- Open access
- Published: 26 February 2019

Stem cells: past, present, and future
- Wojciech Zakrzewski 1 ,
- Maciej Dobrzyński 2 ,
- Maria Szymonowicz 1 &
- Zbigniew Rybak 1
Stem Cell Research & Therapy volume 10 , Article number: 68 ( 2019 ) Cite this article
569k Accesses
857 Citations
54 Altmetric
Metrics details
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory steps of controlled stem cell culturing and derivation. Quality control and teratoma formation assays are important procedures in assessing the properties of the stem cells tested. Derivation methods and the utilization of culturing media are crucial to set proper environmental conditions for controlled differentiation. Among many types of stem tissue applications, the use of graphene scaffolds and the potential of extracellular vesicle-based therapies require attention due to their versatility. The review is summarized by challenges that stem cell therapy must overcome to be accepted worldwide. A wide variety of possibilities makes this cutting edge therapy a turning point in modern medicine, providing hope for untreatable diseases.
Stem cell classification
Stem cells are unspecialized cells of the human body. They are able to differentiate into any cell of an organism and have the ability of self-renewal. Stem cells exist both in embryos and adult cells. There are several steps of specialization. Developmental potency is reduced with each step, which means that a unipotent stem cell is not able to differentiate into as many types of cells as a pluripotent one. This chapter will focus on stem cell classification to make it easier for the reader to comprehend the following chapters.
Totipotent stem cells are able to divide and differentiate into cells of the whole organism. Totipotency has the highest differentiation potential and allows cells to form both embryo and extra-embryonic structures. One example of a totipotent cell is a zygote, which is formed after a sperm fertilizes an egg. These cells can later develop either into any of the three germ layers or form a placenta. After approximately 4 days, the blastocyst’s inner cell mass becomes pluripotent. This structure is the source of pluripotent cells.
Pluripotent stem cells (PSCs) form cells of all germ layers but not extraembryonic structures, such as the placenta. Embryonic stem cells (ESCs) are an example. ESCs are derived from the inner cell mass of preimplantation embryos. Another example is induced pluripotent stem cells (iPSCs) derived from the epiblast layer of implanted embryos. Their pluripotency is a continuum, starting from completely pluripotent cells such as ESCs and iPSCs and ending on representatives with less potency—multi-, oligo- or unipotent cells. One of the methods to assess their activity and spectrum is the teratoma formation assay. iPSCs are artificially generated from somatic cells, and they function similarly to PSCs. Their culturing and utilization are very promising for present and future regenerative medicine.
Multipotent stem cells have a narrower spectrum of differentiation than PSCs, but they can specialize in discrete cells of specific cell lineages. One example is a haematopoietic stem cell, which can develop into several types of blood cells. After differentiation, a haematopoietic stem cell becomes an oligopotent cell. Its differentiation abilities are then restricted to cells of its lineage. However, some multipotent cells are capable of conversion into unrelated cell types, which suggests naming them pluripotent cells.
Oligopotent stem cells can differentiate into several cell types. A myeloid stem cell is an example that can divide into white blood cells but not red blood cells.
Unipotent stem cells are characterized by the narrowest differentiation capabilities and a special property of dividing repeatedly. Their latter feature makes them a promising candidate for therapeutic use in regenerative medicine. These cells are only able to form one cell type, e.g. dermatocytes.
Stem cell biology
A blastocyst is formed after the fusion of sperm and ovum fertilization. Its inner wall is lined with short-lived stem cells, namely, embryonic stem cells. Blastocysts are composed of two distinct cell types: the inner cell mass (ICM), which develops into epiblasts and induces the development of a foetus, and the trophectoderm (TE). Blastocysts are responsible for the regulation of the ICM microenvironment. The TE continues to develop and forms the extraembryonic support structures needed for the successful origin of the embryo, such as the placenta. As the TE begins to form a specialized support structure, the ICM cells remain undifferentiated, fully pluripotent and proliferative [ 1 ]. The pluripotency of stem cells allows them to form any cell of the organism. Human embryonic stem cells (hESCs) are derived from the ICM. During the process of embryogenesis, cells form aggregations called germ layers: endoderm, mesoderm and ectoderm (Fig. 1 ), each eventually giving rise to differentiated cells and tissues of the foetus and, later on, the adult organism [ 2 ]. After hESCs differentiate into one of the germ layers, they become multipotent stem cells, whose potency is limited to only the cells of the germ layer. This process is short in human development. After that, pluripotent stem cells occur all over the organism as undifferentiated cells, and their key abilities are proliferation by the formation of the next generation of stem cells and differentiation into specialized cells under certain physiological conditions.
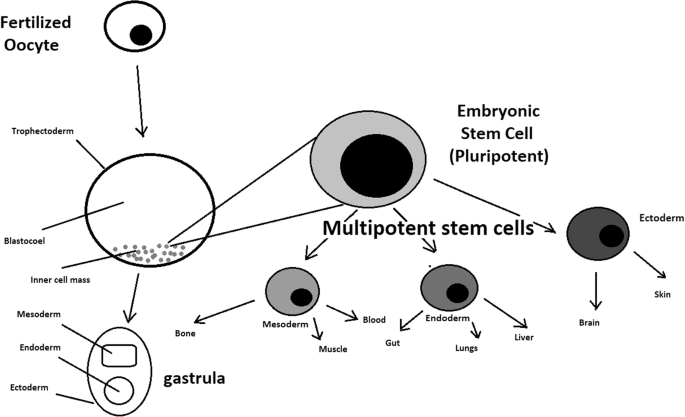
Oocyte development and formation of stem cells: the blastocoel, which is formed from oocytes, consists of embryonic stem cells that later differentiate into mesodermal, ectodermal, or endodermal cells. Blastocoel develops into the gastrula
Signals that influence the stem cell specialization process can be divided into external, such as physical contact between cells or chemical secretion by surrounding tissue, and internal, which are signals controlled by genes in DNA.
Stem cells also act as internal repair systems of the body. The replenishment and formation of new cells are unlimited as long as an organism is alive. Stem cell activity depends on the organ in which they are in; for example, in bone marrow, their division is constant, although in organs such as the pancreas, division only occurs under special physiological conditions.
Stem cell functional division
Whole-body development.
During division, the presence of different stem cells depends on organism development. Somatic stem cell ESCs can be distinguished. Although the derivation of ESCs without separation from the TE is possible, such a combination has growth limits. Because proliferating actions are limited, co-culture of these is usually avoided.
ESCs are derived from the inner cell mass of the blastocyst, which is a stage of pre-implantation embryo ca. 4 days after fertilization. After that, these cells are placed in a culture dish filled with culture medium. Passage is an inefficient but popular process of sub-culturing cells to other dishes. These cells can be described as pluripotent because they are able to eventually differentiate into every cell type in the organism. Since the beginning of their studies, there have been ethical restrictions connected to the medical use of ESCs in therapies. Most embryonic stem cells are developed from eggs that have been fertilized in an in vitro clinic, not from eggs fertilized in vivo.
Somatic or adult stem cells are undifferentiated and found among differentiated cells in the whole body after development. The function of these cells is to enable the healing, growth, and replacement of cells that are lost each day. These cells have a restricted range of differentiation options. Among many types, there are the following:
Mesenchymal stem cells are present in many tissues. In bone marrow, these cells differentiate mainly into the bone, cartilage, and fat cells. As stem cells, they are an exception because they act pluripotently and can specialize in the cells of any germ layer.
Neural cells give rise to nerve cells and their supporting cells—oligodendrocytes and astrocytes.
Haematopoietic stem cells form all kinds of blood cells: red, white, and platelets.
Skin stem cells form, for example, keratinocytes, which form a protective layer of skin.
The proliferation time of somatic stem cells is longer than that of ESCs. It is possible to reprogram adult stem cells back to their pluripotent state. This can be performed by transferring the adult nucleus into the cytoplasm of an oocyte or by fusion with the pluripotent cell. The same technique was used during cloning of the famous Dolly sheep.
hESCs are involved in whole-body development. They can differentiate into pluripotent, totipotent, multipotent, and unipotent cells (Fig. 2 ) [ 2 ].
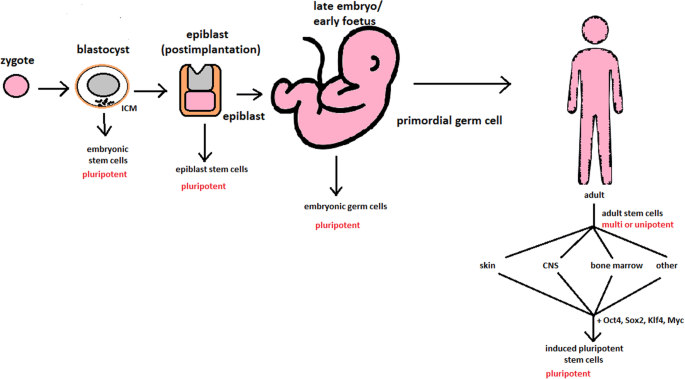
Changes in the potency of stem cells in human body development. Potency ranges from pluripotent cells of the blastocyst to unipotent cells of a specific tissue in a human body such as the skin, CNS, or bone marrow. Reversed pluripotency can be achieved by the formation of induced pluripotent stem cells using either octamer-binding transcription factor (Oct4), sex-determining region Y (Sox2), Kruppel-like factor 4 (Klf4), or the Myc gene
Pluripotent cells can be named totipotent if they can additionally form extraembryonic tissues of the embryo. Multipotent cells are restricted in differentiating to each cell type of given tissue. When tissue contains only one lineage of cells, stem cells that form them are called either called oligo- or unipotent.
iPSC quality control and recognition by morphological differences
The comparability of stem cell lines from different individuals is needed for iPSC lines to be used in therapeutics [ 3 ]. Among critical quality procedures, the following can be distinguished:
Short tandem repeat analysis—This is the comparison of specific loci on the DNA of the samples. It is used in measuring an exact number of repeating units. One unit consists of 2 to 13 nucleotides repeating many times on the DNA strand. A polymerase chain reaction is used to check the lengths of short tandem repeats. The genotyping procedure of source tissue, cells, and iPSC seed and master cell banks is recommended.
Identity analysis—The unintentional switching of lines, resulting in other stem cell line contamination, requires rigorous assay for cell line identification.
Residual vector testing—An appearance of reprogramming vectors integrated into the host genome is hazardous, and testing their presence is a mandatory procedure. It is a commonly used procedure for generating high-quality iPSC lines. An acceptable threshold in high-quality research-grade iPSC line collections is ≤ 1 plasmid copies per 100 cells. During the procedure, 2 different regions, common to all plasmids, should be used as specific targets, such as EBNA and CAG sequences [ 3 ]. To accurately represent the test reactions, a standard curve needs to be prepared in a carrier of gDNA from a well-characterized hPSC line. For calculations of plasmid copies per cell, it is crucial to incorporate internal reference gDNA sequences to allow the quantification of, for example, ribonuclease P (RNaseP) or human telomerase reverse transcriptase (hTERT).
Karyotype—A long-term culture of hESCs can accumulate culture-driven mutations [ 4 ]. Because of that, it is crucial to pay additional attention to genomic integrity. Karyotype tests can be performed by resuscitating representative aliquots and culturing them for 48–72 h before harvesting cells for karyotypic analysis. If abnormalities are found within the first 20 karyotypes, the analysis must be repeated on a fresh sample. When this situation is repeated, the line is evaluated as abnormal. Repeated abnormalities must be recorded. Although karyology is a crucial procedure in stem cell quality control, the single nucleotide polymorphism (SNP) array, discussed later, has approximately 50 times higher resolution.
Viral testing—When assessing the quality of stem cells, all tests for harmful human adventitious agents must be performed (e.g. hepatitis C or human immunodeficiency virus). This procedure must be performed in the case of non-xeno-free culture agents.
Bacteriology—Bacterial or fungal sterility tests can be divided into culture- or broth-based tests. All the procedures must be recommended by pharmacopoeia for the jurisdiction in which the work is performed.
Single nucleotide polymorphism arrays—This procedure is a type of DNA microarray that detects population polymorphisms by enabling the detection of subchromosomal changes and the copy-neutral loss of heterozygosity, as well as an indication of cellular transformation. The SNP assay consists of three components. The first is labelling fragmented nucleic acid sequences with fluorescent dyes. The second is an array that contains immobilized allele-specific oligonucleotide (ASO) probes. The last component detects, records, and eventually interprets the signal.
Flow cytometry—This is a technique that utilizes light to count and profile cells in a heterogeneous fluid mixture. It allows researchers to accurately and rapidly collect data from heterogeneous fluid mixtures with live cells. Cells are passed through a narrow channel one by one. During light illumination, sensors detect light emitted or refracted from the cells. The last step is data analysis, compilation and integration into a comprehensive picture of the sample.
Phenotypic pluripotency assays—Recognizing undifferentiated cells is crucial in successful stem cell therapy. Among other characteristics, stem cells appear to have a distinct morphology with a high nucleus to cytoplasm ratio and a prominent nucleolus. Cells appear to be flat with defined borders, in contrast to differentiating colonies, which appear as loosely located cells with rough borders [ 5 ]. It is important that images of ideal and poor quality colonies for each cell line are kept in laboratories, so whenever there is doubt about the quality of culture, it can always be checked according to the representative image. Embryoid body formation or directed differentiation of monolayer cultures to produce cell types representative of all three embryonic germ layers must be performed. It is important to note that colonies cultured under different conditions may have different morphologies [ 6 ].
Histone modification and DNA methylation—Quality control can be achieved by using epigenetic analysis tools such as histone modification or DNA methylation. When stem cells differentiate, the methylation process silences pluripotency genes, which reduces differentiation potential, although other genes may undergo demethylation to become expressed [ 7 ]. It is important to emphasize that stem cell identity, together with its morphological characteristics, is also related to its epigenetic profile [ 8 , 9 ]. According to Brindley [ 10 ], there is a relationship between epigenetic changes, pluripotency, and cell expansion conditions, which emphasizes that unmethylated regions appear to be serum-dependent.
hESC derivation and media
hESCs can be derived using a variety of methods, from classic culturing to laser-assisted methodologies or microsurgery [ 11 ]. hESC differentiation must be specified to avoid teratoma formation (see Fig. 3 ).
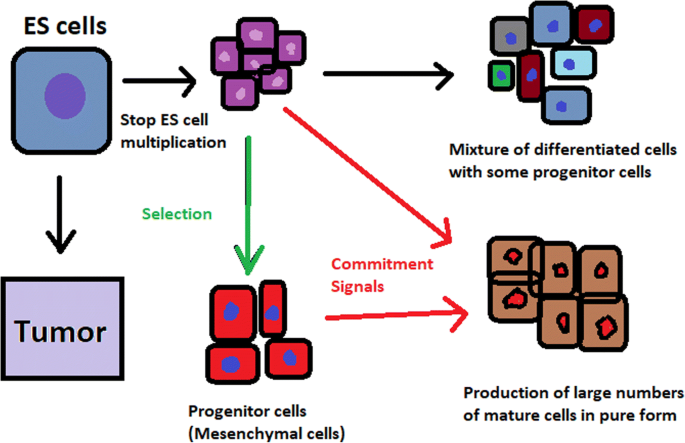
Spontaneous differentiation of hESCs causes the formation of a heterogeneous cell population. There is a different result, however, when commitment signals (in forms of soluble factors and culture conditions) are applied and enable the selection of progenitor cells
hESCs spontaneously differentiate into embryonic bodies (EBs) [ 12 ]. EBs can be studied instead of embryos or animals to predict their effects on early human development. There are many different methods for acquiring EBs, such as bioreactor culture [ 13 ], hanging drop culture [ 12 ], or microwell technology [ 14 , 15 ]. These methods allow specific precursors to form in vitro [ 16 ].
The essential part of these culturing procedures is a separation of inner cell mass to culture future hESCs (Fig. 4 ) [ 17 ]. Rosowski et al. [ 18 ] emphasizes that particular attention must be taken in controlling spontaneous differentiation. When the colony reaches the appropriate size, cells must be separated. The occurrence of pluripotent cells lasts for 1–2 days. Because the classical utilization of hESCs caused ethical concerns about gastrulas used during procedures, Chung et al. [ 19 ] found out that it is also possible to obtain hESCs from four cell embryos, leaving a higher probability of embryo survival. Additionally, Zhang et al. [ 20 ] used only in vitro fertilization growth-arrested cells.
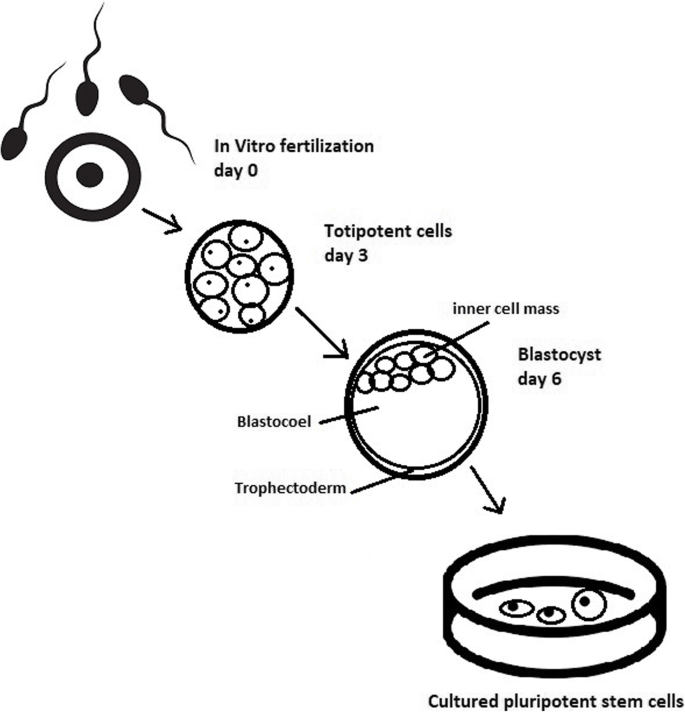
Culturing of pluripotent stem cells in vitro. Three days after fertilization, totipotent cells are formed. Blastocysts with ICM are formed on the sixth day after fertilization. Pluripotent stem cells from ICM can then be successfully transmitted on a dish
Cell passaging is used to form smaller clusters of cells on a new culture surface [ 21 ]. There are four important passaging procedures.
Enzymatic dissociation is a cutting action of enzymes on proteins and adhesion domains that bind the colony. It is a gentler method than the manual passage. It is crucial to not leave hESCs alone after passaging. Solitary cells are more sensitive and can easily undergo cell death; collagenase type IV is an example [ 22 , 23 ].
Manual passage , on the other hand, focuses on using cell scratchers. The selection of certain cells is not necessary. This should be done in the early stages of cell line derivation [ 24 ].
Trypsin utilization allows a healthy, automated hESC passage. Good Manufacturing Practice (GMP)-grade recombinant trypsin is widely available in this procedure [ 24 ]. However, there is a risk of decreasing the pluripotency and viability of stem cells [ 25 ]. Trypsin utilization can be halted with an inhibitor of the protein rho-associated protein kinase (ROCK) [ 26 ].
Ethylenediaminetetraacetic acid ( EDTA ) indirectly suppresses cell-to-cell connections by chelating divalent cations. Their suppression promotes cell dissociation [ 27 ].
Stem cells require a mixture of growth factors and nutrients to differentiate and develop. The medium should be changed each day.
Traditional culture methods used for hESCs are mouse embryonic fibroblasts (MEFs) as a feeder layer and bovine serum [ 28 ] as a medium. Martin et al. [ 29 ] demonstrated that hESCs cultured in the presence of animal products express the non-human sialic acid, N -glycolylneuraminic acid (NeuGc). Feeder layers prevent uncontrolled proliferation with factors such as leukaemia inhibitory factor (LIF) [ 30 ].
First feeder layer-free culture can be supplemented with serum replacement, combined with laminin [ 31 ]. This causes stable karyotypes of stem cells and pluripotency lasting for over a year.
Initial culturing media can be serum (e.g. foetal calf serum FCS), artificial replacement such as synthetic serum substitute (SSS), knockout serum replacement (KOSR), or StemPro [ 32 ]. The simplest culture medium contains only eight essential elements: DMEM/F12 medium, selenium, NaHCO 3, l -ascorbic acid, transferrin, insulin, TGFβ1, and FGF2 [ 33 ]. It is not yet fully known whether culture systems developed for hESCs can be allowed without adaptation in iPSC cultures.
Turning point in stem cell therapy
The turning point in stem cell therapy appeared in 2006, when scientists Shinya Yamanaka, together with Kazutoshi Takahashi, discovered that it is possible to reprogram multipotent adult stem cells to the pluripotent state. This process avoided endangering the foetus’ life in the process. Retrovirus-mediated transduction of mouse fibroblasts with four transcription factors (Oct-3/4, Sox2, KLF4, and c-Myc) [ 34 ] that are mainly expressed in embryonic stem cells could induce the fibroblasts to become pluripotent (Fig. 5 ) [ 35 ]. This new form of stem cells was named iPSCs. One year later, the experiment also succeeded with human cells [ 36 ]. After this success, the method opened a new field in stem cell research with a generation of iPSC lines that can be customized and biocompatible with the patient. Recently, studies have focused on reducing carcinogenesis and improving the conduction system.
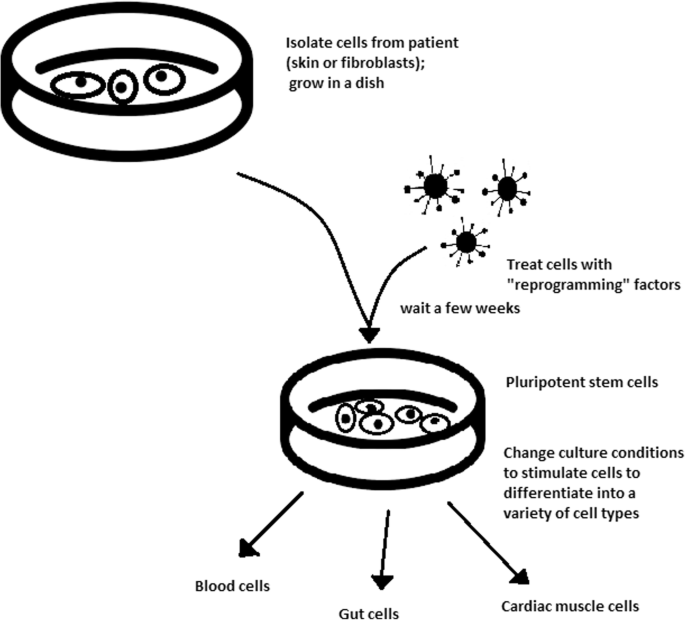
Retroviral-mediated transduction induces pluripotency in isolated patient somatic cells. Target cells lose their role as somatic cells and, once again, become pluripotent and can differentiate into any cell type of human body
The turning point was influenced by former discoveries that happened in 1962 and 1987.
The former discovery was about scientist John Gurdon successfully cloning frogs by transferring a nucleus from a frog’s somatic cells into an oocyte. This caused a complete reversion of somatic cell development [ 37 ]. The results of his experiment became an immense discovery since it was previously believed that cell differentiation is a one-way street only, but his experiment suggested the opposite and demonstrated that it is even possible for a somatic cell to again acquire pluripotency [ 38 ].
The latter was a discovery made by Davis R.L. that focused on fibroblast DNA subtraction. Three genes were found that originally appeared in myoblasts. The enforced expression of only one of the genes, named myogenic differentiation 1 (Myod1), caused the conversion of fibroblasts into myoblasts, showing that reprogramming cells is possible, and it can even be used to transform cells from one lineage to another [ 39 ].
Although pluripotency can occur naturally only in embryonic stem cells, it is possible to induce terminally differentiated cells to become pluripotent again. The process of direct reprogramming converts differentiated somatic cells into iPSC lines that can form all cell types of an organism. Reprogramming focuses on the expression of oncogenes such as Myc and Klf4 (Kruppel-like factor 4). This process is enhanced by a downregulation of genes promoting genome stability, such as p53. Additionally, cell reprogramming involves histone alteration. All these processes can cause potential mutagenic risk and later lead to an increased number of mutations. Quinlan et al. [ 40 ] checked fully pluripotent mouse iPSCs using whole genome DNA sequencing and structural variation (SV) detection algorithms. Based on those studies, it was confirmed that although there were single mutations in the non-genetic region, there were non-retrotransposon insertions. This led to the conclusion that current reprogramming methods can produce fully pluripotent iPSCs without severe genomic alterations.
During the course of development from pluripotent hESCs to differentiated somatic cells, crucial changes appear in the epigenetic structure of these cells. There is a restriction or permission of the transcription of genes relevant to each cell type. When somatic cells are being reprogrammed using transcription factors, all the epigenetic architecture has to be reconditioned to achieve iPSCs with pluripotency [ 41 ]. However, cells of each tissue undergo specific somatic genomic methylation. This influences transcription, which can further cause alterations in induced pluripotency [ 42 ].
Source of iPSCs
Because pluripotent cells can propagate indefinitely and differentiate into any kind of cell, they can be an unlimited source, either for replacing lost or diseased tissues. iPSCs bypass the need for embryos in stem cell therapy. Because they are made from the patient’s own cells, they are autologous and no longer generate any risk of immune rejection.
At first, fibroblasts were used as a source of iPSCs. Because a biopsy was needed to achieve these types of cells, the technique underwent further research. Researchers investigated whether more accessible cells could be used in the method. Further, other cells were used in the process: peripheral blood cells, keratinocytes, and renal epithelial cells found in urine. An alternative strategy to stem cell transplantation can be stimulating a patient’s endogenous stem cells to divide or differentiate, occurring naturally when skin wounds are healing. In 2008, pancreatic exocrine cells were shown to be reprogrammed to functional, insulin-producing beta cells [ 43 ].
The best stem cell source appears to be the fibroblasts, which is more tempting in the case of logistics since its stimulation can be fast and better controlled [ 44 ].
- Teratoma formation assay
The self-renewal and differentiation capabilities of iPSCs have gained significant interest and attention in regenerative medicine sciences. To study their abilities, a quality-control assay is needed, of which one of the most important is the teratoma formation assay. Teratomas are benign tumours. Teratomas are capable of rapid growth in vivo and are characteristic because of their ability to develop into tissues of all three germ layers simultaneously. Because of the high pluripotency of teratomas, this formation assay is considered an assessment of iPSC’s abilities [ 45 ].
Teratoma formation rate, for instance, was observed to be elevated in human iPSCs compared to that in hESCs [ 46 ]. This difference may be connected to different differentiation methods and cell origins. Most commonly, the teratoma assay involves an injection of examined iPSCs subcutaneously or under the testis or kidney capsule in mice, which are immune-deficient [ 47 ]. After injection, an immature but recognizable tissue can be observed, such as the kidney tubules, bone, cartilage, or neuroepithelium [ 30 ]. The injection site may have an impact on the efficiency of teratoma formation [ 48 ].
There are three groups of markers used in this assay to differentiate the cells of germ layers. For endodermal tissue, there is insulin/C-peptide and alpha-1 antitrypsin [ 49 ]. For the mesoderm, derivatives can be used, e.g. cartilage matrix protein for the bone and alcian blue for the cartilage. As ectodermal markers, class III B botulin or keratin can be used for keratinocytes.
Teratoma formation assays are considered the gold standard for demonstrating the pluripotency of human iPSCs, demonstrating their possibilities under physiological conditions. Due to their actual tissue formation, they could be used for the characterization of many cell lineages [ 50 ].
Directed differentiation
To be useful in therapy, stem cells must be converted into desired cell types as necessary or else the whole regenerative medicine process will be pointless. Differentiation of ESCs is crucial because undifferentiated ESCs can cause teratoma formation in vivo. Understanding and using signalling pathways for differentiation is an important method in successful regenerative medicine. In directed differentiation, it is likely to mimic signals that are received by cells when they undergo successive stages of development [ 51 ]. The extracellular microenvironment plays a significant role in controlling cell behaviour. By manipulating the culture conditions, it is possible to restrict specific differentiation pathways and generate cultures that are enriched in certain precursors in vitro. However, achieving a similar effect in vivo is challenging. It is crucial to develop culture conditions that will allow the promotion of homogenous and enhanced differentiation of ESCs into functional and desired tissues.
Regarding the self-renewal of embryonic stem cells, Hwang et al. [ 52 ] noted that the ideal culture method for hESC-based cell and tissue therapy would be a defined culture free of either the feeder layer or animal components. This is because cell and tissue therapy requires the maintenance of large quantities of undifferentiated hESCs, which does not make feeder cells suitable for such tasks.
Most directed differentiation protocols are formed to mimic the development of an inner cell mass during gastrulation. During this process, pluripotent stem cells differentiate into ectodermal, mesodermal, or endodermal progenitors. Mall molecules or growth factors induce the conversion of stem cells into appropriate progenitor cells, which will later give rise to the desired cell type. There is a variety of signal intensities and molecular families that may affect the establishment of germ layers in vivo, such as fibroblast growth factors (FGFs) [ 53 ]; the Wnt family [ 54 ] or superfamily of transforming growth factors—β(TGFβ); and bone morphogenic proteins (BMP) [ 55 ]. Each candidate factor must be tested on various concentrations and additionally applied to various durations because the precise concentrations and times during which developing cells in embryos are influenced during differentiation are unknown. For instance, molecular antagonists of endogenous BMP and Wnt signalling can be used for ESC formation of ectoderm [ 56 ]. However, transient Wnt and lower concentrations of the TGFβ family trigger mesodermal differentiation [ 57 ]. Regarding endoderm formation, a higher activin A concentration may be required [ 58 , 59 ].
There are numerous protocols about the methods of forming progenitors of cells of each of germ layers, such as cardiomyocytes [ 60 ], hepatocytes [ 61 ], renal cells [ 62 ], lung cells [ 63 , 64 ], motor neurons [ 65 ], intestinal cells [ 66 ], or chondrocytes [ 67 ].
Directed differentiation of either iPSCs or ESCs into, e.g. hepatocytes, could influence and develop the study of the molecular mechanisms in human liver development. In addition, it could also provide the possibility to form exogenous hepatocytes for drug toxicity testing [ 68 ].
Levels of concentration and duration of action with a specific signalling molecule can cause a variety of factors. Unfortunately, for now, a high cost of recombinant factors is likely to limit their use on a larger scale in medicine. The more promising technique focuses on the use of small molecules. These can be used for either activating or deactivating specific signalling pathways. They enhance reprogramming efficiency by creating cells that are compatible with the desired type of tissue. It is a cheaper and non-immunogenic method.
One of the successful examples of small-molecule cell therapies is antagonists and agonists of the Hedgehog pathway. They show to be very useful in motor neuron regeneration [ 69 ]. Endogenous small molecules with their function in embryonic development can also be used in in vitro methods to induce the differentiation of cells; for example, retinoic acid, which is responsible for patterning the nervous system in vivo [ 70 ], surprisingly induced retinal cell formation when the laboratory procedure involved hESCs [ 71 ].
The efficacy of differentiation factors depends on functional maturity, efficiency, and, finally, introducing produced cells to their in vivo equivalent. Topography, shear stress, and substrate rigidity are factors influencing the phenotype of future cells [ 72 ].
The control of biophysical and biochemical signals, the biophysical environment, and a proper guide of hESC differentiation are important factors in appropriately cultured stem cells.
Stem cell utilization and their manufacturing standards and culture systems
The European Medicines Agency and the Food and Drug Administration have set Good Manufacturing Practice (GMP) guidelines for safe and appropriate stem cell transplantation. In the past, protocols used for stem cell transplantation required animal-derived products [ 73 ].
The risk of introducing animal antigens or pathogens caused a restriction in their use. Due to such limitations, the technique required an obvious update [ 74 ]. Now, it is essential to use xeno-free equivalents when establishing cell lines that are derived from fresh embryos and cultured from human feeder cell lines [ 75 ]. In this method, it is crucial to replace any non-human materials with xeno-free equivalents [ 76 ].
NutriStem with LN-511, TeSR2 with human recombinant laminin (LN-511), and RegES with human foreskin fibroblasts (HFFs) are commonly used xeno-free culture systems [ 33 ]. There are many organizations and international initiatives, such as the National Stem Cell Bank, that provide stem cell lines for treatment or medical research [ 77 ].
Stem cell use in medicine
Stem cells have great potential to become one of the most important aspects of medicine. In addition to the fact that they play a large role in developing restorative medicine, their study reveals much information about the complex events that happen during human development.
The difference between a stem cell and a differentiated cell is reflected in the cells’ DNA. In the former cell, DNA is arranged loosely with working genes. When signals enter the cell and the differentiation process begins, genes that are no longer needed are shut down, but genes required for the specialized function will remain active. This process can be reversed, and it is known that such pluripotency can be achieved by interaction in gene sequences. Takahashi and Yamanaka [ 78 ] and Loh et al. [ 79 ] discovered that octamer-binding transcription factor 3 and 4 (Oct3/4), sex determining region Y (SRY)-box 2 and Nanog genes function as core transcription factors in maintaining pluripotency. Among them, Oct3/4 and Sox2 are essential for the generation of iPSCs.
Many serious medical conditions, such as birth defects or cancer, are caused by improper differentiation or cell division. Currently, several stem cell therapies are possible, among which are treatments for spinal cord injury, heart failure [ 80 ], retinal and macular degeneration [ 81 ], tendon ruptures, and diabetes type 1 [ 82 ]. Stem cell research can further help in better understanding stem cell physiology. This may result in finding new ways of treating currently incurable diseases.
Haematopoietic stem cell transplantation
Haematopoietic stem cells are important because they are by far the most thoroughly characterized tissue-specific stem cell; after all, they have been experimentally studied for more than 50 years. These stem cells appear to provide an accurate paradigm model system to study tissue-specific stem cells, and they have potential in regenerative medicine.
Multipotent haematopoietic stem cell (HSC) transplantation is currently the most popular stem cell therapy. Target cells are usually derived from the bone marrow, peripheral blood, or umbilical cord blood [ 83 ]. The procedure can be autologous (when the patient’s own cells are used), allogenic (when the stem cell comes from a donor), or syngeneic (from an identical twin). HSCs are responsible for the generation of all functional haematopoietic lineages in blood, including erythrocytes, leukocytes, and platelets. HSC transplantation solves problems that are caused by inappropriate functioning of the haematopoietic system, which includes diseases such as leukaemia and anaemia. However, when conventional sources of HSC are taken into consideration, there are some important limitations. First, there is a limited number of transplantable cells, and an efficient way of gathering them has not yet been found. There is also a problem with finding a fitting antigen-matched donor for transplantation, and viral contamination or any immunoreactions also cause a reduction in efficiency in conventional HSC transplantations. Haematopoietic transplantation should be reserved for patients with life-threatening diseases because it has a multifactorial character and can be a dangerous procedure. iPSC use is crucial in this procedure. The use of a patient’s own unspecialized somatic cells as stem cells provides the greatest immunological compatibility and significantly increases the success of the procedure.
Stem cells as a target for pharmacological testing
Stem cells can be used in new drug tests. Each experiment on living tissue can be performed safely on specific differentiated cells from pluripotent cells. If any undesirable effect appears, drug formulas can be changed until they reach a sufficient level of effectiveness. The drug can enter the pharmacological market without harming any live testers. However, to test the drugs properly, the conditions must be equal when comparing the effects of two drugs. To achieve this goal, researchers need to gain full control of the differentiation process to generate pure populations of differentiated cells.
Stem cells as an alternative for arthroplasty
One of the biggest fears of professional sportsmen is getting an injury, which most often signifies the end of their professional career. This applies especially to tendon injuries, which, due to current treatment options focusing either on conservative or surgical treatment, often do not provide acceptable outcomes. Problems with the tendons start with their regeneration capabilities. Instead of functionally regenerating after an injury, tendons merely heal by forming scar tissues that lack the functionality of healthy tissues. Factors that may cause this failed healing response include hypervascularization, deposition of calcific materials, pain, or swelling [ 84 ].
Additionally, in addition to problems with tendons, there is a high probability of acquiring a pathological condition of joints called osteoarthritis (OA) [ 85 ]. OA is common due to the avascular nature of articular cartilage and its low regenerative capabilities [ 86 ]. Although arthroplasty is currently a common procedure in treating OA, it is not ideal for younger patients because they can outlive the implant and will require several surgical procedures in the future. These are situations where stem cell therapy can help by stopping the onset of OA [ 87 ]. However, these procedures are not well developed, and the long-term maintenance of hyaline cartilage requires further research.
Osteonecrosis of the femoral hip (ONFH) is a refractory disease associated with the collapse of the femoral head and risk of hip arthroplasty in younger populations [ 88 ]. Although total hip arthroplasty (THA) is clinically successful, it is not ideal for young patients, mostly due to the limited lifetime of the prosthesis. An increasing number of clinical studies have evaluated the therapeutic effect of stem cells on ONFH. Most of the authors demonstrated positive outcomes, with reduced pain, improved function, or avoidance of THA [ 89 , 90 , 91 ].
Rejuvenation by cell programming
Ageing is a reversible epigenetic process. The first cell rejuvenation study was published in 2011 [ 92 ]. Cells from aged individuals have different transcriptional signatures, high levels of oxidative stress, dysfunctional mitochondria, and shorter telomeres than in young cells [ 93 ]. There is a hypothesis that when human or mouse adult somatic cells are reprogrammed to iPSCs, their epigenetic age is virtually reset to zero [ 94 ]. This was based on an epigenetic model, which explains that at the time of fertilization, all marks of parenteral ageing are erased from the zygote’s genome and its ageing clock is reset to zero [ 95 ].
In their study, Ocampo et al. [ 96 ] used Oct4, Sox2, Klf4, and C-myc genes (OSKM genes) and affected pancreas and skeletal muscle cells, which have poor regenerative capacity. Their procedure revealed that these genes can also be used for effective regenerative treatment [ 97 ]. The main challenge of their method was the need to employ an approach that does not use transgenic animals and does not require an indefinitely long application. The first clinical approach would be preventive, focused on stopping or slowing the ageing rate. Later, progressive rejuvenation of old individuals can be attempted. In the future, this method may raise some ethical issues, such as overpopulation, leading to lower availability of food and energy.
For now, it is important to learn how to implement cell reprogramming technology in non-transgenic elder animals and humans to erase marks of ageing without removing the epigenetic marks of cell identity.
Cell-based therapies
Stem cells can be induced to become a specific cell type that is required to repair damaged or destroyed tissues (Fig. 6 ). Currently, when the need for transplantable tissues and organs outweighs the possible supply, stem cells appear to be a perfect solution for the problem. The most common conditions that benefit from such therapy are macular degenerations [ 98 ], strokes [ 99 ], osteoarthritis [ 89 , 90 ], neurodegenerative diseases, and diabetes [ 100 ]. Due to this technique, it can become possible to generate healthy heart muscle cells and later transplant them to patients with heart disease.
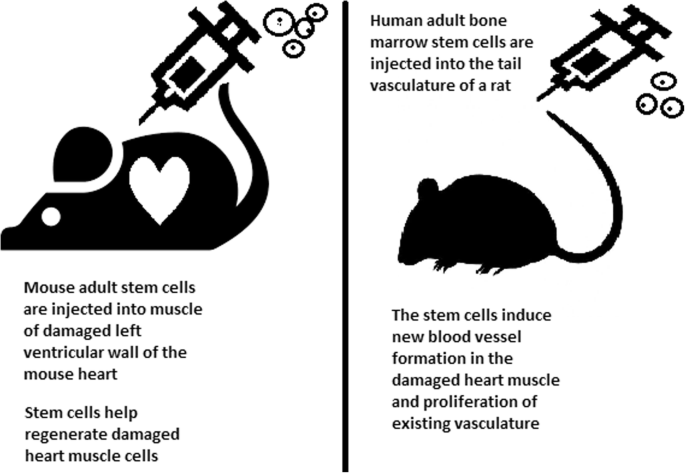
Stem cell experiments on animals. These experiments are one of the many procedures that proved stem cells to be a crucial factor in future regenerative medicine
In the case of type 1 diabetes, insulin-producing cells in the pancreas are destroyed due to an autoimmunological reaction. As an alternative to transplantation therapy, it can be possible to induce stem cells to differentiate into insulin-producing cells [ 101 ].
Stem cells and tissue banks
iPS cells with their theoretically unlimited propagation and differentiation abilities are attractive for the present and future sciences. They can be stored in a tissue bank to be an essential source of human tissue used for medical examination. The problem with conventional differentiated tissue cells held in the laboratory is that their propagation features diminish after time. This does not occur in iPSCs.
The umbilical cord is known to be rich in mesenchymal stem cells. Due to its cryopreservation immediately after birth, its stem cells can be successfully stored and used in therapies to prevent the future life-threatening diseases of a given patient.
Stem cells of human exfoliated deciduous teeth (SHED) found in exfoliated deciduous teeth has the ability to develop into more types of body tissues than other stem cells [ 102 ] (Table 1 ). Techniques of their collection, isolation, and storage are simple and non-invasive. Among the advantages of banking, SHED cells are:
Guaranteed donor-match autologous transplant that causes no immune reaction and rejection of cells [ 103 ]
Simple and painless for both child and parent
Less than one third of the cost of cord blood storage
Not subject to the same ethical concerns as embryonic stem cells [ 104 ]
In contrast to cord blood stem cells, SHED cells are able to regenerate into solid tissues such as connective, neural, dental, or bone tissue [ 105 , 106 ]
SHED can be useful for close relatives of the donor
Fertility diseases
In 2011, two researchers, Katsuhiko Hayashi et al. [ 107 ], showed in an experiment on mice that it is possible to form sperm from iPSCs. They succeeded in delivering healthy and fertile pups in infertile mice. The experiment was also successful for female mice, where iPSCs formed fully functional eggs .
Young adults at risk of losing their spermatogonial stem cells (SSC), mostly cancer patients, are the main target group that can benefit from testicular tissue cryopreservation and autotransplantation. Effective freezing methods for adult and pre-pubertal testicular tissue are available [ 108 ].
Qiuwan et al. [ 109 ] provided important evidence that human amniotic epithelial cell (hAEC) transplantation could effectively improve ovarian function by inhibiting cell apoptosis and reducing inflammation in injured ovarian tissue of mice, and it could be a promising strategy for the management of premature ovarian failure or insufficiency in female cancer survivors.
For now, reaching successful infertility treatments in humans appears to be only a matter of time, but there are several challenges to overcome. First, the process needs to have high efficiency; second, the chances of forming tumours instead of eggs or sperm must be maximally reduced. The last barrier is how to mature human sperm and eggs in the lab without transplanting them to in vivo conditions, which could cause either a tumour risk or an invasive procedure.
Therapy for incurable neurodegenerative diseases
Thanks to stem cell therapy, it is possible not only to delay the progression of incurable neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease (AD), and Huntington disease, but also, most importantly, to remove the source of the problem. In neuroscience, the discovery of neural stem cells (NSCs) has nullified the previous idea that adult CNS were not capable of neurogenesis [ 110 , 111 ]. Neural stem cells are capable of improving cognitive function in preclinical rodent models of AD [ 112 , 113 , 114 ]. Awe et al. [ 115 ] clinically derived relevant human iPSCs from skin punch biopsies to develop a neural stem cell-based approach for treating AD. Neuronal degeneration in Parkinson’s disease (PD) is focal, and dopaminergic neurons can be efficiently generated from hESCs. PD is an ideal disease for iPSC-based cell therapy [ 116 ]. However, this therapy is still in an experimental phase ( https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4539501 /). Brain tissue from aborted foetuses was used on patients with Parkinson’s disease [ 117 ]. Although the results were not uniform, they showed that therapies with pure stem cells are an important and achievable therapy.
Stem cell use in dentistry
Teeth represent a very challenging material for regenerative medicine. They are difficult to recreate because of their function in aspects such as articulation, mastication, or aesthetics due to their complicated structure. Currently, there is a chance for stem cells to become more widely used than synthetic materials. Teeth have a large advantage of being the most natural and non-invasive source of stem cells.
For now, without the use of stem cells, the most common periodontological treatments are either growth factors, grafts, or surgery. For example, there are stem cells in periodontal ligament [ 118 , 119 ], which are capable of differentiating into osteoblasts or cementoblasts, and their functions were also assessed in neural cells [ 120 ]. Tissue engineering is a successful method for treating periodontal diseases. Stem cells of the root apical areas are able to recreate periodontal ligament. One of the possible methods of tissue engineering in periodontology is gene therapy performed using adenoviruses-containing growth factors [ 121 ].
As a result of animal studies, dentin regeneration is an effective process that results in the formation of dentin bridges [ 122 ].
Enamel is more difficult to regenerate than dentin. After the differentiation of ameloblastoma cells into the enamel, the former is destroyed, and reparation is impossible. Medical studies have succeeded in differentiating bone marrow stem cells into ameloblastoma [ 123 ].
Healthy dental tissue has a high amount of regular stem cells, although this number is reduced when tissue is either traumatized or inflamed [ 124 ]. There are several dental stem cell groups that can be isolated (Fig. 7 ).
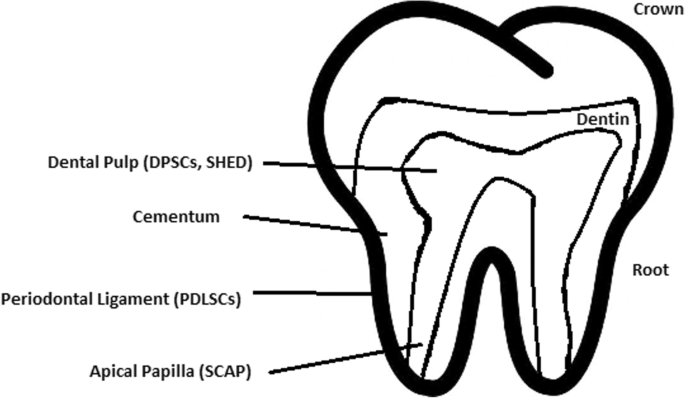
Localization of stem cells in dental tissues. Dental pulp stem cells (DPSCs) and human deciduous teeth stem cells (SHED) are located in the dental pulp. Periodontal ligaments stem cells are located in the periodontal ligament. Apical papilla consists of stem cells from the apical papilla (SCAP)
Dental pulp stem cell (DPSC)
These were the first dental stem cells isolated from the human dental pulp, which were [ 125 ] located inside dental pulp (Table 2 ). They have osteogenic and chondrogenic potential. Mesenchymal stem cells (MSCs) of the dental pulp, when isolated, appear highly clonogenic; they can be isolated from adult tissue (e.g. bone marrow, adipose tissue) and foetal (e.g. umbilical cord) [ 126 ] tissue, and they are able to differentiate densely [ 127 ]. MSCs differentiate into odontoblast-like cells and osteoblasts to form dentin and bone. Their best source locations are the third molars [ 125 ]. DPSCs are the most useful dental source of tissue engineering due to their easy surgical accessibility, cryopreservation possibility, increased production of dentin tissues compared to non-dental stem cells, and their anti-inflammatory abilities. These cells have the potential to be a source for maxillofacial and orthopaedic reconstructions or reconstructions even beyond the oral cavity. DPSCs are able to generate all structures of the developed tooth [ 128 ]. In particular, beneficial results in the use of DPSCs may be achieved when combined with other new therapies, such as periodontal tissue photobiomodulation (laser stimulation), which is an efficient technique in the stimulation of proliferation and differentiation into distinct cell types [ 129 ]. DPSCs can be induced to form neural cells to help treat neurological deficits.
Stem cells of human exfoliated deciduous teeth (SHED) have a faster rate of proliferation than DPSCs and differentiate into an even greater number of cells, e.g. other mesenchymal and non-mesenchymal stem cell derivatives, such as neural cells [ 130 ]. These cells possess one major disadvantage: they form a non-complete dentin/pulp-like complex in vivo. SHED do not undergo the same ethical concerns as embryonic stem cells. Both DPSCs and SHED are able to form bone-like tissues in vivo [ 131 ] and can be used for periodontal, dentin, or pulp regeneration. DPSCs and SHED can be used in treating, for example, neural deficits [ 132 ]. DPSCs alone were tested and successfully applied for alveolar bone and mandible reconstruction [ 133 ].
Periodontal ligament stem cells (PDLSCs)
These cells are used in periodontal ligament or cementum tissue regeneration. They can differentiate into mesenchymal cell lineages to produce collagen-forming cells, adipocytes, cementum tissue, Sharpey’s fibres, and osteoblast-like cells in vitro. PDLSCs exist both on the root and alveolar bone surfaces; however, on the latter, these cells have better differentiation abilities than on the former [ 134 ]. PDLSCs have become the first treatment for periodontal regeneration therapy because of their safety and efficiency [ 135 , 136 ].
Stem cells from apical papilla (SCAP)
These cells are mesenchymal structures located within immature roots. They are isolated from human immature permanent apical papilla. SCAP are the source of odontoblasts and cause apexogenesis. These stem cells can be induced in vitro to form odontoblast-like cells, neuron-like cells, or adipocytes. SCAP have a higher capacity of proliferation than DPSCs, which makes them a better choice for tissue regeneration [ 137 , 138 ].
Dental follicle stem cells (DFCs)
These cells are loose connective tissues surrounding the developing tooth germ. DFCs contain cells that can differentiate into cementoblasts, osteoblasts, and periodontal ligament cells [ 139 , 140 ]. Additionally, these cells proliferate after even more than 30 passages [ 141 ]. DFCs are most commonly extracted from the sac of a third molar. When DFCs are combined with a treated dentin matrix, they can form a root-like tissue with a pulp-dentin complex and eventually form tooth roots [ 141 ]. When DFC sheets are induced by Hertwig’s epithelial root sheath cells, they can produce periodontal tissue; thus, DFCs represent a very promising material for tooth regeneration [ 142 ].
Pulp regeneration in endodontics
Dental pulp stem cells can differentiate into odontoblasts. There are few methods that enable the regeneration of the pulp.
The first is an ex vivo method. Proper stem cells are grown on a scaffold before they are implanted into the root channel [ 143 ].
The second is an in vivo method. This method focuses on injecting stem cells into disinfected root channels after the opening of the in vivo apex. Additionally, the use of a scaffold is necessary to prevent the movement of cells towards other tissues. For now, only pulp-like structures have been created successfully.
Methods of placing stem cells into the root channel constitute are either soft scaffolding [ 144 ] or the application of stem cells in apexogenesis or apexification. Immature teeth are the best source [ 145 ]. Nerve and blood vessel network regeneration are extremely vital to keep pulp tissue healthy.
The potential of dental stem cells is mainly regarding the regeneration of damaged dentin and pulp or the repair of any perforations; in the future, it appears to be even possible to generate the whole tooth. Such an immense success would lead to the gradual replacement of implant treatments. Mandibulary and maxillary defects can be one of the most complicated dental problems for stem cells to address.
Acquiring non-dental tissue cells by dental stem cell differentiation
In 2013, it was reported that it is possible to grow teeth from stem cells obtained extra-orally, e.g. from urine [ 146 ]. Pluripotent stem cells derived from human urine were induced and generated tooth-like structures. The physical properties of the structures were similar to natural ones except for hardness [ 127 ]. Nonetheless, it appears to be a very promising technique because it is non-invasive and relatively low-cost, and somatic cells can be used instead of embryonic cells. More importantly, stem cells derived from urine did not form any tumours, and the use of autologous cells reduces the chances of rejection [ 147 ].
Use of graphene in stem cell therapy
Over recent years, graphene and its derivatives have been increasingly used as scaffold materials to mediate stem cell growth and differentiation [ 148 ]. Both graphene and graphene oxide (GO) represent high in-plane stiffness [ 149 ]. Because graphene has carbon and aromatic network, it works either covalently or non-covalently with biomolecules; in addition to its superior mechanical properties, graphene offers versatile chemistry. Graphene exhibits biocompatibility with cells and their proper adhesion. It also tested positively for enhancing the proliferation or differentiation of stem cells [ 148 ]. After positive experiments, graphene revealed great potential as a scaffold and guide for specific lineages of stem cell differentiation [ 150 ]. Graphene has been successfully used in the transplantation of hMSCs and their guided differentiation to specific cells. The acceleration skills of graphene differentiation and division were also investigated. It was discovered that graphene can serve as a platform with increased adhesion for both growth factors and differentiation chemicals. It was also discovered that π-π binding was responsible for increased adhesion and played a crucial role in inducing hMSC differentiation [ 150 ].
Therapeutic potential of extracellular vesicle-based therapies
Extracellular vesicles (EVs) can be released by virtually every cell of an organism, including stem cells [ 151 ], and are involved in intercellular communication through the delivery of their mRNAs, lipids, and proteins. As Oh et al. [ 152 ] prove, stem cells, together with their paracrine factors—exosomes—can become potential therapeutics in the treatment of, e.g. skin ageing. Exosomes are small membrane vesicles secreted by most cells (30–120 nm in diameter) [ 153 ]. When endosomes fuse with the plasma membrane, they become exosomes that have messenger RNAs (mRNAs) and microRNAs (miRNAs), some classes of non-coding RNAs (IncRNAs) and several proteins that originate from the host cell [ 154 ]. IncRNAs can bind to specific loci and create epigenetic regulators, which leads to the formation of epigenetic modifications in recipient cells. Because of this feature, exosomes are believed to be implicated in cell-to-cell communication and the progression of diseases such as cancer [ 155 ]. Recently, many studies have also shown the therapeutic use of exosomes derived from stem cells, e.g. skin damage and renal or lung injuries [ 156 ].
In skin ageing, the most important factor is exposure to UV light, called “photoageing” [ 157 ], which causes extrinsic skin damage, characterized by dryness, roughness, irregular pigmentation, lesions, and skin cancers. In intrinsic skin ageing, on the other hand, the loss of elasticity is a characteristic feature. The skin dermis consists of fibroblasts, which are responsible for the synthesis of crucial skin elements, such as procollagen or elastic fibres. These elements form either basic framework extracellular matrix constituents of the skin dermis or play a major role in tissue elasticity. Fibroblast efficiency and abundance decrease with ageing [ 158 ]. Stem cells can promote the proliferation of dermal fibroblasts by secreting cytokines such as platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), and basic fibroblast growth factor. Huh et al. [ 159 ] mentioned that a medium of human amniotic fluid-derived stem cells (hAFSC) positively affected skin regeneration after longwave UV-induced (UVA, 315–400 nm) photoageing by increasing the proliferation and migration of dermal fibroblasts. It was discovered that, in addition to the induction of fibroblast physiology, hAFSC transplantation also improved diseases in cases of renal pathology, various cancers, or stroke [ 160 , 161 ].
Oh [ 162 ] also presented another option for the treatment of skin wounds, either caused by physical damage or due to diabetic ulcers. Induced pluripotent stem cell-conditioned medium (iPSC-CM) without any animal-derived components induced dermal fibroblast proliferation and migration.
Natural cutaneous wound healing is divided into three steps: haemostasis/inflammation, proliferation, and remodelling. During the crucial step of proliferation, fibroblasts migrate and increase in number, indicating that it is a critical step in skin repair, and factors such as iPSC-CM that impact it can improve the whole cutaneous wound healing process. Paracrine actions performed by iPSCs are also important for this therapeutic effect [ 163 ]. These actions result in the secretion of cytokines such as TGF-β, interleukin (IL)-6, IL-8, monocyte chemotactic protein-1 (MCP-1), vascular endothelial growth factor (VEGF), platelet-derived growth factor-AA (PDGF-AA), and basic fibroblast growth factor (bFGF). Bae et al. [ 164 ] mentioned that TGF-β induced the migration of keratinocytes. It was also demonstrated that iPSC factors can enhance skin wound healing in vivo and in vitro when Zhou et al. [ 165 ] enhanced wound healing, even after carbon dioxide laser resurfacing in an in vivo study.
Peng et al. [ 166 ] investigated the effects of EVs derived from hESCs on in vitro cultured retinal glial, progenitor Müller cells, which are known to differentiate into retinal neurons. EVs appear heterogeneous in size and can be internalized by cultured Müller cells, and their proteins are involved in the induction and maintenance of stem cell pluripotency. These stem cell-derived vesicles were responsible for the neuronal trans-differentiation of cultured Müller cells exposed to them. However, the research article points out that the procedure was accomplished only on in vitro acquired retina.
Challenges concerning stem cell therapy
Although stem cells appear to be an ideal solution for medicine, there are still many obstacles that need to be overcome in the future. One of the first problems is ethical concern.
The most common pluripotent stem cells are ESCs. Therapies concerning their use at the beginning were, and still are, the source of ethical conflicts. The reason behind it started when, in 1998, scientists discovered the possibility of removing ESCs from human embryos. Stem cell therapy appeared to be very effective in treating many, even previously incurable, diseases. The problem was that when scientists isolated ESCs in the lab, the embryo, which had potential for becoming a human, was destroyed (Fig. 8 ). Because of this, scientists, seeing a large potential in this treatment method, focused their efforts on making it possible to isolate stem cells without endangering their source—the embryo.
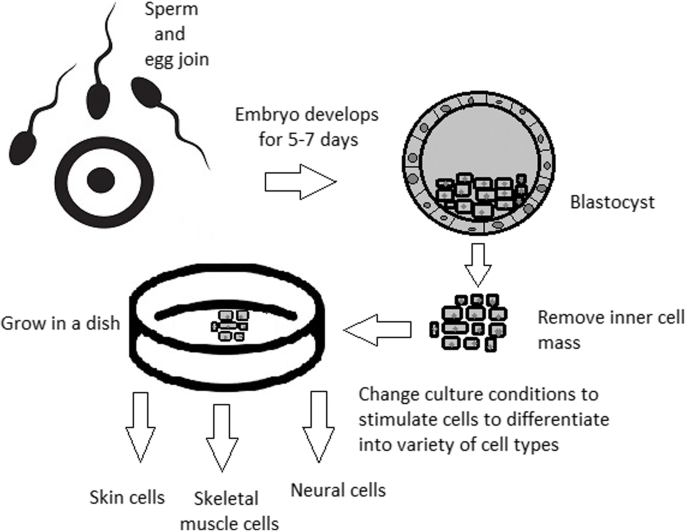
Use of inner cell mass pluripotent stem cells and their stimulation to differentiate into desired cell types
For now, while hESCs still remain an ethically debatable source of cells, they are potentially powerful tools to be used for therapeutic applications of tissue regeneration. Because of the complexity of stem cell control systems, there is still much to be learned through observations in vitro. For stem cells to become a popular and widely accessible procedure, tumour risk must be assessed. The second problem is to achieve successful immunological tolerance between stem cells and the patient’s body. For now, one of the best ideas is to use the patient’s own cells and devolve them into their pluripotent stage of development.
New cells need to have the ability to fully replace lost or malfunctioning natural cells. Additionally, there is a concern about the possibility of obtaining stem cells without the risk of morbidity or pain for either the patient or the donor. Uncontrolled proliferation and differentiation of cells after implementation must also be assessed before its use in a wide variety of regenerative procedures on living patients [ 167 ].
One of the arguments that limit the use of iPSCs is their infamous role in tumourigenicity. There is a risk that the expression of oncogenes may increase when cells are being reprogrammed. In 2008, a technique was discovered that allowed scientists to remove oncogenes after a cell achieved pluripotency, although it is not efficient yet and takes a longer amount of time. The process of reprogramming may be enhanced by deletion of the tumour suppressor gene p53, but this gene also acts as a key regulator of cancer, which makes it impossible to remove in order to avoid more mutations in the reprogrammed cell. The low efficiency of the process is another problem, which is progressively becoming reduced with each year. At first, the rate of somatic cell reprogramming in Yamanaka’s study was up to 0.1%. The use of transcription factors creates a risk of genomic insertion and further mutation of the target cell genome. For now, the only ethically acceptable operation is an injection of hESCs into mouse embryos in the case of pluripotency evaluation [ 168 ].
Stem cell obstacles in the future
Pioneering scientific and medical advances always have to be carefully policed in order to make sure they are both ethical and safe. Because stem cell therapy already has a large impact on many aspects of life, it should not be treated differently.
Currently, there are several challenges concerning stem cells. First, the most important one is about fully understanding the mechanism by which stem cells function first in animal models. This step cannot be avoided. For the widespread, global acceptance of the procedure, fear of the unknown is the greatest challenge to overcome.
The efficiency of stem cell-directed differentiation must be improved to make stem cells more reliable and trustworthy for a regular patient. The scale of the procedure is another challenge. Future stem cell therapies may be a significant obstacle. Transplanting new, fully functional organs made by stem cell therapy would require the creation of millions of working and biologically accurate cooperating cells. Bringing such complicated procedures into general, widespread regenerative medicine will require interdisciplinary and international collaboration.
The identification and proper isolation of stem cells from a patient’s tissues is another challenge. Immunological rejection is a major barrier to successful stem cell transplantation. With certain types of stem cells and procedures, the immune system may recognize transplanted cells as foreign bodies, triggering an immune reaction resulting in transplant or cell rejection.
One of the ideas that can make stem cells a “failsafe” is about implementing a self-destruct option if they become dangerous. Further development and versatility of stem cells may cause reduction of treatment costs for people suffering from currently incurable diseases. When facing certain organ failure, instead of undergoing extraordinarily expensive drug treatment, the patient would be able to utilize stem cell therapy. The effect of a successful operation would be immediate, and the patient would avoid chronic pharmacological treatment and its inevitable side effects.
Although these challenges facing stem cell science can be overwhelming, the field is making great advances each day. Stem cell therapy is already available for treating several diseases and conditions. Their impact on future medicine appears to be significant.
After several decades of experiments, stem cell therapy is becoming a magnificent game changer for medicine. With each experiment, the capabilities of stem cells are growing, although there are still many obstacles to overcome. Regardless, the influence of stem cells in regenerative medicine and transplantology is immense. Currently, untreatable neurodegenerative diseases have the possibility of becoming treatable with stem cell therapy. Induced pluripotency enables the use of a patient’s own cells. Tissue banks are becoming increasingly popular, as they gather cells that are the source of regenerative medicine in a struggle against present and future diseases. With stem cell therapy and all its regenerative benefits, we are better able to prolong human life than at any time in history.
Abbreviations
Basic fibroblast growth factor
Bone morphogenic proteins
Dental follicle stem cells
Dental pulp stem cells
Embryonic bodies
Embryonic stem cells
Fibroblast growth factors
Good Manufacturing Practice
Graphene oxide
Human amniotic fluid-derived stem cells
Human embryonic stem cells
Human foreskin fibroblasts
Inner cell mass
Non-coding RNA
Induced pluripotent stem cells
In vitro fertilization
Knockout serum replacement
Leukaemia inhibitory factor
Monocyte chemotactic protein-1
Fibroblasts
Messenger RNA
Mesenchymal stem cells of dental pulp
Myogenic differentiation
Osteoarthritis
Octamer-binding transcription factor 3 and 4
Platelet-derived growth factor
Platelet-derived growth factor-AA
Periodontal ligament stem cells
Rho-associated protein kinase
Stem cells from apical papilla
Stem cells of human exfoliated deciduous teeth
Synthetic Serum Substitute
Trophectoderm
Vascular endothelial growth factor
Transforming growth factors
Sukoyan MA, Vatolin SY, et al. Embryonic stem cells derived from morulae, inner cell mass, and blastocysts of mink: comparisons of their pluripotencies. Embryo Dev. 1993;36(2):148–58
Larijani B, Esfahani EN, Amini P, Nikbin B, Alimoghaddam K, Amiri S, Malekzadeh R, Yazdi NM, Ghodsi M, Dowlati Y, Sahraian MA, Ghavamzadeh A. Stem cell therapy in treatment of different diseases. Acta Medica Iranica. 2012:79–96 https://www.ncbi.nlm.nih.gov/pubmed/22359076 .
Sullivan S, Stacey GN, Akazawa C, et al. Quality guidelines for clinical-grade human induced pluripotent stem cell lines. Regenerative Med. 2018; https://doi.org/10.2217/rme-2018-0095 .
Amps K, Andrews PW, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat. Biotechnol. 2011; 29 (12):1121–44.
Google Scholar
Amit M, Itskovitz-Eldor J. Atlas of human pluripotent stem cells: derivation and culturing. New York: Humana Press; 2012.
Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–46.
CAS PubMed Google Scholar
Kang MI. Transitional CpG methylation between promoters and retroelements of tissue-specific genes during human mesenchymal cell differentiation. J. Cell Biochem. 2007;102:224–39.
Vaes B, Craeye D, Pinxteren J. Quality control during manufacture of a stem cell therapeutic. BioProcess Int. 2012;10:50–5.
Bloushtain-Qimron N. Epigenetic patterns of embryonic and adult stem cells. Cell Cycle. 2009;8:809–17.
Brindley DA. Peak serum: implications of serum supply for cell therapy manufacturing. Regenerative Medicine. 2012;7:809–17.
Solter D, Knowles BB. Immunosurgery of mouse blastocyst. Proc Natl Acad Sci U S A. 1975;72:5099–102.
CAS PubMed PubMed Central Google Scholar
Hoepfl G, Gassmann M, Desbaillets I. Differentiating embryonic stem cells into embryoid bodies. Methods Mole Biol. 2004;254:79–98 https://doi.org/10.1385/1-59259-741-6:079 .
Lim WF, Inoue-Yokoo T, Tan KS, Lai MI, Sugiyama D. Hematopoietic cell differentiation from embryonic and induced pluripotent stem cells. Stem Cell Res Ther. 2013;4(3):71. https://doi.org/10.1186/scrt222 .
Article CAS PubMed PubMed Central Google Scholar
Mohr JC, de Pablo JJ, Palecek SP. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27(36):6032–42. https://doi.org/10.1016/j.biomaterials.2006.07.012 .
Article CAS PubMed Google Scholar
Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of the visceral yolk sac, blood islands, and myocardium. J Embryol Exp Morphol. 1985;87:27–45.
Kurosawa HY. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103:389–98.
Heins N, Englund MC, Sjoblom C, Dahl U, Tonning A, Bergh C, Lindahl A, Hanson C, Semb H. Derivation, characterization, and differentiation of human embryonic stem cells. Stem Cells. 2004;22:367–76.
Rosowski KA, Mertz AF, Norcross S, Dufresne ER, Horsley V. Edges of human embryonic stem cell colonies display distinct mechanical properties and differentiation potential. Sci Rep. 2015;5:Article number:14218.
PubMed Google Scholar
Chung Y, Klimanskaya I, Becker S, Li T, Maserati M, Lu SJ, Zdravkovic T, Ilic D, Genbacev O, Fisher S, Krtolica A, Lanza R. Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell. 2008;2:113–7.
Zhang X, Stojkovic P, Przyborski S, Cooke M, Armstrong L, Lako M, Stojkovic M. Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells. 2006;24:2669–76.
Beers J, Gulbranson DR, George N, Siniscalchi LI, Jones J, Thomson JA, Chen G. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat Protoc. 2012;7:2029–40.
Ellerström C, Hyllner J, Strehl R. single cell enzymatic dissociation of human embryonic stem cells: a straightforward, robust, and standardized culture method. In: Turksen K, editor. Human embryonic stem cell protocols. Methods in molecular biology: Humana Press; 2009. p. 584.
Heng BC, Liu H, Ge Z, Cao T. Mechanical dissociation of human embryonic stem cell colonies by manual scraping after collagenase treatment is much more detrimental to cellular viability than is trypsinization with gentle pipetting. Biotechnol Appl Biochem. 2010;47(1):33–7.
Ellerstrom C, Strehl R, Noaksson K, Hyllner J, Semb H. Facilitated expansion of human embryonic stem cells by single-cell enzymatic dissociation. Stem Cells. 2007;25:1690–6.
Brimble SN, Zeng X, Weiler DA, Luo Y, Liu Y, Lyons IG, Freed WJ, Robins AJ, Rao MS, Schulz TC. Karyotypic stability, genotyping, differentiation, feeder-free maintenance, and gene expression sampling in three human embryonic stem cell lines deri. Stem Cells Dev. 2004;13:585–97.
Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–6.
Nie Y, Walsh P, Clarke DL, Rowley JA, Fellner T. Scalable passaging of adherent human pluripotent stem cells. 2014. https://doi.org/10.1371/journal.pone.0088012 .
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7.
Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cellsexpress an immunogenic nonhuman sialic acid. Nat. Med. 2005;11:228–32.
Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336(6200):688–90. https://doi.org/10.1038/336688a0 .
Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnol. 2001;19:971–4. https://doi.org/10.1038/nbt1001-971 .
Article CAS Google Scholar
Weathersbee PS, Pool TB, Ord T. Synthetic serum substitute (SSS): a globulin-enriched protein supplement for human embryo culture. J. Assist Reprod Genet. 1995;12:354–60.
Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods. 2011;8:424–9.
Sommer CA, Mostoslavsky G. Experimental approaches for the generation of induced pluripotent stem cells. Stem Cell Res Ther. 2010;1:26.
PubMed PubMed Central Google Scholar
Takahashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013;140(12):2457–61 https://doi.org/10.1242/dev.092551 .
Shi D, Lu F, Wei Y, et al. Buffalos ( Bubalus bubalis ) cloned by nuclear transfer of somatic cells. Biol. Reprod. 2007;77:285–91. https://doi.org/10.1095/biolreprod.107.060210 .
Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. Development. 1962;10:622–40 http://dev.biologists.org/content/10/4/622 .
CAS Google Scholar
Kain K. The birth of cloning: an interview with John Gurdon. Dis Model Mech. 2009;2(1–2):9–10. https://doi.org/10.1242/dmm.002014 .
Article PubMed Central Google Scholar
Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;24(51(6)):987–1000.
Quinlan AR, Boland MJ, Leibowitz ML, et al. Genome sequencing of mouse induced pluripotent stem cells reveals retroelement stability and infrequent DNA rearrangement during reprogramming. Cell Stem Cell. 2011;9(4):366–73.
Maherali N, Sridharan R, Xie W, Utika LJ, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70.
Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, Qi Z, Downey SL, Manos PD, Rossi DJ, Yu J, Hebrok M, Hochedlinger K, Costello JF, Song JS, Ramalho-Santos M. Incomplete DNA methylation underlines a transcriptional memory of somatic cells in human IPS cells. Nat Cell Biol. 2011;13:541–9.
Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32 https://doi.org/10.1038/nature07314 .
Hilfiker A, Kasper C, Hass R, Haverich A. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbecks Arch Surg. 2011;396:489–97.
Zhang Wendy, Y., de Almeida Patricia, E., and Wu Joseph, C. Teratoma formation: a tool for monitoring pluripotency in stem cell research. StemBook, ed. The Stem Cell Research Community . June 12, 2012. https://doi.org/10.3824/stembook.1.53.1 .
Narsinh KH, Sun N, Sanchez-Freire V, et al. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J Clin Invest. 2011;121(3):1217–21.
Gertow K, Przyborski S, Loring JF, Auerbach JM, Epifano O, Otonkoski T, Damjanov I, AhrlundRichter L. Isolation of human embryonic stem cell-derived teratomas for the assessment of pluripotency. Curr Protoc Stem Cell Biol . 2007, Chapter 1, Unit 1B 4. 3: 1B.4.1-1B.4.29.
Cooke MJ, Stojkovic M, Przyborski SA. Growth of teratomas derived from human pluripotent stem cells is influenced by the graft site. Stem Cells Dev. 2006;15(2):254–9.
Przyborski SA. Differentiation of human embryonic stem cells after transplantation in immune-deficient mice. Stem Cells. 2005;23:1242–50.
Tannenbaum SE, Turetsky TT, Singer O, Aizenman E, Kirshberg S, Ilouz N, Gil Y, Berman-Zaken Y, Perlman TS, Geva N, Levy O, Arbell D, Simon A, Ben-Meir A, Shufaro Y, Laufer N, Reubinoff BE. Derivation of xeno-free and GMP-grade human embryonic stem cells- platforms for future clinical applications. PLoS One. 2012;7:e35325.
Cohen DE, Melton D. Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet. 2011;12:243–52.
Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev. 2007;60(2):199–214. https://doi.org/10.1016/j.addr.2007.08.036 .
Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–29.
Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–806.
Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–714.
Efthymiou AG, Chen G, Rao M, Chen G, Boehm M. Self-renewal and cell lineage differentiation strategies in human embryonic stem cells and induced pluripotent stem cells. Expert Opin Biol Ther. 2014;14:1333–44.
Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDRþembryonic-stem-cell-derived population. Nature. 2008;453:524–8.
Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D’amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52. https://doi.org/10.1038/nbt1393 .
Vallier L, Reynolds D, Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–21.
Burridge PW, Zambidis ET. Highly efficient directed differentiation of human induced pluripotent stem cells into cardiomyocytes. Methods Mol Biol. 2013;997:149–61.
Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, Meng S, Chen Y, Zhou R, Song X, Guo Y, Ding M, Deng H. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–39.
Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH. Directing human embryonic stem cell differentiation towards a renal lineage generates a selforganizing kidney. Nat Cell Biol. 2014;16:118–26.
Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, VunjakNovakovic G, Bhattacharya J, Snoeck HW. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol. 2014;32:84–91.
Kadzik RS, Morrisey EE. Directing lung endoderm differentiation in pluripotent stem cells. Cell Stem Cell. 2012;10:355–61.
Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–97.
Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–9.
Oldershaw RA, Baxter MA, Lowe ET, Bates N, Grady LM, Soncin F, Brison DR, Hardingham TE, Kimber SJ. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 2010;28:1187–94.
Jun Cai, Ann DeLaForest, Joseph Fisher, Amanda Urick, Thomas Wagner, Kirk Twaroski, Max Cayo, Masato Nagaoka, Stephen A Duncan. Protocol for directed differentiation of human pluripotent stem cells toward a hepatocyte fate. 2012. DOI: https://doi.org/10.3824/stembook.1.52.1 .
Frank-Kamenetsky M, Zhang XM, Bottega S, Guicherit O, Wichterle H, Dudek H, Bumcrot D, Wang FY, Jones S, Shulok J, Rubin LL, Porter JA. Small-molecule modulators of hedgehog signaling: identification and characterization of smoothened agonists and antagonists. J Biol. 2002;1:10.
Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S. Mechanosensitive hair celllike cells from embryonic and induced pluripotent stem cells. Cell. 2010;141:704–16.
Osakada F, Jin ZB, Hirami Y, Ikeda H, Danjyo T, Watanabe K, Sasai Y, Takahashi M. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci. 2009;122:3169–79.
Kshitiz PJ, Kim P, Helen W, Engler AJ, Levchenko A, Kim DH. Control of stem cell fate and function by engineering physical microenvironments. Intergr Biol (Camb). 2012;4:1008–18.
Amps K, Andrews PW, Anyfantis G, Armstrong L, Avery S, Baharvand H, Baker J, Baker D, Munoz MB, Beil S, Benvenisty N, Ben-Yosef D, Biancotti JC, Bosman A, Brena RM, Brison D, Caisander G, Camarasa MV, Chen J, ChiaoE CYM, Choo AB, Collins D, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol. 2011;29:1132–44.
Nukaya D, Minami K, Hoshikawa R, Yokoi N, Seino S. Preferential gene expression and epigenetic memory of induced pluripotent stem cells derived from mouse pancreas. Genes Cells. 2015;20:367–81.
Murdoch A, Braude P, Courtney A, Brison D, Hunt C, Lawford-Davies J, Moore H, Stacey G, Sethe S, Procurement Working Group Of National Clinical H, E. S. C. F, National Clinical H, E. S. C. F. The procurement of cells for the derivation of human embryonic stem cell lines for therapeutic use: recommendations for good practice. Stem Cell Rev. 2012;8:91–9.
Hewitson H, Wood V, Kadeva N, Cornwell G, Codognotto S, Stephenson E, Ilic D. Generation of KCL035 research grade human embryonic stem cell line carrying a mutation in HBB gene. Stem Cell Res. 2016;16:210–2.
Daley GQ, Hyun I, Apperley JF, Barker RA, Benvenisty N, Bredenoord AL, Breuer CK, Caulfield T, Cedars MI, Frey-Vasconcells J, Heslop HE, Jin Y, Lee RT, Mccabe C, Munsie M, Murry CE, Piantadosi S, Rao M, Rooke HM, Sipp D, Studer L, Sugarman J, et al. Setting global standards for stem cell research and clinical translation: the 2016 ISSCR guidelines. Stem Cell Rep. 2016;6:787–97.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. https://doi.org/10.1016/j.cell.2006.07.024 .
Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–40.
Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Guillemain R, SuberbielleBoissel C, Tartour E, Desnos M, Larghero J. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J. 2015;36:2011–7.
Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–16.
Ilic D, Ogilvie C. Concise review: human embryonic stem cells-what have we done? What are we doing? Where are we going? Stem Cells. 2017;35:17–25.
Rocha V, et al. Clinical use of umbilical cord blood hematopoietic stem cells. Biol Blood Marrow Transplant. 2006;12(1):34–4.
Longo UG, Ronga M, Maffulli N. Sports Med Arthrosc 17:112–126. Achilles tendinopathy. Sports Med Arthrosc. 2009;17:112–26.
Tempfer H, Lehner C, Grütz M, Gehwolf R, Traweger A. Biological augmentation for tendon repair: lessons to be learned from development, disease, and tendon stem cell research. In: Gimble J, Marolt D, Oreffo R, Redl H, Wolbank S, editors. Cell engineering and regeneration. Reference Series in Biomedical Engineering. Cham: Springer; 2017.
Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34.
Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14:177–82.
Li R, Lin Q-X, Liang X-Z, Liu G-B, et al. Stem cell therapy for treating osteonecrosis of the femoral head: from clinical applications to related basic research. Stem Cell Res Therapy. 2018;9:291 https://doi.org/10.1186/s13287-018-1018-7 .
Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled study. Bone. 2011;49(5):1005–9.
Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50(1):325–30.
Sen RK, Tripathy SK, Aggarwal S, Marwaha N, Sharma RR, Khandelwal N. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: a randomized control study. J Arthroplast. 2012;27(5):679–86.
Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Aït-Hamou N, Leschik J, Pellestor F, Ramirez JM, De Vos J, Lehmann S, Lemaitre JM. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 2011;25:2248–53.
Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–8.
Petkovich DA, Podolskiy DI, Lobanov AV, Lee SG, Miller RA, Gladyshev VN. Using DNA methylation profiling to evaluate biological age and longevity interventions. Cell Metab. 2017;25:954–60 https://doi.org/10.1016/j.cmet.2017.03.016 .
Gerontology, Rejuvenation by cell reprogramming: a new horizon in. Rodolfo G. Goya, Marianne Lehmann, Priscila Chiavellini, Martina Canatelli-Mallat, Claudia B. Hereñú and Oscar A. Brown. Stem Cell Res Therapy . 2018, 9:349. https://doi.org/10.1186/s13287-018-1075-y .
Ocampo A, Reddy P, Martinez-Redondo P, Platero-Luengo A, Hatanaka F, Hishida T, Li M, Lam D, Kurita M, Beyret E, Araoka T, Vazquez-Ferrer E, Donoso D, Roman JLXJ, Rodriguez-Esteban C, Nuñez G, Nuñez Delicado E, Campistol JM, Guillen I, Guillen P, Izpisua. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell. 2016;167:1719–33.
de Lázaro I, Cossu G, Kostarelos K. Transient transcription factor (OSKM) expression is key towards clinical translation of in vivo cell reprogramming. EMBO Mol Med. 2017;9:733–6.
Sun S, Li ZQ, Glencer P, Cai BC, Zhang XM, Yang J, Li XR. Bringing the age-related macular degeneration high-risk allele age-related maculopathy susceptibility 2 into focus with stem cell technology. Stem Cell Res Ther. 2017;8:135 https://doi.org/10.1186/s13287-017-0584-4 .
Liu J. Induced pluripotent stem cell-derived neural stem cells: new hope for stroke? Stem Cell Res Ther. 2013;4:115 https://doi.org/10.1186/scrt326 .
Shahjalal HM, Dayem AA, Lim KM, Jeon TI, Cho SG. Generation of pancreatic β cells for treatment of diabetes: advances and challenges. Stem Cell ResTher. 2018;9:355 https://doi.org/10.1186/s13287-018-1099-3 .
Kroon E, Martinson LA, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26;443–52.
Arora V, Pooja A, Munshi AK. Banking stem cells from human exfoliated deciduous teeth. J Clin Pediatr Dent. 2009;33(4):289–94.
Mao JJ. Stem cells and the future of dental care. New York State Dental J. 2008;74(2):21–4.
Reznick, Jay B. Continuing education: stem cells: emerging medical and dental therapies for the dental Professional. Dentaltown Magazine . 2008, pp. 42–53.
Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26(7):1787–95.
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith A. Dental pulp tissue engineering with stem cells from exfoliated. J Endod. 2008;34(8):962–9.
Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146(4):519–32. https://doi.org/10.1016/j.cell.2011.06.052 .
Sadri-Ardekani H, Atala A. Testicular tissue cryopreservation and spermatogonial stem cell transplantation to restore fertility: from bench to bedside. Stem Cell ResTher. 2014;5:68 https://doi.org/10.1186/scrt457 .
Zhang Q, Xu M, Yao X, Li T, Wang Q, Lai D. Human amniotic epithelial cells inhibit granulosa cell apoptosis induced by chemotherapy and restore the fertility. Stem Cell Res Ther. 2015;6:152 https://doi.org/10.1186/s13287-015-0148-4 .
Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19:672–82. https://doi.org/10.1038/cr.2009.56 .
Dantuma E, Merchant S, Sugaya K. Stem cells for the treatment of neurodegenerative diseases. Stem Cell ResTher. 2010;1:37 https://doi.org/10.1186/scrt37 .
Wang Q, Matsumoto Y, Shindo T, Miyake K, Shindo A, Kawanishi M, Kawai N, Tamiya T, Nagao S. Neural stem cells transplantation in cortex in a mouse model of Alzheimer’s disease. J Med Invest. 2006;53:61–9. https://doi.org/10.2152/jmi.53.61 .
Article PubMed Google Scholar
Moghadam FH, Alaie H, Karbalaie K, Tanhaei S, Nasr Esfahani MH, Baharvand H. Transplantation of primed or unprimed mouse embryonic stem cell-derived neural precursor cells improves cognitive function in Alzheimerian rats. Differentiation. 2009;78:59–68. https://doi.org/10.1016/j.diff.2009.06.005 .
Byrne JA. Developing neural stem cell-based treatments for neurodegenerative diseases. Stem Cell ResTher. 2014;5:72. https://doi.org/10.1186/scrt461 .
Awe JP, Lee PC, Ramathal C, Vega-Crespo A, Durruthy-Durruthy J, Cooper A, Karumbayaram S, Lowry WE, Clark AT, Zack JA, Sebastiano V, Kohn DB, Pyle AD, Martin MG, Lipshutz GS, Phelps PE, Pera RA, Byrne JA. Generation and characterization of transgene-free human induced pluripotent stem cells and conversion to putative clinical-grade status. Stem Cell Res Ther. 2013;4:87. https://doi.org/10.1186/scrt246 .
Peng J, Zeng X. The role of induced pluripotent stem cells in regenerative medicine: neurodegenerative diseases. Stem Cell ResTher. 2011;2:32. https://doi.org/10.1186/scrt73 .
Wright BL, Barker RA. Established and emerging therapies for Huntington’s disease. 2007;7(6):579–87 https://www.ncbi.nlm.nih.gov/pubmed/17896994/579-87 .
Lin NH, Gronthos S, Bartold PM. Stem cells and periodontal regeneration. Aust Dent J. 2008;53:108–21.
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55.
Ramseier CA, Abramson ZR, Jin Q, Giannobile WV. Gene therapeutics for periodontal regenerative medicine. Dent Clin North Am. 2006;50:245–63.
Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. OrthodCraniofac Res. 2005;8:191–9.
Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein. J Dent Res. 2004;83:590–5.
Hu B, Unda F, Bopp-Kuchler S, Jimenez L, Wang XJ, Haikel Y, et al. Bone marrow cells can give rise to ameloblast-like cells. J Dent Res. 2006;85:416–21.
Liu Y, Liu W, Hu C, Xue Z, Wang G, Ding B, Luo H, Tang L, Kong X, Chen X, Liu N, Ding Y, Jin Y. MiR-17 modulates osteogenic differentiation through a coherent feed-forward loop in mesenchymal stem cells isolated from periodontal ligaments of patients with periodontitis. Stem Cells. 2011;29(11):1804–16. https://doi.org/10.1002/stem.728 .
Raspini G, Wolff J, Helminen M, Raspini G, Raspini M, Sándor GK. Dental stem cells harvested from third molars combined with bioactive glass can induce signs of bone formation in vitro. J Oral Maxillofac Res. 2018;9(1):e2. Published 2018 Mar 31. https://doi.org/10.5037/jomr.2018.9102 .
Christodoulou I, Goulielmaki M, Devetzi M, Panagiotidis M, Koliakos G, Zoumpourlis V. Mesenchymal stem cells in preclinical cancer cytotherapy: a systematic review. Stem Cell Res Ther. 2018;9(1;336). https://doi.org/10.1186/s13287-018-1078-8 .
Bansal R, Jain A. Current overview on dental stem cells applications in regenerative dentistry. J Nat Sci Biol Med. 2015;6(1):29–34. https://doi.org/10.4103/0976-9668.149074 .
Article PubMed PubMed Central Google Scholar
Edgar Ledesma-Martínez, Víctor Manuel Mendoza-Núñez, Edelmiro Santiago-Osorio. Mesenchymal stem cells derived from dental pulp: a review. Stem Cells Int . 2016, 4,709,572, p. doi: https://doi.org/10.1155/2016/4709572 ].
Grzech-Leśniak K. Making use of lasers in periodontal treatment: a new gold standard? Photomed Laser Surg. 2017;35(10):513–4.
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–12. https://doi.org/10.1073/pnas.0937635100 .
Yasui T, Mabuchi Y, Toriumi H, Ebine T, Niibe K, Houlihan DD, Morikawa S, Onizawa K, Kawana H, Akazawa C, Suzuki N, Nakagawa T, Okano H, Matsuzaki Y. Purified human dental pulp stem cells promote osteogenic regeneration. J Dent Res. 2016;95(2):206–14. https://doi.org/10.1177/0022034515610748 .
Yamamoto A, Sakai K, Matsubara K, Kano F, Ueda M. Multifaceted neuro-regenerative activities of human dental pulp stem cells for functional recovery after spinal cord injury. Neurosci Res. 2014;78:16–20. https://doi.org/10.1016/j.neures.2013.10.010 .
d’Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, Desiderio V, Laino G, Papaccio G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;12, PMID: 19908196:75–83.
Wang L, Shen H, Zheng W, Tang L, Yang Z, Gao Y, Yang Q, Wang C, Duan Y, Jin Y. Characterization of stem cells from alveolar periodontal ligament. Tissue Eng. Part A. 2011;17(7–8):1015–26. https://doi.org/10.1089/ten.tea.2010.0140 .
Iwata T, Yamato M, Zhang Z, Mukobata S, Washio K, Ando T, Feijen J, Okano T, Ishikawa I. Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J Clin Periodontol. 2010;37(12):1088–99. https://doi.org/10.1111/j.1600-051X.2010.01597.x .
Chen F-M, Gao L-N, Tian B-M, Zhang X-Y, Zhang Y-J, Dong G-Y, Lu H, et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7:33. https://doi.org/10.1186/s13287-016-0288-1 .
Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, Geurtsen W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol. 2011;56(7):709–21. https://doi.org/10.1016/j.archoralbio.2010.12.008 .
Han C, Yang Z, Zhou W, Jin F, Song Y, Wang Y, Huo N, Chen L, Qian H, Hou R, Duan Y, Jin Y. Periapical follicle stem cell: a promising candidate for cementum/periodontal ligament regeneration and bio-root engineering. Stem Cells Dev. 2010;19(9):1405–15. https://doi.org/10.1089/scd.2009.0277 .
Luan X, Ito Y, Dangaria S, Diekwisch TG. Dental follicle progenitor cell heterogeneity in the developing mouse periodontium. Stem Cells Dev. 2006;15(4):595–608. https://doi.org/10.1089/scd.2006.15.595 .
Handa K, Saito M, Tsunoda A, Yamauchi M, Hattori S, Sato S, Toyoda M, Teranaka T, Narayanan AS. Progenitor cells from dental follicle are able to form cementum matrix in vivo. Connect Tissue Res. 2002;43(2–3):406–8 PMID: 12489190.
Guo W, Chen L, Gong K, Ding B, Duan Y, Jin Y. Heterogeneous dental follicle cells and the regeneration of complex periodontal tissues. Tissue Engineering. Part A. 2012;18(5–6):459–70 https://doi.org/10.1089/ten.tea.2011.0261 .
Bai, Yudi et al. Cementum- and periodontal ligament-like tissue formation by dental follicle cell sheets co-cultured with Hertwig’s epithelial root sheath cells. Bone. 2011, 48, Issue 6, pp. 1417–1426, https://doi.org/10.1016/j.bone.2011.02.016 .
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. 2008, 34, pp. 962–969.
Dobie K, Smith G, Sloan AJ, Smith AJ. Effects of alginate, hydrogels and TGF-beta 1 on human dental pulp repair in vitro. Connect Tissue Res 2. 2002;43:387–90.
Friedlander LT, Cullinan MP, Love RM. Dental stem cells and their potential role in apexogenesis and apexification. Int Endod J. 2009;42:955–62.
Cai J, Zhang Y, Liu P, Chen S, Wu X, Sun Y, Li A, Huang K, Luo R, Wang L, Liu Y, Zhou T, Wei S, Pan G, Pei D, Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regen (Lond). July 30, 2013, 2(1), pp. 6, doi: https://doi.org/10.1186/2045-9769-2-6 .
Craig J. Taylor, Eleanor M. Bolton, and J. Andrew Bradley 2011 Aug 12 and https://doi.org/10.1098/rstb.2011.0030 ], 366(1575): 2312–2322. [doi:. Immunological considerations for embryonic and induced pluripotent stem cell banking,. Philos Trans R SocLond B Biol Sci. 2011, 366(1575), pp. 2312–2322, doi: https://doi.org/10.1098/rstb.2011.0030 .
T.R. Nayak, H. Andersen, V.S. Makam, C. Khaw, S. Bae, X.F. Xu, P.L.R. Ee, J.H. Ahn, B.H. Hong, G. Pastorin, B. Ozyilmaz, ACS Nano, 5 (6) (2011), pp. 4. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells,. ACS Nano. 2011, pp. 4670–4678.
Lee WC, Lim C, Shi H, Tang LAL, Wang Y, Lim CT, Loh KP. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano. 2011;5(9):7334–41.
Kenry LWC, Loh KP, Lim CT. When stem cells meet graphene: opportunities and challenges in regenerative medicine. Biomaterials. 2018;155:236–50.
Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, et al. Transfer of microRNAs by embryonic stem cell microvesicles. 2009. 2009, 4(3), p. https://doi.org/10.1371/journal.pone . 0004722.
Oh, Myeongsik, et al. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int. J. Mol. Sci. 2018, 19(6), p. 1715.
Ramirez MI. et al. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10:881–906.
Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–9.
Mateescu B, et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—an ISEV position paper. J. Extracell. Vesicles. 2017;6(1). https://doi.org/10.1080/20013078.2017.1286095 .
Nawaz M, et al. Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. 2016;2016:17. Article ID 1073140.
Helfrich, Y.R., Sachs, D.L. and Voorhees, J.J. Overview of skin aging and photoaging. Dermatol. Nurs. 20, pp. 177–183, https://www.ncbi.nlm.nih.gov/pubmed/18649702 .
Julia Tigges, Jean Krutmann, Ellen Fritsche, Judith Haendeler, Heiner Schaal, Jens W. Fischer, Faiza Kalfalah, Hans Reinke, Guido Reifenberger, Kai Stühler, Natascia Ventura, Sabrina Gundermann, Petra Boukamp, Fritz Boege. The hallmarks of fibroblast ageing, mechanisms of ageing and development, 138, 2014, Pages 26–44. 2014, 138, pp. 26–44, ISSN 0047–6374, https://doi.org/10.1016/j.mad.2014.03.004 .
Huh MI, Kim MS, Kim HK, et al. Effect of conditioned media collected from human amniotic fluid-derived stem cells (hAFSCs) on skin regeneration and photo-aging. Tissue Eng Regen Med. 2014;11:171 https://doi.org/10.1007/s13770-014-0412-1 .
Togel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31.
Liu J, Han G, Liu H, et al. Suppression of cholangiocarcinoma cell growth by human umbilical cord mesenchymal stem cells: a possible role of Wnt and Akt signaling. PLoS One. 2013;8:e62844.
Oh M, et al. Promotive effects of human induced pluripotent stem cell-conditioned medium on the proliferation and migration of dermal fibroblasts. Biotechnol. Bioprocess Eng. 2017;22:561–8.
Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PloS One. 2008;3:e1886.
Bae J-S, Lee S-H, Kim J-E, Choi J-Y, Park R-W, Park JY, Park H-S, Sohn Y-S, Lee D-S, Lee EB. βig-h3 supports keratinocyte adhesion, migration, and proliferation through α3β1 integrin. Biochem. Biophys. Res. Commun. 2002;294:940–8.
Zhou B-R, Xu Y, Guo S-L, Xu Y, Wang Y, Zhu F, Permatasari F, Wu D, Yin Z-Q, Luo D. The effect of conditioned media of adipose-derived stem cells on wound healing after ablative fractional carbon dioxide laser resurfacing. BioMed Res. Int. 2013;519:126.
Peng Y, Baulier E, Ke Y, Young A, Ahmedli NB, Schwartz SD, et al. Human embryonic stem cells extracellular vesicles and their effects on immortalized human retinal Müller cells. PLoS ONE. 2018, 13(3), p. https://doi.org/10.1371/journal.pone.019400 .
Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998–1003.
Mascetti VL, Pedersen RA. Human-mouse chimerism validates human stem cell pluripotency. Cell Stem Cell. 2016;18:67–72.
Gandia C, Armiñan A, García-Verdugo JM, Lledó E, Ruiz A, Miñana MD, Sanchez-Torrijos J, Payá R, Mirabet V, Carbonell-Uberos F, Llop M, Montero JA, Sepúlveda P. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2007;26(3):638–45.
Perry BC, Zhou D, Wu X, Yang FC, Byers MA, Chu TM, Hockema JJ, Woods EJ, Goebel WS. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods. 2008;14(2):149–56.
Garcia-Olmo D, Garcia-Arranz M, Herreros D, et al. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–23.
de Mendonça CA, Bueno DF, Martins MT, Kerkis I, Kerkis A, Fanganiello RD, Cerruti H, Alonso N, Passos-Bueno MR. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J Craniofac Surg. 2008;19(1):204–10.
Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, Lee JS, Shi S. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008;14(5):428–34.
Abbas, Diakonov I., Sharpe P. Neural crest origin of dental stem cells. Pan European Federation of the International Association for Dental Research (PEF IADR). 2008, Vols. Seq #96 - Oral Stem Cells.
Kerkis I, Ambrosio CE, Kerkis A, Martins DS, Gaiad TP, Morini AC, Vieira NM, Marina P, et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs. J Transl Med. 2008;6:35.
Xianrui Yang, Li Li, Li Xiao, Donghui Zhang. Recycle the dental fairy’s package: overview of dental pulp stem cells. Stem Cell Res Ther . 2018, 9, 1, 1. https://doi.org/10.1186/s13287-018-1094-8 .
Wang J, Wang X, Sun Z, Wang X, Yang H, Shi S, Wang S. Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev. 2010;19:1375–83.
Wang J, et al. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly (L-lactic acid) scaffolds in vitro and in vivo. Acta Biomater. 2010;6(10):3856–63.
Davies OG, Cooper PR, Shelton RM, Smith AJ, Scheven BA. A comparison of the in vitro mineralisation and dentinogenic potential of mesenchymal stem cells derived from adipose tissue, bone marrow and dental pulp. J Bone Miner Metab. 2015;33:371–82.
Huang GT-J, Shagramanova K, Chan SW. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J Endod. 2006;32:1066–73.
Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29(6):532–9.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30.
Nuti N, Corallo C, Chan BMF, Ferrari M, Gerami-Naini B. Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Rev Rep. 2016;12:511–23.
Ferro F, et al. Dental pulp stem cells differentiation reveals new insights in Oct4A dynamics. PloS One. 2012;7(7):e41774.
Conde MCM, Chisini LA, Grazioli G, Francia A, Carvalho RVd, Alcázar JCB, Tarquinio SBC, Demarco FF. Does cryopreservation affect the biological properties of stem cells from dental tissues? A systematic review. Braz Dent J. 2016;1210(6):633-40. https://doi.org/10.1590/0103-6440201600980 .
Papaccio G, Graziano A, d’Aquino R, Graziano MF, Pirozzi G, Menditti D, De Rosa A, Carinci F, Laino G. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol. 2006;208:319–25.
Alge DL, Zhou D, Adams LL, et. al. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med. 2010;4(1):73–81.
Jo Y-Y, Lee H-J, Kook S-Y, Choung H-W, Park J-Y, Chung J-H, Choung Y-H, Kim E-S, Yang H-C, Choung P-H. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13:767–73.
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5.
Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, Pirozzi G, Papaccio G. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res. 2005;20:1394–402.
Zainal A, Shahrul H, et al. In vitro chondrogenesis transformation study of mouse dental pulp stem cells. Sci World J. 2012;2012:827149.
Wei X, et al. Expression of mineralization markers in dental pulp cells. J Endod. 2007;33(6):703–8.
Dai J, et al. The effect of co-culturing costal chondrocytes and dental pulp stem cells combined with exogenous FGF9 protein on chondrogenesis and ossification in engineered cartilage. Biomaterials. 2012;33(31):7699–711.
Vasandan AB, et al. Functional differences in mesenchymal stromal cells from human dental pulp and periodontal ligament. J Cell Mol Med. 2014;18(2):344–54.
Werle SB, et al. Carious deciduous teeth are a potential source for dental pulp stem cells. Clin Oral Investig. 2015;20:75–81.
Nemeth CL, et al. Enhanced chondrogenic differentiation of dental pulp stem cells using nanopatterned PEG-GelMA-HA hydrogels. Tissue Eng A. 2014;20(21–22):2817–29.
Paino F, Ricci G, De Rosa A, D’Aquino R, Laino L, Pirozzi G, et al. Ecto-mesenchymal stem cells from dental pulp are committed to differentiate into active melanocytes. Eur. Cell Mater. 2010;20:295–305.
Ferro F, Spelat R, Baheney CS. Dental pulp stem cell (DPSC) isolation, characterization, and differentiation. In: Kioussi C, editor. Stem cells and tissue repair. Methods in molecular biology (methods and protocols): Humana Press. 2014;1210.
Ishkitiev N, Yaegaki K, Imai T, Tanaka T, Nakahara T, Ishikawa H, Mitev V, Haapasalo M. High-purity hepatic lineage differentiated from dental pulp stem cells in serum-free medium. J Endod. 2012;38:475–80.
Download references
Acknowledgements
Not applicable.
This work is supported by Wrocław Medical University in Poland.
Availability of data and materials
Please contact author for data requests.
Author information
Authors and affiliations.
Department of Experimental Surgery and Biomaterials Research, Wroclaw Medical University, Bujwida 44, Wrocław, 50-345, Poland
Wojciech Zakrzewski, Maria Szymonowicz & Zbigniew Rybak
Department of Conservative Dentistry and Pedodontics, Krakowska 26, Wrocław, 50-425, Poland
Maciej Dobrzyński
You can also search for this author in PubMed Google Scholar
Contributions
WZ is the principal author and was responsible for the first draft of the manuscript. WZ and ZR were responsible for the concept of the review. MS, MD, and ZR were responsible for revising the article and for data acquisition. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Wojciech Zakrzewski .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Zakrzewski, W., Dobrzyński, M., Szymonowicz, M. et al. Stem cells: past, present, and future. Stem Cell Res Ther 10 , 68 (2019). https://doi.org/10.1186/s13287-019-1165-5
Download citation
Published : 26 February 2019
DOI : https://doi.org/10.1186/s13287-019-1165-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Differentiation
- Pluripotency
- Induced pluripotent stem cell (iPSC)
- Stem cell derivation
- Growth media
- Tissue banks
- Tissue transplantation
Stem Cell Research & Therapy
ISSN: 1757-6512
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

Stem Cells and the Future of Regenerative Medicine (2002)
Chapter: executive summary, executive summary.

S tem cell research offers unprecedented opportunities for developing new medical therapies for debilitating diseases and a new way to explore fundamental questions of biology. Stem cells are unspecialized cells that can self-renew indefinitely and also differentiate into more mature cells with specialized functions. Research on human embryonic stem cells, however, is controversial, given the diverse views held in our society about the moral and legal status of the early embryo. The controversy has encouraged provocative and conflicting claims both inside and outside the scientific community about the biology and biomedical potential of both adult and embryonic stem cells.
The National Research Council and Institute of Medicine formed the Committee on the Biological and Biomedical Applications of Stem Cell Research to address the potential of stem cell research. The committee organized a workshop that was held on June 22, 2001. At the workshop, the committee heard from many leading scientists who are engaged in stem cell research and from philosophers, ethicists, and legal scholars. (Audio files of the speakers’ presentations are available until December 31, 2002, at the workshop Web site, www.nationalacademies.org/stemcells .)
The participants discussed the science of stem cells and a variety of ethical and other arguments relevant to public policy as it applies to stem cells. The committee considered the
information presented, explored the literature on its own, and contemplated the substance and importance of the preliminary data from recent stem cell experiments. The committee’s deliberations on the issues led to the following conclusions and recommendations.
Experiments in mice and other animals are necessary, but not sufficient, for realizing the potential of stem cells to develop tissue-replacement therapies that will restore lost function in damaged organs. Because of the substantial biological differences between nonhuman animal and human development and between animal and human stem cells, studies with human stem cells are essential to make progress in the development of treatments for human disease, and this research should continue.
There are important biological differences between adult and embryonic stem cells and among adult stem cells found in different types of tissue. The implications of these biological differences for therapeutic uses are not yet clear, and additional data are needed on all stem cell types. Adult stem cells from bone marrow have so far provided most of the examples of successful therapies for replacement of diseased or destroyed cells. Despite the enthusiasm generated by recent reports, the potential of adult stem cells to differentiate fully into other cell types (such as brain, nerve, pancreas cells) is still poorly understood and remains to be clarified. In contrast, studies of human embryonic stem cells have shown that they can develop into multiple tissue types and exhibit long-term self-renewal in culture, features that have not yet been demonstrated with many human adult stem cells. The application of stem cell research to therapies for human disease will require much more knowledge about the biological properties of all types of stem cells. Although stem cell research is on the cutting edge of biological science today, it is still in its infancy. Studies of both embryonic and adult human stem cells will be required to most efficiently advance the scientific and therapeutic potential of regenerative medicine. Moreover, research on embryonic stem cells will be important to inform research on
adult stem cells, and vice versa. Research on both adult and embryonic human stem cells should be pursued.
Over time, all cell lines in tissue culture change, typically accumulating harmful genetic mutations. There is no reason to expect stem cell lines to behave differently. In addition, most existing stem cell lines have been cultured in the presence of non-human cells or serum that could lead to potential human health risks. Consequently, while there is much that can be learned using existing stem cell lines if they are made widely available for research, such concerns necessitate continued monitoring of these cells as well as the development of new stem cell lines in the future.
High-quality, publicly funded research is the wellspring of medical breakthroughs. Although private, for-profit research plays a critical role in translating the fruits of basic research into medical advances that are broadly available to the public, stem cell research is far from the point of providing therapeutic products. Without public funding of basic research on stem cells, progress toward medical therapies is likely to be hindered. In addition, public funding offers greater opportunities for regulatory oversight and public scrutiny of stem cell research. Stem cell research that is publicly funded and conducted under established standards of open scientific exchange, peer review, and public oversight offers the most efficient and responsible means of fulfilling the promise of stem cells to meet the need for regenerative medical therapies.
Conflicting ethical perspectives surround the use of embryonic stem cells in medical research, particularly where the moral and legal status of human embryos is concerned. The use of embryonic stem cells is not the first biomedical research activity to raise ethical and social issues among the public. Restrictions and guidelines for the conduct of controversial research have been developed to address such concerns in other instances. For example, when recombinant-DNA techniques raised questions and were subject to intense debate and public scrutiny, a national advisory body, the Recombinant DNA Advisory Committee, was established at the National Institutes of Health (NIH) to ensure that
the research met the highest scientific and ethical standards. If the federal government chooses to fund research on human embryonic stem cells, a similar national advisory group composed of exceptional researchers, ethicists, and other stakeholders should be established at NIH to oversee it. Such a group should ensure that proposals to work on human embryonic stem cells are scientifically justified and should scrutinize such proposals for compliance with federally mandated ethical guidelines.
Regenerative medicine is likely to involve the implantation of new tissue in patients with damaged or diseased organs. A substantial obstacle to the success of transplantation of any cells, including stem cells and their derivatives, is the immune-mediated rejection of foreign tissue by the recipient’s body. In current stem cell transplantation procedures with bone marrow and blood, success can hinge on obtaining a close match between donor and recipient tissues and on the use of immunosuppressive drugs, which often have severe and life-threatening side effects. To ensure that stem cell-based therapies can be broadly applicable for many conditions and individuals, new means to overcome the problem of tissue rejection must be found. Although ethically controversial, somatic cell nuclear transfer, a technique that produces a lineage of stem cells that are genetically identical to the donor, promises such an advantage. Other options for this purpose include genetic manipulation of the stem cells and the development of a very large bank of embryonic stem cell lines. In conjunction with research on stem cell biology and the development of stem cell therapies, research on approaches that prevent immune rejection of stem cells and stem cell-derived tissues should be actively pursued.
The committee is aware of and respectful of the wide array of social, political, legal, ethical, and economic issues that must be considered in policy-making in a democracy. And it is impressed by the commitment of all parties in this debate to life and health, regardless of the different conclusions they draw. The committee hopes that this report, by clarifying what is known about the scientific potential of stem cells and how that potential can best be realized, will be a useful contribution to the
debate and to the enhancement of treatments for disabling human diseases and injuries. On August 9, 2001, when President Bush announced a new federal policy permitting limited use of human embryonic stem cells for research, this report was already in review. Because this report presents the committee’s interpretation of the state of the science of stem cells independent of any specific policy, only minor modifications to refer to the new policy have been made in the report.
Recent scientific breakthroughs, celebrity patient advocates, and conflicting religious beliefs have come together to bring the state of stem cell research—specifically embryonic stem cell research—into the political crosshairs. President Bush's watershed policy statement allows federal funding for embryonic stem cell research but only on a limited number of stem cell lines. Millions of Americans could be affected by the continuing political debate among policymakers and the public.
Stem Cells and the Future of Regenerative Medicine provides a deeper exploration of the biological, ethical, and funding questions prompted by the therapeutic potential of undifferentiated human cells. In terms accessible to lay readers, the book summarizes what we know about adult and embryonic stem cells and discusses how to go about the transition from mouse studies to research that has therapeutic implications for people.
Perhaps most important, Stem Cells and the Future of Regenerative Medicine also provides an overview of the moral and ethical problems that arise from the use of embryonic stem cells. This timely book compares the impact of public and private research funding and discusses approaches to appropriate research oversight.
Based on the insights of leading scientists, ethicists, and other authorities, the book offers authoritative recommendations regarding the use of existing stem cell lines versus new lines in research, the important role of the federal government in this field of research, and other fundamental issues.
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
Understanding Stem Cell Research
What's a stem cell?
The foundational building blocks of living organisms, stem cells are defined by two characteristics:
- They can make copies of themselves, or self-renew
- They can differentiate , or develop into more specialized cells
In humans, there are different types of stem cells that originate from different parts of the body and emerge during different times in our lives. These include embryonic stem cells, which only exist during the earliest stages of development, and tissue-specific stem cells, which arise during fetal development and persist throughout life.
"Stem cells are the foundation of organisms, the stalk from which everything buds and branches.” Alexander Capron Bioethicist, World Health Organization
What are the different types of stem cells?
Tissue-Specific Stem Cells
Tissue-specific stem cells, sometimes referred to as adult or somatic stem cells, contribute to the body’s ability to create new cells that can restore damaged organs and tissues. They also replace cells that are lost in normal day-to-day living.
Scientists now know that small populations of tissue-specific stem cells reside in many organs and tissues, including the brain, skeletal muscle, skin, heart, intestines and liver. While tissue-specific stem cells can help the body repair by producing all the cell types within their designated tissues, their ability to regenerate is limited and decreases over time.
Blood stem cells, also known as hematopoietic stem cells, are found in the bone marrow and generate the different kinds of blood cells needed over a person’s lifespan. This includes red blood cells, white blood cells — also known as immune cells — and platelets. Transplants of blood stem cells have been used to treat patients with blood and immune diseases such as leukemia and aplastic anemia for more than 50 years. Today, the sources of blood stem cells used for transplantation include bone marrow, umbilical cord blood and peripheral blood.
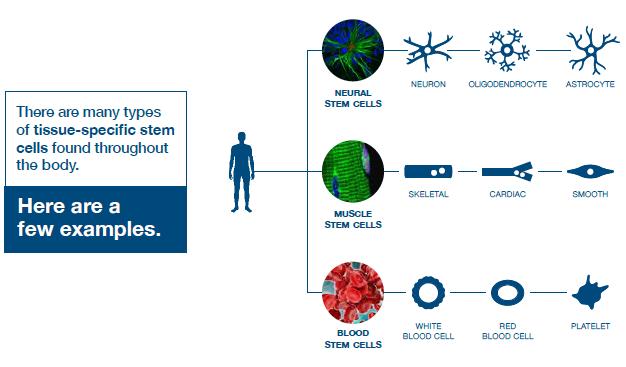
Human Embryonic Stem Cells
In 1998, scientists succeeded in isolating human embryonic stem cells, or hESCs, for the first time. Since then, the more versatile and flexible regenerative potential of hESCs has proved vital to scientific research, enabling scientists to learn about human developmental processes that would otherwise be inaccessible. Unlike tissue-specific stem cells, hESCs have two distinct capabilities: They can replicate indefinitely, and they’re pluripotent, meaning they can produce the more than 200 cell types found in the human body through a process called differentiation.
Induced Pluripotent Stem Cells
In 2006, a team in Japan showed it was possible to induce pluripotency in mature cells, creating what are known as induced pluripotent stem cells, or iPSCs. Shortly after, center members Drs. Kathrin Plath , William Lowry , Amander Clark and April Pyle were the first in California to report the successful generation of iPSCs.
iPSCs originate from cells — such as skin or blood — that are removed from a person and reprogrammed back to a pluripotent state. Like hESCs, these reprogrammed cells can replicate indefinitely as well as differentiate into any cell type in the human body. Since iPSCs are made from a patient’s own cells, therapies created from these cells could potentially be perfectly matched to the patient.
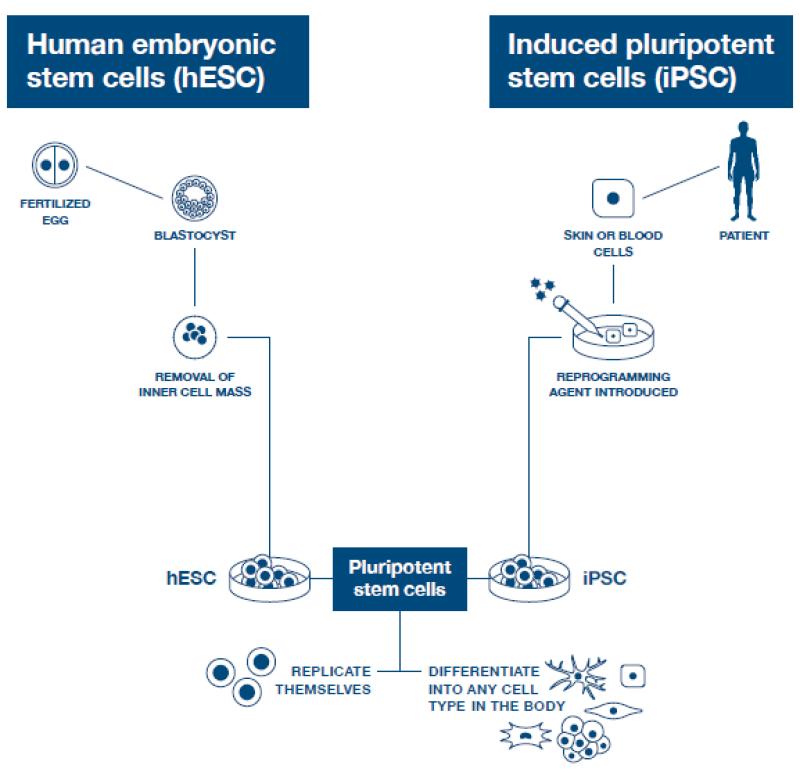
How are stem cells used in research?
Visit our Research Area pages to learn how our multidisciplinary teams of scientists, clinicians and engineers are conducting stem cell research that’s driving medical and scientific knowledge in new directions, fundamentally changing the understanding and treatment of disease.
Where do we get stem cells for research?
All of the human embryonic stem cells currently used in research come from cell lines that have been approved for research and registered with the National Institutes of Health . The original hESCs used to establish these lines come from embryos that are donated from consenting individuals or couples who have unused embryos after in vitro fertilization, or IVF, procedures. The embryonic stem cells are isolated from the inner cell mass of the blastocyst, a stage in early human embryonic development that occurs within the first four to six days after fertilization. Once hESCs are isolated, the cells may be kept alive and multiplied under specific laboratory conditions. Visit the California Institute for Regenerative Medicine's page on stem cell research to learn more about this process.
The human tissue — typically skin or blood — used to create induced pluripotent stem cell lines is donated by consenting individuals under specific research protocols.
For information about stem cell therapies and the availability of stem cell clinical trials at and beyond UCLA, visit our For Patients and Families page.
- History, Facts & Figures
- YSM Dean & Deputy Deans
- YSM Administration
- Department Chairs
- YSM Executive Group
- YSM Board of Permanent Officers
- FAC Documents
- Current FAC Members
- Appointments & Promotions Committees
- Ad Hoc Committees and Working Groups
- Chair Searches
- Leadership Searches
- Organization Charts
- Faculty Demographic Data
- Professionalism Reporting Data
- 2022 Diversity Engagement Survey
- State of the School Archive
- Faculty Climate Survey: YSM Results
- Strategic Planning
- Mission Statement & Process
- Beyond Sterling Hall
- COVID-19 Series Workshops
- Previous Workshops
- Departments & Centers
- Find People
- Biomedical Data Science
- Health Equity
- Inflammation
- Neuroscience
- Global Health
- Diabetes and Metabolism
- Policies & Procedures
- Media Relations
- A to Z YSM Lab Websites
- A-Z Faculty List
- A-Z Staff List
- A to Z Abbreviations
- Dept. Diversity Vice Chairs & Champions
- Dean’s Advisory Council on Lesbian, Gay, Bisexual, Transgender, Queer and Intersex Affairs Website
- Minority Organization for Retention and Expansion Website
- Office for Women in Medicine and Science
- Committee on the Status of Women in Medicine Website
- Director of Scientist Diversity and Inclusion
- Diversity Supplements
- Frequently Asked Questions
- Recruitment
- By Department & Program
- News & Events
- Executive Committee
- Aperture: Women in Medicine
- Self-Reflection
- Portraits of Strength
- Mindful: Mental Health Through Art
- Event Photo Galleries
- Additional Support
- MD-PhD Program
- PA Online Program
- Joint MD Programs
- How to Apply
- Advanced Health Sciences Research
- Clinical Informatics & Data Science
- Clinical Investigation
- Medical Education
- Visiting Student Programs
- Special Programs & Student Opportunities
- Residency & Fellowship Programs
- Center for Med Ed
- Organizational Chart
- Leadership & Staff
- Committee Procedural Info (Login Required)
- Faculty Affairs Department Teams
- Recent Appointments & Promotions
- Academic Clinician Track
- Clinician Educator-Scholar Track
- Clinican-Scientist Track
- Investigator Track
- Traditional Track
- Research Ranks
- Instructor/Lecturer
- Social Work Ranks
- Voluntary Ranks
- Adjunct Ranks
- Other Appt Types
- Appointments
- Reappointments
- Transfer of Track
- Term Extensions
- Timeline for A&P Processes
- Interfolio Faculty Search
- Interfolio A&P Processes
- Yale CV Part 1 (CV1)
- Yale CV Part 2 (CV2)
- Samples of Scholarship
- Teaching Evaluations
- Letters of Evaluation
- Dept A&P Narrative
- A&P Voting
- Faculty Affairs Staff Pages
- OAPD Faculty Workshops
- Leadership & Development Seminars
- List of Faculty Mentors
- Incoming Faculty Orientation
- Faculty Onboarding
- Past YSM Award Recipients
- Past PA Award Recipients
- Past YM Award Recipients
- International Award Recipients
- Nominations Calendar
- OAPD Newsletter
- Fostering a Shared Vision of Professionalism
- Academic Integrity
- Addressing Professionalism Concerns
- Consultation Support for Chairs & Section Chiefs
- Policies & Codes of Conduct
- First Fridays
- Fund for Physician-Scientist Mentorship
- Grant Library
- Grant Writing Course
- Mock Study Section
- Research Paper Writing
- Establishing a Thriving Research Program
- Funding Opportunities
- Join Our Voluntary Faculty
- Child Mental Health: Fostering Wellness in Children
- Faculty Resources
- Research by Keyword
- Research by Department
- Research by Global Location
- Translational Research
- Research Cores & Services
- Program for the Promotion of Interdisciplinary Team Science (POINTS)
- CEnR Steering Committee
- Experiential Learning Subcommittee
- Goals & Objectives
- Issues List
- Print Magazine PDFs
- Print Newsletter PDFs
- YSM Events Newsletter
- Social Media
- Patient Care
INFORMATION FOR
- Residents & Fellows
- Researchers
Stem Cell Research Uncovers Clues to Tissue Repair That Could Help Heal the Uterus and More
Stem cells play a vital role in repairing damaged tissue, whether it’s a scraped knee or a scarred uterus following pregnancy. New stem cell research has identified the molecules that the cells produce to promote the healing process. The finding could pave the way for the development of new, more effective drugs for injuries or various diseases, including conditions related to reproductive health such as Asherman syndrome, a gynecologic condition in which the uterus scars and becomes fibrotic.
Scientists believed in the past that stem cells served as backup cells that repaired tissues by differentiating into new cells that repopulated the site of injury. Now, they have learned that it is rare for stem cells to completely replace injured tissue. But they still don’t fully understand how the cells are able to help damaged areas regenerate.
We found the molecules that stem cells make to help heal and repair tissue, and we hope that understanding this will be potentially useful as a medication in the future. Hugh Taylor, MD
In the uterus, stem cells play a number of roles, including helping it to expand during pregnancy and to regenerate and repair after childbirth. This new study identified several microRNAs (miRNAs) secreted by the stem cells that helped drive the growth and proliferation of cells in uterine tissue. The researchers published their findings in Stem Cell Research & Therapy on May 1.
“We found the molecules that stem cells make to help heal and repair tissue, and we hope that understanding this will be potentially useful as a medication in the future,” says Hugh Taylor, MD , chair and Anita O’Keeffe Young Professor of Obstetrics, Gynecology & Reproductive Sciences at Yale School of Medicine and the study’s principal investigator.
Stem cells secrete miRNAs that support cell proliferation
Exosomes are extracellular vesicles, which contain various bioactive molecules and allow cells to communicate with one another. In their new study, Taylor’s team isolated exosomes secreted by stem cells from human bone marrow. They then used RNA sequencing to characterize all of the miRNA contained in the vesicles and identified those that were most abundant. Then researchers took the most prominent miRNAs and introduced them into human uterine tissue.
The team found that the miRNAs significantly increased the growth and proliferation of the uterine cells. They also studied their effect on the cells’ decidualization in the endometrium. (Decidualization is the differentiation process uterine cells undergo that prepares the uterus to support an embryo.) The study showed that the miRNAs blocked decidualization.
“In a uterus, once a cell becomes differentiated to support pregnancy, it can no longer repair and regenerate. It’s permanently locked in that state and often is shed through menstruation later on,” says Taylor. “By blocking this process, it allows the cells to focus on proliferating and turns on these reparative processes.”
Turning miRNAs into drugs for tissue repair
The study offers insight into how stem cells promote reparative processes without replacing the tissue itself. Taylor hopes that as researchers continue to gain a greater understanding about how miRNAs work, they could one day be used as drugs for repairing various damaged tissue.
Asherman syndrome, for example, typically occurs after pregnancy, when the supply of stem cells may not be adequate to help the organ heal properly, which can hinder fertility in the future. “The idea is that these miRNAs could be used as a medication that is much more readily available and practical,” says Taylor. “We could potentially deliver them to help prepare the uterus in the critical window when it is damaged and may be vulnerable.”
The finding could also have significance beyond the uterus. In future stem cell research, Taylor’s team plans to study how miRNAs respond to other types of traumatic tissue injury in animal models. “We studied the uterus, but the implications are beyond reproduction, potentially including many other conditions where stem cells are involved in repair and regeneration, whether that’s injury due to trauma or degenerative diseases,” says Taylor.
Featured in this article
- Hugh Taylor, MD Anita O'Keeffe Young Professor of Obstetrics, Gynecology, and Reproductive Sciences and Professor of Molecular, Cellular, and Developmental Biology; Chair, Obstetrics, Gynecology & Reproductive Sciences; Chief , Obstetrics and Gynecology, Yale New Haven Hospital

Cultural Relativity and Acceptance of Embryonic Stem Cell Research
Article sidebar.
Main Article Content
There is a debate about the ethical implications of using human embryos in stem cell research, which can be influenced by cultural, moral, and social values. This paper argues for an adaptable framework to accommodate diverse cultural and religious perspectives. By using an adaptive ethics model, research protections can reflect various populations and foster growth in stem cell research possibilities.
INTRODUCTION
Stem cell research combines biology, medicine, and technology, promising to alter health care and the understanding of human development. Yet, ethical contention exists because of individuals’ perceptions of using human embryos based on their various cultural, moral, and social values. While these disagreements concerning policy, use, and general acceptance have prompted the development of an international ethics policy, such a uniform approach can overlook the nuanced ethical landscapes between cultures. With diverse viewpoints in public health, a single global policy, especially one reflecting Western ethics or the ethics prevalent in high-income countries, is impractical. This paper argues for a culturally sensitive, adaptable framework for the use of embryonic stem cells. Stem cell policy should accommodate varying ethical viewpoints and promote an effective global dialogue. With an extension of an ethics model that can adapt to various cultures, we recommend localized guidelines that reflect the moral views of the people those guidelines serve.
Stem cells, characterized by their unique ability to differentiate into various cell types, enable the repair or replacement of damaged tissues. Two primary types of stem cells are somatic stem cells (adult stem cells) and embryonic stem cells. Adult stem cells exist in developed tissues and maintain the body’s repair processes. [1] Embryonic stem cells (ESC) are remarkably pluripotent or versatile, making them valuable in research. [2] However, the use of ESCs has sparked ethics debates. Considering the potential of embryonic stem cells, research guidelines are essential. The International Society for Stem Cell Research (ISSCR) provides international stem cell research guidelines. They call for “public conversations touching on the scientific significance as well as the societal and ethical issues raised by ESC research.” [3] The ISSCR also publishes updates about culturing human embryos 14 days post fertilization, suggesting local policies and regulations should continue to evolve as ESC research develops. [4] Like the ISSCR, which calls for local law and policy to adapt to developing stem cell research given cultural acceptance, this paper highlights the importance of local social factors such as religion and culture.
I. Global Cultural Perspective of Embryonic Stem Cells
Views on ESCs vary throughout the world. Some countries readily embrace stem cell research and therapies, while others have stricter regulations due to ethical concerns surrounding embryonic stem cells and when an embryo becomes entitled to moral consideration. The philosophical issue of when the “someone” begins to be a human after fertilization, in the morally relevant sense, [5] impacts when an embryo becomes not just worthy of protection but morally entitled to it. The process of creating embryonic stem cell lines involves the destruction of the embryos for research. [6] Consequently, global engagement in ESC research depends on social-cultural acceptability.
a. US and Rights-Based Cultures
In the United States, attitudes toward stem cell therapies are diverse. The ethics and social approaches, which value individualism, [7] trigger debates regarding the destruction of human embryos, creating a complex regulatory environment. For example, the 1996 Dickey-Wicker Amendment prohibited federal funding for the creation of embryos for research and the destruction of embryos for “more than allowed for research on fetuses in utero.” [8] Following suit, in 2001, the Bush Administration heavily restricted stem cell lines for research. However, the Stem Cell Research Enhancement Act of 2005 was proposed to help develop ESC research but was ultimately vetoed. [9] Under the Obama administration, in 2009, an executive order lifted restrictions allowing for more development in this field. [10] The flux of research capacity and funding parallels the different cultural perceptions of human dignity of the embryo and how it is socially presented within the country’s research culture. [11]
b. Ubuntu and Collective Cultures
African bioethics differs from Western individualism because of the different traditions and values. African traditions, as described by individuals from South Africa and supported by some studies in other African countries, including Ghana and Kenya, follow the African moral philosophies of Ubuntu or Botho and Ukama , which “advocates for a form of wholeness that comes through one’s relationship and connectedness with other people in the society,” [12] making autonomy a socially collective concept. In this context, for the community to act autonomously, individuals would come together to decide what is best for the collective. Thus, stem cell research would require examining the value of the research to society as a whole and the use of the embryos as a collective societal resource. If society views the source as part of the collective whole, and opposes using stem cells, compromising the cultural values to pursue research may cause social detachment and stunt research growth. [13] Based on local culture and moral philosophy, the permissibility of stem cell research depends on how embryo, stem cell, and cell line therapies relate to the community as a whole . Ubuntu is the expression of humanness, with the person’s identity drawn from the “’I am because we are’” value. [14] The decision in a collectivistic culture becomes one born of cultural context, and individual decisions give deference to others in the society.
Consent differs in cultures where thought and moral philosophy are based on a collective paradigm. So, applying Western bioethical concepts is unrealistic. For one, Africa is a diverse continent with many countries with different belief systems, access to health care, and reliance on traditional or Western medicines. Where traditional medicine is the primary treatment, the “’restrictive focus on biomedically-related bioethics’” [is] problematic in African contexts because it neglects bioethical issues raised by traditional systems.” [15] No single approach applies in all areas or contexts. Rather than evaluating the permissibility of ESC research according to Western concepts such as the four principles approach, different ethics approaches should prevail.
Another consideration is the socio-economic standing of countries. In parts of South Africa, researchers have not focused heavily on contributing to the stem cell discourse, either because it is not considered health care or a health science priority or because resources are unavailable. [16] Each country’s priorities differ given different social, political, and economic factors. In South Africa, for instance, areas such as maternal mortality, non-communicable diseases, telemedicine, and the strength of health systems need improvement and require more focus. [17] Stem cell research could benefit the population, but it also could divert resources from basic medical care. Researchers in South Africa adhere to the National Health Act and Medicines Control Act in South Africa and international guidelines; however, the Act is not strictly enforced, and there is no clear legislation for research conduct or ethical guidelines. [18]
Some parts of Africa condemn stem cell research. For example, 98.2 percent of the Tunisian population is Muslim. [19] Tunisia does not permit stem cell research because of moral conflict with a Fatwa. Religion heavily saturates the regulation and direction of research. [20] Stem cell use became permissible for reproductive purposes only recently, with tight restrictions preventing cells from being used in any research other than procedures concerning ART/IVF. Their use is conditioned on consent, and available only to married couples. [21] The community's receptiveness to stem cell research depends on including communitarian African ethics.
c. Asia
Some Asian countries also have a collective model of ethics and decision making. [22] In China, the ethics model promotes a sincere respect for life or human dignity, [23] based on protective medicine. This model, influenced by Traditional Chinese Medicine (TCM), [24] recognizes Qi as the vital energy delivered via the meridians of the body; it connects illness to body systems, the body’s entire constitution, and the universe for a holistic bond of nature, health, and quality of life. [25] Following a protective ethics model, and traditional customs of wholeness, investment in stem cell research is heavily desired for its applications in regenerative therapies, disease modeling, and protective medicines. In a survey of medical students and healthcare practitioners, 30.8 percent considered stem cell research morally unacceptable while 63.5 percent accepted medical research using human embryonic stem cells. Of these individuals, 89.9 percent supported increased funding for stem cell research. [26] The scientific community might not reflect the overall population. From 1997 to 2019, China spent a total of $576 million (USD) on stem cell research at 8,050 stem cell programs, increased published presence from 0.6 percent to 14.01 percent of total global stem cell publications as of 2014, and made significant strides in cell-based therapies for various medical conditions. [27] However, while China has made substantial investments in stem cell research and achieved notable progress in clinical applications, concerns linger regarding ethical oversight and transparency. [28] For example, the China Biosecurity Law, promoted by the National Health Commission and China Hospital Association, attempted to mitigate risks by introducing an institutional review board (IRB) in the regulatory bodies. 5800 IRBs registered with the Chinese Clinical Trial Registry since 2021. [29] However, issues still need to be addressed in implementing effective IRB review and approval procedures.
The substantial government funding and focus on scientific advancement have sometimes overshadowed considerations of regional cultures, ethnic minorities, and individual perspectives, particularly evident during the one-child policy era. As government policy adapts to promote public stability, such as the change from the one-child to the two-child policy, [30] research ethics should also adapt to ensure respect for the values of its represented peoples.
Japan is also relatively supportive of stem cell research and therapies. Japan has a more transparent regulatory framework, allowing for faster approval of regenerative medicine products, which has led to several advanced clinical trials and therapies. [31] South Korea is also actively engaged in stem cell research and has a history of breakthroughs in cloning and embryonic stem cells. [32] However, the field is controversial, and there are issues of scientific integrity. For example, the Korean FDA fast-tracked products for approval, [33] and in another instance, the oocyte source was unclear and possibly violated ethical standards. [34] Trust is important in research, as it builds collaborative foundations between colleagues, trial participant comfort, open-mindedness for complicated and sensitive discussions, and supports regulatory procedures for stakeholders. There is a need to respect the culture’s interest, engagement, and for research and clinical trials to be transparent and have ethical oversight to promote global research discourse and trust.
d. Middle East
Countries in the Middle East have varying degrees of acceptance of or restrictions to policies related to using embryonic stem cells due to cultural and religious influences. Saudi Arabia has made significant contributions to stem cell research, and conducts research based on international guidelines for ethical conduct and under strict adherence to guidelines in accordance with Islamic principles. Specifically, the Saudi government and people require ESC research to adhere to Sharia law. In addition to umbilical and placental stem cells, [35] Saudi Arabia permits the use of embryonic stem cells as long as they come from miscarriages, therapeutic abortions permissible by Sharia law, or are left over from in vitro fertilization and donated to research. [36] Laws and ethical guidelines for stem cell research allow the development of research institutions such as the King Abdullah International Medical Research Center, which has a cord blood bank and a stem cell registry with nearly 10,000 donors. [37] Such volume and acceptance are due to the ethical ‘permissibility’ of the donor sources, which do not conflict with religious pillars. However, some researchers err on the side of caution, choosing not to use embryos or fetal tissue as they feel it is unethical to do so. [38]
Jordan has a positive research ethics culture. [39] However, there is a significant issue of lack of trust in researchers, with 45.23 percent (38.66 percent agreeing and 6.57 percent strongly agreeing) of Jordanians holding a low level of trust in researchers, compared to 81.34 percent of Jordanians agreeing that they feel safe to participate in a research trial. [40] Safety testifies to the feeling of confidence that adequate measures are in place to protect participants from harm, whereas trust in researchers could represent the confidence in researchers to act in the participants’ best interests, adhere to ethical guidelines, provide accurate information, and respect participants’ rights and dignity. One method to improve trust would be to address communication issues relevant to ESC. Legislation surrounding stem cell research has adopted specific language, especially concerning clarification “between ‘stem cells’ and ‘embryonic stem cells’” in translation. [41] Furthermore, legislation “mandates the creation of a national committee… laying out specific regulations for stem-cell banking in accordance with international standards.” [42] This broad regulation opens the door for future global engagement and maintains transparency. However, these regulations may also constrain the influence of research direction, pace, and accessibility of research outcomes.
e. Europe
In the European Union (EU), ethics is also principle-based, but the principles of autonomy, dignity, integrity, and vulnerability are interconnected. [43] As such, the opportunity for cohesion and concessions between individuals’ thoughts and ideals allows for a more adaptable ethics model due to the flexible principles that relate to the human experience The EU has put forth a framework in its Convention for the Protection of Human Rights and Dignity of the Human Being allowing member states to take different approaches. Each European state applies these principles to its specific conventions, leading to or reflecting different acceptance levels of stem cell research. [44]
For example, in Germany, Lebenzusammenhang , or the coherence of life, references integrity in the unity of human culture. Namely, the personal sphere “should not be subject to external intervention.” [45] Stem cell interventions could affect this concept of bodily completeness, leading to heavy restrictions. Under the Grundgesetz, human dignity and the right to life with physical integrity are paramount. [46] The Embryo Protection Act of 1991 made producing cell lines illegal. Cell lines can be imported if approved by the Central Ethics Commission for Stem Cell Research only if they were derived before May 2007. [47] Stem cell research respects the integrity of life for the embryo with heavy specifications and intense oversight. This is vastly different in Finland, where the regulatory bodies find research more permissible in IVF excess, but only up to 14 days after fertilization. [48] Spain’s approach differs still, with a comprehensive regulatory framework. [49] Thus, research regulation can be culture-specific due to variations in applied principles. Diverse cultures call for various approaches to ethical permissibility. [50] Only an adaptive-deliberative model can address the cultural constructions of self and achieve positive, culturally sensitive stem cell research practices. [51]
II. Religious Perspectives on ESC
Embryonic stem cell sources are the main consideration within religious contexts. While individuals may not regard their own religious texts as authoritative or factual, religion can shape their foundations or perspectives.
The Qur'an states:
“And indeed We created man from a quintessence of clay. Then We placed within him a small quantity of nutfa (sperm to fertilize) in a safe place. Then We have fashioned the nutfa into an ‘alaqa (clinging clot or cell cluster), then We developed the ‘alaqa into mudgha (a lump of flesh), and We made mudgha into bones, and clothed the bones with flesh, then We brought it into being as a new creation. So Blessed is Allah, the Best of Creators.” [52]
Many scholars of Islam estimate the time of soul installment, marked by the angel breathing in the soul to bring the individual into creation, as 120 days from conception. [53] Personhood begins at this point, and the value of life would prohibit research or experimentation that could harm the individual. If the fetus is more than 120 days old, the time ensoulment is interpreted to occur according to Islamic law, abortion is no longer permissible. [54] There are a few opposing opinions about early embryos in Islamic traditions. According to some Islamic theologians, there is no ensoulment of the early embryo, which is the source of stem cells for ESC research. [55]
In Buddhism, the stance on stem cell research is not settled. The main tenets, the prohibition against harming or destroying others (ahimsa) and the pursuit of knowledge (prajña) and compassion (karuna), leave Buddhist scholars and communities divided. [56] Some scholars argue stem cell research is in accordance with the Buddhist tenet of seeking knowledge and ending human suffering. Others feel it violates the principle of not harming others. Finding the balance between these two points relies on the karmic burden of Buddhist morality. In trying to prevent ahimsa towards the embryo, Buddhist scholars suggest that to comply with Buddhist tenets, research cannot be done as the embryo has personhood at the moment of conception and would reincarnate immediately, harming the individual's ability to build their karmic burden. [57] On the other hand, the Bodhisattvas, those considered to be on the path to enlightenment or Nirvana, have given organs and flesh to others to help alleviate grieving and to benefit all. [58] Acceptance varies on applied beliefs and interpretations.
Catholicism does not support embryonic stem cell research, as it entails creation or destruction of human embryos. This destruction conflicts with the belief in the sanctity of life. For example, in the Old Testament, Genesis describes humanity as being created in God’s image and multiplying on the Earth, referencing the sacred rights to human conception and the purpose of development and life. In the Ten Commandments, the tenet that one should not kill has numerous interpretations where killing could mean murder or shedding of the sanctity of life, demonstrating the high value of human personhood. In other books, the theological conception of when life begins is interpreted as in utero, [59] highlighting the inviolability of life and its formation in vivo to make a religious point for accepting such research as relatively limited, if at all. [60] The Vatican has released ethical directives to help apply a theological basis to modern-day conflicts. The Magisterium of the Church states that “unless there is a moral certainty of not causing harm,” experimentation on fetuses, fertilized cells, stem cells, or embryos constitutes a crime. [61] Such procedures would not respect the human person who exists at these stages, according to Catholicism. Damages to the embryo are considered gravely immoral and illicit. [62] Although the Catholic Church officially opposes abortion, surveys demonstrate that many Catholic people hold pro-choice views, whether due to the context of conception, stage of pregnancy, threat to the mother’s life, or for other reasons, demonstrating that practicing members can also accept some but not all tenets. [63]
Some major Jewish denominations, such as the Reform, Conservative, and Reconstructionist movements, are open to supporting ESC use or research as long as it is for saving a life. [64] Within Judaism, the Talmud, or study, gives personhood to the child at birth and emphasizes that life does not begin at conception: [65]
“If she is found pregnant, until the fortieth day it is mere fluid,” [66]
Whereas most religions prioritize the status of human embryos, the Halakah (Jewish religious law) states that to save one life, most other religious laws can be ignored because it is in pursuit of preservation. [67] Stem cell research is accepted due to application of these religious laws.
We recognize that all religions contain subsets and sects. The variety of environmental and cultural differences within religious groups requires further analysis to respect the flexibility of religious thoughts and practices. We make no presumptions that all cultures require notions of autonomy or morality as under the common morality theory , which asserts a set of universal moral norms that all individuals share provides moral reasoning and guides ethical decisions. [68] We only wish to show that the interaction with morality varies between cultures and countries.
III. A Flexible Ethical Approach
The plurality of different moral approaches described above demonstrates that there can be no universally acceptable uniform law for ESC on a global scale. Instead of developing one standard, flexible ethical applications must be continued. We recommend local guidelines that incorporate important cultural and ethical priorities.
While the Declaration of Helsinki is more relevant to people in clinical trials receiving ESC products, in keeping with the tradition of protections for research subjects, consent of the donor is an ethical requirement for ESC donation in many jurisdictions including the US, Canada, and Europe. [69] The Declaration of Helsinki provides a reference point for regulatory standards and could potentially be used as a universal baseline for obtaining consent prior to gamete or embryo donation.
For instance, in Columbia University’s egg donor program for stem cell research, donors followed standard screening protocols and “underwent counseling sessions that included information as to the purpose of oocyte donation for research, what the oocytes would be used for, the risks and benefits of donation, and process of oocyte stimulation” to ensure transparency for consent. [70] The program helped advance stem cell research and provided clear and safe research methods with paid participants. Though paid participation or covering costs of incidental expenses may not be socially acceptable in every culture or context, [71] and creating embryos for ESC research is illegal in many jurisdictions, Columbia’s program was effective because of the clear and honest communications with donors, IRBs, and related stakeholders. This example demonstrates that cultural acceptance of scientific research and of the idea that an egg or embryo does not have personhood is likely behind societal acceptance of donating eggs for ESC research. As noted, many countries do not permit the creation of embryos for research.
Proper communication and education regarding the process and purpose of stem cell research may bolster comprehension and garner more acceptance. “Given the sensitive subject material, a complete consent process can support voluntary participation through trust, understanding, and ethical norms from the cultures and morals participants value. This can be hard for researchers entering countries of different socioeconomic stability, with different languages and different societal values. [72]
An adequate moral foundation in medical ethics is derived from the cultural and religious basis that informs knowledge and actions. [73] Understanding local cultural and religious values and their impact on research could help researchers develop humility and promote inclusion.
IV. Concerns
Some may argue that if researchers all adhere to one ethics standard, protection will be satisfied across all borders, and the global public will trust researchers. However, defining what needs to be protected and how to define such research standards is very specific to the people to which standards are applied. We suggest that applying one uniform guide cannot accurately protect each individual because we all possess our own perceptions and interpretations of social values. [74] Therefore, the issue of not adjusting to the moral pluralism between peoples in applying one standard of ethics can be resolved by building out ethics models that can be adapted to different cultures and religions.
Other concerns include medical tourism, which may promote health inequities. [75] Some countries may develop and approve products derived from ESC research before others, compromising research ethics or drug approval processes. There are also concerns about the sale of unauthorized stem cell treatments, for example, those without FDA approval in the United States. Countries with robust research infrastructures may be tempted to attract medical tourists, and some customers will have false hopes based on aggressive publicity of unproven treatments. [76]
For example, in China, stem cell clinics can market to foreign clients who are not protected under the regulatory regimes. Companies employ a marketing strategy of “ethically friendly” therapies. Specifically, in the case of Beike, China’s leading stem cell tourism company and sprouting network, ethical oversight of administrators or health bureaus at one site has “the unintended consequence of shifting questionable activities to another node in Beike's diffuse network.” [77] In contrast, Jordan is aware of stem cell research’s potential abuse and its own status as a “health-care hub.” Jordan’s expanded regulations include preserving the interests of individuals in clinical trials and banning private companies from ESC research to preserve transparency and the integrity of research practices. [78]
The social priorities of the community are also a concern. The ISSCR explicitly states that guidelines “should be periodically revised to accommodate scientific advances, new challenges, and evolving social priorities.” [79] The adaptable ethics model extends this consideration further by addressing whether research is warranted given the varying degrees of socioeconomic conditions, political stability, and healthcare accessibilities and limitations. An ethical approach would require discussion about resource allocation and appropriate distribution of funds. [80]
While some religions emphasize the sanctity of life from conception, which may lead to public opposition to ESC research, others encourage ESC research due to its potential for healing and alleviating human pain. Many countries have special regulations that balance local views on embryonic personhood, the benefits of research as individual or societal goods, and the protection of human research subjects. To foster understanding and constructive dialogue, global policy frameworks should prioritize the protection of universal human rights, transparency, and informed consent. In addition to these foundational global policies, we recommend tailoring local guidelines to reflect the diverse cultural and religious perspectives of the populations they govern. Ethics models should be adapted to local populations to effectively establish research protections, growth, and possibilities of stem cell research.
For example, in countries with strong beliefs in the moral sanctity of embryos or heavy religious restrictions, an adaptive model can allow for discussion instead of immediate rejection. In countries with limited individual rights and voice in science policy, an adaptive model ensures cultural, moral, and religious views are taken into consideration, thereby building social inclusion. While this ethical consideration by the government may not give a complete voice to every individual, it will help balance policies and maintain the diverse perspectives of those it affects. Embracing an adaptive ethics model of ESC research promotes open-minded dialogue and respect for the importance of human belief and tradition. By actively engaging with cultural and religious values, researchers can better handle disagreements and promote ethical research practices that benefit each society.
This brief exploration of the religious and cultural differences that impact ESC research reveals the nuances of relative ethics and highlights a need for local policymakers to apply a more intense adaptive model.
[1] Poliwoda, S., Noor, N., Downs, E., Schaaf, A., Cantwell, A., Ganti, L., Kaye, A. D., Mosel, L. I., Carroll, C. B., Viswanath, O., & Urits, I. (2022). Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthopedic reviews , 14 (3), 37498. https://doi.org/10.52965/001c.37498
[2] Poliwoda, S., Noor, N., Downs, E., Schaaf, A., Cantwell, A., Ganti, L., Kaye, A. D., Mosel, L. I., Carroll, C. B., Viswanath, O., & Urits, I. (2022). Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthopedic reviews , 14 (3), 37498. https://doi.org/10.52965/001c.37498
[3] International Society for Stem Cell Research. (2023). Laboratory-based human embryonic stem cell research, embryo research, and related research activities . International Society for Stem Cell Research. https://www.isscr.org/guidelines/blog-post-title-one-ed2td-6fcdk ; Kimmelman, J., Hyun, I., Benvenisty, N. et al. Policy: Global standards for stem-cell research. Nature 533 , 311–313 (2016). https://doi.org/10.1038/533311a
[4] International Society for Stem Cell Research. (2023). Laboratory-based human embryonic stem cell research, embryo research, and related research activities . International Society for Stem Cell Research. https://www.isscr.org/guidelines/blog-post-title-one-ed2td-6fcdk
[5] Concerning the moral philosophies of stem cell research, our paper does not posit a personal moral stance nor delve into the “when” of human life begins. To read further about the philosophical debate, consider the following sources:
Sandel M. J. (2004). Embryo ethics--the moral logic of stem-cell research. The New England journal of medicine , 351 (3), 207–209. https://doi.org/10.1056/NEJMp048145 ; George, R. P., & Lee, P. (2020, September 26). Acorns and Embryos . The New Atlantis. https://www.thenewatlantis.com/publications/acorns-and-embryos ; Sagan, A., & Singer, P. (2007). The moral status of stem cells. Metaphilosophy , 38 (2/3), 264–284. http://www.jstor.org/stable/24439776 ; McHugh P. R. (2004). Zygote and "clonote"--the ethical use of embryonic stem cells. The New England journal of medicine , 351 (3), 209–211. https://doi.org/10.1056/NEJMp048147 ; Kurjak, A., & Tripalo, A. (2004). The facts and doubts about beginning of the human life and personality. Bosnian journal of basic medical sciences , 4 (1), 5–14. https://doi.org/10.17305/bjbms.2004.3453
[6] Vazin, T., & Freed, W. J. (2010). Human embryonic stem cells: derivation, culture, and differentiation: a review. Restorative neurology and neuroscience , 28 (4), 589–603. https://doi.org/10.3233/RNN-2010-0543
[7] Socially, at its core, the Western approach to ethics is widely principle-based, autonomy being one of the key factors to ensure a fundamental respect for persons within research. For information regarding autonomy in research, see: Department of Health, Education, and Welfare, & National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (1978). The Belmont Report. Ethical principles and guidelines for the protection of human subjects of research.; For a more in-depth review of autonomy within the US, see: Beauchamp, T. L., & Childress, J. F. (1994). Principles of Biomedical Ethics . Oxford University Press.
[8] Sherley v. Sebelius , 644 F.3d 388 (D.C. Cir. 2011), citing 45 C.F.R. 46.204(b) and [42 U.S.C. § 289g(b)]. https://www.cadc.uscourts.gov/internet/opinions.nsf/6c690438a9b43dd685257a64004ebf99/$file/11-5241-1391178.pdf
[9] Stem Cell Research Enhancement Act of 2005, H. R. 810, 109 th Cong. (2001). https://www.govtrack.us/congress/bills/109/hr810/text ; Bush, G. W. (2006, July 19). Message to the House of Representatives . National Archives and Records Administration. https://georgewbush-whitehouse.archives.gov/news/releases/2006/07/20060719-5.html
[10] National Archives and Records Administration. (2009, March 9). Executive order 13505 -- removing barriers to responsible scientific research involving human stem cells . National Archives and Records Administration. https://obamawhitehouse.archives.gov/the-press-office/removing-barriers-responsible-scientific-research-involving-human-stem-cells
[11] Hurlbut, W. B. (2006). Science, Religion, and the Politics of Stem Cells. Social Research , 73 (3), 819–834. http://www.jstor.org/stable/40971854
[12] Akpa-Inyang, Francis & Chima, Sylvester. (2021). South African traditional values and beliefs regarding informed consent and limitations of the principle of respect for autonomy in African communities: a cross-cultural qualitative study. BMC Medical Ethics . 22. 10.1186/s12910-021-00678-4.
[13] Source for further reading: Tangwa G. B. (2007). Moral status of embryonic stem cells: perspective of an African villager. Bioethics , 21(8), 449–457. https://doi.org/10.1111/j.1467-8519.2007.00582.x , see also Mnisi, F. M. (2020). An African analysis based on ethics of Ubuntu - are human embryonic stem cell patents morally justifiable? African Insight , 49 (4).
[14] Jecker, N. S., & Atuire, C. (2021). Bioethics in Africa: A contextually enlightened analysis of three cases. Developing World Bioethics , 22 (2), 112–122. https://doi.org/10.1111/dewb.12324
[15] Jecker, N. S., & Atuire, C. (2021). Bioethics in Africa: A contextually enlightened analysis of three cases. Developing World Bioethics, 22(2), 112–122. https://doi.org/10.1111/dewb.12324
[16] Jackson, C.S., Pepper, M.S. Opportunities and barriers to establishing a cell therapy programme in South Africa. Stem Cell Res Ther 4 , 54 (2013). https://doi.org/10.1186/scrt204 ; Pew Research Center. (2014, May 1). Public health a major priority in African nations . Pew Research Center’s Global Attitudes Project. https://www.pewresearch.org/global/2014/05/01/public-health-a-major-priority-in-african-nations/
[17] Department of Health Republic of South Africa. (2021). Health Research Priorities (revised) for South Africa 2021-2024 . National Health Research Strategy. https://www.health.gov.za/wp-content/uploads/2022/05/National-Health-Research-Priorities-2021-2024.pdf
[18] Oosthuizen, H. (2013). Legal and Ethical Issues in Stem Cell Research in South Africa. In: Beran, R. (eds) Legal and Forensic Medicine. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-32338-6_80 , see also: Gaobotse G (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[19] United States Bureau of Citizenship and Immigration Services. (1998). Tunisia: Information on the status of Christian conversions in Tunisia . UNHCR Web Archive. https://webarchive.archive.unhcr.org/20230522142618/https://www.refworld.org/docid/3df0be9a2.html
[20] Gaobotse, G. (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[21] Kooli, C. Review of assisted reproduction techniques, laws, and regulations in Muslim countries. Middle East Fertil Soc J 24 , 8 (2020). https://doi.org/10.1186/s43043-019-0011-0 ; Gaobotse, G. (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[22] Pang M. C. (1999). Protective truthfulness: the Chinese way of safeguarding patients in informed treatment decisions. Journal of medical ethics , 25(3), 247–253. https://doi.org/10.1136/jme.25.3.247
[23] Wang, L., Wang, F., & Zhang, W. (2021). Bioethics in China’s biosecurity law: Forms, effects, and unsettled issues. Journal of law and the biosciences , 8(1). https://doi.org/10.1093/jlb/lsab019 https://academic.oup.com/jlb/article/8/1/lsab019/6299199
[24] Wang, Y., Xue, Y., & Guo, H. D. (2022). Intervention effects of traditional Chinese medicine on stem cell therapy of myocardial infarction. Frontiers in pharmacology , 13 , 1013740. https://doi.org/10.3389/fphar.2022.1013740
[25] Li, X.-T., & Zhao, J. (2012). Chapter 4: An Approach to the Nature of Qi in TCM- Qi and Bioenergy. In Recent Advances in Theories and Practice of Chinese Medicine (p. 79). InTech.
[26] Luo, D., Xu, Z., Wang, Z., & Ran, W. (2021). China's Stem Cell Research and Knowledge Levels of Medical Practitioners and Students. Stem cells international , 2021 , 6667743. https://doi.org/10.1155/2021/6667743
[27] Luo, D., Xu, Z., Wang, Z., & Ran, W. (2021). China's Stem Cell Research and Knowledge Levels of Medical Practitioners and Students. Stem cells international , 2021 , 6667743. https://doi.org/10.1155/2021/6667743
[28] Zhang, J. Y. (2017). Lost in translation? accountability and governance of Clinical Stem Cell Research in China. Regenerative Medicine , 12 (6), 647–656. https://doi.org/10.2217/rme-2017-0035
[29] Wang, L., Wang, F., & Zhang, W. (2021). Bioethics in China’s biosecurity law: Forms, effects, and unsettled issues. Journal of law and the biosciences , 8(1). https://doi.org/10.1093/jlb/lsab019 https://academic.oup.com/jlb/article/8/1/lsab019/6299199
[30] Chen, H., Wei, T., Wang, H. et al. Association of China’s two-child policy with changes in number of births and birth defects rate, 2008–2017. BMC Public Health 22 , 434 (2022). https://doi.org/10.1186/s12889-022-12839-0
[31] Azuma, K. Regulatory Landscape of Regenerative Medicine in Japan. Curr Stem Cell Rep 1 , 118–128 (2015). https://doi.org/10.1007/s40778-015-0012-6
[32] Harris, R. (2005, May 19). Researchers Report Advance in Stem Cell Production . NPR. https://www.npr.org/2005/05/19/4658967/researchers-report-advance-in-stem-cell-production
[33] Park, S. (2012). South Korea steps up stem-cell work. Nature . https://doi.org/10.1038/nature.2012.10565
[34] Resnik, D. B., Shamoo, A. E., & Krimsky, S. (2006). Fraudulent human embryonic stem cell research in South Korea: lessons learned. Accountability in research , 13 (1), 101–109. https://doi.org/10.1080/08989620600634193 .
[35] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: interviews with researchers from Saudi Arabia. BMC medical ethics, 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6
[36] Association for the Advancement of Blood and Biotherapies. https://www.aabb.org/regulatory-and-advocacy/regulatory-affairs/regulatory-for-cellular-therapies/international-competent-authorities/saudi-arabia
[37] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: Interviews with researchers from Saudi Arabia. BMC medical ethics , 21 (1), 35. https://doi.org/10.1186/s12910-020-00482-6
[38] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: Interviews with researchers from Saudi Arabia. BMC medical ethics , 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6
Culturally, autonomy practices follow a relational autonomy approach based on a paternalistic deontological health care model. The adherence to strict international research policies and religious pillars within the regulatory environment is a great foundation for research ethics. However, there is a need to develop locally targeted ethics approaches for research (as called for in Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: interviews with researchers from Saudi Arabia. BMC medical ethics, 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6), this decision-making approach may help advise a research decision model. For more on the clinical cultural autonomy approaches, see: Alabdullah, Y. Y., Alzaid, E., Alsaad, S., Alamri, T., Alolayan, S. W., Bah, S., & Aljoudi, A. S. (2022). Autonomy and paternalism in Shared decision‐making in a Saudi Arabian tertiary hospital: A cross‐sectional study. Developing World Bioethics , 23 (3), 260–268. https://doi.org/10.1111/dewb.12355 ; Bukhari, A. A. (2017). Universal Principles of Bioethics and Patient Rights in Saudi Arabia (Doctoral dissertation, Duquesne University). https://dsc.duq.edu/etd/124; Ladha, S., Nakshawani, S. A., Alzaidy, A., & Tarab, B. (2023, October 26). Islam and Bioethics: What We All Need to Know . Columbia University School of Professional Studies. https://sps.columbia.edu/events/islam-and-bioethics-what-we-all-need-know
[39] Ababneh, M. A., Al-Azzam, S. I., Alzoubi, K., Rababa’h, A., & Al Demour, S. (2021). Understanding and attitudes of the Jordanian public about clinical research ethics. Research Ethics , 17 (2), 228-241. https://doi.org/10.1177/1747016120966779
[40] Ababneh, M. A., Al-Azzam, S. I., Alzoubi, K., Rababa’h, A., & Al Demour, S. (2021). Understanding and attitudes of the Jordanian public about clinical research ethics. Research Ethics , 17 (2), 228-241. https://doi.org/10.1177/1747016120966779
[41] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[42] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[43] The EU’s definition of autonomy relates to the capacity for creating ideas, moral insight, decisions, and actions without constraint, personal responsibility, and informed consent. However, the EU views autonomy as not completely able to protect individuals and depends on other principles, such as dignity, which “expresses the intrinsic worth and fundamental equality of all human beings.” Rendtorff, J.D., Kemp, P. (2019). Four Ethical Principles in European Bioethics and Biolaw: Autonomy, Dignity, Integrity and Vulnerability. In: Valdés, E., Lecaros, J. (eds) Biolaw and Policy in the Twenty-First Century. International Library of Ethics, Law, and the New Medicine, vol 78. Springer, Cham. https://doi.org/10.1007/978-3-030-05903-3_3
[44] Council of Europe. Convention for the protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine (ETS No. 164) https://www.coe.int/en/web/conventions/full-list?module=treaty-detail&treatynum=164 (forbidding the creation of embryos for research purposes only, and suggests embryos in vitro have protections.); Also see Drabiak-Syed B. K. (2013). New President, New Human Embryonic Stem Cell Research Policy: Comparative International Perspectives and Embryonic Stem Cell Research Laws in France. Biotechnology Law Report , 32 (6), 349–356. https://doi.org/10.1089/blr.2013.9865
[45] Rendtorff, J.D., Kemp, P. (2019). Four Ethical Principles in European Bioethics and Biolaw: Autonomy, Dignity, Integrity and Vulnerability. In: Valdés, E., Lecaros, J. (eds) Biolaw and Policy in the Twenty-First Century. International Library of Ethics, Law, and the New Medicine, vol 78. Springer, Cham. https://doi.org/10.1007/978-3-030-05903-3_3
[46] Tomuschat, C., Currie, D. P., Kommers, D. P., & Kerr, R. (Trans.). (1949, May 23). Basic law for the Federal Republic of Germany. https://www.btg-bestellservice.de/pdf/80201000.pdf
[47] Regulation of Stem Cell Research in Germany . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-germany
[48] Regulation of Stem Cell Research in Finland . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-finland
[49] Regulation of Stem Cell Research in Spain . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-spain
[50] Some sources to consider regarding ethics models or regulatory oversights of other cultures not covered:
Kara MA. Applicability of the principle of respect for autonomy: the perspective of Turkey. J Med Ethics. 2007 Nov;33(11):627-30. doi: 10.1136/jme.2006.017400. PMID: 17971462; PMCID: PMC2598110.
Ugarte, O. N., & Acioly, M. A. (2014). The principle of autonomy in Brazil: one needs to discuss it ... Revista do Colegio Brasileiro de Cirurgioes , 41 (5), 374–377. https://doi.org/10.1590/0100-69912014005013
Bharadwaj, A., & Glasner, P. E. (2012). Local cells, global science: The rise of embryonic stem cell research in India . Routledge.
For further research on specific European countries regarding ethical and regulatory framework, we recommend this database: Regulation of Stem Cell Research in Europe . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-europe
[51] Klitzman, R. (2006). Complications of culture in obtaining informed consent. The American Journal of Bioethics, 6(1), 20–21. https://doi.org/10.1080/15265160500394671 see also: Ekmekci, P. E., & Arda, B. (2017). Interculturalism and Informed Consent: Respecting Cultural Differences without Breaching Human Rights. Cultura (Iasi, Romania) , 14 (2), 159–172.; For why trust is important in research, see also: Gray, B., Hilder, J., Macdonald, L., Tester, R., Dowell, A., & Stubbe, M. (2017). Are research ethics guidelines culturally competent? Research Ethics , 13 (1), 23-41. https://doi.org/10.1177/1747016116650235
[52] The Qur'an (M. Khattab, Trans.). (1965). Al-Mu’minun, 23: 12-14. https://quran.com/23
[53] Lenfest, Y. (2017, December 8). Islam and the beginning of human life . Bill of Health. https://blog.petrieflom.law.harvard.edu/2017/12/08/islam-and-the-beginning-of-human-life/
[54] Aksoy, S. (2005). Making regulations and drawing up legislation in Islamic countries under conditions of uncertainty, with special reference to embryonic stem cell research. Journal of Medical Ethics , 31: 399-403.; see also: Mahmoud, Azza. "Islamic Bioethics: National Regulations and Guidelines of Human Stem Cell Research in the Muslim World." Master's thesis, Chapman University, 2022. https://doi.org/10.36837/ chapman.000386
[55] Rashid, R. (2022). When does Ensoulment occur in the Human Foetus. Journal of the British Islamic Medical Association , 12 (4). ISSN 2634 8071. https://www.jbima.com/wp-content/uploads/2023/01/2-Ethics-3_-Ensoulment_Rafaqat.pdf.
[56] Sivaraman, M. & Noor, S. (2017). Ethics of embryonic stem cell research according to Buddhist, Hindu, Catholic, and Islamic religions: perspective from Malaysia. Asian Biomedicine,8(1) 43-52. https://doi.org/10.5372/1905-7415.0801.260
[57] Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[58] Lecso, P. A. (1991). The Bodhisattva Ideal and Organ Transplantation. Journal of Religion and Health , 30 (1), 35–41. http://www.jstor.org/stable/27510629 ; Bodhisattva, S. (n.d.). The Key of Becoming a Bodhisattva . A Guide to the Bodhisattva Way of Life. http://www.buddhism.org/Sutras/2/BodhisattvaWay.htm
[59] There is no explicit religious reference to when life begins or how to conduct research that interacts with the concept of life. However, these are relevant verses pertaining to how the fetus is viewed. (( King James Bible . (1999). Oxford University Press. (original work published 1769))
Jerimiah 1: 5 “Before I formed thee in the belly I knew thee; and before thou camest forth out of the womb I sanctified thee…”
In prophet Jerimiah’s insight, God set him apart as a person known before childbirth, a theme carried within the Psalm of David.
Psalm 139: 13-14 “…Thou hast covered me in my mother's womb. I will praise thee; for I am fearfully and wonderfully made…”
These verses demonstrate David’s respect for God as an entity that would know of all man’s thoughts and doings even before birth.
[60] It should be noted that abortion is not supported as well.
[61] The Vatican. (1987, February 22). Instruction on Respect for Human Life in Its Origin and on the Dignity of Procreation Replies to Certain Questions of the Day . Congregation For the Doctrine of the Faith. https://www.vatican.va/roman_curia/congregations/cfaith/documents/rc_con_cfaith_doc_19870222_respect-for-human-life_en.html
[62] The Vatican. (2000, August 25). Declaration On the Production and the Scientific and Therapeutic Use of Human Embryonic Stem Cells . Pontifical Academy for Life. https://www.vatican.va/roman_curia/pontifical_academies/acdlife/documents/rc_pa_acdlife_doc_20000824_cellule-staminali_en.html ; Ohara, N. (2003). Ethical Consideration of Experimentation Using Living Human Embryos: The Catholic Church’s Position on Human Embryonic Stem Cell Research and Human Cloning. Department of Obstetrics and Gynecology . Retrieved from https://article.imrpress.com/journal/CEOG/30/2-3/pii/2003018/77-81.pdf.
[63] Smith, G. A. (2022, May 23). Like Americans overall, Catholics vary in their abortion views, with regular mass attenders most opposed . Pew Research Center. https://www.pewresearch.org/short-reads/2022/05/23/like-americans-overall-catholics-vary-in-their-abortion-views-with-regular-mass-attenders-most-opposed/
[64] Rosner, F., & Reichman, E. (2002). Embryonic stem cell research in Jewish law. Journal of halacha and contemporary society , (43), 49–68.; Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[65] Schenker J. G. (2008). The beginning of human life: status of embryo. Perspectives in Halakha (Jewish Religious Law). Journal of assisted reproduction and genetics , 25 (6), 271–276. https://doi.org/10.1007/s10815-008-9221-6
[66] Ruttenberg, D. (2020, May 5). The Torah of Abortion Justice (annotated source sheet) . Sefaria. https://www.sefaria.org/sheets/234926.7?lang=bi&with=all&lang2=en
[67] Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[68] Gert, B. (2007). Common morality: Deciding what to do . Oxford Univ. Press.
[69] World Medical Association (2013). World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA , 310(20), 2191–2194. https://doi.org/10.1001/jama.2013.281053 Declaration of Helsinki – WMA – The World Medical Association .; see also: National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. (1979). The Belmont report: Ethical principles and guidelines for the protection of human subjects of research . U.S. Department of Health and Human Services. https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html
[70] Zakarin Safier, L., Gumer, A., Kline, M., Egli, D., & Sauer, M. V. (2018). Compensating human subjects providing oocytes for stem cell research: 9-year experience and outcomes. Journal of assisted reproduction and genetics , 35 (7), 1219–1225. https://doi.org/10.1007/s10815-018-1171-z https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6063839/ see also: Riordan, N. H., & Paz Rodríguez, J. (2021). Addressing concerns regarding associated costs, transparency, and integrity of research in recent stem cell trial. Stem Cells Translational Medicine , 10 (12), 1715–1716. https://doi.org/10.1002/sctm.21-0234
[71] Klitzman, R., & Sauer, M. V. (2009). Payment of egg donors in stem cell research in the USA. Reproductive biomedicine online , 18 (5), 603–608. https://doi.org/10.1016/s1472-6483(10)60002-8
[72] Krosin, M. T., Klitzman, R., Levin, B., Cheng, J., & Ranney, M. L. (2006). Problems in comprehension of informed consent in rural and peri-urban Mali, West Africa. Clinical trials (London, England) , 3 (3), 306–313. https://doi.org/10.1191/1740774506cn150oa
[73] Veatch, Robert M. Hippocratic, Religious, and Secular Medical Ethics: The Points of Conflict . Georgetown University Press, 2012.
[74] Msoroka, M. S., & Amundsen, D. (2018). One size fits not quite all: Universal research ethics with diversity. Research Ethics , 14 (3), 1-17. https://doi.org/10.1177/1747016117739939
[75] Pirzada, N. (2022). The Expansion of Turkey’s Medical Tourism Industry. Voices in Bioethics , 8 . https://doi.org/10.52214/vib.v8i.9894
[76] Stem Cell Tourism: False Hope for Real Money . Harvard Stem Cell Institute (HSCI). (2023). https://hsci.harvard.edu/stem-cell-tourism , See also: Bissassar, M. (2017). Transnational Stem Cell Tourism: An ethical analysis. Voices in Bioethics , 3 . https://doi.org/10.7916/vib.v3i.6027
[77] Song, P. (2011) The proliferation of stem cell therapies in post-Mao China: problematizing ethical regulation, New Genetics and Society , 30:2, 141-153, DOI: 10.1080/14636778.2011.574375
[78] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[79] International Society for Stem Cell Research. (2024). Standards in stem cell research . International Society for Stem Cell Research. https://www.isscr.org/guidelines/5-standards-in-stem-cell-research
[80] Benjamin, R. (2013). People’s science bodies and rights on the Stem Cell Frontier . Stanford University Press.
Mifrah Hayath
SM Candidate Harvard Medical School, MS Biotechnology Johns Hopkins University
Olivia Bowers
MS Bioethics Columbia University (Disclosure: affiliated with Voices in Bioethics)
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License .
share this!
May 30, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
Stem cell research reveals new clues to tissue repair that could help heal the uterus and more
by Isabella Backman, Yale University
Stem cells play a vital role in repairing damaged tissue, whether it's a scraped knee or a scarred uterus following pregnancy. New stem cell research has identified the molecules that the cells produce to promote the healing process.
The finding could pave the way for the development of new, more effective drugs for injuries or various diseases, including conditions related to reproductive health such as Asherman syndrome, a gynecologic condition in which the uterus scars and becomes fibrotic.
Scientists believed in the past that stem cells served as backup cells that repaired tissues by differentiating into new cells that repopulated the site of injury. Now, they have learned that it is rare for stem cells to completely replace injured tissue. But they still don't fully understand how the cells are able to help damaged areas regenerate.
In the uterus, stem cells play a number of roles, including helping it to expand during pregnancy and to regenerate and repair after childbirth. This new study identified several microRNAs (miRNAs) secreted by the stem cells that helped drive the growth and proliferation of cells in uterine tissue. The researchers published their findings in Stem Cell Research & Therapy on May 1.
"We found the molecules that stem cells make to help heal and repair tissue, and we hope that understanding this will be potentially useful as a medication in the future," says Hugh Taylor, MD, chair and Anita O'Keeffe Young Professor of Obstetrics, Gynecology & Reproductive Sciences at Yale School of Medicine and the study's principal investigator.
Stem cells secrete miRNAs that support cell proliferation
Exosomes are extracellular vesicles, which contain various bioactive molecules and allow cells to communicate with one another. In their new study, Taylor's team isolated exosomes secreted by stem cells from human bone marrow. They then used RNA sequencing to characterize all of the miRNA contained in the vesicles and identified those that were most abundant. Then researchers took the most prominent miRNAs and introduced them into human uterine tissue.
The team found that the miRNAs significantly increased the growth and proliferation of the uterine cells. They also studied their effect on the cells' decidualization in the endometrium. (Decidualization is the differentiation process uterine cells undergo that prepares the uterus to support an embryo.) The study showed that the miRNAs blocked decidualization.
"In a uterus, once a cell becomes differentiated to support pregnancy, it can no longer repair and regenerate. It's permanently locked in that state and often is shed through menstruation later on," says Taylor. "By blocking this process, it allows the cells to focus on proliferating and turns on these reparative processes."
Turning miRNAs into drugs for tissue repair
The study offers insight into how stem cells promote reparative processes without replacing the tissue itself. Taylor hopes that as researchers continue to gain a greater understanding about how miRNAs work, they could one day be used as drugs for repairing various damaged tissue.
Asherman syndrome, for example, typically occurs after pregnancy, when the supply of stem cells may not be adequate to help the organ heal properly, which can hinder fertility in the future.
"The idea is that these miRNAs could be used as a medication that is much more readily available and practical," says Taylor. "We could potentially deliver them to help prepare the uterus in the critical window when it is damaged and may be vulnerable."
The finding could also have significance beyond the uterus. In future stem cell research , Taylor's team plans to study how miRNAs respond to other types of traumatic tissue injury in animal models.
"We studied the uterus , but the implications are beyond reproduction, potentially including many other conditions where stem cells are involved in repair and regeneration, whether that's injury due to trauma or degenerative diseases ," says Taylor.
Provided by Yale University
Explore further
Feedback to editors

Others' words, not firsthand experience, shape scientific and religious belief formation, study finds
15 minutes ago

Earliest cattle herds in northern Europe found in the Netherlands

Real-time flood risk visualization via server-based mixed reality enhances accessibility and public safety
29 minutes ago

Rocky shores of Pacific Northwest show low resilience to changes in climate
41 minutes ago
Researchers discover disordered clock protein that sheds new light on circadian rhythms
47 minutes ago

Observing ultrafast photoinduced dynamics in a halogen-bonded supramolecular system
49 minutes ago

Study shows climate change boosts olive tree-devouring bacteria in the Mediterranean

How can we make good decisions by observing others? A videogame and computational model have the answer

New method for safe and efficient cell transfection developed by researchers
Do we have more empathy for people who are similar to us? New research suggests it's not that simple
Relevant physicsforums posts, a dna animation.
May 29, 2024
Probability, genetic disorder related
May 28, 2024
Looking For Today's DNA Knowledge
May 27, 2024
Covid Vaccines Reducing Infections
Human sperm, egg cells mass-generated using ips, and now, here comes covid-19 version ba.2, ba.4, ba.5,....
May 25, 2024
More from Biology and Medical
Related Stories
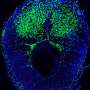
Bone marrow may be the missing piece of the fertility puzzle
Sep 12, 2019

Stem cells 'migrate' to repair damaged lung cells, study shows
Feb 22, 2024
Researchers identify stem cell source of key process in female reproduction
May 29, 2019

Scientists identify G-Exos as a nanocarrier for miRNA transfer to stimulate neural differentiation of stem cells
Oct 28, 2021
Study reveals that dental pulp stem cells and their products could help regenerate peripheral nerves and more
Oct 26, 2023
Discovery of how stem cell niche guides differentiation into functional cells is a significant step towards therapies
Aug 22, 2023
Recommended for you

Scientists push single-molecule DNA sequencing to the next level
Molecular stop signal identified: The surveillance system of cell division
2 hours ago

How symbiotic bacteria adapt to big environmental changes

Scientists develop new method to match genes to their molecular 'switches'
21 hours ago
Study identifies fungus that breaks down ocean plastic
Jun 3, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Aerobic glycolysis of vascular endothelial cells: a novel perspective in cancer therapy
- Open access
- Published: 01 June 2024
- Volume 51 , article number 717 , ( 2024 )
Cite this article
You have full access to this open access article

- Shenhao Xu 1 na1 ,
- Jiahao Liao 1 na1 ,
- Bing Liu 1 na1 ,
- Cheng Zhang 1 &
6 Altmetric
Explore all metrics
Vascular endothelial cells (ECs) are monolayers of cells arranged in the inner walls of blood vessels. Under normal physiological conditions, ECs play an essential role in angiogenesis, homeostasis and immune response. Emerging evidence suggests that abnormalities in EC metabolism, especially aerobic glycolysis, are associated with the initiation and progression of various diseases, including multiple cancers. In this review, we discuss the differences in aerobic glycolysis of vascular ECs under normal and pathological conditions, focusing on the recent research progress of aerobic glycolysis in tumor vascular ECs and potential strategies for cancer therapy.
Similar content being viewed by others
Endothelial cell metabolism: parallels and divergences with cancer cell metabolism

The Implication of Anti-angiogenic Treatment of Malignancies on Human Metabolism

Endothelial Cells (ECs) Metabolism: A Valuable Piece to Disentangle Cancer Biology
Avoid common mistakes on your manuscript.
Introduction
Endothelial cells (ECs) form a monolayer of cells arranged along the inner lining of blood vessels. Under normal physiological conditions, ECs play a pivotal role in maintaining oxygen and nutrient supply to all bodily tissues [ 1 ]. ECs are critical for upholding the internal environment, its homeostasis, and immune function. In recent years, research on EC metabolism has gained significant momentum. A growing body of evidence has shown that EC metabolism undergoes significant alterations under various pathological conditions, profoundly impacting disease onset and progression. As an crucial link in cellular energy metabolism, aerobic glycolysis plays a remarkable role in EC activities and could offer novel targets for disease treatment. This paper aims to explore recent advances in glycolysis in ECs under normal and pathological conditions, with a particular emphasis on human cancers.
The function of ECs in physiological state
Angiogenesis.
ECs are typically quiescent in healthy state but can be rapidly activated in response to pathological changes, facilitating the delivery of oxygen and nutrients to hypoxic tissues through the formation of new blood vessels, a process known as angiogenesis.
Angiogenesis is accomplished through the interaction among three distinct EC subtypes: tip cells, stalk cells, and phalanx cells, each fulfilling a specific role in the process. Neovascularization commences with the migration of tip cells, succeeded by the proliferation of stalk cells, which generate new vascular sprouts. Subsequently, phalanx cells, characterized by a nonproliferating phenotype, continue to align established vessels, facilitating the formation of mature vessels with the functions of regulating vascular homeostasis and establishing endothelial barrier [ 2 ].
The key cytokine that dynamically regulates the differentiation of ECs into one of these subtypes is vascular endothelial growth factor (VEGF), which binds to VEGF receptor 2 (VEGFR2) on ECs and induces their differentiation into tip cells. Tip cells express delta-like ligand 4 (DLL4), which binds to Notch receptors on neighboring ECs, initiating their differentiation into stalk cells. In stalk cells, DLL4 signaling induces cleavage of the Notch intracellular structural domain (NICD), which then induces the cell to stimulating the expression of produce VEGF receptor 1 (VEGFR1). VEGFR1 is substantially less sensitive to VEGF compared to VEGFR2, further ensuring that ECs adjacent to tip cells become stalk cells [ 3 ].
This seemingly strict interaction between tip and stalk cells is actually dynamic, and this dynamic competition ensures that cells with the highest VEGFR2/VEGFR1 ratio become tip cells [ 4 ].
Maintaining internal environmental and its homeostasis
ECs serve as crucial intermediaries between human blood circulation and organ tissues, playing an indispensable role in the regulation of body functions. These functions include maintaining coagulation, regulating blood pressure, and facilitating the exchange of substances both within and outside of blood vessels, with particular significance in the regulation of coagulation. For instance, ECs regulate blood pressure and maintain the balance between coagulation and anticoagulation in blood flow by releasing vasodilatory substances such as prostaglandin I2 (PGI2) and nitric oxide (NO), as well as vasoconstrictive substances like endothelin 1 (ET-1) and angiotensin II (AngII) [ 5 ]. Moreover, ECs can produce nitric oxide synthase (NOS) in response to hormonal and chemical signal stimulation, and NO synthesized by endothelial NOS (eNOS) regulates peripheral vascular tone by stimulating NO-sensitive guanylate cyclase, which plays a vital role in maintaining vascular homeostasis and controlling blood pressure [ 6 ].
Immune response
As the type of cells that are in direct contact with the circulation, ECs are pivotal participants and regulators of the inflammatory response. Their recognition and response to substances like invading circulating microorganisms are crucial for activating the body’s early immune system [ 7 ]. For instance, ECs express Nod1 to trigger the release of the inflammatory factor IL-8 [ 8 ]. There is also an inextricable link between the inflammatory response and the angiogenic process. In chronic inflammation, ECs can respond to angiogenic factors like VEGFA to sustain inflammatory angiogenesis [ 7 ].
EC Metabolism under physiological conditions
Glucose, fatty acids (FA) and amino acids (AA) are the three primary substrates for adenosine triphosphate (ATP) and biomass production in ECs, and their roles have been extensively studied and summarized. The following section will summarize the role of EC metabolism in maintaining endothelial function and disease pathogenesis, with a specific focus on glycolysis in tumor vascular ECs.
The glycolysis and bypasses of ECs
Under physiological conditions, glycolysis serves as the primary mode of energy production in ECs, exhibiting a higher proportion compared to other cells in the body. Similar to many cancer cells, up to 85% of ATP is produced by glycolysis in ECs. This phenomenon can be attributed to two possible reasons. Firstly, ECs protect themselves from oxidative stress by keeping reactive oxygen species (ROS) at a low level. Secondly, ECs would like to increase the oxygen supply to their surrounding tissues by means of anaerobic metabolism [ 9 ]. It has been demonstrated that when exposed to a low glucose environment or when glucose is competed by 2-deoxy-d-glucose (2-DG), a structural analogue of glucose, for the glycolytic process, ECs trigger ROS-induced autophagy, suggesting that ECs reduce oxygen dependence by preferentially utilizing glycolytic capacity, and thereby decrease ROS production [ 9 , 10 ]. Additionally, the angiogenesis process in hypoxic tissues demands a faster rate of ATP production through glycolysis to expedite the re-establishment of blood circulation at hypoxic sites. This necessity also contributes to the high glycolytic rate observed in ECs.
In the process of angiogenesis, the initiation of the glycolytic pathway first involves the uptake of glucose into ECs via the membrane-bound glucose transporter protein (GLUT) [ 11 ]. The glucose-based glycolytic pathway is regulated by three key enzymes: phosphofructokinase-1 (PFK1), hexokinase 2 (HK2) and pyruvate kinase (PK).
PFK1 is an important rate-limiting enzyme in the glycolytic pathway, converting fructose-6-phosphate (F6P) into fructose-1,6-bisphosphate (F1,6P2). 6-phosphofructo-2-kinase/fructose-2,6bisphosphatase3 (PFKFB3), an efficient glycolytic activator, can variably activate PFK1 by producing fructose-2,6-bisphosphate (F2,6P2). PFKFB3 not only regulates the proliferation of ECs but also controls the formation and directional migration of filopodium and lamellipodium. Therefore, the deletion of PFKFB3 impairs the angiogenic function of ECs [ 12 ]. In contrast, overexpression of PFKFB3 leads to elevatedglycolysis levels in ECs exhibiting a pro-tip cell phenotype. It even inhibits Notch1 signaling in pro-stalk cells during retinal vascular development, further underscoring the importance of cellular glycolysis levels in ECs in determining the tip cell phenotype [ 12 ]. As previously previously, VEGF operates through the Notch signaling pathway to ensure that cells with the highest VEGFR2/VEGFR1 ratio become the tip cells. Similarly, it is reported that stalk cells can display higher glycolytic activity when expressing higher levels of PFKFB3, effectively taking the place of tip cells [ 12 ].
HK2 is another glycolytic rate-limiting enzyme responsible for catalyzing the initial step of glycolysis in ECs, which involves the phosphorylation of glucose in ECs to glucose-6-phosphate (G6P). Increasing evidence suggests that HK2 is upregulated in various types of tumors, correlating with enhanced aerobic glycolysis.The upregulation of HK2 expression can be attributed to fibroblast growth factor (FGF), which enhances the expression of MYC, one of the most frequently dysregulated driver genes in human cancers. Although MYC was previously deemed “undruggable” due to the lack of a suitable pocket for high-affinity binding of low-molecular-weight inhibitors, recent preclinical trials have shown significant anticancer effects of some MYC-targeted inhibitors. This suggests that such anticancer effects may also arise from the inhibition of aerobic glycolysis in tumor vascular ECs [ 13 ]. For example, it has recently been found that OTUB1 blocks MYC protein degradation through deubiquitination, thereby promoting HK2-mediated glycolysis and the development of breast cancer [ 14 ].
PK is also a glycolytic rate-limiting enzyme that converts phosphoenolpyruvate (PEP) to pyruvate. PKM2, one of the four tissue-specific isoforms of PK, exists as a dimer or tetramer in normal, malignant, and embryonic cells. The affinity of dimeric PKM2 for PEP is lower than that of tetrameric PKM2, resulting in less conversion to pyruvate, which inhibits glycolysis and leads glucose more into the glycolytic bypass for biomass synthesis. In highly proliferative endothelial cells, PKM2 tends to shift towards its dimeric form to promote biomass synthesis, aligning with their heightened proliferative properties [ 15 , 16 , 17 ]. As a glycolytic rate-limiting enzyme, silencing PKM2 in ECs inhibits neovascularization. Furthermore, Protein JMJD8 has been identified as associated with PKM2 to regulate the angiogenic process. Knocking down JMJD8 inhibits EC metabolism and angiogenesis, although the exact mechanism by which JMJD8 regulates PKM2 remains uncertain [ 18 ].
Several environmental factors and signaling proteins have been identified that can influence the glycolytic level and angiogenic capacity of ECs by regulating the three key enzymes mentioned above and their regulators. For example, mechanical signaling in the circulation can also affect angiogenesis in a metabolism-dependent manner, as evidenced by the activation of Krüppel-like factor 2 (KLF2) in response to laminar shear stress, which inhibits PFKFB3, HK2, and several other glycolytic genes [ 19 ]. The FOXO family is highly enriched in ECs. The transcription factor FOXO1 was found to inhibit glycolysis in ECs by suppressing MYC and PFKFB3 levels [ 20 , 21 ]. Additionally, FOXO1 exhibits environment-dependent activity, promoting the germination and migration of lymphatic ECs by upregulating the purinergic receptor P2RY1 upon exposure to ATP [ 20 ]. Meanwhile, impairment of vascular growth and germination function was also observed after silencing PFKFB3 and HK2 in animal models, further suggesting the essential role of glycolysisl for ECs and angiogenesis in vivo [ 12 , 22 ]. Furthermore, the availability of sufficient glucose in the microenvironment for uptake plays a decisive role in the level of glycolysis of ECs. Glycogen synthesis in ECs increases with sufficient glucose supply, leading to accumulation of intracellular glycogen, while deprivation of glucose causes the inhibition of glycogen phosphorylase (GP), which catalyzes glycogenolysis in ECs, impairing the ability of ECs to migrate and survive [ 23 ]. This evidence suggests that ECs utilize glycogen as reserve energy when carrying out angiogenic pro-cesses in a glucose-deficient environment.
The pentose phosphate pathway (PPP) is a bypass of glycolysis that facilitates the synthesis of raw nucleotide material and the homeostasis of redox balance by diverting G6P generated from glucose to produce nicotinamide adenine dinucleotide phosphate (NADPH) and ribose 5-phosphate (R5P). NADPH, in turn, can regenerate a common cellular antioxidant, reduced glutathione [ 24 , 25 , 26 ]. Additionally, NADPH also participates in fatty acid and NO production. The NADPH generated by the pentose phosphate pathway is directly involved in fatty acid synthesis, thus linking the PPP of ECs to fatty acid metabolism. Moreover, NO promotes the angiogenic activity of ECs [ 27 ]. Glucose 6-phosphate dehydrogenase (G6PD) is one of the rate-limiting enzymes of PPP. Overexpression of G6PD stimulates EC proliferation and migration by boosting NO and NADPH production. Another rate-limiting enzyme of PPP in the reversible non-oxidative pathway is transketolase. Inhibition of either of these two rate-limiting enzymes has been shown to significantly suppress EC survival [ 23 , 28 ].
Another bypass of glycolysis is known as the hexosamine biosynthetic pathway (HBP). Although it contributes to a relatively small proportion of glucose metabolism, HBP is closely linked to post-translational protein modifications via glycosylation, including N-glycosylation and O-glycosylation. .N-glycosylation enhances the stability, membrane expression, and signaling activity of VEGFR2, while O-glycosylation influences interactions related to NOTCH signaling ligands. Consequently, HBP is implicated in determining the apical cell phenotype of ECs, underscoring its unique role in EC functions [ 29 , 30 , 31 , 32 ]. Further investigations are warranted to elucidate the mechanisms by which HBP impacts angiogenesis.
Other metabolic pathways of ECs
Fa metabolism of ecs.
Generally, mitochondria are the primary site of ATP production, and acetyl coenzyme A derived from fatty acids FA is utilized in the tricarboxylic acid (TCA) cycle to main-tain ATP production. While in proliferating endothelial cells (PECs), the main function of mitochondria is biosynthesis. The dependence of dNTP synthesis in PECs on the carbon source in fatty acids is now well established. FA is metabolized to acetyl coenzyme A to maintain the TCA cycle. In addition to the production of ATP, the TCA cycle provides precursors for the synthesis of dNTP necessary for proliferation, thereby promoting DNA synthesis and thus aiding PECs proliferation [ 33 ]. Furthermore, surprisingly, quiescent endothelial cells (QECs) regenerate NADPH for redox homeostasis by enhancing FAO levels [ 34 ].
Carnitine palmitoyltransferase 1a (CPT1a) is a rate-limiting enzyme in the FA metabolic pathway that transfers FA into mitochondria. It has been discovered that CPT1a deficiency not only leads to attenuated proliferation of PECs and defective neovascularization in vitro and in vivo, but also promotes QECs dysfunction by increasing endothelial oxidative stress. Supplementation with acetyl coenzyme A precursors, which promote the tricarboxylic acid (TCA) cycle, restored cellular dNTP levels and alleviated oxidative stress-induced EC dysfunction in CPT1a-deficient ECs. Neither glucose nor glutamine metabolism could compensate for this metabolic defect of Fatty acid oxidation (FAO) [ 33 , 34 ]. These findings indicates that ECs primarily utilize fatty acid-derived carbon for biosynthesis rather than energy generation through FA metabolism. This metabolic pathway supports cell proliferation during angiogenesis and facilitates the regeneration of NADPH to maintain cellular redox balance.
Glutamine metabolism of ECs
Glutamine, the most abundant non-essential amino acid (NEAA) in circulation, plays a crucial role in maintaining proliferation and vasodilation in ECs through its metabolism. In ECs, approximately 30% of the carbon source for the TCA cycle is derived from glutamine, a proportion comparable to glycolysis and FAO-derived carbon. Glutaminase 1 (GLS1) replenishes glutamine as a carbon source for the TCA cycle, supporting protein and nucleotide synthesis. Additionally, maintaining the dynamic redox balance requires glutathione (GSH), a reducing agent produced from glutamine. Thus, glutamine depletion not only impairs biomass synthesis in ECs but also renders them more susceptible to damage induced by ROS [ 35 ]. Silencing GLS1 leads to a reduced tendency for ECs to differentiate into a tip cell phenotype [ 36 ]. However, EC-specific deletion of glutamine synthetase (GS), the enzyme that converts glutamate and ammonia to glutamine, markedly impairs EC migration but not proliferation [ 37 ]. These findings suggest that glutamine metabolism plays a vital role in regulating the phenotype of ECs during vascular sprouting.
In summary, various metabolic pathways may have significant impacts on the for-mation of different subtypes of ECs. Multiple studies have shown that The level of glycolysis mainly affects the ability of ECs to differentiate towards tip cells, while FA metabolism is closely related to the proliferation of stalk cells. Additionally, glutamine metabolism differentially affects the phenotype of ECs under different conditions.

Glucose metabolism characteristics of tumor endothelial cells
Hypoxia of the tumor microenvironment.
Hypoxia is one of the characteristics of the tumor microenvironment where neovascularization is usually exposed to relatively low oxygen, and tumor endothelial cells (TECs) need to adjust their metabolism to adapt to the hypoxic tumor environment [ 38 , 39 ]. In general, cancer cells are characterized by their high metabolism, thus stimulating angiogenesis to supply oxygen and nutrients; however, unlike normal vascular endothelium, blood vessels in tumors are not only disorganized due to abnormal development but also have structural and functional defects due to the lack of tissue hierarchy of normal vascular beds [ 1 ]. As a result, the tumor has uneven gaps between vascular endothelial cells, and poor perfusion makes the tumor hypoxic, thereby promoting metastatic escape of cancer cells [ 40 , 41 ]. In turn, the hypoxic environment stimulates EC proliferation and angiogenesis [ 42 , 43 ], which creates a vicious cycle of hypoxia and tumor development.
The effects of hypoxia on cancer cell metabolism have become clearer. Briefly, restricted and intermittent blood supply in tumor tissues leads to periodic hypoxia and re-oxygenation, resulting in oxidative stress and hypoxia in the tumor microenvironment. Hypoxic cancer cell metabolism under hypoxic conditions upregulates the expression of genes that promote glycolysis, such as GLUT1, GLUT3, PFKFB3, and HK1-3, while it decreases FAO, promotes FA synthesis, and decreases glutamine carboxylation; all of which contribute to increased glycolytic flux and FA synthesis while downregulating aerobic respiration in hypoxic cancer cells. However, the role of hypoxia and HIF signaling in EC metabolism is still not fully elucidated [ 44 ].
Classification of hypoxic conditions has identified acute hypoxia as selective splicing and upregulation of genes involved in pyruvate metabolism and glucose transport [ 45 ]. In contrast, the effect of chronic hypoxia on EC metabolism involves the regulation of the activity of multiple metabolic pathways, such as glycolysis, amino acid biosynthesis, carbon metabolism, PPP, and cysteine/methionine metabolism. However, this chronic hypoxia may be different from the intermittent hypoxic conditions of the tumor hypoxic response described previously. Therefore, the changes in EC metabolism observed during chronic hypoxia need to be confirmed in an intermittent hypoxic environment.
Hypoxia and HIF signaling in angiogenic function of TECs
The prolyl hydroxylase family (PHD) hydroxylates the alpha subunit of HIF. The enzymatic activity of PHD requires oxygen for activation, and the hydroxylated HIF alpha subunit is targeted by the von Hippel‒Lindau (VHL) factor and undergoes ubiquitination and proteasomal degradation [ 46 ]. In a hypoxic environment, the enzymatic activity of PHD is inhibited, and VHL factors are unable to degrade HIF-α, leading to the activation of HIF-related pathways and functions, including the induction of angiogenesis, glucose metabolism, and tumor growth, invasion, and metastasis [ 43 ]. Among the HIFα subunit family, HIF-1α remains in a stable state during acute hypoxia and is the major subunit that induces glycolysis and other metabolic changes [ 47 ].
By focusing on HIF signaling in the study of the tumor hypoxic microenvironment, it has been found that HIF directly induces an increase in VEGF levels in ECs [ 48 ], VEGF contains a hypoxia response element (HRE) that can be activated in response to reduced oxygen, causing hypoxic tissues and macrophages to express VEGF and stimulate angiogenesis to restore oxygen and nutrient supply to hypoxic regions. VEGF directly promotes angiogenesis while inducing subsequent activation of other pro-angiogenic growth factors, such as placental growth factor (PlGF) and FGF [ 49 ]. Thus, the induction of angiogenesis by hypoxia and HIF is largely dependent on VEGF. Hypoxia and HIF signaling affect EC function and angiogenesis in multiple ways. First, hypoxia induces transcriptional activation of multiple angiogenic factors, including VEGF, PlGF, and angiopoietin (ANGPT1 and ANGPT2) [ 50 ]. These vascular growth factors modulate proangiogenic chemokines and receptors that induce EC stem cells to migrate to angiogenic sites [ 51 ]. In addition, hypoxia stabilizes these vascular growth factors and promotes PFKFB3 expression, thereby increasing glycolytic flux in ECs [ 12 ]. Hypoxia also directly stimulates EC proliferation and neovascular germination as well as promotes neovascular stabilization by remodeling the extracellular matrix [ 48 , 52 , 53 , 54 ]. In addition, hypoxia and HIF signaling also modulate vascular function by increasing the production and release of NO to promote vasodilation [ 55 ].
Stabilization of HIF-1α and HIF-2α has different regulatory effects on angiogenesis and normalization of blood vessels. Specifically, HIF1α deficiency in the tumor endothelium reduces the number of tumor vessels, which reduces tumor growth but increases tumor necrosis; roxarsenical (Rox), an organoarsenic compound, has a significant pro-tumor effect in vivo due to its ability to increase HIF-1α [ 56 , 57 ]. In contrast, the lack of tumor endothelial HIF2α leads to reduced vascular integrity in tumor models, increasing the likelihood of tumor metastasis but reducing tumor growth [ 58 , 59 ]. Functional and structural damage to the tumor vasculature increases the likelihood of metastasis, and poor local blood supply also hinders the full efficacy of chemotherapy and immunotherapy. Defective PHD2 mediates the stabilization of HIF2α in ECs, normalizes TECs, reverses tumor hypoxia, and reduces cancer cell metastasis [ 60 ]. Here, we draw a schematic diagram to summarize this chapter. (Fig. 1 ). Thus, targeting the PHD2-HIF2α signaling pathway may be a future tumor treatment strategy based on EC metabolism with antiangiogenesis as the goal.
Glycolytic characteristics of TECs
Endothelial cells in tumor vasculature have higher glycolytic flux to produce ATP compared to normal endothelial cells, and single-cell RNA sequencing has shown that the glycolytic flux of TECs is 2–4 times higher than that of normal ECs [ 61 ]. TECs exhibit a highly glycolytic cellular phenotype, such as enhanced expression of the GLUT1 glucose transporter and the PFKFB3 glycolytic activator [ 62 , 63 ], which may be attributed to the hypoxia-dependent altered expression of glycolytic enzymes and increased secretion of proangiogenic growth factors, such as VEGF. In response to the high glycolytic phenotype of TECs, the hypoxic tumor microenvironment (TME) and the stimulation of inflammatory factors may be critical for the upregulation of PKFBF3 expression [ 63 ]. Reducing the glycolysis of tumor endothelial cells by inhibiting the function of PFKFB3 inhibits their proliferation and thus normalizes the tumor vasculature as evidenced by regular TEC alignment, a dense tumor vascular barrier, and unobstructed blood perfusion. The aim of this therapeutic strategy is not to completely remove the glycolytic flux, which would lead to endothelial cell death and tumor vascular disintegration, but rather to reduce the glycolytic level to that of normal endothelial cells, inhibit their metastasis, and prevent the development of advanced cancer [ 64 , 65 ]. In addition, the pentose phosphate and serine biosynthetic pathways for biosynthesis are highly activated in tumor endothelial cells compared to healthy endothelial cells [ 63 ].
The expression of PKM2, one of the key enzymes of the glycolytic pathway, is traditionally thought to be associated with the proliferation of endothelial cells, especially TECs; however, it has been shown that PKM2 is not required for EC proliferation as PKM2-deficient ECs do not significantly differ from controls in vitro or in vivo, and cell cycle arrest in the absence of PKM2 may be driven by compensatory PKM1 upregulation [ 17 ]. PKM2 accumulates at the junctions of VE-cadherin-expressing endothelial cells and the area near F-actin-rich filopodium and lamellipodium, and VE-cadherin is a major regulator of EC junction formation and stability. After knockdown of PKM2, ECs exhibit unstable intercellular junctions and decreased migration distance, demonstrating that the migration ability of ECs and intercellular junction ability are related to the expression of PKM2, which is closely related to the migration ability of ECs and intercellular connectivity. Although the importance of the glycolytic pathway in maintaining EC function and normalizing TECs is well established, only the PFKBF3 glycolytic enzyme has been shown to regulate inter-EC connections by some unknown mechanism [ 63 ]. In addition to glycolytic enzymes, extracellular vesicles (H-EVs) have recently been found to promote adhesion of cancer cells to endothelial cells in triple-negative breast cancer, and Circulating galectin-3(cirGal-3) enhances this proadhesive effect by a mechanism that may be related to cirGal-3-induced increased expression of ICAM-1, leading to upregulation of glycolysis in endothelial cells [ 66 ]. This special function of PKM2 in maintaining the integrity of the endothelial barrier during migration and thus reducing tumor metastasis provides a new therapeutic strategy for antitumor treatment, namely, targeting PKM2 to inhibit tumor metastasis [ 67 ].
As mentioned previously, the PPP is one of the bypasses of glycolysis and achieves redox homeostasis in ECs by synthesizing NADPH, and G6PD is an important rate-limiting enzyme for the PPP. Due to the hypoxic conditions in tumors, high levels of reactive oxygen species (ROS) in the TME stimulate endoplasmic reticulum (ER) stress due to insufficient reduction flux generated by the pentose phosphate pathway (PPP), which activates the IRE-1 and PERK signaling pathways, thereby increasing autophagy, while in turn, the PPP can also play a role in autophagy regulation due to changes in G6PD activity [ 68 ]. ROS play a role in cancer suppression, but it has been shown that autophagy promotes tumor angiogenesis by activating the JAK2/STAT3 pathway and targeting VEGF; in addition, autophagy regulates the ability of cancer stem cells (CSCs) to differentiate into TECs [ 69 , 70 , 71 , 72 ].
Although the glycolytic processes in TEC proliferation and tumor angiogenesis are important, it has recently been reported that microvascular endothelial cells may not be glucose-dependent in their early growth phase as they exhibit good growth capacity even in a glucose-deficient environment. Evidence suggests that glutamine metabolism plays a more important role in the early growth of microvascular endothelial cells [ 73 ]. This phenomenon suggests that the metabolism of TECs may be different from one species to another, and tumor treatment strategies targeting TEC metabolism may need to change accordingly with their different metabolic profiles.
Lactic acid metabolism
In addition to having a direct effect on ECs, the hypoxic microenvironment of tumors can also lead to EC uptake of lactate through lactate accumulation, and the effect of lactate on TECs varies. First, TECs increase the expression of lactate dehydrogenase B, which converts lactate taken up by TECs into pyruvate to enter the TCA cycle to promote biosynthesis and energy supply, thus promoting TEC proliferation and angiogenesis. Second, lactate uptake by ECs induces ROS-mediated activation of the NF-kappaB/IL-8 pathway, promoting angiogenesis [ 74 ]. In addition, lactate directly regulates tyrosine kinase receptors in ECs and stabilizes N-Myc downstream regulatory gene 3 (NDRG3), thus promoting angiogenesis under hypoxic conditions [ 75 , 76 ]. Finally, lactate, as a signaling molecule, also enhances angiogenesis by activating the HIF-1a and PI3K/AKT pathways, thereby promoting angiogenesis [ 75 , 77 , 78 ]. Overall, lactate independently stimulates angiogenesis, and lactate accumulation is associated with disease progression in tumors.
Although the acidic environment due to high lactic acid is not conducive to cell survival, TECs rapidly proliferate in a high lactic acid environment, allowing tumor angiogenesis and survival. The reason for this phenomenon is that TECs upregulate the expression of carbonic anhydrase 2 (CAII). Knockdown of CAII reduces the survival of TECs under lactic acidosis and nutrient-adequate conditions [ 79 ]. In normal ECs, vascular endothelial growth factor A (VEGFA) induces CAII expression, which is an indirect proangiogenic mechanism of VEGFA. Interestingly, the carbonic anhydrase inhibitor, acetazolamide, does not significantly reduce tumor angiogenesis but instead promotes vascular maturation in tumors, thereby reducing metastasis [ 79 ].
Monocarboxylate transporter (MCT) protein, as a lactate transporter protein, is responsible for transporting lactate inside and outside the cell. MCT4 is the predominant regulator of lactate transport in tumor cells and endothelial cells, which are high glycolytic donors; MCT4 promotes EC migration as well as tumor proliferation and invasion under tumor cell and EC coculture conditions, while the MCT-specific blocker, 7ACC1, reverses the tumor-promoting effect of MCT. However, these findings have only been demonstrated in vitro [ 80 ].
Interactions between different cell types in TME
Based on recent studies, we understand that the level of glycolysis in TECs is inter-cellularly regulated by other cell types in the tumor microenvironment as demonstrated by a 2016 report on tumor-associated macrophages (TAMs) affecting EC glycolytic function. Hypoxia upregulates the expression of regulated in development and DNA damage responses-1 (REDD1), which is an inhibitor of mTOR activation. Knockdown of REDD1 in mice results in an mTOR-dependent enhancement of glycolysis levels in TAMs, and competition between TAMs and TECs for extracellular glucose results in a reduction in glycolytic flux in TECs, indirectly promoting normalization of tumor vasculature [ 81 ]. Because HIF1α deficiency reduces CXCL1-mediated macrophage recruitment, hypoxia in TECs affects TAM behavior and recruitment, thus altering the interaction between TAMs and TECs in the tumor microenvironment [ 82 ].
Cancer associated fibroblasts (CAFs) in the TME maintain a relatively high glycolytic flux in the resting state to maintain basal cell function, and their glycolysis levels are doubled when proliferating [ 83 ]. The reason for this is that the activity of PHD proteins in CAFs is inhibited by high levels of ROS from neighboring cancer cells, which subsequently cause autophagic degradation of caveolin-1 by stabilizing HIF-1α. Caveolin-1 is an NO inhibitory protein whose degradation leads to excessive NO production. These high levels of NO lead to mitochondrial dysfunction of CAFs, resulting in the removal of CAFs by mitochondrial autophagy; thus, CAFs require high levels of glycolysis to produce energy and thus supply lactate, which cancer cells use in the TCA cycle along with converted pyruvate for ATP production [ 84 , 85 ]. CAFs increase glycolytic flux to maintain a specific link with cancer cells. This phenomenon contradicts the mainstream view of the “Warburg effect” in tumors and has been termed the “reverse Warburg effect”. It is reasonable to speculate that highly glycolytic TECs, similar to CAFs, have this special relationship with cancer cells in terms of lactic acid supply.
TECs preserve functional mitochondria
Although TECs have a highly glycolytic profile similar to that of CAFs, the mitochondria within TECs are not as dysfunctional as those in CAFs; instead, TECs retain functional mitochondria [ 86 ]. The majority of pyruvate is converted to lactate at the end of glycolysis and transported out of the TECs and less than 1% of glucose-derived pyruvate is transported to the mitochondria for the subsequent TCA cycle [ 87 ]. However, this oxidative phosphorylation process within the mitochondria not only makes the TEC energy supply more flexible but also maintains TEC proliferation and promotes neovascular sprouting by increasing the amount of biosynthesis. Therefore, the oxidative phosphorylation process in mitochondria is crucial for highly proliferating tumor endothelial cells. Although inhibition of mitochondrial respiration induces death of highly proliferating tumor endothelial cells, there is no significant damage to quiescent normal endothelial cells [ 88 , 89 , 90 , 91 , 92 ]. If the oxidative respiratory chain is interrupted by inactivating the ubiqui-none-binding protein, QPC, a subunit of mitochondrial complex III, then amino acid levels in ECs will not be maintained; although the migratory function of ECs is not affected, their ability to proliferate will be greatly reduced, which further emphasizes the importance of mitochondrial function for EC proliferation [ 93 ]. This mechanism of cell death through inhibition of mitochondrial respiration may be related to ROS production and uncoupling of mitochondrial membrane potential. Thus, inhibition of mitochondrial function is an antitumor strategy that has not been explored in depth and deserves further investigation.
Translational implications
Many current targeted antiangiogenic therapies aim to inhibit the VEGF signaling pathway in ECs, which is clinically associated with a high rate of drug resistance. In addition, single antiangiogenic therapies may also increase the potential for tumor metastasis due to hypoxia in the tumor microenvironment [ 94 ]. Compared to the genetic stability of normal cells, tumor cells are genetically unstable and have a high mutation rate, making it difficult to advance therapies targeting tumor cell metabolism. It is reasonable to assume that combination therapy would not only benefit the long-term survival of cancer patients but also attenuate the side effects caused by targeted therapies and improve the quality of life. For example, the PFKFB3 inhibitor, PFK15, has been shown to have significant antitumor proliferation and proapoptotic effects in mice [ 93 ], and it synergistically inhibits the proliferation and migration of human umbilical vein endothelial cells (HUVECs) when combined with the tyrosine kinase inhibitor, sunitinib [ 95 , 96 ]. Regrettably, there is little research related to TEC metabolism, and further understanding of the mechanisms involved in the role and effects of TEC glycolysis will facilitate the development of new therapeutic approaches to treat cancer. Here, we present in table form a list of potentially promising therapeutic agents or substances that currently target the TEC glycolytic pathway (Table 1 ) [ 63 , 64 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 ].
Conclusions
The regulation of glycolytic metabolism in endothelial cells (ECs) has gained significant attention, particularly in the context of pathological angiogenesis within the tumor microenvironment. This review provides a comprehensive summary of the differences in glycolytic features between normal ECs and tumor endothelial cells (TECs), as well as the factors that affect their regulation. A promising approach for cancer treatment could be targeting the regulation of glycolytic metabolism in TECs, in combination with classical antiangiogenic therapies. Although many agents targeting aerobic glycolysis of ECs are still in the preclinical or early clinical trial stages, with the continuous exploration in this field, it is optimistic that these emerging drugs may provide new options for cancer treatment in the future.
Data availability
No datasets were generated or analysed during the current study.
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473(7347):298–307 Epub 2011/05/20. doi: 10.1038/nature10144. PubMed PMID: 21593862; PubMed Central PMCID: PMCPMC4049445
Article CAS PubMed PubMed Central Google Scholar
Potente M, Gerhardt H, Carmeliet P (2011) Basic and therapeutic aspects of angiogenesis. Cell 146(6):873–887 Epub 2011/09/20. https://doi.org/10.1016/j.cell.2011.08.039
Article CAS PubMed Google Scholar
Phng LK, Gerhardt H (2009) Angiogenesis: a team effort coordinated by notch. Dev Cell 16(2):196–208 Epub 2009/02/17. https://doi.org/10.1016/j.devcel.2009.01.015
Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM et al (2010) Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol 12(10):943–953 Epub 2010/09/28. https://doi.org/10.1038/ncb2103
Förstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33(7):829–837 837a-837d. Epub 2011/09/06. https://doi.org/10.1093/eurheartj/ehr304
Leo F, Suvorava T, Heuser SK, Li J, LoBue A, Barbarino F et al (2021) Red blood cell and endothelial eNOS independently Reg-Ulate circulating nitric oxide metabolites and blood pressure. Circulation 144(11):870–889 Epub 2021/07/08. https://doi.org/10.1161/circulationaha.120.049606
Pober JS, Sessa WC (2007) Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. ;7(10):803–815. Epub 2007/09/26. https://doi.org/10.1038/nri2171 . PubMed PMID: 17893694
Opitz B, Förster S, Hocke AC, Maass M, Schmeck B, Hippenstiel S et al (2005) Nod1-mediated endothelial cell activation by Chlamydophila pneumoniae. Circ Res 96(3):319–326 Epub 2005/01/18. https://doi.org/10.1161/01.RES.0000155721.83594.2c
Wang J, Alexanian A, Ying R, Kizhakekuttu TJ, Dharmashankar K, Vasquez-Vivar J et al (2012) Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: role for AMP kinase. Arterioscler Thromb Vasc Biol 32(3):712–720 Epub 2011/12/31. https://doi.org/10.1161/atvbaha.111.227389
Wang Q, Liang B, Shirwany NA, Zou MH (2011) 2-Deoxy-D-glucose treatment of endothelial cells induces autophagy by reactive oxygen species-mediated activation of the AMP-activated protein kinase. PLoS ONE 6(2):e17234 Epub 2011/03/10. https://doi.org/10.1371/journal.pone.0017234
Uldry M, Thorens B (2004) The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch 447(5):480–489 Epub 2003/05/17. https://doi.org/10.1007/s00424-003-1085-0
De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR et al (2013) Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154(3):651–663 Epub 2013/08/06. https://doi.org/10.1016/j.cell.2013.06.037
Duffy MJ, O’Grady S, Tang M, Crown J (2021) MYC as a target for cancer treatment. Cancer Treat Rev 94:102154 Epub 2021/02/02. https://doi.org/10.1016/j.ctrv.2021.102154
Han X, Ren C, Lu C, Qiao P, Yang T, Yu Z (2022) Deubiquitination of MYC by OTUB1 contributes to HK2 mediated glycolysis and breast tumorigenesis. Cell Death Differ 29(9):1864–1873 Epub 2022/03/18. https://doi.org/10.1038/s41418-022-00971-8
Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC (2008) Pyruvate kinase M2 is a phosphotyrosine-binding pro-tein. Nature 452(7184):181–186 Epub 2008/03/14. https://doi.org/10.1038/nature06667
Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K et al (2009) Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal 2(97):ra73 Epub 2009/11/19. https://doi.org/10.1126/scisignal.2000431
Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G et al (2013) PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell 155(2):397–409 Epub 2013/10/15. https://doi.org/10.1016/j.cell.2013.09.025
Boeckel JN, Derlet A, Glaser SF, Luczak A, Lucas T, Heumüller AW et al (2016) JMJD8 regulates angiogenic sprouting and cel-lular metabolism by interacting with pyruvate kinase M2 in endothelial cells. Arterioscler Thromb Vasc Biol 36(7):1425–1433 Epub 2016/05/21. https://doi.org/10.1161/atvbaha.116.307695
Doddaballapur A, Michalik KM, Manavski Y, Lucas T, Houtkooper RH, You X et al (2015) Laminar shear stress inhibits endothe-lial cell metabolism via KLF2-mediated repression of PFKFB3. Arterioscler Thromb Vasc Biol 35(1):137–145 Epub 2014/11/02. https://doi.org/10.1161/atvbaha.114.304277
Niimi K, Ueda M, Fukumoto M, Kohara M, Sawano T, Tsuchihashi R et al (2017) Transcription factor FOXO1 promotes cell Mi-Gration toward exogenous ATP via controlling P2Y1 receptor expression in lymphatic endothelial cells. Biochem Biophys Res Commun 489(4):413–419 Epub 2017/06/01. https://doi.org/10.1016/j.bbrc.2017.05.156
Wilhelm K, Happel K, Eelen G, Schoors S, Oellerich MF, Lim R et al (2016) FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature 529(7585):216–220 Epub 2016/01/07. https://doi.org/10.1038/nature16498
Yu P, Wilhelm K, Dubrac A, Tung JK, Alves TC, Fang JS et al (2017) FGF-dependent metabolic control of vascular development. Nature 545(7653):224–228 Epub 2017/05/04. https://doi.org/10.1038/nature22322
Vizán P, Sánchez-Tena S, Alcarraz-Vizán G, Soler M, Messeguer R, Pujol MD et al (2009) Characterization of the metabolic changes underlying growth factor angiogenic activation: identification of new potential therapeutic targets. Carcinogenesis 30(6):946–952 Epub 2009/04/17. https://doi.org/10.1093/carcin/bgp083
Jongkind JF, Verkerk A, Baggen RG (1989) Glutathione metabolism of human vascular endothelial cells under peroxidative stress. Free Radic Biol Med 7(5):507–512 Epub 1989/01/01. https://doi.org/10.1016/0891-5849(89)90026-9
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7(1):11–20 Epub 2008/01/08. https://doi.org/10.1016/j.cmet.2007.10.002
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324(5930):1029–1033 Epub 2009/05/23. https://doi.org/10.1126/science.1160809
Ghesquière B, Wong BW, Kuchnio A, Carmeliet P (2014) Metabolism of stromal and immune cells in health and disease. Nature 511(7508):167–176 Epub 2014/07/11. https://doi.org/10.1038/nature13312
Lorenzi M (2007) The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res 2007:61038 Epub 2008/01/29. https://doi.org/10.1155/2007/61038
Wells L, Vosseller K, Hart GW (2001) Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science 291(5512):2376–2378 Epub 2001/03/28. https://doi.org/10.1126/science.1058714
Vosseller K, Sakabe K, Wells L, Hart GW (2002) Diverse regulation of protein function by O-GlcNAc: a nuclear and cytoplasmic carbohydrate post-translational modification. Curr Opin Chem Biol 6(6):851–857 Epub 2002/12/10. https://doi.org/10.1016/s1367-5931(02)00384-8
Croci DO, Cerliani JP, Dalotto-Moreno T, Méndez-Huergo SP, Mascanfroni ID, Dergan-Dylon S et al (2014) Glycosyla-tion-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell 156(4):744–758 Epub 2014/02/18. https://doi.org/10.1016/j.cell.2014.01.043
Rahimi N, Costello CE (2015) Emerging roles of post-translational modifications in signal transduction and angiogenesis. Pro-teomics 15(2–3):300–309 Epub 2014/08/28. https://doi.org/10.1002/pmic.201400183
Article CAS Google Scholar
Schoors S, Bruning U, Missiaen R, Queiroz KC, Borgers G, Elia I et al (2015) Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature 520(7546):192–197 Epub 2015/04/02. https://doi.org/10.1038/nature14362
Kalucka J, Bierhansl L, Conchinha NV, Missiaen R, Elia I, Brüning U et al (2018) Quiescent endothelial cells upregulate fatty acid β-Oxidation for Vasculoprotection via Redox Homeostasis. Cell Metab 28(6):881–894 .e813. Epub 2018/08/28. https://doi.org/10.1016/j.cmet.2018.07.016
Kim B, Li J, Jang C, Arany Z (2017) Glutamine fuels proliferation but not migration of endothelial cells. Embo j 36(16):2321–2333 Epub 2017/07/01. https://doi.org/10.15252/embj.201796436
Huang H, Vandekeere S, Kalucka J, Bierhansl L, Zecchin A, Brüning U et al (2017) Role of glutamine and interlinked asparagine metabolism in vessel formation. Embo j 36(16):2334–2352 Epub 2017/07/01. https://doi.org/10.15252/embj.201695518
Eelen G, Dubois C, Cantelmo AR, Goveia J, Brüning U, DeRan M et al (2018) Role of glutamine synthetase in angiogenesis beyond glutamine synthesis. Nature 561(7721):63–69 Epub 2018/08/31. https://doi.org/10.1038/s41586-018-0466-7
Brown JM, Wilson WR (2004) Exploiting tumor hypoxia in cancer treatment. Nat Rev Cancer. ;4(6):437–447. Epub 2004/06/02. https://doi.org/10.1038/nrc1367 . PubMed PMID: 15170446
Bache M, Kappler M, Said HM, Staab A, Vordermark D (2008) Detection and specific targeting of hypoxic regions within solid tumors: current preclinical and clinical strategies. Curr Med Chem 15(4):322–338 Epub 2008/02/22. doi: 10.2174/092986708783497391. PubMed PMID: 18288988
DeClerck K, Elble RC (2010) The role of hypoxia and acidosis in promoting metastasis and resistance to chemotherapy. Front Biosci (Landmark Ed) 15(1):213–225 Epub 2009/12/29. doi: 10.2741/3616. PubMed PMID: 20036816
Jain RK (2014) Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 26(5):605–622 Epub 2014/12/18. https://doi.org/10.1016/j.ccell.2014.10.006
Clementi E, Brown GC, Feelisch M, Moncada S (1998) Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci U S A 95(13):7631–7636 Epub 1998/06/24. https://doi.org/10.1073/pnas.95.13.7631
Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer. ;3(10):721–732. Epub 2003/09/18. https://doi.org/10.1038/nrc1187 . PubMed PMID: 13130303
Wong BW, Marsch E, Treps L, Baes M, Carmeliet P (2017) Endothelial cell metabolism in health and disease: impact of hypoxia. Embo j 36(15):2187–2203 Epub 2017/06/24. https://doi.org/10.15252/embj.201696150
Weigand JE, Boeckel JN, Gellert P, Dimmeler S (2012) Hypoxia-induced alternative splicing in endothelial cells. PLoS ONE 7(8):e42697 Epub 2012/08/10. https://doi.org/10.1371/journal.pone.0042697
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR et al (2001) C. Elegans EGL-9 and mammalian homo-logs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107(1):43–54 Epub 2001/10/12. https://doi.org/10.1016/s0092-8674(01)00507-4
Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC (2003) Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol 23(24):9361–9374 Epub 2003/12/04. https://doi.org/10.1128/mcb.23.24.9361-9374.2003
Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ et al (2005) Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105(2):659–669 Epub 2004/09/18. https://doi.org/10.1182/blood-2004-07-2958
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD et al (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16(9):4604–4613 Epub 1996/09/01. https://doi.org/10.1128/mcb.16.9.4604
Semenza GL (2003) Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med 54:17–28 Epub 2002/10/03. https://doi.org/10.1146/annurev.med.54.101601.152418
Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME et al (2004) Progenitor cell trafficking is regu-lated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10(8):858–864 Epub 2004/07/06. https://doi.org/10.1038/nm1075
Faller DV, Weng H, Choi SY (1997) Activation of collagenase IV gene expression and enzymatic activity by the Moloney murine leukemia virus long terminal repeat. Virology 227(2):331–342 Epub 1997/01/20. https://doi.org/10.1006/viro.1996.8345
Abidia A (2000) Endothelial cell responses to hypoxic stress. Clin Exp Pharmacol Physiol. ;27(8):630. Epub 2000/07/20. https://doi.org/10.1046/j.1440-1681.2000.03310.x . PubMed PMID: 10901394
Li W, Petrimpol M, Molle KD, Hall MN, Battegay EJ, Humar R (2007) Hypoxia-induced endothelial proliferation requires both mTORC1 and mTORC2. Circ Res. ;100(1):79–87. Epub 2006/11/18. https://doi.org/10.1161/01.RES.0000253094.03023.3f . PubMed PMID: 17110594
Chan CK, Vanhoutte PM (2013) Hypoxia, vascular smooth muscles and endothelium. Acta Pharm Sinica B 3(1):1–7. https://doi.org/10.1016/j.apsb.2012.12.007
Article Google Scholar
Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP et al (2004) Loss of HIF-1alpha in endothelial cells disrupts a hypox-ia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell 6(5):485–495 Epub 2004/11/16. https://doi.org/10.1016/j.ccr.2004.09.026
Chen X, Zhang M, Chen L, Zhou Z, Chen B, Wang C et al (2021) Roxarsone Promotes Glycolysis and Angiogenesis by Inducing Hypoxia-Inducible Factor-1α In Vitro and In Vivo. ACS Omega. ;6(14):9559–9566. Epub 2021/04/20. https://doi.org/10.1021/acsomega.1c00072 . PubMed PMID: 33869936; PubMed Central PMCID: PMCPMC8047655
Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S et al (2009) Endothelial deletion of hypoxia-inducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood 114(2):469–477 Epub 2009/05/15. https://doi.org/10.1182/blood-2008-12-193581
Skuli N, Majmundar AJ, Krock BL, Mesquita RC, Mathew LK, Quinn ZL et al (2012) Endothelial HIF-2α regulates murine patho-logical angiogenesis and revascularization processes. J Clin Invest 122(4):1427–1443 Epub 2012/03/20. https://doi.org/10.1172/jci57322
Mazzone M, Dettori D, de Oliveira RL, Loges S, Schmidt T, Jonckx B et al (2009) Heterozygous deficiency of PHD2 restores Tu-mor oxygenation and inhibits metastasis via endothelial normalization. Cell 136(5):839–851 Epub 2009/02/17. https://doi.org/10.1016/j.cell.2009.01.020
Rohlenova K, Goveia J, García-Caballero M, Subramanian A, Kalucka J, Treps L et al (2020) Single-cell RNA sequencing maps endothelial metabolic plasticity in pathological angiogenesis. Cell Metab 31(4):862–877 .e814. Epub 2020/04/09. https://doi.org/10.1016/j.cmet.2020.03.009
Yeh WL, Lin CJ, Fu WM (2008) Enhancement of glucose transporter expression of brain endothelial cells by vascular endothelial growth factor derived from glioma exposed to hypoxia. Mol Pharmacol 73(1):170–177 Epub 2007/10/19. https://doi.org/10.1124/mol.107.038851
Cantelmo AR, Conradi LC, Brajic A, Goveia J, Kalucka J, Pircher A et al (2016) Inhibition of the glycolytic activator PFKFB3 in Endothelium induces Tumor Vessel normalization, Impairs Metastasis, and improves chemotherapy. Cancer Cell 30(6):968–985 Epub 2016/11/22. https://doi.org/10.1016/j.ccell.2016.10.006
Schoors S, De Bock K, Cantelmo AR, Georgiadou M, Ghesquière B, Cauwenberghs S et al (2014) Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab 19(1):37–48 PubMed PMID: 24332967
Conradi LC, Brajic A, Cantelmo AR, Bouché A, Kalucka J, Pircher A et al (2017) Tumor vessel disintegration by maximum tol-erable PFKFB3 blockade. Angiogenesis 20(4):599–613 Epub 2017/09/07. https://doi.org/10.1007/s10456-017-9573-6
Wang L, Du DD, Zheng ZX, Shang PF, Yang XX, Sun C et al (2022) Circulating galectin-3 promotes tumor-endothelium-adhesion by upregulating ICAM-1 in endothelium-derived extracellular vesicles. Front Pharmacol 13:979474 Epub 2022/11/18. https://doi.org/10.3389/fphar.2022.979474
Gómez-Escudero J, Clemente C, García-Weber D, Acín-Pérez R, Millán J, Enríquez JA et al (2019) PKM2 regulates endothelial cell junction dynamics and angiogenesis via ATP production. Sci Rep 9(1):15022 Epub 2019/10/23. https://doi.org/10.1038/s41598-019-50866-x
Mele L, la Noce M, Paino F, Regad T, Wagner S, Liccardo D et al (2019) Glucose-6-phosphate dehydrogenase blockade potenti-ates tyrosine kinase inhibitor effect on breast cancer cells through autophagy perturbation. J Exp Clin Cancer Res 38(1):160 Epub 2019/04/17. https://doi.org/10.1186/s13046-019-1164-5
Article PubMed PubMed Central Google Scholar
Tsai HC, Tzeng HE, Huang CY, Huang YL, Tsai CH, Wang SW et al (2017) WISP-1 positively regulates angiogenesis by control-Ling VEGF-A expression in human osteosarcoma. Cell Death Dis 8(4):e2750 Epub 2017/04/14. https://doi.org/10.1038/cddis.2016.421
Carbajo-Pescador S, Ordoñez R, Benet M, Jover R, García-Palomo A, Mauriz JL et al (2013) Inhibition of VEGF expression through blockade of Hif1α and STAT3 signalling mediates the anti-angiogenic effect of melatonin in HepG2 liver cancer cells. Br J Cancer 109(1):83–91 Epub 2013/06/13. https://doi.org/10.1038/bjc.2013.285
Huynh J, Etemadi N, Hollande F, Ernst M, Buchert M (2017) The JAK/STAT3 axis: a comprehensive drug target for solid malig-nancies. Semin Cancer Biol 45:13–22 Epub 2017/06/26. https://doi.org/10.1016/j.semcancer.2017.06.001
Hassanpour M, Rezabakhsh A, Pezeshkian M, Rahbarghazi R, Nouri M (2018) Distinct role of autophagy on angiogenesis: high-lights on the effect of autophagy in endothelial lineage and progenitor cells. Stem Cell Res Ther 9(1):305. https://doi.org/10.1186/s13287-018-1060-5 PubMed PMID: 30409213; PubMed Central PMCID: PMCPMC6225658 Epub 2018/11/10
Ocaña MC, Martínez-Poveda B, Quesada AR, Medina M (2019) Highly Glycolytic Immortalized Human Dermal Microvascular Endothelial Cells are Able to Grow in Glucose-Starved Conditions. Biomolecules. ;9(8). Epub 2019/08/04. https://doi.org/10.3390/biom9080332 . PubMed PMID: 31374952; PubMed Central PMCID: PMCPMC6723428
Végran F, Boidot R, Michiels C, Sonveaux P, Feron O (2011) Lactate influx through the endothelial cell monocarboxylate trans-porter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res 71(7):2550–2560 Epub 2011/02/09. https://doi.org/10.1158/0008-5472.Can-10-2828
Article PubMed Google Scholar
Ruan GX, Kazlauskas A (2013) Lactate engages receptor tyrosine kinases Axl, Tie2, and vascular endothelial growth factor re-ceptor 2 to activate phosphoinositide 3-kinase/Akt and promote angiogenesis. J Biol Chem 288(29):21161–21172 Epub 2013/06/12. https://doi.org/10.1074/jbc.M113.474619
Lee DC, Sohn HA, Park ZY, Oh S, Kang YK, Lee KM et al (2015) A lactate-induced response to hypoxia. Cell 161(3):595–609 PubMed PMID: 25892225
Hunt TK, Aslam RS, Beckert S, Wagner S, Ghani QP, Hussain MZ et al (2007) Aerobically derived lactate stimulates Revasculari-Zation and tissue repair via redox mechanisms. Antioxid Redox Signal 9(8):1115–1124 Epub 2007/06/15. https://doi.org/10.1089/ars.2007.1674
Porporato PE, Payen VL, De Saedeleer CJ, Préat V, Thissen JP, Feron O et al (2012) Lactate stimulates angiogenesis and acceler-ates the healing of superficial and ischemic wounds in mice. Angiogenesis 15(4):581–592 Epub 2012/06/05. https://doi.org/10.1007/s10456-012-9282-0
Annan DA, Maishi N, Soga T, Dawood R, Li C, Kikuchi H et al (2019) Carbonic anhydrase 2 (CAII) supports tumor blood endo-thelial cell survival under lactic acidosis in the tumor microenvironment. Cell Commun Signal 17(1):169 Epub 2019/12/19. https://doi.org/10.1186/s12964-019-0478-4
Guo C, Huang T, Wang QH, Li H, Khanal A, Kang EH et al (2019) Monocarboxylate transporter 1 and monocarboxylate trans-porter 4 in cancer-endothelial co-culturing microenvironments promote proliferation, migration, and invasion of renal cancer cells. Cancer Cell Int 19:170 Epub 2019/07/13. https://doi.org/10.1186/s12935-019-0889-8
Wenes M, Shang M, Di Matteo M, Goveia J, Martín-Pérez R, Serneels J et al (2016) Macrophage metabolism controls tumor blood vessel morphogenesis and metastasis. Cell Metab 24(5):701–715 Epub 2016/10/25. https://doi.org/10.1016/j.cmet.2016.09.008
Akhtar S, Hartmann P, Karshovska E, Rinderknecht FA, Subramanian P, Gremse F et al (2015) Endothelial hypoxia-inducible Factor-1α promotes atherosclerosis and monocyte recruitment by upregulating MicroRNA-19a. Hypertension 66(6):1220–1226 Epub 2015/10/21. https://doi.org/10.1161/hypertensionaha.115.05886
Lemons JM, Feng XJ, Bennett BD, Legesse-Miller A, Johnson EL, Raitman I et al (2010) Quiescent fibroblasts exhibit high meta-bolic activity. PLoS Biol 8(10):e1000514 Epub 2010/11/05. https://doi.org/10.1371/journal.pbio.1000514
Pavlides S, Vera I, Gandara R, Sneddon S, Pestell RG, Mercier I et al (2012) Warburg meets autophagy: cancer-associated fibro-blasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxid Redox Signal 16(11):1264–1284 Epub 2011/09/03. https://doi.org/10.1089/ars.2011.4243
Lisanti MP, Martinez-Outschoorn UE, Chiavarina B, Pavlides S, Whitaker-Menezes D, Tsirigos A et al (2010) Understanding the lethal drivers of tumor-stroma co-evolution: emerging role(s) for hypoxia, oxidative stress and autophagy/mitophagy in the tumor micro-environment. Cancer Biol Ther 10(6):537–542 Epub 2010/09/24. https://doi.org/10.4161/cbt.10.6.13370
Koziel A, Woyda-Ploszczyca A, Kicinska A, Jarmuszkiewicz W (2012) The influence of high glucose on the aerobic metabolism of endothelial EA.hy926 cells. Pflugers Arch 464(6):657–669 Epub 2012/10/12. https://doi.org/10.1007/s00424-012-1156-1
De Bock K, Georgiadou M, Carmeliet P (2013) Role of endothelial cell metabolism in vessel sprouting. Cell Metab 18(5):634–647 Epub 2013/08/27. https://doi.org/10.1016/j.cmet.2013.08
Blecha J, Novais SM, Rohlenova K, Novotna E, Lettlova S, Schmitt S et al (2017) Antioxidant defense in quiescent cells deter-mines selectivity of electron transport chain inhibition-induced cell death. Free Radic Biol Med 112:253–266 Epub 2017/08/05. https://doi.org/10.1016/j.freeradbiomed.2017.07.033
Coutelle O, Hornig-Do HT, Witt A, Andree M, Schiffmann LM, Piekarek M et al (2014) Embelin inhibits endothelial mitochon-drial respiration and impairs neoangiogenesis during tumor growth and wound healing. EMBO Mol Med 6(5):624–639 Epub 2014/03/22. https://doi.org/10.1002/emmm.201303016
Rohlena J, Dong LF, Kluckova K, Zobalova R, Goodwin J, Tilly D et al (2011) Mitochondrially targeted α-tocopheryl succinate is antiangiogenic: potential benefit against tumor angiogenesis but caution against wound healing. Antioxid Redox Signal 15(12):2923–2935 Epub 2011/09/10. https://doi.org/10.1089/ars.2011.4192
Don AS, Kisker O, Dilda P, Donoghue N, Zhao X, Decollogne S et al (2003) A peptide trivalent arsenical inhibits tumor angio-genesis by perturbing mitochondrial function in angiogenic endothelial cells. Cancer Cell 3(5):497–509 Epub 2003/06/05. https://doi.org/10.1016/s1535-6108(03)00109-0
Orecchioni S, Reggiani F, Talarico G, Mancuso P, Calleri A, Gregato G et al (2015) The biguanides metformin and phenformin inhibit angiogenesis, local and metastatic growth of breast cancer by targeting both neoplastic and microenvironment cells. Int J Cancer 136(6):E534–544 Epub 2014/09/10. https://doi.org/10.1002/ijc.29193
Diebold LP, Gil HJ, Gao P, Martinez CA, Weinberg SE, Chandel NS (2019) Mitochondrial complex III is necessary for endothelial cell proliferation during angiogenesis. Nat Metab 1(1):158–171 Epub 2019/05/21. https://doi.org/10.1038/s42255-018-0011-x
Ribatti D, Annese T, Ruggieri S, Tamma R, Crivellato E (2019) Limitations of anti-angiogenic treatment of tumors. Transl On-col 12(7):981–986 Epub 2019/05/24. https://doi.org/10.1016/j.tranon.2019.04.022
Horváthová J, Moravčík R, Matúšková M, Šišovský V, Boháč A, Zeman M (2021) Inhibition of Glycolysis Suppresses Cell Prolif-eration and Tumor Progression In Vivo: Perspectives for Chronotherapy. Int J Mol Sci. ;22(9). Epub 2021/05/01. https://doi.org/10.3390/ijms22094390 . PubMed PMID: 33922320; PubMed Central PMCID: PMCPMC8122821
Zlacká J, Murár M, Addová G, Moravčík R, Boháč A, Zeman M (2022) Synthesis of glycolysis inhibitor PFK15 and its synergistic action with an approved Multikinase Antiangiogenic Drug on Human Endothelial Cell Migration and Proliferation. Int J Mol Sci 23(22). https://doi.org/10.3390/ijms232214295 PubMed PMID: 36430773; PubMed Central PMCID: PMCPMC9697023 Epub 2022/11/27
Kaji K, Nishimura N, Seki K, Sato S, Saikawa S, Nakanishi K et al (2018) Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer 142(8):1712–1722 Epub 2017/12/06. https://doi.org/10.1002/ijc.31193
Ocaña MC, Martínez-Poveda B, Marí-Beffa M, Quesada AR, Medina M (2020) Fasentin diminishes endothelial cell proliferation, differentiation and invasion in a glucose metabolism-independent manner. Sci Rep 10(1):6132 Epub 2020/04/11. https://doi.org/10.1038/s41598-020-63232-z
Wu CH, Ho YS, Tsai CY, Wang YJ, Tseng H, Wei PL et al (2009) In vitro and in vivo study of phloretin-induced apoptosis in human liver cancer cells involving inhibition of type II glucose transporter. Int J Cancer 124(9):2210–2219 Epub 2009/01/07. https://doi.org/10.1002/ijc.24189
Fu Z, Chen X, Guan S, Yan Y, Lin H, Hua ZC (2015) Curcumin inhibits angiogenesis and improves defective hematopoiesis in-duced by tumor-derived VEGF in tumor model through modulating VEGF-VEGFR2 signaling pathway. Oncotarget 6(23):19469–19482 Epub 2015/08/09. https://doi.org/10.18632/oncotarget.3625
Zheng L, Li D, Xiang X, Tong L, Qi M, Pu J et al (2013) Methyl jasmonate abolishes the migration, invasion and angiogenesis of gastric cancer cells through down-regulation of matrix metalloproteinase 14. BMC Cancer 13:74 Epub 2013/02/12. https://doi.org/10.1186/1471-2407-13-74
Huang CC, Wang SY, Lin LL, Wang PW, Chen TY, Hsu WM et al (2015) Glycolytic inhibitor 2-deoxyglucose simultaneously targets cancer and endothelial cells to suppress neuroblastoma growth in mice. Dis Model Mech 8(10):1247–1254 Epub 2015/09/24. https://doi.org/10.1242/dmm.021667
Singh S, Pandey S, Chawla AS, Bhatt AN, Roy BG, Saluja D et al (2019) Dietary 2-deoxy-D-glucose impairs tumor growth and metastasis by inhibiting angiogenesis. Eur J Cancer 123:11–24 Epub 2019/11/02. https://doi.org/10.1016/j.ejca.2019.09.005
Merchan JR, Kovács K, Railsback JW, Kurtoglu M, Jing Y, Piña Y et al (2010) Antiangiogenic activity of 2-deoxy-D-glucose. PLoS ONE 5(10):e13699. https://doi.org/10.1371/journal.pone.0013699 PubMed PMID: 21060881; PubMed Central PMCID: PMCPMC2965179 Epub 2010/11/10
Agnihotri S, Mansouri S, Burrell K, Li M, Mamatjan Y, Liu J et al (2019) Ketoconazole and Posaconazole selectively Target HK2-expressing Glioblastoma cells. Clin Cancer Res 25(2):844–855 Epub 2018/10/17. https://doi.org/10.1158/1078-0432.Ccr-18-1854
Clem BF, O’Neal J, Tapolsky G, Clem AL, Imbert-Fernandez Y, Kerr DA 2 et al (2013) Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther 12(8):1461–1470 Epub 2013/05/16. https://doi.org/10.1158/1535-7163.Mct-13-0097
Clem B, Telang S, Clem A, Yalcin A, Meier J, Simmons A et al (2008) Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Mol Cancer Ther 7(1):110–120 Epub 2008/01/19. https://doi.org/10.1158/1535-7163.Mct-07-0482
Porporato PE, Dhup S, Dadhich RK, Copetti T, Sonveaux P (2011) Anticancer targets in the glycolytic metabolism of tumors: a comprehensive review. Front Pharmacol 2:49 Epub 2011/09/10. https://doi.org/10.3389/fphar.2011.00049
Lin H, Ma X, Yang X, Chen Q, Wen Z, Yang M et al (2022) Natural shikonin and acetyl-shikonin improve intestinal microbial and protein composition to alleviate colitis-associated colorectal cancer. Int Immunopharmacol 111:109097 Epub 2022/08/12. https://doi.org/10.1016/j.intimp.2022.109097
Dai Y, Liu Y, Li J, Jin M, Yang H, Huang G (2022) Shikonin inhibited glycolysis and sensitized cisplatin treatment in non-small cell lung cancer cells via the exosomal pyruvate kinase M2 pathway. Bioengineered 13(5):13906–13918 PubMed PMID: 35706397; PubMed Central PMCID: PMCPMC9275963
Wang Y, Hao F, Nan Y, Qu L, Na W, Jia C et al (2018) PKM2 inhibitor shikonin overcomes the cisplatin resistance in bladder Cancer by inducing necroptosis. Int J Biol Sci 14(13):1883–1891 Epub 2018/11/18. https://doi.org/10.7150/ijbs.27854
Yang W, Liu J, Hou L, Chen Q, Liu Y (2021) Shikonin differentially regulates glucose metabolism via PKM2 and HIF1α to over-come apoptosis in a refractory HCC cell line. Life Sci 265:118796 Epub 2020/11/22. https://doi.org/10.1016/j.lfs.2020.118796
Zhou S, Li D, Xiao D, Wu T, Hu X, Zhang Y et al (2022) Inhibition of PKM2 enhances sensitivity of Olaparib to Ovarian Cancer cells and induces DNA damage. Int J Biol Sci 18(4):1555–1568 Epub 2022/03/15. https://doi.org/10.7150/ijbs.62947
Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Trarbach T, Folprecht G et al (2011) Prognostic and predictive role of lactate dehydrogenase 5 expression in colorectal cancer patients treated with PTK787/ZK 222584 (vatalanib) antiangiogenic ther-apy. Clin Cancer Res 17(14):4892–4900 Epub 2011/06/03. https://doi.org/10.1158/1078-0432.Ccr-10-2918
Pang X, Wu Y, Wu Y, Lu B, Chen J, Wang J et al (2011) (-)-Gossypol suppresses the growth of human prostate cancer xenografts via modulating VEGF signaling-mediated angiogenesis. Mol Cancer Ther 10(5):795–805 Epub 2011/03/05. https://doi.org/10.1158/1535-7163.Mct-10-0936
El-Sisi AE, Sokar SS, Abu-Risha SE, El-Mahrouk SR (2017) Oxamate potentiates taxol chemotherapeutic efficacy in experimental-ly-induced solid ehrlich carcinoma (SEC) in mice. Biomed Pharmacother 95:1565–1573 Epub 2017/09/28. https://doi.org/10.1016/j.biopha.2017.09.090
Download references
This study was supported by grants from the National Natural Science Foundation of China (82172597), Zhejiang Provincial Natural Science Foundation (LY22H160027), and Beijing Bethune Charitable Foundation.
Author information
Shenhao Xu, Jiahao Liao and Bing Liu contributed equally to this work.
Authors and Affiliations
Department of urology, the Fourth Affiliated Hospital of School of Medicine, and International School of Medicine, International Institutes of Medicine, Zhejiang University, Yiwu, 322000, China
Shenhao Xu, Jiahao Liao, Bing Liu & Cheng Zhang
The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, 310000, China
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization and supervision, C.Z. and X.X.; writing—original draft preparation, S.X., J.L. and B.L.; writing—review and editing, S.X.; funding acquisition, X.X. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Correspondence to Cheng Zhang or Xin Xu .
Ethics declarations
Ethical approval.
The authors declare no conflict of interest. This study did not involve Human Participants and/or Animals. All authors have read and agreed to the published version of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Xu, S., Liao, J., Liu, B. et al. Aerobic glycolysis of vascular endothelial cells: a novel perspective in cancer therapy. Mol Biol Rep 51 , 717 (2024). https://doi.org/10.1007/s11033-024-09588-1
Download citation
Received : 07 March 2024
Accepted : 25 April 2024
Published : 01 June 2024
DOI : https://doi.org/10.1007/s11033-024-09588-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Vascular endothelial cells
- Aerobic glycolysis
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 31 May 2024
CRISPR/Cas-based CAR-T cells: production and application
- Ping Song 1 na1 ,
- Qiqi Zhang 2 na1 ,
- Zhiyong Xu 3 na1 ,
- Yueli Shi 3 ,
- Ruirui Jing 2 &
- Dingcun Luo 1 , 4
Biomarker Research volume 12 , Article number: 54 ( 2024 ) Cite this article
208 Accesses
Metrics details
Chimeric antigen receptor T cell (CAR-T) therapy has revolutionized the treatment approach for cancer, autoimmune disease, and heart disease. The integration of CAR into T cells is typically facilitated by retroviral or lentiviral vectors. However, the random insertion of CARs can lead to issues like clonal expansion, oncogenic transformation, variegated transgene expression, and transcriptional silencing. The advent of precise gene editing technology, like Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), allows for controlled and precise genome modification, facilitating the translation of CAR-T research to the clinical applications. This review aims to provide a comprehensive analysis of the application of CRISPR gene editing techniques in the context of precise deletion and insertion methodologies, with a specific focus on their potential for enhancing the development and utilization of CAR-T cell therapy.
Introduction
Overview of car-t cell therapy.
Chimeric antigen receptor T cell (CAR-T) cell therapy has demonstrated remarkable efficacy and safety for the treatment of hematological malignancies in recent years. CAR constructs, which consist of an extracellular antigen-binding domain (single-chain fragment variable, scFv), transmembrane hinges, and intracellular signal domains (such as CD3ζ chain and costimulatory domain), enable CAR-T cells to specifically identify, activate, and eradicate tumor cells in an antigen-specific and MHC-independent manner [ 1 ]. So far, six CAR-T products leveraging this mechanism have been approved for the therapeutic management of B-cell acute lymphoblastic leukemia/Non-Hodgkin Lymphoma (B-ALL/NHL) or Multiple Myeloma (MM). However, the production of all six products involves the introduction of CAR genes into human primary T cells through infection with lentivirus (LV) or retroviral vector (RV). Consequently, this integration process may result in clone amplification, carcinogenic transformation, mutated transgenic expression, and transcriptional silencing. Additionally, CAR-T cell exhaustion, toxicity concerns, and limited autologous cell availability have hindered widespread adoption.
Briefs of gene editing technologies
Gene editing technologies play a crucial role in the production and optimization of CAR-T cells for anti-tumor purposes. These technologies, including transcription activator-like effector nucleases (TALENs), zinc-finger nucleases (ZFNs), and clustered regularly interspaced short palindromic repeats (CRISPR), facilitate precise modification and manipulation of genes in CAR-T cell engineering [ 2 ].
ZFNs and TALENs are chimeric nucleases comprising a modular DNA-binding domain and a sequence-independent cleavage domain derived from the FokΙ restriction enzyme [ 3 ]. Utilizing a zinc finger protein or transcriptional activator-like effect (TALE) domain, they recognize and bind to DNA at a specific sequence. Subsequently, they introduce an endonuclease to cleave the sequence, resulting in a DNA double-stranded break (DSB) at the targeted locus. However, the broad application of TALENs and ZFNs is hindered by the time-consuming and complex process involved, as a specific editing protein is required for each version of genome editing [ 4 , 5 ]. Following the DSB, the eukaryotic cellular DNA repair system repairs the DSBs through either the homology-directed repair (HDR) or non-homologous end joining (NHEJ) pathways [ 6 ], leading to targeted integration or disruption of genes, depending on the pathway utilized.
In contrast, the CRISPR/Cas system is widely recognized as a powerful gene editing tool due to its simple design and high efficiency, offering promising prospects for cancer treatment. CRISPR/Cas system has greatly simplified the gene editing process and is now extensively applied in cell therapy, with recent progress toward clinical applications. Initially reported in E. coli , CRISPRs were later identified as an intrinsic adaptive immune system in eukaryotic cells, providing defense against foreign DNA. Currently, the most used systems are CRISPR/Cas9 and CRISPR/Cas12a. The CRISPR/Cas9 technology involves a 20-base pair single guide RNA (sgRNA) that guides the DNA endonuclease to the desired cutting site. This site is specified by a protospacer adjacent motif (PAM) sequence located downstream of the cleavage site within the target DNA [ 7 ]. On the other hand, the CRISPR/Cas12a system recognizes the TTTV sequence on the genome and requires only a single crRNA to cut the genomic DNA. This process produces sticky ends that are repaired similarly to CRISPR/Cas9. MEGA-CRISPR harnesses Cas13d’s RNA-directed editing capabilities through tailored guide RNA (gRNA) design, enabling precise recognition and cleavage of target RNA sequences for editing [ 8 ]. Here is a comparison of the advantages and drawbacks of CRISPR/Cas9, CRISPR/Cas12a, and CRISPR/Cas13d in CAR-T therapy, presented in Table 1 . These characteristics help better understand the strengths and limitations of each system in the context of CAR-T therapy. The CRISPR/dCas9 system is utilized to modulate transcriptional activities by recruiting transcriptional activators or repressors to specific loci, known as CRISPR activation (CRISPRa) and CRISPR interference (CRISPRi), respectively. Provided below is an in-depth exploration regarding the generation of CAR-T cell therapy leveraging the aforementioned gene editing approaches [ 9 ].
The production of CRISPR/Cas-based CAR-T cells
Currently, there are three primary approaches for generating CAR-T cells utilizing the CRISPR system, with the most conventional being the CRISPR/Cas9 system, alongside CRISPR/Cas12a and CRISPR/Cas13d. The procedures for generating CAR-T cells utilizing these systems will be elaborated upon in the following sections.
Current state of research on the production of CAR-T cells using the CRISPR/Cas9 system
Over the years, extensive research has been undertaken to deliver the CRISPR system into human primary T cells in three different forms: (i) Viral delivery of CRISPR vectors, such as LV or adeno-associated virus (AAV), (ii) Cas9 mRNA combined with synthetic guide RNA, (iii) Binding of Cas9 protein and synthetic guide RNA to form RNP complex [ 10 , 11 ] (Fig. 1 ). The efficiency of gene knockout using CRISPR/Cas9 gene editing is relatively high, with the efficiency at the PD-1 locus exceeding 75%. Previous researchers have integrated guide RNA and CAR into a single vector, subsequently electro-transferring Cas9 mRNA to create gene-edited CAR-T cells. The efficacy of CRISPR/Cas9-mediated knockout hinges on the choice of target and guide RNA. To enhance knockout efficiency, researchers introduced MS (2’-Omethyl 3’-phosphorothioate) or MSP (2’-O-methyl 3’-thio PACE) modifications to the guide RNA. After binding the modified guide RNA to the Cas9 protein, they electrotransfected it into human primary T cells and CD34 + hematopoietic stem cells simultaneously. The results demonstrated a 2.4-fold increase in indel frequencies for MS-modified sgRNAs compared to unmodified ones (30.7% vs. 12.8%), significantly improving genome editing efficiency [ 12 ].

CRISPR mediate gene KO and KI strategy in CAR/TCR-T cell therapy. To achieve the formation of CAR/TCR-T cells, sgRNA and Cas9 protein are co-transposed into T cells, while CAR/TCR can enter T cells through two primary methods, eventually resulting in CAR/TCR-T cells. (1) Random insertion via LV/RV: The CAR or TCR is randomly inserted into T cells using LV or RV. (2) Precise insertion: This method facilitated by a donor template. Various forms of templates such as dsDNA, ssDNA, pDNA, or AAV are employed for site-specific integration of CAR or TCR into the T cells. sgRNA, single guide RNA; LV, lentivirus; RV, retrovirus; KO, knockout; KI, knockin; dsDNA, double strain DNA; ssDNA, single strain DNA; pDNA, plasmid DNA; AAV, Adeno-associated virus
Current research on CAR-T cell production using the CRISPR/Cas12a system
Cas12a (Cpf1) has two major isoforms, AsCpf1 and LbCpf1, known for higher specificity toward human cells compared to Cas9. It is now understood that Cas12a cleaves genomic DNA, generating sticky ends and displaying greater susceptibility to homologous recombination repair [ 13 ]. AAV6 vectors have been employed to engineer CrTRAC, crPDCD1, and CD19 CAR into one vector, achieving a simultaneous knockin efficiency of 37%, seven times that of the CRISPR/Cas9 system. The AAV-Cpf1 KIKO system established a precedent for the efficient expression of two CARs in the same T cell, facilitating the clinical application of bispecific CAR-T cells. Despite high gene knockin and knockout efficiency, Cas12a RNP cleavage efficiency was relatively low [ 14 ]. Researchers addressed this by developing a mutated version, AsCas12a Ultra, carrying M537R and F870L mutations. These mutations significantly enhanced knockout and knockin efficiency, especially in T cells, with single transgene knockin reaching up to 60% and double knockin up to 40% [ 15 ].
Current research on CAR-T cell production using the CRISPR/Cas13d system
Tieu et al. introduced MEGA-CRISPR, a CRISPR/Cas13d-based tool [ 8 ], which utilizes tailored gRNA design to edit target RNA sequences with precision. This technology shows promise in enhancing CAR-T cell therapy efficacy by addressing T cell exhaustion and improving anti-cancer capabilities within the tumor microenvironment. Empirical validation in murine models demonstrates MEGA-CRISPR’s ability to enhance tumor cell killing efficiency, leading to significant tumor suppression and prolonged survival. Despite its potential, challenges such as complex technology and safety assessment hinder its widespread use. Further research and clinical validation are necessary to optimize MEGA-CRISPR for clinical applications.
CRISPR/Cas system-mediated loci-specific knockin in CAR-T cells
To date, CAR-T cells have been primarily transduced using γ-retroviral vectors or lentiviral vectors. However, these methods result in random DNA integration in T cells and carry the risk of malignant transformation. To overcome this problem, one intriguing strategy is site-specific gene integration. By utilizing target-directed nucleases to create a double-strand break at a specific genomic locus, CAR transgenes can be integrated into the T cell genome via homologous recombination.
In 2017, Michel Sadelain’s group employed knockin techniques to insert the CD19 CAR gene into the TRAC locus, generating TRAC CAR-T cells. In comparison to CAR-T cells infected with retroviral vectors, the CD19 CAR knockin CAR-T cells exhibited diminished differentiation and depletion, while demonstrating significantly improved anti-tumor effects in mouse models [ 16 ]. In a melanoma mouse model, TCR-T cells generated with linear double-stranded DNA (dsDNA) as an HDR template exhibited more pronounced inhibition of melanoma growth compared to TCR-T cells generated with lentiviral vectors [ 10 ]. By utilizing non-viral, gene-specific targeted CAR-T cells through CRISPR-Cas9 at the PD-1 locus, it was demonstrated that non-viral, gene-specific integrated CAR-T cells offer both high safety and efficacy. This provides an innovative technology for CAR-T cell therapy of B-ALL [ 17 ]. A novel approach was devised to create targeted knockin CAR-T cells by employing modified plasmid DNA as a donor (referred to as pTRAC-CAR-T cells). In a murine leukemia model, the anti-tumor efficacy of these pTRAC-CAR-T cells was assessed and compared with CAR-T cells generated using AAV as the donor template. Results indicated that both variants of CAR-T cells demonstrated comparable anti-tumor effects. The well-established and cost-effective GMP (Good Manufacturing Practice) production of plasmid vectors highlights the feasibility of leveraging pTRAC-CAR-T cells generated from plasmid templates through the CRISPR/Cas9 system for prompt integration into future clinical trials [ 18 ].
In summary, there are presently three primary HDR template types for targeted gene delivery into primary T cells using the CRISPR system: (1) AAV-dependent target gene delivery [ 14 , 16 ]; (2) linear ssDNA/dsDNA target gene delivery [ 10 , 19 ]; and (3) plasmid DNA target gene delivery (Fig. 1 ). When employing AAV6 for target genes, one method involves co-transferring synthetic guide RNA and Cas9 mRNA via electrotransfer into human primary T cells, specifically targeting the TRAC site, followed by the introduction of AAV6 carrying the CAR gene. This approach achieves an editing efficiency of up to 45.6% [ 16 ]. An alternative strategy involves designing the guide RNA, purifying the Cas9 protein, creating an RNP complex in vitro, electrotransfecting it into T cells, and subsequently introducing AAV6 carrying the CAR gene. Using this method, the knockin efficiency can reach approximately 50% in T cells. In instances where linear dsDNA is utilized for knockin into the TRAC or PD-1 locus of human primary T cells, the knockin efficiency of the CAR/TCR gene is approximately 10-20% [ 10 , 17 ]. Plasmid vectors, employed as HDR templates with the CRISPR/Cas9 system, necessitate the incorporation of a guide RNA sequence at each end of the homologous arm of the target gene, in addition to vectors containing guide RNA. The presence of the vector containing guide RNA significantly enhances the knockin efficiency, resulting in a 4-8-fold increase compared to the vector without guide RNA [ 18 ]. Both the advantage and disadvantage were showed in Table 2 .
The application of CRISPR/Cas-based CAR-T cells
The utilization of CRISPR-based CAR-T cells encompasses several key facets, including the generation of universal CAR-T cells, overcoming immune checkpoint inhibition, and mitigating CAR-T cell fratricide. Subsequently, a detailed exploration of the application of CRISPR-based CAR-T cells in both scientific investigation and clinical settings will be provided.
Generation of universal CAR-T cells
Currently, most CAR-T cell manufacturing relies on T cells sourced from autologous peripheral blood mononuclear cells (PBMCs). However, the costly and time-consuming production process may impede the accessibility of CAR-T cell therapy for individuals in urgent need, including those with rapidly progressing diseases or those unable to obtain potent autologous T cells due to inherent T cell defects [ 20 ]. In such scenarios, the utilization of off-the-shelf CAR-T products derived from healthy donors could potentially address these challenges. Nevertheless, the significant obstacle of acute and chronic graft-versus-host disease (GVHD) looms over this intriguing concept. To mitigate the risk of GVHD, CAR-T cells can be derived either from the patient’s previous HLA-matched hematopoietic stem cell transplant (HSCT) donor or through the genetic modification of CAR-T cells. Researchers have turned to gene editing technology to disrupt genes encoding the T cell receptor (TCR) and major histocompatibility complex (MHC), both of which contribute to alloreactivity. Two critical genes, TRAC and TRBC, encode endogenous TCR chains, with the TRAC locus serving as an ideal target for gene knockout and CAR knockin.
In addition, Georgiadis et al. pioneered the creation of TCR-knockout CAR-T cells by integrating a self-inactivating lentiviral platform with the CRISPR/Cas system. Their study demonstrated that these TT CAR-T cells exhibit superior potency compared to TCR-positive CAR-T cells. Another promising approach to diminish the allogeneic response involves the ablation of MHC class I by targeting B2M. Researchers have successfully generated TRAC, B2M, and PDCD1 multiplex knockout CAR-T cells targeting CD19 or prostate stem cell antigen (PSCA), exhibiting reduced alloreactivity coupled with enhanced anti-tumor activity [ 21 ]. (Fig. 2 )
The gene editing site and application used by CRISPR system to enhance CAR-T cell function
Disrupting immune checkpoint inhibitors
T cells express inhibitory receptors on their surface, contributing to T cell exhaustion, including PD-1, CTLA4, TIGIT, LAG-3, CD244, CD160, TIM3, and others. The suppressive tumor microenvironment and tumor cells can induce T cell anergy and exhaustion by upregulating inhibitory immune checkpoint signaling [ 22 ]. Repeated encounters with tumor cells lead CAR-T cells to adopt an exhausted phenotype primarily due to the upregulation of immune inhibitory receptors by tumor cells [ 22 , 23 , 24 ]. Knocking out these receptors enhances T cells’ ability to recognize tumor antigens. The PD-1/PD-L1 signaling pathway modulates T cell proliferation, activation, exhaustion, and immune tolerance [ 25 ]. Blocking the PD-1/PD-L1 axis on T cells has been documented to enhance CAR-T cell function [ 26 , 27 ]. Inhibiting the expression of immunosuppressive receptors like PD-1 has been extensively studied in hematologic and solid tumors. Current evidence suggests that PD-1 knockout activates the T cell immune response against tumors, particularly in lung cancer. Additionally, PD-1 knockout has demonstrated increased anti-tumor activity in CD19 CAR-T cells for hematological malignancies, GPC3 CAR-T cells for liver cancer, and mesothelin CAR-T cells for human ductal adenocarcinoma. Knocking out molecular markers associated with T cell exhaustion, like PD-1 and CTLA4, in Universal CARs improved their tumor-killing activity. Taken together, the above findings suggest that CAR-T cell therapies designed based on immune checkpoints offer potential advantages in controlling solid tumors, presenting a novel strategy for adoptive T cell therapies.
Combining immunotherapy with CAR-T cells and immune checkpoint blockade has shown tumor regression. However, systematic administration of immune checkpoint/ligand monoclonal antibodies poses a risk of immune-related adverse events (IRAEs) [ 28 ]. Genetically disrupting intrinsic PD-1 signaling using CRISPR/Cas9 can minimize toxicity while preserving CAR-T cells’ effector function. Extensive evidence supports the idea that abrogating PD-1 with CRISPR/Cas9 enhances the anti-tumor potency of both allogeneic and autologous CAR-T cells in hematological malignancies and solid tumors during preclinical and clinical evaluations [ 11 , 29 ].
To counterbalance the negative impact of the Fas/FasL axis on T cell survival, Ren et al. developed a practical one-shot CRISPR system. They incorporated multiple gRNAs into a lentiviral vector along with a CAR transgene, resulting in the generation of Fas-resistant universal CAR-T cells and PD-1/CTLA-4 dual-resistant universal CAR-T cells. Despite a decrease in knockout efficacy with an increased number of targeted genes, Fas-deficient CAR-T cells exhibited enhanced resistance to AICD, leading to prolonged persistence [ 30 ]. This finding was supported by Zhang et al., who reported robust efficacy of LAG-3-deficient CAR-T cells in a preclinical model [ 31 ]. (Fig. 2 )
Avoiding fratricide in CAR-T cell therapy targeting T cell malignancy
While CAR-T cell therapy has demonstrated remarkable success in treating advanced B-cell malignancies and adult relapsed/refractory multiple myeloma, its effectiveness is currently limited, and treatment options for refractory and relapsed T cell-related tumors remain scarce. A significant challenge in CAR-T cell therapy lies in the presence of targeted T cell-pan markers on CAR-T cells, potentially resulting in self-activation, fratricide, and impaired functionality of CAR-T cells. These factors significantly impact the efficacy of CAR-T cell therapy for T cell-related tumors.
CD5 and CD7 are transmembrane proteins that are highly expressed in T cell malignancies [ 32 , 33 ], with restricted expression mainly to T cells, NK cells, and B1 cells, making them attractive targets for CAR-T cell therapy. However, the presence of shared antigens on tumor cells and CAR-T cells could lead to fratricide. To mitigate this issue, researchers have explored genetic editing of the CD5 and CD7 genes in CAR-T cells using the CRISPR/Cas9 system [ 34 ].
In the development of CD5-targeted CAR-T cells for T cell malignancies, researchers encountered a challenge of self-mutilation during the in vitro generation process. To address this, they employed the CRISPR/Cas9 system to knockout CD5 on CAR-T cells. This approach resulted in reduced levels of CAR-T cell activation while significantly increasing the expression of CD5 CARs [ 35 ]. In an experiment conducted by the Carl June group, CD5 knockout CAR-T cells injected into a mouse Jurkat T tumor model led to a significant extension of the mice’s survival. Another target, CD7, exhibited high expression not only in T lymphoma cells but also in normal T cells. Knocking out CD7 using the CRISPR/Cas9 system did not impact T cell proliferation or killing ability. In an AML mouse model, tumors largely disappeared when mice were injected with CD7-knockout CAR-T cells. TCR-, β2M-, and CD7-knockout universal CAR-T cell therapy has been investigated in clinical trials for treating T cell acute lymphoblastic leukemia (T-ALL). (Fig. 2 )
CRISPR/Cas9-based gene-knockout enhances CAR-T cell function
T cells express a variety of inhibitory receptors on their surface, including PD-1, CTLA4, TIGIT, LAG-3, CD244, CD160, and TIM3, contributing to T cell exhaustion [ 22 , 23 , 36 ]. Knocking out these receptors has been shown to enhance T cells’ ability to recognize tumor antigens. In this respect, the suppression of PD-1 expression has been extensively studied in hematological and solid tumors [ 25 ]. PD-1 knockout activates the T cell immune response against tumors, providing a potential treatment for lung cancer [ 37 ]. Moreover, PD-1 knockout enhances the anti-tumor activity of CD19 CAR-T cells in hematological malignancies. To address the negative effect of the Fas/FasL axis on T cell survival, Ren et al. developed a practical one-shot CRISPR system. Multiple gRNAs were incorporated into a lentiviral vector along with the CAR transgene, resulting in the generation of Fas-resistant universal CAR-T cells and PD-1/CTLA-4 dual-resistant universal CAR-T cells. Despite a decrease in knockout efficacy with an increasing number of targeted genes, Fas-deficient CAR-T cells demonstrated greater resistance to AICD and prolonged persistence [ 30 ]. Similarly, Zhang et al. reported robust efficacy of LAG-3-deficient CAR-T cells in preclinical models [ 31 ].
Beyond immune checkpoints, there are other molecules whose knockout can improve CAR-T cell function or reduce side effects in therapy. Fas, a member of tumor necrosis factor-alpha (TNF-α), mediates cell death through the Fas-FasL signaling-induced activation-induced cell death (AICD), potentially reducing CAR-T cell activation. Producing anti-Fas CAR-T cells using the CRISPR/Cas9 system can improve CAR-T cell tolerance to AICD and prolong the survival of tumor-bearing mice. TGF-β, binding to the TGF-β receptor (TGFBRI) on the T cell membrane, activates downstream signaling pathways SMAD2 and SMAD3, leading to reduced cytokine production and increased cytotoxicity [ 38 ]. Knocking out TGF receptor II (TGFBR2) in CAR-T cells using the CRISPR-Cas9 system promotes the differentiation of CAR-T cells into central memory and effector cells, enhancing tumor clearance in solid tumor models [ 39 ].
It has been established that adenosine, an immunosuppressive factor, activates the adenosine A2A receptor, inhibiting the activation of multiple immune cells and suppressing the anti-tumor immune response. Using CRISPR/Cas9 to knock out the adenosine A2A receptor was found to enhance the anti-tumor effects of Her2-targeted CAR-T cells in breast cancer [ 40 ]. Glycerol diglyceride kinase (DGK) metabolizes glycerol diesters into phosphatidic acid. Knocking out DGK enhances TCR signaling, increasing the killing capacity of T cells in glioma [ 41 ]. Granulocyte macrophage colony-stimulating factor (GM-CSF), mainly produced by T cells and macrophages, has been targeted in clinical trials involving leukemia patients. GM-CSF knockout, coupled with the generation of CAR-T cells targeting IL6, has been shown to reduce autocrine production of IL-1 and IL-6, subsequently decreasing cytokine release syndrome (CRS) in patients. GM-CSF knockout has also been found to enhance the anti-tumor efficacy of CD19-targeting CAR-T cells in mice, prolonging the survival time of tumor-bearing mice [ 42 , 43 ]. (Fig. 2 )
Gain of CAR-T cell function by CRISPR screening
Zhang Feng’s research group has advanced CRISPRa and CRISPRi technologies, increasingly employed for screening and modifying genes related to T cell function [ 44 , 45 , 46 ]. These technologies entail the introduction of CRISPRa and CRISPRi libraries into T cells using AAV or LV vectors. Through in vitro and in vivo experiments, target genes associated with T cell cytotoxicity are identified, enhancing the tumor-killing ability of CAR-T cells through single or multiple gene editing. This innovative technology opens new possibilities for T cell therapy (Fig. 3 ).
The flow chart of CRISPR screening process to screen genes to enhance CAR-T cell function. (1) CRISPRa or CRISPRi components (including guide RNA library) can be delivered into T cells using viral vectors such as AAV or lentivirus. (2) Depending on the phenotypes of interest, either in vitro or in vivo assays can be utilized for guide RNA selection. (3) Next-generation sequencing is then conducted to assess guide RNA enrichment or deletion. (4) Target gene editing is performed in T cells using CRISPR/Cas technology. (5) The ultimate objective of these screens is to evaluate the capacity to enhance the recognition and killing of tumor cells by CAR-T cells. CRISPRa, CRISPR activation; CRISPRi, CRISPR interference; AAV, Adeno-associated virus
Sidi Chen has developed a hybrid genetic screening system in which Sleeping Beauty (SB) transposons and a sgRNA cassette are nested in adeno-associated virus (AAV) [ 47 ]. This system enables efficient gene editing in primary murine T cells and provides a screening readout. In vivo, AAV–SB-CRISPR screens were conducted to identify membrane protein targets in CD8 + T cells in mouse models of glioblastoma (GBM). The screen hits, including PDIA3, MGAT5, EMP1, and LAG3 gene editing, were validated through the adoptive transfer of CD8 + T cells, enhancing the survival of GBM-bearing mice in both syngeneic and T cell receptor transgenic models [ 47 ]. In another study by Hongbo Chi et al., an in vivo pooled CRISPR-Cas9 screening system was employed to target REGNASE-1 in CD8 + T cells. The results demonstrated that CD8 + T cells with REGNASE-1 knockout exhibited long-lived effector cells with extensive accumulation, better persistence, and robust effector function in tumors [ 48 ]. Further research showed that knockout of REGNASE-1 enhances CAR-T cell persistence and CAR-T-mediated antitumor immunity in murine and human xenograft B-ALL models. This was achieved by targeting TCF7 mRNA to inhibit the formation of precursors of exhausted T cells (T PEX ) [ 49 ].
Alexander Marson conducted multiple genome-wide CRISPR knock-out screens under different immunosuppressive conditions to identify genes that can be targeted to prevent T cell dysfunction. These screens revealed that RASA2, a RAS GTPase-activating protein (RasGAP), serves as a signaling checkpoint. It has been established that RASA2 is downregulated upon acute T cell receptor stimulation and gradually increases with chronic antigen exposure. Ablation of RASA2 was found to enhance MAPK signaling and CAR-T cell cytolytic activity in response to the target antigen [ 50 ]. CRISPR screening also identified that inactivating MED12 or CCNC in CAR-T cells increased T cell expansion and metabolic fitness, ultimately enhancing T cell effector activity [ 51 ].
Jeremy N. Rich et al. utilized CRISPR screening in CAR-T cells to identify the knockout of TLE4 and IKZF2, which enhanced the efficacy of CAR-T cells against glioblastoma [ 52 ]. Douglas R. Green et al. employed CRISPR screening to identify inhibitors of antigen-specific memory T cell generation in vivo. Their study revealed the crucial role of the cBAF complex in T cell fate decisions and CAR-T cells [ 53 ]. Sidi Chen’s lab devised a dead-guide RNA (dgRNA)-based CRISPR activation screen system in primary CD8 + T cells. Through this system, they identified gain-of-function targets for CAR-T engineering. They demonstrated that overexpressing PRODH2, which takes part in proline metabolism, enhances CAR-T-based killing and in vivo efficacy in various cancer models. These findings not only present a method for identifying immune boosters with gain-of-function, but also highlight PRODH2 as a target to enhance CAR-T efficacy by reshaping gene expression and metabolic programs [ 54 ].
Sidi Chen developed a system called CLASH that harnesses Cas12a/Cpf1 mRNA and pooled adeno-associated viruses to facilitate simultaneous gene editing and precise transgene knockin using massively parallel homology-directed repair. This system generates a pool of stably integrated mutant variants, each with targeted gene editing. They applied this technology in primary human T cells and observed that mutation of PRDM1 in CAR-T cells resulted in increased proliferation, stem-like properties, central memory, and longevity. Consequently, these cells demonstrated higher efficacy in vivo in CD19 + CD22 + NALM6 cancer models and in the HER2 + HT29 tumor model [ 55 ]. (Fig. 3 )
The clinical trial for CRISPR-based CAR-T cells
In a Phase I study, CTA101, universal CD19/CD22-targeting CAR-T cells, were infused into patients with r/r ALL. These CAR-T cells featured a CRISPR/Cas9-disrupted TRAC region and CD52 gene to prevent host immune-mediated rejection. On day 28 post-infusion, the complete remission (CR) rate was 83.3%. With a median follow-up of 4.3 months, these CRISPR/Cas9-engineered CAR-T cells displayed a manageable safety profile and significant antileukemia activity. The technology achieved highly efficient, high-fidelity gene editing, resulting in the production of universal CAR-T cells without observable genotoxicity or chromosomal translocations [ 56 ].
Utilizing next-generation CRISPR/Cas9 editing, CAR expression was linked to multiplexed DNA editing of TRAC and CD52 by incorporating self-duplicating CRISPR guide RNA expression cassettes within the 3’ long terminal repeat of a CAR19 lentiviral vector. In a study treating children with relapsed/refractory CD19-positive B cell acute lymphoblastic leukemia (B-ALL), six patients received TT52CAR19 T cells. Four of these patients exhibited cell expansion, achieved flow cytometric remission, and subsequently underwent allogeneic stem cell transplantation. While two patients experienced grade II cytokine release syndrome requiring intervention, one patient developed transient grade IV neurotoxicity, and another developed skin GVHD, resolving after transplant conditioning. This study demonstrated the feasibility, safety, and therapeutic potential of CRISPR-engineered immunotherapy [ 57 ].
Furthermore, TCR and B2M double-disrupted universal CAR T cells were generated from healthy donor T cells using lentivirus and CRISPR/Cas9 genome-editing technology to treat DLBCL. Although the study had limitations regarding safety and clinical response, the pooled analysis represents a significant advancement in the development of universal CAR T cells for improving safety, efficacy, and feasibility in patients with hematological malignancies [ 58 ].
In a single-arm phase I dose-escalation clinical trial evaluating PD1-19bbz in adult patients with r/r B-NHL, twenty-one patients received PD1-19bbz infusion. Most patients had advanced disease stages and intermediate or worse risk stratifications. Notably, some participants exhibited high levels of programmed death ligand-1 (PD-L1) expression in pre-treatment tumor samples. PD1-19bbz demonstrated promising efficacy with a manageable toxicity profile in this first-in-human study of non-viral specifically integrated CAR-T products. A phase I/II trial of PD1-19bbz in a larger patient cohort is currently underway [ 17 ].
PD-1-mediated immunosuppression likely limits the efficacy of CAR-T cells in solid tumors. Researchers utilized CRISPR/Cas9 to create PD-1 and TCR deficient mesothelin-specific CAR-T (MPTK-CAR-T) cells and assessed them in a dose-escalation study with 15 patients. No dose-limiting toxicity or unexpected adverse events occurred. Only two patients showed stable disease as the best overall response. Circulating MPTK-CAR-T cells peaked at days 7–14 and declined thereafter. TCR-positive CAR-T cells were predominantly detected post-infusion. Animal models also confirmed the reduced persistence of TCR-deficient CAR-T cells. These findings establish the feasibility and safety of CRISPR-engineered CAR-T cells with PD-1 disruption and underscore the role of natural TCR in CAR-T cell persistence in solid tumor therapy [ 59 ].
CAR-T therapy for T cell malignancies faces challenges such as CAR-T cell fratricide and blast contamination. Allogeneic CAR-T cells from healthy donors offer blast-free products but risk graft-versus-host disease (GvHD) and rejection. We developed CD7-targeting CAR-T cells (RD13-01) from healthy donors with genetic modifications for fratricide resistance, GvHD prevention, and enhanced antitumor function. In a phase I trial (NCT04538599) with twelve patients (eleven with T cell leukemia/lymphoma, one with CD7-expressing AML), all met endpoints, with eleven proceeding to efficacy evaluation. No dose-limiting toxicity, GvHD, neurotoxicity, or severe cytokine release syndrome (grade ≥ 3) occurred. At 28 days post-infusion, 81.8% showed objective responses, with a complete response rate of 63.6% (including the AML patient). Three patients underwent allogeneic stem cell transplantation, and four remained in complete remission at a median follow-up of 10.5 months. CMV/EBV reactivation was observed, and one patient died from EBV-associated DLBCL. Expansion of CD7-negative T cells was detected post-infusion. This Phase I trial demonstrates the safety and efficacy of RD13-01 allogeneic CAR-T cells for CD7 + tumors [ 60 ].
In Table 3 , we summarize the clinical trials of CRISPR- based CAR-T therapies. The clinical trials demonstrate the potential of CRISPR-based CAR-T cell therapy in treating hematological malignancies and solid tumors, but it also presents several challenges in terms of safety and effectiveness.
Conclusion and prospects
The development of the CRISPR system used in human primary T cells has undergone rapid progression over the past decade, especially for gene knockout applications, with relatively high efficiency for both single and multiple gene targeting. Knockout of molecules involved in T cell exhaustion and suppression of T cell function by the CRISPR system can significantly enhance the function of CAR-T cells, providing a new approach for CAR-T cell applications in solid tumors and hematological malignancies.
While CRISPR-based CAR-T cell therapy presents great promise, its application in preclinical studies or clinical trials is fraught with challenges, particularly concerning safety and efficacy. Here, we explore the pivotal safety considerations associated with CRISPR technology and propose potential solutions. (1) Mitigation of Off-Target Effects: The CRISPR system can sometimes induce unwanted mutations at off-target sites within the genome. By employing advanced bioinformatics tools for designing gRNAs and utilizing CRISPR variants with enhanced specificity, the occurrence of off-target effects can be controlled. (2) Immune Response and Immunogenicity: CRISPR-edited cells may trigger immune responses in recipients, potentially leading to rejection or adverse reactions. Strategies to reduce immunogenicity include the selection of non-immunogenic CRISPR components or the use of immunomodulatory agents, which are currently under investigation. (3) Insertional Mutagenesis: Viral vectors used in CRISPR delivery could integrate the CAR gene into the host genome, thereby posing risks of insertional mutagenesis and oncogenesis. Employing non-integrating delivery methods such as mRNA-based or PiggyBac transposon-based approaches can mitigate these risks. (4) Genomic Stability: Ensuring the genomic stability of CRISPR-edited cells is crucial to prevent unintended genetic alterations. Periodic genomic profiling and long-term monitoring of treated patients are essential to assess the stability of edited genomes and to detect any potential abnormalities. (5) Long-Term Effects: Long-term follow-up studies are necessary to evaluate the durability of the therapeutic response and monitor any late-onset adverse events associated with CRISPR-based CAR-T cell therapies. This includes assessing potential long-term effects on the immune system, hematopoiesis, and overall health. Addressing these safety concerns requires rigorous preclinical evaluation, careful patient selection, and continuous monitoring during clinical trials. Another challenge lies in ensuring the efficacy of the treatment, including stable expression of CAR-T cells in the body and their ability to recognize and eliminate tumor cells. This necessitates consideration of the complexities of cellular engineering in the design and optimization of therapeutic protocols and appropriate evaluation and adjustments during clinical trials. Furthermore, the cost and complexity of manufacturing CRISPR-based CAR-T cell therapies pose additional challenges. Optimizing production processes, enhancing the standardization of technology, and reducing manufacturing costs are key factors in advancing this field. In summary, CRISPR-based CAR-T cell therapies hold immense potential in the treatment of various diseases, particularly cancer. While CRISPR/Cas9 remains the most explored system due to its efficiency and relatively better understood characteristics, both CRISPR/Cas12a and CRISPR/Cas13d show promise, each with their unique advantages. The safety and efficacy of these therapies are being actively investigated, and with further research, they can be optimized to provide safer and more effective treatments in the future.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
Chimeric Antigen Receptor T cell
Single-Chain Fragment Variable
B-cell Acute Lymphoblastic Leukemia
Non-Hodgkin Lymphoma
Multiple Myeloma
Lenti-Virus
Retroviral Vector
Adeno-Associated Virus
Transcription Activator-Like Effector Nucleases
Zinc-Finger Nucleases
Clustered Regularly Interspaced Short Palindromic Repeats
Double-Stranded Break
Homology-Directed Repair
Non-Homologous End Joining
Single Guide RNA
Protospacer Adjacent Motif
CRISPR Activation
CRISPR Interference
Peripheral Blood Mononuclear Cells
Graft-Versus-Host Disease
Hematopoietic Stem Cell Transplant
T Cell Receptor
Major Histocompatibility Complex
Prostate Stem Cell Antigen
Immune-Related Adverse Events
T cell Acute Lymphoblastic Leukemia
Tumor Necrosis Factor Alpha
Activation-Induced Cell Death
TGF-β Binds to the TGF-β Receptor
TGF Receptor II
Glycerol Diglyceride Kinase
Granulocyte Macrophage Colony Stimulating Factor
Cytokine Release Syndrome
Double-Stranded DNA
Sleeping Beauty
Glioblastoma
Precursor Exhausted T Cells
RAS GTPase-Activating Protein
Dead-Guide RNA
Obligate Mobile Element-Guided Activity Omega
Good Manufacturing Practice
Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–4.
Article CAS PubMed PubMed Central Google Scholar
Bailey SR, Maus MV. Gene editing for immune cell therapies. Nat Biotechnol. 2019;37:1425–34.
Article CAS PubMed Google Scholar
Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11:636–46.
Katsuyama T, Akmammedov A, Seimiya M, Hess SC, Sievers C, Paro R. An efficient strategy for TALEN-mediated genome engineering in Drosophila. Nucleic Acids Res. 2013;41:e163.
Palpant NJ, Dudzinski D. Zinc finger nucleases: looking toward translation. Gene Ther. 2013;20:121–7.
Gaj T, Gersbach CA, Barbas CF 3. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405.
Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–78.
Tieu V, Sotillo E, Bjelajac JR, Chen C, Malipatlolla M, Guerrero JA, Xu P, Quinn PJ, Fisher C, Klysz D, et al. A versatile CRISPR-Cas13d platform for multiplexed transcriptomic regulation and metabolic engineering in primary human T cells. Cell. 2024;187:1278–e12951220.
Dong MB, Tang K, Zhou X, Zhou JJ, Chen S. Tumor immunology CRISPR screening: present, past, and future. Trends Cancer. 2022;8:210–25.
Roth TL, Puig-Saus C, Yu R, Shifrut E, Carnevale J, Li PJ, Hiatt J, Saco J, Krystofinski P, Li H, et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature. 2018;559:405–9.
Choi BD, Yu X, Castano AP, Darr H, Henderson DB, Bouffard AA, Larson RC, Scarfo I, Bailey SR, Gerhard GM, et al. CRISPR-Cas9 disruption of PD-1 enhances activity of universal EGFRvIII CAR T cells in a preclinical model of human glioblastoma. J Immunother Cancer. 2019;7:304.
Article PubMed PubMed Central Google Scholar
Hendel A, Bak RO, Clark JT, Kennedy AB, Ryan DE, Roy S, Steinfeld I, Lunstad BD, Kaiser RJ, Wilkens AB, et al. Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nat Biotechnol. 2015;33:985–9.
Kim HK, Song M, Lee J, Menon AV, Jung S, Kang YM, Choi JW, Woo E, Koh HC, Nam JW, Kim H. In vivo high-throughput profiling of CRISPR-Cpf1 activity. Nat Methods. 2017;14:153–9.
Dai X, Park JJ, Du Y, Kim HR, Wang G, Errami Y, Chen S. One-step generation of modular CAR-T cells with AAV-Cpf1. Nat Methods. 2019;16:247–54.
Zhang L, Zuris JA, Viswanathan R, Edelstein JN, Turk R, Thommandru B, Rube HT, Glenn SE, Collingwood MA, Bode NM, et al. AsCas12a ultra nuclease facilitates the rapid generation of therapeutic cell medicines. Nat Commun. 2021;12:3908.
Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJ, Hamieh M, Cunanan KM, Odak A, Gonen M, Sadelain M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–7.
Zhang J, Hu Y, Yang J, Li W, Zhang M, Wang Q, Zhang L, Wei G, Tian Y, Zhao K, et al. Non-viral, specifically targeted CAR-T cells achieve high safety and efficacy in B-NHL. Nature. 2022;609:369–74.
Jing R, Jiao P, Chen J, Meng X, Wu X, Duan Y, Shang K, Qian L, Huang Y, Liu J, et al. Cas9-Cleavage sequences in size-reduced plasmids enhance nonviral genome targeting of CARs in primary human T cells. Small Methods. 2021;5:e2100071.
Article PubMed Google Scholar
Nguyen DN, Roth TL, Li PJ, Chen PA, Apathy R, Mamedov MR, Vo LT, Tobin VR, Goodman D, Shifrut E, et al. Polymer-stabilized Cas9 nanoparticles and modified repair templates increase genome editing efficiency. Nat Biotechnol. 2020;38:44–9.
Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19:185–99.
Stenger D, Stief TA, Kaeuferle T, Willier S, Rataj F, Schober K, Vick B, Lotfi R, Wagner B, Grunewald TGP, et al. Endogenous TCR promotes in vivo persistence of CD19-CAR-T cells compared to a CRISPR/Cas9-mediated TCR knockout CAR. Blood. 2020;136:1407–18.
Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130–44.
Amezquita RA, Kaech SM. Immunology: the chronicles of T-cell exhaustion. Nature. 2017;543:190–1.
Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–99.
Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory pathways in Immunotherapy for Cancer. Annu Rev Immunol. 2016;34:539–73.
Cao Y, Lu W, Sun R, Jin X, Cheng L, He X, Wang L, Yuan T, Lyu C, Zhao M. Anti-CD19 Chimeric Antigen Receptor T Cells in Combination with Nivolumab are safe and effective against Relapsed/Refractory B-Cell non-hodgkin Lymphoma. Front Oncol. 2019;9:767.
Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, June CH, Schuster SJ. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood. 2017;129:1039–41.
Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chavez A, Keegan N, Khamashta MA, Lambotte O, Mariette X, Prat A, Suarez-Almazor ME. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38.
Hu W, Zi Z, Jin Y, Li G, Shao K, Cai Q, Ma X, Wei F. CRISPR/Cas9-mediated PD-1 disruption enhances human mesothelin-targeted CAR T cell effector functions. Cancer Immunol Immunother. 2019;68:365–77.
Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, Zhao Y. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget. 2017;8:17002–11.
Zhang Y, Zhang X, Cheng C, Mu W, Liu X, Li N, Wei X, Liu X, Xia C, Wang H. CRISPR-Cas9 mediated LAG-3 disruption in CAR-T cells. Front Med. 2017;11:554–62.
Campana D, van Dongen JJ, Mehta A, Coustan-Smith E, Wolvers-Tettero IL, Ganeshaguru K, Janossy G. Stages of T-cell receptor protein expression in T-cell acute lymphoblastic leukemia. Blood. 1991;77:1546–54.
Chen KH, Wada M, Pinz KG, Liu H, Lin KW, Jares A, Firor AE, Shuai X, Salman H, Golightly M, et al. Preclinical targeting of aggressive T-cell malignancies using anti-CD5 chimeric antigen receptor. Leukemia. 2017;31:2151–60.
Dai Z, Mu W, Zhao Y, Cheng J, Lin H, Ouyang K, Jia X, Liu J, Wei Q, Wang M, et al. T cells expressing CD5/CD7 bispecific chimeric antigen receptors with fully human heavy-chain-only domains mitigate tumor antigen escape. Signal Transduct Target Ther. 2022;7:85.
Dai Z, Mu W, Zhao Y, Jia X, Liu J, Wei Q, Tan T, Zhou J. The rational development of CD5-targeting biepitopic CARs with fully human heavy-chain-only antigen recognition domains. Mol Ther. 2021;29:2707–22.
Benyahia B, Bensaid Y, Ammar F, Dhobb M, Benjelloun A, Benabderrazik T. [Arteriopathies of the lower limbs in adults under 40 years of age]. Chirurgie. 1989;115(Suppl 1):18–26. discussion 26 – 17.
PubMed Google Scholar
Lu Y, Xue J, Deng T, Zhou X, Yu K, Deng L, Huang M, Yi X, Liang M, Wang Y, et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat Med. 2020;26:732–40.
Pickup M, Novitskiy S, Moses HL. The roles of TGFbeta in the tumour microenvironment. Nat Rev Cancer. 2013;13:788–99.
Tang N, Cheng C, Zhang X, Qiao M, Li N, Mu W, Wei XF, Han W, Wang H. TGF-beta inhibition via CRISPR promotes the long-term efficacy of CAR T cells against solid tumors. JCI Insight 2020, 5.
Giuffrida L, Sek K, Henderson MA, Lai J, Chen AXY, Meyran D, Todd KL, Petley EV, Mardiana S, Molck C, et al. CRISPR/Cas9 mediated deletion of the adenosine A2A receptor enhances CAR T cell efficacy. Nat Commun. 2021;12:3236.
Jung IY, Kim YY, Yu HS, Lee M, Kim S, Lee J. CRISPR/Cas9-Mediated knockout of DGK improves Antitumor activities of Human T cells. Cancer Res. 2018;78:4692–703.
Sterner RM, Sakemura R, Cox MJ, Yang N, Khadka RH, Forsman CL, Hansen MJ, Jin F, Ayasoufi K, Hefazi M, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133:697–709.
Yi Y, Chai X, Zheng L, Zhang Y, Shen J, Hu B, Tao G. CRISPR-edited CART with GM-CSF knockout and auto secretion of IL6 and IL1 blockers in patients with hematologic malignancy. Cell Discov. 2021;7:27.
Wang D, Zhang F, Gao G. CRISPR-Based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell. 2020;181:136–50.
Patel SJ, Sanjana NE, Kishton RJ, Eidizadeh A, Vodnala SK, Cam M, Gartner JJ, Jia L, Steinberg SM, Yamamoto TN, et al. Identification of essential genes for cancer immunotherapy. Nature. 2017;548:537–42.
Joung J, Konermann S, Gootenberg JS, Abudayyeh OO, Platt RJ, Brigham MD, Sanjana NE, Zhang F. Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening. Nat Protoc. 2017;12:828–63.
Ye L, Park JJ, Dong MB, Yang Q, Chow RD, Peng L, Du Y, Guo J, Dai X, Wang G, et al. In vivo CRISPR screening in CD8 T cells with AAV-Sleeping Beauty hybrid vectors identifies membrane targets for improving immunotherapy for glioblastoma. Nat Biotechnol. 2019;37:1302–13.
Wei J, Long L, Zheng W, Dhungana Y, Lim SA, Guy C, Wang Y, Wang YD, Qian C, Xu B, et al. Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature. 2019;576:471–6.
Zheng W, Wei J, Zebley CC, Jones LL, Dhungana Y, Wang YD, Mavuluri J, Long L, Fan Y, Youngblood B, et al. Regnase-1 suppresses TCF-1 + precursor exhausted T-cell formation to limit CAR-T-cell responses against ALL. Blood. 2021;138:122–35.
Carnevale J, Shifrut E, Kale N, Nyberg WA, Blaeschke F, Chen YY, Li Z, Bapat SP, Diolaiti ME, O’Leary P, et al. RASA2 ablation in T cells boosts antigen sensitivity and long-term function. Nature. 2022;609:174–82.
Freitas KA, Belk JA, Sotillo E, Quinn PJ, Ramello MC, Malipatlolla M, Daniel B, Sandor K, Klysz D, Bjelajac J, et al. Enhanced T cell effector activity by targeting the mediator kinase module. Science. 2022;378:eabn5647.
Wang D, Prager BC, Gimple RC, Aguilar B, Alizadeh D, Tang H, Lv D, Starr R, Brito A, Wu Q, et al. CRISPR Screening of CAR T cells and Cancer stem cells reveals critical dependencies for cell-based therapies. Cancer Discov. 2021;11:1192–211.
Guo A, Huang H, Zhu Z, Chen MJ, Shi H, Yuan S, Sharma P, Connelly JP, Liedmann S, Dhungana Y, et al. cBAF complex components and MYC cooperate early in CD8(+) T cell fate. Nature. 2022;607:135–41.
Ye L, Park JJ, Peng L, Yang Q, Chow RD, Dong MB, Lam SZ, Guo J, Tang E, Zhang Y, et al. A genome-scale gain-of-function CRISPR screen in CD8 T cells identifies proline metabolism as a means to enhance CAR-T therapy. Cell Metab. 2022;34:595–e614514.
Dai X, Park JJ, Du Y, Na Z, Lam SZ, Chow RD, Renauer PA, Gu J, Xin S, Chu Z et al. Massively parallel knock-in engineering of human T cells. Nat Biotechnol 2023.
Hu Y, Zhou Y, Zhang M, Ge W, Li Y, Yang L, Wei G, Han L, Wang H, Yu S, et al. CRISPR/Cas9-Engineered Universal CD19/CD22 Dual-targeted CAR-T cell therapy for Relapsed/Refractory B-cell Acute Lymphoblastic Leukemia. Clin Cancer Res. 2021;27:2764–72.
Ottaviano G, Georgiadis C, Gkazi SA, Syed F, Zhan H, Etuk A, Preece R, Chu J, Kubat A, Adams S, et al. Phase 1 clinical trial of CRISPR-engineered CAR19 universal T cells for treatment of children with refractory B cell leukemia. Sci Transl Med. 2022;14:eabq3010.
Guo Y, Tong C, Su L, Zhang W, Jia H, Liu Y, Yang Q, Wu Z, Wang Y, Han W. CRISPR/Cas9 genome-edited universal CAR T cells in patients with relapsed/refractory lymphoma. Blood Adv. 2022;6:2695–9.
Wang Z, Li N, Feng K, Chen M, Zhang Y, Liu Y, Yang Q, Nie J, Tang N, Zhang X, et al. Phase I study of CAR-T cells with PD-1 and TCR disruption in mesothelin-positive solid tumors. Cell Mol Immunol. 2021;18:2188–98.
Hu Y, Zhou Y, Zhang M, Zhao H, Wei G, Ge W, Cui Q, Mu Q, Chen G, Han L, et al. Genetically modified CD7-targeting allogeneic CAR-T cell therapy with enhanced efficacy for relapsed/refractory CD7-positive hematological malignancies: a phase I clinical study. Cell Res. 2022;32:995–1007.
Download references
Acknowledgements
Not applicable.
This study was funded by the medical and health research project of Zhejiang province (grant numbers: 2024KY1312), Zhejiang Provincial Basic Public Welfare Research Project (grant number: LGF22H160082), Zhejiang Medical and Health Science and Technology Plan Project (grant number:2022KY939) and Hangzhou Medical and Health Science and Technology Major Project (grant number: Z20210025).
Author information
Ping Song, Qiqi Zhang and Zhiyong Xu contributed equally to this work.
Authors and Affiliations
Department of Surgical Oncology, Affiliated Hangzhou First People’s Hospital, Westlake University School of Medicine, No. 261, Huansha Road, Shangcheng district, Hangzhou 310006, Zhejiang, P. R. China
Ping Song & Dingcun Luo
Bone Marrow Transplantation Center, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Qiqi Zhang & Ruirui Jing
Department of Respiratory Medicine, The Fourth Affiliated Hospital, International Institutes of Medicine, Zhejiang University School of Medicine, Yiwu City, China
Zhiyong Xu & Yueli Shi
The Fourth Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou 310006, Zhejiang, China
Dingcun Luo
You can also search for this author in PubMed Google Scholar
Contributions
D.L. and R.J. initiated the project and devised the main conceptual ideas. P.S., R.J., Q.Z., Z.X., and Y.S. drafted and revised the manuscript. All authors reviewed and approved the submitted version of the manuscript.
Corresponding author
Correspondence to Dingcun Luo .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Song, P., Zhang, Q., Xu, Z. et al. CRISPR/Cas-based CAR-T cells: production and application. Biomark Res 12 , 54 (2024). https://doi.org/10.1186/s40364-024-00602-z
Download citation
Received : 25 December 2023
Accepted : 21 May 2024
Published : 31 May 2024
DOI : https://doi.org/10.1186/s40364-024-00602-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Immunotherapy
- CAR-T cell therapy
- Gene editing
- CRISPR/Cas9
Biomarker Research
ISSN: 2050-7771
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 30 August 2023
Enhancing reproducibility in human stem cell research
- Stylianos Lefkopoulos 1
Nature Cell Biology volume 25 , pages 1237–1239 ( 2023 ) Cite this article
946 Accesses
2 Citations
7 Altmetric
Metrics details
In June 2023, the International Society for Stem Cell Research (ISSCR) released a report detailing standards for human stem cell research . We spoke to the co-chairs of the Steering Committee, Tenneille Ludwig, Senior Scientist and Director of the WiCell Stem Cell Bank, and Peter W. Andrews, Emeritus Professor at the University of Sheffield, and discussed the purpose and some of the basic aspects of these standards.
This is a preview of subscription content, access via your institution
Relevant articles
Open Access articles citing this article.
Advancements in Human Embryonic Stem Cell Research: Clinical Applications and Ethical Issues
- Soo Jin Park
- , Yoon Young Kim
- … Seung-Yup Ku
Tissue Engineering and Regenerative Medicine Open Access 19 March 2024
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Author information
Authors and affiliations.
Nature Cell Biology https://www.nature.com/ncb/
Stylianos Lefkopoulos
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Stylianos Lefkopoulos .
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Lefkopoulos, S. Enhancing reproducibility in human stem cell research. Nat Cell Biol 25 , 1237–1239 (2023). https://doi.org/10.1038/s41556-023-01218-5
Download citation
Published : 30 August 2023
Issue Date : September 2023
DOI : https://doi.org/10.1038/s41556-023-01218-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
- Yoon Young Kim
- Seung-Yup Ku
Tissue Engineering and Regenerative Medicine (2024)
Setting standards for stem cells
Nature Methods (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Research Council (US) and Institute of Medicine (US) Committee on the Biological and Biomedical Applications of Stem Cell Research. Stem Cells and the Future of Regenerative Medicine. Washington (DC): National Academies Press (US); 2002.

Stem Cells and the Future of Regenerative Medicine.
- Hardcopy Version at National Academies Press
Executive Summary
S tem cell research offers unprecedented opportunities for developing new medical therapies for debilitating diseases and a new way to explore fundamental questions of biology. Stem cells are unspecialized cells that can self-renew indefinitely and also differentiate into more mature cells with specialized functions. Research on human embryonic stem cells, however, is controversial, given the diverse views held in our society about the moral and legal status of the early embryo. The controversy has encouraged provocative and conflicting claims both inside and outside the scientific community about the biology and biomedical potential of both adult and embryonic stem cells.
The National Research Council and Institute of Medicine formed the Committee on the Biological and Biomedical Applications of Stem Cell Research to address the potential of stem cell research. The committee organized a workshop that was held on June 22, 2001. At the workshop, the committee heard from many leading scientists who are engaged in stem cell research and from philosophers, ethicists, and legal scholars. (Audio files of the speakers' presentations are available until December 31, 2002, at the workshop Web site, www.nationalacademies.org/stemcells .)
The participants discussed the science of stem cells and a variety of ethical and other arguments relevant to public policy as it applies to stem cells. The committee considered the information presented, explored the literature on its own, and contemplated the substance and importance of the preliminary data from recent stem cell experiments. The committee's deliberations on the issues led to the following conclusions and recommendations.
- Experiments in mice and other animals are necessary, but not sufficient, for realizing the potential of stem cells to develop tissue-replacement therapies that will restore lost function in damaged organs. Because of the substantial biological differences between nonhuman animal and human development and between animal and human stem cells, studies with human stem cells are essential to make progress in the development of treatments for human disease, and this research should continue.
- There are important biological differences between adult and embryonic stem cells and among adult stem cells found in different types of tissue. The implications of these biological differences for therapeutic uses are not yet clear, and additional data are needed on all stem cell types. Adult stem cells from bone marrow have so far provided most of the examples of successful therapies for replacement of diseased or destroyed cells. Despite the enthusiasm generated by recent reports, the potential of adult stem cells to differentiate fully into other cell types (such as brain, nerve, pancreas cells) is still poorly understood and remains to be clarified. In contrast, studies of human embryonic stem cells have shown that they can develop into multiple tissue types and exhibit long-term self-renewal in culture, features that have not yet been demonstrated with many human adult stem cells. The application of stem cell research to therapies for human disease will require much more knowledge about the biological properties of all types of stem cells. Although stem cell research is on the cutting edge of biological science today, it is still in its infancy. Studies of both embryonic and adult human stem cells will be required to most efficiently advance the scientific and therapeutic potential of regenerative medicine. Moreover, research on embryonic stem cells will be important to inform research on adult stem cells, and vice versa. Research on both adult and embryonic human stem cells should be pursued.
- Over time, all cell lines in tissue culture change, typically accumulating harmful genetic mutations. There is no reason to expect stem cell lines to behave differently. In addition, most existing stem cell lines have been cultured in the presence of non-human cells or serum that could lead to potential human health risks. Consequently, while there is much that can be learned using existing stem cell lines if they are made widely available for research, such concerns necessitate continued monitoring of these cells as well as the development of new stem cell lines in the future.
- High-quality, publicly funded research is the wellspring of medical breakthroughs. Although private, for-profit research plays a critical role in translating the fruits of basic research into medical advances that are broadly available to the public, stem cell research is far from the point of providing therapeutic products. Without public funding of basic research on stem cells, progress toward medical therapies is likely to be hindered. In addition, public funding offers greater opportunities for regulatory oversight and public scrutiny of stem cell research. Stem cell research that is publicly funded and conducted under established standards of open scientific exchange, peer review, and public oversight offers the most efficient and responsible means of fulfilling the promise of stem cells to meet the need for regenerative medical therapies.
- Conflicting ethical perspectives surround the use of embryonic stem cells in medical research, particularly where the moral and legal status of human embryos is concerned. The use of embryonic stem cells is not the first biomedical research activity to raise ethical and social issues among the public. Restrictions and guidelines for the conduct of controversial research have been developed to address such concerns in other instances. For example, when recombinant-DNA techniques raised questions and were subject to intense debate and public scrutiny, a national advisory body, the Recombinant DNA Advisory Committee, was established at the National Institutes of Health (NIH) to ensure that the research met the highest scientific and ethical standards. If the federal government chooses to fund research on human embryonic stem cells, a similar national advisory group composed of exceptional researchers, ethicists, and other stakeholders should be established at NIH to oversee it. Such a group should ensure that proposals to work on human embryonic stem cells are scientifically justified and should scrutinize such proposals for compliance with federally mandated ethical guidelines.
- Regenerative medicine is likely to involve the implantation of new tissue in patients with damaged or diseased organs. A substantial obstacle to the success of transplantation of any cells, including stem cells and their derivatives, is the immune-mediated rejection of foreign tissue by the recipient's body. In current stem cell transplantation procedures with bone marrow and blood, success can hinge on obtaining a close match between donor and recipient tissues and on the use of immunosuppressive drugs, which often have severe and life-threatening side effects. To ensure that stem cell-based therapies can be broadly applicable for many conditions and individuals, new means to overcome the problem of tissue rejection must be found. Although ethically controversial, somatic cell nuclear transfer, a technique that produces a lineage of stem cells that are genetically identical to the donor, promises such an advantage. Other options for this purpose include genetic manipulation of the stem cells and the development of a very large bank of embryonic stem cell lines. In conjunction with research on stem cell biology and the development of stem cell therapies, research on approaches that prevent immune rejection of stem cells and stem cell-derived tissues should be actively pursued.
The committee is aware of and respectful of the wide array of social, political, legal, ethical, and economic issues that must be considered in policy-making in a democracy. And it is impressed by the commitment of all parties in this debate to life and health, regardless of the different conclusions they draw. The committee hopes that this report, by clarifying what is known about the scientific potential of stem cells and how that potential can best be realized, will be a useful contribution to the debate and to the enhancement of treatments for disabling human diseases and injuries. On August 9, 2001, when President Bush announced a new federal policy permitting limited use of human embryonic stem cells for research, this report was already in review. Because this report presents the committee's interpretation of the state of the science of stem cells independent of any specific policy, only minor modifications to refer to the new policy have been made in the report.
RECOMMENDATIONS
Studies with human stem cells are essential to make progress in the development of treatments for human disease, and this research should continue.
Although stem cell research is on the cutting edge of biological science today, it is still in its infancy. Studies of both embryonic and adult human stem cells will be required to most efficiently advance the scientific and therapeutic potential of regenerative medicine. Research on both adult and embryonic human stem cells should be pursued.
While there is much that can be learned using existing stem cell lines if they are made widely available for research, concerns about changing genetic and biological properties of these stem cell lines necessitate continued monitoring as well as the development of new stem cell lines in the future.
Human stem cell research that is publicly funded and conducted under established standards of open scientific exchange, peer review, and public oversight offers the most efficient and responsible means to fulfill the promise of stem cells to meet the need for regenerative medical therapies.
If the federal government chooses to fund human stem cell research, proposals to work on human embryonic stem cells should be required to justify the decision on scientific grounds and should be strictly scrutinized for compliance with existing and future federally mandated ethical guidelines.
A national advisory group composed of exceptional researchers, ethicists, and other stakeholders should be established at the National Institutes of Health (NIH) to oversee research on human embryonic stem cells. The group should include leading experts in the most current scientific knowledge relevant to stem cell research who can evaluate the technical merit of any proposed research on human embryonic stem cells. Other roles for the group could include evaluation of potential risks to research subjects and ensuring compliance with all legal requirements and ethical standards.
In conjunction with research on stem cell biology and the development of potential stem cell therapies, research on approaches that prevent immune rejection of stem cells and stem cell-derived tissues should be actively pursued. These scientific efforts include the use of a number of techniques to manipulate the genetic makeup of stem cells, including somatic cell nuclear transfer.
- Cite this Page National Research Council (US) and Institute of Medicine (US) Committee on the Biological and Biomedical Applications of Stem Cell Research. Stem Cells and the Future of Regenerative Medicine. Washington (DC): National Academies Press (US); 2002. Executive Summary.
- PDF version of this title (5.0M)
Recent Activity
- Executive Summary - Stem Cells and the Future of Regenerative Medicine Executive Summary - Stem Cells and the Future of Regenerative Medicine
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Open access
- Published: 03 June 2024
Extracellular vesicles activated cancer-associated fibroblasts promote lung cancer metastasis through mitophagy and mtDNA transfer
- Zhuan Zhou 1 , 2 , 3 na1 ,
- Chunhui Qu 1 , 2 na1 ,
- Peijun Zhou 1 , 2 ,
- Qin Zhou 1 ,
- Xia Wu 2 , 5 &
- Lifang Yang ORCID: orcid.org/0000-0002-3012-8350 1 , 2
Journal of Experimental & Clinical Cancer Research volume 43 , Article number: 158 ( 2024 ) Cite this article
Metrics details
Studies have shown that oxidative stress and its resistance plays important roles in the process of tumor metastasis, and mitochondrial dysfunction caused by mitochondrial DNA (mtDNA) damage is an important molecular event in oxidative stress. In lung cancer, the normal fibroblasts (NFs) are activated as cancer-associated fibroblasts (CAFs), and act in the realms of the tumor microenvironment (TME) with consequences for tumor growth and metastasis. However, its activation mechanism and whether it participates in tumor metastasis through antioxidative stress remain unclear.
The role and signaling pathways of tumor cell derived extracellular vesicles (EVs) activating NFs and the characteristic of induced CAFs (iCAFs) were measured by the transmission electron microscopy, nanoparticle tracking analysis, immunofluorescence, collagen contraction assay, quantitative PCR, immunoblotting, luciferase reporter assay and mitochondrial membrane potential detection. Mitochondrial genome and single nucleotide polymorphism sequencing were used to investigate the transport of mtDNA from iCAFs to ρ 0 cells, which were tumor cells with mitochondrial dysfunction caused by depletion of mtDNA. Further, the effects of iCAFs on mitochondrial function, growth and metastasis of tumor cells were analysed in co-culture models both in vitro and in vivo, using succinate dehydrogenase, glutathione and oxygen consumption rate measurements, CCK-8 assay, transwell assay, xenotransplantation and metastasis experiments as well as in situ hybridization and immunohistochemistry.
Our findings revealed that EVs derived from high-metastatic lung cancer cells packaged miR-1290 that directly targets MT1G, leading to activation of AKT signaling in NFs and inducing NFs conversion to CAFs. The iCAFs exhibit higher levels of autophagy and mitophagy and more mtDNA release, and reactive oxygen species (ROS) could further promote this process. After cocultured with the conditioned medium (CM) of iCAFs, the ρ 0 cells may restore its mitochondrial function by acquisition of mtDNA from CAFs, and further promotes tumor metastasis.
Conclusions
These results elucidate a novel mechanism that CAFs activated by tumor-derived EVs can promote metastasis by transferring mtDNA and restoring mitochondrial function of tumor cells which result in resistance of oxidative stress, and provide a new therapeutic target for lung cancer metastasis.
Lung cancer remains the highest incidence of mortality and the second highest incidence of morbidity among all tumors [ 1 ]. In recent years, although many advances such as surgery, radiation, chemotherapy and immunotherapies have been made in the treatment of lung cancer, locally advanced or distally metastatic tumors are still major causes of poor prognosis [ 2 ]. Therefore, the elucidation of metastatic pathways and mechanisms will provide a novel target for prediction and precisely treatment of lung cancer.
Metastasis, a multistep process involving dissemination of cancer cells from a primary site to distant tissues and organs. During the initiation of metastasis, most tumor cells undergo oxidative stress when they detach from the local extracellular matrix (ECM), resulting in cell death and the inefficiency of the metastasis [ 3 ]. However, Piskounova et al. show that circulating tumor cells (CTCs) isolated from blood and from metastatic sites with increased antioxidant capacity are able to suppress tumor cell death and promote melanoma cell metastasis [ 4 ]. Laoukili et al. demonstrate that the BRAF V600E oncogene increases the capacity of disseminated tumor cells (DTCs) to withstand metastasis associated oxidative stress by stimulating glutathione synthesis. This pathway promotes the formation of liver and lung metastases [ 5 ]. Thus, metastatic tumor cells not only keep reactive oxygen species (ROS) levels higher than in primary tumor cells, but also maintain an increased antioxidant capacity, as they travel through the bloodstream and initiate new metastatic lesions [ 6 ]. Therefore, understanding how oxidative stress and its resistance in tumor cells lead to tumor metastasis will provide important evidence for elucidating the mechanism of tumor metastasis.
Mitochondria are not only the center of cellular energy metabolism, but also the main organelles involved in oxidative stress within cells. ROS can reduce the antioxidant function of mitochondria, lead to disorder of free radical metabolism, and trigger cell apoptosis by inducing mitochondrial DNA (mtDNA) damage and mediating downstream signaling [ 7 ]. In recent years, research have found that mtDNA damage is a key molecular event in oxidative stress and lead to mitochondrial dysfunction during cancer metastasis [ 8 ]. In microsatellite stabilized colorectal cancer, increasing the copy number of mtDNA can promote tumor metastasis by elevated mitochondrial oxidative phosphorylation [ 9 ]. These studies demonstrate that maintaining the integrity of mtDNA and normal mitochondrial function is essential for tumor cells during the process of metastasis [ 10 ].
Tumor metastasis is closely related to the tumor microenvironment (TME), and the cancer-associated fibroblasts (CAFs) are one of the most abundant stromal components in the TME and it critically involved in cancer progression and metastasis [ 11 , 12 , 13 ]. Research shows that in many types of tumors, such as breast cancer, prostate cancer, head and neck cancer and lymphoma, the ROS produced by oxidative stress in tumor cells can cause CAFs metabolic reprogramming, increase autophagy, and produce nutrients such as lactate, ketone bodies, fatty acids, glutamine, and other amino acids to support tumor cell growth and metastasis [ 12 , 13 ]. Our previous studies had shown that the oncogenic protein LMP1 loaded on extracellular vesicles (EVs) secreted by nasopharyngeal carcinoma cells promotes the activation of CAFs, enhances the autophagy level of CAFs, and couples the produced metabolites with tumor cell metabolism, thereby promoting tumor metastasis and antiradiation [ 14 ]. This CAFs metabolic reprogramming phenomenon known as the reverse Warburg effect and it can be reversed after antioxidant treatment [ 15 , 16 ]. Therefore, it is meaningful to study the oxidative stress resistance during tumor cell metastasis from the perspective of tumor microenvironment, especially the activation and function of CAFs.
In present study, the critical miR-1290 packaged by EVs was identified and its role of promoting normal fibroblasts (NFs) conversion to CAFs in lung cancer was demonstrated. Moreover, we found that cancer cells with damaged mitochondria could acquire mtDNA, which released by CAFs through mitophagy, resulting in recovery of mitochondria function, thereby resisting oxidative stress and promoting tumor metastasis.
Materials and methods
Cell culture and reagents.
95C and 95D cells were obtained from the Shanghai Institute of Cell Biology (Shanghai, China). The paired cells are human giant cell lung carcinoma cell lines, and have the same genetic background and varied metastatic capacity. 95D cells had the higher metastatic capacity [ 17 ]. The cells were cultured in DMEM (Gibco BRL, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT, USA). A549 (CRM-CCL-185) and H1299 (CRL-5803) cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). A549 was cultured in DMEM/F12 (Gibco BRL) supplemented with 10% FBS, and H1299 was cultured in DMEM supplemented with 10% FBS at 37 °C in a 5% CO 2 incubator.
Primary NFs and CAFs were cultured in our laboratory [ 18 ]. For the preparation of ρ 0 cells which was depleted of mitochondrial DNA, parental tumor cell lines A549 and H1299 cells were cultured for 30 or 60 days in low-dose Ethidium bromide (EtBr, 50 ng/mL) supplemented with 100 μg/mL pyruvate and 50 μg/mL uridine. After 60 days of culture, ρ 0 cells were transfer to medium lacking EtBr [ 19 ]. EtBr (E1510), pyruvate (P5280), uridine (U3003), DNaseI (D7291), Carbonyl cyanide 3-chlorophenylhydrazone (CCCP, C2759) and hydrogen peroxide (H 2 O 2 , 516813) were purchased from Merck Millipore (Billerica, MA, USA).
EVs isolation
EVs-depleted FBS was purchased from SBI (Palo Alto, CA, USA). Cells were cultured in DMEM with 10% EVs-depleted FBS at 37 °C for 48 h, and their culture media were collected for EVs isolation. The isolation of EVs was performed using ExoQuick-TC™ kit (EXOTC10A-1, SBI). BCA Assay reagent (23225, Pierce™ Thermo Fisher, Waltham, MA, USA) was used to measure the concentration of isolated EVs. The purified EVs resuspended in PBS were stored at − 80 °C.
Transmission electron microscopy (TEM)
The morphology of EVs was evaluated using a TEM (H-600, Hitachi, Tokyo, Japan) at a voltage of 75 kV. EVs sample was diluted with PBS and dropped on a carbon-coated copper grid. To remove unattached EVs, the grid was washed with ddH 2 O. After staining with a 2% phosphotungstic acid, the grids were air-dried and subjected to TEM assay.
Nanoparticle tracking analysis (NTA)
NTA of EVs samples was carried out using a Zeta View PMX 120 (Particle Metrix, Meerbusch, Germany) as previously described [ 14 ]. Briefly, EVs were diluted (v/v, 1:4000) with ddH 2 O, and their size distributions and particle concentrations were analyzed using the parameters (min area 5, max area 1000, min brightness 20, and camera 0.713 μm/px) at 25 °C.
Intracellular uptake of EVs
PKH67 (MINI67, Sigma-Aldrich, St. Louis MO, USA) fluorescent staining was performed to label EVs. The PKH67-labeled EVs were cocultured with NFs for 24 h in FBS-free DMEM medium at 37 °C. The cells were fixed with 4% paraformaldehyde solution for 10 min, and then the cells were incubated with phalloidin (C2207S, Beyotime, Shanghai, China) for 30 min. DAPI staining solution (E607303, BBI Life Sciences, Shanghai, China) was used to stain the cell nucleus. The stained cells were observed under a laser scanning confocal microscopy (LSM900, Zeiss, Oberkochen, Germany).
Transfection
The sequences of the mimics (miR-1290, miR-652, miR-222), inhibitors (miR-1290), and negative control were all synthesized from Ribobio Company (Guangzhou, China). The pDONR233-MT1G plasmid and empty plasmid were purchased from Youbio (G152804, Hunan, China). For the transient transfection of plasmid and miRNA-mimic/inhibitor, Lipofectamine 3000 reagent (L3000015, Invitrogen, Carlsbad, CA, USA) was used to according to the manufacturer's instructions.
Quantitative PCR (qPCR)
Trizol reagent was purchased from Thermo Fisher Scientific (15596026) to extract total RNA from cells and EVs according to the manufacturer’s instructions, and cel-miR-39 (Ribobio) was added into each EVs sample at a final concentration of 10 pmol/μL acting as external reference. Reverse transcription was performed using Mir-X™ miRNA First-Strand Synthesis Kit for miRNAs (638315, Clontech, Mountain View, CA, USA) or RevertAid First Strand cDNA Synthesis Kit (K1621, Thermo Fisher Scientific) for general genes. For mitochondrial DNA isolation, DNA was extracted from cells or cell-free supernatants with QIAamp DNA Blood Mini Kit (Qiagen, Duesseldorf, Germany). DNA was extracted from formaldehyde-fixed mouse tissue using the TIANamp FFPE DNA Kit (TIANGEN, DP331, Beijing, China) according to the manufacturer’s instructions. qPCR was conducted using SYBR Green (4309155, Life Technologies Corporation, Gaithersburg, MD, USA) and performed on ABI 7500 PCR detection system (Foster City, CA, USA). The sequences of all indicated primers were listed in Table S1-S2. The relative expression levels of mRNAs, miRNA and mtDNA were calculated with 2 –ΔΔCt method. Actin was selected as the housekeeping gene. U6 and miR-39 were selected as the housekeeping gene of miRNA for cells and EVs, respectively.
Immunoblotting
The cells were lysed in IP buffer (87787, Thermo Fisher Scientific) containing inhibitors cocktail (4693116001, Roche, Basel, Switzerland). The protein concentration was measured by BCA Assay reagent (23225, Pierce Chemical). The protein was separated by SDS-PAGE and then transferred to PVDF membrane (Merck Millipore). The membrane was incubated with first antibody at 4 °C overnight, and then incubated with the second antibody 1 h. The bands were observed with an enhanced chemiluminescence detection kit (36208-A, Yeasen, Shanghai, China) and analyzed by ChemiDoc XRS system (Bio-Rad, Hercules, CA, USA).
The antibodies were used as follows, α-SMA (14395–1-AP), Vimentin (10366–1-AP), PINK1 (23274–1-AP), AKT (60203–2-IG), p-AKT (66444–1-IG), Calnexin (10427–2-AP), E-cadherin (20874–1-AP) and Snail (13099–1-AP) were purchased from Proteintech (Wuhan, China). Actin (AC026), GAPDH (AC002), and FAP (A6349) were purchased from Abclonal (Wuhan, China). MT1G (CSB-PA17384A0Rb) was purchased from Cusabio (Wuhan, China). LC3 (L7543) was purchased from Sigma-Aldrich. BNIP3 (Ab10433), CD63 (Ab134045) and TSG101 (Ab125011) were purchased from Abcam (Cambridge, MA, USA).
Immunofluorescence (IF)
The cells were fixed with 4% paraformaldehyde solution for 10 min, and then blocked in 5% donkey serum in PBS for 1 h and incubated with the primary antibody (α-SMA, 55135–1-AP, Proteintech) at 4˚C overnight. The cells were incubated with fluorochrome-conjugated secondary antibody (Anti-Rabbit, SAB4600234, Sigma-Aldrich) for 45 min. DAPI staining solution (E607303, BBI Life Sciences) was used to stain the cell nucleus. For fluorescence analysis, cell samples were visualized on a laser scanning confocal microscopy (LSM900, Zeiss).
Collagen contraction assay
A total of 2 × 10 5 NFs were suspended in 100 μL DMEM. Then the cell suspension was mixed with 400 μL of cold collagen gel working solution (Cell Contraction Assay, CBA-201, Cell Biolabs, San Diego, CA, USA), added to one well of 24-well plates and allowed to solidify for 60 min at 37 °C. After collagen polymerization, 1.0 mL of culture medium containing EVs is added atop each collagen gel lattice. After incubation two days with medium, gently release collagen gels from the sides of the culture dishes with a sterile spatula, the gels were photographed by digital camera.
Luciferase reporter assay
The 3′ UTR segments of the MT1G genes were amplified by PCR and inserted into the vector pmiR-RB-REPORT™ (Ribobio). Co-transfections of MT1G 3′ UTR plasmids with miR-1290 mimic into the cells were accomplished by using Lipofectamine 3000 (L3000075, Invitrogen). Luciferase activity was measured 48 h after transfection by the Dual-Luciferase Reporter Assay System (E1910, Promega, Madison, WI, USA).
Mitochondrial membrane potential (MMP) detection
After 2 × 10 4 cells were treated in a 6-well plate, JC-1 dye (J6004L, Uelandy, Suzhou, China) was added for 20 min at 37 °C. MMP was detected and analyzed using relative fluorescence ratio staining. The red to green fluorescence ratio was lower in damaged mitochondria cells than in normal cells. The fluorescence intensity was measured by BioTek Cytaiton1 (Hercules, CA, USA).
Succinate dehydrogenase (SDH) and Glutathione (GSH) assay
SDH activity was measured using Succinate Dehydrogenase Activity Assay Kit (D799375, Sangon, Shanghai, China). GSH concentration was measured using GSH Content Assay Kit (D799614, Sangon). All assays were performed following the manufacturer’s instruction. SDH activity was measured by absorbance at 600 nm and GSH was detected by absorbance at 412 nm using BioTek Cytaiton1 (Hercules).
Oxygen consumption rate (OCR) assay
Cells were seeded in XFe 96-well microplates (6000 cells/well) (Agilent Technologies, Sana Clara, USA), followed by indicated stimulation for a further 48 h. Cells were washed and incubated in base medium (Agilent Technologies) at 37 °C for 1 h. OCR was measured in real-time with Mito Stress Test Kit (103015–100, Agilent Technologies) using the Seahorse XFe96 Analyser (Agilent Technologies) following manufacturer’s instructions.
Reactive oxygen species (ROS) assay
Cells after treated were collected, washed with PBS twice, and incubated with DCFH-DA (S0033S, Beyotime) at a final concentration of 10 μM in FBS-free DMEM for 15 min at 37 °C in the dark, and then washed three times with FBS-free DMEM. The cells were collected, and ROS levels were analyzed using flow cytometer (FlowSight, Millipore, Billerica, MA, USA).
Mitochondrial genome sequencing
Total DNA was extracted from cells with QIAamp DNA Blood Mini Kit (Qiagen) according to the manufacturer’s recommendations. The DNA samples were quantified with NanoDrop 2000 (Thermo Fisher Scientific) and ascertained with electrophoresis. The mitochondria genome was sequenced by Genesky Biotechnologies (Shanghai, China).
Single nucleotide polymorphism (SNP) sequencing
Using H1299ρ 0 cells as control, NFs treated with 95D-EVs and H1299ρ 0 cells cocultured with conditional medium (CM) from NFs treated with 95D-EVs, DNA of these three groups was extracted with QIAamp DNA Blood Mini Kit (Qiagen) and sequenced by Sangon Biotech (Shanghai, China) to analyze the variation of single nucleotide in the genome.
Mitochondrial function
Cells were incubated with Mito-Red (M7512, Invitrogen) at a final concentration of 25 nM for 15 min at 37 °C, and subsequently stained with DAPI (E607303, BBI). Next, images were captured and analyzed using a laser scanning confocal microscopy (LSM900, Zeiss).
According to the manufacturer's instructions, the cell viability was measured through the CCK8 kit (C0005, Targetmol, Boston, MA). The cells of the control group or the treatment group were seeded in a 96-well plate for an appropriate time, the viability was measured OD450 after 2 h incubation with determination solution using Microplate Reader (BioTek ELx800, Winooski, VT).
Transwell assay
1 × 10 5 lung cancer cells were suspended in serum-free medium and seeded into the transwell chambers with inserts of 8-μm pore size (353097, Falcon, NY, USA). The medium with 10% FBS was placed into the bottom chamber (353,047, Falcon). After 24 h, the cells that had migrated through the membrane and stuck to the lower surface of the membrane were stained with crystal violet solution (V5265, Sigma-Aldrich) and were measured by light microscope (AMEX-1200, AMG).
Animal experiment
H1299 (2 × 10 6 ), H1299ρ 0 cells (2 × 10 6 ), the mixture of 2 × 10 6 H1299ρ 0 cells with 5 × 10 5 NFs or 5 × 10 5 induced CAFs (iCAFs, that originated from NFs treatment with 95D-EVs) cells (4:1) was resuspended in 100 μL PBS and injected subcutaneously into 5-week-old female BALB/C nude mice as previously described [ 18 ]. Tumor formation was examined every 3 days. The tumor volume was calculated as volume (mm 3 ) = d 2 × D/2, where d and D were the shortest and the longest diameters, respectively.
To examine the roles of EVs in metastasis, 5 × 10 5 H1299 cells were intravenously injected into 5-week-old female BALB/c nude mice through the tail vein. Subsequently, mice were randomly divided into groups and intravenously injected with equal numbers of EVs from different tumor cells twice a week for 1 month [ 20 ].
For lung metastasis experiments, the nude mice were randomly divided into four groups, H1299 (5 × 10 5 ), H1299ρ 0 cells (5 × 10 5 ), the mixture of 5 × 10 5 H1299ρ 0 cells with 5 × 10 5 NFs or 5 × 10 5 iCAFs cells (1:1) were injected 5-week-old female BALB/c nude mice via the tail vein, respectively [ 21 ]. The nude mice were sacrificed by cervical dislocation at the indicated timepoint, and lung tissue was removed, imaged and the number of nodules on the surface of the lung was recorded to assess tumor metastasis. Lung tissues were then fixed with 4% paraformaldehyde for hematoxylin–eosin (H&E).
In situ hybridization (ISH) and immunohistochemistry (IHC)
Tissue sections of 41 clinical lung cancer pathological sections from the Department of Pathology of the second Xiangya Hospital (2019–2021) were collected (Table S3). For ISH, the miR-1290 miRCURY LNA detection probe (Exiqon, Denmark) was used by the manufacturer’s protocol. IHC was performed using universal two-step detection kit (PV-9000, ZSGB-Bio, Beijing). The sections were stained with DAB (ZLI-9017, ZSGB-Bio, Beijing) for 1–3 min, and lightly counterstained with Mayer hematoxylin (ZLI-9610, ZSGB-Bio). The expression of each protein was semi-quantitatively evaluated using the method previously described [ 14 ]. TOM20 (11802–1-AP, Proteintech) was used in this experiment.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8 software. Quantitative values of all experiments were expressed as the mean ± SD. Differences among/between sample groups were analyzed by one-way ANOVA or the independent samples T test. Pearson’s correlation coefficient was used to measure the relationship between miR-1290, α-SMA and BNIP3. P < 0.05 was considered to be statistically significant.
High metastatic potential lung cancer cells derived EVs packaged miR-1290 activates NFs to CAFs
CAFs have been demonstrated to participate in the tumor metastasis progression. Therefore, it was meaningful to further understand the activation of CAFs caused by tumor cells derived EVs. The primary isolated NFs and CAFs from lung tissue were constructed in our previous study [ 18 ]. Both NFs and CAFs expressed vimentin, while NFs had decreased expression of the CAFs markers a-SMA and FAP proteins (Fig. S1). To determine the difference of EVs derived from low metastatic potential 95C and high metastatic potential 95D cells in CAFs activation, firstly, EVs derived from cells were isolated and purified, the result showed that both isolated EVs exhibited typical lipid bilayer membrane encapsulation and cup-shaped morphology by TEM analysis (Fig. 1 A). The size distribution of EVs ranged from 30 to 300 nm, and the peak was approximately 110 nm by NTA (Fig. 1 B). Additionally, immunoblotting revealed that the EVs were positive for EVs markers CD63 and TSG101 (Fig. 1 C). To identify the internalization of EVs by NFs, the tumor-cell derived EVs and NFs were labeled with PKH67 (green) or Phalloidin (red), respectively. As shown in Fig. 1 D, EVs were internalized and accumulated around the nuclei of NFs after incubation, suggesting that EVs were effectively delivered to NFs. Meanwhile, the expression of α-SMA and FAP in NFs were significantly higher in the 95D-derived EVs treatment groups than in the 95C-derived EVs groups (Fig. 1 E, Fig. S2). These data indicated that NFs were activated by 95D-derived EVs. It is known that activated fibroblasts have enhanced matrix adhesions, resulting in increased ability of collagen gel contraction [ 14 ]. Further data showed that the contraction abilities of NFs were markedly enhanced after treatment with 95D-derived EVs in comparison with 95C-derived EVs ( P < 0.001) (Fig. 1 F). These results suggested that 95D-derived EVs could effectively activate NFs to CAF-like cells.
95D-derived EVs packaged miR-1290 activated NFs to CAFs. A The representative images of EVs derived from 95C and 95D were analyzed by TEM. Scale bar, 100 nm. B EVs were detected by NTA analysis. C Immunoblotting analyses for EVs markers CD63, TSG101 and negative control Calnexin. D NFs were pre-treated with 95C or 95D derived EVs for 24 h. Confocal imaging showed the delivery of PKH67-labeled EVs (green) to Phalloidin labelled NFs (red) and the localization of DAPI-stained nuclei (blue). Yellow arrows represented delivered EVs. Scale bar, 20 μm. E After NFs were incubated with 95C or 95D derived EVs for 48 h, the protein levels of α-SMA and FAP were analyzed by immunoblotting. F Representative images of the contraction ability were analyzed by collagen contraction assay. Isolated primary CAFs were used as the positive control group. G-H After NFs transfected with indicated mimics, the mRNA levels of α-SMA and FAP were analyzed by qPCR. Negative control (NC), Untreated (UN). I The protein levels of α-SMA and FAP were detected by immunoblotting. J Immunoblotting analysis of α-SMA and FAP in NFs treated with blank control (PBS) or EVs derived from 95C/vec and 95C/miR-1290 or 95D/control and 95D/anti-miR-1290. K Representative images and quantification analysis of IF of α-SMA in NFs treated as indicated. Scale bar, 20 μm. L Collagen contraction assay was assessed for the contraction ability of NFs treated as indicated. Data were shown as the mean ± SD of at least three independent experiments. *** P < 0.001, ns: not significant
miRNAs encapsulated in EVs are abundant and have an important role in cell–cell communication. Previously, we conducted microarrays to generate miRNAs profiles between the 95D and 95C cells and found 16 miRNAs with significantly different expression levels [ 17 ]. To identify the specific miRNAs involved NFs activation, qPCR was used to examine the expression of these 16 miRNAs in the EVs, the results showed that miR-222, miR-487b, miR-652, miR-487a, miR-1290 and miR-663 were highly expressed in 95D cell derived EVs (Fig. S3A). Meanwhile, these 6 miRNAs were detected in NFs and CAFs cells, and the results showed that miR-222, miR-652 and miR-1290 were highly expressed in CAFs, suggesting that these 3 miRNAs might be associated with the activation of NFs (Fig. S3B). To confirm this, the expression of α-SMA and FAP at both mRNA and protein levels after transfecting miR-222, miR-652, miR-1290-mimics in NFs cells were detected, the results suggested that the miR-1290-mimics could promote the expression of α-SMA and FAP (Fig. 1 G, H, I). Further, the stable cell lines which overexpressing miR-1290 in 95C cell or knockdown miR-1290 in 95D cells were established (Fig. S4). After NFs cocultured with EVs, the data demonstrate that 95C/miR-1290-derived EVs could increase the expression of α-SMA and FAP, while EVs secreted from 95D/anti-miR-1290 could decrease two protein expression (Fig. 1 J). These results were further confirmed by IF assays for α-SMA (Fig. 1 K). Furthermore, collagen contraction assays also revealed that EVs packaged miR-1290 enhanced the collagen contraction ability of NFs (Fig. 1 L). These results suggest that EVs packaged miR-1290 could activate NFs to CAFs.
EVs packaged miR-1290 activates NFs to CAFs via MT1G/AKT and promotes lung cancer metastasis
A study in lung cancer showed that Metallothionein 1G (MT1G), as a target for miR-1290, have critical roles in regulating tumor growth and metastasis [ 22 ]. Consistent with previous study, we confirmed that miR-1290 could mediate MT1G expression through luciferase reporter assay (Fig. 2 A, B). Further, qPCR results showed that MT1G expression could be downregulated, and α-SMA and FAP expression were upregulated in NFs treated with miR-1290 mimics compared to controls (Fig. 2 C). The previous study had reported that MT1G could inhibit AKT signaling [ 23 ], and AKT signaling pathway was responsible for NFs activation [ 24 ]. As shown in Fig. 2 D, miR-1290 mimics could suppress the expression of MT1G, meanwhile activating the AKT and upregulating the expression of α- SMA and FAP in NFs. IF results also showed that overexpressed miR-1290 in NFs could promoted α-SMA expression (Fig. 2 E). Furthermore, to explore the importance of MT1G/AKT in the activation of CAFs, functional rescue experiments showed that restoration the expression of MT1G in miR-1290 mimics-treated NFs could suppress the expression of the p-AKT, α-SMA and FAP. In addition, 95C/miR-1290-EVs increased the protein level of p-AKT, α-SMA and FAP, while restoration of MT1G expression abrogated these effects (Fig. 2 F, G). Similar results were observed in IF staining experiments (Fig. 2 H). Moreover, over-expressed MT1G in CAFs decreased the protein level of p-AKT, α-SMA, FAP and the intensity of α-SMA (Fig. S5A, B, C).
EVs packaged miR-1290 activates NFs to CAFs via MT1G/AKT and promotes lung cancer metastasis. A The binding sites and corresponding mutation sites between miR-1290 and the target gene MT1G. B Luciferase reporter assay in 293 T cells cotransfected with the wild or mutant-type MTIG 3' UTR and miR-1290 mimics. Untreated (UN). Blank Vector (Control). C qPCR analysis of MT1G, α-SMA and FAP expression in NFs treated with miR-1290 mimics. Negative control (NC). D Immunoblotting analysis of MT1G, p-AKT, AKT, α-SMA and FAP in NFs transfected with miR-1290 mimics. E IF analysis and quantification data of α-SMA in NFs treated as indicated. Scale bar, 20 μm. F–H pDONR233-MT1G plasmid was transfected in NFs treated with miR-1290 mimics and 95C/miR-1290-EVs, respectively, F qPCR assays for MT1G, α-SMA and FAP, G Immunoblotting analysis of MT1G, p-AKT, AKT, α-SMA and FAP, H IF analysis for α-SMA. Scale bar, 20 μm. I-J H1299 was cocultured with conditional medium (CM) from different groups indicated, I CCK8 assay for cell proliferation, and J Transwell assays for the cell migration analysis. Scale bar, 100 μm. K Schematic representation of the establishment process of the EVs-educated tumor metastasis model. L The representative tissue images and HE images of lung foci. Scale bar, 200 μm. M Quantitative metastatic area in lung metastatic foci. N ISH images of miR-1290 and IHC images of MT1G, p-AKT and α-SMA in lung foci of different experimental groups. Red arrow: positive signal in the stroma; Black arrow: positive signal in the tumor. Data were shown as the mean ± SD of at least three independent experiments.** P < 0.01, ** *P < 0.001, ns: not significant
To explore the function of EVs-packaged miR-1290 in tumor metastasis, NFs cells were firstly treated with EVs derived from 95C/vec, 95C/miR-1290 and 95D cells, then co-culture the collected CM with H1299 cells. CCK8 and Transwell assays revealed that 95C/miR-1290 and 95D-derived EVs could notably promote the proliferation and migration of cancer cells in vitro (Fig. 2 I, J). For in vivo lung metastatic mouse model, H1299 was injected in mice tail vein firstly, then EVs derived from 95C/vec, 95C/miR-1290, and 95D were injected respectively, lung metastasis foci were observed after 8 weeks (Fig. 2 K). The results showed that, compared with 95C/vec-EVs group, 95C/miR-1290 as well as 95D-derived EVs promoted the formation of lung metastases more strongly (Fig. 2 L, M). In addition, compared with that in 95C/vec-EVs-treated group, a dramatically upregulated expression of miR-1290, p-AKT and α-SMA was observed in lung from mice implanted 95C/miR-1290 derived EVs, while MT1G was decreased (Fig. 2 N). Collectively, these results suggest that EVs-packaged miR-1290 promoted the activation of NFs to CAFs by targeting MT1G/AKT, and accelerated the metastatic homing of lung cancer cells.
ROS enhances mitophagy and increases release of mtDNA in iCAFs
To investigate the autophagic status of iCAFs that originated from NFs treatment with 95D-EVs, autophagy-related protein LC3 and mitophagy-related proteins BNIP3 and PINK1 were detected. The result showed that the expression of LC3, BNIP3 and PINK1 was overexpressed in iCAFs (Fig. 3 A). MMP was regard as a key indicator of mitochondrial function [ 25 ]. Here, the loss of MMP levels was observed in iCAFs compared with NFs cocultured with 95C-EVs, indicating that iCAFs had a weaker mitochondrial function (Fig. 3 B). Further, qPCR analysis for ND1, COX1 and D-Loop was used to detect the released mtDNA in cell culture medium, the results showed that more mtDNA was released in iCAFs group (Fig. 3 C). Further, treatment with 95C/miR-1290-EVs dramatically promoted mitophagy status, while treatment with 95D/anti-miR-1290-EVs significantly decreased the mitophagy of iCAFs (Fig. 3 D). Consistently, the loss of MMP levels was observed in NFs treated 95C/miR-1290-EVs, and reversed expression pattern was found in NFs cocultured with 95D/anti-miR-1290-EVs (Fig. 3 E). qPCR data showed that NFs treatment with 95C/miR-1290-EVs released more mtDNA, while 95D/anti-miR-1290-EVs blocked the effect of mtDNA release from iCAFs (Fig. 3 F). Study had demonstrated that cells stimulated by hydrogen peroxide (H 2 O 2 ) could generate high levels of ROS that can perturb the normal redox balance and shift cells into a state of oxidative stress [ 26 ]. Therefore, H 2 O 2 was used to established a model of oxidative stress in vitro. It was shown that the mitophagy in iCAFs was enhanced in a concentration dependent manner (Fig.S6A). Meanwhile, the result showed that CCCP, a mitophagy inducer, could significantly increase mitophagy of iCAFs (Fig. S6B). Interestingly, iCAFs dramatically elevated mitophagy status compared with NFs cocultured with 95C-EVs, and H 2 O 2 can further enhance mitophagy of iCAFs (Fig. S6C). As shown in Fig. 3 G and H, H 2 O 2 treatment of iCAFs promoted the mitophagy and decreased the level of MMP, which is the same as the result of positive control CCCP treatment. Similarly, qPCR data showed that iCAFs released more mtDNA compared to NFs, and H 2 O 2 treatment further promoted the release of mtDNA (Fig. 3 I). In addition, primary human lung cancer tissues were examined to seek evidence for tumor derived miR-1290 activated CAFs as well as the consequent high mitophagy. miR-1290 levels of tumor and stromal was positively correlated with stromal α-SMA, whereas in the stroma, a strong correlation between α-SMA and BNIP3 (Fig. 3 J). These data suggested that iCAFs exhibited higher levels of autophagy and mitophagy and more mtDNA released, and ROS could further enhance this process.
ROS enhances mitophagy and increases release of mtDNA in iCAFs. A-C NFs were treated with EVs derived from 95C or 95D for 48 h. Isolated NFs and CAFs were used as blank control and positive control, respectively. A Immunoblotting analysis of PINK1, BNIP3 and LC3 in the indicated groups. B The fluorescence intensity reflecting the MMP in the different groups. C qPCR analyses for ND1, COX1, D-Loop in the supernatants of indicated cells. D Immunoblotting analysis of PINK1, BNIP3 and LC3 in NFs treated with blank control (PBS) or EVs derived from 95C/vec and 95C/miR-1290 or 95D/control and 95D/anti-miR-1290. E The fluorescence intensity reflecting the MMP in NFs treated as indicated. F qPCR analyses for ND1, COX1, D-Loop in the supernatants of indicated cell. G-I NFs were treated with 95D-derived EVs for 48 h, and then exposed to H 2 O 2 (0.25 mM) or CCCP (10 μM), respectively. G Immunoblotting analysis of PINK1, BNIP3 and LC3 in the indicated groups. H The fluorescence intensity reflecting the MMP in the different groups. I qPCR analyses for ND1, COX1, D-Loop in the supernatants of indicated cells. J ISH images of miR-1290 and IHC images of α-SMA and BNIP3 in lung cancer tissue sections. Stromal and tumor are separated by dashed lines (left). The correlation analysis (right) between miR-1290 and α-SMA (upper), and between α-SMA and BNIP3 (lower). Data were shown as the mean ± SD of at least three independent experiments. * P < 0.05, ** P < 0.01, *** P < 0.001, ns: not significant
iCAFs transports mtDNA to tumor cells with damaged mitochondria
To construct tumor cells with damaged mitochondria, lung cancer cells H1299 and A549 cells were treated with low-dose EtBr in order to generating mtDNA depletion cell (H1299ρ 0 and A549ρ 0 cells). After 30 and 60 days of cell treatment, morphological observation showed gradually enhanced cytoplasmic shrinkage (Fig. 4 A). qPCR was performed to detect the expression of mtDNA-encoded genes including 12S, 16S, D-loop, ND1, ND2, ND3, ND4, ND4L, ND5, ND6, COX1, COX2, COX3, CYTB, ATP6, ATP8. The results demonstrated that the vast majority genes were obviously decreased in H1299ρ 0 and A549ρ 0 cells (Fig. 4 B). Then, cellular OCR and oxidative stress-related enzymes GSH and SDH was detected, the results showed that the level of the OCR, SDH activity and GSH concentration in H1299ρ 0 and A549ρ 0 cells were lower than that in parent tumor cells (Fig. 4 C, D, E). Studies have demonstrated that the ROS levels in EtBr-treated cells were significantly higher than in the controls [ 27 ]. Our data is consistent with previous studies that cellular ROS were increased in H1299ρ 0 and A549ρ 0 cells (Fig. 4 F). The above results showed that tumor cell with depleted mtDNA exhibit mitochondrial dysfunction.
The differences between normal and mitochondria damaged lung cancer cells. A The representative macrographs of parental cells and cells treated with EtBr for 30 or 60 days. B qPCR analysis of 16 genes (12S, 16S, D-Loop, ND1, ND2, ND3, ND4, ND4L, ND5, ND6, COX1, COX2, COX3, CYTB, ATP6, ATP8) in cells . Untreated (UN). C OCR was detected by seahorse mitochondrial stress in indicated groups. ρ 0 cells: cells were depleted of mitochondrial DNA after treated with EtBr 60 days. D, E The activity of SDH and the concentration of GSH were detected. F The level of ROS in cells was analyzed by flow cytometry. Data were shown as the mean ± SD of at least three independent experiments. ** P < 0.01, *** P < 0.001
To determine whether the iCAFs transmitted mtDNA to mitochondria damaged cells, the whole mitochondrial DNA sequencing was performed for H1299, H1299ρ 0 , NFs, iCAFs and primary CAFs. The results showed that the sequences of mtDNA in H1299ρ 0 cells was not obvious different from that in H1299, and the mtDNA from stromal cells (NFs, iCAFs and CAFs) had different degrees of mutations compared to H1299. In addition, the mtDNA of iCAFs also showed a basically consistent mutation frequency compared with CAFs (Fig. 5 A). To elucidate whether tumor cells can obtain mtDNA from CAFs, the CM from iCAFs was isolated and cocultured with H1299ρ 0 cells (Fig. 5 B), the results showed that the expression of ND2 and CYTB in tumor cells was restored (Fig. 5 C, D). Further, mtDNA SNP sequencing of H1299ρ 0 , iCAFs and H1299ρ 0 cocultured with iCAFs-CM was performed, the results displayed that compared to H1299ρ 0 , both ND2 as well as CYTB had three mutant sites in the iCAFs group, and specially, H1299ρ 0 cocultured with iCAFs-CM possessed these mutant sites as well (Fig. 5 E), indicating that mtDNA from iCAFs could be transmitted to H1299ρ 0 cells. Furthermore, mito-Red was used to label mitochondria to measure its function. Compared with H1299ρ 0 /A549ρ 0 , H1299/A549 had higher mitochondrial activity. And H1299ρ 0 /A549ρ 0 cocultured with iCAFs-CM also possessed more mitochondrial activity than H1299ρ 0 /A549ρ 0 treated with NFs-CM (Fig. 5 F). In addition, H1299ρ 0 /A549ρ 0 cocultured with iCAFs-CM also possessed more MMP levels than H1299ρ 0 /A549ρ 0 treated with NFs-CM (Fig. 5 G). Taken together, these data demonstrated the recovery of mtDNA in mitochondria damaged cells was from iCAFs-mediated mtDNA transfer but not an induction of mtDNA endogenous expression.
iCAFs transports mtDNA to tumor cells with mitochondria damaged. A Mitochondrial genome sequencing was performed in H1299, H1299ρ 0 , NFs, iCAFs (NFs treated with 95D-EVs for 48 h) and isolated primary CAFs. B Diagram of experimental procedure for inducing CAFs and coculturing with H1299ρ 0 cells. C, D PCR and qPCR were used to analyze the ND2 and CYTB in the indicated groups. E SNP sequencing of the ND2 and CTYB was performed. F Confocal imaging showed mitochondria labeled with mito-Red and the localization of DAPI-stained nuclei (blue) in the indicated groups. Mean fluorescence intensity (Mito-Red) data was shown. Scale bar, 10 μm. G The fluorescence intensity reflecting the MMP in the different groups. Data were shown as the mean ± SD of at least three independent experiments. ** P < 0.01, *** P < 0.001
Mitochondria damaged tumor cells restores mitochondrial function, the ability of cell proliferation, migration and EMT through uptaking mtDNA released by iCAFs
To investigate the effect of mtDNA from CAFs on mitochondria damaged tumor cells, after H1299ρ 0 /A549ρ 0 cells coculture with NFs-CM or iCAFs-CM, meanwhile CM was treated with DNaseI enzyme to hydrolyze mtDNA and followed by heating to inactivate enzyme [ 28 ], the OCR, SDH activity and GSH concentration were examined. The data found that H1299ρ 0 /A549ρ 0 cells coculture with iCAFs-CM could restore the mitochondrial functions. Heating treatment does not affect this effect, whereas the addition of DNaseI into iCAFs-CM to degrade the DNA resulted in impaired mitochondrial function recovery (Fig. 6 A, B, C). Further, CCK8 and transwell assays results revealed that iCAFs-CM significantly enhanced cell proliferation and migration compare with NFs-CM, and the addition of DNaseI could weaken this ability (Fig. 6 D, E). In addition, the immunoblotting results showed that compared to control groups, the epithelial-mesenchymal transition (EMT) marker Vimentin and Snail were upregulated in H1299ρ 0 /A549ρ 0 cocultured with iCAFs-CM, while the expression of epithelial marker E-cadherin was downregulated. However, under DNaseI treatment, this regulation was blocked (Fig. 6 F, Fig. S7). These results suggest that mtDNA from CAFs can be taken up by tumor cell depleted of mtDNA, thus contributing to its mitochondrial function, the ability of cell proliferation and migration and EMT recovery.
Mitochondria damaged tumor cells restores mitochondrial function, the ability of cell proliferation, migration and EMT through uptaking mtDNA released by iCAFs. Used H1299, A549, H1299ρ 0 and A549ρ 0 cells as control groups, cocultured H1299ρ 0 and A549ρ 0 cells with NFs-CM or iCAFs-CM for 48 h, respectively, and the CM was treated with DNaseI enzyme (0.1 mg/mL) in 37 °C for 1 h to hydrolyze mtDNA and followed by heating (70 °C for 10 min) to inactivate enzyme. A OCR was detected by seahorse mitochondrial stress. B, C The activity of SDH and the concentration of GSH were detected. D CCK8 assay for cell proliferation. E Transwell assays for the cell migration analysis. Scale bar, 100 μm. F Immunoblotting analysis of E-cadherin, Vimentin and Snail expression. Data were shown as the mean ± SD of at least three independent experiments. ** P < 0.01, *** P < 0.001
iCAFs promote the growth and metastasis of mitochondria damaged tumor cells in vivo
To confirm the iCAFs could promote the growth and metastasis of mitochondria damaged tumor cells in vivo, subcutaneous xenograft experiment was performed by using H1299, H1299ρ 0 and H1299ρ 0 co-injected with NFs/iCAFs cells in the axilla of nude mice. And the results showed that the tumorigenic ability of H1299ρ 0 was significantly weaker than that of H1299 cells, while co-injection with NFs slightly increased the tumorigenic capacity of H1299ρ 0 , but it is significantly weaker than the group co-injected with iCAFs (Fig. 7 A, B). The same conclusion was obtained in the tumor weight results (Fig. 7 C). TOM20 is widely used to monitor the mitochondrial mass, and a decreased expression of TOM20 is considered to indicate a reduced mitochondrial mass and upregulated mitophagy [ 29 ]. To demonstrate the transfer of mtDNA and the restoration of mitochondrial function in mitochondria damaged tumor cells in vivo, the PCR and IHC results showed that the group H1299ρ 0 co-injected with iCAFs restores the mtDNA expression and exhibits higher level of mitochondria marker TOM20 compared with co-injection with NFs, which is closely related to their metastatic mtDNA and the restoration of tumor cell function (Fig. 7 D, E). Moreover, H1299, H1299ρ 0 cells and the mixture of H1299ρ 0 cells with NFs or iCAFs cells were injected nude mice via the tail vein to conduct the lung cancer metastasis formation model, respectively. The results demonstrated that the metastatic foci in the lungs of the group co-injected with H1299ρ 0 and iCAFs were more significant than those in the group co-injected with NFs (Fig. 7 F,G). To determine the effect of iCAFs in lung metastatic foci, the IHC results showed that the expression of α-SMA and TOM20 was significantly higher in mice engrafted with H1299ρ 0 treated with iCAFs compared with NFs (Fig. 7 H). Together, these results suggest that iCAFs could promote the growth and metastasis of mitochondria damaged tumor cells in vivo.
iCAFs promote the growth and metastasis of mitochondria damaged tumor cells in vivo. A-C After H1299 (2 × 10 6 ), H1299ρ 0 cells (2 × 10 6 ), the mixture of 2 × 10 6 H1299ρ 0 cells with 5 × 10 5 NFs or 5 × 10 5 CAFs cells (4:1) were injected subcutaneously into 5-week-old nude mice, A the tumor growth, B tumor volume and C tumor weight was monitored. D PCR was used to detect the ND2 and CYTB in the indicated groups. E IHC analysis of TOM20 was performed in mouse xenograft tissues. F–H After H1299 (5 × 10 5 ), H1299ρ 0 cells (5 × 10 5 ), the mixture of 5 × 10 5 H1299ρ 0 cells with 5 × 10 5 NFs or 5 × 10 5 iCAFs cells (1:1) were injected into the lateral tail vein of 5-week-old nude mice, F the representative tissue macrographs and HE images of lung foci, and G quantitative analysis of lung metastatic foci. Scale bar, 200 μm. H IHC images of α-SMA and TOM20 in lung foci of different experimental groups. Data were shown as the mean ± SD of at least three independent experiments. ** P < 0.01, *** P < 0.001
It has been found that tumor cells undergoing oxidative stress can lead to mitochondrial dysfunction, which is closely associated with reduce of mtDNA copy number [ 8 , 9 ]. Since Spees et al. has proved that mitochondria or mtDNA can move between cells [ 30 ], studies further point out that mitochondria or mtDNA could be transported between stromal cell and cancer cells, thereby restoring mitochondrial function in mitochondria damaged tumor cells and promoting tumor progress. For example, the study showed a preferential transfer of mitochondria from endothelial to cancer cells resulting in acquisition of chemoresistance [ 31 ]. Mitochondria damaged cancer cells that acquire mtDNA from host stroma restore their mitochondria function, thereby exerting a pro-cancer function [ 19 ]. And the mtDNA can be transferred via EVs from stromal cells to cancer cells to sustain OXPHOS potential and mediate an exit from hormonal therapy-induced metabolic dormancy in breast cancer [ 32 ]. In present study, our data showed that tumor cells with compromised respiration due to mtDNA depletion have the following characteristics: decreased oxygen consumption, downregulated levels of oxidative stress related enzymes and increased levels of ROS. Specially, the mitochondrial genome sequencing data show that the mtDNA mutation in cancer cell was different from CAFs, the mtDNA SNP sequencing results further support that mtDNA could be the paracrine-signaling molecule generated by CAFs and could be absorbed by cancer cell with mtDNA deletion. And the rescue of mitochondrial function in cancer cell with mtDNA deletion cocultured with CAFs-CM was demonstrated.
CAFs are one of the most prominent and active components in the lung cancer microenvironment, and the enhancement of autophagy in CAFs has been shown to play a role in the malignant phenotype of human tumors. Autophagy promotes tumor progress not only by providing nutrients to the cancerous cells but also by inducing EMT, angiogenesis, stemness, and metastatic dissemination of the cancer cells [ 33 ]. Yuan et al. demonstrated that the nucleosides are secreted by CAFs through autophagy increased glucose utilization and promoted growth of pancreatic ductal adenocarcinoma [ 34 ]. Li et al. report shows that rocuronium bromide could repress PI3K/AKT/mTOR signaling pathway and autophagy to block the CXCL12 expression in CAFs, thereby weakening the cytokines CXCL12-mediated esophageal cancer progression [ 35 ]. A well-studied mechanism that triggers the reverse warburg effect is ROS-induced mitophagy in CAFs. Martinez et al. showed that cancer cells triggered oxidative stress-induced mitophagy in neighboring fibroblasts by secreting hydrogen peroxide, thereby facilitating stromal aerobic glycolysis [ 36 ]. ITGB4-overexpressing triple negative breast cancer cells provided CAFs with ITGB4 proteins via exosomes, which induced BNIP3L-dependent mitophagy and lactate production, and then promoted the proliferation, EMT and invasion of breast cancer cells [ 37 ]. Our results showed that iCAFs exhibited a higher level of autophagy and mitophagy, and ROS could further enhance mitophagy and increase release of mtDNA in CAFs. This further prove the communication between CAFs and tumor cells through autophagy and ROS.
Numerous studies have shown that tumor derived EVs play important roles in various events of tumor development including the activation of NFs, through the proteins, DNA and RNA substances they carry. The molecules that induce CAF activation vary in different tumor cells [ 38 ]. The exosomal miR-210 derived from tumor cells induces the reprogramming of NFs to CAFs through TET, further promotes tumor angiogenesis and progress by producing MMP9, FGF2 and VEGF [ 39 ]. Lung cancer cell derived-EVs packaged lncRNA HOTIAR could motivate the transformation of NFs into CAFs, thereby promote the tumor metastasis [ 40 ]. In our study, we analyzed the different profiles of miRNAs carried by EVs between high-metastatic cancer cells and low-metastatic lung cancer cells, and identified that miR-1290 expression was higher both in high-metastatic cancer cells and EVs. Previous studies have shown that serum exosomal miR-1290 could be a potential diagnostic and prognostic biomarker for lung adenocarcinoma [ 41 ], and is a crucial driver for tumor initiation and cancer progression in human non-small cell lung cancer [ 22 ]. A study showed that hypoxic lung adenocarcinoma cell-derived exosomes overexpressing miR-1290 can polarize M2 macrophages by targeting SOCS3, which further promote tumor progression [ 42 , 43 ]. And recent report has found that exosomal miR-1290 improves CAFs activation through target COX-2, further enhance tumor growth [ 42 , 43 ]. Here, we found that miR-1290 directly transferred from high-metastatic cancer cells to NFs via EVs, and further activate NFs to CAFs through MT1G/AKT pathway. However, it should be noted that miR-1290 is not the only cargos packaged in EVs, other components also have a certain effect, for example, 95C-EVs with low miR-1290 expression could activate NFs to a certain extent, these still need further clarification.
In summary, our results indicate that high metastatic lung cancer cells derived EVs packaged miR-1290 promote the activation of NFs to CAFs. The activated CAFs exhibit higher levels of autophagy and mitophagy and more mtDNA release, and ROS could further promote this process. More importantly, tumor cells with compromised mitochondrial due to mtDNA depletion, could absorb the mtDNA provided by CAFs, thus promote the recovery of mitochondrial function and resistance to oxidative stress, thereby promote tumor metastasis (Fig. 8 ). Our study elucidates a novel mechanism by which intercellular crosstalk between tumor cells and CAFs control lung cancer metastasis, providing potential efficient prevention and therapeutic strategies for lung cancer.
Schematic diagram . The role and mechanism of EVs activated CAFs in regulating lung cancer metastasis through mitophagy and mtDNA transfer
Availability of data and materials
All the data supporting the findings of this study are available within the article and its additional files.
Abbreviations
Mitochondrial DNA
Normal fibroblasts
- Cancer-associated fibroblasts
Tumor microenvironment
- Extracellular vesicles
Induced CAFs
Reactive oxygen species
Conditioned medium
Extracellular matrix
Circulating tumor cells
Disseminated tumor cells
Fetal bovine serum
Ethidium bromide
Hydrogen peroxide
Carbonyl cyanide 3-chlorophenylhydrazone
Transmission electron microscopy
Nanoparticle tracking analysis
Immunofluorescence
Quantitative PCR
Mitochondrial membrane potential
Succinate dehydrogenase
Glutathione
Oxygen consumption rate
Single nucleotide polymorphism
In situ hybridization
Immunohistochemistry
Metallothionein 1G
Epithelial-mesenchymal transition
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Article PubMed Google Scholar
Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, Paz-Ares L. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389:299–311.
Article CAS PubMed Google Scholar
Gill JG, Piskounova E, Morrison SJ. Cancer, oxidative stress, and metastasis. Cold Spring Harb Symp Quant Biol. 2016;81:163–75.
Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ, Morrison SJ. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–91.
Article CAS PubMed PubMed Central Google Scholar
Laoukili J, van Schelven S, Kucukkose E, Verheem A, Goey K, Koopman M, Borel Rinkes I, Kranenburg O. BRAF(V600E) in colorectal cancer reduces sensitivity to oxidative stress and promotes site-specific metastasis by stimulating glutathione synthesis. Cell Rep. 2022;41:111728.
Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell. 2020;38:167–97.
Arfin S, Jha NK, Jha SK, Kesari KK, Ruokolainen J, Roychoudhury S, Rathi B, Kumar D. Oxidative stress in cancer cell metabolism. Antioxidants (Basel). 2021;10:642.
Luo Y, Ma J, Lu W. The significance of mitochondrial dysfunction in cancer. Int J Mol Sci. 2020;21:5598.
Sun X, Zhan L, Chen Y, Wang G, He L, Wang Q, Zhou F, Yang F, Wu J, Wu Y, et al. Increased mtDNA copy number promotes cancer progression by enhancing mitochondrial oxidative phosphorylation in microsatellite-stable colorectal cancer. Signal Transduct Target Ther. 2018;3:8.
Article PubMed PubMed Central Google Scholar
Scheid AD, Beadnell TC, Welch DR. Roles of mitochondria in the hallmarks of metastasis. Br J Cancer. 2021;124:124–35.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–98.
Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18:99–115.
Chen Y, McAndrews KM, Kalluri R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol. 2021;18:792–804.
Wu X, Zhou Z, Xu S, Liao C, Chen X, Li B, Peng J, Li D, Yang L. Extracellular vesicle packaged LMP1-activated fibroblasts promote tumor progression via autophagy and stroma-tumor metabolism coupling. Cancer Lett. 2020;478:93–106.
Liang L, Li W, Li X, Jin X, Liao Q, Li Y, Zhou Y. “Reverse Warburg effect” of cancer-associated fibroblasts (Review). Int J Oncol. 2022;60:67.
Martinez-Outschoorn UE, Lisanti MP, Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol. 2014;25:47–60.
Liu C, Yang H, Xu Z, Li D, Zhou M, Xiao K, Shi Z, Zhu L, Yang L, Zhou R. microRNA-548l is involved in the migration and invasion of non-small cell lung cancer by targeting the AKT1 signaling pathway. J Cancer Res Clin Oncol. 2015;141:431–41.
Zhou Z, Zhou Q, Wu X, Xu S, Hu X, Tao X, Li B, Peng J, Li D, Shen L, et al. VCAM-1 secreted from cancer-associated fibroblasts enhances the growth and invasion of lung cancer cells through AKT and MAPK signaling. Cancer Lett. 2020;473:62–73.
Tan AS, Baty JW, Dong LF, Bezawork-Geleta A, Endaya B, Goodwin J, Bajzikova M, Kovarova J, Peterka M, Yan B, et al. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. 2015;21:81–94.
Zhou Y, Ren H, Dai B, Li J, Shang L, Huang J, Shi X. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J Exp Clin Cancer Res. 2018;37:324.
Neri S, Ishii G, Hashimoto H, Kuwata T, Nagai K, Date H, Ochiai A. Podoplanin-expressing cancer-associated fibroblasts lead and enhance the local invasion of cancer cells in lung adenocarcinoma. Int J Cancer. 2015;137:784–96.
Zhang WC, Chin TM, Yang H, Nga ME, Lunny DP, Lim EK, Sun LL, Pang YH, Leow YN, Malusay SR, et al. Tumour-initiating cell-specific miR-1246 and miR-1290 expression converge to promote non-small cell lung cancer progression. Nat Commun. 2016;7:11702.
Fu J, Lv H, Guan H, Ma X, Ji M, He N, Shi B, Hou P. Metallothionein 1G functions as a tumor suppressor in thyroid cancer through modulating the PI3K/Akt signaling pathway. BMC Cancer. 2013;13:462.
Wu F, Yang J, Liu J, Wang Y, Mu J, Zeng Q, Deng S, Zhou H. Signaling pathways in cancer-associated fibroblasts and targeted therapy for cancer. Signal Transduct Target Ther. 2021;6:218.
Ren W, Ji A, Karmach O, Carter DG, Martins-Green MM, Ai HW. A membrane-activatable near-infrared fluorescent probe with ultra-photostability for mitochondrial membrane potentials. Analyst. 2016;141:3679–85.
Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47.
Nacarelli T, Azar A, Sell C. Inhibition of mTOR prevents ROS production initiated by ethidium bromide-induced mitochondrial DNA depletion. Front Endocrinol (Lausanne). 2014;5:122.
Haldar S, Mishra R, Billet S, Thiruvalluvan M, Placencio-Hickok VR, Madhav A, Duong F, Angara B, Agarwal P, Tighiouart M, et al. Cancer epithelia-derived mitochondrial DNA is a targetable initiator of a paracrine signaling loop that confers taxane resistance. Proc Natl Acad Sci U S A. 2020;117:8515–23.
Zheng J, Chen L, Lu T, Zhang Y, Sui X, Li Y, Huang X, He L, Cai J, Zhou C, et al. MSCs ameliorate hepatocellular apoptosis mediated by PINK1-dependent mitophagy in liver ischemia/reperfusion injury through AMPKalpha activation. Cell Death Dis. 2020;11:256.
Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–8.
Pasquier J, Guerrouahen BS, Al Thawadi H, Ghiabi P, Maleki M, Abu-Kaoud N, Jacob A, Mirshahi M, Galas L, Rafii S, et al. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. J Transl Med. 2013;11:94.
Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A, Stepanova A, Iommarini L, Mastroleo C, Daly L, et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc Natl Acad Sci U S A. 2017;114:E9066–75.
Chen Y, Zhang X, Yang H, Liang T, Bai X. The “Self-eating” of cancer-associated fibroblast: a potential target for cancer. Biomed Pharmacother. 2023;163:114762.
Yuan M, Tu B, Li H, Pang H, Zhang N, Fan M, Bai J, Wang W, Shu Z, DuFort CC, et al. Cancer-associated fibroblasts employ NUFIP1-dependent autophagy to secrete nucleosides and support pancreatic tumor growth. Nat Cancer. 2022;3:945–60.
Li J, Gu X, Wan G, Wang Y, Chen K, Chen Q, Lu C. Rocuronium bromide suppresses esophageal cancer via blocking the secretion of C-X-C motif chemokine ligand 12 from cancer associated fibroblasts. J Transl Med. 2023;21:248.
Martinez-Outschoorn UE, Lin Z, Trimmer C, Flomenberg N, Wang C, Pavlides S, Pestell RG, Howell A, Sotgia F, Lisanti MP. Cancer cells metabolically “fertilize” the tumor microenvironment with hydrogen peroxide, driving the Warburg effect: implications for PET imaging of human tumors. Cell Cycle. 2011;10:2504–20.
Sung JS, Kang CW, Kang S, Jang Y, Chae YC, Kim BG, Cho NH. ITGB4-mediated metabolic reprogramming of cancer-associated fibroblasts. Oncogene. 2020;39:664–76.
Naito Y, Yoshioka Y, Ochiya T. Intercellular crosstalk between cancer cells and cancer-associated fibroblasts via extracellular vesicles. Cancer Cell Int. 2022;22:367.
Fan J, Xu G, Chang Z, Zhu L, Yao J. miR-210 transferred by lung cancer cell-derived exosomes may act as proangiogenic factor in cancer-associated fibroblasts by modulating JAK2/STAT3 pathway. Clin Sci (Lond). 2020;134:807–25.
Zhang X, Zhang Y, Qiu X, Cai J, Yang Z, Song F. Extracellular vesicles derived from lung cancer cells induce transformation of normal fibroblasts into lung cancer-associated fibroblasts and promote metastasis of lung cancer by delivering lncRNA HOTAIR. Stem Cells Int. 2022;2022:3805013.
Wu Y, Wei J, Zhang W, Xie M, Wang X, Xu J. Serum exosomal miR-1290 is a potential biomarker for lung adenocarcinoma. Onco Targets Ther. 2020;13:7809–18.
Gu J, Yang S, Wang X, Wu Y, Wei J, Xu J. Hypoxic lung adenocarcinoma-derived exosomal miR-1290 induces M2 macrophage polarization by targeting SOCS3. Cancer Med. 2023;12:12639–52.
Bai X, Shao J, Duan T, Liu X, Wang M, Li X, You Q, Zhang Z, Pan J. Exo-miR-1290-induced by COX-2 overexpression promotes cancer-associated fibroblasts activation and tumor progression by CUL3-Nrf2 pathway in lung adenocarcinoma. Cell Commun Signal. 2023;21:242.
Download references
This work was supported by the grants from the National Natural Science Foundation of China (No. 82103471,82372683), the Natural Science Foundation of Changsha, China (kq2208453), the Natural Science Foundation of Hunan Province, China (No: 2023JJ40067) and the Fundamental Research Funds for the Central Universities of Central South University (No. 1053320215872).
Author information
Zhuan Zhou and Chunhui Qu contributed equally to this work.
Authors and Affiliations
Department of Oncology, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, 410078, China
Zhuan Zhou, Chunhui Qu, Peijun Zhou, Qin Zhou & Lifang Yang
Cancer Research Institute, School of Basic Medicine Science, Central South University, Xiangya Road 110, Changsha, 410078, China
Zhuan Zhou, Chunhui Qu, Peijun Zhou, Xia Wu & Lifang Yang
Department of Laboratory Medicine, The Affiliated Changsha Hospital of Xiangya School of Medicine, Central South University, Changsha, 410078, China
Department of Life Science, College of Biology, Hunan University, Changsha, 410012, China
Department of Pathology, The Second Xiangya Hospital, Central South University, Renmin Middle Road 139, Changsha, 410011, China
You can also search for this author in PubMed Google Scholar
Contributions
Z. Zhou, X. Wu and L. Yang designed the study. Z. Zhou, C. Qu, P. Zhou and Q. Zhou obtained and assembled data. Z. Zhou, C. Qu, D. Li, X. Wu and L. Yang analysed and interpreted the data. Z. Zhou and L. Yang wrote the article, which was edited by all authors. The order of the co-first authors was assigned by their relative contributions to the study. All authors read and approved the final manuscript.
Corresponding authors
Correspondence to Xia Wu or Lifang Yang .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Ethics Committee of Second Xiangya Hospital of Central South University and conducted in accordance with the Declaration of Helsinki. All animals were raised and operated in accordance with the guidelines established by the Medical Research Animal Ethics Committee of Central South University.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zhou, Z., Qu, C., Zhou, P. et al. Extracellular vesicles activated cancer-associated fibroblasts promote lung cancer metastasis through mitophagy and mtDNA transfer. J Exp Clin Cancer Res 43 , 158 (2024). https://doi.org/10.1186/s13046-024-03077-w
Download citation
Received : 07 December 2023
Accepted : 21 May 2024
Published : 03 June 2024
DOI : https://doi.org/10.1186/s13046-024-03077-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Lung cancer
Journal of Experimental & Clinical Cancer Research
ISSN: 1756-9966
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Conclusions. Stem cell therapy is becoming a tangible reality by the day, thanks to the mounting research conducted over the past decade. With every research conducted the possibilities of stem cells applications increased in spite of the many challenges faced. ... Although, challenges might seem daunting, stem cell research is advancing ...
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory steps of controlled stem cell culturing and derivation.
Conclusion. Stem cells are diverse in their differentiation capacity as well as their source of origin. As we can see from this review, there are similarities and differences when these cells are extracted from different sources. ... Lo B, Parham L. Ethical Issues in Stem Cell Research. Endocrine Reviews. 2009;30(3):204-213. doi:10.1210/er.2008 ...
The potential impact of stem cell technology on medical and dental practice is vast. Stem cell research will not only provide the foundation for future therapies, but also reveal unique insights into basic disease mechanisms. ... Conclusion. Stem cell technology is unique in that it possesses both diagnostic and therapeutic potential. iPS cells ...
Stem Cell Research & Therapy (2023) Despite many reports of putative stem-cell-based treatments in genetic and degenerative disorders or severe injuries, the number of proven stem cell therapies ...
First, muscle stem cells (MuSCs) are a heterogeneous population that diverges over time and in response to disease or ageing. Targeting the functional subset of MuSCs is an unmet challenge. Second ...
The discovery of hPSCs, including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), has revolutionized stem cell research and cell-based therapy. 98 hESCs were ...
The ethical implications of stem cell research are often discussed in terms of risks, side effects, and safety, which are examples of hard impacts. In this article, Assen and colleagues argue that to understand the broader spectrum of ethical implications of stem cell research on science and society, it is important to recognize the so-called soft impacts.
The committee's deliberations on the issues led to the following conclusions and recommendations. Experiments in mice and other animals are necessary, but not sufficient, for realizing the potential of stem cells to develop tissue-replacement therapies that will restore lost function in damaged organs. ... Stem cell research that is publicly ...
Stem cells are noted for their ability to self-renew and differentiate into a variety of cell types. Some stem cells, described as totipotent cells, have tremendous capacity to self-renew and differentiate. Embryonic stem cells have pluripotent capacity, able to form tissues of all 3 germ layers but unable to form an entire live being. Research with embryonic stem cells has enabled ...
Blood stem cells, also known as hematopoietic stem cells, are found in the bone marrow and generate the different kinds of blood cells needed over a person's lifespan. This includes red blood cells, white blood cells — also known as immune cells — and platelets. Transplants of blood stem cells have been used to treat patients with blood ...
The field of stem cell research has undergone a significant transformation with the advent of human embryonic stem cells (hESCs). Since their pioneering isolation in 1998, hESCs have been at the forefront of scientific inquiry due to their unique ability for self-renewal and pluripotency [1, 2].This comprehensive review article delves into the advancements, challenges, and ethical ...
C.H. Jamieson and I.L. WeissmanN Engl J Med 2023;389:1310-1319. Stem cells can renew themselves without differentiating. Aging, inflammation, and environmental exposures may drive phenotypic ...
In 2009 the U.S. Food and Drug Administration approved the first clinical trial designed to test a human embryonic stem cell-based therapy, but the trial was halted in late 2011 because of a lack of funding and a change in lead American biotech company Geron's business directives. The therapy to be tested was known as GRNOPC1, which consisted of progenitor cells (partially differentiated ...
Primary stem cells have long been used therapeutically for applications such as bone marrow transplantation. This Review discusses how cell-engineering approaches are enabling the development of ...
In the past 60 years, stem cell research has gone through a long development stage. Beginning with the description of the first stem cells by Drs. James Edgar Till and Ernest A. McCulloch in 1961, stem cell research has developed into a promising field of application. Researchers have defined five basic stem cell types: embryonic stem cells ...
New stem cell research has identified the molecules that the cells produce to promote the healing process. The finding could pave the way for the development of new, more effective drugs for injuries or various diseases, including conditions related to reproductive health such as Asherman syndrome, a gynecologic condition in which the uterus ...
Stem cell research respects the integrity of life for the embryo with heavy specifications and intense oversight. This is vastly different in Finland, where the regulatory bodies find research more permissible in IVF excess, but only up to 14 days after fertilization.[48] ... CONCLUSION While some religions emphasize the sanctity of life from ...
Stem cell research can further help in better understanding stem cell physiology. This may result in finding new ways of treating currently incurable diseases. ... Conclusion. After several decades of experiments, stem cell therapy is becoming a magnificent game changer for medicine. With each experiment, the capabilities of stem cells are ...
Stem cell research reveals new clues to tissue repair that could help heal the uterus and more. by Isabella Backman, Yale University. Inhibition of decidualization of eSFs in vitro by miR-100-5p ...
The most common types of stem cells used in biological research are embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and adult stem cells (ASCs). These cells can be transformed into a variety of terminally-differentiated adult cell types, which can then be used for disease modeling or toxicity testing [ 24 ].
Stem cell research offers unprecedented opportunities for developing new treatments for debilitating diseases for which there are few or no cures. Stem cells also present a new way to explore fundamental questions of biology, such as determining the basic mechanisms of tissue development and specialization, which will be required for the development of therapies.
Nara Institute of Science and Technology. "Tackling the hurdle of tumor formation in stem cell therapies." ScienceDaily. ScienceDaily, 31 May 2024. <www.sciencedaily.com / releases / 2024 / 05 ...
Vascular endothelial cells (ECs) are monolayers of cells arranged in the inner walls of blood vessels. Under normal physiological conditions, ECs play an essential role in angiogenesis, homeostasis and immune response. Emerging evidence suggests that abnormalities in EC metabolism, especially aerobic glycolysis, are associated with the initiation and progression of various diseases, including ...
Stem-cell research articles from across Nature Portfolio. Stem-cell research is the area of research that studies the properties of stem cells and their potential use in medicine. As stem cells ...
The cytokines secreted by T cells and macrophages were more of an immune reserve against pathogenic microorganisms. In the inflammatory state, the spectrum of cytokines secreted by various types of cells in the dental pulp tended to be identical, such that it mainly resisted pathogenic microorganisms. Conclusions
Chimeric antigen receptor T cell (CAR-T) therapy has revolutionized the treatment approach for cancer, autoimmune disease, and heart disease. The integration of CAR into T cells is typically facilitated by retroviral or lentiviral vectors. However, the random insertion of CARs can lead to issues like clonal expansion, oncogenic transformation, variegated transgene expression, and ...
In June 2023, the International Society for Stem Cell Research (ISSCR) released a report detailing standards for human stem cell research . We spoke to the co-chairs of the Steering Committee ...
Stem cell research offers unprecedented opportunities for developing new medical therapies for debilitating diseases and a new way to explore fundamental questions of biology. Stem cells are unspecialized cells that can self-renew indefinitely and also differentiate into more mature cells with specialized functions. Research on human embryonic stem cells, however, is controversial, given the ...
Cell culture and reagents. 95C and 95D cells were obtained from the Shanghai Institute of Cell Biology (Shanghai, China). The paired cells are human giant cell lung carcinoma cell lines, and have the same genetic background and varied metastatic capacity. 95D cells had the higher metastatic capacity [].The cells were cultured in DMEM (Gibco BRL, Gaithersburg, MD, USA) supplemented with 10% ...