Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 03 March 2010

Reconsidering reproductive benefit through newborn screening: a systematic review of guidelines on preconception, prenatal and newborn screening
- Yvonne Bombard 1 ,
- Fiona A Miller 1 ,
- Robin Z Hayeems 1 ,
- Denise Avard 2 &
- Bartha M Knoppers 2
European Journal of Human Genetics volume 18 , pages 751–760 ( 2010 ) Cite this article
1924 Accesses
41 Citations
Metrics details
- Genetic services
- Health policy
- Population screening
- Reproductive biology
The expansion of newborn screening (NBS) has been accompanied by debate about what benefits should be achieved and the role of parental discretion in their pursuit. The opportunity to inform parents of reproductive risks is among the most valued additional benefits gained through NBS, and assumes prominence where the primary goal of identifying a treatable condition is not assured. We reviewed 53 unique guidelines addressing prenatal, preconception and newborn screening to examine: (1) how generating reproductive risk information is construed as a benefit of screening; and (2) what conditions support the realization of this benefit. Most preconception and prenatal guidelines – where generating reproductive risk information is described as a primary benefit – required that individuals be given a ‘cascade of choices’, ensuring that each step in the decision-making process was well informed, from deciding to pursue information about reproductive risks to deciding how to manage them. With the exception of three guidelines, NBS policy infrequently attended to the potential for reproductive benefits; further, most guidelines that acknowledged such benefits construed voluntarism narrowly, without attention to the choices attendant on receiving reproductive risk information. This review suggests that prenatal and preconception guidance identifies a coherent framework to support the pursuit of reproductive benefits through population screening programmes. Interestingly, attention to reproductive benefits is increasing among NBS guidance, yet reflection on how such benefits ought to be pursued remains limited. Traditional norms for NBS may require reconsideration where the remit of screening exceeds the primary goal of clinical benefits for infants.
Similar content being viewed by others

Perception of genomic newborn screening among peripartum mothers

Non-invasive prenatal testing (NIPT) and pregnant women’s views on good motherhood: a qualitative study
Routinization of prenatal screening with the non-invasive prenatal test: pregnant women’s perspectives
Introduction.
Newborn screening (NBS) is a premier example of the application of genomic discoveries to population health benefits. Traditionally, NBS programmes identified serious conditions where early detection and urgent presymptomatic treatment were necessary to avert serious clinical harm. The classic example is phenylketonuria, where the immediate detection of affected babies resulted in clinical benefit through lifelong dietary management, effectively preventing neurological devastation. In this context, NBS operated under the rubric of a ‘public health emergency’ model, 1 in which screening was mandatory or consent was otherwise implied.
Although these clinical goals remain, increased technological capacity means that expanded NBS programmes can now identify a broader range of conditions, including those for which treatment is not established, as well as benign carrier states or variants of uncertain clinical significance. 2 In consequence, expansion has been accompanied by debates about the nature of benefit to be achieved. Advocates of expanded infant screening programmes argue for a wider interpretation of the notion of benefit. 3 They maintain that screening for conditions in which clinical outcomes are unproven or limited provides information, and permits access to programmes that offer education and support. 4 , 5 The opportunity to inform parents and infants of future reproductive risks is among the most valued additional benefits to be achieved. 4 , 5 , 6 , 7 , 8
Historically, ‘reproductive benefit’ – that is, the potential benefit of learning reproductive risk information to support family planning – arose as a secondary outcome of the primary goal of identifying a treatable condition, and thus little attention was given to how such a benefit should be realized. Yet, there are several ways in which expanded NBS upsets this hierarchy of benefits. 9 The first is in the case of expanded NBS panels that include conditions for which clear evidence of medical benefit is not established, 2 such that the identification of reproductive risks assumes greater prominence. 3 , 4 A second way pertains to certain rare conditions, such as Duchenne muscular dystrophy, for which there is no medical treatment but for which early diagnosis permits the identification of reproductive risks. 10 , 11 , 12 Finally, NBS can also detect healthy infants who are carriers; routine disclosure of this information can identify reproductive risks in parents and future adults.
Where the traditional outcome of NBS is not assured, the opportunity to acquire reproductive risk information may assume greater prominence, increasing the need to reconsider the relevance of parental discretion in pursuing such benefits. To this end, we turned to guidance from complementary paradigms – preconception (PCS) (community-based screening of at-risk groups) and prenatal screening (PNS) programmes – where the pursuit of reproductive risk information is generally the primary goal. We conducted a systematic review of relevant policies and position papers on PCS, PNS and NBS guidelines to examine: how guidelines construed generating reproductive risk information as a potential benefit of screening; and what conditions were seen to support the realization of this benefit.
Data sources
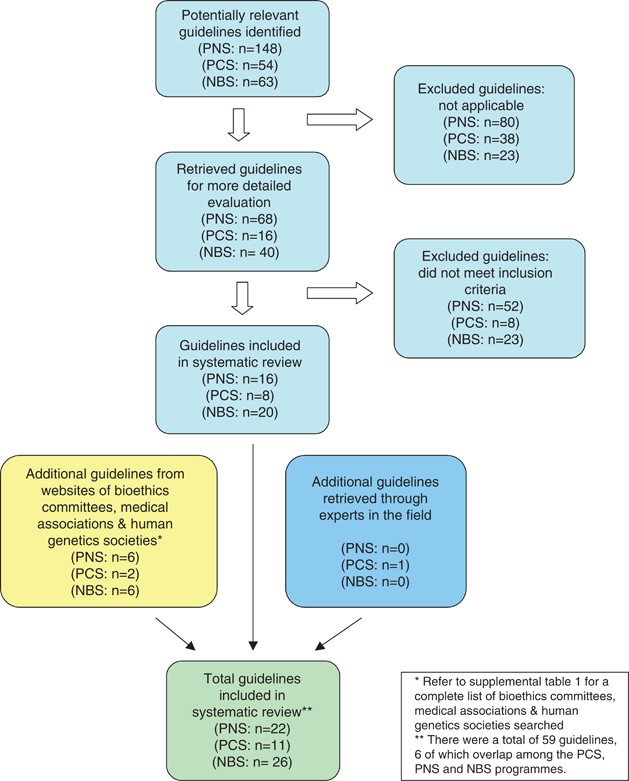
In accordance with the core principles of systematic review methodology, 13 we conducted a review of relevant guidelines using HUMGEN (a database of laws and policies related to human genetics, which uses other databases, including PubMed, Google Scholar and others – www.humgen.org/int/_ressources/Method_en.pdf ) ( Figure 1 ). In addition, we searched websites of key organizations catalogued in HumGen, including the WHO, UNESCO, Council of Europe, national bioethics committees, human genetics societies and national medical associations ( Supplementary Table 1 ). Additional guidelines were obtained from experts in the field. Our review included international and regional governmental and nongovernmental health organizations. In addition, guidance from national organizations limited to Europe, North America, the United Kingdom and Australasia were included, to represent jurisdictions that shared similar health-care and public health infrastructures. We searched the databases and websites using the following search terms: ‘preconception’ [or] ‘reproductive’ [or] ‘pre-pregnancy’ [and] ‘genetic screening’ [or] ‘screening’ [or] ‘testing’; ‘prenatal’ [or] ‘pregnancy’ [and] ‘genetic screening’ [or] ‘screening’ [or] ‘testing’; and ‘newborn’ [or] ‘neonatal’ [or] ‘neonate’ [and] ‘genetic screening’ [or] ‘screening’ [or] ‘testing’.
Study selection.
Study selection
Policies were eligible for inclusion in our review if they were available position papers, reports or if they contained guidelines or statements produced by international, national and regional governmental and nongovernmental health organizations, bioethics committees or professional associations that explicitly addressed (1) newborn screening, (2) preconception screening or (3) prenatal screening. Only guidelines written in English or translated into English from relevant organizations were eligible for inclusion.
We excluded guidelines that did not explicitly include a statement that described the goals or purpose of the screening programme and that were published before 1996. This date restriction reflected the time period during which most relevant guidelines were produced. Guidelines focused on prenatal diagnosis, and those emanating from subnational organizations (eg, provinces or states) were also excluded. Finally, guidelines that focused on technical, organizational, laboratory or cost issues were ineligible. Any uncertainties regarding inclusion were discussed and agreed upon by 2–3 members of the team.
Data extraction and synthesis
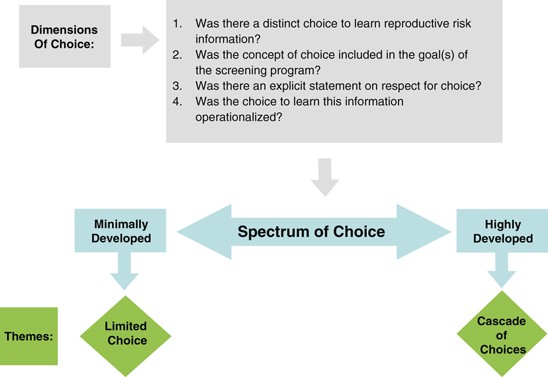
We used a qualitative content analytical approach to examine selected guidelines, drawing on the principles of qualitative description 14 and constant comparison. 15 First, guidelines were examined for an explicit description of the goals and benefits of the respective screening programme, noting the ways in which the generation of reproductive risk information was construed. The guidelines were then examined for an explicit description of how these benefits were to be realized. Specifically, we noted whether the screening programme was to be voluntary and whether informed consent was required. Focusing first on selected PCS and PNS guidelines, we developed an analytical framework describing the conditions or processes considered to support the pursuit of reproductive benefit. The analytical framework ( Figure 2 ) included four dimensions of choice, which were then used to distil guidelines into a two-part spectrum with regard to the orientation towards voluntarism, from highly to minimally developed. Finally, this framework was used to examine the NBS guidelines, to identify similarities and differences in their orientation towards voluntarism.
Analytical framework.
We retrieved a total of 59 guidelines ( Table 1 ) from 31 different organizations (six guidelines overlapped among PCS, PNS and NBS, yielding 53 unique guidelines). The guidelines originated from government-affiliated institutions ( n =7), government advisory bodies ( n =11), medical research agencies ( n =3) and nongovernmental professional organizations ( n =32). Altogether, 11 guidelines related to PCS, 22 on PNS and 26 pertained to NBS. The majority emanated from national organizations ( n =49), with fewer from international ( n =1) and supranational ( n =3) bodies.
Most preconception and prenatal guidelines – where generating reproductive risk information was a primary benefit of screening – required that individuals be given a ‘cascade of choices’ regarding reproductive risk information. By contrast, guidance for NBS infrequently attended to the potential for reproductive benefits as a primary or secondary goal. Further, most guidelines that acknowledged such benefits construed voluntarism narrowly, offering ‘limited choices’ that did not attend to the specific choices arising from the pursuit of reproductive risk information.
Recognizing reproductive benefit
It is not surprising that a sharp contrast was apparent in how PCS and PNS guidance approached reproductive benefit when compared with how this benefit was considered in NBS policies. All PCS and PNS guidelines stated that the pursuit of reproductive risk information was the primary benefit of screening (PCS: n =11 of 11, PNS: n =22 of 22), whereas few ( n =3) NBS guidelines identified reproductive benefit as an intended or unintended benefit of screening ( Table 1 ).
For example, in its statement entitled ‘Essentially Yours: The Protection of Human Genetic Information in Australia’ 16 the Australian Law Reform Commission clearly stipulated that the purpose of PCS is ‘to alert individuals to their carrier status so that they are able to make informed decisions about reproduction’ (note 24.30). Similarly, in the PNS context, guidelines explicitly oriented the programmes towards the immediate benefit of this risk information.
Conversely, few of the NBS guidelines ( n =3 of 26) identified the generation of reproductive information as a benefit of NBS: it was seen as a primary aim in one guideline and as an ‘indirect’ benefit to the infant and family in the other two. The Human Genetics Commission's statement on ‘Making Babies: Reproductive Decisions and Genetic Technologies’ 17 acknowledged reproductive benefit as an indirect and limited end, stating that newborn screens are ‘not simply relevant to the care of the child involved. But because they may lead to the diagnosis of a genetic condition they can have implications for future reproductive decision-making by parents’ (pp 41). However, the other two guidelines pointed to ways in which reproductive benefits may achieve elevated significance in this context. In their statement on ‘Newborn Screening’, 18 the Health Council of the Netherlands acknowledged both the existence of an ‘indirect’ reproductive benefit, and the potential for this benefit to assume primary importance in cases in which the typical goal of clinical benefit cannot be achieved:
The identification of patients with a hereditary disorder also brings to light parent carriers. This discovery allows future family planning choices to be made in families with what are usually serious hereditary disorders… The opportunity to make choices is a benefit for the family, and sometimes also for the newborn child… The indirect benefits for the patient and the benefits for the family may result in screening being contemplated where there is little if any direct benefit to be had. Patients’ organisations have taken the view that screening should not automatically be ruled out even if no treatment is available. The Committee shares this opinion. (pp 29)
Importantly, the Center for Disease Control's recommendations with regard to NBS for cystic fibrosis (CF) 19 included ‘reproductive benefits to families’ as one of the primary benefits, alongside the other primary goals of disease detection and avoidance of the diagnostic odyssey, as illustrated in Figure 3 :
The benefits of screening flow from early, asymptomatic detection and can be classified in terms of health benefits to the affected person and psychosocial benefits to persons and families… Another potential benefit to parents from a diagnosis of CF by newborn screening is the ability to make informed decisions related to further childbearing, because the diagnosis might occur 1 year earlier on average compared with conventional diagnosis (0.5 and 14.5 months, respectively). (pp 10 and 23)
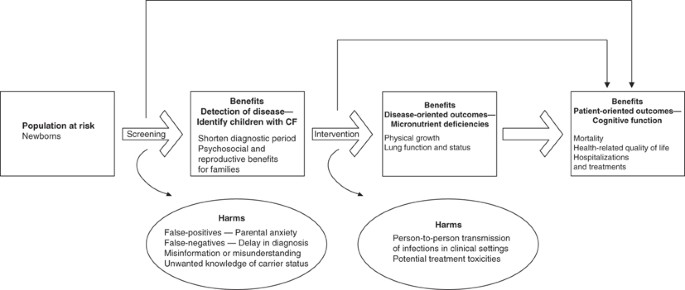
Potential benefits and harms of newborn screening for cystic fibrosis, as identified by Grosse and colleagues 19 (figure reproduced with permission).
All PCS and PNS guidelines positioned the generation of reproductive risk information as the primary goal and benefit of screening programmes, and did so uniformly. Reproductive benefit in NBS guidelines, by contrast, was noted in only three guidelines. However, although clearly framed as ‘indirect’ to the main goal of identifying treatable disorders in two of these, the potential of this indirect goal to assume primary significance was acknowledged; further, it was seen as a primary benefit in the other guideline.
Achieving the benefits of screening
All PCS and PNS guidelines emphasized voluntarism as the means of achieving the benefits of screening, requiring that individuals be given the choice to learn of their reproductive risk information (PCS: n =11 of 11; PNS: n =22 of 22). It is interesting that 15 out of 26 of the NBS guidelines advocated voluntarism in pursuing the traditional clinical benefits of screening; however, only one of the three NBS policies that recognized reproductive benefit highlighted voluntarism as the means of pursuing this specific benefit. Consequently, we identified a spectrum through which the choice to pursue reproductive benefit was described across the three screening programmes, with some guidelines emphasizing a fulsome ‘cascade of choices’ and others construing voluntarism more narrowly as a set of ‘limited choices’ ( Tables 1 and 2 ).
Cascade of choices
The majority of PCS (9 of 11) and PNS (16 of 22) guidelines clearly presented a cascade of choices in pursuing reproductive benefit. Voluntarism within these guidelines involved a set of nested decisions, each one preceding and enabling action on the other, by sequentially referring to the following: (i) the choice ‘to pursue’ reproductive risk information; (ii) the choice ‘to know’ diagnostic information in light of the risk information received; and where relevant; (iii) the choice ‘to act,’ specifically, whether to continue a pregnancy. The New Zealand's Ministry of Health PNS guideline 20 draws a clear distinction between the elements in this cascade of choices: (i) to pursue reproductive risk information (‘who choose to have this information’); (ii) ‘to make informed decisions about whether to have diagnostic testing’; and, (iii) ‘to make informed decisions about whether to continue or terminate the pregnancy’ (pp 17). Importantly, these distinct choices are stipulated as part of the goals of the programme. Similarly, the National Society of Genetic Counsellors’ ‘Preconception/Prenatal Genetic Screening’ guidelines 21 emphasize this cascade of choices:
Individuals/couples considering screening should be provided with accurate, balanced information about the condition for which screening is being offered. They should be informed of the specificity, sensitivity, accuracy, risks, benefits and limitations of the screening tests offered and of any follow-up diagnostic tests as well as their reproductive options given a positive diagnostic test result (pp 3).
Guidelines offering a cascade of choices emphasized a high degree of respect for individuals’ sequential choices in pursuing reproductive benefit. For example, the overall principle within New Zealand's statement on PNS 20 focused on an ‘unconditional acceptance and support’ for women's choices ‘at each stage of the screening and diagnostic pathway’ (pp 3). Within the United Kingdom's Human Genetics Commission guidelines in reference to PNS, 17 the choice to participate in screening is emphasized in their discussion on the ‘ethos of offering screening.’ They stressed the importance of explaining the ‘aims of screening’ before booking the screening appointment to ensure that the ‘offer of screening’ was seen as a ‘real option’ rather than as a ‘default option’ so as ‘to minimize any sense of guilt or attribution of blame for a decision not to participate’ (pp 12).
In the NBS context where reproductive benefit was recognized as a discrete benefit in only three guidelines, 17 , 18 , 19 the Health Council of the Netherlands 18 published the only policy that advanced a cascade of choices with regard to reproductive benefit. Although reproductive benefit was framed as an ‘indirect’ benefit, they acknowledged that:
Special attention needs to be paid to providing information about the possibility of screening revealing that a newborn is a carrier. This practically always means that one or both parents are also carriers. As with parents of an affected child, if required, adequate information must also be available on what being a carrier entails and on the disorder concerned. (pp 16)
They required that a choice be offered as to whether parents want to learn incidentally generated reproductive risk information (ie, child's carrier status), and restricted the pursuit of this benefit to ‘medically indicated’ cases:
Parents ought therefore to be given the option of forgoing information about carrier status at the point in time when the information [about NBS in general] is provided (during pregnancy, as advocated in Section 4.4.3). If, however, they should ask for carrier screening after receiving the information, then this request can be satisfied if this is medically indicated (owing to a family history of the disorder in question or, in the case of hemoglobinopathy, the geographical origin of the affected individuals). (pp 78)
Guidelines in this category paid significant attention to the voluntary pursuit of reproductive risk information, as well as to the respect for individuals’ choices. They offered a distinct choice to learn reproductive risk information and distinguished between the sequential or nested nature of the choices inherent in pursuing such information.
Limited choice
Of the guidelines recognizing reproductive benefit, a few of the PCS (2 of 11) and PNS (6 of 22) policies presented voluntarism as a limited array of choices; where voluntarism was construed more narrowly, guidelines paid little attention to the sequential nature of choices afforded to individuals in pursuing reproductive risk information. Similarly, two of the remaining three NBS guidelines that identified the possibility of reproductive benefit did not reflect on voluntarism at all, and importantly, the choices specific to reproductive risk information were entirely absent.
Several of the PCS and PNS policies presented limited choices in the pursuit of reproductive benefit. The PCS guideline by the US Preventive Services Task Force, 22 for example, highlighted the need for education and counselling without an explicit distinction between the types of choices offered (‘informed reproductive choices by receiving genetic counseling’). Among others, voluntarism was referred to abstractly or was otherwise absent. For example, in the PNS guideline prepared by the Royal Australian and New Zealand College of Obstetricians and Gynaecologists, 23 the condition to achieve voluntarism seems to refer to one point in time, rather than to a process encompassing several stages:
As with any test or procedure, these investigations should only be undertaken with the informed consent of the patient after adequate and appropriate counseling as to the implications, limitations and consequences of such investigation.
The choice regarding reproductive risk information was largely ignored in the two remaining NBS guidelines that recognize reproductive benefit. 17 , 19 The CDC's 19 guidelines on NBS for CF advocated voluntarism only with respect to the choice to participate in the NBS programme as a whole, without attention to the specific choices attendant on receiving reproductive risk information. It asserted:
Documentation of consent might not be necessary. The focus should be on providing thorough, easily understood information to parents about screening for CF and other conditions, especially before delivery, to reduce misunderstanding and provide parents with an opportunity to make informed choices, consistent with state laws. (pp 28)
The Human Genetics Commission's recommendations 17 did not mention voluntarism in relation to either participating in NBS programmes generally or pursing reproductive risk information through such programmes, more specifically.
Guidelines in this category reflected only partially on the conditions supporting choice in the receipt of reproductive risk information. Where voluntarism was addressed, it was characterized by broad statements gesturing to the need for choice in achieving the benefits of screening, but without attention to an initial choice to receive reproductive risk information, nor to the sequential, nested choices of acting on that information. Importantly, of the two remaining NBS guidelines that identified the potential for reproductive benefits, only one supported any form of voluntarism. However, the voluntarism advocated was solely for participating in screening as a whole and not for the pursuit of reproductive risk information; in the final case, no form of voluntarism was identified.
The diversity of NBS programmes along with rapid technological advances requires an assessment of the direction of NBS programmes. These issues are not without controversy and there is considerable international dissensus on the appropriate scope of NBS panels and the types of discretion to be afforded to parents. 24 , 25 , 26 , 27 Central to these debates is the balance of risks and benefits, which also remains equivocal. Although the potential risks – namely, psychosocial harms from false-positive results, 28 overmedicalization, 29 , 30 misattributed paternity, 31 stigma and discrimination 32 – have remained the same, notions of benefit have evolved. Benefits, as argued by some, extend beyond the strictly medical model to include benefits previously considered secondary, such as early intervention, avoidance of the ‘diagnostic odyssey’ and guidance for reproductive decision making. 3 , 5 , 6 , 7 , 27 Indeed, NBS practice is changing, as an increasing number of jurisdictions have embraced the potential value of these broader benefits and have expanded their panels accordingly. 27 , 33 , 34 Guidelines, however, have not evolved in tandem to reflect on the realization of these expanded benefits, nor on the choices regarding the pursuit of reproductive benefits.
Given that NBS typically operates as a mandatory or implied consent programme, reproductive risk information becomes packaged into this programme, effectively requiring parents to receive reproductive risk information, 35 which is at odds with the principles of voluntarism and nondirectiveness underpinning the PCS and PNS programmes. 36 Automatic disclosure of this risk information in the context of NBS is surely appropriate in cases in which infants can be expected to receive health benefits. Yet, it might be seen to violate parents’ autonomy, 35 in cases in which the benefit of reproductive risk information achieves particular prominence. This may occur in three specific ways including: (1) screening for rare conditions (eg, Duchenne muscular dystrophy) that do not have accepted treatment, yet early diagnosis and assisting reproductive decision making are considered important benefits; 10 , 11 , 12 (2) expanded NBS panels that identify conditions without clear evidence of clinical benefit for affected infants, 33 wherein the benefits of acquiring reproductive risk information are assuming greater, if not equivalent, importance, blurring the lines between primary and secondary benefits; 9 and (3) incidental results (eg, carrier status) that are clinically benign for the infant, yet (potentially) immediately useful to his or her parents in planning future pregnancies. In these scenarios, the significance and relevance of this information for reproductive planning raises anew questions on how to pursue reproductive benefit.
The PCS and PNS guidelines offer direction for considering the realization of reproductive benefit through NBS. These guidelines are sensitive to the issues inherent in screening for the purpose of reproductive benefit. In addressing such a benefit, PCS and PNS guidance seeks to mobilize people's capacity to make sequential choices, and typically emphasizes the importance of a fulsome ‘cascade of choices’ to ensure a well-informed decision-making process, which differentiates between the choice to pursue reproductive risk information, the choice to know how this risk information pertains to a specific pregnancy and the choice to act on a diagnosis. By contrast, although many NBS guidelines support voluntarism regarding participation in the programme as a whole, only two of those that identified the potential for reproductive benefits supported any form of voluntarism. Whereas one guideline supported a cascade of choices, in another, the pursuit of reproductive risk information was collapsed into the choice to participate in screening as a whole, thus highlighting challenges in the ethical pursuit of reproductive benefit through NBS.
Although it is widely agreed that the fundamental goal of NBS is to benefit the infant, 37 and further, that the ethical norm of respect for persons necessitates that infants not be treated as a means to an end, debate continues as to whether the fact that NBS can inform parents about their reproductive risks is, in itself, a justification for an expanded panel or for a routine disclosure of incidental results. 4 , 5 , 9 , 38 , 39 Indeed, an inherent tension exists between the obligations of serving the primary health interests of children, respecting autonomy and reducing potential harms in view of the current routine provision of most NBS programmes. The automatic disclosure of reproductive risk information in the absence of a fulsome consent process defies each of these obligations equally. 7 , 40 , 41 , 42 This is especially salient in light of recent evidence suggesting that the broader benefits encouraged by expanded NBS programmes may effect subtle and complex familial and social harms. 43 PCS and PNS programmes, conversely, provide alternative methods of achieving reproductive benefits without imposing the myriad of moral burdens identified here. Further, such programmes offer a more fulsome cascade of choices to parents as illustrated by this review.
Limitations
Typical of systematic reviews of literature, our review has omitted guidance that may have provided additional insight into how to pursue reproductive benefit in the context of NBS. Although beyond the scope of the paper, guidelines produced by consumer groups and disease-centred organizations were omitted because of our focus on guidance produced by professional, health organizations or committees. The deliberate exclusion of guidelines pertaining to prenatal diagnosis, for the sake of direct comparison, may have also limited the scope of insights gleaned with regard to the extent of parental discretion afforded in such programmes. Finally, our interpretation of the nature of consent presented in these guidelines offers a typology that was restricted by the lens we adopted to answer our particular research question. The interpretations and typology are thus tentative and we are unable to immediately generalize to all guidelines related to these programmes.
Future research
Recognizing that guidelines are meant to be reflections on the appropriate use of particular health technologies, it is unknown how such guidance actually affects those it is meant to serve. Further research on stakeholders’ perspectives on these expanded notions of benefit may illuminate their opinions on the risk/benefit ratio of NBS and when parental discretion may be important. Appropriate consent models should also be explored with stakeholders, noting both preferences and capacity for its provision. 44 Finally, a fulsome study of stakeholders’ informed preferences for PCS or prenatal versus NBS approaches in pursuing reproductive benefit is also warranted. Ultimately, further discussion and analysis is required to address the implications of providing reproductive risk information as a primary benefit of a population-screening programme. Salient questions arise, including whether consent for and/or education on pursuing reproductive risk information is included in the context of NBS; who should be responsible for this process; and who should ensure that the future-adult infant is informed of his or her carrier status. Further, once such a benefit is pursued as part of a population intervention, should parents and other family members (eg, minors) be invited to undergo carrier testing, and how should cascade testing be carried out?
Conclusions
This review suggests that guidance from prenatal and preconception contexts identifies a coherent framework for the pursuit of reproductive benefits through population screening. Thus, when NBS no longer serves the primary goal of clinical benefit for affected infants (eg, in cases in which infant carrier results are generated, or in the absence of demonstrated clinical benefit for an affected infant), it should seek to introduce a ‘cascade of choices’ in pursuing reproductive benefits. Alternatively, achieving reproductive benefit may be best pursued through PCS and PNS.
Grosse SD, Boyle CA, Kenneson A, Khoury MJ, Wilfond BS : From Public Health Emergency to Public Health Service: the implications of evolving criteria for newborn screening panels. Pediatrics 2006; 117 : 923–929.
Article Google Scholar
American College of Medical Genetics: Newborn Screening: Toward a Uniform Screening Panel and System . Bethesda: American College of Medical Genetics, 2005.
Bailey DB, Skinner D, Warren SF : Newborn screening for developmental disabilities: reframing presumptive benefit. Am J Public Health 2005; 95 : 1889–1893.
Alexander D, van Dyck PC : A vision of the future of newborn screening. Pediatrics 2006; 117 : 350–354.
Bailey DB, Beskow LM, Davis AM, Skinner D : Changing perspectives on the benefits of newborn screening. Ment Retard Dev Disabil 2006; 12 : 10.
Laird L, Dezateux C, Anionwu EN : Neonatal screening for sickle cell disorders: what about the carrier infants? Br Med J 1996; 313 : 407–411.
Article CAS Google Scholar
Andrews LB, Fullarton JE, Holtzman NA, Motulsky AG : Assessing Genetic Risks: Implications for Health and Social Policy . Washington: National Academy Press, 1994.
Google Scholar
Green NS, Dolan SM, Murray TH : Newborn screening: complexities in universal genetic testing. Am J Public Health 2006; 96 : 1955–1959.
Bombard Y, Miller FA, Hayeems RZ et al : The expansion of newborn screening: is reproductive benefit an appropriate pursuit? Nat Rev Genet 2009; 10 : 666–667.
Ross LF : Screening for conditions that do not meet the Wilson and Jungner criteria: the case of Duchenne muscular dystrophy. Am J Med Genet A 2006; 140 : 914–922.
Bradley DM, Parsons EP, Clarke AJ : Experience with screening newborns for Duchenne muscular dystrophy in Wales. BMJ 1993; 306 : 357–360.
Scheuerbrandt G, Lundin A, Lovgren T, Mortier W : Screening for Duchenne muscular dystrophy: an improved screening test for creatine kinase and its application in an infant screening program. Muscle Nerve 1986; 9 : 11–23.
Lavis J, Davies H, Oxman A, Denis JL, Golden-Biddle K, Ferlie E : Towards systematic reviews that inform health care management and policy-making. J Health Serv Res Policy 2005; 10 (Suppl 1): 35–48.
Sandelowski M : Whatever happened to qualitative description? Res Nurs Health 2000; 23 : 334–340.
Strauss A, Corbin J : Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory , 2nd edn. Thousand Oaks: Sage Publications, 1998.
Australian Law Reform Commission: Essentially Yours: The Protection of Human Genetic Information in Australia . Sydney: Australian Law Reform Commission, 2003.
Human Genetics Commission: Making Babies: Reproductive Decisions and Genetic Technologies . London: Human Genetics Commission, 2006.
Health Council of the Netherlands: Neonatal Screening . The Hague: Health Council of the Netherlands, 2005.
Scott D, Grosse PD, Coleen A et al : Newborn screening for cystic fibrosis: evaluation of benefits and risks and recommendations for state newborn screening programs. Morb Mortal Wkly Rep 2004; 53 : 1–36.
New Zealand Ministry of Health: Antenatal Down Syndrome Screening in New Zealand . Wellington: New Zealand Ministry of Health, 2007.
National Society of Genetic Counselors: Preconception/Prenatal Genetic Screening . Chicago: National Society of Genetic Counselors, 2005.
U.S. Preventative Services Task Force: Congenital Disorders-Screening for Hemaglobinopathies . Rockville: Agency for Healthcare Research and Quality, 1996.
The Royal Australian and New Zealand College of Obstetricians and Gynaecologists: Antenatal Screening Tests . East Melbourne: The Royal Australian and New Zealand College of Obstetricians and Gynaecologists, 2006.
Mandl KD, Feit S, Larson C, Kohane IS : Newborn screening program practices in the United States: notification, research, and consent. Pediatrics 2002; 109 : 269–273.
Hiller EH, Landenburger G, Natowicz MR : Public participation in medical policy-making and the status of consumer autonomy: the example of newborn-screening programs in the United States. Am J Public Health 1997; 87 : 1280–1288.
Campbell ED, Ross LF : Incorporating newborn screening into prenatal care. Am J Obstet Gynecol 2004; 190 : 1.
Pollitt RJ : Introducing new screens: why are we all doing different things? J Inherit Metab Dis 2007; 30 : 423–429.
Hewlett J, Waisbren S : A review of the psychosocial effects of false-positive results on parents and current communication practices in newborn screening. J Inherit Metab Dis 2006; 29 : 677–682.
Marteau TM, van Duijn M, Ellis I : Effects of genetic screening on perceptions of health: a pilot study. J Med Genet 1992; 29 : 24–26.
Miller FA, Paynter M, Hayeems RZ et al : Understanding sickle cell carrier status identified through newborn screening: a qualitative study. Eur J Hum Genet 2009; 18 : 303–308.
Lucassen A, Parker M : Revealing false paternity: some ethical considerations. The Lancet 2001; 357 : 1033–1035.
Bailey DB, Skinner D, Davis AM, Whitmarsh I, Powell C : Ethical, legal, and social concerns about expanded newborn screening: fragile X syndrome as a prototype for emerging issues. Pediatrics 2008; 121 : e693–e704.
Watson MS, Mann MY, Lloyd-Puryear MA, Rinaldo P, Howell RR, American College of Medical Genetics Newborn Screening Expert G: Newborn screening: toward a uniform screening panel and system – executive summary. Pediatrics 2006; 117 : S296–S307.
Ontario Ministry of Health and Long Term Care: McGuinty Government Expands Newborn Screening, 2006. Accessed 3 March 2008.
Miller FA, Robert JS, Hayeems RZ : Questioning the consensus: managing carrier status results generated by newborn screening. Am J Public Health 2009; 99 : 210.
Fraser FC : Genetic counseling. Am J Hum Genet 1974; 26 : 636–659.
CAS PubMed PubMed Central Google Scholar
Wilson JMG, Jungner G : Principles and Practice of Screening for Disease . Geneva: World Health Organization, 1968.
Miller FA, Robert JS, Hayeems RZ : Questioning the consensus: managing carrier status results generated by newborn screening. Am J Public Health 2009; 99 : 210–215.
Health Council of the Netherlands: Screening: Between Hope and Hype . The Hague: Health Council of the Netherlands, 2008.
Takala T, Hayry M : Genetic ignorance, moral obligations and social duties. J Med Philos 2000; 25 : 107–113; discussion 114–120.
Borry P, Fryns JP, Schotsmans P, Dierickx K : Carrier testing in minors: a systematic review of guidelines and position papers. Eur J Hum Genet 2006; 14 : 133–138.
European Society of Human Genetics: Genetic testing in asymptomatic minors: recommendations of the European Society of Human Genetics. Eur J Hum Genet 2009; 17 : 720–721.
Grob R : Is my sick child healthy? Is my healthy child sick?: changing parental experiences of cystic fibrosis in the age of expanded newborn screening. Soc Sci Med 2008; 67 : 1056–1064.
Miller FA, Hayeems RZ, Carroll JC et al : Consent for newborn screening: the attitudes of health care providers. Public Health Genomics 2009; e-pub ahead of print 22 September 2009.
Download references
Acknowledgements
We acknowledge the financial support provided for the following individuals: Yvonne Bombard was supported by a Postdoctoral fellowship from Apogee-Net, a network funded by the Canadian Institutes of Health Research (CIHR); Fiona A Miller is supported by a New Investigator Award from the Institute of Health Services and Policy Research of the CIHR (FRN # 80495); Robin Z Hayeems is supported by a CADRE Postdoctoral Fellowship from the Canadian Institutes of Health Research and the Canadian Health Services Research Foundation; Bartha Maria Knoppers and Denise Avard are supported by Genome Quebec and Genome Canada.
Author information
Authors and affiliations.
Department of Health Policy, Management and Evaluation, Faculty of Medicine, University of Toronto, Toronto, ON, Canada
Yvonne Bombard, Fiona A Miller & Robin Z Hayeems
Department of Human Genetics, Center of Genomics and Policy, Faculty of Medicine, McGill University, Montr, é, al, QC, Canada
Denise Avard & Bartha M Knoppers
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Yvonne Bombard .
Ethics declarations
Competing interests.
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on European Journal of Human Genetics website
Supplementary information
Supplementary table 1 (doc 33 kb), table 1 (pdf 100 kb), references to table 1 (pdf 87 kb), rights and permissions.
Reprints and permissions
About this article
Cite this article.
Bombard, Y., Miller, F., Hayeems, R. et al. Reconsidering reproductive benefit through newborn screening: a systematic review of guidelines on preconception, prenatal and newborn screening. Eur J Hum Genet 18 , 751–760 (2010). https://doi.org/10.1038/ejhg.2010.13
Download citation
Received : 06 October 2009
Revised : 14 December 2009
Accepted : 29 December 2009
Published : 03 March 2010
Issue Date : July 2010
DOI : https://doi.org/10.1038/ejhg.2010.13
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- newborn screening
- reproductive risk information
- informed decision making
This article is cited by
Expanding the australian newborn blood spot screening program using genomic sequencing: do we want it and are we ready.
- Stephanie White
- Tamara Mossfield
- Veronica Wiley
European Journal of Human Genetics (2023)
The perception of parents with a child with sickle cell disease in Ghana towards prenatal diagnosis
- Menford Owusu Ampomah
- Kate Flemming
Journal of Community Genetics (2022)
Neonatal and carrier screening for rare diseases: how innovation challenges screening criteria worldwide
- Martina C. Cornel
- Tessel Rigter
- Lidewij Henneman
Journal of Community Genetics (2021)
Primary care provider perspectives on using genomic sequencing in the care of healthy children
- Chloe Mighton
- Yvonne Bombard
European Journal of Human Genetics (2020)
International differences in the evaluation of conditions for newborn bloodspot screening: a review of scientific literature and policy documents
- Marleen E Jansen
- Selina C Metternick-Jones
- Karla J Lister
European Journal of Human Genetics (2017)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
First and second trimester ultrasound in pregnancy: A systematic review and metasynthesis of the views and experiences of pregnant women, partners, and health workers
Roles Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Research in Childbirth and Health Group, THRIVE Centre, University of Central Lancashire, Preston, United Kingdom
Roles Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing
Roles Investigation, Writing – review & editing
Affiliation School of Health and Community Studies, University of Central Lancashire, Preston, United Kingdom
Affiliation Applied Health Research Hub, University of Central Lancashire, Preston, United Kingdom
Roles Conceptualization, Project administration
Affiliation UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction, Department of Sexual and Reproductive Health and Research, World Health Organization, Geneva, Switzerland
Roles Conceptualization, Project administration, Writing – review & editing
Roles Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing
- Gill Moncrieff,
- Kenneth Finlayson,
- Sarah Cordey,
- Rebekah McCrimmon,
- Catherine Harris,
- Maria Barreix,
- Özge Tunçalp,

- Published: December 14, 2021
- https://doi.org/10.1371/journal.pone.0261096
- Peer Review
- Reader Comments
The World Health Organization (WHO) recommends one ultrasound scan before 24 weeks gestation as part of routine antenatal care (WHO 2016). We explored influences on provision and uptake through views and experiences of pregnant women, partners, and health workers.
We undertook a systematic review (PROSPERO CRD42021230926). We derived summaries of findings and overarching themes using metasynthesis methods. We searched MEDLINE, CINAHL, PsycINFO, SocIndex, LILACS, and AIM (Nov 25th 2020) for qualitative studies reporting views and experiences of routine ultrasound provision to 24 weeks gestation, with no language or date restriction. After quality assessment, data were logged and analysed in Excel. We assessed confidence in the findings using Grade-CERQual.
From 7076 hits, we included 80 papers (1994–2020, 23 countries, 16 LICs/MICs, over 1500 participants). We identified 17 review findings, (moderate or high confidence: 14/17), and four themes: sociocultural influences and expectations; the power of visual technology; joy and devastation : consequences of ultrasound findings; the significance of relationship in the ultrasound encounter . Providing or receiving ultrasound was positive for most, reportedly increasing parental-fetal engagement. However, abnormal findings were often shocking. Some reported changing future reproductive decisions after equivocal results, even when the eventual diagnosis was positive. Attitudes and behaviours of sonographers influenced service user experience. Ultrasound providers expressed concern about making mistakes, recognising their need for education, training, and adequate time with women. Ultrasound sex determination influenced female feticide in some contexts, in others, termination was not socially acceptable. Overuse was noted to reduce clinical antenatal skills as well as the use and uptake of other forms of antenatal care. These factors influenced utility and equity of ultrasound in some settings.
Though antenatal ultrasound was largely seen as positive, long-term adverse psychological and reproductive consequences were reported for some. Gender inequity may be reinforced by female feticide following ultrasound in some contexts. Provider attitudes and behaviours, time to engage fully with service users, social norms, access to follow up, and the potential for overuse all need to be considered.
Citation: Moncrieff G, Finlayson K, Cordey S, McCrimmon R, Harris C, Barreix M, et al. (2021) First and second trimester ultrasound in pregnancy: A systematic review and metasynthesis of the views and experiences of pregnant women, partners, and health workers. PLoS ONE 16(12): e0261096. https://doi.org/10.1371/journal.pone.0261096
Editor: Carla Betina Andreucci Polido, Universidade Estadual de Campinas, BRAZIL
Received: September 17, 2021; Accepted: November 22, 2021; Published: December 14, 2021
Copyright: © 2021 Moncrieff et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files.
Funding: The work was commissioned to the University of Central Lancashire by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), a cosponsored program executed by the World Health Organization (WHO). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Antenatal ultrasound is a routine and established component of antenatal care within high-income countries [ 1 ]. In low- and middle-income countries ultrasound scanning in pregnancy is more recent [ 2 ]. In many of these settings, provision is not universal [ 3 ], and it is often restricted to high level and/or private facilities, limiting access for many [ 2 , 4 ]. In 2016, the World Health Organization first recommended ultrasound as a routine aspect of antenatal care [ 5 ]. This recommendation was for one ultrasound scan before 24 weeks gestation, to estimate gestational age, improve detection of fetal anomalies and multiple pregnancies, reduce induction of labour for post-term pregnancy, and improve a woman’s pregnancy experience. Part of the rationale for the establishment of this recommendation within guidelines was to better regulate the use of antenatal ultrasound, and to increase equitable access for pregnant women in low- and middle-income settings.
For many expectant parents, antenatal ultrasound provides a positive experience [ 6 ]. Health workers value its use for gestational age estimation, multiple pregnancy identification and assessment of physiological or potentially pathological fetal growth [ 1 ]. Identification of fetal anomalies is also an intrinsic part of ultrasound examination in early pregnancy [ 1 ]. As imaging has become more sophisticated, there has been increasing potential to identify markers of uncertain significance [ 7 ]. This can bring many benefits, but it has also resulted in concerns relating to overdiagnosis as well as the psychological risks for women, birthing people, and partners when the implications of these markers are not clear [ 8 , 9 ]. Some have expressed eugenic concerns, as ultrasound-identified fetal abnormalities force parents to decide between giving birth to a child with disabilities, or termination [ 10 ], while in some social, cultural and religious contexts, termination is not an option [ 11 ]. In some social settings, ultrasound sex determination is associated with female feticide [ 12 ], and possibly sex distribution skew [ 13 ], raising moral, ethical, and gender equity issues.
Because of the rapid technical improvements in first and second trimester ultrasound, and the spread in routine use, the WHO recommended updating of their early ultrasound recommendation. This qualitative systematic review was carried out to inform the update, enabling the consideration of values and preferences, and acceptability, feasibility, and equity implications, and the opportunity to share insights into successful implementation and service provision. These considerations are integral to implementation of antenatal ultrasound where it is not yet a routine component of antenatal care, as well as the improvement of existing services.
We undertook a rapid scoping search of the existing literature but did not identify any previous systematic reviews of experiences of first and second trimester ultrasound that were suitable to inform WHO guidelines on this subject. There is one previous systematic review on experiences of antenatal ultrasound, but this was published in 2002. It did not include the perspectives of health workers, or studies from low- or middle-income countries [ 6 ].
To inform guidelines and practice in the area of first and second trimester ultrasound we aimed to examine the following questions, for maternity service users (including birth companions), health workers, policy makers and funders in all settings:
a. What views, beliefs, concerns and experiences have been reported in relation to routine ultrasound examination in pregnancy?
b. What are the influencing factors associated with appropriate or inappropriate use of routine antenatal ultrasound scanning?
Search strategy and selection criteria
We undertook a systematic review using thematic synthesis to develop our review findings and analytic themes [ 14 ]. The study protocol is registered on PROSPERO (CRD42021230926).
We undertook searches in Medline (Ovid), CINAHL, PsycINFO, and SocIndex (via EBSCO), and LILACS and AIM (via Global Index Medicus) on Nov 25th and 26th 2020, with no language or date restrictions. Additional relevant papers were identified through searching reference lists and citation searches of included studies. A log was used to record inclusion/exclusion at each stage of selection. One member of the review team (CH) undertook the searches, and de-duplication of results using both automated and manual methods in EndNote.
Inclusion criteria.
Our protocol specified searches for qualitative, survey, and mixed-methods studies. For this paper, we report on findings from qualitative studies. We included papers addressing routine use of ultrasound during antenatal care, including to detect fetal viability, gestational age, fetal growth, fetal abnormality, multiple pregnancy, and any other routine application, where this was a standard part of the routine ultrasound offer for the population in the country(ies) where the study was set.
Included participants were pregnant or postnatal women, families of such women, and related community members, antenatal health workers, managers, funders, or policy makers involved in the receipt, provision, management or funding of routine antenatal ultrasound scanning.
We included all settings (low-, high- and middle-income), and all types of health care design and provision (including public, private and mixed models of provision), and localities (hospital facilities, birth centres, or local communities).
Exclusion criteria.
We excluded papers if ultrasound was undertaken for specific indications, for example following IVF procedures, or after women’s reports of reduced fetal movements.
We excluded controlled studies, cohort studies, and epidemiological studies.
Initial screening by title and abstract was refined through blind screening 100 records in two teams to ensure agreement in the screening process. Uncertainties were discussed amongst the review team, and a further 100 hits were then screened until sufficient agreement was reached. For full text screening, batches of ten records were screened in each team until sufficient agreement was reached, after which three members of the review team (GM, SC, RM) screened the remaining records independently.
Data extraction and analysis
Studies assessed as eligible for inclusion were quality assessed [ 15 ]. Quality assessment was undertaken by GM, SC, RM and KF. SD independently assessed 10% of studies to calibrate the assessments of the teams. Very low-quality studies were logged for transparency but were not included in the analysis.
The authors name, the date, characteristics, and setting of included papers, and the key findings, were logged on the study-specific Excel file. Translation of non-English studies was carried out using Google translate.
Analytic procedure.
We initially derived review findings and overarching themes using a thematic synthesis approach [ 14 ]. We started by logging themes and findings highlighted by the authors, or, where these were not clear, reviewer generated findings from the quote material and author narratives (GM, SC, RM). As each subsequent paper was coded, themes were generated (GM, KF, SD) and entered iteratively onto a separate worksheet of the study Excel file, resulting in an initial thematic framework. The findings continued to develop as the data from each paper were added. This included looking for what was similar between papers and for what contradicted (‘disconfirmed’) the review findings. All authors involved in the primary analysis (GM, KF, SD), consciously looked for data that would contradict our prior beliefs and views.
Confidence in each finding was assessed using GRADE-CERQual [ 16 ]. Review findings were graded using a classification system ranging from ‘high’ to ‘moderate’ to ‘low’ to ‘very low’ confidence. Following CERQual assessment the review findings were grouped into higher order analytic themes and the final framework was agreed by consensus amongst the authors.
Analysis of subgroups or subsets.
Findings were logged by country income status (HIC vs LMIC), and by trimester of scan (first, second, or both). Interpretation of the findings and themes includes these subgroups where they can be clearly differentiated in the data.
Reflexive statement.
Based on our collective and individual experiences (as midwives, academics, service users, and researchers), we anticipated that the findings of our review would reveal that women and their partners generally look forward to ultrasound but may be unprepared for it to reveal abnormalities; that health workers like to use it as it gives them a sense of certainty in diagnosis; and that policy makers and funders see it as a useful source of revenue and/or of attracting women to use facilities. We maintained awareness of these prior beliefs and their potential impact on our analysis to ensure we were not over-interpreting data that supported our prior beliefs, or over-looking disconfirming data.
Of the 7076 records generated by our search,181 studies met the initial inclusion criteria to be included in our synthesis. 4656 records were excluded at the initial abstract screening stage, primarily because they were unrelated to the focus of this review. Full text screening excluded 574 studies, primarily because they did not focus on perceptions/experiences of routine ultrasound. Of the 181 studies initially identified as being eligible for inclusion, 80 were qualitative and 98 were quantitative or mixed methods studies. Due to the large number of qualitative papers identified, the decision was made to focus on the qualitative studies, and to analyse the qualitative/mixed methods studies separately. Eighty qualitative papers were therefore included before quality screening, and three more were identified from reference lists of the included papers. Following quality appraisal, 3 studies were rated D and excluded. Fig 1 outlines the screening and selection process.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0261096.g001
Of the 80 studies included in our review, eight were rated A, 52 B, and 20 were rated C. They were published between 1994 and 2020 and were from 23 different countries, with 16 studies from LICs/MICs. They represent the views of over 1500 participants. The majority of papers reported the views of women or women and their partners; 19 reported provider perspectives; seven reported the views of both. There were no eligible studies that included the views of funders or policy makers. Study characteristics and quality appraisal grades are presented in Table 1 .
https://doi.org/10.1371/journal.pone.0261096.t001
Our analysis generated 17 review findings, synthesised into four over-arching analytic themes. Three findings represent the views of women and their partners only, three represent the views of healthcare professionals only, and 11 describe findings from both groups. Most were graded moderate or high confidence. The Summary of Findings and CERQual assessment are provided in Table 2 .
https://doi.org/10.1371/journal.pone.0261096.t002
Sociocultural influences and expectations. For many women, ultrasound was seen as an integral part of pregnancy and an opportunity not to be missed [ 17 – 26 ]. It offered parents the chance to ‘meet’ their baby and receive an image of the scan that they could share with friends and family [ 21 , 25 , 27 , 28 ]. Fathers’ attendance was seen as a demonstration of their commitment to their family and to facilitate involvement with the pregnancy [ 19 , 25 , 28 , 29 – 34 ]. For health workers however, these views sometimes conflicted with their role in providing a medical assessment and potential diagnosis [ 35 – 37 ]. It also sometimes conflicted with parent’s autonomy in terms of whether attending ultrasound was seen as a choice, or a decision to be made [ 17 , 18 , 21 , 22 , 38 – 42 ]. Some felt that they had not been offered an actual choice due to the routine nature of ultrasound in antenatal care, whilst others felt they should follow the authoritative advice of health professionals to ensure wellbeing of their baby [ 43 – 47 ]. In some contexts, healthcare professionals actively directed women towards ultrasound with the belief that this would inevitably result in better outcomes, and women were seen as irresponsible if they declined the offer of a scan [ 39 , 44 , 48 – 52 ].
‘Yes I’m sure it is (optional) but I think everybody else does it … well maybe not … but anyway I wouldn’t miss it .’ (Sweden) [ 25 ] ‘ I don’t know if it is good or bad . They provide it for us so we use it .’ (Australia) [ 46 ] ‘ The ones that choose not to are far more informed than the ones that choose to–because you have to go against the system .’ (Australia) [ 50 ]
In some low-income settings, access to ultrasound was limited due to lack of staff and other resources, as well as the costs incurred for women and the distance they would have to travel to attend appointments [ 53 – 56 ]. Some midwives in these contexts expressed the desire for training in the use of ultrasound, so that they could make decisions when other staff were not available [ 55 , 57 ]. There were varying beliefs in relation to the safety of ultrasound as well as the diagnosis that could be made through its use [ 19 , 34 , 41 , 49 , 52 , 58 ]. In some contexts, social and religious beliefs influenced the utility of a diagnosis if the only solution to a finding of fetal abnormality was termination [ 44 , 59 – 61 ].
‘She [pregnant woman] didn’t go for ultrasound even though she was told to do so , she refused because of the cost . ’ (Tanzania) [ 54 ] ’We perceive that it is not out our job , but our wish as midwives is to be able to perform ultrasound so that we can play a role in the mother’s care and make decisions without necessarily waiting for the availability of the doctor . ’ (Rwanda) [ 55 ] ‘In our society it would be too late to do anything about that because the woman is not allowed , according to our religion , to have an abortion . Hence there is no point in doing tests during pregnancy . It’s only a waste of time , money and effort . ’ (Israel) [ 60 ]
For some, beliefs about what was important to know during pregnancy, the value placed on ultrasound, and the impact of a diagnosis, appeared to be influenced by the vicarious experiences of friends, family and community members [ 17 , 19 , 28 , 62 , 63 ]. Information about the provision and nature of the ultrasound assessment appeared to also be mediated through community members in some cases, rather than healthcare professionals [ 29 , 64 , 65 ]. This extended to support after the scan which was often provided by friends and family [ 66 – 68 ].
’I needed help to sort out all my feelings and questions , my husband was a great support to me , but I would have liked to talk to my midwife .’ (Iceland) [ 65 ]
Finding out the fetal sex was important for respondents in a range of contexts, in terms of imaging their future baby, and practical planning [ 28 , 45 , 48 , 68 – 70 ]. However, in some circumstances, this knowledge had negative consequences [ 30 , 71 ]. As reported by both health workers and community members, this was particularly (but not only) apparent in cultures where there is a preference for male babies. In these contexts, the disclosure of female fetal sex through ultrasound could result in feticide [ 19 , 53 , 71 , 72 ]. To avoid this potential outcome there was a policy of non-disclosure relating to fetal sex to avoid this outcome [ 19 , 53 , 54 , 71 , 72 ].
‘USG is done to know the sex of the child and then abortion is done if its female child . ’ (India) [ 71 ] There is this stigma between girls and boys , in some communities they want to know if it’s a boy or a girl so that they may be able to either prevent the pregnancy from going on . ’ (Tanzania) [ 53 ] ‘… via USG people can know about sex of the baby and can get the girl child aborted . ’ (India) [ 71 ]
The power of visual technology.
For most respondents, ultrasound was seen as central to antenatal care. Women generally trusted it as a valued technology that could provide confirmation of their pregnancy and reassurance of fetal wellbeing [ 19 , 28 , 30 , 43 , 64 , 66 , 73 ]. For providers, it was an important tool, particularly for the detection and management of complications [ 39 , 43 , 53 , 57 , 74 – 76 ]. However, some respondents reported that a reliance on ultrasound results in the potential for overuse, and consequent neglect of other forms of antenatal care [ 19 , 53 , 74 ]. Some participants felt compelled towards ultrasound to visualise their baby and for reassurance [ 19 , 31 , 43 , 47 , 61 ]. For some women and healthcare professionals, ultrasound held greater value than other forms of antenatal assessment. The overuse of ultrasound was felt to result in reduced clinical skills and the potential to miss complications that were not picked up through this form of assessment [ 38 , 43 , 48 , 55 , 74 ].
‘ The scan is very necessary; there is no point in visiting the doctor without seeing the fetus and knowing how well it is doing . You would not benefit at all !’ (Syria) [ 19 ] ‘ Initially , I can say it came as an extra tool without really knowing why I have to do this . But , through getting used to the tools and doing it regularly , I came to get used to it and think right now I can say it is something we feel like we cannot do without . ’ (Kenya) [ 57 ] ‘I think that in Vietnam nowadays , obstetric ultrasound is the most important investigation to monitor the pregnancy . Some other investigations like blood test , urine test also have importance but they cannot be compared to the obstetric ultrasound . ’ (Vietnam) [ 75 ] ‘ I think it’s a very useful tool , I think we’re getting to the situation where many people can do nothing without an ultrasound , so those clinical skills have gone to a large extent .’ (Australia) [ 74 ]
For many women and healthcare professionals, the power of the ultrasound image was significant [ 32 , 32 , 43 , 50 , 54 , 66 , 73 , 75 – 77 ]. Some women appeared to lack trust that they were pregnant until they were able to visualise the image of their baby [ 21 , 25 , 44 , 52 , 68 , 72 , 78 ]. The capacity for visualisation was particularly valued by fathers and other parents [ 21 , 28 , 61 , 78 ]. The scan image offered the chance to visualise the future together as family. For some, it represented an opportunity to construct their child’s future personality and characteristics [ 32 , 21 , 73 , 79 ]. However, this sense of connection also complicated decisions around termination of pregnancy [ 18 , 30 , 34 , 68 ].
‘ Before I found out I was pregnant I’d always said if I knew I was having a handicapped baby , I’d have a termination , but then when I went for the very first scan and saw the baby moving about and saw his heart beating , I thought afterwards I don’t know whether I could do it now , because he’s alive , it’s a person .’ (England) [ 18 ]
Some providers were concerned that the clarity of the ultrasound image meant that all complications should be visible and identified [ 39 ]. Some feared the potential for consequences for both the mother, and for their professional security, if abnormalities were missed [ 36 , 39 , 76 ]. In some LMIC contexts, concerns were also expressed about the lack of appropriate training and the potential for this to result in missed complications or misdiagnosis [ 38 , 39 , 76 , 80 ]. Some respondents described professional and moral dilemmas around prioritising either mother or fetus in their clinical assessments [ 35 , 40 , 81 , 82 ], as well ethical concerns when parents made decisions that did not fit with personal or professional beliefs [ 55 , 80 , 82 ]. Some also expressed concern that women would go to any lengths to protect the wellbeing of their baby, even when this was to their own detriment [ 38 , 75 , 81 ].
‘No special training on ultrasound , that’s the limitation , that’s why you can sometimes miss some complications if I find something I am not understanding .’ (Rwanda) [ 76 ] ‘ I have never met an expectant mother who has hesitated to expose herself to something that might be harmful to her health as long as it benefits the fetus .’ (Sweden) [ 38 ]
Both joy and devastation; consequences of ultrasound findings.
The scan appointment was a source of great excitement, joy and relief for many couples, providing a chance to bond with their baby, whilst also instilling a sense of responsibility, particularly amongst fathers and other co-parents [ 19 , 21 , 32 , 41 , 45 , 68 , 77 , 83 ]. For some, it also offered the potential for choice and the opportunity to plan when complications were detected [ 22 , 68 , 84 , 85 ]. However, for many, the identification of abnormalities was completely unexpected [ 17 , 18 , 20 , 24 , 65 , 69 , 73 , 80 , 86 – 88 ]. Some reported deep shock and distress on hearing this news [ 17 , 65 , 67 , 69 , 73 , 86 – 89 ]. Both service users and healthcare professionals reflected on how this shock could be compounded by couples’ expectations that the scan appointment is a happy event that would provide confirmation of wellbeing [ 24 , 36 , 65 , 83 ]. The difficulty in getting the balance right in preparing couples for potential consequences of the scan was also discussed by healthcare professionals. Some felt that they lacked time to do this, amongst all the other issues to be discussed in an appointment, and they struggled to get the balance between discussing risk and maintaining a sense of normality prior to the scan [ 27 , 37 , 90 ].
… it’s making sure that they know enough but not frightening them or making them feel very negative about the pregnancy … not put too much emphasis on the possibility of problems . ’ (England) [ 27 ] ‘We were so naive . We thought we were going to see the baby and get a nice photo .’ (Canada) [ 24 ] “ It was a shock like this , because what we expect is that it will be everything perfect ” (Brazil) [ 69 ] ‘You come to find out the sex of the baby and have the bomb dropped on you . ’ (USA) [ 87 ]
Uncertain findings that could, but may not, indicate abnormality, were particularly difficult for many couples, resulting in feelings of having lost their pregnancy, and a shift to a new tentative, risky state [ 18 , 20 , 29 , 91 ]. Some women reported detaching themselves from their pregnancy and/or baby while also experiencing constant worry in relation to their baby’s wellbeing [ 17 , 18 , 22 ]. This state persisted into the long term for some, even after a follow-up diagnosis that all was well [ 18 , 20 , 91 ]. In some cases, this concern persisted even into infanthood, with, at the extreme, the decision not to pursue previously planned future pregnancies [ 18 , 20 , 91 ]. Some health professionals were acutely aware of the impact of uncertain findings on parents, resulting in dilemmas around whether these should be disclosed [ 36 , 74 , 81 ]. Parents were also conflicted about the benefits versus the harms of disclosing these findings [ 17 , 29 ]. Some expressed regret in retrospect about the negative impact on their pregnancy [ 20 , 87 , 65 , 67 ].
‘ Because of this I wouldn’t have a third child … I’m not putting myself through this stress again ever , and I would have gone on to have a third one . We’re stopping at two .’ (England) [ 18 ] ‘ The more you see sometimes the more uncertain things get . And you can ruin a pregnancy quite a bit like that . So I’m not sure whether it’s always good .’ (Australia) [ 74 ]
The significance of relationship in the ultrasound encounter.
Women and partners expressed a desire for scan providers to recognise the unique nature of the scan experience for them, to make them feel welcome, and to provide information and the opportunity to ask questions [ 21 , 22 , 25 , 76 , 65 , 88 ]. Their actual experiences ranged from health workers being cold, disinterested, and lacking time to provide information, to those who were warm and engaging, and actively fostered questions and interest in the scan [ 18 , 19 , 22 , 72 , 80 , 92 ]. In some contexts, women reported that they were unable to ask questions and that their experience was completely in the hands of the healthcare professional [ 19 , 92 ]. Some women and their partners reported being completely excluded from their scan experience, unable to see the image of their baby, and left in silence to guess through body language what might be happening [ 18 , 19 , 22 , 87 ].
‘ He was staring for a long time at the screen . You see he is very good . He keeps looking [she waves as if she is reading from a book] , and he keeps explaining . He told me about the [amniotic fluid] . My previous doctor was different . She does the scan very quickly and tells you : ‘Hey stand up… you have nothing’ and that’s all . I tell you , I felt the difference between those two doctors .’ (Syria) [ 19 ]
For some health workers supporting women through difficult findings was a rewarding aspect of their role; but they expressed the desire for more training in the communication of abnormal results, as well as more professional support to confirm findings [ 36 , 37 , 93 – 95 ]. A lack of time to form relationships and properly communicate results meant that some providers felt the need to distance themselves, in order to protect their own emotions and to enable them to perform consecutive scans within a limited time period [ 36 , 90 , 95 ].
‘ It’s the responsibility of being alone in such a small place , I’m the only one looking . . . I miss a colleague , so I could say “Could you take a look with me , let’s discuss this together .’ (Norway) [ 95 ] ‘You’ve got to protect yourself , you’ve got to … not harden your heart , but you do have to protect yourself and not get too emotionally involved , because otherwise you wouldn’t survive very long in our job . ’ (England) [ 36 ]
In 2019, the WHO maternal and perinatal health steering group prioritised updating their early ultrasound scan recommendations [ 5 ]. This systematic review informs the subsequent recommendations and will inform living guideline updates of this recommendation [ 96 ]. The potential drivers for appropriate or inappropriate use of ultrasound were captured in the four study themes.
In line with other studies [ 6 ], the experience of providing or receiving ultrasound was generally seen as positive in our analysis [ 21 , 25 , 34 , 38 , 39 , 41 , 97 ], generating high demand for scans [ 19 , 39 , 43 , 49 , 50 , 55 , 64 , 74 ], but the consequences of adverse findings was sometimes devastating [ 18 , 20 , 50 , 65 , 67 , 73 , 74 , 87 ]. Importantly, in this review, we found that even when an initial concern was later ruled out, there were very significant long-term adverse consequences for some service users [ 17 , 18 , 20 , 67 , 91 ]. Respondents also reported overuse, with implications for the provision of other antenatal assessments and potential loss of clinical skills [ 19 , 38 , 48 , 53 , 55 , 74 , 82 ]. This reinforces previously published survey data from a range of settings [ 98 – 100 ].
Provider attitudes and behaviours were influential in the service user experience [ 18 , 19 , 21 , 22 , 72 , 86 , 88 ], as were local social norms [ 18 , 21 , 25 , 34 , 41 , 52 , 58 , 60 , 61 ] and access to follow up investigations and support [ 21 , 22 , 67 , 86 , 87 ]. Providers reported concerns around missing important features of the scan [ 38 , 39 , 75 , 96 ], and a lack of sufficient time and training to appropriately carry out ultrasound assessments [ 36 , 38 , 76 , 90 , 95 ].
Previous survey research has found mixed evidence about the impact of ultrasound screening on maternal anxiety [ 101 ]. Our data suggest possible drivers for the varying perceptions of ultrasound screening. The power of the visual in making the fetus ‘real’ is evident in our analysis [ 21 , 23 , 28 , 32 , 35 , 43 , 44 , 50 , 73 ], reinforcing the validity of concepts of what has been termed the ‘ tentative pregnancy’ , in which women put their sense of being pregnant on hold until they have visual evidence of the fetus, and of its wellbeing [ 102 ]. Our data show that visual markers with unknown provenance or meaning can be unsettling for health workers as well as for service users [ 17 , 18 , 20 , 38 , 50 , 74 , 81 ]. The value of diagnosing abnormality was less clear in contexts where termination was not an option [ 58 , 60 , 61 ]. The critical, ethical and equity issue of female feticide reported in some settings underpins growing concerns about sex selection, linked to a much lower female-male sex ratio than would be expected in some countries [ 13 , 68 , 69 , 103 ].
Our findings raise questions about the utility of ultrasound in pregnancy as a screening tool in settings where the implications of features on the scans are not always understood by practitioners or service users [ 100 , 104 – 107 ], and/or if there are no effective follow up, treatment, or solution to some ultrasound findings [ 108 – 110 ]. They raise concerns about the use of ultrasound as a deliberate ‘draw’ to bring women into antenatal care, if the consequence is overuse by undertrained staff, without time to undertaken the scan effectively, including provision of tailored information and psychosocial support where needed; and without effective, affordable, equitable referral pathways.
The strengths of this review include the comprehensive search that was not restricted by language or date, and the inclusion of 80 qualitative studies covering countries from most regions of the world. Fourteen of the 17 review findings were assessed as high or moderate confidence evidence using the GRADE CERQual approach [ 16 ]. We have included the experiences and perspectives of women and their partners, as well as health workers, from low-, middle- and high-income countries. Limitations include that we were unable to distinguish between first and second trimester ultrasound in our findings, as the findings were not clearly separated, or they were similar in both trimesters. We were also unable to include the views of policy makers or funders, as our search did not retrieve any eligible studies that included this perspective. Furthermore, many of the findings relate to identification and diagnosis of abnormality, rather than to assessment of gestational age, fetal growth, or multiple pregnancy. The majority of studies in our review are from high-income countries, which was anticipated, but the inclusion of more studies from low-income settings may have provided further implications for the use of ultrasound services in this context. Thirteen of the included studies were from the CROss-Country Ultrasound Study (CROCUS). However, these studies explored the views of both providers and service users, from a number of different low-, middle-, and high-income countries.
This review offers a critical insight into how countries can introduce and maintain optimal routine antenatal ultrasound services. The findings reinforce the psychological and emotional benefits of such services from the point of view of most women and their partners, and the clinical benefits as perceived by service providers. However, there are implications for implementation in settings where antenatal ultrasound is not yet a routine component of antenatal care, and improvements that can be made in other settings where use of this technology is already established. In all settings, and particularly those with restricted resources, adequate education and training in both the use of obstetric ultrasound and in positive interactions with service users is essential, as well the allowance of sufficient time to undertake the scan effectively and with attention to the needs of the parents. Mitigation against overuse is important, to ensure that the use of ultrasound is appropriately balanced with the provision of expert clinical antenatal care. The potential for, and consequences of fetal sex disclosure must also be considered, especially in contexts where there is sociocultural bias towards male sex. Improvements can be made in all settings to ensure that women and their partners make autonomous informed decisions relating to the uptake of antenatal ultrasound; that they are adequately involved during the scanning procedure; and that information relating to the results is provided in a timely and supportive manner.
Future research should consider the ways in which ultrasound might be implemented to ensure equity of access, follow up, and longer term social and psychological support where this is needed, so that the positive aspects are maintained, while limiting the potential for overuse and for adverse impacts. There is a need to determine what is necessary and optimal to disclose with regard to markers of unclear significance and to consider how couples can be optimally supported through uncertain findings, and through to future reproductive decision making. Consideration should be given to the whole maternity and health care system into which ultrasound is introduced. Research into the use of portable ultrasound may be relevant for all settings, but particularly within LMICs, where this may be a requirement for rural and remote provision of ultrasound. This would require the ability to produce scan images of sufficient quality, as well as consideration of the findings of this review.
Supporting information
S1 checklist. prisma 2020 checklist..
https://doi.org/10.1371/journal.pone.0261096.s001
S1 Table. Search strategy.
https://doi.org/10.1371/journal.pone.0261096.s002
- View Article
- PubMed/NCBI
- Google Scholar
- 5. World Health Organisation. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience; 2016.
- 102. Rothman BK. The Tentative Pregnancy: Prenatal Diagnosis and the Future of Motherhood. Penguin Books; 1987.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Prenatal screening.
Felisha L. Kitchen ; Brian W. Jack .
Affiliations
Last Update: July 4, 2023 .
- Continuing Education Activity
Historically, pregnancy was a very tenuous part of a woman's life because of the high mortality rate associated with gestation. In the early 1900s, maternal mortality was 6 to 9 out of 1000 live births, and 100 per 1000 live-born infants died within the first 12 months of life. In 2000, maternal mortality declined to less than 0.1 out of 1000 live births, and infant mortality declined to 7 out of 1000 live births. Improvements in nutrition and advances in clinical medicine are key factors in the decline in mortality. Today, prevention of mortality is not the only goal but also the prevention of morbidity. Prenatal screening helps practitioners recognize the physical needs of a gestating mother to prevent both morbidity and mortality. This activity reviews the considerations for the use of prenatal screening and the role of the interprofessional team in performing prenatal screening.
- Review the epidemiology of childbirth mortality.
- Explain the cause of a decline in maternal and infant mortality during childbirth.
- Summarize how prenatal screening can help improve morbidity and mortality during pregnancy.
- Outline the considerations for the use of prenatal screening and the role of the interprofessional team in performing prenatal screening.
- Introduction
Historically, pregnancy was a very tenuous part of a woman's life because of the high mortality rate associated with gestation. In the early 1900s, maternal mortality was 6 to 9 out of 1000 live births, and 100 per 1000 live-born infants died within the first 12 months of life. In 2000, maternal mortality declined to less than 0.1 out of 1000 live births, and infant mortality declined to 7 out of 1000 live births. Improvements in nutrition and advances in clinical medicine are key factors in the decline in mortality. Today, prevention of mortality is not the only goal but also the prevention of morbidity. Prenatal screening helps practitioners understand the physical needs of a gestating mother to prevent both morbidity and mortality. [1] [2] [3]
First Trimester
The first 14 weeks of pregnancy are exciting for parents but an important time for screening by their practitioners. The first obstetrical visit should include a general physical examination and pelvic examination. Baseline vital signs along with the physical examination aid a practitioner in assessing the overall physical wellbeing of the gestating mother. At this time, chronic medical and psychological problems can be addressed by the practitioner as well as a prior obstetrical/gynecologic history. The pelvic examination should include cervical cytology (i.e., Pap smear) if one has not been collected within the past 12 months and the female patient is greater than or equal to 21 years old. Testing for Neisseria gonorrhea and Chlamydia trachomatis should also be performed. The uterus, adnexa, and cervix should be palpated in a bimanual pelvic examination to determine if masses or other abnormalities are present.
One of the most important things to determine during the first trimester is a gestational age and estimated due date. Often, the gestational age is calculated from the last menstrual period if it is known by the patient. If the last menstrual period is not known or the uterine size does not correlate with the estimated due date by the last menstrual period, an ultrasound for dating takes place. Ultrasound in the first trimester gives the most accurate gestational age and estimated due date. The ultrasonographer will assess live fetal status by measuring cardiac activity as well as assess the number of fetuses, amniotic sacs, and placentas. The ultrasonographer will calculate an estimated gestational age by measuring the crown-rump length of the fetus. This examination will also detail if uterine or adnexal abnormalities are present.
Laboratory tests are often performed during the first prenatal visit. An assessment of Rh factor and abnormal antibodies, serological tests for syphilis and rubella and either a hemoglobin or hematocrit are required in most institutions. The American College of Obstetricians and Gynecologists (ACOG) also recommends screening for hepatitis B and HIV. Patients with chronic medical conditions should have additional testing specific for the evaluation of that condition. Aneuploidy screening can begin as early as ten weeks gestation with cell-free DNA in women with prior aneuploidy and women =35 years old. First-trimester aneuploidy screening generally occurs between weeks 11-13 and includes nuchal translucency measurement on ultrasound and maternal serum-free BhCG and PAPP-A to detect Down's syndrome and Trisomy 18.
Second Trimester
Laboratory and ultrasound assessments of fetal genetic disorders and structural abnormalities typically take place between 15-20 weeks gestation in the second trimester. Since 1984, screening for Down's syndrome has been primarily maternal serum alpha protein levels (MSAFP) levels since a report then found that there was lower MSAFP found in women carrying a Down's syndrome affected fetus. Elevated hCG, elevated inhibin A and lower levels of unconjugated estriol are also linked with an increased risk of a Down's syndrome affected fetus creating the quad screen. Elevated MSAFP is associated with fetuses affected by open spina bifida. More recently, screening for genetic disorders trisomies 13, 18 and 21 have been performed using maternal serum cell-free fetal DNA which is considered a non-invasive prenatal test (NIPT). In 2012, ACOG published guidelines on NIPT use which includes women with prior aneuploidy and women =35 years old.
According the American Institute of Ultrasound in Medicine (AIUM), second trimester ultrasound assessments should include: (1) fetal cardiac activity, number, presentation; (2) estimate of amniotic fluid volume; (3) placental location, appearance, and relationship to the internal cervical os; (4) imaging of the umbilical cord, and the number of vessels in the cord; (5) gestational (menstrual) age (via biparietal diameter, head circumference, femoral diaphysis length, abdominal circumference, or average abdominal diameter); (6) fetal weight estimation; (7) maternal anatomy (uterus, adnexal structures, cervix); (8) and fetal anatomic survey. The fetal anatomic survey includes assessment of: head, face, neck, chest (including a four-chamber view of the fetal heart), abdomen, stomach, kidneys, bladder, abdominal cord insertion site, spine, extremities, and sex.
In the late second trimester (26-28 weeks gestation), most institutions will perform and oral glucose tolerance test (OGTT) to screen for gestational diabetes. The testing can be done with either a 1 hour 50-gram glucose load or a diagnostic 2 hour 75-gram glucose load.
Third Trimester
Third-trimester prenatal testing generally focuses on maternal wellbeing and reducing fetal morbidity/mortality. Streptococcus agalactiae (group B streptococci beta-hemolytic, GBS) is a major cause of neonatal morbidity and mortality. Vaginal screening for GBS typically takes place in the late third trimester (34-37 weeks gestation) so that mothers positive for the bacterium can receive treatment during labor before delivery. Many institutions also require repeat evaluations of hemoglobin/hematocrit, syphilis serology, and HIV screening. [4] [5] [6]
- Issues of Concern
Patients with a higher risk of genetic disorders based on ethnicity also should be screened in the first trimester. Cystic fibrosis (CF) is one of the most common genetic diseases in Caucasians and relatively common in Ashkenazi Jews and Hispanics. The National Institutes of Health (NIH) issued a consensus statement in 1997 indicating that CF testing should be offered to adults with a family history of CF, to partners of people with CF, to all couples planning a pregnancy, and to couples seeking prenatal testing. Patients of Ashkenazi Jew descent are predisposed to several genetic disorders. Both the American College of Obstetricians and Gynecologists (ACOG) and the American College of Medical Genetics and Genomics (ACMG) recommend carrier testing for three carrier tests: Tay-Sachs disease, Canavan disease, and familial dysautonomia along with CF testing in this population. The ACMG also recommends screening for Fanconi anemia group C, Niemann-Pick type A, Bloom syndrome, mucolipidosis type IV, and Gaucher disease to all Ashkenazi Jewish couples. Screening for sickle cell disease should be offered to individuals of African, African-American, Mediterranean basin, Middle Eastern, and Indian descent.
Third Trimester
Before delivery, an assessment of fetal position and estimated fetal weight should be documented. [7] [8] [9]
- Clinical Significance
At every prenatal visit in the first trimester, maternal vital signs, maternal weight, and urinalysis for glucose, protein, and leukocytes should be evaluated.
At every prenatal visit in the second trimester, maternal vital signs, maternal weight, fetal heart rate, uterine fundal height and urinalysis for glucose, protein, and leukocytes should be evaluated.
At every prenatal visit in the third trimester, maternal vital signs, maternal weight, fetal heart rate, uterine fundal height and urinalysis for glucose, protein, and leukocytes should be evaluated.
- Enhancing Healthcare Team Outcomes
The key to prenatal screening is patient education provided by an interprofessional team. Besides the general healthcare provider, the obstetrician, dietitian and prenatal nurse should educate the patient on the benefits of prenatal screening. In order for patients to make informed decisions about the wide number of test, they need to understand the limitations and benefits of the testing. The patient should also be informed about the risk of some prenatal tests like amniocentesis or chorionic villus sampling. It is also important to educate the patient on the possibility of false positives and false negative results especially when testing for genetic or chromosomal disorders. Finally, genetic counselors should always be available when a diagnosis of a chromosomal disorder is diagnosed prenatally. The decision to continue with the pregnancy, adopt or choosing to end the pregnancy is not always easy and is best done with the help of genetic and mental health counselors. [10] [11] (Level V)
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Felisha Kitchen declares no relevant financial relationships with ineligible companies.
Disclosure: Brian Jack declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Kitchen FL, Jack BW. Prenatal Screening. [Updated 2023 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Effect of cleansing the birth canal with antiseptic solution on maternal and newborn morbidity and mortality in Malawi: clinical trial. [BMJ. 1997] Effect of cleansing the birth canal with antiseptic solution on maternal and newborn morbidity and mortality in Malawi: clinical trial. Taha TE, Biggar RJ, Broadhead RL, Mtimavalye LA, Justesen AB, Liomba GN, Chiphangwi JD, Miotti PG. BMJ. 1997 Jul 26; 315(7102):216-9; discussion 220.
- CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors--United States, 2005-2013. [MMWR Suppl. 2014] CDC National Health Report: leading causes of morbidity and mortality and associated behavioral risk and protective factors--United States, 2005-2013. Johnson NB, Hayes LD, Brown K, Hoo EC, Ethier KA, Centers for Disease Control and Prevention (CDC). MMWR Suppl. 2014 Oct 31; 63(4):3-27.
- The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. [Int J Gynaecol Obstet. 2019] The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Poon LC, Shennan A, Hyett JA, Kapur A, Hadar E, Divakar H, McAuliffe F, da Silva Costa F, von Dadelszen P, McIntyre HD, et al. Int J Gynaecol Obstet. 2019 May; 145 Suppl 1(Suppl 1):1-33.
- Review Annual summary of vital statistics--1995. [Pediatrics. 1996] Review Annual summary of vital statistics--1995. Guyer B, Strobino DM, Ventura SJ, MacDorman M, Martin JA. Pediatrics. 1996 Dec; 98(6 Pt 1):1007-19.
- Review Infant mortality statistics from the 2004 period linked birth/infant death data set. [Natl Vital Stat Rep. 2007] Review Infant mortality statistics from the 2004 period linked birth/infant death data set. Mathews TJ, MacDorman MF. Natl Vital Stat Rep. 2007 May 2; 55(14):1-32.
Recent Activity
- Prenatal Screening - StatPearls Prenatal Screening - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Advertisement
A systematic review of screening for perinatal depression and anxiety in community-based settings
- Review Article
- Published: 11 July 2021
- Volume 25 , pages 33–49, ( 2022 )
Cite this article

- Amritha Bhat ORCID: orcid.org/0000-0002-9284-0650 1 ,
- Arjun Nanda 2 ,
- Lauren Murphy 3 ,
- Andrea L. Ball 4 ,
- John Fortney 1 &
- Jodie Katon 5 , 6
2429 Accesses
11 Citations
7 Altmetric
Explore all metrics
Screening for perinatal depression and anxiety in community-based maternal and child health settings may help close the detection and treatment gap among women at higher risk for these conditions. We aim to review perinatal depression and anxiety screening tools, timing, and follow-up processes for positive screens in community-based settings. We conducted a systematic review of the literature to identify papers describing screening and interventions for perinatal depression and anxiety in community-based settings. We identified 49 papers describing 47 studies of perinatal depression or anxiety screening in community-based settings. The Edinburgh Postnatal Depression Scale (EPDS) was the most frequently used screening tool. Referral and referral tracking for those who screened positive for symptoms were inadequately described. Types of training and technical assistance provided for screening varied widely. It is feasible and acceptable to screen for perinatal depression in community settings, but there is a need for systematic research examining which screening tools to use, the ideal frequency of screening, and referral completion rates. There is a lack of information regarding perinatal anxiety screening and a lack of uniformity in training regarding screening in community-based settings. Future studies should compare the efficacy of screening in community-based settings to screening in healthcare settings.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
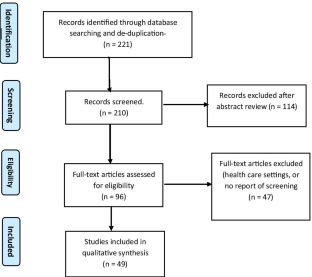
Similar content being viewed by others
The impact of COVID-19 lockdown on child and adolescent mental health: systematic review

The management of ADHD in children and adolescents: bringing evidence to the clinic: perspective from the European ADHD Guidelines Group (EAGG)

Anxiety Disorders in the DSM-5: Changes, Controversies, and Future Directions
Agrati D, Browne D, Jonas W, Meaney M, Atkinson L, Steiner M, Fleming AS, M. r. team (2015) Maternal anxiety from pregnancy to 2 years postpartum: transactional patterns of maternal early adversity and child temperament. Archives of Women’s Mental Health 18(5):693–705
Article PubMed Google Scholar
Ajzen I (1991) The theory of planned behavior. Organ Behav Hum Decis Process 50(2):179–211
Article Google Scholar
Alder J, Fink N, Bitzer J, Hösli I, Holzgreve W (2007) Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. J Matern Fetal Neonatal Med 20(3):189–209
Ali NS, Ali BS, Azam IS, Khuwaja AK (2010) Effectiveness of counseling for anxiety and depression in mothers of children ages 0–30 months by community workers in Karachi, Pakistan: a quasi experimental study. BMC Psychiatry 10:57
Article PubMed PubMed Central Google Scholar
Almond P, Lathlean J (2011) Inequity in provision of and access to health visiting postnatal depression services. J Adv Nurs 67(11):2350–2362
American College of Obstetricians and Gynecologists (2015). "Screening for perinatal depression." Obstet Gynecol(Committee Opinion No. 630.)
American College of Obstetrics and Gynecology (2017) "Guidelines for perinatal care." Guidelines - Obstetric
Anderson CM, Robins CS, Greeno CG, Cahalane H, Copeland VC, Andrews RM (2006) Why lower income mothers do not engage with the formal mental health care system: perceived barriers to care. Qual Health Res 16(7):926–943
Appleby L, Hirst E, Marshall S, Keeling F, Brind J, Butterworth T, Lole J (2003) The treatment of postnatal depression by health visitors: impact of brief training on skills and clinical practice. J Affect Disord 77(3):261–266
Arosemena FA, Fox L, Lichtveld MY (2013) Reproductive health assessment after disasters: embedding a toolkit within the disaster management workforce to address health inequalities among Gulf-Coast women. J Health Care Poor Underserved 24(4 Suppl):17–28
PubMed Google Scholar
Ashford MT, Ayers S, Olander EK (2017) Supporting women with postpartum anxiety: exploring views and experiences of specialist community public health nurses in the UK. Health Soc Care Community 25(3):1257–1264
Bennett IM, Marcus SC, Palmer SC, Coyne JC (2010) Pregnancy-related discontinuation of antidepressants and depression care visits among Medicaid recipients. Psychiatr Serv 61(4):386–391
Brunton RJ, Dryer R, Saliba A, Kohlhoff J (2015) Pregnancy anxiety: a systematic review of current scales. J Affect Disord 176:24–34
Byatt N, Levin LL, Ziedonis D, Moore Simas TA, Allison J (2015) Enhancing participation in depression care in outpatient perinatal care settings: a systematic review. Obstet Gynecol 126(5):1048–1058
Chowdhary N, Sikander S, Atif N, Singh N, Ahmad I, Fuhr DC, Rahman A, Patel V (2014) The content and delivery of psychological interventions for perinatal depression by non-specialist health workers in low and middle income countries: a systematic review. Best Pract Res Clin Obstet Gynaecol 28(1):113–133
Cooper PJ, Tomlinson M, Swartz L, Woolgar M, Murray L, Molteno C (1999) Post-partum depression and the mother-infant relationship in a South African peri-urban settlement. Br J Psychiatry 175:554–558
Article PubMed CAS Google Scholar
Cooper PJ, Landman M, Tomlinson M, Molteno C, Swartz L, Murray L (2002) Impact of a mother-infant intervention in an indigent peri-urban South African context: pilot study. Br J Psychiatry 180:76–81
Cox JL, Holden JM, Sagovsky R (1987) Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 150(6):782–786
Article CAS Google Scholar
Craig E, Judd F, Hodgins G (2005) Therapeutic group programme for women with postnatal depression in rural Victoria: a pilot study. Australas Psychiatry 13(3):291–295
Cullinan R (1991) Health visitor intervention in postnatal depression. Health Visit 64(12):412–414
PubMed CAS Google Scholar
Davies BR, Howells S, Jenkins M (2003) Early detection and treatment of postnatal depression in primary care. J Adv Nurs 44(3):248–255
Dodge KA, Goodman WB, Murphy RA, O’Donnell K, Sato J, Guptill S (2014) Implementation and randomized controlled trial evaluation of universal postnatal nurse home visiting. Am J Public Health 104(Suppl 1):S136-143
Earls MF, C. o. P. A. o. Child and F. Health (2010) Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics 126(5):1032–1039
Emond A, Pollock J, Deave T, Bonnell S, Peters TJ, Harvey I (2002) An evaluation of the First Parent Health Visitor Scheme. Arch Dis Child 86(3):150–157
Article PubMed PubMed Central CAS Google Scholar
Fuggle P, Haydon K (2000) Estimating resources for setting up an HV service for postnatal depression. Br J Community Nurs 5(7):348–351
Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T (2005) Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol 106(5.1):1071–1083
George C, Kumar A, Girish N (2020) Effectiveness of a group intervention led by lay health workers in reducing the incidence of postpartum depression in South India. Asian journal of psychiatry 47:101864
Glavin K (2012) Preventing and treating postpartum depression in women–a municipality model. J Res Nurs 17(2):142–156
Glavin K, Leahy-Warren P (2015) Examining practices of identification and assessment of PND in primary health care settings. Archives of Women’s Mental Health 18(2):298–299
Google Scholar
Glavin K, Ellefsen B, Erdal B (2010a) Norwegian public health nurses’ experience using a screening protocol for postpartum depression. Public Health Nurs 27(3):255–262
Glavin K, Smith L, Sorum R, Ellefsen B (2010b) Redesigned community postpartum care to prevent and treat postpartum depression in women–a one-year follow-up study. J Clin Nurs 19(21–22):3051–3062
Glavin K, Smith L, Sørum R, Ellefsen B (2010c) Supportive counselling by public health nurses for women with postpartum depression. J Adv Nurs 66(6):1317–1327
Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ (2010) A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry 67(10):1012–1024
Hall ES, Goyal NK, Ammerman RT, Miller MM, Jones DE, Short JA, Van Ginkel JB (2014) Development of a linked perinatal data resource from state administrative and community-based program data. Matern Child Health J 18(1):316–325
Hung KJ, Tomlinson M, le Roux IM, Dewing S, Chopra M, Tsai AC (2014) Community-based prenatal screening for postpartum depression in a South African township. Int J Gynaecol Obstet 126(1):74–77
Jones CC, Jomeen J, Hayter M (2015) A Home-Start peer support scheme for women with low mood following childbirth. Community Pract 88(9):41–44
Keller C, Ainsworth B, Records K, Todd M, Belyea M, Vega-Lopez S, Permana P, Coonrod D, Nagle-Williams A (2014) A comparison of a social support physical activity intervention in weight management among post-partum Latinas. BMC Public Health 14:971
Kendig S, Keats JP, Hoffman MC, Kay LB, Miller ES, Simas TAM, Frieder A, Hackley B, Indman P, Raines C (2017) Consensus bundle on maternal mental health: perinatal depression and anxiety. J Obstet Gynecol Neonatal Nurs 46(2):272–281
Kieffer EC, Caldwell CH, Welmerink DB, Welch KB, Sinco BR, Guzmán JR (2013) Effect of the Healthy MOMs Lifestyle Intervention on reducing depressive symptoms among pregnant Latinas. Am J Community Psychol 51(1–2):76–89
Kohrt BA, Asher L, Bhardwaj A, Fazel M, Jordans MJ, Mutamba BB, Nadkarni A, Pedersen GA, Singla DR, Patel V (2018) The role of communities in mental health care in low-and middle-income countries: a meta-review of components and competencies. Int J Environ Res Public Health 15(6):1279
Article PubMed Central Google Scholar
Kozhimannil KB, Trinacty CM, Busch AB, Huskamp HA, Adams AS (2011) Racial and ethnic disparities in postpartum depression care among low-income women. Psychiatr Serv 62(6):619–625
le Roux KW, Almirol E, Rezvan PH, Le Roux IM, Mbewu N, Dippenaar E, Rotheram-Borus MJ (2020) Community health workers impact on maternal and child health outcomes in rural South Africa–a non-randomized two-group comparison study. BMC public health 20(1):1–14
Long MM, Cramer RJ, Jenkins J, Bennington L, Paulson JF (2019) A systematic review of interventions for healthcare professionals to improve screening and referral for perinatal mood and anxiety disorders. Archives of Women’s Mental Health 22(1):25–36
Luca DL, Garlow N, Staatz C, Margiotta C, Zivin K (2019) Societal costs of untreated perinatal mood and anxiety disorders in the United States, Mathematica Policy Research
Lutenbacher M, Elkins T, Dietrich MS, Riggs A (2018) The efficacy of using peer mentors to improve maternal and infant health outcomes in Hispanic families: findings from a randomized clinical trial. Maternal and child health journal 22(1):92–104
Målqvist M, Clarke K, Matsebula T, Bergman M, Tomlinson M (2016) Screening for antepartum depression through community health outreach in Swaziland. J Community Health 41(5):946–952
Mangla K, Hoffman MC, Trumpff C, O’Grady S, Monk C (2019) Maternal self-harm deaths: an unrecognized and preventable outcome. Am J Obstet Gynecol 221(4):295–303
Matthey S, Henshaw C, Elliott S, Barnett B (2006) Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale–implications for clinical and research practice. Archives of Women’s Mental Health 9(6):309–315
McCabe-Beane JE, Segre LS, Perkhounkova Y, Stuart S, O’Hara MW (2016) The identification of severity ranges for the Edinburgh Postnatal Depression Scale. J Reprod Infant Psychol 34(3):293–303
Molenaar NM, Kamperman AM, Boyce P, Bergink V (2018) Guidelines on treatment of perinatal depression with antidepressants: an international review. Aust N Z J Psychiatry 52(4):320–327
Mosack V, Shore ER (2006) Screening for depression among pregnant and postpartum women. J Community Health Nurs 23(1):37–47
Mundorf C, Shankar A, Peng T, Hassan A, Lichtveld M (2017) Therapeutic relationship and study adherence in a community health worker-led intervention. J Community Health 42(1):21–29
Mundorf C, Shankar A, Moran T, Heller S, Hassan A, Harville E, Lichtveld M (2018) Reducing the risk of postpartum depression in a low-income community through a community health worker intervention. Matern Child Health J 22(4):520–528
Munodawafa M, Lund C, Schneider M (2017) A process evaluation exploring the lay counsellor experience of delivering a task shared psycho-social intervention for perinatal depression in Khayelitsha, South Africa. BMC Psychiatry 17(1):236
Naysmith C, Wells M, Newson S, Webb J (2015) Development and outcomes of a therapeutic group for women with postnatal depression. Community Pract 88(3):35–38
Nyström K, Öhrling K (2004) Parenthood experiences during the child’s first year: literature review. J Adv Nurs 46(3):319–330
O’Connor E, Rossom RC, Henninger M, Groom HC, Burda BU (2016) Primary care screening for and treatment of depression in pregnant and postpartum women: evidence report and systematic review for the US Preventive Services Task Force. JAMA 315(4):388–406
Painter A (1995) Health visitor identification of postnatal depression. Health Visit 68(4):138–140
Putnam KT, Wilcox M, Robertson-Blackmore E, Sharkey K, Bergink V, Munk-Olsen T, Deligiannidis KM, Payne J, Altemus M, Newport J (2017) Clinical phenotypes of perinatal depression and time of symptom onset: analysis of data from an international consortium. The Lancet Psychiatry 4(6):477–485
Rahman A (2007) Challenges and opportunities in developing a psychological intervention for perinatal depression in rural Pakistan–a multi-method study. Arch Womens Ment Health 10(5):211–219
Rahman A, Malik A, Sikander S, Roberts C, Creed F (2008) Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. Lancet 372(9642):902–909
Rahman A, Fisher J, Bower P, Luchters S, Tran T, Yasamy MT, Saxena S, Waheed W (2013) Interventions for common perinatal mental disorders in women in low- and middle-income countries: a systematic review and meta-analysis. Bull World Health Organ 91(8):593-601I
Reeves RV, Krause E (2019) The effects of maternal depression on early childhood development and implications for economic mobility. The Brookings Institution, Washington, DC
Roman LA, Lindsay JK, Moore JS, Duthie PA, Peck C, Barton LR, Gebben MR, Baer LJ (2007) Addressing mental health and stress in Medicaid-insured pregnant women using a nurse-community health worker home visiting team. Public Health Nurs 24(3):239–248
Roman LA, Gardiner JC, Lindsay JK, Moore JS, Luo Z, Baer LJ, Goddeeris JH, Shoemaker AL, Barton LR, Fitzgerald HE, Paneth N (2009) Alleviating perinatal depressive symptoms and stress: a nurse-community health worker randomized trial. Arch Womens Ment Health 12(6):379–391
Rossiter C, Fowler C, McMahon C, Kowalenko N (2012) Supporting depressed mothers at home: their views on an innovative relationship-based intervention. Contemp Nurse 41(1):90–100
Rotheram-Borus MJ, le Roux IM, Tomlinson M, Mbewu N, Comulada WS, le Roux K, Stewart J, O’Connor MJ, Hartley M, Desmond K, Greco E, Worthman CM, Idemundia F, Swendeman D (2011) Philani Plus (+): a Mentor Mother community health worker home visiting program to improve maternal and infants’ outcomes. Prev Sci 12(4):372–388
Samankasikornm W, Pierce B, St. Ivany A, Gwon SH, Schminkey D, Bullock L (2016) Effect of home visiting with pregnant teens on maternal health. MCN Am J Matern Child Nurs 41(3):162–167
Segre LS, Brock RL, O’Hara MW, Gorman LL, Engeldinger J (2011) Disseminating perinatal depression screening as a public health initiative: a train-the-trainer approach. Matern Child Health J 15(6):814–821
Segre LS, O’Hara MW, Fisher SD (2013) Perinatal depression screening in healthy start: an evaluation of the acceptability of technical assistance consultation. Community Ment Health J 49(4):407–411
Sidebottom A, Vacquier M, LaRusso E, Erickson D, Hardeman R (2020) "Perinatal depression screening practices in a large health system: identifying current state and assessing opportunities to provide more equitable care." Archives of women’s mental health
Silverstein M, Diaz-Linhart Y, Cabral H, Beardslee W, Hegel M, Haile W, Sander J, Patts G, Feinberg E (2017) Efficacy of a maternal depression prevention strategy in Head Start: a randomized clinical trial. JAMA Psychiat 74(8):781–789
Siu, A. L., U. S. Preventive Services Task Force (2016) Screening for depression in adults: US preventive services task force recommendation statement. JAMA 315(4):380–387
Smithbattle L, Lorenz R, Leander S (2013) Listening with care: using narrative methods to cultivate nurses’ responsive relationships in a home visiting intervention with teen mothers. Nurs Inq 20(3):188–198
Tabb KM, Choi S, Pineros-Leano M, Meline B, McDonald HG, Kester R, Huang H (2015) Perinatal depression screening in a women, infants, and children (WIC) program: perception of feasibility and acceptability among a multidisciplinary staff. Gen Hosp Psychiatry 37(4):305–309
Tachibana Y, Koizumi N, Akanuma C, Tarui H, Ishii E, Hoshina T, Ito H (2019) Integrated mental health care in a multidisciplinary maternal and child health service in the community: the findings from the Suzaka trial. BMC pregnancy and childbirth 19(1):1–11
Tandon D, Mackrain M, Beeber L, Topping-Tailby N, Raska M, Arbour M (2020) Addressing maternal depression in home visiting: findings from the home visiting collaborative improvement and innovation network. PloS one 15(4):e0230211
Tomlinson M, Rotheram-Borus MJ, Harwood J, le Roux IM, O’Connor M, Worthman C (2015) Community health workers can improve child growth of antenatally-depressed, South African mothers: a cluster randomized controlled trial. BMC Psychiatry 15:225
Tomlinson M, Rotheram-Borus MJ, le Roux IM, Youssef M, Nelson SH, Scheffler A, Weiss RE, O’Connor M, Worthman CM (2016a) Thirty-six-month outcomes of a generalist paraprofessional perinatal home visiting intervention in South Africa on maternal health and child health and development. Prev Sci 17(8):937–948
Torres A, Gelabert E, Roca A, Navarro P, Plaza A, Subirà S, Martin-Santos R, Ascaso C, Garcia-Esteve L (2019) Course of a major postpartum depressive episode: a prospective 2 years naturalistic follow-up study. J Affect Disord 245:965–970
Tsai AC, Tomlinson M, Dewing S, le Roux IM, Harwood JM, Chopra M, Rotheram-Borus MJ (2014) Antenatal depression case finding by community health workers in South Africa: feasibility of a mobile phone application. Arch Womens Ment Health 17(5):423–431
Turner KM, Chew-Graham C, Folkes L, Sharp D (2010) Women’s experiences of health visitor delivered listening visits as a treatment for postnatal depression: a qualitative study. Patient Educ Couns 78(2):234–239
Ueda M, Yamashita H, Yoshida K (2006) Impact of infant health problems on postnatal depression: pilot study to evaluate a health visiting system. Psychiatry Clin Neurosci 60(2):182–189
U.S. Department of Heath and Human Services (2016) Demonstrating improvement in the maternal, infant, and early childhood home visiting program a report to congress
Van Lieshout RJ, Layton H, Feller A, Ferro MA, Biscaro A, Bieling PJ (2020) Public health nurse delivered group cognitive behavioral therapy (CBT) for postpartum depression: A pilot study. Public Health Nursing 37(1):50–55
Vik K, Aass IM, Willumsen AB, Hafting M (2009) “It’s about focusing on the mother’s mental health”: screening for postnatal depression seen from the health visitors’ perspective–a qualitative study. Scand J Public Health 37(3):239–245
Ward B (1999) Improving the detection of postnatal depression. Prof Nurse 15(1):15–18
Weir S, Posner HE, Zhang J, Willis G, Baxter JD, Clark RE (2011) Predictors of prenatal and postpartum care adequacy in a Medicaid managed care population. Women’s Health Issues 21(4):277–285
Wisner KL, Logsdon MC, Shanahan BR (2008) Web-based education for postpartum depression: conceptual development and impact. Archives of Women’s Mental Health 11(5–6):377
Yonkers KA, Wisner KL, Stewart DE, Oberlander TF, Dell DL, Stotland N, Ramin S, Chaudron L, Lockwood C (2009) The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry 31(5):403–413
Download references
Author information
Authors and affiliations.
Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA, USA
Amritha Bhat & John Fortney
Department of Child and Adolescent Psychiatry, Keck School of Medicine at University of Southern California, Los Angeles, CA, USA
Arjun Nanda
College of Medicine - Tucson, The University of Arizona, Tucson, AZ, USA
Lauren Murphy
MultiCare Institute for Research and Innovation, Tacoma, WA, USA
Andrea L. Ball
VA Puget Sound Health Care System, WA, Seattle, USA
Jodie Katon
Department of Health Services, University of Washington, Seattle, WA, USA
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Amritha Bhat .
Ethics declarations
Conflict of interest.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 13 KB)
Rights and permissions.
Reprints and permissions
About this article
Bhat, A., Nanda, A., Murphy, L. et al. A systematic review of screening for perinatal depression and anxiety in community-based settings. Arch Womens Ment Health 25 , 33–49 (2022). https://doi.org/10.1007/s00737-021-01151-2
Download citation
Received : 08 December 2020
Accepted : 24 May 2021
Published : 11 July 2021
Issue Date : February 2022
DOI : https://doi.org/10.1007/s00737-021-01151-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Perinatal depression
- Perinatal anxiety
- Community-based
- Find a journal
- Publish with us
- Track your research
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Factors Affecting Improved Prenatal Screening: A Narrative Review

2015, Global Journal of Health Science
Related Papers
The Journal of Obstetrics and Gynecology of India
Hend Shalaby
Women's reproductive health
Michele Hoffnung
Nature Reviews Genetics
Antina De Jong
European Journal of Human Genetics
Fiona Miller , Robin Hayeems
Millenium - Journal of Education, Technologies, and Health
Dirce Guilhem
Wahied Khawar Balwan
Prenatal Diagnosis means diagnosis before birth. The fundamental philosophy of prenatal diagnosis is to provide reassurance to couples at risk that they may selectively have unaffected children even if their perspective risk for having defective offspring might be unacceptably high. The incidence of birth defects or genetic disorders in pregnancy is approximately 3%. Some will be found to have congenital or genetic defect during childhood or early adulthood. Recent advances in technology have enabled the development of a wide range of methods for prenatal diagnosis. The desire of every couple is to have a ‘perfect’ healthy normal baby. Prenatal testing and its perceived benefits have been in focus for a long time. There has been widespread debate and passionate arguments on both sides. In India, where the law allows termination of pregnancy before 20 weeks, there are several prenatal tests that are available only after 20 weeks. This dichotomy produces considerable anxiety and stres...
Prenatal Diagnosis
Jorge Arturo Burgos
Journal of Clinical Nursing
kafiye Eroğlu
Talya Miron-shatz
STUDY QUESTION: Do clinicians manage pregnancies conceived by assisted reproductive technologies (ART) differently from spontaneous pregnancies? SUMMARY ANSWER: Clinicians' decisions about prenatal testing during pregnancy depend, at least partially, on the method of conception. WHAT IS KNOWN ALREADY: Research thus far has shown that patients' decisions regarding prenatal screening are different in ART pregnancies compared with spontaneous ones, such that ART pregnancies may be considered more valuable or 'precious' than pregnancies conceived without treatment. STUDY DESIGN, SIZE AND DURATION: In this cross-sectional study, preformed during the year 2011, 163 obstetricians and gynecologists in Israel completed an anonymous online questionnaire. PARTICIPANTS, SETTING, METHODS: Clinicians were randomly assigned to read one of two versions of a vignette describing the case of a pregnant woman. The two versions differed only with regard to the method of conception (ART; n = 78 versus spontaneous; n = 85). Clinicians were asked to provide their recommendations regarding amniocentesis. MAIN RESULTS AND THE ROLE OF CHANCE: The response rate among all clinicians invited to complete the questionnaire was 16.7%. Of the 85 clinicians presented with the spontaneous pregnancy scenario, 37 (43.5%) recommended amniocentesis. In contrast, of the 78 clinicians presented with the ART pregnancy scenario, only 15 (19.2%) recommended the test. Clinicians were 3.2 (95% confidence interval [CI]: 1.6-6.6) times more likely to recommend amniocentesis for a spontaneous pregnancy than for an ART pregnancy. LIMITATIONS AND REASONS FOR CAUTION: The study is limited by a low response rate, the relatively small sample and the hypothetical nature of the decision, as clinician recommendations may have differed in an actual clinical setting. WIDER IMPLICATIONS OF THE FINDINGS: Our findings show that fertility history and use of ART may affect clinicians' recommendations regarding amniocentesis following receipt of screening test results. This raises the question of how subjective factors influence clinicians' decisions regarding other aspects of pregnancy management. STUDY FUNDING AND COMPETING INTEREST: There was no funding source to this study. The authors declare no conflicts of interest.
RELATED PAPERS
Can Tho University Journal of Science
Bui Thi Buu Hue 000019
Environmental Toxicology and Chemistry
Daniel Villeneuve
Seibutsu Butsuri
Ans van Kemenade
Journal of Computational Physics
Jens Strackeljan
Maria Arroz
Alenka Gracheva
Plant molecular biology
Antje Rohde
Proceedings - Irrigation, Society and Landscape. Tribute to Thomas F. Glick
Jorge Nunez
American Journal of Obstetrics and Gynecology
Rodney Wright
Yılmaz Aydın Azteklər
Fikrat " S O N N E N K Ö N İ G " Hajizada
Pesquisa Operacional
Luiz Flavio Autran Monteiro Gomes
ATHENS JOURNAL OF SCIENCES
Eberhard Kranich
Psicologia: Reflexão e Crítica
Matilde Vieira
Sean Bambrough
The Wordsworth Circle
marilyn gaull
American Journal of Respiratory and Critical Care Medicine
Quality Production Improvement - QPI
Renata Stasiak-Betlejewska
Fumio Imazeki
arXiv (Cornell University)
Srinath Srinivasa
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- Share full article
Advertisement
Supported by
When They Warn of Rare Disorders, These Prenatal Tests Are Usually Wrong
Some of the tests look for missing snippets of chromosomes. For every 15 times they correctly find a problem ...
… they are wrong 85 times
By Sarah Kliff and Aatish Bhatia
Leer en español
After a year of fertility treatments, Yael Geller was thrilled when she found out she was pregnant in November 2020. Following a normal ultrasound , she was confident enough to tell her 3-year-old son his “brother or sister” was in her belly.
But a few weeks later, as she was driving her son home from school, her doctor’s office called. A prenatal blood test indicated her fetus might be missing part of a chromosome, which could lead to serious ailments and mental illness.
Sitting on the couch that evening with her husband, she cried as she explained they might be facing a decision on terminating the pregnancy. He sat quietly with the news. “How is this happening to me?” Ms. Geller, 32, recalled thinking.
The next day, doctors used a long, painful needle to retrieve a small piece of her placenta. It was tested and showed the initial result was wrong. She now has a 6-month-old, Emmanuel, who shows no signs of the condition he screened positive for.

Ms. Geller had been misled by a wondrous promise that Silicon Valley technology has made to expectant mothers: that a few vials of their blood, drawn in the first trimester, can allow companies to detect serious developmental problems in the DNA of the fetus with remarkable accuracy.
In just over a decade, the tests have gone from laboratory experiments to an industry that serves more than a third of the pregnant women in America, luring major companies like Labcorp and Quest Diagnostics into the business, alongside many start-ups.
The tests initially looked for Down syndrome and worked very well. But as manufacturers tried to outsell each other, they began offering additional screenings for increasingly rare conditions.
The grave predictions made by those newer tests are usually wrong, an examination by The New York Times has found.
That includes the screening that came back positive for Ms. Geller, which looks for Prader-Willi syndrome, a condition that offers little chance of living independently as an adult. Studies have found its positive results are incorrect more than 90 percent of the time.
Nonetheless, on product brochures and test result sheets, companies describe the tests to pregnant women and their doctors as near certain. They advertise their findings as “reliable” and “highly accurate,” offering “total confidence” and “peace of mind” for patients who want to know as much as possible.

Quest Diagnostics
Myriad Genetics
Examples of
for prenatal
blood tests.

Some of the companies offer tests without publishing any data on how well they perform, or point to numbers for their best screenings while leaving out weaker ones. Others base their claims on studies in which only one or two pregnancies actually had the condition in question.
This isn’t the first time Silicon Valley technology has been used to build a business around blood tests. Years before the first prenatal testing company opened, another start-up, Theranos, made claims that it could run more than a thousand tests on a tiny blood sample, before it collapsed amid allegations of fraud.
In contrast with Theranos, the science behind these companies’ ability to test blood for common disorders is not in question. Experts say it has revolutionized Down syndrome screening, significantly reducing the need for riskier tests.
However, the same technology — known as noninvasive prenatal testing, or NIPT — performs much worse when it looks for less common conditions. Most are caused by small missing pieces of chromosomes called microdeletions. Others stem from missing or extra copies of entire chromosomes. They can have a wide range of symptoms, including intellectual disability, heart defects, a shortened life span or a high infant mortality rate.
Not every patient is screened for every condition; doctors decide what to order, and most companies sell microdeletion testing as an optional add-on to the Down screening. Most test makers don’t say how often their microdeletion tests are being performed.
But it is clear some of the tests are in widespread use. One large test maker, Natera, said that in 2020 it performed more than 400,000 screenings for one microdeletion — the equivalent of testing roughly 10 percent of pregnant women in America.
To evaluate the newer tests, The Times interviewed researchers and then combined data from multiple studies to produce the best estimates available of how well the five most common microdeletion tests perform.
The analysis showed that positive results on those tests are incorrect about 85 percent of the time.
For These Five Tests, Positive Results Are Often Wrong
As prenatal tests have expanded to more rare conditions, a larger share of their positive results are incorrect. Some of the worst-performing tests look for microdeletions, which are small missing snippets of chromosomes.
DiGeorge syndrome
Affects 1 in 4,000 births
Can cause heart defects and delayed language acquisition. (May appear on lab reports as “22q.”)
1p36 deletion
1 in 5,000 births
Can cause seizures, low muscle tone and intellectual disability.
Cri-du-chat syndrome
1 in 15,000 births
Can cause difficulty walking and delayed speech development.
Wolf-Hirschhorn syndrome
1 in 20,000 births
Can cause seizures, growth delays and intellectual disability.
Prader-Willi and Angelman syndromes
Can cause seizures and an inability to control food consumption.

Why these five tests?
Testing companies currently offer seven microdeletion screenings. But two syndromes — Langer-Giedion and Jacobsen — are so rare that there is not enough data to understand how well the tests work. A few other tests for conditions that are not caused by microdeletions are also widely offered, with varying degrees of reliability. The screenings for Patau syndrome (which often appears on lab reports as “trisomy 13”) and Turner syndrome (“monosomy X”) also generate a large percentage of incorrect positives, while the screenings for Down syndrome (“trisomy 21”) and Edwards syndrome (“trisomy 18”) work well, according to experts.
Experts say there is no single threshold for how often a test needs to get positive results right to be worth offering. They note that when the tests do accurately identify an abnormality, it can give expectant parents time to learn about and prepare for challenges to come. Some said one common microdeletion screening, for a condition called DiGeorge syndrome, has the most potential to do good.
But there are hundreds of microdeletion syndromes, and the most expansive tests look for between five and seven, meaning women shouldn’t take a negative result as proof their baby doesn’t have a genetic disorder. For patients who are especially worried, obstetricians who study these screenings currently recommend other types of testing, which come with a small risk of miscarriage but are more reliable.
Some said the blood screenings that look for the rarest conditions are good for little more than bolstering testing companies’ bottom lines.
“It’s a little like running mammograms on kids,” said Mary Norton, an obstetrician and geneticist at the University of California, San Francisco. “The chance of breast cancer is so low, so why are you doing it? I think it’s purely a marketing thing.”
There are few restrictions on what test makers can offer. The Food and Drug Administration often requires evaluations of how frequently other consequential medical tests are right and whether shortfalls are clearly explained to patients and doctors. But the F.D.A. does not regulate this type of test .
Alberto Gutierrez, the former director of the F.D.A. office that oversees many medical tests, reviewed marketing materials from three testing companies and described them as “problematic.”
“I think the information they provide is misleading,” he said.
Patients who receive a positive result are supposed to pursue follow-up testing, which often requires a drawing of amniotic fluid or a sample of placental tissue. Those tests can cost thousands of dollars, come with a small risk of miscarriage and can’t be performed until later in pregnancy — in some states, past the point where abortions are legal.
The companies have known for years that the follow-up testing doesn’t always happen. A 2014 study found that 6 percent of patients who screened positive obtained an abortion without getting another test to confirm the result. That same year The Boston Globe quoted a doctor describing three terminations following unconfirmed positive results.
Three geneticists recounted more recent examples in interviews with The Times. One described a case in which the follow-up testing revealed the fetus was healthy. But by the time the results came, the patient had already ended her pregnancy.
After being presented with some of The Times’s reporting, half a dozen of the largest prenatal testing companies declined interview requests. They issued written statements that said patients should always review results with a doctor, and cautioned that the tests are meant not to diagnose a condition but rather to identify high-risk patients in need of additional testing.
In interviews, 14 patients who got false positives said the experience was agonizing. They recalled frantically researching conditions they’d never heard of, followed by sleepless nights and days hiding their bulging bellies from friends. Eight said they never received any information about the possibility of a false positive, and five recalled that their doctor treated the test results as definitive.
When Meredith Bannon’s pregnancy tested positive for DiGeorge syndrome, a nurse called and told her she and her husband would soon face “tough decisions” related to their child’s “quality of life,” which Ms. Bannon took to mean a choice about whether to end the pregnancy.
The call came as Ms. Bannon was driving to her parents’ house, with her son in the back seat wearing a “big brother” T-shirt. “I was coming home to tell them that I was pregnant, but instead I had to tell them the news I got this horrible result back,” Ms. Bannon recalled.
Further testing revealed that the result was wrong. Her baby is due in April.
Some women began tentatively planning abortions after receiving positive screenings.
“I couldn’t help but have termination on my mind,” said Allison Mihalich, 33, whose screening incorrectly indicated her baby might have Turner syndrome, which can cause infertility and heart defects. ( Studies show that the test’s positive results are wrong 74 percent of the time.) She lived in Indiana at the time and recalled scrambling to arrange follow-up testing before the state’s 22-week abortion ban.
A big market for rare conditions
Between 2011 and 2013, a small California-based biotech company, Sequenom, tripled in size. The key to its success: MaterniT21, a new prenatal screening test that did remarkably well at detecting Down syndrome.
Older screening tests took months and required multiple blood tests. This new one generated fewer false positives with a single blood draw.
The test could also determine the sex of a fetus. It quickly became a hit. “You had people walking in saying, ‘I want this sex test,’” recalled Dr. Anjali Kaimal, a maternal-fetal medicine specialist at Massachusetts General Hospital.
Competitors began launching their own tests. Today, analyst estimates of the market’s size range from $600 million into the billions, and the number of women taking these tests is expected to double by 2025.
As companies began looking for ways to differentiate their products, many decided to start screening for more and rarer disorders. All the screenings could run on the same blood draw, and doctors already order many tests during short prenatal care visits, meaning some probably thought little of tacking on a few more.
For the testing company, however, adding microdeletions can double what an insurer pays — from an average of $695 for the basic tests to $1,349 for the expanded panel, according to the health data company Concert Genetics. (Patients whose insurance didn’t fully cover the tests describe being billed wildly different figures, ranging from a few hundred to thousands of dollars.)
But these conditions were so rare that there were few instances for the tests to find.
Take Natera, which ran 400,000 tests in 2020 for DiGeorge syndrome, a disorder associated with heart defects and intellectual disability.
The 400,000 tests would be expected to identify about 200 actual cases of the disorder.
In a recent study, Natera said that its latest algorithm would identify about an equal number of false positives.
But that same study also included the results from when the tests were actually taken. Those numbers suggest there would be three times as many false positives as actual cases.
At least six percent of the tests include the full panel of microdeletions. Those would be expected to find about eight true positives and between 17 and 134 false ones.
Natera declined an interview request after The Times presented its reporting. In statements, it said that the early detection of DiGeorge syndrome can “profoundly improve” patient outcomes and stressed how infrequently it identifies some of the other conditions. (It said the screening that gave a false positive for Prader-Willi syndrome in Ms. Geller’s pregnancy, for example, had returned positive results only 113 times since 2015.) It pointed to its recent study of 20,000 pregnant women that found DiGeorge syndrome occurs in 1 in 1,600 births — twice as common as other estimates.
The company offers free genetic counseling to patients who screen positive. Natera also publishes data on how often its positive results are right and includes that information on patient results sheets.
Other companies release little information about how many tests they sell, and far less research on how well their screenings work.
Myriad Genetics’s prenatal test, Prequel, offers five microdeletion screenings, even though its study on the test includes just two confirmed cases of microdeletions.
In a statement, Myriad estimated that only one in 9,000 of its patients screens positive for a microdeletion. It said its data showed a “very small fraction” of those are wrong, but declined to provide specific figures.
Some companies test for conditions so rare that there are few known examples for comparison.
Both Labcorp, which purchased Sequenom, and Myriad Genetics offer screenings for one disorder that is so rare its prevalence is unknown, and another, called Jacobsen syndrome, that affects 1 in 100,000 births.
Dr. Diana Bianchi runs a National Institutes of Health laboratory studying prenatal blood screenings. She said of Jacobsen syndrome, “I’ve never seen a case of that in my 20-plus years of practicing genetics.”
Here’s why a test that works well for Down syndrome can be much less useful for rarer conditions.
If 20,000 women take a test of the same quality as the better prenatal blood screenings, there would be about 20 false positives.
And if the test is screening pregnant women in their late 30s for Down syndrome, it would identify about 100 real cases.
DiGeorge syndrome is 20 times as rare. An equally good test would get a similar number of false positives. But it would find only five actual cases.
And Prader-Willi syndrome is even more rare. That test would be expected to find one case.
The positive results would be wrong around 95 percent of the time.
‘Total confidence in every result’
Those shortfalls are rarely referenced when companies explain the tests to doctors and patients.
A Labcorp MaterniT21 lab report tells patients the test “detected” a problem, even though most studies show positives on that screening are usually wrong. Myriad Genetics advertised “total confidence in every result” on its prenatal testing website but said nothing about how often false positives can occur.
After The Times inquired about these tests, Myriad took down that language.
The Times reviewed 17 patient and doctor brochures from eight of the testing companies, including Natera, Labcorp, Quest and smaller competitors. Ten of the brochures never mention that a false positive can happen. Only one mentioned how often each test gets positive results wrong.

Examples of positive
test results.
Labcorp MaterniT21
Tests for these conditions
usually get positives wrong.
Roche Harmony
A footnote defines “high probability”
as “1% or greater.”

Genetic counselors who have dealt with false positives say some doctors may not understand how poorly the tests work. And even when caregivers do correctly interpret the information, patients may still be inclined to believe the confident-sounding results sheets.
When Cloey Canida, 25, got a positive result from Roche’s Harmony test in September, the result sheet seemed clear: It said her daughter had a “greater than 99/100” probability of being born with Patau syndrome, a condition that babies often do not survive beyond a week.
Her obstetrician tried to reassure her, citing independent data showing that for a woman her age, 93 percent of positives turn out to be wrong.
But Ms. Canida couldn’t stop thinking about the result sheet. She recalls crying during an ultrasound, thinking it was one of the few times she’d see her child moving.
After spending $1,200 on follow-up tests, she learned that her pregnancy was healthy, and that her daughter would not be born with Patau syndrome. She is now in her third trimester.
“I wish that we would have been informed of the false positive rate before I agreed to the test,” she said. “I was given zero information about that.”
Roche, which recently sold the Harmony test to another company, said in a statement that “all women should discuss their results with their health care provider” before making any decisions based on screening results.
Three experts reviewed marketing materials and results sheets for The Times and identified obvious reasons a patient would be confused.
“These numbers are meaningless,” said Mr. Gutierrez, the former F.D.A. official, after reviewing an advertisement for the Quest Diagnostics QNatal Advanced Test.
The test is advertised as getting positive microdeletion results right 75 percent of the time. But that figure comes from a single study that included nine confirmed cases of microdeletions, for a test that screens for seven such disorders. The company doesn’t specify how the tests perform individually, and declined to provide that data. (In a statement, Quest said its test has “excellent performance.”)
The F.D.A. considered regulating these tests a decade ago, but backed away. If the agency had oversight, Mr. Gutierrez said, Quest would be required to publish a brochure, but “it would not look like this.”
Nonetheless, companies are charging ahead, viewing microdeletions as a major business opportunity — especially if they can persuade more doctors to order them and more insurers to cover them.
Myriad Genetics, which owns the Prequel test, has told investors that it plans to start a “next-generation” microdeletion screening in 2022, and that it will lobby the professional society for obstetricians to begin recommending the test to its members.
Natera has performed more than two million screenings for Down syndrome since 2013. It went public in 2015, and the value of its stock has grown to $8.8 billion.
With its expanded panel of screenings, the company sees more growth ahead. “This is a really significant moment for the microdeletions business,” the company’s chief executive, Steve Chapman, said at an investor conference last January.
The company’s 2020 revenues were $391 million, and it projected its 2021 revenues to exceed $615 million. But if more insurers begin paying for microdeletion tests, Mr. Chapman said, the potential is “enormous” — it could bring in up to another $300 million every year.
Kitty Bennett contributed research.
About the analysis
To estimate the performance of microdeletion screening tests, The Times interviewed genetic counselors and experts on medical testing and prenatal care, then searched for peer-reviewed studies of screenings by U.S.-based labs that included follow-up diagnostic testing. Six studies met these criteria: three from diagnostic testing labs, and three studies funded by one of the test makers, Natera. An additional 2021 report by Natera was added as it included results from a recent clinical trial of its microdeletion test. (An eighth study , published in 2015, was excluded because experts identified multiple problems with its methodology.) Reporters then combined the data from these studies and estimated the tests’ overall positive predictive value to be 15 percent. Two researchers reviewed the resulting analysis.
Three of the four Natera studies include projected performance numbers that are based on re-analyzing the blood samples they collected with a modified version of the original test, a practice that can help improve results. At times, the company could not replicate those projections in subsequent studies. To be conservative, The Times used Natera’s higher projected numbers in its estimates; using the initial data instead would decrease the calculated positive predictive value from 15 percent to 12 percent.
An earlier version of this article misstated the location of the company Sequenom. It was based in San Diego, not Silicon Valley. Although many of the noninvasive prenatal blood tests currently in use were developed in Silicon Valley, not all of the testing firms have current headquarters there. The earlier version also referred imprecisely to projected numbers from Natera’s newest algorithms. The company said those figures were included in its clinical studies.
How we handle corrections
More about Sarah Kliff
Pregnancy, Childbirth and Postpartum Experiences
Aspirin’s Benefits: Not enough pregnant women at risk of developing pre-eclampsia, life-threatening high blood pressure, know that low-dose aspirin can help lower that risk. Leading experts are hoping to change that.
‘A Chance to Live’: Cases of trisomy 18 may rise as many states restrict abortion. Some women have chosen to have these babies , love them tenderly and care for them devotedly.
Teen Pregnancies: A large study in Canada found that women who were pregnant as teenagers were more likely to die before turning 31 .
Weight-Loss Drugs: Doctors say they are seeing more women try weight-loss medications in the hopes of having a healthy pregnancy. But little is known about the impact of those drugs on a fetus.
Premature Births: After years of steady decline, premature births rose sharply in the United States between 2014 and 2022. Experts said the shift might be partly the result of a growing prevalence of health complications among mothers .
Depression and Suicide: Women who experience depression during pregnancy or in the year after giving birth have a greater risk of suicide and attempted suicide .
- Open access
- Published: 16 May 2024
Women’s country of birth and failure to catch up an overdue cervical cancer cytological screening participation during pregnancy in France, an observational study based on survey sources
- Elisabeth Lyonnais 1 ,
- Solène Vigoureux 1 ,
- Béatrice Blondel 1 ,
- Sophie Wylomanski 2 &
- Elie Azria 1 , 2
BMC Cancer volume 24 , Article number: 595 ( 2024 ) Cite this article
105 Accesses
Metrics details
Cervical cancer is the fourth most common cancer among women worldwide, both for incidence and mortality. Prevention relies on screening with a Pap test to detect precancerous lesions, which can then be treated. Access to this screening is currently both improvable and inequitable. Pregnancy may be an ideal moment for women to catch up on their overdue cervical cancer screening. In the general population, women's risk of not being screened is associated with their place of birth and other social factors; this may be true as well among pregnant women. Our objective was to study the association between women's place of birth and their failure to catch up with this screening during pregnancy.
The 2016 French National Perinatal Survey included 13,147 women who gave birth after 21 weeks of gestation. The association between their place of birth and failure to catch up on this screening (defined by the absence of a Pap test during pregnancy for women overdue for it) was adjusted for age, parity, education level, health insurance, and when they began prenatal care with logistic regression models.
Among the women for whom screening was then recommended, 49% were not up to date at the start of pregnancy, and of these, 53% were not caught up before delivery. After adjustment for other risk factors, maternal place of birth was not associated with a higher risk of failure to catch up with this screening during pregnancy. However, factors identified as associated with this risk included a low education level and late start of prenatal care.
About half of women overdue for cervical cancer screening did not catch up with it during their pregnancy. Professionals should pay special attention to women with lower education levels and late initiation of prenatal care, who constitute a group at high risk of not catching up on this screening during pregnancy.
Peer Review reports
The World Health Organization (WHO) reports that the incidence of cervical cancer was 660,000 worldwide in 2022 and that more than 350,000 women died from it that year [ 1 ].
Prevention relies on screening with a Pap test to detect precancerous lesions that can then be treated. In Europe, screening and treating early neoplasia have substantially reduced the incidence and mortality of cervical cancer since 1960 [ 2 , 3 , 4 , 5 ]. In this area in 2021, rates of women aged 30–49 who reported ever having had a cervical cancer test varied from 42.0% (in Romania) to 98.4% in Finland, according to the WHO [ 6 ].In other high-income countries, rates were higher: 88% in the USA, 91% in Canada, and 95% in Australia had ever undergo Pap tests [ 7 ].
From 2010 to 2019, French guidelines recommended that women have a Pap test every 3 years between the ages of 25 and 65 years, after they have had two normal Pap smear results one year apart [ 8 ]. These guidelines, however, have been poorly implemented in France: only 58.7% of women were screened every three years between 2015 and 2017 in France [ 9 ].
Low participation rates in screening programs increases the risk of dying from invasive cervical cancer, and every year in France, 60% to 70% of the new cases of this cancer are diagnosed among women aged from 35 to 69 years who are unscreened or underscreened [ 8 ]. Recent studies show that risk factors for such non-screening or underscreening include a low education level and/or low income, living alone, unemployment, and lack of medical insurance, compared with women living in more privileged environments [ 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 ]. Some studies have also found that migrant women are screened less often than native women [ 11 , 22 , 23 , 24 , 25 , 26 , 27 ]. In Canada, migrant women have an adjusted RR 1.32; 95% CI 1.20–1.45) for an overdue Pap test compared to Canadian-born women [ 25 ]. Several French studies found that foreign women born to foreign parents underwent recommended cervical cancer screening less often than French women born to foreign parents, who themselves were less likely to be screened than French women born to French parents [ 12 , 28 , 29 ]. In Norway, Enden and al. showed that, despite a global increase of cervical cancer screening participation between 2012 et 2017, this increase was significantly smaller among immigrant women compared to Norwegian-born women [ 24 ].
Because pregnancy is a privileged moment for access to health care, it might be a good time to catch up with gynecologic follow-up for women not receiving regular triennial screening [ 8 ].
Although the performance of this screening has not been evaluated in pregnant women, the French Health Authority has recommended since 2007 Pap tests for all woman at the beginning of pregnancy if their last test took place more than three years earlier [ 30 ]. Maternal health inequalities according to maternal place of birth have been described in high-income countries, specifically in France [ 31 , 32 ]. It is important to know if these inequalities also affect cervical cancer screening during pregnancy.
The objectives of this study were to describe the association between mothers' place of birth and their failure to catch up on cervical cancer screening during pregnancy and to identify whether some other social characteristics might be risk factors for this among a national sample of women giving birth in France.
Data sources
The study population came from the French National Perinatal Survey conducted in March 2016. These surveys are fairly regular population-based cross-sectional studies using the same methodology and including all births (live births and stillbirths) after 21 weeks’ gestation or with a birthweight of at least 500 g during a one-week period in all maternity units in France [ 33 ].
For each birth, data were collected by a face-to-face interview and the collection of information from the medical records by a midwife. Maternal socioeconomic characteristics and prenatal care were obtained during the interview. Each woman was asked about a Pap test during pregnancy and over the past three years.
The perinatal survey database included 13,893 women (Fig. 1 Study population). The study population included all women who gave birth in mainland France, were more than 25 years old, and were interviewed and answered the question about a Pap test during pregnancy and over the past 3 years.
Study population
Outcome measurement
The outcome was the performance of a Pap test during pregnancy for women aged 26 years or older who were not up to date for this screening.
We considered women to be “up to date” for screening when they reported having had a Pap test in the previous three years. Women were considered overdue for it when they reported that their last Pap test took place more than three years earlier or that its date (if any) was unknown.
Among the overdue women, those who answered “Yes” to the question about a Pap test during pregnancy are classified as “caught up” and those who answered “No” or did not remember having had a Pap test during pregnancy were considered as “not caught up”.
Exposure measurement
Maternal place of birth was classified in five categories: France, other European countries, North Africa, other African countries, and elsewhere in the world.
Social and demographic characteristics
Maternal age was divided into three categories: 26–30 years old, 31–35 years old, or 36 years older or more.
Education level was the highest level of education, again in three categories: Middle school or less, high school and beyond high school.
Socioeconomic situation was defined by several characteristics:
Employment status during pregnancy: Employed, unemployed and/or looking for work, homemaker or student or other.
Personal housing during the last trimester of pregnancy, as a binary variable: yes or no.
No work-related household income, as a binary variable: yes or no.
Standard health insurance coverage at the beginning of pregnancy, as a binary variable: yes or no.
Living with a partner as a binary variable: yes or no.
Inadequate prenatal care utilization
We used the indicator described by Gonthier et al. [ 34 ] to assess the adherence of prenatal care to current French recommendations. It covers late initiation of care (started later than 12 weeks of gestation) and too few appointments (i.e. < 7 prenatal visits and 3 ultrasound examinations for full term pregnancies) and is defined specifically as:
Late initiation of care
Fewer than half the number of prenatal visits expected according to the duration of pregnancy
Insufficient number of ultrasound screenings: missing either the first-trimester ultrasound examination or both the second- and third-trimester examinations
Statistical analyses
The population was described by comparing the women overdue at the start of pregnancy who were and were not "caught up" by its end for proportions of categorical variables. To study the association between each social factors and failure to catch up on screening, we performed a bivariate analysis. Then, we constructed a multivariate logistic regression model adjusted for maternal place of birth, age, parity, education level, health insurance coverage, and timing of prenatal care initiation. Associations between failure to catch up, mother’s place of birth, and covariates were expressed as crude odds ratios (OR) and adjusted odds ratios (AOR) and their 95% confidence intervals (CIs).
STATA 15.0 software was used to perform the analyses.
Description
Women included in the National Perinatal Survey and who gave birth in mainland France, were more than 25 years old (eligible for cervical cancer screening), and answered the question about a Pap test during pregnancy and over the past 3 years were 9,638 (Fig. 1 Study population). Among the latter, 4,840 women reported they were up to date for cervical cancer screening because they had had a Pap test in the previous three years, while 4,739 women (49%) were overdue. Among these overdue women, 2,243 (47%) answered the question about a Pap test during pregnancy positively and are considered caught up, while 1,862 (53%) answered negatively or did not remember and were considered not caught up.
Not caught up women were younger (chi-square p test = 0.001), less well educated ( p < 0.0001), and less often employed ( p = 0.002) than women who were caught up (Table 1 ). They also initiated prenatal care later than caught-up women and had an inadequate prenatal care utilization more frequently ( p < 0.0001).
Factors associated with not catching up: bivariate and multivariate analyses
We did not observe with the bivariate analysis any association between maternal place of birth and failure to catch up during pregnancy (Table 2 ). The analysis however showed that several factors were associated with failing to catch up, including having non-standard (versus standard) health insurance at the beginning of pregnancy (Crude OR 1.34 95% CI [1.14–1.57]) and a middle school or high school education level (versus beyond high school level) (Crude OR 1.33 95% CI [1.15–1.53] and 1.27 95% CI [1.10–1.48] respectively).
In the multivariate analysis, after adjustment for age, parity, level of education, health insurance and late initiation of prenatal care, the maternal place of birth was not significantly associated with the risk of not being caught up. On the other hand, having a middle school or high school education level was significantly associated with not catching up (AOR 1.24 95% CI [1.06–1.45] and AOR 1.21 95% CI [1.04–1.41] respectively), compared with women with higher qualifications. Late initiation of antenatal care was strongly associated with failure to catch up (AOR = 2.13 95% CI [1.46–3.10]).
The proportion of missing data was less than 2% for each variable. Observations containing missing data were excluded from the multivariate analysis which was performed on 4,601 complete observations out of 4,739.
This analysis of the 2016 French National Perinatal Survey shows that 49% of the women eligible for cervical screening were overdue for it, and among this group, 53% did not catch up with this screening during their pregnancy, despite national guidelines strongly recommending it. Maternal place of birth was not associated with this failure to catch up during pregnancy, although an age of 26–30 years, a lower education level, a start of prenatal care later, compared with overdue women who were caught up, were associated with it.
Strengths and limitations
One of the strengths of this analysis is the large number of women included and the low rate of missing data; these factors together provide good statistical power and limit the risk of bias. The survey's design also ensures the sample's representativity. The participation of nearly every maternity unit in France resulted in a number of births very close to that expected according to the INSEE statistics; at the same time, the characteristics of the mothers, deliveries, and newborns were similar to those already known through hospital discharge summaries (PMSI) [ 33 ].
Nonetheless, women not speaking French well did not have face-to-face interviews and were thus excluded from this analysis. They accounted for almost 4% of the women aged 26 years or older. Most of them were immigrants and perhaps among the most deprived individuals in our sample. This selection bias might have led us to underestimate the strength of the associations between social factors and failure to catch up. On the other hand, excluding these women from the study and analysis might have prevented us from being able to highlight an existing association between immigration and catch-up failure.
Another limitation is related to the quality of the data collected about prenatal care. Women may have forgotten, omitted, or misunderstood some questions. They may confuse Pap tests with simple vaginal samples. A few studies suggested that women over report Pap tests, partly by equating any examination of the pelvic area to a Pap test [ 9 , 35 , 36 , 37 ]. Women with a low level of education or with a language barrier may therefore have more often misunderstood this question; some women may not have been considered caught up although they had had a Pap test, or the inverse might be true.
While many authors have asked if social and economic status influences the rate of reporting the response is not unanimous: some authors find over-reporting among the most disadvantaged, others among the most advantaged, while still others find no association between social background and reporting [ 35 , 36 , 38 ]. Lastly, during the National Perinatal Survey, the interview was carried out by a midwife, who could help women remember this test and could have limited memorization bias.
Interpretation of results
Failure to catch up.
First, almost half of all pregnant women were overdue for cervical cancer screening in France in 2016, and slightly more than half did not catch up during pregnancy. French hospital-based studies have found similar rates of failure to catch up during pregnancy (from 53 to 61%) [ 39 , 40 , 41 ], but our work is the first study to describe this phenomenon among a national sample of pregnant women. In the UK, Coleridge et al. found that nearly half (47.3%) of a sample of 260 pregnant women were overdue for cervical screening and 74% were not caught up during either their pregnancy or the first 6 months postpartum [ 42 ]. In Brazil, Terlan and Cesar have observed that, despite prenatal visits, 21.6% pregnant women did not undergo the Pap smears they should have had [ 43 ].
Despite these inadequate catch-up rates during pregnancy, some countries have shown that this period does indeed present an important opportunity for health care professionals to help women to catch up with overdue screening. A Norwegian cohort study including more than 2 million women showed that pregnant women were almost five times more likely to have a Pap smear test within one year compared to the non-pregnant women [ 44 ]. A Polish hospital-based study found that 7.5% of women older than 25 years reported that the Pap test performed during pregnancy, in accordance with local guidelines, was the first they had ever had.
Maternal place of birth
In our analysis, maternal place of birth was not associated with failure to catch up with cervical cancer screening during pregnancy. To our knowledge, this study is the first to assess specifically the association between maternal place of birth and this screening during pregnancy. Moreover, we have not found studies that investigated the associations between maternal nationality or ethnicity and cervical cancer screening. Most studies concern associations between women’s place of birth or ethnicity in general populations.
Several Canadian studies have shown significant cervical cancer screening inequalities based on age, income, immigration status, and world region of origin [ 25 , 27 ]. A review of the literature conducted in 2019 showed that women from sub-Saharan Africa and living in Canada origin had the lowest cervical cancer screening rates [ 45 ].
In Norway, women from North and sub-Saharan Africa had lower rates of participation in cervical cancer screening programs than Norwegian-born women (adjusted OR 0.61, 95% CI [0.56–0.67]) [ 46 ]. In Denmark, migrant women have the lowest rate of participation in the national screening program, even after adjustment for other social characteristics. The authors suggest that this result might be due to a language barrier, some difficulties in understanding the screening invitation (written in Danish), and poor health literacy — all barriers to seeking care or understanding and adhering to prevention and screening [ 11 , 47 ]. According to Idehen et al., Russians, Somalis and Kurds women living in Finland are less screened than Finnish women [ 26 ].
In France, Sassenou et al. observed in 2023 that women residing in France and born in European countries other than France were screened less often than native women [ 29 ]. The lack of association between maternal place of birth and catch-up screening during pregnancy, analyzed in a selected population of overdue pregnant women, does not however reflect an association that would exist outside pregnancy between place of birth and access to cervical cancer screening.
Age was also associated with failure to catch up. In our study, the youngest pregnant women had had fewer Pap tests than those older than 30 years. In the Polish study by Kusczborska et al., age was the only factor associated with Pap tests both before and during the current pregnancy, but it enrolled women younger than 25 years, who are normally not subject to Polish screening guidelines [ 48 ]. In Brazil, Monteiro et al. and Cesar et al. found that young age (younger than 35 years old, respectively) was associated with lower Pap testing rates during pregnancy [ 49 , 50 ].
Adherence to medical guidelines
Late initiation of prenatal care was associated with failure to catch up on screening. This may be due to the care provider's concern about performing a Pap test after the first trimester and suggests poor knowledge of current guidelines. The French Health Authority guidelines, the French Public Health Code, and the guidelines of the French National College of Gynecologists and Obstetricians state that a Pap test can be performed at any time during pregnancy, especially for women without regular gynecological follow-up [ 40 , 51 ]. Nonetheless, among a sample of French midwives interviewed in 2018, 29% reported that they would perform a Pap test at 25 weeks of gestation, compared with more than 90% at 10 weeks [ 52 ]. In a study that took place in 2009–2010 in a University Hospital Center of France, the proportion of adequate screening (defined by performing a Pap test during pregnancy if the last one was more than two years earlier or if its result was unknown) was significantly higher when the first prenatal visit occurred during the first trimester rather than during the second or the third trimester (48% versus 12%) [ 41 ]. According to Saulneron et al. most Pap tests performed during pregnancy take place during the first trimester (86.7%) [ 40 ]. In the Norwegian cohort of Nygard et al., most Pap smears from pregnant women were taken during the first 4 months of pregnancy [ 44 ].
A Pap test can also be proposed during the postnatal visit, but several studies have shown that 68% to 83% of women do not attend this visit, in particular, those in situations of social deprivation [ 53 , 54 , 55 ]. This non-adherence results in missing the opportunity for these women to be caught up with this important preventive care, in particular, those with a poor access to gynecological care [ 56 , 57 ].
Strong public health policies could reduce the late initiation of prenatal care and thereby have a positive impact on cervical cancer screening during pregnancy. In Norway, the high rate of participation of pregnant women in the national screening program has improved its coverage throughout the female population [ 44 ].
In 2019, the French Health Authority (HAS) published new recommendations on cervical cancer screening, advising an HPV test every five years for women over 30, rather than Pap tests [ 58 ]. These new guidelines, if well disseminated to and adhered to by health care providers, may improve screening of pregnant woman overdue for cervical cancer screening.
Individual factors play a moderate role in failed catch up of women overdue for screening during pregnancy. A better understanding of why recommendations are so poorly implemented requires a study of the knowledge, attitudes, and practices of all health care providers.
Despite guidelines, nearly half of all pregnant women are overdue for cervical cancer screening, and catch-up will not occur for 53% of them during pregnancy. A young age (younger than 30 years), a low education level, and late initiation of prenatal care are factors associated with failure to catch up, but maternal place of birth does not appear to be an independent risk factor. Health care professionals must be made aware of these factors, so that women who are overdue for screening, particularly those most at risk, can catch up. It is important that professionals involved in prenatal care understand the new screening procedures well and can implement them, even for women whose prenatal care begin late.
Availability of data and materials
A description of the study is available from: enp.inserm.fr. The data are partially accessible from the following link http://quetelet.progedo.fr/.
WHO. Cervical cancer. Available from: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer .
Defossez G, Le Guyader-Peyrou S, Uhry Z, Grosclaude P, Colonna M, Dantonny E, et al. Estimations nationales de l’incidence et de la mortalité par cancer en France métropolitaine entre 1990 et 2018 - Tumeurs solides : Étude à partir des registres des cancers du réseau Francim. Available from: https://www.santepubliquefrance.fr/import/estimations-nationales-de-l-incidence-et-de-la-mortalite-par-cancer-en-france-metropolitaine-entre-1990-et-2018-tumeurs-solides-etude-a-partir . Cited 2024 Apr 2.
Lapôtre-Ledoux B, Remontet L, Uhry Z, Dantonny E, Grosclaude P, Molinié F, et al. Incidence des principaux Cancers en France Métropolitaine en 2023 et tendances depuis 1990. Bull Épidémiol Hebd. 2023;12–13:188–204.
Google Scholar
Jansen EEL, Zielonke N, Gini A, Anttila A, Segnan N, Vokó Z, et al. Effect of organised cervical cancer screening on cervical cancer mortality in Europe: a systematic review. Eur J Cancer. 2020;127:207–23.
Article PubMed Google Scholar
Pedersen K, Fogelberg S, Thamsborg LH, Clements M, Nygård M, Kristiansen IS, et al. An overview of cervical cancer epidemiology and prevention in Scandinavia. Acta Obstet Gynecol Scand. 2018;97(7):795–807.
Williams J, Rakovac I, Victoria J, Tatarinova T, Corbex M, Barr B, et al. Cervical cancer testing among women aged 30–49 years in the WHO European Region. Eur J Pub Health. 2021;31(4):884–9.
Article Google Scholar
WHO. Complete cervical cancer profile sets. 2021. Available from: https://www.who.int/publications/m/item/cervical-cancer-country-profiles . Cited 2024 Apr 11
État des lieux et recommandations pour le dépistage du cancer du col de l’utérus en France. HAS; 2010. Available from: https://www.has-sante.fr/jcms/c_1009772/fr/etat-des-lieux-et-recommandations-pour-le-depistage-du-cancer-du-col-de-l-uterus-en-france .
Hamers F, Jezeweski-Serra D. Couverture du dépistage du cancer du col de l’utérus en France, 2012–2017. Revue de Biologie Médicale/N. 2020;353:67–74.
Levinson KL, Jernigan AM, Flocke SA, Tergas AI, Gunderson CC, Huh WK, et al. Intimate partner violence and barriers to cervical cancer screening: a gynecologic oncology fellow research network study. J Low Genit Tract Dis. 2016;20(1):47–51.
Article PubMed PubMed Central Google Scholar
Harder E, Juul KE, Jensen SM, Thomsen LT, Frederiksen K, Kjaer SK. Factors associated with non-participation in cervical cancer screening–a nationwide study of nearly half a million women in Denmark. Prev Med. 2018;111:94–100.
Grillo F, Vallée J, Chauvin P. Inequalities in cervical cancer screening for women with or without a regular consulting in primary care for gynaecological health, in Paris, France. Prevent Med. 2012;54(3–4):259–65.
Konopka AM, Barnay T, Billaudeau N, Sevilla-Dedieu C. Les déterminants du recours au dépistage du cancer du col de l’utérus: une analyse départementale. Econ Prév. 2019;2:43–63.
Ricardo-Rodrigues I, Jiménez-García R, Hernández-Barrera V, Carrasco-Garrido P, Jiménez-Trujillo I, de Andrés AL. Social disparities in access to breast and cervical cancer screening by women living in Spain. Public Health. 2015;129(7):881–8.
Article CAS PubMed Google Scholar
Petkeviciene J, Ivanauskiene R, Klumbiene J. Sociodemographic and lifestyle determinants of non-attendance for cervical cancer screening in Lithuania, 2006–2014. Public Health. 2018;156:79–86.
Guthmann JP, Pelat C, Parent du Chatelet I, Duport N, Lévy-Bruhl D, Institut de Veille Sanitaire (InVS). Déterminants socio-économiques de vaccination et de dépistage du cancer du col par frottis cervico-utérin (FCU). Analyse de l’Enquête santé et protection sociale (ESPS), 2012. 2014. Available from: https://www.santepubliquefrance.fr/determinants-de-sante/vaccination/documents/rapport-synthese/determinants-socio-economiques-de-vaccination-et-de-depistage-du-cancer-du-col-par-frottis-cervico-uterin-fcu-.-analyse-de-l-enquete-sante-et-prot .
Barré S, Massetti M, Leleu H, Catajar N, De Bels F. Caractérisation des femmes ne réalisant pas de dépistage du cancer du col de l’utérus par frottis cervico-utérin en France. Bull Epidémiol Hebd. 2017;2–3:39.
Sassenou J, Ringa V, Zins M, Ozguler A, Paquet S, Panjo H, et al. Women with obesity in cervical cancer screening. The double penalty: Underscreening and income inequalities. Obes Res Clin Pract. 2021;15(3):212–5.
Le Bihan-Benjamin C, Marchadier A, Audiger C, Khati I, Barré-Pierrel S. Quel déploiement du Programme national de dépistage organisé du cancer du col de l’utérus en France en 2022 ? Bull Épidémiol Hebd. 2024;5:82–91.
Bryere J, Dejardin O, Launay L, Grosclaude P, Launoy G, Réseau Français des regristres de cancers Francim. Environnement socioéconomique et incidence des cancers en France. Bull Épidémiol Hebd. 2017;4:68–77.
Traoré M, Vallée J, Chauvin P. Risk of late cervical cancer screening in the Paris region according to social deprivation and medical densities in daily visited neighborhoods. Int J Health Geogr. 2020;19(1):18.
Kristensson JH, Sander BB, von Euler-Chelpin M, Lynge E. Predictors of non-participation in cervical screening in Denmark. Cancer Epidemiol. 2014;38(2):174–80.
Rondet C, Lapostolle A, Soler M, Grillo F, Parizot I, Chauvin P. Are immigrants and nationals born to immigrants at higher risk for delayed or no lifetime breast and cervical cancer screening? The results from a population-based survey in Paris metropolitan area in 2010. PLoS ONE. 2014;9(1):e87046.
Enden MR, Møen K, Igland J, Diaz E. Trends in cervical cancer screening in Norway 2012–2017: a comparison study of non-immigrant and immigrant women. Scand J Public Health. 2024J;2:14034948231217636.
Bacal V, Blinder H, Momoli F, Wu KY, McFaul S. Is immigrant status associated with cervical cancer screening among women in Canada? Results from a cross-sectional study. J Obstet Gynaecol Can. 2019;41(6):824-831.e1.
Idehen EE, Koponen P, Härkänen T, Kangasniemi M, Pietilä AM, Korhonen T. Disparities in cervical screening participation: a comparison of Russian, Somali and Kurdish immigrants with the general finnish population. Int J Equity Health. 2018;17(1):56.
Vahabi M, Lofters AK, Kopp A, Glazier RH. Correlates of non-adherence to breast, cervical, and colorectal cancer screening among screen-eligible women: a population-based cohort study in Ontario, Canada. Cancer Causes Control. 2021F;32(2):147–55.
Crampe-Casnabet C, Franck JE, Ringa V, Coeuret-Pellicer M, Chauvin P, Menvielle G. Role of obesity in differences in cervical cancer screening rates by migration history. The Constances survey. Cancer Epidemiol. 2019;58:98–103.
Sassenou J, Ringa V, Zins M, Ozguler A, Paquet S, Panjo H, et al. Combined influence of immigration status and income on cervical cancer screening uptake. Prev Med Rep. 2023;36:102363.
Suivi et orientation des femmes enceintes en fonction des situations à risque identifiées. HAS; 2007. Available from: http://www.cfef.org/archives/bricabrac/suivigrossesse.pdf .
Eslier M, Azria E, Chatzistergiou K, Stewart Z, Dechartres A, Deneux-Tharaux C. Association between migration and severe maternal outcomes in high-income countries: Systematic review and meta-analysis. PLoS Med. 2023;20(6):e1004257.
Eslier M, Deneux-Tharaux C, Sauvegrain P, Schmitz T, Luton D, Mandelbrot L, et al. Severe maternal morbidity among undocumented migrant women in the PreCARE prospective cohort study. BJOG. 2022;129(10):1762–71.
Blondel B, Coulm B, Bonnet C, Goffinet F, Le Ray C. National coordination group of the national perinatal surveys. Trends in perinatal health in metropolitan France from 1995 to 2016: results from the French national perinatal surveys. J Gynecol Obstet Hum Reprod. 2017;46(10):701–13.
Gonthier C, Estellat C, Deneux-Tharaux C, Blondel B, Alfaiate T, Schmitz T, et al. Association between maternal social deprivation and prenatal care utilization: the PreCARE cohort study. BMC Pregnancy Childbirth. 2017;17:126.
Aranda E, Franck JE, Ringa V, Sassenou J, Coeuret-Pellicer M, Rigal L, et al. Social inequalities in participation in cancer screening: does the mode of data collection matter? The CONSTANCES cohort. European Journal of Public Health. 2021;31(3):602–8.
Lofters A, Vahabi M, Glazier RH. The validity of self-reported cancer screening history and the role of social disadvantage in Ontario, Canada. BMC Public Health. 2015;15(1):28.
Anderson J, Bourne D, Peterson K, Mackey K. Evidence brief: accuracy of self-report for cervical and breast cancer screening. Washington (DC): Department of Veterans Affairs (US). 2019.
Lofters AK, Moineddin R, Hwang SW, Glazier RH. Does social disadvantage affect the validity of self-report for cervical cancer screening? Int J Womens Health. 2013;5:29–33.
Moussier M. Réalisation du frottis cervico-utérin de rattrapage pendant la grossesse : profil des patientes concernées et informations reçues à propos du dépistage du cancer du col de l’utérus : étude observationnelle multicentrique dans le département des Alpes-Maritimes. 2019. Available from: https://dumas.ccsd.cnrs.fr/dumas-02271416/document . Cited 2020 Jul 22.
Saulneron M, Descamps-Mollier M, Carcopino X. Analyse de la pratique du frottis cervico-utérin de dépistage pendant la grossesse en France : étude bicentrique rétrospective de cohorte. J Gynecol Obstet Biol Reprod. 2015;44(6):516–23.
Article CAS Google Scholar
Gauchotte E. Frottis cervico-vaginaux pendant la grossesse : évaluation des pratiques professsionnelles et suivi des anomalies. 2011.
Coleridge SL, Wiggans A, Nelissen E, Bethune R, Blackwell R, Bryant A, et al. Improving the uptake of cervical screening in pregnant and recently postnatal women: a quality improvement project. BMJ Open Qual. 2022;11(2):e001709.
Terlan RJ, Cesar JA. Non-performance of Pap smears among pregnant women in the Extreme South of Brazil: prevalence and associated factors. Cien Saude Colet. 2018;23(11):3557–66.
Nygård M, Daltveit AK, Thoresen SØ, Nygård JF. Effect of an antepartum Pap smear on the coverage of a cervical cancer screening programme: a population-based prospective study. BMC Health Serv Res. 2007;7(1):10.
Nnorom O, Findlay N, Lee-Foon NK, Jain AA, Ziegler CP, Scott FE, et al. Dying to learn: a scoping review of breast and cervical cancer studies focusing on black Canadian women. J Health Care Poor Underserved. 2019;30(4):1331–59.
Møen KA, Kumar B, Qureshi S, Diaz E. Differences in cervical cancer screening between immigrants and nonimmigrants in Norway: a primary healthcare register-based study. Eur J Cancer Prev. 2017;26(6):521–7.
Ruel J, Moreau AC, Ndengeyingoma A, Arwidson P, Allaire C. Littératie en santé et prévention du cancer. Sante Publique. 2019;S2(HS2):75–8.
Kuczborska K, Kacperczyk-Bartnik J, Wolska M, Pluta M, Bartnik P, Dobrowolska-Redo A, et al. Secondary cervical cancer prevention in routine prenatal care — coverage, results and lessons for the future. Ginekol Pol. 2019;90(7):396–402.
Monteiro PB, Monteiro Filho MP, De Figueiredo JT, Saintrain MVDL, Bruno ZV, Carvalho FHC. Cytology-based screening during antenatal care as a method for preventing cervical cancer. Asian Pac J Cancer Prev. 2017S 1;18(9):2513–8.
PubMed PubMed Central Google Scholar
Cesar JA, dos Santos GB, Sutil AT, Cunha CF, Dumith SD. [Pap smears among pregnant women in Southern Brazil: a representative cross-sectional survey]. Rev Bras Ginecol Obstet. 2012;34(11):518–23.
Recommandations pour la pratique clinique : Prévention du cancer du col de l’utérus. Collège national des gynécologues et obstétriciens français; 2007 Dec p. 391–404. Available from: http://www.cngof.fr/pratiques-cliniques/recommandations-pour-la-pratique-clinique/apercu?path=RPC%2BCOLLEGE%252F2007%252Frpc_prev-K-col2007.pdf&i=21959 Cited 2020 Jul 22.
Kervella L, Berveiller P, Bourdillon M, Rousseau A. Midwives’ practices related to cervical cancer screening during pregnancy: A vignette-based study. Sex Reprod Healthc. 2020;26:100539.
Puech M. La consultation du post-partum: une consultation négligée? [PhD Thesis]. France: Université Toulouse III-Paul Sabatier; 2019.
Coget C. Connaissances, pratiques et attentes des femmes concernant la visite post-natale. Étude quantitative [PhD Thesis]. Faculté Mixte de Médecine et de Pharmacie de Rouen; 2018. Disponible sur: https://dumas.ccsd.cnrs.fr/dumas-02047837 .
Polk S, Edwardson J, Lawson S, Valenzuela D, Hobbins E, Prichett L, et al. Bridging the postpartum gap: a randomized controlled trial to improve postpartum visit attendance among low-income women with limited English Proficiency. Womens Health Rep (New Rochelle). 2021;2(1):381–8.
PubMed Google Scholar
Généralisation du dépistage du cancer du col de l’utérus /Étude médico-économique /Phase 1,appui à la décision [Internet]. INCA; 2015. Available from: https://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Generalisation-du-depistage-du-cancer-du-col-de-l-uterus-etude-medico-economique-Phase-1
Brun-Micaleff E, Coffy A, Rey V, Didelot MN, Combecal J, Doutre S, et al. Cervical cancer screening by cytology and human papillomavirus testing during pregnancy in French women with poor adhesion to regular cervical screening. J Med Virol. 2014;86(3):536–45.
HAS. Évaluation de la recherche des papillomavirus humains (HPV) en dépistage primaire des lésions précancéreuses et cancéreuses du col de l’utérus et de la place du double immuno-marquage p16/Ki67. 2019. Available from: https://www.has-sante.fr/jcms/c_2806160/fr/evaluation-de-la-recherche-des-papillomavirus-humains-hpv-en-depistage-primaire-des-lesions-precancereuses-et-cancereuses-du-col-de-l-uterus-et-de-la-place-du-double-immuno-marquage-p16/ki67 . Cited 2020 Jun 8.
Download references
Acknowledgements
The authors thank the department head in each maternity unit who agreed to have the survey performed in their unit, the investigators and all the women who agreed to participate. EL would particularly like to thank Dr. J. Merrer, Midwife, PhD for her assistance with the data analysis and writing of the article.
The 2016 National Perinatal Survey was developed and implemented by the French National Institute of Health and Medical Research (INSERM), three directorates of the Ministry of Social Affairs and Health and the French National Public Health Agency (Santé Publique France).
Author information
Authors and affiliations.
Obstetrical, Perinatal and Pediatric Epidemiology (EPOPé Research Team), FHU Prema, Université Paris Cité – INSERM, 75014, Paris, France
Elisabeth Lyonnais, Solène Vigoureux, Béatrice Blondel & Elie Azria
Maternity Unit, Paris Saint Joseph Hospital, Paris, France
Sophie Wylomanski & Elie Azria
You can also search for this author in PubMed Google Scholar
Contributions
BB contributed to the conception of the National Perinatal Survey and was responsible for the data collection and dissemination of the main results. EL, SV, and EA performed the analyses and drafted the manuscript. SW critically reviewed the manuscript and contributed to substantial improvements. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Elisabeth Lyonnais .
Ethics declarations
Ethics approval and consent to participate.
The 2016 French National Perinatal Survey was approved by the French Data Protection Authority (Commission Nationale de l’Informatique et des Libertés, approval no.: 915197), the National Council on Statistical Information (Comité du Label, approval no.: 2016X703SA), and the French Institute of Health and Medical Research Ethics Committee (INSERM Ethics Committee approval no.: IRB00003888 no. 14–191). Oral informed consent was obtained from women for survey participation before the interview.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lyonnais, E., Vigoureux, S., Blondel, B. et al. Women’s country of birth and failure to catch up an overdue cervical cancer cytological screening participation during pregnancy in France, an observational study based on survey sources. BMC Cancer 24 , 595 (2024). https://doi.org/10.1186/s12885-024-12335-1
Download citation
Received : 25 January 2024
Accepted : 03 May 2024
Published : 16 May 2024
DOI : https://doi.org/10.1186/s12885-024-12335-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cervical cancer screening
- National perinatal survey
- Health inequalities
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Essay on Prenatal Screening Tests
Every parent, regardless of biological background, has a strong desire for a healthy child. However, abnormalities during fetus growth have become a common phenomenon due to physical, chemical, and radiation factors. Biological factors such as genetic mutation, which causes increased mental retardation and abnormal body growth, bring trouble to the child’s future growth and development. Scientific innovations and modern technologies in the medical field have developed different screening techniques to detect fetus abnormality before baby delivery. Blood testing and ultrasound techniques of prenatal screening tests are essential in determining blood type, Rh factor, and fetus abnormality, although such screening tests can place a pregnancy at a high risk of infection, premature rupture of membranes, bleeding, and even loss of the pregnancy.
Prenatal screening such as blood and ultrasound tests helps detect the blood type and Rh factor’s compatibility of the mother and the fetus. Blood testing prevents pregnancy complications caused by an antigen-antibody reaction of the Rh factors group of the mother and that attached to the fetus, which can result in fetus health complications such as hemolytic anemia (Nshimyumukiza et al. 12). Besides, blood testing helps in detecting blood-borne diseases such as Hepatitis B, HIV, and Rubella. Ultrasound can help in determining the growth and development of a fetus during pregnancy. According to Chitty medical experts can use Ultrasound to detect structural defects such as anencephaly and spinal Bifida, cleft lip, congenital heart, kidney malformations, and gastrointestinal defects. When some health complications are detected through prenatal screening tests, immunosuppressed drugs can help boost the fetus’s immune system.
Prenatal testing comes with more genetic risks due to its invasive nature. Blood and Ultrasound tests can place a pregnancy at a high risk of premature rupture of membranes, loss of the pregnancy, excessive bleeding, and infections. A research study conducted on the effects of prenatal screening tests among Latino Americans women found that prenatal testing may cause increased stress and anxiety among parents if abnormalities pose significant risks to the pregnancy (Chitty et al. 161). Parents may feel angry, anxious, guilty, or depressed about their prenatal results. In some instances, prenatal testing such as blood testing can create increased tension and negative relationship because the results can reveal unknown information about family identities. Prenatal testing procedures that require a buccal smear or blood sample carry real risks of miscarriage since it involves extraction of amniotic fluid sample around the fetus.
To have or not to have prenatal tests wholly lies on the mother’s decision. Nshimyumukiza et al., denotes that women may decide not to have prenatal tests because they may not get adequate information about prenatal testing, particularly invasive tests that are likely to harm the fetus. Some women may skip prenatal tests after learning about the risks such as premature rupture of membranes, loss of the pregnancy, excessive bleeding, and infections. Prenatal testing may jeopardize people’s physical health, privacy, and financial well-being.
In summary, prenatal testing techniques such as blood and Ultrasound tests provide a suitable background for understanding fetus blood type, Rh factor, and other abnormalities—that may put the future of mother and fetus at a higher risk. However, some of the risks associated with prenatal testing, such as loss of the pregnancy, excessive bleeding, and infections, can make a woman have a false impression and negative attitude towards prenatal screening tests. Medical experts need to provide suitable answers about the importance of having prenatal tests to help parents better decide whether or not to have prenatal screening tests.
Works Cited
Chitty, Lyn S., Louanne Hudgins, And Mary E. Norton. “Current Controversies in Prenatal Diagnosis 2: Cell‐Free DNA Prenatal Screening Should Be Used to Identify All Chromosome Abnormalities.” Prenatal Diagnosis 38.3 (2018): 160-165.
Nshimyumukiza, L., et al. “Cell‐Free DNA Noninvasive Prenatal Screening for Aneuploidy Versus Conventional Screening: A Systematic Review of Economic Evaluations.” Clinical Genetics 94.1 (2018): 3-21.
Cite this page
Similar essay samples.
- Culpability: An appeal to reduce the age at which children are deemed ...
- A strategic analysis of Eni
- Essay on Onboarding Program for Virtual Learners
- Essay on Australian Civil Procedure
- Essay on Nursing Individualised Care
- Amazon Financial Analysis
- Open access
- Published: 11 March 2024
Equity in prenatal healthcare services globally: an umbrella review
- Zeenat Ladak 1 , 4 ,
- Nagma Grewal 2 ,
- Minji Olivia Kim 2 ,
- Stephanie Small 1 ,
- Alexia Leber 1 ,
- Mehdiya Hemani 3 ,
- Qiuyu Sun 2 ,
- Deena M. Hamza 2 ,
- Celia Laur 1 , 4 ,
- Noah M. Ivers 1 , 4 , 5 ,
- Olesya Falenchuk 1 &
- Richard Volpe 1
BMC Pregnancy and Childbirth volume 24 , Article number: 191 ( 2024 ) Cite this article
1061 Accesses
11 Altmetric
Metrics details
Timely, appropriate, and equitable access to quality healthcare during pregnancy is proven to contribute to better health outcomes of birthing individuals and infants following birth. Equity is conceptualized as the absence of differences in healthcare access and quality among population groups. Healthcare policies are guides for front-line practices, and despite merits of contemporary policies striving to foster equitable healthcare, inequities persist. The purpose of this umbrella review is to identify prenatal healthcare practices, summarize how equities/inequities are reported in relation to patient experiences or health outcomes when accessing or using services, and collate equity reporting characteristics.
For this umbrella review, six electronic databases were searched (Medline, EMBASE, APA PsychInfo, CINAHL, International Bibliography of the Social Sciences, and Cochrane Library). Included studies were extracted for publication and study characteristics, equity reporting, primary outcomes (prenatal care influenced by equity/inequity) and secondary outcomes (infant health influenced by equity/inequity during pregnancy). Data was analyzed deductively using the PROGRESS-Plus equity framework and by summative content analysis for equity reporting characteristics. The included articles were assessed for quality using the Risk of Bias Assessment Tool for Systematic Reviews.
The search identified 8065 articles and 236 underwent full-text screening. Of the 236, 68 systematic reviews were included with first authors representing 20 different countries. The population focus of included studies ranged across prenatal only ( n = 14), perinatal ( n = 25), maternal ( n = 2), maternal and child ( n = 19), and a general population ( n = 8). Barriers to equity in prenatal care included travel and financial burden, culturally insensitive practices that deterred care engagement and continuity, and discriminatory behaviour that reduced care access and satisfaction. Facilitators to achieve equity included innovations such as community health workers, home visitation programs, conditional cash transfer programs, virtual care, and cross-cultural training, to enhance patient experiences and increase their access to, and use of health services. There was overlap across PROGRESS-Plus factors.
Conclusions
This umbrella review collated inequities present in prenatal healthcare services, globally. Further, this synthesis contributes to future solution and action-oriented research and practice by assembling evidence-informed opportunities, innovations, and approaches that may foster equitable prenatal health services to all members of diverse communities.
Peer Review reports
Introduction
Timely, quality healthcare should be available and accessible to all individuals, and the policies that guide healthcare decision making should foster equitable care. However, globally, achieving this has proved to be challenging [ 1 , 2 , 3 ]. Broadly, equity is conceptualized as the absence of differences in healthcare access and use among population groups, and that all population groups can achieve the health outcomes of the most socially advantaged [ 4 , 5 ]. Prominently, evidence suggests that healthcare inequities disproportionately affect women’s, maternal, birthing individuals’ health, infant development, and family wellbeing [ 3 , 6 ].
Globally, major health organizations have categorized prenatal health as encompassing overall maternal health during pregnancy [ 3 , 7 ]. The prenatal period is defined as the time from conception of pregnancy up to delivery. Evidence suggests that suboptimal health outcomes during this life-stage stem from inequitable access to and subsequent engagement in prenatal care services [ 8 , 9 ]. Studies have identified that inadequate prenatal care can result in a higher risk of complications during and after pregnancy for the birthing individual and infant [ 10 , 11 , 12 , 13 , 14 , 15 , 16 ]. Our review focuses on the prenatal period as adequacy of care during this time can influence subsequent physiological and psychological experiences during birth and the postpartum period [ 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 ]. Inequities are rooted in systemic factors such as institutional racism, and social and economic inequities that influence one’s social determinants of health [ 18 ]. Common patient reported challenges include geographical proximity (e.g., rural and remote settings), communication barriers, financial barriers, lack of cultural safety, and a lack of known services. These challenges have been exacerbated during the COVID-19 pandemic [ 1 , 3 , 6 ].
Despite the merit of contemporary policies that strive to foster the conditions for health equity for all, inequities in maternal healthcare persist. For example, inequities can be observed in access to services such as consultation with a healthcare professional (i.e., general practitioner, obstetrician, gynecologist, midwife), timely prenatal screening, and prevention and early intervention for maternal mental health needs [ 1 , 3 ]. Complicating matters further, evidence suggests that individual practitioners’ interpretations of policies may contribute to variability in application of these guidelines resulting in inconsistent implementation of everyday practices with diverse populations [ 19 , 20 ]. As such, research aimed at addressing birthing individuals’ access to and use of prenatal healthcare is necessary at practice and policy levels, to ensure that care is both equitable and effective in improving the health of prenatal patients [ 1 , 3 , 21 ]. Prenatal healthcare services include care provided to a birthing individual, to prevent complications of pregnancy and to ensure the wellbeing of the birthing individual and infant following birth [ 17 , 22 , 23 ]. Examples of these services include, but are not limited to, visiting a healthcare professional or community health worker in person or through virtual care for a physical exam, a fetal ultrasound, prenatal genetic testing or screening, gestational diabetes screening, birth planning, nutrition, substance use and mental health consults [ 22 , 23 ].
The aim of this umbrella review was to identify and summarize practices within prenatal healthcare services as they relate to equity/inequity and explore barriers and facilitators of how equities/inequities influence the patient experience or health outcomes when accessing/using services, and to review how equity is reported. We intended to identify both qualitative and quantitative systematic reviews that investigated primary studies for practices in prenatal healthcare. This review provides an overarching scan of existing evidence of prenatal healthcare practices globally and a platform to critically discuss their contribution to reduce inequity present in prenatal healthcare, and plausible solutions to improve equity.
Umbrella review methodology
There has been an influx of systematic reviews on the topic of equity influencing access and use of prenatal care. It is becoming increasingly difficult for healthcare professionals, policy makers, and researchers to review the volume of evidence-generating literature to guide evidence-informed actions. An umbrella review (also termed “overview of reviews” or “review of reviews”) consolidates the content captured in systematic reviews and meta-analyses [ 24 , 25 , 26 , 27 , 28 ]. The umbrella review provides a solution by packaging mass information into a synthesized and focused document for decision-makers, including healthcare professionals and policy makers, to efficiently incorporate evidence into their own contexts [ 24 , 26 , 27 , 28 ]. Further, the umbrella review methodology allows us to capture the way in which equity is conceptualized and reported in the included studies [ 29 , 30 , 31 ]. There is still much variability in how equity is reported in systematic reviews; we used the recommended Campbell and Cochrane Collaboration’s PRISMA-Equity extension (Supplementary file 1 ) as a guide to encourage more standardized data extraction and reporting [ 31 ]. To ensure a thorough review of equity factors, we also used the PROGRESS-Plus equity framework to guide this work as it offers a comprehensive set of factors to consider as potential sources of inequity population-wide, and it is meant to complement the PRISMA-Equity extension [ 31 , 32 ].
The protocol for this umbrella review was registered with PROSPERO (CRD42022301574) [ 33 ]. Any changes to the protocol were documented and can be viewed online.
Eligibility criteria
The eligibility criteria for this review followed the PICOS (population, intervention, comparison, outcome, and study design) framework. Detailed inclusion and exclusion criteria are listed in Table 1 . A significant aspect of our inclusion criteria was that outcomes were required to include an explanation of how equity/inequity influenced prenatal patient experience or health outcomes; a factual/statistical relation/association was not sufficient to be included in this review. The rationale for this was the need to develop a greater understanding of the mechanisms involved that lead to equity/inequity in different contexts and how decision-makers can adapt them to improve health equity in prenatal care.
Search strategy and selection
A systematic search strategy was developed with support from two librarians (EM, JW) and was used to retrieve relevant systematic reviews and meta-analyses [ 34 , 35 ] from six electronic databases (Ovid: Medline, Embase, APA PsycInfo; EBSCO: CINAHL; ProQuest: International Bibliography of the Social Sciences; Cochrane Database of Systematic Reviews). Search terms included: prenatal, antenatal, prepartum, pregnancy, equity, and inequity ; truncations and variations were used where relevant. No limitations on date were used during the search. Hand searching of reference lists was also completed for all studies included in the review to ensure any systematic reviews that may have been missed from the systematic search, were included. The complete search strategy is available in Supplementary file 2 . The original search was performed in January 2022 and updated in August 2022.
Covidence is a web-based collaboration software platform that streamlines the production of systematic and other literature reviews [ 36 ] and was used to organize and carry out the screening process accurately. All identified articles were uploaded to Covidence and duplicates were removed. Title, abstract, and full text screening were completed independently, in duplicate, by two reviewers (ZL, NG, MOK, SS, AL, MH, QS); the second reviewer was always the lead author (ZL). Any conflicts were resolved through discussion. Tracking of included articles and reasons for excluded articles was done through Covidence and later recorded manually using a Microsoft Excel spreadsheet.
Data extraction, analysis, and synthesis
A Microsoft Excel spreadsheet was used for data extraction and was designed through an iterative process, and included revisions between two authors (ZL, NG). To pilot the data extraction sheet, three full text articles were extracted independently, in duplicate, by two authors (ZL, NG). Any conflicts were resolved through discussion and the data extraction sheet was adjusted and optimized as appropriate. After the data extraction sheet was finalized, each full text article was extracted independently by one reviewer (NG, SS, MH, AL, MOK, QS), and all extractions were reviewed for accuracy by the lead author (ZL).
Extracted data included publication characteristics (author, country, year of publication, journal, funding source, title), study characteristics (research question, population, intervention, comparators, study designs), equity reporting (definition, when and where equity is mentioned, equity related frameworks), primary outcomes, and secondary outcomes. The primary outcome included how an equity factor influenced the prenatal patient experience or health outcome, while the secondary outcome included how equity/inequity during pregnancy impacted subsequent infant/child health and development.
The primary and secondary outcomes were deductively analyzed and mapped to the PROGRESS-Plus framework as barriers and facilitators to equity in health services. PROGRESS refers to place of residence, race/ethnicity/culture/language, occupation, gender/sex, religion, education, socioeconomic status, and social capital. Plus refers to other equity factors not listed such as age [ 32 ]. Inductive themes were also generated. Deductive and inductive themes are illustrated through maximum variation to capture themes within and across the studies [ 37 ].
The primary objective to review equity reporting characteristics was analyzed by summative content analysis techniques [ 38 , 39 ]. The terms ‘equity’ and ‘inequity’ and their truncated equivalents (equit*, inequit*) were searched and counted in each included systematic review. Counts were analyzed using descriptive statistics in Microsoft Excel. Additionally, any equity/inequity definitions were sought and compared.
Quality assessment
Each included systematic review was assessed for quality by the lead author (ZL) using the Risk of Bias Assessment Tool for Systematic Reviews (ROBIS), which evaluates the risk of bias within systematic reviews [ 40 ]. The ROBIS includes four domains and a final overall review of risk of bias. The scoring of each domain was categorized into low, moderate, and high risk of bias. Specific scoring categories and scores for each included study can be seen in Supplementary file 3 .
The systematic search captured 8065 articles (Fig. 1 ). Of the 236 articles reviewed during secondary, full-text screening, 68 systematic reviews were included in this umbrella review (Fig. 1 ). A condensed summary of study characteristics and outcomes for each included review can be viewed in Supplementary file 4 and all excluded studies with reason for exclusion can be viewed in Supplementary file 5 . To address the aims of this review, we present findings in six categories: Study Characteristics; Study Foci, to identify and summarize practices within prenatal healthcare services; Impact of Equity/Inequity on Prenatal Care and Other Factors Impacting Access/Use of Prenatal Care, to explore barriers and facilitators of how equities/inequities influence the patient experience or health outcomes when accessing/using services; Equity Reporting Characteristics to review how equity is reported; and Quality Assessment.
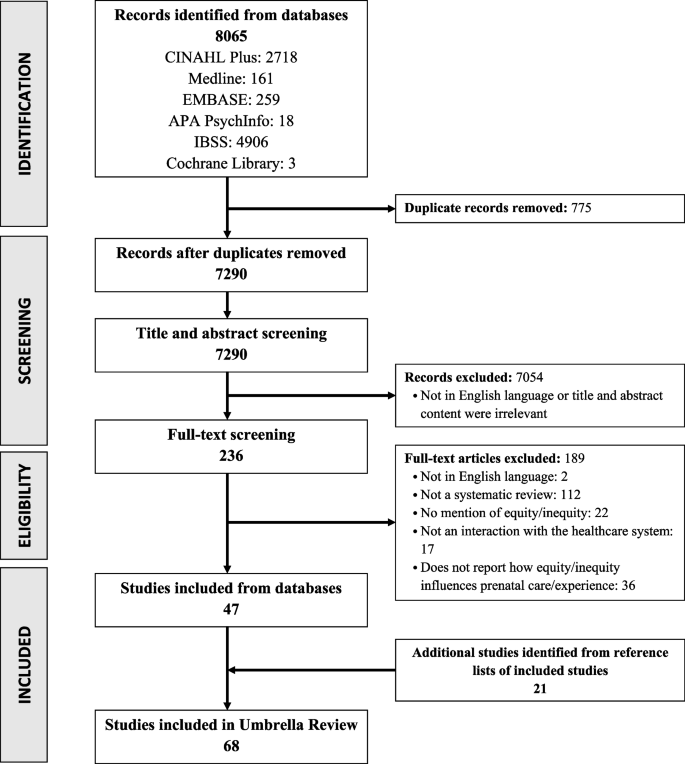
PRISMA flow diagram of literature search and selection process
Study characteristics
Of the 68 included systematic reviews, 13 were meta-analyses. The methodology of included studies within the reviews varied; 33 included mixed methods studies, 23 included quantitative studies only, 10 included qualitative studies only, and two reviews did not report their methods clearly. The majority of study first authors were from the United Kingdom ( n = 23), Australia ( n = 18), Canada ( n = 7), and United States of America ( n = 6). The studies analyzed within the included reviews were distributed across the globe (Fig. 2 , Supplemental file 4 ), with the largest proportion of studies from Africa ( n = 310), followed by Asia ( n = 225). Studies from Oceania ( n = 30) and South America ( n = 46) were the least represented. All included systematic reviews were published in or after 2003, with 15 published during or after the year 2020. Of these, four included analyses of studies during or after the year 2020 [ 41 , 42 , 43 , 44 ] (Fig. 3 ). Figure 3 also shows the distribution across time of the publication year of all studies within the included reviews, with the earliest being in 1976 and the most recent in 2021.
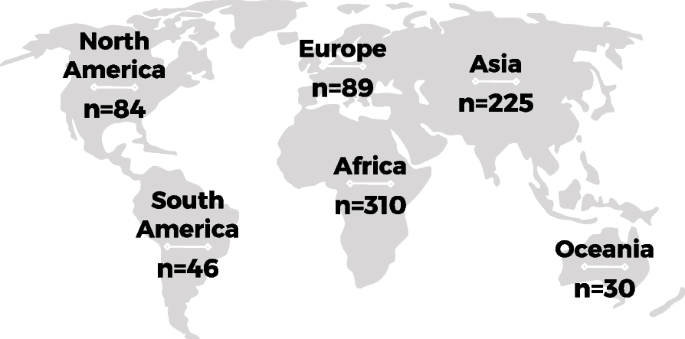
Global distribution of studies within included systematic reviews. Values represent the number of studies within the included systematic reviews that were published within the labeled continent. Studies not reported: Malqvist 2012, Jhaveri 2021
Publication timeline distribution of studies within included systematic reviews. Horizontal lines represent the publication year range from earliest to latest of studies within the included systematic reviews. Studies not reported: Malqvist 2012, Victoria 2012, Vanstone 2019, Jhaveri 2021
To identify and summarize practices within prenatal healthcare services, we reviewed the population and health service topic of focus for each included study. The included systematic reviews focused on various populations including prenatal only ( n = 14), perinatal ( n = 25), maternal ( n = 2), maternal and child ( n = 19), and a general unspecified population ( n = 8). The reviews also focused on a range of topics including prenatal healthcare services such as prenatal testing/screening, smoking cessation, mobile-health (mhealth)/virtual health, lay/community health workers (CHWs), and mental health, and other services associated with prenatal healthcare such as conditional cash transfer (CCT, i.e., income subsidies) and faith-based/community organizations. A condensed summary of healthcare services for each included review can be viewed in Supplementary file 4 .
Impact of equity/inequity on prenatal care
The included systematic reviews provided insight on how barriers and facilitators of equity/inequity in prenatal healthcare impacts the patient experience or health outcomes when accessing/using care, and subsequently infant and child health. Much of the data overlaps across PROGRESS-Plus factors, however, Tables 2 and 3 summarize the data into each of the factors. A condensed summary of equity related outcomes for each included review can be viewed in Supplementary file 4 .
Place of residence
Transportation was a challenge for individuals living in remote or rural areas globally leading to a lack of access and use of services and a greater chance of adverse pregnancy outcomes (e.g., maternal mortality and morbidities, preterm birth, low birth weight, stillbirth), especially during emergencies. However, there were many facilitators with the potential to reduce this challenge, such as resources brought directly to communities and patients, including CHWs and home visiting programs. Virtual care was a facilitator identified commonly in Asian and African countries along with CCT strategies which were useful in reducing transport fees. The mention of multipurpose healthcare professionals was only identified in one study which analyzed settings across Asia, Africa, and South America (Table 2 ) [ 53 ]. The positive impact of CHWs also extended to infant health as utilization of services increased because of the reduced transportation barrier (Table 3 ).
Race, ethnicity, culture, language and religion
For the purpose of this review, we combined two PROGRESS-Plus factors, Race/Ethnicity/Culture/Language and Religion as most of the relevant data was associated with all or most of these factors. There was evidence of prenatal patients encountering discrimination when accessing and receiving care, poor service and care quality if offered at all, stigmatizing behaviour, and a lack of cultural appreciation, which led to a greater risk of adverse outcomes and lower utilization of care. These experiences were mostly associated with those in North America, Europe, and Oceania, who were non-White/European, immigrants, unfamiliar with the common language or western medicine culture, and of minority religions. Much of the data speaks to patients feeling unsupported, devalued, and even fearful, and leads them to avoid accessing care all together. Globally, studies identified facilitators to achieving equity. This included CHWs to improve health education among minority individuals and virtual innovations to incorporate local languages. Many studies mentioned adapting healthcare services to meet patient expectations by incorporating cross-cultural training to reduce patient anxiety and increase a sense of cultural safety (Table 2 ). This adaptation and tailoring of innovations also reduced the incidence of adverse infant or child outcomes (e.g., neonatal mortality, neonatal morbidities, stunting) in Asian, African, and South American countries (Table 3 ).
The review captured information from South America, Asia, and Africa about unemployed patients and their partners booking late or fewer prenatal appointments, while employed individuals faced barriers in taking time off work due to financial constraints or for family obligations (Table 2 ). There were no facilitators of equity identified from the data.
Gender norms globally (i.e., women cannot travel alone, cannot make decisions, or they must stay home to take care of their children) contributed to delays or underuse of care because of powerlessness in decision-making processes. The underrepresentation of women in both healthcare (i.e., staff or healthcare professionals) and personal support systems (i.e., peers or family members) was found to deter some individuals from accessing prenatal health services across continents. Additionally, the lack of healthcare professionals’ knowledge or inclusivity of LGBTQ2S+ groups led to distressing experiences for patients in North America, Europe, and Oceania (Table 2 ). To overcome these barriers, studies explained the use of innovations that encouraged men and partners to support and promote the birthing individual’s autonomy which improved health education, care use, reduced adverse outcomes of pregnancy and infant health, improved newborn care, and improved maternal and infant nutrition (Tables 2 and 3 ). Home visitation programs in Asia, Africa, and South America were also useful in providing information to women who were disadvantaged by gender norms. In North America, Europe, and Oceania, the use of inclusive strategies (e.g., the use of gender-neutral pronouns) was mentioned to support LGBTQ2S+ patients in feeling comfortable and improve the patient experience (Table 2 ).
Lower levels of patient or partner education were associated with a lack of health education and led to delayed initiation or reduced use of care across the globe. A lack of health education was reported frequently as a cause for underutilization and adverse pregnancy outcomes (Table 2 ). Additionally, even with health education it was common for misinformation to be provided to patients, which increased the risk of adverse outcomes for patients and their newborns (Tables 2 and 3 ). CHWs, birth preparedness, and home visitation programs have been used to improve patient education and self-confidence which prevented adverse outcomes. Home-based records are paper or electronic documents that pregnant women and caregivers can use in the household to monitor their health and the health of their children [ 90 ]. Home-based records have also been implemented to improve health education and readiness during pregnancy and for newborn care (Tables 2 and 3 ).
Socioeconomic Status (SES)
Patients of low SES across the globe reported a reduced uptake of prenatal care because of stress surrounding loss of income, cost of services, and experiencing stigmatizing behaviour from healthcare professionals (Table 2 ). Innovations that overcame these barriers, including CCTs, reducing user fees, or public assistance programs, led to increased use of services by patients with strained financial status and improved health education, health outcomes during pregnancy, and health outcomes for newborns (Tables 2 and 3 ). These innovations also empowered patients to seek care in Asian and African settings. CHWs in South America, Asia, and Africa assisted by actively connecting patients of low SES to care during pregnancy (Table 2 ). Despite availability of innovations including CCT and CHWs, financially secure populations were prioritized over populations with lower SES, but the motivation and rationale for this was not included in the reviews (Table 2 ).
Social capital
Social capital barriers across the globe included personal/family priorities and lack of family support that may conflict with accessing care. Not knowing a health professional directly or limited personal networks were reported as factors leading to reduced opportunities to access prenatal healthcare services in Asia, Africa, and South America (Table 2 ). In similar settings, faith-based and community organizations have been successful in improving access to care for those that may be socially reserved or excluded; they increased referrals, improved prenatal attendance, and improved health outcomes (Tables 2 and 3 ). These organizations were more successful when families were involved. In general, the findings indicate that in-person or virtual innovations encouraging significant relationships and psychosocial support improved pregnancy and infant health outcomes (Tables 2 and 3 ).
Patients older or younger than the average reproductive age (i.e., 15–49 years) had different experiences during pregnancy in Asia and Europe. In some Asian cultures, older age was associated with greater authority if patients had previous experience with pregnancy, while younger aged patients received biased treatment. In Africa, Electronic health innovations (e.g., virtual health, mobile innovations) have been helpful in facilitating patient retention for those that were under 18 years of age (Table 2 ).
Other Factors Impacting Access/Use of Prenatal Care
Across the PROGRESS-Plus factors, this study identified integrated themes that impacted access and use of prenatal care, that may or may not have been influenced by equity. Integrated themes include adequate prenatal care, patient-centred care, team-based care, continuity of care, multiple innovations, privacy and confidentiality, healthcare professionals’ assumptions, health system challenges, and care not benefiting the most in need when interventions are spread and scaled. The concept of adequate or inadequate prenatal care was mentioned in included studies which spanned analysis across all continents [ 45 , 67 , 71 , 104 ], but definitions of ‘adequate’ varied or were not defined at all. Patient-centred care globally took the form of healthcare professionals’ attitudes, behaviours, and targeted care. All of which influenced whether patients would seek care or be satisfied with the care they received [ 42 , 43 , 44 , 45 , 46 , 49 , 51 , 52 , 60 , 61 , 71 , 72 , 73 , 74 , 77 , 78 , 79 , 81 , 82 , 88 , 89 , 91 , 108 ]. Team-based or interprofessional care was a common theme across studies that included North American, European, and Australian settings; many explained how shared care increased quality and use of services and enhanced comprehensive care for patients [ 61 , 67 ]. Continuity of care was also a recurring theme across continents, predominantly in North American, European, and Australian settings; it was important for patients to know that their healthcare professionals understood their journey, which further built a meaningful relationship [ 43 , 49 , 73 , 74 ]. The approach of using multiple interventions to achieve equity was successful in studies across the globe, with an emphasis in African, Asian, and South American contexts, to ensure that patients received support from different avenues, as equity is complex and it is likely that more than one factor influenced their care [ 56 , 59 , 62 , 63 , 103 , 108 ]. Privacy and confidentiality were also brought up as concerns in the data, specifically for electronic health innovations. Patients were uncertain of how their health data was stored and used; this was also influenced by technological literacy and was identified in studies that included countries across all continents [ 78 , 91 ].
Stereotyped inequities of patients related to culture, religion, or ethnicity were included as barriers related to healthcare professionals’ assumptions, which led to unfair treatment in the United Kingdom. Examples of assumptions included that Muslim individuals did not want prenatal care or some cultures would be against terminating an affected pregnancy and hence these populations were less likely to be offered services including prenatal screening [ 66 ]. Studies with analyses predominantly in South America, Asia, and Africa acknowledged healthcare system challenges that increased opportunity for inequity including capacity burdens of health facilities and overworked healthcare professionals that led to deterioration in service quality [ 47 , 49 , 50 , 52 , 54 , 72 , 82 , 99 ]. A consistent theme across the data, and most common in studies that included Asian and African countries, was the notion that interventions that attempted to overcome health inequities were not effective in reaching marginalized populations. Within this theme, studies suggested an increased need to explore implementation and evaluation characteristics to uncover how to better target innovations in different contexts, for patients with different circumstances, to ensure successful spread and scale and to avoid further contribution to equity gaps [ 47 , 54 , 60 , 75 , 90 , 92 , 93 , 94 , 96 , 97 , 101 ].
Equity reporting characteristics
Equity reporting characteristics of the included reviews were assessed based on the use and frequency of the term equity or inequity, or truncated equivalents (Table 4 ). On average, included reviews mentioned the terms equity or inequity 11.9 times in their articles, with 120 being the greatest and one being the least frequent, which depicts the variation in the significance of the use of the terms. The mode presented as two mentions of the terms across all included studies. Only seven of the studies included equity/inequity as part of their article title, and 36 included it in their abstracts. The majority of equity/inequity counts were identified in the discussion section of the papers. When exploring the use of the terms in the entirety of the reviews, 19 articles mentioned equity/inequity only in the introduction, discussion, and/or conclusion sections, while only nine used the term in all sections. We also explored whether reviews defined equity/inequity or health equity/inequity and only five of the 68 included reviews provided definitions [ 46 , 47 , 79 , 89 , 94 ]. From the included studies, 51 were published on or after 2013, and of these, only three used the PRISMA-Equity 2012 checklist to guide their review [ 46 , 47 , 70 ]. Other frameworks related to equity that were a part of the reviews included PROGRESS-Plus [ 46 , 47 , 103 , 109 ], an Indigenous Māori analytical framework [ 64 ], the Access to Care Framework [ 82 ], and the Stigma Action Framework [ 43 ].
The ROBIS quality appraisal tool was used to assess the included systematic reviews. Majority of the included reviews presented with low to moderate risk of bias. In domain 1 (eligibility criteria), four reviews showed high risk of bias. Two studies showed a high risk of bias for domain 2 (identification and selection) and 12 studies showed a high risk of bias in data collection and appraisal (domain 3). Synthesis and findings (domain 4) and the final overall review of risk of bias only included one article in each with a high risk of bias. The specific scoring of each domain for each included systematic review can be seen in Supplementary file 3 .
This umbrella review identified and summarized practices within prenatal healthcare services as they related to equity/inequity, consolidated barriers and facilitators of equity/inequity factors and summarized how these factors influence the prenatal patient experience or health outcomes when accessing/using health services, globally. The included studies represent 20 different countries. In addition to reporting on types and reasons for inequities as described in the included studies, this review consolidates practices that are suggested to facilitate the conditions necessary for health equity in prenatal care (e.g., CHWs, home visitation programs, CCT programs, virtual care options, and cross-cultural training). Additionally, this review explored how equity is presented in each of the systematic reviews, and if the authors of each review provided a working definition or conceptualization of the term.
Our study aligns with recent literature highlighting how inequities lead to suboptimal healthcare for prenatal patients [ 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 ]. For example, studies investigating access and uptake of prenatal screening services in Canada and New Zealand have identified similar challenges for patients in navigating services. This includes cost of services, remote living, low maternal age, being an ethnic minority, or having a recent immigrant status [ 110 , 121 ]. In both countries, coverage of basic prenatal screening services is publicly insured for residents [ 110 , 121 ]. Comparable to our findings, patients lacked knowledge and awareness of available services which was an inherent barrier of accessing care [ 110 , 121 ]. Disparities in prenatal healthcare have been reported in urban areas in Southern Brazil and rural areas in China, such as the inadequate use and uptake of prenatal supplements (e.g., folic acid or iron) to support the health and development of birthing individuals and their fetus [ 112 , 113 , 118 ]. Indeed, the study in China by Liu et al. reported that despite government recommendations, there was a barrier to uptake of prenatal supplements by pregnant women that had lower levels of education, were an ethnic minority, or were unemployed [ 112 ]. A study by Yaya et al. conducted in rural areas of Nigeria uncovered the challenge of gender inequality in accessing healthcare services. Similar to our review, they identified the cultural norm of women having less decision-making power in a relationship and therefore were restricted in accessing quality care by their partner, usually identified as a man [ 116 ]. Our review identified that patient-centred care influenced patients’ satisfaction with the care they received. Complementing this finding, a recent study by King et al. found that education and a non-white ethnicity were inversely related to the perceived quality of patient-centred care in a cohort of prenatal patients at a provincial health centre in Canada [ 122 ].
This umbrella review also identified facilitators to health equity that led to a greater perceived quality of prenatal healthcare. Although these findings are not as common, recent literature has identified strategies towards achieving health equity in prenatal care [ 123 , 124 ]. An established prenatal care program in Mexico, a low-middle income country, which targets populations from rural areas with low SES used shared-care between general practitioners, obstetricians, and other specialized health professionals, to ensure a multidisciplinary approach to care, similar to the team-based findings from our review [ 123 ]. In the United States of America, a high income country, a study evaluated the effect of trauma-informed care for adolescents receiving prenatal care services at an established adolescent maternity program and found that this strategy led to equitable pregnancy outcomes across racial and ethnic groups, which is comparable to our findings of cross-cultural training for professionals as a strategy to reduce patient anxiety [ 124 ].
A gap in our findings was the association between equity/inequity and the implementation climate of practices, which is important to consider for longevity and sustainability of equitable practices [ 125 ]. Implementation climate is defined by the surrounding context of where an intervention is to be incorporated; this can include the people, the physical environment, or social or cultural norms [ 126 , 127 ]. Cultural norms of the implementation climate should be a priori of consideration when establishing how to implement a practice and how inequities may play a role. For example, research from China, Nigeria, and South Africa have investigated the SES of different regions and how this affected the adoption of prenatal healthcare services. The studies depicted that generalizability, spread and scale, are not always possible [ 111 , 112 , 128 ]. Interestingly, Linhares et al. found an inverse inequality distribution where supplements had a greater uptake in urban Southern Brazilian prenatal populations of low income or education level, which depicts how context matters [ 113 ]. A recent United States ethnography study of the clinical environment in prenatal care discussed the difference in site specific factors for care that led to differing perspectives of service by patients and healthcare professionals. For example, waiting time was a great disruption in the patient journey. Those who were of low SES, non-white, often of immigrant status or non-English speaking were expected to accommodate their own schedules to the demands of health service centres [ 115 ]. A timely example of an implementation climate which influences equitable access to prenatal care is the COVID-19 pandemic. During the pandemic, healthcare services related to obstetrics and gynecology were overlooked, leading to an increase in prenatal morbidity, mortality, and an overall decline in wellbeing [ 6 , 129 , 130 ]. There is limited data in the literature to explain the effects of inequity on access and use of care for this population during and since the COVID-19 pandemic [ 131 ]. This umbrella review provides a global perspective of how equity/inequity may influence prenatal care; it is important to consider how the context and implementation climate of different countries plays a role in this influence.
As part of the review, we also examined how equity was reported. Surprisingly, the majority of studies did not define equity and none defined inequity, which adds to the confusion of the use of the terms and how they may be perceived by different researchers and decision-makers [ 29 , 30 ]. The studies that did define equity [ 46 , 47 , 79 , 89 , 94 ] were quite consistent; they each mentioned terminology surrounding the inclusion of every person or population including those that are vulnerable or disadvantaged, and the necessity of healthcare to be fair. The inconsistency of definitions became apparent when discussing what constructs were recognized as factors of equity and the spectrum from inequity to equity. Most of the included studies did not use an equity related framework to guide their methods which contributes to the variability in reporting. A relevant study by Hartwell and colleagues from 2022 explored equity reporting characteristics of systematic reviews and meta-analyses that focused on the COVID-19 pandemic, and maternal and childbirth outcomes [ 131 ]. This study also used PROGRESS-Plus as a guiding framework and only identified factual relations between outcomes and equity factors. Our umbrella review presents data prior to and during the pandemic, and narrows its focus to the prenatal population to enhance the specificity of how equity influences care in this population.
Limitations
There are limitations to this umbrella review. We limited our inclusion criteria to English language studies only, which was a decision made due to resource constraints. Prior studies have identified that this limitation does not lead to significant bias within medical research [ 132 ]. We also limited our inclusion criteria to studies that mentioned equit*/inequit* because Cochrane’s PRISMA-Equity checklist identifies ‘equity’ in the title as a category [ 31 ]. We extended this category to anywhere in the article. Articles that discuss equity without using the term explicitly may have been missed. As we only identified five explicit definitions, this means that there could still be much discrepancy of the use of the term ‘equity’ or ‘inequity’ in healthcare and research, adding to the challenge of effective goal setting and action in health systems change [ 29 , 30 ]. Further, our analysis of these terms did not separate equity and inequity; the combined analysis may impede the clarity of which of these terms were featured more or less in the studies.
We used a maximum variation technique in our analysis to ensure we captured patterns that emerged within and across the studies, which presented great heterogeneity in context and settings. With this technique, we did not correlate themes specific to context, rather across them. To overcome this barrier, we have provided details on countries which the included studies analyzed to provide insight into context relevant to our data (Supplemental file 4 ). Additionally, a challenge we faced was extracting data specific to the prenatal period as many of our studies ranged from prenatal specific populations to general unspecified populations and maternal healthcare more broadly. During data extraction, we ensured to only extract data that was relevant to pregnancy before delivery. Data extracted must have identified the population of focus as prenatal. When this was not possible, we did include data that was applicable across the perinatal period, from conception to following birth, which still included a prenatal population. We treated this data in the same way, although Supplementary file 4 does identify which systematic reviews have a prenatal only population.
In this umbrella review, we explored reported barriers and facilitators to health equity/inequity across the globe and their impact on prenatal care and subsequently infant health and development. The review highlights how equity/inequity influences prenatal patients’ access and use of care within prenatal healthcare practices and collates potential solutions to gaps in health equity for this population. The findings highly overlapped across PROGRESS-Plus equity factors and the barriers and facilitators that we identified are likely much more complex and intertwined [ 133 ]. This study adds value to the literature as it shows how current innovations, some of which are common across the globe, are utilized to overcome barriers to achieving equity. The data also speaks to how barriers and potential facilitators or solutions are common across countries. Decision-makers and knowledge-users from across the globe, including healthcare professionals, healthcare administrators, and policy-makers, can apply these findings in their own contexts to improve equity in the access and use of prenatal healthcare services.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
Conditional cash transfer
Community health worker
Coronavirus disease of 2019
Lesbian, Gay, Bisexual, Transgender, Queer or Questioning, Two-Spirit, Plus
Mobile-health
Preferred Reporting Items for Systematic Reviews and Meta-Analyses—Equity
Place of residence, Race/ethnicity/culture/language, Occupation, Gender/sex, Religion, Education, Socioeconomic status, Social capital, Plus
Risk of Bias Assessment Tool for Systematic Reviews
Socioeconomic status
Higginbottom GM, Safipour J, Yohani S, O’Brien B, Mumtaz Z, Paton P, et al. An ethnographic investigation of the maternity healthcare experience of immigrants in rural and urban Alberta, Canada. BMC Pregnancy Childbirth. 2016;16(1):20.
Article PubMed PubMed Central Google Scholar
Fernandez Turienzo C, Newburn M, Agyepong A, Buabeng R, Dignam A, Abe C, et al. Addressing inequities in maternal health among women living in communities of social disadvantage and ethnic diversity. BMC Public Health. 2021;21(1):1–5.
Article Google Scholar
Sharma S, Kolahdooz F, Launier K, Nader F, June Yi K, Baker P, et al. Canadian Indigenous womens perspectives of maternal health and health care services: a systematic review. Divers Equal Health Care. 2016;13(5):335.
Kringos DS, Boerma WG, Bourgueil Y, Cartier T, Hasvold T, Hutchinson A, et al. The European primary care monitor: structure, process and outcome indicators. BMC Fam Pract. 2010;11:1–8.
England N, Improvement N. Equity and equality: guidance for local maternity systems. 2021.
Davenport MH, Meyer S, Meah VL, Strynadka MC, Khurana R. Moms are not OK: COVID-19 and maternal mental health. Front Glob Womens Health. 2020;1:1.
WHO. Health topics: maternal health. 2021. Available from: https://www.who.int/health-topics/maternal-health#tab=tab_1 . [cited 2021 Dec 15].
Buultjens M, Farouque A, Karimi L, Whitby L, Milgrom J, Erbas B. The contribution of group prenatal care to maternal psychological health outcomes: a systematic review. Women and Birth. 2021;34:e631-42. Elsevier B.V.
Article PubMed Google Scholar
Yan J. The effects of prenatal care utilization on maternal health and health behaviors. Health Econ. 2017;26(8):1001–18.
McDonald S, Kehler H, Bayrampour H, Fraser-Lee N, Tough S. Risk and protective factors in early child development: results from the All Our Babies (AOB) pregnancy cohort. Res Dev Disabil. 2016;58:20–30.
Petitclerc A, Richard, Tremblay E. Childhood disruptive behaviour disorders: review of their origin, development, and prevention. Can J Psychiatry. 2009;54(4):222–31.
Berry OO, Tobón AL, Wanjikũ, Njoroge FM. Social determinants of health: the impact of racism on early childhood mental health. Curr Psychiatry Rep. 1920;23(23):1–10. https://doi.org/10.1007/s11920-021-01240-0 .
Enns JE, Nickel NC, Chartier M, Chateau D, Campbell R, Phillips-Beck W, et al. An unconditional prenatal income supplement is associated with improved birth and early childhood outcomes among First Nations children in Manitoba, Canada: a population-based cohort study. BMC Pregnancy Childbirth. 2021;21(1):1.
Puthussery S. Perinatal outcomes among migrant mothers in the United Kingdom: is it a matter of biology, behaviour, policy, social determinants or access to health care? Best Pract Res Clin Obstet Gynaecol. 2016;32:39–49.
Evans A, Ray JG, Austin PC, Lu H, Gandhi S, Guttmann A. Receipt of adequate prenatal care for privately sponsored versus government-assisted refugees in Ontario, Canada: a population-based cohort study. CMAJ. 2023;195(13):E469–78.
Varner CE, Park AL, Ray JG. Maternal emergency department use before pregnancy and infant emergency department use after birth. JAMA Netw Open. 2023;6(3):e232931.
Dragonas T, Christodoulou GN. Prenatal Care. Clin Psychol Rev. 1998;18(2):127–42.
Article CAS PubMed Google Scholar
Crear-Perry J, Correa-De-Araujo R, Lewis Johnson T, Mclemore MR, Neilson E, Wallace M. Social and structural determinants of health inequities in maternal health. J Womens Health. 2021;30(2):230–5.
Langell JT. Evidence-based medicine: a data-driven approach to lean healthcare operations. Int J Healthc Manag. 2021;14(1):226–9.
Michaels JA. Potential for epistemic injustice in evidence-based healthcare policy and guidance. J Med Ethics. 2021;47:417–22. BMJ Publishing Group.
Buxton M, Hanney S. How can payback from health services research be assessed? The strategic importance of assessing payback. J Health Serv Res. 1996;1:35–43.
Article CAS Google Scholar
Public Health Agency of Canada. Government of Canada. 2019. Family-centred maternity and newborn care: National guidelines. Available from: https://www.canada.ca/en/public-health/services/publications/healthy-living/maternity-newborn-care-guidelines-chapter-3.html . [cited 2022 Jan 27].
Shmerling A, Hoss M, Malam N, Staton EW, Lyon C. Prenatal care via telehealth. Prim Care Clin Office Pract. 2022;49:609–19. W.B. Saunders.
Booth A. EVIDENT guidance for reviewing the evidence: a compendium of methodological literature and websites. University of Sheffield. 2016. Available from: www.academia.edu/21598179/EVIDENT_Guidance_for_Reviewing_the_Evidence_a_compendium_of_methodological_literature_and_websites . [cited 2024 Mar 6].
Tsagris M, Fragkos KC. Umbrella reviews, overviews of reviews, and meta-epidemiologic studies: similarities and differences. In: Umbrella reviews: evidence synthesis with overviews of reviews and meta-epidemiologic studies. Switzerland: Springer International Publishing; 2016. p. 43–54.
Aromataris E, Fernandez RS, Godfrey C, Holly C, Khalil H. Methodology for JBI umbrella reviews. 2014.
Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inform Lib J. 2009;26:91–108.
Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132–40.
Braveman P, Gruskin S. Defining equity in health. J Epidemiol Community Health. 2003;57(4):254–8.
Article CAS PubMed PubMed Central Google Scholar
Braveman P, Arkin E, Orleans T, Proctor D, Acker J, Plough A. What is health equity? Behav Sci Policy. 2018;4:1–4.
Welch V, Petticrew M, Tugwell P, Moher D, O’Neill J, Waters E, et al. PRISMA-equity 2012 extension: reporting guidelines for systematic reviews with a focus on health equity. PLoS Med. 2012;9(10):e1001333.
O’Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: Using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol. 2014;67(1):56–64.
Ladak Z, Grewal N, Small S, Hemani M, Leber A, Kim O, et al. PROSPERO International prospective register of systematic reviews Citation. 2022. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022301574 .
Montori VM, Wilczynski NL, Morgan D, Haynes RB. Optimal search strategies for retrieving systematic reviews from Medline: analytical survey. Br Med J. 2005;330:68–71.
Wilczynski NL, Haynes RB. EMBASE search strategies achieved high sensitivity and specificity for retrieving methodologically sound systematic reviews. J Clin Epidemiol. 2007;60(1):29–33.
Veritas Health Innovation, Melbourne, Australia. Covidence systematic review software. Available from: www.covidence.org . [cited 2023 Nov 16].
Patton MQ. Designing Qualitative Studies. In: Qualitative research & evaluation methods: Integrating theory and practice. Sage publications. 2014;(4):244–326
Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88.
Zhang Y, Wildemuth BM. Qualitative analysis of content. Hum Brain Mapp. 2005;30(7):2197–206.
Google Scholar
Whiting P, Savović J, Higgins JPT, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–34.
Moncrieff G, Finlayson K, Cordey S, McCrimmon R, Harris C, Barreix M, et al. First and second trimester ultrasound in pregnancy: a systematic review and metasynthesis of the views and experiences of pregnant women, partners, and health workers. PLoS One. 2021;16:e0261096. Public Library of Science.
Kirubarajan A, Barker LC, Leung S, Ross LE, Zaheer J, Park B, et al. LGBTQ2S+ childbearing individuals and perinatal mental health: a systematic review. BJOG. 2022;129:1630–43. John Wiley and Sons Inc.
Lyall V, Wolfson L, Reid N, Poole N, Moritz KM, Egert S, et al. “The problem is that we hear a bit of everything … ”: a qualitative systematic review of factors associated with alcohol use, reduction, and abstinence in pregnancy. Int J Environ Res Public Health. 2021;18:3445. MDPI AG.
Toh RKC, Shorey S. Experiences and needs of women from ethnic minorities in maternity healthcare: a qualitative systematic review and meta-aggregation. Women Birth. 2023;36:30–8. Elsevier B.V.
Murray SF, Hunter Msc BM, Bisht R, Ensor T, Bick D. Demand-side financing measures to increase maternal health service utilisation and improve health outcomes: a systematic review of evidence from low-and middle-income countries Executive summary Background. JBI Libr Syst Rev. 2012;10(58):4165–567.
PubMed Google Scholar
McCollum R, Gomez W, Theobald S, Taegtmeyer M. How equitable are community health worker programmes and which programme features influence equity of community health worker services? A systematic review. BMC Public Health. 2016;16:1–6. BioMed Central Ltd.
Blanchard AK, Prost A, Houweling TAJ. Effects of community health worker interventions on socioeconomic inequities in maternal and newborn health in low-income and middle-income countries: a mixed-methods systematic review. BMJ Glob Health. 2019;4(3):e001308.
Banke-Thomas A, Wright K, Collins L. Assessing geographical distribution and accessibility of emergency obstetric care in sub- Saharan Africa: a systematic review. J Glob Health. 2019;9(1):010414.
Watson H, Harrop D, Walton E, Young A, Soltani H. A systematic review of ethnic minority women’s experiences of perinatal mental health conditions and services in Europe. PLoS One. 2019;14(1):e0210587.
Dahab R, Sakellariou D. Barriers to accessing maternal care in low income countries in Africa: a systematic review. Int J Environ Res Public Health. 2020;17:1–17. MDPI AG.
Chando S, Tong A, Howell M, Dickson M, Craig JC, DeLacy J, et al. Stakeholder perspectives on the implementation and impact of Indigenous health interventions: a systematic review of qualitative studies. Health Expect. 2021;24:731–43. Blackwell Publishing Ltd.
Sidze EM, Wekesah FM, Kisia L, Abajobir A. Inequalities in access and utilization of maternal, newborn and child health services in sub-Saharan Africa: a special focus on urban settings. Matern Child Health J. 2022;26:250–79. Springer.
Say L, Raine R. A systematic review of inequalities in the use of maternal health care in developing countries: examining the scale of the problem and the importance of context. Bull World Health Org. 2007;85:812–9.
Lee ACC, Lawn JE, Cousens S, Kumar V, Osrin D, Bhutta ZA, et al. Linking families and facilities for care at birth: what works to avert intrapartum-related deaths? Int J Gynecol Obstet. 2009;107:S65–88. John Wiley and Sons Ltd.
Lewin S, Munabi-Babigumira S, Glenton C, Daniels K, Bosch-Capblanch X, van Wyk BE, et al. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database Syst Rev. 2010;2010(3):CD004015.
Byrne A, Hodge A, Jimenez-Soto E, Morgan A. What works? Strategies to increase reproductive, maternal and child health in difficult to access mountainous locations: a systematic literature review. PLoS One. 2014;9:e87683.
Article ADS PubMed PubMed Central Google Scholar
Gopalan SS, Mutasa R, Friedman J, Das A. Health sector demand-side financial incentives in low- and middle-income countries: a systematic review on demand- and supply-side effects. Soc Sci Med. 2014;100:72–83.
Hunter BM, Murray SF. Demand-side financing for maternal and newborn health: What do we know about factors that affect implementation of cash transfers and voucher programmes? BMC Pregnancy Childbirth. 2017;17(1):262.
Wekesah FM, Mbada CE, Muula AS, Kabiru CW, Muthuri SK, Izugbara CO. Effective non-drug interventions for improving outcomes and quality of maternal health care in sub-Saharan Africa: a systematic review. Syst Rev. 2016;5(1):1–8.
Palmer MJ, Henschke N, Bergman H, Villanueva G, Maayan N, Tamrat T, et al. Targeted client communication via mobile devices for improving maternal, neonatal, and child health. Cochrane Database Syst Rev. 2020;2020(8):CD013679.
Hollowell J, Oakley L, Kurinczuk JJ, Brocklehurst P, Gray R. The effectiveness of antenatal care programmes to reduce infant mortality and preterm birth in socially disadvantaged and vulnerable women in high-income countries: a systematic review. BMC Pregnancy Childbirth. 2011;11:13.
Mcarthur A, Lockwood C. Maternal mortality in Cambodia, Thailand, Malaysia and Sri Lanka: a systematic review of local and national policy and practice initiatives Executive summary Background. JBI Database Syst Rev Implement Rep. 2013;11:1–10. JBL000409.
Lassi ZS, Middleton PF, Bhutta ZA, Crowther C. Strategies for improving health care seeking for maternal and newborn illnesses in low- and middle-income countries: a systematic review and meta-analysis. Global Health Act. 2016;9:31408. Co-Action Publishing.
Walker RC, Graham A, Palmer SC, Jagroop A, Tipene-Leach DC. Understanding the experiences, perspectives and values of indigenous women around smoking cessation in pregnancy: systematic review and thematic synthesis of qualitative studies. Int J Equity Health. 2019;18:744. BioMed Central Ltd.
Rowe RE, Garcia J. Social class, ethnicity and attendance for antenatal care in the United Kingdom: a systematic review. J Public Health Med. 2003;25:113–9.
Rowe RE, Garcia J, Davidson LL. Social and ethnic inequalities in the offer and uptake of prenatal screening and diagnosis in the UK: a systematic review. Public Health. 2004;118:177–89.
Feijen-De Jong EI, Jansen DE, Baarveld F, Van Der Schans CP, Schellevis FG, Reijneveld SA. Determinants of late and/or inadequate use of prenatal healthcare in high-income countries: a systematic review. Eur J Public Health. 2012;22:904–13.
Yu J. A systematic review of issues around antenatal screening and prenatal diagnostic testing for genetic disorders: women of Asian origin in western countries. Health Soc Care Commun. 2012;20:329–46.
Mutambudzi M, Meyer JD, Reisine S, Warren N. A review of recent literature on materialist and psychosocial models for racial and ethnic disparities in birth outcomes in the US, 2000–2014. Ethn Health. 2017;22(3):311–32.
Prady SL, Endacott C, Dickerson J, Bywater TJ, Blower SL. Inequalities in the identification and management of common mental disorders in the perinatal period: an equity focused reanalysis of a systematic review. Public Lib Sci. 2021;16:329–46. Public Library of Science.
Balaam MC, Akerjordet K, Lyberg A, Kaiser B, Schoening E, Fredriksen AM, et al. A qualitative review of migrant women’s perceptions of their needs and experiences related to pregnancy and childbirth. J Adv Nurs. 2013;69:1919–30.
Knight HE, Self A, Kennedy SH. Why are women dying when they reach hospital on time? A systematic review of the “Third Delay.” PLoS One. 2013;8(5):e63846.
Article ADS CAS PubMed PubMed Central Google Scholar
Small R, Roth C, Raval M, Shafiei M, Korfker D, Heaman M, et al. Immigrant and non-immigrant women’s experiences of maternity care: a systematic and comparative review of studies in five countries. BMC Pregnancy Childbirth. 2014;14:152.
Higginbottom GMA, Evans C, Morgan M, Bharj KK, Eldridge J, Hussain B. Experience of and access to maternity care in the UK by immigrant women: a narrative synthesis systematic review. BMJ Open. 2019;9:e029478. BMJ Publishing Group.
Fair F, Raben L, Watson H, Vivilaki V, van den Muijsenbergh M, Soltani H. Migrant women’s experiences of pregnancy, childbirth and maternity care in European countries: a systematic review. PLoS One. 2020;15:e0228378. Public Library of Science.
Firdous T, Darwin Z, Hassan SM. Muslim women’s experiences of maternity services in the UK: qualitative systematic review and thematic synthesis. BMC Pregnancy Childbirth. 2020;20(1):115.
Kyei-Nimakoh M, Carolan-Olah M, McCann TV. Access barriers to obstetric care at health facilities in sub-Saharan Africa-a systematic review. Syst Rev. 2017;6(1):110.
Ames HMR, Glenton C, Lewin S, Tamrat T, Akama E, Leon N. Clients’ perceptions and experiences of targeted digital communication accessible via mobile devices for reproductive, maternal, newborn, child, and adolescent health: a qualitative evidence synthesis. Cochrane Database Syst Rev. 2019;2019:CD013447. John Wiley and Sons Ltd.
PubMed Central Google Scholar
Målqvist M, Hoa DTP, Thomsen S. Causes and determinants of inequity in maternal and child health in Vietnam. BMC Public Health. 2012;12:641.
De Maio FG. Immigration as pathogenic: a systematic review of the health of immigrants to Canada. Int J Equity Health. 2010;9:27.
Ag Ahmed MA, Gagnon MP, Hamelin-Brabant L, Mbemba GIC, Alami H. A mixed methods systematic review of success factors of mhealth and telehealth for maternal health in Sub-Saharan Africa. Mhealth. 2017;3:22.
Byrne A, Hodge A, Jimenez-Soto E, Morgan A. Looking beyond supply: A systematic literature review of demand-side barriers to health service utilization in the mountains of Nepal. Asia Pac J Public Health. 2013;25:438–51. SAGE Publications Inc.
Muralidharan A, Fehringer J, Pappa S, Rottach E, Das M, Mandal M. Transforming gender norms, roles, and power dynamics for better health: evidence from a systematic review of gender-integrated health programs in low- and middle-income countries. Washington: United States Agency for International Development USAID; 2015.
Benova L, Campbell OMR, Ploubidis GB. Socio-economic gradients in maternal and child health-seeking behaviours in Egypt: systematic literature review and evidence synthesis. PLoS One. 2014;9(3):e93032.
Mengist B, Desta M, Tura AK, Habtewold TD, Abajobir A. Maternal near miss in Ethiopia: protective role of antenatal care and disparity in socioeconomic inequities: a systematic review and meta-analysis. Int J Afr Nurs Sci. 2021;15:100332. Elsevier Ltd.
Darmstadt GL, Lee ACC, Cousens S, Sibley L, Bhutta ZA, Donnay F, et al. 60 Million non-facility births: who can deliver in community settings to reduce intrapartum-related deaths? Int J Gynecol Obstet. 2009;107:S89–112. John Wiley and Sons Ltd.
Ehiri JE, Gunn JKL, Center KE, Li Y, Rouhani M, Ezeanolue EE. Training and deployment of lay refugee/internally displaced persons to provide basic health services in camps: a systematic review. Global Health Act. 2014;7:23902. Co-Action Publishing.
Bovill M, Chamberlain C, Bar-Zeev Y, Gruppetta M, Gould GS. Ngu - Ng - gi - La - nha (to exchange) knowledge. How is Aboriginal and Torres Strait Islander people’s empowerment being upheld and reported in smoking cessation interventions during pregnancy: a systematic review. Austr J Prim Health. 2019;25:395–401. CSIRO.
Beatson R, Molloy C, Perini N, Harrop C, Goldfeld S. Systematic review: an exploration of core componentry characterizing effective sustained nurse home visiting programs. J Adv Nurs. 2021;77:2581–94. Blackwell Publishing Ltd.
Magwood O, Kpadé V, Thavorn K, Oliver S, Mayhew AD, Pottie K. Effectiveness of home-based records on maternal, newborn and child health outcomes: a systematic review and meta-analysis. PLoS One. 2019;14(1):e0209278.
Magwood O, Kpadé V, Afza R, Oraka C, McWhirter J, Oliver S, et al. Understanding women’s, caregivers’, and providers’ experiences with home-based records: a systematic review of qualitative studies. PLoS One. 2019;13:e0204966. Public Library of Science.
Çalışkan Z, Kılıç D, Öztürk S, Atılgan E. Equity in maternal health care service utilization: a systematic review for developing countries. Int J Public Health. 2015;60:815–25. Birkhauser Verlag AG.
Tokhi M, Comrie-Thomson L, Davis J, Portela A, Chersich M, Luchters S. Involving men to improve maternal and newborn health: a systematic review of the effectiveness of interventions. PLoS One. 2018;13(1):e0191620.
Victoria C, Barros F, Assunção M, Restrepo-Méndez M, Matijasevich A, Martorell R. Scaling up maternal nutrition programs to improve birth outcomes: a review of implementation issues. Food Nutr Bull. 2012;33(2):S6–26.
Cormick G, Betran A, Romero I, Lombardo C, Gulmezoglu A, Ciapponi A, et al. Global inequities in dietary calcium intake duringpregnancy: a systematic review and meta-analysis. BJOG. 2019;126:444–56.
Ogundele OJ, Pavlova M, Groot W. Socioeconomic inequalities in reproductive health care services across Sub-Saharan Africa. A systematic review and meta-analysis. Sex Reprod Healthc. 2020;25:100536.
Vanstone M, Cernat A, Majid U, Trivedi F, De Freitas C. Perspectives of pregnant people and clinicians on noninvasive prenatal testing: a systematic review and qualitative meta-synthesis. Ont Health Technol Assess Ser. 2019;19:1–38.
PubMed PubMed Central Google Scholar
Jhaveri R, Yee LM, Antala S, Murphy M, Grobman WA, Shah SK. Responsible inclusion of pregnant individuals in eradicating HCV. Hepatology. 2021;74(3):2021.
Lagarde M, Haines A, Palmer N. Conditional cash transfers for improving uptake of health interventions in low-and middle-income countries: a systematic review. JAMA. 2007;298(16):1900–10. American Medical Association.
Lagarde M, Palmer N. The impact of user fees on health service utilization in low- and middle-income countries: how strong is the evidence? Bull World Health Organ. 2008;86(11):839–48.
Glassman A, Duran D, Fleisher L, Singer D, Sturke R, Angeles G, et al. Impact of conditional cash transfers on maternal and newborn health. J Health Popul Nutr. 2013;31(4):48–66.
Målqvist M, Yuan B, Trygg N, Selling K, Thomsen S. Targeted interventions for improved equity in maternal and child health in low- and middle-income settings: a systematic review and meta-analysis. PLoS One. 2013;8(6):e66453.
Yuan B, Målqvist M, Trygg N, Qian X, Ng N, Thomsen S. What interventions are effective on reducing inequalities in maternal and child health in low- and middle-income settings? A systematic review. BMC Public Health. 2014;14:634. BioMed Central Ltd.
Hunter BM, Harrison S, Portela A, Bick D. The effects of cash transfers and vouchers on the use and quality of maternity care services: a systematic review. PLoS One. 2017;12:e0173068. Public Library of Science.
Yaya S, Sanogo AN. Universal health coverage and facilitation of equitable access to care in Africa: a systematic review. Front Public Health. 2019;7:102. Frontiers Media S.A.
Widmer M, Betran AP, Merialdi M, Requejo J, Karpf T. The role of faith-based organizations in maternal and newborn health care in Africa. Int J Gynecol Obstet. 2011;114:218–22. John Wiley and Sons Ltd.
Lassi ZS, Haider BA, Bhutta ZA. Community-based intervention packages for reducing maternal morbidity and mortality and improving neonatal outcomes. J Dev Effect. 2012;4:151–87.
Chamberlain C, Mara-Eves A, Oliver S, Caird J, Perlen S, Eades S, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst Rev. 2013;10(10):CD001055.
Saad A, Magwood O, Aubry T, Alkhateeb Q, Hashmi SS, Hakim J, et al. Mobile interventions targeting common mental disorders among pregnant and postpartum women: an equity-focused systematic review. PLoS One. 2021;16:e0259474.
Payne O, Pillai A, Wise M, Stone P. Inequity in timing of prenatal screening in New Zealand: who are our most vulnerable? Aust N Z J Obstet Gynaecol. 2017;57(6):609–16.
Ngandu NK, Van Malderen C, Goga A, Speybroeck N. Wealth-related inequality in early uptake of HIV testing among pregnant women: an analysis of data from a national cross-sectional survey, South Africa. BMJ Open. 2017;7(7):e013362.
Liu M, Chen J, Liu J, Zhang S, Wang Q, Shen H, et al. Socioeconomic inequality in periconceptional folic acid supplementation in China: a census of 0.9 million women in their first trimester of pregnancy. BMC Pregnancy Childbirth. 2017;17(1):422.
Linhares AO, da Linhares RS, Cesar JA. Inequality in iron sulfate supplementation among pregnant women in Southern Brazil. Rev Bras Epidemiol. 2017;20(4):650–60.
Noah AJ, Yang TC, Wang WL. The black-white disparity in sexually transmitted diseases during pregnancy: how do racial segregation and income inequality matter? Sex Transm Dis. 2018;45(5):301–6.
Andaya E. Race-ing time: clinical temporalities and inequality in public prenatal care. Med Anthropol. 2019;38(8):651–63.
Yaya S, Okonofua F, Ntoimo L, Udenige O, Bishwajit G. Gender inequity as a barrier to women’s access to skilled pregnancy care in rural Nigeria: a qualitative study. Int Health. 2019;11(6):551–60.
Vedam S, Stoll K, Taiwo TK, Rubashkin N, Cheyney M, Strauss N, et al. The Giving Voice to Mothers study: inequity and mistreatment during pregnancy and childbirth in the United States. Reprod Health. 2019;16(1):77.
Miranda VIA, da Silva Dal Pizzol T, Silveira MPT, Mengue SS, da Silveira MF, Lutz BH, et al. The use of folic acid, iron salts and other vitamins by pregnant women in the 2015 Pelotas birth cohort: is there socioeconomic inequality? BMC Public Health. 2019;19(1):889.
Chirwa GC, Mazalale J, Likupe G, Nkhoma D, Chiwaula L, Chintsanya J. An evolution of socioeconomic related inequality in teenage pregnancy and childbearing in Malawi. PLoS One. 2019;14(11):e0225374.
Vilda D, Wallace M, Dyer L, Harville E, Theall K. Income inequality and racial disparities in pregnancy-related mortality in the US. SSM Popul Health. 2019;9:100477.
Hayeems RZ, Campitelli M, Ma X, Huang T, Walker M, Guttmann A. Rates of prenatal screening across health care regions in Ontario, Canada: a retrospective cohort study. CMAJ Open. 2015;3(2):E236–43.
King A, Piccinini-Vallis H. Patient-perceived patient-centeredness during pregnancy. J Obstet Gynaecol Can. 2023;45:102194.
de la Bermudez Rojas ML, Medina Jimenez V, Manzanares Cuadros JI, Diaz Martínez DA, Padilla Raygoza N, Lara Lona E. Universal prenatal screening: a initiative from Guanajuato, Mexico to improve equity in perinatal healthcare. Front Med (Lausanne). 2023;10:1127802.
Noroña-Zhou AN, Ashby BD, Richardson G, Ehmer A, Scott SM, Dardar S, et al. Rates of preterm birth and low birth weight in an adolescent obstetric clinic: achieving health equity through trauma-informed care. Health Equity. 2023;7(1):562–9.
Hamza DM, Regehr G. Eco-normalization: evaluating the longevity of an innovation in context. Acad Med. 2021;96:S48–53. NLM (Medline).
Damschroder LJ, Reardon CM, OpraWiderquist MA, Lowery J. Conceptualizing outcomes for use with the Consolidated Framework for Implementation Research (CFIR): the CFIR Outcomes Addendum. Implement Sci. 2022;17(1):7.
Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50.
Muhammad FM, Majdzadeh R, Nedjat S, Sajadi HS, Parsaeian M. Socioeconomic inequality in intermittent preventive treatment using Sulphadoxine pyrimethamine among pregnant women in Nigeria. BMC Public Health. 2020;20(1):1860.
Barbosa-Leiker C, Smith CL, Crespi EJ, Brooks O, Burduli E, Ranjo S, et al. Stressors, coping, and resources needed during the COVID-19 pandemic in a sample of perinatal women. BMC Pregnancy Childbirth. 2021;21(1):50.
Sarwer A, Javed B, Soto EB, ur Mashwani ZR. Impact of the COVID-19 pandemic on maternal health services in Pakistan. Int J Health Plan Manag. 2020;35(6):1306–10.
Hartwell M, Lin V, Gatewood A, Sajjadi NB, Garrett M, Reddy AK, et al. Health disparities, COVID-19, and maternal and childbirth outcomes: a meta-epidemiological study of equity reporting in systematic reviews. J Matern Fetal Neonatal Med. 2022;35(25):9622–30.
Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28:138–44.
Harris LM, Forson-Dare Z, Gallagher PG. Critical disparities in perinatal health—understanding risks and changing the outcomes. J Perinatol. 2021;14:181–2. Springer Nature.
Download references
Acknowledgements
We would like to acknowledge the guidance and mentorship of Dr. Betty Onyura, PhD, CE (Director of Knowledge Mobilization & Associate Scientist, Centre for Addictions and Mental Health; Scientist, Wilson Centre; Assistant Professor, University of Toronto; Toronto, Canada), and the systematic expertise of Emilia Main, MI (Library & Information Services, University Health Network, Toronto, Canada) and Jenaya Webb, MA, MI (Library Director, Ontario Institute for Studies in Education, University of Toronto, Toronto, Canada).
The primary author (ZL) was funded during this study as a PhD student through 1) the department of Applied Psychology and Human Development at the University of Toronto’s Ontario Institute for Studies in Education, 2) the Canadian Institute for Health Research, Health Systems Impact Fellowship, and 3) the Ontario Graduate Scholarship, Government of Ontario. Funders did not play a role in the design of the study, collection, analysis, interpretation of data, or in writing the manuscript.
Author information
Authors and affiliations.
University of Toronto, Toronto, Canada
Zeenat Ladak, Stephanie Small, Alexia Leber, Celia Laur, Noah M. Ivers, Olesya Falenchuk & Richard Volpe
University of Alberta, Edmonton, Canada
Nagma Grewal, Minji Olivia Kim, Qiuyu Sun & Deena M. Hamza
McMaster University, Hamilton, Canada
Mehdiya Hemani
Women’s College Hospital Institute for Health System Solutions & Virtual Care, Toronto, Canada
Zeenat Ladak, Celia Laur & Noah M. Ivers
Women’s College Hospital, Toronto, Canada
Noah M. Ivers
You can also search for this author in PubMed Google Scholar
Contributions
ZL conceptualized the study design, developed the protocol, performed the search strategy, extracted and analyzed data, and prepared the manuscript draft. NG contributed to the conceptualization of the study design, extracted and analyzed data. MOK, SS, AL, MH, and QS extracted and analyzed data. DMH supported study conceptualization and provided methodological support and expertise. CL provided methodological support and expertise. NI and OF supported study conceptualization, provided methodological support and expertise, and study oversight. RV provided funding, supported study conceptualization provided methodological support and expertise, and study oversight. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Zeenat Ladak .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Prisma-Equity Checklist. Completed PRISMA-Equity checklist to guide the umbrella review.
Additional file 2.
Search Strategy. Complete search strategy for all electronic data bases searched for this review.
Additional file 3.
ROBIS Quality Appraisal of Included Studies. Complete list of included studies in umbrella review with ROBIS quality appraisal scoring.
Additional file 4.
List of included studies and study characteristics. Complete list of included studies in umbrella review and study characteristics (author, year of publication, title, aim, countries of studies analyzed within included reviews, population, health services focus, outcomes, funding).
Additional file 5.
List of excluded studies and reason for exclusion. Complete list of excluded studies during search and screening process with reason for exclusion.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ladak, Z., Grewal, N., Kim, M.O. et al. Equity in prenatal healthcare services globally: an umbrella review. BMC Pregnancy Childbirth 24 , 191 (2024). https://doi.org/10.1186/s12884-024-06388-0
Download citation
Received : 30 November 2023
Accepted : 04 March 2024
Published : 11 March 2024
DOI : https://doi.org/10.1186/s12884-024-06388-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Health services
- Health equity
- Umbrella review
- Review of reviews
- PROGRESS-plus
BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]

Prenatal Screening and Diagnostics
- • Tests that can provide valuable information about a baby's health
- • Detects possible birth defects or genetic disorders
- • Different screening tests are performed at certain time points during the pregnancy
- • Involves Laboratory Medicine, High-Risk Pregnancy Program, and Prenatal Genetic Diagnosis Program
- First Trimester Screening
- Prenatal Testing
- Nuchal Translucency Ultrasound
- Amniocentesis
- Prenatal Genetics
What is prenatal screening?
What is the benefit of prenatal testing, what is a prenatal cell-free dna (cfdna) screening test, which tests are performed to determine potential birth defects, who is first-trimester risk assessment for, what happens at a first-trimester risk assessment, what makes yale medicine's approach to prenatal screening unique.
Pregnant and expecting mothers have the opportunity to learn a lot about their unborn child's health with today's genetic screening and diagnostic tests. Prenatal genetic testing allows the expectant mother and her health care team to provide the best health care for the baby. At Yale Medicine, prenatal screening and diagnostic tests are completed in a clinical chemistry lab. Scientists and researchers work with doctors caring for expecting mothers to help monitor pregnancies and ensure that mothers and their loved ones are identifying any possible challenges early.
"One of the important distinctions to make is whether the test is for screening or diagnosis," says Yale Medicine's Katherine Harper Campbell, MD, MPH , medical director of the Yale Medicine's Maternal-Fetal Medicine section. A screening test allows the mother and her care team to know if a baby has a higher risk of developing a condition, Dr. Campbell explains. "A diagnostic test is more invasive and can convey a clear 'yes' or 'no' about a condition or disease a baby has," Dr. Campbell says.
At Yale Medicine, a team of experts in diverse areas, including ultrasound, fetal care and genetic counseling, work together to provide the highest level of personalized care for all expectant mothers.
By taking biochemical and ultrasound measurements, physicians can identify pregnancies at a serious risk of birth defects. With the results, healthcare providers can calculate specific risks of Down’s syndrome, a genetic disorder that can lead to mild to severe intellectual disability and occurs in about 1 in 700 infants. A test is also possible for Trisomy 18, a severe genetic disorder, as well as spinal birth defects.
Prenatal testing involves both screening tests and diagnostic tests. Screening tests are not diagnostic. They can provide information regarding the risk of a baby having a certain disorder or condition. Only diagnostic tests are definitive and can identify if a baby does have a birth defect.
The tests can be especially helpful for pregnant women who are older, as the results can help them to decide whether to have further diagnostic testing relatively early in their pregnancies. Risk assessment includes not only blood test results and ultrasound measurements, but also incorporate factors such as age and ethnicity to determine risk of pregnancy abnormalities.
"We also recommend that expectant mothers undergo carrier screening for the status of any common recessive genetic diseases, including cystic fibrosis, spinal muscular atrophy and Fragile X syndrome," Dr. Campbell says. The best time for carrier screening is prior to conception or early in the pregnancy.
This is a blood test that can determine the sex of the baby and provide information regarding the risk of a child having a chromosomal disorder. This test is recommended for women who are at least 10 weeks pregnant. "The biggest appeal of this test is that it can be done from a simple blood sample," Dr. Campbell says.
The following are prenatal tests frequently performed at Yale Medicine. Speak with your doctor about potential false-positive results.
- First-Trimester Screen (nuchal translucency, hCG, and PAPP-A) : This test is usually performed during weeks 11-13 of pregnancy. It's a noninvasive evaluation combining a mother's blood screening with an ultrasound of the fetus. The test includes nuchal translucency, a portion that can help discover other potential abnormalities, including heart disorders. Primarily, the test is for chromosomal abnormalities, including Down syndrome or Trisomy-18.
- Chorionic Villus Sampling (CVS) : This test can be administered during weeks 10-13 of pregnancy. A sample of tissue is taken from the placenta and evaluated to identify chromosomal abnormalities and other inherited disorders, including Down syndrome and cystic fibrosis.
- Second-Trimester Quad Screen (AFP, hCG, Estriol, Inhibin-A), Integrated Screens : This screen can be done during weeks 16-18 of pregnancy. This blood test measures levels of alpha-fetoprotein (AFP), a fetus-produced protein; human chorionic gonadotropin (hCG), a hormone made in the placenta; estriol, an estrogen formed by the mother and the placenta; and inhibin-A, a protein produced in the placenta and in the ovaries. It tests for Down syndrome, Trisomy-18, and other chromosomal abnormalities. It can also help evaluate the chance for neural tube defects, such as spina bifida, and abdominal wall defects, such as omphalocele.
- Amniocentesis (also referred to as amniotic fluid test or AFT) : This diagnostic test is performed during weeks 15-20 of pregnancy. A small amount of amniotic fluid is sampled from the amniotic sac that surrounds the fetus.
The first trimester lasts between weeks 1-12 and all pregnant women should consider screening tests during this time. Every baby has a tiny risk of having Down syndrome—even babies of two young parents.
Noninvasive screening methods are used, including ultrasound and blood analysis. Blood is typically drawn before the patient’s appointment, so expecting mothers can receive results immediately.
The quad screen, also called the quadruple marker test or second-trimester screen, is recommended for women between 15 and 20 weeks of pregnancy to measure serum alpha-fetoprotein (AFP) and determine risk for open fetal spinal defect (or spina bifida).
Through our epidemiological monitoring program, we share data with obstetricians and physicians and work closely with them to track performance. We monitor trends in overall test results and review them with our clinicians every quarter to ensure that pregnant patients are receiving the best possible care, as well as the most up-to-date prenatal testing.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Talking with Your Healthcare Provider
- Birth Defects Statistics
- Birth Defects Resources
- Birth Defects Awareness Month
- Living with Down Syndrome
- Conversation Tips
- Growth Charts for Down Syndrome
- Accessing NBDPS and BD-STEPS Data
- Birth Defects Awareness Month Social Media Resources
- About Alcohol Use During Pregnancy
About Down Syndrome
- Down syndrome is a genetic condition where a person is born with an extra chromosome.
- This can affect how their brain and body develop.
- People diagnosed with Down syndrome can lead healthy lives with supportive care.
Down syndrome is a condition in which a person has an extra copy of chromosome 21. Chromosomes are small "packages" of genes in the body's cells, which determine how the body forms and functions.
When babies are growing, the extra chromosome changes how their body and brain develop. This can cause both physical and mental challenges.
People with Down syndrome often have developmental challenges, such as being slower to learn to speak than other children.
Distinct physical signs of Down syndrome are usually present at birth and become more apparent as the baby grows. They can include facial features, such as:
- A flattened face, especially the bridge of the nose
- Almond-shaped eyes that slant up
- A tongue that tends to stick out of the mouth
Other physical signs can include:
- A short neck
- Small ears, hands, and feet
- A single line across the palm of the hand (palmar crease)
- Small pinky fingers
- Poor muscle tone or loose joints
- Shorter-than-average height
Some people with Down syndrome have other medical problems as well. Common health problems include:
- Congenital heart defects
- Hearing loss
- Obstructive sleep apnea
Down syndrome is the most common chromosomal condition diagnosed in the United States. Each year, about 5,700 babies born in the US have Down syndrome. 1

There are three types of Down syndrome. The physical features and behaviors are similar for all three types.
With Trisomy 21, each cell in the body has three separate copies of chromosome 21. About 95% of people with Down syndrome have Trisomy 21.
Translocation Down syndrome
In this type, an extra part or a whole extra chromosome 21 is present. However, the extra chromosome is attached or "trans-located" to a different chromosome rather than being a separate chromosome 21. This type accounts for about 3% of people with Down syndrome.
Mosaic Down syndrome
Mosaic means mixture or combination. In this type, some cells have three copies of chromosome 21, but other cells have the typical two copies. People with mosaic Down syndrome may have fewer features of the condition. This type accounts for about 2% of people with Down syndrome.
Risk factors
We don't know for sure why Down syndrome occurs or how many different factors play a role. We do know that some things can affect your risk of having a baby with Down syndrome.
One factor is your age when you get pregnant. The risk of having a baby with Down syndrome increases with age, especially if you are 35 years or older when you get pregnant. 2 3 4
However, the majority of babies with Down syndrome are still born to mothers less than 35 years old. This is because there are many more births among younger women. 5 6
Regardless of age, parents who have one child with Down syndrome are at an increased risk of having another child with Down syndrome. 7
Screening and diagnosis
There are two types of tests available to detect Down syndrome during pregnancy: screening tests and diagnostic tests. A screening test can tell you if your pregnancy has a higher chance of being affected Down syndrome. Screening tests don't provide an absolute diagnosis.
Diagnostic tests can typically detect if a baby will have Down syndrome, but they carry more risk. Neither screening nor diagnostic tests can predict the full impact of Down syndrome on a baby.
The views of these organizations are their own and do not reflect the official position of CDC.
Down Syndrome Resource Foundation (DSRF) : The DSRF supports people living with Down syndrome and their families with individualized and leading-edge educational programs, health services, information resources, and rich social connections so each person can flourish in their own right.
GiGi's Playhouse : GiGi's Playhouse provides free educational, therapeutic-based, and career development programs for individuals with Down syndrome, their families, and the community, through a replicable playhouse model.
Global Down Syndrome Foundation : This foundation is dedicated to significantly improving the lives of people with Down syndrome through research, medical care, education and advocacy.
National Association for Down Syndrome : The National Association for Down Syndrome supports all persons with Down syndrome in achieving their full potential. They seek to help families, educate the public, address social issues and challenges, and facilitate active participation.
National Down Syndrome Society (NDSS) : NDSS seeks to increase awareness and acceptance of those with Down syndrome.
- Stallings, E. B., Isenburg, J. L., Rutkowski, R. E., Kirby, R. S., Nembhard, W.N., Sandidge, T., Villavicencio, S., Nguyen, H. H., McMahon, D. M., Nestoridi, E., Pabst, L. J., for the National Birth Defects Prevention Network. National population-based estimates for major birth defects, 2016–2020. Birth Defects Research. 2024 Jan;116(1), e2301.
- Allen EG, Freeman SB, Druschel C, et al. Maternal age and risk for trisomy 21 assessed by the origin of chromosome nondisjunction: a report from the Atlanta and National Down Syndrome Projects. Hum Genet. 2009 Feb;125(1):41-52.
- Ghosh S, Feingold E, Dey SK. Etiology of Down syndrome: Evidence for consistent association among altered meiotic recombination, nondisjunction, and maternal age across populations. Am J Med Genet A. 2009 Jul;149A(7):1415-20.
- Sherman SL, Allen EG, Bean LH, Freeman SB. Epidemiology of Down syndrome. Ment Retard Dev Disabil Res Rev. 2007;13(3):221-7.
- Olsen CL, Cross PK, Gensburg LJ, Hughes JP. The effects of prenatal diagnosis, population ageing, and changing fertility rates on the live birth prevalence of Down syndrome in New York State, 1983-1992. Prenat Diagn. 1996 Nov;16(11):991-1002.
- Adams MM, Erickson JD, Layde PM, Oakley GP. Down's syndrome. Recent trends in the United States. JAMA. 1981 Aug 14;246(7):758-60.
- Morris JK, Mutton DE, Alberman E. Recurrences of free trisomy 21: analysis of data from the National Down Syndrome Cytogenetic Register. Prenatal Diagnosis: Published in Affiliation With the International Society for Prenatal Diagnosis. 2005 Dec 15;25(12):1120-8.
Birth Defects
About one in every 33 babies is born with a birth defect. Although not all birth defects can be prevented, people can increase their chances of having a healthy baby by managing health conditions and adopting healthy behaviors before becoming pregnant.
For Everyone
Health care providers, public health.

IMAGES
VIDEO
COMMENTS
Abstract. This review examines the factors that affect the decision-making process of parental couples evaluating prenatal screening and diagnostic tests. A systematic search was performed using PubMed and PsycInfo databases. The 46 included studies had to: investigate the decision-making process about prenatal testing; focus on tests detecting ...
Prenatal and Newborn Screening: A Case Study. Prenatal screening began with broad implementation of amniocentesis in the 1970s and '80s, and it has since expanded to multiple forms of genetic and genomic testing, including the chromosomal microarray analysis that Allison Werner-Lin, Judith McCoyd, and Barbara Bernhardt write about in one essay in this special report and Barbara Biesecker in ...
Later review of studies in the period of 2000 to 2017 indicated higher risks of miscarriage—0.35% (95% CI 0.07 to 0.63%) and 0.35% (95% CI −0.31 to 1.00%)—for amniocentesis and CVS, respectively 45. If this tendency continues, the training and maintaining of skillful clinicians will be a challenge for future prenatal care.
Background Non-invasive prenatal testing (NIPT) is a widely adopted maternal blood test that analyses foetal originating DNA to screen for foetal chromosomal conditions, including Down's syndrome (DS). The introduction of this test, which may have implications for important decisions made during pregnancy, requires continual monitoring and evaluation. This systematic review aims to assess ...
An early scan at 11-13 + 6 weeks offers the ideal opportunity for a first detailed anatomical assessment of the fetus. Non-invasive prenatal testing (NIPT) is superior to the combined test to screen for common trisomies, especially trisomy 21, in both singleton and twin pregnancies.
This review suggests that prenatal and preconception guidance identifies a coherent framework to support the pursuit of reproductive benefits through population screening programmes.
Noninvasive prenatal testing (NIPT) has increased the number of conditions that can be screened. However, the prevalence of conditions assessed by NIPT has remained stable. The "prevalence threshold," a novel epidemiological concept, uses a test's sensitivity and specificity to determine the prevalence below which a test's positive ...
Academia.edu is a platform for academics to share research papers. Factors Affecting Improved Prenatal Screening: A Narrative Review (PDF) Factors Affecting Improved Prenatal Screening: A Narrative Review | Zeinab Hamzehgardeshi - Academia.edu
Establishing the diagnosis of a fetal genetic disease in utero expands decision-making opportunities for individuals during pregnancy and enables providers to tailor prenatal care and surveillance to disease-specific risks. The selection of prenatal genetic tests is guided by key details from fetal imaging, family and obstetrical history, suspected diagnoses and mechanisms of disease, an ...
2021. TLDR. Non-invasive prenatal testing (NIPT) for trisomy 21, 13 and 18 is a highly efficient screening method and has been applied as a first-line or a contingent screening approach all over the world since 2012, in some countries without a systematic screening program. Expand.
Since 2011, a new non-invasive prenatal screening test that uses fetal cell-free DNA (NIPT) obtained from circulating maternal blood is available (Lo et al., 1997).It can be performed starting from the 9th week of pregnancy, as it involves a single blood draw with a result turnaround time of about 2 weeks (Scott et al., 2018).NIPT holds no risk of miscarriage and offers clinical benefits over ...
Explore the latest full-text research PDFs, articles, conference papers, preprints and more on PRENATAL SCREENING. Find methods information, sources, references or conduct a literature review on ...
Prenatal and Newborn Screening: A Case Study. Prenatal screening began with broad implementation of amniocentesis in the 1970s and '80s, and it has since expanded to multiple forms of genetic and genomic testing, including the chromosomal microarray analysis that Allison Werner-Lin, Judith McCoyd, and Barbara Bernhardt write about in one ...
The alternative of moving prenatal testing outside the healthcare system into the private sector is problematic, as it makes these tests accessible only to those who can afford to pay for it. New developments in prenatal screening will have to be assessed in terms of whether and to what extent they either contribute to or undermine the stated ...
Diagram to show the flow of screening stages and number of papers at each stage of the systematic review. It includes details from both the initial search up to 10th May 2022, and the updated search on 29th March 2023. ... Sridhar S, Rote M, Hung A, et al. Non-invasive prenatal testing: A review of international implementation and challenges ...
In this review we separate prenatal testing into screening and diagnostic testing. On the one hand, screening testing is noninvasive and does not have an increased risk for miscarriage. ... Search Heart failure treatment Papers Topics Collections Effects of Sodium-Glucose Cotransporter 2 Inhibitors for the Treatment of Patients With Heart ...
Findings. From 7076 hits, we included 80 papers (1994-2020, 23 countries, 16 LICs/MICs, over 1500 participants). We identified 17 review findings, (moderate or high confidence: 14/17), and four themes: sociocultural influences and expectations; the power of visual technology; joy and devastation: consequences of ultrasound findings; the significance of relationship in the ultrasound encounter.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers. ... Non-invasive prenatal testing (NIPT) has become a routine practice in screening for common aneuploidies of chromosomes 21, 18, and 13 and gonosomes X and Y in fetuses worldwide since 2015 ...
Introduction. Prenatal ultrasound is a useful clinical screening tool to monitor fetal development and detect anomalies that may indicate further diagnostic testing or assist with clinical decision making ().In addition, ultrasounds are a psychologically appealing "window into the womb" (Edvardsson et al., 2015) offering pregnant women and their partners a chance to see their baby ...
Historically, pregnancy was a very tenuous part of a woman's life because of the high mortality rate associated with gestation. In the early 1900s, maternal mortality was 6 to 9 out of 1000 live births, and 100 per 1000 live-born infants died within the first 12 months of life. In 2000, maternal mortality declined to less than 0.1 out of 1000 live births, and infant mortality declined to 7 out ...
Screening for perinatal depression and anxiety in community-based maternal and child health settings may help close the detection and treatment gap among women at higher risk for these conditions. We aim to review perinatal depression and anxiety screening tools, timing, and follow-up processes for positive screens in community-based settings. We conducted a systematic review of the literature ...
The results of a review study using data from articles on amniocentesis and chorionic villus sampling showed that poor skills in using prenatal screening equipment and underestimating the real risks of screening have adverse consequences (Mujezinovic & Alfirevic, 2007).Thus it is recommended for health care providers to use screening services ...
The key to its success: MaterniT21, a new prenatal screening test that did remarkably well at detecting Down syndrome. Older screening tests took months and required multiple blood tests. This new ...
Description. Women included in the National Perinatal Survey and who gave birth in mainland France, were more than 25 years old (eligible for cervical cancer screening), and answered the question about a Pap test during pregnancy and over the past 3 years were 9,638 (Fig. 1 Study population). Among the latter, 4,840 women reported they were up to date for cervical cancer screening because they ...
Prenatal testing comes with more genetic risks due to its invasive nature. Blood and Ultrasound tests can place a pregnancy at a high risk of premature rupture of membranes, loss of the pregnancy, excessive bleeding, and infections. A research study conducted on the effects of prenatal screening tests among Latino Americans women found that ...
The systematic search captured 8065 articles (Fig. 1).Of the 236 articles reviewed during secondary, full-text screening, 68 systematic reviews were included in this umbrella review (Fig. 1).A condensed summary of study characteristics and outcomes for each included review can be viewed in Supplementary file 4 and all excluded studies with reason for exclusion can be viewed in Supplementary ...
The following are prenatal tests frequently performed at Yale Medicine. Speak with your doctor about potential false-positive results. First-Trimester Screen (nuchal translucency, hCG, and PAPP-A): This test is usually performed during weeks 11-13 of pregnancy.It's a noninvasive evaluation combining a mother's blood screening with an ultrasound of the fetus.
Screening tests don't provide an absolute diagnosis. See Also: Screening for Birth Defects. Diagnostic tests can typically detect if a baby will have Down syndrome, but they carry more risk. ... Olsen CL, Cross PK, Gensburg LJ, Hughes JP. The effects of prenatal diagnosis, population ageing, and changing fertility rates on the live birth ...