Sciencing_Icons_Science SCIENCE
Sciencing_icons_biology biology, sciencing_icons_cells cells, sciencing_icons_molecular molecular, sciencing_icons_microorganisms microorganisms, sciencing_icons_genetics genetics, sciencing_icons_human body human body, sciencing_icons_ecology ecology, sciencing_icons_chemistry chemistry, sciencing_icons_atomic & molecular structure atomic & molecular structure, sciencing_icons_bonds bonds, sciencing_icons_reactions reactions, sciencing_icons_stoichiometry stoichiometry, sciencing_icons_solutions solutions, sciencing_icons_acids & bases acids & bases, sciencing_icons_thermodynamics thermodynamics, sciencing_icons_organic chemistry organic chemistry, sciencing_icons_physics physics, sciencing_icons_fundamentals-physics fundamentals, sciencing_icons_electronics electronics, sciencing_icons_waves waves, sciencing_icons_energy energy, sciencing_icons_fluid fluid, sciencing_icons_astronomy astronomy, sciencing_icons_geology geology, sciencing_icons_fundamentals-geology fundamentals, sciencing_icons_minerals & rocks minerals & rocks, sciencing_icons_earth scructure earth structure, sciencing_icons_fossils fossils, sciencing_icons_natural disasters natural disasters, sciencing_icons_nature nature, sciencing_icons_ecosystems ecosystems, sciencing_icons_environment environment, sciencing_icons_insects insects, sciencing_icons_plants & mushrooms plants & mushrooms, sciencing_icons_animals animals, sciencing_icons_math math, sciencing_icons_arithmetic arithmetic, sciencing_icons_addition & subtraction addition & subtraction, sciencing_icons_multiplication & division multiplication & division, sciencing_icons_decimals decimals, sciencing_icons_fractions fractions, sciencing_icons_conversions conversions, sciencing_icons_algebra algebra, sciencing_icons_working with units working with units, sciencing_icons_equations & expressions equations & expressions, sciencing_icons_ratios & proportions ratios & proportions, sciencing_icons_inequalities inequalities, sciencing_icons_exponents & logarithms exponents & logarithms, sciencing_icons_factorization factorization, sciencing_icons_functions functions, sciencing_icons_linear equations linear equations, sciencing_icons_graphs graphs, sciencing_icons_quadratics quadratics, sciencing_icons_polynomials polynomials, sciencing_icons_geometry geometry, sciencing_icons_fundamentals-geometry fundamentals, sciencing_icons_cartesian cartesian, sciencing_icons_circles circles, sciencing_icons_solids solids, sciencing_icons_trigonometry trigonometry, sciencing_icons_probability-statistics probability & statistics, sciencing_icons_mean-median-mode mean/median/mode, sciencing_icons_independent-dependent variables independent/dependent variables, sciencing_icons_deviation deviation, sciencing_icons_correlation correlation, sciencing_icons_sampling sampling, sciencing_icons_distributions distributions, sciencing_icons_probability probability, sciencing_icons_calculus calculus, sciencing_icons_differentiation-integration differentiation/integration, sciencing_icons_application application, sciencing_icons_projects projects, sciencing_icons_news news.
- Share Tweet Email Print
- Home ⋅
- Science ⋅
- Chemistry ⋅

Examples of Chemical Synthesis

What is a Synthesis Reaction?
Studying chemical reactions and the conditions that affect their progression is the chief concern of the entire discipline of chemistry. A chemical reaction is the creation of one or more new substances from one or more existing ones; the former are called products and the latter reactants.
Often, the aim of chemists is to, well, make things. Formally, this process is known as synthesis, and it occurs with no human input in the body in the form of biosynthesis. The reactions associated with creating a new compound from multiple existing substances are called chemical synthesis reactions , and examples of these abound in everyday life, from industrial settings to academic chemistry laboratories.
Types of Chemical Reactions
The five basic types of chemical reactions are briefly detailed below. Some reactions can be of more than one type, as you'll soon see. The basic principles of any chemical reaction dictate that it must be balanced in elements and charge, meaning that these elements and charge are conserved before and after the reaction. These reactions might take place in a variety of situations. Some might happen spontaneously while others might require a catalyst to initiate the reaction.
- Decomposition reaction: This kind of reaction has the general form: AB → A + B . An example is the decomposition of hydrogen peroxide into water and oxygen: 2H 2 O 2 → 2H 2 O + O 2
- Combustion reaction: In these reactions, one reactant, called a fuel, reacts with oxygen gas, O 2 (one oxygen atom bonded to another). Often the result is visible as fire
There are two types of displacement reactions. They are also sometimes called single or double replacement reactions.
- Single displacement (or single replacement) reaction: These reactions involve one reactant "poaching" from another. The general form is A + BC → AB + C
- Double displacement (or double replacement) reaction: These are "trades" of the form AB + CD → AD + BC
- Synthesis reaction (also called a composition or combination reaction): A reaction of the general form A + B → AB . In the general example, it creates a single product from multiple inputs. An example is the synthesis of calcium carbonate from calcium oxide and carbon dioxide: CaO + CO 2 → CaCO 3
The process of rearranging elements and quantities to get a balanced chemical equation is called stoichiometry. This makes sure the the chemical compounds on either side of a reaction make up the same amount of reactants and products.
Synthesis Reaction: General Equation
As you saw, the synthesis reaction "word equation" (using letters to represent unspecified atoms or molecules) is A + B → AB. A and B can consist of single elements, either alone as ions (such as Na + ) or as diatomic molecules (such as Cl 2 ). One or both can also consist of molecular groups, e.g., NO< 3 - ).
Seven elements are naturally found as diatomic molecules: H 2 , N 2 , O 2 , F 2 , Cl 2 , Br 2 , and I 2 >.
Occasionally, composition reactions will include more than two elements and assume the form A + B + C → ABC.
Synthesis Reactions in Everyday Life
If someone asked for a list of chemical reactions in everyday life, or even, say, just a list of single-replacement reaction examples in everyday life, an experienced chemist could go on for a very long time! But limiting the discussion to synthesis reaction examples alone hardly spoils the fun. A few common such reactions are described below.
- Two of the substances you have already seen combine to form ordinary table salt, sodium chloride: 2Na + Cl 2 > → 2NaCl
- The reaction of calcium oxide and carbon dioxide above is an example of a class of synthesis reactions that involve metal oxides combining with CO 2 to produce the corresponding metallic carbonate
- Magnesium metal and oxygen gas combine to form magnesium oxide: 2Mg + O 2 → 2MgO . This is also a combustion reaction and applies to various other metals
- The synthesis of zinc oxide (handy for preventing nose sunburns) is a synthesis reaction that also involves oxidation, or the loss of electrons, often as a result of interacting with oxygen. The balanced reaction for this synthesis is 2Zn + O 2 → 2ZnO
- Potassium chloride reacts with ordinary oxygen gas to produce potassium chlorate. This is also an example of a general process – the combination of oxygen and a binary chloride to produce a chlorate of the metal: KCl + O 2 → KClO 3 .
Organic Synthesis
When chemical reactions involve carbon, they are generally considered to fall under the umbrella of organic chemistry. When multiple organic compounds and reactions are combined together to create a new element this is an organic example of a synthesis reaction.
Photosynthesis is likely the most famous example of this type of reaction; simple inputs of carbon-dioxide, water, and energy (sunlight) are used to create complex molecules and complex products. The reaction mechanisms involved include many types of reactions, but the predominant mechanism of photosynthesis creates natural products from natural reagents – organic synthesis.
The chemical bonds and more complex particles store this input energy in the form of glucose and other organic molecules.
Related Articles
How to identify the 6 types of chemical reactions, examples of single replacement reactions, how to predict products in chemical reactions, how to complete chemical reactions, chemical properties of benzoic acid, how to prepare potassium chloride, three types of aqueous reactions, what is a combustion reaction, when does a hydrolysis reaction occur, what happens in a lewis acid base reaction, what happens to the oxidation number when an atom in..., how to write a chemical compound formula, how to find heat reaction when zn reacts with hcl, how to calculate the stoichiometric ratio, what is a monatomic ion, safe combustion reaction experiments, what are the reactants & products in a combustion reaction, what is being oxidized and what is being reduced in..., what kind of reaction happens with hydrochloric acid....
- LibreTexts Chemistry: Types of Chemical Reactions
- Rhode Island College Chemistry: Chemical Reactions
- Open Stax | Classifying Chemical Reactions
About the Author
Kevin Beck holds a bachelor's degree in physics with minors in math and chemistry from the University of Vermont. Formerly with ScienceBlogs.com and the editor of "Run Strong," he has written for Runner's World, Men's Fitness, Competitor, and a variety of other publications. More about Kevin and links to his professional work can be found at www.kemibe.com.
Find Your Next Great Science Fair Project! GO
10 Examples of Synthesis Reactions
Synthesis reactions are those in which two or more compounds react to certain conditions to form one or more new products.
In a generic way, the reaction can be represented as the form: A + B → C.
The synthesis reactions are very important for science, because thanks to these methods can be made various materials, medicines and products that we use in everyday life.
Examples of synthesis reactions
Production of ammonia (nh3).
Nitrogen molecules contain two atoms of this element. Hydrogen appears the same in this way, so when combined in the right proportions and under the right conditions of pressure and temperature, ammonia is produced, according to the following reaction.
N2 + 3H2 → 2NH3
Sulfuric acid
This is produced from sulfur trioxide and a water molecule. It is a highly corrosive product and its main use is in the fertilizer industry. It is obtained from the following reaction.
SO3 + H2O → H2SO4
Table salt (sodium chloride)
This salt is one of the best known by all for its great domestic use. It is obtained from sodium and chlorine, and although it can be obtained by the following reaction, it is very easy to find it naturally.
Na + Cl → NaCl
The formula for synthesizing methanol is like two moles of diatomic hydrogen and carbon monoxide. The result is methanol (CH3OH).
However, to produce this process is not strictly followed and there are several intermediate steps to obtain the final product. Methanol serves as a solvent and is used in industries for various processes.
This is one of the most important reactions for life to exist as we know it. Plants use carbon dioxide and water from the environment in sunlight to produce glucose and oxygen.
The reaction in a very general way can be seen below, but it is important to understand that behind it there are several reactions and mechanisms to make this possible.
6CO2 + 6H2O → C6H12O6 + O2
This synthesis reaction occurs in living organisms and occurs when glucose is polymerized with fructose. Due to its structure, these two molecules interact and the final result is sucrose and water, as can be seen in the following equation:
C6H12O6 + C6H12O6 → C12H22O11 + H2O
Magnesium sulphate
It can be produced from a very simple reaction consisting of magnesium and sulfuric acid. It is very difficult to find it in nature without water.
Mg + H2SO4 → H2 + MgSO4
Carbon dioxide
It happens naturally in several processes, when the diatomic oxygen molecule is found with carbon carbon dioxide is produced.
It is present in natural processes such as respiration, as a reagent in photosynthesis and it occurs easily in combustion reactions.
C + O2 → CO2
Hydrochloric acid
Hydrochloric acid is widely used as a cheap acid and as a reactive agent for the synthesis of other compounds.
Cl2 + H2 → 2HCl
Calcium carbonate
It is widely known as a very abundant agent in nature, mainly in rocks, minerals and shells in the sea. Its reaction is based on the interaction of calcium oxide with carbon dioxide.
CaO + CO2 → CaCO3
- House, H. O. (1978). Modern reactions of organic synthesis . Mexico; Barcelona;: Reverté.
- Diaz, J. C., Fontal, B., Combita, D., Martinez, C., & Corma, A. (2013). Synthesis of nano-au supported in metal oxides and its catalytic activity in oxidation reactions of co. Latin American Journal of Metallurgy and Materials, 33 (1), 43-53.
- Rivera-Rivera, L. A. (2004). Synthesis, characterization, reactions and mechanisms of (dihapto- [carbon (60)] fullerene) (dihapto-bidentate ligand) tungsten (0) tricarbonyl
- Carriedo, G. A. (2010). Inorganic chemistry in reactions . Madrid: Synthesis.
- Chang, R. (1997). chemistry i . Mexico: McGraw-Hill.
Recent Posts
Brain Snack
Tired of ads, cite this source, logging out…, logging out....
You've been inactive for a while, logging you out in a few seconds...
W hy's T his F unny?

19 Synthesis Reaction Example: Detailed Explanations
In this article, “synthesis reaction example”, different types of synthesis (Williamson synthesis, balanced synthesis and peptide synthesis) example with detailed explanations are discusses briefly.
The examples are-
Synthesis of ethyl methyl ether
Synthesis of anisole, synthesis of 2-ethoxynaphthalene, synthesis of phenyl propyl ether.
- Synthesis of Benzyl-tert butyl ether
Synthesis of tert-butyl methyl ether
Synthesis of ethoxy benzene, synthesis of cyclopentyl methyl ether, synthesis of water, synthesis of carbon-dioxide, synthesis of ammonia.
- Synthesis of Aluminium Oxide
Synthesis of Iron Sulfide
Synthesis of potassium chloride, formation of rust, synthesis of calcium carbonate, synthesis of zinc oxide.
- Synthesis of dipeptide (Gly-Ala)
Solid Phase Peptide Synthesis
What is synthesis reaction.
Synthesis reaction is one type of chemical reaction in which two different atoms involve in the reaction, react with each other to form a totally different molecular compound. In most of the synthesis reaction, energy is released from the reaction medium and known as exothermic reaction.

Example of Williamson Synthesis
Williamson synthesis process is the best method to synthesis ethyl methyl ether (CH 3 -O- CH 2 CH 3 ). This reaction proceeds through SN 2 pathway. To obtain ethyl methyl ether as the synthesized product, sodium methoxide (CH 3 ONa) and ethyl chloride (C 2 H 5 Cl) reacts with each other. Sodium methoxide acts as nucleophile and attacks the electrophilic centre of ethyl chloride to eliminate the leaving group (Cl – ). Ethyl methyl ether is obtained as the Williamson synthesized product.

This ether can also be synthesized by Williamson ether synthesis . To obtain anisole, sodium phenoxide (C 5 H 5 ONa) will react with methyl iodide (CH 3 I) and sodium phenoxide (nucleophile) attacks the electrophilic centre of methyl iodide. Iodide (I – ) will be eliminated as it is a good leaving group and anisole is formed.

To know more please check: 12+ Exothermic Reaction Examples: Detailed Explanations
To proceed this reaction, hydroxyl group should be inserted at the 2 position of naphthalene group and reacts with bromoethane. The reaction medium should be basis. Thus, sodium hydride (NaH) is used. Nucleophilic oxygen atom of OH group in naphthalene attacks the CH 2 centre of CH 3 CH 2 Br and Br – is eliminated as the leaving group.

To synthesis phenyl propyl ether the reactants that are chosen are phenol, sodium metal and n-propyl bromide. Solvent that is used in this synthesis reaction is a polar aprotic solvent. The first step is to react phenol with sodium to form sodium phenoxide (active nucleophile). This nucleophile reacts with n-propyl bromide (electrophile) to synthesize phenyl propyl ether after elimination of bromide ion.

To know more please follow: 11+ First Order Reaction Example: Detailed Explanations
Synthesis of Benzyl-tertbutyl ether
William synthesis pathway is followed for the formation of benzyl-tertbutyl ether. Sodium tert-butoxide and benzyl bromide is taken as the reactants. O – ion from sodium tert-butoxide attacks the electron deficient centre of benzyl bromide Br – is eliminated as the leaving group to form the desired product.

This synthesis process almost similar to the synthesis of benzyl tert-butyl ether. One of the reactants is also same, sodium tert-butoxide and the another reactant is methyl bromide (CH 3 Br). Tertiary sodium tert butoxide reacts as nucleophile and attacks the methyl carbon center to eliminate bromide ion.

To know more please go through: 10+ Covalent bond types of elements: Detailed Insights And Facts
In this process of synthesis of ethoxy benzene, Williamson synthesis process is followed. Sodium ethoxide reacts with phenyl bromide to form ethoxy benzene. O – attacks the electrophilic centre of phenyl bromide and ethoxy benzene is obtained.

In this Williamson ether synthesis, cyclopentanol and methyl bromide is reacted with each other in a basic medium. In presence of base, hydrogen in O-H bond is eliminated and O – attacks the methyl bromide to form the cyclopentyl methyl ether.

Example of Balanced Synthesis
Hydrogen and oxygen-these two gases are the two main reactants of this synthesis. Water molecule that is formed is also in gaseous state. Two molecules of hydrogen react with one molecule of oxygen to form water molecule. Dissociation of water is taken place by passing electric through water.
2H 2 +O 2 = 2H 2 O
Electrolysis of water results-
- Reduction at cathode: 2H + (aq) +2e – = H 2 (g)
- Oxidation at anode: 2H 2 O = O 2 (g) + 4H + (aq) + 4e –
- Net balanced equation: 2H 2 O= 2H 2 + O 2
Carbon dioxide is synthesized during the different decay process of various different material and fermentation of sugars. It can be produced by combustion process of wood or other organic materials. Another procedure is to react metal carbonates with dilute acid for the formation of water. For example, carbon dioxide can be synthesized by the reaction between sodium carbonate with dilute HCl .
Haber-Bosch process is the most well known process to synthesize ammonia. High pressure and high temperature is the two most important driving force of ammonia production. It is an exothermic process (del H= -91.8 KJ/mol). Ammonia is widely used as fertilizer.
N 2 (g) + 3H 2 (g) = 2NH 3
To know more please check: Disulfide reduction: How, What, Methods and Several Facts
Synthesis of Aluminium oxide
Aluminium hydroxide is the main reactant for the formation of aluminium oxide. Solid Al(OH) 3 is decomposed over 1100 0 C and form aluminium oxide (Al 2 O 3 ). Besides that aluminium is oxidized in presence of oxygen to form aluminium oxide.
2Al(OH) 3 = (Al 2 O 3 ) + 3H 2 O
4al (s) + 3o 2 (g)= 2al 2 o 3.
Iron after reaction with sulfur forms iron sulfide (pyrrhotite) in presence of heat energy. Iron sulphide (FeS) has totally different physical and chemical properties with respect to two reactants, iron and sulphur. The ratio of iron with sulphur is 1:1. Equal amount of iron is reacted with equal amount of sulphur to form iron sulphide.
Fe + S = FeS
Potassium chloride is basically an ionic salt. It can be synthesized by the reaction bases of potassium like potassium hydroxide (KOH) with strong acid, hydrochloric acid . In this synthesis reaction, strong acid (HCl) is completely neutralized by strong base (KOH). Water is also produced along with the KCl.
KOH (aq) + HCl (aq) = KCl (s) + H 2 O (liq)
Rust is reddish brown iron oxide formed by reacting iron with oxygen. Water or air takes part in this synthesis reaction as catalyst. Chemical formula of rust is Fe 2 O 3 .Nh 2 O and iron oxide hydroxide (FeO (OH),Fe(OH) 3 ).
- Fe(OH) 2 = FeO + H 2 O
- Fe(OH) 3 = FeO(OH) + H 2 O
- 2FeO(OH) = Fe 2 O 3 +H 2 O
In this synthesis reaction, calcium oxide (CaO) and carbon dioxide is reacted to form calcium carbonate. At first step, calcium hydroxide is prepared by the reaction between calcium oxide with water. After that Ca(OH) 2 is reacted with carbon dioxide and as a product calcium carbonate is obtained.
- CaO +H 2 O = Ca(OH) 2
- Ca(OH) 2 +CO 2 = CaCO 3 +H 2 O
High temperature is one of the most important driving forces. Zinc vapour is reacted with air (oxygen) at 910 0 C. It is mainly an oxidation process and ZnO is produced.
Example of Peptide Synthesis
Synthesis of dipeptide ( gly – ala).
To synthesis of a dipeptide the following steps should be followed-
- At first alpha amino group of glycine should be blocked by tert-butyloxycarbonylchloride.
- After giving the protection to alpha amino acid of glycine, alanine will react with the previously formed compound.
- Then the tert-butyloxycarbonylchloride group is eliminated by reacting with dilute acid and dipeptide (ala-gly) is obtained as the final product.

This synthesis procedure is known as Merrifield synthesis discovered by scientist R.Bruce Merrifield. In this peptide synthesis procedure, homogenous solution is not used for deprotection. This deprotection is carried out at the surface of an insoluble polymer or any solid support.
The carboxyl terminal amino acid is covalently linked with the Merrifield Resin and the length of the peptide chain is increased. Reagents are used to remove the resin with the soluble by products from the peptide chain and at the end desired peptide chain is obtained.

- Maillard reaction
- First order reaction example
- Is oxidation a redox reaction
- Light independent reaction in photosynthesis
- Nuclear fission reaction
- Addition reaction example
- Oxidation reaction example
- Decomposition reaction example
- Redox reactions
- Nuclear fusion reaction

Hello, I am Aditi Ray, a chemistry SME on this platform. I have completed graduation in Chemistry from the University of Calcutta and post graduation from Techno India University with a specialization in Inorganic Chemistry. I am very happy to be a part of the Lambdageeks family and I would like to explain the subject in a simplistic way. Let’s connect through LinkedIn-https://www.linkedin.com/in/aditi-ray-a7a946202
Synthesis Reactions
Synthesis reactions, also known as combination reactions, are chemical reactions in which two or more reactants combine to form a single product. This type of reaction is represented by the general equation: A + B → AB
Formation of a new substance: Synthesis reactions result in creating a new compound or molecule from simpler reactants.
Energy absorption: These reactions often require an input of energy, typically in the form of heat or light, to initiate the reaction.
Common examples of synthesis reactions include the formation of water (2H 2 + O 2 → 2H 2 O), the synthesis of ammonia (3H₂ + N₂ → 2NH₃), and photosynthesis (6CO 2 + 6H 2 O → C 6 H 12 O 6 + 6O 2 + 6H 2 ).
- Anatomy & Physiology
- Astrophysics
- Earth Science
- Environmental Science
- Organic Chemistry
- Precalculus
- Trigonometry
- English Grammar
- U.S. History
- World History
... and beyond
- Socratic Meta
- Featured Answers

What are some examples of synthesis reactions?


- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Examples of Chemical Reactions in Everyday Life

Chemical reactions occur everywhere in the world around you, not just in a chemistry lab. Here are 20 examples of chemical reactions in everyday life and a closer look at what’s happening on a molecular level.
How to Recognize a Chemical Reaction
The first step to recognizing chemical reactions in the world around you is identifying when a reaction is taking place. Chemical reactions cause chemical changes . In other words, substances interact and form new products . Not every change in matter is a chemical reaction . For example, melting ice, tearing a sheet of paper into strips, and dissolving sugar in water are physical changes that don’t change the chemical identity of matter .
Here are some signs of a chemical reaction. If more than one sign is present, it’s likely a reaction has occurred:
- Temperature change
- Color change
- Bubbling or gas production
- Formation of a solid called a precipitate when liquids are mixed
20 Examples of Chemical Reactions in Everyday Life
Here are some broad examples of chemical reactions in daily life:

Photosynthesis
- Aerobic cellular respiration
- Anaerobic respiration (including fermentation )
- Oxidation (including rust)
- Metathesis reactions (such as baking soda and vinegar)
- Electrochemistry (including chemical batteries)
- Soap and detergent reactions
- Acid-base reactions
- Rotting of food
- Electroplating metals
- Disinfecting surfaces and contact lenses
- Leaves changing color with seasons
- Salt keeping ice off roads and helping to freeze ice cream

Examples of Organic Compounds
Some chemicals are inorganic, while those with carbon and hydrogen are organic. Here are examples in everyday life.
A Closer Look at Chemical Reactions in Daily Life
Here is a closer look at some everyday reactions, along with some chemical equations.
You experience combustion reactions when you strike a match, burn a candle, start a campfire, or light a grill. In a combustion reaction, a fuel reacts with oxygen from air to produce water and carbon dioxide. Here is the reaction for the combustion of propane, a fuel used in gas grills and some fireplaces: C 3 H 8 + 5O 2 → 4H 2 O + 3CO 2 + energy
Plants use a chemical reaction called photosynthesis to convert carbon dioxide and water into food (glucose) and oxygen. It’s a key reaction because it generates oxygen and yields food for plants and animals. The overall chemical reaction for photosynthesis is: 6 CO 2 + 6 H 2 O + light → C 6 H 12 O 6 + 6 O 2
Aerobic Cellular Respiration
Animals use the oxygen provided by plants to perform essentially the reverse reaction of photosynthesis to get energy for cells. Aerobic respiration reacts glucose and oxygen to form water and chemical energy in the form of adenosine triphosphate ( ATP ). Here is the overall equation for aerobic cellular respiration: C 6 H 12 O 6 + 6O 2 → 6CO 2 + 6H 2 O + energy (36 ATP)
Anaerobic Cellular Respiration
Organisms also have ways of getting energy without oxygen. Humans use anaerobic respiration during intense or prolonged exercise to get enough energy to muscle cells. Yeast and bacteria use anerobic respiration in the form of fermentation to make everyday products, such as wine, vinegar, yogurt, bread, cheese, and beer. The equation for one form of anerobic respiration is: C 6 H 12 O 6 → 2C 2 H 5 OH + 2CO 2 + energy
Rust, verdigris, and tarnish are all examples of common oxidation reactions. When iron rusts, it changes color and texture to form a flake coating called rust. The reaction also releases heat, but it usually occurs too slowly for this to be noticeable. Here is the chemical equation for the rusting of iron: Fe + O 2 + H 2 O → Fe 2 O 3 . XH 2 O
Electrochemistry
Electrochemical reactions are redox (oxidation and reduction) reactions that convert chemical energy into electrical energy. The type of reaction depends on the battery. Spontaneous reactions occur in galvanic cells, while nonspontaneous reactions take place in electrolytic cells.
Digestion is a complex process that involves thousands of chemical reactions. When you put food in your mouth, water and the enzyme amylase breaks down sugar and other carbohydrates into simpler molecules. Hydrochloric acid and enzymes break down proteins in your stomach. Sodium bicarbonate released into the small intestine neutralizes the acid and protects the digestive tract from dissolving itself.
Soap and Detergent Reactions
Washing your hands with water isn’t a chemical reaction because you’re just mechanically rinsing away grime. But, when you add soap or detergent, chemical reactions occur that emulsify grease and lower surface tension so you can remove oily grime. Even more reactions occur in laundry detergent, which may contain enzymes to break apart proteins and whiteners to prevent clothes from looking dingy.
Just mixing dry ingredients usually doesn’t result in a chemical reaction. But, adding a liquid ingredient often results in a reaction. Cooking with heat also causes reactions. Mixing flour, sugar, and salt is not a chemical reaction. Neither is mixing oil and vinegar. Cooking an egg is a chemical reaction because heat polymerizes proteins in egg white, while the hydrogen and sulfur in the yolk can react to form hydrogen sulfide gas. When you heat sugar, a reaction called caramelization occurs. When you heat meat, it browns due to the Maillard reaction . Baked goods rise due to carbon dioxide bubbles formed by the reaction between baking powder or soda and liquid ingredients.
Acid-Base Reactions
Acid-base reactions occur anytime you mix an acid (e.g., lemon juice, vinegar, muriatic acid, battery acid, carbonic acid from carbonated beverages) with a base (e.g., baking soda, ammonia, lye). A good example of an acid-base reaction is the reaction between baking soda and vinegar to form sodium acetate, water, and carbon dioxide gas: NaHCO 3 + HC 2 H 3 O 2 → NaC 2 H 3 O 2 + H 2 O + CO 2 In general, a reaction between an acid and a base produces a salt and water. For example, if you react muriatic acid (HCl) and lye (NaOH), you get table salt (NaCl) and water (H 2 O): HCl + NaOH → NaCl + H 2 O In this reaction, two clear liquids form another clear liquid, but you can tell a reaction occurs because it releases a lot of heat.
Related Posts
Examples of Chemical Reactions in Everyday Life
ThoughtCo / Emily Roberts
- Chemistry In Everyday Life
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Chemistry happens in the world around you, not just in a lab. Matter interacts to form new products through a process called a chemical reaction or chemical change. Every time you cook or clean, it's chemistry in action. Your body lives and grows thanks to chemical reactions. There are reactions when you take medications, light a match, and draw a breath.
These examples of chemical reactions from everyday life are a small sampling of the hundreds of thousands of reactions you experience as you go about your day.
Key Takeaways: Chemical Reactions in Everyday Life
- Chemical reactions are common in daily life, but you may not recognize them.
- Look for signs of a reaction. Chemical reactions often involve color changes, temperature changes, gas production, or precipitant formation.
- Simple examples of everyday reactions include digestion, combustion, and cooking.
- What Is a Chemical Reaction?
A chemical change , often called a chemical reaction , occurs when substances transform into new and distinct substances. Essentially, it involves the rearrangement of atoms. Generally, chemical changes can be identified by temperature changes, light emission, bubble formation, precipitate formation, color changes, and odor release. These effects signify a change in composition, but they may not always be immediately apparent.
Usually, chemical changes are permanent, so they cannot be undone. Conversely, physical changes do not create new substances and can be reversed. Understanding these distinctions is fundamental to the study of chemistry .
Photosynthesis
Frank Krahmer / Getty Images
Plants apply a chemical reaction called photosynthesis to convert carbon dioxide and water into food (glucose) and oxygen. It's one of the most common everyday chemical reactions and also one of the most important because this is how plants produce food for themselves (and animals) and convert carbon dioxide into oxygen. The equation for the reaction is:
6 CO 2 + 6 H 2 O + light → C 6 H 12 O 6 + 6 O 2
Aerobic Cellular Respiration
Kateryna Kon/Science Photo Library / Getty Images
Aerobic cellular respiration is the opposite process of photosynthesis in that energy molecules are combined with the oxygen we breathe to release the energy needed by our cells plus carbon dioxide and water. Energy used by cells is chemical energy in the form of ATP, or adenosine triphosphate .
Here is the overall equation for aerobic cellular respiration:
C 6 H 12 O 6 + 6O 2 → 6CO 2 + 6H 2 O + energy (36 ATPs)
Anaerobic Respiration
Tastyart Ltd Rob White / Getty Images
Anaerobic respiration is a set of chemical reactions that allows cells to gain energy from complex molecules without oxygen. Your muscle cells perform anaerobic respiration whenever you exhaust the oxygen being delivered to them, such as during intense or prolonged exercise. Anaerobic respiration by yeast and bacteria is harnessed for fermentation to produce ethanol, carbon dioxide, and other chemicals that make cheese, wine, beer, yogurt, bread, and many other common products.
The overall chemical equation for one form of anaerobic respiration is:
C 6 H 12 O 6 → 2C 2 H 5 OH + 2CO 2 + energy
Every time you strike a match, burn a candle, build a fire, or light a grill, you see the combustion reaction. Combustion combines energetic molecules with oxygen to produce carbon dioxide and water.
For example, the equation for the combustion reaction of propane, found in gas grills and some fireplaces, is:
C 3 H 8 + 5O 2 → 4H 2 O + 3CO 2 + energy
Alex Dowden/EyeEm / Getty Images
Over time, iron develops a red, flaky coating called rust. This is an example of an oxidation reaction . Other everyday examples include the formation of verdigris on copper and the tarnishing of silver.
Here is the chemical equation for the rusting of iron:
Fe + O 2 + H 2 O → Fe 2 O 3 . XH 2 O
If you combine vinegar and baking soda for a chemical volcano or milk with baking powder in a recipe, you experience a double displacement , or metathesis reaction (plus some others.) The ingredients recombine to produce carbon dioxide gas and water. The carbon dioxide forms bubbles in the volcano and helps baked goods rise .
These reactions seem simple in practice but often consist of multiple steps. Here is the overall chemical equation for the reaction between baking soda and vinegar:
HC 2 H 3 O 2 (aq) + NaHCO 3 (aq) → NaC 2 H 3 O 2 (aq) + H 2 O(l) + CO 2 (g)
Electrochemistry
Batteries use electrochemical or redox reactions to convert chemical energy into electrical energy. Spontaneous redox reactions occur in galvanic cells , while nonspontaneous chemical reactions take place in electrolytic cells .
Peter Dazeley/Photographer's Choice / Getty Images
Thousands of chemical reactions take place during digestion. As soon as you put food in your mouth, an enzyme in your saliva called amylase starts to break down sugars and other carbohydrates into simpler forms your body can absorb. Hydrochloric acid in your stomach reacts with food to further break it down, while enzymes cleave proteins and fats so they can be absorbed into your bloodstream through the walls of the intestines.
Acid-Base Reactions
Lumina Imaging / Getty Images
Whenever you combine an acid (e.g., vinegar, lemon juice, sulfuric acid , or muriatic acid ) with a base (e.g., baking soda , soap, ammonia, or acetone), you are performing an acid-base reaction. These reactions neutralize the acid and base to yield salt and water.
Sodium chloride isn't the only salt that can be formed. For example, here is the chemical equation for an acid-base reaction that produces potassium chloride, a common table salt substitute:
HCl + KOH → KCl + H 2 O
Soap and Detergent Reactions
JGI/Jamie Grill / Getty Images
Soaps and detergents clean by way of chemical reactions . Soap emulsifies grime, which means oily stains bind to the soap so they can be lifted away with water. Detergents act as surfactants, lowering the surface tension of water so it can interact with oils, isolate them, and rinse them away.
Cooking uses heat to cause chemical changes in food. For example, when you hard boil an egg, the hydrogen sulfide produced by heating the egg white can react with iron from the egg yolk to form a grayish-green ring around the yolk . When you brown meat or baked goods, the Maillard reaction between amino acids and sugars produces a brown color and a desirable flavor.
More Examples of Chemistry in Everyday Life
Chemical reactions are everywhere, and in a way, chemistry really makes up everything. From the emotions you feel to peculiar questions such as, "Can bottled water go bad?" Here are some examples of chemistry in everyday life.
- Simple Chemical Reactions
- Types of Chemical Reactions
- What Are the Products of Photosynthesis?
- Understanding Endothermic and Exothermic Reactions
- 10 Fascinating Photosynthesis Facts
- Equation for the Reaction Between Baking Soda and Vinegar
- The Balanced Chemical Equation for Photosynthesis
- How Many Types of Chemical Reactions Are There?
- Combustion Definition in Chemistry
- An Introduction to Types of Respiration
- Calvin Cycle Steps and Diagram
- Chemical Change Examples
- Citric Acid Cycle or Krebs Cycle Overview
- Combustion Reactions in Chemistry
- Chemical Change Definition in Chemistry

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

7.11: Application: Useful SN2 Reactions
- Last updated
- Save as PDF
- Page ID 28176

- Layne Morsch
- University of Illinois Springfield
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
SAM methyltransferases
Some of the most important examples of S N 2 reactions in biochemistry are those catalyzed by S-adenosyl methionine (SAM) – dependent methyltransferase enzymes. We have already seen, in chapter 6 and again in chapter 8, how a methyl group is transferred in an S N 2 reaction from SAM to the amine group on the nucleotide base adenosine:
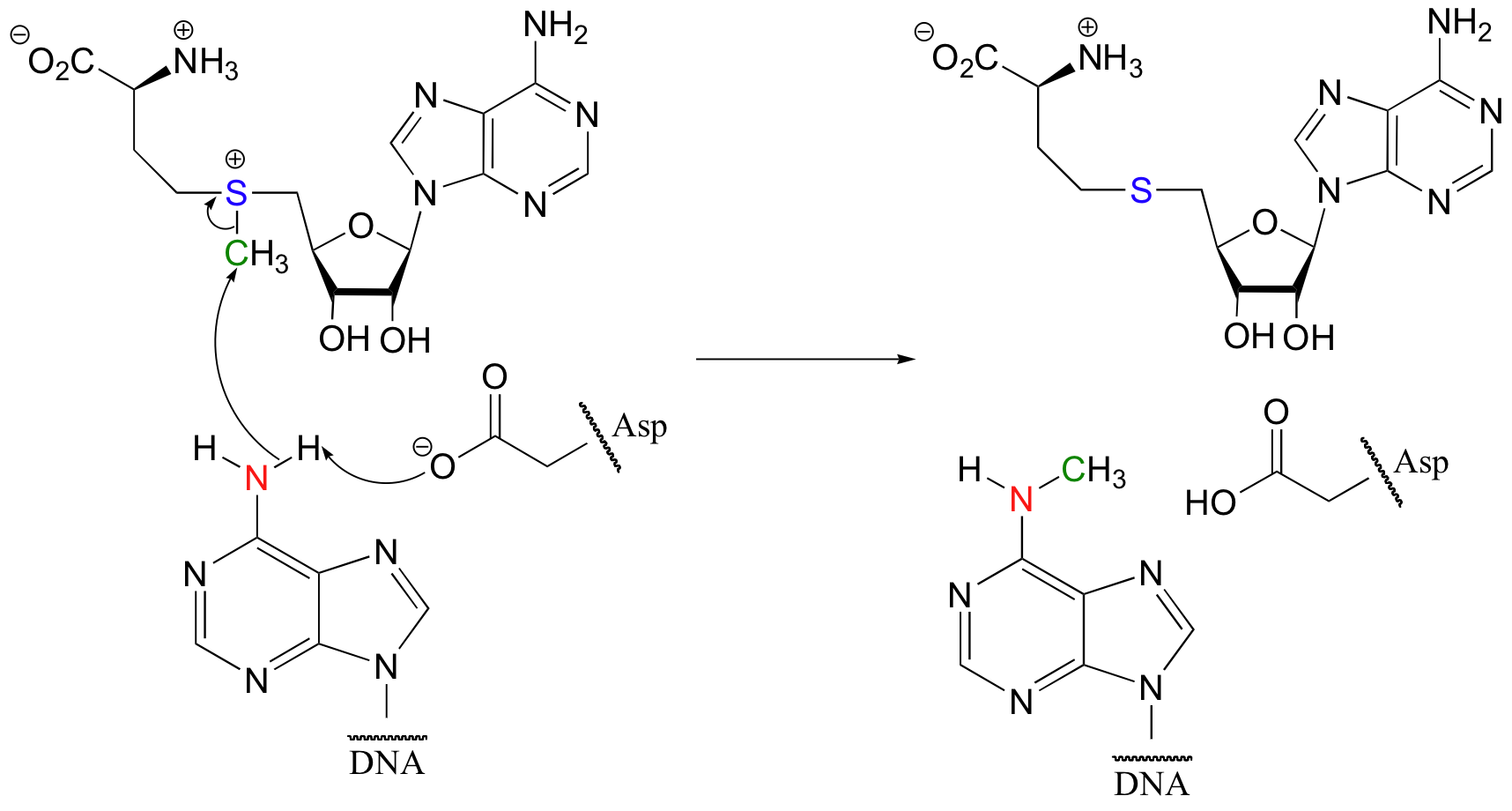
( Nucleic Acids Res . 2000 , 28 , 3950 ).
Another SAM-dependent methylation reaction is catalyzed by an enzyme called catechol-O-methyltransferase. The substrate here is epinephrine, also known as adrenaline.
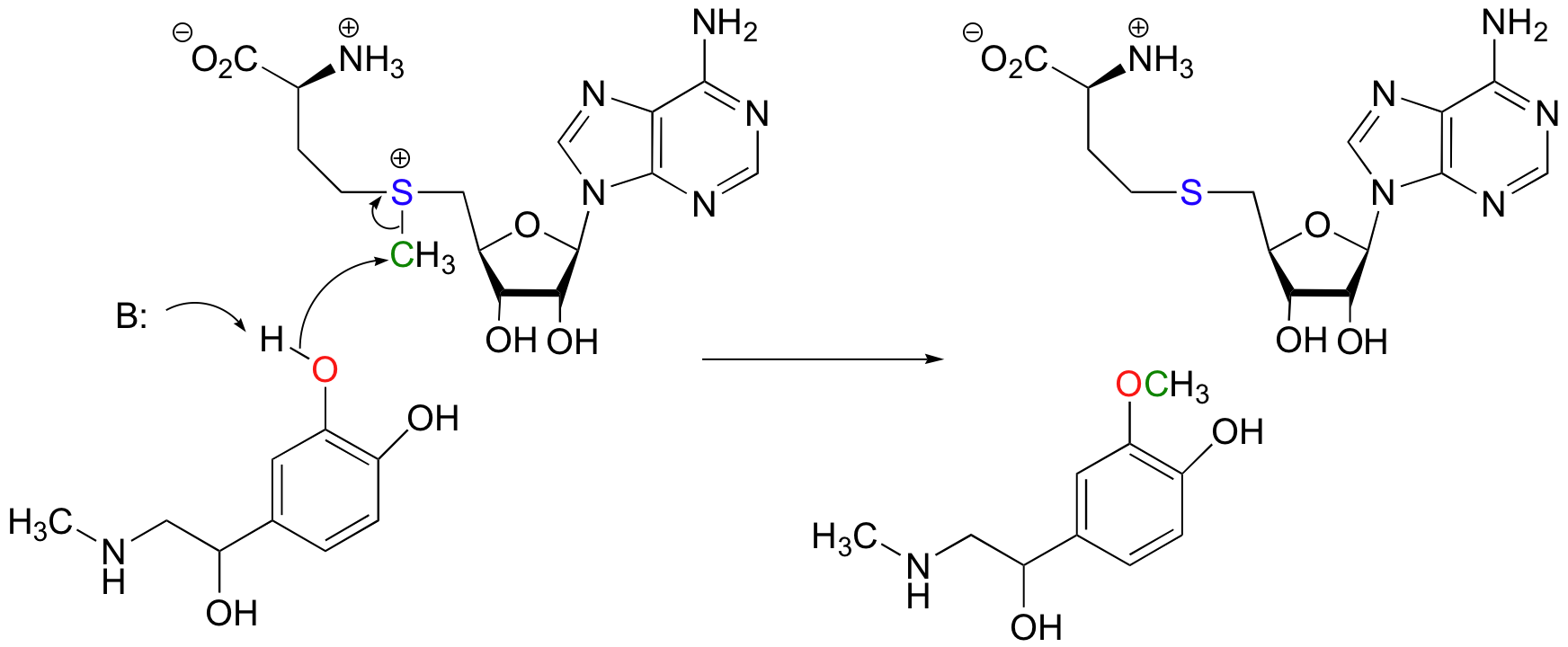
Notice that in this example, the attacking nucleophile is an alcohol rather than an amine (that’s why the enzyme is called an O-methyltransferase). In both cases, though, a basic amino acid side chain is positioned in the active site in just the right place to deprotonate the nucleophilic group as it attacks, increasing its nucleophilicity. The electrophile in both reactions is a methyl carbon, so there is little steric hindrance to slow down the nucleophilic attack. The methyl carbon is electrophilic because it is bonded to a positively-charged sulfur, which is a powerful electron withdrawing group. The positive charge on the sulfur also makes it an excellent leaving group, as the resulting product will be a neutral and very stable sulfide. All in all, in both reactions we have a reasonably good nucleophile, an electron-poor, unhindered electrophile, and an excellent leaving group.
Because the electrophilic carbon in these reactions is a methyl carbon, a stepwise S N 1-like mechanism is extremely unlikely: a methyl carbocation is very high in energy and thus is not a reasonable intermediate to propose. We can confidently predict that this reaction is S N 2. Does this S N 2 reaction occur, as expected, with inversion of stereochemistry? Of course, the electrophilic methyl carbon in these reactions is achiral, so inversion is not apparent. To demonstrate inversion, the following experiment has been carried out with catechol-O-methyltransferase:
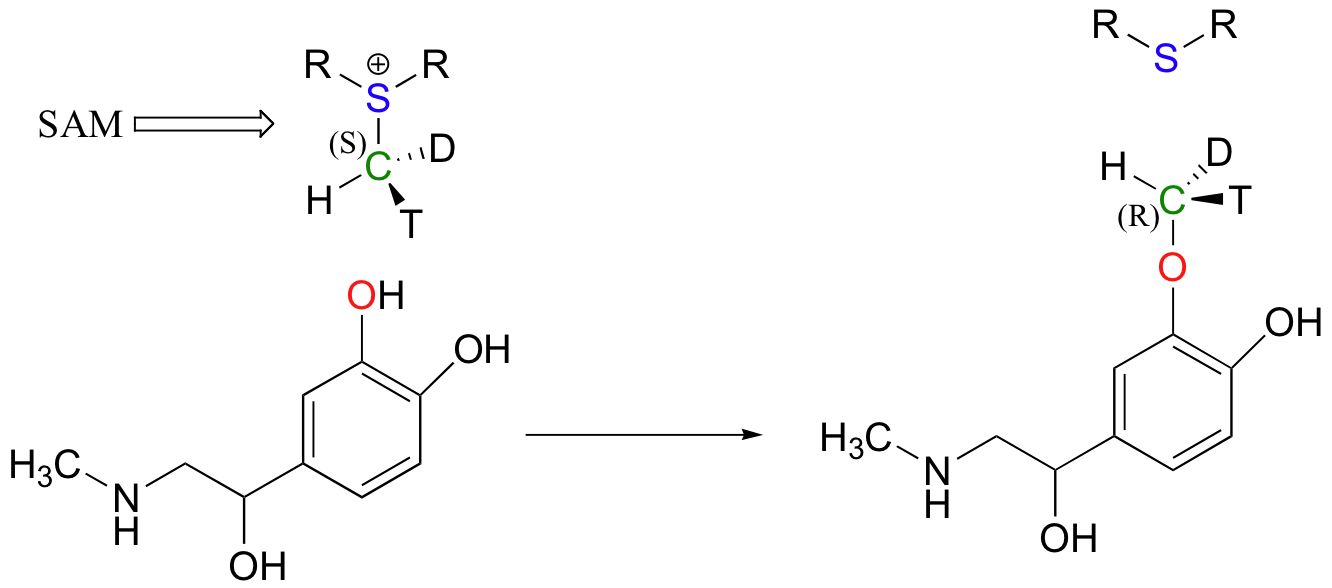
Here, the methyl group of SAM was made to be chiral by incorporating hydrogen isotopes tritium ( 3 H, T) and deuterium ( 2 H, D). The researchers determined that the reaction occurred with inversion of configuration, as expected for an S N 2 displacement ( J. Biol. Chem . 1980, 255 , 9124 ).
Exercise 9.1 : SAM is formed by a nucleophilic substitution reaction between methionine and adenosine triphosphate (ATP). Propose a mechanism for this reaction.
9.1B: Synthetic parallel – the Williamson ether synthesis
Synthetic organic chemists often use reactions in the laboratory that are conceptually very similar to the SAM-dependent methylation reactions described above. In what is referred to as "O-methylation", an alcohol is first deprotonated by a strong base, typically sodium hydride (this is essentially an irreversible deprotonation, as the hydrogen gas produced can easily be removed from the reaction vessel).
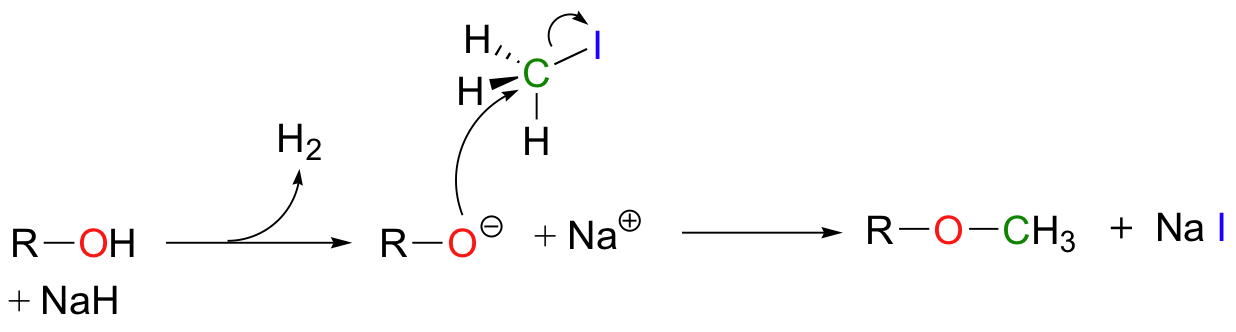
The alkoxide ion, a powerful nucleophile, is then allowed to attack the electrophilic carbon in iodomethane, displacing iodide in an S N 2 reaction. Recall ( section 7.3A ) that iodide ion is the least basic - and thus the best leaving group - among the halogens commonly used in the synthetic lab. CH 3 I is one of the most commonly used lab reagents for methyl transfer reactions, and is the lab equivalent of SAM. In the synthesis of a modified analog of the signaling molecule myo -inisitol triphosphate, an alcohol group was methylated using sodium hydride and iodomethane ( Carbohydrate Research 2004, 339 , 51 ):
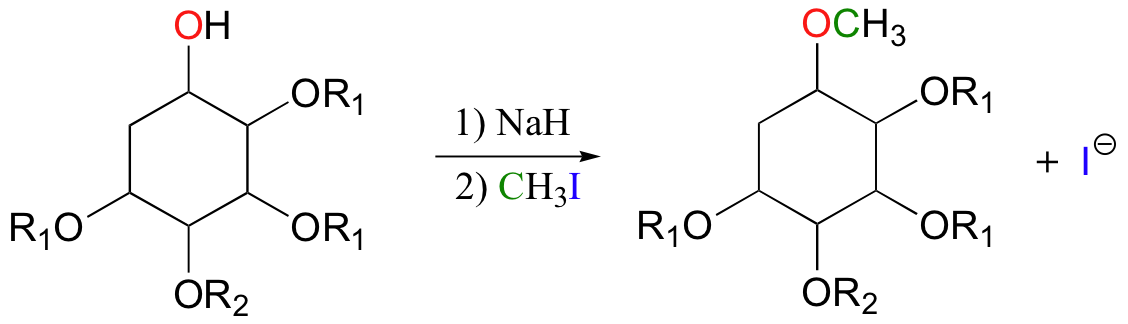
Methylation of amines (N-methylation) by iodomethane is also possible. A recent study concerning the optimization of peptide synthesis methods involved the following reaction ( J. Org. Chem . 2000 65 , 2309 ):
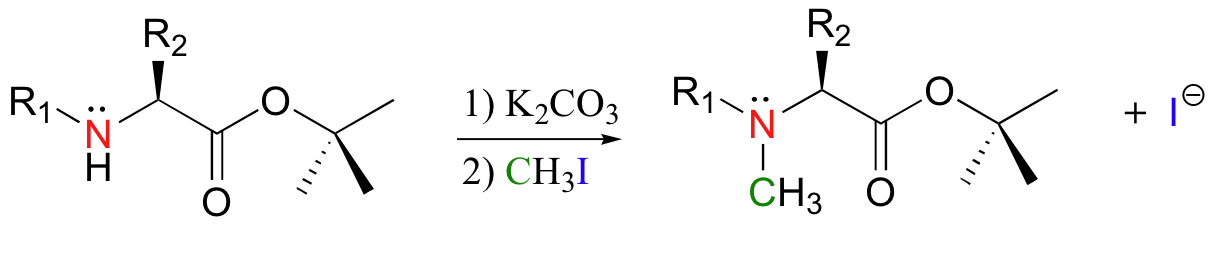
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
Chemistry Learner
It's all about chemistry.
- Chemical Bonds
- Chemical Reactions
- Materials Chemistry
- Organic Chemistry
- Periodic Trends
- Periodic Table Groups
- How to Read Periodic Table
- Naming Covalent Compounds Worksheets
- Net Ionic Equation Worksheets
- Types of Chemical Reactions Worksheets
- Word Equations Worksheets
- Valence Electrons Worksheets
- Graphing Periodic Trends Worksheets
- Periodic Trends Ionization Energy Worksheets
- Atomic Structure And Isotopes Worksheets
Decomposition Reaction
What is a decomposition reaction, what occurs during a decomposition reaction, characteristics of decomposition reaction, examples of decomposition reaction, how to balance decomposition reaction, types of decomposition reaction, decomposition reaction examples in real life.
A decomposition reaction is a type of chemical reaction in which a compound breaks down into two or more substances. It is the opposite of a combination reaction . Sometimes, a complex chemical species breaks down into simple parts. The starting substance is called the reactant, and the resulting substances are called products [1-5] .
General Equation
The following equation represents a general decomposition reaction.
When writing an actual reaction, the reaction must be balanced.

During a decomposition reaction, the bonds between the atoms break down in the starting substance. The atoms then rearrange to form new bonds, resulting in new substances with properties different from the starting material.
- One single reactant and two or more products
- Requires energy
An example of a decomposition reaction is the breakdown of carbonic acid (H 2 CO 3 ) to carbon dioxide (CO 2 ) and water (H 2 O) [2] .
H 2 CO 3 (aq.) → CO 2 (g) + H 2 O (l)
This reaction is significant and is responsible for the fizz in soda. Below are some more examples:

Let us take the example of the breakdown of potassium chlorate (KClO 3 ) into potassium chloride (KCl) and oxygen (O 2 ).
KClO 3 (s) → KCl (s) + O 2 (g)
This equation is unbalanced. In order to balance the equation, we inspect the number of oxygen (O) atoms on the right-hand side of the equation and compare it to the left. We notice that there are two oxygen atoms on the right and three on the left. So, we multiply the right-O by 3 and the left-O by 2. Note that we are going to multiply the entire compound KClO 3 by 2 and balance the oxygen.
2 KClO 3 (s) → KCl (s) + 3 O 2 (g)
Now, we notice that the potassium (K) and chlorine (Cl) are not balanced. On the left-hand side of the equation, there are two atoms of K and Cl, whereas, on the right, there is only one atom of each. So, we multiply the compound KCl by 2 and obtain the final equation.
2 KClO 3 (s) → 2 KCl (s) + 3 O 2 (g)
There are three types of decomposition reactions [1-3] .
1. Thermal Decomposition
In this reaction, energy in the form of heat is required to break the bonds of the compound. The reaction is generally endothermic, i.e., heat is added to the reaction.
Rules for Thermal Decomposition
- Carbonates decompose into carbon dioxide and an oxide.
- Chlorates decompose into oxygen gas and a chloride.
- Hydroxides decompose into water and an oxide.
- Acids containing oxygen decompose into water and a molecular oxide.
- Oxides decompose into oxygen and another element.
- Calcium carbonate (CaCO 3 ) decomposes into calcium oxide (CaO) and carbon dioxide (CO 2 ) gas when heated.
CaCO 3 (s) + heat → CaO (s) + CO 2 (g)
- At high temperatures, magnesium carbonate (MgCO 3 ) decomposes to magnesium oxide (MgO) and carbon dioxide (CO 2 ).
- Thermal decomposition of phosphoric acid (H 3 PO 4 ) produces diphosphoric acid (H 4 P 2 O 7 ) and water (H 2 O).
2 H 3 PO 4 → H 4 P 2 O 7 + H 2 O
- Ammonium dichromate (NH 4 ) 2 Cr 2 O 7 decomposes on heating to produce nitrogen gas, water vapor, and solid chromium (III) oxide (Cr 2 O 3 ) [6]
(NH 4 ) 2 Cr 2 O 7 (s) + heat → Cr 2 O 3 (s) + N 2 (g) + 4 H 2 O (g)
2. Electrolytic Decomposition
In this reaction, decomposition occurs when an electric current is passed through an aqueous solution of the compound.
- Electrolysis of water (H 2 O) to give hydrogen (H 2 ) and oxygen (O 2 ) gases
2 H 2 O (l) → 2 H 2 (g) + O 2 (g)
- Electrolysis of sodium chloride (NaCl) solution gives molten sodium (Na) and chlorine (Cl 2 ) gas
2 NaCl (l) → 2 Na (l) + Cl 2 (g)
3. Photo or Photochemical Decomposition
In this reaction, light (photons) is used to decompose the compound.
- Silver chloride (AgCl) decomposes into silver (Ag) and chlorine (Cl 2 ) in the presence of sunlight
2 AgCl (s) + sunlight → 2 Ag (s) + Cl 2 (g)
- In the presence of light, hydrogen peroxide (H 2 O 2 ) decomposes into water (H 2 O) and oxygen (O 2 ).
2 H 2 O 2 (l) → 2 H 2 O (l) + O 2 (g)
Another Type of Decomposition Reaction
Catalytic decomposition.
In this type of decomposition, the reaction occurs with the aid of a catalyst.
- The decomposition of hydrogen peroxide (H 2 O 2 ) to water (H 2 O) and oxygen (O 2 ) is catalyzed by an enzyme called catalase [7] .
2 H 2 O 2 (l) + catalase (enzyme) → 2 H 2 O (l) + O 2 (g)
The decomposition reaction has a few applications in the industry and daily life.
- Production of calcium oxide or quicklime
- Production of lithium oxide
- Preparation of oxygen and carbon dioxide
- In metallurgy, for the extraction of metals from their oxides and chlorides through electrolytic decomposition
- When a soda bottle is opened, carbonic acid breaks down to produce water and carbon dioxide, which causes the fizz.
- During the digestion of food in our body, carbohydrates, fats, and proteins decompose to form many simpler substances.
- Photographic films have a coating of silver bromide, which on exposure to light splits into silver and bromine.
- When hydrogen peroxide is applied onto a cut or wound, the peroxide decomposes and forms oxygen bubbles and bursts.
- While baking a cake with baking powder (sodium bicarbonate), decomposition leads to the formation of carbon dioxide and sodium hydroxide.
- Many organic decompositions are observed in food like fermentation and fouling. Rotting of food and vegetable peels produce excellent nutrients and manure for the soil.
- In several analytical techniques like mass spectrometry, gravimetric analysis, and thermogravimetric analysis
Ans. Most decomposition reactions require energy either in the form of heat, light, or electricity. Absorption of energy causes the breaking of the bonds present in the reacting substance which decomposes to give the product.
Ans. In a combination reaction, two or more substances combine to form a single product. However, in decomposition reaction, a single substance breaks down to give two or more substances. Therefore, the two are opposite to each other. Generally, combination reactions are mostly exothermic, while decomposition reactions are endothermic.
Ans. No. Not all decomposition are redox reactions.
Ans. No. The decomposition of aspirin is a double-replacement reaction.
- Amrita.olabs.edu.in
- Chem.libretexts.org
- Opentextbc.ca
- Cpanhd.sitehost.iu.edu
- Chem.wisc.edu
- Angelo.edu York.ac.uk
Examples of Redox Reactions in Everyday Life
Redox reactions reactions play a crucial role in various chemical processes, both in nature and in human-made systems. These reactions involve the transfer of electrons between reactants, leading to changes in the oxidation states of the elements involved.
This article discusses redox reactions, their significance in everyday life, examples, identification, and their impact on environmental stability.
What are Redox Reactions?
Redox reactions , or (oxidation-reduction reactions), are chemical reactions in which there is a transfer of electrons between two reactants. These reactions involve the oxidation of one substance (loss of electrons) and the simultaneous reduction of another substance (gain of electrons).
In a redox reaction, the substance that undergoes oxidation is called the reducing agent because it causes the reduction of another substance by donating electrons. Conversely, the substance that undergoes reduction is called the oxidizing agent because it causes the oxidation of another substance by accepting electrons.
Real-Life Examples of Redox Reactions
Some common examples of redox reactions that occur in our daily lives are discussed below:
Rust Formation
An iron or steel exposed to the air may have a reddish-brown rust coating, which is an example of redox reaction. When iron comes into contact with oxygen and moisture in the air, it undergoes oxidation to form iron oxide (rust). This reaction is known as rusting of iron and can be represented as:
4Fe(s) + 3O 2 (g) → 2Fe 2 O 3 (s)
Here, iron (Fe) is oxidized from its elemental state to Fe 2 O 3 , while oxygen (O 2 ) is reduced.
Redox reaction is the main point of the process of charge and discharge of battery. For example, in alkaline batteries, as the current flows through the zinc and manganese dioxide, an electrochemical reaction leading to the production of electricity occurs.
Just like in lithium-ion battery, the redox reactions between lithium ions and electrodes are responsible for their working.
Photosynthesis
Photosynthesis, which is the reaction in which the green plant, algae, and some of the bacteria transform light energy into chemical energy in the form of glucose, is considered as an irreversible redox reaction.
Plants, during photosynthesis, absorb light energy which is then converted to chemical energy by the process of reduction of carbon dioxide to glucose using the chlorophyll. Likewise, water molecules are stripped away of their oxygen atom. Therefore, Photosynthesis is one of the key stages for the production of oxygen as well as synthesis of organic compounds that are important for the growth, development and nutrient uptake of the plants.
Corrosion of metals
Corrosion is the gradual degradation of metals due to chemical reactions with the environment, often involves redox processes. For instance, when silver tarnishes in the presence of sulfur compounds in the air, silver metal is oxidized to silver sulfide:
4 Ag ( s ) + 2 H 2 S ( g ) + O 2 ( g ) → 2 Ag 2 S ( s ) + 2 H 2 O ( l )
In this reaction, silver ( Ag ) is oxidized, while sulfur compounds are reduced.
The process of cooking also sometimes involves redox reactions. In this case, when meat is grilled or roasted, a conversion of amino acids and reducing sugar takes place which is a reaction between amino acids and reducing sugars that are responsible for the changes in the flavor, color, and aroma of the cooked food.
Cleaning Agents
Many common domestic cleaning agents (like bleaches for instance) undergo redox reactions which help in the removal of dirt and disinfection of surfaces. An oxidizing agent like bleach kills organic stains by introducing oxidation.
Redox reactions, the types of reactions that involve the transfer of electrons, perform some crucial functions within the digestion process. As an example, enzymes of the digestive system perform the breaking of carbohydrates, proteins, and fats into more straightforward molecules. Oxidation-reduction reactions that follow then lead to a release of energy that is utilized by the body for different functions.
Effects of Oxidation Reactions in Everyday Life Importance of Chemistry in Everyday Life Importance of pH in Everyday Life
In this article we discussed various examples that illustrate the diverse range of redox reactions that occur in everyday life, from the rusting of metals to the generation of energy in living organisms. Understanding these processes is essential for appreciating the role of redox chemistry in both natural and technological systems.
Frequently Asked Questions: FAQs on Examples of Redox Reactions
What are some common examples of redox reactions in everyday life.
Redox reactions are important in daily life. Some common examples include rusting of iron, combustion of fuels like gasoline or wood, respiration in living organisms, corrosion of metals, and photosynthesis in plants.
How does rusting of iron demonstrate a redox reaction?
Rusting of iron occurs when iron reacts with oxygen and water in the air to form iron oxide (rust). In this process, iron undergoes oxidation (loses electrons) to form iron ions, while oxygen is reduced (gains electrons) to form oxide ions. The overall reaction involves the transfer of electrons between iron and oxygen.
Is combustion an example of a redox reaction?
Yes, combustion is a classic example of a redox reaction. For instance, when a hydrocarbon fuel like methane or gasoline burns in the presence of oxygen, it undergoes oxidation to produce carbon dioxide and water vapor. The fuel is oxidized (loses electrons), while oxygen is reduced (gains electrons).
How are redox reactions involved in cellular respiration?
Cellular respiration is a series of metabolic reactions that occur in cells to produce energy. During aerobic respiration, glucose is oxidized to carbon dioxide and water, releasing energy that is used to produce ATP. Oxygen serves as the final electron acceptor, undergoing reduction to form water.
How does photosynthesis demonstrate a redox reaction?
Photosynthesis is a complex process in which plants convert light energy into chemical energy. In the light-dependent reactions, water molecules are oxidized to produce oxygen gas, protons, and electrons. The electrons released from water are used to drive the synthesis of ATP and NADPH, while oxygen is released as a byproduct.
Please Login to comment...
Similar reads.
- Real life example of
- School Chemistry
- School Learning
Improve your Coding Skills with Practice
What kind of Experience do you want to share?

COMMENTS
A synthesis reaction or direct combination reaction reacts two or more simple elements or compounds to form a more complex product. A synthesis reaction is one of the four main types of chemical reactions, along with decomposition, single replacement, and double replacement reactions. Here is the synthesis reaction definition, examples of the reaction using elements and compounds, a look at ...
Example of Synthesis Reaction. An example of a combination reaction is the combination of aluminum (Al) and oxygen (O 2) to aluminum oxide (Al 2 O 3). ... There are a few examples of the synthesis reaction in real and daily life. Almost all real-life examples are seen in the industry. During industrial production, the synthesis reaction plays a ...
Synthesis Reaction Examples. In the simplest synthesis reactions, two elements combine to form a binary compound (a compound made of two elements). The combination of iron and sulfur to form iron (II) sulfide is an example of a synthesis reaction : 8 Fe + S 8 → 8 FeS. Another example of a synthesis reaction is the formation of potassium ...
Recognizing Synthesis Reactions . The hallmark of a synthesis reaction is that a more complex product is formed from the reactants. One easy-to-recognize type of synthesis reaction occurs when two or more elements combine to form a compound. The other type of synthesis reaction happens when an element and a compound combine to form a new compound.
About the Author. It is easy to find synthesis reaction examples in everyday life, such as the formation of table salt, NaCl, from its constituent elements Na (sodium) and Cl (chlorine). The general form of these reactions is A + B → AB. These reactions may fall into other chemical reaction types.
The synthesis reactions are very important for science, because thanks to these methods can be made various materials, medicines and products that we use in everyday life. Examples of synthesis reactions Production of ammonia (NH3) Nitrogen molecules contain two atoms of this element. Hydrogen appears the same in this way, so when combined in ...
A combination reaction is a reaction in which two or more substances combine to form a single new substance. Combination reactions can also be called synthesis reactions. The general form of a combination reaction is: A + B → AB A + B → AB. One combination reaction is two elements combining to form a compound.
Synthesis Reaction Examples in Real Life. In our everyday lives, we encounter synthesis reactions without even realizing it. One common example is the formation of water through the combination of hydrogen and oxygen gases. This reaction, represented by the chemical equation H2 + O2 → H2O, ...
Yes No. Synthesis reactions (also called combination reactions) are the simplest type of chemical reaction. In a synthesis reaction two or more substances undergo a chemical reaction producing a new substance.
We've already seen one real life example of a combination example in this guide: sodium chloride synthesis. 2 Na (s) + Cl 2 (g) → 2 NaCl (s) ... Common examples of synthesis reactions are when a metal or non-metal reacts with oxygen to form an oxide. Synthesis reactions can also occur when a metal or non-metal combine to form a binary compound.
In this synthesis reaction, calcium oxide (CaO) and carbon dioxide is reacted to form calcium carbonate. At first step, calcium hydroxide is prepared by the reaction between calcium oxide with water. After that Ca (OH) 2 is reacted with carbon dioxide and as a product calcium carbonate is obtained. CaO +H2O = Ca (OH)2.
A decomposition reaction is the opposite of a synthesis reaction. During a decomposition reaction, a more complex compound breaks down into multiple simpler compounds. A classic example of this type of reaction is the decomposition of hydrogen peroxide into oxygen and hydrogen gas: \[H_2O_2 (l) \rightarrow H_2 (g) + O_2 (g) \nonumber \]
A synthesis reaction is one in which a single product is made from two or more reactants. It follows the pattern A+ B → C. Explanation: There are many practical processes that are synthesis reactions. Here are a couple: N 2 + 3H 2 → 2N H 3. The Haber process was a Nobel-prize winning advance which demonstrated how to fix atmospheric ...
Energy absorption: These reactions often require an input of energy, typically in the form of heat or light, to initiate the reaction. Examples. Common examples of synthesis reactions include the formation of water (2H 2 + O 2 → 2H 2 O), the synthesis of ammonia (3H₂ + N₂ → 2NH₃), and photosynthesis (6CO 2 + 6H 2 O → C 6 H 12 O 6 ...
A synthesis reaction, also known as a composition reaction, is characterized by the reaction of two or more substances chemically joining to form a single product. Here are three examples Magnesium metal reacts with oxygen to produce magnesium oxide 2 Mg + O_2 -> 2MgO In this next example, sodium reacts with chloride to form table salt. 2Na + Cl_2 -> 2 NaCl In the examples above, two different ...
chemical synthesis, the construction of complex chemical compounds from simpler ones. It is the process by which many substances important to daily life are obtained. It is applied to all types of chemical compounds, but most syntheses are of organic molecules. Chemists synthesize chemical compounds that occur in nature in order to gain a ...
A synthesis reaction occurs when two or more reactants combine to form a single product. This type of reaction is represented by the general equation: A + B → AB. An example of a synthesis reaction is the combination of sodium (Na) and chlorine (Cl) to produce sodium chloride (NaCl). This reaction is represented by the chemical equation: 2Na ...
Here are some broad examples of chemical reactions in daily life: Combustion. Photosynthesis. Aerobic cellular respiration. Anaerobic respiration (including fermentation) Oxidation (including rust) Metathesis reactions (such as baking soda and vinegar) Electrochemistry (including chemical batteries) Digestion.
Examples of Synthesis Reactions Another example of a synthesis reaction is the combination of sodium (Na) and chlorine (Cl) to produce sodium chloride (NaCl). This reaction is represented by the chemical equation: 2Na + Cl 2! 2NaCl Sodium is a highly reactive metal, and chlorine is a poisonous gas. Both elements are pictured in the Figure1.1. The
Alex Dowden/EyeEm / Getty Images. Over time, iron develops a red, flaky coating called rust. This is an example of an oxidation reaction. Other everyday examples include the formation of verdigris on copper and the tarnishing of silver. Here is the chemical equation for the rusting of iron: Fe + O 2 + H 2 O → Fe 2 O 3.
Real life example: The white precipitate formed by acid rain on a marble statue: \[CaCO_3(aq)+H_2SO_4(aq) \rightarrow CaSO_4(s)+H_2O(l)+CO_2(g) \nonumber \] ... Synthesis Reactions. A synthesis reaction occurs when one or more compounds combines to form a complex compound. The simplest equation of synthesis reaction is illustrated below.
The alkoxide ion, a powerful nucleophile, is then allowed to attack the electrophilic carbon in iodomethane, displacing iodide in an S N 2 reaction. Recall ( section 7.3A) that iodide ion is the least basic - and thus the best leaving group - among the halogens commonly used in the synthetic lab. CH 3 I is one of the most commonly used lab ...
Example. The decomposition of hydrogen peroxide (H 2 O 2) to water (H 2 O) and oxygen (O 2) is catalyzed by an enzyme called catalase [7]. 2 H 2 O 2 (l) + catalase (enzyme) → 2 H 2 O (l) + O 2 (g) Decomposition Reaction Examples in Real Life. The decomposition reaction has a few applications in the industry and daily life. Industry
4Ag(s) + 2H 2 S(g) + O 2 (g) → 2Ag 2 S(s) + 2H 2 O(l). In this reaction, silver (Ag) is oxidized, while sulfur compounds are reduced.Cooking. The process of cooking also sometimes involves redox reactions. In this case, when meat is grilled or roasted, a conversion of amino acids and reducing sugar takes place which is a reaction between amino acids and reducing sugars that are responsible ...