Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 23 June 2023

Environmental risk factors of systemic lupus erythematosus: a case–control study
- Rania H. Refai ORCID: orcid.org/0000-0002-9524-1346 1 ,
- Mohammed F. Hussein ORCID: orcid.org/0000-0003-3775-6226 2 ,
- Mamdouh H. Abdou 2 &
- Anna N. Abou-Raya 3
Scientific Reports volume 13 , Article number: 10219 ( 2023 ) Cite this article
2900 Accesses
3 Citations
1 Altmetric
Metrics details
- Rheumatology
- Risk factors
Systemic lupus erythematosus (SLE) is a complicated chronic autoimmune disorder. Several genetic and environmental factors were suggested to be implicated in its pathogenesis. The main objective of this study was to examine how exposure to selected environmental factors was associated with SLE risk to support the development of disease preventive strategies. A case–control study was conducted at the Rheumatology outpatient clinic of Alexandria Main University Hospital, in Alexandria, Egypt. The study sample consisted of 29 female SLE patients, and 27 healthy female controls, who matched the cases on age and parity. Data were collected by a structured interviewing questionnaire. Blood levels of lead, cadmium, and zinc of all participants were assessed by flame atomic absorption spectrometry. The multivariate stepwise logistic regression model revealed that five factors showed significant association with SLE, namely living near agricultural areas, passive smoking, blood lead levels ≥ 0.075 mg/L, and exposure to sunlight (odds ratio (OR) 58.556, 95% confidence interval (CI) 1.897–1807.759, OR 24.116, 95% CI 1.763–329.799, OR 18.981, 95% CI 1.228–293.364, OR 9.549, 95% CI 1.299–70.224, respectively). Whereas walking or doing exercise were significantly protective factors (P = 0.006). The findings of this study add to the evidence that SLE can be environmentally induced. Preventive measures should be taken to address the environmental risk factors of SLE.
Similar content being viewed by others

Alzheimer’s disease risk reduction in clinical practice: a priority in the emerging field of preventive neurology

Genome-wide association studies

The serotonin theory of depression: a systematic umbrella review of the evidence
Introduction.
Systemic lupus erythematosus (SLE) is a chronic rheumatic autoimmune disorder that could be manifested by many symptoms. It is a multi-system disease that may involve nearly any organ resulting in serious organ complications and even death 1 , 2 . It predominantly affects women in the child-bearing ages 3 . SLE was suggested to be a condition of multifactorial etiology; including genetics, hormones, and environmental exposures.
It is currently known that different environmental factors could trigger SLE onset and flares in genetically susceptible individuals 4 , 5 . Owing to the increasing prevalence and overall SLE burden, efforts have been made to recognize these genetic and non-genetic factors 6 .
The role of the environment was more prominent by the fact that SLE concordance among identical monozygotic twins is below 25% 7 . In addition, the contribution of environmental factors to SLE risk has been evaluated to constitute 56% 8 .
Smoking, silica dust, UV radiation, infections, stress, air pollution, pesticides, and heavy metals are the major environmental risk factors having some evidence for association with SLE 9 .
In recent years, heavy metal pollution has become a significant health issue; continuous exposure to low levels of these toxic trace elements may result in bioaccumulation and produce a wide variety of biological effects on human beings 10 .
Lead and cadmium are known to pose serious risks to human health. Toxicity of these agents is evidenced by being identified in the top 10 environmental hazards by the Agency for Toxic Substances and Disease Registry 11 .
Environmental sources of lead include inhalation of airborne dusts containing lead and ingestion through food or water contaminated by lead. Old deteriorating household paints, and lead use in some traditional medicines and cosmetics can also be a source of lead exposure 12 . In addition, active and passive smoking were found to be associated with increased blood lead levels 13 .
Cadmium is present in cigarette smoke, air, food, and water. It can enter human bodies through inhalation, ingestion and dermal contact 14 .
Experimental studies of lead and cadmium exposure in rodent models proposed that metals may play a causative role in SLE 15 , 16 . There is relatively little data pertaining to lead or cadmium exposure with the risk of SLE in humans; exposure to stained or leaded glass as a hobby was found to be more common among SLE cases than controls 17 .
Trace elements such as zinc play a crucial role in growth and development of all organisms. Zinc is the second most abundant trace metal in the human body after iron, but zinc cannot be stored and has to be taken up daily via food to guarantee sufficient supply. However, it was stated that zinc excess as well as zinc deficiency may result in severe disturbances in immune cell numbers and activities leading to immune dysfunction 18 .
A study by Sahebari et al. 19 found that serum Zn values were lower in SLE patients than healthy age and sex-matched controls. On the other hand, zinc-deficient diets retarded autoantibody production and enhanced survival in mice 20 . In their review, Constantin et al. 21 advised to restrict consumption of some minerals such as zinc and sodium.
As the data about the relation between environmental risk factors and SLE in Egypt are lacking and the incidence is increasing, the present study was proposed to determine the association between some environmental exposures with SLE risk; in order to assess the extent to which SLE is environmentally induced.
Estimation of SLE risk in relation to some socio-demographic characteristics
Analysis of data regarding the socio-demographic characteristics revealed that patients and controls were similar in terms of demographic background, no statistical significant difference between cases and controls except for the education level, the occupation, and the residence near agricultural areas (Tables 1 , 2 ).
Regarding the educational level, there was a statistically significant difference between cases and controls (P < 0.05). The percentage of illiterate cases was (31%) and constituted more than double their percentage in the control group (14.8%). Also, the percentage of cases with higher education was only (3.4%), which is very low compared to controls (14.8%). The findings showed that females with SLE who were in the primary or preparatory educational level were significantly more likely to be at risk of SLE (OR 14.67, 95% CI 1.16–185.23) compared to controls.
With regard to occupation, there was a statistically significant difference between cases and controls (P < 0.05), the majority of cases were housewives (86.2%) compared to (33.3%) in the controls group.
Estimation of SLE risk in relation to some lifestyle factors
Lack of physical activity, exposure to domestic animals, or to sun light showed significant results (Table 3 ). As regards walking and physical activity, a high proportion of SLE patients (89.7%) do not like to walk or perform any physical activity compared to (40.7%) in the control group.
Sedentary lifestyle increased SLE risk by 12.61 folds (OR 12.61, 95% CI 3.05–52.17). Dealing with domestic animals was another risk factor that was tested in the current study; the results showed that 58.6% of cases were exposed to animals more than controls (29.6%). This may be because 48.3% of cases live in rural areas compared to 7.4% of controls. There was a statistically significant increase in SLE risk when dealing with farm animals (sheep, chicken) (OR 3.36, 95% CI 1.11–10.19).
Results about UV radiation exposure and SLE risk showed a statistically significant increase of SLE risk (OR 13.714, 95% CI 3.768–49.920).
Estimation of SLE risk in relation to some indoor environmental risk factors
The risk values for the association between some indoor environmental exposures and SLE were statistically not significant except for the type of drinking water, the filled tube cooker as the fuel used at home, and passive smoking (Table 4 ); 62.1% of SLE patients use tap water for drinking compared to 22.2% of controls, meanwhile, the majority of controls (74.1%) use filtered water compared to 37.9% of cases. The use of filtered water for drinking was assumed to have protective effects against SLE.
A statistically significant difference (X 2 = 16.021, P = 0.001) between cases and controls with regard to fuel used at home, where 79.3% of cases reported the use of gas cylinders compared to 25.9% of controls. The use of gas cylinders constituted 10.95 times more risk to SLE than the use of natural gas pipes (OR 10.95, 95% CI 3.16–38.01).
Regarding passive smoking at home, which was assessed by the number of active smokers who were residing in the same house, (62.1%) of cases were exposed to Environmental Tobacco Smoke (ETS) versus (29.6%) of controls, there was a statistically significant difference between cases and controls with regard to exposure to ETS (OR 3.886, 95% CI 1.273–11.861).
Moreover, SLE risk increased with the increase in the number of cigarettes smoked per day (the risk was 2.96 and 10.36 times for light and heavy passive smoking (OR 2.96, 95% CI 0.9–9.75; OR 10.36, 95% CI 1.1–97.69 respectively). A significant trend for risk was noticed with increased passive smoking (X 2 for trend = 5.87, P = 0.015) concluding that ETS may be an important risk factor for SLE.
Estimation of SLE risk in relation to a family history of any auto-immune disease
The study found a statistically significant difference between cases and controls regarding the family history of any auto immune disease as illustrated in (Table 5 ), 33.3% of controls had surprisingly a family history of an auto immune disease including SLE compared to 3.4% of cases.
Estimation of SLE risk in relation to hormonal factors
The findings of the present study showed the prevalence of menstrual disorders and hormonal disturbances among SLE patients as demonstrated in Table 5 . Early menopause was present in (31%) of SLE patients compared to (0%) of controls, irregular menstrual cycle was prevalent in (40%) of SLE patients compared to (8%) of controls, (37.9%) of cases used hormonal therapy compared to (11.1%) of controls, and (31%) of cases had problems in uterus versus (7.4%) of controls.
There was a statistically significant increase in SLE risk with early menopause, menstrual irregularities, use of hormonal therapy, and presence of problems in uterus (OR 31.26, 95% CI 1.7–575.54; OR 7.67, 95% CI 1.3–45.29; OR 4.889, 95% CI 1.19–20.13; OR 5.625, 95% CI 1.09–29.03 respectively).
Estimation of SLE risk in relation to blood levels of lead, cadmium and zinc
As shown in (Table 6 ), the range of values of blood lead levels (Pb) in the cases group was very extensive from minimum 0.0237 mg/L to maximum 0.6951 mg/L and it is higher than the range of values in the controls group. The median concentration of blood lead in the cases group (0.115 ± 0.165) was significantly higher than in the controls group (0.067 ± 0.077). There were significantly higher blood lead levels in the cases group compared to the controls group (U = 210, P = 0.003).
After categorization of the levels of blood lead in the sample, the SLE risk associated with blood lead levels ≥ 0.09 mg/L was higher and statistically significant (OR 7.71, 95% CI 1.85–32.21) in comparison with subjects having blood lead levels < 0.05 mg/L. A statistically significant trend was computed (Chi-square trend for odds = 7.77, P value = 0.005) showing that the more the increase in blood lead level, the more the chance of SLE occurrence.
The median levels of blood cadmium (Cd) in cases group (0.059 ± 0.102 mg/L) were significantly higher than in the controls group (0.017 ± 0.042 mg/L) as U = 216, P = 0.004.
As presented in (Table 6 ), the level of blood cadmium in the sample was categorized into groups, females having blood cadmium levels from 0.03 to less than 0.07 and ≥ 0.07 mg/L blood had 6.68 and 4.45 times more risk to develop SLE in comparison with females having blood cadmium levels < 0.03 mg/L blood and the risks for these upper two categories were statistically significant (95% CI 1.58–28.29; 1.18–16.8 respectively). The observed increased trend was found to be statistically significant (X 2 trend for odds = 4.98, P = 0.026).
Regarding blood zinc levels (Zn), the median concentration of zinc in the controls group (2.275 ± 0.707 mg/L blood) was lower than the median concentration of zinc in the cases group (2.660 ± 0.970 mg/L blood), but the median blood zinc values in the cases and controls groups did not differ significantly (U = 290.5, P > 0.05).
Pearson’s correlation coefficient was calculated to demonstrate the relationship between lead and cadmium blood levels with each other and yielded a result of r = 0.548, P = 0.002, portraying a positive, moderate, linear, and significant correlation between lead and cadmium blood levels for the cases group (Fig. 1 ), whereas a non-significant correlation was observed in the controls group (r = 0.123, P = 0.542).
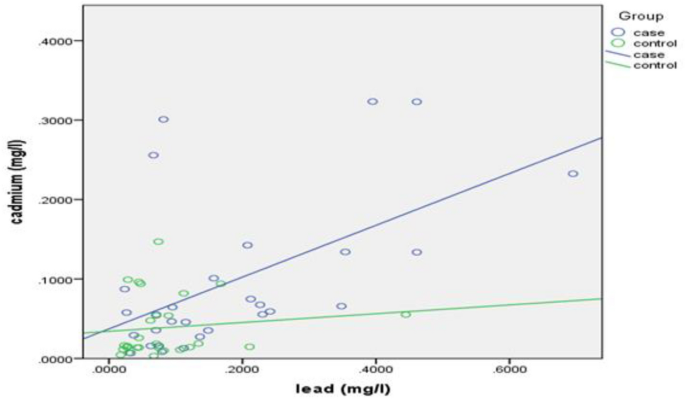
Correlation between blood lead and cadmium levels for cases and controls (Rheumatology outpatient clinic, Alexandria University Hospital, Alexandria, 2020). Performed through SPSS version 21.
Analysis of risk factors affecting SLE risk by stepwise logistic regression
A multivariate stepwise logistic regression model was built in order to determine which of these predictors really contribute to predicting SLE risk, and exclude those who do not.
Negelkerke R Square suggests that the model explains 78.4% of the variance in the outcome. The accuracy of the model was 87.5%, depicting that the model can correctly classify 87.5% of the cases. The sensitivity and specificity of the model were calculated, and they were 86.2%, and 88.9%, respectively.
As illustrated in (Table 7 ), the final model revealed that only five factors showed significant association with SLE. The risk was highest for subjects living near agricultural areas with an OR of 58.556 (95% CI 1.897–1807.759), followed by subjects exposed to passive smoking with an OR of 24.116 (95% CI 1.763–329.799), then subjects having blood lead levels ≥ 0.075 mg/L who were 18.981 times (95% CI 1.228–293.364) more likely to have SLE than those having blood lead levels < 0.075 mg/L, followed by subjects exposed to the sunlight who were at increased risk of SLE by 9.549 (95% CI 1.299–70.224). Whereas walking or doing exercise were significantly protective against SLE (P = 0.006); the table demonstrated a negative B coefficient (− 5.246) which indicates that a decrease in the walking and exercise is associated with a greater likelihood of SLE risk.
The current study purpose was to gain insights into the etiology of SLE and some possible risk factors.
The statistically significant difference between cases and controls regarding the education level assumes that lower education level—which is an indicator of socioeconomic status—may constitute a risk for SLE. That is similar to other studies that showed the association between lower education level and SLE onset and flare 22 , 23 .
The distribution of the study sample according to the residence area revealed that 48.3% of cases reported living near agricultural areas compared to 7.4% of controls, with 11.67 times more SLE risk (OR 11.67, 95% CI 2.32–58.6) compared to those not living near these areas. This may be attributed to some environmental exposures such as exposure to sunlight and pesticides used in agriculture, in addition to lower socioeconomic status, lower educational level, and poverty. This finding is in accordance with Pons-Estel et al. (2012) who concluded that rural residence was associated with high levels of disease activity at diagnosis and with renal disease occurrence in a Latin American multi-ethnic cohort (OR 1.65, 95% CI 1.06–2.57; OR 1.77, 95% CI 1.00–3.11) 24 .
Our data showed an increase of SLE risk due to sedentary lifestyle which is in accordance with a cross sectional study that showed that a high proportion of SLE patients were physically inactive with a long daily sedentary time 25 .
The statistically significant increase in SLE risk when dealing with animals or birds was in the same line with a case control study in Southern Sweden that reported a statistically significant difference between cases and controls regarding close contact with sheep 26 . This was not in the same line with the results of a recent study which supported the idea that exposures related to childhood farm residence and livestock farming may decrease susceptibility to developing SLE and that contact with livestock may confer protection against SLE 27 .
Current study findings support the role of sun exposure as a trigger for SLE, adding evidence to experimental and human studies that have shown that it can trigger disease onset and induce disease flares in SLE patients 26 , 28 , 29 .
ETS as a risk factor for SLE was not enormously previously discussed except for a cross sectional study of Brazilian SLE patients that assessed the association between smoking and SLE and confirmed that never smokers confer a 22% relative SLE risk reduction compared to ever smokers (including second hand smokers) 30 .
In addition, data from a cohort of SLE patients and controls suggested that secondhand smoke during childhood may be a risk factor for SLE (OR 1.81, 95% CI 1.13–2.89) 31 . Whereas, a US prospective cohort study concluded that Early-life exposure to cigarette smoke due to mothers’ or fathers’ smoking did not increase the risk of adult-onset SLE (RR 0.9, 95% CI 0.6–1.4; RR 1.0, 95% CI 0.8–1.3 respectively) 32 .
Our data revealed that the highest proportion of the study patients (96.6%) had negative family history of SLE. This supports the great contribution of other risk factors rather than the genetic factors in the development of SLE; suggesting the influence of environmental triggers on disease expression 8 , 17 . This finding was in line with a study that reported that autoimmune diseases in family members have not been associated with SLE 33 . On the other hand, a previous case control study reported the prevalence of auto-immune disease in first degree relatives in cases more than controls (53% vs 39%) and stated that a family history of any auto-immune disease was associated with increased risk of SLE (OR 6.8, 95% CI 1.4–32) 26 . This finding is also in contrast with other previous studies that stated that family history of an auto immune disease in first degree relatives (parents or siblings) was associated with increased SLE risk 34 , 35 .
The results of the current study regarding SLE risk in relation to hormonal factors was in concordance with a cohort study that revealed that menstrual irregularity was associated with an increased SLE 36 . In the same line comes a cross sectional study of 61 SLE patients, in which 49.2% of the patients had menstrual irregularities, of which 60% had sustained amenorrhoea (premature menopause) compared to the control group (16.7%) 37 . Moreover, a cross sectional study (N = 87) showed menstrual alterations in 37.9% of SLE patients and amenorrhea in 11.5% of patients which was higher than the general population 38 . Additionally, an increased SLE risk was reported in the NHS (Nurses’ Health Study) with the use of estrogen replacement therapy 39 . Moreover, a population‐based nested case control study found that the current use of COCs (Combined Oral Contraceptives) was associated with an increased SLE risk (RR 1.54, 95% CI 1.15–2.07) 40 .
Other studies contradicted the findings of the current study and stated that the use of HRT (Hormone Replacement Therapy) in postmenopausal SLE women did not appear to increase the rate of lupus flares and appeared to be well tolerated and safe in postmenopausal SLE patients 41 , 42 .
Data from experimental studies suggested that heavy metals may enhance systemic autoimmunity or accelerate disease progression in experimental models of lupus and that co-exposure to certain heavy metals may increase the risk associated with other exposures 5 .
Detailed studies on SLE onset and flares with reference to lead, cadmium, and zinc are scanty. Therefore, the current study was conducted to evaluate the role of lead, cadmium, and zinc in SLE. All blood samples were found to have lead, cadmium, and zinc concentrations.
It was observed that the median blood lead levels in the cases group as well as the controls group in this study were higher than the CDC permissible range of less than 5 µg/dL of lead in children and adults (0.05 mg/L) and this is indicative of the extent of environmental lead pollution 43 . It was also noticed that the median blood cadmium levels for both cases and controls in this study were higher than the WHO permissible range of 0.03–0.12 µg/dL of Cd 44 . This suggests more protection measures to be taken into consideration in order to avoid the toxic effects of lead and cadmium.
It was observed that the median blood zinc levels were not consistent with reference ranges of zinc in blood (70–120 µg/dL) 45 , requiring further consideration of zinc levels to avoid overdose and toxicity.
In contrast to the current study is a recent similar case control study that reported that SLE diagnosis was associated with lower serum Zn (P = 0.003), and Pb (P = 0.020) 46 . Other studies reported similar results 47 , 48 . However, some studies did not observe a significant difference in serum Zn concentrations between SLE patients and healthy controls which is similar to the finding of the present study 49 , 50 .
A case control study reported a positive correlation between lead and cadmium blood levels for the exposed group (ρ = 0.39, P = 0.023) which was consistent with the finding of the current study, whereas blood zinc levels correlated negatively with both lead (ρ = − 0.41, P = 0.015) and cadmium blood levels (ρ = − 0.44, P = 0.009). In addition, no correlations between the studied metals were found in the control group and the study suggested that zinc insufficiency is more likely to occur in cases of combined exposure to cadmium and lead, because of competition between similar ions for receptors involved in absorption, transport, storage or function, and this would explain the negative correlation in the controls group between zinc blood levels with either lead and cadmium levels 51 .
Limitations of the study
The potential of case control studies for recall bias and misclassification error; as most exposure information were based on self-reported history so some inaccuracies can be expected. The design of the study also limited the ability to ascribe causal relationships to the associations detected and to control for all potential confounders.
Absence of genetic information for participants, so the potential effects of genetic heterogeneity on the association between risk factors and SLE risk could not be determined.
Human population is rarely exposed to a single agent over time, and there may be a significant delay between exposure and the onset of the disease.
The small sample size may make it difficult to determine if a particular outcome is a true finding and in some cases no difference between the study groups is reported.
Conclusion and recommendations
To date, our knowledge about the etiology of SLE is still unclear and limited; genetic and environmental interactions were suggested.
From the present case control study, it is concluded that the risk portion attributed to unsafe and unhealthy environment was found to be quite significant, showing how the environment can play an important role in SLE occurrence.
Exposure to potential environmental risk factors specifically heavy metals should not be under-estimated. Hence, there is an urgent need for interventions to reduce environmental risk factors exposure in order to achieve substantial public health gains.
Increasing the awareness of patients about the environmental pollutants and the ways to protect their health is necessary. As well as the awareness of health care providers about environmental risk factors, so they can advise the patients about the ways of reduction of exposure to environmental hazards. Educational awareness programs to patients and their family should be carried out through media and non-governmental organizations (NGOs) to raise their knowledge about environmental hazards and how to minimize the sources of their exposure and their consequent negative impacts.
Additional experimental and epidemiological studies are required to determine the causative role of several environmental exposures, to confirm data from case control studies.
This case control study was conducted at the Rheumatology outpatient clinic of Alexandria Main University Hospital. The Inclusion criteria were female patients diagnosed with idiopathic SLE according to SLICC criteria 52 . The controls included healthy females who accompanied the SLE patients who came from remote rural areas in their visit to the Rheumatology outpatient clinic. The cases and controls were matched for age and parity.
Exclusion criteria included (1) male SLE patients “they were excluded mainly to avoid gender bias that may affect the results due to the hormonal effect. Besides, the disease affects mainly females”; (2) drug-induced Lupus; (3) overlap syndrome as lupus and rheumatoid arthritis; (4) any other rheumatic diseases; (5) coexisting morbidity not related to SLE, e.g. diabetes, hypertension; (6) cancer; (7) dementia or psychosis; (8) intake of nutritional supplements in the 6 months prior to the blood collection.
Sample size
Based on a previous case control study, the mean of serum Zinc among systemic lupus erythematosus (SLE) was 700.61 ± 135.91 and among controls was 860.45 ± 123.74, using an alpha error of 0.05 and power 98% 48 . The minimum required sample size was estimated to be 46 adults, 23 for each group, which was increased to 56 adults, 29 cases and 27 controls. The sample size was calculated using G. Power software.
Data collection methods and tools
A pre-designed pre-coded structured interviewing questionnaire was used to collect data from all participants (cases and controls). It included personal and socio-demographic data, data about occupation, lifestyle factors, the medical history, the smoking history including exposure to passive smoking, and some possible indoor environmental risk factors aiming to ascertain exposure to some environmental risk factors suspected to affect SLE risk. Regarding the evaluation of poor water quality, the participants were asked about the availability and quality of drinking water, any problems concerning drinking water (clarity, taste, smell), the type of drinking water pipes (with or without lead) by asking them whether they are old or newly installed, and the drinking water source (filtered, bottled, or tap water). Whereas for sun exposure, they were asked about the daily exposure to the sun, duration of exposure, wearing of protective clothes, and use of sunscreen, as well as whether there is an occupational sun exposure. Concerning the evaluation of sedentary lifestyle, they were questioned whether they perform any physical activity and the duration per week, whether they prefer walking or taking the car, and the duration of watching TV.
Questions of the questionnaire were taken from similar previously validated Arabic and English research questionnaires 26 , 53 . In addition, it was assessed by an expert at the faculty of medicine, Alexandria University. The English version of the questionnaire underwent a forward and back translation by native speakers whom are experts in public health.
Laboratory investigation
A blood sample (5 mL) was drawn from each participant to measure blood levels of lead, cadmium, and zinc. The samples were transferred into heparinized collection tubes. Cadmium, lead, and zinc were extracted from the blood samples using the conventional wet acid digestion method using concentrated nitric acid (HNO 3 ). A blank using deionized water instead of blood was done for each batch of analysis for comparison 44 , 54 . The digested samples were filtered and were subjected to elemental analysis using flame atomic absorption spectrophotometer (Shimadzu model AA-6650) at the central laboratory of the High Institute of Public Health (HIPH), Alexandria University.
Statistical design
SPSS version 21 was used for data entry and analysis. Qualitative variables were described through number and percentages of cases. For quantitative continuous variables, tests of normality were done. Mean and standard deviation (mean ± SD) were calculated if the variable follows normal distribution, and median and interquartile range (median ± IQR) if it does not follow normal distribution. “Pearson’s Chi-square test (X 2 )” was used to calculate significant differences between cases and controls for the categorical data. If the assumptions were violated, “Fisher’s exact test” (if 2*2 table) or “Monte Carlo test” (if m*n table) were used.
Differences between the means of the two groups were examined using independent t test for the continuous, normally distributed variables. The Mann–Whitney–Wilcoxon non-parametric test (U), for the continuous, non-normally distributed variables. Odds ratio (OR) was calculated to measure SLE risk. Increasing trends in SLE risk concerning some risk factors were tested using “Chi-square for trend”. Pearson’s correlation coefficient was used to test the association between quantitative variables.
A multivariate stepwise logistic regression analysis was used to see if there were significant associations between specific exposures and SLE and to adjust for some potential confounders. Negelkerke R 2 was calculated to tell the amount of variation in SLE risk which is explained by the model.
Ethical approval
Approval of the Ethics Committee of the High Institute of Public Health for conducting the research was obtained. Approval for conducting the study at the Rheumatology Outpatient Clinic of Alexandria Main University Hospital was obtained from the hospital outpatient clinics director after a formal written request for that. The study was performed in accordance with the ethical standards in the Declaration of Helsinki. A written informed consent was taken from all study participants after explanation of the purpose and benefits of the research. Anonymity and confidentiality were ensured. All methods were performed in accordance with the relevant guidelines and regulations.
Data availability
The datasets used and/or analyzed during the current study are not publicly due to privacy and ethical concerns but are available from the corresponding author on reasonable request.
Ferenkeh-Koroma, A. Systemic lupus erythematosus: Nurse and patient education. Nurs. Stand. 26 (39), 49–57. https://doi.org/10.7748/ns2012.05.26.39.49.c9134 (2012).
Article PubMed Google Scholar
Zucchi, D. et al. Systemic lupus erythematosus: One year in review 2023. Clin. Exp. Rheumatol. 41 (5), 997–1008. https://doi.org/10.55563/clinexprheumatol/4uc7e8 (2023) ( Epub 2023 May 3 ).
Lai, Y. et al. Different pregnancy outcomes in patients with systemic lupus erythematosus treated with belimumab. Lupus 32 (1), 149–154. https://doi.org/10.1177/09612033221141805 (2023) ( Epub 2022 Dec 5 ).
Article CAS PubMed Google Scholar
Woo, J. M. P., Parks, C. G., Jacobsen, S., Costen-bader, K. H. & Bernatsky, S. The role of environmental exposures and gene–environment interactions in the etiology of systemic lupus erythematous. J. Intern. Med. 291 , 755–778 (2022).
Barbhaiya, M. & Costenbader, K. H. Environmental exposures and the development of systemic lupus erythematosus. Curr. Opin. Rheumatol. 28 (5), 497–505. https://doi.org/10.1097/BOR.0000000000000318 (2016).
Article CAS PubMed PubMed Central Google Scholar
Gergianak, I. et al. Epidemiology and burden of systemic lupus erythematosus in a Southern European population: Data from the community-based lupus registry of Crete, Greece. Ann. Rheum. Dis. 76 , 1992–2000. https://doi.org/10.1136/annrheumdis-2017-211206 (2017).
Article Google Scholar
Bhaskar, L. V. K. S. & Nagaraju, G. P. Clinical and immunogenetic aspects of systemic lupus erythematosus. Crit. Rev. Immunol. 39 (5), 343–360. https://doi.org/10.1615/CritRevImmunol.2020033247 (2019).
Leffers, H. C. B., Lange, T., Collins, C., Ulff-Moller, C. J. & Jacobsen, S. The study of interactions between genome and exposome in the development of systemic lupus erythematosus. Autoimmun. Rev. 18 (4), 382–392. https://doi.org/10.1016/j.autrev.2018.11.005 (2019).
Gulati, G. & Brunner, H. I. Environmental triggers in systemic lupus erythematosus. Semin. Arthritis Rheum. 47 (5), 710–717. https://doi.org/10.1016/j.semarthrit.2017.10.001 (2018).
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B. & Beeregowda, K. N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 7 (2), 60–72. https://doi.org/10.2478/intox-2014-0009 (2014).
ATSDR [internet]. ATSDR’s Substance Priority List. 2022. https://www.atsdr.cdc.gov/spl/index.html .
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K. & Sutton, D. J. Heavy metal toxicity and the environment. Exp. Suppl. 101 , 133–164. https://doi.org/10.1007/978-3-7643-8340-4_6 (2012).
Mannino, D. M., Homa, D. M., Matte, T. & Hernandez-Avila, M. Active and passive smoking and blood lead levels in US adults: Data from the Third National Health and Nutrition Examination Survey. Nicotine Tob. Res. 7 (4), 557–564. https://doi.org/10.1080/14622200500185264 (2005).
Virani, S. et al. DNA methylation is differentially associated with environmental cadmium exposure based on sex and smoking status. Chemosphere 145 , 284–290. https://doi.org/10.1016/j.chemosphere.2015.10.123 (2016).
Article ADS CAS PubMed Google Scholar
Hudson, C. A., Cao, L., Kasten-Jolly, J., Kirkwood, J. N. & Lawrence, D. A. Susceptibility of lupus-prone NZM mouse strains to lead exacerbation of systemic lupus erythematosus symptoms. J. Toxicol. Environ. Health Part A 66 (10), 895–918. https://doi.org/10.1080/15287390306456 (2003).
Article CAS Google Scholar
Leffel, E. K., Wolf, C., Poklis, A. & White, K. L. Drinking water exposure to cadmium, an environmental contaminant, results in the exacerbation of autoimmune disease in the murine model. Toxicology 188 (2–3), 233–250. https://doi.org/10.1016/s0300-483x(03)00092-1 (2003).
Kamen, D. L. Environmental influences on systemic lupus erythematosus expression. Rheum. Dis. Clin. N. Am. 40 (3), 401–412. https://doi.org/10.1016/j.rdc.2014.05.003 (2014).
Maywald, M., Wessels, I. & Rink, L. Zinc signals and immunity. Int. J. Mol. Sci. 18 (10), 2222. https://doi.org/10.3390/ijms18102222 (2017).
Sahebari, M. et al. Association between serum trace element concentrations and the disease activity of systemic lupus erythematosus. Lupus 23 (8), 793–801. https://doi.org/10.1177/0961203314530792 (2014).
Beach, R. S., Gershwin, M. E. & Hurley, L. S. Nutritional factors and autoimmunity. II. Prolongation of survival in zinc-deprived/W mice. J. Immunol. 128 (1), 308–313 (1982).
Constantin, M. M. et al. Significance and impact of dietary factors on systemic lupus erythematosus pathogenesis. Exp. Ther. Med. 17 (2), 1085–1090. https://doi.org/10.3892/etm.2018.6986 (2019).
Ward, M. M. Education level and mortality in systemic lupus erythematosus (SLE): Evidence of underascertainment of deaths due to SLE in ethnic minorities with low education levels. Arthritis Rheumatol. 51 (4), 616–624. https://doi.org/10.1002/art.20526 (2004).
Zhang, L. et al. Lack of patient education is risk factor of disease flare in patients with systemic lupus erythematosus in China. BMC health Serv. Res. 19 (1), 378. https://doi.org/10.1186/s12913-019-4206-y (2019).
Article PubMed PubMed Central Google Scholar
Pons-Estel, G. J. et al. The impact of rural residency on the expression and outcome of systemic lupus erythematosus: Data from a multiethnic Latin American cohort. Lupus 21 (13), 1397–1404. https://doi.org/10.1177/0961203312458465 (2012).
Margiotta, D. P. E. et al. Physical activity and sedentary behavior in patients with Systemic Lupus Erythematosus. PLoS One 13 (3), e0193728. https://doi.org/10.1371/journal.pone.0193728 (2018).
Bengtsson, A. A., Rylander, L., Hagmar, L., Nived, O. & Sturfelt, G. Risk factors for developing systemic lupus erythematosus: A case–control study in southern Sweden. J. Rheumatol. (Oxf). 41 (5), 563–571. https://doi.org/10.1093/rheumatology/41.5.563 (2002).
Parks, C., Long, S., Beane-Freeman, L., Jonathan, H. & Dale, S. Systemic lupus erythematosus and Sjögren’s syndrome in the agricultural health study: Lower risk associated with childhood farm residence and raising livestock [abstract]. Arthritis Rheumatol. 71 , 10 (2019).
Google Scholar
Mak, A. & Tay, S. H. Environmental factors, toxicants and systemic lupus erythematosus. Int. J. Mol. Sci. 15 (9), 16043–16056. https://doi.org/10.3390/ijms150916043 (2014).
Cooper, G. S. et al. Occupational and environmental exposures and risk of systemic lupus erythematosus: Silica, sunlight, solvents. Rheumatology (Oxford) 49 (11), 2172–2180. https://doi.org/10.1093/rheumatology/keq214 (2010).
Montes, R. A. et al. Smoking and its association with morbidity in systemic lupus erythematosus evaluated by the systemic lupus international collaborating clinics/American College of Rheumatology Damage Index: Preliminary Data and Systematic Review. Arthritis Rheumatol. 68 (2), 441–448. https://doi.org/10.1002/art.39427 (2016).
Minkin, S. J., Slan, S. N., Gilkeson, G. S. & Kamen, D. L. Smoking and secondhand smoke among patients with systemic lupus erythematosus and controls: Associations with disease and disease damage. Arthritis Res. Ther. 16 (Suppl 1), A40. https://doi.org/10.1186/ar4656 (2014).
Article PubMed Central Google Scholar
Simard, J. F., Costenbader, K. H., Liang, M. H., Karlson, E. W. & Mittleman, M. A. Early-life exposure to cigarette smoke and adult-onset SLE. Lupus 18 (5), 431–435. https://doi.org/10.1177/0961203308098186 (2009).
Grimaldi-Bensouda, L. et al. The risk of systemic lupus erythematosus associated with vaccines: An international case–control study. Arthritis Rheumatol. 66 (6), 1559–1567. https://doi.org/10.1002/art.38429 (2014).
Cooper, G. S., Dooley, M. A., Treadwell, E. L., StClair, E. W. & Gilkeson, G. S. Hormonal and reproductive risk factors for development of systemic lupus erythematosus: Results of a population-based, case–control study. Arthritis Rheumatol. 46 (7), 1830–1839. https://doi.org/10.1002/art.10365 (2002).
Kuo, C. F. et al. Familial aggregation of systemic lupus erythematosus and coaggregation of autoimmune diseases in affected families. JAMA Intern. Med. 175 (9), 1518–1526. https://doi.org/10.1001/jamainternmed.2015.3528 (2015).
Costenbader, K. H., Feskanich, D., Stampfer, M. J. & Karlson, E. W. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheumatol. 56 (4), 1251–1262. https://doi.org/10.1002/art.22510 (2007).
Fatnoon, N. N., Azarisman, S. M. & Zainal, D. Prevalence and risk factors for menstrual disorders among systemic lupus erythematosus patients. Singap. Med. J. 49 (5), 413–418 (2008).
CAS Google Scholar
Nonato, D. R. et al. Menstrual disturbances in systemic lupus erythematosus patients using immunossuppressants. Rev. Bras. Reumatol. 50 (5), 501–515 (2010).
Sanchez-Guerrero, J., Liang, M. H., Karlson, E. W., Hunter, D. J. & Colditz, G. A. Postmenopausal estrogen therapy and the risk for developing systemic lupus erythematosus. Ann. Intern. Med. 122 (6), 430–433. https://doi.org/10.7326/0003-4819-122-6-199503150-00005 (1995).
Bernier, M. O., Mikaeloff, Y., Hudson, M. & Suissa, S. Combined oral contraceptive use and the risk of systemic lupus erythematosus. Arthritis Rheumatol. 61 (4), 476–481. https://doi.org/10.1002/art.24398 (2009).
Buyon, J. P. et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: A randomized trial. Ann. Intern. Med. 142 (12 Pt 1), 953–962. https://doi.org/10.7326/0003-4819-142-12_part_1-200506210-00004 (2005).
Mok, C. C. et al. Safety of hormonal replacement therapy in postmenopausal patients with systemic lupus erythematosus. Scand. J. Rheumatol. 27 (5), 342–346. https://doi.org/10.1080/03009749850154357 (1998).
ATSDR. Lead Toxicity. What Are U.S. Standards for Lead Levels? [2019-b]. https://www.atsdr.cdc.gov/csem/csem.asp?csem=34&po=8 .
Alli, L. A. Blood level of cadmium and lead in occupationally exposed persons in Gwagwalada, Abuja, Nigeria. Interdiscip. Toxicol. 8 (3), 146–150. https://doi.org/10.1515/intox-2015-0022 (2015).
Mashhadi, M. A., Bakhshipour, A., Zakeri, Z. & Ansari- Moghadam, A. Reference range for zinc level in young healthy population in Southeast of Iran. Health Scope 6 , 1. https://doi.org/10.17795/jhealthscope-18181 (2016).
Pedro, E. M. et al. Trace elements associated with systemic lupus erythematosus and insulin resistance. Biol. Trace Elem. Res. 191 (1), 34–44. https://doi.org/10.1007/s12011-018-1592-7 (2019).
Yilmaz, A., Sari, R. A., Gundogdu, M., Kose, N. & Dag, E. Trace elements and some extracellular antioxidant proteins levels in serum of patients with systemic lupus erythematosus. Clin. Rheumatol. 24 (4), 331–335. https://doi.org/10.1007/s10067-004-1028-y (2005).
Toth, C. N. et al. Elemental analysis of whole and protein separated blood serum of patients with systemic lupus erythematosus and Sjogren’s syndrome. Biol. Trace Elem. Res. 179 (1), 14–22. https://doi.org/10.1007/s12011-017-0945-y (2017).
Almroth, G., Westberg, N. G. & Sandstrom, B. M. Normal zinc and selenium levels in patients with systemic lupus erythematosus. J. Rheumatol. 12 (3), 633–634 (1985).
CAS PubMed Google Scholar
Nossent, J., Lester, S., Rischmueller, M. & Zalewski, P. No zinc deficiency but a putative immunosuppressive role for labile Zn in patients with systemic autoimmune disease. Curr. Rheumatol. Rev. 13 (1), 59–64. https://doi.org/10.2174/1573397111666151026223501 (2017).
Gidikova, P. L. Blood lead, cadmium and zinc correlations in elderly rural residents. Folia Med. (Plovdiv). 61 (1), 113–119. https://doi.org/10.2478/folmed-2018-0051 (2019).
Petri, M. et al. Derivation and validation of systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 64 (8), 2677–2686. https://doi.org/10.1002/art.34473 (2012).
Hussein, M. F. Some Environmental Risk Factors of Autism Spectrum Disorder (Unpublished Doctoral Dissertation) (High Institute of Public Health, 2017).
Lemos, V. A. & de Carvalho, A. L. Determination of cadmium and lead in human biological samples by spectrometric techniques: A review. Environ. Monit. Assess. 171 (1–4), 255–265. https://doi.org/10.1007/s10661-009-1276-z (2010).
Download references
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and affiliations.
Department of Medicine Supply and Pharmacy, Alexandria University Hospitals, Alexandria University, Alexandria, Egypt
Rania H. Refai
Department of Occupational Health and Industrial Medicine, High Institute of Public Health, Alexandria University, Alexandria, Egypt
Mohammed F. Hussein & Mamdouh H. Abdou
Department of Internal Medicine, Rheumatology & Clinical Immunology, Faculty of Medicine, Alexandria University, Alexandria, Egypt
Anna N. Abou-Raya
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization and design of the work: R.R., M.H., M.A., and A.A.; methodology: R.R., M.H., and M.A.; selection of cases and controls: A.A.; data analysis: R.R., and M.H.; data curation: R.R., and M.H.; writing—original draft preparation, R.R.; writing—review and editing: R.R., M.H., M.A., and A.A.; visualization: R.R. All authors read and approved the final submitted revised manuscript and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Correspondence to Rania H. Refai .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Refai, R.H., Hussein, M.F., Abdou, M.H. et al. Environmental risk factors of systemic lupus erythematosus: a case–control study. Sci Rep 13 , 10219 (2023). https://doi.org/10.1038/s41598-023-36901-y
Download citation
Received : 17 January 2022
Accepted : 12 June 2023
Published : 23 June 2023
DOI : https://doi.org/10.1038/s41598-023-36901-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
Contributor Disclosures
Please read the Disclaimer at the end of this page.
INTRODUCTION — Systemic lupus erythematosus (SLE) is a chronic autoimmune disease of unknown cause that can affect virtually any organ of the body. Immunologic abnormalities, especially the production of a number of antinuclear antibodies (ANA), are a prominent feature of the disease.
Patients present with variable clinical features ranging from mild joint and skin involvement to life-threatening kidney, hematologic, or central nervous system involvement. The clinical heterogeneity of SLE and the lack of pathognomonic features or tests pose a diagnostic challenge for the clinician. To complicate matters, patients may present with only a few clinical features of SLE, which can resemble other autoimmune, infectious, or hematologic diseases.
The diagnosis of SLE is generally based on clinical and laboratory findings after excluding alternative diagnoses. In the absence of SLE diagnostic criteria, SLE classification criteria are often used by clinicians as guidance to help identify some of the salient clinical features when making the diagnosis. Serologic findings are important in suggesting the possibility of SLE, with some antibodies (eg, anti-double-stranded deoxyribonucleic acid [anti-dsDNA] and anti-Smith [anti-Sm]) highly associated with this condition.
The clinical manifestations and an approach to the diagnosis of SLE will be reviewed here. Separate topic reviews related to SLE in adults include the following:
● (See "Systemic lupus erythematosus in adults: Overview of the management and prognosis" .)
● (See "Epidemiology and pathogenesis of systemic lupus erythematosus" .)
● (See "Drug-induced lupus" .)
The diagnosis and treatment of specific clinical manifestations of SLE are discussed separately:
● (See "Overview of cutaneous lupus erythematosus" .)
● (See "Arthritis and other musculoskeletal manifestations of systemic lupus erythematosus" .)
● (See "Lupus nephritis: Diagnosis and classification" .)
● (See "Lupus nephritis: Therapy of lupus membranous nephropathy" .)
● (See "Lupus nephritis: Initial and subsequent therapy for focal or diffuse lupus nephritis" .)
● (See "Hematologic manifestations of systemic lupus erythematosus" .)
● (See "Gastrointestinal manifestations of systemic lupus erythematosus" .)
● (See "Coronary artery disease in systemic lupus erythematosus" .)
● (See "Non-coronary cardiac manifestations of systemic lupus erythematosus in adults" .)
● (See "Neurologic and neuropsychiatric manifestations of systemic lupus erythematosus" .)
● (See "Manifestations of systemic lupus erythematosus affecting the peripheral nervous system" .)
The approach to contraception and pregnancy in females with SLE is covered separately:
● (See "Approach to contraception in women with systemic lupus erythematosus" .)
● (See "Pregnancy in women with systemic lupus erythematosus" .)
CLINICAL MANIFESTATIONS
Major clinical features and organ involvement
Constitutional symptoms — Constitutional symptoms such as fatigue, fever, and weight loss are present in most patients with systemic lupus erythematosus (SLE) at some point during the course of the disease.
● Fatigue – Fatigue is the most common complaint, occurring in 80 to 100 percent of patients, and can sometimes be disabling. Its presence is not clearly correlated with other measures of disease activity and is more frequently associated with depression, sleep disturbances, and concomitant fibromyalgia [ 1-5 ].
● Fever – Fever can be a manifestation of active disease and is seen in over 50 percent of patients with SLE [ 6 ]. However, in clinical practice, distinguishing fever associated with a lupus flare from other causes of fever, such as infection, a drug reaction, or malignancy, can be difficult. Clinically, there are no specific features that definitively distinguish fever due to SLE from fever due to other causes. The history may be helpful in determining the cause of the fever. As an example, fever in the setting of moderate or high doses of glucocorticoids should lead one to strongly suspect new infection, particularly if other signs of active disease are not present. Fever that does not respond to nonsteroidal antiinflammatory drugs (NSAIDs), acetaminophen , and/or low to moderate doses of glucocorticoids should raise the suspicion of an infectious or drug-related etiology, since most fevers due to active SLE will remit with use of these agents [ 7 ]. In addition, a low white blood cell (WBC) count in the setting of fever would be more consistent with lupus activity rather than infection. Infection should clearly be ruled out in a patient with SLE presenting with fever.
Serious infections are a major cause of morbidity among patients and should be considered in all immunocompromised SLE patients with fever. (See 'Clinical manifestations' above.)
● Myalgia – Myalgia is also common among patients with SLE, whereas severe muscle weakness or myositis is relatively uncommon. Myalgia and muscle weakness are discussed in more detail separately. (See "Arthritis and other musculoskeletal manifestations of systemic lupus erythematosus", section on 'Muscle involvement' .)
● Weight change – Weight changes are frequent in patients with SLE and may be related to the disease or to its treatment. Weight loss often occurs prior to the diagnosis of SLE. Unintentional weight loss may be due to decreased appetite, side effects of medications (particularly diuretics and occasionally hydroxychloroquine ), and gastrointestinal disease (eg, gastroesophageal reflux, abdominal pain, peptic ulcer disease, or pancreatitis) (see "Gastrointestinal manifestations of systemic lupus erythematosus" ). Weight gain in SLE may be due to salt and water retention associated with hypoalbuminemia (eg, due to nephrotic syndrome or protein-losing enteropathy) or, alternatively, due to increased appetite associated with the use of glucocorticoids. (See "Overview of heavy proteinuria and the nephrotic syndrome" and "Gastrointestinal manifestations of systemic lupus erythematosus", section on 'Protein-losing enteropathy' .)
Arthritis and arthralgias — Arthritis and arthralgias occur in over 90 percent of patients with SLE and are often one of the earliest manifestations [ 8 ]. Arthritis, with demonstrable inflammation, occurs in 65 to 70 percent of patients and tends to be migratory, polyarticular, and symmetrical. The arthritis is moderately painful, usually does not cause erosion, and is rarely deforming ( picture 1A-B ). However, occasionally patients with SLE also develop a deforming erosive arthritis, which is similar to that of rheumatoid arthritis (RA) [ 9 ]. The clinical characteristics and management of arthritis and arthralgias in SLE are discussed in detail elsewhere. (See "Arthritis and other musculoskeletal manifestations of systemic lupus erythematosus", section on 'Joint involvement' .)
Mucocutaneous involvement — Most patients develop skin and mucous membrane lesions at some point during the course of their disease. There is tremendous variability in the type of skin involvement in SLE. The most common lesion is a facial eruption that characterizes acute cutaneous lupus erythema (also known as "the butterfly rash") that presents as erythema in a malar distribution over the cheeks and nose (but sparing the nasolabial folds) that appears after sun exposure ( picture 2A-B ). Some patients may develop discoid lesions, which are more inflammatory and which have a tendency to scar ( picture 3A-B ). Photosensitivity is also a common theme for skin lesions associated with SLE. The various cutaneous manifestations of SLE are presented in detail separately. (See "Overview of cutaneous lupus erythematosus" .)
Many patients develop oral and/or nasal ulcers, which are usually painless in contrast to herpetic chancre blisters. Nasal ulcers may lead to nasal septal perforation. Nonscarring alopecia is also observed in many SLE patients at some point during the course of their disease. Scarring alopecia can occur in patients with discoid lupus erythematosus. (See "Overview of cutaneous lupus erythematosus", section on 'Discoid lupus erythematosus' .)
Cardiac involvement and vascular manifestations — A variety of cardiac and vascular abnormalities can occur in patients with SLE.
● Cardiac disease among patients with SLE is common and can involve the pericardium, myocardium, valves, conduction system, and coronary arteries. Pericarditis, with or without an effusion, is the most common cardiac manifestation of SLE, occurring in approximately 25 percent of patients at some point during their disease course [ 10 ]. Verrucous (Libman-Sacks) endocarditis is usually clinically silent, but it can produce valvular insufficiency and can serve as a source of emboli ( picture 4 ). Myocarditis is uncommon but may be severe. Patients with SLE also have an increased risk of coronary artery disease. (See "Non-coronary cardiac manifestations of systemic lupus erythematosus in adults" and "Coronary artery disease in systemic lupus erythematosus" .)
Neonatal lupus, which can occur in babies of women with SLE expressing anti-Ro/SSA and anti-La/SSB, can cause heart block of varying degrees that may be noted in utero and/or that may present as congenital heart block and is discussed separately. (See "Neonatal lupus: Epidemiology, pathogenesis, clinical manifestations, and diagnosis" .)
● Raynaud phenomenon – Raynaud phenomenon in SLE is a vasospastic process induced by cold that occurs in up to 50 percent of patients with SLE ( picture 5 ) [ 6 ]. Raynaud phenomenon is characterized by intermittent acral pallor followed by cyanosis and erythroderma [ 11 ]. Raynaud phenomenon is discussed in detail separately. (See "Clinical manifestations and diagnosis of Raynaud phenomenon" .)
● Vasculitis – Estimates of the prevalence of vasculitis among SLE patients from large cohorts range from 11 to 36 percent [ 12 ]. The clinical spectrum of vasculitis in the setting of SLE is broad due to the potential for inflammatory involvement of vessels of all sizes. Small vessel involvement is the most common, often manifesting as cutaneous lesions; however, medium- and large-vessel involvement have also been observed. Cutaneous small-vessel vasculitis can manifest as palpable purpura, petechiae, papulonodular lesions, livedo reticularis, panniculitis, splinter hemorrhages, and superficial ulcerations (see "Evaluation of adults with cutaneous lesions of vasculitis" ). As an example, a large series of 670 SLE patients identified vasculitis among 11 percent of patients [ 13 ]. Cutaneous lesions were the main clinical presentation of vasculitis, present in 89 percent of patients. The remaining 11 percent of patients with vasculitis had visceral involvement (eg, peripheral nerves, lung, pancreas, and kidney).
Other specific types of vasculitic involvement in SLE include mesenteric vasculitis, hepatic vasculitis, pancreatic vasculitis, coronary vasculitis, pulmonary vasculitis, and retinal vasculitis, as well as vasculitis of the peripheral or central nervous system. A few cases of aortitis, similar to that seen in Takayasu arteritis, have been reported [ 14 ]. (See "Overview of cutaneous lupus erythematosus" and "Gastrointestinal manifestations of systemic lupus erythematosus", section on 'Autoimmune hepatitis' and "Gastrointestinal manifestations of systemic lupus erythematosus", section on 'Acute pancreatitis' and "Gastrointestinal manifestations of systemic lupus erythematosus", section on 'Mesenteric vasculitis/ischemia' and "Manifestations of systemic lupus erythematosus affecting the peripheral nervous system" and "Retinal vasculitis associated with systemic disorders and infections", section on 'Systemic immune-mediated causes' .)
● Thromboembolic disease – Thromboembolic disease can complicate SLE, particularly in the context of antiphospholipid antibodies. Although the precise mechanism is unknown, thromboembolic disease can affect both the venous and arterial circulations [ 15,16 ]. As an example, in a large observational cohort of 554 newly diagnosed SLE patients followed for a median of 6.3 years, an arterial thrombotic event (ATE) occurred in 11 percent, a venous thrombotic event (VTE) occurred in 5 percent, and the estimated 10-year risks were 10 percent for VTE, 26 percent for ATE, and 33 percent for any thrombotic event [ 16 ]. Antimalarials may be protective for the development of thromboembolic disease in SLE [ 17 ].
Kidney involvement — Kidney involvement is clinically apparent in approximately 50 percent of SLE patients and is a significant cause of morbidity and mortality [ 18 ]. Thus, periodic screening for the presence of lupus nephritis with urinalyses, quantitation of proteinuria, and estimation of the glomerular filtration rate is an important component of the ongoing management of SLE patients. Several forms of glomerulonephritis can occur, and kidney biopsy is useful to define the type and extent of kidney involvement. The clinical presentation of lupus nephritis is highly variable, ranging from asymptomatic hematuria and/or proteinuria to nephrotic syndrome and rapidly progressive glomerulonephritis with loss of kidney function. Some patients with lupus nephritis also have hypertension. (See "Lupus nephritis: Diagnosis and classification" .)
Gastrointestinal involvement — Gastrointestinal symptoms are common in SLE patients, occurring in up to 40 percent of patients. The majority of gastrointestinal symptoms are caused by adverse medication reactions and viral or bacterial infections [ 19 ]. SLE-related gastrointestinal abnormalities can involve almost any organ along the gastrointestinal tract and include esophagitis, intestinal pseudo-obstruction, protein-losing enteropathy, lupus hepatitis, acute pancreatitis, mesenteric vasculitis or ischemia, and peritonitis. The gastrointestinal manifestations of SLE are discussed in detail elsewhere. (See "Gastrointestinal manifestations of systemic lupus erythematosus" .)
Pulmonary involvement — During the course of their disease, many patients develop symptoms secondary to pulmonary involvement of SLE. Pulmonary manifestations of SLE include pleuritis (with or without effusion), pneumonitis, interstitial lung disease, pulmonary hypertension, shrinking lung syndrome, and alveolar hemorrhage. Respiratory symptoms must also be distinguished from infection, particularly in patients on immunosuppressive therapy. The risk of thromboembolic involvement is increased in those with antiphospholipid antibodies or with lupus anticoagulant. (See "Pulmonary manifestations of systemic lupus erythematosus in adults" .)
Neurologic and neuropsychiatric involvement — Neuropsychiatric involvement of SLE consists of a broad range of neurologic and psychiatric manifestations, including stroke, seizures, cognitive dysfunction, delirium, psychosis, and/or peripheral neuropathies. Other less common problems are movement disorders, cranial neuropathies, myelitis, and meningitis. (See "Neurologic and neuropsychiatric manifestations of systemic lupus erythematosus" and "Manifestations of systemic lupus erythematosus affecting the peripheral nervous system" .)
Thromboembolic events, often in association with antiphospholipid antibodies or with lupus anticoagulant, may occur in a substantial minority (20 percent) of patients with SLE [ 20 ]. Arterial thromboemboli may cause focal neurologic problems, such as stroke or seizures and/or more diffuse cognitive defects [ 20 ]. (See "Clinical manifestations of antiphospholipid syndrome" .)
Hematologic abnormalities — Hematologic abnormalities are common in SLE, and all three blood cell lines can be affected. Anemia of chronic disease (also called anemia of inflammation and anemia of chronic inflammation) is the most common type of anemia among patients with SLE. Leukopenia is common in SLE patients, occurring in approximately 50 percent of patients [ 21 ]. Leukopenia can be due to lymphopenia and/or secondary neutropenia and generally correlates with clinically active disease. Neutropenia may also result from toxicity due to immunosuppressive medications. Mild thrombocytopenia is also a common hematologic abnormality. Rarely, severe thrombocytopenia can occur and requires treatment. Autoimmune hemolytic anemia is also relatively rare but can be severe, requiring immediate therapy. A more detailed discussion of the hematologic manifestations of SLE is presented separately. (See "Hematologic manifestations of systemic lupus erythematosus" .)
Lymph node enlargement commonly occurs in association with active SLE and usually involves the cervical, axillary, and inguinal regions. Splenomegaly can also be observed among SLE patients, particularly with active disease. (See "Hematologic manifestations of systemic lupus erythematosus", section on 'Lymphadenopathy' .)
Ophthalmologic involvement — Any structure of the eye can be involved in SLE, with keratoconjunctivitis sicca being the most common manifestation as a result of secondary Sjögren's disease [ 22 ] (see "Clinical manifestations of Sjögren's disease: Exocrine gland disease" ). The next most common pathologic condition involving the eye in lupus patients is retinal vasculopathy in the form of cotton wool spots. (See "Retinal vasculitis associated with systemic disorders and infections", section on 'Systemic immune-mediated causes' .)
Other less common ophthalmologic manifestations of SLE include optic neuropathy, choroidopathy, episcleritis, scleritis, and anterior uveitis (iritis, iridocyclitis). (See "Optic neuropathies", section on 'Systemic autoimmune disease' and "Episcleritis" and "Clinical manifestations and diagnosis of scleritis" and "Uveitis: Etiology, clinical manifestations, and diagnosis", section on 'Systemic inflammatory diseases' .)
Orbital tissues such as the lacrimal gland (typically resulting in sicca), extraocular muscles, and other orbital tissues may also be involved in SLE, leading to pain, proptosis, lid swelling, and diplopia [ 23 ]. In addition, there are specific ocular toxicities secondary to medications seen in patients with SLE, including glucocorticoid-induced glaucoma and retinal toxicity due to antimalarial therapy.
Other associated conditions and complications — A number of comorbid medical conditions that are related to either the underlying disease or therapy can occur in patients with SLE.
● Immunodeficiencies – Hereditary angioedema is a rare genetic disorder primarily caused by a defect in the C1 inhibitor. It can be associated with some inflammatory and autoimmune disorders, including SLE [ 24 ]. (See "Hereditary angioedema (due to C1 inhibitor deficiency): Pathogenesis and diagnosis" .)
Patients with other forms of complement deficiency like C2 also have forms of SLE. Often the manifestations depend on whether such deficiencies are homozygous. Patients with complete C4 deficiency and C1q deficiency often present with SLE [ 25 ]. Inherited C4 deficiency is discussed in detail separately (see "Inherited disorders of the complement system", section on 'C4 deficiency' ). Acquired low immunoglobulin levels can also be observed in patients with SLE [ 26 ].
● Antiphospholipid syndrome – Antiphospholipid antibodies are detected in 40 percent of patients with SLE [ 27 ]. However, the development of antiphospholipid syndrome is much less common. (See "Diagnosis of antiphospholipid syndrome" and "Clinical manifestations of antiphospholipid syndrome" .)
● Fibromyalgia – Patients with SLE, as well as several other systemic rheumatic diseases, have a higher prevalence of fibromyalgia than the general population [ 28 ]. (See "Arthritis and other musculoskeletal manifestations of systemic lupus erythematosus", section on 'Fibromyalgia' .)
● Osteonecrosis – The estimated risk of osteonecrosis, which can present with severe joint pain among patients with SLE, varies widely, ranging from 3 to 40 percent [ 29 ]. The increased risk is thought to be related to the underlying disease as well as the concomitant use of glucocorticoids. (See "Arthritis and other musculoskeletal manifestations of systemic lupus erythematosus", section on 'Osteonecrosis' .)
● Osteoporosis – Osteoporosis is a common complication of SLE and is discussed in detail separately. (See "Arthritis and other musculoskeletal manifestations of systemic lupus erythematosus", section on 'Osteoporosis' .)
● Infection – Serious infectious complications, especially of the skin, respiratory, and urinary systems, develop in up to 50 percent of SLE patients [ 6,30-33 ]. A large cohort from a Medicaid database of 33,565 SLE patients, 7113 of whom had lupus nephritis, found that the incidence rate (per 100 person-years) of serious infections requiring hospitalization was 10.8 in the SLE cohort and 23.9 in the lupus nephritis subcohort [ 34 ]. A large majority of infections (approximately 80 percent) are due to pathogenic bacteria [ 33 ]. Opportunistic infections, including those due to fungi, can be related to the use of immunosuppressive therapy and are a common cause of death [ 35-38 ]. Consequently, ascribing fever to SLE in an immunocompromised patient should be done only after reasonable efforts have been made to exclude infection.
Risk factors for infection include active SLE disease [ 39 ], long-term disease damage, neutropenia, lymphopenia, hypocomplementemia, hypogammaglobulinemia, kidney involvement, neuropsychiatric manifestations, and the use of glucocorticoids and other immunosuppressive drugs [ 33,40 ]. In nationwide cohort study, risk for infection was increased in Black Americans and for male sex; antimalarials have been found to be protective [ 34 ]. Viral infections are also common, including parvovirus B19 (which can cause a lupus-like syndrome), Epstein-Barr virus, cytomegalovirus, varicella-zoster virus, and human papillomavirus. Mycobacterial infections, including non-tuberculosis, have been noted to be more frequent in patients with SLE [ 30,33 ].
● Other autoimmune diseases – There is an increased prevalence of thyroid disease among patients with SLE, usually in the form of Hashimoto's thyroiditis. Myasthenia gravis has also been reported to co-occur in patients with SLE. There is a high prevalence of autoimmune diseases among relatives of patients with SLE [ 41-43 ]. (See "Clinical manifestations of myasthenia gravis", section on 'Epidemiology' .)
When to suspect SLE — The initial diagnosis of systemic lupus erythematosus (SLE) depends on the manner of presentation and the exclusion of alternative diagnoses. Given the heterogeneity of clinical presentations, there are some patients for whom the constellation of presenting clinical features and supportive laboratory studies makes the diagnosis of SLE relatively straightforward. By contrast, there are others who present with isolated complaints or infrequent disease characteristics and represent more of a diagnostic challenge. Demographics should also be taken into account when evaluating a patient for SLE, since it occurs primarily in young women of childbearing age. In addition, SLE occurs more commonly in certain racial and ethnic groups, particularly Black, Asian, and Hispanic populations compared with White populations [ 44 ]. (See "Epidemiology and pathogenesis of systemic lupus erythematosus", section on 'Epidemiology' .)
As an example, the diagnosis of SLE is more likely to be present in a young woman who develops fatigue, arthralgia, and pleuritic chest pain and is found to have hypertension, a malar rash, a pleural friction rub, several tender and swollen joints, and mild peripheral edema. Laboratory testing may reveal leukopenia, anemia, an elevated serum creatinine, hypoalbuminemia, proteinuria, an active urinary sediment, hypocomplementemia, and positive tests for antinuclear antibodies (ANA), including those to double-stranded DNA (dsDNA) and the Smith (Sm) antigen. By contrast, another patient may present with fatigue and arthralgias without evidence of organ involvement in the setting of a positive ANA test. Such patients may or may not subsequently develop characteristic multisystem features of SLE in the following months or years. (See 'Clinical manifestations' above.)
Thus, the initial evaluation requires a careful history and physical exam, along with selected laboratory testing to identify features that are characteristic of SLE or that suggest an alternative diagnosis. Patients presenting with symptoms for a shorter duration of time will need close follow-up, as the frequency with which various features of SLE are observed differs according to stage of disease [ 45-49 ].
History and physical examination — We perform a thorough medical history, with particular attention to the following symptoms and signs:
● Constitutional symptoms, such as fever, fatigue, lymphadenopathy, or weight loss
● Photosensitive skin lesions, such as a malar rash
● Painless oral or nasal ulcers
● Hair loss that is patchy or frontal/peripheral
● Raynaud phenomenon
● Joint pain or swelling, which can be migratory or symmetrical
● Dyspnea or pleuritic chest pain suggestive of serositis
● Chest pain suggestive of pericarditis
● Lower-extremity edema
● Neurologic symptoms, such as seizures or psychosis
● Recurrent miscarriages (see "Pregnancy in women with systemic lupus erythematosus" )
● Exposure to medications associated with drug-induced lupus (see "Drug-induced lupus" )
Given the broad range of clinical manifestations of SLE, it is helpful to consider the various features according to frequency at disease onset ( table 1 ).
A complete physical examination is indicated, since any organ system can be involved in SLE. Pertinent physical examination findings include the following:
● Skin lesions consistent with a malar rash or discoid lesions
● Scarring or nonscarring patchy alopecia
● Oral or nasopharyngeal ulcers, nasal septal perforation
● Polyarticular arthritis, which is often symmetric
● Subluxation at the metacarpophalangeal joints and rheumatoid-like swan neck deformities in the hands
● Decreased or abnormal breath sounds that may indicate a pleural effusion, pneumonitis, or interstitial lung disease
● Lower-extremity edema and hypertension
Laboratory testing — We obtain the following routine laboratory tests, which may provide diagnostically useful information:
● Complete blood count and differential may reveal leukopenia, mild anemia, and/or thrombocytopenia
● Elevated serum creatinine may be suggestive of kidney dysfunction
● Urinalysis with urine sediment may reveal hematuria, pyuria, proteinuria, and/or cellular casts
● Serum protein electrophoresis may demonstrate a hypergammaglobulinemia that is suggestive of a systemic inflammatory process
In addition to the routine laboratories described above, we perform the following laboratory tests, which support the diagnosis of SLE if abnormal:
● ANA (ideally by indirect immunofluorescence testing)
● Anti-double-stranded DNA (anti-dsDNA)
● Antiphospholipid antibodies (lupus anticoagulant [LA], immunoglobulin [Ig] G and IgM anticardiolipin [aCL] antibodies, and IgG and IgM anti-beta2-glycoprotein [GP] 1)
● C3 and C4 or CH50 complement levels
● Erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP) levels
● Urine protein-to-creatinine ratio
The ANA test is positive in virtually all patients with SLE at some time in the course of their disease (see "Measurement and clinical significance of antinuclear antibodies" ). If the ANA is positive, one should test for other specific antibodies, such as anti-dsDNA, anti-Smith (anti-Sm), Ro/SSA, La/SSB, and U1 ribonucleoprotein (RNP), which are described further below. In some labs, a positive ANA test by indirect immunofluorescence will automatically result in testing for such additional ANA that are often present in patients with SLE. However, a positive ANA must also be interpreted in the setting of other clinical and laboratory findings. Almost 15 percent of the population in the United States has been found to have a positive ANA of at least 1:80 by indirect immunofluorescence, but only 10 percent have a true autoimmune disorder [ 50 ]. A more detailed discussion related to measurement and interpretation of ANA testing can be found elsewhere. (See "Measurement and clinical significance of antinuclear antibodies" .)
● Anti-dsDNA and anti-Sm antibodies are highly specific for SLE, but anti-Sm antibodies lack sensitivity [ 51,52 ]. Anti-dsDNA and anti-Sm antibodies are seen in approximately 70 and 30 percent of patients with SLE, respectively. (See "Antibodies to double-stranded (ds)DNA, Sm, and U1 RNP" .)
● Anti-Ro/SSA and anti-La/SSB antibodies are present in approximately 30 and 20 percent of patients with SLE, respectively; however, both antibodies are more commonly associated with Sjögren's disease [ 51 ]. (See "The anti-Ro/SSA and anti-La/SSB antigen-antibody systems" .)
● Anti-U1 RNP antibodies are observed in approximately 25 percent of patients with SLE, but they also occur in patients with other conditions, and high levels are almost always present in patients with mixed connective tissue disease (MCTD) [ 51,52 ]. (See "Antibodies to double-stranded (ds)DNA, Sm, and U1 RNP" .)
● Antiribosomal P protein antibodies have a high specificity for SLE but low sensitivity for SLE. They also lack specificity for involvement of a particular organ system or disease manifestation. (See "Antiribosomal P protein antibodies", section on 'Clinical utility of antiribosomal P antibodies' .)
If the initial ANA test is negative but the clinical suspicion of SLE is high, then additional antibody testing may still be appropriate. This is partly related to the differences in the sensitivity and specificity among the methods used to detect ANA. A more detailed discussion on the techniques used to detect ANA and the reasons behind some of the variability in test results is presented separately. (See 'ANA-negative lupus' below.)
Additional information regarding the interpretation of abnormalities of the ESR and CRP in patients with SLE can be found elsewhere. (See "Systemic lupus erythematosus in adults: Overview of the management and prognosis", section on 'Laboratory evaluation' .)
We perform the following additional laboratory tests in selected patients:
● Rheumatoid factor and anti-cyclic citrullinated peptide antibodies – In patients with predominant arthralgias or arthritis, rheumatoid factor (RF) and anti-cyclic citrullinated peptide (CCP) antibodies may help exclude a diagnosis of rheumatoid arthritis (RA). RF has less diagnostic utility, since 20 to 30 percent of people with SLE have a positive RF. Anti-CCP antibodies, however, have a much higher specificity for RA and may be more useful for distinguishing the arthritis associated with RA. (See "Biologic markers in the assessment of rheumatoid arthritis", section on 'Rheumatoid factors' and "Biologic markers in the assessment of rheumatoid arthritis", section on 'Anti-citrullinated peptide antibodies' .)
● Serologic studies for infection – In patients with a brief history (eg, less than six weeks) of predominant arthralgias or arthritis, we perform serologic testing for human parvovirus B19. We also perform serologic testing for hepatitis B virus and hepatitis C virus in patients with multisystemic clinical findings. In areas endemic for Lyme disease, we may send serologic studies for Borrelia as well. Testing for Epstein-Barr virus infection may also be indicated in the appropriate clinical setting. (See "Diagnosis of Lyme disease" and "Viral arthritis: Causes and approach to evaluation and management" .)
● Creatine kinase – An elevated creatine kinase (CK) may reflect myositis, which is relatively uncommon in patients with SLE. Myositis may also suggest an alternative diagnosis, such as MCTD, polymyositis, or dermatomyositis.
● 24-hour urine collection – If the spot urine protein-to-creatinine ratio is above 0.05 g/mmol, then a 24-hour urine collection should be performed, as the spot urine collection may not reflect the true amount of proteinuria [ 53 ]. (See "Assessment of urinary protein excretion and evaluation of isolated non-nephrotic proteinuria in adults", section on '24-hour versus spot urine collection' .)
Additional testing in selected patients — Biopsy of an involved organ (eg, skin or kidney) is necessary in some cases. Typical histologic findings in various organs in SLE are discussed in topic reviews devoted to the particular sites of involvement. (See "Lupus nephritis: Diagnosis and classification" and "Overview of cutaneous lupus erythematosus" .)
Other tests that may be necessary are typically dictated by the clinical presentation and associated differential diagnostic possibilities. Examples include:
● Electrocardiography in the assessment of chest pain that may be due to pericarditis or to myocardial ischemia
● Tests to assess for pulmonary embolism in a patient with pleuritic chest pain and dyspnea
● Diffusing capacity for carbon monoxide to assess for suspected pulmonary hemorrhage and to estimate the severity of interstitial lung disease
Diagnostic imaging may be valuable but is not routinely obtained unless indicated by the presence of symptoms, clinical findings, or laboratory abnormalities. Examples include:
● Plain radiographs (eg, of swollen joints; unlike affected joints in RA, erosions are observed infrequently in SLE [ 54 ]).
● Musculoskeletal ultrasonography (eg, of painful joints to detect synovitis and tenosynovitis in the hands and wrists [ 55,56 ]).
● Kidney ultrasonography (eg, to assess kidney size and to rule out urinary tract obstruction when there is evidence of kidney impairment).
● Chest radiography (eg, for suspected pleural effusion, interstitial lung disease, cardiomegaly).
● Echocardiography (eg, for suspected pericardial involvement, to assess for a source of emboli, or noninvasive estimation of pulmonary artery pressure; and for evaluation of suspected valvular lesions, such as verrucae).
● Computed tomography (CT; eg, for abdominal pain, suspected pancreatitis, interstitial lung disease).
● Magnetic resonance imaging (MRI; eg, for focal neurologic deficits or cognitive dysfunction).
CLASSIFICATION CRITERIA — Several classification criteria have been developed for systemic lupus erythematosus (SLE) as a means of categorizing patients for inclusion in research studies. These criteria can be useful for clinicians in systematically documenting key disease features, but their imperfect sensitivity and specificity limit their use for diagnostic purposes.
● 2019 EULAR/ACR criteria – The European Alliance of Associations for Rheumatology (EULAR; formerly known as European League Against Rheumatism)/American College of Rheumatology (ACR) classification criteria for SLE were developed to improve detection of early- or new-onset SLE as well as improve the sensitivity and specificity compared with previous criteria ( table 2A-B ) [ 57,58 ]. The classification for SLE requires the presence of a positive antinuclear antibodies (ANA) as an entry criterion. Additive criteria consist of seven clinical (ie, constitutional, hematologic, neuropsychiatric, mucocutaneous, serosal, musculoskeletal, renal) and three immunologic (ie, antiphospholipid antibodies, complement proteins, SLE-specific antibodies) categories, each of which are weighted from 2 to 10. Patients are classified as having SLE with a score of 10 or more points. In the validation cohort, which included patients with early disease, the EULAR/ACR criteria had a sensitivity of 96.1 percent and a specificity of 93.4 percent, compared with the 96.7 percent sensitivity and 83.7 percent specificity of the Systemic Lupus International Collaborating Clinics (SLICC) criteria and the 82.8 percent sensitivity and 93.4 percent specificity of the ACR criteria.
The performance of the EULAR/ACR classification criteria was evaluated using the combined derivation and validation cohorts consisting of 1197 patients with SLE and 1074 patients without SLE, but with a variety of conditions mimicking SLE, and reported a sensitivity and specificity of a positive ANA of 99.5 and 19.5 percent, respectively [ 59 ]. The sensitivities of the specific criteria items were highly variable, but the specificities were more consistently high (eg, at least 80 percent).
● 2012 SLICC criteria – In 2012, the SLICC proposed classification criteria that were developed to address inherent weaknesses of the 1997 ACR classification criteria ( table 3 ) [ 60 ]. As an example, one of the major limitations of the 1997 ACR criteria is that patients with biopsy-confirmed lupus nephritis could still fail to fulfill criteria. Other concerns regarding the ACR criteria included the possible duplication of highly correlated cutaneous features (such as malar rash and photosensitivity), the lack of inclusion of other cutaneous manifestations (such as maculopapular or polycyclic rash), and the omission of many neurologic manifestations of SLE (such as myelitis). The ACR criteria also did not include relevant immunologic information such as low serum levels of complement components.
Classification as having SLE by the SLICC criteria requires either that a patient satisfy at least 4 of 17 criteria, including at least 1 of the 11 clinical criteria and one of the six immunologic criteria, or that the patient has biopsy-proven nephritis compatible with SLE in the presence of ANA or anti-double-stranded DNA (anti-dsDNA) antibodies.
The SLICC criteria were validated by analysis of 690 patients with SLE or other rheumatic diseases. In this initial validation testing, the SLICC revised criteria had greater sensitivity but lower specificity than the 1997 ACR classification criteria (sensitivity of 97 versus 83 percent and specificity of 84 versus 96 percent, respectively).
Despite the improved sensitivity compared with the ACR criteria, the SLICC criteria might delay the diagnosis of SLE in a significant number of patients, and some patients might not be classified at all. These situations were demonstrated in a study in which patients with SLE from two large cohorts were grouped according to whether the SLICC criteria were met before, at the same time as, or after the ACR criteria, and the groups were then compared [ 61 ]. Among the patients diagnosed later with the SLICC criteria, in the majority of cases, the delay was due to the fact that the combination of malar rash and photosensitivity both fall within the acute cutaneous SLE category and thus only count as one criterion.
● 1997 ACR criteria – The classification criteria that were developed by the American Rheumatism Association (ARA, now the ACR) for the classification of SLE were established by cluster analyses, primarily in academic centers and in White American patients [ 62-64 ].
The patient is classified with SLE using the ACR criteria if four or more of the manifestations are present, either serially or simultaneously, during any interval of observations ( table 3 ) [ 62,63 ]. A positive lupus erythematosus cell test, used in older criteria, was replaced by the presence of antiphospholipid antibodies [ 62 ]. When tested against other rheumatic diseases, these criteria have a sensitivity and specificity of approximately 96 percent.
DIAGNOSIS — The diagnosis of systemic lupus erythematosus (SLE) is based upon the judgment of an experienced clinician who recognizes characteristic constellations of symptoms and signs in the setting of supportive serologic studies after excluding alternative diagnoses. This is often challenging due to the great variability in the expression of SLE. Although the classification criteria were designed for research purposes, many clinicians refer to aspects of these criteria when making the diagnosis of SLE. (See 'Classification criteria' above.)
In the absence of existing "diagnostic criteria," we describe our general approach to the diagnosis that takes into consideration the strengths of the 1997 American College of Rheumatology (ACR) criteria, the 2012 Systemic Lupus International Collaborating Clinics (SLICC) criteria ( table 3 ), or the 2019 European Alliance of Associations for Rheumatology (EULAR)/ACR classification criteria ( table 2A ). (See 'Classification criteria' above.)
Note that our general diagnostic approach does not adequately address the myriad manifestations or subtleties of some clinical features, nor does it substitute for clinical judgment. Thus, it is often appropriate to refer the patient in whom the diagnosis of SLE is suspected to a rheumatologist with experience in this disease [ 65 ].
Our diagnostic criteria
Definite SLE — After excluding alternative diagnoses, we diagnose SLE in the patient who fulfills the 1997 ACR criteria, the 2012 SLICC criteria ( table 3 ), or the 2019 EULAR/ACR criteria ( table 2A ). As previously mentioned, the ACR criteria require that a patient satisfy at least 4 of 11 criteria. The SLICC criteria require either that a patient satisfy at least 4 of 17 criteria, including at least 1 of the 11 clinical criteria and one of the six immunologic criteria, or that the patient has biopsy-proven nephritis compatible with SLE in the presence of antinuclear antibodies (ANA) or anti-double-stranded DNA (anti-dsDNA) antibodies. According to the EULAR/ACR criteria, a patient can be classified as having SLE if they have a positive ANA ≥1:80 and score 10 or more points. This number has been shown to be valid in a number of cohorts. (See 'Classification criteria' above.)
Probable SLE — There are patients who do not fulfill the classification criteria for SLE but in whom we still diagnose the disorder. These patients include those presenting with an inadequate number of ACR or SLICC criteria or those who have other SLE manifestations not included in either classification criteria.
As a loose guide, we diagnose SLE in patients who have two or three of the ACR or SLICC criteria, along with at least one other feature that may be associated with but is not specific for SLE. Some of these features include the following [ 66 ]:
● Optic neuritis, aseptic meningitis
● Glomerular hematuria
● Pneumonitis, pulmonary hemorrhage, pulmonary hypertension, interstitial lung disease
● Myocarditis, verrucous endocarditis (Libman-Sacks endocarditis)
● Abdominal vasculitis
● Elevated acute phase reactants (eg, erythrocyte sedimentation rate [ESR] and C-reactive protein [CRP])
Possible SLE — We consider SLE a possible diagnosis in individuals who have only one of the ACR/SLICC criteria, in addition to at least one or two of the other features listed above. Other terms that have been used to describe patients with probable or possible SLE include incipient or latent lupus, which has been defined as patients who have three or fewer of the ACR or SLICC criteria [ 67 ].
In general, patients with either probable or possible SLE are managed similarly to patients with SLE and treated according to their predominant symptoms and manifestations. Over time, the symptoms in these patients may persist, evolve into definite SLE or a related connective tissue disorder, or even resolve. In addition, these patients may develop positive serology over time and become more clearly diagnosable as SLE. However, patients treated with high doses of prednisone may "lose" their antibodies, and it may be more difficult to diagnose the underlying disease.
Undifferentiated connective tissue disease — Other patients who have even fewer features suggestive of SLE may be classified as having undifferentiated connective tissue disease (UCTD). This term is used to describe patients with signs and symptoms suggestive of a systemic autoimmune disease but who do not meet the ACR criteria for SLE or another defined connective tissues disease [ 68 ]. (See "Undifferentiated systemic rheumatic (connective tissue) disease and overlap syndromes" .)
Case series have been published that summarize the outcome of patients who have UCTD at presentation [ 69-73 ]. Up to one-third of patients have all symptoms and signs disappear over a 10-year follow-up period. Anywhere from 40 to 60 percent of patients continue to exhibit their initial clinical features, while 5 to 30 percent evolve and meet classification criteria for a definite disease, such as SLE, rheumatoid arthritis, scleroderma, or an inflammatory myopathy (myositis) [ 69-73 ] (see "Undifferentiated systemic rheumatic (connective tissue) disease and overlap syndromes" ). Thus, patients with UCTD should be followed carefully, encouraged to report new symptoms, and have periodic laboratory testing to assess for the emergence of new clinical features or laboratory findings. Appropriate testing should include the laboratory testing described above. (See 'Laboratory testing' above.)
ANA-negative lupus — ANA-negative SLE has been recognized since the 1970s but was later shown to be influenced by the testing methods used to detect ANA. At that time, it was estimated that about 5 percent of patients with SLE were ANA-negative by indirect immunofluorescence [ 74 ]. However, this negative finding occurred because sera were tested using rodent, not human, tissues as the substrate for the indirect immunofluorescence test for ANA [ 75 ]. By comparison, anti-Ro/SSA antibodies were found in many of these patients when a human cell line extract was used as the substrate for anti-Ro/SSA antibody testing.
The subsequent substitution of human epithelial type 2 (Hep-2) cells (a human cell line) for rodent tissue sections in the indirect immunofluorescence ANA assay has resulted in even fewer SLE patients with negative ANA by indirect immunofluorescence. Nevertheless, on rare occasions, the presence of anti-Ro/SSA antibodies may suggest a systemic autoimmune disease despite the presence of a negative ANA indirect immunofluorescence. As an example, in one study in Sweden, among 4025 sera tested for ANA, 64 patients with negative ANA by indirect immunofluorescence had anti-Ro/SSA antibodies [ 76 ]. Of these 64 patients, 12 had SLE and 5 had cutaneous lupus erythematosus.
The clinician should understand the technique used to detect the ANA, since this can influence the result. As an example, a negative ANA by indirect immunofluorescence is clinically useful as it dramatically decreases the likelihood of SLE. On the other hand, in a patient with a strong clinical suspicion for SLE and a negative ANA result by a solid-phase assay, the test should be repeated using indirect immunofluorescence method with Hep-2 cells, given the increased risk of a false-negative result for the initial ANA test by solid-phase assay. A detailed discussion of the methods used to detect ANA is presented separately. (See "Measurement and clinical significance of antinuclear antibodies" .)
Other factors that may also influence ANA negativity in SLE patients include disease duration and treatment exposure [ 77 ]. In our experience, the frequency of ANA-negative SLE is lower in patients presenting at an early stage of their disease. In addition, SLE patients who have longstanding disease and/or have undergone treatment may lose ANA reactivity and become serologically negative over time.
DIFFERENTIAL DIAGNOSIS — Given the protean manifestations of systemic lupus erythematosus (SLE), the differential diagnosis is correspondingly broad. While it is beyond the scope of this review to provide a comprehensive list of all possible alternative diagnoses, we present several here.
● Rheumatoid arthritis – Early rheumatoid arthritis (RA) may be difficult to distinguish from the arthritis of SLE, since both conditions cause joint tenderness and swelling ( table 4 ). Features such as swan neck deformities, ulnar deviation, and soft tissue laxity, which are observed in later stages of RA in patients with more destructive disease, can also be seen in some patients with SLE. However, an important distinguishing features is that the joint deformities in SLE are often reducible and infrequently erosive on plain radiographs.
Some extraarticular RA manifestations, including serositis, sicca symptoms, subcutaneous nodules, anemia, and fatigue, are other features that may also be observed in SLE. These features are more common in RA patients with more severe or advanced disease. Serologic abnormalities, such as the presence of anti-cyclic citrullinated peptides (CCP), are more supportive of the diagnosis of RA and can help distinguish the diseases. It should be recognized that the antinuclear antibodies (ANA) may be positive in up to one-half of patients with RA. Conversely, rheumatoid factor (RF) may be present in approximately one-third of SLE patients. Also, anti-CCP can be present in 5 to 10 percent of patients with SLE [ 78 ]. (See "Diagnosis and differential diagnosis of rheumatoid arthritis" .)
● Rhupus – The term rhupus has been used to describe patients with overlapping features of both SLE and RA. Whether rhupus is clinically and immunologically a distinct entity, a true overlap of SLE and RA, or a subset of patients with SLE remains a matter of debate. In addition to having serologies consistent with both SLE and RA, some patients classified as rhupus may have an erosive arthropathy that is atypical for SLE. (See "Undifferentiated systemic rheumatic (connective tissue) disease and overlap syndromes", section on 'Diagnostic challenges' .)
● Mixed connective tissue disease – Mixed connective tissue disease (MCTD) is characterized by overlapping features of SLE, systemic sclerosis (SSc), and polymyositis (PM), and by the presence of high titers of antibodies against U1 ribonucleoprotein (RNP). However, the diagnosis of MCTD is often complicated, since many of its characteristic features occur sequentially, often over a period of years. In addition, some patients with MCTD may evolve into another connective tissue disease, including SLE, during the clinical course [ 79 ]. (See "Mixed connective tissue disease" .)
● Undifferentiated connective tissue disease – As mentioned above, patients with undifferentiated connective tissue disease (UCTD) have signs and symptoms suggestive of a systemic autoimmune disease but do not satisfy the classification criteria for a defined connective tissue disease such as SLE or MCTD. These patients may have symptoms such as arthritis and arthralgias, Raynaud phenomenon, and serologic findings that are difficult to distinguish from early phases of SLE. The majority of patients with UCTD maintain an undefined profile and have a mild disease course [ 80 ]. (See "Undifferentiated systemic rheumatic (connective tissue) disease and overlap syndromes" .)
● Systemic sclerosis – The coexistence of Raynaud phenomenon and gastroesophageal reflux is typically observed in SSc; however, these findings are nonspecific and may be seen in patients with SLE or healthy individuals. By contrast, sclerodactyly, telangiectasia, calcinosis, and malignant hypertension with acute kidney failure are more consistent with SSc rather than SLE. Further, a positive ANA is present in most patients with SSc, while other serologies, such as anti-double-stranded DNA (anti-dsDNA) and anti-Smith (anti-Sm) antibodies that are more specific for SLE, are not commonly observed in SSc. Correspondingly, patients with SSc commonly express antibodies to an antigen called Scl-70 (topoisomerase I) or antibodies to centromere proteins. Distinguishing SSc from SLE can be particularly difficult in cases in which there is overlap of these diseases, such as in MCTD. (See "Clinical manifestations and diagnosis of systemic sclerosis (scleroderma) in adults" .)
● Sjögren's disease – Patients with Sjögren's disease may have extraglandular manifestations that can be observed in SLE, such as neurologic and pulmonary abnormalities. However, patients with Sjögren's disease should have objective signs of keratoconjunctivitis sicca and xerostomia and characteristic findings on salivary gland biopsy that are not typical of SLE. Patients with Sjögren's disease commonly express antibodies to Ro and La antigens. Also, some patients may have SLE with associated Sjögren's disease. (See "Diagnosis and classification of Sjögren's disease" .)
● Vasculitis – Patients with medium- and small-vessel vasculitides, such as polyarteritis nodosa, granulomatosis with polyangiitis, or microscopic polyangiitis, may present with overlapping features of SLE, including constitutional symptoms, skin lesions, neuropathy, and kidney dysfunction. However, patients with these types of vasculitides are usually ANA-negative. Rather, these patients frequently display antibodies to neutrophil cytoplasmic antigens. (See "Granulomatosis with polyangiitis and microscopic polyangiitis: Clinical manifestations and diagnosis" and "Clinical manifestations and diagnosis of polyarteritis nodosa in adults" .)
● Behçet syndrome – Oral aphthae are present in almost all patients with Behçet syndrome and may be observed in patients with SLE. Other overlapping features include inflammatory eye disease, neurologic disease, vascular disease, and arthritis. However, the oral aphthae in Behçet syndrome are typically painful, and patients with Behçet syndrome are more commonly male and ANA-negative. Also, vascular involvement of any size (small, medium, large) is more commonly a feature of Behçet syndrome than SLE. (See "Clinical manifestations and diagnosis of Behçet syndrome" .)
● Dermatomyositis and polymyositis – Patients with SLE can present with a low-grade myositis, whereas patients with dermatomyositis (DM) and PM generally demonstrate more overt proximal muscle weakness. A positive ANA is observed in approximately 30 percent of patients with DM and PM, compared with almost all patients in SLE. Patients with DM may have characteristic skin findings, including Gottron's papules, a heliotrope eruption, and photodistributed poikiloderma (including the shawl and V signs). Clinical findings characteristic of SLE, such as oral ulcers, arthritis, nephritis, and hematologic abnormalities, are absent in DM and PM. Patients with DM or PM may also express myositis-specific antibodies, such as anti-Jo-1. (See "Clinical manifestations of dermatomyositis and polymyositis in adults" .)
● Adult-onset Still's disease – Some of the clinical manifestations observed in adult-onset Still's disease (AOSD), such as fever, arthritis or arthralgias, and lymphadenopathy, are not unusual for patients with SLE. However, patients with AOSD often present with a leukocytosis rather than the leukopenia observed in SLE, and they are typically negative for ANA. Markedly elevated serum ferritin concentrations are also more frequently observed in AOSD. (See "Clinical manifestations and diagnosis of adult-onset Still's disease" .)
● Kikuchi disease – Kikuchi disease is a benign and usually self-limited form of histiocytic-necrotizing lymphadenitis. Clinical features at presentation include lymphadenopathy, fever, myalgias, arthralgias, and, less commonly, hepatosplenomegaly. Associations with SLE have been reported, but the clinical course is usually favorable, with spontaneous remission often occurring within four months. The diagnosis of Kikuchi disease is based on a lymph node biopsy, which reveals a histiocytic cellular infiltrate. (See "Kikuchi disease" .)
● Serum sickness – Many of the clinical features observed in serum sickness, such as fever, lymphadenopathy, cutaneous eruptions, and arthralgias, are often observed in SLE. Furthermore, during severe episodes, complement measurements including C3 and C4 can be depressed, as in SLE. Unlike SLE, however, ANA are typically negative, and the course tends to be self-limited. (See "Serum sickness and serum sickness-like reactions" .)
● Fibromyalgia – Patients with SLE may present with generalized arthralgias, myalgias, and fatigue, much like patients with fibromyalgia. However, other characteristic features of SLE, such as a photosensitive rash, arthritis, and multisystem organ involvement, are absent. However, fibromyalgia occurs more commonly in patients with systemic rheumatic diseases than in the general population. Concomitant fibromyalgia has been reported in at least 22 percent of patients with SLE [ 81 ]. (See "Clinical manifestations and diagnosis of fibromyalgia in adults" and "Overview of chronic widespread (centralized) pain in the rheumatic diseases", section on 'Systemic lupus erythematosus and Sjögren's disease' .)
● Infections – Several viral infections can produce signs and symptoms present in SLE, including cytomegalovirus and Epstein-Barr virus. In addition, Epstein-Barr virus infection may lead to a positive ANA [ 82,83 ]. Human parvovirus B19 can cause flu-like symptoms and hematologic abnormalities such as leukopenia and thrombocytopenia, which can be observed in SLE, and patients may present with arthralgias or arthritis.
Other viral infections that may present with multisystem involvement include human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus. However, serologic assays can be diagnostic for many of these viruses. Some bacterial infections such as Salmonella or tuberculosis should also be considered, if appropriate.
● Multiple sclerosis – Although rare, patients with SLE can present with cranial neuropathies that must be distinguished from multiple sclerosis (MS). Unilateral optic neuritis and pyramidal syndrome, with lesions detected by MRI suggesting dissemination in space and time, are characteristic of MS. (See "Evaluation and diagnosis of multiple sclerosis in adults" .)
● Malignancies – Leukemia or myelodysplastic syndromes may present with hematologic and constitutional symptoms similar to those observed in SLE. However, monoclonal expansion of B and T cells (as assessed by immunophenotyping), monocytosis, or macrocytosis can distinguish these malignancies from SLE. Patients with lymphoma also typically have additional findings such as splenomegaly, lymphadenopathy, or increased lactate dehydrogenase levels. Patients with angioimmunoblastic T cell lymphoma may be distinguished by findings on an excisional tissue biopsy, most commonly a lymph node.
● Thrombotic thrombocytopenic purpura – Although both patients with thrombotic thrombocytopenic purpura (TTP) and SLE may have fever and thrombocytopenia, patients with TTP also have microangiopathic hemolytic anemia, acute kidney insufficiency, fluctuating neurologic manifestations, and/or low levels of ADAMSTS13. (See "Diagnosis of immune TTP" .)
● Other – Some patients with psychiatric disorders (eg, bipolar disorder, substance use disorders) are thought to have SLE on the basis of a positive ANA and leukopenia that may actually be drug- or medication-induced. Thus, these patients should be evaluated for other clinical features consistent with SLE. (See "Drug-induced lupus" and "Drug-induced neutropenia and agranulocytosis" .)
SOCIETY GUIDELINE LINKS — Links to society and government-sponsored guidelines from selected countries and regions around the world are provided separately. (See "Society guideline links: Systemic lupus erythematosus" .)
INFORMATION FOR PATIENTS — UpToDate offers two types of patient education materials, "The Basics" and "Beyond the Basics." The Basics patient education pieces are written in plain language, at the 5 th to 6 th grade reading level, and they answer the four or five key questions a patient might have about a given condition. These articles are best for patients who want a general overview and who prefer short, easy-to-read materials. Beyond the Basics patient education pieces are longer, more sophisticated, and more detailed. These articles are written at the 10 th to 12 th grade reading level and are best for patients who want in-depth information and are comfortable with some medical jargon.
Here are the patient education articles that are relevant to this topic. We encourage you to print or e-mail these topics to your patients. (You can also locate patient education articles on a variety of subjects by searching on "patient info" and the keyword(s) of interest.)
● Basics topics (see "Patient education: Lupus (The Basics)" )
● Beyond the Basics topics (see "Patient education: Antinuclear antibodies (ANA) (Beyond the Basics)" and "Patient education: Systemic lupus erythematosus (Beyond the Basics)" )
SUMMARY AND RECOMMENDATIONS
● Clinical manifestations – Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease with a wide range of clinical and serologic manifestations that can affect virtually any organ. The disease course is often marked by remissions and relapses and may vary from mild to severe. (See 'Introduction' above.)
Major clinical manifestations of SLE include the following:
• Constitutional symptoms – Constitutional symptoms, such as fatigue, fever, and weight loss, are present in most patients with SLE at some point during the course of the disease. (See 'Constitutional symptoms' above.)
• Arthritis and arthralgias – Arthritis and arthralgias occur in over 90 percent of patients with SLE and are often one of the earliest manifestations. (See 'Arthritis and arthralgias' above.)
• Mucocutaneous involvement – Many patients also have skin and/or mucous membrane lesions. (See 'Mucocutaneous involvement' above.)
• Cardiovascular involvement – Cardiac disease among patients with SLE is common and can involve the pericardium, myocardium, valves, conduction system, and coronary arteries. Several vascular abnormalities, including Raynaud phenomenon, vasculitis, microvascular angina, and thromboembolic disease, can also be observed. (See 'Cardiac involvement and vascular manifestations' above.)
• Kidney involvement – Kidney involvement is clinically apparent in approximately 40 percent of patients and is a significant cause of morbidity and mortality. (See 'Kidney involvement' above.)
• Gastrointestinal involvement – Gastrointestinal abnormalities can involve almost any organ along the gastrointestinal tract. However, the majority of symptoms are related to adverse medication reactions or infection. (See 'Gastrointestinal involvement' above.)
• Pulmonary involvement – Pulmonary manifestations of SLE include pleuritis (with or without effusion), pneumonitis, interstitial lung disease, pulmonary hypertension, shrinking lung syndrome, and alveolar hemorrhage. (See 'Pulmonary involvement' above.)
• Neuropsychiatric involvement – Neuropsychiatric involvement of SLE consists of a broad range of neurologic and psychiatric manifestations, including cognitive dysfunction, organic brain syndromes, delirium, psychosis, seizures, headache, and/or peripheral neuropathies. (See 'Neurologic and neuropsychiatric involvement' above.)
• Hematologic abnormalities – Hematologic abnormalities can include anemia, leukopenia, and thrombocytopenia. Lymphadenopathy and splenomegaly can also be observed. (See 'Hematologic abnormalities' above.)
● Evaluation – As part of the medical history and physical examination, we pay particular attention to the following symptoms and signs (see 'Evaluation' above):
• Photosensitive skin lesions, such as a malar rash or discoid lesions
• Painless oral or nasal ulcers
• Hair loss that is patchy or frontal/peripheral
• Raynaud phenomenon
• Joint pain or swelling, which can be migratory or symmetrical
• Symptoms of serositis/pericarditis
• Fatigue/aching/fever
We also ask about exposure to medications associated with drug-induced lupus (eg, hydralazine ). (See "Drug-induced lupus" .)
● Laboratory testing – We obtain a complete blood count and differential as well as serum creatinine level and urinalysis. We also test for antinuclear antibodies (ANA; and if positive, other specific autoantibodies, such as anti-double-stranded DNA [anti-dsDNA] and anti-Smith [anti-Sm]), antiphospholipid antibodies, C3 and C4 or CH50 complement levels, erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP) levels, and the urine protein-to-creatinine ratio. (See 'Laboratory testing' above.)
● Additional testing in selected patients – Additional studies, such as diagnostic imaging or biopsy of an involved organ, may be necessary; such testing is dictated by the clinical presentation and associated differential diagnostic possibilities. (See 'Additional testing in selected patients' above.)
● Classification criteria – Classification criteria have been developed for SLE as a means of categorizing patients for study purposes. These criteria can be useful for clinicians in systematically documenting key disease features. (See 'Classification criteria' above.)
● Diagnosis – In the absence of existing "diagnostic criteria," our general approach to the diagnosis of SLE is as follows (see 'Our diagnostic criteria' above):
• Definite SLE – After excluding alternative diagnoses, we diagnose SLE in the patient who fulfills the 1997 American College of Rheumatology (ACR) criteria, the 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria ( table 3 ), or the 2019 European Alliance of Associations for Rheumatology (EULAR)/ACR criteria ( table 2A ). (See 'Definite SLE' above and 'Classification criteria' above.)
• Probable SLE – There are patients who do not fulfill the classification criteria for SLE but in whom we still diagnose the disorder. These patients include those presenting with an inadequate number of ACR or SLICC criteria or those who have other SLE manifestations not included in either classification criteria. (See 'Probable SLE' above.)
As a loose guide, we diagnose SLE in patients who have two or three of the ACR or SLICC criteria, along with at least one other feature that may be associated with but is not specific for SLE. Some of these features include:
- Optic neuritis, aseptic meningitis
- Glomerular hematuria
- Pneumonitis, pulmonary hemorrhage, or pulmonary hypertension, interstitial lung disease
- Myocarditis, verrucous endocarditis (Libman-Sacks endocarditis)
- Abdominal vasculitis
- Raynaud phenomenon
- Elevated acute phase reactants (eg, ESR and CRP)
• Possible SLE – We consider SLE a possible diagnosis in individuals who have only one of the ACR/SLICC criteria, in addition to at least one or two of the uncommon features listed above. (See 'Possible SLE' above.)
• Undifferentiated connective tissue disease – Other patients who have even fewer features suggestive of SLE may be classified as undifferentiated connective tissue disease (UCTD). (See 'Undifferentiated connective tissue disease' above.)
• ANA-negative SLE – Less than 5 percent of patients with SLE are negative for ANA as detected by indirect immunofluorescence. (See 'ANA-negative lupus' above.)
ACKNOWLEDGMENT — The UpToDate editorial staff acknowledges Peter Schur, MD, who contributed to an earlier version of this topic review.
- McKinley PS, Ouellette SC, Winkel GH. The contributions of disease activity, sleep patterns, and depression to fatigue in systemic lupus erythematosus. A proposed model. Arthritis Rheum 1995; 38:826.
- Tench CM, McCurdie I, White PD, D'Cruz DP. The prevalence and associations of fatigue in systemic lupus erythematosus. Rheumatology (Oxford) 2000; 39:1249.
- Jump RL, Robinson ME, Armstrong AE, et al. Fatigue in systemic lupus erythematosus: contributions of disease activity, pain, depression, and perceived social support. J Rheumatol 2005; 32:1699.
- Wang B, Gladman DD, Urowitz MB. Fatigue in lupus is not correlated with disease activity. J Rheumatol 1998; 25:892.
- Iaboni A, Ibanez D, Gladman DD, et al. Fatigue in systemic lupus erythematosus: contributions of disordered sleep, sleepiness, and depression. J Rheumatol 2006; 33:2453.
- Cervera R, Khamashta MA, Font J, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003; 82:299.
- Rovin BH, Tang Y, Sun J, et al. Clinical significance of fever in the systemic lupus erythematosus patient receiving steroid therapy. Kidney Int 2005; 68:747.
- Greco CM, Rudy TE, Manzi S. Adaptation to chronic pain in systemic lupus erythematosus: applicability of the multidimensional pain inventory. Pain Med 2003; 4:39.
- Budhram A, Chu R, Rusta-Sallehy S, et al. Anti-cyclic citrullinated peptide antibody as a marker of erosive arthritis in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Lupus 2014; 23:1156.
- Miner JJ, Kim AH. Cardiac manifestations of systemic lupus erythematosus. Rheum Dis Clin North Am 2014; 40:51.
- Richter JG, Sander O, Schneider M, Klein-Weigel P. Diagnostic algorithm for Raynaud's phenomenon and vascular skin lesions in systemic lupus erythematosus. Lupus 2010; 19:1087.
- Barile-Fabris L, Hernández-Cabrera MF, Barragan-Garfias JA. Vasculitis in systemic lupus erythematosus. Curr Rheumatol Rep 2014; 16:440.
- Ramos-Casals M, Nardi N, Lagrutta M, et al. Vasculitis in systemic lupus erythematosus: Prevalence and clinical characteristics in 670 patients. Medicine (Baltimore) 2006; 85:95.
- Sokalski DG, Copsey Spring TR, Roberts WN. Large artery inflammation in systemic lupus erythematosus. Lupus 2013; 22:953.
- Dhillon PK, Adams MJ. Thrombosis in systemic lupus erythematosus: role of impaired fibrinolysis. Semin Thromb Hemost 2013; 39:434.
- Sarabi ZS, Chang E, Bobba R, et al. Incidence rates of arterial and venous thrombosis after diagnosis of systemic lupus erythematosus. Arthritis Rheum 2005; 53:609.
- Jung H, Bobba R, Su J, et al. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum 2010; 62:863.
- Danila MI, Pons-Estel GJ, Zhang J, et al. Renal damage is the most important predictor of mortality within the damage index: data from LUMINA LXIV, a multiethnic US cohort. Rheumatology (Oxford) 2009; 48:542.
- Tian XP, Zhang X. Gastrointestinal involvement in systemic lupus erythematosus: insight into pathogenesis, diagnosis and treatment. World J Gastroenterol 2010; 16:2971.
- Romero-Díaz J, García-Sosa I, Sánchez-Guerrero J. Thrombosis in systemic lupus erythematosus and other autoimmune diseases of recent onset. J Rheumatol 2009; 36:68.
- Newman K, Owlia MB, El-Hemaidi I, Akhtari M. Management of immune cytopenias in patients with systemic lupus erythematosus - Old and new. Autoimmun Rev 2013; 12:784.
- Silpa-archa S, Lee JJ, Foster CS. Ocular manifestations in systemic lupus erythematosus. Br J Ophthalmol 2016; 100:135.
- Rosenbaum JT, Trune DR, Barkhuizen A, et al. Ocular, aural, and oral manifestations. In: Dubois' Lupus Erythematosus and Related Syndromes, 8th ed, Wallace DJ, Hahn BH (Eds), Elsevier, Philadelphia 2013. p.393.
- Gallais Sérézal I, Bouillet L, Dhôte R, et al. Hereditary angioedema and lupus: A French retrospective study and literature review. Autoimmun Rev 2015; 14:564.
- Sawada T, Fujimori D, Yamamoto Y. Systemic lupus erythematosus and immunodeficiency. Immunol Med 2019; 42:1.
- Almaghlouth I, Su J, Johnson SR, et al. Acquired low immunoglobulin levels and risk of clinically relevant infection in adult patients with systemic lupus erythematosus: a cohort study. Rheumatology (Oxford) 2021; 60:1456.
- Abu-Shakra M, Gladman DD, Urowitz MB, Farewell V. Anticardiolipin antibodies in systemic lupus erythematosus: clinical and laboratory correlations. Am J Med 1995; 99:624.
- Haliloglu S, Carlioglu A, Akdeniz D, et al. Fibromyalgia in patients with other rheumatic diseases: prevalence and relationship with disease activity. Rheumatol Int 2014; 34:1275.
- Ehmke TA, Cherian JJ, Wu ES, et al. Treatment of osteonecrosis in systemic lupus erythematosus: a review. Curr Rheumatol Rep 2014; 16:441.
- Zhou WJ, Yang CD. The causes and clinical significance of fever in systemic lupus erythematosus: a retrospective study of 487 hospitalised patients. Lupus 2009; 18:807.
- Nived O, Sturfelt G, Wollheim F. Systemic lupus erythematosus and infection: a controlled and prospective study including an epidemiological group. Q J Med 1985; 55:271.
- Hidalgo-Tenorio C, Jiménez-Alonso J, de Dios Luna J, et al. Urinary tract infections and lupus erythematosus. Ann Rheum Dis 2004; 63:431.
- Cuchacovich R, Gedalia A. Pathophysiology and clinical spectrum of infections in systemic lupus erythematosus. Rheum Dis Clin North Am 2009; 35:75.
- Feldman CH, Hiraki LT, Winkelmayer WC, et al. Serious infections among adult Medicaid beneficiaries with systemic lupus erythematosus and lupus nephritis. Arthritis Rheumatol 2015; 67:1577.
- Hellmann DB, Petri M, Whiting-O'Keefe Q. Fatal infections in systemic lupus erythematosus: the role of opportunistic organisms. Medicine (Baltimore) 1987; 66:341.
- Zandman-Goddard G, Shoenfeld Y. SLE and infections. Clin Rev Allergy Immunol 2003; 25:29.
- Chen HS, Tsai WP, Leu HS, et al. Invasive fungal infection in systemic lupus erythematosus: an analysis of 15 cases and a literature review. Rheumatology (Oxford) 2007; 46:539.
- Barber CE, Barnabe C. Another consequence of severe lupus: invasive fungal disease. J Rheumatol 2012; 39:1772.
- Gladman DD, Hussain F, Ibañez D, Urowitz MB. The nature and outcome of infection in systemic lupus erythematosus. Lupus 2002; 11:234.
- Rodziewicz M, Dyball S, Lunt M, et al. Early infection risk in patients with systemic lupus erythematosus treated with rituximab or belimumab from the British Isles Lupus Assessment Group Biologics Register (BILAG-BR): a prospective longitudinal study. Lancet Rheumatol 2023; 5:e284.
- Ferrari SM, Elia G, Virili C, et al. Systemic Lupus Erythematosus and Thyroid Autoimmunity. Front Endocrinol (Lausanne) 2017; 8:138.
- Yun JS, Bae JM, Kim KJ, et al. Increased risk of thyroid diseases in patients with systemic lupus erythematosus: A nationwide population-based Study in Korea. PLoS One 2017; 12:e0179088.
- Kuo CF, Grainge MJ, Valdes AM, et al. Familial Aggregation of Systemic Lupus Erythematosus and Coaggregation of Autoimmune Diseases in Affected Families. JAMA Intern Med 2015; 175:1518.
- Lim SS, Drenkard C. Epidemiology of lupus: an update. Curr Opin Rheumatol 2015; 27:427.
- Cervera R, Khamashta MA, Font J, et al. Systemic lupus erythematosus: clinical and immunologic patterns of disease expression in a cohort of 1,000 patients. The European Working Party on Systemic Lupus Erythematosus. Medicine (Baltimore) 1993; 72:113.
- Estes D, Christian CL. The natural history of systemic lupus erythematosus by prospective analysis. Medicine (Baltimore) 1971; 50:85.
- Font J, Cervera R, Ramos-Casals M, et al. Clusters of clinical and immunologic features in systemic lupus erythematosus: analysis of 600 patients from a single center. Semin Arthritis Rheum 2004; 33:217.
- Pons-Estel BA, Catoggio LJ, Cardiel MH, et al. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among "Hispanics". Medicine (Baltimore) 2004; 83:1.
- Nossent J, Kiss E, Rozman B, et al. Disease activity and damage accrual during the early disease course in a multinational inception cohort of patients with systemic lupus erythematosus. Lupus 2010; 19:949.
- Satoh M, Chan EK, Ho LA, et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum 2012; 64:2319.
- Riemakasten G, Hiepe F. Autoantibodies. In: Dubois' Lupus Erythematosus and Related Syndromes, 8th ed, Wallace DJ, Hahn BH (Eds), Elsevier, Philadelphia 2013. p.282.
- Benito-Garcia E, Schur PH, Lahita R, American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines. Guidelines for immunologic laboratory testing in the rheumatic diseases: anti-Sm and anti-RNP antibody tests. Arthritis Rheum 2004; 51:1030.
- Medina-Rosas J, Gladman DD, Su J, et al. Utility of untimed single urine protein/creatinine ratio as a substitute for 24-h proteinuria for assessment of proteinuria in systemic lupus erythematosus. Arthritis Res Ther 2015; 17:296.
- Weissman BN, Rappoport AS, Sosman JL, Schur PH. Radiographic findings in the hands in patients with systemic lupus erythematosus. Radiology 1978; 126:313.
- Ruano CA, Malheiro R, Oliveira JF, et al. Ultrasound detects subclinical joint inflammation in the hands and wrists of patients with systemic lupus erythematosus without musculoskeletal symptoms. Lupus Sci Med 2017; 4:e000184.
- Lins CF, Lima de Sá Ribeiro D, Dourado Santos WG, et al. Ultrasound Findings on Hands and Wrists of Patients with Systemic Lupus Erythematosus: Relationship with Physical Examination. Ultrasound Med Biol 2017; 43:1764.
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis 2019; 78:1151.
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol 2019; 71:1400.
- Aringer M, Brinks R, Dörner T, et al. European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) SLE classification criteria item performance. Ann Rheum Dis 2021; 80:775.
- Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012; 64:2677.
- Pons-Estel GJ, Wojdyla D, McGwin G Jr, et al. The American College of Rheumatology and the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus in two multiethnic cohorts: a commentary. Lupus 2014; 23:3.
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40:1725.
- Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25:1271.
- Petri M, Magder L. Classification criteria for systemic lupus erythematosus: a review. Lupus 2004; 13:829.
- Guidelines for referral and management of systemic lupus erythematosus in adults. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Arthritis Rheum 1999; 42:1785.
- Bertsias GK, Pamfil C, Fanouriakis A, Boumpas DT. Diagnostic criteria for systemic lupus erythematosus: has the time come? Nat Rev Rheumatol 2013; 9:687.
- Ganczarczyk L, Urowitz MB, Gladman DD. "Latent lupus". J Rheumatol 1989; 16:475.
- Alarcón GS, Williams GV, Singer JZ, et al. Early undifferentiated connective tissue disease. I. Early clinical manifestation in a large cohort of patients with undifferentiated connective tissue diseases compared with cohorts of well established connective tissue disease. J Rheumatol 1991; 18:1332.
- Greer JM, Panush RS. Incomplete lupus erythematosus. Arch Intern Med 1989; 149:2473.
- Lom-Orta H, Alarcon-Segovia D, Diaz-Jouanen E. Systemic lupus erythematosus. Differences between patients who do, and who do not, fulfill classification criteria at the time of diagnosis. J Rheumatol 1980; 7:831.
- Bodolay E, Csiki Z, Szekanecz Z, et al. Five-year follow-up of 665 Hungarian patients with undifferentiated connective tissue disease (UCTD). Clin Exp Rheumatol 2003; 21:313.
- Ståhl Hallengren C, Nived O, Sturfelt G. Outcome of incomplete systemic lupus erythematosus after 10 years. Lupus 2004; 13:85.
- Mosca M, Tani C, Bombardieri S. A case of undifferentiated connective tissue disease: is it a distinct clinical entity? Nat Clin Pract Rheumatol 2008; 4:328.
- Maddison PJ, Provost TT, Reichlin M. Serological findings in patients with "ANA-negative" systemic lupus erythematosus. Medicine (Baltimore) 1981; 60:87.
- Cross LS, Aslam A, Misbah SA. Antinuclear antibody-negative lupus as a distinct diagnostic entity--does it no longer exist? QJM 2004; 97:303.
- Blomberg S, Ronnblom L, Wallgren AC, et al. Anti-SSA/Ro antibody determination by enzyme-linked immunosorbent assay as a supplement to standard immunofluorescence in antinuclear antibody screening. Scand J Immunol 2000; 51:612.
- Heller CA, Schur PH. Serological and clinical remission in systemic lupus erythematosus. J Rheumatol 1985; 12:916.
- Sauerland U, Becker H, Seidel M, et al. Clinical utility of the anti-CCP assay: experiences with 700 patients. Ann N Y Acad Sci 2005; 1050:314.
- Cappelli S, Bellando Randone S, Martinović D, et al. "To be or not to be," ten years after: evidence for mixed connective tissue disease as a distinct entity. Semin Arthritis Rheum 2012; 41:589.
- Mosca M, Tani C, Neri C, et al. Undifferentiated connective tissue diseases (UCTD). Autoimmun Rev 2006; 6:1.
- Gladman DD, Urowitz MB, Gough J, MacKinnon A. Fibromyalgia is a major contributor to quality of life in lupus. J Rheumatol 1997; 24:2145.
- Sculley DG, Sculley TB, Pope JH. Reactions of sera from patients with rheumatoid arthritis, systemic lupus erythematosus and infectious mononucleosis to Epstein-Barr virus-induced polypeptides. J Gen Virol 1986; 67 ( Pt 10):2253.
- Al-Jitawi SA, Hakooz BA, Kazimi SM. False positive Monospot test in systemic lupus erythematosus. Br J Rheumatol 1987; 26:71.
Systemic lupus erythematosus: a case-based presentation of renal, neurologic, and hematologic emergencies
Affiliations.
- 1 a Internal Medicine Resident, Department of Medicine, Cumming School of Medicine , University of Calgary , Calgary , Alberta , Canada.
- 2 b Professor of Medicine, Division of Rheumatology, The Arthritis Society Chair in Rheumatic Diseases, Cumming School of Medicine , University of Calgary , Calgary , Alberta , Canada.
- 3 c Solovy Arthritis Research Society Professor of Medicine, Department of Medicine/Rheumatology , Northwestern University Feinberg School of Medicine , Chicago , IL , USA.
- PMID: 30173578
- DOI: 10.1080/1744666X.2018.1518132
Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder with systemic manifestations and multiorgan involvement. Although primarily diagnosed, and managed in the outpatient setting, it can occasionally present with life-threatening complications that require rapid assessment and urgent aggressive therapy. Areas covered: In our review, we explore three organ systems that are often affected in SLE, but have the potential to present as medical emergencies; these are the kidney, the central nervous system, and the hematologic system. We take a case-based approach to each clinical scenario, with information given sequentially in order to reflect "real-life" situations where management decisions need to be made with limited information. We review the acute management, pathophysiology, diagnostic approach, and treatment along with a review of the literature, for lupus nephritis presenting as rapidly progressive glomerulonephritis, acute lupus transverse myelitis, and refractory antiphospholipid syndrome. Expert commentary: At the conclusion of each section, we provide an expert commentary regarding each issue, relating to diagnosis, early management, and current evidence behind treatment recommendations.
Keywords: Systemic lupus erythematosus; acute transverse myelitis; antiphospholipid syndrome; lupus nephritis; rapidly progressive glomerulonephritis.
Publication types
- Case Reports
- Antiphospholipid Syndrome* / diagnosis
- Antiphospholipid Syndrome* / etiology
- Antiphospholipid Syndrome* / therapy
- Emergencies
- Lupus Erythematosus, Systemic / complications*
- Lupus Nephritis* / diagnosis
- Lupus Nephritis* / therapy
- Lupus Vasculitis, Central Nervous System* / diagnosis
- Lupus Vasculitis, Central Nervous System* / therapy
Complete Your CE
Course case studies, external link, this link leads outside of the netce site to:.
While we have selected sites that we believe offer good, reliable information, we are not responsible for the content provided. Furthermore, these links do not constitute an endorsement of these organizations or their programs by NetCE, and none should be inferred.
Systemic Lupus Erythematosus
Course #34464 - $30 -

#34464: Systemic Lupus Erythematosus
Your certificate(s) of completion have been emailed to
- Back to Course Home
- Review the course material online or in print.
- Complete the course evaluation.
- Review your Transcript to view and print your Certificate of Completion. Your date of completion will be the date (Pacific Time) the course was electronically submitted for credit, with no exceptions. Partial credit is not available.
Patient A is a woman, 20 years of age, living in a small, rural town. In October, she suddenly begins experiencing fatigue, anxiety, and heart palpitations. She has recently given birth to her second child, a daughter. She contacts her physician, who believes the symptoms are related to the stress of having given birth in addition to caring for her toddler son. The physician recommends that Patient A rest and obtain some help caring for her two small children.
Patient A's symptoms worsen and then gradually resolve. Her family encourages her to take her physician's advice and try to reduce the stress in her life. Approximately two years after the initial symptoms, Patient A begins experiencing abdominal pain. Her rural family physician refers her to an internist in a large metropolitan city 50 miles from her home. The internist examines her and preliminarily determines that due to the patient's age and symptoms, her gallbladder is most likely causing the abdominal pain. The internist prescribes a low-fat diet and advises Patient A to adopt healthy lifestyle practices, including increased physical activity.
Five years after the symptoms initially began, Patient A becomes pregnant with her third child. During the sixth month of her pregnancy, the patient begins to experience problems. She has premature contractions, increased fatigue, headaches, and swelling in her legs. Her physician prescribes bed rest due to overexertion. During the last three months of pregnancy, Patient A remains on bed rest and the pregnancy is monitored with bi-weekly visits and ultrasounds. Her family plays a central role in helping her with housekeeping and childcare for her two children. However, she is forced to take a leave of absence from her job due to the premature labor. She delivers a healthy baby girl in September.
Shortly after the birth of her third child, Patient A begins experiencing new and puzzling symptoms. Her ankles and knees begin to swell, and the edema is noted bilaterally. She also starts to complain of joint pain in her ankles, knees, elbows, wrists, and fingers. Patient A has difficulty climbing a flight of steps or dancing. Rest and over-the-counter pain medication relieve her symptoms, but it is difficult for her to find time for much rest due to the responsibilities of caring for a family and working full-time. Her family is very concerned about her health and wonders why the physician is not able to find a cause for her problems.
The winter brings a new intolerance to low temperatures. While Patient A has never liked cold weather, suddenly she is having a problem with her hands and feet becoming painful and discolored when she is exposed to cold. Her extremities became painful, stiff, and altered in color when exposed to cold temperatures. Patient A finally returns to her rural family physician in March. He is perplexed; he is not sure what was causing the young woman's problems. The physician decides to send Patient A to see a rheumatologist in the same metropolitan area as the internist.
The rheumatologist examines the patient and runs several blood tests. Patient A's ANA test is positive at 1:640. Her lupus erythematosus test or LE cell prep is negative (normal: negative test with no LE cells noted.) The rheumatoid arthritis factor is negative (normal: negative with <60 U/mL), and her sedimentation rate is 62 mm/hour (normal: up to 20 mm/hour for women).
The rheumatologist tells Patient A that he cannot be sure of her condition, but that he is considering the possibility that it could be lupus. He emphatically tells her, however, that it is not a positive diagnosis, and he certainly does not want to label her with such a devastating disease unless he is certain. He prescribes an anti-inflammatory medication, naproxen, and tells her to return home and rest. Patient A is frustrated—no one has been able to find an answer to why she feels so sick. She tries to talk to her family and friends, but they do not seem to understand what she is going through. Even the physicians do not seem to hear what she is telling them.
While medication reduces the pain and swelling in her joints, Patient A continues to experience fatigue, abdominal pain, and intolerance to cold weather. She is frustrated and feels as if no one is listening to her complaints. Her spouse, family, and friends do not understand why she feels so bad when she looks as if there is nothing really wrong with her. To Patient A, it seems as if she will never feel healthy again.
In the summer of the following year, Patient A experiences a strange red, raised rash with itching after having been out in the sun. She has always enjoyed the outdoors, and while she has been sunburned in the past, she has never had a rash. In addition, she begins to develop small, raised sores on her legs and arms. The joint pain, swelling, and fatigue continue.
Convinced that there must be something wrong with her, Patient A begins researching information on rheumatologic conditions. Based on her symptoms and the lab test results performed in the past, she begins to suspect that she has lupus. She begins to inquire around the town in which she lives to determine if anyone knows of a good rheumatologist that they like and trust. She finally locates a rheumatologist and makes an appointment as soon as possible.
At the first visit to the new rheumatologist's office, the physician elicits the patient's long medical history and description of her numerous symptoms. He examines her and obtains lab work, including a CBC, ANA, anti-DNA antibody test, and complement series, as well as a skin biopsy of the lesions on her legs. The skin biopsy results indicate small vessel vasculitis. The following lab results are recorded:
ANA: 1:640 (normal: No ANA detected in a titer with a dilution 1:32)
Anti-DNA antibody test: Elevated (normal: low or none)
Complement assay: Decreased C 3 level at 43 mg/dL (normal: 55–120 mg/dL) and decreased C 4 level at 14 mg/dL (normal: 20–50 mg/dL)
Red blood cell count: 3.8 million/mm 3 (normal: 4.2–5.4 million/mm 3 for women)
Hemoglobin: 10.5 g/dL (normal: 12–16 g/dL for women)
Hematocrit: 35% (normal: 37% to 47% for women)
White blood cell count: 6,000/mm 3 (normal: 5,000–10,000/mm 3 for women)
Platelets: 138,000/mm 3 (normal: 150,000–400,000/mm 3 )
After completing all of the tests, the rheumatologist sits with the patient and her spouse and tells them that although the tests are pending, he is certain that she has systemic lupus erythematosus. She meets the entry criterion of an ANA titer >1:80 of the EULAR/ACR criteria for diagnosis and has the following additive criteria: butterfly rash/facial erythema (acute cutaneous lupus, 6 points), nonerosive arthritis (joint involvement, 6 points), hematologic or blood disorder (autoimmune hemolytic anemia, 4 points), immunologic disorder (abnormal anti-DNA antibody test, 6 points), for a total of 22 points. Patient A has a positive diagnosis with the entry criterion of ANA titer being met, at least one of the clinical domains being met, and a score of greater than 10 points [28] .
The physician describes the disease in great detail to Patient A and her husband and answers all of their questions. The physician believes that the patient has had lupus for more than seven years, beginning with her initial symptoms. A one-month course of prednisone with tapered doses is prescribed. Nabumetone, an anti-inflammatory, is added to the regimen prior to the prednisone being weaned off. He reassures Patient A as she leaves his office that he will be available for her and will help her manage the disease.
As Patient A leaves the rheumatologist's office, her feelings are mixed. She is thankful to finally know she is not crazy and to have a diagnosis. She is also angry and bitter that it took seven years and four physicians to finally find a cause for her symptoms. She is relieved that she can finally tell her family and friends why she has felt so sick. She is also very afraid because she is not certain what her future will hold now that she has been diagnosed with the chronic disease lupus.
This case study provides one example of the physical and psychological experiences associated with lupus. The struggles Patient A experiences in an attempt to diagnose her chronic illness are documented, as well as the vagueness of her symptoms. This woman's experience provides a brief glimpse into the challenges of obtaining a diagnosis and of living with lupus.
- About NetCE
- About TRC Healthcare
- Do Not Sell My Personal Information
Copyright © 2024 NetCE · Contact Us
- Qazi Corner
- Biosimilars Spotlight
- DME & nAMD On-Demand Presentation

- Multimedia Series
- Conferences
- Advisory Board
Lupus Case Scenario: Patient History and Presentation
Anne E. Winkler, MD, PhD, MACP: Let’s go ahead and switch gears a little bit and start talking about a hypothetical case. And even though it’s hypothetical, I will tell you I’ve had patients just like this. So, this is a 24-year-old Afro American computer programmer. She was referred to rheumatologist for a sensitive rash and joint pain. On exam, she had a rash in the Malar regions of the face and forearms, she was tendering in some of the small joints of the hands and the wrist. Normal blood pressure, labs did show however a low C3 of 63, elevated double stranded DNA antibody of over 300, her UPCR was 0.2 and she was initially treated with hydroxychloroquine and leflunomide. Again, pretty typical kind of scenario we would start. She comes back and every three months she’s getting UPCR which of course, we’re big on these days because we know already because young woman of color, low C3, high double stranded DNA antibody, she’s at high risk for lupus nephritis and on a routine visit, because again, I’ve seen this happen, no other symptoms that maybe some fatigue. Her UPCR now is four, so obviously she’s developed lupus nephritis. Kind of if you can comment a little bit on what you think and how you would approach this patient.
Kristi V. Mizelle, MD, MPH, FACR : Yeah, I’d like to actually take it back to the initial presentation. So when I see a patient, and the concern is potentially for lupus, most- a lot of people ask, well, what’s the ANA? That’s the first thing we want to know. And yes, the ANA is important. But I’d also want to get a little bit more of a history, I’d also want to see what the examination findings are. These are very important things because there’s some things that can mimic manifestations of lupus that we have to make sure that we are clearly seeing things that look like lupus. For example, patient presents with malar rash, OK, a lot of people have rosacea, a lot of rosacea is called malar rash. So it’s very important to understand the difference between rosacea and malar rash, looking for if there’s fine capillaries present? Does it spare the nasal labial folds? Is it raised, is it painful, or uncomfortable on the face? Those things are very important because we may be chasing something that’s not necessarily a Lupus manifestation. And so it’s important to distinguish those things when we do see patients. For example, you mentioned she had some joint pain. Well, joint pain in and of itself is not enough to make a Lupus diagnosis and patients can have arthralgias, but diagnostic and classification criteria actually speak to inflammatory arthritis. Is there synovitis at the joints? When we examine the joints and do X-rays, is there erosion? If there’s erosion, then that makes us take a step back and say maybe this isn’t truly lupus because lupus very rarely, usually does not cause erosions at the joints. So, we have to make sure that we’re very clear in our clinical approach and that we’re very detail oriented as we take the history, because on its face when you give this presentation for an initial visit with the patient, oh, it’s like lupus, slam dunk. But I can’t tell you the number of patients who’ve come in and I’ve had similar findings, and I say, you know what? These things are not technically meeting what I would expect the criteria to be in order for us to say it’s truly a manifestation of lupus. And so we have to have our minds open. I’ve had patients come in with an ANA one 180, one to 160 and have joint inflammation and we check their inflammatory markers as well as their lupus and rheumatoid arthritis studies and they have a high CCP or they have discoid lupus that’s biopsy proven, but no ANA, and then they have inflammatory joint changes with erosions. I have some patients who have discoid lupus and an seropositive erosive rheumatoid arthritis. So, we have to make sure we understand the diagnosis because we have to- that then is so-
Anne E. Winkler, MD, PhD, MACP: You just froze again, you have a patient with seropositive, I think is where you stopped.
Kristi V. Mizelle, MD, MPH, FACR : Seropositive rheumatoid arthritis. We have to make sure that we’re very clear on our initial diagnosis before we start running and putting on treatments, because our treatment could directly flow from what the diagnosis is and we may treat or choose inappropriately as far as choice of drug if we don’t have the right diagnosis. When that patient presents to me, like you said, and I’d probe on some of those things that she kind of came in with. She’s got a very good history with UV light exposure, which can sometimes activate or be a trigger potentially for lupus. The history sounds like it could work, a young woman of reproductive age sounds like it could work, African American, increased likelihood, because African Americans have an increased risk of lupus as compared to other races and ethnicities. A lot of those things are suggestive, but we still have to make sure we do the work to understand clearly, do the various manifestations meet what we would expect as sort of the clinical and or laboratory findings that would speak more to a Lupus diagnosis? Getting the right diagnosis, super, super important. I can’t tell you how many patients I’ve seen who were evaluated by other rheumatologist and come in and I say, I want to start from the beginning. Let’s make sure we have the right diagnosis. We’re just going to go back and rethink it all. Let’s go get the old labs. Let’s go back and take the history. And then let’s come back and see are we arriving at the same point, same diagnosis? If we are, great. If we’re not, OK, well, what is it that we think is going on? And what would we do differently based upon that? It’s important to be a good diagnostician when we’re thinking about lupus patients and to take the time to be detailed. When you present that case, to me, the first thing is, let’s make sure we got the right diagnosis.
Transcript edited for clarity

Emerging Agents in the Treatment of Rheumatic Diseases

Inclusive Trials, Informed Treatments: Prioritizing Diversity in Clinical Research

Challenges with Step Therapy Protocols in Treating Rheumatoid Arthritis

Prolonged Medical Fasting May Benefit Pain Symptoms in Fibromyalgia

Secukinumab 300 mg May Improve PsA or Active Psoriasis More Than 150 mg
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Tracking down the genetic causes of lupus to personalize treatment
Genetic screen identifies many mutations associated with autoimmune diseases that could help target therapy
By Robert Sanders
Image courtesy of Lupus Research Alliance
May 23, 2024
Lupus is a lifelong, often painful and occasionally lethal autoimmune disease. Few treatments exist today beyond powerful steroids to knock down a patient’s immune system — a therapy that has its own serious risks.
The good news is that new and promising treatments are in clinical trials. But the term lupus belies the fact that the disease has a variety of causes, which means that treatments will have to be highly personalized to guarantee that each patient is given the drug that targets the specific genetic mutation responsible for their variety of lupus.
Researchers are just now beginning to link specific genetic mutations to subsets of lupus patients, allowing physicians to target therapies to those who will benefit most. In the latest advance, researchers at the University of California, Berkeley, report in a new paper the discovery of two sets of patients with genetic mutations that are nearly identical to mutations that the researchers had earlier pinpointed in mouse and cell lines as linked to autoimmune disease.
These two genetic links are among several dozen mutations that the UC Berkeley team recently discovered and linked to lupus, all in one gene that regulates a prime suspect in a subset of lupus patients — proteins called toll-like receptors (TLR), which enable immune cells to recognize foreign DNA and RNA.
According to study leader Gregory Barton, UC Berkeley professor of molecular and cell biology, identifying these mutations could help doctors deliver a personalized treatment to patients with oversensitive TLRs and, in particular, oversensitive TLR7 receptors.
This is exciting because the drug will be orally available and is in clinical trials now. Victoria Rael, graduate student
“We basically have a map now,” said Barton, who is also an investigator in the Howard Hughes Medical Institute. “It’s not like everybody that has lupus has a mutation in the gene that causes overactivation of TLRs and TLR7. But there are drugs coming online that very specifically inhibit TLR7. As we sequence more and more people, it will become easier to identify those patients and put them on those drugs. That’s a lot better than the current course of therapy for lupus, which is brutal.”
“This is exciting because the drug will be orally available and is in clinical trials now,” said Victoria Rael, a UC Berkeley graduate student who, with fellow graduate student Julian Yano, is a co-first author of the paper.
The results of the genetic screens and details of the patients’ mutations were published today (May 23) in the Journal of Experimental Medicine .
A problem recognizing ‘self’
Autoimmune diseases, which range from rheumatoid arthritis and Crohn’s disease to scleroderma and numerous thyroid conditions, stem from attacks by the immune system on the body’s own cells that destroy normal, healthy tissue.
Victoria Rael and Gregory Barton, UC Berkeley
Many studies have linked at least two types of autoimmune disease, lupus and psoriasis, to TLRs, which are part of the innate immune system that initially detects foreign invaders, such as viruses and bacteria, and stimulates a first line of attack. Normally, TLRs are delicately tuned to react only to foreign DNA and RNA, but if that tuning is off, they can react to a body’s own nucleic acids and proteins associated with nucleic acids, which look much like those of pathogens.
What makes this autoimmune reaction so deadly is that the TLRs also activate the body’s second-line defense, the more powerful adaptive immune response, mobilizing T and B cells, macrophages, and other cells. These cells then mount a sustained attack that destroys the body’s healthy tissue and causes chronic inflammation.
The most common form, systemic lupus erythematosus (SLE), for example, is characterized initially by skin rashes — in particular, a butterfly-shaped rash on the face — but later by damage to joints, muscles, organs and skin, causing pain and fatigue. It’s most commonly seen in females, often starting during the teen years. Lupus, in general, is two to three times more prevalent among women from many ethnic and racial minority groups than among white women.
… in some people, the receptor is more responsive, so now levels of self-nucleic acids that otherwise wouldn’t stimulate the receptor in a normal person activate the receptor. Gregory Barton, professor of molecular and cell biology
“We think the way the system works is that if nucleic acids find these receptors, most likely they’re going to be from a virus,” Barton said. “But in some people, the receptor is more responsive, so now levels of self-nucleic acids that otherwise wouldn’t stimulate the receptor in a normal person activate the receptor. We think that one of the ways that these mutations are working is that they’re making levels of self-nucleic acids that normally wouldn’t be stimulatory, stimulatory.”
Barton and his lab colleagues have been investigating the role of TLRs that are misregulated in lupus, and in particular, one of the main proteins that regulates them: UNC93B1, or UNC for short. Several years ago, a team of postdoctoral fellows and graduate students in his lab screened in cell culture more than 100 genetic mutations in the UNC gene to see which ones overstimulated TLRs and would be good targets for further study. While they published some details in earlier papers, they didn’t publish the complete list because there seemed to be little point — almost no data was available on the genome sequences of lupus patients to compare with the mutations that overstimulated TLRs.
But that has changed in recent years, thanks to a plunge in the cost of genome sequencing. That’s how the mother of a young girl with severe autoimmune disease found Barton. Her daughter’s DNA had been sequenced and showed a mutation in a region of UNC that Barton’s team had noted in an earlier paper.
Lupus in the family
Rael and undergraduate Madeleine Weiss used the same cell culture screening technique to test the novel mutation from the young girl and found that it had an overstimulating effect, similar to the effect of other mutations in that area of the UNC gene. Surprisingly, the patient had the genetic mutation on only one of the two UNC alleles, meaning that she had one normal UNC gene, yet she still suffered severe autoimmune symptoms.

Courtesy of U.S. Centers for Disease Control and Prevention
Barton and his team also connected with a family of five afflicted with lupus. All had mutations on one UNC allele in another area of the UNC protein that Barton’s team had previously identified. That mutation, when screened in cell lines, also produced overactive TLRs.
“We were skeptical that just one copy of a gene would be sufficient to cause a disease,” Rael said. “It wasn’t until we put the patients’ mutations into cell lines and saw that they led to very convincing TLR hyper-responsiveness that we realized they had the possibility of being sufficient to be disease-causing.”
Rael and Yano then repeated the screening work previously performed in the lab and confirmed that 32 distinct mutations in the UNC gene — about one-third of the mutations tested — increased the sensitivity of TLR7 to nucleic acids at least twofold. About another five mutants increased TLR7 sensitivity but to a lesser degree. Before these screens, only two mutations in the UNC protein had been linked to increased sensitivity of TLR7 in mice, though three additional human mutations were reported within the last two months.
Barton is hopeful that by publishing the complete list of TLR hypersensitivity mutations, doctors can identify other lupus patients who could benefit from the anti-TLR drugs now in clinical trials. One drug, M5049, or Enpatoran, appears to work by latching onto two human receptors, TLR7 and TLR8, and preventing them from binding nucleic acids.
Rael, Yano and other members of Barton’s lab are investigating further how these unique UNC mutations affect the way a patient manifests the disease. They have recreated these patients’ mutations in mice so that they can model human lupus.
“With mouse models, you can start thinking about how, even though the mutations are in the same protein, the different mechanisms of TLR regulation break down, which immune cells get activated as a result, and how this can lead to differences in the symptoms patients suffer from,” Rael said.
The lab also is trying to understand how UNC tunes TLRs, which may be by regulating the number and arrangement of TLRs on immune cells. More TLRs may make a person more sensitive to the small number of self-nucleic acids circulating in the body.
“UNC93B1 is important for getting the receptors to the place where they can function, but it also is important for regulating them when they get there,” Barton said. “The protein is a very baroque way of trying to make decisions about whether the nucleic acid that you just bound to a TLR is from a virus or from one of your own cells.”
He hopes that physicians add this gene to the list of lupus-associated genes, “so if they see a mutation like these, even a heterozygous mutation, they will investigate further.”
Other senior authors of the paper are Bo Liu of the Chinese Academy of Sciences in Shanghai and Olivia Majer of the Max Planck Institute for Infection Biology in Berlin, Germany. The co-authors also include physicians from UC San Francisco, Stanford University, and hospitals in Missouri, North Carolina and Washington.
The work was funded in part by the Lupus Research Alliance , formerly the Lupus Research Institute, and the National Institutes of Health (R01AI072429).
RELATED INFORMATION
- Large scale mutational analysis identifies UNC93B1 variants that drive TLR-mediated autoimmunity in mice and humans ( JEM )
- Gregory Barton’s lab website
- Breastfeeding is good for yet another reason, researchers discover (2016)

Horrific nightmares may signal initial onset of these chronic diseases, study says
Sign up for CNN’s Sleep, But Better newsletter series. Our seven-part guide has helpful hints to achieve better sleep .
The nightmares are intense and often horrifying, sometimes lasting well into the day.
“There’s a serial killer after me and the last few years I have the same one,” according to a Canadian patient. “He’s got my legs or something I can still feel something on my legs even when I’m then awake.”
Another English patient described nightmares “where I can’t breathe and where someone is sitting on my chest.” Yet another shared stories of “really nasty” violent visions in their sleep.
“Horrific, like murders, like skin coming off people,” said one Irish patient about his nightmares. “I think it’s like when I’m overwhelmed which could be the lupus being bad … so I think the more stress my body is under then the more vivid and bad the dreaming would be.”
Nightmares and “daymares,” dreamlike hallucinations that appear when awake, may be little-known signs of the onset of lupus and other systemic autoimmune diseases such as rheumatoid arthritis, according to a new study published Monday in the journal eClinicalMedicine.
Such unusual symptoms may also be a signal that an established disease may be about to intensely worsen or “flare” and require medical treatment, said lead study author Melanie Sloan, a researcher in the department of public health and primary care at the University of Cambridge in the United Kingdom.
“This is particularly the case in a disease like lupus, which is well known for affecting multiple organs including the brain, but we also found these patterns of symptoms in the other rheumatological diseases, like rheumatoid arthritis, Sjogren’s syndrome, and systemic sclerosis,” Sloan said in an email.
Lupus is a long-term disease in which the body’s immune system goes haywire, attacking healthy tissue and causing inflammation and pain in any part of the body, including blood cells, the brain, heart, joints and muscles, kidneys, liver, and lungs.
“Cognitive problems and many of these other neuropsychiatric symptoms we studied can have a huge influence on people’s lives, ability to work, to socialize, and just to have as much of a normal life as possible,” she said.
“These symptoms are often invisible and (currently) untestable but that shouldn’t make them any less important to be considered for treatment and support.”
Jennifer Mundt, an assistant professor of sleep medicine, psychiatry and behavioral sciences at Northwestern University’s Feinberg School of Medicine in Chicago who was not involved in the study, said in an email she was pleased the study focused on nightmares.
“Although nightmares are a very distressing problem in many medical and psychiatric conditions, they rarely get focused on except in the context of PTSD (post-traumatic stress syndrome),” Mundt said.
“A recent study showed that 18% of people with long-COVID have (frequent) nightmares, and this compares to a general population prevalence of about 5%,” she said. “Hearing the patient perspective is critical so that research and clinical care can be guided by what is most important to patients themselves.”
Doctors and patients need to know
While research in the field is rather new, a March 2019 study found patients with inflammatory arthritis and other autoimmune and inflammatory diseases also experienced nightmares and other REM sleep disorders such as sleep paralysis. REM is short for rapid eye movement, the stage of sleep in which people dream and information and information and experiences are consolidated and stored in memory.
In that study, one 57-year-old man recalled being “threatened by feral birds of prey” in his nightmares, while a 70-year-old woman dreamed her nephew was in grave danger but she could do nothing to help him.
The new study surveyed 400 doctors and 676 people living with lupus and also conducted detailed interviews with 50 clinicians and 69 people living with systemic autoimmune rheumatic diseases, including lupus.
Researchers found 3 in 5 lupus patients, and 1 in 3 patients with other rheumatology-related diseases, had increasingly vivid and distressing nightmares just before their hallucinations. These nightmares often involved falling or being attacked, trapped, or crushed or committing murder.
“I’d be riding a horse, going around cutting people out with my sword. One of them was somebody attacking me and I ended up slitting their throat,” the English patient said.
“I’m not a violent person at all. I don’t even kill an insect,” the patient continued. “And I came to the conclusion that’s probably me fighting my own (autoimmune) system. … I’m probably attacking myself, that’s the only thing I can logically make sense out of it.
Systemic autoimmune diseases often have a range of symptoms, called prodromes, that appear as signs of a sudden and possibly dangerous worsening of the condition. In lupus, for example, headaches, an increase in fatigue, painful, swollen joints, rashes, dizziness and a fever without an infection are well-known signs of an upcoming flare.
Recognizing these warning signs are important, Sloan said, because they allow “earlier detection and therefore treatment of flares, some of which can be organ damaging and even fatal in lupus patients.”
However, unique warning symptoms such as nightmares and daymares are not in the diagnostic criteria for lupus or other diseases, Sloan said. The study found doctors infrequently ask about such experiences, and patients often avoid talking about them to their physicians.
“We are strongly encouraging more doctors to ask about nightmares and other neuropsychiatric symptoms — thought to be unusual, but actually very common in systemic autoimmunity — to help us detect disease flares earlier,” said senior study author David D’Cruz, a consultant rheumatologist at Guy’s Hospital and Kings College London.
Connect the dots to autoimmune disease
On first glance, it would make sense that such neurological manifestations as nightmares would occur if the autoimmune disease impacts the brain, which lupus often does, Sloan said. But that’s not what the study uncovered.
“Interestingly, we found that lupus patients who were classified as having organ involvement other than the brain, such as kidneys or lungs, often also reported a variety of neuropsychiatric symptoms in the lead up to their kidney/lung flare,” Sloan said via email.
“This suggests that monitoring these symptoms — such as nightmares and changing mood — as well as the usual rashes and protein in the urine (due to inflammation in the kidneys ), etc., may help with earlier flare detection in many patients, not just those who go on to develop major brain involvement,” she said.
However, there is no reason for people with occasional nightmares or daytime dreams to be worried they may have an inflammatory autoimmune disease, said sleep disorder specialist Dr. Carlos Schenck, a professor and senior staff psychiatrist at the Hennepin County Medical Center at the University of Minnesota in Minneapolis.
“This study could alarm the general public into believing or worrying about whether they have lupus or a related autoimmune disorder if they have nightmares or hallucinations, which are what doctors call ‘nonspecific symptoms,’ meaning that a variety of conditions (medical and psychiatric) can manifest with these symptoms,” Schenck said in an email.
It is indeed “perfectly normal” to have occasional nightmares and even daymares, or hallucinations, which “are also more common than we think,” Sloan said.
However, if those are intense, upsetting and occur around other symptoms such as extreme fatigue, headaches and other signs of autoimmune disorders , they “should be discussed with a doctor,” Sloan said.
“People shouldn’t be afraid or embarrassed to talk about these symptoms,” she said. “In some cases, reporting these symptoms earlier, even if they seem strange and unconnected, may lead to the doctor being able to ‘join the dot’s’ to diagnose an autoimmune disease.”
For more CNN news and newsletters create an account at CNN.com

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Mediterr J Rheumatol
- v.31(3); 2020 Sep

A Rare Case with Systemic Lupus Erythematosus Manifested by two Different Neurologic Entities; Guillain Barre Syndrome and Posterior Reversible Encephalopathy Syndrome
Firdevs ulutaş.
1 Department of Rheumatology
Veli Çobankara
Uğur karasu.
2 Department of Internal Medicine
Ismail Hakkı Akbudak
3 Department of Intensive Care Unit, Faculty of Medicine, Pamukkale University, Denizli, Turkey
Systemic lupus erythematosus (SLE) is an immune-mediated, lifelong disease characterized by quite heterogeneous neuropsychiatric manifestations. Herewith, we report the first rare co-incidental case with posterior reversible encephalopathy syndrome (PRES), Guillain Barre Syndrome (GBS), and (SLE). The coexistence of these neurological conditions in SLE patients could lead to delayed diagnosis and treatment due to this rare coalescence and clinical diversity. Currently, there are no specific, diagnostic radiological or laboratory biomarkers for neurological involvement in SLE. Awareness and, early recognition of neuropsychiatric involvements of the disease are important for timely appropriate treatment. Delayed treatment may cause permanent damage, poor prognosis, long term morbidity, and even death.
INTRODUCTION
Systemic lupus erythematosus (SLE) is an immune-mediated, lifelong disease. Vasculitis of small vessels, deposition of immune complexes, and autoantibody production and deposition in various organs play a role in the disease pathogenesis. Neuropsychiatric manifestations of SLE, including headaches, seizures, psychosis, and delirium, are quite heterogeneous and may occur at the onset of lupus or later in the course of the disease. The frequency of neurologic involvement ranges between 12%–95% in SLE patients. 1 Peripheral nervous system involvement is in less than 10% of all nervous system manifestations. 2 The simultaneous prevalence of SLE with Guillain Barre Syndrome (GBS) has been reported to be between 0.6% and 1.7%, whereas the prevalence of posterior reversible encephalopathy syndrome (PRES) is 0.69% in SLE. 3 The coexistence of PRES and GBS in SLE patients has not been reported in the past. Prompt diagnosis and treatment of patients can be delayed due to this rare coalescence and clinical diversity. Also, many confusing clinical conditions including hypertensive and uremic encephalopathy, delirium, use of cytotoxic drugs, ischemic or haemorrhagic cerebrovascular events, and infections may have added to the neurological involvement. 4 Although it is difficult to find overlapping effects of predisposing factors in these patients, making the distinction of the above clinical conditions is very important for treatment modalities. In the literature, this is the first educational case where GBS and PRES occurred together in an SLE patient.
CASE REPORT
A 39-year-old man with a previous history of urogenital infection one year ago, was admitted to the inpatient clinic of Neurology at Pamukkale University, Denizli. He complained of acute onset lower extremity pain, numbness and progressive weakness, stiffness in the ankles, and walking instability for the previous two weeks. At presentation, he was afebrile and normotensive. He was unable to stand alone. He had no malar rash, no oral ulcers, no weight loss, no alcohol or illicit drug use, and no high-risk sexual behaviour. On physical exam, the muscle strength in his legs was 3/5 proximally and 2/5 distally and his deep tendon reflexes were absent. The pattern in the distal limbs resembled acute onset symmetric ascending paraparesis. Bilateral axillary and inguinal 1–2 cm superficial lymphadenopathies were noted. Cerebrospinal fluid and nerve conduction studies showed albuminocytologic dissociation and findings of dysautonomic inflammatory demyelinating polyneuropathy, respectively. He was firstly treated with standard therapies including intravenous immunoglobulin (IVIG) and plasmapheresis sessions for the diagnosis of Guillain Barre Syndrome, but did not respond well to these therapies. Brain and whole spine magnetic resonance imaging was normal and inguinal excisional lymph node biopsy resulted in reactive lymphoid hyperplasia. Serum angiotensin converting enzyme (ACE) level was normal, and no lymph node or parenchymal involvement was detected in lung tomography. After excluding sarcoidosis, infections, malignancies, and antiphospholipid antibody syndrome, he was diagnosed as SLE with the presence of symmetrical arthralgia and positive immunological markers including positive antinuclear antibody, anti-dsDNA antibody, anti-Smith antibody, and anti-SSA antibody with low complement levels, meeting the minimum 4 of 11 classification criteria created by the American College of Rheumatology. Laboratory tests revealed no hematological or renal involvement. Intravenous pulse methylprednisolone (1000 mg per day, 5 consecutive days) and cyclophosphamide (1000 mg single dose per month) were given. After intensive immunosuppressive treatments, he had tonic clonic seizures and severe headaches. Hypertension was observed (160/110). Concurrent infections and cerebrovascular insults were excluded. His brain magnetic resonance imaging (MRI) was consistent with hyperintense white matter vasogenic edema, predominantly the right side of the posterior brain on T2 weighted images ( Figure 1 ). He was treated with antiepileptic and antiedema therapies. Parenteral antihypertensive agents were titrated for adequate blood pressure control. Post-treatment, complete resolution of vasogenic edema was observed on fluid-attenuated inversion recovery (FLAIR) MRI images ( Figure 2 ). Thus the diagnosis of Posterior Reversible Encephalopathy Syndrome (PRES) was made. Despite aggressive intensive care management, no clinical improvement was seen and the patient subsequently died as a result of the neurological insult.

T2 weighted image of brain MRI
39-year-old man with systemic lupus erythematosus, presenting with headache and seizures after cytotoxic treatment and hypertension. Hyperintense lesions, posterior subcortical right side predominant vasogenic white matter edema is seen indicating PRES.

FLAIR MRI; complete resolution of vasogenic edema after supportive treatments
This is the first original educational case where GBS and PRES occurred together in an SLE patient. Only 15 cases with PRES and GBS have been reported in the recent literature, with the vast majority of patients being female and older than the age of 55. 5 PRES can develop in association with autoimmune diseases like SLE, GBS, and polyarteritis nodosa. Although the association between PRES and GBS are poorly understood, underlying possible mechanisms leading to PRES in GBS patients may include dysautonomia, autoimmunity, IVIG therapy, and activation of the sympathetic nervous system. Dysautonomic cardiac and cerebrovascular complications of GBS include tachy-bradycardia, hypo-hypertension, and PRES, respectively. 6 The rare coexistence of PRES and GBS has also been reported after spinal surgery, 7 in association with hyponatremia and IVIG therapy 8 and head injury. 9
The 75% of patients with SLE can present with many different neuropsychiatric manifestations from headache to stroke throughout the course of the disease. 10 In patients with GBS, autoantibodies target peripheral nervous system cells. As a result, damaged nerves cannot transmit signals from the brain to the muscles. Many studies showed that antecedent viral or bacterial infectious agents like Campylobacter play a role in the etiology and may trigger autoimmune peripheral neuropathy. The recent cases of GBS and SLE in the literature were treated successfully with a combination of IVIG, corticosteroids, plasma exchange, and/or intravenous cyclophosphamide (CyC). The response rate of treatments was stated as 77.4% of patients with different types of peripheral nervous system involvement. 11 Although multiple clinical trials demonstrated the significant benefits of intravenous immunoglobulin and plasma exchange for the treatment of GBS, our patient did not respond to initial treatment modalities and his clinical condition worsened. 12 During the intensive immunosuppressive treatment including CyC and pulse steroid, he developed posterior reversible encephalopathy syndrome (PRES). This was first described in 1996 by Hinchey. 13 This neuroradiological disease is characterized by classical symptoms like headache, altered mental function, visual symptoms, vomiting, seizures, and with typical bilateral posterior subcortical brain edema on magnetic resonance imaging. PRES is completely reversible with supportive treatments and does not require immunosuppressive drugs. The rate in SLE patients was 18% in a case series of 120 patients with PRES and patients diagnosed with SLE and PRES were analyzed retrospectively. Concurrent hypertension, treatment with high dose steroids, and CyC have also been reported as risk factors. 14 CyC is a mainstay drug for neurolupus and lupus nephritis and may trigger PRES via direct endothelial cytotoxic effects at the blood brain barrier. 15 In another study, renal insufficiency and high SLE Disease Activity Index (SLEDAI) were also shown to be risk factors for the development of PRES. 16 Cui Hw et al. stated that SLE patients with PRES had more early disease onset with predominantly seizures, and higher mortality rates than controls. 17 Severe hypertension disrupts the autoregulation of brain blood flow. Also, interleukin-6 (IL-6)-related inflammation and endothelial damage are thought to cause hyperperfusion induced vasogenic edema and brain injury in SLE, which show a high mortality rate. 18 Male gender, atypic presentation with GBS, early disease onset, and unresponsiveness to previous treatment modalities were poor prognostic factors for our patient.
Herewith, we report the first rare coincidental case with PRES, GBS, and SLE. Underlying possible conditions in this patient were not clear. The underlying autoimmune diseases including SLE and GBS, concurrent hypertension, the use of cyclophosphamide (CyC), and IVIG may be predisposing causes for the development of PRES. We did not have a chance for further distinction due to the lethal outcome of the disease. Nevertheless, we surmise that cyclophosphamide-related PRES developed rather than active lupus disease. The patient already had been treated with intensive immunosuppressive drugs for active disease.
CONCLUSIONS
Currently, there are no specific, diagnostic radiological or laboratory biomarkers for neurological involvement in SLE. Awareness and early recognition of neuropsychiatric involvements of the disease are important for timely and appropriate treatment. Delayed treatment may cause permanent damage, poor prognosis, long term morbidity, and even death. We hope that this case could raise awareness of atypical presentations of neuropsychiatric involvement in SLE patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Horrific nightmares may signal oncoming flare of these chronic diseases, study says
By sandee lamotte, cnn | posted - may 21, 2024 at 2:33 p.m., vivid, disturbing nightmares may be a sign of a newly developing autoimmune disorder or an upcoming flare of existing disease, experts say. (getty images).
Estimated read time: 5-6 minutes
ATLANTA — The nightmares are intense and often horrifying, sometimes lasting well into the day.
Nightmares and "daymares," dreamlike hallucinations that appear when awake, may be little-known signs of the onset of lupus and other systemic autoimmune diseases such as rheumatoid arthritis, according to a new study published Monday in the journal eClinicalMedicine.
Such unusual symptoms may also be a signal that an established disease may be about to intensely worsen or "flare" and require medical treatment, said lead study author Melanie Sloan, a researcher in the Department of Public Health and Primary Care at the University of Cambridge in the United Kingdom.
"This is particularly the case in a disease like lupus, which is well known for affecting multiple organs including the brain, but we also found these patterns of symptoms in the other rheumatological diseases, like rheumatoid arthritis, Sjogren's syndrome and systemic sclerosis," Sloan said in an email.
"Cognitive problems and many of these other neuropsychiatric symptoms we studied can have a huge influence on people's lives, ability to work, to socialize and just to have as much of a normal life as possible," she said.
"These symptoms are often invisible and (currently) untestable but that shouldn't make them any less important to be considered for treatment and support."
Jennifer Mundt, an assistant professor of sleep medicine, psychiatry and behavioral sciences at Northwestern University's Feinberg School of Medicine in Chicago who was not involved in the study, said in an email she was pleased the study focused on nightmares.
"Although nightmares are a very distressing problem in many medical and psychiatric conditions, they rarely get focused on except in the context of PTSD," Mundt said.
"A recent study showed that 18% of people with long-COVID have (frequent) nightmares, and this compares to a general population prevalence of about 5%," she said. "Hearing the patient perspective is critical so that research and clinical care can be guided by what is most important to patients themselves."
Doctors and patients need to know
While research in the field is rather new, a March 2019 study found patients with inflammatory arthritis and other autoimmune and inflammatory diseases also experienced nightmares and other sleep disorders such as sleep paralysis.
In that study, one 57-year-old man recalled being "threatened by feral birds of prey" in his nightmares, while a 70-year-old woman dreamed her nephew was in grave danger but she could do nothing to help him.
Researchers found 3 in 5 lupus patients, and 1 in 3 patients with other rheumatology-related diseases, had increasingly vivid and distressing nightmares just before their hallucinations. These nightmares often involved falling or being attacked, trapped, or crushed or committing murder.
"I'd be riding a horse, going around cutting people out with my sword," the English patient said.
"I'm not a violent person at all. I don't even kill an insect," the patient continued. "And I came to the conclusion that's probably me fighting my own (autoimmune) system. … I'm probably attacking myself, that's the only thing I can logically make sense out of it.
Recognizing warning signs of lupus is important, Sloan said, because they allow "earlier detection and therefore treatment of flares, some of which can be organ damaging and even fatal in lupus patients."
Unique warning symptoms such as nightmares and daymares are not in the diagnostic criteria for lupus or other diseases, Sloan said. The study found doctors infrequently ask about such experiences, and patients often avoid talking about them to their physicians.
"We are strongly encouraging more doctors to ask about nightmares and other neuropsychiatric symptoms — thought to be unusual, but actually very common in systemic autoimmunity — to help us detect disease flares earlier," said senior study author David D'Cruz, a consultant rheumatologist at Guy's Hospital and Kings College London.
Connect the dots to autoimmune disease
It would make sense that such neurological manifestations as nightmares would occur if the autoimmune disease impacts the brain, which lupus often does, Sloan said. But that's not what the study uncovered.
"Interestingly, we found that lupus patients who were classified as having organ involvement other than the brain, such as kidneys or lungs, often also reported a variety of neuropsychiatric symptoms in the lead up to their kidney/lung flare," Sloan said via email.
"This suggests that monitoring these symptoms — such as nightmares and changing mood — as well as the usual rashes and protein in the urine (due to inflammation in the kidneys), etc., may help with earlier flare detection in many patients, not just those who go on to develop major brain involvement," she said.
There is no reason for people with occasional nightmares or daytime dreams to be worried they may have an inflammatory autoimmune disease, said sleep disorder specialist Dr. Carlos Schenck, a professor and senior staff psychiatrist at the Hennepin County Medical Center at the University of Minnesota in Minneapolis.
"This study could alarm the general public into believing or worrying about whether they have lupus or a related autoimmune disorder if they have nightmares or hallucinations, which are what doctors call 'nonspecific symptoms,' meaning that a variety of conditions (medical and psychiatric) can manifest with these symptoms," Schenck said in an email.
It is indeed "perfectly normal" to have occasional nightmares and even daymares, or hallucinations, which "are also more common than we think," Sloan said.
"People shouldn't be afraid or embarrassed to talk about these symptoms," she said. "In some cases, reporting these symptoms earlier, even if they seem strange and unconnected, may lead to the doctor being able to 'join the dots' to diagnose an autoimmune disease."
Most recent Health stories
Utah junior high student diagnosed with brain cancer finds joy in track and field.
- Study says 72 hours not long enough before removing life support
University of Utah breaks ground on new medical innovation center
Related topics, more stories you may be interested in.

Genetic profile may predict best response to weight-loss drug Wegovy

Food for thought: Do storage containers keep your fruits and veggies fresh longer?
Most viewed.
- American missionary couple killed by gang in Haiti, family says
- Toddler who nearly drowned is out of coma; first responders recall rescue as 'organized chaos'
- Grayson Murray dies at age 30 a day after withdrawing from Colonial, PGA Tour says
- Juvenile dies in West Jordan motorcycle crash on Mountain View Corridor
- Mountain biker recounts aggressive badger standoff in Draper
- Utah 7th grader helps friend cross the finish line of school race
- Massage parlor owner sentenced for prostitution, unlawful activity
- Town of Brighton makes first step in potential purchase of 'icon' Silver Fork Lodge
- Man accused of kidnapping, killing Kearns woman pleads guilty
STAY IN THE KNOW

KSL Weather Forecast

- Updated Terms of Use
- New Privacy Policy
- Your Privacy Choices
- Closed Caption Policy
- Accessibility Statement
This material may not be published, broadcast, rewritten, or redistributed. ©2024 FOX News Network, LLC. All rights reserved. Quotes displayed in real-time or delayed by at least 15 minutes. Market data provided by Factset . Powered and implemented by FactSet Digital Solutions . Legal Statement . Mutual Fund and ETF data provided by Refinitiv Lipper .
8 of the biggest health stories from this week in case you missed them
Catch up here on supplement side effects, crisis skills, sleep signs and much more.

Fox News Flash top headlines for May 25
Fox News Flash top headlines are here. Check out what's clicking on Foxnews.com.
Fox News Digital publishes a range of health pieces every day of the week to keep you up-to-date on the most important wellness news.
Cutting-edge medical research, breakthrough medications, mental health challenges, personal medical dramas and more are all covered.
In case you missed them, here are a few of the biggest health stories from this week.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
As always, you can see a full list of recent health pieces at http://www.foxnews/health .
Check out these eight key stories.
1. Certain supplements could increase heart attack, stroke risk
A new study suggests that taking a popular form of supplements could make a certain group of people more susceptible to experiencing heart disease and strokes.
A cardiologist and nutritionist weighed in. Click here to get the story.

"Further studies are needed to determine the precise mechanisms for the development and prognosis of cardiovascular disease events with regular use of fish oil supplements," the authors of a new study wrote. (iStock)
2. Half of Americans are ill-equipped to help in a crisis
Only 51% of polled Americans know how to perform hands-only CPR, and only 49% could assist with serious bleeding.
ER doctors shared tips on how people can be better prepared. Click here to get the story.

"When you’re trained in these lifesaving skills, you’ll know how to recognize the signs that someone needs help and buy time until the [first] responders can get there," a doctor said. (iStock)
3. Many patients taken off life support may have survived, study suggests
Families may want to wait before making the "irreversible decision" to take loved ones off life support after a traumatic brain injury, some doctors and researchers say. Click here to get the story.

Many patients who died after traumatic brain injuries may have survived and recovered if their families had waited to take them off life support, a new study has found. (iStock)
4. Three women share their best longevity tips
For Women’s Health Month, three mothers and grandmothers — ages 41, 55 and 64 — revealed how they're defying their chronological ages. Click here to get the story.

Left to right, Julie Gibson Clark, Amy Hardison and Lil Eskey all shared the lifestyle habits that are helping them slow down biological aging. (James Lee, Amy Hardison, Lil Eskey)
5. Lupus expert debunks 7 common myths
Dr. Brooke Goldner of Cornell University, who lives with lupus, has dedicated her life to raising awareness of the disease. She shared the truths behind some of the biggest misconceptions. Click here to get the story.

Dr. Brooke Goldner, a board-certified medical doctor and an autoimmune professor at Cornell University, pictured at right, is committed to debunking lupus myths and misconceptions. (iStock/Dr. Brooke Goldner)
6. Heart attack risk could spike during election season
Research from Massachusetts General Hospital found that people who have specific genetic traits, paired with anxiety or depression, are at a "significantly higher heart attack risk" during periods of social or political stress. Click here to get the story.
"The mind-heart connection is strong, and this study highlights that not only our bodies, but also our minds, need rest and care," a doctor said. (Lorenzo Bevilaqua/ABC via Getty Images; iStock)
7. Disrupted sleep, plus nightmares, could be linked to autoimmune diseases
Those who experience vivid nightmares and odd hallucinations might be at a higher risk of lupus, a new study suggests. Researchers and doctors revealed the link. Click here to get the story.

The study looked at not only the issues surrounding sleep, but also when the issues for participants began. (iStock)
CLICK HERE TO GET THE FOX NEWS APP
8. Paralyzed patients could find new hope in spinal cord stimulation
Ninety percent of paralyzed patients regained strength or function in their upper limbs after receiving an experimental therapy, a new study found. Experts weighed in on why this could be a "game-changer" for some patients. Click here to get the story.

Some of this week's top health stories include supplement risks, emergency skills, sleep disorder ramifications and more. (iStock)
For more Health articles, visit www.foxnews.com/health .
Melissa Rudy is health editor and a member of the lifestyle team at Fox News Digital. Story tips can be sent to [email protected].

Stay up-to-date on the biggest health and wellness news with our weekly recap.
You've successfully subscribed to this newsletter!

IMAGES
VIDEO
COMMENTS
This patient presented with systemic lupus erythematosus (SLE), rapidly progressing glomerulonephritis caused by lupus nephritis, and TMA. TMA can be caused by HUS, thrombotic thrombocytopenic purpura (TTP), malignant hypertension, or medications.
Learn about lupus diagnosis and management through traditional and interactive case studies. The Lupus Initiative is a program from the American College of Rheumatology that provides lupus awareness and education.
Autoimmune myelofibrosis is a rare complication of SLE. Autoimmune myelofibrosis could be the first and only presenting feature of SLE. It is sensible to recognize this relationship, as prompt diagnosis and treatment is crucial. Corticosteroids have been shown to be useful in treating both SLE and the associated autoimmune myelofibrosis.
Studies have suggested that a positive ANA may disappear in some SLE patients over time, with sensitivity dropping to 76% and positivity dropping from 98% to 71% in patients with established SLE . Khajehdehi et al. reported a rare case of class III lupus nephritis in a male who developed clinical flare-up of SLE despite being ANA-negative.
Systemic lupus erythematosus (SLE) is a worldwide chronic autoimmune disease which may affect every organ and tissue. Genetic predisposition, environmental triggers, and the hormonal milieu, interplay in disease development and activity. Clinical manifestations and the pattern of organ involvement are widely heterogenous, reflecting the complex ...
Limbic encephalitis with phenotypic NMDA receptor antibodies in patients with de novo diagnosis of Systemic Lupus Erythematosus. Case report☆, Colombian Journal of Anesthesiology, 45, (59-65 ...
Systemic lupus erythematosus (SLE) is a chronic rheumatic autoimmune condition that can cause a wide range of symptoms and problems that may affect the health-related quality of life. The main ...
Case Study: Systemic Lupus Erythematosus and Antiphospholipid Syndrome in Valvular Heart Disease Roxana Tabrizi1 2and Kamran Shamsa , M.D. 1University of California, ... studies on systemic lupus erythematosus and rheumatoid arthritis. Mol Biol Rep. 2012 Dec;39(12):1062735. doi: - 10.1007/s11033-012-1952-x. Epub 2012 Oct 7.
YF Case Discussion Guide Key Learning Objectives • List the differential diagnosis for a systemic disease presentation. • Describe the key manifestations of disease on physical exam in a multi-system disease presentation. • Discuss the approach to the diagnosis of systemic lupus erythematosus.
The proposed study is a randomized, double-blind, Phase 2 study in patients with systemic lupus erythematosus (SLE), a rare disease with a high unmet need. Patients are to be randomized to one of
Sedentary lifestyle increased SLE risk by 12.61 folds (OR 12.61, 95% CI 3.05-52.17). Dealing with domestic animals was another risk factor that was tested in the current study; the results ...
Case Reports. Systemic lupus erythematosus (SLE) is an autoimmune disorder with a wide range of systemic manifestations. Though skin, renal, joint, and hematologic involvement are often associated with SLE, hepatitis is not a common manifestation. While clinically significant hepatopathy in SLE is rare, asymptoma ….
The mainstay of SLE treatment is an immunosuppressive drug regimen including corticosteroids. In Colombia, retrospective case-control research revealed that a 12-month cumulative steroid dose of <1830 mg nearly increased the risk of TB. In a cohort study conducted in Taiwan, cyclophosphamide was linked to an almost 3-fold increased risk of TB.
Case series have been published that summarize the outcome of patients who have UCTD at presentation ... Dhôte R, et al. Hereditary angioedema and lupus: A French retrospective study and literature review. Autoimmun Rev 2015; 14:564. Sawada T, Fujimori D, Yamamoto Y. Systemic lupus erythematosus and immunodeficiency. Immunol Med 2019; 42:1.
Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder with systemic manifestations and multiorgan involvement. ... We take a case-based approach to each clinical scenario, with information given sequentially in order to reflect "real-life" situations where management decisions need to be made with limited information. We review ...
Case Study 87 Systemic Lupus Erythematosus. D. is a 25-year-old married woman with three children under 5 years old. She came to her physician 7 months ago with vague complaints of intermittent fatigue, joint pain, low-grade fever, and unintentional weight loss. Her physician noted small, patchy areas of vitiligo and a scaly rash across her ...
The classification of a patient's lupus nephritis through renal biopsy is typically used to guide therapy. 4 Ongoing efforts by clinicians worldwide are focused on continuing to revise and standardize the classification and nomenclature used to diagnose lupus nephritis, through evidence-based diagnostic, therapeutic, and outcome studies. 2,9 ...
CASE STUDY. Patient A is a woman, 20 years of age, living in a small, rural town. In October, she suddenly begins experiencing fatigue, anxiety, and heart palpitations. ... Her lupus erythematosus test or LE cell prep is negative (normal: negative test with no LE cells noted.) The rheumatoid arthritis factor is negative (normal: negative with ...
Lupus Case Scenario: Patient History and Presentation. Anne E. Winkler, MD, PhD, ... one to 160 and have joint inflammation and we check their inflammatory markers as well as their lupus and rheumatoid arthritis studies and they have a high CCP or they have discoid lupus that's biopsy proven, but no ANA, and then they have inflammatory joint ...
with serologically positive systemic lupus erythematosus (Belimumab in Subjects with Systemic Lupus Erythemato-sus [BLISS]-52 and BLISS-76). Further details regarding BLISS-52 and BLISS-76 are provided below.5,6 Briefly, dosing with the study agent occurred on Days 0, 14, and 28, then every 28 days through 48 weeks for BLISS-52 and 72 weeks ...
A recent study published in the New England Journal of Medicine, suggests CAR T-cell therapy could become a highly effective treatment for SLE patients who do not respond to current lupus therapeutics. The study included 15 patients — eight with lupus, four with systemic sclerosis (scleroderma), and three with idiopathic inflammatory myositis ...
Lupus is a lifelong, often painful and occasionally lethal autoimmune disease. Few treatments exist today beyond powerful steroids to knock down a patient's immune system — a therapy that has its own serious risks. ... According to study leader Gregory Barton, UC Berkeley professor of molecular and cell biology, identifying these mutations ...
Risk of bias assessment of cohort studies and case-control studies using NOS The NOS scale is a risk of bias tool for the assessment of cohorts and case control studies based on their performance in three grouping items namely the selection of population, the comparability and the outcomes/exposures of the respective study [1].
The new study surveyed 400 doctors and 676 people living with lupus and also conducted detailed interviews with 50 clinicians and 69 people living with systemic autoimmune rheumatic diseases ...
Systemic lupus erythematosus (SLE) is an autoimmune disorder classically associated with malar rash, arthralgias, cytopenias, serositis, renal failure, endocarditis, and antiphospholipid syndrome [ 1 ]. However, with the pathophysiology of the disease being based in the production of antinuclear antibodies (ANA) and antibodies against various ...
The new study surveyed 400 doctors and 676 people living with lupus and also conducted detailed interviews with 50 clinicians and 69 people living with systemic autoimmune rheumatic diseases ...
Systemic lupus erythematosus (SLE) patients present a high prevalence of cardiometabolic risk, associated with worse clinical manifestations and mortality. ... A case-control study was conducted on 394 unrelated Mexican-mestizo women: 199 with SLE according to the 1997 SLE-ACR criteria and 196 control subjects (CS). Folic acid and homocysteine ...
Concurrent hypertension, treatment with high dose steroids, and CyC have also been reported as risk factors. 14 CyC is a mainstay drug for neurolupus and lupus nephritis and may trigger PRES via direct endothelial cytotoxic effects at the blood brain barrier. 15 In another study, renal insufficiency and high SLE Disease Activity Index (SLEDAI ...
"This study could alarm the general public into believing or worrying about whether they have lupus or a related autoimmune disorder if they have nightmares or hallucinations, which are what ...
Check out these eight key stories. 1. Certain supplements could increase heart attack, stroke risk. A new study suggests that taking a popular form of supplements could make a certain group of ...