- Sign In to save searches and organize your favorite content.
- Not registered? Sign up

Recently viewed (0)
- Save Search
- Subscriptions
- Join E-mail List
Patient Case Studies and Panel Discussion: Leukemia – Rare and Emerging Subtypes
- Get Citation Alerts
- Download PDF to Print
Rare and emerging subtypes of leukemia can be incredibly challenging to diagnose and even more challenging to treat. At the NCCN 2019 Annual Congress: Hematologic Malignancies, a panel of experts, moderated by Andrew D. Zelenetz, MD, PhD, were presented with particularly challenging cases in these malignancies and asked to discuss best approaches to treatment.
- Patient Case Study 1
In the first case study, a 77-year-old woman presented with multiple nodular lesions and plaques on her face, chest, and back. She had a history of type 2 diabetes, stage 3 hypertension, hyperlipidemia, coronary heart disease, cerebral infarction, glaucoma, lens extracapsular extraction and posterior chamber intraocular lens implantation, Sjögren syndrome, rheumatoid arthritis, and left axillary vein and brachial vein thrombosis.
She had previously received a conventional therapy of Chinese medicine, but her condition did not improve. Her clinicians performed a bone marrow biopsy and an aspiration biopsy of a nodule on the right side of her face, and immunostaining results revealed the following immunophenotype: CD4+, CD123+, CD43+, CD56+, with Ki-67 level of 30% to 40%.
The patient was diagnosed with blastic plasmacytoid dendritic cell neoplasm, which is a rare blood cancer in the myeloid malignancies family. Andrew D. Zelenetz, MD, PhD, Memorial Sloan Kettering Cancer Center, noted that this disease used to be classified as a variant of acute lymphoblastic leukemia (ALL) and has a distinctive immunophenotype and clinical appearance, characterized by purple skin lesions.
He said a helpful tool for remembering the immunophenotype of this disease is to think “123456”: CD123, CD4, and CD56. Conversely, Nitin Jain, MD, The University of Texas MD Anderson Cancer Center, noted that although this rule of thumb can be helpful, it is important to keep in mind that approximately 10% of patients with this malignancy are actually CD56-negative.
Daniel A. Pollyea, MD, MS, University of Colorado Cancer Center, emphasized the unique phenotypic expression pattern in this malignancy, and the risk of cytopenias due to bone marrow involvement. “Certainly there are patients with bone marrow involvement who don't have cytopenias and have predominant expression of these skin manifestations,” he said. “But I think the CD123 is really the key, because this is a very, very difficult diagnosis to make, and that can be the linchpin.” He added that CD123 expression status is important to know not only for diagnostic purposes but also from a therapeutic perspective. However, many clinical pathologists do not possess the capabilities to test for CD123, so if a diagnosis of blastic plasmacytoid dendritic cell neoplasm is even being entertained, a discussion with a pathologist regarding testing for CD123 is critical.
The nodule on the right side of the patient’s face was surgically excised, and she was treated with gemcitabine, nedaplatin (a second-generation platinum drug used in China that is not approved by the FDA; it is similar to carboplatin and cisplatin), and bleomycin. The patient experienced an initial response to therapy but subsequently developed additional nodular lesions on her arm.
According to Dr. Pollyea, regardless of what transpired with this particular patient, surgical resection of skin lesions did not have a role in this case. “Typically, if the disease is going to respond, the skin lesions are very, very sensitive,” he said. “So there are issues with wound healing if you perform a large resection.”
The panel then discussed tagraxofusp-erzs, a recently approved drug for the treatment of this disorder that has been shown to be highly effective. 1 Dr. Pollyea noted that the mechanism of action of this drug is “quite brilliant.”
“You're taking one of nature's most potent toxins and delivering it directly to a cell population of critical importance in this disease, and potentially the precursor or primitive population of the disease,” he said.
A trial of tagraxofusp treatment in patients with blastic plasmacytoid dendritic cell neoplasms led to durable responses and high complete response rates, particularly in the first-line setting (72%). 1 In relapsed/refractory disease, it was less effective, but “still very effective,” according to Dr. Zelenetz, with a complete response rate of 38%. However, significant toxicity was seen, with capillary leak syndrome a fatal toxicity.
Jae Park, MD, Memorial Sloan Kettering Cancer Center, noted that because of the limited clinical experience with this agent, it is critical to administer the drug in an inpatient setting whenever possible and to closely monitor any patient-related physical changes, including weight fluctuations, kidney function, and respiratory status.
William G. Wierda, MD, PhD, The University of Texas MD Anderson Cancer Center, agreed, adding that he actually treated patients with this compound on a clinical trial before its approval. “During the trial, we were closely monitoring daily weight, albumin, and [liver function], and making daily adjustments in dosing based on what was happening with patients clinically,” he said. “So it's important to be very familiar with the prescribing information.”
Given this particular patient’s age, history, and comorbidities, stem cell transplantation was not an option. However, according to Dr. Park, allotransplant should be considered in these cases whenever possible, and earlier rather than later. “Even with a good response, it becomes difficult to continue this regimen,” he said. “And after [patients] relapse, there are very few treatment options available.”
- Patient Case Study 2
A 28-year-old woman presented with fatigue and lymphadenopathy. Her initial WBC count was 11.1 k/uL with 40% blasts, and she showed hypercellular bone marrow. Her immunophenotype included the following: 88.0% CD45+/–, CD34+, CD19+, CD10+ (variable), CD20– (∼4% of cells stain), sCD22+, CD13–, CD33–, CD38+, CD56–, CD2+/–, CD3–, CD4–, CD8–, CD7–, CD5–, CD117, HLA-DR+, sIg light chain–, cCD79a+, cCD22+, MPO–, cIgM+, and TdT+. After noting the complexity of the patient’s immunophenotype, Dr. Pollyea emphasized the importance of working with a skilled hematopathologist in cases such as this.
The patient was diagnosed with B-cell ALL and treated with the CALGB 10403 regimen. 2 At day 30, bone marrow biopsy showed residual disease with 16% blasts by flow. As her next course of treatment, the patient received blinatumomab for one cycle.
Dr. Jain agreed that this was a reasonable next step, but added that an additional cycle of chemotherapy would also have been feasible. Although the patient was high-risk, he would not yet say treatment had failed after only one treatment cycle.
“I think on the adult side we have to take our cues from the pediatricians who have been so incredibly successful with this disease,” said Dr. Pollyea. “And CALGB 10403 is a regimen that attempts to apply the pediatric regimens to an adolescent/young adult population.” 2
He added that pediatricians tend to stick to protocol, and the protocol for this particular regimen allows for a more extended induction period. “So at this point you should have a lot of concerns about this patient, but I think the protocol allows you to continue.”
About 4 weeks after starting blinatumomab, the patient experienced complete remission confirmed by bone marrow biopsy. She also received 6 cycles of intrathecal chemotherapy throughout the course of her treatment and showed no evidence of central nervous system involvement.
A month later, she presented with enlarged lymph nodes in her groin and neck, and bone marrow biopsy confirmed 63% blasts with an ALL phenotype. A same-day inguinal lymph node biopsy was consistent with lymphoblastic leukemia involvement.
Although the patient experienced a complete remission initially, Dr. Park noted that minimal residual disease (MRD) status was never confirmed. This factor is critical in assessing a patient’s depth of remission, and MRD-positive patients should receive additional therapy sooner rather than later to get to MRD-negative status, he said.
Dr. Jain said that additional diagnostic testing in the form of RNA sequencing would be appropriate in this case, but noted a caveat of the limited availability of this type of testing. The patient underwent next-generation sequencing (NGS), which revealed the following: DIAPH1-PDGFRB fusion; CDKN2A/B - p14 ARF loss exon 1 and CDKN2b loss; PIK3R1 splice site 1746-2A>6; and TP53 N288fs*60.
According to Dr. Park, interpreting NGS data can be difficult, and misinterpretation can lead to the wrong choice of treatment. This again underlines the importance of consulting with a skilled pathologist or other experienced ALL expert to assist in interpreting mutation profiles.
The patient was determined to have Ph-like ALL (a newly recognized entity of Ph-negative ALL with a poor prognosis) and was enrolled in the KTE-CA19 CAR-T (axicabtagene ciloleucel [axi-cel]) trial ( ClinicalTrials.gov identifier: NCT02614066). She received cytoreductive chemotherapy with hyperCVAD part A before apheresis for CAR-T generation, and experienced favorable cytoreduction (she received fludarabine/cyclophosphamide for lymphodepletion). She then received a post–CAR-T infusion and showed no response; her blast count increased from 0.42 to 80.35 within a week.
“This is just a tough case,” said Dr. Park, noting the unusually refractory nature of the disease. “Initial response rates to CAR-T cell therapy are approximately 80%, so she’s already in the very unlucky 20% of cases,” he said.
Dr. Jain described 2 subtypes of Ph-like ALL: approximately half are CRLF2 -rearranged, 3 and these patients should ideally be referred to a clinical trial. The other half are nonrearranged, 3 and these patients should be referred for RNA sequencing to determine fusion genes.
No response was seen to further treatment, and the patient chose to continue care in hospice.
According to Dr. Zelenetz, incorporation of comprehensive genetic analysis and fluorescence in situ hybridization testing is important to identify high-risk patients (such as those with Ph-like phenotype) and plan for allogeneic hematopoietic stem cell transplantation (alloHSCT) or referral to clinical trials as early as possible.
MRD assessment by flow and/or NGS is critical to assess depth of response, modification of therapy, and candidacy for early alloHSCT. Dr. Park noted that both gene sequencing tests are validated, so patient preference should take priority.
Incorporation of tyrosine kinase inhibitors (TKIs) in Ph-like ALL is being investigated in clinical trials, and patients with this disease should be referred earlier rather than later, added Dr. Zelenetz. “But the nuance to that is understanding how to integrate TKIs into this entity, which is going to be dependent on understanding the mechanisms involved in the disease,” he said. “It won’t be just one TKI [that everyone receives]; it's much more complicated than that, unfortunately.”
Dr. Jain added that although Ph-like ALL has been established as high risk in the setting of chemotherapy, its classification remains to be determined in the new era of targeted therapies. “Some emerging data suggest that blinatumomab, inotuzumab, and CAR-T-cell therapy may overcome the negative prognostication of Ph-like ALL,” he said. “So those are some data we’ll hopefully see at the ASH Annual Meeting.”
Jarrod Holmes, MD, Annadel Medical Group, also participated in the panel discussion.
Pemmaraju N , Lane AA , Sweet KL , et al. . Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm . N Engl J Med 2019 ; 380 : 1628 – 1637 .
- Search Google Scholar
- Export Citation
Stock W , Luger SW , Advani AS , et al. . A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403 . Blood 2016 ; 133 : 1548 – 1559 .
Jain N , Roberts KG , Jabbour E , et al. . Ph-like acute lymphoblastic leukemia: a high-risk subtype in adults . Blood 2017 ; 129 : 572 – 581 .
Disclosures: Dr. Zelenetz has disclosed that he receives research support from Genentech/Roche, Gilead, MEI, and BeiGene; he has been a consultant for Celegene/JUNO, Genentech/Roche, Gilead, BeiGene, Pharmacyclics, Jansen, Amgen, Astra‐Zeneca, Novartis, and MEI Pharma; and he is on the Scientific Advisory Board of the Lymphoma Research Foundation and Adaptive Biotechnologies. Dr. Jain has disclosed that he is a consultant for AbbVie, Inc., AstraZeneca Pharmaceuticals LP, Genentech, Inc., Janssen Pharmaceutica Products, LP, Adaptive Biotechnologies, Precision Biosciences, Verastem, and Pharmacyclics; receives grant/research support from AbbVie, Inc., AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, Genentech, Inc., Incyte Corporation, Adaptive Biotechnologies, ADC Therapeutics, Cellectis, Precision Biosciences, Servier, Verastem, Pfizer, Inc., and Pharmacyclics; is a scientific advisor for AbbVie, Inc., AstraZeneca Pharmaceuticals LP, Genentech, Inc., Janssen Pharmaceutica Products, LP, Adaptive Biotechnologies, Precision Biosciences, Verastem, and Pharmacyclics; and has received honoraria from AbbVie, Inc., AstraZeneca Pharmaceuticals LP, Genentech, Inc., Janssen Pharmaceutica Products, LP, Adaptive Biotechnologies, Precision Biosciences, Verastem, and Pharmacyclics. Dr. Park has disclosed that he receives grant/research support from Amgen Inc., Genentech, Inc., Incyte Corporation, Juno Therapeutics, Inc., Kite Pharma, Novartis Pharmaceuticals Corporation, and Servier; and is a scientific advisor for from Amgen Inc., AstraZeneca Pharmaceuticals LP, GlaxoSmithKline, Incyte Corporation, Kite Pharma, Novartis Pharmaceuticals Corporation, Allogene Therapeutics, Autolus Therapeutics plc, and Takeda Pharmaceuticals North America, Inc. Dr. Pollyea has disclosed that he is a scientific advisor for AbbVie, Inc., Agios, Inc., Celgene Corporation, Daiichi-Sankyo Co., Forty Seven, Inc., Janssen Pharmaceutica Products, LP, Pfizer Inc., and Takeda Pharmaceuticals North America, Inc. Dr. Wierda has disclosed that he is a consultant for Genzyme Corporation and receives grant/research support from AbbVie, Inc., Acerta Pharma, Genentech, Inc., Gilead Sciences, Inc., Janssen Pharmaceutica Products, LP, Juno Therapeutics, Inc., Karyopharm Therapeutics, Kite Pharma, Cyclacel Pharmaceuticals, Inc., GlaxoSmithKline/Novartis Pharmaceuticals Corporation, Loxo Oncology, Inc., miRagen Therapeutics, Inc., Oncternal Therapeutics, Inc., Xencor, Inc., Pharmacyclics, and Sunesis Pharmaceuticals, Inc. Dr. Holmes has disclosed that he has no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products discussed in this article or their competitors.
Article Sections
Article information.
- Get Permissions
- Similar articles in PubMed
Google Scholar
Related articles.
- Advertising
- Terms of Use
- Privacy Policy
- Permissions

© 2019-2024 National Comprehensive Cancer Network
Powered by:
- [66.249.64.20|185.66.14.236]
- 185.66.14.236
Character limit 500 /500
- Case report
- Open access
- Published: 05 February 2022
A case report of pediatric acute lymphoblastic leukemia with e8a2 BCR/ABL1 fusion transcript
- Aleksandra Mroczkowska ORCID: orcid.org/0000-0002-8837-6517 1 ,
- Bożena Jaźwiec 1 , 2 ,
- Justyna Urbańska-Rakus 3 ,
- Sylwia Szymanowska 1 ,
- Anna Tessmann 1 ,
- Sonia Pająk 3 ,
- Katarzyna Machnik 3 ,
- Olga Haus 4 &
- Tomasz Wróbel 2
BMC Medical Genomics volume 15 , Article number: 20 ( 2022 ) Cite this article
8394 Accesses
4 Citations
Metrics details
Acute lymphoblastic leukemia is the most common type of cancer in children. Most often it affects the age group between 2 and 5 years of age. Studies have shown an improvement in general survivability, more than 90% 5-year overall survival (OS). Current treatment protocols for acute lymphoblastic leukemia require verification of the presence of favorable and unfavorable genetic abnormalities, which help qualify patients to the appropriate risk group and select a more suitable treatment. The presence of the BCR/ABL1 fusion gene stratifies the patient into a high-risk group and requires special treatment with tyrosine kinase inhibitors (TKI). The three dominant mRNA transcripts are e1a2, e13a2, and e14a2. Nevertheless, cases of atypical BCR/ABL1 transcripts have also been reported.
Case presentation
This paper presents the case of a pediatric patient with Ph + B-cell precursor acute lymphoblastic leukemia with rare atypical e8a2 BCR/ABL1 fusion transcript. Our patient achieved complete remission after 33 days of treatment. Molecular and cytogenetic studies in TP1 did not reveal the presence of the BCR/ABL1 transcript. The PCR-MRD test in TP1b was negative, the patient did not require hematopoietic stem cell transplantation.
Genetic evaluation of the bone marrow sample is crucial in the initial stage of the diagnosis. Fluorescent in situ hybridization and reverse transcriptase polymerase chain reaction with Sanger sequencing are the appropriate methods used in the detection of rare variants of BCR/ABL1 transcripts.
Peer Review reports
Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy. ALL is a heterogeneous neoplasm derived from the precursors of the lymphoid lineage. About 80–85% of cases are B-cell precursor leukemias, while T-lineage leukemias are about 15–20%. The ALL diagnoses are based on certain criteria including clinical presentation, laboratory tests, a bone marrow biopsy, immunophenotypic analysis and genetic tests. Currently, cytogenetic and molecular tests play a very important role in determining prognosis and stratification for suitable treatment of pediatric ALL [ 1 , 2 ]. The typical recurrent translocations occurring in ALL are t(12;21)(p13;q22) causing ETV6/RUNX1 , t(1;19)(q23;p13) causing TCF3/PBX1 , t(9;22)(q34;q11.2) causing BCR/ABL1 , and the most common rearrangement of KMT2A gene, t(4;11)(q21;q23) causing KMT2A/AFF1 . ETV6/RUNX1 is associated with a favorable prognosis and the last three genetic abnormalities have unfavorable outcomes [ 3 , 4 ].
BCR/ABL1 fusion transcripts occur approximately in 2–5% cases of childhood ALL and the frequency of BCR/ABL1(+)ALL increases with the patient’s age [ 5 ]. ALL cases with this genetic abnormality are associated with poor outcome and are qualified to the high risk group. Due to the introduction of tyrosine kinase inhibitors to the therapy, the prognosis of Ph + patients has improved.
The most common mRNA transcripts of BCR/ABL1: e1a2, e13a2, e14a2, occur in about 99% of Ph + cases. Approximately 70% of Ph + ALL patients have an e1a2 transcript and more than 25% e13a2 or e14a2. 1% of patients with Ph + shows atypical transcripts like e19a2, e6a2, e1a3, e13a3, e14a3 and e8a2 [ 6 ].
We present here a case of a pediatric patient with Ph + BCP-ALL (B cell precursor ALL) with an e8a2 BCR/ABL1 transcript.
An 11-year-old boy was admitted to the Unit of Pediatric Hematology and Oncology, City Hospital, Chorzów, Poland due to a suspicion of acute leukemia. Five days before admission to the hospital, he developed a severe and difficult to stop nosebleed. Since then, the boy was experienced weakness, lethargy, lack of appetite. Additionally he developed abdominal pain, a headache and nausea. Physical examination revealed pale skin with petechiae, inflammation of the gingiva, tooth decay and splenomegaly. Lymphadenopathy, hepatomegaly and the presence of a Central Nervous System (CNS) disease/leukemia were not observed. Family Health History has no indication of any genetic, hematologic or cancerous diseases. Patient was not exposed to any physical (i.e. ionic radiation) or chemical factors (organic solvents, pesticides, herbicides, paints, lacquers) during childhood nor fetal period. He was born out of second pregnancy, first childbirth (first pregnancy ended due to spontaneous miscarriage around eighth week). Weight at birth 2400 g. Mother's age at birth: 19, father: 21. Patient has younger step-sister (same mother, different father), showing no symptoms of ALL or any other hematological disorders.
By the time of diagnosis of ALL, the patient had been sick sporadically and had no routine blood tests—including morphology. The patient has not taken any medications on a permanent basis.
The laboratory results showed: white blood cell 206,900/µl, platelet count 142,000/µl and hemoglobin level 10.2 g/dl. The bone marrow was highly cellular, represented by a homogeneous population of small blasts with lymphoid morphology (88.5%). Flow cytometric analysis showed BCP-ALL phenotype: CD45dim + , CD38 + , CD34(+), CD81(+), CD24(+), CD19(+), CD79a(+), TdT(+), CD10(+), CDdim33(+), CD20dim(+), CD22dim(+), CD15(-), CD117(-). The boy was diagnosed with common B-cell precursor ALL and qualified for treatment according to the AIEOP-BFM ALL 2017 protocol.
The cytogenetic and molecular examinations of the patient's bone marrow were performed by the Laboratory of Molecular Biology and Cytogenetics at the University Clinical Hospital in Wroclaw. Karyotype analysis and fluorescence in situ hybridization (FISH) was performed on the bone marrow sample according to the AIEOP-BFM ALL 2017 protocol. According to the protocol, tests for genetic diagnostics were performed. By day 6, a FISH test was performed to obtain a result for the presence of the Philadelphia chromosome. Up to day 33, the FISH test was performed for the frequent genetic aberrations: ETV6/RUNX1 translocation and rearrangements in the KMT2A and TCF3 genes. At the same time, molecular tests were carried out using the RT-PCR method for the presence of the BCR/ABL1 and KMT2A/AFF1 fusion gene. G-banded chromosome analysis revealed an abnormal male karyotype 46,XY,t(9;22)(q34;q11) [ 11 ]/46,XY [ 9 ] (Fig. 1 A). The FISH study showed no rearrangements in ETV6/RUNX1, TCF3 (MetaSystems Probes, Germany) or KMT2A (Vysis, Abbott Molecular, Illinois, USA). The FISH study performed with the BCR/ABL1 dual color, dual fusion translocation probe (Vysis, Abbott Molecular, Illinois, USA) disclosed a typical translocation pattern 2 green/orange BCR/ABL1 fusion signals, 1 green BCR signal, and one orange ABL1 signal in 90% of the interphase cells (Fig. 1 B). Reverse transcription-polymerase chain reaction (RT-PCR) was performed to detect the presence or absence of the KMT2A /AFF1 and BCR/ABL1 fusion gene using primers as per JJM van Dongen et al. [ 7 ]. The test was negative in both cases. Due to the positive result of the FISH test for BCR/ABL1 , another RT-PCR was performed in order to search for atypical BCR/ABL1 transcripts. New RT-PCR analysis was performed based on primers BCR-6 and ABL-3 published by T. Burmeister and R. Reinhardt [ 6 ]. Electrophoresis showed a band of ~ 489 bp (Fig. 2 A). Sanger sequencing confirmed the direct junction between exon 8 of BCR (NM_004327.4) and exon 2 of ABL1 (NM_005157.6) (Fig. 2 B). The Sanger sequencing was important because this method determined the type of transcript by analyzing the direct junction between exons. Transcript type information is crucial for monitoring the presence of BCR/ABL1 transcript by RT-PCR method.
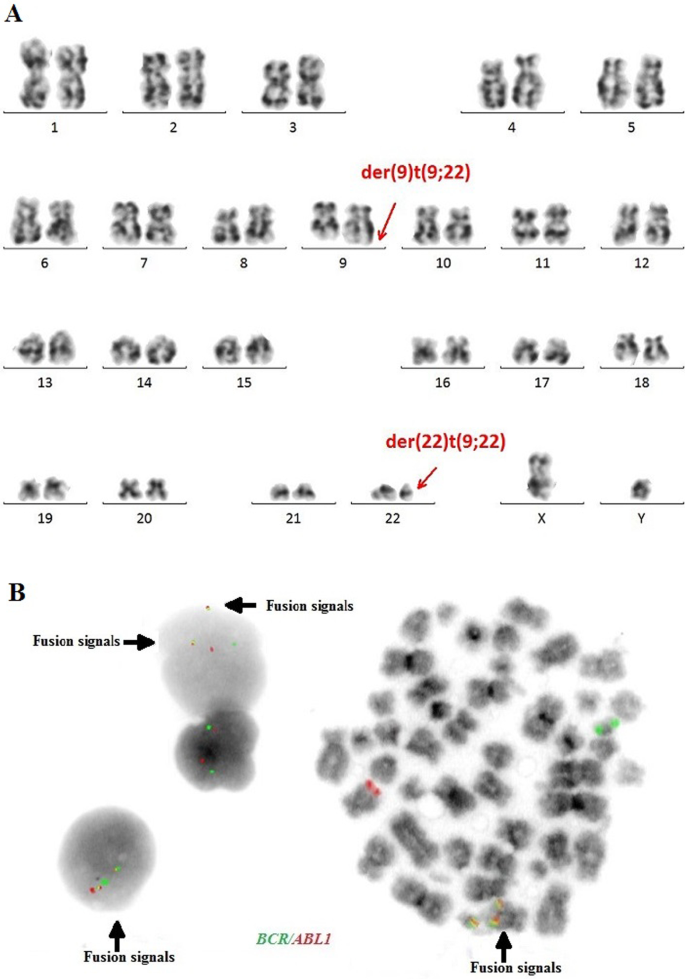
A —Conventional G-banding karyotype analysis showing typical translocation between chromosome 9 and 22. B —FISH analysis on interphase and metaphase with LSI BCR/ABL1 Dual Color, Dual Fusion Translocation Probe
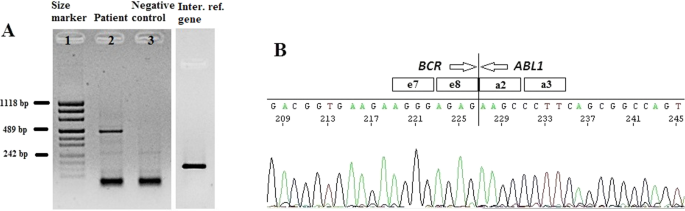
A —Detection of e8a2 BCR/ABL1 transcript by RT-PCR. Lane 1: size marker; lane 2: patient sample; lane 3: negative control, lane 4: internal reference gene—ABL1. B —Sanger sequencing demonstrating the direct junction between BCR exon e8 and ABL1 exon a2
The patient’s induction therapy started according to the protocol IA-Pred. On the 8th day of treatment, the patient had a poor response to prednisone. Due to the presence of the BCR /ABL1 fusion gene, further treatment was performed according to the EsPhALL 2009 protocol (European intergroup study of post-induction treatment of Philadelphia-chromosome-positive ALL). Imatinib at a dose of 300 mg/m 2 daily was started on day 15 of treatment, but on day 28 was withheld due to hepatotoxicity (WHO grade III). Evolution of peripheral blood cell counts during therapy is presented in Table 1 . In accordance to protocol, the patient's bone marrow was collected on days 15 and 33 of treatment. Examination of the bone marrow sample on day 15 revealed 15.4% blast cells in bone marrow morphology. Flow cytometry (FCM) revealed 23.48% of blasts. On day 33 (TP1), the bone marrow was already aplastic. Nevertheless, a PCR-MRD (Minimal Residual Disease) result was obtained. MRD in TP1 was low-positive (< 10 −4 ). Bone marrow smear revealed a total of 2.6% of blasts. Despite the poor quality of the material in TP1, it was also possible to perform a FISH study (Fig. 3 A) and RT-PCR test (Fig. 3 B). Both molecular and cytogenetic tests were negative. According to the EsPhALL 2009 protocol the boy should have been classified as poor risk Ph(+) ALL group because of PPR (prednisone poor responder) on the 8th day, but due to complete remission on day 33 (LBL 1.2%, PC-MRD < 10 –4 ) he was classified as good risk Ph(+) ALL group. From about day 32 of treatment, the patient reported abdominal pain, constipation, nausea and vomiting. Physical examinations showed hepatomegaly and lazy intestinal peristalsis. The symptoms were most likely caused by paralytic intestinal obstruction after chemotherapy. Additionally, the patient developed a fungal infection of the bladder.

A —FISH study on day 33 of treatment, B —RT-PCR test on day 33 of treatment. Lane 1: size marker; lane 2: positive control; lane 3: patient; lane 4: negative control, lane 5: internal reference gene—ABL1
Due to the general condition of the patient, consolidation treatment was delayed by 25 days. After this time, according to the EsPhALL 2009 protocol the IB protocol was started and Imatinib was resumed. Another PCR-MRD test was performed on day 17 of treatment (TP1b) of the IB protocol. PCR-MRD in TP1b was negative. Therefore the patient continued chemotherapy without qualification for HSCT (Hematopoietic Stem Cell Transplantation). The patient after consolidation therapy was in haematological remission of ALL. The patient remains without a transplant for 8 months after diagnosis.
Discussion and conclusions
The very rare e8a2 transcript (about 8% from 1% of non-typical BCR/ABL1 transcripts) has been reported mainly in cases of chronic myeloid leukemia (CML) [ 8 , 9 , 10 , 11 , 12 , 13 , 18 ]. Two cases have been reported in adult ALL [ 14 , 15 ]. The e8a2 BCR/ABL1 transcript could be associated with worse prognosis than the e13a2 or the e14a2 transcripts in CML patients. However, there were cases of good response to treatment with imatinib with an achievement of a major molecular response [ 8 , 10 , 12 ]. CML cases with this transcript that have been reported so far, additionally had insertions from ABL1 intron 1b or 1a, from BCR intron 8 or another gene such as PRDM12 , MAST2 [ 11 , 16 , 17 ]. Only one patient with CML and e8a2 BCR/ABL1 transcript had no additional insertions and after treatment with imatinib achieved a complete cytogenetic response [ 12 ]. In adult acute lymphoblastic leukemia one case was reported with insertion of 2 nucleotides from ABL1 intron 1a [ 14 ]. One adult ALL woman that had RALGPS1 exon 8 inserted into the fusion, was treated with FLAG-Ida (fludarabine, cytarabine, granulocyte-colony stimulating factor [G-CSF], idarubicin) and dasatinib and after re-induction therapy achieved hematological, cytogenetic and molecular remission [ 15 ]. Unfortunately, the e8a2 variant in adult ALL patients is so rare, that its impact on outcome remains unknown. To the best of our knowledge, our patient is the first pediatric ALL case with e8a2 BCR/ABL1 transcript. Our case sequencing analysis revealed e8a2 BCR/ABL1 transcript without any insertion. Creation of the e8a2 transcript by the exact fusion of BCR exon e8 to ABL1 exon a2 could encode an oncogenic protein, therefore our patient was qualified for treatment with the EsPhALL 2009 protocol. Our patient achieved complete remission after 33 days of treatment. Molecular and cytogenetic studies in TP1 did not reveal the presence of the BCR/ABL1 transcript. The PCR-MRD test in TP1b was negative, the patient did not require hematopoietic stem cell transplantation.
The presence of the BCR/ABL1 fusion gene is considered an unfavorable genetic abnormality and is associated with poor prognosis but survival has improved with the development of TKI. Our case shows that atypical transcripts of BCR/ABL1 also occur in cases other than CML or adult ALL. RT-PCR and sequencing are appropriate methods for identifying these atypical transcripts. Using both conventional cytogenetics and molecular methods, we are able to detect many genetic changes occurring in leukemias. It is important to identify them accurately and use this information to monitor the patient’s treatments. The monitoring of the presence and quantity of the BCR/ABL1 transcript using the RT-qPCR method is a gold standard in monitoring of Ph + patients with chronic myeloid leukemia. This method can also be used in monitoring of Ph + ALL patients to assess treatment efficiency. For proper patient monitoring it is important to evaluate the type of transcript at the time of diagnosis. Detection of a rare atypical transcript may affect the patient's treatment and may be associated with a worse prognosis.
Availability of data and materials
The Sanger Sequencing data generated in the study has been submitted to NCBI GenBank BankIt with the accession number OL672741; https://www.ncbi.nlm.nih.gov/nuccore/OL672741 . Reference sequences used in this study are available in the following link: https://www.ncbi.nlm.nih.gov/nuccore/NM_004327.4 ; https://www.ncbi.nlm.nih.gov/nuccore/NM_005157.6 . https://www.ncbi.nlm.nih.gov/nuccore/MF925339.1/ .
Abbreviations
Overall survival
Tyrosine kinase inhibitors
B cell precursor Acute Lymphoblastic Leukemia
Central Nervous System
Fluorescence in situ hybridization
Reverse transcription-polymerase chain reaction
Hematopoietic Stem Cell Transplantation
Chronic myeloid leukemia
Granulocyte-colony stimulating factor
Tasian S, Loh M, Hunger S. Childhood acute lymphoblastic leukemia: integrating genomics into therapy. Cancer. 2015;121(20):3577–90. https://doi.org/10.1002/cncr.29573 .
Article PubMed Google Scholar
Moorman A. The clinical relevance of chromosomal and genomic abnormalities in B-cell precursor acute lymphoblastic leukaemia. Blood Rev. 2012;26(3):123–35. https://doi.org/10.1016/j.blre.2012.01.001 .
Article CAS PubMed Google Scholar
Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7(6): e577. https://doi.org/10.1038/bcj.2017.53 .
Article CAS PubMed PubMed Central Google Scholar
Inaba H, Mullighan CG. Pediatric acute lymphoblastic leukemia. Haematologica. 2020;105(11):2524–39. https://doi.org/10.3324/haematol.2020.247031 .
Mohseni M, Uludag H, Brandwein JM. Advances in biology of acute lymphoblastic leukemia (ALL) and therapeutic implications. Am J Blood Res. 2018;8(4):29–56.
CAS PubMed PubMed Central Google Scholar
Burmeister T, Reinhardt R. A multiplex PCR for improved detection of typical and atypical BCR-ABL fusion transcripts. Leuk Res. 2008;32(4):579–85. https://doi.org/10.1016/j.leukres.2007.08.017 .
Van Dongen JJ, Macintyre EA, Gabert JA, Delabesse E, Rossi V, Saglio G, et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Report of the BIOMED-1 Concerted Action: investigation of minimal residual disease in acute leukemia. Leukemia. 1999;13(12):1901–28. https://doi.org/10.1038/sj.leu.2401592 .
Cayuela JM, Rousselot P, Nicolini F, Espinouse D, Ollagnier C, Bui-Thi MH, et al. Identification of a rare e8a2 BCR–ABL fusion gene in three novel chronic myeloid leukemia patients treated with imatinib. Leukemia. 2005;19(12):2334–6. https://doi.org/10.1038/sj.leu.2403986 .
Demehri S, Paschka P, Schultheis B, Lange T, Koizumi T, Sugimoto T, et al. e8a2 BCR–ABL: more frequent than other atypical BCR–ABL variants? Leukemia. 2005;19(4):681–4. https://doi.org/10.1038/sj.leu.2403604 .
Tchirkov A, Couderc JL, Perissel B, Goumy C, Regnier A, Uhrham-mer N, et al. Major molecular response to imatinib in a patient with chronic myeloid leukemia expressing a novel form of e8a2 BCR–ABL transcript. Leukemia. 2006;20(1):167–8. https://doi.org/10.1038/sj.leu.2404012 .
Park IJ, Lim YA, Lee WG, Park JS, Kim HC, Lee H-J, Cho SR. A case of chronic myelogenous leukemia with e8a2 fusion transcript. Cancer Genet Cytogenet. 2008;185(2):106–8. https://doi.org/10.1016/j.cancergencyto.2008.06.001 .
Jin C, Zhu X, Xiao M, Liu S, Liu X, Liu J, Xu X, et al. A novel e8a2BCR-ABL1 fusion transcript without insertion sequence in a patient with chronic myeloid leukemia. Ann Lab Med. 2018;38(2):169–71.
Article Google Scholar
Branford S, Rudzki Z, Hughes TP. A novel BCR-ABL transcript (e8a2) with the insertion of an inverted sequence of ABL intron 1b in a patient with Philadelphia-positive chronic myeloid leukaemia. Br J Haematol. 2000;109(3):635–7. https://doi.org/10.1046/j.1365-2141.2000.02042 .
Kim MJ, Yoon HJ, Park TS. The e8a2 fusion transcript in B lymphoblastic leukemia with BCR-ABL1 rearrangement. Korean J Hematol. 2012;47(3):161. https://doi.org/10.5045/kjh.2012.47.3.161 .
Article PubMed PubMed Central Google Scholar
McCarron SL, Kelly J, Coen N, McCabe S, Fay M, O’Dwyer M, et al. A novel e8a2 BCR-ABL1 fusion with insertion of RALGPS1 exon 8 in a patient with relapsed Philadelphia chromosome-positive acute lymphoblastic leukemia. Leuk Lymphoma. 2011;52(5):919–21. https://doi.org/10.3109/10428194.2011.555025 .
Riva E, Manrique Arechavaleta G, De Almeida C, Costa V, Fernandez Del Campo M, Ifran González S, Uriarte R. A novel e8a2 BCR-ABL1 fusion with insertion of MAST2 exon 2 in a four-way translocation t (1;17;9;22) (p35;q24;q44;q11) in a patient with chronic myeloid leukemia. Leuk Lymphoma. 2016;57(1):203–5. https://doi.org/10.3109/10428194.2015.1043549 .
Reid AG, Nacheva EP. A potential role for PRDM12 in the pathogenesis of chronic myeloid leukaemia with derivative chromosome 9 deletion. Leukemia. 2004;18(1):178–80. https://doi.org/10.1038/sj.leu.2403162 .
Qin YZ, Jiang Q, Jiang H, Lai Y-Y, Shi H-X, Chen W-M, et al. Prevalence and outcomes of uncommon BCR/ABL1 fusion transcripts in patients with chronic myeloid leukaemia: data from a single centre. Br J Haematol. 2018;182(5):693–700. https://doi.org/10.1111/bjh.15453 .
Download references
Acknowledgements
Not applicable.
No funding was obtained for this study.
Author information
Authors and affiliations.
Laboratory of Molecular Biology and Cytogenetics, Department of Hematology, Blood Neoplasms and Bone Marrow Transplantation, Wroclaw Medical University, Wrocław, Poland
Aleksandra Mroczkowska, Bożena Jaźwiec, Sylwia Szymanowska & Anna Tessmann
Department of Hematology, Blood Neoplasms and Bone Marrow Transplantation, Wroclaw Medical University, Wrocław, Poland
Bożena Jaźwiec & Tomasz Wróbel
Unit of Pediatric Hematology and Oncology, City Hospital, Chorzow, Poland
Justyna Urbańska-Rakus, Sonia Pająk & Katarzyna Machnik
Department of Clinical Genetics, Faculty of Medicine, Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Torun, Bydgoszcz, Poland
You can also search for this author in PubMed Google Scholar
Contributions
AM wrote the manuscript with support from TW and OH. BJ, AM conducted molecular genetics experiments and interpreted the Sanger sequencing data. SS, AT performed cytogenetical experiments. JU-R, SP, KM contributed to the clinical part of the study, prepared a clinical data and edited a clinical part of manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Correspondence to Aleksandra Mroczkowska .
Ethics declarations
Ethics approval and consent to participate.
This study was approved by the ethics committee of Wroclaw Medical University, Poland (committee’s reference number: KB 716/2018). Written consent to participate was obtained from patient which served as negative control.
Consent for publication
Written informed consent was obtained from the patient’s parents for publication of this case report and any accompanying images. Written consent for publication was also obtained from patient which served as negative control.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Mroczkowska, A., Jaźwiec, B., Urbańska-Rakus, J. et al. A case report of pediatric acute lymphoblastic leukemia with e8a2 BCR/ABL1 fusion transcript. BMC Med Genomics 15 , 20 (2022). https://doi.org/10.1186/s12920-022-01169-0
Download citation
Received : 01 July 2021
Accepted : 27 January 2022
Published : 05 February 2022
DOI : https://doi.org/10.1186/s12920-022-01169-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Acute lymphoblastic leukemia
BMC Medical Genomics
ISSN: 1755-8794
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Case report
- Open access
- Published: 11 April 2022
A challenging case of an adolescent and young adult patient with high-risk acute lymphoblastic leukemia: the need for a multidisciplinary approach: a case report
- Izabela Kranjčec ORCID: orcid.org/0000-0003-2860-5805 1 ,
- Nuša Matijašić 1 ,
- Slaven Abdović 2 ,
- Iva Hižar Gašpar 3 ,
- Lavinia La Grasta Sabolić 4 &
- Filip Jadrijević-Cvrlje 1
Journal of Medical Case Reports volume 16 , Article number: 147 ( 2022 ) Cite this article
2428 Accesses
2 Citations
1 Altmetric
Metrics details
Adolescents and young adults diagnosed with acute lymphoblastic leukemia are treated according to pediatric-based regimens to achieve better results. However, implementation of intensive chemotherapy protocols in this age group is associated with increased treatment-related toxicities, affecting almost every organ and system. In this case, the focus of our interest was on rather rare entities: steroid-induced psychosis that seldom develops in children and adolescents, and choroid plexus hemosiderosis, infrequently identified as a first sign of iron overload.
Case presentation
The aim of this paper is to present a challenging case of a 15-year-old Caucasian male patient treated for high-risk acute lymphoblastic leukemia and who experienced various adverse incidents during intensive chemotherapy, thus necessitating a high-quality multidisciplinary approach. Slow minimal residual disease clearance was an additional concerning issue. Induction and re-induction were complicated by steroid-induced hyperglycemia that required multiple-week insulin. During consolidation, acute kidney injury on the basis of chronic kidney disease was verified, demanding subsequent drug dose modifications. By the end of re-induction, after dexamethasone cessation, infrequent steroid-induced psychosis, presented as incoherent speech, aggressive behavior, and mood swings, required intensive psychiatric support. Neurological evaluation of seizures revealed uncommon choroid plexus hemosiderosis by brain magnetic resonance imaging, warranting appropriate selection of iron chelation therapy in the context of preexisting nephropathy. Ultimately, iron deposits of moderate intensity were verified by liver magnetic resonance imaging, while heart tissue remained intact. The early diagnosis and adequate treatment of aforementioned difficult toxicities resulted in complete recovery of the patient.
Conclusions
Treating adolescents with high-risk acute leukemia and multiple therapy-related morbidities remains a challenge, even in the era of extensive and effective supportive therapy. Superior survival rates might be achieved by prompt recognition of both frequent and rarely encountered adverse episodes, as well as well-timed and appropriate management by a well-coordinated multidisciplinary team.
Peer Review reports
Adolescents and young adults (AYA) diagnosed with acute lymphoblastic leukemia (ALL) have faced poorer survival rates compared with the history of this illness treatment in children [ 1 ]. However, several European and US studies have reported improved outcomes for AYA patients treated with pediatric-based protocols [ 2 , 3 , 4 ]. however, AYA patients receiving pediatric regimens and doses, unlike children, have disproportionately increased toxicities affecting almost every organ and system [ 5 ], most likely due to pubertal changes, inadequate nutritional status, and altered drug metabolism [ 6 ]. The most common nonhematological toxicities in AYA patients during induction include hyperglycemia, febrile neutropenia, and transaminitis [ 3 ].
The aim of this paper is to present the case of an adolescent with high-risk ALL who experienced various adverse episodes throughout the intensive chemotherapy, including multiple frequent toxicities mentioned above. However, the focus of our interest is on rather rare entities, such as steroid-induced psychosis that seldom develops in children and adolescents, and choroid plexus hemosiderosis, infrequently identified as a first sign of iron overload.
A 15-year-old Caucasian male presented with painless cervical lymphadenopathy and excessive sweating. The patient’s family and psychosocial history was unremarkable. Moreover, no relevant past interventions were recorded in the adolescent’s medical history. Normocytic anemia (Hemoglobin 86 g/L Mean corpuscular volume 93.6fL), thrombocytopenia (Plt 49 × 10 9 /L), and blasts (36%) dominated in the peripheral blood. Bone marrow analysis by flow cytometry revealed the diagnosis of precursor B-ALL (60% of aberrant “common” B-cells by European Group for Immunological Classification of Leukemias (EGIL) classification; TdT+, CD19+, CD10+, CD79a+, citIgM−). A favorable hyperdiploid clone (55, XY, X, +4, +6, +10, +14, +17, +18, +21, +21/46, XY) was detected by classical cytogenetic technique (G-banding). PBX1 gene duplication and tetrasomy of chromosome 21 were verified by fluorescence in situ hybridization (FISH). Clonal IgH and T-cell receptor (TCR) gene rearrangements were confirmed by molecular analysis (real-time polymerase chain reaction). No unfavorable cytogenetic or molecular disease features (for example, bcr/ abl , KTM2A ) were discovered. Additionally, next-generation sequencing (NGS) investigation of the tumor DNA revealed NRAS and CBL mutations but without potential therapeutic implications. No leukocytes or blasts were discovered in cerebrospinal fluid, and initial brain magnetic resonance (MR) was normal, thus central nervous system (CNS) was free of disease (CNS1 status). Diagnostic assessment was carried out according to the protocol’s standards, and no special (for example, financial) work-up or therapeutic challenges were encountered.
Chemotherapy according to the ALL-Intercontinental Berlin–Frankfurt–Münster (IC BFM) 2009 protocol was initiated, consisting of induction (prednisone, vincristine, daunorubicin, PEG-asparaginase, intrathecal methotrexate), early intensification (cyclophosphamide, cytarabine, 6-mercaptopurine, intrathecal methotrexate), consolidation (combination of dexamethasone, vincristine, vindensine HD-cytarabine, HD-methotrexate, cyclophosphamide, ifosfamide, PEG-asparaginase, etoposide, intrathecal therapy), and re-induction therapy (dexamethasone, vincristine, doxorubicin, PEG-asparaginase, cyclophosphamide, cytarabine, 6-thioguanine), followed by maintenance (6-mercaptopurine, methotrexate). While good prednisone response (peripheral absolute blast count < 1000/µL) was achieved by day 8 (peripheral absolute blast count 237/µL), flow cytometry minimal residual disease (FC-MRD) on day 15 and 33 was 28.9% and 0.03%, respectively. Solely due to high FC-MRD percentage of blasts (> 10%) on day 15, the patient was classified into high-risk (HR) disease group. Persistent minimal residual disease (MRD) (0.0012%) was detected by day 78, no MRD (0%) status was achieved prior to second high-risk block (consolidation), and the patient remained disease-free through further intensive chemotherapy course. Following the decision of the national transplantation team, the patient was not eligible for allogeneic hematopoietic transplantation.
Throughout the 10-month intensive chemotherapy, the patient experienced multiple toxicities of various degrees. Treatment-related adverse events of moderate to higher grade, according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.03, are listed in Table 1 . The most troublesome complications warranting multidisciplinary approach are described in more detail below.
Endocrine system
Hyperglycemia (serum glucose 12.1 mmol/L) was first noted on the third day of induction, during increase of prednisone dose (beginning with 25% of the calculated dose, 25% daily increments, full dose of 60 mg/m 2 /day reached on the 4th day), when nutritional advice was sought. As serum glucose levels (17.3 mmol/L) had risen additionally by day 9, intensive insulin therapy was initiated, intermittent-scanning continuous glucose monitoring system was applied, and education of the patient and his mother was performed in detail. Further workup (Glycated hemoglobin, C-peptide, diabetes related autoantibodies) excluded the possibility of type 1 diabetes, and hyperglycemia had resolved after 31 days of insulin therapy. Short-term steroid use (dexamethasone 20 mg/m 2 /day over 5 days) during consolidation demanded solely nutritional adjustments. During the re-induction, hyperglycemia (serum glucose 14.5 mmol/L) appeared the third day of dexamethasone use (10 mg/m 2 /day), necessitating multiple daily insulin injections (basal-bolus regimen) for 34 days. Adrenal insufficiency (cortisol 63 nmol/L) and central hypothyroidism (triiodothyronine < 0.80 nmol/L, thyroxine 47 nmol/L, thyroid stimulating hormone 0.04 mU/L) were detected by the end of the fourth week of induction and third week of steroid therapy in re-induction, warranting 2-week hormone replacement therapy (levothyroxine, hydrocortisone) with prolonged tapering.
Renal system
As evaluated by Pediatric Risk, Injury, Failure, Loss, End Stage Renal Disease (pRIFLE) criteria [ 7 ], our patient developed acute kidney injury (AKI) on three occasions, details of which are presented in Fig. 1 .
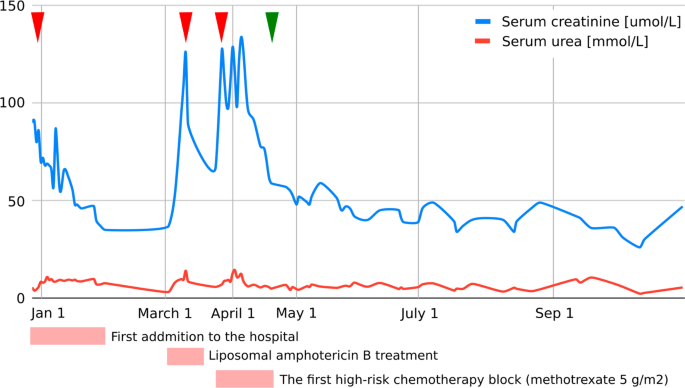
Graphic presentation of the renal function (serum creatinine, serum urea) during intensive treatment. First acute kidney injury was diagnosed when the patient was initially admitted to the hospital with creatinine levels of 90 µmol/L, which decreased to reference values for age with an intensive rehydration regimen (left arrowhead). At that time, kidney morphology was evaluated with ultrasound, which showed normal dimensions and echomorphology without dilatation of the urinary tract. Second (middle arrowhead) and third episodes (right arrowhead) were classified as acute kidney failure and occurred during liposomal amphotericin B treatment and during the first high-risk chemotherapy block when a significant delay (198-hour) in high-dose methotrexate (5 g/m 2 ) metabolite excretion was noticed, resulting in transient rise of creatinine and cystatin C levels up to 125 µmol/L and 2.36 g/L, respectively (estimated Glomerula Filtration Rate 30 mL/min/1.73 m 2 ). Creatinine levels returned to normal when replacing amphotericin B with voriconazole and monitoring complete methotrexate elimination. Apart from urine alkalinization, increased hydration, and administration of leucovorin, no other treatments were necessary to resolve acute kidney injury. Renal Tc-99m Diethyl Triamine Penta-Acetic scintigraphy scan revealed decreased clearance of radiopharmaceutical material (75 mL/min/1.73 m 2 ), and chronic kidney disease grade 2 was diagnosed. The patient had previously (at age of 3) been followed by pediatric nephrologist due to congenital hydronephrosis, but renal function and morphology were reported normal. We presume the patient initially had reduced renal parenchymal reserve and was more prone to acute kidney injury during precipitating factors (dehydration and unadjusted drug doses). Further cytostatic and symptomatic therapy dose corrections (75% of the total methotrexate dose and avoidance of all nephrotoxic drugs) were consistently undertaken, and laboratory parameters carefully monitored (starting from green arrowhead), so no additional kidney function deterioration was observed
Nervous system and psychological status
One day after a 3-week dexamethasone course (10 mg/m 2 /day) with 1-week tapering, at the end of the first part of re-induction, a bizarre behavior pattern was observed. The patient’s speech was incoherent, and aggressive outbursts were replaced by manic-depressive mood swings. A sudden-onset qualitative consciousness disturbance accompanied by short tonic–clonic convulsions demanded prompt neurological evaluation. No electrolyte disorders, abnormal glucose levels, or high blood pressure readings were detected. Urine toxicological screening was negative. Urgent head computed tomography (CT) scan was unremarkable, and repeated electroencephalogram (EEG) recordings were normal. Magnetic resonance imaging (MRI) of the brain revealed choroid plexus hemosiderosis, providing no explanation for psychological status alteration. Polymerase chain reaction (PCR) encephalitis and meningitis panels (Borrelia, tick-borne encephalitis, herpes simplex virus type 1 and type 2, varicella-zoster virus, cytomegalovirus, Epstein-Barr virus, measles, mumps, Human herpesvirus 6) in cerebrospinal fluid were negative, as were autoimmune encephalitis autoantibodies. A child psychiatrist diagnosed the patient with steroid-induced psychosis and introduced medications (risperidone, promazine), along with intensive psychological support. Within a week, all symptoms ceased, and the patient’s psychological status remained stable even after the drugs’ discontinuation. No new cerebral events were described, and follow-up EEG and neurological status were satisfactory.
- Iron overload
Hemosiderosis, due to repeated blood transfusion, was revealed during MRI of the brain and confirmed with high ferritin levels and MRI of the liver (Fig. 2 ). The baseline ferritin level of 323 µg/L (serum iron 20 µmol/L, total iron-binding capacity 43 µmol/L, unsaturated iron-binding capacity 23 µmol/L) in our patient had risen to 5143 µg/L after 9 months of intensive chemotherapy and then spontaneously fallen to 2994 µg/L at 1 month after (ferritin reference range 10.3–55.8 µg/L). During intensive chemotherapy, the patient received a total of 43 doses of blood (red cell) transfusions. After only a month of chelation therapy (deferasirox 20 mg/kg/day), a significant decrease to 1664 µg/L was noted with no deterioration in kidney function observed.
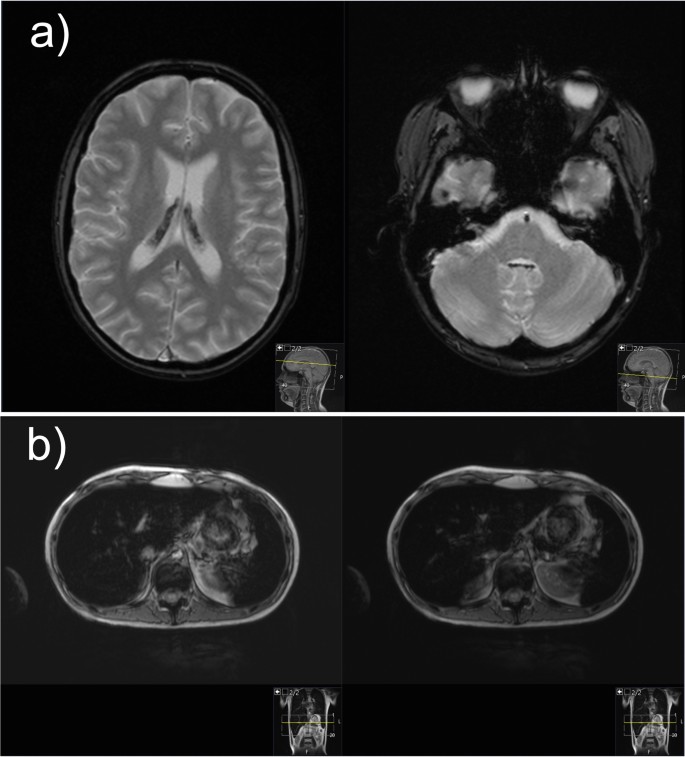
Magnetic resonance images of the brain and liver demonstrating iron overload. a Magnetic resonance imaging of the brain: axial T2-weighted gradient echo images demonstrate presence of hypointense hemosiderin deposits in the choroid plexus of both the lateral ventricles and fourth ventricle. b Magnetic resonance imaging of the liver: axial gradient echo sequences T2-weighted magnetic resonance image shows the liver hypointensity that is due to iron overload
Discussion and conclusions
B-precursor HR-ALL AYA patients are known to have inferior outcomes and increased treatment-related toxicities compared with children [ 8 ]. Superior outcomes in AYA patients are, however, achieved by implementing more intensive, pediatric-type protocols, with survival rates reaching 70% [ 9 ]. Lower event-free and survival rates in AYA patients are, among other factors, due to unfavorable tumor biology. The frequency of Philadelphia Chromosome positive acute lymphoblastic leukemia (Ph+ALL) and other HR abnormalities increases with age, in contrast to favorable cytogenetics in younger patients [ 9 ]. Although our patient displayed no disadvantageous genetic features, persistent MRD, known to be of extreme prognostic relevance, raised great concern. While MRD clearance, although slow, was still achieved in consolidation and allogeneic transplant avoided, a multitude of newly arising toxicities remained a challenge.
One in ten pediatric patients experiences hyperglycemia as a common side effect of ALL treatment [ 6 ]. Grade 3–4 hyperglycemia, a major concern in our patient throughout the treatment, was observed in almost 30% of AYA population treated with Children's Oncology Group (COG) pediatric-inspired chemotherapy regimens [ 10 ]. Higher proportion of steroid and asparaginase-related hyperglycemia in AYA patients might be the result of postpubertal hormonal changes, and given the former contradictory results, the correlation with infection predilection remains to be determined [ 6 ].
Although the frequency of febrile neutropenia (FN) in AYA patients is reported lower compared with younger individuals, our patient developed fever during hematologic aplasia in every stage of intensive chemotherapy. However, an association to hyperglycemia was not apparent [ 8 ]. Nevertheless, malnutrition has been repeatedly described as a predisposing factor for FN [ 11 , 12 , 13 ].
Although less than 10% of AYA patients are malnourished at time of diagnosis, almost half of them experience more than 5% weight loss during cancer treatment [ 14 ]. Not only was our patient severely underweight (Body mass index, BMI 15.9 kg/m 2 ) when first faced with hematologic malignancy, but significant weight loss (14%) was also observed during induction. High-risk disease and hyperglycemia, both present in our patient, among other features, are recognized as risk factors for negative weight trend during induction [ 15 ]. However, an early dietitian referral and specialist gastroenterologist involvement, along with timely enteral nutrition and supplement introduction, resulted in desirable weight gain at the end of intensive chemotherapy regimen (+4 kg).
Hepatotoxicity, sometimes severe, is a common side effect of contemporary pediatric ALL regimens, in one-fourth of cases occurring during induction, with obesity and age (> 15 years) being regarded as risk factors [ 16 ]. Transitory rise in transaminases in our patient followed every administration and was related to PEG-asparaginase exposure but never required any specific interventions nor treatment postponement (max ALT 342 U/L, AST 265 U/L, GGT 482 U/L). Other PEG-asparaginase attributable toxicities, such as hypersensitivity reactions, pancreatitis, and thromboembolic events, were not described in our patient [ 17 ]. However, therapeutic drug monitoring (TDM) was performed and dose modification conducted, possibly decreasing the occurrence of asparaginase-related adverse events.
Increased risk of high-dose methotrexate (MTX) renal toxicity was found to be correlated with delayed MTX elimination [ 18 , 19 ]. Since serum creatinine levels and concentration of MTX after 48 hours had excellent value in predicting AKI, with area under the curve (AUC) of 89.2% and 96.8%, respectively, it is necessary to follow-up these values and initiate intravenous hydration, urine alkalization, and if necessary, renal replacement therapy on time.
Patients with HR-ALL are at higher risk for iron overload, accompanied by endocrine and liver dysfunction, compared with other risk groups, yet no regular iron status monitoring is routinely performed in many pediatric oncology centers [ 20 , 21 ], a practice that our center should consider implementing. Accumulation of iron in the liver correlates with the amount of transfused iron, occurring rather early, after as much as ten transfusions, while for the iron to be loaded in the heart and endocrine organs, high transferrin saturations are needed [ 22 ]. Moreover, choroid plexus iron depositions, at any stage of treatment, have rarely been described in literature [ 23 ]. In our patient, the peak ferritin level correlated with neurological and psychological deterioration and characteristic brain MRI findings of iron overload (Fig. 2 ). Iron deposits of moderate intensity were verified by liver MRI, while heart tissue remained intact. Hereditary hemochromatosis gene (HFE) mutations, which aggravate iron overload in ALL patients, were not detected in our case [ 24 ]. Hepatic dysfunction, dysglycemia, and endocrine disorders, such as primary thyroid gland hypofunction, are organ abnormalities commonly related to hemosiderosis in literature [ 25 ]. Occasional hepatotoxicity, central hypothyroidism, and hypocortisolism in our patient were not considered to be related to hemosiderosis, as explained above, but preexisting nephropathy played an important role in iron chelation selection. Orally active once-daily deferasirox is a frequently preferred iron chelator, especially in the outpatient setting [ 25 , 26 ], usually being well tolerated [ 27 ], as in our case. Nevertheless, regarding clinical presentation, neurological symptomatology could not be interpreted based on choroid plexus hemochromatosis, as it is mostly asymptomatic [ 23 ], so further elucidation was sought.
Steroid-induced psychosis, a variety of neuropsychiatric symptoms related to glucocorticoid use, is frequently described in adult populations rather than children, with fewer than 20 cases reported worldwide [ 28 ]. All routes of corticosteroid administration (oral, intravenous, inhalation) at any time point of treatment may provoke psychotic symptoms, but clear risk factors (for example, type and dose of glucocorticoid) have not yet been established [ 29 ]. Hippocampal injury caused by glucocorticoids, resulting in behavioral and emotional dysfunction, in pediatric patients with cancer might be aggravated by synergistic toxicity of other chemotherapeutic agents [ 30 ]. The hallucinations that arose in our patient with negative personal and family history of psychiatric disorders and that caused him significant stress and impairment were associated with recent steroid exposure, while infection, severe electrolyte, and serum glucose disorders were ruled out. Combination of an antipsychotic drug (for example, haloperidol) with steroid dose reduction or discontinuation is generally an effective treatment strategy [ 31 ]. Risperidone, a preferred antipsychotic in literature [ 28 , 32 ], was also our child psychiatrist’s medication of choice, leading to complete symptom resolution. An optimal medicamental prophylactic approach (for example, carbamazepine, chlorpromazine, etc.) still needs to be determined [ 31 ].
In conclusion, treating an AYA patient with high-risk leukemia and multiple therapy-related morbidities remains a challenge, even in the era of abundant and effective supportive treatment. A timely and appropriate multidisciplinary approach is mandatory to ensure no significant delays, and modification in scheduled therapy is required, late effects diminished, and quality of life preserved, to achieve optimal treatment outcomes.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Acute kidney injury
Acute lymphoblastic leukemia
Acute Lymphoblastic Leukemia Intercontinental Berlin–Frankfurt–Münster
Alanine aminotransferase
Aspartate aminotransferase
Adolescents and young adults
Body mass index
Central nervous system
Children's Oncology Group
Computed tomography
Common Terminology Criteria for Adverse Events
Diethyl Triamine Penta-Acetic scintigraphy
Electroencephalogram
Estimated glomerula filtration rate
European Group for Immunological Classification of Leukemias
Fluorescence in situ hybridization
Gamma-glutamyl transferase
Gradient echo sequences
Febrile neutropenia
Flow cytometry minimal residual disease
Hereditary hemochromatosis
Magnetic resonance
Minimal residual disease
Magnetic resonance imaging
Methotrexate
Polymerase chain reaction
Philadelphia Chromosome positive acute lymphoblastic leukemia
Pediatric Risk, Injury, Failure, Loss, End Stage Renal Disease
Therapeutic drug monitoring
Wolfson JA, Richman JS, Sun CL, Landier W, Leung K, Smith EP, et al . Causes of inferior outcome in adolescents and young adults with acute lymphoblastic leukemia: across oncology services and regardless of clinical trial enrollment. Cancer Epidemiol Biomark Prev. 2018;27(10):1133–41. https://doi.org/10.1158/1055-9965.EPI-18-0430 .
Article Google Scholar
Rytting ME, Jabbour EJ, Jorgensen JL, Ravandi F, Franklin AR, Kadia TM, et al . Final results of a single institution experience with a pediatric-based regimen, the augmented Berlin-Frankfurt-Münster, in adolescents and young adults with acute lymphoblastic leukemia, and comparison to the hyper-CVAD regimen. Am J Hematol. 2016;91(8):819–23. https://doi.org/10.1002/ajh.24419 .
Article PubMed PubMed Central Google Scholar
Stock W, Luger SM, Advani AS, Yin J, Harvey RC, Mullighan CG, et al . A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB. Blood. 2019;133(14):1548–59. https://doi.org/10.1182/blood-2018-10-881961 ( published correction appears in Blood . 2019;134(13):1111 ).
Article CAS PubMed PubMed Central Google Scholar
Siegel SE, Stock W, Johnson RH, Advani A, Muffly L, Douer D, et al . Pediatric-inspired treatment regimens for adolescents and young adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: a review. JAMA Oncol. 2018;4(5):725–34. https://doi.org/10.1001/jamaoncol.2017.5305 .
Gupta A, Damania RC, Talati R, O’Riordan MA, Matloub YH, Ahuja SP. Increased toxicity among adolescents and young adults compared with children hospitalized with acute lymphoblastic leukemia at children’s hospitals in the United States. J Adolesc Young Adult Oncol. 2021;10(6):645–53. https://doi.org/10.1089/jayao.2020.0154 .
Article PubMed Google Scholar
Gramatges MM, Rabin KR. The adolescent and young adult with cancer: state of the art—acute leukemias. Curr Oncol Rep. 2013;15(4):317–24. https://doi.org/10.1007/s11912-013-0325-5 .
Bresolin N, Bianchini AP, Haas CA. Pediatric acute kidney injury assessed by pRIFLE as a prognostic factor in the intensive care unit. Pediatr Nephrol. 2013;28(3):485–92. https://doi.org/10.1007/s00467-012-2357-8 .
Larsen EC, Salzer W, Nachman J, Devidas M, Freyer DR, Raetz EA, et al . Treatment toxicity in adolescents and young adult (AYA) patients compared with younger patients treated for high risk B-precursor acute lymphoblastic leukemia (HR-ALL): a report from the children’s oncology group study AALL0232. Blood. 2011;118(21):1510. https://doi.org/10.1182/blood.V118.21.1510.1510 .
Carobolante F, Chiaretti S, Skert C, Bassan R. Practical guidance for the management of acute lymphoblastic leukemia in the adolescent and young adult population. Ther Adv Hematol. 2020;11:2040620720903531. https://doi.org/10.1177/2040620720903531 .
Advani AS, Sanford B, Luger S, Devidas M, Larsen EC, Liedtke M, et al . Frontline-treatment of acute lymphoblastic leukemia (ALL) in older adolescents and young adults (AYA) using a pediatric regimen is feasible: toxicity results of the prospective US intergroup trial C10403 (alliance). Blood. 2013;122(21):3903. https://doi.org/10.1182/blood.V122.21.3903.3903 .
Agnes M, Widjajanto P, Damayanti W. Impact of malnutrition on febrile neutropenia in children with acute lymphoblastic leukemia during induction phase chemotherapy. Paediatr Indones. 2018;58(6):298–304. https://doi.org/10.14238/pi58.6.2018.298-304 .
Sonowal R, Gupta V. Nutritional status in children with acute lymphoblastic leukemia, and its correlation with severe infection. Indian J Cancer. 2021;58(2):190–4.
PubMed Google Scholar
Ramamoorthy JG, Radhakrishnan V, Ganesan P, Dhanushkodi M, Ganesan TS, Sagar TG. Malnutrition is a predisposing factor for developing recurrent fever following febrile neutropenia in children with acute lymphoblastic leukemia. Pediatr Hematol Oncol J. 2020;5(3):75–9. https://doi.org/10.1016/j.phoj.2020.06.002 .
van der Haak N, Edwards S, Perem M, Landorf E, Osborn M. Nutritional status at diagnosis, during, and after treatment in adolescents and young adults with cancer. J Adolesc Young Adult Oncol. 2021;10(6):668–74. https://doi.org/10.1089/jayao.2020.0197 .
Hill R, Hamby T, Johnson D, Boren C, Downs H, Ray A. Prevalence and predictors of weight loss during induction therapy for childhood acute lymphoblastic leukemia. Nutrition. 2021;81: 110937. https://doi.org/10.1016/j.nut.2020.110937 .
Denton CC, Rawlins YA, Oberley MJ, Bhojwani D, Orgel E. Predictors of hepatotoxicity and pancreatitis in children and adolescents with acute lymphoblastic leukemia treated according to contemporary regimens. Pediatr Blood Cancer. 2018;65(3): e26891. https://doi.org/10.1002/pbc.26891 .
Article CAS Google Scholar
Rytting ME, Jabbour EJ, O’Brien SM, Kantarjian HM. Acute lymphoblastic leukemia in adolescents and young adults. Cancer. 2017;123(13):2398–403. https://doi.org/10.1002/cncr.30624 .
Cheng DH, Lu H, Liu TT, Zou XQ, Pang HM. Identification of risk factors in high-dose methotrexate-induced acute kidney injury in childhood acute lymphoblastic leukemia. Chemotherapy. 2018;63(2):101–7. https://doi.org/10.1159/000486823 .
Article CAS PubMed Google Scholar
Li H, Xu Q, Wang Y, Chen K, Li J. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for predicting high dose methotrexate associated acute kidney injury in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2020;85(1):95–103. https://doi.org/10.1007/s00280-019-03980-6 .
Cacciotti C, Athale U. Transfusion-related iron overload in children with leukemia. J Pediatr Hematol Oncol. 2021;43(1):18–23. https://doi.org/10.1097/MPH.0000000000001849 .
Eng J, Fish JD. Insidious iron burden in pediatric patients with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;56(3):368–71. https://doi.org/10.1002/pbc.22851 .
Reitman AJ, Coates TD, Freyer DR. Early cardiac iron overload in a child on treatment of acute lymphoblastic leukemia. Pediatrics. 2015;136(3):e697–700. https://doi.org/10.1542/peds.2014-3770 .
Kim MS, Lee HY, Lim MK, Kang YH, Kim JH, Lee KH. Transfusional iron overload and choroid plexus hemosiderosis in a pediatric patient: brain magnetic resonance imaging findings. Investig Magn Reson Imaging. 2019;23(4):390–4. https://doi.org/10.13104/imri.2019.23.4.390 .
El-Rashedi FH, El-Hawy MA, El-Hefnawy SM, Mohammed MM. HFE gene mutation and iron overload in Egyptian pediatric acute lymphoblastic leukemia survivors: a single-center study. Hematology. 2017;22(7):398–404. https://doi.org/10.1080/10245332.2017.1289324 .
Yassin MA, Soliman A, De Sanctis V, Hmissi SM, Abdulla MAJ, Ekeibed Y, et al . The impact of iron overload in patients with acute leukemia and myelodysplastic syndrome on hepatic and endocrine functions. Acta Biomed. 2018;89(3-S):18–22. https://doi.org/10.23750/abm.v89i3-S.7213 .
Olcay L, Hazirolan T, Yildirmak Y, Erdemli E, Terzi YK, Arda K, et al . Biochemical, radiologic, ultrastructural, and genetic evaluation of iron overload in acute leukemia and iron-chelation therapy. J Pediatr Hematol Oncol. 2014;36(4):281–92. https://doi.org/10.1097/MPH.0b013e3182a11698 .
Poggiali E, Cassinerio E, Zanaboni L, Cappellini MD. An update on iron chelation therapy. Blood Transfus. 2012;10(4):411–22. https://doi.org/10.2450/2012.0008-12 .
Hodgins GE, Saltz SB, Gibbs EP, Gonzalez R, Regan J, Nemeroff C. Steroid-induced psychosis in the pediatric population: a new case and review of the literature. J Child Adolesc Psychopharmacol. 2018;28(5):354–9. https://doi.org/10.1089/cap.2018.0017 .
Stuart FA, Segal TY, Keady S. Adverse psychological effects of corticosteroids in children and adolescents. Arch Dis Child. 2005;90(5):500–6. https://doi.org/10.1136/adc.2003.041541 .
Hochhauser CJ, Lewis M, Kamen BA, Cole PD. Steroid-induced alterations of mood and behavior in children during treatment for acute lymphoblastic leukemia. Support Care Cancer. 2005;13(12):967–74. https://doi.org/10.1007/s00520-005-0882-8 .
Huynh G, Reinert JP. Pharmacological management of steroid-induced psychosis: a review of patient cases. J Pharm Technol. 2021;37(2):120–6. https://doi.org/10.1177/8755122520978534 .
Bag O, Erdoğan I, Onder ZS, Altinoz S, Ozturk A. Steroid-induced psychosis in a child: treatment with risperidone. Gen Hosp Psychiatry. 2012;34(1):103.e5-103.e6. https://doi.org/10.1016/j.genhosppsych.2011.09.003 .
Download references
Acknowledgements
Not applicable.
No funding was received for this article.
Author information
Authors and affiliations.
Department of Oncology and Hematology, Children’s Hospital Zagreb, Klaićeva 16, 10000, Zagreb, Croatia
Izabela Kranjčec, Nuša Matijašić & Filip Jadrijević-Cvrlje
Division of Nephrology, Department of Pediatrics, Children’s Hospital Zagreb, Zagreb, Croatia
Slaven Abdović
Department of Pediatrics, Children’s Hospital Zagreb, Zagreb, Croatia
Iva Hižar Gašpar
Department of Pediatric Endocrinology, Diabetes and Metabolism, University Hospital Center Sestre milosrdnice, Zagreb, Croatia
Lavinia La Grasta Sabolić
You can also search for this author in PubMed Google Scholar
Contributions
IK and NM undertook patient care, designed this case report, obtained informed consent, and prepared and wrote the manuscript. SA undertook patient care, analyzed and interpreted the patient’s data, and wrote the manuscript. IHG helped draft and edited the manuscript, and performed literature review. LLS helped draft and revise the manuscript. FJC produced the images used in the manuscript and helped draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Izabela Kranjčec .
Ethics declarations
Ethics approval and consent to participate, consent for publication.
Written informed consent was obtained from the patient’s legal guardian for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Kranjčec, I., Matijašić, N., Abdović, S. et al. A challenging case of an adolescent and young adult patient with high-risk acute lymphoblastic leukemia: the need for a multidisciplinary approach: a case report. J Med Case Reports 16 , 147 (2022). https://doi.org/10.1186/s13256-022-03366-y
Download citation
Received : 28 January 2022
Accepted : 10 March 2022
Published : 11 April 2022
DOI : https://doi.org/10.1186/s13256-022-03366-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Acute lymphoid leukemia
- Hyperglycemia
- Chronic kidney diseases
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Outcomes of older adults in the era of conventional chemotherapy
Novel approaches to philadelphia chromosome–negative all in older adults, novel approaches to ph+ all in older adults, additional issues in advancing care for older adults with all, conflict-of-interest disclosure, off-label drug use, acute lymphoblastic leukemia in older adults: curtain call for conventional chemotherapy.
- Split-Screen
- Request Permissions
- Cite Icon Cite
- Search Site
- Open the PDF for in another window
Marlise R. Luskin; Acute lymphoblastic leukemia in older adults: curtain call for conventional chemotherapy?. Hematology Am Soc Hematol Educ Program 2021; 2021 (1): 7–14. doi: https://doi.org/10.1182/hematology.2021000226
Download citation file:
- Ris (Zotero)
- Reference Manager
Visual Abstract

Unlike younger adults with acute lymphoblastic leukemia (ALL), older adults are rarely cured due to a combination of intrinsic disease resistance and treatment-related toxicities. Novel therapeutics such as inotuzumab ozogamicin, blinatumomab, venetoclax, and ABL kinase inhibitors have high activity in ALL and are well tolerated by older adults. Frontline treatment regimens for older adults using novel therapeutics with reduction or omission of conventional chemotherapy are being developed with early results demonstrating high remission rates and lower toxicity, but long-term efficacy and toxicity data are lacking. Collaboration between academic and pharmaceutical stakeholders is needed to develop clinical trials to define the optimal treatment regimens for older adults with ALL.
Understand the role of novel therapeutics vs CC in initial treatment of older adults with Ph-negative ALL
Understand the role of tyrosine kinase inhibitor treatment with or without CC or novel therapeutics in initial treatment of adults with Ph-positive ALL
For most of history, acute lymphoblastic leukemia (ALL) has been an uncompromising, deadly illness regardless of age, comorbidities, or social circumstance. Then, in 1948, Sidney Farber announced 5 temporary remissions among 16 children with acute leukemia treated with the folic acid antagonist, aminopterin. 1 Now, after decades of effort by numerous clinicians, scientists, and cooperative groups, more than 90% of children in resourced settings are cured of ALL with chemotherapy. 2 Although celebrated, these pediatric ALL chemotherapy regimens are notable for length, complexity, and toxicity.
Traditionally thought of as a pediatric disease, approximately half of ALL diagnoses occur in younger (18-49 years) and older (≥50 years) adults in roughly equal porportions. 3 Unfortunately, the outstanding outcomes in children have not been replicated in older cohorts. The standard approach to treating ALL in adults has been conventional chemotherapy (CC) programs adapted from pediatric schedules but with more myelosuppressive agents and a deemphasis on the noncytotoxic agents that feature prominently in pediatric regimens (corticosteroids, vincristine, and asparaginase). Results in young adults have improved due to the use of pediatric-like therapy, with more than 70% now achieving long-term survival, but older adults aged 55 to 60 or more years have consistently fared more poorly, with less than 20% cured ( Table 1 ). 4-9 Older patients experience more toxicity, leading to dose reductions, treatment delays, and high rates of early death and treatment-related mortality in remission. 4-7 , 11 , 12 In addition, high-risk genetic features are more common, leading to fewer remissions and frequent relapse. 10 , 16 Age-based dose modifications and prospective studies of CC designed specifically for older adults have not improved outcomes. 4 , 5 , 12-14 , 17 There remains no accepted standard-of-care CC regimen for older adults with ALL.
Outcomes of older adults treated with CC, select trials (see also Gökbuget 10 )
DFCI, Dana-Farber Cancer Institute.
Clinicians caring for older adults with ALL perceive limited benefit of CC. A 2019 US Medicare analysis revealed that only 53.5% of patients with ALL aged 66 or more years received any treatment within 90 days of diagnosis. 18 A 2017 US Surveillance, Epidemiology, and End Results analysis reported a median overall survival (OS) of just 4 months among adults 60 years or older diagnosed with ALL since 1980, with minimal progress over 3 decades (3-year OS increasing from 10% to 16%). 19 In summary, the chemotherapy regimens that cure children are not a good match for older adults. 10 , 16
A 78-year-old man with coronary artery disease, hypertension, and hyperlipidemia developed several weeks of fatigue and weight loss. Prior to illness, he visited the gym regularly engaging in vigorous exercise. Laboratory work revealed pancytopenia (white blood cell count, 2.9 K/µL; hemoglobin, 11.2 g/dL; platelets, 86 K/µL) with 7% circulating blasts. He was diagnosed with B-cell acute lymphoblastic leukemia (B-ALL). Immunophenotype was CD45+, CD34+, HLA-DR+, TdT+, CD19+ (variable), CD22+, CD10−, and CD20−. Karyotype was complex with no evidence of the Philadelphia chromosome. Next-generation sequencing revealed a pathogenic mutation in TP53 . In 2019, he was referred to our academic center.
The development of novel chemotherapy agents—blinatumomab and inotuzumab ozogamicin (IO)—for relapsed B-ALL has led to a reimagining of the treatment for older adults with ALL. IO is a CD22 monoclonal antibody covalently linked to calicheamicin, a cytotoxic agent (antibody–drug conjugate). Blinatumomab is a bispecific T-cell engager targeting CD19 and CD3. Each is now approved by the US Food and Drug Administration (FDA) for relapsed and refractory (R/R) ALL based on phase 3 randomized trial data showing superiority of the novel agent to salvage cytotoxic chemotherapy in the R/R setting. 20 , 21 It is hoped that using IO and blinatumomab in the first-line treatment of older adults will allow toxic CC to be de-escalated or omitted, resulting in both improved response rates and less toxicity.
Investigators at MD Anderson Cancer Center (MDACC) have been testing IO plus mini-hyper-CVD (cyclophosphamide, vincristine, dexamethasone), a lower-intensity CC regimen, in older adults (≥60 years) with untreated Philadelphia chromosome–negative (Ph–) CD22+ B-ALL in a single-center phase 2 trial (NCT01371630, Table 2 ). In the original design, IO was administered on day 3 of the first 4 of 8 planned CC cycles followed by 36 months of POMP (6-mercaptopurine, vincristine, methotrexate, prednisone) maintenance. In 2018, MDACC investigators reported high response rates (98% overall response rate [ORR], 96% minimal residual disease-negativity [MRD-negativity]) and no early mortality in the first 52 patients treated. 22 An update of the trial in 2020 (with 70 patients enrolled) was notable for continued high response rates (98% ORR, 96% MRD negativity, with no early deaths) and an encouraging 3-year complete remission (CR) duration and OS of 79% and 56%, respectively. 23
Novel approaches to Ph– ALL in older adults: published and ongoing trials *
Results at most recent publication.
Blina, blinatumomab; CCR, continuous complete remission; CRi, complete remission with incomplete count recovery; NR, not reached.
Notable toxicities associated with the IO plus mini-hyper-CVD regimen have included frequent prolonged thrombocytopenia (81%), hepatotoxicity (17% grade ≥3 hyperbilirubinemia, 9% veno-occlusive disease), and infections (41% during induction, 70% during consolidation). 23 Death in remission due to treatment-related toxicity has been the major challenge. At the most recent update, 34% (24/70) of responding patients had died in CR due to sepsis, veno-occlusive disease, or secondary myeloid malignancies. Mortality was more common in patients 70 years or older who comprised ~40% of enrolled patients. In response to an initial experience with toxicity, protocol modifications have included IO dose fractionation and reduction (to address liver toxicity) and a drastic decrease in the number of recommended CC cycles (originally 8, decreased to 4 for patients aged 60-69 years and to 2 for patients aged ≥70 years).
The European phase 2 EWALL (European Working Group for Adult ALL)-INO study (NCT03249870) is also exploring IO in combination with low-intensity chemotherapy for untreated patients 55 years or older with CD22+ ALL. Patients receive IO plus low-intensity chemotherapy induction for 2 cycles followed by IO-free chemotherapy consolidation and maintenance.
In contrast to the combination approach taken by MDACC and EWALL, the German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia (GMALL) study group is studying a sequential approach. In the phase 2 INITIAL-1 study (NCT03460522), untreated older adults (aged >55 years) receive 3 cycles of IO monotherapy followed by CC consolidation ( Table 2 ). A 100% CR was reported among the first 31 patients treated (78% MRD negative), with the majority (29/31) able to complete all 3 IO induction cycles with no early deaths. 24 Long-term efficacy and toxicity of the regimen are not yet known.
Venetoclax plus CC
The BCL2 inhibitor venetoclax, an agent with demonstrated preclinical and clinical activity in relapsed ALL, is also being studied in the frontline in combination with mini-hyper-CVD (NCT03319901, Table 2 ). 27 , 28 A phase 1b study of venetoclax plus mini-hyper-CVD for relapsed/refractory (R/R) (n = 8) and newly diagnosed patients 60 years or older (n = 11) with ALL demonstrated safety (no dose-limiting toxicities (DLTs) or early mortality), with venetoclax 600 mg daily declared the recommended phase 2 dose. 25 Notably, there was no evidence of prolonged cytopenias or liver toxicity. The regimen was particularly effective in newly diagnosed patients, with 91% (10/11) achieving an MRD-negative CR, including 6 patients with a TP53 mutation. Because 9 of 10 responders were consolidated with allogeneic hematopoietic stem cell transplant (HSCT), there are limited data on late toxicity. The trial has now moved to phase 2 (with venetoclax 400 mg) and is currently enrolling adults 60 years or older with newly diagnosed Ph– ALL. A distinct advantage of this regimen is that it can be offered to patients with both B- and T-cell ALL, in contrast to approaches that rely on B-lineage restricted antibodies and antibody–drug conjugates. Venetoclax is not approved by the FDA for ALL.
Closing the curtain on chemotherapy for Ph– ALL?
Given the efficacy of novel agents, a chemotherapy-free approach may be possible. The US National Clinical Trial Network (NCTN) trial Southwest Oncology Group (SWOG) 1318 phase 2 study of blinatumomab induction followed by blinatumomab consolidation for newly diagnosed Ph– B-ALL (aged >65 years) reported a 66% CR rate (92% MRD negative) among the first 29 patients with no early deaths (NCT02143414, Table 2 ). 26 Another phase 2 NCTN trial (Alliance 041703 NCT03739814) is investigating IO induction followed by blinatumomab consolidation ( Table 2 ). This approach seeks to use each novel agent to maximum advantage. IO is active regardless of disease burden, making it an ideal induction agent, whereas blinatumomab is more effective and less toxic in settings of lower disease burden and thus a good agent for consolidation therapy. 15 , 29
Encore for chemotherapy
Blinatumomab and IO achieve high response rates in the R/R setting but with short durability. 20 , 21 Although the durability of single-agent novel therapy in the upfront setting is not known, relapses driven by selective pressure would be expected. It is hypothesized that low-intensity CC integrated into induction and/or consolidation (as being pursued by EWALL-INO and GMALL INITIAL-1 studies) may continue to benefit older patients receiving novel agents by applying broader antileukemic pressure and diminishing opportunities for clonal escape. Novel combination approaches (such as being studied in A041703) may offer the best of both worlds via a multipronged attack without chemotherapy toxicities. MDACC has added blinatumomab consolidation to their IO plus mini-hyper-CVD protocol. Venetoclax plus IO will also soon be tested in R/R B-ALL due to nonoverlapping toxicities and preclinical evidence for synergy. 30 If shown to be safe, it may be useful as a frontline regimen. For T-cell ALL, a chemotherapy-free option does not yet exist. More research to identify therapeutic vulnerabilities and novel therapeutics for this subtype is needed.
CASE 1 (continued)
The patient was treated with venetoclax plus mini-hyper-CVD on an investigational protocol and achieved an MRD-negative CR after 1 cycle. He completed 4 cycles of venetoclax plus mini-hyper-CVD, after which he was bridged to venetoclax plus POMP maintenance due to toxicity from CC. He continued POMP maintenance for 2 years and remains in an ongoing remission. (See Figure 1 for approach to Ph– ALL in older adults.)

How I treat Ph– ALL in older adults.
A 73-year-old woman with a history of hypertension and rheumatoid arthritis was asymptomatic but found to have an abnormal complete blood count on routine testing: white blood cells (19 K/µL) with 39% circulating blasts that are CD45+, CD34+, HLA-DR+, TdT+, CD19+, CD10+, and CD20−. Karyotype shows t(9;22) with additional abnormalities. Reverse transcription polymerase chain reaction shows p210 transcript more than 50% international scale (IS).
The Philadelphia chromosome is present in approximately half of ALLs in older adults. 31 Historically associated with an adverse prognosis, the development of ABL kinase inhibitors has resulted in frequent cures in younger adults and in older adults has enabled prolonged remissions, negating the adverse prognosis. 32-34 Older adults with ALL are ideal candidates for low-intensity CC and chemotherapy-free tyrosine kinase inhibitor (TKI)–based protocols.
TKI plus CC
The EWALL has studied TKI plus low-intensity CC in adults 55 years or older (NCT028889777, Table 3 ). Second-generation TKIs were combined with low-intensity induction and consolidation CC in the consecutive EWALL-PH01 (dasatinib) and EWALL-PH02 (nilotinib) protocols. Both protocols resulted in high CR rates (>95%) without induction mortality. Long-term survival was particularly encouraging in the EWALL-PH02, in which allogeneic HSCT was pursued in 30% (24/79) of patients (4-year OS, 47%; 61% and 39% in transplanted vs nontransplanted, respectively). 35 , 36 Importantly, the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAAPH)-2005 trial, which enrolled younger adults (18-59 years), demonstrated that reduced-intensity CC induction was associated with fewer induction deaths and equivalent efficacy compared with standard-intensity CC induction, questioning the benefit of intensive CC induction for any patient (regardless of age) with Ph+ ALL in the TKI era. 37
Novel approaches to Ph+ ALL in older adults: published and ongoing trials *
CMR, complete molecular response; DFS, disease-free survival; EFS, event-free survival.
Curtain call on chemotherapy for Ph± ALL?
The Italian Gruppo Italiano Malattie EMatologiche dell’Adulto (GIMEMA) group has pioneered a chemotherapy-free induction approach for Ph+ ALL ( Table 3 ). Imatinib plus corticosteroids induced universal hematologic remissions, although with limited depth (only 1 of 29 achieved a complete molecular remission) and durability (median duration of hematologic response of 8 months). 38 Subsequently, the more potent TKI dasatinib was combined with corticosteroids, with more patients achieving deep molecular remissions (52.1% achieved BCR-ABL level <10−3 by day 85). 39 , 40 The original GIMEMA studies were induction-only studies, with subsequent consolidation therapy for each patient dictated by the treating physician. The US NCTN Alliance 10701 phase 2 study (NCT01256398, Table 3 ) tested a chemotherapy-free dasatinib plus steroid induction followed by dasatinib plus CC consolidation and then transplant (allogeneic vs autologous HSCT or chemotherapy based on availability of an human leukocyte antigen (HLA)-matched donor and fitness). 41 Deep remissions were consistently obtained (97% CR, 75% ultimately achieving a complete molecular response) with 3-year OS of 55% (49% in patients aged >60 years).
Although the GRAAPH-2005 study showed that intensive CC induction was not advantageous compared with lower-intensity CC induction in the context of TKIs, the benefit of adding CC to TKI-based consolidation, particularly in patients bridging to allogeneic HSCT, is not known.
Approaches to prevent relapse in Ph± ALL
Despite improvements with TKI-based therapy, relapse remains common, with T315I-driven relapses a particular menace. 35 , 39 Approaches to address this issue include use of ponatinib, which is active against T315I; dual ABL kinase inhibition with dasatinib and asciminib; and addition of novel agent consolidation with blinatumomab.
The GIMEMA group has extended its chemotherapy-free induction approach to ponatinib plus steroids in older adults (aged ≥60 years), with encouraging early results (NCT01641107, Table 3 ). 40 Although toxicity appears reasonable with dose reductions (only 15/42 remained at 45 mg daily at week 24), the risk of cardiovascular toxicity with ponatinib remains a concern in older patients. The planned EWALL-03 study (NCT04688983) is randomizing patients to imatinib vs ponatinib in combination with low-intensity CC and may provide insight regarding risk/benefit ratio of later-generation TKIs. A “risk-adapted” approach using early response milestones to select which patients require escalation of therapy to more potent (and more toxic) later-generation TKIs would be attractive.
Combination ABL kinase inhibition is being explored in a phase 1 study treating newly diagnosed adults 50 years or older with Ph+ ALL with dasatinib and asciminib, a novel myristoyl binding pocket inhibitor (NCT03595917, Table 3 ). The hypothesis is that dual ABL inhibition may induce deeper remissions and prevent T315I relapses. Thus far, the regimen appears well tolerated, with asymptomatic amylase and lipase elevations being the most notable adverse event. 42 This study is ongoing to establish tolerability and recommended dose.
The GIMEMA D-ALBA trial added dasatinib-blinatumomab consolidation to dasatinib-steroid induction with promising early results (NCT02744768, Table 3 ). 43 The US NCTN SWOG 1318 Cohort 2 (NCT02143414) is studying a similar approach of dasatinib-steroid induction followed by blinatumomab consolidation. A study of ponatinib-steroid induction followed by blinatumomab consolidation is also planned (NCT04722848). An open question is whether blinatumomab consolidation should be applied to all patients or used in a risk-adapted manner. Frequency of central nervous system (CNS) relapse will also need to be monitored over time in patients treated entirely with TKIs and novel agents (with only intrathecal (IT) chemotherapy administered to prevent CNS relapse).
Other active agents in ALL, including IO and venetoclax, also require evaluation as part of treatment of Ph+ ALL. The Alliance 041703 is planning to add an arm for Ph+ patients testing an IO plus ponatinib induction.
The US NCTN is currently accruing to EA9181 (NCT04530565), a phase 3 study of patients up to age 70 years who receive chemotherapy-free induction of TKI plus steroids and then are randomized to TKI (dasatinib or ponatinib) plus hyper-cyclophosphamide, vincristine, doxorubicin, dexamethasone (CVAD) vs TKI plus blinatumomab consolidation to compare CC vs novel agent consolidation. Eligible patients undergo transplantation when MRD negative, and all patients receive high-dose methotrexate for CNS prophylaxis.
CASE 2 (continued)
The patient was treated with dasatinib and asciminib on a clinical trial. She tolerated therapy well initially but had an inadequate response after 3 cycles of therapy (CR but MRD+ by flow cytometry, BCR/ABL 6.3% IS). She required multiple dasatinib dose reductions due to recurrent pleural effusions. She was transitioned to ponatinib 30 mg daily and continues in remission (BCR-ABL p210 transcript undetectable) now 2 years from diagnosis. (See Figure 2 for approach to Ph– ALL in older adults.)

How I treat Ph+ ALL in older adults.
The outlook for older adults diagnosed with ALL is improving rapidly. Additional issues facing the field are summarized in Figure 3 .

Important components for ALL clinical trials in older adults.
Curability of older adults
Although survival is poor among older adults treated with CC, a few fit older patients may tolerate and be cured by CC regimens. 5 , 11 , 12 , 44 , 45 In contrast, there is no evidence yet that novel agent approaches are curative, although they may induce durable MRD-negative remissions. Patients responding to novel approaches may become eligible for curative-potential allogeneic HSCT, but the role of HSCT is not clear and has not been confirmed to be superior to CC for older adults with Ph– ALL in first CR in the CC era. 46 The Alliance/CALGB 10701 study has attempted to address this question for Ph+ ALL by assigning adult patients (aged 18-69 years) to reduced intensity conditioning (RIC) allogeneic HSCT vs autologous HSCT based on availability of a fully matched donor ( Table 3 ). 41 Although 3-year OS was similar between those receiving allogeneic HSCT and other consolidation approaches, patients assigned to the allogeneic approach were less likely to relapse.
Need for randomized comparison
Results of novel agent–based regimens tested in nonrandomized studies are extremely encouraging, with strong evidence for superiority compared with historical CC regimens based on high response rates and low toxicity. The next step will be to formally compare a novel and a “standard” CC approach in Ph– ALL, and thus the NCTN is designing a randomized phase 2 study of IO plus mini-hyper-CVD (the novel regimen with the most experience to date) vs age-adjusted hyper-CVAD (Alliance 042001). The objective of this trial will be to establish a novel agent–based benchmark regimen to which other novel agent regimens can be subsequently compared ( Figure 4 ).

Treating Ph-negative B-cell ALL in older adults: future trial landscape.
Surrogate end points and risk-adapted approaches
Early response end points (such as MRD negativity) should be studied to confirm validity as surrogates for survival in older adults. This could facilitate rapid completion of trials as well as development of risk-adapted approaches.
Novel agents are expensive and likely to increase disparities between resourced and underresourced settings. Advocacy for improved access is needed.
Representative enrollment and geriatric assessment
Almost every older adult has a medical problem other than ALL. Trial eligibility should be designed to allow representative enrollment. Unnecessarily restrictive inclusion criteria that exclude patients based on advanced age, mildly compromised organ function, or “clinically insignificant” concurrent malignancies should be avoided. Geriatric assessment (GA) should be routinely incorporated into ALL trials of older adults (with financial support to ensure completion). GA has been shown to be feasible in cooperative group cancer trials, including in an Alliance trial for acute myeloid leukemia. 47 , 48 The phase 2 NCTN trial in development plans to incorporate GA into its study design.
Quality-of-life assessment
Quality-of-life end points should be given importance. Incremental improvements in efficacy may not be “worth it” to an older adult if it is associated with significant toxicity and treatment complexity. Similarly, new regimens with equivalent responses but better tolerability may warrant further development.
Cooperation to clobber the conventional approach
By far the biggest challenge in adult ALL will be to efficiently design and conduct logical, sequential studies to answer key questions and make sure patient care “keeps up” with scientific advances. In a rare disease, this is no small task. Trials will need to be developed collaboratively, opened widely with equity considered, and conducted efficiently so that questions can be iteratively asked and answered for the betterment of patients. In the United States, a NCTN working group involving representatives from Alliance, ECOG, and SWOG has formed to coordinate efforts.
An older adult diagnosed with ALL in 2021 has more promising treatment options than an adult diagnosed even 5 years ago. The first task is to expeditiously replace the unacceptable traditional CC approaches with novel approaches via practice changing clinical trials. Then we must work to further define the optimal treatment for all patients considering individual (age, comorbidities, and frailty) and disease (immunophenotype, genetics, response milestones) features and personal preferences.
Marlise R. Luskin: discloses no relevant conflict of interest.
Marlise R. Luskin: only clinical trials discussed.
- Previous Article
- Next Article
Email alerts
Affiliations, american society of hematology.
- 2021 L Street NW, Suite 900
- Washington, DC 20036
- TEL +1 202-776-0544
- FAX +1 202-776-0545
ASH Publications
- Blood Advances
- Blood Neoplasia
- Blood Vessels, Thrombosis & Hemostasis
- Hematology, ASH Education Program
- ASH Clinical News
- The Hematologist
- Publications
- Privacy Policy
- Cookie Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Open access
- Published: 02 December 2022
Prognostic value and outcome for acute lymphocytic leukemia in children with MLL rearrangement: a case-control study
- Kun-yin Qiu 1 , 2 na1 ,
- Dun-hua Zhou 1 , 2 na1 ,
- Xiong-yu Liao 1 , 2 na1 ,
- Ke Huang 1 , 2 ,
- Yang Li 1 , 2 ,
- Hong-gui Xu 1 , 2 ,
- Wen-jun Weng 1 , 2 ,
- Lu-hong Xu 1 , 2 &
- Jian-pei Fang 1 , 2
BMC Cancer volume 22 , Article number: 1257 ( 2022 ) Cite this article
2011 Accesses
4 Citations
Metrics details
To evaluate the prognostic factors and outcome for acute lymphoblastic leukemia (ALL) in children with MLL rearrangement (MLL-r).
A total of 124 pediatric patients who were diagnosed with ALL were classified into two groups based on the MLL-r status by using a retrospective case-control study method from June 2008 to June 2020.
The prevalence of MLL-r positive in the whole cohort was 4.9%. The complete remission (CR) rate on Day 33 in the MLL-r positive group was not statistically different from the negative group (96.8% vs 97.8%, P = 0.736). Multivariate analysis showed that T-cell, white blood cell counts (WBC) ≥ 50 × 10 9 /L, MLL-AF4, and D15 minimal residual disease (MRD) positive were independent risk factors affecting the prognosis of MLL-r positive children. Stem cell transplantation (SCT) was a favorable independent prognostic factor affecting event-free survival (EFS) in MLL-r positive patients ( P = 0.027), and there was a trend toward an independent prognostic effect on overall survival (OS) ( P = 0.065). The 10-year predicted EFS for patients with MLL-AF4, MLL-PTD, MLL-ENL, other MLL partner genes, and MLL-r negative cases were 46.67 ± 28.61%, 85.71 ± 22.37%, 75 ± 32.41%, 75 ± 32.41%, and 77.33 ± 10.81%, respectively ( P = 0.048). The 10-year predicted OS were 46.67 ± 28.61%, 85.71 ± 22.37%, 75 ± 32.41%, 75 ± 32.41%, and 85.2 ± 9.77%, respectively ( P = 0.049). The 124 patients with ALL were followed up and eventually 5 (4%) cases relapsed, with a median relapse time of 3.9 years.
Patients with MLL-r positive ALL have moderate remission rates, but are prone to relapse with low overall survival. The outcome of MLL-r positive ALL was closely related to the partner genes, and clinical attention should be paid to screening for MLL partner genes and combining them with other prognostic factors for accurate risk stratification.
Peer Review reports
Introduction
The mixed lineage leukemia (MLL) gene, located on the long arm of chromosome 11, region 2, band 3 (11q23), which is fused to a variety of translocation partner genes (TPG), is known to identify 135 different MLL rearrangements, and its rearrangements produce MLL fusion proteins can cause abnormal self-renewal and epigenetic deregulation of hematopoietic progenitor cells, leading to the development of malignant leukemia [ 1 ]. Previous literature showed that the prevalence of MLL gene rearrangement (MLL-r) in childhood acute lymphoblasitc leukemia (ALL) is 2.5 to 5% [ 2 , 3 ]. Compared with non-MLL leukemia, children with MLL-r positive ALL have clinical features that are different from other types of leukemia, mainly high white blood cell count (WBC) at disease onset, insensitivity to traditional chemotherapeutic agents, low complete remission rate (CR), and short survival and poor outcome [ 4 , 5 , 6 ], which is why the South China Children’s Leukemia Group [ 7 ] classified children with MLL-r positive ALL as a high-risk group.
The children with ALL who have fused the N-terminal gene and any of the different partner genes to produce a new MLL fusion protein within the framework combine with different fusion partner genes [ 5 ]. The prognosis is different, but the prognosis is usually poor. MLL fusion gene is related to age and sex. The incidence rate of MLL-AF4 in infants and adults is higher than that in children, the incidence rate of MLL-ENL in infants and children is higher than that in adults, the incidence of AF10 in children is higher, and PTD tends to children and adults. MLL-AF10 appears more frequently in male patients, while female patients are more affected by MLL-AF4 fusion [ 4 , 5 , 6 , 7 ].
Currently, although a number of studies have reported MLL-r positive ALL, there are only a few literature reports focusing on partner genes. Hence, in this study, we collected data from 637 children with ALL in our hospital over the past ten years, tested MLL gene rearrangements and their partner gene types, retrospectively analyzed the clinical characteristics and laboratory data of children with MLL-r positive ALL, and evaluate the outcome and prognostic value of this type of children.
Patients and methods
Study participants.
All cases were enrolled from 637 children with ALL who were initially diagnosed in the Department of Hematology at our Children’s Medical Center from June 2008 to June 2020, and 31 cases were included in the MLL-r positive group (case group) and 93 cases from four common fusion genes (ETV6/RUNX1, BCR-ABL1, MLL gene rearrangement, and E2A-PBX1 fusion gene) were negative. The 93 children were randomly selected by systematic sampling from 465 children with ALL as the MLL-r negative group (control group). The systematic sampling method was as follows: 465 children with ALL were numbered 1–465 according to the time of diagnosis, in groups of 5, and a random number 2 was generated by applying a random number list, and 93 cases were selected from 2, 7, 12, 17, 22, 27, 32, 37, 42, 47, 52, and so on to 465 to form the control group. The study was conducted in accordance with the principles set down in the Declaration of Helsinki and was approved by the Ethics Committee of Sun Yat-sen Memorial Hospital. All the patients’ parents or guardians, provided written informed consent.
Clinical characteristics such as age, gender, WBC, Hemoglobin (Hb), platelet (PLT), risk stratification and immunophenotype at the time of initial diagnosis were analyzed in the whole cohort, and the response to prednisone treatment, BM smear and minimal residual disease (MRD) on Day 15 and Day 33 of induction chemotherapy were also monitored.
Inclusion criteria: ① Age ≤ 18 years; ② Clinical presentation consistent with ALL and definite diagnosis of ALL by BM cytomorphology, immunophenotype, cytogenetics and molecular biology (ETV6/RUNX1, BCR-ABL1, MLL gene rearrangement and E2A-PBX1 fusion gene as routine tests); ③ First-episode children (not undergoing any ALL before coming to the hospital for related treatment). All the patients were screened for relevant fusion genes by FISH (fluorescence in situ hybridization) or quantitative PCR (Polymerase Chain Reaction) at the time of initial diagnosis.
Exclusion criteria: (i) T-lineage, mature B or acute mixed leukemia; (ii) Secondary to immunodeficiency disease; (iii) Second tumor; (iv) Definite CML (chronic myelocytic leukemia) acute change; (v) ALL with Down’s syndrome; (vi) Glucocorticoid use for more than 1 week in the month before enrollment; (vii) MLL data missing.
Chemotherapy protocol
Children diagnosed between February 2008 to September 2016 were treated according to the Guangdong Children’s Leukemia Group-ALL-2008 (GD-ALL-2008) [ 8 ] protocol; and children diagnosed between October 2016 to June 2020 were treated according to the South China Children’s Leukemia Group-ALL-2016 (SCCLG-ALL-2016) [ 7 ] protocol.
Treatment response
Prednisone treatment is beneficial to risk stratification and prognosis evaluation of ALL patients. ① Prednisone response (PR): those with absolute peripheral blood naive lymphocyte count < 1000 × 10 6 /L on Day 8 of prednisone induction were considered prednisone good responders (PGR), while those ≥1000 × 10 6 /L were considered prednisone poor responders (PPR); ② Morphological evaluation of bone marrow smear was performed on Day-15 and Day-33. Patients were classified according to their blast cells amount, with M1 (blast cells< 5%), M2 (5% to< 25%), or M3 (> 25%); (3) Relapse: including BM relapse, central nervous system (CNS) relapse, testicular relapse and combined relapse.BM relapse means that after complete remission (CR) of ALL, the ratio of primitive plus naive cells in bone marrow is > 25% on review. CNS relapse means that primitive plus naive cells are detected in cerebrospinal fluid by centrifugal smear or CNS infiltration is present and other causes cannot be explained or CT/MRI showed brain or meningeal lesions, testicular relapse i.e. ultrasound or biopsy confirmed unilateral or bilateral testicular leukemic cell infiltration, combined relapse i.e. extramedullary relapse (central and/or testicular relapse) with > 5% leukemic cells in the BM; ④ Events included: persistent non-CR, relapse, development of a second tumor, death and so on.
Risk classification
Stand-risk (SR) group: Patients with B-cell precursor ALL were (1) between 1and 6 years of age and had a WBC count at diagnosis < 20 × 10 9 /L and (2) show a good early response, including a good response to prednisone on day 8 or M1/M2 marrow on day 15 and (3) M1 marrow on day 33.
Intermediate-risk (IR) group: Patients had (1) T-lineage ALL or below 1or after 6 years of age and had a WBC count at diagnosis>20 × 10 9 /L or (2) show a good early response, including a good response to prednisone on day 8 or M1/M2 marrow on day 15 and (3) M1 marrow on day 33 (4) CNSL (central nervous system leukemia).
High-risk (HR) group: Patients who had (1) show a poor early response, including a poor response to prednisone on day 8 or M3 marrow on day 15 or (2) M2/M3 marrow on day 33 or (3) t(9:22)(BCR/ABL) or had t(4:11) MLL/AF4 or (4) Testicular leukemia. The final risk group was determined based on the treatment response.
All cases were followed up by outpatient review or telephone, and children receiving the GD-ALL 2008 protocol were followed up until June 30, 2018, while children receiving the SCCLG-ALL 2016 protocol were followed up until July 31, 2020, with study endpoints set as death, lost to follow-up, or follow-up cutoff, and those lost to follow-up. The overall survival (OS) period was the time from the start of treatment to death or the end of follow-up. The event-free survival (EFS) period was the time from the start of treatment to the occurrence of any event, including death from any cause, second tumor, disease progression, relapse, or missed follow-up. Patients were observed for general condition, relapse and interventions, survival, and monitoring of BM.
Statistical analyses
Baseline characteristics were grouped by MLL status and presented as mean ± SD for continuous variables and as frequency (%) for categorical variables. Chi-square test was used for categorical variables, when the number of samples is small, we use Fisher’s exact test. In addition, analysis of variance or Kruskal-Wallis test was used for continuous variables. In order to assess the clinical outcome, we used the following concepts: complete remission (CR, was defifined as less than 5% lymphoblasts in active hematopoietic bone marrow at the end of induction), event-free survival (EFS, defined as the start of the study to the timing of events for the first time, including induced failure, progress, or any cause of death and recurrence), and overall survival (OS, defined as the time between the beginning of learning and death from any cause). Cox proportional hazards models were used to test the associations between EFS or OS and baseline covariates, with results presented as HRs with 95% CIs. Similarly, the HRs and 95% CIs of EFS or OS in each MLL status were estimated, and their interactions were tested. EFS and OS were evaluated by Kaplan-Meier method and compared by log-rank test. All statistical analysis by SPSS statistical software version 22.0 and EmpowerStats ( http://www.empowerstats.cn/ ). P < 0.05 was considered statistically significant.
Baseline characteristics of pediatric ALL patients
Thirty-one MLL-r positive cases were detected among 637 children with initial ALL, with a positive rate of 4.9% (31/637). Thirty-one MLL-r positive children included 9 positive MLL-PTD (Partial Tandem Duplications), 8 positive MLL-AF4, 5 positive MLL-ENL, 3 positive MLL-AF10, 3 positive MLL-AF9, 2 positive MLL-AF6, and 1 positive MLL-ELL. For statistical convenience, the partner genes were divided into MLL-PTD group, MLL-AF4 group, and MLL-ENL group, and the remaining partner genes were uniformly classified as other groups (Table 1 ).
Comparison between MLL-r-positive and MLL-r-negative ALL
The differences between the MLL-r positive group and the negative group were not statistically significant ( P > 0.05) in terms of chemotherapy protocol, gender, Hb and PLT (Table 2 ). The median age of the 124 children was 3.9 (0.4–14.9) years, including 74 males (59.7%) and 50 females (40.3%).
By comparing the MLL-r positive and negative groups, the median age of onset was 3.1(0.4–12.5) and 4.5(1.4–14.9) years, respectively, with statistically significant differences between the two groups( P = 0.019). The MLL-r positive group were mostly seen in common-B, immature-B and T, when compared with the negative group ( P < 0.001). The proportion of patients with risk factors (age < 1 year, WBC ≥50 × 10 9 /L) at initial diagnosis was significantly higher in the MLL-r positive group than in the negative group (12.9% versus 0, P = 0.002; 41.9% versus 10.8%, P < 0.001). In the final clinical risk group, a significantly higher proportion of MLL-r positive cases was included in the high-risk group than in the negative group (64.5% versus 23.7%, P < 0.001).
Further comparing the response of PR between MLL-r positive and negative groups (Table 2 ), the proportion of PGR in MLL-r positive group was lower than that in negative group, but there was no significant difference (80.6% versus 87.1%, P = 0.377). When the BM was detected on Day 15 of induction chemotherapy, the proportion of M1 in the MLL-r positive group was significantly lower than that in the negative group (67.7% versus 84.9%, P = 0.036), while the BM was monitored on Day 33 of induction chemotherapy, the M1 in the MLL-r positive group was not significantly different from that in the negative group (96.8% versus 97.8%, P = 0.736). A total of 110 children were tested for D15 MRD in this study, and 65 (59.1%) were positive for D15 MRD, with a higher proportion of positive D15 MRD in the MLL-r positive group than in the MLL-r negative group (70.6% versus 57%, P = 0.294). Finally, 112 children were tested for D33 MRD, and 14 (12.5%) were positive for D33 MRD, with a higher proportion of positive D33 MRD in the MLL-r positive group than in the MLL-r negative group. The CR rate on Day 33 was 96.8% (30/31) in the MLL-r positive group and 97.8% (91/93) in the MLL-r negative group, with no statistical difference between the two CR rates ( P = 0.736).
Prognostic significance of the overall cohort among pediatric ALL
Univariate analysis of risk factors that had statistically significant effects on the whole cohort with ALL were selected for inclusion in the multivariate analysis, and the results showed that WBC ≥50 × 10 9 /L (EFS: HR = 2.7, 95% CI (1.1–7.1), P = 0.039; OS: HR = 4.4, 95% CI (1.1–19.6), P = 0.048) and MLL-AF4 (EFS: HR = 7.5, 95% CI (1.6–35.5), P = 0.012; OS: HR = 7.8, 95% CI (1.1–53.1), P = 0.036) were both independent risk factors for the prognosis of patients with ALL. In contrast, MLL-r positive was not an independent prognostic risk factor (EFS: HR = 1.6, 95% CI (0.6–4.5), P = 0.343; OS: HR = 1.4, 95% CI (0.4–5.1), P = 0.608).
Subgroup analysis of prognostic significance for MLL-r positive patients and survival analysis
Analysis of possible risk factors affecting EFS in MLL-r positive children by applying a columnar chi-square test showed that gender, WBC, immunophenotype, MLL partner gene type, D15 MRD and SCT were all associated factors affecting EFS and OS in MLL-r positive patients ( P values < 0.05) (Table 3 ). Interaction tests were done between MLL status and EFS or OS (Supplementary Table 1 and Supplementary Table 2 ).
Cox model multivariate regression analysis showed that T-cell phenotype (EFS: HR = 5.0, 95% CI (1.5–17.5), P = 0.011; OS: HR = 6.7, 95% CI (1.5–29.6), P = 0.012), WBC ≥50 × 10 9 /L(EFS: HR = 6.5, 95% CI (1.6–24.8), P = 0.007; OS: HR = 3.6, 95% CI (1.1–12.4), P = 0.041), MLL-AF4 (EFS: HR = 3.5, 95% CI (1.1–11.5), P = 0.035; OS: HR = 4.6, 95% CI (1.5–14.4), P = 0.008), and D15 MRD positive (EFS: HR = 4.8, 95% CI (1.4–17.1), P = 0.015; OS: HR = 2.6, 95% CI (1.1–6.0), P = 0.025) were the independent risk factors affecting the outcome of MLL-r positive patients. Interestingly, SCT was a favorable independent factor on EFS( HR = 0.4, 95% CI (0.0–0.9), P = 0.027), and tended to have an independent effect on OS ( HR = 0.2, 95% CI: 0.0–1.1, P = 0.065) (Table 4 ).
Further comparison of the K-M survival curves of the 31 MLL-r positive and 93 MLL-r negative patients on standardized treatment showed that the 10-year predicted EFS rate was significantly lower in the MLL-r positive than in the negative group (EFS: 56.01 ± 16.89% versus 77.33 ± 10.81%, P = 0.022) (Fig. 1 A). While the 10-year predicted OS rate in the positive group had a tendency to be lower in the positive group than in the negative group (OS: 73.32 ± 16.6% versus 85.2 ± 9.77%, P = 0.11) (Fig. 1 B). Moreover, our K-M survival analysis of children with ALL receiving chemotherapy-only showed that the 10-year predicted EFS rate was significantly lower in MLL-r positive cases receiving chemotherapy-only than in the negative group (54.32 ± 16.89% versus 76.78 ± 11.01%, P = 0.018) (Fig. 2 A), and the 10-year predicted OS rate also tended to be lower in the positive group than in the negative group (72.19 ± 16.88% versus 84.84 ± 9.97%, P = 0.11) (Fig. 2 B). Among the MLL-r positive cases, the 10-year predicted EFS rate was significantly higher in those who received SCT than in those who received chemotherapy-alone (100% versus 54.32 ± 16.89%, P = 0.038) (Fig. 3 A). While the 10-year predicted OS rate in children who received SCT showed a trend to be higher than in those who received chemotherapy-only (100% versus 72.19 ± 16.88%, P = 0.053) (Fig. 3 B).
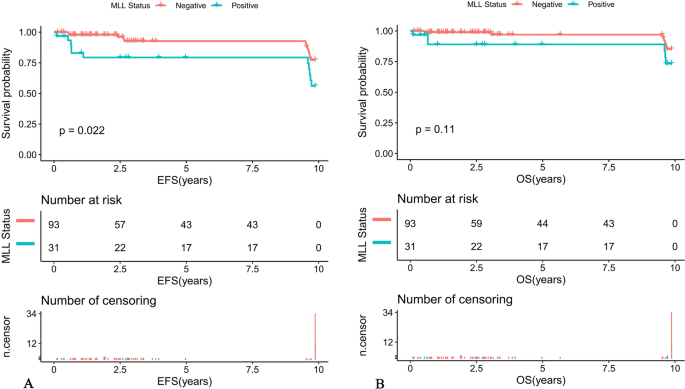
Survival cures of pediatric ALL according to different MLL status. A The 10-year EFS of MLL-r positive and MLL-r negative ALL patients. B The 10-year OS of MLL-r positive and MLL-r negative ALL patients
Survival cures of pediatric ALL with chemotherapy-only according to different MLL status. A The 10-year EFS of pediatric ALL with chemotherapy-only according to different MLL status. B The 10-year OS of pediatric ALL with chemotherapy-only according to different MLL status
Survival curves of MLL-r positive pediatric ALL patients according to SCT status. A Probability of EFS for MLL-r positive patients according to SCT status. B Probability of OS for MLL-r positive patients according to SCT status
Among patients with ALL, the 10-year predicted EFS for MLL-AF4 positive, MLL-PTD positive, MLL-ENL positive, other MLL gene partner positive and MLL-r negative children were 46.67 ± 28.61%, 85.71 ± 22.37%, 75 ± 32.41%, 75 ± 32.41% and 77.33 ± 10.81%, respectively ( P = 0.048) (Fig. 4 A). The 10-year predicted OS was 46.67 ± 28.61%, 85.71 ± 22.37%, 75 ± 32.41%, 75 ± 32.41%, and 85.2 ± 9.77%, respectively ( P = 0.049) (Fig. 4 B).
Survival curves of different MLL partner gene among pediatric ALL patients ( A ) The 10-year EFS of different MLL partner gene among ALL patients. B The 10-year OS of different MLL partner gene among ALL patients
Relapse analysis
Long-term follow-up of 124 children with ALL resulted in relapse in 5 (4%) children with a median relapse time of 3.9 (0.1–9.9) years. The median time to relapse was 3.4 years in the MLL-r negative group and 9.6 (0.1–9.9) years in the MLL-r-positive group, with no statistical difference between the two groups. There was no statistical difference between the two relapse times ( P = 0.502). The four children with MLL-r positive relapse were on the GD-ALL 2008 protocol and the partner genotypes included one MLL-AF4, one MLL-PTD, and two MLL-ENL, all with solo BM relapse and high risk factors for poor prognosis such as WBC ≥50 × 10 9 /L, T-cell phenotype, or D15 MRD positive (Table 5 ).
Previous literature reported that MLL-r positive ALL can develop among children at all ages, accounting for 2.5–5% of children with initial ALL [ 2 , 3 ], and the detection rate of MLL-r positive ALL in infants younger than 1 year of age is even as high as 23.8–79% [ 9 , 10 , 11 ]. The detection rate of MLL-r positive ALL patients in this study was 4.9%, which was basically consistent with the literature. However, the detection rate in infants younger than 1 year of age was only 3.2%, which was much lower than the above mentioned literature, and the reason for this might be related to the sample size of MLL-r positive patients included in different studies.
Compared with the MLL-r negative group of patients, the clinical characteristics of MLL-r positive cases in this study included: a young age of onset, with a median age of onset of 3.1 years, and 12.9% of infant leukemia, which was much higher than that of the MLL-r negative group. In addition, a high WBC of onset, with a significantly higher proportion of initial WBC greater than 50 × 10 9 /L than that of the negative group and a high percentage of 64.5% in the high-risk group, which were consistent with previous studies. The MLL-r, as one of the subgroups of ALL, may be associated with certain immunophenotypic characteristics. Moorman et al [ 12 ] showed that MLL-r positive ALL was mostly of B-cell lineage and common B or pro-B immunophenotypes were more common, accounting for 62% of the cases. Peterson et al [ 13 ] retrospectively found that in 806 children with T-lineage ALL, 27 (3.3%) MLL-r positive cases were detected. In contrast, our findings showed that the immunophenotype of MLL-r positive children was more prevalent in common-B, immature B and T cells compared to the MLL-r negative group, which was basically consistent with Moorman and Peterson’s report. In China, it has been reported that the immunophenotype pro-B is a risk prognostic factor in MLL-r positive cases [ 14 ]. In contrast, the results of the multivariate analysis in this study showed that the T-lineage immunophenotype was an independent prognostic factor affecting MLL-r positive ALL, which was inconsistent with the above-mentioned studies and we implied that it might be related to the small number of reported cases in China (only 6 cases).
Some studies [ 10 , 11 , 14 , 15 ] reported that MLL-r positive compared to MLL-r negative children had a higher proportion of patients with poor early treatment response in addition to more risk factors at initial diagnosis. In the current study, we also found that the proportion of M1 patients on Day 15 of induction chemotherapy in MLL-r positive children with ALL was significantly lower than that in the negative group, and the proportion of MRD positive patients on Day 33 was significantly higher than that in the negative group, suggesting that the poor early treatment response in MLL-r positive patients may be due to the dominant clone of MLL-r positive leukemia cells being insensitive or resistant to chemotherapy at the time of initial diagnosis, rather than chemotherapy “selected” for the inferior clone [ 16 , 17 ]. However, after one course of standardized induction chemotherapy, the percentage of MLL-r positive patients with CR of BM could reach 96.8%, which is similar to the 97.6% CR rate reported in China [ 10 ], which might be related to the early strong chemotherapy.
In this present study, only 13 of 31 MLL-r positive ALL cases were successfully detected for BM karyotype, and 8 cases were normal karyotype, and the CCA compliance rate was only 38.5%, which may be due to the fact that most of the chromosomes in MLL-r positive children belong to normal. On the other hand, it may also be due to the small 11q23 broken fragment and the influence of the quality of the split phase and the cryptic chromosomal translocation, so there was a missed detection [ 11 ]. FISH technique can detect basically all cases with MLL-r positive and had higher resolution, stronger sensitivity and shorter cycle time, but it cannot detect MLL partner genes and MLL-PTD due to the limitation of probes. Combined with PCR method, our department can detect MLL-AF4, MLL-AF6, MLL-AF9, MLL- AF10, MLL-ELL, MLL-ENL, MLL-AF1q, MLL-AF17, and MLL-PTD, which are common MLL-associated fusion genes, thus compensating for the inadequacy of FISH methods [ 18 ]. Thus, we suggested the clinical use of a combination of the three above-mentioned assays, which will help to improve the detection rate of MLL-r positive patients.
Meyer et al. [ 1 ] analyzed 1420 MLL-r positive children with ALL, and the most common partner genes were MLL-AF4 (57%), MLL-ENL (18%), MLL-AF9 (13%), MLL-AF10 (4%), MLL-EPS15 (2%), and MLL-AF6 (2%). In China, the statistical results of Sun et al [ 19 ] on 57 cases of 11q/23 MLL-r pediatric patients with ALL showed that the MLL-AF4 accounted for 29.8% and MLL-PTD accounted for 26.3%, followed by MLL-AF9 (22.8%), MLL-ENL (12.3%) and MLL-AF10 (8.8%), respectively. In our study, MLL-PTD (29%), MLL-AF4 (25.8%), and MLL-ENL (16.1%) were predominant among the 31 MLL-r positive cases, which were consistent with that reported by Sun Yulan et al. and different from that reported by Meyer et al. We speculated that it might be due to the racial differences. Although the detection rate of MLL-r positive ALL children was inconsistent among different studies, the most common partner gene was still MLL-AF4. Some clinical reports [ 1 ] showed that MLL partner genes were the main determinants of leukemia phenotype, and MLL-AF4 was mainly associated with lymphoid malignancies, while MLL-AF9 was more likely to cause myeloid malignancies, which might may explain why MLL-AF4 was most common in ALL.
It has been reported in the literature [ 7 , 20 ] that WBC and MLL-r positive at disease onset were important factors affecting the prognosis of children with ALL, and the results of the multivariate analysis of 124 children with ALL in our study also showed that WBC ≥50 × 10 9 /L was an independent risk factor affecting the outcome of patients with ALL, which was consistent with the literature. However, our study also observed that MLL-r positive was not a prognostic factor for patients with ALL, but its partner gene MLL-AF4 was an independent risk factor for the prognosis of children with ALL, suggesting that the prognosis of our children with ALL may be closely related to the MLL-r partner gene type.
There was a consensus that the overall prognosis of MLL gene rearrangement leukemia is poor, and a large pediatric leukemia collaborative group [ 8 , 20 ] has shown that the 5-year EFS and OS of MLL-r positive children with ALL were 60–65% and 68–74%, respectively, while our K-M survival analysis showed that the EFS of MLL-r positive children were 56.01 ± 16.89% and OS were 73.32 ± 16.6%, which were significantly lower than the MLL-r negative group and basically close to those reported by the collaborative group, and both were lower than the 5-year EFS (72–80%) and OS (83–85%) levels in domestic patients with ALL, which laterally confirmed that concomitant MLL gene rearrangement positive was a more malignant type of childhood ALL. Therefore, this study further analyzed the prognostic factors affecting MLL-r positive ALL, and the results of Cox model multivariate analysis showed that T-cell phenotype, WBC ≥50 × 10 9 /L and D15 MRD positive were independent risk factors affecting MLL-r positive children, which were consistent with the findings of Tomizawa et al [ 21 ] With the increasing maturity of conventional chemotherapy, most children with MLL-r ALL were able to achieve CR with treatment, and the mainstream treatment regimen was still chemotherapy. Our survival analysis of patients with ALL who received only chemotherapy showed that the 10-year EFS and OS of MLL-r positive children who received only chemotherapy were lower than those of the negative group, indicating that although children could enter remission with conventional chemotherapy, some children still experienced relapse after remission, resulting in a significant decrease in EFS, and although the relapse rate of children with MLL-r associated leukemia could be reduced by increasing the intensity of chemotherapy, the treatment-related relapse rate was significantly lower. Although the relapse rate of children with MLL-r associated leukemia could be reduced by increasing the intensity of chemotherapy, the OS due to treatment-related mortality and infection-based complications decreased accordingly. This suggested that chemotherapy-only may be less effective in MLL-r positive cases.
The role of SCT in the treatment of MLL-r-positive leukemia has been controversial. The results showed that the 5-year EFS of 53 children treated with SCT and 47 children treated with chemotherapy alone were 48.8 and 48.7%, respectively, with no statistical difference ( P = 0.6), indicating that SCT for MLL-r-positive ALL did not show any advantage. ALL did not show any advantage [ 22 ]. In contrast, a recent study by the Japanese Pediatric Leukemia/Lymphoma Collaborative Group [ 23 ] of 43 MLL-r-positive high-risk children (age < 6 months and/or CNS leukemia) who received SCT showed a 3-year EFS and OS of 56.8 and 80.2%, respectively, demonstrating a good prognosis, suggesting that SCT has more therapeutic advantages over conventional chemotherapy. The 10-year EFS rate and OS of children who received SCT in this study were 100%, which were higher than those who received chemotherapy alone at 54.32 ± 16.89% and 72.19 ± 16.88%, respectively, and SCT seemed to be a good independent prognostic factor affecting MLL-r-positive children. However, due to the small sample size, we cannot conclude that SCT can effectively treat MLL-r positive patients.
It has been suggested that alterations in MLL-r play an important role in the activation of oncogenes, while the role of partner genes fused to them is unclear, while some studies revealed that ALL with MLL-r positive were similar in most morphological and histochemical features, and childhood ALL with MLL-r positive, regardless of the type of partner gene, had an extremely poor prognosis [ 24 ]. Previous studies have shown that MLL-AF4 fusions in ALL were associated with poorer survival [ 25 ]. A large ( n = 756), multicenter, retrospective Nordic study analyzing the prognosis of various types of MLL fusion gene leukemia showed that MLL-AF4 and MLL-AF6 had a very poor prognosis with 10-year EFS of 29 and 11% and 10-year OS of 27 and 22%, respectively, while MLL-AF9 had 10-year EFS and OS of 50 and 63%, with a relatively good prognosis [ 26 ]. Previous studies [ 10 , 11 , 19 ] had also shown that MLL-AF4 had a worse prognosis than non-MLL-AF4 partner genes, and the above findings strongly suggested that different partner genes had distinct effects on the prognosis of patients with MLL-r positive leukemia. In the present study, we found that partner genes such as MLL-AF4, MLL-PTD and MLL-ENL were more common in MLL-r positive ALL, and further comparison of EFS and OS among the three groups showed statistically significant differences, indicating that MLL-AF4 positive ALL had the worst prognosis, and the results also indicated that the MLL fusion gene type (MLL-AF4) as an independent risk prognostic factor. Inconsistent with other reports in the literature, the most reported partner gene in our study was MLL-PTD, which had a 10-year EFS and OS of 85.71 ± 22.37%, showing a good prognosis, suggesting that MLL-PTD may be an indicator of relatively good prognosis, but most of the literature has not analyzed MLL-PTD, and the limited number of cases included in this study was not enough to reveal this, and the overall prognosis of children in this group deserves our further attention. We conducted long-term follow-up of 124 children with ALL included in this study, and eventually 5 children relapsed, with a significantly higher proportion of MLL-r positive children relapsing than in the negative group, and all of them relapsed in bone marrow alone and had high-risk factors for poor prognosis such as WBC ≥ 50 × 10 9 /L, T-cell phenotype or positive D15 MRD, consistent with the literature [ 10 , 11 , 12 , 13 , 14 , 15 ].
Based on these findings, we believe that MLL-r positive should not be uniformly classified as a high-risk group in clinical practice, but that screening for these MLL partner genes is needed for accurate risk stratification at diagnosis: those positive for MLL-AF4 can be treated as a high-risk group, while other MLL partner genes need to be specifically combined with other prognostic factors (T-cell phenotype, WBC ≥ 50 × 10 9 /L and D15 MRD positive) for a comprehensive evaluation. Moreover, it seemed that the relapse time for the MLL-r positive group is longer than MLL-r negative group (9.6 vs 3.9). We implied that the reason for the late relapse of MLL-r positive children was that the combination of drug chemotherapy will not kill the MLL gene clone formed in the fetal period of ALL children. The clone will undergo secondary transformation after treatment, which will eventually lead to the late relapse of ALL [ 12 , 13 , 14 , 15 ].
In conclusion, the remission rate of MLL-r positive ALL in children was moderate, but prone to relapse with low overall survival, and poor prognosis for those treated with chemotherapy-only. The prognosis of children with MLL-r positive ALL were closely related to the type of MLL-r, and clinical attention should be paid to screening for MLL partner genes and combining them with other prognostic factors for accurate risk stratification.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due personal privacy but are available from the corresponding author on reasonable request.
Meyer C, Burmeister T, Gröger D, Tsaur G, Fechina L, Renneville A, et al. The MLL Recombinome of acute Leukemias in 2017. Leukemia. 2018;32:273–84.
Article CAS PubMed Google Scholar
Valentine MC, Linabery AM, Chasnoff S, Hughes AE, Mallaney C, Sanchez N, et al. Excess congenital non-synonymous variation in leukemia-associated genes in MLLinfant leukemia: a Children's oncology group report. Leukemia. 2014;28(6):1235–41. https://doi.org/10.1038/leu.2013.367 Epub 2013 Dec 4. PMID: 24301523; PMCID: PMC4045651.
Article CAS PubMed PubMed Central Google Scholar
Meyer C, Hofmann J, Burmeister T, Gröger D, Park TS, Emerenciano M, et al. The MLL recombinome of acute leukemias in 2013. Leukemia. 2013;27(11):2165–76. https://doi.org/10.1038/leu.2013.135 Epub 2013 Apr 30. PMID: 23628958; PMCID: PMC3826032.
Cimino G, Cenfra N, Elia L, Sica S, Luppi M, Meloni G, et al. The therapeutic response and clinical outcome of adults with ALL1(MLL)/AF4 fusion positive acute lymphoblastic leukemia according to the GIMEMA experience. Haematologica. 2010;95(5):837–40. https://doi.org/10.3324/haematol.2009.009035 Epub 2010 Jan 27. PMID: 20107154; PMCID: PMC2864392.
Smith E, Lin C, Shilatifard A. The super elongation complex (SEC) and MLL in development and disease. Genes Dev. 2011;25(7):661–72. https://doi.org/10.1101/gad.2015411 PMID: 21460034; PMCID: PMC3070929.
Sun YN, Hu YX, Gao L, Xiao PF, Lu J, Wu SY, et al. The therapeutic efficacy of pediatric ALL patients with MLL gene rearrangement treated with CCLG-ALL2008 protocol. Eur Rev Med Pharmacol Sci. 2018;22(18):6020–9. https://doi.org/10.26355/eurrev_201809_15938 PMID: 30280786.
Article PubMed Google Scholar
Qiu KY, Xu HG, Luo XQ, Mai HR, Liao N, Yang LH, et al. Prognostic value and outcome for ETV6/RUNX1-positive pediatric acute lymphoblastic leukemia: a report from the South China Children's leukemia group. Front Oncol. 2021;11:797194. https://doi.org/10.3389/fonc.2021.797194 PMID: 34988026; PMCID: PMC8722219.
Article PubMed PubMed Central Google Scholar
Li XY, Li JQ, Luo XQ, Wu XD, Sun X, Xu HG, et al. Reduced intensity of early intensification does not increase the risk of relapse in children with standard risk acute lymphoblastic leukemia - a multi-centric clinical study of GD-2008-ALL protocol. BMC Cancer. 2021;21(1):59. https://doi.org/10.1186/s12885-020-07752-x PMID: 33435902; PMCID: PMC7805214.
Jansen MW, Corral L, van der Velden VH, Panzer-Grümayer R, Schrappe M, Schrauder A, et al. Immunobiological diversity in infant acute lymphoblastic leukemia is related to the occurrence and type of MLL gene rearrangement. Leukemia. 2007;21(4):633–41. https://doi.org/10.1038/sj.leu.2404578 Epub 2007 Feb 1. PMID: 17268512.
Liu Q, Zhang N, Shao JB, et al. Clinical characteristics and prognosis of pediatric acute leukemia patients with MLL gene rearrangements. J Shanghai Jiaotong Univ (Medical Science). 2021;(7). https://doi.org/10.3969/j.issn.1674-8115.2021.07.009 .
Wang Y. Clinical analysis of newly diagnosed acute lymphoblastic leukemia with 11q23/MLL gene rearrangement. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2020;37(10):1092–6. https://doi.org/10.3760/cma.j.cn511374-20190116-00015 Chinese. PMID: 32924108.
Moorman AV, Ensor HM, Richards SM, Chilton L, Schwab C, Kinsey SE, et al. Prognostic effect of chromosomal abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: results from the UK Medical Research Council ALL97/99 randomised trial. Lancet Oncol. 2010;11(5):429–38. https://doi.org/10.1016/S1470-2045(10)70066-8 Epub 2010 Apr 19. Erratum in: Lancet Oncol.2010 Jun;11(6):516. PMID: 20409752.
Peterson JF, Baughn LB, Pearce KE, Williamson CM, Benevides Demasi JC, Olson RM, et al. Ketterling RP.KMT2A (MLL) rearrangements observed in pediatric/young adult T-lymphoblastic leukemia/lymphoma: a 10-year review from a single cytogenetic laboratory. Genes Chromosom Cancer. 2018;57(11):541–6. https://doi.org/10.1002/gcc.22666 Epub 2018 Sep 10. PMID: 30203571.
Gong XY, Wang Y, Liu BC, Wei H, Zhou CL, Lin D, et al. Clinical features and prognosis in CD10(−) pre-B acute lymphoblastic leukemia. Zhonghua Xue Ye Xue Za Zhi. 2017;38(1):17–21Chinese. https://doi.org/10.3760/cma.j.issn.0253-2727.2017.01.004 .
Sun YL, He HL, Zhao WL, et al. Clinical analysis of childhood acute lymphoblastic leukemia with MLL gene rearrangement. J China Pediatr Blood Cancer. 2013;(3). https://doi.org/10.3969/j.issn.1673-5323.2013.03.003 .
van der Linden MH, Seslija L, Schneider P, Driessen EM, Castro PG, Stumpel DJ, et al. Identification of genes transcriptionally responsive to the loss of MLL fusions in MLL-rearranged acute lymphoblastic leukemia. PLoS One. 2015;10(3):e0120326. https://doi.org/10.1371/journal.pone.0120326 PMID: 25793396; PMCID: PMC4368425.
Zoghbi A, Zur Stadt U, Winkler B, Müller I, Escherich G. Lineage switch under blinatumomab treatment of relapsed common acute lymphoblastic leukemia without MLL rearrangement. Pediatr Blood Cancer. 2017;64(11). https://doi.org/10.1002/pbc.26594 Epub 2017 Apr 28. PMID: 28453885.
Balgobind BV, Zwaan CM, Pieters R, Van den Heuvel-Eibrink MM. The heterogeneity of pediatric MLL-rearranged acute myeloid leukemia. Leukemia. 2011;25(8):1239–48. https://doi.org/10.1038/leu.2011.90 Epub 2011 May 13. PMID: 21566656.
Sun YL, He HL, Shao XJ, et al. Clinical and molecular biologic characteristics of childhood acute leukemia with mixed lineage leukemia gene rearrangement. J Appl Clin Pediatr. 2013;(3). https://doi.org/10.3760/cma.j.issn.2095-428X.2013.03.014 .
Cui L, Li ZG, Chai YH, et al. Outcome of children with newly diagnosed acute lymphoblastic leukemia treated with CCLG-ALL 2008: the first nationwide prospective multicenter study in China. Am J Hematol. 2018;93(7):913–20.
Tomizawa D, Miyamura T, Imamura T, Watanabe T, Moriya Saito A, Ogawa A, et al. A risk-stratified therapy for infants with acute lymphoblastic leukemia: a report from the JPLSG MLL-10 trial. Blood. 2020;136(16):1813–23. https://doi.org/10.1182/blood.2019004741 PMID: 32845001.
Dreyer ZE, Dinndorf PA, Camitta B, Sather H, La MK, Devidas M, et al. Analysis of the role of hematopoietic stem-cell transplantation in infants with acute lymphoblastic leukemia in first remission and MLL gene rearrangements: a report from the Children's oncology group. J Clin Oncol. 2011;29(2):214–22. https://doi.org/10.1200/JCO.2009.26.8938 Epub 2010 Dec 6. PMID: 21135279; PMCID: PMC3058277.
Takachi T, Watanabe T, Miyamura T, Moriya Saito A, Deguchi T, Hori T, et al. Hematopoietic stem cell transplantation for infants with high-risk KMT2A gene-rearranged acute lymphoblastic leukemia. Blood Adv. 2021;5(19):3891–9. https://doi.org/10.1182/bloodadvances.2020004157 PMID: 34500465; PMCID: PMC8679679.
van der Linden MH, Boer JM, Schneider P, Willekes M, Seslija L, De Lorenzo P, et al. Clinical and molecular genetic characterization of wild-type MLL infant acute lymphoblastic leukemia identifies few recurrent abnormalities. Haematologica. 2016;101(3):e95–8. https://doi.org/10.3324/haematol.2014.122119 Epub 2015 Dec 17. PMID: 26681762; PMCID: PMC4815736.
Britten O, Ragusa D, Tosi S, Kamel YM. MLL -rearranged acute leukemia with t(4;11)(q21;q23)-current treatment options. Is there a role for CAR-T cell therapy? Cells. 2019;8(11):1341. https://doi.org/10.3390/cells8111341 PMID: 31671855; PMCID: PMC6912830.
Balgobind BV, Raimondi SC, Harbott J, Zimmermann M, Alonzo TA, Auvrignon A, et al. Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: results of an international retrospective study. Blood. 2009;114(12):2489–96. https://doi.org/10.1182/blood-2009-04-215152 Epub 2009 Jun 15. PMID: 19528532; PMCID: PMC2927031.
Download references
Acknowledgements
Authors gratefully acknowledge the patients and the guardian.
This work was supported by the Guangzhou Science and Technology Program key projects (No.201803010032), Beijing New Sunshine Charity Foundation, Sun Yat-sen Scientific Research Project(No.SYSKY-2022-103-01), Bethune Medical Scientific Research Fund Project (No.SCE111DS), Guangdong Basic and Applied Basic Research Fund (No.2021A1515011809), Basic Research Project (Dengfeng Hospital) jointly funded by Universities (Institutes) in Guangzhou(No.202201020310) and Sun Yat-sen Scientific Research Sailing Project(No.YXQH202205).
Author information
Kun-yin Qiu, Dun-hua Zhou and Xiong-yu Liao contributed equally to this work.
Authors and Affiliations
Children’s Medical Center, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, 510120, People’s Republic of China
Kun-yin Qiu, Dun-hua Zhou, Xiong-yu Liao, Ke Huang, Yang Li, Hong-gui Xu, Wen-jun Weng, Lu-hong Xu & Jian-pei Fang
Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, 510120, People’s Republic of China
You can also search for this author in PubMed Google Scholar
Contributions
Kun-yin Qiu and Dun-hua Zhou wrote the draft. Xiong-yu Liao analysis the data. Ke Huang, Yang Li, Hong-gui Xu, Wen-jun Weng and Lu-hong Xu collect the data. Jian-pei Fang review the manuscript and afford the funding support. The work reported in the paper has been performed by the authors, unless clearly specified in the text. The authors read and approved the final manuscript.
Corresponding author
Correspondence to Jian-pei Fang .
Ethics declarations
Ethics approval and consent to participate.
All methods were carried out in accordance with Declaration of Helsinki.
All experimental protocols were approved by the ethics committee of Sun Yat-sen Memorial Hospital of Sun Yat-sen University.
Informed consent was obtained from the participants’ legal guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: supplementary table 1..
Interaction terms tested between MLL status and EFS.

Additional file 2: Supplementary Table 2.
Interaction terms tested between MLL status and OS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Qiu, Ky., Zhou, Dh., Liao, Xy. et al. Prognostic value and outcome for acute lymphocytic leukemia in children with MLL rearrangement: a case-control study. BMC Cancer 22 , 1257 (2022). https://doi.org/10.1186/s12885-022-10378-w
Download citation
Received : 26 June 2022
Accepted : 28 November 2022
Published : 02 December 2022
DOI : https://doi.org/10.1186/s12885-022-10378-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Acute lymphoblastic leukemia
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
Acute Lymphoblastic Leukemia (ALL)
(acute lymphocytic leukemia).
- Pathophysiology |
- Classification |
- Symptoms and Signs |
- Diagnosis |
- Treatment |
- Prognosis |
- Key Points |
- More Information |
Acute lymphoblastic leukemia (ALL) is the most common pediatric cancer; it also strikes adults of all ages. Malignant transformation and uncontrolled proliferation of an abnormally differentiated, long-lived hematopoietic progenitor cell results in a high circulating number of blasts, replacement of normal marrow by malignant cells, and the potential for leukemic infiltration of the central nervous system (CNS) and testes. Symptoms include fatigue, pallor, infection, bone pain, CNS symptoms (eg, headache), easy bruising, and bleeding. Examination of peripheral blood smear and bone marrow is usually diagnostic. Treatment typically includes combination chemotherapy to achieve remission, intrathecal and systemic chemotherapy and/or corticosteroids for CNS prophylaxis, and sometimes cerebral irradiation for intracerebral leukemic infiltration, consolidation chemotherapy with or without stem cell transplantation, and maintenance chemotherapy for up to 3 years to avoid relapse.
(See also Overview of Leukemia .)
The American Cancer Society estimates that in the United States in 2023 there will be over 6500 new cases of acute lymphoblastic leukemia (ALL) and almost 1400 deaths will have occurred. Sixty percent of all ALL cases occur in children, with a peak incidence at age 2 to 5 years; a second peak occurs after age 50. ALL is the most common cancer in children, and represents about 75% of leukemias among children ARID5B gene.
Pathophysiology of ALL
Similar to acute myeloid leukemia , acute lymphoblastic leukemia is caused by a series of acquired genetic aberrations. Malignant transformation usually occurs at the pluripotent stem cell level, although it sometimes involves a committed stem cell with more limited capacity for self-renewal. Abnormal proliferation, clonal expansion, aberrant differentiation, and diminished apoptosis (programmed cell death) lead to replacement of normal blood elements with malignant cells.
Classification of ALL
In acute lymphoblastic leukemia, the precursor lymphoid neoplasms are broadly categorized based on their lineage into
B-lymphoblastic leukemia/lymphoma (B-ALL/LBL)
T-lymphoblastic leukemia/lymphoma (T-ALL/LBL)
Disease can manifest as a leukemia when neoplastic cells (lymphoblasts) involve blood and bone marrow (defined as > 20% bone marrow blasts) or as a lymphoma when blasts infiltrate mainly extramedullary tissue.
The 2016 World Health Organization (WHO) classification of lymphoid neoplasms incorporates genetic data, clinical features, cell morphology, and immunophenotype, all of which have important implications for disease prognosis and management.
Symptoms and Signs of ALL
Symptoms and signs of acute lymphoblastic leukemia may be present for only days to weeks before diagnosis.
The most common presenting symptoms are due to disrupted hematopoiesis with ensuing
Thrombocytopenia
Granulocytopenia
Anemia can manifest with fatigue, weakness, pallor, malaise, dyspnea on exertion, tachycardia, and exertional chest pain.
Thrombocytopenia can cause mucosal bleeding, easy bruising, petechiae/purpura, epistaxis, bleeding gums, and heavy menstrual bleeding. Hematuria and gastrointestinal bleeding are uncommon. Patients can present with spontaneous hemorrhage, including intracranial or intra-abdominal hematomas.
Granulocytopenia or neutropenia can lead to a high risk of infections, including those of bacterial, fungal, and viral etiologies. Patients may present with fevers and a severe and/or recurrent infection.
Organ infiltration by leukemic cells results in enlargement of the liver, spleen, and lymph nodes. Bone marrow and periosteal infiltration may cause bone and joint pain, especially in children with ALL. CNS penetration and meningeal infiltration are common and can result in cranial nerve palsies, headache, visual or auditory symptoms, altered mental status, and transient ischemic attack/stroke.
Diagnosis of ALL
Complete blood count (CBC) and peripheral blood smear
Bone marrow examination
Histochemical studies, cytogenetics, and immunophenotyping
A diagnosis of acute lymphoblastic leukemia is made when blast cells of lymphoid origin are ≥ 20% of marrow nucleated cells or ≥ 20% of non-erythroid cells when the erythroid component is > 50%. If marrow cells are insufficient or unavailable, diagnosis can be made by the same criteria using a peripheral blood sample.
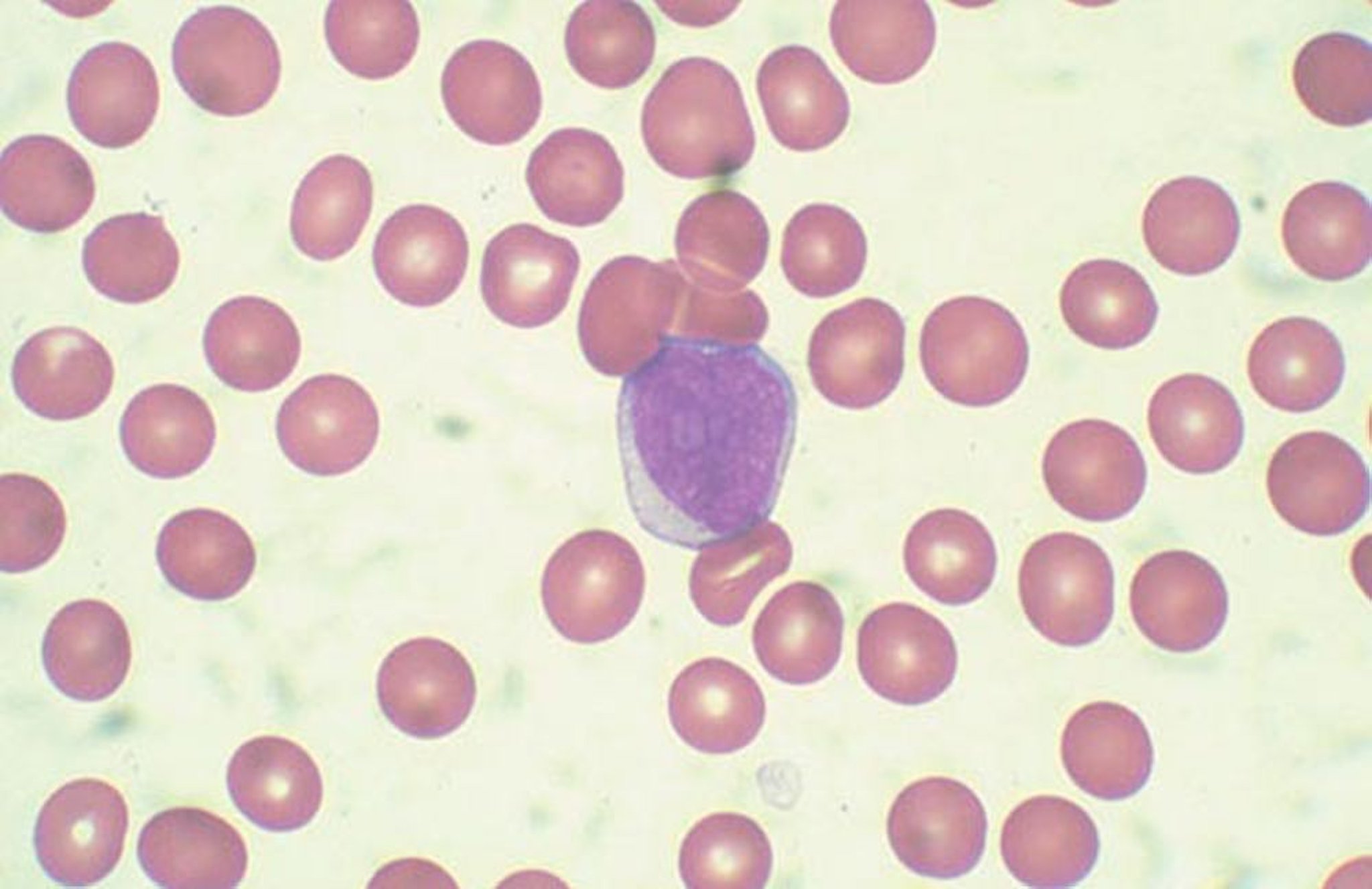
By permission of the publisher. From Chang K, Forman S. In Atlas of Clinical Hematology . Edited by JO Armitage. Philadelphia, Current Medicine, 2004.
CBC and peripheral smear are the first tests done; pancytopenia and peripheral blasts suggest acute leukemia. Blast cells in the peripheral smear may approach 90% of the white blood cell (WBC) count. Aplastic anemia , viral infections such as infectious mononucleosis , and vitamin B12 deficiency , and folate deficiency should be considered in the differential diagnosis of severe pancytopenia. Unlike in AML, Auer rods (linear azurophilic inclusions in the cytoplasm of blast cells) are never present in acute lymphoblastic leukemia.
Bone marrow examination (aspiration and needle biopsy) is routinely done. Blast cells in the bone marrow are typically between 25 and 95% in patients with ALL.
Histochemical studies, cytogenetics, and immunophenotyping studies help distinguish the blasts of ALL from those of AML or other disease processes. Histochemical studies include staining for terminal deoxynucleotidyl transferase (TdT), which is positive in cells of lymphoid origin. Detection of specific immunophenotypic markers such as CD3 (for lymphoid cells of T cell origin) and CD19, CD20, and CD22 (for lymphoid cells of B cell origin) is essential in classifying the acute leukemias. Common cytogenetic abnormalities in ALL include t(9;22) in adults and t(12;21) and high hyperdiploidy in children (see table Common Cytogenetic Abnormalities in ALL ).
Less common cytogenetic abnormalities include the following:
t(v;11q23) / MLL or KMT2A rearranged, including t(4;11)/ KMT2A-AF4
t(1;19)/ E2A-PBX1 ( TCF3-PBX1 )
t(5;14)/ IL3-IGH
t(8;14), t(8;22), t(2;8)/ C-MYC rearranged
BCR-ABL -like acute lymphoblastic leukemia overlaps phenotypically with ALL in which the Philadelphia chromosome [a reciprocal balanced translocation between chromosomes 9 and 22, t(9;22)] is present (Ph+ ALL).
Other laboratory findings may include hyperuricemia, hyperphosphatemia , hyperkalemia , hypocalcemia , and elevated lactate dehydrogenase (LDH), which indicate a tumor lysis syndrome . Elevated serum levels of hepatic transaminases or creatinine, and hypoglycemia may also be present. Patients with Ph+ ALL and patients with t( v ;11q23) involving MLL rearrangements often present with hyperleukocytosis.
CT of the head is done in patients with CNS symptoms. CT of the chest and abdomen should be done to detect mediastinal masses and lymphadenopathy and may also detect hepatosplenomegaly. Echocardiography or multi-gated acquisition (MUGA) scanning is typically done to assess baseline cardiac function (prior to administration of anthracyclines, which are cardiotoxic).
Treatment of ALL
Systemic chemotherapy
Prophylactic CNS chemotherapy and sometimes CNS radiation
For Ph+ ALL, also a tyrosine kinase inhibitor
Supportive care
Sometimes immunotherapy , targeted therapy , stem cell transplantation , and/or radiation therapy
Treatment for newly diagnosed acute lymphoblastic leukemia generally consists of 3 to 4 cycles of chemotherapy blocks of non–cross-resistant chemotherapy for the first 9 to 12 months, followed by 2.5 to 3 years of maintenance chemotherapy.
Chemotherapy
The 4 general phases of chemotherapy for acute lymphoblastic leukemia include
Remission induction
Postremission consolidation
Interim maintenance and intensification
Maintenance
The goal of induction treatment is complete remission, defined as 1000/mcL (> 1 × 10 9 /L), a platelet count > 100,000/mcL (> 100 × 10 9 /L), and no need for blood transfusion. In patients with complete remission, a low measurable residual disease (also known as minimal residual disease or MRD) is the most important prognostic factor ( 1 ). Measurable or minimal residual disease is microscopic disease that is not detected by standard assays but can be measured by more sensitive assays. A low measurable residual disease (MRD negativity) is defined variably (based on the assay used) as
Components of induction therapy include
The goal of consolidation is to prevent leukemic regrowth. Consolidation therapy usually lasts a few months and combines regimen-specific courses of non–cross-resistant drugs that have different mechanisms of action. For adults with Ph+ ALL, allogeneic stem cell transplantation is recommended as consolidation therapy.
Interim maintenance and late/delayed intensification therapy are used after consolidation therapy. These phases of therapy incorporate a variety of chemotherapeutic agents with different doses and schedules that are less intense than induction and consolidation.
Most regimens include maintenance therapy
CNS prophylaxis
Medically frail patients with ALL
About one third of patients with acute lymphoblastic leukemia are older adults (> 65). Older ALL patients are more likely to have precursor B-cell ALL and have higher risk and more complex cytogenetics, including Philadelphia chromosome positive (Ph+) or t( v ;11q23) MLL or KMT2A rearranged disease.
hematopoietic stem cell transplantation is an option.
Targeted immunotherapy drugs that are available for treatment of relapsed or refractory ALL are increasingly used for treatment of older patients with ALL in clinical trials or clinical practice.
Older patients with ALL probably tolerate asparaginase more poorly than younger patients do.
Relapsed or refractory ALL
Leukemic cells may reappear in the bone marrow, CNS, testes, or other sites. Bone marrow relapse is particularly ominous. Although a new round of chemotherapy may induce a second remission in the majority of children and about one third of adults, subsequent remissions tend to be brief. Chemotherapy helps only a few patients with early bone marrow relapse to achieve long disease-free second remissions or cure.
Chimeric antigen receptor T (CAR-T) cells, engineered and generated from the patient's T cells, induce remission in patients with relapsed ALL with remarkable efficacy, albeit with significant toxicity ( 2 ).
Available immunotherapies for relapsed or refractory ALL include
a biospecific CD19-directed CD3 T-cell engager, prolongs overall survival for children and adults with relapsed or refractory B-cell precursor ALL, whether Ph+ or Ph-. Life-threatening toxicities may include cytokine release syndrome 3 ).
4 ). Inotuzumab may cause hepatotoxicity, including fatal and life-threatening veno-occlusive disease and is associated with higher post-transplant non-relapse mortality.
a CD19-directed genetically modified autologous T-cell immunotherapy, is available for the treatment of patients up to 25 years of age with B-cell precursor ALL that is refractory or in a 2nd or later relapse. Life-threatening toxicities may include cytokine release syndrome and neurologic toxicities ( 5 ).
Brexucabtagene autoleucel , a CD19-directed genetically modified autologous T cell immunotherapy, can be used to treat adult patients with relapsed or refractory B-cell precursor ALL. Complications, including cytokine release syndrome and neurologic toxicities, may be life threatening.
Other agents have been available, but clinically meaningful outcomes have not been convincingly demonstrated (ie, the approvals were based upon response rate but there were no trials verifying an improvement in disease-related symptoms or increased survival) for these. Examples include:
Stem cell transplantation following reinduction chemotherapy or immunotherapy offers the greatest hope of long-term remission or cure if an HLA-matched sibling is available. Cells from other relatives or from matched, unrelated donors are sometimes used. Transplantation is rarely used for patients > 65 years because it is much less likely to be successful and because adverse effects are much more likely to be fatal.
CNS relapse
Testicular relapse may be evidenced clinically by painless firm swelling of a testis or may be identified on biopsy. If unilateral testicular involvement is clinically evident, the apparently uninvolved testis should undergo biopsy. Treatment is radiation therapy of the involved testis and administration of systemic reinduction therapy.
Supportive care is similar in the acute leukemias and may include
Transfusions
Antimicrobials
Hydration and urine alkalinization
Psychologic support
Transfusions of red blood cells and sometimes platelets are administered as needed to patients with bleeding or anemia. Prophylactic platelet transfusion is done when platelets fall to < 10,000/mcL ( 9 /L). Anemia (hemoglobin < 7 or 8 g/dL [
Antimicrobials are often needed for prophylaxis and treatment because patients are immunosuppressed; in such patients, infections can progress quickly with little clinical prodrome. After appropriate studies and cultures have been done, febrile patients with neutrophil counts < 500/mcL ( < 0.5 × 10 9 Pneumocystis jirovecii infection or a viral infection should be suspected and confirmed by bronchoscopy and bronchoalveolar lavage and treated appropriately.
Aspergillus and Candida P. jirovecii
Hydration, tumor lysis syndrome G6PD deficiency
Psychologic support may help patients and their families with the shock of illness and the rigors of treatment for a potentially life-threatening condition.
Treatment references
1. Berry DA, Zhou S, Higley H, et al : Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: A meta-analysis. JAMA Oncol 3(7): e170580, 2017. doi:10.1001/jamaoncol.2017.0580
2. Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al : T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385(9967) :517–528, 2015.
3. Kantarjian H, Stein A, Gökbuget N, et al : Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med 376(9):836–847, 2017.
4. Kantarjian HM, DeAngelo DJ, Stelljes M, et al : Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med 375(8):740–753, 2016.
5. Maude SL, Laetsch TW, Buechner J, et al : Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378(5):439–448, 2018.
Prognosis for ALL
Prognostic factors help determine treatment protocol and intensity.
Favorable prognostic factors are
Age 3 to 9 years
WBC count < 25,000/mcL ( 9 /L) or 9 /L) in children
Leukemic cell karyotype with high hyperdiploidy (51 to 65 chromosomes), t(1;19), and t(12;21)
No CNS disease at diagnosis
Unfavorable factors include
Leukemic cell karyotype with 23 chromosomes (haploidy), with
Leukemic cell karyotype with t( v ;11q23) MLL ( KMT2A ) rearranged, including t(4;11)/ KMT2A-AF4
Leukemic cell karyotype t(5;14)/ IL3-IG
Leukemic cell karyotype t(8;14), t(8;22), t(2;8) C-MYC rearranged
Presence of the Philadelphia (Ph) chromosome t(9;22) BCR-ABL1
Increased age in adults
BCR/ABL1 -like molecular signature
Regardless of prognostic factors, the likelihood of initial remission is ≥ 95% in children and 70 to 90% in adults. Of children, > 80% have continuous disease-free survival for 5 years and appear to be cured. Of adults,
Less ability to tolerate intensive chemotherapy
More frequent and severe comorbidities
Higher risk ALL genetics that confer chemotherapy resistance
Poorer adherence to ALL treatment regimens, which include frequent (often daily or weekly) out-patient chemotherapy and doctor visits
Less frequent use of pediatric-inspired treatment regimens
Most investigatory protocols select patients with poor prognostic factors for more intense therapy because the increased risk of and toxicity from treatment are outweighed by the greater risk of treatment failure leading to death.
Acute lymphoblastic leukemia (ALL) is the most common cancer in children but also occurs in adults.
Central nervous system (CNS) involvement is common; most patients receive intrathecal chemotherapy and corticosteroids and sometimes CNS radiation therapy.
Response to treatment is good in children, with cure possible in > 80% of children but in
Repeat induction chemotherapy, immunotherapy, and stem cell transplantation may be helpful for relapse.
More Information
The following English-language resource may be useful. Please note that THE MANUAL is not responsible for the content of this resource.
Leukemia and Lymphoma Society: Resources for Healthcare Professionals : Provides information on education programs and conferences and resources for referrals to specialty care

- Cookie Preferences

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
CASE REPORT article
A case report of secondary b-cell acute lymphoblastic leukemia treated with a combination of flt3 inhibitor and decitabine.

- 1 Department of Hematology, The First Affiliated Hospital of Yangtze University, Jingzhou, Hubei, China
- 2 Department of Endocrinology, The First Affiliated Hospital of Yangtze University, Jingzhou, Hubei, China
- 3 Department of Hubei Provincial Clinical Research Center for Personalized Diagnosis and Treatment of Cancer, The First Affiliated Hospital of Yangtze University, Jingzhou, Hubei, China
Secondary acute lymphoblastic leukemia (s-ALL) refers to acute lymphoblastic leukemia that occurs after a previous malignant tumor, including therapy-related acute lymphoblastic leukemia (t-ALL) and prior malignant tumor acute lymphoblastic leukemia (pm-ALL). We report a case of a 51-year-old female patient who developed acute lymphoblastic leukemia 14 years after being diagnosed with diffuse large B-cell lymphoma (DLBCL). The patient was unresponsive to conventional chemotherapy for acute lymphoblastic leukemia (ALL) and achieved remission with a combination of sorafenib and decitabine based on the molecular biology characteristics of her B-ALL.
1 Introduction
The therapy related acute lymphoblastic leukemia (t-ALL) is defined as malignant tumor patients who have been exposed to radiation and/or chemotherapy in the past ( 1 ). Due to the rarity of the disease, our understanding of t-ALL is limited, but some current studies suggest that t-ALL is a unique entity with poor genetic characteristics and clinical outcomes ( 2 , 3 ). There is currently no standard treatment for t-ALL, and the response rate to traditional therapies is low with poor survival outcomes. The application of molecular research and next-generation sequencing technologies to identify genetic characteristics may be helpful in selecting treatment plans, but long-term survival still depends on allogeneic hematopoietic stem cell transplantation.
2 Case presentation
The patient is a 51-year-old female who initially presented with an upper abdominal mass in 2009. A surgical resection of the mass was performed and subsequent pathological examination revealed a diagnosis of diffuse large B-cell lymphoma (DLBCL), stage IA, with an International Prognostic Index (IPI) score of 1 point, placing her in the low-risk group. Following six cycles of cyclophosphamide, pirarubicin, vindesine, and dexamethasone (CHOP) chemotherapy, she achieved complete remission. In June 2013, a relapse of DLBCL in the breast occurred. The patient underwent an additional 6 cycles of CHOP chemotherapy and received intermittent intrathecal injections of methotrexate, cytarabine, and dexamethasone to prevent central nervous system infiltration of lymphoma. Once again, the patient achieved complete remission.
In August 2019, the patient was diagnosed with central nervous system lymphoma. Further diagnostic procedures, including marrow aspiration cytology and flow cytometry, confirmed the diagnosis as DLBCL stage IV, with an IPI scored of 2, placing the patient in the medium-low risk group. The treatment plan involved craniospinal irradiation, which resulted in a significant reduction of intracranial lesions as observed in a follow-up MRI conducted one month later. Subsequently, from January 2020 onwards, the patient underwent 7 cycles of a combination of Rituximab and lenalidomide (R2), followed by maintenance therapy involving lenalidomide.
In April 2023, the patient’s blood cell count revealed a total white blood cell count of 21.59x10^9/L, platelet count of 70x10^9/L, hemoglobin of 108.00g/L, lymphocyte percentage of 44.40%, monocyte percentage of 52.30%, and neutrophil count of 0.64x10^9/L. Notably, bone marrow cytology analysis demonstrated that abnormal lymphocytes comprised 94% of nucleated cells, suggesting a new diagnosis of acute lymphoblastic leukemia ( Figure 1 ). Flow cytometry analysis also confirmed the presence of abnormal lymphocytes expressing HLA-DR, CD19, CD33, CD34, CD38, CD58, CD123, cCD79a, and TdT ( Figure 2 ), indicating a diagnosis of acute B lymphoblastic leukemia (pro-B-ALL possible). BCR::ABL1 p190 was found in the screening of 56 common fusion genes, with a quantification of 0.03%. Chromosomal karyotype analysis showed a normal female karyotype, 46 XX [20]. The Next-generation sequencing (NGS) detected four class I mutations: FLT3 (p.Y572C and p.I836delI variants, with mutation allele frequencies (MAF) of 21.28% and 5.18% respectively), BCORL1 (p.D1495Efs7 and p.R1090X variants, with MAFs of 22.63% and 0.78% respectively), XBP1 (p.R168Sfs196 variant, with a MAF of 44.56%), and DNMT3A (c.1015-3_1015-2delinsAG variant, with a MAF of 42.88%). After being hospitalized, the patient received induction chemotherapy consisting of dexamethasone 10mg + vindesine 4mg, with the addition of imatinib treatment. One week later, a review of bone marrow cytology revealed that primitive and immature lymphocytes still accounted for 70.5% of the total. The treatment plan was adjusted to include a combination of decitabine 20mg/m 2 on days 1-5, sorafenib 0.4g twice daily, and dasatinib 70mg once daily. One week after completing this chemotherapy regimen, a follow-up bone marrow aspiration cytology showed that the proportion of primitive cells had decreased to 0.5%. Minimal residual disease (MRD) detection indicated approximately 0.4% of abnormal B lymphocytes. In May 2023, the patient’s BCR::ABL1 fusion gene and MRD were negative, at which point we discontinued the use of TKIS. Up to now, the detection of BCR::ABL1 emains negative. During the course of treatment with decitabine and sorafenib, the patient experienced transient leukopenia and localized erythema and edema of the fingers. Currently, the patient has completed 8 cycles of decitabine and sorafenib, and remains in complete remission.
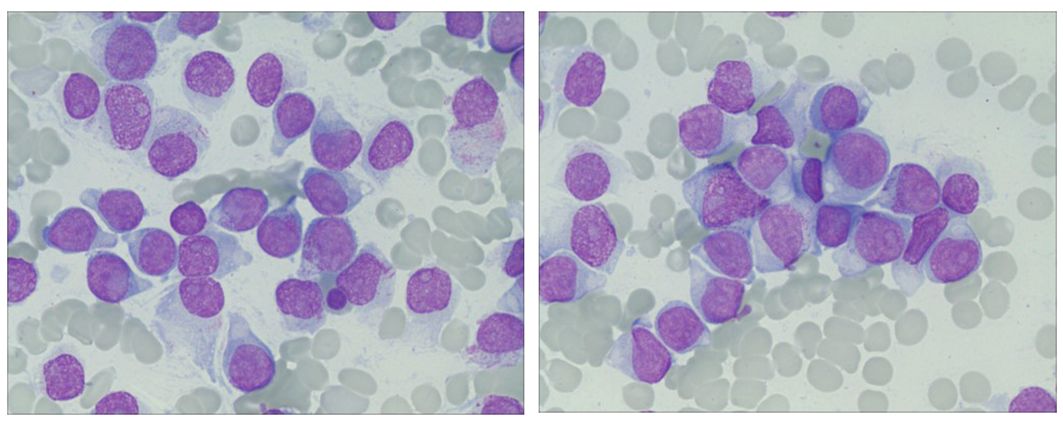
Figure 1 Ring sideroblasts in bone marrow. The bone marrow aspirate from the patient showed abnormal lymphocytes occupying 94% of the nucleated cells.
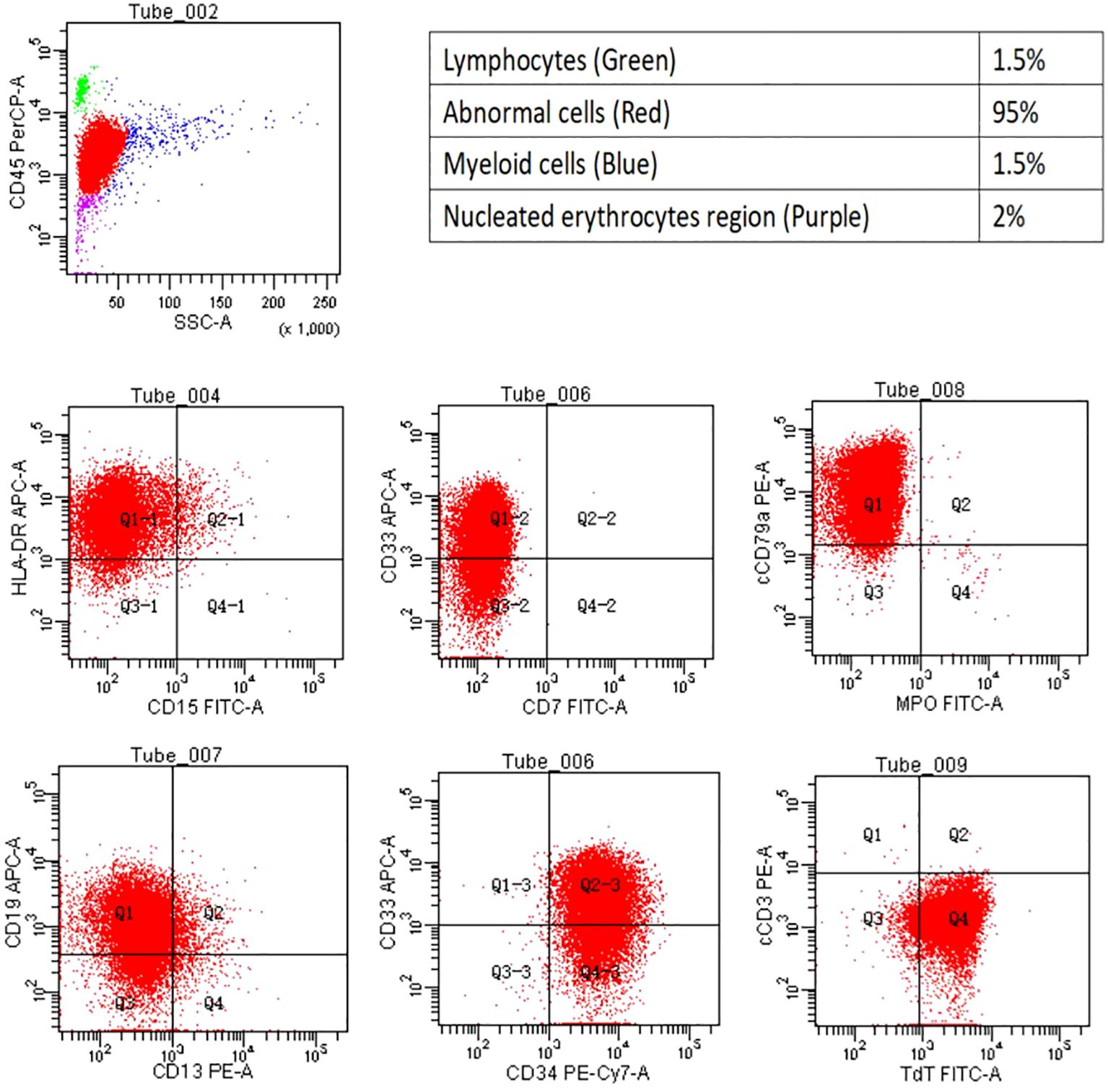
Figure 2 Flow cytometry. The flow cytometry analysis revealed the cell population proportion of nucleated cells and their positivity for HLA- DR, CD19, CD33, CD34, cCD79a, and TDT.
3 Discussion
The incidence of t-ALL accounts for 3%-9% of all cases of ALL ( 4 ). It is further categorized into alkylating agent/radiotherapy-related leukemia and leukemia that occurs after treatment with DNA topoisomerase II inhibitors ( 5 ). Similar to treatment-related acute myeloid leukemia (t-AML) or treatment-related myelodysplastic syndrome (t-MDS), the pathogenesis of t-ALL is also attribute to the genotoxic effects of cytotoxic therapy on hematopoietic stem cells. However, the exact mechanism underlying t-ALL development is not as clear as that of treatment-related myeloid tumors ( 6 ). The median latency period for t-ALL diagnosis is approximately 6.8 years. It is commonly associated with advanced age, female gender, a previous history of breast neoplasms, and central nervous system tumors ( 2 , 3 , 7 ). Studies have reported that maintenance therapy involving lenalidomide and thalidomide may contribute to the development of t-ALL ( 7 – 9 ). The mechanism may involve lenalidomide inducing the proteasomal degradation of the transcription factor Ikaros encoded by IKZF1, while mutations or deletions in IKZF1 have been shown to play a driving role in the development of ALL ( 4 ). The median time for lenalidomide-induced t-ALL onset is approximately 32.5 months ( 10 ).
Compared to newly diagnosed ALL (dn ALL), the occurrence of high-risk cytogenetic and molecular features is higher in t-ALL. These features include BCR::ABL1 positivity, MLL rearrangements, and hyperdiploid ( 3 , 7 , 11 , 12 ). Several studies have also indicated that the incidence of BCR::ABL1 positivity is similar between these two types of leukemia ( 3 , 13 ). The most commonly mutated genes in dn ALL were IKZF1 (37%), CDKN2A (14%), SETD2 (13%), and CDKN2B (11%). On the other hand, TP53 (38%) and RB1 (25%) were the most frequently mutated genes in t-ALL ( 3 ). One study has reported that that most t-ALL patients had mutations more commonly found in myeloid malignancies, such as DNMT3A, RUNX1, and ASXL1. Some patients also had ALL-type mutations, like CDKN2A and IKZF1, or mutations in other cancer susceptibility genes, such as BRCA2 ( 2 ).
In this case, the patient is a middle-aged woman who was initially diagnosed with DLBCL. The patient had undergone radiation therapy and multiple rounds of chemotherapy, she also had a history of breast and central nervous system lymphoma and she had been exposed to lenalidomide for 27 months, all of which are consistent with the characteristics of t-ALL. Moreover, the second-generation sequencing did not reveal mutations in commonly observed genes in DLBCL, and flow cytometry analysis of lymphocytes revealed an immunophenotype consistent with pro-B-cell ALL. Therefore, it is considered that the patient has developed treatment-related lymphocytic leukemia. Due to the prolonged duration and complex treatment process of this patient, it is difficult to ascertain whether any single drug or radiotherapy-induced secondary lymphocytic leukemia during treatment. In this particular case, four Class I mutations detected (FLT3, DNMT3A, XBP1, BCORL1) are not commonly observed in patients with ALL. The incidence of FLT3 gene mutation in adult and pediatric B-ALL patients is 12.3% and 2%, respectively ( 14 ). This mutation is associated with a poor prognosis, however, patients with this mutation have shown potential responses to tyrosine kinase inhibitors ( 15 ). Based on this premise, the FLT3 inhibitor sorafenib was chosen for the treatment regimen of the patient. DNMT3A mutations are known to be associated with a poor prognosis in acute myeloid leukemia (AML) and T-lymphoblastic leukemia (T-ALL), but the expression level and prognostic significance of DNMT3A in B-ALL remain unclear ( 16 ). DNMT3A is one of the commonly mutated genes in Clonal Hematopoiesis of Indeterminate Potential (CHIP) ( 17 ). It may promote irregular cell proliferation, increasing the risk of hematological ( 17 , 18 ). Previous data from AML indicate that DNMT3A gene mutations increase sensitivity to hypomethylating agents, which provides a rationale for utilizing decitabine in this patient.
Previous reports indicate that patients with t-ALL can achieve a similar remission rate to dn ALL after conventional chemotherapy ( 1 , 7 , 11 ). However, t-ALL patients exhibit a significantly lower overall survival (OS) rate, with median OS ranging from 20 weeks to 13.6 months based on various studies ( 12 ). The presence of s-ALL is considered to be an unfavorable independent prognostic factor, potentially due to its adverse cytogenetic and molecular characteristics ( 12 , 19 , 20 ). Other factors contributing to poorer OS in t-ALL patients include advanced age at diagnosis and prior exposure to radiation therapy or chemotherapy, which may potentially induce mutations associated with secondary malignancies ( 21 – 24 ). Currently, there is limited research on post-conventional chemotherapy failure treatment. According to the NCCN guidelines for Acute Lymphoblastic Leukemia, chemotherapy is not the first-line treatment for refractory/relapsed Ph-negative B-ALL. The preferred treatment options recommended by the guidelines include blinatumomab, inotuzumab ozogamicin, tisagenlecleucel, and brexucabtagene autoleucel. Other recommended regimens include inotuzumab ozogamicin + mini-hyper-CVD ± blinatumomab (cyclophosphamide, dexamethasone, vincristine, alternating with methotrexate, cytarabine), clofarabine alone or in combination, MOpAD regimen, fludarabine-based regimens, cytarabine-containing regimens, and alkylator combination regimens, and more ( 25 ). Chimeric antigen receptor-engineered T (CAR-T) cell therapy has achieved a complete remission rate of 60%-90% in adult and pediatric relapsed/refractory ALL ( 26 ). But these only achieve short-term remission. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) shows promise in improving long-term outcomes for t-ALL patients ( 1 , 13 ). For those undergoing allo-HSCT, factors such as exposure to certain agents or genetic anomalies do not exhibit an adverse correlation with relapse-free survival (RFS) or OS. This emphasizes the potential of allo-HSCT in mitigating the negative impact associated with these factors ( 1 ). For this case, Blinatumomab and CAR-T cell therapy may present as feasible therapeutic strategies. However, she has declined both treatment options. Therefore, in response to the aforementioned gene mutations and BCR::ABL1 fusion gene positivity, second-generation ABL kinase inhibitor dasatinib, FLT3 inhibitor sorafenib, and demethylating agent decitabine were selected. Following one cycle of treatment, the patient achieved complete remission. It is noteworthy that the quantification of this patient’s BCR::ABL1 fusion gene was exceptionally low, which we believe could be attributed to it being a subclone. Following treatment and disease remission, this subclone was eliminated.
4 Conclusions
In summary, t-ALL is a relatively rare type of leukemia. At present, there is no standard treatment regimen, and the efficacy of conventional chemotherapy regimens is not satisfactory. Personalized treatment plans based on patient-specific gene mutations and immunological targets have shown some promise in achieving certain levels of effectiveness. However, the remission period tends to be short, and the long-term survival rate remains low. Allo-HSCT may be a viable treatment option. Further research is still required to explore the potential relationship between t-ALL and specific chemotherapy or radiotherapy regimens, as well as the genetic and molecular characteristics of t-ALL, in order to develop more appropriate and effective treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of the First People’s Hospital of Jingzhou. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MH: Writing – original draft. WL: Writing – original draft. PL: Writing – original draft. JT: Writing – review & editing. YW: Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Grant from the Doctoral Research Initiation Fund Project of the First People's Hospital of Jingzhou (Grant No. 2023DIF02).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
DLBCL, diffuse large B-cell lymphoma; ALL, acute lymphoblastic leukemia.
1. Riazat-Kesh Y, Mascarenhas J, Bar-Natan M. ‘Secondary’ Acute lymphoblastic/lymphocytic leukemia - done playing second fiddle? Blood Rev . (2023) 60:101070. doi: 10.1016/j.blre.2023.101070
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Saygin C, Kishtagari A, Cassaday RD, Reizine N, Yurkiewicz I, Liedtke M, et al. Therapy-related acute lymphoblastic leukemia is a distinct entity with adverse genetic features and clinical outcomes. Blood Adv . (2019) 3:4228–37. doi: 10.1182/bloodadvances.2019000925
3. Kook HW, Kim JJ, Park MR, Jang JE, Min YH, Lee ST, et al. Therapy-related acute lymphoblastic leukaemia has a unique genetic profile compared to de novo acute lymphoblastic leukaemia. J Cancer . (2022) 13:3326–32. doi: 10.7150/jca.76719
4. Parrondo RD, Rahman ZA, Heckman MG, Wieczorek M, Jiang L, Alkhateeb HB, et al. Unique characteristics and outcomes of therapy-related acute lymphoblastic leukemia following treatment for multiple myeloma. Blood Cancer J . (2022) 12:87. doi: 10.1038/s41408-022-00680-y
5. Douet-Guilbert N, Eveillard JR, Meyer C, Ugo V, Le Bris MJ, Basinko A, et al. Mll partner genes in secondary acute lymphoblastic leukemia: report of a new partner prrc1 and review of the literature. Leukemia Res . (2014) 38:1316–9. doi: 10.1016/j.leukres.2014.08.011
CrossRef Full Text | Google Scholar
6. Ribera JM. Therapy-related acute lymphoblastic leukemia. Haematologica . (2018) 103:1581–3. doi: 10.3324/haematol.2018.200311
7. Khan DSR, Tariq DM, Fayyaz DSM, Soomar SM, Moosajee DM. Lenalidomide induced secondary acute lymphoblastic leukemia in a multiple myeloma patient: A case-report. Leukemia Res Rep . (2022) 17:100315. doi: 10.1016/j.lrr.2022.100315
8. Liu J, Huang B, Gu J, Li J. Clinical characteristics and prognosis of secondary acute lymphoblastic leukemia in patients with multiple myeloma during long-term thalidomide maintenance. J Personalized Med . (2023) 13. doi: 10.3390/jpm13030412
9. Germans SK, Kulak O, Koduru P, Oliver D, Gagan J, Patel P, et al. Lenalidomide-associated secondary B-lymphoblastic leukemia/lymphoma-a unique entity. Am J Clin Pathol . (2020) 154:816–27. doi: 10.1093/ajcp/aqaa109
10. Kallen ME, Koka R, Singh ZN, Ning Y, Kocoglu MH, Badros AZ, et al. Therapy-related B-lymphoblastic leukemia after multiple myeloma. Leukemia Res Rep . (2022) 18:100358. doi: 10.1016/j.lrr.2022.100358
11. Kelleher N, Gallardo D, Gonzalez-Campos J, Hernandez-Rivas JM, Montesinos P, Sarra J, et al. Incidence, clinical and biological characteristics and outcome of secondary acute lymphoblastic leukemia after solid organ or hematologic Malignancy. Leukemia Lymphoma . (2016) 57:86–91. doi: 10.3109/10428194.2015.1040013
12. Giri S, Chi M, Johnson B, McCormick D, Jamy O, Bhatt VR, et al. Secondary acute lymphoblastic leukemia is an independent predictor of poor prognosis. Leukemia Res . (2015) 39:1342–6. doi: 10.1016/j.leukres.2015.09.011
13. Aldoss I, Douer D, Pullarkat V. Therapy-related acute lymphoblastic leukemia: where do we stand with regards to its definition and characterization? Blood Rev . (2019) 37:100584. doi: 10.1016/j.blre.2019.06.001
14. Messina M, Chiaretti S, Wang J, Fedullo AL, Peragine N, Gianfelici V, et al. Prognostic and therapeutic role of targetable lesions in B-lineage acute lymphoblastic leukemia without recurrent fusion genes. Oncotarget . (2016) 7:13886–901. doi: 10.18632/oncotarget.v7i12
15. Aladily TN, Qiqieh JF, Alshorman A, Alhyari S, Khader M. Pax5 and tdt-negative B-acute lymphoblastic leukemia with unusual genetic mutations: A case report. Avicenna J Med . (2022) 12:186–90. doi: 10.1055/s-0042-1758387
16. Li W, Liu S, Wang C, Cui L, Zhao X, Liu W, et al. Dnmt3a low-expression is correlated to poor prognosis in childhood B-all and confers resistance to daunorubicin on leukemic cells. BMC Cancer . (2023) 23:255. doi: 10.1186/s12885-023-10724-6
17. Cen YX, Li Y. [Clinical characteristics and prognostic significance of bcor/bcorl1 gene mutation in patients with myelodysplastic syndromes]. Zhongguo Shi Yan Xue Ye Xue Za Zhi . (2020) 28:2004–10. doi: 10.19746/j.cnki.issn.1009-2137.2020.06.034
18. Chaudry SF, Chevassut TJ. Epigenetic guardian: A review of the DNA methyltransferase dnmt3a in acute myeloid leukaemia and clonal haematopoiesis. BioMed Res Int . (2017) 2017:5473197. doi: 10.1155/2017/5473197
19. Chen W, Wang E, Lu Y, Gaal KK, Huang Q. Therapy-related acute lymphoblastic leukemia without 11q23 abnormality: report of six cases and a literature review. Am J Clin Pathol . (2010) 133:75–82. doi: 10.1309/AJCPYWC6AQC7BAVJ
20. Abdulwahab A, Sykes J, Kamel-Reid S, Chang H, Brandwein JM. Therapy-related acute lymphoblastic leukemia is more frequent than previously recognized and has a poor prognosis. Cancer . (2012) 118:3962–7. doi: 10.1002/cncr.26735
21. Annino L, Vegna ML, Camera A, Specchia G, Visani G, Fioritoni G, et al. Treatment of adult acute lymphoblastic leukemia (All): long-term follow-up of the gimema all 0288 randomized study. Blood . (2002) 99:863–71. doi: 10.1182/blood.V99.3.863
22. Swaika A, Ailawadhi S, Yang D, Finn LE, Chanan-Khan A, Foran JM. Secondary acute lymphoblastic leukemia after primary solid organ Malignancy: A seer analysis of incidence and outcomes. Blood . (2014) 124:935–. doi: 10.1182/blood.V124.21.935.935
23. Pagano L, Pulsoni A, Tosti ME, Annino L, Mele A, Camera A, et al. Acute lymphoblastic leukaemia occurring as second Malignancy: report of the gimema archive of adult acute leukaemia. Gruppo italiano malattie ematologiche Maligne dell’adulto. Br J Haematol . (1999) 106:1037–40. doi: 10.1046/j.1365-2141.1999.01636.x
24. Bloomfield CD, Archer KJ, Mrozek K, Lillington DM, Kaneko Y, Head DR, et al. 11q23 balanced chromosome aberrations in treatment-related myelodysplastic syndromes and acute leukemia: report from an international workshop. Genes Chromosomes Cancer . (2002) 33:362–78. doi: 10.1002/gcc.10046
25. Brown PA, Shah B, Advani A, Aoun P, Boyer MW, Burke PW, et al. Acute lymphoblastic leukemia, version 2.2021, nccn clinical practice guidelines in oncology. J Natl Compr Cancer Network . (2021) 19:1079–109. doi: 10.6004/jnccn.2021.0042
26. Sheth VS, Gauthier J. Taming the beast: crs and icans after car T-cell therapy for all. Bone Marrow Transplantation . (2021) 56:552–66. doi: 10.1038/s41409-020-01134-4
Keywords: diffuse large B-cell lymphoma, acute lymphoblastic leukemia, secondary acute lymphoblastic leukemia, FLT3, myelodysplastic syndrome
Citation: Hu M, Li W, Li P, Tan J and Wang Y (2024) A case report of secondary B-cell acute lymphoblastic leukemia treated with a combination of FLT3 inhibitor and decitabine. Front. Oncol. 14:1329279. doi: 10.3389/fonc.2024.1329279
Received: 28 October 2023; Accepted: 15 April 2024; Published: 26 April 2024.
Reviewed by:
Copyright © 2024 Hu, Li, Li, Tan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Tan, [email protected] ; Ya Wang, [email protected]
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Campus Directory
- Current Students
- Faculty & Staff

Acute Lymphocytic Leukemia - Case Summary
2. Noah's symptoms at the time of his return visit to his pediatrician were not alarming. He was very tired; his mother assumed this was due to a recent Strep infection. He did have some bleeding and bone pain. Symptoms in ALL come on abruptly and include fatigue (caused by anemia), bleeding (resulting from thrombocytopenia), and fever due to infection. Bone pain is often present due to the infiltration of leukemic cells. If the cerebral or spinal meninges have been infiltrated, the patient may exhibit neurological symptoms including a headache, vomiting, and blurred vision.
3. Acute leukemias are diagnosed on clinical and laboratory findings. Noah's pediatrician made the initial diagnoses based on the peripheral blood smear which showed the presence of blasts. The pathologist confirmed the diagnosis from a bone marrow examination which showed a hypercellular bone marrow with greater than 30% blasts. Cytochemical stains and immunologic marker studies are performed to differentiate the different leukemias.
4. Treatment consists of chemotherapy and supportive therapy such as blood transfusions and antibiotics. In ALL, intrathecal chemotherapy, spinal radiation, or a combination of both are usually needed. Bone marrow transplants are now being used successfully, usually in patients who have a recurrence of leukemia.
5. The five-year survival rate for children diagnosed with leukemia and subsequently treated is approximately 70%. The response to treatment varies greatly for each individual. Recovery rates are constantly improving with continuing research of new and better methods to treat leukemia.
6. Although the cause of leukemia is not known, certain risk factors have been identified which increase the chance of developing leukemia. Risk factors include exposure to high-energy radiation, electromagnetic fields, and certain chemicals such as benzene. Avoidance of these environmental risk factors may aid in prevention. Other risk factors may be genetic and/or viral in nature and at this point cannot be prevented.
7. Many health professionals work together to treat a child with leukemia. In this case, the pediatrician made the initial diagnosis and then referred the child to a pediatric oncologist who specializes in the treatment of childhood diseases of the blood. Nurses are the main link between the health care team and the family. They care for the child during hospital stays performing a multitude of duties including checking vital signs, starting I.V.'s, giving medications, and making sure the child is comfortable. The patient often receives chemotherapy in the oncologist's office. Nurses work with the oncologist to deliver chemotherapy and educate cancer patients about their disease. Medical laboratory technologists perform laboratory testing which aids in the diagnosis and continual assessment of the child's condition. The pathologist obtains and analyzes bone marrow samples. The secondary complication of pneumonia was treated by respiratory therapists who assessed breathing capacity and administered oxygen. The radiological technicians obtained chest x-rays, which allowed the radiologist to diagnose life-threatening pneumonia. All of the health care team worked together in diagnosing and treating this child.
HemaBlog Issue 3: A Case Study of Acute Lymphoblastic Leukemia (ALL)

Clinical information
A 37-year-old male patient sought medical assistance at the hospital due to persistent asthenia, soreness in both lower limbs, as well as discomfort in the left hand and left shoulder. Subsequently, he was admitted to undergo diagnostic procedures for acute lymphoblastic leukemia (ALL).
CBC results
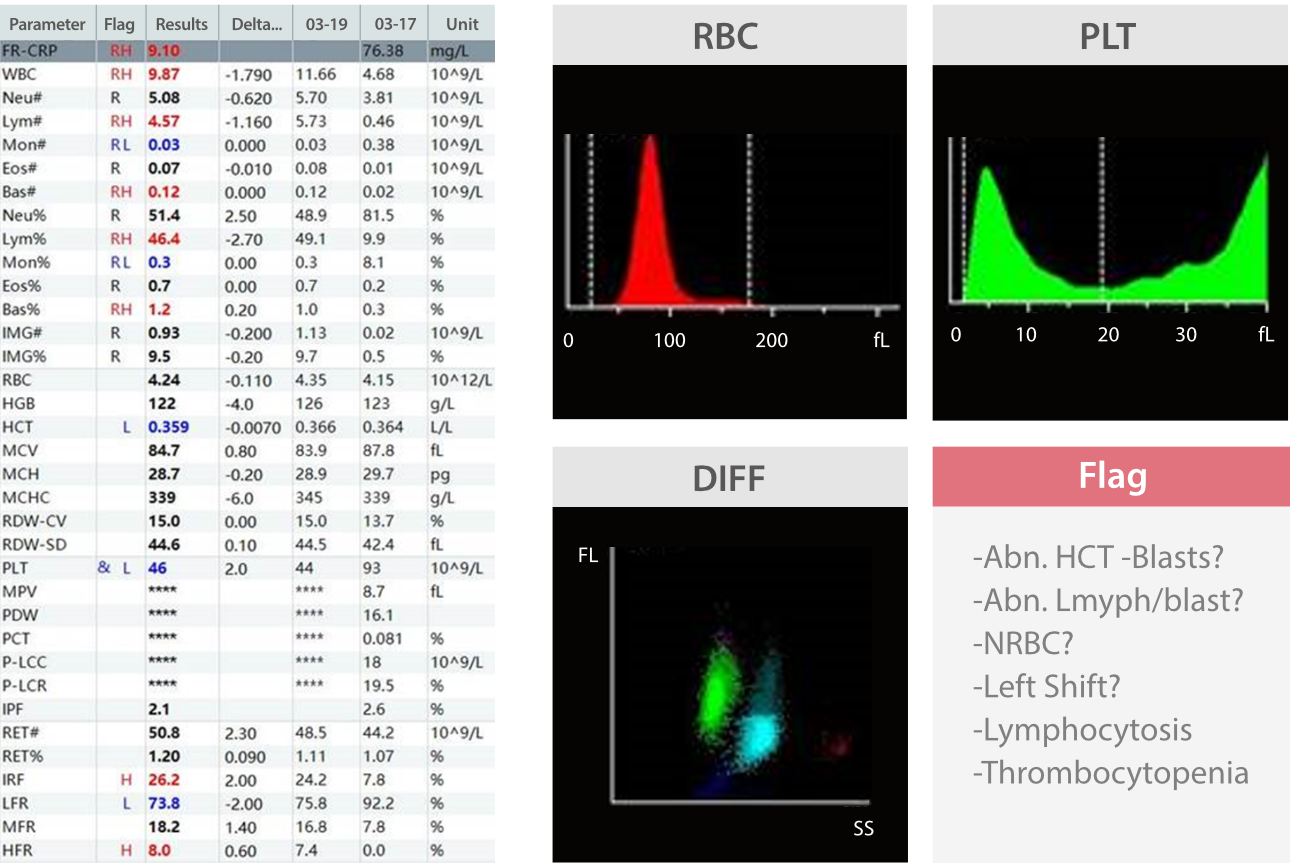
The patient's white blood cell (WBC) count and differential were within normal ranges, as were the red blood cell (RBC) parameters. However, the patient exhibited a low platelet count (PLT).
On the DIFF scattergram, there was an extension of neutrophil particles towards the high-fluorescence region, indicating the presence of immature granulocytes. The lymphocyte region appeared significantly dense, with some particles extending towards the high-fluorescence region. The instrument indicated poor reliability in classification.
As a result, re-examination rules were triggered, and the slides were thoroughly re-examined and the images reviewed.
Peripheral blood morphology examination
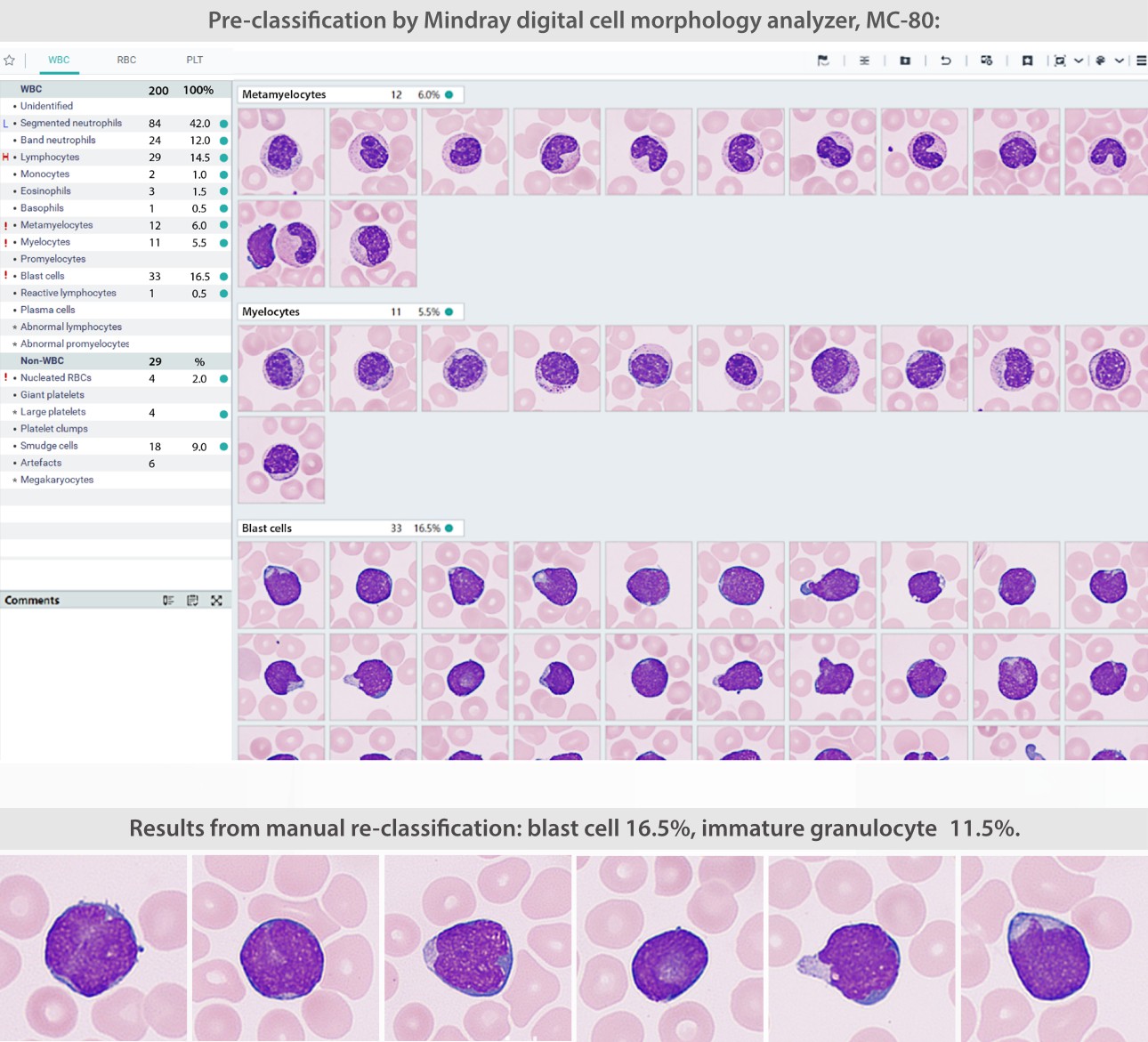
Case analysis
This is a case of a patient who has undergone treatment for acute lymphoblastic leukemia (ALL).
What is Acute Lymphoblastic Leukemia (ALL)
Acute lymphoblastic leukemia (ALL) is the most common type of cancer in children, accounting for 75-80% of childhood leukemias. It is characterized by two peaks in incidence: one occurring in children aged 2-5 years and the other in individuals aged 50 years or older.
According to the WHO Classification of Hematological Malignancies (2001), Burkitt's lymphoma and ALL-L3 are two stages of the same disease. In most newly diagnosed patients, there is a significant increase in peripheral white blood cells (WBCs), normocytic anemia, decreased platelet count, and a high ratio of myeloblasts and promyelocytes. The scattergram typically exhibits a distinctive baseball bat shape (Figure 1).
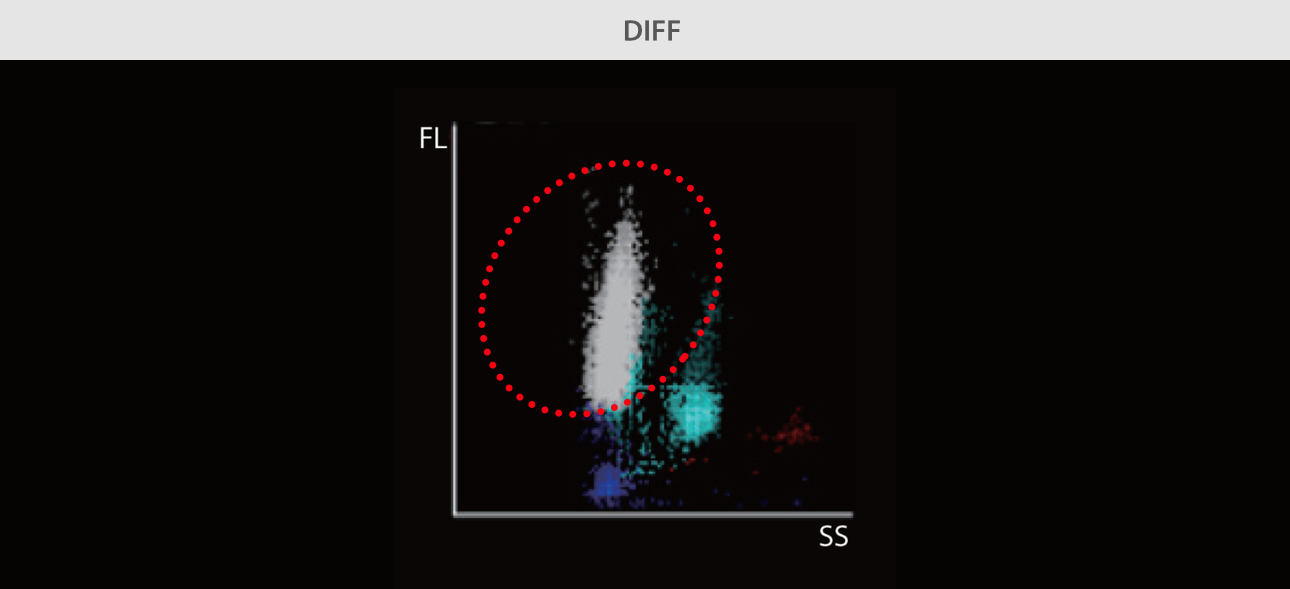
However, in post-ALL-treatment cases, the abnormal lymphocytes decrease, resulting in the absence of typical scattergram characteristics (Figure 2).
Therefore, alongside the scattergram analysis, it is crucial to give attention to instrument alarms. An excessively high WBC count at the initial disease onset often indicates a poor prognosis.
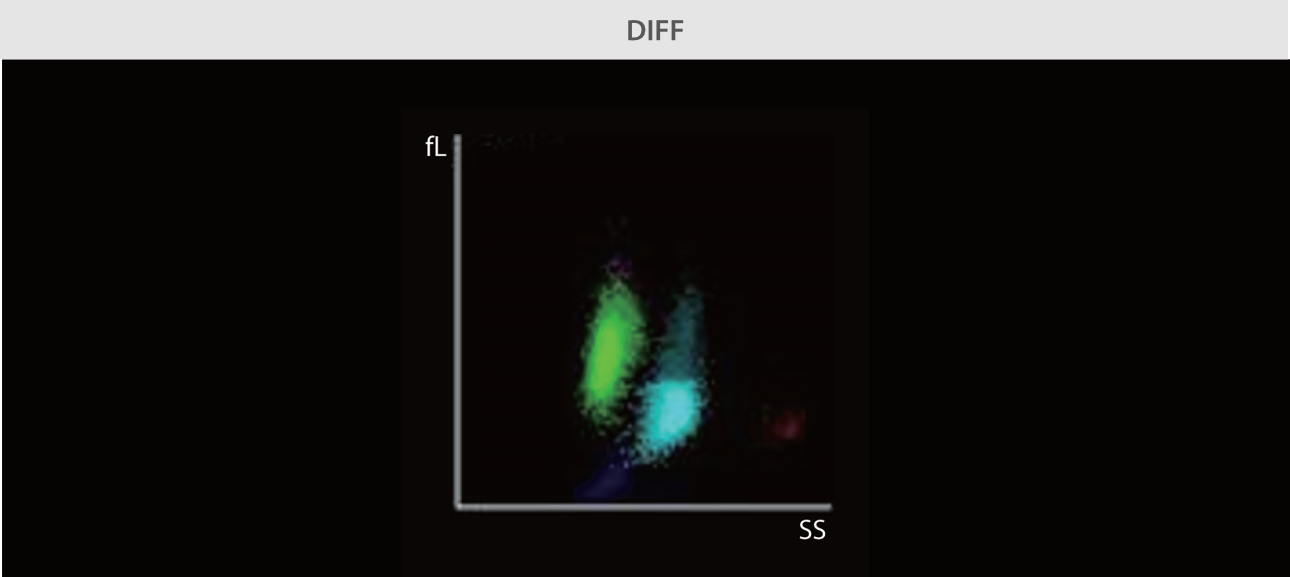
Call for case studies
- Do you want to share your case with us? We will share your case in the upcoming issues with acknowledgement.
- IVD Cases: You are recommended to submit any typical, misdiagnosed, special or rare cases that received a definite diagnosis or successful treatment. Cases that involved effective communication between the laboratory and clinical departments are particularly encouraged.
- Format: You are required to include four sections in your entry: "Case Introduction, Case Details, Case Analysis, and an Executive Summary". The Case Details section must include: primary medical history, positive/negative test results that aided in the diagnosis, and the diagnosis and treatment process.
- Please submit your entry in a Word document (in English) by sending it to [email protected] by December 5, 2023.
- The email should come in a subject that reads: country+name of the entry + name of the applicant.

- Patient Care & Health Information
- Diseases & Conditions
- Acute lymphocytic leukemia
Acute lymphocytic leukemia (ALL) is a type of cancer of the blood and bone marrow — the spongy tissue inside bones where blood cells are made.
The word "acute" in acute lymphocytic leukemia comes from the fact that the disease progresses rapidly and creates immature blood cells, rather than mature ones. The word "lymphocytic" in acute lymphocytic leukemia refers to the white blood cells called lymphocytes, which ALL affects. Acute lymphocytic leukemia is also known as acute lymphoblastic leukemia.
Acute lymphocytic leukemia is the most common type of cancer in children, and treatments result in a good chance for a cure. Acute lymphocytic leukemia can also occur in adults, though the chance of a cure is greatly reduced.
Products & Services
- Children’s Book: My Life Beyond Leukemia
- Mayo Clinic Comprehensive Cancer Center
Signs and symptoms of acute lymphocytic leukemia may include:
- Bleeding from the gums
- Frequent infections
- Frequent or severe nosebleeds
- Lumps caused by swollen lymph nodes in and around the neck, armpits, abdomen or groin
- Shortness of breath
- Weakness, fatigue or a general decrease in energy
When to see a doctor
Make an appointment with your doctor or your child's doctor if you notice any persistent signs and symptoms that concern you.
Many signs and symptoms of acute lymphocytic leukemia mimic those of the flu. However, flu signs and symptoms eventually improve. If signs and symptoms don't improve as expected, make an appointment with your doctor.
Acute lymphocytic leukemia occurs when a bone marrow cell develops changes (mutations) in its genetic material or DNA. A cell's DNA contains the instructions that tell a cell what to do. Normally, the DNA tells the cell to grow at a set rate and to die at a set time. In acute lymphocytic leukemia, the mutations tell the bone marrow cell to continue growing and dividing.
When this happens, blood cell production becomes out of control. The bone marrow produces immature cells that develop into leukemic white blood cells called lymphoblasts. These abnormal cells are unable to function properly, and they can build up and crowd out healthy cells.
It's not clear what causes the DNA mutations that can lead to acute lymphocytic leukemia.
Risk factors
Factors that may increase the risk of acute lymphocytic leukemia include:
- Previous cancer treatment. Children and adults who've had certain types of chemotherapy and radiation therapy for other kinds of cancer may have an increased risk of developing acute lymphocytic leukemia.
- Exposure to radiation. People exposed to very high levels of radiation, such as survivors of a nuclear reactor accident, have an increased risk of developing acute lymphocytic leukemia.
- Genetic disorders. Certain genetic disorders, such as Down syndrome, are associated with an increased risk of acute lymphocytic leukemia.
Acute lymphocytic leukemia care at Mayo Clinic
Living with acute lymphocytic leukemia?
Connect with others like you for support and answers to your questions in the Blood Cancers & Disorders support group on Mayo Clinic Connect, a patient community.
Blood Cancers & Disorders Discussions

362 Replies Tue, Apr 30, 2024

34 Replies Mon, Apr 29, 2024

21 Replies Fri, Apr 26, 2024
- AskMayoExpert. Acute lymphoblastic leukemia (child). Mayo Clinic; 2020.
- Pediatric acute lymphoblastic leukemia. National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed Oct. 2, 2020.
- Acute lymphoblastic leukemia. National Comprehensive Cancer Network. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed Oct. 2, 2020.
- Hoffman R, et al. Clinical manifestations and treatment of childhood acute lymphoblastic leukemia. In: Hematology: Basic Principles and Practice. 7th ed. Elsevier; 2018. https://www.clinicalkey.com. Accessed Oct. 2, 2020.
- Childhood acute lymphoblastic leukemia treatment (PDQ). National Cancer Institute. https://www.cancer.gov/types/leukemia/patient/child-all-treatment-pdq. Accessed Oct. 2, 2020.
- Types of complementary therapies. Cancer.net. https://www.cancer.net/navigating-cancer-care/how-cancer-treated/integrative-medicine/types-complementary-therapies. Accessed Oct. 7, 2020.
- Warner KJ. Allscripts EPSi. Mayo Clinic. July 9, 2020.
Associated Procedures
- Bone marrow biopsy
- Bone marrow transplant
- Chemotherapy
- Lumbar puncture (spinal tap)
- Radiation therapy
News from Mayo Clinic
- 3 facts about acute lymphocytic leukemia in children Sept. 21, 2023, 03:04 p.m. CDT
Mayo Clinic in Rochester, Minnesota, Mayo Clinic in Jacksonville, Florida, and Mayo Clinic in Phoenix/Scottsdale, Arizona, have been recognized among the top Cancer hospitals in the nation for 2023-2024 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
- Care at Mayo Clinic
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Make twice the impact
Your gift can go twice as far to advance cancer research and care!
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 30 June 2017
Acute lymphoblastic leukemia: a comprehensive review and 2017 update
- T Terwilliger 1 &
- M Abdul-Hay 1 , 2
Blood Cancer Journal volume 7 , page e577 ( 2017 ) Cite this article
128k Accesses
692 Citations
73 Altmetric
Metrics details
- Acute lymphocytic leukaemia
- Targeted therapies
Acute lymphoblastic leukemia (ALL) is the second most common acute leukemia in adults, with an incidence of over 6500 cases per year in the United States alone. The hallmark of ALL is chromosomal abnormalities and genetic alterations involved in differentiation and proliferation of lymphoid precursor cells. In adults, 75% of cases develop from precursors of the B-cell lineage, with the remainder of cases consisting of malignant T-cell precursors. Traditionally, risk stratification has been based on clinical factors such age, white blood cell count and response to chemotherapy; however, the identification of recurrent genetic alterations has helped refine individual prognosis and guide management. Despite advances in management, the backbone of therapy remains multi-agent chemotherapy with vincristine, corticosteroids and an anthracycline with allogeneic stem cell transplantation for eligible candidates. Elderly patients are often unable to tolerate such regimens and carry a particularly poor prognosis. Here, we review the major recent advances in the treatment of ALL.
Similar content being viewed by others

Updated risk-oriented strategy for acute lymphoblastic leukemia in adult patients 18–65 years: NILG ALL 10/07
Temporal changes in incidence of relapse and outcome after relapse of childhood acute lymphoblastic leukemia over three decades; a Nordic population-based cohort study

Relapsed acute myeloid leukemia in children and adolescents: current treatment options and future strategies
Introduction.
Acute lymphoblastic leukemia (ALL) is a malignant transformation and proliferation of lymphoid progenitor cells in the bone marrow, blood and extramedullary sites. While 80% of ALL occurs in children, it represents a devastating disease when it occurs in adults. Within the United States, the incidence of ALL is estimated at 1.6 per 100 000 population. 1 In 2016 alone, an estimated 6590 new cases were diagnosed, with over 1400 deaths due to ALL (American Cancer Society). The incidence of ALL follows a bimodal distribution, with the first peak occurring in childhood and a second peak occurring around the age of 50. 2 While dose-intensification strategies have led to a significant improvement in outcomes for pediatric patients, prognosis for the elderly remains very poor. Despite a high rate of response to induction chemotherapy, only 30–40% of adult patients with ALL will achieve long-term remission. 3
Pathophysiology
The pathogenesis of ALL involves the abnormal proliferation and differentiation of a clonal population of lymphoid cells. Studies in the pediatric population have identified genetic syndromes that predispose to a minority of cases of ALL, such as Down syndrome, Fanconi anemia, Bloom syndrome, ataxia telangiectasia and Nijmegen breakdown syndrome. 4 , 5 , 6 , 7 Other predisposing factors include exposure to ionizing radiation, pesticides, certain solvents or viruses such as Epstein-Barr Virus and Human Immunodeficiency Virus. 8 , 9 , 10 However, in the majority of cases, it appears as a de novo malignancy in previously healthy individuals. Chromosomal aberrations are the hallmark of ALL, but are not sufficient to generate leukemia. Characteristic translocations include t(12;21) [ ETV6-RUNX1 ], t(1;19) [ TCF3-PBX1 ], t(9;22) [ BCR-ABL1 ] and rearrangement of MLL . 11 More recently, a variant with a similar gene expression profile to (Philadelphia) Ph-positive ALL but without the BCR-ABL1 rearrangement has been identified. In more than 80% of cases of this so-called Ph-like ALL, the variant possesses deletions in key transcription factors involved in B-cell development including IKAROS family zinc finger 1 (IKZF1), transcription factor 3 (E2A), early B-cell factor 1 (EBF1) and paired box 5 (PAX5). 12 Similarly, kinase-activating mutations are seen in 90% of the Ph-like ALL. The most common of these include rearrangements involving ABL1, JAK2, PDGFRB, CRLF2 and EPOR, activating mutations of IL7R and FLT3 and deletion of SH2B3, which encodes the JAK2-negative regulator LNK. 13 This has significant therapeutic implications as it suggests that Ph-like ALL, which tends to carry a worse prognosis, may respond to kinase inhibitors. In fact, Roberts et al. 14 showed that cell lines and human leukemic cells expressing ABL1, ABL2, CSF1R and PDGFRB were sensitive in vitro and in vivo human xenograft models to second-generation TKIs (for example, dasatinib.); those with EPOR and JAK2 rearrangements were sensitive to JAK kinase inhibitors (for example, ruxolitinib); and those with ETV6-NTRK3 fusion were sensitive to ALK inhibitors crizotinib. Furthermore, Holmfeldt et al. 15 recently described the genetic basis of another subset with poor outcomes, hypodiploid ALL. In near-haploid (24–31 chromosomes) ALL, alterations in tyrosine kinase or Ras signaling was seen in 71% of cases and in IKAROS family zinc finger 3 (IKZF3) in 13% of cases. In contrast, low-hypodiploid (32–39 chromosomes) ALL, alterations in p53 (91%), IKZF2 (53%) and RB1 (41%) were more common. Both near-haploid and low-hypodiploid exhibited activation of Ras- and PI3K-signaling pathways, suggesting that these pathways may be a target for therapy in aggressive hypodiploid ALL. 15
Most of the clinical manifestations of ALL reflect the accumulation of malignant, poorly differentiated lymphoid cells within the bone marrow, peripheral blood, and, extramedullary sites. Presentation can be nonspecific, with a combination of constitutional symptoms and signs of bone marrow failure (anemia, thrombocytopenia, leukopenia). Common symptoms include ‘B symptoms’ (fever, weight loss, night sweats), easy bleeding or bruising, fatigue, dyspnea and infection. Involvement of extramedullary sites commonly occurs and can cause lymphadenopathy, splenomegaly or hepatomegaly in 20% of patients. 16 , 17 CNS involvement at time of diagnosis occurs in 5–8% of patients and present most commonly as cranial nerve deficits or meningismus. 3 T-cell ALL also may present with a mediastinal mass.
Diagnosis is established by the presence of 20% or more lymphoblasts in the bone marrow or peripheral blood. 16 Evaluation for morphology, flow cytometry, Immunophenotyping and cytogenetic testing is valuable both for confirming the diagnosis and risk stratification. Lumbar puncture with CSF analysis is standard of care at the time of diagnosis to evaluate for CNS involvement. If the CNS is involved, brain MRI should be performed. Other evaluation includes complete blood count with differential and smear to evaluate the other hematopoietic cell lines, coagulation profiles and serum chemistries. Baseline uric acid, calcium, phosphate and lactate dehydrogenase should be recorded to monitor for tumor lysis syndrome.
Classification
The first attempt at classifying ALL was the French American British (FAB) morphological criteria that divided ALL into 3 subtypes (L1, L2 and L3) based on cell size, cytoplasm, nucleoli, vacuolation and basophilia. 18 In 1997, the World Health Organization proposed a composite classification in attempt to account for morphology and cytogenetic profile of the leukemic blasts and identified three types of ALL: B lymphoblastic, T lymphoblastic and Burkitt-cell Leukemia. 19 Later revised in 2008, Burkitt-cell Leukemia was eliminated as it is no longer seen as a separate entity from Burkitt Lymphoma, and B-lymphoblastic leukemia was divided into two subtypes: B-ALL with recurrent genetic abnormalities and B-ALL not otherwise specified. B-ALL with recurrent genetic abnormalities is further delineated based on the specific chromosomal rearrangement present ( Table 1 ). 20 In 2016, two new provisional entities were added to the list of recurrent genetic abnormalities and the hypodiploid was redefined as either low hypodiploid or hypodiploid with TP53 mutations. 21 In adults, B-cell ALL accounts for ~75% of cases while T-cell ALL comprises the remaining cases.
Prognostic factors
Accurate assessment of prognosis is central to the management of ALL. Risk stratification allows the physician to determine the most appropriate initial treatment regimen as well as when to consider allogeneic stem cell transplantation (Allo-SCT). Historically, age and white blood cell count at the time of diagnosis have been used to risk stratify patients. Increasing age portends a worsening prognosis. Patients over the age of 60 have particularly poor outcomes, with only 10–15% long-term survival. 22 Age is at least in part a surrogate for other prognosticators as the elderly tend to have disease with intrinsic unfavorable biology (for example, Philadelphia chromosome positive, hypodiploidy and complex karyotype), more medical comorbidities and inability to tolerate standard chemotherapy regimens but helps guide therapy nonetheless. In the largest prospective trial to determine optimal treatment, MRC UKALL XII/ECOG E2993 found a significant difference of disease-free (DFS) and overall survival (OS) based on age using a cutoff of 35 in Ph-negative disease. 23 Similarly, they found an elevated white blood cell count at diagnosis, defined as >30 × 10 9 for B-ALL or >100 10 9 for T-ALL, was an independent prognostic factor for DFS and OS. On the basis of these results, Ph-negative disease could be categorized as low risk (no risk factors based on age or WBC count), intermediate risk (age >35 or elevated WBC count), or high risk (age >35 and elevated WBC count). The 5-year OS rates based on these risk categories were 55, 34 and 5%, respectively. 23
Although clinical factors play an important role in guiding therapy, cytogenetic changes have a significant role in risk determination. The cytogenetic aberration with the greatest impact on prognosis and treatment is the presence of the Philadelphia chromosome, t(9;22). The prevalence of t(9;22) in adult ALL can range from 15–50% and increases with age. 24 Ph-positivity has implications both in terms of prognosis and for treatment. Historically, Ph-positive ALL has a 1-year survival of around 10%. However, with the development of TKIs, survival has improved and thus the Ph-status of all patients must be obtained prior to starting therapy. Subsequent analysis of MRC UKALL XII/ECOG E2993, identified cytogenetic subgroups of Ph-negative disease with inferior outcomes. These included t(4;11), KMT2A translocation, t(8;14), complex karyotype ( ⩾ 5 chromosomal abnormalities) and low hypodiploidy (30–39 chromosomes)/near triploidy (60–78 chromosomes). In contrast, patients with hyperdiploidy and del(9p) had a significantly better outcome. 25 In a later study, the Southwest Oncology Group (SWOG) showed that among the 200 study patients, cytogenetic profile was a more important prognostic factor than age or WBC count. 26 More recently, a subset of high-risk ALL without t(9;22) has been identified with a genetic profile similar to that of Ph-positive ALL. This so called, Ph-like ALL has been associated with poor response to induction chemotherapy, elevated minimal residual disease and poor survival. 13 , 14 , 27
In addition to disease characteristics at the outset, it has long been recognized that response to initial therapy predicts outcome. Historically, treatment response was evaluated morphologically. Recently, it has become standard practice to evaluate patients for minimal residual disease (MRD) using molecular techniques such as flow cytometry and PCR. 28 Several studies have shown the importance of MRD in assigning risk. 29 , 30 , 31 , 32 , 33 , 34 Bruggemann et al. 29 re-stratified standard-risk patients to low risk, intermediate risk and high risk with relapse rates of 0%, 47% and 94%, respectively, based on the persistence of elevated MRD, defined as >10 −4 . In a multivariate analysis of 326 adolescent and adult patients with high-risk Ph-negative ALL treated in The Programa Espanol de Tratamientos en Hematologia (PETHEMA ALL-AR-03), Ribera et al. 35 showed that poor MRD clearance, defined as levels >1 × 10 −3 after induction and levels >5 × 10 −4 after early consolidation by flow cytometry, was the only significant prognostic factor for disease-free and overall survival.
On the basis of what is known about prognostic factors in adult ALL, the National Comprehensive Cancer Network (NCCN) has developed recommendations to approach risk stratification. 16 The National Cancer Institutes defines adolescent and young adults (AYA) to be those aged 15–39 years. The NCCN recognizes that AYA may benefit from treatment with pediatric-inspired regimens and thus are considered separately from adults >40 years. 36 , 37 Both age groups are then stratified into high-risk Ph-positive and standard-risk Ph-negative subgroups. The Ph-negative subgroup can further be categorized as high-risk based on the presence of MRD, elevated WBC (defined above) or unfavorable cytogenetics (defined above).
Established treatments
The structure of treatment of adult ALL has been adapted from pediatric protocols. Unfortunately, while long-term survival approaches 90% for standard-risk pediatric ALL, the success rate is much more modest in adults. Chemotherapy consists of induction, consolidation and long-term maintenance, with CNS prophylaxis given at intervals throughout therapy. The goal of induction therapy is to achieve complete remission and to restore normal hematopoiesis. The backbone of induction therapy typically includes vincristine, corticosteroids and an anthracycline. 38 , 39 In the Cancer and Leukemia Group B 8811 trial, Larsen et al. 40 achieved a complete response rate of 85% and a median survival of 36 months. The 4-week long induction schedule consists of cyclophosphamide on day 1, 3 consecutive days of daunorubicin, weekly vincristine, biweekly l -asparaginase and 3 weeks of prednisone. 40 Due to high induction-related mortality, one-third dose reductions of cyclophosphamide and daunorubicin were implemented for patients older than 60 and the duration of prednisone was shortened to 7 days in this age group. The role of L-asparaginase, while standard in pediatric protocols, is a challenge in adults at times due to the increased rate of adverse events. 41 In fact, in the UKALL 14 Trial, Patel et al. 42 , 43 demonstrated that asparaginase toxicity was the leading cause of induction-related mortality and the protocol was amended to omit asparaginase for patients over the age of 40. The MRC UKALL XII/ECOG 2993 23 regimen utilizes a similar structure to CALGB 8811. Induction is divided into two phases of four weeks. In contrast to CALGB 8811, cyclophosphamide is omitted in phase I of induction, but a single dose of intrathecal methotrexate is added for CNS prophylaxis. In phase II of induction, cyclophosphamide is introduced along with cytarabine, oral 6-mercaptopurine (6-MP), four additional intrathecal doses of methotrexate, and cranial radiation if CNS is positive. After induction therapy, patients received three cycles of intensification therapy of methotrexate with leucovorin rescue and l -asparaginase. Eligible patients with high-risk disease and a matched donor, then underwent Allo-SCT. All others were randomized to standard consolidation/maintenance or autologous stem cell transplant. This study yielded a complete response rate of 91% and an overall 5-year survival of 38%. 23
The Hyper-CVAD (HCVAD)/ Methotrexate-cytarabine regimen is utilizes an alternative structure to the approaches described above. It consists of four cycles of hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone alternated with four cycles of high dose cytarabine and methotrexate. 44 CNS prophylaxis with 4-16 doses of intrathecal chemotherapy depending on predetermined risk of CNS disease. HCVAD has demonstrated similar efficacy to the ECOG trial with a 92% complete response rate and 32% 5-year disease-free survival. 44 Several studies have suggested a benefit to using dexamethasone as opposed to prednisone due to the ability of dexamethasone to achieve higher concentrations in the CNS. Despite a reduction in CNS relapse and improved event-free survival, dexamethasone has increased risk of adverse events compared to prednisone. Since there have been no studies comparing overall survival, the benefit of one corticosteroid over the other has not been established. 45 , 46
After induction, eligible patients may go on to Allo-SCT while all others go on to intensification/consolidation and maintenance. 47 Consolidation varies in the different protocols, but generally utilize similar agents to induction and includes intrathecal chemotherapy and cranial radiation for CNS prophylaxis at times. Maintenance therapy consists of daily 6-MP, weekly methotrexate, and vincristine and a 5-day prednisone pulse every 3 months. Maintenance is administered for 2–3 years after induction, beyond which it has not been shown to have benefit. 17 , 47
Special consideration must be made in the treatment of Ph-positive ALL. Historically, Ph-positive ALL was a very bad player with 5-year survival ~5–20% and Allo-SCT being the only chance for cure. 48 , 49 Various studies have found that matched-sibling Allo-SCT may improve long-term survival to 35–55%, however, availability of matched donors represents a significant limitation. 49 , 50 , 51 The advent of TKIs marked a turning point in the treatment of Ph-positive ALL. Thomas et al. 52 , 53 showed that when added to traditional HCVAD, imatinib resulted in improvement in 3-year OS (54 vs 15%). Despite these promising results, some patients fails treatment due to resistance or relapse, particularly in the CNS where imatinib has limited penetration. 54 Second-generation ABL kinase inhibitor, dasatinib, was developed as a dual src/abl kinase inhibitor for chronic myeloid leukemia with a superior resistance profile to imatinib. Dasatinib was also shown to penetrate the blood-brain barrier and was effective at treating CNS disease in a mouse model and pediatric Ph-positive ALL. 55 In the first study of dasatinib in Ph-positive ALL, Ravandi et al. 56 found a CR rate of 96% when dasatinib was combined with HCVAD, and a 5-year OS of 46%. In a subsequent, multi-center trial HCVAD plus dasatinib achieve a 3-year OS of 71% in adult patients younger than 60. 57 In addition, prior resistance to imatinib did not preclude a response to dasatinib. 58 In addition, dasatinib was shown to be effective in inducing complete remission when used in combination with prednisone and intrathecal methotrexate. 59 In the GIMEMA LAL1205 study, 59 it was noted that the most common cause of relapse was a T315I mutation in the ABL kinase doman. Ponatinib, a third-generation TKI with the ability to inhibit most BCR-ABL1 kinase domain mutations, has recently gained approval for resistant Ph-positive ALL. The PACE trial 60 demonstrated the ability of ponatinib to generate a cytogenetic response in 47% of Ph-positve ALL patients after dasatinib failure. When compared head-to-head with dasatinib, ponatinib achieved significantly better 3-year EFS and OS when used as frontline therapy. 42 , 61 , 62 These data suggest that ponatinib may soon have a role in the frontline therapy of Ph-positive ALL.
Recent studies have suggested that the AYA population, defined as aged 15–39, may benefit from treatment on pediatric-inspired protocols. In an analysis of 262 AYA patients aged 16–21 on pediatric protocol CCG 1961, Nachman et al. 63 reported a 5-year EFS of 68%. Furthermore, patients in the study that were treated on augmented intensity therapy performed better. In a prospective study, Stock et al. 64 treated 317 patients aged 17–39 on Children’s Oncology Group AALL0232 protocol. Median EFS approached 60 months, which was statistically higher than the null hypothesis of 32 months. OS at 2-years was 78%. 64 Similarly, The Group for Research on Adult Acute Lymphoblastic Leukemia (GRAAL), compared 225 patients up to the age of 60 who were treated on pediatric-inspired regimen and historical data from 712 adults treated on standard adult regimen LALA-93. 36 They observed a significant improvement in CR, EFS and OS, which was most marked in patients younger than age of 45 years. In fact, in patients older than 45 years, there was a significantly higher rate of chemotherapy-related events compared to younger patients, suggesting that an age cutoff for pediatric-inspired regimens is appropriate. However still one of the adult regimens is still considered for AYA patients is HCVAD±rituximab. An MD Anderson Cancer Center study revealed no significant difference in CR rate or OS in AYA patients treated with HCVAD±rituximab vs an augmented-Berlin-Frankfurt-Munster regimen. 65
Refractory/relapsed disease
While 85–90% of patients go into remission after induction therapy, there are subsets that are refractory to induction therapy. In addition, a majority of patients that do achieve CR go on to relapse. Options of salvage therapy for relapsed/refractory (r/r) Ph-negative disease include augmented cytotoxic chemotherapy, reformulated single-agent chemotherapy and novel monoclonal antibodies. Augmented-HCVAD for salvage therapy was inspired by pediatric regimens that employ intensified doses of vincristine, corticosteroids and asparaginase in frontline therapy. Faderl et al. 66 treated 90 patients (median age 34) with relapsed or refractory disease with HCVAD in which the dosing of vincristine, dexamethasone and asparaginase where intensified as follows: vincristine 2 mg i.v. weekly on days 1, 8 and 15; dexamethasone 80 mg i.v. or orally (p.o.) on days 1–4 and 15–18, and pegaspargase 2500 units/m 2 i.v. on day 1 of the hyper-CVAD courses (1, 3, 5 and 7) and day 5 of the methotrexate/cytarabine courses (2, 4, 6 and 8). The majority of patients were in first salvage and ten patients were primary refractory, and patients with prior exposure to HCVAD were not excluded. Complete response was observed in 47% of the patients, with a median duration of 5 months. Median DFS and OS were 6.2 and 6 months respectively. 66 It was also noted that the addition of rituximab to HCVAD for B-ALL with high CD20 expression to improve the activity of this salvage regimen.
In patients with relapsed/refractory ALL, particularly those with multiple relapses, toxicity of multi-agent cytotoxic therapy may be limiting. Therefore, attempts have been made at salvage therapy with a single agent. In subgroup analysis of 70 patients receiving second salvage therapy with a single agent (most commonly vinorelbine (6), clofarabine (5), nelarabine (4) and topotecan (4)), only 3 achieved a complete response. 67 , 68 Vincristine sulfate liposomes injection (VSLI) was developed to overcome the dosing and pharmacokinetic limitations of nonliposomal vincristine (VCR). In a phase II study in adults with Ph-negative ALL in their second or greater relapse, VSLI was administered weekly at a dose of 2.25 mg/m 2 . 69 Of the 65 adults enrolled, 20% achieved complete response with a median duration of 23 weeks (range 5–66). Twelve patients were bridged to Allo-SCT, with five long-term survivors. 69 This study led to the accelerated approval of VSLI for salvage therapy in 2012. VSLI was well tolerated with a side effect profile similar to standard-formulation VCR, despite the massive cumulative doses of VCR achieved.
Despite the modest ability of cytotoxic chemotherapy to prolong survival, the only hope for long-term survival in these regimens remains Allo-SCT. However, recently novel monoclonal antibodies have transformed the landscape of salvage therapy by offering a chance at cure may be without Allo-SCT. The first of these is the bispecific anti-T-cell receptor/anti-CD19 antibody, blinatumomab. The proposed mechanism of action of blinatumomab is that it engages T cells to activate a B-cell specific inflammatory and cytolytic response. 70 Blinatumomab was first studied in patients with MRD positive ALL. In one trial, 80% of patients became MRD negative after the first cycle of blinatumomab, with 60% of patients remaining in CR at a median follow-up of 33 months. 71 Importantly, in a multi-center trial (BLAST), Gokbuget et al. 72 confirmed the ability of blinatumomab to eliminate MRD and showed no difference in OS or relapse-free survival (RFS) between patients who received Allo-SCT during the first CR (CR1) and those who did not. Based on these results, blinatumomab was studied for relapsed/refractory Ph-negative ALL. The landmark study was a multi-center, single-arm, open-label phase 2 trial in which 189 patients with primary refractory and relapsed ALL received single-agent therapy with blinatumomab. CR was achieved after 2 cycles in 43 with 82% achieving MRD negativity. The median response duration and the overall survival were 9 and 6 months, respectively. 73 Based on these results, blinatumomab was approved by the FDA for relapsed and refractory ALL in 2016. Subsequently, blinatumomab was compared to investigator’s choice of chemotherapy for r/r Ph-negative ALL in the phase 3 randomized trial (TOWER study). The blinatumomab study group ( n =271) had a median survival of 7.7 months (95% confidence interval (CI): 5.6, 9.6) versus 4.0 months (95% CI: 2.9, 5.3) for standard of care ( n =134) ( P =0.012, hazards ratio (HR), 0.71). 74 The study was terminated early for efficacy based on these results. Blinatumomab has also been investigated for r/r Ph-positive disease. In the ALCANTARA trial, standard dose blinatumomab was given for up to 5 cycles in 45 patients. CR was observed in 36 and 88% of whom were MRD negative, and with a median follow-up of 9 months, the median OS was 7.1 months. 75 Future investigation is planned for the frontline use of blinatumomab for Ph-positive ALL in conjunction with TKIs. 76 The toxicity profile of blinatumomab is acceptable. The most frequent adverse events include fever, chills, neutropenia, anemia and hypogammaglobulinemia. 3 More significant adverse events are rare, but include cytokine release syndrome, altered mental status and seizures. 73 Death from sepsis that is thought to be treatment-related has been reported.
Frontline therapy is the same for B-cell ALL and T-cell ALL. However, owing to different biology of the two subtypes, T-cell ALL is not amenable to salvage treatment with blinatumomab. Fortunately, alternative options for salvage therapy exist. Nelarabine is a T-cell specific purine nucleoside analog that is FDA approved for r/r T-cell ALL. Nelarabine accumulates in T cells at a high rate and incorporates into DNA causing an inhibition of DNA synthesis and subsequent apoptosis. 77 In a phase 2, open-label, multi-center trial, nelarabine was administered on alternate day schedule (days 1, 3 and 5) at 1.5 g/m 2 /day for r/r T-cell ALL. Cycles were repeated every 22 days. The rate of complete remission was 31% (95% CI, 17, 48%), the median DFS and OS were 20 weeks with a 1-year OS of 28%. 77 However, there is still more that needs to be done to achieve a better response and overall survival in patients with relapsed/refractory B- and T-cell ALL.
Future therapies
1-monoclonal antibodies, a-cd22-directed therapy.
CD22 is a B-lineage differentiation antigen expressed in B-cell ALL in 50–100% of adults and 90% of children. 78 , 79 , 80 Upon binding of an antibody, CD22 is rapidly internalized, thus making it an attractive target for delivering immunotoxin to leukemic cells. 81
Epratuzumab
Epratuzumab is an unconjugated monoclonal antibody targeting CD22 that has been studied in pediatric and adult relapsed/refractory ALL. Epratuzumab was evaluated in 15 pediatric patients as part of a salvage therapy regimen. The antibody was administered as a single-agent followed by the antibody in combination with standard re-induction chemotherapy. The treatment resulted in a CR in 9 of the patients, with 7 achieving complete MRD clearance at the end of re-induction. 82 A phase 2 study in adults with relapsed/refractory disease evaluated the addition of epratuzumab to clofaribine/cytarabine. The study demonstrated a superior response rate when compared to historical data of clofaribine/cytarabine alone. 83 More recently, epratuzumab conjugated to the topoisomerase I inhibitor, SN-38, has been shown to have activity against B-cell lymphoma and leukemia cell lines in in vitro and in vivo preclinical studies. 84
Inotuzumab ozogamicin
Inotuzumab ozogamicin (InO) is a monoclonal antibody against CD22 that is conjugated to calicheamicin, a potent cytotoxic compound that induces double-strand DNA breaks. 85 Upon internalization of the immunoconjugate, calicheamicin binds DNA and causes double-stranded DNA breaks, which induces apoptosis. Preclinical studies showed that calicheamicin conjugated to an anti-CD22 antibody resulted in potent cytotoxicity leading to regression of B-cell lymphoma and prevention of xenograft establishment at picomolar concentrations. 86 Phase 1 studies in non-hodgkin lymphoma (NHL) established a maximum tolerated dose of 1.8 mg/m 2 InO given intravenously every 3 to 4 weeks. 87 Subsequently, InO was studied in adults with relapsed/refractory ALL. 88 In this phase 2 trial, 90 patients were treated with either a single infusion every 3 to 4 weeks or weekly InO infusions. Cumulative doses were equivalent among the two treatment strategies. Overall response rate was 58%, with similar response between the two dosing schedules. Median survival was 6.2 months, with a non-significant benefit seen in weekly dosing. However, toxicity was greatly improved by weekly dosing, with a significant reduction in fever, hepatotoxicity and veno-occlusive disease. 89 A second phase 2 study of 35 patients with CD22+ ALL in second salvage or later showed similar complete response rate (66%) and median overall survival (7.4 months). 90 Based on these results, Kantarjian et al. 91 compared weekly dosing of InO to standard chemotherapy for relapsed/refractory ALL. The rate of complete remission was significantly higher in the InO group versus standard chemotherapy 80.7% (95% CI, 72.1–87.7) vs 29.4% (95% CI, 21.0–38.8), P <.001). 91 Progression-free survival (5 months vs 1.8 months) and overall survival (7.7 months vs 6.7 months) were also significantly prolonged with InO compared to standard chemotherapy. The most common adverse events of InO treatment included thrombocytopenia and neutropenia. Veno-occlusive liver disease occurred in 11% of patients treated with InO compared to 1% of those receiving standard chemotherapy. 91 Based on these results, InO was granted Breakthrough Therapy status by the FDA in 2015 and is a strong candidate for expedited approval for relapsed/refractory ALL.
InO has also been studied in frontline therapy in combination with low-intensity HCVAD for elderly patients >60 years. 92 These patients are prone to adverse events from chemotherapy and have poorer outcomes than their younger counterparts. In attempt to reduce toxicity, doxorubicin was eliminated from induction therapy, and cyclophosphamide, prednisone, methotrexate and cytarabine were given at reduced doses. InO was given during each of the first four courses. The regimen was well tolerated and produced superior 1-year OS as compared to historical data among similar patient population (78 vs 60%). 92
Moxetumomab pasudodotox
A third anti-CD22 monoclonal antibody, moxetumomab, is currently in development for treatment of pediatric and adult ALL. Moxetumomab is a reformulation of an older study drug, BL22, which was composed the variable region (F v ) of an anti-CD22 monoclonal antibody fused to Pseudomonas aeruginosa exotoxin A. 93 BL22 was shown to be highly active against Hairy Cell Leukemia in a phase 2 trial. 94 In a phase 1 trial of children with relapsed/refractory ALL, BL22 was well tolerated and exhibited anti-leukemic activity at all doses, but clinical benefits were transient and modest. 95 Therefore, BL22 was reformulated as moxetumomab to contain a F v fragment with greater affinity for CD22. In phase 1 trials, moxetumomab showed an overall activity rate of 70% in children with relapsed/refractory ALL. 96 Enrollment is ongoing for a phase 1/2 trial of moxetumomab pasudodotox for treatment of relapsed/refractory ALL in adults. 97
Combotox is a combination immunotoxin that contains a 1:1 mixture of anti-CD19 and anti-CD22 antibodies, both conjugated to the cytotoxin deglycosylated ricin-A chain. In pediatric patients with relapsed/refractory ALL, combotox led to a CR in 3 of 17 patients. In addition, six additional patients experienced a >95% reduction in peripheral blasts. 98 In adults with relapsed/refractory disease, combotox led to reduction of peripheral blasts in all patients; however, a durable response was not seen as blast count rebounded quickly after the final dose of combotox. 99 A phase I trial is recruiting patients to evaluate combotox in combination with cytarabine for adults with relapsed/refractory ALL (NCT01408160).
CD20 is a B-lineage specific antigen expressed at nearly all stages of differentiation on the surface of both normal and malignant B-cells. Signaling through CD20 plays a role in cell cycle progression, differentiation pathways and regulation of apoptosis. CD20 is expressed in 40–50% of precursor lymphoblasts, and confers a worse prognosis. 100 Moreover, CD20-positive leukemia responds poorly to dose intensification, highlighting the need for targeted therapy. The addition of rituximab, a first-generation anti-CD20 monoclonal antibody, has improved outcomes in these patients, but resistance to rituximab represents a limitation to its use.
Ofatumumab is a second-generation anti-CD20 antibody with a distinct binding site from that of rituximab. Ofatumumab was first showed to have benefit in fludarabine-refractory chronic lymphocytic leukemia, irrespective of prior rituximab exposure. 101 Ofatumumab induces higher levels of complement-dependent cytotoxicity (CDC) and has a slower dissociation rate than rituximab, and thus holds promise for CD20+ lymphoid malignancies both as frontline therapy and as salvage for rituximab-refractory disease. 102 , 103 In a phase 2 study, ofatumumab was used in combination with HCVAD in patients with either newly diagnosed pre-B CD20+ ALL or those who had completed a single course of chemotherapy. In all study patients, CD20+ expression was >1%. 104 Ofatumumab was administered at a dose of 2 grams on days 1 and 11 of the first 4 cycles of induction therapy. All but one patient (98%) achieved CR after cycle 1 and 93% of patients were negative for MRD at end induction. The 3-year CR and OS rates were 78% and 68%, respectively. 105 This is similar to benefits seen when rituximab was used as frontline therapy in CD20+ ALL. 106 Ofatumumab represents a potential alternative frontline therapy for CD20+ pre-B-ALL and an option for patients who failed a rituximab-based regimen.
Obinutuzumab
Another novel anti-CD20 monoclonal antibody, obinutuzumab, has shown promise in preclinical trials for CD20-positive B-ALL. Obinutuzumab was engineered to have enhanced affinity for the FcγRIIIa receptor on effector cells and thus enhanced antibody-dependent cell-mediated cytotoxicity (ADCC). 107 This compromises the ability of obinutuzumab to activate complement and predictably, CDC was inferior to that of rituximab and ofatumumab in vitro . However, obinutuzumab induced direct cell death and ADCC more rapidly and effectively. When all three mechanisms of cell death were evaluated together in B-cell depletion assays, obinutuzumab was more effective than either rituximab or ofatumumab achieving higher maximal depletion and lower EC 50 . Furthermore, obinutuzumab was superior in inhibiting growth in NHL xenograft models. 107 Awasthi et al. 108 compared obinutuzumab to ritixumab in pre-B-ALL cell lines and found obinutuzumab to be superior in inducing cell death and ADCC. In a pre-B-ALL xenograft model, overall survival was improved with obinutuzumab compared to ritixumab. 108 In clinical trials, obinutuzumab has been added to chlorambucil for treatment of adults with CLL and shown to prolong progression-free survival and improve complete response rate when compared to rituximab and chlorambucil. 109 Taken together, these results suggest a role for obinutuzumab in CD20+ pre-B-ALL.
REGN1979 is a biallelic monoclonal antibody targeting CD20 and CD3. The theory of REGN1979 is similar to that of blinatumumab, to engage T cells and B-cells thus resulting in activation of T-cell immune response against B-cells. REGN1979 prevented the establishment of lymphoma xenografts and led to complete tumor regression in murine models. 110 In addition, in a primate model, REGN1979 led to a complete and durable depletion of B-cells. When compared to treatment with rituximab, treatment with REGN1979 led to significantly more profound depletion of B-cells. 110 The safety of REGN1979 was established in a phase 1 trial of 25 patients with NHL and CLL. Dose-dependent antitumor activity was observed. The most significant adverse events include cytokine release syndrome (CRS) and hypotension. 111 A phase 2 trial of REGN1979 in relapsed/refractory ALL is currently open for recruitment (NCT02651662).
CD19 is the most widely expressed B-lineage specific antigen, expressed during all stages of differentiation, but lost on maturation to plasma cells. CD19 serves as a co-receptor for the B-cell surface immunoglobulin and its activation triggers a phosphorylation cascade involving src-family kinases and PI3K as well as the activation of c-myc, leading to proliferation and differentiation. 112 , 113 , 114 CD19 is expressed in nearly all B-cell leukemias, and is rapidly internalized upon binding of an antibody, making it an ideal candidate for immunoconjugate therapy. 115
Coltuximab ravtansine (SAR3419)
Coltuximab ravtansine is an anti-CD19 humanized monoclonal antibody conjugated to a semisynthetic maytansinoid compound, an anti-tubulin molecule similar to vincristine. Maytansinoids are more potent than vinca alkaloids, and thus have been of limited use in systemic therapy due to unacceptable toxicity. 116 However, this potency makes them attractive candidates for targeted delivery. In preclinical studies, SAR3419 monotherapy delayed progression in pre-B-ALL xenografts and provided objective response. When used in combination with a chemotherapy regimen that mimicked pediatric induction protocols, SAR3419 was effective at prolonging the duration of remission. 117 SAR3419 was then evaluated in a Phase 1 clinical trial with CD19+ B-cell lymphoma. Dose-limiting toxicities were reversible blurred vision and neuropathy. A maximum tolerated dose (MTD) of 160 mg/m 2 administered once every three weeks was established. Reduction of tumor size was seen in 74% of patients, including 47% of patients with rituximab-resistant disease. 118 An initial phase 2 clinical trial was terminated early due to low response rate of 25%. 119
Denintuzumab mafodotin (SGN-CD19A)
A second anti-CD19 conjugated monoclonal antibody, denintuzumab mafodotin, is currently in development. In this case, the antibody is linked to the microtubule-disrupting agent monomethyl auristatin F (MMAF). In a phase 1 study of patients with relapsed/refractory B-ALL or aggressive B-cell lymphomas, a complete response rate of 35% was observed. 120 Dosing interval of 3 weeks was shown to be superior to weekly dosing. An MTD was identified at 5 mg/kg q3wk. Interestingly, among Ph-positive B-ALL, the response rate was 63%, leading to recruitment of Ph-positive patients for an expansion cohort. These results warrant further evaluation of denintuzumab mafodotin for relapsed/refractory ALL.
ADCT-402 is the newest anti-CD19 monoclonal antibody to enter development. It is a humanized monoclonal antibody conjugated to a pyrrolobenzodiazepine (PBD). PBDs are a class of natural antibiotics derived from actinomycetes bacteria that inhibit cell division by binding in the minor groove of DNA and cross-linking strands of DNA. In vivo studies show superior antitumor activity of ADCT-402 against CD19-positive lymphoma than maytansinoid or auristatin based therapy. 121 A phase 1 trial of ADCT-402 for relapsed/refractory ALL is underway (NCT02669264).
CD25 is a cell surface antigen and component of the Interleukin-2 receptor (IL-2 R) heterotrimer. 122 Binding of IL-2 R by its ligand activates JAK/STAT, MAP kinase and phosphoinositide 3-kinase (PI3K) signaling pathways, leading to cell proliferation. IL-2 R is rapidly recycled upon binding of its ligand. 123 The IL-2 R signaling pathway is particularly activated in T-cell immune response, and has thus been an attractive target for post-transplant immunosuppression. In some studies, CD25 expression has been as high as 30% of pre-B-ALL lymphoblasts, including 100% expression among the Ph-positive subset. 124
ADCT-301 is a monoclonal antibody against CD25 conjugated to a PBD. In preclinical studies, ADCT-301 has been shown to be potently cytotoxic to CD25-positive anaplastic large cell lymphoma and Hodgkin lymphoma cell lines. In vivo , ADCT-301 exhibited antitumor activity in xenograft and disseminated mouse models. 122 A phase 1 trial is recruiting participants for ADCT-301 in relapsed/refractory AML and ALL (NCT02588092).
2-Proteasome inhibitor (Bortezomib)
Bortezomib, a proteasome inhibitor, was first approved for the treatment of multiple myeloma. Preclinical studies have suggested a synergistic role of bortezomib with dexamethasone and additive effects to standard chemotherapy agents in acute leukemias. 125 As a single agent, bortezomib did not produce durable responses in patients with relapsed/refractory ALL, despite demonstrable proteasomal inhibition. 126 However, in a phase 2 study, bortezomib in combination with vincristine, dexamethasone, pegylated asparaginase and doxorubicin produced a response rate of 80% in children with relapsed/refractory pre-B-ALL. 127 In a recent phase 2 COG trial, re-induction chemotherapy plus bortezomib resulted in a complete response in 68% of children with relapsed pre-B-ALL. 128 Due to it’s ability to inhibit the NF-κB and NOTCH1 signaling pathways, bortezomib is being studied as frontline therapy in T-cell ALL. Recruitment is ongoing for a phase 3 trial of standard chemotherapy with or without bortezomib in children and young adults (age 2–30) with newly diagnosed T-cell ALL or T-cell lymphoblastic lymphoma (NCT02112916). In adults, recruitment has begun for a phase 2 trial of bortezomib with combination chemotherapy in relapsed/refractory ALL (NCT01769209).
3-JAK inhibitor (Ruxolitinib)
The JAK/STAT signaling pathway has been identified as a significant mechanism by which leukemic cells bypass normal growth and proliferation restrictions. 13 In particular, Ph-like ALL appears to be dependent on JAK signaling. The most common rearrangements in Ph-like ALL involve the transmembrane receptor CRLF2, which signals through downstream JAK kinases. Many cytokine receptors, including IL-7 R, act through JAK kinases as well. In addition, JAK1 and JAK2 mutations are found in approximately half of CRLF2-rearranged Ph-like ALL. 12 , 13 , 14 Preclinical studies have suggested benefit of ruxolitinib for the treatment of Ph-like ALL and CRLF2-rearranged ALL. 129 , 130 In addition, ruxolitinib inhibited tumor growth in in vitro and in vivo models of T-ALL with a gain of function in IL-7 R-alpha subunit. 131 A phase 2 trial of ruxolitinib with standard multi-agent chemotherapy is currently open for recruitment of children, adolescents and adults with newly diagnosed high-risk B-ALL with CRLF2 rearrangements (NCT02723994).
4-Hypomethylating agent (Decitabine)
DNA methylation is an important epigenetic modification that regulates gene expression. It has long been reported that DNA methylation may play a role in the development of ALL and that methylation status may be used as part of risk stratification. 132 , 133 , 134 , 135 Decitabine is a cytosine analog that inhibits DNA methyltransferase by targeting it for degredation, thus causing hypomethylation of key regulatory domains on DNA. This leads to differentiation and suppression of tumor growth. 136 Decitabine is currently approved for the treatment of myelodysplastic syndrome (MDS). In a case report, a young girl with her third-relapse of ALL was treated with a decitabine and dexamethasone regimen based on MDS dosing. She was able to undergo Allo-SCT after CR was achieved with re-induction therapy and remained in CR 8 months after transplant. 137 In a MD Anderson phase I trial of decitabine for relapsed/refractory ALL, decitabine was shown to have efficacy when used in combination with Hyper-CVAD for re-induction therapy. 138 In addition, decitabine monotherapy is well tolerated and thus offers a potential treatment option for relapsed disease in patients that cannot tolerate multi-agent chemotherapy. In a phase 2 study, decitabine and vorinostat (a histone deacetylase inhibitor) were given prior to vincristine, prednisone, PEG-L-asparaginase and doxorubicin for relapsed/refractory ALL. 139 Results were promising with a CR rate of 50% (95% CI, 15.7–84.3%) and the OR rate 75% (95% CI, 34.9–96.8%). Decitabine has also been studied in preclinical trials of early T-cell precursor ALL (ETP-ALL), where it has been shown to be synergistic to conventional chemotherapy. 140 Decitabine is currently being studied in the post-Allo-SCT setting (NCT02264873) and in combination with clofarabine, idarubicin and cytarabine for relapsed/refractory AML and ALL (NCT01794702).
5-PI3K/mTOR Inhibitors
The phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) and mammalian target of rapamycin (mTOR) pathways are shown to be constitutively activated in 50–75% of T-ALL. 141 Preclinical studies suggest that inhibition of the PI3K/AKT/mTOR pathways may be an effective treatment for T-ALL. 142 , 143 , 144 , 145 A dual PI3K/mTOR inhibitor, NVP-BEZ235, potently inhibited the proliferation ALL cells in vitro , causing G 0 /G 1 arrest. Moreover, inhibition of proliferation was synergistic when NVP-BEZ235 was combined with cytotoxic agents. 144 On the basis of this promising preclinical data, several clinical trials are underway to evaluate the use of mTOR and PI3K inhibitors in combination with multi-agent chemotherapy in the frontline and relapsed/refractory setting (NCT01756118, NCT02484430, NCT01523977, NCT01403415, NCT01614197 and NCT01184885).
6-Chimeric antigen receptor (CAR) T cells
Chimeric antigen receptor-modified (CAR) T cells are genetically engineered T cells that express the antigen-binding domain of an immunoglobulin linked via transmembrane domains to the intracellular T-cell receptor signaling moieties. 146 This allows the T cells to recognize unprocessed antigens and to be activated in a major histocompatibility complex (MHC)-independent manner. First generation CAR-Ts contain intracellular signaling moieties derived only from the T-cell receptor/CD3 complex. In contrast, second- and third-generation CAR-Ts include co-stimulatory signals in the CAR gene constructs. More recently, fourth-generation CAR-Ts have been engineered to include a cytokine-expressing cassette.
The process of CAR T-cell therapy involves collecting T cells, introducing the CAR construct, and then an autologous transplant of the modified T cells back into the patient. Options for gene delivery methods include viral vectors and RNA-based methods. 147 Using a viral vector has the benefit of inducing permanent gene expression and thus offering antitumor activity for as long as the transduced T cells persist. Theoretic risks of this method include malignant transformation of the engineered T cells if the CAR construct is inserted in such a way that it deregulates the expression of an oncogene. 148 Another method of gene delivery involves direct transfer of an mRNA construct through electroporation. 149 As no DNA is inserted into the genome of the T-cell, this eliminates the risk of malignant transformation. Given the high replicative potential of these T cells, this methods also offers the advantage of a profound antitumor response. 150 However, the effects of direct mRNA insertion are transient and antitumor activity rarely persists beyond 7 days. Preclinical studies have suggested a role for RNA-based methods with multiple infusions; however, all current clinical trials utilize a viral vector to deliver the CAR construct. 150
As mentioned above, CD19 is an ideal target for immunotherapy against B-cell ALL due to its near universal expression on B-lymphoblasts. In a pilot study at the Children’s Hospital of Philadelphia, Grupp et al. 151 treated 53 children with relapsed/refractory ALL with lymphocyte depleting chemotherapy followed by CD19-directed CAR-Ts. A CR was observed in 50 patients (94%), with a 12-month EFS rate of 45% (95% CI, 31–66%) and OS rate of 78% (95% CI, 67–91%). The CAR-Ts were persistent at 6 months in 68% of the patients. Nearly all of the patients developed cytokine release syndrome (CRS). The 15 patients in which CRS was severe were effectively treated with the anti-IL-6-receptor antibody, tocilizumab. 152 Important causes of treatment failure included the loss of circulating CAR-Ts and the expansion of a CD19-negative clone. CAR-Ts have also shown activity in adults with relapsed/refractory B-ALL. Davila et al. 153 treated 16 adults at Memorial Sloan Kettering Cancer Center (MSKCC) with conditioning chemotherapy followed by CD19-directed CAR T-cell infusion. CR was observed in 88% of patients, with a 1–3 month persistence of CAR-Ts. Lee et al. 154 reported a 66.7%% CR rate in a National Cancer Institute (NCI) intent-to-treat analysis of 20 children and young adults with ALL, with a median CAR-T persistence of 68 days. These data suggest a role for CAR-Ts in the treatment of relapsed/refractory ALL as a bridge to Allo-SCT or to produce durable remission. Limitations include the expansion of CD19-negative clones, the lack of long-term persistence of CAR-Ts after a single infusion, and the risk of CRS. Studies are ongoing to identify factors associated with the development of severe CRS and predict patients that would benefit from pretreatment. 155 , 156
Recently, the application of CAR-T cells has been expanded to CD22-positive B-ALL. Early preclinical studies have showed antitumor activity of CD22-directed CAR-Ts in in vitro and in vivo models that approximates that of CD19-directed CAR-Ts. 157 Based on these findings, phase 1 trials using CD22-directed CAR-Ts are in the recruiting stages (NCT02650414). Preliminary results of nine patients have demonstrated that therapy is well tolerated and produced a sustained remission at 3 months in all three patients treated with a dose level of 1 × 10 6 transduced T cells/kg. 158
Hematopoietic stem cell transplantation
After achieving complete response, treatment options include consolidation and maintenance chemotherapy or Allo-SCT for eligible patients. For high-risk patients and patients with relapsed/refractory disease, Allo-SCT has long been considered the standard of care and best chance for a durable response. While criteria differ between studies, in general high-risk disease is defined as Ph-positive ALL, elevated WBC count, CNS disease, high-risk gene rearrangements, or hypodiploidy. The LALA-94 and City of Hope and Stanford University series have shown a benefit of Allo-SCT over standard chemotherapy in these high-risk patients. 49 , 159 , 160 It is therefore recommended that all high-risk young adults with an available donor undergo Allo-SCT during their first CR (CR1). Recent studies have suggested that patients with ETP-ALL and Ph-like ALL be treated as high-risk and be offered Allo-SCT during CR1 as well. 161 , 162 The role of Allo-SCT in standard-risk adults is less clearly defined. In general, MRD has emerged as a prognostic marker that can restratify patients to high-risk, making them candidates for Allo-SCT. Studies 32 found that MRD-positivity is an independent risk factor for decreased relapse-free and overall survival. Subsequently, other studies 163 evaluated the risk factors in patients treated with Allo-SCT versus standard chemotherapy after CR1. In patients with positive MRD, Allo-SCT was associated with improved relapse-free survival. However, in patients with a complete MRD response, there was no survival benefit to Allo-SCT over standard chemotherapy. 163
Allo-SCT also should be considered in all patients that relapse, optimally after achieving a second CR (CR2). The LALA-94 trial showed a 5-year OS of 33% in patients who were able to undergo Allo-SCT during CR2 compared to 8% in patients who underwent Allo-SCT during active relapse. 164 Patients who are unable to achieve CR2 by conventional methods should be considered for clinical trials with novel agents as a bridge to Allo-SCT. In the MRC/ECOG 2993 study, 5-year survival was highest in the group receiving a sibling donor Allo-SCT compared to unmatched donor or chemotherapy alone (23%, 16% and 4%, respectively). 165
Acute lymphoblastic leukemia has been touted as a major success story in pediatric oncology through the implementation of dose-intensification chemotherapy and Allo-SCT. However, due to high-risk disease characteristics and significant toxicity associated with chemotherapy in adults, outcomes are far less encouraging. There remains much uncertainty about how best to treat adults with ALL, as some studies have shown benefit of pediatric-inspired regimens. However, not all adults are able to tolerate such dose intensification and the exact subset of patients who are likely to benefit has not clearly been defined. Furthermore, elderly patients are particularly susceptible to the dose-limiting toxicities of these agents and are often excluded from Allo-SCT on the basis of performance status and medical comorbidities. Novel targeted therapies offer the promise of effective anti-leukemic activity with reduced toxicity from off-target effects. Given the diverse molecular and genetic alterations occurring in ALL, it is unlikely that a single agent will be effective for all patients with ALL. However, with the ability to characterize the immunophenotype and genotype of each patient’s leukemia, targeted therapy can be expected to lead to improvements in remission and survival as part of individualized treatment strategies. The successes from tyrosine kinase inhibition in CML have been translated to Ph-positive ALL, and second and third generation TKIs are being studied for use in high-risk Ph-like disease. Other signaling pathways, such as PI3K/AKT/mTOR pathway, are also promising targets for small molecule inhibition. In addition to targeting intracellular pathways, monoclonal antibodies recognize cell surface antigens. Immunoconjugates, such as inotuzumab ozogamicin, bind to leukemic cells, are internalized and release a cytotoxin that kills the leukemic cell; whereas dual-specific antibodies, such as blinatumumab, cause the direct activation of T cells against blasts. CAR-Ts involve a similar mechanism, in which a patient’s own T cells are genetically programmed to recognize leukemic cells, inducing an anti-leukemic immune response. Finally, existing agents, such as bortezomib, decitabine and ruxolitinib that are well tolerated in the treatment of various malignancies are now being studied for application in ALL. As the role of these novel agents is further defined and integrated into new treatment strategies, adult ALL may follow pediatric ALL as a major success story in the near future.
National Cancer Institute. SEER cancer statistics review, 1975-2013:Leukemia, annual incidence rates (acute lymphocytic leukemia).
Paul S, Kantarjian H, Jabbour EJ . Adult Acute Lymphoblastic Leukemia. Mayo Clin Proc 2016; 91 : 1645–1666.
PubMed Google Scholar
Jabbour E, O'Brien S, Konopleva M, Kantarjian H . New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia. Cancer 2015; 121 : 2517–2528.
Shah A, John BM, Sondhi V . Acute lymphoblastic leukemia with treatment—naive Fanconi anemia. Indian Pediatr 2013; 50 : 508–510.
German J . Bloom's syndrome. XX. The first 100 cancers. Cancer Genet Cytogenet 1997; 93 : 100–106.
CAS PubMed Google Scholar
Bielorai B, Fisher T, Waldman D, Lerenthal Y, Nissenkorn A, Tohami T et al . Acute lymphoblastic leukemia in early childhood as the presenting sign of ataxia-telangiectasia variant. Pediatr Hematol Oncol 2013; 30 : 574–582.
Chessells J, Harrison G, Richards S, Bailey C, Hill F, Gibson B et al . Down's syndrome and acute lymphoblastic leukaemia: clinical features and response to treatment. Arch Dis Child 2001; 85 : 321–325.
CAS PubMed PubMed Central Google Scholar
Spector LG, R J, Robison LL, Bhatia S . Epidemiology and Etiology Childhood Leukemias , 2nd edition. Cambridge University Press, pp 48–66.
Sehgal S, Mujtaba S, Gupta D, Aggarwal R, Marwaha RK . High incidence of Epstein Barr virus infection in childhood acute lymphocytic leukemia: a preliminary study. Indian J Pathol Microbiol 2010; 53 : 63–67.
Geriniere L, Bastion Y, Dumontet C, Salles G, Espinouse D, Coiffier B . Heterogeneity of acute lymphoblastic leukemia in HIV-seropositive patients. Ann Oncol 1994; 5 : 437–440.
Mullighan CG, Collins-Underwood JR, Phillips LA, Loudin ML, Liu W, Zhang J et al . Rearrangement of CRLF2 in B-progenitor and down syndrome associated acute lymphoblastic leukemia. Nat Genet 2009; 41 : 1243–1246.
Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD et al . Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature 2007; 446 : 758–764.
Roberts KG, Morin RD, Zhang J, Hirst M, Zhao Y, Su X et al . Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell 2012; 22 : 153–166.
Roberts KG, Li Y, Payne-Turner D, Harvey RC, Yang YL, Pei D et al . Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med 2014; 371 : 1005–1015.
PubMed PubMed Central Google Scholar
Holmfeldt L, Wei L, Diaz-Flores E, Walsh M, Zhang J, Ding L et al . The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat Genet 2013; 45 : 242–252.
Alvarnas JC, Brown PA, Aoun P, Ballen KK, Barta SK, Borate U et al . Acute lymphoid leukemia (version 2.2015). Natl Comprehens Cancer Netw 2015; 13 : 1240–1279.
CAS Google Scholar
Jabbour EJ, Faderl S, Kantarjian HM . Adult acute lymphoblastic leukemia. Mayo Clin Proc 2005; 80 : 1517–1527.
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR et al . Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol 1976; 33 : 451–458.
Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J et al . World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol 1999; 17 : 3835–3849.
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A et al . The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114 : 937–951.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM et al . The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127 : 2391–2405.
Rowe JM . Prognostic factors in adult acute lymphoblastic leukaemia. Br J Haematol 2010; 150 : 389–405.
Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM et al . Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood 2005; 106 : 3760–3767.
Faderl, S HM Kantarjian, et al , T.U.o.T.M.D.A.C.C. Department of Leukemia, Houston, Texas, T.U.o.T.M.D.A.C.C. Department of Leukemia, P.O. Box 428, 1515 Holcombe Blvd., Houston, TX 77030, S. Jeha, T.U.o.T.M.D.A.C.C. Department of Leukemia, Houston, Texas, The biology and therapy of adult acute lymphoblastic leukemia. Cancer, 2017; 98 : 1337–1354.
Moorman AV, Harrison CJ, Buck GA, Richards SM, Secker-Walker LM, Martineau M et al . Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood 2007; 109 : 3189–3197.
Pullarkat V, Slovak ML, Kopecky KJ, Forman SJ, Appelbaum FR . Impact of cytogenetics on the outcome of adult acute lymphoblastic leukemia: results of Southwest Oncology Group 9400 study. Blood 2008; 111 : 2563–2572.
Hunger SP, Mullighan CG . Redefining ALL classification: toward detecting high-risk ALL and implementing precision medicine. Blood 2015; 125 : 3977–3987.
Dongen JJMv, v.d. Velden VHJ, Brüggemann M, Orfao A . Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood 2015; 125 : 3996–4009.
Bruggemann M, Raff T, Flohr T, Gokbuget N, Nakao M, Droese J et al . Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood 2006; 107 : 1116–1123.
Jacquy C, Delepaut B, Van Daele S, Vaerman JL, Zenebergh A, Brichard B et al . A prospective study of minimal residual disease in childhood B-lineage acute lymphoblastic leukaemia: MRD level at the end of induction is a strong predictive factor of relapse. Br J Haematol 1997; 98 : 140–146.
Sutton R, Venn NC, Tolisano J, Bahar AY, Giles JE, Ashton LJ et al . Clinical significance of minimal residual disease at day 15 and at the end of therapy in childhood acute lymphoblastic leukaemia. Br J Haematol 2009; 146 : 292–299.
Bassan R, Spinelli O, Oldani E, Intermesoli T, Tosi M, Peruta B et al . Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood 2009; 113 : 4153–4162.
Campana D . Minimal residual disease in acute lymphoblastic leukemia. Semin Hematol 2009; 46 : 100–106.
Raff T, Gökbuget N, Lüschen S, Reutzel R, Ritgen M, Irmer S et al . Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials. Blood 2007; 109 : 910–915.
Ribera JM, Oriol A, Morgades M, Montesinos P, Sarra J, Gonzalez-Campos J et al . Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial. J Clin Oncol 2014; 32 : 1595–1604.
Huguet F, Leguay T, Raffoux E, Thomas X, Beldjord K, Delabesse E et al . Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol 2009; 27 : 911–918.
Stock W, La M, Sanford B, Bloomfield CD, Vardiman JW, Gaynon P et al . What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood 2008; 112 : 1646–1654.
Scavino HF, George JN, Sears DA . Remission induction in adult acute lymphocytic leukemia. Use of vincristine and prednisone alone. Cancer 1976; 38 : 672–677.
Gottlieb A, Weinberg V, Ellison R, Henderson E, Terebelo H, Rafla S et al . Efficacy of daunorubicin in the therapy of adult acute lymphocytic leukemia: a prospective randomized trial by cancer and leukemia group B. Blood 1984; 64 : 267–274.
Larson RA, Dodge RK, Burns CP, Lee EJ, Stone RM, Schulman P et al . A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811. Blood 1995; 85 : 2025–2037.
Nagura E, Kimura K, Yamada K, Ota K, Maekawa T, Takaku F et al . Nation-wide randomized comparative study of doxorubicin, vincristine and prednisolone combination therapy with and without L-asparaginase for adult acute lymphoblastic leukemia. Cancer Chemother Pharmacol 1994; 33 : 359–365.
Short NJ, Jabbour E, Sasaki K, Patel K, O'Brien SM, Cortes JE et al . Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 2016; 128 : 504–507.
Patel B, Kirkwood A, Dey A, Rowntree C, McMillan A, Marks D et al . Feasibility Of pegylated-asparaginase (PEG-ASP) during induction in adults with acute lymphoblastic leukaemia (ALL): results from the UK Phase 3 Multicentre Trial UKALL 14. 2013.
Kantarjian HM, O'Brien S, Smith TL, Cortes J, Giles FJ, Beran M et al . Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol 2000; 18 : 547–561.
Hurwitz CA, Silverman LB, Schorin MA, Clavell LA, Dalton VK, Glick KM et al . Substituting dexamethasone for prednisone complicates remission induction in children with acute lymphoblastic leukemia. Cancer 2000; 88 : 1964–1969.
Jones B, Freeman AI, Shuster JJ, Jacquillat C, Weil M, Pochedly C et al . Lower incidence of meningeal leukemia when prednisone is replaced by dexamethasone in the treatment of acute lymphocytic leukemia. Med Pediatr Oncol 1991; 19 : 269–275.
Narayanan S, Shami PJ . Treatment of acute lymphoblastic leukemia in adults. Crit Rev Oncol Hematol 2012; 81 : 94–102.
Faderl S, Kantarjian HM, Thomas DA, Cortes J, Giles F, Pierce S et al . Outcome of Philadelphia chromosome-positive adult acute lymphoblastic leukemia. Leuk Lymphoma 2000; 36 : 263–273.
Dombret H, Gabert J, Boiron JM, Rigal-Huguet F, Blaise D, Thomas X et al . Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia—results of the prospective multicenter LALA-94 trial. Blood 2002; 100 : 2357–2366.
Laport GG, Alvarnas JC, Palmer JM, Snyder DS, Slovak ML, Cherry AM et al . Long-term remission of Philadelphia chromosome-positive acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation from matched sibling donors: a 20-year experience with the fractionated total body irradiation-etoposide regimen. Blood 2008; 112 : 903–909.
Fielding AK, Rowe JM, Richards SM, Buck G, Moorman AV, Durrant IJ et al . Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood 2009; 113 : 4489–4496.
Thomas DA, Faderl S, Ravandi Kashani F, Wierda WG, Andreeff M, Garris RS et al . Long-term outcome after hyper-CVAD and imatinib (IM) for de novo or minimally treated Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph-ALL)[abstract]. 2010; 28(15 s):abstr 8506:[Available from http://meetinglibrary.asco.org/content/52502-74 .
Thomas DA, Faderl S, Cortes J, O'Brien S, Giles FJ, Kornblau SM et al . Treatment of Philadelphia chromosome–positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood 2004; 103 : 4396–4407.
Jones D, Thomas D, Yin CC, O'Brien S, Cortes JE, Jabbour E et al . Kinase domain point mutations in Philadelphia chromosome-positive acute lymphoblastic leukemia emerge after therapy with BCR-ABL kinase inhibitors. Cancer 2008; 113 : 985–994.
Porkka K, Koskenvesa P, Lundan T, Rimpilainen J, Mustjoki S, Smykla R et al . Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood 2008; 112 : 1005–1012.
Ravandi F, O'Brien SM, Cortes JE, Thomas DM, Garris R, Faderl S et al . Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer 2015; 121 : 4158–4164.
Ravandi F, Othus M, O'Brien S, Forman SJ, Ha CS, Wong JYC et al . Multi-Center US Intergroup Study of Intensive Chemotherapy Plus Dasatinib Followed By Allogeneic Stem Cell Transplant in Patients with Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia Younger Than 60 . 2015.
Ottmann O, Dombret H, Martinelli G, Simonsson B, Guilhot F, Larson RA et al . Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood 2007; 110 : 2309–2315.
Foà R, Vitale A, Vignetti M, Meloni G, Guarini A, Propris MSD et al . Dasatinib as first-line treatment for adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia. Blood 2011; 118 : 6521–6528.
Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C et al . A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med 2013; 369 : 1783–1796.
Sasaki K, Jabbour EJ, Ravandi F, Short NJ, Thomas DA, Garcia-Manero G et al . Hyper-CVAD plus ponatinib versus hyper-CVAD plus dasatinib as frontline therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: A propensity score analysis. Cancer 2016; 122 : 3650–3656.
Sasaki Koji, Ravandi Farhad, Thomas Deborah A, Cortes Jorge E, Pemmaraju Naveen, Kadia Tapan M et al . Updated results from phase II study of combination of hyper-CVAD (HCVAD) with ponatinib in frontline therapy of patients (pts) with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL)[abstract]. 2016; 34 s; abstr 7036: Available from http://meetinglibrary.asco.org/content/167324-176 .
Nachman J, Siebel N, Sather H, Steinherz P, DeLaat C, Fryer D et al . Outcome for Adolescent and Young Adults 16–21 years of age (AYA) with Acute Lymphoblastic Leukemia (ALL) Treated on the Children’ s Cancer Group (CCG) 1961 Study . 2004.
Stock W, Luger SM, Advani AS, Geyer S, Harvey RC, Mullighan CG et al . Favorable Outcomes for Older Adolescents and Young Adults (AYA) with Acute Lymphoblastic Leukemia (ALL): Early Results of US Intergroup Trial C10403 , 2014.
Rytting ME, Jabbour EJ, Jorgensen JL, Ravandi F, Franklin AR, Kadia TM et al . Final results of a single institution experience with a pediatric-based regimen, the augmented Berlin-Frankfurt-Munster, in adolescents and young adults with acute lymphoblastic leukemia, and comparison to the hyper-CVAD regimen. Am J Hematol 2016; 91 : 819–823.
Faderl S, Thomas DA, O'Brien S, Ravandi F, Garcia-Manero G, Borthakur G et al . Augmented hyper-CVAD based on dose-intensified vincristine, dexamethasone, and asparaginase in adult acute lymphoblastic leukemia salvage therapy. Clin Lymphoma Myeloma Leuk 2011; 11 : 54–59.
O’Brien S, Thomas D, Ravandi F, Faderl S, Cortes J, Borthakur G et al . Outcome of Adults With Acute Lymphocytic Leukemia After Second Salvage Therapy. Cancer 2008; 113 : 3186–3191.
Deitcher OR, O'Brien S, Deitcher SR, Thomas DA, Kantarjian HM . Single-Agent Vincristine Sulfate Liposomes Injection (Marqibo) Compared to Historical Single-Agent Therapy for Adults with Advanced, Relapsed and/or Refractory Philadelphia Chromosome Negative Acute Lymphoblastic Leukemia , 2011.
O'Brien S, Schiller G, Lister J, Damon L, Goldberg S, Aulitzky W et al . High-Dose Vincristine Sulfate Liposome Injection for Advanced, Relapsed, and Refractory Adult Philadelphia Chromosome–Negative Acute Lymphoblastic Leukemia. in J Clin Oncol 2013; 31 : 676–683.
Nagorsen D, Kufer P, Baeuerle PA, Bargou R . Blinatumomab: a historical perspective. Pharmacol Ther 2012; 136 : 334–342.
Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S et al . Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 2011; 29 : 2493–2498.
Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C et al . Long-Term Outcomes after Blinatumomab Treatment: Follow-up of a Phase 2 Study in Patients (Pts) with Minimal Residual Disease (MRD) Positive B-Cell Precursor Acute Lymphoblastic Leukemia (ALL). Blood 2015; 126 : 680–680.
Google Scholar
Topp MS, Gokbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC et al . Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2015; 16 : 57–66.
Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera J-M et al . Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. NEJM 2017; 376 : 836–847.
Martinelli G, Dombret H, Chevallier P, Ottmann OG, Goekbuget N, Topp MS et al . Complete Molecular and Hematologic Response in Adult Patients with Relapsed/Refractory (R/R) Philadelphia Chromosome-Positive B-Precursor Acute Lymphoblastic Leukemia (ALL) Following Treatment with Blinatumomab: Results from a Phase 2 Single-Arm, Multicenter Study (ALCANTARA)[Abstract]. Blood 2015; 126 : 679.
D-ALBA Frontline Sequential Dasatinib and Blinatumomab in Adult Philadelphia Positive Acute Lymphoblastic Leukemia - Full Text View - ClinicalTrials.gov. 2017; Available at: https://clinicaltrials.gov/ct2/show/NCT02744768 .
DeAngelo DJ, Yu D, Johnson JL, Coutre SE, Stone RM, Stopeck AT et al . Nelarabine induces complete remissions in adults with relapsed or refractory T-lineage acute lymphoblastic leukemia or lymphoblastic lymphoma: Cancer and Leukemia Group B study 19801. Blood 2007; 109 : 5136–5142.
Shah NN, Stevenson MS, Yuan CM, Richards K, Delbrook C, Kreitman RJ et al . Characterization of CD22 expression in acute lymphoblastic leukemia. Pediatr Blood Cancer 2015; 62 : 964–969.
Piccaluga PP, Arpinati M, Candoni A, Laterza C, Paolini S, Gazzola A et al . Surface antigens analysis reveals significant expression of candidate targets for immunotherapy in adult acute lymphoid leukemia. Leuk Lymphoma 2011; 52 : 325–327.
Chevallier P, Robillard N, Houille G, Ayari S, Guillaume T, Delaunay J et al . Simultaneous study of five candidate target antigens (CD20, CD22, CD33, CD52, HER2) for antibody-based immunotherapy in B-ALL: a monocentric study of 44 cases in Leukemia 2009; England pp 806–807.
Carnahan J, Wang P, Kendall R, Chen C, Hu S, Boone T et al . Epratuzumab, a humanized monoclonal antibody targeting CD22: characterization of in vitro properties. Clin Cancer Res 2003; 9 (10 Pt 2): 3982s–3990ss.
Raetz EA, Cairo MS, Borowitz MJ, Blaney SM, Krailo MD, Leil TA et al . Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children's Oncology Group Pilot Study. J Clin Oncol 2008; 26 : 3756–3762.
Advani AS, McDonough S, Coutre S, Wood B, Radich J, Mims M et al . SWOG S0910: a phase 2 trial of clofarabine/cytarabine/epratuzumab for relapsed/refractory acute lymphocytic leukaemia. Br J Haematol 2014; 165 : 504–509.
Sharkey RM, Govindan SV, Cardillo TM, Goldenberg DM . Epratuzumab–SN-38: A New Antibody–Drug Conjugate for the Therapy of Hematologic Malignancies. Mol Cancer Ther 2012; 11 : 224–234.
Hinman LM, Hamann PR, Wallace R, Menendez AT, Durr FE, Upeslacis J . Preparation and characterization of monoclonal antibody conjugates of the calicheamicins: a novel and potent family of antitumor antibiotics. Cancer Res 1993; 53 : 3336–3342.
DiJoseph JF, Armellino DC, Boghaert ER, Khandke K, Dougher MM, Sridharan L et al . Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood 2004; 103 : 1807–1814.
Advani A, Coiffier B, Czuczman MS, Dreyling M, Foran J, Gine E et al . Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin's lymphoma: results of a phase I study. J Clin Oncol 2010; 28 : 2085–2093.
Kantarjian H, Thomas D, Jorgensen J, Jabbour E, Kebriaei P, Rytting M et al . Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol 2012; 13 : 403–411.
Kantarjian H, Thomas D, Jorgensen J, Kebriaei P, Jabbour E, Rytting M et al . Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer 2013; 119 : 2728–2736.
Advani AS, Stein AS, Kantarjian HM, Shustov AR, DeAngelo DJ, Ananthakrishnan R et al . A Phase II Study of Weekly Inotuzumab Ozogamicin (InO) in Adult Patients with CD22-Positive Acute Lymphoblastic Leukemia (ALL) in Second or Later Salvage. Blood 2014; 124 : 2255.
Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W et al . Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med 2016; 375 : 740–753.
Jabbour E, O’Brien S, Thomas D, Sasaki K, Garcia-Manero G, Ravandi F et al . Inotuzumab Ozogamicin (IO) in Combination with Low-Intensity Chemotherapy (mini-hyper-CVD) as Frontline Therapy for Older Patients (pts) and as Salvage Therapy for Adult with Relapsed/Refractory (R/R) Acute Lymphoblastic Leukemia (ALL). Clin Lymphoma Myeloma Leuk 2015, 15.
Kreitman RJ, Pastan I . Antibody Fusion Proteins: Anti-CD22 Recombinant Immunotoxin Moxetumomab Pasudotox. Clin Cancer Res 2011; 17 : 6398–6405.
Kreitman RJ, Stetler-Stevenson M, Margulies I, Noel P, FitzGerald DJP, Wilson WH et al . Phase II Trial of Recombinant Immunotoxin RFB4(dsFv)-PE38 (BL22) in Patients With Hairy Cell Leukemia. J Clin Oncol 2009; 27 : 2983–2990.
Wayne AS, Kreitman RJ, Findley HW, Lew G, Delbrook C, Steinberg SM et al . Anti-CD22 Immunotoxin RFB4(dsFv)-PE38 (BL22) for CD22-Positive Hematologic Malignancies of Childhood: Preclinical Studies and Phase I Clinical Trial. Clin Cancer Res 2010; 16 : 1894–1903.
Wayne AS, Bhojwani D, Silverman LB, Richards K, Stetler-Stevenson M, Shah NN et al . A Novel Anti-CD22 Immunotoxin, Moxetumomab Pasudotox: Phase I Study in Pediatric Acute Lymphoblastic Leukemia (ALL). Blood 2011; 118 : 248.
Ravandi F, Kantarjian HM, Goswami T, Wang F, Ibrahim R . Design Of a Phase 1/2 Study Of Moxetumomab Pasudotox In Adult Patients With Relapsed and/Or Refractory Acute Lymphoblastic Leukemia (ALL). Blood 2013; 122 : 5021.
Herrera L, Bostrom B, Gore L, Sandler E, Lew G, Schlegel PG et al . A phase 1 study of Combotox in pediatric patients with refractory B-lineage acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2009; 31 : 936–941.
Schindler J, Gajavelli S, Ravandi F, Shen Y, Parekh S, Braunchweig I et al . A Phase I Study of a Combination of anti-CD19 and anti-CD22 Immunotoxins (Combotox) in Adult Patients with Refractory B-Lineage Acute Lymphoblastic Leukaemia. Br J Haematol 2011; 154 : 471–476.
Thomas DA, O'Brien S, Jorgensen JL, Cortes J, Faderl S, Garcia-Manero G et al . Prognostic significance of CD20 expression in adults with de novo precursor B-lineage acute lymphoblastic leukemia. Blood 13 : 6330–6337.
Wierda WG, Padmanabhan S, Chan GW, Gupta IV, Lisby S, Österborg A et al . Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase 2 international study. Blood 2011; 118 : 5126–5129.
Teeling JL, French RR, Cragg MS, v.d. Brakel J, Pluyter M, Huang H et al . Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 2004; 104 : 1793–1800.
Pawluczkowycz AW, Beurskens FJ, Beum PV, Lindorfer MA, v.d. Winkel JGJ, Parren PWHI et al . Binding of Submaximal C1q Promotes Complement-Dependent Cytotoxicity (CDC) of B Cells Opsonized with Anti-CD20 mAbs Ofatumumab (OFA) or Rituximab (RTX): Considerably Higher Levels of CDC Are Induced by OFA than by RTX. J Immunol 2009; 183 : 749–758.
Jabbour E, Hagop K, Thomas D, Garcia-Manero G, Hoehn D, Garris R et al . Phase II Study Of The Hyper-CVAD Regimen In Combination With Ofatumumab As Frontline Therapy For Adults With CD-20 Positive Acute Lymphoblastic Leukemia (ALL). Blood 2013; 122 : 2664.
Sasaki K, Kantarjian HM, Ravandi F, Daver N, Kadia TM, Khouri RB et al . Frontline Ofatumumab in Combination with Hyper-CVAD for Adult Patients with CD-20 Positive Acute Lymphoblastic Leukemia (ALL): Interim Result of a Phase II Clinical Trial. Poster presented at the American Society of Hematology 58th Annual Meeting and Exposition . 2 December 2016, San Diego, CA, USA, 2016.
Thomas DA, O'Brien S, Faderl S, Garcia-Manero G, Ferrajoli A, Wierda W et al . Chemoimmunotherapy With a Modified Hyper-CVAD and Rituximab Regimen Improves Outcome in De Novo Philadelphia Chromosome–Negative Precursor B-Lineage Acute Lymphoblastic Leukemia. J Clin Oncol 2010; 28 : 3880–3889.
Herter S, Herting F, Mundigl O, Waldhauer I, Weinzierl T, Fauti T et al . Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther 2013; 12 : 2031–2042.
Awasthi A, Ayello J, Van de Ven C, Elmacken M, Sabulski A, Barth MJ et al . Obinutuzumab (GA101) compared to rituximab significantly enhances cell death and antibody-dependent cytotoxicity and improves overall survival against CD20(+) rituximab-sensitive/-resistant Burkitt lymphoma (BL) and precursor B-acute lymphoblastic leukaemia (pre-B-ALL): potential targeted therapy in patients with poor risk CD20(+) BL and pre-B-ALL. Br J Haematol 2015; 171 : 763–775.
Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM et al . Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions. N Engl J Med 2014; 370 : 1101–1110.
Smith EJ, Olson K, Haber LJ, Varghese B, Duramad P, Tustian AD et al . A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys. Scientific Reports, Published online 2015; 5 : 17943, 2015.
Bannerji R, Brown JR, Advani RH, Arnason J, O'Brien SM, Allan JN et al . Phase 1 Study of REGN1979, an Anti-CD20 x Anti-CD3 Bispecific Monoclonal Antibody, in Patients with CD20+ B-Cell Malignancies Previously Treated with CD20-Directed Antibody Therapy. Blood 2016; 128 : 621.
Fujimoto M, Poe JC, Jansen PJ, Sato S, Tedder TF . CD19 amplifies B lymphocyte signal transduction by regulating Src-family protein tyrosine kinase activation. J Immunol 1999; 162 : 7088–7094.
Otero DC, Omori SA, Rickert RC . CD19-dependent Activation of Akt Kinase in B-lymphocytes. J Biol Chem 2001; 276 : 1474–1478.
Chung EY, Psathas JN, Yu D, Li Y, Weiss MJ, Thomas-Tikhonenko A . CD19 is a major B cell receptor-independent activator of MYC-driven B-lymphomagenesis. J Clin Invest 2012; 122 : 2257–2266.
Ning BT, Tang YM, Chen YH, Shen HQ, Qian BQ . Comparison between CD19 and CD20 expression patterns on acute leukemic cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2005; 13 : 943–947.
Widdison Wayne C, Wilhelm Sharon D, Cavanagh Emily E, Whiteman Kathleen R, Leece Barbara A, Kovtun Yelena et al . Semisynthetic Maytansine Analogues for the Targeted Treatment of Cancer. J Med Chem 2006; 49 : 4392–4408.
Carol H, Szymanska B, Evans K, Boehm I, Houghton PJ, Smith MA et al . The Anti-CD19 Antibody–Drug Conjugate SAR3419 Prevents Hematolymphoid Relapse Postinduction Therapy in Preclinical Models of Pediatric Acute Lymphoblastic Leukemia. Clin Cancer Res 2013; 19 : 1795–1805.
Younes A, Kim S, Romaguera J, Copeland A, Farial Sdc, Kwak LW et al . Phase I Multidose-Escalation Study of the Anti-CD19 Maytansinoid Immunoconjugate SAR3419 Administered by Intravenous Infusion Every 3 Weeks to Patients With Relapsed/Refractory B-Cell Lymphoma. Blood 2009; 114 : 585.
Kantarjian HM, Lioure B, Kim SK, Atallah E, Leguay T, Kelly K et al . A Phase II Study of Coltuximab Ravtansine (SAR3419) Monotherapy in Patients With Relapsed or Refractory Acute Lymphoblastic Leukemia. Clin Lymphoma Myeloma Leuk 2016; 16 : 139–145.
Fathi AT, Borate U, DeAngelo DJ, O'Brien MM, Trippett T, Shah BD et al . A Phase 1 Study of Denintuzumab Mafodotin (SGN-CD19A) in Adults with Relapsed or Refractory B-Lineage Acute Leukemia (B-ALL) and Highly Aggressive Lymphoma. Blood 2015; 126 : 1328.
Zammarchi F, Williams DG, Adams L, Havenith K, Chivers S, D'Hooge F et al . Pre-Clinical Development of Adct-402, a Novel Pyrrolobenzodiazepine (PBD)-Based Antibody Drug Conjugate (ADC) Targeting CD19-Expressing B-Cell Malignancies. Blood 2015; 126 : 1564.
Flynn MJ, Pv Berkel, Zammarchi F, Levy J-N, Tiberghien A, Masterson LA et al . Pre-Clinical Activity of Adct-301, a Novel Pyrrolobenzodiazepine (PBD) Dimer-Containing Antibody Drug Conjugate (ADC) Targeting CD25-Expressing Hematological Malignancies. Blood 2014; 124 : 4491.
Flynn MJ, Berkel PHv, Zammarchi F, Tyrer PC, Akarca AU, Janghra N et al . Pre-Clinical Activity of Adct-301, a Novel Pyrrolobenzodiazepine (PBD) Dimer-Containing Antibody Drug Conjugate (ADC) Targeting CD25-Expressing Hematological Malignancies. Blood 2014; 124 : 4491.
Owaidah TM, Rawas FI, Al Khayatt MF, Elkum NB . Expression of CD66c and CD25 in acute lymphoblastic leukemia as a predictor of the presence of BCR/ABL rearrangement. Hematol Oncol Stem Cell Ther 2008; 1 : 34–37.
Horton TM, Gannavarapu A, Blaney SM, D'Argenio DZ, Plon SE, Berg SL . Bortezomib interactions with chemotherapy agents in acute leukemia in vitro . Cancer Chemother Pharmacol 2006; 58 : 13–23.
Cortes J, Thomas D, Koller C, Giles F, Estey E, Faderl S et al . Phase I Study of Bortezomib in Refractory or Relapsed Acute Leukemias. Clin Cancer Res 2004; 10 : 3371–3376.
Messinger YH, Gaynon PS, Sposto R, v.d. Giessen J, Eckroth E, Malvar J et al . Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study. Blood 2012; 120 : 285–290.
Horton Terzah M, O'Brien Maureen Megan, Borowitz Michael J, Devidas Meenakshi, Raetz Elizabeth A, Brown Patrick Andrew et al . Bortezomib reinduction therapy to improve response rates in pediatric ALL in first relapse: A Childrenâ?'s Oncology Group (COG) study (AALL07P1) [Abstract]. 2013; Available at: http://meetinglibrary.asco.org/content/111816-132 .
Maude SL, Tasian SK, Vincent T, Hall JW, Sheen C, Roberts KG et al . Targeting JAK1/2 and mTOR in murine xenograft models of Ph-like acute lymphoblastic leukemia. Blood 2012; 120 : 3510–3518.
Tasian SK, Doral MY, Borowitz MJ, Wood BL, Chen IM, Harvey RC et al . Aberrant STAT5 and PI3K/mTOR pathway signaling occurs in human CRLF2-rearranged B-precursor acute lymphoblastic leukemia. Blood 2012; 120 : 833–842.
Senkevitch E, Hixon J, Andrews C, Barata JT, Li W, Durum S . The JAK Inhibitor Ruxolitinib Is Effective in Treating T Cell Acute Lymphoblastic Leukemia with Gain of Function Mutations in IL-7R Alpha. Blood 2015; 126 : 1330.
Nordlund J, Milani L, Lundmark A, Lonnerholm G, Syvanen AC . DNA methylation analysis of bone marrow cells at diagnosis of acute lymphoblastic leukemia and at remission. PLoS One 2012; 7 : e34513.
Nordlund J, Backlin CL, Zachariadis V, Cavelier L, Dahlberg J, Ofverholm I et al . DNA methylation-based subtype prediction for pediatric acute lymphoblastic leukemia. Clin Epigenetics 2015; 7 : 11.
Chatterton Z, Morenos L, Mechinaud F, Ashley DM, Craig JM, Sexton-Oates A et al . Epigenetic deregulation in pediatric acute lymphoblastic leukemia. Epigenetics 2014; 9 : 459–467.
Milani L, Lundmark A, Kiialainen A, Nordlund J, Flaegstad T, Forestier E et al . DNA methylation for subtype classification and prediction of treatment outcome in patients with childhood acute lymphoblastic leukemia. Blood 2010; 115 : 1214–1225.
Issa J-PJ . DNA Methylation as a Therapeutic Target in Cancer. Clin Cancer Res 2007; 13 : 1634–1637.
Yánez L, Bermúdez A, Richard C, Bureo E, Iriondo A . Successful induction therapy with decitabine in refractory childhood acute lymphoblastic leukemia. Leukemia 2009; 23 : 1342–1343.
Benton CB, Thomas DA, Yang H, Ravandi F, Rytting M, O’Brien S et al . Safety and clinical activity of 5-aza-2’-deoxycytidine (decitabine) with or without Hyper-CVAD in relapsed/refractory acute lymphocytic leukaemia. Br J Haematol 2014; 167 : 356–365.
Burke MJ, Lamba JK, Pounds S, Cao X, Ghodke-Puranik Y, Lindgren BR et al . A therapeutic trial of decitabine and vorinostat in combination with chemotherapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol 2014; 89 : 889–895.
Lu BY, Thanawala SU, Zochowski KC, Burke MJ, Carroll WL, Bhatla T . Decitabine enhances chemosensitivity of early T-cell precursor-acute lymphoblastic leukemia cell lines and patient-derived samples. Leuk Lymphoma 2016; 57 : 1938–1941.
Zhao WL . Targeted therapy in T-cell malignancies: dysregulation of the cellular signaling pathways. Leukemia 2010; 24 : 13–21.
Chiarini F, Fala F, Tazzari PL, Ricci F, Astolfi A, Pession A et al . Dual inhibition of class IA phosphatidylinositol 3-kinase and mammalian target of rapamycin as a new therapeutic option for T-cell acute lymphoblastic leukemia. Cancer Res 2009; 69 : 3520–3528.
Bressanin D, Evangelisti C, Ricci F, Tabellini G, Chiarini F, Tazzari PL et al . Harnessing the PI3K/Akt/mTOR pathway in T-cell acute lymphoblastic leukemia: Eliminating activity by targeting at different levels. Oncotarget 2012; 3 : 811–823.
Schult C, Dahlhaus M, Glass A, Fischer K, Lange S, Freund M et al . The dual kinase inhibitor NVP-BEZ235 in combination with cytotoxic drugs exerts anti-proliferative activity towards acute lymphoblastic leukemia cells. Anticancer Res 2012; 32 : 463–474.
Saunders P, Cisterne A, Weiss J, Bradstock KF, Bendall LJ . The mammalian target of rapamycin inhibitor RAD001 (everolimus) synergizes with chemotherapeutic agents, ionizing radiation and proteasome inhibitors in pre-B acute lymphocytic leukemia. Haematologica 2011; 96 : 69–77.
Dai H, Wang Y, Lu X, Han W . Chimeric Antigen Receptors Modified T-Cells for Cancer Therapy. Natl Cancer Inst 2016; 108 : pii djv439.
Maude SL, Teachey DT, Porter DL, Grupp SA . CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 2015; 125 : 4017–4023.
Suerth JD, Schambach A, Baum C . Genetic modification of lymphocytes by retrovirus-based vectors. Curr Opin Immunol 2012; 24 : 598–608.
Riet T, Holzinger A, Dorrie J, Schaft N, Schuler G, Abken H . Nonviral RNA transfection to transiently modify T cells with chimeric antigen receptors for adoptive therapy. Methods Mol Biol 2013; 969 : 187–201.
Barrett DM, Liu X, Jiang S, June CH, Grupp SA, Zhao Y . Regimen-specific effects of RNA-modified chimeric antigen receptor T cells in mice with advanced leukemia. Hum Gene Ther 2013; 24 : 717–727.
Grupp SA, Maude SL, Shaw PA, Aplenc R, Barrett DM, Callahan C et al . Durable Remissions in Children with Relapsed/Refractory ALL Treated with T Cells Engineered with a CD19-Targeted Chimeric Antigen Receptor (CTL019). Blood 2015; 126 : 681–681.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ et al . Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371 : 1507–1517.
Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K et al . Efficacy and Toxicity Management of 19-28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Sci Transl Med 2014; 6 : 224ra25.
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA et al . T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385 : 517–528.
Maude SL, Barrett D, Teachey DT, Grupp. SA . Managing Cytokine Release Syndrome Associated With Novel T Cell-Engaging Therapies. Cancer J 2014; 20 : 119–122.
Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M et al . Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014; 124 : 188–195.
Haso W, Lee DW, Shah NN, Stetler-Stevenson M, Yuan CM, Pastan IH et al . Anti-CD22–chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 2013, 1165–1174.
Shah NN, Stetler-Stevenson M, Yuan CM, Shalabi H, Yates B, Delbrook C et al . Minimal Residual Disease Negative Complete Remissions Following Anti-CD22 Chimeric Antigen Receptor (CAR) in Children and Young Adults with Relapsed/Refractory Acute Lymphoblastic Leukemia (ALL). Blood 2016; 128 : 650.
Jamieson CH, Amylon MD, Wong RM, Blume KG . Allogeneic hematopoietic cell transplantation for patients with high-risk acute lymphoblastic leukemia in first or second complete remission using fractionated total-body irradiation and high-dose etoposide: a 15-year experience. Exp Hematol 2003; 31 : 981–986.
Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N et al . Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol 2004; 22 : 4075–4086.
Jain N, Lamb AV, O'Brien S, Ravandi F, Konopleva M, Jabbour E et al . Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood 2016; 127 : 1863–1869.
Gokbuget N, Kneba M, Raff T, Trautmann H, Bartram CR, Arnold R et al . Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood 2012; 120 : 1868–1876.
Dhedin N, Huynh A, Maury S, Tabrizi R, Beldjord K, Asnafi V et al . Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood 2015; 125 : 2486–2496, quiz 2586.
Tavernier E, Boiron JM, Huguet F, Bradstock K, Vey N, Kovacsovics T et al . Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia 2007; 21 : 1907–1914.
Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G et al . Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007; 109 : 944–950.
Download references
Author information
Authors and affiliations.
New York University School of Medicine, New York, USA
T Terwilliger & M Abdul-Hay
Department of Hematology, New York University Perlmutter Cancer Center, New York, USA
M Abdul-Hay
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to M Abdul-Hay .
Ethics declarations
Competing interests.
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Reprints and permissions
About this article
Cite this article.
Terwilliger, T., Abdul-Hay, M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 7 , e577 (2017). https://doi.org/10.1038/bcj.2017.53
Download citation
Received : 28 March 2017
Accepted : 21 April 2017
Published : 30 June 2017
Issue Date : June 2017
DOI : https://doi.org/10.1038/bcj.2017.53
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Targeting ferroptosis for leukemia therapy: exploring novel strategies from its mechanisms and role in leukemia based on nanotechnology.
- Muhammad Hossein Ashoub
- Razieh Razavi
- Mahnaz Amiri
European Journal of Medical Research (2024)
Plasma-derived exosomal miR-326, a prognostic biomarker and novel candidate for treatment of drug resistant pediatric acute lymphoblastic leukemia
- Neda Saffari
- Soheila Rahgozar
- Fikrettin Sahin
Scientific Reports (2024)
Multimodal probing of T-cell recognition with hexapod heterostructures
- Xiaodan Huang
- Lingyuan Meng
Nature Methods (2024)
A prognostic score system in adult T‐cell acute lymphoblastic leukemia after hematopoietic stem cell transplantation
- Mengyu Xiao
- Jianying Zhou
- Xiaohui Zhang
Bone Marrow Transplantation (2024)
Study of the intestinal microbiota composition and the effect of treatment with intensive chemotherapy in patients recovered from acute leukemia
- Xenia Vázquez
- Pilar Lumbreras-Iglesias
- Carlos Sabater
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- ASH Foundation
- Log in or create an account
- Publications
- Diversity Equity and Inclusion
- Global Initiatives
- Resources for Hematology Fellows
- American Society of Hematology
- Hematopoiesis Case Studies
Case Study: 47-Year-Old Woman With New-Onset AML and Leukostasis
- Agenda for Nematology Research
- Precision Medicine
- Genome Editing and Gene Therapy
- Immunologic Treatment
- Research Support and Funding
A 47-year-old woman presents to the emergency department complaining of fatigue and shortness of breath. She reports a two-week history of worsening exercise tolerance and a rather abrupt onset of shortness of breath over the past several hours. The patient has no major past medical history and works as an architect. Prior to this illness, she exercised three to four times weekly. Her breathing appears somewhat labored. Physical examination is notable for tachycardia, tachypnea, an erythematous rash on her chest and back, and scattered ecchymosis on the extremities. Her laboratory results reveal the following:
White blood cell (WBC) differential is notable for 89 percent blasts. Peripheral blood smear shows a vast majority of cells are large blasts with occasional cytoplasmic granules and pseudopodia. Bone marrow aspiration and biopsy is performed, revealing a hypercellular marrow involved with monocytic-appearing blasts comprising 80 percent of bone marrow cellularity. Cytogenetics reveal t(6;11)(q27;q23) present in 19 out of 20 metaphase cells. Molecular studies show wild-type CEPBA and NPM1 genes and a FLT3-ITD mutation (FMS-like tyrosine kinase 3, internal tandem duplication) is present. She is admitted to the hospital to initiate induction chemotherapy for acute myeloid leukemia (AML).
Following acute cytoreductive strategies to treat pulmonary complications of leukostasis, which of the following FDA-approved induction regimens is most likely to result in long-term overall survival?
- 7+3 chemotherapy with infusional cytarabine and an anthracycline (daunorubicin or idarubicin) plus gemtuzumab ozogamicin
- 7+3 chemotherapy with infusional cytarabine and an anthracycline (daunorubicin or idarubicin), plus etoposide
- 7+3 chemotherapy with infusional cytarabine and an anthracycline (daunorubicin or idarubicin), plus midostaurin
- 7+3 chemotherapy with infusional cytarabine and an anthracycline (daunorubicin or idarubicin), plus sorafenib
The correct answer is (C), 7+3 chemotherapy with infusional cytarabine and an anthracycline (daunorubicin or idarubicin), plus midostaurin. The patient is a younger adult woman with no prior medical history who presents with de novo AML with t(6;11) as well as a FLT3 -ITD mutation. Her clinical presentation is explained by her anemia (fatigue), thrombocytopenia (ecchymoses), and extreme leukocytosis (pulmonary leukostasis). Her cytogenetics reveal an 11q23 translocation, associated with therapy-related AML secondary to topoisomerase II inhibitors (such as etoposide and anthracyclines), which she does not have given her lack of prior history of such exposures, and monocytic differentiation of the leukemia, which she does have on the basis of her morphology. Monocytic differentiation may increase the chance of leukemic blasts infiltrating into tissues, which may result in leukemia cutis (likely based on her exam), gingival hyperplasia, and a higher likelihood of central nervous system involvement. Her very high WBC count is likely a result of her FLT3 -ITD mutation, which is associated with extreme elevations in the WBC count at presentation, a shorter WBC doubling time, and an increased likelihood of relapse following consolidation therapy. Midostaurin is a newly developed inhibitor of FLT3 that is FDA-approved, along with standard 7+3 combination chemotherapy, for the induction therapy of FLT3 mutation-positive AML. This is based on a multicenter phase III trial of 717 adult patients with newly-diagnosed FLT3 mutation-positive AML who were randomized to either standard 7+3 induction chemotherapy plus placebo or 7+3 induction chemotherapy plus midostaurin (on days 8 through 21, following chemotherapy). After a median of 59 months of follow-up, median overall survival was superior in the midostaurin group (75 months vs. 26 months), with a hazard ratio for death of 0.78.
While induction therapy with midostaurin has not been directly compared to such therapy with gemtuzumab ozogamicin (answer choice A), etoposide (answer choice B), or sorafenib (answer choice D), studies have examined the impact of adding etoposide to 7+3 and no benefit over 7+3 alone has been found. Sorafenib is a multi-tyrosine kinase inhibitor with activity against FLT3, and small studies have suggested a possible role for this drug in the management of patients with FLT3 mutation-positive AML, but more investigation is necessary, and the agent is not currently FDA-approved for this purpose. Gemtuzumab ozogamicin is a recombinant anti-CD33 monoclonal antibody linked to a cytotoxic agent. It had initially been approved by the FDA for use in older adults (age >60) with AML in first relapse, but it has since been pulled from the U.S. market following a more recent randomized trial showing no benefit from adding gemtuzumab ozogamicin to standard induction in younger adults with newly diagnosed AML. Trials are ongoing investigating other possible uses of this agent.
Case study submitted by Hanny Al-Samkari, MD, of Massachusetts General Hospital, Dana-Farber Cancer Institute, Harvard University, Boston, MA
- Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation . N Engl J Med. 2017; doi:10.1056/NEJMoa1614359. [Epub ahead of print].
- Bishop JF, Lowenthal RM, Joshua D, et al. Etoposide in acute nonlymphocytic leukemia. Australian Leukemia Study Group . Blood. 1990; 75:27-32.
- Röllig C, Serve H, Hüttmann A, et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial . Lancet Oncol. 2015; 16:1691-1699.
- Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia . Blood. 2013; 121:4854-4860.
American Society of Hematology. (1). Case Study: 47-Year-Old Woman With New-Onset AML and Leukostasis. Retrieved from https://www.hematology.org/education/trainees/fellows/case-studies/female-with-new-onset-aml-and-leukostasis .
American Society of Hematology. "Case Study: 47-Year-Old Woman With New-Onset AML and Leukostasis." Hematology.org. https://www.hematology.org/education/trainees/fellows/case-studies/female-with-new-onset-aml-and-leukostasis (label-accessed May 01, 2024).
"American Society of Hematology." Case Study: 47-Year-Old Woman With New-Onset AML and Leukostasis, 01 May. 2024 , https://www.hematology.org/education/trainees/fellows/case-studies/female-with-new-onset-aml-and-leukostasis .
Citation Manager Formats
[Obesity and risk of relapse in patients with Acute Lymphoblastic Leukemia: A retrospective study]
Affiliations.
- 1 Servicio de Hematología, Hospital General de México, Ciudad de México, México.
- 2 Escuela Superior de Medicina, Hospital General de México, Ciudad de México, México.
- 3 Instituto Politécnico Nacional, Ciudad de México, México.
- 4 Hospital General de México, Ciudad de México, México.
- PMID: 38687542
- DOI: 10.4067/s0034-98872023000500600
Background: Obesity has been associated with a low-grade proinflammatory state, and it has been related to the development of cancer in general, including hematologic cancer.
Aim: The present work aimed to identify the association of the diagnosis of obesity according to the body mass index (BMI) with prognostic factors of adult patients with Acute Lymphoblastic Leukemia (ALL).
Patients and method: This observational, retrospective study included hospitalized patients diagnosed with ALL of the B-cell lineages. BMI was estimated based on the weight and height registered on clinical records at the admission of the patients. The relapse risk and bone marrow relapse were determined, and the survival rate was measured. The statistical analysis included the Kaplan-Meier method using the log-Rank test.
Results: This study included 128 clinical records of patients. Weight had no significant association with relapse risk. The frequency of bone marrow relapse was 43.8%. Obesity did not impact overall survival (p = 0.640) or disease-free survival (p = 0.527). The presence of obesity does not behave as a relapse risk variable (p = 0.873). BMI with a 30 kg/m2 cut-off point did not influence relapse risk (OR 1.078).
Conclusion: Obesity is not an independent risk factor for the prognosis of adult patients with Acute Lymphoblastic Leukemia B-lineage.
Publication types
- Observational Study
- English Abstract
- Body Mass Index*
- Disease-Free Survival
- Kaplan-Meier Estimate
- Middle Aged
- Obesity* / complications
- Precursor Cell Lymphoblastic Leukemia-Lymphoma* / complications
- Precursor Cell Lymphoblastic Leukemia-Lymphoma* / mortality
- Retrospective Studies
- Risk Factors
- Young Adult

IMAGES
VIDEO
COMMENTS
Rare and emerging subtypes of leukemia can be incredibly challenging to diagnose and even more challenging to treat. At the NCCN 2019 Annual Congress: Hematologic Malignancies, a panel of experts, moderated by Andrew D. Zelenetz, MD, PhD, were presented with particularly challenging cases in these malignancies and asked to discuss best approaches to treatment.
Discussion. Acute lymphoblastic leukaemia (ALL) is the most common malignancy in the paediatric age group. 1 Symptoms of ALL include anaemia, fever, bleeding tendency and fatigue. 2 Hypercalcaemia and osteolytic lesions are rare in B-ALL in contrast to their incidence in some other lymphoid malignancies like adult T-cell leukaemia/lymphoma, myeloma and LCH. 2-4 However, in our case, due to ...
Background Acute lymphoblastic leukemia is the most common type of cancer in children. Most often it affects the age group between 2 and 5 years of age. Studies have shown an improvement in general survivability, more than 90% 5-year overall survival (OS). Current treatment protocols for acute lymphoblastic leukemia require verification of the presence of favorable and unfavorable genetic ...
Acute lymphocytic leukemia is a disease with low incidence overall in population studies. The incidence of acute lymphocytic leukemia is about 3.3 cases per 100,000 children. Survival rates for ALL have improved dramatically since the 1980s, with a current five-year overall survival rate estimated at greater than 85 percent.
Case Study: Prognostic Factors in Acute Lymphocytic Leukemia. A 48-year-old female presented to the emergency department with severe headaches, dyspnea on exertion, and petechiae on the lower extremities. A CBC was drawn that showed the following: WBC=56 x10 3 /µL, Hgb=9.0 g/dL, Hct=23, MCV=97 fl, plt=15 x10 9 /µL, ANC=0.7x10 3 /µL.
Acute lymphoblastic leukemia (ALL) is the second most common acute leukemia in adults, with an incidence of over 6500 cases per year in the United States alone. ... is currently in development. In this case, the antibody is linked to the microtubule-disrupting agent monomethyl auristatin F (MMAF). ... Phase I Study in Pediatric Acute ...
Adolescents and young adults (AYA) diagnosed with acute lymphoblastic leukemia (ALL) have faced poorer survival rates compared with the history of this illness treatment in children [].However, several European and US studies have reported improved outcomes for AYA patients treated with pediatric-based protocols [2,3,4]. however, AYA patients receiving pediatric regimens and doses, unlike ...
A recent retrospective study analyzed data from 12 patients with relapsed or refractory T-cell ALL or T-cell lymphoblastic lymphoma (median age, 14 years) who received donor CD7 CAR T-cell therapy ...
Acute Lymphocytic Leukemia Case Study. Leukemia is a cancer of white blood cells. In acute leukemia, the abnormal cells divide rapidly, quickly overtaking functional white and red blood cells. The most common form of cancer in children 0-14 years of age is acute lymphocytic leukemia (ALL). The survival rate in children has improved more than 50 ...
However, we recently reported a single case of T-cell acute lymphoblastic leukemia related to GT in the analysis on long-term safety and efficacy of 43 ADA-SCID patients who received retroviral ex ...
Acute lymphoblastic leukaemia develops in both children and adults, with a peak incidence between 1 year and 4 years. Most acute lymphoblastic leukaemia arises in healthy individuals, and predisposing factors such as inherited genetic susceptibility or environmental exposure have been identified in only a few patients. It is characterised by chromosomal abnormalities and genetic alterations ...
Background. Major advances in the treatment of acute lymphoblastic leukemia (ALL) over the past decade have resulted in 5-year overall survival (OS) rates of 80% in mature B cell ALL, 50% in precursor B cell ALL, 50% to 60% in T cell ALL, and 60% to 70% in Philadelphia chromosome-positive (Ph+) ALL, as reported in studies from large, specialized centers.
In contrast to the combination approach taken by MDACC and EWALL, the German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia (GMALL) study group is studying a sequential approach. In the phase 2 INITIAL-1 study (NCT03460522), untreated older adults (aged >55 years) receive 3 cycles of IO monotherapy followed by CC consolidation ...
To evaluate the prognostic factors and outcome for acute lymphoblastic leukemia (ALL) in children with MLL rearrangement (MLL-r). A total of 124 pediatric patients who were diagnosed with ALL were classified into two groups based on the MLL-r status by using a retrospective case-control study method from June 2008 to June 2020. The prevalence of MLL-r positive in the whole cohort was 4.9%.
Acute lymphoblastic leukemia (ALL) is the most common pediatric cancer; it also strikes adults of all ages. Malignant transformation and uncontrolled proliferation of an abnormally differentiated, long-lived hematopoietic progenitor cell results in a high circulating number of blasts, replacement of normal marrow by malignant cells, and the potential for leukemic infiltration of the central ...
1 Introduction. The therapy related acute lymphoblastic leukemia (t-ALL) is defined as malignant tumor patients who have been exposed to radiation and/or chemotherapy in the past ().Due to the rarity of the disease, our understanding of t-ALL is limited, but some current studies suggest that t-ALL is a unique entity with poor genetic characteristics and clinical outcomes (2, 3).
A Case Study of Child with Acute Lymphoblastic Leukemia Treated in Intensive Care 47 The initial observation time w as not less than 24 ho urs from the moment of nding the basic trunk are exia.
Acute Lymphocytic Leukemia - Case Summary. 1. Acute lymphocytic leukemia is the most common type of leukemia found in children. ALL accounts for about two-thirds of all cases. In ALL there is a proliferation of lymphoblasts, causing a decrease in other cell types. 2.
Acute lymphoblastic leukemia (ALL) is the most common type of cancer in children, accounting for 75-80% of childhood leukemias. It is characterized by two peaks in incidence: one occurring in children aged 2-5 years and the other in individuals aged 50 years or older. According to the WHO Classification of Hematological Malignancies (2001 ...
Causes. Acute lymphocytic leukemia occurs when a bone marrow cell develops changes (mutations) in its genetic material or DNA. A cell's DNA contains the instructions that tell a cell what to do. Normally, the DNA tells the cell to grow at a set rate and to die at a set time. In acute lymphocytic leukemia, the mutations tell the bone marrow cell ...
Acute lymphoblastic leukemia (ALL) represents 20% of adult leukemias ().Although the cure rate of pediatric ALL is >90%, older adults with ALL have inferior long-term survival due to the adverse molecular characteristics of their disease and the overall frailty that precludes the use of intensive pediatric therapy ().It is imperative to understand the unique leukemogenic pathways driving ALL ...
Acute lymphoblastic leukemia (ALL) is the second most common acute leukemia in adults, with an incidence of over 6500 cases per year in the United States alone. ... In this case, the antibody is ...
Educational Case: Diagnostic studies for B-cell acute lymphoblastic leukemia. The following fictional case is intended as a learning tool within the Pathology Competencies for Medical Education (PCME), a set of national standards for teaching pathology. These are divided into three basic competencies: Disease Mechanisms and Processes, Organ ...
Current drug development in upfront therapy for adult patients with acute lymphoblastic leukemia (ALL) focuses on prevention of relapse, as most newly diagnosed adult ALL patients may achieve complete remission (CR) upon intensive induction therapy. ... OS at 5 years was estimated at 61 ± 4% for patients in both study arms. TABLE 1. HOVON-100 ...
Acute Lymphoblastic Leukemia History of Present Problem: April Peters is a 10-year-old female with acute lymphoblastic leukemia (ALL) who presents to the emergency department with a temperature of 38 degrees C. (101 F.) and a complaint of a sore throat. She has been receiving chemotherapy since her diagnosis three months ago.
A diagnosis of ALL generally requires that at least 20% of the cells in the bone marrow are blasts. Under normal circumstances, blasts don't make up more than 5% of bone marrow cells. Sometimes just counting and looking at the cells doesn't provide a definite diagnosis, and other lab tests are needed. Cytochemistry: In cytochemistry tests ...
Introduction. Acute lymphoblastic leukemia (ALL) is one of the most common acute hematologic malignancies, whose annual incidence increased from 49,070 in 1990 to 64,190 in 2017 around the world [Citation 1].ALL is caused by the dysregulated growth of clonal lymphoid cells, among which pre-B cells are the most commonly affected, followed by T cells and mature B cells [Citation 2, Citation 3].
Blood. 2013; 121:4854-4860. A 47-year-old woman presents to the emergency department complaining of fatigue and shortness of breath. She reports a two-week history of worsening exercise tolerance and a rather abrupt onset of shortness of breath over the past several hours.
Background: Obesity has been associated with a low-grade proinflammatory state, and it has been related to the development of cancer in general, including hematologic cancer. Aim: The present work aimed to identify the association of the diagnosis of obesity according to the body mass index (BMI) with prognostic factors of adult patients with Acute Lymphoblastic Leukemia (ALL).
Childhood acute lymphoblastic leukemia (ALL) is a hematological malignancy that arises from immature B- and less commonly T-lymphoid immune cells. 1 ALL is the most common type of cancer in children (age 0-14 y), representing nearly 80% of childhood leukemia cases and 20%-30% of all childhood cancer cases. 1-3 Incidence of ALL typically peaks in children age 2-4 y, 1,4 indicating that ...