An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.


StatPearls [Internet].
Brigham J. Merrell ; John P. McMurry .
Affiliations
Last Update: August 8, 2023 .
- Continuing Education Activity
Folic acid (vitamin B9) is a water-soluble vitamin used to manage and treat megaloblastic anemia. Folic acid has FDA approval for treating megaloblastic and macrocytic anemias due to folic deficiency. This activity describes the indications, mechanism of action, and contraindications for folic acid as a valuable agent in managing megaloblastic anemia and preventing other disorders. One of the beneficial roles of folate appears to be its ability to reduce homocysteine levels in neural tube defects. In addition, this activity will highlight the adverse event profile and other key factors (eg, off-label uses, dosing, monitoring, relevant interactions) pertinent to healthcare teams dealing with conditions that can benefit from supplemental folic acid.
- Identify appropriate indications for folic acid supplementation based on patient characteristics and medical conditions.
- Screen patients at risk of folate deficiency, such as those with chronic alcoholism, malnutrition, or specific medical conditions.
- Assess patients' folate status through appropriate laboratory tests and interpret the results to guide folic acid therapy.
- Communicate effectively with patients, providing education about the benefits, potential side effects, and importance of adherence to folic acid therapy.
- Indications
Folate is a generic term that typically refers to a group of water-soluble compounds that play an essential role in deoxyribonucleic acid (DNA) biosynthesis. [1] It is also known as vitamin B9 and differs from folinic acid or leucovorin, which is more technically known as 5-formyltetrahydrofolate (5-FTHF). Folic acid is the synthetic form of folate. Folate converts into tetrahydrofolic acid (THF). This compound undergoes several transfer/methylation reactions that are important for synthesizing nitrogenous bases in DNA and ribonucleic acid (RNA) and are necessary for the maturation of red blood cells (RBCs).
There are small folic acid reserve pools in the liver and kidney. A folic acid deficiency can result in macrocytic megaloblastic anemia, usually from chronic alcoholism, malabsorption disorders, hemolytic anemia, or increased requirements during pregnancy. Folate appears naturally in some food sources and must be ingested regularly since humans, and all other animals, cannot synthesize it. Sources of folate in the diet include leafy green vegetables like spinach, broccoli, lettuce, meats (liver), eggs, and milk. However, despite the need for regular folate amounts, daily intake levels are frequently lower than the recommended dosage given by national health authorities. [2]
One of the most important indications for folate use is considering the development of the central nervous system. Women planning on getting pregnant should take folic acid supplements to reduce the risk of neural tube defects (NTDs), such as spina bifida, in the developing fetus. Some have proposed that the mechanism by which neural tube defects form in the absence of folate involves the increased ubiquitination of neural tube closure–related genes, thereby affecting their expression. [3]
One of the beneficial roles of folate appears to be its ability to reduce homocysteine levels in neural tube defects. [4] The period of greatest vulnerability is during the fourth week of development, when a woman may be unaware that she is pregnant. For this reason, women of childbearing age should take folic acid supplements if they are sexually active, especially when planning to conceive. If the pregnant mother were to take 4 mg of folic acid daily, it could take 20 weeks for her body to reach optimal folate levels to reduce the risk of a neural tube defect. Because of this, supplementation should be initiated 5 to 6 months before conception. [5] Adequate folic acid is also associated with a decreased risk of preterm birth. [6]
Many other therapeutic uses of folic acid exist, though these uses are less impactful than those already mentioned. Folic acid can help protect against neoplasia in ulcerative colitis, prevent cervical dysplasia, treat vitiligo, restore hematopoiesis in macrocytic anemia due to a folate deficiency, and increase gingival resistance to local irritants, thereby reducing inflammation. [4] [7]
Of these uses, the treatment of megaloblastic anemia is the only indication recognized by the FDA, including preventing NTDs. Folic acid is also an alternative to leucovorin calcium and serves as adjunctive therapy in methanol toxicity. When homocysteine levels increase above baseline, they can reduce global cognition, especially in older adults. [8]
Some research has shown that a combination of vitamin B12 and folate can significantly improve cognitive performance and is superior to either folate or B12 administration alone. [9] Hyperhomocysteinemia has detrimental cardiovascular effects and is a complication of chronic kidney disease (CKD). While there is currently a lack of definitive proof that folic acid or vitamin B12 administration in these situations is directly beneficial, it would be reasonable to consider them appropriate as adjunctive therapy. [10]
- Mechanism of Action
Folate is mainly concentrated in the liver. [11] The synthetic form, folic acid, is given as dihydrofolate (DHF) and is converted to THF by the action of the dihydrofolate reductase enzyme, which depends on nicotinamide adenine dinucleotide phosphate hydrogen (NADPH). THF then converts to 5-10-methylenetetrahydrofolate (5-10-MTHF), which can diverge down different paths: toward DNA synthesis via dTMP or methionine synthesis. [12]
For DNA synthesis, deoxyuridine monophosphate (dUMP) accepts one methyl group from 5-10-MTHF—via thymidylate synthase, which accepts the other—to become deoxythymidine monophosphate (dTMP) and allows the cell cycle to continue while simultaneously regenerating DHF. Drugs used in cancer chemotherapy disrupt this process by inhibiting vital enzymes necessary for cell cycle progression. Methotrexate, for example, inhibits dihydrofolate reductase. By reducing available THF and its downstream components, methotrexate indirectly deprives the thymidylate synthase of its substrates. [13] Humans cannot create dTMP in the presence of methotrexate, and the DNA pool becomes unbalanced, resulting in cell death.
Methionine is a byproduct synthesized as folate reduces homocysteine levels in the blood; 5-10-MTHF donates a methyl group to an enzyme, methyl-tetrahydrofolate reductase (MTHFR), and then becomes 5-methyl THF. [13] 5-methyl THF donates its remaining methyl group to homocysteine via methionine synthase, converting homocysteine to methionine. This transfer of both methyl groups from the original 5-10-MTHF regenerates THF and re-enters the cycle. Vitamin B12 is a crucial cofactor for methionine synthase, and B12 deficiency can lead to macrocytic megaloblastic anemia, similar to folate deficiency, but with additional clinical symptoms beyond the scope of this article. [14]
- Administration
Adult Dosing
- Folic acid is often administered as an oral supplement. Dosing is usually dependent on the disorder. The recommended daily requirement of folic acid for an adult is 400 mcg. [7]
- The World Health Organization recommends a daily dose of 400 to 800 mcg to prevent neural tube defects in pregnancy. Clinicians generally prescribe iron-folic acid supplements for prenatal vitamins during and before pregnancy. [15] [16] Most of these include 1 mg of folate, which is more than enough to meet this criterion. [17] Again, for maximum effect, this supplementation must begin in the earliest stages of pregnancy, if not months before conception.
- Folic acid may be given orally, intravenously, or subcutaneously for macrocytic anemia. Oral recommendations are 1 mg to 5 mg once daily, but doses up to 15 mg once daily have also been recommended.
- To avoid folic acid deficiency in patients on hemodialysis, the recommended dose is estimated to range from 1 mg to 5 mg daily. For intravenous administration, 5 mg or less of undiluted folic acid may be infused over at least 1 minute or combined with 50 mL of either normal saline (NS) or dextrose 5% in water (D5W) and infused over 30 minutes. Folic acid may also be given as an infusion when added to other IV maintenance solutions. To avoid folic acid deficiency in patients on hemodialysis, the recommended dose is estimated to range from 1 mg to 5 mg daily. [18]
- Patients treated with methotrexate should be prescribed folic acid supplements to reduce the adverse events associated with methotrexate therapy.
Renal and Hepatic Dosing: Folic acid dose adjustments are undefined in patients with impaired renal or hepatic function.
Pediatric Patients: Pediatric dosing for megaloblastic anemia is as follows:
- 1 to 11 months: 30 to 45 mcg orally, subcutaneously, intramuscularly, or intravenously daily. Start at 15 mcg/kg/dose daily until achieving hematological correction.
- 1 to 10 years: 0.1 to 4 mg orally daily. Start at 15 mcg/kg/dose daily. Start at 1 mg daily until achieving hematological correction. Max dosage 5 mg daily. Doses over 1 mg are rarely more effective. May use IM, SQ, or IV routes in cases of malabsorption of oral dosing.
- 11 and older: same as ages 1 to 10; the maintenance dose for pregnant or breastfeeding patients is 0.8 mg orally daily.
Pregnancy and Breastfeeding Considerations : Folic acid may be used for supplementation during pregnancy and breastfeeding.
- RDA for supplementation during pregnancy is 600 mcg orally daily. There is no known risk of fetal harm.
- RDA for supplementation during pregnancy is 500 mcg orally daily. There is no known risk of infant harm or adverse effects on milk production.
- Adverse Effects
For the general population, a diet that contains a daily amount of folic acid below the established upper intake level of 1000 mcg has not been demonstrated to result conclusively in any adverse health outcomes. The U.S. National Toxicology Program (NTP) examined areas of previous concern, including cognition (relating to vitamin B12 deficiency), cancer, diabetes- and thyroid-related disorders, and hypersensitivity-related outcomes.
Researchers identified these areas from previous reports of patients receiving more than 400 mcg daily. Overall, the NTP report concluded that no definitive evidence exists for the areas considered for adverse effects due to folic acid. [19] [20] However, reports exist of rare instances of GI upset. [21] This report and other literature reviews performed since drawing their conclusions while still emphasizing the need for further investigation. But, overall, the benefits that stand to be gained from folic acid intake justify any potential risk that might be encountered. Furthermore, the mandatory folic acid fortification program guidelines in countries worldwide have yielded no established risks for adverse effects.
- Contraindications
Hypersensitivity to folic acid or its formulation is a potential contraindication to its administration. One must recall that research has yet to establish hypersensitivity reactions to folic acid, but a history of an anaphylactic reaction from any substance must deter the administration of the offending agent.
Folate deficiency can manifest in numerous ways. The measurement of deficient folate levels in the blood renders a definitive diagnosis, but other signs exist.
Low levels of folate lead to macrocytic megaloblastic anemia. A simple blood smear of an individual with a folate deficiency will reveal erythrocyte macrocytosis and hyper-segmented polymorphonuclear cells (PMNs). [22] This abnormal morphology results from impaired DNA synthesis, which causes precursor cells in the bone marrow to have immature nuclei relative to their cytoplasm.
Additionally, oral ulcers may appear without neurological symptoms [as opposed to a vitamin B12 deficiency, which causes subacute combined degeneration (SCD)]. [23]
A folate deficiency in pregnancy contributes heavily to fetal neural tube defects.
The interruption of DNA synthesis due to a folate deficiency will result in elevated homocysteine levels. Hyperhomocysteinemia is also present in vitamin B12 deficiency, but B12 lack also has elevated methylmalonic acid levels, and the neurological signs associated with SCD are absent in folate deficiency. Therefore, clinicians must rule out a concurrent B12 deficiency before administering folic acid in apparent folate-deficiency anemia. The rationale is that folic acid administration will address the anemia aspect of B12 deficiency, but methylmalonic acid levels will remain elevated and cause toxic neurological effects. Therefore, a simple measurement of B12 levels before folic acid administration is advisable to avoid potential SCD development.
Deficient folate levels have been detected in up to 16% of patients on antiepileptic drugs, including gabapentin, phenytoin, carbamazepine, valproate, and primidone. [24] Women using antiepileptic drugs may develop a folic acid deficiency during pregnancy (valproic acid impairs folic acid absorption) and require a higher dose to maintain adequate treatment levels. However, the recommendation is to reduce valproic acid to the minimum effective dose and increase the dose of folic acid supplementation to achieve maximum protective effect against NTD formation in the fetus. The research found that many women use antiepileptic drugs for non-epileptic disorders like migraines. Sexually active women of reproductive age who are not using contraception are encouraged to use anti-epilepsy drugs only to treat epilepsy. [25] [26] Together, these consequences of folate deficiency can help the examiner start a workup to look for folate deficiency.
Like other water-soluble vitamins, folic acid does not have significant storage in the body; hence toxicity is not a common concern. However, infrequent neurologic side effects have been noted in the context of folate supplementation in individuals with pernicious anemia. [27]
The direct toxicity of folate only contributes minimally. Instead, the neurologic effects are more directly caused by the masking of SCD, resulting from a vitamin B12 deficiency that continues to destroy neuronal cells despite folate supplementation appearing to resolve the anemic aspect seen in pernicious anemia. There is one published case report of fatal poisoning, but the authors acknowledge that the findings may be a unique manifestation of folic acid toxicity in humans. [21]
- Enhancing Healthcare Team Outcomes
The relative safety of folic acid allows healthcare providers to administer it to patients with little concern for adverse effects. However, specific best practice techniques should be considered to ensure positive outcomes. To increase folate levels, the primary care provider should counsel patients to consume a healthy diet, including vegetables, eggs, and milk. Dieticians can consult on the case to ensure inpatients receive appropriate food selections to enhance folate delivery. Pregnant women should be made aware of folate deficiency risks and take supplements. Healthcare providers should refer to the American College of Obstetricians and Gynecologists for prepregnancy counseling for women of childbearing age and recommend folic acid supplements based on the patient's health condition.
In contrast, pregnant women should be prescribed antiepileptic drugs for only epilepsy in the smallest dose possible to prevent low folic acid level complications. Nurses and pharmacists can alert clinicians that anti-epilepsy medications are on the pregnant patient's list of medicines. The source of macrocytic megaloblastic anemia must be determined before supplement administration begins, as folate and vitamin B12 deficiency can present similarly in patients. Therefore, clinicians should consider verifying the vitamin B12 level of the patients to optimize treatment plans and improve patient outcomes when prescribing folic acid. As depicted above, all physicians, advanced practice practitioners, nursing staff, pharmacists, and dieticians should work collaboratively as an interprofessional team and communicate when dealing with patients that can benefit from folic acid supplementation.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Brigham Merrell declares no relevant financial relationships with ineligible companies.
Disclosure: John McMurry declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Merrell BJ, McMurry JP. Folic Acid. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Folic acid with or without vitamin B12 for cognition and dementia. [Cochrane Database Syst Rev. 2003] Review Folic acid with or without vitamin B12 for cognition and dementia. Malouf M, Grimley EJ, Areosa SA. Cochrane Database Syst Rev. 2003; (4):CD004514.
- Comparative Assessment of Vitamin-B12, Folic Acid and Homocysteine Levels in Relation to p53 Expression in Megaloblastic Anemia. [PLoS One. 2016] Comparative Assessment of Vitamin-B12, Folic Acid and Homocysteine Levels in Relation to p53 Expression in Megaloblastic Anemia. Yadav MK, Manoli NM, Madhunapantula SV. PLoS One. 2016; 11(10):e0164559. Epub 2016 Oct 25.
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. [Cochrane Database Syst Rev. 2022] Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas. Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Review The role of folic acid in deficiency states and prevention of disease. [J Fam Pract. 1997] Review The role of folic acid in deficiency states and prevention of disease. Swain RA, St Clair L. J Fam Pract. 1997 Feb; 44(2):138-44.
- Review Chapter 30: historical aspects of the major neurological vitamin deficiency disorders: the water-soluble B vitamins. [Handb Clin Neurol. 2010] Review Chapter 30: historical aspects of the major neurological vitamin deficiency disorders: the water-soluble B vitamins. Lanska DJ. Handb Clin Neurol. 2010; 95:445-76.
Recent Activity
- Folic Acid - StatPearls Folic Acid - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Case report
- Open access
- Published: 13 September 2021
Critical iron deficiency anemia with record low hemoglobin: a case report
- Audrey L. Chai ORCID: orcid.org/0000-0002-5009-0468 1 ,
- Owen Y. Huang 1 ,
- Rastko Rakočević 2 &
- Peter Chung 2
Journal of Medical Case Reports volume 15 , Article number: 472 ( 2021 ) Cite this article
30k Accesses
6 Citations
2 Altmetric
Metrics details
Anemia is a serious global health problem that affects individuals of all ages but particularly women of reproductive age. Iron deficiency anemia is one of the most common causes of anemia seen in women, with menstruation being one of the leading causes. Excessive, prolonged, and irregular uterine bleeding, also known as menometrorrhagia, can lead to severe anemia. In this case report, we present a case of a premenopausal woman with menometrorrhagia leading to severe iron deficiency anemia with record low hemoglobin.
Case presentation
A 42-year-old Hispanic woman with no known past medical history presented with a chief complaint of increasing fatigue and dizziness for 2 weeks. Initial vitals revealed temperature of 36.1 °C, blood pressure 107/47 mmHg, heart rate 87 beats/minute, respiratory rate 17 breaths/minute, and oxygen saturation 100% on room air. She was fully alert and oriented without any neurological deficits. Physical examination was otherwise notable for findings typical of anemia, including: marked pallor with pale mucous membranes and conjunctiva, a systolic flow murmur, and koilonychia of her fingernails. Her initial laboratory results showed a critically low hemoglobin of 1.4 g/dL and severe iron deficiency. After further diagnostic workup, her profound anemia was likely attributed to a long history of menometrorrhagia, and her remarkably stable presentation was due to impressive, years-long compensation. Over the course of her hospital stay, she received blood transfusions and intravenous iron repletion. Her symptoms of fatigue and dizziness resolved by the end of her hospital course, and she returned to her baseline ambulatory and activity level upon discharge.
Conclusions
Critically low hemoglobin levels are typically associated with significant symptoms, physical examination findings, and hemodynamic instability. To our knowledge, this is the lowest recorded hemoglobin in a hemodynamically stable patient not requiring cardiac or supplemental oxygen support.
Peer Review reports
Anemia and menometrorrhagia are common and co-occurring conditions in women of premenopausal age [ 1 , 2 ]. Analysis of the global anemia burden from 1990 to 2010 revealed that the prevalence of iron deficiency anemia, although declining every year, remained significantly high, affecting almost one in every five women [ 1 ]. Menstruation is considered largely responsible for the depletion of body iron stores in premenopausal women, and it has been estimated that the proportion of menstruating women in the USA who have minimal-to-absent iron reserves ranges from 20% to 65% [ 3 ]. Studies have quantified that a premenopausal woman’s iron storage levels could be approximately two to three times lower than those in a woman 10 years post-menopause [ 4 ]. Excessive and prolonged uterine bleeding that occurs at irregular and frequent intervals (menometrorrhagia) can be seen in almost a quarter of women who are 40–50 years old [ 2 ]. Women with menometrorrhagia usually bleed more than 80 mL, or 3 ounces, during a menstrual cycle and are therefore at greater risk for developing iron deficiency and iron deficiency anemia. Here, we report an unusual case of a 42-year-old woman with a long history of menometrorrhagia who presented with severe anemia and was found to have a record low hemoglobin level.
A 42-year-old Hispanic woman with no known past medical history presented to our emergency department with the chief complaint of increasing fatigue and dizziness for 2 weeks and mechanical fall at home on day of presentation.
On physical examination, she was afebrile (36.1 °C), blood pressure was 107/47 mmHg with a mean arterial pressure of 69 mmHg, heart rate was 87 beats per minute (bpm), respiratory rate was 17 breaths per minute, and oxygen saturation was 100% on room air. Her height was 143 cm and weight was 45 kg (body mass index 22). She was fully alert and oriented to person, place, time, and situation without any neurological deficits and was speaking in clear, full sentences. She had marked pallor with pale mucous membranes and conjunctiva. She had no palpable lymphadenopathy. She was breathing comfortably on room air and displayed no signs of shortness of breath. Her cardiac examination was notable for a grade 2 systolic flow murmur. Her abdominal examination was unremarkable without palpable masses. On musculoskeletal examination, her extremities were thin, and her fingernails demonstrated koilonychia (Fig. 1 ). She had full strength in lower and upper extremities bilaterally, even though she required assistance with ambulation secondary to weakness and used a wheelchair for mobility for 2 weeks prior to admission. She declined a pelvic examination. No bleeding was noted in any part of her physical examination.
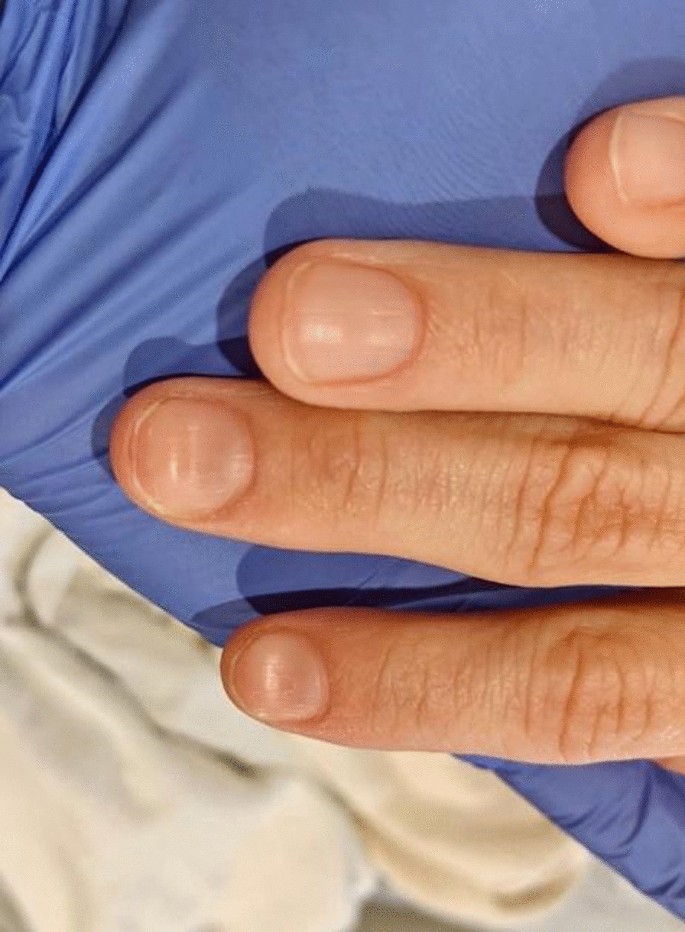
Koilonychia, as seen in our patient above, is a nail disease commonly seen in hypochromic anemia, especially iron deficiency anemia, and refers to abnormally thin nails that have lost their convexity, becoming flat and sometimes concave in shape
She was admitted directly to the intensive care unit after her hemoglobin was found to be critically low at 1.4 g/dL on two consecutive measurements with an unclear etiology of blood loss at the time of presentation. Note that no intravenous fluids were administered prior to obtaining the hemoglobin levels. Upon collecting further history from the patient, she revealed that she has had a lifetime history of extremely heavy menstrual periods: Since menarche at the age of 10 years when her periods started, she has been having irregular menstruation, with periods occurring every 2–3 weeks, sometimes more often. She bled heavily for the entire 5–7 day duration of her periods; she quantified soaking at least seven heavy flow pads each day with bright red blood as well as large-sized blood clots. Since the age of 30 years, her periods had also become increasingly heavier, with intermittent bleeding in between cycles, stating that lately she bled for “half of the month.” She denied any other sources of bleeding.
Initial laboratory data are summarized in Table 1 . Her hemoglobin (Hgb) level was critically low at 1.4 g/dL on arrival, with a low mean corpuscular volume (MCV) of < 50.0 fL. Hematocrit was also critically low at 5.8%. Red blood cell distribution width (RDW) was elevated to 34.5%, and absolute reticulocyte count was elevated to 31 × 10 9 /L. Iron panel results were consistent with iron deficiency anemia, showing a low serum iron level of 9 μg/dL, elevated total iron-binding capacity (TIBC) of 441 μg/dL, low Fe Sat of 2%, and low ferritin of 4 ng/mL. Vitamin B12, folate, hemolysis labs [lactate dehydrogenase (LDH), haptoglobin, bilirubin], and disseminated intravascular coagulation (DIC) labs [prothrombin time (PT), partial thromboplastin time (PTT), fibrinogen, d -dimer] were all unremarkable. Platelet count was 232,000/mm 3 . Peripheral smear showed erythrocytes with marked microcytosis, anisocytosis, and hypochromia (Fig. 2 ). Of note, the patient did have a positive indirect antiglobulin test (IAT); however, she denied any history of pregnancy, prior transfusions, intravenous drug use, or intravenous immunoglobulin (IVIG). Her direct antiglobulin test (DAT) was negative.
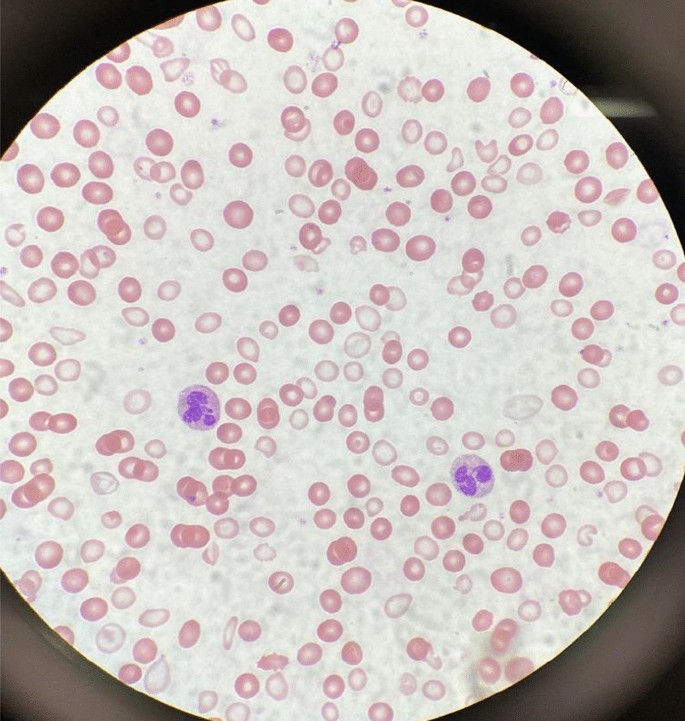
A peripheral smear from the patient after receiving one packed red blood cell transfusion is shown. Small microcytic red blood cells are seen, many of which are hypochromic and have a large zone of pallor with a thin pink peripheral rim. A few characteristic poikilocytes (small elongated red cells also known as pencil cells) are also seen in addition to normal red blood cells (RBCs) likely from transfusion
A transvaginal ultrasound and endometrial biopsy were offered, but the patient declined. Instead, a computed tomography (CT) abdomen and pelvis with contrast was performed, which showed a 3.5-cm mass protruding into the endometrium, favored to represent an intracavitary submucosal leiomyoma (Fig. 3 ). Aside from her abnormal uterine bleeding (AUB), the patient was without any other significant personal history, family history, or lab abnormalities to explain her severe anemia.
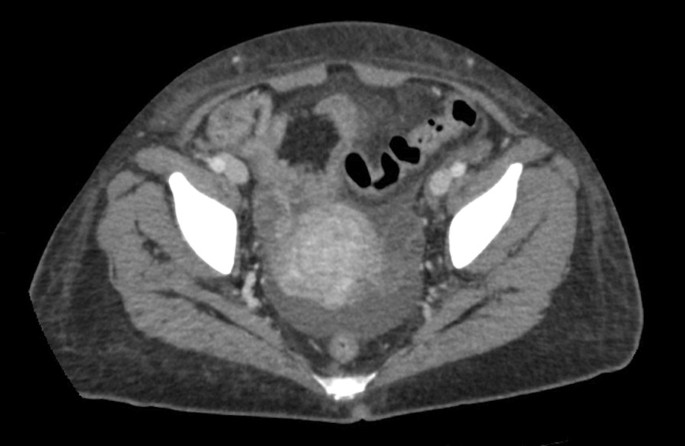
Computed tomography (CT) of the abdomen and pelvis with contrast was obtained revealing an approximately 3.5 × 3.0 cm heterogeneously enhancing mass protruding into the endometrial canal favored to represent an intracavitary submucosal leiomyoma
The patient’s presenting symptoms of fatigue and dizziness are common and nonspecific symptoms with a wide range of etiologies. Based on her physical presentation—overall well-appearing nature with normal vital signs—as well as the duration of her symptoms, we focused our investigation on chronic subacute causes of fatigue and dizziness rather than acute medical causes. We initially considered a range of chronic medical conditions from cardiopulmonary to endocrinologic, metabolic, malignancy, rheumatologic, and neurological conditions, especially given her reported history of fall. However, once the patient’s lab work revealed a significantly abnormal complete blood count and iron panel, the direction of our workup shifted towards evaluating hematologic causes.
With such a critically low Hgb on presentation (1.4 g/dL), we evaluated for potential sources of blood loss and wanted to first rule out emergent, dangerous causes: the patient’s physical examination and reported history did not elicit any concern for traumatic hemorrhage or common gastrointestinal bleeding. She denied recent or current pregnancy. Her CT scan of abdomen and pelvis was unremarkable for any pathology other than a uterine fibroid. The microcytic nature of her anemia pointed away from nutritional deficiencies, and she lacked any other medical comorbidities such as alcohol use disorder, liver disease, or history of substance use. There was also no personal or family history of autoimmune disorders, and the patient denied any history of gastrointestinal or extraintestinal signs and/or symptoms concerning for absorptive disorders such as celiac disease. We also eliminated hemolytic causes of anemia as hemolysis labs were all normal. We considered the possibility of inherited or acquired bleeding disorders, but the patient denied any prior signs or symptoms of bleeding diatheses in her or her family. The patient’s reported history of menometrorrhagia led to the likely cause of her significant microcytic anemia as chronic blood loss from menstruation leading to iron deficiency.
Over the course of her 4-day hospital stay, she was transfused 5 units of packed red blood cells and received 2 g of intravenous iron dextran. Hematology and Gynecology were consulted, and the patient was administered a medroxyprogesterone (150 mg) intramuscular injection on hospital day 2. On hospital day 4, she was discharged home with follow-up plans. Her hemoglobin and hematocrit on discharge were 8.1 g/dL and 24.3%, respectively. Her symptoms of fatigue and dizziness had resolved, and she was back to her normal baseline ambulatory and activity level.
Discussion and conclusions
This patient presented with all the classic signs and symptoms of iron deficiency: anemia, fatigue, pallor, koilonychia, and labs revealing marked iron deficiency, microcytosis, elevated RDW, and low hemoglobin. To the best of our knowledge, this is the lowest recorded hemoglobin in an awake and alert patient breathing ambient air. There have been previous reports describing patients with critically low Hgb levels of < 2 g/dL: A case of a 21-year old woman with a history of long-lasting menorrhagia who presented with a Hgb of 1.7 g/dL was reported in 2013 [ 5 ]. This woman, although younger than our patient, was more hemodynamically unstable with a heart rate (HR) of 125 beats per minute. Her menorrhagia was also shorter lasting and presumably of larger volume, leading to this hemoglobin level. It is likely that her physiological regulatory mechanisms did not have a chance to fully compensate. A 29-year-old woman with celiac disease and bulimia nervosa was found to have a Hgb of 1.7 g/dL: she presented more dramatically with severe fatigue, abdominal pain and inability to stand or ambulate. She had a body mass index (BMI) of 15 along with other vitamin and micronutrient deficiencies, leading to a mixed picture of iron deficiency and non-iron deficiency anemia [ 6 ]. Both of these cases were of reproductive-age females; however, our patient was notably older (age difference of > 20 years) and had a longer period for physiologic adjustment and compensation.
Lower hemoglobin, though in the intraoperative setting, has also been reported in two cases—a patient undergoing cadaveric liver transplantation who suffered massive bleeding with associated hemodilution leading to a Hgb of 0.6 g/dL [ 7 ] and a patient with hemorrhagic shock and extreme hemodilution secondary to multiple stab wounds leading to a Hgb of 0.7 g/dL [ 8 ]. Both patients were hemodynamically unstable requiring inotropic and vasopressor support, had higher preoperative hemoglobin, and were resuscitated with large volumes of colloids and crystalloids leading to significant hemodilution. Both were intubated and received 100% supplemental oxygen, increasing both hemoglobin-bound and dissolved oxygen. Furthermore, it should be emphasized that the deep anesthesia and decreased body temperature in both these patients minimized oxygen consumption and increased the available oxygen in arterial blood [ 9 ].
Our case is remarkably unique with the lowest recorded hemoglobin not requiring cardiac or supplemental oxygen support. The patient was hemodynamically stable with a critically low hemoglobin likely due to chronic, decades-long iron deficiency anemia of blood loss. Confirmatory workup in the outpatient setting is ongoing. The degree of compensation our patient had undergone is impressive as she reported living a very active lifestyle prior to the onset of her symptoms (2 weeks prior to presentation), she routinely biked to work every day, and maintained a high level of daily physical activity without issue.
In addition, while the first priority during our patient’s hospital stay was treating her severe anemia, her education became an equally important component of her treatment plan. Our institution is the county hospital for the most populous county in the USA and serves as a safety-net hospital for many vulnerable populations, most of whom have low health literacy and a lack of awareness of when to seek care. This patient had been experiencing irregular menstrual periods for more than three decades and never sought care for her heavy bleeding. She, in fact, had not seen a primary care doctor for many years nor visited a gynecologist before. We emphasized the importance of close follow-up, self-monitoring of her symptoms, and risks with continued heavy bleeding. It is important to note that, despite the compensatory mechanisms, complications of chronic anemia left untreated are not minor and can negatively impact cardiovascular function, cause worsening of chronic conditions, and eventually lead to the development of multiorgan failure and even death [ 10 , 11 ].
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Kassebaum NJ. The global burden of anemia. Hematol Oncol Clin. 2016;30(2):247–308.
Article Google Scholar
Donnez J. Menometrorrhagia during the premenopause: an overview. Gynecol Endocrinol. 2011;27(sup1):1114–9.
Cook JD, Skikne BS, Lynch SR, Reusser ME. Estimates of iron sufficiency in the US population. Blood. 1986;68(3):726–31.
Article CAS Google Scholar
Palacios S. The management of iron deficiency in menometrorrhagia. Gynecol Endocrinol. 2011;27(sup1):1126–30.
Can Ç, Gulactı U, Kurtoglu E. An extremely low hemoglobin level due to menorrhagia and iron deficiency anemia in a patient with mental retardation. Int Med J. 2013;20(6):735–6.
Google Scholar
Jost PJ, Stengel SM, Huber W, Sarbia M, Peschel C, Duyster J. Very severe iron-deficiency anemia in a patient with celiac disease and bulimia nervosa: a case report. Int J Hematol. 2005;82(4):310–1.
Kariya T, Ito N, Kitamura T, Yamada Y. Recovery from extreme hemodilution (hemoglobin level of 0.6 g/dL) in cadaveric liver transplantation. A A Case Rep. 2015;4(10):132.
Dai J, Tu W, Yang Z, Lin R. Intraoperative management of extreme hemodilution in a patient with a severed axillary artery. Anesth Analg. 2010;111(5):1204–6.
Koehntop DE, Belani KG. Acute severe hemodilution to a hemoglobin of 1.3 g/dl tolerated in the presence of mild hypothermia. J Am Soc Anesthesiol. 1999;90(6):1798–9.
Georgieva Z, Georgieva M. Compensatory and adaptive changes in microcirculation and left ventricular function of patients with chronic iron-deficiency anaemia. Clin Hemorheol Microcirc. 1997;17(1):21–30.
CAS PubMed Google Scholar
Lanier JB, Park JJ, Callahan RC. Anemia in older adults. Am Fam Phys. 2018;98(7):437–42.
Download references
Acknowledgements
Not applicable.
No funding to be declared.
Author information
Authors and affiliations.
Department of Medicine, University of Southern California, Los Angeles, CA, USA
Audrey L. Chai & Owen Y. Huang
Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, University of Southern California, Los Angeles, CA, USA
Rastko Rakočević & Peter Chung
You can also search for this author in PubMed Google Scholar
Contributions
AC, OH, RR, and PC managed the presented case. AC performed the literature search. AC, OH, and RR collected all data and images. AC and OH drafted the article. RR and PC provided critical revision of the article. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Audrey L. Chai .
Ethics declarations
Ethics approval and consent to participate, consent for publication.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Chai, A.L., Huang, O.Y., Rakočević, R. et al. Critical iron deficiency anemia with record low hemoglobin: a case report. J Med Case Reports 15 , 472 (2021). https://doi.org/10.1186/s13256-021-03024-9
Download citation
Received : 25 March 2021
Accepted : 21 July 2021
Published : 13 September 2021
DOI : https://doi.org/10.1186/s13256-021-03024-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Menometrorrhagia
- Iron deficiency
- Critical care
- Transfusion

Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

Search Your Topic
Case study-folic acid deficiency, case details .
A 44-year-old man who had lost his job because of absenteeism presented to his physician complaining of loss of appetite, fatigue, muscle weakness, and emotional depression. The physical examination revealed a somewhat enlarged liver that was firm and nodular, and there was a hint of jaundice in the sclerae and a hint of alcohol in his breath. The initial laboratory profile included a hematological analysis that showed that he had anemia with enlarged red blood cells (macrocytic). A bone marrow aspirate confirmed the suspicion that he had megaloblastic anemia because it showed a greater than a normal number of red and white blood cell precursors, most of which were larger than normal. Further analyses revealed that his serum folic acid level was1.2 ng/mL (normal 2.5 to20), his serum B12 level was 253 ng/mL (normal 200 to 900), but his serum iron level was normal.
What is the cause of megaloblastic anemia in this patient? What is its correlation with alcoholism?
Case discussion
The megaloblastic anemias are a group of disorders characterized by the presence of distinctive morphological appearances of the developing red cells in the bone marrow. The cause is usually a deficiency of either cobalamin (vitamin B 12 ) or folate, but megaloblastic anemia may arise because of genetic or acquired abnormalities affecting the metabolism of these vitamins or because of defects in DNA synthesis not related to cobalamin or folate. Megaloblastic anemia in folate deficiency is identical to anemia resulting from vitamin B 12 deficiency However, the serum vitamin B 12 level is normal in this patient so that rules out vitamin B 12 deficiency. Alcoholics, in particular, are at risk for folate deficiency because of impaired gastrointestinal absorption and poor nutrition. Folate is essential for many biochemical processes in the body, including DNA synthesis and red blood cell synthesis.
Megaloblastic anemia due to folate deficiency
The prevalence of folic acid deficiency has decreased since the United States and Canada introduced a mandatory folic acid food fortification program in November 1998. People with excessive alcohol intake and malnutrition are still at high risk of folic acid deficiency.
Biochemical Basis of Megaloblastic Anemia
The common feature of all megaloblastic anemias is a defect in DNA synthesis that affects rapidly dividing cells in the bone marrow. All conditions that give rise to megaloblastic changes share in common a disparity in the rate of synthesis or availability of the four immediate precursors of DNA: the deoxyribonucleoside triphosphates (dNTPs): dA(adenine)TP and dG(guanine)TP (purines), dT(thymine)TP and dC(cytosine)TP (pyrimidines). In deficiencies of either folate or cobalamin, there is a failure to convert deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP), the precursor of dTTP. This is because folate is needed as the coenzyme 5, 10-methylene-THF polyglutamate for the conversion of dUMP to dTMP; the availability of 5, 10-methylene-THF is reduced in either cobalamin or folate deficiency (Figure-1).
Cobalamin-Folate Relations
Folate is required for many reactions in mammalian tissues. Only two reactions in the body are known to require cobalamin. Methylmalonyl CoA isomerization, which requires Adenosylcobalamin and the methylation of homocysteine to methionine, requires both methylcobalamin and both 5-MTHF. In cobalamin deficiency, MTHF accumulates in plasma, while intracellular folate concentrations fall due to failure of formation of THF, the substrate on which folate polyglutamate is built. This has been termed THF starvation, or the methylfolate trap (Figure-1). This theory explains the abnormalities of folate metabolism that occur in cobalamin deficiency and also why the anemia of cobalamin deficiency will respond to folic acid in large doses.
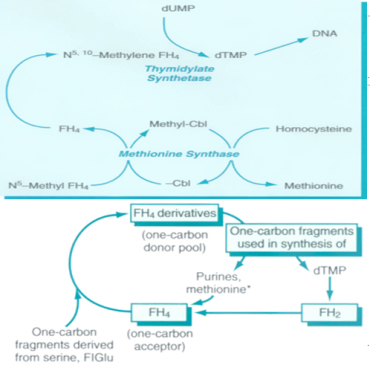
Figure-1 showing the role of folic acid in DNA synthesis and methionine metabolism
Causes of folate deficiency
Folate deficiency can result from several possible causes, including inadequate ingestion, impaired absorption, impaired metabolism leading to inability to utilize folate that is absorbed, increased requirement, increased excretion, and increased destruction.
- Inadequate ingestion of folate-containing foods: Poor nutrition is prevalent among people with alcoholism and patients with psychiatric morbidities, as well as elderly people (due to conditions such as ill-fitting dentures, physical disabilities, and social isolation). Because folates are destroyed by prolonged exposure to heat, people of certain cultures that involve traditionally cooking food in kettles of boiling water may be predisposed to folate deficiency. Moreover, for patients with renal and liver failure, anorexia and restriction of foods rich in protein, potassium, and phosphate contribute to decreased folate intake.
- A decreased absorptive area due to small bowel resection or mesenteric vascular insufficiency would decrease folate absorption.
- Celiac disease and tropical sprue cause villous atrophy.
- The process of aging causes shorter and broader villi in 25% of the elderly population.
- Achlorhydria leads to elevation of gastric pH above the optimal level (ie, pH of 5) for folate absorption.
- Anticonvulsant drugs, such as Dilantin, interfere with mucosal conjugase, hence impairing folate absorption.
- Zinc deficiency also decreases folate absorption because zinc is required to activate mucosal conjugase.
- Bacterial overgrowth in blind loops, stricture formation, or jejunal diverticula likewise would decrease folate absorption.
- Impaired metabolism, leading to an inability to utilize absorbed folate: Antimetabolites that are structurally analogous to the folate molecule can competitively antagonize folate utilization. Methotrexate and trimethoprim both are folate antagonists that inhibit dihydrofolate reductase. Hypothyroidism has been known to decrease hepatic levels of dihydrofolate reductase as well as Methylene THFA reductase. Furthermore, congenital deficiency involving the enzymes of folate metabolism also can show impaired folate utilization. People with alcoholism can have very active alcohol dehydrogenase that binds up folate and thus interferes with folate utilization.
- Increased requirement: Factors that increase the metabolic rate can increase the folic requirement. Infancy (a period of rapid growth), pregnancy (rapid fetal growth), lactation (uptake of folate into breast milk), malignancy (increased cell turnover), concurrent infection (Immuno proliferative response), and chronic hemolytic anemia (increased hematopoiesis) all can result in an increased folate requirement.
- Increased excretion/loss: Increased excretion of folate can occur subsequent to vitamin B-12 deficiency. During the course of vitamin B-12 deficiency, methylene THFA is known to accumulate in the serum, which is known as the folate trap phenomenon. In turn, large amounts of folate filter through the glomerulus, and urine excretion occurs.
- Increased destruction: Superoxide, an active metabolite of ethanol metabolism, is known to inactivate folate by splitting the folate molecule in half between the C9 and N10 position. The relationship between cigarette smoking and low folate levels has been noted as possibly due to folate inactivation in exposed tissue.
Clinical manifestations
- History- In folate deficiency, the patient’s history is important because it may reveal the underlying disorder. Very often, a patient presents with a history of excessive alcohol intake with concurrent poor diet intake. Other times, patients may be pregnant or lactating; may take certain drugs, such as phenytoin, sulfonamides, or methotrexate; may have chronic hemolytic anemia, or may have underlying malabsorption.
- Oral lesions – Some patients complain of a sore tongue or pain upon swallowing. The tongue may appear swollen, beefy, red, or shiny, usually around the edges and tips initially (Figure-2). Angular stomatitis also may be observed (Figure-3). These oral lesions typically occur at the time when folate depletion is severe enough to cause megaloblastic anemia, although, occasionally, lesions may occur before the anemia.
- GI symptoms – Patients may present with GI symptoms, such as nausea, vomiting, abdominal pain, and diarrhea, especially after meals. Anorexia also is common and, in combination with the above symptoms, may lead to marked weight loss. However, an underlying malabsorption disorder could be causing these symptoms, as well as folate depletion. The lack of folate itself may not be the culprit.
- Hyper pigmentation- Patients with folate deficiency may have darkening of the skin and mucous membranes, particularly at the dorsal surfaces of the fingers, toes, and creases of palms and soles. Distribution typically is patchy. Fortunately, the hyperpigmentation gradually should resolve after weeks or months of folate treatment.
- A modest temperature elevation (<102°F) is common in patients who are folate deficient, despite the absence of any infection. Although the underlying mechanism is obscure, the temperature typically falls within 24-48 hours of vitamin treatment and returns to normal within a few days.
- Hematological manifestations – Folate deficiency can cause anemia. The presentation typically consists of macrocytosis and hyper segmented polymorph nuclear leucocytes (PMNs). The anemia usually progresses over several months, and the patient typically does not express symptoms as such until the hematocrit level reaches less than 20%. At that point, symptoms such as weakness, fatigue, difficulty concentrating, irritability, headache, palpitations, and shortness of breath can occur. Furthermore, heart failure can develop in light of high-output cardiac compensation for decreased tissue oxygenation. Angina pectoris may occur in predisposed individuals due to increased cardiac work demand. Tachycardia, postural hypotension, and lactic acidosis are other common findings. Less commonly, neutropenia and thrombocytopenia also will occur, although it usually will not be as severe as the anemia. In rare cases, the absolute neutrophil count can drop below 1000/mL and the platelet count below 50,000/mL.
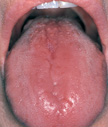
Figure-2- Glossitis in folic acid deficiency
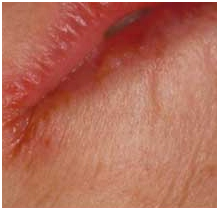
Figure-3- Angular stomatitis in folic acid deficiency
Laboratory Investigations
- As the initial test, ruling out cobalamin deficiency is very important because folate treatment will not improve neurologic abnormalities due to cobalamin deficiency.
- Additional follow-up tests include serum homocysteine (reference range 5-16 mmol/L), which is elevated in B-12 and folate deficiency, and serum methylmalonic acid (reference range 70-270 mmol/L), which is elevated in B-12 deficiency only.
- Red blood cell folate levels (reference range >140 ng/mL) tend to reflect chronic folate status rather than acute changes in folate that are reflected in serum folate levels,
- Hematological Findings (Peripheral Blood film)
Oval macrocytes, usually with considerable anisocytosis and poikilocytosis, are the main feature (Figure-4). The MCV is usually >100 fL unless a cause of microcytosis (e.g., iron deficiency or thalassemia trait) is present. Some of the neutrophils are hyper segmented (more than five nuclear lobes). There may be leucopenia due to a reduction in granulocytes and lymphocytes, but this is usually >1.5 x 10 9 /L; the platelet count may be moderately reduced, rarely to <40 x 10 9 /L. The severity of all these changes parallels the degree of anemia. In the nonanemic patient, the presence of a few macrocytes and hyper segmented neutrophils in the peripheral blood may be the only indication of the underlying disorder.
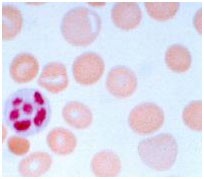
Figure – 4 – showing peripheral blood film in macrocytic anemia. Macrocytes are observed and some of the red blood cells show ovalocytosis. A 6-lobed polymorph nuclear leucocyte is present.
- Bone Marrow
Bone marrow morphology is characteristically abnormal. Marked erythroid hyperplasia is present as a response to defective red blood cell production (ineffective erythropoiesis). Megaloblastic changes in the erythroid series include abnormally large cell size and asynchronous maturation of the nucleus and cytoplasm—ie, cytoplasmic maturation continues while impaired DNA synthesis causes retarded nuclear development.
- Ineffective Hematopoiesis- There is an accumulation of unconjugated bilirubin in the plasma due to the death of nucleated red cells in the marrow (ineffective erythropoiesis).
Mortality/Morbidity
Elevated serum homocysteine and atherosclerosis
Folate in the 5-methyl THFA form is a cosubstrate required by methionine synthase when it converts homocysteine to methionine. As a result, in the scenario of folate deficiency, homocysteine accumulates. Several recent clinical studies have indicated that mild-to-moderate hyperhomocysteinemia is highly associated with atherosclerotic vascular diseases such as coronary artery disease (CAD) and stroke. In this case, mild hyperhomocysteinemia is defined as the total plasma concentration of 15-25 mmol/L and moderate hyperhomocysteinemia is defined as 26-50 mmol/L. Elevated homocysteine levels might act as an atherogenic factor by converting a stable plaque into an unstable, potentially occlusive, lesion. Homocysteine is believed to have atherogenic and prothrombotic properties via multiple mechanisms.
Fruits and vegetables constitute the primary dietary source of folic acid. The minimal daily requirement is about 50 mcg, but this may be increased several-fold during periods of enhanced metabolic demand such as pregnancy.
Recommended Daily Allowance
- Males: 400 mcg/d
- Females: 400 mcg/d
- Pregnant: 600 mcg/d
- Nursing: 500 mcg/d
Deficiency treatment
0.4-1 mg per day. Large doses of 5–15 mg folic acid daily are given in patients with severe absorption. It is essential to exclude cobalamin deficiency before large doses of folic acid are given, and deficiency if present must be corrected, otherwise, cobalamin neuropathy may develop, despite a response of the anemia of cobalamin deficiency to folate therapy.
Patients whose folic acid deficiency is related to dietary factors should be counseled to include green vegetables and fruit in their diet. Prophylactic treatment of pregnant patients and patients with chronic hemolytic anemias can prevent folic acid deficiency due to the increased requirement for folate in these conditions.
Supplements and food fortification
Folic acid is added to a variety of foods, the most important of which are flour, salt, breakfast cereals and beverages, soft drinks and baby foods. Folic acid is available as oral preparations, alone or in combination with other vitamins or minerals (e.g. iron), and as an aqueous solution for injection. As folic acid is only poorly soluble in water, folate salts are used to prepare liquid dosage forms. Folinic acid (also known as leucovorin or citrovorum factor) is a derivative of folic acid administered by intramuscular injection to circumvent the action of dihydrofolate reductase inhibitors, such as methotrexate. It is not otherwise indicated for the prevention or treatment of folic acid deficiency.
- deoxythymidine monophosphate (dTMP)
- deoxyuridine monophosphate (dUMP)
- DNA synthesis
- megaloblastic anemia
- methylfolate trap
- polyglutamate
- THF starvation
- vitamin B12
Reference Books By Dr. Namrata Chhabra
Advertisement
Introduction and epidemiology
Outcomes/consequences of anemia during pregnancy, iron requirements in pregnancy, diagnosis of iron-deficiency anemia, returning to patient 1: diagnosis and management, folic acid and vitamin b12 (cobalamin) deficiency, diagnostic tests, returning to patient 2: diagnosis and management, management of folate deficiency, cobalamin deficiency, returning to patient 3: diagnosis and management, rbc transfusions in pregnancy, acknowledgment, how i treat anemia in pregnancy: iron, cobalamin, and folate.
- Split-Screen
- Request Permissions
- Cite Icon Cite
- Search Site
- Open the PDF for in another window
Maureen M. Achebe , Anat Gafter-Gvili; How I treat anemia in pregnancy: iron, cobalamin, and folate. Blood 2017; 129 (8): 940–949. doi: https://doi.org/10.1182/blood-2016-08-672246
Download citation file:
- Ris (Zotero)
- Reference Manager
Anemia of pregnancy, an important risk factor for fetal and maternal morbidity, is considered a global health problem, affecting almost 50% of pregnant women. In this article, diagnosis and management of iron, cobalamin, and folate deficiencies, the most frequent causes of anemia in pregnancy, are discussed. Three clinical cases are considered. Iron deficiency is the most common cause. Laboratory tests defining iron deficiency, the recognition of developmental delays and cognitive abnormalities in iron-deficient neonates, and literature addressing the efficacy and safety of IV iron in pregnancy are reviewed. An algorithm is proposed to help clinicians diagnose and treat iron deficiency, recommending oral iron in the first trimester and IV iron later. Association of folate deficiency with neural tube defects and impact of fortification programs are discussed. With increased obesity and bariatric surgery rates, prevalence of cobalamin deficiency in pregnancy is rising. Low maternal cobalamin may be associated with fetal growth retardation, fetal insulin resistance, and excess adiposity. The importance of treating cobalamin deficiency in pregnancy is considered. A case of malarial anemia emphasizes the complex relationship between iron deficiency, iron treatment, and malaria infection in endemic areas; the heightened impact of combined etiologies on anemia severity is highlighted.
Anemia of pregnancy is a well-recognized global health problem, affecting almost half of pregnant women. 1 The World Health Organization (WHO) defines anemia of pregnancy as hemoglobin (Hb) <11 g/dL, or hematocrit <33%, at any time during the pregnancy. 1 The Centers for Disease Control and Prevention (CDC) define anemia of pregnancy as Hb <11 g/dL, or hematocrit <33% during the first and third trimesters, and <10.5 g/dL or a hematocrit <32% in the second trimester. 2 The WHO defines severe anemia in all persons as a Hb of <7 g/dL and very severe anemia as a Hb of <4 g/dL. 3
Physiologic anemia of pregnancy reflects an expansion of plasma volume of 50% relative to the increase in the red blood cell (RBC) mass of 25%. 4 Globally, the most common cause for anemia of pregnancy is iron deficiency, arising from maternal-fetal transfer of iron, frequently aggravated by decreased maternal iron reserves. 5 The Nutrition Impact Model Study, a systematic analysis of 257 population-representative data sources from 107 countries, estimated the global prevalence of anemia in pregnancy at 43% in 1995 and 38% in 2011. 6
Anemia is an important risk factor for both maternal and fetal morbidity. Iron-deficiency anemia is associated with higher rates of preterm birth, low birth weight (LBW), and small-for-gestational age (SGA) newborns. 7 Maternal iron deficiency affects iron concentrations in umbilical cord blood. 8 Fetal-neonatal iron deficiency causes diminished auditory recognition memory in infants, a reflection of its impact on the developing hippocampus. 9 Children born to iron-deficient mothers demonstrate learning and memory impairments that may persist into adulthood. 10 Folic acid deficiency, especially at the time of conception, is strongly correlated with increased neural tube defects (NTDs). 11 Low maternal RBC folate is also associated with LBW, and an increased risk for SGA. 12 Maternal vitamin B12 (cobalamin) status affects fetal growth and development. Low cobalamin is associated with an increased fetal risk of low lean mass and excess adiposity, increased insulin resistance, and impaired neurodevelopment. 13 Maternal risks include fatigue, pallor, tachycardia, poor exercise tolerance, and suboptimal work performance. 14 Depleted blood reserves during delivery may increase the need for blood transfusion, 15 preeclampsia, 16 placental abruption, 17 cardiac failure, and related death. 18 In this article, we present 3 cases to address how we treat the most common nutritional causes of anemia of pregnancy: iron, cobalamin, and folate deficiencies.
A 35-year-old woman presented to clinic 35 weeks pregnant, with fatigue that started early in pregnancy, dyspnea on exertion, and restless sleep. There was a history of Crohn ileitis, longstanding menorrhagia, and a previous preterm delivery due to severe preeclampsia. Her pulse was 109; her blood pressure was 145/96 mmHg. Complete blood count revealed: leukocytes, 10.9 × 10 9 /L; Hb, 8.8 g/dL; hematocrit (Hct), 28.1%; mean corpuscular volume (MCV), 71 fL; platelets, 270 × 10 9 /L; red cell distribution width, 17.1. A month earlier (for Hb, 8.5g/dL; Hct, 26.9%; and MCV, 76 fL), oral iron was started by an obstetrician and caused severe constipation.
A 28-year-old Gravida 2 para 1 woman at 29 weeks’ gestation presented with chronic fatigue, dyspnea, and palpitations. Her counts were: Hb, 9.1 g/dL; Hct, 28.0%; MCV, 83 fL; and platelets, 192 × 10 9 /L. There was a history of conversion of an adjustable gastric band to Roux-en-Y gastric bypass 1 year before, complicated by persistent nausea. Physical examination revealed a gravid uterus but was otherwise unremarkable.
A 27-year-old woman at 34 weeks’ gestation was admitted to a hospital in Jos, Nigeria with 1 week of fever, vomiting, and chills. She had completed prenatal antimalarial prophylaxis with sulphadoxine-pyrimethamine. No prenatal iron was prescribed. She was febrile, pale, and icteric, tachycardic (110 beats per minute), tachypneic (24 breaths per minute), and had splenomegaly. Her counts were: Hb, 5.3 g/dL; MCV, 80 fL, platelets, 480 × 10 9 /L; and white blood cells, 18.0 × 10 9 /L.
In a typical pregnancy, maternal iron requirements include 300 to 350 mg for the fetus and the placenta, 500 mg for the expansion of the maternal RBC mass, and 250 mg associated with blood loss during labor and delivery. 19 The requirement for iron increases gradually from 0.8 mg per day in the first trimester to 7.5 mg per day in the third. 20 Yet, the average daily absorption of iron from western diets is only 1 to 5 mg. 5 Therefore, women cannot fulfill their iron needs from normal food intake, and must draw upon iron stores, increasing the risk of iron-deficiency anemia. The CDC recommends that all pregnant women begin a 30 mg per day iron supplement at the first prenatal visit, 2 the WHO suggests 60 mg per day for all pregnant women, 1 whereas British guidelines do not recommend any routine iron supplementation in pregnancy. 21
The laboratory diagnosis of iron-deficiency anemia may be especially difficult during pregnancy because the changes in maternal physiology may affect the serum levels of biochemical markers of iron status ( Figure 1 ).

Algorithm of suggested approach to diagnosis and management of iron-deficiency anemia in pregnancy. **Oral iron treatment should not be interrupted once normal Hb values are achieved, but rather supplementation should continue to replenish iron stores (generally for at least 2-3 months, and until 6 weeks postpartum). BID, twice a day; IDA, iron-deficiency anemia; PRBC, packed red blood cells.
Hemoglobin level
Maternal Hb declines progressively during pregnancy due to hemodilution and may be accentuated by iron-deficient erythropoiesis, with a nadir reached at 24 to 32 weeks’ gestation. 4 , 22 Due to considerable variation in Hb level, it cannot be used as a single parameter to estimate iron status.
Ferritin reflects total body iron stores. Iron deficiency is the only clinical situation associated with extremely low values of ferritin. Ferritin declines gradually during pregnancy, reaches a nadir during weeks 35 to 38, and increases during the month before delivery. The nadir is about 15 ng/mL without iron supplementation and 20 ng/mL with it. 23 Studies correlating the presence or absence of stainable marrow iron with serum ferritin indicate that the 12 ng/mL threshold of ferritin is only 25% sensitive for detecting iron deficiency. 24 Instead, a ferritin of 30 ng/mL or less has a 92% sensitivity and 98% specificity for diagnosing iron deficiency. 24 Ferritin is a more sensitive and specific marker for iron deficiency than serum iron, transferrin saturation, and erythrocyte protoporphyrin values 25 and is the best test for iron deficiency in pregnancy if low.
In the absence of active comorbidity, ferritin values >100 ng/mL indicate adequate iron stores and a low likelihood of iron-deficiency anemia. 26
Mean corpuscular volume
MCV is an unreliable marker of iron deficiency in pregnancy. Stimulation of erythropoiesis leads to a physiologic increase in MCV during gestation that counterbalances the microcytosis of iron deficiency. 27 A low MCV, defined as an MCV <80 fL, is highly sensitive, 26 but not specific, for iron-deficiency anemia.
Iron, transferrin, and transferrin saturation
Serum iron circulates bound to its transport protein, transferrin. The serum iron reflects both iron recycling from macrophages and iron absorbed from the diet. It demonstrates diurnal variation, with a rise in the morning and fall at night 28 ; serum iron is also influenced by recently ingested meals. Therefore, no single value is diagnostic of iron deficiency. 29 Serum iron should be drawn after an overnight fast. Total iron-binding capacity (TIBC) and transferrin are measurements of iron transport proteins that increase in iron deficiency. Inflammation, chronic infection, malignancies, liver disease, nephrotic syndrome, and malnutrition can lower TIBC, whereas pregnancy can raise it, in the absence of iron deficiency. 27
Plasma transferrin saturation is the ratio of plasma iron to transferrin. A saturation of <15% suggests an inadequate supply of iron, 30 either because of low total body iron (iron deficiency) or due to trapping of iron in macrophages (anemia of inflammation).
Soluble transferrin receptor
The soluble transferrin receptor (sTfR) is a truncated fragment of the membrane receptor. In iron deficiency, synthesis of transferrin receptors, and sTfR, is increased. 31 Unlike TIBC and ferritin, sTfR concentrations are not affected by inflammation. 24 A meta-analysis of 10 studies of sTfR showed that the assay had a sensitivity of 86% and a specificity of 75%. 32 However, the assay is not standardized and is not used in routine diagnosis of iron-deficiency anemia.
Hepcidin is the master regulator of systemic iron bioavailability. Hepcidin decreases as pregnancy progresses, with the lowest hepcidin levels seen in the third trimester. 33 Pregnant women with undetectable serum hepcidin transfer more maternally ingested iron to their fetus than women with detectable hepcidin, indicating that maternal hepcidin in part determines the iron bioavailability to the fetus. 34 Hepcidin is currently being evaluated as a biomarker in pregnancy. 35
In summary, Hb, the percentage of transferrin saturation, and plasma ferritin are adequate to assess iron status in the majority of pregnant women, and the combination of anemia and ferritin <15 to 30 ng/mL is diagnostic of iron deficiency. 1
Additional laboratory data in our 35-year-old patient at 36 weeks’ gestation included: serum iron, 24 μg/dL; TIBC, 623 μg/dL; and ferritin, 6 μg/L (ng/mL), establishing a diagnosis of iron-deficiency anemia.
Iron repletion can be achieved with either oral or IV iron. The choice of therapy depends on the degree of anemia, the stage of pregnancy, and factors that influence gastrointestinal absorption of iron.
Oral iron is the frontline therapy for iron-deficiency anemia. It is inexpensive, readily available, and effective. However, up to 70% of patients experience significant gastrointestinal side effects (nausea, constipation, diarrhea, indigestion, and metallic taste) that prevent adherence to treatment. 36 In pregnancy, decreased bowel motility caused by elevated progesterone and the enlarging uterus pressing on the rectum is made worse by oral iron. 37 , 38
Recommendations for dosing oral iron vary from 60 to 200 mg of elemental iron per day. 2 , 21 This can be achieved with 325-mg tablets (each containing 50-65 mg of elemental iron) given once to 3 times daily. The acid pH of the stomach favors solubility of iron by the conversion of ferric (Fe 3+ ) to ferrous (Fe 2+ ) iron for duodenal uptake. Iron absorption is facilitated by ascorbate (which facilitates Fe 3+ to Fe 2+ ), amino acids, and iron deficiency, and is retarded by phytates, tannins, antacids, and iron overload. The most commonly prescribed iron preparations are ferrous sulfate, ferrous gluconate, and ferrous fumarate. Prolonged-released ferrous sulfate (ferrous sulfate–polymeric complex) is the best tolerated oral preparation, and is associated with good compliance, 39 although delayed release compromises absorption. An iron-deficient patient absorbs up to 28% of oral iron, if taken without food. 40 The total iron absorbed increases with increasing doses to a maximum of 160 mg per day. However, oral iron acutely increases hepcidin and recent data suggest that twice and thrice daily supplementation may have little added benefit over once-daily dosing. 41 Two weeks after starting oral iron, a Hb increase of 1 g or more suggests adequate absorption. 42 Replacement should be continued until iron stores are replenished (generally 2-3 months), and 6 weeks postpartum.
IV iron circumvents gastrointestinal absorption and is therefore the preferred agent for patients with gluten sensitivity, inflammatory bowel disease, gastrointestinal malabsorption, after gastric bypass surgery, hyperemesis gravidarum, or a history of oral iron intolerance. IV iron is superior to oral iron in achieving a sustained Hb response, reducing the need for packed RBC transfusions and improving quality of life for chronic heart failure, 43 inflammatory bowel disease, 44 chronic kidney diseases and hemodialysis, 45 and cancer-related anemia. 46
Several authors have reported that parenteral iron therapy in pregnancy and postpartum is associated with a more rapid increase in Hb and/or better replenishment of iron stores than is oral therapy. 18 , 47-54 Patient 1 had Crohn ileitis, a history of menorrhagia, and may have started the pregnancy with suboptimal iron stores. At 32 weeks’ gestation, we would recommend treatment with IV iron.
In the first trimester, we treat iron deficiency with oral iron, reserving IV iron for after the 13th week. This is in keeping with recommendations of the European Medicine Agency’s Committee of Medicinal Products for Human Use (CHMP). 55 The US Food and Drug Administration (FDA) does not explicitly restrict the use of IV iron until after the first trimester. Because IV iron has been shown to improve Hb more rapidly than oral iron, 45 , 51 we preferentially treat patients with IV iron in the second half of pregnancy. Some investigators report additional advantages of IV over oral iron beyond the more rapid increase in Hb. Breymann and colleagues enrolled 252 woman in the second and third trimester (weeks 16-33), randomly assigning them to oral ferrous sulfate or IV ferric carboxymaltose (FCM). Hb improvements and newborn outcomes were similar in both groups, but vitality and social functioning were better with IV iron. 53
All available IV iron formulations consist of iron-carbohydrate complexes of small spheroidal iron-carbohydrate particles ( Table 1 ). The carbohydrates serve as a shell around a core iron-hydroxide gel, permitting slow release of elemental iron while the remaining particles stay in colloid suspension. 56 , 57 Currently available IV iron formulations are of acceptable safety and equivalent effectiveness in the general population. 58 , 59 All IV formulations may be associated with allergic reactions characterized by nausea, hypotension, tachycardia, chest pain, dyspnea, and edema of the extremities that mostly occur within 24 hours of the infusion. These minor infusion reactions are self-limited, do not require treatment, 60 and should not be misread as anaphylaxis, 61 and they rarely recur with rechallenge. Empiric use of steroids prior to retreatment may diminish minor reactions that occur the next day. 62 Patients may also experience self-limited arthralgia, myalgias, and/or headache within a few days of infusion that generally respond to nonsteroidal anti-inflammatory drugs.
IV iron preparations
FDA pregnancy categories: B, Animal reproduction studies have failed to demonstrate a risk to the fetus and there are no adequate and well-controlled studies in pregnant women. C, Animal reproductive studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks.
TDI, total dose infusion.
A meta-analysis of 103 randomized controlled trials, comparing 10 391 patients treated with IV iron to 4044 who received oral iron, 1329 with no iron, and 3335 with placebo showed that neither serious adverse events nor infections were increased with IV iron. 63
Iron sucrose and sodium ferric gluconate are assigned to FDA pregnancy category B based on safety studies in pregnancy. Both high-molecular weight (HMW) and low-molecular-weight (LMW) iron dextrans retain a pregnancy category C designation despite evidence suggesting that adverse events ascribed to iron dextran are mostly associated with the HMW formulation. 64 Notably, these studies did not include pregnant women. HMW iron dextran has been linked to an increased risk of anaphylaxis and is no longer available. 65 An observational study of 189 women treated with LMW iron dextran in second and third trimester reported no severe adverse events and only 2% transient infusion reactions. 66 These results corroborated outcomes in other studies showing safety of LMW iron dextran, 67 , 68 which allows complete replacement of IV iron in a single infusion over 15 to 60 minutes. Despite well-established safety, LMW iron dextran still requires a test dose.
The newer IV iron formulations, ferumoxytol, FCM, and iron isomaltoside are all based on carbohydrates with reduced immunogenic properties; although it is not established that these decreased allergic reactions, a test dose is not required. Nevertheless, FCM and ferumoxytol are also assigned FDA pregnancy category C. FCM has been shown to be efficacious and safe in pregnancy ( Table 2 ). In a prospective study, Froessler and colleagues treated 65 pregnant women with FCM and reported no serious adverse effects and no change in fetal heart monitoring. 69 Furthermore, Christoph et al evaluated 206 pregnant women in a comparison study of iron sucrose and FCM and showed equivalent safety profiles in both drugs. 70 We recommend that pregnant women with any degree of iron deficiency be treated to correct anemia and replete ferritin as early in pregnancy as possible.
Examples of trials of IV iron formulations and dosing in pregnancy
IM, intramuscular; IS, iron sucrose; LMWID, LMW iron dextran; RCT, randomized controlled trial.
Iron formula deficit: weight × (target Hb − actual Hb) × 0.24 + 500 mg.
Myers shows safety of FCM and LMWID in pregnancy.
Patient 1 was 35 weeks pregnant and was treated with LMW iron dextran without incident. Had she presented before the 13th week of pregnancy and been able to tolerate oral iron, we would have treated with oral iron with follow-up 2 weeks later (and after the 13th week) for an assessment of response and to determine the need for IV iron. Tables 1 and 2 show the characteristics and dosing schedules of IV iron formulations with safety in pregnancy.
Prior to nationwide mandatory folate fortification programs, folate deficiency was the second most common cause of anemia during pregnancy. 71 The prevalence of folate deficiency in pregnancy varies from 1% to 50%, and is higher in economically deprived regions of the world. Numerous studies illustrate that the prevalence of both folic acid and cobalamin deficiency increase with advancing gestation. 72
Folate and cobalamin are involved in tetrahydrofolate metabolism, and are necessary for DNA synthesis for fetal growth and maternal tissue growth. 73 Dietary folate is absorbed in the jejunum. Poor nutrition, intestinal malabsorption, and increased requirements for fetal growth may contribute to folate deficiency. Cobalamin is present in animal protein and absorbed in the terminal ileum. R-protein (haptocorrin), secreted by salivary glands, binds cobalamin in the stomach and transports cobalamin to the duodenum where pancreatic proteases degrade the R-protein. Cobalamin is then released and binds to intrinsic factor released from gastric parietal cells. The cobalamin-intrinsic factor complex subsequently binds to receptors on ileal enterocytes. Atrophic gastritis, proton pump inhibitors, and malabsorption all increase the risk of cobalamin deficiency. 74
Bariatric surgery in the United States increased by 800% between 1998 and 2005, with women accounting for 83% of procedures in the 18- to 45-year age group. 75 In a retrospective study, anemia was detected in 17% of patients undergoing bariatric surgery, low ferritin in 15%, low cobalamin in 11%, and low RBC folate in 12%. 76
Most pregnant women with folate or cobalamin deficiency do not exhibit macrocytosis, 74 , 77 which may be masked by iron deficiency or by an underlying minor thalassemic phenotype. Furthermore, 2% to 5% of pregnant women with normocytic anemia have mild megaloblastic changes in the bone marrow that resolve with folic acid supplementation. 74
Serum cobalamin measures cobalamin bound to 2 circulating binding proteins, haptocorrin and transcobalamin. In nonpregnant patients, serum cobalamin <200 pg/mL (<148 pmol/L) is diagnostic of cobalamin deficiency, whereas levels above 300 are considered normal. Levels in the range of 200 to 300 pg/mL are borderline, and cobalamin deficiency is possible. 78 Notably, there is no difference in levels of the metabolites homocysteine and methylmalonic acid in pregnant women with subnormal cobalamin levels when compared with pregnant women with normal levels, 79 , 80 suggesting that low cobalamin in pregnancy may not reflect true tissue deficiency. A “physiologic” decline in cobalamin is seen in up to 20% of pregnant women that is indistinguishable from frank deficiency using routine laboratory studies. 79 , 81 , 82 Holotranscobalamin (biologically active cobalamin bound to transcobalamin) does not decline in pregnancy and has been suggested as a marker for cobalamin deficiency in pregnancy. 83 Holotranscobalamin is not available for clinical use. Serum folic acid concentrations <2 ng/mL are diagnostic of folic acid deficiency, whereas levels above 4 ng/mL effectively rule out deficiency. Levels in the range of 2 to 4 ng/mL are borderline. 84 Serum folate may be affected by recent oral/dietary intake, limiting the value of a single test. Although RBC folate is not so influenced, serum folate is more readily available and, in most instances, RBC folate measurement could be reserved for patients with borderline low serum values.
Further blood work in case 2 showed: serum iron, 25 μg/dL; TIBC, 395 μg/dL; ferritin, 4 μg/dL; folate, 21.5 ng/mL; and cobalamin, 113 pg/mL, confirming combined cobalamin and iron deficiency.
Because of the significant consequences of folate deficiency on neural tube development, folate supplementation is a standard component of antenatal care in the United States and Canada. National-scale public health initiatives requiring fortification of flour with folic acid in the United States and Canada have been effective in substantially reducing the prevalence of NTDs. 85 By contrast, Khoshnood et al, in an observational study of 11 353 cases of NTD in ∼12.5 million births in 19 countries in Europe, showed no change in prevalence of NTDs between 1991 and 2011, despite longstanding recommendations promoting folic acid supplementation and the existence of voluntary folic acid fortification. 86 Absent mandatory fortification, the prevalence of NTD in Europe has remained unchanged.
Folate fortification of foods in the United States is recommended because the neural tube closes around day 26 of gestation, a time when most women do not yet know they are pregnant. The C677T single nucleotide polymorphism (SNP) of MTHFR confers higher risk of NTD and such mothers have higher folate requirements. Yet, the SNP may confer greater protection against development of anemia and perhaps even maternal survival. This might explain the high allele frequency of this polymorphism in some populations through selective pressure. 87
The WHO recommends folate supplementation for pregnant women, 400 μg per day from early pregnancy to 3 months postpartum. The US Public Health Service and CDC recommend the same for all women of childbearing age (15-45 years of age) to prevent spina bifida and anencephaly. 88
Most prenatal vitamins contain 1 mg of folate, which is more than sufficient to meet the increased needs of pregnancy. A higher supplementation dose, 5 mg per day, is recommended in women who have increased demands for folate (multiple pregnancies, hemolytic disorders, folate metabolism disorders) and in women who are at an increased risk of NTDs (personal or family history of NTD, pregestational diabetes, epilepsy on valproate or carbamazepine).
Owing to the relatively large amounts of cobalamin that are stored in the human body, cobalamin deficiency in pregnancy is far less common than folate deficiency. However, with more pregnant women having undergone bariatric surgery, the risk of cobalamin deficiency is increased. Mead et al examined 113 women with a history of gastric bypass surgery delivering 150 babies and showed low cobalamin in over 10% of patients after biliopancreatic diversion, Roux-en-Y gastric bypass, or sleeve gastrectomy. 89
The WHO and US National Institutes of Health (NIH) recommend a higher daily allowance of cobalamin in pregnant women than in nonpregnant women (2.6 vs 2.4 μg per day) 90 , 91 to support fetal neurologic development. Growth retardation, general hypotonia, and loss of neuromotor skills have been described in infants of mothers with cobalamin deficiency. 92 Furthermore, cobalamin supplementation improves the motor functioning and regurgitation of cobalamin-deficient infants. 93 Notably, hematologic abnormalities caused by cobalamin deficiency may respond to folate supplementation, leaving other consequences of cobalamin deficiency unchecked. Therefore, prompt recognition of cobalamin deficiency and rapid treatment are of great significance.
Patient 2 was at risk of cobalamin (and iron) deficiency as a result of her bariatric procedures. She received intramuscular cobalamin 1000 mcg every 4 weeks, through pregnancy and the puerperium, with recommendation for lifelong replacement therapy. Our practice is to treat all pregnant women with laboratory data suggestive of cobalamin deficiency, irrespective of the cause.
Treatment of cobalamin deficiency in pregnancy is similar to that outside of pregnancy and can be achieved through oral or parenteral replacement. When oral vitamin cobalamin 1000 mcg daily is used, serum levels should be monitored to ensure adequate repletion. For patients who have had bariatric surgery, or other conditions that might interfere with intestinal absorption, sublingual cobalamin is an alternative to oral, 94 and in patients with neurological features attributable to cobalamin deficiency, parenteral treatment is preferred.
Additional results in the 27-year-old febrile Nigerian patient included: serum iron, 20 μg/dL; TIBC, 600 μg/dL; ferritin, 4 μg/L; and cobalamin, 113 pg/mL. Malaria parasites were seen on blood smear. HIV, hepatitis B and C were negative. Lactate dehydrogenase and bilirubin were normal. Absolute reticulocyte count was 20 000/μL. A diagnosis of anemia due to combined iron deficiency, cobalamin deficiency, and malaria was established. Malaria treatment was given. She received 6 units of packed RBCs and intramuscular hydroxycobalamin 1000 mcg daily for 1 week. After resolution of parasitemia, ferrous sulfate 200 mg and folic acid 5 mg daily were given.
Malaria poses a significant threat to pregnant women’s health. Each year, 23 million women conceive in malaria-endemic zones in Africa. 95 The prevalence is higher in primigravidas than in nonpregnant women. Plasmodium falciparum is the predominant species. Malaria contracted during pregnancy increases the mother’s risk of becoming anemic, infects the fetus (congenital malaria), and is associated with LBW and increased infant mortality. 96
Malaria prevention reduces neonatal mortality. 97 For pregnant women in areas with medium and high malaria transmission, WHO recommends intermittent preventive treatment during pregnancy, with at least 3 doses of sulphadoxine-pyrimethamine in the second and third trimesters, 98 in conjunction with the use of insecticide-treated mosquito nets. The patient in case 3 received sulphadoxine-pyrimethamine as recommended.
Severe anemia is seen in areas of very high malarial transmission and is most common in young children and pregnant women. 99 The prevalence of malarial anemia in endemic areas of Africa is 60% to 80% in pregnant women. 100 The pathogenesis includes direct destruction of parasitized and nonparazitized RBCs (extravascular and intravascular hemolysis), splenic and hepatic sequestration of RBCs, bone marrow suppression, and dyserythropoiesis. 101
The relationship between iron deficiency and malaria has been a focus of intense study because the 2 often coexist. In vitro, P falciparum infects erythrocytes from iron-deficient individuals less efficiently than erythrocytes from iron-replete humans, 102 a result supported by clinical studies. A cross-sectional study of 445 Tanzanian pregnant women reported that iron deficiency decreased the risk of placental malaria. 103 In a case control study in Malawi, iron deficiency was less frequent in 112 women with placental malaria than in 110 women without. 104 In a 2016 Cochrane systematic review of 35 randomized controlled trials assessing iron supplementation in children living in areas with hyperendemic or holoendemic malaria transmission, iron did not cause an excess of clinical malaria. 105 This review did not include pregnant women. Provision of supplemental iron to pregnant women in malaria-endemic areas has been controversial due to concerns that iron therapy may exacerbate infections. Oral iron has been shown ex vivo to promote bacterial growth. 106 In the 2 studies of pregnant women in countries with malaria, there was no difference in placental malaria and parasitemia between those who received iron to those who did not. 107
The third case illustrates the complexity of anemia in pregnancy in areas with malaria.
Patient 3 received blood. Alongside preventive measures, rapid access to safe blood products is critical to reducing anemia-related mortality in women in developing countries. 108 The most common indications for blood in sub-Saharan Africa are maternal hemorrhage, trauma, and malaria-associated anemia. 109 In a study of blood transfusion services in Malawi, the mean Hb of transfused patients was 4.8 g∕dL, and 17% (18 of 104) were given to pregnant women. 110
Guidelines for the management of postpartum hemorrhage have been published by a number of obstetric societies. The most recent French guidelines recommend transfusion in the setting of postpartum hemorrhage in order to maintain a Hb concentration >8 g/dL. 111
The latest transfusion guidelines of the AABB (formerly the American Association of Blood Banks) are based on 12 587 patients enrolled in 31 eligible randomized controlled trials in nonobstetric settings. They recommend using a restrictive Hb transfusion threshold of 7 g/dL for hemodynamically stable hospitalized adult patients. The evidence base that supports this approach in obstetrics is limited. Therefore, clinicians should consider the Hb, overall clinical context, patient preferences, and alternative therapies when making transfusion decisions for a patient. 112
Anemia in pregnancy is a significant global health problem. Symptoms mimic those of normal pregnancy therefore active surveillance is required for early diagnosis. Effective treatment improves maternal health and prevents deleterious effects on the child.
The authors thank Amaka Ocheke for providing a case and related input for this “How I Treat” article.
Contribution: M.M.A. and A.G.-G. wrote and revised portions of the manuscript and reviewed the manuscript.
Conflict-of-interest disclosure: M.M.A. is a consultant for Luitpold Pharmaceuticals, Inc. A.G.-G. declares no competing financial interests.
Correspondence: Maureen M. Achebe, Division of Hematology, Brigham and Women’s Hospital, 75 Francis St, Mid-Campus 3, Boston, MA 02115; e-mail: [email protected] .
This feature is available to Subscribers Only
- Previous Article
- Next Article
Email alerts
Affiliations.
- Current Issue
- First edition
- Collections
- Submit to Blood
- About Blood
- Subscriptions
- Public Access
- Permissions
- Blood Classifieds
- Advertising in Blood
- Terms and Conditions
American Society of Hematology
- 2021 L Street NW, Suite 900
- Washington, DC 20036
- TEL +1 202-776-0544
- FAX +1 202-776-0545
ASH Publications
- Blood Advances
- Blood Neoplasia
- Blood Vessels, Thrombosis & Hemostasis
- Hematology, ASH Education Program
- ASH Clinical News
- The Hematologist
- Publications
- Privacy Policy
- Cookie Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account

COMMENTS
Introduction: Macrocytic anemia can be associated with chronic alcohol use, vitamin B12 and folic acid deficiency. However, it is very uncommon to see severe anemia in developed world, especially in a young healthy patient without any medical issues. Folate deficiency is rarely reported to cause hemolytic anemia.
A Brief Review on Vitamin B 12 Deficiency Looking at Some Case Study Reports in Adults. Elena ... an intensive search for the active factor in liver extracts that prevents pernicious anemia showed that both folate and vitamin B 12 ... (four women aged 78 years, 84 years, 77 years and 87 years, 84 years old, and two men 71 and 75 years old ...
Folate is an essential water-soluble vitamin, naturally present in food, especially in fruits, green leafy vegetables, and liver.[1][2] Folic acid is the synthesized form of folate present in fortified foods and supplements and has a higher bioavailability than naturally occurring folate. Folate has been added to grains in the United States to prevent congenital disabilities, especially neural ...
Elevated methylmalonic acid (MMA) and homocysteine levels are also supportive of vitamin B 12 deficiency (MMA will be normal in folate deficiency). 3. To diagnose pernicious anemia as the etiology of a vitamin B 12 deficiency, additional testing for antibodies against intrinsic factor is needed. Although rarely performed today, the Schilling ...
We report a case of severe pancytopenia in a 15-year-old patient due to a severe deficiency in vitamin B12 and folic acid, probably of nutritional origin. The clinical and biological course was favorable after vitamin supplementation. With this case, we discuss the diagnostic approach of pancytopenia with megaloblastic anemia in children and adolescents, as well as the mechanisms involved in ...
Emily Zerlang Case Study 87 Folic Acid Deficiency Anemia 1. Docusate Sodium is a stool softener taken for constipation and a lot of older adults experience this because of their sedentary lifestyle and lack of fiber in their diet. There are also changes in the GI tract with age, less movement and decreased GI excretions. The other factor for this pt is his medications, the diuretic and the ...
Folate deficiency anemia can occur when you don't have enough vitamin B9 in your diet. This shortage affects red blood cell production and causes weakness and fatigue. It's most often the result of not eating a balanced diet or having an underlying health condition. Treatment with a vitamin B9 supplement usually restores red blood cells and ...
Folate-deficiency anemia is the lack of folic acid in the blood. In most cases it is caused by a lack of folic acid in the diet. Leafy vegetables, citrus fruits, beans, and whole grains are natural sources of folic acid. Folate-deficiency anemia in pregnancy may cause a neural tube defect. This is when the brain or spinal cord doesn't develop ...
The present study includes a review of this subject and a report of a patient with sickle-cell anemia in whom documentation of folic acid deficiency was obtained and the response to "minute" or ...
Case Study 87: Folic Acid Deficiency Anemia. Disease Summary; Case Study 88: Iron Deficiency Anemia. Disease Summary; Case Study 89: Sickle Cell Anemia. Disease Summary; Case Study 90: Vitamin B12 Deficiency Anemia. Disease Summary; Part 15: Disorders of the Eyes, Ears, Nose, and Throat. Case Study 91: Acute Otitis Media.
World Health Organization (WHO) thresholds were established in 1968 in a cohort of persons <65 years old, defining anemia as a hemoglobin (Hb) level of <130 g/L in men and <120 g/L in women. 10 However, Hb levels decline with age and are distinct in different ethnic groups. So far, the WHO definition 10 of anemia has been applied in the majority of studies at older age.
Case Study 1 A 54 year ... Stage IV—clinical folate deficiency anemia. 10/27/2016 5 Management Initial dosage Maintenance dosage Route of administration of B12 Oral 1,000 to 2,000 mcg per day for one to two weeks 1,000 mcg per day for life Intramuscular 100 to 1,000 mcg every day or every
a. glove and stocking parasthesia. b. wobbly gait. c. tachycardia. d. mild icterus. c. tachycardia. This is most likely iron deficiency anemia based on the physical exam findings. The other options were symptoms of B12 deficiency anemia. A 30 year old male patient presents with chief complaint of fatigue for the past month. During the history ...
Case Study A patient with folic acid deficiency anemia p. 1027 1. The pathophysiologic basis for Mrs. Matthews's abnormal vital signs during her initial assessment is due to her 20 lb weight loss since the death of her husband. She lacks proper nutrition including lack of fluid intake. The most common cause of folate deficiency is because of inadequate dietary intake.
Folic acid (vitamin B9) is a water-soluble vitamin used to manage and treat megaloblastic anemia. Folic acid has FDA approval for treating megaloblastic and macrocytic anemias due to folic deficiency. This activity describes the indications, mechanism of action, and contraindications for folic acid as a valuable agent in managing megaloblastic anemia and preventing other disorders. One of the ...
Megaloblastic anemia causes macrocytic anemia from ineffective red blood cell production and intramedullary hemolysis. The most common causes are folate (vitamin B9) deficiency and cobalamin (vitamin B12) deficiency. Megaloblastic anemia can be diagnosed based on characteristic morphologic and laboratory findings. However, other benign and neoplastic diseases need to be considered ...
The diagnosis of a vitamin B 12 deficiency is first made with a CBC, peripheral blood smear, and vitamin B 12 and folate levels, demonstrating a megaloblastic macrocytic anemia and low serum vitamin B 12 (<200 pg/mL), but a normal folate level (necessary to rule out folate deficiency, which may be comorbid).
A number of cases with iron deficiency anemia (IDA) are associated with low vitamin B12 (LB12). However, LB12 is not synonymous with B12 deficien ... Iron Deficiency and Vitamin B12/Folate Levels. A Case-Control Study. Angel F. Remacha, MD, Angel F. Remacha, MD ... LB12 was observed in 17.8% of IDA cases (87 cases), including 8.2% with B12 ...
DS87-4 Case Study 87 Folic Acid Deficiency Anemia Diagnosis: Clinical Manifestations and Laboratory Tests Diagnosis of FADA generally is straightforward and determined from a comprehensive patient medical history, clinical manifestations, and selected laboratory blood tests. With folate defi- ciency, the patient's history is important because it may reveal the underlying cause of defi- ciency.
Background Anemia is a serious global health problem that affects individuals of all ages but particularly women of reproductive age. Iron deficiency anemia is one of the most common causes of anemia seen in women, with menstruation being one of the leading causes. Excessive, prolonged, and irregular uterine bleeding, also known as menometrorrhagia, can lead to severe anemia. In this case ...
This has been termed THF starvation, or the methylfolate trap (Figure-1). This theory explains the abnormalities of folate metabolism that occur in cobalamin deficiency and also why the anemia of cobalamin deficiency will respond to folic acid in large doses. Figure-1 showing the role of folic acid in DNA synthesis and methionine metabolism.
Physiologic anemia of pregnancy reflects an expansion of plasma volume of 50% relative to the increase in the red blood cell (RBC) mass of 25%. 4 Globally, the most common cause for anemia of pregnancy is iron deficiency, arising from maternal-fetal transfer of iron, frequently aggravated by decreased maternal iron reserves. 5 The Nutrition Impact Model Study, a systematic analysis of 257 ...
In Cox proportional hazard analysis, osimertinib was associated with a 50% higher risk of vitamin B deficiency (Hazard ratio, 1.52 [95% CI: 1.07-2.16]) compared to other EGFR-TKIs. Consistent with this observation, osimertinib was associated with an increased risk of vitamin B12 deficiency anemia and a tendency towards folate deficiency anemia.
View case study# 87.docx from NURSING 112 at California State University, Fresno. Chanpreet Kaur Case Study#87 1. ... DS - Folic acid deficiency anemia. Barry University. BMS 510. notes. Final Information Care (1).pdf. Chamberlain University College of Nursing. NR 565. 2020 pharm.docx. Solutions Available. Walden University.
Serious B12 deficiency may cause anemia and nerve damage. There are several B12 supplements available, and some (like B12 tablets) have high dosages far above the daily recommended value. ... The study analyzed deficiency rates among different age groups; the vitamin deficiency in the age group that included the elderly varied between 11% to 90 ...
e15648 Background: Colorectal carcinoma (CRC) is third most commonly diagnosed cancer in males and second in females. Implications of CRC with coexisting anemia and its outcomes of hospitalization have not been studied. We aim to evaluate the outcomes of CRC hospitalization with and without coexisting nutritional anemia. Methods: A total of 504,515 patients (male: 52.61%, female: 47.39%), aged ...