Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic

RELATED TOPICS
INTRODUCTION
The following topic will outline issues related to the management of hepatitis B through the use of cases studies that incorporate patient-specific clinical information and test results. Our approach to treatment is generally consistent with guidelines from the European Association for the Study of the Liver guidelines, Asian-Pacific Association for the Study of the Liver guidelines, and American Association for the Study of Liver Diseases Practice Guidelines and Guidance [ 1-5 ].
Additional topic reviews that address the diagnosis and management of HBV include:
● (See "Hepatitis B and pregnancy" .)
● (See "Clinical manifestations and diagnosis of hepatitis B virus infection in children and adolescents" and "Management of hepatitis B virus infection in children and adolescents" .)
Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State
Viral Hepatitis: A Case Study
Contributors.
- Aisha Prioleau-Johnson
- Beth Mengstu
- Matt Kilboy
Our rationale for choosing viral hepatitis
Viral hepatitis is an infection of the liver that comes in several forms. The different forms of hepatitis (A,B,C,D,E) determine how it was contracted, how it is treated and the prognosis. Research has allowed the development of vaccines for hepatitis A and B, however, with the increase in the anti-vaccination movement and IV drug use, the prevalence of Hepatitis has not decreased as one would expect. Professionals in healthcare must remain aware of this preventable disease so we can educate patients about the effective methods in reducing its prevalence.
- Open supplemental data
- Reference Manager
- Simple TEXT file
People also looked at
Case report article, case report: a case of severe acute hepatitis of unknown origin.

- 1 The Key Laboratory of Molecular Biology of Infectious Diseases Designated by the Chinese Ministry of Education, Chongqing Medical University, Chongqing, China
- 2 Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Child Infection and Immunity, Department of Infectious Disease, National Clinical Research Center for Child Health and Disorders, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 3 Department of Endocrine and Breast Surgery, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 4 Department of Pathology, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 5 Department of Radiology, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 6 Chongqing Municipal Center for Disease Control and Prevention, Chongqing, China
- 7 Key Laboratory of Laboratory Medical Diagnostics, Chinese Ministry of Education, Chongqing Medical University, Chongqing, China
According to analyses of etiology, clinical features, diagnostic methods, and treatment strategies by summarizing a case of unexplained acute hepatitis recently experienced, we are aiming to provide some information to enrich the clinical experience in diagnosis and treatment of severe acute hepatitis of unknown etiology in young children. A boy, aged 10 years and 6 months old, was admitted to the hospital due to acute abdominal pain, jaundice, and exceptionally high levels of ALT and AST. A range of measures, including patient history, physical examination, and routine laboratory testing, were performed. Furthermore, strategies such as trio-based next-generation sequencing (Trio-NGS) and liver biopsy, as well as metagenomic NGS (mNGS) of blood and liver samples were also performed. In summary, this case was an acute severe non-A–E hepatitis that is a probable case with hepatitis of unknown origin. Immunohistochemical analysis showed an immune injury in liver tissues. Torque teno virus (TTV) sequences were detected by mNGS assay. As for treatment strategies, in addition to general treatment, this patient also underwent plasmapheresis and methylprednisolone treatment due to disease deterioration. The patient’s liver function was improved afterward and discharged after one month of treatment. Taken together, this work reported the clinical feature and treatment of severe acute hepatitis with non-A–E hepatitis in detail. The potential mechanism of liver damage might be due to an immune attack in which TTV might play a role as a co-factor.
Introduction
Liver disease of unknown etiology refers to liver diseases that cannot be clearly diagnosed by patient history, physical examination, and routine laboratory testing. Due to differences in race and region, the reported incidence of unexplained liver disease in pediatric patients is variable, ranging from 10 to 50% ( 1 – 4 ). The etiologies of unexplained liver disease can be roughly divided into infectious and non-infectious categories. Although the common viruses (hepatitis viruses A, B, C, D, and E) that cause acute viral hepatitis are undetectable, infectious etiologies, including Epstein–Barr virus (EBV), cytomegalovirus (CMV), herpes simplex viruses (HSV), bacteria, fungi, and parasites, are still common causes of liver injury ( 5 – 9 ). In addition, non-infectious etiologies, such as non-alcoholic fatty liver disease (NAFLD), drug-induced liver injury (DILI), autoimmune hepatitis (AIH), and inherited metabolic liver disease, also account for a relatively large proportion of hepatitis cases ( 10 – 13 ).
Since the first few cases of severe acute hepatitis of unknown origin were reported among young children in Scotland in March ( 14 ), increasing numbers of cases have been reported worldwide. As of 26 May 2022, at least 650 cases of unexplained hepatitis in children ranging from ages 1 month to 16 years have been reported in 33 countries, including England, Spain, Israel, the United States, and Denmark ( 15 ). The clinical manifestation is acute hepatic dysfunction with significantly elevated aminotransferase levels. Most of the affected children have jaundice, abdominal pain, nausea, vomiting, and diarrhea but no fever. Thirty-eight patients had received liver transplantation, and at least nine deaths were reported ( 15 ). According to the World Health Organization (WHO), the case definition of Confirmed is N/A at present. The case definition of Probable is a person presenting with an acute hepatitis (non-hepA–E*) with serum transaminase > 500 IU/L (AST or ALT), who is 16 years and younger, since 1 October 2021 ( 15 ).
The cause of the pediatric liver disease has not been revealed yet. However, a leading hypothesis is that an infectious agent is the underlying cause or a risk factor. Given the presence in about three-fourths of the investigated cases, adenovirus type 41 was initially suspected to be the causative pathogen ( 16 , 17 ). Nevertheless, adenovirus does not fully explain the increased severity of the cases. In addition, adenovirus is a seasonally transmitted virus with a peak period of infection from February to April. It can cause severe infection in multiple organs, including the liver, in immunocompromised children ( 18 ); however, it is rarely able to lead to severe infection in immunocompetent children ( 19 , 20 ). Thus, in the updated technical note released by WHO, adenovirus positivity was considered more likely to be a coincidental factor ( 21 ).
Our team recently experienced a case with severe acute non-A–E hepatitis. Here, we supplement a few noteworthy points based on this case, hoping to provide more information for pediatricians about the current hepatitis of unknown origin in children.
Case description
A boy who was 10 years and 6 months old was admitted to the hospital due to abdominal pain that persisted for 4 days. The patient also vomited three times without concomitant fever or diarrhea. He was found to have jaundice when referred to a local hospital. The biochemical tests showed exceptionally high levels of alanine aminotransferase [(ALT) 2330 U/L], aspartate aminotransferase [(AST) 1326 U/L], and total bilirubin [(TB) 74.2 μmol/L]. The patient received no intervention or treatment before being transferred to our hospital. He was previously in good health, denied taking any medications or having a history of exposure to patients with COVID-19, and received the second dose of inactivated COVID-19 vaccine 3 months before admission. At admission, the physical examination revealed that the child had scleral icterus, jaundice, hepatosplenomegaly, upper abdominal tenderness, and a positive Murphy’s sign.
The patient underwent infectious pathogen screening. Evidence of hepatitis A, B, C, and E viruses, CMV, HSV, and EBV infection is not found by serology (Hepatitis A, B, C, and E virus, CMV, and EBV), immunohistochemical (EBV), and PCR (HSV and EBV) analyses. The autoimmune hepatitis-related antibodies (17 common antibodies including ANA, SSA, AMA M2, and ss-DNA) and metabolism disease-related tests (including blood glucose, blood ammonia, lactate, alpha-fetoprotein, and ceruloplasmin) were also negative. Abdominal ultrasound indicated hepatosplenomegaly, pericholecystic edema, and thickening of the gallbladder wall. Magnetic resonance cholangiopancreatography (MRCP) showed edema of the first porta hepatis, the periportal region, and the gallbladder, as well as stenosis of the choledoch ( Figures 1A–C,G ). To further clarify the potential cause, the patient underwent trio-based next-generation sequencing (Trio-NGS) and liver biopsy. Trio-NGS did not detect any abnormalities, while liver biopsy showed mild interfacial hepatitis and fibrosis of portal area (G1S1) ( Figures 2A,B ), and further classification of the intrahepatic lymphocytes predominantly suggested CD8 + cells ( Figure 2C ). Tests for HBsAg, HBcAg, CMV, IgG4, and EBER in liver tissue remained negative. Although some interlobular bile ducts were very small, the number of intrahepatic bile ducts was normal. Finally, torque teno virus (TTV) sequences were detected by metagenomic NGS (mNGS) in whole blood sample ( Figure 3A ) and liver tissue ( Figure 3B ). The result of electron microscopy ( Figure 2D ) indicated chronic active hepatitis, which was shown as follows: hepatic cells were swollen, rough endoplasmic reticulum and smooth endoplasmic reticulum were slightly expanded, and lipid droplets were observed in hepatocytes (upper image), as well as lymphocytosis were observed in hepatic sinusoidal (lower image). However, no viral inclusions were found.
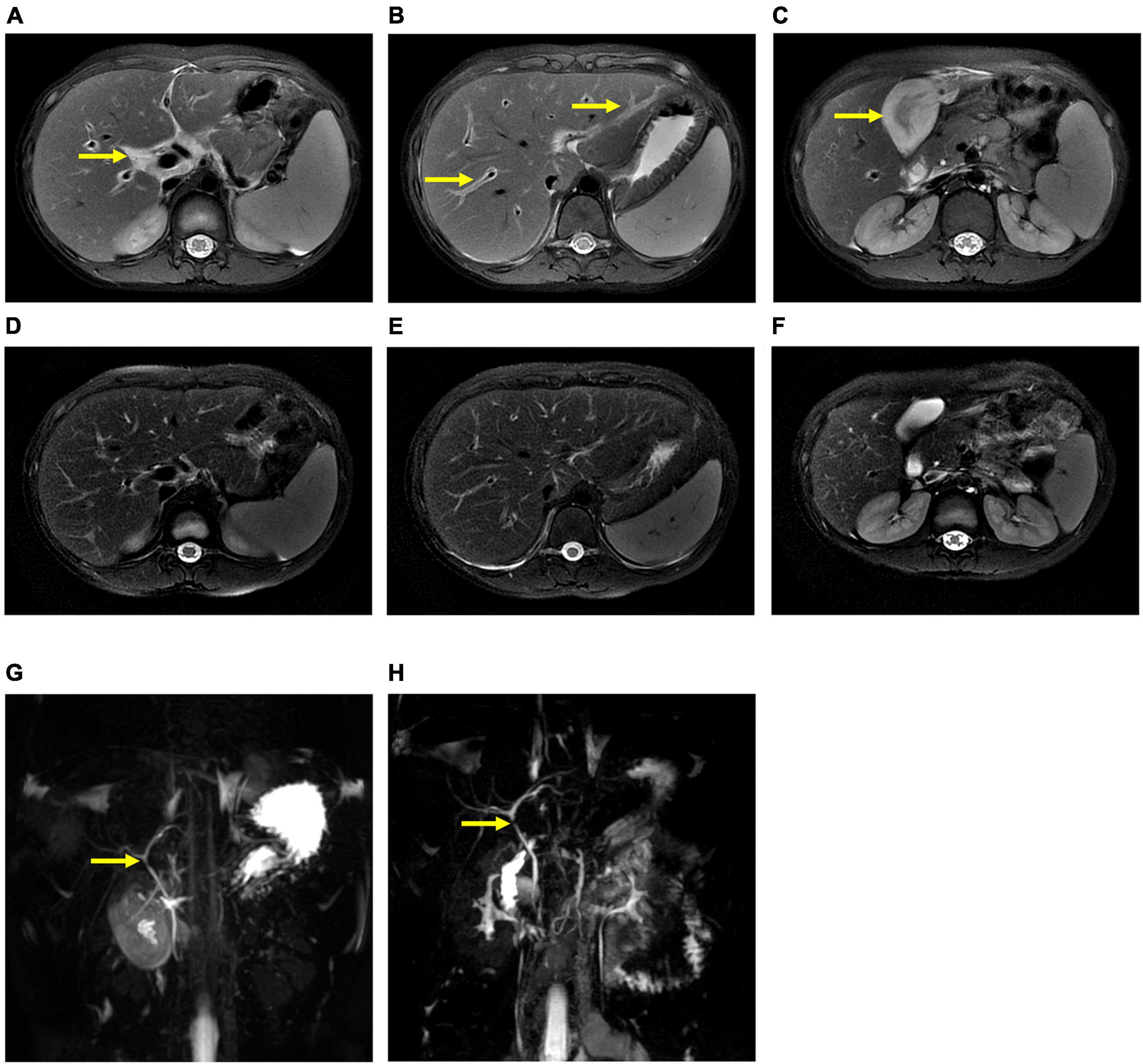
Figure 1. Magnetic resonance cholangiopancreatography (MRCP) results of the patient. MRCP showed edema of the first porta hepatis and the periportal region (A,B) and thickness of the gallbladder wall (C) at the beginning of treatment (thick arrow), and they were resolved after treatment (D–F) . However, MRCP showed a narrow bile duct that was not relieved during hospitalization (G,H) .
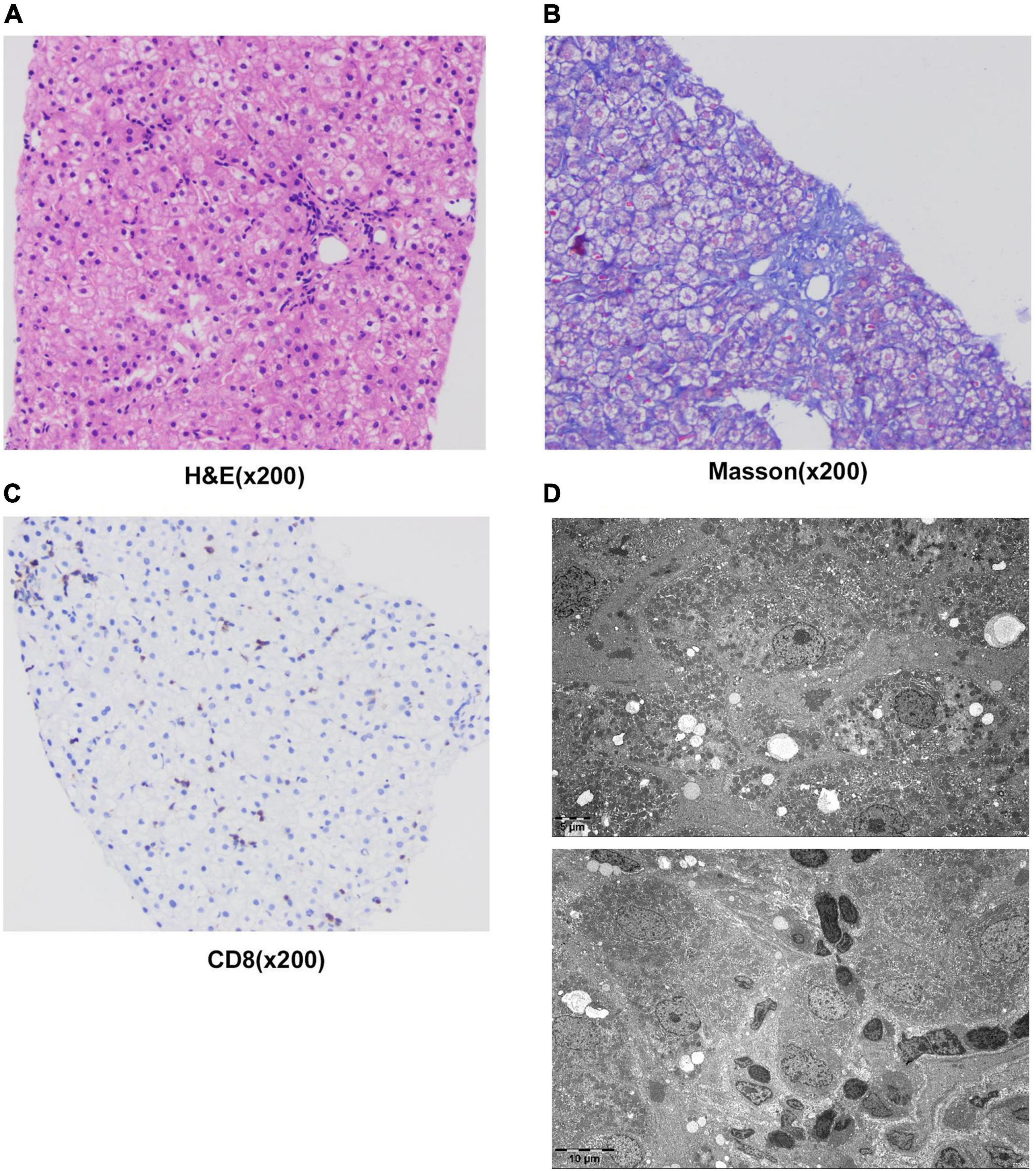
Figure 2. Results of liver histology of the patient. (A–C) Liver biopsy results. H&E staining of liver tissue showed swollen hepatocytes and inflammatory cells infiltrating into portal duct areas, and there was mild interfacial inflammation (A) . Masson staining indicated mild hepatic fibrosis in the portal area (B) . Immunohistochemical staining of CD8 + T-lymphocytes (C) . (D) The result of electron microscopy showed chronic active hepatitis. No viral inclusions were found.
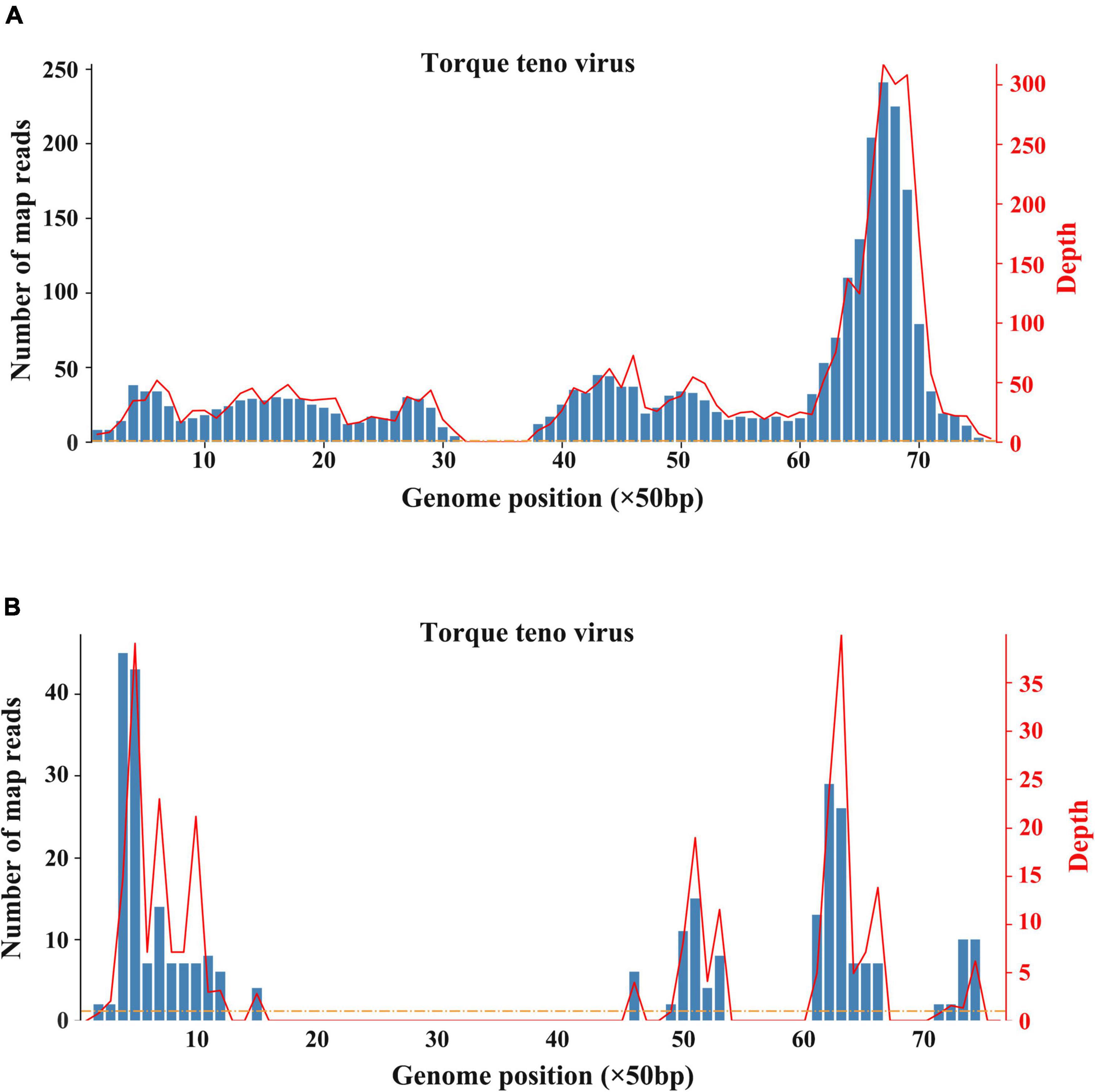
Figure 3. Results of metagenomic sequencing. (A) Sequencing results of whole blood. (B) Sequencing results of liver tissue.
After hospitalization, the patient received ceftazidime (based on cholecystitis), lipid-soluble vitamins, and reduced glutathione treatment. Unfortunately, the patient’s situation deteriorated with rapid progressive jaundice (peak TB: 173 μmol/L), underwent plasmapheresis once, and was administered methylprednisolone. The patient’s liver function was improved after the administration of steroids ( Figures 4A–C ). MRCP tests showed that the narrow bile duct was not relieved, but the hepatosplenomegaly and gallbladder edema had disappeared ( Figures 1D–F,H ). The patient was discharged after one month of treatment. Two weeks post-discharge, he revisited the hospital. He was clinically stable without any adverse and unanticipated events. The biochemical tests showed that ALT was 163 U/L, AST was 60 U/L, and TB was 11.6 μmol/L.
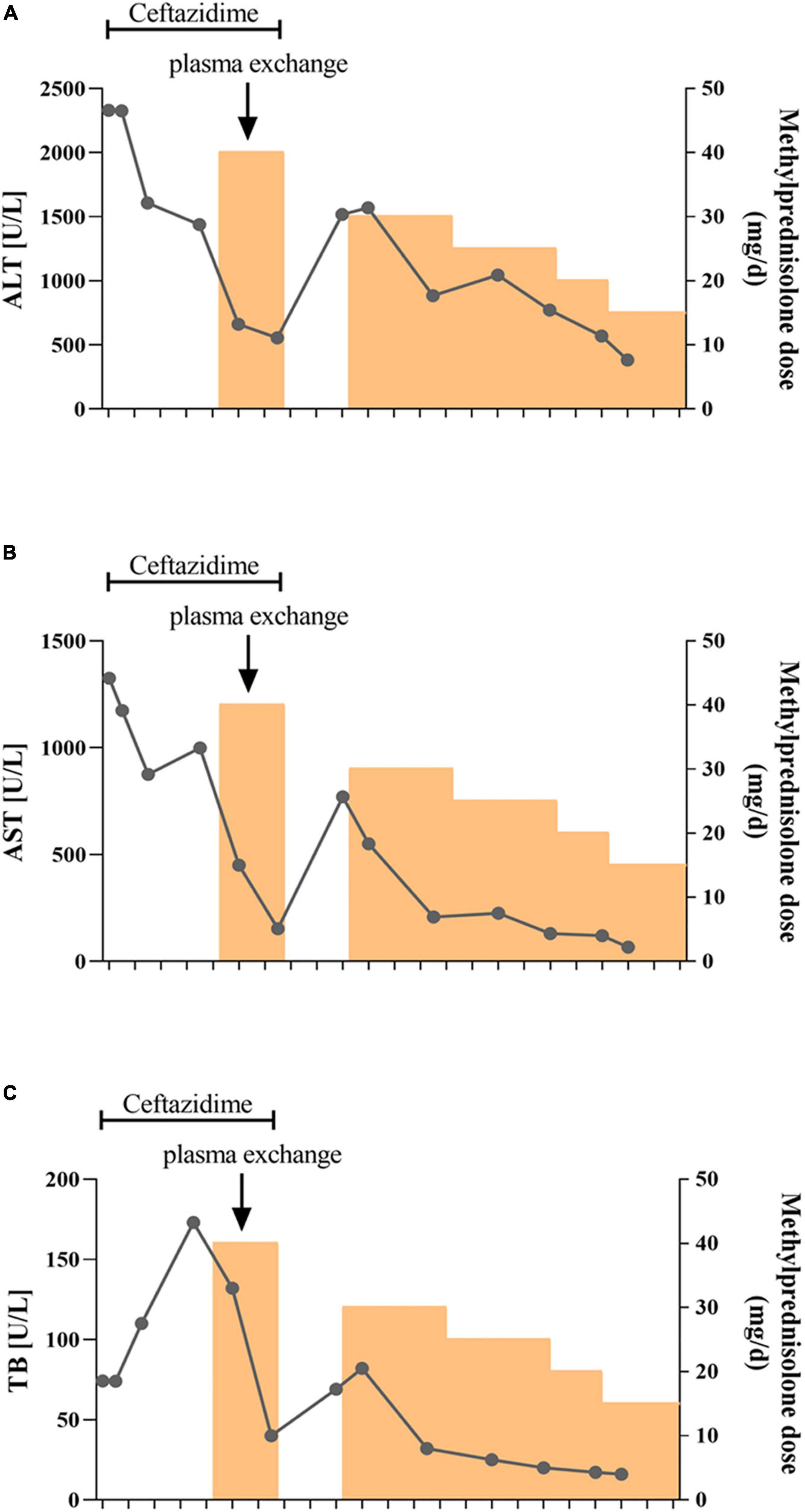
Figure 4. Schematic diagram of the transaminase and bilirubin fluctuations during treatment. The alanine aminotransferase (A) , aspartate aminotransferase (B) , and total bilirubin (C) levels were tested every 3–5 days during treatment. Ceftazidime was used for the first two weeks followed by methylprednisolone treatment. The patient also received plasma exchange once due to rapid aggravation of jaundice.
As a whole, this case report follows the CARE Case Report Guidelines. Our adherence to these reporting guidelines has been listed in the Supplementary material .
Patient in this case report presented with gastrointestinal symptoms of abdominal pain and vomiting, elevated transaminases, and jaundice at the onset. He was healthy before disease onset and had no history of taking certain drugs or exposure to poisons. The results of viral hepatitis serology screen were negative, indicating that this case was consistent with the definition of a Probable case by the WHO ( 15 ).
Viral hepatitis due to hepatitis viruses or to occasionally hepatotropic viruses is one of the main etiological groups of acute or chronic hepatitis in children ( 22 ). Recently, with the popularity of hepatitis virus vaccines and the improved detection methods, non-hepatotropic virus (including CMV, EBV, and coxsackievirus) infection-induced acute hepatitis in young children has gained increased attention ( 23 ). In the recent outbreak of acute and severe hepatitis of unknown etiology in children, some clues suggested that adenovirus and SARS-CoV-2 might be the etiologies ( 24 ). However, until now, there is still a lack of definite evidence on associated mechanisms or causative relationships. As for this case we reported, evidence of adenovirus and SARS-CoV-2 infection was negative, indicating that such two virus infections are unlikely. However, both blood sample and liver tissues were tested positive for torque teno virus (TTV) according to mNGS analysis. TTV is a small single-stranded DNA virus that was discovered in the late 20th century. TTV has an extremely high prevalence worldwide, which is frequently detectable in healthy infants, healthy adults, patients with HBV/HCV, and cases of hepatitis without an obvious viral agent ( 25 – 27 ). Previous studies indicate that TTV is hepatotropic, and TTV infection-induced liver damage could present a diverse spectrum of pathological damage, including ballooning, acidophilia degeneration, formation of apoptosis bodies and focus of necrosis, and mild inflammation in the lobule and portal area ( 28 ). Nevertheless, there was no significant difference of TTV DNA positivity in patients with hepatitis when compared to that in healthy controls ( 28 ). Moreover, due to the lack of reliable cell culture and animal models, the pathogenicity of TTV remains controversial ( 27 ). Notably, most studies assumed that TTV is non-pathogenic. A published article by Okamura et al. reported that genotype 1a of TTV might play a role in the pathogenesis of fulminate hepatitis and chronic liver disease in children liver disease of unknown etiology ( 29 ), indicating that some specific genotype of TTV may be pathogenic in children. In the current case, TTV was monitored by mNGS, and its expression level is not high enough to identify the genotype. In addition, no TTV virus particles were observed in liver tissues by electron microscopy. Therefore, before well-grounded evidence emerges, we cannot determine the pathogenicity of TTV. On the contrary, the immunohistochemical analysis showed that IgG4 staining was negative, but the majority of infiltrating inflammatory cells were CD8 + lymphocytes. More importantly, the response of this patient to hormones treatment was good, implying that it is more likely to be an immune injury. Given the uncertainty about the pathogenicity of TTV, we consider that TTV is more likely a co-factor responsible for the inappropriate immune response.
Besides infectious factors, given the immune-mediated hepatic damage, as well as the well-response to methylprednisolone treatment, AIH could not be ruled out yet in this case report. Even though majority of common autoimmune hepatitis-related antibodies were negative, we might also have to consider the possibility of autoantibody-negative autoimmune hepatitis. It has been suggested that seronegative AIH accounts for less than 5% of all adult patients with AIH ( 30 ). However, little information is available in children. A retrospective study conducted by Islek et al. found that seven of 54 patients with AIH under 18 years of age were seronegativity persisted during treatment ( 31 ), indicating that seronegative AIH could not be ignored in clinical practice. As for this child in our report, he has no documented history of other autoimmune diseases and no typical histologic features of AIH. We considered that the mild interfacial hepatitis is not enough to explain his severe liver damage. Thus, before more substantial evidence emerges, seronegative autoimmune hepatitis cannot be determined.
There were some limitations in the exploration of etiologies in this study. First, the depth and breadth of laboratory testing are not comprehensive enough. Some investigations such as multiplex PCR for respiratory viruses, multiplex PCR gastrointestinal viruses panel, and stool culture for common bacterial enteropathogens are not performed, which might cause the loss of some clinical data. Second, the detection of pathogens and histology was performed after the condition was stable, which is not conducive to the search for etiology. Similarly, no typical manifestations of the acute phase were observed in liver biopsy, which might influence the clinical assessment. Third, this case is a single case report. Continuous follow-up is required to further clarify the clinical characteristics and etiology of such liver diseases in young children.
Collectively, we report a case with severe acute hepatitis of unknown origin. Based on laboratory examinations and treatment response, we suspect the etiology of this case may be due to an immune injury in which TTV might play a role as a co-factor. We suggest liver biopsy for patients with severe acute hepatitis of unknown origin and trial steroid therapy when the liver damage is similar to autoimmune hepatitis. By reporting this case, we expect to add further support to the notion that immune dysfunction might be the main cause of liver damage in children with acute hepatitis of unknown origin.
Data availability statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Children’s Hospital of Chongqing Medical University (2022-177). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
JC and Z-ZZ were involved in the interpretation of the data, conceptualized the manuscript, and participated in the revisions. JC, Z-ZZ, and FL provided the financial support. JZ, TA, and KZ were involved in the acquisition of all the clinical data. Y-JZ, H-YG, and Q-QT were involved in the drafting of the manuscript and analyzed the data. B-YX, QW, and A-LH participated to the revisions. All authors approved the final version of the manuscript.
This study was supported by the Program for Youth Innovation in Future Medicine, Chongqing Medical University (Z-ZZ); Chongqing Natural Science Foundation (Grant no. cstc2021jcyj-msxmX0139 to Z-ZZ); and the Medical Scientific Research Project of Chongqing (Grant no. 2021MSXM067 to FL).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.975628/full#supplementary-material
1. Larson-Nath C, Vitola B. Pediatric acute liver failure. Crit Care Clin. (2022) 38:301–15. doi: 10.1016/j.ccc.2021.11.015
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Quirós E, Piédrola G, Maroto C. GB virus C in patients with liver disease of unknown etiology. J Clin Lab Anal. (2000) 14:70–2. doi: 10.1002/(SICI)1098-2825(2000)14:2<70::AID-JCLA6>3.0.CO;2-M
CrossRef Full Text | Google Scholar
3. Chiou FK, Logarajah V, Ho CWW, Goh LS, Karthik SV, Aw MM, et al. Demographics, aetiology and outcome of paediatric acute liver failure in Singapore. Singapore Med J. (2021). [Epub ahead of print]. doi: 10.11622/smedj.2021138
4. Narkewicz MR, Dell Olio D, Karpen SJ, Murray KF, Schwarz K, Yazigi N, et al. Pattern of diagnostic evaluation for the causes of pediatric acute liver failure: an opportunity for quality improvement. J Pediatr. (2009) 155:801–6.e1. doi: 10.1016/j.jpeds.2009.06.005
5. Raimondo G, Locarnini S, Pollicino T, Levrero M, Zoulim F, Lok AS, et al. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. (2019) 71:397–408. doi: 10.1016/j.jhep.2019.03.034
6. Mrzljak A, Tabain I, Premac H, Bogdanic M, Barbic L, Savic V, et al. The role of emerging and neglected viruses in the etiology of hepatitis. Curr Infect Dis Rep. (2019) 21:51. doi: 10.1007/s11908-019-0709-2
7. Styczynski J, Czyzewski K, Wysocki M, Gryniewicz-Kwiatkowska O, Kolodziejczyk-Gietka A, Salamonowicz M, et al. Increased risk of infections and infection-related mortality in children undergoing haematopoietic stem cell transplantation compared to conventional anticancer therapy: a multicentre nationwide study. Clin Microbiol Infect. (2016) 22:179.e1–10. doi: 10.1016/j.cmi.2015.10.017
8. Pelland-Marcotte MC, Hwee J, Pole JD, Nathan PC, Sung L. Incidence of infections after therapy completion in children with acute lymphoblastic leukemia or acute myeloid leukemia: a systematic review of the literature. Leuk Lymphoma. (2019) 60:2104–14. doi: 10.1080/10428194.2019.1573369
9. Koley S, Datta J, Sa SK, Tarafdar D. Scabies involving palms in older children and adults: a changing scenario. Int J Dermatol. (2021) 60:605–10. doi: 10.1111/ijd.15383
10. Yodoshi T, Orkin S, Arce-Clachar AC, Bramlage K, Xanthakos SA, Valentino PL, et al. Alternative etiologies of liver disease in children with suspected NAFLD. Pediatrics. (2021) 147:e2020009829. doi: 10.1542/peds.2020-009829
11. Hegarty R, Hadzic N, Gissen P, Dhawan A. Inherited metabolic disorders presenting as acute liver failure in newborns and young children: king’s College Hospital experience. Eur J Pediatr. (2015) 174:1387–92. doi: 10.1007/s00431-015-2540-6
12. Stravitz RT, Lefkowitch JH, Fontana RJ, Gershwin ME, Leung PS, Sterling RK, et al. Autoimmune acute liver failure: proposed clinical and histological criteria. Hepatology. (2011) 53:517–26. doi: 10.1002/hep.24080
13. Zhang C, Wu Y, Yuan S, Dou X, Sheng Q, Wang J, et al. Characteristics of drug-induced liver injury in northeast china: disease spectrum and drug types. Dig Dis Sci. (2020) 65:3360–8. doi: 10.1007/s10620-019-06030-6
14. Uk Health Security Agency. Increase In Acute Hepatitis Cases Of Unknown Aetiology In Children. (2022). Available online at: https://www.gov.uk/government/publications/hepatitis-increase-in-acute-cases-of-unknown-aetiology-in-children/increase-in-acute-hepatitis-cases-of-unknown-aetiology-in-children . (accessed May 16, 2022).
Google Scholar
15. World Health Organization [WHO]. Acute Hepatitis Of Unknown Aetiology In Children - Multi-Country. Geneva: World Health Organization (2022).
16. Sallam M, Mahafzah A, Şahin GÖ. On behalf of escmid study group for viral hepatitis-esgvh. hepatitis of unknown origin and etiology (Acute Non HepA-E Hepatitis) among children in 2021/2022: review of the current findings. Healthcare. (2022) 10:973. doi: 10.3390/healthcare10060973
17. Baker JM, Buchfellner M, Britt W, Sanchez V, Potter JL, Ingram LA, et al. Acute hepatitis and adenovirus infection among children - Alabama, October 2021–February 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:638–40. doi: 10.15585/mmwr.mm7118e1
18. Kiwan P, Kamel R, Kamel R, Hamod D. Adenoviral hepatitis in an immunocompetent child: case report. J Pediatr Neonatal Care. (2017) 7:00290. doi: 10.15406/jpnc.2017.07.00290
19. Hierholzer JC. Adenoviruses in the immunocompromised host. Clin Microbiol Rev. (1992) 5:262–74. doi: 10.1128/CMR.5.3.262
20. Munoz FM, Piedra PA, Demmler GJ. Disseminated adenovirus disease in immunocompromised and immunocompetent children. Clin Infect Dis. (1998) 27:1194–200. doi: 10.1086/514978
21. Pan American Health Organization. Acute, Severe Hepatitis Of Unknown Origin In Children. 10 May 2022. (2022). Available online at: https://www.paho.org/en/documents/acute-severe-hepatitis-unknown-origin-children-10-may-2022 . (accessed May 16, 2022).
22. Maggiore G, Socie G, Sciveres M, Roque-Afonso AM, Nastasio S, Johanet C, et al. Seronegative autoimmune hepatitis in children: spectrum of disorders. Dig Liver Dis. (2016) 48:785–91. doi: 10.1016/j.dld.2016.03.015
23. Tsunoda T, Inui A, Iwasawa K, Oikawa M, Sogo T, Komatsu H, et al. Acute liver dysfunction not resulting from hepatitis virus in immunocompetent children. Pediatr Int. (2017) 59:551–6. doi: 10.1111/ped.13249
24. The Lancet Infectious Diseases. Explaining the unexplained hepatitis in children. Lancet Infect Dis. (2022) 22:743. doi: 10.1016/S1473-3099(22)00296-1
25. Hsieh SY, Wu YH, Ho YP, Tsao KC, Yeh CT, Liaw YF. High prevalence of TT virus infection in healthy children and adults and in patients with liver disease in Taiwan. J Clin Microbiol. (1999) 37:1829–31. doi: 10.1128/JCM.37.6.1829-1831.1999
26. Reshetnyak VI, Maev IV, Burmistrov AI, Chekmazov IA, Karlovich TI. Torque teno virus in liver diseases: on the way towards unity of view. World J Gastroenterol. (2020) 26:1691–707. doi: 10.3748/wjg.v26.i15.1691
27. Webb B, Rakibuzzaman A, Ramamoorthy S. Torque teno viruses in health and disease. Virus Res. (2020) 285:198013. doi: 10.1016/j.virusres.2020.198013
28. Hu ZJ, Lang ZW, Zhou YS, Yan HP, Huang DZ, Chen WR, et al. Clinicopathological study on TTV infection in hepatitis of unknown etiology. World J Gastroenterol. (2002) 8:288–93. doi: 10.3748/wjg.v8.i2.288
29. Okamura A, Yoshioka M, Kikuta H, Kubota M, Ma X, Hayashi A, et al. Detection of TT virus sequences in children with liver disease of unknown etiology. J Med Virol. (2000) 62:104–8. doi: 10.1002/1096-9071(200009)62:1<104::AID-JMV16>3.0.CO;2-P
30. Mieli-Vergani G, Vergani D, Baumann U, Czubkowski P, Debray D, Dezsofi A, et al. Diagnosis and management of pediatric autoimmune liver disease: ESPGHAN hepatology committee position statement. J Pediatr Gastroenterol Nutr. (2018) 66:345–60. doi: 10.1097/MPG.0000000000001801
31. Islek A, Keskin H. Seronegative autoimmune hepatitis in children: a single-center experience. Acta Gastroenterol Belg. (2021) 84:305–10. doi: 10.51821/84.2.305
Keywords : case report, unexplained acute hepatitis, torque teno virus, immune injury, methylprednisolone
Citation: Zhou Y-J, Gu H-Y, Tang Q-Q, Li F, Zhu J, Ai T, Zhu K, Xu B-Y, Wang Q, Huang A-L, Chen J and Zhang Z-Z (2022) Case report: A case of severe acute hepatitis of unknown origin. Front. Pediatr. 10:975628. doi: 10.3389/fped.2022.975628
Received: 22 June 2022; Accepted: 12 September 2022; Published: 05 October 2022.
Reviewed by:
Copyright © 2022 Zhou, Gu, Tang, Li, Zhu, Ai, Zhu, Xu, Wang, Huang, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Chen, [email protected] ; Zhen-Zhen Zhang, [email protected]
† These authors have contributed equally to this work
This article is part of the Research Topic
Case Reports in Pediatric Gastroenterology, Hepatology and Nutrition 2022

Clinical Cases in Hepatology
- © 2022
- Nora V. Bergasa 0
Department of Medicine, H+H/Metropolitan Physician Affiliate Group of New York, New York, USA
You can also search for this editor in PubMed Google Scholar
Features case presentations of how to appropriately approach treating patients with liver disease
Describes the epidemiological characteristics of a variety of liver diseases
Details techniques for interpreting experimental data and techniques for conducting clinical trials
22k Accesses
5 Citations
2 Altmetric
This is a preview of subscription content, log in via an institution to check access.
Access this book
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
About this book
This book provides a comprehensive resource for clinical hepatology. It details the systematic approach to patients with liver disease in outpatient and inpatient medical settings. A variety of case studies in hepatology including chronic viral hepatitis, and metabolic, autoimmune, and alcohol related liver disease are presented. The book enables the reader to develop a thorough understanding of the clinical presentation, natural history, epidemiology, genetics, and therapeutic options for the liver diseases that clinicians must recognize and manage from the first encounter with the patient through the years of follow up.
Similar content being viewed by others

The Hepatological Curiosities

Diseases of the Liver

- Ischemic hepatitis
- Autoimmune hepatitis
- Fatty liver disease
- Biliary cirrhosis
Table of contents (15 chapters)
Front matter.
Nora V. Bergasa
Approach to the Patient with Liver Disease
Primary biliary cholangitis, autoimmune hepatitis, primary sclerosing cholangitis, chronic hepatitis c, chronic hepatitis b, alcohol induced liver disease, nonalcoholic fatty liver disease, alpha-1-antitrypsin deficiency, hemochromatosis, wilson’s disease, tumors of the liver, drug induced liver injury, complications of liver disease, back matter, editors and affiliations, about the editor.
Dr. Nora V. Bergasa is Professor of Medicine Emerita at New York Medical College and Chairman Emerita of the Department of Medicine at New York City (NYC) Health + Hospitals (H+H)/ Metropolitan and a retired member of the Physician Affiliate Group of New York. She serves as Hepatology Attending at NYC, H+H/Woodhull. She graduated from medical school from the Universidad Central del Este in the Dominican Republic, did her internal medicine residency and gastroenterology fellowship at the State University of New York (SUNY) at Downstate, and completed her clinical and research training in hepatology in the Liver Diseases Section of the National Institutes of Health. She has conducted basic and clinical investigations in several areas of hepatology, including cholestasis. Her major research field has concerned the pruritus of liver disease for which she is internationally recognized.
Bibliographic Information
Book Title : Clinical Cases in Hepatology
Editors : Nora V. Bergasa
DOI : https://doi.org/10.1007/978-1-4471-4715-2
Publisher : Springer London
eBook Packages : Medicine , Medicine (R0)
Copyright Information : Springer-Verlag London Ltd., part of Springer Nature 2022
Hardcover ISBN : 978-1-4471-4714-5 Published: 26 October 2021
eBook ISBN : 978-1-4471-4715-2 Published: 25 October 2021
Edition Number : 1
Number of Pages : XII, 497
Number of Illustrations : 3 b/w illustrations, 34 illustrations in colour
Topics : Hepatology
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Quick links
- Find funding
- Find publications
- News centre
- Outcomes of funding rounds
- Sapphire Login
- Impact case studies
Protecting against hepatitis: Case study
Over the past century and throughout the world, viral hepatitis emerged as a significant public health issue afflicting hundreds of millions of people and causing severe ill health, liver damage, cancer and death. 1
In collaboration with international colleagues, the National Health and Medical Research Council (NHMRC) funded researchers at The University of Western Australia, Fairfield Hospital's Epidemiological Research Unit (now the Burnet Institute) and Monash University made key contributions to the identification and control of viral hepatitis.
A landscape format version of this case study is available as a PDF (see the Downloads section below).
'Hepatitis' means liver inflammation and its health consequences may include fever, fatigue, nausea, vomiting, jaundice, cirrhosis of the liver and liver cancer. Hepatitis is most commonly caused by certain viruses. However, there are other causes including heavy alcohol use, some medications, toxins, other infections and autoimmune diseases.
Disease outbreaks resembling viral hepatitis have been known since ancient times 2 and have especially occurred during wars. 3 By the time of the Second World War, however, medical research had advanced to a stage that it was being conducted internationally on such outbreaks.
By 1946, the evidence indicated that there were at least 2 types of viral hepatitis. 4 One of these was infectious and caused epidemics, had a short incubation period and was transmitted through ingestion of contaminated food or water. The other was most commonly transmitted through exposure to infected blood and had a longer incubation period. By 1953, the World Health Organization (WHO) had named these types (respectively) hepatitis A and hepatitis B (HB). 5
In 1957, NHMRC recommended that hepatitis be proclaimed a notifiable disease in all states of Australia. 6
A notifiable disease is required by law to be reported to government authorities, to allow authorities to monitor the disease and provide early warning of possible outbreaks.
However – and unlike other major infectious diseases – at this time the causative agent(s) for hepatitis had yet to be discovered 7 and no blood tests for hepatitis were available. 8 Medical research was required before progress could be made in preventing and controlling this disease.
Grants and investment
NHMRC-funded research at 2 different institutions contributed to international efforts to identify and control the hepatitis A and B viruses (HAV and HBV).
Commencing in 1950, Robert Kirk, a population geneticist at The University of Western Australia, received NHMRC grants to support basic research on blood proteins.
Also commencing in 1950, NHMRC funded a team of researchers based at the Epidemiological Research Unit of the Fairfield Infectious Diseases Hospital in Melbourne.
The unit included Alan Ferris, Ian Gust, Noreen Lehmann, Jacov Kaldor and Stephen Locarnini. Later, Ferris moved to Monash University and worked with the Fairfield team from there, along with electron microscopist Geoff Cross.
Results – hepatitis B
Kirk was investigating the different forms (polymorphisms) of various types of blood protein. The best known of these were the red blood cell antigens (that is, blood groups: A, B, AB, O) but Kirk was also interested in haptoglobins, transferrins, gamma globulins 10 and lipoproteins. 11
An antigen is a substance that generates an immune response.
This work was inherently collaborative – there were too many polymorphisms for a single researcher to investigate 11 – and involved both other Australian 11 and overseas researchers. 10 Consequently, Kirk shared blood samples collected in Australia and South-East Asia 8 with researchers at the US National Institutes of Health (NIH). 11
Kirk had thought that his work might lead to an improved understanding of rheumatoid arthritis. However, in 1964, NIH researchers discovered that one of Kirk's samples contained a novel protein 11 which, by 1968, had been confirmed to be the surface protein of HBV. 13
This protein became known as 'Australia antigen' and its discovery provided a test for the presence of HBV. An immediate and important use for such a test was to confirm the safety of blood donations. The protein was also used to create the world’s first HBV vaccine, which was patented in 1972. 14
The routine testing of blood donors to detect Australia antigen began recently ... Use of the test offers some promise of controlling the transmission of serum hepatitis through blood transfusion. If the test indicates the presence of Australia antigen, the blood concerned is rejected as unsuitable for use for blood transfusion purposes. Director-General of Health Annual Report 1970–71 15
Results – hepatitis A
In 1965, when Gust commenced work at Fairfield Hospital, it was one of the largest infectious diseases hospitals in the world. Viral hepatitis was a very common disease in Melbourne at the time and, since Fairfield admitted 700 to 800 patients with the disease each year, identifying and treating hepatitis was a focus for research efforts there. 16
In 1969, equipped with samples of Australia antigen donated to Gust by NIH-funded groups, researchers at Fairfield began seeking to identify HAV, which they could now distinguish from HBV. Since HAV was known to be shed in faeces, they tested faecal extracts from patients with naturally acquired acute non-HB using blood serum that was thought to contain HAV antibodies. This test caused any HAV particles to cluster, making them visible when viewed through an electron microscope. 16 Using this technique, in March 1974 they identified HAV, independently of but just after a team at NIH, who reported their findings in December 1973. 5
An antibody is a blood protein that binds to a specific antigen.
In 1976, Gust joined the NIH team and brought with him faecal and blood samples selected by Lehmann from a group of Fairfield patients infected with HAV. The strain of HAV contained in one of these samples (code named HM175) was found to be particularly easy to work with. It was the first HAV strain to be isolated in cell cultures and sequenced and remains a standard HAV strain used in many laboratories today. Eventually, HM175 became the basis for the first commercial hepatitis A vaccine (Havrix™) which was licensed in 1991. 16 , 17
In 1979, Locarnini and his colleagues reported the discovery of an HAV-specific antibody and a simple technique for the detection of HAV antibodies. 18
Health outcomes and impact
Testing for Australia antigen quickly became routine in blood banks in many countries. 14 The risk of acquiring hepatitis B from transfusion in Australia is now considered negligible, that is, less than 1 in 1 million per unit transfused. 19 The test for antibodies developed by the Fairfield team remains the standard approach used internationally. 20
Not only was the first-generation HB vaccine produced using Australia antigen but so are current vaccines. To prevent HBV infection, WHO recommends that all infants receive the HB vaccine as soon as possible after birth followed by 2 or 3 further doses to complete the vaccination series. Protection lasts at least 20 years and is probably lifelong. 21
Vaccines for hepatitis A and B are both very effective and very safe. 20 , 21 In those countries where these vaccines are widely available, these diseases have virtually disappeared. 16
In 1983, Gust participated in a WHO Consultative Group that helped to shape the WHO Programme for the Control of Viral hepatitis. 22 In 1986, Gust and 10 other international experts established an independent International Task Force on hepatitis B whose objective was to demonstrate that HB vaccination could be successfully added to WHO's Expanded Program on Immunization (EPI). The Task Force raised funding from a range of sources, including the Australian Government, to help it initiate 5 model immunisation programs – in Indonesia, Thailand, China, Kenya and Cameroon. 23
The project in Lombok, Indonesia (1987–1991), was the first demonstration of the usefulness of HB vaccination in a developing country's immunisation program and contributed to the models used now by international aid organisations. 24 As a consequence of the project, in 1992 the HB vaccine was recommended for inclusion in the WHO EPI. 23
Researcher profiles
Dr robert kirk.
Robert Louis Kirk (1921–2010) was a chemist and zoologist at The University of Western Australia for over a decade, then became head of the Human Biology Department at the John Curtin School of Medical Research at The Australian National University (ANU, 1967–1987). In 1964, Kirk was appointed the national convenor of the International Biological Program – Human Adaptability study and was on the Australian Academy of Science subcommittee responsible for the study.
The blood samples that Kirk collected are a key resource managed by the ANU's National Centre for Indigenous Genomics.
Allan Ferris
Allan Aveling Ferris (1912–1997) was a member of the Australian Army Medical Corps during WWII. From 1940 to 1945, he was Officer-in-charge of the 104 th Mobile Bacteriological Laboratory in New Guinea and later Pathologist to the 2/2 Australian General Hospital. He was Assistant Director of the Public Health Laboratory and Senior Lecturer in Microbiology at The University of Melbourne (1945–1948) then a pathologist and Director of the Epidemiological Research Unit at Fairfield Hospital (1948–1970) and reader in microbiology at Monash University (1970–1977). Ferris was President of the Australian Society for Microbiology (1974–1975).
Professor Ian Gust AO
Ian David Gust (1941-) studied medicine at the Universities of Melbourne and London, then became Director of the Virology Laboratory at Fairfield Hospital (1970–1990). Gust was Chief Commonwealth Medical and Scientific Adviser on AIDS and was the inaugural Director of the Macfarlane Burnet Centre for Medical Research (now the Burnet Institute). Gust was Director of Research and Development at CSL Ltd (1990–2000) and was President of the Australian Society of Microbiology (1998–2000). In 1992, Gust was appointed an Officer of the Order of Australia for service to public health, particularly in the prevention of hepatitis and AIDS.
Noreen Lehmann BSc
Noreen I Lehmann (1932-2020) graduated in science from The University of Melbourne in 1957 and in 1958 was recruited to join the Epidemiological Research Unit at Fairfield Hospital. At Fairfield, Lehmann was responsible for isolating a wide range of respiratory and enteric (intestinal) viruses as well as viruses responsible for childhood rashes. She was the first person in Australia to isolate rubella virus in cell culture and to establish a sensitive assay for rubella antibodies. She also undertook quality assurance of rubella tests performed by other laboratories.
Professor Stephen Locarnini AM
Stephen A Locarnini is Head of Unit, Research and Molecular Development (1998–2023) and Director Laboratory Services (1990–1998) at the Victorian Infectious Diseases Reference Laboratory (VIDRL). VIDRL was initially the Department of Pathology at Fairfield Hospital and is now part of the Peter Doherty Institute for Infection and Immunity. Locarnini is current Director of the WHO Collaborating Centre for Virus Reference and Research which is based at VIDRL.
In 2023, Locarnini was appointed a Member of the Order of Australia for significant service to medicine as a virologist, and to medical research.
Dr Geoff Cross
Geoff Cross was an electron microscopist at Monash University.
Dr Jacov Kaldor
Jacov Kaldor was Senior Biochemist within the Biochemistry Department at Fairfield Hospital.
Professor Ron Lucas
Charles Ronald Lucas (1932–2009) graduated in Medicine from The University of Melbourne, then commenced work as a physician at Fairfield Hospital in 1964, where he remained until his retirement in the early 1990s.
This case study was developed with input from Professor Ian Gust and in partnership with the Burnet Institute, The University of Western Australia and Monash University.
The information and images from which NHMRC Impact Case Studies are produced may be obtained from sources including our case study partner, NHMRC's internal records and publicly available materials.
The following sources were consulted for this case study:
- Division of Viral Hepatitis, National Center for HIV, Viral Hepatitis, STD, and TB Prevention. Viral Hepatitis. Centres for Disease Control and Prevention. Atlanta, United States. 19 July 2021 .
- Thomas E, Yoneda M, Schiff ER. Viral hepatitis: past and future of HBV and HDV. Cold Spring Harbor Perspectives in Medicine. 2015; 5(2):a021345
- Trepo C. A brief history of hepatitis milestones. Liver International. 2014; 34:29-37
- Maccallum FO. Homologous Serum Hepatitis. Proceedings of the Royal Society of Medicine. 1946; 39(10):655-7
- Feinstone SM. History of the discovery of the hepatitis A virus. Cold Spring Harbour Perspectives in Medicine 2019; 9:a031740
- National Health and Medical Research Council. Report of the 43rd Session of the National Health and Medical Research Council. Australia: NHMRC; May 1957, Volume 1, p16
- Other types of hepatitis viruses have subsequently been identified: hepatitis C (1989), hepatitis D (1977) and hepatitis E (1978).
- National Health and Medical Research Council. National Health and Medical Research Council: Report for 1970. Parliamentary Paper No. 122. The Parliament of the Commonwealth of Australia: 1971, p203
- National Health and Medical Research Council. National Health and Medical Research Council: Report for 1971. Parliamentary Paper No. 242. The Parliament of the Commonwealth of Australia: 1972, p180
- National Health and Medical Research Council. Report of the 51st Session of the National Health and Medical Research Council. Australia: NHMRC; May 1961, Volume 1, p17
- Blumberg BS. Hepatitis B—The hunt for a killer virus. Princeton University Press. 2003. p79
- National Health and Medical Research Council. Report upon the work done under the Medical Research Endowment Act during the year 1959. Commonwealth of Australia: 1960, pp16-17
- Gust ID. Recent developments in hepatitis A. Pathology. 1978; 10(4):299-306
- Blumberg BS. Australia antigen and the biology of hepatitis B. Science. 1977; 197(4298):17-25
- Director-General of Health. Annual Report for year 1970-71. Parliamentary Paper No. 191. The Parliament of The Commonwealth of Australia. 1971, p92
- Gust ID. Viral hepatitis: Some reflections on a personal journey. Human Vaccines. 2009; 5(5):357-360
- Gust I, Kennett M. Generous mentor to two generations of scientists and physicians. The Sydney Morning Herald. Obituaries. Noreen Lehmann 1932-2020. 15 June 2020
- Locarnini SA, Coulepis AG, Stratton AM, Kaldor JA, Gust ID. Solid-phase enzyme-linked immunosorbent assay for detection of hepatitis A-specific immunoglobulin M. Journal of Clinical Microbiology. 1979; 9(4):459-65
- Australian Red Cross Lifeblood. Hep B transfusion risk lower. 19 January 2017.
- World Health Organization. Hepatitis A fact sheet. 24 June 2022 .
- World Health Organization. Hepatitis B fact sheet. 24 June 2022 .
- National Health and Medical Research Council. Report of the Ninety-sixth session: 26-27 October 1983. NHMRC: Canberra, October 1983, p504
- Ruff TA, Muller N, Gust ID. Hepatitis B control: lessons from the International Task Force on Hepatitis B Immunization and the Lombok Hepatitis B Model Immunization Project. Progress in Liver Diseases. 1993; 11:179-201
- Van Der Weyden MB. Bench‐to‐bedside research in Australian research institutes: a snapshot. Medical Journal of Australia. 2003; 179(11):603-10

Related resources
- Targeted Calls for Research

10 of the best, 10 years on: Reversing the effects of diabetes

Reducing trips and slips: healthy exercises to prevent falls as we age
- Ideas Grants 2022 Peer reviewer briefing webinar introductions and presentation
- Indigenous Research Excellence Criteria video transcript
- Funding agreement and deed of agreement
- Institution approvals
- Institutional Annual Compliance Report
- Vary your grant
- Financial reporting
- Progress, final and additional reporting
- Direct Research Cost Guidelines
- Previous Personnel Support Package rates
- Previous Salary Support Package rates
- Institutional approvals and grant conditions
- Funding Agreement
- Financial Reports
- Scientific reporting and milestones
- Direct Research Costs and Personnel and Salary Support Packages
- Eligibility
- Peer review
- Research funding data
- Analysis of Australian health and medical research publications
- Medical Research Future Fund
- International Collaborative Health Research Funding
- Funding calendar
- Working together to support research
- Statements of Expectations
- Capacity and capability building and strengthening
- Health Advice and Publications
- Health effects of water fluoridation
- Preventing infection
- How NHMRC develops public health guidelines
- Complementary medicines
- Electronic cigarettes
- Myalgic Encephalomyelitis and Chronic Fatigue Syndrome
- Lead blood levels
- Recreational Water Quality Advisory Committee
- Australian Guidelines to Reduce Health Risks from Drinking Alcohol - Public submissions
- National Statement on Ethical Conduct in Human Research
- Genomics resources for clinicians and researchers
- Vitamin K for newborns
- Guidelines for Guidelines
- COVID-19 impacts
- Clinical trials reform
- Determining whether an embryo model is regulated by the ERLC
- Information for applicants
- Information for Licence Holders
- Mitochondrial Donation Licensing Scheme
- Training and Quality Assurance activities
- Import and export of cell lines
- Database of Licences issued
- Training and Quality Assurance
- Use of animals in NHMRC funded research
- The Human Research Ethics Applications (HREA)
- Ethical issues and further resources
- National Certification Scheme
- Human research ethics committees
- Clinical ethics
- Ethical guidelines for Assisted Reproductive Technology
- Ethical guidelines for research with Aboriginal and Torres Strait Islander Peoples
- NHMRC ethical guidelines on organ and tissue donation and transplantation
- Guideline development
- International engagement
- Research quality
- Research translation
- Research impact
- Dementia research
- NHMRC Special Initiative in Mental Health
- NHMRC’s role in addressing health implications of environmental change
- Submission of Targeted Calls for Research online pathway
- NHMRC health priorities 2021–2024
- Framework for Identifying and Prioritising Targeted Calls for Research
- Targeted Calls for Research Prioritisation Criteria Rubric
- Guide for Proposing Targeted Calls for Research
- Administering Institution Policy
- NHMRC Gender Equity Strategy 2022-2025
- Statement on sex and gender in health and medical research
- Structural priority funding and gender equity
- Accountability and reporting
- Fifteenth edition
- Fourteenth edition
- Ten of the Best Archive
- Ten of the Best
- 2022 Research Excellence Awards
- 2023 Research Excellence Awards
- Health Innovation Advisory Committee 2015-2018
- Legislative basis to NHMRC
- Senior executive and leadership team
- Indigenous Research Ethics Guidelines Review Working Committee
- Natural Therapies Working Committee
- Publications
- Probity Event - Additional Guidance
- NHMRC Complaints Policy
- Temporary Employment Register
- Working at NHMRC
- How we select our people
- Indigenous internship program
- Freedom of information
- Child Safe Policy
- Annual reports and corporate plans
- Consumer and community engagement
- About the review
- Consumer Statement review
- Consumer and community representative involvement in the peer review process of Targeted Calls for Research
Complete Your CE
Course case studies, external link, this link leads outside of the netce site to:.
While we have selected sites that we believe offer good, reliable information, we are not responsible for the content provided. Furthermore, these links do not constitute an endorsement of these organizations or their programs by NetCE, and none should be inferred.
Viral Hepatitis
Course #94994 - $30 -
#94994: Viral Hepatitis
Your certificate(s) of completion have been emailed to
- Back to Course Home
- Review the course material online or in print.
- Complete the course evaluation.
- Review your Transcript to view and print your Certificate of Completion. Your date of completion will be the date (Pacific Time) the course was electronically submitted for credit, with no exceptions. Partial credit is not available.
HEPATITIS A
Patient A is 19 years of age and a college sophomore who presented to her physician's office with mild jaundice. The patient reports being in good health until a week before, at which time she began having flu-like symptoms of headache, low-grade fever, nausea, loss of appetite, and malaise. She self-treated the fever with acetaminophen. The symptoms persisted. Upon awakening this morning, she noticed that her eyes were yellow. She therefore contacted her physician's office.
In response to her physician's questions, she indicated that her urine has been darker than usual and she has been experiencing joint pain for the last three days. She also acknowledged that her stools have been lighter than usual.
Her medical history is positive for mild exercise-induced asthma, for which she uses a prophylactic bronchodilating inhaler. Her only other routine medication is a daily vitamin/mineral supplement. She reports no surgeries. Family history is positive for cardiovascular disease (father and both sets of grandparents) and breast cancer (mother).
Other significant history includes that she was immunized against hepatitis B at 12 years of age and she recently participated in a two-week mission trip to Central America. Although she was very cautious about the foods she ingested during the mission trip, the patient indicated that a primary recreational activity after the day's work was to swim in the lagoon near the village. The lagoon was fed both by the stream in which the natives washed their clothes and the adjacent bay. Rainfall averaged 2–3 inches per day. Patient A returned to the United States five weeks ago.
Physical examination revealed a well-developed, well-nourished female who was alert and oriented. Her temperature was 99.7°F; other vital signs were within normal limits. Abnormal physical findings included mild icterus of sclera and skin, abdominal tenderness, hepatomegaly, and palpable spleen. Results of laboratory tests are indicated in Table 2 .
PATIENT A LABORATORY TEST RESULTS
Case Study Discussion
Patient A has presented with classic signs and symptoms of acute hepatitis. Based on her past history, travel, and exposure history, the most likely diagnosis is acute hepatitis A infection. The hepatic chemistry profile and serologic studies confirm this diagnosis. Exposure probably resulted from accidental ingestion of contaminated water while swimming in the lagoon.
Because acute viral hepatitis is usually a self-limited disease and Patient A is alert with no evidence of coagulopathy, she can be managed as an outpatient with close follow-up. Liver enzymes and PT should be monitored every 5 to 7 days for the first two weeks, then, if convalescence is satisfactory, at 14-day intervals until function test results have returned to normal. Bed rest is not indicated, but the patient should avoid strenuous activity. She should eat a well-balanced diet and abstain from alcohol for the duration of the illness. Because acetaminophen can be toxic to the liver, ibuprofen would be a better alternative for controlling fever. No other alterations in the patient's medications are necessary at this point. If nausea precludes the patient from ingesting food and fluids, IV replacement of fluids and electrolytes may be necessary. In the event the patient develops bleeding tendencies or signs of encephalopathy, she should immediately be taken to the hospital or her physician's office.
Hepatitis A virus is a reportable disease. The health department should be informed of the case immediately. Because the exposure probably occurred outside the geographical area, follow-up will be limited to those with similar exposure (i.e., persons who were also on the mission trip) and to her intimate and/or household contacts. A single dose of HAV immunoglobulin is recommended for close contacts. If immunoglobulin is not available, administration of hepatitis A vaccine may prevent illness or lessen the severity of the contact's symptoms if infection does occur. Immunoglobulin is not recommended for those who may have been exposed on the mission trip, as those exposures occurred more than two weeks prior to the diagnosis. Follow-up with these persons is primarily to determine if they too are experiencing symptoms and are possible sources of spreading the disease.
CHRONIC HEPATITIS C
Patient B is a paramedic, 48 years of age. Laboratory work obtained during his annual physical examination reveals hyperlipidemia; CBC, glucose, BUN, and electrolytes were within normal range. With the exception of his weight (15 lbs heavier than indicated for his height), his exam identifies no abnormalities.
After two months of a diet and exercise program, his cholesterol level is 256. Therefore, his physician elects to begin a lipid-lowering agent. A baseline liver profile is drawn prior to initiation of the medication. Because the patient is in a profession that is high-risk for bloodborne pathogen exposure, an HCV antibody test with reflex to qualitative HCV RNA is ordered. The liver profile reveals an AST of 226 Units/L and an ALT of 282 Units/L. HCV antibody and qualitative HCV RNA are both positive.
The physician reviews Patient B's history and medications. He has been a paramedic for 25 years. He was immunized against HBV in 1988. During his career, he has experienced several exposures to blood (usually blood splashes, but also two needlesticks from IV needles), most before the advent of Standard Precautions. His most recent exposure was two years ago. An HIV test six months post-exposure was negative. He does not recall hepatitis testing being performed at that time.
Patient B's surgical history includes a hernia repair in childhood and removal of skin lesions three times in the past eight years. He has had no transfusions. He is the widowed father of two teenage children. His wife died six years ago from ovarian cancer.
The patient has never smoked. He drinks about six beers per week and rarely drinks hard liquor. He denies any history of illegal drug use. Although the patient has no current prescription medications, he uses several herbal preparations including garlic, ginkgo, and an antioxidant preparation. The patient takes ibuprofen for pain, consuming 6 to 10 tablets (200 mg each) per month.
Although alcohol consumption and herbal antioxidants can both cause liver inflammation, the degree of his liver inflammation is much higher than would be expected from limited use of these two factors. The patient is diagnosed with chronic HCV infection.
In order to evaluate the extent of liver damage and determine an appropriate treatment plan, the physician orders an HCV RNA quantitative PCR and genotype as well as a PT. A gastroenterology specialist is consulted for further evaluation and possible liver biopsy and to co-manage the patient. The PT is within normal range. The liver biopsy reveals chronic inflammatory infiltration of the portal areas with minimal fibrosis. Genotype identifies the virus as type 3. HCV RNA viral load is 350,000 phages/cc.
Treatment options appropriate for HCV genotype 3, and the timing of therapy in relation to biopsy findings and anticipated progression of disease are discussed with Patient B. He is advised to eat a nutritious, balanced diet and abstain completely from alcohol. Although he is not currently sexually active, the patient is educated about the low but present risk of sexual transmission of HCV and how to minimize the risk of transmission. A test for HAV antibody is found to be negative. Immunization against HAV is also recommended, as acquiring an acute case of HAV in a patient with pre-existing chronic hepatitis can be much more serious that either condition alone. Because of uncertainty as to how recently he acquired the infection, the decision is made to defer treatment for three to four months while monitoring the course of the infection.
Four months after the initial diagnosis, there has been no improvement in Patient B's liver function tests: the ALT is 356 Units/L and AST is 418 Units/L. The HCV RNA remains detectable in the blood, and the viral load has increased to 450,000 phages/cc. He is advised to begin antiviral treatment; therapeutic options are discussed in relation to efficacy, potential drug interactions, and cost reimbursement priorities, bearing in mind that he is a treatment-naïve patient with no evidence of cirrhosis. The recommended course of therapy is the 12-week, two-drug oral regimen of sofosbuvir (400 mg) and velpatasvir (100 mg) for a duration of 12 weeks (reported SVR rate: 95% in clinical trials for genotype 3).
On treatment, the patient experiences transient nausea and persistent mild fatigue, but is compliant with the recommended duration of therapy. At 12 weeks, the ALT and AST are both within normal range and HCV RNA is undetectable. Treatment is discontinued and Patient B is asked to return in three months, six months, and one year after cessation of therapy to repeat HCV viral load and confirm a sustained virologic response.
HIV AND CHRONIC HBV COINFECTION
Patient C is a man, 32 years of age, with a history of injection drug use, who participated in a free HIV testing day. His screening test was found to be positive. A confirmatory test conducted at the health department was also positive. He has therefore been referred to the Infectious Disease Clinic of a large university medical center for follow up.
During his first visit, the patient indicates that he injected drugs off and on beginning at 19 years of age. His first two experiences with rehabilitation failed, but he has been "clean" for two years, since his best friend died of an overdose. He reports that he also snorted cocaine occasionally during the years he used injected drugs.
The patient's medical history includes a hospitalization for a motorcycle accident at age 24, with surgery on his right leg both on that admission and again about a year later. He received 2 units of blood during the first admission. The patient denies a history of heart disease, neurologic disorders, or endocrine disorders. He has had pneumonia both in adolescence and again last year.
The patient's parents are living and in good health. Grandparents all have hypertension, and maternal grandmother has type 2 diabetes. The patient smokes 1/2 to 1 pack of cigarettes per day and consumes two or three drinks per day. The patient's current medications include acetaminophen or ibuprofen as needed for leg pain and paroxetine for anxiety and depression.
Physical examination reveals no acute distress. Vital signs are within normal limits, and sclerae are non-icteric. Oral cavity is free from thrush and leukoplakia. Cervical lymph nodes are palpable but moveable and nontender. Heart sounds are normal; lungs are clear. Abdomen is soft; both liver and spleen are palpable. Neurologic exam is normal. The patient has full function in upper extremities and left leg; right leg has a slight decrease in strength and a moderate decrease in range of motion.
Initial laboratory tests ordered by the nurse practitioner (NP) include an HIV PCR viral load, a CD4 count, a CBC, a chemistry panel, and a liver profile. Because of the high incidence of HCV and/or HBV coinfection in persons whose HIV was acquired percutaneously, the NP also orders a hepatitis profile. Baseline tuberculosis testing is also recommended for persons with HIV who are entering care. Therefore, a T-SPOT interferon gamma release assay is also ordered. The patient is instructed to return in 72 hours to review lab results and formulate a treatment plan.
Upon his return, all results except the HIV PCR are available. His CD4 count is 246. Hematocrit is 44%, hemoglobin 15 gm/dL, and WBC is 3,800. The liver profile reveals an alkaline phosphatase of 143 Units/mL, AST 358 Units/L, ALT 383 Units/L, total bilirubin 1.2 mg/dL, and albumin 2.8 gm/dL. The remainder of the chemistry panel is unremarkable. Hepatitis profile is positive for HBsAg, HBeAg, and total anti-HBc. The anti-HAV, anti-HCV and anti-HBc IgM are negative. The PPD is negative.
The NP informs Patient C that he is coinfected with HIV and HBV and instructs him about the problems associated with HIV/HBV coinfection. He is given HAV and pneumococcal immunizations and options for antiretroviral therapy are discussed. Because of its effectiveness against both HIV and HBV, a medication regimen including tenofovir with lamivudine or tenofovir with emtricitabine should be utilized. A third medication for HIV viral suppression should be added, with consideration of the hepatotoxicity profile of the medication. After discussing available options with limited hepatotoxicity, an integrase inhibitor is selected as the third active agent in the combination. A single tablet medication containing bictegravir, emtricitabine, and tenofovir alafenamide in a once daily formulation was therefore selected to treat both HIV and HBV.
Information is provided to Patient C regarding safe sex practices. He is also instructed to abstain from alcohol and to use ibuprofen (or no more than 2 g acetaminophen in 24 hours) for pain control. The NP also orders a PT to be drawn; in addition, the patient is referred to hepatology for a liver biopsy to be performed in order to evaluate the progression of the liver disease. The patient is scheduled for a follow-up visit in four weeks, with a repeat HIV PCR performed at that time. In the interim, his baseline HIV PCR is found to be 123,000.
Upon his return to the office, Patient C is advised that the liver biopsy revealed periportal inflammation with focal necrosis and bridging fibrosis. PT is 15.6 seconds (control: 12 seconds). These findings indicate severe, advanced liver disease and the guarded prognosis. Because of the severity of his liver disease, he is not a good candidate for PegIFN therapy. The patient's current HIV status precludes his being a transplant candidate at the time. The recommended treatment plan for Patient C is to maximize his HIV suppression while minimizing his continued liver damage. If he is compliant with his therapy, he should be able to maintain a fairly good quality of life for three years or more. Prolonging the time until liver failure also provides the opportunity to improve immunocompetency. Some liver transplant centers now accept HIV-positive patients, provided that HIV viral loads are undetectable and CD4 counts are sufficiently high (usually >500). Patient C's future, therefore, depends upon his tolerance of the regimen, his compliance with the treatment plan, and his body's response to therapy.
The patient will initially be followed on a monthly basis. The viral load will be checked one month after the initiation of therapy, then every three months thereafter. Liver profile, CBC, and amylase will be assessed after one month, then bimonthly. After three months, HIV and HBV quantitative PCRs will be measured. If both are well suppressed, follow-up will be extended to every two to three months. If the patient's liver function significantly deteriorates, supportive therapy for end-stage liver disease will be instituted.
- About NetCE
- About TRC Healthcare
- Do Not Sell My Personal Information
Copyright © 2024 NetCE · Contact Us
- Campus Directory
- Current Students
- Faculty & Staff

Hepatitis A and B Case Study
Jon and Laura Green are siblings sharing similar symptoms. Both have a yellow tinge to their skin and the whites of their eyes. Both have flu-like symptoms. Jon, however, has been living in Brazil while Laura has been in San Francisco. Is this a weird sibling connection? Find out what’s causing their unusual symptoms in Case #5.
Mudule 10: Hepatitis
This case is actually two separate cases that address viral hepatitis. This format was selected...
Hepatitis - Page 1
When Jon arrived home, Jon and Laura’s father arranged for both of his children to see Dr. Lyon...
Hepatitis - Page 2
Dr. Lyon ordered the following laboratory tests on both Jon and Laura Green...
Hepatitis - Page 3
Since Jon's diagnosis was confirmed by his immune response to the presence of the virus, the physicians agreed...
Hepatitis - Page 4

Case Summary
Summary of the Case
Hepatitis - Summary

Answers to Case Questions
Hepatitis - Answers

Professionals
Health Professionals Introduced in Case
Hepatitis - Professionals
Additonal Links
Optional links to explore further
Hepatitis - Links
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Hepatitis A Surveillance Guidance
Uses of surveillance data, cases and clusters of potential public health importance, interpretation of laboratory test results, recommended reportable laboratory markers, surveillance case definition, case ascertainment, case investigation, case reporting and national notification.
Hepatitis A is typically a self-limited disease caused by hepatitis A virus (HAV), primarily transmitted fecal-orally after close contact with an infected person or consumption of contaminated food or water ( 31 ). Clinical symptoms are indistinguishable from acute hepatitis B and hepatitis C. Hepatitis A is an acute illness and does not result in chronic disease. The United States is considered a low endemicity country with most infections occurring among adults reporting risk behaviors or exposures such as SUD, homelessness, sexual practices resulting in fecal-oral contact, and international travel to hepatitis A-endemic countries ( 3 , 32 ).
A safe and effective hepatitis A vaccine was licensed in 1995 ( 33 ). Prior to vaccine licensure and use, the number of reported hepatitis A cases was around 21,000 annually, and infections were common among children ( 34 , 35 ). With the widespread adoption of the universal childhood vaccination recommendations in 2006, the overall incidence rate of hepatitis A decreased by 95% across all age groups from 1995 through 2014 ( 3 , 33 ). However, the incidence rate of hepatitis A increased during 2016–2019 due to widespread person-to-person outbreaks, primarily among PWUD and people experiencing homelessness ( 3 ). Increases in hepatitis A have also been reported among MSM ( 36 ). A study published in 2020 showed that approximately three-fourths of US-born adults ≥20 years of age were susceptible to hepatitis A during 2007–2016 ( 37 ). During 2016–2018, approximately 15,000 hepatitis A cases were reported to CDC, representing a 294% increase compared with 2013–2015 ( 38 ). In 2019, the number of hepatitis A cases reported to CDC peaked at 18,846 cases, corresponding to 37,700 estimated infections after adjusting for case under-ascertainment and underreporting ( 3 ). The annual number of hepatitis A cases reported to CDC has since declined as more states declared an end to their outbreaks. Disruptions to health care access and health department surveillance capacity during the COVID-19 pandemic may have affected the ability to detect and report all hepatitis A cases.
The purpose of this section is to provide jurisdictional guidance to implement and improve hepatitis A surveillance. Information about reporting requirements, collection of relevant laboratory data, and case investigation is provided. Given that current systems for surveillance differ by jurisdiction, the standards outlined in this document are designed to provide models for best practices based on jurisdictional resources, recognizing that not every jurisdiction is able to meet those standards with available resources.
Top of Page
Hepatitis A surveillance data can be used to inform and improve public health interventions in the following ways:
- Monitoring trends in disease incidence and determining risk behaviors or exposures . Hepatitis A surveillance data should be analyzed at a minimum of weekly by person, place, and time to monitor disease incidence. The proportion of cases reporting specific risk behaviors or exposures should be determined to monitor disease transmission patterns.
- Identifying outbreaks. The identification of a hepatitis A geotemporal cluster or increase in incidence can be an early signal of an outbreak and should prompt further investigation. This investigation should include collection of additional information, including risk behaviors or exposures for person-to-person transmission (e.g., non-injection and injection drug use, homelessness, and sexual and other practices leading to fecal-oral contact) or potential exposures to a common-source (e.g., suspected foods and infected food handler). Surveillance data should be analyzed to determine affected areas (e.g., rates by local jurisdiction or zip code) and groups (e.g., age-specific incidence rates and frequencies of reported risk behaviors or exposures). Prospective surveillance should be conducted to identify additional outbreak cases, identify candidates for post-exposure prophylaxis (if indicated), enhance vaccination efforts for populations at risk, and inform communication and infection control measures. If an outbreak is identified, DVH staff are available to provide consultation.
- Identifying cases among people who might expose others. The identification of a hepatitis A case in someone in a certain occupation (e.g., food handler) or congregate living situations is important because of the potential to expose additional people. This information can facilitate prompt contact tracing and coordination of postexposure prophylaxis.
- Molecular sequencing of viral isolates might help guide response measures. When investigating a possible outbreak, in some instances, collecting sera from patients for diagnosis and molecular characterization (genome sequencing and genotype identification) might provide additional information to guide control efforts and identify outbreaks within outbreaks (e.g., foodborne-related cases during person-to-person outbreak). Public health professionals who need guidance regarding use of nucleic acid testing (NAT) for the investigation of hepatitis A outbreaks should contact CDC’s DVH at [email protected] .
- Assessing missed opportunities for prevention. Patients whose infection source was reported as a household or sexual contact with suspected or confirmed hepatitis A should be investigated to determine if the patient received post-exposure prophylaxis when the source case was identified. In addition, surveillance data can be used to provide information about people at high risk for infection to provide education and awareness about the importance of vaccinating populations as recommended by the Advisory Committee on Immunization Practices (ACIP).
- Assessing the impact of vaccination programs. Age-specific incidence rates for the priority groups and the community as a whole can be compared to historical rates for the same age groups to assess the impact of routine vaccination programs.
Jurisdictions should review and analyze hepatitis A data regularly to identify cases and clusters of hepatitis A that merit further investigation. Ideally, all cases of reported hepatitis A should be investigated. In jurisdictions with limited resources, cases and clusters should be prioritized for investigation in accordance with the degree of public health importance. The following are examples of cases that are high priority for further follow-up:
- Cases in people who are in higher risk groups (e.g., PWUD, people experiencing homelessness) or who live in congregate settings (e.g., shelters, correctional facilities) to assure that interventions to prevent further spread are implemented in a timely fashion
- Cases who were previously vaccinated to characterize possible vaccine failures (see Section 1.10 ).
- Cases of hepatitis A in people born after 2005 to distinguish between failure of vaccine and failure to vaccinate
- Cases without common risk behaviors or exposures
- Two or more cases among patrons at the same store or food service establishment
Immunoglobulin M antibody to hepatitis A virus (anti-HAV IgM) and viremia identified by HAV NAT using polymerase chain reaction (PCR) can detect recent or current acute infection with HAV. A description of hepatitis A laboratory markers can be found in Appendix B . Figure 2-1 illustrates the typical serologic course of HAV infection and recovery.
Figure 2-1. Typical serologic course of hepatitis A virus infection and recovery
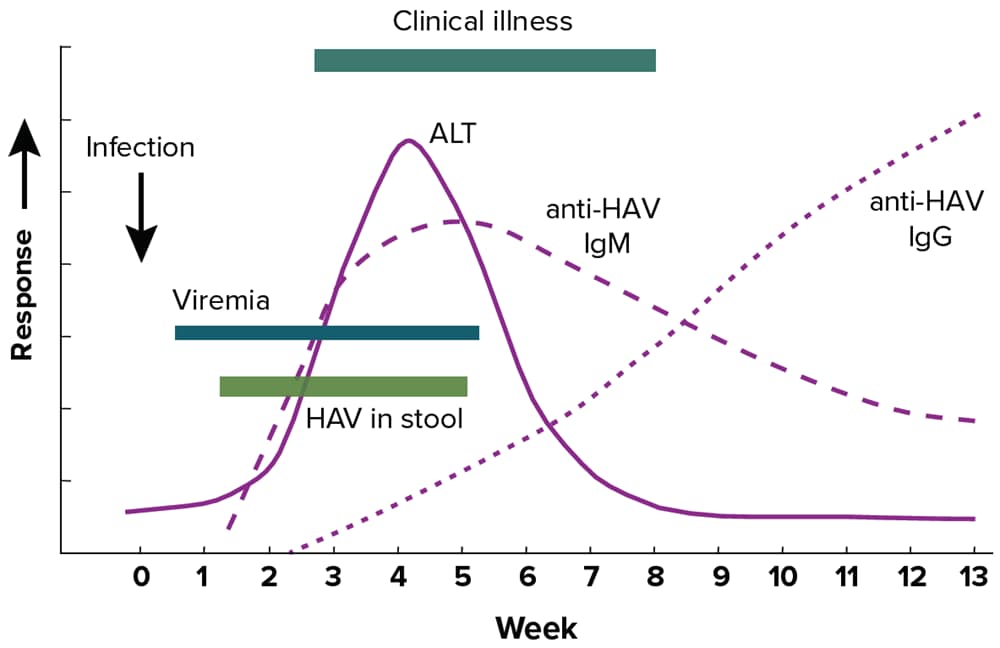
Figure obtained from https://www.cdc.gov/mmwr/pdf/rr/rr5304.pdf . Downloads of this figure: PDF | PPT
Caution should be exercised when interpreting a positive anti-HAV IgM laboratory result, as positive tests can occur in people >1 year after infection and false-positive tests can also occur in those without clinical or epidemiologic evidence of recent infection ( 39 ). A person with a positive anti-HAV IgM result may also be positive for anti-HAV IgG and total anti-HAV. Because of the risk of misinterpreting positive results, anti-HAV IgM testing should be limited to people with clinical presentation of hepatitis who are suspected of having hepatitis A. Anti-HAV IgM testing should not be used as a screening tool or as part of testing panels in the workup of abnormal liver function tests. Some conditions might cause cross-reactivity with anti-HAV IgM tests, including infection with the Epstein-Barr virus ( 40 ) and hepatitis C virus ( 41 ). Furthermore, some infected people might initially test negative for anti-HAV IgM during the first few days of symptoms ( 42 ). If there is high clinical suspicion of hepatitis A in a person who has a negative test for anti-HAV IgM early in their clinical course, repeat testing may be indicated ( 42 ). One study found that the optimal time for repeat testing is at least 2 days after ALT levels have peaked ( 42 ).
Table 2-1 interprets the combinations of total anti-HAV and anti-HAV IgM laboratory results frequently available in viral hepatitis test panels, following the biomarker changes over the course of infection as shown in Figure 2-1 . If HAV RNA testing is performed, a detectable HAV RNA level indicates the presence of infection.
Table 2-1. Interpretation of hepatitis A laboratory results
*Ingestion of high levels of biotin can significantly interfere with certain commonly used biotinylated immunoassays, such as those used to detect anti-HAV, and cause false-positive or false-negative laboratory test results. Currently, the US Food and Drug Administration (FDA) is investigating thresholds associated with false-positive and false-negative tests. This section will be updated as more information becomes available. Reference: https://www.fda.gov/medical-devices/safety-communications/update-fda-warns-biotin-may-interfere-lab-tests-fda-safety-communication . Downloads of this table: PDF | PPT
To aid in hepatitis A surveillance, the following laboratory markers should be reported to public health agencies:
- Positive anti-HAV IgM;
- Positive/detectable HAV RNA (including qualitative, quantitative, or genotype testing); and
- All concurrent ALT and total bilirubin results reported with positive hepatitis A laboratory results, which can also be helpful in classifying hepatitis A cases that do not have an HAV RNA laboratory result.
Table 2-2 specifies the surveillance case definition for hepatitis A, adopted by CSTE and CDC in 2019. This definition should be used for hepatitis A case classification and national notification ( 12 , 43 ). See Appendix C for classification scenarios of cases of hepatitis A.
Table 2-2. US Centers for Disease Control and Prevention (CDC) and Council of State and Territorial Epidemiologists (CSTE) case definition for hepatitis A, 2019
Up to 10% of people with hepatitis A might experience a relapse of symptoms during the 6 months after acute illness. Cases of relapsing hepatitis A should not be enumerated as new cases. In addition, a case should not be counted as a hepatitis A case if there is an alternate, more likely diagnosis.
The primary method of ascertaining cases is by reviewing reports from clinical laboratories, health care facilities, and health care providers. All states should have rules or regulations requiring that these facilities report evidence of hepatitis A to public health agencies. See Section 1.6. and Section 2.5. for information on the recommended reporting requirements for hepatitis A.
Laboratory Reporting
All states require clinical laboratories to report hepatitis A laboratory markers, such as positive anti-HAV IgM and positive HAV RNA results.
Health Care Facility and Provider Reporting
All states require health care facilities and providers to report hepatitis A diagnoses.
Additional sources that will facilitate case ascertainment and case characterization include medical records, hospital discharge databases, and death certificates. Section 5.4 describes the usefulness of select data sources in supplementing case ascertainment.
Figure 2-2 illustrates one approach for hepatitis A case ascertainment and classification. Specific procedures might vary by jurisdiction, but should generally follow the scheme outlined in Figure 2-2 , in accordance with the CDC/CSTE Position Statement for the 2019 hepatitis A case definition ( 12 , 43 ). See Appendix C for case classification scenarios for hepatitis A.
Figure 2-2. Process for hepatitis A case ascertainment and classification
Reports from laboratories, health care providers, and other data sources indicative of hepatitis A should be submitted to HDs (as specified by local regulations) and investigated as soon as possible to ensure adequate time to implement preventive measures (e.g., vaccination of contacts). Suspected cases of hepatitis A should be reported with appropriate laboratory results and clinical information ( Table 2-2 ). For general information on conducting viral hepatitis case investigations, see Section 1.10. The following is a description of the follow-up activities that should be conducted for reported hepatitis A cases:
Information from the Laboratory
Positive anti-HAV IgM and positive/detectable HAV RNA laboratory results should be reported to the HD and investigated immediately. Other laboratory information that can assist with case classification includes ALT and total bilirubin levels.
Information from the Provider or Medical Records
The following information might be available from medical records to confirm the diagnosis, inform case classification, and determine public health priority.
- Diagnostic test results. Hepatitis A laboratory markers (e.g., positive anti-HAV IgM and positive/detectable HAV RNA) should be reportable to the HD. If additional laboratory testing (e.g., for ALT and total bilirubin levels) is needed to classify the case, HD staff will work with the provider to obtain these test results.
- Clinical features. Includes reason for testing, illness onset date, clinical signs and symptoms (including the presence of jaundice), coinfections, hospitalization status and date of death, and whether hepatitis A or an alternate diagnosis is suspected.
- Demographic information. Includes name, date of birth, sex at birth, current gender, race, ethnicity, and residential address (including zip code).
- Patients who deny known risk behaviors or exposures for infection can be interviewed with a supplemental food history questionnaire.
- At the earliest possible point, information regarding whether the patient is in a sensitive occupation (e.g., food handler) or an attendee or resident of a congregate setting should be obtained.
- Occupation. While no documented evidence indicates that food handlers or health care workers are at higher risk for infection than people in other occupations, jurisdictions routinely obtain this information to inform contact tracing. Special attention should be given to the job duties of people in sensitive occupations, including whether the patient was symptomatic while at work, which symptoms (if any) were experienced while at work, and the patient’s work schedule during their infectious period. Food handlers should be restricted from working in a food handling capacity while infectious, and patrons from food service establishments or health care providers should be notified as appropriate ( 44 ).
- Vaccination information . Hepatitis A vaccination has been recommended for infants since 2006 in all US states. Depending on age of the HAV-infected person, some cases of hepatitis A should have been vaccinated in childhood, whereas others should have been vaccinated as adults because they met specific risk criteria. Though rare, recent vaccination might result in transient anti-HAV IgM positivity. Obtaining vaccination history can be done via the patient’s provider or state immunization registries and is useful in identifying vaccine failure or transient anti-HAV IgM positivity.
Information from the Patient
Resources permitting, all patients with hepatitis A should be contacted for an interview using the jurisdiction-specific case investigation form. At a minimum, all patients who are classified as “confirmed” per the CDC/CSTE case definition should be interviewed. The patient interview should ideally include the following components:
- Epidemiologic link. For all laboratory-confirmed cases of hepatitis A, obtain information on contacts where exposure occurred 15–50 days prior to the onset of symptoms and investigate whether contacts met the clinical criteria.
- Risk behaviors or exposures. To determine the most likely mode of transmission, ask patients about behaviors and exposures during the 15–50 days prior to illness onset. Patients who deny other risks for infection should be interviewed with a supplemental food history questionnaire.
- Education and referral for follow-up. Assess whether the patient requires education or other medical follow-up services, including hepatitis B vaccination, as appropriate. People with hepatitis A should be counseled on how to prevent transmission to others.
- Identification of contacts requiring post-exposure prophylaxis. If resources allow, identify contacts and coordinate referral for post-exposure prophylaxis if contact occurred within 14 days. Information regarding hepatitis A vaccination and post-exposure prophylaxis can be found on the Hepatitis A ACIP Vaccine Recommendations website .
Special Considerations when Investigating Certain Populations or Settings at Risk for Rapid Disease Transmission
Certain populations and settings are associated with increased risk for rapid transmission of hepatitis A. Considerations when investigating hepatitis A cases among people experiencing homelessness, PWUD, people engaging in high-risk sexual practices, and people in correctional facilities are provided in Section 1.10.
Outbreak Reporting and Notification
All hepatitis A outbreaks should be reported to the appropriate local authorities for further investigation within the timeframe each jurisdiction has specified. The reporting timeframe to local authorities varies by jurisdiction. Notification to CDC is done through NNDSS and by contacting [email protected] , as indicated in CDC-RFA-PS21-2103. See Section 5.3 for guidance on reporting outbreak source to NNDSS.
Cases of hepatitis A should be reported to HDs as specified by state, territorial, and local regulations. Hepatitis A is a nationally notifiable condition ( 9 ). Hepatitis A cases are identified using an event code ( Table 1-2 ). Data are sent weekly or more frequently, depending on the infrastructure of the jurisdiction sending the data. Cases might be re-classified or removed as needed after the initial transmission to CDC, if the changes occur before surveillance data are finalized each year.
- Overview of Viral Hepatitis
- Statistics & Surveillance
- Populations & Settings
- State and Local Partners & Grantees
- Policy, Programs, and Science
- Resource Center
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
We have a new app!
Take the Access library with you wherever you go—easy access to books, videos, images, podcasts, personalized features, and more.
Download the Access App here: iOS and Android . Learn more here!
- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

24: Hepatitis C
Lindsey Childs-Kean
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Patient presentation.
- Full Chapter
- Supplementary Content
Chief Complaint
“I want to get this Hepatitis C cured.”
History of Present Illness
PD is a 45-year-old Caucasian male who presents to the infectious diseases clinic seeking treatment for his chronic hepatitis C infection. He states that he was diagnosed with the disease a few years ago, but he’s not sure when he contracted it. He doesn’t remember having any symptoms related to the infection. He has never received any treatment for his hepatitis C.
Past Medical History
HTN, anxiety, dyslipidemia, GERD
Surgical History
Family history.
Father and mother both alive with HTN and dyslipidemia; sister is alive with no known medical issues.
Social History
Former IV drug user and alcohol drinker, but clean and sober for 7+ years. No tobacco. Works repairing wheelchairs and scooters. Married with 2 children (12 and 4 years). Enjoys playing sports and doing outdoor activities with wife and children.
Home Medications
Amlodipine 10 mg PO daily
Alprazolam 1 mg PO TID PRN for anxiety
Simvastatin 20 mg PO daily
Omeprazole 40 mg PO daily
Multivitamin PO daily
Acetaminophen 325 mg PO PRN for headache
Physical Examination
Vital signs.
Temp 98.8°F, P 82 bpm, RR 18 breaths per minute, BP 127/78 mm Hg, pO 2 99%, Ht 6′, Wt 101 kg
Normal appearing male in no apparent distress, well groomed
Nomocephalic, atraumatic, PERRLA, EOMI, b/l sclera anicteric, moist mucous membranes, poor dentition
Clear to auscultation, no use of accessory muscles, no cracks or wheezes
Cardiovascular
NSR, no m/r/g
Soft, non-distended, non-tender, normal bowel sounds
Genitourinary
Normal male genitalia, no complaints of dysuria or hematuria
Alert and oriented × 3, CN 2-12 grossly intact
Extremities
No edema, cyanosis, or clubbing
Intact, w/o rashes or lesions, or erythema
Laboratory Findings
Sign in or create a free Access profile below to access even more exclusive content.
With an Access profile, you can save and manage favorites from your personal dashboard, complete case quizzes, review Q&A, and take these feature on the go with our Access app.
Pop-up div Successfully Displayed
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
A case-control study of risk factors for hepatitis B infection: A regional report among Isfahanian adults
Affiliations.
- 1 Department of Infectious Diseases, Isfahan University of Medical Sciences, Isfahan, Iran.
- 2 Baghiatallah Research Center for Gastroenterology and Liver Diseases, Baghiatallah University of Medical Sciences, Tehran, Iran.
- 3 Isfahan Medical School Research Committee, Isfahan University of Medical Sciences, Isfahan, Iran.
- 4 Department of Anatomical Sciences, Isfahan University of Medical Sciences, Isfahan, Iran.
- 5 Isfahan University of Medical Sciences, Isfahan, Iran.
- PMID: 31007692
- PMCID: PMC6450138
- DOI: 10.4103/jrms.JRMS_761_18
Background: Hepatitis B is one of the major causes of mortality among viral diseases. To reduce morbidity rate and increase knowledge of people about potential risk factors, the aim of this study was to determine the prevalence of hepatitis B among the general population and the risk factors associated with hepatitis B virus (HBV) infection in Isfahan, Iran.
Materials and methods: In a case-control study, 314 HBV-infected patients and 557 healthy participants were recruited. Data on demographics, immunization history, medical history, family medical history, life history, therapeutic factors, and behavioral risk factors were collected through a standard checklist. Chi-square and logistic regression were used for univariate and multivariable analyses.
Results: Our results showed that among sociodemographic variables, higher age, being male, lower economic status, and lower educational attainments increased the risk of affecting by HBV (odds ratio [OR] >1, P < 0.001); furthermore, Iranian and no immigrant people showed higher significant risk of being affected by HBV. Multivariable logistic regression showed among medical, blood, and behavioral risk factors, family history of hepatitis (OR: 10.56; 95% confidence interval [CI]: 4.56-24.86), dental treatment history (OR: 4.30; 95% CI: 1.41-13.10), and hospitalization (OR: 2.94; 95% CI: 1.72-5.00).
Conclusion: Our results demonstrated that there are still several risk factors for hepatitis B surface antigen infection among the Iranian adult population. Immunization programs should continue and focus on high-risk adults, and interventions should be directed toward to reduce risk factors associated with hepatitis B.
Keywords: Hepatitis-B virus; risk factors; viral infection.
- Case Report
- Open access
- Published: 27 May 2024
A complex case study: coexistence of multi-drug-resistant pulmonary tuberculosis, HBV-related liver failure, and disseminated cryptococcal infection in an AIDS patient
- Wei Fu 1 , 2 na1 ,
- Zi Wei Deng 3 na1 ,
- Pei Wang 1 ,
- Zhen Wang Zhu 1 ,
- Zhi Bing Xie 1 ,
- Yong Zhong Li 1 &
- Hong Ying Yu 1
BMC Infectious Diseases volume 24 , Article number: 533 ( 2024 ) Cite this article
294 Accesses
1 Altmetric
Metrics details
Hepatitis B virus (HBV) infection can cause liver failure, while individuals with Acquired Immunodeficiency Virus Disease (AIDS) are highly susceptible to various opportunistic infections, which can occur concurrently. The treatment process is further complicated by the potential occurrence of immune reconstitution inflammatory syndrome (IRIS), which presents significant challenges and contributes to elevated mortality rates.
Case presentation
The 50-year-old male with a history of chronic hepatitis B and untreated human immunodeficiency virus (HIV) infection presented to the hospital with a mild cough and expectoration, revealing multi-drug resistant pulmonary tuberculosis (MDR-PTB), which was confirmed by XpertMTB/RIF PCR testing and tuberculosis culture of bronchoalveolar lavage fluid (BALF). The patient was treated with a regimen consisting of linezolid, moxifloxacin, cycloserine, pyrazinamide, and ethambutol for tuberculosis, as well as a combination of bictegravir/tenofovir alafenamide/emtricitabine (BIC/TAF/FTC) for HBV and HIV viral suppression. After three months of treatment, the patient discontinued all medications, leading to hepatitis B virus reactivation and subsequent liver failure. During the subsequent treatment for AIDS, HBV, and drug-resistant tuberculosis, the patient developed disseminated cryptococcal disease. The patient’s condition worsened during treatment with liposomal amphotericin B and fluconazole, which was ultimately attributed to IRIS. Fortunately, the patient achieved successful recovery after appropriate management.
Enhancing medical compliance is crucial for AIDS patients, particularly those co-infected with HBV, to prevent HBV reactivation and subsequent liver failure. Furthermore, conducting a comprehensive assessment of potential infections in patients before resuming antiviral therapy is essential to prevent the occurrence of IRIS. Early intervention plays a pivotal role in improving survival rates.
Peer Review reports
HIV infection remains a significant global public health concern, with a cumulative death toll of 40 million individuals [ 1 ]. In 2021 alone, there were 650,000 deaths worldwide attributed to AIDS-related causes. As of the end of 2021, approximately 38 million individuals were living with HIV, and there were 1.5 million new HIV infections reported annually on a global scale [ 2 ]. Co-infection with HBV and HIV is prevalent due to their similar transmission routes, affecting around 8% of HIV-infected individuals worldwide who also have chronic HBV infection [ 3 ]. Compared to those with HBV infection alone, individuals co-infected with HIV/HBV exhibit higher HBV DNA levels and a greater risk of reactivation [ 4 ]. Opportunistic infections, such as Pneumocystis jirovecii pneumonia, Toxoplasma encephalitis, cytomegalovirus retinitis, cryptococcal meningitis (CM), tuberculosis, disseminated Mycobacterium avium complex disease, pneumococcal pneumonia, Kaposi’s sarcoma, and central nervous system lymphoma, are commonly observed due to HIV-induced immunodeficiency [ 5 ]. Tuberculosis not only contributes to the overall mortality rate in HIV-infected individuals but also leads to a rise in the number of drug-resistant tuberculosis cases and transmission of drug-resistant strains. Disseminated cryptococcal infection is a severe opportunistic infection in AIDS patients [ 6 ], and compared to other opportunistic infections, there is a higher incidence of IRIS in patients with cryptococcal infection following antiviral and antifungal therapy [ 7 ]. This article presents a rare case of an HIV/HBV co-infected patient who presented with MDR-PTB and discontinued all medications during the initial treatment for HIV, HBV, and tuberculosis. During the subsequent re-anti-HBV/HIV treatment, the patient experienced two episodes of IRIS associated with cryptococcal infection. One episode was classified as “unmasking” IRIS, where previously subclinical cryptococcal infection became apparent with immune improvement. The other episode was categorized as “paradoxical” IRIS, characterized by the worsening of pre-existing cryptococcal infection despite immune restoration [ 8 ]. Fortunately, both episodes were effectively treated.
A 50-year-old male patient, who is self-employed, presented to our hospital in January 2022 with a chief complaint of a persistent cough for the past 2 months, without significant shortness of breath, palpitations, or fever. His medical history revealed a previous hepatitis B infection, which resulted in hepatic failure 10 years ago. Additionally, he was diagnosed with HIV infection. However, he ceased taking antiviral treatment with the medications provided free of charge by the Chinese government for a period of three years. During this hospital visit, his CD4 + T-cell count was found to be 26/μL (normal range: 500–1612/μL), HIV-1 RNA was 1.1 × 10 5 copies/ml, and HBV-DNA was negative. Chest computed tomography (CT) scan revealed nodular and patchy lung lesions (Fig. 1 ). The BALF shows positive acid-fast staining. Further assessment of the BALF using XpertMTB/RIF PCR revealed resistance to rifampicin, and the tuberculosis drug susceptibility test of the BALF (liquid culture, medium MGIT 960) indicated resistance to rifampicin, isoniazid, and streptomycin. Considering the World Health Organization (WHO) guidelines for drug-resistant tuberculosis, the patient’s drug susceptibility results, and the co-infection of HIV and HBV, an individualized treatment plan was tailored for him. The treatment plan included BIC/TAF/FTC (50 mg/25 mg/200 mg per day) for HBV and HIV antiviral therapy, as well as linezolid (0.6 g/day), cycloserine (0.5 g/day), moxifloxacin (0.4 g/day), pyrazinamide (1.5 g/day), and ethambutol (0.75 g/day) for anti-tuberculosis treatment, along with supportive care.
The patient’s pulmonary CT scan shows patchy and nodular lesions accompanied by a small amount of pleural effusion, later confirmed to be MDR-PTB
Unfortunately, after 3 months of follow-up, the patient discontinued all medications due to inaccessibility of the drugs. He returned to our hospital (Nov 12, 2022, day 0) after discontinuing medication for six months, with a complaint of poor appetite for the past 10 days. Elevated liver enzymes were observed, with an alanine aminotransferase level of 295 IU/L (normal range: 0–40 IU/L) and a total bilirubin(TBIL) level of 1.8 mg/dL (normal range: 0–1 mg/dL). His HBV viral load increased to 5.5 × 10 9 copies/ml. Considering the liver impairment, elevated HBV-DNA and the incomplete anti-tuberculosis treatment regimen (Fig. 2 A), we discontinued pyrazinamide and initiated treatment with linezolid, cycloserine, levofloxacin, and ethambutol for anti-tuberculosis therapy, along with BIC/TAF/FTC for HIV and HBV antiviral treatment. Additionally, enhanced liver protection and supportive management were provided, involving hepatoprotective effects of medications such as glutathione, magnesium isoglycyrrhizinate, and bicyclol. However, the patient’s TBIL levels continued to rise progressively, reaching 4.4 mg/dL on day 10 (Fig. 3 B). Suspecting drug-related factors, we discontinued all anti-tuberculosis medications while maintaining BIC/TAF/FTC for antiviral therapy, the patient’s TBIL levels continued to rise persistently. We ruled out other viral hepatitis and found no significant evidence of obstructive lesions on magnetic resonance cholangiopancreatography. Starting from the day 19, due to the patient’s elevated TBIL levels of 12.5 mg/dL, a decrease in prothrombin activity (PTA) to 52% (Fig. 3 D), and the emergence of evident symptoms such as abdominal distension and poor appetite, we initiated aggressive treatment methods. Unfortunately, on day 38, his hemoglobin level dropped to 65 g/L (normal range: 120–170 g/L, Fig. 3 A), and his platelet count decreased to 23 × 10 9 /L (normal range: 125–300 × 10 9 /L, Fig. 3 C). Based on a score of 7 on the Naranjo Scale, it was highly suspected that “Linezolid” was the cause of these hematological abnormalities. Therefore, we had to discontinue Linezolid for the anti-tuberculosis treatment. Subsequently, on day 50, the patient developed recurrent fever, a follow-up chest CT scan revealed enlarged nodules in the lungs (Fig. 2 B). The patient also reported mild dizziness and a worsening cough. On day 61, the previous blood culture results reported the growth of Cryptococcus. A lumbar puncture was performed on the same day, and the cerebrospinal fluid (CSF) opening pressure was measured at 130 mmH 2 O. India ink staining of the CSF showed typical encapsulated yeast cells suggestive of Cryptococcus. Other CSF results indicated mild leukocytosis and mildly elevated protein levels, while chloride and glucose levels were within normal limits. Subsequently, the patient received a fungal treatment regimen consisting of liposomal amphotericin B (3 mg/kg·d −1 ) in combination with fluconazole(600 mg/d). After 5 days of antifungal therapy, the patient’s fever symptoms were well controlled. Despite experiencing bone marrow suppression, including thrombocytopenia and worsening anemia, during this period, proactive symptom management, such as the use of erythropoietin, granulocyte colony-stimulating factor, and thrombopoietin, along with high-calorie dietary management, even reducing the dosage of liposomal amphotericin B to 2 mg/kg/day for 10 days at the peak of severity, successfully controlled the bone marrow suppression. However, within the following week, the patient experienced fever again, accompanied by a worsened cough, increased sputum production, and dyspnea. Nevertheless, the bilirubin levels did not show a significant increase. On day 78 the patient’s lung CT revealed patchy infiltrates and an increased amount of pleural effusion (Fig. 2 C). The CD4 + T-cell count was 89/μL (normal range: 500–700/μL), indicating a significant improvement in immune function compared to the previous stage, and C-reactive protein was significantly elevated, reflecting the inflammatory state, other inflammatory markers such as IL-6 and γ-IFN were also significantly elevated. On day 84, Considering the possibility of IRIS, the patient began taking methylprednisolone 30 mg once a day as part of an effort to control his excessive inflammation. Following the administration of methylprednisolone, the man experienced an immediate improvement in his fever. Additionally, symptoms such as cough, sputum production, dyspnea, and poor appetite gradually subsided over time. A follow-up lung CT showed significant improvement, indicating a positive response to the treatment. After 28 days of treatment with liposomal amphotericin B in combination with fluconazole, liposomal amphotericin B was discontinued, and the patient continued with fluconazole to consolidate the antifungal therapy for Cryptococcus. Considering the patient’s ongoing immunodeficiency, the dosage of methylprednisolone was gradually reduced by 4 mg every week. After improvement in liver function, the patient’s anti-tuberculosis treatment regimen was adjusted to include bedaquiline, contezolid, cycloserine, moxifloxacin, and ethambutol. The patient’s condition was well controlled, and a follow-up lung CT on day 117 indicated a significant improvement in lung lesions (Fig. 2 D).
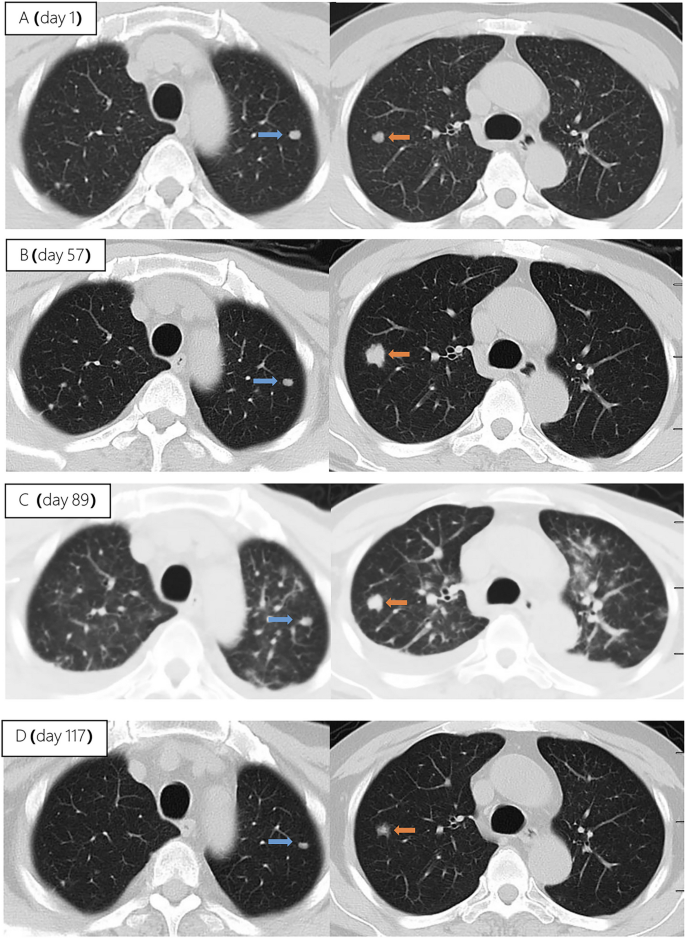
Upon second hospitalization admission ( A ), nodular lesions were already present in the lungs, and their size gradually increased after the initiation of ART ( B , C ). Notably, the lung lesions became more pronounced following the commencement of anti-cryptococcal therapy, coinciding with the occurrence of pleural effusion ( C ). However, with the continuation of antifungal treatment and the addition of glucocorticoids, there was a significant absorption and reduction of both the pleural effusion and pulmonary nodules ( D )
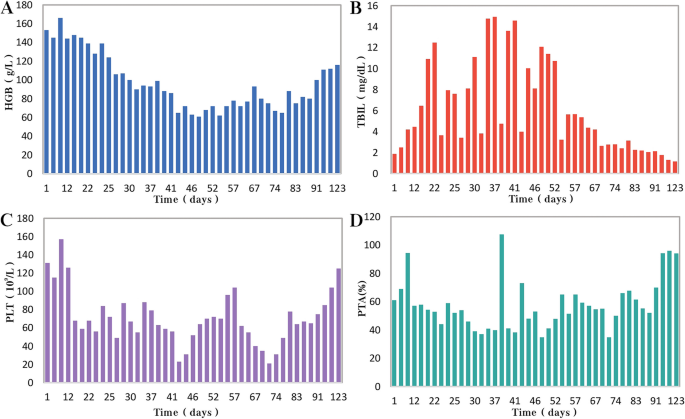
During the patient's second hospitalization, as the anti-tuberculosis treatment progressed and liver failure developed, the patient’s HGB levels gradually decreased ( A ), while TBIL levels increased ( B ). Additionally, there was a gradual decrease in PLT count ( C ) and a reduction in prothrombin activity (PTA) ( D ), indicating impaired clotting function. Moreover, myelosuppression was observed during the anti-cryptococcal treatment ( C )
People living with HIV/AIDS are susceptible to various opportunistic infections, which pose the greatest threat to their survival [ 5 ]. Pulmonary tuberculosis and disseminated cryptococcosis remain opportunistic infections with high mortality rates among AIDS patients [ 9 , 10 ]. These infections occurring on the basis of liver failure not only increase diagnostic difficulty but also present challenges in treatment. Furthermore, as the patient’s immune function and liver function recover, the occurrence of IRIS seems inevitable.
HIV and HBV co-infected patients are at a higher risk of HBV reactivation following the discontinuation of antiviral drugs
In this case, the patient presented with both HIV and HBV infections. Although the HBV DNA test was negative upon admission. However, due to the patient’s self-discontinuation of antiretroviral therapy (ART), HBV virologic and immunologic reactivation occurred six months later, leading to a rapid increase in viral load and subsequent hepatic failure. Charles Hannoun et al. also reported similar cases in 2001, where two HIV-infected patients with positive HBsAg experienced HBV reactivation and a rapid increase in HBV DNA levels after discontinuing antiretroviral and antiviral therapy, ultimately resulting in severe liver failure [ 11 ]. The European AIDS Clinical Society (EACS) also emphasize that abrupt discontinuation of antiviral therapy in patients co-infected with HBV and HIV can trigger HBV reactivation, which, although rare, can potentially result in liver failure [ 12 ].
Diagnosing disseminated Cryptococcus becomes more challenging in AIDS patients with liver failure, and the selection of antifungal medications is significantly restricted
In HIV-infected individuals, cryptococcal disease typically manifests as subacute meningitis or meningoencephalitis, often accompanied by fever, headache, and neck stiffness. The onset of symptoms usually occurs approximately two weeks after infection, with typical signs and symptoms including meningeal signs such as neck stiffness and photophobia. Some patients may also experience encephalopathy symptoms like somnolence, mental changes, personality changes, and memory loss, which are often associated with increased intracranial pressure (ICP) [ 13 ]. The presentation of cryptococcal disease in this patient was atypical, as there were no prominent symptoms such as high fever or rigors, nor were there any signs of increased ICP such as somnolence, headache, or vomiting. The presence of pre-existing pulmonary tuberculosis further complicated the early diagnosis, potentially leading to the clinical oversight of recognizing the presence of cryptococcus. In addition to the diagnostic challenges, treating a patient with underlying liver disease, multidrug-resistant tuberculosis, and concurrent cryptococcal infection poses significant challenges. It requires considering both the hepatotoxicity of antifungal agents and potential drug interactions. EACS and global guideline for the diagnosis and management of cryptococcosis suggest that liposomal amphotericin B (3 mg/kg·d −1 ) in combination with flucytosine (100 mg/kg·d −1 ) or fluconazole (800 mg/d) is the preferred induction therapy for CM for 14 days [ 12 , 14 ]. Flucytosine has hepatotoxicity and myelosuppressive effects, and it is contraindicated in patients with severe liver dysfunction. The antiviral drug bictegravir is a substrate for hepatic metabolism by CYP3A and UGT1A1 enzymes [ 15 ], while fluconazole inhibits hepatic enzymes CYP3A4 and CYP2C9 [ 16 ]. Due to the patient's liver failure and bone marrow suppression, we reduced the dosage of liposomal amphotericin B and fluconazole during the induction period. Considering the hepatotoxicity of fluconazole and its interaction with bictegravir, we decreased the dosage of fluconazole to 600 mg/d, while extending the duration of induction therapy to 28 days.
During re-antiviral treatment, maintaining vigilance for the development of IRIS remains crucial
IRIS refers to a series of inflammatory diseases that occur in HIV-infected individuals after initiating ART. It is associated with the paradoxical worsening of pre-existing infections, which may have been previously diagnosed and treated or may have been subclinical but become apparent due to the host regaining the ability to mount an inflammatory response. Currently, there is no universally accepted definition of IRIS. However, the following conditions are generally considered necessary for diagnosing IRIS: worsening of a diagnosed or previously unrecognized pre-existing infection with immune improvement (referred to as “paradoxical” IRIS) or the unmasking of a previously subclinical infection (referred to as “unmasking” IRIS) [ 8 ]. It is estimated that 10% to 30% of HIV-infected individuals with CM will develop IRIS after initiating or restarting effective ART [ 7 , 17 ]. In the guidelines of the WHO and EACS, it is recommended to delay the initiation of antiviral treatment for patients with CM for a minimum of 4 weeks to reduce the incidence of IRIS. Since we accurately identified the presence of multidrug-resistant pulmonary tuberculosis in the patient during the early stage, we promptly initiated antiretroviral and anti-hepatitis B virus treatment during the second hospitalization. However, subsequent treatment revealed that the patient experienced at least two episodes of IRIS. The first episode was classified as “unmasking” IRIS, as supported by the enlargement of pulmonary nodules observed on the chest CT scan following the initiation of ART (Fig. 2 A). Considering the morphological changes of the nodules on the chest CT before antifungal therapy, the subsequent emergence of disseminated cryptococcal infection, and the subsequent reduction in the size of the lung nodules after antifungal treatment, although there is no definitive microbiological evidence, we believe that the initial enlargement of the lung nodules was caused by cryptococcal pneumonia. As ART treatment progressed, the patient experienced disseminated cryptococcosis involving the blood and central nervous system, representing the first episode. Following the initiation of antifungal therapy for cryptococcosis, the patient encountered a second episode characterized by fever and worsening pulmonary lesions. Given the upward trend in CD4 + T-cell count, we attributed this to the second episode of IRIS, the “paradoxical” type. The patient exhibited a prompt response to low-dose corticosteroids, further supporting our hypothesis. Additionally, the occurrence of cryptococcal IRIS in the lungs, rather than the central nervous system, is relatively uncommon among HIV patients [ 17 ].

Conclusions
From the initial case of AIDS combined with chronic hepatitis B, through the diagnosis and treatment of multidrug-resistant tuberculosis, the development of liver failure and disseminated cryptococcosis, and ultimately the concurrent occurrence of IRIS, the entire process was tortuous but ultimately resulted in a good outcome (Fig. 4 ). Treatment challenges arose due to drug interactions, myelosuppression, and the need to manage both infectious and inflammatory conditions. Despite these hurdles, a tailored treatment regimen involving antifungal and antiretroviral therapies, along with corticosteroids, led to significant clinical improvement. While CM is relatively common among immunocompromised individuals, especially those with acquired immunodeficiency syndrome (AIDS) [ 13 ], reports of disseminated cryptococcal infection on the background of AIDS complicated with liver failure are extremely rare, with a very high mortality rate.
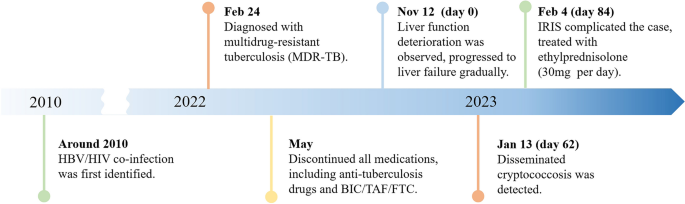
A brief timeline of the patient's medical condition progression and evolution
Through managing this patient, we have also gained valuable insights. (1) Swift and accurate diagnosis, along with timely and effective treatment, can improve prognosis, reduce mortality, and lower disability rates. Whether it's the discovery and early intervention of liver failure, the identification and treatment of disseminated cryptococcosis, or the detection and management of IRIS, all these interventions are crucially timely. They are essential for the successful treatment of such complex and critically ill patients.
(2) Patients who exhibit significant drug reactions, reducing the dosage of relevant medications and prolonging the treatment duration can improve treatment success rates with fewer side effects. In this case, the dosages of liposomal amphotericin B and fluconazole are lower than the recommended dosages by the World Health Organization and EACS guidelines. Fortunately, after 28 days of induction therapy, repeat CSF cultures showed negative results for Cryptococcus, and the improvement of related symptoms also indicates that the patient has achieved satisfactory treatment outcomes. (3) When cryptococcal infection in the bloodstream or lungs is detected, prompt lumbar puncture should be performed to screen for central nervous system cryptococcal infection. Despite the absence of neurological symptoms, the presence of Cryptococcus neoformans in the cerebrospinal fluid detected through lumbar puncture suggests the possibility of subclinical or latent CM, especially in late-stage HIV-infected patients.
We also encountered several challenges and identified certain issues that deserve attention. Limitations: (1) The withdrawal of antiviral drugs is a critical factor in the occurrence and progression of subsequent diseases in patients. Improved medical education is needed to raise awareness and prevent catastrophic consequences. (2) Prior to re-initiating antiviral therapy, a thorough evaluation of possible infections in the patient is necessary. Caution should be exercised, particularly in the case of diseases prone to IRIS, such as cryptococcal infection. (3) There is limited evidence on the use of reduced fluconazole dosage (600 mg daily) during antifungal therapy, and the potential interactions between daily fluconazole (600 mg) and the antiviral drug bictegravir and other tuberculosis medications have not been extensively studied. (4) Further observation is needed to assess the impact of early-stage limitations in the selection of anti-tuberculosis drugs on the treatment outcome of tuberculosis in this patient, considering the presence of liver failure.
In conclusion, managing opportunistic infections in HIV patients remains a complex and challenging task, particularly when multiple opportunistic infections are compounded by underlying liver failure. Further research efforts are needed in this area.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
Hepatitis B virus
Acquired immunodeficiency virus disease
Immune reconstitution inflammatory syndrome
Human immunodeficiency virus
Multi-drug resistant pulmonary tuberculosis
Bronchoalveolar lavage fluid
Bictegravir/tenofovir alafenamide/emtricitabine
Cryptococcal meningitis
World Health Organization
Computed tomography
Total bilirubin
Cerebrospinal fluid
European AIDS Clinical Society
Intracranial pressure
Antiretroviral therapy
Prothrombin activity
Bekker L-G, Beyrer C, Mgodi N, Lewin SR, Delany-Moretlwe S, Taiwo B, et al. HIV infection. Nat Rev Dis Primer. 2023;9:1–21.
Google Scholar
Data on the size of the HIV epidemic. https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/data-on-the-size-of-the-hiv-aids-epidemic?lang=en . Accessed 3 May 2023.
Leumi S, Bigna JJ, Amougou MA, Ngouo A, Nyaga UF, Noubiap JJ. Global burden of hepatitis B infection in people living with human immunodeficiency virus: a systematic review and meta-analysis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020;71:2799–806.
Article Google Scholar
McGovern BH. The epidemiology, natural history and prevention of hepatitis B: implications of HIV coinfection. Antivir Ther. 2007;12(Suppl 3):H3-13.
Article CAS PubMed Google Scholar
Kaplan JE, Masur H, Holmes KK, Wilfert CM, Sperling R, Baker SA, et al. USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus: an overview. USPHS/IDSA Prevention of Opportunistic Infections Working Group. Clin Infect Dis Off Publ Infect Dis Soc Am. 1995;21 Suppl 1:S12-31.
Article CAS Google Scholar
Bamba S, Lortholary O, Sawadogo A, Millogo A, Guiguemdé RT, Bretagne S. Decreasing incidence of cryptococcal meningitis in West Africa in the era of highly active antiretroviral therapy. AIDS Lond Engl. 2012;26:1039–41.
Müller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M, et al. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–61.
Article PubMed PubMed Central Google Scholar
Haddow LJ, Easterbrook PJ, Mosam A, Khanyile NG, Parboosing R, Moodley P, et al. Defining immune reconstitution inflammatory syndrome: evaluation of expert opinion versus 2 case definitions in a South African cohort. Clin Infect Dis Off Publ Infect Dis Soc Am. 2009;49:1424–32.
Obeagu E, Onuoha E. Tuberculosis among HIV patients: a review of Prevalence and Associated Factors. Int J Adv Res Biol Sci. 2023;10:128–34.
Rajasingham R, Govender NP, Jordan A, Loyse A, Shroufi A, Denning DW, et al. The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect Dis. 2022;22:1748–55.
Manegold C, Hannoun C, Wywiol A, Dietrich M, Polywka S, Chiwakata CB, et al. Reactivation of hepatitis B virus replication accompanied by acute hepatitis in patients receiving highly active antiretroviral therapy. Clin Infect Dis Off Publ Infect Dis Soc Am. 2001;32:144–8.
EACS Guidelines | EACSociety. https://www.eacsociety.org/guidelines/eacs-guidelines/ . Accessed 7 May 2023.
Cryptococcosis | NIH. 2021. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/cryptococcosis . Accessed 6 May 2023.
Chang CC, Harrison TS, Bicanic TA, Chayakulkeeree M, Sorrell TC, Warris A, et al. Global guideline for the diagnosis and management of cryptococcosis: an initiative of the ECMM and ISHAM in cooperation with the ASM. Lancet Infect Dis. 2024;10:S1473-3099(23)00731-4.
Deeks ED. Bictegravir/emtricitabine/tenofovir alafenamide: a review in HIV-1 infection. Drugs. 2018;78:1817–28.
Article CAS PubMed PubMed Central Google Scholar
Bellmann R, Smuszkiewicz P. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection. 2017;45:737–79.
Shelburne SA, Darcourt J, White AC, Greenberg SB, Hamill RJ, Atmar RL, et al. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin Infect Dis Off Publ Infect Dis Soc Am. 2005;40:1049–52.
Download references
Acknowledgements
We express our sincere gratitude for the unwavering trust bestowed upon our medical team by the patient throughout the entire treatment process.
This work was supported by the Scientific Research Project of Hunan Public Health Alliance with the approval No. ky2022-002.
Author information
Wei Fu and Zi Wei Deng contributed equally to this work.
Authors and Affiliations
Center for Infectious Diseases, Hunan University of Medicine General Hospital, Huaihua, Hunan, China
Wei Fu, Pei Wang, Zhen Wang Zhu, Ye Pu, Zhi Bing Xie, Yong Zhong Li & Hong Ying Yu
Department of Tuberculosis, The First Affiliated Hospital of Xinxiang Medical University, XinXiang, Henan, China
Department of Clinical Pharmacy, Hunan University of Medicine General Hospital, Huaihua, Hunan, China
Zi Wei Deng
You can also search for this author in PubMed Google Scholar
Contributions
WF and ZWD integrated the data and wrote the manuscript, YHY contributed the revision of the manuscript, PW and YP provided necessary assistance and provided key suggestions, ZWZ, YZL and ZBX contributed data acquisition and interpretation for etiological diagnosis. All authors reviewed and approved the final manuscript.
Corresponding author
Correspondence to Hong Ying Yu .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Ethics Committee of the Hunan University of Medicine General Hospital (HYZY-EC-202306-C1), and with the informed consent of the patient.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Fu, W., Deng, Z.W., Wang, P. et al. A complex case study: coexistence of multi-drug-resistant pulmonary tuberculosis, HBV-related liver failure, and disseminated cryptococcal infection in an AIDS patient. BMC Infect Dis 24 , 533 (2024). https://doi.org/10.1186/s12879-024-09431-9
Download citation
Received : 30 June 2023
Accepted : 24 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1186/s12879-024-09431-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Liver failure
- Disseminated cryptococcal disease
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
- Site Navigation
- HCV Biology

- View all Course Modules
Self-Study Module 4th Edition CNE/CME Available
- Screening and Diagnosis: Self-Study CNE/CME
- HCV Epidemiology in the United States
- Recommendations for Hepatitis C Screening
- Hepatitis C Diagnostic Testing
- Counseling for Prevention of HCV Transmission
- Diagnosis of Acute HCV Infection
- Certificate Requirements CNE/CME
Quick Reference 4th Edition
- Screening and Diagnosis of Hepatitis C Infection: Overview
- Evaluation, Staging, and Monitoring: Self-Study CNE/CME
- Initial Evaluation of Persons with Chronic HCV
- Natural History of HCV Infection
- Counseling Persons with Chronic HCV Infection
- Evaluation and Staging of Liver Fibrosis
- Evaluation and Prognosis of Persons with Cirrhosis
- Surveillance for Hepatocellular Carcinoma
- Extrahepatic Conditions Related to HCV Infection
- Evaluation, Staging, and Monitoring of Chronic Hepatitis C: Overview
- Management of Cirrhosis-Related Complications: Self-Study CNE/CME
- Diagnosis and Management of Ascites
- Recognition and Management of Spontaneous Bacterial Peritonitis
- Screening for Varices and Prevention of Bleeding
- Diagnosis and Management of Hepatic Encephalopathy
- Referral for Liver Transplantation
- Management of Cirrhosis-Related Complications: Overview
- Evaluation and Preparation for Treatment: Self-Study CNE/CME
- Goals and Benefits with HCV Treatment
- Making a Decision on When to Initiate HCV Therapy
- Addressing Structural Barriers to HCV Treatment
- Evaluation and Preparation for Hepatitis C Treatment: Overview
- Treatment of HCV: Self-Study CNE/CME
- Simplified HCV Treatment for All HCV Genotypes
- Retreatment of Patients with Prior HCV Treatment Experience
- Monitoring During and After HCV Treatment
- Treatment of HCV in Persons with Cirrhosis
- Treatment of Acute HCV Infection
- Treatment of Hepatitis C Infection: Overview
- Treatment of Key Populations and Unique Situations: Self-Study CNE/CME
- Treatment of HCV in Persons with HIV Coinfection
- Treatment of HCV in Persons with Renal Impairment
- Treatment of HCV in Persons with Substance Use
- Treatment of HCV in a Correctional Setting
- Management of Health Care Personnel Exposed to HCV
- Perinatal HCV Transmission
- Treatment of Key Populations and Unique Situations: Overview
- CTP Calculator Child-Turcotte-Pugh (CTP) Calculator
- APRI Calculator AST to Platelet Ratio Index (APRI) Calculator
- BMI Calculator Body Mass Index (BMI) Calculator
- CrCl Calculator Creatinine Clearance (CrCl) Calculator
- FIB-4 Calculator Fibrosis-4 (FIB-4) Calculator
- Glasgow Coma Scale Glasgow Coma Scale
- GFR Calculator Glomerular Filtration Rate (GFR) Estimate by MDRD 4-Variable Equation
- MELD Calculator Model for End-Stage Liver Disease (MELD) for ages 12 and older
- SAAG Calculator Serum-Ascites Albumin Gradient (SAAG)
- Alcohol: AUDIT-C Alcohol Use Disorders Identification Test (AUDIT-C)
- Alcohol: CAGE CAGE Alcohol Questionnaire
- Opioid: Risk Tool Opioid Risk Tool (ORT)
- Clinical Consultation
- Clinical Challenges
- Mini- Lectures
- Search Search for Use this Search to quickly find items or navigate the site.
- Screening and Diagnosis of Hepatitis C Infection
- Module 1 Overview Screening and Diagnosis of Hepatitis C Infection
- 1A. Core Concepts
- 2A. Core Concepts
- 2B. CDC: Know More Hepatitis
- 3A. Core Concepts
- 3B. CDC HCV Testing Sequence for Current Infection
- 4A. Core Concepts
- 4B. CDC: Recommendations for Prevention and Control of Hepatitis C Virus
- 4C. HCV Transmission FAQs
- 5A. Core Concepts
- 5B. CDC: Surveillance Guidelines for Acute Hepatitis C
- Progress Tracker
Lesson 1. HCV Epidemiology in the United States
Learning objective performance indicators.
- Describe the epidemiologic profile of the HCV epidemic in the United States
- Discuss application of HCV epidemiological data in public health planning
- Identify key risk factors and their mitigation for transmission of HCV
- Describe the impact of the ongoing opioid epidemic on trends in HCV epidemiology
- Summarize Centers for Disease Control and Prevention (CDC) case definitions for acute and chronic HCV infection
Definitions of HCV Incidence
Method of estimating hcv incidence, hcv incidence data, importance of hcv incidence data, definition of hcv prevalence, hcv prevalence estimates, newly reported chronic hepatitis c cases, awareness of hcv infection status, hcv genotype, hcv-related deaths, overview of risk factors for hcv acquisition, injection drug use, noninjection drug use, sexual exposure, chronic hemodialysis, receipt of clotting factor concentrates, receipt of transfusion of blood products, receipt of immune globulin, organ and tissue transplantation, household contact, tattoos and piercings, cdc hepatitis c case definitions.
- Acute Hepatitis C—2020 Case Definition
- Chronic Hepatitis C Virus Infection—2020 Case Definition
- Perinatal Infection Hepatitis C Infection—2018 Case Definition
Reporting Criteria
Summary points, additional references, hcv incidence in the united states.
The incidence of hepatitis C virus (HCV) infection is defined as the number of new infections in a specific region over a specific time period; the incidence data is typically reported for a 1-year period, often in conjunction with cumulative and comparative multiyear data ( Illustration: David H. Spach, MD " class="linked-doc-1 style-text-width-below document has-link-relation link-relation-below" href="//cdn.hepatitisc.uw.edu/doc/447-6/concept-hcv-incidence.jpg">Figure 1 ). The Centers for Disease Control and Prevention (CDC) defines the incidence of HCV in the United States (or in each state) as the number of acute HCV cases that occur per year, which is the closest proxy of actual new infections. The incidence rate is the number of cases per total population (typically defined as the number per 100,000 persons).
Most individuals with acute HCV infection do not have a clinically evident illness, and most do not seek medical care. In addition, many cases of diagnosed acute hepatitis C are not reported. Thus, determining the true incidence of new HCV infections per year based on the number of reported cases requires highly complex epidemiology modeling techniques. [ 1 ] For each new acute HCV case that is reported in the United States, the CDC estimates there are approximately 13.9 actual new acute HCV cases (reported and unreported) that have occurred. [ 1 , 2 ] This high ratio (total estimated cases to actual reported cases) is primarily a result of the large proportion of persons with acute HCV who have asymptomatic or minimally symptomatic infection and do not seek medical care, or they have undiagnosed HCV infection ( Source: modified from Klevens RM, Liu S, Roberts H, Jiles RB, Holmberg SD. Estimating acute viral hepatitis infections from nationally reported cases. Am J Public Health. 2014;104:482-7. " class="linked-doc-2 style-text-width-below document has-link-relation link-relation-below" href="//cdn.hepatitisc.uw.edu/doc/458-7/conceptual-view-reported-cases-versus-actual-infections.jpg">Figure 2 ); the passive HCV reporting system likely also contributes to the low number of reported acute HCV cases. The CDC provides several numbers related to the incidence of hepatitis C in the United States, including number of reported acute cases, estimated number of acute clinical cases, estimated number of new infections, and rates per 100,000 persons at the state and national level. [ 2 ]
In 2021, a total of 5,023 new cases of acute hepatitis C were reported to the CDC from 44 states; based on this number, the CDC estimated there was a total of 69,800 new acute cases of HCV in 2021. [ 2 ] From 2010 to 2021, the number of estimated annual acute HCV infections has steadily increased, with an overall increase of 492%. [ 2 ] The increase in new HCV infections from 2010 to 2021 is primarily attributed to the opioid epidemic and associated injection drug use, particularly among young adults. [ 3 , 4 , 5 , 6 ] The following summarizes CDC HCV surveillance data for 2021 based on specific groups and demographic factors ( Source: Centers for Disease Control and Prevention (CDC). 2021 Viral Hepatitis Surveillance Report—Hepatitis C. Published August 2023. " class="linked-doc-3 style-text-width-split document document-series has-link-relation link-relation-bottom" href="//cdn.hepatitisc.uw.edu/doc/390-8/acute-cases-hcv-united-states-2010-2021.jpg" onclick="return openFigure(3,this)">Figure 3 ). [ 2 ]
- Gender : The number of reported cases of acute HCV infections was higher in males (3,348) than in females (1,669), with males accounting for two-thirds of the acute HCV infections. [ 2 ]
- Age Group : Most reported acute infections involved persons 20-49 years of age, with the highest number among persons 30-39 years of age. [ 2 ]
- Race/Ethnicity : The highest number of reported cases of acute HCV by race/ethnicity occurred in White persons, followed by Hispanic and Black persons. [ 2 ] White persons comprised 72% (3,097 of the 4,329) cases for which race/ethnicity data was reported. [ 2 ] The highest rate of acute HCV (cases per 100,000 population) was in American Indian/Alaska Native persons, followed by White persons; this has been consistent since 2005. [ 2 ]
- Urban/Rural : Although 84% of cases were diagnosed in Urban areas, the rate of acute HCV (cases per 100,000 population) was slightly higher in rural areas (1.7) versus urban areas (1.3). [ 2 ]
The United States HCV incidence data provide important information for monitoring trends in transmission patterns, developing hepatitis C prevention strategies, monitoring the effectiveness of implemented prevention plans, and identifying focal outbreaks or regional patterns of infection. In addition, valuable information emerges when data is categorized by age group, gender, race/ethnicity, and risk factor for acquiring HCV, as these data may inform major population-specific prevention strategies.
HCV Prevalence in United States
The HCV prevalence is defined as the number (or percent) of persons in the total population living with HCV. Typically, HCV prevalence specifically refers to persons with active (HCV RNA-positive) HCV infection. The HCV prevalence in the United States is dynamic and is impacted by five factors: (1) number of new HCV infections, (2) number of persons who experience a spontaneous HCV cure, (3) number of treatment cures, (4) number of deaths, and (5) number of persons cured who acquire HCV again ( Source: Illustration by David H. Spach, MD " class="linked-doc-4 style-text-width document has-link-relation link-relation-below" href="//cdn.hepatitisc.uw.edu/doc/392-4/dynamics-hcv-prevalence-united-states.jpg">Figure 4 ). Less frequently, the HCV prevalence data is given for all individuals who have ever been infected with HCV, which includes those with active HCV, persons who spontaneously resolved HCV, and those with HCV treatment-related cure. The prevalence rate of chronic hepatitis C is the number of persons living with HCV per population (typically defined as number of persons per 100,000 population).
In the United States, HCV infection is the most common bloodborne infection. The best estimates of HCV prevalence derive from analysis of serum specimens taken from participants in the National Health and Nutrition Examination Survey (NHANES). [ 7 , 8 , 9 , 10 , 11 , 12 ] The NHANES surveys require some adjustments since they do not sample certain populations, such as persons who are incarcerated, persons without housing, nursing home residents, and persons on active military duty. The most recent estimate of HCV prevalence in the United States was generated from analyses of 2017 to 2020 NHANES survey data. [ 12 ] In this time period, there were an estimated 2.2 million persons who were HCV RNA positive (0.9% prevalence among adults). [ 12 ] The following summarizes NHANES HCV prevalence estimate by sex, age group, and race ethnicity ( Source: Lewis KC, Barker LK, Jiles R, Gupta N. Estimated prevalence and awareness of hepatitis C virus infection among U.S. adults- National Health and Nutrition Examination Survey, January 2017-March 2020. Clin Infect Dis. 2023 Jul 7;ciad411. " class="linked-doc-5 style-text-width-split document document-series has-link-relation link-relation-bottom" href="//cdn.hepatitisc.uw.edu/doc/457-3/nhanes-estimates-hcv-prevalence-2017-2020-gender.jpg" onclick="return openFigure(5,this)">Figure 5 ). [ 12 ]
- Sex : In the United States, the estimated HCV prevalence for adult males is 1.4%, which is 2.8-fold higher than the 0.5% prevalence in adult females; among people living with HCV, males account for 73% and females 27%. [ 12 ]
- Age Group : More than 50% of people living with HCV are 55 years of age or older. Persons 55-64 years of age account for the highest number of people living with HCV, with an HCV prevalence of 2.4% . [ 12 ]
- Race/Ethnicity : Based on race/ethnicity, White people have the highest HCV prevalence, accounting for roughly 80% of people living with HCV. [ 12 ] The prevalence rate among White people was 5.0%, which is only slightly higher than the 4.8% prevalence rate in Black people. [ 12 ]
- State and Region : In the United States, 9 states each have more than 65,000 persons living with HCV; these states are California (318,900), Texas (202,500), Florida (151,000), New York (116,000), Pennsylvania (93,900), Ohio (89,600), Michigan (69,100), Tennessee (69,100), and North Carolina (66,400). [ 13 ] Five of these states (New York, Pennsylvania, Ohio, Tennessee, and North Carolina) are in the Appalachian region. [ 13 ] Together, these 9 states account for an estimated 52% of all persons living with HCV nationally. [ 13 ]
In 2021, the CDC reported 107,540 persons in the United States with a new diagnosis of chronic HCV. [ 2 ] Persons 30-39 years of age had the highest numbers of reported new diagnoses of HCV, followed by persons 60 years of age and older. [ 14 ] Among the newly reported chronic HCV cases, 64% were male and 36% female. [ 14 ] ( Source: Centers for Disease Control and Prevention (CDC). 2021 Viral Hepatitis Surveillance Report—Hepatitis C. Published August 2023. " class="linked-doc-6 style-text-width-below document flourish has-link-relation link-relation-below" href="//cdn.hepatitisc.uw.edu/doc/506-1/newly-reported-cases-chronic-hcv-infection--united-states-2021.jpg">Figure 6 )
Using the most recent NHANES data, investigators estimated only 56% of persons infected with HCV in the United States were aware of their HCV infection status. [ 15 ] Awareness of infection status was lower in persons who were foreign-born and in persons with income below the poverty level. [ 15 ] These most recent NHANES data do not show a major improvement from a prior analysis of NHANES data (from 2001-2008) that reported 50% of persons infected with HCV were aware of their HCV infection status. [ 16 ] Awareness of HCV status may be higher among persons engaged in the private health care sector—a study involving persons with access to medical care in four private health care organizations during the years 2006 to 2008 showed an estimated 57% were aware of their HCV infection. [ 17 ]
In the United States, approximately 75% of chronic HCV infections are caused by hepatitis C genotype 1 (subtypes 1a or 1b), 15 to 20% by genotype 2 or 3, and less than 5% by genotypes 4, 5, or 6. [ 18 , 19 , 20 ] Among the genotype 1 infections, genotype 1a is more common than 1b. [ 20 ]
The CDC reports for HCV-related deaths in the United States are based on death certificate data and likely underestimate the true number of HCV-related deaths. [ 21 ] During the years 2010 to 2021, the annual deaths attributed to HCV in the United States peaked in 2014 and 2015, followed by an overall decline from 2016 through 2021. [ 2 ] In 2021, there were 13,385 deaths with hepatitis C listed as the cause of death, and 71% of these deaths occurred in males. [ 2 ] The total number of HCV-related deaths was highest in White persons. [ 2 ] ( Source: Centers for Disease Control and Prevention (CDC). 2021 Viral Hepatitis Surveillance Report—Hepatitis C. Published August 2023. " class="linked-doc-8 style-text-width-split document document-series has-link-relation link-relation-below" href="//cdn.hepatitisc.uw.edu/doc/26-7/annual-hcv-related-deaths-2010-to-2021.jpg" onclick="return openFigure(8,this)">Figure 8 )
Risk Factors for Acquiring HCV
Investigators and public health officials have identified multiple risk factors for acquiring HCV in the United States: injection drug use, history of receiving a blood product transfusion prior to July 1992, receipt of solid-organ transplantation, hemophilia with receipt of factor concentrates made before 1987, male-to-male sex, body tattoos, and intranasal cocaine use. [ 18 , 22 , 23 , 24 ] Among these, injection drug use is the most common and important risk factor for acquiring HCV in the United States. In the 1970s and 1980s, receipt of HCV-infected blood products or organs accounted for nearly 50% of new cases of HCV, but after the discovery of HCV as the cause of non-A, non-B hepatitis in 1989 and the introduction of blood screening tests in the early 1990s, the proportion of new HCV cases caused by contaminated blood or organs dramatically declined. [ 7 , 23 , 25 , 26 ]
Injection drug use remains the most common risk factor for acquiring HCV in the United States, and in 2021, among the 1,449 reported cases of acute HCV for which risk factor information on drug use was available, 57% reported injection drug use as their risk factor for acquiring HCV. [ 2 ] Approximately 20 to 30% of persons who inject drugs become infected with HCV within the first 2 years of starting to inject drugs, and 50% within 5 years. [ 27 ] Transmission risk is greatest with “direct sharing” of needles and syringes, but may also occur indirectly via sharing injection paraphernalia, such as water, cookers, and cotton filters. [ 28 , 29 , 30 ] The incidence of HCV in persons who inject drugs markedly declined from early 1992-2002, likely due to the increased availability of needle exchange programs that arose in response to the HIV epidemic. [ 29 , 31 ] Since 2002, however, multiple reports have documented a surge in HCV infections in the United States among young persons who inject drugs, owing to the ongoing opioid epidemic and continued high rates of receptive needle and syringe sharing ( Source: Handanagic S, Finlayson T, Burnett JC, Broz D, Wejnert C. HIV Infection and HIV-Associated Behaviors Among Persons Who Inject Drugs - 23 Metropolitan Statistical Areas, United States, 2018. MMWR Morb Mortal Wkly Rep. 2021;70:1459-65. " class="linked-doc-7 style-text-width document flourish has-link-relation link-relation-below" href="//cdn.hepatitisc.uw.edu/doc/508-1/receptive-syringe-or-needle-sharing-among-people-who-inject-drugs-pwid-united-states.jpg">Figure 7 ). [ 3 , 4 , 5 , 32 , 33 ]
The role of noninjection drug use, such as snorting crack cocaine, powder cocaine, methamphetamines, or heroin, as a risk factor for acquiring hepatitis C remains controversial. [ 34 ] The risk of acquiring HCV is plausible with use of pipes that may cause burns in the oral mucosa (with possible open mouth sores) or use of straws or tubing that causes erosion of nasal membranes (with bleeding in the nasal passage). Sharing these blood-contaminated devices could then lead to HCV transmission. The prevalence of HCV in people who use noninjection drugs ranges from 2.3 to 35.3%. In some people, the use of noninjection drugs is a surrogate for other risk activities associated with HCV acquisition.
The risk of acquiring HCV through sexual contact with a person who has HCV infection depends on one’s sex partners and the type of sex they engage in. Overall, sexual transmission has accounted for up to 15% of cases of HCV in the United States, but very close interrogation revealed that most cases of sexual transmission also involved injection drug use as a risk factor. Multiple studies involving monogamous heterosexual couples have shown a rate of HCV transmission of less than 1% per year, including one study among 500 serodifferent monogamous couples that reported a sexual transmission rate of 0.07% per year. [ 35 , 36 , 37 , 38 , 39 ] In recent years, however, multiple reports have identified cases of sexual transmission among men who have sex with men (MSM), particularly men with HIV who have sex with other men. [ 40 , 41 , 42 , 43 ] Specific factors identified with HCV transmission among MSM include coinfection with HIV, use of recreational drugs during sex, and certain sex practices that result in rectal bleeding or damage to the rectal mucosa, including fisting and sharing sex toys. [ 40 , 44 ]
The prevalence of HCV infection in persons receiving hemodialysis is approximately 8%, which is nearly 5-fold higher than the general United States population. [ 45 ] Several risk factors have been identified for dialysis patients acquiring HCV, including number of blood transfusions received, number of years on dialysis, mode of dialysis (hemodialysis poses a greater risk than peritoneal dialysis), and the prevalence of HCV in the dialysis unit. In the United States, multiple dialysis-associated HCV outbreaks have occurred. In most cases, HCV transmission likely resulted from inadequate infection control practices, particularly in situations when patients received dialysis immediately after a patient with HCV infection received dialysis. [ 46 , 47 , 48 , 49 ]
In the late 1970s through the mid-1980s, most persons with hemophilia acquired HCV infection via the receipt of contaminated plasma clotting factor concentrates. [ 50 , 51 , 52 ] In 1985, several companies introduced virus inactivation procedures for hemophilia blood products, and by 1987, these procedures were uniformly used, virtually eliminating the risk of transmission of HCV via clotting factor concentrates. Use of recombinant factor concentrate also provides an option for providing factor concentrate with no risk of viral contamination.
In the 1960s, the risk of acquiring HCV from a blood transfusion was approximately 33%. The universal screening of blood and organ donors with the routine use of second-generation HCV antibody tests in 1992 nearly eliminated the subsequent risk of transfusion-associated HCV. [ 26 , 31 , 53 ] In 1999, the introduction of HCV nucleic acid testing (NAT) as a supplement to HCV antibody testing of blood products further reduced the risk of acquiring HCV from a blood transfusion. [ 26 , 54 , 55 ] The estimated risk of acquiring HCV from a blood transfusion decreased further following the introduction, in 1999, of HCV nucleic acid testing (NAT) as a supplement to HCV antibody testing of blood products. [ 54 , 55 ] Blood banks in the United States now use a combination of the third-generation enzyme-linked immunosorbent assay (ELISA) and NAT screening of mini-pool testing (16 samples). The current estimated risk of acquiring HCV from a transfusion in the United States is approximately 1 in 2 million.
In the 1990s, scattered cases and outbreaks of HCV transmission via gamma globulin occurred in the United States and Europe. [ 56 , 57 , 58 ] Subsequently, advances in virus inactivation procedures have nearly eliminated any risk of HCV transmission with immune globulin, and the manufacturers of intravenous immune globulin use vigorous viral inactivation and removal procedures.
Rare cases of inadvertent HCV transmission via organ or tissue transplantation have occurred in the United States. [ 59 , 60 ] Most cases of transmission have involved HCV antibody-negative, HCV RNA-positive donors. [ 59 , 60 ] In 2013, the U.S. Public Health Service drafted updated guidelines for preventing HCV transmission through organ transplantation, and these guidelines recommend HCV RNA testing of all organ and tissue donors, which has further reduced the risk of inadvertent HCV transmission. [ 61 ] It should be noted, however, that owing to the safety profile and effectiveness of direct-acting antivirals for HCV, some institutions now allow for HCV-positive organ donations, with a plan to treat organ recipients post-transplant.
In the United States, due to the opioid epidemic, there has been a significant increase in HCV infection in recent years among women of childbearing age; this trend has raised concerns about a potentially significant increase in perinatal HCV infections. [ 62 , 63 , 64 ] Among pregnant women with chronic HCV, approximately 6% will transmit HCV to their child. [ 65 , 66 , 67 ] Nearly all cases of perinatal HCV transmission have involved mothers who had detectable HCV RNA in plasma during pregnancy. Women coinfected with HIV and HCV have an approximately twofold higher risk of perinatal HCV transmission when compared with women who have HCV monoinfection. [ 65 ] The risk of HCV transmission via breastfeeding appears to be negligible. [ 65 , 66 , 67 ]
Acquiring HCV via nonsexual household contact with a person infected with HCV can occur, but the number of documented cases is extremely low. [ 68 , 69 ] Transmission in this setting would most likely involve sharing a razor or toothbrush, since this process could involve transmission via a blood-tainted device.
In the United States, the risk of acquiring HCV from a licensed, regulated professional tattoo or piercing center is extremely low. [ 70 ] Tattoos performed in unregulated and unlicensed tattoo centers, such as with tattoos applied by friends or in prison, increase the risk for HCV acquisition. [ 70 , 71 ]
CDC Case Definitions and Reporting
The CDC has established case definitions and reporting criteria for acute and for past (resolved) or present (chronic) hepatitis C infection.
Acute Hepatitis C—2020 Case Definition
The CDC 2020 Case Definition for Acute Hepatitis C infection includes clinical and laboratory criteria ( Source: Centers for Disease Control and Prevention (CDC) " class="linked-doc-9 style-text-width-below document has-link-relation link-relation-below" href="//cdn.hepatitisc.uw.edu/doc/23-8/acute-hepatitis-c-2020-case-definitionclinical-laboratory-criteria.jpg">Figure 9 ), along with a case classification as probable or confirmed. [ 72 ] Of note, a patient can have a confirmed case of acute HCV based on laboratory data alone (a hepatitis test conversion documented by a negative HCV antibody, HCV antigen, or NAT laboratory test result, followed within 12 months by a positive result with any of these tests). Symptomatic cases often go unreported for multiple reasons: many persons with symptomatic acute HCV do not seek medical care, the diagnosis may be missed, and medical providers may fail to report diagnosed cases. It is important not to report cases that have already been reported. A more detailed discussion of the Acute Hepatitis C 2020 Case Definition is included in the lesson Diagnosis of Acute HCV Infection .
Chronic Hepatitis C Virus Infection—2020 Case Definition
The CDC 2020 Case Definition for Chronic Hepatitis C includes clinical and laboratory criteria ( Source: Centers for Disease Control and Prevention (CDC) " class="linked-doc-10 style-text-width-below document has-link-relation link-relation-below" href="//cdn.hepatitisc.uw.edu/doc/24-5/chronic-hepatitis-c-2020-case-definitionclinical-laboratory-criteria.jpg">Figure 10 ), as well as a case classification. [ 73 ] These cases pertain to persons with current (active) HCV infection and do not represent persons who spontaneously cleared HCV infection. Also, it is important not to report cases that have already been reported.
Perinatal Infection Hepatitis C Infection—2018 Case Definition
The CDC 2018 Case Definition for Hepatitis C, Perinatal Infection includes clinical criteria, laboratory criteria for diagnosis, criteria to distinguish a new case from an existing case, and a case classification. [ 74 ] A confirmed case requires the following:
- Infant who has a positive test for HCV RNA nucleic acid amplification test (NAAT), HCV antigen, or detectable HCV genotype at ≥2 months and ≤36 months of age and is not known to have been exposed to HCV via a mechanism other than perinatal.
Persons identified with acute HCV should undergo an interview to determine an identifiable risk factor in the 2-week to 6-month time frame that preceded the onset of their illness. Similarly, the individual with past (resolved) or present (chronic) HCV infection should be interviewed to determine lifetime risk factors for HCV. The Viral Hepatitis Case Report form should be filled out for persons identified with either acute HCV infection or past/present HCV. Cases of HCV should be reported to a health department, which in turn submits reporting data to the CDC via the Nationally Notifiable Diseases Surveillance System (NNDSS).
- The estimated number of annual acute HCV infections in the United States increased significantly from 11,800 in 2010 to 69,800 in 2021.
- The recent increases in new HCV infections have primarily resulted from the ongoing opioid epidemic and the associated injection drug use.
- In 2021, the highest number of new HCV infections occurred in persons 30-39 years of age.
- In 2021, American Indian/Alaskan Native, White, and Black individuals were among the top three groups with the greatest prevalence rate of new HCV infections.
- Based on CDC estimates, there are 2.2 million persons living with HCV infection, corresponding to a 0.9% HCV prevalence among the adult population in the United States.
- The HCV prevalence is dynamic and impacted by the number of new HCV infections, the number of persons that spontaneously resolve their infection, the number of treatment cures, the number of deaths, and the number of cured persons who become reinfected.
- It is estimated that approximately 56% of persons with HCV infection are aware of their infection in the United States.
- Injection drug use is the most common risk factor for HCV acquisition; sexual transmission of HCV can occur, but this most often involves MSM; the risk among MSM is substantially higher in those with HIV.
- The number of annual deaths attributed to HCV has declined significantly in recent years, falling from 19,613 in 2014 to 13,895 in 2021, with most of the deaths involving males.
- The CDC has established a clear definition of acute, chronic, and perinatal HCV infection for reporting purposes as well as reporting guidelines.
- 1. Klevens RM, Liu S, Roberts H, Jiles RB, Holmberg SD. Estimating acute viral hepatitis infections from nationally reported cases. Am J Public Health. 2014;104:482-7. [ PubMed Abstract ] -
- 2. Centers for Disease Control and Prevention (CDC). Hepatitis C Surveillance 2021. Published August 2023. [ CDC ] -
- 3. Zibbell JE, Asher AK, Patel RC, et al. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am J Public Health. 2018;108:175-181. [ PubMed Abstract ] -
- 4. Zibbell JE, Hart-Malloy R, Barry J, Fan L, Flanigan C. Risk factors for HCV infection among young adults in rural New York who inject prescription opioid analgesics. Am J Public Health. 2014;104:2226-32. [ PubMed Abstract ] -
- 5. Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006-2012. MMWR Morb Mortal Wkly Rep. 2015;64:453-8. [ PubMed Abstract ] -
- 6. Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006-2012. Clin Infect Dis. 2014;59:1411-9. [ PubMed Abstract ] -
- 7. Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2013;60:691-8. [ PubMed Abstract ] -
- 8. Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, Holmberg SD. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160:293-300. [ PubMed Abstract ] -
- 9. Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705-14. [ PubMed Abstract ] -
- 10. Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556-62. [ PubMed Abstract ] -
- 11. Hofmeister MG, Rosenthal EM, Barker LK, et al. Estimating Prevalence of Hepatitis C Virus Infection in the United States, 2013-2016. Hepatology. 2019;69:1020-31. [ PubMed Abstract ] -
- 12. Lewis KC, Barker LK, Jiles RB, Gupta N. Estimated Prevalence and Awareness of Hepatitis C Virus Infection Among US Adults: National Health and Nutrition Examination Survey, January 2017-March 2020. Clin Infect Dis. 2023;77:1413-5. [ PubMed Abstract ] -
- 13. Bradley H, Hall EW, Rosenthal EM, Sullivan PS, Ryerson AB, Rosenberg ES. Hepatitis C Virus Prevalence in 50 U.S. States and D.C. by Sex, Birth Cohort, and Race: 2013-2016. Hepatol Commun. 2020;4:355-70. [ PubMed Abstract ] -
- 14. Centers for Disease Control and Prevention (CDC). 2019 Viral Hepatitis Surveillance Report—Hepatitis C. Published May 2021. [ CDC ] -
- 15. Kim HS, Yang JD, El-Serag HB, Kanwal F. Awareness of chronic viral hepatitis in the United States: An update from the National Health and Nutrition Examination Survey. J Viral Hepat. 2019;26:596-602. [ PubMed Abstract ] -
- 16. Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001-2008. Hepatology. 2012;55:1652-61. [ PubMed Abstract ] -
- 17. Spradling PR, Rupp L, Moorman AC, et al. Hepatitis B and C Virus Infection Among 1.2 Million Persons With Access to Care: Factors Associated With Testing and Infection Prevalence. Clin Infect Dis. 2012;55:1047-55. [ PubMed Abstract ] -
- 18. Guss D, Sherigar J, Rosen P, Mohanty SR. Diagnosis and Management of Hepatitis C Infection in Primary Care Settings. J Gen Intern Med. 2018;33:551-7. [ PubMed Abstract ] -
- 19. Germer JJ, Mandrekar JN, Bendel JL, Mitchell PS, Yao JD. Hepatitis C virus genotypes in clinical specimens tested at a national reference testing laboratory in the United States. J Clin Microbiol. 2011;49:3040-3. [ PubMed Abstract ] -
- 20. Manos MM, Shvachko VA, Murphy RC, Arduino JM, Shire NJ. Distribution of hepatitis C virus genotypes in a diverse US integrated health care population. J Med Virol. 2012;84:1744-50. [ PubMed Abstract ] -
- 21. Mahajan R, Xing J, Liu SJ, et al. Mortality among persons in care with hepatitis C virus infection: the Chronic Hepatitis Cohort Study (CHeCS), 2006-2010. Clin Infect Dis. 2014;58:1055-61. [ PubMed Abstract ] -
- 22. Bragg DA, Crowl A, Manlove E. Hepatitis C: A New Era. Prim Care. 2017;44:631-642. [ PubMed Abstract ] -
- 23. Williams I. Epidemiology of hepatitis C in the United States. Am J Med. 1999;107(6B):2S-9S. [ PubMed Abstract ] -
- 24. Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26(3 Suppl 1):62S-65S. [ PubMed Abstract ] -
- 25. Stramer SL. Current risks of transfusion-transmitted agents: a review. Arch Pathol Lab Med. 2007;131:702-7. [ PubMed Abstract ] -
- 26. Alter HJ, Houghton M. Clinical Medical Research Award. Hepatitis C virus and eliminating post-transfusion hepatitis. Nat Med. 2000;6:1082-6. [ PubMed Abstract ] -
- 27. Hagan H, Pouget ER, Des Jarlais DC, Lelutiu-Weinberger C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am J Epidemiol. 2008;168:1099-109. [ PubMed Abstract ] -
- 28. Pouget ER, Hagan H, Des Jarlais DC. Meta-analysis of hepatitis C seroconversion in relation to shared syringes and drug preparation equipment. Addiction. 2012;107:1057-65. [ PubMed Abstract ] -
- 29. Holtzman D, Barry V, Ouellet LJ, et al. The influence of needle exchange programs on injection risk behaviors and infection with hepatitis C virus among young injection drug users in select cities in the United States, 1994-2004. Prev Med. 2009;49:68-73. [ PubMed Abstract ] -
- 30. Hagan H, Thiede H, Weiss NS, Hopkins SG, Duchin JS, Alexander ER. Sharing of drug preparation equipment as a risk factor for hepatitis C. Am J Public Health. 2001;91:42-6. [ PubMed Abstract ] -
- 31. Klevens RM, Hu DJ, Jiles R, Holmberg SD. Evolving epidemiology of hepatitis C virus in the United States. Clin Infect Dis. 2012;55 Suppl 1:S3-9. [ PubMed Abstract ] -
- 32. Centers for Disease Control and Prevention (CDC). Hepatitis C virus infection among adolescents and young adults: Massachusetts, 2002-2009. MMWR Morb Mortal Wkly Rep. 2011;60:537-41. [ PubMed Abstract ] -
- 33. Handanagic S, Finlayson T, Burnett JC, Broz D, Wejnert C. HIV Infection and HIV-Associated Behaviors Among Persons Who Inject Drugs - 23 Metropolitan Statistical Areas, United States, 2018. MMWR Morb Mortal Wkly Rep. 2021;70:1459-65. [ PubMed Abstract ] -
- 34. Scheinmann R, Hagan H, Lelutiu-Weinberger C, Stern R, Des Jarlais DC, Flom PL, Strauss S. Non-injection drug use and hepatitis C virus: a systematic review. Drug Alcohol Depend. 2007;89:1-12. [ PubMed Abstract ] -
- 35. Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36(5 Suppl 1):S99-105. [ PubMed Abstract ] -
- 36. Kao JH, Liu CJ, Chen PJ, Chen W, Lai MY, Chen DS. Low incidence of hepatitis C virus transmission between spouses: a prospective study. J Gastroenterol Hepatol. 2000;15:391-5. [ PubMed Abstract ] -
- 37. Tahan V, Karaca C, Yildirim B, et al. Sexual transmission of HCV between spouses. Am J Gastroenterol. 2005;100:821-4. [ PubMed Abstract ] -
- 38. Vandelli C, Renzo F, Romanò L, et al. Lack of evidence of sexual transmission of hepatitis C among monogamous couples: results of a 10-year prospective follow-up study. Am J Gastroenterol. 2004;99:855-9. [ PubMed Abstract ] -
- 39. Terrault NA, Dodge JL, Murphy EL, Tavis JE, Kiss A, Levin TR, Gish RG, Busch MP, Reingold AL, Alter MJ. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology. 2013;57:881-9. [ PubMed Abstract ] -
- 40. Centers for Disease Control and Prevention (CDC). Sexual transmission of hepatitis C virus among HIV-infected men who have sex with men--New York City, 2005-2010. MMWR Morb Mortal Wkly Rep. 2011;60:945-50. [ PubMed Abstract ] -
- 41. Hoornenborg E, Achterbergh RCA, Schim van der Loeff MF, et al. MSM starting preexposure prophylaxis are at risk of hepatitis C virus infection. AIDS. 2017;31:1603-1610. [ PubMed Abstract ] -
- 42. Jin F, Matthews GV, Grulich AE. Sexual transmission of hepatitis C virus among gay and bisexual men: a systematic review. Sex Health. 2017;14:28-41. [ PubMed Abstract ] -
- 43. Urbanus AT, Van De Laar TJ, Geskus R, et al. Trends in hepatitis C virus infections among MSM attending a sexually transmitted infection clinic; 1995-2010. AIDS. 2014;28:781-90. [ PubMed Abstract ] -
- 44. Gorgos L. Sexual transmission of viral hepatitis. Infect Dis Clin North Am. 2013;27:811-36. [ PubMed Abstract ] -
- 45. Patel PR, Thompson ND, Kallen AJ, Arduino MJ. Epidemiology, surveillance, and prevention of hepatitis C virus infections in hemodialysis patients. Am J Kidney Dis. 2010;56:371-8. [ PubMed Abstract ] -
- 46. Thompson ND, Novak RT, Datta D, et al. Hepatitis C virus transmission in hemodialysis units: importance of infection control practices and aseptic technique. Infect Control Hosp Epidemiol. 2009;30:900-3. [ PubMed Abstract ] -
- 47. Nguyen DB, Gutowski J, Ghiselli M, et al. A Large Outbreak of Hepatitis C Virus Infections in a Hemodialysis Clinic. Infect Control Hosp Epidemiol. 2016;37:125-33. [ PubMed Abstract ] -
- 48. Centers for Disease Control and Prevention (CDC). Hepatitis C virus transmission at an outpatient hemodialysis unit--New York, 2001-2008. MMWR Morb Mortal Wkly Rep. 2009;58:189-94. [ PubMed Abstract ] -
- 49. Muleta D, Kainer MA, Moore-Moravian L, et al. Notes from the Field: Hepatitis C Outbreak in a Dialysis Clinic--Tennessee, 2014. MMWR Morb Mortal Wkly Rep. 2016;64:1386-7. [ PubMed Abstract ] -
- 50. Zoulim F, Bailly F. New approaches to the management of hepatitis C in haemophilia in 2012. Haemophilia. 2012;18 Suppl 4:28-33. [ PubMed Abstract ] -
- 51. Witkop ML, Peerlinck K, Luxon BA. Medical co-morbidities of patients with haemophilia: pain, obesity and hepatitis C. Haemophilia. 2016;22 Suppl 5:47-53. [ PubMed Abstract ] -
- 52. Troisi CL, Hollinger FB, Hoots WK, et al. A multicenter study of viral hepatitis in a United States hemophilic population. Blood. 1993;81:412-8. [ PubMed Abstract ] -
- 53. Donahue JG, Muñoz A, Ness PM, et al. The declining risk of post-transfusion hepatitis C virus infection. N Engl J Med. 1992;327:369-73. [ PubMed Abstract ] -
- 54. Busch MP. Transfusion-transmitted viral infections: building bridges to transfusion medicine to reduce risks and understand epidemiology and pathogenesis. Transfusion. 2006;46:1624-40. [ PubMed Abstract ] -
- 55. Perkins HA, Busch MP. Transfusion-associated infections: 50 years of relentless challenges and remarkable progress. Transfusion. 2010;50:2080-99. [ PubMed Abstract ] -
- 56. Bresee JS, Mast EE, Coleman PJ, et al. Hepatitis C virus infection associated with administration of intravenous immune globulin. A cohort study. JAMA. 1996;276:1563-7. [ PubMed Abstract ] -
- 57. Bjøro K, Frøland SS, Yun Z, Samdal HH, Haaland T. Hepatitis C infection in patients with primary hypogammaglobulinemia after treatment with contaminated immune globulin. N Engl J Med. 1994;331:1607-11. [ PubMed Abstract ] -
- 58. Centers for Disease Control and Prevention (CDC). Outbreak of hepatitis C associated with intravenous immunoglobulin administration--United States, October 1993-June 1994. MMWR Morb Mortal Wkly Rep. 1994;43:505-9. [ PubMed Abstract ] -
- 59. Centers for Disease Control and Prevention (CDC). Transmission of hepatitis C virus through transplanted organs and tissue--Kentucky and Massachusetts, 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1697-700. [ PubMed Abstract ] -
- 60. Tugwell BD, Patel PR, Williams IT, et al. Transmission of hepatitis C virus to several organ and tissue recipients from an antibody-negative donor. Ann Intern Med. 2005;143:648-54. [ PubMed Abstract ] -
- 61. Seem DL, Lee I, Umscheid CA, Kuehnert MJ. PHS guideline for reducing human immunodeficiency virus, hepatitis B virus, and hepatitis C virus transmission through organ transplantation. Public Health Rep. 2013;128:247-343. [ PubMed Abstract ] -
- 62. Ly KN, Jiles RB, Teshale EH, Foster MA, Pesano RL, Holmberg SD. Hepatitis C Virus Infection Among Reproductive-Aged Women and Children in the United States, 2006 to 2014. Ann Intern Med. 2017;166:775-782. [ PubMed Abstract ] -
- 63. Koneru A, Nelson N, Hariri S, et al. Increased Hepatitis C Virus (HCV) Detection in Women of Childbearing Age and Potential Risk for Vertical Transmission - United States and Kentucky, 2011-2014. MMWR Morb Mortal Wkly Rep. 2016;65:705-10. [ PubMed Abstract ] -
- 64. Panagiotakopoulos L, Sandul AL, Conners EE, Foster MA, Nelson NP, Wester C. CDC Recommendations for Hepatitis C Testing Among Perinatally Exposed Infants and Children - United States, 2023. MMWR Recomm Rep. 2023;72:1-21. [ PubMed Abstract ] -
- 65. Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis. 2014;59:765-73. [ PubMed Abstract ] -
- 66. Page CM, Hughes BL, Rhee EHJ, Kuller JA. Hepatitis C in Pregnancy: Review of Current Knowledge and Updated Recommendations for Management. Obstet Gynecol Surv. 2017;72:347-355. [ PubMed Abstract ] -
- 67. Post JJ. Update on hepatitis C and implications for pregnancy. Obstet Med. 2017;10:157-160. [ PubMed Abstract ] -
- 68. Diago M, Zapater R, Tuset C, et al. Intrafamily transmission of hepatitis C virus: sexual and non-sexual contacts. J Hepatol. 1996;25:125-8. [ PubMed Abstract ] -
- 69. Ackerman Z, Ackerman E, Paltiel O. Intrafamilial transmission of hepatitis C virus: a systematic review. J Viral Hepat. 2000;7:93-103. [ PubMed Abstract ] -
- 70. Tohme RA, Holmberg SD. Transmission of hepatitis C virus infection through tattooing and piercing: a critical review. Clin Infect Dis. 2012;54:1167-78. [ PubMed Abstract ] -
- 71. Carney K, Dhalla S, Aytaman A, Tenner CT, Francois F. Association of tattooing and hepatitis C virus infection: a multicenter case-control study. Hepatology. 2013;57:2117-23. [ PubMed Abstract ] -
- 72. Centers for Disease Control and Prevention (CDC). National Notifiable Diseases Surveillance System (NNDSS). Hepatitis C, Acute: 2020 Case Definition. [ CDC and NNDSS ] -
- 73. Centers for Disease Control and Prevention (CDC). National Notifiable Diseases Surveillance System (NNDSS). Hepatitis C, Chronic: 2020 Case Definition. [ CDC and NNDSS ] -
- 74. Centers for Disease Control and Prevention (CDC). National Notifiable Diseases Surveillance System (NNDSS). Hepatitis C, Perinatal Infection: 2018 Case Definition. [ CDC and NNDSS ] -
- Binka M, Paintsil E, Patel A, Lindenbach BD, Heimer R. Survival of Hepatitis C Virus in Syringes Is Dependent on the Design of the Syringe-Needle and Dead Space Volume. PLoS One. 2015;10:e0139737. [ PubMed Abstract ] -
- Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090-101. [ PubMed Abstract ] -
- Chhatwal J, Wang X, Ayer T, et al. Hepatitis C Disease Burden in the United States in the era of oral direct-acting antivirals. Hepatology. 2016;64:1442-1450. [ PubMed Abstract ] -
- Durham DP, Skrip LA, Bruce RD, et al. The Impact of Enhanced Screening and Treatment on Hepatitis C in the United States. Clin Infect Dis. 2015;62:298-304. [ PubMed Abstract ] -
- Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62:1353-63. [ PubMed Abstract ] -
- Gish RG, Cohen CA, Block JM, et al. Data supporting updating estimates of the prevalence of chronic hepatitis B and C in the United States. Hepatology. 2015;62:1339-41. [ PubMed Abstract ] -
- Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017. pii: S0168-8278(17)32273-0. [ PubMed Abstract ] -
- Ly KN, Hughes EM, Jiles RB, Holmberg SD. Rising Mortality Associated With Hepatitis C Virus in the United States, 2003-2013. Clin Infect Dis. 2016;62:1287-8. [ PubMed Abstract ] -
- Paintsil E, Binka M, Patel A, Lindenbach BD, Heimer R. Hepatitis C virus maintains infectivity for weeks after drying on inanimate surfaces at room temperature: implications for risks of transmission. J Infect Dis. 2014;209:1205-11. [ PubMed Abstract ] -
- Rosenberg ES, Rosenthal EM, Hall EW, et al. Prevalence of Hepatitis C Virus Infection in US States and the District of Columbia, 2013 to 2016. JAMA Netw Open. 2018;1:e186371. [ PubMed Abstract ] -
- Singer AW, Reddy KR, Telep LE, et al. Direct-acting antiviral treatment for hepatitis C virus infection and risk of incident liver cancer: a retrospective cohort study. Aliment Pharmacol Ther. 2018;47:1278-1287. [ PubMed Abstract ] -

Share by e-mail
You must be signed in to customize your interaction with these questions..
- Elbasvir-Grazoprevir ( Zepatier )
- Glecaprevir-Pibrentasvir ( Mavyret )
- Ledipasvir-Sofosbuvir ( Harvoni )
- Ribavirin ( Copegus, Rebetol, Ribasphere )
- Sofosbuvir ( Sovaldi )
- Sofosbuvir-Velpatasvir ( Epclusa )
- Sofosbuvir-Velpatasvir-Voxilaprevir ( Vosevi )
- Evaluation, Staging, and Monitoring of Chronic Hepatitis C
- Management of Cirrhosis-Related Complications
- Evaluation and Preparation for Hepatitis C Treatment
- Treatment of Hepatitis C Infection
- Treatment of Key Populations and Unique Situations
- Clinical Calculators
- Substance Use Screening Tools
Since you've received 80% or better on this quiz, you may claim continuing education credit.
You seem to have a popup blocker enabled. If you want to skip this dialog please Always allow popup windows for the online course .
- Open access
- Published: 24 May 2024
Integration of case-based learning and three-dimensional printing for tetralogy of fallot instruction in clinical medical undergraduates: a randomized controlled trial
- Jian Zhao 1 na1 ,
- Xin Gong 1 na1 ,
- Jian Ding 1 ,
- Kepin Xiong 2 ,
- Kangle Zhuang 3 ,
- Rui Huang 1 ,
- Shu Li 4 &
- Huachun Miao 1
BMC Medical Education volume 24 , Article number: 571 ( 2024 ) Cite this article
272 Accesses
Metrics details
Case-based learning (CBL) methods have gained prominence in medical education, proving especially effective for preclinical training in undergraduate medical education. Tetralogy of Fallot (TOF) is a congenital heart disease characterized by four malformations, presenting a challenge in medical education due to the complexity of its anatomical pathology. Three-dimensional printing (3DP), generating physical replicas from data, offers a valuable tool for illustrating intricate anatomical structures and spatial relationships in the classroom. This study explores the integration of 3DP with CBL teaching for clinical medical undergraduates.
Sixty senior clinical medical undergraduates were randomly assigned to the CBL group and the CBL-3DP group. Computed tomography imaging data from a typical TOF case were exported, processed, and utilized to create four TOF models with a color 3D printer. The CBL group employed CBL teaching methods, while the CBL-3DP group combined CBL with 3D-printed models. Post-class exams and questionnaires assessed the teaching effectiveness of both groups.
The CBL-3DP group exhibited improved performance in post-class examinations, particularly in pathological anatomy and TOF imaging data analysis ( P < 0.05). Questionnaire responses from the CBL-3DP group indicated enhanced satisfaction with teaching mode, promotion of diagnostic skills, bolstering of self-assurance in managing TOF cases, and cultivation of critical thinking and clinical reasoning abilities ( P < 0.05). These findings underscore the potential of 3D printed models to augment the effectiveness of CBL, aiding students in mastering instructional content and bolstering their interest and self-confidence in learning.
The fusion of CBL with 3D printing models is feasible and effective in TOF instruction to clinical medical undergraduates, and worthy of popularization and application in medical education, especially for courses involving intricate anatomical components.
Peer Review reports
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart disease(CHD) [ 1 ]. Characterized by four structural anomalies: ventricular septal defect (VSD), pulmonary stenosis (PS), right ventricular hypertrophy (RVH), and overriding aorta (OA), TOF is a focal point and challenge in medical education. Understanding anatomical spatial structures is pivotal for learning and mastering TOF [ 2 ]. Given the constraints of course duration, medical school educators aim to provide students with a comprehensive and intuitive understanding of the disease within a limited timeframe [ 3 ].
The case-based learning (CBL) teaching model incorporates a case-based instructional approach that emphasizes typical clinical cases as a guide in student-centered and teacher-facilitated group discussions [ 4 ]. The CBL instructional methods have garnered widespread attention in medical education as they are particularly appropriate for preclinical training in undergraduate medical education [ 5 , 6 ]. The collection of case data, including medical records and examination results, is essential for case construction [ 7 ]. The anatomical and hemodynamic consequences of TOF can be determined using ultrasonography, computed tomography (CT), and magnetic resonance imaging techniques. However, understanding the anatomical structures from imaging data is a slow and challenging psychological reconstruction process for undergraduate medical students [ 8 ]. Three-dimensional (3D) visualization is valuable for depicting anatomical structures [ 9 ]. 3D printing (3DP), which creates physical replicas based on data, facilitates the demonstration of complex anatomical structures and spatial relationships in the classroom [ 10 ].
During the classroom session, 3D-printed models offer a convenient means for hands-on demonstration and communication, similar to facing a patient, enhancing the efficiency and specificity of intra-team communication and discussion [ 11 ]. In this study, we printed TOF models based on case imaging data, integrated them into CBL teaching, and assessed the effectiveness of classroom instruction.
Research participants
The study employed a prospective, randomized controlled design which received approval from the institutional ethics committee. Senior undergraduate students majoring in clinical medicine at Wannan Medical College were recruited for participation based on predefined inclusion criteria. The researchers implemented recruitment according to the recruitment criteria by contacting the class leaders of the target classes they had previously taught. Notably, these students were in their third year of medical education, with anticipation of progressing to clinical courses in the fourth year, encompassing Internal Medicine, Surgery, Obstetrics, Gynecology, and Pediatrics. Inclusion criteria for participants encompassed the following: (1) proficient communication and comprehension abilities, (2) consistent attendance without absenteeism or truancy, (3) absence of failing grades in prior examinations, and (4) capability to conscientiously fulfill assigned learning tasks. Exclusion criteria were (1) absence from lectures, (2) failure to complete pre-and post-tests, and (3) inadequate completion of questionnaires. For their participation in the study, Students were provided access to the e-book “Localized Anatomy,” authored by the investigators, as an incentive for their participation. Voluntary and anonymous participation was emphasized, with participants retaining the right to withdraw from the study at any time without providing a reason.
The study was conducted between May 1st, 2023, and June 30, 2023, from recruitment to completion of data collection. Drawing upon insights gained from a previous analogous investigation which yielded an effect size of 0.95 [ 10 ]. Sample size was computed, guided by a statistical consultant, with the aim of 0.85 power value, predicated on an effect size of 0.8 and a margin of error set at 0.05. A minimum of 30 participants per group was calculated using G*Power software (latest ver. 3.1.9.7; Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany), resulting in the recruitment of a total of 60 undergraduate students. Each participant was assigned an identification number, with codes placed in boxes. Codes drawn from the boxes determined allocation to either the CBL group or the CBL-3DP group. Subsequently, participants were randomly assigned to either the CBL group, receiving instruction utilizing the CBL methodology, or the CBL-3DP group, which received instruction integrating both CBL and 3D Printed models.
Printing of TOF models
Figure 1 A shows the printing flowchart of the TOF models. A typical TOF case was collected from the Yijishan Hospital of Wannan Medical College. The CT angiography imaging data of the case was exported. Mimics Research 20.0 software (Mimics Innovation Suite version 20, Materialize, Belgium) was used for data processing. The cardiovascular module of the CT-Heart tool was employed to adjust the threshold range, independently obtain the cardiac chambers and vessels, post-process the chambers and vessels to generate a hollow blood pool, and merge it with the myocardial volume to construct a complete heart model. The file was imported into Magics 24.0 software (version 24.0; Materialize, Belgium) for correction using the Shell tool page. After repairs, the model entered the smoothing page, where tools such as triangular surface simplification, local smoothing, refinement and smoothing, subdivision of components, and mesh painting were utilized to achieve varying degrees of smoothness. Finally, optimized data were obtained and exported as stereolithography (STL) files. An experienced cardiothoracic surgeon validated the anatomical accuracy of the digital model.
The STL files were imported into a 3D printer (J401Pro; Sailner 3D Technology, China) for model printing. This printer can produce full-color medical models using different materials. The models were fabricated using two distinct materials: rigid and flexible. Both materials are suitable for the observational discussion of the teaching objectives outlined in our study. From the perspective of observing pathological changes in the TOF, there is no significant difference between the two materials.
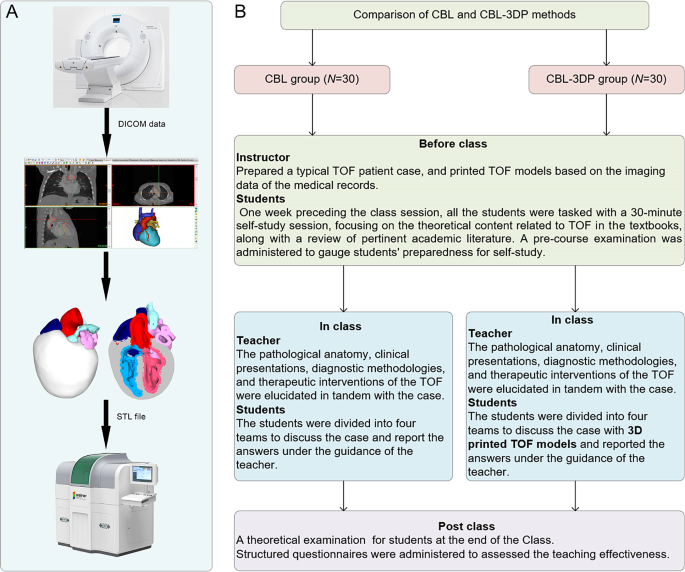
Experimental flow chart of this study. A TOF model printing flow chart. B The instructional framework
Teaching implementation
Figure 1 B illustrates the instructional framework employed in this study. One week preceding the class session, all the students were tasked with a 30-minute self-study session, focusing on the theoretical content related to TOF as outlined in the Pediatrics and Surgery textbooks, along with a review of pertinent academic literature. Both groups received co-supervision from two basic medicine lecturers boasting over a decade of teaching experience, alongside a senior cardiothoracic surgeon. Teaching conditions remained consistent across groups, encompassing uniform assessment criteria and adherence to predefined teaching time frames, all conducted in a Project-Based Learning (PBL) classroom at Wannan Medical College. Additionally, a pre-course examination was administered to gauge students’ preparedness for self-study.
In adherence to the curriculum guidelines, the teaching objectives aimed to empower students to master TOF’s clinical manifestations, diagnostic modalities, and differential diagnoses, while acquainting them with treatment principles and surgical methodologies. Additionally, the objectives sought to cultivate students’ clinical reasoning abilities and problem-solving skills. the duration of instruction for the TOF theory session was standardized to 25 min. The didactic content was integrated with the TOF case study to construct a coherent pedagogical structure.
During the instructional session, both groups underwent teaching utilizing the CBL methodology. Clinical manifestations and case details of TOF cases were presented to stimulate students’ interest and curiosity. Subsequently, the theory of TOF, including its etiology, pathogenesis, pathologic anatomy, clinical manifestations, diagnostic methods, and therapeutic interventions, was briefly elucidated. Emphasis was then placed on the case, wherein selected typical TOF cases were explained, guiding students in analysis and discussion. Students were organized into four teams under the instructors’ supervision, fostering cooperative learning and communication, thereby deepening their understanding of the disease through continuous inquiry and exploration (Fig. 2 L). In the routinely equipped PBL classroom with standard heart models (Fig. 2 J, K), all students had prior exposure to human anatomy and were familiar with these models. Both groups were provided with four standard heart models for reference, while the CBL-3DP group received additional four 3D-printed models depicting TOF anomalies, enriching their learning experience (Fig. 2 D, G). After the lesson, summarization, and feedback sessions were conducted to consolidate group discussions’ outcomes, evaluate teaching effectiveness, and assess learning outcomes.
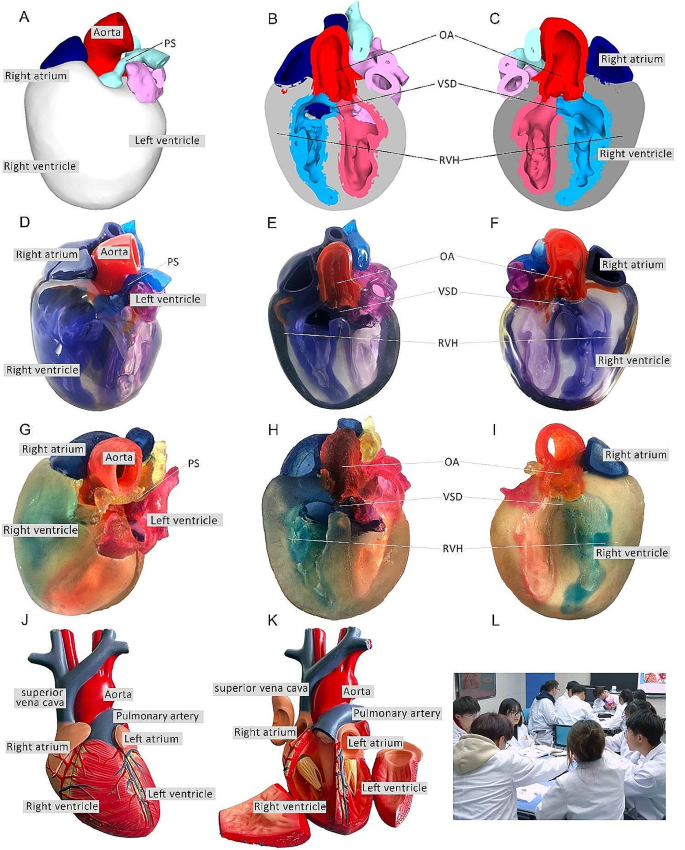
Heart models utilized in instructional sessions. A External perspective of 3D digital models. B, C Cross-sectional views following trans-septal sagittal dissection of the 3D digital model (PS: Pulmonary Stenosis; OA: Overriding Aorta; VSD: Ventricular Septal Defect; RVH: Right Ventricular Hypertrophy). D External depiction of rigid 3D printed model. E, F Sagittal sections of the rigid 3D printed model. G External portrayal of flexible 3D printed model. H, I Sagittal sections of the flexible 3D printed model. J, K The normal heart model employed in the instruction of the CBL group. L Ongoing classroom session
Teaching effectiveness assessment
Following the instructional session, participants from the two groups underwent a theoretical examination to assess their comprehension of the taught material. This assessment covered domains such as pathological anatomy, clinical manifestations, imaging data interpretation, diagnosis, and treatment relevant to TOF. Additionally, structured questionnaires were administered to evaluate the efficacy of the pedagogical approach employed. The questionnaire consisted of six questions designed to gauge participants’ understanding of the teaching content, enhancement of diagnostic skills, cultivation of critical thinking and clinical reasoning abilities, bolstering of confidence in managing TOF cases, satisfaction with the teaching mode, and satisfaction with the CBL methodology.
The questionnaire employed a 5-point Likert scale to gauge responses, with 5 indicating “strongly satisfied/agree,” 4 for “satisfied/agree,” 3 denoting “neutral,” 2 reflecting “dissatisfied/disagree,” and 1 indicating “strongly dissatisfied/disagree.” It comprised six questions, with the initial two probing participants’ knowledge acquisition, questions 3 and 4 exploring satisfaction regarding enhanced competence, and the final two assessing satisfaction with teaching methods and modes. Additionally, participants were encouraged to provide suggestions at the end of the questionnaire. To ensure the questionnaire’s validity, five esteemed lecturers in basic medical sciences with more than 10 years of experience verified its content and assessed its Content Validity Ratio and Content Validity Index to ensure alignment with the study’s objectives.
Statistical analysis
Statistical analyses were conducted utilizing GraphPad Prism 9.0 software. Aggregate score data for both groups were presented as mean ± standard deviation (x ± s). The gender comparisons were analyzed with the chi-square (χ2) test, while the other variables were compared using the Mann-Whitney U test. The threshold for determining statistical significance was set at P < 0.05.
Three-dimensional printing models
After configuring the structural colors of each component (Fig. 2 A, B, C), we printed four color TOF models using both rigid and flexible materials, resulting in four life-sized TOF models. Two color TOF models were created using rigid materials (Fig. 2 D, E, F). These models, exhibiting resistance to deformation, and with a firm texture, smooth and glossy surface, and good transparency, allowing visibility of the internal structures, were deemed conducive to teaching and observation. We also fabricated two color TOF models using flexible materials (Fig. 2 G, H, I), characterized by soft texture, opacity, and deformability, allowing for easy manipulation and cutting. It has potential utility beyond observational purposes. It can serve as a valuable tool for simulating surgical interventions and may be employed to create tomographic anatomical specimens. In this study, both material models were suitable for observation in the classroom. The participants were able to discern the four pathological changes characteristic of TOF from surface examination or cross-sectional analysis.
Baseline characteristics of the students
In total, 60 students were included in this study. The CBL group comprised 30 students (14 males and 16 females), with an average age of (21.20 ± 0.76) years. The CBL-3DP group consisted of 30 students (17 males and 13 females) with an average age of 20.96 years. All the students completed the study procedures. There were no significant differences in age, sex ratio, or pre-class exam scores between the two groups ( P > 0.05), indicating that the baseline scores between the two groups were comparable (Table 1 ).
Theoretical examination results
All students completed the research procedures as planned. The post-class theoretical examination encompassed assessment of pathological anatomy, clinical presentations, imaging data interpretation, diagnosis, and treatment pertinent to TOF. Notably, no statistically significant disparities were observed in the scores on clinical manifestations, diagnosis and treatment components between the cohorts, as delineated in Table 2 . Conversely, discernible distinctions were evident whereby the CBL-3DP group outperformed the CBL group notably in pathological anatomy, imaging data interpretation, and overall aggregate scores ( P < 0.05).
Results of the questionnaires
All the 60 participants submitted the questionnaire. Comparing the CBL and CBL-3DP groups, the scores from the CBL-3DP group showed significant improvements in many areas. This included satisfaction with the teaching mode, promotion of diagnostic skills, bolstering of self-assurance in managing TOF cases, and cultivation of critical thinking and clinical reasoning abilities (Fig. 3 B, C, D, E). All of which improved significantly ( P < 0.05 for the first aspects and P < 0.01 for the rest). However, the two groups were not comparable ( P > 0.05) in terms of understanding of the teaching content and Satisfaction with the CBL methodology (Fig. 3 A, F).
Upon completion of the questionnaires, participants were invited to proffer recommendations. Notably, in the CBL group, seven students expressed challenges in comprehending TOF and indicated a need for additional time for consolidation to enhance understanding. Conversely, within the CBL-3DP group, twelve students advocated for the augmentation of model repertoire and the expansion of disease-related data collection to bolster pedagogical efficacy across other didactic domains.
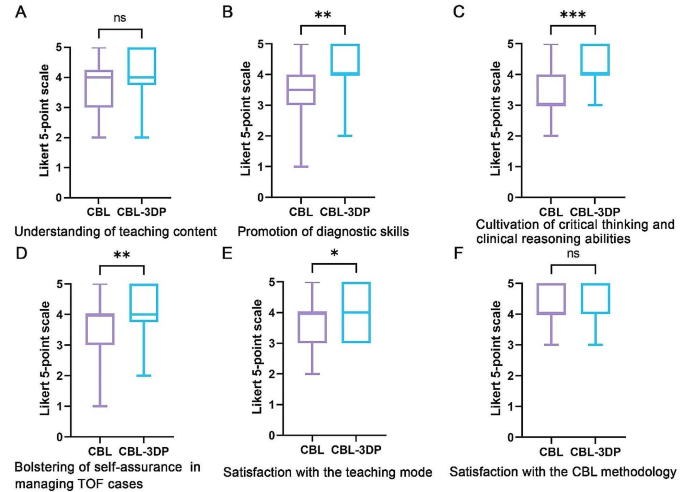
Five-point Likert scores of students’ attitudes in CBL ( n = 30) and CBL-3DP ( n = 30) groups. A Understanding of teaching content. B Promotion of diagnostic skills. C Cultivation of critical thinking and clinical reasoning abilities. D Bolstering of self-assurance in managing TOF cases. E Satisfaction with the teaching mode. F Satisfaction with the CBL methodology. ns No significant difference, * p < 0.05, ** p < 0.01, *** p < 0.001
TOF presents a significant challenge in clinical practice, necessitating a comprehensive understanding for effective diagnosis and treatment [ 12 ]. Traditional teaching methods in medical schools have relied on conventional resources such as textbooks, 2D illustrations, cadaver dissections, and radiographic materials to impart knowledge about complex conditions like TOF [ 13 ]. However, the limitations of these methods in fully engaging students and bridging the gap between theoretical knowledge and practical application have prompted a need for innovative instructional approaches.
CBL has emerged as a valuable tool in medical education, offering students opportunities to engage with authentic clinical cases through group discussions and inquiry-based learning [ 14 ]. By actively involving students in problem-solving and decision-making processes, CBL facilitates the application of theoretical knowledge to real-world scenarios, thus better-preparing students for future clinical practice [ 15 ]. Our investigation revealed that both groups of students exhibited comparable levels of satisfaction with the CBL methodology, devoid of discernible disparities.
CHD presents a formidable challenge due to the intricate nature of anatomical anomalies, the diverse spectrum of conditions, and individual variations [ 16 ]. Utilizing 3D-printed physical models, derived from patient imaging data, can significantly enhance comprehension of complex anatomical structures [ 17 ]. These models have proven invaluable in guiding surgical planning, providing training for junior or inexperienced pediatric residents, and educating healthcare professionals and parents of patients [ 18 ]. Studies indicate that as much as 50% of pediatric surgical decisions can be influenced by the insights gained from 3D printed models [ 19 ]. By providing tangible, anatomically accurate models, 3D printing offers a unique opportunity for people to visualize complex structures and enhance their understanding of anatomical intricacies. Our study utilized full-color, to-scale 3D printed models to illustrate the structural abnormalities associated with TOF, thereby enriching classroom sessions and facilitating a deeper comprehension of the condition.
Comparative analysis between the CBL-3DP group and the CBL group revealed significant improvements in post-test performance, particularly in pathological anatomy and imaging data interpretation. Additionally, questionnaire responses indicated higher levels of satisfaction and confidence among students in the CBL-3DP group, highlighting the positive impact of incorporating 3D printed models into the learning environment, improving the effectiveness of CBL classroom instruction. Despite the merits, our study has limitations. Primarily, participants were exclusively drawn from the same grade level within a single college, possibly engendering bias owing to shared learning backgrounds. Future research could further strengthen these findings by expanding the sample size and including long-term follow-up to assess the retention of knowledge and skills. Additionally, the influence of the 3D models depicting a normal heart on the learning process and its potential to introduce bias into the results warrants consideration, highlighting a need for scrutiny in subsequent studies.
As medical science continues to advance, the need for effective teaching methods becomes increasingly paramount. Our study underscores the potential of combining active learning approaches like CBL with innovative technologies such as 3D printing to enhance teaching effectiveness, improve knowledge acquisition, and foster students’ confidence and enthusiasm in pursuing clinical careers. Moving forward, further research and integration of such methodologies are essential for meeting the evolving demands of medical education, especially in areas involving complex anatomical understanding.
Conclusions
Integrating 3D-printed models with the CBL method is feasible and effective in TOF instruction. The demonstrated success of this method warrants broad implementation in medical education, particularly for complex anatomical topics.
Data availability
All data supporting the conclusions of this research are available upon reasonable request from the corresponding author.
Apitz C, Webb GD, Redington AN. Tetralogy of Fallot. Lancet. 2009;374:1462–71.
Article Google Scholar
Ghosh RM, Jolley MA, Mascio CE, Chen JM, Fuller S, Rome JJ, et al. Clinical 3D modeling to guide pediatric cardiothoracic surgery and intervention using 3D printed anatomic models, computer aided design and virtual reality. 3D Print Med. 2022;8:11.
Chakrabarti R, Wardle K, Wright T, Bennie T, Gishen F. Approaching an undergraduate medical curriculum map: challenges and expectations. BMC Med Educ. 2021;21:341.
Donkin R, Yule H, Fyfe T. Online case-based learning in medical education: a scoping review. BMC Med Educ. 2023;23:564.
Novack JP. Designing cases for case-based immunology teaching in large medical school classes. Front Immunol. 2020;11:995.
Chen HC, Van Den Broek WES, Ten Cate O. The case for use of entrustable professional activities in undergraduate medical education. Acad Med. 2015;90:431–6.
Wang M, Sun Z, Jia M, Wang Y, Wang H, Zhu X, et al. Intelligent virtual case learning system based on real medical records and natural language processing. BMC Med Inf Decis Mak. 2022;22:60.
Yoo S-J, Thabit O, Kim EK, Ide H, Yim D, Dragulescu A, et al. 3D printing in medicine of congenital heart diseases. 3D Print Med. 2015;2:3.
Yammine K, Violato C. A meta-analysis of the educational effectiveness of three-dimensional visualization technologies in teaching anatomy. Anat Sci Educ. 2015;8:525–38.
Miao H, Ding J, Gong X, Zhao J, Li H, Xiong K, et al. Application of 3D-printed pulmonary segment specimens in experimental teaching of sectional anatomy. BMC Surg. 2023;23:109.
Sun Z, Wong YH, Yeong CH. Patient-specific 3D-printed low-cost models in medical education and clinical practice. Micromachines (Basel). 2023;14:464.
Downing TE, Kim YY. Tetralogy of Fallot: general principles of management. Cardiol Clin. 2015;33:531–41. vii–viii.
Jia X, Zeng W, Zhang Q. Combined administration of problem- and lecture-based learning teaching models in medical education in China: a meta-analysis of randomized controlled trials. Med (Baltim). 2018;97:e11366.
McLean SF. Case-based learning and its application in medical and health-care fields: a review of worldwide literature. J Med Educ Curric Dev. 2016;3:JMECD.S20377.
Zeng N, Lu H, Li S, Yang Q, Liu F, Pan H, et al. Application of the combination of CBL teaching method and SEGUE framework to improve the doctor-patient communication skills of resident physicians in otolaryngology department. Bmc Med Educ. 2024;24:201.
Sun Z. Patient-specific 3D-printed models in pediatric congenital heart disease. Children. 2023;10:319.
Meyer-Szary J, Luis MS, Mikulski S, Patel A, Schulz F, Tretiakow D, et al. The role of 3D printing in planning complex medical procedures and training of medical professionals—cross-sectional multispecialty review. IJERPH. 2022;19:3331.
Sun Z, Wee C. 3D printed models in cardiovascular disease: an exciting future to deliver personalized medicine. Micromachines-basel. 2022;13:1575.
Valverde I, Gomez-Ciriza G, Hussain T, Suarez-Mejias C, Velasco-Forte MN, Byrne N, et al. Three-dimensional printed models for surgical planning of complex congenital heart defects: an international multicentre study. Eur J Cardio-thorac. 2017;52:1139–48.
Download references
Acknowledgements
We extend our sincere appreciation to the instructors and students whose invaluable participated in this study.
This paper received support from the Education Department of Anhui Province, China (Grant Numbers 2022jyxm1693, 2022jyxm1694, 2022xskc103, 2018jyxm1280).
Author information
Jian Zhao and Xin Gong are joint first authors.
Authors and Affiliations
Department of Human Anatomy, Wannan Medical College, No.22 West Wenchang Road, Wuhu, 241002, China
Jian Zhao, Xin Gong, Jian Ding, Rui Huang & Huachun Miao
Department of Cardio-Thoracic Surgery, Yijishan Hospital of Wannan Medical College, Wuhu, China
Kepin Xiong
Zhuhai Sailner 3D Technology Co., Ltd., Zhuhai, China
Kangle Zhuang
School of Basic Medical Sciences, Wannan Medical College, Wuhu, China
You can also search for this author in PubMed Google Scholar
Contributions
Jian Zhao and Huachun Miao designed the research. Jian Zhao, Xin Gong, Jian Ding, Kepin Xiong designed the tests and questionnaires. Kangle Zhuang processed the imaging data and printed the models. Xing Gong and Kepin Xiong implemented the teaching. Jian Zhao and Rui Huang collected the data and performed the statistical analysis. Jian Zhao and Huachun Miao prepared the manuscript. Shu Li and Huachun Miao revised the manuscript. Shu Li provided the Funding acquisition. All authors reviewed and approved the final manuscript.
Corresponding authors
Correspondence to Shu Li or Huachun Miao .
Ethics declarations
Ethics approval and consent to participate.
This investigation received ethical approval from the Ethical Committee of School of Basic Medical Sciences, Wannan Medical College (ECBMSWMC2022-1-12). All methodologies adhered strictly to established protocols and guidelines. Written informed consent was obtained from the study participants to take part in the study.
Consent for publication
Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, supplementary material 3, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zhao, J., Gong, X., Ding, J. et al. Integration of case-based learning and three-dimensional printing for tetralogy of fallot instruction in clinical medical undergraduates: a randomized controlled trial. BMC Med Educ 24 , 571 (2024). https://doi.org/10.1186/s12909-024-05583-z
Download citation
Received : 03 March 2024
Accepted : 21 May 2024
Published : 24 May 2024
DOI : https://doi.org/10.1186/s12909-024-05583-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Medical education
- Case-based learning
- 3D printing
- Tetralogy of fallot
- Medical undergraduates
BMC Medical Education
ISSN: 1472-6920
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Acad Pathol
- v.9(1); 2022
Educational Case: Evaluating a patient with cirrhosis
The following fictional case is intended as a learning tool within the Pathology Competencies for Medical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, Organ System Pathology, and Diagnostic Medicine and Therapeutic Pathology. For additional information, and a full list of learning objectives for all three competencies, see https://www.journals.elsevier.com/academic-pathology/news/pathology-competencies-for-medical-education-pcme . 1
Primary objective
Objective HB1.6: Cirrhosis. Classify types of cirrhosis, in terms of etiology, pathogenesis, morphologic pattern (gross and microscopic),and their relationship to neoplasia.
Competency 2: Organ system pathology; Topic: Hepatobiliary (HB); Learning goal 1: Hepatitis.
Secondary objectives
Objective HB3.4: Radiology of cirrhosis. Identify the major space occupying lesions that may be seen on radiographic imaging of the normal and cirrhotic liver, and discuss the complications of cirrhosis.
Competency 2: Organ system pathology; Topic: Hepatobiliary (HB); Learning goal 3: Hepatic neoplasms.
Objective CHEM1.4: Liver and gastrointestinal disease. Discuss the clinical presentation and the pathophysiologic bases of liver and gastrointestinal diseases including the efficient use of laboratory tests to make a definitive diagnosis and manage the disease.
Competency 3: Diagnostic medicine and therapeutic pathology; Topic: Chemistry (CHEM); Learning goal 1: Pathogenesis, diagnosis, and treatment of common disorders.
Patient presentation
A 56-year-old man accompanied by his wife presents to the clinic with chief concern of vague abdominal pain for the past two weeks. The patient has also experienced progressive shortness of breath, bloating, and fatigue during this time frame. His medical history is significant for obesity and lifestyle-controlled diabetes mellitus. Surgical history is significant for repair of a femoral fracture following a motor vehicle accident in 1990, which required a blood transfusion. The patient is unsure of his vaccination status. He takes no medications other than over-the-counter acetaminophen for a recent cold. He works as a marketing executive and recently traveled to several European and Southeast Asian countries on business. He does not report using tobacco products or illicit drugs. The patient states that he drinks occasionally, at which point his wife informs the physician that he consumes a six-pack of beer after work each day and more on the weekends.
Diagnostic findings, Part 1
Vital signs include a temperature of 97 °F, a heart rate of 87 beats per min, a respiratory rate of 18 breaths per min, an oxygen saturation of 94%, and a blood pressure of 137/86 mm Hg. Physical examination shows an uncomfortable-appearing male in no acute distress. The cardiac exam demonstrates regular rate and rhythm, with no rubs, or gallops. Lung auscultation demonstrates bilateral basilar crackles. Abdominal examination reveals a soft, protuberant abdomen with shifting dullness to percussion. There is pitting edema present to the mid-tibia bilaterally, with multiple bruises on the lower extremities.
Questions/discussion points, Part 1
What is the differential diagnosis for this patient's history and physical examination findings.
The differential diagnosis for abdominal pain with associated peripheral edema, fatigue, and shortness of breath is broad and includes heart failure, liver failure, renal failure, nephrotic syndrome, malnutrition, malabsorption, myxedema, lymphatic obstruction, and trauma. 2 The patient has specific risk factors for heart failure (obesity, diabetes mellitus, high alcohol consumption), liver disease (obesity, high alcohol consumption, hepatitis risk from travel or transfusion, and acetaminophen use), coagulopathy (bruises), and renal failure (diabetes mellitus).
Diagnostic findings, Part 2
Given the patient's broad differential, a comprehensive work-up is initiated including an electrocardiogram (EKG), B-type natriuretic peptide (BNP), complete blood count (CBC), coagulation panel, and complete metabolic panel (CMP). EKG findings are within normal limits, and the remaining laboratory results are displayed in Table 1 , Table 2 , Table 3 .
Table 1
Complete blood cell count with differential.
Table 2
Coagulation panel.
Table 3
Complete metabolic panel with B-type natriuretic peptide.
Questions/discussion points, Part 2
How do this patient's laboratory results help to differentiate between causes of coagulopathy.
The patient's elevated prothrombin time (PT), activated thromboplastin time (aPTT), and international normalized ratio (INR) are significant and suggestive of coagulopathy. These measurements are used to evaluate the integrity of the patient's clotting cascade. 2 , 3 PT is a measurement of clotting speed via the extrinsic or tissue factor and common pathways. 2 , 3 PT is used to assess the activity of clotting factors VII, V, X, II, and fibrinogen and can be prolonged if these are deficient or if there is an inhibitor. Deficiency could occur in the case of vitamin K deficiency, warfarin therapy, liver disease, and disseminated intravascular coagulation. 2 , 3 INR is a calculated standardization of the PT, used similarly to PT for assessment of extrinsic and common pathway clotting time and for monitoring the effects of warfarin pharmacotherapy. 2 aPTT measures the clotting time of the intrinsic and common pathways of the clotting cascade and is elevated in the setting of deficiencies in factors XII, XI, IX,VIII, V, X, II, or fibrinogen. aPTT can be elevated in some types of von Willebrand disease due to stabilization of factor VIII, antiphospholipid syndrome, in the presence of inhibitors, and in disseminated intravascular coagulation (DIC), severe vitamin K deficiency, or liver disease. 2 aPTT is also used in monitoring the effects of heparin pharmacotherapy. 2 Notably, vitamin K deficiency, DIC, and liver disease affect both PT and aPTT. 2 The presence of coagulopathy affecting both arms of the clotting cascade as reflected by the abnormal PT and aPTT measurements should be considered in context of the synthetic function of the liver.
The patient's CBC is also significant for low red blood cell count (RBC), low hemoglobin and hematocrit (Hgb and Hct, respectively), an elevated mean corpuscular volume (MCV), and an elevated red cell distribution width (RDW), all of which suggest megaloblastic anemia. Megaloblastic anemia can occur secondary to a micronutrient vitamin B 12 or folate deficiency. 4 , 5 , 6 In patients who consume large quantities of alcohol to the exclusion of more nutritious food may develop nutrient deficiencies. Folate and vitamin B 12 deficiencies impair hematopoiesis and primarily cause a decrease in number of RBCs. More severe cases can cause a significant decrease in WBCs and platelets as well, resulting in a pancytopenia. 4 It is likely that megaloblastic anemia is contributing to this patient's symptoms of fatigue and shortness of breath.
How do this patient's laboratory results help to differentiate between causes of liver disease?
CMP demonstrates low total protein and low albumin, both of which are indicators of the synthetic capacity of hepactocytes. 7 , 8 The major contributors to the total protein measurement are globulin and albumin fractions. 7 The globulin fraction includes enzymes, including clotting factors produced by hepatocytes, as well as immunoglobulins produced by plasma cells, and the albumin fraction consists exclusively of albumin produced by hepatocytes. 7 Damage to hepatocytes that results in decreased synthetic capacity is thus revealed by decreased albumin and total proteins measurements in the setting of coagulopathy. 7 , 8 Hepatocyte integrity can be further assessed with the measurement of serum levels of hepatocellular enzymes aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and should be considered in relation to biliary excretory function measured with alkaline phosphatase (ALP) and bilirubin levels. 8
This patient's metabolic laboratory studies demonstrate a hepatocellular pattern of liver injury as suggested by markedly elevated hepatocellular enzymes AST and ALT out of proportion to an also elevated ALP. 8 , 9 , 10 A cholestatic pattern of liver injury would be more likely if the ALP was elevated out of proportion to the AST and ALT, accompanied by a more severe hyperbilirubinemia. This pattern would warrant further consideration of biliary obstructive and non-hepatic etiologies. 8 , 9 , 10 The hepatocellular pattern of liver injury can be further characterized by the AST:ALT ratio, which is > 1 in this case. 8 , 9 The AST:ALT ratio should be < 1 in a normal person without elevations, and elevated values with an AST:ALT ratio > 1 are strongly suggestive of alcoholic liver disease, due to a variety of reasons related to alcohol metabolism in hepatocytes. 9 Hepatocellular patterns of liver injury closer to an AST:ALT ratio of < 1 are more suggestive of metabolic-associated fatty liver disease (MAFLD). 9 , 11 AST and ALT levels can decline in chronic liver disease as the severity progresses to end-stage liver disease and hepatocyte death removes the source of these enzymes. 12
How do this patient's laboratory studies help to differentiate between causes of hyponatremia?
The patient's CMP is also significant for low sodium, which in the context of physical exam findings of peripheral edema and shifting dullness to abdominal percussion indicating ascites, are suggestive of a hypervolemic state. This is consistent with hypervolemic hyponatremia, in which inappropriate water retention results in a dilutional hyponatremia, often secondary to renal failure, nephrotic syndrome, congestive heart failure, or cirrhosis. 13 , 14 , 15 In cirrhotic patients, the lack of oncotic pressure secondary to hypoalbuminemia results in a volume shift from intravascular spaces to extracellular compartments, as demonstrated by this patient's ascites and peripheral edema. 14 , 16 , 17 It is also significant that the patient's brain natriuretic peptide (BNP) is above normal limits. BNP is a hormone released in response to the increased cardiac ventricular wall stress experienced in a state of increased ventricular blood volume. It stimulates natriuresis, diuresis, and systemic vasodilation, while inhibiting the renin–angiotensin–aldosterone system to decrease blood pressure and increase cardiac ejection fraction. 18 , 19 This patient's slightly elevated BNP is evidence of hypervolemia, but is significantly less than would be expected in decompensated heart failure. 20 , 21 , 22 Thus, heart failure is unlikely to be the underlying etiology for this patient's hypervolemic hyponatremia. Renal failure is also made less likely by the patient's low creatinine, which is removed by the kidney and elevated in the setting of acute kidney injury and renal failure. 8 , 23 Creatinine is generated through the metabolism of creatine by hepatocytes, so chronic liver disease and loss of hepatocyte synthetic function is commonly associated with lower levels of creatinine. 16 , 24
Diagnostic findings, Part 3
It is concluded that the patient's symptoms are likely due to a hepatic disease process, and he is given a preliminary diagnosis of cryptogenic liver failure, with alcoholic liver disease as the most likely etiology. The remainder of the differential for liver disease includes the following: viral hepatitis, autoimmune hepatitis, drug- and toxin-induced liver injuries, metabolic-associated fatty liver disease, hemochromatosis, Wilson disease, and α1-antitrypsin deficiency. 8 Appropriate laboratory tests and an abdominal ultrasound with sonoelastography are ordered. The results are displayed Table 4 , Table 5 , Table 6 . 25 , 26 , 27 , 28
Table 4
Hepatitis panel.
Table 5
Ultrasonography findings.
Table 6
Sonoelastography.
Questions/discussion points, Part 3
How do this patient's additional laboratory results narrow the differential diagnosis.
The results of the hepatitis panel are significant for positivity for hepatitis B surface antibody (HBsAb) and negativity for the remainder of the tested antigens and antibodies. HBsAb positivity suggests previous exposure to the hepatitis B surface glycoproteins, which occurs during vaccination or infection. 29 During infection by the hepatitis B virus (HBV), the host's immune system is also exposed to the hepatitis B core proteins (HBc) and mounts an immune response that results in hepatitis B IgM core antibody (HBcAb-IgM) positivity during acute infection. This is followed by hepatitis B IgG core antibody (HBcAb-IgG) positivity later in the disease course and following resolution. 29 Hepatitis B type e antigen (HBeAg) detection is associated with a high level of active viral replication resulting in increased infectivity. 29 Detectable HBsAb with undetectable levels of other HBV antigens and antibodies suggests that the patient was successfully vaccinated against HBV and is unlikely to have been infected previously. 29 Undetectable levels of hepatitis A IgM, IgG, and hepatitis C antibodies (HA Ab-IgM, -IgG and, HC Ab, respectively) suggests that the patient has neither been nor is currently infected with the hepatitis A or C viruses. 29 Thus the patient's current presentation is unlikely to be related to a viral hepatitis.
What focal and diffuse liver lesions can be demonstrated with ultrasonography?
Abdominal ultrasound is a commonly utilized tool for detecting and characterizing lesions of the liver. 30 Normal liver parenchyma is echogenic with a homogenously porous appearance and visibly branching vasculature. 30 Abnormal ultrasonographic findings can be indicative of focal and diffuse liver disease depending on the morphologic pattern. 27 , 30 A hepatic cyst, the most common and often incidentally found space-occupying lesion of the liver, can be visualized as a round anechoic space with a variable degree of septation depending on lesion complexity. 30 , 31 A hepatic abscess secondary to infectious etiology can have a variable presentation on ultrasonography including but not limited to septations, debris, and the presence of gas demonstrated as bubbles or an air-fluid level. 32 Hemangiomas are the most common benign liver tumor and appear as well-defined hyperechoic lesions with vascular activity visible with use of color-Doppler. 33 Focal nodular hyperplasia (FNH) is the second most common benign liver tumor and is thought to result from a hyperplastic response to an arteriovenous malformation. 34 Ultrasonographic features of FNH are variable, but approximately 20% of cases demonstrate a central scar with disruption of surrounding vasculature. 35 A hepatocellular adenoma is a benign neoplasm of the liver associated with oral contraceptive use that is usually asymptomatic unless the neoplasm ruptures and bleeds. 36 This neoplasm is often characterized on ultrasonography as a solitary, well-circumscribed mass of variable echogenicity. 37
Diffuse liver disease includes etiologies such as the various viral and immune hepatitides, storage diseases, hepatic steatosis, fibrosis, and cirrhosis. 38 Ultrasonography findings are often non-specific in the case of diffuse liver disease, and biopsy is usually required to differentiate between the various etiologies. 38 In cases of acute hepatitis, the most common finding is hepatomegaly with diffusely decreased echogenicity. 38 Chronic hepatitis can be demonstrated by the presence of focal or diffuse hepatic steatosis and fibrosis developed in response to prolonged periods of parenchymal damage and regeneration. 38 Diffuse hepatic steatosis can also occur in cases of alcohol abuse, metabolic-associated steatohepatitis, chronic hepatitis, glycogen storage diseases, and various drug therapies. 39 Heterogenous or coarsened hepatic echotexture on ultrasonography is evidence of a loss of parenchymal uniformity as seen in cases of cirrhosis, metabolic storage diseases, and chronic hepatitis. 40 , 41 Surface nodularity can be a useful finding in differentiating cirrhosis from other forms of diffuse liver disease, as metastatic tumor nodules are the only other notable disease process that demonstrates this ultrasonographic finding. 38
A feared complication of diffuse liver disease, hepatocellular carcinoma (HCC) is the most common primary liver cancer in adults and can present as a focal, multifocal, or diffuse lesions on ultrasonography. 27 , 38 Over 80% of new cases of HCC occur in the setting of cirrhosis. 42 Thus cirrhotic patients are currently recommended to undergo biannual abdominal ultrasonography as a non-invasive and cost-effective screening modality. 42 In addition to cirrhosis, risk factors for HCC include any disease process which results in chronic hepatic injury, such as the immune and viral hepatitides, alcohol consumption, storage diseases, and metabolic-associated steatohepatitis. 43 , 44 Focal HCC lesions tend to be hypoechoic compared to surrounding hepatic parenchyma. As focal HCC lesions grow, they become increasingly heterogenous and echogenic, demonstrating features of fat accumulation, necrosis, and calcification, or development of a central scar that can be misinterpreted as FNH. 38 Advanced HCC usually presents in patients with underlying diffuse liver disease as multifocal lesions with variable echogenicity, and can appear similar to metastases on imaging. 38 , 43 , 44 HCC foci developing within regenerative nodules demonstrate a nodule-in-nodule appearance on magnetic resonance imaging. 45 Diffuse or infiltrative HCC presents with scattered hepatic nodularity, rather than distinct masses, that is difficult to differentiate from cirrhosis based on advanced imaging alone. 46
How do this patient's ultrasonography findings affect the differential diagnosis?
Abdominal ultrasound findings of mild hepatosplenomegaly, liver surface nodularity with heterogenous echotexture, and segmental hypertrophy and atrophy are consistent with cirrhosis. 26 Abnormal blood flow resultant from the disruption of normal hepatic cellular architecture results in segmental hypertrophy of the caudate lobe and lateral segments of the left lobe, with concurrent segmental atrophy of the right lobe and medial segments of the left lobe. 47 Nodule formation occurs secondary to fibrotic and regenerative processes involving both stromal and parenchymal hepatocytes, and is seen as the nodular surface and heterogenous texture on this patient's abdominal ultrasound. 8 , 47 Diffusely increased echogenicity of the hepatic parenchyma when compared to the right kidney is indicative of abnormal accumulation of lipids within hepatocytes, that occurs in response to chronic disease processes. 38 Hepatic echogenicity is normally similar to or greater than that of the renal cortex, which serves as a standard of comparison in determination of lipid accumulation. 38 Additional findings of an enlarged splenic vein and ascites are suggestive of portal hypertension and hypoalbuminemic edema, both of which are complications of cirrhosis. 26 Sonoelastography utilizes sonography and the application of mechanical pressure to evaluate tissue stiffness and elasticity, and it has been demonstrated to have a high negative predictive value when ruling out cirrhosis. 48 Stiffness and elasticity are measured in kilopascals (kPa), with increasing values correlating to higher stages of fibrosis. 48 , 49 Sonoelastography values over 10 kPa suggest advanced chronic liver disease, over 12.5 kPa suggest cirrhosis, and those exceeding 21 kPa suggest clinically significant portal hypertension. 49 , 50 This patient's sonoelastography findings suggest the presence of cirrhosis with clinically significant portal hypertension, which is further supported by previous physical exam, laboratory, and ultrasound findings. Despite recent and improved non-invasive modalities for assessment of liver fibrosis and cirrhosis, liver biopsy remains the gold standard for identification and classification of cirrhosis. 49
Diagnostic findings, Part 4
An ultrasound-guided liver biopsy is performed, and the results are in Fig. 1 , Fig. 2 , Fig. 3 .

Liver biopsy. At low power, the liver biopsy shows steatosis throughout, and inflammation (upper right and middle left). Bands of fibrosis are visible at low power (arrows). H&E, 40x.

Liver biopsy. At higher power, bands of fibrosis surrounding a nodule are seen (arrows). The hepatocytes contain numerous clear fat droplets, and there is predominantly lymphocytic portal inflammation in the upper right. H&E, 200x

Liver biopsy. Trichrome stain highlights in blue, the circumferential bands of fibrosis surrounding regenerative nodules. Trichrome stain, 100x.
Questions/discussion points, Part 4
What is the cellular architecture of the liver.
Hepatic micro-architecture is structured in terms of 1- to 2-mm diameter lobules composed of portal triads of hepatic arteries, portal veins, and bile ducts, surrounding plates of hepatocytes which radiate towards a central vein at the center. 8 , 51 Hepatocytic trabeculae are separated by intervening sinusoidal spaces in which a mixture of portal venous and hepatic arterial blood flows from the portal tract to the central vein. 8 , 51 Alternatively, the micro-architecture can be classified into acini made of three zones according to distance from the portal tract, which correlate to their respective degrees of oxygenation. 8 , 51 , 52 The area adjacent to the portal tract is zone 1, which is the most well-oxygenated region, the intermediate zone between the portal tract and the central vein is zone 2, and the area adjacent to the central vein is zone 3, which is the least oxygenated. 8 , 51 Non-parenchymal cells of the liver include liver sinusoidal endothelial cells (LSECs), Kupffer cells (KCs), and hepatic stellate cells (HSCs). 8 , 51 , 52 Fenestrated LSECs separate the sinusoidal spaces from the space of Disse which is occupied by hepatocytic microvilli responsible for transportation of nutrients from sinusoidal blood to the hepatocytes. 8 , 51 KCs are phagocytic monocytes present on the luminal aspect of LSECs. 8 , 51 , 52 HSCs occupy the space of Disse and are the primary mediators of hepatic fibrosis. 8 , 51 , 52 Bile canaliculi border hepatocytes and drain ultimately into the terminal bile ducts of the portal tracts. 8 , 51 , 52
What is the process of hepatocyte damage?
Irreversible injury to hepatocytes results in either apoptotic or necrotic cell death. 8 , 52 The former commonly occurs secondary to viral, autoimmune, or drug- and toxin-induced hepatitides, and the latter often occurs secondary to ischemic and hypoxic injury. 8 , 52 Hepatocyte apoptosis is a form of cell death whereby caspase cascades are activated, initiating an organized process of pyknosis, karyorrhexis, and cellular fragmentation into acidophil bodies, which appear intensely eosinophilic on staining. 8 Hepatocyte necrosis occurs when a hepatocyte is unable to maintain osmotic regulation, so it swells, and ruptures, releasing cytoplasmic contents, including pro-inflammatory cytokines, into the extracellular compartment. 8 Confluent necrosis is a phenomenon in which areas of necrosis are localized to one or more lobular acinar zones. Hepatocyte drop-out begins near the central vein in zone 3, where oxygenation is at its lowest within the acinus, and extends contiguously toward the penetrating hepatic vessels through zone 2 and zone 1. When necrosis involves multiple adjacent acini of multiple lobules it is described as bridging necrosis. 8 Confluent and bridging necrosis are the result of acute or severe chronic hepatocyte injury. 8
What is the response to hepatocyte apoptosis and necrosis?
KCs lining fenestrated LSECs are activated in response to hepatocyte injury. 8 , 17 , 52 KCs contribute to the cellular response to hepatocyte damage by producing the following cytokines: platelet-derived growth factor (PDGF), tumor necrosis factor alpha (TNF-a), endothelin-1 (ET-1), monocyte chemoattractant protein-1 (MCP-1), and transforming growth factor beta (TGF-b). PDGF and TNF-alpha stimulate HSC proliferation and activation of previously quiescent HSCs. ET-1 stimulates vasoconstriction, TGF-b stimulates fibrogenesis, and MCP-1 and PDGF stimulate chemotaxis of activated HSCs to the site of injury. 8 , 17 , 52 KCs further contribute to the activation of previously quiescent HSCs by degrading collagen type IV in the space of Disse. Additionally, KCs phagocytize hepatocyte apoptotic bodies and produce pro-apoptotic ligands, such as Fas. 8 , 17 , 52 The net effects of KC activation and function include hepatocyte dysfunction and death, HSC activation and chemotaxis, and stimulation of fibrogenesis. 8 , 17 , 52
Quiescent vitamin A-rich HSCs located in the space of Disse function as lipid-storing cells until they become active and convert to myofibroblasts. 8 , 17 , 52 Sources of activation include cytokines and chemokines produced by KCs and other non-parenchymal cells, pro-inflammatory cytokines associated with chronic inflammation, direct stimulation by toxins, and disruption of the extracellular matrix. 8 Myofibroblasts are highly fibrogenic and contribute significantly to the generation of types I and III collagen, which form fibrolysis-resistant collagen bundles through cross-linking and deposition of extracellular matrix within the space of Disse. 53 In a state of chronic liver disease, fibrogenesis is perpetuated and fibrous septa formed at the sites of parenchymal loss begin to surround regenerating hepatocytes, distorting the microarchitecture of the hepatic lobules and producing the nodularity of cirrhosis. 1 , 3 , 8 Fibrosis and fibrolysis are concurrent bidirectional processes, with active liver disease favoring the former and remitting liver disease favoring the latter. 8 , 17 , 52 Regression of cirrhotic nodularity with reversal of fibrotic scar formation has been demonstrated in cases where the insulting factor is removed before permanent microarchitectural change takes place. 54 Vascular architecture is also disrupted by this remodeling process, resulting in intralobular vascular shunts within the fibrous septa. 8 , 17 , 52
What are the types of cirrhosis and how can they be differentiated on histopathology?
Cirrhosis can be classified based on both the morphologic findings and the underlying etiology. 55 Morphological categories are separated into the following: micronodular, macronodular, and mixed. 55 In micronodular cirrhosis, nodules are uniform and less than 3 mm in diameter. 51 They are irregular and more than 3 mm in diameter in macronodular cirrhosis, and varied sizes with features of both in mixed cirrhosis. 51 Mixed cirrhosis is often an intermediate through which micronodular cirrhosis progresses to macronodular cirrhosis. 55 A micronodular pattern of cirrhosis can be seen in cases of cirrhosis secondary to alcoholic liver disease, hemochromatosis, and hepatic venous and biliary outflow obstructions. 55 Macronodular cirrhosis is more commonly seen in cases of chronic viral hepatitis, primary biliary cholangitis, Wilson disease, and α-1 antitrypsin deficiency. 8 , 55 Etiologies of cirrhosis can also be classified based on their pattern of hepatocyte damage and subsequent fibrosis. 55 For example, cirrhosis secondary to alcoholic liver disease and MAFLD demonstrate an initial centrilobular and perivenular pattern of fibrosis that advances toward the periportal acinar zones with progression of disease. Other etiologies of chronic liver disease that result in cirrhosis, including viral hepatitides, autoimmune hepatitis, chronic cholestatic disease, and metabolic and storage diseases, can be distinguished by a predominance of periportal fibrosis. 56
What are the histopathologic features of alcoholic liver disease?
Alcoholic liver disease is characterized by macrovesicular steatosis most prominent in acinar zone 3, or the perivenular area, continuing outward through acinar zones 2 and 1 in increasingly severe disease states. 57 Alcohol-metabolizing cytochromes are present in disproportionate amounts in the centrilobular hepatocytes when compared to the periportal hepatocytes. 56 Metabolism of alcohol and byproduct formation place additional stress on these centrilobular hepatocytes and are responsible for the hepatocellular ballooning pattern of hepatocellular injury seen on histopathological evaluation of alcoholic liver disease. 56 Hepatocellular ballooning and subsequent necrosis constitutes the primary mechanism of injury in alcoholic liver disease, which progresses to a micronodular cirrhosis with perivenular and pericellular fibrosis, visible as blue-stained collagen on Masson trichrome stain. 56 Mallory-Denk bodies are damaged filamentous inclusions in hepatocytes observable on hematoxylin and eosin (H&E) and chromotrope aniline stains in chronic liver disease, including alcoholic liver disease and cirrhosis. 56 The presence of centrilobular fibrosis accompanied by obliteration of the central vein, and perivenular necrosis with Mallory-Denk bodies constitutes sclerosing hyaline necrosis, a histopathological finding consistent with alcoholic cirrhosis. 58 Megamitochondria may also be seen on H&E stain in cases of alcoholic cirrhosis. 57 Metabolic-associated fatty liver disease demonstrates a similar histopathological pattern to that of alcoholic liver disease. Notable differences that exist between MAFLD and alcoholic liver disease include more significant steatosis in the former than the latter and more significant inflammation in latter than the former. 59 While also present in MAFLD, perivenular fibrosis tends to be more significant in alcoholic liver disease. 59 This patient's liver biopsy findings are consistent with alcoholic cirrhosis.
How does the pathophysiology of cirrhosis explain its clinical presentation and complications?
The symptomatic presentation of cirrhosis and its complications occurs secondary to increased intrahepatic vascular resistance, loss of synthetic and metabolic functions of hepatocytes, and prolonged regenerative processes. 60 Prolonged hepatocyte injury and subsequent fibrogenesis results in increased formation of collagen in the space of Disse and the loss of endothelial fenestration, a process which is referred to as sinusoidal capillarization. 60 Vascular reorganization is accompanied by fibrotic expansion of the portal tract and fibrosis of the central vein which results in portal hypertension. 60 Decreased synthetic capacity of the liver due to loss of functional hepatocytes results in diminished nitric oxide production and contributes to increased intrahepatic vascular resistance and portal hypertension. 61 This leads to the development of intrahepatic portal-systemic shunts that results in collateral circulation which bypasses normal liver flow. 17 , 52 , 60 While these shunts help to reduce portal hypertension, they enlarge over time and result in many serious and often life-threatening complications of cirrhosis. 17 , 52 , 60 Esophageal varices, a major complication of portal hypertension and vascular congestion, are abnormally enlarged veins in the esophagus that pose a significant bleeding risk to cirrhotic patients. 61
Intrahepatic shunting also leads to decreased metabolism of ammonia by functional hepatocytes and subsequently increased bioavailable ammonia in systemic circulation. 17 , 52 , 60 Increased flow of nitrogen-rich blood to the brain leads to the development of hepatic encephalopathy, a neuropsychiatric condition characterized by memory impairment, asterixis, myoclonus, and personality and behavioral changes. 62 Increased blood flow from portal-systemic shunting further contributes to the development of hepatopulmonary and hepatorenal syndromes. 17 , 52 , 60 In hepatopulmonary syndrome, pulmonary capillary dilation causes a ventilation–perfusion mismatch presenting with dyspnea, platypnea, and hypoxemia, such as in this patient's case. 63 Hepatorenal syndrome occurs when splanchnic vasodilation secondary to intrahepatic shunting leads to renal hypoperfusion and subsequent activation of the renin-angiotensin-aldosterone system, ultimately progressing to renal failure. 64 , 65 Vascular congestion secondary to portal hypertension and shunting also contributes to the development of splenomegaly and caput medusa, two prominent physical exam findings in cirrhotic patients. 60
Both portal hypertension and the decreased synthesis of albumin by hepatocytes contribute to the formation of ascites, the accumulation of protein-rich fluid in the abdominal cavity. 60 Ascites is a common finding in cirrhotic patients and can become complicated by infection resulting in spontaneous bacterial peritonitis. 66 This commonly presents with fever and altered mental status which can be accompanied by chills, abdominal pain, nausea, and vomiting. 66 Loss of hepatocytes contributes to decreased metabolic function, including bilirubin and estradiol metabolism. Reduced excretion of bilirubin results in intrahepatic cholestasis and jaundice, and reduced estradiol metabolism leads to hyperestrogenism and its effect of palmar erythema, gynecomastia, and spider angiomata, which are most prevalent on the upper trunk and face. 60
What are the next steps in management for this patient?
Management of this patient begins with determination of whether the cirrhosis is compensated or decompensated. 67 Compensated cirrhosis is an early asymptomatic stage of the disease process without complications of portal hypertension and liver dysfunction. 67 Decompensated cirrhosis is an acute deterioration in liver function with the presence of complications, and it can be precipitated by many etiologies such as medications, infections, hypovolemia, electrolyte imbalance, and alcohol use. 68 , 69 In this patient, the presence of complications suggests decompensated cirrhosis. 68 In the absence of life-threatening complications, such as variceal hemorrhage, management is directed toward symptomatic treatment, initiation of relevant surveillance programs, risk reduction, and patient education. 68 The presence of new-onset ascites warrants a diagnostic paracentesis with analysis of cell count, total protein, albumin, and bacterial culture with sensitivity testing. 69 Cirrhotic patients are effectively immunosuppressed and frequently decompensate due to infections such as spontaneous bacterial peritonitis. 68 In the absence of a known prior episode of spontaneous bacterial peritonitis, results from the diagnostic paracentesis will be used to determine if the patient requires oral antibiotic prophylaxis. 69 Reduction of ascites is accomplished with salt restriction and diuretic pharmacotherapy such as spironolactone with or without loop diuretics. 68 , 69 Hepatic encephalopathy, which is not present in this patient, is managed with the administration of lactulose with or without rifaximin to encourage stooling and elimination of nitrogen from the systemic circulation. 69
Health surveillance for this patient will include regular screenings for HCC and esophageal varices. 44 , 61 , 69 Screening for HCC is recommended every six months for cirrhotic patients and is performed via right upper quadrant ultrasonography. 69 Screening for esophageal varices via endoscopic gastroduodenoscopy (EGD) is indicated in newly diagnosed compensated cirrhosis, unless sonoelastography demonstrates a liver stiffness of at least 20 kPa and CBC demonstrates a platelet count of at least 150,000. 70 If no varices are detected on initial screening, EGD is repeated every three years in the absence of ongoing liver injury and every two years in the presence of ongoing liver injury. 70 For patients with decompensated cirrhosis, EGD is recommended at the time of diagnosis followed by repeat screening every year. 71 If present, esophageal varices are risk-stratified based on size and count. Primary prevention of variceal hemorrhage includes nonselective beta blockers and/or endoscopic band ligation depending on risk. 69 Acute variceal hemorrhage is a life-threatening emergency managed with vasoconstrictors, endoscopic band ligation, balloon tamponade, and surgical intervention. 72 Placement of a transjugular intrahepatic portosystemic shunt (TIPS) can be performed as a salvage treatment to reduce portal hypertension and variceal hemorrhage risk, but its use must be considered alongside potentially worsening hepatic encephalopathy secondary to increased intrahepatic shunting. 72
The model for end-stage liver disease (MELD) and Child–Pugh scores are two clinically useful calculations that use patient laboratory values and symptom severity to stratify risk and estimate disease severity in cirrhotic patients. 73 The MELD score is commonly used in transplant allocation. 74 Due to the progressive nature of chronic liver disease, liver transplant remains the definitive treatment option for advanced cirrhosis. 74 This patient's initial laboratory work-up demonstrates a MELD score of 20 and an estimated three-month mortality of 19.6%. 73 Patient education is an important intervention in the management of cirrhosis and includes counselling on alcohol reduction or abstinence, signs and symptoms of serious complications, and the importance of continued surveillance and long-term follow up. 67
Teaching points
- • Cirrhosis is characterized by hepatic fibrosis, prolonged hepatocellular regeneration, and disruption of hepatic microarchitecture that occurs secondary to chronic liver diseases. It is commonly classified by morphology or etiology.
- • Morphologic classifications include both macroscopic and microscopic patterns. Macroscopic patterns include micronodular, macronodular, and mixed types of cirrhosis. Microscopic patterns vary by etiology but can be categorized as biliary and nonbiliary types of cirrhosis.
- • Etiologies of cirrhosis include infectious (predominately viral) and autoimmune hepatitides, storage disorders, alcoholic and metabolic-associated steatohepatitides, and biliary dysfunction.
- • The loss of hepatocytes results in decreased metabolic and synthetic capacity of the liver leading to coagulopathies, jaundice, hepatic encephalopathy, hyperestrogenism, hypoalbuminemia, and ascites, which can be further complicated by spontaneous bacterial peritonitis.
- • Fibrosis and vascular reorganization in cirrhosis results in the development of portal hypertension and subsequent intrahepatic shunting leading to complications including hepatic encephalopathy, hepatopulmonary syndrome, hepatorenal syndrome, and esophageal varices.
- • Loss of hepatic parenchyma in the setting of cirrhosis is demonstrated by elevated liver enzymes (specifically AST ALT, ALP) in acute liver disease and declining levels in end-stage chronic liver disease. Interpretation of hepatocellular and cholestatic patterns of liver enzyme elevations can be used to differentiate liver disease etiology and direct further management.
- • Various identifiable patterns of AST, ALT, and ALP elevation are suggestive of different types of liver injury. A normal AST:ALT ratio is < 1 whereas an AST:ALT ratio > 1 is highly suggestive of liver injury. Predominant elevations of AST and ALT with or without notable ALP elevation suggest a hepatocellular pattern of liver injury. A hepatocellular pattern with an AST:ALT ratio >1 is strongly suggestive of an alcoholic etiology of liver damage while a ratio of < 1 suggests metabolic-associated fatty liver disease. Predominantly elevated ALP with or without significant AST and/or ALT elevations suggests a cholestatic pattern of liver injury.
- • Ultrasonography and sonoelastography are the most commonly used imaging modalities in the characterization of liver disease and cirrhosis. Ultrasonography characterizes hepatic parenchyma and can differentiate etiologies of focal and diffuse liver disease. Sonoelastography measures the stiffness and elasticity of hepatic tissue to determine the extent of hepatic fibrosis and can be used to risk stratify related complications.
- • Ultrasonographic evidence of cirrhosis includes heterogenous echotexture, nodularity, hepatosplenomegaly, segmental hypertrophy and atrophy, and vascular abnormalities.
- • Normal liver parenchyma appears homogenous and porous with an echogenic texture that is similar to that of the renal cortex. Abnormal ultrasonographic findings vary depending on the etiology of focal or diffuse liver disease.
- • Prolonged hepatocyte regeneration in the setting of chronic liver disease significantly increases the risk of developing hepatocellular carcinoma in cirrhotic patients.
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Authors’ note
The opinions expressed herein are those of the authors and are not necessarily representative of those of the Uniformed Services University of the Health Sciences (USUHS), the Department of Defense (DOD), or the United States Army, Navy, or Air Force, or the Henry M. Jackson Foundation.
Declaration of competing interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.

IMAGES
VIDEO
COMMENTS
As of June 13, 2022, the U.K. Health Security Agency has reported 260 cases of non-A-E hepatitis in children younger than 16 years of age (since January 1, 2022) 23; 156 of 241 patients (64.7% ...
We herein report an elderly patient with acute hepatitis B caused by a rare subgenotype (D1) of HBV. To our knowledge, this is the first report of a case of subgenotype D1 HBV infection in Japan. In the present case, we made a diagnosis of acute hepatitis B based on the extremely high titer of IgM-HBcAb (21.0 S/CO).
The following topic will outline issues related to the management of hepatitis B through the use of cases studies that incorporate patient-specific clinical information and test results. Our approach to treatment is generally consistent with guidelines from the European Association for the Study of the Liver guidelines, Asian-Pacific ...
Abstract. The following fictional case is intended as a learning tool within the Pathology Competencies for Medical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, Organ System Pathology, and Diagnostic Medicine and Therapeutic Pathology.
Patient Case Presentation. Our patient, Mr. Smith, is a 43 year old caucasian male who came in today with complaints of fatigue, anorexia, malaise, nausea, vomiting, abdominal pain, and low grade fever for the past month, and recently has been alarmed by the discoloration of his skin and sclera turning yellow.
Abstract. Hepatitis A is a common worldwide cause of acute hepatitis. It has been classically associated with epidemics and is increasingly prevalent in the developing world. Generally, the illness is self-limited and only requires supportive management, reassurance, and proper hygiene instructions. This case involves a male in his early 30s ...
A Case Study. Use Enter or Space to activate links. Use appropriate arrow key to open or close submenus. Patient History; Viral Hepatitis Pathophysiology ... Review Questions. Review Question Answers; Patient Education Videos; References; Posts Viral Hepatitis: A Case Study. October 14, 2019 at 2:41pm October 26, 2019 by Matthew Kilboy ...
According to analyses of etiology, clinical features, diagnostic methods, and treatment strategies by summarizing a case of unexplained acute hepatitis recently experienced, we are aiming to provide some information to enrich the clinical experience in diagnosis and treatment of severe acute hepatitis of unknown etiology in young children. A boy, aged 10 years and 6 months old, was admitted to ...
Here we describe an acute hepatitis B infection in a patient who received five hepatitis B vaccinations. Although his initial response to vaccination was moderate, he finally reached an excellent hepatitis B surface antibody level (anti-HBs) titres of more than 1000 IU/l in response to a booster vaccination with a recombinant DNA vaccine.
About this book. This book provides a comprehensive resource for clinical hepatology. It details the systematic approach to patients with liver disease in outpatient and inpatient medical settings. A variety of case studies in hepatology including chronic viral hepatitis, and metabolic, autoimmune, and alcohol related liver disease are presented.
case, stabilizing the patient was crucial before considering transplantation and the best chance of achieving SVR may be in treating the patient post-transplant; SOF/VEL/VOX may be an option in that scenario. Case study: managing cryoglobulinemic vasculitis in hepatitis C virus. Paul Clark, Princess Alexandra Hospital, Brisbane Case study
File type. Size. Protecting against hepatitis case study - Poster. pdf. 0.38 MB. Download. In collaboration with international colleagues, the National Health and Medical Research Council (NHMRC) funded researchers at The University of Western Australia, Fairfield Hospital's Epidemiological Research Unit (now the Burnet Institute) and Monash ...
44 yo man with longstanding HIV infection, stage 2 with nadir CD4 220 and chronic hepatitis B infection, e-Ag positive with high baseline HBV viral level and probable cirrhosis. • Persistent HBV viremia in 5 log10 IU/mL range on lamivudine/adefovir (along with various antiretroviral agents) for many years. Case 1 - Extensive Treatment ...
HEPATITIS A. Patient A is 19 years of age and a college sophomore who presented to her physician's office with mild jaundice. The patient reports being in good health until a week before, at which time she began having flu-like symptoms of headache, low-grade fever, nausea, loss of appetite, and malaise.
Hepatitis A and B Case Study. Jon and Laura Green are siblings sharing similar symptoms. Both have a yellow tinge to their skin and the whites of their eyes. Both have flu-like symptoms. Jon, however, has been living in Brazil while Laura has been in San Francisco. Is this a weird sibling connection?
Case ID: _____ Legacy Case ID:_____ DEMOGRAPHIC INFORMATION RACE: (check all that apply) Amer Indian or Alaska Native Black or African American White Asian Native Hawaiian or Pacific Islander Other Race, specify _____ ETHNICITY:
Epidemiology. First discovered in 1973 by Feinstone, a spherical 27 nanometer particle was seen on immune electron microscopy in the fecal sample of hepatitis A patients[].A member of the picornavirus family, the hepatitis A virus (HAV) is an RNA virus responsible for 1.4 million cases per year globally[], with an estimated 7134 deaths in 2016; almost half of these cases were reported in Asia[].
A study published in 2020 showed that approximately three-fourths of US-born adults ≥20 years of age were susceptible to hepatitis A during 2007-2016 ... Additional sources that will facilitate case ascertainment and case characterization include medical records, hospital discharge databases, and death certificates. ... Process for ...
Read chapter 24 of Infectious Diseases: A Case Study Approach online now, exclusively on AccessPharmacy. AccessPharmacy is a subscription-based resource from McGraw Hill that features trusted pharmacy content from the best minds in the field.
Materials and methods: In a case-control study, 314 HBV-infected patients and 557 healthy participants were recruited. Data on demographics, immunization history, medical history, family medical history, life history, therapeutic factors, and behavioral risk factors were collected through a standard checklist.
The HEP 5-Step HCV MCM Model (Figure 2) provides. framework for medical case managers as they support. client's movement along the HCV continuum of care as shown above. These steps include: 1) meeting the client; 2) intake and assessment; 3) linkage to care; 4) engagement in care; and 5) post-treatment support.
From the initial case of AIDS combined with chronic hepatitis B, through the diagnosis and treatment of multidrug-resistant tuberculosis, the development of liver failure and disseminated cryptococcosis, and ultimately the concurrent occurrence of IRIS, the entire process was tortuous but ultimately resulted in a good outcome (Fig. 4 ...
Methods: This single-center case-control study, conducted at Peking Union Medical College Hospital, enrolled 33 patients with HBV-HCC, 26 patients with CHB, 30 healthy controls, and 33 controls with other types of cancer. Urine proteomes were analyzed using liquid chromatography with tandem mass spectrometry by data-dependent acquisition or ...
The hepatitis C virus (HCV) causes acute hepatitis C and is commonly detected via HCV antibody testing. However, delayed seroconversion of HCV antibodies and non-specific symptoms may hinder the diagnosis of this disease. A 71-year-old woman developed acute hepatitis while hospitalised for back pain. An HCV antibody test was negative, and she ...
CDC Hepatitis C Case Definitions. ... Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001-2008. ... Hoots WK, et al. A multicenter study of viral hepatitis in a United States hemophilic population. Blood. 1993;81:412-8 ...
Background Case-based learning (CBL) methods have gained prominence in medical education, proving especially effective for preclinical training in undergraduate medical education. Tetralogy of Fallot (TOF) is a congenital heart disease characterized by four malformations, presenting a challenge in medical education due to the complexity of its anatomical pathology. Three-dimensional printing ...
Educational Case: Evaluating a patient with cirrhosis. The following fictional case is intended as a learning tool within the Pathology Competencies for Medical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, Organ System Pathology, and ...
This case-control study enrolled 41 children and adolescents (aged 8-18 years) with asthma and their age and gender-matched controls in 2019-2020. Serum Calcium, phosphate, vitamin D, and bone mineral density were measured. ... Shiraz University of Medical Science Ethics Committee and vice-chancellor of research approved the study with the ...