Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 12 September 2019

The effects of plant-based diets on the body and the brain: a systematic review
- Evelyn Medawar ORCID: orcid.org/0000-0001-5011-8275 1 , 2 , 3 ,
- Sebastian Huhn 4 ,
- Arno Villringer 1 , 2 , 3 &
- A. Veronica Witte 1
Translational Psychiatry volume 9 , Article number: 226 ( 2019 ) Cite this article
311k Accesses
189 Citations
1438 Altmetric
Metrics details
- Human behaviour
- Molecular neuroscience
- Psychiatric disorders
Western societies notice an increasing interest in plant-based eating patterns such as vegetarian and vegan, yet potential effects on the body and brain are a matter of debate. Therefore, we systematically reviewed existing human interventional studies on putative effects of a plant-based diet on the metabolism and cognition, and what is known about the underlying mechanisms. Using the search terms “plant-based OR vegan OR vegetarian AND diet AND intervention” in PubMed filtered for clinical trials in humans retrieved 205 studies out of which 27, plus an additional search extending the selection to another five studies, were eligible for inclusion based on three independent ratings. We found robust evidence for short- to moderate-term beneficial effects of plant-based diets versus conventional diets (duration ≤ 24 months) on weight status, energy metabolism and systemic inflammation in healthy participants, obese and type-2 diabetes patients. Initial experimental studies proposed novel microbiome-related pathways, by which plant-based diets modulate the gut microbiome towards a favorable diversity of bacteria species, yet a functional “bottom up” signaling of plant-based diet-induced microbial changes remains highly speculative. In addition, little is known, based on interventional studies about cognitive effects linked to plant-based diets. Thus, a causal impact of plant-based diets on cognitive functions, mental and neurological health and respective underlying mechanisms has yet to be demonstrated. In sum, the increasing interest for plant-based diets raises the opportunity for developing novel preventive and therapeutic strategies against obesity, eating disorders and related comorbidities. Still, putative effects of plant-based diets on brain health and cognitive functions as well as the underlying mechanisms remain largely unexplored and new studies need to address these questions.
Similar content being viewed by others

Microdosing with psilocybin mushrooms: a double-blind placebo-controlled study

Gut microbiome remodeling and metabolomic profile improves in response to protein pacing with intermittent fasting versus continuous caloric restriction

Temporal dynamics of the multi-omic response to endurance exercise training
Introduction.
Western societies notice an increasing interest in plant-based eating patterns such as avoiding meat or fish or fully excluding animal products (vegetarian or vegan, see Fig. 1 ). In 2015, around 0.4−3.4% US adults, 1−2% British adults, and 5−10% of German adults were reported to eat largely plant-based diets 1 , 2 , 3 , 4 , due to various reasons (reviewed in ref. 5 ). Likewise, the number of scientific publications on PubMed (Fig. 2 ) and the public popularity as depicted by Google Trends (Fig. 3 ) underscore the increased interest in plant-based diets. This increasing awareness calls for a better scientific understanding of how plant-based diets affect human health, in particular with regard to potentially relevant effects on mental health and cognitive functions.
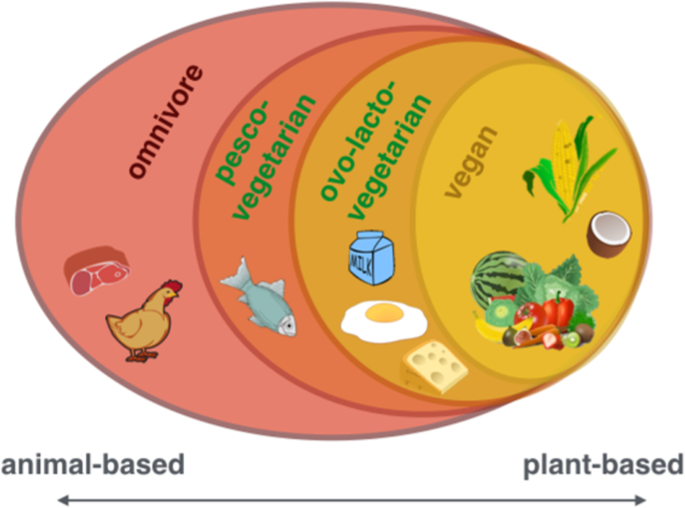
From left to right: including all food items (omnivore), including all except for meat (pesco-vegetarian) or meat and fish (ovo-lacto-vegetarian) to including only plant-based items (vegan)
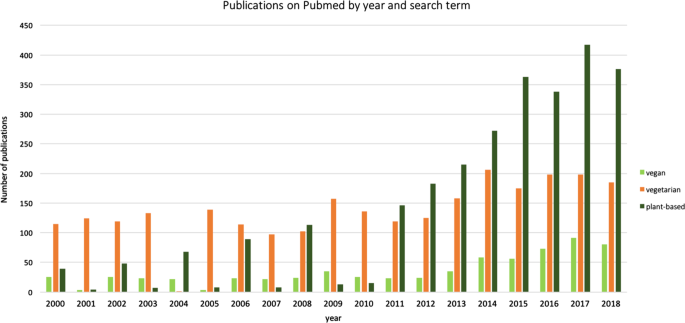
Frequency of publications on PubMed including the search terms “vegan” (in light green), vegetarian (in orange) and plant-based (dark green)—accessed on 19 April 2019
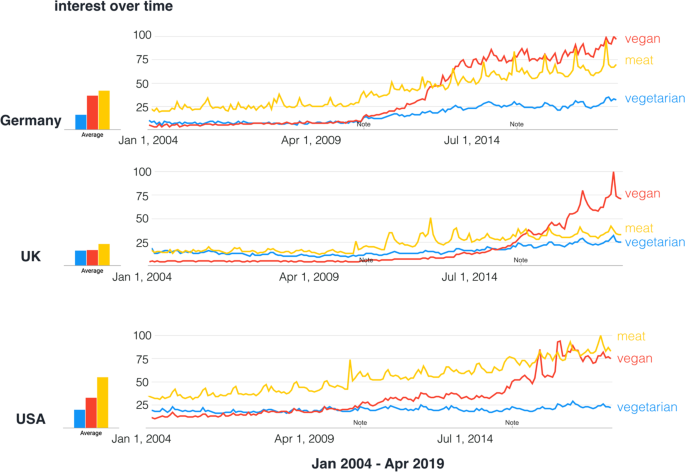
Note indicates technical improvements implemented by Google Trends. Data source: Google Trends . Search performed on 18 April 2019
A potential effect of plant-based diets on mortality rate remains controversial: large epidemiological studies like the Adventist studies ( n = 22,000−96,000) show a link between plant-based diets, lower all-cause mortality and cardiovascular diseases 6 , 7 , while other studies like the EPIC-Oxford study and the “45 and Up Study” ( n = 64,000−267,000) show none 8 , 9 . Yet, many, but not all, epidemiological and interventional human studies in the last decades have suggested that plant-based diets exert beneficial health effects with regard to obesity-related metabolic dysfunction, type 2 diabetes mellitus (T2DM) and chronic low-grade inflammation (e.g. refs. 6 , 7 , 10 , 11 , for reviews, see refs. 12 , 13 , 14 , 15 , 16 , 17 , 18 ). However, while a putative link between such metabolic alterations and brain health through pathways which might include diet-related neurotransmitter precursors, inflammatory pathways and the gut microbiome 19 becomes increasingly recognized, the notion that plant-based diets exert influence on mental health and cognitive functions appears less documented and controversial 20 , 21 , 22 , 23 , 24 . We therefore systematically reviewed the current evidence based on available controlled interventional trials, regarded as the gold standard to assess causality, on potential effects of plant-based diets on (a) metabolic factors including the microbiome and (b) neurological or psychiatric health and brain functions. In addition, we aimed to evaluate potential underlying mechanisms and related implications for cognition.
We performed a systematic PubMed search with the following search terms “plant-based OR vegan OR vegetarian AND diet AND intervention” with the filter “clinical trial” and “humans”, preregistered at PROSPERO (CRD42018111856; https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=111856 ) (Suppl. Fig. 1 ). PubMed was used as search engine because it was esteemed to yield the majority of relevant human clinical trials from a medical perspective. Exclusion criteria were insufficient design quality (such as lack of a control group), interventions without a plant-based or vegetarian or vegan diet condition, intervention with multiple factors (such as exercise and diet), and the exclusive report of main outcomes of no interest, such as dietary compliance, nutrient intake (such as vitamins or fiber intake), or nonmetabolic (i.e., not concerning glucose metabolism, lipid profile, gastrointestinal hormones or inflammatory markers) or non-neurological/psychiatric disease outcomes (e.g. cancer, caries).
Studies were independently rated for eligibility into the systematic review by three authors based on reading the abstract and, if needed, methods or other parts of the publication. If opinions differed, a consensus was reached through discussion of the individual study. This yielded 27 eligible out of 205 publications; see Table 1 for details. To increase the search radius for studies dealing with microbial and neurological/psychiatric outcomes, we deleted the search term “intervention”, which increased the number of studies by around one third, and checked for studies with “microbiome/microbiota”, “mental”, “cognitive/cognition” or “psychological/psychology” in the resulting records. Through this, we retrieved another five studies included in Table 1 . Further related studies were reviewed based on additional nonsystematic literature search.
Section I: Effects of plant-based diets on body and brain outcomes
Results based on interventional studies on metabolism, microbiota and brain function.
Overall, the vast majority of studies included in this systematic review reported a short-term beneficial effect of plant-based dietary interventions (study duration 3−24 months) on weight status, glucose, insulin and/or plasma lipids and inflammatory markers, whereas studies investigating whether plant-based diets affect microbial or neurological/psychiatric disease status and other brain functions were scarce and rather inconclusive (Table 1 ).
More specifically, 19 out of 32 studies dealing with T2DM and/or obese subjects and seven out of 32 dealing with healthy subjects observed a more pronounced weight loss and metabolic improvements, such as lowering of glycated hemoglobin (HbA1c)—a long-term marker for glucose levels—decreased serum levels of low-density (LDL) and high-density lipoproteins (HDL) and total cholesterol (TC), after a plant-based diet compared to an omnivore diet. This is largely in line with recent meta-analyses indicating beneficial metabolic changes after a plant-based diet 25 , 26 , 27 .
For example, Lee et al. found a significantly larger reduction of HbA1c and lower waist circumference after vegan compared to conventional dieting 28 . Jenkins et al. found a disease-attenuating effect in hyperlipidemic patients after 6 months adopting a low-carbohydrate plant-based diet compared to a high-carbohydrate lacto-ovo-vegetarian diet 29 , 30 . However, lower energy intake in the vegan dieters might have contributed to these effects. Yet, while a plant-based diet per se might lead to lower caloric intake, other studies observed nonsignificant trends toward higher effect sizes on metabolic parameters after a vegan diet, even when caloric intake was comparable: two studies in T2DM patients 31 , 32 compared calorie-unrestricted vegan or vegetarian to calorie-restricted conventional diets over periods of 6 months and 1.5 years, respectively, in moderate sample sizes ( n ~ 75−99) with similar caloric intake achieved in both diet groups. Both studies indicated stronger effects of plant-based diets on disease status, such as reduced medication, improved weight status and increased glucose/insulin sensitivity, proposing a diabetes-preventive potential of plant-based diets. Further, a five-arm study comparing four types of plant-based diets (vegan, vegetarian, pesco-vegetarian, semi-vegetarian) to an omnivore diet (total n = 63) in obese participants found the most pronounced effect on weight loss for a vegan diet (−7.5 ± 4.5% of total body weight) 33 . Here, inflammation markers conceptualized as the dietary inflammatory index were also found to be lower in vegan, vegetarian and pesco-vegetarian compared to semi-vegetarian overweight to obese dieters 33 .
Intriguingly, these results 28 , 29 , 30 , 31 , 32 , 33 cohesively suggest that although caloric intake was similar across groups, participants who had followed a vegan diet showed higher weight loss and improved metabolic status.
As a limitation, all of the reviewed intervention studies were carried out in moderate sample sizes and over a period of less than 2 years, disregarding that long-term success of dietary interventions stabilizes after 2−5 years only 34 . Future studies with larger sample sizes and tight control of dietary intake need to confirm these results.
Through our systematic review we retrieved only one study that added the gut microbiome as novel outcome for clinical trials investigating the effects of animal-based diets compared to plant-based diets. While the sample size was relatively low ( n = 10, cross-over within subject design), it showed that changing animal- to plant based diet changed gut microbial activity towards a trade-off between carbohydrate and protein fermentation processes within only 5 days 35 . This is in line with another controlled-feeding study where microbial composition changes already occurred 24 h after changing diet (not exclusively plant-based) 36 . However, future studies incorporating larger sample sizes and a uniform analysis approach of microbial features need to further confirm the hypothesis that a plant-based diet ameliorates microbial diversity and health-related bacteria species.
Considering neurological or psychiatric diseases and brain functions, the systematic review yielded in six clinical trials of diverse clinical groups, i.e. migraine, multiple sclerosis, fibromyalgia and rheumatoid arthritis. Here, mild to moderate improvement, e.g. measured by antibody levels, symptom improvement or pain frequency, was reported in five out of six studies, sometimes accompanied by weight loss 37 , 38 , 39 , 40 (Table 1 ). However, given the pilot character of these studies, indicated by small sample sizes ( n = 32−66), lack of randomization 37 , or that the plant-based diet was additionally free of gluten 40 , the evidence is largely anecdotal. One study in moderately obese women showed no effects on psychological outcomes 41 , two studies with obese and nonobese healthy adults indicated improvements in anxiety, stress and depressive symptom scores 23 , 24 . Taken together, the current evidence based on interventional trials regarding improvements of cognitive and emotional markers and in disease treatment for central nervous system disorders such as multiple sclerosis or fibromyalgia remains considerably fragmentary for plant-based diets.
Among observational studies, a recent large cross-sectional study showed a higher occurrence of depressive symptoms for vegetarian dieters compared to nonvegetarians 20 . Conversely, another observational study with a sample of about 80% women found a beneficial association between a vegan diet and mood disturbance 24 .
Overall, the relationship between mental health (i.e. depression) and restrictive eating patterns has been the focus of recent research 20 , 21 , 22 , 24 , 42 ; however, causal relationships remain uninvestigated due to the observational design.
Underlying mechanisms linking macronutrient intake to metabolic processes
On the one hand, nutrient sources as well as their intake ratios considerably differ between plant-based and omnivore diets (Suppl. Table 1 ), and on the other hand, dietary micro- and macromolecules as well as their metabolic substrates affect a diversity of physiological functions, pointing to complex interdependencies. Thus, it seems difficult to nail down the proposed beneficial effects of a plant-based diet on metabolic status to one specific component or characteristic, and it seems unlikely that the usually low amount of calories in plant-based diets could explain all observed effects. Rather, plant-based diets might act through multiple pathways, including better glycemic control 43 , lower inflammatory activity 44 and altered neurotransmitter metabolism via dietary intake 45 or intestinal activity 46 (Fig. 4 ).
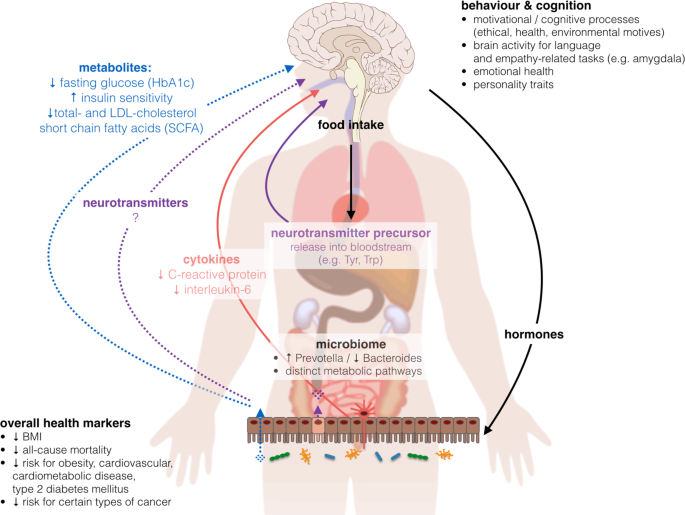
BMI body-mass-index, HbA1c hemoglobin A1c, LDL-cholesterol low-density lipoprotein cholesterol, Trp tryptophan, Tyr tyrosine. Images from commons.wikimedia.org , “Brain human sagittal section” by Lynch 2006 and “Complete GI tract” by Häggström 2008, “Anatomy Figure Vector Clipart” by http://moziru.com
On the macronutrient level, plant-based diets feature different types of fatty acids (mono- and poly-unsaturated versus saturated and trans) and sugars (complex and unrefined versus simple and refined), which might both be important players for mediating beneficial health effects 18 . On the micronutrient level, the EPIC-Oxford study provided the largest sample of vegan dieters worldwide ( n (vegan) = 2396, n (total) = 65,429) and showed on the one hand lower intake of saturated fatty acids (SFA), retinol, vitamin B12 and D, calcium, zinc and protein, and on the other hand higher intake of fiber, magnesium, iron, folic acid, vitamin B1, C and E in vegan compared to omnivore dieters 47 . Other studies confirmed the variance of nutrient intake across dietary groups, i.e. omnivores, vegetarians and vegans, showing the occurrence of critical nutrients for each group 48 , 49 . Not only the amount of SFA but also its source and profile might be important factors regulating metabolic control (reviewed in ref. 14 ), for example through contributing to systemic hyperlipidemia and subsequent cardiovascular risk. Recently, it has been shown in a 4-week intervention trial that short-term dietary changes favoring a diet high in animal-based protein may lead to an increased risk for cardiovascular derangements mediated by higher levels of trimethylamine N-oxide (TMAO), which is a metabolite of gut bacteria-driven metabolic pathways 50 .
Secondly, high fiber intake from legumes, grains, vegetables and fruits is a prominent feature of plant-based diets (Table 1 ), which could induce beneficial metabolic processes like upregulated carbohydrate fermentation and downregulated protein fermentation 35 , improved gut hormonal-driven appetite regulation 51 , 52 , 53 , 54 , 55 , and might prevent chronic diseases such as obesity and T2DM by slowing down digestion and improving lipid control 56 . A comprehensive review including evidence from 185 prospective studies and 58 clinical trials concluded that risk reduction for a myriad of diseases (incl. CVD, T2DM, stroke incidence) was greatest for daily fiber intake between 25 and 29 g 57 . Precise evidence for underlying mechanisms is missing; however, more recently it has been suggested that high fiber intake induces changes on the microbial level leading to lower long-term weight gain 58 , a mechanism discussed below.
The reason for lower systemic inflammation in plant-based dieters could be due to the abundance of antiinflammatory molecule intake and/or avoidance of proinflammatory animal-derived molecules. Assessing systemic inflammation is particularly relevant for medical conditions such as obesity, where it has been proposed to increase the risk for cardiovascular disease 59 , 60 . In addition, higher C-reactive protein (CRP) and interleukin-6 (IL-6) levels have been linked with measures of brain microstructure, such as microstructural integrity and white matter lesions 61 , 62 , 63 and higher risk of dementia 64 , and recent studies point out that a diet-related low inflammatory index might also directly affect healthy brain ageing 65 , 66 .
Interventional studies that focus on plant- versus meat-based proteins or micronutrients and potential effects on the body and brain are lacking. A meta-analysis including seven RCTs and one cross-sectional studies on physical performance and dietary habits concluded that a vegetarian diet did not adversely influence physical performance compared to an omnivore diet 67 . An epidemiological study by Song et al. 11 estimated that statistically replacing 3% of animal protein, especially from red meat or eggs, with plant protein would significantly improve mortality rates. This beneficial effect might however not be explained by the protein source itself, but possibly by detrimental components found in meat (e.g. heme-iron or nitrosamines, antibiotics, see below).
Some studies further hypothesized that health benefits observed in a plant-based diet stem from higher levels of fruits and vegetables providing phytochemicals or vitamin C that might boost immune function and eventually prevent certain types of cancer 68 , 69 , 70 . A meta-analysis on the effect of phytochemical intake concluded a beneficial effect on CVD, cancer, overweight, body composition, glucose tolerance, digestion and mental health 71 . Looking further on the impact of micronutrients and single dietary compounds, there is room for speculation that molecules, that are commonly avoided in plant-based diets, might affect metabolic status and overall health, such as opioid-peptides derived from casein 72 , pre- and probiotics 73 , 74 , carry-over antibiotics found in animal products 75 , 76 or food-related carcinogenic toxins, such as dioxin found in eggs or nitrosamines found in red and processed meat 77 , 78 . Although conclusive evidence is missing, these findings propose indirect beneficial effects on health deriving from plant-based compared to animal-based foods, with a potential role for nonprotein substances in mediating those effects 18 . While data regarding chemical contaminant levels (such as crop pesticides, herbicides or heavy metals) in different food items are fragmentary only, certain potentially harmful compounds may be more (or less) frequently consumed in plant-based diets compared to more animal-based diets 79 . Whether these differences lead to systematic health effects need to be explored.
Taken together, the reviewed studies indicating effects of plant-based diets through macro- and micronutrient intake reveal both the potential of single ingredients or food groups (low SFA, high fiber) and the immense complexity of diet-related mechanisms for metabolic health. As proposed by several authors, benefits on health related to diet can probably not be viewed in isolation for the intake (or nonintake) of specific foods, but rather by additive or even synergistic effects between them (reviewed in refs. 12 , 80 ). Even if it remains a challenging task to design long-term RCTs that control macro- and micronutrient levels across dietary intervention groups, technological advancements such as more fine-tuned diagnostic measurements and automated self-monitoring tools, e.g. automatic food recognition systems 81 and urine-related measures of dietary intake 82 , could help to push the field forward.
Nutrients of particular interest in plant-based diets
As described above, plant-based diets have been shown to convey nutritional benefits 48 , 49 , in particular increased fiber, beta carotene, vitamin K and C, folate, magnesium, and potassium intake and an improved dietary health index 83 . However, a major criticism of plant-based diets is the risk of nutrient deficiencies for specific micronutrients, especially vitamin B12, a mainly animal-derived nutrient, which is missing entirely in vegan diets unless supplemented or provided in B12-fortified products, and which seems detrimental for neurological and cognitive health when intake is low. In the EPIC-Oxford study about 50% of the vegan dieters showed serum levels indicating vitamin B12 deficiency 84 . Along other risk factors such as age 85 , diet, and plant-based diets in particular, seem to be the main risk factor for vitamin B12 deficiency (reviewed in ref. 86 ), and therefore supplementing vitamin B12 for these risk groups is highly recommended 87 . Vitamin B12 is a crucial component involved in early brain development, in maintaining normal central nervous system function 88 and suggested to be neuroprotective, particularly for memory performance and hippocampal microstructure 89 . One hypothesis is that high levels of homocysteine, that is associated with vitamin B12 deficiency, might be harmful to the body. Vitamin B12 is the essential cofactor required for the conversion of homocysteine into nonharmful components and serves as a cofactor in different enzymatic reactions. A person suffering from vitamin B12 insufficiency accumulates homocysteine, lastly promoting the formation of plaques in arteries and thereby increasing atherothrombotic risk 90 , possibly facilitating symptoms in patients of Alzheimer’s disease 91 . A meta-analysis found that vitamin B12 deficiency was associated with stroke, Alzheimer’s disease, vascular dementia, Parkinson’s disease and in even lower concentrations with cognitive impairment 92 , supporting the claim of its high potential for disease prevention when avoided or treated 93 . Further investigations and longitudinal studies are needed, possibly measuring holotranscobalamin (the active form of vitamin B12) as a more specific and sensitive marker for vitamin B12 status 94 , to examine in how far nonsupplementing vegan dieters could be at risk for cardiovascular and cognitive impairment.
Similar health dangers can stem from iron deficiency, another commonly assumed risk for plant-based dieters and other risk groups such as young women. A meta-analysis on 24 studies proposes that although serum ferritin levels were lower in vegetarians on average, it is recommended to sustain an optimal ferritin level (neither too low nor too high), calling for well-monitored supplementation strategies 95 . Iron deficiency is not only dependent on iron intake as such but also on complimentary dietary factors influencing its bioavailability (discussed in ref. 95 ). The picture remains complex: on the one hand iron deficiency may lead to detrimental health effects, such as impairments in early brain development and cognitive functions in adults and in children carried by iron-deficient mothers 96 and a possible role for iron overload in the brain on cognitive impairment on the other hand 97 . One study showed that attention, memory and learning were impaired in iron-deficient compared to iron-sufficient women, which could be restored after a 4-month oral iron supplementation ( n = 118) 98 . Iron deficiency-related impairments could be attributed to anemia as an underlying cause, possibly leading to fatigue, or an undersupply of blood to the brain or alterations in neurobiological and neuronal systems 99 provoking impaired cognitive functioning.
This leads to the general recommendation to monitor health status by frequent blood tests, to consult a dietician to live healthily on a plant-based diet and to consider supplements to avoid nutrient deficiencies or nutrient-overdose-related toxicity. All in all, organizations such as the Academy of Nutrition and Dietetics 100 and the German Nutrition Society do not judge iron as a major risk factor for plant-based dieters 101 .
Section II: Effects of diet on the gut microbiome
The link between diet and microbial diversity.
Another putative mechanistic pathway of how plant-based diets can affect health may involve the gut microbiome which has increasingly received scientific and popular interest, lastly not only through initiatives such as the Human Microbiome Project 102 . A common measure for characterizing the gut community is enterotyping, which is a way to stratify individuals according to their gut bacterial diversity, by calculating the ratio between bacterial genera, such as Prevotella and Bacteroides 103 . While interventional controlled trials are still scarce, this ratio has been shown to be conclusive for differentiating plant-based from animal-based microbial profiles 36 . Specifically, in a sample of 98 individuals, Wu et al. 36 found that a diet high in protein and animal fats was related to more Bacteroides, whereas a diet high in carbohydrates, representing a plant-based one, was associated with more Prevotella. Moreover, the authors showed that a change in diet to high-fat/low-fiber or to low-fat/high-fiber in ten individuals elicited a change in gut microbial enterotype with a time delay of 24 h only and remained stable over 10 days, however not being able to switch completely to another enterotype 36 . Another strictly controlled 30-day cross-over interventional study showed that a change in diet to either an exclusively animal-based or plant-based diet promoted gut microbiota diversity and genetic expression to change within 5 days 35 . Particularly, in response to adopting an animal-based diet, microbial diversity increased rapidly, even overshadowing individual microbial gene expression. Beyond large shifts in overall diet, already modest dietary modifications such as the daily consumption of 43 g of walnuts, were able to promote probiotic- and butyric acid-producing bacterial species in two RCTs, after 3 and 8 weeks respectively 104 , 105 , highlighting the high adaptability of the gut microbiome to dietary components. The Prevotella to Bacteroides ratio (P/B) has been shown to be involved in the success of dietary interventions targeting weight loss, with larger weight loss in high P/B compared to low P/B in a 6-month whole-grain diet compared to a conventional diet 106 . Only recently, other microbial communities, such as the salivary microbiome, have been shown to be different between omnivores and vegan dieters 107 , opening new avenues for research on adaptable mechanisms related to dietary intake.
A continuum in microbial diversity dependent on diet
Plant-based diets are supposed to be linked to a specific microbial profile, with a vegan profile being most different from an omnivore, but not always different from a vegetarian profile (reviewed in ref. 15 ). Some specifically vegan gut microbial characteristics have also been found in a small sample of six obese subjects after 1 month following a vegetarian diet, namely less pathobionts, more protective bacterial species improving lipid metabolism and a reduced level of intestinal inflammation 108 . Investigating long-term dietary patterns a study found a dose-dependent effect for altered gut microbiota in vegetarians and vegans compared to omnivores depending on the quantity of animal products 109 . The authors showed that gut microbial profiles of plant-based diets feature the same total number but lower counts of Bacteroides, Bifidobacterium, E. coli and Enterobacteriaceae compared to omnivores, with the biggest difference to vegans. Still today it remains unclear, what this shift in bacterial composition means in functional terms, prompting the field to develop more functional analyses.
In a 30-day intervention study, David et al. found that fermentation processes linked to fat and carbohydrate decomposition were related to the abundance of certain microbial species 35 . They found a strong correlation between fiber intake and Prevotella abundance in the microbial gut. More recently, Prevotella has been associated with plant-based diets 110 that are comparable to low-fat/high-fiber diets 111 and might be linked to the increased synthesis of short-chain fatty acids (SCFA) 112 . SCFAs are discussed as putative signaling molecules between the gut microbiome and the receptors, i.e. free fatty acid receptor 2 (FFA2) 51 , found in host cells across different tissues 113 and could therefore be one potential mechanism of microbiome−host communication.
The underlying mechanisms of nutrient decomposition by Prevotella and whether abundant Prevotella populations in the gut are beneficial for overall health remain unknown. Yet it seems possible that an increased fiber intake and therefore higher Prevotella abundance such as associated with plant-based diets is beneficial for regulating glycemic control and keeping inflammatory processes within normal levels, possibly due to reduced appetite and lower energy intake mediated by a higher fiber content 114 . Moreover, it has been brought forward that the microbiome might influence bodily homeostatic control, suggesting a role for the gut microbiota in whole-body control mechanisms on the systemic level. Novel strategies aim to develop gut-microbiota-based therapies to improve bodily states, e.g. glycemic control 115 , based on inducing microbial changes and thereby eliciting higher-level changes in homeostasis. While highly speculative, such strategies could in theory also exert changes on the brain level, which will be discussed next in the light of a bi-directional feedback between the gut and the brain.
Effects on cognition and behavior linking diet and cognition via the microbiome−gut−brain axis
While the number of interventional studies focusing on cognitive and mental health outcomes after adopting plant-based diets overall is very limited (see Section I above), one underlying mechanism of how plant-based diets may affect mood could involve signaling pathways on the microbiome−gut−brain axis 116 , 117 , 118 , 119 . A recent 4-week intervention RCT showed that probiotic administration compared to placebo and no intervention modulated brain activity during emotional decision-making and emotional recognition tasks 117 . In chronic depression it has been proposed that immunoglobulin A and M antibodies are synthesized by the host in response to gut commensals and are linked to depressive symptoms 120 . Whether the identified gram-negative bacteria might also play a role in plant-based diets remains to be explored. A meta-analysis on five studies concluded that probiotics may mediate an alleviating effect on depression symptomatic 121 —however, sample sizes remained rather small ( n < 100) and no long-term effects were tested (up to 8 weeks).
Currently, several studies aim to identify microbial profiles in relation to disease and how microbial data can be used on a multimodal way to improve functional resolution, e.g. characterizing microbial profiles of individuals suffering from type-1 diabetes 122 . Yet, evidence for specific effects of diet on cognitive functions and behavior through changes in the microbiome remains scarce. A recent study indicated the possibility that our food choices determine the quantity and quality of neurotransmitter-precursor levels that we ingest, which in turn might influence behavior, as shown by lower fairness during a money-redistribution task, called the ultimatum game, after a high-carbohydrate/protein ratio breakfast than after a low-ratio breakfast 123 . Strang et al. found that precursor forms of serotonin and dopamine, measured in blood serum, predicted behavior in this task, and precursor concentrations were dependent on the nutrient profile of the consumed meal before the task. Also on a cross-sectional level tryptophan metabolites from fecal samples have been associated with amygdala-reward network functional connectivity 124 . On top of the dietary composition per se, the microbiota largely contributes to neurotransmitter precursor concentrations; thus, in addition to measuring neurotransmitter precursors in the serum, metabolomics on fecal samples would be helpful to further understand the functional role of the gut microbiota in neurotransmitter biosynthesis and regulation 125 .
Indicating the relevance of gut microbiota for cognition, a first human study assessing cognitive tests and brain imaging could distinguish obese from nonobese individuals using a microbial profile 126 . The authors found a specific microbiotic profile, particularly defined by Actinobacteria phylum abundance, that was associated with microstructural properties in the hypothalamus and in the caudate nucleus. Further, a preclinical study tested whether probiotics could enhance cognitive function in healthy subjects, showing small effects on improved memory performance and reduced stress levels 127 .
A recent study could show that microbial composition influences cerebral amyloidogenesis in a mouse model for Alzheimer’s disease 128 . Health status of the donor mouse seemingly mattered: fecal transplants from transgenic mice had a larger impact on amyloid beta proliferation in the brain compared to wild-type feces. Translational interpretations to humans should be done with caution if at all—yet the results remain elucidative for showing a link between the gut microbiome and brain metabolism.
The evidence for effects of strictly plant-based diets on cognition is very limited. For other plant-based diets such as the Mediterranean diet or DASH diet, there are more available studies that indicate protective effects on cardiovascular and brain health in the aging population (reviewed in refs. 129 , 130 ). Several attempts have been made to clarify potential underlying mechanisms, for example using supplementary plant polyphenols, fish/fish-oil consumption or whole dietary pattern change in RCTs 131 , 132 , 133 , 134 , 135 , 136 , 137 , yet results are not always equivocal and large-scale intervention studies have yet to be completed.
The overall findings of this paragraph add to the evidence that microbial diversity may be associated with brain health, although underlying mechanisms and candidate signaling molecules remain unknown.
Based on this systematic review of randomized clinical trials, there is an overall robust support for beneficial effects of a plant-based diet on metabolic measures in health and disease. However, the evidence for cognitive and mental effects of a plant-based diet is still inconclusive. Also, it is not clear whether putative effects are due to the diet per se, certain nutrients of the diet (or the avoidance of certain animal-based nutrients) or other factors associated with vegetarian/vegan diets. Evolving concepts argue that emotional distress and mental illnesses are linked to the role of microbiota in neurological function and can be potentially treated via microbial intervention strategies 19 . Moreover, it has been claimed that certain diseases, such as obesity, are caused by a specific microbial composition 138 , and that a balanced gut microbiome is related to healthy ageing 111 . In this light, it seems possible that a plant-based diet is able to influence brain function by still unclear underlying mechanisms of an altered microbial status and systemic metabolic alterations. However, to our knowledge there are no studies linking plant-based diets and cognitive abilities on a neural level, which are urgently needed, due to the hidden potential as a dietary therapeutic tool. Also, further studies are needed to disentangle motivational beliefs on a psychological level that lead to a change in diet from causal effects on the body and the brain mediated e.g., by metabolic alterations or a change in the gut microbiome.
GOV.UK. National Diet and Nutrition Survey: headline results from years 1, 2 and 3 (combined) of the rolling programme 2008/09–2010/11. https://www.gov.uk/government/statistics/national-diet-and-nutrition-survey-headline-results-from-years-1-2-and-3-combined-of-the-rolling-programme-200809-201011 (2012).
V. E. B. U. Deutschland & Joy, S. Anzahl der Veganer und Vegetarier in Deutschland. Stand 31 , 2016 (2015).
Mensink, G., Barbosa, C. L. & Brettschneider, A.-K. Verbreitung der vegetarischen Ernährungsweise in Deutschland 1 , (2016).
The Vegetarian Resource Group. How many adults in the U.S. are vegetarian and vegan? http://www.vrg.org/nutshell/Polls/2016_adults_veg.htm (2016).
Rosenfeld, D. L. & Burrow, A. L. Vegetarian on purpose: understanding the motivations of plant-based dieters. Appetite 116 , 456–463 (2017).
Article PubMed Google Scholar
Orlich, M. J. et al. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern. Med. 173 , 1230–1238 (2013).
Article CAS PubMed PubMed Central Google Scholar
Le, L. T. & Sabaté, J. Beyond meatless, the health effects of vegan diets: findings from the Adventist cohorts. Nutrients 6 , 2131–2147 (2014).
Article PubMed PubMed Central Google Scholar
Mihrshahi, S. et al. Vegetarian diet and all-cause mortality: evidence from a large population-based Australian cohort-the 45 and up study. Prev. Med. 97 , 1–7 (2017).
Key, T. J. et al. Mortality in British vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am. J. Clin. Nutr. 89 , 1613S–1619S (2009).
Article CAS PubMed Google Scholar
Fung, T. T. et al. Low-carbohydrate diets and all-cause and cause-specific mortalitytwo cohort studies. Ann. Intern. Med. 153 , 289–298 (2010).
Song, M. et al. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern. Med. 176 , 1453–1463 (2016).
Hu, F. B. Plant-based foods and prevention of cardiovascular disease: an overview. Am. J. Clin. Nutr. 78 , 544S–551S (2003).
Tonstad, S., Butler, T., Yan, R. & Fraser, G. E. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care 32 , 791–796 (2009).
McEvoy, C. T., Temple, N. & Woodside, J. V. Vegetarian diets, low-meat diets and health: a review. Public Health Nutr. 15 , 2287–2294 (2012).
Glick-Bauer, M. & Yeh, M.-C. The health advantage of a vegan diet: exploring the gut microbiota connection. Nutrients 6 , 4822–4838 (2014).
Appleby, P. N. & Key, T. J. The long-term health of vegetarians and vegans. Proc. Nutr. Soc. 75 , 287–293 (2016).
Eichelmann, F., Schwingshackl, L., Fedirko, V. & Aleksandrova, K. Effect of plant‐based diets on obesity‐related inflammatory profiles: a systematic review and meta‐analysis of intervention trials. Obes. Rev. 17 , 1067–1079 (2016).
McMacken, M. & Shah, S. A plant-based diet for the prevention and treatment of type 2 diabetes. J. Geriatr. Cardiol. 14 , 342 (2017).
CAS PubMed PubMed Central Google Scholar
Rogers, G. B. et al. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol. Psychiatry 21 , 738–748 (2016).
Hibbeln, J. R., Northstone, K., Evans, J. & Golding, J. Vegetarian diets and depressive symptoms among men. J. Affect Disord. 225 , 13–17 (2018).
Forestell, C. A. & Nezlek, J. B. Vegetarianism, depression, and the five factor model of personality. Ecol. Food Nutr. 57 , 246–259 (2018).
Matta, J. et al. Depressive symptoms and vegetarian diets: results from the constances cohort. Nutrients 10 , 1695 (2018).
Article PubMed Central Google Scholar
Agarwal, U. et al. A multicenter randomized controlled trial of a nutrition intervention program in a multiethnic adult population in the corporate setting reduces depression and anxiety and improves quality of life: the GEICO study. Am. J. Health Promot. 29 , 245–254 (2015).
Beezhold, B., Radnitz, C., Rinne, A. & DiMatteo, J. Vegans report less stress and anxiety than omnivores. Nutr. Neurosci. 18 , 289–296 (2015).
Barnard, N. D., Levin, S. M. & Yokoyama, Y. A systematic review and meta-analysis of changes in body weight in clinical trials of vegetarian diets. J. Acad. Nutr. Diet. 115 , 954–969 (2015).
Huang, R.-Y., Huang, C.-C., Hu, F. B. & Chavarro, J. E. Vegetarian diets and weight reduction: a meta-analysis of randomized controlled trials. J. Gen. Intern. Med. 31 , 109–116 (2016).
Benatar, J. R. & Stewart, R. A. H. Cardiometabolic risk factors in vegans: a meta-analysis of observational studies. PLoS ONE 13 , e0209086 (2018).
Lee, Y.-M. et al. Effect of a brown rice based vegan diet and conventional diabetic diet on glycemic control of patients with type 2 diabetes: a 12-week randomized clinical trial. PLoS ONE 11 , e0155918 (2016).
Article PubMed PubMed Central CAS Google Scholar
Jenkins, D. J. A. et al. Effect of a 6-month vegan low-carbohydrate (‘Eco-Atkins’) diet on cardiovascular risk factors and body weight in hyperlipidaemic adults: a randomised controlled trial. BMJ Open 4 , e003505 (2014).
Jenkins, D. J. A. et al. The effect of a plant-based low-carbohydrate (“Eco-Atkins”) diet on body weight and blood lipid concentrations in hyperlipidemic subjects. Arch. Intern. Med. 169 , 1046–1054 (2009).
Barnard, N. D. et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am. J. Clin. Nutr. https://doi.org/10.3945/ajcn.2009.26736H (2009).
Kahleova, H., Hill M. & Pelikánova, T. Vegetarian vs. conventional diabetic diet—a 1-year follow-up. Cor Vasa 56 . https://doi.org/10.1016/j.crvasa.2013.12.004 (2016).
Article Google Scholar
Turner-McGrievy, G. M., Davidson, C. R., Wingard, E. E., Wilcox, S. & Frongillo, E. A. Comparative effectiveness of plant-based diets for weight loss: a randomized controlled trial of five different diets. Nutrition 31 , 350–358 (2015).
Wing, R. R. & Phelan, S. Long-term weight loss maintenance. Am. J. Clin. Nutr. 82 , 222S–225S (2005).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505 , 559–563 (2014).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science (80-) 334 , 105–108 (2011).
Article CAS Google Scholar
Kaartinen, K. et al. Vegan diet alleviates fibromyalgia symptoms. Scand. J. Rheumatol. 29 , 308–313 (2000).
Yadav, V. et al. Low-fat, plant-based diet in multiple sclerosis: a randomized controlled trial. Mult. Scler. Relat. Disord. 9 , 80–90 (2016).
Rauma, A. L., Nenonen, M., Helve, T. & Hänninen, O. Effect of a strict vegan diet on energy and nutrient intakes by Finnish rheumatoid patients. Eur. J. Clin. Nutr. 47 , 747–749 (1993).
CAS PubMed Google Scholar
Elkan, A.-C. et al. Gluten-free vegan diet induces decreased LDL and oxidized LDL levels and raised atheroprotective natural antibodies against phosphorylcholine in patients with rheumatoid arthritis: a randomized study. Arthritis Res. Ther. 10 , R34 (2008).
Karlsson, J. et al. Predictors and effects of long-term dieting on mental well-being and weight loss in obese women. Appetite 23 , 15–26 (1994).
Beezhold, B. L. & Johnston, C. S. Restriction of meat, fish, and poultry in omnivores improves mood: a pilot randomized controlled trial. Nutr. J. 11 , 9 (2012).
Yokoyama, Y., Barnard, N. D., Levin, S. M. & Watanabe, M. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc. Diagn. Ther. 4 , 373–382 (2014).
PubMed PubMed Central Google Scholar
Sutliffe, J. T., Wilson, L. D., de Heer, H. D., Foster, R. L. & Carnot, M. J. C-reactive protein response to a vegan lifestyle intervention. Complement Ther. Med. 23 , 32–37 (2015).
Strasser, B., Gostner, J. M. & Fuchs, D. Mood, food, and cognition: role of tryptophan and serotonin. Curr. Opin. Clin. Nutr. Metab. Care 19 , 55–61 (2016).
O’Mahony, S. M., Clarke, G., Borre, Y. E., Dinan, T. G. & Cryan, J. F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 277 , 32–48 (2015).
Article PubMed CAS Google Scholar
Davey, G. K. et al. EPIC–Oxford: lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31 546 non meat-eaters in the UK. Public Health Nutr. 6 , 259–268 (2003).
Schüpbach, R., Wegmüller, R., Berguerand, C., Bui, M. & Herter-Aeberli, I. Micronutrient status and intake in omnivores, vegetarians and vegans in Switzerland. Eur. J. Nutr. 56 , 283–293 (2017).
Clarys, P. et al. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 6 , 1318–1332 (2014).
Park, J. E., Miller, M., Rhyne, J., Wang, Z. & Hazen, S. L. Differential effect of short-term popular diets on TMAO and other cardio-metabolic risk markers. Nutr. Metab. Cardiovasc. Dis. 29 , 513–517 (2019).
Psichas, A. et al. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J. Obes. 39 , 424 (2015).
Lin, H. V. et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 7 , e35240 (2012).
Canfora, E. E., Jocken, J. W. & Blaak, E. E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 11 , 577 (2015).
Guo, Y. et al. Physiological evidence for the involvement of peptide YY in the regulation of energy homeostasis in humans. Obesity 14 , 1562–1570 (2006).
Holzer, P., Reichmann, F. & Farzi, A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut–brain axis. Neuropeptides 46 , 261–274 (2012).
Kendall, C. W. C., Esfahani, A. & Jenkins, D. J. A. The link between dietary fibre and human health. Food Hydrocoll. 24 , 42–48 (2010).
Reynolds, A. et al. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet . https://doi.org/10.1016/S0140-6736(18)31809-9 (2019).
Menni, C. et al. Gut microbiome diversity and high-fibre intake are related to lower long-term weight gain. Int. J. Obes. 41 , 1099 (2017).
Van Gaal, L. F., Mertens, I. L. & Christophe, E. Mechanisms linking obesity with cardiovascular disease. Nature 444 , 875 (2006).
Ferreira, C. M. et al. The central role of the gut microbiota in chronic inflammatory diseases. J. Immunol. Res. 2014 , https://doi.org/10.1155/2014/689492 (2014).
Wersching, H. et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology 74 , 1022–1029 (2010).
Gu, Y. et al. Circulating inflammatory biomarkers in relation to brain structural measurements in a non-demented elderly population. Brain Behav. Immun. 65 , 150–160 (2017).
Lampe, L. et al. Visceral obesity relates to deep white matter hyperintensities via inflammation. Ann. Neurol. 85 , 194–203 (2018).
Google Scholar
Schmidt, R. et al. Early inflammation and dementia: a 25‐year follow‐up of the Honolulu‐Asia Aging Study. Ann. Neurol. 52 , 168–174 (2002).
Rosano, C., Marsland, A. L. & Gianaros, P. J. Maintaining brain health by monitoring inflammatory processes: a mechanism to promote successful aging. Aging Dis. 3 , 16 (2012).
PubMed Google Scholar
Tangney, C. C. et al. Relation of DASH-and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology 83 , 1410–1416 (2014).
Craddock, J. C., Probst, Y. & Peoples, G. Vegetarian nutrition—comparing physical performance of omnivorous and vegetarian athletes. J. Nutr. Intermed. Metab. 4 , 19 (2016).
Liu, R. H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 78 , 517S–520S (2003).
Boffetta, P. et al. Fruit and vegetable intake and overall cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC). J. Natl. Cancer Inst. 102 , 529–537 (2010).
Reczek, C. R. & Chandel, N. S. Revisiting vitamin C and cancer. Science (80-) 350 , 1317–1318 (2015).
Probst, Y. C., Guan, V. X. & Kent, K. Dietary phytochemical intake from foods and health outcomes: a systematic review protocol and preliminary scoping. BMJ Open 7 , e013337 (2017).
Hartmann, R. & Meisel, H. Food-derived peptides with biological activity: from research to food applications. Curr. Opin. Biotechnol. 18 , 163–169 (2007).
Tillisch, K. et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 144 , 1394–1401 (2013).
Gibson, G. R. et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on thedefinition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14 , 491 (2017).
Nisha, A. R. Antibiotic residues-a global health hazard. Vet. World 1 , 375–377 (2008).
Wang, H. et al. Antibiotic residues in meat, milk and aquatic products in Shanghai and human exposure assessment. Food Control 80 , 217–225 (2017).
Bertazzi, P. A. et al. Health effects of dioxin exposure: a 20-year mortality study. Am. J. Epidemiol. 153 , 1031–1044 (2001).
Bouvard, V. et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 16 , 1599–1600 (2015).
Van Audenhaege, M. et al. Impact of food consumption habits on the pesticide dietary intake: comparison between a French vegetarian and the general population. Food Addit. Contam . 26 , 1372–1388 (2009).
Jacobs, D. R. & Tapsell, L. C. Food synergy: the key to a healthy diet. Proc. Nutr. Soc. 72 , 200–206 (2013).
Kawano, Y. & Yanai, K. Foodcam: a real-time food recognition system on a smartphone. Multimed. Tools Appl. 74 , 5263–5287 (2015).
Garcia-Perez, I. et al. Objective assessment of dietary patterns by use of metabolic phenotyping: a randomised, controlled, crossover trial. Lancet Diabetes Endocrinol. 5 , 184–195 (2017).
Turner-McGrievy, G. M. et al. Changes in nutrient intake and dietary quality among participants with type 2 diabetes following a low-fat vegan diet or a conventional diabetes diet for 22 weeks. J. Am. Diet. Assoc. 108 , 1636–1645 (2008).
Gilsing, A. M. J. et al. Serum concentrations of vitamin B12 and folate in British male omnivores, vegetarians and vegans: results from a cross-sectional analysis of the EPIC-Oxford cohort study. Eur. J. Clin. Nutr. 64 , 933–939 (2010).
Allen, L. H. How common is vitamin B-12 deficiency? Am. J. Clin. Nutr. 89 , 693S–696S (2008).
Pawlak, R., Parrott, S. J., Raj, S., Cullum-Dugan, D. & Lucus, D. How prevalent is vitamin B12 deficiency among vegetarians? Nutr. Rev. 71 , 110–117 (2013).
Rizzo, G. et al. Vitamin B12 among vegetarians: status, assessment and supplementation. Nutrients 8 , 767 (2016).
Article PubMed Central CAS Google Scholar
Stabler, S. P. Vitamin B12 deficiency. N. Engl. J. Med . 368 , 149–160 (2013).
Köbe, T. et al. Vitamin B-12 concentration, memory performance, and hippocampal structure in patients with mild cognitive impairment, 2. Am. J. Clin. Nutr. 103 , 1045–1054 (2016).
Ganguly, P. & Alam, S. F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 14 , 6 (2015).
McCaddon, A., Regland, B., Hudson, P. & Davies, G. Functional vitamin B12 deficiency and Alzheimer disease. Neurology 58 , 1395–1399 (2002).
Moore, E. et al. Cognitive impairment and vitamin B12: a review. Int. Psychogeriatr. 24 , 541–556 (2012).
Spence, J. D. Metabolic vitamin B12 deficiency: a missed opportunity to prevent dementia and stroke. Nutr. Res. 36 , 109–116 (2016).
Nexo, E. & Hoffmann-Lücke, E. Holotranscobalamin, a marker of vitamin B-12 status: analytical aspects and clinical utility. Am. J. Clin. Nutr. 94 , 359S–365S (2011).
Haider, L. M., Schwingshackl, L., Hoffmann, G. & Ekmekcioglu, C. The effect of vegetarian diets on iron status in adults: a systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 58 , 1359–1374 (2018).
Lozoff, B. & Georgieff, M. K. et al. Iron deficiency and brain development. Semin. Pediatr. Neurol. 13 , 158–165 (2006).
Ayton, S. et al. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol. Psychiatry 1 , https://doi.org/10.1038/s41380-019-0375-7 (2019).
Murray-Kolb, L. E. & Beard, J. L. Iron treatment normalizes cognitive functioning in young women. Am. J. Clin. Nutr. 85 , 778–787 (2007).
Beard, J. Iron deficiency alters brain development and functioning. J. Nutr. 133 , 1468S–1472S (2003).
Melina, V., Craig, W. & Levin, S. Position of the Academy of Nutrition and Dietetics: vegetarian diets. J. Acad. Nutr. Diet. 116 , 1970–1980 (2016).
Richter, M. et al. For the German Nutrition Society (DGE)(2016) Vegan diet. Position of the German Nutrition Society (DGE). Ernaehrungsumschau 63 , 92–102 (2016).
Peterson, J. et al. The NIH human microbiome project. Genome Res. 19 , 2317–2323 (2009).
Arumugam, M. et al. Enterotypes of the human gut microbiome. Nature 473 , 174–180 (2013).
Bamberger, C. et al. A walnut-enriched diet affects gut microbiome in healthy Caucasian subjects: a randomized, controlled trial. Nutrients 10 , 244 (2018).
Holscher, H. D. et al. Walnut consumption alters the gastrointestinal microbiota, microbially derived secondary bile acids, and health markers in healthy adults: a randomized controlled trial. J. Nutr. 148 , 861–867 (2018).
Hjorth, M. F. et al. Pre-treatment microbial Prevotella-to-Bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int J. Obes. 42 , 580 (2018).
Hansen, T. H. et al. Impact of a vegan diet on the human salivary microbiota. Sci. Rep. 8 , 5847 (2018).
Kim, M., Hwang, S., Park, E. & Bae, J. Strict vegetarian diet improves the risk factors associated with metabolic diseases by modulating gut microbiota and reducing intestinal inflammation. Environ. Microbiol. Rep. 5 , 765–775 (2013).
Zimmer, J. et al. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr. 66 , 53–60 (2012).
De Filippis, F., Pellegrini, N., Laghi, L., Gobbetti, M. & Ercolini, D. Unusual sub-genus associations of faecal Prevotella and Bacteroides with specific dietary patterns. Microbiome 4 , 57 (2016).
Kumar, M., Babaei, P., Ji, B. & Nielsen, J. Human gut microbiota and healthy aging: Recent developments and future prospective. Nutr. Health Aging 4 , 3–16 (2016).
Wu, G. D. et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 65 , 63–72 (2014).
Morrison, D. J. & Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7 , 189–200 (2016).
Wanders, A. J. et al. Effects of dietary fibre on subjective appetite, energy intake and body weight: a systematic review of randomized controlled trials. Obes. Rev. 12 , 724–739 (2011).
Brunkwall, L. & Orho-Melander, M. The gut microbiome as a target for prevention and treatment of hyperglycaemia in type 2 diabetes: from current human evidence to future possibilities. Diabetologia 60 , 943–951 (2017).
Lach, G., Schellekens, H., Dinan, T. G. & Cryan, J. F. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics 15 , 36–59 (2018).
Bagga, D. et al. Influence of 4-week multi-strain probiotic administration on resting-state functional connectivity in healthy volunteers. Eur. J. Nutr. 58 , 1821–1827 (2018).
Foster, J. A. & Neufeld, K.-A. M. Gut–brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 36 , 305–312 (2013).
Saulnier, D. M. et al. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes 4 , 17–27 (2013).
Maes, M., Kubera, M., Leunis, J.-C. & Berk, M. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J. Affect Disord. 141 , 55–62 (2012).
Huang, R., Wang, K. & Hu, J. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients 8 , 483 (2016).
Heintz-Buschart, A. et al. Integrated multi-omics of the human gut microbiome in a case study of familial type 1 diabetes. Nat. Microbiol. 2 , 16180 (2016).
Strang, S. et al. Impact of nutrition on social decision making. Proc. Natl Acad. Sci. 114 , 6510–6514 (2017).
Osadchiy, V. et al. Correlation of tryptophan metabolites with connectivity of extended central reward network in healthy subjects. PLoS ONE 13 , e0201772 (2018).
Franzosa, E. A. et al. Sequencing and beyond: integrating molecular’omics’ for microbial community profiling. Nat. Rev. Microbiol. 13 , 360–372 (2015).
Fernandez-Real, J.-M. et al. Gut microbiota interacts with brain microstructure and function. J. Clin. Endocrinol. Metab. 100 , 4505–4513 (2015).
Allen, A. P. et al. Bifidobacterium longum 1714 as a translational psychobiotic: modulation of stress, electrophysiology and neurocognition in healthy volunteers. Transl. Psychiatry 6 , e939 (2016).
Harach, T. et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 7 , 41802 (2017).
Huhn, S., Masouleh, S. K., Stumvoll, M., Villringer, A. & Witte, A. V. Components of a Mediterranean diet and their impact on cognitive functions in aging. Front Aging Neurosci 7 , 132 (2015).
Larsson, S. C., Wallin, A. & Wolk, A. Dietary approaches to stop hypertension diet and incidence of stroke: results from 2 prospective cohorts. Stroke 47 , 986–990 (2016).
van de Rest, O. et al. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology 71 , 430–438 (2008).
Witte, A. V. et al. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb. Cortex 24 , 3059–3068 (2013).
Witte, A. V., Kerti, L., Margulies, D. S. & Flöel, A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 34 , 7862–7870 (2014).
Brickman, A. M. et al. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci. 17 , 1798 (2014).
Martínez-González, M. A. et al. Benefits of the Mediterranean diet: insights from the PREDIMED study. Prog. Cardiovasc. Dis. 58 , 50–60 (2015).
Huhn, S. et al. Effects of resveratrol on memory performance, hippocampus connectivity and microstructure in older adults—a randomized controlled trial. Neuroimage (2018).
Rosenberg, A. et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: The FINGER trial. Alzheimer’s. Dement. 14 , 263–270 (2018).
Turnbaugh, P. J. Microbes and diet-induced obesity: fast, cheap, and out of control. Cell Host Microbe 21 , 278–281 (2017).
Turner-Mc Grievy, G. M., Barnard, N. D. & Scialli, A. R. A two-year randomized weight loss trial comparing a vegan diet to a more moderate low-fat diet*. Obesity 15 , 2276–2281 (2007).
Burke, L. E. et al. A randomized clinical trial of a standard versus vegetarian diet for weight loss: the impact of treatment preference. Int. J. Obes. 32 , 166–176 (2008).
Barnard, N. D. et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am. J. Clin. Nutr. 89 , 1588S–1596S (2009).
Marniemi, J., Seppänen, A. & Hakala, P. Long-term effects on lipid metabolism of weight reduction on lactovegetarian and mixed diet. Int. J. Obes. 14 , 113–125 (1990).
Acharya, S. D., Brooks, M. M., Evans, R. W., Linkov, F. & Burke, L. E. Weight loss is more important than the diet type in improving adiponectin levels among overweight/obese adults. J. Am. Coll. Nutr. 32 , 264–271 (2013).
Wright, N., Wilson, L., Smith, M., Duncan, B. & McHugh, P. The BROAD study: A randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr. Diabetes 7 , e256 (2017).
Turner-McGrievy, G. M., Davidson, C. R., Wingard, E. E. & Billings, D. L. Low glycemic index vegan or low-calorie weight loss diets for women with polycystic ovary syndrome: a randomized controlled feasibility study. Nutr. Res. 34 , 552–558 (2014).
Kahleova, H. et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet. Med 28 , 549–559 (2011).
Ferdowsian, H. R. et al. A multicomponent intervention reduces body weight and cardiovascular risk at a GEICO corporate site. Am. J. Heal. Promot 24 , 384–387 (2010).
Mishra, S. et al. A multicenter randomized controlled trial of a plant-based nutrition program to reduce body weight and cardiovascular risk in the corporate setting: the GEICO study. Eur. J. Clin. Nutr. 67 , 718 (2013).
Agarwal, U. et al. A multicenter randomized controlled trial of a nutrition intervention program in a multiethnic adult population in the corporate setting reduces depression and anxiety and improves quality of life: the GEICO study. Am. J. Heal. Promot 29 , 245–254 (2015).
Kahleova, H., Dort, S., Holubkov, R. & Barnard, N. A plant-based high-carbohydrate, low-fat diet in overweight individuals in a 16-week randomized clinical trial: the role of carbohydrates. Nutrients 10 , 1302 (2018).
Barnard, N., Scialli, A. R., Bertron, P., Hurlock, D. & Edmonds, K. Acceptability of a therapeutic low-fat, vegan diet in premenopausal women. J. Nutr. Educ. 32 , 314–319 (2000).
Gardner, C. D. et al. The effect of a plant-based diet on plasma lipids in hypercholesterolemic adults: a randomized trial. Ann. Intern. Med. 142 , 733 (2005).
Macknin, M. et al. Plant-based, no-added-fat or American Heart Association diets: impact on cardiovascular risk in obese children with hypercholesterolemia and their parents. J. Pediatr. 166 , 953–959 (2015).
Sciarrone, S. E. et al. Biochemical and neurohormonal responses to the introduction of a lacto-ovovegetarian diet. J. Hypertens. 11 , 849–860 (1993).
Alleman, R. J., Harvey, I. C., Farney, T. M. & Bloomer, R. J. Both a traditional and modified Daniel Fast improve the cardio-metabolic profile in men and women. Lipids Health Dis. 12 , 114 (2013).
Neacsu, M., Fyfe, C., Horgan, G. & Johnstone, A. M. Appetite control and biomarkers of satiety with vegetarian (soy) and meat-based high-protein diets for weight loss in obese men: a randomized crossover trial–. Am. J. Clin. Nutr. 100 , 548–558 (2014).
Koebnick, C. et al. Double-blind, randomized feedback control fails to improve the hypocholesterolemic effect of a plant-based low-fat diet in patients with moderately elevated total cholesterol levels. Eur. J. Clin. Nutr. 58 , 1402 (2004).
Kjeldsen-Kragh, J., Haugen, M., Førre, Ø., Laache, H. & Malt, U. F. Vegetarian diet for patients with rheumatoid arthritis: can the clinical effects be explained by the psychological characteristics of the patients? Rheumatology 33 , 569–575 (1994).
Bunner, A. E., Agarwal, U., Gonzales, J. F., Valente, F. & Barnard, N. D. Nutrition intervention for migraine: a randomized crossover trial. J. Headache Pain. 15 , 69 (2014).
Kahleova, H., Hrachovinova, T., Hill, M. & Pelikanova, T. Vegetarian diet in type 2 diabetes–improvement in quality of life, mood and eating behaviour. Diabet. Med 30 , 127–129 (2013).
Turner-McGrievy, G. M. et al. Randomization to plant-based dietary approaches leads to larger short-term improvements in Dietary Inflammatory Index scores and macronutrient intake compared with diets that contain meat. Nutr. Res. 35 , 97–106 (2015).
Download references
Acknowledgements
This work was supported by a scholarship (E.M.) by the German Federal Environmental Foundation and by the grants of the German Research Foundation contract grant number CRC 1052 “Obesity mechanisms” Project A1 (AV) and WI 3342/3-1 (A.V.W.).
Author information
Authors and affiliations.
Department of Neurology, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany
Evelyn Medawar, Arno Villringer & A. Veronica Witte
Berlin School of Mind and Brain, Humboldt-Universität zu Berlin, Berlin, Germany
Evelyn Medawar & Arno Villringer
Charité—Universitätsmedizin Berlin, Humboldt-Universität zu Berlin, Berlin, Germany
Helmholtz Centre for Environmental Research GmbH—UFZ, Leipzig, Germany
Sebastian Huhn
You can also search for this author in PubMed Google Scholar
Contributions
E.M., A.V. and A.V.W. designed research; E.M. conducted research; E.M., S.H. and A.V.W. analyzed data; E.M. and A.V.W. wrote the paper; E.M., A.V. and A.V.W. had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Evelyn Medawar .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Suppl. table 1, suppl. figure 1, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Medawar, E., Huhn, S., Villringer, A. et al. The effects of plant-based diets on the body and the brain: a systematic review. Transl Psychiatry 9 , 226 (2019). https://doi.org/10.1038/s41398-019-0552-0
Download citation
Received : 20 February 2019
Revised : 22 June 2019
Accepted : 17 July 2019
Published : 12 September 2019
DOI : https://doi.org/10.1038/s41398-019-0552-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Nutrient scoring for the degs1-ffq – from food intake to nutrient intake.
- Ronja Thieleking
- Lennard Schneidewind
- Evelyn Medawar
BMC Nutrition (2023)
Environmental pressures and pesticide exposure associated with an increase in the share of plant-based foods in the diet
- Emmanuelle Kesse-Guyot
- Benjamin Allès
- Julia Baudry
Scientific Reports (2023)
Animal protein intake is directly associated with serum level of pentraxin 3 in hemodialysis patients
- Fatemeh Navab
- Sahar Foshati
- Mohammad Hossein Rouhani
Integrated multiomic wastewater-based epidemiology can elucidate population-level dietary behaviour and inform public health nutrition assessments
- Devin A. Bowes
- Erin M. Driver
- Rolf U. Halden
Nature Food (2023)
Influence of the vegan, vegetarian and omnivore diet on the oral health status in adults: a systematic review and meta-analysis
- Luana Giò Azzola
- Nicolas Fankhauser
- Murali Srinivasan
Evidence-Based Dentistry (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Research article
- Open access
- Published: 08 September 2013
Long-term health benefits of physical activity – a systematic review of longitudinal studies
- Miriam Reiner 1 ,
- Christina Niermann 2 ,
- Darko Jekauc 2 &
- Alexander Woll 1
BMC Public Health volume 13 , Article number: 813 ( 2013 ) Cite this article
207k Accesses
805 Citations
567 Altmetric
Metrics details
The treatment of noncommunicable diseases (NCD), like coronary heart disease or type 2 diabetes mellitus, causes rising costs for the health system. Physical activity is supposed to reduce the risk for these diseases. Results of cross-sectional studies showed that physical activity is associated with better health, and that physical activity could prevent the development of these diseases. The purpose of this review is to summarize existing evidence for the long-term (>5 years) relationship between physical activity and weight gain, obesity, coronary heart disease, type 2 diabetes mellitus, Alzheimer’s disease and dementia.
Fifteen longitudinal studies with at least 5-year follow up times and a total of 288,724 subjects (>500 participants in each study), aged between 18 and 85 years, were identified using digital databases. Only studies published in English, about healthy adults at baseline, intentional physical activity and the listed NCDs were included.
The results of these studies show that physical activity appears to have a positive long-term influence on all selected diseases.
Conclusions
This review revealed a paucity of long-term studies on the relationship between physical activity and the incidence of NCD.
Peer Review reports
Especially in the last century, most Western countries have experienced significant demographic changes with a continuing increase in the number of older people who face medical and functional challenges, as well as diseases that are age-specific but have often originated in people’s younger years [ 1 – 4 ]. Most of these diseases including obesity, cardiovascular heart diseases (CHD) or type 2 diabetes mellitus are caused by civilisation [ 1 , 2 , 5 ]. The World Health Organisation has identified these three diseases as the most severe noncommunicable diseases (NCD) causing problems in today’s Western world [ 6 ]. Noncommunicable diseases are mostly diseases of slow progression and normally of long duration. The WHO identified for main types of NCDs: cardiovascular diseases, cancer, chronic respiratory diseases and diabetes [ 7 ].
Most NCDs primarily result from unhealthy lifestyles including the consumption of too much or unhealthy food [ 1 , 6 , 8 , 9 ], too much alcohol [ 1 , 8 , 10 ] and excessive smoking habits [ 1 , 8 , 11 ], combined with physical inactivity [ 1 , 2 , 8 , 12 ]. More specifically, inactivity and unhealthy eating habits are associated with weight gain, overweight and obesity are the major underlying causes for modern diseases such as CHD or type 2 diabetes mellitus [ 13 – 15 ]. Many cross-sectional and intervention studies have focused on the relationship between an unhealthy lifestyle, e.g. physical inactivity, unhealthy eating behaviour, smoking and alcohol consumption, and diseases in different study groups, e.g. high risk groups or different age groups [ 14 ]. All in all, cross-sectional studies suggest that physical activity may be an important factor for improving the general health and preventing the development of among others the above mentioned NCDs [ 1 ]. Because NCDs develop, not only by definition, over a long period of time and may have many causes, understanding the development of these diseases and their association with habitual factors such as physical activity is important for developing long-term prevention programs and guidelines. To investigate the development of these diseases, longitudinal studies with healthy persons, i.e. persons without obvious diseases at baseline examination, and a long term epidemiological view are necessary. It is important to follow the general population and not specific subgroups, e.g. high risk groups, persons with indications of NCD (e.g. hypertension or obesity / high body weight) or top athletes, to discover the general progression of the researched complaints in the general population.
Although these diseases are very prominent in many western countries, only few longitudinal studies exist that focus on their development during a person’s lifetime and their association with other habitual factors such as physical activity.
Many cross sectional studies have researched the relationship between physical activity and health outcomes - these results are summarized in quite a number of reviews. As opposed to this, only few long-term studies about the effect of physical activity on diseases exist, and to date there are no reviews that concentrate on long-term results in an epidemiologic view.
Therefore, the purpose of this article was to review long-term effects of physical activity on the development of weight gain and obesity, CHD and type 2 diabetes mellitus in healthy adults.
Furthermore, dementia and Alzheimer’s disease, two diseases which are of rising importance in modern societies and which develop over a long period of time, are regarded in the context of the long-term influences of physical activity. There is some evidence which indicates that physical activity has a positive effect against the development and progress of these two diseases.
To determine the importance of physical activity for the above described common health problems [ 8 ], only studies investigating the effect of physical activity on weight gain and obesity , CHD , type 2 diabetes mellitus and dementia and Alzheimer’s disease were included in this review. We searched the electronic databases Pubmed, BASE and OVID for articles published between January 1980 and May 2012 using the following search terms (without “and” or “or” and with longitudinal as well as long-term as a keyword to reduce the selection to such studies alone): “longitudinal / long-term, physical activity, adult” (3708 articles); “longitudinal / long-term, physical activity, adult, weight gain” (180 articles); “longitudinal / long-term, physical activity, adult, obesity” (483 articles); “longitudinal / long-term, physical activity, adult, CHD / coronary heart disease” (224 articles); “longitudinal / long-term, physical activity, adult, t2dm / type 2 diabetes mellitus” (87 articles); “longitudinal / long-term, physical activity, adult, dementia” (103 articles); and “longitudinal / long-term, physical activity, adult, Alzheimer’s disease” (60 articles) (Figure 1 Selection criteria and number of excluded and included papers / studies.).
Selection criteria and number of excluded and included papers/studies.
From these studies, only longitudinal studies with five or more years of follow-up time were included to show the intermediate to long-term effects of physical activity rather than short-term effects of physical activity. In addition, only studies involving adults were included to show the disease development in adulthood and old age. To show the development in the general population, not in subgroups, only large epidemiological studies with more than 500 participants were included.
Further, only epidemiologic longitudinal studies involving healthy adult participants at the baseline examination were included to determine the impact of normal daily activities performed by the general population. Clinical trials, cross-sectional studies, studies involving patients, and reviews and overviews were excluded. Publications using the same study population were included as long as they held more information or investigated other topics as well.
Only those studies were included that referred to intentional physical activity , e.g. playing soccer, or intentional activities of daily living, e.g. take the bike for shopping, to determine the impact of leisure time physical activity in the general population. Instead of this, activities of daily living, that are necessary to live a normal self-determined life, e.g. getting up from a chair or climbing stairs, are excluded.
Finally only studies published in English were included in this review.
Overall, 4,845 articles were identified with our search strategy; of these, 4,827 were excluded from the review (Figure 1 ) because of the above mentioned reasons. A total of 292,278 subjects were involved at baseline (268,885 subjects at follow-up). Four publications, involving 17,329 subjects, studied the effect of physical activity on weight gain and obesity [ 16 – 19 ]. Six publications, involving 134,188 subjects, investigated the effect of physical activity on CHD [ 20 – 26 ]. Five publications, involving 84,647 subjects, studied the effect of physical activity on type 2 diabetes mellitus [ 27 – 31 ]. Six publications, involving 15,006 subjects, investigated the effect of physical activity on Alzheimer’s disease and dementia [ 32 – 37 ]. Some studies included more than one disease accounting for the discrepancy in the overall number of subjects and included studies. The maximum follow-up time ranged from 6 to 60 years.
Effect of physical activity on weight gain and obesity
Overall, the studies included in this review showed a negative relationship between physical activity and weight gain or obesity over time. Additional file 1 : Table S1 summarises the examination data and the used survey sizes for the included studies on the long-term relationship between physical activity and weight gain and obesity.
An important study analysing the development of obesity depending on physical activity is the Aerobics Center Longitudinal Study (ACLS) conducted by the Cooper Clinic, Texas [ 16 ]. Between 1970 and 1998, DiPietro et al. [ 16 ] examined 2,501 healthy men aged between 22 and 55 years at baseline and five years later. The daily physical activity level was negatively related to the weight gain during the follow-up time. Those people who reduced their daily physical activity level gained a considerable amount of weight, while those people who maintained the same level of activity during the study did not gain weight. Further, those people who increased their physical activity level during the study experienced weight loss. DiPietro et al. [ 16 ] reported that a daily physical activity level with a metabolic rate of at least 60% above the resting metabolic rate is necessary for losing weight. Hence 45 to 60 minutes of brisk walking, gardening or cycling should be included in the daily routine to maintain weight in middle-aged men.
Gordon-Larsen et al. [ 17 ] investigated the relationship between walking and weight gain. In the Coronary Artery Risk Development in Young Adults (CARDIA) Study, they examined 4,995 women and men aged between 18 and 30 years at baseline (1985/1986) who were re-examined 2, 5, 7, 10 and 15 years later. After 15 years, there was a negative association between 30 minutes walking per day and weight gain depending on the percentile of baseline weight. Data for people in the 25 th percentile of baseline weight showed no significant relation between walking duration and weight gain. In contrast, data for people in the 50 th percentile of baseline weight revealed that for every 30 minutes of daily walking the weight gain was 0.15 kg per year less for men and 0.29 kg per year less for women. Finally, data for people in the 75 th percentile of baseline weight showed the smallest weight gain: for every 30 minutes of walking per day, men reduced their weight gain by about 0.25 kg per year and women by about 0.53 kg per year without making any other changes to their habitual lifestyle. Hence, the results of this study indicate that participants with a higher baseline weight benefit more from being physically active (for instance, for women: the total weight gain in 15 years was 13 kg for inactive women compared to only 5 kg for active women).
Hankinson et al. [ 18 ] used the same study population (CARDIA) to investigate the physical activity level in relation to a 20-year weight gain. Of 1,561 men and women, those with high habitual activity at the 20-year follow-up had a smaller increase in mean BMI, waist circumference and weight per year compared than those with low habitual activity. Men and women maintaining higher activity gained 2.6 and 6.1 kg less weight over the 20-year period than men and women with low activity, respectively. In addition, the results of that study indicated that women benefit more from maintaining a higher physical activity level than men and that maintaining higher activity levels during adulthood may lessen weight gain during the course of their life.
The Copenhagen City Heart Study by Petersen, Schnohr and Sorensen [ 19 ] linked cross-sectional and 10 year long-term analyses, to determine the development of weight gain. They examined 3,653 women and 2,626 men at three measurement points at 5-year intervals. The participants were aged between 20 and 78 years at baseline. Results of the three cross-sectional examinations (1 st at baseline, 2 nd after five years, 3 rd after 10 years) also showed a negative relationship between physical activity and weight. The preventing effects of medium leisure time physical activity (LTPA) on obesity were lower than those of high LTPA for both genders. The longitudinal analysis revealed a significant direct correlation between the level of LTPA and the risk of becoming obese for men but not for women. In contrast to the results of the cross-sectional analysis, the more active participants had a higher risk of becoming obese. Moreover, the results of that study indicate that obesity may lead to physical inactivity.
Therefore, the results of the first three studies [ 16 – 18 ] suggest a negative correlation between physical activity and weight gain after several years of follow-up (greater physical activity leads to less weight gain). In contrast, the fourth study [ 19 ] provided evidence that being more physically active leads to a greater risk of becoming obese. They suggest that obesity influences the development of physical inactivity; however they did not discuss possible causes and effect relations. These results raise the question of the causality of the relationship between physical activity and weight gain. Detailed information, results and limitations of each study are presented in Additional file 1 : Table S1.
Effect of physical activity on coronary heart disease (CHD)
Of all modern diseases, coronary heart disease (CHD) has received the most scientific scrutiny. Overall, most studies reported a negative relationship between physical activity and the occurrence of CHD for physical activity levels above the minimum energy expenditure. Additional file 2 : Table S2 summarises the examination data and the used survey sizes of the included studies addressing the longitudinal relationship between physical activity and coronary heart diseases.
In 1948, the National Heart, Lung and Blood Institute founded by Kannel et al. established the Framingham Heart Study. This research group investigated the general causes and the development of coronary heart disease in 5,209 men and women, aged 30 to 62 years at baseline [ 38 ]. The results revealed a negative association between the physical activity level and the emergence of CHD events and overall cardiovascular mortality [ 38 – 40 ]. Lee and Paffenbarger [ 20 ] compared the results of the Framingham Heart Study with data for 18,835 men who graduated from Harvard University between 1916 and 1950 and established the Harvard Alumni Health Study. In five mail-back surveys, researchers investigated the association between physical activity and stroke [ 20 ] and other CHD [ 21 ].
The relationship between energy expenditure and the incidence of stroke showed a u-shape pattern [ 20 ]. Specifically, spending at least 2,000 to 3,000 kcal additional energy per week on physical activity was necessary for reducing the risk of stroke. These results were reassessed for all CHD [ 21 ] in 12,516 Harvard Alumni over the course of 16 years (from 1977 through 1996). For CHD in general, the relationship between energy expenditure and the incidence of CHD showed the same u-shape pattern but the curve was shifted towards lower additional energy expenditure: spending at least 1,000 kcal additional energy per week on physical activity was necessary to reduce the risk of CHD. Hence, moderate to vigorous additional physical activity of about 2,000 to 3,000 kcal (min. 1,000 to 2,000 kcal) per week appear to reduce the overall risk for CHD, stroke and other diseases (e.g. hypertension).
Comparable results were also reported by the Honolulu Heart Program [ 22 , 23 ] including 8,006 men of Japanese ancestry aged 45 to 68 years at baseline who lived in Oahu, Hawaii. After 16 years, the physical activity reported at baseline was negatively related to CHD events and mortality. However, it is important to note that these results were partially mediated through the effects of hypertension, diabetes mellitus, cholesterol and BMI.
The studies cited in the next section had similar results but also featured the following additional findings.
The Alameda County Health Study by Kaplan et al. [ 24 ] reported the dependency of CHD mortality on several health factors and behaviour by quantifying the relative risks of various covariates (age, sex, perceived health, mobility impairment, heart problems, high blood pressure, diabetes mellitus, shortness of breath, current smoking, low BMI and social isolation) in 6928 men and women. After including all covariates, a protective effect of LTPA is still noticeable.
Gillum et al. [ 25 ] investigated the relationship between physical activity and stroke incidence in The National Health and Nutrition Examination Study I Epidemic Follow-Up Study on 5,852 persons aged 24 to 74 years at baseline and reported comparable results as above studies [ 20 – 23 ]. However, while the u-shaped relationship between physical activity and the incidence of stroke was confirmed for men, for women greater physical activity was negatively linearly associated with the incidence of stroke. In addition, recreational physical activity was not associated with the incidence of stroke in African American subjects, yet a significant interaction between heart rate and the incidence of stroke was observed only for African American subjects. The authors provided limited discussion of these differing results between Caucasian and African Americans.
To investigate the link between obesity and associated diseases, Li et al. [ 26 ] quantified the relative risk of developing CHD dependent on obesity and physical activity. They followed 88,393 Nurses aged 34 to 59 in their Nurses’ Health Study from 1980 to 2000. Being overweight and obese was significantly associated with increased risk of CHD. In addition, increased levels of physical activity were related to a graded reduction in CHD risk. Further, greater absolute mass (in kg) gained during adulthood predicted a higher CHD risk. The study concluded that obesity and physical inactivity contribute independently to the development of CHD in women.
Overall, all studies included in this review section showed a predicted negative relation between physical activity and the risk of CHD over time. Two studies [ 20 , 21 ] showed that a minimum additional energy expenditure of 1,000 to 2,000 kcal per week is necessary to achieve health related results. Limitations of these studies comprise the inclusion of very specific and selected participants (e.g. Harvard Alumni in the Harvard Alumni Heart Study and Nurses in the Nurses’ Health Study). In addition, these results cannot be generalized for the general public because of the selected social and ethnic backgrounds of participants and unbalanced gender distributions. In addition, most studies used Caucasian subjects alone. Hence, additional research on other ethnicities is necessary to obtain generalizable results. Moreover, the summarized studies were not designed to clarify the causality of the relationship between physical activity and CHD events. Additional research on the impact of other lifestyle factors as mediators or moderators of the relationship between physical activity and CHD is necessary. Detailed information, results and limitations of each study are presented in Additional file 2 : Table S2.
Effect of physical activity on type 2 diabetes mellitus
While the incidence of type 2 diabetes mellitus in older people has increased rapidly [ 1 ], all studies reported a negative relation between physical activity and the risk of type 2 diabetes mellitus. Additional file 3 : Table S3 summarizes the results of the included studies that investigated the long-term relationship between physical activity and type 2 diabetes mellitus.
In their Nurses’ Health Study involving 70,120 nurses aged 40 to 64, which has been on-going since 1976, Hu et al. [ 27 ] investigated the relationship between participants’ physical activity level and the development of the relative risks for type 2 diabetes mellitus. Physical activity was negatively related to the incidence of type 2 diabetes mellitus even after adjusting for BMI where participants with higher physical activity levels had a lower relative risk of acquiring type 2 diabetes mellitus than those who with a lower physical activity level.
Berenzen et al. [ 28 ] and Demakakos et al. [ 29 ] reported generally comparable results in 653 men and women in the Copenhagen City Heart Study and in the English Longitudinal Study of Ageing covering different age groups, respectively. In addition to the negative relation between physical activity and the incidence of type 2 diabetes mellitus, Demakakos et al. [ 29 ] showed that moderate to vigorous physical activity (performed at least once per week) is necessary to achieve a positive effect on health and to reduce risk of type 2 diabetes mellitus. Stratifying their results by age revealed that with increasing age a higher intensity per training session or even several sessions per week are required to achieve the same risk reduction.
A high body weight or obesity, often described by the relation between body weight and body height (body mass index—BMI), and socioeconomic status are strong covariates for the relationship between physical activity and the incidence of type 2 diabetes mellitus. For instance, Katzmarzyk et al. [ 30 ] analysed the association between obesity, physical activity, cardiorespiratory fitness and the incidence of type 2 diabetes mellitus in their Physical Activity Longitudinal Study involving 1,543 men and women. Obesity and physical fitness, but not physical activity, were significant predictors of the incidence of type 2 diabetes mellitus. Mozaffarin et al. [ 31 ] added lifestyle factors in their analysis of the risk of type 2 diabetes mellitus in 4,883 participants of the Cardiovascular Health Study. Low-risk lifestyle factors included physical activity above the median level, dietary score in the upper two quintiles, having never smoked, no alcohol, a body mass index below 25 kg/m 2 and a waist circumference below 88 cm for women or below 92 cm for men. With every healthy lifestyle factor the incidence for type 2 diabetes mellitus decreased by 35%. For people scoring lowest (that is, were the healthiest) in every lifestyle factor, an 82% lower risk for type 2 diabetes mellitus was predicted compared to all other patients. In addition, it was predicted that if these associations were causal, 8 of 10 cases of type 2 diabetes mellitus could be prevented.
All studies [ 28 – 31 ] reported a negative relationship between physical activity and the incident risk of type 2 diabetes mellitus. However, there are other factors than physical activity that are important in the development of type 2 diabetes mellitus. For instance, the results of the Physical Activity Longitudinal Study by Katzmarzyk et al. [ 31 ] suggest that not only the presence or absence of physical activity is a determining health factor but that the level of obesity and physical fitness also has an influence on the relationship between physical activity and the state of health. However, it is difficult to confirm these conclusions because of the small number of longitudinal studies that consider physical fitness and other lifestyle factors. In addition, the precise mechanism of how physical activity acts to reduce the risk of type 2 diabetes mellitus, such as through altered insulin sensitivity or altered insulin production, is still unknown.
Effect of physical activity on Alzheimer’s disease and dementia
The relationship between physical activity and dementia, particularly Alzheimer’s disease, is important for the general public because the incidence of dementia increases with increasing age [ 1 ]. Additional file 4 : Table S4 summarizes the results of the included longitudinal studies on the relationship between physical activity and Alzheimer’s disease and dementia.
The few existing studies [ 32 – 37 ] found that physical activity is negatively related to the incidence of Alzheimer’s disease and dementia in healthy men and women. Physically active people are at a lower risk of developing cognitive impairment and have a higher cognitive ability score. Interestingly, activities with low intensity, such as walking, are negatively related to the incidence of dementia and Alzheimer’s disease [ 32 ]. These results indicate that regular physical activity may be an important and potent factor preventing cognitive decline and dementia in healthy older people. Most studies on Alzheimer’s disease and dementia originate in the field of Psychology. The link between physical activity and Alzheimer’s disease and dementia in healthy participants at baseline has only been reported in very few studies [ 32 – 37 ], further emphasizing the overall lack of studies and specifically the lack of long-term studies that include people without dementia or Alzheimer’s disease. Most studies included people who had already been diagnosed with dementia or Alzheimer’s disease to research the development of the diseases. Detailed information, results and limitations of all included studies on physical activity and Alzheimer’s disease and dementia are presented in additional file 4 : Table S4.
The results of the reviewed studies indicate that physical activity seems to be an important factor that can have beneficial effects for the reviewed noncommunicable diseases weight gain and obesity, CHD and type 2 diabetes mellitus, the risk factors weight gain and obesity and the age-related diseases dementia and Alzheimer’s disease.
Two of the three longitudinal studies with at least 5-year follow-up focusing on the development of obesity over time showed a negative relationship between physical activity and obesity [ 16 , 17 ]. Surprisingly, results of one study indicated that high leisure-time physical activity increased the risk of becoming obese in the following ten years for men [ 19 ]. The reason for this remains unexplained. Overall, the results of the studies included in this review are inconclusive regarding the required minimum level of physical activity for preventing obesity. There is no evidence for the type, intensity and frequency of activities that lead to positive health results.
Several studies [ 20 – 26 , 38 ] investigated the longitudinal effect of physical activity on the development of coronary heart diseases. Overall, the results showed a positive long-term effect where people who were physically active had a lower risk of suffering from a CHD later in their life. A minimum additional 1,000 kcal energy expenditure per week spent on physical activity has been found to be necessary for preventing overall CHD [ 21 ]. However, information on the type, intensity and frequency of activities necessary for reducing the incidence of CHD are unknown.
The results of studies [ 28 – 31 ] examining the effect of physical activity on the risk of suffering from type 2 diabetes mellitus showed a negative relation where higher rates of physical activity were associated with a lower risk of developing a type 2 diabetes mellitus. A higher level of physical activity appears to be required, that is a higher intensity per training session or even several sessions per week are needed, for achieving health benefits [ 29 ]. Presumably, not only physical activity level but also weight and fitness status, and their association, play a role in the development of type 2 diabetes mellitus [ 30 ].
Finally, six studies [ 32 – 37 ] focused on the relationship between physical activity and the incidence of dementia and Alzheimer’s disease. Results of these studies emphasized the importance of regular physical activity, but no information was provided about the type, intensity and frequency of physical activity that has the greatest health benefit.
However, several problems in the reviewed studies have become apparent.
First: There are only few long-term studies on the relationship between physical activity and the incidence of NCD, which stresses the general paucity of longitudinal research in this area. More long-term studies, following the development of diseases and the impact of lifestyle, especially physical activity, are needed. Further longitudinal studies are needed that differentiate between ethnic groups, genders and groups with different social backgrounds. The results presented in this review only encompass the relationship between physical activity and the incidence of NCD in western countries and mainly for Caucasian participants. In addition, only adults were included in these studies, and hence the results cannot be generalized to other groups. Many age related diseases, such as type 2 diabetes mellitus, CHD or certain types of cancer, develop over a long time before they are diagnosed by a physician. To identify this development in detail, longitudinal studies involving healthy participants at baseline should be conducted and these participants should be followed into older age when the disease occurs. To understand this lifelong development of NCD, studies following children throughout their lifespan are desirable. To the best of our knowledge, there are only very few studies that follow children through their adolescence into their adulthood [ 41 , 42 ]. The realization of this approach is very difficult, so the research should be as long as possible and about different groups, e.g. different age-related cohorts.
Second: However, more research is necessary on the clinical picture and the development of NCD. Clearly, a thorough insight into these aspects is a prerequisite for the design and development of effective prevention programs. In addition, the relationship between physical activity and the development of NCD must be better understood including the role of other parameters, such as, for instance, nutrition, body composition, alcohol consumption and smoking behaviour. Indeed, results of some of the included studies [ 17 , 18 , 20 , 21 , 23 , 24 , 26 , 27 ],[ 29 , 31 – 33 , 36 , 37 ] suggested that other factors including eating behaviour and food intake, smoking habits and a general activity level or disease specific risk factors such as hypertension are involved in the correlations of physical activity and health outcomes. It is almost impossible to explain the impact of just one factor, e.g. just physical activity, on the development of a lifestyle related complains like CHD - other factors are always involved, e.g. genetic constitution, other diseases, for instance obesity in the relationship with type 2 diabetes mellitus, personal behaviour or individual factors, like cognitive, motivational, volitional or emotional aspects.
Third: Most studies [ 16 – 24 , 26 , 27 , 29 , 31 – 38 ] only used self-reported/estimated physical activity for measuring participant’s physical activity. However, some studies [ 30 , 43 ] (this study was excluded from the review because they researched just physical fitness, not intentional physical activity), showed that the correlation of physical activity and health benefits are mediated through the physical fitness level. The quality and relevance of findings could be improved by the use of an objectively assessed variable, such as the physical fitness level measured by a fitness test or the physical activity level monitored by an accelerometer to become independent of subjective estimates and social desirability [ 44 ]. Another limitation of using self-reported physical activity alone is the fact that most questionnaires only feature the actual physical activity at the time of the examination, and hence are unable to assess physical activity performed between questionnaire administrations. However, this information is critical for determining the importance of continuous physical activity in a healthy and active lifestyle and its benefits for health [ 45 , 46 ].
The reviewed studies have shown that physical activity could help in the prevention of non-communicable and age-related diseases. The studies have shown that it is necessary to include physical activity into prevention programs for NCD and to inform the patients and the population in general about its virtues. To achieve this, a closer cooperation between physicians, research and sport facilities is needed. Research and physical activity service providers, e.g. gyms or sports clubs, health insurances or public providers (e.g. adult education centers) have to cooperate together to improve the general health. In addition, the knowledge about the causes and the development of modern diseases in the population should be improved. Instead of treating with medicine alone, physicians should advise patients to be more physically active within their limits. Children and adolescents should generally be encouraged to maintain a healthy lifestyle throughout their lives. In addition, public health projects that are targeted at improving the general health during adulthood and older age should focus on effective disease prevention starting during childhood.
It is important to highlight the limitations of this review in order to provide a context for the results. First, the assessment is limited to published work and may be subject to publication bias. Second, the influence of several confounders, as age, the lag between baseline and follow-up, or attrition rate, could affect conclusions of this review. Third, the work contained in this review is limited to English-written journals and thus the results cannot generalize to studies conducted and published in other languages. Fourth, we included only studies with more than 500 participants. Fifth, the literature reviewed consisted of self-reported physical activity. Finally, the review is limited to the search terms and data-bases contained in our “Methods” section. Studies that have not been abstracted with these key words will be missing from our review.
This review indicates the relative lack of epidemiologic longitudinal studies on the effects of physical activity in addition to non-communicable diseases. The presented studies exclusively illustrate positive results. To the best of our knowledge no other studies reporting no or negative results over time exist.
To show the longitudinal improvements of physical activity in addition to the presented non-communicable diseases of a large number of adults within normal communities, no studies with subsamples or unhealthy participants alone were considered. This review just focuses on studies with more than 500 healthy participants. Other studies [e.g. [ 47 ] following smaller samples of participants were not included in this review; however they too contribute to the long term understanding of the development of non-communicable diseases.
Overall, the results of the reviewed articles provide a general view about the longitudinal relationship between physical activity and the incidence of NCD and health problems. Physical activity seems to be a relevant factor for preventing age-related diseases; however more long-term research is necessary.
Dishman RK, Washburn RA, Heath GW: Physical Activity Epidemiology. 2004, Champaign, IL: Human Kinetics
Google Scholar
Schuit J: Physical activity, body composition and healthy ageing. Sci & Sports. 2006, 21: 209-213. 10.1016/j.scispo.2006.06.004.
Article Google Scholar
Raebel MA, Malone DC, Conner DA, Xu S, Porter JA, Lanty FA: Health services use and health care costs of obese and nonobese individuals. Arch Intern Med. 2004, 164: 2135-3140. 10.1001/archinte.164.19.2135.
Article PubMed Google Scholar
Chan R, Woo J: Prevention of overweight and obesity: how effective is the current public health approach. Int J Environ Res Public Health. 2010, 7: 765-783. 10.3390/ijerph7030765.
Article PubMed PubMed Central Google Scholar
Booth FW, Chakravarthy MV: Cost and Consequences of Sedentary Living: New Battleground for an Old Enemy. 2002, Washington DC: President’s Council on Physical Fitness and Sports
Chai W, Nigg CR, Pagano IS, Motl RW, Horwath C, Dishman RK: Associations of quality of life with physical activity, fruit and vegetable consumption, and physical inactivity in a free living, multiethnic population in Hawaii: a longitudinal study. The international journal of behavioral nutrition and physical activity. 2010, 7: 83-10.1186/1479-5868-7-83.
World Health Organization: Global status report on noncommunicable diseases 2010. World Health Organisation. 2011, Geneva, Switzerland: WHO Press
World Health Organization: Global Health Risks - Mortality and burden of disease attributable to selected major risks. 2009, World Health Organization, http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf ,
Astrup A, Dyerberg J, Selleck M, Stender S: Nutrition transition and its relationship to the development of obesity and related chronic diseases. Obes Rev. 2008, 9: 48-52. 10.1111/j.1467-789X.2007.00438.x.
Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J: Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009, 373: 2223-2233. 10.1016/S0140-6736(09)60746-7.
Ambrose JA, Barua RS: The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004, 43: 1731-1737. 10.1016/j.jacc.2003.12.047.
Article CAS PubMed Google Scholar
Bijnen FCH, Caspersen CJ, Mosterd WL: Physical inactivity as a risk factor for coronary heart disease: a WHO and international society and federation of cardiology position statement. Bull World Health Organ. 1994, 72: 1-4.
CAS PubMed PubMed Central Google Scholar
de Berrington Gonzaley A, Hartge P, Cerhan JR: Body mass index and mortality among 1.43 million white adults. N Engl J Med. 2010, 363: 2211-10.1056/NEJMoa1000367.
Vogel T, Brechat P-H, Leprâtre P-M, Kaltenbach G, Berthel M, Lonsdorfer J: Health benefits of physical activity in older patients: a review. The Int J od Clin Pract. 2009, 63: 303-320. 10.1111/j.1742-1241.2008.01957.x.
Article CAS Google Scholar
Warburton DE, Nicol CW, Bredin SS: Health benefits of physical activity: the evidence. CMAJ. 2006, 174: 801-809.
Di Pietro L, Dziura J, Blair SN: Estimated change in physical activity level (PAL) and prediction of 5-year weight change in men: the aerobics center longitudinal study. Int J Obes Relat Metab Disord. 2004, 28: 1541-1547. 10.1038/sj.ijo.0802821.
Gordon-Larsen P, Hou N, Sidney S, Sternfeld B, Lewis CE, Jacobs DR, Popkin BM: Fifteen-year longitudinal trends in walking patterns and their impact on weight change. Am J Clin Nutr. 2009, 89: 16-26.
Hankinson AL, Daviglus ML, Bouchard C, Carnethon M, Lewis CE, Schreiner PJ, Liu K, Sidney S: Maintaining a high physical activity level over 20 years and weight gain. J Am Med Assoc. 2010, 304: 2603-2610. 10.1001/jama.2010.1843.
Petersen L, Schnohr P, Sorensen TI: Longitudinal study of the long-term relation between physical activity and obesity in adults. Int J Obes Relat Metab Disord. 2004, 28: 105-112. 10.1038/sj.ijo.0802548.
Lee I-M, Paffenbarger RS: Physical activity and stroke incidence - the Harvard alumni health study. Stroke. 1998, 29: 2049-2054. 10.1161/01.STR.29.10.2049.
Sesso H, Paffenbarger RS, Lee I-M: Physical activity and coronary heart disease in men - the Harvard alumni health study. Circulation. 2000, 102: 975-980. 10.1161/01.CIR.102.9.975.
Donahue R, Abbott R, Reed D, Katshuiko Y: Physical activity and coronary heart diseases in middle-aged and elderly men: the Honolulu heart program. J Public Health. 1988, 78: 683-685. 10.2105/AJPH.78.6.683.
CAS Google Scholar
Rodriguez BL, Curb JD, Burchfield CM, Abbott RD, Petrovich H, Masaki K, Chiu D: Physical activity and 23 year incidence of coronary heart disease morbidity and mortality among middle-aged men - the Honolulu heart program. Circulation. 1994, 89: 2540-2544. 10.1161/01.CIR.89.6.2540.
Kaplan GA, Strawbridge WJ, Cohen RD, Hungerford LR: Natural history of leisure-time physical activity and its correlates: associations with mortality from all-causes and cardiovascular disease over 28 years. Am J Epidemiol. 1996, 144: 793-797. 10.1093/oxfordjournals.aje.a009003.
Gillum RF, Mussolino ME, Ingram DD: Physical activity and stroke incidence in women and men - the NHANES I epidemic follow up study. Am J Epidemiol. 1996, 143: 860-869. 10.1093/oxfordjournals.aje.a008829.
Li TY, Rana JS, Manson JE, Willett WC, Stampfer MJ, Colditz GA, Rexrode KM, Hu FB: Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation. 2006, 113: 499-506. 10.1161/CIRCULATIONAHA.105.574087.
Hu FB, Sigal R, Rich-Edwards JW, Colditz GA, Solomon CG, Wilett WC, Speizer FE, MJ E: Walking compared with vigorous physical activity and risk of type 2 diabetes in women - a prospecttive study. J Am Med Assoc. 1999, 282: 1433-1439. 10.1001/jama.282.15.1433.
Berenzen T, Petersen L, Pedersen O, Black E, Astrup A, Sorensen TI: Long-term effects of leisure time physical activity on risk of insulin resistance and impaired glucose tolerance, allowing for body weight history, in Danish men. Diabet Med. 2007, 26: 63-72.
Demakakos P, Hamer M, Stamatakis E, Steptoe A: Low-intensity physical activity is associated with reduced risk of incident type 2 diabetes in older adults: evidence from the English longitudinal study of ageing. Diabetologia. 2010, 53: 1877-1885. 10.1007/s00125-010-1785-x.
Katzmarzyk PT, Craig CL, Gauvin L: Adiposity, physical fitness and incident diabetes: the physical activity longitudinal study. Diabetologia. 2007, 50: 538-544. 10.1007/s00125-006-0554-3.
Mozzafarian D, Kamineni A, Carnethon M, Djousse L, Mukamal K, Siscovick D: Lifestyle risk factors and new-onset diabetes mellitus in older adults. Arch Intern Med. 2009, 169: 798-807. 10.1001/archinternmed.2009.21.
Abbott RD, White LR, Ross GW, Masaki K, Curb JD, Petrovich H: Walking and dementia in physically capable elderly men. J Am Med Assoc. 2004, 292: 1447-1453. 10.1001/jama.292.12.1447.
Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W: Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006, 144: 73-81. 10.7326/0003-4819-144-2-200601170-00004.
Rovio S, Kåreholt I, Helkala E-L, Viitanen M, Winblad B, Tuomilehto J, Soininen H, Nissinen A, Kivipelto M: Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. The Lancet Neurology. 2005, 4: 705-711. 10.1016/S1474-4422(05)70198-8.
Laurin D, Verreault R, Linday J, MacPerson K, Rockwood K: Physical Activity and Risk of cognitive impairment and dementia in Elderly Persons. Arch Neurol. 2001, 58: 498-504. 10.1001/archneur.58.3.498.
Chang M, Jonsson PV, Snaedal J, Bjornsson S, Saczynski JS, Aspelund T, Eriksdottir G, Jonsdottir MK, Lopez OJ, Harris TB, et al: The Effect of midlife physical activity on cognitive function among older adults: AGES - Reykjavik Study. J Gerontol. 2010, 65: 1369-1374.
Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OJ, Carlson M, Lyketsos CG: Physical activity, APOE genotype and dementia risk: findings from the cardiovascular health cognition study. Am J Epidemiol. 2005, 161: 639-651. 10.1093/aje/kwi092.
Sherman S, D’Agostino R, Cobb J: Does exercise reduce mortality rates in the elderly? experience from the Framingham heart study. Am Heart Study. 1997, 128: 962-972.
Kannel W, Sorley P: Some health benefits of physical activity. Arch Intern Med. 1979, 112: 820-825.
Kannel W, Belange A, D’Agostino R, Israel I: Physical activity and physical demand on the job and risk of cardiovascular diseases and death: the Framingham study. Am Heart Study. 1986, 112: 820-825. 10.1016/0002-8703(86)90480-1.
Must A, Jaques PF, Dallai GE, Bajema CJ, Dietz WH: Long-term morbidity and mortality of overweight adolescents — a follow-up of the Harvard growth study of 1922 to 1935. N Engl J Med. 1992, 327: 1350-1355. 10.1056/NEJM199211053271904.
Juonala M, Järvisalo MJ, Mäki-Torkko N, Kähönen M, Viikari JSA, Raitakari OT: Risk factors identified in childhood and decreased carotid artery elasticity in adulthood - the cardiovascular risk in young Finns study. Circulation. 2005, 112: 1486-1493. 10.1161/CIRCULATIONAHA.104.502161.
Sui X, Lamonte MJ, Ladika JN, Hardin JW, Chase N, Hooker SP, Blair SN: Cardiorespiratory fitness and adiposity as mortality predictors in older adults. J Am Med Assoc. 2007, 298: 2507-2516. 10.1001/jama.298.21.2507.
Adams SA, Matthews CE, Ebbeling CB, Moore CG, Cunningham JE, Fulton J, Herbert JR: The effect of social desirability and social approval on self-reports of physical activity. Am J Epidemiol. 2005, 161: 389-398. 10.1093/aje/kwi054.
Klein T, Becker S: Is there really a reduction in physical activity in the life-span? an analysis of age-and cohort-related differences in physical activity. Z Soziol. 2008, 37: 226-245.
Klaperski S, Seelig H, Fuchs R: Physical activity as a stress buffer. Z Sportpsychol. 2012, 19: 80-90. 10.1026/1612-5010/a000061.
Drygas W, Kostka T, Jegier A, Kuński H: Long-term effects of different physical activity levels on coronary heart disease risk factors in middle-aged men. Int J Sports Med. 2000, 21: 235-241. 10.1055/s-2000-309.
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2458/13/813/prepub
Download references
Acknowledgements
We would also like to express our appreciation to PD Dr. Annegret Mündermann, Julia Everke-Buchanan, Sven Henrich and Andreas Nothardt for their writing assistance on behalf of the authors.
Author information
Authors and affiliations.
Institute of Sport and Sport Science, Karlsruhe Institute of Technology, Engler-Bunte Ring 15, 76131, Karlsruhe, Germany
Miriam Reiner & Alexander Woll
Institute of Sport Science. University of Konstanz, Universitätsstr. 10, 78467, Konstanz, Germany
Christina Niermann & Darko Jekauc
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Miriam Reiner .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors’ contributions
MR searched for relevant literature and wrote the manuscript. CN and DJ helped writing and drafting the manuscript. AW has given final approval of the version to be published. All authors read and appropriated the final manuscript.
Miriam Reiner, Christina Niermann, Darko Jekauc and Alexander Woll contributed equally to this work.
Electronic supplementary material
12889_2012_5766_moesm1_esm.pdf.
Additional file 1: Table S1: Description of studies on the association between physical activity and weight gain / obesity. Description of dataset: Table S1 describes the included studies on the association between physical activity and weight gain or obesity (Author and year, Name of the study, Baseline and measuring points, Follow up time, Baseline sample and Age at baseline, Drop out, sample size in the survey, Operationalization of physical activity and the outcome variables, Results, Limitations). (PDF 81 KB)
12889_2012_5766_MOESM2_ESM.pdf
Additional file 2: Table S2: Description of studies of the association between physical activity and coronary heart diseases. Description of dataset: Table S2 describes the included studies on the association between physical activity and coronary heart diseases (Author and year, Name of the study, Baseline and measuring points, Follow up time, Baseline sample and Age at baseline, Drop out, sample size in the survey, Operationalization of physical activity and the outcome variables, Results, Limitations). (PDF 45 KB)
12889_2012_5766_MOESM3_ESM.pdf
Additional file 3: Table S3: Description of studies on the association between physical activity and type 2 diabetes mellitus. Description of dataset: Table S3 describes the included studies on the association between physical activity and type 2 diabetes mellitus (Author and year, Name of the study, Baseline and measuring points, Follow up time, Baseline sample and Age at baseline, Drop out, sample size in the survey, Operationalization of physical activity and the outcome variables, Results, Limitations). (PDF 58 KB)
12889_2012_5766_MOESM4_ESM.pdf
Additional file 4: Table S4: Description of studies on the association between physical activity and Alzheimer’s disease and dementia. Description of dataset: Table S4 describes the included studies on the association between physical activity and Alzheimer’s disease and dementia (Author and year, Name of the study, Baseline and measuring points, Follow up time, Baseline sample and Age at baseline, Drop out, sample size in the survey, Operationalization of physical activity and the outcome variables, Results, Limitations). (PDF 64 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Reiner, M., Niermann, C., Jekauc, D. et al. Long-term health benefits of physical activity – a systematic review of longitudinal studies. BMC Public Health 13 , 813 (2013). https://doi.org/10.1186/1471-2458-13-813
Download citation
Received : 06 July 2012
Accepted : 04 September 2013
Published : 08 September 2013
DOI : https://doi.org/10.1186/1471-2458-13-813
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Physical activity
- Weight gain
- Type 2 diabetes mellitus
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]
- Subscribe to journal Subscribe
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Potential Health Benefits
Singletary, Keith PhD
Keith Singletary, PhD, is professor emeritus of nutrition in the Department of Food Science and Human Nutrition at the University of Illinois. From 2001 to 2004, he was the director of the Functional Foods for Health Program, an interdisciplinary program between the Chicago and Urbana-Champaign campuses of the University of Illinois. Dr Singletary received bachelor's and master's degrees in microbiology from Michigan State University and his PhD in nutritional sciences from the University of Illinois. Dr Singletary's primary research interests are in molecular carcinogenesis and cancer chemoprevention, specifically identifying and determining the mechanism of action of phytochemicals in fruits, vegetables, and spices as cancer-protective agents. He has been recognized with the Senior Faculty Award for Excellence in Research by the College of Agricultural, Consumer and Environmental Sciences at the University of Illinois. Dr Singletary currently resides in Florida.
Funding for the preparation of this manuscript was provided by McCormick and Co.
The authors have no conflicts of interest to disclose.
Correspondence: Keith Singletary, PhD, Department of Food Science and Human Nutrition, University of Illinois, 905 South Goodwin Ave, Urbana, IL 61801 ( [email protected] ).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site ( www.nutritiontodayonline.com ).
This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND) , where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal.
For centuries, Curcuma longa (turmeric) was used as a spice in Asian cuisine and as a medicinal herb for treatment of inflammation, pain, wound healing, and digestive disorders, to name a few. Considerable preclinical research found that turmeric and its bioactive curcuminoid polyphenols can affect a variety of chronic conditions. Poor oral bioavailability of these curcuminoids hindered human trials investigating the efficacy of oral turmeric and its curcuminoids in treating various diseases and disorders. However, with the development of absorption-enhanced curcuminoid formulations in the past decade, dozens of clinical studies were conducted examining this spice's actions toward inflammatory conditions and glucose/lipid dysregulation. This narrative review of human trials addresses the scientific evidence for potential health benefits of turmeric and its curcuminoids in the treatment of arthritis, diabetes, and the metabolic syndrome and discusses recommendations for future research.
The spice turmeric is a botanical that is used widely in the Middle East and Asia, not only to impart a distinctive flavor to foods, but also purportedly to provide health benefits as a component of traditional medicines. Recently, it has been added to nutraceuticals, beverages, and processed foods. Turmeric is obtained from the rhizome of Curcuma longa L. (Zingiberaceae family) ( Figure 1 ). Three curcuminoids, curcumin, demethoxycurcumin, and bisdemethoxycurcumin ( Figure 2 ), of which curcumin is the most prevalent, are among many bioactive ingredients in turmeric. The yellow pigment curcumin or diferuloylmethane makes up 60% to 70% of crude turmeric extracts and is the principal curcuminoid evaluated for health-promoting activities. 1 In addition, turmeric contains sugars, proteins, resins, and volatile oils, such as turmerone, atlantone, and zingiberene, some of which may have bioactivity as well. 1–4 Numerous preclinical investigations identified a variety of potential health benefits, including treatment for heart disease, arthritis, Alzheimer's disease, gastrointestinal disorders, and the metabolic syndrome (MetS). 5 Initial human trials examining the biological actions of oral curcumin given as raw turmeric powder ( Figure 3 ) were confronted with its poor water solubility, low intestinal absorption, and rapid metabolic degradation, which limited its systemic distribution and bioavailability. 1 Consumption of gram quantities of curcumin powder by human subjects was needed to obtain measurable amounts of circulating curcumin from which discernable biological actions were inconsistently observed. 1,6–10 Over the previous decade, considerable research led to improvements in curcumin's bioavailability, which has contributed to the appearance of numerous clinical studies evaluating various possible health benefits of both turmeric and curcuminoids using these new delivery approaches. Such methods include inclusion of piperine, a phytochemical that enhances intestinal uptake of curcumin, and use of novel delivery systems that complex curcuminoids within diverse matrices. For example, curcumin can be incorporated into micelles, microemulsions, liposomes, nanoparticles, and in other lipid and biopolymer particles. 11–13 Curcuminoids encapsulated with turmeric essential oils, particularly with turmerone, which enhances intestinal permeability, also have been prepared. Noticeable improvements in oral bioavailability in human subjects were reported using these novel formulations, 14–22 and comparative efficacies have been reported for some strategies. 23–27 This narrative review examines human investigations into the effects of turmeric and its extracts and novel formulations on subjects with signs and symptoms of arthritis, type 2 diabetes mellitus (T2DM) and MetS. It may provide new insights into emerging potential health benefits of turmeric from the recent and growing number of human studies.

A search of the PubMed, ClinicalTrials.gov, and Biomed Central databases identified more than 150 preclinical and clinical reports published prior to 2019 that examined the impact of turmeric or its constituents on the biochemical and physiological characteristics of MetS, T2DM, and arthritis and on the prevention or treatment of these conditions. Search terms included C. longa L., curcumin, curcuminoids, turmeric, and diferuloylmethane. Full reports of English-language publications and English-language abstracts of foreign-language articles from peer-reviewed journals were the primary sources of information. The human studies identified were inconsistent in methodological rigor and differed, for example, in inclusion of appropriate controls, extent of blinding, completeness of participant descriptions, randomization, procedures for statistical analysis of data, and chemical characterization of intervention treatments. Nonetheless, all published English-language human investigations identified were included in this narrative review so that the totality and diversity of information can be evaluated, and issues for future research can be identified. Additional information was gleaned from bibliographies within these sources. Studies examining curcumin within multi-ingredient preparations were not included in this review.
The composition of turmeric or curcumin samples used in published human studies routinely is not well characterized. These “curcumin” products likely are curcuminoid mixtures that consist of varying amounts not only of curcumin but also of demethoxycurcumin and bisdemethoxycurcumin in the turmeric extracts. Therefore, unless a trial specifically identifies a treatment sample as isolated diferuloylmethane, the terms curcuminoids (C) or C-fraction will be used in tables and text in the place of the term curcumin . Also, in this regard, chemical analyses of curcuminoid content in samples will be included in supplementary tables if reported in an article.
Details of 21 clinical trials assessing the effectiveness of turmeric powder or curcuminoid-containing supplements on signs and symptoms of arthritis are included in Supplemental Digital Content 1, https://links.lww.com/NT/A27 . A summary of these studies is presented in Table 1 . Most of these trials evaluated patients with knee osteoarthritis, and the patients enrolled were predominantly females (75%). Patient populations were derived primarily from the Middle East and Asia. The trials measured both intensity of pain and improvements in physical functioning using several indices. The Western Ontario and McMaster Universities Osteoarthritis Index, the Lequesne's Pain Functional Index, and the Japanese Knee Osteoarthritis Measure evaluate improvements in pain and functionality, whereas the visual analog scale measures severity of pain. The Disease Activity Score measures the number of swollen and tender joints; blood markers of inflammation, such as erythrocyte sedimentation rate and C-reactive protein (CRP); and the patient's global assessment of health. The American College of Rheumatology survey measures similar criteria as the Disease Activity Score. The Clinician Global Impression of Change measures joint tenderness, crepitus, alignment, movement, and muscle wasting.

The curcuminoids were administered orally in various formulations. Six trials evaluated the efficacy of simple curcuminoid formulations, 28–33 which essentially were turmeric powder or dried organic solvent (usually ethanol) extractions from this powder that may or may not have had the volatile oil portion removed. These samples contained varying ratios of curcuminoids and were administered at doses of 90 mg/d to 1.2 g/d for periods of 2 weeks to 4 months. When compared with baseline values, significant improvements in arthritic symptoms, such as stiffness, walking pain, or Western Ontario and McMaster Universities Osteoarthritis Index scores, were recorded for those receiving curcuminoids alone. 28,29,32 In comparisons of efficacy between curcuminoids and positive controls (nonsteroidal anti-inflammatory drugs [NSAIDs]), the potency of NSAID treatment was not superior to (not significantly different than) that of the curcuminoid preparations.
More than a dozen arthritis trials were performed using a variety of proprietary curcuminoid preparations engineered to improve oral bioavailability. All studies demonstrated some improvement in patients' arthritis symptoms, although changes in specific clinical measures varied among trials. Taken together, the trials that provided statistical comparisons of curcuminoid outcomes with those of placebo controls 34,38,42,43,45,47,48 reported significant improvement in either pain or physical function. For those trials evaluating curcuminoid formulations in comparison to an NSAID control group, comparable efficacy was observed. When curcuminoids were provided as adjuvants with diclofenac (a nonsteroidal anti-inflammatory agent) dosing, patient improvement was not consistently observed, compared with those treated with diclofenac alone. 30,33,41,44 Patients treated with curcuminoids in several trials 34,38,45,47 reported decreased use of rescue pain medications. It is noteworthy that a curcuminoid-free polysaccharide fraction isolated from turmeric (Turmacin) significantly decreased arthritis symptom scores, compared with placebo. 47 Polysaccharides from C longa have been reported to have anti-inflammatory and immunomodulatory activities, as have sesquiterpenoids in water extracts. 49–51 Systematic reviews of arthritis trials indicate that minor adverse gastrointestinal disturbances were the most common adverse effects of turmeric and curcuminoid administration, although curcuminoids generally exhibited a lower risk of these events compared with prescribed drugs. 52,53
To obtain insights into possible mechanisms of action, blood levels of CRP and inflammation-associated cytokines were measured in 7 trials, 35–39,42,43 but no consistent pattern of change was observed. One study 31 examined knee joint aspirations from patients and found that, when compared with baseline values, the administration of either curcuminoids or diclofenac resulted in similar, not significantly different, suppression of cyclooxygenase-2 secretion by synovial fluid monocytes. In this study, no data were provided for changes in physical symptoms; thus, determining if these changes in a biomarker for joint inflammation actually translated to relief of arthritic symptoms was not possible. This is important because the dose of curcuminoids (90 mg/d) given in this trial was low, compared with other trials.
Systematic reviews and meta-analyses of select arthritis trials provide evidence that supports the efficacy of curcuminoids in treating arthritis with fewer adverse effects than with NSAIDs. 35,43,52–58 However, a variety of trial limitations and shortcomings noted in these systematic reviews make recommendations for clinical use difficult to provide. The relatively small number of carefully performed randomized controlled trials (RCTs) is a significant limitation. The designs and methodological qualities of the trials varied considerably. Most often statistical analyses of treatment responses were performed by evaluating within-group differences between baseline and posttreatment values rather than between treatment and control or placebo groups. Some trials lacked controls altogether. Moreover, patient populations were small, the durations of treatments were short, long-term follow-up was lacking (eg, frequency of arthritic flare-ups and remissions), and baseline symptoms of patients among trials varied in severity. Chemical analyses of the formulations and extracts were not consistently provided. Nonetheless, collectively, these meta-analyses suggest that there is potential for curcuminoids to improve the quality of life of arthritis patients. Additional large, well-controlled trials are clearly warranted.
MetS and T2DM
In Supplemental Digital Content 2, https://links.lww.com/NT/A28 , the details of 62 clinical trials that evaluated the effects of turmeric powder or curcuminoid extracts on glucose and insulin regulation and serum lipid profiles are listed. Prior to 2009, only 6 trials were identified. Today, novel delivery systems increase the bioavailability of curcumin. Because of this, in the last 10 years, more than 50 reports have been published of trials investigating the impact of different forms and doses of oral turmeric and curcuminoids in healthy adults, as well as in those with T2DM and those with MetS. These trials are summarized in Table 2 . Human studies evaluating similar endpoints in subjects with nonalcoholic fatty liver disease (NAFLD) are included in Supplemental Digital Content 3, https://links.lww.com/NT/A29 .

In healthy adults, 6 studies evaluated curcuminoids at doses as high as 6 g/d for periods as long as 6 months. 59–64 Outcomes were inconsistent for measurements of blood glucose levels and lipid profiles. Similar disparate effects were observed in healthy adults when curcuminoids were provided in bioavailability-enhanced forms such as in solid lipid particles or as a phosphatidylcholine-phytosome complex. 65–67
In obese individuals, findings from 10 reports 68–77 show no consistent influence of curcuminoid treatment on oxidative stress biomarkers, serum levels of cytokines, growth factors, and hormones, or on blood pressure and blood glucose and lipid concentrations, compared with controls. Dose and form of curcuminoids as well as age and gender of subjects varied considerably among trials.
For those trials investigating subjects with prediabetes or hypercholesterolemia, 19,78–82 the limited number of studies precludes making generalities about the effectiveness of different turmeric or curcuminoid forms. However, of note, an alcohol extract of turmeric (1.5 g/d) given to prediabetic individuals for 9 months 78 significantly decreased fasting blood glucose (FBG), hemoglobin A 1c (HbA 1c ), and insulin resistance (IR), compared with controls. It also reduced the prevalence of newly diagnosed T2DM patients. This is one of the longest trials evaluating a health benefit of curcuminoids and underscores the importance of the long-term examination of this phytochemical's actions, especially if early stages in the progression to disease are evaluated. Also, when turmeric powder was given as a tea preparation in 1 trial, 79 improvements in blood lipid profile were observed, compared with controls, suggesting that curcuminoid administration as a beverage may be worthwhile to more routinely examine.
In 20 investigations, a variety of curcuminoid preparations were administered to subjects with T2DM. 83–102 For those trials evaluating raw turmeric powder or curcuminoid fractions, no consistent effects were noted for measures of FBG, HbA 1c , lipid profiles, antioxidant status, and IR. Doses administered were from 66 mg/d to 1.3 g/d for periods of 4 weeks to 6 months. Determination of doses was problematic, because some studies did not provide curcuminoid content within the powder or extracted samples. Two studies examined curcuminoids as an adjunct to drugs for treating hyperglycemia. In combination with metformin, curcuminoids decreased blood low-density lipoprotein (LDL) levels and had no effect on FBG, postprandial blood glucose, HbA 1c , and IR, compared with those given metformin alone. 91 In combination with the sulfonylurea glyburide, curcuminoid administration resulted in significant improvements in postprandial blood glucose and blood lipids, compared with glyburide alone, and also increased the total area under the first moment of the concentration-time curve for glyburide. 90 Use of curcuminoids as an adjunct to these types of drugs deserves more scrutiny, as does determining whether curcuminoids also can affect drug bioavailability. Of interest, 3 investigations 84,86,98 observed beneficial effects of these proprietary curcuminoid formulations on diabetic pathologies. This included decreases in foot microangiopathy, foot edema, and retinal edema, as well as in functional impairments. Microcirculation, visual acuity and blood flow improved. In light of these findings, future studies should document changes in diabetic pathologies.
The influence of curcuminoids on MetS was described in 14 reports. 103–116 For those studies of MetS in which powdered turmeric or curcuminoid fractions were evaluated, no consistent improvements in blood lipid and glucose levels or in insulin sensitivity were observed. 104,105,115,116 Of note, 1 trial measured liver morphology and detected a lower level of liver steatosis after 12 months of curcuminoid dosing, 101 compared with baseline. For those 13 MetS trials administering bioavailability-enhanced preparations, no consistent changes were observed in blood glucose and lipid levels, as well as for body mass index and biomarkers of antioxidant status, compared with controls.
For subjects with NAFLD, 5 different trials were identified in which 4 different turmeric preparations were separately evaluated (Supplemental Digital Content 3, https://links.lww.com/NT/A29 ). Compared with subjects administered placebo, NAFLD patients administered curcuminoids showed significant improvements in FBG for 3 of 4 trials, in HbA 1c for 2 of 3 trials, and in IR for 2 of 3 trials. 118–122 For 3 of 4 studies, serum total cholesterol (TC), triglycerides (TGs), and LDL significantly decreased, compared with placebo. No benefits of curcuminoid dosing on serum alanine aminotransferase and aspartate aminotransferase concentrations were observed. In a recent RCT, NAFLD patients were given 250 mg of a phospholipid form of curcuminoids for 8 weeks while examining changes in nuclear magnetic resonance spectroscopy (NMR)-based serum metabolic profiles. Compared with placebo, curcuminoid treatment variously altered levels of specific amino acids, tricarboxylic acid cycle intermediates, bile acids, and gut microbiota. 123 This may be a complementary approach to use in understanding the mechanisms of action of curcuminoids in this disorder.
Two trials of subjects with coronary artery disease showed no benefit of curcuminoid administration on serum lipid profiles or on blood glucose levels. 124,125
Recent systematic reviews and meta-analyses of select trials (that were judged to be of sufficient quality for analysis) shown in Table 2 reached different conclusions regarding the efficacy of curcuminoids for T2DM and MetS. Based on the meta-analysis of Azhdari et al 126 of MetS RCTs, 104–107,116,127 curcuminoid intake was associated with improvements in FBG, TG, and high-density lipoprotein (HDL) levels and diastolic blood pressure. Another meta-analysis 128 of prediabetes and T2DM trials, which were typically of 2- to 3-month duration, 78,83,85,88,89,92,93,129 found that curcuminoids significantly decreased HbA 1c levels in prediabetes and T2DM and decreased FBG levels in T2DM, but no significant improvement in lipid profiles was observed. It is worth noting that in this meta-analysis these authors conducted a separate sensitivity analysis to assess the statistical robustness of their findings. To do so, they removed a larger T2DM trial of longer duration (>3 months) from the analysis and found that the beneficial effects on glycemic outcomes in T2DM were no longer evident. Based on this assessment, the authors suggested that curcuminoid administration for shorter periods (2- to 3-month duration) might not be adequate to detect improvements in glycemic control in T2DM.
A meta-analysis of 7 trials 130 with patients demonstrating cardiovascular risk factors determined that turmeric and curcuminoids significantly decreased blood LDL and TG levels, but did not improve HDL or TC levels. This meta-analysis was not in agreement with the prior meta-analysis by Sahebkar, 131 which found no significant effect of curcuminoid dosing on any of the blood lipid parameters measured. Another meta-analysis of 26 RCTs of patients with MetS and related disorders 132 determined that curcuminoid administration was associated with significant decreases in FBG, IR, HbA 1c , TG, and TC, but not in LDL and HDL levels.
Several factors likely contributed to inconsistencies in outcomes among the trials shown in Table 2 , as well as in the conclusions from systematic reviews and meta-analyses. Rigor of statistical analyses of data varied considerably among RCTs. In some studies, outcomes measured in treatment and placebo groups at the conclusion of interventions were compared with baseline values within groups. In contrast, other trials statistically analyzed treatment group outcomes with those of appropriate controls. Trial populations were generally small and frequently from South East Asia, India, Iran, and Pakistan. Moreover, there was substantial heterogeneity among studies in participants' baseline characteristics, stage of disease at enrollment, gender, comorbidities, comedications (often not reported), trial duration, doses administered, characteristics of curcuminoid formulations, and subjects' diets and lifestyles. 126,128,130,132 It may be of value to analyze treatment effectiveness based on gender, because 2 trials detected different male versus female responses for blood ghrelin 103 and HbA 1c levels. 107
Mechanisms of Action
The mechanisms of action of curcumin in humans are not well understood. Inflammatory cytokines and biochemical markers of oxidative status were measured in some arthritis trials, and there is preliminary evidence from meta-analyses that curcuminoids may decrease biomarkers of systematic inflammation and increase serum adiponectin levels. 133–136 For trials of T2DM and MetS, there is preliminary evidence from meta-analyses that curcuminoids may reduce serum levels of CRP and leptin. 137,138
Preclinical experiments indicate that changes in cellular inflammatory responses, oxidative stress, chondrocyte destruction and renewal, and in osteoclastogenesis contribute to the antiarthritic action of curcuminoids. 139,140 Preclinical studies of glucose and lipid homeostasis indicate that curcuminoids can modulate insulin action, lipolysis, adipogenesis, lipoprotein function, and nutrient absorption. 126,132,136,141–143
Turmeric, its essential oils, and oleoresins are generally recognized as safe by the US Food and Drug Administration. A similar designation for curcuminoids has not yet been published by the Food and Drug Administration. Studies in animals indicate that curcuminoids have relatively low potential for toxicity. 54,144–146 In humans, the intake of turmeric powder as high as 8 g/d has apparently been tolerated with only minor adverse consequences, mainly gastrointestinal distress. 147 However, safe doses of more bioavailable formulations have not been established and possibly may be different and substantially less. Thus, more careful documentation in human trials of adverse effects of these novel curcuminoid delivery methods needs to be compiled. In this regard, it has been cautioned that a new bioavailable form of curcuminoid might “narrow its therapeutic window and lead to off-target toxicity.” 1 Additionally, the impact of curcuminoid dose on bioavailability and bioactivity of other ingested dietary components has received little attention. For example, data on curcuminoid intake and mineral absorption are conflicting. 148,149 There also needs to be an examination of potential drug interactions following curcuminoid administration, because both inhibitory and enhancing effects on drug efficacy in humans have been reported. 2,150–157 As with any herbal supplement, an awareness of reports of adverse consumer responses is important. Recently, Belgium's Federal Agency for Food Chain Safety and Italy's National Institute of Health warned consumers to avoid specific turmeric-containing supplements associated with an outbreak of acute cholestatic hepatitis ( https://www.nutraingredients.com/Article/2019/07/11/Belgium-recall-same-curcumin-based-supplement-linked-to-Italian-hepatitis-cases?utm_source=newsletter_daily# ).
Future Studies
Research findings on curcuminoids for the treatment of arthritis and possibly of T2DM are promising. A number of issues can be addressed in future investigations to improve their quality, reproducibility, and usefulness for crafting recommendations regarding curcuminoid's potential health benefits. Larger and longer-duration, high-quality RCTs are needed that study different population ages, gender and ethnic representation, baseline disease severity, and adverse effects and that use multiple dosing levels of chemically defined samples. As indicated previously, the bioavailability of curcuminoids from raw turmeric is exceptionally low, and the actual content of curcuminoids within the treatment sample, generally considered to be approximately 3%, is often not reported. The new delivery forms of curcuminoids may improve relief of disease symptoms compared with poorly absorbed raw turmeric and its simple, solvent extracts and thus deserve closer scrutiny. 130,131 Although different forms of bioavailable curcuminoids were administered in trials, no supportive data were provided substantiating that absorption and distribution necessarily improved in these patient populations. This is important because high interindividual variability in pharmacokinetics and nonlinear dose dependency were observed for one trial of a novel formulation. 158 Serum or urine levels of curcuminoids and/or their metabolites need to be measured in trials so as to assess relative bioavailability and compliance along with efficacy. 54,159 In addition, the influence of comedications on curcuminoid efficacy should be clarified. The potential of curcuminoids when used in combination with NSAIDs and hypoglycemic drugs to improve efficacy, reduce doses needed, and lower adverse effects of drugs warrants more thorough characterization.
In light of the evidence that the small amounts of curcuminoids in turmeric powder are very poorly bioavailable, culinary quantities of turmeric powder added to foods for sensory purposes are unlikely to provide meaningful health benefits for the conditions reviewed. Based on current, preliminary evidence from human trials, curcuminoid extracts and other novel formulations may have potential to help manage symptoms of T2DM, MetS, and especially arthritis. Yet, due to inconsistent findings from trials that differ substantially in quality, and due to incomplete understanding of curcuminoids' effective doses and duration and of their safety and their interactions with comedications, it is premature to recommend their supplemental use to improve health in a clinical setting or in the general population. Future larger, longer, high-quality RCTs are needed to better characterize any potential health benefits of this spice's bioactive constituents.
- Cited Here |
- Google Scholar
Supplemental Digital Content
- NT_00_00_2020_01_09_SINGLETARY_19045_SDC3.docx; [Word] (15 KB)
- NT_00_00_2020_01_09_SINGLETARY_19045_SDC1.docx; [Word] (26 KB)
- NT_00_00_2020_01_09_SINGLETARY_19045_SDC2.docx; [Word] (44 KB)
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Turmeric: an overview of potential health benefits, raw milk consumption: risks and benefits, body mass index: obesity, bmi, and health: a critical review, clove: overview of potential health benefits, red pepper: overview of potential health benefits.
Advertisement
Herbal Teas and their Health Benefits: A Scoping Review
- Review Article
- Published: 26 June 2019
- Volume 74 , pages 266–276, ( 2019 )
Cite this article

- Fatima S. Poswal 1 ,
- Grace Russell 1 ,
- Marion Mackonochie 2 ,
- Euan MacLennan 2 ,
- Emmanuel C. Adukwu 3 &
- Vivien Rolfe ORCID: orcid.org/0000-0002-5489-6194 2
9256 Accesses
83 Citations
87 Altmetric
12 Mentions
Explore all metrics
Herbal teas are used as therapeutic vehicles in many forms of traditional medicine and are a popular global beverage. The purpose of this scoping review was to examine the evidence relating to the clinical efficacy and safety of herbal teas, and to identify the main research themes and gaps in knowledge to inform further work. A scoping review methodology was followed that set out the research question and described the sourcing, selection and analysis of studies. Overall, a total of 145 research publications were retrieved from global bibliographic databases, and after applying exclusion criteria, 21 remained. These studies looked at herbal tea use in female health, diabetes, heart disease and weight loss, with plant species including lavender, chamomile, fenugreek, stinging nettle, spearmint, hibiscus, yerba maté, echinacea and combinations of herbs. Observational studies explored associations between herbal tea consumption and cancer risk, liver health, and the risks linked to the consumption of environmental contaminants in the plant material. Despite plant materials being the basis for drug discovery, and the popularity of herbal teas, the number of articles exploring clinical efficacy and safety is small. In this review we discuss how herbal teas may be beneficial in some areas of clinical and preventative health, and what further research is required to understand whether regular consumption can contribute to healthy living more generally.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
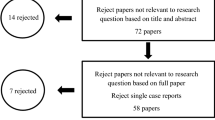
Impact of soft drinks to health and economy: a critical review

Ultra-processed Food and Obesity: What Is the Evidence?
Influence of diet on the gut microbiome and implications for human health.
World Health Organisation (2012) The top 10 causes of death. World Health Organisation. http://www.who.int/mediacentre/factsheets/fs310/en/ . Accessed 11 June 2019
Calder PC, Carding SR, Christopher G, Kuh D, Langley-Evans SC, McNulty H (2018) A holistic approach to healthy ageing: how can people live longer, healthier lives? J Hum Nutr Diet 31:439–450. https://doi.org/10.1111/jhn.12566
Article CAS PubMed Google Scholar
Schulze MB, Martínez-González MA, Fung TT, Lichtenstein AH, Forouhi NG (2018) Food based dietary patterns and chronic disease prevention. BMJ 361:k2396. https://doi.org/10.1136/bmj.k2396
Article PubMed PubMed Central Google Scholar
Lachat C, Raneri JE, Smith KW, Kolsteren P, Van Damme P, Verzelen K, Penafiel D, Vanhove W, Kennedy G, Hunter D, Odhiambo FO (2018) Dietary species richness as a measure of food biodiversity and nutritional quality of diets. Proc Natl Acad Sci 115:127–132. https://doi.org/10.1073/pnas.1709194115
Kromhout D, Spaaij CJK, De Goede J, Weggemans RM (2015) The 2015 Dutch food-based dietary guidelines. Eur J Clin Nutr 70:869–878. https://doi.org/10.1038/ejcn.2016.52
Article CAS Google Scholar
Khan N, Mukhtar H (2013) Tea and health: studies in humans. Curr Pharm Des 19:6141–6147. https://doi.org/10.2174/1381612811319340008
Article CAS PubMed PubMed Central Google Scholar
Suzuki Y, Miyoshi N, Isemura M (2012) Health-promoting effects of green tea. Proc Jpn Acad Ser B 88:88–101. https://doi.org/10.2183/pjab.88.88
Yang YC, Lu FH, Wu JS, Wu CH, Chang CJ (2004) The protective effect of habitual tea consumption on hypertension. Arch Intern Med 164:1534–1540. https://doi.org/10.1001/archinte.164.14.1534
Article PubMed Google Scholar
Tandon N, Yadav SS (2017) Contributions of Indian Council of Medical Research (ICMR) in the area of medicinal plants/traditional medicine. Ethnopharmacol 197:39–45. https://doi.org/10.1016/j.jep.2016.07.064
Article Google Scholar
Ritch-Krc EM, Thomas S, Turner NJ, Towers GHN (1996) Carrier herbal medicine: traditional and contemporary plant use. J Ethnopharmacol 52:85–94. https://doi.org/10.1016/0378-8741(96)01392-X
McKay DL, Blumberg JB (2006) A review of the bioactivity and potential health benefits of peppermint tea ( Mentha piperita L.). Phytother Res 20:619–633. https://doi.org/10.1002/ptr.1936
McKay DL, Blumberg JB (2006) A review of the bioactivity and potential health benefits of chamomile tea ( Matricaria recutita L.). Phytother Res 20:519–530. https://doi.org/10.1002/ptr.1900
World Health Organisation (2013) WHO traditional medicine strategy 2014–2023. World Health Organization. https://www.who.int/medicines/publications/traditional/trm_strategy14_23/en/ . Accessed 11 June 2019
Zhang AL, Xue CC, Fong HHS (2011) Integration of herbal medicine into evidence-based clinical practice. In: Benzie IFF, Wachtel-Galor S (eds) Herbal Medicine: biomolecular and clinical aspects, 2 nd edn. CRC Press, Taylor Francis
Das S, de Oliveira LM, da Silva E, Liu Y, Ma LQ (2017) Fluoride concentrations in traditional and herbal teas: health risk assessment. Environ Pollut 231:779–784. https://doi.org/10.1016/j.envpol.2017.08.083
Black C, Haughey SA, Chevallier OP, Galvin-King P, Elliott CT (2016) A comprehensive strategy to detect the fraudulent adulteration of herbs: the oregano approach. Food Chem 210:551–557. https://doi.org/10.1016/j.foodchem.2016.05.004
Cacchione PZ (2016) The evolving methodology of scoping reviews. Clin Nurs Res 25(2):115–119. https://doi.org/10.1177/1054773816637493
Tricco AC, Lillie E, Zarin W, O’Brien K, Colquhoun H, Kastner M, Levac D, Ng C, Sharpe JP, Wilson K, Kenny M (2016) A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol 16(1):15. https://doi.org/10.1186/s12874-016-0116-4
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269. https://doi.org/10.1371/journal.pmed.1000097
Narahari SR, Aggithaya MG, Suraj KR (2010) A protocol for systematic reviews of Ayurveda treatments. Int J Ayurveda Res 1:254–267. https://doi.org/10.4103/0974-7788.76791
World Health Organisation (2018) Constitution of WHO: principles. World Health Organisation. https://www.who.int/about/mission/en/ . Accessed 11 June 2019
Vidya TJ, Kulkarni KS (2002) Using herbal tea in the treatment modality: special reference to slimtea in overweight individuals. Anc Sci Life 21(3):202–204
CAS PubMed PubMed Central Google Scholar
Jayawardena MHS, De Alwis NMW, Hettigoda V, Fernando DJS (2005) A double blind randomised placebo controlled cross over study of a herbal preparation containing Salacia reticulata in the treatment of type 2 diabetes. J Ethnopharmacol 97(2):215–218. https://doi.org/10.1016/j.jep.2004.10.026
McKay DL, Chen CYO, Saltzman E, Blumberg JB (2009) Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. J Nutr 140(2):298–303. https://doi.org/10.3945/jn.109.115097
Mozaffari-Khosravi H, Jalali-Khanabadi BA, Afkhami-Ardekani M, Fatehi F, Noori-Shadkam M (2009) The effects of sour tea (Hibiscus sabdariffa) on hypertension in patients with type II diabetes. J Hum Hypertens 23(1):48–54. https://doi.org/10.1038/jhh.2008.100
Ryan EA, Imes S, Wallace C, Jones S (2000) Herbal tea in the treatment of diabetes mellitus. Clin Investig Med 23(5):311–317
CAS Google Scholar
Zemestani M, Rafraf M, Asghari-Jafarabadi M (2016) Chamomile tea improves glycemic indices and antioxidants status in patients with type 2 diabetes mellitus. Nutrition 32(1):66–72. https://doi.org/10.1016/j.nut.2015.07.011
Grant P (2010) Spearmint herbal tea has significant anti-androgen effects in polycystic ovarian syndrome. A randomized controlled trial. Phytother Res 24(2):186–188. https://doi.org/10.1002/ptr.2900
Akdoğan M, Tamer MN, Cüre E, Cüre MC, Köroğlu BK, Delibaş N (2007) Effect of spearmint ( Mentha spicata Labiatae) teas on androgen levels in women with hirsutism. Phytother Res 21(5):444–447. https://doi.org/10.1002/ptr.2074
Chen S, Chen C (2015) Effects of lavender tea on fatigue, depression, and maternal-infant attachment in sleep-disturbed postnatal women. Worldviews Evid Based Nurs 12(6):370–379. https://doi.org/10.1111/wvn.12122
Chang S, Chen C (2016) Effects of an intervention with drinking chamomile tea on sleep quality and depression in sleep disturbed postnatal women: a randomized controlled trial. J Adv Nurs 72(2):306–315. https://doi.org/10.1111/jan.12836
Ozalkaya E, Aslandoğdu Z, Özkoral A, Topcuoğlu S, Karatekin G (2018) Effect of a galactagogue herbal tea on breast milk production and prolactin secretion by mothers of preterm babies. Niger J Clin Pract 21(1):38–42. https://doi.org/10.4103/1119-3077.224788
Turkyılmaz C, Onal E, Hirfanoglu IM, Turan O, Koç E, Ergenekon E, Atalay Y (2011) The effect of galactagogue herbal tea on breast milk production and short-term catch-up of birth weight in the first week of life. J Altern Complement Med 17(2):139–142. https://doi.org/10.1089/acm.2010.0090
Kavurt S, Bas AY, Aydemir O, Yucel H, Isıkoglu S, Demirel N (2013) The effect of galactagogue herbal tea on oxidant and anti-oxidant status of human milk. J Matern Fetal Neonatal Med 26(10):1048–1051. https://doi.org/10.3109/14767058.2013.766690
Denzer MY, Kirsch F, Buettner A (2014) Are odorant constituents of herbal tea transferred into human milk? J Agric Food Chem 63(1):104–111. https://doi.org/10.1021/jf504073d
Maufrais C, Sarafian D, Dulloo A, Montani JP (2018) Cardiovascular and metabolic responses to the ingestion of caffeinated herbal tea: drink it hot or cold? Front Physiol 9:315. https://doi.org/10.3389/fphys.2018.00315
Lindenmuth GF, Lindenmuth EB (2000) The efficacy of echinacea compound herbal tea preparation on the severity and duration of upper respiratory and flu symptoms: a randomized, double-blind placebo-controlled study. J Altern Complement Med 6(4):327–334. https://doi.org/10.1089/10755530050120691
Chio PH, Zaroff CM (2015) Traditional Chinese medicinal herbal tea consumption, self-reported somatization, and alexithymia. Asia Pac Psychiatry 7(2):127–134. https://doi.org/10.1111/appy.12161
Riza E, Linos A, Petralias A, de Martinis L, Duntas L, Linos D (2015) The effect of Greek herbal tea consumption on thyroid cancer: a case-control study. Euro J Public Health 25(6):1001–1005. https://doi.org/10.1093/eurpub/ckv063
Colapinto CK, Arbuckle TE, Dubois L, Fraser W (2016) Is there a relationship between tea intake and maternal whole blood heavy metal concentrations? J Expo Sci Environ Epidemiol 26(5):503–509. https://doi.org/10.1038/jes.2015.86
Colapinto CK, Arbuckle TE, Dubois L, Fraser W (2015) Tea consumption in pregnancy as a predictor of pesticide exposure and adverse birth outcomes: the MIREC study. Environ Res 142:77–83. https://doi.org/10.1016/j.envres.2015.06.020
Alferink LJ, Fittipaldi J, Kiefte-de Jong JC, Taimr P, Hansen BE, Metselaar HJ, Schoufour JD, Ikram MA, Janssen HL, Franco OH, Murad SD (2017) Coffee and herbal tea consumption is associated with lower liver stiffness in the general population: the Rotterdam study. J Hepatol 67(2):339–348. https://doi.org/10.1016/j.jhep.2017.03.013
Gertsch J (2016) The metabolic plant feedback hypothesis: how plant secondary metabolites nonspecifically impact human health. Planta Med 82(11–12):920–929. https://doi.org/10.1055/s-0042-108340
Foti MC (2007) Antioxidant properties of phenols. J Pharm Pharmacol 59(12):1673–1685. https://doi.org/10.1211/jpp.59.12.0010
Pengelly A (2004) The constituents of medicinal plants: an introduction to the chemistry and therapeutics of herbal medicine. CABI Publishing
Booker A, Frommenwiler D, Johnston D, Umealajekwu C, Reich E, Heinrich M (2014) Chemical variability along the value chains of turmeric ( Curcuma longa ): a comparison of nuclear magnetic resonance spectroscopy and high performance thin layer chromatography. J Ethnopharmacol 152(2):292–301. https://doi.org/10.1016/j.jep.2013.12.042
Periche A, Koutsidis G, Escriche I (2014) Composition of antioxidants and amino acids in Stevia leaf infusions. Plant Foods Hum Nutr 69(1):1–7. https://doi.org/10.1007/s11130-013-0398-1
Moss M, Oliver L (2012) Plasma 1, 8-cineole correlates with cognitive performance following exposure to rosemary essential oil aroma. Ther Adv Psychopharmacol 2(3):103–113. https://doi.org/10.1177/2045125312436573
Horžić D, Komes D, Belščak A, Ganić KK, Iveković D, Karlović D (2009) The composition of polyphenols and methylxanthines in teas and herbal infusions. Food Chem 115(2):441–448. https://doi.org/10.1016/j.foodchem.2008.12.022
Bhebhe M, Chipurura B, Muchuweti M (2015) Determination and comparison of phenolic compound content and antioxidant activity of selected local Zimbabwean herbal teas with exotic Aspalathus linearis . S Afr J Bot 100:213–218. https://doi.org/10.1016/j.sajb.2015.06.006
Guimarães R, Barros L, Carvalho AM, Ferreira IC (2011) Infusions and decoctions of mixed herbs used in folk medicine: synergism in antioxidant potential. Phytother Res 25(8):1209–1214. https://doi.org/10.1002/ptr.3366
Yin SY, Wei WC, Jian FY, Yang NS (2013) Therapeutic applications of herbal medicines for cancer patients. Evid-Based Complement Alternat Med. https://doi.org/10.1155/2013/302426
Yin SY, Yang NS, Lin TJ (2017) Phytochemicals approach for developing cancer immune-therapeutics. Front Pharmacol 8:386. https://doi.org/10.3389/fphar.2017.00386
Tschiggerl C, Bucar F (2012) Guaianolides and volatile compounds in chamomile tea. Plant Foods Hum Nutr 67(2):129–135. https://doi.org/10.1007/s11130-012-0277-1
Singh O, Khanam Z, Misra N, Srivastava MK (2011) Chamomile ( Matricaria chamomilla L.): an overview. Pharmacogn Rev 5:82–95. https://doi.org/10.4103/0973-7847.79103
Seow YX, Yeo CR, Chung HL, Yuk HG (2014) Plant essential oils as active antimicrobial agents. Crit Rev Food Sci Nutr 54(5):625–644. https://doi.org/10.1080/10408398.2011.599504
Abdul Qadir M, Shahzadi SK, Bashir A, Munir A, Shahzad S (2017) Evaluation of phenolic compounds and antioxidant and antimicrobial activities of some common herbs. Int J Anal Chem. https://doi.org/10.1155/2017/3475738
Schoop R, Klein P, Suter A, Johnston SL (2006) Echinacea in the prevention of induced rhinovirus colds: a meta-analysis. Clin Ther 28(2):174–183. https://doi.org/10.1016/j.clinthera.2006.02.001
Schapowal A, Klein P, Johnston SL (2015) Echinacea reduces the risk of recurrent respiratory tract infections and complications: a meta-analysis of randomized controlled trials. Adv Ther 32(3):187–200. https://doi.org/10.1007/s12325-015-0194-4
Denner SS (2009) Lavandula angustifolia Miller: english lavender. Holist Nurs Pract 23(1):57–64. https://doi.org/10.1097/01.HNP.0000343210.56710.fc
Burns EE, Blamey C, Ersser SJ, Barnetson L, Lloyd AJ (2000) An investigation into the use of aromatherapy in intrapartum midwifery practice. J Altern Complement Med 6(2):141–147. https://doi.org/10.1089/acm.2000.6.141
Koulivand PH, Khaleghi Ghadiri M, Gorji A (2013) Lavender and the nervous system. Evid Based Complement Alternat Med 2013:1–10. https://doi.org/10.1155/2013/681304
Chrubasik JE, Roufogalis BD, Wagner H, Chrubasik S (2007) A comprehensive review on the stinging nettle effect and efficacy profiles. Part II: Urticae radix . Phytomedicine 14(7–8):568–579. https://doi.org/10.1016/j.phymed.2007.03.014
Wani SA, Kumar P (2018) Fenugreek: a review on its nutraceutical properties and utilization in various food products. J Saudi Soc Agric Sci 17(2):97–106. https://doi.org/10.1016/j.jssas.2016.01.007
Shawahna R, Qiblawi S, Ghanayem H (2018) Which benefits and harms of using fenugreek as a galactagogue need to be discussed during clinical consultations? A Delphi study among breastfeeding women, gynecologists, pediatricians, family physicians, lactation consultants, and pharmacists. Evid-Based Complementary Altern Med vol 2018, article ID 2418673. https://doi.org/10.1155/2018/2418673
Grant P, Ramasamy S (2012) An update on plant derived anti-androgens. Int J Endocrinol Metab 10(2):497–502. https://doi.org/10.5812/ijem.3644
Medagama AB (2015) Salacia reticulata ( Kothala himbutu ) revisited; a missed opportunity to treat diabetes and obesity? Nutr J 14:21. https://doi.org/10.1186/s12937-015-0013-4
Büyükbalci A, El SN (2008) Determination of in vitro antidiabetic effects, antioxidant activities and phenol contents of some herbal teas. Plant Foods Hum Nutr 63(1):27–33. https://doi.org/10.1007/s11130-007-0065-5
García-Conesa MT, Chambers K, Combet E, Pinto P, Garcia-Aloy M, Andrés-Lacueva C, de Pascual-Teresa S, Mena P, Konic Ristic A, Hollands W, Kroon P (2018) Meta-analysis of the effects of foods and derived products containing ellagitannins and anthocyanins on cardiometabolic biomarkers: analysis of factors influencing variability of the individual responses. Int J Mol Sci 19(3):E694. https://doi.org/10.3390/ijms19030694
Reis JF, Monteiro VVS, Gomes RS, Carmo MM, Costa GV, Ribera PC, Monteiro MC (2016) Action mechanism and cardiovascular effect of anthocyanins: a systematic review of animal and human studies. J Transl Med 14(1):315. https://doi.org/10.1186/s12967-016-1076-5
Daily JW, Yang M, Park S (2016) Efficacy of turmeric extracts and curcumin for alleviating the symptoms of joint arthritis: a systematic review and meta-analysis of randomized clinical trials. J Med Food 19(8):717–729. https://doi.org/10.1089/jmf.2016.3705
Kongkeaw C, Dilokthornsakul P, Thanarangsarit P, Limpeanchob N, Scholfield CN (2014) Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract. J Ethnopharmacol 151(1):528–535. https://doi.org/10.1016/j.jep.2013.11.008
Paredes-López O, Cervantes-Ceja ML, Vigna-Pérez M, Hernández-Pérez T (2010) Berries: improving human health and healthy aging, and promoting quality life—a review. Plant Foods Hum Nutr 65(3):299–308. https://doi.org/10.1007/s11130-010-0177-1
Makanjuola SA, Enujiugha VN (2017) Enhancing sensory perception of plant based nutraceutical drinks by combining plants from different sources: a preliminary study of tea and ginger blend. Prev Nutr Food Sci 22(4):372–375. https://doi.org/10.3746/pnf.2017.22.4.372
Son HU, Lee S, Heo JC, Lee SH (2017) The solid-state fermentation of Artemisia capillaris leaves with Ganoderma lucidum enhances the anti-inflammatory effects in a model of atopic dermatitis. Int J Mol Med 39(5):1233–1241. https://doi.org/10.3892/ijmm.2017.2945
Sheih IC, Fang TJ, Wu TK, Chang CH, Chen RY (2011) Purification and properties of a novel phenolic antioxidant from Radix astragali fermented by Aspergillus oryzae M29. J Agric Food Chem 59(12):6520–6525. https://doi.org/10.1021/jf2011547
Rolfe V (2018) Herbal tea safety and efficacy review. Figshare Dataset. https://doi.org/10.6084/m9.figshare.7398593.v1
Download references
Acknowledgements
This research was co-funded between the University of the West of England Internship Scheme and Pukka Herbs.
Author information
Authors and affiliations.
Department of Applied Sciences, University of the West of England, Frenchay Campus, Bristol, BS16 1QY, UK
Fatima S. Poswal & Grace Russell
Pukka Herbs, The Chocolate Factory, Bristol, BS31 2GN, UK
Marion Mackonochie, Euan MacLennan & Vivien Rolfe
Centre for Research in Biosciences, University of the West of England, Frenchay Campus, Bristol, BS16 1QY, UK
Emmanuel C. Adukwu
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Vivien Rolfe .
Ethics declarations
Conflict of interest.
Pukka Herbs is a manufacturer of herbal teas and supplements. The data relating to this research is shared openly for transparency.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Poswal, F.S., Russell, G., Mackonochie, M. et al. Herbal Teas and their Health Benefits: A Scoping Review. Plant Foods Hum Nutr 74 , 266–276 (2019). https://doi.org/10.1007/s11130-019-00750-w
Download citation
Published : 26 June 2019
Issue Date : 15 September 2019
DOI : https://doi.org/10.1007/s11130-019-00750-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Plant medicine
- Phytochemicals
- Plant biodiversity
- Find a journal
- Publish with us
- Track your research
Loading metrics
Open Access
Peer-reviewed
Research Article
Estimating impact of food choices on life expectancy: A modeling study
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations Department of Global Public Health and Primary Care, University of Bergen, Norway, Bergen Addiction Research, Department of Addiction Medicine, Haukeland University Hospital, Bergen, Norway
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing
Affiliations Department of Global Public Health and Primary Care, University of Bergen, Norway, Bergen Center for Ethics and Priority Setting, University of Bergen, Norway
Contributed equally to this work with: Øystein A. Haaland, Kjell Arne Johansson
Roles Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing
Roles Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing
Affiliations Department of Global Public Health and Primary Care, University of Bergen, Norway, Bergen Addiction Research, Department of Addiction Medicine, Haukeland University Hospital, Bergen, Norway, Bergen Center for Ethics and Priority Setting, University of Bergen, Norway
- Lars T. Fadnes,
- Jan-Magnus Økland,
- Øystein A. Haaland,
- Kjell Arne Johansson

- Published: February 8, 2022
- https://doi.org/10.1371/journal.pmed.1003889
- Peer Review
- Reader Comments
25 Mar 2022: Fadnes LT, Økland JM, Haaland ØA, Johansson KA (2022) Correction: Estimating impact of food choices on life expectancy: A modeling study. PLOS Medicine 19(3): e1003962. https://doi.org/10.1371/journal.pmed.1003962 View correction
Interpreting and utilizing the findings of nutritional research can be challenging to clinicians, policy makers, and even researchers. To make better decisions about diet, innovative methods that integrate best evidence are needed. We have developed a decision support model that predicts how dietary choices affect life expectancy (LE).
Methods and findings
Based on meta-analyses and data from the Global Burden of Disease study (2019), we used life table methodology to estimate how LE changes with sustained changes in the intake of fruits, vegetables, whole grains, refined grains, nuts, legumes, fish, eggs, milk/dairy, red meat, processed meat, and sugar-sweetened beverages. We present estimates (with 95% uncertainty intervals [95% UIs]) for an optimized diet and a feasibility approach diet. An optimal diet had substantially higher intake than a typical diet of whole grains, legumes, fish, fruits, vegetables, and included a handful of nuts, while reducing red and processed meats, sugar-sweetened beverages, and refined grains. A feasibility approach diet was a midpoint between an optimal and a typical Western diet. A sustained change from a typical Western diet to the optimal diet from age 20 years would increase LE by more than a decade for women from the United States (10.7 [95% UI 8.4 to 12.3] years) and men (13.0 [95% UI 9.4 to 14.3] years). The largest gains would be made by eating more legumes (females: 2.2 [95% UI 1.1 to 3.4]; males: 2.5 [95% UI 1.1 to 3.9]), whole grains (females: 2.0 [95% UI 1.3 to 2.7]; males: 2.3 [95% UI 1.6 to 3.0]), and nuts (females: 1.7 [95% UI 1.5 to 2.0]; males: 2.0 [95% UI 1.7 to 2.3]), and less red meat (females: 1.6 [95% UI 1.5 to 1.8]; males: 1.9 [95% UI 1.7 to 2.1]) and processed meat (females: 1.6 [95% UI 1.5 to 1.8]; males: 1.9 [95% UI 1.7 to 2.1]). Changing from a typical diet to the optimized diet at age 60 years would increase LE by 8.0 (95% UI 6.2 to 9.3) years for women and 8.8 (95% UI 6.8 to 10.0) years for men, and 80-year-olds would gain 3.4 years (95% UI females: 2.6 to 3.8/males: 2.7 to 3.9). Change from typical to feasibility approach diet would increase LE by 6.2 (95% UI 3.5 to 8.1) years for 20-year-old women from the United States and 7.3 (95% UI 4.7 to 9.5) years for men. Using NutriGrade, the overall quality of evidence was assessed as moderate. The methodology provides population estimates under given assumptions and is not meant as individualized forecasting, with study limitations that include uncertainty for time to achieve full effects, the effect of eggs, white meat, and oils, individual variation in protective and risk factors, uncertainties for future development of medical treatments; and changes in lifestyle.

Conclusions
A sustained dietary change may give substantial health gains for people of all ages both for optimized and feasible changes. Gains are predicted to be larger the earlier the dietary changes are initiated in life. The Food4HealthyLife calculator that we provide online could be useful for clinicians, policy makers, and laypeople to understand the health impact of dietary choices.
Author summary
Why was this study done.
- Food is fundamental for health, and globally dietary risk factors are estimated to cause 11 million deaths and 255 million disability-adjusted life years annually.
- The Global Burden of Diseases, Injuries, and Risk Factors study (GBD) provides summary measures of population health that are relevant when comparing health systems but does not estimate the impact of alterations in food group composition and respective health benefits.
- The EAT–Lancet commission did present a planetary diet, but it gives limited information on the health impact of other diets, and few people are able to adhere to strict health maximization approaches.
What did the researchers do and find?
- Our modeling methodology using meta-analyses, data from the Global Burden of Disease study and life table methodology showed that life expectancy (LE) gains for prolonged changes from typical Western to optimizing diets could translate into more than a decade for young adults.
- The largest gains would be made by eating more legumes, whole grains and nuts, and less red and processed meat.
- For older people, the gains would be smaller but substantial. Even the feasibility approach diet indicates increased LE by 7% or more for both sexes across age groups.
What do these findings mean?
- The online Food4HealthyLife calculator ( https://food4healthylife.org/ ) enables the instant estimation of the effect on LE of a range of dietary changes.
- Understanding the relative health potential of different food groups could enable people to make feasible and significant health gains.
- The Food4HealthyLife calculator could be a useful tool for clinicians, policy makers, and laypeople to understand the health impact of dietary choices.
Citation: Fadnes LT, Økland J-M, Haaland ØA, Johansson KA (2022) Estimating impact of food choices on life expectancy: A modeling study. PLoS Med 19(2): e1003889. https://doi.org/10.1371/journal.pmed.1003889
Academic Editor: Luigi Fontana, The University of Sydney, AUSTRALIA
Received: September 20, 2021; Accepted: December 11, 2021; Published: February 8, 2022
Copyright: © 2022 Fadnes et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting information files.
Funding: The authors received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations: 95% UI, 95% uncertainty interval; FA, feasibility approach diet; GBD, Global Burden of Disease study; HR a , alternative hazard ratio; LE, life expectancy; OD, optimized diet; TW, typical Western diet
Introduction
Food is fundamental for health. Globally, dietary risk factors are estimated to cause 11 million deaths and 255 million disability-adjusted life years annually [ 1 ]. Still, navigating within the nutritional research field can be overwhelming to clinicians, policy makers, and even researchers. Since 2017, about 250,000 scientific articles on nutritionally related topics have been published ( S1 Text ). Fortunately, several recent meta-analyses have summarized the impact on the risk of premature deaths for various food groups, including fruits, vegetables, whole grains and refined grains, nuts and legumes, fish, eggs, milk/dairy, red and processed meats, and sugar-sweetened beverages [ 2 – 6 ].
The Global Burden of Disease study (GBD) provides summary measures of population health that are relevant when comparing health systems [ 7 ]. GBD includes population-level estimates for life years lost due to some dietary risk factors [ 8 ], but such aggregated health metrics have little relevance when making individual decisions. The EAT–Lancet commission did present a planetary diet that presented a diet balancing health and environmental perspectives [ 9 ], but it gives limited information on the health impact of other diets, and few people are able to adhere to strict health maximization approaches [ 10 ]. Although the planetary diet and GBD risk factor estimates indicate directions of changes in food intake that are useful, more comprehensive models estimating the impact of various dietary choices on lifetime health are needed.
To better understand the impact on health of dietary choices, we have developed methodology that integrates and presents current knowledge. The availability of such methodology is essential in order to make informed dietary choices at all levels from individuals to policy makers [ 11 ]. In this paper, we present new methodology that allows for the estimation of how different diets affect sex- and age-specific life expectancy (LE).
The LE at a certain age is the number of years an individual at that age is expected to live before they die given a set of age-specific mortality rates. We used mortality rates extracted from GBD 2019 (published in 2020) [ 12 ]. Johansson and colleagues presented a framework for measuring LE from disease onset for specific conditions [ 13 ]. We modified this approach by considering “change in diet” as a condition that may have both a positive and a negative health impact. Conceptually, our approach can be summed up as follows:
- Let LE age (D) be the age-specific LE with prolonged change to diet D.
- LE age (D) is calculated using standard age-specific lifetable methodology, where annual mortality rates are adjusted according to the selected diet (i.e., mortality rates after the age when the diet is changed are multiplied with the hazard rate corresponding to the change). The baseline diet yields LE age (D 0 ).
- Life years gained (or lost) because of change from the baseline diet to diet D is now LE age (D)–LE age (D 0 ).
A more detailed description of the methodology to estimate background LE is given in Johansson and colleagues’ paper [ 13 ].
Recent meta-analyses provided dose–response data on the impact of various food groups on mortality for the following food groups: whole grains, fruits, vegetables, nuts, legumes, fish, eggs, milk/dairy, refined grains, red meat, processed meat, and sugar-sweetened beverages [ 2 – 5 ]. To identify meta-analyses on these food groups, a search in PubMed dated 26 April 2021 was screened and data extracted from these (see search string in S2 Text ). When several meta-analyses were available, we opted for the most comprehensive (usually the latest meta-analyses) with dose–response relationship data unless later less comprehensive meta-analyses argued well for excluding studies. For white meat, we did not have a complete dose–response curve, but a meta-analysis has suggested that the effect on mortality is neutral [ 14 ], which also was the case for small amounts of added oils [ 15 ]. Most of the studies were adjusted for intake of other food groups and factors such as smoking, exercise, body mass index, age, and sex. Each of the food groups were considered as individual protective or risk factors.
Diets vary between individuals and settings, but as the baseline in our model, we used a “typical Western diet” (TW) based on consumption data from the United States and Europe ( S3 Text ). The optimized diet (OD) values were set where dose–response data on consumption indicated no additional mortality gain in further increasing or decreasing intake (i.e., the impact on mortality plateaued). As a compromise between the TW and the optimal diet, we also considered a feasibility approach diet (FA), which was chosen as the midpoint for each food group between the typical diet and OD.
In each case, dietary intake was improved from the TW through feasible to optimal levels (rounded off):
- Whole grains (fresh weight): TW 50 g, FA 137.5 g, and OD 225 g (e.g., 2 thin slices of rye bread and 1 small bowl of whole grain cereal, and some whole grain rice). For whole grains, 225 g of fresh weight corresponds to about 75 g dry weight, equivalent of 7 servings/day);
- Vegetables: TW 250 g, FA 325 g, and OD 400 g (5 servings, e.g., 1 big tomato, 1 sweet pepper, mixed salad leaves, a half avocado, and a small bowl of vegetable soup);
- Fruits: TW 200 g, 300 g, and OD 400 g (5 servings, e.g., 1 apple, banana, orange, kiwi, and a handful of berries);
- Nuts: TW 0 g, FA 12.5 g, and OD 25 g (1 handful of nuts);
- Legumes: TW 0 g, FA 100 g, and OD 200 g (e.g., 1 big cup of soaked beans/lentils/peas);
- Fish: TW 50 g, FA 125 g, and OD 200 g (e.g., 1 big slice of herring);
- Eggs: TW 50 g, FA 37.5 g, and OD 25 g (half an egg);
- Milk/dairy: TW 300 g, FA 250 g, and OD 200 g (e.g., 1 cup of yoghurt);
- Refined grains: TW 150 g, FA 100 g, OD 50 g (e.g., refined grains in bread if mixed whole/refined bread);
- Red meat: TW 100 g, FA 50 g, and OD 0 g;
- Processed meat: TW 50 g, FA 25 g, and OD 0 g;
- White meat: TW 75 g, FA 62.5 g, and OD 50 g;
- Sugar-sweetened beverages: TW 500 g, FA 250 g, and OD 0 g;
- Added plant oils: TW 25 g, FA 25 g, and OD 25 g.
Other food groups were not considered. To avoid reporting estimates for insufficiently studied and unsustainable diet alternatives, the model does not report estimates if the total energy consumption for the diet input was below 4,000 kJ/day or above 16,000 kJ/day. Energy estimates per food group were obtained from a food content database [ 16 ]. The energy estimates were 8,085 kJ/day for TW, 7,850 kJ/day for FA, and 7,615 kJ/day for OD. The effect of energy restriction on longevity was not considered.
Health gains from diet changes are generally linked to reduction in cardiovascular disease, cancer, and diabetes mortality [ 2 – 5 ], all among the leading causes of mortality globally [ 17 ]. It has earlier been assumed that reversing the process of cardiovascular disease following reductions in major cardiovascular risk factors would require decades, but it has later been argued that cardiovascular disease mortality can change more quickly within a few years [ 18 , 19 ]. For cancers, the time perspective is likely to be longer. It has been indicated for associations between fruit and vegetable consumption and risk of lung cancer that associations for studies with more than 10 years of follow-up on fruits and vegetables are stronger than those with less than 10 years [ 20 ]. More evidence on the time perspective is available for risk factors such as tobacco, where meta-analyses for duration of smoking has indicated that associations between duration of tobacco smoking and risk of lung cancer is substantially higher with 50 years of smoking than 20 years of smoking [ 21 ]. To balance between the time perspectives related to both cardiovascular disease and cancer while weighting in the morbidity burden, we assumed that time to full effect was 10 years with a gradual, linear increase in effect (e.g., the effect was 20% of maximum after 2 years). We also conducted sensitivity analyses with 5 years, 30 years, and 50 years to full effect.
In addition to the 95% UIs, we report sensitivity adjusted uncertainty intervals where the central estimate of the model is based on HR 0 (i.e., m = 1), the lower interval is when m = 0.5 as and similarly the upper interval when m = 1.5.
Data on background mortality from 2019 for specific countries and regions were obtained from the freely available GBD cause of death database [ 12 ]. We extracted data for the United States, China, and Europe, as these are the regions from where most of the nutritional studies providing mortality estimates originate. Region-specific estimates on total mortality rates in 5-year age groups were also available from GBD. These were converted to single-year age-specific mortality rates in our model.
To assess the quality of evidence for each food group from the meta-analyses, we use NutriGrade, a version of GRADE adapted to nutritional studies [ 22 ]. Certainty of evidence is categorized as “very low” (0 to 3.99), “low” (4 to 5.99), “moderate” (6 to 7.99), or “high” (8 to 10). The quality of evidence was “high” for whole grains (NutriGrade score: 8), “moderate” for fish (7.75), processed meat (7.5), nuts (7), red meat (6.5), legumes (6), and dairy (6), “low” for vegetables (5.8), fruits (5.8), SSBs (5.5), and refined grains (5), and “very low” for eggs (3.8) and white meat (2). We further constructed an overall quality score by taking the mean of the NutriGrade scores for each of the food groups weighted by their absolute contribution to LE. The quality of the meta-analyses was assessed with the AMSTAR–2 tool [ 23 ]. The quality of the meta-analyses was rated as high for studies on all included meta-analyses [ 2 – 5 , 15 ], except for the meta-analysis on white meat that was rated as moderate [ 14 ].
We used the R package Shiny to create a web application ( https://food4healthylife.org/ ) that enables the estimation of the effect of a range of dietary changes ( S1 Fig ). In the left food panel (i.e., the diet before change), the defaults are set to the “typical diet.” The right food panel represents diet after change. Clicking the “ Optimal ” or “ Feasible ” button, the right panel of sliders are adjusted to the 2 OD and FA diet patterns. In this paper, we present estimated gain in LE when changing from a typical diet to OD or FA for 20-, 40-, 60-, and 80-year-old adults from the United States, China, and Europe. Graphs including forest plots are calculated in Stata SE 17.0 (including the admetan package).
Only publicly available data sources have been used, and thus no ethical permission is required. We adhered to the transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD; see S1 TRIPOD Checklist ) [ 24 ].
In this section, we will focus on the United States, but the results for China and Europe were generally very similar (can be found in S2 – S15 Figs). Table 1 and Fig 1 estimate the life expectancies at different ages associated with a typical Western diet, a feasibility approach diet, and an optimized diet. As seen, an increase in LE of up to 13.0 years (95% UI 9.4 to 14.3) is possible for male 20-year-olds from the United States by sustained dietary changes, and even for 80-year-olds, gains of 3.4 years (95% UI 2.7 to 3.9) are possible. Corresponding numbers for 20- and 80-year-old females are 10.7 years (95% UI 8.4 to 12.3) and 3.4 years (95% UI 2.6 to 3.8). Still, prolonged dietary changes at age 20 years would give about 48% higher gain in LE as changes starting from age 60 years, and 3 times the gains when compared with changes starting at age 80 years (Figs 2 and 3 ). Similar findings were seen for China and the United States. Changing from a typical diet to the feasibility approach diet would also give substantial gains for all age groups.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Gain in LE when changing from a typical Western diet to a feasibility approach or optimized diet is also indicated.
https://doi.org/10.1371/journal.pmed.1003889.t001
Estimates per food groups and total change in LE is presented with uncertainty intervals (UI). *The meta-evidence is high for whole grains; moderate for fish, nuts, legumes, processed and red meat, and sugar-sweetened beverages; and low for and very low for white meat. LE, life expectancy; 95% UI, 95% uncertainty interval.
https://doi.org/10.1371/journal.pmed.1003889.g001
https://doi.org/10.1371/journal.pmed.1003889.g002
Right plot presents similar estimates with a feasible approach* diet (time to full effect: 10 years). * For the optimal diet and feasibility approach diet, the following intakes were used: 225 g and 137.5 g whole grains (fresh weight), 400 g and 325 g vegetables, 400 g and/ 300 g fruits, 25 g and 12.5 g nuts, 200 g and/ 100 g legumes, 200 g and 100 g fish, 25 g and 37.5 g eggs, 200 g and 250 g milk/dairy, 50 g and 100 g refined grains, 0 g and 50 g red meat, 0 g and 25 g processed meat, 50 g and 62.5 g white meat, 0 g and 250 g sugar-sweetened beverages, and 25 g and 25 g added plant oils. Note that lines for LE for red and processed meat changes are overlapping and similarly also for white meat and added oils. LE, life expectancy.
https://doi.org/10.1371/journal.pmed.1003889.g003
When changing from a typical Western to an optimized diet, the largest gains in LE could be made by eating more legumes, whole grains, and nuts, as well as eating less red meat and processed meat, with gradual reduction in effect with increasing age ( Fig 2 and S2 Table ). For a 20-year-old from the United States, LE would increase by more than 1 year for each of these food groups. Fruits and vegetables as well as fish had substantial positive impact, but the intake in a typical diet is closer to an optimal intake than for legumes, whole grains, and nuts.
S3 Table indicates that when increasing time to full effect from 10 years to 30 years, gains in LE were reduced by less than 1 year for 20-year-olds (i.e., by 4% to 7%), but the gains for 60-year-olds and 80-year-olds were reduced by 35% to 71%. Conversely, decreasing time to full effect from 10 years to 5 years ( S16 Fig , S3 Table ), health gains for 20-year-old females and males increased by 0.1 to 0.2 years (i.e., by 1% to 2%), whereas gains increased by 0.5 to 0.8 years for 60-year-olds (i.e., 6% to 9%) and 1.2 to 1.3 years for 80-year-olds (i.e., 35% to 38%) ( S17 Fig ).
The overall quality of evidence was moderate for the optimized diet (NutriGrade score: 6.5) and identical for the feasibility approach diet (NutriGrade score: 6.5).
In this paper, we present a method for estimating the impact of food choices on LE. This method has been implemented in a tool that is freely available online—the Food4HealthyLife calculator. Our results indicate that for individuals with a typical Western diet, sustained dietary changes at any age may give substantial health benefits, although the gains are the largest if changes start early in life.
Eating more legumes, whole grains, and nuts, and eating less red meat and processed meats were estimated to be the most effective ways to increase LE for individuals with a typical diet. This reflects a combination of the health effect for each food group combined with the difference between typical and optimal intakes. Meta-analyses have also shown strong positive health effects from fruits, vegetables, and fish [ 2 , 5 ]. However, for these food groups, the typical intake was closer to optimal intake than for other food groups, particularly for vegetables. One could argue that for some food groups such as legumes, an optimal diet requires large intake and that such intakes might be unfeasible for many. Thus, we have also presented feasibility approach diet estimates that are closer to what we may realistically expect from diet changes of most people in most settings where ideals often are difficult to follow in practice. However, for most food groups, our estimates in the feasibility approach are within ranges that are common in cohort studies. There are also substantial individual variations in diet profile, which has impact on the potential health gain for each food group. As an example, some people have diets that are relatively similar to optimized diets and can expect less additional benefits from optimizing diets compared to individuals with a typical Western diet. Our food outcome calculation could take such variations at baseline into account by using different assumptions on nutrition starting points beyond what is presented here as default for a typical “Western diet.”
For several of the food groups, more than one meta-analysis is available. For red and processed meats, a more recent meta-analysis from 2019 than the one used in our estimates has been published [ 6 ]. However, this did not present dose–response data for red and processed meats separately, and the supplemental data for these groups combined indicated similar results as for the meta-analysis by Schwingshackl and colleagues. It is worthy to note that meta-analyses indicate worse outcomes on LE from processed meat than nonprocessed red meat when compared by weight, but if the consumption of unprocessed red meat consumption is double as high as for processed meat, the total effect is probably similar. For fish, whole grains, and legumes, more recent but smaller and less comprehensive meta-analyses were omitted from our data [ 25 – 27 ]. These also provided similar effect estimates to the estimates we used. For some food groups such as dairy products, fruits, and vegetables, systematic reviews of meta-analyses were available and supported the selection of the data sources [ 28 , 29 ]. For added oils, there were mixed results depending on type of oil, where monounsaturated fatty acids such as olive oil have been reported to have beneficial effects [ 15 , 30 , 31 ]. As most added oils contain a combination of different types of fatty acids, the general trend for health impact of added oils is often neutral [ 15 ]. Many of the background studies were adjusted for other food groups. It can be argued that food groups are interrelated and thus not independent. Studies presenting outcome measures with and without adjustment for other food groups have generally indicated minimal changes in the outcome measures [ 32 – 34 ]. To account for this possibility, we added sensitivity analyses model adjustment.
Our method has several strengths. First, our food impact estimates are from the most comprehensive and recent meta-analyses presenting dose–response data on diet patterns and mortality. We also have developed methodology that integrates different aspects such as time to full effects and potentially some degree of overlapping with sensitivity analyses and uncertainty intervals.
Our method also has several limitations. Meta-analyses present associations and some caution must be used when interpreting these. Still, meta-analyses are in many cases the best available evidence available as trials on diets could be challenging and, in several cases, could be unethical. Thus, emphasized several sensitivity analyses. For some food groups, meta-analyses presenting dose–response data were not available, which yield more uncertainty in model output.
The meta-analyses used in these data had high quality [ 23 ], while the meta-evidence ranged from very low (eggs and white meat) to high (whole grains) with most in the moderate quality category [ 22 ]. The overall meta-evidence was estimated as moderate for the optimal and feasibility approach diets. Still, the quality of the evidence for diet changes mostly involving eggs and white meat would be lower than when diet changes are dominated by whole grains, fish, processed meat, and nuts. This is reported in the tool for transparency. For added oils, it is likely that olive oils that are rich in monounsaturated fatty acids have beneficial effects and are probably superior to several other added oils [ 15 , 30 , 31 ]. However, we did not have sufficient data to present different oils separately.
GBD provides background epidemiological data for the populations we have presented but involves a combination of background data and modeling. We have no information on the impact on past morbidity experienced due to disease, and this was therefore not included in the model, although different health profiles may be associated with different impact of food choices. Thus, our estimates are based on population distributions of health indicators and do not account for differences in risk factors nor genetic vulnerability. The time perspective of diet change adds another layer of uncertainty. The duration of changes in the studies varies, and it is likely that short-term changes yield weaker effects than those presented in this article. We assumed 10 years to achieve full effects while conducting sensitivity analyses for both 5, 30, and 50 years. Still, progress in development of medical treatments and continuous changes in lifestyle can affect the impact of diet on LE and thus add uncertainty to our estimates [ 35 ]. Thus, the methodology is not meant as individualized forecasting of life years gained, but rather population estimates under certain assumptions.
Even though the diet approaches were relatively similar in energy, energy differences may have played a role in the relationships presented, and meta-analyses indicate that patterns in line with the optimal diet are likely to reduce the risk of obesity/overweight [ 36 ]. From the literature, we also know that one’s diet has a large impact on health-related quality of life [ 2 – 4 , 36 – 39 ]. Although we do not model nonfatal effects, LE is correlated with healthy life years. Most of the background data are adjusted for factors such as smoking, exercise, age, and sex. However, some residual confounding may still affect the estimates. Further, we have not considered any long-term health consequences that are due to sustained excessive intake of food with high levels of toxins, such as dioxins and polychlorinated biphenyls, which are relevant for some types of fish and sea foods [ 40 , 41 ]. This is more likely to overestimate than underestimate effect sizes. There is also a risk of overadjustment as some of the studies included in meta-analyses adjusted for potential intermediate factors. This may contribute to underestimating the full impact on dietary changes on health. Model development often have iterative improvements that will gradually give more precise estimates; however, the main messages are likely to be robust. Our sensitivity analyses indicate how the estimated changes in LE due to dietary changes vary if the true effects are over- or underestimated. Even the most conservative approaches indicate strong effects.
In conclusion, sustained change from a typical to an optimized diet from early age could translate into an increase in LE of more than 10 years. Gains are reduced substantially with delayed initiation of changes, particularly when approaching the age of 80 years. An increase in the intake of legumes, whole grains, and nuts, and a reduction in the intake of red meat and processed meats, contributed most to these gains. Fruits and vegetables also have a positive health impact, but for these food groups, the intake in a typical Western diet is closer to the optimal intake than for the other food groups. The Food4HealthyLife calculator could be a useful tool for both clinicians, policy makers, and laypeople to understand impact of various food choices.
Supporting information
S1 text. medline/pubmed search to estimate number of nutritional articles per year..
https://doi.org/10.1371/journal.pmed.1003889.s001
S2 Text. String used in PubMed to identify meta-analyses for setting hazard ratios.
https://doi.org/10.1371/journal.pmed.1003889.s002
S3 Text. Estimated intake of various food groups in the United States and Norway.
https://doi.org/10.1371/journal.pmed.1003889.s003
S1 Table. Hazard ratios (with uncertainty intervals) for various food groups with uncertainty limits (orange/red labels).
https://doi.org/10.1371/journal.pmed.1003889.s004
S2 Table. Increase in LE for each food group change for 20- and 60-year-old female and male adults from the United States, who change from a TW to OD or FA.
FA, feasibility approach diet; LE, life expectancy; OD, optimized diet; TW, typical Western diet.
https://doi.org/10.1371/journal.pmed.1003889.s005
S3 Table. Absolute and relative change in LE with delay to full effects of 10 (default), 5, 30, and 50 years for 20-, 40-, 60-, and 80-year-old females and males from the United States.
LE, life expectancy.
https://doi.org/10.1371/journal.pmed.1003889.s006
S1 Fig. Example of calculator input and output.
https://doi.org/10.1371/journal.pmed.1003889.s007
S2 Fig. Expected life years gained for 20-year-old female adults from China who change from a typical Western diet to an optimized or feasible approach diet with changes indicated in grams.
https://doi.org/10.1371/journal.pmed.1003889.s008
S3 Fig. Expected life years gained for 20-year-old male adults from China who change from a typical Western diet to an optimized or feasible approach diet with changes indicated in grams.
Estimates per food group and change in LE are presented with 95% UIs. LE, life expectancy; 95% UI, 95% uncertainty interval.
https://doi.org/10.1371/journal.pmed.1003889.s009
S4 Fig. Expected life years gained for 20-year-old female adults from Europe who change from a typical Western diet to an optimized or feasible approach diet with changes indicated in grams.
https://doi.org/10.1371/journal.pmed.1003889.s010
S5 Fig. Expected life years gained for 20-year-old male adults from Europe who change from a typical Western diet to an optimized or feasible approach diet with changes indicated in grams.
https://doi.org/10.1371/journal.pmed.1003889.s011
S6 Fig. Expected life years gained for 20-year-old female adults from the United States who change from a typical Western diet to an optimized or feasible approach diet.
https://doi.org/10.1371/journal.pmed.1003889.s012
S7 Fig. Expected life years gained for 20-year-old male adults from the United States who change from a typical Western diet to an optimized or feasible approach diet.
https://doi.org/10.1371/journal.pmed.1003889.s013
S8 Fig. Expected life years gained for 40-year-old female adults from the United States who change from a typical Western diet to an optimized or feasible approach diet.
https://doi.org/10.1371/journal.pmed.1003889.s014
S9 Fig. Expected life years gained for 40-year-old male adults from the United States who change from a typical Western diet to an optimized or feasible approach diet.
https://doi.org/10.1371/journal.pmed.1003889.s015
S10 Fig. Expected life years gained for 60-year-old female adults from the United States who change from a typical Western diet to an optimized or feasible approach diet.
https://doi.org/10.1371/journal.pmed.1003889.s016
S11 Fig. Expected life years gained for 60-year-old male adults from the United States who change from a typical Western diet to an optimized or feasible approach diet.
https://doi.org/10.1371/journal.pmed.1003889.s017
S12 Fig. Expected life years gained for 80-year-old female adults from the United States who change from a typical Western diet to an optimized or feasible approach diet.
https://doi.org/10.1371/journal.pmed.1003889.s018
S13 Fig. Expected life years gained for 80-year-old male adults from the United States who change from a typical Western diet to an optimized or feasible approach diet.
https://doi.org/10.1371/journal.pmed.1003889.s019
S14 Fig. Expected life years gained for 20-, 40-, 60-, and 80-year-old male and female adults from the US, China, and EU, who change from a typical Western diet to an optimized* (labeled “Optimal”) or a feasibility approach diet* (labeled “Feasible”).
Estimates for change in LE is presented with 95% UIs. *For the optimal diet and feasibility approach diet, the following intakes were used: 225/137.5 g whole grains (fresh weight), 400/325 g vegetables, 400/300 g fruits, 25/12.5 g nuts, 200/100 g legumes, 200/125 g fish, 25/37.5 g eggs, 200/250 g milk/dairy, 50/100 g refined grains, 0/50 g red meat, 0/25 g processed meat, 50/62.5 g white meat, 0/250 g sugar-sweetened beverages, and 25/25 g added plant oils. **F20 indicates 20-year-old females, and M60 indicates 60-year-old males. Uncertainty intervals for some food groups have rounding differences compared to corresponding S2 Table due to symmetrical adjustment in the admetan package in Stata. EU, Europe; LE, life expectancy; US, United States; 95% UI, 95% uncertainty interval.
https://doi.org/10.1371/journal.pmed.1003889.s020
S15 Fig. Expected life years gained for 20-, 40-, 60-, and 80-year-old male and female adults from the US, China, and EU, who change from a typical Western diet to an optimized* (labeled “Optimal”) or a feasibility approach diet* (labeled “Feasible”).
Estimates for change in LE is presented with sensitivity adjusted uncertainty intervals using lower interval as model adjustment of 0.5 and upper interval as model adjustment of 1.5. *For the optimal diet and feasibility approach diet, the following intakes were used: 225/137.5 g whole grains (fresh weight), 400/325 g vegetables, 400/300 g fruits, 25/12.5 g nuts, 200/100 g legumes, 200/125 g fish, 25/37.5 g eggs, 200/250 g milk/dairy, 50/100 g refined grains, 0/50 g red meat, 0/25 g processed meat, 50/62.5 g white meat, 0/250 g sugar-sweetened beverages, and 25/25 g added plant oils. **F20 indicates 20-year-old females, and M60 indicates 60-year-old males. Uncertainty intervals for some food groups have rounding differences compared to corresponding S2 Table due to symmetrical adjustment in the admetan package in Stata. EU, Europe; LE, life expectancy; US, United States.
https://doi.org/10.1371/journal.pmed.1003889.s021
S16 Fig. Expected increase in LE for optimizing different food groups with diet changes initiating from various ages between 20 and 80 years of age (time to full effect: 5 years).
* For the optimal diet and feasibility approach diet, the following intakes were used: 225 g and 137.5 g whole grains (fresh weight), 400 g and 325 g vegetables, 400 g and/ 300 g fruits, 25 g and 12.5 g nuts, 200 g and/ 100 g legumes, 200 g and 100 g fish, 25 g and 37.5 g eggs, 200 g and 250 g milk/dairy, 50 g and 100 g refined grains, 0 g and 50 g red meat, 0 g and 25 g processed meat, 50 g and 62.5 g white meat, 0 g and 250 g sugar-sweetened beverages, and 25 g and 25 g added plant oils. LE, life expectancy.
https://doi.org/10.1371/journal.pmed.1003889.s022
S17 Fig. Expected increase in LE for optimizing different food groups with diet changes initiating from various ages between 20 and 80 years of age (time to full effect: 30 years).
https://doi.org/10.1371/journal.pmed.1003889.s023
S1 TRIPOD Checklist. Checklist for prediction model development.
https://doi.org/10.1371/journal.pmed.1003889.s024
Acknowledgments
Thanks to Arngeir Berge for assistance with images.
- View Article
- PubMed/NCBI
- Google Scholar
- 8. Institute for Health Metrics and Evaluation and collaborators. Global Burden of Disease study (2017, 2016, 2015, 2013 and 2010). Lancet. 2018, 2017, 2016, 2014, 2012.
- 12. The Institute for Health Metrics and Evaluation (IHME). The Global Health Data Exchange (GHDx) Institute for Health Metrics and Evaluation, University of Washington. http://ghdx.healthdata.org/gbd-results-tool .
- 16. Mattilsynet. Matvaretabellen. https://www.matvaretabellen.no/?language=en .
- Open access
- Published: 03 March 2015
Health research improves healthcare: now we have the evidence and the chance to help the WHO spread such benefits globally
- Stephen R Hanney 1 &
- Miguel A González-Block 2
Health Research Policy and Systems volume 13 , Article number: 12 ( 2015 ) Cite this article
12k Accesses
21 Citations
16 Altmetric
Metrics details
There has been a dramatic increase in the body of evidence demonstrating the benefits that come from health research. In 2014, the funding bodies for higher education in the UK conducted an assessment of research using an approach termed the Research Excellence Framework (REF). As one element of the REF, universities and medical schools in the UK submitted 1,621 case studies claiming to show the impact of their health and other life sciences research conducted over the last 20 years. The recently published results show many case studies were judged positively as providing examples of the wide range and extensive nature of the benefits from such research, including the development of new treatments and screening programmes that resulted in considerable reductions in mortality and morbidity.
Analysis of specific case studies yet again illustrates the international dimension of progress in health research; however, as has also long been argued, not all populations fully share the benefits. In recognition of this, in May 2013 the World Health Assembly requested the World Health Organization (WHO) to establish a Global Observatory on Health Research and Development (R&D) as part of a strategic work-plan to promote innovation, build capacity, improve access, and mobilise resources to address diseases that disproportionately affect the world’s poorest countries.
As editors of Health Research Policy and Systems ( HARPS ), we are delighted that our journal has been invited to help inform the establishment of the WHO Global Observatory through a Call for Papers covering a range of topics relevant to the Observatory, including topics on which HARPS has published articles over the last few months, such as approaches to assessing research results, measuring expenditure data with a focus on R&D, and landscape analyses of platforms for implementing R&D. Topics related to research capacity building may also be considered. The task of establishing a Global Observatory on Health R&D to achieve the specified objectives will not be easy; nevertheless, this Call for Papers is well timed – it comes just at the point where the evidence of the benefits from health research has been considerably strengthened.
The start of 2015 sees a dramatic increase in the body of evidence demonstrating the benefits arising from health research. Throughout 2014, the higher education funding bodies in the UK conducted an assessment of research, termed the Research Excellence Framework (REF), in which, for the first time, account was taken of the impact on society of the research undertaken. As part of this, UK universities and medical schools produced 1,621 case studies that aimed to show the benefits, such as improved healthcare, arising from examples of their health and other life sciences research conducted over the last 20 years. Panels of experts, including leading academics from many countries, published their assessments of these case studies in December 2014 [ 1 ], with the full case studies and an analysis of the results being made public in January 2015 [ 2 , 3 ].
As we recently anticipated [ 4 ], the expert panels concluded that the case studies did indeed overwhelmingly illustrate the wide range and extensive nature of the benefits from health research. Main Panel A covered the range of life sciences and its overview report states: “ MPA [Main Panel A] believes that the collection of impact case studies provide a unique and powerful illustration of the outstanding contribution that research in the fields covered by this panel is making to health, wellbeing, wealth creation and society within and beyond the UK ” [ 3 ], p. 1. The section of the report covering public health and health services research also notes that: “ Outstanding examples included cases focused on national screening programmes for the selection and early diagnosis of conditions ” [ 3 ], p. 30. In their section of the report, the international experts say of the REF2014: “ It is the boldest, largest, and most comprehensive exercise of its kind of any country’s assessment of its science ” [ 3 ], p. 20.
The REF2014 is therefore attracting wide international attention. Indeed, some of the methods used are already informing studies in other countries, including, for example, an innovative assessment recently published in Health Research Policy and Systems ( HARPS ) identifying the beneficial effects made on healthcare policies and practice in Australia by intervention studies funded by the National Health and Medical Research Council [ 5 ].
The REF also illustrates that, even when focusing on the research from one country, there are examples of studies in which there has been international collaboration and which have built on research conducted elsewhere. For example, one REF case study on screening describes how a major UK randomised controlled trial of screening for abdominal aortic aneurysms (AAA) involving 67,800 men [ 6 , 7 ] was the most significant trial globally. The trial provided the main evidence for the policy to introduce national screening programmes for AAA for men reaching 65 throughout the UK [ 2 ]. The importance of this trial lay partly in its size, given that it accounted for over 50% of the men included in the meta-analyses performed in the 2007 Cochrane review [ 8 ] and the 2009 practice guideline from the US Society for Vascular Surgery [ 9 ]. Nevertheless, two of the three smaller studies that were also included in these two meta-analyses came from outside the UK, specifically from Denmark [ 10 ] and Australia [ 11 ].
Moreover, a recent paper published in HARPS also included descriptions of how the research contributing to new interventions often comes from more than one country. These accounts are included in a separate set of seven extensive case studies constructed to illustrate innovative ways to measure the time that can elapse between research being conducted and its translation into improved health [ 12 ]. While being a separate set of case studies, one of them does, nevertheless, explore the international timelines involved in research on screening for AAA, and, in addition to highlighting the key role of the UK research, it also highlights that the pioneering first screening study using ultrasound had been conducted in 1983 on 73 patients in a US Army medical base [ 13 ].
These case studies therefore further reinforce the well-established argument that health research progress often involves contributions from various countries. However, as has long been argued, not all populations fully share the benefits. In recognition of this, in May 2013, the World Health Assembly requested the World Health Organization (WHO), in its resolution 66.22, to establish a Global Observatory on Health Research and Development as part of a strategic work-plan to promote innovation, build capacity, improve access, and mobilise resources to address diseases that disproportionately affect the world’s poorest countries [ 14 ].
As editors of HARPS , we are delighted that our journal has been invited to help inform the establishment of the WHO Global Observatory by publishing a series of papers whose publication costs will be funded by the WHO. In support of this WHO initiative, Taghreed Adam, John-Arne Røttingen, and Marie-Paule Kieny recently published a Call for Papers for this series [ 15 ], which can be accessed through the HARPS webpage.
The aim of the series is “ to contribute state-of-the-art knowledge and innovative approaches to analyse, interpret, and report on health R&D information… [and] to serve as a key resource to inform the future WHO-convened coordination mechanism, which will be utilized to generate evidence-informed priorities for new R&D investments to be financed through a proposed new global financing and coordination mechanism for health R&D ” [ 15 ], p. 1. The Call for Papers covers a range of topics relevant to the aims of the Global Observatory. These include ones on which HARPS has published articles in the last few months, such as approaches to assessing research results, as seen in the Australian article described above [ 5 ]; papers measuring expenditure data with a focus on R&D, as described in a recent Commentary by Young et al. [ 16 ]; and landscape analyses of platforms for implementing R&D, as described in the article by Ongolo-Zogo et al. [ 17 ], analysing knowledge translation platforms in Cameroon and Uganda, and partially in the article by Yazdizadeh et al. [ 18 ], relaying lessons learnt from knowledge networks in Iran.
Adam et al. also make clear that the topics listed in the Call for Papers are examples and that the series editors are also willing to consider other areas [ 15 ]. Indeed, in the Introduction to the Call for Papers, the importance of capacity building is highlighted. This, too, is a topic described in recent papers in HARPS , such as those by Ager and Zarowsky [ 19 ], analysing the experiences of the Health Research Capacity Strengthening initiative’s Global Learning program of work across sub-Saharan Africa, and by Hunter et al. [ 20 ], describing needs assessment to strengthen capacity in water and sanitation research in Africa.
Finally, as we noted in our earlier editorial [ 4 ], the World Health Report 2013: Health Research for Universal Coverage showed how the demonstration of the benefits from health research could be a strong motivation for further funding of such research. As the Report states, “ adding impetus to do more research is a growing body of evidence on the returns on investments … there is mounting quantitative proof of the benefits of research to health, society and the economy ” [ 21 ]. We noted, too, that since the Report’s publication in 2013, there had been further examples from many countries of the benefits from medical research. The REF2014 in the UK signifies an additional major boost to the evidence that a wide range of health research does contribute to improved health and other social benefits. The results of such evaluations highlight the appropriateness of the WHO’s actions in attempting to ensure all populations share the benefits of health research endeavours by creating the Global Observatory on Health Research and Development. This will not be an easy task, but we welcome the opportunity afforded by the current Call for Papers for researchers and other stakeholders to engage with this process and influence it [ 15 ].
Abbreviations
Abdominal aortic aneurysms
Health Research Policy and Systems
Main Panel A
Research and development
Research Excellence Framework
- World Health Organization
Higher Education Funding Council for England. Research Excellence Framework 2014: The results. 2014. http://www.ref.ac.uk/pubs/201401/ . Accessed 20 Feb 2015.
Higher Education Funding Council for England. Research Excellence Framework 2014: Results and submissions. 2015. http://results.ref.ac.uk/ . Accessed 20 Feb 2015.
Higher Education Funding Council for England. REF 2014 Panel overview reports: Main Panel A and sub-panels 1–6. 2015. http://www.ref.ac.uk/media/ref/content/expanel/member/Main%20Panel%20A%20overview%20report.pdf . Accessed 20 Feb 2015.
Hanney SR, González-Block MA. Four centuries on from Bacon: progress in building health research systems to improve health systems? Health Res Policy Syst. 2014;12:56.
Article PubMed PubMed Central Google Scholar
Cohen G, Schroeder J, Newson R, King L, Rychetnik L, Milat AJ, et al. Does health intervention research have real world policy and practice impacts: testing a new impact assessment tool. Health Res Policy Syst. 2015;13:3.
Scott RAP, Ashton HA, Buxton M, Day NE, Kim LG, Marteau TM, et al. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360:1531–9.
Article PubMed Google Scholar
Buxton M, Ashton H, Campbell H, Day NE, Kim LG, Marteau TM, et al. Multicentre aneurysm screening study (MASS): cost effectiveness analysis of screening for abdominal aortic aneurysms based on four year results from randomised controlled trial. BMJ. 2002;325:1135–8.
Article Google Scholar
Cosford PA, Leng GC, Thomas J: Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev 2007; (2)CD002945.
Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. The care of patients with an abdominal aortic aneurysm: the Society for Vascular Surgery practice guidelines. J Vasc Surg. 2009;50(4 Suppl):S2–49.
Lindholt JS, Juul S, Fasting H, Henneberg EW. Hospital costs and benefits of screening for abdominal aortic aneurysms. Results from a randomised population screening trial. Eur J Vasc Endovasc Surg. 2002;23:55–60.
Article CAS PubMed Google Scholar
Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ. 2004;329(7477):1259–64.
Hanney SR, Castle-Clarke S, Grant J, Guthrie S, Henshall C, Mestre-Ferrandiz J, et al. How long does biomedical research take? Studying the time taken between biomedical and health research and its translation into products, policy, and practice. Health Res Policy Syst. 2015;13:1.
Cabellon S, Moncrief CL, Pierre DR, Cavenaugh DG. Incidence of abdominal aortic aneurysms in patients with artheromatous arterial disease. Am J Surg. 1983;146:575–6.
World Health Organization. WHA resolution 66.22: Follow up of the report of the Consultative Expert Working Group on Research and Development: financing and coordination. Geneva: WHO; 2013. http://www.who.int/phi/resolution_WHA-66.22.pdf . Accessed 20 Feb 2015.
Google Scholar
Adam T, Røttingen JA, Kieny MP. Informing the establishment of the WHO Global Observatory on Health Research and Development: a call for papers. Health Res Policy Syst. 2015;13:9.
Young AJ, Terry RF, Røttingen JA, Viergever RF. Global trends in health research and development expenditures – the challenge of making reliable estimates for international comparison. Health Res Policy Syst. 2015;13:7.
Ongolo-Zogo P, Lavis JN, Tomson G, Sewankambo NK. Climate for evidence informed health system policymaking in Cameroon and Uganda before and after the introduction of knowledge translation platforms: a structured review of governmental policy documents. Health Res Policy Syst. 2015;13:2.
Yazdizadeh B, Majdzadeh R, Alami A, Amrolalaei S. How can we establish more successful knowledge networks in developing countries? Lessons learnt from knowledge networks in Iran. Health Res Policy Syst. 2014;12:63.
Ager A, Zarowsky C. Balancing the personal, local, institutional, and global: multiple case study and multidimensional scaling analysis of African experiences in addressing complexity and political economy in health research capacity strengthening. Health Res Policy Syst. 2015;13:5.
Hunter PR, Abdelrahman SH, Antwi-Agyei P, Awuah E, Cairncross S, Chappell E, et al. Needs assessment to strengthen capacity in water and sanitation research in Africa: experiences of the African SNOWS consortium. Health Res Policy Syst. 2014;12:68.
The World Health Organization. The World Health Report 2013: Research for Universal Health Coverage. Geneva: WHO; 2013.
Download references
Acknowledgements
The authors thank Bryony Soper for most helpful comments on an earlier draft.
Author information
Authors and affiliations.
Health Economics Research Group, Brunel University London, Kingston Lane, Uxbridge, UB8 3PH, UK
Stephen R Hanney
Universidad Anáhuac, Av. Universidad Anáhuac 46, Lomas Anáhuac, 52786 Huixquilucan, Mexico City, Mexico
Miguel A González-Block
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Stephen R Hanney .
Additional information
Competing interests.
The authors are co-Chief Editors of Health Research Policy and Systems.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/4.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Hanney, S.R., González-Block, M.A. Health research improves healthcare: now we have the evidence and the chance to help the WHO spread such benefits globally. Health Res Policy Sys 13 , 12 (2015). https://doi.org/10.1186/s12961-015-0006-y
Download citation
Received : 20 February 2015
Accepted : 20 February 2015
Published : 03 March 2015
DOI : https://doi.org/10.1186/s12961-015-0006-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Assessing research benefits
- Capacity building
- Diseases of poorest countries
- Global health
- Global Observatory
- Platforms for research implementation
- Research expenditure
- Screening for abdominal aortic aneurysms
- World Health Report 2013
ISSN: 1478-4505
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
Health-promoting potential of millet: a review.

Graphical Abstract
1. Introduction
2. methodology, 3. nutritional quality of millets, 4. beneficial features of millet, 4.1. antioxidant activity, 4.2. anti-hyperglycemic effects, 4.3. anti-cholesterol effects, 4.4. anti-hypertensive effects, 4.5. anthropometric effects, 4.6. effects on gut microbiota composition, 5. conclusions, author contributions, data availability statement, acknowledgments, conflicts of interest, abbreviations.
- Tripathi, M.K.; Jadam, R.S.; Kumar, A. Quality Management System in Millet and Sorghum. In Millets and Millet Technology ; Springer: Berlin/Heidelberg, Germany, 2021; pp. 363–379. [ Google Scholar ] [ CrossRef ]
- FAO. World Food and Agriculture—Statistical Yearbook ; FAO: Rome, Italy, 2020. [ Google Scholar ]
- Saini, S.; Saxena, S.; Samtiya, M.; Puniya, M.; Dhewa, T. Potential of underutilized millets as Nutri-cereal: An overview. J. Food Sci. Technol. 2021 , 58 , 4465–4477. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ertop, M.H.; Bektaş, M. Enhancement of bioavailable micronutrients and reduction of antinutrients in foods with some processes. Food Health 2018 , 4 , 159–165. [ Google Scholar ] [ CrossRef ]
- Yin, D.; Yin, X.; Wang, X.; Lei, Z.; Wang, M.; Guo, Y.; Aggrey, S.E.; Nie, W.; Yuan, J. Supplementation of amylase combined with glucoamylase or protease changes intestinal microbiota diversity and benefits for broilers fed a diet of newly harvested corn. J. Anim. Sci. Biotechnol. 2018 , 9 , 24. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Sharma, N.; Niranjan, K. Foxtail millet: Properties, processing, health benefits, and uses. Food Rev. Int. 2017 , 34 , 329–363. [ Google Scholar ] [ CrossRef ]
- Asrani, P.; Ali, A.; Tiwari, K. Millets as an alternative diet for gluten-sensitive individuals: A critical review on nutritional components, sensitivities and popularity of wheat and millets among consumers. Food Rev. Int. 2022 , 38 , 1–30. [ Google Scholar ] [ CrossRef ]
- Yang, X.; Zhang, S.; He, C.; Xue, P.; Zhang, L.; He, Z.; Zang, L.; Feng, B.; Sun, J.; Zheng, M. METTL14 suppresses proliferation and metastasis of colorectal cancer by down-regulating oncogenic long non-coding RNA XIST. Mol. Cancer 2020 , 19 , 46. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ren, X.; Chen, J.; Wang, C.; Molla, M.M.; Diao, X.; Shen, Q. In vitro starch digestibility, degree of gelatinization and estimated glycemic index of foxtail millet-derived products: Effect of freezing and frozen storage. J. Cereal Sci. 2016 , 69 , 166–173. [ Google Scholar ] [ CrossRef ]
- Akinola, S.A.; Badejo, A.A.; Osundahunsi, O.F.; Edema, M.O. Effect of preprocessing techniques on pearl millet flour and changes in technological properties. Int. J. Food Sci. Technol. 2017 , 52 , 992–999. [ Google Scholar ] [ CrossRef ]
- Ganguly, S.; Sabikhi, L.; Singh, A.K. Effect of whey-pearl millet-barley based probiotic beverage on Shigella-induced pathogenicity in murine model. J. Funct. Foods 2019 , 54 , 498–505. [ Google Scholar ] [ CrossRef ]
- Sarita; Singh, E. Potential of Millets: Nutrients Composition and Health Benefits. J. Sci. Innov. Res. 2016 , 5 , 46–50. [ Google Scholar ] [ CrossRef ]
- Srilekha, K.; Kamalaja, T.; Maheswari, K.U.; Rani, R.N. Nutritional Composition of Little Millet Flour. Int. Res. J. Pure Appl. Chem. 2019 , 31 , 1–4. [ Google Scholar ] [ CrossRef ]
- Tyl, C.; Marti, A.; Hayek, J.; Anderson, J.; Ismail, B.P. Effect of growing location and variety on nutritional and functional properties of proso millet ( Panicum miliaceum ) grown as a double crop. Cereal Chem. 2018 , 95 , 288–301. [ Google Scholar ] [ CrossRef ]
- Das, S.; Khound, R.; Santra, M.; Santra, D.K. Beyond Bird Feed: Proso Millet for Human Health and Environment. Agriculture 2019 , 9 , 64. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Almaski, A.; Thondre, S.; Lightowler, H.; Coe, S. Determination of the polyphenol and antioxidant activity of different types and forms of millet. Proc. Nutr. Soc. 2017 , 76 , E5. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ramadoss, D.P.; Sivalingam, N. Vanillin extracted from Proso and Barnyard millets induce apoptotic cell death in HT-29 human colon cancer cell line. Nutr. Cancer 2019 , 72 , 1422–1437. [ Google Scholar ] [ CrossRef ]
- Anis, M.A.; Sreerama, Y.N. Inhibition of protein glycoxidation and advanced glycation end-product formation by barnyard millet (Echinochloa frumentacea) phenolics. Food Chem. 2020 , 315 , 126265. [ Google Scholar ] [ CrossRef ]
- Tripathi, M.K.; Mohapatra, D.; Jadam, R.S.; Pandey, S.; Singh, V.; Kumar, V.; Kumar, A. Nutritional Composition of Millets. In Millets and Millet Technology ; Springer: Berlin/Heidelberg, Germany, 2021; pp. 101–119. [ Google Scholar ]
- Abah, C.R.; Ishiwu, C.N.; Obiegbuna, J.E.; Oladejo, A.A. Nutritional Composition, Functional Properties and Food Applications of Millet Grains. Asian Food Sci. J. 2020 , 14 , 9–19. [ Google Scholar ] [ CrossRef ]
- Saleh, A.S.; Zhang, Q.; Chen, J.; Shen, Q. Millet Grains: Nutritional Quality, Processing, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2013 , 12 , 281–295. [ Google Scholar ] [ CrossRef ]
- Amadou, I.; Gounga, M.E.; Le, G.-W. Millets: Nutritional Composition, Some Health Benefits and Processing—A Review. Emir. J. Food Agric. 2013 , 25 , 501–508. [ Google Scholar ] [ CrossRef ]
- Molla, M.M. Effect of Foxtail Millet Diet on Liver Injury and Blood Lipid Profile Induced by D-Galactosamine in Mice. Ph.D. Thesis, China Agricultural University (CAU), Beijing, China, 2016. [ Google Scholar ]
- Ciulu, M.; Cádiz-Gurrea, M.d.l.L.; Segura-Carretero, A. Extraction and Analysis of Phenolic Compounds in Rice: A Review. Molecules 2018 , 23 , 2890. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Xiang, J.; Apea-Bah, F.B.; Ndolo, V.U.; Katundu, M.C.; Beta, T. Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chem. 2018 , 275 , 361–368. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Slama, A.; Cherif, A.; Sakouhi, F.; Boukhchina, S.; Radhouane, L. Fatty acids, phytochemical composition and antioxidant potential of pearl millet oil. J. Consum. Prot. Food Saf. 2019 , 15 , 145–151. [ Google Scholar ] [ CrossRef ]
- Yuan, Y.; Xiang, J.; Zheng, B.; Sun, J.; Luo, D.; Li, P.; Fan, J. Diversity of phenolics including hydroxycinnamic acid amide derivatives, phenolic acids contribute to antioxidant properties of proso millet. Lwt 2021 , 154 , 112611. [ Google Scholar ] [ CrossRef ]
- Xiang, J.; Zhang, M.; Apea-Bah, F.B.; Beta, T. Hydroxycinnamic acid amide (HCAA) derivatives, flavonoid C-glycosides, phenolic acids and antioxidant properties of foxtail millet. Food Chem. 2019 , 295 , 214–223. [ Google Scholar ] [ CrossRef ]
- Saha, D.; Gowda, M.V.C.; Arya, L.; Verma, M.; Bansal, K.C. Genetic and Genomic Resources of Small Millets. Crit. Rev. Plant Sci. 2016 , 35 , 56–79. [ Google Scholar ] [ CrossRef ]
- Chandrasekara, A.; Naczk, M.; Shahidi, F. Effect of processing on the antioxidant activity of millet grains. Food Chem. 2011 , 133 , 1–9. [ Google Scholar ] [ CrossRef ]
- Abedin, J.; Abdullah, A.T.M.; Satter, M.A.; Farzana, T. Physical, functional, nutritional and antioxidant properties of foxtail millet in Bangladesh. Heliyon 2022 , 8 , e11186. [ Google Scholar ] [ CrossRef ]
- Liang, S.; Liang, K. Millet grain as a candidate antioxidant food resource: A review. Int. J. Food Prop. 2019 , 22 , 1652–1661. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Sharma, B.; Gujral, H.S. Influence of nutritional and antinutritional components on dough rheology and in vitro protein & starch digestibility of minor millets. Food Chem. 2019 , 299 , 125115. [ Google Scholar ] [ CrossRef ]
- Anitha, S.; Kane-Potaka, J.; Tsusaka, T.W.; Botha, R.; Rajendran, A.; Givens, D.I.; Parasannanavar, D.J.; Subramaniam, K.; Prasad, K.D.V.; Vetriventhan, M.; et al. A Systematic Review and Meta-Analysis of the Potential of Millets for Managing and Reducing the Risk of Developing Diabetes Mellitus. Front. Nutr. 2021 , 8 , 386. [ Google Scholar ] [ CrossRef ]
- Ren, X.; Yin, R.; Hou, D.; Xue, Y.; Zhang, M.; Diao, X.; Zhang, Y.; Wu, J.; Hu, J.; Hu, X.; et al. The Glucose-Lowering Effect of Foxtail Millet in Subjects with Impaired Glucose Tolerance: A Self-Controlled Clinical Trial. Nutrients 2018 , 10 , 1509. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Shobana, S.; Harsha, M.R.; Platel, K.; Srinivasan, K.; Malleshi, N.G. Amelioration of Hyperglycaemia and Its Associated Complications by Finger Millet ( Eleusine Coracana L.) Seed Coat Matter in Streptozotocin-Induced Diabetic Rats. Br. J. Nutr. 2010 , 104 , 1787–1795. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Fu, Y.; Yin, R.; Liu, Z.; Niu, Y.; Guo, E.; Cheng, R.; Diao, X.; Xue, Y.; Shen, Q. Hypoglycemic Effect of Prolamin from Cooked Foxtail Millet ( Setaria italic ) on Streptozotocin-Induced Diabetic Mice. Nutrients 2020 , 12 , 3452. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Seo, K.-H.; Ra, J.-E.; Lee, S.-J.; Lee, J.H.; Kim, S.R.; Lee, J.H.; Seo, W.D. Anti-hyperglycemic activity of polyphenols isolated from barnyard millet ( Echinochloa utilis L.) and their role inhibiting α-glucosidase. J. Korean Soc. Appl. Biol. Chem. 2015 , 58 , 571–579. [ Google Scholar ] [ CrossRef ]
- Krishnan, V.; Verma, P.; Saha, S.; Singh, B.; Vinutha, T.; Kumar, R.R.; Kulshreshta, A.; Singh, S.P.; Sathyavathi, T.; Sachdev, A.; et al. Polyphenol-Enriched Extract from Pearl Millet (Pennisetum Glaucum) Inhibits Key Enzymes Involved in Post Prandial Hyper Glycemia (α-Amylase, α-Glucosidase) and Regulates Hepatic Glucose Uptake. Biocatal. Agric. Biotechnol. 2022 , 43 , 102411. [ Google Scholar ] [ CrossRef ]
- Alzahrani, N.S.; Alshammari, G.M.; El-Ansary, A.; Yagoub, A.E.A.; Amina, M.; Saleh, A.; Yahya, M.A. Anti-Hyperlipidemia, Hypoglycemic, and Hepatoprotective Impacts of Pearl Millet (Pennisetum Glaucum L.) Grains and Their Ethanol Extract on Rats Fed a High-Fat Diet. Nutrients 2022 , 14 , 1791. [ Google Scholar ] [ CrossRef ]
- Park, K.-O.; Ito, Y.; Nagasawa, T.; Choi, M.-R.; Nishizawa, N. Effects of Dietary Korean Proso-Millet Protein on Plasma Adiponectin, HDL Cholesterol, Insulin Levels, and Gene Expression in Obese Type 2 Diabetic Mice. Biosci. Biotechnol. Biochem. 2008 , 72 , 2918–2925. [ Google Scholar ] [ CrossRef ]
- Anitha, S.; Botha, R.; Kane-Potaka, J.; Givens, D.I.; Rajendran, A.; Tsusaka, T.W.; Bhandari, R.K. Can Millet Consumption Help Manage Hyperlipidemia and Obesity? A Systematic Review and Meta-Analysis. Front. Nutr. 2021 , 8 , 478. [ Google Scholar ] [ CrossRef ]
- Lee, S.H.; Chung, I.-M.; Cha, Y.-S.; Park, Y. Millet consumption decreased serum concentration of triglyceride and C-reactive protein but not oxidative status in hyperlipidemic rats. Nutr. Res. 2010 , 30 , 290–296. [ Google Scholar ] [ CrossRef ]
- Nishizawa, N.; Togawa, T.; Park, K.-O.; Sato, D.; Miyakoshi, Y.; Inagaki, K.; Ohmori, N.; Ito, Y.; Nagasawa, T. Dietary Japanese Millet Protein Ameliorates Plasma Levels of Adiponectin, Glucose, and Lipids in Type 2 Diabetic Mice. Biosci. Biotechnol. Biochem. 2009 , 73 , 351–360. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Choi, Y.-Y.; Osada, K.; Ito, Y.; Nagasawa, T.; Choi, M.-R.; Nishizawa, N. Effects of Dietary Protein of Korean Foxtail Millet on Plasma Adiponectin, HDL-Cholesterol, and Insulin Levels in Genetically Type 2 Diabetic Mice. Biosci. Biotechnol. Biochem. 2005 , 69 , 31–37. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lidon, F.; Silva, M.M. An Overview on Applications and Side Effects of Antioxidant Food Additives. Emir. J. Food Agric. 2016 , 28 , 823. [ Google Scholar ] [ CrossRef ]
- Gul, K.; Yousuf, B.; Singh, A.K.; Singh, P.; Wani, A.A. Rice bran: Nutritional values and its emerging potential for development of functional food—A review. Bioact. Carbohydr. Diet. Fibre 2015 , 6 , 24–30. [ Google Scholar ] [ CrossRef ]
- Nishizawa, N.; Oikawa, M.; Hareyama, S. Effect of Dietary Protein from Proso Millet on the Plasma Cholesterol Metabolism in Rats. Agric. Biol. Chem. 1990 , 54 , 229–230. [ Google Scholar ]
- Hou, D.; Chen, J.; Ren, X.; Wang, C.; Diao, X.; Hu, X.; Zhang, Y.; Shen, Q. A whole foxtail millet diet reduces blood pressure in subjects with mild hypertension. J. Cereal Sci. 2018 , 84 , 13–19. [ Google Scholar ] [ CrossRef ]
- Damsgaard, C.T.; Biltoft-Jensen, A.; Tetens, I.; Michaelsen, K.F.; Lind, M.V.; Astrup, A.; Landberg, R. Whole-Grain Intake, Reflected by Dietary Records and Biomarkers, Is Inversely Associated with Circulating Insulin and Other Cardiometabolic Markers in 8-to 11-Year-Old Children. J. Nutr. 2017 , 147 , 816–824. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Chen, J.; Duan, W.; Ren, X.; Wang, C.; Pan, Z.; Diao, X.; Shen, Q. Effect of foxtail millet protein hydrolysates on lowering blood pressure in spontaneously hypertensive rats. Eur. J. Nutr. 2016 , 56 , 2129–2138. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021 , 10 , 839. [ Google Scholar ] [ CrossRef ]
- Hou, D.; Zhao, Q.; Yousaf, L.; Khan, J.; Xue, Y.; Shen, Q. Consumption of mung bean ( Vigna radiata L.) attenuates obesity, ameliorates lipid metabolic disorders and modifies the gut microbiota composition in mice fed a high-fat diet. J. Funct. Foods 2019 , 64 , 103687. [ Google Scholar ] [ CrossRef ]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean Diet: The Role of Long-Chain ω-3 Fatty Acids in Fish; Polyphenols in Fruits, Vegetables, Cereals, Coffee, Tea, Cacao and Wine; Probiotics and Vitamins in Prevention of Stroke, Age-Related Cognitive Decline, and Alzheimer Disease. Rev. Neurol. 2019 , 175 , 724–741. [ Google Scholar ] [ CrossRef ]
- Liu, B.; Ding, C.; Tang, W.; Zhang, C.; Gu, Y.; Wang, Z.; Yu, T.; Li, Z. Hepatic ROS Mediated Macrophage Activation Is Responsible for Irinotecan Induced Liver Injury. Cells 2022 , 11 , 3791. [ Google Scholar ] [ CrossRef ]
- Molla, M.M.; Ren, X.; Rahman, E.; Kamal, M.; Sabuz, A.A.; Khatun, A.; Chao, W.; Shen, Q. Use of Chou’s 5-steps Rule to Study the Effect of Cereal Dietary Protein on Liver and Coronary Heart Disease Prevention. Curr. Nutr. Food Sci. 2020 , 17 , 11–27. [ Google Scholar ] [ CrossRef ]
- Tuan, N.T.; Adair, L.S.; Stevens, J.; Popkin, B.M. Prediction of hypertension by different anthropometric indices in adults: The change in estimate approach. Public Health Nutr. 2009 , 13 , 639–646. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Moon, J.; Koh, G. Clinical Evidence and Mechanisms of High-Protein Diet-Induced Weight Loss. J. Obes. Metab. Syndr. 2020 , 29 , 166–173. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Wu, Y.; Li, B.; Li, L.; Mitchell, S.E.; Green, C.L.; D’Agostino, G.; Wang, G.; Wang, L.; Li, M.; Li, J.; et al. Very-Low-Protein Diets Lead to Reduced Food Intake and Weight Loss, Linked to Inhibition of Hypothalamic MTOR Signaling, in Mice. Cell Metab. 2021 , 33 , 888–904. [ Google Scholar ] [ CrossRef ]
- Chauhan, M.; Sonawane, S.K.; Arya, S.S. Nutritional and Nutraceutical Properties of Millets: A Review. Clin. J. Nutr. Diet. 2018 , 1 , 1–10. [ Google Scholar ]
- Shi, D.; Lv, L.; Fang, D.; Wu, W.; Hu, C.; Xu, L.; Chen, Y.; Guo, J.; Hu, X.; Li, A.; et al. Administration of Lactobacillus salivarius LI01 or Pediococcus pentosaceus LI05 prevents CCl4-induced liver cirrhosis by protecting the intestinal barrier in rats. Sci. Rep. 2017 , 7 , 6927. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Massier, L.; Blüher, M.; Kovacs, P.; Chakaroun, R.M. Impaired Intestinal Barrier and Tissue Bacteria: Pathomechanisms for Metabolic Diseases. Front. Endocrinol. 2021 , 12 , 616506. [ Google Scholar ] [ CrossRef ]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007 , 114 , 97–109. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019 , 16 , 690–704. [ Google Scholar ] [ CrossRef ]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019 , 11 , 2393. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Murphy, E.F.; Cotter, P.D.; Hogan, A.; O’Sullivan, O.; Joyce, A.; Fouhy, F.; Clarke, S.F.; Marques, T.M.; O’Toole, P.W.; Stanton, C.; et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut 2013 , 62 , 220–226. [ Google Scholar ] [ CrossRef ]
- Costa, M.C.; Arroyo, L.G.; Allen-Vercoe, E.; Stämpfli, H.R.; Kim, P.T.; Sturgeon, A.; Weese, J.S. Comparison of the Fecal Microbiota of Healthy Horses and Horses with Colitis by High Throughput Sequencing of the V3-V5 Region of the 16S rRNA Gene. PLoS ONE 2012 , 7 , e41484. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Middelbos, I.S.; Vester Boler, B.M.; Qu, A.; White, B.A.; Swanson, K.S.; Fahey Jr, G.C. Phylogenetic Characterization of Fecal Microbial Communities of Dogs Fed Diets with or without Supplemental Dietary Fiber Using 454 Pyrosequencing. PLoS ONE 2010 , 5 , e9768. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Liu, Y.-M.; Shen, J.-D.; Xu, L.-P.; Li, H.-B.; Li, Y.-C.; Yi, L.-T. Ferulic acid inhibits neuro-inflammation in mice exposed to chronic unpredictable mild stress. Int. Immunopharmacol. 2017 , 45 , 128–134. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Chung, W.S.F.; Walker, A.W.; Bosscher, D.; Garcia-Campayo, V.; Wagner, J.; Parkhill, J.; Duncan, S.H.; Flint, H.J. Relative Abundance of the Prevotella Genus within the Human Gut Microbiota of Elderly Volunteers Determines the Inter-Individual Responses to Dietary Supplementation with Wheat Bran Arabinoxylan-Oligosaccharides. BMC Microbiol. 2020 , 20 , 283. [ Google Scholar ] [ CrossRef ]
- Zhang, Y.; Tan, L.; Li, C.; Wu, H.; Ran, D.; Zhang, Z. Sulforaphane alter the microbiota and mitigate colitis severity on mice ulcerative colitis induced by DSS. AMB Express 2020 , 10 , 119. [ Google Scholar ] [ CrossRef ]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020 , 8 , 1715. [ Google Scholar ] [ CrossRef ]
| Millet Type | Features | References |
|---|---|---|
| Foxtail millet | Reduces risk of colon cancer. Lessen cholesterol and possesses anti-diabetic capability. Attenuates ethanol-induced hepatic damage. | [ , ] |
| Pearl millet | Gluten-free property averts celiac disease. The immune system improves by inhibiting pathogenicity induced by Shigella. | [ , ] |
| Finger millet | Reduces damage to soft tissue and facilitates the healing process. Reduces plasma triglycerides, thus reducing the risk of cardiovascular disease. | [ ] |
| Kodo millet | Minimize glycemic index and diabetes occurrence, and have antioxidant actions as well. | [ ] |
| Proso millet | Celiac disease can be prevented due to gluten-free properties. Being a low-glycemic index (GI) food reduces type 2 diabetes risks. | [ , ] |
| Little millet | Polyphenol content helps to prevent various metabolic disorders. | [ , ] |
| Barnyard millet | Damaging apoptotic cells reduces colorectal cancer risk. Inhibits protein glycation and glycoxidation, which improves the state of diabetes. | [ , ] |
| Food Grain | Arg | His | Lys | Trp | Phe | Tyr | Met | Cys | Thr | Leu | Ile | Val |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rice | 480 | 130 | 230 | 80 | 280 | 290 | 150 | 90 | 230 | 500 | 300 | 280 |
| Wheat | 290 | 130 | 170 | 70 | 280 | 180 | 90 | 140 | 180 | 410 | 220 | 280 |
| Bajra | 300 | 140 | 190 | 110 | 290 | 200 | 150 | 110 | 140 | 750 | 260 | 330 |
| Sorghum | 240 | 160 | 150 | 70 | 300 | 180 | 100 | 90 | 210 | 880 | 270 | 340 |
| Finger millet | 300 | 130 | 220 | 100 | 310 | 220 | 210 | 140 | 240 | 690 | 400 | 480 |
| Kodo millet | 270 | 130 | 150 | 50 | 430 | - | 180 | 110 | 197 | 648 | 360 | 410 |
| Proso millet | 290 | 110 | 190 | 50 | 310 | - | 160 | - | 150 | 760 | 410 | 410 |
| Foxtail millet | 220 | 130 | 140 | 60 | 420 | - | 180 | 100 | 190 | 1040 | 480 | 430 |
| Little millet | 250 | 120 | 110 | 60 | 330 | - | 180 | 90 | 190 | 760 | 370 | 350 |
| Barnyard millet | 270 | 120 | 150 | 50 | 430 | - | 180 | 110 | 200 | 650 | 360 | 410 |
| Food Grain | Phenolic Acid (mg/100 g) | Reducing Capacity (%) | Reference | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vanillic | Proto Catechuic | P-Hydroxy Benzoic | Syringic | Gentisic | Gallic | Coumaric | Caffeic | Ferulic | Sinapinic | Cinnamic | |||
| Rice | 0.54 | 0.90 | 0.49 | 0.55 | NA | 1.38 | 1.62 | 0.61 | 11.48 | 2.08 | 0.30 | - | [ ] |
| Finger millet | 1.52 | 2.31 | 0.89 | 0.77 | 6.15 | NA | 5.69 | 1.66 | 67.97 | NA | 3.50 | 5.7 ± 1.05 | [ ] |
| Pearl millet | 1.63 | 1.18 | 2.20 | 1.73 | 9.63 | NA | 26.82 | 2.13 | 38.70 | NA | 34.53 | - | [ ] |
| Proso millet | NA | NA | NA | 3.05 | NA | NA | 8.35 | 7.55 | 23.56 | NA | NA | 2.6 ± 0.20 | [ ] |
| Foxtail millet | 8.71 | NA | 1.46 | 9.36 | 2.15 | NA | 213.37 | 1.06 | 75.58 | NA | 78.17 | 4.8 ± 1.15 | [ ] |
| Food Grain | CHO (g) | Protein (g) | Fat (g) | Crude Fiber (g) | Dietary Fiber (g) | Energy (Kcal) | Mineral (g) | Calcium (mg) | Potassium (mg) | Iron (mg) | Phosphorus (mg) | Magnesium (mg) | Sodium (mg) | Zinc (mg) | Thiamin (mg) | Niacin (mg) | Riboflavin (mg) | Carotene (mg) | VB6 (mg) | Folic Acid | VE (mg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rice | 78.2 | 6.8 | 0.5 | 0.2 | 5.2 | 345 | 0.6 | 10 | 160 | 0.7 | 160 | 90 | - | 1.4 | 0.41 | 4.3 | 0.04 | 0 | - | 8.0 | - |
| Wheat | 71.2 | 11.8 | 1.5 | 1.2 | 12.9 | 346 | 1.5 | 41 | 306 | 5.3 | 306 | 138 | 17.1 | 2.7 | 0.41 | 5.1 | 0.1 | 64 | 0.57 | 36.6 | - |
| Bajra | 67.5 | 11.6 | 5.0 | 1.2 | - | 361 | 2.3 | 42 | 296 | 8.0 | 307 | 137 | 10.9 | 3.1 | 0.38 | 2.8 | 0.21 | 132 | - | 45.5 | 19.0 |
| Sorghum | 72.6 | 10.4 | 1.3 | 1.6 | 14.3 | 349 | 1.6 | 25 | 222 | 4.1 | 266 | 171 | 7.3 | 1.6 | 0.38 | 4.3 | 0.15 | 47 | 0.21 | 20.0 | 12.0 |
| Finger millet | 72.0 | 7.3 | 1.3 | 3.6 | 18.8 | 328 | 2.7 | 344 | 283 | 3.9 | 283 | 137 | 11.0 | 2.3 | 0.42 | 1.1 | 0.19 | 42 | - | 18.3 | 22.0 |
| Kodo millet | 65.9 | 8.3 | 1.4 | 9.0 | 15 | 309 | 2.6 | 27 | 188 | 0.5 | 188 | 147 | 4.6 | 0.7 | 0.15 | 2.0 | 0.09 | 0 | - | 23.1 | - |
| Proso millet | 70.4 | 12.5 | 1.1 | 2.2 | 14.2 | 341 | 1.9 | 14 | 206 | 0.8 | 206 | 153 | 8.2 | 1.4 | 0.41 | 4.5 | 0.28 | 0 | - | - | - |
| Foxtail millet | 60.9 | 12.3 | 4.3 | 8.0 | 14 | 331 | 3.3 | 31 | 290 | 2.8 | 290 | 81 | 4.6 | 2.4 | 0.59 | 3.2 | 0.11 | 32 | - | 15.0 | 31.0 |
| Little millet | 67.0 | 7.7 | 4.7 | 7.6 | 12.2 | 341 | 1.5 | 17 | 220 | 9.3 | 220 | 133 | 8.1 | 3.7 | 0.30 | 3.2 | 0.09 | 0 | - | 9.0 | - |
| Barnyard millet | 65.5 | 6.2 | 2.2 | 9.8 | 13.7 | 307 | 4.4 | 20 | 280 | 5.0 | 280 | 82 | - | 3.0 | 0.33 | 4.2 | 0.1 | 0 | - | - | - |
| Diet Group | Fasting Blood Glucose (mmol/L) | Insulin (mu/L) | Islet β Cell Function (HOMA-β) | Blood Glucose 0, 30, 60 and 120 min (mmol/L) | Area under Curve (AUC) | Serum Triglyceride (mmol/L) | Liver Triglyceride (TG) (mmol/L) | Aspartate Amino-Transferase (U/L) | Alanine Amino-Transferase (U/L) |
|---|---|---|---|---|---|---|---|---|---|
| NC | 7.20 | 16.20 | 135.45 | 5, 10, 8, 7 | 18.00 | 0.70 | 1.20 | 56.00 | 30.00 |
| MC | 27.30 | 16.35 | 15.24 | 27, 32, 31, 30 | 60.00 | 1.60 | 3.00 | 80.00 | 77.00 |
| PCFM | 23.40 | 18.25 | 20.23 | 21, 29, 25, 23 | 56.00 | 1.00 | 2.00 | 54.00 | 56.00 |
| Parameter | Dietary Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G0T0 | GNT0 | GNT1 | G1T1 | G2T2 | G3T3 | G4T4 | G5T5 | G6T6 | ||
| Activities of serum enzyme (U/L) | AST | 340.83 ± 34.94 | 113.33 ± 25.21 | 261.25 ± 21.13 | 241.10 ± 7.30 | 243.16 ± 211.32 | 81.33 ± 5.24 | 94.66 ± 3.14 | 67.66 ± 14.27 | 85.50 ± 11.39 |
| ALT | 55.0 ± 18.56 | 48.33 ± 23.44 | 50.33 ± 10.59 | 50.33 ± 21.36 | 51.66 ± 3.14 | 18.16 ± 7.38 | 22.16 ± 2.40 | 13.0 ± 4.0 | 21.66 ± 1.50 | |
| LDH | 2832.16 ± 347.53 | 1531.83 ± 307.60 | 2585.16 ± 1020.30 | 2232.5 ± 461.59 | 2298.66 ± 259.77 | 1114.33 ± 314.91 | 1283.83 ± 87.44 | 1047.0 ± 95.33 | 1107.66 ± 193.95 | |
| Plasma components (mmol/L) | TC | 4.34 ± 0.19 | 3.45 ± 021 | 4.12 ± 1.39 | 3.98 ± 0.42 | 3.47 ± 0.28 | 2.62 ± 0.16 | 3.06 ± 0.07 | 2.05 ± 0.32 | 2.85 ± 0.10 |
| TG | 1.25 ± 0.20 | 1.13 ± 0.23 | 1.20 ± 0.15 | 0.84 ± 0.14 | 0.85 ± 0.08 | 0.82 ± 0.08 | 0.83 ± 0.15 | 0.81 ± 0.16 | 0.83 ± 0.07 | |
| HDL-C | 0.72 ± 0.54 | 1.34 ± 1.19 | 0.75 ± 0.35 | 1.55 ± 1.63 | 1.67 ± 1.80 | 2.11 ± 1.58 | 2.07 ± 1.46 | 3.17 ± 1.48 | 2.96 ± 1.83 | |
| LDL-C | 2.81 ± 1.28 | 1.81 ± 1.28 | 2.33 ± 1.13 | 1.94 ± 1.03 | 2.01 ± 1.03 | 0.48 ± 0.39 | 0.68 ± 0.60 | 0.47 ± 0.22 | 0.68 ± 0.23 | |
| Liver lipids (mmol/L) | TC | 0.92 ± 0.56 | 0.44 ± 0.14 | 0.51 ± 0.36 | 0.42 ± 0.15 | 0.38 ± 0.23 | 0.36 ± 0.14 | 0.36 ± 0.22 | 0.16 ± 0.15 | 0.26 ± 0.16 |
| TG | 1.50 ± 0.98 | 1.08 ± 0.60 | 1.67 ± 1.15 | 0.92 ± 0.56 | 1.27 ± 1.48 | 0.74 ± 0.34 | 0.85 ± 0.30 | 0.38 ± 0.26 | 0.76 ± 0.55 | |
| Liver MDA (nmol/mg protein) | 5.21 ± 0.65 | 4.35 ± 1.46 | 4.56 ± 0.67 | 4.68 ± 1.13 | 4.58 ± 1.37 | 2.55 ± 1.65 | 2.89 ± 0.32 | 0.68 ± 0.21 | 2.79 ± 0.08 | |
| Parameter | Dietary Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G0T0 | GNT0 | GNT1 | G1T1 | G2T2 | G3T3 | G4T4 | G5T5 | G6T6 | ||
| Activities of serum enzyme (U/L) | AST | 133.16 ± 56.33 | 126.00 ± 23.09 | 128.66 ± 34.56 | 125.66 ± 38.68 | 111.83 ± 36.21 | 121.16 ± 22.56 | 120.33 ± 24.32 | 106.33 ± 27.50 | 102.66 ± 21.78 |
| ALT | 62.16 ± 12.44 | 43.00 ± 52.50 | 48.50 ± 17.62 | 40.83 ± 11.97 | 42.83 ± 40.57 | 34.50 ± 12.58 | 29.16 ± 15.31 | 25.50 ± 12.37 | 32.66 ± 10.30 | |
| LDH | 1338.66 ± 323.80 | 1210.16 ± 286.14 | 1334.50 ± 328.57 | 1104.66 ± 277.13 | 1117.66 ± 226.02 | 904.33 ± 425.28 | 960.83 ± 151.03 | 903.16 ± 95.10 | 1019.50 ± 244.42 | |
| Plasma components (mmol/L) | TC | 3.79 ± 0.62 | 3.11 ± 0.33 | 3.44 ± 0.53 | 3.03 ± 0.50 | 3.04 ± 1.04 | 2.46 ± 0.69 | 2.68 ± 0.36 | 2.11 ± 0.17 | 2.61 ± 0.92 |
| TG | 1.50 ± 0.74 | 1.27 ± 0.80 | 1.28 ± 0.45 | 1.21 ± 0.42 | 1.20 ± 0.12 | 0.95 ± 0.22 | 1.00 ± 0.14 | 0.87 ± 0.22 | 0.93 ± 0.27 | |
| HDL-C | 2.75 ± 0.64 | 3.10 ± 0.45 | 3.05 ± 1.01 | 3.17 ± 0.45 | 3.12 ± 2.48 | 4.11 ± 0.81 | 4.08 ± 1.05 | 8.06 ± 6.97 | 7.65 ± 2.34 | |
| LDL-C | 10.63 ± 7.61 | 10.41 ± 4.05 | 10.51 ± 3.26 | 5.59 ± 1.85 | 4.90 ± 3.05 | 0.20 ± 0.04 | 0.26 ± 0.09 | 0.10 ± 0.0 | 0.14 ± 0.05 | |
| Liver lipids(mmol/L) | TC | 0.91 ± 0.28 | 0.77 ± 0.15 | 0.83 ± 0.16 | 0.81 ± 0.20 | 0.76 ± 0.21 | 0.62 ± 0.06 | 0.72 ± 0.16 | 0.54 ± 0.28 | 0.72 ± 0.16 |
| TG | 6.74 ± 0.50 | 3.51 ± 1.03 | 4.61 ± 2.66 | 3.74 ± 2.26 | 5.26 ± 6.04 | 1.30 ± 1.03 | 1.68 ± 0.61 | 0.52 ± 0.60 | 0.96 ± 0.80 | |
| Liver MDA (nmol/mg protein) | 5.31 ± 1.22 | 3.03 ± 1.16 | 3.13 ± 1.58 | 3.43 ± 1.46 | 3.47 ± 1.43 | 1.38 ± 0.87 | 1.81 ± 0.77 | 0.58 ± 0.14 | 1.27 ± 0.84 | |
| Diet | Bacteroidetes % | Firmicutes % | Verrucomicrobia % | Proteobacteria % | Actinobacteria % | Cyanobacteria % | Tenericutes % | Other % |
|---|---|---|---|---|---|---|---|---|
| Normal | 54.82 | 31.23 | 9.24 | 2.9 | 1.05 | 0.11 | 0 | 0.65 |
| Casein | 85.94 | 12.61 | 0.01 | 0.39 | 0.09 | 0.01 | 0.01 | 0.94 |
| Control | 57.89 | 16.80 | 24.44 | 0.30 | 0.33 | 0.01 | 0.03 | 0.20 |
| UC FM flour | 46.10 | 49.88 | - | 0.87 | 0.84 | 0.01 | - | 2.30 |
| C FM flour | 80.81 | 5.63 | - | 3.65 | 8.30 | - | - | 1.61 |
| UCE FM protein | 68.07 | 12.87 | 0.04 | 18.18 | 0.67 | 0.01 | - | 0.16 |
| CE FM protein | 79.35 | 14.26 | - | 5.62 | 0.14 | 0.01 | - | 0.62 |
| CEE FM protein | 69.46 | 22.48 | 4.53 | 1.79 | 0.58 | - | - | 1.16 |
| UCEE FM protein | 0.96 | 34.55 | 63.40 | 0.50 | 0.57 | - | - | 0.02 |
| Diet | Akkermansia (%) | Allobaculum (%) | Prevotella (%) | Bacteroides (%) | Lactobacillus (%) | Oscilliospria (%) | Anaerotruncus (%) | Sutterella (%) |
|---|---|---|---|---|---|---|---|---|
| Normal | 9.24 | 24.14 | 0.85 | 2.99 | 1.84% | 0.34 | 0.29 | - |
| Casein | 0.01 | 0.01 | 10.96 | 5.53 | 0.19 | 0.65 | 0.01 | - |
| Control | 24.45 | - | 1.68 | 1.21 | 10.93 | 0.38 | 0.04 | 0.12 |
| UC FM flour | - | 38.39 | 0.84 | 0.13 | 4.96 | 0.17 | 0.04 | 0.26 |
| C FM flour | - | 0.91 | 12.05 | 0.81 | 0.70 | 0.14 | 0.01 | 3.52 |
| UCE FM protein | 0.04 | - | 6.33 | 4.82 | 0.01 | 4.84 | 2.13 | 14.02 |
| CE FM protein | - | - | 0.51 | 29.87 | - | 1.94 | 0.43 | 0.18 |
| CEEFM protein | 4.53 | - | 54.92 | 3.64 | - | 0.54 | 1.12 | 1.56 |
| UCEE FM protein | 63.40 | - | - | 0.25 | 0.01 | 1.50 | 0.38 | 0.01 |
| The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
Share and Cite
Sabuz, A.A.; Rana, M.R.; Ahmed, T.; Molla, M.M.; Islam, N.; Khan, H.H.; Chowdhury, G.F.; Zhao, Q.; Shen, Q. Health-Promoting Potential of Millet: A Review. Separations 2023 , 10 , 80. https://doi.org/10.3390/separations10020080
Sabuz AA, Rana MR, Ahmed T, Molla MM, Islam N, Khan HH, Chowdhury GF, Zhao Q, Shen Q. Health-Promoting Potential of Millet: A Review. Separations . 2023; 10(2):80. https://doi.org/10.3390/separations10020080
Sabuz, Ashfak Ahmed, Md Rahmatuzzaman Rana, Tanvir Ahmed, Mohammad Mainuddin Molla, Nazmul Islam, Hafizul Haque Khan, Golam Ferdous Chowdhury, Qingyu Zhao, and Qun Shen. 2023. "Health-Promoting Potential of Millet: A Review" Separations 10, no. 2: 80. https://doi.org/10.3390/separations10020080
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- Biochemistry and Molecular Biology
- Biostatistics
- Environmental Health and Engineering
- Epidemiology
- Health Policy and Management
- Health, Behavior and Society
- International Health
- Mental Health
- Molecular Microbiology and Immunology
- Population, Family and Reproductive Health
- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?
research@BSPH
The School’s research endeavors aim to improve the public’s health in the U.S. and throughout the world.
- Funding Opportunities and Support
- Faculty Innovation Award Winners
Conducting Research That Addresses Public Health Issues Worldwide
Systematic and rigorous inquiry allows us to discover the fundamental mechanisms and causes of disease and disparities. At our Office of Research ( research@BSPH), we translate that knowledge to develop, evaluate, and disseminate treatment and prevention strategies and inform public health practice. Research along this entire spectrum represents a fundamental mission of the Johns Hopkins Bloomberg School of Public Health.
From laboratories at Baltimore’s Wolfe Street building, to Bangladesh maternity wards in densely packed neighborhoods, to field studies in rural Botswana, Bloomberg School faculty lead research that directly addresses the most critical public health issues worldwide. Research spans from molecules to societies and relies on methodologies as diverse as bench science and epidemiology. That research is translated into impact, from discovering ways to eliminate malaria, increase healthy behavior, reduce the toll of chronic disease, improve the health of mothers and infants, or change the biology of aging.
120+ countries
engaged in research activity by BSPH faculty and teams.
of all federal grants and contracts awarded to schools of public health are awarded to BSPH.
citations on publications where BSPH was listed in the authors' affiliation in 2019-2023.
publications where BSPH was listed in the authors' affiliation in 2019-2023.
Departments
Our 10 departments offer faculty and students the flexibility to focus on a variety of public health disciplines
Centers and Institutes Directory
Our 80+ Centers and Institutes provide a unique combination of breadth and depth, and rich opportunities for collaboration
Institutional Review Board (IRB)
The Institutional Review Board (IRB) oversees two IRBs registered with the U.S. Office of Human Research Protections, IRB X and IRB FC, which meet weekly to review human subjects research applications for Bloomberg School faculty and students
Generosity helps our community think outside the traditional boundaries of public health, working across disciplines and industries, to translate research into innovative health interventions and practices
Introducing the research@BSPH Ecosystem
The research@BSPH ecosystem aims to foster an interdependent sense of community among faculty researchers, their research teams, administration, and staff that leverages knowledge and develops shared responses to challenges. The ultimate goal is to work collectively to reduce administrative and bureaucratic barriers related to conducting experiments, recruiting participants, analyzing data, hiring staff, and more, so that faculty can focus on their core academic pursuits.
Research at the Bloomberg School is a team sport.
In order to provide extensive guidance, infrastructure, and support in pursuit of its research mission, research@BSPH employs three core areas: strategy and development, implementation and impact, and integrity and oversight. Our exceptional research teams comprised of faculty, postdoctoral fellows, students, and committed staff are united in our collaborative, collegial, and entrepreneurial approach to problem solving. T he Bloomberg School ensures that our research is accomplished according to the highest ethical standards and complies with all regulatory requirements. In addition to our institutional review board (IRB) which provides oversight for human subjects research, basic science studies employee techniques to ensure the reproducibility of research.
Research@BSPH in the News
Four bloomberg school faculty elected to national academy of medicine.
Considered one of the highest honors in the fields of health and medicine, NAM membership recognizes outstanding professional achievements and commitment to service.
The Maryland Maternal Health Innovation Program Grant Renewed with Johns Hopkins
Lerner center for public health advocacy announces inaugural sommer klag advocacy impact award winners.
Bloomberg School faculty Nadia Akseer and Cass Crifasi selected winners at Advocacy Impact Awards Pitch Competition
COVID-19: Long-term effects
Some people continue to experience health problems long after having COVID-19. Understand the possible symptoms and risk factors for post-COVID-19 syndrome.
Most people who get coronavirus disease 2019 (COVID-19) recover within a few weeks. But some people — even those who had mild versions of the disease — might have symptoms that last a long time afterward. These ongoing health problems are sometimes called post- COVID-19 syndrome, post- COVID conditions, long COVID-19 , long-haul COVID-19 , and post acute sequelae of SARS COV-2 infection (PASC).
What is post-COVID-19 syndrome and how common is it?
Post- COVID-19 syndrome involves a variety of new, returning or ongoing symptoms that people experience more than four weeks after getting COVID-19 . In some people, post- COVID-19 syndrome lasts months or years or causes disability.
Research suggests that between one month and one year after having COVID-19 , 1 in 5 people ages 18 to 64 has at least one medical condition that might be due to COVID-19 . Among people age 65 and older, 1 in 4 has at least one medical condition that might be due to COVID-19 .
What are the symptoms of post-COVID-19 syndrome?
The most commonly reported symptoms of post- COVID-19 syndrome include:
- Symptoms that get worse after physical or mental effort
- Lung (respiratory) symptoms, including difficulty breathing or shortness of breath and cough
Other possible symptoms include:
- Neurological symptoms or mental health conditions, including difficulty thinking or concentrating, headache, sleep problems, dizziness when you stand, pins-and-needles feeling, loss of smell or taste, and depression or anxiety
- Joint or muscle pain
- Heart symptoms or conditions, including chest pain and fast or pounding heartbeat
- Digestive symptoms, including diarrhea and stomach pain
- Blood clots and blood vessel (vascular) issues, including a blood clot that travels to the lungs from deep veins in the legs and blocks blood flow to the lungs (pulmonary embolism)
- Other symptoms, such as a rash and changes in the menstrual cycle
Keep in mind that it can be hard to tell if you are having symptoms due to COVID-19 or another cause, such as a preexisting medical condition.
It's also not clear if post- COVID-19 syndrome is new and unique to COVID-19 . Some symptoms are similar to those caused by chronic fatigue syndrome and other chronic illnesses that develop after infections. Chronic fatigue syndrome involves extreme fatigue that worsens with physical or mental activity, but doesn't improve with rest.
Why does COVID-19 cause ongoing health problems?
Organ damage could play a role. People who had severe illness with COVID-19 might experience organ damage affecting the heart, kidneys, skin and brain. Inflammation and problems with the immune system can also happen. It isn't clear how long these effects might last. The effects also could lead to the development of new conditions, such as diabetes or a heart or nervous system condition.
The experience of having severe COVID-19 might be another factor. People with severe symptoms of COVID-19 often need to be treated in a hospital intensive care unit. This can result in extreme weakness and post-traumatic stress disorder, a mental health condition triggered by a terrifying event.
What are the risk factors for post-COVID-19 syndrome?
You might be more likely to have post- COVID-19 syndrome if:
- You had severe illness with COVID-19 , especially if you were hospitalized or needed intensive care.
- You had certain medical conditions before getting the COVID-19 virus.
- You had a condition affecting your organs and tissues (multisystem inflammatory syndrome) while sick with COVID-19 or afterward.
Post- COVID-19 syndrome also appears to be more common in adults than in children and teens. However, anyone who gets COVID-19 can have long-term effects, including people with no symptoms or mild illness with COVID-19 .
What should you do if you have post-COVID-19 syndrome symptoms?
If you're having symptoms of post- COVID-19 syndrome, talk to your health care provider. To prepare for your appointment, write down:
- When your symptoms started
- What makes your symptoms worse
- How often you experience symptoms
- How your symptoms affect your activities
Your health care provider might do lab tests, such as a complete blood count or liver function test. You might have other tests or procedures, such as chest X-rays, based on your symptoms. The information you provide and any test results will help your health care provider come up with a treatment plan.
In addition, you might benefit from connecting with others in a support group and sharing resources.
- Long COVID or post-COVID conditions. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects.html. Accessed May 6, 2022.
- Post-COVID conditions: Overview for healthcare providers. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html. Accessed May 6, 2022.
- Mikkelsen ME, et al. COVID-19: Evaluation and management of adults following acute viral illness. https://www.uptodate.com/contents/search. Accessed May 6, 2022.
- Saeed S, et al. Coronavirus disease 2019 and cardiovascular complications: Focused clinical review. Journal of Hypertension. 2021; doi:10.1097/HJH.0000000000002819.
- AskMayoExpert. Post-COVID-19 syndrome. Mayo Clinic; 2022.
- Multisystem inflammatory syndrome (MIS). Centers for Disease Control and Prevention. https://www.cdc.gov/mis/index.html. Accessed May 24, 2022.
- Patient tips: Healthcare provider appointments for post-COVID conditions. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/post-covid-appointment/index.html. Accessed May 24, 2022.
- Bull-Otterson L, et al. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years — United States, March 2020 — November 2021. MMWR Morbidity and Mortality Weekly Report. 2022; doi:10.15585/mmwr.mm7121e1.
Products and Services
- A Book: Endemic - A Post-Pandemic Playbook
- Begin Exploring Women's Health Solutions at Mayo Clinic Store
- A Book: Future Care
- Antibiotics: Are you misusing them?
- COVID-19 and vitamin D
- Convalescent plasma therapy
- Coronavirus disease 2019 (COVID-19)
- COVID-19: How can I protect myself?
- Herd immunity and respiratory illness
- COVID-19 and pets
- COVID-19 and your mental health
- COVID-19 antibody testing
- COVID-19, cold, allergies and the flu
- COVID-19 tests
- COVID-19 drugs: Are there any that work?
- COVID-19 in babies and children
- Coronavirus infection by race
- COVID-19 travel advice
- COVID-19 vaccine: Should I reschedule my mammogram?
- COVID-19 vaccines for kids: What you need to know
- COVID-19 vaccines
- COVID-19 variant
- COVID-19 vs. flu: Similarities and differences
- COVID-19: Who's at higher risk of serious symptoms?
- Debunking coronavirus myths
- Different COVID-19 vaccines
- Extracorporeal membrane oxygenation (ECMO)
- Fever: First aid
- Fever treatment: Quick guide to treating a fever
- Fight coronavirus (COVID-19) transmission at home
- Honey: An effective cough remedy?
- How do COVID-19 antibody tests differ from diagnostic tests?
- How to measure your respiratory rate
- How to take your pulse
- How to take your temperature
- How well do face masks protect against COVID-19?
- Is hydroxychloroquine a treatment for COVID-19?
- Loss of smell
- Mayo Clinic Minute: You're washing your hands all wrong
- Mayo Clinic Minute: How dirty are common surfaces?
- Multisystem inflammatory syndrome in children (MIS-C)
- Nausea and vomiting
- Pregnancy and COVID-19
- Safe outdoor activities during the COVID-19 pandemic
- Safety tips for attending school during COVID-19
- Sex and COVID-19
- Shortness of breath
- Thermometers: Understand the options
- Treating COVID-19 at home
- Unusual symptoms of coronavirus
- Vaccine guidance from Mayo Clinic
- Watery eyes
Related information
- Post-COVID Recovery & COVID-19 Support Group - Related information Post-COVID Recovery & COVID-19 Support Group
- Rehabilitation after COVID-19 - Related information Rehabilitation after COVID-19
- Post-COVID-19 syndrome could be a long haul (podcast) - Related information Post-COVID-19 syndrome could be a long haul (podcast)
- COVID-19 Coronavirus Long-term effects
We’re transforming healthcare
Make a gift now and help create new and better solutions for more than 1.3 million patients who turn to Mayo Clinic each year.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.15(1); 2023 Jan

Role of Physical Activity on Mental Health and Well-Being: A Review
Aditya mahindru.
1 Department of Psychiatry, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, Wardha, IND
Pradeep Patil
Varun agrawal.
In addition to the apparent physical health benefits, physical activity also affects mental health positively. Physically inactive individuals have been reported to have higher rates of morbidity and healthcare expenditures. Commonly, exercise therapy is recommended to combat these challenges and preserve mental wellness. According to empirical investigations, physical activity is positively associated with certain mental health traits. In nonclinical investigations, the most significant effects of physical exercise have been on self-concept and body image. An attempt to review the current understanding of the physiological and psychological mechanisms by which exercise improves mental health is presented in this review article. Regular physical activity improves the functioning of the hypothalamus-pituitary-adrenal axis. Depression and anxiety appear to be influenced by physical exercise, but to a smaller extent in the population than in clinical patients. Numerous hypotheses attempt to explain the connection between physical fitness and mental wellness. Physical activity was shown to help with sleep and improve various psychiatric disorders. Exercise in general is associated with a better mood and improved quality of life. Physical exercise and yoga may help in the management of cravings for substances, especially in people who may not have access to other forms of therapy. Evidence suggests that increased physical activity can help attenuate some psychotic symptoms and treat medical comorbidities that accompany psychotic disorders. The dearth of literature in the Indian context also indicated that more research was needed to evaluate and implement interventions for physical activity tailored to the Indian context.
Introduction and background
Physical activity has its origins in ancient history. It is thought that the Indus Valley civilization created the foundation of modern yoga in approximately 3000 B.C. during the early Bronze Age [ 1 ]. The beneficial role of physical activity in healthy living and preventing and managing health disorders is well documented in the literature. Physical activity provides various significant health benefits. Mechanical stress and repeated exposure to gravitational forces created by frequent physical exercise increase a variety of characteristics, including physical strength, endurance, bone mineral density, and neuromusculoskeletal fitness, all of which contribute to a functional and independent existence. Exercise, defined as planned, systematic, and repetitive physical activity, enhances athletic performance by improving body composition, fitness, and motor abilities [ 2 ]. The function of physical activity in preventing a wide range of chronic illnesses and premature mortality has been extensively examined and studied. Adequate evidence links medical conditions such as cardiovascular disease and individual lifestyle behaviours, particularly exercise [ 3 ]. Regular exercise lowered the incidence of cardiometabolic illness, breast and colon cancer, and osteoporosis [ 4 ]. In addition to improving the quality of life for those with nonpsychiatric diseases such as peripheral artery occlusive disease and fibromyalgia, regular physical activity may help alleviate the discomforts of these particular diseases [ 5 ]. Exercise also helps with various substance use disorders, such as reducing or quitting smoking. As physical exercise strongly impacts health, worldwide standards prescribe a weekly allowance of "150 minutes" of modest to vigorous physical exercise in clinical and non-clinical populations [ 6 ]. When these recommendations are followed, many chronic diseases can be reduced by 20%-30%. Furthermore, thorough evaluations of global studies have discovered that a small amount of physical exercise is sufficient to provide health benefits [ 7 ].
Methodology
In this review article, a current understanding of the underlying physiological and psychological processes during exercise or physical activity that are implicated in improving mental health is presented. Search terms like "exercise" or "physical activity" and "mental health", "exercise" or "physical activity" and "depression", "exercise" or "physical activity" and "stress", "exercise" or "physical activity" and "anxiety", "exercise" or "physical activity" and "psychosis," "exercise" or "physical activity" and "addiction" were used as search terms in PubMed, Google Scholar, and Medline. An overwhelming majority of references come from works published within the past decade.
The impact of physical health on mental health
There is an increasing amount of evidence documenting the beneficial impacts of physical activity on mental health, with studies examining the effects of both brief bouts of exercise and more extended periods of activity. Systematic evaluations have indicated better outcomes for mental diseases with physical activity. Numerous psychological effects, such as self-esteem, cognitive function, mood, depression, and quality of life, have been studied [ 8 ]. According to general results, exercise enhances mood and self-esteem while decreasing stress tendencies, a factor known to aggravate mental and physical diseases [ 9 ]. Studies show that people who exercise regularly have a better frame of mind. However, it should be highlighted that a consistent link between mood enhancement and exercise in healthy individuals has not been established.
Additionally, human beings produce more of these two neurochemicals when they engage in physical activity. Human bodies manufacture opioids and endocannabinoids that are linked to pleasure, anxiolytic effects, sleepiness, and reduced pain sensitivity [ 10 ]. It has been shown that exercise can improve attention, focus, memory, cognition, language fluency, and decision-making for up to two hours [ 11 ]. Researchers state that regular physical activity improves the functioning of the hypothalamus-pituitary-adrenal (HPA) axis, lowering cortisol secretion and restoring the balance of leptin and ghrelin (Figure (Figure1) 1 ) [ 12 ].

HPA: hypothalamus-pituitary-adrenal
This image has been created by the authors.
Regular exercise has immunomodulatory effects such as optimising catecholamine, lowering cortisol levels, and lowering systemic inflammation. Physical activity has been shown to increase plasma brain-derived neurotrophic factor (BDNF), which is thought to reduce amyloid-beta toxicity linked to Alzheimer's disease progression [ 13 ].
Although no causal correlations have been proven, methodologically sound research has discovered a related improvement in mentally and physically ill populations. These findings are based on research and studies conducted all across the globe, particularly in the Western Hemisphere. In order to address a widespread health problem in India, it is useful to do a literature review that draws on research conducted in a variety of settings. In addition, the prevalence of these mental illnesses and the benefits of exercise as a complementary therapy might be made clear by a meta-analysis of research undertaken in India [ 14 ].
This review also analysed published literature from India to understand the effects of exercise on mental health and the implications for disease management and treatment in the Indian context. Results from Indian studies were consistent with those found in global meta-analyses. The Indian government has made public data on interventions, such as the effects of different amounts of physical exercise. Exercising and yoga have been shown to be effective adjunct therapies for a variety of mental health conditions [ 12 ]. Though yoga may not require a lot of effort to perform, other aspects of the program, such as breathing or relaxation exercises, may have an impact on a practitioner's mental health at the same time. Due to its cultural significance as a common physical practice among Indians and its low to moderate activity level, yoga would be an appropriate activity for this assessment [ 15 ].
Yoga as an adjunctive treatment
Although yoga is a centuries-old Hindu practice, its possible therapeutic effects have recently been studied in the West. Mind-body approaches have been the subject of a lot of studies, and some of the findings suggest they may aid with mental health issues on the neurosis spectrum. As defined by the National Center for Complementary and Alternative Medicine, "mind-body interventions" aim to increase the mind's potential to alter bodily functions [ 16 ]. Due to its beneficial effects on the mind-body connection, yoga is used as a treatment for a wide range of conditions. Possible therapeutic benefits of yoga include the activation of antagonistic neuromuscular systems, stimulation of the limbic system, and a reduction in sympathetic tone.
Anxiety and depression sufferers might benefit from practising yoga. Yoga is generally safe for most people and seldom causes unintended negative consequences. Adding yoga to traditional treatment for mental health issues may be beneficial. Many of the studies on yoga included meditation as an integral part of their methodology. Meditation and other forms of focused mental practice may set off a physiological reaction known as the relaxation response. Functional imaging has been used to implicate certain regions of the brain that show activity during meditation. According to a wealth of anatomical and neurochemical evidence, meditation has been shown to have far-reaching physiological effects, including changes in attention and autonomic nervous system modulation [ 17 ]. Left anterior brain activity, which is associated with happiness, was shown to rise considerably during meditation. There's also some evidence that meditation might worsen psychosis by elevating dopamine levels [ 18 - 20 ]. We do not yet know enough about the possible downsides of meditation for patients with mental illness, since this research lacks randomised controlled trials.
Physical activity and schizophrenia
Schizophrenia is a debilitating mental disorder that often manifests in one's early years of productive life (late second decade). Remission of this disorder occurs in just a small fraction of cases. More than 60% will have relapses, and they might occur with or without noticeable deficits. Apart from delusions, hallucinations, and formal thought disorders, many patients exhibit cognitive deficits that emerge in the early stages of the disease and do not respond adequately to therapy [ 21 ].
Treatment for schizophrenia is challenging to master. Extrapyramidal side effects are a problem with first-generation antipsychotic drugs. Obesity and dyslipidemia have been related to second-generation drugs, which may cause or exacerbate these conditions. The majority of patients do not achieve complete remission, and many do not even experience satisfactory symptom relief. Even though certain antipsychotic medications may alleviate or even exacerbate negative and cognitive symptoms, these responses are far less common. This means that patients may benefit from cognitive rehabilitation. Because of their illness or a negative reaction to their medicine, they may also have depressive symptoms. This would make their condition even more disabling. Many patients also deal with clinical and emotional complications. Tardive extrapyramidal illnesses, metabolic syndromes, defect states, and attempted suicide are all in this category. Patient compliance with treatment plans is often poor. The caregivers take on a lot of stress and often get exhausted as a result.
Evidence suggests that increased physical activity can aid in attenuating some psychotic symptoms and treating medical comorbidities that accompany psychotic disorders, particularly those subject to the metabolic adverse effects of antipsychotics. Physically inactive people with mental disorders have increased morbidity and healthcare costs. Exercise solutions are commonly recommended to counteract these difficulties and maintain mental and physical wellness [ 22 ].
The failure of current medications to effectively treat schizophrenia and the lack of improvement in cognitive or negative symptoms with just medication is an argument in favour of utilising yoga as a complementary therapy for schizophrenia. Even without concomitant medication therapy, co-occurring psychosis and obesity, or metabolic syndrome, are possible. The endocrine and reproductive systems of drug abusers undergo subtle alterations. Numerous studies have shown that yoga may improve endocrine function, leading to improvements in weight management, cognitive performance, and menstrual regularity, among other benefits. In this context, the role of yoga in the treatment of schizophrenia has been conceptualized. However, yoga has only been studied for its potential efficacy as a therapy in a tiny number of studies. There might be several reasons for this. To begin with, many yoga academies frown against the practice being adapted into a medical modality. The second misconception is that people with schizophrenia cannot benefit from the mental and physical aspects of yoga practised in the ways that are recommended. Third, scientists may be hesitant to recommend yoga to these patients because of their lack of knowledge and treatment compliance.
In a randomised controlled experiment with a yoga group (n = 21) and an exercise group (n = 20), the yoga group exhibited a statistically significant reduction in negative symptoms [ 2 ]. In accordance with the most recent recommendations of the National Institute for Health and Care Excellence (NICE), the above research provides substantial evidence for the use of yoga in the treatment of schizophrenia. According to a meta-analysis of 17 distinct studies [ 23 ] on the subject, frequent physical activity reduces the negative symptoms associated with schizophrenia considerably.
Physical activity and alcohol dependence syndrome
Substance abuse, namely alcohol abuse, may have devastating effects on a person's mental and physical health. Tolerance and an inability to control drinking are some hallmarks of alcoholism. Research shows that physical activity is an effective supplement in the fight against alcohol use disorder. In addition to perhaps acting centrally on the neurotransmitter systems, physical exercise may mitigate the deleterious health consequences of drinking. Evidence suggests that persons with alcohol use disorder are not physically active and have low cardiorespiratory fitness. A wide number of medical comorbidities, like diabetes mellitus, hypertension, and other cardiovascular illnesses, occur with alcohol use disorders. Physical exercise may be highly useful in aiding the management of these comorbidities [ 24 ].
Physical exercise and yoga may help in the management of cravings for substances when other forms of therapy, such as counselling or medication for craving management are not feasible or acceptable. Physical exercise has been shown to have beneficial effects on mental health, relieve stress, and provide an enjoyable replacement for the substance. However, the patient must take an active role in physical activity-based therapies rather than passively accept the process as it is, which is in stark contrast to the approach used by conventional medicine. Since most substance use patients lack motivation and commitment to change, it is recommended that physical activity-based therapies be supplemented with therapies focusing on motivation to change to maximise therapeutic outcomes.
One hundred seventeen persons with alcohol use disorder participated in a single-arm, exploratory trial that involved a 12-minute fitness test using a cycle ergometer as an intervention. Statistically, significantly fewer cravings were experienced by 40% [ 24 ]. Exercise programmes were found to significantly reduce alcohol intake and binge drinking in people with alcohol use disorder in a meta-analysis and comprehensive review of the effects of such therapies [ 25 ].
Physical activity and sleep
Despite widespread agreement that they should prioritise their health by making time for exercise and sufficient sleep, many individuals fail to do so. Sleep deprivation has negative impacts on immune system function, mood, glucose metabolism, and cognitive ability. Slumber is a glycogenetic process that replenishes glucose storage in neurons, in contrast to the waking state, which is organised for the recurrent breakdown of glycogen. Considering these findings, it seems that sleep has endocrine effects on the brain that are unrelated to the hormonal control of metabolism and waste clearance at the cellular level. Several factors have been proposed as potential triggers for this chain reaction: changes in core body temperature, cytokine concentrations, energy expenditure and metabolic rate, central nervous system fatigue, mood, and anxiety symptoms, heart rate and heart rate variability, growth hormone and brain-derived neurotrophic factor secretion, fitness level, and body composition [ 26 ].
After 12 weeks of fitness training, one study indicated that both the quantity and quality of sleep in adolescents improved. Studies using polysomnography indicated that regular exercise lowered NREM stage N1 (very light sleep) and raised REM sleep (and REM sleep continuity and performance) [ 22 ]. As people age, both short- and long-term activities have increasingly deleterious effects on sleep. In general, both short- and long-term exercise were found to have a favourable effect on sleep quality; however, the degree of this benefit varied substantially among different sleep components. On measures of sleep quality, including total sleep time, slow-wave sleep, sleep onset latency, and REM sleep reduction, acute exercise had no effect. But both moderate and strenuous exercise has been shown to increase sleep quality [ 27 ]. According to a meta-analysis of randomised controlled trials, exercise has shown a statistically significant effect on sleep quality in adults with mental illness [ 28 ]. These findings emphasise the importance that exercise plays in improving outcomes for people suffering from mental illnesses.
Physical activity in depressive and anxiety disorders
Depression is the leading cause of disability worldwide and is a major contributor to the global burden of disease, as per the World Health Organization. However, only 10%-25% of depressed people actually seek therapy, maybe due to a lack of money, a lack of trained doctors, or the stigma associated with depression [ 29 ]. For those with less severe forms of mental illness, such as depression and anxiety, regular physical exercise may be a crucial part of their treatment and management. Exercise and physical activity might improve depressive symptoms in a way that is comparable to, if not more effective than, traditional antidepressants. However, research connecting exercise to a decreased risk of depression has not been analysed in depth [ 30 ]. Endorphins, like opiates, are opioid polypeptide compounds produced by the hypothalamus-pituitary system in vertebrates in response to extreme physical exertion, emotional arousal, or physical pain. The opioid system may mediate analgesia, social bonding, and depression due to the link between b-endorphins and depressive symptoms (Figure (Figure2 2 ).

The "endorphin hypothesis" states that physical activity causes the brain to produce more endogenous opioid peptides, which reduce pain and boost mood. The latter reduces feelings of worry and hopelessness. A recent study that demonstrated endorphins favourably improved mood during exercise, and provided support for these theories suggested that further research into the endorphin theory is required [ 31 ].
Physical activity and exercise have been shown to improve depressive symptoms and overall mood in people of all ages. Exercise has been implicated in lowering depressive and anxious symptoms in children and adolescents as well [ 32 ]. Pooled research worldwide has revealed that physical exercise is more effective than a control group and is a viable remedy for depression [ 33 ]. Most forms of yoga that start with a focus on breathing exercises, self-awareness, and relaxation techniques have a positive effect on depression and well-being [ 34 ]. Despite claims that exercise boosts mood, the optimal kind or amount of exercise required to have this effect remains unclear and seems to depend on a number of factors [ 35 ].
Exercise as a therapy for unipolar depression was studied in a meta-analysis of 23 randomised controlled trials involving 977 subjects. The effect of exercise on depression was small and not statistically significant at follow-up, although it was moderate in the initial setting. When compared to no intervention, the effect size of exercise was large and significant, and when compared to normal care, it was moderate but still noteworthy [ 36 ]. A systematic evaluation of randomised controlled trials evaluating exercise therapies for anxiety disorders indicated that exercise appeared useful as an adjuvant treatment for anxiety disorders but was less effective than antidepressant treatment [ 37 ].
Conclusions
The effects of exercise on mental health have been shown to be beneficial. Among persons with schizophrenia, yoga was shown to have more positive effects with exercise when compared with no intervention. Consistent physical activity may also improve sleep quality significantly. Patients with alcohol dependence syndrome benefit from a combination of medical therapy and regular exercise since it motivates them to battle addiction by decreasing the craving. There is also adequate evidence to suggest that physical exercise improves depressive and anxiety symptoms. Translating the evidence of the benefits of physical exercise on mental health into clinical practice is of paramount importance. Future implications of this include developing a structured exercise therapy and training professionals to deliver it. The dearth of literature in the Indian context also indicates that more research is required to evaluate and implement interventions involving physical activity that is tailored to the Indian context.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.

COMMENTS
In this paper, we intend to describe sport's physiological and psychosocial health benefits, stemming both from physical activity and from sport participation per se. This narrative review summarizes research and presents health-related data from Swedish authorities.
A recent paper modeled the frequency of salad consumption using residential property values as a predictor of dietary behavior (Drewnowski, Buszkiewicz, and Aggarwal Citation ... Clinical research demonstrates a protective role of strawberries, pomegranate juice, ... many health benefits chronic conditions are interrelated (e.g., microbiota ...
Research suggests that "healthy" food choices such as eating fruits and vegetables have not only physical but also mental health benefits and might be a long-term investment in future well-being.
Since then, research on the health benefits of dietary fibre has continued apace, ... Of 376 papers, 25 datasets were included in the analysis, ... The population-wide health benefits would likely be substantial (particularly for less educated people who are generally at increased risk of health problems), based on the wealth of evidence ...
As described above, plant-based diets have been shown to convey nutritional benefits 48,49, in particular increased fiber, beta carotene, vitamin K and C, folate, magnesium, and potassium intake and an improved dietary health index 83. However, a major criticism of plant-based diets is the risk of nutrient deficiencies for specific ...
Background Sedentary lifestyle is a major risk factor for noncommunicable diseases such as cardiovascular diseases, cancer and diabetes. It has been estimated that approximately 3.2 million deaths each year are attributable to insufficient levels of physical activity. We evaluated the available evidence from Cochrane systematic reviews (CSRs) on the effectiveness of exercise/physical activity ...
Level of physical activity. Recommendations regarding the level of PA required for physical health benefits and the prevention of chronic disease, across the lifespan, are well established (U.S. Department of Health and Human Services, Citation 2018).It is recommended that children and adolescents aged 6-17 years engage in PA of moderate to vigorous intensity for a minimum of 60 min per day.
Introduction. A transition toward healthy and environmentally sustainable food is among major global challenges. Replacing animal sources, namely red meat and milk, with plant-based sources has the potential to impact on cutting greenhouse gas emissions (Springmann et al. Citation 2018).That is a reason for the growing popularity of diets eliminating or reducing meat, milk, dairy, and eggs ...
Overall, the relationship between mental health (i.e. depression) and restrictive eating patterns has been the focus of recent research 20,21,22,24,42; however, causal relationships remain ...
This paper provides an up-to-date summary of research progress with respect to grape, contributing to improved understanding of the relationship between grape, as well as its main bioactive compounds, and human health, in addition to promoting the application of grape in the food industry. ... In this paper, we will focus on the health benefits ...
Background The treatment of noncommunicable diseases (NCD), like coronary heart disease or type 2 diabetes mellitus, causes rising costs for the health system. Physical activity is supposed to reduce the risk for these diseases. Results of cross-sectional studies showed that physical activity is associated with better health, and that physical activity could prevent the development of these ...
Since ancient times, the health benefits of regular physical activity/exercise have been recognized and the classic studies of Morris and Paffenbarger provided the epidemiological evidence in support of such an association. Cardiorespiratory fitness, often measured by maximal oxygen uptake, and habitual physical activity levels are inversely related to mortality. Thus, studies exploring the ...
Considering these health benefits of probiotics, now it has been applied to different food materials which are designated as functional food. This review explored a portrait of the beneficial effects of probiotics on human health. ... Another research with VSL3 on this group of patients has reported that this bacterium could reduce aspartate ...
Abstract. Exercise has long been recognized for its physical health benefits, but its impact on mental health has gained increasing attention in recent years. This paper provides a comprehensive ...
Abstract. For centuries, Curcuma longa (turmeric) was used as a spice in Asian cuisine and as a medicinal herb for treatment of inflammation, pain, wound healing, and digestive disorders, to name a few. Considerable preclinical research found that turmeric and its bioactive curcuminoid polyphenols can affect a variety of chronic conditions.
Herbal teas are used as therapeutic vehicles in many forms of traditional medicine and are a popular global beverage. The purpose of this scoping review was to examine the evidence relating to the clinical efficacy and safety of herbal teas, and to identify the main research themes and gaps in knowledge to inform further work. A scoping review methodology was followed that set out the research ...
Direct evidence supporting kombucha's benefits for human health is lacking. A systematic review published in 2003 found no clinical studies related to kombucha [18]. The purpose of our systematic review is to identify the empirical health benefits of kombucha from human subjects research and characterize opportunities for future research. Methods
A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the ...
improve physical fitness, relieve stress, and enhance quality of life. In addition, they may be. addressing specific health conditions, such as back pain, neck pain, arthritis, and anxiety ...
Abstract. There is increasing evidence that gardening provides substantial human health benefits. However, no formal statistical assessment has been conducted to test this assertion. Here, we present the results of a meta-analysis of research examining the effects of gardening, including horticultural therapy, on health.
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing * E-mail: [email protected] Affiliations Department of Global Public Health and Primary Care, University of Bergen, Norway, Bergen Addiction Research, Department of Addiction ...
health benefits associated with the consumption of ghee. Consumption of ghee in an adequate amount, imparts various health benefits such as binds toxins, enhances complexion and glow of the face ...
1. Introduction. Ginger (Zingiber officinale Roscoe), a well-known herbaceous plant, has been widely used as a flavoring agent and herbal medicine for centuries.Furthermore, the consumption of the ginger rhizome is a typical traditional remedy to relieve common health problems, including pain, nausea, and vomiting [].Notably, a prominent number of randomized clinical trials (RCTs) have been ...
In two of our recent papers, transcriptomics was performed on the triceps muscle ... While future research should determine whether the FNDC5 cleavage-product was produced locally in hippocampal neurons or was secreted into the circulation, this finding eloquently displays one mechanism responsible for brain health benefits following exercise ...
INTRODUCTION. Sleep is vital for health and well-being in children, adolescents, and adults. 1-3 Healthy sleep is important for cognitive functioning, mood, mental health, and cardiovascular, cerebrovascular, and metabolic health. 4 Adequate quantity and quality of sleep also play a role in reducing the risk of accidents and injuries caused by sleepiness and fatigue, including workplace ...
There has been a dramatic increase in the body of evidence demonstrating the benefits that come from health research. In 2014, the funding bodies for higher education in the UK conducted an assessment of research using an approach termed the Research Excellence Framework (REF). As one element of the REF, universities and medical schools in the UK submitted 1,621 case studies claiming to show ...
Being a key source of animal food, millet production has been sharply increasing over the last few years in order to cope with the dietary requirements of the ever-increasing world population. It is a splendid source of essential nutrients such as protein, carbohydrates, fat, minerals, vitamins, and also some other bioactive compounds that eventually help through multiple biological activities ...
Research at the Bloomberg School is a team sport. In order to provide extensive guidance, infrastructure, and support in pursuit of its research mission, research@BSPH employs three core areas: strategy and development, implementation and impact, and integrity and oversight. Our exceptional research teams comprised of faculty, postdoctoral ...
People who had severe illness with COVID-19 might experience organ damage affecting the heart, kidneys, skin and brain. Inflammation and problems with the immune system can also happen. It isn't clear how long these effects might last. The effects also could lead to the development of new conditions, such as diabetes or a heart or nervous ...
In order to address a widespread health problem in India, it is useful to do a literature review that draws on research conducted in a variety of settings. In addition, the prevalence of these mental illnesses and the benefits of exercise as a complementary therapy might be made clear by a meta-analysis of research undertaken in India [ 14 ].