History of the Periodic Table Reading, Questions and Answers


Also included in

Description
History of the Periodic Table
Included in the resource:
- An informative reading passage
- A student question sheet
- An answer key
Reading passage:
Includes information on five key scientist of the Periodic Table: Dalton, Dobereiner, Newlands, Mendeleev and Moseley, the dates of their work and a concise summary of their findings.
Question sheet:
Tiered as recall, explain and apply, the questions follow the reading passage. They challenge what a student has read and how well the information has been processed. Some questions are simple information extraction whilst others require thinking around the topic and explanations.
Answer key:
Included is a comprehensive set of answers for all questions.
Ideal as an independent in-class activity, homework or revision. I have found this resource to be a great activity to introduce the scientists of the Periodic Table to a wide range of students. Please feel free to ask any questions before purchasing.
You may also like my resources ...
CLICK >>
Scientists of the Periodic Table Cut and Paste
History of the Periodic Table Mendeleev Full Lesson
Questions & Answers
Science house.
- We're hiring
- Help & FAQ
- Privacy policy
- Student privacy
- Terms of service
- Tell us what you think
- International
- Schools directory
- Resources Jobs Schools directory News Search

History of the Periodic Table Reading Passage
Subject: Chemistry
Age range: 14-16
Resource type: Worksheet/Activity
Last updated
9 November 2022
- Share through email
- Share through twitter
- Share through linkedin
- Share through facebook
- Share through pinterest

History of the Periodic Table
Included in the resource:
- An informative reading passage
- A student question sheet
- An answer key
Reading passage:
Includes information on five key scientist of the Periodic Table: Dalton, Dobereiner, Newlands, Mendeleev and Moseley, the dates of their work and a concise summary of their findings.
Question sheet:
Tiered as recall, explain and apply, the questions follow the reading passage. They challenge what a student has read and how well the information has been processed. Some questions are simple information extraction whilst others require thinking around the topic and explanations.
Answer key:
Included is a comprehensive set of answers for all questions.
Ideal as an independent in-class activity, homework or revision. I have found this resource to be a great activity to introduce the scientists of the Periodic Table to a wide range of students. Please feel free to ask any questions before purchasing.
Tes paid licence How can I reuse this?
Your rating is required to reflect your happiness.
It's good to leave some feedback.
Something went wrong, please try again later.
Emmajepson6
Incorrect use of the word atomic mass when protons hadn't been discovered yet so was arranged in atomic mass.
Empty reply does not make any sense for the end user
Report this resource to let us know if it violates our terms and conditions. Our customer service team will review your report and will be in touch.
Not quite what you were looking for? Search by keyword to find the right resource:
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Post-lockdown teaching support
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- RSC Education News
- Supporting teacher training
- Interest groups

- More from navigation items
International Year of the Periodic Table activities
- 1 IYPT 2019
- 2 History of the periodic table
- 3 Build an atom simulator activity sheet
- 4 Periodic table bingo
History of the periodic table
- Five out of five
- No comments
Enthuse students with the history and development of the periodic table by using this engaging and interactive classroom activity. It can be differentiated to varying abilities and adapted to different levels or specifications
The timeline for the development of the model of the atom is clearly outlined in many schemes of work, so this activity follows that timeline with the addition of information relating to the development of the periodic table. Students do not necessarily have to know about the existence of atoms, or the atomic model before starting the exercise. Ages 7-14, there are hint cards for scaffolding (possibly up to age 16 depending on the group and use of stretch and challenge Qs). The teacher can be more specific with the objectives by using the blank summary sheet at the end.
History of the Periodic Table activity sheet
Additional information.
These activities have been created by the Royal Society of Chemistry to help celebrate the International Year of the Periodic Table. Find out more at: www.rsc.org/iypt

Build an atom simulator activity sheet

Periodic table bingo
- 11-14 years
- 14-16 years
- 16-18 years
- Elements and the periodic table
Specification
- Elements in the Periodic Table are arranged in order of increasing atomic number.
- Elements are arranged in the periodic table in order of increasing atomic number.
- participate in discussions, debates and interviews, for example debate disposing of polymers by incineration or landfill, or discuss the environmental effects of fossil fuel combustion;
- 1.6.1 describe how Mendeleev arranged the elements in the Periodic Table and left gaps for elements that had not been discovered at that time, and how this enabled him to predict properties of undiscovered elements;
- 1.6.2 demonstrate knowledge and understanding of how scientific ideas have changed over time in terms of the differences and similarities between Mendeleev’s Periodic Table and the modern Periodic Table;
- 1.6.4 demonstrate knowledge and understanding that a group is a vertical column in the Periodic Table and a period is a horizontal row; and
- interpret, analyse and present information in oral, written and ICT formats, for example prepare a PowerPoint presentation or a poster about acid rain and its causes, its environmental impact and society’s responsibility to minimise it on an…
- 1. Appreciate how scientists work and how ideas are modified over time.
- 2. Develop and use models to describe the nature of matter; demonstrate how they provide a simple way to to account for the conservation of mass, changes of state, physical change, chemical change, mixtures, and their separation.
- History of the idea of elements, including the contributions of the Greeks, Boyle, Davy and Moseley.
- History of the periodic table, including the contributions of Dobereiner, Newlands, Mendeleev and Moseley.
- Comparison of Mendeleev’s table with the modern periodic table.
- Very brief outline of the historical development of atomic theory (outline principles only; mathematical treatment not required): Dalton: atomic theory;
- C1.2a describe how and why the atomic model has changed over time
- C2.2i explain in terms of atomic number how Mendeleev’s arrangement was refined into the modern Periodic Table
- C2.1.1 describe how and why the atomic model has changed over time to include the main ideas of Dalton, Thomson, Rutherford and Bohr
- C2.2.3 describe how discovery of new elements and the ordering elements by atomic number supports Mendeleev’s decisions to leave gaps and reorder some elements
- New experimental evidence may lead to a scientific model being changed or replaced.
- Niels Bohr adapted the nuclear model by suggesting that electrons orbit the nucleus at specific distances. The theoretical calculations of Bohr agreed with experimental observations.
- Later experiments led to the idea that the positive charge of any nucleus could be subdivided into a whole number of smaller particles, each particle having the same amount of positive charge. The name proton was given to these particles.
- The early periodic tables were incomplete and some elements were placed in inappropriate groups if the strict order of atomic weights was followed.
- Mendeleev overcame some of the problems by leaving gaps for elements that he thought had not been discovered and in some places changed the order based on atomic weights.
- Describe how and why the atomic model has changed over time.
- 1.1 Describe how the Dalton model of an atom has changed over time because of the discovery of subatomic particles
- 1.13 Describe how Mendeleev arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds
- 1.14 Describe how Mendeleev used his table to predict the existence and properties of some elements not then discovered
- 1.15 Explain that Mendeleev thought he had arranged elements in order of increasing relative atomic mass but this was not always true because of the relative abundance of isotopes of some pairs of elements in the periodic table
- 1.17a Describe that in the periodic table: elements are arranged in order of increasing atomic number, in rows called periods
- 1.17b elements with similar properties are placed in the same vertical columns called groups
- Appreciate that knowledge and understanding of atomic structure has evolved over time.
Related articles

Representing elements and compounds | Review my learning worksheets | 14–16 years
By Lyn Nicholls
Identify learning gaps and misconceptions with this set of worksheets offering three levels of support

Everything you need to teach atomic structure and periodicity at post-16
2023-11-09T05:00:00Z By Johanne Brolly
Top tips and teaching ideas to help your students get to grips with the periodic table and its trends

Boost younger students’ grasp of atomic structure
2023-07-12T07:35:00Z By Louise Hussein
How to go beyond the simplistic when teaching this topic at 11–14, to help students achieve success at 14–16
No comments yet
Only registered users can comment on this article., more from resources.

Fractional distillation and hydrocarbons | Review my learning worksheets | 14–16 years

Chromatography | Review my learning worksheets | 14–16 years
2024-05-10T13:33:00Z By Lyn Nicholls

Solubility | Review my learning worksheets | 14–16 years
- Contributors
- Email alerts
Site powered by Webvision Cloud

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

7.1: The History of the Periodic Table
- Last updated
- Save as PDF
- Page ID 349430

\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
- To become familiar with the history of the periodic table
The modern periodic table has evolved through a long history of attempts by chemists to arrange the elements according to their properties as an aid in predicting chemical behavior. One of the first to suggest such an arrangement was the German chemist Johannes Dobereiner (1780–1849), who noticed that many of the known elements could be grouped in triads A set of three elements that have similar properties. , sets of three elements that have similar properties—for example, chlorine, bromine, and iodine; or copper, silver, and gold. Dobereiner proposed that all elements could be grouped in such triads, but subsequent attempts to expand his concept were unsuccessful. We now know that portions of the periodic table—the d block in particular—contain triads of elements with substantial similarities. The middle three members of most of the other columns, such as sulfur, selenium, and tellurium in group 16 or aluminum, gallium, and indium in group 13, also have remarkably similar chemistry.
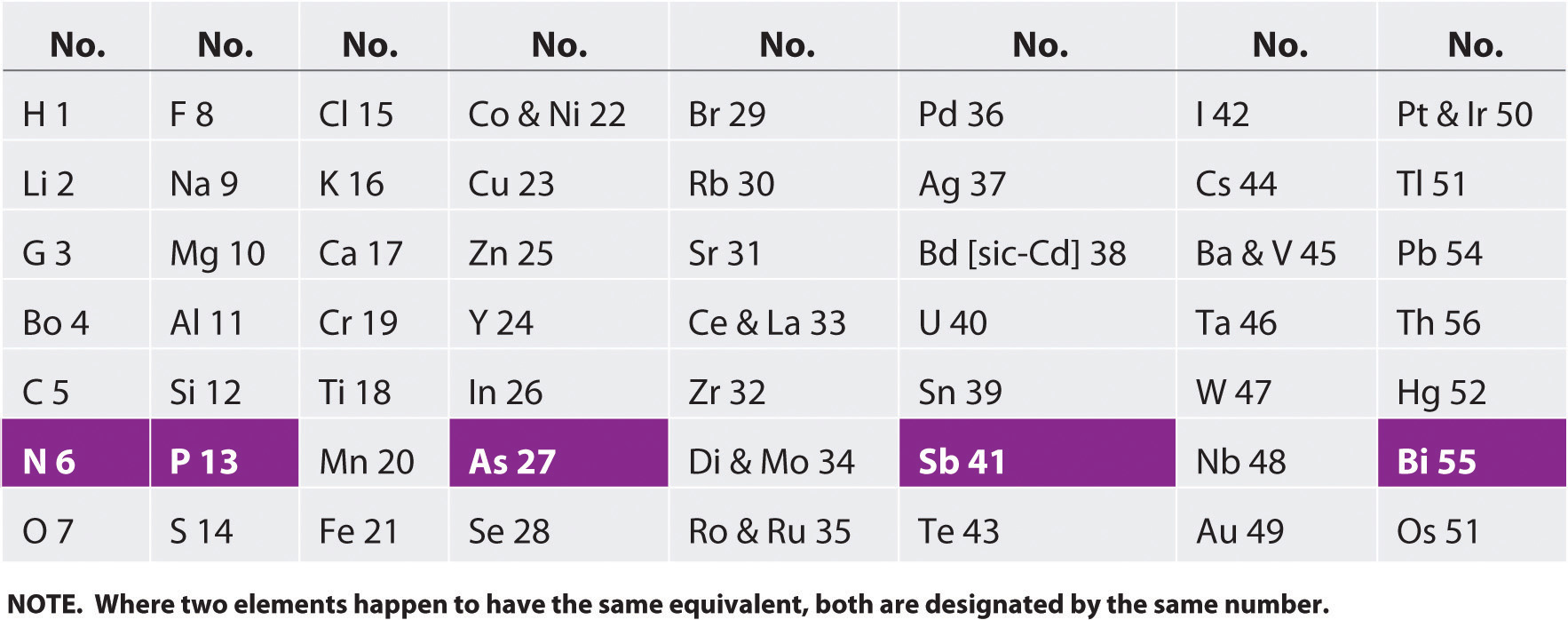
By the mid-19th century, the atomic masses of many of the elements had been determined. The English chemist John Newlands (1838–1898), hypothesizing that the chemistry of the elements might be related to their masses, arranged the known elements in order of increasing atomic mass and discovered that every seventh element had similar properties ( Figure 3.1.1 ). Newlands therefore suggested that the elements could be classified into octaves . He described octaves as a group of seven elements, which correspond to the horizontal rows in the main groups of today's periodic table. There were seven elements because the noble gases were not known at the time. Unfortunately, Newlands’s “law of octaves” did not seem to work for elements heavier than calcium, and his idea was publicly ridiculed. At one scientific meeting, Newlands was asked why he didn’t arrange the elements in alphabetical order instead of by atomic mass, since that would make just as much sense! Actually, Newlands was on the right track—with only a few exceptions, atomic mass does increase with atomic number, and similar properties occur every time a set of ns 2 np 6 subshells is filled. Despite the fact that Newlands’s table had no logical place for the d -block elements, he was honored for his idea by the Royal Society of London in 1887.
John Newlands (1838–1898)
Newlands noticed that elemental properties repeated every seventh (or multiple of seven) element, as musical notes repeat every eighth note.
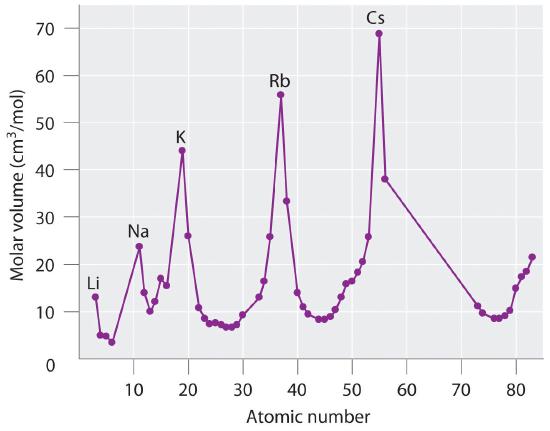
The periodic table achieved its modern form through the work of the German chemist Julius Lothar Meyer (1830–1895) and the Russian chemist Dimitri Mendeleev (1834–1907), both of whom focused on the relationships between atomic mass and various physical and chemical properties. In 1869, they independently proposed essentially identical arrangements of the elements. Meyer aligned the elements in his table according to periodic variations in simple atomic properties, such as “atomic volume” ( Figure 3.1.2 ), which he obtained by dividing the atomic mass (molar mass) in grams per mole by the density of the element in grams per cubic centimeter. This property is equivalent to what is today defined as molar volume, t he molar mass of an element divided by its density, (measured in cubic centimeters per mole):
\[ \frac{molar\; mass\left ( \cancel{g}/mol \right )}{density\left ( \cancel{g}/cm^{3} \right )}=molar\; volume\left ( cm^{3}/mol \right ) \tag{3.1.1}\]
As shown in Figure 3.1.2 , the alkali metals have the highest molar volumes of the solid elements. In Meyer’s plot of atomic volume versus atomic mass, the nonmetals occur on the rising portion of the graph, and metals occur at the peaks, in the valleys, and on the downslopes.
Dimitri Mendeleev (1834–1907)
When his family’s glass factory was destroyed by fire, Mendeleev moved to St. Petersburg, Russia, to study science. He became ill and was not expected to recover, but he finished his PhD with the help of his professors and fellow students. In addition to the periodic table, another of Mendeleev’s contributions to science was an outstanding textbook, The Principles of Chemistry , which was used for many years.
Mendeleev’s Periodic Table
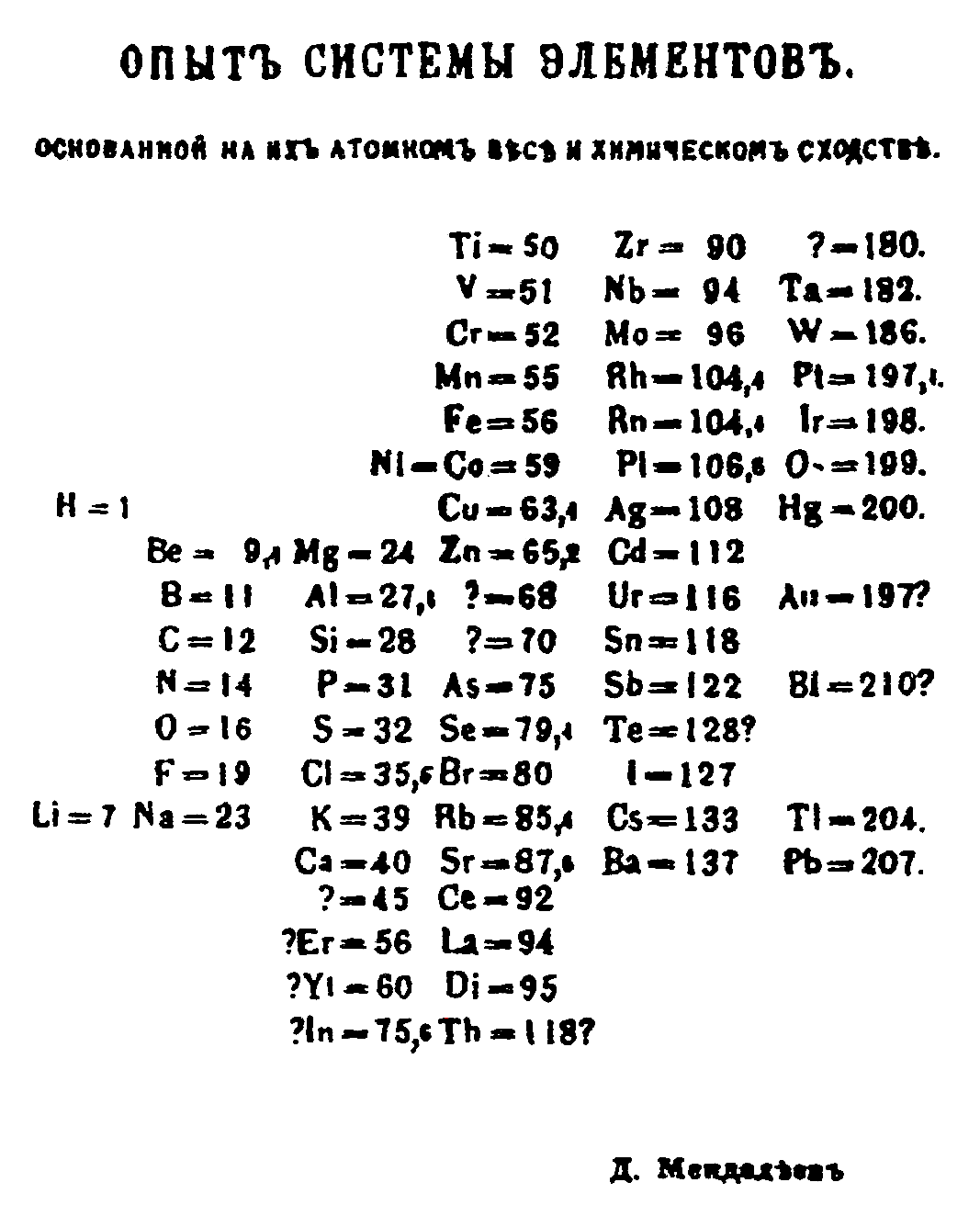
Mendeleev, who first published his periodic table in 1869 ( Figure 3.1.3 ), is usually credited with the origin of the modern periodic table. The key difference between his arrangement of the elements and that of Meyer and others is that Mendeleev did not assume that all the elements had been discovered (actually, only about two-thirds of the naturally occurring elements were known at the time). Instead, he deliberately left blanks in his table at atomic masses 44, 68, 72, and 100, in the expectation that elements with those atomic masses would be discovered. Those blanks correspond to the elements we now know as scandium, gallium, germanium, and technetium.
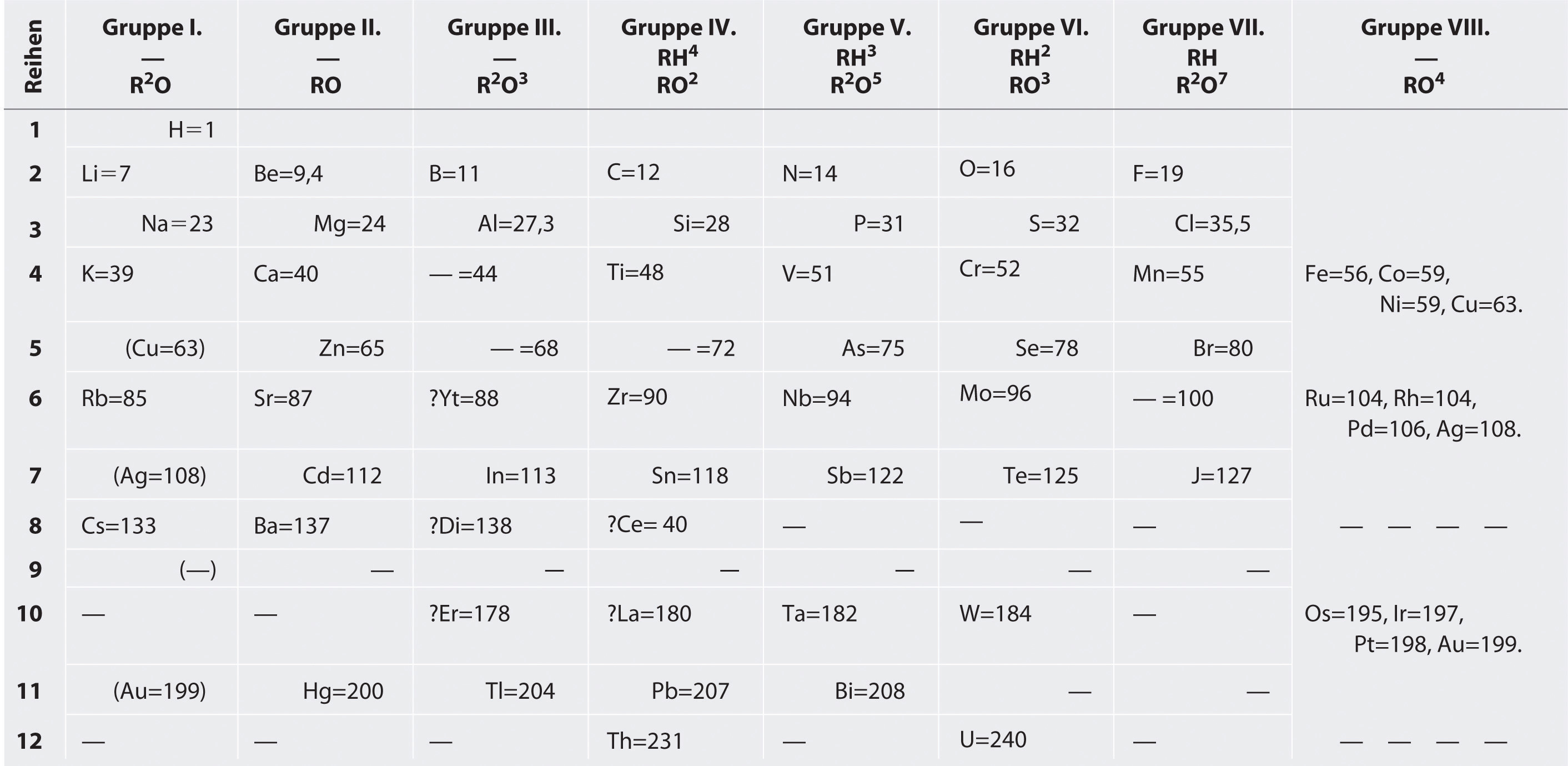
The groups in Mendeleev's table are determined by how many oxygen or hydrogen atoms are needed to form compounds with each element. For example, in Group I, two atoms of hydrogen, lithium, Li, sodium, Na, and potassium form compounds with one atom of oxygen. In Group VII, one atom of fluorine, F, chlorine, Cl, and bromine, Br, react with one atom of hydrogen. Notice how this approach has trouble with the transition metals. Until roughly 1960, a rectangular table developed from Mendeleev's table and based on reactivity was standard at the front of chemistry lecture halls.
The most convincing evidence in support of Mendeleev’s arrangement of the elements was the discovery of two previously unknown elements whose properties closely corresponded with his predictions ( Table 3.1.1 ). Two of the blanks Mendeleev had left in his original table were below aluminum and silicon, awaiting the discovery of two as-yet-unknown elements, eka -aluminum and eka -silicon (from the Sanskrit eka , meaning “one,” as in “one beyond aluminum”). The observed properties of gallium and germanium matched those of eka -aluminum and eka -silicon so well that once they were discovered, Mendeleev’s periodic table rapidly gained acceptance.
Table 3.1.1 Comparison of the Properties Predicted by Mendeleev in 1869 for eka -Aluminum and eka -Silicon with the Properties of Gallium (Discovered in 1875) and Germanium (Discovered in 1886)
When the chemical properties of an element suggested that it might have been assigned the wrong place in earlier tables, Mendeleev carefully reexamined its atomic mass. He discovered, for example, that the atomic masses previously reported for beryllium, indium, and uranium were incorrect. The atomic mass of indium had originally been reported as 75.6, based on an assumed stoichiometry of InO for its oxide. If this atomic mass were correct, then indium would have to be placed in the middle of the nonmetals, between arsenic (atomic mass 75) and selenium (atomic mass 78). Because elemental indium is a silvery-white metal , however, Mendeleev postulated that the stoichiometry of its oxide was really In 2 O 3 rather than InO. This would mean that indium’s atomic mass was actually 113, placing the element between two other metals, cadmium and tin.
One group of elements that absent from Mendeleev’s table is the noble gases, all of which were discovered more than 20 years later, between 1894 and 1898, by Sir William Ramsay (1852–1916; Nobel Prize in Chemistry 1904). Initially, Ramsay did not know where to place these elements in the periodic table. Argon, the first to be discovered, had an atomic mass of 40. This was greater than chlorine’s and comparable to that of potassium, so Ramsay, using the same kind of reasoning as Mendeleev, decided to place the noble gases between the halogens and the alkali metals.
The Role of the Atomic Number in the Periodic Table
Despite its usefulness, Mendeleev’s periodic table was based entirely on empirical observation supported by very little understanding. It was not until 1913, when a young British physicist, H. G. J. Moseley (1887–1915), while analyzing the frequencies of x-rays emitted by the elements, discovered that the underlying foundation of the order of the elements was by the atomic number , not the atomic mass. Moseley hypothesized that the placement of each element in his series corresponded to its atomic number Z , which is the number of positive charges (protons) in its nucleus. Argon, for example, although having an atomic mass greater than that of potassium (39.9 amu versus 39.1 amu, respectively), was placed before potassium in the periodic table. While analyzing the frequencies of the emitted x-rays, Moseley noticed that the atomic number of argon is 18, whereas that of potassium is 19, which indicated that they were indeed placed correctly. Moseley also noticed three gaps in his table of x-ray frequencies, so he predicted the existence of three unknown elements: technetium ( Z = 43), discovered in 1937; promethium ( Z = 61), discovered in 1945; and rhenium ( Z = 75), discovered in 1925.
H. G. J. Moseley (1887–1915)
Moseley left his research work at the University of Oxford to join the British army as a telecommunications officer during World War I. He was killed during the Battle of Gallipoli in Turkey.
Example 3.1.1
Before its discovery in 1999, some theoreticians believed that an element with a Z of 114 existed in nature. Use Mendeleev’s reasoning to name element 114 as eka -______; then identify the known element whose chemistry you predict would be most similar to that of element 114.
Given: atomic number
Asked for: name using prefix eka -
A Using the periodic table locate the n = 7 row. Identify the location of the unknown element with Z = 114; then identify the known element that is directly above this location.
B Name the unknown element by using the prefix eka - before the name of the known element.
A The n = 7 row can be filled in by assuming the existence of elements with atomic numbers greater than 112, which is underneath mercury (Hg). Counting three boxes to the right gives element 114, which lies directly below lead (Pb). B If Mendeleev were alive today, he would call element 114 eka -lead.
Use Mendeleev’s reasoning to name element 112 as eka -______; then identify the known element whose chemistry you predict would be most similar to that of element 112.
Answer: eka -mercury
The periodic table arranges the elements according to their electron configurations, such that elements in the same column have the same valence electron configurations. Periodic variations in size and chemical properties are important factors in dictating the types of chemical reactions the elements undergo and the kinds of chemical compounds they form. The modern periodic table was based on empirical correlations of properties such as atomic mass; early models using limited data noted the existence of triads and octaves of elements with similar properties. The periodic table achieved its current form through the work of Dimitri Mendeleev and Julius Lothar Meyer, who both focused on the relationship between atomic mass and chemical properties. Meyer arranged the elements by their atomic volume, which today is equivalent to the molar volume , defined as molar mass divided by molar density. The correlation with the electronic structure of atoms was made when H. G. J. Moseley showed that the periodic arrangement of the elements was determined by atomic number, not atomic mass.

Key Takeaway
- The elements in the periodic table are arranged according to their properties, and the periodic table serves as an aid in predicting chemical behavior.
Conceptual Problems
Johannes Dobereiner is credited with developing the concept of chemical triads. Which of the group 15 elements would you expect to compose a triad? Would you expect B, Al, and Ga to act as a triad? Justify your answers.
Despite the fact that Dobereiner, Newlands, Meyer, and Mendeleev all contributed to the development of the modern periodic table, Mendeleev is credited with its origin. Why was Mendeleev’s periodic table accepted so rapidly?
How did Moseley’s contribution to the development of the periodic table explain the location of the noble gases?
The eka - naming scheme devised by Mendeleev was used to describe undiscovered elements.
- Use this naming method to predict the atomic number of eka -mercury, eka -astatine, eka -thallium, and eka -hafnium.
- Using the eka -prefix, identify the elements with these atomic numbers: 79, 40, 51, 117, and 121.
Numerical Problem
Based on the data given, complete the table.
Plot molar volume versus molar mass for these substances. According to Meyer, which would be considered metals and which would be considered nonmetals?
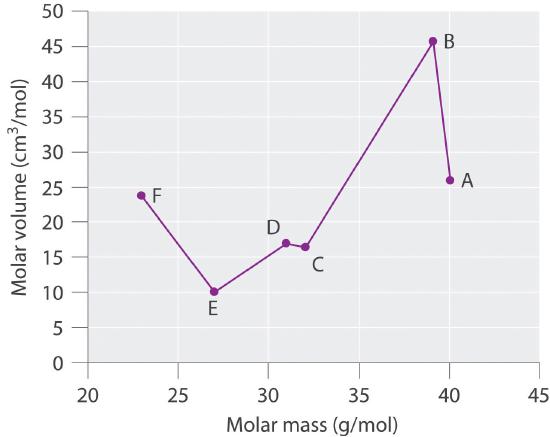
Meyer found that the alkali metals had the highest molar volumes, and that molar volumes decreased steadily with increasing atomic mass, then leveled off, and finally rose again. The elements located on the rising portion of a plot of molar volume versus molar mass were typically nonmetals. If we look at the plot of the data in the table, we can immediately identify those elements with the largest molar volumes (A, B, F) as metals located on the left side of the periodic table. The element with the smallest molar volume (E) is aluminum. The plot shows that the subsequent elements (C, D) have molar volumes that are larger than that of E, but smaller than those of A and B. Thus, C and D are most likely to be nonmetals (which is the case: C = sulfur, D = phosphorus).
Contributors
Modified by Joshua Halpern ( Howard University )
Genius of Mendelev by TED Ed on YouTube
A brief history of the periodic table

The periodic table of elements is a common sight in classrooms, campus hallways and libraries, but it is more than a tabular organization of pure substances. Scientists can use the table to analyze reactivity among elements, predict chemical reactions, understand trends in periodic properties among different elements and speculate on the properties of those yet to be discovered.
The modern periodic table arranges the elements by their atomic numbers and periodic properties. Several scientists worked over almost a century to assemble the elements into this format.
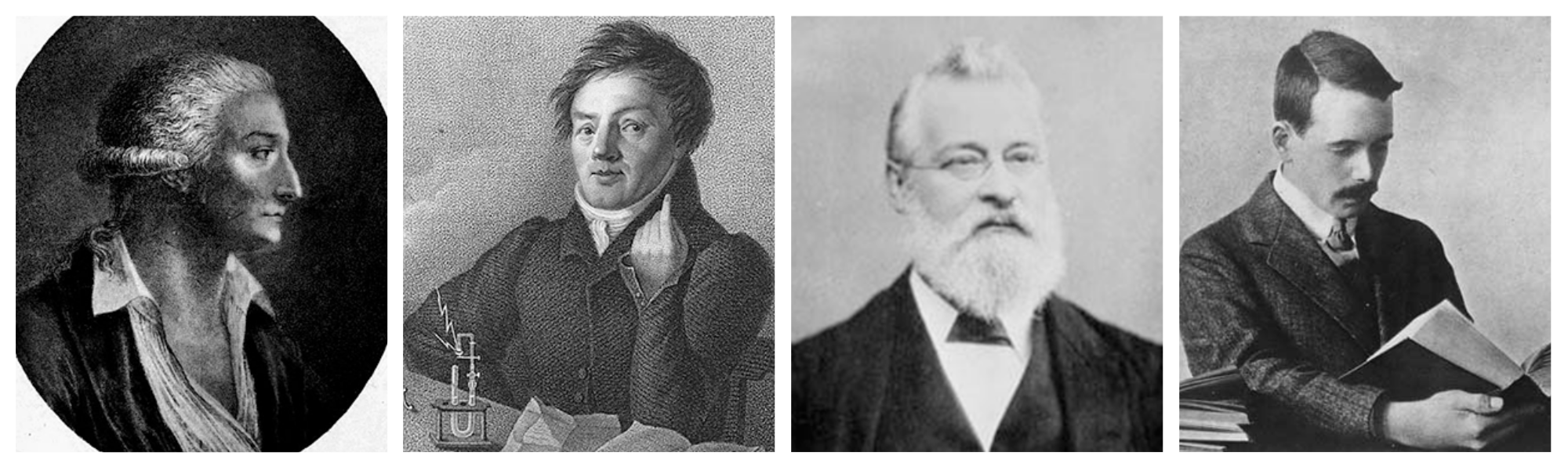
In 1789, French chemist Antoine Lavoisier tried grouping the elements as metals and nonmetals. Forty years later, German physicist Johann Wolfang Döbereiner observed similarities in physical and chemical properties of certain elements. He arranged them in groups of three in increasing order of atomic weight and called them triads, observing that some properties of the middle element, such as atomic weight and density, approximated the average value of these properties in the other two in each triad.
A breakthrough came with the publication of a revised list of elements and their atomic masses at the first international conference of chemistry in Karlsruhe, Germany, in 1860. They concluded that hydrogen would be assigned the atomic weight of 1 and the atomic weight of other elements would be decided by comparison with hydrogen. For example, carbon, being12 times heavier than hydrogen, would have an atomic weight of 12.

British chemist John Newlands was the first to arrange the elements into a periodic table with increasing order of atomic masses. He found that every eight elements had similar properties and called this the law of octaves. He arranged the elements in eight groups but left no gaps for undiscovered elements.
In 1869, Russian chemist Dmitri Mendeleev created the framework that became the modern periodic table, leaving gaps for elements that were yet to be discovered. While arranging the elements according to their atomic weight, if he found that they did not fit into the group he would rearrange them. Mendeleev predicted the properties of some undiscovered elements and gave them names such as "eka-aluminium" for an element with properties similar to aluminium. Later eka-aluminium was discovered as gallium. Some discrepancies remained; the position of certain elements, such as iodine and tellurium, could not be explained.
German chemist Lothar Meyer produced a version of the periodic table similar to Mendeleev’s in 1870. He left gaps for undiscovered elements but never predicted their properties. The Royal Society of London awarded the Davy Medal in 1882 to both Mendeleev and Meyer. The later discovery of elements predicted by Mendeleev, including gallium (1875), scandium (1879) and germanium (1886), verified his predictions and his periodic table won universal recognition. In 1955 the 101st element was named mendelevium in his honor.
The concept of sub-atomic particles did not exist in the 19 th century. In 1913, English physicist Henry Moseley used X-rays to measure the wavelengths of elements and correlated these measurements to their atomic numbers. He then rearranged the elements in the periodic table on the basis of atomic numbers. This helped explain disparities in earlier versions that had used atomic masses.
In the periodic table, the horizontal rows are called periods, with metals in the extreme left and nonmetals on the right. The vertical columns, called groups, consist of elements with similar chemical properties. The periodic table provides information about the atomic structure of the elements and the chemical similarities or dissimilarities between them. Scientists use the table to study chemicals and design experiments. It is used to develop chemicals used in the pharmaceutical and cosmetics industries and batteries used in technological devices.
UNESCO named 2019 the International Year of the Periodic Table to mark the 150 th anniversary of Mendeleev’s publication. Researchers and teachers worldwide took this opportunity to reflect on the importance of the periodic table and spread awareness about it in classrooms and beyond. Workshops and conferences encouraged people to use the knowledge of the periodic table to solve problems in health, technology, agriculture, environment and education. Publication houses organized monthly activities such as quiz contests, podcasts, personal story sections and industry site tours. These initiatives demonstrated how the elements are integral to our daily lives in medicines, pesticides and lithium batteries.
On its website marking the celebration, UNESCO wrote, “The Periodic Table of Chemical Elements is more than just a guide or catalogue of the entire known atoms in the universe; it is essentially a window on the universe, helping to expand our understanding of the world around us.”
Enjoy reading ASBMB Today?
Become a member to receive the print edition monthly and the digital edition weekly.
Deboleena M. Guharay earned her Ph.D. in chemistry from Virginia Commonwealth University. She is very enthusiastic and passionate about science communication.
Related articles
Get the latest from asbmb today.
Enter your email address, and we’ll send you a weekly email with recent articles, interviews and more.
Latest in Science
Science highlights or most popular articles.
Universal tool for tracking cell-to-cell interactions
A team of researchers has developed LIPSTIC, which can lay the groundwork for a dynamic map tracking physical interactions between different cells — the elusive cellular interactome.

Weedy rice gets competitive boost from its wild neighbors
Rice feeds the world. But researchers have found that a look-alike weed has many ways of getting ahead.
From the journals: JLR
A “T” makes a difference in blood clotting. High cholesterol: two screens are better than one. Biomarkers for cardiovascular risk. Statin-induced changes to the HDL lipidome. Read about recent papers on these topics.
Decoding microglial language
Emory University scientists characterize extracellular vesicles that facilitate intercellular communication.

What is metabolism?
A biochemist explains how different people convert energy differently – and why that matters for your health.

What’s next in the Ozempic era?
Diabetes, weight loss and now heart health: A new family of drugs is changing the way scientists are thinking about obesity — and more uses are on the horizon.

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Chemistry Worksheets and Handouts (PDF for Printing)

This is a collection of free chemistry worksheets and handouts to print. Most of the printables are PDF files, although some are available as JPG or PNG files. All of these worksheets print cleanly on normal printer paper, plus you can resize them to fit your needs.
Here is a list of worksheets. This site also has articles explaining these topics in detail.
- Label Parts of the Atom [ Google Apps worksheet ][ worksheet PDF ][ worksheet PNG ][ answers PNG ]
- Acid formulas [ PDF ][ Answers ]
- Balancing equations Worksheet #1 [ PDF ][ Answers ] Worksheet #2 [ PDF ][ Answers ] Worksheet #3 [ PDF ][ Answers ] Worksheet #4 [ PDF ][ Answers ]
- Chemical and Physical Changes [ PDF ][ Answers ]
- Chemistry scavenger hunt [ PDF clues ][ Answers ]
- Element names crossword [ PDF ][ Answers ]
- Element symbols – Symbols that make words [ PDF worksheet ][ Answers ]
- Element symbols – Countries of the world [ PDF ][ Answers ]
- More element symbol worksheets
- Homogeneous or Heterogeneous Mixtures [ PDF ][ Answers ]
- Intensive and Extensive Properties [ Worksheet ][ Answer Key ]
- Intrinsic and Extrinsic Properties [ PDF ][ Answers ]
- Ionic and Covalent Compounds (Names and Identification) [ PDF Worksheet ][ Answer Key ]
- Ionic Compound Names and Formulas [ PDF Worksheet ][ Answer Key ]
- Metric to English Unit Conversions [ PDF Worksheet ][ Answer Key ]
- Mixtures [ PDF ][ Answers ]
- Periodic table scavenger hunt [ PDF clues ][ Answers ]
- Reading a meniscus [ PDF ][ Answers ]
- Reading periodic table element information Worksheet #1 [ PDF ][ Answers ] Worksheet #2 [ PDF ][ Answers ]
- Scientific Notation [ PDF ][ Answers ]
- Significant digits Rules [ PDF ][ Answers ] Addition and subtraction [ PDF ][ Answers ] Multiplication and division [ PDF ][ Answers ]
- Types of Chemical Reactions [ Worksheet ][ Answers ]
In addition to these chemistry worksheets, there is a collection of word search puzzles .
Chemistry Handouts
These chemistry handouts illustrate chemistry concepts and offer examples.
- Amino acid side chains [ PDF ]
- Antimatter examples [ PNG ]
- Atom facts [ PNG ]
- Chemical properties [ JPG ]
- Colligative properties [ JPG ]
- Electron configurations [ PDF ]
- Element electronegativities [ PDF ]
- 118 Element Flash Cards [ PDF ]
- Element list [ PDF ]
- Endothermic reactions [ PNG ]
- Error calculations [ JPG ]
- Exothermic reactions [ JPG ]
- Heterogeneous mixtures [ JPG ]
- Hydrocarbon prefixes [ JPG ]
- Ionic compound properties [ PNG ]
- Genetic codons [ PDF ]
- Lewis structures [ JPG ]
- Litmus test [ PNG ]
- Magnetic vs non-magnetic metals [ JPG ]
- Mole ratio [ JPG ]
- Organic vs inorganic [ JPG ]
- Oxidation numbers [ JPG ]
- Periodic table Bingo game [ PDF ]
- pH indicators [ PNG ]
- Physical change [ JPG ]
- Physical properties [JPG ]
- Noble metals [ JPG ]
- Reactants and products [ JPG ]
- RNA vs DNA [ JPG ]
- States of matter [ JPG ]
- Visible spectrum [ JPG ]
Periodic Tables
There’s a printable periodic table for just about any purpose, but some of the most popular are listed here.

- 118 element vibrant periodic table [ PNG ]
- Actinides [ JPG ]
- Blank periodic table [ PDF ]
- Element charges [ JPG ]
- Element density [ PDF ]
- Element electrical conductivity [ PDF ]
- Element state of matter [ PDF ]
- Muted color 118 element periodic table [ PDF ]
- Native elements [ JPG ]
- Valence [ JPG ]

Biology Worksheets and Handouts
Is biology more your thing? We’ve got similar resources for the life sciences, including biology, biochemistry, cell biology, and anatomy.
Chemistry Worksheets Terms of Use
You are welcome to print these resources for personal or classroom use. They may be used as handouts or posters. They may not be posted elsewhere online, sold, or used on products for sale.
This page doesn’t include all of the assets on the Science Notes site. If there’s a table or worksheet you need but don’t see, just let us know!
Related Posts
Log In | Join AACT | Renew Membership
Save Your Favorite AACT Resources! ×
Log in or join now to start building your personalized "My Favorites" page. Easily save all the resources you love by logging in and clicking on the star icon next to any resource title.
- AACT member benefits »
- Forgot User Name or Password?
Ptable.com Investigations Mark as Favorite (138 Favorites)
ACTIVITY in Physical Properties , History , Periodic Table , Atoms , Model of the Atom , Isotopes , Electron Configuration , Valence Electrons , Atomic Mass , Subatomic Particles , Atomic Radius , Chemical Properties , Electrons , Orbitals , Unlocked Resources . Last updated January 05, 2022.
In this activity, students will use the online periodic table, ptable.com , to investigate a number of chemistry concepts. Students will use this online resource to explore information about the elements, including historical data, physical properties, periodic trends and more.
Grade Level
High or middle school
By the end of this lesson, students should be able to
- Better understand how the periodic table is organized.
- Identify several trends on the periodic table.
- Classify elements by family name, group number and period number.
- Understand the periodic trend of atomic radius.
Chemistry Topics
- Periodic Table
- Periodic Trends
- Physical Properties
Teacher Preparation : minimal
Lesson : 30-60 minutes (time will vary depending on activity chosen)
- Computer, tablet or mobile device (internet access is needed)
- Student handouts
- No specific safety precautions need to be observed for this activity.
Teacher Notes
- The Scavenger Hunt: This lesson will allow students to become familiar with ptable.com. It questions students as it walks them through the many features of the website. Students will investigate a large number of topics, from physical properties to history of the elements, and will conclude the activity by watching several videos available on the website.
- Periodic Table Investigation: This lesson is a self-guided introduction to the basics of the periodic table, including grouping, family names and period assignments. Students will also investigate common properties within a family, identify numbers of valence electrons, and create Bohr models for elements.
- Atomic Radius Exploration: This lesson is designed as a self-guided investigation for students to explore the trend in atomic radius on the periodic table. Students will collect and interpret data from this interact website to support their understanding of this trend.
- There is an answer key provided for each of the student resources for the teacher’s reference.
- Teachers should be actively involved in checking the students work, reviewing answers to ensure accuracy and elaborating on concepts when needed.
- www.ptable.com would be a valuable resource for many activities found in the AACT resource library, such as: Periodic Trends Investigation and Element Skit .

- Classification of Elements and Periodicity in Properties
- Development Of Modern Periodic Table
History of the Periodic Table
We should get familiar with the word periodic table before we start up with the history of the modern periodic table.
Table of Contents
What is a periodic table, recommended videos, doberiener’s triads, newland’s octaves, mendeleev’s periodic table, limitations of mendeleev’s periodic table.
The periodic table is basically a table in which elements are arranged on the basis of the atomic number . Elements having similar chemical properties fall under the same vertical column. These vertical columns are known as the groups and the horizontal rows are called periods.
Modern Periodic Table
Higher Elements Periodic Table

The German chemist, Johann Dobereiner in 1800 first observed similarities in the elements on the basis of their properties. He saw that there are groups consisting of three elements (triads) that have similar chemical and physical properties. In every group, the atomic weight of the middle element was half of the sum of the atomic weight of the other two elements. Properties of the middle element were also in the halfway of both the elements. Dobereiner called this grouping method as the law of triads. Later on, it was found that this law was not true for every element and hence it was not successful.
For example:
Atomic weight of Na
(Atomic weight of Li + Atomic weight of K )/2 = (7+39)/2 =23
In 1865, after the failure of Doberiener’s triad the English chemist, John Alexander Newlands gave the law of octaves. According to him, elements can be arranged in ascending order of their atomic weights . He also said that in this arrangement every eighth element of a row had similar properties to that of the first element of the same row, depicting the octaves of music. This law was also dismissed as it was only true for elements of up to calcium.
The real development in the periodic table took place after the development of Mendeleev’s periodic table . He gave a law which states that “The properties of an element are the periodic function of their atomic masses” . He arranged elements in periods (horizontal rows) and groups (vertical columns) in the increasing order of atomic weights. The vertical column consists of elements that have similar properties.

- It did not provide a clear idea about the structure of the atom.
- In order to arrange elements in a group, the order of atomic weight was reversed several times.
The discovery of new elements and their subsequent addition to the periodic table keeps revealing new dimensions in periodicity. To explore more about the periodic table please visit the BYJU’S YouTube channel.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.


IMAGES
VIDEO
COMMENTS
Those blanks correspond to the elements we now know as scandium, gallium, germanium, and technetium. Figure 3.4.3 Mendeleev's Periodic Table, as Published in the German Journal Annalen der Chemie und Pharmacie in 1872 The column headings "Reihen" and "Gruppe" are German for "row" and "group.". Formulas indicate the type of ...
Study with Quizlet and memorize flashcards containing terms like What is the correct chronological order of scientists contributing to the modern periodic table? Lavoisier, Newlands, Moseley, Dobereiner, and Mendeleev Lavoisier, Dobereiner, Newlands, Mendeleev, and Moseley Moseley, Mendeleev, Newlands, Dobereiner, and Lavoisier, One of the following properties was originally used to arrange ...
An answer key. Reading passage: Includes information on five key scientist of the Periodic Table: Dalton, Dobereiner, Newlands, Mendeleev and Moseley, the dates of their work and a concise summary of their findings. Question sheet: Tiered as recall, explain and apply, the questions follow the reading passage.
Independent Reading Passage: "History of the Periodic Table" This passage provides a detailed overview of how and why the Periodic Table has changed over time. Purpose . General: The purpose of the reading lesson is to support students' access to science content through the effective use of reading comprehension strategies. It is . not
An answer key. Reading passage: Includes information on five key scientist of the Periodic Table: Dalton, Dobereiner, Newlands, Mendeleev and Moseley, the dates of their work and a concise summary of their findings. Question sheet: Tiered as recall, explain and apply, the questions follow the reading passage.
The Periodic Table is for many the symbol of Chemistry. It is a single image that contains all of the known elements in the universe combined into an easily readable table. There are many patterns present in the table as well. All of the elements seem to fit together and connect to form a readable table and in turn the image of chemistry.
Early Attempts to Organize Elements. By the year 1700, only a handful of elements had been identified and isolated. Several of these, such as copper and lead, had been known since ancient times. As scientific methods improved, the rate of discovery dramatically increased. With the ever-increasing number of elements, chemists recognized that ...
History of the periodic table. Enthuse students with the history and development of the periodic table by using this engaging and interactive classroom activity. It can be differentiated to varying abilities and adapted to different levels or specifications. The timeline for the development of the model of the atom is clearly outlined in many ...
Video Transcript. In this video, we will learn how to describe the history and development of the periodic table, name the key people involved, and outline their contribution. Many scientists have attempted to organize the known elements to make sense of their properties and relationships. In 1789, French scientist Antoine Lavoisier published ...
Mendeleev Reading and Writing Activity (30 min): Handout copies of the periodic table and article on Mendeleev. Students read part 1 of the article on Mendeleev and answer questions on the worksheet below (Appendix A & B). Have students Think-Pair-Share. After working on the reading and questions on their own, have them discuss their answers with a
Learn about the history of the atom and how different models evolved over time, from Dalton to Bohr and beyond. This revision note covers the AQA GCSE Chemistry syllabus and explains the key concepts and experiments that shaped our understanding of atomic structure and the periodic table.
After reading Lesson 6.2, answer the following questions. Reading the Periodic Table 1. Label the sample square from the periodic table below. Use the labels element name, element symbol, atomic number, and average atomic mass. 12 Mg Magnesium 24.305 2. List three things, other than the name, symbol, atomic number, and average atomic
The groups in Mendeleev's table are determined by how many oxygen or hydrogen atoms are needed to form compounds with each element. For example, in Group I, two atoms of hydrogen, lithium, Li, sodium, Na, and potassium form compounds with one atom of oxygen. In Group VII, one atom of fluorine, F, chlorine, Cl, and bromine, Br, react with one ...
The modern periodic table arranges the elements by their atomic numbers and periodic properties. Several scientists worked over almost a century to assemble the elements into this format. Among the scientists who worked to created a table of the elements were, from left, Antoine Lavoisier, Johann Wolfang Döbereiner, John Newlands and Henry ...
Our resource for Pearson Chemistry includes answers to chapter exercises, as well as detailed information to walk you through the process step by step. With Expert Solutions for thousands of practice problems, you can take the guesswork out of studying and move forward with confidence. Find step-by-step solutions and answers to Pearson ...
Of course, the most basic question is "What is the periodic table?". The simple answer is that it is a chart that shows all of the chemical elements and basic facts about them, that orders the elements by increasing atomic number and common properties. The atomic number is the number of protons in every atom of the element.
The periodic table is an arrangement of the chemical elements, structured by their atomic number, electron configuration and recurring chemical properties.In the basic form, elements are presented in order of increasing atomic number, in the reading sequence. Then, rows and columns are created by starting new rows and inserting blank cells, so that rows and columns show elements with recurring ...
Print free chemistry worksheets and handouts to enhance student learning. This is a collection of free chemistry worksheets and handouts to print. Most of the printables are PDF files, although some are available as JPG or PNG files. All of these worksheets print cleanly on normal printer paper, plus you can resize them to fit your needs.
Chem 5.2 - Electron Configuration + The Periodic Table. 23 terms. theadrianperono. Preview. Chemistry Section Review 5.2. 31 terms. haleydc_. Preview. Study with Quizlet and memorize flashcards containing terms like Mendeleev attempted to organize the chemical elements based on their..., Mendeleev noticed that properties of elements appeared at ...
Chapter 4, Lesson 2: The Periodic Table Key Concepts • The periodic table is a chart containing information about the atoms that make up all matter. • An element is a substance made up of only one type of atom. • The atomic number of an atom is equal to the number of protons in its nucleus.
Periodic Table Investigation: This lesson is a self-guided introduction to the basics of the periodic table, including grouping, family names and period assignments. Students will also investigate common properties within a family, identify numbers of valence electrons, and create Bohr models for elements. Atomic Radius Exploration: This lesson ...
Mendeleev's Periodic Table. The real development in the periodic table took place after the development of Mendeleev's periodic table. He gave a law which states that "The properties of an element are the periodic function of their atomic masses". He arranged elements in periods (horizontal rows) and groups (vertical columns) in the ...
Formative Work. Reading Assignment over 5.1. Get Organized! A Periodic Table Webquest -> KEY. Periodic Table Worksheet -> KEY. Metal/Nonmetal lab -> VIDEO -> SAMPLE DATA. Metal or Nonmetal Worksheet —> KEY. Reading Assignment over 5.3.