An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Shanghai Arch Psychiatry
- v.29(6); 2017 Dec 25
Language: English | Chinese

A Case Report of A Patient with Treatment-Resistant Depression Successfully Treated with Repeated Intravenous Injections of A Low Dosage of Ketamine
低剂量氯胺酮重复静脉注射成功治疗难治性抑郁症患者 1 例.
Depression is a highly prevalent and severely disabling disease. The treatment effects, intensity and onset time of antidepressants have been highlighted in many studies. Recent studies on the rapid-onset of antidepressant response focused on the effect of a single low dose of intravenous ketamine. However, there are still some problems with treatment, including safety, efficacy, ethics, dose, frequency of administration and their effect in treatment-resistant depression. In the present study, we treated one case of treatment resistant depression with repeated intravenous injections with a low dosage of ketamine.
概述
抑郁症是一类患病率高且危害严重的疾病,抗抑郁药物的疗效强度及起效时间一直备受关注,目前在抗抑郁治疗的快速起效方面研究热点是以低剂量氯胺酮静脉注射治疗,但在氯胺酮使用过程中仍有较多问题,包括安全性、疗效、伦理、给药剂量及频次、在难治性抑郁症患者中的应用等。本研究采用重复低剂量氯胺酮静脉注射的方式成功治疗了一例难治性抑郁症患者,结果安全有效。
1. Introduction
Depression is a kind of disorder with core symptoms of low mood, decreased interests and anhedonia, and can also be accompanied by a variety of somatic symptoms as well as negative thoughts and behaviors. However, the current rate of effectiveness for antidepressants is only 60% to 80%, [ 1 ] and it takes about two weeks for them to be effective; furthermore, residues of symptoms are common after treatment. Therefore, achieving rapid relief of depression symptoms within a short period of time has attracted increasing attention in the current research field. Previous research [ 2 ] has stated that the antagonist of N-methyl-D-aspartic acid receptor (NMDA) represented by ketamine can relieve the depression symptoms of patients effectively, but mostly with a single intravenous injection of a low dose; even though the curative effect is guaranteed, it does not last long. We treated one case of treatment-resistant depression with repeated intravenous injections of low dose ketamine in our department with satisfying results. Now the case is reported as below:
2. Medical history
The patient was a 27-year old single, male, with a junior high education, surnamed Wang. Wang was a blue collar worker from Huzhou, Zhejiang province. Patient’s depression had a duration of over 8 years. He was admitted to our hospital on 29 th August 2016 with principle complaints being “trouble sleeping, feeling unhappy, worried and fatigued”. Over 8 years ago this patient gradually developed sleep difficulties, as well as low mood, loss of interest, speaking less, loss of movement and excessive worries. At that time he sought treatment in our out-patient department, and was diagnosed with depressive disorder. After taking Seroxat 40 mg every morning, his symptoms improved with normal intermissions. However, after he stopped taking medication on his own, he started to feel unable to be happy, have no motivation to do things, have prominent anxiety and a tendency to uncontrollably over-think situations. He returned to our hospital for treatments multiple times, and during the treatment, he took many medications, such as “paroxetine (Seroxat), Sertraline (Zoloft), Fluvoxamine (Luvox), Venlafaxine (Efexor RX), clorimipramine, etc.” He tried both single-drug-use and multiple-drug-use of antidepressant medications with different mechanisms, and “Olanzapine (Zyprexa) or Quetiapine (Seroquel)” were also used as supplemental drugs to improve the curative effect. Even though during the period of treatment, he was treated with a systematic antidepressant procedure accompanied with sufficient doses and time, his symptoms were not fully relieved. Moreover, they became greatly aggravated in the month leading up to the treatment reported here. He became depressed all day, was fatigued, had no motivation to do anything, had decreased interest in life, and experienced difficulties working normally, and felt distressed. Therefore, he was hospitalized with a diagnosis of “recurrent depression disorder, current as major depressive episode without psychotic symptoms”. The patient had an appendicitis operation two years ago, and denied any other major medical conditions or a history of allergies. The patient was an only child and reported a normal childhood and development. He had a middle school education, and reports being an average student. After growing up the patient began working in a delivery company and reports having good relationships with his coworkers and no problems with his work ability. Client denied any drug or alcohol use, and is a non-smoker. Individual history was unremarkable and family history of mental illness was negative.
Physical examination at the time of hospitalization revealed that his height was 181 cm and his weight was 80 kg. His vital signs were all normal, and no abnormal results were found from his cardiopulmonary auscultation. There was a 10 cm long surgical scar on his lower right quadrant. The result of the neurological examination was negative. The mental status examination indicated that his consciousness was clear, that he was cooperative during contact, and that he was able to stay on topic during conversations. There were no signs of hallucinations and delusions. His mood was low, and his facial expressions were sad. He showed obvious anxiety, and he was worried that he could not get better. Decreased interests were present. He had little energy all day long, and felt difficulty concentrating. He had low self-assessment and distressed feelings. Even though negative thoughts were present, he did not show any negative behavior. His volitional behavior was diminished, but his insight about the illness existed.
After hospitalization, his results of routine blood tests, blood biochemistry tests, thyroid function tests, electrocardiogram, chest radiograph, abdominal color Doppler ultrasound and head CT were all within normal limits. The initial treatment was Venlafaxine release capsules (150 mg every morning) and Olanzapine tablets (2.5 mg every evening) orally. After two weeks of treatment, the effect was not satisfactory. Therefore, Escitalopram (the dose was increased to 20 mg/d for 2 weeks) tablets were added; but the effect was still not significant. The patient lay and sighed with sad expressions all day long. Then he was given with 6 consecutive sessions of MECT with propofol anesthesia (3 times a week), but it did not improve his symptoms either. His mood and interests were low, and his movements diminished. His HAMD-17 score was 28. And his score on the Beck Scale for Suicide Ideation-Chinese version (BSI-CV) [ 3 ] was 11. During his hospitalization, he was discussed as a difficult case multiple times, and the diagnosis was always treatment-resistant depressions. [ 4 ] Considering that regular drugs did not improve symptoms and he did not react well to MECT, we planned to have an anesthesiologist conduct MECT with ketamine intravenous injection anesthesia under supervision after the patient and his family provided written consent. The initial injection was a one-minute-long fast intravenous injection with a subclinical dose of 70 mg (<1 mg/kg). [ 5 ] A few minutes later, the patient’s depressive symptoms were completely gone; instead, he experienced comfort which he had never felt before. He commented that “this is so much better than electroshock therapy”, and that “now we have entered the 25 th century” and so on. His expression became happy, and he felt relaxed. Hence, the following MECT electrical stimulation was canceled and he was put on observation. At the 40, 80, 120, 230 minutes, the first and the second day after the injection, the scores of HAMD-17 were consistently under 7, and those of BSI were consistently under 5. The patient was able to communicate with other patients in the ward, but he still worried that the effect of the drug would not last. Three days later, he was given another 40 mg (the dose was based on 0.5 mg/kg) [ 2 ] ketamine intravenous injection using a micro pump and lasted for 40 minutes. He was injected every other day and three times in total. Evaluations with HAMD-17 and BSI-CV were conducted before and after every ketamine infection. After the injection treatment, his symptoms disappeared and he was discharged from the hospital with a prescription of “Venlafaxine release capsules 150 mg every morning and Escitalopram tablets 20 mg every morning”. During the ketamine injection treatment, no other adverse reactions other than light dizziness were found. No cognitive impairment was detected in the neuropsychological tests. However, the patient reported that the pleasant experiences felt after the last three ketamine injections were far less than what he felt after the first injection. During that time, he wanted to accept fast intravenous injection treatment of ketamine again, but this request was denied because ketamine has a potential risk of being abused and addictive. [ 6 ] In the outpatient follow-ups during the two weeks after he was discharged, his symptoms of depression were improved, and he was able to work. Besides worrying about the recurrence of depression disorder, he did not show any other symptoms.
3. Discussion
Ketamine is a kind of phencyclidine drug with a complex mechanism, and it may involve NMDA receptors, opioid receptors, monoamine receptors, acetylcholine receptors, voltage-gated channels and so on. Due to its rapid and strong effect against depression, it has become a hot research topic in recent years. The mechanism of this drug’s rapid effect on improving depression remains unclear, but it may be related to the biological factors including neural plasticity (slow waved electrical activities of brain, brain-derived neurotrophic factor (BDNF), Val166Met gene polymorphism, Shank 3 protein expression), neurological factors (anterior cingulate gyrus activities, glutamate/glutamine concentration), inflammatory factors (IL-6 concentration), and metabolic factors (VitB12 concentration, D-/L-serine, mitochondria β-fatty acid oxidation changes). [ 7 ] Because of the potential psychoactive effect of this drug and the ethical issues involved, the clinical use of it is limited. [ 8 ] However, the current evidence-based medicine studies have shown that using it to treat major or treatment-resistant depression does not violate medical ethics. [ 9 ]
This patient had multiple antidepressant treatments with adequate dosage and duration, and six consecutive MECT treatments, but his depressive symptoms did not improve significantly. In this situation, employing ketamine intravenous injection treatment produced a satisfying outcome. His depressive symptoms were relieved to the clinically cured level, and there was a significant improvement in his self-experience. However, this patient was followed up for only two weeks outside the hospital. In order to explore the effect that ketamine has on treating difficult-to-treat depressive disorder, future studies should include larger samples and longer follow-up durations.
During the ketamine injection treatment, the patient was able to experience a rapid relief of negative emotions. What was different about this case was that this patient received ketamine treatments with two different dosages and injection methods. During the initial rapid intravenous anesthesia with 70 mg ketamine before MECT, this patient experienced the relief of his depressive symptoms; in the meantime, he also experienced pleasure that he had never experienced before. However, when he was treated three times with micro-pump intravenous injections with 0.5 mg/kg ketamine as it was indicated in the literature on ketamine, this phenomenon did not appear again. Furthermore, it was not seen among other depressed patients who also received ketamine treatments. This suggests that high dosage treatments are highly likely to cause dissociative symptoms and an increase in blood pressure. Moreover, it can also make patients want to seek “pleasure” again, [ 10 ] thereby potentially leading to drug-seeking or drug-abusing behavior. Therefore, we recommend using slow intravenous injections with low dosages for treatment, and there is research recommending an even lower ketamine dosage (0.1 mg/kg) to treat difficult-to-treat depressive disorder. [ 11 ] This should be noted for future clinical research or practice.
Adverse reactions to ketamine in clinical practice are usually hallucinations, delirium, nightmares, fear or cataleptic state. But in this case, the patient only felt a light dizziness during ketamine injection treatment, which was tolerable. Moreover, the adverse reaction disappeared after bed rest. This indicates that the adverse reaction of this drug is related to the dosage and tolerance of the individual. In fact, if the dosage of ketamine for antidepressant use is the dosage (0.5 mg/kg) indicated by the current published research, the patient’s consciousness will be clear, and there will not be obvious respiratory effect or cognitive impairment. Furthermore, in terms of improving depressed emotion and negative thoughts, its effect is strong and rapid, and it may be more effective than MECT in some cases. Therefore, under careful observation and with full informed consent, this could be an alternative treatment for treatment -resistant depression.
Shikai Wang graduated from the Medical School of Peking University in June 2007 with a master’s degree in psychiatry. He is currently a psychiatry PhD candidate at the Anding Hospital of Capital Medical University. He has been working in the Huzhou Third People’s Hospital in Zhejiang Province since March 2008. He is currently the director of the Open Psychiatry Department in the Huzhou Third People’s Hospital. His research interests are biological psychiatry and psychopharmacology.
Funding statement
Huzhou division of science and technology public welfare technology application project (2014GZ12)
Conflicts of interests statement
Informed consent
The patient and his family provided written informed consent.
Authors’ contribution
Shikai Wang was responsible for coordinating the study and writing up the paper. Mincai Qian participated in the revision of the paper. Liang Li was responsible for delivering the specific treatment. Qi Yang was responsible for the evaluations with scales during treatment.
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Experimental depression treatment is nearly 80% effective in controlled study
In a double-blind controlled study, high doses of magnetic brain stimulation, given on an accelerated timeline and individually targeted, caused remission in 79% of trial participants with severe depression.
October 28, 2021 - By Mandy Erickson

Since receiving an experimental depression treatment at Stanford, Tommy Van Brocklin has been walking Scout for "the sheer joy of it." Nellie Van Brocklin
A new type of magnetic brain stimulation brought rapid remission to almost 80% of participants with severe depression in a study conducted at the Stanford University School of Medicine .
The treatment, known as Stanford accelerated intelligent neuromodulation therapy (SAINT) or simply Stanford neuromodulation therapy, is an intensive, individualized form of transcranial magnetic stimulation. In the study, remission typically occurred within days and lasted months. The only side effects were temporary fatigue and headaches.
“It works well, it works quickly and it’s noninvasive,” said Nolan Williams , MD, an assistant professor of psychiatry and behavioral sciences. “It could be a game changer.” Williams is the senior author of the study, which was published Oct. 29 in the American Journal of Psychiatry .
Twenty-nine people with treatment-resistant depression participated in the study: About half received SAINT, and the rest underwent a placebo procedure that mimicked the real treatment. After five days of treatment, 78.6% of the participants in the treatment group were no longer depressed, according to several standard methods of evaluation. “It’s quite a dramatic effect, and it’s quite sustained,” said Alan Schatzberg , MD, the Kenneth T. Norris, Jr. Professor in Psychiatry and Behavioral Sciences, who was a co-author of the study.
A lifetime of depression
Tommy Van Brocklin, 60, has suffered from depression since he was 15. “In 1975, they didn’t have the medication and understanding they do now,” he said. “I was told I wasn’t trying hard enough.”
“I’ve functioned all these years, but it’s been very difficult at times,” the civil engineer added. Talk therapy helped “for about half a day after an appointment.” When selective serotonin reuptake inhibitors became available in the 1990s, he started on paroxetine, commonly sold under the brand name Paxil.
“It worked like a miracle drug,” he said, but after 10 or 15 years it started to lose its effect. After 25 years, it stopped working entirely. He tried other medications, but none helped; one even made him suicidal.
His sister, who lives near Stanford, connected him with the researchers studying SAINT. He flew from his home in Memphis, Tennessee, and underwent the treatment in September. He felt nothing the first day; on day two, he began feeling emotional — “I felt the struggle of what I’d been through all these years.”
“The next day, all of a sudden, it broke through,” he said. “I felt so much better, and it’s stuck with me.”
Specialized magnetic stimulation
The transcranial magnetic stimulation treatment currently approved by the Food and Drug Administration requires six weeks of once-daily sessions. Only about half of patients who undergo the treatment improve, and only about a third experience remission from depression.
SAINT advances that treatment by targeting the magnetic pulses according to each patient’s neurocircuitry and providing a greater number of pulses at a faster pace.
In the study, the researchers first used MRI to locate the best location to target within each participant’s dorsolateral prefrontal cortex, which regulates executive functions, such as problem solving and inhibiting unwanted responses. They applied the stimulation in a subregion that has the strongest relationship with the subgenual cingulate, a part of the brain that is overactive in people experiencing depression. The transcranial magnetic stimulation strengthens the connection between the two regions, facilitating dorsolateral prefrontal cortex control of the activity in the subgenual cingulate.
The researchers also used 1,800 pulses per session instead of 600. (The larger amount has been used safely in other forms of brain stimulation for neurological disorders such as Parkinson’s disease.) And instead of providing one treatment a day, they gave participants 10 10-minute treatments, with 50-minute breaks in between.
For the control group, the researchers disguised the treatment with a magnetic coil that mimicked the experience of the magnetic pulse; both the control and active treatment groups wore noise-canceling earphones and received a topical ointment to dull sensation. Neither the researcher administering the procedure nor the participant knew whether the participant was receiving real treatment.
A hard-to-treat group
The trial participants ranged in age from 22 to 80; on average, they had suffered depression for nine years. They had tried medications, but either they had had no effect or they had stopped working. During the trial, participants who were on medication maintained their regular dosage; participants who weren’t taking medications did not start any.

Nolan Williams demonstrates SAINT, the magnetic brain stimulation therapy he and his colleagues developed, on Deirdre Lehman, a participant in a previous study of the treatment. Steve Fisch
Within four weeks after treatment, 12 of the 14 participants who had received the treatment improved, and 11 of them met FDA criteria for remission. In contrast, only two of the 15 participants who had received the placebo met the criteria for remission.
Because the study participants typically felt better within days of starting SAINT, the researchers are hoping it can be used to quickly treat patients who are at a crisis point. Patients who start taking medication for depression typically don’t experience any reduction of symptoms for a month.
“We want to get this into emergency departments and psychiatric wards where we can treat people who are in a psychiatric emergency,” Williams said. “The period right after hospitalization is when there’s the highest risk of suicide.”
Van Brocklin said that since he returned home following treatment, he’s made some radical changes. “I have a really strong desire to get my life together,” he said.
“I don’t procrastinate anymore,” he added. “I’m sleeping better. I completely quit alcohol. I’m walking my dog and playing the guitar again, for nothing more than the sheer joy of it.”
Most importantly, he said, “I’m remaining positive and being respectful of others. These are big changes in my life.”
Other Stanford scientists who contributed to the study are former postdoctoral scholars Eleanor Cole, PhD, and Angela Phillips, PhD; Brandon Bentzley, MD, PhD, David Carreon, MD, Jennifer Keller, PhD, Kristin Raj, MD, and Flint Espil, PhD, all clinical assistant professors of psychiatry and behavioral sciences; clinical research coordinators Katy Stimpson, Romina Nejad, Clive Veerapal, Nicole Odenwald and Maureen Chang; former clinical research coordinators Fahim Barmak, MD, Naushaba Khan and Rachel Rapier; postdoctoral scholars Kirsten Cherian, PhD, James Bishop, PhD, Azeezat Azeez, PhD, and John Coetzee, PhD; life science research professional Heather Pankow; clinical research manager Jessica Hawkins; Charles DeBattista, MD, professor of psychiatry and behavioral sciences; and Booil Jo, PhD, associate professor of psychiatry and behavioral sciences.
Scientists from the U.S. Department of Veterans Affairs; Palo Alto University; the Centre for Neuroimaging and Cognitive Genomics at the National University of Ireland; and the School of Medicine at Southern Illinois University, Carbondale, contributed to the research.
The research was funded by a Brain and Behavior Research Foundation Young Investigator Award, Charles R. Schwab, the David and Amanda Chao Fund II, the Amy Roth PhD Fund, the Neuromodulation Research Fund, the Lehman Family, the Still Charitable Trust, the Marshall and Dee Ann Payne Fund, and the Gordie Brookstone Fund.
Stanford’s Department of Psychiatry and Behavioral Sciences also contributed to the work.
If you're interested in participating in a study, please email [email protected] .

About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Hope amid crisis
Psychiatry’s new frontiers


Case 1: Newly Diagnosed Treatment Resistant Depression
Lisa Harding, MD, and Angelos Halaris, MD, PhD, APA, ACNP, CINP, review a case of a 26-year-old male patient who was recently diagnosed with treatment resistant depression
EP: 1 . Case 1: Newly Diagnosed Treatment Resistant Depression
Ep: 2 . case 1: an overview of treatment resistant depression, ep: 3 . case 2: prescribing intranasal esketamine, ep: 4 . case 2: starting a patient on intranasal esketamine, ep: 5 . targeting the glutamatergic system for treatment resistant depression, ep: 6 . phase 3 transform trial, ep: 7 . intransal esketamine and rems, ep: 8 . health care requirements to be aware of for intranasal esketamine, ep: 9 . advising clinicians on using intranasal esketamine.
Lisa Harding, MD: Welcome to this Psychiatric Times® Case-Based Psych Perspectives titled “Managing Patients With Treatment-Resistant Depression.” I’m Dr Lisa Harding, a board-certified psychiatrist and a clinical instructor of psychiatry at the Yale School of Medicine in New Haven, Connecticut. Joining me is the esteemed Dr Angelos Halaris, a board-certified psychiatrist and a professor of psychiatry at Loyola University Chicago’s Stritch School of Medicine in Maywood, Illinois. The goal of our discussion is to share insights in diagnosing treatment-resistant depression [TRD] and reasons for inadequate treatment response with antidepressant therapy, as well as to provide a brief overview of available treatment options and to offer recommendations on how treat patients with this disorder. Welcome, Dr Halaris.
Angelos Halaris, MD, PhD, APA, ACNP, CINP: Thank you so much for inviting me. I’m honored by your invitation and glad to be here.
Lisa Harding, MD: It’s nice to see you again. We’ll start by reviewing a couple of case scenarios. The first case presentation, No. 1, is a patient newly diagnosed with treatment-resistant depression. A 26-year-old man with a history of major depressive disorder for over 7 years presents with complaints of trouble sleeping as well as feeling unhappy, worried, and fatigued. He gradually developed sleeping difficulty as well as low mood and loss of interest. He tried multiple treatments, including escitalopram, fluoxetine, venlafaxine, and bupropion. However, his symptoms weren’t fully relieved. He was subsequently diagnosed with treatment-resistant depression. The patient reports having an average childhood, being an average student, and having good relationships with coworkers and no problems at work. He was always involved in psychotherapy, and denied any drug or alcohol use.
My overall impression of this case is this is a young patient who’s supposed to be living the life actuation part of his life, and he has now tried and failed more than 2 antidepressants. One of the things coming to mind was, is he stopping these antidepressants because of adverse effects, as I see in my clinical practice? What was the adequate dose of the adequate trial in terms of these medications that he was prescribed? Dr Halaris, what are your overall impressions of the case?
Angelos Halaris, MD, PhD, APA, ACNP, CINP: Much like what you just said, Lisa, as presented, this brief case scenario leaves many more unanswered questions, some of which you already touched upon. I’d like to reinforce your own questions and add a few of mine as well. First and foremost, what kind of work-up was done prior to diagnosing the patient and then treating him with the list of mainly SSRIs [selective serotonin reuptake inhibitors] and SNRIs [serotonin and norepinephrine reuptake inhibitors]? By that, I mean where other factors that are known to contribute to depression, and especially TRD, had they been carefully assessed by means of a thorough psychiatric diagnostic evaluation and the pretty much established blood work that we know is essential, such as ruling out endocrinopathies, assessing HPA [hypothalamic-pituitary-adrenal] function, looking at diabetes, inflammatory conditions, any chronic medical illnesses that invariably lead to chronic inflammation, including neuroinflammation. Because if there’s an inflammatory focus elsewhere in the body, these pro-inflammatory substances known as cytokines invariably make their way into the brain parenchyma and stimulate microglia and astrocytes to also become inflamed. So we have a relocation of the peripheral inflammation into the brain leading to neuroinflammation. These are all factors that I’d like to see addressed.
Other issues are obviously vitamin deficiencies, notably vitamin D, especially during the winter months. But this also happens in summer months in susceptible individuals. I’m amazed at the frequency of vitamin D deficiency, including in young people. Unless we make a point to check these issues routinely at the initial evaluation, some of the symptoms of vitamin D deficiency resemble symptoms of depressive disorder with anxiety, low energy, low motivation, sense of desperation, attention-focusing issues and so on. The good news is that it’s fixable by administering the right supplementation of vitamin D.
This transcript has been edited for clarity.

Mood Disorders in the News

Blue Light Blockers: A Behavior Therapy for Mania

Postpartum Depression and Psychosis in Psychiatric Times

Treating ‘Morally Objectionable’ Patients

The 2024 APA Annual Meeting: Sunday, May 5
Semaglutide and Depression: What Is the Relationship?
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Treatment-Resistant Depression in Real-World Clinical Practice: A Systematic Literature Review of Data from 2012 to 2022
- Open access
- Published: 26 October 2023
- Volume 41 , pages 34–64, ( 2024 )
Cite this article
You have full access to this open access article

- Albino J. Oliveira-Maia ORCID: orcid.org/0000-0001-5071-3007 1 , 2 ,
- Ania Bobrowska 3 ,
- Eric Constant ORCID: orcid.org/0000-0001-8023-1535 4 , 5 ,
- Tetsuro Ito ORCID: orcid.org/0000-0003-0802-638X 6 ,
- Yerkebulan Kambarov ORCID: orcid.org/0000-0003-0362-8373 7 ,
- Hannah Luedke ORCID: orcid.org/0009-0006-8290-600X 8 ,
- Siobhán Mulhern-Haughey ORCID: orcid.org/0000-0002-9708-4356 9 &
- Christian von Holt 10
1 Altmetric
Explore all metrics
Real-world evidence in treatment-resistant depression (TRD; commonly defined as non-response to ≥ 2 consecutive treatments at adequate dosage and duration) is lacking. A systematic literature review was conducted to understand disease burden and treatment outcomes for patients with TRD, studied in a real-world setting over the last decade.
Data Sources
A literature search was conducted in May 2022 in MEDLINE, Embase, The Cochrane Libraries and PsycINFO, comprising studies published from 2012 to 2022. Bibliographies of all relevant identified systematic reviews and relevant conference proceedings from 2020 to 2022 were manually hand-searched.
Study Selection
Real-world studies, including cohort, cross-sectional, case–control, chart review and registry studies, published in English and reporting outcomes in adults with TRD, were included.
Data Extraction
Extracted data included study and baseline disease characteristics, treatment type, treatment response, clinical outcomes and health-related quality of life.
Twenty studies were included. Criteria for TRD varied, but patients typically experienced long-lasting depression (range 1.4 to 16.5 years). Across studies, mean disease severity scores demonstrated moderate to severe depression, reflecting a high burden of disease at baseline. Remission rates were typically low but generally increased with longer follow-up durations. However, the heterogeneity of interventions, follow-up durations (range 2 weeks to 9.4 years) and assessment tools precluded their quantitative synthesis. Studies were frequently limited by low sample size (range 14 to 411 patients) and health-related quality of life was infrequently assessed.
Conclusions
There is a lack of clinical consensus regarding the definition, assessment and monitoring of TRD in real-world practice. Nevertheless, TRD carries a high burden of illness and there is an unmet need for faster and more effective treatments. To better understand the personal burden of affected patients, future studies would benefit from standardisation of severity assessment and measures of treatment effectiveness, as well as greater consideration of health-related quality of life.
Plain Language Summary
Many people continue to experience depression even after trying two or more medications. This is called treatment-resistant depression (TRD). Most of the information we have on TRD comes from clinical trials, which take place under tightly-controlled conditions. It is important to understand the effects of TRD and TRD treatments on people in their day-to-day lives. Researchers studying people’s day-to-day lives call this researching in a “real-world setting”. We searched for studies carried out in real-world settings in the last 10 years. We found 20 relevant studies. As these studies were in real-world settings, there were many differences between them, including differences in how TRD was diagnosed, the treatments used, how long people were monitored and how results were measured. This made it difficult to compare how successful different treatments were. Most studies included a small number of people and monitored them for a relatively short time. We found people with TRD had usually lived with it for many years and their symptoms were moderate or severe. Only two studies asked people how TRD affected their lives. These two studies found health-related quality of life and work productivity was low. Most studies found lots of people still had symptoms of depression after treatment. However, symptoms typically improved more when studies monitored people for a longer time. To improve our knowledge of TRD, future studies should monitor more people for longer and use the same ways of measuring results. They should also ask how TRD affects people’s daily lives.
Similar content being viewed by others

Clinical guidelines for the management of treatment-resistant depression: French recommendations from experts, the French Association for Biological Psychiatry and Neuropsychopharmacology and the fondation FondaMental

Optimising first- and second-line treatment strategies for untreated major depressive disorder — the SUN☺D study: a pragmatic, multi-centre, assessor-blinded randomised controlled trial
Standardisation framework for the maudsley staging method for treatment resistance in depression.
Avoid common mistakes on your manuscript.
Introduction
Major depressive disorder (MDD) is one of the most common, yet debilitating, psychiatric disorders, characterised by persistently low mood and energy; anhedonia; changes in appetite, weight and sleep; fatigue; and suicidality, among other symptoms [ 1 , 2 ]. The lifetime prevalence of MDD among the general population is estimated to be ~ 13 to 15%, with first-line treatments consisting of antidepressant medications, behavioural psychotherapy, or a combination thereof [ 3 , 4 ]. Many patients with MDD, however, do not experience a sufficient response to initial antidepressant treatments and may develop treatment-resistant depression (TRD) [ 5 ]. TRD is most commonly defined as non-response to two or more different pharmacological treatments, taken for an adequate duration and at an adequate dosage [ 6 , 7 ].
TRD affects approximately one-third of patients with MDD and is associated with functional and physical decline, resulting in diminished health-related quality of life (HRQoL) [ 3 , 8 , 9 ]. Indeed, a considerable proportion of patients living with TRD are reported to be on long-term sick leave or unemployed [ 10 ]. The burden of illness is substantially greater, both to the individual and to society, for patients with TRD than it is for patients with MDD who respond to initial treatment [ 11 ]. Furthermore, the burden of TRD increases with the duration of the disorder, culminating in rates of hospitalisation for general medical and depression-related causes that are double those reported in patients with treatment-responsive MDD [ 12 , 13 ]. Even in the absence of treatment resistance, patients who do not achieve remission experience increased risk of relapse and an increased personal burden arising from residual symptoms [ 14 ]. Moreover, while long-term remission is the primary goal of antidepressant treatment, the probability of achieving remission after experiencing non-response to two adequate trials of medication decreases with each subsequent treatment, and those who require more treatment steps also demonstrate higher rates of relapse during follow-up [ 5 , 14 , 15 ]. Furthermore, residual symptoms in patients who do not achieve complete remission result in an increased risk of relapse, with lower levels of social and psychological functioning, and greater rates of physical morbidity and mortality [ 14 ].
Pharmacological treatment of TRD can employ all approved antidepressant drugs, including selective serotonin- and serotonin and norepinephrine-reuptake inhibitors (SSRIs/SNRIs), tricyclic antidepressants (TCAs), monoamine oxidase inhibitors and other types of antidepressants. Several medications that are not approved for antidepressant monotherapy in MDD and do not have direct antidepressant activity, such as lithium, thyroid hormone and some atypical antipsychotic drugs, may be used to augment antidepressant treatments [ 16 , 17 ]. However, advances in the development of specific treatments for TRD have been slow. Currently, in Europe, the only treatment approved specifically for TRD, as it is defined above, is esketamine, an N -methyl- d -aspartate receptor antagonist, which is administered as a nasal spray in combination with an SSRI/SNRI [ 18 ]. In the US, in addition to esketamine, a combination of olanzapine and fluoxetine hydrochloride (Symbyax ® ) is also approved [ 19 , 20 ].
Advances in the development of novel treatments for TRD have been slow, with current strategies involving the switching, combining and augmenting of medications approved for the treatment of MDD [ 10 ]. Beyond the confines of clinical trials, there is a dearth of evidence assessing the characteristics of, treatment strategies employed for, and outcomes experienced by, patients with TRD in the real world, where populations are more diverse, have more comorbidities and may be less adherent to treatments [ 16 ]. Such real-world data are essential to draw a more realistic picture of the treatment landscape, long-term outcomes and the personal burden of disease for patients with TRD. Similarly, it is important to establish how outcomes experienced by patients with TRD are assessed and monitored in real-life clinical practice, and the timescales over which outcomes are reported, in order to support greater comparability of future studies.
The purpose of this systematic literature review (SLR) was therefore to assess real-world evidence in TRD, in order to understand and summarise available evidence regarding current treatment strategies and outcomes. Specifically, the objectives of this SLR were: (1) to further understand the clinical and patient-reported disease burden for patients with TRD in the real-world, and (2) to explore real-world effectiveness of available treatments and the unmet need for better treatment options for these patients. Given the rapid transformation of real-world treatment settings and practices, and in order to provide data relevant to current real-world practice, this systematic review was restricted to the decade prior to the review date.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Eligibility Criteria, Selection Process and Outcomes
This SLR was conducted according to a pre-specified protocol. Studies were included if they reported outcomes for adult patients with treatment-resistant MDD [with MDD being diagnosed by the Diagnostic and Statistical Manual of Mental Disorders (DSM) Edition 3 or above, or the International Classification of Diseases (ICD) Edition 9 or above], in which treatment resistance was defined as failure to adequately respond to at least two treatments given at an adequate dose during the same major depressive episode (MDE). Any definition of failure or inadequate response to a treatment as provided by the study authors was deemed eligible for inclusion. Eligible studies included those reporting on both pharmacological and non-pharmacological interventions but excluded non-medical interventions such as traditional Chinese medicine and nutritional supplements. While the search strategy also included studies reporting outcomes related to psychiatric emergencies in patients with MDD (MDD-PE), only articles specific to the TRD population are included within this publication.
Data were collected on prevalence of TRD, treatment types and effectiveness, HRQoL and patient characteristics. Studies reporting the following outcomes for the patient population of interest were included: improvement of depression severity as measured by one of the following validated scales: Montgomery–Åsberg Depression Rating Scale (MADRS), Hamilton Depression Rating Scale (HAM-D), Beck Depression Inventory (BDI), Clinical Global Impression–Change (CGI-C), Clinical Global Impression–Severity (CGI-S), 9-question Patient Health Questionnaire (PHQ-9); improvement in HRQoL as measured by any valid instrument; and rates of remission and/or response and/or non-remitters and/or non-responders, according to any of the aforementioned validated scales.
Only real-world non-interventional studies were included. This included cohort studies, cross-sectional studies, case–control studies, chart reviews and registry studies, but excluded case reports or case series, due to their high potential for selection bias. Interventional studies, such as randomised controlled trials, were also excluded. The search included studies published between January 2012 and up to and including May 2022, and conference proceedings published between January 2020 and up to and including June 2022. Full eligibility criteria are reported in Table 1 .
All titles were reviewed by a single senior reviewer (AB or HL) and 10% of each reviewer's excluded decisions were checked by a second reviewer. All articles included after the title review were reviewed by two independent reviewers at title, abstract and full-text stages. For the reviews at abstract and full-text stage, disagreements were resolved by discussion until a consensus was met. If necessary, a third reviewer made the final decision.
Search Strategy
A search was conducted using the electronic databases in MEDLINE (including MEDLINE In-Process, MEDLINE Daily and MEDLINE Epub Ahead of Print), Embase, The Cochrane Libraries [including Cochrane Database of Systematic Reviews (CDSR) and Cochrane Central Register of Controlled Trials (CENTRAL)] and PsycINFO. MEDLINE, MEDLINE In-Process, MEDLINE Epub Ahead of Print and Embase were searched simultaneously via the Ovid SP platform (11/05/2022). The full list of search terms used for the Ovid SP platform is presented in Supplementary Table S1 . CDSR and CENTRAL were searched via The Cochrane Library, via the Wiley Online platform (11/05/2022; Supplementary Table S2). PsycINFO was searched via the American Psychological Association (APA) website (10/05/2022; Supplementary Table S3).
In addition, the bibliographies of all relevant SLRs identified during the literature review were hand-searched for any additional relevant studies. Furthermore, conference proceedings for 2020 to 2022 from the European College of Neuropsychopharmacology Congress, European Congress of Psychiatry, American Psychiatric Association Annual Meeting, American College of Neuropsychopharmacology Annual Meeting and Psych Congress were also searched.
Data Collection Process and Data Items
Data extractions and quality assessments were performed by a single researcher, with a second researcher independently verifying the extracted information. When necessary, a third individual was enlisted to arbitrate the final decision. The quality of all included studies was assessed using the Alberta Heritage Foundation for Medical Research (AHFMR) tool (Supplementary Figure S1 ), which was found to be the tool most suited to the heterogeneous nature of the study designs and outcomes collected.
Data were extracted for predefined outcomes. Extracted study characteristics included: the definition of TRD used, patient inclusion and exclusion criteria, total number of patients included and number of patients of relevance (patients with TRD) included, duration of follow-up and investigational treatment type. For baseline participant characteristics, we recorded: age, sex, education level, marital status, employment status, race or ethnicity, disease severity, disease duration, number and type of previous therapeutic interventions that did not result in adequate response and existing treatment within the current MDE. Treatment outcomes were extracted for: change in depression severity and rates of remission and response over time, as measured by MADRS, HAM-D, BDI, CGI-C, CGI-S or PHQ-9 score, as well as change in HRQoL over time, as measured by any validated instrument.
Included Studies
A total of 8,030 records were identified through database searches, with a further 5,296 identified through supplementary searches. Following title, abstract and full-text review, 22 publications were included in the SLR (Fig. 1 ). The 22 publications reported on 20 unique studies, including 13 prospective cohort studies, 5 retrospective cohort studies, 1 chart review and 1 case–control study.
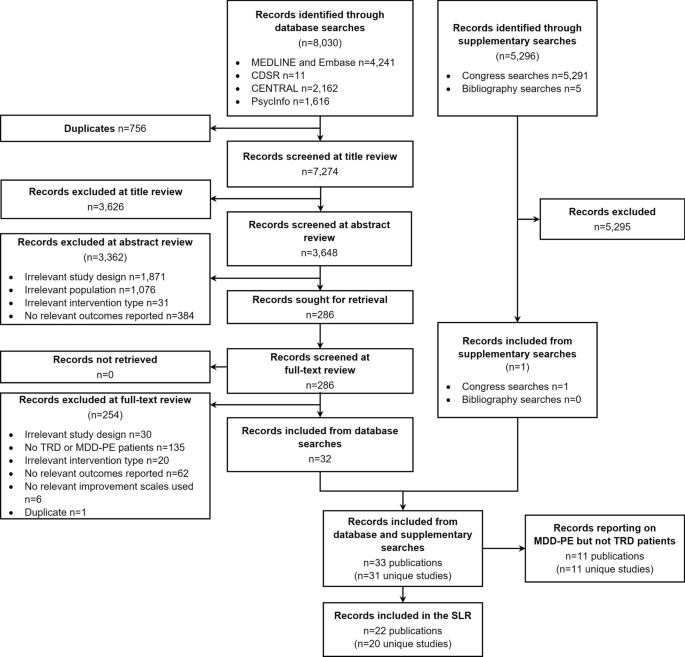
Flowchart of studies included and excluded in the systematic review process. CDSR Cochrane Database of Systematic Reviews, CENTRAL Cochrane Central Register of Controlled Trials, MDD-PE major depressive disorder-psychiatric emergency, TRD treatment resistant depression, SLR systematic literature review
The key characteristics of the included studies reporting on the TRD population are detailed in Table 2 . Non-pharmacological treatments consisted of transcranial magnetic stimulation (TMS; n = 7) [ 21 , 22 , 23 , 24 , 25 , 26 , 27 ], and electroconvulsive therapy (ECT; n = 1) [ 28 ]. Studies of specific pharmacological treatments comprised ketamine and/or esketamine ( n = 3) [ 29 , 30 , 31 ], onabotulinum toxin ( n = 1) [ 32 ], valproate ( n = 1)[ 33 ], pramipexole ( n = 1) [ 34 ] and tranylcypromine and amitriptyline ( n = 1) [ 35 ]. Other studies employed combinations of multiple pharmacological and/or non-pharmacological treatments ( n = 5) [ 16 , 36 , 37 , 38 , 39 , 40 ]. The majority of included studies (13/20) were prospective cohort investigations and reported a wide range of follow-up durations (range: 2 weeks to 9.4 years). The number of patients of relevance included in each study ranged from 14 to 411 (Table 2 ). Just over half of the included studies (12/20) had less than 50 patients with TRD, with only three studies including more than 100 patients.
Of the 19 studies reporting the number of sites, most were undertaken at a single site (14/20), with the remaining five being multicentre studies. The majority of studies were conducted in North America (8/20), Europe (5/20) or Asia (5/20), with only one study reporting data from multiple countries [ 16 ]. The most common countries of study location were Canada (4/20), the United States (4/20), India (3/20) and Italy (3/20).
Definition of TRD
There was considerable variation in the definition of TRD used within the inclusion criteria of studies (Table 2 ). The minimum number of previously failed treatments for classification as treatment-resistant ranged from at least two (as per the study inclusion criteria for this SLR) to at least four. Only some studies (7/20) specified the necessary minimum duration of treatment administration considered to be adequate; this ranged from ‘at least 3 weeks’ to ‘at least 6 to 8 weeks’. Only one study explicitly reported a quantitative value to define inadequate improvement with treatment (≤ 25% improvement on best day Massachusetts General Hospital–Antidepressant Treatment Response Questionnaire [MGH-ATRQ] score) [ 16 ]. Other studies employed different levels of responsiveness, with variations in the language used to define treatment failure, including ‘failure to remit’, ‘insufficient response’ and ‘demonstrated inadequacy’.
Baseline Characteristics
The level of descriptive characteristic data captured at baseline varied between studies (Table 3 ). Included patients with TRD were typically middle-aged (range of mean age: 41.2 to 64.5 years) and overall were approximately balanced for sex (range of percentage female: 27.3 to 66.1%).
Disease severity was reported by 18/20 studies, with the HAM-D scale being used most frequently ( n = 11). Other rating scales used included MADRS ( n = 7), CGI-S ( n = 4), BDI ( n = 3) and the PHQ-9 ( n = 3). All studies that included a measure of disease severity at baseline reported mean scores that could be classified as either moderate, moderate to severe, or severe, according to previously defined thresholds for the HAM-D [ 41 ], MADRS [ 42 ], BDI [ 43 ] and PHQ-9 [ 44 ] scales (Fig. 2 ). Mean disease duration was reported by 9/20 studies, ranging from 1.4 to 16.5 years in the overall cohorts of included studies, with 6/9 of these studies reporting mean durations > 10 years. The duration of the current depressive episode was reported by 6/20 studies, with mean durations ranging from 0.8 to 12.5 years. Three of the included studies reported the mean number of previously failed treatments during the current depressive episode, ranging from 2.9 to 5.9 in the main study cohorts, with one study reporting a mean of 6.4 previously failed treatments in a subset of patients who did not respond to TMS treatment [ 21 ].
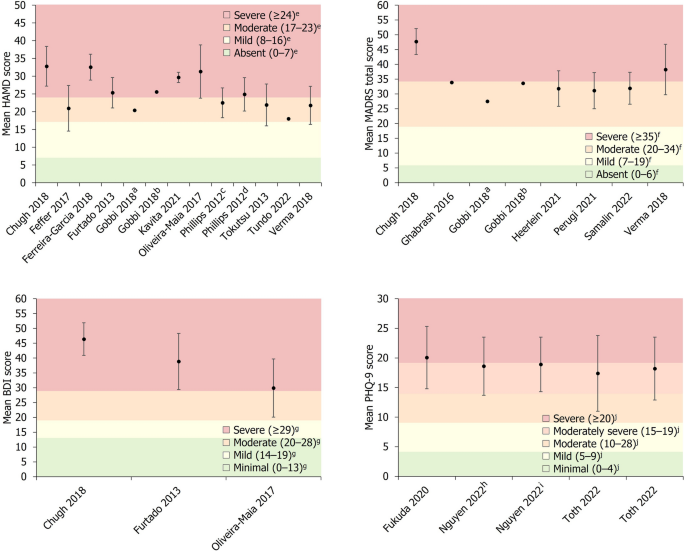
Baseline depression severity scores. Data are presented as mean ± SD (where available). a Group receiving antidepressant treatment. b Group receiving second-generation antipsychotic plus antidepressant treatment. c Patients who subsequently experienced remission. d Patients who did not subsequently achieve remission. e Severity classification for HAM-D score defined by Zimmerman et al. [ 41 ]. f Severity classification for MADRS score defined by Muller et al. [ 42 ]. g Severity classification for BDI score defined by Beck et al. [ 43 ]. h Patients who were THC-positive. I Patients who were THC-negative. j Severity classification for PHQ-9 score defined by Kroenke et al. [ 44 ]. BDI Beck Depression Inventory, HAM-D Hamilton Depression Rating Scale, MADRS Montgomery–Åsberg Depression Rating Scale, PHQ-9 9-question Patient Health Questionnaire, SD standard deviation, THC Tetrahydrocannabinol
Clinical Outcomes
Responsiveness to treatment was assessed most frequently by a version of the HAM-D scale ( n = 10), followed by MADRS ( n = 9), CGI-S ( n = 4), BDI ( n = 3), CGI-C ( n = 2) and PHQ-9 ( n = 2). Studies typically reported follow-up data across a relatively short period, with 11/20 studies featuring a follow-up period of 12 weeks or less and only 5/20 studies reporting follow-up data over a period of 12 months or more. Of studies reporting HAM-D scores, 7/10 studies reported the number of patients experiencing at least one level of response (remission, response, partial response or non-response), 8/10 reported absolute scores and 6/10 reported either an absolute or relative change from baseline. Of studies reporting MADRS scores, 6/9 studies reported the number of patients experiencing at least one level of response, 7/9 reported absolute scores and 4/9 reported either an absolute or relative change from baseline.
For studies reporting absolute or relative changes in a depression severity rating scale, a summary of the mean change in depression severity score from baseline to the final pre-specified timepoint is presented in Table 4 . Studies reporting absolute mean HAM-D (8/20), MADRS (7/20) or BDI (2/20) scores over time are presented alongside previously described thresholds for severity classification and remission in Fig. 3 [ 41 , 42 , 43 , 45 , 46 ].
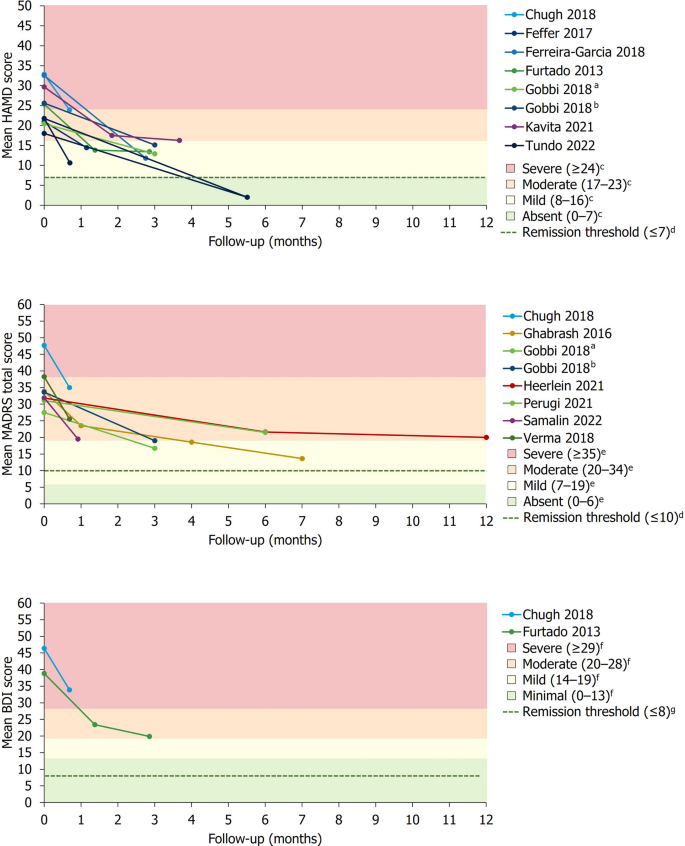
Change in mean absolute depression severity scores over time. a Group receiving antidepressant treatment. b Group receiving second-generation antipsychotic plus antidepressant treatment. c Severity classification for HAM-D score defined by Zimmerman et al. [ 41 ]. d Remission thresholds for HAM-D and MADRS previously defined by Zimmerman et al. [ 45 ]. e Severity classification for MADRS score defined by Muller et al. [ 42 ]. f Severity classification for BDI score defined by Beck et al. [ 43 ]. g Remission threshold for BDI previously defined by Riedel et al. [ 46 ]
In studies reporting the proportion of patients achieving remission and/or response, no single treatment type exhibited a marked pattern of higher rates of treatment responsiveness. However, remission and response rates broadly increased over time.
In the acute setting (≤ 8 weeks), explicitly reported remission rates using HAM-D (2/20) or MADRS (1/20) scores ranged from 0 to 18% in the overall study populations, while rates of response without remission (only reported using HAM-D data) ranged from 0 to 57.1%. Of studies explicitly reporting medium-term remission rates (> 8 weeks to ≤ 6 months) using MADRS (2/20) or HAM-D (1/20) scores, rates of remission and response were generally higher than those reported in the acute setting, ranging from 16.7 to 70.9% in the overall study populations. In these studies, rates of response without remission ranged from 9.8 to 80.6%. Long-term (≥ 12 months) rates of remission and/or response were reported by 2/20 studies using MADRS and 1/20 studies using HAM-D. Long-term remission rates ranged from 19.2 to 54.8% in the overall study populations, while rates of response without remission ranged from 11.6 to 15.9% (only reported using MADRS data).
Health-Related Quality of Life Outcomes
Two studies, Heerlein et al. and Perugi et al., reported HRQoL data, the latter reporting on an Italian subset of patients in the study of the former [ 16 , 38 , 40 ]. These studies assessed HRQoL at baseline and after 6 months using the European Quality of Life Group, 5-Dimension 5-Level Scale (EQ-5D-5L), whereby an index score of 1 represents perfect health, 0 represents a health state equivalent to death and < 0 represents a state worse than death [ 38 ]. Heerlein et al . reported a mean baseline EQ-5D-5L index of 0.41 in 397 patients [ 38 ]. In a separate publication reporting on the same study, after 6 months of receiving various treatments, the EQ-5D-5L score had increased by 0.11 in patients who did not respond to treatment, by 0.26 in those who experienced response without remission and by 0.34 in those who experienced remission [ 16 ]. After 12 months, the improvements from baseline were 0.11, 0.31 and 0.35, respectively [ 16 ]. Perugi et al . similarly reported a mean baseline EQ-5D-5L index of 0.4 in 121 patients [ 40 ]. After 6-months of receiving various treatments, the EQ-5D-5L index improved to 0.6 ( n = 85) in patients remaining in the study, but was lower in those who did not respond to treatment (0.2; n = 61) versus those who responded (0.7; n = 8) or reached remission (0.9; n = 16).
Heerlein et al. also reported the impact of TRD on functioning [Sheehan Disability Scale (SDS)] and work productivity (WPAI). At baseline, according to the SDS, 61.6% of these patients experienced marked or extreme work impairment (mean SDS total score: 22.4), with WPAI scores revealing overall mean impairment of work and activity to be 60.5% and 73.3%, respectively. After 6 and 12 months of treatment, mean change from baseline in total SDS score was − 2.67 and − 2.91, respectively, in those who did not respond to treatment, − 7.58 and − 7.00 in those with response without remission and − 12.53 and − 14.44 in those who experienced remission. Change in WPAI scores were not reported.
Quality Assessment of Included Studies
The quality of the included studies, as indicated by the AHFMR quality assessment checklist, was moderate to good (Supplementary Fig. 1). Of the included studies, the description of subjects and settings was generally appropriate, with just two studies providing only a partially adequate description. While only three studies included an appropriate sample size for the study design and target population, all studies provided an adequate description of the statistical analysis methods employed. There was a consistent lack of adjustment for confounding, which was either not done or not reported in almost all (19/20) studies.
This systematic review has identified 20 real-world studies, comprising a variety of pharmacological and non-pharmacological treatments, reporting baseline characteristics and clinical outcomes in patients with TRD. There was substantial heterogeneity in the definition of TRD and the means of assessment, and manner of reporting, on the burden of illness and treatment outcomes, preventing the quantitative synthesis of results. Nevertheless, patients with TRD consistently presented with moderate to severe depression, long durations of illness and poor HRQoL. Only two studies assessed the latter, suggesting that greater emphasis is placed on clinical outcomes than patient-centred outcomes in real-world studies. Treatment outcomes varied greatly. While many patients typically experienced a level of response by the end of the included studies’ follow-up period, rates of remission were generally low. Studies predominantly involved relatively small sample sizes, followed-up over relatively short durations, highlighting the need for larger-scale, longer-term studies.
In their criteria for TRD, studies did not consistently define what constitutes response failure, nor adequate improvement, with the latter ranging from remission to response. Despite the definition of TRD being centred around the number of prior treatment failures and the well-established negative relationship between the number of prior treatment failures and the probability of relapse from acute response over time, very few studies reported the absolute number of prior treatments received [ 5 ]. The tools used to assess disease severity were varied, with most studies only reporting outcomes using a single tool. As the clinical tools developed to assess TRD focus on different elements of the disease and use a range of assessment methods, a more complete picture of the disease and its burden could be developed by consistently using multiple tools within individual studies. Similarly, the sample size and follow-up duration of the included studies was wide ranging, but studies typically featured relatively small sample sizes monitored over a period of several weeks to a few months, potentially reducing the robustness of the findings. Of the included studies, the most frequently utilised treatment was TMS, while several studies also reported on patients receiving multiple treatments, often comprising two or more different treatment types. Collectively, the heterogeneity of the included studies suggests a lack of clinical consensus and standardisation in the severity classification and monitoring of TRD in real-world practice [ 11 ].
Applying previously defined cut-offs, the severity of depression at baseline, which has been identified as the most important prognostic factor for TRD [ 47 ], ranged from moderate to severe. Disease duration at baseline was typically greater than 10 years, with the current MDE frequently spanning several years. These findings are consistent with an earlier review of the burden of TRD, which reported that, on average, patients with TRD had MDD durations of 4.4 years and had completed 4.7 unsuccessful drug treatments [ 11 ]. Taken together, the common concurrence of MDD that spans many years, with severe and prolonged MDEs, is indicative of the substantial and often unremitting burden imposed by TRD, which exceeds that of MDD alone [ 3 ].
Definitions of remission and response varied between studies. In those studies reporting rates of responsiveness to treatment in the acute setting, remission rates were generally low. Given the established propensity among patients with TRD for severe and long-lasting MDD and MDEs, it follows that longer-term interventions and follow-up periods are likely to be required for many patients to achieve remission. Indeed, studies of medium- and long-term follow-up durations typically reported higher rates of treatment responsiveness than those of acute interventions, but were nevertheless highly variable, with many patients not reaching remission after 12 months of treatment. Heerlein et al . reported that, after 6 months of initiating a new antidepressant treatment, only 16.7% of patients with TRD achieved remission, rising only to 19.2% at 12 months [ 16 ]. Similarly, Perugi et al., reporting on a subset of the previous study, demonstrated that, after 6 months of receiving various treatments, only 18% of patients had experienced remission, rising only marginally to 22.7% at 12 months [ 40 ]. This is in agreement with an earlier review of studies of patients with TRD, which captured data from nearly 60,000 patients and reported wide-ranging rates of remission and response, averaging 20% and 36%, respectively [ 11 ]. Indicative of the severe burden and unmet need for effective treatments experienced by patients with TRD, the aforementioned study also reported a 17% prevalence of prior suicide attempts in this population. Collectively, these studies demonstrate that existing treatment strategies are often insufficiently effective to enable patients with TRD to experience remission, particularly over short time durations of treatment.
Health-related quality of life was rarely assessed within the included studies, suggesting that, in real-world practice, greater emphasis is still placed on clinical outcomes than patient-centred outcomes. However, it should also be considered that there are few disease-specific tools for the assessment of HRQoL in patients with MDD, which may contribute to the infrequency of HRQoL evaluation. Nevertheless, it has been previously demonstrated that, when compared with MDD patients without treatment resistance, patients with TRD experience significantly lower HRQoL and a greater impairment of work activity and productivity [ 3 ]. Both studies that reported HRQoL data demonstrated low HRQoL scores in the TRD population [ 16 , 38 , 40 ]. In these studies, patients with TRD exhibited mean baseline EQ-5D-5L index scores (0.4 and 0.41) that were substantially lower than the general adult population, which typically ranges between ~ 0.70 and 0.95 globally [ 38 , 40 , 48 ]. Patients with TRD were also likely to be experiencing significant work and activity impairment, culminating in high levels of absenteeism and presenteeism [ 10 ]. In these two studies, patients who did not achieve remission during the study period experienced further declines or minimal improvement in HRQoL. Importantly, although remission rates were low in these studies, those patients with TRD who did experience remission also experienced substantial increases in HRQoL, emphasising the merit of remission as the primary treatment goal [ 16 , 40 ]. Future studies would benefit from increased assessment and continued monitoring of HRQoL to better capture the personal burden of TRD and to assess the efficacy of treatment options on functioning and productivity. This, alongside consistent use of a range of clinical outcome assessment tools, could build a more comprehensive picture of the TRD population and specific unmet needs that patients experience in their day-to-day lives.
This SLR was conducted in accordance with best practice guidelines, such as the use of two independent systematic reviewers to review abstracts and full-text articles against the inclusion and eligibility criteria [ 49 ]. Nevertheless, conclusions drawn from this systematic review are naturally limited by the information available in, and the methodological quality of, the included published literature. While the present study’s evaluation of real-world evidence enables greater confidence in the relevance and applicability of findings to the patient population, the lack of standardisation prevented quantitative synthesis of the findings. Owing to the inclusion of only real-world evidence studies, and the heterogeneity of the interventions and study designs employed, safety outcomes were not captured. This decision was made since, outside randomised controlled trials, safety outcomes are inconsistently monitored and reported, thus limiting comparability. Nevertheless, it must be considered that treatment side effects are likely to influence subsequent treatment decisions, clinical outcomes and patient-centred outcomes, such as HRQoL and functioning. As such, it may be of value to investigate real-world safety patterns of therapies used for TRD in future studies.
This review demonstrates that there is a paucity of studies investigating real-world treatment of patients with TRD. Those that do are heterogeneous in their definition, assessment and monitoring of TRD and feature a wide range of treatment types and durations. The lack of evidence, together with the heterogeneity of studies that are available, make drawing specific conclusions about this patient population in the real world challenging. However, more broadly, studies found in this review show that patients with TRD had typically experienced long-lasting MDD, with moderate or severe MDEs spanning multiple years. Rates of response to treatment varied greatly between studies, but remission rates were typically low. Few studies had investigated long-term treatment outcomes in this patient population, for whom response to treatment is notoriously elusive. Longer durations of study intervention and follow-up were associated with marginally greater gains in favourable treatment outcomes, but remission rates typically remained low even after a year of treatment. Health-related quality of life was seldom measured, suggesting that greater emphasis is typically placed on the reporting of clinical outcomes over patient outcomes. When it was measured, HRQoL was reported to be particularly low in patients with TRD. Thus, while there is a lack of clinical consensus on the definition of TRD, the condition doubtless carries a high burden of illness and there exists an unmet need for more effective treatment options. Furthermore, future real-world studies would benefit from the application of standardised modalities of assessment, monitoring and reporting of treatment effectiveness, including greater consideration of HRQoL outcomes, to better understand the burden on patients affected by this condition.
Data Availability
Data available from the corresponding author upon reasonable request.
Salahudeen MS, Wright CM, Peterson GM. Esketamine: new hope for the treatment of treatment-resistant depression? A narrative review. Ther Adv Drug Saf. 2020;11:2042098620937899.
Article PubMed PubMed Central Google Scholar
Fava M, Kendler KS. Major Depressive Disorder. Neuron. 2000;28(2):335–41.
Article CAS PubMed Google Scholar
Jaffe DH, Rive B, Denee TR. The humanistic and economic burden of treatment-resistant depression in Europe: a cross-sectional study. BMC Psychiatry. 2019;19(1):247.
National Institute for Health and Care Excellence. Depression in adults: treatment and management (NG222) 2022. https://www.nice.org.uk/guidance/ng222/resources/depression-in-adults-treatment-and-management-pdf-66143832307909 . Accessed 7 Dec 2022.
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–17.
Article PubMed Google Scholar
Souery D, Amsterdam J, de Montigny C, Lecrubier Y, Montgomery S, Lipp O, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. 1999;9(1–2):83–91.
European Medicines Agency. Guideline on clinical investigation of medicinal products in the treatment of depression. EMA/CHMP/185423/2010 Rev 2 2013. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-depression_en.pdf . Accessed 18 Aug 2022.
Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–88.
Thase ME. Treatment-resistant depression: prevalence, risk factors, and treatment strategies. J Clin Psychiatry. 2011;72(5): e18.
Heerlein K, Young AH, Otte C, Frodl T, Degraeve G, Hagedoorn W, Oliveira-Maia AJ, Perez Sola V, Rathod S, Rosso G. Real-world evidence from a European cohort study of patients with treatment resistant depression: Baseline patient characteristics. J Affect Disord. 2021;283:115–22.
Mrazek DA, Hornberger JC, Altar CA, Degtiar I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatr Serv (Washington, DC). 2014;65(8):977–87.
Article Google Scholar
Halaris A, Sohl E, Whitham EA. Treatment-resistant depression revisited: a glimmer of hope. J Pers Med. 2021;11(2).
Crown WH, Finkelstein S, Berndt ER, Ling D, Poret AW, Rush AJ, et al. The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry. 2002;63(11):963–71.
Mendlewicz J. Towards achieving remission in the treatment of depression. Dialogues Clin Neurosci. 2008;10(4):371–5.
Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush JA. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Services. 2009;60(11):1439–45.
Heerlein K, Perugi G, Otte C, Frodl T, Degraeve G, Hagedoorn W, et al. Real-world evidence from a European cohort study of patients with treatment resistant depression: treatment patterns and clinical outcomes. J Affect Disord. 2021;290:334–44.
Ionescu DF, Rosenbaum JF, Alpert JE. Pharmacological approaches to the challenge of treatment-resistant depression. Dialogues Clin Neurosci. 2015;17(2):111–26.
European Medicines Agency. Esketamine Nasal Spray Summary of Product Characteristics 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/spravato . Accessed 18 Aug 2022.
Food and Drug Administration. Spravato Prescribing Information 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211243lbl.pdf . Accessed 18 Aug 2022.
Food and Drug Administration. Symbyax Prescribing Information 2009. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021520s022lbl.pdf . Accessed 7 Dec 2022.
Furtado CP, Hoy KE, Maller JJ, Savage G, Daskalakis ZJ, Fitzgerald PB. An investigation of medial temporal lobe changes and cognition following antidepressant response: a prospective rTMS study. Brain Stimul. 2013;6(3):346–54.
Feffer K, Lapidus KAB, Braw Y, Bloch Y, Kron S, Netzer R, et al. Factors associated with response after deep transcranial magnetic stimulation in a real-world clinical setting: results from the first 40 cases of treatment-resistant depression. Eur Psychiatry. 2017;44:61–7.
Oliveira-Maia AJ, Press D, Pascual-Leone A. Modulation of motor cortex excitability predicts antidepressant response to prefrontal cortex repetitive transcranial magnetic stimulation. Brain Stimul. 2017;10(4):787–94.
Feffer K, Fettes P, Giacobbe P, Daskalakis ZJ, Blumberger DM, Downar J. 1 Hz rTMS of the right orbitofrontal cortex for major depression: safety, tolerability and clinical outcomes. Eur Neuropsychopharmacol. 2018;28(1):109–17.
Verma R, Kumar N, Kumar S. Effectiveness of adjunctive repetitive transcranial magnetic stimulation in management of treatment-resistant depression: a retrospective analysis. Indian J Psychiatry. 2018;60(3):329–33.
Fukuda AM, Hindley LE, Kang JWD, Tirrell E, Tyrka AR, Ayala A, et al. Peripheral vascular endothelial growth factor changes after transcranial magnetic stimulation in treatment-resistant depression. NeuroReport. 2020;31(16):1121–7.
Article CAS PubMed PubMed Central Google Scholar
Toth C, King Johnson ML, Heinzerling A, Trapp N. Response to TMS treatment for depression associated with higher levels of psychological well-being. J Psychiatr Res. 2022;150:142–6.
Tokutsu Y, Umene-Nakano W, Shinkai T, Yoshimura R, Okamoto T, Katsuki A, et al. Follow-up study on electroconvulsive therapy in treatment-resistant depressed patients after remission: a chart review. Clin Psychopharmacol Neurosci. 2013;11(1):34–8.
Slupski J, Cubala WJ, Gorska N, Slupska A, Galuszko-Wegielnik M. Copper concentrations in ketamine therapy for treatment-resistant depression. Brain Sci. 2020;10(12):1–9.
Nguyen J, Boerth J, Lacro J. Influence of cannabis on effectiveness of esketamine and ketamine for treatment resistant depression. In: College of Psychiatric and Neurologic Pharmacists (CPNP) Annual Meeting. 2022.
Samalin L, Rotharmel M, Mekaoui L, Gaudre-Wattinne E, Codet MA, Bouju S, et al. Esketamine nasal spray in patients with treatment-resistant depression: the real-world experience in the French cohort early-access programme. Int J Psychiatry Clin Pract. 2022;26(4):352–62.
Chugh S, Chhabria A, Jung S, Kruger THC, Wollmer MA. Botulinum toxin as a treatment for depression in a real-world setting. J Psychiatr Pract. 2018;24(1):15–20.
Ghabrash MF, Comai S, Tabaka J, Saint-Laurent M, Booij L, Gobbi G. Valproate augmentation in a subgroup of patients with treatment-resistant unipolar depression. World J Biol Psychiatry. 2016;17(2):165–70.
Tundo A, Betro S, Iommi M, de Filippis R. Efficacy and safety of 24-week pramipexole augmentation in patients with treatment resistant depression. A retrospective cohort study. Prog Neuropsychopharmacol Biol Psychiatry. 2022;112:110425.
Ferreira-Garcia R, Da Rocha Freire RC, Appolinario JC, Levitan MN, Halkjaer-Lassen RD, Bueno JR, et al. Tranylcypromine plus amitriptyline for electroconvulsive therapy-resistant depression. J Clin Psychopharmacol. 2018;38(5):502–4.
Phillips JL, Batten LA, Aldosary F, Tremblay P, Blier P. Brain-volume increase with sustained remission in patients with treatment-resistant unipolar depression. J Clin Psychopharmacol. 2012;73(5):625–31.
Google Scholar
Gobbi G, Ghabrash MF, Nunez N, Tabaka J, Di Sante J, Saint-Laurent M, et al. Antidepressant combination versus antidepressants plus second-generation antipsychotic augmentation in treatment-resistant unipolar depression. Int Clin Psychopharmacol. 2018;33(1):34–43.
Heerlein K, Young AH, Otte C, Frodl T, Degraeve G, Hagedoorn W, et al. Real-world evidence from a European cohort study of patients with treatment resistant depression: baseline patient characteristics. J Affect Disord. 2021;283:115–22.
Kavita, Sahu MK, Dhone PG, Tiwari RK, Hishikar R. A prospective observational study on the effects of antidepressant treatment on hypothalamic-pituitary-adrenal axis regulation in treatment-resistant depression. J Clin Diagn Res. 2021;15(4):FC4–7.
Perugi G, Calo P, De Filippis S, Rosso G, Vita A, Adami M, et al. Clinical features and outcomes of 124 Italian patients with treatment resistant depression: a real-world, prospective study. Front Psychiatry. 2021;12: 769693.
Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton Depression Rating Scale. J Affect Disord. 2013;150(2):384–8.
Müller MJ, Szegedi A, Wetzel H, Benkert O. Moderate and severe depression. Gradations for the Montgomery-Asberg Depression Rating Scale. J Affect Disord. 2000;60(2):137–40.
Beck AT, Steer RA, Brown GK. BDI-II, Beck Depression Inventory: manual: Psychological Corp; 1996.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
Zimmerman M, Posternak MA, Chelminski I. Derivation of a definition of remission on the Montgomery-Asberg Depression Rating Scale corresponding to the definition of remission on the Hamilton Rating Scale for depression. J Psychiatr Res. 2004;38(6):577–82.
Riedel M, Möller HJ, Obermeier M, Schennach-Wolff R, Bauer M, Adli M, et al. Response and remission criteria in major depression–a validation of current practice. J Psychiatr Res. 2010;44(15):1063–8.
Lundberg J, Cars T, Lööv S-Å, Söderling J, Sundström J, Tiihonen J, et al. Association of treatment-resistant depression with patient outcomes and health care resource utilization in a population-wide study. JAMA Psychiat. 2022;80(2):167–75.
Janssen B, Szende A. Population norms for the EQ-5D. In: Szende A, Janssen B, Cabases J, editors. Self-reported population health: an international perspective based on EQ-5D. Dordrecht: Springer; 2014. p. 19–30.
Chapter Google Scholar
Lefebvre C GJ, Briscoe S, Featherstone R, Littlewood A, Marshall C, Metzendorf M-I, Noel-Storr A, Paynter R, Rader T, Thomas J, Wieland LS. Chapter 4: Searching for and selecting studies. 2022. In: Cochrane handbook for systematic reviews of interventions [Internet]. Cochrane. Available from: www.training.cochrane.org/handbook . Accessed 2 June 2023.
Download references
Medical Writing/Editorial Assistance
The authors also acknowledge Andrew Wilhelmsen, PhD, and Jessica Patel, PhD, from Costello Medical, UK, for medical writing and editorial assistance based on the authors’ input and direction. Third-party writing assistance was funded by Janssen EMEA.
This study was sponsored Janssen EMEA. Support for third-party writing assistance for this article, provided by Andrew Wilhelmsen, PhD, and Jessica Patel, PhD, from Costello Medical, UK, was funded Janssen EMEA in accordance with Good Publication Practice (GPP3) guidelines ( http://www.ismpp.org/gpp3 ). Rapid Service and Open Access Fees were also funded by Janssen EMEA.
Author information
Authors and affiliations.
Champalimaud Research and Clinical Centre, Champalimaud Foundation, Lisbon, Portugal
Albino J. Oliveira-Maia
Faculdade de Ciências Médicas, NOVA Medical School, NMS, FCM, Universidade NOVA de Lisboa, Lisbon, Portugal
Costello Medical, Cambridge, UK
Ania Bobrowska
Centre Hospitalier Spécialisé Notre-Dame des Anges, Liège, Belgium
Eric Constant
Université Catholique de Louvain, Brussels, Université de Liège, Liège, Belgium
Janssen EMEA, High Wycombe, UK
Tetsuro Ito
Janssen Pharmaceutica NV, Beerse, Belgium
Yerkebulan Kambarov
Costello Medical, London, UK
Hannah Luedke
Janssen EMEA, Dublin, Ireland
Siobhán Mulhern-Haughey
Janssen EMEA, Neuss, Germany
Christian von Holt
You can also search for this author in PubMed Google Scholar
Contributions
Substantial contributions to study conception and design: Albino J. Oliveira-Maia, Ania Bobrowska, Eric Constant, Tetsuro Ito, Yerkebulan Kambarov, Hannah Luedke, Siobhán Mulhern-Haughey, Christian von Holt; literature review and data extraction: Ania Bobrowska, Hannah Luedke; substantial contributions to analysis and interpretation of the data: Albino J. Oliveira-Maia, Ania Bobrowska, Eric Constant, Tetsuro Ito, Yerkebulan Kambarov, Hannah Luedke, Siobhán Mulhern-Haughey, Christian von Holt; drafting the article or revising it critically for important intellectual content: Albino J Oliveira-Maia, Ania Bobrowska, Eric Constant, Tetsuro Ito, Yerkebulan Kambarov, Hannah Luedke, Siobhán Mulhern-Haughey, Christian von Holt; final approval of the version of the article to be published: Albino J Oliveira-Maia, Ania Bobrowska, Eric Constant, Tetsuro Ito, Yerkebulan Kambarov, Hannah Luedke, Siobhán Mulhern-Haughey, Christian von Holt.
Corresponding author
Correspondence to Yerkebulan Kambarov .
Ethics declarations
Conflicts of interest.
Albino J. Oliveira-Maia: Received grants from Schuhfried GmBH, Janssen and Compass Pathways, Ltd; received payment, honoraria, travel support or advisory board fees from MSD, Janssen, Angelini, Neurolite AG, and the European Monitoring Centre for Drugs and Drug Addiction; investigator-driven research funded by Fundação para Ciência e Tecnologia (PTDC/SAU-NUT/3507/2021; PTDC/MED-NEU/1552/2021; PTDC/MED-NEU/31331/2017), Fundação para Ciência e Tecnologia and FEDER (PTDC/MED-NEU/30845/2017_LISBOA-01–0145-FEDER-030845; FCT-PTDC/MEC-PSQ/30302/2017-IC&DTLISBOA-01-0145-FEDER), the European Commission Horizon 2020 program (H2020-SC1-2017-CNECT-2-777167-ΒΟUNCE; H2020-SC1-DTH-2019-875358-FAITH), the European Research Council (grant ERC-2020-STG-Grant agreement 950357) and the European Joint Programme in Rare Diseases (Joint Translational Call 2019) through Fundação para Ciência e Tecnologia (EJPRD/0001/2020); Vice-President of the Portuguese Society for Psychiatry and Mental Health; Head of the Psychiatry Working Group for the National Board of Medical Examination (GPNA) at the Portuguese Medical Association and Portuguese Ministry of Health. Eric Constant: Received honoraria, advisory board fees and travel support from AstraZeneca, BMS, Lilly, Wyeth, Servier, Janssen Cilag, Pfizer, Lundbeck, Viatris and Teva. Tetsuro Ito, Yerkebulan Kambarov, Siobhán Mulhern-Haughey, Christian von Holt: Employees of Janssen; hold Johnson & Johnson company stocks/stock options. Ania Bobrowska, Hannah Luedke: Employees of Costello Medical, UK.
Ethical Approval
Supplementary information.
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 134 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
Oliveira-Maia, A.J., Bobrowska, A., Constant, E. et al. Treatment-Resistant Depression in Real-World Clinical Practice: A Systematic Literature Review of Data from 2012 to 2022. Adv Ther 41 , 34–64 (2024). https://doi.org/10.1007/s12325-023-02700-0
Download citation
Received : 25 August 2023
Accepted : 28 September 2023
Published : 26 October 2023
Issue Date : January 2024
DOI : https://doi.org/10.1007/s12325-023-02700-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Major depressive disorder
- Real-world evidence
- Systematic literature review
- Treatment-resistant depression
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article-Invited
- Open access
- Published: 03 April 2019
Prognosis and improved outcomes in major depression: a review
- Christoph Kraus ORCID: orcid.org/0000-0002-7144-2282 1 , 2 ,
- Bashkim Kadriu ORCID: orcid.org/0000-0002-3809-9451 2 ,
- Rupert Lanzenberger ORCID: orcid.org/0000-0003-4641-9539 1 ,
- Carlos A. Zarate Jr. 2 &
- Siegfried Kasper ORCID: orcid.org/0000-0001-8278-191X 1
Translational Psychiatry volume 9 , Article number: 127 ( 2019 ) Cite this article
240 Citations
26 Altmetric
Metrics details
- Human behaviour
- Predictive markers
- Scientific community
Treatment outcomes for major depressive disorder (MDD) need to be improved. Presently, no clinically relevant tools have been established for stratifying subgroups or predicting outcomes. This literature review sought to investigate factors closely linked to outcome and summarize existing and novel strategies for improvement. The results show that early recognition and treatment are crucial, as duration of untreated depression correlates with worse outcomes. Early improvement is associated with response and remission, while comorbidities prolong course of illness. Potential biomarkers have been explored, including hippocampal volumes, neuronal activity of the anterior cingulate cortex, and levels of brain-derived neurotrophic factor (BDNF) and central and peripheral inflammatory markers (e.g., translocator protein (TSPO), interleukin-6 (IL-6), C-reactive protein (CRP), tumor necrosis factor alpha (TNFα)). However, their integration into routine clinical care has not yet been fully elucidated, and more research is needed in this regard. Genetic findings suggest that testing for CYP450 isoenzyme activity may improve treatment outcomes. Strategies such as managing risk factors, improving clinical trial methodology, and designing structured step-by-step treatments are also beneficial. Finally, drawing on existing guidelines, we outline a sequential treatment optimization paradigm for selecting first-, second-, and third-line treatments for acute and chronically ill patients. Well-established treatments such as electroconvulsive therapy (ECT) are clinically relevant for treatment-resistant populations, and novel transcranial stimulation methods such as theta-burst stimulation (TBS) and magnetic seizure therapy (MST) have shown promising results. Novel rapid-acting antidepressants, such as ketamine, may also constitute a paradigm shift in treatment optimization for MDD.
Similar content being viewed by others

Psilocybin microdosers demonstrate greater observed improvements in mood and mental health at one month relative to non-microdosing controls

The serotonin theory of depression: a systematic umbrella review of the evidence

Major depressive disorder: hypothesis, mechanism, prevention and treatment
Depression: a major and relentless burden.
Major depressive disorder (MDD) is the most common psychiatric disease and a worldwide leading cause of years lived with disability 1 , 2 . In addition, the bulk of suicides are linked to a diagnosis of MDD. Despite the high prevalence rate of MDD and ongoing efforts to increase knowledge and skills for healthcare providers, the illness remains both underdiagnosed and undertreated 3 . Many novel strategies with potentially broad impact are not yet ready for ‘prime time’, as they are either in early experimental stages or undergoing regulatory processes for approval. This review sought to: (1) provide a synopsis of key factors associated with outcomes in MDD, and (2) synthesize the existing literature on novel treatment strategies for depression. A literature search was conducted using the search terms ‘depression’, ‘antidepressant’, ‘outcome’, ‘predictor’, ‘(bio)marker’, ‘treatment-resistant depression (TRD)’, and ‘chronic depression’ in addition to combinations of these terms. The search was conducted in PubMed, Scopus, and Google Scholar with no restrictions on time period and concluded in October 2018. Notably, we defined ‘outcomes’ loosely, as either disease course (i.e., treatment resistance, chronic depression) or response/remission to treatment.
Prognostic variables for treatment outcomes in MDD
Clinical variables.
Clear evidence of an inverse relationship between duration of episode and treatment outcome (either response or remission) underscores the importance of early intervention in MDD 4 (Table 1 ). In particular, replicable prospective and retrospective studies indicate that shorter duration of untreated disease—both in terms of first and recurrent episodes—is a prognostic factor indicating better treatment response and better long-term outcomes 5 , 6 , 7 , 8 , 9 , 10 , although not all studies have found such an association 11 . Another important clinical variable is time to antidepressant response. For instance, one meta-analysis found that early improvement was positively linked to antidepressant treatment outcome in 15 of 16 studies 9 . Early response to antidepressant treatment appears to occur independently of treatment modality 12 , 13 or outcome parameters 14 , 15 . Another study found that early improvement in work productivity was a significant positive predictor of higher remission rates after three and seven months of treatment 16 . Similarly, imaging studies found that early response to treatment correlated with default mode network deactivation in the posterior cingulate 17 , as well as thickening of gray matter in the anterior cingulate cortex (ACC) 18 . Interestingly, two recent meta-analyses found that initial improvement was linked to response and outcome but failed to be associated with treatment resistance 19 , 20 . This suggests that TRD—defined loosely here as non-response to at least two adequate antidepressant trials—and chronic depression (roughly defined here as non-response to any treatment) may have similar response slopes in the earliest treatment stages.
In addition, lower baseline function and quality of life—including longer duration of the current index episode—have been associated with lower remission rates to various types of antidepressant treatments 21 , 22 . This is in line with results from a previous study that found that baseline function predicted antidepressant response in TRD patients 23 . Worse outcomes in more severely ill patients at baseline were also reported in elderly patients treated in primary-care settings 24 . In contrast, several controlled clinical studies found that elevated baseline severity correlated with improved response and remission rates 25 . Two naturalistic studies with broad inclusion criteria similarly found that remission correlated with higher baseline scores 4 , 26 . However, this discrepancy might be explained by variations in outcome according to parameter. It was noted earlier that studies that defined remission as percent change of baseline values might be biased in favor of higher baseline scores, while absolute endpoints (e.g., remission defined below a cutoff score) favor less sick patients 4 .
Psychosocial variables
The influence of sociodemographic factors such as age, age of onset, gender, and number of previous episodes on treatment outcome has been investigated with mixed results 4 , 27 , 28 . One study found that females had higher remission rates 21 , but this was not confirmed by another prospective study 27 . Others have found that stress related to high occupational levels might impair outcomes 29 . The European “Group for the Study of Resistant Depression” (GSRD) multi-site study found that age at first treatment (i.e., early-onset and early treatment), age, timespan between first and last episode (i.e., duration of illness), suicidality, and education level were all important variables for outcome 30 . Notably, authors of long-lasting longitudinal studies have suggested that recall bias may influence the age of onset variable 31 , 32 ; given the cognitive deficits associated with acute episodes of MDD, retrospective studies must hence address the factor of memory bias in data collection.
Environmental stress and stressful life events (SLEs)
High stress levels significantly influence outcomes in MDD patients who are prone to vulnerable states, such as those with high levels of neuroticism 33 , 34 . A meta-analysis found that history of childhood maltreatment was associated with elevated risk of developing recurrent and persistent depressive episodes, as well as with lack of response or remission during treatment 35 . Another meta-analysis confirmed the detrimental impact of childhood maltreatment (emotional physical or sexual maltreatment or neglect) as a predisposing risk factor for severe, early-onset, and treatment-resistant depression 36 , 37 . Studies also found gender-specific effects; in particular, at lower stress levels females were at higher risk of MDD than males 34 . Moreover, twin studies have suggested a differential reactivity of gender in response to type of SLE 38 . For instance, a treatment study using escitalopram and nortriptyline investigated the association between number of SLEs (e.g., job loss, psychological trauma, loss of a loved one) and antidepressant treatment. Subjects with more SLEs exhibited greater cognitive symptoms at baseline but not significantly more mood or neurovegetative symptoms. These patients also had greater cognitive symptom reduction in response to escitalopram but not nortriptyline 39 . This suggests that SLEs may have a cognitive domain-specific impact in MDD, but more data are needed to elucidate this issue.
Psychiatric and physical comorbidities
Psychiatric comorbidity has been shown to influence outcome in both treated and untreated patients 40 , 41 . Studies have found that elevated baseline anxiety symptoms or comorbid anxiety disorder are associated with worse antidepressant response to first-line selective serotonin reuptake inhibitors (SSRIs) or second-line treatment strategies 42 , 43 . Worse outcomes have also been reported for MDD patients with comorbid drug or alcohol use disorders, post-traumatic stress disorder (PTSD), and “double depression” (depression and dysthymia) 26 , 41 . Data from the Sequential Treatment Alternatives to Relieve Depression (STAR*D) study, which included patients who were seeking medical care in routine medical or psychiatric outpatient treatment, indicate that roughly one-third (34.8%) of all MDD patients are free of any comorbidity; the most frequent comorbid Axis-I disorders are social phobia (31.3%), generalized anxiety disorder (23.6%), PTSD (20.6%), and obsessive-compulsive disorder (14.3%) 21 . A large recent study found that clinically diagnosed personality disorder was associated with negative outcomes (with regard to remission and persistent depressive symptoms) six months after diagnosis in MDD subjects enrolled in primary care 44 . Moreover, meta-analytic studies indicate that comorbid personality disorder increases the likelihood of poorer outcomes 45 , 46 ; it should be noted, though, that negative studies have also been reported 40 .
MDD and several physical diseases—including cardiovascular disease and diabetes—appear to have bidirectional effects on disease trajectory 47 , 48 , yet pathophysiologic links are most likely complex and have to be elucidated. In addition, depression appears to be linked to hormonal diseases, including hypothyroidism 49 . A number of physical disabilities and medical comorbidities have been shown to significantly impact outcome measures in MDD 50 , particularly in elderly subjects 51 . This connection appears to be relevant at any stage of the disease, as number of physical comorbidities did not separate TRD from non-TRD patients 52 . Links between MDD and pain have also been noted; subjects with elevated levels of baseline pain due to chronic conditions had longer depressive episodes, delayed remission 53 and, most importantly, elevated suicide risk 54 , 55 . Interestingly, a prospective, 12-month study of older patients found that elderly patients with atrial fibrillation exhibited better remission rates 56 . Patients with chronic pulmonary diseases had worse outcomes in uncontrolled treatment settings than those without these diseases. This difference was absent in the intervention group, in which depression care managers helped physicians with guideline-concordant recommendations and helped patients adhere to treatment 56 . Further longitudinal studies on shared pathophysiology with physical diseases are needed to confirm such associations.
Neuroimaging markers of treatment outcomes
Structural markers of antidepressant treatment outcomes suggest that hippocampal volumes are related to response and remission 57 , 58 . One study found that low baseline hippocampal volumes were related to impaired treatment outcomes after 3 years 59 ; a meta-analysis confirmed that low baseline hippocampal volumes are associated with negative outcomes 60 . However, negative studies have also been reported 61 , 62 . The volume of other brain regions, including the anterior cingulate or orbitofrontal cortices, have also been shown to be decreased in MDD subjects 63 , but more longitudinal neuroimaging trials with antidepressants are needed to clarify this association. Interestingly, several studies, including one meta-analysis 64 , found significant hippocampal volume increases after ECT 65 , 66 , 67 , although the relationship to antidepressant response has yet to be confirmed 64 , 68 .
The largest functional magnetic resonance imaging (fMRI) study of MDD patients conducted to date reported neurophysiological subtypes based on connectivity patterns within limbic and frontostriatal brain areas 69 . In subset analyses, connectivity patterns plus subtype classifications predicted response to repetitive transcranial magnetic stimulation (rTMS) treatment with higher accuracy (89.6%) than clinical characteristics alone. Other task-based and resting-state fMRI studies found that ACC activity (including pregenual activity) predicted treatment response 70 , a finding corroborated by an expanded electroencephalography study 71 as well as a meta-analysis 60 . While these interesting results suggest that fMRI measures could ultimately help classify biological subtypes of depression, these methods are far from ready for clinical application and results will have to be reproduced. However, given its easy implementation and the short time needed to acquire measurements, fMRI appears to be a promising tool for identifying imaging biomarkers.
Positron emission tomography (PET) studies have identified altered serotonin-1A (5-HT 1A ) receptor and 5-HT transporter (SERT) binding potentials, an index of protein concentration, at baseline and in TRD patients 72 , 73 , 74 , 75 . Most of these results found reduced baseline SERT levels and elevated baseline 5-HT 1A heteroreceptors in MDD patients (depending on PET methodology for 5-HT 1A ); non-remitters had lower 5-HT 1A autoreceptor binding in the serotonergic raphe nuclei 75 , as well as lower SERT 76 . Reduced global 5-HT 1A receptor binding has also been observed after ECT 77 . High costs, technical and methodological challenges, lack of dedicated PET centers with 11 C-radiochemistry, small sample sizes, small effect sizes, and unclear cutoff values have heretofore prevented the broader clinical application of these tools in MDD compared to disorders such as Alzheimer’s and Parkinson’s disease. An earlier [ 18 F]FDG PET study of unmedicated MDD patients was consistent with the aforementioned fMRI results, demonstrating increased glucose turnover in the orbitofrontal and posterior cingulate cortices and amygdala and decreased turnover in the subgenual ACC and dorsolateral prefrontal cortex 78 . A later study corroborated these results and found that glucose turnover was differentially affected by cognitive behavioral therapy or venlafaxine 79 . Interestingly, several studies detected microglial activation by labeling translocator protein (TSPO) with PET, using TSPO radioligands like 18 F-FEPPA. Microglial activation is closely linked to brain tissue damage, traumatic brain injury, neuroinflammation, and increased metabolic demands. Increased TSPO binding in MDD patients has been observed in the ACC, insula, and prefrontal cortex 80 . In addition, TSPO binding has also been shown to positively correlate with length of illness and time without antidepressant treatment, and to negatively correlate with SSRI treatment 80 . Elevated TSPO levels in unmedicated, acutely ill MDD patients have now been reported in at least two independent datasets 81 , 82 . However, TSPO-positive MDD patients may reflect a specific subtype (i.e., associated with neuroinflammation) and may, thus, respond better to treatments that target neuroinflammation. For a graphical summary of these findings see Fig. 1 .
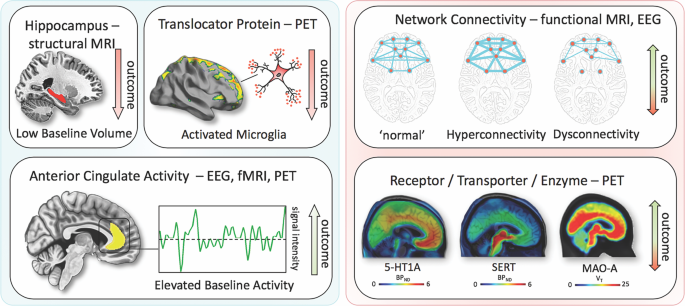
Imaging findings exhibiting unidirectional (left) relationships with outcome in MDD vs. bidirectional (right). fMRI, functional magnetic resonance imaging; PET, positron emission tomography; EEG electroencephalography; 5-HT1A, serotonin-1A receptor; SERT, serotonin transporter; MAO-A monoamine oxidase-A; BP ND , nondisplaceable binding potential; V T , volume of distribution
Blood-based markers of disease outcomes
Consistent with neuroinflammatory processes, elevated levels of C-reactive protein (CRP), tumor necrosis factor alpha (TNFα), and interleukin-6 (IL-6) have been reported in a subset of MDD patients. In particular, elevated levels of CRP, a well-established marker of increased proinflammatory state in blood, was shown to be associated with MDD and increased risk for psychological distress in cross-sectional samples of the general population 83 . A longitudinal study found that lower CRP levels were associated with quicker response to SSRIs, an association not observed for SSRI-bupropion combination therapy 84 . Interestingly, elevated CRP levels have been shown to be more pronounced in female versus male MDD patients 85 . Similar findings have been observed for IL-6 and TNFα. One meta-analysis found that all three were significantly elevated at baseline in MDD patients, but their treatment trajectories differed 86 ; IL-6 levels decreased with antidepressant treatment, but outcomes were indistinguishable. In the same meta-analysis, persistingly high TNFα levels identified TRD patients 86 . Notably, heterogeneity was high within the pooled studies. Another study noted that levels of acute phase protein complement C3 significantly differentiated between atypical and melancholic MDD subtypes 87 . MDD patients have also been shown to have altered levels of peripheral adipokines and bone inflammatory markers; these deficits were corrected with ketamine treatment 88 , 89 .
Given the importance of neuroplasticity in the pathophysiology and treatment of depression, interest has grown in studying brain-derived neurotrophic factor (BDNF), a neurotrophin involved in the structural adaptation of neuronal networks and a prerequisite for neuronal reactions to stressors. BDNF blood levels most likely stem from peripheral tissue. While these peripheral levels are linked to central levels, the question of whether BDNF is actively transported through the blood–brain barrier remains controversial 90 . Compelling evidence suggests that BDNF levels are decreased at baseline in MDD patients and elevated in response to pharmacological 90 , 91 treatments as well as ECT 92 . A meta-analysis found that increased BDNF levels in response to treatment successfully stratified responders and remitters compared to non-responders 93 .
Outcome and genetic and epigenetic links
Heritable risk for MDD is between 30 and 40%, with higher rates in women. A large, collaborative genome-wide association study (GWAS) detected 44 significant loci associated with MDD 94 . Specific analyses identified neuronal genes (but not microglia or astrocytes), gene-expression regulating genes (such as RBFOX1 ), genes involved in gene-splicing, as well as genes that are the targets of antidepressant treatment. The authors suggested that alternative splicing could lead to shifts in the proportion of isoforms and altered biological functions of these proteins 94 .
Hypothesis-driven approaches with candidate genes have provided initial insights into the influence of single-nucleotide polymorphisms (SNPs). It is beyond the scope of this manuscript to review the large number of candidate genes; here, we outline only several representative genes (see Table 1 for meta-analytic evidence of treatment outcomes). These include synaptic proteins involved in stress response, antidepressant binding structures, or neuroplasticity (e.g., CRH receptor 1 ( CRHR1 )), the sodium-dependent serotonin transporter ( SLC6A4 ), and BDNF 95 . The aforementioned multicenter GSRD study also found that combining clinical and genetic variables explained antidepressant response better than SNPs alone in a random forest algorithm 96 . In that study, regulatory proteins such as ZNF804A (associated with response) and CREB1 (associated with remission), as well as a cell adhesion molecule (CHL1, associated with lower risk of TRD), were linked to antidepressant treatment outcomes. Another interesting candidate gene is FK506 binding protein 5 ( FKBP5 ), which was found to moderate the influence of standard treatments in an algorithm lasting up to 14 weeks 97 ; FKBP5 is known to influence HPA axis reactivity 98 , treatment response 99 , and epigenetic mechanisms in response to environmental stressors 100 . Another relevant avenue of research is drug-drug interactions and gene isoforms in the cytochrome P450 pathway (CYP450), which could account for insufficient amounts of a given drug reaching the brain or, conversely, result in exceedingly high plasma values, making subjects more vulnerable to treatment side effects 101 , 102 . Several commercially available kits categorize patients according to their phenotypic status (e.g., CYP2D6, 2C19, CYP3A4). This led to the introduction of phenotype categories—“poor”, “intermediate”, “extensive (normal)”, and “ultrarapid” metabolizers—based on CYP450 isoenzyme status and their relationship to plasma levels at fixed doses 102 . A large naturalistic study of CYP2C19 isoforms found that treatment success with escitalopram was less frequent in “poor” (CYP2C19Null/Null) and “ultrarapid” metabolizers (CYP2C19*1/*17 or CYP2C19*17/*17) 103 .
Clinical subgroups, TRD, and treatment outcomes
While some studies have suggested that depressive subtypes in MDD—including anxious, mixed, melancholic, atypical, and psychotic depression—respond differently to antidepressant treatment, this literature is mixed. For instance, some studies found that melancholic patients initially present with high levels of severity and may respond less well to SSRI treatment than to venlafaxine or tricyclic antidepressants 104 , but other studies did not support this finding 105 . No association was found between subgroups and clinical outcomes in a parallel design, uncontrolled study investigating sertraline, citalopram, and venlafaxine 106 , which found that near equal percentages of patients who met criteria for a pure-form subtype (39%) also had more than one subtype (36%), making these psychopathological subtypes difficult to classify.
It should be noted that treatment success might have more discriminatory power for identifying subgroups than psychopathological subgroup stratification. Although a wide range of definitions exists specifying the number of failed trials necessary to diagnose TRD 107 , the core definition of TRD centers around a lack of improvement in response to consecutive, adequate antidepressant treatments. Resistance occurs at alarmingly high rates and is thought to affect 50–60% of all treated patients 107 . Unsurprisingly, this group of patients has dramatically worse outcomes than those who respond to antidepressants, and factors that are associated with TRD overlap with many of those presented above 28 . Cross-sectional data from the GSRD 108 identified a number of risk factors linked to TRD, including comorbidity (particularly anxiety and personality disorders), suicide risk, episode severity, number of hospitalizations, episode recurrence, early-onset, melancholic features, and non-response at first treatment 28 . Most importantly, TRD is life-threatening, and associated with a two- to threefold increased risk of suicide attempts compared to responding patients, and a 15-fold increased risk compared to the general population 109 . Taken together, the evidence indicates that TRD patients need special attention, as outcomes in these individuals are significantly worse.
Novel and existing strategies to improve treatment outcomes
Early identification, prevention, and early treatment.
Numerous programs for suicide prevention exist 110 , and recognizing acute depressive symptoms is just one of many important facets of such work. Screening tools for early identification of depressed patients can be helpful 111 , and such instruments can start with as few as two items—for instance, the Patient Health Questionnaire-2 112 or Ask Suicide-Screening Questions (asQ’em) 113 —and proceed to more detailed instruments if initial screens are positive. Positive screening should be followed by a diagnostic interview to determine whether patients meet criteria for MDD 111 . In the general population, two large independent studies that used only clinical variables were nevertheless able to accurately predict depression within 1–3 years 114 . In addition, long-term monitoring of vulnerable subjects with known SLEs may further improve the ability to identify at-risk individuals early in their course of illness. As noted above, duration of untreated disease is a negative predictor of treatment outcomes. Because the advantages of early intervention in MDD have been demonstrated 115 , efforts to achieve early treatment might also help slow disease progression in individuals with TRD; however, this hypothesis has not been sufficiently tested.
Modeling environmental impact on predisposition
As noted above, severe SLEs constitute an important risk factor. Elegantly designed studies have demonstrated that genetic predisposition, in concert with SLEs, might account for increased vulnerability to MDD 100 . In this manner, the presence of ‘weak alleles’ in candidate genes such as BDNF, SERT , and others would be increasingly detrimental in the presence of SLEs 116 , 117 . However, studies have been quite inconsistent and yielded small effect sizes, including a negative result in 252 patients enrolled in the GSRD study 118 . It should be noted that counter-regulatory mechanisms or resilience factors, such as social support, may exist that counter SLEs. Nevertheless, preliminary research suggests that the impact of SLEs on MDD may depend on measurable factors such as gender and the timing of exposure 119 . Both genes and the environment are complex systems with frequent opportunity for interaction and elaborate compensatory mechanisms. While the complexity of genetic susceptibility in MDD can be tackled through enormous collaborative projects 94 , the interactions between genetic susceptibility and environmental factors have yet to be determined. Properly powered gene×environment interaction projects may exceed current research capabilities, and large longitudinal studies will certainly be needed 120 .
Developing markers for subgroup identification and disease course
Pioneering research on biological differences—for instance, between patients with atypical versus melancholic depression—suggests differential HPA axis or autonomous nervous system reactivity 121 , 122 , though the subtype results have been only moderately consistent across time and are prone to low group specificity 123 , 124 , 125 . However, at least one study demonstrated the more reliable stability of extreme types over a 2-year period 87 . Interestingly, one study found that individuals with atypical depression had significantly higher body-mass index, waist circumference, levels of inflammatory markers, and triglyceride levels, and lower levels of high-density lipid cholesterol than those with melancholic depression or controls 126 . Using fMRI and biological variables, another study found that MDD subjects could be divided into low/high appetite groups with distinctive correlations between neuronal activity and endocrine, metabolic, and immune states 127 . Other research groups have tried to overcome conventional psychopathological subgroups and model biotypes using resting-state fMRI 69 . Molecular and functional neuroimaging, as well as epigenetic studies, are promising approaches for separating subgroups and may be better suited to identifying screening markers (see Fig. 2 ) that are exclusively valid in certain subgroups with higher predictive power.
These approaches highlight the feasibility of linking and stratifying psychopathological categories with biological variables, a goal further supported by the Research Domain Criteria (RDoc), which seek to link dimensions of observable behavior with neurobiological systems 128 . In the search for biomarkers, subgroup- or domain-specific classifications using unidimensional variables might improve subgroup stratification 129 . Moreover, applying markers to other categories could boost the utility of existing markers that have failed in any given category (see Fig. 2 for established markers). As a field, the focus is largely on staging and prediction markers, but ‘predisposition’ or ‘recurrence’ markers may equally be worth investigating. Presently, however, the relative lack of biologically defined MDD subgroups and their stratification are key obstacles to finding and establishing treament outcome predictors appropriate for broader clinical applications.
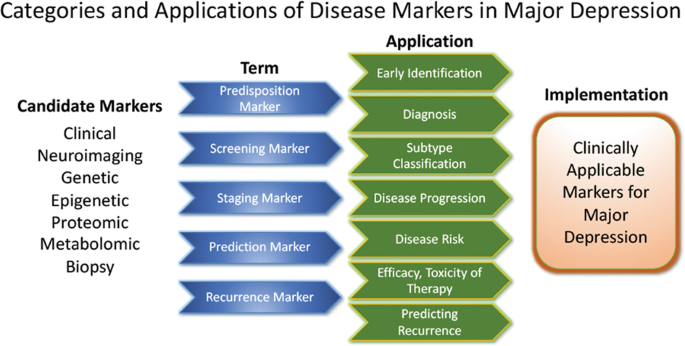
Candidate disease markers can be applied in clinically meaningful ways. While only candidate markers are presently available, sorting these according to their potential applications may facilitate the development of clinically applicable disease markers. The outline follows the classification of markers as suggested by others 200 (modified and reprinted with permission from Springer)
The most important outcome of successful subgroup stratification and staging markers would be that patients and their relatives would receive valuable information at treatment onset about how their disease is likely to improve or worsen. Toward this end, the development of staging methods provides promising solutions. Currently, at least five different methods exist 130 that, to date, have not been evaluated thoroughly enough for clinical implementation. Continuous variables—as obtained by the Maudsley Method and Massachusetts General Hospital Staging Model—appear to provide greater staging advantages than categorical variables. It should be noted here that data indicate that research in severely ill, suicidal, and TRD subjects is safe to conduct in controlled inpatient settings 131 . Presently, patients in various stages of disease and/or treatment history are lumped together and compared in statistical analyses. We propose that staging should be more thoroughly integrated into clinical trial design.
Algorithm- and guideline-based treatments
Despite the availability and distribution of a variety of expert-based guidelines, only a fraction of patients are actually treated according to guidelines 132 (see Table 2 for current guidelines (≤10 years)). New guidelines – particularly for TRD – and more rigorous implementation of guideline-based care are needed. Improvements in currently available treatments have been conducted using treatment algorithms and following sequential treatment strategies, with standardized instructions for therapeutic decision-making. In the past two decades, large, collaborative studies using treatment-based algorithms have introduced standardized, sequential treatments; these include the Texas Medication Algorithm Project 133 , the STAR*D trial 21 , and the German algorithm project 134 . Indeed, evidence suggests that algorithm-based treatments improve treatment outcomes 135 and are cost effective 136 . Here, we considered current clinical treatment guidelines to create a sequential treatment optimization scheme of recommended treatments. While there is no fixed time-frame, first- and second-line treatments are recommended sequentially during the first episode and within 3 months (see Fig. 3 , which also illustrates the need for more third- and fourth-stage treatment options). Figure 4 , illustrates potential reasons for “pseudoresistance” 42 that should be ruled out during this time-frame.
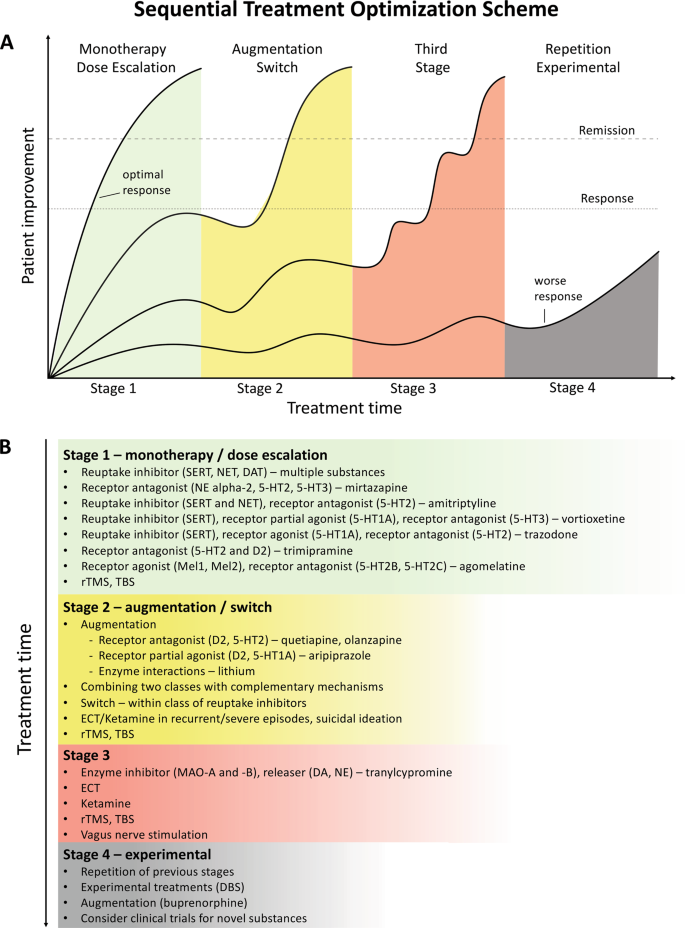
A sequential treatment optimization scheme was generated based on antidepressant treatment guidelines (see Table 2 ). Treatment optimization is possible for patients being treated for the first time but also for patients with insufficient response to first- or second-stage therapies. a Treatment response curves for four common types of patients highlight the importance of sequentially introducing the next step upon non-response to previous steps. b Currently available treatments are listed in neuroscience-based nomenclature 201 with treatment lines corresponding to improvement curves in a . Although current classifications vary, patients classified as having treatment-resistant depression (TRD) are eligible for second- or third-stage therapies. 5-HT1A and similar: serotonin receptor subtypes; DBS: deep brain stimulation; DAT: dopamine transporter; D2: dopamine receptor D2; ECT: electroconvulsive therapy; MAO: monoamine oxidase; NET: noradrenaline transporter; SERT: serotonin transporter; TBS: theta-burst stimulation; rTMS: repetitive transcranial magnetic stimulation; DA: dopamine; NE: norepinephrine.
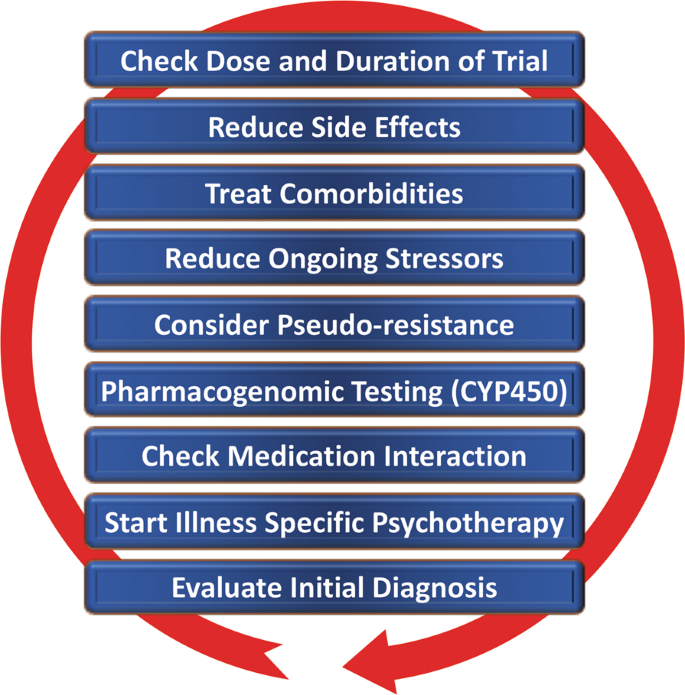
Points—in random order—follow earlier suggestions by Dold and Kasper (2017) 202
Reducing placebo response in clinical trials while harnessing placebo effects in clinical treatment
The issue of placebo response in antidepressant trials has become increasingly important 137 , 138 . Indeed, the contribution of placebo effects to early response needs to be systematically studied in order to disentangle biological therapy-induced effects from psychologically induced effects. Strikingly, in the brain, anatomically similar regions that mediate placebo response are affected by MDD (for a comprehensive review, see ref. 139 ). Several mechanisms contribute to placebo response, including patients’ expectations of benefits, behavioral conditions, and the quality of patient-physician interactions 139 . Strategies for reducing placebo response could lead to better discrimination between effective treatments in clinical trials; such strategies include extending trial duration, excluding placebo responders by including a placebo run-in, or using randomized run-in and withdrawal periods 138 , 139 . Others have suggested using more thorough criteria to select study participants 140 . On the other hand, when antidepressant agents are used clinically, placebo effects must be taken advantage of by harnessing patients’ expectations and learning mechanisms to improve treatment outcomes 141 .
Novel antidepressant treatments
The recent discovery that glutamatergic-based drugs are uniquely capable of rapidly and robustly treating mood disorders has ushered in a new era in the quest to develop novel and effective antidepressants 142 , 143 , 144 . In this regard, the prototypic glutamatergic modulator ketamine has catalyzed research into new mechanistic approaches and offered hope for the development of novel, fast-acting antidepressants. While ketamine’s underlying mechanism of action remains the subject of active investigation, several theories have been propsed 144 . These include N-methyl- d -aspartate receptor (NMDAR)-dependent mechanisms, such as the inhibition of NMDARs on gamma aminobutyric acid (GABA)-ergic interneurons, the inhibition of spontaneous NMDAR-mediated transmission, the inhibition of extrasynaptic NMDARs, the inhibition of lateral habenula neurons, and GABA B receptor expression/function 144 . Substantial evidence also supports additional NMDAR-independent mechanisms, including the stabilization of glutamate release/excitatory transmission, active metabolites such as hydroxynorketamine, regulation of the dopaminergic system, G-alpha subunit translocation, and activation of cyclic adenosine monophosphate, as well as potential sigma-1 and mu-opioid receptor activation 145 . Among those theories, a leading hypothesis remains that NMDAR antagonism increases BDNF synthesis, a process mediated by decreased phosphorylation of eukaryotic elongation factor-2 and the subsequent activation of the mammalian target of rapamycin pathway by BDNF activation of the TrkB receptor 146 , 147 . These putative mechanisms of action are not mutually exclusive and may complement each other to induce potentiation of excitatory synapses in affective-regulating brain circuits, resulting in improved depressive symptoms.
The initial serendipitous discovery that a single, subanesthetic-dose ketamine infusion has rapid-acting antidepressant effects in MDD 148 , a finding subsequently confirmed by numerous randomized trials, has been hailed as one of the most important discoveries in psychiatry in the last decades 149 . The initial proof-of-concept studies demonstrated that a single dose of ketamine (0.5 mg/kg, IV) administered over 40 min led to rapid, robust, and relatively sustained antidepressant effects in TRD—both MDD 150 , 151 , 152 , 153 and bipolar depression 154 , 155 . In research settings, studies of TRD patients found response rates of >70% within 24 h post-infusion 153 , with about 50–70% of participants exhibiting a variable duration of response 156 , 157 . Ketamine has also been shown to be superior to any blinding counterpart 158 . Off-label ketamine use has also been associated with significant and rapid (one to four hours) antisuicidal effects 150 , 159 , 160 , a finding supported by a large, recent metanalysis showing that ketamine exerted rapid (within hours) and sustained (up to 7 days) improvements in suicidal thoughts compared to placebo 161 .
Esketamine hydrochloride
The ketamine enantiomer esketamine received approval by the FDA for TRD and is currently undergoing further Phase III clinical trials. A Phase II, 10-week, clinical trial of flexibly dosed intranasal esketamine (28 mg/56 mg or 84 mg) found that, in TRD patients, this agent demonstrated rapid and clinically relevant improvements in depressive symptoms compared to placebo 162 . Strikingly, 65% of TRD patients met response criteria through Day 57. In another Phase II proof-of-concept, multi-site, 4-week, double-blind study, standard treatment plus intranasal esketamine (84 mg) was compared to standard treatment plus placebo in individuals with MDD at imminent risk of suicide 163 . The authors found a rapid antisuicidal effect, as assessed via the Montgomery-Åsberg Depression Rating Scale Suicide Item score at 4 h.
Other rapid acting and novel antidepressants
Based on the success of ketamine, other rapid-acting or novel antidepressant substances within the glutamatergic/GABA neurotransmitter systems are being developed, several of which are in Phase III clinical trials. A prototype novel substance is AV-101 (L-4-cholorkynurenine). This is a potent selective antagonist at the glycine-binding site of the NMDAR NR1 subunit and has demonstrated antidepressant-like effects in animal models, while human Phase II studies are currently ongoing 164 . Brexanolone is a formulation of the endogenous neurosteroid allopregnanolone, which modulates neuronal activation of GABA A receptors and has met positive endpoints in Phase III, leading to FDA approval for postpartum depression. A comparable substance is under development for MDD 165 . In addition, serotonergic agonists have been studied as our understanding of their mechanism of action (e.g., their effects on glutamate release or plasticity) has increased 166 . Encouraging results have been seen for the serotonin 2A receptor agonist psilocybin 167 , but these findings need to be replicated in larger systematic clinical trials. Initial positive trials of add-on agents—such as buprenorphine 168 , 169 , rapastinel 170 , or scopolamine 145 —have also been conducted. However, it is beyond the scope of this manuscript to review all of these findings, and we refer the interested reader to recent comprehensive reviews of this subject 144 , 145 , 165 , 171 .
Transcranial stimulation paradigms
In contrast to pharmaceutical treatments that exert their efficacy at the molecular level, electrical stimulation techniques target entire neuronal circuits. TMS of the (left) dorsolateral prefrontal cortex has been FDA-approved since 2008 to treat depression in patients who failed to respond to one standard antidepressant treatment. Apart from transient local skin and muscle irritation at the stimulation site and headaches, it is a very safe technique with few side effects. Studies have repeatedly demonstrated the superiority of rTMS over sham procedures, though effect sizes have been moderate 172 , 173 , 174 . Initial studies suggest that rTMS is also effective in TRD but the data are too few to draw definitive conclusions 175 , 176 . Improvements in rTMS techniques known as theta-burst stimulation (TBS) provide significantly shortened treatment times (3 min for TBS versus 37 min for rTMS) and hence allow more patients to be treated per day. A large non-inferiority trial of 414 moderately resistant MDD patients found that TBS was at least as effective as rTMS in reducing depressive symptoms 177 .
Electroconvulsive therapy (ECT)
Regarded as the ‘gold standard’, ECT has been successfully used for many years to treat severe TRD and exhibits both relatively rapid and sustained onset of efficacy; approximately 50% of all patients reach response criteria at the third treatment, typically within 1 week. It is also one of the most effective antidepressant therapies 178 , yielding response rates of ~80%, remission rates of ~75% 179 , and antisuicidal effects 180 . Remission is achieved by about 30% of patients within six ECT sessions 179 . ECT also reduces the risk of readmission 181 and is likewise safe to use in depressed elderly subjects 182 . The side effects of ECT include intermediate disorientation, impaired learning, and retrograde amnesia, all of which usually resolve 183 . The optimal anatomic location of the stimulus electrodes is a topic of current debate 184 , 185 . Recent evidence suggests that all three methods for electrode placement (bifrontal, bitemporal, and unilateral) show clinically significant effects 186 . While no difference in cognitive side effects was observed, bitemporal placement should be considered the first-line choice for urgent clinical situations. Despite its clinical efficacy, ECT remains underutilized. Its use is declining 187 because it needs to be administered in hospital settings under anesthesia, and partly because of misleading portrayals of the procedure itself. Adjusting the dose of electrical stimuli (e.g., through refined electrode placement or individually adjusted pulse amplitudes) may improve ECT’s side effect profile.
Magnetic seizure therapy (MST)
MST uses high doses of rTMS to induce seizures 188 . The electromagnetically induced electrical field generated by MST is unifocal and variable, as there are individual differences in the degree to which the skull provides electrical resistance 189 . As an advantage over ECT, MST is associated with a more superficial stimulation, which exerts less impact on the medial-temporal lobe where cognitive side effects are thought to be elicited. To date, few research sites across the world have used MST, with a concomitant dearth of open-label trials. Nevertheless, the preliminary treatment data suggest that results obtained with MST are similar to those obtained with ECT but with a more favorable side effect profile 190 , 191 .
Vagus nerve stimulation (VNS)
VNS is a surgically implanted pacemaker-like device attached to a stimulating wire threaded along the left vagus nerve. Since 2005, the FDA has approved VNS use for the adjunctive long-term treatment of long-lasting recurrent depression in patients 18 years and older who are experiencing a major depressive episode and have failed to respond to four or more previous adequate standard antidepressant treatment trials. In such cases, it has been shown to have superior long-term effects over conventional psychopharmacological treatment 192 . A recent, large, observational, adjunctive, open-label, naturalistic study followed TRD patients over 5 years 193 . In this group, adjunctive VNS led to significantly better clinical outcomes and higher remission rates than treatment as usual (67.6% vs. 40.9%, respectively).
Deep-brain stimulation (DBS)
DBS involves the neurosurgical implantation of electrodes and has become clinically routine in the treatment of Parkinson’s disease and Dystonia. The technique is safe, removable, and does not cause lasting neuronal lesions. In TRD, anatomical targets include the subgenual cingulate, nucleus accumbens, habenula, and medial forebrain bundle. Clinical trials typically only enroll severely ill TRD patients whose current episode has lasted >12 months, whose age of onset is <45 years, and who have failed to respond to at least four adequate prior treatment trials of standard antidepressants, ECT, and/or psychotherapy. Initial open-label or single-blind trials found that DBS had both rapid and sustained antidepressant effects 194 , 195 , 196 . In contrast, one large and one smaller sham-controlled clinical study both failed to achieve their primary endpoints of symptom reduction 197 , 198 . To date, the number of MDD patients treated with DBS has been very small compared to other treatment options, including ECT and TMS. Nevertheless, brain-electrode interfaces are evolving quickly and it is possible that next generation brain-responsive stimulation devices will be able to adjust stimulation on-demand only when abnormal biological marker impulses (e.g., pulse amplitude) are detected 199 .

Conclusions
Although enormous progress has been made in measuring, predicting, and improving outcomes, depression remains a relentless disease that places a heavy burden on both individuals and society. The research reviewed above indicates that early recognition and early adequate treatment at illness onset are preferable to watch-and-wait strategies. The studies reviewed above also underscore the manner in which SLEs, as well as physical and psychiatric comorbidities, contribute to impaired outcomes. Together, these factors contribute toward treatment resistance, which has gained a substantial amount of importance as a patient-stratifying variable.
This paper also reviewed biological markers, where research has grown exponentially to encompass enormous projects with potentially tens of thousands of subjects enrolled in real world studies. In parallel, studies exploring the underlying genetics of depression have evolved from early candidate gene studies of neurotransmitters, stress, or gene-regulatory systems to large GWAS that help reveal potential new pathways and treatment targets. Moreover, the burgeoning field of proteomics has found promising target molecules. Nevertheless, despite the wealth of recent work in this area, no single biomarker has yet been used in clinical applications. A substantial need exists for replication and, because many biomarker studies are currently open-label, for controlled studies. In combination with neuroimaging techniques such as fMRI, genes or blood-based markers have a high potential of future implementation in stratification of MDD or serve as prognostic marker on treatment outcome.
Above, we also outlined efforts to optimize outcomes. We argue that disease-inherent heterogeneity, in concert with inaccurate group stratification tools, might have contributed to the lack of clinically applicable stratification and response prediction markers. Successful subgroup identification, and the ability to use this information in clinical settings, is crucial to improving future treatment paradigms. While recent research has increasingly focused on TRD, we wish to reiterate that no standard definition of TRD presently exists. Thus, based on currently available guidelines, we have outlined a sequential treatment optimization scheme that includes options for TRD; such work highlights the substantial need to develop and improve “third-line-and-beyond” therapeutics. In this context, this manuscript also reviews novel treatments and brain stimulation techniques that have demonstrated rapid antidepressant effects in TRD, including ketamine, esketamine, ECT, MST, TMS/TBS, VNS, DBS, and others. When treating TRD patients, physicians should consider illness severity, the chronicity of past and recent depressive episodes, the side effect profile of available treatment options, as well as previous refractoriness to particular treatment approaches. If acuity supersedes chronicity, one could consider fast-acting interventions such as ketamine or ECT/MST.
This review, though comprehensive, was not able to consider several lines of evidence on outcome prediction and treatment improvement. In particular, we focused on clinical outcomes in humans and were, thus, unable to fully explore the highly valuable advances made in translational science. Similarly, it was beyond the scope of this manuscript to review the richness of results from animal research and their relevance to MDD. Moreover, given the amount of literature, we were not able to incorporate many proteomic, genetic, or psychopharmacological findings.
Taken together, this review outlines important clinical, psychosocial, and biological factors associated with response and remission to antidepressant treatment (see Table 3 ). Recent studies have led to important insights into neurobiological disease markers that could result in improved disease stratification and response prediction in the near future. Key discoveries into novel rapid-acting substances, in concert with improvements in brain stimulation techniques, may also result in significantly improved treatment outcomes in formerly hard-to-treat patients.
Wittchen, H. U. et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 21 , 655–679 (2011).
Article CAS Google Scholar
Lim, S. S. et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380 , 2224–2260 (2012).
Article PubMed PubMed Central Google Scholar
Lecrubier, Y. Widespread underrecognition and undertreatment of anxiety and mood disorders: results from 3 European studies. J . Clin . Psychiatry 68 Suppl 2 , 36–41 (2007).
Riedel, M. et al. Clinical predictors of response and remission in inpatients with depressive syndromes. J. Affect. Disord. 133 , 137–149 (2011).
Article PubMed Google Scholar
Rost, K. et al. Persistently poor outcomes of undetected major depression in primary care. Gen. Hosp. Psychiatry 20 , 12–20 (1998).
Article CAS PubMed Google Scholar
Ghio, L., Gotelli, S., Marcenaro, M., Amore, M. & Natta, W. Duration of untreated illness and outcomes in unipolar depression: a systematic review and meta-analysis. J. Affect. Disord. 152–154 , 45–51 (2014).
Hung, C. I., Liu, C. Y. & Yang, C. H. Untreated duration predicted the severity of depression at the two-year follow-up point. PLoS ONE 12 , e0185119 (2017).
Article PubMed PubMed Central CAS Google Scholar
Bukh, J. D., Bock, C., Vinberg, M. & Kessing, L. V. The effect of prolonged duration of untreated depression on antidepressant treatment outcome. J. Affect. Disord. 145 , 42–48 (2013).
Habert, J. et al. Functional recovery in major depressive disorder: Focus on early optimized treatment. Prim Care Companion CNS Disord. 18 (2016).
Kautzky, A. et al. Clinical factors predicting treatment resistant depression: affirmative results from the European multicenter study. Acta Psychiatr. Scand. 139 , 78–88 (2018).
Furukawa, T. A., Kitamura, T. & Takahashi, K. Time to recovery of an inception cohort with hitherto untreated unipolar major depressive episodes. Br. J. Psychiatry.: J. Ment. Sci. 177 , 331–335 (2000).
Feffer, K. et al. Early symptom improvement at 10 sessions as a predictor of rTMS treatment outcome in major depression. Brain Stimul. 11 , 181–189 (2018).
Martinez-Amoros, E. et al. Early improvement as a predictor of final remission in major depressive disorder: New insights in electroconvulsive therapy. J. Affect. Disord. 235 , 169–175 (2018).
Soares, C. N., Endicott, J., Boucher, M., Fayyad, R. S. & Guico-Pabia, C. J. Predictors of functional response and remission with desvenlafaxine 50 mg/d in patients with major depressive disorder. CNS Spectr. 19 , 519–527 (2014).
Lam, R. W. et al. Predictors of functional improvement in employed adults with major depressive disorder treated with desvenlafaxine. Int Clin. Psychopharmacol. 29 , 239–251 (2014).
Jha, M. K. et al. Early improvement in work productivity predicts future clinical course in depressed outpatients: Findings from the CO-MED trial. Am. J. Psychiatry 173 , 1196–1204 (2016).
Spies, M. et al. Default mode network deactivation during emotion processing predicts early antidepressant response. Transl. Psychiatry 7 , e1008 (2017).
Article CAS PubMed PubMed Central Google Scholar
Bartlett, E. A. et al. Pretreatment and early-treatment cortical thickness is associated with SSRI treatment response in major depressive disorder. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 43 , 2221–2230 (2018).
Olgiati, P. et al. Early improvement and response to antidepressant medications in adults with major depressive disorder. Meta-analysis and study of a sample with treatment-resistant depression. J. Affect Disord. 227 , 777–786 (2018).
de Vries, Y. A. et al. Predicting antidepressant response by monitoring early improvement of individual symptoms of depression: individual patient data meta-analysis. Br. J. Psychiatry.: J. Ment. Sci. 214 , 1–7 (2018).
Google Scholar
Trivedi, M. H. et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry 163 , 28–40 (2006).
Blom, M. B. et al. Severity and duration of depression, not personality factors, predict short term outcome in the treatment of major depression. J. Affect. Disord. 104 , 119–126 (2007).
Kautzky, A. et al. Refining prediction in treatment-resistant depression: results of machine learning analyses in the TRD III sample. J. Clin. Psychiatry 79 , 16m11385 (2018).
Katon, W., Unutzer, J. & Russo, J. Major depression: the importance of clinical characteristics and treatment response to prognosis. Depress Anxiety 27 , 19–26 (2010).
Papakostas, G. I. Surrogate markers of treatment outcome in major depressive disorder. Int. J. Neuropsychopharmacol./Off. Sci. J. Coll. Int. Neuropsychopharmacol. 15 , 841–854 (2012).
Friedman, E. S. et al. Baseline depression severity as a predictor of single and combination antidepressant treatment outcome: results from the CO-MED trial. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 22 , 183–199 (2012).
Balestri, M. et al. Socio-demographic and clinical predictors of treatment resistant depression: A prospective European multicenter study. J. Affect Disord. 189 , 224–232 (2016).
Souery, D. et al. Clinical factors associated with treatment resistance in major depressive disorder: results from a European multicenter study. J. Clin. Psychiatry 68 , 1062–1070 (2007).
Mandelli, L. et al. Opinion paper: poor response to treatment of depression in people in high occupational levels. Psychol. Med. 49 , 49–54 (2019).
Kautzky, A. et al. A new prediction model for evaluating treatment-resistant depression. J. Clin. Psychiatry 78 , 215–222 (2017).
Paksarian, D. et al. Stability and change in reported age of onset of depression, back pain, and smoking over 29 years in a prospective cohort study. J. Psychiatr. Res. 88 , 105–112 (2017).
Wells, K. et al. Five-year impact of quality improvement for depression: results of a group-level randomized controlled trial. Arch. Gen. Psychiatry 61 , 378–386 (2004).
Vinkers, C. H. et al. Stress exposure across the life span cumulatively increases depression risk and is moderated by neuroticism. Depress Anxiety 31 , 737–745 (2014).
Kendler, K. S., Kuhn, J. & Prescott, C. A. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am. J. Psychiatry 161 , 631–636 (2004).
Nanni, V., Uher, R. & Danese, A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am. J. Psychiatry 169 , 141–151 (2012).
Thompson, A. E. & Kaplan, C. A. Childhood emotional abuse. Br. J. Psychiatry.: J. Ment. Sci. 168 , 143–148 (1996).
Nelson, J., Klumparendt, A., Doebler, P. & Ehring, T. Childhood maltreatment and characteristics of adult depression: meta-analysis. Br. J. Psychiatry.: J. Ment. Sci. 210 , 96–104 (2017).
Article Google Scholar
Kendler, K. S. & Gardner, C. O. Sex differences in the pathways to major depression: a study of opposite-sex twin pairs. Am. J. Psychiatry 171 , 426–435 (2014).
Keers, R. et al. Stressful life events, cognitive symptoms of depression and response to antidepressants in GENDEP. J. Affect. Disord. 127 , 337–342 (2010).
Henriksen, C. A. et al. Identifying factors that predict longitudinal outcomes of untreated common mental disorders. Psychiatr. Serv. 66 , 163–170 (2015).
Dennehy, E. B., Marangell, L. B., Martinez, J., Balasubramani, G. K. & Wisniewski, S. R. Clinical and functional outcomes of patients who experience partial response to citalopram: secondary analysis of STAR*D. J. Psychiatr. Pract. 20 , 178–187 (2014).
Dold, M. et al. Clinical characteristics and treatment outcomes of patients with major depressive disorder and comorbid anxiety disorders—results from a European multicenter study. J. Psychiatr. Res. 91 , 1–13 (2017).
Fava, M. et al. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am. J. Psychiatry 165 , 342–351 (2008).
Angstman, K. B. et al. Personality disorders in primary care: impact on depression outcomes within collaborative care. J. Prim. Care Community Health 8 , 233–238 (2017).
Zeeck, A. et al. Prognostic and prescriptive predictors of improvement in a naturalistic study on inpatient and day hospital treatment of depression. J. Affect. Disord. 197 , 205–214 (2016).
Newton-Howes, G., Tyrer, P. & Johnson, T. Personality disorder and the outcome of depression: meta-analysis of published studies. Br. J. Psychiatry.: J. Ment. Sci. 188 , 13–20 (2006).
Whooley, M. A. et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA 300 , 2379–2388 (2008).
Ducat, L., Philipson, L. H. & Anderson, B. J. The mental health comorbidities of diabetes. JAMA 312 , 691–692 (2014).
Fugger, G. et al. Comorbid thyroid disease in patients with major depressive disorder—results from the European Group for the Study of Resistant Depression (GSRD). Eur. Neuropsychopharmacol. 28 , 752–760 (2018).
Iosifescu, D. V. et al. The impact of medical comorbidity on acute treatment in major depressive disorder. Am. J. Psychiatry 160 , 2122–2127 (2003).
Oslin, D. W. et al. Association between medical comorbidity and treatment outcomes in late-life depression. J. Am. Geriatr. Soc. 50 , 823–828 (2002).
Amital, D. et al. Physical co-morbidity among treatment resistant vs. treatment responsive patients with major depressive disorder. Eur. Neuropsychopharmacol. 23 , 895–901 (2013).
Karp, J. F. et al. Pain predicts longer time to remission during treatment of recurrent depression. J. Clin. Psychiatry 66 , 591–597 (2005).
Ohayon, M. M. & Schatzberg, A. F. Using chronic pain to predict depressive morbidity in the general population. Arch. Gen. Psychiatry 60 , 39–47 (2003).
Racine, M. Chronic pain and suicide risk: A comprehensive review. Prog. Neuropsychopharmacol. Biol. Psychiatry 87 , 269–280 (2018).
Bogner, H. R. et al. The role of medical comorbidity in outcome of major depression in primary care: the PROSPECT study. Am. J. Geriatr. Psychiatry 13 , 861–868 (2005).
MacQueen, G. M., Yucel, K., Taylor, V. H., Macdonald, K. & Joffe, R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol. Psychiatry 64 , 880–883 (2008).
Phillips, J. L., Batten, L. A., Tremblay, P., Aldosary, F. & Blier, P. A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment-resistant depression. Int. J. Neuropsychopharmacol. / Off. Sci. J. Coll. Int. Neuropsychopharmacol. 18 , pyv037 (2015).
Frodl, T. et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J. Psychiatry Neurosci. 33 , 423–430 (2008).
PubMed PubMed Central Google Scholar
Fu, C. H., Steiner, H. & Costafreda, S. G. Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol. Dis. 52 , 75–83 (2013).
Frodl, T. et al. Reduced hippocampal volumes associated with the long variant of the serotonin transporter polymorphism in major depression. Arch. Gen. Psychiatry 61 , 177–183 (2004).
Vythilingam, M. et al. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biol. Psychiatry 56 , 101–112 (2004).
Schmaal, L. et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry 22 , 900–909 (2017).
Wilkinson, S. T., Sanacora, G. & Bloch, M. H. Hippocampal volume changes following electroconvulsive therapy: a systematic review and meta-analysis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2 , 327–335 (2017).
Nordanskog, P. et al. Increase in hippocampal volume after electroconvulsive therapy in patients with depression: a volumetric magnetic resonance imaging study. J. ECT 26 , 62–67 (2010).
Dukart, J. et al. Electroconvulsive therapy-induced brain plasticity determines therapeutic outcome in mood disorders. Proc. Natl Acad. Sci. USA 111 , 1156–1161 (2014).
Gryglewski, G. et al. Structural changes in amygdala nuclei, hippocampal subfields and cortical thickness following electroconvulsive therapy in treatment-resistant depression: longitudinal analysis. Br. J. Psychiatr 214 , 159–167 (2019).
Oltedal, L. et al. Volume of the human hippocampus and clinical response following electroconvulsive therapy. Biol. Psychiatry 84 , 574–581 (2018).
Drysdale, A. T. et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 23 , 28–38 (2017).
Dunlop, B. W. et al. Functional connectivity of the subcallosal cingulate cortex and differential outcomes to treatment with cognitive-behavioral therapy or antidepressant medication for major depressive disorder. Am. J. Psychiatry 174 , 533–545 (2017).
Pizzagalli, D. A. et al. Pretreatment rostral anterior cingulate cortex theta activity in relation to symptom improvement in depression: A randomized clinical trial. JAMA Psychiatry 75 , 547–554 (2018).
Gryglewski, G., Lanzenberger, R., Kranz, G. S. & Cumming, P. Meta-analysis of molecular imaging of serotonin transporters in major depression. J. Cereb. Blood Flow. Metab. 34 , 1096–1103 (2014).
Spies, M., Knudsen, G. M., Lanzenberger, R. & Kasper, S. The serotonin transporter in psychiatric disorders: insights from PET imaging. Lancet Psychiatry 2 , 743–755 (2015).
Wang, L. et al. Serotonin-1A receptor alterations in depression: a meta-analysis of molecular imaging studies. BMC Psychiatry 16 , 319 (2016).
Miller, J. M. et al. Brain serotonin 1A receptor binding as a predictor of treatment outcome in major depressive disorder. Biol. Psychiatry 74 , 760–767 (2013).
Miller, J. M., Oquendo, M. A., Ogden, R. T., Mann, J. J. & Parsey, R. V. Serotonin transporter binding as a possible predictor of one-year remission in major depressive disorder. J. Psychiatr. Res. 42 , 1137–1144 (2008).
Lanzenberger, R. et al. Prediction of SSRI treatment response in major depression based on serotonin transporter interplay between median raphe nucleus and projection areas. NeuroImage 63 , 874–881 (2012).
Drevets, W. C. Neuroimaging studies of mood disorders. Biol. Psychiatry 48 , 813–829 (2000).
Kennedy, S. H. et al. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am. J. Psychiatry 164 , 778–788 (2007).
Setiawan, E. et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72 , 268–275 (2015).
Richards, E. M. et al. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res. 8 , 57 (2018).
Holmes, S. E. et al. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biol. Psychiatry 83 , 61–69 (2018).
Wium-Andersen, M. K., Orsted, D. D. & Nordestgaard, B. G. Elevated plasma fibrinogen, psychological distress, antidepressant use, and hospitalization with depression: two large population-based studies. Psychoneuroendocrinology 38 , 638–647 (2013).
Jha, M. K. et al. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology 78 , 105–113 (2017).
Kohler-Forsberg, O. et al. Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression. Brain Behav. Immun. 62 , 344–350 (2017).
Strawbridge, R. et al. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 25 , 1532–1543 (2015).
Lamers, F. et al. Serum proteomic profiles of depressive subtypes. Transl. Psychiatry 6 , e851 (2016).
Kadriu, B. et al. Acute ketamine administration corrects abnormal inflammatory bone markers in major depressive disorder. Mol. Psychiatry 23 , 1626–1631 (2017).
Machado-Vieira, R. et al. The role of adipokines in the rapid antidepressant effects of ketamine. Mol. Psychiatry 22 , 127–133 (2017).
Schmidt, H. D., Shelton, R. C. & Duman, R. S. Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology 36 , 2375–2394 (2011).
Molendijk, M. L. et al. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N = 9484). Mol. Psychiatry 19 , 791–800 (2014).
Brunoni, A. R., Baeken, C., Machado-Vieira, R., Gattaz, W. F. & Vanderhasselt, M. A. BDNF blood levels after electroconvulsive therapy in patients with mood disorders: a systematic review and meta-analysis. World J. Biol. Psychiatry 15 , 411–418 (2014).
Polyakova, M. et al. BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. J. Affect. Disord. 174 , 432–440 (2015).
Wray, N. R. et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 50 , 668–681 (2018).
Fabbri, C. et al. Consensus paper of the WFSBP Task Force on Genetics: Genetics, epigenetics and gene expression markers of major depressive disorder and antidepressant response. World J. Biol. Psychiatry 18 , 5–28 (2017).
Kautzky, A. et al. The combined effect of genetic polymorphisms and clinical parameters on treatment outcome in treatment-resistant depression. Eur. Neuropsychopharmacol. 25 , 441–453 (2015).
Stamm, T. J. et al. The FKBP5 polymorphism rs1360780 influences the effect of an algorithm-based antidepressant treatment and is associated with remission in patients with major depression. J. Psychopharmacol. 30 , 40–47 (2016).
Binder, E. B. et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 36 , 1319–1325 (2004).
Fabbri, C. et al. Pleiotropic genes in psychiatry: Calcium channels and the stress-related FKBP5 gene in antidepressant resistance. Prog. Neuropsychopharmacol. Biol. Psychiatry 81 , 203–210 (2018).
Klengel, T. & Binder, E. B. Gene x environment interactions in the prediction of response to antidepressant treatment. Int. J. Neuropsychopharmacol./Off. Sci. J. Coll. Int. Neuropsychopharmacol. 16 , 701–711 (2013).
CAS Google Scholar
Schosser, A. & Kasper, S. The role of pharmacogenetics in the treatment of depression and anxiety disorders. Int. Clin. Psychopharmacol. 24 , 277–288 (2009).
Zeier, Z. et al. Clinical implementation of pharmacogenetic decision support tools for antidepressant drug prescribing. Am. J. Psychiatry 175 , 873–886 (2018).
Jukic, M. M., Haslemo, T., Molden, E. & Ingelman-Sundberg, M. Impact of CYP2C19 genotype on escitalopram exposure and therapeutic failure: A retrospective study based on 2,087 patients. Am. J. Psychiatry 175 , 463–470 (2018).
Bauer, M. et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders, part 1: update 2013 on the acute and continuation treatment of unipolar depressive disorders. World J. Biol. Psychiatry 14 , 334–385 (2013).
Uher, R. et al. Melancholic, atypical and anxious depression subtypes and outcome of treatment with escitalopram and nortriptyline. J. Affect. Disord. 132 , 112–120 (2011).
Arnow, B. A. et al. Depression subtypes in predicting antidepressant response: A report from the iSPOT-D trial. Am. J. Psychiatry 172 , 743–750 (2015).
Kasper, S. & Montgomery, S. A. Ohio Library and Information Network, Wiley Online Library (Online service). Treatment-resistant Depression , 1 online resource (2013).
Schosser, A. et al. European Group for the Study of Resistant Depression (GSRD)–where have we gone so far: review of clinical and genetic findings. Eur. Neuropsychopharmacol. 22 , 453–468 (2012).
Bergfeld, I. O. et al. Treatment-resistant depression and suicidality. J. Affect. Disord. 235 , 362–367 (2018).
Mann, J. J. et al. Suicide prevention strategies: a systematic review. JAMA 294 , 2064–2074 (2005).
Nimalasuriya, K., Compton, M. T. & Guillory, V. J., Prevention Practice Committee of the American College of Preventive M. Screening adults for depression in primary care: A position statement of the American College of Preventive Medicine. J. Fam. Pract. 58 , 535–538 (2009).
PubMed Google Scholar
Kroenke, K., Spitzer, R. L. & Williams, J. B. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med. Care 41 , 1284–1292 (2003).
Horowitz, L. M. et al. Ask suicide-screening questions to everyone in medical settings: the asQ’em Quality Improvement Project. Psychosomatics 54 , 239–247 (2013).
King, M. et al. Predicting onset of major depression in general practice attendees in Europe: extending the application of the predictD risk algorithm from 12 to 24 months. Psychol. Med. 43 , 1929–1939 (2013).
Kupfer, D. J., Frank, E. & Perel, J. M. The advantage of early treatment intervention in recurrent depression. Arch. Gen. Psychiatry 46 , 771–775 (1989).
Lopizzo, N. et al. Gene-environment interaction in major depression: focus on experience-dependent biological systems. Front. Psychiatry 6 , 68 (2015).
Gutierrez, B. et al. The risk for major depression conferred by childhood maltreatment is multiplied by BDNF and SERT genetic vulnerability: a replication study. J. Psychiatry Neurosci.: JPN 40 , 187–196 (2015).
Serretti, A. et al. The impact of adverse life events on clinical features and interaction with gene variants in mood disorder patients. Psychopathology 46 , 384–389 (2013).
Herbison, C. E., Allen, K., Robinson, M., Newnham, J. & Pennell, C. The impact of life stress on adult depression and anxiety is dependent on gender and timing of exposure. Dev. Psychopathol. 29 , 1443–1454 (2017).
Keers, R. & Uher, R. Gene-environment interaction in major depression and antidepressant treatment response. Curr. Psychiatry Rep. 14 , 129–137 (2012).
Gold, P. W. & Chrousos, G. P. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol. Psychiatry 7 , 254–275 (2002).
Carroll, B. J. et al. A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch. Gen. Psychiatry 38 , 15–22 (1981).
Musil, R. et al. Subtypes of depression and their overlap in a naturalistic inpatient sample of major depressive disorder. Int. J. Methods Psychiatr. Res . 27 (2018).
Angst J., Gamma A., Benazzi F., Ajdacic V., Rossler W. Melancholia and atypical depression in the Zurich study: epidemiology, clinical characteristics, course, comorbidity and personality. Acta Psychiatr. Scand. Suppl. 72–84 (2007).
Ionescu, D. F., Niciu, M. J., Henter, I. D. & Zarate, C. A. Defining anxious depression: a review of the literature. CNS Spectr. 18 , 252–260 (2013).
Lamers, F. et al. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry 18 , 692–699 (2013).
Simmons, W. K. et al. Appetite changes reveal depression subgroups with distinct endocrine, metabolic, and immune states. Mol. Psychiatry , https://doi.org/10.1038/s41380-018-0093-6 (2018).
Woody, M. L. & Gibb, B. E. Integrating NIMH research domain criteria (RDoC) into depression. Res. Curr. Opin. Psychol. 4 , 6–12 (2015).
Ballard, E. D. et al. Parsing the heterogeneity of depression: An exploratory factor analysis across commonly used depression rating scales. J. Affect. Disord. 231 , 51–57 (2018).
Ruhe, H. G., van Rooijen, G., Spijker, J., Peeters, F. P. & Schene, A. H. Staging methods for treatment resistant depression. A systematic review. J. Affect. Disord. 137 , 35–45 (2012).
Nugent, A. C. et al. Safety of research into severe and treatment-resistant mood disorders: analysis of outcome data from 12 years of clinical trials at the US National Institute of Mental Health. Lancet Psychiatry 3 , 436–442 (2016).
Herzog, D. P. et al. Guideline adherence of antidepressant treatment in outpatients with major depressive disorder: a naturalistic study. Eur. Arch. Psychiatry Clin. Neurosci. 267 , 711–721 (2017).
Trivedi, M. H. et al. Clinical results for patients with major depressive disorder in the Texas Medication Algorithm Project. Arch. Gen. Psychiatry 61 , 669–680 (2004).
Adli, M. et al. How effective is algorithm-guided treatment for depressed inpatients? results from the randomized controlled multicenter german algorithm project 3 trial. Int J. Neuropsychopharmacol. 20 , 721–730 (2017).
Bauer, M. et al. Efficacy of an algorithm-guided treatment compared with treatment as usual: a randomized, controlled study of inpatients with depression. J. Clin. Psychopharmacol. 29 , 327–333 (2009).
Ricken, R. et al. Algorithm-guided treatment of depression reduces treatment costs–results from the randomized controlled German Algorithm Project (GAPII). J. Affect. Disord. 134 , 249–256 (2011).
Khan, A. & Brown, W. A. Antidepressants versus placebo in major depression: an overview. World Psychiatry 14 , 294–300 (2015).
Fava, M., Evins, A. E., Dorer, D. J. & Schoenfeld, D. A. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother. Psychosom. 72 , 115–127 (2003).
Enck, P., Bingel, U., Schedlowski, M. & Rief, W. The placebo response in medicine: minimize, maximize or personalize? Nat. Rev. Drug Discov. 12 , 191–204 (2013).
Desseilles, M. et al. Massachusetts general hospital SAFER criteria for clinical trials and research. Harv. Rev. Psychiatry 21 , 269–274 (2013).
Finniss, D. G., Kaptchuk, T. J., Miller, F. & Benedetti, F. Biological, clinical, and ethical advances of placebo effects. Lancet 375 , 686–695 (2010).
Henter, I. D., de Sousa, R. T. & Zarate, C. A. Jr. Glutamatergic modulators in depression. Harv. Rev. Psychiatry 26 , 307–319 (2018).
Ionescu, D. F. & Papakostas, G. I. Current trends in identifying rapidly acting treatments for depression. Curr. Behav. Neurosci. Rep. 3 , 185–191 (2016).
Kadriu, B. et al. Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int. J. Neuropsychopharmacol. / Off. Sci. J. Coll. Int. Neuropsychopharmacol. 22 , 119–135 (2018).
Zanos, P. et al. Convergent mechanisms underlying rapid antidepressant action. CNS Drugs 32 , 197–227 (2018).
Li, N. et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329 , 959–964 (2010).
Autry, A. E. et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475 , 91–95 (2011).
Berman, R. M. et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47 , 351–354 (2000).
Duman, R. S. & Aghajanian, G. K. Synaptic dysfunction in depression: potential therapeutic targets. Science 338 , 68–72 (2012).
Diazgranados, N. et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J. Clin. Psychiatry 71 , 1605–1611 (2010).
Iadarola, N. D. et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther. Adv. Chronic Dis. 6 , 97–114 (2015).
Ibrahim, L. et al. Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Progress. neuro-Psychopharmacol. Biol. Psychiatry 35 , 1155–1159 (2011).
Zarate, C. A. et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63 , 856–864 (2006).
Diazgranados, N. et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry 67 , 793–802 (2010).
Zarate, C. A. et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol. Psychiatry 71 , 939–946 (2012).
aan het Rot, M. et al. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatry 67 , 139–145 (2010).
Wan, L. B. et al. Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J. Clin. Psychiatry 76 , 247–252 (2015).
Murrough, J. W. et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol. Psychiatry 74 , 250–256 (2013).
Murrough, J. W. et al. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol. Med. 45 , 3571–3580 (2015).
Price, R. B., Nock, M. K., Charney, D. S. & Mathew, S. J. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry 66 , 522–526 (2009).
Wilkinson, S. T. et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am. J. Psychiatry 175 , 150–158 (2018).
Daly, E. J. et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry 75 , 139–148 (2018).
Canuso, C. M. et al. Efficacy and safety of intranasal esketamine for the rapid reduction of symptoms of depression and suicidality in patients at imminent risk for suicide: results of a double-blind, randomized, placebo-controlled study. Am. J. Psychiatry 175 , 620–630 (2018).
Zanos, P. et al. The prodrug 4-chlorokynurenine causes ketamine-like antidepressant effects, but not side effects, by NMDA/glycineB-site inhibition. J. Pharmacol. Exp. Ther. 355 , 76–85 (2015).
Wilkinson, S. T. & Sanacora, G. A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov. Today 24 , 606–615 (2018).
Article PubMed CAS PubMed Central Google Scholar
Vollenweider, F. X. & Kometer, M. The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat. Rev. Neurosci. 11 , 642–651 (2010).
Carhart-Harris, R. L. et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. Lancet Psychiatry 3 , 619–627 (2016).
Karp, J. F. et al. Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adults. J. Clin. Psychiatry 75 , e785–e793 (2014).
Fava, M. et al. Opioid modulation with buprenorphine/samidorphan as adjunctive treatment for inadequate response to antidepressants: A randomized double-blind placebo-controlled trial. Am. J. Psychiatry 173 , 499–508 (2016).
Preskorn, S. et al. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J. Psychiatr. Pract. 21 , 140–149 (2015).
Garay, R. P. et al. Investigational drugs in recent clinical trials for treatment-resistant depression. Expert Rev. Neurother. 17 , 593–609 (2017).
Kolbinger, H. M., Hoflich, G., Hufnagel, A., Moller, H. J. & Kasper, S. Transcranial magnetic stimulation (TMS) in the treatment of major depression—a pilot study. Human. Psychopharmacol. 10 , 305–310 (1995).
Lisanby, S. H. et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacol.: Off. Publ. Am. Coll. Neuropsychopharmacol. 34 , 522–534 (2009).
Brunoni, A. R. et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: A systematic review with network meta-analysis. JAMA Psychiatry 74 , 143–152 (2017).
Benadhira, R. et al. A randomized, sham-controlled study of maintenance rTMS for treatment-resistant depression (TRD). Psychiatry Res 258 , 226–233 (2017).
Cusin, C. & Dougherty, D. D. Somatic therapies for treatment-resistant depression: ECT, TMS, VNS, DBS. Biol. Mood Anxiety Disord. 2 , 14 (2012).
Blumberger, D. M. et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet 391 , 1683–1692 (2018).
Fava, M. Diagnosis and definition of treatment-resistant depression. Biol. Psychiatry 53 , 649–659 (2003).
Husain, M. M. et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J. Clin. Psychiatry 65 , 485–491 (2004).
Kellner, C. H. et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am. J. Psychiatry 162 , 977–982 (2005).
Slade, E. P., Jahn, D. R., Regenold, W. T. & Case, B. G. Association of electroconvulsive therapy with psychiatric readmissions in US hospitals. JAMA Psychiatry 74 , 798–804 (2017).
Kellner, C. H. et al. Right unilateral ultrabrief pulse ECT in geriatric depression: phase 1 of the PRIDE study. Am. J. Psychiatry 173 , 1101–1109 (2016).
McClintock, S. M. et al. Multifactorial determinants of the neurocognitive effects of electroconvulsive therapy. J. ECT 30 , 165–176 (2014).
Fink, M. & Taylor, M. A. Electroconvulsive therapy: evidence and challenges. JAmA 298 , 330–332 (2007).
American Psychiatric Association. Task Force on Electroconvulsive Therapy. The practice of ECT: recommendations for treatment, training and privileging. Convuls. Ther. 6 , 85–120 (1990).
Kellner, C. H. et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br. J. Psychiatry.: J. Ment. Sci. 196 , 226–234 (2010).
Wilkinson, S. T., Agbese, E., Leslie, D. L. & Rosenheck, R. A. Identifying recipients of electroconvulsive therapy: data from privately insured Americans. Psychiatr. Serv. 69 , 542–548 (2018).
Lisanby, S. H., Schlaepfer, T. E., Fisch, H. U. & Sackeim, H. A. Magnetic seizure therapy of major depression. Arch. Gen. Psychiatry 58 , 303–305 (2001).
Deng, Z. -D., Lisanby, S. H. & Peterchev, A. V. Electric field strength and focality in electroconvulsive therapy and magnetic seizure therapy: a finite element simulation study. J. Neural Eng. 8 , 016007 (2011).
Fitzgerald, P. B. et al. Pilot study of the clinical and cognitive effects of high-frequency magnetic seizure therapy in major depressive disorder. Depress Anxiety 30 , 129–136 (2013).
Kayser, S. et al. Magnetic seizure therapy in treatment-resistant depression: clinical, neuropsychological and metabolic effects. Psychol. Med. 45 , 1073–1092 (2015).
Nahas, Z. et al. Two-year outcome of vagus nerve stimulation (VNS) for treatment of major depressive episodes. J. Clin. Psychiatry 66 , 1097–1104 (2005).
Aaronson, S. T. et al. A 5-Year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: comparison of response, remission, and suicidality. Am. J. Psychiatry 174 , 640–648 (2017).
Schlaepfer, T. E., Bewernick, B. H., Kayser, S., Madler, B. & Coenen, V. A. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol. Psychiatry 73 , 1204–1212 (2013).
Bewernick, B. H. et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol. Psychiatry 67 , 110–116 (2010).
Bergfeld, I. O. et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: A randomized clinical trial. JAMA Psychiatry 73 , 456–464 (2016).
Dougherty, D. D. et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol. Psychiatry 78 , 240–248 (2015).
Holtzheimer, P. E. et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry 4 , 839–849 (2017).
Widge, A. S., Malone, D. A. Jr. & Dougherty, D. D. Closing the loop on deep brain stimulation for treatment-resistant depression. Front Neurosci. 12 , 175 (2018).
Jain, K. K. in The Handbook of Biomarkers . 1–26 (Springer New York, New York, NY, 2017).
Zohar, J. et al. A review of the current nomenclature for psychotropic agents and an introduction to the Neuroscience-based Nomenclature. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 25 , 2318–2325 (2015).
Dold, M. & Kasper, S. Evidence-based pharmacotherapy of treatment-resistant unipolar depression. Int J. Psychiatry Clin. Pract. 21 , 13–23 (2017).
Colle, R. et al. BDNF/TRKB/P75NTR polymorphisms and their consequences on antidepressant efficacy in depressed patients. Pharmacogenomics 16 , 997–1013 (2015).
Porcelli, S., Fabbri, C. & Serretti, A. Meta-analysis of serotonin transporter gene promoter polymorphism (5-HTTLPR) association with antidepressant efficacy. Eur. Neuropsychopharmacol.: J. Eur. Coll. Neuropsychopharmacol. 22 , 239–258 (2012).
Download references
Acknowledgements
We thank the 7SE research unit and staff for their support. Ioline Henter (NIMH) provided invaluable editorial assistance. We also thank E. Acevedo-Diaz, Z.D. Deng, and J.W. Evans for scientific input.
Author information
Authors and affiliations.
Department of Psychiatry and Psychotherapy, Medical University of Vienna, Vienna, Austria
Christoph Kraus, Rupert Lanzenberger & Siegfried Kasper
Section on Neurobiology and Treatment of Mood Disorders, Intramural Research Program, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, USA
Christoph Kraus, Bashkim Kadriu & Carlos A. Zarate Jr.
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Siegfried Kasper .
Ethics declarations
Conflict of interest.
Funding for this work was supported in part by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA MH002927). All support given to authors was not related to the design of the manuscript or the ideas stated in this review. Dr. Kasper received grants/research support, consulting fees, and/or honoraria within the last 3 years from Angelini, AOP Orphan Pharmaceuticals AG, AstraZeneca, Eli Lilly, Janssen, KRKA-Pharma, Lundbeck, Neuraxpharm, Pfizer, Pierre Fabre, Schwabe, and Servier. Dr. Lanzenberger received travel grants and/or conference speaker honoraria from AstraZeneca, Lundbeck A/S, Dr. Willmar Schwabe GmbH, Orphan Pharmaceuticals AG, Janssen-Cilag Pharma GmbH, and Roche Austria GmbH. Dr. Kraus has received travel grants from Roche Austria GmbH and AOP Orphan. Dr. Zarate is a full-time U.S government employee. He is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2 R ,6 R )-hydroxynorketamine, ( S )-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of ( R,S )-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2 R ,6 R )-hydroxynorketamine and (2 S ,6 S )-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kraus, C., Kadriu, B., Lanzenberger, R. et al. Prognosis and improved outcomes in major depression: a review. Transl Psychiatry 9 , 127 (2019). https://doi.org/10.1038/s41398-019-0460-3
Download citation
Received : 07 November 2018
Revised : 10 January 2019
Accepted : 11 February 2019
Published : 03 April 2019
DOI : https://doi.org/10.1038/s41398-019-0460-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Cannabinoid type 2 receptor inhibition enhances the antidepressant and proneurogenic effects of physical exercise after chronic stress.
- R. S. Rodrigues
- J. B. Moreira
Translational Psychiatry (2024)
Developing an individualized treatment rule for Veterans with major depressive disorder using electronic health records
- Nur Hani Zainal
- Robert M. Bossarte
- Ronald C. Kessler
Molecular Psychiatry (2024)
Multiomics and blood-based biomarkers of electroconvulsive therapy in severe and treatment-resistant depression: study protocol of the DetECT study
- Iven-Alex von Mücke-Heim
- Julius C. Pape
- Elisabeth B. Binder
European Archives of Psychiatry and Clinical Neuroscience (2024)
Perspectives in treatment-resistant depression: esketamine and electroconvulsive therapy
- Pia Baldinger-Melich
- Marie Spies
- Richard Frey
Wiener klinische Wochenschrift (2024)
Functional and structural alterations in different durations of untreated illness in the frontal and parietal lobe in major depressive disorder
- Xiaowei Jiang
- Yanqing Tang
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Call for papers
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 36, Issue 6
- Acute effect of twice-daily 15 mA transcranial alternating current stimulation on treatment-resistant depression: a case series study
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Wenfeng Zhao 1 ,
- Huang Wang 1 ,
- Haixia Leng 1 ,
- Qing Xue 1 ,
- Mao Peng 1 ,
- Xiukun Jin 1 ,
- Liucen Tan 1 ,
- Xuedi Wang 1 ,
- Jie Wang 2 , 3 ,
- Keming Gao 4 ,
- http://orcid.org/0000-0003-3326-382X Xiangyang Zhang 5 and
- http://orcid.org/0000-0002-7942-9330 Hongxing Wang 1 , 6 , 7
- 1 Department of Neurology, Beijing Psychosomatic Disease Consultation Center, National Center for Neurological Disorders, National Clinical Research Center for Geriatric Diseases , Xuanwu Hospital Capital Medical University , Beijing , China
- 2 Key Laboratory of Magnetic Resonance in Biological Systems, State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, National Center for Magnetic Resonance in Wuhan, Wuhan Institute of Physics and Mathematics, Innovation Academy for Precision Measurement Science and Technology , Chinese Academy of Sciences-Wuhan National Laboratory for Optoelectronics , Wuhan , China
- 3 University of the Chinese Academy of Sciences , Beijing , China
- 4 Department of Psychiatry , University Hospitals Cleveland Medical Center , Cleveland , Ohio , USA
- 5 CAS Key Laboratory of Mental Health , Chinese Academy of Sciences , Beijing , China
- 6 Beijing Institute for Brain Disorders , Beijing , China
- 7 Institute of Special Medical Sciences, School of Forensic Medicine , Shanxi Medical University , Taiyuan , China
- Correspondence to Hongxing Wang; hxwang8888{at}126.com
https://doi.org/10.1136/gpsych-2023-101278
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
- depressive disorder, treatment-resistant
- neuropsychiatry
WHAT IS ALREADY KNOWN ON THIS TOPIC
Depression is a major cause of disability worldwide, and about one third of depressive patients have treatment-resistant depression (TRD). New treatments for depression that are safe, tolerable, and effective are urgently needed.
WHAT THIS STUDY ADDS
Our case series study found that 40 sessions of twice-daily 15 mA, 77.5 Hz transcranial alternating current stimulation (tACS) via the forehead and both mastoids given 5 days a week during a 4-week treatment offers therapeutic potential for the acute treatment of TRD by reducing the severity of the depressive symptoms.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
More exploration of twice-daily 15 mA tACS for TRD in large clinical studies could strengthen our preliminary results indicating that it is a promising alternative intervention with practical treatment implications for TRD.
Introduction
Major depressive disorder (MDD) is a principal cause of disability worldwide and is often associated with high morbidity and mortality rates. Although there are several therapies available for the treatment of depression, about one-third of patients with MMD will not respond to two or more antidepressant drugs with different mechanisms; the patients are then referred to as having treatment-resistant depression (TRD). 1 Patients with TRD have a poorer quality of life, greater economic burden and increased suicidal behaviours. 1 Therefore, new antidepressant treatments that are effective, safe, long-lasting and tolerable are needed. Ketamine infusion, intranasal esketamine and transcranial magnetic stimulation (TMS) have been used to treat early stage TRD. 2 A recent review suggests that electroconvulsive therapy (ECT) may be superior to ketamine for reducing depression severity in the acute treatment of TRD. 3 Another review found that ECT was more effective in treating TRD than repetitive TMS (rTMS). 1
Similar to the electrical current used in ECT, alternating current is also utilised in transcranial alternating current stimulation (tACS), 8 a unique form of non-invasive brain stimulation. tACS has shown efficacy and safety in chronic insomnia, 9 MDD 7 and other treatment-resistant psychiatric conditions such as clozapine-resistant schizophrenia and treatment-resistant obsessive-compulsive disorder. 10 Currently, however, there is no consensus on tACS procedures and parameters for different brain disorders in clinical practice and research. 7 11 The electrical current frequency and amplitude, treatment sessions and electrode locations have varied widely. 7 11 One of our recent studies revealed that a single session of 77.5 Hz tACS with a current amplitude of 15 mA via the forehead and both mastoids given 5 days a week for 4 weeks, totalling 20 sessions, (referred to as a once-daily protocol) is effective in reducing depressive symptoms in first episode and drug-naïve MDD. 7 However, whether15 mA, 77.5 Hz tACS is effective for treating TRD remains unclear.
Due to the refractory nature of TRD, we hypothesised that more sessions of tACS may effectively treat TRD. Therefore, in this initial study, we used the same amplitude and frequency of tACS as our previous studies 7 but changed the treatment course from once a day to twice a day. In doing so, the patients received 40 sessions (twice-daily tACS), instead of 20 sessions, during the 4-week treatment phase. Accordingly, this study aimed to examine the acute therapeutic potential of twice-daily tACS in TRD.
Study design
Our study was a 4-week case series prospective study performed at Xuanwu Hospital, Capital Medical University in Beijing, China between September 2019 and January 2022. It comprised a 2-week screening/baseline period followed by a 4-week treatment phase of twice-daily tACS (twice per day, 5 days a week).
The outcome assessments were conducted by blinded raters at baseline and week 4. Assessment scales include the 17-item Hamilton Rating Scale for Depression (HAMD-17), 7 the Montgomery-Åsberg Depression Rating Scale (MADRS), 12 an 18-item common questionnaire on cranial electrical stimulation made by our study group, 7 and the Young Mania Rating Scale (YMRS). 7
Participants
Patients were enrolled from the outpatient clinic of Xuanwu Hospital, Capital Medical University. The inclusion criteria were: (1) male or female, aged 18–65 years; (2) able to provide written informed consent voluntarily; (3) a diagnosis of MDD (recurrent episodes) without psychotic features in compliance with the criteria from the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition-Text Revised (DSM-IV-TR) and confirmed by the Mini-International Neuropsychiatric Interview, Chinese V.5.0; (4) the depressive episode duration ≥2 years; (5) failure of response to at least two antidepressant medication trials based on the Massachusetts General Hospital Antidepressant Treatment Response Questionnaire; (6) ongoing antidepressant treatment at a fixed dose for at least 8 weeks prior to baseline assessment; (7) a baseline score of >20 on HAMD-17.
Exclusion criteria were: (1) axis I psychiatric disorders, including schizophrenia, bipolar disorder, manic episode, anxiety disorders (panic disorder, generalised anxiety disorder and social anxiety disorder), post-traumatic stress disorder, obsessive compulsive disorder, anorexia nervosa, bulimia nervosa, psychosis over the previous 6 months and any axis II disorders (borderline personality disorder, antisocial personality disorder, schizotypal personality disorder and narcissistic personality disorder); (2) a treatment history of ECT, modified ECT, transcranial direct current stimulation, tACS, deep brain stimulation, or TMS; (3) risk for suicide (defined as a score of ≥3 on the suicide item of HAMD-17); (4) known allergy to electrode materials; (5) inability to communicate with researchers fluently; (6) traumatic brain injury; (7) cerebrovascular or cardiovascular stents; (8) substance use disorder (abuse or dependence, as defined by DSM-IV-TR) in the previous 6 months; (9) females who are pregnant, breast feeding or have the potential for childbearing but refuse to use reliable contraceptive methods during the study.
At baseline, demographic and clinical data on study participants were collected. Depressive symptom severity was assessed with the Chinese versions of HAMD-17 7 and MADRS 12 at baseline and week 4. Similarly, an 18-item common questionnaire on cranial electrical stimulation 7 and the YMRS 7 were administered and a 30 min electroencephalogram (EEG) with an internationally recognised 10/20 system of electrode placement method was carried out to monitor safety and identify manic/hypomanic symptoms (defined as a YMRS score >8) and potential epileptiform discharges at the 4-week efficacy assessment. The EEG was recorded by two experienced EEG technicians.
Twice-daily 15 mA tACS intervention
The tACS procedure was similar to that published in our recent report. 7 Briefly, patients sat in a comfortable chair quietly while receiving Chinese National Medical Products Administration-approved tACS (Nexalin Technology). Three Nexalin conductive electrodes were attached to the scalp. One 4.45 cm×9.53 cm electrode was placed on the scalp on the forehead, equated to Fpz, Fp1 and Fp2. The other two 3.18 cm×3.81 cm electrodes were put on each side of the mastoid area. All participants were scheduled to receive 40 sessions with stimulation at 77.5 Hz and 15 mA during four consecutive weeks. The stimulation was provided twice daily (09:00–11:00 and 14:00–17:00) from Monday to Friday, and each session lasted 40 min.
Permitted medication
Patients were required to continue taking their ongoing antidepressants throughtout the treatment period. Their medication history is shown in online supplemental table 1 .
Supplemental material
Data analysis.
Data were described as the mean and standard deviation (SD) for continuous variables. The percentage was used for the categorical variable. The paired sample Wilcoxon signed rank tests were used to determine the significance of changes from baseline to week 4. Statistical analyses were carried out by IBM SPSS Statistics V.26.0 (SPSS). The significance level was set at p<0.05.
Patient characteristics
A total of 45 participants were screened for eligibility. Thirty-eight were excluded (34 did not meet eligibility criteria, and 4 declined to participate in the study). Seven were eligible for the study and were all female ( online supplemental figure 1 ). The mean (SD) age was 60.6 (5.6) years. The cumulative disease duration was 6.0 (1.9) years. The demographic and clinical features of the study subjects are illustrated in table 1 .
- View inline
Clinical characteristics and outcomes of study participants
Clinical outcomes
HAMD-17 total scores were significantly lower at week 4 than baseline (Z=−2.410, p = 0.016), as shown in table 1 and figure 1A . The changes in the MADRS total scores were also significant, as shown in table 1 (Z=−2.414, p=0.016). All patients achieved a response (defined as HAMD-17/MADRS scores that decreased by 50% or more from the baseline), but no patients achieved remission (defined as HAMD-17 score ≤7) at week 4, as shown in table 1 .
- Download figure
- Open in new tab
- Download powerpoint
Clinical outcomes. (A) The mean HAMD-17 total scores significantly decreased from baseline to week 4. The error bars represent the SD. The asterisk (*) indicates a statistically significant difference with p<0.05. (B) Total YMRS scores at baseline and week 4. HAMD-17, 17-item Hamilton Rating Scale for Depression; No. number; SD, standard deviation; YMRS, Young Mania Rating Scale.
There were no serious complications with the stimulation in this case series study. The two patients had non-serious adverse reactions including headache (patient 1), tinnitus cerebri (patient 1), dizziness (patient 2) and flickering (patient 1) during the twice-daily tACS treatment period. None of the subjects experienced manic symptoms, seizures, fatigue, itchiness or loss of consciousness throughout the duration of the treatment ( online supplemental table 2 and figure 1B ).
Main findings
To the best of our knowledge, the current study was the first to use twice-daily tACS to assess its acute therapeutic potential in patients with TRD. The safety results suggest that twice-daily 77.5 Hz, 15 mA tACS, 5 days a week for 4 weeks is safe and well-tolerated. The efficacy results indicate that the twice-daily tACS has an acute effect in reducing depressive symptoms in patients with TRD. The case series data support the conduction of large, randomised, sham-controlled trials of twice-daily tACS for treating patients with TRD and assessing its usefulness as adjunctive therapy for TRD.
Our study showed that all patients with TRD had a significant reduction in depression symptoms after a 4-week treatment, and all of them achieved a clinical response. Noteworthy, the duration for most clinical trials for depression is 6–8 weeks. Although it is difficult to compare the efficacy of twice-daily tACS with other adjunctive therapies for TRD due to the minimal sample size in the current study, our results indicate that twice-daily tACS may produce an acute effect in TRD.
In a STAR*D study, about 50% of remitters on citalopram achieved remission of depressive symptoms within 6 weeks of treatment; the other half of the remitters achieved remission only after 12 weeks of treatment. 13 14 Therefore, a longer duration of the twice-daily tACS for TRD might produce a larger reduction in depression symptoms. In the current study, no patients reached remission with a 4-week treatment, suggesting that the duration of future studies of twice-daily tACS for TRD should target longer than 4 weeks. A 3-week active versus sham study of TRD with a symmetrical rectangular biphasic current of 1–4 mA, 40 V stimulations over two dorsolateral prefrontal cortex areas did not show a significant difference between the two groups, 15 also supporting the importance of longer study durations when using cranial electrical stimulations.
Limitations
It is important to consider the limitations of this study when interpreting the results. First, there was no control group in our research, and it is not possible to exclude the possibility of a placebo effect entirely. In addition to a shorter study duration than most clinical trials, the small sample size of our case-series study does not allow us to generalise the acute effect we found to all patients with TRD. However, the study showed some strengths of twice-daily tACS, and the questionnaire on cranial electrical stimulation can be used in future studies.
Implications
This case series study showed that twice-daily 15mA tACS, a unique form of non-invasive brain stimulation, offers an acute effective intervention for patients with TRD. A large randomised, sham-controlled study of twice-daily tACS in patients with TRD is warranted to support the findings observed in this case series study.
Ethics statements
Patient consent for publication.
Consent obtained directly from patient(s).
Ethics approval
This study was approved by Xuanwu Hospital, Capital Medical University (LYS(2018)008). Participants gave informed consent to participate in the study before taking part.
- Crowell AL ,
- Riva-Posse P ,
- Holtzheimer PE , et al
- Davila MC ,
- Louviere A , et al
- Forester BP , et al
- Pérez-Balaguer A ,
- Sánchez-Rivero I
- Fairchild JK , et al
- Levkovitz Y ,
- Isserles M ,
- Padberg F , et al
- Xue Q , et al
- Yang Y , et al
- Zhang W-R , et al
- Elyamany O ,
- Herrmann CS , et al
- Alexander ML ,
- Alagapan S ,
- Lugo CE , et al
- Xiang Y-T ,
- Lei H , et al
- Trivedi MH ,
- Wisniewski SR , et al
- Mischoulon D ,
- De Jong MF ,
- Vitolo OV , et al
Wenfeng Zhao is currently a PhD student at Xuanwu Hospital, Capital Medical University in Beijing, China. She obtained her bachelor’s degree in 2019 and completed her master's study in 2022 from Capital Medical University in China. Her main research interests include non-pharmacological intervention in neuropsychiatric disorders and functional neurological disorders, as well as the neurobiological basis of mood disorders such as depression.
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
Contributors WZ and HW conceptualised the study and drafted the original manuscript. WZ, Huang W, HL, QX, MP, XJ, LT and NP interviewed the participants and collected the data. XW, JW, KG and XZ contributed to data analysis. KG and HW critically revised the manuscript. All authors reviewed and approved the final manuscript.
Funding The study was supported by the National Natural Science Foundation of China (82371490), the National Key R&D Program of China (2022YFC2503901, 2022YFC2503900), the Beijing Hundred, Thousand, and Ten Thousand Talents Project (2017-CXYF-09), and Beijing Health System Leading Talent Grant (2022-02-10).
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
- Case report
- Open access
- Published: 16 September 2021
The use of esketamine in comorbid treatment resistant depression and obsessive compulsive disorder following extensive pharmacogenomic testing: a case report
- Marcatili Matteo ORCID: orcid.org/0000-0002-1436-1961 1 , 2 ,
- Pellicioli Cristian 2 ,
- Maggioni Laura 2 ,
- Motta Federico 2 ,
- Redaelli Chiara 2 ,
- Ghelfi Lorenzo 3 ,
- Krivosova Michaela 4 ,
- Matteo Sibilla 2 ,
- Nava Roberto 1 ,
- Colmegna Fabrizia 1 ,
- Dakanalis Antonios 2 ,
- Caldiroli Alice 1 ,
- Capuzzi Enrico 1 ,
- Benatti Beatrice 5 , 6 ,
- Bertola Francesca 7 ,
- Villa Nicoletta 7 ,
- Piperno Alberto 2 , 7 ,
- Ippolito Silvia 8 &
- Clerici Massimo 1 , 2
Annals of General Psychiatry volume 20 , Article number: 43 ( 2021 ) Cite this article
5 Citations
2 Altmetric
Metrics details
Major depressive disorder (MDD) patients not responding to two or more different antidepressant treatments are currently considered to suffer from treatment resistant depression (TRD). Recently, intranasal esketamine has been approved by both the American Food and Drug Administration and European Medicines Agency for TRD and, more recently, in moderate to severe episode of MDD, as acute short-term treatment for the rapid reduction of depressive symptoms, which, according to clinical judgement, constitute a psychiatric emergency. There is currently no indication for obsessive–compulsive disorder (OCD) although recently published studies have already shown a rapid and significant reduction of OCD-like symptoms following ketamine administration. The etiology of OCD has not yet been fully elucidated but there is a growing evidence that glutamate signaling dysfunction in the cortico-striatal–thalamo-cortical circuitry plays an essential role. This case report exemplifies possible clinical effects of esketamine on both depressive and OCD symptoms.
Case presentation
We present the case of a 39-year-old man suffering from TRD. During the first evaluation at our clinic, he also reported the presence of OCD spectrum symptoms, causing him to perform time-consuming mental rituals due to pathological doubts regarding the relationship with his wife as well as intrusive thoughts regarding his mental conditions. He underwent psychometric evaluations, therapeutic drug monitoring analysis, and pharmacogenomic tests. The overall results helped to explain patient’s treatment-resistance. Moreover, we observed a significant reduction in both depressive and OCD symptoms after administration of esketamine.
This case underlines the importance of pharmacogenomic tests in profiling TRD patients and confirms the possible use of esketamine in the treatment of comorbid OCD.
Major depressive disorder (MDD) is a common mental disorder and a leading cause of disability, affecting more than 264 million people worldwide [ 1 , 2 ]. The STAR*D study demonstrated that only one third of patients achieved remission following the first antidepressant treatment and, even after 1 year of therapy with a sequence of four antidepressants administered for 12 weeks each, only two-third of patients achieved symptoms remission [ 3 ]. Although no single definition of treatment resistant depression (TRD) exists, it generally indicates patients who failed to respond to two or more trials of antidepressants, at adequate dosage and treatment duration [ 4 ]. As TRD patients seem not to respond sufficiently to traditional monoaminergic antidepressants, new treatment strategies acting on glutamatergic, cholinergic, and opioid systems are currently under investigation [ 5 ]. Pharmacogenomic testing (PGx) represents a decision-support tool that has been recently introduced into the clinical practice in psychiatry. Such personalized approach is especially useful in patients with conditions resistant to standard treatments due to genetic predisposition to poor psychopharmacological response or high susceptibility to severe side effects. PGx has several benefits: it could both lower the latency to clinical response or remission and increase patient’s compliance by reducing side effects impact and cost-effectiveness of the whole clinical management. The comorbidity of depression with other psychiatric disorders has been described in the past, and one of the most common comorbidities is represented by Obsessive–Compulsive Disorder (OCD) [ 6 , 7 , 8 ]. The coexistence of the two disorders seems to lead to a greater symptoms severity, less satisfactory response to treatment and an overall less favorable prognosis [ 8 ]. The disorders share common psychopathological characteristics and, in some cases, also treatment response [ 9 , 10 , 11 ]. Dysregulation of glutamate signaling in the cortico-striatal–thalamo-cortical circuitry appears to play a role in OCD as supported by preclinical, neuroimaging, and genetic studies [ 12 , 13 , 14 , 15 , 16 , 17 , 18 ].
Intranasal esketamine has been approved by both the American Food and Drug Administration and the European Medicines Agency for TRD in adults and, more recently, in moderate to severe episode of MDD, as acute short-term treatment for the rapid reduction of depressive symptoms, which according to clinical judgement constitute a psychiatric emergency. Esketamine is the (S) enantiomer of ketamine, a non-competitive N -methyl- d -aspartate (NMDA) glutamate receptor antagonist that was introduced in clinics as an anesthetic and analgesic more than 50 years ago [ 19 , 20 ]. The mechanism of antidepressant action of esketamine has not been fully clarified yet but modulation of different signaling pathways implicated in the pathophysiology of MDD, such as synaptogenesis and neuroplasticity pathways, may play a role [ 21 , 22 ]. Although off-label, encouraging results have emerged from the use of intravenous ketamine in treatment resistant OCD as reported by previous clinical studies and case reports [ 23 , 24 , 25 , 26 ], hence the growing interest in the use of intranasal esketamine in treatment resistant OCD.
The aims of this case report were to support the role of pharmacogenomic testing in psychiatry, especially in TRD patients, and to evaluate the effects of intranasal esketamine in the treatment of TRD with comorbid OCD.
We hereby present the case of M.G., a 39-year-old male married engineer, who presented at our clinic for a major depressive episode in the context of a TRD. He had a positive psychiatric family history, since his mother suffered from MDD, while his father had an alcohol use disorder.
MDD onset in this patient had occurred at the age of 27, with a substantial recovery with the introduction of sertraline (50 mg/day) in combination with psychodynamic psychotherapy.
Despite a long disease-free period, in 2019, after his first son’s birth, M.G. experienced a relapse of MDD, characterized by a significant mood deflection, emotional lability, and severe fatigue. Hence, multiple pharmacological trials were made (Table 1 ), with only partial benefit (Fig. 1 ).

Mood variations from the first MDD episode to present
In November 2020, due to the persistence of depressive symptoms, M.G. was referred to our Treatment-Resistant Disorders Clinic at San Gerardo Hospital, Monza, Italy by his private psychiatrist. During our first assessment, M.G. reported the persistence of anhedonia, low energy, asthenia, remarkable levels of anxiety and cognitive impairment (e.g.: persistent poor concentration and attentional deficits), which led to poor performance at work. The patient also reported to be emotionally detached from his family, friends, and environment.
Together with typical MDD symptoms, M.G. showed disabling symptoms related to OCD spectrum that led to the additional diagnosis of OCD according to the DSM-5 criteria [ 27 , 28 ].
Indeed, over the last 2 years the patient had developed intrusive and egodystonic obsessions, consisting mainly in pathological doubts regarding his wife. Specifically, even if physically attracted to his partner, he felt forced to spend a considerable amount of time engaged in mental rituals, consisting of repetitively glancing at his partner to check her body features, such as her nose or chin and subsequently questioning himself about the meaning of these compulsions (e.g.: “Am I continuously checking her chin or nose because I don’t love her anymore?”, “I like her, so why do I have so many doubts about her?”). Such obsessive preoccupations, intrusive thoughts and rituals are what is commonly referred to as relationship obsessive–compulsive disorder [ 8 , 29 ].
In addition, he also reported the presence of ritualistic intrusive and pervasive doubts regarding his mental health conditions. This implied the need to perform time-consuming mental rituals every morning (e.g.: independently from his psychopathological state, he used to repeat analytic checklists monitoring his conditions with precise order: “Am I feeling alright?”, “Am I depressed?”, “Am I happy?”, “Why did I cry?”, “Is it depression or something else?”, “If I feel I have little strength, does it mean that I am depressed?”).
When M.G. first came to our clinic, his therapy consisted of venlafaxine 225 mg/die, bupropion 300 mg/die, lamotrigine 150 mg/die, and olanzapine 5 mg/die.
In line with our Treatment-Resistant Disorders clinic protocol, clinical consultation, psychometric assessment (Table 2 ), Therapeutic Drug Monitoring (TDM) (Table 3 ) and Pharmacogenomic analysis were performed (Table 4 ).
Even though the patient resulted to have moderate depressive and obsessive symptomatology at psychometric evaluations, the patient’s quality of life was deeply affected, as he suffered from frequent crying fits, inability to concentrate at work and to engage in leisurable activities. Following the clinical interview, the patient was diagnosed with TRD with OCD symptoms and enrolled for intranasal esketamine treatment. A standard administration scheme was followed (Table 5 ), maintaining current patient treatment.
As a result of esketamine introduction, the patient showed a rapid resolution of depressive symptoms during the induction phase and a significant reduction of OCD symptoms during the maintenance phase (Fig. 2 ).

Variations of psychometric scales score. BPRS Brief Psychiatric Rating Scale, MADRS Montgomery-Åsberg Depression Rating Scale, YBOCS Yale–Brown obsessive–compulsive scale
By the time of the ninth esketamine administration, in consideration of the evident clinical improvement, olanzapine and lamotrigine were stopped after appropriate tapering. Some residual anxious symptoms were managed thanks to the introduction of pregabalin titrated up to 225 mg/die with further clinical benefit.
Conclusions and discussion
The present case report emphasizes the need for thorough diagnostic investigation to optimize the management of treatment-resistant cases. Indeed, TDM and genetic profiling are of relevant importance to determine optimal treatment.
In this case, serum levels of venlafaxine were 484.4 ng/mL, which is above the therapeutic range according to the TDM guidelines in neuropsychopharmacology [ 30 ]. Thus, the abnormal biotransformation of the drug was not considered as an explanation for the treatment resistance.
Considering the results of pharmacogenomic analysis, the presence of 2 common single nucleotide polymorphisms in methylenetetrahydrofolate reductase (MTHFR) gene in this patient, specifically C677T and A1298C, might have contributed to his vulnerability to psychiatric disorders. MTHFR is an enzyme catalyzing the conversion of folic acid into its active form, methylfolate, which plays an essential role in monoamine biosynthesis [ 31 ]. T allele in C677T and C allele in A1298C might lead to decreased enzymatic activity, thus, as previously reported, an increased risk of affective disorders, such as MDD or other psychiatric disorders [ 32 , 33 , 34 ].
In addition, the genetic profiling also showed the S/S 5-HTTLPR genotype in SLC6A4 gene. SLC6A4 is a serotonin transporter responsible for serotonin reuptake. Such variations ( s allele), previously described in the literature, are linked to decreased serotonin transporter expression in neurons, leading to higher susceptibility to depression, as well as poorer response to Selective serotonin reuptake inhibitors (SSRIs) [ 35 , 36 , 37 , 38 ]. This might also explain the patient's non-response to previous treatments with first-line antidepressants.
A prominent role in the brain control of stress plays GABA [ 39 ]. GABRA6 gene encodes the alpha6 subunit of GABA-A receptor and, according to previous studies, when exposed to recent negative stressful events, T allele carriers were at greater risk of depression- and anxiety-related symptoms which could also enhance suicidal risk [ 40 ]. Another study looked at the different types of recent life stressors and found that T/T genotype in patients, which was present in this case, interacts significantly with recent illness and personal problems stressors in influencing depression [ 41 ]. C allele carriers of another gene related to GABA, pi subunit of the GABA-A receptor (GABRP), were associated with good response to single antidepressant (either SSRI or venlafaxine) administered for at least 6 weeks [ 42 ]. In our patient the T/T genotype was found, representing a possible factor to his treatment refractoriness.
In addition, the patient was a CYP2B6 and CYP2C19 rapid metabolizer, potentially contributing to the inefficacy of bupropion and other SSRI. The predominant metabolic pathway of bupropion that leads to formation of its active metabolite hydroxybupropion is CYP2B6 enzyme-mediated. In case of rapid metabolizers, the therapeutic outcome of bupropion therapy is strongly affected [ 43 ].
Moreover, this case not only validates the rapidity of esketamine in reverting depressive symptoms, but it also shows encouraging findings about the possible use of esketamine in treating OCD symptoms.
Indeed, during the maintenance phase, the patient showed a significant reduction in his OCD symptomatology as showed by the Yale–Brown Obsessive–Compulsive Disorder (YBOCS) score reduction (Fig. 2 , green line): after initial symptoms’ worsening due to a new-onset pathological doubt regarding treatment efficacy and side effects, the YBOCS score showed a 46.67% reduction (from 15 to 8). Since the pre-existing pharmacological treatment was not changed at our clinic, the reduction of the OCD symptoms might be directly referred to the use of esketamine.
It is worth noting that the time-ratio required to relieve OCD symptomatology maintained the 3:1 ratio usually seen with the use of serotonergic antidepressants [ 44 , 45 ].
Literature regarding the possible use of ketamine in OCD remains sparse with some pre-clinical [ 46 ] and clinical studies [ 25 , 26 ] showing a rapid reduction of OCD symptoms after the drug administration. Specifically, human studies indicated that ketamine could quickly and transiently decrease OCD behaviors. Nonetheless, these studies showed multiple limitations, mainly regarding small sample sizes and short-term observations. Thus, further investigation in the form of double-blind, randomized controlled trials is warranted.
Abbreviations
Major depressive disorder
N -Methyl- d -aspartate
- Obsessive–compulsive disorder
Pharmacogenomic testing
Selective serotonin reuptake inhibitors
Therapeutic drug monitoring
- Treatment resistant depression
Yale–Brown obsessive–compulsive disorder
Rehm J, Shield KD. Global burden of disease and the impact of mental and addictive disorders. Curr Psychiatry Rep. 2019;21(2):10.
Article PubMed Google Scholar
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–858.
Article Google Scholar
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–17.
Gaynes BN, Lux L, Gartlehner G, Asher G, Forman-Hoffman V, Green J, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37(2):134–45.
Papakostas GI, Ionescu DF. Towards new mechanisms: an update on therapeutics for treatment-resistant major depressive disorder. Mol Psychiatry. 2015;20(10):1142–50.
Article CAS PubMed Google Scholar
Bolhuis K, Mcadams TA, Monzani B, Gregory AM, Mataix-Cols D, Stringaris A, et al. Aetiological overlap between obsessive-compulsive and depressive symptoms: a longitudinal twin study in adolescents and adults. Psychol Med. 2014;44(7):1439–49.
Denys D, Tenney N, van Megen HJGM, de Geus F, Westenberg HGM. Axis I and II comorbidity in a large sample of patients with obsessive–compulsive disorder. J Affect Disord. 2004;80(2–3):155–62.
Quarantini LC, Torres AR, Sampaio AS, Fossaluza V, de Mathis MA, do Rosário MC, et al. Comorbid major depression in obsessive-compulsive disorder patients. Compr Psychiatry. 2011;52(4):386–93.
de Almeida AG, Quarantini LC, Góis CR, Santos-Jesus R, Miranda-Scippa ÂMA, de Oliveira IR, et al. Obsessive-compulsive disorder: an open-label pilot trial of escitalopram. CNS Spectr. 2007;12(7):519–24.
McGrath PJ, Khan AY, Trivedi MH, Stewart JW, Morris DW, Wisniewski SR, et al. Response to a selective serotonin reuptake inhibitor (citalopram) in major depressive disorder with melancholic features: a STAR*D report. J Clin Psychiatry. 2008;69(12):1847–55.
Moritz S, Meier B, Hand I, Schick M, Jahn H. Dimensional structure of the Hamilton Depression Rating Scale in patients with obsessive-compulsive disorder. Psychiatry Res. 2004;125(2):171–80.
Rosenberg DR, Macmaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ. Decrease in caudate glutamatergic concentrations in pediatric obsessive–compulsive disorder patients taking paroxetine. J Am Acad Child Adolesc Psychiatry. 2000;39(9):1096–103.
Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee SP, et al. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. J Am Acad Child Adolesc Psychiatry. 2004;43(9):1146–53.
Chakrabarty K, Bhattacharyya S, Christopher R, Khanna S. Glutamatergic dysfunction in OCD. Neuropsychopharmacology. 2005;30(9):1735–40.
Yücel M, Wood SJ, Wellard RM, Harrison BJ, Fornito A, Pujol J, et al. Anterior cingulate glutamate-glutamine levels predict symptom severity in women with obsessive–compulsive disorder. Aust N Z J Psychiatry. 2008;42(6):467–77.
Starck G, Ljungberg M, Nilsson M, Jönsson L, Lundberg S, Ivarsson T, et al. A 1H magnetic resonance spectroscopy study in adults with obsessive compulsive disorder: relationship between metabolite concentrations and symptom severity. J Neural Transm. 2008;115(7):1051–62.
Bhattacharyya S, Khanna S, Chakrabarty K, Mahadevan A, Christopher R, Shankar SK. Anti-brain autoantibodies and altered excitatory neurotransmitters in obsessive–compulsive disorder. Neuropsychopharmacology. 2009;34(12):2489–96.
Pittenger C, Bloch MH, Williams K. Glutamate abnormalities in obsessive compulsive disorder: neurobiology, pathophysiology, and treatment. Pharmacol Ther. 2011;132(3):314–32.
Article CAS PubMed PubMed Central Google Scholar
Hashimoto K. Rapid-acting antidepressant ketamine, its metabolites and other candidates: a historical overview and future perspective. Psychiatry Clin Neurosci. 2019;73(10):613–27.
Jelen LA, Young AH, Stone JM. Ketamine: a tale of two enantiomers. J Psychopharmacol. 2021;35(2):109–23.
Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(3):238–49.
Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, et al. Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biol Psychiatry. 2017;81(10):886–97.
Rodriguez CI, Kegeles LS, Flood P, Simpson HB. Rapid resolution of obsessions after an infusion of intravenous ketamine in a patient with treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry. 2011;72(4):567–9.
Article PubMed PubMed Central Google Scholar
Bloch MH, Wasylink S, Landeros-Weisenberger A, Panza KE, Billingslea E, Leckman JF, et al. Effects of ketamine in treatment-refractory obsessive–compulsive disorder. Biol Psychiatry. 2012;72(11):964–70.
Rodriguez CI, Kegeles LS, Levinson A, Feng T, Marcus SM, Vermes D, et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013;38(12):2475–83.
Sharma LP, Thamby A, Balachander S, Janardhanan CN, Jaisoorya TS, Arumugham SS, et al. Clinical utility of repeated intravenous ketamine treatment for resistant obsessive-compulsive disorder. Asian J Psychiatr. 2020;52: 102183.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM-5). 2013. https://doi.org/10.1176/appi.books.9780890425596
Franklin ME, Budzyn S, Freeman H. OCD Spectrum Disorders. In: Olatunji BO, editor. The Cambridge handbook of anxiety and related disorders. Cambridge: Cambridge University Press; 2019. p. 603–23.
Google Scholar
Doron G, Derby D, Szepsenwol O, Nahaloni E, Moulding R. Relationship obsessive–compulsive disorder: interference, symptoms, and maladaptive beliefs. Front Psychiatry. 2016;7:58.
Hiemke C, Bergemann N, Clement HW, Conca A, Deckert J, Domschke K, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1–02):9–62.
CAS PubMed Google Scholar
Stahl SM. L-methylfolate: a vitamin for your monoamines. J Clin Psychiatry. 2008;69(9):1352–3.
López-León S, Janssens ACJW, González-Zuloeta Ladd AM, Del-Favero J, Claes SJ, Oostra BA, et al. Meta-analyses of genetic studies on major depressive disorder. Mol Psychiatry. 2008;13(8):772–85.
Article PubMed CAS Google Scholar
Peerbooms OLJ, van Os J, Drukker M, Kenis G, Hoogveld L, de Hert M, et al. Meta-analysis of MTHFR gene variants in schizophrenia, bipolar disorder and unipolar depressive disorder: evidence for a common genetic vulnerability? Brain Behav Immun. 2011;25(8):1530–43.
Evinova A, Babusikova E, Straka S, Ondrejka I, Lehotsky J. Analysis of genetic polymorphisms of brain-derived neurotrophic factor and methylenetetrahydrofolate reductase in depressed patients in a Slovak (Caucasian) population. Gen Physiol Biophys. 2012;31(4):415–22.
Zanardi R, Serretti A, Rossini D, Franchini L, Cusin C, Lattuada E, et al. Factors affecting fluvoxamine antidepressant activity: influence of pindolol and 5-HTTLPR in delusional and nondelusional depression. Biol Psychiatry. 2001;50(5):323–30.
Arias B, Catalán R, Gastó C, Gutiérrez B, Fañanás L. 5-HTTLPR polymorphism of the serotonin transporter gene predicts non-remission in major depression patients treated with citalopram in a 12-weeks follow up study. J Clin Psychopharmacology. 2003;23(6):563–7.
Article CAS Google Scholar
Serretti A, Cusin C, Rossini D, Artioli P, Dotoli D, Zanardi R. Further evidence of a combined effect of SERTPR and TPH on SSRIs response in mood disorders. Am J Med Genet Neuropsychiatr Genet. 2004;129B(1):36–40.
Cervilla JA, Rivera M, Molina E, Torres-González F, Bellón JA, Moreno B, et al. The 5-HTTLPR s/s genotype at the serotonin transporter gene (SLC6A4) increases the risk for depression in a large cohort of primary care attendees: the PREDICT-gene study. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(8):912–7.
Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16(4):383–406.
Gonda X, Sarginson J, Eszlari N, Petschner P, Toth ZG, Baksa D, et al. A new stress sensor and risk factor for suicide: the T allele of the functional genetic variant in the GABRA6 gene. Sci Rep. 2017;7(1):12887.
Article PubMed PubMed Central CAS Google Scholar
Gonda X, Petschner P, Eszlari N, Sutori S, Gal Z, Koncz S, et al. Effects of different stressors are modulated by different neurobiological systems: the role of GABA-A versus CB1 receptor gene variants in anxiety and depression. Front Cell Neurosci. 2019;13:138.
Pu M, Zhang Z, Xu Z, Shi Y, Geng L, Yuan Y, et al. Influence of genetic polymorphisms in the glutamatergic and GABAergic systems and their interactions with environmental stressors on antidepressant response. Pharmacogenomics. 2013;14(3):277–88.
Tran AX, Ho TT, Varghese GS. Role of CYP2B6 pharmacogenomics in bupropion-mediated smoking cessation. J Clin Pharm Ther. 2019;44(2):174–9.
Pittenger C, Bloch MH. Pharmacological treatment of obsessive-compulsive disorder. Psychiatr Clin North Am. 2014;37(3):375–91.
Kayser RR. Pharmacotherapy for treatment-resistant obsessive–compulsive disorder. J Clin Psychiatry. 2020;81(5): 19ac13182.
Tosta CL, Silote GP, Fracalossi MP, Sartim AG, Andreatini R, Joca SRL, et al. S-ketamine reduces marble burying behaviour: involvement of ventromedial orbitofrontal cortex and AMPA receptors. Neuropharmacology. 2019;144:233–43.
Download references
Acknowledgements
We would like to pay our gratitude and our respects to our friend and colleague, Dr. Matteo Sibilla. After contributing to this paper, Dr. Matteo Sibilla passed away in August of 2021. He was a dedicated clinician, a passionate researcher and an outstanding colleague. He will be deeply missed.
This work has been supported by a grant (n° 2019–3396 to DA) from the Italian Cariplo Foundation, which had not any involvement in manuscript preparation, or decision to submit the article for publication.
Author information
Authors and affiliations.
Psychiatric Department, San Gerardo Hospital, ASST Monza, Monza, Italy
Marcatili Matteo, Nava Roberto, Colmegna Fabrizia, Caldiroli Alice, Capuzzi Enrico & Clerici Massimo
Department of Medicine and Surgery, University of Milano Bicocca, Monza, Italy
Marcatili Matteo, Pellicioli Cristian, Maggioni Laura, Motta Federico, Redaelli Chiara, Matteo Sibilla, Dakanalis Antonios, Piperno Alberto & Clerici Massimo
Faculty of Medicine, University Vita-Salute San Raffaele, Milan, Italy
Ghelfi Lorenzo
Department of Pharmacology, Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, Martin, Slovakia
Krivosova Michaela
Psychiatry Unit, Department of Biomedical and Clinical Sciences “Luigi Sacco”, University of Milan, Milan, Italy
Benatti Beatrice
CRC “Aldo Ravelli” for Neurotechnology and Experimental Brain Therapeutics, University of Milan, Milan, Italy
Cytogenetics and Medical Genetics Unit, Centre for Disorders of Iron Metabolism, San Gerardo Hospital, ASST Monza, Monza, Italy
Bertola Francesca, Villa Nicoletta & Piperno Alberto
Clinical Chemistry Laboratory, San Gerardo Hospital, ASST Monza, Monza, Italy
Ippolito Silvia
You can also search for this author in PubMed Google Scholar
Contributions
MM, NR, CF, PC, ML, MF, RC, SM, and GL were responsible for clinical consultations, psychometric analyses, and esketamine administrations. BF, VN, and PA were responsible for pharmacogenetic analyses along with MM and KM for their interpretation. IS performed TDM analysis. Major contributors for writing the report were MM, PC, ML, MF, RC, GL, and KM. The final revision was made by MM, BB, CA, CE, DA, and CM. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Marcatili Matteo .
Ethics declarations
Availability of data and materials.
The data sets generated and/or analysed during the current study are not publicly available due to privacy reasons.
Ethics approval and consent to participate
Written informed consent for the use of the anonymous clinical data was obtained.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Matteo, M., Cristian, P., Laura, M. et al. The use of esketamine in comorbid treatment resistant depression and obsessive compulsive disorder following extensive pharmacogenomic testing: a case report. Ann Gen Psychiatry 20 , 43 (2021). https://doi.org/10.1186/s12991-021-00365-z
Download citation
Received : 31 May 2021
Accepted : 25 August 2021
Published : 16 September 2021
DOI : https://doi.org/10.1186/s12991-021-00365-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Annals of General Psychiatry
ISSN: 1744-859X
- Submission enquiries: [email protected]
CASE REPORT article
Case report: treatment-resistant depression, multiple trauma exposure and suicidality in an adolescent female with previously undiagnosed autism spectrum disorder.

- 1 Section of Child and Adolescent Neuropsychiatry, Department of Public Health and Pediatric Sciences, University of Turin, Turin, Italy
- 2 Service of Child and Adolescent Psychiatry, Department of Psychiatry, Lausanne University Hospital and the University of Lausanne, Lausanne, Switzerland
- 3 Psychiatric Liaison Service, Department of Psychiatry, Lausanne University Hospital, Lausanne, Switzerland
- 4 Center for Psychiatric Neuroscience, Department of Psychiatry, Lausanne University Hospital and the University of Lausanne, Lausanne, Switzerland
High rates of co-occurring depression are commonly reported in youth with Autism Spectrum Disorder (ASD), especially in individuals without intellectual disability (ID). Depression in ASD undermines adaptive behavior and is associated with a higher risk of suicidality. Females with ASD may be particularly vulnerable due to their greater use of camouflaging strategies. Indeed, in comparison to males, ASD is underdiagnosed in females, despite higher rates of internalizing symptoms and suicidality. Trauma exposure may also play a role in the development of depressive symptoms in this population. Moreover, evidence for effective treatments of depression in autistic youth are lacking, with ASD individuals frequently experiencing low efficacy and side effects. We present the case of an adolescent female with previously undiagnosed ASD without ID, admitted for active suicidal plans and a treatment-resistant depression (TRD), occurred after a COVID-19 lockdown in the context of cumulative exposure to stressful life events. Comprehensive clinical assessments performed at intake confirmed severe depression with suicidality. Intensive psychotherapy and different changes in medications were carried out (SSRI, SNRI, SNRI + NaSSA, SNRI + aripiprazole), all of which were ineffective, with persistent suicidal thoughts, often requiring intensive individual monitoring. The patient was finally successfully treated with lithium augmentation of fluoxetine, with no side effects. During hospitalization she was also evaluated by an ASD specialized center, where a diagnosis of ASD was made according to the Autism Diagnostic Observation Schedule (ADOS) and the Autism Diagnostic Interview-Revised (ADI-R) scores, as well as to clinical judgment of a senior psychiatrist. The present case report shows that clinicians should not overlook undiagnosed autism as a possible cause of TRD, especially in females without ID, where higher rates of under diagnosis may be in part related to their greater use of camouflage. It also suggests that ASD underdiagnosis and resulting unmet needs may be involved in vulnerability to stressful experiences, depression, and suicidality. Furthermore, it shows the complexity of providing care to TRD in youth with autism, suggesting that an augmentation therapy with lithium, a commonly recommended therapeutic strategy for refractory depression in typically developing samples, may also be effective in this population.
Introduction
Autism Spectrum Disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social communication and interaction, and by restricted, repetitive patterns of behaviors and /or interests ( 1 ). High rates of co-occurring depression are commonly reported in youth with ASD ( 2 , 3 ), with a lifetime prevalence rate estimated at 20.2% ( 4 ). ASD individuals are up to 4 times more likely to develop depressive symptoms than neurotypical subjects, with an increasing trend from adolescence to middle adulthood ( 5 ). Depression in ASD undermines adaptive behavior and is associated with a higher risk of suicidality and an increased healthcare burden ( 3 ).
A recent body of literature on adolescent and adult samples suggests that females with ASD may experience higher rates of depression and other internalizing symptoms compared to males, including anxiety, suicidality and eating disorders ( 5 – 7 ), although others studies found no sex differences ( 8 , 9 ). Females with ASD also show higher rates of completed suicide than their male counterpart, in contrast to what is reported in non-ASD samples (i.e., completed suicide more frequent in males) ( 10 ). Moreover, evidence suggests that ASD is potentially underdiagnosed in females ( 11 ), who are also later diagnosed than their male counterpart and frequently misdiagnosed with other mental disorders, especially personality disorders ( 12 – 15 ). In line with this, some authors suggest that there's a female autism phenotype, including female specific manifestations of autism less likely to be captured by current diagnostic tools ( 16 , 17 ). Also, females seem to mask more their autistic traits than males, a phenomenon known as camouflage ( 18 ), which has been associated to higher rates of distress, depression and suicidality in both adolescents and adults with ASD ( 19 – 21 ). Conversely, an earlier ASD diagnosis has shown a protective effect on depression and self-harm behaviors ( 22 , 23 ), potentially enabling timely interventions and social support, and reducing the risk of traumatic experiences ( 24 ), that have been linked to mood symptoms and suicidality in ASD ( 25 , 26 ).
Since current evidence is heavily based on male samples, providing information on the female autism phenotype could reduce mis- and missed-diagnosis rates and prevent secondary comorbidities in this population.
Moreover, evidence for efficacious pharmacological interventions is lacking and therapeutic strategies used for neurotypical patients may not be effective in ASD population ( 27 , 28 ). Some authors suggest considering ASD in case of treatment-resistant depression (TRD) ( 29 ), which is defined by the presence of persistent depressive symptoms despite at least two trials of antidepressants at an appropriate dosage and duration. Lithium augmentation is a first-line treatment strategy for TRD in neurotypical samples ( 30 ). Moreover, lithium has demonstrated an anti-suicidal effect ( 31 ). Despite its potential use, to date, no randomized controlled trials have studied lithium's use in ASD, however, evidence from previous studies suggested a potential efficacy in this population. A preclinical study by Wu et al. ( 32 ) found an improvement in anxiety and depressive symptoms in rats with isolation-induced autistic behaviors. Previous chart reviews of youth and adults with ASD suggested a potential efficacy on mood dysregulation and maladaptive behaviors ( 33 , 34 ). A previous case report showed a completed remission of catatonia and regression in two people with ASD with SHANK3 mutation treated with lithium ( 35 ). Epperson et al. ( 36 ) reported significant improvements in social relatedness and aggressivity with lithium augmentation of fluvoxamine in a man with ASD. Another case report documented a marked reduction in self-injury and aggressive behavior with lithium augmentation in a depressed adolescent male with intellectual disability (ID) ( 37 ). However, to our knowledge, this is the first case report of lithium augmentation efficacy in TRD with suicidality in an adolescent female with ASD, without ID. She was admitted multiple times for chronic suicidal plans and a TRD occurring after COVID-19 lockdown and exposure to traumatic events. Despite different changes in antidepressant treatments, all were ineffective. Eventually, she was diagnosed with ASD during her 4th hospital stay and successfully treated with lithium augmentation, without side effects.
Case description
Patient information.
A. is a 16-year-old girl who was admitted four times in our adolescent psychiatric inpatient unit for depressive symptoms and active suicidal plans. The early development was normal, except for a mild language delay (i.e., she did not speak single words until 2.5 years and had poor speech until 4 years), that benefited from speech therapy. Since early childhood she showed difficulties in interacting with peers, isolation, peculiar interests (i.e., drawing letters and numbers), and was described by teachers as “being in her world,” which was interpreted as a reaction to the sudden death of her father when she was 5 years old. Moreover, she presented poor cognitive flexibility and a marked sense of justice, especially concerning school rules. Her peers frequently bullied her due to this. Also, the school context required her great effort due to her noise sensitivity. During adolescence, she reported a sense of being inadequate in social situations, so that she had to constantly monitor and adjust her reactions to fit into peer contexts. All her activities were organized with a rigid timetable, with a large amount of time dedicated to study and sport training, achieving brilliant sport and school results. Interactions with peers were mostly around these specific interests. Depressive symptoms started during the COVID-19 pandemic lockdown and the interruption of her high-performance sport training due to a sports failure. She presented low mood, fatigue, anhedonia, increased social withdrawal and ruminations about her father's death and the sports failure. She was first referred to an outpatient psychiatric unit, where she was treated with psychotherapy. Despite an initial mild improvement, she was later hospitalized four times for recurrent suicidal plans and diagnosed with major depressive disorder (MDD) according to DSM-5 criteria. During the first three admissions, several changes in medications were carried out: she was first treated with sertraline, up to 200 mg daily, that was then switched to fluoxetine, up to 20 mg daily, that was finally switched to duloxetine, up to 60 mg daily. She was also treated with mirtazapine up to 15 mg daily for her sleep disturbances, with a positive effect. The antidepressant treatments showed only a partial and temporary improvement, with a rapid recrudescence of suicidal plans after each hospitalization. A suspicion of ASD was also hypothesized during her first hospitalization, and the patient was put on a waiting list for outpatient specialized evaluations.
Family history
There was no family history of psychiatric disorders, except a suspicion of autism in her small brother, who had never been investigated.
Clinical findings
At the present hospitalization (i.e., the 4th one), a clinical interview was conducted at intake by a senior psychiatrist, confirming the diagnosis of MDD with active suicidal plans. She was also evaluated by the Prodromal Questionnaire-16 ( 38 ), with a score of 6 (cut-off 7) and by the Health of the Nation Outcome Scales for Children and Adolescents, self-rated version (HoNOSCA-SR) ( 39 ), suggesting severe depressive symptoms with suicidality (HoNOSCA-SR: “ Have you done anything to injure or harm your-self on purpose”- 4/5; “Have you been feeling in a low or anxious mood, or troubled by fears, obsessions or rituals”- 5/5 ). During interviews, she reported depressed mood, guilt, anhedonia, apathy, sleep and appetite disturbances. She struggled to express emotions and her affect was blunted with limited eye contact, neutral facial expressions, and monotone speech. The intelligence quotient was not evaluated due to symptoms severity, however, she demonstrated good cognitive and linguistic competence, as evidenced by her school results and sophisticated vocabulary.
Therapeutic intervention, follow-up, and outcomes
The current hospitalization was complicated by recurrent non-suicidal self-injuries (NSSI) (e.g., self-cutting) and suicidal threats, including an interrupted suicide attempt, requiring intensive individual monitoring during high-risk periods. A. also developed severe food restriction, initially describing it as her only way to die. She subsequently lost significant weight, reaching a body mass index of 16, and ultimately met all diagnostic criteria for anorexia nervosa (DSM-5), necessitating a nasogastric feeding tube.
Concerning pharmacological treatment, duloxetine was initially raised up to 120 mg daily, with no clinical response. Thus, an augmentation treatment, first with mirtazapine (up to 30 mg daily), and then with aripiprazole (up to 15 mg daily), was tried in combination with duloxetine, showing no clinical improvements. Duloxetine was then switched to fluoxetine, augmented up to 40 mg daily (since it had only been previously tried at a low dose), showing no clinical improvements. Lithium was finally added to fluoxetine as an augmentation treatment. Blood tests were conducted periodically to monitor thyroid, parathyroid, and renal function, as well as lithium plasma concentration, to determine the optimal dose and detect possible side effects. The therapeutic dose was 12 mmol daily (corresponding to a lithium plasma level of 0.5 mmol/L), split into two administrations, resulting in a rapid and satisfactory improvement in mood and suicidality, without side effects. A. also showed a parallel improvement in food intake restriction that allowed the removal of the nasogastric tube. During hospitalization, she was evaluated by an ASD specialized center ( Centre Cantonal de l'Autisme) and diagnosed with ASD based on ADOS-2 and ADI-R scores ( 40 , 41 ) ( Table 1 ), as well as the clinical judgment of a senior psychiatrist. A brief psychoeducational intervention followed to help her elaborate the diagnosis. A. also had several ergotherapy sessions focused on recognizing and managing emotions. At hospital discharge, we observed a clinical improvement that was confirmed by the HoNOSCA-SR (with a score of 3/5 as regards to mood and anxiety symptoms, and a score of 3/5 as regards to suicidal thoughts). The patient was addressed to an outpatient clinic for psychological and psychiatric follow-up, as well as to a social skills training group for ASD. Clinically relevant data and medication history are shown in Figures 1 , 2 .
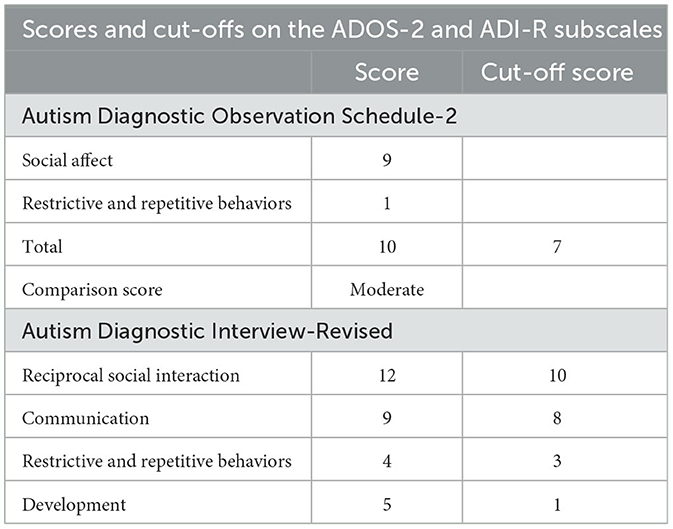
Table 1 . Scores on the Autism Diagnostic Observation Schedule-2 (ADOS-2) and Autism Diagnostic Interview-Revised (ADI-R), with cut-off values.
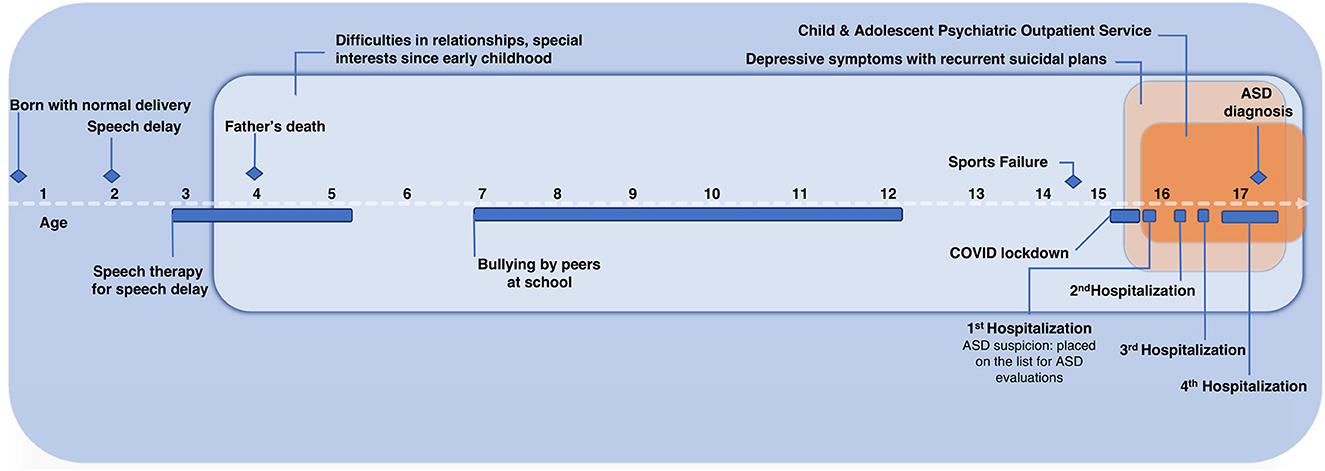
Figure 1 . Case report timeline with relevant data.
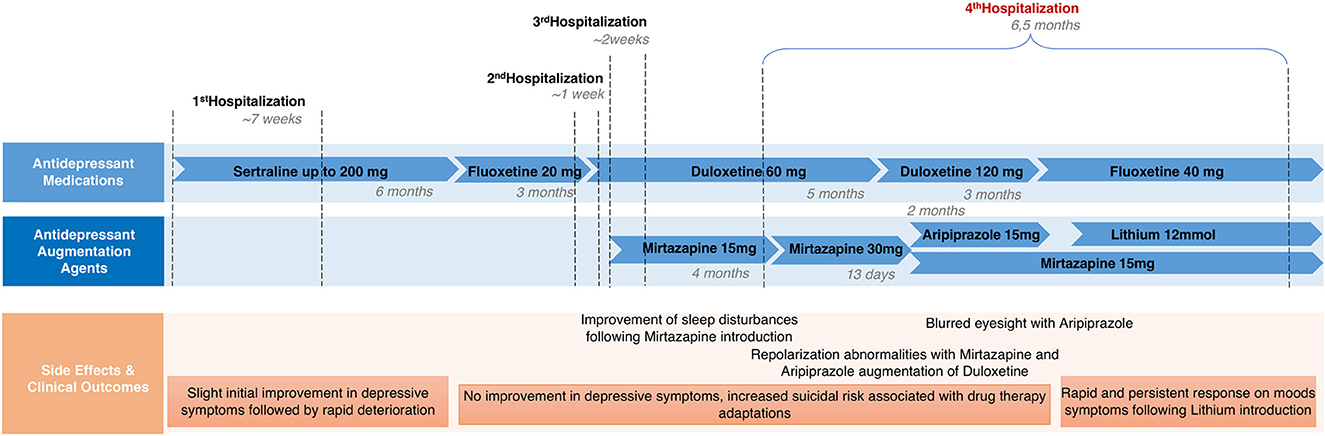
Figure 2 . Patient's medication history.
We present the case of a TRD with NSSI, suicidal behaviors and anorexia nervosa in a 16-year-old-girl with comorbid previously undiagnosed ASD, without ID. The strength of our case report is that it highlights the clinical challenges of females with ASD, describing the factors possibly involved in both autism misdiagnosis and in the development of TRD with suicidality. To our knowledge, this is the first case report specifically exploring lithium augmentation's efficacy for TRD with suicidality in an adolescent female with ASD, without ID, suggesting a potential effectiveness. The limitation of our case report is that the evaluations for ASD were performed during hospitalization (albeit together with the improvement of depressive symptoms), therefore, the possible influence of depression on the scores may not be completely excluded. However, ASD suspicions preceded the current patient's hospitalization and the patient's history was thoroughly explored by clinicians with the aid of the ADI-R, a standardized tool providing a developmental perspective.
Our case is in line with the evidence suggesting a greater incidence of depressive symptoms, suicidality and eating disorders in ASD population, especially in females without ID ( 5 , 42 ). Also, A. showed many typical characteristics of the female autism presentation, including a late ASD diagnosis, being initially referred for depression and using camouflage to fit in with peers ( 12 , 16 , 17 ). Additionally, she displayed “socially acceptable” repetitive interests (e.g., sport and school performance), consistent with studies indicating that females with ASD exhibit less bizarre interests and externalizing behaviors than their male counterpart, contributing to fewer mental health concerns and missed diagnosis ( 43 , 44 ). Camouflaging strategies adopted by the patient, described as “constantly monitoring and adjusting her reactions to fit in social contexts,” may both have contributed to ASD misrecognition, and represented a risk factor for depression and suicidality, as evidence highlighted that camouflage is a risk factor for a wide range of mental health problems ( 16 , 45 ). Despite being “exhausting and distressing,” qualitative studies in adolescent girls with ASD highlighted that camouflage was aimed at “fitting into” neurotypical contexts and avoiding bullying experiences ( 46 ). Traumatic experiences also seem to play a major role in the development of secondary comorbidities in ASD, including depression and suicidality ( 26 , 47 ). Indeed, evidence show that individuals with ASD reported higher rates of trauma, such as bullying victimization and marginalization, compared to neurotypical peers, especially in females with ASD ( 48 ). Furthermore, typical autistic traits, including ruminative thinking, unusual sensory processing and need for predictability, may alter the appraisal of stressful events and hinder coping with changes. This may lead to a wider range of traumatic experiences and a greater impact on mental health ( 47 , 49 ).
Multiple traumatic events in our patient's history, including sports failure and COVID-19 school closures, preceded the onset of depressive symptoms, and disrupted her routine, highlighting the impact of trauma and COVID-19 pandemic in depression development in ASD samples, as evidenced in previous studies and reports ( 26 , 50 – 52 ). Also, COVID-19 lockdown may have exacerbated maladaptive coping strategies, such as ruminations on past traumatic experiences.
In sum, we hypothesize that the delayed diagnosis due to the female-typical ASD presentation, combined with exposure to multiple traumatic events, may have increased the risk of depression and suicidality in our patient. Furthermore, in line with literature findings ( 22 ), an earlier ASD diagnosis may have been a protective factor in reducing the patient's exposure to social stressors and in providing appropriate interventions and support. Indeed, evidence-based psychological treatments adapted for autism have shown efficacy for depression ( 53 , 54 ).
Regarding pharmacological interventions, current evidence for effective treatments for depression in ASD is limited ( 27 , 28 ). Studies exploring SSRI efficacy in reducing ASD core symptoms suggest that ASD youth may experience more adverse side effects than typically developing peers, especially behavioral activation ( 55 , 56 ). In general population samples, current treatment guidelines recommend augmentation strategies after the failure of two antidepressants or a partial response with a primary antidepressant ( 57 ), with atypical antipsychotics and lithium commonly used as augmentation agents in both adults and adolescents ( 58 , 59 ). Despite our patient's lack of response to various medications, including SSRI and SNRI monotherapy, as well as aripiprazole or mirtazapine augmentation, she showed improvement in depressive symptoms and suicidality with lithium augmentation. Limited evidence supports lithium's use in ASD, but previous clinical and pre-clinical studies documented its potential efficacy for mood symptoms, catatonia, social relatedness, and maladaptive behaviors in this population ( 32 , 34 , 36 , 37 ). Our case also suggests lithium as a potential effective strategy for treating TRD and reducing suicide risk in youth with ASD.
Conclusions
This case report has several clinical implications. First, it provides evidence on the female-typical ASD presentation, which could help recognize ASD in females and prevent secondary comorbidities. Second, it suggests that clinicians should not overlook undiagnosed autism as a possible cause of TRD with suicidality, especially in females without ID. Third, it suggests that an augmentation therapy with lithium, an agent commonly recommended for TRD in neurotypical samples, may also be considered for refractory depression in youth with ASD. Given the high rates of depression and suicidality in ASD and limited evidence for effective interventions, further research is needed to evaluate the efficacy of lithium augmentation strategy for TRD in this population. To address this gap in knowledge, prospective controlled trials with larger samples seem necessary.
Patient perspective
The patient identified with the ASD diagnosis, describing difficulties in understanding other people's feelings or intentions since childhood, expressing relief when we discussed the increased prevalence of bullying among youth with autism. Also, she recognized the benefits of the augmentation therapy with lithium and was satisfied with the psychoeducational intervention that provided her with practical techniques to improve emotions recognition and expression.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
IS contributed to the conceptualization and writing of the manuscript. LP worked with the patient and her family and contributed to the writing. AC, LP, PK, and AN worked with the patient and her family, contributed to the conceptualization, and supervised the work. CK and MA contributed to the conceptualization and supervised the work. All authors have approved the submitted version.
Open access funding was provided by the University of Lausanne.
Acknowledgments
The authors express thanks to the patient and her family for their cooperation and the permission to publish their experience.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ASD, Autism Spectrum Disorder; MDD, major depressive disorder; TRD, treatment-resistant depression; NSSI, non-suicidal self-injuries; SSRI, selective serotonin re-uptake inhibitors; SNRI, selective noradrenaline re-uptake inhibitors; NaSSA, noradrenergic and specific serotonergic antidepressants; DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; ID, intellectual disability; ADOS-2, Autism Diagnostic Observation Schedule; ADI-R, Autism Diagnostic Interview-Revised; HoNOSCA-SR, Health of the Nation Outcome Scale for Children and Adolescents—Self rated.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5 ® ) . Washington, DC: American Psychiatric Pub (2013). doi: 10.1176/appi.books.9780890425596
CrossRef Full Text | Google Scholar
2. Bougeard C, Picarel-Blanchot F, Schmid R, Campbell R, Buitelaar J. Prevalence of Autism spectrum disorder and co-morbidities in children and adolescents: a systematic literature review. Front Psychiatry. (2021) 12:744709. doi: 10.3389/fpsyt.2021.744709
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Pezzimenti F, Han GT, Vasa RA, Gotham K. Depression in youth with autism spectrum disorder. Child Adolesc Psychiatr Clin N Am. (2019) 28:397–409. doi: 10.1016/j.chc.2019.02.009
4. Greenlee JL, Mosley AS, Shui AM, Veenstra-VanderWeele J, Gotham KO. Medical and behavioral correlates of depression history in children and adolescents with autism spectrum disorder. Pediatrics . (2016) 137(Suppl. 2):S105–14. doi: 10.1542/peds.2015-2851I
5. Uljarević M, Hedley D, Rose-Foley K, Magiati I, Cai RY, Dissanayake C, et al. Anxiety and depression from adolescence to old age in autism spectrum disorder. J Autism Dev Disord . (2020) 50:3155–65. doi: 10.1007/s10803-019-04084-z
6. Schwartzman JM, Williams ZJ, Corbett BA. Diagnostic- and sex-based differences in depression symptoms in autistic and neurotypical early adolescents. Autism. (2022) 26:256–69. doi: 10.1177/13623613211025895
7. Westwood H, Tchanturia K. Autism spectrum disorder in anorexia nervosa: an updated literature review. Curr Psychiatry Rep. (2017) 19:41. doi: 10.1007/s11920-017-0791-9
8. Gotham K, Brunwasser SM, Lord C. Depressive and anxiety symptom trajectories from school age through young adulthood in samples with autism spectrum disorder and developmental delay. J Am Acad Child Adolesc Psychiatry . (2015) 54:369–76.e3. doi: 10.1016/j.jaac.2015.02.005
9. Nasca BC, Lopata C, Donnelly JP, Rodgers JD, Thomeer ML. Sex differences in externalizing and internalizing symptoms of children with ASD. J Autism Dev Disord. (2020) 50:3245–52. doi: 10.1007/s10803-019-04132-8
10. Hirvikoski T, Mittendorfer-Rutz E, Boman M, Larsson H, Lichtenstein P, Bölte S. Premature mortality in autism spectrum disorder. Br J Psychiatry. (2016) 208:232–8. doi: 10.1192/bjp.bp.114.160192
11. Dworzynski K, Ronald A, Bolton P, Happé F. How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J Am Acad Child Adolesc Psychiatry . (2012) 51:788–97. doi: 10.1016/j.jaac.2012.05.018
12. Gesi C, Migliarese G, Torriero S, Capellazzi M, Omboni AC, Cerveri G, et al. Gender differences in misdiagnosis and delayed diagnosis among adults with autism spectrum disorder with no language or intellectual disability. Brain Sci. (2021) 11:912. doi: 10.3390/brainsci11070912
13. Huang Y, Arnold SRC, Foley K, Lawson LP, Richdale AL, Trollor JN. Factors associated with age at autism diagnosis in a community sample of Australian adults. Autism Research. (2021) 14:2677–87. doi: 10.1002/aur.2610
14. Kirby AV, Bakian AV, Zhang Y, Bilder DA, Keeshin BR, Coon H. A 20-year study of suicide death in a statewide autism population. Autism Res. (2019) 12:658–66. doi: 10.1002/aur.2076
15. South M, Beck JS, Lundwall R, Christensen M, Cutrer EA, Gabrielsen TP, et al. unrelenting depression and suicidality in women with autistic traits. J Autism Dev Disord. (2020) 50:3606–19. doi: 10.1007/s10803-019-04324-2
16. Bargiela S, Steward R, Mandy W. The experiences of late-diagnosed women with autism spectrum conditions: an investigation of the female autism phenotype. J Autism Dev Disord. (2016) 46:3281–94. doi: 10.1007/s10803-016-2872-8
17. Hull L, Petrides KV, Mandy W. The female autism phenotype and camouflaging: a narrative review. Rev J Autism Dev Disord. (2020) 7:306–17. doi: 10.1007/s40489-020-00197-9
18. Attwood T. The Complete Guide to Asperger's Syndrome, 1st ed . London, Philadelphia: Jessica Kingsley Publishers (2006).
Google Scholar
19. Bernardin CJ, Mason E, Lewis T, Kanne S. “You must become a chameleon to survive”: adolescent experiences of camouflaging. J Autism Dev Disord. (2021) 51:4422–35. doi: 10.1007/s10803-021-04912-1
20. Cage E, Troxell-Whitman Z. Understanding the reasons, contexts and costs of camouflaging for autistic adults. J Autism Dev Disord. (2019) 49:1899–911. doi: 10.1007/s10803-018-03878-x
21. Cassidy S, Bradley L, Shaw R, Baron-Cohen S. Risk markers for suicidality in autistic adults. Mol Autism. (2018) 9:42. doi: 10.1186/s13229-018-0226-4
22. Hosozawa M, Sacker A, Cable N. Timing of diagnosis, depression and self-harm in adolescents with autism spectrum disorder. Autism. (2021) 25:70–8. doi: 10.1177/1362361320945540
23. Jadav N, Bal VH. Associations between co-occurring conditions and age of autism diagnosis: implications for mental health training and adult autism research. Autism Res. (2022) 15:2112–25. doi: 10.1002/aur.2808
24. Zuckerman K, Lindly OJ, Chavez AE. Timeliness of autism spectrum disorder diagnosis and use of services among U.S. Element Schl Aged Child Psychiatr Serv. (2017) 68, 33–40. doi: 10.1176/appi.ps.201500549
25. Holden R, Mueller J, McGowan J, Sanyal J, Kikoler M, Simonoff E, et al. Investigating bullying as a predictor of suicidality in a clinical sample of adolescents with autism spectrum disorder. Autism Res. (2020) 13:988–97. doi: 10.1002/aur.2292
26. Taylor JL, Gotham KO. Cumulative life events, traumatic experiences, and psychiatric symptomatology in transition-aged youth with autism spectrum disorder. J Neurodev Disord. (2016) 8:28. doi: 10.1186/s11689-016-9160-y
27. Deb S, Roy M, Lee R, Majid M, Limbu B, Santambrogio J, et al. Randomised controlled trials of antidepressant and anti-anxiety medications for people with autism spectrum disorder: systematic review and meta-analysis. BJPsych Open. (2021) 7:e179. doi: 10.1192/bjo.2021.1003
28. Menezes M, Harkins C, Robinson MF, Mazurek MO. Treatment of depression in individuals with autism spectrum disorder: a systematic review. Res Autism Spectr Disord. (2020) 78:101639. doi: 10.1016/j.rasd.2020.101639
29. White MJ. Treatment-resistant depression: consider autism. Br J Gen Pract. (2019) 69:14. doi: 10.3399/bjgp19X700373
30. Bauer M, Adli M, Baethge C, Berghöfer A, Sasse J, Heinz A, et al. (2003). Lithium augmentation therapy in refractory depression: clinical evidence and neurobiological mechanisms. Can J Psychiatr. 48:440–8. doi: 10.1177/070674370304800703
31. Smith KA, Cipriani A. Lithium and suicide in mood disorders: updated meta-review of the scientific literature. Bipolar Disord. (2017) 19:575–86. doi: 10.1111/bdi.12543
32. Wu X, Bai Y, Tan T, Li H, Xia S, Chang X, et al. Lithium ameliorates autistic-like behaviors induced by neonatal isolation in rats. Front Behav Neurosci. (2014) 8:234. doi: 10.3389/fnbeh.2014.00234
33. Siegel M, Beresford CA, Bunker M, Verdi M, Vishnevetsky D, Karlsson C, et al. Preliminary investigation of lithium for mood disorder symptoms in children and adolescents with autism spectrum disorder. J Child Adolesc Psychopharmacol. (2014) 24:399–402. doi: 10.1089/cap.2014.0019
34. Mintz M, Hollenberg E. Revisiting lithium: utility for behavioral stabilization in adolescents and adults with autism spectrum disorder. Psychopharmacol Bull. (2019)49:28.
PubMed Abstract | Google Scholar
35. Serret S, Thümmler S, Dor E, Vesperini S, Santos A, Askenazy F. Lithium as a rescue therapy for regression and catatonia features in two SHANK3 patients with autism spectrum disorder: case reports. BMC Psychiatry. (2015) 15:107. doi: 10.1186/s12888-015-0490-1
36. Epperson CN, McDougle CJ, Anand A, Marek GJ, Naylor ST, Volkmar FR, et al. Lithium augmentation of fluvoxamine in autistic disorder: a case report. J Child Adolesc Psychopharmacol. (1994) 4:201–7. doi: 10.1089/cap.1994.4.201
37. Clarke D, Baxter M, Perry D, Prasher V. The diagnosis of affective and psychotic disorders in adults with autism: seven case reports. Autism. (1999) 3:149–64. doi: 10.1177/1362361399003002005
38. Ising HK, Veling W, Loewy RL, Rietveld MW, Rietdijk J, Dragt S, et al. The validity of the 16-item version of the prodromal questionnaire (PQ-16) to screen for ultra high risk of developing psychosis in the general help-seeking population. Schizophr Bull. (2012) 38:1288–96. doi: 10.1093/schbul/sbs068
39. Gowers S, Levine W, Bailey-Rogers S, Shore A, Burhouse E. Use of a routine, self-report outcome measure (HoNOSCA–SR) in two adolescent mental health services. Br J Psychiatry. (2002) 180:266–9. doi: 10.1192/bjp.180.3.266
40. Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, et al. Austism diagnostic observation schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. (1989) 19:185–212. doi: 10.1007/BF02211841
41. Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. (1994) 24:659–85. doi: 10.1007/BF02172145
42. Oswald TM, Winter-Messiers MA, Gibson B, Schmidt AM, Herr CM, Solomon M. Sex differences in internalizing problems during adolescence in autism spectrum disorder. J Autism Dev Disord. (2016) 46:624–36. doi: 10.1007/s10803-015-2608-1
43. Lai M-C, Szatmari P. Sex and gender impacts on the behavioural presentation and recognition of autism. Curr Opin Psychiatry. (2020) 33:117–23. doi: 10.1097/YCO.0000000000000575
44. Stephenson KG, Norris M, Butter EM. Sex-based differences in autism symptoms in a large, clinically-referred sample of preschool-aged children with ASD. J Autism Dev Disord . (2021) 53:624–32. doi: 10.1007/s10803-020-04836-2
45. Leedham A, Thompson AR, Smith R, Freeth M. ‘I was exhausted trying to figure it out': the experiences of females receiving an autism diagnosis in middle to late adulthood. Autism. (2020) 24:135–46. doi: 10.1177/1362361319853442
46. Halsall J, Clarke C, Crane L. “Camouflaging” by adolescent autistic girls who attend both mainstream and specialist resource classes: perspectives of girls, their mothers and their educators. Autism. (2021) 25:2074–86. doi: 10.1177/13623613211012819
47. Dell'Osso L, Carpita B, Cremone IM, Muti D, Diadema E, Barberi FM, et al. The mediating effect of trauma and stressor related symptoms and ruminations on the relationship between autistic traits and mood spectrum. Psychiatry Res . (2019) 279:123–9. doi: 10.1016/j.psychres.2018.10.040
48. Haruvi-Lamdan N, Horesh D, Zohar S, Kraus M, Golan O. Autism spectrum disorder and post-traumatic stress disorder: an unexplored co-occurrence of conditions. Autism. (2020) 24:884–98. doi: 10.1177/1362361320912143
49. Kerns CM, Lankenau S, Shattuck PT, Robins DL, Newschaffer CJ, Berkowitz SJ. Exploring potential sources of childhood trauma: a qualitative study with autistic adults and caregivers. Autism. (2022) 26:1987–98. doi: 10.1177/13623613211070637
50. Carmassi C, Bertelloni CA, Salarpi G, Diadema E, Avella MT, Dell'Oste V, et al. Is there a major role for undetected autism spectrum disorder with childhood trauma in a patient with a diagnosis of bipolar disorder, self-injuring, and multiple comorbidities? Case Rep Psychiatry . (2019) 2019:1–6. doi: 10.1155/2019/4703795
51. Lew-Koralewicz A. Psychosocial Functioning and the Educational Experiences of Students with ASD during the COVID-19 Pandemic in Poland. Int J Environ Res Public Health. (2022) 19:9468. doi: 10.3390/ijerph19159468
52. Oomen D, Nijhof AD, Wiersema JR. The psychological impact of the COVID-19 pandemic on adults with autism: a survey study across three countries. Mol Autism. (2021) 12:21. doi: 10.1186/s13229-021-00424-y
53. Cooper K, Loades ME, Russell A. Adapting psychological therapies for autism. Res Autism Spectr Disord. (2018) 45:43–50. doi: 10.1016/j.rasd.2017.11.002
54. Linden A, Best L, Elise F, Roberts D, Branagan A, Tay YBE, et al. Benefits and harms of interventions to improve anxiety, depression, and other mental health outcomes for autistic people: a systematic review and network meta-analysis of randomised controlled trials. Autism. (2022) 2022:136236132211179. doi: 10.1177/13623613221117931
55. Persico AM, Ricciardello A, Lamberti M, Turriziani L, Cucinotta F, Brogna C, et al. The pediatric psychopharmacology of autism spectrum disorder: a systematic review - part I: the past and the present. Progress Neuro Psychopharmacol Biol Psychiatry. (2021) 110:110326. doi: 10.1016/j.pnpbp.2021.110326
56. Williams K, Brignell A, Randall M, Silove N, Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Cochrane Database Syst Rev . (2013) 8. doi: 10.1002/14651858.CD004677.pub3
57. Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological Treat Can J Psychiatry. (2016) 61:540–60. doi: 10.1177/0706743716659417
58. Grunze H, Vieta E, Goodwin GM, Bowden C, Licht RW, Azorin J-M, et al. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: acute and long-term treatment of mixed states in bipolar disorder. World J Biol Psychiatry. (2018) 19:2–58. doi: 10.1080/15622975.2017.1384850
59. Mullen S. Major depressive disorder in children and adolescents. Mental Health Clin. (2018) 8:275–83. doi: 10.9740/mhc.2018.11.275
Keywords: Autism Spectrum Disorder, depression, adolescent, case report, female, lithium, COVID-19, suicidality
Citation: Secci I, Petigas L, Cuenod A, Klauser P, Kapp C, Novatti A and Armando M (2023) Case report: Treatment-resistant depression, multiple trauma exposure and suicidality in an adolescent female with previously undiagnosed Autism Spectrum Disorder. Front. Psychiatry 14:1151293. doi: 10.3389/fpsyt.2023.1151293
Received: 25 January 2023; Accepted: 03 April 2023; Published: 26 April 2023.
Reviewed by:
Copyright © 2023 Secci, Petigas, Cuenod, Klauser, Kapp, Novatti and Armando. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ilaria Secci, ilaria.secci@unito.it
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

IMAGES
VIDEO
COMMENTS
However, there are still some problems with treatment, including safety, efficacy, ethics, dose, frequency of administration and their effect in treatment-resistant depression. In the present study, we treated one case of treatment resistant depression with repeated intravenous injections with a low dosage of ketamine.
A case of treatment-resistant depression in an older adult and a discussion of treatment options - Volume 46 Issue 6 ... CBT, intensive short-term dynamic psychotherapy, interpersonal therapy and group dialectic behavioural therapy. The study concluded that there is moderate-quality evidence to support the efficacy of psychological therapies in ...
Twenty-nine people with treatment-resistant depression participated in the study: About half received SAINT, and the rest underwent a placebo procedure that mimicked the real treatment. After five days of treatment, 78.6% of the participants in the treatment group were no longer depressed, according to several standard methods of evaluation.
A commonly accepted definition of treatment-resistant depression is a lack of improvement despite adequate trials of two different classes of antidepressants for at least 8 weeks. •. Assessment ...
Abstract. Depression is a common mental disorder affecting more than 264 million people worldwide. The first-line treatment for most cases of depression are selective serotonin reuptake inhibitors ...
The first case presentation, No. 1, is a patient newly diagnosed with treatment-resistant depression. A 26-year-old man with a history of major depressive disorder for over 7 years presents with complaints of trouble sleeping as well as feeling unhappy, worried, and fatigued. He gradually developed sleeping difficulty as well as low mood and ...
Treatment-resistant depression and risk of autoimmune diseases: evidence from a population-based cohort and nested case-control study
Treatment-resistant depression is a prevalent and persistent disorder associated with significant morbidity and mortality in older adults. ... This case demonstrates the complexity of treatment-resistant depression in an older adult where various pathologic processes may intersect. ... The present study is an investigation of cholinergic system ...
Real-world evidence in treatment-resistant depression (TRD; commonly defined as non-response to ≥ 2 consecutive treatments at adequate dosage and duration) is lacking. ... 1 chart review and 1 case-control study. Fig. 1. Flowchart of studies included and excluded in the systematic review process. CDSR Cochrane Database of Systematic Reviews
Sara, a 35-year-old married female. Sara was referred to treatment after having a stillbirth. Sara showed symptoms of grief, or complicated bereavement, and was diagnosed with major depression, recurrent. The clinician recommended interpersonal psychotherapy (IPT) for a duration of 12 weeks. Bleiberg, K.L., & Markowitz, J.C. (2008).
Psilocybin is being studied for use in treatment-resistant depression. In this phase 2 double-blind trial, we randomly assigned adults with treatment-resistant depression to receive a single dose o...
The European "Group for the Study of Resistant Depression" (GSRD) multi-site study found that age at first treatment (i.e., early-onset and early treatment), age, timespan between first and ...
#### WHAT IS ALREADY KNOWN ON THIS TOPIC #### WHAT THIS STUDY ADDS #### HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY Major depressive disorder (MDD) is a principal cause of disability worldwide and is often associated with high morbidity and mortality rates. Although there are several therapies available for the treatment of depression, about one-third of patients with MMD will not ...
Major depressive disorder (MDD) patients not responding to two or more different antidepressant treatments are currently considered to suffer from treatment resistant depression (TRD). Recently, intranasal esketamine has been approved by both the American Food and Drug Administration and European Medicines Agency for TRD and, more recently, in moderate to severe episode of MDD, as acute short ...
Background and aims: Treatment‐resistant depression (TRD), defined as inadequate treatment response after at least two adequate treatment trials, is common among patients initiating antidepressant treatment. ... Design: Nested case-control study. Setting: Nation‐wide governmental health‐care registers in Sweden. Cases and controls: Data ...
Effective and specific treatments for treatment-resistant depression are urgently needed. 10 In clinical practice, pharmacologic treatments approved for major depressive disorder, including oral ...
Citation: Secci I, Petigas L, Cuenod A, Klauser P, Kapp C, Novatti A and Armando M (2023) Case report: Treatment-resistant depression, multiple trauma exposure and suicidality in an adolescent female with previously undiagnosed Autism Spectrum Disorder. Front. Psychiatry 14:1151293. doi: 10.3389/fpsyt.2023.1151293
Treatment-resistant depression (TRD), defined as inadequate treatment response after at least two adequate treatment trials, is common among patients initiating antidepressant treatment. ... In this nested case-control study of antidepressant-treated patients with depression, we found that SUD before start of, or during, treatment increases ...