygen species scavenging activity of the edible tuber of “Greater Yam” ( Dioscorea alata L.) from North-east India. Asian J Pharm Clin Res. 2012;5:74–84.
Maithili V, Dhanabal SP, Mahendran S, Vadivelan R. Antidiabetic activity of ethanolic extract of tubers of Dioscorea alata in alloxan induced diabetic rats. Indian J Pharmacol. 2011;43:455–9.
Nono NR, Nzowa KL, Barboni L, Tapondjou AL. Drymaria cordata (Linn.) willd (caryophyllaceae): ethnobotany, pharmacology and phytochemistry. Adv Biol Chem. 2014;4:160–7. https://doi.org/10.4236/abc.2014.42020 .
Liu HB, Zhang CR, Dong SH, Yang SP, Sun Q, Geng MY, Yue JM. Sesquiterpenes from Dysoxylum oliganthum and Dysoxylum excelsum . J Asian Nat Prod Res. 2012;14:224–34. https://doi.org/10.1080/10286020.2011.645810 .
Jayashree R, Albert S, Minaram N. Medicinal plants of North Cachar Hills district of Assam used by the Dimasa tribe. Indian J Tradit Knowl. 2012;11:520–7. http://nopr.niscair.res.in/handle/123456789/14395.
Sharma BK, Ramashanker SG, Rahaman L, Nath N, Kaipeng DL. Plant based folk treatments from North East India for jaundice (an overview). J Med Plants Stud. 2016;4:234–47.
Lasekan O, Buettner A, Christlbauer M. Investigation of important odorants of palm wine ( Elaeis guineensis ). Food Chem. 2007;105:15–23. https://doi.org/10.1016/j.foodchem.2006.12.052 .
Owoyele BV, Owolabi GO. Traditional oil palm (Elaeis guineensis Jacq.) and its medicinal uses: a review. Tang Human Med. 2014. https://doi.org/10.5667/tang.2014.0004 .
Yin NGS, Abdullah S, Phin CK. Phytochemical constituents from leaves of Elaeis guineensis and their antioxidant and antimicrobial activities. Int J Pharm Pharm Sci. 2013;5:137–40.
Shivprasad M, Varsha J. GC-MS screening of some bioactive compounds from methanolic extract of medicinally relevant wild edible plant parts. Int J Sci Res Sci Tech. 2018;4:49–56.
Sethiya NK, Brahmbhat K, Chauhan B, Mishra SH. Pharmacognostic and phytochemical investigation of Ensete superbum (Roxb.) Cheesman pseudostem. Indian J Nat Prod Resour. 2016;7:51–8. http://nopr.niscair.res.in/handle/123456789/34105.
Akter K, Barnes EC, Loa-Kum-Cheung WL, Yin P, Kichu M, Brophy JJ, Barrow RA, Imchen I, Vemulpad SR, Jamie JF. Antimicrobial and antioxidant activity and chemical characterisation of Erythrina stricta Roxb. (Fabaceae). J Ethnopharmacol. 2016;185:171–81. https://doi.org/10.1016/j.jep.2016.03.011 .
Araújo-Júnior JX, de Oliveira MS, Aquino PG, Alexandre-Moreira MS, Sant’Ana AE. A phytochemical and ethnopharmacological review of the genus Erythrina in Phytochemicals. In: Rao V, editors. A global perspective of their role in nutrition and health. London: InTech; 2012. p. 327–49.
Subhashini N, Purnima S, Devi JA, Thirupathi AT, Lavanya N. Anti-inflammatory activity of Erythrina stricta Roxb. in albino rats. Int J Pharm Tech Res. 2011;3:1014–8.
Hada S, Yadav DK, Roat P, Kumari N. Eulophia Nuda : a review of its traditional uses, phytochemistry and pharmacology. Pharm Chem J. 2020;54:40–5. https://doi.org/10.1007/s11094-020-02152-8 .
Jain JB, Kumane SC, Bhattacharya S. Medicinal flora of Madhya Pradesh and Chattisgarh—a review. Indian J Tradit Knowl. 2006;5:237–42. http://nopr.niscair.res.in/handle/123456789/6845.
Pascal OA, Bertrand AEV, Esaïe T, Sylvie HAM, Eloi AY. A review of the ethnomedical uses, phytochemistry and pharmacology of the Euphorbia genus. J Pharm Innov. 2017;6:34–9.
Rauf A, Muhammad N, Qaisar M, Uddin G, Hussain I. Preliminary antinociceptive studies of methanol extract of Euphorbia milli . Middle-East J Med Plants Res. 2012;1:68–70. https://doi.org/10.5829/idosi.mejmpr.2011.1.3.1115 .
Saleem H, Zengin G, Locatelli M, Mollica A, Ahmad I, Mahomoodally FM, Abidin SAZ, Ahemad N. In vitro biological propensities and chemical profiling of Euphorbia milii Des Moul (Euphorbiaceae): a novel source for bioactive agents. Ind Crops Prod. 2019;130:9–15. https://doi.org/10.1016/j.indcrop.2018.12.062 .
Wang P, Xie C, An L, Yang X, Xi Y, Yuan S, Zhang C, Tuerhong M, Jin DQ, Lee D, Zhang J. Bioactive diterpenoids from the stems of Euphorbia royleana . J Nat Prod. 2019;82:183–93. https://doi.org/10.1021/acs.jnatprod.8b00493 .
Ashraf A, Sarfraz RA, Rashid MA, Shahi M. Antioxidant, antimicrobial, antitumor, and cytotoxic activities of an important medicinal plant ( Euphorbia royleana ) from Pakistan. J Food Drug Anal. 2015;23:109–15. https://doi.org/10.1016/j.jfda.2014.05.007 .
Bani S, Kaul A, Jaggi BS, Suri KA, Suri OP, Sharma OP. Anti-inflammatory activity of the hydrosoluble fraction of Euphorbia royleana latex. Fitoterapia. 2000;71:655–62. https://doi.org/10.1016/S0367-326X(00)00225-2 .
Wang GC, Liang JP, Wang Y, Li Q, Ye WC. Chemical constituents from Flueggea virosa . Chin J Nat Med. 2008;6:251–3. https://doi.org/10.1016/S1875-5364(09)60022-4 .
Chao CH, Cheng JC, Shen DY, Huang HC, Wu YC, Wu TS. Terpenoids from Flueggea virosa and their anti-hepatitis C virus activity. Phytochemistry. 2016;128:60–70. https://doi.org/10.1016/j.phytochem.2016.04.003 .
Ezeonwumelu JOC, Omar AN, Ajayi AM, Okoruwa AG, Tanayen JK, Kiplagat DM, Okpanachi OA, Abba S, Ezekiel I, Onchweri AN, Okonkwo CO. Phytochemical screening, acute toxicity, anti-inflammatory and anti-pyretic studies of aqueous extract of the root of Flueggea virosa (Roxb. ex Willd.) in rats. Int J Pharm Biomed Sci. 2012;3:128–35.
Wang GC, Li T, Deng FY, Li YL, Ye WC. Five new phenolic glycosides from Hedyotis scandens . Bioorganic Med Chem Lett. 2013;5:1379–82. https://doi.org/10.1016/j.bmcl.2012.12.077 .
Rahman MA, Uddin SB, Wilcock CC. Medicinal plants used by Chakma tribe in Hill Tracts districts of Bangladesh. Indian J Tradit Knowl. 2007;6:508–17. http://nopr.niscair.res.in/handle/123456789/991
Subba B, Basne P. Antimicrobial activity of some medicinal plants from east and central part of Nepal. Int J Appl Sci Biotechnol. 2014;2:88–92. https://doi.org/10.3126/ijasbt.v2i1.9697 .
Jadhav VM, Thorat RM, Kadam VJ, Sathe NS. Hibiscus rosa sinensis Linn – ‘“Rudrapuspa”’: a review. J Pharm Res. 2009;2:1168–73.
Mak YM, Chuah LO, Ahmad R, Bhat R. Antioxidant and antibacterial activities of hibiscus ( Hibiscus rosa-sinensis L.) and Cassia ( Senna bicapsularis L.) flower extracts. J King Saud Univ Sci. 2013;25(4):275–82. https://doi.org/10.1016/j.jksus.2012.12.003 .
Fu J, Dai L, Lin Z, Lu H. Houttuynia cordata Thunb: a review of phytochemistry and pharmacology and quality control. Chinese Med. 2013;4:101–23. https://doi.org/10.4236/cm.2013.43015 .
Mohanraj R, Sivasankar S. Sweet potato ( Ipomoea batatas [L.] Lam)—a valuable medicinal food: a review. J Med Food. 2014;17:733–41. https://doi.org/10.1089/jmf.2013.2818 .
Hossain S, Ahmed R, Bhowmick S, Al Mamun A, Hashimoto M. Proximate composition and fatty acid analysis of Lablab purpureus (L.) legume seed: implicates to both protein and essential fatty acid supplementation. Springer Plus. 2016;5:1899. https://doi.org/10.1186/s40064-016-3587-1 .
Al-Snafi AE. The pharmacology and medical importance of Dolichos lablab ( Lablab purpureus )—a review. IOSR J Pharm. 2017;7:22–30.
Wei JC, Wang PC, Zhou XI. The caffeoyl phenylethanoid glycosides from Lindernia ruellioides and their anti-HBV effects. J Asian Nat Prod Res. 2018;20:757–62. https://doi.org/10.1080/10286020.2017.1357549 .
Das AK, Nongmaithem R. Phytochemical study of selected medicinal plants used by the maring tribe of Chandel district, Manipur. India J Pharmacogn Phytochem. 2019;8:2155–60.
Kuruvilla GR, Neeraja M, Srikrishna A, Rao SGRS. A new quinone from Maesa indica ( Roxb) A DC, (Myrsinaceae). Indian J Chem Sect B. 2010;49:1637–41.
Patil A, Jadhav V, Arvindekar A, More T. Antidiabetic activity of Maesa indica (Roxb). stem bark in Streptozotoc in induced diabetic rats. American J Phytomed Clin Ther. 2014;2:957–62.
Wei L, Wee W, Siong J, Syamsumir D. Characterization of antimicrobial, antioxidant, anticancer property and chemical composition of Michelia champaca seed and flower extracts. Stamford J Pharm Sci. 2011;4:19–24. https://doi.org/10.3329/sjps.v4i1.8862 .
Vimala R, Nagarajan S, Alam M, Susan T, Joy S. Anti-inflammatory and antipyretic activity of Michelia champaca Linn., (White variety), Ixora Brachiata Roxb. and Rhynchosia Cana (Willd.) D.C. flower extract. Indian J Exp Biol. 1997;35:1310–4.
Dan GD, Yi Z, Wei LE, Tao W, Min HL. Chemical constituents of Mangifera indica leaves (I). Zhong Cao Yao. 2011;42:428–31. http://www.ceps.com.tw/ec/ecJnlIntro.aspx?Jnliid=2790.
Parvez GMM. Pharmacological activities of mango ( Mangifera indica ): a review. J Pharmacogn Phytochem. 2016;5:1–7.
Pandey AK, Rai MK, Acharya D. Chemical composition and antimycotic activity of the essential oils of corn mint ( Mentha arvensis ) and lemon grass ( Cymbopogon flexuosus ) against human pathogenic fungi. Pharm Biol. 2003;41:421–5. https://doi.org/10.1076/phbi.41.6.421.17825 .
Thawkar BS, Jawarkar AG, Kalamkar PV, Pawar KP, Kale MK. Phytochemical and pharmacological review of Mentha arvensis . Int J Green Pharm. 2016;10:2. https://doi.org/10.22377/ijgp.v10i2.643 .
Chow YL, Quon HH. Chemical constituents of the heartwood of Mesua ferrea . Phytochemistry. 1968;7:1871–4. https://doi.org/10.1016/S0031-9422(00)86662-5 .
Chahar MK, Sanjaya KDS, Geetha L, Lokesh T, Manohara KP. Mesua ferrea L.: a review of the medical evidence for its phytochemistry and pharmacological actions. Afr J Pharm Pharmacol. 2013;7:211–9. https://doi.org/10.5897/AJPP12.895 .
Shao H, Nan P, Peng S, Zhang C. Study of chemical constituents of essential oil from flowers of Mikania micrantha . Zhong Yao Cai. 2001;24:341–2.
Dev UK, Md Hossain T, Md IZ. Phytochemical investigation, antioxidant activity and antihelmintic activity of Mikania micrantha leaves. World J Pharma Res. 2015;4:121–33.
Lentz DL, Clark AM, Hufford CD, Meurer-Grimes B, Passreiter CM, Cordero J, Ibrahimi O, Okunade AL. Antimicrobial properties of Honduran medicinal plants. J Ethnopharmacol. 1998;63:253–63. https://doi.org/10.1016/S0378-8741(98)00100-7 .
Yuan K, Lü JL, Yin MW. Chemical constituents of C-glycosylflavones from Mimosa pudica . Yao Xue Xue Bao. 2006;41:435–8.
Joseph B, George J, Mohan J. Pharmacology and traditional uses of Mimosa pudica . Int J Pharma Sci Drug Res. 2013;5:41–4.
Farrag EK, Kassem MES, Bayoumi D, Shaker SE, Afifi MS. Phytochemical study, phenolic profile and antigastric ulcer activity of Morus macroura Miq. fruits extract. J Appl Pharm Sci. 2017;7:152–60. https://doi.org/10.7324/JAPS.2017.70527 .
Sidhu JS, Zafar TA. Bioactive compounds in banana fruits and their health benefits. Food Qual Saf. 2018;2:183–8. https://doi.org/10.1093/fqsafe/fyy019 .
Jyothirmayi N, Rao NM. Banana medicinal uses. J Med Sci Tech. 2015;4:152–60.
Mathew NS, Negi PS. Traditional uses, phytochemistry and pharmacology of wild banana ( Musa acuminata Colla): a review. J Ethnopharmacol. 2017;196:124–40. https://doi.org/10.1016/j.jep.2016.12.009 .
Kim NC, Desjardins AE, Wu CD, Kinghorn AD. Activity of triterpenoid glycosides from the root bark of Mussaenda macrophylla against two oral pathogens. J Nat Prod. 1999;62:1379–84. https://doi.org/10.1021/np9901579 .
Chowdhury SR, Akter S, Sharmin T, Islam F, Quadery TM. Antimicrobial activity of five medicinal plants of Bangladesh. J Pharmacogn Phytochem. 2013;2:164–70.
Shadia E, El-Aziz A, Omer EA, Sabra AS. Chemical composition of Ocimum americanum essential oil and its biological effects against, agrotis ipsilon, (Lepidoptera: Noctuidae). Res J Agric Biol Sci. 2007;3:740–7.
Hakkim FL, Arivazhagan G, Boopathy R. Antioxidant property of selected Ocimum species and their secondary metabolite content. J Med Plant Res. 2008;2:250–7.
Thaweboon S, Thaweboon B. In vitro antimicrobial activity of Ocimum americanum L. essential oil against oral microorganisms. Southeast Asian J Trop Med Public Health. 2009;40:1025–33.
Ahad A, Ganai AA, Sareer O, Najm MZ, Kausar MA, Mujeeb M, Siddiqui WA. Therapeutic potential of oroxylum indicum : a review. J Pharma Res Opinion. 2012;2:163–72.
Singh HV, Chaudhary AK. A review on the taxonomy, ethnobotany, chemistry and pharmacology of Oroxylum indicum Vent. Indian J Pharm Sci. 2011;73:483–90.
Hussain A, Oves M, Alajmi MF, Hussain I, Amir S, Ahmed J, Rehman MT, El-Seedi HR, Ali I. Biogenesis of ZnO nanoparticles using Pandanus odorifer leaf extract: anticancer and antimicrobial activities. RSC Adv. 2019;9:15357–69. https://doi.org/10.1039/C9RA01659G .
Angami T, Bhagawati R, Touthang L, Makdoh B, Bharati KA, Silambarasan R, Ayyanar M. Traditional uses, phytochemistry and biological activities of Parkia timoriana (DC.) Merr., an underutilized multipurpose tree bean: a review. Genet Resour Crop Evol. 2018;65:679–92. https://doi.org/10.1007/s10722-017-0595-0 .
Gaire BP, Subedi L. Phytochemistry, pharmacology and medicinal properties of Phyllanthus emblica Linn. Chin J Integr Med. 2014. https://doi.org/10.1007/s11655-014-1984-2 .
Lin HY, Yuan CY, Xin WY, Peng LD, Juan CW, Lei LJ, Lai LF. Chemical constituents of Picria fel-terrae . Guangxi Zhiwu Guihaia. 2010;30:887–90.
Kumarasingha R, Karpe AV, Preston S, Yeo TC, Lim DS, Tu CL, Luu J, Simpson KJ, Shaw JM, Gasser RB, Beale DJ. Metabolic profiling and in vitro assessment of anthelmintic fractions of Picria fel-terrae Lour. Int J Parasitol Drugs Drug Resist. 2016;6:171–8. https://doi.org/10.1016/j.ijpddr.2016.08.002 .
Satria D, Silalahi J, Haro G, Ilyas S, Hsb PAZ. Antioxidant and antiproliferative activities of an ethylacetate fraction of Picria fel-terrae Lour. Herbs. Asian Pac J Cancer Prev. 2017;18:399–403.
PubMed PubMed Central Google Scholar
Samuelsen AB. The traditional uses, chemical constituents and biological activities of Plantago major L. a review. J Ethnopharmacol. 2000;71:1–21. https://doi.org/10.13040/IJPSR.0975-8232.11(4).1549-56 .
Begum S, Hassan SI, Ali SN, Siddiqui BS. Chemical constituents from the leaves of Psidium guajava . Nat Prod Res. 2004;18:135–40. https://doi.org/10.1080/14786410310001608019 .
Martha R, Gutiérrez P, Mitchell S, Solis RS. Psidium guajava : a review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2008;117:1–27. https://doi.org/10.1016/j.jep.2008.01.025 .
Tai Z, Zhang F, Cai L, Shi J, Cao Q, Ding Z. Flavonol glycosides of Pseudodrynaria coronans and their antioxidant activity. Chem Nat Compd. 2012;48:221–4. https://doi.org/10.1007/s10600-012-0209-1 .
Jasuja ND, Saxena R, Chandra S, Sharma R. Pharmacological characterization and beneficial uses of Punica granatum . Asain J Plant Sci. 2012;11:251–67.
Djakpo O, Yao W. Rhus chinensis and Galla chinensis—Folklore to modern evidence: review. Phytother Res. 2010;24:1739–47. https://doi.org/10.1002/ptr.3215 .
Rishi P, Bimala S. Extraction and isolation of chemical constituents from Schima wallichii Bark. Int J Eng Sci Res Tech. 2014;3:175–80.
Das S, Ghosh LK. Evaluation of analgesic, antipyretic and anti-inflammatory activity of different fractions of Schima wallichii barks. Pharmacologia. 2013;4:400–3. https://doi.org/10.5567/pharmacologia.2013.400.403 .
Sarbadhikary SB, Bhowmik S, Datta BK, Mandal NC. Antimicrobial and antioxidant activity of leaf extracts of two indigenous angiosperm species of Tripura. Int J Curr Microbiol Appl Sci. 2015;4:643–55.
Wang D, Huang L, Chen S. Senecio scandens Buch-Ham.: a review on its ethnopharmacology, phytochemistry, pharmacology, and toxicity. J Ethnopharmacol. 2013;149:1–23. https://doi.org/10.1016/j.jep.2013.05.048 .
Hua S, Zhang Y, Liu J, Dong L, Huang J, Lin D, Fu X. Ethnomedicine, phytochemistry and pharmacology of Smilax glabra : an important traditional Chinese medicine. Am J Chin Med. 2018;46:261–97. https://doi.org/10.1142/S0192415X18500143 .
Biao CY, Ming ZD, Shan YS. Chemical constituents of Smilax perfoliata . Acta Bot Sin. 2004;46:618–20.
Borkataky M. Antimcrobial and antioxidant activity of Smilax perfoliate Lour. Der Pharm Lett. 2014;6:246–50.
Zhao Y, Liu F, Lou HX. Studies on the chemical constituents of Solanum nigrum . Zhong Yao Cai. 2010;33:555–6.
Hameed HI, Calixto CMR, Yahya HM. A review: Solanum nigrum L. antimicrobial, antioxidant properties, hepatoprotective effects and analysis of bioactive natural compounds. Res J Pharm Tech. 2017;10:4063–8. https://doi.org/10.5958/0974-360X.2017.00737.5 .
Jain RAS, Gupta SSPI, Gabrani R. Solanum nigrum : current perspectives on therapeutic properties. Altern Med Rev. 2011;16:78–85.
Chang FR, Yen CT, El-Shazly M, Yu CY, Yen MH, Cheng YB, Chen SL, Wu YC. Spirostanoids with 1,4-dien-3-one or 3β,7α-diol-5,6-ene moieties from Solanum violaceum . Bioorg Med Chem Lett. 2013;23:2738–42. https://doi.org/10.1016/j.bmcl.2013.02.060 .
Karim A, Islam B, Tareq SM, Islam MT. Antinociceptive and antipyretic activities of Solanum violaceum Ortega. Int J Med. 2017;5:90–3. https://doi.org/10.14419/ijm.v5i1.7282 .
Mahaldar K, SaifuzMahaldar K, Saifuzzaman M, Irin T, Barman AK, Islam MK, Rahman MM, Islam MA. Analgesic, anthelmintic and toxicity studies of Solanum violaceum Linn. leaves. Orient Pharm Exp Med. 2016;16:147–52. https://doi.org/10.1007/s13596-016-0227-9 .
Bora NS, Kakoti BB, Gogoi B, Goswami AK. Ethno-medicinal claims, phytochemistry and pharmacology of Spondias pinnata : a review. Int J pharm Sci Res. 2014;5:1138–45. https://doi.org/10.13040/IJPSR.0975-8232.5(4) .
Goswami DV, Nirmal SA, Patil MJ, Dighe NS, Laware NS, Patta SR. An overview of Tectona grandis : chemistry and pharmacological profile. Phcog Rev. 2009;3:181–5.
Vyas P, Yadav DK, Khandelwal P. Tectona grandis (Teak)—a review on its phytochemical and therapeutic potential. Nat Prod Res. 2019;33:2338–54.
El-Ghorab A, El-Massry KF, Shibamoto T. Chemical composition of the volatile extract and antioxidant activities of the volatile and nonvolatile extracts of egyptian corn silk ( Zea mays L.). J Agric Food Chem. 2007;55:9124–7. https://doi.org/10.1021/jf071646e .
Mendoza-Díaz S, del Carmen Ortiz-Valerio M, Castaño-Tostado E, de Dios Figueroa-Cardenas J, Reynoso-Camacho R, Ramos-Gómez M, Campos-Vega R, Loarca-Piña G. Antioxidant capacity and antimutagenic activity of anthocyanin and carotenoid extracts from nixtamalized pigmented creole maize races ( Zea mays L.). Plant Foods Hum Nutr. 2012;67:442–9. https://doi.org/10.1007/s11130-012-0326-9 .
Nessa F, Ismail Z, Mohamed N. Antimicrobial activities of extracts and flavonoid glycosides of corn silk ( Zea mays L.). Int J Biotechnol Wellness Ind. 2012;1:115–21.
Gupta SK, Sharma A. Medicinal properties of Zingiber officinale Roscoe—a review. IOSR J Pharm Biol Sci. 2011;5:124–9.
Rehman R, Akram M, Akhtar N, Jabeen Q, Shah SA, Ahmed K, Shaheen G, Asif HM. Zingiber officinale Roscoe (Parmacological activity). J Med Plants Res. 2011;5:344–8.
Çakılcıoğlu U, Khatun S, Türkoğlu I, Hayta S. Ethnopharmacological survey of medicinal plants in Maden (Elazığ–Turkey). J Ethnopharmacol. 2011;137:469–86. https://doi.org/10.1016/j.jep.2011.05.046 .
Akindele AJ, Ibe IF, Adeyemi OO. Analgesic and antipyretic activities of Drymaria cordata (Linn.) Willd (Caryophyllaceae) extract. Afr J Tradit Complement Altern Med. 2012;9:25–35.
Proma JJ, Faruque MO, Rahman S, Bashar ABMA, Rahmatullah M. Analgesic potential and phytochemical screening of Lablab purpureus aerial parts. World J Pharm Pharmaceut Sci. 2014;3:165–73.
Bakir M, Facey PC, Hassan I, Mulder WH, Porter RB. Mikanolide from Jamaican Mikania micrantha . Acta Crystallogr C. 2004;6:11. https://doi.org/10.1107/S0108270104017809 .
Koriem KM, Arbid MS, Saleh HN. Antidiarrheal and protein conservative activities of Psidium guajava in diarrheal rats. J Integr Med. 2019;17:57–65. https://doi.org/10.1016/j.joim.2018.12.001 .
Salama AA, El-Kassaby MI, Hassan A. Anti-urolithiatic activity of Solanum nigrum hydroalcoholic extract in ethylene glycol-induced urolithiasis in rats. Egypt Pharmaceut J. 2019;18:311. https://www.epj.eg.net/text.asp?2019/18/4/311/272269 .
Yirga G, Teferi M, Gidey G, Zerabruk S. An ethnoveterinary survey of medicinal plants used to treat livestock diseases in Seharti-Samre district, Northern Ethiopia. Afr J Plant Sci. 2012;6:113–9. https://doi.org/10.5897/AJPS11.242 .
Parthiban R, Vijayakumar S, Prabhu S, Gnanaselvam J, Yabesh EM. Quantitative traditional knowledge of medicinal plants used to treat livestock diseases from Kudavasal taluk of Thiruvarur district, Tamil Nadu, India. Rev Bras Farmacogn. 2016;26:109–21. https://doi.org/10.1016/j.bjp.2015.07.016 .
Jadid N, Kurniawan E, Himayani CES, Prasetyowati I, Purwani KI, Muslihatin W, Hidayati D, Tjahjaningrum ITD. An ethnobotanical study of medicinal plants used by the Tengger tribe in Ngadisari village, Indonesia. PLoS ONE. 2020;15:7. https://doi.org/10.1371/journal.pone.0235886 .
Moerman DE. Symbols and selectivity: a statistical analysis of native American medical ethnobotany. J Ethnopharmacol. 1979;1:111–9. https://doi.org/10.1016/0378-8741(79)90002-3 .
Kutal DH, Kunwar RM, Uprety Y, Adhikari YP, Bhattarai S, Adhikari B, Kunwar LM, Bhatt MD, Bussmann RW. Selection of medicinal plants for traditional medicines in Nepal. J Eethnobiol Ethnomed. 2021;17(1):1–11. https://doi.org/10.1186/s13002-021-00486-5 .
Addo-Fordjour P, Kofi Anning A, Durosimi Belford EJ, Akonnor D. Diversity and conservation of medicinal plants in the Bomaa community of the Brong Ahafo region, Ghana. J Med Plants Res. 2008;2:226–33.
Ghorbani A. Studies on pharmaceutical ethnobotany in the region of Turkmen Sahra, north of Iran (Part 1): general results. J Ethnopharmacol. 2005;102:58–68. https://doi.org/10.1016/j.jep.2005.05.035 .
Poonam K, Singh GS. Ethnobotanical study of medicinal plants used by the Taungya community in Terai Arc Landscape, India. J Ethnopharmacol. 2009;123:167–76. https://doi.org/10.1016/j.jep.2009.02.037 .
Luseba D, Tshisikhawe MP. Medicinal plants used in the treatment of live-stock diseases in Vhembe region, Limpopo province. S Afr J Med Plants Res. 2014;7:593–601. https://doi.org/10.5897/JMPR12.1213 .
Mahwasane ST, Middleton L, Boaduo N. An ethnobotanical survey of indigenous knowledge on medicinal plants used by the traditional healers of the Lwamondo area, Limpopo province, South Africa. S Afr J Bot. 2013;88:69–75. https://doi.org/10.1016/j.sajb.2013.05.004 .
Saha MR, Sarker DD, Sen A. Ethnoveterinary practices among the tribal community of Malda district of West Bengal, India. Indian J Tradit Knowl. 2014;13: 359–67. http://nopr.niscair.res.in/handle/123456789/27931
Verma RK, Kumari P, Maurya RK, Kumar V, Verma RB, Singh RK. Medicinal properties of turmeric ( Curcuma longa L.): a review. Int J Chem Stud. 2018;6(4):1354–7.
Singh AG, Kumar A, Tewari DD. An ethnobotanical survey of medicinal plants used in Terai forest of western Nepal. J Ethnobiol Ethnomedicine. 2012;8:19. https://doi.org/10.1186/1746-4269-8-19 .
Chaudhary MI, He Q, Cheng YY, Xiao PG. Ethnobotany of medicinal plants from Tian Mu Shan biosphere reserve, Zhejiang-Province, China. Asian J Plant Sci. 2006;5:646–53.
Çakılcıoğlu U, Türkoğlu I. An ethnobotanical survey of medicinal plants in Sivrice (Elazığ-Turkey). J Ethnopharmacol. 2010;132:165–75. https://doi.org/10.1016/j.jep.2010.08.017 .
Rajakumar N, Shivanna MB. Ethnomedicinal application of plants in the eastern region of Shimoga District, Karnataka. India J Ethnopharmacol. 2009;126:64–73. https://doi.org/10.1016/j.jep.2009.08.010 .
Download references
Acknowledgements
The authors were thankful to the department of Horticulture, Aromatic and Medicinal Plant, Mizoram University, for providing the necessary facilities to complete our work. We also offer our heartfelt gratitude to all the local people of Champhai district, Mizoram, who shared their valuable knowledge and precious time for this research.
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and affiliations.
Department of Horticulture, Aromatic and Medicinal Plants, School of Earth Sciences and Natural Resources Management, Mizoram University, Aizawl, 796004, India
T. B. C. Laldingliani, Nurpen Meitei Thangjam, Laldingngheti Bawitlung & Awadhesh Kumar
Department of Botany, School of Life Science, Mizoram University, Aizawl, 796004, India
R. Zomuanawma
Bioprospection and Product Development, CSIR-Central Institute of Medicinal and Aromatic Plants, CIMAP, Lucknow, 226015, India
Anirban Pal
You can also search for this author in PubMed Google Scholar
Contributions
TBCL and AK carry out ethnobotanical survey, write the manuscript and analyse the data; TBCL, NMT, RZ, LB and AK were study proposer, design the questionnaire and revise the manuscript; NMT and AP design the graphical abstract; RZ and AP were proof reader. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Awadhesh Kumar .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Laldingliani, T.B.C., Thangjam, N.M., Zomuanawma, R. et al. Ethnomedicinal study of medicinal plants used by Mizo tribes in Champhai district of Mizoram, India. J Ethnobiology Ethnomedicine 18 , 22 (2022). https://doi.org/10.1186/s13002-022-00520-0
Download citation
Received : 26 October 2021
Accepted : 14 March 2022
Published : 24 March 2022
DOI : https://doi.org/10.1186/s13002-022-00520-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ethnomedicinal
- Indo-Burma hotspot
Journal of Ethnobiology and Ethnomedicine
ISSN: 1746-4269
- General enquiries: [email protected]

An ethnobotanical study of traditionally used medicinal plants: Case study from Assam, India
- Meghali Kalita ABS Intern, Assam State Biodiversity Board
- Saklain Mustak Alam
- Shah Nawaz Jelil
Background : Traditional knowledge of medicinal plants, their application, and conservation is very likely to be disappearing. From the perspective of conservation planning and management, it is crucial to assemble information about the use of medicinal herbs by various ethnic groups and at various spatial scales. Therefore, with this viewpoint in mind, this study was carried out, which included the ethnobotanical analysis of medicinal plants in Dimoria, Assam, which contributed to the locally based traditional healthcare system of the people living there.
Methods : The study was conducted with 140 traditional knowledgeable people (78 male and 62 female) being interviewed purposively. Household questionnaire surveys (HQS), key informant interviews (KII), and focus group discussions (FGD) were used for data collection. The use value (UV) score of species, informant consensus factor (ICF), and fidelity level (FL) were computed as standard ethnobotanical data analytical methods.
Results : The current study compiled information on the traditional use of 80 plant species from 45 different botanical groups to treat 56 distinct human diseases. The families that have the most species were Fabaceae followed by Rutaceae, Lamiaceae, Acanthaceae, Amaranthaceae, etc. Leaves (60%) were found the most commonly used plant parts for the treatment of various health ailments. The average UV score was found to be 0.64 (0.40 – 0.89). Based on UV scores, the most used species was Zingiber officinale (UV 0.89) and the least used species were Cereus repandus (UV 0.40). The FL of plant species ranged from 67.09 % to 94.44 % for the eleven categories chosen in the study area. The maximum FL of 94.44 % was found for Azadirachta indica for the dermatological disorder category. In our study, ICF factors for each category of usage ranged from 0.69 to 0.94, with cardiovascular disorder exhibiting the highest ICF value of 0.94.
Conclusions : The results of the study demonstrated that Dimoria is a repository for indigenous knowledge and a highly varied range of reliable plant species. Furthermore, additional research is required to determine the safety and efficacy of described ethnomedicinal plants in treating a variety of diseases, as the findings presented in this paper are preliminary in nature. As a result, the study highlights the potential of ethnomedical research as well as the significance of recording customary knowledge about the use of medicinal plants by the community for the benefit of all people.
Keywords : Ethnobotany; Indigenous; Medicinal plants; Traditional knowledge; Assam
How to Cite
- Endnote/Zotero/Mendeley (RIS)
All articles are copyrighted by the first author and are published online by license from the first author. Articles are intended for free public distribution and discussion without charge. Accuracy of the content is the responsibility of the authors.
Current Issue
Information.
- For Readers
- For Authors
- For Librarians
Make a Submission

- Open access
- Published: 21 May 2024
Ethnomedicine, antibacterial activity, antioxidant potential and phytochemical screening of selected medicinal plants in Dibatie district, Metekel zone, western Ethiopia
- Baressa Anbessa 1 , 2 ,
- Ermias Lulekal 1 ,
- Ariaya Hymete 3 ,
- Asfaw Debella 4 ,
- Eyob Debebe 5 ,
- Abiy Abebe 5 &
- Sileshi Degu 5
BMC Complementary Medicine and Therapies volume 24 , Article number: 199 ( 2024 ) Cite this article
241 Accesses
Metrics details
Medicinal plants play a major role in the delivery of healthcare, particularly among the rural population of Ethiopia. Plant extracts and their bioactive compounds have been utilized for the treatment of several diseases. This study was aimed at evaluating the antibacterial activity, antioxidant capacity, and phytochemical content of selected medicinal plants used in Dibatie district, western Ethiopia.
Study plants were collected, shade dried, pulverized, extracted by maceration in 80% ethanol, and subjected to antibacterial, antioxidant, and phytochemical tests. Minimum inhibitory concentration (MIC) was determined using 96-well microplates and nutrient broth microdilution. Antioxidant activity was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay. Phytochemical screening was conducted using standard test methods.
The ethanolic extract of Polystachya steudneri Rchb.f. pseudobulbs was the most active against gram-negative Proteus mirabilis , Salmonella typhimurium , Klebsiella pneumoniae , Escherichia coli , and Shigella flexneri , with MIC values of 8 ± 0, 11 ± 5, 3 ± 1, 3 ± 1, and 2 ± 0 mg/mL, respectively. The ethanolic extract of P. steudneri was also the most effective against gram-positive Staphylococcus aureus , Staphylococcus epidermidis , Streptococcus agalactiae , and Enterococcus faecalis , with MIC values of 8 ± 0, 8 ± 0, 3 ± 1, and 16 ± 0 mg/mL, respectively. Ethanolic extracts of Gnidia involucrata Steud. ex A.Rich. stems and roots were effective antioxidants, with respective 50% DPPH free radical inhibitory concentrations (IC 50 ) of 168.68 and 181.79 µg/mL, followed by that of P. steudneri (IC 50 = 203.11 µg/mL). The study plants contained alkaloids, anthocyanins, anthraquinones, cardiac glycosides, coumarins, flavonoids, phenols, saponins, steroids, tannins, and terpenoids.
Conclusions
This study confirmed the antibiotic, antioxidant, and phytochemical constituents of the investigated plants and suggested further investigations that may lead to bioactive lead compounds.
Peer Review reports
Infectious diseases are the most common causes of mortality and morbidity among human beings throughout the world [ 1 ]. Recently, the rapid emergence and spread of multidrug-resistant pathogens have been considered major challenges for the treatment of several infectious diseases [ 2 , 3 ]. The multiple drug resistance mechanisms include drug uptake limitation, drug target modification, drug inactivation, and active drug efflux, and the resistance processes differ based on microbial types [ 4 ]. Besides, certain pathogenic bacteria form biofilms through quorum sensing and develop drug resistance [ 5 ]. Hence, there is a need for the discovery of new drugs against multidrug-resistant pathogenic microorganisms.
Plant extracts and their bioactive compounds have been utilized for the treatment of several diseases since ancient times [ 5 ]. Medicinal plants were the major sources of bioactive compounds that could be used as potential alternatives to conventional antimicrobials [ 6 ]. The antibacterial activity of the plants could be ensured either by inhibiting the growth of bacteria or by disturbing the cell-to-cell communication system between the bacteria through anti-quorum sensing (AQS) [ 7 ], in which the latter is currently preferable, especially against antimicrobial-resistant bacteria. Hence, plant-derived medicines have been considered convenient therapies due to their fewer side effects and greater pharmacological efficacy [ 8 ]. Medicinal plants contain natural phytochemicals such as alkaloids, flavonoids, saponins, tannins, terpenoids, steroids, resins, cardiac glycosides, coumarins, and phenolic compounds, among others, that could have a multitude of biological activities [ 9 ].
Moreover, medicinal plants are potent antioxidants and play an important role in sequestering reactive oxygen species (ROS) in living cells owing to the presence of various phytochemicals [ 10 ]. Polyphenols from plants scavenge free radicals and inhibit enzymes that are responsible for the formation and accumulation of reactive oxygen species (ROS) [ 11 ]. Antioxidant phenolic compounds reduce the level of free radicals in living cells, thereby preventing the oxidation of cellular components by donating hydrogen atoms to free radicals and forming stable, nontoxic compounds like phenoxyl radicals [ 12 ]. These compounds prevent or treat diseases related to oxidative stress, such as cancer, diabetes, cardiovascular diseases, inflammatory joint diseases, dementia, asthma, eye diseases, and atherosclerosis [ 10 ]. Additionally, plant-derived phenolic compounds capture and neutralize free radicals in human cells to protect them from aging [ 13 ].
Ethiopia is a center for plant diversity, diverse topography, and multiple ethnic groups, languages, cultures, and beliefs, which enhance the practice of using medicinal plants. Particularly, the Metekel zone in Benishangul Gumuz Regional State has various ethnic groups (e.g., Agaw, Amhara, Gumuz, Oromo, and Shinasha), multiple cultures, and a diversity of medicinal plants. For instance, Asparagus flagellaris (Kunth) Baker, Brucea antidysenterica J. F. Mill., Celosia trigyna L., Crepis rueppellii Sch. Bip., Gnidia involucrata Steud. ex A.Rich., Polystachya steudneri Rchb.f., and Sauromatum venosum (Aiton) Kunth are traditionally used to treat toothache, leishmaniasis, tapeworm, diarrhea, gonorrhea, wounds, and amoeba, respectively, in the Dibatie district of the Metekel zone, western Ethiopia. However, there are limited reports yet on the ethnomedicine, antimicrobial activity, antioxidant properties, and phytochemical profiles of these plants in Ethiopia as a whole and in the Dibatie district of the Metekel zone in particular. Therefore, the current study was aimed at evaluating the antibacterial activity, antioxidant potential, and phytochemical constituents of the above medicinal plants.
Study period, study design and area
This study involved a preliminary ethnomedicinal survey through a semi-structured interview [ 14 ], which was conducted from April 2021 to June 2022. Following the ethnomedicinal investigation, the medicinal plants were selected, and the laboratory samples were collected in November 2022. Then, the laboratory work was carried out from February to June 2023. The field ethnobotanical data and sample collection were conducted in the Dibatie district of the Metekel zone, Benishangul Gumuz Regional State, western Ethiopia. Residents from eleven kebeles (sub-districts) such as Berber, Dibatie-02, Donben, Galessa, Gipho, Jan, Lega-buna, Parzeyit, Qorqa, Sombo-sire, and Tuski-gambela participated in the interview process. The plant material preparation, extraction, antibacterial test, antioxidant assay, and phytochemical screening were carried out at the laboratory of the Directorate of Modern and Traditional Medicine Research at the Ethiopian Public Health Institute, Addis Ababa, Ethiopia.
Plants selection
Medicinal plants were selected for laboratory work depending on the prior ethnobotanical survey of their traditional medicinal uses. The selection criteria were mainly based on the medicinal plants traditionally used to treat human ailments such as amoeba, diarrhea, gonorrhea, leishmaniasis, tapeworm, toothache, and wounds. Because these diseases were caused or aggravated due to the infestation by bacteria, protozoans, or helminthes. The selection of the study plants also emphasized the relative curing potential of each plant species (percentage of fidelity level) to heal the above ailments. The percentage of fidelity level helps to give a hint for further investigations on the medicinal efficacy of bioactive constituents. The percentage of fidelity level (FL%) was calculated using the formula: FL% = Ip/Iu × 100, where Ip is the number of respondents who indicated the use of a species for the same major ailments and Iu is the total number of respondents who mentioned the plant for any major ailments indicated [ 15 ]. Accordingly, plants with a fidelity level greater than 45% were selected for the laboratory investigations (Table 1 ). In addition, the selected plants were prioritized since they were not studied using the same methods as the present study so far.
Sample collection and preparation
Based on the above criteria, roots of Asparagus flagellaris , fruits of Brucea antidysenterica , inflorescence having seeds of Celosia trigyna , roots of Crepis rueppellii , roots and stems of Gnidia involucrata , pseudobulbs of Polystachya steudneri , and tubers of Sauromatum venosum were collected from Berber, Galessa, Jan, Lega-buna, Sombo-sire, and Tuski-gambela sub-districts. The collected plants were identified by Dr. Ermias Lulekal and Mr. Baressa Anbessa in the department of plant biology and biodiversity management at Addis Ababa University. In addition, the scientific names were checked by referring to the website Plants of the World Online (POWO). Voucher specimens were deposited at the National Herbarium of Addis Ababa University (ETH).
Fruits, seeds, and inflorescences of the indicated plants (Table 1 ) were shade dried without washing since their dust content was negligible. Roots, stems, and pseudobulbs were washed with tap water, rinsed with distilled water to remove dust, and shade dried in a solar drier. Dried samples were pulverized using an electric grinder to a moderately fine powder and kept in the refrigerator at 4 ºC until extraction.
Extraction process
As the local community usually uses water as a solvent, aqueous 80% ethanol was used for effective extraction of bioactive compounds from medicinal plant materials. The reason is that aqueous-alcoholic (80% ethanol) extracts are better in phytochemical (e.g., phenolics, flavonoids, tannins, etc.) content and antioxidant activity [ 16 , 17 ]. The extraction was carried out by macerating 50 g of powdered plant parts in 500 mL of 80% ethanol and continuously shaking for 24 h using a magnetic stirrer. The mixture was filtrated using Whatman number 1 filter paper. The residue was re-macerated for 24 h and filtered. The filtrates were combined and concentrated in vacuo using a rotary evaporator (BUCHI R-300 Rotavapor, Switzerland). The concentrated extracts were dried in a water bath at 40 ºC and kept in desiccators with active silica gel until they dried well.
Antibacterial assay
The test microorganisms were from the American Type Culture Collection (ATCC). Ethanolic extracts of each sample were tested in vitro against the active pathogenic bacterial strains existing in the laboratory. These include the gram-negative ( Proteus mirabilis ATCC-35,659, Salmonella typhimurium ATCC-13,311, Klebsiella pneumoniae ATCC-700,603, Escherichia coli ATCC-25,922, and Shigella flexneri ATCC-12,022) and the gram-positive ( Staphylococcus aureus ATCC-25,923, Staphylococcus epidermidis ATCC-12,228, Streptococcus agalactiae ATCC-12,386, and Enterococcus faecalis ATCC-1,829,212) bacterial strains.
Nutrient broth and Mueller-Hinton agar were used for microorganism sub-culturing and growing. For that purpose, 13 g of nutrient broth was dissolved in 1000 mL of distilled water, well mixed, and autoclaved at 121 °C and 15 pounds per square inch (psi) for 15 min. Mueller-Hinton agar (38 g) was also dissolved in 1000 mL of distilled water, well mixed, boiled on a hot plate, and autoclaved.
Mueller-Hinton agar for bacteria was used for the subculturing of microorganisms. In this regard, 3–5 well-isolated colonies of the same morphological type from the refreshed agar plate culture were selected. The bacterial colonies were inoculated on sterilized plates containing Mueller-Hinton agar, followed by incubation at 37 °C for 24 h. Later, the bacterial colonies were transferred to the nutrient broth using the sterilized inoculating loop.
The minimum inhibitory concentration (MIC) was determined using 96-well microplates by the nutrient broth microdilution method. Tween 80 was used to dissolve the extracts since it is a low-toxicity surfactant that increases the solubility of bioactive phytochemicals [ 18 ]. The ethanolic extract of each sample was dissolved in 5% Tween 80 to an end concentration of 32 mg/mL, which needs to be engaged in serial dilutions. An aliquot of 100 µL of each extract was subjected to serial dilutions in nutrient broth to concentrations of 16, 8, 4, 2, 1, 0.50, 0.25, and 0.13 mg/mL. A standard reference (ciprofloxacin) was taken as a positive control in concentrations of 10, 5, 2.50, 1.25, 0.63, 0.31, 0.16, and 0.08 µg/mL. Tween 80 (5%) was used as a negative control. The microorganism suspension was standardized to 1 × 10 8 CFU/mL (0.08 to 1.00 turbidity) using a UV-Vis spectrophotometer at 625 nm. An aliquot of 100 µL of standardized microorganisms was inoculated into each well containing serially diluted extracts and controls (positive, negative, and growth controls), except for sterility control. Then, plates were incubated at 37 °C for 18–24 h. In order to read the microorganism growth, 40 µL of 2, 3, 5 tripenyl tetrazolium chloride (TTC) with a concentration of 0.40 mg/mL was added into each well and incubated at 37 °C for 30 min. The development of pink color in the microplate well indicated the presence of living cells (microorganisms), and the reverse result showed inhibition of microbial growth. The lowest concentration of each extract displaying no visible pink color was recorded as the MIC.
During the antibacterial test, we followed various safety practices to avoid any potential hazards. Bacterial cultures were treated as potential pathogens. All materials, media, tubes, plates, loops, needles, pipettes, and other items used were sterilized by autoclaving or using commercially sterilized products. Work spaces were thoroughly cleaned using 70% ethanol or 10% bleach both before and after usage. Mouth pipetting was avoided by staying away from food and drink in the laboratory and washing hands with disinfectant soap before and after working. Labeling everything clearly, autoclaving or disinfecting all waste material, and cleaning up spills with care were also the other safety precautions that we followed during the experiments. Additionally, all necessary personal protective equipment and biological safety cabinets (class II) were used to avoid contamination.
Antioxidant (2,2-diphenyl-1-picrylhydrazyl (DPPH)) assay
The free radical scavenging ability of ethanolic extracts was determined by using a 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay [ 19 ]. Briefly, a fresh 0.1 mM DPPH solution was prepared in 80% ethanol. Ethanolic extracts and ascorbic acid (a positive control) were kept in test tubes at different concentrations (15.63–500 µg/mL) through serial dilution in 80% ethanol. Then, 1 mL of DPPH solution was mixed with 1 mL of each extract and a positive reference in the test tube. The mixtures were shaken thoroughly and incubated in the dark for 30 min at room temperature. The mixture of 1 mL of 80% ethanol and 1 mL of DPPH solution was considered a blank. The absorbance of each mixture was measured at 517 nm against a blank using a UV-VIS spectrophotometer (UV-1800 SHIMADZU).
The percentage of inhibition was calculated using the formula: % Inhibition = [(Ab - As) / Ab] x 100, where Ab is the absorbance of the blank and As is the absorbance of the sample. Later, the 50% inhibition concentration (IC 50 ) was calculated for ascorbic acid and extracts of medicinal plants by using the slope equation: Y = mx + c [ 10 ].
Phytochemical screening
The ethanolic extracts were employed for preliminary screening of phytochemicals such as alkaloids, anthocyanins, anthraquinones, cardiac glycosides, coumarins, flavonoids, phenols, saponins, steroids, tannins, and terpenoids following the standardized protocols [ 5 , 13 , 20 , 21 , 22 ]. The results were expressed as (+) for the presence and (-) for the absence of phytochemical compounds.
Statistical data analysis
The percentage of fidelity level was computed based on the ethnobotanical data to assess the healing potential of each plant species against the corresponding disease. The minimum inhibitory concentration (MIC) data were described as the means ± standard deviation of triplicate analyses. Depending on the MIC values, the principal component analysis (PCA) was computed to indicate variations in the antibiotic effect of medicinal samples and the susceptibility of bacterial strains using R-statistical packages (ggplot2 and grid). The DPPH free radical scavenging activity and the 50% inhibition concentration (IC 50 ) were expressed as means of triplicate determinations. Qualitative phytochemical profiles were expressed as the presence (+) and absence (-) of phytochemical constituents. Microsoft Excel version 2013 was also used for the data analysis.
Antibacterial activities of medicinal plants
Minimum inhibitory concentration (MIC) values of selected medicinal plants were evaluated against gram-negative ( Proteus mirabilis , Salmonella typhimurium , Klebsiella pneumoniae , Escherichia coli , and Shigella flexneri ) and gram-positive ( Staphylococcus aureus , Staphylococcus epidermidis , Streptococcus agalactiae , and Enterococcus faecalis ) bacterial strains at concentrations less than or equal to 16 mg/mL.
MIC values of plant extracts against gram-negative bacteria
The ethanolic extract of P. steudneri pseudobulbs showed the highest antibacterial activity against gram-negative bacterial strains by inhibiting P. mirabilis , S. typhimurium , K. pneumoniae , E. coli , and S. flexneri with MIC values of 8 ± 0, 11 ± 5, 3 ± 1, 3 ± 1, and 2 ± 0 mg/mL, respectively. Whereas the ethanolic extract of A. flagellaris roots exhibited the lowest antibacterial activity as it inhibited both S. typhimurium and E. coli at MIC values of 16 ± 0 mg/mL, and P. mirabilis, K. pneumoniae , and S. flexneri at MIC values > 16 mg/mL each (Table 2 ).
MIC values of plant extracts against gram-positive bacteria
The ethanolic extracts of P. steudneri pseudobulbs and G. involucrata stems were the most active against gram-positive bacterial strains. The extract of P. steudneri inhibited S. aureus , S. epidermidis , S. agalactiae , and E. faecalis at MIC values of 8 ± 0, 8 ± 0, 3 ± 1, and 16 ± 0 mg/mL, and that of G. involucrata stems inhibited these bacteria at MIC values of 3 ± 1, 16 ± 0, 2 ± 0, and 16 ± 0 mg/mL, respectively. On the other hand, ethanolic extracts of A. flagellaris roots and S. venosum tubers were active only against S. agalactiae , with MIC values of 4 ± 0 and 2 ± 0 mg/mL, respectively (Table 3 ).
Coordinates of study plants and bacterial strains based on MIC values
The results of principal component analysis (PCA) revealed that the ethanolic extract of P. steudneri pseudobulb was the most effective antibacterial, closest to the positive reference (ciprofloxacin). The extracts of A. flagellaris root and S. venosum tuber were the least effective antibacterials among the studied medicinal plant species. The results of PCA also showed that S. agalactiae was the most susceptible bacterial strain, illustrated with the longest arrow, while P. mirabilis was found to be the least susceptible (Fig. 1 ).

The PCA illustrating the positions of medicinal samples based on their MIC values against the tested bacterial strains
Antioxidant activity of the study plants
Dpph free radical scavenging ability.
The deep purple color of the DPPH solution changed to colorless when it was mixed with plant extracts having antioxidant properties and ascorbic acid (positive reference). In contrast, the purple color was retained when the DPPH solution was mixed with extracts of plants with less antioxidant activity and the negative control. In this perspective, following the ascorbic acid, ethanolic extracts of G. involucrata stems and roots exhibited the highest DPPH free radical scavenging activity, while extracts of C. rueppellii roots showed the lowest (Fig. 2 ).

Percentage of DPPH free radical scavenging activity
The 50% inhibitory concentration (IC 50 )
Relative to the positive reference (IC 50 = 53.76 µg/mL), ethanolic extracts of G. involucrata stems showed IC 50 value of 168.68 µg/mL, followed by extracts of G. involucrata roots (IC 50 = 181.79 µg/mL). Ethanolic extracts of the remaining plants, such as P. steudneri, B. antidysenterica, C. trigyna, S. venosum, A. flagellaris , and C. rueppellii , exhibited IC 50 values of 203.11, 293.56, 366.15, 387.82, 459.55, and 527.57 µg/mL, respectively (Fig. 3 ).

50% inhibition concentration (IC 50 ) of the medicinal samples
Phytochemical screening of medicinal plants
Qualitative phytochemical screening was carried out to determine the presence or absence of alkaloids, anthocyanins, anthraquinones, cardiac glycosides, coumarins, flavonoids, phenols, saponins, steroids, tannins, and terpenoids in the ethanolic extracts of the studied medicinal plants, and a summary of the findings is presented in Table 4 . Accordingly, root extracts of A. flagellaris were confirmed to have the tested compounds apart from anthraquinones and steroids. Extracts of B. antidysenterica fruits contained all the other tested phytoconstituents except anthocyanins, anthraquinones, cardiac glycosides, and saponins. Except for anthraquinones, the tested phytochemicals were detected in extracts of C. trigyna inflorescence with seeds. The root extracts of C. rueppellii were observed to contain alkaloids, cardiac glycosides, coumarins, and terpenoids. Extracts of G. involucrata roots and stems contained almost similar phytochemicals (anthocyanins, anthraquinones, cardiac glycosides, flavonoids, phenols, tannins, and terpenoids) except for the presence of saponins in roots but not in stems, and the reverse was true for steroids. The tested phytochemicals were observed in the extracts of P. steudneri pseudobulbs, except for alkaloids, saponins, and terpenoids. Tubers of S. venosum contained the other tested constituents except anthocyanins, anthraquinones, saponins, and terpenoids.
In the ethnomedicinal perspective, leaves and stems of A. flagellaris were reported to be used against gonorrhea and syphilis in Nigeria [ 23 ], its fruits for eye diseases, and its roots for measles in Uganda [ 24 ]. Leaves of B. antidysenterica were used to treat wounds in Zuway Dugda district [ 25 ] and diarrhea surrounding the Gullele Botanic Garden in central Ethiopia [ 26 ]. Whole parts of Celosia trigyna were reported to heal arthritis, diarrhea, and dysentery in Kafa Zone [ 27 ], and seeds were used to treat tapeworm in Libo Kemkem district, northwest Ethiopia [ 28 ]. Leaves and roots of Crepis rueppellii were used to cure dysentery by residents on the Dek Island of Lake Tana, northwest Ethiopia [ 29 ]. Roots of Gnidia involucrata were reported to treat gonorrhea and ascaris in the Bule Hora district of southern Ethiopia. The tubers of S. venosum were traditionally used to treat ascaris [ 29 ] and hemorrhoids [ 30 ] in northwest Ethiopia. Hence, the literature supports the ethnomedicinal data in the present study and the effectiveness of the study plants against several infectious diseases, except for P. steudneri , which has not been studied yet.
In the current study, it was observed that the minimum inhibitory concentrations (MIC) of the investigated medicinal plants were dependent on the types of bacterial strains. The investigated medicinal plants exhibited various MIC values against different gram-negative and gram-positive bacterial strains. Similar to the current findings, the previous studies reported P. mirabilis as an antimicrobial-resistant bacterial strain [ 31 , 32 ]. In line with the present study, other previous studies also reported the antimicrobial resistance of S. epidermidis among gram-positive bacteria [ 33 , 34 ]. This indicates the multiple antibiotic resistance of both P. mirabilis and S. epidermidis .
An earlier study conducted by Taye et al. [ 35 ] reported that the methanolic extracts of B. antidysenterica root showed a MIC value of 15.63 mg/mL against S. aureus , while the ethanolic extracts of its fruits inhibited the same bacterial strain at a MIC value of 4.00 mg/mL in the present study. Here, variation in the antibacterial activity of B. antidysenterica might be due to differences in the extraction solvents used or the bioactivity of the tested plant parts. A similar study conducted by Kalbessa et al. [ 36 ] reported the highest efficacy of the ethyl acetate extracts of G. involucrata root bark against S. aureus compared to the other bacterial strains. However, the current findings revealed the most sensitive bacteria, S. agalactiae , to the ethanolic extracts of G. involucrata roots than S. aureus . On the other hand, the study conducted by Zakerifar et al. [ 37 ] showed that S. agalactiae was reported to be resistant to certain antibiotics like erythromycin, levofloxacin, ofloxacin, quinupristin, and tetracycline and susceptible to chloramphenicol, gentamicin, linezolid, penicillin, and vancomycin. In this respect, there are variations in the antibacterial efficacy of medicinal plants and differences in the degree of susceptibility of bacterial strains. Thus, MIC values varied among the extracts of different medicinal plants in the present study. This might be linked to the difference in the biologically active phytochemicals they contain [ 38 ]. Besides, MIC values differed among different bacterial strains owing to their variation in antibiotic resistance.
The study conducted by Odeja et al. [ 23 ] showed that the leaf essential oil of A. flagellaris had high antioxidant activity, with 90.74% inhibition of DPPH free radicals at a concentration of 500 µg/mL. In the present findings, however, the ethanolic extracts of its root exhibited 51.28% inhibition at the same concentration. In this case, the variation in the antioxidant activity of A. flagellaris might be because of the extraction methods employed or the excess of phytoconstituents in leaves rather than roots. The other study conducted by Kalbessa et al. [ 36 ] indicated that ethyl acetate extracts of G. involucrata root bark and its isolated compound exhibited 70.70 and 85.80% inhibition at concentrations of 100 µg/mL, respectively. This is slightly comparable with the current findings, in which the ethanolic extracts of G. involucrata stem showed 68.91% inhibition at 125 µg/mL. This confirms the antioxidant potential of different parts of G. involucrata to reduce risks related to free radicals.
The antibacterial and antioxidant activities of medicinal plants depend on their phytochemical constituents. This is due to the fact that the phytochemical constituents of the medicinal plants are associated with their antioxidant and antibacterial activities [ 9 , 39 ]. Medicinal plants contain mainly phenolic antioxidants like ß-carotene, flavonoids, phenolic acids, terpenes, tocopherols, vitamin C, and so on [ 40 ]. Antioxidant phenolic compounds scavenge free radicals and prevent oxidation of cellular components either by donating hydrogen atoms to free radicals to form stable, harmless compounds [ 12 ] or by inhibiting enzymes responsible for the production of reactive oxygen species [ 11 ]. Thus, they take part in the prevention or treatment of oxidative stress-related diseases, for example, atherosclerosis, biliary diseases, cancer, dementia, diabetes, hypertension, kidney disease, macular degeneration, neurodegenerative diseases, and obesity [ 41 ].
The phytochemical screening of ethanolic extracts showed that the selected medicinal plants contain important phytochemicals, which could play crucial roles in their bioactivities. Phytochemical constituents, mainly bioactive secondary metabolites, play significant roles in the bioactivities of medicinal plants by eliciting a definite and specific action on the human body [ 42 ]. The current results showed the presence of steroids in the ethanolic extracts of B. antidysenterica fruits, C. trigyna inflorescence with seeds, G. involucrata stems, P. steudneri pseudobulbs, and S. venosum tubers. Steroids are used to treat rheumatism, asthma, allergies, skin infections, and inflammations [ 43 ] and to relieve inflammation and swelling in cancer patients [ 42 , 43 ]. Cardioactive steroids, for example, cardenolides, improve heart function, although they are highly toxic and received at a therapeutic dose of 60% of the lethal dose [ 44 ]. Results of phytochemical screening revealed the presence of alkaloids in the extracts of A. flagellaris , B. antidysenterica , C. trigyna , C. rueppellii , and S. venosum . Isoquinoline alkaloids are found in higher plants and are known to have antispasmodic, antiviral, antifungal, anticancer, antioxidant, and enzyme inhibitory activities [ 45 ]. Besides, diterpenoid alkaloids are potent to treat various cancers as new drugs [ 46 ].
Flavonoids, tannins, and phenols were identified from the ethanolic extracts of all investigated medicinal plants except that of C. rueppellii . Flavonoids have antioxidant, anti-inflammatory, and antimicrobial activities; hence, they attribute to the medicinal properties of different medicinal plants [ 43 ]. For instance, plants like Zingiber , Curcuma , and Acorus were reported as sources of antibacterial and antiseptic agents owing to their flavonoid content [ 47 ]. Tannins were described as healing agents for inflammation, hemorrhoids, and gonorrhea [ 42 ] and were known to have anticancer [ 47 ] and antidiabetic [ 48 ] effects. Polyphenolic compounds have been beneficial as antioxidants, anti-inflammatory, and antibacterial agents and reduce blood pressure and heart disease [ 47 ]. Out of the examined plants, saponins were found in extracts of A. flagellaris roots, C. trigyna inflorescence with seeds, and G. involucrata roots. Saponins were stated to treat different human diseases, such as skin infections, liver diseases, trauma, chronic venous insufficiency, and kidney diseases [ 49 ].
The tested medicinal plants were positive for cardiac glycosides, except for B. antidysenterica . In agreement with the current findings, the studies conducted by Liu et al. [ 50 ] and Ravi et al. [ 51 ] reported the existence of cardiac glycosides in many medicinal plants. Cardiac glycosides have beneficial effects for the heart [ 47 ] in that they treat congestive heart failure and cardiac arrhythmia by inhibiting the Na + /K + pump and increasing the level of calcium ions (Ca + ), which enhances the contraction of heart muscles and reduces swelling [ 13 ]. Terpenoids were detected in ethanolic extracts of A. flagellaris roots, B. antidysenterica fruits, C. trigyna inflorescence with seeds, C. rueppellii roots, and G. involucrata roots and stems. Medicinally, they provide significant actions such as antiviral, antibacterial, antimalarial, anti-inflammatory, anticancer, and inhibition of cholesterol synthesis [ 42 ]. Coumarins are among the essential phytochemical compounds found in medicinal plants [ 42 ]. In this regard, results from the current study indicated the presence of coumarins in ethanolic extracts of the investigated medicinal plants, except in the roots and stems of G. involucrata . Medicinally, coumarins were appreciated to treat microbial infections, cancers, tuberculosis, inflammatory diseases, malaria, and AIDS-acquired immunodeficiency syndrome [ 52 ].
The qualitative phytochemical screening revealed that anthraquinones were identified from ethanolic extracts of G. involucrata roots and stems and P. steudneri pseudobulbs. The plant-derived natural anthraquinones were reported to have antiviral potential against different infectious viruses [ 53 ]. Results also showed that the examined medicinal plants, such as A. flagellaris, C. trigyna, G. involucrata, and P. steudneri , were positive for the anthocyanins test. Plant-based anthocyanins have antioxidant properties that play important roles in health and therapeutic effects [ 54 ]. Furthermore, the presence of phytochemicals such as alkaloids, flavonoids, glycosides, phenolic compounds, saponins, tannins, and triterpenoids promotes the anthelmintic properties of medicinal plants [ 55 ].
In the present study, a comparative investigation was carried out between the extracts of G. involucrata roots and stems, though local people traditionally use its roots. The extract of G. involucrata stems showed even higher antibacterial activity, antioxidant capacity, and phytochemical contents than the root extract. This might be due to the distribution of secondary metabolites from the areas of synthesis (leaves) to the areas of sink (roots and stems) via phloem tissue. Hence, results from the current study suggest the use of G. involucrata stems instead of its roots since root harvesting is usually destructive for the sustainable use of medicinal plants.
Ethanolic extracts of the investigated medicinal plants were active against different gram-negative and gram-positive bacterial strains at various concentrations. Additionally, the ethanolic extracts exhibited considerable antioxidant activity compared to ascorbic acid. The qualitative phytochemical screening revealed the presence of important bioactive compounds in the tested medicinal plants. Hence, the findings from this study support the traditional medicinal use of the investigated plants. The study was restricted to their 80% ethanolic extracts. Thus, further investigations using different solvents of various polarities will be required to extract lead compounds for the development of appropriate drugs. Besides, toxicity studies will be necessary to encourage their further use.
Availability of data and materials
The data that support the findings reported herein are available from the corresponding author upon reasonable request.
Abbreviations
American type culture collection
2,2-diphenyl-1-picrylhydrazyl
Fidelity level
50% inhibition concentration
Minimum inhibitory concentration
Principal Component Analysis
Ultra-Violet Visible
Teka A, Rondevaldova J, Asfaw Z, Demissew S, Van Damme P, Kokoska L, et al. In vitro antimicrobial activity of plants used in traditional medicine in Gurage and Silti Zones, south central Ethiopia. BMC Complement Altern Med. 2015;15:286.
Article PubMed PubMed Central Google Scholar
Acharjee M, Zerin N, Ishma T, Mahmud MR. In vitro antibacterial activity of medicinal plants against Urinary Tract Infection (UTI) causing bacteria along with their synergistic effects with commercially available antibiotics. New Microbes New Infect. 2023;51:101076.
Article CAS PubMed Google Scholar
Singh A, Kumar J, Sharma VK, Singh DK, Kumari P, Nishad JH, et al. Phytochemical analysis and antimicrobial activity of an endophytic Fusarium proliferatum (ACQR8), isolated from a folk medicinal plant Cissus quadrangularis L. South Afr J Bot. 2021;140:87–94.
Article CAS Google Scholar
Qadri H, Haseeb Shah A, Mudasir Ahmad S, Alshehri B, Almilaibary A, Ahmad Mir M. Natural products and their semi-synthetic derivatives against antimicrobial-resistant human pathogenic bacteria and fungi. Saudi J Biol Sci. 2022;29:103376.
Article CAS PubMed PubMed Central Google Scholar
Prabu SL, Umamaheswari A, GF SJ. Investigation on the biofilm eradication potential of selected medicinal plants against methicillin-resistant Staphylococcus aureus. Biotechnol Rep. 2020;28:e00523.
Article Google Scholar
Rajput M, Kumar N. Medicinal plants: a potential source of novel bioactive compounds showing antimicrobial efficacy against pathogens infecting hair and scalp. Gene Rep. 2020;21:100879.
Baloyi IT, Cosa S, Combrinck S, Leonard CM, Viljoen AM. Anti-quorum sensing and antimicrobial activities of South African medicinal plants against uropathogens. S Afr J Bot. 2019;122:484–91.
Nazar S, Hussain MA, Khan A, Muhammad G, Bukhari SNA. Alkaloid-rich plant Tylophora indica ; current trends in isolation strategies, chemical profiling and medicinal applications. Arab J Chem. 2020;13:6348–65.
Mapfumari S, Nogbou N-D, Musyoki A, Gololo S, Mothibe M, Bassey K. Phytochemical screening, antioxidant and antibacterial properties of extracts of Viscum Continuum E. Mey. Ex Sprague, a South African mistletoe. Plants. 2022;11:2094.
Jafri SAA, Khalid ZM, Khan MR, Ashraf S, Ahmad N, Karami AM, et al. Evaluation of some essential traditional medicinal plants for their potential free scavenging and antioxidant properties. J King Saud Univ Sci. 2023;35:102562.
Atta S, Waseem D, Naz I, Rasheed F, Phull AR, Ur-Rehman T, et al. Polyphenolic characterization and evaluation of multimode antioxidant, cytotoxic, biocompatibility and antimicrobial potential of selected ethno-medicinal plant extracts. Arab J Chem. 2023;16:104474.
Alu’datt MH, Rababah T, Alhamad MN, Gammoh S, Al-Mahasneh MA, Tranchant CC, et al. Pharmaceutical, nutraceutical and therapeutic properties of selected wild medicinal plants: thyme, spearmint, and rosemary. In: Ther Probiotic Unconv Foods. 2018. p. 275–90. https://linkinghub.elsevier.com/retrieve/pii/B9780128146255000145 . Accessed 10 May 2023.
Iqbal E, Salim KA, Lim LBL. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus Velutinus (Airy Shaw) from Brunei Darussalam. J King Saud Univ Sci. 2015;27:224–32.
Martin GJ. Ethnobotany: a methods manual. 1st ed. London, New York: Chapman & Hall; 1995.
Book Google Scholar
Tahir M, Asnake H, Beyene T, Van Damme P, Mohammed A. Ethnobotanical study of medicinal plants in Asagirt District, Northeastern Ethiopia. Trop Med Health. 2023;51:1.
Gonfa T, Teketle S, Kiros T. Effect of extraction solvent on qualitative and quantitative analysis of major phyto-constituents and in vitro antioxidant activity evaluation of Cadaba rotundifolia Forssk leaf extracts. Yildiz F, editor. Cogent Food Agric. 2020;6:1853867.
Sultana B, Anwar F, Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167–80.
Iswandana R, Olisia S, Adriyani M, Jufri M, Munim A. Application of tween 80 and tween 20 for microwave-assisted extraction of oxyresveratrol from mulberry ( Morus alba L.) twigs. J Appl Pharm Sci. 2020;10:93–100.
Kızıl G, Kızıl M, Yavuz M, Emen S, Hakimoğlu F. Antioxidant activities of ethanol extracts of Hypericum triquetrifolium . and Hypericum scabroides . Pharm Biol. 2008;46:231–42.
Bargale SS, Tripathy TB, Shashirekha HK. Phyto physicochemical profile of Withania somnifera Dunal (Solanaceae). J Drug Deliv Ther. 2019;9:263–8.
Google Scholar
Mechqoq H, Hourfane S, Yaagoubi ME, Hamdaoui AE, Msanda F, Almeida JRG da S, et al. Phytochemical screening, and in vitro evaluation of the antioxidant and dermocosmetic activities of four Moroccan plants: Halimium antiatlanticum, Adenocarpus artemisiifolius, Pistacia lentiscus and Leonotis nepetifolia. Cosmetics. 2022;9:94.
Shaikh JR, Patil M. Qualitative tests for preliminary phytochemical screening: an overview. Int J Chem Stud. 2020;8:603–8.
Odeja OO, Ibok MG, Okpala EO. Composition and biological assays of the leaf essential oil of Asparagus flagellaris (Kunth) Bak. Clin Phytosci. 2021;7:12.
Masters ET. Medicinal plants of the upper Aswa River catchment of northern Uganda - a cultural crossroads. J Ethnobiol Ethnomed. 2023;19:48.
Megersa M, Nedi T, Belachew S. Ethnobotanical study of medicinal plants used against human diseases in Zuway Dugda District, Ethiopia. Gorzalczany S, editor. Evid Based Complement Alternat Med. 2023;2023:1–22.
Woldeamanuel MM, Geda MK, Mohapatra S, Bastia TK, Rath P, Panda AK. Ethnobotanical study of endemic and non-endemic medicinal plants used by indigenous people in environs of Gullele botanical garden Addis Ababa, central Ethiopia: a major focus on Asteraceae family. Front Pharmacol. 2022;13:1020097.
Gosa BB, Wana TW. Evaluation of the phytochemical and antibacterial activity of four selected plant extracts against some pathogenic bacteria. Int J Environ Agric Biotechnol. 2022;7:265–76.
Chekole G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-Gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia. J Ethnobiol Ethnomed. 2015;11:4.
Teklehaymanot T. Ethnobotanical study of knowledge and medicinal plants use by the people in Dek Island in Ethiopia. J Ethnopharmacol. 2009;124:69–78.
Article PubMed Google Scholar
Giday M, Teklehaymanot T, Animut A, Mekonnen Y. Medicinal plants of the Shinasha, Agew-Awi and Amhara peoples in northwest Ethiopia. J Ethnopharmacol. 2007;110:516–25.
Liu L, Dong Z, Ai S, Chen S, Dong M, Li Q, et al. Virulence-related factors and antimicrobial resistance in Proteus mirabilis isolated from domestic and stray dogs. Front Microbiol. 2023;14:1141418.
Tumbarello M, Trecarichi EM, Fiori B, Losito AR, D’Inzeo T, Campana L, et al. Multidrug-resistant Proteus mirabilis bloodstream infections: risk factors and outcomes. Antimicrob Agents Chemother. 2012;56:3224–31.
Chabi R, Momtaz H. Virulence factors and antibiotic resistance properties of the Staphylococcus epidermidis strains isolated from hospital infections in Ahvaz, Iran. Trop Med Health. 2019;47:56.
Eladli MG, Alharbi NS, Khaled JM, Kadaikunnan S, Alobaidi AS, Alyahya SA. Antibiotic-resistant Staphylococcus epidermidis isolated from patients and healthy students comparing with antibiotic-resistant bacteria isolated from pasteurized milk. Saudi J Biol Sci. 2019;26:1285–90.
Taye B, Giday M, Animut A, Seid J. Antibacterial activities of selected medicinal plants in traditional treatment of human wounds in Ethiopia. Asian Pac J Trop Biomed. 2011;1:370–5.
Kalbessa A, Dekebo A, Tesso H, Abdo T, Abdissa N, Melaku Y. Chemical constituents of root barks of Gnidia involucrata and evaluation for antibacterial and antioxidant activities. J Trop Med. 2019;2019:1–8.
Zakerifar M, Kaboosi H, Goli HR, Rahmani Z, Peyravii Ghadikolaii F. Antibiotic resistance genes and molecular typing of Streptococcus agalactiae isolated from pregnant women. BMC Pregnancy Childbirth. 2023;23:43.
Guadie A, Dakone D, Unbushe D, Wang A, Xia S. Antibacterial activity of selected medicinal plants used by traditional healers in Genta Meyche (Southern Ethiopia) for the treatment of gastrointestinal disorders. J Herb Med. 2020;22:100338.
El Hachlafi N, Benkhaira N, Al-Mijalli SH, Mrabti HN, Abdnim R, Abdallah EM, et al. Phytochemical analysis and evaluation of antimicrobial, antioxidant, and antidiabetic activities of essential oils from Moroccan medicinal plants: Mentha suaveolens, Lavandula stoechas , and Ammi visnaga . Biomed Pharmacother. 2023;164:114937.
Škrovánková S, Mišurcová L, Machů L. Antioxidant activity and protecting health effects of common medicinal plants. Adv Food Nutr Res. 2012:75–139. https://linkinghub.elsevier.com/retrieve/pii/B9780123945983000034 . Accessed 20 Nov 2023.
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757–72.
Agidew MG. Phytochemical analysis of some selected traditional medicinal plants in Ethiopia. Bull Natl Res Cent. 2022;46:87.
Mlozi SH. The role of natural products from medicinal plants against COVID-19: traditional medicine practice in Tanzania. Heliyon. 2022;8:e09739.
Nagorny P, Cichowicz N. New strategy based on sequential Michael/Aldol reactions for the asymmetric synthesis of cardenolides. Strateg Tactics Org Synth. 2016:237–67. https://linkinghub.elsevier.com/retrieve/pii/B9780081007563000091 . Accessed 24 Jul 2023.
Dey P, Kundu A, Kumar A, Gupta M, Lee BM, Bhakta T, et al. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). Recent Adv Nat Prod Anal. 2020:505–67. https://linkinghub.elsevier.com/retrieve/pii/B9780128164556000159 . Accessed 2 Aug 2023.
Shen Y, Liang W-J, Shi Y-N, Kennelly EJ, Zhao D-K. Structural diversity, bioactivities, and biosynthesis of natural diterpenoid alkaloids. Nat Prod Rep. 2020;37:763–96.
Sharma T, Pandey B, Shrestha BK, Koju GM, Thusa R, Karki N. Phytochemical screening of medicinal plants and study of the effect of phytoconstituents in seed germination. Tribhuvan Univ J. 2020;35:1–11.
Bouyahya A, El Omari N, Elmenyiy N, Guaouguaou FE, Balahbib A, Belmehdi O, et al. Moroccan antidiabetic medicinal plants: ethnobotanical studies, phytochemical bioactive compounds, preclinical investigations, toxicological validations and clinical evidences; challenges, guidance and perspectives for future management of diabetes worldwide. Trends Food Sci Technol. 2021;115:147–254.
Bailly C, Vergoten G. Esculentosides: insights into the potential health benefits, mechanisms of action and molecular targets. Phytomedicine. 2020;79:153343.
Liu R, Su B, Huang F, Ru M, Zhang H, Qin Z, et al. Identification and analysis of cardiac glycosides in Loranthaceae parasites Taxillus chinensis (DC.) Danser and Scurrula Parasitica Linn. and their host Nerium Indicum Mill. J Pharm Biomed Anal. 2019;174:450–9.
Ravi BG, Guardian MGE, Dickman R, Wang ZQ. High-resolution tandem mass spectrometry dataset reveals fragmentation patterns of cardiac glycosides in leaves of the foxglove plants. Data Brief. 2020;30:105464.
Patil SB. Medicinal significance of novel coumarin analogs: recent studies. Results Chem. 2022;4:100313.
Ntemafack A, Singh RV, Ali S, Kuiate JR, Hassan QP. Antiviral potential of anthraquinones from Polygonaceae, Rubiaceae and Asphodelaceae: potent candidates in the treatment of SARS-COVID-19, a comprehensive review. S Afr J Bot. 2022;151:146–55.
Netravati GS, Pathrose B, N MR, Kuruvila PMJ. Comparative evaluation of anthocyanin pigment yield and its attributes from Butterfly pea ( Clitorea Ternatea L.) flowers as prospective food colorant using different extraction methods. Future Foods. 2022;6:100199.
Kancherla N, Dhakshinamoothi A, Chitra K, Komaram RB. Preliminary analysis of phytoconstituents and evaluation of anthelminthic property of Cayratia auriculata (in vitro). Maedica. 2019;14:350.
PubMed PubMed Central Google Scholar
Download references
Acknowledgements
The authors are grateful to the Office of Postgraduate Program, Office of the Research Directorate, College of Natural and Computational Sciences, and Department of Plant Biology and Biodiversity Management of Addis Ababa University for making the financial support for this work available through the Thematic Project entitled ‘Ethnobotany of the medicinal and wild edible plants of the Dibatie people, and antimicrobial activity study of plants against infectious diseases of the Dibatie district, Metekel zone, Benishangul Gumuz Regional State, Western Ethiopia’. Likewise, we would like to thank Directorate of the Modern and Traditional Medicine Research of Ethiopian Public Health Institute for part of financial and chemical support, and laboratory facilities. Field assistants, informants and administrative leaders in the study area are acknowledged for their support during ethnobotanical survey and sample collection.
This study was funded by the Office of Postgraduate Program and Office of the Directorate for Research in Addis Ababa University, and by Directorate of the Modern and Traditional Medicine Research in Ethiopian Public Health Institute.
Author information
Authors and affiliations.
Department of Plant Biology and Biodiversity Management, College of Natural and Computational Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Baressa Anbessa & Ermias Lulekal
Department of Biology, College of Natural and Computational Sciences, Bule Hora University, Bule Hora, Ethiopia
Baressa Anbessa
Department of Pharmaceutical Chemistry and Pharmacognosy, School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Ariaya Hymete
Directorate of Modern and Traditional Medicine Research, Ethiopian Public Health Institute, Addis Ababa, Ethiopia
Asfaw Debella
Traditional and Modern Medicine Research and Development Directorate, Armauer Hansen Research Institute, Addis Ababa, Ethiopia
Eyob Debebe, Abiy Abebe & Sileshi Degu
You can also search for this author in PubMed Google Scholar
Contributions
B.A. conducted the ethnobotanical survey, selected and identified the plants, collected samples and conducted the laboratory work, analyzed the data, and wrote the original draft. E.L. identified the plants, supervised the field and laboratory works, and reviewed the manuscript. A.H. and A.D. supervised the field and laboratory works, and reviewed the manuscript. E.D. participated in the plant extraction, phytochemical screening, and antioxidant assay. A.A. and S.D. carried out the antibicterial assay.
Corresponding author
Correspondence to Baressa Anbessa .
Ethics declarations
Ethics approval and consent to participate.

Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Anbessa, B., Lulekal, E., Hymete, A. et al. Ethnomedicine, antibacterial activity, antioxidant potential and phytochemical screening of selected medicinal plants in Dibatie district, Metekel zone, western Ethiopia. BMC Complement Med Ther 24 , 199 (2024). https://doi.org/10.1186/s12906-024-04499-x
Download citation
Received : 03 December 2023
Accepted : 10 May 2024
Published : 21 May 2024
DOI : https://doi.org/10.1186/s12906-024-04499-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Antibacterial
- Antioxidant
- Phytochemical
- Medicinal plants
BMC Complementary Medicine and Therapies
ISSN: 2662-7671
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Iran J Pharm Res
- v.11(1); Winter 2012

Introduction of Medicinal Plants Species with the Most Traditional Usage in Alamut Region
Maryam ahvazi.
a Department of Herbarium, Institute of Medicinal Plants, ACECR, Karaj, Iran.
Farahnaz Khalighi-Sigaroodi
b Department of Pharmacognosy and Pharmaceutics, Institute of Medicinal Plants, ACECR, Karaj, Iran
Mohammad Mahdi Charkhchiyan
c Research Institute of Forests and Rangeland, Ghazvin.
Faraz Mojab
d School of Pharmacy and Pharmaceutical Sciences Research Center, Shahid Beheshty University of Medical Sciences, Tehran, Iran.
Vali-Allah Mozaffarian
e Research Institute of Forests and Rangeland, Tehran, Iran.
Hamideh Zakeri
f Cell Line Engineering, Sigma Aldrich Biotechnology Division. St Louis, USA.
The ethnobotany of the medicinal plants of Alamut region is important in understanding the cultures and traditions of Alamut people. This study documents 16 medicinal plant species, most commonly used by the indigenous people of Alamut region (Ghazvin Province), northwest, Iran. The botanical name, family name, vernacular name, part used, and the application of the plants have been provided in this paper. Alamut region was divided into different villages with the aid of maps. We recorded traditional knowledge and use of medicinal plants from herbal practitioners and village seniors in Alamut. The plants were gathered from different sites. The fully dried specimens were then mounted on herbarium sheets. We found 16 medicinal plants belonging to 11 families which were traditionally used in Alamut. Finally, we describe traditional usages by the native people in the Alamut region. The obtained results were compared with data on the herb’s clinical effects. A set of voucher specimens were deposited to the Institute of Medicinal Plants Herbarium (IMPH).
Introduction
Before the introduction of chemical medicines, man relied on the healing properties of medicinal plants. Some people value these plants due to the ancient belief which says plants are created to supply man with food, medical treatment, and other effects. It is thought that about 80% of the 5.2 billion people of the world live in the less developed countries and the World Health Organization estimates that about 80% of these people rely almost exclusively on traditional medicine for their primary healthcare needs. Medicinal plants are the “backbone” of traditional medicine, which means more than 3.3 billion people in the less developed countries utilize medicinal plants on a regular basis ( 1 ). There are nearly 2000 ethnic groups in the world, and almost every group has its own traditional medical knowledge and experiences ( 2 , 3 ). Iran is home to several indigenous tribes with a rich heritage of knowledge on the uses of medicinal plants. Iran has varied climates and geographical regions that have caused a wide distribution of individual medicinal plant species such that each tribe has its own plants and customs. Alamut is one of the most important geographic regions in Iran because of its ancient history of cultivating traditional medicinal plants. Alamut region and the several villages it encompasses are secluded from other cities in Iran, which is why the people living in this region have relied on indigenous medical knowledge and medicinal plants. In this study, we analyzed the medicinal plants with most therapeutic usage in the region.
Experimental
Geographic and climatic overview
Alamut mountainous region is situated in the central Alborz Mountains, between 36˚24´ and 36˚46´ northern latitudes and 50˚30´ and 50˚51´ eastern longitudes with an altitude ranging from 2140 to 4175 m. The region is located on the northeast of Ghazvin Province and is bounded to the north by the Mazandaran Province in Tonekabon and bounded on the east by Tehran Province in the Taleghan mountains. Annually, it rains 368.03 mm and the average temperature is 14°C. Topography is distinctly marked with several mountains, springs, rivulets, and rivers. This area is geographically located in the Irano-Turanian region ( Figure 1 ).

Study area: Iran map and Alamut in Ghazvin Province
The ethnic composition of the region is quite diverse and almost 90% of its population resides in rural areas. The language of the inhabitants is known as Deylamite. People of Alamut have a long history of exporting medicinal plants to other regions of Iran. Roadways have increased communication among the rural natives in Alamut and have also increased tourism to the region because of its several ancient castles. Because of good quality of medicinal plants in this region and more immethodical pick of them, some of species have become extinct. For this reason, an important aim of this study is to protect the preservation of the region’s plants. Other aims include:
Documenting the traditional knowledge of medicinal plants from the natives.
Assessing the most commonly used local medicinal plants.
Promoting the potential benefits of medicinal plants.
Data collection
We first prepared a map with a scale of 1:25,000 from the region to identify the number of villages, roads, and vegetations. We visited the region and spoke to herbal practitioners and village seniors. A questionnaire was used to obtain information on the types of ailments treated using traditional medicinal plant species. Sometimes informants were asked to come to the field and introduce us to the plants. When this was not possible, plants were collected around the villages of the informants and were shown to them to confirm the plant names. This investigation took over 2 years and information was collected 1-2 days per week. Voucher samples were also collected for each plant and were identified using floristic, taxonomic references. Flora Iranica and a dictionary of Iranian plant names were used for identification purposes ( 4 , 5 ). Plants were deposited at the herbarium of Institute of Medicinal Plants (IMPH).
Although ancient sages through trial and error methods have developed herbal medicines, the reported uses of plant species do not certify their efficacy ( 6 ). Reports on ethnomedicinal uses of plant species require pharmacological screenings, chemical analyses, and tests for their bioactive activities. Pharmacological screening of plant extracts provides insight to both their therapeutic and toxic properties as well as helps in eliminating the medicinal plants or practices that may be harmful ( 7 ).
This study provides information on 16 medicinal plants belonging to 12 families that are most commonly used for traditional medicine in Alamut region. Botanical names of plants were sorted alphabetically, and for each species and the following information was hence represented: family, vernacular name, part used ( Table 1 ). Traditional use and preparation was compared with other references ( Table 2 ).
Medicinal plants collected from Alamut region
Comparison of problems due to hot flash in studied groups during the study base on HFQ.
Among these medicinal plants, Apiaceae, Lamiaceae , and Boraginaceae were the most dominant families with 4, 2, 2 species belonging to 4, 2, 2 genera of medicinal plants, respectively.
Of the 16 medicinal plants, 8 species had similar effects in traditional and medicinal uses when comparing Alamut with other references. Achillea millefolium had antibacterial effects; Capparis spinosa is used for headache, renal complaints and stimulating tonic; Echium amoenum is used for common cold and had sedative effects; Ferula persica is used for gout; Juglans regia is used for diabetes; Smyrnium cordifolium is edible and used as tonic; Viola odorata is used for fever and migraine; Ziziphora clinopodioides is used for cold, infections and stomachache.
Some effects which are mentioned in traditional medicine of Alamut region were important with no scientific information about them. For example, Berberis integerrima and Hippophae rhamnoides had good effect on lowering of serum lipids and blood sugar and hypertension. Malva neglecta is used for mouth fungal infection in children and Stachys lavandulifolia is used for headache and renal calculus. Other researches can perform experiments to discover their components and effects.
All of the medicinal plants were collected from the wild or in the native people’s gardens. Some medicinal plants can no longer be found in the region and are only cultivated in the native people’s gardens. For example, Echium amoenum is an endemic plants in Iran with historically wide spread in the region, but because of frequent picking, the species is now just cultivated in the native people’s gardens.
Different parts of medicinal plants were used by the inhabitants of Alamut region as medicine for treating ailments. The most common parts used were flowers (25%). The use of aerial parts, leaves, fruits and roots were the same (15%). Use of the stems (7%), seeds, and blooms (4%) were lower than the others ( Figure 2 ). The 16 medicinal plant species were used in treating 27 different types of ailment ( Table 3 ).

Plants part use and their percentage
Medicinal plant species were used in treating different types of ailment
Acknowledgment
This work was supported by grants from Institute of Medicinal Plants and the Iranian Academic Center for Education, Culture, and Research (ACECR). The authors would like to thank Ghazvin Research Institute of Forests and Rangelands for their sincere cooperation.

IMAGES
VIDEO
COMMENTS
Download full-text PDF Read full-text. ... This Special Issue consists of 13 papers dealing with the phytochemical investigation ... the research was done using medicinal plants in "article ...
The Rigveda (5000 BC) has recorded 67 medicinal plants, the Yajurveda 81 species, the Atharvaveda (4500-2500 BC) 290 species, and the Charak Samhita (700 BC) and Sushrut Samhita (200 BC) have described properties and uses of 1100 and 1270 species, respectively, to compound the drugs and use.
Figure 5. Temporal evolution on medical plants publications for Top 12 countries. The first group is the leaders of this research, China and India, with between 800 and 1100 publications per year. China led the research from 1996 to 2010, and from this year to 2016, the leader was India, after which it returned to China.
A medicinal plant is any plant which, in one or more of its organs, contains substances that can be used for therapeutic purposes, or which are precursors for chemo-pharmaceutical semi-synthesis ...
The history of medicinal plant utilization for therapeutic purposes dates back to antiquity. Medicinal plants form the mainstay of herbal drugs. These plants have been an integral part of the ancient traditional medicine systems, e.g. Chinese, Egyptian and Ayurvedic (Sarker and Nahar 2007). Medicinal plant products have
School of Sciences, IGNOU, New Delhi, India. Medicinal plants: Future source of new drugs. Arvind Kumar Shakya. Abstract. India has a long history and strong base for Ayurveda, w hich is the ...
Nevertheless, medicinal plants still have a hopeful future, as the phytochemical composition and the potential health benefits of many species have not yet been studied or still need to be more deeply investigated [4]. This Special Issue consists of 13 papers dealing with the phytochemical investigation and biological properties of medicinal ...
two drugs derived from a wild plant native to Madagascar. But we still know little about the treasure trove inhabiting our wild places. As of 1995, less than 1 percent of all tropical plant species had been screened for potential pharmaceutical applications. As medicinal plants receive increased scientific and commercial attention, there is
The disciplines of ethnobotany and ethnopharmacology define "medicinal plant" as those species used in traditional medicine that contain beneficial elements in healing diseases in humans and/or animals. The objective of ethnopharmacology is to develop a drug to treat patients, and ultimately to validate traditional use of medicinal plants.
Craib, a traditional Asian medicinal plant. The findings of this research are useful for the quality control of this plant drug [18]. Therefore, if well conducted, Ethnobotany research and the interaction between aca-demics and traditional communities can help to preserve biodiversity, improve the local
The research paradigm of medicinal plant genome and evolution is evolving, and the use of omics techniques is reshaping the landscape of this dynamic field. ... Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. ... PDF/ePub View PDF/ePub. Get access. Access options. If you have ...
1. Introduction. Ethnobotany is defined as the study of local people's interaction with the natural environment: how they classify, manage, and use plants available around them (Getaneh, Citation 2019; Limenih et al., Citation 2015).Over centuries, indigenous people have developed their locality specific knowledge on medicinal plant use, management, and conservation (Duguma & Mesele ...
This book is designed to provide pharmacologists and researchers of natural products a comprehensive review of 200 medicinal plants, ... Download chapter PDF ... He also served as Director of Pharmacy College Research Center, Qassim University, Saudi Arabia. He has many research papers published in international journals, book chapters on beta ...
Academia Journal of Medicinal Plants 9(8): 100-117, August 2021 DOI: 10.15413/ajmp.2021.0112 ISSN: 2315-7720 ©2021 Academia Publishing Research Paper Indigenous uses of medicinal plants in Tarikhet block of Kumaun Himalaya, India Accepted th7 April, 2021 ABSTRACT Over 80% of the world population relies on vegetation for health care; more than
Plants with medicinal properties play an increasingly important role in food and pharmaceutical industries for their functions on disease prevention and treatment. This study characterizes the ...
Background Plant-derived products have an imperative biological role against certain pathogenic organisms and were considered to be a major source of modern drugs. Rural people residing in developing countries are relying on traditional herbal medical system due to their strong believe and minimum access to allopathic medicines. Hence, ethnomedicinal knowledge is useful for the maintenance of ...
Medicinal plants will be useful for Maternal and Child health care, as essential drugs, in food and nutrition, for common illnesses and injury, for endemic infectious diseases, mental health and oral health. Medicinal plants also fit perfectly into the modelling for priorities in Primary Health Care as proposed by McDonald and Ollerenshaw (2011).
A large number of people in both developing and developed countries rely on medicinal plant products to maintain their health or treat illnesses. Available evidence suggests that medicinal plant consumption will remain stable or increase in the short to medium term. Knowledge on what factors determine medicinal plant consumption is, however, scattered across many disciplines, impeding, for ...
Phytochemical investigations from these medicinal plants have shown a large number of organic complex and biologically active compounds. The results of the qualitative phytochemicals analysis showed that the leaf extracts of Lippia adonis var. koseret also indicated the presence of tannins, flavonoids, polyphenols, alkaloids and saponins, while in the case of ethyl acetate alkaloids were not ...
1. Introduction. Medicinal plants have been a vital source of both curative and preventive medical therapy preparations for human beings, which also has been used for the extraction of important bioactive compounds [1,2,3].It is estimated that almost 80% of the world's total population, regularly, depends on traditional medicine and products for its healthcare needs especially in third world ...
More than 100 field days are spent for the current study. Some of the regular cereals, pulses, vegetables and fruits are considered as medicinal plants. 540 species are herbs (mostly annuals), 100 ...
4. Worldwide Worldwide research research on on medical medical plants. plants. The first group is the leaders of this research, China and India, with between 800 and 1100 publications per year. China led the research from 1996 to 2010, and from this year to 2016, the leader was India, after which it returned to China.
Background Medicinal plants have been used countless times for curing diseases mainly in developing countries. They are easily available with little to no side effects when compared to modern medicine. This manuscript encompasses information on ethnomedicinal plants in Champhai district, located in the North East Region (NER) of India. The region lies within Indo-Burma biodiversity hotspot ...
Background: Traditional knowledge of medicinal plants, their application, and conservation is very likely to be disappearing. From the perspective of conservation planning and management, it is crucial to assemble information about the use of medicinal herbs by various ethnic groups and at various spatial scales. Therefore, with this viewpoint in mind, this study was carried out, which ...
Medicinal plants play a major role in the delivery of healthcare, particularly among the rural population of Ethiopia. Plant extracts and their bioactive compounds have been utilized for the treatment of several diseases. This study was aimed at evaluating the antibacterial activity, antioxidant capacity, and phytochemical content of selected medicinal plants used in Dibatie district, western ...
The use of medicinal plants has been done since ancient times and may even be considered the origin of modern medicine. Compounds of plant origin have been and still are an important source of compounds for drugs. In this study a bibliometric study of all the works indexed in the Scopus database until 2019 has been carried out, analyzing more than 100,000 publications. On the one hand, the ...
In recent years, there has been a growing interest in the use of medicinal plants and phytochemicals as potential treatments for acne vulgaris. This condition, characterized by chronic inflammation, predominantly affects adolescents and young adults. Conventional treatment typically targets the key factors contributing to its development: the proliferation of Cutibacterium acnes and the ...
This study documents 16 medicinal plant species, most commonly used by the indigenous people of Alamut region (Ghazvin Province), northwest, Iran. The botanical name, family name, vernacular name, part used, and the application of the plants have been provided in this paper. Alamut region was divided into different villages with the aid of maps.