ENCYCLOPEDIC ENTRY
Photosynthesis.
Photosynthesis is the process by which plants use sunlight, water, and carbon dioxide to create oxygen and energy in the form of sugar.

Loading ...
Learning materials, instructional links.
- Photosynthesis (Google doc)
Most life on Earth depends on photosynthesis .The process is carried out by plants, algae, and some types of bacteria, which capture energy from sunlight to produce oxygen (O 2 ) and chemical energy stored in glucose (a sugar). Herbivores then obtain this energy by eating plants, and carnivores obtain it by eating herbivores.
The process
During photosynthesis, plants take in carbon dioxide (CO 2 ) and water (H 2 O) from the air and soil. Within the plant cell, the water is oxidized, meaning it loses electrons, while the carbon dioxide is reduced, meaning it gains electrons. This transforms the water into oxygen and the carbon dioxide into glucose. The plant then releases the oxygen back into the air, and stores energy within the glucose molecules.
Chlorophyll
Inside the plant cell are small organelles called chloroplasts , which store the energy of sunlight. Within the thylakoid membranes of the chloroplast is a light-absorbing pigment called chlorophyll , which is responsible for giving the plant its green color. During photosynthesis , chlorophyll absorbs energy from blue- and red-light waves, and reflects green-light waves, making the plant appear green.
Light-dependent Reactions vs. Light-independent Reactions
While there are many steps behind the process of photosynthesis, it can be broken down into two major stages: light-dependent reactions and light-independent reactions. The light-dependent reaction takes place within the thylakoid membrane and requires a steady stream of sunlight, hence the name light- dependent reaction. The chlorophyll absorbs energy from the light waves, which is converted into chemical energy in the form of the molecules ATP and NADPH . The light-independent stage, also known as the Calvin cycle , takes place in the stroma , the space between the thylakoid membranes and the chloroplast membranes, and does not require light, hence the name light- independent reaction. During this stage, energy from the ATP and NADPH molecules is used to assemble carbohydrate molecules, like glucose, from carbon dioxide.
C3 and C4 Photosynthesis
Not all forms of photosynthesis are created equal, however. There are different types of photosynthesis, including C3 photosynthesis and C4 photosynthesis. C3 photosynthesis is used by the majority of plants. It involves producing a three-carbon compound called 3-phosphoglyceric acid during the Calvin Cycle, which goes on to become glucose. C4 photosynthesis, on the other hand, produces a four-carbon intermediate compound, which splits into carbon dioxide and a three-carbon compound during the Calvin Cycle. A benefit of C4 photosynthesis is that by producing higher levels of carbon, it allows plants to thrive in environments without much light or water. The National Geographic Society is making this content available under a Creative Commons CC-BY-NC-SA license . The License excludes the National Geographic Logo (meaning the words National Geographic + the Yellow Border Logo) and any images that are included as part of each content piece. For clarity the Logo and images may not be removed, altered, or changed in any way.
Media Credits
The audio, illustrations, photos, and videos are credited beneath the media asset, except for promotional images, which generally link to another page that contains the media credit. The Rights Holder for media is the person or group credited.
Production Managers
Program specialists, last updated.
March 20, 2024
User Permissions
For information on user permissions, please read our Terms of Service. If you have questions about how to cite anything on our website in your project or classroom presentation, please contact your teacher. They will best know the preferred format. When you reach out to them, you will need the page title, URL, and the date you accessed the resource.
If a media asset is downloadable, a download button appears in the corner of the media viewer. If no button appears, you cannot download or save the media.
Text on this page is printable and can be used according to our Terms of Service .
Interactives
Any interactives on this page can only be played while you are visiting our website. You cannot download interactives.
Related Resources
It’s a wonderful world — and universe — out there.
Come explore with us!
Science News Explores
Explainer: how photosynthesis works.
Plants make sugar and oxygen with the power of water, carbon dioxide and sunlight

Green plants take in light from the sun and turn water and carbon dioxide into the oxygen we breathe and the sugars we eat.
Jeja/E+/Getty Images
Share this:
- Google Classroom
By Bethany Brookshire
October 28, 2020 at 6:30 am
Take a deep breath. Then thank a plant. If you eat fruit, vegetables, grains or potatoes, thank a plant too. Plants and algae provide us with the oxygen we need to survive, as well as the carbohydrates we use for energy. They do it all through photosynthesis.
Photosynthesis is the process of creating sugar and oxygen from carbon dioxide, water and sunlight. It happens through a long series of chemical reactions. But it can be summarized like this: Carbon dioxide, water and light go in. Glucose, water and oxygen come out. (Glucose is a simple sugar.)
Photosynthesis can be split into two processes. The “photo” part refers to reactions triggered by light. “Synthesis” — the making of the sugar — is a separate process called the Calvin cycle.
Both processes happen inside a chloroplast. This is a specialized structure, or organelle, in a plant cell. The structure contains stacks of membranes called thylakoid membranes. That’s where the light reaction begins.
Let the light shine in
When light hits a plant’s leaves, it shines on chloroplasts and into their thylakoid membranes. Those membranes are filled with chlorophyll , a green pigment. This pigment absorbs light energy. Light travels as electromagnetic waves . The wavelength — distance between waves — determines energy level. Some of those wavelengths are visible to us as the colors we see . If a molecule, such as chlorophyll, has the right shape, it can absorb the energy from some wavelengths of light.
Chlorophyll can absorb light we see as blue and red. That’s why we see plants as green. Green is the wavelength plants reflect, not the color they absorb.
While light travels as a wave, it also can be a particle called a photon . Photons have no mass. They do, however, have a small amount of light energy.
When a photon of light from the sun bounces into a leaf, its energy excites a chlorophyll molecule. That photon starts a process that splits a molecule of water. The oxygen atom that splits off from the water instantly bonds with another, creating a molecule of oxygen, or O 2 . The chemical reaction also produces a molecule called ATP and another molecule called NADPH. Both of these allow a cell to store energy. The ATP and NADPH also will take part in the synthesis part of photosynthesis.
Notice that the light reaction makes no sugar. Instead, it supplies energy — stored in the ATP and NADPH — that gets plugged into the Calvin cycle. This is where sugar is made.
But the light reaction does produce something we use: oxygen. All the oxygen we breathe is the result of this step in photosynthesis, carried out by plants and algae (which are not plants ) the world over.
Give me some sugar
The next step takes the energy from the light reaction and applies it to a process called the Calvin cycle. The cycle is named for Melvin Calvin, the man who discovered it.
The Calvin cycle is sometimes also called the dark reaction because none of its steps require light. But it still happens during the day. That’s because it needs the energy produced by the light reaction that comes before it.
While the light reaction takes place in the thylakoid membranes, the ATP and NADPH it produces end up in the stroma. This is the space inside the chloroplast but outside the thylakoid membranes.
The Calvin cycle has four major steps:
- carbon fixation : Here, the plant brings in CO 2 and attaches it to another carbon molecule, using rubisco. This is an enzyme , or chemical that makes reactions move faster. This step is so important that rubisco is the most common protein in a chloroplast — and on Earth. Rubisco attaches the carbon in CO 2 to a five-carbon molecule called ribulose 1,5-bisphosphate (or RuBP). This creates a six-carbon molecule, which immediately splits into two chemicals, each with three carbons.
- reduction : The ATP and NADPH from the light reaction pop in and transform the two three-carbon molecules into two small sugar molecules. The sugar molecules are called G3P. That’s short for glyceraldehyde 3-phosphate (GLIH- sur-AAL-duh-hide 3-FOS-fayt).
- carbohydrate formation : Some of that G3P leaves the cycle to be converted into bigger sugars such as glucose (C 6 H 12 O 6 ).
- regeneration : With more ATP from the continuing light reaction, leftover G3P picks up two more carbons to become RuBP. This RuBP pairs up with rubisco again. They are now ready to start the Calvin cycle again when the next molecule of CO 2 arrives.
At the end of photosynthesis, a plant ends up with glucose (C 6 H 12 O 6 ), oxygen (O 2 ) and water (H 2 O). The glucose molecule goes on to bigger things. It can become part of a long-chain molecule, such as cellulose; that’s the chemical that makes up cell walls. Plants also can store the energy packed in a glucose molecule within larger starch molecules. They can even put the glucose into other sugars — such as fructose — to make a plant’s fruit sweet.
All of these molecules are carbohydrates — chemicals containing carbon, oxygen and hydrogen. (CarbOHydrate makes it easy to remember.) The plant uses the bonds in these chemicals to store energy. But we use the these chemicals too. Carbohydrates are an important part of the foods we eat, particularly grains, potatoes, fruits and vegetables.
More Stories from Science News Explores on Plants

Let’s learn about photosynthesis

Experiment: Can plants stop soil erosion?

On hot summer days, this thistle stays cool to the touch

Rampaging vines are slowly strangling tropical forests

This urban gardener is mimicking nature to create healthier plants

To spy this palm’s blooms and fruits, start digging underground

Here’s why blueberries aren’t blue — but appear to be

Scientists Say: Marcescence

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

8.1: Overview of Photosynthesis
- Last updated
- Save as PDF
- Page ID 1864

\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Skills to Develop
- Explain the relevance of photosynthesis to other living things
- Describe the main structures involved in photosynthesis
- Identify the substrates and products of photosynthesis
- Summarize the process of photosynthesis
Photosynthesis is essential to all life on earth; both plants and animals depend on it. It is the only biological process that can capture energy that originates in outer space (sunlight) and convert it into chemical compounds (carbohydrates) that every organism uses to power its metabolism. In brief, the energy of sunlight is captured and used to energize electrons, which are then stored in the covalent bonds of sugar molecules. How long lasting and stable are those covalent bonds? The energy extracted today by the burning of coal and petroleum products represents sunlight energy captured and stored by photosynthesis almost 200 million years ago.
Plants, algae, and a group of bacteria called cyanobacteria are the only organisms capable of performing photosynthesis (Figure \(\PageIndex{1}\)). Because they use light to manufacture their own food, they are called photoautotrophs (literally, “self-feeders using light”). Other organisms, such as animals, fungi, and most other bacteria, are termed heterotrophs (“other feeders”), because they must rely on the sugars produced by photosynthetic organisms for their energy needs. A third very interesting group of bacteria synthesize sugars, not by using sunlight’s energy, but by extracting energy from inorganic chemical compounds; hence, they are referred to as chemoautotrophs .
The importance of photosynthesis is not just that it can capture sunlight’s energy. A lizard sunning itself on a cold day can use the sun’s energy to warm up. Photosynthesis is vital because it evolved as a way to store the energy in solar radiation (the “photo-” part) as high-energy electrons in the carbon-carbon bonds of carbohydrate molecules (the “-synthesis” part). Those carbohydrates are the energy source that heterotrophs use to power the synthesis of ATP via respiration. Therefore, photosynthesis powers 99 percent of Earth’s ecosystems. When a top predator, such as a wolf, preys on a deer (Figure \(\PageIndex{2}\)), the wolf is at the end of an energy path that went from nuclear reactions on the surface of the sun, to light, to photosynthesis, to vegetation, to deer, and finally to wolf.

Main Structures and Summary of Photosynthesis
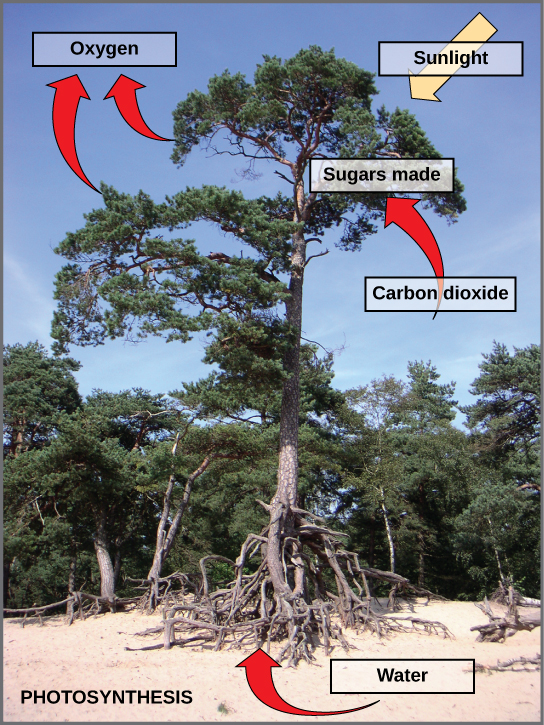
Photosynthesis is a multi-step process that requires sunlight, carbon dioxide (which is low in energy), and water as substrates (Figure \(\PageIndex{3}\)). After the process is complete, it releases oxygen and produces glyceraldehyde-3-phosphate (GA3P), simple carbohydrate molecules (which are high in energy) that can subsequently be converted into glucose, sucrose, or any of dozens of other sugar molecules. These sugar molecules contain energy and the energized carbon that all living things need to survive.
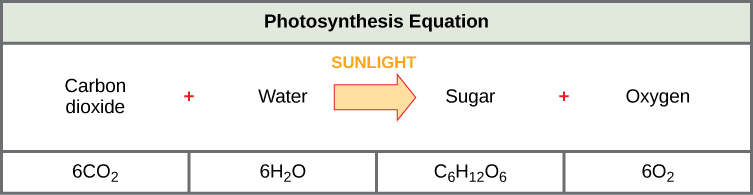
The following is the chemical equation for photosynthesis (Figure \(\PageIndex{4}\)):
Although the equation looks simple, the many steps that take place during photosynthesis are actually quite complex. Before learning the details of how photoautotrophs turn sunlight into food, it is important to become familiar with the structures involved.
In plants, photosynthesis generally takes place in leaves, which consist of several layers of cells. The process of photosynthesis occurs in a middle layer called the mesophyll . The gas exchange of carbon dioxide and oxygen occurs through small, regulated openings called stomata (singular: stoma), which also play roles in the regulation of gas exchange and water balance. The stomata are typically located on the underside of the leaf, which helps to minimize water loss. Each stoma is flanked by guard cells that regulate the opening and closing of the stomata by swelling or shrinking in response to osmotic changes.
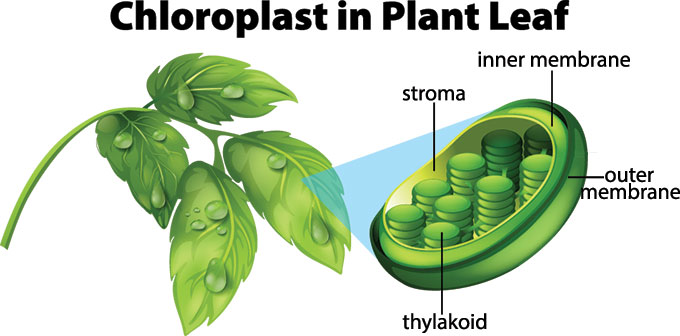
In all autotrophic eukaryotes, photosynthesis takes place inside an organelle called a chloroplast . For plants, chloroplast-containing cells exist in the mesophyll. Chloroplasts have a double membrane envelope (composed of an outer membrane and an inner membrane). Within the chloroplast are stacked, disc-shaped structures called thylakoids . Embedded in the thylakoid membrane is chlorophyll, a pigment (molecule that absorbs light) responsible for the initial interaction between light and plant material, and numerous proteins that make up the electron transport chain. The thylakoid membrane encloses an internal space called the thylakoid lumen . As shown in Figure \(\PageIndex{5}\), a stack of thylakoids is called a granum , and the liquid-filled space surrounding the granum is called stroma or “bed” (not to be confused with stoma or “mouth,” an opening on the leaf epidermis).
Art Connection
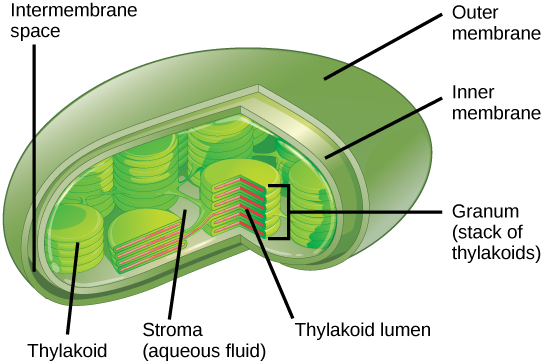
On a hot, dry day, plants close their stomata to conserve water. What impact will this have on photosynthesis?
The Two Parts of Photosynthesis
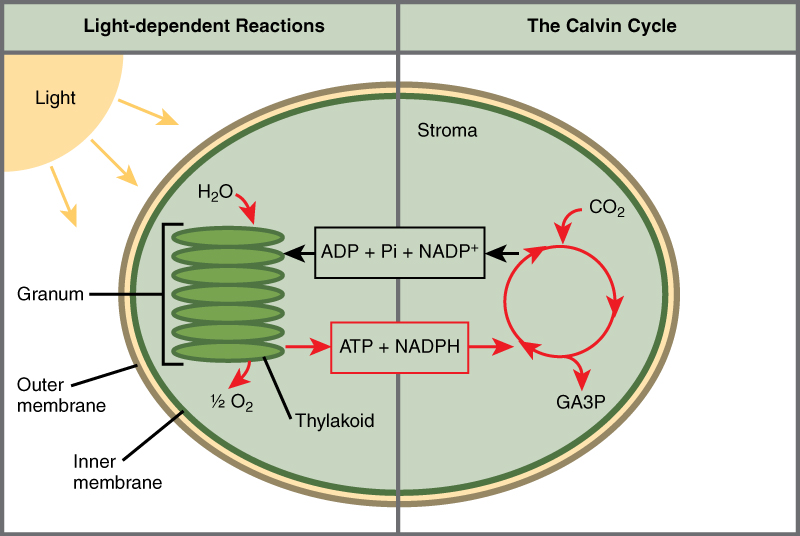
Photosynthesis takes place in two sequential stages: the light-dependent reactions and the light independent-reactions. In the light-dependent reactions , energy from sunlight is absorbed by chlorophyll and that energy is converted into stored chemical energy. In the light-independent reactions , the chemical energy harvested during the light-dependent reactions drive the assembly of sugar molecules from carbon dioxide. Therefore, although the light-independent reactions do not use light as a reactant, they require the products of the light-dependent reactions to function. In addition, several enzymes of the light-independent reactions are activated by light. The light-dependent reactions utilize certain molecules to temporarily store the energy: These are referred to as energy carriers. The energy carriers that move energy from light-dependent reactions to light-independent reactions can be thought of as “full” because they are rich in energy. After the energy is released, the “empty” energy carriers return to the light-dependent reaction to obtain more energy. Figure\(\PageIndex{6}\) illustrates the components inside the chloroplast where the light-dependent and light-independent reactions take place.
Link to Learning

Click the link to learn more about photosynthesis.
Everyday Connection: Photosynthesis at the Grocery Store

Major grocery stores in the United States are organized into departments, such as dairy, meats, produce, bread, cereals, and so forth. Each aisle (Figure \(\PageIndex{7}\)) contains hundreds, if not thousands, of different products for customers to buy and consume.
Although there is a large variety, each item links back to photosynthesis. Meats and dairy link, because the animals were fed plant-based foods. The breads, cereals, and pastas come largely from starchy grains, which are the seeds of photosynthesis-dependent plants. What about desserts and drinks? All of these products contain sugar—sucrose is a plant product, a disaccharide, a carbohydrate molecule, which is built directly from photosynthesis. Moreover, many items are less obviously derived from plants: For instance, paper goods are generally plant products, and many plastics (abundant as products and packaging) are derived from algae. Virtually every spice and flavoring in the spice aisle was produced by a plant as a leaf, root, bark, flower, fruit, or stem. Ultimately, photosynthesis connects to every meal and every food a person consumes.
Summary
The process of photosynthesis transformed life on Earth. By harnessing energy from the sun, photosynthesis evolved to allow living things access to enormous amounts of energy. Because of photosynthesis, living things gained access to sufficient energy that allowed them to build new structures and achieve the biodiversity evident today.
Only certain organisms, called photoautotrophs, can perform photosynthesis; they require the presence of chlorophyll, a specialized pigment that absorbs certain portions of the visible spectrum and can capture energy from sunlight. Photosynthesis uses carbon dioxide and water to assemble carbohydrate molecules and release oxygen as a waste product into the atmosphere. Eukaryotic autotrophs, such as plants and algae, have organelles called chloroplasts in which photosynthesis takes place, and starch accumulates. In prokaryotes, such as cyanobacteria, the process is less localized and occurs within folded membranes, extensions of the plasma membrane, and in the cytoplasm.
Art Connections
Figure \(\PageIndex{5}\): On a hot, dry day, plants close their stomata to conserve water. What impact will this have on photosynthesis?
Levels of carbon dioxide (a necessary photosynthetic substrate) will immediately fall. As a result, the rate of photosynthesis will be inhibited.
- Biology Article
Photosynthesis
Photosynthesis is a process by which phototrophs convert light energy into chemical energy, which is later used to fuel cellular activities. The chemical energy is stored in the form of sugars, which are created from water and carbon dioxide.

Table of Contents
- What is Photosynthesis?
- Site of photosynthesis
Photosynthesis definition states that the process exclusively takes place in the chloroplasts through photosynthetic pigments such as chlorophyll a, chlorophyll b, carotene and xanthophyll. All green plants and a few other autotrophic organisms utilize photosynthesis to synthesize nutrients by using carbon dioxide, water and sunlight. The by-product of the photosynthesis process is oxygen.Let us have a detailed look at the process, reaction and importance of photosynthesis.
What Is Photosynthesis in Biology?
The word “ photosynthesis ” is derived from the Greek words phōs (pronounced: “fos”) and σύνθεσις (pronounced: “synthesis “) Phōs means “light” and σύνθεσις means, “combining together.” This means “ combining together with the help of light .”
Photosynthesis also applies to other organisms besides green plants. These include several prokaryotes such as cyanobacteria, purple bacteria and green sulfur bacteria. These organisms exhibit photosynthesis just like green plants.The glucose produced during photosynthesis is then used to fuel various cellular activities. The by-product of this physio-chemical process is oxygen.
A visual representation of the photosynthesis reaction
- Photosynthesis is also used by algae to convert solar energy into chemical energy. Oxygen is liberated as a by-product and light is considered as a major factor to complete the process of photosynthesis.
- Photosynthesis occurs when plants use light energy to convert carbon dioxide and water into glucose and oxygen. Leaves contain microscopic cellular organelles known as chloroplasts.
- Each chloroplast contains a green-coloured pigment called chlorophyll. Light energy is absorbed by chlorophyll molecules whereas carbon dioxide and oxygen enter through the tiny pores of stomata located in the epidermis of leaves.
- Another by-product of photosynthesis is sugars such as glucose and fructose.
- These sugars are then sent to the roots, stems, leaves, fruits, flowers and seeds. In other words, these sugars are used by the plants as an energy source, which helps them to grow. These sugar molecules then combine with each other to form more complex carbohydrates like cellulose and starch. The cellulose is considered as the structural material that is used in plant cell walls.
Where Does This Process Occur?
Chloroplasts are the sites of photosynthesis in plants and blue-green algae. All green parts of a plant, including the green stems, green leaves, and sepals – floral parts comprise of chloroplasts – green colour plastids. These cell organelles are present only in plant cells and are located within the mesophyll cells of leaves.
Also Read: Photosynthesis Early Experiments
Photosynthesis Equation
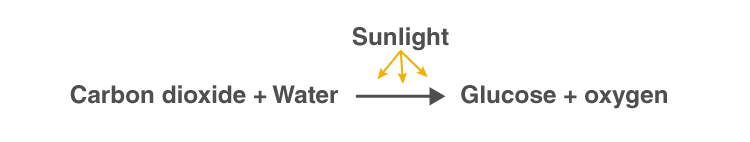
Photosynthesis reaction involves two reactants, carbon dioxide and water. These two reactants yield two products, namely, oxygen and glucose. Hence, the photosynthesis reaction is considered to be an endothermic reaction. Following is the photosynthesis formula:
Unlike plants, certain bacteria that perform photosynthesis do not produce oxygen as the by-product of photosynthesis. Such bacteria are called anoxygenic photosynthetic bacteria. The bacteria that do produce oxygen as a by-product of photosynthesis are called oxygenic photosynthetic bacteria.
Structure Of Chlorophyll
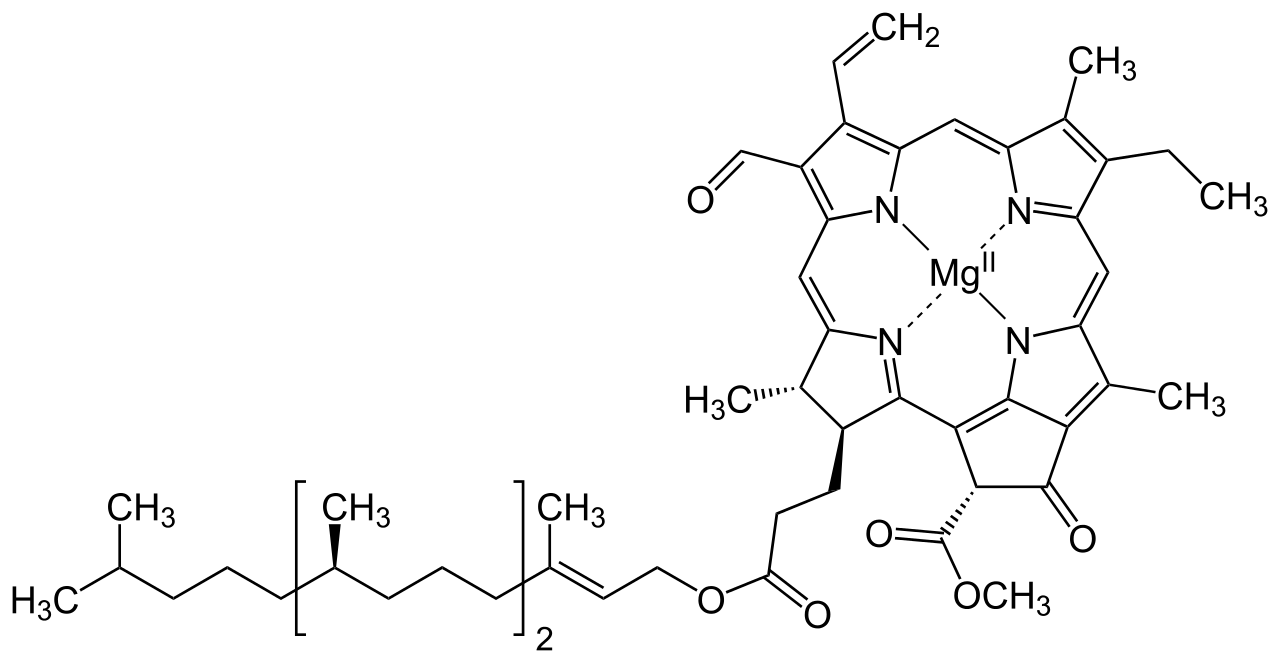
The structure of Chlorophyll consists of 4 nitrogen atoms that surround a magnesium atom. A hydrocarbon tail is also present. Pictured above is chlorophyll- f, which is more effective in near-infrared light than chlorophyll- a
Chlorophyll is a green pigment found in the chloroplasts of the plant cell and in the mesosomes of cyanobacteria. This green colour pigment plays a vital role in the process of photosynthesis by permitting plants to absorb energy from sunlight. Chlorophyll is a mixture of chlorophyll- a and chlorophyll- b .Besides green plants, other organisms that perform photosynthesis contain various other forms of chlorophyll such as chlorophyll- c1 , chlorophyll- c2 , chlorophyll- d and chlorophyll- f .
Also Read: Biological Pigments
Process Of Photosynthesis
At the cellular level, the photosynthesis process takes place in cell organelles called chloroplasts. These organelles contain a green-coloured pigment called chlorophyll, which is responsible for the characteristic green colouration of the leaves.
As already stated, photosynthesis occurs in the leaves and the specialized cell organelles responsible for this process is called the chloroplast. Structurally, a leaf comprises a petiole, epidermis and a lamina. The lamina is used for absorption of sunlight and carbon dioxide during photosynthesis.
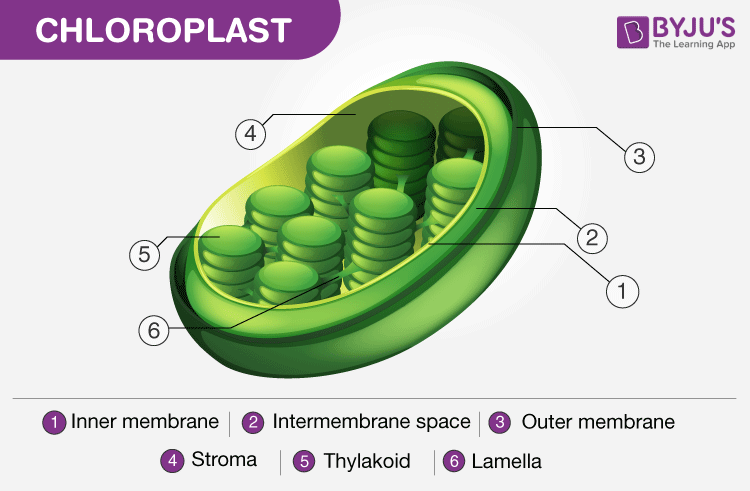
Structure of Chloroplast. Note the presence of the thylakoid
“Photosynthesis Steps:”
- During the process of photosynthesis, carbon dioxide enters through the stomata, water is absorbed by the root hairs from the soil and is carried to the leaves through the xylem vessels. Chlorophyll absorbs the light energy from the sun to split water molecules into hydrogen and oxygen.
- The hydrogen from water molecules and carbon dioxide absorbed from the air are used in the production of glucose. Furthermore, oxygen is liberated out into the atmosphere through the leaves as a waste product.
- Glucose is a source of food for plants that provide energy for growth and development , while the rest is stored in the roots, leaves and fruits, for their later use.
- Pigments are other fundamental cellular components of photosynthesis. They are the molecules that impart colour and they absorb light at some specific wavelength and reflect back the unabsorbed light. All green plants mainly contain chlorophyll a, chlorophyll b and carotenoids which are present in the thylakoids of chloroplasts. It is primarily used to capture light energy. Chlorophyll-a is the main pigment.
The process of photosynthesis occurs in two stages:
- Light-dependent reaction or light reaction
- Light independent reaction or dark reaction
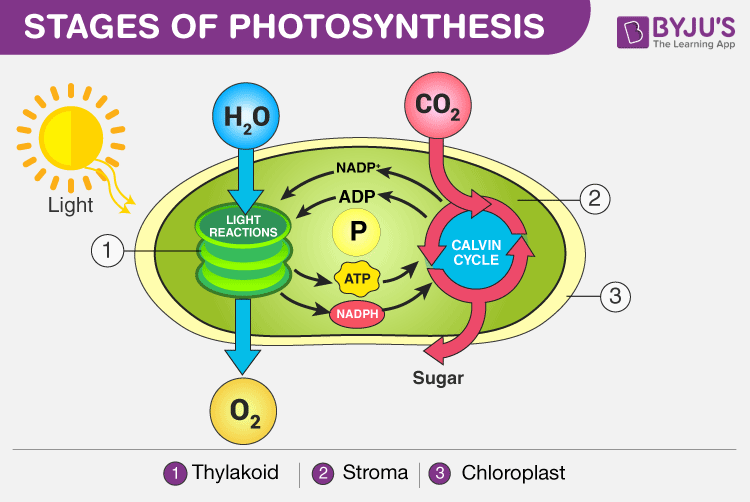
Stages of Photosynthesis in Plants depicting the two phases – Light reaction and Dark reaction
Light Reaction of Photosynthesis (or) Light-dependent Reaction
- Photosynthesis begins with the light reaction which is carried out only during the day in the presence of sunlight. In plants, the light-dependent reaction takes place in the thylakoid membranes of chloroplasts.
- The Grana, membrane-bound sacs like structures present inside the thylakoid functions by gathering light and is called photosystems.
- These photosystems have large complexes of pigment and proteins molecules present within the plant cells, which play the primary role during the process of light reactions of photosynthesis.
- There are two types of photosystems: photosystem I and photosystem II.
- Under the light-dependent reactions, the light energy is converted to ATP and NADPH, which are used in the second phase of photosynthesis.
- During the light reactions, ATP and NADPH are generated by two electron-transport chains, water is used and oxygen is produced.
The chemical equation in the light reaction of photosynthesis can be reduced to:
2H 2 O + 2NADP+ + 3ADP + 3Pi → O 2 + 2NADPH + 3ATP
Dark Reaction of Photosynthesis (or) Light-independent Reaction
- Dark reaction is also called carbon-fixing reaction.
- It is a light-independent process in which sugar molecules are formed from the water and carbon dioxide molecules.
- The dark reaction occurs in the stroma of the chloroplast where they utilize the NADPH and ATP products of the light reaction.
- Plants capture the carbon dioxide from the atmosphere through stomata and proceed to the Calvin photosynthesis cycle.
- In the Calvin cycle , the ATP and NADPH formed during light reaction drive the reaction and convert 6 molecules of carbon dioxide into one sugar molecule or glucose.
The chemical equation for the dark reaction can be reduced to:
3CO 2 + 6 NADPH + 5H 2 O + 9ATP → G3P + 2H+ + 6 NADP+ + 9 ADP + 8 Pi
* G3P – glyceraldehyde-3-phosphate
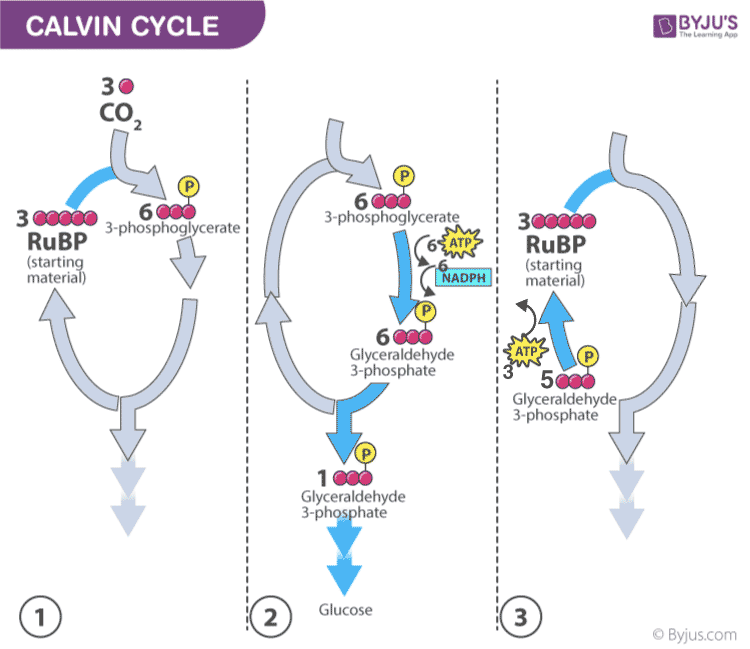
Calvin photosynthesis Cycle (Dark Reaction)
Also Read: Cyclic And Non-Cyclic Photophosphorylation
Importance of Photosynthesis
- Photosynthesis is essential for the existence of all life on earth. It serves a crucial role in the food chain – the plants create their food using this process, thereby, forming the primary producers.
- Photosynthesis is also responsible for the production of oxygen – which is needed by most organisms for their survival.
Frequently Asked Questions
1. what is photosynthesis explain the process of photosynthesis., 2. what is the significance of photosynthesis, 3. list out the factors influencing photosynthesis., 4. what are the different stages of photosynthesis, 5. what is the calvin cycle, 6. write down the photosynthesis equation..

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Biology related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
very useful
It’s very helpful ☺️
Please What Is Meant By 300-400 PPM
PPM stands for Parts-Per-Million. It corresponds to saying that 300 PPM of carbon dioxide indicates that if one million gas molecules are counted, 300 out of them would be carbon dioxide. The remaining nine hundred ninety-nine thousand seven hundred are other gas molecules.
Thank you very much Byju’s! I couldn’t find the answer anywhere. But luckily I hit upon this website. Awesome explanation and illustration.
byjus = Wow!
It helps me a lot thank you
Thanks in a million I love Byjus!
Super Byjus
Thanks helped a lot
Very interesting and helpful site.
Nice it is very uesful
It’s very useful 👍 Thank you Byju’s
Thank you very much Byju’s! I couldn’t find the answer anywhere. But luckily I hit upon this website. Awesome explanation and illustration.
Thank you BYJU’S for helping me in further clarifying my concepts
Excellent material easy to understand
Indeed, it’s precise and understandable. I like it.
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

- Next Article
Cover Image

- PDF Icon PDF Link Table of Contents
- PDF Icon PDF Link Editorial Board
An overview of photosynthesis
How the photosystems work, other electron transfer chain components, abbreviations, competing interests, recommended reading and key publications, photosynthesis.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- Open the PDF for in another window
- Cite Icon Cite
- Get Permissions
Matthew P. Johnson; Photosynthesis. Essays Biochem 31 October 2016; 60 (3): 255–273. doi: https://doi.org/10.1042/EBC20160016
Download citation file:
- Ris (Zotero)
- Reference Manager
Photosynthesis sustains virtually all life on planet Earth providing the oxygen we breathe and the food we eat; it forms the basis of global food chains and meets the majority of humankind's current energy needs through fossilized photosynthetic fuels. The process of photosynthesis in plants is based on two reactions that are carried out by separate parts of the chloroplast. The light reactions occur in the chloroplast thylakoid membrane and involve the splitting of water into oxygen, protons and electrons. The protons and electrons are then transferred through the thylakoid membrane to create the energy storage molecules adenosine triphosphate (ATP) and nicotinomide–adenine dinucleotide phosphate (NADPH). The ATP and NADPH are then utilized by the enzymes of the Calvin–Benson cycle (the dark reactions), which converts CO 2 into carbohydrate in the chloroplast stroma. The basic principles of solar energy capture, energy, electron and proton transfer and the biochemical basis of carbon fixation are explained and their significance is discussed.
Introduction
Photosynthesis is the ultimate source of all of humankind's food and oxygen, whereas fossilized photosynthetic fuels provide ∼87% of the world's energy. It is the biochemical process that sustains the biosphere as the basis for the food chain. The oxygen produced as a by-product of photosynthesis allowed the formation of the ozone layer, the evolution of aerobic respiration and thus complex multicellular life.
Oxygenic photosynthesis involves the conversion of water and CO 2 into complex organic molecules such as carbohydrates and oxygen. Photosynthesis may be split into the ‘light’ and ‘dark’ reactions. In the light reactions, water is split using light into oxygen, protons and electrons, and in the dark reactions, the protons and electrons are used to reduce CO 2 to carbohydrate (given here by the general formula CH 2 O). The two processes can be summarized thus:
Light reactions:

Dark reactions:

The positive sign of the standard free energy change of the reaction (Δ G °) given above means that the reaction requires energy ( an endergonic reaction ). The energy required is provided by absorbed solar energy, which is converted into the chemical bond energy of the products ( Box 1 ).

Photosynthesis converts ∼200 billion tonnes of CO 2 into complex organic compounds annually and produces ∼140 billion tonnes of oxygen into the atmosphere. By facilitating conversion of solar energy into chemical energy, photosynthesis acts as the primary energy input into the global food chain. Nearly all living organisms use the complex organic compounds derived from photosynthesis as a source of energy. The breakdown of these organic compounds occurs via the process of aerobic respiration, which of course also requires the oxygen produced by photosynthesis.

Unlike photosynthesis, aerobic respiration is an exergonic process (negative Δ G °) with the energy released being used by the organism to power biosynthetic processes that allow growth and renewal, mechanical work (such as muscle contraction or flagella rotation) and facilitating changes in chemical concentrations within the cell (e.g. accumulation of nutrients and expulsion of waste). The use of exergonic reactions to power endergonic ones associated with biosynthesis and housekeeping in biological organisms such that the overall free energy change is negative is known as ‘ coupling’.
Photosynthesis and respiration are thus seemingly the reverse of one another, with the important caveat that both oxygen formation during photosynthesis and its utilization during respiration result in its liberation or incorporation respectively into water rather than CO 2 . In addition, glucose is one of several possible products of photosynthesis with amino acids and lipids also being synthesized rapidly from the primary photosynthetic products.
The consideration of photosynthesis and respiration as opposing processes helps us to appreciate their role in shaping our environment. The fixation of CO 2 by photosynthesis and its release during breakdown of organic molecules during respiration, decay and combustion of organic matter and fossil fuels can be visualized as the global carbon cycle ( Figure 1 ).
The global carbon cycle

The relationship between respiration, photosynthesis and global CO 2 and O 2 levels.
At present, this cycle may be considered to be in a state of imbalance due to the burning of fossil fuels (fossilized photosynthesis), which is increasing the proportion of CO 2 entering the Earth's atmosphere, leading to the so-called ‘greenhouse effect’ and human-made climate change.
Oxygenic photosynthesis is thought to have evolved only once during Earth's history in the cyanobacteria. All other organisms, such as plants, algae and diatoms, which perform oxygenic photosynthesis actually do so via cyanobacterial endosymbionts or ‘chloroplasts’. An endosymbiotoic event between an ancestral eukaryotic cell and a cyanobacterium that gave rise to plants is estimated to have occurred ∼1.5 billion years ago. Free-living cyanobacteria still exist today and are responsible for ∼50% of the world's photosynthesis. Cyanobacteria themselves are thought to have evolved from simpler photosynthetic bacteria that use either organic or inorganic compounds such a hydrogen sulfide as a source of electrons rather than water and thus do not produce oxygen.
The site of photosynthesis in plants
In land plants, the principal organs of photosynthesis are the leaves ( Figure 2 A). Leaves have evolved to expose the largest possible area of green tissue to light and entry of CO 2 to the leaf is controlled by small holes in the lower epidermis called stomata ( Figure 2 B). The size of the stomatal openings is variable and regulated by a pair of guard cells, which respond to the turgor pressure (water content) of the leaf, thus when the leaf is hydrated, the stomata can open to allow CO 2 in. In contrast, when water is scarce, the guard cells lose turgor pressure and close, preventing the escape of water from the leaf via transpiration.
Location of the photosynthetic machinery

( A ) The model plant Arabidopsis thaliana . ( B ) Basic structure of a leaf shown in cross-section. Chloroplasts are shown as green dots within the cells. ( C ) An electron micrograph of an Arabidopsis chloroplast within the leaf. ( D ) Close-up region of the chloroplast showing the stacked structure of the thylakoid membrane.
Within the green tissue of the leaf (mainly the mesophyll) each cell (∼100 μm in length) contains ∼100 chloroplasts (2–3 μm in length), the tiny organelles where photosynthesis takes place. The chloroplast has a complex structure ( Figure 2 C, D) with two outer membranes (the envelope), which are colourless and do not participate in photosynthesis, enclosing an aqueous space (the stroma) wherein sits a third membrane known as the thylakoid, which in turn encloses a single continuous aqueous space called the lumen.
The light reactions of photosynthesis involve light-driven electron and proton transfers, which occur in the thylakoid membrane, whereas the dark reactions involve the fixation of CO 2 into carbohydrate, via the Calvin–Benson cycle, which occurs in the stroma ( Figure 3 ). The light reactions involve electron transfer from water to NADP + to form NADPH and these reactions are coupled to proton transfers that lead to the phosphorylation of adenosine diphosphate (ADP) into ATP. The Calvin–Benson cycle uses ATP and NADPH to convert CO 2 into carbohydrates ( Figure 3 ), regenerating ADP and NADP + . The light and dark reactions are therefore mutually dependent on one another.
Division of labour within the chloroplast

The light reactions of photosynthesis take place in the thylakoid membrane, whereas the dark reactions are located in the chloroplast stroma.
Photosynthetic electron and proton transfer chain
The light-driven electron transfer reactions of photosynthesis begin with the splitting of water by Photosystem II (PSII). PSII is a chlorophyll–protein complex embedded in the thylakoid membrane that uses light to oxidize water to oxygen and reduce the electron acceptor plastoquinone to plastoquinol. Plastoquinol in turn carries the electrons derived from water to another thylakoid-embedded protein complex called cytochrome b 6 f (cyt b 6 f ). cyt b 6 f oxidizes plastoquinol to plastoquinone and reduces a small water-soluble electron carrier protein plastocyanin, which resides in the lumen. A second light-driven reaction is then carried out by another chlorophyll protein complex called Photosystem I (PSI). PSI oxidizes plastocyanin and reduces another soluble electron carrier protein ferredoxin that resides in the stroma. Ferredoxin can then be used by the ferredoxin–NADP + reductase (FNR) enzyme to reduce NADP + to NADPH. This scheme is known as the linear electron transfer pathway or Z-scheme ( Figure 4 ).
The photosynthetic electron and proton transfer chain

The linear electron transfer pathway from water to NADP + to form NADPH results in the formation of a proton gradient across the thylakoid membrane that is used by the ATP synthase enzyme to make ATP.
The Z-scheme, so-called since it resembles the letter ‘Z’ when turned on its side ( Figure 5 ), thus shows how the electrons move from the water–oxygen couple (+820 mV) via a chain of redox carriers to NADP + /NADPH (−320 mV) during photosynthetic electron transfer. Generally, electrons are transferred from redox couples with low potentials (good reductants) to those with higher potentials (good oxidants) (e.g. during respiratory electron transfer in mitochondria) since this process is exergonic (see Box 2 ). However, photosynthetic electron transfer also involves two endergonic steps, which occur at PSII and at PSI and require an energy input in the form of light. The light energy is used to excite an electron within a chlorophyll molecule residing in PSII or PSI to a higher energy level; this excited chlorophyll is then able to reduce the subsequent acceptors in the chain. The oxidized chlorophyll is then reduced by water in the case of PSII and plastocyanin in the case of PSI.
Z-scheme of photosynthetic electron transfer

The main components of the linear electron transfer pathway are shown on a scale of redox potential to illustrate how two separate inputs of light energy at PSI and PSII result in the endergonic transfer of electrons from water to NADP + .
The water-splitting reaction at PSII and plastoquinol oxidation at cyt b 6 f result in the release of protons into the lumen, resulting in a build-up of protons in this compartment relative to the stroma. The difference in the proton concentration between the two sides of the membrane is called a proton gradient. The proton gradient is a store of free energy (similar to a gradient of ions in a battery) that is utilized by a molecular mechanical motor ATP synthase, which resides in the thylakoid membrane ( Figure 4 ). The ATP synthase allows the protons to move down their concentration gradient from the lumen (high H + concentration) to the stroma (low H + concentration). This exergonic reaction is used to power the endergonic synthesis of ATP from ADP and inorganic phosphate (P i ). This process of photophosphorylation is thus essentially similar to oxidative phosphorylation, which occurs in the inner mitochondrial membrane during respiration.
An alternative electron transfer pathway exists in plants and algae, known as cyclic electron flow. Cyclic electron flow involves the recycling of electrons from ferredoxin to plastoquinone, with the result that there is no net production of NADPH; however, since protons are still transferred into the lumen by oxidation of plastoquinol by cyt b 6 f , ATP can still be formed. Thus photosynthetic organisms can control the ratio of NADPH/ATP to meet metabolic need by controlling the relative amounts of cyclic and linear electron transfer.

Light absorption by pigments
Photosynthesis begins with the absorption of light by pigments molecules located in the thylakoid membrane. The most well-known of these is chlorophyll, but there are also carotenoids and, in cyanobacteria and some algae, bilins. These pigments all have in common within their chemical structures an alternating series of carbon single and double bonds, which form a conjugated system π–electron system ( Figure 6 ).
Major photosynthetic pigments in plants

The chemical structures of the chlorophyll and carotenoid pigments present in the thylakoid membrane. Note the presence in each of a conjugated system of carbon–carbon double bonds that is responsible for light absorption.
The variety of pigments present within each type of photosynthetic organism reflects the light environment in which it lives; plants on land contain chlorophylls a and b and carotenoids such as β-carotene, lutein, zeaxanthin, violaxanthin, antheraxanthin and neoxanthin ( Figure 6 ). The chlorophylls absorb blue and red light and so appear green in colour, whereas carotenoids absorb light only in the blue and so appear yellow/red ( Figure 7 ), colours more obvious in the autumn as chlorophyll is the first pigment to be broken down in decaying leaves.
Basic absorption spectra of the major chlorophyll and carotenoid pigments found in plants

Chlorophylls absorb light energy in the red and blue part of the visible spectrum, whereas carotenoids only absorb light in the blue/green.
Light, or electromagnetic radiation, has the properties of both a wave and a stream of particles (light quanta). Each quantum of light contains a discrete amount of energy that can be calculated by multiplying Planck's constant, h (6.626×10 −34 J·s) by ν, the frequency of the radiation in cycles per second (s −1 ):

The frequency (ν) of the light and so its energy varies with its colour, thus blue photons (∼450 nm) are more energetic than red photons (∼650 nm). The frequency (ν) and wavelength (λ) of light are related by:

where c is the velocity of light (3.0×10 8 m·s −1 ), and the energy of a particular wavelength (λ) of light is given by:

Thus 1 mol of 680 nm photons of red light has an energy of 176 kJ·mol −1 .
The electrons within the delocalized π system of the pigment have the ability to jump up from the lowest occupied molecular orbital (ground state) to higher unoccupied molecular electron orbitals (excited states) via the absorption of specific wavelengths of light in the visible range (400–725 nm). Chlorophyll has two excited states known as S 1 and S 2 and, upon interaction of the molecule with a photon of light, one of its π electrons is promoted from the ground state (S 0 ) to an excited state, a process taking just 10 −15 s ( Figure 8 ). The energy gap between the S 0 and S 1 states is spanned by the energy provided by a red photon (∼600–700 nm), whereas the energy gap between the S 0 and S 2 states is larger and therefore requires a more energetic (shorter wavelength, higher frequency) blue photon (∼400–500 nm) to span the energy gap.
Jablonski diagram of chlorophyll showing the possible fates of the S 1 and S 2 excited states and timescales of the transitions involved

Photons with slightly different energies (colours) excite each of the vibrational substates of each excited state (as shown by variation in the size and colour of the arrows).
Upon excitation, the electron in the S 2 state quickly undergoes losses of energy as heat through molecular vibration and undergoes conversion into the energy of the S 1 state by a process called internal conversion. Internal conversion occurs on a timescale of 10 −12 s. The energy of a blue photon is thus rapidly degraded to that of a red photon. Excitation of the molecule with a red photon would lead to promotion of an electron to the S 1 state directly. Once the electron resides in the S 1 state, it is lower in energy and thus stable on a somewhat longer timescale (10 −9 s). The energy of the excited electron in the S 1 state can have one of several fates: it could return to the ground state (S 0 ) by emission of the energy as a photon of light (fluorescence), or it could be lost as heat due to internal conversion between S 1 and S 0 . Alternatively, if another chlorophyll is nearby, a process known as excitation energy transfer (EET) can result in the non-radiative exchange of energy between the two molecules ( Figure 9 ). For this to occur, the two chlorophylls must be close by (<7 nm), have a specific orientation with respect to one another, and excited state energies that overlap (are resonant) with one another. If these conditions are met, the energy is exchanged, resulting in a mirror S 0 →S 1 transition in the acceptor molecule and a S 1 →S 0 transition in the other.
Basic mechanism of excitation energy transfer between chlorophyll molecules

Two chlorophyll molecules with resonant S 1 states undergo a mirror transition resulting in the non-radiative transfer of excitation energy between them.
Light-harvesting complexes
In photosynthetic systems, chlorophylls and carotenoids are found attached to membrane-embedded proteins known as light-harvesting complexes (LHCs). Through careful binding and orientation of the pigment molecules, absorbed energy can be transferred among them by EET. Each pigment is bound to the protein by a series of non-covalent bonding interactions (such as, hydrogen bonds, van der Waals interactions, hydrophobic interaction and co-ordination bonds between lone pair electrons of residues such as histidine in the protein and the Mg 2+ ion in chlorophyll); the protein structure is such that each bound pigment experiences a slightly different environment in terms of the surrounding amino acid side chains, lipids, etc., meaning that the S 1 and S 2 energy levels are shifted in energy with respect to that of other neighbouring pigment molecules. The effect is to create a range of pigment energies that act to ‘funnel’ the energy on to the lowest-energy pigments in the LHC by EET.
Reaction centres
A photosystem consists of numerous LHCs that form an antenna of hundreds of pigment molecules. The antenna pigments act to collect and concentrate excitation energy and transfer it towards a ‘special pair’ of chlorophyll molecules that reside in the reaction centre (RC) ( Figure 10 ). Unlike the antenna pigments, the special pair of chlorophylls are ‘redox-active’ in the sense that they can return to the ground state (S 0 ) by the transfer of the electron residing in the S 1 excited state (Chl*) to another species. This process is known as charge separation and result in formation of an oxidized special pair (Chl + ) and a reduced acceptor (A − ). The acceptor in PSII is plastoquinone and in PSI it is ferredoxin. If the RC is to go on functioning, the electron deficiency on the special pair must be made good, in PSII the electron donor is water and in PSI it is plastocyanin.
Basic structure of a photosystem

Light energy is captured by the antenna pigments and transferred to the special pair of RC chlorophylls which undergo a redox reaction leading to reduction of an acceptor molecule. The oxidized special pair is regenerated by an electron donor.
It is worth asking why photosynthetic organisms bother to have a large antenna of pigments serving an RC rather than more numerous RCs. The answer lies in the fact that the special pair of chlorophylls alone have a rather small spatial and spectral cross-section, meaning that there is a limit to the amount of light they can efficiently absorb. The amount of light they can practically absorb is around two orders of magnitude smaller than their maximum possible turnover rate, Thus LHCs act to increase the spatial (hundreds of pigments) and spectral (several types of pigments with different light absorption characteristics) cross-section of the RC special pair ensuring that its turnover rate runs much closer to capacity.
Photosystem II
PSII is a light-driven water–plastoquinone oxidoreductase and is the only enzyme in Nature that is capable of performing the difficult chemistry of splitting water into protons, electrons and oxygen ( Figure 11 ). In principle, water is an extremely poor electron donor since the redox potential of the water–oxygen couple is +820 mV. PSII uses light energy to excite a special pair of chlorophylls, known as P680 due to their 680 nm absorption peak in the red part of the spectrum. P680* undergoes charge separation that results in the formation of an extremely oxidizing species P680 + which has a redox potential of +1200 mV, sufficient to oxidize water. Nonetheless, since water splitting involves four electron chemistry and charge separation only involves transfer of one electron, four separate charge separations (turnovers of PSII) are required to drive formation of one molecule of O 2 from two molecules of water. The initial electron donation to generate the P680 from P680 + is therefore provided by a cluster of manganese ions within the oxygen-evolving complex (OEC), which is attached to the lumen side of PSII ( Figure 12 ). Manganese is a transition metal that can exist in a range of oxidation states from +1 to +5 and thus accumulates the positive charges derived from each light-driven turnover of P680. Progressive extraction of electrons from the manganese cluster is driven by the oxidation of P680 within PSII by light and is known as the S-state cycle ( Figure 12 ). After the fourth turnover of P680, sufficient positive charge is built up in the manganese cluster to permit the splitting of water into electrons, which regenerate the original state of the manganese cluster, protons, which are released into the lumen and contribute to the proton gradient used for ATP synthesis, and the by-product O 2 . Thus charge separation at P680 provides the thermodynamic driving force, whereas the manganese cluster acts as a catalyst for the water-splitting reaction.
Basic structure of the PSII–LHCII supercomplex from spinach

The organization of PSII and its light-harvesting antenna. Protein is shown in grey, with chlorophylls in green and carotenoids in orange. Drawn from PDB code 3JCU
S-state cycle of water oxidation by the manganese cluster (shown as circles with roman numerals representing the manganese ion oxidation states) within the PSII oxygen-evolving complex

Progressive extraction of electrons from the manganese cluster is driven by the oxidation of P680 within PSII by light. Each of the electrons given up by the cluster is eventually repaid at the S 4 to S 0 transition when molecular oxygen (O 2 ) is formed. The protons extracted from water during the process are deposited into the lumen and contribute to the protonmotive force.
The electrons yielded by P680* following charge separation are not passed directly to plastoquinone, but rather via another acceptor called pheophytin, a porphyrin molecule lacking the central magnesium ion as in chlorophyll. Plastoquinone reduction to plastoquinol requires two electrons and thus two molecules of plastoquinol are formed per O 2 molecule evolved by PSII. Two protons are also taken up upon formation of plastoquinol and these are derived from the stroma. PSII is found within the thylakoid membrane of plants as a dimeric RC complex surrounded by a peripheral antenna of six minor monomeric antenna LHC complexes and two to eight trimeric LHC complexes, which together form a PSII–LHCII supercomplex ( Figure 11 ).
Photosystem I
PSI is a light-driven plastocyanin–ferredoxin oxidoreductase ( Figure 13 ). In PSI, the special pair of chlorophylls are known as P700 due to their 700 nm absorption peak in the red part of the spectrum. P700* is an extremely strong reductant that is able to reduce ferredoxin which has a redox potential of −450 mV (and is thus is, in principle, a poor electron acceptor). Reduced ferredoxin is then used to generate NADPH for the Calvin–Benson cycle at a separate complex known as FNR. The electron from P700* is donated via another chlorophyll molecule and a bound quinone to a series of iron–sulfur clusters at the stromal side of the complex, whereupon the electron is donated to ferredoxin. The P700 species is regenerated form P700 + via donation of an electron from the soluble electron carrier protein plastocyanin.
Basic structure of the PSI–LHCI supercomplex from pea

The organization of PSI and its light-harvesting antenna. Protein is shown in grey, with chlorophylls in green and carotenoids in orange. Drawn from PDB code 4XK8.
PSI is found within the thylakoid membrane as a monomeric RC surrounded on one side by four LHC complexes known as LHCI. The PSI–LHCI supercomplex is found mainly in the unstacked regions of the thylakoid membrane ( Figure 13 ).
Plastoquinone/plastoquinol
Plastoquinone is a small lipophilic electron carrier molecule that resides within the thylakoid membrane and carries two electrons and two protons from PSII to the cyt b 6 f complex. It has a very similar structure to that of the molecule ubiquinone (coenzyme Q 10 ) in the mitochondrial inner membrane.
Cytochrome b 6 f complex
The cyt b 6 f complex is a plastoquinol–plastocyanin oxidoreductase and possess a similar structure to that of the cytochrome bc 1 complex (complex III) in mitochondria ( Figure 14 A). As with Complex III, cyt b 6 f exists as a dimer in the membrane and carries out both the oxidation and reduction of quinones via the so-called Q-cycle. The Q-cycle ( Figure 14 B) involves oxidation of one plastoquinol molecule at the Qp site of the complex, both protons from this molecule are deposited in the lumen and contribute to the proton gradient for ATP synthesis. The two electrons, however, have different fates. The first is transferred via an iron–sulfur cluster and a haem cofactor to the soluble electron carrier plastocyanin (see below). The second electron derived from plastoquinol is passed via two separate haem cofactors to another molecule of plastoquinone bound to a separate site (Qn) on the complex, thus reducing it to a semiquinone. When a second plastoquinol molecule is oxidized at Qp, a second molecule of plastocyanin is reduced and two further protons are deposited in the lumen. The second electron reduces the semiquinone at the Qn site which, concomitant with uptake of two protons from the stroma, causes its reduction to plastoquinol. Thus for each pair of plastoquinol molecules oxidized by the complex, one is regenerated, yet all four protons are deposited into the lumen. The Q-cycle thus doubles the number of protons transferred from the stroma to the lumen per plastoquinol molecule oxidized.

( A ) Structure drawn from PDB code 1Q90. ( B ) The protonmotive Q-cycle showing how electrons from plastoquinol are passed to both plastocyanin and plastoquinone, doubling the protons deposited in the lumen for every plastoquinol molecule oxidized by the complex.
Plastocyanin
Plastocyanin is a small soluble electron carrier protein that resides in the thylakoid lumen. The active site of the plastocyanin protein binds a copper ion, which cycles between the Cu 2+ and Cu + oxidation states following its oxidation by PSI and reduction by cyt b 6 f respectively.
Ferredoxin is a small soluble electron carrier protein that resides in the chloroplast stroma. The active site of the ferredoxin protein binds an iron–sulfur cluster, which cycles between the Fe 2+ and Fe 3+ oxidation states following its reduction by PSI and oxidation by the FNR complex respectively.
Ferredoxin–NADP + reductase
The FNR complex is found in both soluble and thylakoid membrane-bound forms. The complex binds a flavin–adenine dinucleotide (FAD) cofactor at its active site, which accepts two electrons from two molecules of ferredoxin before using them reduce NADP + to NADPH.
ATP synthase
The ATP synthase enzyme is responsible for making ATP from ADP and P i ; this endergonic reaction is powered by the energy contained within the protonmotive force. According to the structure, 4.67 H + are required for every ATP molecule synthesized by the chloroplast ATP synthase. The enzyme is a rotary motor which contains two domains: the membrane-spanning F O portion which conducts protons from the lumen to the stroma, and the F 1 catalytic domain that couples this exergonic proton movement to ATP synthesis.
Membrane stacking and the regulation of photosynthesis
Within the thylakoid membrane, PSII–LHCII supercomplexes are packed together into domains known as the grana, which associate with one another to form grana stacks. PSI and ATP synthase are excluded from these stacked PSII–LHCII regions by steric constraints and thus PSII and PSI are segregated in the thylakoid membrane between the stacked and unstacked regions ( Figure 15 ). The cyt b 6 f complex, in contrast, is evenly distributed throughout the grana and stromal lamellae. The evolutionary advantage of membrane stacking is believed to be a higher efficiency of electron transport by preventing the fast energy trap PSI from ‘stealing’ excitation energy from the slower trap PSII, a phenomenon known as spillover. Another possible advantage of membrane stacking in thylakoids may be the segregation of the linear and cyclic electron transfer pathways, which might otherwise compete to reduce plastoquinone. In this view, PSII, cyt b 6 f and a sub-fraction of PSI closest to the grana is involved in linear flow, whereas PSI and cyt b 6 f in the stromal lamellae participates in cyclic flow. The cyclic electron transfer pathway recycles electrons from ferredoxin back to plastoquinone and thus allows protonmotive force generation (and ATP synthesis) without net NADPH production. Cyclic electron transfer thereby provides the additional ATP required for the Calvin–Benson cycle (see below).
Lateral heterogeneity in thylakoid membrane organization

( A ) Electron micrograph of the thylakoid membrane showing stacked grana and unstacked stromal lamellae regions. ( B ) Model showing the distribution of the major complexes of photosynthetic electron and proton transfer between the stacked grana and unstacked stromal lamellae regions.
‘Dark’ reactions: the Calvin–Benson cycle
CO 2 is fixed into carbohydrate via the Calvin–Benson cycle in plants, which consumes the ATP and NADPH produced during the light reactions and thus in turn regenerates ADP, P i and NADP + . In the first step of the Calvin–Benson cycle ( Figure 16 ), CO 2 is combined with a 5-carbon (5C) sugar, ribulose 1,5-bisphosphate in a reaction catalysed by the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco). The reaction forms an unstable 6C intermediate that immediately splits into two molecules of 3-phosphoglycerate. 3-Phosphoglycerate is first phosphorylated by 3-phosphoglycerate kinase using ATP to form 1,3-bisphosphoglycerate. 1,3-Bisphosphoglycerate is then reduced by glyceraldehyde 3-phosphate dehydrogenase using NADPH to form glyceraldehyde 3-phosphate (GAP, a triose or 3C sugar) in reactions, which are the reverse of glycolysis. For every three CO 2 molecules initially combined with ribulose 1,5-bisphopshate, six molecules of GAP are produced by the subsequent steps. However only one of these six molecules can be considered as a product of the Calvin–Benson cycle since the remaining five are required to regenerate ribulose 1,5-bisphosphate in a complex series of reactions that also require ATP. The one molecule of GAP that is produced for each turn of the cycle can be quickly converted by a range of metabolic pathways into amino acids, lipids or sugars such as glucose. Glucose in turn may be stored as the polymer starch as large granules within chloroplasts.
The Calvin–Benson cycle

Overview of the biochemical pathway for the fixation of CO 2 into carbohydrate in plants.
A complex biochemical ‘dance’ ( Figure 16 ) is then involved in the regeneration of three ribulose 1,5-bisphosphate (5C) from the remaining five GAP (3C) molecules. The regeneration begins with the conversion of two molecules of GAP into dihydroxyacetone phosphate (DHAP) by triose phosphate isomerase; one of the DHAP molecules is the combined with another GAP molecule to make fructose 1,6-bisphosphate (6C) by aldolase. The fructose 1,6-bisphosphate is then dephosphorylated by fructose-1,6-bisphosphatase to yield fructose 6-phosphate (6C) and releasing P i . Two carbons are then removed from fructose 6-phosphate by transketolase, generating erythrose 4-phosphate (4C); the two carbons are transferred to another molecule of GAP generating xylulose 5-phosphate (5C). Another DHAP molecule, formed from GAP by triose phosphate isomerase is then combined with the erythrose 4-phosphate by aldolase to form sedoheptulose 1,7-bisphosphate (7C). Sedoheptulose 1,7-bisphosphate is then dephosphorylated to sedoheptulose 7-phosphate (7C) by sedoheptulose-1,7-bisphosphatase releasing P i . Sedoheptulose 7-phosphate has two carbons removed by transketolase to produce ribose 5-phosphate (5C) and the two carbons are transferred to another GAP molecule producing another xylulose 5-phosphate (5C). Ribose 5-phosphate and the two molecules of xylulose 5-phosphate (5C) are then converted by phosphopentose isomerase to three molecules of ribulose 5-phosphate (5C). The three ribulose 5-phosphate molecules are then phosphorylated using three ATP by phosphoribulokinase to regenerate three ribulose 1,5-bisphosphate (5C).
Overall the synthesis of 1 mol of GAP requires 9 mol of ATP and 6 mol of NADPH, a required ratio of 1.5 ATP/NADPH. Linear electron transfer is generally thought to supply ATP/NADPH in a ratio of 1.28 (assuming an H + /ATP ratio of 4.67) with the shortfall of ATP believed to be provided by cyclic electron transfer reactions. Since the product of the Calvin cycle is GAP (a 3C sugar) the pathway is often referred to as C 3 photosynthesis and plants that utilize it are called C 3 plants and include many of the world's major crops such as rice, wheat and potato.
Many of the enzymes involved in the Calvin–Benson cycle (e.g. transketolase, glyceraldehyde-3-phosphate dehydrogenase and aldolase) are also involved in the glycolysis pathway of carbohydrate degradation and their activity must therefore be carefully regulated to avoid futile cycling when light is present, i.e. the unwanted degradation of carbohydrate. The regulation of the Calvin–Benson cycle enzymes is achieved by the activity of the light reactions, which modify the environment of the dark reactions (i.e. the stroma). Proton gradient formation across the thylakoid membrane during the light reactions increases the pH and also increases the Mg 2+ concentration in the stroma (as Mg 2+ flows out of the lumen as H + flows in to compensate for the influx of positive charges). In addition, by reducing ferredoxin and NADP + , PSI changes the redox state of the stroma, which is sensed by the regulatory protein thioredoxin. Thioredoxin, pH and Mg 2+ concentration play a key role in regulating the activity of the Calvin–Benson cycle enzymes, ensuring the activity of the light and dark reactions is closely co-ordinated.
It is noteworthy that, despite the complexity of the dark reactions outlined above, the carbon fixation step itself (i.e. the incorporation of CO 2 into carbohydrate) is carried out by a single enzyme, Rubisco. Rubisco is a large multisubunit soluble protein complex found in the chloroplast stroma. The complex consists of eight large (56 kDa) subunits, which contain both catalytic and regulatory domains, and eight small subunits (14 kDa), which enhance the catalytic function of the L subunits ( Figure 17 A). The carboxylation reaction carried out by Rubisco is highly exergonic (Δ G °=−51.9 kJ·mol- 1 ), yet kinetically very slow (just 3 s −1 ) and begins with the protonation of ribulose 1,5-bisphosphate to form an enediolate intermediate which can be combined with CO 2 to form an unstable 6C intermediate that is quickly hydrolysed to yield two 3C 3-phosphoglycerate molecules. The active site in the Rubisco enzyme contains a key lysine residue, which reacts with another (non-substrate) molecule of CO 2 to form a carbamate anion that is then able to bind Mg 2+ . The Mg 2+ in the active site is essential for the catalytic function of Rubisco, playing a key role in binding ribulose 1,5-bisphosphate and activating it such that it readily reacts with CO 2.. Rubisco activity is co-ordinated with that of the light reactions since carbamate formation requires both high Mg 2+ concentration and alkaline conditions, which are provided by the light-driven changes in the stromal environment discussed above ( Figure 17 B).

( A ) Structure of the Rubisco enzyme (the large subunits are shown in blue and the small subunits in green); four of each type of subunit are visible in the image. Drawn from PDB code 1RXO. ( B ) Activation of the lysine residue within the active site of Rubisco occurs via elevated stromal pH and Mg 2+ concentration as a result of the activity of the light reactions.
In addition to carboxylation, Rubisco also catalyses a competitive oxygenation reaction, known as photorespiration, that results in the combination of ribulose 1,5-bisphosphate with O 2 rather than CO 2 . In the oxygenation reaction, one rather than two molecules of 3-phosphoglycerate and one molecule of a 2C sugar known as phosphoglycolate are produced by Rubisco. The phosphoglycolate must be converted in a series of reactions that regenerate one molecule of 3-phosphoglycerate and one molecule of CO 2 . These reactions consume additional ATP and thus result in an energy loss to the plant. Although the oxygenation reaction of Rubisco is much less favourable than the carboxylation reaction, the relatively high concentration of O 2 in the leaf (250 μM) compared with CO 2 (10 μM) means that a significant amount of photorespiration is always occurring. Under normal conditions, the ratio of carboxylation to oxygenation is between 3:1 and 4:1. However, this ratio can be decreased with increasing temperature due to decreased CO 2 concentration in the leaf, a decrease in the affinity of Rubisco for CO 2 compared with O 2 and an increase in the maximum rate of the oxygenation reaction compared with the carboxylation reaction. The inefficiencies of the Rubisco enzyme mean that plants must produce it in very large amounts (∼30–50% of total soluble protein in a spinach leaf) to achieve the maximal photosynthetic rate.
CO 2 -concentrating mechanisms
To counter photorespiration, plants, algae and cyanobacteria have evolved different CO 2 -concentrating mechanisms CCMs that aim to increase the concentration of CO 2 relative to O 2 in the vicinity of Rubisco. One such CCM is C 4 photosynthesis that is found in plants such as maize, sugar cane and savanna grasses. C 4 plants show a specialized leaf anatomy: Kranz anatomy ( Figure 18 ). Kranz, German for wreath, refers to a bundle sheath of cells that surrounds the central vein within the leaf, which in turn are surrounded by the mesophyll cells. The mesophyll cells in such leaves are rich in the enzyme phosphoenolpyruvate (PEP) carboxylase, which fixes CO 2 into a 4C carboxylic acid: oxaloaceatate. The oxaloacetate formed by the mesophyll cells is reduced using NADPH to malate, another 4C acid: malate. The malate is then exported from the mesophyll cells to the bundle sheath cells, where it is decarboxylated to pyruvate thus regenerating NADPH and CO 2 . The CO 2 is then utilized by Rubisco in the Calvin cycle. The pyruvate is in turn returned to the mesophyll cells where it is phosphorylated using ATP to reform PEP ( Figure 19 ). The advantage of C 4 photosynthesis is that CO 2 accumulates at a very high concentration in the bundle sheath cells that is then sufficient to allow Rubisco to operate efficiently.
Diagram of a C 4 plant leaf showing Kranz anatomy

The C 4 pathway (NADP + –malic enzyme type) for fixation of CO 2

Plants growing in hot, bright and dry conditions inevitably have to have their stomata closed for large parts of the day to avoid excessive water loss and wilting. The net result is that the internal CO 2 concentration in the leaf is very low, meaning that C 3 photosynthesis is not possible. To counter this limitation, another CCM is found in succulent plants such as cacti. The Crassulaceae fix CO 2 into malate during the day via PEP carboxylase, store it within the vacuole of the plant cell at night and then release it within their tissues by day to be fixed via normal C 3 photosynthesis. This is termed crassulacean acid metabolism (CAM).
This article is a reviewed, revised and updated version of the following ‘Biochemistry Across the School Curriculum’ (BASC) booklet: Weaire, P.J. (1994) Photosynthesis . For further information and to provide feedback on this or any other Biochemical Society education resource, please contact [email protected]. For further information on other Biochemical Society publications, please visit www.biochemistry.org/publications .
adenosine diphosphate
adenosine triphosphate
carbohydrate
cytochrome b 6 f
dihydroxyacetone phosphate
excitation energy transfer
ferredoxin–NADP + reductase
glyceraldehyde 3-phosphate
light-harvesting complex
nicotinomide–adenine dinucleotide phosphate
phosphoenolpyruvate
inorganic phosphate
reaction centre
ribulose-1,5-bisphosphate carboxylase/oxygenase
I thank Professor Colin Osborne (University of Sheffield, Sheffield, U.K.) for useful discussions on the article, Dr Dan Canniffe (Penn State University, Pennsylvania, PA, U.S.A.) for providing pure pigment spectra and Dr P.J. Weaire (Kingston University, Kingston-upon-Thames, U.K.) for his original Photosynthesis BASC article (1994) on which this essay is partly based.
The Author declares that there are no competing interests associated with this article.
Get Email Alerts
- Online ISSN 1744-1358
- Print ISSN 0071-1365
- Submit Your Work
- Language-editing services
- Recommend to Your Librarian
- Accessibility
- Sign up for alerts
- Sign up to our mailing list
- Biochemical Society Membership
- Publishing Life Cycle
- Biochemical Society Events
- Sponsored award winners
- About Portland Press
- Portland Press Tel
- +44 (0)20 3880 2795
- Portland Press Company no. 02453983
- Biochemical Society Tel
- +44 (0)20 3880 2793
- Email: [email protected]
- Biochemical Society Company no. 00892796
- Registered Charity no. 253894
- VAT no. GB 523 2392 69
- Privacy and cookies
- © Copyright 2024 Portland Press
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Distance Learning
- Director's Circle
- Sustainability
- Smithsonian Science for the Classroom
- Smithsonian Science for Global Goals
- Smithsonian Science Stories
- STC Curriculum
- Explore Smithsonian
- Free Resources
- Smithsonian Science for Makerspaces
- Girls and Women in STEM
- Smithsonian Science for Computational Thinking
- Women in STEM eBook Series
- Space STEM Resources
- Space STEM Career Resources
- Smithsonian Science for NC and SC Classrooms
- Smithsonian Science Education Academies for Teachers
- Good Thinking!
- Quick Tips for Teachers
- English Learners in STEM
- Upcoming Events
- Action Planning Institute
- Building Awareness for Science Education
- Strategic Planning Institute
- Next Steps Institute
- STEM Diversity
- Zero Barriers in STEM
- Network for Emergent Socio-Scientific Thinking (NESST)
- Where We Are
- Smithsonian Science for Summer School (S4)
- Always Thinking Like A Scientist (ATLAS)
- STEM Literacy Project
- Success Stories
- France in Focus
- Smithsonian Science for Global Goals Research
- Smithsonian Youth STEM Exchange
- STEMvisions Blog
- What is Photosynthesis
When you get hungry, you grab a snack from your fridge or pantry. But what can plants do when they get hungry? You are probably aware that plants need sunlight, water, and a home (like soil) to grow, but where do they get their food? They make it themselves!
Plants are called autotrophs because they can use energy from light to synthesize, or make, their own food source. Many people believe they are “feeding” a plant when they put it in soil, water it, or place it outside in the Sun, but none of these things are considered food. Rather, plants use sunlight, water, and the gases in the air to make glucose, which is a form of sugar that plants need to survive. This process is called photosynthesis and is performed by all plants, algae, and even some microorganisms. To perform photosynthesis, plants need three things: carbon dioxide, water, and sunlight.
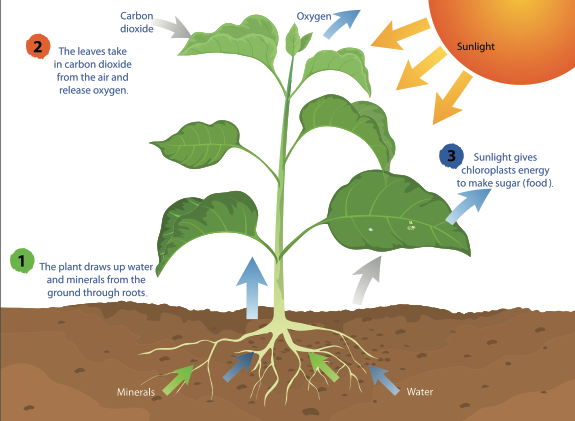
Just like you, plants need to take in gases in order to live. Animals take in gases through a process called respiration. During the respiration process, animals inhale all of the gases in the atmosphere, but the only gas that is retained and not immediately exhaled is oxygen. Plants, however, take in and use carbon dioxide gas for photosynthesis. Carbon dioxide enters through tiny holes in a plant’s leaves, flowers, branches, stems, and roots. Plants also require water to make their food. Depending on the environment, a plant’s access to water will vary. For example, desert plants, like a cactus, have less available water than a lilypad in a pond, but every photosynthetic organism has some sort of adaptation, or special structure, designed to collect water. For most plants, roots are responsible for absorbing water.
The last requirement for photosynthesis is an important one because it provides the energy to make sugar. How does a plant take carbon dioxide and water molecules and make a food molecule? The Sun! The energy from light causes a chemical reaction that breaks down the molecules of carbon dioxide and water and reorganizes them to make the sugar (glucose) and oxygen gas. After the sugar is produced, it is then broken down by the mitochondria into energy that can be used for growth and repair. The oxygen that is produced is released from the same tiny holes through which the carbon dioxide entered. Even the oxygen that is released serves another purpose. Other organisms, such as animals, use oxygen to aid in their survival.
If we were to write a formula for photosynthesis, it would look like this:
6CO 2 + 6H 2 O + Light energy → C 6 H 12 O 6 (sugar) + 6O 2
The whole process of photosynthesis is a transfer of energy from the Sun to a plant. In each sugar molecule created, there is a little bit of the energy from the Sun, which the plant can either use or store for later.
Imagine a pea plant. If that pea plant is forming new pods, it requires a large amount of sugar energy to grow larger. This is similar to how you eat food to grow taller and stronger. But rather than going to the store and buying groceries, the pea plant will use sunlight to obtain the energy to build sugar. When the pea pods are fully grown, the plant may no longer need as much sugar and will store it in its cells. A hungry rabbit comes along and decides to eat some of the plant, which provides the energy that allows the rabbit to hop back to its home. Where did the rabbit’s energy come from? Consider the process of photosynthesis. With the help of carbon dioxide and water, the pea pod used the energy from sunlight to construct the sugar molecules. When the rabbit ate the pea pod, it indirectly received energy from sunlight, which was stored in the sugar molecules in the plant.

Humans, other animals, fungi, and some microorganisms cannot make food in their own bodies like autotrophs, but they still rely on photosynthesis. Through the transfer of energy from the Sun to plants, plants build sugars that humans consume to drive our daily activities. Even when we eat things like chicken or fish, we are transferring energy from the Sun into our bodies because, at some point, one organism consumed a photosynthetic organism (e.g., the fish ate algae). So the next time you grab a snack to replenish your energy, thank the Sun for it!
This is an excerpt from the Structure and Function unit of our curriculum product line, Science and Technology Concepts TM (STC). Please visit our publisher, Carolina Biological , to learn more.
[BONUS FOR TEACHERS] Watch "Photosynthesis: Blinded by the Light" to explore student misconceptions about matter and energy in photosynthesis and strategies for eliciting student ideas to address or build on them.
Related Tags
View the discussion thread.
- Behind the Scenes
Popular Posts
- It’s All About the Tilt: Seasons Misconceptions Debunked
- Are All Snowflakes Really Different? The Science of Winter
- What Are Clouds?
- What is the Winter Solstice?
Featured Authors
- Brian Mandell, PhD
- Ashley Deese
- Kate Echevarria
- Katya Vines, PhD
- Jean Flanagan
- October (1)
- November (1)
- December (2)
- January (1)
- February (2)
- September (3)
- October (7)
- November (4)
- December (3)
- January (4)
- February (4)
- September (4)
- October (2)
- November (2)
- December (1)
- January (3)
- September (6)
- October (3)
- January (2)
- October (6)
- November (5)
- December (4)
- February (6)
- September (2)
- February (1)
- December (5)
- September (1)
- October (5)
- February (5)
- Terms of Use
- Google Plus

Essay on Photosynthesis
Students are often asked to write an essay on Photosynthesis in their schools and colleges. And if you’re also looking for the same, we have created 100-word, 250-word, and 500-word essays on the topic.
Let’s take a look…
100 Words Essay on Photosynthesis
What is photosynthesis.
Photosynthesis is how plants make their own food using sunlight. It happens in the leaves of plants. Tiny parts inside the leaves, called chloroplasts, use sunlight to turn water and carbon dioxide from the air into sugar and oxygen. The sugar is food for the plant.
The Ingredients
The main things needed for photosynthesis are sunlight, water, and carbon dioxide. Roots soak up water from the soil. Leaves take in carbon dioxide from the air. Then, using sunlight, plants create food and release oxygen.
The Process
In the chloroplasts, sunlight energy is changed into chemical energy. This energy turns water and carbon dioxide into glucose, a type of sugar. Oxygen is made too, which goes into the air for us to breathe.
Why It’s Important
Photosynthesis is vital for life on Earth. It gives us food and oxygen. Without it, there would be no plants, and without plants, animals and people would not survive. It also helps take in carbon dioxide, which is good for the Earth.
250 Words Essay on Photosynthesis
Photosynthesis is a process used by plants, algae, and some bacteria to turn sunlight, water, and carbon dioxide into food and oxygen. Think of it like a recipe that plants use to make their own food. This happens in the leaves of plants, which have a green substance called chlorophyll.
Why is Photosynthesis Important?
This process is very important because it is the main way plants make food for themselves and for us, too. Without photosynthesis, plants could not grow, and without plants, animals and humans would not have oxygen to breathe or food to eat.
How Photosynthesis Works
Photosynthesis happens in two main stages. In the first stage, the plant captures sunlight with its leaves. The sunlight gives the plant energy to split water inside its leaves into hydrogen and oxygen. The oxygen is released into the air, and the hydrogen is used in the next stage.
In the second stage, the plant mixes the hydrogen with carbon dioxide from the air to make glucose, which is a type of sugar that plants use for energy. This energy helps the plant to grow, make flowers, and produce seeds.
The Cycle of Life
Photosynthesis is a key part of the cycle of life on Earth. By making food and oxygen, plants support life for all creatures. When animals eat plants, they get the energy from the plants, and when animals breathe, they use the oxygen that plants release. It’s a beautiful cycle that keeps the planet alive.
500 Words Essay on Photosynthesis
Photosynthesis is a process used by plants, algae, and some bacteria to turn sunlight, water, and carbon dioxide into food and oxygen. This happens in the green parts of plants, mainly the leaves. The green color comes from chlorophyll, a special substance in the leaves that captures sunlight.
The Ingredients of Photosynthesis
To make their food, plants need three main things: sunlight, water, and carbon dioxide. Sunlight is the energy plants use to create their food. They get water from the ground through their roots. Carbon dioxide, a gas found in the air, is taken in through tiny holes in the leaves called stomata.
The Photosynthesis Recipe
When sunlight hits the leaves, the chlorophyll captures it and starts the food-making process. The energy from the sunlight turns water and carbon dioxide into glucose, a type of sugar that plants use for energy, and oxygen, which is released into the air. This process is like a recipe that plants follow to make their own food.
The Importance of Photosynthesis
Photosynthesis is very important for life on Earth. It gives us oxygen, which we need to breathe. Plants use the glucose they make for growth and to build other important substances like cellulose, which they use to make their cell walls. Without photosynthesis, there would be no food for animals or people, and no oxygen to breathe.
The Benefits to the Environment
Photosynthesis also helps the environment. Plants take in carbon dioxide, which is a gas that can make the Earth warmer when there is too much of it in the air. By using carbon dioxide to make food, plants help keep the air clean and the Earth’s temperature just right.
Photosynthesis and the Food Chain
All living things need energy to survive, and this energy usually comes from food. Plants are at the bottom of the food chain because they can make their own food using photosynthesis. Animals that eat plants get energy from the glucose in the plants. Then, animals that eat other animals get this energy too. So, photosynthesis is the start of the food chain that feeds almost every living thing on Earth.
Photosynthesis in Our Lives
Photosynthesis affects our lives in many ways. It gives us fruits, vegetables, and grains to eat. Trees and plants also give us wood, paper, and other materials. Plus, they provide shade and help make the air fresh and clean.
In conclusion, photosynthesis is a vital process that allows plants to make food and oxygen using sunlight, water, and carbon dioxide. It is the foundation of the food chain and has a big impact on the environment and our lives. Understanding photosynthesis helps us appreciate how important plants are and why we need to take care of them and the environment they live in.
That’s it! I hope the essay helped you.
If you’re looking for more, here are essays on other interesting topics:
- Essay on Gender Equality And Women’s Empowerment
- Essay on Gender Equality And Sustainable Development
- Essay on Exciting Cricket Match
Apart from these, you can look at all the essays by clicking here .
Happy studying!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

The Process of Photosynthesis
Introduction.
Photosynthesis is fundamental to the energy flow process in living organisms. “Plants are the primary producers and they make use of sunlight to produce sugars for energy production.” (Govindjee, 1997, p. 45) Excess nutrients are stored and the plants are eaten up by herbivores and omnivores which rely on the energy stored in the plant cells to keep alive. The herbivores are subsequently consumed by the omnivores and carnivores; this process continues from one living organism to another creating a food chain that sustains life on earth.
Problem statement
The energy flow process is fundamental to the sustenance of life on earth. For survival, each organism requires nutrition; the nutrition is sourced from another organism as food substrates except for the green plants which manufacture their food using sunlight and minerals. Many types of research have been conducted and reveal that the process of photosynthesis is the main process that sustains life. This experimental study is designed to ascertain how the process of photosynthesis leads to energy production and how it is affected by variation in light intensity and wavelength.
Relevance of the question
This study is essential for a proper understanding of the role of plants as primary producers in the food chain process.

Literature review
The photosynthesis process occurs primarily in the leaves with little taking place in the stem for some plants. The main parts of the leaf in which are involved in the process include; “the upper and lower epidermis, the mesophyll, the vascular bundles and the stomates.” (Photosynthesis, 2000) The epidermis lacks chlorophyll and therefore photosynthesis does not occur there, the epidermis only acts to protect the internal part of the leaf. “The stomates are tiny holes in the epidermis through which gaseous exchange takes place.” (Photosynthesis, 2000, para.2)
Through the stomates, Co2 enters, and O2 leaves. “The vascular bundles constitute the plants transport system through which water and other nutrients are moved around the plant.” (Photosynthesis, 2000, para.2)

Chlorophyll is composed of the outer and inner membranes, “there is also an inter membrane space stroma and thylakoids which are stacked in grana. The chlorophyll is normally built in the membrane of the thylakoids.” (Photosynthesis, 2000, para.3)
Thus, photosynthesis is the main process in which the “radiant light energy is absorbed by the chloroplast’s pigment, chlorophyll and converted into chemical energy in the molecular form of ATP and NADH.” (Photosynthesis, 2000) The energy is then utilized in driving the Calvin cycle in the production of three-carbon sugars. The three-carbon sugars are subsequently converted to various types of carbohydrates.
According to Govindjee, it is currently possible to carry out photolysis in the laboratory. The reaction was first performed by Robert Hill in 1937 and thus became known as the Hill Reaction. The Hill Reaction requires only intact isolated chloroplasts rather than the entire intact plant cell. However, since isolated chloroplasts do not reduce carbon dioxide directly, it is essential to provide a hydrogen acceptor for the reduction process and permit electron transport to take place. The hydrogen acceptor that was chosen for this experiment is the synthetic compound 2,6-dichlorophenol-indophenol or DPIP , which is blue in the oxidized form and colorless when reduced. As indicated above, the source of hydrogen for the reduction in water. This hydrogen can reduce DPIP and turn it from a blue substance to a colorless one.
Oxygen is also formed, but it is not monitored in this experiment. The change in color of the DPIP solution, which is directly proportional to the number of hydrogen produced in the Hill Reaction, can be assayed using colorimetric spectrophotometry. The more hydrogen produced, the more DPIP that is reduced and the more colorless the solution containing DPIP becomes. Therefore, the course of this reaction can be assessed by the bleaching of the artificial hydrogen acceptor. (1997, p. 144))
Light + CO2 + 2H2O → n (CH2O) + H2O + O2
The experiment entailed the use of both boiled and fresh chloroplast suspension. The boiled chloroplast was used as a control and photosynthetic activities were monitored in the fresh chloroplast. The results were measured using a spectrophotometer and tabulated. The same procedure was repeated with variations in the light intensity and wavelength.
- Spectrophotometer
- Spectrophotometer cuvettes
- Phosphate buffer (pH6.5)
- Chloroplast suspension
- Sodium hydrosulfite
- Distilled water
- Wrapping foil
- Digital timer
- DPIP solution
The source of hydrogen for the reduction in water. This hydrogen can reduce DPIP and turn it from a blue substance to a colorless one. Oxygen is also formed, but it is not monitored in this experiment. The change in color of the DPIP solution, which is directly proportional to the number of hydrogen produced in the Hill Reaction, can be assayed using colorimetric spectrophotometry. The more hydrogen produced, the more DPIP that is reduced and the more colorless the solution containing DPIP becomes. Therefore, the course of this reaction can be assessed by the bleaching of the artificial hydrogen acceptor. The experimental procedure was selected because it has a high specificity and therefore results will have a low error margin. The chemicals used are readily available were purchased in various forms and reconstituted in the laboratory before the practice according to the manufacture’s guidelines.
- Obtain fresh and boiled chloroplast suspension prepared before the practical time. The boiling of the suspension will render the chloroplasts non-functional and will serve as one of the experimental controls. Allow the solution to return to room temperature before use. The chloroplast is boiled preferably at 100 degrees for 5 to 7 minutes.
- Obtain four clean spectrophotometer cuvettes. Label them 1-4 and prepare them as follows: Cuvette 1: 3.0 ml of phosphate buffer (pH 6.5) and 1.0 ml of boiled chloroplast suspension, Cuvette 2: 3.0 ml of phosphate buffer (pH 6.5) and 1.0 ml of chloroplast suspension, Cuvette 3: 3.0 ml of phosphate buffer (pH 6.5) and 1.0 ml of chloroplast suspension, Cuvette 4: 3.0 ml of phosphate buffer (pH 6.5) and 1.0 ml of chloroplast suspension
- Add 50 μl of the 0.1% DPIP solution to cuvette 4 only. Shake it to mix the solution well. Then add a few (very few) crystals of sodium hydrosulfite (Na2S2O4) to it. Sodium hydrosulfite reduces the DPIP and removes the blue color.
- Be sure that the spectrophotometer is set to 600nm. Use the ‘reduced’ cuvette 4 as a blank to zero the spectrophotometer.
- Add the same amount of DPIP that you added to cuvette 4 to cuvette 1. Mix the solution well and take a reading as quickly as possible using the spectrophotometer, because cuvettes with functional chloroplasts should immediately start producing hydrogen that reduces the DPIP. Record this value as ‘time zero’. Start the digital timer.
- Repeat step 5 for cuvettes 2 and 3. Record the ‘time zero’ values and their starting time points.
- Immediately after taking the first readings, wrap cuvette 3 completely in aluminum foil and place cuvettes 1-3 in a beaker of water at room temperature. Set the light source 10 inches from the cuvettes and turn it on to the highest setting. Note that the water in the beaker serves as a thermal buffer to prevent any experimental artifact due to warming by the source light.
- Every two minutes record the optical density of cuvettes 1 and 2. Leave cuvette 3 in the dark until the end of the procedure.
- Continue taking readings until the optical density reading of cuvette 2 does not change.
- Remove cuvette 3 from its foil wrapping. Quickly read the optical density of this cuvette. Take two-minute readings until the optical density reading does not change for two or three-time points. Make a graphical plot with time as the independent variable and optical density as the dependent variable.
Note: Different colors of light indicate the difference in wavelength thus the use of various colors of cellophane to measure wavelength.
Dependent, independent, and controlled variables
The independent variable is the time of exposure whereby the duration of the reaction is changed to determine the effect of time on the DPIP reduction (visualized as the clearing of the blue color). The dependent variable is the optical density which varies depending on the duration the cuvette was left to react. The controlled variables include the use of the same amount of constituent chemical for each tube, temperature of the reactions where all the tubes are reacted at room temperature.
Threat reduction to internal validity was done by:
Taking measurements immediately after the timed duration had expired; was done to prevent errors in the measurement of the optical density. Providing the same conditions for all measurements taken i.e. the spectrophotometer was blanked by the same solution for all the readings which was done at 600nm. All the necessary conditions for the materials were provided prior to and during the experiment.
Plants form an important part of the food chain. “Green plants manufacture their on food using the photosynthesis process.” (Photosynthesis, 2000, para.1) Sunlight is an important aspect of this process; the practical examines the process of photosynthesis, particularly the role played by light. Therefore:
HA: Light is a fundamental factor for the photosynthetic process where it’s used to reduce carbon dioxide and break the water molecule. In this experiment, the hydrogen from the water molecule reacts with DCIP to reduce its blue color. The spectrophotometer is used to measure the intensity of the reduced color.
Ho: Light is not a fundamental factor in the process of photosynthesis and does not reduce carbon dioxide or break the water molecule to produce hydrogen used in the experiment to reduce DCIP’s blue color that is measured using a spectrophotometer.
Use of appropriate methods, tools, and technologies to collect quantitative data
In this experiment quantitative data was collected as optical density readings. The readings were taken at 600 nm after the spectrophotometer was blanked using cuvette 4 which had 3.0 ml of phosphate buffer (pH 6.5) 1.0 ml of chloroplast suspension and 50 μl of the 0.1% DPIP solution added and the reaction left to complete( color clears). The optical density reading for each cuvette was recorded against the time duration in the lab notebook.
Data Collection
Data collection is an important aspect for the success of any experiment, for this particular experiment, the data was collected by the use of a spectrophotometer. Every reaction was timed according to the manual and on expiry of the time duration, the optical density readings were taken using the spectrophotometer and recorded in the laboratory notebook for use in the tabulation and plotting of graphs for analysis.
Experiment: the hill reaction.
Results of the Experiment
The results for the experimental were plotted as below
This experiment was designed to investigate the photosynthesis process in which light is utilized for energy production. In the hill reaction, the rate of photosynthesis remained the same for cuvettes 1 and 3, there was no evidence of photosynthetic activity in the fourth cuvette. In cuvette 5, the photosynthetic activity decreased with time. From the experiments, it is deduced that photosynthesis occurs in two stages, the light-dependent, and the light-independent stage. Generally, “in the first stage energy is captured and stored in the form of ATP and NADPH.” (Photosynthesis, 2000)
In the second stage light-independent stage the energy stored is used to process sugars using carbon. The results of this experiment agree with the hypothesis that light is a fundamental factor for the photosynthetic process where it’s used to reduce carbon dioxide and break the water molecule. In this experiment, the hydrogen from the water molecule reacts with DCIP to reduce its blue color. The spectrophotometer is used to measure the intensity of the reduced color
The experiment was carried out successfully and the results indicate that indeed plants manufacture their own food using sunlight as a source of energy. Therefore sunlight is an important factor in this process. However, the success of the practical was due to the experimental design which provided all the necessary material and conditions for the laboratory model of photosynthesis. A good experimental design gives results with minimal errors and thus can be used to conclude. “Validity refers how closeness the values are for repeated measures.”(Govindjee, 1997, p. 67) Replication of the above experiment gives results from which comparison can be drawn to give a test of validity for this experimental design.
The experiment can be replicated by another person provided he/she formulates a suitable experimental design that will include the identification, manipulation, and measurement of all the parameters in the investigation. The experimental design must be reliable and carefully selected to reduce the error margin to minimum values as this is an important aspect of this experiment.
Reference list
Govindjee, G. (1997). Experiments in plant biology. Berlin: Springer.
Photosynthesis. (2000). Web.
Cite this paper
- Chicago (N-B)
- Chicago (A-D)
StudyCorgi. (2021, November 30). The Process of Photosynthesis. https://studycorgi.com/the-process-of-photosynthesis/
"The Process of Photosynthesis." StudyCorgi , 30 Nov. 2021, studycorgi.com/the-process-of-photosynthesis/.
StudyCorgi . (2021) 'The Process of Photosynthesis'. 30 November.
1. StudyCorgi . "The Process of Photosynthesis." November 30, 2021. https://studycorgi.com/the-process-of-photosynthesis/.
Bibliography
StudyCorgi . "The Process of Photosynthesis." November 30, 2021. https://studycorgi.com/the-process-of-photosynthesis/.
StudyCorgi . 2021. "The Process of Photosynthesis." November 30, 2021. https://studycorgi.com/the-process-of-photosynthesis/.
This paper, “The Process of Photosynthesis”, was written and voluntary submitted to our free essay database by a straight-A student. Please ensure you properly reference the paper if you're using it to write your assignment.
Before publication, the StudyCorgi editorial team proofread and checked the paper to make sure it meets the highest standards in terms of grammar, punctuation, style, fact accuracy, copyright issues, and inclusive language. Last updated: November 30, 2021 .
If you are the author of this paper and no longer wish to have it published on StudyCorgi, request the removal . Please use the “ Donate your paper ” form to submit an essay.
- Search Menu
- Sign in through your institution
- Advance Articles
- Editor's Choice
- Commentaries
- Special Issues
- Rapid Papers
- Author Guidelines
- Submission Site
- Open Access Options
- Author Benefits
- Benefits of Publishing Open Access
- About Plant and Cell Physiology
- About the Japanese Society of Plant Physiologists
- Editorial Board
- Permissions
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
Photosynthetic research in plant science.
- Article contents
- Figures & tables
- Supplementary Data
Ayumi Tanaka, Amane Makino, Photosynthetic Research in Plant Science, Plant and Cell Physiology , Volume 50, Issue 4, April 2009, Pages 681–683, https://doi.org/10.1093/pcp/pcp040
- Permissions Icon Permissions
Photosynthesis is a highly regulated, multistep process. It encompasses the harvest of solar energy, transfer of excitation energy, energy conversion, electron transfer from water to NADP + , ATP generation and a series of enzymatic reactions that assimilate carbon dioxide and synthesize carbohydrate.
Photosynthesis has a unique place in the history of plant science, as its central concepts were established by the middle of the last century, and the detailed mechanisms have since been elucidated. For example, measurements of photosynthetic efficiency (quantum yield) at different wavelengths of light (Emerson and Lews 1943 ) led to the insight that two distinct forms of Chl must be excited in oxygenic photosynthesis. These results suggested the concept of two photochemical systems. The reaction center pigments of PSII and PSI (P680 and P700, respectively) were found by studying changes in light absorbance in the red region (Kok 1959 , Döring et al. 1969 ). Chls with absorbance maxima corresponding to these specific wavelengths were proposed as the final light sink. These Chls were shown to drive electron transfer by charge separation. The linkage of electron transfer and CO 2 assimilation was suggested by studies on Hill oxidant (Hill 1937 ). A linear electron transport system with two light-driven reactions (Z scheme) was proposed based upon observations of the redox state of cytochromes (Hill and Bendall 1960 , Duysens et al. 1961 ), and photophosphorylation was found to be associated with thylakoid fragments (Arnon et al. 1954 ). The metabolic pathway that assimilates carbon by fixation of CO 2 was discovered by Calvin's group who used 14 CO 2 radioactive tracers in the 1950s (Bassham and Calvin 1957 ). This was the first significant discovery in biochemistry made using radioactive tracers. The primary reaction of CO 2 fixation is catalyzed by Rubisco (Weissbach et al. 1956 ), initially called Fraction 1 protein (Wildman and Bonner 1947 ). Rubisco is the most abundant protein in the world, largely because it is also the most inefficient with the lowest catalytic turnover rate (1–3 s –1 ). Another CO 2 fixation pathway was then found in sugarcane (Kortschak et al. 1964, Hatch and Slack 1965) and named C 4 photosynthesis.
Although photosynthesis plays the central role in the energy metabolism of plants, historically there have not been strong interactions between photosynthesis research and other fields of plant science. Many techniques and tools developed for photosynthesis research have not been widely used in other fields because they were developed to examine phenomena unique to photosynthesis. For example, excitation energy transfer and charge separation are fundamental but unique processes of photosynthesis. Another reason for the historic isolation of photosynthesis research within plant science is that it was long believed that CO 2 fixation and carbohydrate production are the sole function of photosynthesis, with carbohydrates representing the only link between photosynthesis and other biological phenomena.
However, this situation has begun to change. Recent research has revealed that photosynthesis is closely related to a variety of other physiological processes. It is a major system for controlling the redox state of cells, playing an important role in regulating enzyme activity and many other cellular processes (Buchanan and Balmer 2005 , Hisabori et al. 2007 ). Photosynthesis also generates reactive oxygen species, which are now appreciated as being regulatory factors for many biological processes rather than inevitable by-products of photosynthesis (Wagner et al. 2004 , Beck 2005 ). Precursor molecules of Chl, which are a major component of photosynthesis, act as a chloroplast-derived signal, and are involved in regulating the cell cycle (Kobayashi et al. 2009 ). In light of this new information, it seems important to re-evaluate the function(s), both potential and demonstrated, of photosynthesis from a variety of view points. Photosynthesis research now employs the methods and tools of molecular biology and genetics, which are central methods for plant science in general. Meanwhile, Chl fluorescence and gas exchange measurements, developed especially for photosynthesis research, are now widely used in stress biology and ecology.
Photosynthesis research also contributes to our understanding of ecological phenomena and even the global environments (Farquhar et al. 1980 , de Pury and Farquhar 1997 , Monsi and Saeki 2005 ). Indeed, photosynthesis is now an integral component of simulation models used to predict the future of our planet. Improving the efficiency of photosynthesis by artificial modification of photosynthetic proteins and pathways has long been considered impossible or at best problematic, because, over evolutionary time, photosynthesis has become complex and tightly regulated. However, recent advances have made it possible to manipulate photosynthesis using molecular genetic technology (Andrews and Whiney 2003 , Raines 2006 ). These advances may have positive influences on crop productivity (Parry et al. 2007 ) as photosynthetic rates have frequently been correlated with biomass accretion (Kruger and Volin 2006 ). Thus, we can expect many more novel concepts to be added to this history of photosynthetic research.
As photosynthesis research tackles new challenges, we should also continue to re-evaluate past research. Oxygen evolution, energy dissipation and cyclic electron transport are crucial processes during photosynthesis, yet their mechanisms still remain to be clarified. We have very limited knowledge of the formation and degradation of photosynthetic apparatus. Also, although photosynthesis plays a central role in C and N metabolism in plants, we do not yet understand how potential photosynthesis is related to crop productivity.
Plant and Cell Physiology would like to contribute to the development of novel concepts, pioneering new fields and solving the unanswered questions of photosynthesis. This special issue covers a wide range of topics in photosynthesis research. Terashima et al. (pp. 684–697) readdress the enigmatic question of why leaves are green. They show that the light profile through a leaf is steeper than that of photosynthesis, and that the green wavelengths in white light are more effective in driving photosynthesis than red light. Evans (pp. 698–706) proposes a new model using Chl fluorescence to explore modifications in quantum yield with leaf depth. This new multilayered model can be applied to study variations in light absorption profiles, photosynthetic capacity and calculation of chloroplastic CO 2 concentration at different depths through the leaf.
Singlet oxygen, 1 O 2 , is produced by the photosystem and Chl pigments. 1 O 2 not only causes physiological damage but also activates stress response programs. The flu mutant of Arabidopsis thaliana overaccumulates protochlorophyllide that upon illumination generates singlet oxygen, causing growth cessation and cell death. Coll et al. (pp. 707–718) have isolated suppressor mutants, dubbed ‘singlet oxygen-linked death activator’ (soldat), that specifically abrogate 1 O 2 -mediated stress responses in young flu seedlings, and they discuss the processes of acclimation to stresses. Phephorbide a is a degradation product of Chl and one of the most powerful photosensitzing molecules. Mutants defective in pheophorbide a oxygenase, which converts phephorbide a to open tetrapyrrole, accumulate pheophorbide a and display cell death in a light-dependent manner. Hirashima et al. (pp. 719–729) report that pheophorbide a is involved in this light-independent cell death.
Plants regulate the redox level of the plastoquinone pool in response to the light environment. In acclimation to high-light conditions, the redox level is kept in an oxidized state by the plastoquinone oxidation system (POS). Miyake et al. (pp. 730–743) investigated the mechanism of POS using the Chl fluorescence parameter, qL.
Nagai and Makino (pp. 744–755) examine in detail the differences between rice and wheat, the two most commercially important crops, in the temperature responses of CO 2 assimilation and plant growth. They find that the difference in biomass production between the two species at the level of the whole plant depends on the difference in N-use efficiency in leaf photosynthesis and growth rate. Sage and Sage (pp. 756–772) examine chlorenchyma structure in rice and related Oryza species in relation to photosynthetic function. They find that rice chlorenchyma architecture includes adaptations to maximize the scavenging of photorespired CO 2 and to enhance the diffusive conductance of CO 2 . In addition, they consider that the introduction of Kranz anatomy does not require radical anatomical alterations in engineering C 4 rice.
Bioinformatics has become a powerful tool, especially in photosynthetic research, because photosynthetic organisms have a wide taxonomic distribution among prokaryotes and eukaryotes. Ishikawa et al. (pp. 773–788) present the results of a pilot study of functional orthogenomics, combining bioinformatic and experimental analyses to identify nuclear-encoded chloroplast proteins of endosymbiontic origin (CRENDOs). They conclude that phylogenetic profiling is useful in finding CPRENDOs, although the physiological functions of orthologous genes may be different in chloroplasts and cyanobacteria.
We hope you enjoy this special issue, and would like to invite you to submit more excellent papers to Plant and Cell Physiology in the field of photosynthesis.
Google Scholar
Google Preview
Email alerts
Citing articles via.
- Recommend to Your Librarian
Affiliations
- Online ISSN 1471-9053
- Copyright © 2024 Japanese Society of Plant Physiologists
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
AP®︎/College Biology
Course: ap®︎/college biology > unit 3.
- Photosynthesis
- Intro to photosynthesis
- Breaking down photosynthesis stages
- Conceptual overview of light dependent reactions
The light-dependent reactions
- The Calvin cycle
- Photosynthesis evolution
- Photosynthesis review
Introduction
- Plants carry out a form of photosynthesis called oxygenic photosynthesis . In oxygenic photosynthesis, water molecules are split to provide a source of electrons for the electron transport chain, and oxygen gas is released as a byproduct. Plants organize their photosynthetic pigments into two separate complexes called photosystems (photosystems I and II), and they use chlorophylls as their reaction center pigments.
- Purple sulfur bacteria, in contrast, carry out anoxygenic photosynthesis , meaning that water is not used as an electron source and oxygen gas is not produced. Instead, these bacteria use hydrogen sulfide ( H 2 S ) as an electron source and produce elemental sulfur as a byproduct. In addition, purple sulfur bacteria have only one photosystem, and they use chlorophyll-like molecules called bacteriochlorophylls as reaction center pigments 1 , 2 , 3 .
Overview of the light-dependent reactions
- Light absorption in PSII. When light is absorbed by one of the many pigments in photosystem II, energy is passed inward from pigment to pigment until it reaches the reaction center. There, energy is transferred to P680, boosting an electron to a high energy level. The high-energy electron is passed to an acceptor molecule and replaced with an electron from water. This splitting of water releases the O 2 we breathe.
- ATP synthesis. The high-energy electron travels down an electron transport chain, losing energy as it goes. Some of the released energy drives pumping of H + ions from the stroma into the thylakoid interior, building a gradient. ( H + ions from the splitting of water also add to the gradient.) As H + ions flow down their gradient and into the stroma, they pass through ATP synthase, driving ATP production in a process known as chemiosmosis .
- Light absorption in PSI. The electron arrives at photosystem I and joins the P700 special pair of chlorophylls in the reaction center. When light energy is absorbed by pigments and passed inward to the reaction center, the electron in P700 is boosted to a very high energy level and transferred to an acceptor molecule. The special pair's missing electron is replaced by a new electron from PSII (arriving via the electron transport chain).
- NADPH formation. The high-energy electron travels down a short second leg of the electron transport chain. At the end of the chain, the electron is passed to NADP + (along with a second electron from the same pathway) to make NADPH.
What is a photosystem?
Photosystem i vs. photosystem ii.
- Special pairs. The chlorophyll a special pairs of the two photosystems absorb different wavelengths of light. The PSII special pair absorbs best at 680 nm, while the PSI special absorbs best at 700 nm. Because of this, the special pairs are called P680 and P700 , respectively.
- Primary acceptor . The special pair of each photosystem passes electrons to a different primary acceptor. The primary electron acceptor of PSII is pheophytin, an organic molecule that resembles chlorophyll, while the primary electron acceptor of PSI is a chlorophyll called A 0 7 , 8 .
- Source of electrons . Once an electron is lost, each photosystem is replenished by electrons from a different source. The PSII reaction center gets electrons from water, while the PSI reaction center is replenished by electrons that flow down an electron transport chain from PSII.
Photosystem II
Electron transport chains and photosystem i, some electrons flow cyclically, attribution:, works cited:.
- Lodish, H., Berk, A., Zipursky, S. L., Matsudaira, P., Baltimore, D., and Darnell, J. (2000). Molecular analysis of photosystems. In Molecular cell biology (4th ed., section 16.4). New York, NY: W. H. Freeman. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK21484/ .
- Boundless. (2015, July 21). Anoxygenic photosynthetic bacteria. In Boundless microbiology . Retrieved from https://www.boundless.com/microbiology/textbooks/boundless-microbiology-textbook/microbial-evolution-phylogeny-and-diversity-8/nonproteobacteria-gram-negative-bacteria-105/anoxygenic-photosynthetic-bacteria-551-7338/ .
- Purple sulfur bacteria. (2015, July 16). Retrieved October 24, 2015 from Wikipedia: https://en.wikipedia.org/wiki/Purple_sulfur_bacteria .
- Soda lake. (2015, September 26). Retrieved October 24, 2015 from Wikipedia: https://en.wikipedia.org/wiki/Soda_lake .
- Gutierrez, R. Bio41 Week 7 Biochemistry Lectures 11 and 12. Bio41. 2009.
- Berg, J. M., Tymoczko, J. L., and Stryer, L. (2002). Accessory pigments funnel energy into reaction centers. In Biochemistry (5th ed., section 19.5). New York, NY: W. H. Freeman. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK22604/ .
- Pheophytin. (2015, February 11). Retrieved October 28, 2015 from Wikipedia: https://en.wikipedia.org/wiki/Pheophytin .
- Photosystem I. (2016, June 25). Retrieved from Wikipedia on July 22, 2016: https://en.wikipedia.org/wiki/Photosystem_I .
- Berg, J. M., Tymoczko, J. L., and Stryer, L. (2002). Two photosystems generate a proton gradient and NADPH in oxygenic photosynthesis. In Biochemistry (5th ed., section 19.3). New York, NY: W. H. Freeman. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK22538/#_A2681_ .
- Joliot, P. and Johnson, G. N. (2011). Regulation of cyclic and linear electron flow in higher plants. PNAS, 108(32), 13317-13322. http://dx.doi.org/10.1073/pnas.1110189108 .
- Johnson, Giles N. (2011). Physiology of PSI cyclic electron transport in higher plants. Biochimica et Biophysica Acta - Bioenergetics , 1807 (8), 906-911. http://dx.doi.org/doi:10.1016/j.bbabio.2010.11.009 .
- Berg, J. M., Tymoczko, J. L., and Stryer, L. (2002). A proton gradient across the thylakoid membrane drives ATP synthesis. In Biochemistry (5th ed., section 19.4). New York, NY: W. H. Freeman. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK22519/ .
- Takahashi, S., Milward, S. E., Fan, D.-Y., Chow, W. S., and Badger, M. R. (2008). How does cyclic electron flow alleviate photoinhibition in Arabidopsis? Plant Physiology , 149 (3), 1560-1567. http://dx.doi.org/10.1104/pp.108.134122 .
Additional references:
Want to join the conversation.
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Home — Essay Samples — Science — Photosynthesis — Photosynthesis and Cellular Respiration
Photosynthesis and Cellular Respiration
- Categories: Photosynthesis
About this sample

Words: 573 |
Published: Feb 12, 2019
Words: 573 | Page: 1 | 3 min read
Table of contents
Prompt examples for the "photosynthesis" essays, photosynthesis essay example.
- The Process of Photosynthesis: Breaking It Down Explain the process of photosynthesis in detail, breaking down each step, the key resources involved (light energy, carbon dioxide, water), and the outcomes (glucose and oxygen). How does photosynthesis enable plants to create their own food?
- Photosynthesis vs. Cellular Respiration: Understanding the Differences Compare and contrast photosynthesis and cellular respiration. What are the key distinctions between these two processes? How do plants use these processes differently, and why is it essential for plants to perform photosynthesis during the day and cellular respiration at night?
- The Importance of Photosynthesis for Plant Survival Discuss the critical role of photosynthesis in a plant's survival. How does it provide plants with the necessary energy and nutrients? Explore the potential consequences if a plant were unable to perform photosynthesis.
- Common Misconceptions About Photosynthesis Address common misconceptions or incorrect claims about photosynthesis, such as those mentioned by Mika in the essay. Provide clear explanations to refute these misconceptions and offer accurate information about when photosynthesis and cellular respiration occur in plant cells.
- The Energy Acquisition Strategies of Plants and Animals Compare how plants and animals acquire and utilize energy. Explain the fundamental differences in their energy sources and processes. Why do plants rely on photosynthesis, while animals need to consume other organisms for energy?
Works Cited
- Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., & Walter, P. (2014). Molecular Biology of the Cell. Garland Science.
- Campbell, N. A., & Reece, J. B. (2017). Biology. Pearson.
- Cox, M. M., & Doudna, J. A. (2017). Principles of Molecular Biology. W. H. Freeman.
- Freeman, S., Quillin, K., Allison, L., Black, M., Taylor, E., & Podgorski, G. (2017). Biological Science. Pearson.
- Lodish, H., Berk, A., Zipursky, S. L., Matsudaira, P., Baltimore, D., & Darnell, J. (2016). Molecular Cell Biology. W. H. Freeman.
- National Science Teachers Association. (2016). Photosynthesis and cellular respiration. NSTA.
- Raven, P. H., Evert, R. F., & Eichhorn, S. E. (2017). Biology of Plants. W. H. Freeman.
- Reece, J. B., Urry, L. A., Cain, M. L., Wasserman, S. A., Minorsky, P. V., & Jackson, R. B. (2014). Campbell Biology. Pearson.
- Sadava, D. E., Hillis, D. M., Heller, H. C., & Berenbaum, M. R. (2014). Life: The Science of Biology. W. H. Freeman.
- Taiz, L., & Zeiger, E. (2013). Plant Physiology. Sinauer Associates.

Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Dr Jacklynne
Verified writer
- Expert in: Science

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
1 pages / 506 words
1 pages / 522 words
1 pages / 407 words
1 pages / 293 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online

Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Related Essays on Photosynthesis
Photosynthesis happens in the light. Cellular respiration happens in the dark. In the process of photosynthesis, the plants need sunlight for energy. Photosynthesis is the process that plants use light energy from the sun to [...]
In this experiment, we were testing the rate of photosynthesis in elodea. For a plant to photosynthesize, it needs carbon dioxide and water and sunlight, a factor of photosynthesis. In order for us to measure the rate of [...]
Photosynthesis is the process described by this equation This equation shows the complex 2 steps process that takes place in the chloroplast of green plants. The end product is glucose, but the complex organic molecule such as [...]
We as heterotrophs rely on photosynthetic organisms for nearly all the organic plant matter that we consume for energy. Photosynthesis is one of the oldest and one of the most fundamental processes of life. ("BIO 1510 Laboratory [...]
Many illnesses are hereditary; for instance, Huntington's and Cystic Fibrosis. Other illnesses affected by genes, such as diabetes and cancer. Many advancements make it workable for a solution to target such hereditarily [...]
It is no secret that Darwin's theory of Natural Selection and Evolution, put forth in Origin of Species, has been applied to social theory, giving rise to Social Darwinism. But are we correct to assume that Social Darwinism is [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Portland Press Opt2Pay

Photosynthesis
Matthew p. johnson.
Department of Molecular Biology and Biotechnology, University of Sheffield, Firth Court, Western Bank, Sheffield S10 2TN, U.K.
Photosynthesis sustains virtually all life on planet Earth providing the oxygen we breathe and the food we eat; it forms the basis of global food chains and meets the majority of humankind's current energy needs through fossilized photosynthetic fuels. The process of photosynthesis in plants is based on two reactions that are carried out by separate parts of the chloroplast. The light reactions occur in the chloroplast thylakoid membrane and involve the splitting of water into oxygen, protons and electrons. The protons and electrons are then transferred through the thylakoid membrane to create the energy storage molecules adenosine triphosphate (ATP) and nicotinomide–adenine dinucleotide phosphate (NADPH). The ATP and NADPH are then utilized by the enzymes of the Calvin–Benson cycle (the dark reactions), which converts CO 2 into carbohydrate in the chloroplast stroma. The basic principles of solar energy capture, energy, electron and proton transfer and the biochemical basis of carbon fixation are explained and their significance is discussed.
An overview of photosynthesis
Introduction.
Photosynthesis is the ultimate source of all of humankind's food and oxygen, whereas fossilized photosynthetic fuels provide ∼87% of the world's energy. It is the biochemical process that sustains the biosphere as the basis for the food chain. The oxygen produced as a by-product of photosynthesis allowed the formation of the ozone layer, the evolution of aerobic respiration and thus complex multicellular life.
Oxygenic photosynthesis involves the conversion of water and CO 2 into complex organic molecules such as carbohydrates and oxygen. Photosynthesis may be split into the ‘light’ and ‘dark’ reactions. In the light reactions, water is split using light into oxygen, protons and electrons, and in the dark reactions, the protons and electrons are used to reduce CO 2 to carbohydrate (given here by the general formula CH 2 O). The two processes can be summarized thus:
Light reactions:
Dark reactions:
The positive sign of the standard free energy change of the reaction (Δ G °) given above means that the reaction requires energy ( an endergonic reaction ). The energy required is provided by absorbed solar energy, which is converted into the chemical bond energy of the products ( Box 1 ).
Standard free energy change

Photosynthesis converts ∼200 billion tonnes of CO 2 into complex organic compounds annually and produces ∼140 billion tonnes of oxygen into the atmosphere. By facilitating conversion of solar energy into chemical energy, photosynthesis acts as the primary energy input into the global food chain. Nearly all living organisms use the complex organic compounds derived from photosynthesis as a source of energy. The breakdown of these organic compounds occurs via the process of aerobic respiration, which of course also requires the oxygen produced by photosynthesis.
Unlike photosynthesis, aerobic respiration is an exergonic process (negative Δ G °) with the energy released being used by the organism to power biosynthetic processes that allow growth and renewal, mechanical work (such as muscle contraction or flagella rotation) and facilitating changes in chemical concentrations within the cell (e.g. accumulation of nutrients and expulsion of waste). The use of exergonic reactions to power endergonic ones associated with biosynthesis and housekeeping in biological organisms such that the overall free energy change is negative is known as ‘ coupling’.
Photosynthesis and respiration are thus seemingly the reverse of one another, with the important caveat that both oxygen formation during photosynthesis and its utilization during respiration result in its liberation or incorporation respectively into water rather than CO 2 . In addition, glucose is one of several possible products of photosynthesis with amino acids and lipids also being synthesized rapidly from the primary photosynthetic products.
The consideration of photosynthesis and respiration as opposing processes helps us to appreciate their role in shaping our environment. The fixation of CO 2 by photosynthesis and its release during breakdown of organic molecules during respiration, decay and combustion of organic matter and fossil fuels can be visualized as the global carbon cycle ( Figure 1 ).

The relationship between respiration, photosynthesis and global CO 2 and O 2 levels.
At present, this cycle may be considered to be in a state of imbalance due to the burning of fossil fuels (fossilized photosynthesis), which is increasing the proportion of CO 2 entering the Earth's atmosphere, leading to the so-called ‘greenhouse effect’ and human-made climate change.
Oxygenic photosynthesis is thought to have evolved only once during Earth's history in the cyanobacteria. All other organisms, such as plants, algae and diatoms, which perform oxygenic photosynthesis actually do so via cyanobacterial endosymbionts or ‘chloroplasts’. An endosymbiotoic event between an ancestral eukaryotic cell and a cyanobacterium that gave rise to plants is estimated to have occurred ∼1.5 billion years ago. Free-living cyanobacteria still exist today and are responsible for ∼50% of the world's photosynthesis. Cyanobacteria themselves are thought to have evolved from simpler photosynthetic bacteria that use either organic or inorganic compounds such a hydrogen sulfide as a source of electrons rather than water and thus do not produce oxygen.
The site of photosynthesis in plants
In land plants, the principal organs of photosynthesis are the leaves ( Figure 2 A). Leaves have evolved to expose the largest possible area of green tissue to light and entry of CO 2 to the leaf is controlled by small holes in the lower epidermis called stomata ( Figure 2 B). The size of the stomatal openings is variable and regulated by a pair of guard cells, which respond to the turgor pressure (water content) of the leaf, thus when the leaf is hydrated, the stomata can open to allow CO 2 in. In contrast, when water is scarce, the guard cells lose turgor pressure and close, preventing the escape of water from the leaf via transpiration.

( A ) The model plant Arabidopsis thaliana . ( B ) Basic structure of a leaf shown in cross-section. Chloroplasts are shown as green dots within the cells. ( C ) An electron micrograph of an Arabidopsis chloroplast within the leaf. ( D ) Close-up region of the chloroplast showing the stacked structure of the thylakoid membrane.
Within the green tissue of the leaf (mainly the mesophyll) each cell (∼100 μm in length) contains ∼100 chloroplasts (2–3 μm in length), the tiny organelles where photosynthesis takes place. The chloroplast has a complex structure ( Figure 2 C, D) with two outer membranes (the envelope), which are colourless and do not participate in photosynthesis, enclosing an aqueous space (the stroma) wherein sits a third membrane known as the thylakoid, which in turn encloses a single continuous aqueous space called the lumen.
The light reactions of photosynthesis involve light-driven electron and proton transfers, which occur in the thylakoid membrane, whereas the dark reactions involve the fixation of CO 2 into carbohydrate, via the Calvin–Benson cycle, which occurs in the stroma ( Figure 3 ). The light reactions involve electron transfer from water to NADP + to form NADPH and these reactions are coupled to proton transfers that lead to the phosphorylation of adenosine diphosphate (ADP) into ATP. The Calvin–Benson cycle uses ATP and NADPH to convert CO 2 into carbohydrates ( Figure 3 ), regenerating ADP and NADP + . The light and dark reactions are therefore mutually dependent on one another.

The light reactions of photosynthesis take place in the thylakoid membrane, whereas the dark reactions are located in the chloroplast stroma.
Photosynthetic electron and proton transfer chain
The light-driven electron transfer reactions of photosynthesis begin with the splitting of water by Photosystem II (PSII). PSII is a chlorophyll–protein complex embedded in the thylakoid membrane that uses light to oxidize water to oxygen and reduce the electron acceptor plastoquinone to plastoquinol. Plastoquinol in turn carries the electrons derived from water to another thylakoid-embedded protein complex called cytochrome b 6 f (cyt b 6 f ). cyt b 6 f oxidizes plastoquinol to plastoquinone and reduces a small water-soluble electron carrier protein plastocyanin, which resides in the lumen. A second light-driven reaction is then carried out by another chlorophyll protein complex called Photosystem I (PSI). PSI oxidizes plastocyanin and reduces another soluble electron carrier protein ferredoxin that resides in the stroma. Ferredoxin can then be used by the ferredoxin–NADP + reductase (FNR) enzyme to reduce NADP + to NADPH. This scheme is known as the linear electron transfer pathway or Z-scheme ( Figure 4 ).

The linear electron transfer pathway from water to NADP + to form NADPH results in the formation of a proton gradient across the thylakoid membrane that is used by the ATP synthase enzyme to make ATP.
The Z-scheme, so-called since it resembles the letter ‘Z’ when turned on its side ( Figure 5 ), thus shows how the electrons move from the water–oxygen couple (+820 mV) via a chain of redox carriers to NADP + /NADPH (−320 mV) during photosynthetic electron transfer. Generally, electrons are transferred from redox couples with low potentials (good reductants) to those with higher potentials (good oxidants) (e.g. during respiratory electron transfer in mitochondria) since this process is exergonic (see Box 2 ). However, photosynthetic electron transfer also involves two endergonic steps, which occur at PSII and at PSI and require an energy input in the form of light. The light energy is used to excite an electron within a chlorophyll molecule residing in PSII or PSI to a higher energy level; this excited chlorophyll is then able to reduce the subsequent acceptors in the chain. The oxidized chlorophyll is then reduced by water in the case of PSII and plastocyanin in the case of PSI.

The main components of the linear electron transfer pathway are shown on a scale of redox potential to illustrate how two separate inputs of light energy at PSI and PSII result in the endergonic transfer of electrons from water to NADP + .
Relationship between redox potentials and standard free energy changes

The water-splitting reaction at PSII and plastoquinol oxidation at cyt b 6 f result in the release of protons into the lumen, resulting in a build-up of protons in this compartment relative to the stroma. The difference in the proton concentration between the two sides of the membrane is called a proton gradient. The proton gradient is a store of free energy (similar to a gradient of ions in a battery) that is utilized by a molecular mechanical motor ATP synthase, which resides in the thylakoid membrane ( Figure 4 ). The ATP synthase allows the protons to move down their concentration gradient from the lumen (high H + concentration) to the stroma (low H + concentration). This exergonic reaction is used to power the endergonic synthesis of ATP from ADP and inorganic phosphate (P i ). This process of photophosphorylation is thus essentially similar to oxidative phosphorylation, which occurs in the inner mitochondrial membrane during respiration.
An alternative electron transfer pathway exists in plants and algae, known as cyclic electron flow. Cyclic electron flow involves the recycling of electrons from ferredoxin to plastoquinone, with the result that there is no net production of NADPH; however, since protons are still transferred into the lumen by oxidation of plastoquinol by cyt b 6 f , ATP can still be formed. Thus photosynthetic organisms can control the ratio of NADPH/ATP to meet metabolic need by controlling the relative amounts of cyclic and linear electron transfer.
How the photosystems work
Light absorption by pigments.
Photosynthesis begins with the absorption of light by pigments molecules located in the thylakoid membrane. The most well-known of these is chlorophyll, but there are also carotenoids and, in cyanobacteria and some algae, bilins. These pigments all have in common within their chemical structures an alternating series of carbon single and double bonds, which form a conjugated system π–electron system ( Figure 6 ).

The chemical structures of the chlorophyll and carotenoid pigments present in the thylakoid membrane. Note the presence in each of a conjugated system of carbon–carbon double bonds that is responsible for light absorption.
The variety of pigments present within each type of photosynthetic organism reflects the light environment in which it lives; plants on land contain chlorophylls a and b and carotenoids such as β-carotene, lutein, zeaxanthin, violaxanthin, antheraxanthin and neoxanthin ( Figure 6 ). The chlorophylls absorb blue and red light and so appear green in colour, whereas carotenoids absorb light only in the blue and so appear yellow/red ( Figure 7 ), colours more obvious in the autumn as chlorophyll is the first pigment to be broken down in decaying leaves.

Chlorophylls absorb light energy in the red and blue part of the visible spectrum, whereas carotenoids only absorb light in the blue/green.
Light, or electromagnetic radiation, has the properties of both a wave and a stream of particles (light quanta). Each quantum of light contains a discrete amount of energy that can be calculated by multiplying Planck's constant, h (6.626×10 −34 J·s) by ν, the frequency of the radiation in cycles per second (s −1 ):
The frequency (ν) of the light and so its energy varies with its colour, thus blue photons (∼450 nm) are more energetic than red photons (∼650 nm). The frequency (ν) and wavelength (λ) of light are related by:
where c is the velocity of light (3.0×10 8 m·s −1 ), and the energy of a particular wavelength (λ) of light is given by:
Thus 1 mol of 680 nm photons of red light has an energy of 176 kJ·mol −1 .
The electrons within the delocalized π system of the pigment have the ability to jump up from the lowest occupied molecular orbital (ground state) to higher unoccupied molecular electron orbitals (excited states) via the absorption of specific wavelengths of light in the visible range (400–725 nm). Chlorophyll has two excited states known as S 1 and S 2 and, upon interaction of the molecule with a photon of light, one of its π electrons is promoted from the ground state (S 0 ) to an excited state, a process taking just 10 −15 s ( Figure 8 ). The energy gap between the S 0 and S 1 states is spanned by the energy provided by a red photon (∼600–700 nm), whereas the energy gap between the S 0 and S 2 states is larger and therefore requires a more energetic (shorter wavelength, higher frequency) blue photon (∼400–500 nm) to span the energy gap.

Photons with slightly different energies (colours) excite each of the vibrational substates of each excited state (as shown by variation in the size and colour of the arrows).
Upon excitation, the electron in the S 2 state quickly undergoes losses of energy as heat through molecular vibration and undergoes conversion into the energy of the S 1 state by a process called internal conversion. Internal conversion occurs on a timescale of 10 −12 s. The energy of a blue photon is thus rapidly degraded to that of a red photon. Excitation of the molecule with a red photon would lead to promotion of an electron to the S 1 state directly. Once the electron resides in the S 1 state, it is lower in energy and thus stable on a somewhat longer timescale (10 −9 s). The energy of the excited electron in the S 1 state can have one of several fates: it could return to the ground state (S 0 ) by emission of the energy as a photon of light (fluorescence), or it could be lost as heat due to internal conversion between S 1 and S 0 . Alternatively, if another chlorophyll is nearby, a process known as excitation energy transfer (EET) can result in the non-radiative exchange of energy between the two molecules ( Figure 9 ). For this to occur, the two chlorophylls must be close by (<7 nm), have a specific orientation with respect to one another, and excited state energies that overlap (are resonant) with one another. If these conditions are met, the energy is exchanged, resulting in a mirror S 0 →S 1 transition in the acceptor molecule and a S 1 →S 0 transition in the other.

Two chlorophyll molecules with resonant S 1 states undergo a mirror transition resulting in the non-radiative transfer of excitation energy between them.
Light-harvesting complexes
In photosynthetic systems, chlorophylls and carotenoids are found attached to membrane-embedded proteins known as light-harvesting complexes (LHCs). Through careful binding and orientation of the pigment molecules, absorbed energy can be transferred among them by EET. Each pigment is bound to the protein by a series of non-covalent bonding interactions (such as, hydrogen bonds, van der Waals interactions, hydrophobic interaction and co-ordination bonds between lone pair electrons of residues such as histidine in the protein and the Mg 2+ ion in chlorophyll); the protein structure is such that each bound pigment experiences a slightly different environment in terms of the surrounding amino acid side chains, lipids, etc., meaning that the S 1 and S 2 energy levels are shifted in energy with respect to that of other neighbouring pigment molecules. The effect is to create a range of pigment energies that act to ‘funnel’ the energy on to the lowest-energy pigments in the LHC by EET.
Reaction centres
A photosystem consists of numerous LHCs that form an antenna of hundreds of pigment molecules. The antenna pigments act to collect and concentrate excitation energy and transfer it towards a ‘special pair’ of chlorophyll molecules that reside in the reaction centre (RC) ( Figure 10 ). Unlike the antenna pigments, the special pair of chlorophylls are ‘redox-active’ in the sense that they can return to the ground state (S 0 ) by the transfer of the electron residing in the S 1 excited state (Chl*) to another species. This process is known as charge separation and result in formation of an oxidized special pair (Chl + ) and a reduced acceptor (A − ). The acceptor in PSII is plastoquinone and in PSI it is ferredoxin. If the RC is to go on functioning, the electron deficiency on the special pair must be made good, in PSII the electron donor is water and in PSI it is plastocyanin.

Light energy is captured by the antenna pigments and transferred to the special pair of RC chlorophylls which undergo a redox reaction leading to reduction of an acceptor molecule. The oxidized special pair is regenerated by an electron donor.
It is worth asking why photosynthetic organisms bother to have a large antenna of pigments serving an RC rather than more numerous RCs. The answer lies in the fact that the special pair of chlorophylls alone have a rather small spatial and spectral cross-section, meaning that there is a limit to the amount of light they can efficiently absorb. The amount of light they can practically absorb is around two orders of magnitude smaller than their maximum possible turnover rate, Thus LHCs act to increase the spatial (hundreds of pigments) and spectral (several types of pigments with different light absorption characteristics) cross-section of the RC special pair ensuring that its turnover rate runs much closer to capacity.
Photosystem II
PSII is a light-driven water–plastoquinone oxidoreductase and is the only enzyme in Nature that is capable of performing the difficult chemistry of splitting water into protons, electrons and oxygen ( Figure 11 ). In principle, water is an extremely poor electron donor since the redox potential of the water–oxygen couple is +820 mV. PSII uses light energy to excite a special pair of chlorophylls, known as P680 due to their 680 nm absorption peak in the red part of the spectrum. P680* undergoes charge separation that results in the formation of an extremely oxidizing species P680 + which has a redox potential of +1200 mV, sufficient to oxidize water. Nonetheless, since water splitting involves four electron chemistry and charge separation only involves transfer of one electron, four separate charge separations (turnovers of PSII) are required to drive formation of one molecule of O 2 from two molecules of water. The initial electron donation to generate the P680 from P680 + is therefore provided by a cluster of manganese ions within the oxygen-evolving complex (OEC), which is attached to the lumen side of PSII ( Figure 12 ). Manganese is a transition metal that can exist in a range of oxidation states from +1 to +5 and thus accumulates the positive charges derived from each light-driven turnover of P680. Progressive extraction of electrons from the manganese cluster is driven by the oxidation of P680 within PSII by light and is known as the S-state cycle ( Figure 12 ). After the fourth turnover of P680, sufficient positive charge is built up in the manganese cluster to permit the splitting of water into electrons, which regenerate the original state of the manganese cluster, protons, which are released into the lumen and contribute to the proton gradient used for ATP synthesis, and the by-product O 2 . Thus charge separation at P680 provides the thermodynamic driving force, whereas the manganese cluster acts as a catalyst for the water-splitting reaction.

The organization of PSII and its light-harvesting antenna. Protein is shown in grey, with chlorophylls in green and carotenoids in orange. Drawn from PDB code 3JCU

Progressive extraction of electrons from the manganese cluster is driven by the oxidation of P680 within PSII by light. Each of the electrons given up by the cluster is eventually repaid at the S 4 to S 0 transition when molecular oxygen (O 2 ) is formed. The protons extracted from water during the process are deposited into the lumen and contribute to the protonmotive force.
The electrons yielded by P680* following charge separation are not passed directly to plastoquinone, but rather via another acceptor called pheophytin, a porphyrin molecule lacking the central magnesium ion as in chlorophyll. Plastoquinone reduction to plastoquinol requires two electrons and thus two molecules of plastoquinol are formed per O 2 molecule evolved by PSII. Two protons are also taken up upon formation of plastoquinol and these are derived from the stroma. PSII is found within the thylakoid membrane of plants as a dimeric RC complex surrounded by a peripheral antenna of six minor monomeric antenna LHC complexes and two to eight trimeric LHC complexes, which together form a PSII–LHCII supercomplex ( Figure 11 ).
Photosystem I
PSI is a light-driven plastocyanin–ferredoxin oxidoreductase ( Figure 13 ). In PSI, the special pair of chlorophylls are known as P700 due to their 700 nm absorption peak in the red part of the spectrum. P700* is an extremely strong reductant that is able to reduce ferredoxin which has a redox potential of −450 mV (and is thus is, in principle, a poor electron acceptor). Reduced ferredoxin is then used to generate NADPH for the Calvin–Benson cycle at a separate complex known as FNR. The electron from P700* is donated via another chlorophyll molecule and a bound quinone to a series of iron–sulfur clusters at the stromal side of the complex, whereupon the electron is donated to ferredoxin. The P700 species is regenerated form P700 + via donation of an electron from the soluble electron carrier protein plastocyanin.

The organization of PSI and its light-harvesting antenna. Protein is shown in grey, with chlorophylls in green and carotenoids in orange. Drawn from PDB code 4XK8.
PSI is found within the thylakoid membrane as a monomeric RC surrounded on one side by four LHC complexes known as LHCI. The PSI–LHCI supercomplex is found mainly in the unstacked regions of the thylakoid membrane ( Figure 13 ).
Other electron transfer chain components
Plastoquinone/plastoquinol.
Plastoquinone is a small lipophilic electron carrier molecule that resides within the thylakoid membrane and carries two electrons and two protons from PSII to the cyt b 6 f complex. It has a very similar structure to that of the molecule ubiquinone (coenzyme Q 10 ) in the mitochondrial inner membrane.
Cytochrome b 6 f complex
The cyt b 6 f complex is a plastoquinol–plastocyanin oxidoreductase and possess a similar structure to that of the cytochrome bc 1 complex (complex III) in mitochondria ( Figure 14 A). As with Complex III, cyt b 6 f exists as a dimer in the membrane and carries out both the oxidation and reduction of quinones via the so-called Q-cycle. The Q-cycle ( Figure 14 B) involves oxidation of one plastoquinol molecule at the Qp site of the complex, both protons from this molecule are deposited in the lumen and contribute to the proton gradient for ATP synthesis. The two electrons, however, have different fates. The first is transferred via an iron–sulfur cluster and a haem cofactor to the soluble electron carrier plastocyanin (see below). The second electron derived from plastoquinol is passed via two separate haem cofactors to another molecule of plastoquinone bound to a separate site (Qn) on the complex, thus reducing it to a semiquinone. When a second plastoquinol molecule is oxidized at Qp, a second molecule of plastocyanin is reduced and two further protons are deposited in the lumen. The second electron reduces the semiquinone at the Qn site which, concomitant with uptake of two protons from the stroma, causes its reduction to plastoquinol. Thus for each pair of plastoquinol molecules oxidized by the complex, one is regenerated, yet all four protons are deposited into the lumen. The Q-cycle thus doubles the number of protons transferred from the stroma to the lumen per plastoquinol molecule oxidized.

( A ) Structure drawn from PDB code 1Q90. ( B ) The protonmotive Q-cycle showing how electrons from plastoquinol are passed to both plastocyanin and plastoquinone, doubling the protons deposited in the lumen for every plastoquinol molecule oxidized by the complex.
Plastocyanin
Plastocyanin is a small soluble electron carrier protein that resides in the thylakoid lumen. The active site of the plastocyanin protein binds a copper ion, which cycles between the Cu 2+ and Cu + oxidation states following its oxidation by PSI and reduction by cyt b 6 f respectively.
Ferredoxin is a small soluble electron carrier protein that resides in the chloroplast stroma. The active site of the ferredoxin protein binds an iron–sulfur cluster, which cycles between the Fe 2+ and Fe 3+ oxidation states following its reduction by PSI and oxidation by the FNR complex respectively.
Ferredoxin–NADP + reductase
The FNR complex is found in both soluble and thylakoid membrane-bound forms. The complex binds a flavin–adenine dinucleotide (FAD) cofactor at its active site, which accepts two electrons from two molecules of ferredoxin before using them reduce NADP + to NADPH.
ATP synthase
The ATP synthase enzyme is responsible for making ATP from ADP and P i ; this endergonic reaction is powered by the energy contained within the protonmotive force. According to the structure, 4.67 H + are required for every ATP molecule synthesized by the chloroplast ATP synthase. The enzyme is a rotary motor which contains two domains: the membrane-spanning F O portion which conducts protons from the lumen to the stroma, and the F 1 catalytic domain that couples this exergonic proton movement to ATP synthesis.
Membrane stacking and the regulation of photosynthesis
Within the thylakoid membrane, PSII–LHCII supercomplexes are packed together into domains known as the grana, which associate with one another to form grana stacks. PSI and ATP synthase are excluded from these stacked PSII–LHCII regions by steric constraints and thus PSII and PSI are segregated in the thylakoid membrane between the stacked and unstacked regions ( Figure 15 ). The cyt b 6 f complex, in contrast, is evenly distributed throughout the grana and stromal lamellae. The evolutionary advantage of membrane stacking is believed to be a higher efficiency of electron transport by preventing the fast energy trap PSI from ‘stealing’ excitation energy from the slower trap PSII, a phenomenon known as spillover. Another possible advantage of membrane stacking in thylakoids may be the segregation of the linear and cyclic electron transfer pathways, which might otherwise compete to reduce plastoquinone. In this view, PSII, cyt b 6 f and a sub-fraction of PSI closest to the grana is involved in linear flow, whereas PSI and cyt b 6 f in the stromal lamellae participates in cyclic flow. The cyclic electron transfer pathway recycles electrons from ferredoxin back to plastoquinone and thus allows protonmotive force generation (and ATP synthesis) without net NADPH production. Cyclic electron transfer thereby provides the additional ATP required for the Calvin–Benson cycle (see below).

( A ) Electron micrograph of the thylakoid membrane showing stacked grana and unstacked stromal lamellae regions. ( B ) Model showing the distribution of the major complexes of photosynthetic electron and proton transfer between the stacked grana and unstacked stromal lamellae regions.
‘Dark’ reactions: the Calvin–Benson cycle
CO 2 is fixed into carbohydrate via the Calvin–Benson cycle in plants, which consumes the ATP and NADPH produced during the light reactions and thus in turn regenerates ADP, P i and NADP + . In the first step of the Calvin–Benson cycle ( Figure 16 ), CO 2 is combined with a 5-carbon (5C) sugar, ribulose 1,5-bisphosphate in a reaction catalysed by the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco). The reaction forms an unstable 6C intermediate that immediately splits into two molecules of 3-phosphoglycerate. 3-Phosphoglycerate is first phosphorylated by 3-phosphoglycerate kinase using ATP to form 1,3-bisphosphoglycerate. 1,3-Bisphosphoglycerate is then reduced by glyceraldehyde 3-phosphate dehydrogenase using NADPH to form glyceraldehyde 3-phosphate (GAP, a triose or 3C sugar) in reactions, which are the reverse of glycolysis. For every three CO 2 molecules initially combined with ribulose 1,5-bisphopshate, six molecules of GAP are produced by the subsequent steps. However only one of these six molecules can be considered as a product of the Calvin–Benson cycle since the remaining five are required to regenerate ribulose 1,5-bisphosphate in a complex series of reactions that also require ATP. The one molecule of GAP that is produced for each turn of the cycle can be quickly converted by a range of metabolic pathways into amino acids, lipids or sugars such as glucose. Glucose in turn may be stored as the polymer starch as large granules within chloroplasts.

Overview of the biochemical pathway for the fixation of CO 2 into carbohydrate in plants.
A complex biochemical ‘dance’ ( Figure 16 ) is then involved in the regeneration of three ribulose 1,5-bisphosphate (5C) from the remaining five GAP (3C) molecules. The regeneration begins with the conversion of two molecules of GAP into dihydroxyacetone phosphate (DHAP) by triose phosphate isomerase; one of the DHAP molecules is the combined with another GAP molecule to make fructose 1,6-bisphosphate (6C) by aldolase. The fructose 1,6-bisphosphate is then dephosphorylated by fructose-1,6-bisphosphatase to yield fructose 6-phosphate (6C) and releasing P i . Two carbons are then removed from fructose 6-phosphate by transketolase, generating erythrose 4-phosphate (4C); the two carbons are transferred to another molecule of GAP generating xylulose 5-phosphate (5C). Another DHAP molecule, formed from GAP by triose phosphate isomerase is then combined with the erythrose 4-phosphate by aldolase to form sedoheptulose 1,7-bisphosphate (7C). Sedoheptulose 1,7-bisphosphate is then dephosphorylated to sedoheptulose 7-phosphate (7C) by sedoheptulose-1,7-bisphosphatase releasing P i . Sedoheptulose 7-phosphate has two carbons removed by transketolase to produce ribose 5-phosphate (5C) and the two carbons are transferred to another GAP molecule producing another xylulose 5-phosphate (5C). Ribose 5-phosphate and the two molecules of xylulose 5-phosphate (5C) are then converted by phosphopentose isomerase to three molecules of ribulose 5-phosphate (5C). The three ribulose 5-phosphate molecules are then phosphorylated using three ATP by phosphoribulokinase to regenerate three ribulose 1,5-bisphosphate (5C).
Overall the synthesis of 1 mol of GAP requires 9 mol of ATP and 6 mol of NADPH, a required ratio of 1.5 ATP/NADPH. Linear electron transfer is generally thought to supply ATP/NADPH in a ratio of 1.28 (assuming an H + /ATP ratio of 4.67) with the shortfall of ATP believed to be provided by cyclic electron transfer reactions. Since the product of the Calvin cycle is GAP (a 3C sugar) the pathway is often referred to as C 3 photosynthesis and plants that utilize it are called C 3 plants and include many of the world's major crops such as rice, wheat and potato.
Many of the enzymes involved in the Calvin–Benson cycle (e.g. transketolase, glyceraldehyde-3-phosphate dehydrogenase and aldolase) are also involved in the glycolysis pathway of carbohydrate degradation and their activity must therefore be carefully regulated to avoid futile cycling when light is present, i.e. the unwanted degradation of carbohydrate. The regulation of the Calvin–Benson cycle enzymes is achieved by the activity of the light reactions, which modify the environment of the dark reactions (i.e. the stroma). Proton gradient formation across the thylakoid membrane during the light reactions increases the pH and also increases the Mg 2+ concentration in the stroma (as Mg 2+ flows out of the lumen as H + flows in to compensate for the influx of positive charges). In addition, by reducing ferredoxin and NADP + , PSI changes the redox state of the stroma, which is sensed by the regulatory protein thioredoxin. Thioredoxin, pH and Mg 2+ concentration play a key role in regulating the activity of the Calvin–Benson cycle enzymes, ensuring the activity of the light and dark reactions is closely co-ordinated.
It is noteworthy that, despite the complexity of the dark reactions outlined above, the carbon fixation step itself (i.e. the incorporation of CO 2 into carbohydrate) is carried out by a single enzyme, Rubisco. Rubisco is a large multisubunit soluble protein complex found in the chloroplast stroma. The complex consists of eight large (56 kDa) subunits, which contain both catalytic and regulatory domains, and eight small subunits (14 kDa), which enhance the catalytic function of the L subunits ( Figure 17 A). The carboxylation reaction carried out by Rubisco is highly exergonic (Δ G °=−51.9 kJ·mol- 1 ), yet kinetically very slow (just 3 s −1 ) and begins with the protonation of ribulose 1,5-bisphosphate to form an enediolate intermediate which can be combined with CO 2 to form an unstable 6C intermediate that is quickly hydrolysed to yield two 3C 3-phosphoglycerate molecules. The active site in the Rubisco enzyme contains a key lysine residue, which reacts with another (non-substrate) molecule of CO 2 to form a carbamate anion that is then able to bind Mg 2+ . The Mg 2+ in the active site is essential for the catalytic function of Rubisco, playing a key role in binding ribulose 1,5-bisphosphate and activating it such that it readily reacts with CO 2.. Rubisco activity is co-ordinated with that of the light reactions since carbamate formation requires both high Mg 2+ concentration and alkaline conditions, which are provided by the light-driven changes in the stromal environment discussed above ( Figure 17 B).

( A ) Structure of the Rubisco enzyme (the large subunits are shown in blue and the small subunits in green); four of each type of subunit are visible in the image. Drawn from PDB code 1RXO. ( B ) Activation of the lysine residue within the active site of Rubisco occurs via elevated stromal pH and Mg 2+ concentration as a result of the activity of the light reactions.
In addition to carboxylation, Rubisco also catalyses a competitive oxygenation reaction, known as photorespiration, that results in the combination of ribulose 1,5-bisphosphate with O 2 rather than CO 2 . In the oxygenation reaction, one rather than two molecules of 3-phosphoglycerate and one molecule of a 2C sugar known as phosphoglycolate are produced by Rubisco. The phosphoglycolate must be converted in a series of reactions that regenerate one molecule of 3-phosphoglycerate and one molecule of CO 2 . These reactions consume additional ATP and thus result in an energy loss to the plant. Although the oxygenation reaction of Rubisco is much less favourable than the carboxylation reaction, the relatively high concentration of O 2 in the leaf (250 μM) compared with CO 2 (10 μM) means that a significant amount of photorespiration is always occurring. Under normal conditions, the ratio of carboxylation to oxygenation is between 3:1 and 4:1. However, this ratio can be decreased with increasing temperature due to decreased CO 2 concentration in the leaf, a decrease in the affinity of Rubisco for CO 2 compared with O 2 and an increase in the maximum rate of the oxygenation reaction compared with the carboxylation reaction. The inefficiencies of the Rubisco enzyme mean that plants must produce it in very large amounts (∼30–50% of total soluble protein in a spinach leaf) to achieve the maximal photosynthetic rate.
CO 2 -concentrating mechanisms
To counter photorespiration, plants, algae and cyanobacteria have evolved different CO 2 -concentrating mechanisms CCMs that aim to increase the concentration of CO 2 relative to O 2 in the vicinity of Rubisco. One such CCM is C 4 photosynthesis that is found in plants such as maize, sugar cane and savanna grasses. C 4 plants show a specialized leaf anatomy: Kranz anatomy ( Figure 18 ). Kranz, German for wreath, refers to a bundle sheath of cells that surrounds the central vein within the leaf, which in turn are surrounded by the mesophyll cells. The mesophyll cells in such leaves are rich in the enzyme phosphoenolpyruvate (PEP) carboxylase, which fixes CO 2 into a 4C carboxylic acid: oxaloaceatate. The oxaloacetate formed by the mesophyll cells is reduced using NADPH to malate, another 4C acid: malate. The malate is then exported from the mesophyll cells to the bundle sheath cells, where it is decarboxylated to pyruvate thus regenerating NADPH and CO 2 . The CO 2 is then utilized by Rubisco in the Calvin cycle. The pyruvate is in turn returned to the mesophyll cells where it is phosphorylated using ATP to reform PEP ( Figure 19 ). The advantage of C 4 photosynthesis is that CO 2 accumulates at a very high concentration in the bundle sheath cells that is then sufficient to allow Rubisco to operate efficiently.

Plants growing in hot, bright and dry conditions inevitably have to have their stomata closed for large parts of the day to avoid excessive water loss and wilting. The net result is that the internal CO 2 concentration in the leaf is very low, meaning that C 3 photosynthesis is not possible. To counter this limitation, another CCM is found in succulent plants such as cacti. The Crassulaceae fix CO 2 into malate during the day via PEP carboxylase, store it within the vacuole of the plant cell at night and then release it within their tissues by day to be fixed via normal C 3 photosynthesis. This is termed crassulacean acid metabolism (CAM).
Acknowledgments
I thank Professor Colin Osborne (University of Sheffield, Sheffield, U.K.) for useful discussions on the article, Dr Dan Canniffe (Penn State University, Pennsylvania, PA, U.S.A.) for providing pure pigment spectra and Dr P.J. Weaire (Kingston University, Kingston-upon-Thames, U.K.) for his original Photosynthesis BASC article (1994) on which this essay is partly based.
Abbreviations
This article is a reviewed, revised and updated version of the following ‘Biochemistry Across the School Curriculum’ (BASC) booklet: Weaire, P.J. (1994) Photosynthesis . For further information and to provide feedback on this or any other Biochemical Society education resource, please contact [email protected]. For further information on other Biochemical Society publications, please visit www.biochemistry.org/publications .
Competing Interests
The Author declares that there are no competing interests associated with this article.
Recommended reading and key publications
- Blankenship R.E. Early evolution of photosynthesis. Plant Physiol. 2010; 154 :434–438. doi: 10.1104/pp.110.161687. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Blankenship R.E. Molecular Mechanisms of Photosynthesis. Chichester: Wiley–Blackwell Publishing; 2014. [ Google Scholar ]
- Nelson N., Ben Shem A. The complex architecture of oxygenic photosynthesis. Nat. Rev. 2004; 5 :1–12. doi: 10.1038/nrm1525. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Raines C. The Calvin cycle revisited. Photosynth. Res. 2003; 75 :1–10. doi: 10.1023/A:1022421515027. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ruban A.V. Evolution under the sun: optimizing light harvesting in photosynthesis. J. Exp. Bot. 2015; 66 :7–23. doi: 10.1093/jxb/eru400. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sage R.F. The evolution of C 4 photosynthesis. New Phytol. 2004; 161 :341–370. doi: 10.1111/j.1469-8137.2004.00974.x. [ PubMed ] [ CrossRef ] [ Google Scholar ]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 27 May 2024
Potential decoupling of CO 2 and Hg uptake process by global vegetation in the 21st century
- Tengfei Yuan 1 ,
- Shaojian Huang 1 ,
- Peng Zhang 1 ,
- Zhengcheng Song 1 , 2 , 3 ,
- Jun Ge ORCID: orcid.org/0000-0002-8876-1650 1 , 3 ,
- Xin Miao ORCID: orcid.org/0000-0002-5162-402X 1 ,
- Yujuan Wang ORCID: orcid.org/0000-0002-5907-0578 1 ,
- Qiaotong Pang 1 ,
- Dong Peng 1 ,
- Peipei Wu 1 ,
- Junjiong Shao ORCID: orcid.org/0000-0002-2412-2892 4 ,
- Peipei Zhang 5 ,
- Yabo Wang 6 ,
- Hongyan Guo 7 ,
- Weidong Guo ORCID: orcid.org/0000-0003-0299-6393 1 &
- Yanxu Zhang ORCID: orcid.org/0000-0001-7770-3466 1 , 2 , 3
Nature Communications volume 15 , Article number: 4490 ( 2024 ) Cite this article
174 Accesses
3 Altmetric
Metrics details
- Biogeochemistry
- Environmental sciences
- Plant sciences
Mercury (Hg), a potent neurotoxin posing risks to human health, is cycled through vegetation uptake, which is susceptible to climate change impacts. However, the extent and pattern of these impacts are largely unknown, obstructing predictions of Hg’s fate in terrestrial ecosystems. Here, we evaluate the effects of climate change on vegetation elemental Hg [Hg(0)] uptake using a state-of-the-art global terrestrial Hg model (CLM5-Hg) that incorporates plant physiology. In a business-as-usual scenario, the terrestrial Hg(0) sink is predicted to decrease by 1870 Mg yr −1 in 2100, that is ~60% lower than the present-day condition. We find a potential decoupling between the trends of CO 2 assimilation and Hg(0) uptake process by vegetation in the 21st century, caused by the decreased stomatal conductance with increasing CO 2 . This implies a substantial influx of Hg into aquatic ecosystems, posing an elevated threat that warrants consideration during the evaluation of the effectiveness of the Minamata Convention.
Similar content being viewed by others
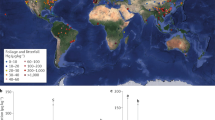
Vegetation uptake of mercury and impacts on global cycling
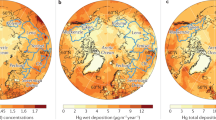
Arctic mercury cycling

Coastal vegetation and estuaries are collectively a greenhouse gas sink
Introduction.
Mercury (Hg) is a pervasive toxic pollutant causing adverse effects on human health at a global scale 1 . Additionally, it endangers ecosystems by bioaccumulating in food chains, affecting biodiversity and disrupting ecological balances 2 . Anthropogenic activities, such as fossil fuel combustion and metal mining, have significantly increased Hg emissions and caused widespread environmental Hg contamination since the industrial era 3 , 4 . Vegetation within terrestrial ecosystems can absorb large amounts of atmospheric gaseous elementary Hg [Hg(0)] (2200–3600 Mg year −1 ), acting as a major sink for the atmosphere in the present-day Hg cycles 5 , 6 . Long-living vegetation not only stores present-day Hg but also Hg emitted into the atmosphere decades ago 7 . Climate-related factors, such as rising temperatures and elevated carbon dioxide (eCO 2 ), are profoundly affecting the growth and physiological processes of vegetation 8 , 9 . However, their cascading effects on the terrestrial Hg cycle, especially the uptake of Hg(0) by vegetation, remain unclear. Here, we evaluate this effect by using a coupled climate–land–mercury model running for the twenty-first century.
Previous research has revealed complex impact pathways of climate change on vegetation Hg(0) uptake. The higher temperature was found to boost vegetation’s ability to absorb atmospheric Hg(0) in glacier retreat areas 10 . Yet, the rising temperatures often lead to localized droughts, which are likely to weaken the Hg sink in terrestrial ecosystems 11 . Furthermore, the alteration in global precipitation patterns can affect the Hg sink of forests 12 . The future increases in vegetation density driven by CO 2 fertilization are expected to enhance the Hg(0) dry deposition velocity 13 , 14 . Stomatal uptake of Hg(0) by foliage was found to be inhibited under high vapor pressure deficit (VPD) conditions and proved to be sensitive to extreme climate events 15 , 16 , 17 . However, previous studies have not systematically considered the interaction of climate change, vegetation dynamics, and Hg processes, and some have primarily focused only on specific regions 14 , 18 .
Therefore, our research aims to exam how vegetation-regulated atmospheric Hg(0) deposition will change under the impact of future climate change. We hypothesize that the climate will influence global vegetation Hg(0) uptake by altering the plant physiology such as the stomatal activities, with CO 2 and other meteorological factors as important driver factors. This study uses the Community Land Model–Hg (CLM5-Hg) within the Community Earth System Model (CESM), which incorporates a dynamic plant growth framework that includes the stomatal uptake process of Hg(0) and various plant functional types (PFTs) 19 . This model also encompasses the comprehensive biogeochemical cycling of Hg in terrestrial ecosystems. It is forced by different future climate scenarios throughout the twenty-first century: (i) the Shared Socioeconomic Pathway (SSP) 1-2.6, a.k.a. the “2 °C scenario” representing a sustainability framework; (ii) the SSP3-7.0, representing a medium-high reference within the socio-economic context of “regional rivalry”; (iii) SSP5-8.5, a.k.a. the “business-as-usual”, considered as the worst-case scenario within a high fossil fuel-intensive world 20 . These future scenarios are compared against the present-day simulation (baseline case). We also include a pre-industrial scenario (ca. 1850) for comparison. Furthermore, we design sensitivity experiments by alternatively changing specific climate-related factors for the SSP5-8.5 scenario while maintaining others consistent with the baseline scenario (Supplementary Table 1 ). These experiments can diagnose and compare the influence of individual factors on the uptake of Hg(0) by vegetation in terrestrial ecosystems. We consider factors including atmospheric CO 2 concentration (with a focus on biogeochemical effects only), precipitation, temperature, humidity, pressure, radiation, and wind. We keep the anthropogenic Hg emissions and the atmospheric Hg concentrations constant for all scenarios to highlight the impact of climate factors, and remove the effects of land use and land cover change (LULCC) and aerosols (see “Methods”).
Results and discussion
Reduced hg(0) uptake.
Our results indicated that, under future climate change scenarios, the biogeochemical effects of elevated CO 2 emerge as the dominant factor influencing vegetation Hg(0) uptake. This uptake represents the gross uptake of atmospheric Hg(0) through both stomatal and cuticular (non-stomatal) processes and does not include the immediate re-emission from foliage. In the SSP5-8.5 (business-as-usual) scenario where only CO 2 concentration is altered and other climatological factors kept as present day, the global Hg(0) uptake decreases by 1870 Mg year −1 or 59.6% in 2100, in comparison to the present-day condition of 3138 Mg year −1 (Fig. 1 ). The most significant changes were simulated in East Asian and the Amazon forests, attributed to their high Hg(0) assimilation compared to other regions 19 . The global vegetation Hg(0) uptake was predicted to further decrease by only 88 Mg year −1 while accounting for the changes of all factors in the SSP5-8.5 scenarios (Supplementary Fig. 2 ). Other climate change factors, such as changing temperature, precipitation, radiation, pressure, and humidity account for a much smaller effect than the biogeochemical effects of eCO 2 alone (Supplementary Figs. 3 and 4b ). We also found no significant interaction between the eCO 2 effect and other factors (Supplementary Fig. 5 ).

a Hg(0) vegetation uptake flux at present day, the value represents the gross uptake of atmospheric Hg(0) through both stomatal and cuticular (non-stomatal) processes and does not include the immediate re-emission from foliage. Observations (represented by rhombuses) are obtained from the global vegetation measurements database (see Materials and Methods). b Change in Hg(0) vegetation uptake flux caused by the biogeochemical effects of eCO 2 between 2100 and the present day under a business-as-usual scenario (SSP5-8.5). The numbers in the figure represent the global total values.
We predicted a higher global vegetation Hg(0) uptake for the other two future scenarios with lower CO 2 levels: 2685 Mg year −1 for the SSP1-2.6 (2 °C) scenario (CO 2 = 445.6 p.p.m.) and 1570 Mg year −1 for SSP3-7.0 (regional rivalry) scenario (CO 2 = 867.2 p.p.m.) vs. 1268 Mg year −1 for SSP5-8.5 (business-as-usual) scenario (CO 2 = 1135.2 p.p.m.) (Fig. 2 ). A slight increase in Hg(0) uptake was simulated during the pre-industrial era: 3324 Mg year −1 when the global average CO 2 is lower at 288 p.p.m. Unlike the future scenarios, we noted the largest impact is contributed by the lower atmospheric humidity in the pre-industrial era (Supplementary Figs. 3d and 4a ). Changes in precipitation and temperature, as well as its interaction with the biogeochemical effects of eCO 2 , significantly affect the uptake of Hg(0) by global vegetation (see Supplementary Figs. 4a and 5a ). This suggested that the biogeochemical impact of eCO 2 on Hg(0) uptake has not yet become dominant when compared with other climate change factors in the pre-industrial era. When all scenarios were considered together, we observe a continuous decrease in the potential of vegetation to uptake Hg(0) in the future as CO 2 levels increase (Supplementary Fig. 12 ).

SSP1-2.6 represents the lowest scenario, termed the “2 °C scenario,” which aims for a sustainable future. SSP3-7.0 represents a moderate scenario, described as a medium-high reference scenario within the socio-economic context of “regional rivalry.” SSP5-8.5 represents the highest scenario, also known as “business-as-usual,” considered the worst-case scenario in a high fossil fuel-intensive world. Solid lines represent atmospheric CO 2 levels during 1850–2000, shaded lines represent the atmospheric CO 2 levels during 2000–2100 under different scenarios.
Direct field evidence that integrated the various processes of terrestrial Hg cycling, and was responsive to multiple climate change factors across different spatiotemporal scales, remained scarce. Previous manipulative experiments focusing on CO 2 enrichment have primarily concentrated on the biogeochemical impact of eCO 2 on vegetation Hg concentrations (Fig. 3a ). The CO 2 concentrations were usually enhanced to 360–610 p.p.m. in these experiments, similar to our future scenarios. Data on Hg veg (Hg flux or Hg concentration of plant) from six eCO 2 experimental studies were integrated into four eCO 2 conditions based on the levels of increased CO 2 concentration (Supplementary Fig. 10 ). Under experimental conditions of a 150 and 200 p.p.m. increase in CO 2 concentration, Hg veg showed a significant decreasing trend ( P < 0.05, Supplementary Fig. 10a ). Despite the lack of significant differences at 253 and 360 p.p.m., even showing an opposite trend at 253 p.p.m. (Supplementary Fig. 10b ), the overall effect of our meta-analysis ( P < 0.01) suggested that eCO 2 had a suppressive effect on vegetation Hg levels (Fig. 3b ). The meta-analysis revealed a significant decrease in vegetation Hg levels as a result of eCO 2 , showing an average decrease in foliage Hg levels or Hg uptake of 5.87% per 100 p.p.m. increase (95% CI, −6.5% to −5.3%). If translated to the increased CO 2 concentration level under our model’s SSP5-8.5 scenario, this change rate reached nearly 50%. The change in terrestrial Hg(0) sink (~60%) simulated by our model was fairly close to the experimentally observed values. Overall, our findings are consistent with existing evidence synthesized from experimental data worldwide, as atmospheric uptake is the major source of Hg in foliage 6 , 21 . Indeed, a multitude of studies, including those employing isotopic techniques, have demonstrated that a predominant portion of atmospheric Hg(0) is assimilated into terrestrial ecosystems via vegetation, primarily through stomatal uptake, accounting for over 80% of the total uptake 22 , 23 .
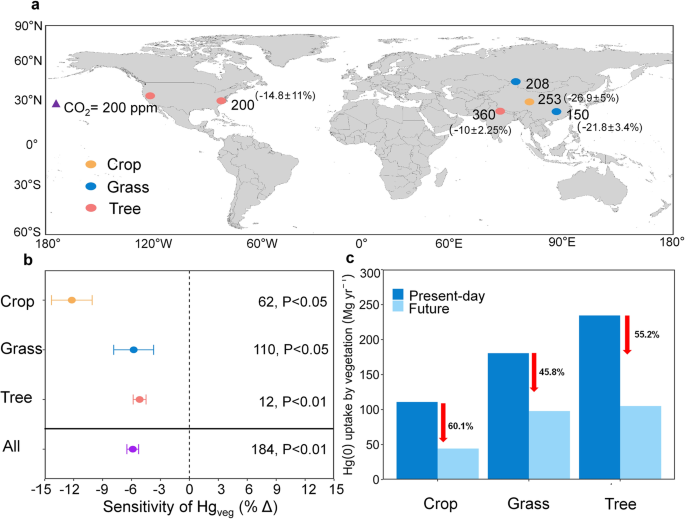
a Global distribution of eCO 2 experiments included in this meta-analysis. Circles of orange, blue, and red indicate experiments on crops, grass, and tree, respectively. The numbers outside the parentheses represent the increase in CO 2 concentration, while the numbers inside the parentheses indicate the corresponding change rate in Hg veg (based on the four integrated Δ CO 2 levels). b The sensitivity of vegetation Hg in response to eCO 2 in different types of plants across different experimental studies. The triangle represents one unit of eCO 2 (100 p.p.m. increase). Each data point represents the weighted mean values; error bars indicate the 95% confidence intervals. The numbers indicate sample sizes with the number of stars representing significant levels. c Modeled annual global Hg(0) uptake by different types of vegetation under present day and future CO 2 levels under the SSP5-8.5 scenario.
We found that this effect varies across different PFTs. The most substantial suppression by eCO 2 was observed in crops (−12.2 ± 1.1% per 100 p.p.m. CO 2 ), while the suppression in grasses (−5.8 ± 1.1% per 100 p.p.m. CO 2 ) and trees (−5.2 ± 0.3% per 100 p.p.m. CO 2 ) was relatively similar (Fig. 3b ). In our model, similar pattern was simulated. We also extracted the Plant Functional Types (PFTs) corresponding to the species used in the meta-analysis experimental data. We found that the modeled global Hg(0) uptake of trees, crops, and grasses in 2100 all showed a substantial decrease under the SSP5-8.5 scenario (Fig. 3c ). Although trees still dominated the reduction of total Hg(0) uptake due to their largest global coverage (Supplementary Fig. 11 ), crops were the most affected by eCO 2 , showing a decline of nearly 60%. Indeed, massive grass species belong to C 4 photosynthetic processes of carbon fixation in plants (C 4 plants), while trees and crops are predominantly C 3 photosynthetic processes of carbon fixation in plants (C 3 plants) 24 . The photosynthesis of the former is less limited by ambient atmospheric CO 2 concentrations and subsequently responds less to eCO 2 than the latter 25 . As C 3 plants dominate global vegetation and account for most of the Earth’s current plant life 26 , an overall significant weakening of global vegetation Hg(0) uptake by the biogeochemical effects of eCO 2 was found (Supplementary Fig. 12 ).
Decoupled CO 2 and Hg
We found the reduced Hg uptake predicted by the model for the future was caused by a decrease in stomatal conductance due to eCO 2 . Vegetation uptakes Hg(0) via diffusion through stomatal pores, which is subsequently fixed by foliage 19 , 23 , 27 . Stomatal conductance depends on its aperture and is associated with plant physiological activities 28 , 29 , 30 (see Eq. 1 in “Methods”). The Medlyn model in our CLM5-Hg model can effectively simulate the response of stomatal conductance to eCO 2 (see Supplementary Figs. 7 and 8 , details in the model validation section of the Supplementary Information). A 42.6% decrease in the stomatal conductance of global vegetation was projected for the year 2100 with eCO 2 under the SSP5-8.5 (business-as-usual) scenario, compared to present-day levels (Fig. 4 ). Our analysis identified that the sunlit stomatal conductance emerges as the key driving factor influencing the observed reduction in Hg(0) uptake. Specifically, the changes of conductance on the sunlit side of leaves were more consistent with the distribution of vegetation Hg(0) uptake than the shaded sides, indicating the changes in the sunlit side as a primary factor contributing to the diminished global vegetation Hg(0) uptake (Fig. 1 ). In general, the sunlit side of leaves receives more direct sunlight and heat, prone to stomatal closure 31 . Conversely, the shaded side has a higher stomatal conductance density and is reserved for gas exchange with relatively stable aperture 32 .

a Changes in global sunlit stomatal conductance (ΔG s _sun) caused by eCO 2 between present day and 2100. b Changes in global shaded stomatal conductance (ΔG s _sha) caused by the eCO 2 between present day and 2100. c Changes in global leaf area index (ΔLAI) caused by the eCO 2 between present day and 2100. d Changes in evapotranspiration from canopy (ΔET c ) caused by the eCO 2 between present day and 2100.
Our study illustrated a complex and extensive feedback mechanism between the terrestrial Hg, water and carbon cycles. The enhancement of photosynthesis is caused by the biogeochemical effect of eCO 2 is accompanied by the loss of plant water content 33 . During this process, plants adjust the stomatal aperture to reduce water transpiration and maximize water use efficiency 34 , 35 . The Medlyn model in our CLM5-Hg model is consistent with this optimal stomatal theory. With eCO 2 , the CO 2 partial pressure at the leaf surface ( C S ) also increased accordingly, leading to enhanced leaf photosynthesis ( A n ) (Eq. ( 1 )). However, A n is constrained by water potential while C s continues to increase in CLM5 29 . This resulted in reduced stomatal conductance ( g s ) with eCO 2 (Supplementary Fig. 13 ), leading to decreased evapotranspiration (Fig. 4d ). This process induced increased soil water storage, enhancing water use efficiency (Supplementary Fig. 14 ). This adaptive mechanism ultimately led to a nonlinear relationship between atmospheric CO 2 concentration and stomatal conductance. Stomatal conductance is directly related to vegetation uptake of Hg(0) in our model (Eqs. ( 1 )–( 4 ), for details refer to “Methods”). This relationship explained the gradual reduction in vegetation uptake of Hg(0). We observed this reduction from the pre-industrial and present-day periods to the SSP1-2.6 scenario (Supplementary Fig. 12 ). Yet, there was a significant decline in the Hg(0) uptake by vegetation from SSP1-2.6 to SSP3-7.0 due to the dramatic increase in atmospheric CO 2 concentration (Fig. 2 ). Intriguingly, the sensitivity of stomata to eCO 2 diminished gradually under the influence of long-term eCO 2 conditions. This occurred because guard cells and mesophyll tissues, which mediate stomatal movements, lead to decreases in stomatal aperture and size, culminating in physiological adaptation to higher concentrations 36 , 37 . Consequently, this resulted in a less pronounced decline from the SSP3-7.0 to SSP5-8.5 scenarios (Fig. 2 ).
A tight coupling between carbon and Hg in terrestrial ecosystems has been observed as a paradigm over the past two decades 10 , 38 . Conventionally, it has been postulated that rising atmospheric CO 2 levels would increase vegetation’s photosynthesis rate, leading to the beneficial impact on plant growth, known as the CO 2 fertilization effect 11 . This effect is believed to enhance the concurrent absorption of both CO 2 and Hg(0), as suggested by Jiskra et al. 39 , Obrist 40 , and Schaefer et al. 41 . For example, atmospheric Hg and CO 2 have similar seasonal fluctuation patterns in both hemispheres, regulated by vegetation photosynthetic activity. An increase in terrestrial net primary production has been also speculated to contribute to a diminishing trend in atmospheric Hg(0) levels in the Northern Hemisphere over the past two decades 39 , 42 , 43 . Contrarily, we predicted a potential decoupling between the trends of CO 2 assimilation and Hg(0) uptake process by vegetation in the twenty-first century, when considering the dynamic response of vegetation physiological activities to climate change. The CLM5 model projected an increased greening of vegetation in many regions in the twenty-first century resulting from eCO 2 (a.k.a. fertilization effect), evidenced by the increased leaf area index (LAI) in the northern mid-to-low latitudes and certain regions of the Southern Hemisphere (Fig. 4c ). The increase in photosynthesis can simultaneously induce a state of water deficit and nutrient saturation within the plant’s internal environment 44 . Therefore, under climate change, the increase in vegetation LAI may only represent an increase in leaf density or even stomatal numbers, but stomatal conductance may not necessarily increase accordingly. However, our model suggested a discernible decrease in the flux of Hg(0) uptake by vegetation in these areas (Fig. 1 ), reflecting the differences in CO 2 and Hg element during plant physiological processes especially those related to water dynamics in terrestrial ecosystems 45 .
Uncertainties
We noted significant uncertainties in our model results. First, various future climate forcing may introduce uncertainties in CLM5 simulations, such as underestimating the phenology and photosynthesis of future plants 46 . This occurred in part because the anomaly forcing method assumes that future changes (anomalies) can overlay present-day variability. Different sub-monthly variations may not accurately represent all facets of future climate changes, particularly in the presence of non-linear interactions or crossed thresholds 29 , 47 . Additionally, it is important to note that some data sources in our meta-analysis originate from seedling experiments. There were inherent physiological and morphological differences between young seedlings and fully mature plants, which could potentially influence the study’s outcomes. However, young seedlings often exhibit more pronounced responses to environmental changes, making them suitable for detecting initial patterns and mechanisms in plant response to elevated CO 2 . Additionally, there will be differences associated with different plant species. Thus, we suggest that future research should focus on this aspect, aiming to bridge the knowledge gap by including experiments across various growth stages and more species.
Given that vegetative stomatal absorption is a key mechanism in our model, we conducted an uncertainty analysis for the parameterization of g s . We included the sensitivity analysis of five ecologically significant parameters (Medlyn_slope, slatop, leafCN, psi50, and stem_leaf) under four levels of perturbation (Supplementary Table 3 ). We found that the global vegetation Hg(0) uptake and g s range 1160–1370 Mg year −1 and 29,600–41,900 μmol H 2 O m −2 s −1 , respectively, with an uncertainty ratio of 17% and 21%, respectively (Supplementary Fig. 15 ). The coefficient of variation (CV, defined as the relative degree of change in the model output compared to the proportion of parameter changes) can reflect the magnitude of an individual parameter’s contribution to uncertainty 48 . The sensitivity analysis revealed that the parameter “Medlyn slope” has the highest CV (1.01 and 1.32 for vegetation Hg(0) uptake and g s , respectively) (Supplementary Fig. 16 and Supplementary Table 4 ). In the Medlyn model within CLM5, the Medlyn slope, denoted as “ g 1 ,” plays a crucial role in controlling how stomata respond to CO 2 levels. It does this by determining the extent to which stomata open, based on the assimilation capacity, CO 2 concentration, and VPD 47 . However, the CLM5 model does not differentiate this parameter for different climate types, which induces relatively large uncertainties (6 ± 1.2%). Additionally, the stomatal conductance simulated by our model is slightly lower than the observed values (Supplementary Fig. 9 ), which could be caused by the uncertainty associated with this parameter. Indeed, Kauwe et al. 49 found a ~30% reduction of the annual transpiration fluxes after better constraining this parameter. This implies that the actual future decrease in Hg(0) uptake could potentially be even higher. More vegetation physiological parameters and Hg observations for different PFTs are thus needed to better constrain our model. There are also likely interaction effects among parameters.
There are still considerable uncertainties regarding the model representation of the land–atmosphere exchange of Hg at present day, which serves as a baseline for our prediction for the future. The atmospheric Hg concentrations and deposition were specified as a boundary condition, not yet dynamically modeled in a two-way coupled fashion. The feedback between land Hg emissions and their atmospheric abundance and subsequent deposition onto the land are also not considered. Although our current framework can well diagnose the direct impact of changing climate on these exchange fluxes, an online land-atmosphere coupled model will be needed to reveal a more comprehensive and accurate changes in global Hg budget in future works. Additionally, current isotopic evidence indicates that the photoreduction process is related to the re-emission of Hg(0) by vegetation leaves, with this re-emission ratio reaching nearly 30% in subtropical forest areas 50 . However, for the majority of other regions worldwide, we lack sufficient observational data to make estimates. In our model, we have only used median values as the reduction parameter 19 . Therefore, in future research, we need to utilize more measured data to refine our parameterization scheme. Our model also did not consider the absorption of Hg from underground root systems and root secretions 51 . Indeed, Hg is hard to enter the plants via the root, as most previous studies have shown 52 , 53 , 54 . Meanwhile, our model did not account for the translocation of Hg among plant tissue organs. A recent study suggested that a significant proportion of Hg in roots may originate from absorption by leaves and subsequent translocation, with an estimation of up to 300 Mg year −1 of atmospheric Hg° stored in roots 55 , but the specific migration and distribution mechanisms are still unclear. Furthermore, the model simplified the soil Hg processes, following GTMM (Global Terrestrial Mercury Model) 38 , by focusing mainly on the microbial reduction process. It did not account for other processes like the radiative transfer in soil, photo-reduction, and other abiotic reduction processes 56 , 57 . These processes also have a potential influence on the amount of Hg(0) uptake by the vegetation, and could be incorporated in our model when more data is available.
In our CLM5-Hg model, throughfall primarily originates from the washing off of atmospheric divalent mercury (sum of the Hg(II) dry deposition onto the canopy surface and the Hg(II) wet deposition that has not been reduced) 38 , 58 . Recent studies indicated that epiphytic vegetation on canopies absorbs atmospheric Hg(0) and decomposes into humus, adhering to tree trunks and canopies, where mercury is subsequently washed into throughfall by precipitation 59 . Additionally, research indicated that the temporal scale and frequency of sampling for throughfall mercury measurements can impact the accuracy of their estimates 60 . Therefore, our model has limitations in this part, and more extensive experimental data covering broader spatiotemporal scales is needed to further constrain the model (e.g., flux measurements or isotope compositions). The anthropogenic and legacy Hg emissions from land and ocean also remained unchanged in this study. Additional uncertainties also aroused from our current understanding of the biogeochemical response of plants to eCO 2 and their possible adaptability to long-term changes 24 . Overall, these uncertainties necessitate further calibration of the model when more data is available and can be effectively addressed as scientific knowledge evolves. The model should be interpreted as a diagnostic tool designed to unveil the influence of individual factors. It serves as a foundation for a more realistic and comprehensive prediction that takes into account factors such as the connection between future Hg and greenhouse gas emissions 1 , 61 , the enhanced soil microbial activity 41 , ocean warming and acidification 62 , and amid many others 12 , 63 .
Implications
We found that, in the climate change scenario, the atmospheric Hg(0) uptake by terrestrial vegetation in 2100 will be likely to decrease by more than half compared to present-day conditions. The atmospheric CO 2 concentration is an important factor that will impact vegetation Hg uptake in the future. The continuous increase in CO 2 concentration will lead to a warming effect, which alters global precipitation patterns 64 , 65 . This could potentially increase VPD and cause drought in many regions, consequently affecting the Hg processes in terrestrial ecosystems. In addition, the composition of global plant communities could be modified over extended time scales 66 . For instance, persistent severe drought could lead to widespread vegetation mortality and shifts in the composition of tropical forest tree 67 . Consequently, this affects the distribution pattern and magnitude of vegetation Hg flux 68 .
Our findings revealed a suppression of atmospheric Hg(0) uptake by plants across most regions in the twenty-first century due to reduced stomatal conductance in vegetation caused by increased CO 2 . With climate change, the bypassing of atmospheric Hg(0) sequestration by plants and the deposition of foliar Hg to the soil lead to increasing concentrations in the atmosphere. This Hg can then be converted to HgII, which is deposited in aquatic ecosystems and can subsequently be methylated 6 , 18 , 54 . Furthermore, these inorganic Hg compounds are transformed into methylmercury by microbes. This process leads to the enrichment of methylmercury in riverine and marine food chains. As a result, a substantial threat to human health arises through the consumption of inland aquatic animals and seafood, including commercial fish 69 , 70 , 71 . These processes coincide with the changes in land use/land cover, such as the potential shift from Amazon rainforest to savannah, which also decreases the land Hg sink and contributes to an additional movement of Hg into the ocean 18 , construing an additional climate change penalty via Hg cycles. Furthermore, although the impact of anthropogenic source emissions was not the focus of this study, some estimations indicated that global anthropogenic emissions of Hg will increase in the forthcoming decades under the current legislative scenario 61 , 72 , 73 . Therefore, under future climate change scenarios, it is possible that there will be a greater threat to human health. We did not consider the impacts LULCC in this study. Under global warming, vegetative succession following melting and increased precipitation intensity is likely to lead to an increase in vegetative biomass and, consequently, an increase in Hg(0) uptake by vegetation 10 . Indeed, the interactive effects of climate change combined with changes in LULCC worth further examination.
The terrestrial ecosystem, recognized as a significant Hg sink, may face disruptions under future climate change scenarios, particularly with rising atmospheric CO 2 concentrations 5 , 74 . Therefore, it becomes crucial to comprehensively consider the tight coupling among Hg, CO 2 , and water cycles when assessing the effectiveness of the Minamata Convention within the context of climate change. From the perspective of the global Hg cycle, considering only the air-land exchange process is insufficient to achieve global mass balance. Both anthropogenic releases and the air-ocean exchange of Hg can potentially affect atmospheric Hg levels, thus influencing the air-land exchange process. Therefore, future research should aim to further incorporate time-varying anthropogenic emissions and develop a fully coupled land-atmosphere-ocean global Hg model within CESM2. This would enable a comprehensive understanding of the complete pathway of Hg from emission to deposition.
We applied a state-of-the-art global terrestrial Hg model to explore the impact of climate change on global vegetation Hg(0) uptake. We used the CLM5-Hg model, which comprehensively contains the biogeophysical and biogeochemical processes that control terrestrial Hg cycling (Supplementary Fig. 1 ). CLM5-Hg was tested against observational field data by Yuan et al. 19 . The model simulates the migration, transformation, accumulation, and emission processes of Hg in terrestrial ecosystems. This includes processes such as the stomatal and non-stomatal uptake of Hg(0) in leaves, the throughfall of divalent Hg [Hg(II)], and the formation of litter and soil Hg. It also includes processes such as the leaching of soil Hg, photo-reduction, as well as microbial decomposition, thermal evaporation, and emissions from wildfires.
The CLM5 model represents its surface heterogeneity with multi-layer nested grid cells. The first layer of the sub-grid consists of land units, including five types: vegetation, lakes, cities, glaciers, and crops. The second layer of the sub-grid represents soil columns, indicating the state changes of the soil within the same land unit. The third layer of the sub-grid is plant functional types (PFTs) with different biogeochemical processes. Sub-grids within the same model grid use the same atmospheric forcing dataset, but the diagnostic variables for each sub-grid are simulated independently. The vegetation covering the land surface is composed of 16 different PFTs (temperate-needleleaf evergreen tree, boreal-needleleaf evergreen tree, boreal-needleleaf deciduous tree, tropical-broadleaf evergreen tree, temperate-broadleaf evergreen tree, tropical-broadleaf deciduous tree, temperate-broadleaf deciduous tree, boreal-broadleaf deciduous tree, temperate- broadleaf evergreen shrub, temperate-Broadleaf deciduous shrub, boreal-broadleaf deciduous shrub, C 3 arctic grass, C 3 grass, C 4 grass, and crops) 19 . Their differences in leaves and stems determine the uniqueness of different vegetation in reflection, transmittance, and solar radiation absorption. Root distribution parameters control soil moisture absorption, aerodynamic parameters determine thermal resistance, moisture, and momentum transfer, and photosynthetic parameters determine stomatal resistance, photosynthesis, and evapotranspiration processes. All PFTs are divided into three different types of phenology: perennial evergreen types, seasonal deciduous types determined by temperature and daylight length, and multi-seasonal stress deciduous types determined by temperature and soil moisture 29 .
We used the offline version of CLM5 (0.90° latitude × 1.25° longitude) with coupled biogeochemical cycles (BGC), which is forced by the dataset of Global Soil Wetness Project (GSWPS), a 3-hourly 0.5° global forcing product based on 20th Century Reanalysis version. The biogeochemical model was run for 200 years, and the CLM5-Hg was run for 10 years in each scenario simulation (1841–1850 for the pre-industrial era, 1991–2000 for the present day, and 2091-2100 for the future). The results for the last year of each simulation were used for data analysis, as this time point reached a steady state, as indicated by two representative variables (see Supplementary Fig. 17 ). We used the simulated atmospheric Hg(0) concentrations and the dry and wet deposition fluxes of Hg(II) from the CAM6-Chem model as its upper boundary conditions 75 . To isolate the impact of climate change, anthropogenic Hg emissions were maintained at the present-day level 76 .
Stomatal model
In CLM5-Hg, maximum stomatal conductance was obtained from the Medlyn “empirical‐optimal” conductance model 30 . This stomatal model calculates stomatal conductance (g s ) based on net leaf photosynthesis, the CO 2 concentration at the leaf surface, and the VPD. The stomatal resistance of the leaf is:
where r s is the stomatal resistance, g 0 is the minimum stomatal conductance, A n is leaf net photosynthesis, C s is the CO 2 partial pressure at the leaf surface, P atm is the atmospheric pressure, and D is the VPD at the leaf surface. The value of g 1 depends on the PFTs following the CABLE model 49 . The model further have corrected r s by partitioning to sunlit and shaded side leaf stomatal resistance and the condition of snow cover 29 :
where R s represents the adjusted stomatal resistance, f sun is the sunlit fraction of canopy, elai represents one-sided LAI buried by snow, \({r}_{{{{{\rm{s}}}}}}^{{sun}}\) is the sunlit leaf stomatal resistance, and \({r}^{{{sha}}}_{{s}}\) is the shaded leaf stomatal resistance.
The dry deposition flux is used to calculate the absorption of atmospheric Hg(0) by global vegetation 77 :
where F d (Z) represents the Hg(0) dry deposition flux at height z, C (z) is the atmospheric Hg(0) concentration, and v d is the dry deposition velocity calculated following the Wesely scheme 78 :
where R a is the aerodynamic resistance between a specific height and the surface, R b is the quasi-laminar sublayer resistance, and R c is the bulk surface resistance. R c is mainly determined by the adjusted stomatal resistance (R s ).
Experiment design
We simulated the terrestrial Hg cycling that is representative of the present-day conditions to serve as the baseline, and 1850 as the pre-industrial climate condition. We selected three different SSPs to represent three different future CO 2 emission scenarios in 2100: SSP1-2.6 represents the lowest, SSP5-8.5 represents the highest, and SSP3-7.0 represents a moderate scenario. To better unravel the effects of individual factors on global vegetation uptake of Hg(0), a suite of simulations was performed in the pre-industrial era and the hypothesized twenty-first century under the SSP5-8.5 scenario. To mitigate high computational costs, we adopted an alternative approach known as “anomaly forcing” for land-only simulations in CLM5 in line with future climate projections, enabling the generation of climate data to drive CLM5 79 . This method used data from a fully coupled simulation to produce monthly changes in near-surface atmospheric states and fluxes, relative to current conditions 79 . We isolated the effects of individual factors by sequentially altering only one variable to reflect future states while maintaining other variables in line with present-day conditions. To eliminate the impact of land use and land cover changes, as well as aerosols, we preprocessed the vegetation patterns and aerosol deposition in all simulation scenarios using the specified file provided by CLM5. These climate change factors specifically include atmospheric CO 2 concentration (limited to its biogeochemical effects and its climate effects are reflected in other factors), precipitation, radiation, meteorological factors such as temperature, humidity, pressure, and wind. In the “all” scenario, all these factors were modified simultaneously to simulate the overall effect. Additionally, we examined the interactions between the biogeochemical effect of CO 2 and temperature and precipitation. We also have intentionally maintained constant levels of anthropogenic emissions and atmospheric Hg concentrations across all scenarios. This methodological choice was made to isolate and underscore the influence of climatic variables on Hg dynamics. By controlling for anthropogenic inputs, we aim to provide a clear assessment of how climate change alone can affect the biogeochemical cycling of Hg.
Data and meta-analysis
We utilized a global vegetation Hg(0) flux dataset to constrain and validate our simulated global vegetation assimilation of Hg(0) at present day. This database contained 79 publications with measurements ranging from 1987 to 2020 18 . These data were from 37 different measurement sites across the world covering the East Asia, Western Europe, and North America regions. The modeled Hg(0) vegetation uptake flux was constrained by the 60 individual data points and agrees well with the observations ( r 2 = 0.38) 19 . The datasets of global stomatal conductance were used to validate the accuracy of stomatal uptake process by CLM5-Hg 37 .
We used meta-analysis to assess the biogeochemical effects of experimentally elevated CO 2 (eCO 2 ) on foliar Hg concentration. We searched for journal articles using the ISI Web of Science with the following keyword combinations: (elevated CO 2 concentration OR CO 2 enrichment OR increasing CO 2 concentration) AND (mercury OR mercury concentration OR mercury uptake) AND (tree OR grass OR plants OR vegetation OR leaf OR leaves) from 1990 to 2023. Papers have to meet the following criteria to be included in our dataset: (i) eCO 2 experiments were conducted in terrestrial ecosystems; (ii) initial environmental factors in control plots were the same as those in eCO 2 plots; (iii) at least two CO 2 concentration regimes were compared. Finally, our dataset included 368 observations from six studies 80 , 81 , 82 , 83 , 84 , 85 . To make sure we include all important studies, we did another search using Google Scholar and sort the studies based on their relevance.
The weighted mean response ratio (lnRR) is employed to analyze the treatment effect on vegetation mercury (Hg) 37 , 86 , the effect size is estimated as:
where \({\overline{x}}_{e{{CO}}_{2}}\) and \({\overline{x}}_{a{{CO}}_{2}}\) represent the mean of the elevated CO 2 level and ambient CO 2 level, respectively.
The individual observations are assigned weights calculated from the experimental replications:
where \({n}_{e{{CO}}_{2}}\) and \({n}_{a{{CO}}_{2}}\) indicate the numbers of replications at elevated CO 2 level and ambient CO 2 level, respectively.
Finally, lnRR is transformed to percentage change (%) as:
The natural log-transformed foliar Hg concentration or flux (Hg veg ) sensitivity (lnSens) is calculated as:
where lnRR represents the natural log-transformed response ration, and the \(\Delta\) is the magnitude of eCO 2 (per 100 p.p.m. increase). The weighted means of lnSens and percentage sensitivity are calculated using equations similar to those presented in Eqs. ( 6 ) and ( 7 ) above.
A negative effect size indicate a decline in the Hg veg (response variable) for the treatment plots compared to the control plots. A variable is considered significantly different between the treatment and control plots ( P < 0.05) if the 95% confidence intervals (CI) of the effect size for that variable does not overlap with zero. Differences between subgroups are deemed significant if their CIs do not overlap. Furthermore, due to the limitations of the data, it is not feasible to specifically subdivide PFTs for a direct one-to-one comparison between the model output and the observational data. Therefore, we have roughly categorized these studies into three subgroups: tree, grass, and crop (Supplementary Table 2 ). This categorization aims to observe the impact of eCO 2 on plants from different PFTs, enabling comparison with our model results.
Uncertainty analysis
We conducted an uncertainty analysis on the Hg(0) uptake and g s using a perturbation experiment. This was based on the process of stomatal uptake, which is a crucial part in the assimilation of atmospheric Hg(0) by global vegetation 55 . Our focus was on a set of five plant physiology-related parameters within the stomatal conductance model (Medlyn model in CLM5), selected due to their mechanical impacts on responses to elevated CO 2 levels and their significance in representing important ecological processes in vegetation 47 (Supplementary Table 3 ). The Medlyn_slope is the slope of the Medlyn model (Eq. 1 ), where the slope parameter dictates the extent of stomatal opening based on a given mix of assimilation capacity, CO 2 concentration, and VPD. Slatop measures the leaf area per gram of leaf biomass. Higher SLA values, indicating thinner and more efficient leaves, lead to a greater LAI from the same biomass. Leaf_cn denotes the ideal leaf carbon to nitrogen (C:N) ratio, with higher leaf nitrogen enhancing photosynthesis but increasing respiration costs. Psi50 is the water potential at which there is a 50% loss of conductivity, a hydraulic trait of plants that effectively reflects the vegetation’s state in response to water-deficient conditions. Stem_leaf determines the biomass distribution between stem and leaves. As this ratio increases, it diminishes the achievable LAI per unit of carbon and nitrogen dedicated to growth, while concurrently increasing the equilibrium woody biomass. These parameters were selected from the broader set of CLM5 parameters through a method primarily guided by the model’s structure and thorough iterative testing during CLM5’s development 47 . To simplify understanding and pinpointing model behavior changes due to parameter variations, we employed one-at-a-time (OAAT) perturbations from the default settings. This approach, unlike a comprehensive global parameter sensitivity analysis, enables easier visualization and interpretation of results. We assessed the sensitivity of simulations in the CLM5-Hg model under a standard scenario, testing four levels for each parameter as outlined in Supplementary Table 3 , resulting in a total of 20 distinct physical perturbation ensembles. In addition, we also considered a condition in which all parameters are altered simultaneously at each level. We estimated the relative uncertainty in Hg(0) uptake and stomatal conductance using the CVs, defined as the relative degree of change in the model output compared to the proportion of parameter changes 48 .
Model validation
We curated a dataset within the CO 2 concentration ranges projected by our model under three SSP scenarios, which included 462 paired observations of treated versus control groups for various species across different vegetation biomes (Supplementary Figs. 6 and 7 ). First, the comparison results under the current scenario indicated that the simulation can captures the global pattern of stomatal conductance ( r > 0.5, Supplementary Fig. 8 ). Second, we performed point-by-point validations against matched observational data for projected eCO 2 concentrations in each scenario, achieving an r value of 0.50 (Supplementary Fig. 9 ).
Our previous research has shown that the CLM5-Hg model can capture the global distribution of vegetative Hg and litter Hg concentrations well ( r > 0.6) via the Hg(0) dataset and a large number of observational datasets related to vegetative Hg tissue concentration 19 .
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated or analyzed are available in the main text, the Supplementary information, and the research group website: https://www.ebmg.online/mercury . GSWP3 climate dataset for CESM2: https://svn-ccsm-inputdata.cgd.ucar.edu/trunk/inputdata/atm/datm7/ . The data from the uncertainty analysis and the sensitivity experiment of model generated in this study are provided in the Supplementary Information/Source data file. Source data are provided with this paper.
Code availability
All core CLM5-Hg model code is available at the research group website: https://www.ebmg.online/mercury . The CESM2 code: https://github.com/ESCOMP/CESM . The CLM5 code: https://github.com/ESCOMP/CTSM/tree/master/src .
Zhang, Y. et al. Global health effects of future atmospheric mercury emissions. Nat. Commun. 12 , 3035 (2021).
Article ADS CAS PubMed PubMed Central Google Scholar
Eagles-Smith, C. A. et al. Mercury in western North America: a synthesis of environmental contamination, fluxes, bioaccumulation, and risk to fish and wildlife. Sci. Total Environ. 568 , 1213–1226 (2016).
Article ADS CAS PubMed Google Scholar
Amos, H. M., Jacob, D. J., Streets, D. G. & Sunderland, E. M. Legacy impacts of all-time anthropogenic emissions on the global mercury cycle. Glob. Biogeochem. Cycles 27 , 410–421 (2013).
Article ADS CAS Google Scholar
Gerson, J. R. et al. Amazon forests capture high levels of atmospheric mercury pollution from artisanal gold mining. Nat. Commun. 13 , 1–10 (2022).
Article Google Scholar
Daniel, O. et al. Previously unaccounted atmospheric mercury deposition in a midlatitude deciduous forest. Proc. Natl Acad. Sci. USA 118 , e2105477118 (2021).
Zhou, J., Obrist, D., Dastoor, A., Jiskra, M. & Ryjkov, A. Vegetation uptake of mercury and impacts on global cycling. Nat. Rev. Earth Environ. 2 , 269–284 (2021).
Article ADS Google Scholar
Bargagli, R. Moss and lichen biomonitoring of atmospheric mercury: a review. Sci. Total Environ. 572 , 216–231 (2016).
Higgins, S. I., Conradi, T. & Muhoko, E. Shifts in vegetation activity of terrestrial ecosystems attributable to climate trends. Nat. Geosci. 16 , 147–153 (2023).
Seddon, A. W. R., Macias-Fauria, M., Long, P. R., Benz, D. & Willis, K. J. Sensitivity of global terrestrial ecosystems to climate variability. Nature 531 , 229–232 (2016).
Wang, X. et al. Global warming accelerates uptake of atmospheric mercury in regions experiencing glacier retreat. Proc. Natl Acad. Sci. USA 117 , 2049–2055 (2020).
Liu, Y. et al. Field-experiment constraints on the enhancement of the terrestrial carbon sink by CO 2 fertilization. Nat. Geosci. 12 , 809–814 (2019).
Sonke, J. E. et al. Global change effects on biogeochemical mercury cycling. Ambio 52 , 853–876 (2023).
Wu, S., Mickley, L. J., Kaplan, J. O. & Jacob, D. J. Impacts of changes in land use and land cover on atmospheric chemistry and air quality over the 21st century. Atmos. Chem. Phys. 12 , 1597–1609 (2012).
Zhang, H., Holmes, C. D. & Wu, S. Impacts of changes in climate, land use and land cover on atmospheric mercury. Atmos. Environ. 141 , 230–244 (2016).
Wohlgemuth, L. et al. Physiological and climate controls on foliar mercury uptake by European tree species. Biogeosciences 19 , 1335–1353 (2022).
Wohlgemuth, L., Feinberg, A., Buras, A. & Jiskra, M. A spatial assessment of current and future foliar Hg uptake fluxes across European forests. Glob. Biogeochem. Cycles 37 , e2023GB007833 (2023).
Damour, G., Simonneau, T., Cochard, H. & Urban, L. An overview of models of stomatal conductance at the leaf level. Plant Cell Environ. 33 , 1419–1438 (2010).
PubMed Google Scholar
Feinberg, A., Dlamini, T., Jiskra, M., Shah, V. & Selin, N. E. Evaluating atmospheric mercury (Hg) uptake by vegetation in a chemistry-transport model. Environ. Sci. Process. Impacts 24 , 1303–1318 (2022).
Article CAS PubMed PubMed Central Google Scholar
Yuan, T. et al. Buffering effect of global vegetation on the air-land exchange of mercury: insights from a novel terrestrial mercury model based on CESM2-CLM5. Environ. Int. 174 , 107904 (2023).
Article CAS PubMed Google Scholar
Meinshausen, M. et al. The shared socio-economic pathway (SSP) greenhouse gas concentrations and their extensions to 2500. Geosci. Model Dev. 13 , 3571–3605 (2020).
Fu, X. et al. Depletion of atmospheric gaseous elemental mercury by plant uptake at Mt. Changbai, Northeast China. Atmos. Chem. Phys. 16 , 12861–12873 (2016).
Laacouri, A., Nater, E. A. & Kolka, R. K. Distribution and uptake dynamics of mercury in leaves of common deciduous tree species in Minnesota, U.S.A. Environ. Sci. Technol. 47 , 10462–10470 (2013).
Liu, Y. et al. Understanding foliar accumulation of atmospheric Hg in terrestrial vegetation: progress and challenges. Crit. Rev. Environ. Sci. Technol. 52 , 4331–4352 (2021).
Reich, P. B., Hobbie, S. E., Lee, T. D. & Pastore, M. A. Unexpected reversal of C3 versus C4 grass response to elevated CO 2 during a 20-year field experiment. Science 360 , 317–320 (2018).
SAGE, R. F. & KUBIEN, D. S. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 30 , 1086–1106 (2007).
Still, C. J., Berry, J. A., Collatz, G. J. & DeFries, R. S. Global distribution of C3 and C4 vegetation: carbon cycle implications. Glob. Biogeochem. Cycles 17 , 6–14 (2003).
Obrist, D. et al. Tundra uptake of atmospheric elemental mercury drives Arctic mercury pollution. Nature 547 , 201–204 (2017).
Franks, P. J. et al. Sensitivity of plants to changing atmospheric CO 2 concentration: from the geological past to the next century. N. Phytol. 197 , 1077–1094 (2013).
Article CAS Google Scholar
Lawrence, D. M. et al. The community land model version 5: description of new features, benchmarking, and impact of forcing uncertainty. J. Adv. Model. Earth Syst. 11 , 4245–4287 (2019).
Medlyn, B. E. et al. Reconciling the optimal and empirical approaches to modelling stomatal conductance. Glob. Change Biol. 18 , 3476 (2012).
Savvides, A., Fanourakis, D. & van Ieperen, W. Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J. Exp. Bot. 63 , 1135–1143 (2012).
Haworth, M. et al. Allocation of the epidermis to stomata relates to stomatal physiological control: stomatal factors involved in the evolutionary diversification of the angiosperms and development of amphistomaty. Environ. Exp. Bot. 151 , 55–63 (2018).
Katul, G. G., Oren, R., Manzoni, S., Higgins, C. & Parlange, M. B. Evapotranspiration: a process driving mass transport and energy exchange in the soil-plant-atmosphere-climate system. Rev. Geophys. https://doi.org/10.1029/2011RG000366 (2012).
Gardner, A. et al. Optimal stomatal theory predicts CO 2 responses of stomatal conductance in both gymnosperm and angiosperm trees. N. Phytol. 237 , 1229–1241 (2023).
Hsiao, J., Swann, A. L. S. & Kim, S.-H. Maize yield under a changing climate: the hidden role of vapor pressure deficit. Agric. For. Meteorol. 279 , 107692 (2019).
Engineer, C. B. et al. CO 2 sensing and CO 2 regulation of stomatal conductance: advances and open questions. Trends Plant Sci. 21 , 16–30 (2016).
Liang, X. et al. Stomatal responses of terrestrial plants to global change. Nat. Commun. 14 , 2188 (2023).
Smith-Downey, N. V., Sunderland, E. M. & Jacob, D. J. Anthropogenic impacts on global storage and emissions of mercury from terrestrial soils: insights from a new global model. J. Geophys. Res. Biogeosci. https://doi.org/10.1029/2009JG001124 (2010).
Jiskra, M. et al. A vegetation control on seasonal variations in global atmospheric mercury concentrations. Nat. Geosci. 11 , 244–250 (2018).
Obrist, D. Atmospheric mercury pollution due to losses of terrestrial carbon pools? Biogeochemistry 85 , 119–123 (2007).
Schaefer, K. et al. Potential impacts of mercury released from thawing permafrost. Nat. Commun. 11 , 4650 (2020).
Fu, X. et al. Significant seasonal variations in isotopic composition of atmospheric total gaseous mercury at forest sites in China caused by vegetation and mercury sources. Environ. Sci. Technol. 53 , 13748–13756 (2019).
St. Louis, V. L. et al. Atmospheric concentrations and wet/dry loadings of mercury at the remote Experimental Lakes Area, Northwestern Ontario, Canada. Environ. Sci. Technol. 53 , 8017–8026 (2019).
Victoria, G., Jean-louis, D. & François, G. Water deficit and nitrogen nutrition of crops. A review. Agron. Sustain. Dev. 30 , 529–544 (2010).
Wang, X., Yuan, W., Lin, C.-J. & Feng, X. Mercury cycling and isotopic fractionation in global forests. Crit. Rev. Environ. Sci. Technol. 52 , 3763–3786 (2021).
Lu, Y. & Yang, X. Using the anomaly forcing Community Land Model (CLM 4.5) for crop yield projections. Geosci. Model Dev. 14 , 1253–1265 (2021).
Fisher, R. A. et al. Parametric controls on vegetation responses to biogeochemical forcing in the CLM5. J. Adv. Model. Earth Syst. 11 , 2879–2895 (2019).
Cui, J. et al. Nitrogen cycles in global croplands altered by elevated CO 2 . Nat. Sustain. 6 , 1166–1176 (2023).
De Kauwe, M. G. et al. A test of an optimal stomatal conductance scheme within the CABLE land surface model. Geosci. Model Dev. 8 , 431–452 (2015).
Yuan, W. et al. Stable isotope evidence shows re-emission of elemental mercury vapor occurring after reductive loss from foliage. Environ. Sci. Technol. 53 , 651–660 (2019).
Keuper, F. et al. Carbon loss from northern circumpolar permafrost soils amplified by rhizosphere priming. Nat. Geosci. 13 , 560–565 (2020).
Chiarantini, L. et al. Black pine ( Pinus nigra ) barks as biomonitors of airborne mercury pollution. Sci. Total Environ. 569–570 , 105–113 (2016).
Article ADS PubMed Google Scholar
Siwik, E. I. H., Campbell, L. M. & Mierle, G. Distribution and trends of mercury in deciduous tree cores. Environ. Pollut. 158 , 2067–2073 (2010).
Arnold, J., Gustin, M. S. & Weisberg, P. J. Evidence for nonstomatal uptake of Hg by aspen and translocation of Hg from foliage to tree rings in Austrian pine. Environ. Sci. Technol. 52 , 1174–1182 (2018).
Zhou, J. & Obrist, D. Global mercury assimilation by vegetation. Environ. Sci. Technol. 55 , 14245–14257 (2021).
Fritsche, J. et al. Elemental mercury fluxes over a sub-alpine grassland determined with two micrometeorological methods. Atmos. Environ. 42 , 2922–2933 (2008).
Wang, X. et al. Emission-dominated gas exchange of elemental mercury vapor over natural surfaces in China. Atmos. Chem. Phys. 16 , 11125–11143 (2016).
Paige Wright, L., Zhang, L. & Marsik, F. J. Overview of mercury dry deposition, litterfall, and throughfall studies. Atmos. Chem. Phys. 16 , 13399–13416 (2016).
Wang, X. et al. Underestimated sink of atmospheric mercury in a deglaciated forest chronosequence. Environ. Sci. Technol. 54 , 8083–8093 (2020).
Choi, H. D., Sharac, T. J. & Holsen, T. M. Mercury deposition in the Adirondacks: a comparison between precipitation and throughfall. Atmos. Environ. 42 , 1818–1827 (2008).
Pacyna, J. M. et al. Current and future levels of mercury atmospheric pollution on a global scale. Atmos. Chem. Phys. 16 , 12495–12511 (2016).
Wang, Y., Wu, P. & Zhang, Y. Climate-driven changes of global marine mercury cycles in 2100. Proc. Natl Acad. Sci. USA 120 , e2202488120 (2023).
Obrist, D. et al. A review of global environmental mercury processes in response to human and natural perturbations: changes of emissions, climate, and land use. Ambio 47 , 116–140 (2018).
Article ADS PubMed PubMed Central Google Scholar
Christian, J. I. et al. Global distribution, trends, and drivers of flash drought occurrence. Nat. Commun. 12 , 6330 (2021).
Shekhar, A., Hörtnagl, L., Buchmann, N. & Gharun, M. Long-term changes in forest response to extreme atmospheric dryness. Glob. Change Biol. 29 , 5379–5396 (2023).
Allen, C. D., Breshears, D. D. & McDowell, N. G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6 , art129 (2015).
Zhao, M. & Running, S. W. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science 329 , 940–943 (2010).
Sun, T. et al. Mercury transport, transformation and mass balance on a perspective of hydrological processes in a subtropical forest of China. Environ. Pollut. 254 , 113065 (2019).
Zhang, Y., Soerensen, A. L., Schartup, A. T. & Sunderland, E. M. A global model for methylmercury formation and uptake at the base of marine food webs. Glob. Biogeochem. Cycles 34 , e2019GB006348 (2020).
Sunderland, E. M. & Mason, R. P. Human impacts on open ocean mercury concentrations. Glob. Biogeochem. Cycles https://doi.org/10.1029/2006GB002876 (2007).
DiMento, B. P. & Mason, R. P. Factors controlling the photochemical degradation of methylmercury in coastal and oceanic waters. Mar. Chem. 196 , 116–125 (2017).
Rafaj, P., Bertok, I., Cofala, J. & Schöpp, W. Scenarios of global mercury emissions from anthropogenic sources. Atmos. Environ. 79 , 472–479 (2013).
Brocza, F. M., Rafaj, P., Sander, R., Wagner, F. & Jones, J. M. Global scenarios of anthropogenic mercury emissions. Preprint at EGUsphere https://doi.org/10.5194/egusphere-2024-41 (2024).
Zhang, Y. et al. An updated global mercury budget from a coupled atmosphere-land-ocean model: 40% more re-emissions buffer the effect of primary emission reductions. One Earth 6 , 316–325 (2023).
Zhang, P. & Zhang, Y. Earth system modeling of mercury using CESM2 – Part 1: Atmospheric model CAM6-Chem/Hg v1.0. Geosci. Model Dev. 15 , 3587–3601 (2022).
Zhang, Y. et al. Observed decrease in atmospheric mercury explained by global decline in anthropogenic emissions. Proc. Natl Acad. Sci. USA 113 , 526–531 (2016).
Khan, T. R., Obrist, D., Agnan, Y., Selin, N. E. & Perlinger, J. A. Atmosphere-terrestrial exchange of gaseous elemental mercury: parameterization improvement through direct comparison with measured ecosystem fluxes. Environ. Sci. Process. Impacts 21 , 1699–1712 (2019).
Wesely, M. L. Parameterization of surface resistances to gaseous dry deposition in regional-scale numerical models. Atmos. Environ. 23 , 1293–1304 (1989).
Lawrence, D. M., Koven, C. D., Swenson, S. C., Riley, W. J. & Slater, A. G. Permafrost thaw and resulting soil moisture changes regulate projected high-latitude CO 2 and CH4 emissions. Environ. Res. Lett. 10 , 94011 (2015).
Tang, B., Chen, J., Wang, Z., Qin, P. & Zhang, X. Mercury accumulation response of rice plant ( Oryza sativa L.) to elevated atmospheric mercury and carbon dioxide. Ecotoxicol. Environ. Saf. 224 , 11628 (2021).
Stamenkovic, J. & Gustin, M. S. Nonstomatal versus stomatal uptake of atmospheric mercury. Environ. Sci. Technol. 43 , 1367–1372 (2009).
Millhollen, A. G., Obrist, D. & Gustin, M. S. Mercury accumulation in grass and forb species as a function of atmospheric carbon dioxide concentrations and mercury exposures in air and soil. Chemosphere 65 , 889–897 (2006).
Natali, S. M. et al. Increased mercury in forest soils under elevated carbon dioxide. Oecologia 158 , 343–354 (2008).
Benjamin, D. et al. Plant-soil distribution of potentially toxic elements in response to elevated atmospheric CO 2 . Environ. Sci. Technol. 45 , 2570–2574 (2011).
Demers, J. D., Blum, J. D. & Zak, D. R. Mercury isotopes in a forested ecosystem: implications for air-surface exchange dynamics and the global mercury cycle. Glob. Biogeochem. Cycles 27 , 222–238 (2013).
Gu, B. et al. Cost-effective mitigation of nitrogen pollution from global croplands. Nature 613 , 77–84 (2023).
Download references
Acknowledgements
We thank Guiyao Zhou, Wenbin Chen, Huimin Zhou, Xizhen Xia, Chao Zhang, and Xiaojiang Liu for the helpful discussions and suggestions. This work was supported by the the National Natural Science Foundation of China (NSFC) 42394094, the “GeoX” Interdisciplinary Research Funds for the Frontiers Science Center for Critical Earth Material Cycling, Nanjing University, the Fundamental Research Funds for the Central Universities (grant nos. 14380188, 14380168), the Frontiers Science Center for Critical Earth Material Cycling, and the Collaborative Innovation Center of Climate Change, Jiangsu Province.
Author information
Authors and affiliations.
School of Atmospheric Sciences, Nanjing University, Nanjing, Jiangsu, China
Tengfei Yuan, Shaojian Huang, Peng Zhang, Zhengcheng Song, Jun Ge, Xin Miao, Yujuan Wang, Qiaotong Pang, Dong Peng, Peipei Wu, Weidong Guo & Yanxu Zhang
Frontiers Science Center for Critical Earth Material Cycling, Nanjing University, Nanjing, Jiangsu, China
Zhengcheng Song & Yanxu Zhang
Joint International Research Laboratory of Atmospheric and Earth System Sciences, Nanjing University, Nanjing, Nanjing, Jiangsu, China
Zhengcheng Song, Jun Ge & Yanxu Zhang
State Key Laboratory of Subtropical Silviculture, College of Forestry and Biotechnology, Zhejiang A&F University, Hangzhou, China
Junjiong Shao
CAS Key Laboratory of Mountain Ecological Restoration and Bioresource Utilization & Ecological Restoration and Biodiversity Conservation Key Laboratory of Sichuan Province, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, China
Peipei Zhang
College of Environmental Science and Engineering, Yangzhou University, Yangzhou, China
State Key Laboratory of Pollution Control and Resource Reuse, School of the Environment, Nanjing University, Nanjing, China
Hongyan Guo
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: T.Y., Y.Z., S.H. Methodology: T.Y., Peng Zhang, Y.Z., J.G., P.W., Yujuan Wang, W.G. Investigation: T.Y., J.S., Peipei Zhang, Z.S., X.M., D.P., Q.P., Y.Z., Yabo Wang. Visualization: T.Y., Peng Zhang, Yujuan Wang, Y.Z., S.H. Funding acquisition: Y.Z. Project administration: Y.Z. Supervision: Y.Z. Writing—original draft: T.Y, Y.Z. Writing—review and editing: T.Y., Y.Z, J.G., Z.S., X.M., J.S., Peng Zhang, H.G., W.G.
Corresponding author
Correspondence to Yanxu Zhang .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Agnieszka Jędruch and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supporting information, peer review file, reporting summary, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Yuan, T., Huang, S., Zhang, P. et al. Potential decoupling of CO 2 and Hg uptake process by global vegetation in the 21st century. Nat Commun 15 , 4490 (2024). https://doi.org/10.1038/s41467-024-48849-2
Download citation
Received : 05 February 2024
Accepted : 15 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1038/s41467-024-48849-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
trending now in US News

Fetterman dramatically whips off Harvard hood at Yeshiva...

Agitated Biden snaps at reporter over Kamala Harris question:...

Florida man, 30, sets himself on fire inside of local grocery...

Maniac screams 'I'm gonna kill all the Jews' as he tries to run...

Here's what could happen if Donald Trump is found guilty at his...

Passenger dead after jumping off the world's largest cruise ship

Judge stunned when man with suspended license joins court Zoom...

'Bad Breath Rapist' on the run for 16 years caught after cops...
Process server felt ‘legitimately threatened’ trying to serve kristi noem lawsuit over texas dental practice endorsement.
- View Author Archive
- Get author RSS feed
Thanks for contacting us. We've received your submission.
The consumer advocacy group behind a recently dismissed lawsuit against Kristi Noem said Tuesday that it plans to continue litigation over her peculiar social media endorsement of a Texas dental practice, noting that the South Dakota Republican governor was “highly evasive” and “threatening” when it tried to serve her legal papers.
The lawsuit, filed by Travelers United in the Superior Court of the District of Columbia, accused Noem of posting an “undisclosed advertisement” on her various social media accounts when she endorsed Smile Texas – the Texas dental practice that put in her veneers – in a five-minute-long infomercial-style testimonial.
The complaint was dismissed last week on the grounds that Travelers United did not present the court with proof that Noem was served with the lawsuit.

But the group claims it did serve the governor and that Noem’s recent anecdote about killing her “untrainable” 14-month-old hunting dog lended credence to the process server’s concerns.
“Ms. Noem has recently been in the news for shooting and killing her dog,” a Tuesday filing by Lauren Wolfe, counsel for Travelers United, states. “The process server felt legitimately threatened.”
Wolfe noted that the process server also alleged that the governor was “highly evasive,” “threatening” and that her staff “were hostile” throughout the process.
Even so, Wolfe argued that Noem was “successfully served” and that the case should not be dismissed, including notes from the process server indicating that the paperwork was eventually left with Noem’s staff.
“The baseless lawsuit filed by Travelers United against me was dismissed by the court last week,” Noem wrote on X Tuesday. “Their actions have exposed them as a fake watchdog group filing frivolous claims to smear me.”

“To be clear, I never received compensation for any alleged ‘advertisements,’” she added.
“If Travelers United continues to engage in baseless lawfare, then I will use every resource at my disposal to hold them accountable,” Noem wrote in a separate post .
Wolfe told The Post that Travelers United “100%” plans to press forward with litigation.

“We would be happy to dismiss this case if we got a receipt showing that Gov. Noem paid in full [for the out-of-state dental procedure],” she said. “We have made that clear to her lawyers and we have yet to receive any sort of documentation showing that.”
It is unclear why Noem cut the testimonial for Smile Dental and posted it widely on social media.
Her office did not respond to The Post’s request for comment.
Travelers United had 14 days from May 20 to contest the dismissal of the lawsuit.
Share this article:

Advertisement

Kristi Noem slammed as process server alleges threats during lawsuit delivery regarding Texas dental practice endorsement
W ASHINGTON, DC: On Tuesday, May 28, the consumer advocacy group, whose recent lawsuit against Kristi Noem was dismissed, announced its intention to pursue legal action regarding her unusual support of a Texas dental practice on social media, as per the New York Post.
The group notably claimed that the Republican Governor of South Dakota was "extremely evasive" and "threatening" when they attempted to serve Noem with legal papers.
Process server claims Noem was 'threatening' as it attempted to serve her with legal papers
Travelers United filed a lawsuit in the Superior Court of the District of Columbia, alleging that Kristi Noem had posted an "undisclosed advertisement" on her social media accounts.
The lawsuit claims that she gave a five-minute testimonial, styled like an infomercial, endorsing Smile Texas, the Texas dental office that placed her veneers.
The complaint was dismissed last week due to Travelers United's failure to provide the court with evidence that Noem had received a copy of the case.
However, the organization asserts that it did serve the governor, and the process server's worries were validated by Noem's previous story of murdering her "untrainable" 14-month-old hunting dog.
A Tuesday filing by Lauren Wolfe, counsel for Travelers United, states, "Ms. Noem has recently been in the news for shooting and killing her dog. The process server felt legitimately threatened."
Moreover, according to Wolfe, the process server also stated that the governor was "extremely evasive" and "threatening," and that her staff "were hostile" during the proceedings.
Nevertheless, citing notes from the process server stating that the documents were ultimately placed with Noem's staff, Wolfe maintained that Noem was "successfully served" and that the matter should not be dropped.
Noem wrote on X Tuesday, "The baseless lawsuit filed by Travelers United against me was dismissed by the court last week. Their actions have exposed them as a fake watchdog group filing frivolous claims to smear me."
She further added, "To be clear, I never received compensation for any alleged ‘advertisements.'"
In a separate post, she mentioned, "If Travelers United continues to engage in baseless lawfare, then I will use every resource at my disposal to hold them accountable".
She informed the New York Post, "We would be happy to dismiss this case if we got a receipt showing that Gov Noem paid in full [for the out-of-state dental procedure]. We have made that clear to her lawyers and we have yet to receive any sort of documentation showing that."
Internet slams Kristi Noem as process server claims she was 'threatening' as it attempted to serve her with legal papers
Several internet users slammed Kristi Noem as the process server claimed that the Republican governor of South Dakota was "extremely evasive" and "threatening" when it attempted to serve Noem with legal papers.
A user posted on X, "She is a menace," while one added, "Maybe they should send a male server next time…"
A person also stated, "She's so yesterdays trash," whereas one mentioned, "I'll bet she can become quite prickly if cornered." [sic]
An online comment read, "Noem apparently thinks she can do whatever. Half of the states have now voted to legalize marijuana and so it happened. When South Dakota voted that way Noem went to work. She said the voters didn't know what they were doing. She got the vote thrown out and overturned."
This article contains remarks made on the Internet by individual people and organizations. MEAWW cannot confirm them independently and does not support claims or opinions being made online.

Main Navigation
- Contact NeurIPS
- Code of Ethics
- Code of Conduct
- Create Profile
- Journal To Conference Track
- Diversity & Inclusion
- Proceedings
- Future Meetings
- Exhibitor Information
- Privacy Policy
NeurIPS 2024 Datasets and Benchmarks Track
If you'd like to become a reviewer for the track, or recommend someone, please use this form .
The Datasets and Benchmarks track serves as a venue for high-quality publications, talks, and posters on highly valuable machine learning datasets and benchmarks, as well as a forum for discussions on how to improve dataset development. Datasets and benchmarks are crucial for the development of machine learning methods, but also require their own publishing and reviewing guidelines. For instance, datasets can often not be reviewed in a double-blind fashion, and hence full anonymization will not be required. On the other hand, they do require additional specific checks, such as a proper description of how the data was collected, whether they show intrinsic bias, and whether they will remain accessible. The Datasets and Benchmarks track is proud to support the open source movement by encouraging submissions of open-source libraries and tools that enable or accelerate ML research.
The previous editions of the Datasets and Benchmarks track were highly successful; you can view the accepted papers from 2021 , 2002 , and 2023 , and the winners of the best paper awards 2021 , 2022 and 2023
CRITERIA. W e are aiming for an equally stringent review as the main conference, yet better suited to datasets and benchmarks. Submissions to this track will be reviewed according to a set of criteria and best practices specifically designed for datasets and benchmarks , as described below. A key criterion is accessibility: datasets should be available and accessible , i.e. the data can be found and obtained without a personal request to the PI, and any required code should be open source. We encourage the authors to use Croissant format ( https://mlcommons.org/working-groups/data/croissant/ ) to document their datasets in machine readable way. Next to a scientific paper, authors should also submit supplementary materials such as detail on how the data was collected and organised, what kind of information it contains, how it should be used ethically and responsibly, as well as how it will be made available and maintained.
RELATIONSHIP TO NeurIPS. Submissions to the track will be part of the main NeurIPS conference , presented alongside the main conference papers. Accepted papers will be officially published in the NeurIPS proceedings .
SUBMISSIONS. There will be one deadline this year. It is also still possible to submit datasets and benchmarks to the main conference (under the usual review process), but dual submission to both is not allowed (unless you retracted your paper from the main conference). We also cannot transfer papers from the main track to the D&B track. Authors can choose to submit either single-blind or double-blind . If it is possible to properly review the submission double-blind, i.e., reviewers do not need access to non-anonymous repositories to review the work, then authors can also choose to submit the work anonymously. Papers will not be publicly visible during the review process. Only accepted papers will become visible afterward. The reviews themselves are not visible during the review phase but will be published after decisions have been made. The datasets themselves should be accessible to reviewers but can be publicly released at a later date (see below). New authors cannot be added after the abstract deadline and they should have an OpenReview profile by the paper deadline. NeurIPS does not tolerate any collusion whereby authors secretly cooperate with reviewers, ACs or SACs to obtain favourable reviews.
SCOPE. This track welcomes all work on data-centric machine learning research (DMLR) and open-source libraries and tools that enable or accelerate ML research, covering ML datasets and benchmarks as well as algorithms, tools, methods, and analyses for working with ML data. This includes but is not limited to:
- New datasets, or carefully and thoughtfully designed (collections of) datasets based on previously available data.
- Data generators and reinforcement learning environments.
- Data-centric AI methods and tools, e.g. to measure and improve data quality or utility, or studies in data-centric AI that bring important new insight.
- Advanced practices in data collection and curation that are of general interest even if the data itself cannot be shared.
- Frameworks for responsible dataset development, audits of existing datasets, identifying significant problems with existing datasets and their use
- Benchmarks on new or existing datasets, as well as benchmarking tools.
- In-depth analyses of machine learning challenges and competitions (by organisers and/or participants) that yield important new insight.
- Systematic analyses of existing systems on novel datasets yielding important new insight.
Read our original blog post for more about why we started this track.
Important dates
- Abstract submission deadline: May 29, 2024
- Full paper submission and co-author registration deadline: Jun 5, 2024
- Supplementary materials submission deadline: Jun 12, 2024
- Review deadline - Jul 24, 2024
- Release of reviews and start of Author discussions on OpenReview: Aug 07, 2024
- End of author/reviewer discussions on OpenReview: Aug 31, 2024
- Author notification: Sep 26, 2024
- Camera-ready deadline: Oct 30, 2024 AOE
Note: The site will start accepting submissions on April 1 5 , 2024.
FREQUENTLY ASKED QUESTIONS
Q: My work is in scope for this track but possibly also for the main conference. Where should I submit it?
A: This is ultimately your choice. Consider the main contribution of the submission and how it should be reviewed. If the main contribution is a new dataset, benchmark, or other work that falls into the scope of the track (see above), then it is ideally reviewed accordingly. As discussed in our blog post, the reviewing procedures of the main conference are focused on algorithmic advances, analysis, and applications, while the reviewing in this track is equally stringent but designed to properly assess datasets and benchmarks. Other, more practical considerations are that this track allows single-blind reviewing (since anonymization is often impossible for hosted datasets) and intended audience, i.e., make your work more visible for people looking for datasets and benchmarks.
Q: How will paper accepted to this track be cited?
A: Accepted papers will appear as part of the official NeurIPS proceedings.
Q: Do I need to submit an abstract beforehand?
A: Yes, please check the important dates section for more information.
Q: My dataset requires open credentialized access. Can I submit to this track?
A: This will be possible on the condition that a credentialization is necessary for the public good (e.g. because of ethically sensitive medical data), and that an established credentialization procedure is in place that is 1) open to a large section of the public, 2) provides rapid response and access to the data, and 3) is guaranteed to be maintained for many years. A good example here is PhysioNet Credentialing, where users must first understand how to handle data with human subjects, yet is open to anyone who has learned and agrees with the rules. This should be seen as an exceptional measure, and NOT as a way to limit access to data for other reasons (e.g. to shield data behind a Data Transfer Agreement). Misuse would be grounds for desk rejection. During submission, you can indicate that your dataset involves open credentialized access, in which case the necessity, openness, and efficiency of the credentialization process itself will also be checked.
SUBMISSION INSTRUCTIONS
A submission consists of:
- Please carefully follow the Latex template for this track when preparing proposals. We follow the NeurIPS format, but with the appropriate headings, and without hiding the names of the authors. Download the template as a bundle here .
- Papers should be submitted via OpenReview
- Reviewing is in principle single-blind, hence the paper should not be anonymized. In cases where the work can be reviewed equally well anonymously, anonymous submission is also allowed.
- During submission, you can add a public link to the dataset or benchmark data. If the dataset can only be released later, you must include instructions for reviewers on how to access the dataset. This can only be done after the first submission by sending an official note to the reviewers in OpenReview. We highly recommend making the dataset publicly available immediately or before the start of the NeurIPS conference. In select cases, requiring solid motivation, the release date can be stretched up to a year after the submission deadline.
- Dataset documentation and intended uses. Recommended documentation frameworks include datasheets for datasets , dataset nutrition labels , data statements for NLP , data cards , and accountability frameworks .
- URL to website/platform where the dataset/benchmark can be viewed and downloaded by the reviewers.
- URL to Croissant metadata record documenting the dataset/benchmark available for viewing and downloading by the reviewers. You can create your Croissant metadata using e.g. the Python library available here: https://github.com/mlcommons/croissant
- Author statement that they bear all responsibility in case of violation of rights, etc., and confirmation of the data license.
- Hosting, licensing, and maintenance plan. The choice of hosting platform is yours, as long as you ensure access to the data (possibly through a curated interface) and will provide the necessary maintenance.
- Links to access the dataset and its metadata. This can be hidden upon submission if the dataset is not yet publicly available but must be added in the camera-ready version. In select cases, e.g when the data can only be released at a later date, this can be added afterward (up to a year after the submission deadline). Simulation environments should link to open source code repositories
- The dataset itself should ideally use an open and widely used data format. Provide a detailed explanation on how the dataset can be read. For simulation environments, use existing frameworks or explain how they can be used.
- Long-term preservation: It must be clear that the dataset will be available for a long time, either by uploading to a data repository or by explaining how the authors themselves will ensure this
- Explicit license: Authors must choose a license, ideally a CC license for datasets, or an open source license for code (e.g. RL environments). An overview of licenses can be found here: https://paperswithcode.com/datasets/license
- Add structured metadata to a dataset's meta-data page using Web standards (like schema.org and DCAT ): This allows it to be discovered and organized by anyone. A guide can be found here: https://developers.google.com/search/docs/data-types/dataset . If you use an existing data repository, this is often done automatically.
- Highly recommended: a persistent dereferenceable identifier (e.g. a DOI minted by a data repository or a prefix on identifiers.org ) for datasets, or a code repository (e.g. GitHub, GitLab,...) for code. If this is not possible or useful, please explain why.
- For benchmarks, the supplementary materials must ensure that all results are easily reproducible. Where possible, use a reproducibility framework such as the ML reproducibility checklist , or otherwise guarantee that all results can be easily reproduced, i.e. all necessary datasets, code, and evaluation procedures must be accessible and documented.
- For papers introducing best practices in creating or curating datasets and benchmarks, the above supplementary materials are not required.
- For papers resubmitted after being retracted from another venue: a brief discussion on the main concerns raised by previous reviewers and how you addressed them. You do not need to share the original reviews.
- For the dual submission and archiving, the policy follows the NeurIPS main track paper guideline .
Use of Large Language Models (LLMs): We welcome authors to use any tool that is suitable for preparing high-quality papers and research. However, we ask authors to keep in mind two important criteria. First, we expect papers to fully describe their methodology, and any tool that is important to that methodology, including the use of LLMs, should be described also. For example, authors should mention tools (including LLMs) that were used for data processing or filtering, visualization, facilitating or running experiments, and proving theorems. It may also be advisable to describe the use of LLMs in implementing the method (if this corresponds to an important, original, or non-standard component of the approach). Second, authors are responsible for the entire content of the paper, including all text and figures, so while authors are welcome to use any tool they wish for writing the paper, they must ensure that all text is correct and original.
REVIEWING AND SELECTION PROCESS
Reviewing will be single-blind, although authors can also submit anonymously if the submission allows that. A datasets and benchmarks program committee will be formed, consisting of experts on machine learning, dataset curation, and ethics. We will ensure diversity in the program committee, both in terms of background as well as technical expertise (e.g., data, ML, data ethics, social science expertise). Each paper will be reviewed by the members of the committee. In select cases where ethical concerns are flagged by reviewers, an ethics review may be performed as well.
Papers will not be publicly visible during the review process. Only accepted papers will become visible afterward. The reviews themselves are also not visible during the review phase but will be published after decisions have been made. Authors can choose to keep the datasets themselves hidden until a later release date, as long as reviewers have access.
The factors that will be considered when evaluating papers include:
- Utility and quality of the submission: Impact, originality, novelty, relevance to the NeurIPS community will all be considered.
- Reproducibility: All submissions should be accompanied by sufficient information to reproduce the results described i.e. all necessary datasets, code, and evaluation procedures must be accessible and documented. We encourage the use of a reproducibility framework such as the ML reproducibility checklist to guarantee that all results can be easily reproduced. Benchmark submissions in particular should take care to ensure sufficient details are provided to ensure reproducibility. If submissions include code, please refer to the NeurIPS code submission guidelines .
- Was code provided (e.g. in the supplementary material)? If provided, did you look at the code? Did you consider it useful in guiding your review? If not provided, did you wish code had been available?
- Ethics: Any ethical implications of the work should be addressed. Authors should rely on NeurIPS ethics guidelines as guidance for understanding ethical concerns.
- Completeness of the relevant documentation: Per NeurIPS ethics guidelines , datasets must be accompanied by documentation communicating the details of the dataset as part of their submissions via structured templates (e.g. TODO). Sufficient detail must be provided on how the data was collected and organized, what kind of information it contains, ethically and responsibly, and how it will be made available and maintained.
- Licensing and access: Per NeurIPS ethics guidelines , authors should provide licenses for any datasets released. These should consider the intended use and limitations of the dataset, and develop licenses and terms of use to prevent misuse or inappropriate use.
- Consent and privacy: Per NeurIPS ethics guidelines , datasets should minimize the exposure of any personally identifiable information, unless informed consent from those individuals is provided to do so. Any paper that chooses to create a dataset with real data of real people should ask for the explicit consent of participants, or explain why they were unable to do so.
- Ethics and responsible use: Any ethical implications of new datasets should be addressed and guidelines for responsible use should be provided where appropriate. Note that, if your submission includes publicly available datasets (e.g. as part of a larger benchmark), you should also check these datasets for ethical issues. You remain responsible for the ethical implications of including existing datasets or other data sources in your work.
- Legal compliance: For datasets, authors should ensure awareness and compliance with regional legal requirements.
ADVISORY COMMITTEE
The following committee will provide advice on the organization of the track over the coming years: Sergio Escalera, Isabelle Guyon, Neil Lawrence, Dina Machuve, Olga Russakovsky, Joaquin Vanschoren, Serena Yeung.
DATASETS AND BENCHMARKS CHAIRS
Lora Aroyo, Google Francesco Locatello, Institute of Science and Technology Austria Lingjuan Lyu, Sony AI
Contact: [email protected]

COMMENTS
In chemical terms, photosynthesis is a light-energized oxidation-reduction process. (Oxidation refers to the removal of electrons from a molecule; reduction refers to the gain of electrons by a molecule.) In plant photosynthesis, the energy of light is used to drive the oxidation of water (H 2 O), producing oxygen gas (O 2 ), hydrogen ions (H ...
Most life on Earth depends on photosynthesis.The process is carried out by plants, algae, and some types of bacteria, which capture energy from sunlight to produce oxygen (O 2) and chemical energy stored in glucose (a sugar). Herbivores then obtain this energy by eating plants, and carnivores obtain it by eating herbivores.. The process. During photosynthesis, plants take in carbon dioxide (CO ...
Cellular respiration happens in the dark. In the process of photosynthesis, the plants need sunlight for energy. Photosynthesis is the process that plants use light energy from the sun to make their own food. Cellular respiration is a chemical process plants use to release the stored chemical energy from glucose as usable chemical energy so it ...
Photosynthesis is the process in which light energy is converted to chemical energy in the form of sugars. In a process driven by light energy, glucose molecules (or other sugars) are constructed from water and carbon dioxide, and oxygen is released as a byproduct. The glucose molecules provide organisms with two crucial resources: energy and ...
Photosynthesis is the process of creating sugar and oxygen from carbon dioxide, water and sunlight. It happens through a long series of chemical reactions. But it can be summarized like this: Carbon dioxide, water and light go in. Glucose, water and oxygen come out. (Glucose is a simple sugar.) Photosynthesis can be split into two processes.
Meaning. Photosynthesis. The process by which plants, algae, and some bacteria convert light energy to chemical energy in the form of sugars. Photoautotroph. An organism that produces its own food using light energy (like plants) ATP. Adenosine triphosphate, the primary energy carrier in living things. Chloroplast.
Main Structures and Summary of Photosynthesis. Photosynthesis is a multi-step process that requires sunlight, carbon dioxide (which is low in energy), and water as substrates (Figure 8.1.3 8.1. 3 ). After the process is complete, it releases oxygen and produces glyceraldehyde-3-phosphate (GA3P), simple carbohydrate molecules (which are high in ...
The chemical equation for the entire process can be seen below. Photosynthesis Equation. 6 CO 2 + 6 H 2 O + Light -> C 6 H 12 O 6 + 6 O 2 + 6 H 2 O. Above is the overall reaction for photosynthesis. Using the energy from light and the hydrogens and electrons from water, the plant combines the carbons found in carbon dioxide into more complex ...
Photosynthesis. Photosynthesis is a process by which phototrophs convert light energy into chemical energy, which is later used to fuel cellular activities. The chemical energy is stored in the form of sugars, which are created from water and carbon dioxide. 3,12,343.
It is the biochemical process that sustains the biosphere as the basis for the food chain. The oxygen produced as a by-product of photosynthesis allowed the formation of the ozone layer, the evolution of aerobic respiration and thus complex multicellular life. Oxygenic photosynthesis involves the conversion of water and CO 2 into complex ...
In the Calvin cycle, carbon atoms from CO 2 are fixed (incorporated into organic molecules) and used to build three-carbon sugars. This process is fueled by, and dependent on, ATP and NADPH from the light reactions. Unlike the light reactions, which take place in the thylakoid membrane, the reactions of the Calvin cycle take place in the stroma ...
The whole process of photosynthesis is a transfer of energy from the Sun to a plant. In each sugar molecule created, there is a little bit of the energy from the Sun, which the plant can either use or store for later. Imagine a pea plant. If that pea plant is forming new pods, it requires a large amount of sugar energy to grow larger.
Photosynthesis is a two stage process. The light dependent reactions, a light-dependent series of reactions which occur in the grana, and require the direct energy of light to make energy-carrier molecules that are used in the second process: • light energy is trapped by chlorophyll to make ATP (photophosphorylation)
500 Words Essay on Photosynthesis What is Photosynthesis? Photosynthesis is a process used by plants, algae, and some bacteria to turn sunlight, water, and carbon dioxide into food and oxygen. This happens in the green parts of plants, mainly the leaves. The green color comes from chlorophyll, a special substance in the leaves that captures ...
The photosynthesis process occurs primarily in the leaves with little taking place in the stem for some plants. The main parts of the leaf in which are involved in the process include; "the upper and lower epidermis, the mesophyll, the vascular bundles and the stomates." (Photosynthesis, 2000) The epidermis lacks chlorophyll and therefore ...
Note that the above global equation of photosynthesis emphasizes that the oxygen molecules released into the atmosphere originate from water oxidation, not from carbon dioxide, as established using 18 O-labelled water (Ruben et al., 1941).. This process starts in the thylakoid membrane (TM) with two light reactions taking place simultaneously at photosystem (PS) II and PSI reaction centres ...
Photosynthesis is a highly regulated, multistep process. It encompasses the harvest of solar energy, transfer of excitation energy, energy conversion, electron transfer from water to NADP +, ATP generation and a series of enzymatic reactions that assimilate carbon dioxide and synthesize carbohydrate.. Photosynthesis has a unique place in the history of plant science, as its central concepts ...
Photosynthesis is the process described by this equation This equation shows the complex 2 steps process that takes place in the chloroplast of green plants. The end product is glucose, but the complex organic molecule such as carbohydrates, amino acid, lipids, and nucleic acids. Photosynthesis is important because it is the biological process ...
Essay # 1. Meaning of Photosynthesis: Although literary meaning of photosynthesis is 'synthesis with the help of light' but this term is usually applied to a very important vital process by which the green plants synthesize organic matter in presence of light.
Photosynthesis is the process in which green plants use sunlight to make their own food. Photosynthesis is necessary for life on Earth. Without it there would be no green plants, and without green plants there would be no animals. Interactive
The light-dependent reactions use light energy to make two molecules needed for the next stage of photosynthesis: the energy storage molecule ATP and the reduced electron carrier NADPH. In plants, the light reactions take place in the thylakoid membranes of organelles called chloroplasts.
The photosynthesis equation is CO2 (carbon dioxide)+H2O (water)+light energy=C6H12O6 (glucose) & O2 (oxygen). Cellular respiration is a process plants use at night for energy. This happens in the mitochondria's of plant cells. The resources needed for this are energy, carbon dioxide, water, and heat. Cellular respiration is the inverse of ...
In their review, Kunz et al. highlight the different ways in which chloroplast ion homeostasis impacts photosynthesis. Ions are necessary for the structure and functioning of pigments and proteins involved in photosynthesis. For example, magnesium (Mg 2+) is the central ion in the porphyrin structure of chlorophyll (Wang & Grimm, 2021).
The process of photosynthesis in plants is based on two reactions that are carried out by separate parts of the chloroplast. The light reactions occur in the chloroplast thylakoid membrane and involve the splitting of water into oxygen, protons and electrons. ... (1994) on which this essay is partly based. ...
Fig. 1: The biogeochemical effect of elevated carbon dioxide on global vegetation uptake of Hg(0). Fig. 2: Global vegetation Hg(0) uptake (bars) via elevated CO 2 under different atmospheric CO 2 ...
"Ms. Noem has recently been in the news for shooting and killing her dog. The process server felt legitimately threatened," a court filing made by Travelers United stated.
Leymus chinensis is a perennial rhizomatous clone plant. It exhibits strong rhizomatous tillering and clonal growth through asexual reproduction. The root system is interdependent with aboveground growth and root growth can regulate aboveground growth and photosynthesis. Melatonin has been shown to regulate root growth and promote photosynthesis. However, it remains unclear whether melatonin ...
Internet slams Kristi Noem as process server claims she was 'threatening' as it attempted to serve her with legal papers. Several internet users slammed Kristi Noem as the process server claimed ...
Call For Papers. Abstract submission deadline: May 15, 2024. Full paper submission deadline, including technical appendices and supplemental material (all authors must have an OpenReview profile when submitting): May 22, 2024. Author notification: Sep 25, 2024. Camera-ready, poster, and video submission: Oct 30, 2024 AOE.
The Datasets and Benchmarks track is proud to support the open source movement by encouraging submissions of open-source libraries and tools that enable or accelerate ML research. The previous editions of the Datasets and Benchmarks track were highly successful; you can view the accepted papers from 2021, 2002, and 2023, and the winners of the ...