Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
The following is a scenario of a patient who experienced ruptured esophageal varices. The patient, Nora Allen, a 55-year-old female presented to the emergency department (ER) on May 5, 2017 just after 10:00 a.m., with complaints of two episodes of melena that morning.
Upon further assessment, her husband expressed concern that his wife had been drinking again because her behavior had seemed “strange”. He had these concerns because his wife had a 20-year history of alcohol abuse and was “under a lot of stress” due to the recent loss of her job. Nora has a history of alcoholism, cirrhosis of the liver, coronary artery disease (CAD) and anemia.
Upon assessment in the ER, her vital signs were 110/76, 85bpm, 95% on room air, 98.2ºF, 22 breaths/min. Neurologically, she was oriented to person, time and situation, but was confused as to where she was. Her pupils were 2mm and sluggish to light. She appeared anxious, was slurring her speech and had visible hand tremors. She reported nausea and melena, and had obvious ascites with a distended firm abdomen. An occult stool was positive and her skin was cool and clammy with poor skin turgor and weak pedal pulses. The remainder of her body systems were within normal limits.
While in the ER, 1 L normal saline (NS) bolus was administered, labs were drawn, a foley catheter was inserted. A blood glucose (finger stick) was taken. She was kept NPO for a scheduled endoscopy later that day.
At 1300, the patient began vomiting and was given an emesis bag. At 1310, Nora’s husband quickly informed the nurse that his wife was violently vomiting bright red blood. On reassessment, the patient’s BP was 94/63 and the HR was 98bpm. The patient was started on octreotide 50mcg bolus. She was intubated for airway protection and to prevent aspiration. She was administered 1 unit of packed red blood cells (PRBCs) and was taken immediately to the gastrointestinal lab for a STAT endoscopy for possible esophageal banding therapy.
The patient was diagnosed with ruptured esophageal varices and sent to the ICU for close observation and monitoring after successful placement of 12 esophageal bands. Overnight, the patient was administered another (1) unit of PRBC, to maintain a hemoglobin greater than 8 g/dL. The following morning at 10:45 a.m. Mrs. Allen was extubated after a successful sedation vacation and spontaneous breathing trial.
When the patient was stable, she was transferred to the medical-surgical floor for observation and case management. On the floor, the patient was educated regarding the reason for the ruptured esophageal varices and taught about her new prescription, propranolol, which, if taken correctly, should decrease the chance of a re-bleed. Lastly, although the patient was resistant to the cessation of alcohol consumption, she met with case management and was educated on alcohol cessation programs and support groups, such as alcoholics anonymous.
Discussion Questions
- If you were the case manager who was educating this unwilling patient about the various rehabilitation facilities, how would you have approached the situation?
- In this scenario, the patient’s vomiting caused the varix to rupture. What type of interventions or nursing assessment tools could have been utilized to prevent vomiting and subsequent rupture of the varix?
- During this scenario, the patient began to vomit large amounts of blood. How would you deal with family members who are in the room who may be shocked about this sudden change in the patient’s condition while also taking care of your patient?
Nursing Case Studies by and for Student Nurses Copyright © by jaimehannans is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.

Share This Book
Patient Education Reference Center - Case Scenario #1 - Esophageal Varices
Nov 13, 2018 • knowledge, information.
Clinical Case Scenario #1
Health Education for a Male Inpatient with Esophageal Varices
Clinical Scenario
Forty five year old G.K. is admitted to the hospital’s Emergency Department. He is vomiting large amounts of blood. He is mildly intoxicated but alert. His pulse is fast and thready. An IV of normal saline 0.9% is started; he is given oxygen by mask, and is prepared to receive multiple units of packed red blood cells as needed (blood transfusions). He is transferred to the endoscopy lab for an immediate procedure to investigate the source of the bleeding.
In the Endoscopy lab it will be determined that he has bleeding esophageal varices, and a procedure will be performed to stop the bleeding. He will then be admitted to the hospital for observation.
Nursing goals include explanation of all procedures he is to undergo, given his level of comprehension, and written handouts for review later when fully conscious.
Searching in Patient Education Reference Center (PERC)
Esophageal varices: The nurse uses PERC to gather the handout she will need for Mr. K. From the Home page, she searches for esophageal varices She chooses the Conditions source type She reads #1: Esophageal varices and checks the paper to glean meaningful information to share with Mr. K. She prints the hospital-customized handout.
Blood transfusion: The nurse searches Procedures & Lab Tests and finds Blood Transfusion for Mr. K.
Endoscopy The nurse returns to the results list and chooses the Procedure source type. She prints Upper GI Endoscopy and shares the paper with Mr. K before he is sedated, answering his questions and providing reassurance.
The gastroenterologist performs the endoscopy and locates the bleeding site. He uses Endoscopic Band Ligation to stop the bleeding. M. K. recovers quickly and is returned to his room.
Mr. K. is visited by the hospital’s social worker to discuss the potentiality of alcoholism and is referred to a community services program for follow up. The Social worker uses PERC to search for alcoholism. She prints Alcoholism and Alcohol Abuse and reviews the paper with Mr. K.
Following his hospital stay, Mr. K is discharged home with Social Services support. The nurse chooses the Discharge Instructions source type and prints both Discharge Instructions for Upper GI endoscopy and Discharge Instructions for Endoscopic Band Ligation . She discusses his instructions which include important follow up instructions such as seeing his doctor within 2 days and avoiding alcohol.
Following Mr. K.’s discharge, the nurse adds all patient education papers to folders and their related subfolders in PERC, to make available to the other members of the multidisciplinary team.
Handouts for Acute Care Folders in PERC
Blood and Blood Products Blood transfusion GI : esophageal varices GI : Procedures Upper GI Endoscopy, Upper Band Ligation GI : Discharge info Discharge Instructions for Upper GI endoscopy, Discharge Instructions for Endoscopic Band Ligation. Psychosocial : Alcoholism


NurseStudy.Net
Nursing Education Site

Esophageal Varices Nursing Diagnosis and Care Plan
Last updated on February 20th, 2023 at 09:15 am
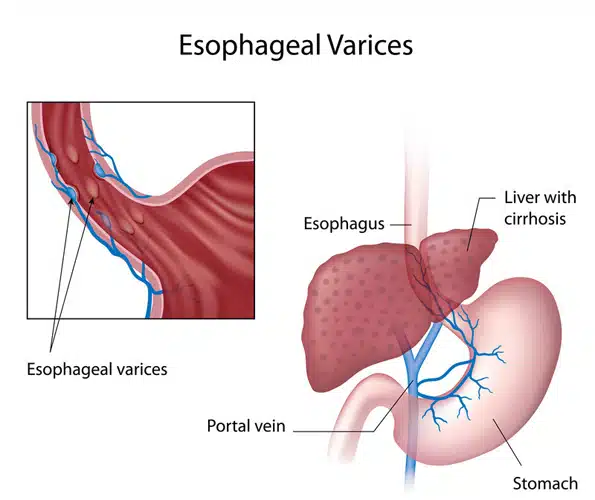
Esophageal varices are veins that are abnormally enlarged and are usually found on the lower two-thirds of the esophagus.
They arise from the blockage of the portal vein of the liver. Instead of flowing through the portal vein, the blood flows through the smaller blood vessels, which eventually causes venous enlargement, leakage, or even rupture.
The treatment plan for esophageal varices is mainly focused on the prevention or stoppage of their rupture and bleeding.
Signs and Symptoms of Esophageal Varices
Unless they are bleeding, esophageal varices may not present any signs or symptoms. A patient with bleeding esophageal varices may show:
- Hematemesis or vomiting large amounts of blood
- Black, tarry or bloody stools
- Lightheadedness
- Loss of consciousness due to severe bleeding
Esophageal varices related to liver disease may have the following symptoms:
- Jaundice or yellow coloration of eyes and skin
- Ascites or abdominal fluid buildup
- Getting easily bruised
Causes and Risk Factors of Esophageal Varices
Liver disease cause scarring of the liver tissue known as cirrhosis of the liver. The liver scar tissues facilitate the backing up of the blood flow, thus increasing the pressure in the liver’s portal vein.
This condition is known as portal hypertension. To compensation for the increased pressure, the blood is forced to flow in smaller veins, including those veins that are found in the esophagus’ lowest part.
Their small size means that they cannot accommodate a large volume of blood. The veins may balloon, rupture, and bleed in time.
Aside from liver cirrhosis, thrombosis or the formation of blood clot in the portal vein or splenic vein may cause esophageal varices.
Parasitic infection of the liver, such as schistosomiasis found in Asia, Africa, Caribbean countries, and South America, can cause liver damage and lead to the formation of esophageal varices.
Alcohol abuse may lead to the rupture and bleeding of esophageal varices.
Complications of Esophageal Varices
Bleeding is the most life-threatening complication of esophageal varices. Internal bleeding may result to shock due to loss of a significant amount of blood, and this is fatal.
Another complication of esophageal varices is the increased risk for another rupture and bleeding episode of other varices.
Diagnosis of Esophageal Varices
- Physical examination and history taking – to check for any hematemesis, black tarry or blood stools, as well as to explore any alcohol abuse or history of liver disease
- Endoscopy – to visualize the gastrointestinal system, looking for any dilation of veins and any presence of red spots or red streaks which may indicate a very high risk of rupture and bleeding. Treatment of bleeding esophageal varices can also be done during this exam.
- Capsule endoscopy – to perform endoscopy but with the use of a capsule that has a camera in it. The patient swallows this like a pill, and the camera takes images of the GI tract as it goes down. This is more expensive than the usual endoscopy, but can be helpful for those who cannot tolerate the endoscope tube
- Imaging – CT scan and ultrasound Doppler of the portal and splenic veins
Treatment of Esophageal Varices
- Portal vein drugs. Beta blockers such as propanolol and nadolol can help treat portal hypertension by lowering the blood pressure in the portal vein. These reduce the risk for esophageal varices rupture and bleeding. After a bleeding episode, drugs like vasopressin and octreotide can be prescribed for up to 5 days to reduce the blood flow in the portal vein.
- Endoscopic band ligation. This procedure can be done while the patient is undergoing endoscopic exam and the doctor finds out that there are esophageal varices that are bleeding, or at high risk of rupture and bleed in the future. The doctor uses an elastic band to tie off the veins that are bleeding.
- Balloon tamponade. To stop the bleeding, this procedure involves inflating a balloon temporarily (for up to 24 hours) in order to place pressure on the esophageal varices.
- Transjugular intrahepatic portosystemic shunt (TIPS). This procedure is used to create a diversion of the blood flow away from the portal vein by means of making a shunt or an opening between the hepatic vein and the portal vein.
Esophageal Varices Nursing Diagnosis
Nursing care plan for esophageal varices 1.
Nursing Diagnosis: Risk for Bleeding secondary to esophageal varices
Desired Outcome : The patient will be able to avoid having any frank or occult bleeding and will remain hemodynamically stable.
Nursing Care Plan for Esophageal Varices 2
Nursing Diagnosis: Imbalanced Nutrition: Less than Body Requirements related to digestive tract bleeding secondary to esophageal varices, as evidenced by hematemesis, weight loss, nausea and vomiting, loss of appetite and dizziness/ lightheadedness
Desired Outcome : The patient will be able to achieve a weight within his/her normal BMI range, demonstrating healthy eating patterns and choices.
Nursing Care Plan for Esophageal Varices 3
Risk for Decreased Cardiac Output
Nursing Diagnosis: Risk for Decreased Cardiac Output related to bleeding secondary to esophageal varices .
Desired Outcomes:
- The patient will exhibit adequate cardiac output after the bleeding is controlled as evidenced by stable vital signs, normal peripheral perfusion, adequate intake and output, warm and dry skin, absence of breathing difficulties, and normal level of consciousness.
- The patient will be able to demonstrate self-care activities to improve gastrointestinal and cardiac health.
Nursing Care Plan for Esophageal Varices 4
Risk for Deficient Fluid Volume
Nursing Diagnosis: Risk for Deficient Fluid Volume related to nausea and hematemesis secondary to esophageal varices.
- The patient will verbalize a decrease in the severity of nausea and vomiting.
- The patient will maintain adequate fluid volume while waiting for treatment as evidenced by stable vital signs, adequate skin perfusion, strong peripheral pulses, alert mental state, and urine output greater than 30ml per hour.
Nursing Care Plan for Esophageal Varices 5
Risk for Injury
Nursing Diagnosis: Risk for Injury related to lightheadedness secondary to bleeding esophageal varices.
- The patient will be able to prevent injury by doing activities within the parameters of limitation and modifying the environment to adapt to the patient’s capacity.
- The patient will be able to perform activities of daily living with minimal supervision and maintain a treatment regimen to regain balance and increase compliance.
More Esophageal Varices Nursing Diagnosis
- Risk for Shock
- Disturbed Body Image
- Deficient Knowledge
Nursing References
Ackley, B. J., Ladwig, G. B., Makic, M. B., Martinez-Kratz, M. R., & Zanotti, M. (2020). Nursing diagnoses handbook: An evidence-based guide to planning care . St. Louis, MO: Elsevier. Buy on Amazon
Gulanick, M., & Myers, J. L. (2022). Nursing care plans: Diagnoses, interventions, & outcomes . St. Louis, MO: Elsevier. Buy on Amazon
Ignatavicius, D. D., Workman, M. L., Rebar, C. R., & Heimgartner, N. M. (2020). Medical-surgical nursing: Concepts for interprofessional collaborative care . St. Louis, MO: Elsevier. Buy on Amazon
Silvestri, L. A. (2020). Saunders comprehensive review for the NCLEX-RN examination . St. Louis, MO: Elsevier. Buy on Amazon
Disclaimer:
Please follow your facilities guidelines and policies and procedures. The medical information on this site is provided as an information resource only and is not to be used or relied on for any diagnostic or treatment purposes. This information is not intended to be nursing education and should not be used as a substitute for professional diagnosis and treatment.
Anna Curran. RN, BSN, PHN
Leave a Comment Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .


The Royal Children's Hospital Melbourne
- My RCH Portal

- Health Professionals
- Patients and Families
- Departments and Services
- Health Professionals
- Departments and Services
- Patients and Families
- Research
Nursing guidelines
- About nursing guidelines
- Nursing guidelines index
- Developing and revising nursing guidelines
- Other useful clinical resources
- Nursing guideline disclaimer
- Contact nursing guidelines
In this section
Acute management of an oesophageal variceal bleed

Introduction
Oesophageal varices (and indeed any varices) are a rare but serious complication of portal hypertension. Portal hypertension is defined by an increase in pressure within the portal circulatory system and is caused by an increase in vascular resistance in blood flow through the liver. In RCH’s patient population, portal hypertension is frequently a result of biliary atresia, but it can also be a manifestation of post-hepatic, pre-hepatic (e.g. portal vein obstruction), or other intra-hepatic problems such as cystic fibrosis, congenital hepatic fibrosis or other cirrhosis. With increased resistance in the portal vascular system, blood begins to shunt through collateral systemic vessels to return to the vena cava. Prolonged elevation in portal vein pressure causes dilatation of the collateral vessels, which can form internal varices in the rectum and varices in the gastro-oesophageal veins. Oesophageal variceal bleeds occur when the variceal wall tension exceeds the wall strength and subsequently rupture. Children with liver disease also have liver dysfunction, which results in clotting cascade problems and a deficit in Vitamin K-dependent factors. This increases the risk of significant haemorrhage in this patient group.
In several large studies of children with portal hypertension, approximately two thirds presented with haematemesis (vomiting blood) or melaena (blood in stool/dark stools caused by upper GI bleeds), usually from rupture of an oesophageal varix. Twenty to thirty percent of children with biliary atresia have variceal bleeds and tend to develop varices early, with an estimated risk of bleeding of fifteen percent before the age of two. Mortality rates associated with large bleeds range from zero to fifteen percent.
The aim of this guideline is to assist nurses and other health professionals in the management of infants and children with oesophageal varices to minimise risk of variceal bleeding. This guideline will also outline the management of an acute oesophageal variceal bleed.
Definition of Terms
- GI - gastrointestinal
- NGT – Nasogastric tube
- PPIs – Proton pump inhibitors (e.g., omeprazole, pantoprazole)
Assessment of Patients with Known or Suspected Varices
- Complete primary assessment as per Clinical Guidelines (Nursing) : Nursing assessment
- Routine observations as per Clinical Guidelines (Nursing): Observation and Continuous Monitoring
- Monitor for signs of hypovolaemia: e.g. tachycardia, hypotension.
- Investigations: FBE, UEC, VBG, Coagulation Screen, Group & hold, blood cultures, BGL, ammonia, see specimen collection .
- Monitor for upper GI bleeding (e.g. haematemesis or coffee ground vomit)
- Monitor for melaena or fresh blood in stools.
- Capture clinical images of melaena & haematemesis to guide volumes of blood loss.
- Monitor for abdominal distension or protruding umbilical veins, especially when seen in combination with other listed symptoms.
- Assess coagulation screen for risk of bleeding.
- Maintain strict fluid balance including measurement or estimation of losses, including urine output.
- Monitor for neurological changes.
Management of Children with Known or Suspected Varices
- Stylet should be removed.
- Insertion should be carried out by senior staff members or medical personnel in high-risk patients. Eg those who have had previous GI bleeds, those with significant coagulopathies, those with high grades varices as identified by treating team.
- NGT should not have a gastric aspirate taken from the tube. Instead, position should be confirmed by x-ray.
- Ensure patient has valid blood product consent on file (see Consent- Informed Procedure )
- Family should be provided with education on varices and at home emergency management plan by Liver & Intestinal Transplant Clinical Nurses Consultants or Gastroenterology team members.
- Patients should have routine surveillance gastroscopies to monitor status and progression of varices. Banding or sclerotherapy can be undertaken as required. Frequency of gastroscopies is dictated by treating gastroenterologist.
- Patients should have the following investigations prior to gastroscopies: FBE, UEC, coagulation screen, group & hold.
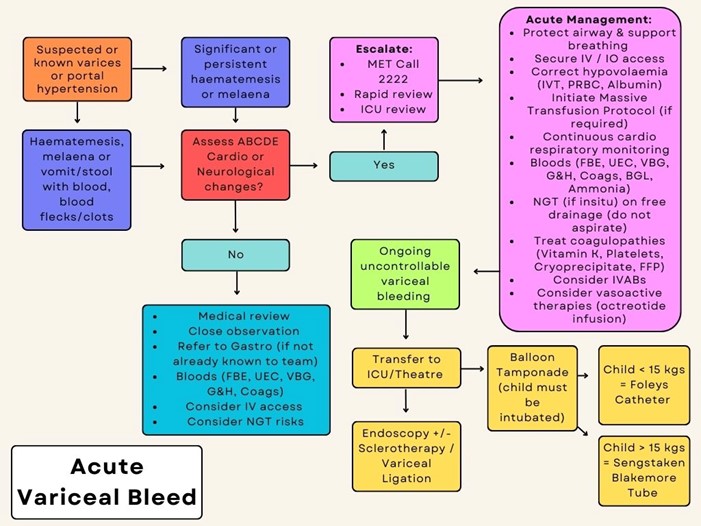
Acute Management of Variceal Bleed (refer to algorithm overleaf)
- Seek urgent medical/ ICU review/ MET (ext. 2222).
- Protect airway, support breathing as required (see Resuscitation guidelines ). Give oxygen to patients with significant circulatory impairment or shock.
- Secure large bore IV access.
- Consider need to activate Massive Haemorrhage and Critical Bleeding Procedure .
- No more than 20mL/kg NaCl bolus. Albumin should also be given if further fluid administration is required.
- RBCs if Hb <70g/L.
- Maintain strict fluid balance.
- Octreotide, IV bolus followed by IV infusion. Refer to Medication Guideline: Octreotide for dosing
- Always discuss with a gastroenterology fellow or consultant before commencing vasoactive therapy.
- Once bleeding controlled, slow wean of octreotide as per Octreotide Medication Guideline
- Monitor BGL 6 hourly whilst on octreotide infusion due to risk of hypoglycaemia and/or hyperglycaemia – escalate any abnormal results to treating team
- Continuous cardiorespiratory monitoring (see Clinical Guidelines (Nursing): Observation and Continuous Monitoring ).
- If NGT in situ, place on free drainage. Do NOT aspirate NGT . NGT should only be inserted under endoscopic guidance or with gastroenterologist consent.
- Consider treating coagulopathies (vitamin K, platelets, cryoprecipitate and FFP).
- If bleeding is ongoing and uncontrollable, patient will require Balloon Tamponade (Foleys Catheter if child <15kg or Sengstaken Blakemore tube if child>15kg). This will ideally be performed in the ICU or theatre environment.
- Transfer to ICU or theatre for management as clinically appropriate
- Consider prophylactic intravenous antibiotics.
- Patient should be kept nil by mouth (NBM) until bleeding controlled and medically cleared for oral intake.
- Whilst NBM, all regular medications should be given IV.
- Consider use of PPI: IV whilst NBM followed by oral once cleared for oral intake.
Management of Patients with Recent Variceal Bleeding/Banding
- Patients should remain nil by mouth (NBM) post variceal bleeding or banding and grade up diet as directed by treating gastroenterologist.
- Ensure patient has a valid group and hold in case requires transfusion.
- Maintain IV access.
- Ensure foley catheter is at bedside in case of re-bleed. If leaving the ward, patient must take catheter with them.
Special considerations
- For CVAD management (see Central Venous Access Device )
- Blood transfusion safety adherence (see Blood Transfusion Procedure ).
- Massive Transfusion Protocol (see Massive Haemorrhage and Critical Bleeding Procedure ).
- If body fluid splash (see Procedure Needlestick Injuries and Blood-Body Fluid Exposures ).
Algorithm for Management of Acute Variceal Bleed
- PICU Guidelines: Liver Protocols
- RCH Liver Transplant Protocol
- RCH Enteral Feeding and Medication Administration CPG
Clinician websites
- American College of Gastroenterology Practice Guideline
- British Society of Gastroenterology Guidelines
- The National Institute for Health and Care Excellence Clinical Guideline
- World Gastroenterology Guideline
- NICE Guideline: Stent Insertion for Bleeding Oesophageal Varices
Information for parents
- Children’s Liver Disease Foundation
- Starship Parent Guide to Portal Hypertension
- Starship Parent Guide to Liver Transplant
*Please remember to read the disclaimer .
The development of this nursing guideline was coordinated by Rachel Horn, CNC, Gastroenterology, and Laura Davies, CNS, Cockatoo, approved by the Nursing Clinical Effectiveness Committee. Updated December 2023.
Evidence table

- Editorial Board
- Special Issues
- Submit Manuscript
- Articles in Press
- Current Issue
- Author Guidelines
- Indexing Completed
Esophageal varices incidences are increasing by nearly 5% every year. Esophageal varices are the causes of bleeding in approximately 18% of hospital admissions for upper GI bleeding.
Objective: The purpose of this study is to understand the cause, clinical manifestations and treatment course for esophageal varices.
Material and methods: Detailed clinical history and physical examination was done. All the pertinent investigations were studied thoroughly of the selected case.
Results: The esophageal varices is confirmed with the help of upper gastrointestinal gastroscopy and repaired by banding.
Conclusion: Esophageal varices usually go undiagnosed due to early common symptoms. Hematemesis is one of the suggestive of esophageal varices and OGD is only the confirmative diagnosis for it. The prognosis depends usually better if treatment received on time.
INTRODUCTION
Esophageal varices are abnormal, enlarged veins in the tube that connects the throat and stomach. Variceal rupture is governed by Laplace's law. Increased wall tension is the end result of increased intravariceal pressure, increased diameter of the varices and reduced wall thickness. The variceal wall thickness can be evaluated visually by the presence of red wale markings. These markings reflect areas where the wall is especially thin [1]. Variceal rupture often occurs at the level of the gastroesophageal junction, where the varices are very superficial and thus have thinner walls. Esophageal varices are the major complication of portal hypertension [2].
RISK FACTORS
· Large esophageal varices
· Red marks on the esophageal varices as seen on a lighted stomach scope (endoscopy)
· Portal hypertension
· Severe cirrhosis
· A bacterial infection
· Excessive alcohol use
· Excessive vomiting
· Constipation
· Severe coughing bouts
DIAGNOSTIC INVESTIGATIONS
· Blood tests
· Endoscopy
· Imaging tests, such as CT and MRI scans
ESOPHAGEAL VARICES SYMPTOMS
· Hematemesis
· Stomach pain
· Light-headedness or loss of consciousness
· Melena (black stools)
· Bloody stools (in severe cases)
· Shock (excessively low blood pressure due to blood loss that can lead to multiple organ damage)
Therapeutic approaches are variceal ligation (banding) and sclerotherapy. Banding is a medical procedure which uses elastic bands for constriction. Banding may be used to tie off blood vessels in order to stop bleeding, as in the treatment of bleeding esophageal varices. The band restricts blood flow to the ligated tissue, so that it eventually dies and sloughs away from the supporting tissue.
Sclerotherapy is a form of treatment where a doctor injects medicine into blood vessels or lymph vessels that causes them to shrink. It is commonly used to treat varicose veins or so-called spider veins. The procedure is non-surgical, requiring only an injection.
An 85 year old patient with known case of diabetes mellitus, hypertension since last 20 years and status post percutaneous transluminal coronary (2002, 2011 and 2015). Patient had no history of any bad habits like cigarette smoking, alcohol consumption or any other drug substance. Patient was found semiconscious at home at night suddenly and when aroused by relatives, patient had hematemesis of around 500 ml at home. Patient was brought to hospital. The patient had complaints of malena and acidity since one week. Patient was investigated in the form of alkaline phosphatase, alpha fetoprotein, serum glutamic pyruvic transaminase, SGOT, bilirubin, glucose, IgG, CBC, USG KUB, ABG, Electrocardiogram, APTT, ESR, USG whole abdomen.
CBC shows Hb less than 7 g/dl. USG whole abdomen was suggestive of reduced size of liver with diffusely altered echo texture and surface irregularity, suggestive of chronic liver parenchymal disease. Few small tortuous mesenteric venous collaterals, could be suggestive of portal hypertension.
Upper gastrointestinal gastroscopy was suggestive of esophageal varices. One band applied endoscopically and further, patient was managed with anti-diabetics, antacid, analgesic, antibiotic, beta blocker, statin and other supportive care.
Esophageal varices usually go undiagnosed until hematemesis occur. Hematemesis is a medical emergency and always occur due to upper GI tract bleeding. The color is usually bright red in color. The hematemesis is treated with somatostatin analogue (e.g. Octreotide) or vasopressin (e.g. Terlipressin); it helps to reduce splanchnic blood flow. The Glasgow-Blatch Ford Bleeding scoring system score is used to determine the risk. This scale is purely based on clinical and biochemical parameters. The warning signs of esophageal varices is dizziness even when awake, weight loss, low Hb level, complaints of acidity, heartburn, hematemesis and malena.
Patients with hypertension and diabetes have poor prognosis as medicines have adverse effects on liver which can lead to liver cirrhosis and portal hypertension as well. At advanced age, the banding is done for symptomatic treatment only as no other option is available at this age.
1. Hilzenrat N, Sherker AH (2012) Esophageal varices: Pathophysiology, approach and clinical dilemmas. Int J Hepatol 2012: 35-40.
2. Maruyama H, Yokosuka O (2012) Pathophysiology of portal hypertension and esophageal varices. Int J Hepatol 2012: 38-42.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
- SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Rheumatology Research (ISSN:2641-6999)
- Journal of Pathology and Toxicology Research
- Advance Research on Alzheimers and Parkinsons Disease
Newsletter signup
- Open Access
- A-Z Journals
- Editor in Chiefs
Journal Groups
- Engineering
- Life Sciences

- Pre-Nursing
- Nursing School
- After Graduation
Esophageal Varices: Patho, Manifestations, & Diagnostics

Jump to Sections
- What is a Varice?
Esophageal Varices Causes
Signs and symptoms of esophageal varices, esophageal varices assessment & diagnostics, esophageal varices nclex question.
Esophageal varices are complications due to liver diseases or dysfunctions. It’s important to identify and treat the underlying cause of them to prevent complications such as bleeding and improve patient outcomes.
You can think of esophageal varices as the result of having a plumbing backup.
What is a Varice? (The Septic Tank Analogy)
Associating esophageal varices with having a backup of septic tank content is helpful since you can compare the liver to the body’s septic tank. If the septic tank becomes clogged or has hardened, there will be an immediate backup from the system.
Toilets and sinks will have a backflow of waste products – water will not drain properly from the shower.
The Liver: The Body’s Septic Tank
As food is chewed inside the mouth, it goes down the esophagus, then into the stomach to be broken down by gastric juices. Digested food now goes inside the duodenum, the first part of the small intestine.
After the duodenum , the contents are sucked into the portal vein that goes through the pancreas. This process is referred to as the first-pass phenomenon concerning medications.
Hardening of the Liver
When the liver hardens due to some type of scarring, for instance:
- Cirrhosis that’s basically the production of scar tissue in the liver
Whatever the condition is, the backing up of blood into the portal vein is the main cause of esophageal varices.
Esophageal varices are primarily caused by increased pressure in the portal vein system, which can result from various underlying medical conditions. Some common causes of esophageal varices include:
- Cirrhosis : Cirrhosis scarring can obstruct blood flow through the liver, leading to increased pressure in the portal vein system.
- Hepatitis : Chronic hepatitis, a viral infection that causes inflammation and scarring of the liver, can also lead to esophageal varices.
- Congenital disorders : Rare genetic disorders, such as Budd-Chiari syndrome or portal vein thrombosis, can obstruct the portal vein and lead to increased pressure in the portal vein system.
- Thrombosis : Blood clots in the portal vein or its branches can cause portal hypertension and lead to the development of esophageal varices.
- Schistosomiasis : A parasitic infection common in parts of Africa and South America, schistosomiasis can cause liver damage and develop esophageal varices.
Since there is the backing up of blood or fluid inside the esophagus, the following manifestations will occur:
- Esophageal bleeding
- Vomiting with blood
- Bloody stools
- Decreased blood pressure (due to decreased volume caused by bleeding)
- A skyrocketing heart rate. Due to the decreased hemoglobin, the heart will compensate by pumping faster to distribute oxygen to the body’s different systems.
- Tachycardia
One of the main diagnostic procedures for patients with esophageal varices is esophagogastroduodenoscopy (EGD) . With EGD, a tube with a camera is inserted to visualize the inside of the esophagus.
Liver function tests are also done to check the status of the liver through abnormalities with alanine transaminase (ALT) and aspartate aminotransferase (AST).
The hemoglobin and hematocrit levels are also tested because if there is bleeding anywhere in the body, the lab results for H&H will be low.
When a patient comes in with perfused bleeding from his mouth, should you do an EGD?
Answer: No . The airway is the priority in any situation. In this case, if the patient is vomiting blood, stopping the bleeding is paramount.
The first thing to do is to relieve the patient of the blood by suctioning its mouth, and once the bleeding ceases, that’s when an EGD is done.
Aside from suctioning, there are other procedures done to stop the bleeding, namely:
- Medications
- Inserting a balloon catheter into the esophagus
Study More in Less Time
Using a study tool can help you keep engaged and at a steady pace when studying conditions like esophageal varices.
SimpleNursing is trusted by nursing students to retain more nursing school material while cutting down on study hours. We have adaptive exams to tailor the learning experience to each individual learner’s needs, interests, and abilities.
Get a better understanding of your nursing material .
Want to ace Nursing School Exams & the NCLEX?
Make topics click with easy-to-understand videos & more. We've helped over 1,000,000 students & we can help you too.
Share this post
Nursing students trust simplenursing.

SimpleNursing Student Testimonial
Most recent posts.

How to Remove a PICC Line
Does the idea of removing a PICC line make you feel uneasy? You’re not alone.…

What to Do If You’re Retaking the NCLEX
Jump to Sections Is the NCLEX harder the second time? What Repeat NCLEX Test Takers…

The NCLEX-RN Test Plan for 2024
Embarking on a nursing career requires not only dedication and passion in school, but also…

How to Calculate Heart Rate on an ECG with the 6 Second Method
If you’ve ever watched a medical drama (any “Grey’s Anatomy” fans here?), you’ve probably seen…
Find what you are interested in
- Patient Care & Health Information
- Diseases & Conditions
- Esophageal varices
- Upper endoscopy
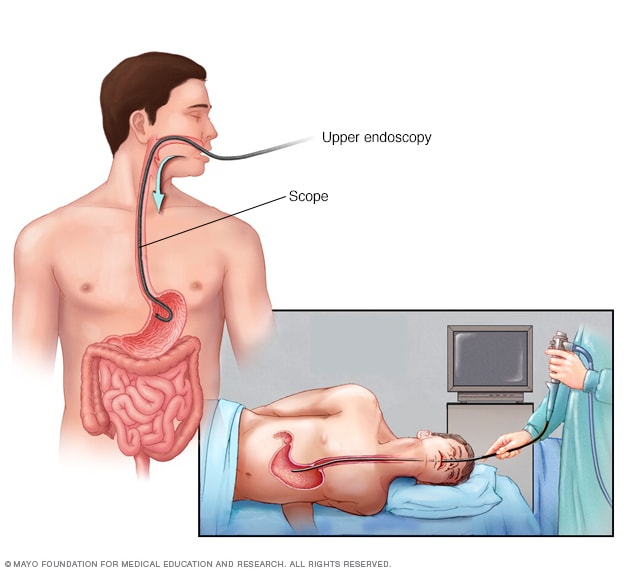
During an upper endoscopy, a healthcare professional inserts a thin, flexible tube equipped with a light and camera down the throat and into the esophagus. The tiny camera provides a view of the esophagus, stomach and the beginning of the small intestine, called the duodenum.
If you have cirrhosis, your health care provider typically screens you for esophageal varices when you're diagnosed. How often you'll have screening tests depends on your condition. Main tests used to diagnose esophageal varices are:
Endoscopic exam. A procedure called upper gastrointestinal endoscopy is the preferred method of screening for esophageal varices. An endoscopy involves inserting a flexible, lighted tube called an endoscope down the throat and into the esophagus. A tiny camera on the end of the endoscope lets your doctor examine your esophagus, stomach and the beginning of your small intestine, called the duodenum.
The provider looks for dilated veins. If found, the enlarged veins are measured and checked for red streaks and red spots, which usually indicate a significant risk of bleeding. Treatment can be performed during the exam.
- Imaging tests. Both abdominal CT scans and Doppler ultrasounds of the splenic and portal veins can suggest the presence of esophageal varices. An ultrasound test called transient elastography may be used to measure scarring in the liver. This can help your provider determine if you have portal hypertension, which may lead to esophageal varices.
More Information
- Capsule endoscopy
The primary aim in treating esophageal varices is to prevent bleeding. Bleeding esophageal varices are life-threatening. If bleeding occurs, treatments are available to try to stop the bleeding.
Treatment to prevent bleeding
Treatments to lower blood pressure in the portal vein may reduce the risk of bleeding esophageal varices. Treatments may include:
- Medicines to reduce pressure in the portal vein. A type of blood pressure drug called a beta blocker may help reduce blood pressure in your portal vein. This can decrease the likelihood of bleeding. Beta blocker medicines include propranolol (Inderal, Innopran XL) and nadolol (Corgard).
Using elastic bands to tie off bleeding veins. If your esophageal varices appear to have a high risk of bleeding, or if you've had bleeding from varices before, your health care provider might recommend a procedure called endoscopic band ligation.
Using an endoscope, the provider uses suction to pull the varices into a chamber at the end of the scope and wraps them with an elastic band. This essentially "strangles" the veins so that they can't bleed. Endoscopic band ligation carries a small risk of complications, such as bleeding and scarring of the esophagus.
Treatment if you're bleeding
Bleeding esophageal varices are life-threatening, and immediate treatment is essential. Treatments used to stop bleeding and reverse the effects of blood loss include:
- Using elastic bands to tie off bleeding veins. Your provider may wrap elastic bands around the esophageal varices during an endoscopy.
- Taking medicines to slow blood flow into the portal vein. Medicines such as octreotide (Sandostatin) and vasopressin (Vasostrict) slow the flow of blood to the portal vein. Medicine is usually continued for up to five days after a bleeding episode.
Diverting blood flow away from the portal vein. If medicine and endoscopy treatments don't stop the bleeding, your provider might recommend a procedure called transjugular intrahepatic portosystemic shunt (TIPS).
The shunt is an opening that is created between the portal vein and the hepatic vein, which carries blood from your liver to your heart. The shunt reduces pressure in the portal vein and often stops bleeding from esophageal varices.
But TIPS can cause serious complications, including liver failure and mental confusion. These symptoms can develop when toxins that the liver normally would filter are passed through the shunt directly into the bloodstream.
TIPS is mainly used when all other treatments have failed or as a temporary measure in people awaiting a liver transplant.
Placing pressure on varices to stop bleeding. If medicine and endoscopy treatments don't work, your provider may try to stop bleeding by applying pressure to the esophageal varices. One way to temporarily stop bleeding is by inflating a balloon to put pressure on the varices for up to 24 hours, a procedure called balloon tamponade. Balloon tamponade is a temporary measure before other treatments can be performed, such as TIPS .
This procedure carries a high risk of bleeding recurrence after the balloon is deflated. Balloon tamponade also may cause serious complications, including a rupture in the esophagus, which can lead to death.
- Restoring blood volume. You might be given a transfusion to replace lost blood and a clotting factor to stop bleeding.
- Preventing infection. There is an increased risk of infection with bleeding, so you'll likely be given an antibiotic to prevent infection.
- Replacing the diseased liver with a healthy one. Liver transplant is an option for people with severe liver disease or those who experience recurrent bleeding of esophageal varices. Although liver transplantation is often successful, the number of people awaiting transplants far outnumbers the available organs.
Re-bleeding
There is a high risk that bleeding might recur in people who've had bleeding from esophageal varices. Beta blockers and endoscopic band ligation are the recommended treatments to help prevent re-bleeding.
After initial banding treatment, your provider typically repeats your upper endoscopy at regular intervals. If necessary, more banding may be done until the esophageal varices are gone or are small enough to reduce the risk of further bleeding.
Potential future treatment
Researchers are exploring an experimental emergency therapy to stop bleeding from esophageal varices that involves spraying an adhesive powder. The hemostatic powder is given through a catheter during an endoscopy. When sprayed on the esophagus, hemostatic powder sticks to the varices and may stop bleeding.
Another potential way to stop bleeding when all other measures fail is to use self-expanding metal stents (SEMS). SEMS can be placed during an endoscopy and stop bleeding by placing pressure on the bleeding esophageal varices.
However, SEMS could damage tissue and can migrate after being placed. The stent is typically removed within seven days and bleeding could recur. This option is experimental and isn't yet widely available.
- Blood transfusion
- Liver transplant
Preparing for your appointment
You might start by seeing your primary health care provider. Or you may be referred immediately to a provider who specializes in digestive disorders, called a gastroenterologist. If you're having symptoms of internal bleeding, call 911 or your local emergency number to be taken to the hospital for urgent care.
Here's some information to help you get ready for an appointment.
What you can do
When you make the appointment, ask if there's anything you need to do in advance, such as fasting before a specific test. Make a list of:
- Your symptoms, including any that seem unrelated to the reason for your appointment.
- Key personal information, including major stresses, recent life changes or recent travels, family and personal medical history, and your alcohol use.
- All medications, vitamins or other supplements you take, including doses.
- Questions to ask your doctor.
Take a family member or friend along, if possible, to help you remember information you're given.
For esophageal varices, questions to ask include:
- What's likely causing my symptoms?
- What other possible causes are there?
- What tests do I need?
- What's the best course of action?
- What are the side effects of the treatments?
- Are my symptoms likely to recur, and what can I do to prevent that?
- I have other health conditions. How can I best manage them together?
- Are there restrictions that I need to follow?
- Should I see a specialist?
- Are there brochures or other printed materials I can have? What websites do you recommend?
Don't hesitate to ask other questions.
What to expect from your doctor
Your provider is likely to ask you questions, such as:
- When did your symptoms begin?
- Have your symptoms stayed the same or gotten worse?
- How severe are your symptoms?
- Have you had signs of bleeding, such as blood in your stools or vomit?
- Have you had hepatitis or yellowing of your eyes or skin (jaundice)?
- Have you traveled recently? Where?
- If you drink alcohol, when did you start and how much do you drink?
What you can do in the meantime
If you develop bloody vomit or stools while you're waiting for your appointment, call 911 or your local emergency number or go to an emergency room immediately.
- Sanyal AJ. Overview of the management of patients with variceal bleeding. https://www.uptodate.com/contents/search. Accessed Jan. 11, 2023.
- Varices. Merck Manual Professional Version. https://www.merckmanuals.com/professional/gastrointestinal-disorders/gastrointestinal-bleeding/varices/?autoredirectid=1083. Accessed Jan. 11, 2023.
- Ferri FF. Esophageal varices. In: Ferri's Clinical Advisor 2023. Elsevier; 2023. https://www.clinicalkey.com. Accessed Jan. 17, 2023.
- Rockey DC. Causes of upper gastrointestinal bleeding in adults. https://www.uptodate.com/contents/search. Accessed Jan. 11, 2023.
- Sanyal AJ, et al. Prediction of variceal hemorrhage in patients with cirrhosis. https://www.uptodate.com/contents/search. Accessed Jan. 11, 2023.
- 13 ways to a healthy liver. American Liver Foundation. https://liverfoundation.org/resource-center/blog/13-ways-to-a-healthy-liver/. Accessed Jan. 17, 2023.
- AskMayoExpert. Esophageal and gastric varices. Mayo Clinic; 2022.
- Zuckerman MJ, et al. Endoscopic treatment of esophageal varices. Clinical Liver Disease. 2022; doi:10.1016/j.cld.2021.08.003.
Associated Procedures
Products & services.
- A Book: Mayo Clinic on Digestive Health
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
You are using an outdated browser. Please upgrade your browser to improve your experience.
- About Journal

Asian Journal of Nursing Education and Research
2349-2996 (Online) 2231-1149 (Print)
Nursing Care in Esophageal Varices
Author(s): Ajaya Ghosh Ru , Athuldev T , Sarika M L
Email(s): [email protected] , [email protected] , [email protected]

Address: Ajaya Ghosh Ru1, Athuldev T2, Sarika M L3 1Nursing Officer, Dept of Nursing, AIIMS Bhubaneswar, Bhubaneswar, Odisha 2Lecturer, Dept of Medical Surgical Nursing, MIMS College of Nursing, Calicut, Kerala 3Medical Surgical Nursing, JIPMER College of Nursing, Puducherry *Corresponding Author
Published In: Volume - 9 , Issue - 2 , Year - 2019

ABSTRACT: Objectives To identify and understand Nursing care in patients with esophageal varices.; Methods to adopt while managing acute variceal bleeding.; Nurses role in prevention of secondary bleeding. Design: used for this article is to review method. Data sources are different types of Medical and Gastroenterology text books, Medical Journals and Medical Surgical Nursing Text Books. The results that can be found; esophageal varices and paraesophageal varices are swollen veins in the lining of the lower esophagus. In most of the cases esophageal varices occur in people who have portal hypertension with variety of etiology. The veins don't enlarge in a uniform fashion. Esophageal varices usually have enlarged, irregularly shaped bulbous regions (varicosities) that are interrupted by narrower regions. These abnormal dilated veins rupture easily and can bleed profusely because, The pressure inside the varices is higher than the pressure inside normal veins; The walls of the varices are thin. About 50% of people who have bleeding from esophageal varices will have the problem return during the first one to two years. It is very much common among severe liver disease and ongoing alcohol consumption. Screening is done by an Upper GI Endoscopy.The preventive method used in patients is to give beta blockers. Bleeding from esophageal varices is an emergency that requires immediate treatment which includes variceal ligation and trans-jugular intra hepatic portosystemic shunt. Vasopressin and somatostatin analogue are main two drugs used to treat active bleeding. Conclusion: this article deals with management of esophageal varices which include both medical and nursing management. With effective and prompt nursing care for varices, the life of patients can be extended.
- Esophageal varices
- variceal ligation and transjugular intrahepatic portosystemic shunt (TIPS)

Cite this article: Ajaya Ghosh Ru, Athuldev T, Sarika M L. Nursing Care in Esophageal Varices. Asian J. Nursing Education and Research. 2019; 9(2):273-277. doi: 10.5958/2349-2996.2019.00059.4 Cite(Electronic): Ajaya Ghosh Ru, Athuldev T, Sarika M L. Nursing Care in Esophageal Varices. Asian J. Nursing Education and Research. 2019; 9(2):273-277. doi: 10.5958/2349-2996.2019.00059.4 Available on: https://ajner.com/AbstractView.aspx?PID=2019-9-2-27
Recomonded Articles:

Asian Journal of Nursing Education and Research (AJNER) is an international, peer-reviewed journal devoted to nursing sciences....... Read more >>>
Quick Links
- Submit Article
- Author's Guidelines
- Paper Template
- Copyright Form
- Cert. of Conflict of Intrest
- Processing Charges
- Indexing Information

Latest Issues
Popular articles, recent articles.

- Background/Objectives:
- technologically
- Nursing"[Mesh])
- "Australia"[Mesh]
- Undergraduate
- SARS-CoV-2.
- Cross-sectional
- Stress Resilience
- Nursing students.
- national-level
- facility-based
- cross-sectional
- Multicollinearity
- Neonatal Mortality
- administration
- schoolchildren
- television/mass
- First aid and safety measures
- school children
- computer assisted Teaching.
- undergraduates
- Research anxiety
- Research methods
- Research usefulness.
- considerations
- transformative
- administrative
- Artificial intelligence
- Clinical Decision Support Systems
- Natural Language Processing
- Workflow Optimization and Resource Management.
- under-nutrition
- non-experimental
- non-probability
- Malnutrition
- School going children.
- pre-experimental
- identification
- Effective Planned Teaching Programme
- Breast Cancer
- Prevention.
- End of life care
- Attitude. Frommelt Attitude Towards Care of Dying (FATCOD).
- evidence-based
- socio-economic
- Pediatric Obesity
- Childhood Obesity
- Nursing Strategies
- Pediatric Wellness.
- Pre-experimental
- Effectiveness of Structured Teaching Program
- Organ Donation
- transportation
- Nurse Practitioner
- Role of Nurse.
- physical/biological
- responsibility
- Premenstrual Syndrome
- Nursing officers
- Coping measures
- Premenstrual dysphoric disorder
- PMS and work performance
- PMS and relationship with patients/co-workers.
- differentiation
- Care giver satisfaction
- Nursing care services
- Care givers.
- self-administered
- confidentiality
- hospitalization
- Individuals
- Prevention strategies.
- Cardiovascular
- disease-causing
- cardiovascular
- community-based
- cardio-vascular
- socio-demographic
- Younger Adults
- Rashtriya Bal Swasthya Karyakram
- District Early Intervention Centre
- Menstrual Cup
- Nursing Students.
- revolutionized
- AI based technology
- Nursing Education
- Nursing Practice.
Esophageal Varices Nursing Management
- Bleeding esophageal varices are hemorrhagic processes involving dialted, tortuous veins in the submucosa of the lower esophagus. .
Risk Factors
- Portal hypertension resulting from obstructed portal venous circulation
Pathophysiology
- In portal hypertension, collateral circulation develops in the lower esophagus as venous blood, which is diverted from the GI tract and spleen because of portal obstruction, seeks an outlet.
- Because of excessive intraluminal pressure, these collateral veins become tortuous, dilated, and fragile. They are particularly prone to ulceration and hemorrhage. Rupture of esophageal varices is the most common cause of death of clients with hepatic cirrhosis.
image by : hepatitisc.uw.edu
Assessment/Clinical Manifestations/Signs And Symptoms
- Hematemesis and melena, if ulcerated massive hemorrhage occurs
- Signs of hepatic encephalopathy
- Dilated abdominal veins
Laboratory and diagnostic study findings
- Endoscopy identifies the cause and site of bleeding
- Ultrasound and computed tomography assist in identifying the site of bleeding
Medical Management
Non-surgical treatment is preferred because of the high mortality associated with emergency surgery to control bleeding from esophageal varices and because of the poor physical condition of most of these patients.
Nonsurgical measures include:
- Pharmacologic therapy: somatostatin, vasopressin, beta-blocker and nitrates
- Balloon tamponade, saline lavage, endoscopic sclerotherapy
- Transjugular intrahepatic portosystemic shunting (TIPS)
- Esophageal banding therapy, variceal band ligation
If necessary, surgery may involve:
- Bypass procedures (e.g. portacaval shunts, splenorenal shunt, mesocaval shunt)
- Devascularization and transaction
Aggressive medical care includes evaluation of extent of bleeding and continuous monitoring of vital signs when hematemesis and melena are present.
Signs of potential hypovolemia are noted; blood volume is monitored with a central venous pressure or arterial catheter.
Oxygen is administered to prevent hypoxia and maintain adequate blood oxygenation, and intravenous fluids and volume expanders are administered to restore fluid volume and replace electrolytes.
Need for blood transfusion is assessed, and intake and output (insert indwelling catheter) are monitored.
Nursing Diagnosis
- Risk for bleeding
- Imbalanced nutrition: less than body requirements
Nursing Management
Provide ongoing assessment.
- Assess for ecchymosis, epistaxis, petechiae, and bleeding gums
- Monitor level of consciousness, vital signs, and urinary output to evaluate fluid balance.
- Monitor the client during blood transfusion administration if prescribed.
Institute measure to address bleeding.
- Use small-gauge needles, and apply pressure or cold for bleeding.
Provide nursing care for the client undergoing a prescribed balloon tamponade to control bleeding.
- Explain the procedure to the client to reduce fear and enhance cooperation with insertion and maintenance of the esophageal tamponade tube.
- Monitor the client closely to prevent accidental removal or displacement of the tube with resultant airway obstruction.
Provide nursing intervention for the client undergoing a prescribed iced saline lavage.
- Ensure nasogastric tube patency to prevent aspiration
- Observe gastric aspirate for evidence of bleeding.
- Protect the client from chilling.
After injection sclerotherapy, assess for:
- Esophageal perforation
- Continued bleeding
After portal-systemic surgical intervention, monitor for complications.
- Development of systemic encephalopathy
- Liver failure
Administer prescribed medications, which may include vasopressin and vitamin K.
Related posts, endocarditis nursing management, magnetic resonance imaging, bone marrow aspiration and biopsy.
Hepatic Cirrhosis

- Hepatic cirrhosis is a chronic hepatic disease characterized by diffuse destruction and fibrotic regeneration of hepatic cells.

Table of Contents
- What is Hepatic Cirrhosis?
Classification
Pathophysiology, statistics and incidences, clinical manifestations, complications, assessment and diagnostic findings, pharmacologic therapy, surgical management, nursing assessment, nursing diagnosis, nursing care planning & goals, nursing interventions, discharge and home care guidelines, documentation guidelines, what is hepatic cirrhosis.
The end-stage of liver disease is called cirrhosis.
- As necrotic tissue yields to fibrosis, this disease alters liver structure and normal vasculature, impairs blood and lymph flow, and ultimately causes hepatic insufficiency.
- The prognosis is better in noncirrhotic forms of hepatic fibrosis, which cause minimal hepatic dysfunction and don’t destroy liver cells.
These clinical types of cirrhosis reflect its diverse etiology:
- Laennec’s cirrhosis. The most common type, this occurs in 30% to 50% of cirrhotic patients, up to 90% of whom have a history of alcoholism.
- Biliary cirrhosis. Biliary cirrhosis results in injury or prolonged obstruction.
- Postnecrotic cirrhosis. Postnecrotic cirrhosis stems from various types of hepatitis .
- Pigment cirrhosis. Pigment cirrhosis may result from disorders such as hemochromatosis.
- Cardiac cirrhosis. Cardiac cirrhosis refers to cirrhosis caused by right-sided heart failure .
- Idiopathic cirrhosis. Idiopathic cirrhosis has no known cause.
Although several factors have been implicated in the etiology of cirrhosis, alcohol consumption is considered the major causative factor.
- Necrosis. Cirrhosis is characterized by episodes of necrosis involving the liver cells.
- Scar tissue. The destroyed liver cells are gradually replaced with a scar tissue.
- Fibrosis. There is diffuse destruction and fibrotic regeneration of hepatic cells.
- Alteration. As necrotic tissue yields to fibrosis, the disease alters the liver structure and normal vasculature, impairs blood and lymph flow, and ultimately causes hepatic insufficiency.
Various types of cirrhosis may occur in different types of individuals.
- The most common, Laennec’s cirrhosis, occurs in 30% to 50% of cirrhotic patients.
- Biliary cirrhosis occurs in 15% to 20% of patients.
- Postnecrotic cirrhosis occurs in 10% to 30% of patients.
- Pigment cirrhosis occurs in 5% to 10% of patients.
- Idiopathic cirrhosis occurs in about 10% of patients.
Different types of cirrhosis have different causes.
- Excessive alcohol consumption. Too much alcohol intake is the most common cause of cirrhosis as liver damage is associated with chronic alcohol consumption.
- Injury. Injury or prolonged obstruction causes biliary cirrhosis.
- Hepatitis . The different types of hepatitis can cause postnecrotic cirrhosis.
- Other diseases. Diseases such as hemochromatosis causes pigment cirrhosis.
- Right-sided heart failure. Cardiac cirrhosis, a rare kind of cirrhosis, is caused by right-sided heart failure.
Clinical manifestations of the different types of cirrhosis are similar, regardless of the cause.
- GI system. Early indicators usually involve gastrointestinal signs and symptoms such as anorexia , indigestion, nausea , vomiting constipation , or diarrhea .
- Respiratory system . Respiratory symptoms occur late as a result of hepatic insufficiency and portal hypertension , such as pleural effusion and limited thoracic expansion due to abdominal ascites, interfering with efficient gas exchange leading to hypoxia.
- Central nervous system . Signs of hepatic encephalopathy also occur as a late sign, and these are lethargy , mental changes, slurred speech, asterixis (flapping tremor), peripheral neuritis, paranoia, hallucinations , extreme obtundation, and ultimately, coma.
- Hematologic. The patient experiences bleeding tendencies and anemia .
- Endocrine. The male patient experiences testicular atrophies, while the female patient may have menstrual irregularities, and gynecomastia and loss of chest and axillary hair .
- Skin. There is severe pruritus, extreme dryness, poor tissue turgor, abnormal pigmentation, spider angiomas, palmar erythema, and possibly jaundice .
- Hepatic. Cirrhosis causes jaundice, ascites, hepatomegaly, edema of the legs, hepatic encephalopathy, and hepatic renal syndrome.
The complications of hepatic cirrhosis include the following:
- Portal hypertension . Portal hypertension is the elevation of pressure in the portal vein that occurs when blood flow meets increased resistance.
- Esophageal varices. Esophageal varices are dilated tortuous veins in submucosa of the lower esophagus .
- Hepatic encephalopathy. Hepatic encephalopathy may manifest as deteriorating mental status and dementia or as physical signs such as abnormal involuntary and voluntary movements.
- Fluid volume excess . Fluid volume excess occurs due to an increased cardiac output and decreased peripheral vascular resistance.
Laboratory findings and imaging studies that are characteristic of cirrhosis include:
- Liver scan. Liver scan shows abnormal thickening and a liver mass.
- Liver biopsy . Liver biopsy is the definitive test for cirrhosis as it detects destruction and fibrosis of the hepatic tissue.
- Liver imaging. Computed tomography scan , ultrasound, and magnetic resonance imaging may confirm the diagnosis of cirrhosis through visualization of masses, abnormal growths, metastases, ans venous malformations.
- Cholecystography and cholangiography. These two visualize the gallbladder and the biliary duct system.
- Splenoportal venography. Splenoportal venography visualizes the portal venous system.
- Percutaneous transhepatic cholangiography. This test differentiates intrahepatic from extrahepatic obstructive jaundice and discloses hepatic pathology and the presence of gallstones .
- Complete blood count . There is decreased white blood cell count, hemoglobin level and hematocrit, albumin, or platelets .
Medical Management
Treatment is designed to remove or alleviate the underlying cause of cirrhosis.
- Diet . The patient may benefit from a high-calorie and a medium to high protein diet , as developing hepatic encephalopathy mandates restricted protein intake.
- Sodium restriction. is usually restricted to 2g/day .
- Fluid restriction. Fluids are restricted to 1 to 1.5 liters/day .
- Activity. Rest and moderate exercise is essential.
- Paracentesis. Paracentesis may help alleviate ascites.
- Sengstaken-Blakemore or Minnesota tube. The Sengstaken-Blakemore or Minnesota tube may also help control hemorrhage by applying pressure on the bleeding site.
Drug therapy requires special caution because the cirrhotic liver cannot detoxify harmful agents effectively.
- Octreotide . If required, octreotide may be prescribed for esophageal varices.
- Diuretics . Diuretics may be given for edema , however, they require careful monitoring because fluid and electrolyte imbalance may precipitate hepatic encephalopathy.
- Lactulose. Encephalopathy is treated with lactulose.
- Antibiotics . Antibiotics are used to decrease intestinal bacteria and reduce ammonia production, one of the causes of encephalopathy.
Surgical procedures for management of hepatic cirrhosis include:
- Transjugular intrahepatic portosystemic shunt (TIPS) procedure. The TIPS procedure is used for the treatment of varices by upper endoscopy with banding to relieve portal hypertension .
Nursing Management
Nursing management for the patient with cirrhosis of the liver should focus on promoting rest, improving nutritional status , providing skin care , reducing risk of injury , and monitoring and managing complications.
Assessment of the patient with cirrhosis should include assessing for:
- Bleeding. Check the patient’s skin, gums, stools, and vomitus for bleeding.
- Fluid retention. To assess for fluid retention, weigh the patient and measure abdominal girth at least once daily.
- Mentation. Assess the patient’s level of consciousness often and observe closely for changes in behavior or personality.
Based on the assessment data, the major nursing diagnosis for the patient are:
- Activity intolerance related to fatigue , lethargy , and malaise.
- Imbalanced nutrition : less than body requirements related to abdominal distention and discomfort and anorexia.
- Impaired skin integrity related to pruritus from jaundice and edema .
- High risk for injury related to altered clotting mechanisms and altered level of consciousness.
- Disturbed body image related to changes in appearance, sexual dysfunction , and role function.
- Chronic pain and discomfort related to enlarged liver and ascites.
- Fluid volume excess related ascites and edema formation.
- Disturbed thought processes and potential for mental deterioration related to abnormal liver function and increased serum ammonia level.
- Ineffective breathing pattern related to ascites and restriction of thoracic excursion secondary to ascites, abdominal distention, and fluid in the thoracic cavity.
Main Article: 8 Liver Cirrhosis Nursing Care Plans
The major goals for a patient with cirrhosis are:
- Report decrease in fatigue and increased ability to participate in activities.
- Maintain a positive nitrogen balance, no further loss of muscle mass, and meet nutritional requirements.
- Decrease potential for pressure ulcer development and breaks in skin integrity .
- Reduce the risk of injury.
- Verbalize feelings consistent with improvement of body image and self-esteem .
- Increase level of comfort .
- Restore normal fluid volume.
- Improve mental status, maintain safety, and ability to cope with cognitive and behavioral changes.
- Improve respiratory status.
The patient with cirrhosis needs close observation, first-class supportive care, and sound nutrition counseling.
Promoting Rest
- Position bed for maximal respiratory efficiency; provide oxygen if needed.
- Initiate efforts to prevent respiratory, circulatory, and vascular disturbances.
- Encourage patient to increase activity gradually and plan rest with activity and mild exercise.
Improving Nutritional Status
- Provide a nutritious, high-protein diet supplemented by B-complex vitamins and others, including A, C, and K.
- Encourage patient to eat: Provide small, frequent meals, consider patient preferences, and provide protein supplements, if indicated.
- Provide nutrients by feeding tube or total PN if needed.
- Provide patients who have fatty stools (steatorrhea) with water-soluble forms of fat-soluble vitamins A, D, and E, and give folic acid and iron to prevent anemia .
- Provide a low-protein diet temporarily if patient shows signs of impending or advancing coma; restrict sodium if needed.
Providing Skin Care
- Change patient’s position frequently.
- Avoid using irritating soaps and adhesive tape.
- Provide lotion to soothe irritated skin; take measures to prevent patient from scratching the skin.
Reducing Risk of Injury
- Use padded side rails if patient becomes agitated or restless.
- Orient to time, place, and procedures to minimize agitation.
- Instruct patient to ask for assistance to get out of bed.
- Carefully evaluate any injury because of the possibility of internal bleeding.
- Provide safety measures to prevent injury or cuts (electric razor, soft toothbrush).
- Apply pressure to venipuncture sites to minimize bleeding.
Monitoring and Managing Complications
- Monitor for bleeding and hemorrhage .
- Monitor the patient’s mental status closely and report changes so that treatment of encephalopathy can be initiated promptly.
- Carefully monitor serum electrolyte levels are and correct if abnormal.
- Administer oxygen if oxygen desaturation occurs; monitor for fever or abdominal pain , which may signal the onset of bacterial peritonitis or other infection .
- Assess cardiovascular and respiratory status; administer diuretics, implement fluid restrictions, and enhance patient positioning , if needed.
- Monitor intake and output , daily weight changes, changes in abdominal girth, and edema formation.
- Monitor for nocturia and, later, for oliguria, because these states indicate increasing severity of liver dysfunction.
Home Management
- Prepare for discharge by providing dietary instruction, including exclusion of alcohol.
- Refer to Alcoholics Anonymous , psychiatric care, counseling, or spiritual advisor if indicated.
- Continue sodium restriction; stress avoidance of raw shellfish.
- Provide written instructions, teaching, support, and reinforcement to patient and family.
- Encourage rest and probably a change in lifestyle (adequate,well-balanced diet and elimination of alcohol).
- Instruct family about symptoms of impending encephalopathy and possibility of bleeding tendencies and infection.
- Offer support and encouragement to the patient and provide positive feedback when the patient experiences successes.
- Refer patient to home care nurse , and assist in transition from hospital to home.
Expected patient outcomes include:
- Reported decrease in fatigue and increased ability to participate in activities.
- Maintained a positive nitrogen balance, no further loss of muscle mass, and meet nutritional requirements.
- Decreased potential for pressure ulcer development and breaks in skin integrity .
- Reduced the risk of injury.
- Verbalized feelings consistent with improvement of body image and self-esteem .
- Increased level of comfort .
- Restored normal fluid volume.
- Improved mental status, maintain safety, and ability to cope with cognitive and behavioral changes.
- Improved respiratory status.
The focus of discharge education is dietary instructions.
- Alcohol restriction. Of greatest importance is the exclusion of alcohol from the diet, so the patient may need referral to Alcoholics Anonymous, psychiatric care, or counseling.
- Sodium restriction. Sodium restriction will continue for considerable time, if not permanently.
- Complication education. The nurse also instructs the patient and family about symptoms of impending encephalopathy, possible bleeding tendencies, and susceptibility to infection.
The focus of documentation may include:
- Level of activity.
- Causative or precipitating factors.
- Vital signs before, during, and following activity.
- Plan of care.
- Response to interventions, teaching, and actions performed.
- Teaching plan.
- Changes to plan of care.
- Attainment or progress toward desired outcome.
- Caloric intake.
- Individual cultural or religious restrictions, personal preferences.
- Availability and use of resources.
- Duration of the problem.
- Perceptio of pain , effects on lifestyle, and expectations of therapeutic regimen .
- Results of laboratory tests, diagnostic studies, and mental status and cognitive evaluation .
Other posts from the site you may like:
- 8 Liver Cirrhosis Nursing Care Plans
- 7 Hepatitis Nursing Care Plans
- Bile Duct Stones (Choledocholithiasis)
1 thought on “Hepatic Cirrhosis”
Good effective management of cirrhosis that will help nurse student to manage patients: well done health team🙏
Leave a Comment Cancel reply

Let’s check in on Mr. Abrams
By now Mrs. Abrams has arrived and is able to provide information about the patient’s home medications, which she has brought with her. You inspect the pill bottles and note Mr. Abrams takes metoprolol, hydralazine, levothyroxine, metformin, furosemide, naproxen, and atorvastatin.
Why does he take metoprolol?
Hypertension
Why does he take hydralazine?
Why does he take levothyroxine?
Hypothyroidism
Why does he take metformin?
Type 2 diabetes
Why does he take furosemide?
To reduce edema and ascites associated with chronic liver disease
Now that we know he takes furosemide, what labs will we double check?
Creatinine and potassium…remember, furosemide is a loop diuretic that causes potassium losses! It also can cause creatinine to increase.
Why does he take naproxen?
Osteoarthritis
Why does he take atorvastatin?
Hyperlipidemia
Which medication is a red flag?
The naproxen is a red flag because of its association with GI bleeding. Not only can it cause peptic ulcers to form and bleed, studies show it can also contribute to the bleeding of esophageal varices. Since esophageal varices develop in patients with liver disease due to portal hypertension, and Mr. Abrams has low platelets and an elevated prothrombin time, we are very concerned about his use of this medication.
What do we want to ask his wife about in regards to this medication?
We want to ask his wife how much naproxen he takes. She tells you he takes it multiple times daily, so now we are now really, really, really concerned!
Physical assessment
You perform a full head-to-toe assessment on Mr. Abrams. Significant findings reveal that he is lethargic, and disoriented to time and situation. You reorient the patient, but repeat assessment shows continued confusion. Heart sounds are normal, pulse is weak and fast in the mid 120’s, capillary refill is 3 seconds. Pt is tachypneic, complaining of shortness of breath, and speaking in three to four word sentences. Accessory muscle use is present and lung sounds are normal. Abdomen is moderately distended, with caput medusae present. Pt shows 2+ edema in bilateral lower extremities. Skin signs reveal jaundice and pallor.
Why is Mr. Abrams lethargic?
Anemia secondary to GI bleed.
Why is Mr. Abrams disoriented?
One reason for confusion or disorientation is that he lost consciousness at home and woke up in the ambulance. This would be disorienting to anyone! However, hypoxia causes confusion, which is definitely a concern for this patient.
Why is his pulse weak?
Low circulating blood volume reduces preload, which reduces cardiac stretch. Reduced cardiac stretch leads to reduced cardiac output and a weak pulse.
Are we concerned about his capillary refill?
This is right at the edge of delayed capillary refill. Any further blood loss is going to affect this even more, so yes, we are concerned.
Why is the patient tachypneic, short of breath, and using accessory muscles?
Low hemoglobin means less oxygen is being delivered to the tissues, so Mr. Abrams is hypoxic. As a compensatory mechanism, his respiratory rate and work of breathing has increased. This also causes him to feel shortness of breath.
Why is Mr. Abrams’ abdomen distended?
Patients with chronic liver disease have increased portal hypertension and low circulating plasma proteins, so fluid is easily lost into the abdomen due to third-spacing (a condition called ascites). Increased vascular pressure can also cause an enlarged spleen, and the liver is likely enlarged as well. Both of these enlarged organs can contribute to abdominal enlargement. Note that when ascites is significant, it also causes shortness of breath, so we want to watch Mr. Abrams closely for increased abdominal distention and associated respiratory compromise.
What is caput medusae?
Caput medusae is associated with portal hypertension and is due to the shunting of blood through umbilical veins, which become engorged and visible on the surface.
Why does the patient have 2+ pitting edema in the BLE?
Mr. Abrams has low serum albumin and therefore, low circulating plasma proteins. This means oncotic pressure is decreased and fluid is able to leak into the interstitial space (third-spacing).
How do we assess for jaundice in Mr. Abrams and what does it indicate?
In individuals with dark or yellow-toned skin, the best place to observe for jaundice is the sclera. Note that if a patient with darker skin has callouses on the palms or soles of their feet, these can appear yellow without jaundice being present. You can also observe for a yellow discoloration of the oral mucosa by looking at the hard palate. Jaundice indicates there is a buildup of bilirubin in the blood.
How do we assess for pallor in Mr. Abrams?
Assessing for pallor in individuals with darker skin tones can be difficult. The mucus membranes of very dark skinned individuals tend to appear ashen or gray while the color is more yellowish-brown in those with brown skin tones. You can also observe the palmar surface which may appear paler than usual. Note that fluorescent lighting can give the skin a bluish tint, so use a halogen lamp or natural light.
Now, back to Mr. Abrams
While you are calling the MD to let her know that Mr. Abrams’ labs have resulted, you hear a scream coming from his bay. You rush in to see Mrs. Abrams shouting, “He’s bleeding, he’s bleeding!” as Mr. Abrams vomits bright red blood.

Thankfully, Dr. Jones has also heard the commotion and is rushing to the bedside. She orders an emergent blood transfusion and calls for a central line kit while you suction the oropharynx and maintain Mr. Abrams in a side-lying position. The central line is inserted and secured just as the four units of unmatched O-negative blood are delivered to his bay. Dr. Jones has ordered a stat chest X-ray and the technician has also just shown up. At this time you notice cyanotic skin signs and the monitor reveals a HR of 132, BP 76/43, RR 28, SpO2 72% on 3L oxymask.
What is the significance of Mr. Abrams vomiting bright red blood?
The blood is bright red because it is fresh, meaning the bleeding is actively occurring. The fact that he is vomiting the blood tells us this is an upper GI bleed, possibly from esophageal varices or a bleeding ulcer.
Why did the blood bank send up O-negative blood?
O-negative blood is the “universal donor” blood and can be administered in emergency situations without a type and screen or type and crossmatch.
What’s going on with his blood pressure?
Mr. Abrams’ blood pressure has dropped significantly due to loss of circulating volume.
Does Mr. Abrams need volume or does he need vasopressors like norepinephrine to improve his blood pressure?
Mr. Abrams needs volume! The blood transfusions will help and he’ll also likely get fluids at the same time. We need to increase circulating volume quickly to avoid circulatory collapse, especially since it appears he is actively hemorrhaging!
Why did Dr. Jones insert a central venous catheter?
A central venous catheter allows for the rapid infusion of blood and fluid. Plus, with a blood pressure in the 70s, getting a peripheral IV is going to be incredibly difficult.
How do you assess for cyanosis in Mr. Abrams, who has dark skin?
In dark skinned individuals, cyanosis is most likely to be noticed as a gray or whitish tint around the mouth or a bluish or gray discoloration of the conjunctiva.
How would you assess for cyanosis in Mr. Abrams if he had yellow skin tones?
In individuals with yellow skin tones, cyanosis presents as a grayish-greenish skin color.
Why did Dr. Jones order a stat chest X-ray?
A chest X-ray is used to verify position of the central venous catheter prior to its use.
What’s going on with his vital signs? What concerns you and what are you going to do about it?
The significant drop in SpO2 is very concerning. A good plan would be to escalate oxygen delivery to a non-rebreather at 100% FiO2. Depending on how he responds, this may be sufficient, or the patient may need intubation and mechanical ventilation.
What’s next for Mr. Abrams?
Luckily Mr. Abrams maintains an adequate oxygen saturation level on the non-rebreather (whew!) and Dr. Jones calls the gastroenterologist on-call to tell him Mr. Abrams needs a STAT EGD (upper endoscopy). The GI specialist orders a pantoprazole infusion and octreotide infusion to be started immediately and tells Dr. Jones the team can be there in 60 minutes.
Though the transfusion and fluids have improved Mr. Abrams’ blood pressure, it still has not risen above 88 systolic and Dr. Jones knows patients can bleed out quickly. She calls for an intubation tray and a Blakemore tube. Mr. Abrams is intubated and then Dr. Jones places the Blakemore tube. Once the nasogastric tube is set to intermittent LWS, you see initial drainage of blood and what looks like coffee grounds, but after a minute, it appears to slow down. Good job! We’ve just bought Mr. Abrams some time.
What is the purpose of pantoprazole in GI bleeds?
Pantoprazole is used in upper GI bleeds to reduce gastric acid and minimize its detrimental effects on platelet aggregation, thereby reducing bleeding.
What is the purpose of octreotide for Mr. Abrams?
Octreotide inhibits vasodilatory hormones, which reduces blood flow in the GI tract along with portal and variceal pressures. Because we suspect Mr. Abrams is bleeding from esophageal varices, this medication may be beneficial.
Why was Mr. Abrams intubated? He seemed to be doing okay with the non-rebreather.
Mr. Abrams was intubated for airway protection.
What is the purpose of a Blakemore tube?
Though not as common as they once were thanks to the use of endoscopic procedures, a Sengstaken-Blakemore tube is a nasogastric tube with two inflatable balloons. Once inflated, the gastric tube in the stomach helps reduce blood flow to the esophageal varices, which can help reduce bleeding. If needed, the other balloon, which is located along the length of the tubing, is inflated to provide gentle steady pressure that helps tamponade bleeding esophageal varices. The tube also has an opening at the bottom for drainage of gastric contents.
Why did Dr. Jones consult the gastroenterologist?
With bleeding, the most important intervention is to make it stop. The Blakemore tube is only a temporary measure and should not be inflated for more than six hours. An esophagogastroduodenoscopy (EGD) enables the gastroenterologist to locate and stop the bleeding through various techniques such as clipping, band ligation, and injection of localized epinephrine.
What’s up with the coffee ground substance from the NG tube?
When blood sits in the stomach it coagulates, giving it a coffee-ground appearance. Sometimes patients will vomit this substance (coffee-ground emesis), so if you see this…think GI bleed!
Help is here to save the day!
At this point, Mr. Abrams is in the hands of the endoscopy team who have elected to perform this life-saving procedure at the bedside in the ER. As the primary nurse, you monitor Mr. Abrams’ vital signs and, since he is intubated, you manage sedation through a continuous infusion of propofol and fentanyl. The procedure takes approximately one hour and you realize as the team finishes up that Mr. Abrams has been lying in the supine position for quite some time. Before transferring him to the ICU, you perform a skin assessment to look for early signs of pressure injury and thankfully don’t find any. You transport Mr. Abrams to the ICU for close monitoring and are happy to see much improved vital signs: HR 84, BP 110/71, RR 18, SpO2 98% on 40% FiO2. You anticipate Mr. Abrams being extubated in the ICU once the propofol wears off and being transferred to the Med Surg floor tomorrow. Good job!
Why are propofol and fentanyl often used together?
Propofol provides sedation, but does not provide any pain control. Fentanyl provides pain control and some sedation. Together, the patient is sedated and pain is managed.
How do you assess for stage 1 pressure injury in a patient with dark skin like Mr. Abrams?
In a patient with lighter skin, you assess for a stage 1 pressure injury by checking for non-blanching erythema. Because darker skin may not show blanching even when healthy, checking for non-blanching erythema will not be beneficial. In fact, erythema may not even be noticeable. Check the area for variations in skin color and note it may be warmer or cooler than surrounding tissue, firmer than surrounding tissue, have noticeable edema or bogginess, or be painful.
A happy ending
Mr. Abrams is extubated later that day and shows no signs of further bleeding or hemodynamic compromise. He is provided education about avoiding NSAIDs and taught to recognize the signs of GI bleeding. He is discharged home after a three day hospital stay. Good job working with Mr. Abrams!
_____________________________________
The information, including but not limited to, audio, video, text, and graphics contained on this website are for educational purposes only. No content on this website is intended to guide nursing practice and does not supersede any individual healthcare provider’s scope of practice or any nursing school curriculum. Additionally, no content on this website is intended to be a substitute for professional medical advice, diagnosis or treatment.
References:
A.D.A.M. (n.d.). Blue discoloration of the skin: MedlinePlus Medical Encyclopedia . Medline Plus. https://medlineplus.gov/ency/article/003215.htm
Alexis, A. F., Woolery-Lloyd, H., Williams, K., Andriessen, A., Desai, S., Han, G., Perez, M., Roberts, W., & Taylor, S. (2021). Racial/Ethnic Variations in Skin Barrier: Implications for Skin Care Recommendations in Skin of Color. Journal of Drugs in Dermatology , 20 (9), 932–938. https://doi.org/10.36849/jdd.6312
American Academy of Dermatology Association. (n.d.). Skin cancer in people of color . American Academy of Dermatology Association. https://www.aad.org/public/diseases/skin-cancer/types/common/melanoma/skin-color
American Nurse. (2011, January 11). Color awareness: A must for patient assessment . American Nurse. https://www.myamericannurse.com/color-awareness-a-must-for-patient-assessment/
Black, J., Cox, J., Capasso, V., Bliss, D. Z., Delmore, B., Iyer, V., Massaro, J., Munro, C., Pittman, J., & Ayello, E. A. (2023). Current Perspectives on Pressure Injuries in Persons with Dark Skin Tones from the National Pressure Injury Advisory Panel. Advances in Skin & Wound Care , 36 (9), 470–480. https://doi.org/10.1097/ASW.0000000000000032
Black, J. M., Brindle, C. T., & Honaker, J. S. (2015). Differential diagnosis of suspected deep tissue injury. International Wound Journal , 13 (4), 531–539. https://doi.org/10.1111/iwj.12471
Chapman, J., Goyal, A., & Azevedo, A. (2024). Splenomegaly. In StatPearls . StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK430907/
De Ledinghen, V., Heresbach, D., Fourdan, O., Bernard, P., Liebaert-Bories, M., Nousbaum, J., Gourlaouen, A., Becker, M., Ribard, D., Ingrand, P., Silvain, C., & Beauchant, M. (1999). Anti-inflammatory drugs and variceal bleeding: a case-control study. Gut , 44 (2), 270–273. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1727398/
Dhoonmoon, L. (2024, May). Skin tone in the management of incontinence-associated dermatitis . Urology & Continence Care Today. https://www.ucc-today.com/journals/issue/launch-edition/article/skin-tone-in-the-management-of-incontinence-associated-dermatitis-ucct
Dhoonmoon, L., & Fletcher, J. (n.d.). Assessing skin tones in practice: Results of an international survey. Clinical Practice .
Doyle, G. R., & McCutcheon, J. A. (2015). 5.4 Signs and Symptoms of Hypoxia . https://opentextbc.ca/clinicalskills/chapter/5-3-causes-of-hypoxemia-2/
Edsberg, L. E., Black, J. M., Goldberg, M., McNichol, L., Moore, L., & Sieggreen, M. (2016). Revised National Pressure Ulcer Advisory Panel Pressure Injury Staging System. Journal of Wound, Ostomy, and Continence Nursing , 43 (6), 585–597. https://doi.org/10.1097/WON.0000000000000281
Everett, J. S., Budescu, M., & Sommers, M. S. (2012). Making Sense of Skin Color in Clinical Care. Clinical Nursing Research , 21 (4), 495–516. https://doi.org/10.1177/1054773812446510
Gupta, A. K., Bharadwaj, M., & Mehrotra, R. (2016). Skin Cancer Concerns in People of Color: Risk Factors and Prevention. Asian Pacific Journal of Cancer Prevention : APJCP , 17 (12), 5257–5264. https://doi.org/10.22034/APJCP.2016.17.12.5257
Haesler, E. (Ed.). (2019). Prevention and treatment of pressure ulcers/injuries: clinical practice guideline: the international guideline (3. edition). Epuap, European Pressure Ulcer Advisory Panel.
Healthwise. (n.d.). Esophageal Varices: Care Instructions . Alberta Canada. Retrieved April 21, 2024, from https://myhealth.alberta.ca/Health/aftercareinformation/pages/conditions.aspx?hwid=ut2735
Ho, B. K., & Robinson, J. K. (2015). Color Bar Tool for Skin Type Self-Identification: a cross sectional study. Journal of the American Academy of Dermatology , 73 (2), 312-313.e1. https://doi.org/10.1016/j.jaad.2015.05.024
Jung, K., & Moon, W. (2019). Role of endoscopy in acute gastrointestinal bleeding in real clinical practice: An evidence-based review. World Journal of Gastrointestinal Endoscopy , 11 (2), 68–83. https://doi.org/10.4253/wjge.v11.i2.68
Kumar, R. (2018). Hepatogenous Diabetes: An Underestimated Problem of Liver Cirrhosis. Indian Journal of Endocrinology and Metabolism , 22 (4), 552–559. https://doi.org/10.4103/ijem.IJEM_79_18
Kundu, R. V., & Patterson, S. (2013a). Dermatologic Conditions in Skin of Color: Part I. Special Considerations for Common Skin Disorders. American Family Physician , 87 (12), 850–856. https://www.aafp.org/pubs/afp/issues/2013/0615/p850.html
Kundu, R. V., & Patterson, S. (2013b). Dermatologic Conditions in Skin of Color: Part I. Special Considerations for Common Skin Disorders. American Family Physician , 87 (12), 850–856. https://www.aafp.org/pubs/afp/issues/2013/0615/p850.html
Lewis, G., Addison, M., Machingaifa, F., & McGuire, R. (2022, May 31). Identifying AEFI in diverse skin colour – . The Melbourne Vaccine Education Centre (MVEC). https://mvec.mcri.edu.au/references/identifying-aefi-in-diverse-skin-colour/
MCCREATH, H. E., BATES-JENSEN, B. M., NAKAGAMI, G., PATLAN, A., BOOTH, H., CONNOLLY, D., TRUONG, C., & WOLDAI, A. (2016). Use of Munsell Color Charts to Measure Skin Tone Objectively in Nursing Home Residents at Risk for Pressure Ulcer Development. Journal of Advanced Nursing , 72 (9), 2077–2085. https://doi.org/10.1111/jan.12974
Mount Sinai. (n.d.). Jaundice Information | Mount Sinai – New York . Mount Sinai. https://www.mountsinai.org/health-library/diseases-conditions/jaundice
Mukwende, M., Tamony, P., & Turner, M. (2020). Mind the Gap (1st ed.). https://www.blackandbrownskin.co.uk/mindthegap
NHS.UK. (2018, January 30). Jaundice . Nhs.Uk. https://www.nhs.uk/conditions/jaundice/
Oozageer Gunowa, N., Brooke, J., Hutchinson, M., & Jackson, D. (2020). Embedding skin tone diversity into undergraduate nurse education: Through the lens of pressure injury. Journal of Clinical Nursing , 29 (21–22), 4358–4367. https://doi.org/10.1111/jocn.15474
openanesthesia. (n.d.). ABCs of Blood Transfusions . OpenAnesthesia. Retrieved April 21, 2024, from https://www.openanesthesia.org/keywords/abcs-of-blood-transfusions/
Peck-Radosavljevic, M. (2017). Thrombocytopenia in chronic liver disease. Liver International: Official Journal of the International Association for the Study of the Liver , 37 (6), 778–793. https://doi.org/10.1111/liv.13317
Powell, M., & Journey, J. D. (2024). Sengstaken-Blakemore Tube. In StatPearls . StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/NBK558924/
Pusey-Reid, E., Quinn, L., Samost, M. E., & Reidy, P. A. (2023a). Skin Assessment in Patients with Dark Skin Tone. AJN The American Journal of Nursing , 123 (3), 36. https://doi.org/10.1097/01.NAJ.0000921800.61980.7e
Pusey-Reid, E., Quinn, L., Samost, M. E., & Reidy, P. A. (2023b). Skin Assessment in Patients with Dark Skin Tone. AJN The American Journal of Nursing , 123 (3), 36. https://doi.org/10.1097/01.NAJ.0000921800.61980.7e
Sen, S., Mridha, K., & Datta, A. (2021). Skin of Color – An Enigma: A Systematic Review. Turkish Journal of Dermatology , 15 (3), 45. https://doi.org/10.4103/tjd.tjd_9_21
Sharma, B., & Raina, S. (2015). Caput medusae. The Indian Journal of Medical Research , 141 (4), 494. https://doi.org/10.4103/0971-5916.159322
Sherrell, Z. (2020, June 29). Hepatomegaly (enlarged liver): Symptoms, causes, and treatment . Medical News Today. https://www.medicalnewstoday.com/articles/hepatomegaly-enlarged-liver
Stillman, A. (1990). Chapter 87 Jaundice. In H. Walker, W. Hall, & J. Hurst (Eds.), Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. (3rd ed., p. chapter 87). Butterworths. https://www.ncbi.nlm.nih.gov/books/NBK413/
Talley Group. (2020). Pressure Ulcers in People with Dark Skin Tones . Talley Group. https://www.talleygroup.com/medias/documents/PPPIA-Pressure-Ulcers-in-People-with-Dark-Skin-Tones-Poster-A3L-0-1604484440.pdf
Thavarajah, D., Vanezis, P., & Perrett, D. (2012). Assessment of bruise age on dark-skinned individuals using tristimulus colorimetry. Medicine, Science and the Law , 52 (1), 6–11. https://doi.org/10.1258/msl.2011.011038
Tully, A. S., Trayes, K. P., & Studdiford, J. S. (2012). Evaluation of Nail Abnormalities. American Family Physician , 85 (8), 779–787. https://www.aafp.org/pubs/afp/issues/2012/0415/p779.html
UCLA Health. (n.d.). Gastrointestinal Bleeding . UCLA Health. Retrieved April 21, 2024, from https://www.uclahealth.org/medical-services/radiology/interventional-radiology/conditions-treated/gastrointestinal-bleeding
UpToDate. (n.d.). Medline ® Abstracts for References 11-13 of “Approach to acute upper gastrointestinal bleeding in adults.” UpToDate. Retrieved April 21, 2024, from https://www.uptodate.com/contents/approach-to-acute-upper-gastrointestinal-bleeding-in-adults/abstract/11-13
Wounds International. (2024). Skin tears and skin tone made easy . Wounds International. https://woundsinternational.com/wp-content/uploads/sites/8/2023/11/ESS23_ME_Skin-Tears_WINT-web.pdf
20 Secrets of Successful Nursing Students
Being a successful nursing student is more than just study tips and test strategies. It’s a way of life.
you may also like...

#349: Assessing Skin Signs Case Study

#330: Do You Know These Medical Terms?

Eponymous Terms to Know

The Doorway Assessment: Episode 277
The page could not be loaded. The CMS.gov Web site currently does not fully support browsers with "JavaScript" disabled. Please enable "JavaScript" and revisit this page or proceed with browsing CMS.gov with "JavaScript" disabled. Instructions for enabling "JavaScript" can be found here. Please note that if you choose to continue without enabling "JavaScript" certain functionalities on this website may not be available.

An official website of the United States government
Here's how you know
The .gov means it's official.
Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure.
The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.

Tracking Sheet
Lower esophageal magnetic sphincter augmentation, document note, note history, contractor information, lcd information, document information.
CPT codes, descriptions and other data only are copyright 2023 American Medical Association. All Rights Reserved. Applicable FARS/HHSARS apply.
Fee schedules, relative value units, conversion factors and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The AMA assumes no liability for data contained or not contained herein.
Current Dental Terminology © 2023 American Dental Association. All rights reserved.
Copyright © 2023 , the American Hospital Association, Chicago, Illinois. Reproduced with permission. No portion of the American Hospital Association (AHA) copyrighted materials contained within this publication may be copied without the express written consent of the AHA. AHA copyrighted materials including the UB‐04 codes and descriptions may not be removed, copied, or utilized within any software, product, service, solution or derivative work without the written consent of the AHA. If an entity wishes to utilize any AHA materials, please contact the AHA at 312‐893‐6816.
Making copies or utilizing the content of the UB‐04 Manual, including the codes and/or descriptions, for internal purposes, resale and/or to be used in any product or publication; creating any modified or derivative work of the UB‐04 Manual and/or codes and descriptions; and/or making any commercial use of UB‐04 Manual or any portion thereof, including the codes and/or descriptions, is only authorized with an express license from the American Hospital Association. The American Hospital Association (the "AHA") has not reviewed, and is not responsible for, the completeness or accuracy of any information contained in this material, nor was the AHA or any of its affiliates, involved in the preparation of this material, or the analysis of information provided in the material. The views and/or positions presented in the material do not necessarily represent the views of the AHA. CMS and its products and services are not endorsed by the AHA or any of its affiliates.
This LCD outlines limited coverage for this service with specific details under Coverage Indications, Limitations and/or Medical Necessity .
No changes between Proposed LCD and Final LCD.
CMS National Coverage Policy
Title XVIII of the Social Security Act (SSA), §1862(a)(1)(A) excludes expenses incurred for items or services which are not reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member.
Coverage Guidance
Lower esophageal magnetic sphincter augmentation (MSA) is considered medically reasonable and necessary when all the following conditions are met:
- Patient is diagnosed with gastroesophageal reflux disease (GERD) defined by abnormal pH testing in which acid exposure time (AET) is greater than 6% 1
- Patient has undergone appropriate endoscopic and esophageal manometric evaluation to rule out extragastrointestinal etiology of symptoms
- Patient has chronic GERD symptoms despite maximum medical therapy for the treatment of reflux defined as maximum (or maximum tolerated) dose of proton pump inhibitors (PPI) for at least 6 months 1
- Implantation of the device is performed by a surgeon with experience in laparoscopic anti-reflux procedures and has received product specific training
Because safety and efficacy has not been established, coverage is excluded for the following:
- Patients with suspected or known allergies to titanium, stainless steel, nickel, or ferrous materials
- Patients with electrical implants such as pacemakers and defibrillators, or other metallic, abdominal implants
- Unrepaired hiatal hernia >3 cm or a paraesophageal hernia
- Barrett’s Esophagus or esophagitis Los Angeles (LA) class C or D
- Scleroderma
- Suspected or confirmed esophageal or gastric cancer
- Prior esophageal or gastric surgery or endoscopic intervention
- Distal esophageal motility less than 35mmHg peristaltic amplitude on wet swallows or <70% (propulsive) peristaltic sequences or a known motility disorder such as Achalasia, Nutcracker Esophagus, and Diffuse Esophageal Spasm or Hypertensive lower esophageal sphincter (LES)
- Symptoms of dysphagia more than once per week within the last 3 months
- Esophageal stricture or gross esophageal anatomic abnormalities (Schatzki’s ring, obstructive lesions, etc.)
- Esophageal or gastric varices
- Lactating, pregnant or plan to become pregnant
- Morbid obesity (body mass index (BMI) >35)
- Age < 21
Definitions:
The LA Classification of GERD 2 :
Grade A-One (or more) mucosal break no longer than 5 mm that does not extend between the tops of 2 mucosal folds
Grade B-One (or more) mucosal break more than 5 mm long that does not extend between the tops of 2 mucosal folds
Grade C-One (or more) mucosal break that is continuous between the tops of 2 or more mucosal folds, but which involve less than 75% of the circumference
Grade D-One (or more) mucosal break which involves at least 75% of the esophageal circumference
The Montreal Consensus defines GERD as "a condition which develops when the reflux of stomach contents causes troublesome symptoms and/or complications". 3 It includes a spectrum of symptoms, including heartburn, regurgitation, dysphagia, laryngitis, dental problems, adult-onset asthma, and aspiration pneumonia. The prevalence of GERD is high and increasing globally. 4
Lifestyle modification and medications are the mainstay of treatment for GERD. Despite proper medical therapy, 10 to 40% of patients continue to have significant symptoms. 5,6 Surgical intervention is generally reserved for patients who have persistent symptoms or develop complications despite optimal medical therapy. Fundoplication is a well-established surgical intervention that dates to the 1950’s. Since then, multiple variations of the fundoplication have been established. The laparoscopic fundoplication (LF) and its variations are considered highly effective and durable but also associated with significant potential for adverse effects, including dysphagia, difficulty in vomiting, and gas bloating. 7
Since the advent of fundoplication, other less invasive options that do not alter the gastric fundus have been developed. MSA is 1 of those options. It is performed using the LINX ® Reflux Management System. This device treats GERD by augmenting the LES with an extraluminal ring consisting of a series of magnets. The magnetic attraction increases the LES closure pressure but permits food passage when swallowing.
Safety and efficacy
Since FDA approval in 2012, numerous studies have evaluated the safety and efficacy of MSA using the LINX ® device. Multiple short to moderate-term studies (6 months to 5 years) have demonstrated reduced GERD symptoms, improved GERD-related quality of life scores, cessation of PPI use, and normalization of objective GERD measurements. More recently, studies have been published with data extending beyond 5 years.
In 2012, Lipham JC et al. performed a multicenter, prospective, single-arm study of 44 patients who underwent laparoscopic placement of the LINX ® System. 8 The AET reduced from 11.9% at baseline to 3.8% at 3 years, with 80% (18/20) of patients achieving pH normalization. At ≥ 4 years, 100% of the patients reported improved quality-of-life measures for GERD, and 80% had complete cessation of PPIs. There were no reported deaths or long-term device-related complications such as migration or erosion.
A retrospective case-control study was done in 2014 by Louie BE et al. It involved consecutive patients undergoing either laparoscopic Nissen fundoplication (LF) or MSA who had chronic GERD and a hiatal hernia of less than 3 cm. 9 Sixty-six patients underwent operations (34 MSA and 32 LF) and were followed for at least 6 months. The groups were similar in reflux characteristics and hernia size. MSA resulted in less gassy and bloated feelings and enabled belching in 67% compared with none of the LF patients. The AET normalized in both groups but was statistically better in the LF group. MSA resulted in similar objective control of GERD, symptom resolution, and improved quality of life compared with LF.
Saino G et al. (2015) evaluated the safety and efficacy of the MSA for 5 years during a prospective, multicenter study. 10 Forty-four patients (ages 18-75 years) had a LINX ® device implanted by laparoscopy, and 33 patients were followed to 5 years. At 5 years, GERD Health-Related Quality of Life (HRQL) questionnaire score, esophageal pH, PPI use, and complications were evaluated. Complete discontinuation of PPIs was achieved by 87.8% of patients. No device erosions or migrations were reported. However, 11 (25%) of the study patients were not followed to the 5-year endpoint. Three patients had device removal.
A 2015 prospective, multicenter study by Riegler M et al. compared MSA and LF in clinical practice. 11 Two hundred forty-nine patients (202 MSA patients and 47 LF patients) had completed one-year follow-up. Discontinuation of PPIs was achieved by 81.8% of patients after MSA and 63.0% after LF. Excessive gas and abdominal bloating were reported by 10.0% of patients after MSA and 31.9% following LF. Following MSA, 91.3% of patients were able to vomit if needed, compared with 44.4% of those undergoing LF. The reoperation rate was 4.0% following MSA and 6.4% following LF.
Ganz RA et al. (2016) performed a prospective study on 100 subjects ages (18-75) that underwent the LINX ® placement, 85 of which were followed for 5 years to evaluate quality of life, reflux control, use of PPIs, and side effects. 12 All patients used PPIs at baseline; this decreased to 15.3% at 5 years. Moderate or severe regurgitation occurred in 57% of subjects before the procedure and 1.2% at 5 years post-surgery. All patients reported the ability to belch and vomit if needed. Bothersome dysphagia was present in 5% at baseline and 6% at 5 years. No device erosions, migrations, or malfunctions were reported in this study. Device removal occurred in 7 patients.
Bell R et al. (2019) prospectively studied 152 patients with GERD and moderate-to-severe regurgitation despite 8 weeks of once-daily PPI therapy. 13 These results indicate 89% (42/47) of treated patients with MSA reported relief of regurgitation compared with 10% (10/101) of the BID PPI group at the 6-month primary endpoint. The same authors published another study in 2020 that offered MSA to patients in the PPI arm of the study who had persistent moderate to severe regurgitation and excess reflux episodes during impedance or pH testing on medication. 14 In this study, 70% (48/69) of patients had pH normalization at study completion. MSA was not associated with peri-operative events, device explants, erosions, or migrations.
The 2019 multicenter, prospective study by Louie BE et al. evaluated patients with pathologic acid reflux confirmed by esophageal pH testing undergoing MSA. 15 A total of 200 patients, ages ranging from 19.7-71.6 years. At 1 year, the mean total AET decreased from 10.0% at baseline to 3.6% and 74.4% of patients had normal esophageal AET. The device removal rate at 1 year was 2.5%. One erosion was reported.
In a long-term retrospective study, Ferrari D et al. (2020) reported on the course of 335 patients, 124 of which were followed from 6 to 12 years after surgery (median 9 years). 16 The mean total GERD-HRQL score significantly improved from 19.9 to 4.01, and PPI use was discontinued by 79% of patients. The mean total percent time with pH < 4 decreased from 9.6% at baseline to 4.1%, with 89% of patients achieving pH normalization. The rate of procedure-related adverse events was 11.6%, with 2.4% requiring a single endoscopic pneumatic dilation due to persistent dysphagia. Thirty-one patients (9.2%) required laparoscopic device removal for the following reasons: erosion (6), regurgitation (6), heartburn (5), dysphagia (5), foreign body sensation (2), odynophagia (1), pharyngodynia (1), chronic cough (1), need for Magnetic Resonance Imaging (MRI) (1).
Addressing postoperative dysphagia, Ganz RA et al. (2013) prospectively assessed 100 patients with GERD before and after sphincter augmentation. 17 The study did not include a concurrent control group. The most frequent adverse event was dysphagia (in 68% of patients postoperatively, in 11% at 1 year, and 4% at 3 years). Serious adverse events occurred in 6 patients, and the device was removed in 6 patients. Ayazi S et al. (2020) performed a retrospective review of prospectively collected data of patients who underwent MSA over 5 years. 18 The preoperative objective evaluation included upper endoscopy, esophagram, high-resolution impedance manometry, and esophageal pH testing. A total of 380 patients underwent MSA; at a mean follow-up of 11.5 months, 15.5% of patients were experiencing persistent dysphagia. The overall response rate to dilation therapy was 67%, and the efficacy of dilation reduced with each subsequent procedure.
Regarding the risk of erosion, Alicuben ET et al. (2018) examined data for all devices placed worldwide from February 2007 through July 2017 using the device registry. 19 In total, 9453 devices were placed, and there were 29 reported cases of erosion. The median time to presentation of erosion was 26 months, most occurring between 1 and 4 years after placement. The risk of erosion was 0.3% at 4 years after device implantation. In this series, smaller devices were associated with higher rates of erosion. Notably, the smaller 12-bead device was responsible for 18/29 (62%) of erosions.
Effectiveness compared to fundoplication
In a systematic review and meta-analysis of 688 patients (273 fundoplication and 415 MSA), Skubleny D et al. (2017) found MSA was statistically superior to LF in preserving patient's ability to belch and vomit. 20 There was no statistically significant difference between MSA and LF in gas/bloating, postoperative dysphagia, and PPI elimination.
Aiolfi A et al. (2018) examined 7 observational cohort studies, including 1211 patients, 686 MSA and 525 LF. 21 Postoperative morbidity ranged from 0 to 3% in the MSA group and from 0 to 7% in the LF group, and there was no mortality reported. Dysphagia requiring endoscopic dilatation occurred in 9.3% of MSA and 6.6% of LF patients. Postoperative PPI use, dysphagia requiring dilatation, and quality of life are similar in the 2 patient groups. MSA was associated with less gas/bloat symptoms and an increased ability to vomit and belch.
Chen M-Y et al. (2017) conducted a meta-analysis of 4 trials that included 624 patients and aimed to evaluate the differences in PPI use, complications, and adverse events. 22 MSA had a shorter operative time and length of stay. Similar PPI use, complication rate, and severe dysphagia for dilation were shown in both groups. Although there was no difference between the MSA and LF in the number of adverse events, the incidence of postoperative gas or bloating favored the MSA group. There was no significant difference in the ability to belch and vomit.
The systematic review by Guidozzi N et al. (2019) identified 6 comparative studies of MSA versus fundoplication and 13 single-cohort studies. 23 Collectively, the study included 1099 patients, 632 receiving MSA and 467 receiving fundoplication. Following MSA, only 13.2% required postoperative PPI therapy, 7.8% dilatation, 3.3% device removal or reoperation, and esophageal erosion was seen in 0.3%. There was no significant difference between the groups in the requirement for postoperative PPI therapy, GERD-HRQOL score, dysphagia, and reoperation. However, when compared to fundoplication, MSA was associated with significantly less gas bloating and a greater ability to belch. Regarding safety, 3.3% of the MSA patients required device removal.
Patient selection
Rona KA, et al. performed a retrospective review of 192 patients. 24 Median follow-up was 20 months (ranging from 3-75 months). Fifty-two patients had a hiatal hernia >3cm. This study reports MSA in patients with large hiatal hernias showed decreased postoperative PPI requirement and mean GERD-HRQL scores compared to patients with smaller hernias.
Buckley FP 3rd et al. (2018) conducted a 3-year multicenter, prospective study of consecutive patients undergoing MSA with the LINX ® device with concurrent repair of paraesophageal and hernias over 3 cm. 25 Non-permanent mesh reinforcement of hiatal repair was performed in 83% of the patients. At 9-month median follow up, complete PPI independence was achieved in 94%, 9.5% required dilation, GERD-HRQL scores improved from 26 preoperatively to 2 postoperatively. There were no device explants, erosions, or migrations reported.
In a retrospective review, Dunn CP et al. (2021) evaluated 79 patients with GERD and hiatal hernias ≥ 3 cm who underwent MSA and hiatal hernia repair over a 7-year period. 26 Seventy-nine patients, with a median age of 65.56 years were included, median follow up was 2.98 years. Five (6.3%) hiatal hernia recurrences occurred, and 1 required re-operation. Median GERD-HRQL scores improved from 21 to 2.
Alicuben ET et al. (2019) and Dunn CP et al. (2021) published very similar retrospective studies showing MSA to be effective at preventing progression of metaplasia/Barrett’s esophagus to dysplasia or neoplasia. 27,28 The studies involved 86 and 87 patients, respectively. In both studies, the effect remained consistent even after 2 years of follow-up.
Warren HF et al. (2018) retrospectively studied clinical, endoscopic, manometric, pH data, and intraoperative factors of 170 patients. 29 Manometric data pre- and post-MSA demonstrated that LESs with 1 defective component were restored to normal in 77% of patients; however, those with 2 or 3 defective components can only be restored to normal in 56%. MSA appears to provide less control of GERD in patients with LES with multiple defects. Using a multivariable analysis, BMI >35, structurally defective LES, and preoperative LES residual pressure were independent negative predictors of excellent/good outcome.
In a 3-year retrospective cohort study, James TJ et al. (2022) evaluated the outcomes of 621 consecutive patients who underwent laparoscopic MSA. 30 Patients were grouped into 4 cohorts according to the World Health Organization body mass index (BMI) classification: BMI < 25 (normal weight), BMI 25-29.9 (overweight), BMI 30-34.9 (obese class I), and BMI > 35 (obese class II-III). Follow-up with endoscopy or video esophagram was available for 361 patients (58%) with a median follow-up of 15.4 months. There were no significant differences in outcomes between normal weight, overweight, and obese patient groups undergoing MSA.
With fundoplication considered the gold standard for surgical treatment of GERD refractory to medical management, numerous studies have evaluated MSA versus fundoplication. Current evidence shows MSA to have similar safety and efficacy when used in appropriately selected patients. The data shows similar quality-of-life improvement scores and rates of PPI cessation in both groups. In contrast, more fundoplication patients are unable to belch and vomit. Compared to fundoplication, MSA generally has shorter operative times and duration of hospital stay. 20-23
Dysphagia is a common adverse symptom that generally decreases over time and has been successfully treated with dilation therapy. 17, 18 The risk of erosion was addressed in a large 2018 study, which found the incidence to be 0.3%. 19 Persistent or recurrent adverse effects such as heartburn, regurgitation, dysphagia, chest pain, and device erosion have resulted in explantation. The likelihood of explantation generally ranges from 3-7% and should be included in the preoperative risk/benefit discussion. 12,31
The criteria for patient selection undergoing MSA are primarily based on the manufacturer's FDA-approved instructions for use, as these guidelines have been used in the majority of studies. Although use outside these parameters is reported, it has only been evaluated in a small number of studies, has limited objective data, and lacks adequate long-term follow-up.
There is limited study in patients with an unrepaired hiatal hernia >3m. However, there are multiple studies that show the effective use of MSA in patients who have a hiatal hernia concurrently repaired at the time of placement. 24-26
Thus far, there is limited data regarding use in patients with more severe GERD including esophagitis LA class C or D and Barrett’s esophagus. Two small retrospective studies evaluated the outcomes of MSA in patients with Barrett’s and showed promising results. 27-28 However, these studies were from the same institutions, had nearly identical numbers, and occurred over a similar timeframe. Although published separately, the results appear consistent with data used from an overlapping patient population. Authors involved in both articles disclosed a paid consulting relationship with the manufacturer, adding limitation to the studies.
The literature suggests that endoscopy and esophageal manometry are valuable in the preoperative evaluation to rule out alternative pathology including cancer, motility disorders, stricture, and anatomic abnormalities. The 2018 Warren study showed structurally defective LES and preoperative LES residual pressure were independent negative predictors of excellent/good outcome. 29
The initial trials that led to the approval of the LINX ® device excluded patients with a BMI > 35, and most studies since then have followed this criterion. James et al. examined the effect of preop BMI on outcomes and found no significant differences between normal-weight patients, overweight, and different classes of obesity. 30 However, the 2018 study by Warren et al. suggests a higher BMI may be a negative predictor. 29 Currently, there is not sufficient evidence for use outside this parameter.
It is important to note that patients >75 years old were excluded from the original FDA trials. Since then, no significant study has addressed its use for patients >75 years old. Although studies have suggested that younger age is a predictor of positive outcomes 19 , age is not listed as an exclusionary criterion. Careful patient selection is critical for both success and safety.
Proposed Process Information
Coding information, bill type codes, revenue codes, cpt/hcpcs codes, icd-10-cm codes that support medical necessity, icd-10-cm codes that do not support medical necessity, additional icd-10 information, general information.
1. Jobe BA, Richter JE, Hoppo T, et al. Preoperative diagnostic workup before antireflux surgery: an evidence and experience-based consensus of the Esophageal Diagnostic Advisory Panel. J Am Coll Surg . 2013;217(4):586-597.
2. SS Sami, K Ragunath. The Los Angeles Classification of gastroesophageal reflux disease, video journal and encyclopedia of GI. Endoscopy , 2013, 1(1): 103-104.
3. Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am J Gastroenterol . 2006;101(8):1900-1943.
4. GBD 2017 Oesophageal Cancer Collaborators. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol . 2020;5(6):582-597.
5. Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol . 2022;117(1):27-56.
6. Yadlapati R, Vaezi MF, Vela MF, et al. Management options for patients with GERD and persistent symptoms on proton pump inhibitors: recommendations from an expert panel. Am J Gastroenterol . 2018;113(7):980-986.
7. Spechler SJ, Hunter JG, Jones KM, et al. Randomized trial of medical versus surgical treatment for refractory heartburn. N Engl J Med . 2019;381(16):1513-1523.
8. Lipham JC, DeMeester TR, Ganz RA, et al. The LINX ® reflux management system: confirmed safety and efficacy now at 4 years. Surg Endosc . 2012;26(10):2944-2949.
9. Louie BE, Farivar AS, Shultz D, Brennan C, Vallières E, Aye RW. Short-term outcomes using magnetic sphincter augmentation versus Nissen fundoplication for medically resistant gastroesophageal reflux disease. Ann Thorac Surg . 2014;98(2):498-505.
10. Saino G, Bonavina L, Lipham JC, Dunn D, Ganz RA. Magnetic sphincter augmentation for gastroesophageal reflux at 5 years: Final results of a pilot study show long-term acid reduction and symptom improvement. J Laparoendosc Adv Surg Tech A . 2015;25(10):787-792.
11. Riegler M, Schoppman SF, Bonavina L, Ashton D, Horbach T, Kemen M. Magnetic sphincter augmentation and fundoplication for GERD in clinical practice: one-year results of a multicenter, prospective observational study. Surg Endosc . 2015;29(5):1123-1129.
12. Ganz RA, Edmundowicz SA, Taiganides PA, et al. Long-term outcomes of patients receiving a magnetic sphincter augmentation device for gastroesophageal reflux. Clin Gastroenterol Hepatol . 2016;14(5):671-677.
13. Bell R, Lipham J, Louie B, et al. Laparoscopic magnetic sphincter augmentation versus double-dose proton pump inhibitors for management of moderate-to-severe regurgitation in GERD: a randomized controlled trial. Gastrointest Endosc . 2019;89(1):14-22.e1.
14. Bell R, Lipham J, Louie BE, et al. Magnetic sphincter augmentation superior to proton pump inhibitors for regurgitation in a 1-year randomized trial. Clin Gastroenterol Hepatol . 2020;18(8):1736-1743.e2.
15. Louie BE, Smith CD, Smith CC, et al. Objective evidence of reflux control after magnetic sphincter augmentation: One year results from a post approval study. Ann Surg . 2019;270(2):302-308.
16. Ferrari D, Asti E, Lazzari V, Siboni S, Bernardi D, Bonavina L. Six to 12-year outcomes of magnetic sphincter augmentation for gastroesophageal reflux disease. Sci Rep . 2020;10(1):13753. Published 2020 Aug 13.
17. Ganz RA, Peters JH, Horgan S. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med . 2013;368(21):2039-2040.
18. Ayazi S, Zheng P, Zaidi AH, et al. Magnetic sphincter augmentation and postoperative dysphagia: Characterization, clinical risk factors, and management. J Gastrointest Surg . 2020;24(1):39-49.
19. Alicuben ET, Bell RCW, Jobe BA, et al. Worldwide experience with erosion of the magnetic sphincter augmentation device. J Gastrointest Surg . 2018;22(8):1442-1447.
20. Skubleny D, Switzer NJ, Dang J, et al. LINX ® magnetic esophageal sphincter augmentation versus Nissen fundoplication for gastroesophageal reflux disease: A systematic review and meta-analysis. Surg Endosc . 2017;31(8):3078-3084.
21. Aiolfi A, Asti E, Bernardi D, et al. Early results of magnetic sphincter augmentation versus fundoplication for gastroesophageal reflux disease: Systematic review and meta-analysis. Int J Surg . 2018;52:82-88.
22. Chen MY, Huang DY, Wu A, et al. Efficacy of magnetic sphincter augmentation versus Nissen fundoplication for gastroesophageal reflux disease in short term: A meta-analysis. Can J Gastroenterol Hepatol . 2017;2017:9596342.
23. Guidozzi N, Wiggins T, Ahmed AR, Hanna GB, Markar SR. Laparoscopic magnetic sphincter augmentation versus fundoplication for gastroesophageal reflux disease: Systematic review and pooled analysis. Dis Esophagus . 2019;32(9):doz031.
24. Rona KA, Reynolds J, Schwameis K, et al. Efficacy of magnetic sphincter augmentation in patients with large hiatal hernias. Surg Endosc . 2017;31(5):2096-2102.
25. Buckley FP 3rd, Bell RCW, Freeman K, Doggett S, Heidrick R. Favorable results from a prospective evaluation of 200 patients with large hiatal hernias undergoing LINX ® magnetic sphincter augmentation. Surg Endosc . 2018;32(4):1762-1768.
26. Dunn CP, Zhao J, Wang JC, et al. Magnetic sphincter augmentation with hiatal hernia repair: Long term outcomes. Surg Endosc . 2021;35(10):5607-5612.
27. Alicuben ET, Tatum JM, Bildzukewicz N, et al. Regression of intestinal metaplasia following magnetic sphincter augmentation device placement. Surg Endosc . 2019;33(2):576-579.
28. Dunn CP, Henning JC, Sterris JA, et al. Regression of Barrett's esophagus after magnetic sphincter augmentation: Intermediate-term results. Surg Endosc . 2021;35(10):5804-5809.
29. Warren HF, Brown LM, Mihura M, Farivar AS, Aye RW, Louie BE. Factors influencing the outcome of magnetic sphincter augmentation for chronic gastroesophageal reflux disease. Surg Endosc . 2018;32(1):405-412.
30. James TJ, Burke JF, Putnam LR, et al. Loosening the belt on magnetic sphincter augmentation indications: does body mass index matter? Surg Endosc . 2022;36(7):4878-4884.
31. Lipham JC, Taiganides PA, Louie BE, Ganz RA, DeMeester TR. Safety analysis of first 1000 patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease. Dis Esophagus . 2015;28(4):305-311.
Revision History Information
Associated documents.
- lower esophageal magnetic sphincter augmentation
- gastroesophageal reflux disease
Read the LCD Disclaimer
Email this document to yourself or someone else
An asterisk ( * ) indicates a required field. This email will be sent from you to the recipient email address(es) you enter. Please do not use this feature to contact CMS. To submit a comment or question to CMS, please use the Feedback/Ask a Question link available at the bottom of every MCD page.
Contractor Detail
Lcd disclaimer, license agreements.
Please review and accept the agreements in order to view Medicare Coverage documents, which may include licensed information and codes.
LICENSE FOR USE OF PHYSICIANS' CURRENT PROCEDURAL TERMINOLOGY, FOURTH EDITION ("CPT")
End User Point and Click Amendment: CPT codes, descriptions and other data only are copyright 2023 American Medical Association. All Rights Reserved (or such other date of publication of CPT). CPT is a trademark of the American Medical Association (AMA).
You, your employees and agents are authorized to use CPT only as agreed upon with the AMA internally within your organization within the United States for the sole use by yourself, employees and agents. Use is limited to use in Medicare, Medicaid or other programs administered by the Centers for Medicare and Medicaid Services (CMS). You agree to take all necessary steps to insure that your employees and agents abide by the terms of this agreement.
Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CPT for resale and/or license, transferring copies of CPT to any party not bound by this agreement, creating any modified or derivative work of CPT, or making any commercial use of CPT. License to use CPT for any use not authorized herein must be obtained through the AMA, CPT Intellectual Property Services, AMA Plaza 330 N. Wabash Ave., Suite 39300, Chicago, IL 60611-5885. Applications are available at the AMA Web site, http://www.ama-assn.org/go/cpt .
Applicable FARS\DFARS Restrictions Apply to Government Use.
AMA Disclaimer of Warranties and Liabilities.
CPT is provided "as is" without warranty of any kind, either expressed or implied, including but not limited to, the implied warranties of merchantability and fitness for a particular purpose. No fee schedules, basic unit, relative values or related listings are included in CPT. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this file/product is with CMS and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this file/product. This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
CMS Disclaimer
The scope of this license is determined by the AMA, the copyright holder. Any questions pertaining to the license or use of the CPT should be addressed to the AMA. End Users do not act for or on behalf of the CMS. CMS DISCLAIMS RESPONSIBILITY FOR ANY LIABILITY ATTRIBUTABLE TO END USER USE OF THE CPT. CMS WILL NOT BE LIABLE FOR ANY CLAIMS ATTRIBUTABLE TO ANY ERRORS, OMISSIONS, OR OTHER INACCURACIES IN THE INFORMATION OR MATERIAL CONTAINED ON THIS PAGE. In no event shall CMS be liable for direct, indirect, special, incidental, or consequential damages arising out of the use of such information or material. Should the foregoing terms and conditions be acceptable to you, please indicate your agreement and acceptance by clicking below on the button labeled "I Accept".
LICENSE FOR USE OF CURRENT DENTAL TERMINOLOGY (CDT TM )
End User License Agreement: These materials contain Current Dental Terminology (CDT TM ), copyright© 2023 American Dental Association (ADA). All rights reserved. CDT is a trademark of the ADA.
The license granted herein is expressly conditioned upon your acceptance of all terms and conditions contained in this agreement. By clicking below on the button labeled "I accept", you hereby acknowledge that you have read, understood and agreed to all terms and conditions set forth in this agreement.
If you do not agree with all terms and conditions set forth herein, click below on the button labeled "I do not accept" and exit from this computer screen.
If you are acting on behalf of an organization, you represent that you are authorized to act on behalf of such organization and that your acceptance of the terms of this agreement creates a legally enforceable obligation of the organization. As used herein, "you" and "your" refer to you and any organization on behalf of which you are acting.
- Subject to the terms and conditions contained in this Agreement, you, your employees and agents are authorized to use CDT only as contained in the following authorized materials and solely for internal use by yourself, employees and agents within your organization within the United States and its territories. Use of CDT is limited to use in programs administered by Centers for Medicare & Medicaid Services (CMS). You agree to take all necessary steps to ensure that your employees and agents abide by the terms of this agreement. You acknowledge that the ADA holds all copyright, trademark and other rights in CDT. You shall not remove, alter, or obscure any ADA copyright notices or other proprietary rights notices included in the materials.
- Any use not authorized herein is prohibited, including by way of illustration and not by way of limitation, making copies of CDT for resale and/or license, transferring copies of CDT to any party not bound by this agreement, creating any modified or derivative work of CDT, or making any commercial use of CDT. License to use CDT for any use not authorized herein must be obtained through the American Dental Association, 211 East Chicago Avenue, Chicago, IL 60611. Applications are available at the American Dental Association web site, http://www.ADA.org .
- Applicable Federal Acquisition Regulation Clauses (FARS)/Department of Defense Federal Acquisition Regulation supplement (DFARS) Restrictions Apply to Government Use. U.S. Government Rights Provisions.
- Organizations who contract with CMS acknowledge that they may have a commercial CDT license with the ADA, and that use of CDT codes as permitted herein for the administration of CMS programs does not extend to any other programs or services the organization may administer and royalties dues for the use of the CDT codes are governed by their commercial license.
- ADA DISCLAIMER OF WARRANTIES AND LIABILITIES. CDT is provided "as is" without warranty of any kind, either expressed or implied, including but not limited to, the implied warranties of merchantability and fitness for a particular purpose. No fee schedules, basic unit, relative values or related listings are included in CDT. The ADA does not directly or indirectly practice medicine or dispense dental services. The sole responsibility for software, including any CDT and other content contained therein, is with CMS; and no endorsement by the ADA is intended or implied. The ADA expressly disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this file/product. This Agreement will terminate upon notice to you if you violate the terms of this Agreement. The ADA is a third party beneficiary to this Agreement.
- CMS DISCLAIMER. The scope of this license is determined by the ADA, the copyright holder. Any questions pertaining to the license or use of the CDT should be addressed to the ADA. End Users do not act for or on behalf of the CMS. CMS disclaims responsibility for any liability attributable to end user use of the CDT. CMS will not be liable for any claims attributable to any errors, omissions, or other inaccuracies in the information or material covered by this license. In no event shall CMS be liable for direct, indirect, special, incidental, or consequential damages arising out of the use of such information or material. The license granted herein is expressly conditioned upon your acceptance of all terms and conditions contained in this agreement. If the foregoing terms and conditions are acceptable to you, please indicate your agreement by clicking below on the button labeled "I Accept". If you do not agree to the terms and conditions, you may not access or use software. Instead you must click below on the button labeled "I Do Not Accept" and exit from this computer screen.
LICENSE FOR NATIONAL UNIFORM BILLING COMMITTEE (NUBC)
American hospital association disclaimer.
The American Hospital Association ("the AHA") has not reviewed, and is not responsible for, the completeness or accuracy of any information contained in this material, nor was the AHA or any of its affiliates, involved in the preparation of this material, or the analysis of information provided in the material. The views and/or positions presented in the material do not necessarily represent the views of the AHA. CMS and its products and services are not endorsed by the AHA or any of its affiliates.
Page Help for LCD - Lower Esophageal Magnetic Sphincter Augmentation (L39780)
Introduction.
This page displays your requested Local Coverage Determination (LCD). The document is broken into multiple sections. You can use the Contents side panel to help navigate the various sections. A Local Coverage Determination (LCD) is a decision made by a Medicare Administrative Contractor (MAC) on whether a particular service or item is reasonable and necessary, and therefore covered by Medicare within the specific jurisdiction that the MAC oversees. Please note that codes (CPT/HCPCS and ICD-10) have moved from LCDs to Billing & Coding Articles.
More information
MACs are Medicare contractors that develop LCDs and process Medicare claims. MACs develop an LCD when there is no national coverage determination (NCD) or when there is a need to further define an NCD for the specific jurisdiction. LCDs outline how the contractor will review claims to ensure that the services provided meet Medicare coverage requirements. Before an LCD becomes final, the MAC publishes Proposed LCDs, which include a public comment period. LCD document IDs begin with the letter "L" (e.g., L12345). Proposed LCD document IDs begin with the letters "DL" (e.g., DL12345). The guidelines for LCD development are provided in Chapter 13 of the Medicare Program Integrity Manual. The Social Security Act, Sections 1869(f)(2)(B) and 1862(l)(5)(D) define LCDs and provide information on the process
The LCD Tracking Sheet is a pop-up modal that is displayed on top of any Proposed LCD that began to appear on the MCD on or after 1/1/2022. The Tracking Sheet provides key details about the Proposed LCD, including a summary of the issue, who requested the new/updated policy, links to key documents, important process-related dates, who to contact with questions about the policy, and the history of previous policy considerations. The information displayed in the Tracking Sheet is pulled from the accompanying Proposed LCD and its correlating Final LCD and will be updated as new data becomes available. The Tracking Sheet modal can be closed and re-opened when viewing a Proposed LCD.
Printing a Document to PDF

Frequently Asked Questions (FAQs)
Are you a provider and have a question about billing or coding.
Please contact your Medicare Administrative Contractor (MAC). MACs can be found in the MAC Contacts Report .
Do you have questions related to the content of a specific Local Coverage Determination (LCD) or an Article?
Are you a beneficiary and have questions about your coverage, are you looking for codes (e.g., cpt/hcpcs, icd-10), local coverage.
For the most part, codes are no longer included in the LCD (policy). You will find them in the Billing & Coding Articles. Try using the MCD Search to find what you're looking for. Enter the code you're looking for in the "Enter keyword, code, or document ID" box. The list of results will include documents which contain the code you entered.
Please Note: For Durable Medical Equipment (DME) MACs only, CPT/HCPCS codes remain located in LCDs. All other Codes (ICD-10, Bill Type, and Revenue) have moved to Articles for DME MACs, as they have for the other Local Coverage MAC types.
National Coverage
NCDs do not contain claims processing information like diagnosis or procedure codes nor do they give instructions to the provider on how to bill Medicare for the service or item. For this supplementary claims processing information we rely on other CMS publications, namely Change Requests (CR) Transmittals and inclusions in the Medicare Fee-For-Service Claims Processing Manual (CPM).
In order for CMS to change billing and claims processing systems to accommodate the coverage conditions within the NCD, we instruct contractors and system maintainers to modify the claims processing systems at the national or local level through CR Transmittals. CRs are not policy, rather CRs are used to relay instructions regarding the edits of the various claims processing systems in very descriptive, technical language usually employing the codes or code combinations likely to be encountered with claims subject to the policy in question. As clinical or administrative codes change or system or policy requirements dictate, CR instructions are updated to ensure the systems are applying the most appropriate claims processing instructions applicable to the policy.
How do I find out if a specific CPT code is covered in my state?
Enter the CPT/HCPCS code in the MCD Search and select your state from the drop down. (You may have to accept the AMA License Agreement.) Look for a Billing and Coding Article in the results and open it. (Or, for DME MACs only, look for an LCD.) Review the article, in particular the Coding Information section.
If you need more information on coverage, contact the Medicare Administrative Contractor (MAC) who published the document. The contractor information can be found at the top of the document in the Contractor Information section (expand the section to see the details).
If you don’t find the Article you are looking for, contact your MAC .
Did you receive a Medicare coverage denial?
Was your Medicare claim denied? Here are some hints to help you find more information:
1) Check out the Beneficiary card on the MCD Search page.
2) Try using the MCD Search and enter your information in the "Enter keyword, code, or document ID" box. Your information could include a keyword or topic you're interested in; a Local Coverage Determination (LCD) policy or Article ID; or a CPT/HCPCS procedure/billing code or an ICD-10-CM diagnosis code. Try entering any of this type of information provided in your denial letter.
3) Contact your MAC .
4) Visit Medicare.gov or call 1-800-Medicare.
It is Thursday and the weekly MCD data isn’t refreshed?
Are you having technical issues with the medicare coverage database (mcd), mcd session expiration warning.
Your MCD session is currently set to expire in 5 minutes due to inactivity. If your session expires, you will lose all items in your basket and any active searches. If you would like to extend your session, you may select the Continue Button.
Reset MCD Search Data
If you are experiencing any technical issues related to the search, selecting the 'OK' button to reset the search data should resolve your issues.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- World J Gastrointest Pharmacol Ther
- v.10(1); 2019 Jan 21
Update on the management of gastrointestinal varices
Umesha boregowda.
Department of Gastroenterology and Hepatology, University of California San Francisco, Fresno, CA 93721, United States
Chandraprakash Umapathy
Nasir halim, madhav desai.
Department of Gastroenterology and Hepatology, Kansas University Medical Center, Kansas City, KS 66160, United States
Arpitha Nanjappa
Subramanyeswara arekapudi.
Department of Medicine, VA Central California Healthcare System, Fresno, CA 93703, United States
Thimmaiah Theethira
Department of Gastroenterology and Hepatology, VA Central California Healthcare System, Fresno, CA 93703, United States
Marina Roytman
Shreyas saligram.
Department of Gastroenterology and Hepatology, VA Central California Healthcare System, Fresno, CA 93703, United States. ude.fscu.onserf@margilass
Corresponding author: Shreyas Saligram MD, MRCP, Assistant Professor, Director, Doctor, Department of Gastroenterology and Hepatology, University of California San Francisco, 2823 Fresno Street, Fresno, CA 93721, United States. ude.fscu.onserf@margilass
Telephone: +1-559-4593821 Fax: +1-559-4593887
Cirrhosis of liver is a major problem in the western world. Portal hypertension is a complication of cirrhosis and can lead to a myriad of pathology of which include the development of porto-systemic collaterals. Gastrointestinal varices are dilated submucosal veins, which often develop at sites near the formation of gastroesophageal collateral circulation. The incidence of varices is on the rise due to alcohol and obesity. The most significant complication of portal hypertension is life-threatening bleeding from gastrointestinal varices, which is associated with substantial morbidity and mortality. In addition, this can cause a significant burden on the health care facility. Gastrointestinal varices can happen in esophagus, stomach or ectopic varices. There has been considerable progress made in the understanding of the natural history, pathophysiology and etiology of portal hypertension. Despite the development of endoscopic and medical treatments, early mortality due to variceal bleeding remains high due to significant illness of the patient. Recurrent variceal bleed is common and in some cases, there is refractory variceal bleed. This article aims to provide a comprehensive review of the management of gastrointestinal varices with an emphasis on endoscopic interventions, strategies to handle refractory variceal bleed and newer endoscopic treatment modalities. Early treatment and improved endoscopic techniques can help in improving morbidity and mortality.
Core tip: Cirrhosis of liver can lead to gastrointestinal varices. Gastrointestinal bleed from varices can be debilitating and can cause morbidity and mortality if not well controlled. This is a detailed review on the endoscopic management of variceal bleed and gives an insight into some of the new endoscopic techniques that can be helpful in treating variceal bleed.
INTRODUCTION
Less than 1% of the United States population have cirrhosis of liver[ 1 ]. In the western world, the most common etiology of portal hypertension is cirrhosis due to alcoholic liver disease, nonalcoholic steatohepatitis (NASH), and hepatitis C infection[ 2 ]. According to a recent estimate 15 million people in the United States have alcohol abuse disorder, nearly 88000 people die annually due to alcohol, and 10%-15% of people with alcoholism develop cirrhosis[ 3 ]. Another 3 million people have chronic hepatitis C infection[ 4 ], and 25%-28% of these patients go on to develop cirrhosis[ 5 , 6 ]. Nonalcoholic fatty liver disease (NAFLD) is a spectrum of chronic liver disease consisting of mild to an advanced form of fatty degeneration of the liver described as NASH. Prevalence of NASH is estimated to be around 3%-8% of the general population, and 10%-25% of these patients progress to cirrhosis[ 7 ]. Moreover, the rate of NASH is rising due to the increasing prevalence of obesity, insulin resistance, and diabetes. NASH is the second most common cause among patients with cirrhosis who are currently waiting for liver transplant. Recent trends have indicated that NAFLD is expected to overtake hepatitis C and alcohol as the most common etiology of liver cirrhosis and indication for liver transplants in the western countries by year 2030[ 8 , 9 ]. Therefore, in order to reduce morbidity and mortality, as well as the overall burden on healthcare, it is essential to develop cost-effective screening and management strategies for portal hypertension related to cirrhosis.
NATURAL HISTORY OF GASTROINTESTINAL VARICES
Gastrointestinal varices are abnormally dilated submucosal veins in the digestive tract due to portal hypertension and can potentially cause life-threatening bleeding. Prevalence of varices increases with the severity of liver disease (Child-Pugh class A 42.7%, class B 70.7% and class C 75.5%)[ 2 , 10 ]. The Child-Pugh score is described in Table Table1. 1 . The incidence of esophageal varices in cirrhotic patients is around 5% at the end of one year and 28% at the end of three years. Small varices progress to large varices at a rate of 10% to 12% annually[ 11 ]. Approximately 50% of all patients with a new diagnosis of cirrhosis have gastrointestinal varices[ 2 ]. Annual risk of variceal bleeding among small and large varices is 5% and 15% respectively[ 12 ]. The six-week mortality rate among patients with index variceal bleeding is approximately 20%[ 13 ]. Risk of rebleeding without endoscopic intervention is almost 60% with an increased mortality rate (33%)[ 14 ].
Child-Pugh scoring and classification
Class A (score 5-6), class B (score 7-9), and class C (score 10-15). PT/INR: Prothrombin time/international standardized ratio.
PATHOPHYSIOLOGY
The development of portal hypertension in cirrhosis is a multifactorial process with changes in both the portal and systemic circulation. This is shown in Figure Figure1. 1 . The majority of patients in western countries with portal hypertension have underlying cirrhosis. Non-cirrhotic portal hypertension is typically less common and encompasses a broad range of pathology, typically vascular in origin[ 15 ]. Portal hypertension is defined as hepatic vein pressure gradient (HVPG) more than 5 mmHg. The HVPG is a surrogate means to measure pressure in the portal veins. Normal HVPG (= hepatic vein wedge pressure - free hepatic vein pressure) is around 3-5 mmHg. Varices usually develop when patients have HVPG >10 mmHg and presence of HVPG > 12 mmHg is a risk factor for variceal bleeding. Reduction in HVPG to less than 12 mmHg or by ≥ 20% from baseline reduces the risk of initial bleeding, and other complications of portal hypertension (ascites, encephalopathy)[ 14 ].

Mechanism of portal hypertension and the development of gastrointestinal varices. VEGF: Vascular endothelial growth factor; PDGF: Platelet-derived growth factor; NO: Nitric oxide; HVPG: Hepatic venous pressure gradient.
Porto-systemic shunting due to portal hypertension causes diversion of the portal blood into systemic circulation and results in variceal formation. Presence of ongoing liver injury due to alcohol, viral hepatitis (hepatitis B and C), or NASH can lead to increase in the size of the varices, whereas elimination of etiological factor can lead to decrease in the size or disappearance of varices in patients with alcoholic cirrhosis[ 16 , 17 ].
Intrahepatic hemodynamics
Architectural distortion : Hepatocellular injury causes transformation of hepatic stellate cells into myofibroblasts. Increased expression of pro-inflammatory genes and fibrotic activity, as a result, promotes neoangiogenesis and interstitial collagen deposition resulting in distortion of the hepatic sinusoidal architecture[ 18 , 19 ]. Architectural damage and regenerative nodules are responsible for nearly 2 nd /3 rd of the increase in intrahepatic resistance.
Increased vascular resistance : In addition to the known anatomical disruption in the sinusoidal architecture, it is now understood that there are changes in the neurohormonal regulation of vascular tone within the portal circulation. The hepatic injury causes increased production of vasoconstrictors (endothelin 1[ 20 , 21 ] and thromboxane A2[ 22 , 23 ]) and reduction in nitric oxide (NO) synthesis due to sinusoidal endothelial dysfunction[ 24 ]. The imbalance in the production of vasoconstrictors and vasodilators causes impaired vasomotor control leading to further increase in resistance and is responsible for approximately 1 st /3 rd of the increase in intrahepatic resistance[ 25 , 26 ].
Extrahepatic hemodynamics
Portal hypertension induces neurohormonal changes in the splanchnic circulation as well. Overproduction of NO from splanchnic endothelium leads to reduced splanchnic and systemic vascular resistance[ 27 - 29 ]. Furthermore, a compensatory activation of the renin-angiotensin mechanism leads to increased cardiac output and hepatic blood flow. Increased portal pressure is also suspected to result in overproduction of angiogenic factors such as vascular endothelial growth factor, platelet-derived growth factor at the microcirculatory level, contributing to angiogenesis and collateral formation resulting in varices[ 30 , 31 ].
Gastrointestinal varices develop as a consequence of portal hypertension. Most common etiology of portal hypertension in the United States is cirrhosis due to alcohol, NASH, and hepatitis C. The exact prevalence of portal hypertension is not known. Causes of portal hypertension are classified as below.
Presinusoidal
Extrahepatic : Portal vein thrombosis, splenic vein thrombosis.
Intrahepatic : Schistosomiasis, congenital hepatic fibrosis, and sarcoidosis. (1) Sinusoidal: Cirrhosis due to viral hepatitis (hepatitis B and C), NASH, alcohol, primary biliary cirrhosis, primary sclerosing cholangitis, hemochromatosis, Wilson’s disease, and cytotoxic drugs; and (2) Postsinusoidal: Budd-Chiari syndrome, caval web, constrictive pericarditis, and veno-occlusive disorders.
MECHANISM OF VARICEAL BLEEDING
Increased blood flow through the portosystemic collaterals due to portal hypertension causes dilation of the submucosal venous plexus resulting in elevated intravariceal pressure and wall tension. The mechanism of variceal rupture can be explained by Frank’s modified Laplace’s law[ 32 ]. This is shown in Figure Figure2 2 .

Mechanism of variceal bleeding. P: Pressure; R: Radius; WT: Wall thickness.
Wall tension (T) = [Transmural pressure (Pvarices-Plumen) × variceal radius (R)]/[Variceal wall thickness (WT)].
RISK STRATIFICATION FOR VARICEAL BLEEDING
Hvpg > 12 mmhg.
Rise in portal pressure causes increased flow through the varices and thus increased intravariceal pressure. In a randomized control trial (RCT) patients with HVPG < 12 mmHg did not develop variceal bleeding[ 33 ], and presence of HVPG > 20 mmHg was associated with high risk of failed hemostasis and death[ 34 ]. Whereas, a decrease in HVPG > 20% from the baseline reduces complications of portal hypertension including bleeding, ascites, encephalopathy, and death[ 35 - 37 ].
Variceal size
Large varices (> 5 mm) have a higher tendency to bleed due to increased wall tension as explained above.
Wall tension
Increased wall tension and the presence of ‘red wale sign’ (dilated capillaries on the variceal wall) indicate a high risk for bleeding.
Other factors
Presence of coagulopathy, infection, and decompensated cirrhosis are other risk factors for variceal bleeding.
DIAGNOSTIC TESTS FOR GASTROINTESTINAL VARICES
Esophago-gastro duodenoscopy (EGD) is the gold standard procedure used in the diagnosis of gastroesophageal varices (GOVs). Based on the endoscopic assessment, GOVs are classified into small (< 5 mm), and large varices(> 5 mm)[ 38 ] for clinical management. Disadvantages of endoscopy include the risk of sedation, higher cost, bleeding and risk of aspiration.
Endoscopic ultrasound
Endoscopic ultrasound (EUS) has been evaluated as a diagnostic tool in assessing GOVs. EUS is better than EGD in detecting gastric varices (GVs), and its ability to evaluate the anatomy of collateral and perforating veins makes it an excellent choice in monitoring treatment response to endoscopic variceal ligation (EVL) and predicting recurrence[ 39 - 41 ]. Currently, EUS is not considered as a primary diagnostic modality due to limited availability of local expertise.
Capsule endoscopy
A recent meta-analysis reviewed the use of capsule endoscopy for the diagnosis and grading of esophageal varices and noted a diagnostic accuracy of 90% with a pooled sensitivity and specificity of 83% and 85%, respectively[ 42 ]. The inability of capsule endoscopy to detect GVs is a significant drawback. Even though capsule endoscopy is relatively less invasive and does not require sedation, the diagnostic sensitivity is not adequate to advocate for index surveillance. It may be a consideration for a select subgroup of high-risk patients who are unwilling to undergo more invasive traditional endoscopic evaluation[ 43 , 44 ]. One study showed that 97% of the patients preferred capsule endoscopy over endoscopy with or without sedation[ 44 ].
Noninvasive testing
Various clinical findings, laboratory tests, and imaging studies have been considered as predictors of clinically significant portal hypertension (CSPH) (HVPG > 12 mmHg); however, they are not accurate enough to either reliably diagnose or exclude CSPH. Specifically, transient elastography, platelet count, spleen size, magnetic resonance elastography, and splenic stiffness are the most commonly used parameters to predict the presence of CSPH and varices in patients with cirrhosis. The presence of portosystemic collaterals on ultrasound, computed tomography, or magnetic resonance imaging is indicative of CSPH and necessitate screening endoscopy[ 38 ]. Liver stiffness measured by transient elastography in combination with platelet count can rule out presence high-risk varices[ 45 ]. A liver stiffness < 20 kPa and platelet count > 150000/μL indicate < 5% chance of having high-risk varices, and screening endoscopy can be safely deferred as long as ongoing clinical monitoring can be assured[ 46 ].
ESOPHAGEAL VARICES
Epidemiology.
Esophageal varices are the most common type of gastrointestinal varices, and their prevalence in Child-Pugh class A is 42.7%, around 70.7% in class B, and 75.5% in class C[ 2 ]. The bleeding risk for small varices and large varices is around 5% and 15% per year respectively. Early mortality rate (6 wk) is approximately 20%[ 47 ] in esophageal varices after index bleeding.
Venous drainage from the sub-mucosal venous plexus of the esophagus drains into the collateral veins around the esophagus. The interconnected collateral venous plexus runs longitudinally along the esophagus and communicates with submucosal venous plexus through perforating veins in the palisading area. The cervical esophagus drains into inferior thyroid vein, the thoracic esophagus drains to azygous, hemizygous, intercostal, and bronchial veins, whereas the abdominal portion of the esophagus drains into the left gastric vein, which in turn empties into the portal vein. Portal hypertension leads to shunting of blood from the portal circulation into these low pressure, thin-walled submucosal systemic veins and manifest as varices.
Modified Paquet classification
Grade I : Varices extending just above the mucosal level.
Grade II : Varices projecting by one-third of the luminal diameter that cannot be compressed with air insufflation.
Grade III : Varices projecting up to 50% of the luminal diameter and in contact with each other.
Screening and surveillance EGD for esophageal VARICES
Shown in Figure Figure3. 3 . All patients who are newly diagnosed with cirrhosis should be screened for esophageal varices. Patients with compensated cirrhosis without varices in the absence of ongoing liver injury, endoscopy should be done every three years. Those who have compensated cirrhosis without varices, but have an ongoing liver injury (alcohol abuse, hepatitis C) and/or other cofactor diseases (alcohol/obesity) screening endoscopy should be repeated every two years.

Screening endoscopy for esophageal varices per practice society guidelines[ 38 , 48 ]. NSBBs: Nonselective beta-blockers; EVL: Endoscopic variceal ligation; EGD: Esophago-gastro duodenoscopy; LS: Liver stiffness; PLT: Platelet.
Patients with small varices without ongoing liver injury or cofactor disease endoscopy is recommended every two years, and every year if either ongoing liver injury or cofactor disease is present. Patients with medium and large size varices should be started on nonselective beta-blockers or considered for EVL. If the patient is on nonselective beta blockers, no further surveillance endoscopy is needed.
On the other hand, if EVL is considered for primary prophylaxis endoscopy should be done every 1-2 wk until eradication and then repeated every 6-12 mo.
Management of patients with esophageal varices that have not bled
Either nonselective beta blockers or EVL (Figure (Figure4) 4 ) can be used as primary prophylaxis of variceal hemorrhage in patients with medium/large esophageal varices. Only approved nonselective beta-blockers are propranolol, nadolol, and carvedilol[ 38 , 48 - 52 ]. The choice should be made based on the cost, contraindications, availability, and patient preference. Nonselective beta-blockers are preferred over EVL due to their low cost, easy availability, ability to reduce the HVPG. Nonselective beta-blockers reduce the risk of hemorrhage and other complications (ascites, encephalopathy, and death) of portal hypertension[ 37 ]. Based on the currently available data, beta-blockers do not prevent the development of varices or their progression from small to large varices, although there is some reported benefit of reduction in risk of bleeding.

Endoscopic variceal ligation for primary prophylaxis. A: Esophageal varices before banding; B: Esophageal varix post banding.
The effect of nadolol on the progression of variceal size was studied in a prospective randomized study. A total of 161 patients were randomized into nadolol ( n = 83) and placebo ( n = 78) groups. All patients had yearly screening endoscopy and with a mean follow up of 36 mo. The cumulative probability of bleeding and progression of small varices was lower in nadolol group (20%) when compared to placebo (51%) ( P < 0.001) (absolute risk difference: 31%; 95%CI: 17%-45%)[ 53 ]. However, this benefit has not been proven in other studies.
In a recent meta-analysis of 6 RCTs, the effect of nonselective beta-blockers in cirrhotic patients with no or small varices was analyzed. The incidence of large varices (OR = 1.05, 95%CI: 0.25-4.36; P = 0.95), first variceal bleeding (OR = 0.59, 95%CI: 0.24-1.47; P = 0.26) and death (OR = 0.70, 95%CI: 0.45-1.10; P = 0.12) were similar in both nonselective beta-blocker group and placebo group. However, the incidence of adverse events in the nonselective beta-blockers group was significantly higher than the placebo group. Notably, nonselective beta-blockers did not reduce the incidence of large varices or prevent the progression of small varices to large varices[ 54 ].
On the other hand, when compared to nonselective beta blockers, EVL has a higher rate of recurrence of varices, lacks the benefit of HVPG reduction, and needs further endoscopic surveillance. EVL has lower but more severe side effects (bleeding, ulcers, and strictures) compared to nonselective beta-blockers (weakness, tiredness, shortness of breath). However, there is no significant difference in the mortality rate between the two[ 46 ].
In a prospective randomized study, the combination of EVL and propranolol was compared to EVL alone among high-risk patients. One hundred forty-four patients in total were randomized into EVL + propranolol ( n = 72) group and EVL alone ( n = 72) group respectively. Addition of propranolol to EVL did not reduce the risk of first variceal bleed (7% vs 11%, P = 0.72) or death (8% vs 15%, P = 0.37). However, the combination group had significant adverse effects due to propranolol in 22% of the patients. Combination of nonselective beta-blockers and EVL is not recommended as primary prophylaxis due to a higher rate of side effects. However, the recurrence of varices was significantly lower when propranolol was added ( P = 0.03)[ 55 ]. Recent practice society guidelines suggest the use of nonselective beta-blockers as a recommended therapy for primary prophylaxis for small varices with high-risk features (presence of ‘red wale’ signs or decompensated cirrhosis)[ 38 , 46 ].
Isosorbide mononitrate, sclerotherapy, glue injection, and transjugular intrahepatic portosystemic stent (TIPS) shunt are not used as primary prophylaxis due to a higher rate of side effects without mortality benefit.
Use of nonselective beta-blockers among patients who have cirrhosis with refractory ascites is controversial. A prospective case study showed that the use of nonselective beta-blockers in this patient group was associated with increased mortality[ 56 ]. Another study also showed the increased risk of renal injury, hospital stay and mortality with the use of nonselective beta-blockers with spontaneous bacterial peritonitis due to post-paracentesis circulatory dysfunction[ 57 ]. However, a meta-analysis of 3 RCTs and 13 observational studies ( n = 8279) showed no significant difference in mortality or incidence of hepatorenal syndrome and spontaneous bacterial peritonitis among cirrhosis patients with refractory ascites, when treated with nonselective beta blockers[ 58 ]. Due to concern for possible deleterious effects in patients with advanced cirrhosis, many physicians now prefer EVL over nonselective beta blockers. Larger RCTs are required before nonselective beta-blockers are considered as a contraindication in this subgroup.
Management of acute esophageal variceal bleeding
General measures: All patients with acute variceal bleeding should be resuscitated at an early stage to protect the airway and achieve hemodynamic stability, preferably in a medical intensive care unit. Prognostic indicators for early mortality due to acute variceal bleeding are HVPG, Child-Pugh score, and model for end-stage liver disease (MELD) score. A MELD score of > 19 showed 20% mortality due to index variceal bleeding[ 34 , 47 , 59 ]. When measured within 24 h of acute bleeding, HVPG > 20 mmHg predicts a high risk of early rebleeding and death[ 38 , 48 , 60 ]. The Child-Pugh score is also a significant predictor of early mortality and can help guide patient risk stratification[ 61 ]. Medical management with vasoactive agents, antibiotics, blood transfusion, combined with EVL is the standard of care in treating acute variceal bleeding.
Restrictive transfusion strategy: All patients with Hb ≤ 7 g/dL should get packed red blood cells to maintain hemoglobin at 7-8 g/dL. Previous RCTs have shown a survival benefit, reduced need for blood transfusion, and a lower rate of adverse events with a restrictive strategy when compared to liberal transfusion[ 62 ].
Most patients with acute variceal bleeding have elevated HVPG (> 12 mmHg). Further elevation of HVPG due to liberal transfusion can increase the risk of rebleeding. In a recent meta-analysis, the incidence of death (OR = 0.52, 95%CI: 0.31-0.87, P = 0.01), blood transfusion requirement (standard mean difference: -0.74, 95%CI: -1.15--0.32, P = 0.0005) and hospital stay (standard mean difference: -0.17, 95%CI: -0.30--0.04, P = 0.009) were significantly lower in the restrictive transfusion group compared to the liberal transfusion group[ 63 ].
Therefore, a restrictive transfusion strategy should be employed in managing patients with acute variceal bleeding. The current practice society guidelines do not recommend routine use of plasma products and platelet transfusion in this setting due to inconsistent data on the use of plasma products and reliability of prothrombin time (PT)/international normalized ratio (INR) in patients with cirrhosis[ 38 , 46 ]. However, platelet and plasma transfusion can be done in select patients who are hemodynamically unstable with active variceal bleeding (goal: platelet count > 50000/ μL and INR < 1.5)[ 47 ].
Vasoactive agents: Vasoactive agents such as octreotide, terlipressin, somatostatin, and vasopressin cause splanchnic vasoconstriction and thus reduce portal pressure. All patients with confirmed or suspected variceal bleeding should be started on vasoactive agents as early as possible and should be continued for 2-5 d. They can be stopped early if the patient undergoes a TIPS procedure.
Terlipressin is a synthetic analog of vasopressin. The role of terlipressin in acute variceal bleeding was analyzed in a meta-analysis involving 1609 patients from 22 studies. Among those 22 studies, seven studies (443 patients) compared the effect of terlipressin to a placebo group. Terlipressin group was noted to have a statistically significant reduction in all-cause mortality (relative risk = 0.66, 95%CI: 0.49-0.88). Remaining studies compared terlipressin to somatostatin, octreotide, vasopressin or balloon tamponade. There was no significant difference in mortality or adverse events between the groups[ 64 , 65 ].
Use of vasoactive agents has been shown to reduce acute bleeding, need for transfusion, and seven-day all-cause mortality[ 66 ]. There was no significant difference in their efficacy or benefits noted between these agents[ 67 ].
Antibiotics: Short-term antibiotics should be started in all patients with suspected or confirmed variceal bleeding to reduce bacterial infection, recurrent bleeding, and mortality[ 38 , 48 , 68 , 69 ]. Bacterial infection is also considered to be an independent risk factor for variceal rupture. Choice of antibiotics should be based on local resistance pattern. However, third-generation cephalosporins with gram-negative coverage are commonly used. Intravenous ceftriaxone 1 g, every 24 h for a maximum of 7 d is preferred over oral fluroquinolones[ 38 , 46 ].
Other considerations: Most patients with variceal bleeding have loss of intravascular volume, and it is paramount to prevent hypotension. Due to the risk of hypotension and hemodynamic deterioration, nonselective beta-blockers should not be started during acute variceal bleeding and should be discontinued if the patient is already taking. However, nonselective beta-blockers should be restarted after the acute event, once hemostasis is achieved and vasoactive agents are discontinued.
Endoscopic management
Endoscopic intervention should be performed as early as possible but should be within 12 h from the time of presentation as per practice society guidelines. The diagnosis of variceal bleeding as the etiology of acute upper gastrointestinal bleeding is made when any of the following is noted on upper endoscopy: (1) Actively bleeding varices (Figure (Figure5); 5 ); (2) Signs of recent bleeding noted on varices or high-risk stigmata; e.g ., telangiectasia, red color signs, platelet-fibrin plug (white nipple sign), red wale marking or varices on varices (Figure (Figure6); 6 ); (3) Presence of varices and blood is noted in the stomach, with no other source of bleeding noted.

Bleeding esophageal varices.

High-risk stigmata of bleeding from esophageal varices. A: Platelet-fibrin plug on esophageal varix (white nipple sign); B: Bleeding esophageal varix post banding.
EVL (Figure (Figure7) 7 ) was first proposed for the treatment of esophageal varices in 1988 by Van Stiegmann et al[ 70 ]. Currently, EVL is considered to be the first line of endoscopic treatment for the management of bleeding esophageal varices. EVL has better hemostasis, a lower rate of side effects (ulcer, stricture), a reduced rate of early rebleeding, and a lower rate of early mortality compared to sclerotherapy. Higher rebleeding in sclerotherapy is thought to be due to sustained elevation of HVPG, whereas HVPG returned to baseline with EVL[ 71 - 73 ]. The slightly higher rate of variceal recurrence after EVL, when compared to sclerotherapy is due to its inability to affect the blood flow through perforators and esophageal collateral veins.

Endoscopic variceal band ligation.
Treatment failure
Sengstaken-Blakemore tube : Whenever variceal bleeding is not controlled by EVL, temporary hemostatic measures should be used as a bridge to more definitive treatment, such as TIPS or variceal shunt surgery. Sengstaken-Blakemore tube is inserted through the mouth or nose and then distended to achieve hemostasis during active variceal bleeding by tamponading varices. The rate of hemostasis with Sengstaken-Blakemore tube varies (47%-80%). It is associated with a high rate of serious adverse events including aspiration, esophageal ulceration, and rarely esophageal rupture. Sengstaken-Blakemore tubes cannot be left in place for more than 24 h due to an increased risk of adverse events and a high rate of rebleeding (50%)[ 72 , 73 ].
Metal stents : Endoscopically placed self-expanding fully covered metal stents (Figure (Figure8) 8 ) can achieve hemostasis in most cases (80%-96%). The stents expand inside the esophagus and tamponade the varices to achieve hemostasis. They can be left in place for up to 2 wk and have a lower rate of serious adverse events and transfusion requirements when compared to balloon tamponade[ 74 , 75 ]. Adverse events associated with this modality of treatment include stent migration (28%), rebleeding (16%) and ulcers. However, there was no significant difference in mortality compared to balloon tamponade[ 76 , 77 ].

Metal stents for the treatment of bleeding esophageal varices. A: Bleeding esophageal varix before stenting; B: Esophageal varix after metal stent.
In a meta-analysis of 12 studies ( n = 155) hemostasis was achieved in 96% (95%CI: 0.90-1.00) of the patients within 24 h with 97% technical success (95%CI: 0.91-1.00). Adverse events (rebleeding, ulceration and stent migration) were noted in 36% (95%CI: 0.23-0.50) of the patients. Pooled susvival rate at 30 d and 60 d were 68% (95%CI: 0.56-0.80) and 64% (95%CI: 0.48-0.78) respectively[ 78 ]. Similar results were noted in another meta analsysis of 5 studies ( n = 80) with technical success of 96.7% (95%CI: 91.6%-99.5%) and hemostasis of 93.9% (95%CI: 82.2%-99.6%). Rebleeding was observed in 13.2% (95%CI: 1.8%-32.8%) and the overall mortality was 34.5% (95%CI: 24.8%-44.8%)[ 79 ].
Based on the above evidence, self-expanding metal stents are a better choice for bridge therapy in uncontrolled esophageal variceal bleeding and should be used whenever available.
Patients with uncontrolled variceal hemorrhage despite the combination of medical and endoscopic treatment should be considered for early TIPS within (24 h) with covered PTFE (polytetrafluoroethylene) stents. TIPS is a shunt created by placing a stent between the portal vein and hepatic vein to reduce the portal pressure and thereby portal hypertension. Also, early rebleeding (within five days of initial bleeding) can be treated with repeat endoscopic intervention or covered TIPS stent[ 38 , 46 , 48 ].
TIPS vs pharmacotherapy and endoscopic treatment
In a meta-analysis of six comparative studies, TIPS was compared with medical and endoscopic treatment for acute variceal bleeding. In this study, the survival rate (HR = 0.55; 95%CI: 0.38-0.812) was better in TIPS patients, and the incidence of bleeding-related death (OR = 0.19; 95%CI: 0.06-0.59) was lower compared to medical/endoscopic treatment. There was no significant increase in hepatic encephalopathy (OR = 1.37; 95%CI: 0.63-2.99) in TIPS patients. Although there was no significant difference in rebleeding rate between the two groups, it was evident that rebleeding in the high-risk patients was higher on subgroup analysis[ 80 ].
Early TIPS vs pharmacotherapy and endoscopy in high-risk patients
Patients with Child-Pugh class B with active bleeding and class C are considered high-risk due to increased risk of treatment failure and rebleeding.
In a 2010 study, early TIPS was compared with pharmacotherapy (vasoactive agents) and EVL in Child-Pugh class C patients and class B patients with a high risk of treatment failure. Sixty-two patients were randomized into the treatment group (early TIPS, n = 32), and control group (pharmacotherapy and EVL, n = 31). Rescue TIPS was used in control group as needed for treatment failure. Rebleeding or failure to control bleeding occurred in one patient in the early TIPS group and 14 patients in the control group ( P = 0.001). The one-year actuarial survival rate was 61% in the control group vs 86% in the early-TIPS group ( P < 0.001)[ 81 ].
In another international multicenter observational study (671 patients from 34 centers) patients who were admitted for acute variceal bleeding with Child-Pugh class C, and Child-Pugh class B with active bleeding were included in the study. Patients were treated with either pharmacotherapy and endoscopic interventions or preemptive TIPS. Preemptive TIPS was associated with significantly lower one-year mortality (22% vs 47%, P = 0.002), treatment failure and rebleeding (92% vs 74%, P = 0.017) when compared to patients treated with pharmacotherapy and endoscopic interventions. TIPS also prevented the development of new ascites or worsening of pre-existing ascites[ 82 ]. Even though these results are encouraging, it was an observational study, and patients were not randomized. Each center chose to treat the patient with either preemptive TIPS or medications and endoscopy at its discretion. Therefore, the results may not be generalized. However, large RCTs can determine the use of preemptive TIPS in this high-risk population[ 82 ].
Complications from TIPS include hepatic encephalopathy, heart failure, and stent stenosis. The incidence of hepatic encephalopathy is close to 50% without a significant difference in mortality[ 83 ]. Absolute contraindications for TIPS include heart failure, severe pulmonary hypertension, severe tricuspid valve regurgitation, sepsis, and unrelieved biliary obstruction. Relative contraindications are portal vein thrombosis, hepatoma, uncorrected coagulopathy, and severe thrombocytopenia (platelet count < 20000/μL).
Direct ultrasound-guided direct intrahepatic porto-caval shunt
Patients who failed TIPS, those who have altered anatomy due to previous surgery or congenital anomaly, or are otherwise not candidates for TIPS, can be treated with direct ultrasound-guided direct intrahepatic porto-caval shunt (DIPS)[ 84 ]. The DIPS is a modified TIPS procedure, and it involves percutaneous ultrasound-guided puncture from the inferior vena cava to the portal vein through the caudate lobe of the liver.
Porto-caval shunt surgeries
Surgical shunts are considered when all other treatment modalities fail. Portocaval surgery has a very high rate of encephalopathy but does have good bleeding control. Most patients who undergo portocaval shunt surgery already have high morbidity and surgery adds to it further[ 85 ]. In a recent RCT, emergency TIPS procedure was compared with emergency portocaval shunt surgery, and shunt surgery was noted to have superior bleeding control, long-term survival (10 years vs 1.99 years) and low rate of encephalopathy. However, this has not been replicated, and more evidence is required before using portocaval shunt surgery as a salvage procedure after failure of first-line treatment with medical therapy and EVL[ 86 ].
Secondary prophylaxis
Patients who were treated with EVL and medical therapy without TIPS are at high risk for rebleeding. Approximately 60% of patients will experience rebleeding during the first year and have a high mortality rate (up to 33%) with no further intervention. Combination therapy with nonselective beta blockers (propranolol and nadolol) and EVL is the first line of treatment for secondary prophylaxis with a goal to eradicate varices and prevent recurrent bleeding[ 87 ]. TIPS should be considered if patients do not tolerate or fail the combination of nonselective beta-blockers and EVL.
A multicenter RCT compared TIPS with the combination of EVL or glue injection and nonselective beta-blockers. Patients in the TIPS group had a significantly lower rebleeding rate (0%) compared to the EVL or glue injection and nonselective beta blockers group (29%) without a significant difference in survival benefit[ 88 ].
GASTRIC VARICES
GVs are less frequent compared to esophageal varices and are reported to be seen in 20% of the patients with portal hypertension[ 38 , 89 ]. Bleeding from GVs account for 20% of all variceal bleeding[ 48 ]. The annual bleeding rate in GVs, which have never bled before is reported to be as low as 16% per year. Sarin et al[ 90 ] classified GVs based on their location.
Sarin classification
Shown in Figure Figure9. 9 . Gastroesophageal varix type 1 (GOV1): Extension of esophageal varices along lesser curvature (most common 75% of GVs); GOV2: Extension of esophageal varices along the greater curvature; Isolated gastric varix type 1 (IGV1): Isolated varices seen in the fundus of the stomach; IGV2: Isolated varices in the stomach (body, pylorus, antrum).

Sarin classification of gastric varices. GOV1: Gastroesophageal varix type 1; GOV2: Gastroesophageal varix type 2; IGV1: Isolated gastric varix type 1; IGV2: Isolated gastric varix type 2.
Predictors of bleeding from GVs
Location (IGV1 > GOV2 > GOV1); The severity of liver disease; Stigmata of high-risk bleeding such as ‘red wale’ sign.
GVs bleed less frequently but have high mortality due to the severity of bleeding. Bleeding from IGV is associated with the highest risk of death[ 38 , 48 , 91 ].
GVs have complex anatomy and understanding the anatomy assists in the endoscopic management of GVs. The most common type of GVs are GOV1 and are usually associated with portal hypertension due to cirrhosis. They are a continuation of esophageal varices along the lesser curvature of the stomach. These are supplied by the esophageal collateral veins and are also treated similarly to esophageal varices.
On the other hand, GOV2 and IGV1 are supplied by the posterior and left gastric vein, which later drains into left renal vein due to porto-systemic shunting. Therefore GOV2 and IGV1 are together called cardiofundal varices[ 91 ]. Isolated IGV1 can be associated with splenic vein thrombosis in the setting of pancreatitis or malignancy.
Diagnosis of GVs
Diagnosis of GVs (Figure (Figure10) 10 ) is commonly done with endoscopy. However, the recent use of EUS has increased the sensitivity of detecting GVs. No guidelines are currently available regarding the use of endoscopy or EUS specifically to diagnose GVs.

Gastric varices.
Management of patients with GVs that have not bled
Primary prophylaxis for GVs is not well established. Currently, nonselective beta-blockers are the first line of treatment as per practice society guidelines, in large part due to their ability to prevent other complications of cirrhosis. The role of endoscopic glue (N-butyl-2-cyanoacrylate) injection and EVL in primary prophylaxis are not clear. One study has shown glue injection was associated with lower bleeding and mortality due to GVs when compared to nonselective beta blockers[ 92 ]. Prophylactic EUS guided injection has also shown to be equally beneficial, and further studies are required to evaluate its role in primary prophylaxis for GVs.
Management of acute gastric variceal bleeding
Medical management of suspected gastric variceal bleeding is similar to esophageal variceal bleeding as described above, including airway protection, admission to the intensive care unit, blood transfusion, vasoactive agents, and antibiotics (Figure (Figure11 11 ).

Algorithm for the management of acute variceal bleed. ICU: Intensive care unit; EGD: Esophago-gastro duodenoscopy; NSBB: Nonselective beta blockers; EVL: Endoscopic variceal ligation; TIPS: Transjugular intrahepatic portosystemic shunt.
Endoscopic interventions
Diagnosis of gastric variceal bleeding can be made based on endoscopic findings. Most practice guidelines recommend endoscopic glue injection as the first line of treatment in the management of acute gastric variceal bleeding. However, glue injection comes with the risk of several complications including venous and systemic thromboembolism (pulmonary embolism, stroke), ulcers, protracted bleeding, splenic and portal vein thrombosis[ 93 ]. Portal vein thrombosis due to embolized glue can render a future plan for TIPS and liver transplantation ineffective. Embolized glue can also act as a nidus of infection and cause recurrent bacteremia[ 94 ]. Successful glue injection requires experience due to gastric anatomy. Because of the drawbacks mentioned above, many centers use TIPS as the first line of treatment in managing acute gastric variceal bleeding.
A RCT compared efficacy and complication of TIPS and glue injection in treating GVs. Rebleeding (11% vs 38%, P = 0.014; OR = 3.6, 95%CI: 1.2-11.1) and transfusion requirements were lower ( P < 0.01) in TIPS compared to endoscopic glue injection with similar initial hemostasis, side effects, and mortality[ 95 ].
Even though initial hemostasis in both glue injection and EVL is similar for GOV1 GVs, rebleeding is high in EVL. So EVL should be avoided[ 96 - 98 ]. Combination of sclerotherapy and EVL is currently not recommended due to a higher rate of complications, and adverse events without mortality benefit[ 99 ]. In a recent RCT, scleroligation (variceal ligation + sclerotherapy) compared to EVL alone, in the management of GOVs, the scleroligation group required a lower number of endoscopic procedures, transfusion, and bands used, without a significant difference in recurrence rate, major side effects, and mortality[ 100 ]. Further research is needed to prove the benefits of scleroligation.
The recent emergence of EUS guided glue and coil injection in treating GVs has shown a lower bleeding rate, transfusion requirements, and mortality when compared to glue injection. When EUS guided coil embolization alone was compared with EUS guided glue injection, both had similar hemostasis rates, but coil embolization had fewer adverse events and required a fewer number of endoscopies[ 101 ]. When these two techniques were combined (glue + coil), the mean number of coils used, mean volume of glue used, and the recurrence rate was lower compared to either of them alone[ 102 ].
Patients with uncontrolled gastric variceal bleeding despite endoscopic intervention should be managed with balloon tamponade with Sengstaken-Blakemore tube or Linton-Nachlas tube as a bridge to definitive treatment. In a controlled trial Sengstaken-Blakemore tube failed to control gastric variceal bleeding in all the cases, and 50% hemostasis was achieved by Linton-Nachlas tube. Types of GVs and their frequency between the two groups was not available[ 73 ]. This difference could be attributed to a larger gastric balloon (500 mL) when compared to smaller gastric balloon in the Sengstaken-Blakemore tube. Therefore, Linton-Nachlas tube should be used whenever possible.
Hemostatic powder
Hemostatic powder (TC 325 - hemospray) and similar products have been used as bridging therapy in controlling acute peptic ulcer bleeding in the past. The hemostatic powder when sprayed at the bleeding site, it absorbs water and creates a mechanical barrier to achieve hemostasis. Recently one study assessed its role in acute variceal bleeding. Hemostasis in the study group was better than the control group, with fewer study group patients requiring rescue endoscopy (12%). Rescue endoscopy was performed if initial hemostasis was not achieved within the first 12 h with hemospray. All patients were later treated with definitive endoscopic intervention after 24 h. Larger RCTs are required to evaluate the role of hemostasis powder, and currently not approved by Food and Drug Administration[ 103 , 104 ].
Patients with GVs who fail to respond to the endoscopic treatment will require TIPS or shunt surgery to control acute variceal bleeding. Recurrent bleeding is noted in 11%-30% of the patients who undergo TIPS.
Balloon-occluded retrograde transvenous obliteration
Patients with GVs and gastro-renal collaterals can be treated with balloon-occluded retrograde transvenous obliteration (BRTO). This procedure involves retrograde cannulation of the outflow channels which drain the GVs through the femoral or jugular vein, and obliteration of the varices and collaterals assisted by balloon occlusion and followed by coil and sclerosant. Various studies have evaluated its efficacy in treating GVs. A recent meta-analysis showed a success rate for obliteration was 97.3%, and 33.3% recurrence. BRTO can be considered as an alternative to TIPS in managing GVs. A retrospective review of 142 consecutive patients treated for acute gastric variceal bleeding comparing the efficacy of BRTO ( n = 95) and TIPS ( n = 47) showed significantly lower rebleeding rate in BRTO (8.6%) group compared to TIPS (19.8%)[ 105 ] at the end of the first year. There was no significant difference in mortality. BRTO is mostly done in Asian countries, but recently it is gaining popularity in the United States[ 38 , 48 , 106 ].
Risk of rebleeding among patients who are treated with glue injection for gastric variceal bleeding was noted to vary from 15%-72%[ 98 , 107 , 108 ]. TIPS is considered to be superior to endoscopic glue injection for secondary prophylaxis of GVs[ 38 ]. However, there is no significant mortality benefit when compared to glue injection. The role of nonselective beta-blockers is not evident in secondary prophylaxis of GVs. Data on EUS guided glue injection and coiling for primary and secondary prophylaxis is lacking. Larger multicenter RCTs will help in understanding the role of EUS in the management of GVs.
ECTOPIC GASTROINTESTINAL VARICES
Gastrointestinal varices can develop in the duodenum, rectum, colon, small bowel, gallbladder and the retroperitoneal areas. The prevalence of ectopic gastrointestinal varices is unknown. According to one estimate, among patients with cirrhosis and portal hypertension who underwent angiography, 40% of patients had duodenal varices. Ectopic varices are responsible for up to 1%-5% of all variceal bleeding. Understanding the complex anatomy of ectopic varices, and their anastomosis with mesenteric veins is essential in managing ectopic varices[ 91 , 109 ].
Duodenal varices
Duodenal varices are more commonly seen in noncirrhotic, extrahepatic portal hypertension ( e.g ., portal vein thrombosis, splenic vein thrombosis) and their prevalence is around 0.4%[ 109 ]. Duodenal varices form due to Porto-mesenteric and Porto-portal anastomosis. Duodenal varices are noted on endoscopy as submucosal dilated veins, usually arising from anastomosis between tributaries of the superior mesenteric vein and portal vein draining into inferior vena cava. EUS is notably superior in diagnosing duodenal varices compared to EGD[ 110 ]. Acute duodenal variceal bleeding is usually treated with endoscopic glue injection. There have been no RCTs evaluating the treatment strategies for duodenal varices owing to their rarity. In the largest case series involving ten patients with duodenal variceal glue injection, 4 out of the five patients who presented with acute bleeding were treated with endoscopic glue injection and had 100% hemostasis rate without recurrence[ 110 ]. Duodenal varices bleed at a lower hepatic venous pressure gradient, and therefore TIPS may not be sufficient to treat duodenal varices and need further definitive treatment with intravascular obliteration with glue injection, or embolization through BRTO. BRTO can also be used for patients who fail endoscopic therapy and are not candidates for TIPS[ 111 ].
Rectal varices
Rectal varices usually arise from portosystemic anastomosis between superior hemorrhoidal veins (a tributary of the inferior mesenteric vein) and the middle or inferior hemorrhoidal veins (tributaries of iliac or pudendal veins). Prevalence of rectal varices patients with portal hypertension varies from 28%-56% in cirrhotic patients[ 112 ], and are more common among patients with extrahepatic portal vein obstruction(up to 90%)[ 113 ]. EUS has a higher sensitivity to detect rectal varices compared to endoscopy. Risk of bleeding from rectal varices is 8%-38%[ 112 ]. Rectal varices bleed at the lower hepatic venous pressure gradient and may not disappear with TIPS. Endoscopic variceal band ligation is the preferred method of treatment for rectal varices compared to endoscopic sclerotherapy or glue injection, but the recurrence rate of rebleeding is high with Endoscopic variceal band ligation. Recurrent bleeding in endoscopic sclerotherapy (33%) was much lower compared to EVL (55.6%)[ 114 ] but not commonly used due to the occurrence of severe ulcers. Endoscopic glue injection can be useful in managing rectal varices, but nearly 0.5%-4.3% of these patients develop embolization. EUS guided coil and glue embolization is also considered useful in large rectal varices that are not amenable to variceal ligation[ 115 ]. Role of BRTO has been evaluated in small case series; no RCTs are available to compare its efficacy. Optimal management of rectal varices is not yet established.
Stomal varices
Stomal varices usually occur at the mucocutaneous junction of the stoma, due to portosystemic shunt between the portal circulation of the bowel and systemic circulation of the abdominal wall. Diagnosis of stomal varices is difficult, on physical exam, they appear as bluish discoloration of the skin. Visibly dilated veins and characteristic raspberry appearance of the stoma should prompt further evaluation for the cause of bleeding. Patients with stomal varices can be treated with a glue injection. Percutaneous sclerotherapy is not recommended due to increased risk of damaging the stoma. Gastrointestinal varices can also form in other parts of the gastrointestinal tract including jejunum, ileum, and colon as well. The actual prevalence of these varices is unknown but considered to be low.
In summary, development, and utilization of newer treatment modalities such as therapeutic EUS, BRTO, and hemospray in managing gastrointestinal varices will help to reduce further- the morbidity and mortality related to variceal bleeding. Further research in understanding the risk factors, mechanism of liver injury, and evaluation of antifibrotic agents to prevent architectural changes to the liver can revolutionize the management of portal hypertension and its complications.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: None of the authors have any conflicts of interest.
Peer-review started: August 27, 2018
First decision: October 5, 2018
Article in press: December 11, 2018
P- Reviewer: Lo GH, Qi XS S- Editor: Ma RY L- Editor: A E- Editor: Bian YN
Contributor Information
Umesha Boregowda, Department of Gastroenterology and Hepatology, University of California San Francisco, Fresno, CA 93721, United States.
Chandraprakash Umapathy, Department of Gastroenterology and Hepatology, University of California San Francisco, Fresno, CA 93721, United States.
Nasir Halim, Department of Gastroenterology and Hepatology, University of California San Francisco, Fresno, CA 93721, United States.
Madhav Desai, Department of Gastroenterology and Hepatology, Kansas University Medical Center, Kansas City, KS 66160, United States.
Arpitha Nanjappa, Department of Gastroenterology and Hepatology, University of California San Francisco, Fresno, CA 93721, United States.
Subramanyeswara Arekapudi, Department of Medicine, VA Central California Healthcare System, Fresno, CA 93703, United States.
Thimmaiah Theethira, Department of Gastroenterology and Hepatology, University of California San Francisco, Fresno, CA 93721, United States.
Helen Wong, Department of Gastroenterology and Hepatology, VA Central California Healthcare System, Fresno, CA 93703, United States.
Marina Roytman, Department of Gastroenterology and Hepatology, University of California San Francisco, Fresno, CA 93721, United States.
Shreyas Saligram, Department of Gastroenterology and Hepatology, University of California San Francisco, Fresno, CA 93721, United States. Department of Gastroenterology and Hepatology, VA Central California Healthcare System, Fresno, CA 93703, United States. ude.fscu.onserf@margilass .

IMAGES
VIDEO
COMMENTS
Nursing Case Studies by and for Student Nurses. 25. The following is a scenario of a patient who experienced ruptured esophageal varices. The patient, Nora Allen, a 55-year-old female presented to the emergency department (ER) on May 5, 2017 just after 10:00 a.m., with complaints of two episodes of melena that morning. ...
Nov 13, 2018 Knowledge. Clinical Case Scenario #1. Health Education for a Male Inpatient with Esophageal Varices. Clinical Scenario. Forty five year old G.K. is admitted to the hospital's Emergency Department. He is vomiting large amounts of blood. He is mildly intoxicated but alert. His pulse is fast and thready.
Nursing Care Plan for Esophageal Varices 2. Nursing Diagnosis: Imbalanced Nutrition: Less than Body Requirements related to digestive tract bleeding secondary to esophageal varices, as evidenced by hematemesis, weight loss, nausea and vomiting, loss of appetite and dizziness/ lightheadedness.
CASE SUMMARY. A 46-year-old woman, who denied a history of liver disease, was admitted to our hospital on presentation of hematemesis. Laboratory examination revealed a hemoglobin level of 83 g/L, and a platelet count of 397 × 10 9 /L. The appearance of gastric and esophageal varices with red colored signs as displayed by an urgent endoscopy was followed by endoscopic variceal ligation and ...
Esophageal varices (EV) are one of the most common causes of acute upper gastrointestinal bleeding (UGIB) with varying prevalence worldwide [1,2]. They are the leading cause of death from UGIB. ... This case study validates the efficacy of the EUS coil/CYA in the treatment of GV. Coil placement, with or without CYA, can have unfavorable ...
Esophageal varices are the most common type of gastro-esophageal varices, with a prevalence of 50-60% among patients with cirrhosis, up to 85% in patients with decompensated cirrhosis. Gastric varices are present in about 20% of patients with cirrhosis and they can be of different types 1. Sarin's classification 2 is the most commonly used ...
The development of this nursing guideline was coordinated by Rachel Horn, CNC, Gastroenterology, and Laura Davies, CNS, Cockatoo, approved by the Nursing Clinical Effectiveness Committee. ... Some small case-series paediatric studies have been undertaken (421). ... Assessment of risk of bleeding from esophageal varices during management of ...
Esophageal varices incidences are increasing by nearly 5% every year. Esophageal varices are the causes of bleeding in approximately 18% of hospital admissions for upper GI bleeding. Objective: The purpose of this study is to understand the cause, clinical manifestations and treatment course for esophageal varices.
Acute variceal bleeding (AVB) is a potentially fatal complication of clinically significant portal hypertension and is one of the most common causes of acute upper gastrointestinal bleeding. Thus, esophagogastric varices represent a major economic and population health issue. Patients with advanced chronic liver disease typically undergo an upper endoscopy to screen for esophagogastric varices ...
Esophageal varices are primarily caused by increased pressure in the portal vein system, which can result from various underlying medical conditions. ... Study More in Less Time. Using a study tool can help you keep engaged and at a steady pace when studying conditions like esophageal varices. SimpleNursing is trusted by nursing students to ...
Esophageal varices are abnormal, enlarged veins in the tube that connects the throat and stomach. Variceal rupture is governed by Laplace's law. Increased wall tension is the end result of increased intravariceal pressure, increased diameter of the varices, and reduced wall thickness. The variceal wall thickness can be evaluated visually by the ...
Main tests used to diagnose esophageal varices are: Endoscopic exam. A procedure called upper gastrointestinal endoscopy is the preferred method of screening for esophageal varices. An endoscopy involves inserting a flexible, lighted tube called an endoscope down the throat and into the esophagus.
Compare and contrast the treatment options and nursing implications for esophageal varices. 7. Discuss the rationale for Mr. Hubert's vasopressin therapy, management of side effects, and nursing concerns. 8. Identify the relevant nursing diagnoses for Mr. Hubert while he is in the ICU. 9.
All the pertinent investigations were studied thoroughly of the selected case. Results: the esophageal varices is confirmed with the help of Upper gastrointestinal gastroscopy and repaired by banding. Conclusion: Esophageal varices is usually goes undiagnosed due to early common symptoms. ... Esophageal Varices: A Case Study. Asian J. Nursing ...
Objectives To identify and understand Nursing care in patients with esophageal varices.; Methods to adopt while managing acute variceal bleeding.; Nurses role in prevention of secondary bleeding. Design: used for this article is to review method. Data sources are different types of Medical and Gastroenterology text books, Medical Journals and Medical Surgical Nursing Text Books.
Aim: This study aimed toassess nurse's performance regarding care of patients with esophageal variceal bleeding. Design A descriptive exploratory design was utilized for the conduction of this study. Setting the study was carried out in intensive care unit of hematemsis at Elmery hospital affiliated to Alexandria University.
Esophageal Varices . ... (15 plus hrs), over 125 study tools and over 800 practice questions and rationales that are sure to clarify complex topics and leave you confident and ready to tackle the exam. NURSING.com teamed up with long-time emergency nursing expert, Dr. Laura Gasparis Vonfrolio, RN, PHD on the review questions that serve as both ...
Definition Bleeding esophageal varices are hemorrhagic processes involving dialted, tortuous veins in the submucosa of the lower esophagus. . Risk Factors Portal hypertension resulting from obstructed portal venous circulation Pathophysiology In portal hypertension, collateral circulation develops in the lower esophagus as venous blood, which is diverted from the GI tract and spleen because of ...
Esophageal varices are dilated submucosal distal esophageal veins connecting the portal and systemic circulations. This happens due to portal hypertension (most commonly a result of cirrhosis), resistance to portal blood flow, and increased portal venous blood inflow. The most common fatal complication of cirrhosis is variceal rupture; the severity of liver disease correlates with the presence ...
The above clinical practice guideline recommendations arise from studies in which patients with a diagnosis of hemophilia were not included, making the approach challenging due to the lack of available evidence. ... UGB in patients with portal hypertension is likely from esophageal varices. Case management is challenging as congenital ...
Nursing management for the patient with cirrhosis of the liver should focus on promoting rest, improving nutritional status, providing skin care, reducing risk of injury, and monitoring and managing complications. Nursing Assessment. Assessment of the patient with cirrhosis should include assessing for: Bleeding.
Case study for GIB, liver disease, and skin assessment. Your patient is Mr. Abrams, a 63 year-old Black male with a history of non-alcoholic liver cirrhosis, esophageal varices, hyperlipidemia, hypertension, type 2 diabetes, osteoarthritis, hypothyroidism, and renal insufficiency. He does not drink alcohol, smoke or use recreational drugs.
Cardiac varices (CVs) in patients with type 1 gastroesophageal varices (GOV1s) usually disappear with treatment for esophageal varices (EVs) by endoscopic injection sclerotherapy (EIS). However, whether this applies to patients treated with endoscopic band ligation (EBL) for EVs remains unclear.
The study suggests that routine esophageal biopsy sampling in individuals with refractory reflux symptoms has low diagnostic yield and recommends selectively obtaining esophageal biopsies, focusing on patients with refractory reflux symptoms accompanied by dysphagia for a more targeted and clinically meaningful diagnostic approach.
Blunt trauma to the head and neck is a rare cause of cervical esophageal perforation. We report a cervical esophageal perforation caused by compression by a shoulder-harness seatbelt during a high-speed motor vehicle crash. We are not aware of a similar case in the trauma literature.
The 2019 multicenter, prospective study by Louie BE et al. evaluated patients with pathologic acid reflux confirmed by esophageal pH testing undergoing MSA. 15 A total of 200 patients, ages ranging from 19.7-71.6 years. At 1 year, the mean total AET decreased from 10.0% at baseline to 3.6% and 74.4% of patients had normal esophageal AET.
Device assisted enteroscopy (DAE) like the double balloon enteroscopy (DBE) and single balloon enteroscopy (SBE) are postulated to ease small bowel examination and performance of therapy. However, studies comparing the effectiveness of these 2 modalities have yielded varying results. The aim of this study is to compare the efficacy and safety ...
A prospective case study showed that the use of nonselective beta-blockers in this patient group was associated with increased mortality ... Smith C, Lieberman G. The natural history of esophageal varices; a study of 115 cirrhotic patients in whom varices were diagnosed prior to bleeding. Am J Med. 1959; 26:228-237. [Google Scholar] 17. ...
Of the ten studies included that reported conducting screening protocols for varices, 1130/1429 (79%) were screened prior to therapy, and 46% reported the presence of EVs and GVs. The incidence of EV bleeding (EVB) was similar between those who received prophylactic therapy (esophageal variceal ligation or NSBB) versus those who did not.
4. Esophageal varices. Treatment. Nursing action: The treatment of esophageal varices aims to prevent bleeding and manage complications. Nursing actions may include monitoring vital signs, particularly blood pressure and heart rate, to detect signs of bleeding or hemodynamic instability.