Advertisement

Introduction
Aml classification, diagnostic procedures, 2022 european leukemianet genetic risk classification at diagnosis, monitoring of measurable residual disease, response criteria and outcome measures, therapy for aml, allogeneic hematopoietic cell transplantation, clinical trials, new therapies, management of special situations and supportive care, supportive care, acknowledgments, diagnosis and management of aml in adults: 2022 recommendations from an international expert panel on behalf of the eln.
- Split-Screen
- Request Permissions
- Cite Icon Cite
- Search Site
- Open the PDF for in another window
Hartmut Döhner , Andrew H. Wei , Frederick R. Appelbaum , Charles Craddock , Courtney D. DiNardo , Hervé Dombret , Benjamin L. Ebert , Pierre Fenaux , Lucy A. Godley , Robert P. Hasserjian , Richard A. Larson , Ross L. Levine , Yasushi Miyazaki , Dietger Niederwieser , Gert Ossenkoppele , Christoph Röllig , Jorge Sierra , Eytan M. Stein , Martin S. Tallman , Hwei-Fang Tien , Jianxiang Wang , Agnieszka Wierzbowska , Bob Löwenberg; Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022; 140 (12): 1345–1377. doi: https://doi.org/10.1182/blood.2022016867
Download citation file:
- Ris (Zotero)
- Reference Manager
The 2010 and 2017 editions of the European LeukemiaNet (ELN) recommendations for diagnosis and management of acute myeloid leukemia (AML) in adults are widely recognized among physicians and investigators. There have been major advances in our understanding of AML, including new knowledge about the molecular pathogenesis of AML, leading to an update of the disease classification, technological progress in genomic diagnostics and assessment of measurable residual disease, and the successful development of new therapeutic agents, such as FLT3, IDH1, IDH2, and BCL2 inhibitors. These advances have prompted this update that includes a revised ELN genetic risk classification, revised response criteria, and treatment recommendations.
Since the 2017 report from the European LeukemiaNet (ELN), 1 there has been substantial progress in our knowledge of acute myeloid leukemia (AML). Recent advances significantly influence clinical practice. These advances include insights into the clinical value of genomic abnormalities for diagnosis and prognosis, the clinical significance of inherited predisposition to AML, technological advancements in the quantitative assessment of measurable residual disease (MRD) and their utility for assessing therapeutic response and disease risk, the development of a range of novel therapeutic agents, and developments in allogeneic hematopoietic cell transplantation (HCT), resulting in new disease classification, 2 diagnostic and prognostic algorithms, and updated therapeutic practices. The current report highlights these advances and updates their implications for the standard of care and for clinical trials in AML.
The panel included international members with recognized clinical and research expertise in AML. Literature and relevant abstract review, categorization of evidence, and arrival at consensus recommendations were developed as previously reported. 1,3 For diagnosis and management of acute promyelocytic leukemia (APL), readers are referred to the respective recommendations. 4
Molecular landscape
Somatic mutations drive the development of AML. Although the epigenetic state of leukemia cells, the bone marrow microenvironment, the health of normal hematopoietic cells, and other features are important for leukemia biology, somatic mutations can be assessed readily with current techniques. Leukemia develops from the serial acquisition of somatic mutations in hematopoietic stem and progenitor cells with the capacity to self-renew and propagate the neoplastic clone. 5,6 Initiating mutations may lead to an expanded clone of cells that is apparent in the peripheral blood, termed clonal hematopoiesis, a common pre-malignant state that increases in prevalence with age. 7 Although some mutations, such as those in DNMT3A , TET2 , and ASXL1 , are more common in clonal hematopoiesis and appear to be relatively early events in leukemogenesis, others tend to be acquired later in the course of leukemia development, including mutations in FLT3 , NRAS , and RUNX1 . The combinations of mutations that ultimately drive leukemogenesis are influenced by biological cooperativity and mutual exclusivity between mutated genes.
General classification
The International Consensus Classification of AML 2,8 that updated the prior revised fourth edition World Health Organization (WHO) classification of AML 9 introduced changes in the blast thresholds and new genetic entities to define AML, further expanding the spectrum of classification identified by cytogenetic and mutational profiles ( Table 1 ). Because of their overriding impact on disease phenotype and disease outcome, genetic aberrations are given priority in defining AML disease classification, with additional predisposing features (therapy-related, prior myelodysplastic syndrome [MDS] or MDS/myeloproliferative neoplasm [MPN], germline predisposition) appended as qualifiers of the primary diagnosis. A summary of the hierarchical classification is depicted in Figure 1 .

Hierarchical classification of the International Consensus Classification of AML. The classification is hierarchical (ie, AML with recurrent genetic abnormalities takes precedence over all other categories); among the remaining categories, AML with mutated TP53 supersedes AML with myelodysplasia-related gene mutations, and the latter supersedes AML with myelodysplasia-related cytogenetic abnormalities. a Myeloblasts, monoblasts, and megakaryoblasts are included in the blast count. Monoblasts and promonocytes, but not abnormal monocytes, are counted as blast equivalents in AML with monocytic or myelomonocytic differentiation, and promyelocytes in the setting of PML :: RARA or variant RARA rearrangement. Cases with prior diagnosis of MPN are excluded and are classified as accelerated (10%-19% blasts) or blast phase (≥20% blasts) MPN. For patients who already have a history of MDS/MPN (eg, CMML), the diagnosis of MDS/MPN should be retained until there are ≥20% blasts/blast equivalents; however, once an AML-defining recurrent genetic abnormality (eg, KMT2A rearrangement or NPM1 mutation) is detected and the blast count is ≥10%, AML-type therapy is recommended. b AML-defining recurrent genetic abnormalities are t(15;17)(q24.1;q21.2)/ PML :: RARA ; t(8;21)(q22;q22.1)/ RUNX1 :: RUNX1T1 ; inv(16)(p13.1q22) or t(16;16)(p13.1;q22)/ CBFB :: MYH11 ; t(9;11)(p21.3;q23.3)/ MLLT3 :: KMT2A ; t(6;9)(p22.3;q34.1)/ DEK :: NUP214 ; inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2)/ GATA2, MECOM ( EVI1 ); mutated NPM1 ; in-frame bZIP mutated CEBPA ; t(9;22)(q34.1;q11.2)/ BCR :: ABL1 ; other recurrent rearrangements involving RARA , KMT2A , MECOM , and other rare rearrangements as listed in Table 1 . The entity is named with the specific genetic abnormality. Cases with BCR::ABL1 rearrangement and 10% to 19% blasts are classified as CML in accelerated phase, and cases with history of CML and ≥20% blasts are classified as CML in myeloid blast phase. c Examples how to append diagnostic qualifiers: AML with myelodysplasia-related cytogenetic abnormality, therapy-related; AML with myelodysplasia-related gene mutation, prior myelodysplastic syndrome; AML with myelodysplasia-related gene mutation, germline RUNX1 mutation (ie, gene or syndrome should be specified).
Changes to the blast thresholds defining AML
All recurrent genetic abnormalities ( Table 1 ) that define specific subtypes of AML, with the exception of AML with t(9;22)(q34.1;q11.2)/ BCR :: ABL1 , are now considered to establish a diagnosis of AML if there are ≥10% blasts in the bone marrow or blood. The clinical behavior of myeloid neoplasms with these rearrangements reflects the specific genetic abnormality, even for cases presenting with <20% blasts. 10-18 This 10% blast threshold aligns with previously AML-defining abnormalities, such as PML :: RARA , CBFB :: MYH11 , and RUNX1 :: RUNX1T1 . 19 To avoid potential overlap with chronic myeloid leukemia in accelerated phase, AML with BCR :: ABL1 still requires ≥20% blasts.
AML and related neoplasms and acute leukemias of ambiguous lineage
| AML and related neoplams . | |
|---|---|
| • APL with t(15;17)(q24.1;q21.2)/ :: A • AML with t(8;21)(q22;q22.1)/ :: • AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22)/ :: • AML with t(9;11)(p21.3;q23.3)/ :: • AML with t(6;9)(p22.3;q34.1)/ :: • AML with inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2)/ , • AML with other rare recurring translocations • AML with mutated • AML with in-frame bZIP mutated • AML with t(9;22)(q34.1;q11.2)/ :: | • Acute undifferentiated leukemia • MPAL with t(9;22)(q34.1;q11.2)/ :: • MPAL with t(v;11q23.3)/ -rearranged • MPAL, B/myeloid, not otherwise specified • MPAL, T/myeloid, not otherwise specified |
| • AML with mutated • AML with myelodysplasia-related gene mutations Defined by mutations in , , , , , , , , and/or • AML with myelodysplasia-related cytogenetic abnormalities • AML not otherwise specified | • Transient abnormal myelopoiesis associated with Down syndrome • Myeloid leukemia associated with Down syndrome |
| Therapy-related • Prior chemotherapy, radiotherapy, immune interventions Progressed from MDS • MDS should be confirmed by standard diagnostics and >3 mo prior to AML diagnosis Progressed from MDS/MPN (specify type) • MDS/MPN should be confirmed by standard diagnostics and >3 mo prior to AML diagnosis Germline predisposition (specify type) | |
| AML and related neoplams . | |
|---|---|
| • APL with t(15;17)(q24.1;q21.2)/ :: A • AML with t(8;21)(q22;q22.1)/ :: • AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22)/ :: • AML with t(9;11)(p21.3;q23.3)/ :: • AML with t(6;9)(p22.3;q34.1)/ :: • AML with inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2)/ , • AML with other rare recurring translocations • AML with mutated • AML with in-frame bZIP mutated • AML with t(9;22)(q34.1;q11.2)/ :: | • Acute undifferentiated leukemia • MPAL with t(9;22)(q34.1;q11.2)/ :: • MPAL with t(v;11q23.3)/ -rearranged • MPAL, B/myeloid, not otherwise specified • MPAL, T/myeloid, not otherwise specified |
| • AML with mutated • AML with myelodysplasia-related gene mutations Defined by mutations in , , , , , , , , and/or • AML with myelodysplasia-related cytogenetic abnormalities • AML not otherwise specified | • Transient abnormal myelopoiesis associated with Down syndrome • Myeloid leukemia associated with Down syndrome |
| Therapy-related • Prior chemotherapy, radiotherapy, immune interventions Progressed from MDS • MDS should be confirmed by standard diagnostics and >3 mo prior to AML diagnosis Progressed from MDS/MPN (specify type) • MDS/MPN should be confirmed by standard diagnostics and >3 mo prior to AML diagnosis Germline predisposition (specify type) | |
Classification adopted from reference 2. BM, bone marrow; MPAL, mixed phenotype acute leukemia.
Bone marrow or peripheral blood blast count of ≥ 10% required, except for AML with t(9;22)(q34.1;q11.2)/ BCR :: ABL1 which requires bone marrow or peripheral blood blast count of ≥ 20% due to its overlap with progression of chronic myeloid leukemia, BCR :: ABL1 -positive.
Other recurring translocations involving RARA should be reported accordingly: eg, APL with t(1;17)(q42.3;q21.2)/ IRF2BP2 :: RARA ; APL with t(5;17)(q35.1;q21.2)/ NPM1 :: RARA ; APL with t(11;17)(q23.2;q21.2)/ ZBTB16 :: RARA ; APL with cryptic inv(17) or del(17)(q21.2q21.2)/ STAT5B :: RARA ; STAT3 :: RARA ; other genes rarely rearranged with RARA: TBL1XR1 (3q26.3); FIP1L1 (4q12); BCOR (Xp11.4).
Other recurring translocations involving KMT2A should be reported accordingly: eg, AML with t(4;11)(q21.3;q23.3)/ AFF1 :: KMT2A ; AML with t(6;11)(q27;q23.3)/ AFDN :: KMT2A ; AML with t(10;11)(p12.3;q23.3)/ MLLT10 :: KMT2A ; AML with t(10;11)(q21.3;q23.3)/ TET1 :: KMT2A ; AML with t(11;19)(q23.3;p13.1)/ KMT2A :: ELL ; AML with t(11;19)(q23.3;p13.3)/ KMT2A :: MLLT1.
Other recurring translocations involving MECOM should be reported accordingly: eg, AML with t(2;3)(p11∼23;q26.2)/ MECOM :: ? ; AML with t(3;8)(q26.2;q24.2)/ MYC , MECOM ; AML with t(3;12)(q26.2;p13.2)/ ETV6 :: MECOM ; AML with t(3;21)(q26.2;q22.1)/ MECOM :: RUNX1 .
Other rare recurring translocations: AML with t(1;3)(p36.3;q21.3)/ PRDM16 :: RPN1 ; AML (megakaryoblastic) with t(1;22)(p13.3;q13.1)/ RBM15 :: MRTFA ; AML with t(3;5)(q25.3;q35.1)/ NPM1 :: MLF1 ; AML with t(5;11)(q35.2;p15.4)/ NUP98 :: NSD1 ; AML with t(7;12)(q36.3;p13.2)/ ETV6 :: MNX1 ; AML with t(8;16)(p11.2;p13.3)/ KAT6A :: CREBBP ; AML with t(10;11)(p12.3;q14.2)/ PICALM :: MLLT10 ; AML with t(11;12)(p15.4;p13.3)/ NUP98 :: KMD5A ; AML with NUP98 and other partners; AML with t(16;21)(p11.2;q22.2)/ FUS :: ERG ; AML with t(16;21)(q24.3;q22.1)/ RUNX1 :: CBFA2T3 ; AML with inv(16)(p13.3q24.3)/ CBFA2T3 :: GLIS2.
AML with in-frame mutation in the bZIP domain of the CEBPA gene, either monoallelic or biallelic.
The presence of a pathogenic somatic TP53 mutation (at a variant allele fraction of at least 10%, with or without loss of the wild-type TP53 allele) defines the entity AML with mutated TP53 .
Cytogenetic abnormalities sufficient for the diagnosis of AML with MDS-related cytogenetic abnormalities and the absence of other AML-defining disease categories. Complex karyotype: ≥3 unrelated chromosome abnormalities in the absence of other class-defining recurring genetic abnormalities; excludes hyperdiploid karyotypes with three or more trisomies (or polysomies) without structural abnormalities. Unbalanced clonal abnormalities: del(5q)/t(5q)/add(5q); −7/del(7q); +8; del(12p)/t(12p)/(add)(12p); i(17q), −17/add(17p) or del(17p); del(20q); and/or idic(X)(q13).
Examples: AML with myelodysplasia-related cytogenetic abnormality, therapy-related; AML with myelodysplasia-related gene mutation, prior myelodysplastic syndrome; AML with myelodysplasia-related gene mutation, germline RUNX1 mutation.
Prior therapy for nonmyeloid neoplasms.
Although all other AML subtypes require ≥20% blasts for diagnosis, a new category of MDS/AML has been introduced in association with defined genomic abnormalities to include cases with 10% to 19% blasts in the bone marrow or blood to recognize the fact that these cases lie on the border between AML and MDS in terms of their biology and prognosis ( Table 1 ). 20-25 Patients diagnosed with MDS/AML should be eligible for either MDS or AML clinical trials and treatment approaches.
Antecedent AML history
An important change to the classification is the removal of the former categories AML with myelodysplasia-related changes (AML-MRC) and therapy-related myeloid neoplasms. Recent data indicate that genetic characteristics, rather than clinical history (de novo, secondary after an antecedent MDS or MDS/MPN, or therapy-related), have most relevance in classifying biologically distinct AML subgroups. 6,26 Dysplastic morphology, currently used as a criterion for AML-MRC, lacks independent prognostic significance. 27-29 Thus, although a prior history of MDS or MDS/MPN and prior exposure to therapy are still important features to note in the diagnosis, they are now applied as diagnostic qualifiers to the AML-defining category ( Table 1 ; Figure 1 ).
AML with recurrent genetic abnormalities
This category has been expanded to include additional variant translocations involving RARA , KMT2A , and MECOM , as well as other rare recurring translocations, which are now recognized as AML-defining entities ( Table 1 ). 14,30,31 Recent studies show that in-frame mutations affecting the basic leucine zipper (bZIP) region of CEBPA confer a favorable outcome, irrespective of their occurrence as biallelic or monoallelic mutations. 32-35 In-frame bZIP variants are found in 90% and 35% of cases with biallelic and monoallelic CEBPA mutations, respectively. Gene expression analyses support a distinct biology associated with CEBPA bZIP mutation in AML. Accordingly, this AML subtype has been redefined to only require an in-frame bZIP CEBPA mutation for classification rather than the previous requirement for biallelic CEBPA abnormalities.
AML with mutated TP53 , AML with myelodysplasia-related gene mutations, and AML with myelodysplasia-related cytogenetic abnormalities
Accumulating evidence indicates that from both a clinical and molecular perspective, TP53 -mutant AML and MDS represent a distinct disease entity. The vast majority of TP53 -mutant cases have complex karyotypes, and in about half, TP53 mutations occur in the absence of other AML-associated gene mutations. Clinically, these myeloid neoplasms are associated with a very poor prognosis. 6,36-41 The presence of a pathogenic TP53 mutation (at a variant allele fraction of at least 10%, with or without loss of the wild-type TP53 allele) defines the new entity AML with mutated TP53 .
Cases lacking TP53 mutation, but with mutations in ASXL1 , BCOR , EZH2 , RUNX1 , SF3B1 , SRSF2 , STAG2 , U2AF1 , and/or ZRSR2, are categorized as AML with myelodysplasia-related gene mutations, irrespective of any prior history of MDS. These mutations are highly associated with AML following prior MDS or MDS/MPN and confer an adverse prognosis even if they occur in de novo AML. 6,26,42-45 AML with myelodysplasia-related gene mutations encompasses the prior provisional category of AML with mutated RUNX1 .
The new category AML with myelodysplasia-related cytogenetic abnormalities now includes cases previously classified as AML-MRC due to the presence of myelodysplasia-associated cytogenetic findings, but lacking TP53 or myelodysplasia-related gene mutations. 46
Of note, the classification is hierarchical ( Figure 1 ); ie, “AML with mutated TP53 ” takes precendence over “AML with myelodysplasia-related gene mutations,” and the latter supercedes “AML with myelodysplasia-related cytogenetic abnormalities.”
The remaining AML cases are categorized as “AML, not otherwise specified” (irrespective of the presence or absence of multilineage dysplasia). The 4 categories described above are designated as AML/MDS if the bone marrow or blood blast count is 10% to 19% and as AML with ≥20% blasts ( Table 1 ; Figure 1 ). Cases that have both a specific AML-defining recurrent genetic abnormality and TP53 mutation and/or myelodysplasia-related gene mutations or cytogenetics should be classified according to the defined recurrent genetic abnormality. Although complex karyotypes and certain co-mutation profiles confer adverse prognosis to some genetic AML subtypes, these are captured in the prognostic stratification scheme and do not affect their primary diagnostic classification.
Therapy-related AML
Currently comprising 10% to 15% of all newly diagnosed AML, the incidence of cases showing relatedness to previous therapy for another disease continues to rise due in part to increasing numbers of cancer survivors at risk. 47 As mentioned above, “therapy-related AML” is no longer considered a disease entity, but the term “therapy-related” is now used as a diagnostic qualifier to the disease entities that are primarily defined by their genetic profile.
These neoplasms have been thought to be the direct consequence of mutational events induced by cytotoxic therapy and/or selection of chemotherapy-resistant clones. 48-50 In general, these AMLs are associated with adverse genetic lesions, and more than 90% show an abnormal karyotype. 51,52 The more common subtype, seen in ∼75% of cases, typically presents 5 to 7 years after first exposure to alkylating agents or radiation, is often preceded by MDS, and is frequently accompanied by chromosome 5 and/or 7 abnormalities, complex karyotype, and TP53 mutations. 48,49,52,53 Some individuals develop AML after treatment with topoisomerase II inhibitors, with breakage at topoisomerase II sites leading to abnormal recombination and balanced translocations involving KMT2A at 11q23.3, RUNX1 at 21q22.1, or RARA at 17q21.2. In these cases, the latency period is shorter, often it is only 1 to 3 years, and antecedent MDS is rare.
Another pathogenetic pathway is represented by cases with a preexisting myeloid clone that is resistant to chemotherapy. 52 Clonal hematopoiesis of indeterminate potential may be the first step in a multi-hit model. 54,55 Cases were identified in which the exact TP53 mutation found at diagnosis was already present at low frequency in blood or bone marrow many years before AML development. 52 These data suggest a model in which hematopoietic stem cells carrying mutations in TP53 or PPM1D undergo positive selection by cytotoxic therapy, ultimately leading to AML. 56,57 Mutations in the RAS/MAPK pathway, alterations in RUNX1 or TP53 , and KMT2A rearrangements are also frequent somatic drivers in pediatric AML related to previous therapy, but unlike in adults, most cases appear to represent independent clones arising as a consequence of cytotoxic therapy and not preexisting minor clones. 50
Deleterious mutations typical of familial cancer predisposition syndromes in the homologous recombination DNA repair pathway, particularly BRCA1 , BRCA2 , PALB2 , TP53 , or CHEK2 , are observed in ∼20% of cases. 58,59 The identification of such preexisting conditions facilitates screening and counseling of patients prior to treatment of their primary disease, family donor selection for allogeneic HCT, cancer/organ surveillance strategies, and cascade testing within families. 60
Germline predisposition
Increasingly, individuals are being recognized as having an inherited germline predisposition to hematopoietic malignancies ( Table 2 ). 61,62 Recognition of such hereditary predispositions impacts patient management, especially if there is consideration for an allogeneic HCT and health surveillance strategies for the patient and relatives who share the causative variant. Clinical testing for these syndromes is difficult for most clinicians given their relative lack of experience regarding these conditions, requirement for obtaining germline DNA for testing ( Table 3 ), and a lack of standardization in the field regarding which patients and which genes should be tested. 63
Myeloid neoplasms with germline predisposition
| Syndrome name . | Gene . | Inheritance . | Age of onset . | Predisposition to other cancers . | Clinical features . |
|---|---|---|---|---|---|
| Germline predisposition due to P/LP variants | AD | Wide range | Not yet described | 2nd allele mutations are common, typically at the 3′ end Without allogeneic HCT, individuals are susceptible to additional malignancies | |
| Germline predisposition due to P/LP variants | AD | Adult > childhood | Likely | Male mutation carriers appear to develop myeloid malignancies more often than female mutation carriers Age of onset of myeloid malignancies similar to the general population R525H hotspot occurs commonly in myeloid malignancies as a somatic mutation | |
| Li-Fraumeni syndrome | AD | Wide age range | Yes | Predisposition to several tumor types | |
| Germline predisposition due to P/LP variants | AD | Wide age range | Myeloid malignancies > T-ALL > B-cell malignancies | Life-long thrombocytopenia and qualitative platelet defects | |
| Germline predisposition due to P/LP variants | AD | Adult > childhood | Not yet described | Life-long thrombocytopenia, various platelet function abnormalities No syndromic features | |
| Germline predisposition due to P/LP variants | AD | Wide age range | ALL > myeloid malignancies | Life-long thrombocytopenia | |
| Germline predisposition due to P/LP variants | AD | Adolescents and young adults | Yes | Associated with immunodeficiencies, lymph edema, and many other phenotypes | |
| Severe congenital neutropenia | AD, AR | Adolescents and young adults | Not yet described | Severe opportunistic infections without growth factor support | |
| Shwachman-Diamond syndrome | AR | Childhood > adult | Not yet described | Exocrine pancreas dysfunction, variable cytopenias, skeletal dysplasia, hepatomegaly and transaminitis in early childhood, may present as nonsyndromic AA or MDS/AML | |
| Fanconi anemia | AR | Childhood > adult | Yes | Congenital malformations, facial dysmorphism, BM failure, squamous cell carcinomas and liver tumors, sensitivity to genotoxic agents | |
| Telomere biology disorders/short telomere syndromes | , , , , , , , , , , , , , | AD, AR, and X-linked | Wide age range | Yes | Mucocutaneous triad of nail/hair abnormalities, skin rash, leukoplakia BM failure, pulmonary fibrosis, liver cirrhosis, vascular anomalies, squamous cell carcinoma May present as nonsyndromic AA or monosomy 7 MDS |
| CBL syndrome | AD | Early childhood | Not yet described | JMML/Noonan syndrome-like: facial dysmorphism, cardiac disease, musculoskeletal anomalies, cognitive deficits, vasculopathy; variable syndrome expressivity | |
| Noonan syndrome | , , | AD | Early childhood | ALL, AML, various non-hematologic cancers | Facial dysmorphism, cardiopathy, chylothorax, hygroma, and later in life short stature |
| Neurofibromatosis type I | AD | Childhood > adult | Yes | Café au lait, neurofibromas Noonan syndrome-like disorder | |
| Germline predisposition due to P/LP variants | AD | Childhood > adult | Not yet described | MIRAGE syndrome: MDS with Infections, Renal abnormalities, Adrenal Insufficiency, Genitourinary anomalies, Enteropathy May present as non-syndromic monosomy 7 MDS or BM failure | |
| Germline predisposition due to P/LP variants | AD | Childhood > adult | Not yet described | Ataxia-pancytopenia syndrome May present as non-syndromic monosomy 7 MDS or BM failure | |
| Bloom syndrome | AR | Childhood > adult | Yes | Prenatal growth deficiency, mild immunodeficiency, excessive photosensitivity with facial lupus-like skin lesions, type 2 diabetes mellitus, hypogonadism | |
| Germline predisposition due to P/LP variants | AD | Adult > childhood | Yes | Predisposition to clonal hematopoiesis and several tumor types | |
| Germline predisposition due to P/LP variants | AR, AD | Adult > childhood | Also associated with lymphoid malignancies | Thrombocytopenia: AR (homozygous and compound heterozygous); thrombocytosis: AD | |
| Germline predisposition due to P/LP variants | AR | Adult > childhood | Yes | Atrophic skin and pigment changes Alopecia, osteopenia, cataracts | |
| Hereditary breast and ovarian cancer | AD | Adult > childhood | Yes | Predisposition to several tumor types | |
| Hereditary breast and ovarian cancer | AD | Adult > childhood | Yes | Predisposition to several tumor types | |
| Lynch syndrome | , , , | AD, AR | Adult > childhood | Yes | Tumors show microsatellite instability |
| Nijmegen breakage syndrome | AR | Childhood > adult | Yes | >90% are homozygous for a 5-base pair deletion founder mutation Microcephaly at birth and progressive with age, dysmorphic facial features, mild growth retardation, intellectual disability, combined cellular and humoral immunodeficiency with recurrent sino-pulmonary infections, females with hypergonadotropic hypogonadism | |
| Wiskott-Aldrich syndrome | X-linked | Adult > childhood | Yes | Immunodeficiency with microthrombocytopenia and neutropenia, eczema, recurrent infections, autoimmunity | |
| Germline predisposition due to CSF3R P/LP variants | AD | Adult > childhood | Not yet described | Full syndrome description awaits publication of additional cases | |
| Germline predisposition due to P/LP variants | AR (homozygous) | Adult > childhood | Not yet described | Full syndrome description awaits publication of additional cases | |
| Germline predisposition due to P/LP variants | AD | Adult > childhood | Not yet described | Associated with thrombocythemia | |
| Germline predisposition due to P/LP variants | AD | Adult > childhood | Likely | Myeloid malignancies have a high mutational rate Somatic mutation of is common | |
| Germline predisposition due to P/LP variants | AD | Childhood > adult | Not yet described | Radioulnar synostosis, clinodactyly, cardiac and renal malformations, presenile hearing loss BM failure, B-cell deficiency | |
| Germline predisposition due to P/LP variants | AD | Childhood > adult | Not yet described | Full syndrome description awaits publication of additional cases | |
| Germline predisposition due to P/LP variants | AD | Adult > childhood | Not yet described | Full syndrome description awaits publication of additional cases | |
| Germline predisposition due to P/LP variants | AD | Wide age range | Not yet described | Full syndrome description awaits publication of additional cases | |
| Germline predisposition due to P/LP variants | AD, AR | Childhood > adult | Not yet described | Full syndrome description awaits publication of additional cases | |
| Syndrome name . | Gene . | Inheritance . | Age of onset . | Predisposition to other cancers . | Clinical features . |
|---|---|---|---|---|---|
| Germline predisposition due to P/LP variants | AD | Wide range | Not yet described | 2nd allele mutations are common, typically at the 3′ end Without allogeneic HCT, individuals are susceptible to additional malignancies | |
| Germline predisposition due to P/LP variants | AD | Adult > childhood | Likely | Male mutation carriers appear to develop myeloid malignancies more often than female mutation carriers Age of onset of myeloid malignancies similar to the general population R525H hotspot occurs commonly in myeloid malignancies as a somatic mutation | |
| Li-Fraumeni syndrome | AD | Wide age range | Yes | Predisposition to several tumor types | |
| Germline predisposition due to P/LP variants | AD | Wide age range | Myeloid malignancies > T-ALL > B-cell malignancies | Life-long thrombocytopenia and qualitative platelet defects | |
| Germline predisposition due to P/LP variants | AD | Adult > childhood | Not yet described | Life-long thrombocytopenia, various platelet function abnormalities No syndromic features | |
| Germline predisposition due to P/LP variants | AD | Wide age range | ALL > myeloid malignancies | Life-long thrombocytopenia | |
| Germline predisposition due to P/LP variants | AD | Adolescents and young adults | Yes | Associated with immunodeficiencies, lymph edema, and many other phenotypes | |
| Severe congenital neutropenia | AD, AR | Adolescents and young adults | Not yet described | Severe opportunistic infections without growth factor support | |
| Shwachman-Diamond syndrome | AR | Childhood > adult | Not yet described | Exocrine pancreas dysfunction, variable cytopenias, skeletal dysplasia, hepatomegaly and transaminitis in early childhood, may present as nonsyndromic AA or MDS/AML | |
| Fanconi anemia | AR | Childhood > adult | Yes | Congenital malformations, facial dysmorphism, BM failure, squamous cell carcinomas and liver tumors, sensitivity to genotoxic agents | |
| Telomere biology disorders/short telomere syndromes | , , , , , , , , , , , , , | AD, AR, and X-linked | Wide age range | Yes | Mucocutaneous triad of nail/hair abnormalities, skin rash, leukoplakia BM failure, pulmonary fibrosis, liver cirrhosis, vascular anomalies, squamous cell carcinoma May present as nonsyndromic AA or monosomy 7 MDS |
| CBL syndrome | AD | Early childhood | Not yet described | JMML/Noonan syndrome-like: facial dysmorphism, cardiac disease, musculoskeletal anomalies, cognitive deficits, vasculopathy; variable syndrome expressivity | |
| Noonan syndrome | , , | AD | Early childhood | ALL, AML, various non-hematologic cancers | Facial dysmorphism, cardiopathy, chylothorax, hygroma, and later in life short stature |
| Neurofibromatosis type I | AD | Childhood > adult | Yes | Café au lait, neurofibromas Noonan syndrome-like disorder | |
| Germline predisposition due to P/LP variants | AD | Childhood > adult | Not yet described | MIRAGE syndrome: MDS with Infections, Renal abnormalities, Adrenal Insufficiency, Genitourinary anomalies, Enteropathy May present as non-syndromic monosomy 7 MDS or BM failure | |
| Germline predisposition due to P/LP variants | AD | Childhood > adult | Not yet described | Ataxia-pancytopenia syndrome May present as non-syndromic monosomy 7 MDS or BM failure | |
| Bloom syndrome | AR | Childhood > adult | Yes | Prenatal growth deficiency, mild immunodeficiency, excessive photosensitivity with facial lupus-like skin lesions, type 2 diabetes mellitus, hypogonadism | |
| Germline predisposition due to P/LP variants | AD | Adult > childhood | Yes | Predisposition to clonal hematopoiesis and several tumor types | |
| Germline predisposition due to P/LP variants | AR, AD | Adult > childhood | Also associated with lymphoid malignancies | Thrombocytopenia: AR (homozygous and compound heterozygous); thrombocytosis: AD | |
| Germline predisposition due to P/LP variants | AR | Adult > childhood | Yes | Atrophic skin and pigment changes Alopecia, osteopenia, cataracts | |
| Hereditary breast and ovarian cancer | AD | Adult > childhood | Yes | Predisposition to several tumor types | |
| Hereditary breast and ovarian cancer | AD | Adult > childhood | Yes | Predisposition to several tumor types | |
| Lynch syndrome | , , , | AD, AR | Adult > childhood | Yes | Tumors show microsatellite instability |
| Nijmegen breakage syndrome | AR | Childhood > adult | Yes | >90% are homozygous for a 5-base pair deletion founder mutation Microcephaly at birth and progressive with age, dysmorphic facial features, mild growth retardation, intellectual disability, combined cellular and humoral immunodeficiency with recurrent sino-pulmonary infections, females with hypergonadotropic hypogonadism | |
| Wiskott-Aldrich syndrome | X-linked | Adult > childhood | Yes | Immunodeficiency with microthrombocytopenia and neutropenia, eczema, recurrent infections, autoimmunity | |
| Germline predisposition due to CSF3R P/LP variants | AD | Adult > childhood | Not yet described | Full syndrome description awaits publication of additional cases | |
| Germline predisposition due to P/LP variants | AR (homozygous) | Adult > childhood | Not yet described | Full syndrome description awaits publication of additional cases | |
| Germline predisposition due to P/LP variants | AD | Adult > childhood | Not yet described | Associated with thrombocythemia | |
| Germline predisposition due to P/LP variants | AD | Adult > childhood | Likely | Myeloid malignancies have a high mutational rate Somatic mutation of is common | |
| Germline predisposition due to P/LP variants | AD | Childhood > adult | Not yet described | Radioulnar synostosis, clinodactyly, cardiac and renal malformations, presenile hearing loss BM failure, B-cell deficiency | |
| Germline predisposition due to P/LP variants | AD | Childhood > adult | Not yet described | Full syndrome description awaits publication of additional cases | |
| Germline predisposition due to P/LP variants | AD | Adult > childhood | Not yet described | Full syndrome description awaits publication of additional cases | |
| Germline predisposition due to P/LP variants | AD | Wide age range | Not yet described | Full syndrome description awaits publication of additional cases | |
| Germline predisposition due to P/LP variants | AD, AR | Childhood > adult | Not yet described | Full syndrome description awaits publication of additional cases | |
AA, aplastic anemia; AD, autosomal dominant; ALL, acute lymphoblastic leukemia; AR, autosomal recessive; JMML, juvenile myelomonocytic leukemia; LP, likely pathogenic; P, pathogenic.
Approximately 10% of patients with bi-allelic CEBPA -mutant AML have one of those alleles as a germline allele, typically the 5′-end mutation, although rare 3′-end germline mutations have been described. Germline 5′-end CEBPA mutations have a penetrance of close to 100%, in contrast to germline 3′-end mutations, which have lower penetrance. Because of the high penetrance of leukemia development in those with 5′ end germline mutations, some advocate pre-emptive allogeneic HCT. Leukemia survival appears to be longer for those with a germline mutation compared with those with two acquired mutations. The presence of the acquired CEBPA mutation serves as a molecular marker of AML, and these 3′-end acquired mutations are distinct in AML that re-emerge in germline CEBPA -mutation carriers, suggesting that they are independent primary AMLs rather than relapses. Therefore, individuals with germline CEBPA mutations who develop AML and are treated only with chemotherapy are at risk for developing independent AML, since their germline mutation remains. For this reason, some argue for allogeneic HCT in first remission for these patients.
Among these, all show phenotypic variability even within the same family. People with germline ANKRD26 mutations generally have the lowest platelet counts. Germline RUNX1 mutations cause myeloid malignancies > T-cell ALL > B-cell malignancies; germline ETV6 mutations cause B-cell ALL > myeloid malignancies; and germline ANKRD26 mutations have been associated only with myeloid malignancies to date.
Thirty percent of germline RUNX1 -mutated patients have clonal hematopoiesis prior to leukemia development, where BCOR mutations predominate. When leukemias develop, somatic mutations in the wild-type RUNX1 allele are seen commonly along with acquired mutations in ASXL1 , FLT3 , GATA2 , PHF6 , SRSF2 , and WT1 .
Emerging disorders are so-named due to limited numbers of cases from the published literature at this time.
Clinical features prompting consideration of clinical testing for a germline predisposition allele(s)
| Clinical features . |
|---|
| Personal history of ≥2 cancers, 1 of which is a hematopoietic malignancy (order does not matter) |
| Personal history of a hematopoietic malignancy plus: • Another relative within two generations with another hematopoietic malignancy, or • Another relative within two generations with a solid tumor diagnosed at age 50 or younger, or • Another relative within two generations with other hematopoietic abnormalities |
| Presence of a deleterious gene variant in tumor profiling that could be a germline allele, especially if that variant is present during remission |
| Age of diagnosis of hematopoietic malignancy at an earlier age than average (eg, MDS diagnosed ≤ 40 y) |
| Germline status of a variant is confirmed by: |
| Its presence in DNA derived from a tissue source not likely to undergo somatic mutation frequently (eg, cultured skin fibroblasts or hair follicles) AND at a variant allele frequency consistent with the germline (generally considered between 30-60%), or |
| Its presence in at least two relatives at a variant allele frequency consistent with the germline |
| Clinical features . |
|---|
| Personal history of ≥2 cancers, 1 of which is a hematopoietic malignancy (order does not matter) |
| Personal history of a hematopoietic malignancy plus: • Another relative within two generations with another hematopoietic malignancy, or • Another relative within two generations with a solid tumor diagnosed at age 50 or younger, or • Another relative within two generations with other hematopoietic abnormalities |
| Presence of a deleterious gene variant in tumor profiling that could be a germline allele, especially if that variant is present during remission |
| Age of diagnosis of hematopoietic malignancy at an earlier age than average (eg, MDS diagnosed ≤ 40 y) |
| Germline status of a variant is confirmed by: |
| Its presence in DNA derived from a tissue source not likely to undergo somatic mutation frequently (eg, cultured skin fibroblasts or hair follicles) AND at a variant allele frequency consistent with the germline (generally considered between 30-60%), or |
| Its presence in at least two relatives at a variant allele frequency consistent with the germline |
Certain gene alleles (eg, CHEK2 I200T and truncating DDX41 variants) are overwhelmingly likely to be germline and should prompt consideration of germline testing when identified even once.
Germline predisposition risk should be considered for all patients diagnosed with a hematopoietic malignancy regardless of age, because some germline predisposition alleles, like those in DDX41 , can drive hematopoietic malignancies in older age. 64,65 When identified, germline predisposing disorders should be applied as diagnostic qualifiers to the specific AML disease category. Key features of the clinical presentation that should prompt consideration of germline testing are given in Table 3 . Clinicians should familiarize themselves with academic and commercial testing options, including the culture and sequencing of skin fibroblasts, thereby excluding somatic mutations in hematopoietic cells, and the panel of genes to be analyzed ( Table 2 ). 63 Germline variants are categorized as pathogenic, likely pathogenic, variant of uncertain significance, likely benign, or benign; only pathogenic and likely pathogenic variants are considered causative of disease and are followed clinically in families. However, gene variant classification can change over time as additional information regarding gene/allele function and/or segregation data from families becomes available, and variants of uncertain significance in particular are often reclassified as likely pathogenic or pathogenic.
Certain germline disorders are associated with specific characteristics that are important for clinicians to recognize ( Table 2 ), those associated with quantitative and qualitative platelet defects: ANKRD26 , ETV6 , and RUNX1 , and those associated with other organ dysfunction: GATA2 with immunodeficiency; Shwachman Diamond syndrome with exocrine pancreas insufficiency and skeletal dysplasia; Fanconi anemia with facial dysmorphism, squamous cell carcinomas, and liver tumors; and dyskeratosis congenita with pulmonary fibrosis, liver cirrhosis, and vascular anomalies; among others. Some disorders are associated only with myeloid malignancies (eg, CEBPA ), whereas others confer risk to a variety of hematopoietic malignancies and solid tumors. The tumor spectrum associated with each disorder may expand over time as more individuals and families are identified. Germline predisposition alleles that confer risk to lymphoid malignancies are emerging and often overlap with the myeloid malignancy risk genes.
Because the treatment plan for many patients with AML includes allogeneic HCT and relatives are the preferred donors, testing for germline risk alleles should be performed as early as possible during clinical management. Use of hematopoietic donor stem cells from carriers of deleterious RUNX1 and CEBPA variants is prohibitive, but we lack data for most predisposition genes and whether any variants are permissive to transplantation. 66 Future studies that lead to a comprehensive list of all predisposition genes will advance our ability to provide the best treatments for patients and their families and will facilitate strategies to maintain health for them throughout their lifetimes.
All tests necessary to establish the diagnosis, risk classification, and the other procedures recommended to be performed at diagnosis are listed in Table 4 .
Tests and procedures at diagnosis for a patient with AML
| Tests and procedures . | ||
|---|---|---|
| Complete physical examination Performance status (ECOG/WHO score) Geriatric assessment (optional) Biochemistry, coagulation tests Hepatitis A, B, C; HIV-1 testing; CMV, EBV, HSV, VZV Serum pregnancy test Eligibility assessment for allogeneic HCT (incl. HLA-typing) Chest x-ray, 12-lead electrocardiogram, echocardiography or MUGA (on indication) Computed tomography of the chest (on indication) Lumbar puncture (on indication) Information on oocyte and sperm cryopreservation Biobanking | ||
| Complete blood count and differential count | ||
| Bone marrow aspirate | ||
| Bone marrow trephine biopsy | ||
| Immunophenotyping by flow cytometry (see ) | ||
| Results preferably available within | ||
| Cytogenetics | • 5-7 d | |
| Screening for gene mutations required for establishing the diagnosis and to identify actionable therapeutic targets | ||
| • , , • • , ; , , , , , , , , | • 3-5 d • 3-5 d • 1st cycle | |
| Screening for gene rearrangements | ||
| • :: , :: , :: , rearrangements, :: , other fusion genes (if available) | • 3-5 d | |
| Additional genes recommended to test at diagnosis • , , , , , , , , , , , , , , , , , , , | ||
| Demographics and medical history | ||
| Detailed family history | ||
| Patient bleeding history | ||
| Analysis of comorbidities | ||
| Tests and procedures . | ||
|---|---|---|
| Complete physical examination Performance status (ECOG/WHO score) Geriatric assessment (optional) Biochemistry, coagulation tests Hepatitis A, B, C; HIV-1 testing; CMV, EBV, HSV, VZV Serum pregnancy test Eligibility assessment for allogeneic HCT (incl. HLA-typing) Chest x-ray, 12-lead electrocardiogram, echocardiography or MUGA (on indication) Computed tomography of the chest (on indication) Lumbar puncture (on indication) Information on oocyte and sperm cryopreservation Biobanking | ||
| Complete blood count and differential count | ||
| Bone marrow aspirate | ||
| Bone marrow trephine biopsy | ||
| Immunophenotyping by flow cytometry (see ) | ||
| Results preferably available within | ||
| Cytogenetics | • 5-7 d | |
| Screening for gene mutations required for establishing the diagnosis and to identify actionable therapeutic targets | ||
| • , , • • , ; , , , , , , , , | • 3-5 d • 3-5 d • 1st cycle | |
| Screening for gene rearrangements | ||
| • :: , :: , :: , rearrangements, :: , other fusion genes (if available) | • 3-5 d | |
| Additional genes recommended to test at diagnosis • , , , , , , , , , , , , , , , , , , , | ||
| Demographics and medical history | ||
| Detailed family history | ||
| Patient bleeding history | ||
| Analysis of comorbidities | ||
CMV, cytomegalovirus; EBV, Epstein-Barr virus; ECOG, Eastern Cooperative Oncology Group; HSV, herpes simplex virus; MUGA, multigated acquisition; VZV, varicella-zoster virus.
Two hundred nucleated cells on blood smears should be counted.
Five hundred nucleated cells on bone marrow smears should be counted. Myeloblasts, monoblasts, and megakaryoblasts are included in the blast count. Monoblasts and promonocytes, but not abnormal monocytes, are counted as blast equivalents in AML with monocytic or myelomonocytic differentiation.
In patients with a dry tap ( punctio sicca ); touch preparations from the core biopsy should be performed if a dry tap is suspected.
At least 20 bone marrow metaphases are needed to define a normal karyotype and recommended to describe an abnormal karyotype. Normal and abnormal karyotypes may be diagnosed from blood specimens with circulating blasts. In case of no analyzable metaphases, fluorescence in-situ hybridization is an alternative method to detect genetic abnormalities like RUNX1 :: RUNX1T1 , CBFB :: MYH11 , KMT2A , and MECOM gene fusions, or myelodysplasia-related chromosome abnormalities, eg, loss of chromosome 5q, 7q, or 17p material.
Screening for gene mutations is an evolving field of research; screening for single genes is increasingly replaced by gene panel diagnostics.
FLT3 : mutational screening should include the analysis of internal tandem duplications (ITD) and of tyrosine kinase domain (TKD) mutations. Longer FLT3 -ITDs may be missed by next-generation sequencing, therefore, we recommend continuing to use capillary electrophoresis.
The report should specify type of mutation: only in-frame mutations affecting the basic leucine zipper (bZIP) region of CEBPA , irrespective whether they occur as monoallelic or biallelic mutations, have been associated with favorable outcome.
Screening for gene rearrangements should be performed if rapid information is needed for recommendation of suitable therapy, if chromosome morphology is of poor quality, or if there is typical morphology but the suspected cytogenetic abnormality is not present.
Results from these genes are not required for establishing the diagnosis or for the identification of actionable therapeutic targets, rather they may be used for subsequent monitoring of the disease by next-generation sequencing-based techniques (with the exception of mutations consistent with pre-malignant clonal hematopoiesis, eg, DNMT3A , TET2 , ASXL1 ); although these techniques are still investigational, this is a rapidly evolving field.
Including race or ethnicity, prior exposure to toxic agents, prior malignancy, therapy for prior malignancy, information on smoking.
Thorough family history needed to identify potential myeloid neoplasms with germline predisposition.
History of bleeding episodes may inform cases of myeloid neoplasms with germline predisposition and preexisting platelet disorders.
Special attention for skin (bleeding symptoms, leukemia cutis, Sweet syndrome), gingival hyperplasia, lymphadenopathy, testis enlargement, signs of infection (eg, pulmonary, perianal, mouth/teeth); symptoms of central nervous system involvement; signs of abnormalities associated with germline predisposition syndromes ( Table 2 ).
Tests for objectively measured physical and cognitive function are particularly useful in the context of trials.
Biochemistry: glucose, sodium, potassium, calcium, creatinine, aspartate amino transferase (AST), alanine amino transferase (ALT), alkaline phosphatase, lactate dehydrogenase (LDH), bilirubin, urea, total protein, uric acid, total cholesterol, total triglycerides, creatinine phosphokinase (CPK). Special attention should be given to tumor lysis syndrome. Coagulation tests: prothrombin time (PTT), international normalized ratio (INR) where indicated, activated partial thromboplastin time (aPTT).
In women with childbearing potential.
HLA typing and CMV testing should be performed in those patients eligible for allogeneic HCT. In patients in whom allogeneic HCT is likely to be indicated, it is also important to commence a search for sibling or volunteer unrelated donor at diagnosis.
If suspicion of pulmonary infection.
Required in patients with clinical symptoms suspicious of central nervous system involvement; patient should be evaluated by imaging study for intracranial bleeding, leptomeningeal disease, and mass lesion; lumbar puncture considered optional in other settings (eg, high white blood cell count).
Cryopreservation to be done in accordance with the wish of the patient.
Pretreatment leukemic bone marrow and blood sample; preferably also normal tissue (eg, skin biopsy, nail clippings).
Immunophenotyping
Immunophenotyping by multiparameter flow cytometry (MFC) is required to diagnose AML accurately by identifying cell surface and intracellular markers ( Table 5 ). Because of the heterogeneity of AML, no marker is expressed in all cases. It is also important to identify leukemia-associated immunophenotypes (LAIP) for subsequent MRD monitoring by MFC. In cases where an aspirate is unobtainable and circulating blasts are absent, myeloid phenotype may be confirmed on a core biopsy using immunohistochemistry.
Expression of cell-surface and cytoplasmic markers for the diagnosis of AML and MPAL
| Diagnosis of AML . | . |
|---|---|
| Diagnosis of AML | |
| Precursor marker | CD34, CD117, HLA-DR |
| Myeloid markers | Cytoplasmic MPO, CD33, CD13 |
| Myeloid maturation markers | CD11b, CD15, CD64, CD65 |
| Monocytic markers | CD14, CD36, CD64, CD4, CD38, CD11c |
| Megakaryocytic markers | CD41 (glycoprotein IIb/IIIa), CD61 (glycoprotein IIIa), CD36 |
| Erythroid markers | CD235a (glycophorin A), CD71, CD36 |
| Diagnosis of MPAL | |
| Myeloid lineage | MPO (flow cytometry, immunohistochemistry or cytochemistry), or monocytic differentiation (at least 2 of the following: non-specific esterase cytochemistry, CD11c, CD14, CD64, lysozyme), or at least two myeloid markers, ie, CD177, CD33, CD13 |
| T-lineage | Strong cytoplasmic CD3 (with antibodies to CD3 ε chain) or surface CD3 |
| B-lineage | Strong CD19 with at least one of the following strongly expressed: cytoplasmic CD79a, cCD22 or CD10, or weak CD19 with at least two of the following strongly expressed: CD79a, cCD22 or CD10 |
| Core MRD markers | |
| CD34, CD117, CD45, CD33, CD13, CD56, CD7, HLA-DR If monocytic: CD64, CD11b, CD4 (in addition) |
| Diagnosis of AML . | . |
|---|---|
| Diagnosis of AML | |
| Precursor marker | CD34, CD117, HLA-DR |
| Myeloid markers | Cytoplasmic MPO, CD33, CD13 |
| Myeloid maturation markers | CD11b, CD15, CD64, CD65 |
| Monocytic markers | CD14, CD36, CD64, CD4, CD38, CD11c |
| Megakaryocytic markers | CD41 (glycoprotein IIb/IIIa), CD61 (glycoprotein IIIa), CD36 |
| Erythroid markers | CD235a (glycophorin A), CD71, CD36 |
| Diagnosis of MPAL | |
| Myeloid lineage | MPO (flow cytometry, immunohistochemistry or cytochemistry), or monocytic differentiation (at least 2 of the following: non-specific esterase cytochemistry, CD11c, CD14, CD64, lysozyme), or at least two myeloid markers, ie, CD177, CD33, CD13 |
| T-lineage | Strong cytoplasmic CD3 (with antibodies to CD3 ε chain) or surface CD3 |
| B-lineage | Strong CD19 with at least one of the following strongly expressed: cytoplasmic CD79a, cCD22 or CD10, or weak CD19 with at least two of the following strongly expressed: CD79a, cCD22 or CD10 |
| Core MRD markers | |
| CD34, CD117, CD45, CD33, CD13, CD56, CD7, HLA-DR If monocytic: CD64, CD11b, CD4 (in addition) |
Cytogenetic and molecular studies
Conventional cytogenetic analysis is mandatory in the evaluation of AML. If conventional cytogenetics fails, fluorescence in situ hybridization is an alternative to detect specific abnormalities like RUNX1 :: RUNX1T1 , CBFB :: MYH11 , KMT2A ( MLL ), and MECOM ( EVI1 ) gene fusions, or myelodysplasia-related chromosome abnormalities, eg, loss of chromosome 5q, 7q, or 17p material ( Table 1 ).
Molecular genetic testing should screen for all the genetic abnormalities that define disease and risk categories or that are needed for targeted treatment modalities ( Table 4 ). These tests can be performed by commercially available gene panel diagnostics or platforms simultaneously testing for mutations and rearrangements. When AML with germline predisposition is suspected, a dedicated gene panel including known predisposing alleles should be used. However, caution should be used in interpreting data from tumor-based panels, because hematopoietic tissues undergo somatic reversion frequently leading to false-negative results, and panel-based testing is often not able to detect germline copy number variants, which are relatively common predisposition alleles.
For patients with mutant NPM1 and core-binding factor (CBF)-AML, it is recommended to perform baseline molecular assessment by quantitative polymerase chain reaction (qPCR) or droplet digital PCR (dPCR) to facilitate MRD monitoring after treatment.
At least in clinical studies, but preferably also outside this context, bone marrow and blood samples should be obtained at time of diagnosis, at remission, and at relapse and stored under appropriate conditions (DNA and RNA stored at −80°C and viable cells stored at −196°C). Broad informed consent should be obtained to allow for performance of correlative laboratory studies. In addition, a sample from healthy tissue should be stored to enable delineation of germline from somatic mutations.
Since 2017, new data have emerged that prompted the need to adjust the risk classification. In addition to baseline genetic characterization, the importance of response to initial therapy and assessment of early MRD in individual risk assignment are highlighted. 67 In clinical practice, a patient with favorable-risk AML may be reclassified as intermediate-risk or vice versa, based on the presence or absence of MRD, respectively. For instance, this is particularly relevant for patients with NPM1 -mutant AML. 68-70
The most important changes made to the previous risk classification are outlined in Table 6 . (1) The FLT3 -ITD allelic ratio is no longer considered in the risk classification; consequently, AML with FLT3 -ITD (without adverse-risk genetic lesions) are now categorized in the intermediate-risk group, irrespective of the allelic ratio or concurrent presence of NPM1 mutation. The reason for this change relates to methodological issues with standardizing the assay to measure the FLT3 -ITD allelic ratio, the modifying impact of midostaurin-based therapy on FLT3 -ITD without NPM1 mutation, 71 and the increasing role of MRD in treatment decisions. (2) AML with myelodysplasia-related gene mutations is now categorized in the adverse-risk group. These mutations, typically associated with AML following an antecedent hematologic disease, are also prevalent in de novo AML and indicate adverse risk even in the absence of myelodysplasia-related cytogenetic abnormalities. 6,26,42,44,45 Beyond the previously considered ASXL1 and/or RUNX1 genes, this category of myelodysplasia-related gene mutations now includes pathologic variants in at least one of the ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, or ZRSR2 genes. (3) The presence of adverse-risk cytogenetic abnormalities in NPM1 -mutated AML now defines adverse risk. A meta-analysis has shown that NPM1 -mutated AML with adverse cytogenetic abnormalities is associated with a poor outcome. 72 Whether other genetic abnormalities (eg, myelodysplasia-related gene mutations) also confer unfavorable outcome to NPM1 -mutated AML is under investigation. (4) As mentioned previously, recent studies have shown that in-frame mutations affecting the basic leucine zipper region of CEBPA confer the favorable outcome, irrespective of their occurrence as biallelic or monoallelic mutations and therefore are now categorized in the favorable-risk group. 32,34,35 (5) Additional disease-defining recurring cytogenetic abnormalities are included in the adverse-risk group, including t(3q26.2;v) involving the MECOM gene, 31,73 or t(8;16)(p11.2;p13.3) associated with KAT6A::CREBBP gene fusion. 14 (6) Finally, hyperdiploid karyotypes with multiple trisomies (or polysomies) are no longer considered complex karyotypes and as adverse risk. 74
2022 ELN risk classification by genetics at initial diagnosis *
| Risk category . | Genetic abnormality . |
|---|---|
| Favorable | • t(8;21)(q22;q22.1)/ :: , • inv(16)(p13.1q22) or t(16;16)(p13.1;q22)/ :: , • Mutated , without -ITD • bZIP in-frame mutated |
| Intermediate | • Mutated , with -ITD • Wild-type with -ITD (without adverse-risk genetic lesions) • t(9;11)(p21.3;q23.3)/ :: , • Cytogenetic and/or molecular abnormalities not classified as favorable or adverse |
| Adverse | • t(6;9)(p23.3;q34.1)/ :: • t(v;11q23.3)/ -rearranged • t(9;22)(q34.1;q11.2)/ :: • t(8;16)(p11.2;p13.3)/ :: • inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2)/ , • t(3q26.2;v)/ ( )-rearranged • −5 or del(5q); −7; −17/abn(17p) • Complex karyotype, monosomal karyotype • Mutated • Mutated |
| Risk category . | Genetic abnormality . |
|---|---|
| Favorable | • t(8;21)(q22;q22.1)/ :: , • inv(16)(p13.1q22) or t(16;16)(p13.1;q22)/ :: , • Mutated , without -ITD • bZIP in-frame mutated |
| Intermediate | • Mutated , with -ITD • Wild-type with -ITD (without adverse-risk genetic lesions) • t(9;11)(p21.3;q23.3)/ :: , • Cytogenetic and/or molecular abnormalities not classified as favorable or adverse |
| Adverse | • t(6;9)(p23.3;q34.1)/ :: • t(v;11q23.3)/ -rearranged • t(9;22)(q34.1;q11.2)/ :: • t(8;16)(p11.2;p13.3)/ :: • inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2)/ , • t(3q26.2;v)/ ( )-rearranged • −5 or del(5q); −7; −17/abn(17p) • Complex karyotype, monosomal karyotype • Mutated • Mutated |
Frequencies, response rates and outcome measures should be reported by risk category, and, if sufficient numbers are available, by specific genetic lesions indicated.
Mainly based on results observed in intensively treated patients. Initial risk assignment may change during the treatment course based on the results from analyses of measurable residual disease.
Concurrent KIT and/or FLT3 gene mutation does not alter risk categorization.
AML with NPM1 mutation and adverse-risk cytogenetic abnormalities are categorized as adverse-risk.
Only in-frame mutations affecting the basic leucine zipper (bZIP) region of CEBPA , irrespective whether they occur as monoallelic or biallelic mutations, have been associated with favorable outcome.
The presence of t(9;11)(p21.3;q23.3) takes precedence over rare, concurrent adverse-risk gene mutations.
Excluding KMT2A partial tandem duplication (PTD).
Complex karyotype: ≥3 unrelated chromosome abnormalities in the absence of other class-defining recurring genetic abnormalities; excludes hyperdiploid karyotypes with three or more trisomies (or polysomies) without structural abnormalities.
Monosomal karyotype: presence of two or more distinct monosomies (excluding loss of X or Y), or one single autosomal monosomy in combination with at least one structural chromosome abnormality (excluding core-binding factor AML).
For the time being, these markers should not be used as an adverse prognostic marker if they co-occur with favorable-risk AML subtypes.
TP53 mutation at a variant allele fraction of at least 10%, irrespective of the TP53 allelic status (mono- or biallelic mutation); TP53 mutations are significantly associated with AML with complex and monosomal karyotype.
Although numerous reports have studied mutations in other genes, for example, IDH1/IDH2 or DNMT3A , current evidence does not yet warrant their assignment to a distinct ELN prognostic group. Also, the emerging therapeutic use of targeted inhibitors might impact prognostic outcome in IDH1 / IDH2 -mutated AML. Finally, it should be emphasized that the ELN AML risk classification has been developed based on data from intensively treated patients and may warrant modifications for patients receiving less intensive therapies.
MRD assessment in AML is used to (1) provide a quantitative methodology to establish a deeper remission status; (2) refine postremission relapse risk assessment; (3) identify impending relapse to enable early intervention; and (4) as a surrogate end point to accelerate drug testing and approval. 75
Currently, the 2 most extensively evaluated methodologies are multiparameter flow cytometry-based MRD (MFC-MRD) and molecular MRD (Mol-MRD) assessed by qPCR. 76 Emerging exploratory technologies are next-generation sequencing (NGS) and dPCR ( Table 7 ). 77 The current update of the ELN recommendation on MRD includes new technical recommendations for standardized MFC-MRD and Mol-MRD analysis, MRD thresholds, definitions of MRD response, and suggestions for clinical implications. 67
Methods for detection of MRD in AML
| . | Method . | Target . | Sensitivity . | Applicable in % of AML . | Turn-around time (days) . | Limitations/problems . |
|---|---|---|---|---|---|---|
| Established | Multi-parameter flow cytometry (MFC) | Leukemia-associated immunophenotype (LAIP) or different from normal (DfN) | 10 to 10 | 85-90 | 2 | Less sensitive, more subjective analysis |
| Established | Real-time quantitative PCR (RT-qPCR) | Robust data: , :: , :: Less validated: :: , :: , :: , | 10 to 10 | 40-50 | 3-5 | Limited applicability |
| Exploratory | Next-generation sequencing (NGS) , | Potentially any somatic mutation | 10 to 10 | ∼100 | 5-10 | Less sensitive, costly, technically challenging |
| Exploratory | Digital PCR (dPCR) | Specific targeted mutations | 10 to 10 | ∼70 | 3-5 | Specific assay necessary for every mutation, limited sensitivity |
| . | Method . | Target . | Sensitivity . | Applicable in % of AML . | Turn-around time (days) . | Limitations/problems . |
|---|---|---|---|---|---|---|
| Established | Multi-parameter flow cytometry (MFC) | Leukemia-associated immunophenotype (LAIP) or different from normal (DfN) | 10 to 10 | 85-90 | 2 | Less sensitive, more subjective analysis |
| Established | Real-time quantitative PCR (RT-qPCR) | Robust data: , :: , :: Less validated: :: , :: , :: , | 10 to 10 | 40-50 | 3-5 | Limited applicability |
| Exploratory | Next-generation sequencing (NGS) , | Potentially any somatic mutation | 10 to 10 | ∼100 | 5-10 | Less sensitive, costly, technically challenging |
| Exploratory | Digital PCR (dPCR) | Specific targeted mutations | 10 to 10 | ∼70 | 3-5 | Specific assay necessary for every mutation, limited sensitivity |
Less frequent in elderly patients with AML.
The NGS-MRD threshold has not been defined for individual mutations; NGS-MRD positivity is provisionally defined as ≥ 0.1% variant allele frequency, excluding mutations related to clonal hematopoiesis and germline mutations.
Common gene mutations consistent with pre-malignant clonal hematopoiesis such as DNMT3A , TET2 , and AXSL1 excluded; further study is required to determine which mutations are truly indicative of residual AML and not clonal hematopoiesis.
Multiparameter flow cytometry
Integration of diagnostic LAIP that distinguish AML cells from normal hematopoietic cells in an individual patient and the more generally defined “different from normal” aberrant immunophenotype (DfN) allow both for tracking of diagnostic and emerging clones and should include core MRD markers ( Table 5 ). 67 MFC-MRD assessment should be performed with a qualified assay based on guidelines for rare event detection. 78 Evaluation of residual leukemic stem cells (LSC) by MFC-MRD is still investigational but is recommended for evaluation in clinical studies. The prognostic value of LSC-MRD has been associated with a higher sensitivity and lower false negativity. 79,80 LSC can be immunophenotypically defined as CD34 + /CD38 low cells combined with an aberrant marker not present on normal HSCs (eg, CD45RA [PTPRC], CLL-1 [CLEC12A], or CD123 [IL3RA]). 81
Molecular MRD
The technique used, including qPCR and dPCR, should reach a limit of detection of at least 10 −3 . Either peripheral blood or bone marrow may be used, although sensitivity in blood is generally lower by an order of magnitude compared with bone marrow. Leukemia-related abnormalities suitable for qPCR monitoring include mutated NPM1 ; CBFB :: MYH11 , RUNX1 :: RUNX1T1 , KMT2A :: MLLT3 , DEK :: NUP214 , and BCR :: ABL1 gene fusions; and WT1 expression. 67 Validation is most robust for NPM1 -mutated, as well as CBFB :: MYH11 and RUNX1 :: RUNX1T1 -positive AML. 82
If using NGS, error-corrected targeted panel-based approaches are preferred. 83 Care must be taken to recognize and exclude germline mutations. Mutations consistent with premalignant clonal hematopoiesis (eg, DNMT3A , TET2 , ASXL1 ) should not be considered as MRD. 84 Further study is required to identify and distinguish mutations truly indicative of residual AML from clonal hematopoiesis related abnormalities. 85,86 It is important to note that NGS-based strategies currently lack standardization as a stand-alone technique for MRD assessment.
Implementation of MRD testing/decision making in AML
The prognostic value of MRD detection in complete remission (CR) or CR with incomplete hematologic recovery (CRi) has been demonstrated both in patients treated with intensive and more recently less-intensive treatment modalities. 87-89 Various studies and a systematic meta-analysis of 81 publications have shown the prognostic value of MRD for relapse and overall survival (OS). 68,87,90-93 Although MRD estimates furnish critical prognostic insights, they are imperfect, because relapse still occurs in a minority of MRD-negative patients. Thus, a negative MRD test result may not indicate complete disease eradication but refers to disease below the MRD test threshold in the tested sample. Conversely, not all patients who are MRD positive will relapse. Of note, Mol-MRD may remain detectable at low levels (CR MRD-LL ) without prognostic significance, and therefore, are called negative operationally if the MRD values are below the threshold linked to prognosis. 67 For instance, in CBF-AML and NPM1 -mutant AML, the transcripts may show persistent low-level expression after treatment, but this is not prognostic of relapse. 68,70,94-96 The presence of detectable MRD before transplant is an independent unfavorable predictor of posttransplant outcome. 97-100 However, there is currently no evidence showing benefit of additional courses of intensive chemotherapy prior to transplant in CR1 patients who are MRD positive. If fit enough, such patients should be considered candidates for a myeloablative conditioning (MAC) regimen or an early taper of posttransplant immunosuppression. 98
Definitions of MRD response categories and molecular relapse are listed in Table 8 . In Figure 2 , the recommended time points for MRD evaluation and clinical decision making are depicted for NPM1 -mutated, CBF-AML, and AML assessed by MFC.
Response criteria in AML
| Category . | Definition . | Comment . |
|---|---|---|
| CR , , | Bone marrow blasts < 5%; absence of circulating blasts; absence of extramedullary disease; ANC ≥ 1.0 × 10 /L (1,000/µL); platelet count ≥ 100 × 10 /L (100 000/µL) | |
| CRh , , | ANC ≥ 0.5 × 10 /L (500/µL) and platelet count ≥ 50 × 10 /L (50 000/µL), otherwise all other CR criteria met | If CRh used, CRi should only include patients not meeting the definition of CRh |
| CRi , , | All CR criteria except for residual neutropenia < 1.0 × 10 /L (1,000/µL) or thrombocytopenia < 100 × 10 /L (100 000/µL) | |
| MLFS | Bone marrow blasts < 5%; absence of circulating blasts; absence of extramedullary disease; no hematologic recovery required | Marrow should not merely be “aplastic”; bone marrow spicules should be present; at least 200 cells should be enumerated in the aspirate or cellularity should be at least 10% in the biopsy. Mainly used in the context of phase 1-2 clinical trials |
| PR | All hematologic criteria of CR; decrease of bone marrow blast percentage to 5% to 25%; and decrease of pre-treatment bone marrow blast percentage by at least 50% | Mainly used in the context of phase 1-2 clinical trials |
| No response | Patients evaluable for response but not meeting the criteria for CR, CRh, CRi, MLFS or PR are categorized as having no response prior to the response landmark. Patients failing to achieve response by the designated landmark are designated as having refractory disease | |
| Nonevaluable for response | Non-evaluable for response will include patients lacking an adequate bone marrow response evaluation. This category will include patients with early death, withdrawal prior to response assessment, or a technically suboptimal bone marrow sample precluding assessment | |
| CR, CRh, or CRi without MRD (CR , CRh or CRi ) | CR, CRh or CRi with MRD below a defined threshold for a genetic marker by qPCR, or by MFC. Response without MRD should be confirmed with a subsequent assessment at least 4 wk apart. The date of response without MRD is the first date in which the MRD was below the defined threshold Response with MRD detection at low-level (CR ) is included in this category of CR, CRh or CRi without MRD. CR is currently only defined for -mutant and CBF-AML | Sensitivities vary by marker tested, and by method used; therefore, test used, tissue source and minimum assay sensitivity for evaluability should be reported; analyses should be done in experienced laboratories (centralized diagnostics) |
| Refractory disease | No CR, CRh or CRi at the response landmark, ie, after 2 courses of intensive induction treatment or a defined landmark, eg, 180 d after commencing less-intensive therapy | Patients not responding to a first cycle of 7 + 3 should be considered for a regimen containing higher doses of cytarabine |
| Relapsed disease (after CR, CRh or CRi) | Bone marrow blasts ≥ 5%; or reappearance of blasts in the blood in at least 2 peripheral blood samples at least one week apart; or development of extramedullary disease | |
| MRD relapse (after CR, CRh or CRi without MRD) | 1. Conversion from MRD negativity to MRD positivity, independent of method, or 2. Increase of MRD copy numbers ≥ 1 log between any two positive samples in patients with CR , CRh or CRi by qPCR The result of 1. or 2. should be rapidly confirmed in a second consecutive sample from the same tissue source | Test methodology, sensitivity of the assay, and cutoff values used must be reported; analyses should be done in experienced laboratories (centralized diagnostics) |
| Category . | Definition . | Comment . |
|---|---|---|
| CR , , | Bone marrow blasts < 5%; absence of circulating blasts; absence of extramedullary disease; ANC ≥ 1.0 × 10 /L (1,000/µL); platelet count ≥ 100 × 10 /L (100 000/µL) | |
| CRh , , | ANC ≥ 0.5 × 10 /L (500/µL) and platelet count ≥ 50 × 10 /L (50 000/µL), otherwise all other CR criteria met | If CRh used, CRi should only include patients not meeting the definition of CRh |
| CRi , , | All CR criteria except for residual neutropenia < 1.0 × 10 /L (1,000/µL) or thrombocytopenia < 100 × 10 /L (100 000/µL) | |
| MLFS | Bone marrow blasts < 5%; absence of circulating blasts; absence of extramedullary disease; no hematologic recovery required | Marrow should not merely be “aplastic”; bone marrow spicules should be present; at least 200 cells should be enumerated in the aspirate or cellularity should be at least 10% in the biopsy. Mainly used in the context of phase 1-2 clinical trials |
| PR | All hematologic criteria of CR; decrease of bone marrow blast percentage to 5% to 25%; and decrease of pre-treatment bone marrow blast percentage by at least 50% | Mainly used in the context of phase 1-2 clinical trials |
| No response | Patients evaluable for response but not meeting the criteria for CR, CRh, CRi, MLFS or PR are categorized as having no response prior to the response landmark. Patients failing to achieve response by the designated landmark are designated as having refractory disease | |
| Nonevaluable for response | Non-evaluable for response will include patients lacking an adequate bone marrow response evaluation. This category will include patients with early death, withdrawal prior to response assessment, or a technically suboptimal bone marrow sample precluding assessment | |
| CR, CRh, or CRi without MRD (CR , CRh or CRi ) | CR, CRh or CRi with MRD below a defined threshold for a genetic marker by qPCR, or by MFC. Response without MRD should be confirmed with a subsequent assessment at least 4 wk apart. The date of response without MRD is the first date in which the MRD was below the defined threshold Response with MRD detection at low-level (CR ) is included in this category of CR, CRh or CRi without MRD. CR is currently only defined for -mutant and CBF-AML | Sensitivities vary by marker tested, and by method used; therefore, test used, tissue source and minimum assay sensitivity for evaluability should be reported; analyses should be done in experienced laboratories (centralized diagnostics) |
| Refractory disease | No CR, CRh or CRi at the response landmark, ie, after 2 courses of intensive induction treatment or a defined landmark, eg, 180 d after commencing less-intensive therapy | Patients not responding to a first cycle of 7 + 3 should be considered for a regimen containing higher doses of cytarabine |
| Relapsed disease (after CR, CRh or CRi) | Bone marrow blasts ≥ 5%; or reappearance of blasts in the blood in at least 2 peripheral blood samples at least one week apart; or development of extramedullary disease | |
| MRD relapse (after CR, CRh or CRi without MRD) | 1. Conversion from MRD negativity to MRD positivity, independent of method, or 2. Increase of MRD copy numbers ≥ 1 log between any two positive samples in patients with CR , CRh or CRi by qPCR The result of 1. or 2. should be rapidly confirmed in a second consecutive sample from the same tissue source | Test methodology, sensitivity of the assay, and cutoff values used must be reported; analyses should be done in experienced laboratories (centralized diagnostics) |
ANC, absolute neutrophil count; CBF, core-binding factor; VAF, variant allele frequency.
To recognize the potential for continuing improvements in blood counts after myelosuppressive therapy, response definitions for patients with marrow blast clearance (< 5%) may be adjusted to reflect the best hematologic response achieved prior to commencement of the next treatment cycle. Aspirate reports that include MLFS, CRh, or CRi should note the potential for post-marrow blood counts to alter the final response designation. Patients should not have received G-CSF, nor platelet transfusions within 7 d prior to hematologic response determination.
For patients with CR, CRh, or CRi, the presence of a low percentage of circulating blasts in the blood may represent a regenerating marrow and should not be interpreted as persistent disease. In such cases the blasts generally disappear within a week.
A response landmark for CR, CRh, or CRi should be stated, eg, after 2 cycles of intensive therapy; this landmark may be longer for nonintensive based treatment options, eg, 180 days.
MFC-MRD positivity is defined as ≥ 0.1% of CD45 expressing cells with the target immunophenotype. MRD test positivity by qPCR is defined as cycling threshold (Ct) < 40 and is negative if Ct ≥ 40 in ≥ 2 of 3 replicates. In NPM1 -mutated and CBF-AML, CR with molecular MRD detectable at low-level (CR MRD-LL ) defined as < 2% is designated as negative for MRD, because when measured at the end of consolidation treatment, is associated with a very low relapse rate.

Algorithm of MRD assessment and time points at which MRD is considered a clinically relevant biomarker. Blue squares indicate timepoints of assessment and source of material; pink squares indicate timepoints for treatment modification based on a clinical relevant biomarker: for example, if the level of molecular MRD as assessed by qPCR is ≥2% or if there is failure to reduce mutant transcript levels by 3 to 4 log after completion of consolidation chemotherapy, treatment modifications (eg, allogeneic hematopoietic cell transplantation) may be considered; similarly, if patients are still MRD positive by MFC after 2 cycles of intensive chemotherapy or at end of treatment. For patients receiving less intensive therapy, timepoints for assessment and clinical decision making are not yet established. Modified from 2021 ELN MRD recommendations 67 BM, bone marrow; CBF, core-binding factor. a MFC as assessed by LAIP or the DfN method.
AML response criteria and outcome measures are summarized in Tables 8 and 9 .
Outcome measures for clinical trials in acute myeloid leukemia
| Category . | Definition . |
|---|---|
| Early death | Death from any cause within a timeframe relevant for the therapy being investigated (eg, 30 and 60 d from commencing therapy) |
| Overall survival | Defined for all patients in a trial; measured from day 1 of randomization or day 1 of registration in non-randomized trials (or from the date of diagnosis, eg, for correlative science studies) to the date of death from any cause; patients not known to have died at last follow-up are censored on the date they were last known to be alive |
| Event-free survival (EFS) | Defined for all patients in a trial; measured from day 1 of randomization or day 1 of registration in non-randomized trials to the date of treatment failure, hematologic relapse from CR/CRh/CRi or death from any cause, whichever occurs first; treatment failure is defined as not achieving either CR, CRh or CRi by a pre-defined landmark (eg, after two cycles of intensive chemotherapy or 180 d for non-intensive therapy); patients evaluable for response but not achieving either CR, CRh or CRi by the defined landmark and patients who die before the defined landmark without response assessments are considered an event at day 1 of randomization; patients alive who are non-evaluable for response should be censored at day 1 of the randomization; patients achieving either CR, CRh or CRi by the defined landmark but do not relapse or die should be censored on the date they were last assessed for response |
| Relapse-free survival (RFS) | Defined only for patients achieving CR, CRh, or CRi; measured from the date of achievement of remission until the date of hematologic relapse or death from any cause; patients not known to have relapsed or died at last follow-up are censored on the date they were last known to be alive |
| Cumulative incidence of relapse (CIR) | Defined for all patients achieving CR, CRh, CRi; measured from the date of achievement of a remission until the date of hematologic relapse; patients not known to have relapsed are censored on the date they were last assessed for response; patients who died without relapse are counted as a competing cause of failure |
| Cumulative incidence of death (CID) | Defined for all patients achieving CR, CRh, CRi; measured from the date of achievement of a remission to death without prior relapse; relapse is considered as competing risk |
| EFS | Measured from day 1 of randomization or day 1 of registration in non-randomized trials to the date of failure to achieve CR, CRh or CRi by a defined landmark (eg, after two cycles of intensive chemotherapy or 180 d for non-intensive therapy), hematologic relapse, MRD relapse (for patients achieving CR, CRh or CRi without MRD) or death from any cause |
| RFS | Measured from the date of achievement of a remission (CR, CRh, or CRi) until the date of hematologic relapse, MRD relapse, or death from any cause |
| CIR | Measured from the date of achievement of a remission (CR, CRh or CRi) until the date of hematologic relapse, or molecular MRD relapse; patients who died without relapse are counted as a competing cause of failure |
| CID | Measured from the date of achievement of a remission (CR, CRh, or CRi) to death without prior relapse; morphologic or molecular MRD relapse is considered as competing risk |
| Category . | Definition . |
|---|---|
| Early death | Death from any cause within a timeframe relevant for the therapy being investigated (eg, 30 and 60 d from commencing therapy) |
| Overall survival | Defined for all patients in a trial; measured from day 1 of randomization or day 1 of registration in non-randomized trials (or from the date of diagnosis, eg, for correlative science studies) to the date of death from any cause; patients not known to have died at last follow-up are censored on the date they were last known to be alive |
| Event-free survival (EFS) | Defined for all patients in a trial; measured from day 1 of randomization or day 1 of registration in non-randomized trials to the date of treatment failure, hematologic relapse from CR/CRh/CRi or death from any cause, whichever occurs first; treatment failure is defined as not achieving either CR, CRh or CRi by a pre-defined landmark (eg, after two cycles of intensive chemotherapy or 180 d for non-intensive therapy); patients evaluable for response but not achieving either CR, CRh or CRi by the defined landmark and patients who die before the defined landmark without response assessments are considered an event at day 1 of randomization; patients alive who are non-evaluable for response should be censored at day 1 of the randomization; patients achieving either CR, CRh or CRi by the defined landmark but do not relapse or die should be censored on the date they were last assessed for response |
| Relapse-free survival (RFS) | Defined only for patients achieving CR, CRh, or CRi; measured from the date of achievement of remission until the date of hematologic relapse or death from any cause; patients not known to have relapsed or died at last follow-up are censored on the date they were last known to be alive |
| Cumulative incidence of relapse (CIR) | Defined for all patients achieving CR, CRh, CRi; measured from the date of achievement of a remission until the date of hematologic relapse; patients not known to have relapsed are censored on the date they were last assessed for response; patients who died without relapse are counted as a competing cause of failure |
| Cumulative incidence of death (CID) | Defined for all patients achieving CR, CRh, CRi; measured from the date of achievement of a remission to death without prior relapse; relapse is considered as competing risk |
| EFS | Measured from day 1 of randomization or day 1 of registration in non-randomized trials to the date of failure to achieve CR, CRh or CRi by a defined landmark (eg, after two cycles of intensive chemotherapy or 180 d for non-intensive therapy), hematologic relapse, MRD relapse (for patients achieving CR, CRh or CRi without MRD) or death from any cause |
| RFS | Measured from the date of achievement of a remission (CR, CRh, or CRi) until the date of hematologic relapse, MRD relapse, or death from any cause |
| CIR | Measured from the date of achievement of a remission (CR, CRh or CRi) until the date of hematologic relapse, or molecular MRD relapse; patients who died without relapse are counted as a competing cause of failure |
| CID | Measured from the date of achievement of a remission (CR, CRh, or CRi) to death without prior relapse; morphologic or molecular MRD relapse is considered as competing risk |
Relapse-free and disease-free survival have been used with the same definition.
Molecular MRD relapse should only consider data for mutated NPM1 , RUNX1 :: RUNX1T1 or CBFB :: MYH11 fusion transcripts as assessed by real-time quantitative PCR.
Response criteria
Cr, cri, partial remission (pr), and morphologic leukemia-free state (mlfs).
The criterion “absence of blasts with Auer rods” was eliminated.
CR with partial hematologic recovery
The term CR with partial hematologic recovery (CRh) has been introduced for patients with morphologic bone marrow blast clearance and partial recovery of both neutrophils (≥0.5 × 10 9 /L [500/µL]) and platelets (≥50 × 10 9 /L [50 000/µL]) because those represent clinical benefit to the patient; other CR criteria need to be met. Thus far, CRh has only been used in the context of trials evaluating less-intensive therapies. It is recommended that future studies validate the role of CRh as a surrogate measure of survival after intensive and less-intensive therapies.
Response criteria with MRD assessment
The 2017 ELN recommendations included the term CR without MRD (CR MRD− ) to recognize the increasing role of MRD technologies in stratifying prognosis of patients in CR. 1 The current response criteria expand MRD classification to include patients achieving CRh or CRi without MRD (CRh MRD− or CRi MRD− ).
Time window for response assessment
To recognize the potential for continuing improvements in blood counts after myelosuppressive therapy, response definitions for patients with marrow blast clearance (<5%) may be adjusted to reflect the best hematologic response achieved prior to commencement of the next treatment cycle. Aspirate reports that include MLFS, CRh, or CRi should note the potential for post-marrow blood counts to alter the final response designation.
No response
Patients evaluable for response but not meeting the criteria for CR, CRh, CRi, MLFS, or PR will be categorized as having “no response.”
Nonevaluable for response
For accurate reporting of response, it is necessary to include all registered/randomized patients on an intention to treat principle. Therefore, patients nonevaluable for response should be included in the denominator of response assessment analyses. This category may include patients yet to have a response assessment, suffering early death, exiting the study early, or those with a technically suboptimal bone marrow sample precluding assessment. Patients previously categorized as having death in aplasia or from indeterminate causes are now designated as nonevaluable for response.
Treatment failure
Relapsed disease is defined as ≥5% leukemic blasts in the bone marrow, reappearance of leukemic blasts in peripheral blood (PB) in at least 2 PB samples at least 1 week apart, or development of new extramedullary disease.
Refractory disease
If a specified response has not been achieved by a defined landmark (ie, failure to achieve response after 2 cycles of intensive chemotherapy or a predetermined landmark, eg, 180 days after commencing less-intensive therapy), the patient will be designated as having refractory disease .
CR, CRh, or CRi with MRD relapse
For patients initially achieving CR, CRh, or CRi without MRD, the term CR, CRh, or CRi with MRD relapse may be applied if there is evidence of MRD relapse as defined by ELN criteria ( Table 8 ). 67
Outcome measures
Systematic reporting of early death (eg, 30 and 60 days) is recommended to enable assessment of treatment-related mortality with new therapies being relevant for the therapy under consideration.
Although the primary end point for registrational studies in AML has historically been OS, the increased availability of poststudy treatment options with potential to confound OS interpretation may encourage adoption of alternative end points, such as event-free survival (EFS; or relapse-free survival [RFS] for postremission studies) as comparative outcome measures in registrational studies (see also “Clinical trials”). In a retrospective patient-level analysis of 8 randomized trials evaluating intensive chemotherapy conducted by the US Food and Drug Administration (FDA), EFS had the best correlation with OS when response was limited to a strict CR ( R 2 = 0.87; 95% confidence interval [CI], 0.47-0.98); EFS with the definition of response broadened to include CRi and CR with incomplete platelet recovery was also shown to correlate, albeit less strongly, with OS ( R 2 = 0.59; 95% CI, 0.13-0.93). 101 Limitations of the analysis included relatively small sample sizes, heterogeneity among trials, and lack of multivariate analyses.
For drugs that add myelosuppression (eg, venetoclax, CPX-351, gemtuzumab ozogamycin), the sole use of a strict CR in the definition of EFS is increasingly challenged. We recommend broadening the definition of EFS to include CRh or CRi in response. Patients not achieving response by the predetermined landmark (refractory disease) should have the event recorded on day 1 of registration in nonrandomized trials (or day 1 of random assignment in randomized trials). Patients who die before reaching the response landmark and prior to/without response assessments are considered treatment failures and should have the event recorded at day 1 of registration/randomization. Patients alive but nonevaluable for response are censored at day 1 of registration/randomization. To enable consistency in trial reporting, a response landmark for failure to achieve response should be prespecified. Furthermore, the response landmark should be relevant for the treatment received; for example, after completion of 2 cycles of intensive therapy or 180 days after commencing less-intensive approaches.
The incorporation of MRD outcomes as a measure of treatment failure necessitates the inclusion of new terms incorporating molecular MRD relapse into time to event definitions for EFS MRD , RFS MRD , and cumulative incidence of relapse (CIR MRD ; Table 9 ). For each study, clear definitions regarding how MRD relapse is determined should be specified in the statistical analysis plan.
The goal of treatment is control and, whenever possible, eradication of disease. This outcome is accomplished ideally by inducing a CR with initial therapy, followed by consolidation and/or maintenance efforts to deepen the remission and maximize response duration. The role of HCT and post-HCT therapies is discussed in the section on allogeneic HCT. Results of genetic analyses should be available as rapidly as possible, preferably within 3 to 5 days, to identify therapeutically actionable targets ( Table 4 ). A short delay in starting treatment to stabilize patients and identify the best treatment option is recommended to optimize clinical outcome. 102 If hyperleukocytosis is present, immediate cytoreduction is advised (see Management of special situations). If a patient cannot tolerate an active intensive or nonintensive treatment option, the purpose of therapy is to optimize quality of life and decrease the incidence of cytopenia-related complications with transfusion and other supportive care measures and early involvement of palliative care services as appropriate.
The survival of patients with AML that are related to previous therapy overall remains poor, which is mainly due to the high frequency of adverse (cyto)genetic features, 103,104 but also to the sequelae of prior therapy and sometimes persistent primary disease. In general, patients should be managed according to the same general therapeutic principles depending on whether they are candidates for intensive or nonintensive therapy and allogeneic HCT. 104,105 CPX-351 offers a new option for the treatment of these patients (see below).
Patients considered fit for intensive therapy
Induction therapy.
Anthracyclines and cytarabine remain the backbone of intensive chemotherapy. Alternative options are fludarabine, cytarabine, granulocyte colony-stimulating factor, and idarubicin (FLAG-IDA) and mitoxantrone-based cytarabine regimens ( Table 10 ). It has become standard to incorporate the kinase inhibitor midostaurin into first-line therapy for patients with FLT3 -mutant AML. Midostaurin improved 4-year OS by 7.1%, from 44.3 to 51.4% when used as an adjunct to daunorubicin-cytarabine induction and high-dose cytarabine consolidation in patients 18 to 59 years of age. 106 Although study treatment incorporated single-agent maintenance for 12 monthly cycles, the value of adding maintenance therapy remains uncertain. 107 In a prospective nonrandomized study, midostaurin also showed a beneficial effect in patients up to 70 years of age in comparison with a historical control group. 108
Selected treatment options for patients fit for intensive chemotherapy
| Fit for intensive chemotherapy . | Induction . | Consolidation . | Maintenance . |
|---|---|---|---|
| AML with mutation | Daunorubicin 60 mg/m IV d1-3; or idarubicin 12 mg/m IV d1-3; and cytarabine 100-200 mg/m /d CIV d1-7; plus midostaurin 50 mg q12h PO d8-21 Re-induction: either 2nd cycle “7 + 3” or regimen containing higher dose of cytarabine, each plus midostaurin, preferable the latter in patients with no response to 1st cycle | 3-4 cycles of IDAC 1000-1500 mg/m IV (500-1000 mg/m if ≥60 y old) over 3h q12h d1-3; plus midostaurin 50 mg q12h PO d8-21 (in all cycles) | Midostaurin 50 mg q12h PO d1-28, q4 wk, over 12 cycles |
| Non- mutant | Daunorubicin 60 mg/m IV d1-3, idarubicin 12 mg/m IV d1-3, or mitoxantrone 12 mg/m IV d1-3; and cytarabine 100-200 mg/m /d CIV d1-7 Re-induction: either 2nd cycle “7 + 3” or regimen containing higher dose of cytarabine, preferable the latter in patients with no response | 3-4 cycles of IDAC 1000-1500 mg/m IV (500-1000 mg/m if ≥60 y old) over 3h q12h d1-3 | Oral azacitidine 300 mg PO daily d1-14, q4 wk, until disease progression |
| Gemtuzumab ozogamicin (GO) for CD33-positive AML, favorable (or intermediate) cytogenetic risk | Daunorubicin 60 mg/m IV d1-3 and cytarabine 100-200 mg/m /d CIV d1-7; plus GO 3 mg/m (maximum dose 5 mg) IV, d1, 4, 7. GO is also widely administered on day 1 of induction only. Re-induction (if not in CR/CRh/CRi) may be with daunorubicin 60 mg/m IV d1-2 and cytarabine 1000 mg/m IV (500-1000 mg/m if ≥60 y old) over 3h q12h d1-3 without GO | 2-4 cycles of IDAC 1000-1500 mg/m IV (500-1000 mg/m if ≥60 y old) over 3h q12h d1-3. GO 3 mg/m may be added on d1 (in up to 2 cycles). Consider omitting GO if allogeneic HCT is planned to reduce the risk of veno-occlusive disease. | |
| CPX-351 for AML with myelodysplasia-related changes or therapy-related AML | CPX-351 100 U/m (daunorubicin 44 mg/cytarabine 100 mg) IV d1, 3, 5 Re-induction (if not in CR/CRh/CRi): CPX-351 100 U/m IV d1, 3 only | 1-2 cycles of CPX-351 65 U/m (daunorubicin 29 mg/cytarabine 65 mg) IV d1, 3 | |
| Gilteritinib (AML with mutation) | Gilteritinib 120 mg PO QD d1-28, q4 wk, until disease progression | ||
| Intermediate-dose cytarabine (with or without anthracycline) | Cytarabine 1000-1500 mg/m IV over 3h q12h d1-3 (500-1000 mg/m in patients ≥ 60y); with or without daunorubicin 60 mg/m IV d1-3; idarubicin 8-10 mg/m IV d3-5; or mitoxantrone 8-10 mg/m IV d1-3 | ||
| FLAG-IDA | Fludarabine 30 mg/m IV d2-6; cytarabine 1500-2000 mg/m IV over 3h, starting 4h after fludarabine infusion, d2-6; idarubicin 10 mg/m IV d2-4; G-CSF 5 µg/kg SC d1-5; additional G-CSF may be administered starting 7 d after end of chemotherapy until WBC count > 0.5 × 10 /L Consider dose reduction in patients ≥60 y: fludarabine 20 mg/m ; cytarabine 500-1000 mg/m ; idarubicin 8 mg/m | ||
| MEC | Mitoxantrone 8 mg/m IV d1-5; etoposide 100 mg/m IV d1-5; cytarabine 1000 mg/m IV d1-5 | ||
| CLAG-M | Cladribine 5 mg/m IV d1–5; cytarabine 2000 mg/m IV d1–5 (starting 2h after cladribine infusion); mitoxantrone 10 mg/m IV d1–3; G-CSF 300 μg SC d0–5 | ||
| Allogeneic HCT | Consider transplantation for patients with primary refractory disease, for patients in second CR (or CRh, CRi) or with major cytoreduction but still active disease following salvage therapy. Consider second transplantation under certain conditions. Perform early HLA typing. | ||
| Gilteritinib (AML with mutation) | 120 mg PO QD d1-28, q4 wk, until disease progression | ||
| Ivosidenib (AML with mutation) | 500 mg PO QD d1-28, q4 wk, until disease progression | ||
| Enasidenib (AML with mutation) | 100 mg PO QD d1-28, q4 wk, until disease progression | ||
| Fit for intensive chemotherapy . | Induction . | Consolidation . | Maintenance . |
|---|---|---|---|
| AML with mutation | Daunorubicin 60 mg/m IV d1-3; or idarubicin 12 mg/m IV d1-3; and cytarabine 100-200 mg/m /d CIV d1-7; plus midostaurin 50 mg q12h PO d8-21 Re-induction: either 2nd cycle “7 + 3” or regimen containing higher dose of cytarabine, each plus midostaurin, preferable the latter in patients with no response to 1st cycle | 3-4 cycles of IDAC 1000-1500 mg/m IV (500-1000 mg/m if ≥60 y old) over 3h q12h d1-3; plus midostaurin 50 mg q12h PO d8-21 (in all cycles) | Midostaurin 50 mg q12h PO d1-28, q4 wk, over 12 cycles |
| Non- mutant | Daunorubicin 60 mg/m IV d1-3, idarubicin 12 mg/m IV d1-3, or mitoxantrone 12 mg/m IV d1-3; and cytarabine 100-200 mg/m /d CIV d1-7 Re-induction: either 2nd cycle “7 + 3” or regimen containing higher dose of cytarabine, preferable the latter in patients with no response | 3-4 cycles of IDAC 1000-1500 mg/m IV (500-1000 mg/m if ≥60 y old) over 3h q12h d1-3 | Oral azacitidine 300 mg PO daily d1-14, q4 wk, until disease progression |
| Gemtuzumab ozogamicin (GO) for CD33-positive AML, favorable (or intermediate) cytogenetic risk | Daunorubicin 60 mg/m IV d1-3 and cytarabine 100-200 mg/m /d CIV d1-7; plus GO 3 mg/m (maximum dose 5 mg) IV, d1, 4, 7. GO is also widely administered on day 1 of induction only. Re-induction (if not in CR/CRh/CRi) may be with daunorubicin 60 mg/m IV d1-2 and cytarabine 1000 mg/m IV (500-1000 mg/m if ≥60 y old) over 3h q12h d1-3 without GO | 2-4 cycles of IDAC 1000-1500 mg/m IV (500-1000 mg/m if ≥60 y old) over 3h q12h d1-3. GO 3 mg/m may be added on d1 (in up to 2 cycles). Consider omitting GO if allogeneic HCT is planned to reduce the risk of veno-occlusive disease. | |
| CPX-351 for AML with myelodysplasia-related changes or therapy-related AML | CPX-351 100 U/m (daunorubicin 44 mg/cytarabine 100 mg) IV d1, 3, 5 Re-induction (if not in CR/CRh/CRi): CPX-351 100 U/m IV d1, 3 only | 1-2 cycles of CPX-351 65 U/m (daunorubicin 29 mg/cytarabine 65 mg) IV d1, 3 | |
| Gilteritinib (AML with mutation) | Gilteritinib 120 mg PO QD d1-28, q4 wk, until disease progression | ||
| Intermediate-dose cytarabine (with or without anthracycline) | Cytarabine 1000-1500 mg/m IV over 3h q12h d1-3 (500-1000 mg/m in patients ≥ 60y); with or without daunorubicin 60 mg/m IV d1-3; idarubicin 8-10 mg/m IV d3-5; or mitoxantrone 8-10 mg/m IV d1-3 | ||
| FLAG-IDA | Fludarabine 30 mg/m IV d2-6; cytarabine 1500-2000 mg/m IV over 3h, starting 4h after fludarabine infusion, d2-6; idarubicin 10 mg/m IV d2-4; G-CSF 5 µg/kg SC d1-5; additional G-CSF may be administered starting 7 d after end of chemotherapy until WBC count > 0.5 × 10 /L Consider dose reduction in patients ≥60 y: fludarabine 20 mg/m ; cytarabine 500-1000 mg/m ; idarubicin 8 mg/m | ||
| MEC | Mitoxantrone 8 mg/m IV d1-5; etoposide 100 mg/m IV d1-5; cytarabine 1000 mg/m IV d1-5 | ||
| CLAG-M | Cladribine 5 mg/m IV d1–5; cytarabine 2000 mg/m IV d1–5 (starting 2h after cladribine infusion); mitoxantrone 10 mg/m IV d1–3; G-CSF 300 μg SC d0–5 | ||
| Allogeneic HCT | Consider transplantation for patients with primary refractory disease, for patients in second CR (or CRh, CRi) or with major cytoreduction but still active disease following salvage therapy. Consider second transplantation under certain conditions. Perform early HLA typing. | ||
| Gilteritinib (AML with mutation) | 120 mg PO QD d1-28, q4 wk, until disease progression | ||
| Ivosidenib (AML with mutation) | 500 mg PO QD d1-28, q4 wk, until disease progression | ||
| Enasidenib (AML with mutation) | 100 mg PO QD d1-28, q4 wk, until disease progression | ||
CIV, continuous IV; IDAC; intermediate-dose cytarabine; PO, per os; QD, once daily; SC, subcutaneously.
Results from assessment of MRD should be taken into account for selecting the appropriate consolidation therapy.
In the trial that led to the regulatory approval of midostaurin for FLT3 -mutated AML, consolidation cycles included high-dose cytarabine at 3000 mg/m 2 , whereas intermediate dose levels of cytarabine (1000-1500 mg/m 2 ) are nowadays more commonly applied in AML therapeutics.
The value of maintenance treatment with midostaurin remains uncertain.
Alternative active frontline induction regimens that are sometimes used include FLAG-IDA (defined below under common salvage regimens).
Data regarding the role of oral azacitidine maintenance therapy in younger patients (< 55 y) or patients with core-binding factor AML are lacking; in addition, data are lacking for oral azacitidine after GO-based or CPX-351 induction/consolidation therapy.
Data in younger adult patients (< 60 y) and for AML post myeloproliferative neoplasm are lacking. No benefit compared with “7 + 3” induction was shown in patients with antecedent MDS with prior hypomethylating agent exposure.
Regimens containing higher doses of cytarabine are generally considered as the best option for patients not responding to a first cycle of “7 + 3.” Single-agent IDAC should not be used in patients relapsing within 6 mo following consolidation with higher doses of cytarabine.
Idarubicin may be replaced by mitoxantrone 10 mg/m 2 IV d2-4 (FLAG-MITO); or by amsacrine 100 mg/m 2 IV d2-4 (FLAG-AMSA).
Gilteritinib as a salvage option has only been validated in a randomized trial after prior intensive chemotherapy.
Based on single-arm data.
Although enasidenib did not show improved overall survival in a randomized study in comparison with conventional therapy in late-stage IDH2 -mutant AML, clinically useful single-agent anti-leukemic activity has been demonstrated.
Newer and potentially more potent FLT3 inhibitors are currently under randomized evaluation as therapeutic alternatives to midostaurin. 109,110 A placebo-controlled phase 3 trial enrolled 539 patients to either quizartinib or placebo in combination with intensive induction and consolidation chemotherapy followed by single agent quizartinib maintenance for up to 36 cycles in patients 18 to 75 years of age with FLT3 -ITD–positive AML. Post-HCT maintenance was permitted. Although peer-reviewed results are not yet available, a preliminary meeting abstract reported prolonged OS for quizartinib compared with placebo. Grade ≥3 treatment-emergent neutropenia was more frequent in the quizartinib arm; early death (≤30 days) was 5.7% and 3.1% in the quizartinib compared with placebo arms, respectively. 111
Gemtuzumab-ozogamicin (GO) is a humanized anti-CD33 IgG4 antibody chemically linked to a calicheamicin-based cytotoxic warhead. Following a history of initial FDA approval followed by retraction based on questionable clinical benefit, a subsequent randomized study demonstrated an EFS advantage among patients 50 to 70 years with de novo AML, with benefit limited to favorable or intermediate cytogenetic risk disease. 112,113 Although 4 other open-label randomized studies individually failed to demonstrate improved survival for GO added to front line therapy in AML, a meta-analysis of all 5 studies indicated a benefit, particularly in patients with CBF-AML. 114 In another randomized study, a reduction of the relapse probability and greater mutant NPM1 molecular clearance was shown in patients with NPM1 -mutated AML, but with no EFS difference. 70,115 GO dosed at 3 mg/m 2 (capped at 5 mg) D1, 4, and 7 of induction and day 1 of consolidation has been approved for patients with previously untreated CD33 antigen positive AML in combination with daunorubicin and cytarabine, but a single dose of GO delivered on day 1 of induction may also be efficacious. 114,116,117
CPX-351 is a dual-drug liposomal formulation that encapsulates cytarabine/daunorubicin in a 5:1 fixed molar ratio. 118 In an open label phase 3 randomized study in newly diagnosed patients aged 60 to 75 years with disease subtypes including therapy-related AML, a history of MDS or CMML, or de novo AML with myelodysplasia-related cytogenetic abnormalities CPX-351 improved the clinical response rate and OS compared with induction with cytarabine-daunorubicin, followed by “5 + 2” consolidation. 119 Five-year OS in the CPX-351 arm was improved from 10% to 18% compared with patients receiving “7 + 3.” 120 CPX-351 delayed the median time to neutrophil and platelet recovery by approximately 7 days and increased the risk of bleeding. Early 30-day mortality, however, was not increased by CPX-351 (5.9%) compared with “7 + 3” (10.6%), and less mucositis was noted. Randomized data are lacking for patients under 60 years and for AML following prior MPN.
Consolidation therapy
After attainment of CR (or CRh/CRi), patients are consolidated ideally with regimens that include intermediate-dose cytarabine. 121 Consecutive administration on days 1 to 3, rather than on alternate days (days 1, 3, and 5) may hasten blood count recovery. 122,123 Although high-dose cytarabine (3000 mg/m 2 ) is still used in some centers, its greater toxicity and failure to improve survival argues against its continued use. 124-126
In addition to baseline risk factors, assessment of MRD in CR (or CRh/CRi) is recommended for patients with nonadverse risk in first remission to inform consolidation treatment choice. For patients with an estimated relapse risk exceeding 35% to 40%, consolidation with allogeneic HCT remains the preferred postremission option. 127 These include patients with adverse-risk AML or nonadverse-risk disease with MRD persistence. Autologous HCT, although not widely used, offers an alternative postremission option for patients with favorable- or intermediate-risk disease with an adequate MRD response or for whom allogeneic HCT is not available. 128 In the subset of patients receiving induction with a FLT3 inhibitor, GO or CPX-351, these agents may be incorporated into consolidation ( Table 10 ).
Maintenance therapy
There is no generally accepted definition of “maintenance therapy.” In most previous trials, maintenance therapy has been administered for a defined period of time in patients who achieved remission after intensive chemotherapy. The FDA defines maintenance therapy for AML as an extended but time-limited course of treatment, that is usually less toxic, given after achievement of CR with the objective of reducing the risk of relapse. Thus, a trial designed to demonstrate the efficacy of maintenance therapy would need to include a specified induction and consolidation treatment followed by randomization to a predefined duration of treatment. 129
The main objective of maintenance therapy is to deliver a minimally toxic therapy capable of reducing the risk of leukemic relapse. In a randomized study in newly diagnosed older patients in first remission after 2 cycles of intensive induction, azacitidine maintenance therapy, administered subcutaneously for up to 12 cycles, improved disease-free survival compared with no maintenance. 130 An orally administered version of azacitidine, CC-486, given over 14 days in 28-day cycles as continuous postremission therapy, was shown subsequently in a randomized placebo-controlled trial to reduce relapse risk and improve median OS (from 14.8 to 24.7 months) among patients ≥ 55 years not considered candidates for allogeneic HCT. 131 Oral azacitidine prolonged OS independently of the MRD status as assessed by MFC (47% of patients were MRD positive and 53% were MRD negative at study entry). 132 Oral azacitidine is approved for continued treatment of patients with AML in first CR/CRi following intensive induction chemotherapy who are not able to complete intensive curative therapy, including allogeneic HCT. However, there are limitations to the trial design that prohibit generalizability of the data. 133 First, data regarding the role of oral azacitidine in younger populations or patients with CBF-AML are lacking; furthermore, only few patients had AML with adverse-risk cytogenetics (14%). Second, because the trial did not specify prior induction and consolidation therapy, there was considerable variability in therapy prior to selection for maintenance (ie, 45% of patients had received 1 consolidation cycle, 31% had 2 cycles, and 20% had no consolidation).
Patients who received midostaurin during induction and consolidation may continue these agents in maintenance in line with the reported phase 3 experience. 106
Patients not considered candidates for intensive therapy
There are no generally accepted or validated criteria to consider a patient ineligible for intensive chemotherapy. In the context of clinical trials, criteria have been used that consider a patient not eligible for intensive chemotherapy (for instance as defined in Table 11 ) that may also offer guidance in routine practice.
Selected treatment options for patients not suitable for intensive chemotherapy *
| Regimen . | Recommended dosing . |
|---|---|
| Azacitidine or decitabine + venetoclax , | Azacitidine 75 mg/m SC/IV d1-7 (alternatively d1-5 + d8-9) or decitabine 20 mg/m IV d1-5; venetoclax dose ramp up: 100 mg d1, 200 mg d2, 400 mg PO QD d3-28 • Adjust venetoclax dose if concurrent strong CYP3A4 inhibitors: 10 mg on d1, 20 mg on d2, 50 mg on d3, 100 mg (or less ) PO QD from d4 • For venetoclax dose modifications and management of myelosuppression see |
| Low-dose cytarabine + venetoclax , | Cytarabine 20 mg/m SC daily, d1-10; venetoclax dose ramp up: 100 mg d1, 200 mg d2, 400 mg d3, 600 mg d4-28 PO • Adjust venetoclax dose if concurrent strong CYP3A4 inhibitors: 10 mg d1, 20 mg d2, 50 mg d3, 100 mg (or less ) PO QD d4-28 • For venetoclax dose modifications and management of myelosuppression see |
| Azacitidine + ivosidenib (AML with mutation) | Azacitidine 75 mg/m SC/IV d1-7 (alternatively d1-5 + d8-9); ivosidenib 500 mg PO QD d1-28; both q4 wk, until progression |
| Ivosidenib (AML with mutation) | For very frail patients, ivosidenib 500 mg PO QD d1-28 as monotherapy, until progression may be considered |
| Best supportive care | Including hydroxyurea; for patients who cannot tolerate any anti-leukemic therapy, or who do not wish any therapy |
| Regimen . | Recommended dosing . |
|---|---|
| Azacitidine or decitabine + venetoclax , | Azacitidine 75 mg/m SC/IV d1-7 (alternatively d1-5 + d8-9) or decitabine 20 mg/m IV d1-5; venetoclax dose ramp up: 100 mg d1, 200 mg d2, 400 mg PO QD d3-28 • Adjust venetoclax dose if concurrent strong CYP3A4 inhibitors: 10 mg on d1, 20 mg on d2, 50 mg on d3, 100 mg (or less ) PO QD from d4 • For venetoclax dose modifications and management of myelosuppression see |
| Low-dose cytarabine + venetoclax , | Cytarabine 20 mg/m SC daily, d1-10; venetoclax dose ramp up: 100 mg d1, 200 mg d2, 400 mg d3, 600 mg d4-28 PO • Adjust venetoclax dose if concurrent strong CYP3A4 inhibitors: 10 mg d1, 20 mg d2, 50 mg d3, 100 mg (or less ) PO QD d4-28 • For venetoclax dose modifications and management of myelosuppression see |
| Azacitidine + ivosidenib (AML with mutation) | Azacitidine 75 mg/m SC/IV d1-7 (alternatively d1-5 + d8-9); ivosidenib 500 mg PO QD d1-28; both q4 wk, until progression |
| Ivosidenib (AML with mutation) | For very frail patients, ivosidenib 500 mg PO QD d1-28 as monotherapy, until progression may be considered |
| Best supportive care | Including hydroxyurea; for patients who cannot tolerate any anti-leukemic therapy, or who do not wish any therapy |
For instance, criteria that have been used in clinical trials to select patients not suitable for intensive chemotherapy have been as follows: (1) age ≥75 y (however, this cannot be an absolute criterion; for instance, patients with more favorable disease and without relevant comorbidities may derive benefit from intensive chemotherapy) or (2) ECOG performance status > 2 and/or age-related comorbidities, such as severe cardiac disorder (eg, congestive heart failure requiring treatment, ejection fraction ≤ 50%, or chronic stable angina), severe pulmonary disorder (eg, DLCO ≤ 65% or FEV1 ≤ 65%), creatinine clearance < 45 mL/min, hepatic disorder with total bilirubin > 1.5 times the upper limit of normal, or any other comorbidity that the physician assesses to be incompatible with intensive chemotherapy.
To reduce the risk of tumor lysis syndrome, the prophylactic use of uric acid lowering drugs, close electrolyte monitoring and cytoreduction of the WBC to < 25 x 10 9 /L or even lower, for patients with high bone marrow blast burden, elevated LDH is recommended.
In the VIALE-A and VIALE-C trials, an adjusted venetoclax dose of 50 mg was used in the presence of a strong CYP3A4 inhibitor. This venetoclax dose is supported by a pharmacokinetic study examining venetoclax in the presence of posaconazole. 207
Substantial progress has been made in the management of patients considered unfit for intensive chemotherapy ( Table 11 ). Compared with azacitidine alone, addition of the BCL2 inhibitor venetoclax improved clinical response (CR/CRi, 66.4% vs 28.3%) and median OS (14.7 vs 9.6 months), establishing a new standard of care for older or unfit patients with AML. 134 To limit prolonged myelosuppression and the risk of tumor lysis syndrome associated with this regimen, management recommendations are outlined in Table 12 . 135 Although not evaluated in randomized clinical trials, phase 1b/2 studies suggest that clinical outcomes with decitabine plus venetoclax are similar to the azacitidine plus venetoclax combination. 136 For patients failing frontline venetoclax-based therapy, prognosis appears very poor. 137 For patients unable to receive a hypomethylating agent (HMA), low-dose cytarabine (LDC) in combination with venetoclax represents an alternative treatment option. 138 Although an open-label randomized study showed improved survival for the hedgehog inhibitor glasdegib in combination with LDC, compared with LDC alone, the relatively low response rate (CR/CRi 24%) with this regimen does not favor its use as an alternative nonintensive option. 139
Novel agents: management of selected adverse events
| Agent . | AE requiring special attention (incidence all grades) . | Management . |
|---|---|---|
| Midostaurin | QT prolongation (10%) | Dose interruption/reduction, substitution of QT prolonging co-medication if possible, otherwise additional ECG controls |
| Gilteritinib | Transaminase elevation (81%) | Dose interruption/reduction (if grade ≥ 3) |
| QT prolongation (9%) | Dose interruption/reduction, substitution of QT prolonging co-medication if possible | |
| PRES (1%) | Discontinuation | |
| Ivosidenib | Differentiation syndrome (25% single agent, 17% in combination with azacitidine) | Dexamethasone, hydroxyurea for co-occurring leukocytosis, Dose interruption/reduction |
| QT prolongation (21% single agent, 26% combination with azacitidine) | Dose interruption/reduction, substitution of QT prolonging co-medication if possible | |
| Enasidenib | Differentiation syndrome (14% single agent, 10% in combination with azacitidine) | Dexamethasone, hydroxyurea for co-occurring leukocytosis, Dose interruption/reduction |
| Bilirubin elevation (81%) | Dose interruption/reduction | |
| Gemtuzumab ozogamicin | Transaminase elevation (24.5%) Bilirubin elevation (13%) | Dose interruption/reduction |
| VOD/SOS (2.9-4.6%) | Dose interruption, supportive care, fluid management, possibly defibrotide | |
| Venetoclax | Neutropenia Thrombocytopenia | Early response assessment, eg, on day 14-21 of cycle 1, if bone marrow blasts < 5%, cease venetoclax for up to 14 d to allow count recovery to ≥ CRh. If neutropenia does not recover with 7 d of ceasing venetoclax, addition of G-CSF may augment recovery. Subsequent cycles: azacitidine 75 mg/m SC/IV d1-7 (or d1-5 + d8-9) or decitabine 20 mg/m IV d1-5 plus venetoclax 400 mg QD, or LDC 20 mg/m SC d1-10 plus venetoclax 600 mg QD q4 wk until progression. Delayed count recovery or recurrent treatment-emergent grade 4 neutropenia/thrombocytopenia lasting ≥ 7 d require reductions in the duration of administered venetoclax (from 28 to 21 or 14 d, or even less) and/or reductions in the dose of azacitidine, decitabine, or LDC if severe bone marrow hypoplasia. |
| Tumor lysis syndrome | Dose ramp up in cycle 1; hydration, the prophylactic use of uric acid lowering drugs, close electrolyte monitoring and reduction of WBC to < 25 × 10 /L (< 25 000/µL) is recommended. | |
| Interaction with CYP3A inhibitors | • Moderate CYP3A inhibitors (eg, ciprofloxacin): reduce the venetoclax dose by at least 50%; ramp-up phase: 50 mg on d1, 100 mg on d2, 200 mg PO QD from d3 • Strong CYP3A inhibitors (eg, posaconazole): ramp-up phase: 10 mg on d1, 20 mg on d2, 50 mg on d3, 100 mg (or less ) QD PO from d4. | |
| Glasdegib | Muscle spams (15%) QT prolongation (8.3%) | Dose interruption/reduction Dose interruption/reduction, substitution of QT prolonging co-medication if possible |
| CPX-351 | Prolonged myelosuppression | Consequent anti-infectious prophylaxis |
| CC-486/oral azacitidine | Neutropenia (44%) Thrombocytopenia (33%) Nausea (65%), vomiting (60%), diarrhea (50%) | Dose interruption/reduction, myeloid growth factors Prophylactic anti-emetics |
| Agent . | AE requiring special attention (incidence all grades) . | Management . |
|---|---|---|
| Midostaurin | QT prolongation (10%) | Dose interruption/reduction, substitution of QT prolonging co-medication if possible, otherwise additional ECG controls |
| Gilteritinib | Transaminase elevation (81%) | Dose interruption/reduction (if grade ≥ 3) |
| QT prolongation (9%) | Dose interruption/reduction, substitution of QT prolonging co-medication if possible | |
| PRES (1%) | Discontinuation | |
| Ivosidenib | Differentiation syndrome (25% single agent, 17% in combination with azacitidine) | Dexamethasone, hydroxyurea for co-occurring leukocytosis, Dose interruption/reduction |
| QT prolongation (21% single agent, 26% combination with azacitidine) | Dose interruption/reduction, substitution of QT prolonging co-medication if possible | |
| Enasidenib | Differentiation syndrome (14% single agent, 10% in combination with azacitidine) | Dexamethasone, hydroxyurea for co-occurring leukocytosis, Dose interruption/reduction |
| Bilirubin elevation (81%) | Dose interruption/reduction | |
| Gemtuzumab ozogamicin | Transaminase elevation (24.5%) Bilirubin elevation (13%) | Dose interruption/reduction |
| VOD/SOS (2.9-4.6%) | Dose interruption, supportive care, fluid management, possibly defibrotide | |
| Venetoclax | Neutropenia Thrombocytopenia | Early response assessment, eg, on day 14-21 of cycle 1, if bone marrow blasts < 5%, cease venetoclax for up to 14 d to allow count recovery to ≥ CRh. If neutropenia does not recover with 7 d of ceasing venetoclax, addition of G-CSF may augment recovery. Subsequent cycles: azacitidine 75 mg/m SC/IV d1-7 (or d1-5 + d8-9) or decitabine 20 mg/m IV d1-5 plus venetoclax 400 mg QD, or LDC 20 mg/m SC d1-10 plus venetoclax 600 mg QD q4 wk until progression. Delayed count recovery or recurrent treatment-emergent grade 4 neutropenia/thrombocytopenia lasting ≥ 7 d require reductions in the duration of administered venetoclax (from 28 to 21 or 14 d, or even less) and/or reductions in the dose of azacitidine, decitabine, or LDC if severe bone marrow hypoplasia. |
| Tumor lysis syndrome | Dose ramp up in cycle 1; hydration, the prophylactic use of uric acid lowering drugs, close electrolyte monitoring and reduction of WBC to < 25 × 10 /L (< 25 000/µL) is recommended. | |
| Interaction with CYP3A inhibitors | • Moderate CYP3A inhibitors (eg, ciprofloxacin): reduce the venetoclax dose by at least 50%; ramp-up phase: 50 mg on d1, 100 mg on d2, 200 mg PO QD from d3 • Strong CYP3A inhibitors (eg, posaconazole): ramp-up phase: 10 mg on d1, 20 mg on d2, 50 mg on d3, 100 mg (or less ) QD PO from d4. | |
| Glasdegib | Muscle spams (15%) QT prolongation (8.3%) | Dose interruption/reduction Dose interruption/reduction, substitution of QT prolonging co-medication if possible |
| CPX-351 | Prolonged myelosuppression | Consequent anti-infectious prophylaxis |
| CC-486/oral azacitidine | Neutropenia (44%) Thrombocytopenia (33%) Nausea (65%), vomiting (60%), diarrhea (50%) | Dose interruption/reduction, myeloid growth factors Prophylactic anti-emetics |
AE, adverse event; LDC, low-dose cytarabine; PRES, posterior reversible encephalopathy syndrome; SmPC, Summary of Product Characteristics; SOS, sinusoidal obstructive syndrome, VOD, veno-occlusive disease.
Single agent.
Median times to absolute neutrophil count ≥ 0.5 × 10 9 /L (≥500/µL) were 35 and 29 days; and median times to platelet count ≥ 50 × 10 9 /L (≥50 000/µL) were 36.5 and 29 days after CPX-351 vs “7 + 3,” respectively, in patients who achieved CR/CRi after initial induction chemotherapy.
For newly diagnosed patients with IDH1 mutation, results from a randomized study indicate that the IDH1 inhibitor ivosidenib plus azacitidine improves EFS (hazard ratio, 0.33; 95% CI, 0.16-0.69), clinical response (CR/CRh, 52.8 vs 17.6%), and median OS (24.0 vs 7.9 months) compared with azacitidine plus placebo. 140 To identify patients suitable for ivosidenib at initial diagnosis, rapid IDH1 mutation screening in older patients with AML is recommended. Patients with IDH1 / 2 -mutated AML who are considered too frail to tolerate HMA-based treatment may be offered best supportive care or monotherapy with targeted IDH1/IDH2 inhibitors. 141
In patients receiving HMA-based combination therapy (with venetoclax, ivosidenib, other investigational agents), response should be assessed early during the first cycle (eg, on day 14-21) due to high rates of early responses seen with HMA combinations and the need to delay or modify dosing in the setting of persistent cytopenias in a leukemia-free marrow ( Table 12 ). A second assessment is commonly performed after 3 cycles and then repeated every 3 cycles for patients in remission or at the discretion of the physician outside of a clinical trial. In the absence of treatment intolerance, nonintensive treatment approaches have commonly been continued until disease progression, but for the time being, there are no data supporting the advantage of an open-ended duration approach over therapy for a confined period.
Relapsed and refractory disease
Common salvage regimens for patients with refractory or relapsed disease are given in Table 10 . At clinical progression, it is important to highlight the potential for clonal evolution and emergence of actionable targets not detected at diagnosis. Currently, these include emergence of IDH1 / IDH2 mutations or new or expanded FLT3 -ITD or FLT3 tyrosine kinase domain clones. 142-146 Therefore, molecular re-evaluation at relapse is important to identify patients who may be suitable for targeted salvage options. In the interest of therapeutic progress, it is recommended to enter these patients into clinical trials whenever possible. Patients failing to achieve remission after 2 cycles of induction (including at least 1 cycle of intermediate-dose cytarabine) are defined as having primary refractory AML. Patients are unlikely to benefit from further cycles of conventional chemotherapy and instead should be referred for consideration of allogeneic HCT or participation in clinical trials. 147
Factors associated with reduced survival at AML relapse include shorter RFS (<6-12 months), nonfavorable risk karyotype at diagnosis, older age (>45-55 years), or prior history of HCT. 148,149 In general, after cytoreduction has been achieved, allogeneic HCT is recommended. If HCT is not a realistic option (eg, in the older patient), disease control using a nonintensive option, such as HMA with or without venetoclax, may be appropriate. For patients with relapsed/refractory FLT3 -mutated disease, the kinase inhibitor gilteritinib has been approved based on a randomized trial showing improved response rates (CR, 21.1% vs 10.5%) and median OS (9.3 vs 5.6 months) in the gilteritinib arm compared with physician’s choice of salvage therapy. 109,150 Although more patients receiving gilteritinib were bridged to HCT (25.5% vs 15.3%) and these patients were permitted to restart gilteritinib 30 to 90 days after HCT, the clinical benefit of post-HCT gilteritinib remains uncertain. In addition, only 5.7% of patients received prior midostaurin as first-line therapy in this study, making generalization of treatment outcomes after this and other FLT3 inhibitors difficult. In a randomized trial evaluating the FLT3 inhibitor quizartinib in patients with relapsed/refractory FLT3 -ITD–positive AML, quizartinib also showed improved OS compared with conventional care regimens. 110 However, after evaluation of the trial data, neither FDA nor the European Medicines Agency granted approval.
For patients with relapsed/refractory IDH1 / IDH2 -mutant AML, salvage with ivosidenib or enasidenib is a possibility because these IDH inhibitors induce CR rates in the range of 20% and overall response, including hematologic improvement in approximately 40%. 151-153 Median time required to attain CR is ∼3 to 4 months, with 80% of cumulative responses attained after completion of 6 cycles of therapy. 151,152 Among responders, molecular clearance with ivosidenib was observed in 21% and was associated with longer remission duration and prolonged survival. 151 Although responders to enasidenib may also achieve molecular clearance, targeting IDH2 in a nonblinded randomized trial did not show improvement in OS compared with conventional care options among patients ≥60 years failing 2 or 3 prior lines of therapy. 154 For management of adverse events associated with novel agents, see the section on supportive care below and Table 12 .
AML is the most frequent indication for allogeneic HCT. 155,156 Advances allowing for the use of partially matched unrelated donors, cord blood, and haplo-identical family members mean that an allogeneic donor can be found for most patients in need. Nonmyeloablative and reduced intensity conditioning (RIC) regimens make allogeneic HCT possible in patients up to age 80 at experienced centers. 157,158 With newly approved methods to prevent and treat both infections and graft-versus-host disease (GVHD), outcomes following transplant continue to improve, leaving disease recurrence as the major cause of treatment failure. 159 Despite its central role in the management of adult AML, only a minority of patients for whom transplantation is indicated undergo the procedure. 156 Reasons for underutilization include biologic factors, personal and physician choice, and lack of access. 160
Indications for allogeneic HCT
The decision to perform allogeneic HCT during first remission depends on the risk-benefit ratio (ie, nonrelapse mortality [NRM] and disability/reduction in relapse risk) based on cytogenetic and molecular genetic features of disease at presentation and response to initial therapy, as well as patient, donor, and transplant factors. Allogeneic HCT should be considered when the relapse probability without the procedure is predicted to be >35% to 40%. 127 For patients with favorable-risk disease, allogeneic HCT in CR1 is generally not recommended except for those with inadequate clearance of MRD. 69,161-163 In contrast, allogeneic HCT is recommended for patients with adverse-risk AML and for the majority of those with intermediate-risk disease, although quite a few centers rely on the presence of MRD to guide their decision based on the predicted risk of relapse. For patients who are age 60 or older, mostly based on retrospective comparisons, allogeneic HCT in first remission is recommended for those with intermediate-risk or adverse-risk disease willing and able to undergo remission-inducing therapy. 164,165 Judicious patient selection is important in patients over 60 especially regarding the presence of comorbidities and support at home. Allogeneic HCT is the only curative therapy for patients with primary refractory disease and offers the best chance for cure in those who relapse after initial chemotherapy. 166 Other factors including comorbidities, donor source, and individual patient goals must be considered.
Comorbidities and risk scores
Several transplant-related models address the impact of comorbidities and disease risk. 167 The HCT comorbidity index (which has been modified to include age) sums a patient’s comorbidities into a single score that predicts the likelihood of NRM following transplantation independent of the disease being treated. 168,169 A disease-risk index based on disease-stage and cytogenetics predicts the likelihood of disease recurrence following transplantation independent of patient comorbidities. 170 The modified European Society of Blood and Marrow Transplantation risk score combines both patient and disease risk factors thus predicting OS rather than NRM or relapse risk. 171
Preparative regimen intensity
Transplant preparative regimens run the gamut from nonmyeloablative, which would result in only mild, temporary depression of blood counts without transplant, to RIC regimens of varying intensity, to high-dose true MAC. Prospective randomized trials yield inconsistent results, but in general, NRM is increased, and relapse rates are diminished with higher-dose regimens. The best evidence supporting the use of MAC regimens in patients aged 18 to 65 years comes from the randomized phase 3 BMT CTN 0901 study, which showed improved survival with MAC compared with RIC because of a marked reduction in disease recurrence. 172,173 In a retrospective analysis, the benefit of MAC was greatest in patients with genomic evidence of residual disease before transplant, as determined by NGS at the time of transplant. 98,100
Donor selection/GVHD prophylaxis
Registry analyses show approximate equivalence in outcomes for patients transplanted using a well-matched unrelated donor compared with those using a matched sibling donor. 174,175 However, many patients lack a suitable sibling or volunteer unrelated donor. The recent demonstration that posttransplant cyclophosphamide GVHD prophylaxis is tolerable and results in encouraging outcomes using mismatched unrelated and haplo-identical donors substantially widens the donor pool. 176-179 The use of single or double cord blood units with a high nucleated cell dose also results in excellent outcomes, particularly in patients with evidence of pretransplant MRD. 179,180 Current data support the utilization of a matched sibling donor or well-matched unrelated donor as the preferred donor option in adults with AML. 177 Recognition of germline predisposition in the patient with AML and family members influences donor selection, and the use of relatives with deleterious germline variants should be avoided (see Germline predisposition). Randomized trials comparing outcome after transplantation using a matched unrelated donor vs a haplo-identical donor are underway.
Pre- and posttransplant strategies to prevent posttransplant relapse
Disease relapse is the major cause of treatment failure in adults allografted for AML. 181 For patients who are in CR1 following 2 cycles of intensive therapy, there is no evidence that additional chemotherapy prior to transplantation reduces the risk of relapse regardless of pretransplant MRD status. There is increasing interest in the use of pharmacological or cellular therapy posttransplant to prevent disease recurrence. In patients allografted for FLT3 -mutated AML, randomized studies show that maintenance with the FLT3 inhibitor sorafenib, although sometimes challenging to deliver, reduces the risk of relapse, suggesting that the use of a FLT3 inhibitor is a reasonable option. 182,183 A randomized trial examining the benefit of posttransplant maintenance with the second-generation FLT3 inhibitor gilteritinib in this patient population is in progress. There is less evidence supporting the use of other agents as posttransplant maintenance in AML. A randomized study of maintenance using subcutaneous azacitidine showed no benefit and is not recommended based on available evidence 184 ; oral azacitidine (CC-486) is currently under study.
Relapse after transplant
Ninety percent of those who relapse after an allogeneic HCT for AML do so by 2 years. The outcome of patients with morphologic relapse within the first 12 months is very poor, although a rapid taper of immunosuppression or donor lymphocyte infusion may salvage a proportion of patients with early molecular or cytogenetic relapse. 185,186 For patients relapsing after an allogeneic HCT for FLT3 -mutated AML, giltertinib is the preferred treatment option with evidence of an emergent FLT3 mutant clone. In the pivotal study, giltertinib improved survival in patients with early relapses and was at least equivalent compared with intensive chemotherapy in relapses occurring beyond 6 months. 109,150 Azacitidine, with or without donor lymphocyte infusion, and venetoclax-based salvage regimens may produce remissions in a small proportion of patients with less toxicity than intensive chemotherapy. 187 Those who achieve a second CR can sometimes still be cured with either donor lymphocyte infusion or a second allograft. 188
It is recommended to enroll patients with AML onto clinical trials whenever a suitable trial opportunity is available. Real-time availability of rapid biomarker screening has become a basic requirement to enable timely enrollment of patients to clinical trials targeting defined AML subpopulations. Routine biobanking of patient samples should be standard practice to maximize clinical research.
Trial design
The execution of clinical trials for drug development in AML has become progressively challenging. There is an increasing number of novel AML therapeutics that warrant evaluation of safety and efficacy, in single agent and combination format, with many requiring prospective allocation to biologically defined genotypes. As AML is already a relatively rare disease, timely completion of adequately powered phase 3 clinical trials within smaller disease subsets has become more challenging, highlighting the growing need for intercontinental trials.
Early-phase clinical development
Innovation in clinical trial design is needed. Phase 1 exploration of new AML drugs in the relapsed/refractory setting remains a formidable task, with high levels of drug resistance, rapid disease progression, and complications related to severe cytopenias representing key hurdles to success. In such settings, a pharmacodynamic primary end point verifying the drug’s proposed mechanism of action may represent an appropriate objective during the single agent dose-finding stage, followed by rapid transition to combination testing to demonstrate clinical efficacy. In phase 2, multiarm biomarker-stratified studies permitting parallel investigation of several drugs simultaneously will facilitate more efficient screening of new drugs and combinations for clinical activity. 189
Phase 3 trials
Randomized trials are the cornerstone of drug approval, especially in newly diagnosed patients. Accelerated recruitment to such trials is of central importance to improvements in outcome for patients with AML and yet paradoxically this remains a notoriously slow process. For example, the regulatory approval of midostaurin in 2017 for FLT3 -mutant AML using OS as the primary study end point took almost a decade. The increasing number of new therapies in AML coupled with genomic stratification is creating significant challenges to the timely recruitment of patients to practice informing trials. As more effective salvage therapies are now available, the OS end point is complicated further by subsequent lines of AML-directed therapy; crossover of patients from the control arm to novel agents has confounded the interpretation of OS increasingly in comparative studies (see “Outcome measures”). EFS as a primary study end point will not only eliminate the confounding effect of poststudy therapies, but as an additional advantage, it will shorten study completion timelines. In this regard, the use of the restricted “traditional” CR as one of the key events in EFS has become subject of debate. Because of frequent myelosuppression with novel drug combinations, and in addition, the need to proceed with therapy before full hematologic recovery, from a therapeutic point, it has become increasingly unrealistic to consider failure to attain CRh/CRi as events in EFS estimates even though the level of survival after CRh/CRi may be below that following CR. 101 Another way of expediting earlier assessment of drug efficacy is to base outcomes on standardized MRD measurements. 67 To facilitate incorporation of MRD as an efficacy end point, CR (or CRh/CRi) with MRD response and EFS with molecular MRD relapse as an event represent promising new study end points. This will allow for direct comparisons between the quantitative depth of response of investigational and reference therapies as indicators of relative therapeutic value.
Acceleration of drug development could also benefit from using a validated control population, thus omitting the concurrent standard control arm so that all patients recruited to the trial receive investigational therapy. To realize such an approach, a well annotated and contemporary external reference cohort is required and efforts to establish real-world databases for this purpose are being explored. Finally, it remains of utmost importance to override geographic and interstudy group barriers and continue efforts to stimulate the formation of “global” alliances and networks to expedite completion of registration-enabling clinical studies within a markedly condensed time window.
Clinical investigation of new therapies and new combinations is of critical importance in continuing to improve AML patient outcomes. 190 Drug development strategies have focused until now primarily on single-agent dose finding studies in the relapsed setting, which have led to successful approvals of targeted therapies, such as FLT3, IDH1, and IDH2 inhibitors, and is the pattern for the current evaluation of menin inhibitors for patients with KMT2A rearrangements or NPM1 mutations. 109,151,152,191
Other agents (ie, epigenetically targeted therapies) and immunotherapy approaches including bi-specific T-cell engaging antibodies, checkpoint inhibitors, and chimeric antigen receptor T cells or natural killer cells are likely to be most effective in the setting of MRD, in frontline, or early salvage combination approaches. 190,192,193 Although of limited single agent activity, the CD47 inhibitor magrolimab has demonstrated preliminary activity in combination with azacitidine in patients with newly diagnosed MDS and AML, even in the setting of TP53 -mutated disease. Trials of various inhibitors of the CD47-SIRP-α macrophage checkpoint are currently under various stages of early clinical evaluation. 194
Due to the changing therapeutic environment, which now includes HMAs in combination with small molecule inhibitors like venetoclax or targeted therapies, future development of frontline combinations is now more complex. The evaluation of so-called “triplet” therapies is an increasingly common clinical trial design for “chemotherapy-ineligible” patients, which involves the evaluation of a third agent (either approved or investigational) to the HMA and venetoclax “backbone.” New combination trials in intensive chemotherapy eligible patients typically involve the incorporation of a new target or agent in combination with standard chemotherapy, such as the ongoing clinical trials of the FLT3 inhibitor gilteritinib with standard “7 + 3” vs “7 + 3” and midostaurin or the spleen tyrosine kinase (SYK) inhibitor with “7 + 3” vs “7 + 3” alone for NPM1 -mutant AML.
In addition, oral formulations of the HMAs are now approved for AML maintenance (oral azacitidine) 131 and high-risk MDS (oral decitabine/cedazuridine), 195 respectively, and given the increased patient convenience of oral formulations, it is likely that these agents will be increasingly used in future HMA-based combination trials.
A white blood cell count (WBC) > 100 × 10 9 /L is generally defined as hyperleukocytosis and associated with increased induction mortality mainly due to hemorrhagic events, tumor lysis syndrome, and the risk for clinical leukostasis syndrome. 196 Hydroxyurea (up to 50-60 mg/kg per day) is most commonly used to lower the WBC below 25 × 10 9 /L, particularly before the commencement of HMA- or venetoclax-based treatments. Clinical leukostasis syndrome is a medical emergency requiring the WBC to be promptly lowered without delay by either hydroxyurea or planned induction therapy and a restrictive transfusion policy for red blood cells. Retrospective studies suggest a beneficial effect of dexamethasone, which may counteract effects of leukostasis. 197 Although leukapheresis may be performed in parallel with chemotherapy in patients with leukostasis syndrome, 198 current evidence does not support the use of leukapheresis in asymptomatic patients with hyperleukocytosis. 199,200
Other special situations requiring therapeutic intervention are the presence of disseminated intravascular coagulation (DIC), tumor lysis syndrome, and differentiation syndrome. DIC can be screened for using a scoring system and is present in 8.5% to 25% of patients with non–APL, with another ∼15% also developing DIC soon after the initiation of chemotherapy. 201 Special attention to tumor lysis syndrome is required in patients with hyperleukocytosis or with venetoclax-based treatments ( Table 12 ). Close monitoring for signs of differentiation syndrome such as unexplained fever, lung edema, weight gain, pulmonary infiltrates, hypoxia, and dyspnea is necessary, particularly in patients on treatment with IDH inhibitors. 202
Anti-infectious prophylaxis
For prophylaxis and treatment of infections, prevailing institutional infectious organisms and their drug resistance pattern should be considered primarily. There is good evidence to recommend antifungal prophylaxis with posaconazole during remission induction therapy, 203 whereas there is not enough evidence from randomized trials on antiviral prophylaxis for herpes simplex virus in patients with acute leukemia, 204 and no evidence for a beneficial effect of Pneumocystis jirovecii pneumonia prophylaxis. For prophylaxis of infectious disease in the setting of allogeneic HCT, we refer to respective guidelines. 205
Vaccination for influenza 206 and COVID-19 viral infections is recommended for all patients to reduce the risk of severe infections.
The use of growth factors is not routinely recommended unless in individual patients (eg, in case of severe infections) or particular treatment settings (eg, to reduce the hematologic recovery times in consolidation cycles). 1,122,123
Transfusions
The availability of several effective novel agents may lead to a higher proportion of patients treated on an outpatient basis. If blood count checks are not possible at regular intervals in the outpatient setting, platelet and hemoglobin transfusion triggers should be elevated to ensure adequate support until the next outpatient visit. Besides the platelet count, mucosal bleeding, infection, severe mucositis, and fever should be considered in the assessment of bleeding risk and should increase the platelet level transfusion threshold. Otherwise, it is generally accepted to keep the hemoglobin level above 8 g/dL, and a platelet count of <10 × 10 9 /L remains the trigger for prophylactic platelet transfusions.
The authors gratefully acknowledge Rüdiger Hehlmann for his continuous generous support of these recommendations on behalf of the European LeukemiaNet and Partrycja Gradowska, Axel Benner, Maral Saadati, and Julia Krzykalla for reviewing the sections “Response criteria” and “Outcome measures.”
H. Döhner is supported by SFB 1074 “Experimental models and clinical translation in leukemia” funded by the Deutsche Forschungsgemeinschaft. A.H.W. is supported by the Australian National Health and Medical Research Council, Victorian Cancer Agency, Metcalf Family Fellowship, and the Medical Research Future Fund. C.D.D. is supported by the LLS Scholar in Clinical Research award.
Clara Bloomfield, Elihu Estey and Francesco Lo-Coco have died since the publication of the 2017 ELN AML Recommendation edition. The authors acknowledge the impressive seminal contributions that Clara Bloomfield, Elihu Estey, and Francesco Lo-Coco made throughout many years to improve the treatment of AML and advance our understanding of the pathobiology of the disease. They were founding coauthors of the ELN AML Recommendation editions that first appeared in 2010 and (pro)actively participated with a high level of commitment in this endeavor. Each one in their own unique way contributed their invaluable clinical expertise, knowledge, and scientific rigor and were instrumental in establishing these recommendations as a widely accepted standard of reference.
Contribution: All authors reviewed the literature and wrote first drafts of specific sections. H. Döhner and B.L. assembled the sections and wrote the final version of the manuscript. All authors reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: H. Döhner had an advisory role with honoraria for AbbVie, Agios, Amgen, Astellas, AstraZeneca, Berlin-Chemie, BMS, Celgene, GEMoaB, Gilead, Janssen, Jazz, Novartis, Servier, and Syndax and received clinical research funding (to institution) from AbbVie, Agios, Amgen, Astellas, Bristol Myers Squibb, Celgene, Jazz Pharmaceuticals, Kronos Bio, and Novartis. A.H.W. had an advisory role with honoraria for AbbVie, Agios, Amgen, Astellas, AstraZeneca, Roche, BMS, Celgene, Gilead, Pfizer, Janssen, Jazz, Novartis, and Servier; received clinical research funding (to institution) from AbbVie, Servier, Celgene-BMS, Astra Zeneca, Amgen, and Novartis; and receives a fraction of royalty payments from the Walter and Eliza Hall Institute of Medical Research related to venetoclax. F.R.A. has consulted for Jasper Biote. C.C. had an advisory role with honoraria for AbbVie, Amgen, Astellas, AstraZeneca, Berlin-Chemie, BMS, Celgene, Daiichi-Sankyo, Eurocept, Gilead, Janssen, Jazz, and Novartis and received clinical research funding from AbbVie, Bristol Myers Squibb, Celgene, and Jazz Pharmaceuticals. C.D.D. received honoraria/consulting fees from AbbVie, Agios/Servier, Astellas, Celgene/BMS, Cleave, Foghorn, Genentech, GenMab, GSK, Novartis, Notable Labs, Takeda and research grants (to institution) from AbbVie, Agios/Servier, Astex, Calithera, Celgene/BMS, Cleave, Foghorn, ImmuneOnc, and Loxo. H. Dombret received honoraria/consulting fees from AbbVie, Amgen, Astellas, Celgene-BMS, Daiichi Sankyo, Incyte, Jazz Pharmaceuticals, Pfizer, and Servier and research funding from Amgen, Astellas, Celgene-BMS, Incyte, Jazz Pharmaceuticals, and Pfizer. B.L.E. received consulting fees from GRAIL and research funding from Celgene, Deerfield, Novartis, and Calico and is a member of the scientific advisory board and shareholder for Neomorph Therapeutics, TenSixteen Bio, Skyhawk Therapeutics, and Exo Therapeutics. P.F. received honoraria and, as French MDS group chairperson, research support from Celgene/BMS, Novartis, AbbVie, Jazz, Janssen, and Agios. R.A.L. has been a consultant or advisor to Amgen, Ariad/Takeda, Astellas, Celgene/BMS, CVS/Caremark, Epizyme, Immunogen, MedPace, MorphoSys, Novartis, and Servier and has received clinical research support to his institution from Astellas, Celgene, Cellectis, Daiichi Sankyo, Forty Seven/Gilead, Novartis, Rafael Pharmaceuticals and royalties from UpToDate. R.L.L. was on the supervisory board of Qiagen and an scientific advisor to Imago, Mission Bio, Zentalis, Ajax, Auron, Prelude, C4 Therapeutics, and Isoplexis, for which he receives equity; received research support from Calico, Constellation, Zentalis, and Ajax; consulted for Incyte, Janssen, Morphosys, and Novartis; and received honoraria from Roche, Lilly, and Amgen for invited lectures and from Gilead for grant reviews. Y.M. received honoraria from Nippon-Shinyaku, Bristol-Myers Squib, Novartis, Sumitomo Pharma, Kyowa-Kirin, AbbVie, Daiichi-Sankyo, Takeda, Janssen Pharmaceutical, Astellas, Pfizer, Eizai, and Otsuka Pharmaceutical and research funding from Sumitomo-Dainippon. G.O. had an advisory role with honoraria for AbbVie, Astellas, Bristol Myers Squibb, Celgene, Gilead, Servier, JAZZ, and Novartis. C.R. had an advisory role with honoraria for AbbVie, Amgen, Astellas, BMS, Celgene, Jazz, Novartis, Pfizer, and Servier and received clinical research funding from AbbVie, Novartis, and Pfizer. J.S. had an advisory role with honoraria for AbbVie, Astellas, AstraZeneca, Berlin-Chemie, Celgene, AvenCell-GEMoaB, Janssen, Jazz Pharmaceuticals, Novartis, and Bristol Myers Squibb and received clinical research funding from AbbVie, Astellas, Jazz Pharmaceuticals, and Jose Carreras International Leukemia Foundation. E.M.S. was on the advisory board for Novartis, PinotBio, Janssen, Bristol Myers Squibb, Agios, Jazz, Menarini, Genentech, Genesis, AbbVie, Neoleukin, Gilead, Syndax, OnCusp, CTI Biopharma, Foghorn, Servier, Calithera, Daiichi, Aptose, Syros, Astellas, Ono Pharma, and Blueprint; received honoraria from Kura; performed safety monitoring for Epizyme and Cellectis; and received research funding for Eisai and Bristol Myers Squibb and equity from Auron. M.S.T. was on the advisory boards for AbbVie, Daiichi-Sankyo, Orsenix, KAHR, Oncolyze, Jazz Pharma, Roche, Biosight, Novartis, Innate Pharmaceuticals, Kura, Syros Pharmaceuticals, Ipsen Biopharmaceuticals, and Cellularity and received research funding from AbbVie, Orsenix, Biosight, Gylcomimetics, Rafael Pharmaceuticals, and Amgen and royalties from UpToDate. H.-F.T. had an advisory role with honoraria for AbbVie, Alexion, Celgene, Daiichi Sankyo, Novartis, and Roche and received research funding from Celgene. J.W. had an advisory role with honoraria for AbbVie. A.W. had an advisory role with honoraria for AbbVie, Astellas, BMS, Celgene, Gilead, Janssen, Jazz Pharmaceuticals, Novartis, and Servier and received clinical research funding from Jazz Pharmaceuticals. B.L. had an advisory role with honoraria for AbbVie, Astellas, Bristol Myers Squibb/Celgene, Catamaran Bio, AvenCell/GEMoaB, Frame Pharmaceuticals, Gilead, Kronos Bio, Oxford Biomedica, and Servier/Agios and received royalties from UpToDate and equity from Frame Pharmaceuticals. All remaining authors declare no competing financial interests.
Correspondence: Hartmut Döhner, Department of Internal Medicine III, Ulm University Hospital, Albert-Einstein-Allee 23, 89081 Ulm, Germany; e-mail: hartmut [email protected] .
There is a Blood Commentary on this article in this issue.
bZIP mutations in CEBPA
This feature is available to subscribers only.
- Previous Article
- Next Article
Email alerts
Affiliations.
- Current Issue
- First edition
- Collections
- Submit to Blood
- About Blood
- Subscriptions
- Public Access
- Permissions
- Blood Classifieds
- Advertising in Blood
- Terms and Conditions
American Society of Hematology
- 2021 L Street NW, Suite 900
- Washington, DC 20036
- TEL +1 202-776-0544
- FAX +1 202-776-0545
ASH Publications
- Blood Advances
- Blood Neoplasia
- Blood Vessels, Thrombosis & Hemostasis
- Hematology, ASH Education Program
- ASH Clinical News
- The Hematologist
- Publications
- Privacy Policy
- Cookie Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Acute myeloid leukemia: Treatment and research outlook for 2021 and the MD Anderson approach
Affiliation.
- 1 Department of Leukemia, MD Anderson Cancer Center, Houston, Texas.
- PMID: 33734442
- DOI: 10.1002/cncr.33477
The unraveling of the pathophysiology of acute myeloid leukemia (AML) has resulted in rapid translation of the information into clinical practice. After more than 40 years of slow progress in AML research, the US Food and Drug Administration has approved nine agents for different AML treatment indications since 2017. In this review, we detail the progress that has been made in the research and treatment of AML, citing key publications related to AML research and therapy in the English literature since 2000. The notable subsets of AML include acute promyelocytic leukemia (APL), core-binding factor AML (CBF-AML), AML in younger patients fit for intensive chemotherapy, and AML in older/unfit patients (usually at the age cutoff of 60-70 years). We also consider within each subset whether the AML is primary or secondary (therapy-related, evolving from untreated or treated myelodysplastic syndrome or myeloproliferative neoplasm). In APL, therapy with all-trans retinoic acid and arsenic trioxide results in estimated 10-year survival rates of ≥80%. Treatment of CBF-AML with fludarabine, high-dose cytarabine, and gemtuzumab ozogamicin (GO) results in estimated 10-year survival rates of ≥75%. In younger/fit patients, the "3+7" regimen (3 days of daunorubicin + 7 days of cytarabine) produces less favorable results (estimated 5-year survival rates of 35%; worse in real-world experience); regimens that incorporate high-dose cytarabine, adenosine nucleoside analogs, and GO are producing better results. Adding venetoclax, FLT3, and IDH inhibitors into these regimens has resulted in encouraging preliminary data. In older/unfit patients, low-intensity therapy with hypomethylating agents (HMAs) and venetoclax is now the new standard of care. Better low-intensity regimens incorporating cladribine, low-dose cytarabine, and other targeted therapies (FLT3 and IDH inhibitors) are emerging. Maintenance therapy now has a definite role in the treatment of AML, and oral HMAs with potential treatment benefits are also available. In conclusion, AML therapy is evolving rapidly and treatment results are improving in all AML subsets as novel agents and strategies are incorporated into traditional AML chemotherapy. LAY SUMMARY: Ongoing research in acute myeloid leukemia (AML) is progressing rapidly. Since 2017, the US Food and Drug Administration has approved 10 drugs for different AML indications. This review updates the research and treatment pathways for AML.
Keywords: acute myelogenous leukemia; new drugs; progress; research; therapy.
© 2021 American Cancer Society.
PubMed Disclaimer
Similar articles
- Venetoclax-based therapies for acute myeloid leukemia. Guerra VA, DiNardo C, Konopleva M. Guerra VA, et al. Best Pract Res Clin Haematol. 2019 Jun;32(2):145-153. doi: 10.1016/j.beha.2019.05.008. Epub 2019 May 24. Best Pract Res Clin Haematol. 2019. PMID: 31203996 Free PMC article. Review.
- Secondary AML Emerging After Therapy with Hypomethylating Agents: Outcomes, Prognostic Factors, and Treatment Options. Richardson DR, Green SD, Foster MC, Zeidner JF. Richardson DR, et al. Curr Hematol Malig Rep. 2021 Feb;16(1):97-111. doi: 10.1007/s11899-021-00608-6. Epub 2021 Feb 20. Curr Hematol Malig Rep. 2021. PMID: 33609248 Review.
- Single-agent and combination biologics in acute myeloid leukemia. Richard-Carpentier G, DiNardo CD. Richard-Carpentier G, et al. Hematology Am Soc Hematol Educ Program. 2019 Dec 6;2019(1):548-556. doi: 10.1182/hematology.2019000059. Hematology Am Soc Hematol Educ Program. 2019. PMID: 31808888 Free PMC article. Review.
- Venetoclax plus intensive chemotherapy with cladribine, idarubicin, and cytarabine in patients with newly diagnosed acute myeloid leukaemia or high-risk myelodysplastic syndrome: a cohort from a single-centre, single-arm, phase 2 trial. Kadia TM, Reville PK, Borthakur G, Yilmaz M, Kornblau S, Alvarado Y, Dinardo CD, Daver N, Jain N, Pemmaraju N, Short N, Wang SA, Tidwell RSS, Islam R, Konopleva M, Garcia-Manero G, Ravandi F, Kantarjian HM. Kadia TM, et al. Lancet Haematol. 2021 Aug;8(8):e552-e561. doi: 10.1016/S2352-3026(21)00192-7. Lancet Haematol. 2021. PMID: 34329576 Free PMC article. Clinical Trial.
- The cure of leukemia through the optimist's prism. Kantarjian HM, Jain N, Garcia-Manero G, Welch MA, Ravandi F, Wierda WG, Jabbour EJ. Kantarjian HM, et al. Cancer. 2022 Jan 15;128(2):240-259. doi: 10.1002/cncr.33933. Epub 2021 Oct 6. Cancer. 2022. PMID: 34614211 Free PMC article. Review.
- On the mechanism of wogonin against acute monocytic leukemia using network pharmacology and experimental validation. Wang X, Wang Y, Chen J, Wang Q, Liu Z, Yin Y, Yang T, Shen T, Sa Y. Wang X, et al. Sci Rep. 2024 May 2;14(1):10114. doi: 10.1038/s41598-024-60859-0. Sci Rep. 2024. PMID: 38698063 Free PMC article.
- Venetoclax Combination Treatment of Acute Myeloid Leukemia in Adolescents and Young Adult Patients. Chatzikalil E, Roka K, Diamantopoulos PT, Rigatou E, Avgerinou G, Kattamis A, Solomou EE. Chatzikalil E, et al. J Clin Med. 2024 Apr 1;13(7):2046. doi: 10.3390/jcm13072046. J Clin Med. 2024. PMID: 38610812 Free PMC article. Review.
- Curcumin in treatment of hematological cancers: Promises and challenges. Entezari M, Tayari A, Paskeh MDA, Kheirabad SK, Naeemi S, Taheriazam A, Dehghani H, Salimimoghadam S, Hashemi M, Mirzaei S, Samarghandian S. Entezari M, et al. J Tradit Complement Med. 2024 Jan 5;14(2):121-134. doi: 10.1016/j.jtcme.2023.10.004. eCollection 2024 Mar. J Tradit Complement Med. 2024. PMID: 38481552 Free PMC article. Review.
- Discovery of 5-trifluoromethyl-2-aminopyrimidine derivatives as potent dual inhibitors of FLT3 and CHK1. Deng M, Gao Y, Wang P, Du W, Xu G, Li J, Zhou Y, Liu T. Deng M, et al. RSC Med Chem. 2023 Dec 7;15(2):539-552. doi: 10.1039/d3md00597f. eCollection 2024 Feb 21. RSC Med Chem. 2023. PMID: 38389894
- Probing the Internalization and Efficacy of Antibody-Drug Conjugate via Site-Specific Fc-Glycan Labelling of a Homogeneous Antibody Targeting SSEA-4 Bearing Tumors. Shivatare VS, Huang HW, Tseng TH, Chuang PK, Zeng YF, Wong CH. Shivatare VS, et al. Isr J Chem. 2023 Oct;63(10-11):e202300042. doi: 10.1002/ijch.202300042. Epub 2023 Apr 22. Isr J Chem. 2023. PMID: 38348405 Free PMC article.
- Kantarjian H. Acute myeloid leukemia-major progress over four decades and glimpses into the future. Am J Hematol. 2016;91:131-145.
- Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392:593-606.
- Kadia TM, Ravandi F, Cortes J, Kantarjian H. Toward individualized therapy in acute myeloid leukemia: a contemporary review. JAMA Oncol. 2015;1:820-828.
- Dohner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med. 2015;373:1136-1152.
- Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249-1259.
Publication types
- Search in MeSH
Related information
- PubChem Compound (MeSH Keyword)
Grants and funding
- CA100632/MD Anderson Cancer Center Leukemia SPORE
- Charif Souki Cancer Research Grant.
- P30 CA016672/CA/NCI NIH HHS/United States
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
Other Literature Sources
- scite Smart Citations
- Genetic Alliance
- MedlinePlus Health Information
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
SYSTEMATIC REVIEW article
Acute myeloid leukemia: from biology to clinical practices through development and pre-clinical therapeutics.

- 1 Inserm EFS BFC, UMR1098 RIGHT, University Bourgogne Franche-Comté, Besançon, France
- 2 Department of Hematology, University Hospital of Besançon, Besançon, France
Recent studies have provided several insights into acute myeloid leukemia. Studies based on molecular biology have identified eight functional mutations involved in leukemogenesis, including driver and passenger mutations. Insight into Leukemia stem cells (LSCs) and assessment of cell surface markers have enabled characterization of LSCs from hematopoietic stem and progenitor cells. Clonal evolution has been described as having an effect similar to that of microenvironment alterations. Such biological findings have enabled the development of new targeted drugs, including drug inhibitors and monoclonal antibodies with blockage functions. Some recently approved targeted drugs have resulted in new therapeutic strategies that enhance standard intensive chemotherapy regimens as well as supportive care regimens. Besides the progress made in adoptive immunotherapy, since allogenic hematopoietic stem cell transplantation enabled the development of new T-cell transfer therapies, such as chimeric antigen receptor T-cell and transgenic TCR T-cell engineering, new promising strategies that are investigated.
Introduction
In 2010 and 2017, the European LeukemiaNet (ELN) international expert panel published recommendation for the diagnosis and management of acute myeloid leukemia (AML) ( 1 , 2 ). Furthermore an update of the WHO classification in 2016 provided a few changes to the previously described disease categories ( 3 ). This new classification of AML is focused on the determination of genetic abnormalities via molecular based investigations, in addition to cytogenetic abnormalities for de novo AML, in contrast to myelodysplasia- and therapy-related AML (MRC-AML and tAML). Moreover, genetic and molecular characterizations of AML resulted in the establishment of the 2017 ELN risk stratification ( 2 ). This article reviews current AML pathogeneses and novel therapies.
Biology and Pathogenesis
Leukemogenesis of aml results from cytogenetic and genetic abnormalities.
During the last decade, some progress has been made towards a better understanding of AML disease pathogenesis ( 4 ). The Cancer Genome Atlas Research Network has described eight functional categories of genes that are commonly mutated in de novo AML ( 5 ): signaling genes (FLT3, KRAS, NRAS and KIT mutations); epigenetic homeostasis genes with 2 subcategories, chromatin-modifying genes (ASXL1 and EZH2 mutations, MLL fusions) and methylation-related genes (DNMT3A, TET2, IDH1, and IDH2 mutations); nucleophosmin gene (NPM1 mutations); spliceosome-complex genes (SRSF2, SF3B1, U2AF1, and ZRSR2 mutations); cohesin-complex genes (RAD21, STAG1, STAG2, SMC1A, SMC3 mutations), myeloid transcription factors (RUNX1, CEBPA, and GATA2 mutations, RUNX1-RUNX1T1, PML-RARA, MYH1-CBFB fusions); and tumor suppressive genes (WT1, TP53 mutations with PTEN and DMM2 deregulations); ( Table 1 ) ( 4 , 6 ). Two or more of these driver mutations have been identified in 86% of the patients. Combinations of these driver mutations may be compartmentalized into 11 classes with different overall survival rates ( 7 ). Thus, two new provisional entities (AML with mutated RUNX1 and AML with BCR-ABL1) have been included in the update of the WHO classification ( 3 ) and mutations in three genes (RUNX1, ASXL1 and TP53) have been added to the risk stratification of the 2017 ELN recommendation ( 2 ), which could guide new therapies ( 8 ). These mutations have been confirmed in the largest mutational study conducted thus far, the Beat AML cohort, with similar frequency of mutations ( 9 ).
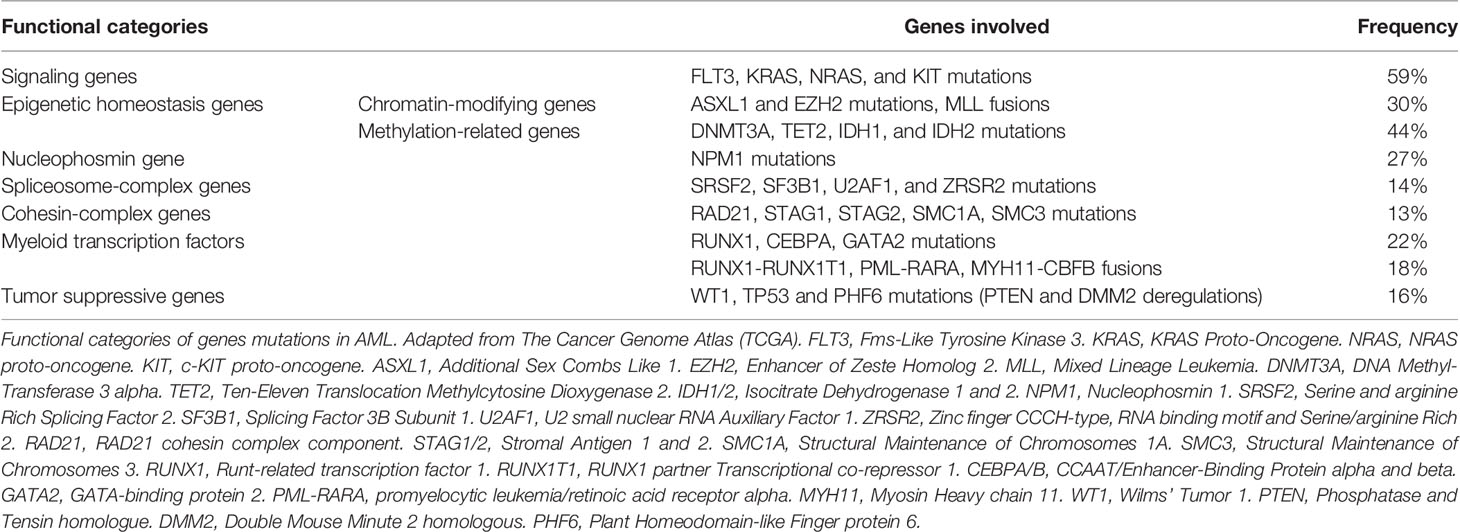
Table 1 Eight functional categories of genes mutations in acute myeloid leukemia (AML).
Most cases of AML present with clonal heterogeneity at the time of diagnosis ( 5 , 8 ). Driver mutations, such as DNMT3A, TET2, and ASXL1, appear early in AML clones ( 5 ) and in the myelodysplastic syndrome (MDS) ( 10 ). Nevertheless, these 3 mutations are also found in healthy donors, and more frequently in elderly individuals along with clonal hematopoiesis of indeterminate potential (CHIP) and age-related clonal hematopoiesis (ARCH) ( 11 ). Most mutations found in AML genomes occur randomly in hematopoietic stem and progenitor cells (HSPC) that result in clonal evolution. These mutations preexist in the background before HSPC acquire the initiating mutations (NPM1, DNMT3A or IDH1) leading to AML pathogenesis ( 12 ). Only a small number of genic mutations are required for AML pathogenesis, and one or two additional cooperative mutations are needed to generate the founding malignant clone. The most frequent associations are NPM1+DNMT3A, NPM1+IDH1, NPM1+FLT3, DNMT3A+IDH1, and DNMT3A+FLT3. Similarly, clonal evolution is also detected when relapsing ( 13 ).
During the course of treatment, AML cells acquire a small number of additional cooperating mutations from the primary clone that contribute to disease progression or relapse ( 12 ). Selection of a dominant clone and/or additional mutations may be caused by inadequate treatment, such as the use of a drug despite apparent drug specific resistance or treatment that is not sufficiently intensive ( 13 ). Patients who are unable to tolerate aggressive consolidation have poorer outcomes ( 2 ). Specific additional mutations may result in resistance to chemotherapy and may play an important role in relapses. Mutational analyses of samples of primary and relapsing tumors indicate that chemotherapy may induce a substantial effect on the mutation spectrum at relapse, probably via DNA damage ( 13 ). Nevertheless, these same results suggest that mutations are neither associated with generalized genomic instability ( 13 ), nor with recurrent cohesin complex gene mutations ( 12 ). Clonal evolution may be caused by a certain type of therapy itself ( 13 ). Therefore, targeted therapies may be used in order to reduce the side effects of mutagenesis, while avoiding the use of cytotoxic drugs. Thus, continuous AML genome evolution in an individual patient would find and eradicate all subclones. Although only a tiny fraction of the total mutations are relevant for pathogenesis, some mutated non-genic regions are also described, suggesting functional properties that need further investigation ( 12 ). Lastly circular RNA profiling has been performed in cytogenetically normal AML as a proof-of-principle and has allowed 3 clusters with clinical and functional significances to be characterized ( 14 ). High levels of KLHL8 and FCHO2 circular RNA are known to be associated with better outcomes.
Recently, AML pathogenesis has been modeled by expression of distinct leukemia-associated mutations ( 15 ). “TYPE-A mutations” (expression of AML-associated fusion genes such as MLL, CBF or RARA fusions) are necessary to maintain transformed phenotypes. “TYPE-B mutations” (constitutively activated kinases by fusion or mutation such as ABL, PDGFR, KIT, FLT3, JAK2, or signaling mediators activating the RAS-MAPK pathway) lead to the development of a lethal myeloproliferative disorder. “TYPE-C mutations” (characterizing clonal hematopoiesis and preleukemic states including point mutations in IDH1/2, DNMT3A, TET2, NPM1c) which are referred to as “seed mutations,” based on their potential. Coexpression of TYPE-A and TYPE-B mutations cooperates to induce AML-like phenotype following a short latency, whereas TYPE-C mutation collaborate with TYPE-A and TYPE-B mutations resulting in AML with high penetrance in mice. Targeting of TYPE-A mutations has been reported as the best path to take in order to cure respective potent driver oncogenes. Although targeting of TYPE-B mutations may be insufficient to eliminate the disease, it may be combined with conventional chemotherapy. Finally, targeting of TYPE-C mutations, such as mutant IDH1/IDH2, has recently demonstrated potential as a promising antileukemic therapeutic strategy ( 16 – 19 ).
Leukemic Stem Cells Develop From Cell-of-Origin of Leukemia Through a Number of Transforming Events
Studies based on mouse models have highlighted that AML, driven by potent oncogenes, such MLL fusion, may have developed via committed myeloid progenitors (CMP) whereas AML without any major cytogenetic abnormalities may emerge due to a combination of preleukemic initiating events arising in the hematopoietic stem cell (HSC) pool ( 15 ). Strong oncogenes mostly originate in CMPs, following which a few cooperating mutations may enable development of AML. In a minor fraction, it may originate in the HSC compartment leading to a particularly invasive and highly resistant phenotype. A high fraction of AML may be produced by the early cooperation of multiple mutations in the HSC compartment, which provides a clonal advantage leading to a preleukemic state, after which the gain of additional mutation may lead to the development of symptomatic AML. Thus, many AML samples show evidence of cellular origin associated with a hierarchical organization model of leukemia, driven by a small population of stem cells, leukemia-initiating cells (LIC) or leukemic stem cells (LSC). These cells are capable of self-renewal in serial mice transplantations and partially differentiate into non-LSC bulk blasts that resemble the original disease, although non-LSC blasts are unable to self-renew ( 20 ). LSC properties are self-renewal, relative quiescence, resistance to apoptosis, and increased drug efflux ( 21 ). Most cases of AML in mice and humans originate from a continuum of early multipotent to more differentiated hematopoietic progenitor cells, but about 10-20% of human AML may arise from more immature cells, the long-term HSCs (LT-HSCs) ( 15 ). The transforming capacity of particularly potent leukemogenic oncogenes may be influenced by the cell of origin. The role of heterogeneity in AML links clonal evolution and LSC ( 22 ).
Xenotransplantation of human AML samples, mostly AML LSCs, in NOD/SCID-gamma null (NSG) mice have highlighted that 80% of CD34 + AML cases, which are defined by the presence of CD34 expression on more than 10% of blasts analyzed, contained 2 expanded cell populations with an immune phenotype of CD38 — CD90 — CD45RA + or CD38 + CD110 + CD123 + CD45RA + both resembling normal, early and more mature hematopoietic progenitor cells (HPCs) rather than HSCs ( 23 ). These two cell populations display leukemia initiating potential upon serial transplantation and are hierarchically ordered; CD38 — cells with a higher number of LSCs resembling normal lymphoid-primed multipotent progenitors (LMPP-like LSC) give rise to leukemic granulocyte-macrophage progenitors (GMP-like LSC), but not vice versa, while CD38 + cells present less LSC. This suggests that the majority of CD34 + AML cells arise from HPCs that have acquired self-renewal properties, rather than from those with a direct HSC origin ( 15 , 20 ). Despite many lymphoid and myeloid antigens aberrantly expressed in AML (CD7 or CD11b) and complex leukemia-associated phenotype shifts at the time of relapse ( 24 ), a number of cell surface markers that are upregulated on CD34 + CD38 — LSC compared with normal CD34 + CD38 — HSPC ( 20 ) have been identified as follows: CD123 (IL-3 Receptor α chain) ( 25 ); CD44 (cell surface glycoprotein ligand for L-selectin) ( 26 ); CD47 (ligand for signal regulatory protein alpha, SIRPα) ( 27 ); TIM-3 (T cell immunoglobulin mucin-3) ( 28 ); CD96 (recognizing the adhesion molecule nectin-like protein-5, necl-5) ( 29 ); CD99 ( 30 ), CD371 as known as CLL-1 (C-type lectin-like molecule-1) ( 31 ); CD32 (Fcγ Receptor II), CD25 (IL-2 receptor); IL1RAP (IL-1 receptor accessory protein) ( 32 , 33 ); GPR56 (G protein-coupled receptor 56) ( 34 ); and CD93 (C-type lectin) ( 35 ). However, their stability is unknown because most have not been studied during relapse ( 20 ) and only few have been targeted ( 36 , 37 ). However concerning the CD123 cell surface maker, at relapse CD123 + CD34 + CD38 — cells were found to increase ( 20 ). In addition, CD123 co-segregates with FLT3-internal tandem duplication (ITD) mutation-positive CD34 + CD38 — cells suggesting that CD123 is a robust LSC marker in FLT3-ITD-mutated AML ( 38 ). Notably, nearly all human AML CD34 + and CD34 — cells are CD33 + in xenotransplantation ( 23 ). On the other hand, above 25% of AML blasts do not express CD34, defined as present on less than 10% of blasts, but are enriched for NPM1 mutation. These are composed of more than 98% of CD34 — cells with multiple and nonhierarchically arranged CD34 — and CD34 + LSC-containing populations ( 39 ). Both CD34 — and CD34 + LSCs represent the same cells with aberrant plasticity of CD34 expression ( 20 , 39 ). CD34 — LSC global transcription profiles seem very similar to those of normal CD34 — GMP. However, unlike mature precursors, they express multiple normal stem cell transcriptional regulators that are implicated in LSC functions. This suggests that the nature of the genetic/epigenetic driver events determines a disordered transcriptional program that results in LSCs with differentiation arrested at either the progenitor or precursor stages of hematopoiesis ( 15 , 20 , 39 ). However, clonal composition changes in the majority of AML xenografts in mice, but could also enable delineation of clonal hierarchies and unmask previously undetectable clones ( 40 ). New tools such as single-cell sequencing may permit a better understanding of LSC hierarchies and functions ( 41 ), and help define specific targeting strategies ( 42 ).
Single-cell gene expression preceded by genome-wide transcriptional analysis of AML and chronic myeloid leukemia (CML) LSCs have highlighted several cell markers, such as IL1RAP and CD25 that are overexpressed ( 42 – 44 ). Nevertheless, these AML and CML LSC cell surface makers are also expressed on normal HSCs ( 45 ). Interestingly, IL1RAP is described to potentiate multiple oncogenic pathways in AML ( 46 ). Targeting the extracellular portion of IL1RAP with monoclonal antibodies, IL1RAP down regulation with short hairpin RNA, and knocking out of Il1rap via genetic deletion inhibits AML growth intrinsically, through the induction of differentiation and apoptosis without affecting healthy hematopoietic cells that present a low expression of IL1RAP, and inhibit AML pathogenesis in vivo . Inhibition of the canonical IL-1 receptor signaling pathway induces the abolition of IRAK1 expression, which reduces MDS and AML leukemic colony formation and prolongs the survival of xenograft models ( 47 ). Indeed the IL-1 receptor complex, formed by IL-1 receptor, IL1RAP, MyD88, IL-1R associated kinases 2 (IRAK2), and IRAK4 ( 48 ), induces the activation of IRAK1 and TRAF6, leading to the activation of the IKK complex, which in turn results in activation of NFκB target genes, as well as JNK and p38 ( 49 ). This pathway has been described in several cancers and its targeting in AML seems promising ( 32 , 49 ). Of note, an active super enhancer has been reported with IL1RAP in AML, in samples that also presented FLT3, NPM1, and IDH1 associated mutations ( 50 ). In addition to the inhibition of the IL-1 receptor pathway, targeting IL1RAP may also inhibit signaling and AML cell growth occurring via the FLT3 and c-KIT pathways with a reduced response to FLT3 ligand, stem cell factor (SCF) and IL-1β ( 46 ). Nevertheless, FLT3 mutations independently induce a constitutive activation of the IL1RAP associated FLT3 pathway, but it has been demonstrated that IL1RAP physically interacts with FTL3 and c-KIT, even although FLT3 is mutated. However IL1RAP overexpression occurs early in LSC pathogenesis ( 43 ) whereas FLT3 and c-KIT activation mutations seem to be a relatively late event in LSC transformation ( 51 , 52 ). Furthermore, chronic inflammation due to exposure to IL-1 impairs blood hemostasis and restricts HSC lineage output ( 53 ). Continuous exposure to IL-1, in association with IL-6, TNF (tumor necrosis factor) and IFNs (interferons), may promote genomic instability and induce a pre-leukemic stage via continuous proliferation, bone marrow niche dysfunction and exposure to reactive oxygen species (ROS), that drive myeloid malignancies such AML ( 54 ). IL1RAP is described as an inflammatory regulator and its overexpression in AML may be linked to a proinflammatory state ( 55 ).
Bone Marrow Microenvironment Sustains Blast Proliferation, and Promotes Resistance to Treatment
HSCs in the bone marrow interact with mesenchymal stem/stromal cells (MSC), sinusoidal endothelial cells, osteoblasts, osteoclasts, macrophages, and immune cells, adipocytes, autonomic neurons, extracellular vesicles, extracellular matrix, and soluble factors, including cytokines and growth factors ( 56 ). In AML the interaction among blasts, stromal cells and immune cells of the bone marrow (BM) microenvironment, promoted by soluble niche factors, create a niche that sustains blast proliferation and confers chemoresistance by remodeling the BM niche via alteration of each cellular constituent ( 57 – 59 ). AML cells induce osteogenic differentiation but inhibit adipogenic differentiation of MSC leukemia growth, whereas normal hematopoiesis is favored by AML-secreted exosomes and pro-inflammatory IL-1, which promote the expansion of AML progenitors and disease progression by activating the IL-1/p38MAPK pathway. Chronic increase of IL-1β with IL1RAP binding expands malignant cells while HSCs are intrinsically depleted ( 33 , 53 ). Indeed, myeloid differentiation of Dnmt3a -mutant LT-HSC seems to be promoted by HSC-extrinsic alterations in aged BM microenvironments, via TNFα and Macrophage Colony-Stimulating Factor (M-CSF), that overcome impaired differentiation ( 60 ).
Interestingly, IL-1β appears to play a key role in cardiovascular diseases of young patients with CHIP ( 11 ). Similarly, overproduction of IL-1β by mutant progenitors damages Schwann cells, leading to neuropathy, increases MSC apoptosis and decreases HSC adhesion molecule expression ( 43 , 59 ). Cytokines and particularly IL-33, a ligand of IL1RAP, released by AML cause the remodeling of vasculature, reduces its ability to support normal hematopoiesis, and compromise vascular integrity by niche cell based stimulation of myeloid cytokine production. Then FoxO1 and β-catenin stimulate osteoblasts that trigger aberrant Notch signaling in HSCs, and induce leukemic transformation ( 58 ). In proteomic and gene expression profiling, the cytokine/chemokine signaling network causes the most striking AML-associated proteomic alteration of BM microenvironment ( 58 ). Pro-inflammatory IL-8 is the key central molecule of this network in AML that may show potential as an attractive therapeutic target. MPIF-1 (CCL23), an inhibitory cytokine that inhibits proliferation and differentiation of myeloid precursor cells, is also upregulated. Increases in the network of proteins that regulate osteoblastic and osteoclastic activities have been highlighted in AML BM. Deregulated expression of CXCL12/CXCR4 by stromal cells may impinge on the ability of normal HSCs to find and reside in their quiescence maintaining niches, and promote exhaustion and progressive malignant clonal dominance of these HSCs ( 59 ). Contact-dependent transfer of functional mitochondria from MSCs to AML cells has been demonstrated ( 61 ). Such mitochondrial transfer is increased under chemotherapy conditions and may lead to resistance to chemotherapy. Similarly, LSCs may highjack the lipolytic role of adipocytes via the fatty acid transporter CD36 ( 62 ). Interestingly, CD36 is associated with a poor prognosis ( 63 ).
Additionally, certain processes that indicate immune dysregulation and leukemia microenvironment remodeling have been described in AML as follows: low neoantigen burden and defective antigen presentation; higher regulatory/suppressive T-cells (Treg) proportion and lower T effector (Teff) proportion; T-cells exhaustion due to upregulation of immune checkpoint ligands and receptors; chronic inflammation and increase of inflammatory macrophage population (M1); increase of myeloid derived suppressor cell population (MDSC) and suppressive macrophage population (M2) derived from both normal progenitors and leukemia cells; and production of immunosuppressive soluble factors and metabolites ( 64 , 65 ). Antigen presentation by MHC class II genes is reportedly downregulated during relapse following allogeneic stem cell transplantation (ASCT) ( 66 ). Although checkpoint blockade is correlated with high mutation and neoantigen burden, the role of checkpoint blockade in AML remains unclear ( 67 ). Indeed, AML is considered to present a low mutational burden and thereby low immunogenicity. Nevertheless TP53-mutated AML should be associated with an increased mutation burden and efficacy of ASCT, indicating sufficient immunogenicity ( 65 ). In addition, bone marrow T-cells overexpress PD1 and CTLA-4 while LAG-3 and T-cell Immunoglobulin Mucin-3 (TIM-3) are not overexpressed ( 57 , 65 , 68 ). Type I and II interferons also induce the expression of PD-L1/2 in AML blasts ( 69 , 70 ). TIM-3, CD84, and LAG-3 are increased at the RNA and protein levels in leukemic marrow ( 58 ). Indeed TIM-3 is involved in an autocrine stimulatory loop in AML LSCs. Besides, MDSC and M2-polarized macrophages are associated with a poor prognosis, in a manner similar to that of high proportions of M1-polarized macrophages ( 57 , 65 ). Fatty acid and lipid mediators also modulate the leukemia microenvironment via immune signaling ( 71 ). Indeed, PGE2 a lipid mediator, has been shown to promote tumor progression via the induction and maintenance of MDSC via PD-L1 inducing expression on tumor associated macrophages and MDSCs ( 72 ).
By contrast, in AML, cytotoxic T-cells (Teff) fail to eliminate leukemic blasts and become senescent via the activity of immunosuppressive cells, such as Treg ( 73 ). Treg levels are correlated with the response to chemotherapy wherein the lowest levels have been observed during hematopoietic recovery following chemotherapy ( 74 ). In addition, an integrated, quantitative immune cell and phenotype profile of AML patients reflects a poor prognosis with a high proportion of M1-polarized macrophages and FOXP3 + helper T-cells, with a similar outcome between bone marrow and peripheral blood samples ( 57 ). Two major immunologic divergent clusters were identified and correlated with age, T-cell receptor (TCR) clonality and survival. Elderly cluster represents higher TCR clonality, higher OX40 expression in cytotoxic and Treg and higher CD45RO + T-cells, as well as lower naïve and central memory CD27 + , memory CD25 + and late-stage CD57 + cytolytic T-cells. Clonality trends were found to be associated with complex karyotypes and higher ELN risk but failed to show a significance due to the insufficient number of patients studied, while an association between T-cell lineage and AML gene mutations has been reported ( 15 , 57 ). Notably, although FLT3-mutated AML reportedly displays dendritic cells and Treg expansion, Teff are able to perform effector functions in the absence of an enriched Treg population ( 75 ). In addition, AML blasts may also present HLA-E and suppress NK cell functions via NKG2A activation ( 76 ). NK cells express NKG2D, an activator receptor for NKG2D ligands (NKG2DL), such as MHC class I polypeptide-related sequence A and B (MICA/B) and UL16-binding protein (ULBP), that are expressed on AML blast but could be shedding ( 77 ).
Current Therapies and Perspectives
Standard cytotoxic chemotherapies remain the main treatment for the induction phase.
For decades, the standard therapy of intensive induction chemotherapy 7 + 3 regimen has been the backbone for younger and fit patients. This therapy involves a combination of continuous infusion of cytarabine (100 or 200 mg/m² per day) for 7 d and anthracycline treatment (daunorubicin 60 mg/m² per day) for 3 d ( 1 , 2 , 4 , 78 ). Despite a complete remission rate of 60%–80% in younger patients and 40%–60% in older patients, the rate of overall survival (OS) is lower, approximating 40% ( 78 ). This highlights the critical issue pertaining to a relapse of leukemia following an initial response. For younger patients who are not undergoing ASCT, a consolidation therapy has been proposed since 1994 ( 78 ). Intermediate cytarabine dose (IDAC) consolidation is recommended for younger and older patients who are not undergoing ASCT ( 2 ). For high-risk older patients, the effect of consolidation is unclear. Interestingly anthracycline-based chemotherapy induces damage associated molecular patterns (DAMPs) such calreticulin, HSP70 and HSP90 and anti-tumor immunity with phagocytosis by antigen-presenting cells, TH1 polarization, cytotoxic CD8 + T-cells and NK cells activation leading to immunogenic cell death ( 79 ). Thus chemotherapy, particularly the 7 + 3 regimen, triggers immunologic response against leukemic cells. The prospects of adding a third drug such as the purine analog, fludarabine or clofarabine, have been investigated ( 78 ). Similar to clofarabine, fludarabine together with the 7 + 3 regimen appears to induce a better response rate in intermediate-risk AML, but OS in younger patients is not improved while toxicity is increased ( 80 – 82 ).
Targeting Epigenetic Alterations
Elderly or “unfit” patients, with multiple comorbidities who are not eligible to receive standard intensive chemotherapy have few options available. In such cases, hypomethylating agents (HMA), such as azacytidine and decitabine, are mainly used. Low dose cytarabine (LDAC) may also be used ( 2 ). Better responses to HMA have been reported with certain subsets of AML, such as azacytidine and TET2 mutation ( 83 ) or decitabine and TP53 mutation ( 84 ). DNA demethylation in myeloid neoplasms reactivates genes and leads to differentiation. Azacytidine can inhibit RNA methyltransferase DNMT2 and induce RNA demethylation ( 68 , 85 ). In addition, HMA may recalibrate the immune microenvironment, via promoting anti-tumor response by upregulation of the antigen presentation pathway, TH1 and TH17 polarization, cytotoxic CD8 T-cells activation, Treg function reduction and immune checkpoint blockade ( 64 , 86 ). Conventional HMA exhibits a complete response (CR) rate above 30% with an overall survival (OS) similar to that of a conventional care regimen ( 87 ). A novel HMA, Guadecitabine (SGI-110), has been highlighted in a phase 1/2 study by way of a composite CR rate associating CR and CRi (CR/CRi, CR and CR with incomplete platelet recovery and incomplete neutrophil recovery), between 50%–59% ( 88 ). Further investigations are ongoing (NCT02348489). Acute promyelocytic leukemia (APL) is considered separately from non-APL. AML, prior to implementation of specific pathophysiology and risk stratification and response, is associated with high cure rates when all-trans retinoic acid- (ATRA) and arsenic trioxide- (ATO) containing therapy is utilized ( 89 ). Nevertheless ATRA and ATO have demonstrated immune modulatory activity which exerts an effect on CD4 + and CD8 + T-cells and dendritic cells development, and also enhances NK cell mediated cytolytic activity via NK ligand upregulation ( 65 , 90 ). In this regard, ATRA and ATO are being investigated together, or in combination with novel therapies, in IDH1/2-mutated AML ( 65 , 91 ). Of note, glasdegib, an inhibitor of the smoothened multi-transmembrane (SMO), a component of the Hedgehog pathway, combined with LDAC, which was approved for newly-diagnosed AML in the elderly, who are ineligible to receive standard induction chemotherapy, has shown an increase in the OS rate (8.8 months versus 4.9 months in LDAC only) ( 92 , 93 ).
Allogenic Hematopoietic Stem Cell Transplantation as Curative AML Treatment and First Immunotherapy
For “fit” patients with ELN-2017 intermediate or high risk disease ( 2 ), performing allogenic hematopoietic stem cell transplantation (ASCT) during first complete response (CR1) offers durable remission and long term survival, whereas no ASCT has a hazard ratio (HR) of 0.80, when weighed against treatment related morbidity (TRM) and mortality ( 94 ). Alternative ASCT, such as haploidentical ASCT, in CR1 confers lower leukemia-free survival (LFS) with a HR of 1.74 for intermediate-risk, whereas no differences were seen in high-risk disease, compared with HLA-matched unrelated donor (MUD) ( 95 ). Other alternative ASCTs, such as cord blood unit (UCB) or mismatched unrelated donor (MMUD), show LFS comparable to haploidentical ASCT ( 96 ). MMUD is still the best alternative option for patients without related HLA-matched donors during CR1 to intermediate-risk AML ( 97 ). However, although haploidentical ASCT is an alternative ASCT with debatable LFS outcomes ( 98 , 99 ), recent results pertaining to myeloablative conditioning (MAC) were at least, either equal or better than MMUD ASCT ( 100 , 101 ), that led to a prospective investigation of MMUD versus haploidentical ASCT (NCT03655145). Notably, in CR1, MAC regimen is associated with a greater risk for infection, particularly bacterial infection, before day 100, compared with reduced-intensity of non-myeloablative conditioning (RIC/NMA) ( 102 ). In addition, MAC and RIC/NMA of various regimens induced different LFS, OS and non-related morbidity and mortality rates. Those outcomes are associated with graft-versus-host disease (GVHD), toxicity and infectious complications ( 103 – 106 ). Then the European Bone Marrow Transplantation society (EBMT) has published a transplant conditioning intensity score (TCI) that provides a better RIC/MAC classification ( 107 ). As same, the EBMT has published recommendations about prophylaxis and management of GVHD ( 108 ). Although, MAC regimens promote better outcomes in high-risk AML, these are associated with greater toxicity and infection risk, whereas RIC/NMA regimens induce higher GVHD rates ( 105 , 106 , 109 ). Currently MAC regimens are recommended for young patients while RIC regimen are recommended for older patients with comorbidities ( 2 ). GVHD prophylaxis is critical for the successful application of ASCT, but still needs to be standardized ( 110 ). Prophylactic donor lymphocyte infusion (DLI) is offered following ASCT. Retrospectively it seems to improve OS only in high-risk AML and/or ASCT beyond CR1 ( 111 ). Relapse following ASCT benefits from DLI or a second ASCT via a MAC or RIC regimen without differences in the 5-year OS. However, relapses less than 6 months after primary ASCT inducing a worse OS, regardless of the treatment prescribed have been observed ( 112 , 113 ). The use of azacytidine for maintenance following ASCT has been proposed. Although well tolerated, azacytidine has failed to demonstrate improvement of OS in phase 3 studies ( 114 ). Moreover, immune pressure may lead to clonal evolution as recently described after ASCT ( 115 ). A deviation of the trajectory of leukemia clonal evolution was suggested that allows to escape immune control by different mechanisms, such as genomic HLA loss. In addition, several deregulations of costimulatory ligands on AML blasts (PD-L1, B7-H3, or CD80) have been also highlighted with concomitant changes in donor T-cells after ASCT. These immune control escape mechanisms could be interesting to target in intermediate- and high-risk AML. Use of IFNγ and immune checkpoint blockade therapies might overcome this two evade mechanisms, respectively.

New Available Therapiesand Perspectives
Improvement of cytotoxicity and specificity of chemotherapy toward aml cells.
Recently, several drugs have been approved by the FDA for AML therapy as follows: a liposomal formulation of daunorubicin and cytarabine (CPX-351); the anti-CD33 antibody drug conjugate gemtuzumab ozogamicin (GO); the IDH1/2 mutant inhibitors ivosidenib and enasidenib; the FLT3 inhibitor midostaurin in combination with chemotherapy and gilteritinib; and the BCL2 inhibitor venetoclax in combination with HMA ( 116 ). Daunorubicin-cytarabine liposome, with a molar ratio at 5:1, reportedly improves the response rate compared to the standard 7 + 3 regimen, with an overall response rate (ORR) of 47.7% versus 33.3%, p=0.016, in 60/65 year-old patients who are newly-diagnosed with MRC-AML and tAML ( 117 ). However, OS is not improved while hematological toxicities are enhanced. Nevertheless, pharmacologically this formulation allows longer half-life and greater AUC, thereby prolonging exposure to leukemic cells that may reduce multidrug resistance ( 118 ), and overcome Pgp-mediated drug resistance ( 116 ).
The anti-CD33 antibody drug conjugate gemtuzumab ozogamicin (GO) was finally approved in 2017 by the FDA following a decade of controversy ( 119 ). CD33 (or Siglec 3) is a transmembrane receptor expressed in myeloid cells but not in normal HSCs that are widespread among AML blasts (>90%) and many AML precursors ( 120 ). When GO binds CD33, this receptor internalizes GO which then releases ozogamicin, a calicheamicin derivative, under the acidic conditions of lysosomes. GO was initially developed as a form of monotherapy involving a single 9 mg/m² dose during the first recurrence of CD33 + AML. CR recovery is 13% with a median recurrence-free survival of 6.4 months. Main complications are grade 3 and 4 neutropenia (98%), thrombopenia (99%), and a few venoocclusive diseases (VOD, 0.9%) ( 121 ). Several studies that were performed did not report an improvement in OS or significant toxicities ( 78 , 119 ). A better understanding may be obtained from the Acute Leukemia French Association (ALFA) 701 study which used a GO fractionated 3 mg/m² dose on days 1, 4, and 7 together with a standard 7 + 3 regimen ( 122 ). A meta-analysis of GO together with induction chemotherapy concluded that addition of GO significantly reduced the risk of a relapse (HR 0.81, p=0.0001) and improved the 5-year OS (HR 0.90, p=0.01) ( 123 ). At 6 years, the survival benefit is especially apparent with favorable and intermediate risks. In addition, in NPM1 mutated AML, GO and chemotherapy regimen was associated with fewer relapses following CR ( 124 ). CD33 expression itself does not appear to exert a marked effect on AML treatment outcome. However, Core Binding Factor (CBF) AML, which appears to benefit from GO treatment, exhibits blasts with relatively low CD33 levels. This could be explained by high chemosensitivity or CBF AML which arises from CD33 + precursors ( 120 ). Notably, CD33 binding could be impaired by the germline CD33 single-nucleotide polymorphism, rs12459419, which is associated with the expression of an alternatively spliced variant of CD33 ( 125 ). The risk of developing VOD is a major toxicity concern. The risk is lower with GO doses no greater than 3 mg/m², but increases in heavily treated patients ( 119 ). However, GO prior to ASCT does not seem to increase VOD risk after ASCT ( 126 ). A similar VOD risk is reported with inotuzumab ozogamicin, an anti-CD22 antibody with the same conjugate drug, suggesting that CD33 independent toxicity may occur ( 127 ). Nevertheless, a new anti-CD33 antibody conjugated with pyrrolobenzodiazepine dimer instead of calicheamicin (SGN-CD33A) indicates that targeting CD33 may also contribute to VOD ( 128 ). Occurrences of VOD stop its development. A different method for targeting CD33 involves using chimeric antigen receptor T-cells (CAR T-cells) that exhibit potent preclinical activity against human AML ( 129 ). However, the efficacy of this method against refractory/relapse (R/R) AML is still under investigation ( 130 ). Safety assessment, particularly concerning the VOD risk, will be vital.
Targeted Therapies
Idh1/2-targeting in relapse/refractory aml or newly diagnosed older non eligible aml.
Blockade of IDH1/2-mutated enzyme by inhibitors induces differentiation of malignant blasts ( 16 ). IDH1/2 mutations induce the production of oncometabolite R-2-Hydroxyglutarate (R-2-HG), which is responsible for a blockade in terminal differentiation via calcium influx reduction, NFAT translocation and proliferation suppression ( 131 , 132 ). Enasidenib and ivosidenib, inhibitors of mutant IDH2 and IDH1 respectively, have recently been approved by the FDA for IDH2-mutated and IDH1-mutated AML. Treatment consisting of the single agent, Enasidenib, resulted in a CR of 19.6% and an overall response rate (ORR) of 38.8% with a median OS of 8.8 months in R/R IDH2-mutated AML ( 17 , 133 ). CR is achieved via the clearance of mutant-IDH2 clones, but interestingly functional mutant-IDH2 neutrophils are detected, suggesting a conversion from undifferentiated to differentiated myeloid cells ( 18 ). In addition, enasidenib specific toxicity profile is corroborated by IDH inhibitor-associated differentiation syndrome (IDH-DS) ( 17 ). Non-responding patients are significantly associated only with FLT3 baseline mutations. Association with NRAS and other MAPK pathway effector mutations remain unclear, but may be associated with non-response to enasidenib ( 18 , 133 ). Interestingly, R-2-HG diminution may also reduce the paracrine hyperleukocytosis and leukemogenesis effect on IDH2 wild-type clones ( 134 ). On the other hand, ivosidenib, a single agent used against R/R IDH1-mutated AML ( 19 ), which has been newly-recognized as ineligible for standard chemotherapy against IDH1-mutated AML ( 135 ), showed a CR/CRi rate of 30.4% and 42.4%, respectively, and an ORR of 41.6% and 54.5%, respectively, with a median OS of 8.8 months and 12.6 months, respectively. The toxicity profile is similar, and IDH-DS has been described. Furtherer associations with azacytidine or venetoclax are under investigation ( 136 , 137 ).
Active Developments in the Area of FLT3 Inhibitors
Several FLT3 tyrosine kinase inhibitors have been developed in the last few years with variable pharmacological and clinical profiles. FLT3 inhibitors are divided into first generation multi-kinase inhibitor (including sorafenib, lestaurtinib, midoastaurine) and next generation inhibitors (including quizartininb, crenolitinib, gilteritinib) ( 138 ). Many trials are ongoing investigating FLT3 inhibitors alone or in combination with standard chemotherapy or HMA, in first line or in relapse. The broad-spectrum FLT3 inhibitor midostaurin has been recently approved in combination with standard of care chemotherapy. Midostaurin, used as a single agent, has reduced or eliminated FTL3-mutated and FTL3-wild-type blasts, but showed limited effect on BM blasts as well as a short therapeutic duration ( 139 ). This suggests the necessity for combining midostaurin with chemotherapy. Midostaurin in association with daunorubicin and cytarabine induction and consolidation chemotherapy, or alone in maintenance, has demonstrated a median OS of 74.7 months as opposed to 25.6 months in the placebo arm, with a HR for death of 0.78 (p=0.009), and a 4-year OS rate of 51.4% and 44.3%, respectively ( 140 ). ASCT, which was performed at the discretion of the investigators in 57% of patients, has shown a 4-year OS rate of 63.7% in the midostaurin arm as opposed to 55.7% in the placebo arm but without significance (p=0.08).
On the other hand, sorafenib used in association with azacytidine in ineligible de novo FTL3-mutated AML and in R/R FLT3-ITD-mutated AML, has shown a CR rate of 26% and 27% respectively and a CR/CRi rate of 70% and 43% ( 141 , 142 ). Association with standard induction chemotherapy improved the median EFS to 21 months versus 9 months in the placebo arm, but was accompanied by a significant increase in toxicity ( 143 ). In maintenance therapy following ASCT, sorafenib permitted a greater relapse-free survival (RFS) than the placebo arm, with a 2-year RFS rate of 85% versus 53.3%, respectively ( 144 ). Despite interesting results, except for significant toxicity and absence of a proven OS benefit, sorafenib is not used as a frontline drug that is combined with standard intensive chemotherapy ( 145 ). However, regardless of the absence of appropriate clinical trials, prospective large-scale studies have made sorafenib a preferred option for maintenance following ASCT. Despite the development of FLT3 inhibitors, the EMBT Acute Leukemia Working Party recommends performing ASCT in their 2017-ELN risk stratification, but with the accompaniment of post-transplantation FLT3 inhibitor maintenance for at least 2 years ( 146 ). Of note in both FLT3-mutated and FLT3-wild type AML, midostaurin has demonstrated immune-modulatory effects as shown by a reduction in the CD4 + CD25 + cells proportion, mRNA levels of FOXP3 and IL-10 and TNFα levels ( 147 ). This decrease in Treg might be possibly linked to FLT3-ITD inhibition.
Gilteritinib has been recently approved for single use in R/R FLT3-mutated AML. Compared with chemotherapy, gilteritinib has demonstrated a greater median OS (9.3 months versus 5.6 months with HR of death of 0.64), CR, and CR/CRI (21.1% versus 10.5% and 34% versus 15.3% respectively), whereas its incidence of exposure-adjusted events was lower (19.24 versus 42.44 respectively); ( 148 ). Interestingly, gilteritinib shows continuous target inhibition despite FLT3-D835 mutation, whereas sorafenib do not show such inhibition. The use of other FLT3 inhibitors still needs to be determined. Despite the highest FLT3 inhibition and an improvement of OS rate (6.2 months versus 4.7 month for salvage chemotherapy), quizartinib is associated with marginal clinical benefits and significant toxicity levels ( 149 ). For this reason quizartinib did not receive FDA drug approval yet ( 145 ). A novel FLT3 inhibitor, crenolanib, presents a FLT3 inhibition despite FLT3-D835 mutation, that might be interesting in relapse, but is currently still under investigation ( 150 ).
Apoptosis Regulators as Novel Anti-Leukemic Strategies
Venetoclax, an inhibitor of BCL2 apoptosis regulation, which was initially used as a single agent in AML relapse, has shown an ORR of 19% in a preclinical study. In elderly, untreated patients with AML who are ineligible for standard induction chemotherapy, venetoclax combined with HMA resulted in a CR/CRi rate of 67%, and a CR/CRi of 91.5%, in NPM1-mutated AML ( 151 ). In TP53-mutated AML, this combination was promising, with CR/CRi of 47% and a median OS of 7.2 months. However, 70% of patients have discontinued the treatment due to disease progression. Recently a phase 3 study confirmed CR/CRi rate improvement with venetoclax adjunction to HMA (66.4% versus 28.3%, p<0.001) with an increasement of median OS (14.7 months versus 9.6 months, HR for death 0.66%, p<0.001) ( 152 ). Similarly, in elderly patients with newly-diagnosed AML who are ineligible for standard induction chemotherapy, a combination of venetoclax and LDAC was associated with a CR/CRi of 54% ( 153 ), while, in a phase 3 study, the same combination showed a median OS of 8.4 months as opposed to 4.1 months with LDAC alone (HR 0.70, p=0.04); ( 154 ). NPM1- or IDH1/2-mutated AML have been associated with higher CR/CRi rate (89% and 72%, respectively) in contrast to TP53- or FLT3-mutated AML (30% and 44% respectively). Venetoclax resistance may be driven by MCL-1 overexpression ( 155 ). Further suggested combinations, including drugs targeting XPO1, CDK9, MDM2, or MEK, await determination via other studies ( 156 ). The association between Venetoclax and chemotherapy is also under investigation ( 157 ). Interestingly, response in NPM1-mutated AML may be linked to tumor-specific immunity recovery of T-cells ( 65 ) and HLA-presentation in the mutant protein of NPM1 (NPM1c) ( 158 ). NPM1c-targeting has also been proposed ( 159 ). In addition, nuclear relocalization of NPM1c, induced via XPO1 inhibition by selinexor, promotes arrest of growth and differentiation ( 160 ). Moreover, a retrospective study of elderly NPM1-mutated AML patients with positive NPM1-measurable residual disease (MRD), indicated that initiation of venetoclax, following induction and consolidation of chemotherapy together with azacytidine or LDAC, induces rapid clearance of residual NPM1-blasts ( 161 ). Of note, the tumor suppressor p53, deficient in TP53-mutated AML, is associated with pro-apoptotic activity following DNA damage, and inhibits BCL2 directly as well as indirectly ( 155 ). Although, BCL-2 inhibitors in TP53-mutated AML appear to be promising, further investigations are required.
Therapies in Development
Epigenetics and tyrosine kinases inhibitors.
Gene expression profiling, which helps identify new targets as well as drug resistance, provides information that is valuable for the development of therapeutic strategies ( Figure 1 ) ( 9 , 82 ). Recent strategies advocate inhibition of other epigenetic modifiers, such as the enhancer of zeste homolog 2 (EZH2) ( 162 ), lysine demethylase 1A (KDM1A or LDS1) ( 163 ) and DOT1-like histone lysine methyltransferase (DOT1L) ( 164 ). The MLL subset interacts with DOT1L and the bromodomain and extra-terminal (BET) family member, bromodomain-containing 4 (BRD4), leading to potent inhibition ( 165 , 166 ). The OTX015 BET inhibitor is currently being clinically investigated following a phase 1 study ( 167 ). By contrast, vorinostat and panobinostat histone deacetylase inhibitors, that were initially promising, either alone or with HMA or cytarabine regimen, have failed to demonstrate benefits in AML ( 168 – 170 ). Several trials of multiples of other epigenetic targets, such as DS-3201b or pinometostat, are ongoing ( 171 ). In addition, many of the several genes that were found to be correlated with drug sensitivity or resistance, are from the BEAT cohort study ( 9 ). TP53 mutations or NRAS and KRAS mutations cause broad patterns of drug resistance, but TP53 mutations trend more sensitivity to elesclemol, in a manner similar to that of NRAS mutation with MAPK inhibitors, such as vemurafenib, pazopanib, or tivozanib. However, sensitivity of KRAS mutations is lower. Mutations in RUNX1 are correlated with sensitivity to PIK3C and mTOR inhibitors, such as dactolisib, and to cediranib, a multi-kinase VEGFR inhibitor. Everolimus is also being investigated ( 172 ). Similarly, FLT3-ITD mutations with or without NPM1 mutation, are significantly sensitive to ibrutinib, an inhibitor of BTK and TEC family kinases, as well as entospletinib, an inhibitor of spleen-associated tyrosine kinase (SYK). SYK interacts with FLT3 ( 173 ), and thus targeting SYK with entospletinib may be effective in FLT3-mutated AML and is currently under investigation (NCT02343939, NCT03135028, NCT03013998) ( Table 2 ). Lastly, BOCR and RUNX1 mutations are sensitive to JAK kinase inhibitors, such as ruxolitinib, whereas BCOR mutations, either alone or in association with DNMT3A or RSF2 are not. However BCOR mutations alone show sensitivity to the multi-kinase inhibitor crizotinib ( 9 ). Interestingly, targeting the IL-1 pathway with the multi-kinase inhibitor, pacritinib, which inhibits IRAK1/4, FLT3, and JAK2, highlights robust sensitivity despite adaptive resistance to therapies and significant AML cell death ( 174 , 175 ). Further investigations are ongoing ( 176 ). IRAK1 inhibitor association with venetoclax also appears to be interesting ( 47 ). In addition, the IRAK4 inhibitor, CA-4948, is under investigation for its effect on AML (NCT04278768).
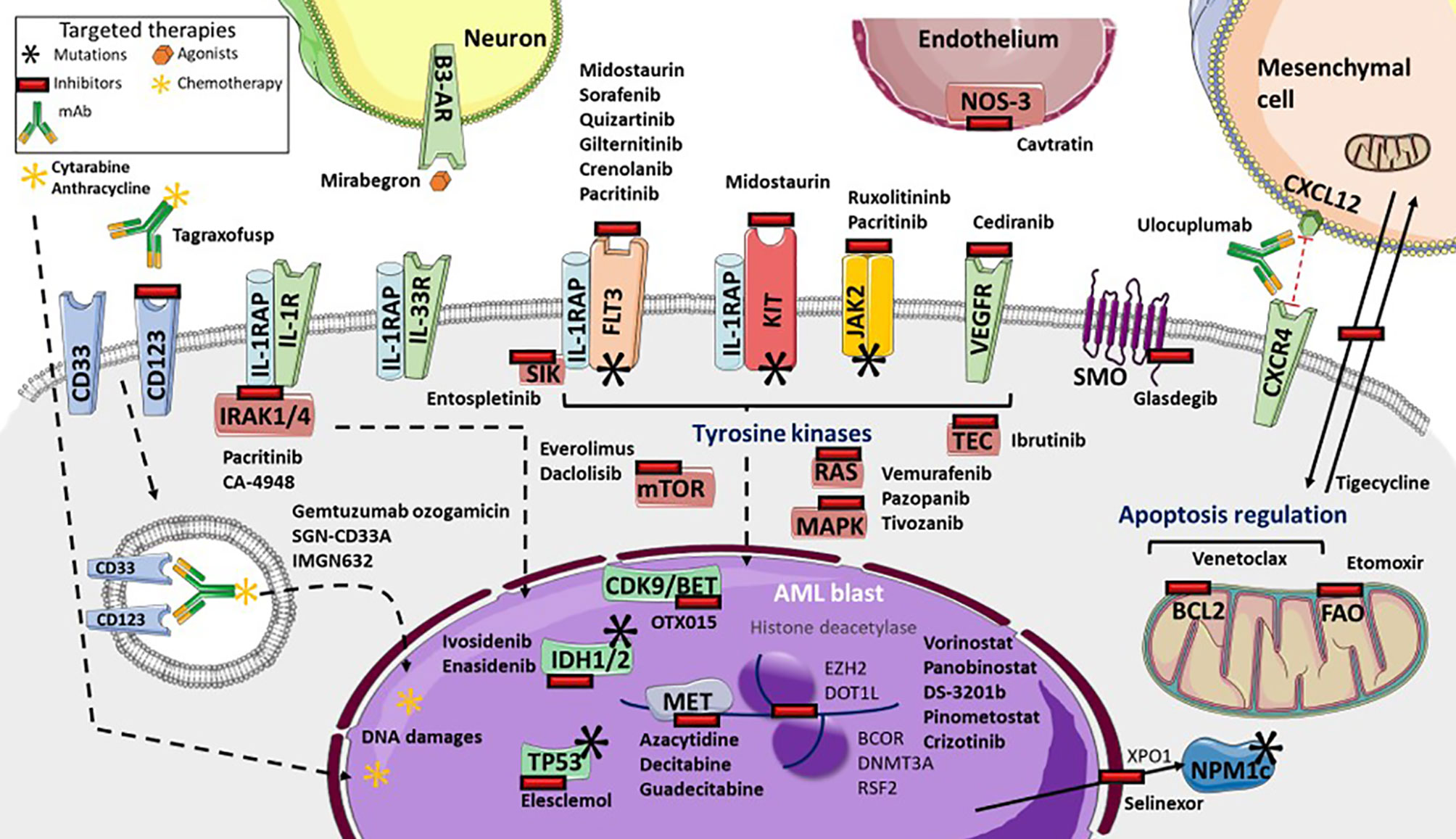
Figure 1 Targeted therapies available and in development in Acute Myeloid Leukemia (AML). Many tyrosine kinases inhibitors are either currently available or in development. These include: Fms-Like Tyrosine Kinase 3 (FLT3) inhibitors (such as sorafenib, quizartinib, gilteritinib, crenolanib), pan-kinase inhibitors (including midostaurin), FLT3 and KIT inhibitors (including pacritinib), Janus Kinase-2 (JAK2) and interleukine-1 Receptor (IL-1R) Associated Kinases 1/4 (IRAK1/4) inhibitors, the IRAK4 inhibitor Ca-4948, the JAK2 inhibitor ruxolitinib, NRAS, KRAS and MAP Kinases (MAPK) inhibitors (such as vemurafenib, pazopanib, tivozanib), mTOR inhibitors (everolimus and dactolisib), TEC kinases inhibitors (including ibrutinib), and vascular and endothelial growth factor receptor (VEGFR) inhibitor cediranib. Several targeted-drugs are available, or in development, for transcription factors: ivosidenib, an Isocitrate Deshydrogenase-1 (IDH1) inhibitor, enasidenib, an IDH2 inhibitor, azacytidine, decitabine, guadecitabine as hypomethylated agents, the histone deacetylase vorinostat and panobinostat, DS-3201b, a zeste 2 polycomb repressive complex 2 subunit (EZH2) inhibitor, pinometostat, a DOT1-like histone lysine methyltransferase (DOT1L) inhibitor, crizotinib, the Bcl6 Corepressor (BCOR) inhibitor, OTX015, a cyclin-dependent kinase 9/bromodomain and extraterminal (CDK9/BET) inhibitor, and elesclomol, a TP53 inhibitor. Selinexor inhibits the XPO1 exporter, which inhibits leukemic activity of mutated NPM1 proteins. Glasdegib inhibits smoothened multi-transmembrane (SMO), a member of the Hedgehog pathway. Tagraxofusp inhibits CD123, whereas IMGN632 transports chemotherapy through CD123 internalization, as does gemtuzumab ozogamicin and SGN-CD33A through CD33. Venetoclax inhibits the BCL2 anti-apoptotic protein. Several microenvironment targeted drug are in development: etomoxir inhibits fatty acid oxidation metabolism, tigecycline inhibits mitochondrial heterocellular transfer, thus inhibiting drug resistance exchange, ulocuplumab inhibits CXCR4/CXCL12 interaction from inducing leukemia myeloid cell migration, mirabegron, an agonist for sympathetic neuropathy β3-adrenergic receptor (β3-AR), and cavtratin, the NO synthase inhibitor.
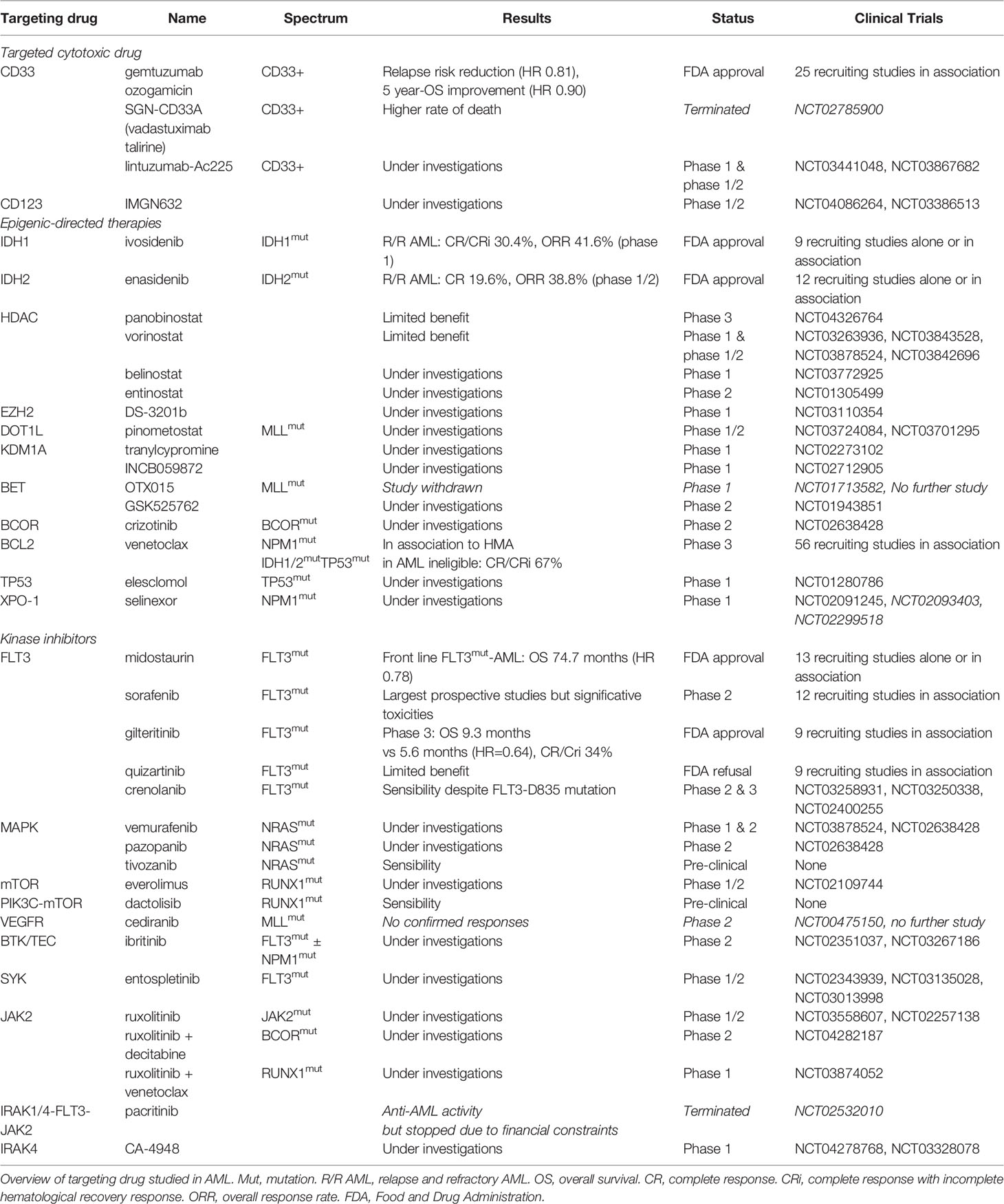
Table 2 Targeting drugs under investigations (based on www.clinicaltrials.gov at 05/25/2020).
Immunotherapies
Monoclonal and bispecific t-cells engagers (bite) antibodies as form of passive immunotherapy.
Cell surface markers of LSC and AML blasts may be targeted using several strategies, including antagonists, monoclonal antibodies (mAb), CAR T-cells or transgenic TCR ( Figure 2 ). Monoclonal antibodies could target one antigen, then induce different response depending on the kind of cells or molecules recruited: direct apoptosis, complement-dependent cytotoxicity, and antibody-dependent cell-mediated cytotoxicity ( 36 ). But technological progress has permitted to develop synthetic antibodies capable of several antigen targeting, such as Bispecific T-cells Engagers (BiTE) or dual-bodies. CD33-targeting with GO is currently the most advanced strategy available, but CD33-targeting is still under investigation, particularly to limit VOD, as described previously. Indeed the effect of several mAbs, with or without a conjugated drug, on CD25, CD38, CD44, CD45, CD47, CD70, CD123, CD157, FLT3, IL1RAP, and CCL-1, are being investigated ( 36 , 177 ). Only a few translations from preclinical models to clinical trials have shown a satisfactory response in AML clinical studies ( Table 3 ). In phase 1, anti-CD25, anti-CD44 and anti-CD47 antibodies failed to obtain a significant response ( 177 – 179 ). Only a few minor studies without clear results for anti-CD45 radiolabeled antibodies have been reported. No results have been reported by the clinical trial investigating the anti-CD157 antibody (NCT02353143) as of yet ( 177 ). Anti-FLT3 antibody shows potent preclinical activity and is being tested as a single agent for efficacy in R/R FLT3-mutated AML (NCT02864290) ( 180 ). The anti-CD70 antibody cusatuzumab has shown a CR/CRi rate of 82% in phase 1 studies ( 181 ). Due to positive preclinical results in AML, the anti-CD38 antibody, daratumumab, is currently under investigation for efficacy as a single agent, in phase 2 studies (NCT03067571) ( 182 ). Its combination with either azacytidine or ATRA is also under investigation (NCT02807558), but preliminary results appear to be disappointing ( 183 ). Interestingly ATRA upregulates CD38 in AML cells ( 184 ).
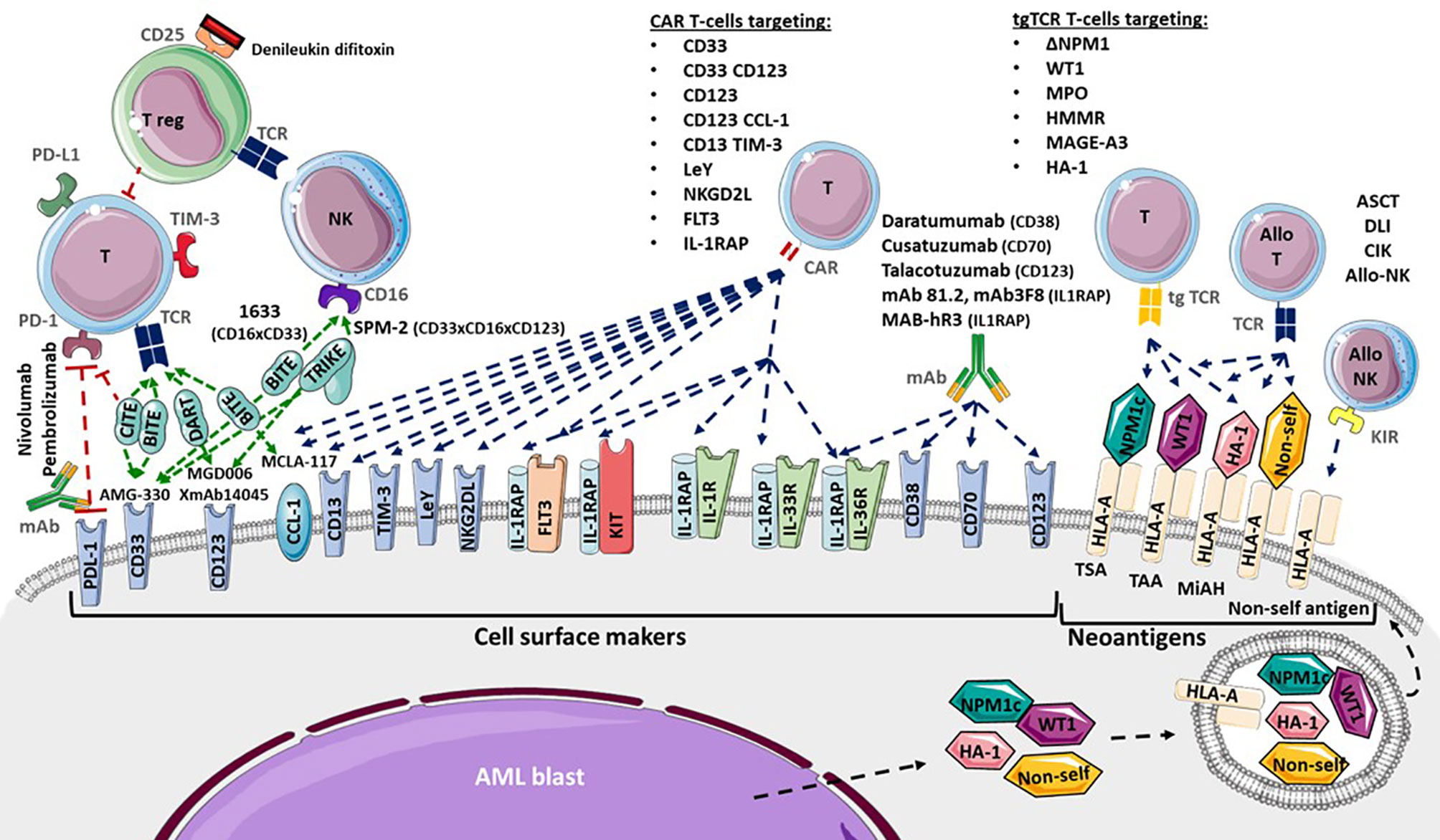
Figure 2 Adoptive immunotherapies available and in development for Acute Myeloid Leukemia (AML). Cytotoxicity functions of T-cells (T) and NK-cells (NK) are investigated in several ways. Allogenic hematopoietic Stem Cell Transplantation (ASCT), Donor Lymphocytes Infusion (DLI), Cytokine-induced Killer cells and donor NK-cells infusion provide allogenic T-cells (allo T) and NK-cells (allo NK) targeting neoantigens including Human Leukocyte Antigen (HLA) Major Histocompatibility Complex (CMH) mismatch, Killer-cell Immunoglobulin-like Receptor (KIR) mismatch, Minor Histocompatibility Antigen (MiHA), Tumor Specific Antigen (TSA) and Tumor Associated Antigen (TAA). Transgenic T-cell receptor (tgTCR) T-cells could target the nucleophosmin-1- (NPM1) mutated antigen (ΔNPM1), Wilms’ Tumor 1 (WT1), Myeloperoxidase (MPO), Hyaluronan-mediated motility receptor- (HMMR/Rhamm), Melanoma Associated Antigen-A3 (MAGE A-3), leukemia-associated minor H antigen 1- (HA-1). Monoclonal antibodies could target CD38 (Daratumumab), CD70 (Cusatuzumab), CD123 (Talacotuzumab) and IL1RAP (mAb 81.2, mAb3F8, MAB-hR3) and induced AML cells lysis. Blockade antibodies could target Program Cell Death 1 (PD-1) and PD-1 ligand (PD-L1). Bispecific T-cells Engagers (BiTE) (AMG-330, XmAb14045, MCLA-117), Dual-Affinity Re-Targeting (DART) (MGD006), Bi- and Tri-specific Killer Engagers (BiKE, TriKE) (1633, SPM-2) and Checkpoint inhibitor T-cell Engager (CiTE) antibodies could engage T-cell targeting toward specific antigens. Debileukin difitoxin could block IL-2 receptor (CD25) and then induce regulatory T-cells (Treg) apoptosis. Chimeric Antigen Receptor (CAR) T-cells could target CD33, CD123, C-type lectin domain family 12 member A (CLEC12A or CCL-1), CD33 and CD123, CD123 and CCL-1 (compound CAR), CD13 and T-cells Immunoglobulin Mucin-3 (TIM-3) (bispecific CAR), CD38, CD44, Lewis Y (LeY), Natural Killer Group 2D Ligand (NKG2DL), B7-H3, Fms-Like Tyrosine Kinase 3 (FLT3), c-KIT (CD117) and interleukine-1 Receptor Accessory Protein (IL1RAP).
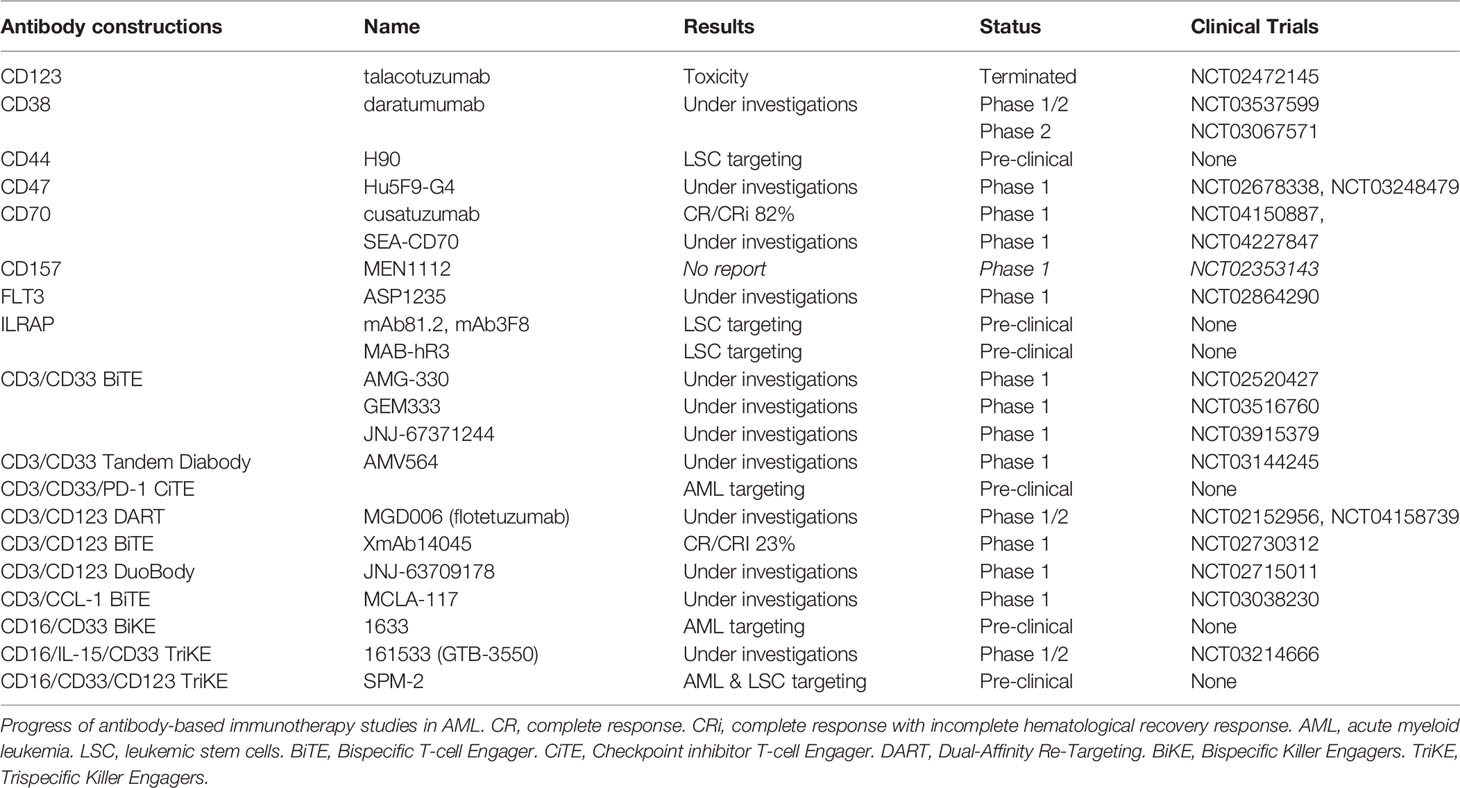
Table 3 Antibody based immunotherapies under investigation (based on www.clinicaltrials.gov at 05/25/2020).
Studies pertaining to anti-CD123 unconjugated antibodies, such as talacotuzumab, have been terminated early because of issues related to toxicity ( 185 ). However, an interim analysis of anti-CD123 conjugated IMGN632 has shown a CR/CRi rate of 33% ( 186 ). A combination of IMGN632 and venetoclax has yielded interesting results in a preclinical model ( 187 ). Moreover a CD123 antagonist, the diphtheria toxin IL-3 fusion protein tagraxofusp, indicates potent activity against AML blasts and is under investigation ( 188 , 189 ). CCL-1- (known as CLEC12A or CD371) targeting combined with CD3-targeting (MCLA-117) using a BiTE antibody is being developed and is at the phase 1 study stage (NCT03038230). In addition, anti-CD3/CD33 BiTE (AMG-330), anti-CD16/CD33 Bispecific Killer Engagers (BiKE) (known as 1633), anti-CD16/IL-15/CD33 Trispecific Killer Engagers (TriKE) (known as 161533), and anti-CD3/CD123 Dual-Affinity Re-Targeting (DART) antibodies (MGD006) are also in development and under investigation for effectiveness in AML ( 36 , 189 ). The anti- CD3/CD123 BITE XmAb14045 phase 1 showed a CR/CRi rate of 23% ( 190 ). Moreover, anti-CD33/CD123/CD16 TriKE is under investigation ( 191 ). Another interesting BiTE combination being investigated for overweight PD-1/PD-L1 exhaustion, is PD-1 extracellular domain (PD-EX) blockade in association with anti-CD3/anti-CD33, which is thus a Checkpoint inhibitor T-cell Engager (CiTE) ( 192 ).
Lastly, IL1RAP (also named IL-1R3) blockade, using mAb81.2 and mAb3F8, which induces antibody-dependent cellular cytotoxicity and IL-1 signaling blockade in AML and CML cells provides evidence that IL1RAP may be a potent target in AML cells ( 33 , 193 ). All functions of the IL-1 family (IL-1α, IL-1β, IL-33, IL-36α, IL-36β, and IL-36γ) are attenuated by IL1RAP blockade with the human IL1RAP antibody, MAB-hR3 ( 194 ). No increase in IL-6 production was observed, and IL1RAP blockade induced a broader anti-inflammatory activity than that associated with IL-1R1 blockade by IL-1Ra antagonist anakinra. Interestingly, chronic IL-1 exposure drives HSC clonal evolution in AML cells ( 53 ), and thus IL1RAP blockade may allow a reduction in bone marrow inflammation associated with AML.
T-Cell Transfer Therapy a New Era in Cancer Immunotherapy
Chimeric antigen receptors (car) t-cells, leaders in t-cell transfer therapy.
A novel adoptive T-cell transfer therapy named Chimeric Antigen Receptor T-cells (CAR T-cells), is recently available in lymphoid neoplasms, using CD19 target with promising results ( 195 – 197 ). T-cells are transduced by a viral supernatant that induces a cell surface chimeric receptor expression. This allows to target a specific antigen expressed at the surface of the tumor cells ( 198 ). Afterward, genetically modified T-cells are infused in order to induce a selective tumor lysis after CAR recognition. Thus, several cell surface markers and transgene constructions are investigated for CAR T-cell engineering in AML ( 130 ). As compared to mAb, CD33, CD123, CCL-1, FLT3, CD38, CD44 variant 6, and NKG2DL targets are investigated in early phase clinical trial, whereas c-KIT (CD117), B7-H3 (as known as CD276), IL1RAP, and CD13 targets are under preclinical investigation ( Table 4 ) ( 36 , 199 ).
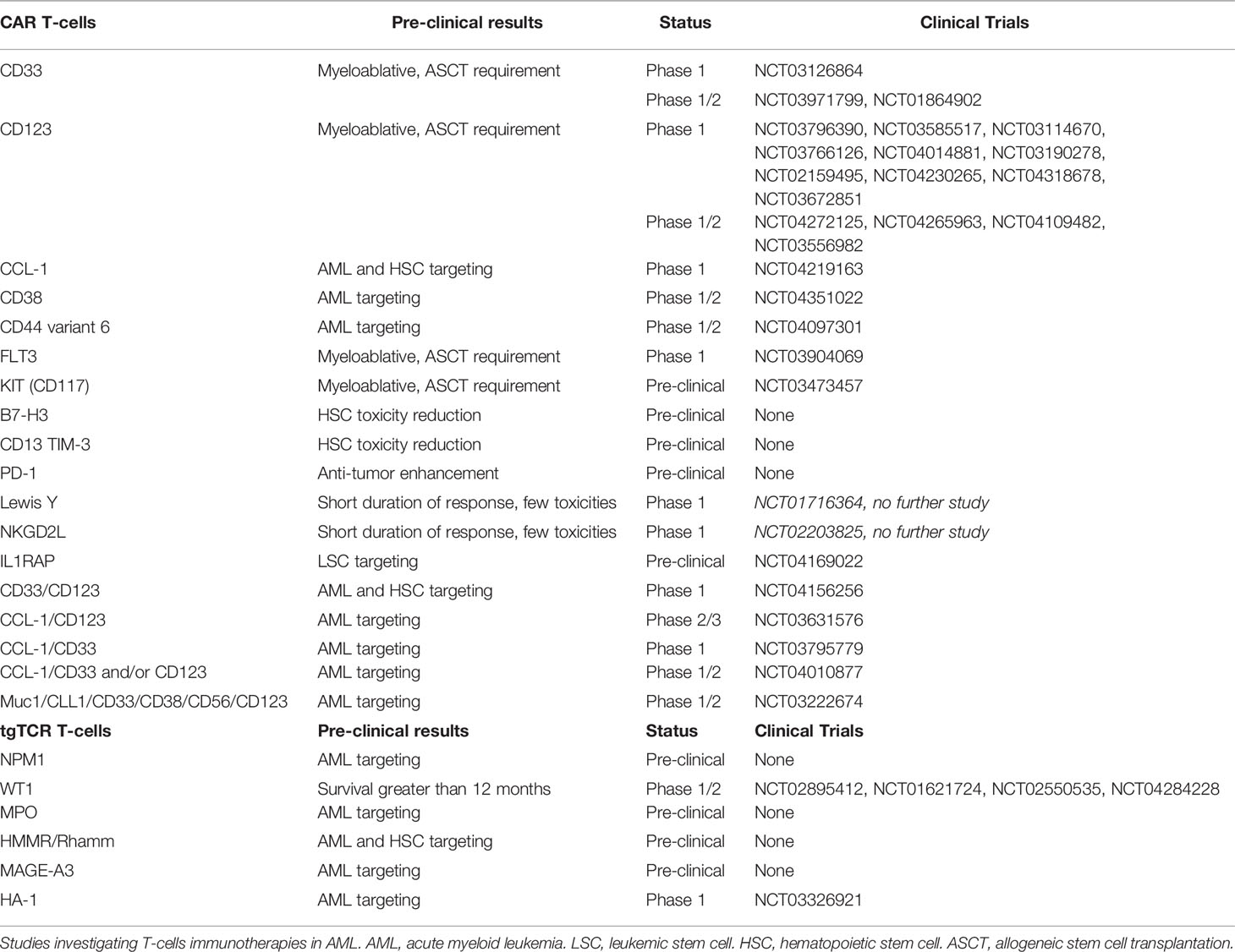
Table 4 T-cells immunotherapies under investigations (based on www.clinicaltrials.gov at 05/25/2020).
The two main targets are CD33 and CD123 with several CAR constructions improvement over time. Addition to a 4-1BBz costimulatory transgene in the CD33 CAR construction have shown better antileukemic activity as well as resistance to exhaustion with an increasing central memory comportment ( 200 ). Despite use as myeloablative conditioning regimen before ASCT ( 201 , 202 ), hematopoietic toxicity is a limitation of CD33 and CD123 CAR T-cells, that conducts to investigate different strategies. Thus, two strategies have been developed: modulation of CAR affinity ( 203 ), and transient transgene expression ( 129 ). Modification by sequence-mutation of anti-CD123 single chain fragment variable (scFv) might conduct to a lesser hematopoietic toxicity ( 201 , 204 , 205 ). Another proposed strategy in order to reduce hematopoietic toxicity is the use of an transiently expressed ARN to induce self-limiting activity against AML cells ( 129 ). Inactivation of the CD33 gene in HSCs prior to transplantation was suggested in order to prevent CD33 induced hematopoietic toxicity of CAR T-cells ( 206 ). Yet, CD123 CAR T-cells in combination with ASCT could be an interesting strategy for treating R/R AML patients ( 189 , 202 ).
Other targets are investigated in the purpose to reduced hematopoietic toxicity. CCL-1 CAR T-cells show interesting preclinical efficacy on LSC and AML blasts without HSC toxicity ( 207 , 208 ). FLT3 or CD117 CAR T-cells are also cytotoxic against LSC and AML blasts but require association with ASCT ( 209 , 210 ). However, targeting of the Lewis Y (LeY) antigen and NKG2DL CAR T-cells have also been proposed, but phase 1 trials have shown short response durations, despite less toxicity ( 211 , 212 ). Combinations using several targets have been also proposed. Compound CAR T-cells targeting CD33 and CD123 are in development, and exhibit pronounced anti-leukemic activity ( 213 ). Nevertheless, CD123 and CCL-1 compound CAR T-cells might be useful for LSC targeting with limited hematopoietic toxicity ( 36 , 177 ). Lastly, IL1RAP CAR T-cells have been described as having a potent effect on LSC in CML, and more recently in AML, without affecting healthy HSCs ( 214 , 215 ). Interestingly this target is shared with AML blasts that overexpress IL1RAP and it is not only associated to IL-1 receptor but also to FLT3, KIT and other pro-inflammatory interleukin receptors ( 46 ). Lastly, integrative approaches using proteomics and transcriptomics could help to identify the better combinatorial CAR T-cells therapy. Some target pairwise combinations hold promise, such as CD33/CD70, CD33/ADGRE2 (CD312), CCL-1/CCR1 (CD191), and CCL-1/LILRB2 (CD85d) combinations ( 216 ), although clinical investigations are required to fully validate this approach.
Furthermore, microenvironment could play a major role in regulation of CAR T-cell functions and toxicities. Indeed, IL-15 may enhance anti-AML activity of CD123 CAR T-cells ( 217 ). Moreover, several costimulatory ligands are deregulated that induce T-cell inhibition ( 115 ). Thus, one proposed strategy was targeting inhibitory ligands expressed by AML in addition to LSC antigens targeting. Bispecific CD13-TIM-3 CAR T-cells reduced HSC toxicity ( 218 ), as well as a B7-H3 CAR T-cells ( 219 ). Besides, B7-H3 pan-cancer target was also studied in solid tumors ( 220 ). Preliminary reports show that PD-1 inhibitors also regulate CAR T-cell response, although few data are available ( 221 ). Furthermore, delivery of PD-1-blocking scFv CAR T-cells in pre-clinical investigations demonstrated interesting anti-tumor efficacy enhancement ( 222 ).
Lastly, several mechanisms were used to secure CAR T-cells administration. In order to avoid uncontrolled toxicity, the use of anti-CD52 alemtuzumab or CD20 protein co-expression in CD123 CAR T-cells for anti-CD20 rituximab targeting, is proposed ( 223 ). Integration to a suicide gene in the CAR construction, using inducible procaspase 9 by rimiducid, has been reported ( 224 ). A major side effect of CAR T-cell administration is cytokine release syndrome (CRS) resulting from the excessive production of those cytokines, particularly IL-6 ( 225 ). Similarly, immune effector cell-associated neurotoxicity syndrome (ICAN) is associated with excessive production of IL-1. Furthermore, the production of IL-1β, IL-6, and TNFα induces IL-10 and TGFβ productions that impair CAR T-cells functions ( 226 ). Anti-IL-1 (anakinra) and anti-IL-6 (tocilizumab) were described to counteract these two immune side effects ( 225 ). Of note, targeting IL1RAP might be an interesting manner to reduce pro-inflammatory side effect by reducing pro-inflammatory interleukin activation ( 194 ), but also by targeting monocytes that are assumed to produce IL-1 and IL-6 in CRS ( 214 , 227 ). However, that suggestion needs to be demonstrated. Several challenges remain to be overcome as recently reported ( 228 ), and further investigations may provide a better understanding.
Transgenic T-Cell Receptor (TCR) T-Cells, Leading Competitors
Another adoptive T-cells transfer therapy that could be proposed is generated T-cells with a transgenic TCR specific to one antigen. A few tumor-specific proteins that act as leukemia-specific antigens (Tumor Specific Antigens, TSA), such as RUNX1-RUNX1T1, FLT3, and NPM1, have been described as being associated with AML ( 229 ), resulting from mutations (mutated TSA) or aberrant expression (aberrantly expressed TSA) from noncoding regions ( 230 ). C-terminal CLAVEEVSLRK sequence of NPM1c (ΔNPM1) binds and presents as HLA-A*02:01 ( 231 ). Anti-tumor activity by ΔNPM1-transgenic -cells receptor (tgTCR) T-cells against AML cells have been recently reported ( 232 ). Further investigations may yield interesting results. Subsequently in vitro T-cell recognition has been demonstrated in IDH1/2, FLT3-ITD and Ras-MAPK pathway mutations, and PML-RARα and DEK-CAN fusion proteins, that are considered as promising targets for CAR T-cells, tgTCR T-cells or a vaccine ( 233 , 234 ). Other antigens such as WT1 that are considered as leukemia-associated antigens (Tumor-Associated Antigens; TAA) are not completely specific to AML cells. A phase 1 study of WT1-tgTCR T-cells highlights interesting survival rates that are greater than 12 months in patients with persistent tgTCR T-cells ( 235 ). Moreover, the use of allogeneic WT1-tgTCR T-cells following ASCT appear to prevent AML relapses ( 236 ). However, only HLA-A*24:02 presents WT1 peptides to T-cell receptors (TCR). Thus, WT1-transgenic TCR T-cells (tgTCR) are restricted to HLA-A*24:02 patients. Subsequently, further investigations are ongoing ( 229 ). WT1 is also a target investigated in vaccination studies, such as the multivalent WT1 peptide vaccine ( 237 ) or the dendritic cell vaccination ( 238 ). Myeloperoxidase- (MPO) targeting by MPO-tgTCR T-cells is also described as having potent anti-AML efficacy, but is restricted to HLA-B*07:02 presentation ( 239 ). Notably, resistance to MPO-tgTCR T-cells may appear via the downregulation of HLA. Hyaluronan-mediated motility receptor- (HMMR/Rhamm) tgTCR T-cells, which are similarly restricted to HLA-A*02:01 presentation, are reported as showing potent cytotoxicity toward not only AML cells, but also HSCs ( 240 ). In addition, overexpression of Melanoma Associated Antigen-A3 (MAGE-A3) in AML and MAGE-A3-targeting have been reported ( 241 , 242 ).
Unlike antigens presented by the HLA major histocompatibility complex (MHC), minor histocompatibility antigens (MiHAs) are encoded by germline polymorphisms. In ASCT, differences between MiHA expression in malignant and normal hematopoietic cells of patients and donors, respectively, result in GVHD but also in graft-versus-leukemia (GVL), due to HLA and Killer-cell Immunoglobulin-like Receptor (KIR) mismatches ( 242 , 243 ). This strategy is used in DLI, Cytokine-induced Killer cell (CIK) and donor NK cell infusions as well. But MiHA-targeting is limited by the low frequency (only 0.5%) of non-synonymous single nucleotide polymorphism (sn-SNP) generating HLA-associated peptides ( 244 ). MiHAs are being investigated in order to identify suitable targets in AML, such as the via the proteomic approach. The most common HLA haplotype presenting MiHAs in European Americans seems to be HLA-A*02:01; B*44:03 ( 245 ). Thus, MiHA-based immunotherapies should target antigens restricted to AML cells, well balanced in the population, but also demonstrating MHC binding and immunogenicity ( 242 ). In fact, the leukemia-associated minor H antigen 1- (HA-1) tgTCR T-cells, restricted to HLA-A*02:01, are used for the most advanced MiHA-based immunotherapy, regarding which further investigations are ongoing (NCT03326921) ( 246 ). Therefore, TSA-, TAA-, and MiHA-based therapies are mainly limited to their HLA restriction in addition to the small number of suitable targets ( 242 ). Lastly, to limit off-target toxicities induced by mispairing between the endogenous and the introduced TCR chains, a reported strategy is knocking-out the endogenous TCR by genome editing, as TALEN or CRISPR-Cas-9 approaches ( 247 ). This strategy is also investigated to generate universal engineered T-cells ( 198 ).
Immune Checkpoint Blockade and Microenvironment Targeted Therapies Enhance Immune Cell Therapies
As previously described, AML blasts present a deregulation of costimulatory ligand with concomitant changes in donor T-cells after ASCT. In addition, several inhibitory receptors are expressed by early differentiated memory and central memory T-cell in bone marrow of AML patients relapsing ( 248 ). Immune checkpoint blockade (ICB) antibodies have demonstrated efficacy in solid tumor with immune synapse restoration and tumor cell eradication ( 249 ). Then, several preclinical studies have investigated ICB in AML ( 36 , 64 ). Superior efficacies of ICB nivolumab (anti-PD1), pembrolizumab (anti-PDL1), and ipilimumab (anti-CTLA4) in combination with azacytidine or other cytotoxic drugs have been reported in relapses following ASCT, or with other ICB ( 64 ). The use of Ipilimumab in AML relapse following ASCT has shown a CR rate of 23%, but use of ICB after ASCT needs particular caution regarding GVHD ( 250 ). Interestingly, response occurs in extramedullary disease. HMAs increase PD-1/PD-L1 expression in AML blasts ( 251 ). Moreover the azacytidine and nivolumab combination in R/R AML induced a CR/CRi rate of 22%, whereas the addition of ipilimumab improved the CR/CRi rate and median OS ( 252 , 253 ). The consensus is that ICB may play an adjunct role to other interventions, and display limited efficiency when used alone ( 68 , 254 , 255 ). Multiple studies in search of further information are ongoing ( 65 , 68 , 256 ). TIM-3 and T Cell Immunoglobulin and Immunoreceptor Tyrosine-Based inhibitory motif domain (TIGIT) blockade are also being investigated. Moreover, ICB and CAR T-cells combination was proposed in solid tumor in the purpose to enhance antitumor activity, with promising results ( 257 ). That strategy could be also interesting to investigate in AML.
Furthermore, in vivo Treg depletion via immunotherapies based on bacterial toxins are also being investigated. A recent study reported preclinical results indicating that denileukin difitoxin, a diphtheria toxin, may target the IL-2 receptor and eliminate Tregs ( 68 , 258 ). Another form of microenvironment targeting is achieved by the interruption of the CXCR4/CXCL12 signaling axis by CXCR4 monoclonal antibody, ulocuplumab, that showed interesting results in phase 1 trials with a CR/CRi rate of 51% ( 259 ). This blockade induces migration of HPCs and LSCs from the BM to peripheral blood. Several investigations in combination with LDAC or chemotherapy are ongoing ( 256 ). Further investigations may reveal novel and useful immunotherapies. In addition, several microenvironmental targets such as IL-1β pathway and IL-1 antagonists or β3-AR agonists for sympathetic neuropathy (such as mirabegron) ( 260 ), mitochondrial heterocellular transfer, and inhibition by tigecycline, fatty acid oxidation (FAO) and FAO inhibitor etomoxir that sensitizes AML cells to therapeutic challenge, NO synthase inhibitors (such as cavtratin) ( 261 , 262 ) and endothelium activation ( 59 ), are under investigation.
Global Strategies for AML Treatment
For patients eligible for intensive chemotherapy, the ELN 2017 panel recommends an induction therapy involving 3 days of treatment with an anthracycline, such as daunorubicin ≥60 mg/m², idarubicin 12 mg/m² or mitoxantrone 12 mg/m², combined with 7 days of continuous cytarabine infusion (100-200 mg/m²). Induction has to be followed by a consolidation therapy depending on ELN risk-stratification and age or comorbidities as follows: an intermediate dose of cytarabine (IDAC) (2-4 cycles of 1,000–1,500 mg/m² twice a day for of 3 days) for younger patients in favorable- and intermediate-risk groups (18–60/65 year-old) and IDAC (2–3 cycles of 500–1,000 mg/m² twice a day for 3 days) for older patients in the favorable-risk group (≥60/65 year-old) ( 2 ). No consolidation therapy has been shown to have an established value for intermediate- and adverse-risk older patients. Addition of midostaurin to intensive chemotherapy should be considered in case of FLT3-mutated AML ( 140 ). ASCT is recommended for intermediate- and adverse-risk younger patients, and for older patients with a low comorbidity index ( 2 ). These recommendations have been recently confirmed by the ESMO Guidelines Committee with a few updates ( 263 ). The benefits of prophylactic or pre-emptive DLI and maintenance with HMA, or other drugs, are still unclear and require further studies. Lastly, an oral HMA has reportedly improved OS during maintenance in ≥55 year-old patients who are in CR1 after intensive chemotherapy ( 264 ). Only tyrosine-kinase inhibitor based maintenance in BCR-ABL-positive AML following ASCT is recommended ( 263 ).
Salvage therapy consists of IDAC with or without anthracycline described as FLAG +/- IDA (fludarabine, cytarabine, idarubicin, and additional granulocyte-stem cells factor). Other salvage regimens, such as mitoxantrone, etoposide and cytarabine (MEC), have shown overlapping results ( 2 ). The sequential transplant conditioning regimen has also been proposed as FLAMSA-RIC (cytarabine/amsacrine salvage regimen and fludarabine based RIC regimen) when either a donor or the donor source (MUD, haploidentical, or UCB) is immediately available ( 263 ). Patients ineligible for intensive chemotherapy as a first line may benefit from HMA (azacytidine or decitabine). The challenging options for such frail patients are low, sub-cutaneous doses of cytarabine (LDAC) or best supportive care.
For adults, new drugs have been recently approved by the FDA and EMA as follows: CPX-351 for AML with MRC-AML or tAML, gemtuzumab ozogamicin in addition to standard induction chemotherapy for CD33-positive AML with favorable- and intermediate-risk, gilteritinib for R/R FLT3-mutated AML and glasdegib in combination with LDAC for newly-diagnosed AML cases who are ≥75 year-old, or have comorbidities. In addition, the FDA has also approved ivosidenib for ≥75 year-old ineligible patients with IDH1-mutated AML or R/R IDH1-mutated AML, enasidenib for R/R IDH2-mutated AML, and venetoclax in combination with azacytidine, decitabine, or LDAC for newly diagnosed ≥75 year-old AML patients who are ineligible for standard intensive chemotherapy. Nevertheless, as discussed by Estey et al., several concerns have been reported as problematic issues confronting the assessment of fitness for standard induction therapy, such as the intensity of compared induction, problematic endpoints and approvals for unstudied populations ( 265 ). Fitness evaluation via a standardized score, which is similar to that for ASCT or geriatric assessment, should be performed ( 266 , 267 ). Moreover, the OS rate and quality of life assessments may be more useful for patients.
Recently, DiNardo et al., proposed that these new therapies should be discussed in detail ( 268 ). Personalized medicine may be offered to many patients depending on fitness or phenotypic and molecular disease characteristics. Development of targeting drugs such molecular inhibitors or mAb may be useful as a third drug in intensive induction regimens, in salvage therapy or in maintenance, depending on specific mutations or cell surface markers. ICB and venetoclax appear to show potential for salvage therapy in association with other drugs, but further studies are required. Concerning maintenance therapy following ASCT, despite the identification of driver mutations and development of inhibitors, strategies adapted to residual disease, monitored by MRD assessment after ASCT, need further investigation, before specific therapies can be proposed at the appropriate moment ( 269 , 270 ). Azacytidine and DLI are the most widely used therapies following ASCT, in addition to immunosuppressive drug adaptation ( 111 , 168 ). In fact, for patients undergoing FLT3 inhibitor treatment prior to ASCT the only therapy the EBMT Acute Leukemia Working Party of recommends is ASCT consolidation and FTL3 inhibitor maintenance for at least 2 years following ASCT ( 146 ).
Finally, no leukemia-specific surface marker common to all patient exists. A major concern in CAR T-cells therapy, where the targeted cells share some phenotypic features with HSCs, is myeloablative activity of CAR-T cells via HSC targeting. The dilemma is reducing the potency of CAR T-cells and may increase the risk of disease escape ( 202 ). CAR T-cells may be considered as a bridge-to-transplant therapy in the face of significant or pan-myeloid cell activity. Indeed, ASCT is proposed in refractory AML but with a poorer outcome due to AML burden ( 2 ). Thus, CAR T-cells therapy may reduce the AML burden and act as an interesting salvage therapy that induces a greater response rate prior to ASCT. Moreover, MRD negative status before ASCT is associated with greater PFS and OS ( 271 ). Therefore, MRD monitoring may guide treatment intensification strategies before ASCT and CAR T-cells use, as well. While developing new targets for CAR T-cells with potent smaller hematopoietic toxicity, an ASCT should be prepared for salvage therapy in case CAR T-cells fail or hematopoietic toxicity is induced. Thus, CAR T-cells are more appropriate for younger and fitter patients. Better assessment and understanding of adverse toxicity risk factors and toxicity management may permit the use of CAR T-cells in R/R AML, high risk AML or older patients. In addition, the use of MiHA-, TSA-, and TAA-based immunotherapies, such as tgTCR, T-cells or vaccination approaches, appear to be more potent in the context of low burden disease ( 242 ). Thus, MRD monitoring studies may provide guidance for earlier use of T-cell therapies ( Figure 3 ).
Figure 3 Acute Myeloid Leukemia (AML) intensive strategy perspectives in younger patients. Standard treatments are in regular small captions, putative investigated treatments are in italics. Standard chemotherapy could be combined with targeted drug inhibitors (targeted inhibitor), such as FLT3 inhibitors or targeted chemotherapeutic agents such as CD33-conjugated antibodies. In the case of first complete response (CR1), intermediate and high-risk patients with or without maintenance therapy are subjected to consolidation via chemotherapy, with or without targeted drug inhibitors, such FLT3 inhibitors, followed by allogeneic hematopoietic stem cell transplantation (ASCT). In the case of primary refractory AML, salvage therapy may be improved via chemotherapy by the addition of a hypomethylated agent (HMA), targeted inhibitors, targeted-drug immunotherapy (immunotherapy) or chimeric antigen receptor (CAR) T-cells, followed by ASCT. Measurable residual disease or low burden relapse may be treated with HMA or a donor lymphocyte infusion (DLI) in case of ASCT, and improved through the addition or the single use of a target inhibitor, immunotherapy or transgenic T-cell receptor (tgTCR) T-cells. Relapses with greater AML burden may be treated via chemotherapy and improved via the addition or single use of a targeted inhibitor, immunotherapy, or CAR T-cells. Next, ASCT following chemotherapy, single or several targeted therapy regimens depending on the response or CAR T-cells may be performed. Consolidation with or without maintenance may also be performed using HMA, donor lymphocyte infusion (DLI) in case of ASCT, or targeted therapy.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
XR, MD, and CF designed the study. XR collected data, wrote the draft manuscript and drew the figures. MN improved the figures. All authors contributed to the article and approved the submitted version.
Association « Nausicaa combat sa Leucémie » (non profit association 1901); Gift 2017-03, 2018 and 2019. SFGMTC/Association Capucine Grant 2013-AO2. Supported by the MiMedi project funded by BPI France (no. DOS0060162/00) and the EU through the European Regional Development Fund of the Region BFC (no. FC0013440). French Blood Center (EFS, DRVI) grants 2018 and 2019.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Editage ( www.editage.com ) for English language editing.
1. Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood (2010) 115:453–74. doi: 10.1182/blood-2009-07-235358
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood (2017) 129:424–47. doi: 10.1182/blood-2016-08-733196
3. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Beau MML, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood (2016) 127:2391–405. doi: 10.1182/blood-2016-03-643544
4. Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N Engl J Med (2015) 373:1136–52. doi: 10.1056/NEJMra1406184
5. Cancer Genome Atlas Research Network, Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med (2013) 368:2059–74. doi: 10.1056/NEJMoa1301689
6. DiNardo CD, Cortes JE. Mutations in AML: prognostic and therapeutic implications. Hematol Am Soc Hematol Educ Program (2016) 2016:348–55. doi: 10.1182/asheducation-2016.1.348
CrossRef Full Text | Google Scholar
7. Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N Engl J Med (2016) 374:2209–21. doi: 10.1056/NEJMoa1516192
8. Bullinger L, Döhner K, Döhner H. Genomics of Acute Myeloid Leukemia Diagnosis and Pathways. J Clin Oncol (2017) 35:934–46. doi: 10.1200/JCO.2016.71.2208
9. Tyner JW, Tognon CE, Bottomly D, Wilmot B, Kurtz SE, Savage SL, et al. Functional genomic landscape of acute myeloid leukaemia. Nature (2018) 562:526–31. doi: 10.1038/s41586-018-0623-z
10. Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Loo PV, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood (2013) 122:3616–27. doi: 10.1182/blood-2013-08-518886
11. Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Blood Adv (2018) 2:3404–10. doi: 10.1182/bloodadvances.2018020222
12. Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, et al. The Origin and Evolution of Mutations in Acute Myeloid Leukemia. Cell (2012) 150:264–78. doi: 10.1016/j.cell.2012.06.023
13. Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature (2012) 481:506–10. doi: 10.1038/nature10738
14. Papaioannou D, Volinia S, Nicolet D, Świerniak M, Petri A, Mrózek K, et al. Clinical and functional significance of circular RNAs in cytogenetically normal AML. Blood Adv (2020) 4:239–51. doi: 10.1182/bloodadvances.2019000568
15. Fisher JN, Kalleda N, Stavropoulou V, Schwaller J. The Impact of the Cellular Origin in Acute Myeloid Leukemia: Learning From Mouse Models. HemaSphere (2019) 3:e152. doi: 10.1097/HS9.0000000000000152
16. Yen K, Travins J, Wang F, David MD, Artin E, Straley K, et al. AG-221, a First-in-Class Therapy Targeting Acute Myeloid Leukemia Harboring Oncogenic IDH2 Mutations. Cancer Discovery (2017) 7:478–93. doi: 10.1158/2159-8290.CD-16-1034
17. Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood (2017) 130:722–31. doi: 10.1182/blood-2017-04-779405
18. Amatangelo MD, Quek L, Shih A, Stein EM, Roshal M, David MD, et al. Enasidenib induces acute myeloid leukemia cell differentiation to promote clinical response. Blood (2017) 130:732–41. doi: 10.1182/blood-2017-04-779447
19. DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N Engl J Med (2018) 378:2386–98. doi: 10.1056/NEJMoa1716984
20. Thomas D, Majeti R. Biology and relevance of human acute myeloid leukemia stem cells. Blood (2017) 129:1577–85. doi: 10.1182/blood-2016-10-696054
21. Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CHM, Jones DL, et al. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res (2006) 66:9339–44. doi: 10.1158/0008-5472.CAN-06-3126
22. Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell (2014) 14:275–91. doi: 10.1016/j.stem.2014.02.006
23. Goardon N, Marchi E, Atzberger A, Quek L, Schuh A, Soneji S, et al. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer Cell (2011) 19:138–52. doi: 10.1016/j.ccr.2010.12.012
24. Béné MC, Nebe T, Bettelheim P, Buldini B, Bumbea H, Kern W, et al. Immunophenotyping of acute leukemia and lymphoproliferative disorders: a consensus proposal of the European LeukemiaNet Work Package 10. Leukemia (2011) 25:567–74. doi: 10.1038/leu.2010.312
25. Jin L, Lee EM, Ramshaw HS, Busfield SJ, Peoppl AG, Wilkinson L, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell (2009) 5:31–42. doi: 10.1016/j.stem.2009.04.018
26. Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med (2006) 12:1167–74. doi: 10.1038/nm1483
27. Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell (2009) 138:286–99. doi: 10.1016/j.cell.2009.05.045
28. Kikushige Y, Shima T, Takayanagi S, Urata S, Miyamoto T, Iwasaki H, et al. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell (2010) 7:708–17. doi: 10.1016/j.stem.2010.11.014
29. Hosen N, Park CY, Tatsumi N, Oji Y, Sugiyama H, Gramatzki M, et al. CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc Natl Acad Sci U.S.A. (2007) 104:11008–13. doi: 10.1073/pnas.0704271104
30. Chung SS, Tavakkoli M, Devlin SM, Park CY. CD99 Is a Therapeutic Target On Disease Stem Cells In Acute Myeloid Leukemia and The Myelodysplastic Syndromes. Blood (2013) 122:2891–1. doi: 10.1182/blood.V122.21.2891.2891
31. van Rhenen A, van Dongen GAMS, Kelder A, Rombouts EJ, Feller N, Moshaver B, et al. The novel AML stem cell–associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood (2007) 110:2659–66. doi: 10.1182/blood-2007-03-083048
32. Askmyr M, Ågerstam H, Hansen N, Gordon S, Arvanitakis A, Rissler M, et al. Selective killing of candidate AML stem cells by antibody targeting of IL1RAP. Blood (2013) 121:3709–13. doi: 10.1182/blood-2012-09-458935
33. Ågerstam H, Hansen N, von Palffy S, Sandén C, Reckzeh K, Karlsson C, et al. IL1RAP antibodies block IL-1–induced expansion of candidate CML stem cells and mediate cell killing in xenograft models. Blood (2016) 128:2683–93. doi: 10.1182/blood-2015-11-679985
34. Pabst C, Bergeron A, Lavallée V-P, Yeh J, Gendron P, Norddahl GL, et al. GPR56 identifies primary human acute myeloid leukemia cells with high repopulating potential in vivo. Blood (2016) 127:2018–27. doi: 10.1182/blood-2015-11-683649
35. Iwasaki M, Liedtke M, Gentles AJ, Cleary ML. CD93 Marks a Non-Quiescent Human Leukemia Stem Cell Population and Is Required for Development of MLL-Rearranged Acute Myeloid Leukemia. Cell Stem Cell (2015) 17:412–21. doi: 10.1016/j.stem.2015.08.008
36. Shang Y, Zhou F. Current Advances in Immunotherapy for Acute Leukemia: An Overview of Antibody, Chimeric Antigen Receptor, Immune Checkpoint, and Natural Killer. Front Oncol (2019) 9:917. doi: 10.3389/fonc.2019.00917
37. Majeti R. Monoclonal antibody therapy directed against human acute myeloid leukemia stem cells. Oncogene (2011) 30:1009–19. doi: 10.1038/onc.2010.511
38. Al-Mawali A, Gillis D, Lewis I. Immunoprofiling of leukemic stem cells CD34+/CD38-/CD123+ delineate FLT3/ITD-positive clones. J Hematol OncolJ Hematol Oncol (2016) 9:61. doi: 10.1186/s13045-016-0292-z
39. Quek L, Otto GW, Garnett C, Lhermitte L, Karamitros D, Stoilova B, et al. Genetically distinct leukemic stem cells in human CD34- acute myeloid leukemia are arrested at a hemopoietic precursor-like stage. J Exp Med (2016) 213:1513–35. doi: 10.1084/jem.20151775
40. Sandén C, Lilljebjörn H, Orsmark Pietras C, Henningsson R, Saba KH, Landberg N, et al. Clonal competition within complex evolutionary hierarchies shapes AML over time. Nat Commun (2020) 11:579. doi: 10.1038/s41467-019-14106-0
41. Wilson NK, Göttgens B. Single-Cell Sequencing in Normal and Malignant Hematopoiesis. HemaSphere (2018) 2:e34. doi: 10.1097/HS9.0000000000000034
42. Warfvinge R, Geironson L, Sommarin MNE, Lang S, Karlsson C, Roschupkina T, et al. Single-cell molecular analysis defines therapy response and immunophenotype of stem cell subpopulations in CML. Blood (2017) 129:2384–94. doi: 10.1182/blood-2016-07-728873
43. Barreyro L, Will B, Bartholdy B, Zhou L, Todorova TI, Stanley RF, et al. Overexpression of IL-1 receptor accessory protein in stem and progenitor cells and outcome correlation in AML and MDS. Blood (2012) 120:1290–8. doi: 10.1182/blood-2012-01-404699
44. Gerber JM, Gucwa JL, Esopi D, Gurel M, Haffner MC, Vala M, et al. Genome-wide comparison of the transcriptomes of highly enriched normal and chronic myeloid leukemia stem and progenitor cell populations. Oncotarget (2013) 4:715–28. doi: 10.18632/oncotarget.990
45. Vetrie D, Helgason GV, Copland M. The leukaemia stem cell: similarities, differences and clinical prospects in CML and AML. Nat Rev Cancer (2020) 20:158–73. doi: 10.1038/s41568-019-0230-9
46. Mitchell K, Barreyro L, Todorova TI, Taylor SJ, Antony-Debré I, Narayanagari S-R, et al. IL1RAP potentiates multiple oncogenic signaling pathways in AML. J Exp Med (2018) 215:1709–27. doi: 10.1084/jem.20180147
47. Rhyasen GW, Bolanos L, Fang J, Jerez A, Wunderlich M, Rigolino C, et al. Targeting IRAK1 as a Therapeutic Approach for Myelodysplastic Syndrome. Cancer Cell (2013) 24:90–104. doi: 10.1016/j.ccr.2013.05.006
48. Brikos C, Wait R, Begum S, O’Neill LAJ, Saklatvala J. Mass Spectrometric Analysis of the Endogenous Type I Interleukin-1 (IL-1) Receptor Signaling Complex Formed after IL-1 Binding Identifies IL-1RAcP, MyD88, and IRAK-4 as the Stable Components. Mol Cell Proteomics (2007) 6:1551–9. doi: 10.1074/mcp.M600455-MCP200
49. Rhyasen GW, Starczynowski DT. IRAK signalling in cancer. Br J Cancer (2015) 112:232–7. doi: 10.1038/bjc.2014.513
50. McKeown MR, Corces MR, Eaton ML, Fiore C, Lee E, Lopez JT, et al. Superenhancer Analysis Defines Novel Epigenomic Subtypes of Non-APL AML, Including an RARα Dependency Targetable by SY-1425, a Potent and Selective RARα Agonist. Cancer Discovery (2017) 7:1136–53. doi: 10.1158/2159-8290.CD-17-0399
51. Paschka P, Du J, Schlenk RF, Gaidzik VI, Bullinger L, Corbacioglu A, et al. Secondary genetic lesions in acute myeloid leukemia with inv(16) or t(16;16): a study of the German-Austrian AML Study Group (AMLSG). Blood (2013) 121:170–7. doi: 10.1182/blood-2012-05-431486
52. Corces-Zimmerman MR, Hong W-J, Weissman IL, Medeiros BC, Majeti R. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc Natl Acad Sci (2014) 111:2548–53. doi: 10.1073/pnas.1324297111
53. Pietras EM, Mirantes-Barbeito C, Fong S, Loeffler D, Kovtonyuk LV, Zhang S, et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat Cell Biol (2016) 18:607–18. doi: 10.1038/ncb3346
54. Pietras EM. Inflammation: a key regulator of hematopoietic stem cell fate in health and disease. Blood (2017) 130:1693–8. doi: 10.1182/blood-2017-06-780882
55. O’Neill LAJ. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol Rev (2008) 226:10–8. doi: 10.1111/j.1600-065X.2008.00701.x
56. Pinho S, Frenette PS. Haematopoietic stem cell activity and interactions with the niche. Nat Rev Mol Cell Biol (2019) 20:303–20. doi: 10.1038/s41580-019-0103-9
57. Brück O, Dufva O, Hohtari H, Blom S, Turkki R, Ilander M, et al. Immune profiles in acute myeloid leukemia bone marrow associate with patient age, T-cell receptor clonality, and survival. Blood Adv (2020) 4:274–86. doi: 10.1182/bloodadvances.2019000792
58. Çelik H, Lindblad KE, Popescu B, Gui G, Goswami M, Valdez J, et al. Highly multiplexed proteomic assessment of human bone marrow in acute myeloid leukemia. Blood Adv (2020) 4:367–79. doi: 10.1182/bloodadvances.2019001124
59. Medyouf H. The microenvironment in human myeloid malignancies: emerging concepts and therapeutic implications. Blood (2017) 129:1617–26. doi: 10.1182/blood-2016-11-696070
60. SanMiguel JM, Loberg M, Heuer S, Stearns T, Young K, Trowbridge J. Cell-Extrinsic Stressors from the Aging Bone Marrow (BM) Microenvironment Promote Dnmt3a-Mutant Clonal Hematopoiesis. Blood (2019) 134:5–5. doi: 10.1182/blood-2019-124511
61. Moschoi R, Imbert V, Nebout M, Chiche J, Mary D, Prebet T, et al. Protective mitochondrial transfer from bone marrow stromal cells to acute myeloid leukemic cells during chemotherapy. Blood (2016) 128:253–64. doi: 10.1182/blood-2015-07-655860
62. Ye H, Adane B, Khan N, Sullivan T, Minhajuddin M, Gasparetto M, et al. Leukemic Stem Cells Evade Chemotherapy by Metabolic Adaptation to an Adipose Tissue Niche. Cell Stem Cell (2016) 19:23–37. doi: 10.1016/j.stem.2016.06.001
63. Perea G, Domingo A, Villamor N, Palacios C, Juncà J, Torres P, et al. Adverse prognostic impact of CD36 and CD2 expression in adult de novo acute myeloid leukemia patients. Leuk Res (2005) 29:1109–16. doi: 10.1016/j.leukres.2005.02.015
64. Lamble AJ, Lind EF. Targeting the Immune Microenvironment in Acute Myeloid Leukemia: A Focus on T Cell Immunity. Front Oncol (2018) 8:213. doi: 10.3389/fonc.2018.00213
65. Mendez LM, Posey RR, Pandolfi PP. The Interplay Between the Genetic and Immune Landscapes of AML: Mechanisms and Implications for Risk Stratification and Therapy. Front Oncol (2019) 9:1162. doi: 10.3389/fonc.2019.01162
66. Christopher MJ, Petti AA, Rettig MP, Miller CA, Chendamarai E, Duncavage EJ, et al. Immune Escape of Relapsed AML Cells after Allogeneic Transplantation. N Engl J Med (2018) 379:2330–41. doi: 10.1056/NEJMoa1808777
67. McGranahan N, Furness AJS, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science (2016) 351:1463–9. doi: 10.1126/science.aaf1490
68. Barrett AJ. Acute myeloid leukaemia and the immune system: implications for immunotherapy. Br J Haematol (2020) 188:147–58. doi: 10.1111/bjh.16310
69. Dolen Y, Esendagli G. Myeloid leukemia cells with a B7-2+ subpopulation provoke Th-cell responses and become immuno-suppressive through the modulation of B7 ligands. Eur J Immunol (2013) 43:747–57. doi: 10.1002/eji.201242814
70. Krönig H, Kremmler L, Haller B, Englert C, Peschel C, Andreesen R, et al. Interferon-induced programmed death-ligand 1 (PD-L1/B7-H1) expression increases on human acute myeloid leukemia blast cells during treatment. Eur J Haematol (2014) 92:195–203. doi: 10.1111/ejh.12228
71. de Jong AJ, Kloppenburg M, Toes REM, Ioan-Facsinay A. Fatty Acids, Lipid Mediators, and T-Cell Function. Front Immunol (2014) 5:483. doi: 10.3389/fimmu.2014.00483
72. Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci (2017) 114:1117–22. doi: 10.1073/pnas.1612920114
73. Le Dieu R, Taussig DC, Ramsay AG, Mitter R, Miraki-Moud F, Fatah R, et al. Peripheral blood T cells in acute myeloid leukemia (AML) patients at diagnosis have abnormal phenotype and genotype and form defective immune synapses with AML blasts. Blood (2009) 114:3909–16. doi: 10.1182/blood-2009-02-206946
74. Szczepanski MJ, Szajnik M, Czystowska M, Mandapathil M, Strauss L, Welsh A, et al. Increased Frequency and Suppression by Regulatory T Cells in Patients with Acute Myelogenous Leukemia. Clin Cancer Res (2009) 15:3325–32. doi: 10.1158/1078-0432.CCR-08-3010
75. Lau CM, Nish SA, Yogev N, Waisman A, Reiner SL, Reizis B. Leukemia-associated activating mutation of Flt3 expands dendritic cells and alters T cell responses. J Exp Med (2016) 213:415–31. doi: 10.1084/jem.20150642
76. Davies JOJ, Stringaris K, Barrett AJ, Rezvani K. Opportunities and limitations of natural killer cells as adoptive therapy for malignant disease. Cytotherapy (2014) 16:1453–66. doi: 10.1016/j.jcyt.2014.03.009
77. Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee H-G, et al. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood (2003) 102:1389–96. doi: 10.1182/blood-2003-01-0019
78. Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood (2016) 127:53–61. doi: 10.1182/blood-2015-08-604520
79. Fucikova J, Truxova I, Hensler M, Becht E, Kasikova L, Moserova I, et al. Calreticulin exposure by malignant blasts correlates with robust anticancer immunity and improved clinical outcome in AML patients. Blood (2016) 128:3113–24. doi: 10.1182/blood-2016-08-731737
80. Burnett AK, Russell NH, Hills RK, Hunter AE, Kjeldsen L, Yin J, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol (2013) 31:3360–8. doi: 10.1200/JCO.2012.47.4874
81. Löwenberg B, Pabst T, Maertens J, van Norden Y, Biemond BJ, Schouten HC, et al. Therapeutic value of clofarabine in younger and middle-aged (18-65 years) adults with newly diagnosed AML. Blood (2017) 129:1636–45. doi: 10.1182/blood-2016-10-740613
82. Estey EH. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am J Hematol (2018) 93:1267–91. doi: 10.1002/ajh.25214
83. Itzykson R, Kosmider O, Cluzeau T, Mansat-De Mas V, Dreyfus F, Beyne-Rauzy O, et al. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia (2011) 25:1147–52. doi: 10.1038/leu.2011.71
84. Welch JS, Petti AA, Miller CA, Fronick CC, O’Laughlin M, Fulton RS, et al. TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. N Engl J Med (2016) 375:2023–36. doi: 10.1056/NEJMoa1605949
85. Schaefer M, Hagemann S, Hanna K, Lyko F. Azacytidine Inhibits RNA Methylation at DNMT2 Target Sites in Human Cancer Cell Lines. Cancer Res (2009) 69:8127–32. doi: 10.1158/0008-5472.CAN-09-0458
86. Luo N, Nixon MJ, Gonzalez-Ericsson PI, Sanchez V, Opalenik SR, Li H, et al. DNA methyltransferase inhibition upregulates MHC-I to potentiate cytotoxic T lymphocyte responses in breast cancer. Nat Commun (2018) 9:1–11. doi: 10.1038/s41467-017-02630-w
87. Stein EM, Tallman MS. Emerging therapeutic drugs for AML. Blood (2016) 127:71–8. doi: 10.1182/blood-2015-07-604538
88. Kantarjian HM, Roboz GJ, Kropf PL, Yee KWL, O’Connell CL, Tibes R, et al. Guadecitabine (SGI-110) in treatment-naive patients with acute myeloid leukaemia: phase 2 results from a multicentre, randomised, phase 1/2 trial. Lancet Oncol (2017) 18:1317–26. doi: 10.1016/S1470-2045(17)30576-4
89. Sanz MA, Fenaux P, Tallman MS, Estey EH, Löwenberg B, Naoe T, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of the European LeukemiaNet. Blood (2019) 133:1630–43. doi: 10.1182/blood-2019-01-894980
90. Alex AA, Ganesan S, Palani HK, Balasundaram N, David S, Lakshmi KM, et al. Arsenic Trioxide Enhances the NK Cell Cytotoxicity Against Acute Promyelocytic Leukemia While Simultaneously Inhibiting Its Bio-Genesis. Front Immunol (2018) 9:1357. doi: 10.3389/fimmu.2018.01357
91. Boutzen H, Saland E, Larrue C, de Toni F, Gales L, Castelli FA, et al. Isocitrate dehydrogenase 1 mutations prime the all-trans retinoic acid myeloid differentiation pathway in acute myeloid leukemia. J Exp Med (2016) 213:483–97. doi: 10.1084/jem.20150736
92. Terao T, Minami Y. Targeting Hedgehog (Hh) Pathway for the Acute Myeloid Leukemia Treatment. Cells (2019) 8(4):312. doi: 10.3390/cells8040312
93. Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia (2019) 33:379–89. doi: 10.1038/s41375-018-0312-9
94. Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia in First Complete Remission: Systematic Review and Meta-analysis of Prospective Clinical Trials. JAMA (2009) 301:2349–61. doi: 10.1001/jama.2009.813
95. Salvatore D, Labopin M, Ruggeri A, Battipaglia G, Ghavamzadeh A, Ciceri F, et al. Outcomes of hematopoietic stem cell transplantation from unmanipulated haploidentical versus matched sibling donor in patients with acute myeloid leukemia in first complete remission with intermediate or high-risk cytogenetics: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica (2018) 103:1317–28. doi: 10.3324/haematol.2018.189258
96. Lorentino F, Labopin M, Bernardi M, Ciceri F, Socié G, Cornelissen JJ, et al. Comparable outcomes of haploidentical, 10/10 and 9/10 unrelated donor transplantation in adverse karyotype AML in first complete remission. Am J Hematol (2018) 93:1236–44. doi: 10.1002/ajh.25231
97. Yano S, Yokoyama H, Yanada M, Mori J, Aoki J, Ohashi K, et al. Role of alternative donor allogeneic hematopoietic stem cell transplantation in patients with intermediate- or poor-risk acute myeloid leukemia in first complete remission. Bone Marrow Transplant (2019) 54:2004–12. doi: 10.1038/s41409-019-0571-8
98. Gagelmann N, Bacigalupo A, Rambaldi A, Hoelzer D, Halter J, Sanz J, et al. Haploidentical Stem Cell Transplantation With Posttransplant Cyclophosphamide Therapy vs Other Donor Transplantations in Adults With Hematologic Cancers: A Systematic Review and Meta-analysis. JAMA Oncol (2019) 5(12):1739–48. doi: 10.1001/jamaoncol.2019.3541
99. Perales M-A, Tomlinson B, Zhang M-J, St Martin A, Beitinjaneh A, Gibson J, et al. Alternative donor transplantation for acute myeloid leukemia in patients aged ≥50 years: young HLA-matched unrelated or haploidentical donor? Haematologica (2020) 105:407–13. doi: 10.3324/haematol.2018.215202
100. Duléry R, Bastos J, Paviglianiti A, Malard F, Brissot E, Battipaglia G, et al. Thiotepa, Busulfan, and Fludarabine Conditioning Regimen in T Cell-Replete HLA-Haploidentical Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2019) 25:1407–15. doi: 10.1016/j.bbmt.2019.02.025
101. Roy DC, Walker I, Maertens J, Lewalle P, Olavarria E, Selleslag D, et al. ATIR101 administered after T-cell-depleted haploidentical HSCT reduces NRM and improves overall survival in acute leukemia. Leukemia (2020) 34:1907–23. doi: 10.1038/s41375-020-0733-0
102. Ustun C, Kim S, Chen M, Beitinjaneh AM, Brown VI, Dahi PB, et al. Increased overall and bacterial infections following myeloablative allogeneic HCT for patients with AML in CR1. Blood Adv (2019) 3:2525–36. doi: 10.1182/bloodadvances.2019000226
103. Gupta V, Lazarus HM, Keating A. Myeloablative conditioning regimens for AML allografts: 30 years later. Bone Marrow Transplant (2003) 32:969–78. doi: 10.1038/sj.bmt.1704285
104. Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood (2014) 124:344–53. doi: 10.1182/blood-2014-02-514778
105. Sengsayadeth S, Savani BN, Blaise D, Malard F, Nagler A, Mohty M. Reduced intensity conditioning allogeneic hematopoietic cell transplantation for adult acute myeloid leukemia in complete remission - a review from the Acute Leukemia Working Party of the EBMT. Haematologica (2015) 100:859–69. doi: 10.3324/haematol.2015.123331
106. Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J Clin Oncol (2017) 35:1154–61. doi: 10.1200/JCO.2016.70.7091
107. Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transplant (2020) 55:1114–25. doi: 10.1038/s41409-020-0803-y
108. Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol (2020) 7:e157–67. doi: 10.1016/S2352-3026(19)30256-X
109. Konuma T, Kondo T, Mizuno S, Doki N, Aoki J, Fukuda T, et al. Conditioning Intensity for Allogeneic Hematopoietic Cell Transplantation in Acute Myeloid Leukemia Patients with Poor-Prognosis Cytogenetics in First Complete Remission. Biol Blood Marrow Transplant (2020) 26:463–71. doi: 10.1016/j.bbmt.2019.09.025
110. Ruutu T, Gratwohl A, de Witte T, Afanasyev B, Apperley J, Bacigalupo A, et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transplant (2014) 49:168–73. doi: 10.1038/bmt.2013.107
111. Schmid C, Labopin M, Schaap N, Veelken H, Schleuning M, Stadler M, et al. Prophylactic donor lymphocyte infusion after allogeneic stem cell transplantation in acute leukaemia - a matched pair analysis by the Acute Leukaemia Working Party of EBMT. Br J Haematol (2019) 184:782–7. doi: 10.1111/bjh.15691
112. Kharfan-Dabaja MA, Labopin M, Polge E, Nishihori T, Bazarbachi A, Finke J, et al. Association of Second Allogeneic Hematopoietic Cell Transplant vs Donor Lymphocyte Infusion With Overall Survival in Patients With Acute Myeloid Leukemia Relapse. JAMA Oncol (2018) 4:1245–53. doi: 10.1001/jamaoncol.2018.2091
113. Gilleece MH, Labopin M, Savani BN, Yakoub-Agha I, Socié G, Gedde-Dahl T, et al. Allogeneic haemopoietic transplantation for acute myeloid leukaemia in second complete remission: a registry report by the Acute Leukaemia Working Party of the EBMT. Leukemia (2020) 34:87–99. doi: 10.1038/s41375-019-0527-4
114. Oran B, de Lima M, Garcia-Manero G, Thall PF, Lin R, Alousi AM, et al. Maintenance with 5-Azacytidine for Acute Myeloid Leukemia and Myelodysplastic Syndrome Patients. Blood (2018) 132:971–1. doi: 10.1182/blood-2018-99-111582
115. Toffalori C, Zito L, Gambacorta V, Riba M, Oliveira G, Bucci G, et al. Immune signature drives leukemia escape and relapse after hematopoietic cell transplantation. Nat Med (2019) 25:603–11. doi: 10.1038/s41591-019-0400-z
116. Wei AH, Tiong IS. Midostaurin, enasidenib, CPX-351, gemtuzumab ozogamicin, and venetoclax bring new hope to AML. Blood (2017) 130:2469–74. doi: 10.1182/blood-2017-08-784066
117. Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J Clin Oncol (2018) 36(26):2684–92. doi: 10.1200/JCO.2017.77.6112
118. Maakaron JE, Mims AS. Daunorubicin-cytarabine liposome (CPX-351) in the management of newly diagnosed secondary AML: A new twist on an old cocktail. Best Pract Res Clin Haematol (2019) 32:127–33. doi: 10.1016/j.beha.2019.05.005
119. Appelbaum FR, Bernstein ID. Gemtuzumab ozogamicin for acute myeloid leukemia. Blood (2017) 130:2373–6. doi: 10.1182/blood-2017-09-797712
120. Walter RB, Appelbaum FR, Estey EH, Bernstein ID. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood (2012) 119:6198–208. doi: 10.1182/blood-2011-11-325050
121. Larson RA, Sievers EL, Stadtmauer EA, Löwenberg B, Estey EH, Dombret H, et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer (2005) 104:1442–52. doi: 10.1002/cncr.21326
122. Castaigne S, Pautas C, Terré C, Raffoux E, Bordessoule D, Bastie J-N, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet (2012) 379:1508–16. doi: 10.1016/S0140-6736(12)60485-1
123. Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol (2014) 15:986–96. doi: 10.1016/S1470-2045(14)70281-5
124. Schlenk RF, Paschka P, Krzykalla J, Weber D, Kapp-Schwoerer S, Gaidzik VI, et al. Gemtuzumab Ozogamicin in NPM1-Mutated Acute Myeloid Leukemia: Early Results From the Prospective Randomized AMLSG 09-09 Phase III Study. J Clin Oncol (2020) 38:623–32. doi: 10.1200/JCO.19.01406
125. Laszlo GS, Harrington KH, Gudgeon CJ, Beddoe ME, Fitzgibbon MP, Ries RE, et al. Expression and functional characterization of CD33 transcript variants in human acute myeloid leukemia. Oncotarget (2016) 7:43281–94. doi: 10.18632/oncotarget.9674
126. Ho VT, St Martin A, Pérez WS, Steinert P, Zhang M-J, Chirnomas D, et al. Prior Gemtuzumab Ozogamicin Exposure in Adults with Acute Myeloid Leukemia Does Not Increase Hepatic Veno-Occlusive Disease Risk after Allogeneic Hematopoietic Cell Transplantation: A Center for International Blood and Marrow Transplant Research Analysis. Biol Blood Marrow Transplant (2019) 26(5):884–92. doi: 10.1016/j.bbmt.2019.12.763
127. Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med (2016) 375:740–53. doi: 10.1056/NEJMoa1509277
128. Godwin CD, McDonald GB, Walter RB. Sinusoidal obstruction syndrome following CD33-targeted therapy in acute myeloid leukemia. Blood (2017) 129:2330–2. doi: 10.1182/blood-2017-01-762419
129. Kenderian SS, Ruella M, Shestova O, Klichinsky M, Aikawa V, Morrissette JJD, et al. CD33-specific chimeric antigen receptor T cells exhibit potent preclinical activity against human acute myeloid leukemia. Leukemia (2015) 29:1637–47. doi: 10.1038/leu.2015.52
130. Hofmann S, Schubert M-L, Wang L, He B, Neuber B, Dreger P, et al. Chimeric Antigen Receptor (CAR) T Cell Therapy in Acute Myeloid Leukemia (AML). J Clin Med (2019) 8(2):200. doi: 10.3390/jcm8020200
131. Kats LM, Reschke M, Taulli R, Pozdnyakova O, Burgess K, Bhargava P, et al. Proto-Oncogenic Role of Mutant IDH2 in Leukemia Initiation and Maintenance. Cell Stem Cell (2014) 14:329–41. doi: 10.1016/j.stem.2013.12.016
132. Bunse L, Pusch S, Bunse T, Sahm F, Sanghvi K, Friedrich M, et al. Suppression of antitumor T cell immunity by the oncometabolite (R )-2-hydroxyglutarate. Nat Med (2018) 24:1192–203. doi: 10.1038/s41591-018-0095-6
133. Stein EM, DiNardo CD, Fathi AT, Pollyea DA, Stone RM, Altman JK, et al. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood (2019) 133:676–87. doi: 10.1182/blood-2018-08-869008
134. Chaturvedi A, Araujo Cruz MM, Jyotsana N, Sharma A, Goparaju R, Schwarzer A, et al. Enantiomer-specific and paracrine leukemogenicity of mutant IDH metabolite 2-hydroxyglutarate. Leukemia (2016) 30:1708–15. doi: 10.1038/leu.2016.71
135. Roboz GJ, DiNardo CD, Stein EM, de Botton S, Mims AS, Prince GT, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood (2020) 135:463–71. doi: 10.1182/blood.2019002140
136. DiNardo CD, Stein AS, Stein EM, Fathi AT, Frankfurt O, Schuh AC, et al. Mutant IDH1 inhibitor ivosidenib (IVO; AG-120) in combination with azacitidine (AZA) for newly diagnosed acute myeloid leukemia (ND AML). J Clin Oncol (2019) 37:7011–1. doi: 10.1200/JCO.2019.37.15_suppl.7011
137. DiNardo C, Takahashi K, Kadia T, Loghavi S, Naqvi K, Bose P, et al. A phase 1b/2 clinical study of targeted IDH1 inhition with ivosidenib, in combination with the BCL-2 inhibitor venetoclax, for paitents with IDH1-mutated (mIDH1) myeloid maligancies. HemaSphere (2019) 3:97. doi: 10.1097/01.HS9.0000559376.17429.23
138. Antar AI, Otrock ZK, Jabbour E, Mohty M, Bazarbachi A. FLT3 inhibitors in acute myeloid leukemia: ten frequently asked questions. Leukemia (2020) 34:682–96. doi: 10.1038/s41375-019-0694-3
139. Fischer T, Stone RM, Deangelo DJ, Galinsky I, Estey E, Lanza C, et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol (2010) 28:4339–45. doi: 10.1200/JCO.2010.28.9678
140. Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med (2017) 377:454–64. doi: 10.1056/NEJMoa1614359
141. Ohanian M, Garcia-Manero G, Levis M, Jabbour E, Daver N, Borthakur G, et al. Sorafenib Combined with 5-azacytidine in Older Patients with Untreated FLT3-ITD Mutated Acute Myeloid Leukemia. Am J Hematol (2018) 93:1136–41. doi: 10.1002/ajh.25198
142. Ravandi F, Alattar ML, Grunwald MR, Rudek MA, Rajkhowa T, Richie MA, et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood (2013) 121:4655–62. doi: 10.1182/blood-2013-01-480228
143. Röllig C, Serve H, Hüttmann A, Noppeney R, Müller-Tidow C, Krug U, et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol (2015) 16:1691–9. doi: 10.1016/S1470-2045(15)00362-9
144. Burchert A, Bug G, Finke J, Stelljes M, Rollig C, Wäsch R, et al. Sorafenib As Maintenance Therapy Post Allogeneic Stem Cell Transplantation for FLT3-ITD Positive AML: Results from the Randomized, Double-Blind, Placebo-Controlled Multicentre Sormain Trial. Blood (2018) 132:661–1. doi: 10.1182/blood-2018-99-112614
145. Perl AE. Availability of FLT3 inhibitors: how do we use them? Blood (2019) 134:741–5. doi: 10.1182/blood.2019876821
146. Bazarbachi A, Bug G, Baron F, Brissot E, Ciceri F, Abou Dalle I, et al. Clinical practice recommendation on hematopoietic stem cell transplantation for acute myeloid leukemia patients with FLT3 internal tandem duplication: a position statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica (2020) 105(6):1507–16. doi: 10.3324/haematol.2019.243410
147. Gutierrez L, Jang M, Zhang T, Akhtari M, Alachkar H. Midostaurin reduces Regulatory T cells markers in Acute Myeloid Leukemia. Sci Rep (2018) 8:1–8. doi: 10.1038/s41598-018-35978-0
148. Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3 -Mutated AML. N Engl J Med (2019) 381:1728–40. doi: 10.1056/NEJMoa1902688
149. Cortes JE, Khaled S, Martinelli G, Perl AE, Ganguly S, Russell N, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol (2019) 20:984–97. doi: 10.1016/S1470-2045(19)30150-0
150. Wang ES, Stone RM, Tallman MS, Walter RB, Eckardt JR, Collins R. Crenolanib, a Type I FLT3 TKI, Can be Safely Combined with Cytarabine and Anthracycline Induction Chemotherapy and Results in High Response Rates in Patients with Newly Diagnosed FLT3 Mutant Acute Myeloid Leukemia (AML). Blood (2016) 128:1071–1. doi: 10.1182/blood.V128.22.1071.1071
151. DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood (2019) 133:7–17. doi: 10.1182/blood-2018-08-868752
152. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med (2020) 383:617–29. doi: 10.1056/NEJMoa2012971
153. Wei AH, Strickland SA, Hou J-Z, Fiedler W, Lin TL, Walter RB, et al. Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study. J Clin Oncol (2019) 37:1277–84. doi: 10.1200/JCO.18.01600
154. Wei AH, Montesinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood (2020) 135:2137–45. doi: 10.1182/blood.2020004856
155. McBride A, Houtmann S, Wilde L, Vigil C, Eischen CM, Kasner M, et al. The Role of Inhibition of Apoptosis in Acute Leukemias and Myelodysplastic Syndrome. Front Oncol (2019) 9:192. doi: 10.3389/fonc.2019.00192
156. Savona MR, Wei AH. Incorporating Precision BH3 Warheads Into the Offensive Against Acute Myeloid Leukemia. J Clin Oncol (2019) 37:1785–9. doi: 10.1200/JCO.19.00400
157. Guerra VA, DiNardo C, Konopleva M. Venetoclax-based therapies for acute myeloid leukemia. Best Pract Res Clin Haematol (2019) 32:145–53. doi: 10.1016/j.beha.2019.05.008
158. Narayan R, Olsson N, Wagar LE, Medeiros BC, Meyer E, Czerwinski D, et al. Acute myeloid leukemia immunopeptidome reveals HLA presentation of mutated nucleophosmin. PloS One (2019) 14(7):e0219547. doi: 10.1371/journal.pone.0219547
159. Van Der Lee DI, Bijen HM, Hagedoorn RS, Honders WM, Kester MGD, van der Steen DM, et al. Mutated NPM1 As Target for Immunotherapy of Acute Myeloid Leukemia. Blood (2017) 130:168–8. doi: 10.1182/blood.V130.Suppl_1.168.168
160. Brunetti L, Gundry MC, Sorcini D, Guzman AG, Huang Y-H, Ramabadran R, et al. Mutant NPM1 Maintains the Leukemic State through HOX Expression. Cancer Cell (2018) 34:499–512.e9. doi: 10.1016/j.ccell.2018.08.005
161. Tiong IS, Dillon R, Ivey A, Teh T-C, Nguyen P, Cummings N, et al. Venetoclax induces rapid elimination of NPM1 mutant measurable residual disease in combination with low-intensity chemotherapy in acute myeloid leukaemia. Br J Haematol (2020). doi: 10.1111/bjh.16722
162. Fiskus W, Wang Y, Sreekumar A, Buckley KM, Shi H, Jillella A, et al. Combined epigenetic therapy with the histone methyltransferase EZH2 inhibitor 3-deazaneplanocin A and the histone deacetylase inhibitor panobinostat against human AML cells. Blood (2009) 114:2733–43. doi: 10.1182/blood-2009-03-213496
163. Schenk T, Chen WC, Göllner S, Howell L, Jin L, Hebestreit K, et al. Inhibition of the LSD1 (KDM1A) demethylase reactivates the all-trans-retinoic acid differentiation pathway in acute myeloid leukemia. Nat Med (2012) 18:605–11. doi: 10.1038/nm.2661
164. Daigle SR, Olhava EJ, Therkelsen CA, Majer CR, Sneeringer CJ, Song J, et al. Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer Cell (2011) 20:53–65. doi: 10.1016/j.ccr.2011.06.009
165. Daigle SR, Olhava EJ, Therkelsen CA, Basavapathruni A, Jin L, Boriack-Sjodin PA, et al. Potent inhibition of DOT1L as treatment of MLL-fusion leukemia. Blood (2013) 122:1017–25. doi: 10.1182/blood-2013-04-497644
166. McCalmont H, Li KL, Jones L, Toubia J, Bray SC, Casolari DA, et al. Efficacy of combined CDK9/BET inhibition in preclinical models of MLL-rearranged acute leukemia. Blood Adv (2020) 4:296–300. doi: 10.1182/bloodadvances.2019000586
167. Berthon C, Raffoux E, Thomas X, Vey N, Gomez-Roca C, Yee K, et al. Bromodomain inhibitor OTX015 in patients with acute leukaemia: a dose-escalation, phase 1 study. Lancet Haematol (2016) 3:e186–195. doi: 10.1016/S2352-3026(15)00247-1
168. Craddock CF, Houlton AE, Quek LS, Ferguson P, Gbandi E, Roberts C, et al. Outcome of Azacitidine Therapy in Acute Myeloid Leukemia Is not Improved by Concurrent Vorinostat Therapy but Is Predicted by a Diagnostic Molecular Signature. Clin Cancer Res (2017) 23:6430–40. doi: 10.1158/1078-0432.CCR-17-1423
169. Garcia-Manero G, Othus M, Pagel JM, Radich JP, Fang M, Rizzieri DA, et al. SWOG S1203: A Randomized Phase III Study of Standard Cytarabine Plus Daunorubicin (7+3) Therapy Versus Idarubicin with High Dose Cytarabine (IA) with or without Vorinostat (IA+V) in Younger Patients with Previously Untreated Acute Myeloid Leukemia (AML). Blood (2016) 128:901–1. doi: 10.1182/blood.V128.22.901.901
170. Garcia-Manero G, Sekeres MA, Egyed M, Breccia M, Graux C, Cavenagh JD, et al. A phase 1b/2b multicenter study of oral panobinostat plus azacitidine in adults with MDS, CMML or AML with ≦̸30% blasts. Leukemia (2017) 31:2799–806. doi: 10.1038/leu.2017.159
171. Pan D, Rampal R, Mascarenhas J. Clinical developments in epigenetic-directed therapies in acute myeloid leukemia. Blood Adv (2020) 4:970–82. doi: 10.1182/bloodadvances.2019001245
172. Schlenk RF, Jaramillo S, Müller-Tidow C. Improving consolidation therapy in acute myeloid leukemia - a tough nut to crack. Haematologica (2018) 103:1579–81. doi: 10.3324/haematol.2018.200485
173. Puissant A, Fenouille N, Alexe G, Pikman Y, Bassil CF, Mehta S, et al. SYK is a critical regulator of FLT3 in acute myeloid leukemia. Cancer Cell (2014) 25:226–42. doi: 10.1016/j.ccr.2014.01.022
174. Hosseini MM, Kurtz SE, Abdelhamed S, Mahmood S, Davare MA, Kaempf A, et al. Inhibition of interleukin-1 receptor-associated kinase-1 is a therapeutic strategy for acute myeloid leukemia subtypes. Leukemia (2018) 32:2374–87. doi: 10.1038/s41375-018-0112-2
175. Melgar K, Walker MM, Jones LM, Bolanos LC, Hueneman K, Wunderlich M, et al. Overcoming adaptive therapy resistance in AML by targeting immune response pathways. Sci Transl Med (2019) 11(508):eaaw8828. doi: 10.1126/scitranslmed.aaw8828
176. Jeon JY, Zhao Q, Buelow DR, Phelps M, Walker AR, Mims AS, et al. Preclinical activity and a pilot phase I study of pacritinib, an oral JAK2/FLT3 inhibitor, and chemotherapy in FLT3-ITD-positive AML. Invest New Drugs (2020) 38:340–9. doi: 10.1007/s10637-019-00786-4
177. Morsink LM, Walter RB. Novel monoclonal antibody-based therapies for acute myeloid leukemia. Best Pract Res Clin Haematol (2019) 32:116–26. doi: 10.1016/j.beha.2019.05.002
178. Goldberg AD, Tallman MS, Solh MM, Ungar D, Rizzieri DA, Walter RB, et al. Results from an Ongoing Phase 1 Study Indicate ACDT-301 (Camidanlumab Tesirine) Is Well-Tolerated in Patients with Relapsed or Refractory CD25-Positive Acute Leukemia. Blood (2017) 130:2662–2. doi: 10.1182/blood.V130.Suppl_1.2662.2662
179. Vey N, Delaunay J, Martinelli G, Fiedler W, Raffoux E, Prebet T, et al. Phase I clinical study of RG7356, an anti-CD44 humanized antibody, in patients with acute myeloid leukemia. Oncotarget (2016) 7:32532–42. doi: 10.18632/oncotarget.8687
180. Rudra-Ganguly N, Lowe C, Virata C, Leavitt M, Jin L, Mendelsohn B, et al. AGS62P1, a Novel Anti-FLT3 Antibody Drug Conjugate, Employing Site Specific Conjugation, Demonstrates Preclinical Anti-Tumor Efficacy in AML Tumor and Patient Derived Xenografts. Blood (2015) 126:3806–6. doi: 10.1182/blood.V126.23.3806.3806
181. Ochsenbein AF, Riether C, Bacher U, Müller R, Höpner S, Banz Y, et al. Argx-110 Targeting CD70, in Combination with Azacitidine, Shows Favorable Safety Profile and Promising Anti-Leukemia Activity in Newly Diagnosed AML Patients in an Ongoing Phase 1/2 Clinical Trial. Blood (2018) 132:2680–0. doi: 10.1182/blood-2018-99-118302
182. Naik J, Themeli M, de Jong-Korlaar R, Ruiter RWJ, Poddighe PJ, Yuan H, et al. CD38 as a therapeutic target for adult acute myeloid leukemia and T-cell acute lymphoblastic leukemia. Haematologica (2019) 104:e100–3. doi: 10.3324/haematol.2018.192757
183. Cook RJ, Moyo T, Liesveld JL, Rizzieri DA, Stein EM, De Botton S, et al. Early Results from a Biomarker-Directed Phase 2 Trial of Sy-1425 in Combination with Azacitidine or Daratumumab in Non-APL Acute Myeloid Leukemia (AML) and Myelodysplastic Syndrome (MDS). Blood (2018) 132:2735–5. doi: 10.1182/blood-2018-99-111285
184. Buteyn NJ, Fatehchand K, Santhanam R, Fang H, Dettorre GM, Gautam S, et al. Anti-leukemic effects of all-trans retinoic acid in combination with Daratumumab in acute myeloid leukemia. Int Immunol (2018) 30:375–83. doi: 10.1093/intimm/dxy040
185. Kubasch AS, Schulze F, Giagounidis A, Götze KS, Krönke J, Sockel K, et al. Single agent talacotuzumab demonstrates limited efficacy but considerable toxicity in elderly high-risk MDS or AML patients failing hypomethylating agents. Leukemia (2020) 34:1182–6. doi: 10.1038/s41375-019-0645-z
186. Daver NG, Erba HP, Papadantonakis N, DeAngelo DJ, Wang ES, Konopleva MY, et al. First-in-Human Study Evaluating the Safety and Preliminary Antileukemia Activity of IMGN632, a Novel CD123-Targeting Antibody-Drug Conjugate, in Patients with Relapsed/Refractory Acute Myeloid Leukemia and Other CD123-Positive Hematologic Malignancies. Blood (2018) 132:27–7. doi: 10.1182/blood-2018-99-112955
187. Adams S, Zhang Q, McCarthy R, Flaherty LJ, Watkins K, Sloss CM, et al. The combinatio of IMGN632, a CD123-targeting ADC, with venetoclax enhances anti-leukemic activity in vitro and prolongs survival in vivo in pre-clinical models of human AML. HemaSphere (2019) 3:53. doi: 10.1097/01.HS9.0000559020.72361.fa
188. Mani R, Goswami S, Gopalakrishnan B, Ramaswamy R, Wasmuth R, Tran M, et al. The interleukin-3 receptor CD123 targeted SL-401 mediates potent cytotoxic activity against CD34+CD123+ cells from acute myeloid leukemia/myelodysplastic syndrome patients and healthy donors. Haematologica (2018) 103:1288–97. doi: 10.3324/haematol.2018.188193
189. Testa U, Pelosi E, Castelli G. CD123 as a Therapeutic Target in the Treatment of Hematological Malignancies. Cancers (2019) 11:1358. doi: 10.3390/cancers11091358
190. Ravandi F, Bashey A, Foran JM, Stock W, Mawad R, Blum W, et al. Complete Responses in Relapsed/Refractory Acute Myeloid Leukemia (AML) Patients on a Weekly Dosing Schedule of XmAb14045, a CD123 x CD3 T Cell-Engaging Bispecific Antibody: Initial Results of a Phase 1 Study. Blood (2018) 132:763–3. doi: 10.1182/blood-2018-99-119786
191. Braciak TA, Roskopf CC, Wildenhain S, Fenn NC, Schiller CB, Schubert IA, et al. Dual-targeting triplebody 33-16-123 (SPM-2) mediates effective redirected lysis of primary blasts from patients with a broad range of AML subtypes in combination with natural killer cells. OncoImmunology (2018) 7:e1472195. doi: 10.1080/2162402X.2018.1472195
192. Herrmann M, Krupka C, Deiser K, Brauchle B, Marcinek A, Ogrinc Wagner A, et al. Bifunctional PD-1 × αCD3 × αCD33 fusion protein reverses adaptive immune escape in acute myeloid leukemia. Blood (2018) 132:2484–94. doi: 10.1182/blood-2018-05-849802
193. Ågerstam H, Karlsson C, Hansen N, Sandén C, Askmyr M, von Palffy S, et al. Antibodies targeting human IL1RAP (IL1R3) show therapeutic effects in xenograft models of acute myeloid leukemia. Proc Natl Acad Sci USA (2015) 112:10786–91. doi: 10.1073/pnas.1422749112
194. Højen JF, Kristensen MLV, McKee AS, Wade MT, Azam T, Lunding LP, et al. IL-1R3 blockade broadly attenuates the functions of six members of the IL-1 family, revealing their contribution to models of disease. Nat Immunol (2019) 20:1138–49. doi: 10.1038/s41590-019-0467-1
195. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med (2017) 377:2531–44. doi: 10.1056/NEJMoa1707447
196. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med (2019) 380:45–56. doi: 10.1056/NEJMoa1804980
197. Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med (2018) 378:449–59. doi: 10.1056/NEJMoa1709919
198. Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol (2020) 17:147–67. doi: 10.1038/s41571-019-0297-y
199. Przespolewski AC, Griffiths EA. BITES and CARS and checkpoints, oh my! Updates regarding immunotherapy for myeloid malignancies from the 2018 annual ASH meeting. Blood Rev (2020) 43:100654. doi: 10.1016/j.blre.2020.100654
200. Li S, Tao Z, Xu Y, Liu J, An N, Wang Y, et al. CD33-Specific Chimeric Antigen Receptor T Cells with Different Co-Stimulators Showed Potent Anti-Leukemia Efficacy and Different Phenotype. Hum Gene Ther (2018) 29:626–39. doi: 10.1089/hum.2017.241
201. Gill S, Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor–modified T cells. Blood (2014) 123:2343–54. doi: 10.1182/blood-2013-09-529537
202. Gill SI. How close are we to CAR T-cell therapy for AML? Best Pract Res Clin Haematol (2019) 32:101104. doi: 10.1016/j.beha.2019.101104
203. Arcangeli S, Rotiroti MC, Bardelli M, Simonelli L, Magnani CF, Biondi A, et al. Balance of Anti-CD123 Chimeric Antigen Receptor Binding Affinity and Density for the Targeting of Acute Myeloid Leukemia. Mol Ther (2017) 25:1933–45. doi: 10.1016/j.ymthe.2017.04.017
204. Mardiros A, Dos Santos C, McDonald T, Brown CE, Wang X, Budde LE, et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood (2013) 122:3138–48. doi: 10.1182/blood-2012-12-474056
205. Thokala R, Olivares S, Mi T, Maiti S, Deniger D, Huls H, et al. Redirecting Specificity of T cells Using the Sleeping Beauty System to Express Chimeric Antigen Receptors by Mix-and-Matching of VL and VH Domains Targeting CD123+ Tumors. PloS One (2016) 11:e0159477. doi: 10.1371/journal.pone.0159477
206. Kim MY, Yu K-R, Kenderian SS, Ruella M, Chen S, Shin T-H, et al. Genetic Inactivation of CD33 in Hematopoietic Stem Cells to Enable CAR T Cell Immunotherapy for Acute Myeloid Leukemia. Cell (2018) 173:1439–53. doi: 10.1016/j.cell.2018.05.013
207. Tashiro H, Sauer T, Shum T, Parikh K, Mamonkin M, Omer B, et al. Treatment of Acute Myeloid Leukemia with T Cells Expressing Chimeric Antigen Receptors Directed to C-type Lectin-like Molecule 1. Mol Ther (2017) 25:2202–13. doi: 10.1016/j.ymthe.2017.05.024
208. Wang J, Chen S, Xiao W, Li W, Wang L, Yang S, et al. CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. J Hematol OncolJ Hematol Oncol (2018) 11:7. doi: 10.1186/s13045-017-0553-5
209. Jetani H, Garcia-Cadenas I, Nerreter T, Thomas S, Rydzek J, Meijide JB, et al. CAR T-cells targeting FLT3 have potent activity against FLT3-ITD+ AML and act synergistically with the FLT3-inhibitor crenolanib. Leukemia (2018) 32:1168–79. doi: 10.1038/s41375-018-0009-0
210. Myburgh R, Kiefer JD, Russkamp NF, Magnani CF, Nuñez N, Simonis A, et al. Anti-human CD117 CAR T-cells efficiently eliminate healthy and malignant CD117-expressing hematopoietic cells. Leukemia (2020) 34:2688–703. doi: 10.1038/s41375-020-0818-9
211. Ritchie DS, Neeson PJ, Khot A, Peinert S, Tai T, Tainton K, et al. Persistence and efficacy of second generation CAR T cell against the LeY antigen in acute myeloid leukemia. Mol Ther (2013) 21:2122–9. doi: 10.1038/mt.2013.154
212. Baumeister SH, Murad J, Werner L, Daley H, Trebeden-Negre H, Gicobi JK, et al. Phase I Trial of Autologous CAR T Cells Targeting NKG2D Ligands in Patients with AML/MDS and Multiple Myeloma. Cancer Immunol Res (2019) 7:100–12. doi: 10.1158/2326-6066.CIR-18-0307
213. Petrov JC, Wada M, Pinz KG, Yan LE, Chen KH, Shuai X, et al. Compound CAR T-cells as a double-pronged approach for treating acute myeloid leukemia. Leukemia (2018) 32:1317–26. doi: 10.1038/s41375-018-0075-3
214. Warda W, Larosa F, Rocha MND, Trad R, Deconinck E, Fajloun Z, et al. CML Hematopoietic Stem Cells Expressing IL1RAP Can Be Targeted by Chimeric Antigen Receptor–Engineered T Cells. Cancer Res (2019) 79:663–75. doi: 10.1158/0008-5472.CAN-18-1078
215. Trad R, Warda W, Neto Da Rocha M, Alcazer V, Berceanu A, Nicod C, et al. Nouvelle alternative thérapeutique dans le traitement de la leucémie aiguë myéloïde : une immunothérapie cellulaire par lymphocytes à récepteur chimérique à l’antigénique ciblant la protéine accessoire au récepteur de l’interleukine 1. Hématologie (2020) 26:193–4. doi: 10.1684/hma.2020.1538
216. Perna F, Berman SH, Soni RK, Mansilla-Soto J, Eyquem J, Hamieh M, et al. Integrating Proteomics and Transcriptomics for Systematic Combinatorial Chimeric Antigen Receptor Therapy of AML. Cancer Cell (2017) 32:506–19.e5. doi: 10.1016/j.ccell.2017.09.004
217. Mu H, Ma H, Vaidya A, Bonifant CL, Gottschalk S, Velasquez MP, et al. IL15 Expressing CD123-Targeted Engager T-Cell Therapy for Adult Acute Myeloid Leukemia. Blood (2018) 132:2724–4. doi: 10.1182/blood-2018-99-116811
218. He X, Feng Z, Ma J, Ling S, Cao Y, Gurung B, et al. Bispecific and split CAR T cells targeting CD13 and TIM3 eradicate acute myeloid leukemia. Blood (2020) 135:713–23. doi: 10.1182/blood.2019002779
219. Lichtman E, Du H, Savoldo B, Ferrone S, Li G, Su L, et al. Pre-Clinical Evaluation of B7-H3-Specific Chimeric Antigen Receptor T-Cells for the Treatment of Acute Myeloid Leukemia. Blood (2018) 132:701–1. doi: 10.1182/blood-2018-99-113468
220. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol (2020) 20:651–68. doi: 10.1038/s41577-020-0306-5
221. American Association for Cancer Research, Doglin E. Augmenting CAR T Cells with PD-1 Blockade. Cancer Discovery (2019) 9:158–8. doi: 10.1158/2159-8290.CD-NB2018-165
222. Rafiq S, Yeku OO, Jackson HJ, Purdon TJ, van Leeuwen DG, Drakes DJ, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol (2018) 36:847–56. doi: 10.1038/nbt.4195
223. Tasian SK, Kenderian SS, Shen F, Ruella M, Shestova O, Kozlowski M, et al. Optimized depletion of chimeric antigen receptor T cells in murine xenograft models of human acute myeloid leukemia. Blood (2017) 129:2395–407. doi: 10.1182/blood-2016-08-736041
224. Di Stasi A, Tey S-K, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med (2011) 365:1673–83. doi: 10.1056/NEJMoa1106152
225. Garcia Borrega J, Gödel P, Rüger MA, Onur ÖA, Shimabukuro-Vornhagen A, Kochanek M, et al. In the Eye of the Storm: Immune-mediated Toxicities Associated With CAR-T Cell Therapy. HemaSphere (2019) 3:e191. doi: 10.1097/HS9.0000000000000191
226. Epperly R, Gottschalk S, Velasquez MP. A Bump in the Road: How the Hostile AML Microenvironment Affects CAR T Cell Therapy. Front Oncol (2020) 10:262. doi: 10.3389/fonc.2020.00262
227. Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med (2018) 24:739. doi: 10.1038/s41591-018-0036-4
228. Mardiana S, Gill S. CAR T Cells for Acute Myeloid Leukemia: State of the Art and Future Directions. Front Oncol (2020) 10:697. doi: 10.3389/fonc.2020.00697
229. Lee JB, Chen B, Vasic D, Law AD, Zhang L. Cellular immunotherapy for acute myeloid leukemia: How specific should it be? Blood Rev (2019) 35:18–31. doi: 10.1016/j.blre.2019.02.001
230. Laumont CM, Vincent K, Hesnard L, Audemard É, Bonneil É, Laverdure J-P, et al. Noncoding regions are the main source of targetable tumor-specific antigens. Sci Transl Med (2018) 10(470):eaau5516. doi: 10.1126/scitranslmed.aau5516
231. Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med (2005) 352:254–66. doi: 10.1056/NEJMoa041974
232. van der Lee DI, Reijmers RM, Honders MW, Hagedoorn RS, de Jong RCM, Kester MGD, et al. Mutated nucleophosmin 1 as immunotherapy target in acute myeloid leukemia. J Clin Invest (2019) 129:774–85. doi: 10.1172/JCI97482
233. Roerden M, Nelde A, Walz JS. Neoantigens in Hematological Malignancies-Ultimate Targets for Immunotherapy? Front Immunol (2019) 10:3004. doi: 10.3389/fimmu.2019.03004
234. Biernacki MA, Bleakley M. Neoantigens in Hematologic Malignancies. Front Immunol (2020) 11:121. doi: 10.3389/fimmu.2020.00121
235. Tawara I, Kageyama S, Miyahara Y, Fujiwara H, Nishida T, Akatsuka Y, et al. Safety and persistence of WT1-specific T-cell receptor gene-transduced lymphocytes in patients with AML and MDS. Blood (2017) 130:1985–94. doi: 10.1182/blood-2017-06-791202
236. Chapuis AG, Egan DN, Bar M, Schmitt TM, McAfee MS, Paulson KG, et al. T cell receptor gene therapy targeting WT1 prevents acute myeloid leukemia relapse post-transplant. Nat Med (2019) 25:1064–72. doi: 10.1038/s41591-019-0472-9
237. Maslak PG, Dao T, Bernal Y, Chanel SM, Zhang R, Frattini M, et al. Phase 2 trial of a multivalent WT1 peptide vaccine (galinpepimut-S) in acute myeloid leukemia. Blood Adv (2018) 2:224–34. doi: 10.1182/bloodadvances.2017014175
238. Anguille S, Van de Velde AL, Smits EL, Van Tendeloo VF, Juliusson G, Cools N, et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood (2017) 130:1713–21. doi: 10.1182/blood-2017-04-780155
239. Klar R, Schober S, Rami M, Mall S, Merl J, Hauck SM, et al. Therapeutic targeting of naturally presented myeloperoxidase-derived HLA peptide ligands on myeloid leukemia cells by TCR-transgenic T cells. Leukemia (2014) 28:2355–66. doi: 10.1038/leu.2014.131
240. Spranger S, Jeremias I, Wilde S, Leisegang M, Stärck L, Mosetter B, et al. TCR-transgenic lymphocytes specific for HMMR/Rhamm limit tumor outgrowth in vivo. Blood (2012) 119:3440–9. doi: 10.1182/blood-2011-06-357939
241. Martínez A, Olarte I, Mergold MA, Gutiérrez M, Rozen E, Collazo J, et al. mRNA expression of MAGE-A3 gene in leukemia cells. Leuk Res (2007) 31:33–7. doi: 10.1016/j.leukres.2006.05.009
242. Janelle V, Rulleau C, Del Testa S, Carli C, Delisle J-S. T-Cell Immunotherapies Targeting Histocompatibility and Tumor Antigens in Hematological Malignancies. Front Immunol (2020) 11:276. doi: 10.3389/fimmu.2020.00276
243. Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet (2009) 373:1550–61. doi: 10.1016/S0140-6736(09)60237-3
244. Granados DP, Sriranganadane D, Daouda T, Zieger A, Laumont CM, Caron-Lizotte O, et al. Impact of genomic polymorphisms on the repertoire of human MHC class I-associated peptides. Nat Commun (2014) 5:3600. doi: 10.1038/ncomms4600
245. Granados DP, Rodenbrock A, Laverdure J-P, Côté C, Caron-Lizotte O, Carli C, et al. Proteogenomic-based discovery of minor histocompatibility antigens with suitable features for immunotherapy of hematologic cancers. Leukemia (2016) 30:1344–54. doi: 10.1038/leu.2016.22
246. Dossa RG, Cunningham T, Sommermeyer D, Medina-Rodriguez I, Biernacki MA, Foster K, et al. Development of T-cell immunotherapy for hematopoietic stem cell transplantation recipients at risk of leukemia relapse. Blood (2018) 131:108–20. doi: 10.1182/blood-2017-07-791608
247. Bonte S, De Munter S, Goetgeluk G, Ingels J, Pille M, Billiet L, et al. T-cells with a single tumor antigen-specific T-cell receptor can be generated in vitro from clinically relevant stem cell sources. Oncoimmunology (2020) 9:1727078. doi: 10.1080/2162402X.2020.1727078
248. Noviello M, Manfredi F, Ruggiero E, Perini T, Oliveira G, Cortesi F, et al. Bone marrow central memory and memory stem T-cell exhaustion in AML patients relapsing after HSCT. Nat Commun (2019) 10:1065. doi: 10.1038/s41467-019-08871-1
249. Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W. CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol (2018) 18:635–47. doi: 10.1038/s41577-018-0044-0
250. Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N Engl J Med (2016) 375:143–53. doi: 10.1056/NEJMoa1601202
251. Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng Q-R, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia (2014) 28:1280–8. doi: 10.1038/leu.2013.355
252. Daver N, Garcia-Manero G, Basu S, Boddu PC, Alfayez M, Cortes JE, et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discovery (2019) 9:370–83. doi: 10.1158/2159-8290.CD-18-0774
253. Daver NG, Garcia-Manero G, Konopleva MY, Alfayez M, Pemmaraju N, Kadia TM, et al. Azacitidine (AZA) with Nivolumab (Nivo), and AZA with Nivo + Ipilimumab (Ipi) in Relapsed/Refractory Acute Myeloid Leukemia: A Non-Randomized, Prospective, Phase 2 Study. Blood (2019) 134:830–0. doi: 10.1182/blood-2019-131494
254. Masarova L, Kantarjian H, Garcia-Mannero G, Ravandi F, Sharma P, Daver N. Harnessing the Immune System Against Leukemia: Monoclonal Antibodies and Checkpoint Strategies for AML. Adv Exp Med Biol (2017) 995:73–95. doi: 10.1007/978-3-319-53156-4_4
255. Bewersdorf JP, Stahl M, Zeidan AM. Immune checkpoint-based therapy in myeloid malignancies: a promise yet to be fulfilled. Expert Rev Anticancer Ther (2019) 19:393–404. doi: 10.1080/14737140.2019.1589374
256. Schürch CM. Therapeutic Antibodies for Myeloid Neoplasms—Current Developments and Future Directions. Front Oncol (2018) 8:152. doi: 10.3389/fonc.2018.00152
257. Grosser R, Cherkassky L, Chintala N, Adusumilli PS. Combination Immunotherapy with CAR T Cells and Checkpoint Blockade for the Treatment of Solid Tumors. Cancer Cell (2019) 36:471–82. doi: 10.1016/j.ccell.2019.09.006
258. Kumar P, Kumar A, Parveen S, Murphy JR, Bishai W. Recent advances with Treg depleting fusion protein toxins for cancer immunotherapy. Immunotherapy (2019) 11:1117–28. doi: 10.2217/imt-2019-0060
259. Becker PS, Foran JM, Altman JK, Yacoub A, Castro JE, Sabbatini P, et al. Targeting the CXCR4 Pathway: Safety, Tolerability and Clinical Activity of Ulocuplumab (BMS-936564), an Anti-CXCR4 Antibody, in Relapsed/Refractory Acute Myeloid Leukemia. Blood (2014) 124:386–6. doi: 10.1182/blood.V124.21.386.386
260. Drexler B, Passweg JR, Tzankov A, Bigler M, Theocharides AP, Cantoni N, et al. The sympathomimetic agonist mirabegron did not lower JAK2-V617F allele burden, but restored nestin-positive cells and reduced reticulin fibrosis in patients with myeloproliferative neoplasms: results of phase II study SAKK 33/14. Haematologica (2019) 104:710–6. doi: 10.3324/haematol.2018.200014
261. Passaro D, Di Tullio A, Abarrategi A, Rouault-Pierre K, Foster K, Ariza-McNaughton L, et al. Increased Vascular Permeability in the Bone Marrow Microenvironment Contributes to Disease Progression and Drug Response in Acute Myeloid Leukemia. Cancer Cell (2017) 32:324–41.e6. doi: 10.1016/j.ccell.2017.08.001
262. Passaro D, Bonnet D. How to say NO to vascular disruption and stem cell mobilization. Expert Opin Ther Targets (2018) 22:563–5. doi: 10.1080/14728222.2018.1486821
263. Heuser M, Ofran Y, Boissel N, Brunet Mauri S, Craddock C, Janssen J, et al. Acute myeloid leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2020) 31:697–712. doi: 10.1016/j.annonc.2020.02.018
264. Wei AH, Döhner H, Pocock C, Montesinos P, Afanasyev B, Dombret H, et al. The QUAZAR AML-001 Maintenance Trial: Results of a Phase III International, Randomized, Double-Blind, Placebo-Controlled Study of CC-486 (Oral Formulation of Azacitidine) in Patients with Acute Myeloid Leukemia (AML) in First Remission. Blood (2019) 134:LBA–3-LBA-3. doi: 10.1182/blood-2019-132405
265. Estey E, Karp JE, Emadi A, Othus M, Gale RP. Recent drug approvals for newly diagnosed acute myeloid leukemia: gifts or a Trojan horse? Leukemia (2020) 34:671–81. doi: 10.1038/s41375-019-0704-5
266. Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood (2005) 106:2912–9. doi: 10.1182/blood-2005-05-2004
267. Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood (2013) 121:2854–63. doi: 10.1182/blood-2012-09-455063
268. DiNardo CD, Wei AH. How I treat acute myeloid leukemia in the era of new drugs. Blood (2020) 135:85–96. doi: 10.1182/blood.2019001239
269. Appelbaum FR. Maintenance therapy after allogeneic hematopoietic cell transplantation for acute myeloid leukemia. Best Pract Res Clin Haematol (2019) 32:101109. doi: 10.1016/j.beha.2019.101109
270. Schuurhuis GJ, Heuser M, Freeman S, Béné M-C, Buccisano F, Cloos J, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood (2018) 131:1275–91. doi: 10.1182/blood-2017-09-801498
271. Buckley SA, Wood BL, Othus M, Hourigan CS, Ustun C, Linden MA, et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: a meta-analysis. Haematologica (2017) 102:865–73. doi: 10.3324/haematol.2016.159343
Keywords: acute myeloid leukemia, immunotherapies, CAR T cells, management, clinical trials
Citation: Roussel X, Daguindau E, Berceanu A, Desbrosses Y, Warda W, Neto da Rocha M, Trad R, Deconinck E, Deschamps M and Ferrand C (2020) Acute Myeloid Leukemia: From Biology to Clinical Practices Through Development and Pre-Clinical Therapeutics. Front. Oncol. 10:599933. doi: 10.3389/fonc.2020.599933
Received: 28 August 2020; Accepted: 02 November 2020; Published: 09 December 2020.
Reviewed by:
Copyright © 2020 Roussel, Daguindau, Berceanu, Desbrosses, Warda, Neto da Rocha, Trad, Deconinck, Deschamps and Ferrand. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christophe Ferrand, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.4(5); 2020 Oct

Chronic Myeloid Leukemia in 2020
New insights have emerged from maturing long-term academic and commercial clinical trials regarding optimum management of chronic myeloid leukemia (CML). Velocity of response has unexpectedly proved less important than hitherto thought, does not predict survival, and is of unclear relevance for treatment-free remission (TFR). Serious and cumulative toxicity has been observed with tyrosine kinase inhibitors that had been expected to replace imatinib. Generic imatinib has become cost-effective first-line treatment in chronic phase despite chronic low-grade side-effects in many patients. Earlier recognition of end-phase by genetic assessment might improve prospects for blast crisis (BC). TFR has become an important new treatment goal of CML. To reflect this new situation ELN has recently revised and updated its recommendations for treating CML. After a brief review of 175 years of CML history this review will focus on recent developments and on current evidence for treating CML in 2020.
Introduction
Twenty-two years after the first patients with chronic myeloid leukemia (CML) were treated with the tyrosine kinase inhibitor (TKI) imatinib, outcome exceeds all expectations: most CML patients achieve a normal life expectancy, some in sustained treatment-free remissions (TFR) may operationally be cured.
Some expectations remain unmet, however. Most patients require life-long maintenance therapy. Also, progression to blast crisis still occurs in 5% to 7% of patients and remains a challenge. CML has not become the model disease for treating other leukemias or cancers. But the principle of elucidation of pathogenesis as a successful approach to treatment of cancer has been impressively shown in CML.
Success came a long way. CML was first described in 1844/5 when Virchow coined the term leukemia (Leukämie). 1 – 5 Bone marrow was proposed early as possible tissue of origin of CML, 6 but a definite diagnosis became possible only 82 years later when the Philadelphia (Ph)-chromosome was discovered and then the translocation t (9;22) was identified as hallmarks of the disease. 7 , 8 The subsequent molecular dissection of the chromosomal breakpoints with identification of the BCR-ABL fusion products laid the groundwork for molecular CML-diagnostics and for targeted therapy with BCR-ABL Tyrosine kinase inhibitors (TKI) as the current treatment principle of choice. Molecular BCR-ABL1 monitoring in CML with derivation of the International Scale (IS) has become the posterchild for molecular monitoring of other leukemias and diseases.
Early palliative treatments were arsenic (Fowler's solution, 5 to 10 drops 3× daily for several weeks) 9 , 10 and splenic irradiation, 11 the mainstays of treatment until 1953 when busulfan was introduced. 12 Hydroxyurea, available since 1963, 13 was easier to handle, had fewer side effects than busulfan and prolonged survival modestly. 14 Bone marrow transplantation was introduced in the late seventies 15 and provided the first cures. 16 At the same time interferon alpha (IFN) was shown to induce complete cytogenetic remissions (CCR) in a substantial minority of patients, 17 usually younger patients. Randomized studies 18 – 20 documented prolongation of survival with IFN which became the treatment of choice, although its exact mechanism of action is still not fully understood. 21
The benefit by IFN had just been recognized (ASH management recommendations 1999) 22 when BCR-ABL tyrosine kinase inhibition was introduced.
The detection of the ABL-oncogene was a byproduct of the search for a human leukemia virus in the 1960s and early 1970s. The first oncogenes (SRC, MYC) were isolated from chicken leukemia viruses. 23 , 24 ABL was isolated from the acutely transforming murine Abelson leukemia virus in 1980. 25 Numerous other oncogenes, isolated from retroviruses and from genomes of normal cells, followed.
Many oncogenes, amongst them SRC and ABL, encoded kinase activities that most notably phosphorylate tyrosine, a rarely phosphorylated amino acid. 25 , 26 This finding gained significance for CML when it was recognized that the human ABL oncogene homologue was located on chromosome 9 at the breakpoint of t (9;22). 27 The discovery of fusion transcripts of ABL with BCR sequences from chromosome 22 28 led to transfection experiments and the observation that BCR-ABL sequences induced leukemia in mice. 29 , 30 Since BCR-ABL1's oncogenic properties were mainly connected to its tyrosine kinase activity, it was the logical next step to define an inhibitor specific for bcr-abl tyrosine kinase suitable for therapeutic use in humans. 31
The first trial with imatinib, a phase I study with poor risk CML patients, started in 1998. 32 The stunning results convinced even skeptics that further studies were indicated. In 1999, a group of international investigators on CML met in Biarritz, France, to discuss the results and to convince Novartis to produce imatinib (at that time still STI571) in sufficient quantities for larger phase II and III trials. A letter sent by the group to Dr Daniel Vasella, then CEO of Novartis, recommending scale-up of the production of imatinib made the difference (The Magic Bullet 33 ).
The development of tyrosine kinase inhibitor (TKI) therapy and of molecular monitoring has been extensively reviewed by ELN 34 – 36 and will not be repeated here. But recent developments of current importance as discussed by ELN in its most recent recommendations, 37 will be highlighted in this review.
Epidemiology
Median age at diagnosis of CML is approximately 56 to 57 years in Western countries as estimated from the EUTOS and SIMPLICITY registries. 38 , 39 Patients older than 70 years make up more than 20%. In developing countries with younger populations median age is less than 50 years. 40 The incidence per year per 100,000 population varies by age and ranges between 1 and 2 depending on the age of the respective populations.
Initial diagnostic workup
The workup at baseline includes the following examinations (Table (Table1 1 ).
Initial Diagnostic Workup.

The preferred risk score for CML in the TKI era is the EUTOS long-term survival (ELTS) score whose accuracy to predict death from CML is higher than the Sokal score (Table (Table2 2 ). 41
Assessment of Risk Score (ELTS score).

Identification of transcript type is important for molecular monitoring, since atypical transcripts may give false negative test results – and is also of prognostic importance. The shorter e13a2 transcript is reportedly associated with shorter survival and a longer time to DMR compared with the longer e14a2 transcript. Based on evidence from a registry of transcript types in 45,503 newly diagnosed patients from 45 countries transcript type may be helpful to predict response to treatment, outcome of treatment, and TFR. 42
Several additional risk factors have been implicated, but so far none has been validated or found useful except reticulin content in a bone marrow biopsy 43 – 45 and high-risk additional chromosomal abnormalities (ACA; Table Table3). 3 ). High-risk ACAs predict poorer response to TKIs and a higher risk of progression. 46 – 48 Whereas the 2013 ELN-recommendations considered ACA a warning sign, 36 the 2020 ELN recommendations upgraded ACA to a high-risk sign for treating patients. 37
Genetically Based Risk Assessment.

First-line treatment
With few exceptions, the current first-line treatment is a TKI. A short course of hydroxyurea may be given in symptomatic patients while a diagnosis of CML is pending. Currently, 4 TKIs are approved for first-line treatment by the FDA and EMA: imatinib (Glivec®, Novartis), dasatinib (Sprycel®, Bristol-Myers Squibb), nilotinib (Tasigna®, Novartis), and bosutinib (Bosulif®, Pfizer). Radotinib (Supect®, Dae Wong Pharma) has been approved in South-Korea only 49 and is not further considered here.
Imatinib is effective in all phases of CML, and therapy has resulted in a normal life expectancy of most patients treated in chronic phase (CP) in clinical trials 50 , 51 and population-based registries. 52 – 54 No serious toxicity has surfaced after more than 20 years of use. 37 , 55 , 56 DMR was achieved in more than 80% of patients which is stable in more than 70% 57 allowing attempts at treatment discontinuation to achieve treatment-free remissions (TFR) 58 , 59 alleviating chronic low-grade side-effects such as fatigue and muscle cramps.
Generic imatinib 60 – 62 is now available worldwide and has become cost-effective initial therapy in CP. 37 , 63 If a generic drug meets the national standards of a country involved in quality, bioavailability and efficacy, generic imatinib is an acceptable alternative to a branded product. The 2020 ELN recommendations 37 state generic and brand product dosing should be the same. Monitoring the response to generics should also be the same as with branded drugs. If there is a change in therapy from a brand to a generic product, enhanced vigilance for the first six months is advised. Patients should continue the same generic brand if possible, to avoid potential side-effects due to changes in drug structure, bioavailability and drug preparation.
Imatinib resistance, second generation TKI, and second-line treatment
Second generation TKIs (2G-TKI, dasatinib, nilotinib, bosutinib) were developed following recognition of imatinib kinase domain (KD) resistance mutations 64 which occur in 4.6% of 1551 CP CML patients over 10 years making it relatively rare. 51 The higher potency of 2G-TKIs resulting in more rapid responses and relief of symptoms compared to imatinib when used in second-line 65 , 66 led to their use also as first-line therapy. By recognizing imatinib resistant mutations, fewer patients progressed to blast crisis (BC). 67 , 68 These positive effects, however, were counterbalanced by drug-induced adverse effects. 5- and 10-year data of randomized trials indicate survival with 2G-TKI first-line is similar to imatinib. The high rates of adverse effects to 2G-TKI (particularly pleural effusions in more than 25% of dasatinib-treated patients and serious vascular events with linear increase to 25% by 10 years in nilotinib-treated patients) argue against the use of 2G-TKI in first-line therapy. 67 – 69
For second-line treatment, patients must be carefully selected considering the comorbidities and the side-effects of 2G-TKI. In the case of failure to imatinib, a change of therapy is mandatory and should be accompanied by investigating BCR-ABL1 KD mutations (Table (Table3). 3 ). In the case of intolerance, the decision to change may be subjective depending in part on the patient, the physician and options of supportive care. Response criteria are the same as for first-line treatment.
Since dasatinib has pleuro-pulmonary toxicity, previous pleuro-pulmonary disease is a strong contraindication. A dose reduction from the approved dose of 100 mg/day in CP to 50 mg/day may reduce toxicity. 70
Because of the cardiovascular toxicity of nilotinib a history of coronary heart disease, cerebrovascular accidents and/or peripheral arterial occlusive disease represent strong contraindications to nilotinib. Also, hypertension, diabetes mellitus, hypercholesterolemia and a history of pancreatitis may be contraindications to using nilotinib. A dose-increase from the approved dose of 300 mg twice daily is not recommended.
No relevant comorbidities and no strong contraindications to bosutinib have been identified. At the approved dose of 400 mg/day annoying, but typically transient diarrhea occurs. Owing to the shorter observation time compared to the other TKI, no firm statement can be made regarding long-term safety.
Selection criteria and dosing of TKI in first- and second-line have been extensively discussed in recent ELN recommendations. 37 , 56
Indications of 2G-TKI and of the 3 rd generation TKI (3G-TKI) ponatinib for second- and third-line treatments based on the most frequent KD resistance mutations are shown in Table Table4 4 .
TKI Indications Based on ABL1 Kinase Domain Mutation Status.

Ponatinib has been approved for patients resistant against 2 TKI and is the only approved TKI with activity against the T315I mutation. 71 , 72 Dosing is critical; safety and efficacy must be considered. 37
2G-TKI and ponatinib are effective against most KD resistance mutations, but cannot overcome resistance from other causes such as clonal evolution with emergence of ACA.
Table Table5 5 summarizes the 5- and 10-year survival results of long term randomized and observational studies with imatinib or 2G-TKI. Similar survival rates are reported by population-based registries. 52 – 54
Five- and Ten-year Survival Results in Clinical Trials.

Current determinants of survival in CML are comorbidities, 75 major route ACA, 76 risk score, smoking 77 and treatment center, but not initial treatment selection. 51
Resistance to imatinib occurs in 10% to 15%, and to 2GTKI in <10% of patients in first-line treatment. In some patients, failure to respond may be related to poor compliance. Mutations account for resistance in about one third of resistant CP patients, and in about two thirds of resistant accelerated phase (AP) and BC patients. Alternative mechanisms of resistance include clonal evolution (emergence of high-risk ACA) and the activation of BCR-ABL1 independent pathways. A cytogenetic risk classification has been proposed to allow risk-based treatment adaptation. 47 , 48 , 78
In about two-thirds of compliant TKI resistant CP patients and in about one third of resistant AP and BC patients, a mutation is neither detected, nor is it the only cause of resistance. Analyzing the genome and expression profiles of resistant CML cells may identify somatic mutations 79 – 81 as early signs of progression, and lead to a genetically-based risk classification with the potential for non-BCR-ABL1 targeted therapy for resistant patients. 82
BCR-ABL1 mutations can be detected with sensitivities of about 20% by Sanger sequencing and in about 3% by NGS. NGS is the recommended technology to detect clinically relevant BCR-ABL1 resistance mutations in patients not responding adequately to TKI. 83 , 84
Allogeneic hematopoietic cell transplantation
Despite the superiority of drug treatment, allogeneic hematopoietic cell transplantation has retained a place in CP CML for patients with disease resistant to multiple TKIs or personal preferences. 85 , 86 In resource poor countries the onetime expense of a transplant may be more economical than life-long treatment with a TKI.
Transplants should be strongly considered in persons resistant to 2G-TKIs. Someone resistant to the initial 2G-TKI therapy has a low chance of achieving a durable response to an alternative TKI and should be assessed early for a transplant. Early transplantation as a rule improves outcome. 87 If the patient has also failed ponatinib, risk of progression is high. Someone progressing to AP under treatment is a candidate for an immediate transplant. For a patient presenting in BC a return to a second CP (CP2) should be attempted. Return to CP2 improves transplantation outcome. 85 , 88 Also, in patients with high-risk ACA and low blast counts early transplantation may improve survival. 48 Transplant mortality in CP is low, 85 but GvHD remains a problem. Transplantation in BC is a high-risk procedure and not advised. 37
Pregnancy and fertility
All TKIs are teratogenic and should be withheld during pregnancy. 89 , 90 Low-level secretion of TKIs in breast milk contraindicates their use during breast-feeding. 91 Sperm quality and morphology are unchanged after treatment with TKI. 92 For more in-depth information see the ELN 2020 recommendations. 37
Response monitoring and milestones
Timely recognition of suboptimal response or resistance to TKI requires regular monitoring. Hematologic and cytogenetic monitoring have been replaced in most instances by the more sensitive molecular monitoring with quantitative PCR-techniques for BCR-ABL1 transcripts. 93 , 94 Transcript levels are reported in a standardized fashion according to the International Scale (IS) 95 – 97 which underlies the response milestones guiding treatment (Table (Table6). 6 ). Complete cytogenetic remission (CCR) has been shown to be equivalent to 0.1% BCR-ABL1 on the IS. 98
Response Milestones Expressed as BCR-ABL1 on the International Scale (IS).

DMR at the MR 4 and MR 4.5 levels is prognostic. Progression of CML is extremely rare at these levels. 57 Patients may be operationally cured and require no further treatment. To test this possibility TKI discontinuation studies have been undertaken to determine optimum duration of treatment and of deep DMR, rate of TFR after discontinuation, and markers predictive of successful discontinuation, 58 , 59 , 99 see paragraph on TFR below.
Quality of life
This is an important evolving field building on survival, but beyond the scope of this review.
In brief, since most patients receive TKIs for many years or even indefinitely, observation of quality of life in these patients and amelioration of chronic low-grade side-effects are important. Current research preferentially addresses tolerability of different TKIs. 100 , 101 Replacement of one TKI by another may improve tolerability, but frequently at the expense of other, potentially more serious toxicity. 102 Dose-reductions of TKIs are an option. 70 , 103 Patient-reported outcome (PRO) questionnaires are encouraged to quantify chronic quality of life issues faced by CML patients. 104
Treatment-free remission (TFR)
TFR is a new significant goal of CML management. A significant proportion of patients will achieve a DMR defined as BCR-ABL1 levels of MR 4 and MR 4.5 on the IS with current TKIs. Benchmark times for molecular response rates with imatinib are shown in Figure Figure1 1 .

Benchmarks for molecular response rates with imatinib . 12-year incidences are 91% for MR2 (equivalent to CCR), 89% for MR3 (MMR), 82% for MR4, 72% for MR4.5 and 54% for MR5. Data updated from CML study IV. (M Pfirrmann, update of Ref. 55).
Median times to MR 4 are 2.9 years, to MR 4.5 4.7 years. 5-year rates are 67% for MR 4 and 53% for MR 4.5 .
Table Table7 7 lists benchmarks of DMR that can be expected by 5 and 10 years after treatment with imatinib, nilotinib and dasatinib. 55 , 57 , 67 – 69 Five-year follow-up of first-line bosutinib is not yet available. 105
Cumulative Incidences of DMR (MR 4 and MR 4.5 ) with Imatinib, Nilotinib and Dasatinib by 5 and 10 Years as Benchmarks.

An attempt at treatment discontinuation can be considered, if sustained DMR of sufficiently long duration has been achieved. An initial observation of 12 patients 94 showed that about half of them in DMR (no detectable BCR-ABL transcripts by PCR) stayed in remission after cessation of imatinib. In a follow-up study of 100 patients (STop IMatinib or STIM study) 38% stayed in TFR after an observation period of 7 years. 58 , 106 Most relapses occurred early within the first 6–12 months. Loss of MMR indicates failure of TFR. 107 Virtually all relapsing patients regained their prior best response level after re-treatment.
A polymyalgia-like TKI withdrawal syndrome of musculo-skeletal pain may occur in a third of patients which is usually self-limited, but may require treatment with acetaminophen, non-steroidal anti-inflammatory drugs or rarely a short course of oral steroids. 108 , 109 A patient study reported that the TKI withdrawal syndrome if unmanaged may cause more morbidity than hitherto thought. 110
Table Table8 8 shows a selection of discontinuation studies after treatment with imatinib or the 2G-TKI dasatinib and nilotinib.
Selected TKI-discontinuation Studies, Update 2020.

The largest of these studies = the Euro-SKI study of 755 mostly imatinib treated CML patients who had been in DMR at the MR 4 level for at least 1 year = showed a TFR rate of 49% after 3 years. 59 Duration of MR 4 was determined as the most important predictor of TFR. Treatment discontinuation is feasible only in CP patients. Patients in advanced phases, particularly in BC, remain a challenge.
After failure of TFR, a second stop after additional treatment can result in a TFR-rate as high as 33% at 4 years, 126 updated at EHA 2019.
Interestingly, dose reduction prior to complete discontinuation to reduce side-effects may improve successful TFR (Destiny study 103 ). Another interesting observation is the finding in the ISAV study, by comparing TFR rates in younger and older patients, of significantly lower TFR rates in patients under 45 years of age 114 , 115 which is in line with the observation of more aggressive disease in adolescents and young adults. 127 , 128
Several studies addressed the issue of changing from imatinib to a 2G-TKI to shorten the interval to DMR and TFR. A more rapid response was generally observed, but toxicity of 2G-TKI limits this approach.
In the TIDEL-II study, the dose of patients receiving imatinib 600 mg/day failing to reach time benchmarks was increased to imatinib 800 mg/day or medication was changed to nilotinib 2 × 400 mg/day. 129 This approach was considered feasible.
In the ENESTcmr study, imatinib-treated patients in CCR were randomized to remain on imatinib or to change to nilotinib. The rate of DMR by 4 years was, as expected, higher in the nilotinib group, but only 57% of nilotinib-treated patients completed 4 years of nilotinib therapy. The study provided no information whether patients in DMR subsequently achieved TFR successfully. 130 , 131 It should be remembered that most patients in durable DMR still harbor residual BCR-ABL1 sequences in their genomic DNA. 132
The ELN 2020 recommendations define the following requirements for TKI discontinuation for successfully achieving TFR (Table (Table9 9 ). 37
Requirements for TKI Discontinuation.

It is recommended to consider TFR in appropriate patients after careful discussion employing the concept of shared decision making. 133 First-line TKI, or a change to a 2G-TKI, for faster DMR are not recommended because of the more serious side-effects of 2G-TKI, their increased costs and absent information about the number of patients who might actually benefit. A change to 2G-TKI to improve the depth of response can be considered in selected patients in whom DMR has not been reached such as the motivated patient with a high priority for TFR, younger patients with low or intermediate risk disease or women who wish to become pregnant.
End phase CML and blast crisis
Outcome of patients in blast crisis (BC) treated with single agents, combination chemotherapy, and TKI alone and in combination with intensive chemotherapy 134 , 135 remains unsatisfactory. Once BC has occurred, survival is generally less than one year with death due to infection or bleeding. New approaches are urgently needed.
Genetically-based risk assessment by ACA and somatic mutations has recently been proposed for a better recognition of patients at risk for progression to end-phase CML and BC. 47 , 48 , 77 , 79 Currently, diagnosis of BC rests on the percentage of blasts (20% or 30%) in blood or marrow, 34 , 136 , 137 but not all patients dying of CML reach the BC-defining blast levels. 138 Earlier recognition of end-phase might enable earlier intervention to improve prospects for BC.
End-phase CML comprises early progression with emerging high-risk ACA and late progression with failing hematopoiesis and blast cell proliferation. 48 Up to 90% of BC patients show chromosomal aberrations in addition to the Ph-chromosome (termed major or minor route by Mitelman depending on their frequency in BC 139 , 140 ) and as many as 80% BCR-ABL1 KD mutations. 141 Also, somatic mutations have been detected in BC and are associated with poor risk disease when detected at diagnosis. 78 , 79 Blast increase in blood or marrow represents the end stage of progression.
High-risk ACA defined as the major route ACA +8, +Ph, i(17q), +19, +21, +17 (the ACA most frequently observed in BC), 140 the minor route ACA -7/7q-, 3q26.2 and 11q23 rearrangements (less frequently observed, but negative impact on prognosis), and complex aberrant karyotypes 47 , 77 herald death by CML in the presence of low blast counts. 48
Somatic mutations observed in BC and in poor risk patients include mutations of genes associated with poor outcome in other malignancies. 142 They also might enable early identification of patients at risk of progression. Frequently mutated genes include RUNX1, ASXL1 and IKZF1 78 , 79 (Table (Table3 3 ).
Patients with suboptimal responses by ELN criteria 34 and with less than DMR after 2 years (less than MR 4 ) should have a genetic evaluation. In patients with high-risk ACA more intensive treatment, for example, by hematopoietic cell transplantation (allo-HCT), may be indicated. Current treatment approaches to end-phase CML are summarized in Figure Figure2. 2 . Treatment depends on disease stage. Elimination of BCR-ABL1 by effective TKI treatment is expected to prevent progression.

Management strategy for end-phase CML. The red arrow indicates progression to the worse. CP2 = second chronic phase.
Cytogenetic monitoring is indicated when response to therapy is unsatisfactory. When high-risk ACA emerge, intensification of treatment should be considered. There is also evidence that earlier transplantation is more successful in patients with high-risk ACA. An appropriate time for a change of treatment may be the emergence of high-risk ACA rather than waiting for an increase of blasts. AP should be treated as high-risk CML. Transplantation is recommended if response to drug treatment is not optimal. Treatment of BC consists of intensive combination chemotherapy based on AML regimens for myeloid, and ALL regimens for lymphoid, BC with or without a TKI, for instance dasatinib at the approved dose 140 mg/day for BC or ponatinib, in preparation for a prompt transplantation if possible. Flow cytometry distinguishes between lymphoid and myeloid BC allowing appropriate selection of treatment. Lymphoid BC has more treatment options and a better outcome than myeloid BC. In patients who cannot tolerate intensive chemotherapy regimens, a more palliative approach with less intensive therapy according to immunophenotype should be considered such as vincristine and prednisone in lymphoid BC.
There is evidence that emergence of high-risk ACA is an indication for a timelier change of treatment with better outcome. 48 Comparing transplantation outcome in early and late end-phase, a clinically relevant, though not statistically significant difference of 30% in 2-year survival suggests that outcome of transplanted patients with high-risk ACA depends on disease stage similar to patients without ACA. 87
Summary and prospects
Based on the results of maturing long-term clinical trials management of CP-CML is again changing profoundly. All randomized studies that compare imatinib 400 mg once daily with 2G-TKIs, imatinib 400 mg with dose increase, or imatinib combined with IFN alpha or low-dose cytarabine have failed to improve OS. Although deeper molecular responses occurred more rapidly with 2G-TKIs, with imatinib dose increase or with imatinib in combination with peg-IFN alpha, these events did not translate into better OS than with imatinib at a standard dose of 400 mg daily. Nevertheless, these studies provided greater insights in the safety and efficacy of the drugs, as well as benchmarks for molecular response as a basis for individualized treatment and eventually treatment discontinuation. The studies showed that survival has moved close to that of the general population. Now more patients die of CML-unrelated causes than from CML. The goal of treatment in these patients is better supportive care and management of side-effects of treatments aiming at best possible quality of life.
A new important development has been recognizing that treatment can be successfully stopped in a substantial minority of patients depending upon whether duration of both treatment and DMR are long enough to make TFR a feasible option. TFR is an important new goal of CML management which should be discussed with appropriate patients.
Regarding changing therapy from imatinib to a 2G-TKI in a patient with stable CCR or MMR, but in whom the level of DMR (< MR 4 ) was insufficient to warrant consideration of discontinuation, no recommendation can be made in view of the high toxicity and costs of 2G-TKI. Also, there is no information about the rate of successful TFR from large randomized trials with different initial treatment regimens addressing this specific issue.
Regarding changing from 2G-TKI to imatinib, this can be considered when no DMR is achieved within 3 years to avoid the risk of serious cumulative toxicity of 2G-TKI.
Current challenges on the path to cure of CML are increasing the proportion of patients in whom treatment can be successfully discontinued, and the further decrease of patients who progress to BC. This can be achieved by optimizing treatment with available drugs, by developing new drugs with better efficacy and by better recognition of patients at risk for progression and of optimum conditions for treatment discontinuation (duration of DMR, duration of treatment, other factors such as risk score, age, gender), and by more intensive treatment of patients not responding well enough, respectively. Of urgency is still the management of refractory disease of those 6% who progress to BC in spite of seemingly adequate treatment. Earlier recognition of such patients seems possible.
Finally, factors causing CML remain of interest. The only established risk factor is still radiation as observed after the atomic bombs on Hiroshima and Nagasaki. Better epidemiologic studies and registries may provide an answer. 143 – 145
Acknowledgements
The author thanks Drs. Richard T Silver and Robert P. Gale for critically reading the manuscript, and Johannes Hehlmann for support.
Citation: Hehlmann R. Chronic Myeloid Leukemia in 2020. HemaSphere , 2020;4:5(e468). http://dx.doi.org/10.1097/HS9.0000000000000468
The author declares no conflicts of interest.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 30 May 2024
Targeting a lineage-specific PI3Kɣ–Akt signaling module in acute myeloid leukemia using a heterobifunctional degrader molecule
- Lois M. Kelly 1 na1 ,
- Justine C. Rutter ORCID: orcid.org/0000-0002-0851-0887 2 na1 ,
- Kevin H. Lin 2 na1 ,
- Frank Ling 1 ,
- Matthieu Duchmann 1 ,
- Emmanuelle Latour 1 ,
- Nadia Arang 3 , 4 ,
- Hélène Pasquer 1 ,
- Duong Ho Nhat 1 ,
- Juliette Charles 1 ,
- Shane T. Killarney ORCID: orcid.org/0000-0002-8471-6641 2 ,
- Hazel X. Ang ORCID: orcid.org/0000-0002-4194-7204 2 ,
- Federica Namor 1 ,
- Cécile Culeux 1 ,
- Bérangère Lombard ORCID: orcid.org/0000-0001-9044-3662 5 ,
- Damarys Loew ORCID: orcid.org/0000-0002-9111-8842 5 ,
- Danielle L. Swaney ORCID: orcid.org/0000-0001-6119-6084 3 , 4 , 6 ,
- Nevan J. Krogan ORCID: orcid.org/0000-0003-4902-337X 3 , 4 , 6 ,
- Luc Brunel 7 ,
- Élodie Carretero 7 ,
- Pascal Verdié 7 ,
- Muriel Amblard 7 ,
- Sofiane Fodil 8 ,
- Tony Huynh 8 ,
- Marie Sebert ORCID: orcid.org/0000-0003-4376-4097 1 , 8 ,
- Lionel Adès ORCID: orcid.org/0000-0002-9020-8766 1 , 8 ,
- Emmanuel Raffoux 1 , 8 ,
- Nina Fenouille 1 ,
- Raphaël Itzykson ORCID: orcid.org/0000-0003-2139-6262 1 , 8 ,
- Camille Lobry ORCID: orcid.org/0000-0003-0550-4921 1 ,
- Lina Benajiba 1 , 9 ,
- Antoine Forget 3 , 4 ,
- Anthony R. Martin ORCID: orcid.org/0000-0001-6187-6979 7 na2 ,
- Kris C. Wood ORCID: orcid.org/0000-0002-5887-2253 2 na2 &
- Alexandre Puissant ORCID: orcid.org/0000-0002-3997-9282 1 na2
Nature Cancer ( 2024 ) Cite this article
688 Accesses
13 Altmetric
Metrics details
- Acute myeloid leukaemia
- Chemical tools
- Targeted therapies
Dose-limiting toxicity poses a major limitation to the clinical utility of targeted cancer therapies, often arising from target engagement in nonmalignant tissues. This obstacle can be minimized by targeting cancer dependencies driven by proteins with tissue-restricted and/or tumor-restricted expression. In line with another recent report, we show here that, in acute myeloid leukemia (AML), suppression of the myeloid-restricted PIK3CG/p110γ – PIK3R5/p101 axis inhibits protein kinase B/Akt signaling and compromises AML cell fitness. Furthermore, silencing the genes encoding PIK3CG/p110γ or PIK3R5/p101 sensitizes AML cells to established AML therapies. Importantly, we find that existing small-molecule inhibitors against PIK3CG are insufficient to achieve a sustained long-term antileukemic effect. To address this concern, we developed a proteolysis-targeting chimera (PROTAC) heterobifunctional molecule that specifically degrades PIK3CG and potently suppresses AML progression alone and in combination with venetoclax in human AML cell lines, primary samples from patients with AML and syngeneic mouse models.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
111,21 € per year
only 9,27 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Cancer therapy with antibodies

Antibody drug conjugate: the “biological missile” for targeted cancer therapy

The present and future of bispecific antibodies for cancer therapy
Data availability.
Gene expression data for normal and malignant tissues were obtained from the GTEx gene expression dataset and accessed through the Gepia portal. Gene dependency data were obtained from the DepMap dependency dataset. Data analyses were performed using R or GraphPad Prism 8. Genetic and clinical features were explored using star counts and clinical annotation from the Beat AML 1.0 cohort. The Beat AML 1.0 cohort was used also to assess the gene expression of patients with AML with available transcriptomic profiling. Raw counts from the Beat AML 1.0 cohort were available from the NIH GDC portal and genetic and clinical annotations were available from the supplementary information provided in the original article reporting this cohort 26 . TCGA data were available from the NIH GDC portal. The RNA-seq-based profiling of the AML cell lines treated with ARM165 is available from GSE260759 . Queried gene sets were from the MSigDB Hallmark and C2 geneset libraries. The H3K SRA database was accessible under accession number SRP103200 . All other data supporting the findings of this study are available from the corresponding author on reasonable request. No custom code was generated in the course of this study. Source data are provided with this paper.
Shimony, S., Stahl, M. & Stone, R. M. Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 98 , 502–526 (2023).
Article PubMed Google Scholar
Appelbaum, F. R. et al. Age and acute myeloid leukemia. Blood 107 , 3481–3485 (2006).
Article CAS PubMed PubMed Central Google Scholar
Bhansali, R. S., Pratz, K. W. & Lai, C. Recent advances in targeted therapies in acute myeloid leukemia. J. Hematol. Oncol. 16 , 29 (2023).
Article PubMed PubMed Central Google Scholar
DiNardo, C. D. et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133 , 7–17 (2019).
DiNardo, C. D. et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med. 383 , 617–629 (2020).
Article CAS PubMed Google Scholar
Tsherniak, A. et al. Defining a cancer dependency map. Cell 170 , 564–576 (2017).
Burger, J. A. Treatment of chronic lymphocytic leukemia. N. Engl. J. Med. 383 , 460–473 (2020).
Jordan, V. C. & O’Malley, B. W. Selective estrogen-receptor modulators and antihormonal resistance in breast cancer. J. Clin. Oncol. 25 , 5815–5824 (2007).
Watson, P. A., Arora, V. K. & Sawyers, C. L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 15 , 701–711 (2015).
Yu, A. L. et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 363 , 1324–1334 (2010).
Burger, J. A. et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 34 , 787–798 (2020).
Millis, S. Z., Ikeda, S., Reddy, S., Gatalica, Z. & Kurzrock, R. Landscape of phosphatidylinositol-3-kinase pathway alterations across 19 784 diverse solid tumors. JAMA Oncol. 2 , 1565–1573 (2016).
Hirai, H. et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol. Cancer Ther. 9 , 1956–1967 (2010).
Maira, S. M. et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol. Cancer Ther. 11 , 317–328 (2012).
Maira, S. M. et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 7 , 1851–1863 (2008).
Di Leo, A. et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 19 , 87–100 (2018).
Hopkins, B. D. et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560 , 499–503 (2018).
Krop, I. E. et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 17 , 811–821 (2016).
Furman, R. R. et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 370 , 997–1007 (2014).
De Henau, O. et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature 539 , 443–447 (2016).
Foubert, P., Kaneda, M. M. & Varner, J. A. PI3Kγ activates integrin α 4 and promotes immune suppressive myeloid cell polarization during tumor progression. Cancer Immunol. Res. 5 , 957–968 (2017).
Schmid, M. C. et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kγ, a single convergent point promoting tumor inflammation and progression. Cancer Cell 19 , 715–727 (2011).
Kaneda, M. M. et al. PI3Kγ is a molecular switch that controls immune suppression. Nature 539 , 437–442 (2016).
Hong, D. S. et al. Eganelisib, a first-in-class PI3Kγ inhibitor, in patients with advanced solid tumors: results of the phase 1/1b MARIO-1 trial. Clin. Cancer Res. 29 , 2210–2219 (2023).
Gu, H. et al. PI3Kγ maintains the self-renewal of acute myeloid leukemia stem cells by regulating the pentose phosphate pathway. Blood 143 , 1965–1979 (2024).
Tyner, J. W. et al. Functional genomic landscape of acute myeloid leukaemia. Nature 562 , 526–531 (2018).
van Galen, P. et al. Single-cell RNA-seq reveals AML hierarchies relevant to disease progression and immunity. Cell 176 , 1265–1281 (2019).
Ng, S. W. et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature 540 , 433–437 (2016).
Cancer Genome Atlas Research, N. et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 368 , 2059–2074, (2013).
Article Google Scholar
Shymanets, A. et al. p87 and p101 subunits are distinct regulators determining class IB phosphoinositide 3-kinase (PI3K) specificity. J. Biol. Chem. 288 , 31059–31068 (2013).
Stephens, L. R. et al. The Gβγ sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell 89 , 105–114 (1997).
Horejsi, Z. et al. CK2 phospho-dependent binding of R2TP complex to TEL2 is essential for mTOR and SMG1 stability. Mol. Cell 39 , 839–850 (2010).
Sarbassov, D. D., Guertin, D. A., Ali, S. M. & Sabatini, D. M. Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science 307 , 1098–1101 (2005).
Cantley, L. C. The phosphoinositide 3-kinase pathway. Science 296 , 1655–1657 (2002).
Pham, L. V. et al. Strategic therapeutic targeting to overcome venetoclax resistance in aggressive B-cell lymphomas. Clin. Cancer Res. 24 , 3967–3980 (2018).
Sathe, A. et al. Parallel PI3K, Akt and mTOR inhibition is required to control feedback loops that limit tumor therapy. PLoS ONE 13 , e0190854 (2018).
Tavor, S. et al. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res. 64 , 2817–2824 (2004).
Fields, T. A. & Casey, P. J. Signalling functions and biochemical properties of pertussis toxin-resistant G-proteins. Biochem. J. 321 , 561–571 (1997).
Houslay, D. M. et al. Coincident signals from GPCRs and receptor tyrosine kinases are uniquely transduced by PI3Kβ in myeloid cells. Sci. Signal. 9 , ra82 (2016).
Bondeson, D. P. et al. Lessons in PROTAC design from selective degradation with a promiscuous warhead. Cell Chem. Biol. 25 , 78–87 (2018).
Buhimschi, A. D. et al. Targeting the C481S ibrutinib-resistance mutation in Bruton’s tyrosine kinase using PROTAC-mediated degradation. Biochemistry 57 , 3564–3575 (2018).
Douglass, E. F. Jr., Miller, C. J., Sparer, G., Shapiro, H. & Spiegel, D. A. A comprehensive mathematical model for three-body binding equilibria. J. Am. Chem. Soc. 135 , 6092–6099 (2013).
Wurz, R. P. et al. Affinity and cooperativity modulate ternary complex formation to drive targeted protein degradation. Nat. Commun. 14 , 4177 (2023).
Lu, J. et al. Hijacking the E3 ubiquitin ligase Cereblon to efficiently target BRD4. Chem. Biol. 22 , 755–763 (2015).
Chua, C. C. et al. Chemotherapy and venetoclax in elderly acute myeloid leukemia trial (CAVEAT): a phase Ib dose-escalation study of venetoclax combined with modified intensive chemotherapy. J. Clin. Oncol. 38 , 3506–3517 (2020).
Rahmani, M. et al. Cotargeting BCL-2 and PI3K induces BAX-dependent mitochondrial apoptosis in AML cells. Cancer Res. 78 , 3075–3086 (2018).
Lin, K. H. et al. Targeting MCL-1/BCL-XL forestalls the acquisition of resistance to ABT-199 in acute myeloid leukemia. Sci. Rep. 6 , 27696 (2016).
Evans, C. A. et al. Discovery of a selective phosphoinositide-3-kinase (PI3K)-γ inhibitor (IPI-549) as an immuno-oncology clinical candidate. ACS Med. Chem. Lett. 7 , 862–867 (2016).
Gangadhara, G. et al. A class of highly selective inhibitors bind to an active state of PI3Kγ. Nat. Chem. Biol. 15 , 348–357 (2019).
Vanhaesebroeck, B., Ali, K., Bilancio, A., Geering, B. & Foukas, L. C. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem. Sci. 30 , 194–204 (2005).
Nürnberg, B. & Beer-Hammer, S.Function, regulation and biological roles of PI3Kγ variants. Biomolecules 9 , 427 (2019).
Perino, A., Ghigo, A., Scott, J. D. & Hirsch, E. Anchoring proteins as regulators of signaling pathways. Circ. Res. 111 , 482–492 (2012).
Patrucco, E. et al. PI3Kγ modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell 118 , 375–387 (2004).
Beretta, M., Bauer, M. & Hirsch, E. PI3K signaling in the pathogenesis of obesity: the cause and the cure. Adv. Biol. Regul. 58 , 1–15 (2015).
Damilano, F., Perino, A. & Hirsch, E. PI3K kinase and scaffold functions in heart. Ann. N. Y. Acad. Sci. 1188 , 39–45 (2010).
Mohan, M. L. & Naga Prasad, S. V. Scaffolding function of PI3Kγ emerges from enzyme’s shadow. J. Mol. Biol. 429 , 763–772 (2017).
Winter, G. E. et al. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 348 , 1376–1381 (2015).
Bai, L. et al. A potent and selective small-molecule degrader of STAT3 achieves complete tumor regression in vivo. Cancer Cell 36 , 498–511 (2019).
Kaneshige, A. et al. A selective small-molecule STAT5 PROTAC degrader capable of achieving tumor regression in vivo. Nat. Chem. Biol. 19 , 703–711 (2023).
Koide, E. et al. Development and characterization of selective FAK inhibitors and PROTACs with in vivo activity. Chembiochem 24 , e202300141 (2023).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10 , R25 (2009).
Ramirez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44 , W160–W165 (2016).
Pohl, A. & Beato, M. bwtool: a tool for bigWig files. Bioinformatics 30 , 1618–1619 (2014).
Martz, C. A. et al. Systematic identification of signaling pathways with potential to confer anticancer drug resistance. Sci. Signal. 7 , ra121 (2014).
Su, A. et al. The folate cycle enzyme MTHFR is a critical regulator of cell response to MYC-targeting therapies. Cancer Discov. 10 , 1894–1911 (2020).
Lin, K. H. et al. P2RY2–Akt activation is a therapeutically actionable consequence of XPO1 inhibition in acute myeloid leukemia. Nat. Cancer 3 , 837–851 (2022).
Brinkman, E. K., Chen, T., Amendola, M. & van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 42 , e168 (2014).
Kuo, Y. H. et al. Cbfβ-SMMHC induces distinct abnormal myeloid progenitors able to develop acute myeloid leukemia. Cancer Cell 9 , 57–68 (2006).
Pimentel, H., Bray, N. L., Puente, S., Melsted, P. & Pachter, L. Differential analysis of RNA-seq incorporating quantification uncertainty. Nat. Methods 14 , 687–690 (2017).
Hanzelmann, S., Castelo, R. & Guinney, J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinf. 14 , 7 (2013).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31 , 455–461 (2010).
Download references
Acknowledgements
We thank the members of the A. Puissant, K. C. Wood and A. R. Martin laboratories for their scientific input. We are indebted to V. Montcuquet, N. Setterblad, C. Doliger and C. Maillard from the Saint-Louis Research Institute Core Facility and to CNRS, University Montpellier and the SynBio3 platform supported by IBiSa and the Chimie Balard Cirimat Carnot Institute. We are grateful to L. H. Castilla for providing us with the Cbfb – MYH11 knock-in mouse model and to M. Wymann for providing us with the PIK3CG -directed antibody and for fruitful suggestions on the project. This work was supported by the ERC Starting and Consolidator programs (AltChem 758848 and DynAML 101088563 to A.P.), the Laurette Fugain association (to A.P.), Amgen Innovations (to A.P.), Fondation ARC (to C.Lo.), ATIP-Avenir 2022 Ligue Nationale contre le Cancer (to L. Be.) and the INCA PLBIO program (PLBIO20-246 to A.P. and C.Lo.; PLBIO20-074 to C.Lo.). This work was supported by NIH R01CA266389 (to K.C.W. and A.P.) and NIH U54CA274502 (to A.F., D.L.S. and N.J.K.). M.D. is supported by the Bettencourt Schueller Foundation (CCA-INSERM-Bettencourt). A.P. is an FSER laureate and a recipient of the Brigitte Mérand, Jean Valade and Tourre awards. A.P., R.I., L.Be. and C.L. are supported by the SIRIC InsiTu program (INCa-DGOS-INSERM-ITMO Cancer_18008).
Author information
These authors contributed equally: Lois M. Kelly, Justine C. Rutter, Kevin H. Lin.
These authors jointly supervised this work: Anthony R. Martin, Kris C. Wood, Alexandre Puissant.
Authors and Affiliations
INSERM UMR 944, IRSL, Saint-Louis Hospital, Paris Cité University, Paris, France
Lois M. Kelly, Frank Ling, Matthieu Duchmann, Emmanuelle Latour, Hélène Pasquer, Duong Ho Nhat, Juliette Charles, Federica Namor, Cécile Culeux, Marie Sebert, Lionel Adès, Emmanuel Raffoux, Nina Fenouille, Raphaël Itzykson, Camille Lobry, Lina Benajiba & Alexandre Puissant
Department of Pharmacology and Cancer Biology, Duke University, Durham, NC, USA
Justine C. Rutter, Kevin H. Lin, Shane T. Killarney, Hazel X. Ang & Kris C. Wood
Quantitative Biosciences Institute (QBI), University of California, San Francisco, San Francisco, CA, USA
Nadia Arang, Danielle L. Swaney, Nevan J. Krogan & Antoine Forget
Department of Cellular and Molecular Pharmacology, University of California San Francisco, San Francisco, CA, USA
Curie Institute, Mass Spectrometry and Proteomics Facility, PSL Research University, Paris, France
Bérangère Lombard & Damarys Loew
Gladstone Institutes, San Francisco, California, USA
Danielle L. Swaney & Nevan J. Krogan
IBMM, University of Montpellier, CNRS, ENSCM, Montpellier, France
Luc Brunel, Élodie Carretero, Pascal Verdié, Muriel Amblard & Anthony R. Martin
Department of Hematology and Immunology, Saint-Louis Hospital, AP-HP, Paris Cité University, Paris, France
Sofiane Fodil, Tony Huynh, Marie Sebert, Lionel Adès, Emmanuel Raffoux & Raphaël Itzykson
Clinical Investigation Center, Saint-Louis Hospital, AP-HP, Paris Cité University, Paris, France
Lina Benajiba
You can also search for this author in PubMed Google Scholar
Contributions
L.M.K., conceptualization, formal analysis, validation, investigation, visualization, methodology and writing—original draft. J.C.R., conceptualization, formal analysis, validation, investigation, visualization and methodology. K.H.L., conceptualization, formal analysis, validation, investigation, visualization and methodology. F.L., investigation and methodology. M.D., resources and investigation. E.L., investigation and methodology. N.A., investigation and methodology. H.P., resources and methodology. D.H.N., investigation and methodology. J.C., investigation. S.T.K., investigation. H.X.A., investigation. F.N., investigation. C.C., resources. B.L., methodology. D.L., methodology. D.L.S., methodology and resources. N.J.K., resources. L. Brunel, methodology and resources. E.C., methodology and resources. P.V., methodology and resources. M.A., methodology and resources. S.F., resources. T.H., resources. M.S., resources. L.A., resources. E.R., resources. N.F., methodology, resources and support. R.I., conceptualization, methodology and investigation. C.L., conceptualization, formal analysis, visualization and methodology. L. Benajiba, conceptualization, investigation, methodology and resources. A.F., conceptualization, investigation, methodology and visualization. A.R.M., conceptualization, formal analysis, supervision, investigation, visualization, methodology and writing—original draft. K.C.W., conceptualization, supervision, funding acquisition, investigation, visualization, methodology, writing—original draft and project administration. A.P., conceptualization, formal analysis, supervision, funding acquisition, investigation, methodology, writing—original draft and project administration.
Corresponding authors
Correspondence to Anthony R. Martin , Kris C. Wood or Alexandre Puissant .
Ethics declarations
Competing interests.
The Krogan Laboratory has received research support from Vir Biotechnology, F. Hoffmann-La Roche and Rezo Therapeutics. N.J.K. has financially compensated consulting agreements with Maze Therapeutics. N.J.K. is the president and on the board of directors of Rezo Therapeutics and a shareholder in Tenaya Therapeutics, Maze Therapeutics, Rezo Therapeutics and Interline Therapeutics. K.C.W. is a cofounder, consultant and equity holder at Tavros Therapeutics and Celldom, is a consultant and equity holder at Simple Therapeutics and Decrypt Biomedicine and has performed consulting work for Guidepoint Global, Bantam Pharmaceuticals and Apple Tree Partners. The other authors declare no competing interests.
Peer review
Peer review information.
Nature Cancer thanks Oliver Hantschel, Ross Levine and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended data fig. 1.
a . Comparison of the expression levels of PIK3CG and PIK3R5 across AML and healthy tissues. P-values calculated using one-way ANOVA. Error bars represent mean ± SD. b-d . Expression levels of PIK3CG and PIK3R5 in AML patients from various FAB ( b ) and genetic ( c ) subcategories, or at diagnosis versus relapse ( d ). Median with lower and upper hinges corresponding to the first and third quartiles. The lower and upper whiskers represent the lowest and largest value within 1.5 times the lower and upper interquartile, respectively. The points represent individual values. P-values calculated using two-sided Wilcoxon test and reported on the figure panel. e and f . Spearman correlation between AML cell differentiation state and the expression of PIK3CG and PIK3R5 . Correlation between PIK3CG or PIK3R5 expression and ssGSVA score were tested using Spearman’s rank correlation. Two-sided p-values were corrected for multi-testing using the Benjamini & Hochberg method. f . The smooth area corresponds to the 95% confidence interval of the linear regression model. b-f . Data generated using the Beat AML cohort. a-d: n values displayed on the graph.
Source data
Extended data fig. 2.
a . DNA sanger sequencing of the PIK3CG and PIK3R5 genomic regions targeted by the CRISPR-Cas9 PIK3CG - and PIK3R5 -directed guides in OCI-AML2 cells. Sequences were aligned using TIDE online tool to determine the relative efficiency of each sgRNA. Repeated twice with similar results. b . Bioluminescence pictures of three representative mice from Fig. 1j injected with OCI-AML2 infected with either a non-targeting control or a PIK3CG -directed sgRNA. Median bioluminescence is depicted in radiance on days 7, 13, 18 and 22.
Extended Data Fig. 3
a and b . Representative growth inhibition curves, and corresponding IC50 and AUC values (n = 4, A, n = 7, B, mean ± SD after three days of seeding) from Fig. 2c of OCI-AML2 cells transduced with either a non-targeting control, two PIK3CG -directed, or two PIK3R5 -directed sgRNAs and treated with increasing concentrations of the indicated targeted therapies or chemotherapy drugs ( a ), FLT3 inhibitors, gilteritinib and sorafenib, or KIT inhibitors, amuvanib and telatinib ( b ). Av. = Averaged. c . Bioluminescence pictures of three representative mice from Fig. 2i injected with OCI-AML2 cells harboring a non-targeting control or PIK3CG -directed sgRNA. Median bioluminescence is depicted in radiance on days 17, 24, 27 and 31.
Extended Data Fig. 4
a . Volcano plots of the PIK3R5-interacting protein pulled down in OCI-AML2, MV4-11, and NOMO-1 cells compared to control. DIA MS data were processed using Spectronaut with default factory setting providing an unpaired t-test p-value for quantified proteins. b . Ranking of proteins with positive infinite ratios (only identified in PIK3R5 pull down) according to the number of peptides identified. Only proteins with a z-test score > 0.95 were included for further analysis. c . Network corresponding to Fig. 3a depicting, in two colors, the PIK3R5-interacting proteins identified in two cell lines (in yellow) or all three cell lines (in orange).
Extended Data Fig. 5
a . Correlation between gene dependency scores of AKT1 and AKT2 versus PIK3CG in a panel of non-AML cell lines. Data from DepMap CRISPR/Cas9 dependency profiling. Pearson correlation coefficient (ρ) provided to demonstrate no correlation. b . MK-2206 sensitizer and resistor topoisomerase-encoding genes, polymerase-encoding genes, and anti-apoptotic protein-encoding genes identified from a pooled drug-modifier screen conducted in OCI-AML2 cells. Gene-level scores were obtained by averaging sgRNA-level comparisons. Red dot denotes BCL2 identified as a sensitizer gene. All other genes included in the analysis are depicted as blue dots. c . Chemokine/receptor expression heatmap indicating upregulation of both CXCL12/CXCR4 in AML relative to normal tissue. Tumor expression data from TCGA database; normal expression data from GeTex expression database. Data accessed through Gepia gene expression portal.
Extended Data Fig. 6
Synthetic scheme for the synthesis of the PIK3CG degrader, ARM165. Reagents and conditions: i) LiOH monohydrate, MeOH, H 2 O, 60 °C, 40 h (94%); ii) Boc-AOc-OH, T3P 50% in ethyl acetate, pyridine, N,N -dimethylformamide, 80 °C, 16 h; iii) TFA, CH 2 Cl 2 , r.t. 2 h; iv) Glutaric anhydride, N,N -diisopropylethylamine, toluene, N,N -dimethylformamide, 110 °C, 2 h (62% over 3 steps, ii-iv); v) Compound I, HATU, N,N -diisopropylethylamine, N,N -dimethylformamide, r.t. 16 h (55%).
Extended Data Fig. 7
a . Synthetic scheme for the synthesis of non-PIK3CG-targeting control compound for ARM165, ARM204. Reagents and conditions are provided below the synthesis scheme. b . Representative growth inhibition curves with IC50s and AUCs (n = 7, mean ± SD), corresponding to the Fig. 5b for AML and non-AML cells treated with increasing doses of ARM204, AZ2, and ARM165.
Extended Data Fig. 8
a . Representative growth inhibition curves, IC50s, and AUC values n = 7, mean ± SD) corresponding to Fig. 6a of indicated AML cells treated with increasing doses of venetoclax in presence of AZ2 or ARM165 after three days of seeding. b-c . Growth inhibition curves, IC50s, and AUC values (n = 3 biological replicates composed of seven technical replicates, mean ± SD), of OCI-AML2 cells treated with increasing doses of cytarabine, daunorubicin, or venetoclax in combination with 500 nM ARM165. P-values calculated using one-way ANOVA and reported on the figure panel.
Extended Data Fig. 9
a-b . Toxicity profile of ARM165 treatment in naive mice. Mice were treated with IV injection of 0.051 mg/kg ARM165 for seven consecutive days. Individual mouse weight was measured daily (n = 5 mice per group) ( a ) and the proportion of each indicated hematopoietic cell fraction ( b ) was established in blood using an MS9 instrument, and in bone marrow (BM) and spleen (SP) by flow cytometry (n = 10 mice per group). Error bars represent mean ± SD. P-values calculated using Mann-Whitney and reported on the figure panel.
Supplementary information
Supplementary information.
Flow cytometry gating strategies.
Reporting Summary
Supplementary table 1.
Supplementary Table 1: Clinical annotations of the nine primary samples of patients with AML used in the study; female = 0, male = 1. Supplementary Table 2: sgRNA sequences. Supplementary Table 3: Antibodies used for western blot. Supplementary Table 4: shRNA sequences. Supplementary Table 5: Antibodies used for flow cytometry analyses.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Source data fig. 3, source data fig. 4, source data fig. 5, source data fig. 6, source data.
Unprocessed western blots.
Source Data Extended Data Fig. 1 and Table 1
Source data extended data fig. 3 and table 3, source data extended data fig. 4 and table 4, source data extended data fig. 5 and table 5, source data extended data fig. 6 and table 6.
Drug development data.
Source Data Extended Data Fig. 7 and Table 7
Statistical source and drug development data.
Source Data Extended Data Fig. 8 and Table 8
Source data extended data fig. 9 and table 9, rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Kelly, L.M., Rutter, J.C., Lin, K.H. et al. Targeting a lineage-specific PI3Kɣ–Akt signaling module in acute myeloid leukemia using a heterobifunctional degrader molecule. Nat Cancer (2024). https://doi.org/10.1038/s43018-024-00782-5
Download citation
Received : 24 October 2023
Accepted : 13 May 2024
Published : 30 May 2024
DOI : https://doi.org/10.1038/s43018-024-00782-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
- DOI: 10.4103/gjtm.gjtm_16_24
- Corpus ID: 270114127
Alteration of Blood Group in a Patient with Chronic Myelogenous Leukemia
- Published in Global Journal of Transfusion… 1 January 2024
4 References
Weakening of a antigen in myelodysplastic syndrome-mimicking a case of wrong blood in tube, loss and reappearance of a antigen after chemotherapy leading to blood group discrepancy in acute myeloid leukemia: a case report., the disappearance of blood group antigens: a clue to the clinical diagnosis of leukemia., loss of red cell a, b, and h antigens is frequent in myeloid malignancies., related papers.
Showing 1 through 3 of 0 Related Papers

IMAGES
VIDEO
COMMENTS
Progress in understanding the pathophysiology and improving the therapy of acute myeloid leukemia (AML) is now occurring at a rapid pace. The discovery of the activity of cytarabine (ara-C) and of ...
Acute myeloid leukemia (AML) is a rapidly progressing myeloid neoplasm characterized by the clonal expansion of immature myeloid-derived cells, known as blasts, in the peripheral blood and bone marrow. This expansion results in ineffective erythropoiesis and megakaryopoiesis, clinically manifesting as relatively rapid bone marrow failure compared to chronic and indolent leukemias. This leads ...
Abstract. Treatment of acute myeloid leukemia (AML) has improved in recent years and several new therapeutic options have been approved. Most of them include mutation-specific approaches (e.g., gilteritinib for AML patients with activating FLT3 mutations), or are restricted to such defined AML subgroups, such as AML-MRC (AML with myeloid-related changes) or therapy-related AML (CPX-351).
Introduction. Understanding of the biology and pathophysiology of acute myeloid leukemia (AML) has accelerated translational discoveries and is contributing to a steady improvement in the outlook and prognosis of AML. 1-4 Recent important transitions into clinical practice include small molecule-targeted therapies such as the fms-like tyrosine kinase 3 (FLT3) and isocitrate dehydrogenase ...
Acute myeloid leukemia (AML) is a malignancy of the stem cell precursors of the myeloid lineage (red blood cells, platelets, and white blood cells other than B and T cells). Like other malignancies, it is due to genetic variations that lead to neoplastic changes and clonal proliferation. AML remains a rare malignancy, accounting for only 1.2% ...
Since the 2017 report from the European LeukemiaNet (ELN), 1 there has been substantial progress in our knowledge of acute myeloid leukemia (AML). Recent advances significantly influence clinical practice. These advances include insights into the clinical value of genomic abnormalities for diagnosis and prognosis, the clinical significance of inherited predisposition to AML, technological ...
Objective: Leukemia is the most common pediatric malignancy and a major cause of morbidity and mortality in children. Among all subtypes, a lack of consensus exists regarding the diagnosis and treatment of acute myeloid leukemia (AML). Patient survival rates have remained modest for the past three decades in AML.
In conclusion, AML therapy is evolving rapidly and treatment results are improving in all AML subsets as novel agents and strategies are incorporated into traditional AML chemotherapy. LAY SUMMARY: Ongoing research in acute myeloid leukemia (AML) is progressing rapidly. Since 2017, the US Food and Drug Administration has approved 10 drugs for ...
1 INTRODUCTION. Acute myeloid leukemia (AML) is a heterogenous disease that arises from uncontrolled proliferation of clonal hematopoietic cells. 1, 2 It is the most common form of acute leukemia in adults, with a median age at diagnosis of 68 years. 3 The estimated 5-year OS is 30% 4 and differs greatly between various age groups, reaching 50% in younger patients but is less than 10% in ...
3. Acute myeloid leukaemia accounts for over 80 000 deaths globally per annum, with this number expected to double over the next two decades. 4. The 5-year relative survival for patients in the USA diagnosed with acute myeloid leukaemia is currently 30·5%, improved from only 18% in the year 2000. 5.
Kantarjian H. Acute myeloid leukemia-major progress over four decades and glimpses into the future. ... left a lasting impact on the authors of this paper and will be greatly missed. This research ...
Acute myeloid leukemia (AML) is an aggressive malignancy characterized by challenges in treatment, including drug resistance and frequent relapse. Recent research highlights the crucial roles of tumor microenvironment (TME) in assisting tumor cell immune escape and promoting tumor aggressiveness. This study delves into the interplay between AML ...
Acute Myeloid Leukemia. Acute myeloid leukemia (AML) is a form of cancer that is characterized by infiltration of the bone marrow, blood, and other tissues by proliferative, clonal, abnormally ...
Introduction. In 2010 and 2017, the European LeukemiaNet (ELN) international expert panel published recommendation for the diagnosis and management of acute myeloid leukemia (AML) (1, 2).Furthermore an update of the WHO classification in 2016 provided a few changes to the previously described disease categories ().This new classification of AML is focused on the determination of genetic ...
Rabin Medical Center and Faculty of Medicine, Tel Aviv University, Tel Aviv-Yafo, Israel. Correspondence. Richard M. Stone, MD, Dana-Farber Cancer. Institute, 450 Brookline Ave, Boston, MA. 02215 ...
Comorbidities and outcomes of patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: a real-world, nationwide, retrospective study from Hungary, Pathology and Oncology ...
Patients with acute myeloid leukemia (AML) may experience extramedullary involvement when disease is present outside of the blood and bone marrow. In particular, the presence of central nervous system (CNS) involvement has traditionally been thought of as a poor prognostic factor.In the presently available literature, there is a paucity of conclusive data surrounding CNS AML given its rarity ...
Abstract. The blast crisis (BC) of chronic myeloid leukemia (CML) has poor efficacy against existing treatments and extremely short survival. However, the molecular mechanism of CML-chronic phase (CP) transformation to CML-BC is not yet fully understood. Here, we show that Lin28B, a RNA binding protein, acted as an activator enhancing the transformation to CML-BC by mediating excessive cell ...
Introduction. Chronic myeloid leukemia (CML), a malignancy originating in hematopoietic stem cells (HSCs) in the chronic phase of the disease (Table 1) and characterized by myeloid cells of various maturation stages in peripheral blood and bone marrow, is caused by the oncoprotein BCR-ABL1, a dysregulated tyrosine kinase 1.In early 2001, the first targeted therapy against an oncoprotein, the ...
In some people with AML, the leukemia cells have a mutation in the IDH1 or IDH2 gene, which stops the cells from maturing properly. IDH inhibitors can help the leukemia cells mature into normal blood cells. Some of these drugs, such as enasidenib (Idhifa), olutasidenib (Rezlidhia), and ivosidenib (Tibsovo), are now approved to treat AML with ...
This model was able to predict AML 6-12 months before diagnosis with a sensitivity of 25.7% and overall specificity of 98.2%. The model performed consistently across different age groups with an ...
Acute myeloid leukemia (AML) is characterized by a block in hematopoietic differentiation where the hematopoietic stem cell (HSC) fails to develop into mature myeloid cells resulting in bone marrow failure. Citation 1 AML arises due to a wide range of molecular abnormalities and genetic mutations, making the disease difficult to treat.
Patients were excluded if they (a) had PML/RARa-positive leukemia, MDS-AML, secondary Acute Myeloid Leukemia (sAML), genetic syndrome, and Trisomy 21 syndrome with AML, and (b) gave up treatment within 7 days of induction therapy initiation because the aim of this study was to evaluate the outcomes of our treatment protocol including dual ...
Introduction. Accurate quantification of the BCR::ABL1 fusion gene in whole blood is pivotal for the clinical management of chronic myeloid leukemia (CML) patients. The fusion protein encoded by BCR::ABL1 can vary in size, depending on the BCR and/or ABL1 gene breakpoint. The vast majority of CML patients have a p210 BCR::ABL1 fusion gene (M-BCR), which can be attributed to the presence of ...
BY Erin Dahlstrom, Ph.D. Leukemia is an overarching term encompassing several subtypes of blood cancers. Blood cells are produced in the bone marrow, the spongy material inside bones. The bone marrow contains immature stem cells that develop and mature to become red blood cells, platelets, or different types of white blood cells.
An evidence-based analysis of the effect of busulfan, hydroxyurea, interferon, and allogeneic bone marrow transplantation in treating the chronic phase of chronic myeloid leukemia: developed for the american society of hematology: presented in part at the ASH Education Session, December 5, 1998, Miami Beach, FL. Blood. 1999; 94:1517-1536.
This study shows that specific low abundant Gram-negative intestinal commensals of the genus Sutterella suppress the anti-leukemia immune response in chronic myeloid leukemia (CML). We found that germ-free and specific opportunistic pathogen-free (SOPF) mice are protected from CML development and that colonization of SOPF mice with Sutterella ...
Puissant and colleagues identify a myeloid-restricted PIK3CG/p110γ-PIK3R5/p101 axis as a therapeutic vulnerability in acute myeloid leukemia and develop a proteolysis-targeting chimera to ...
A male patient diagnosed with acute myeloid leukemia presented with complaints of fever, lethargy, and bleeding manifestations and further workups on follow-up after the commencement of chemotherapy showed that his original blood group was A Rh D positive, in which the A antigen expression was previously masked by the underlying disease condition of the patient.