
AMY CANAVAN, MD, AND BILLY S. ARANT, JR., MD
Am Fam Physician. 2009;80(7):692-696
Author disclosure: Nothing to disclose.
The most useful individual signs for identifying dehydration in children are prolonged capillary refill time, abnormal skin turgor, and abnormal respiratory pattern. However, clinical dehydration scales based on a combination of physical examination findings are better predictors than individual signs. Oral rehydration therapy is the preferred treatment of mild to moderate dehydration caused by diarrhea in children. Appropriate oral rehydration therapy is as effective as intravenous fluid in managing fluid and electrolyte losses and has many advantages. Goals of oral rehydration therapy are restoration of circulating blood volume, restoration of interstitial fluid volume, and maintenance of rehydration. When rehydration is achieved, a normal age-appropriate diet should be initiated.
Clinical dehydration scales based on a combination of physical examination findings are the most specific and sensitive tools for accurately diagnosing dehydration in children and categorizing its severity. Overdiagnosis of dehydration may lead to unnecessary tests and treatment, whereas underdiagnosis may lead to increased morbidity (e.g., protracted vomiting, electrolyte disturbances, acute renal insufficiency).
Among children in the United States, fluid and electrolyte disturbances from acute gastroenteritis result in 1.5 million outpatient visits, 200,000 hospitalizations, and 300 deaths per year. 1 Additionally, children may become dehydrated because of a variety of other illnesses that cause vomiting, diarrhea, or poor fluid intake.

PARENTAL OBSERVATION
Parental report of vomiting, diarrhea, or decreased oral intake is sensitive, but not specific, for identifying dehydration in children. If parents report that the child does not have diarrhea, has normal oral intake, and has normal urine output, the chance of dehydration is low. Likewise, when parents are asked about physical signs of dehydration, a number of positive answers suggest dehydration. However, if the parents report normal tear production, the chance of dehydration is low. 2 , 3
PHYSICAL EXAMINATION
Comparing change in body weight from before and after rehydration is the standard method for diagnosing dehydration. 4 To identify dehydration in infants and children before treatment, a number of symptoms and clinical signs have been evaluated and compared with this standard method. Physical examination findings during dehydration represent desiccation of tissue, the body's compensatory reaction to maintain perfusion, or both. The most useful individual signs for identifying dehydration are prolonged capillary refill time, abnormal skin turgor, and abnormal respiratory pattern. 5 However, clinical dehydration scales based on a combination of physical examination findings are much better predictors than individual signs. 5
In one study, four factors predicted dehydration: capillary refill time of more than two seconds, absence of tears, dry mucous membranes, and ill general appearance; the presence of two or more of these signs indicated a fluid deficit of at least 5 percent. 6 In a similar validated scale, general appearance, degree of sunken eyes, dryness of mucous membranes, and tear production were associated with length of hospital stay and need for intravenous fluids in children with acute gastroenteritis. 7
Capillary refill time is performed in warm ambient temperature, and is measured on the sternum of infants and on a finger or arm held at the level of the heart in older children. The measurement is not affected by fever and should be less than two seconds. 8 Assessment of skin turgor is performed by pinching skin on the lateral abdominal wall at the level of the umbilicus. Turgor (i.e., time required for the skin to recoil) is normally instantaneous and increases linearly with degree of dehydration. 9 Respiratory pattern and heart rate should be compared with age-specific normal values.
LABORATORY ASSESSMENT
Unlike in adults, calculation of the blood urea nitrogen (BUN)/creatinine ratio is not useful in children. Although the normal BUN level is the same for children and adults, the normal serum creatinine level changes with age (0.2 mg per dL [17.68 μmol per L] in infants to 0.8 mg per dL [70.72 μmol per L] in adolescents). BUN alone and urine specific gravity also have poor sensitivity and specificity for predicting dehydration in children. 10
In combination with a clinical dehydration scale, a serum bicarbonate level of less than 17 mEq per L (17 mmol per L) may improve sensitivity of identifying children with moderate to severe hypovolemia. 11 Additionally, a serum bicarbonate level of less than 13 mEq per L (13 mmol per L) is associated with increased risk of failure of outpatient rehydration efforts. 12
PATHOPHYSIOLOGY
Most of the volume loss in dehydration is extracellular fluid. The extracellular fluid space has two components: plasma and lymph as a delivery system, and interstitial fluid for solute exchange. 13 The goal of rehydration therapy is first to restore the circulating blood volume, if necessary; then to restore the interstitial fluid volume; and finally to maintain hydration and replace continuing losses, such as diarrhea and increased insensible losses caused by fever.
ORAL REHYDRATION THERAPY
The American Academy of Pediatrics recommends oral rehydration therapy (ORT) as the preferred treatment of fluid and electrolyte losses caused by diarrhea in children with mild to moderate dehydration. 14 ORT is as effective as intravenous fluid in rehydration of children with mild to moderate dehydration—there is no difference in failure rate or hospital admission rate between the two treatments. 15 Additionally, ORT has many advantages compared with intravenous fluid therapy. It can be administered at home, reducing the need for outpatient and emergency department visits; requires less emergency department staff time; and leads to shorter emergency department stays. Parents are also more satisfied with the visit when ORT had been used. 16 With ORT, the same fluid can be used for rehydration, maintenance, and replacement of stool losses; and ORT can be initiated more quickly than intravenous fluid therapy. 17
The principles of ORT to treat dehydration from gastroenteritis apply to the treatment of dehydration from other causes. Altered mental status with risk of aspiration, abdominal ileus, and underlying intestinal malabsorption are contraindications. Cost to the family may be a deterrent to home ORT; therefore, ORT solution provided by the physician's office or emergency department increases the likelihood that parents will use ORT and reduces unscheduled follow-up visits. 16
Nasogastric rehydration therapy with ORT solution is an alternative to intravenous fluid therapy in patients with poor oral intake. Nasogastric hydration using oral rehydration solution is tolerated as well as ORT. Failure rate of nasogastric tube placement is significantly less than that of intravenous lines, and significant complications of nasogastric tube placement are rare. Nasogastric rehydration therapy is also less expensive than intravenous fluid therapy. 18
As soon as children with acute gastroenteritis are rehydrated, a regular age-appropriate diet should be initiated. This does not worsen the symptoms of mild diarrhea, and may decrease its duration. 14
Preparations . Use of an appropriate ORT solution, such as commercial electrolyte solutions for children (e.g., Pedialyte), corrects and helps prevent electrolyte disturbances caused by gastroenteritis. 17 – 19 The World Health Organization ORT solution contains 90 mEq per L of sodium, mimicking the sodium content of diarrhea caused by cholera. Commercial ORT preparations typically contain around 50 mEq per L of sodium, which is more consistent with the sodium content of diarrhea caused by rotavirus. 20 Commercial ORT solutions contain 25 g per L of dextrose, which helps prevent hypoglycemia without causing osmotic diuresis, 21 and 30 mEq per L of bicarbonate, which leads to less vomiting and more efficient correction of acidosis. 19 Commercial ORT solutions are recommended over homemade solutions because of the risk of preparation errors. 22 Clear sodas and juices should not be used for ORT because hyponatremia may occur. Table 1 compares the electrolyte composition of commercial electrolyte solutions with other clear liquids.
Administration . For mild dehydration, 50 mL per kg of ORT solution should be administered over four hours using a spoon, syringe, or medicine cup 14 ; this can be accomplished by giving 1 mL per kg of the solution to the child every five minutes. Patients may be treated at home. 14 If the child vomits, treatment should be resumed after 30 minutes. 15 After the four-hour treatment period, maintenance fluids should be given and ongoing losses assessed and replaced every two hours. Maintenance therapy includes providing anticipated water and electrolyte needs for the next 24 hours in the child who is now euvolemic with expected normal urine output. The Holliday-Segar method ( Table 2 23 ) is a simple, reliable formula for estimating water needs. 24 Based on average weights of infants and children, this method can be further simplified to provide maintenance ORT at home: 1 oz per hour for infants, 2 oz per hour for toddlers, and 3 oz per hour for older children. To replace ongoing losses, 10 mL per kg for every loose stool and 2 mL per kg for every episode of emesis should be administered.
For moderate dehydration, 100 mL per kg of ORT solution should be given over four hours in the physician's office or emergency department. 14 If treatment is successful and ongoing losses are not excessive, the child may be sent home. At home, caregivers should provide maintenance therapy and replace ongoing losses every two hours as described for mild dehydration. ORT is considered to be unsuccessful if vomiting is severe and persistent (i.e., at least 25 percent of the hourly oral requirement) or if ORT cannot keep up with the volume of stool losses. 17
Severe dehydration should be treated with intravenous fluids until the patient is stabilized (i.e., circulating blood volume is restored). Treatment should include 20 mL per kg of isotonic crystalloid (normal saline or lactated Ringer solution) over 10 to 15 minutes. 25 No other fluid type is currently recommended for volume resuscitation in children. 26 Treatment should be repeated as necessary, with monitoring of the patient's pulse strength, capillary refill time, mental status, and urine output. Stabilization often requires up to 60 mL per kg of fluid within an hour. 25 Electrolyte measurement should be performed in all children with severe dehydration and considered in those with moderate dehydration because it may be difficult to predict which children have significant electrolyte abnormalities. 27 After resuscitation is completed and normal electrolyte levels are achieved, the patient should receive 100 mL per kg of ORT solution over four hours, then maintenance fluid and replacement of ongoing losses. If ORT fails after initial resuscitation of a child with severe dehydration, intravenous fluid therapy should be initiated. First, 100 mL per kg of isotonic crystalloid should be administered over four hours, followed by a maintenance solution. This method also may be used when a child with moderate dehydration fails ORT.
The electrolyte content of intravenous maintenance fluid for infants and children with normal serum electrolyte levels should be 5 percent dextrose and 25 percent normal saline, plus 20 mEq per L of potassium. 23 , 28 , 29 Intake, output, and vital signs must be checked every four hours, and adjustments made to the therapy as necessary (e.g., in the setting of ongoing losses, such as excessive stool output, or persistent fever). If stool output exceeds 30 mL per kg per day, it should be replaced in an equal volume every four hours with an intravenous solution comparable in electrolytes with the stool (50 percent normal saline plus 20 to 30 mEq per L of potassium), in addition to the volume of maintenance fluid, until ORT can be tolerated. Children with persistent fever may require 1 mL per kg per degree centigrade every hour, in addition to the calculated maintenance therapy. Postoperatively and in children with central nervous system infection or injury, 20 to 50 percent less fluid and fluid with higher sodium content may be needed because of abnormal antidiuretic hormone secretion. 28 These adjustments in fluid rates are guided by regular measurement of urine output and vital signs.
MEDICATIONS
Pharmacologic agents are not recommended to decrease diarrhea because of limited evidence and concern for toxicity. Although Lactobacillus has no major toxic effects, its effectiveness in patients with diarrhea has not been demonstrated. 14 A single dose of ondansetron (Zofran) has been shown to facilitate ORT by reducing the incidents and frequency of vomiting and, therefore, reducing the failure of ORT and the need for intravenous fluid therapy. 30 Recurrent dosing of ondansetron has not been studied.
Complications
Hypernatremia, hyponatremia, and hypoglycemia occasionally complicate dehydration. Serum electrolyte levels should be measured in children with severe dehydration and in those with moderate dehydration that presents in atypical ways.
Hypernatremia (serum sodium level of greater than 145 mEq per L [145 mmol per L]) indicates water loss in excess of sodium loss. Because sodium is restricted to the extracellular fluid space, the typical signs of dehydration are less pronounced in the setting of hypernatremia, and significant circulatory disturbance is not likely to be noted until dehydration reaches 10 percent. Findings that may aid in the diagnosis of hypernatremia in children include a “doughy” feeling rather than tenting when testing for skin turgor, increased muscle tone, irritability, and a high-pitched cry. 31 Hyponatremia is often caused by inappropriate use of oral fluids that are low in sodium, such as water, juice, and soda. If severe dehydration is present, a child with hypernatremia or hyponatremia should receive isotonic crystalloid until stabilized. If after initial volume repletion, hyponatremia or hypernatremia remains moderate to severe (serum sodium level of less than 130 mEq per L [130 mmol per L] or greater than 150 mEq per L [150 mmol per L]), replacement of the remaining fluid deficit should be altered, with a principal goal of slow correction.
In one study, blood glucose levels of less then 60 mg per dL (3.33 mmol per L) were detected in 9 percent of children younger than nine years (mean age 18 months) admitted to the hospital with diarrhea. 27 History and physical examination findings did not indicate that these children were at risk; therefore, blood glucose screening may be indicated for toddlers with diarrhea.
King CK, Glass R, Bresee JS, Duggan C for the Centers for Disease Control and Prevention. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52(RR-16):1-16.
Porter SC, Fleisher GR, Kohane IS, Mandl KD. The value of parental report for diagnosis and management of dehydration in the emergency department. Ann Emerg Med. 2003;41(2):196-205.
Armon K, Stephenson T, MacFaul R, Eccleston P, Werneke U. An evidence and consensus based guideline for acute diarrhoea management. Arch Dis Child. 2001;85(2):132-142.
Friedman JN, Goldman RD, Srivastava R, Parkin PC. Development of a clinical dehydration scale for use in children between 1 and 36 months of age. J Pediatr. 2004;145(2):201-207.
Steiner MJ, DeWalt DA, Byerley JS. Is this child dehydrated?. JAMA. 2004;291(22):2746-2754.
Gorelick MH, Shaw KN, Murphy KO. Validity and reliability of clinical signs in the diagnosis of dehydration in children. Pediatrics. 1997;99(5):E6.
Goldman RD, Friedman JN, Parkin PC. Validation of the clinical dehydration scale for children with acute gastroenteritis. Pediatrics. 2008;122(3):545-549.
Gorelick MH, Shaw KN, Murphy KO, Baker MD. Effect of fever on capillary refill time. Pediatr Emerg Care. 1997;13(5):305-307.
Laron Z. Skin turgor as a quantitative index of dehydration in children. Pediatrics. 1957;19(5):816-822.
Teach SJ, Yates EW, Feld LG. Laboratory predictors of fluid deficit in acutely dehydrated children. Clin Pediatr (Phila). 1997;36(7):395-400.
Vega RM, Avner JR. A prospective study of the usefulness of clinical and laboratory parameters for predicting percentage of dehydration in children. Pediatr Emerg Care. 1997;13(3):179-182.
Reid SR, Bonadio WA. Outpatient rapid intravenous rehydration to correct dehydration and resolve vomiting in children with acute gastroenteritis. Ann Emerg Med. 1996;28(3):318-323.
Holliday MA, Friedman AL, Wassner SJ. Extracellular fluid restoration in dehydration: a critique of rapid versus slow. Pediatr Nephrol. 1999;13(4):292-297.
Practice parameter: the management of acute gastroenteritis in young children. American Academy of Pediatrics, Provisional Committee on Quality Improvement, Subcommittee on Acute Gastroenteritis. Pediatrics. 1996;97(3):424-435.
Atherly-John YC, Cunningham SJ, Crain EF. A randomized trial of oral vs intravenous rehydration in a pediatric emergency department. Arch Pediatr Adolesc Med. 2002;156(12):1240-1243.
Duggan C, Lasche J, McCarty M, et al. Oral rehydration solution for acute diarrhea prevents subsequent unscheduled follow-up visits. Pediatrics. 1999;104(3):e29.
Spandorfer PR, Alessandrini EA, Joffe MD, Localio R, Shaw KN. Oral versus intravenous rehydration of moderately dehydrated children: a randomized, controlled trial. Pediatrics. 2005;115(2):295-301.
Nager AL, Wang VJ. Comparison of nasogastric and intravenous methods of rehydration in pediatric patients with acute dehydration. Pediatrics. 2002;109(4):566-572.
Islam MR, Ahmed SM. Oral rehydration solution without bicarbonate. Arch Dis Child. 1984;59(11):1072-1075.
Molla AM, Rahman M, Sarker SA, Sack DA, Molla A. Stool electrolyte content and purging rates in diarrhea caused by rotavirus, enterotoxigenic E. coli , and V. cholerae in children. J Pediatr. 1981;98(5):835-838.
Rahman O, Bennish ML, Alam AN, Salam MA. Rapid intravenous rehydration by means of a single polyelectrolyte solution with or without dextrose. J Pediatr. 1988;113(4):654-660.
Meyers A, Sampson A, Saladino R, Dixit S, Adams W, Mondolfi A. Safety and effectiveness of homemade and reconstituted packet cereal-based oral rehydration solutions: a randomized clinical trial. Pediatrics. 1997;100(5):E3.
Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19(5):823-832.
Holliday MA, Ray PE, Friedman AL. Fluid therapy for children: facts, fashions and questions. Arch Dis Child. 2007;92(6):546-550.
Boluyt N, Bollen CW, Bos AP, Kok JH, Offringa M. Fluid resuscitation in neonatal and pediatric hypovolemic shock: a Dutch Pediatric Society evidence-based clinical practice guideline. Intensive Care Med. 2006;32(7):995-1003.
Pediatric Advanced Life Support Provider Manual Dallas, Tex: American Heart Association; 2006: 232.
Wathen JE, MacKenzie T, Bothner JP. Usefulness of the serum electrolyte panel in the management of pediatric dehydration treated with intravenously administered fluids. Pediatrics. 2004;114(5):1227-1234.
Friedman AL, Ray PE. Maintenance fluid therapy: what it is and what it is not. Pediatr Nephrol. 2008;23(5):677-680.
Assadi F, Copelovitch L. Simplified treatment strategies to fluid therapy in diarrhea [published correction appears in Pediatr Nephrol . 2004;19(3):364]. Pediatr Nephrol. 2003;18(11):1152-1156.
Freedman SB, Adler M, Seshadri R, Powell EC. Oral ondansetron for gastroenteritis in a pediatric emergency department. N Engl J Med. 2006;354(16):1698-1705.
Conley SB. Hypernatremia. Pediatr Clin North Am. 1990;37(2):365-372.
Continue Reading
More in afp, more in pubmed.
Copyright © 2009 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Dehydration in Children
- Pathophysiology |
- Symptoms and Signs |
- Diagnosis |
- Treatment |
- Practical Rehydration Example |
- Procedure |
- More Information |
Dehydration is significant depletion of body water and, to varying degrees, electrolytes. Symptoms and signs include thirst, lethargy, dry mucosa, decreased urine output, and, as the degree of dehydration progresses, tachycardia, hypotension, and shock. Diagnosis is based on history and physical examination. Treatment is with oral or IV replacement of fluid and electrolytes.
Dehydration remains a major cause of morbidity and mortality in infants and young children worldwide. Dehydration is a symptom or sign of another disorder, most commonly diarrhea . Infants are particularly susceptible to the ill effects of dehydration because of their greater baseline fluid requirements (due to a higher metabolic rate), higher evaporative losses (due to a higher ratio of surface area to volume), and inability to communicate thirst or seek fluid.
Etiology of Dehydration in Children
Dehydration results from
Increased fluid loss
Decreased fluid intake
The most common source of increased fluid loss is the gastrointestinal tract—from vomiting , diarrhea , or both (eg, gastroenteritis ). Other sources are renal (eg, diabetic ketoacidosis ), cutaneous (eg, excessive sweating , burns ), and 3rd-space losses (eg, into the intestinal lumen in bowel obstruction or ileus).
Decreased fluid intake is common during mild illnesses such as pharyngitis or during serious illnesses of any kind. Decreased fluid intake is particularly problematic when the child is vomiting or when fever, tachypnea, or both increase insensible losses. It may also be a sign of neglect .
Pathophysiology of Dehydration in Children
All types of lost fluid contain electrolytes in varying concentrations, so fluid loss is always accompanied by some degree of electrolyte loss. The exact amount and type of electrolyte loss varies depending on the cause. For example, significant amounts of bicarbonate may be lost with diarrhea, predisposing to metabolic acidosis ; however, with vomiting, hydrogen ions are lost, predisposing to metabolic alkalosis . However, fluid lost always contains a lower concentration of sodium than the plasma. Thus, in the absence of any fluid replacement, the serum sodium usually rises (hypernatremia).
Hypernatremia causes water to shift from the intracellular and interstitial space into the intravascular space, helping, at least temporarily, to maintain vascular volume. With hypotonic fluid replacement (eg, with plain water), serum sodium may normalize but can also decrease below normal (hyponatremia). Hyponatremia results in some fluid shifting out of the intravascular space into the interstitium at the expense of vascular volume.
Symptoms and Signs of Dehydration in Children
Symptoms and signs of dehydration vary according to degree of deficit (see table Clinical Correlates of Dehydration ) and by the serum sodium level.
Because of the fluid shift out of the interstitium into the vascular space, children with hypernatremia appear more ill (eg, with very dry mucous membranes, a doughy appearance to the skin) for a given degree of water loss than do children with hyponatremia. However, children with hypernatremia have better hemodynamics (eg, less tachycardia and better urine output) than do children with hyponatremia, in whom fluid has shifted out of the vascular space.
Dehydrated children with hyponatremia may appear only mildly dehydrated but are actually closer to hypotension and cardiovascular collapse than are equally dehydrated children with elevated or normal sodium levels.
Diagnosis of Dehydration in Children
Clinical evaluation
In general, dehydration is defined as follows:
Mild: No hemodynamic changes (about 5% body weight in infants and 3% in adolescents)
Moderate: Tachycardia (about 10% body weight in infants and 5 to 6% in adolescents)
Severe: Hypotension with impaired perfusion (about 15% body weight in infants and 7 to 9% in adolescents)
However, using a combination of symptoms and signs to assess dehydration is a more accurate method than using only one sign.
Another way to assess the degree of dehydration in children with acute dehydration is change in body weight; all short-term weight loss > 1%/day is presumed to represent fluid deficit. However, this method depends on knowing a precise, recent preillness weight. Parental estimates are usually inadequate; a 1-kg error in a 10-kg child causes a 10% error in the calculated percentage of dehydration—the difference between mild and severe dehydration.
Laboratory testing is usually reserved for moderately or severely ill children, in whom electrolyte disturbances (eg, hypernatremia , hypokalemia , metabolic acidosis or metabolic alkalosis
Treatment of Dehydration in Children
Fluid replacement (oral if possible)
Treatment of dehydration is best approached by considering the following separately:
Fluid resuscitation requirements
Current deficit
Ongoing losses
Maintenance requirements.
The volume (eg, amount of fluid), composition, and rate of replacement differ for each. Formulas and estimates used to determine treatment parameters provide a starting place, but treatment requires ongoing monitoring of vital signs, clinical appearance, urine output, weight, and sometimes serum electrolyte levels.
The American Academy of Pediatrics and the World Health Organization (WHO) both recommend oral replacement therapy for mild and moderate dehydration. Children with severe dehydration (eg, evidence of circulatory compromise) should receive fluids IV. Children who are unable or unwilling to drink or who have repetitive vomiting can receive fluid replacement orally through frequently repeated small amounts, through an IV, or through a nasogastric tube (see Solutions ).
Resuscitation
Patients with signs of hypoperfusion should receive fluid resuscitation with boluses of isotonic fluid (eg, 0.9% saline or Ringer's lactate). The goal is to restore adequate circulating volume to restore blood pressure and perfusion.
The resuscitation phase should reduce moderate or severe dehydration to a deficit of about 6 to 8% body weight. If dehydration is moderate, 20 mL/kg (2% body weight) is given IV over 20 to 30 minutes, reducing a 10% deficit to 8%. If dehydration is severe, 3 boluses of 20 mL/kg (6% body weight) may be required.
The end point of the fluid resuscitation phase is reached when peripheral perfusion and blood pressure are restored and the heart rate is returned to normal (in an afebrile child).
Deficit replacement
Total deficit volume is estimated clinically as described previously. Sodium deficits are usually about 60 mEq/L (60 mmol/L) of fluid deficit, and potassium deficits are usually about 30 mEq/L (30 mmol/L) of fluid deficit. The resuscitation phase should have reduced moderate or severe dehydration to a deficit of about 6 to 8% body weight; this remaining deficit is typically replaced over the next 24 hours.
Because 0.45% saline has 77 mEq sodium per liter (77 mmol/L), it is usually an appropriate fluid choice, particularly in children with diarrhea, because the electrolyte content of diarrhea is typically 50 to 100 mEq/L (50 to 100 mmol/L) (see table Estimated Electrolyte Deficits by Cause ); 0.9% saline may be used as well.
Potassium replacement (usually by adding 20 to 40 mEq potassium per liter [20 to 40 mmol/L] of replacement fluid) should not begin until adequate urine output is established.
Dehydration in neonates, particularly with significant hypernatremia (eg, serum sodium > 160 mEq/L [> 160 mmol/L]) or hyponatremia (eg, serum sodium < 120 mEq/L [
Volume of ongoing losses should be measured directly (eg, nasogastric tube, catheter, stool measurements) or estimated (eg, 10 mL/kg per diarrheal stool). Replacement should be milliliter for milliliter in time intervals appropriate for the rapidity and extent of the loss.
Ongoing electrolyte losses can be estimated by source or cause (see table Estimated Electrolyte Deficits by Cause ).
Urinary electrolyte losses vary with intake and disease process but can be measured if electrolyte abnormalities fail to respond to replacement therapy.
(See also the American Academy of Pediatrics' clinical practice guideline (2018) for maintenance IV fluids in children.)
Fluid and electrolyte needs from basal metabolism must also be accounted for. Maintenance requirements are related to metabolic rate and affected by body temperature. Insensible losses (evaporative free water losses from the skin and respiratory tract) account for about one third of total maintenance water (slightly more in infants and less in adolescents and adults).
Volume rarely must be exactly determined but generally should aim to provide an amount of water that does not require the kidney to significantly concentrate or dilute the urine. The most common estimate is the Holliday-Segar formula, which uses patient weight to calculate metabolic expenditure in kcal/24 hours, which approximates fluid needs in mL/24 hours (see table Holliday-Segar Formula for Maintenance Fluid Requirements by Weight ). More complex calculations (eg, those using body surface area) are rarely required.
Maintenance fluid volumes can be given as a separate simultaneous infusion, so that the infusion rate for replacing deficits and ongoing losses can be set and adjusted independently of the maintenance infusion rate.
Baseline estimates are affected by fever (increasing by 12% for each degree > 37.8 ° C), hypothermia, and activity (eg, increased for hyperthyroidism or status epilepticus, decreased for coma).
The traditional approach to calculating the composition of maintenance fluids was also based on the Holliday-Segar formula. According to that formula, patients require
Sodium: 3 mEq/100 kcal/24 hours (3 mEq/100 mL/24 hours)
Potassium: 2 mEq/100 kcal/24 hours (2 mEq/100 mL/24 hours)
(NOTE: 2 to 3 mEq/100 mL is equivalent to 20 to 30 mEq/L [20 to 30 mmol/L].)
hyponatremia . This development is likely due to volume-related ADH release as well as to significant amounts of stimuli-related ADH release (eg, from stress, vomiting, dehydration, or hypoglycemia). The ADH causes increased free water retention. Iatrogenic hyponatremia may be a greater problem for more seriously ill children and those who are hospitalized after surgery where stress plays a bigger role.
Because of this possibility of iatrogenic hyponatremia, many centers are now using a more isotonic fluid such as 0.45% or 0.9% saline for maintenance in dehydrated children. The American Academy of Pediatrics' clinical practice guideline (2018)
Practical Rehydration Example
A 7-month-old infant has diarrhea for 3 days with weight loss from 10 kg to 9 kg. The infant is currently producing 1 diarrheal stool every 3 hours and refusing to drink. Clinical findings of dry mucous membranes, poor skin turgor, markedly decreased urine output, and tachycardia with normal blood pressure and capillary refill suggest 10% fluid deficit. Rectal temperature is 37 ° C. Serum measurements are sodium, 136 mEq/L (136 mmol/L); potassium, 4 mEq/L (4 mmol/L); chloride, 104 mEq/L (104 mmol/L); and bicarbonate, 20 mEq/L (20 mmol/L).
Fluid volume is estimated by deficits, ongoing losses, and maintenance requirements.
The total fluid deficit given 1 kg weight loss = 1 L.
Ongoing diarrheal losses are measured as they occur by weighing the infant’s diaper before application and after the diarrheal stool.
Baseline maintenance requirements by the weight-based Holliday-Segar method are 100 mL/kg × 10 kg = 1000 mL/day = 1000 mL/24 hours or 40 mL/hour.
Electrolyte losses resulting from diarrhea in a eunatremic patient (see table Estimated Electrolyte Deficits by Cause ) are an estimated 80 mEq of sodium and 80 mEq of potassium.
Fluid selection
The patient is given an initial bolus of Ringer's lactate 200 mL (20 mL/kg × 10 kg) over 30 minutes. This amount replaces 26 mEq of the estimated 80 mEq sodium deficit.
Residual fluid deficit is 800 mL (1000 initial − 200 mL resuscitation). This residual amount is given over the next 24 hours. Typically, half (400 mL) is given over the first 8 hours (400 ÷ 8 = 50 mL/hour) and the other half is given over the next 16 hours (25 mL/hour).
The estimated residual sodium deficit is 54 mEq (80 − × 77 mEq sodium/L [77 mmol/L] = 62 mEq sodium); the additional 62 mEq of sodium given by using 0.9% saline is not clinically significant as long as renal function is intact.
When urine output is established, potassium is added at a concentration of 20 mEq/L (20 mmol/L; for safety reasons, no attempt is made to replace complete potassium deficit acutely).
Maintenance fluid
More information.
The following English-language resource may be useful. Please note that THE MANUAL is not responsible for the content of this resource.
American Academy of Pediatrics: Clinical practice guideline for maintenance intravenous fluids in children (2018)

- Cookie Preferences

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
Disclaimer » Advertising
- HealthyChildren.org

- Previous Article
- Next Article
Presentation
The condition, patient course, lessons for the clinician, suggested readings, case 6: dehydration and electrolyte abnormalities in an 11-year-old boy.
AUTHOR DISCLOSURE
Drs Almeida, Lopes, Figueiredo, Oliveira, Sousa, and Sequeira have disclosed no financial relationships relevant to this article. This commentary does not contain a discussion of an unapproved/investigative use of a commercial product/device.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Filipa Almeida , Susana Lopes , Margarida Figueiredo , Filipe Oliveira , Susana Sousa , Alexandra Sequeira; Case 6: Dehydration and Electrolyte Abnormalities in an 11-year-old Boy. Pediatr Rev September 2017; 38 (9): 441–442. https://doi.org/10.1542/pir.2015-0150
Download citation file:
- Ris (Zotero)
- Reference Manager
A previously healthy 11-year-old white boy presents to the emergency department with a 3-day history of nausea, anorexia, weakness, abdominal pain, and an episode of vomiting. He has no history of fever, diarrhea, constipation, respiratory or urinary symptoms, or use of laxatives or diuretics.
Physical examination reveals a thinly built boy with signs of dehydration (sunken eyes and slightly dry mucous membranes) and generalized skin hyperpigmentation, especially noticed on the extensor surfaces of the fingers of both hands ( Fig ). He is afebrile, with capillary refill time of less than 2 seconds, blood pressure of 94/68 mm Hg, and a heart rate of 116 beats/min. His weight is 32 kg (70.5 lb) (weight loss of 6% in the previous 3 days). Findings on the rest of the physical examination are normal.

Skin hyperpigmentation on the extensor surfaces of the fingers.
Initial laboratory evaluation reveals the following values: sodium, 118 mEq/L (118 mmol/L) (reference range, 136–145 mEq/L [136–145 mmol/L]); potassium, 6.1 mEq/L (6.1 mmol/L) (reference range, 3.5–5.1 mEq/L [3.5–5.1 mmol/L]); chloride, 92 mEq/L (92 mmol/L) (reference range, 101–111 mEq/L [101–111 mmol/L]); blood urea nitrogen, 109 mg/dL (38.9 mmol/L) (reference range, 0–48 mg/dL [0–17.1 mmol/L]); and serum creatinine, 0.81 mg/dL (71.6 μmol/L) (reference range, 0.1–1.0 mg/dL [8.8–88.4 μmol/L]). He has mild metabolic acidosis (pH 7.33; bicarbonate, 18 mEq/L [18 mmol/L]), and he is normoglycemic (glucose, 94 mg/dL [5.22 mmol/L]). His complete blood cell count and liver enzyme, uric acid, lactate dehydrogenase, and C-reactive protein levels are within normal limits. Results of urine drug screen also are negative.
He is diagnosed as having hyponatremic dehydration and is given an intravenous bolus of 20 mL/kg of isotonic (0.9%) sodium chloride solution. He is hospitalized for monitoring and further evaluation. Right after hospitalization, he develops hypotension and is administered another bolus of normal saline solution. His blood pressure does not improve, and he is, therefore, started on inotropic support with dopamine.
Additional laboratory evaluation reveals the cause of fluid-resistant hypotension and abnormal laboratory results.
In the presence of hypotension unresponsive to initial resuscitation efforts and abnormal electrolyte values, the diagnosis of acute adrenal insufficiency was considered. An early-morning blood sample was collected for hormonal study, and the patient was treated with intravenous hydrocortisone (70 mg/m 2 ). Clinical improvement was noticed. An abdominal computed tomographic scan revealed no abnormalities in the adrenal glands. The measurement of a plasma corticotropin (ACTH) level greater than 1,250 pg/mL (275 pmol/L) (reference range, 9–52 pg/mL [2–11 pmol/L]) and a serum cortisol level of 1 μg/dL (28 nmol/L) (reference range, 3–21 μg/dL [83–579 nmol/L]) confirmed the diagnosis of primary adrenal insufficiency (PAI). His aldosterone level, although within normal limits (2.1 ng/dL [58.3 pmol/L]; reference range, 2–22 ng/dL [55.5–610.3 pmol/L]) was low for the degree of hyperreninemia (plasma renin activity, 8.36 ng/mL per hour; reference range, 0.15–2.33 ng/mL per hour; and renin level >500 μU/mL; reference range, 2.8–40 μU/mL), confirming mineralocorticoid deficiency. Adrenal antibodies were detected (1/160; reference range, <1/10), confirming an autoimmune etiology (Addison disease). Other causes of PAI, such as infections with M tuberculosis, cytomegalovirus, Epstein-Barr virus, and human immunodeficiency virus (HIV), were excluded. Results of the investigation for other autoimmune diseases were negative: calcium, phosphorus, and parathyroid hormone levels were normal; thyroid function was normal, and thyroid peroxidase and thyroglobulin antibodies were negative; anti-tissue transglutaminase immunoglobulin A antibodies specific for celiac disease were also negative.
Addison disease, also known as PAI, is an uncommon disease in children, with an incidence of approximately 0.8 in 100,000 general population. Addison disease results from impaired synthesis and release of adrenocortical hormones (glucocorticoids and/or mineralocorticoids) despite elevated levels of corticotropin. It can be a consequence of the impaired function of the adrenal cortex due to abnormal adrenal development (adrenal hypoplasia congenita), steroidogenesis disorders (congenital adrenal hyperplasia), or adrenal destruction. The adrenal destruction of the adrenal cortex can be caused by autoimmune process, infections, infiltrative or metastatic diseases, hemorrhage of the adrenal gland, metabolic disorders, or the effects of drugs. Autoimmune destruction is the most common cause of Addison disease in children and can occur as an isolated condition or as a component of autoimmune polyglandular syndromes (APSs): APS type 1 consists of the triad of hypoparathyroidism, chronic mucocutaneous candidiasis, and Addison disease, and APS type 2 is defined by Addison disease, thyroiditis, and type 1 diabetes.
Clinical presentation is variable and can be insidious. Symptoms often are nonspecific and include loss of appetite, generalized weakness, nausea, abdominal pain, vomiting, weight loss, and salt craving. These symptoms can be easily confused with a viral gastroenteritis or a psychiatric disorder, especially depression, and, thus, results in delay in the diagnosis. Classic clinical signs include weight loss, orthostatic hypotension, hyperpigmentation, and shock. The skin and mucosal hyperpigmentation is a classic finding and results from increased secretion of melanocyte stimulation hormone (MSH). The pituitary secretes large quantities of corticotropin in response to low cortisol level. Pro-opiomelanocortin is a precursor for both corticotropin and MSH. Thus, when corticotropin production increases, there is increased production of MSH as well. Classic biochemical abnormalities of Addison disease include hyponatremia, hyperkalemia, hypoglycemia, and metabolic acidosis. Acute presentation of Addison disease is a true medical emergency, and the outcome can be fatal if not promptly recognized and treated.
Diagnosis of Addison disease requires a high degree of suspicion, and definitive diagnosis is based on checking hormonal levels.
The initial screening for adrenal insufficiency should include the measurement of electrolytes, early morning cortisol, corticotropin, renin or plasma renin activity, and aldosterone. Diagnosis of PAI is confirmed by an elevated plasma corticotropin concentration (frequently >100 pg/mL [22 pmol/L]) in the presence of a low serum cortisol concentration (usually <10 μg/dL [276 nmol/L]). When the diagnosis is in doubt, a stimulation test with synthetic corticotropin should be performed, and in patients with PAI there will not be an increase in the cortisol level. Mineralocorticoid deficiency is confirmed in the face of a relatively low aldosterone value despite hyperreninemia.
If PAI is diagnosed, further evaluation should be performed to determine the underlying cause. Antibodies should be checked to evaluate for autoimmune PAI. If adrenal antibodies are positive, the patient should be screened for autoimmune polyglandular syndromes by measuring serum calcium, phosphorus, parathyroid hormone level, and antibodies against thyroid and islet cells. In addition, screening for other autoimmune disorders, such as celiac disease and autoimmune atrophic gastritis, should be performed. If autoimmune evaluation is negative, then other causes of PAI, such as infections (tuberculosis, HIV, cytomegalovirus, candidiasis), infiltrative diseases (hemochromatosis, primary amyloidosis), adrenal hemorrhage (associated with sepsis, anticoagulants), and adrenal metastases or tumors should be looked for. A computed tomographic scan of the adrenals may be useful in these cases. Males with negative antibodies should be screened for adrenoleukodystrophy by measuring very-long-chain fatty acids. In early infancy, the most common cause of PAI is congenital adrenal hyperplasia, and 17-OH-progesterone should be measured (a value greater than 1,000 ng/dL is diagnostic for 21-hydroxylase deficiency, the most common cause of congenital adrenal hyperplasia).
Primary adrenal insufficiency in its acute presentation (adrenal crisis) is a life-threatening condition. Even when the diagnosis is uncertain, the treatment should be initiated. Management of acute adrenal insufficiency involves initial fluid resuscitation with a 20-mL/kg bolus of normal saline (repeated bolus may be required) and the administration of intravenous stress doses of hydrocortisone (50–100/m 2 ) as early as possible. Electrolyte abnormalities and hypoglycemia need to be corrected appropriately.
Long-term treatment of Addison disease consists of exogenous corticosteroid replacement. The preferred glucocorticoid replacement is oral hydrocortisone (the current recommendation for a total starting daily dose of hydrocortisone in children is 8 mg/m 2 divided into 3 or 4 doses), and mineralocorticoid replacement is performed with fludrocortisone at a dose of 0.1 to 0.2 mg/day.
The aim of the treatment in children is to control the symptoms of adrenal insufficiency with a dose that allows adequate growth and pubertal development. The current guidelines suggest monitoring adequacy of glucocorticoid replacement by clinical assessment by checking growth velocity, body weight, blood pressure, and energy level.
Patients with adrenal insufficiency are recommended to wear medic-alert jewelry (either bracelet or necklace). They are also provided an “emergency letter” that they are recommended to always carry with them because it details management of their condition in situations of physical stress or illness.
After intravenous hydrocortisone therapy, our patient was started on oral fludrocortisone therapy. He responded well to this therapy, with normalization of serum electrolyte levels. He is being followed by a pediatric endocrinologist. There was a progressive decrease of skin hyperpigmentation, with full resolution after 4 months and a decrease of plasma corticotropin levels (203 pg/mL [45 pmol/L]; reference range, 9–52 pg/mL [2–11 pmol/L]). The plasma renin activity and renin levels also normalized. Approximately 10 months later, adrenal antibody titers had decreased (1/40; reference range, <1/10).
Currently, with 3 years of follow-up, this patient maintains normal growth and pubertal development and has not developed any other autoimmune diseases.
If a patient has symptoms of asthenia, signs of dehydration disproportionate to estimated losses, and hyperpigmentation of skin, the index of suspicion for adrenal insufficiency should be high, even in the absence of the characteristic electrolyte abnormalities.
Any patient with hypotension or shock unresponsive to initial resuscitation efforts and/or an abnormal electrolyte profile (hyponatremia, hyperkalemia, hypoglycemia, or metabolic acidosis) should be considered to have adrenal insufficiency until proved otherwise.
Follow-up of adrenal insufficiency is required not only for monitoring response to therapy and treatment adherence but also for monitoring for development of other autoimmune disorders.
Competing Interests
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- ABP Content Spec Map
- Pediatrics On Call
- Online ISSN 1526-3347
- Print ISSN 0191-9601
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Open access
- Published: 23 May 2024
Prevalence and factors associated with rotavirus diarrhea among children aged 3–24 months after the introduction of the vaccine at a referral hospital in Uganda: a cross-sectional study
- Goretty Laker 1 ,
- Jolly Nankunda 3 ,
- Bernis Maren Melvis 1 ,
- Dickson Kajoba 1 ,
- Martin Nduwimana 1 ,
- Joel Kimera 1 ,
- Richard Justine Odong 1 &
- Isaac Edyedu 2
BMC Pediatrics volume 24 , Article number: 358 ( 2024 ) Cite this article
261 Accesses
1 Altmetric
Metrics details
Rotavirus has a significant morbidity and mortality in children under two years. The burden of rotavirus diarrhea 4 years post introduction of rotavirus vaccine in Uganda is not well established. This study aimed to determine the prevalence, severity of dehydration and factors associated with rotavirus diarrhea among children aged 3 to 24 months after the introduction of the vaccine at Fort Portal Regional Referral hospital.
This was a cross-sectional hospital-based study in which children with acute watery diarrhea were included. A rectal tube was used to collect a stool sample for those unable to provide samples. Stool was tested for rotavirus using rapid immunochromatographic assay. Data was analysed using SPSS version 22 with logistic regression done to determine the factors.
Out of 268 children with acute watery diarrhea, 133 (49.6%) were females. Rotavirus test was positive in 42 (15.7%), majority of whom had some dehydration 28(66.7%). The factors that were independently associated with rotavirus diarrhea were; age < 12 months (AOR = 8.87, P = 0.014), male gender (AOR = 0.08, P = 0.001), coming from a home with another person with diarrhea (AOR = 17.82, P = 0.001) or a home where the water source was a well (AOR = 50.17, P = 0.002).
The prevalence of rotavirus diarrhea was three times less in the post rotavirus vaccination period compared to pre-rota vaccination period. Majority of the participants with rotavirus diarrhea had some dehydration. There is need for provision of safe water sources to all homes. Surveillance to determine the cause of the non rota diarrhea should be done.
Peer Review reports
The rotavirus is a double-stranded RNA virus that belongs to the Reoviridae family [ 1 ]. Rotavirus is composed of three concentric shells that enclose 11 gene segments [ 1 ]. The outermost shell contains two proteins (VP7 0R G-protein and VP4 or P-protein) which are believed to be involved in immune protection and they are responsible for the classification of the rotavirus [ 1 ]. The rotavirus genome segments encodes 6 structural proteins which make up virus particles (Viral protein or VP) and 6 non-structural proteins (NSP). The type of rotavirus detected in this study was rotavirus A [ 1 ].
Children between the ages of 3 and 24 months experience the most symptomatic illness [ 1 ]. Rotavirus diarrhea is due to rotavirus infecting and destroying the enterocytes bringing about malabsorption as the chief mechanism of diarrhea. Rotavirus also induces intestinal secretions through activation of the enteric nervous system and also leads to antigenemia which is associated strongly with manifestation of acute gastroenteritis [ 2 ].
Rotavirus the primary cause of diarrheal illness worldwide is projected to result in 2.4 million hospitalizations and 114 million episodes per year requiring home care [ 3 ]. Although there have been reports of rotavirus spreading through water among young children, rotavirus is typically spread from person to person by the oral-fecal route [ 4 , 5 ]. The most common cause of severe acute diarrhea in the world, rotavirus has a significant morbidity and fatality rate in children under the age of five [ 2 ].
Globally, rotavirus infection was the leading cause of diarrheal deaths, accounting for 19.11% of deaths from diarrhea in 2019. Rotavirus has caused a higher death burden in African, Oceanian, and South Asian countries in the past three decades [ 6 ]. Sub-Saharan Africa contributed 121,000 deaths of 215,000 rotavirus related deaths globally in 2016 [ 6 ]. As a result, it was listed as the third most common infection linked to mortality in children under the age of five [ 7 ].
While there are many different laboratory techniques for diagnosing rotavirus, enzyme-linked immunosorbent tests and quick stool antigen detection assays with reverse transcription polymerase chain reaction are frequently utilized, particularly in research laboratories [ 5 , 8 ]. The identification and classification of dehydration status forms the foundation of care and is crucial to the evaluation of acute diarrhea [ 9 ]. Dehydration status is classified as: no dehydration, some dehydration, or severe dehydration, and is therefore treated according to plans A, B, and C respectively [ 10 ].
As of 2018, more than 92 nations had integrated vaccines into their national immunization programs, following WHO recommendations to utilize 2 rotavirus vaccines in all national vaccination programs worldwide since 2006 [ 11 ]. Uganda introduced the rotavirus vaccine (Rotarix) a 2 dose vaccine into the routine immunization schedule in June 2018, however global supply challenges in 2022 through 2023 necessitated the switch to a new formulation to ensure that children continue to be protected from rotavirus disease [ 6 ]. On the impact of the introduction of rotavirus vaccination on diarrheal fatalities in Africa, there are no any post-rotavirus vaccination population-based data available [ 12 ].
Diarrhea in Uganda is still ranked among the top 10 causes of morbidity, mainly because it is highly contagious and associated with severe dehydration leading to shock and multiorgan failure [ 13 , 14 ]. Studies carried out at Mulago National Hospital in 2010 and 2012 respectively found a pre-rotavirus vaccination prevalence of 45.4% among children aged 3–59 months [ 14 ] and 32.8% among children under 5 years [ 15 ]. However, there is a paucity of data on post-vaccination diarrhea status in the country since its rollout in 2018. This study was done to determine the prevalence of rotavirus, severity of dehydration and factors associated with rotavirus diarrhea among children aged 3 to 24 months fours post introduction of the vaccine at Fort Portal Regional Referral Hospital.
Study design and setting
This was a hospital based cross sectional study done at Fort Portal Regional Referral Hospital (FRRH) in the Pediatric department (OPD, ward and Nutrition unit) from December 2022 – February 2023. FRRH is one of the public regional referrals under the Ministry of health in western Uganda that also serves as a Teaching Hospital for Kampala International University. It is a specialized hospital with specialized services in pediatrics and child health. The pediatric ward has a seventy five-bed capacity. The pediatric ward, OPD and emergency is managed by four pediatricians, three senior housing officers and seven nurses. At Fort Portal Regional Referral Hospital, acute diarrhea is managed according to the WHO guideline [ 16 ].
Study population
All children 3–24 months old who presented with diarrhea at Fort Portal Regional Referral hospital for medical care during the study period were eligible. Three months was the lower limit because at this age rotavirus immunization is complete in Uganda. The upper limit of 24 was chosen because 3–24 months marks the peak of rotavirus infection [ 15 , 17 ].
Eligibility criteria
Inclusion was for children with acute watery diarrhea aged 3–24 months whose caregivers consented. Children with persistent or chronic diarrhea were excluded.
Sample size and sampling
Using open Epi software for calculating sample size for 2 proportions, a sample considering an odds ratio of 2.0 for occurrence of rotavirus infection among children with acute diarrhea in Kenya [ 17 ], considering two-sided confidence level (1- alpha) = 95%, Power = 80%, ratio of controls to cases = 1, least extreme odds ratio to be detected = 2.00, giving a total sample size of 268 participants. Consecutive sampling technique was used until the desired sample size was obtained.
Data collection procedure
At the outpatient clinic of FRRH, patients were triaged for participation. Those admitted were identified using the ward in-patient registers. Criteria for admission were; excessive vomiting associated with some dehydration, severe dehydration and children with acute malnutrition admitted for inpatient therapeutic feeding. Those who did not meet the admission criteria were included in the study from the outpatient department.
After identifying the study participants, time was spent to explain the study protocols in simple language and then consent was sought. For those who provided informed consent, the researcher went ahead and administered the questionnaire to collect information on diarrhea, social demographics, child factors and family social factors. Further the researcher examined the participants for dehydration signs and after which a rectal tube was used to collect the stool sample for rotavirus testing for those unable to provide stool samples.
Those found to have rotavirus were managed as a case for viral diarrhea; antibiotics were stopped for those who were taking them. They were given zinc tablets, and managed according to their level of dehydration. They were advised to continue feeding. They were also advised on the importance of hand washing, and they were encouraged to boil water for drinking.
Data collection instruments
A semi-structured questionnaire with closed ended questions was used to obtain social demographic, child and family social factors associated with rotavirus diarrhea, hydration status and rotavirus test result. This was administered and filled by the principal investigator and research assistant. Every patient who presented with acute diarrhea was evaluated for dehydration status and was further classified as either no dehydration, some dehydration or severe dehydration according to the WHO classification [ 16 ].

Rotavirus testing procedure
This study adopted the methodology used by Sharma (2017) [ 18 ] in his study “comparison of a rapid immunochromatography test with Elisa to detect rotavirus”. Sample collection was done by means of a rectal tube for those unable to provide stool samples. A 5 ml syringe was attached to a rectal tube size 10 which was inserted into the rectum following aseptic technique [ 14 ]. The tube was inserted approximately 2 cm in the rectum while the child was held on the mother’s thighs in prone position and 5mls of stool was aspirated. The sample was placed into a sterile and dry screw-top stool container. The container was labelled with a unique identifier of the study participant.
Assay diluent was taken in a disposable dropper up to the line marked on it and then transferred into the sample collection tube. This was done twice. Sample collection swab was put into the stool sample and then inserted into the tube containing assay diluent (sample collection tube) [ 18 ]. Sample collection swab was then swirled ten times in the sample collection tube until the sample dissolved into the assay diluents. The swab was discarded while squeezing it against the wall of tube. The presence of only control band within the result window indicated a negative result [ 18 ]. The presence of two-color bands as test band (T) and control band (C) within the result window, no matter which band appeared first, indicated a positive result and having only the presence of the test band was considered invalid [ 18 ].
The study used immunochromatographic assay (Fastep kit sensitivity- 90%, specificity- 93%) for the diagnosis of rotavirus diarrhea. This was because this test is easy to perform, it provides rapid results and has a high sensitivity [ 1 ]. A study in tertiary Care Hospital in Bangladesh showed immunochromatographic assay sensitivity of 90.70% and specificity of 93.88% in comparison to ELISA [ 19 ] while in India it had a sensitivity of 95.24% and specificity of 97.47% [ 20 ]. The kit employed in this study used the lateral flow method, took 10–15 min to give results, required fecal specimen and was manufactured by polymed therapeutics inc. Houston, USA. Registration number 4,003,234. Rapid immunochromatographic assay was carried out according to procedure provided by the manufacturer.
Quality assurance
Data quality was assured by collecting data from caregivers of the children who met the inclusion criteria, after stabilizing the unstable patients. The questionnaire was pretested for reliability and validity prior to the commencement of data collection. Research assistants were trained nurses who were trained on how to identify signs of dehydration, collection of stool sample and how to fill the questionnaires. The principal investigator had training on how to carry out the rapid diagnostic test of rotavirus using the immunochromatography by laboratory technologist before commencement of the study. For every 20 samples, one sample was taken to a reference laboratory to check for consistence of the result findings. Each filled questionnaire was cross-checked for consistencies and completeness before the interview was terminated.
Data analysis
Data was entered in Microsoft excel software, cleaned and sorted and thereafter exported to SPSS version 22. To determine the prevalence of rotavirus diarrhea, univariate analysis was done and presented as a pie chart in percentages. To classify the dehydration status of rotavirus diarrhea, univariate analysis was done and presented in a tabular form as frequencies and percentages. To identify factors associated with rotavirus diarrhea, analysis was done at both bivariate and multivariate. At bivariate level, crude odds ratios and P -values were computed and presented in a table with significance set at 95% and any variable with p -value less than 0.2 was subjected to multivariate logistic regression to establish associations presenting adjusted odds ratio and P -value in tables with significance set at 95% ( P -value < 0.05).
Ethical considerations and consent
All methods were carried out in accordance with relevant guidelines and regulations. Ethical approval was sought from the Research and Ethics Committee of Kampala International University Western Campus ( Ref No: KIU-2022-188) . All parents/guardians gave written informed consent.
During the study period, 268 children with diarrhea were enrolled. All the children enrolled had the rapid test for rotavirus done. About half of the children enrolled with diarrhea were females 135(50.4%). Majority of the participants were aged 3–12 months 177 (66.0%). Majority of the participants had diarrhea for less than 5 days 220 (82.1%). In this study, one test result was invalid. Forty-two tests were positive representing a prevalence of 15.7% (Fig. 1 ). Among the study participants with rotavirus diarrhea, majority had some dehydration 28(66.7%) while only 2 children (4.8%) with severe dehydration (Table 1 ).
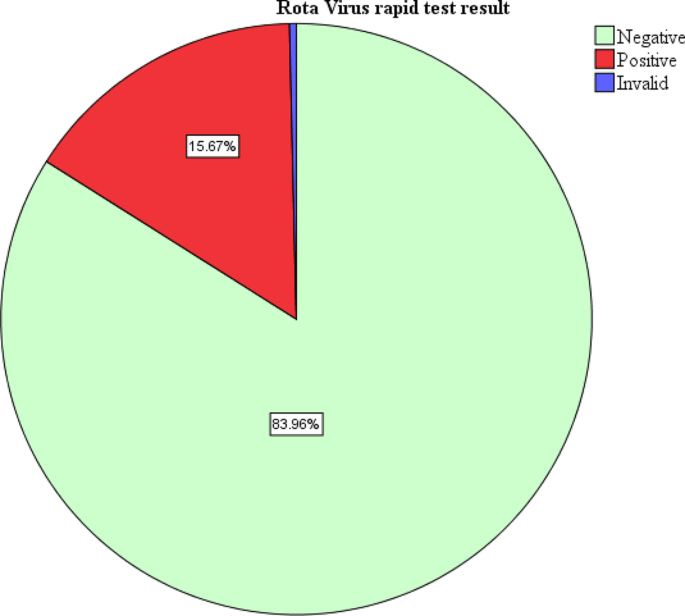
Prevalence of Rota-virus Diarrhea among children aged 3–24 months old attending Fort Portal Regional Referral hospital
At multivariate level of analysis, the variables that were significantly associated with rotavirus infection were child’s age, gender, having another child with diarrhea at home and getting water from a well. In this study, a child aged less than 12 months was 8.87 times more likely to have rotavirus compared to one older than 12 months (AOR = 8.87, CI = 1.55–50.74, P = 0.014). A male child was 0.083 times less likely to have rotavirus infection compared to a female (AOR = 0.08, CI = 0.02–0.36, P = 0.001). A child from a home with another person with diarrhea was 17.82 times more likely to have rotavirus infection (AOR = 17.82, CI = 3.48–91.17, P = 0.001). A child from a home where water for home use was obtained from a well was 50.17 times more likely to have rotavirus infection compared to one from a home where water was obtained from a tap (AOR = 50.17, CI = 4.40-71.97, P = 0.002). The details of bivariate and multivariate analysis are shown in Table 2 . It is also important to note that 15% of the children reported as completely vaccinated had rotavirus.
The study finding established a post rota vaccination era prevalence of 15.67% among infants 3–24 months of age attending Fort Portal Regional Referral Hospital. This study represents a reduction of about three times the finding in Mulago National Referral Hospital [ 14 ] 4 years post- introduction of rotavirus vaccine. This reflects a possible and expected effectiveness of rotavirus vaccination in reducing the burden of rotavirus gastroenteritis [ 21 ]. The study findings are similar to that observed in Nairobi Kenya (15.2%) 5 years post vaccination rollout [ 22 ]. Possibly due to effectiveness of the rotavirus vaccine 5 years post introduction in both countries. Additionally, the studies from both countries were carried out in urban settings which fairly have better environmental hygiene compared to the rural settings. Higher prevalence was seen in; Tanzania (Moshi) 26.4% [ 23 ], possibly due to large sample size, slightly longer study duration (5 months) and also the study was carried out in four different facilities therefore making a larger coverage and increasing the chance of rotavirus diarrhea.
Rotavirus diarrhea is one of the most leading causes of dehydration among all the diarrhea causing organisms. In the present study some dehydration and severe dehydration accounted for 71.5% of the participants with rotavirus gastroenteritis in comparison to those who were rotavirus negative, with some and severe dehydration accounting for 24.9%. This is due to increased loss of water and electrolytes without adequate replacement [ 9 ]. Because of diarrhea, loss of appetite, vomiting and fever associated with rotavirus, fluid replacement is impaired as fluid loss worsens [ 24 ]. The study findings were lower than that observed in a cross sectional study in Bandung, Indonesia where some dehydration and severe dehydration contributed 82.8% [ 25 ]. This could be because the study participants were children ≤ 6 months of age who are highly susceptible to fluid loss. This explains the urgent need to intervene in infants with rotavirus diarrhea with zinc and ORS to prevent the progression to dehydration since more than 1.5 million cases of rotavirus diarrhea become severe enough requiring admission and intravenous fluid management [ 7 , 26 ].
In this study, factors that were found to be significantly associated with rotavirus diarrhea at multivariable level were, child’s age, gender, having another child with diarrhea at home and getting water for domestic use from a well. A child aged less than 12 months was 8.87 times more likely to have rotavirus compared to one older than 12 months. Our study agreed with another study in Uganda and one in Tanzania that there is a higher risk of rotavirus among children less than 12 months compared to those above [ 15 , 27 ] with prevalence of 61.1% and 61.6% for children less than 12 months respectively. This could be because within this age group, the children are within the oral phase of development and everything they find in their environment is put to the mouth thus putting them at a more risk of acquiring rotavirus diarrhea compared to their other counterparts who are older than 12 months [ 28 ]. Also, it could be possible that at this age group, high odds could be related to waning passive immunity from the mother around the time for weaning.
According to this study, a male child was 0.083 times less likely to have rotavirus infection compared to a female one. There are conflicting data on the correlation of rotavirus and gender as some studies show no gender predilection for rotavirus as seen in Baghdad [ 29 ] and Northwestern Angola [ 30 ]. Other reports such as a study in Mulago Regional Referral Hospital and another in Nigeria found that males were more at risk of having rotavirus diarrhea compared to the females [ 15 , 31 ]. Further investigation is required in determining the sex variation in rotavirus infection.
A child from a home with another person with diarrhea was 17.82 times more likely to have rotavirus infection compared to a child from a family where there was no one having diarrhea. This also is in agreement with a study carried out in Maiduguri teaching hospital, Borno state in Nigeria, which found that the source of water supply and presence of persons with gastroenteritis in the household were risk factors for infection acquisition with a statistical significance P < 0.05 [ 32 ]. A similar study in Mwanza, Tanzania also found that living next door to a child with diarrhea as being highly associated with rotavirus infection ( p = 0.036) [ 33 ]. This can be explained by the fact that rotavirus is an infectious disease that is transmitted through the feco–oral route. This means that close contact plays a big role in the transmission of the infection.
This study also noted that a child from a home where water for home use was obtained from a well was 50.17 times more likely to have rotavirus infection compared to one from a home where water was obtained from a tap. This finding was also in line with a finding from a study in Nigeria about the risk factors associated with rotavirus gastroenteritis among under five children where source of water was also associated with rotavirus gastroenteritis with a P < 0.05 [ 32 ]. Another study finding that concurs with our findings is that in Pader district northern Uganda, where use of unprotected water sources such as wells contributed significantly to watery diarrhea in children less than 5 years, P < 0.001 [ 34 ]. In this study however causation was not determined but since it is the most common cause of diarrhea it could have been a key contributor to the diarrhea. This could be explained by the fact that rotavirus diarrhea is transmitted orofecally therefore use of water for domestic use from unprotected sources such as wells may carry rotavirus into the water from either animal or human excreta, therefore leading to acquisition of infection if the water is not well treated or boiled.
It is important to note that 15% of the children reported as completely vaccinated had rotavirus and though the explanation for this is not clear, it is possible that some of these children could have been miss classified as having completed rotavirus vaccination give that some mothers/caretakers did not have the vaccination card and the information on the vaccination status was obtained by verbally asking the mother who replied in accordance to what they recall which could have been affected by recall bias.
Limitations of the study
The study was conducted in a hospital setting, this might not reflect the true burden in the community. However, the researcher also included participants who were attending care as outpatients. The study was carried out within a short period of 3 months therefore the season variations of rotavirus could have affected the study findings. Nutritional status was not studied as an independent factor. The study used an immunochromatographic assay as a rapid diagnostic test which is not the gold standard as compared to RT-PCR. There is a possibility that some of the children documented as having been fully vaccinated could have been wrongly classified since some caretakers/mothers did not have their Childs’ immunization card, and only verbally informed about the immunization status. More so, during data collection, we did not indicate which information about vaccination was card-verified.
Strength of the study
This was the first study to describe the prevalence of rotavirus diarrhea post introduction of rotavirus vaccination in Fort-portal and probably in Uganda.
The prevalence of rotavirus diarrhea was high in the post rotavirus vaccination period.
Majority of the participants with rotavirus diarrhea had some dehydration. The factors that were significantly associated with rotavirus diarrhea were child’s age, gender, having another person at home with diarrhea and use of water from the well for domestic purpose. This study demonstrates the need for provision of safe water sources and encouraging treating water for domestic use in the communities in order to reduce the cases of rotavirus.
There is need to carry out more surveillance to find out the possible cause of the diarrhea cases that were seen other than rotavirus.
Given the dehydration distribution with a substantial fraction having ‘no’ dehydration, this suggests that the trigger for hospital referral for diarrhoea is low. Any future study of this subject could include questions regarding distance travelled to reach the hospital or be supplemented by a community health utilization survey (referral behavior relative to the signs/symptoms of diarrhoea and dehydration).
Data availability
Data is available upon request. Requests should be sent to [email protected] (GL).
Abbreviations
Oral Rehydration Salts
FortPortal Regional Referral Hospital
Bass ES, Pappano DA, Sharon G. Rotavirus Gastrointest Disord. 2021;28(5).
Crawford SE, Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, et al. Rotavirus infection. Nat Rev Dis Prim. 2018;3(17083):1–39.
Google Scholar
Aliabadi N, Antoni S, Mwenda JM, Weldegebriel G, Biey JNM, Cheikh D, et al. Global impact of rotavirus vaccine introduction on rotavirus hospitalisations among children under 5 years of age, 2008–16: findings from the Global Rotavirus Surveillance Network. Lancet Glob Heal. 2019;7(7):e893–903.
Article Google Scholar
Kiulia NM, Hofstra N, Vermeulen LC, Obara MA, Medema G, Rose JB. Global occurrence and Emission of rotaviruses to Surface Waters. Pathogens. 2015;4:229–55.
Article PubMed PubMed Central Google Scholar
Hameed AR, Mohamed BY, Abakar AD, Ali KS. Prevalence of Rotavirus among Iraqi children with Diarrhea in Diyala Province. Int J Sci Res Biol Sci. 2021;8(2):20–3.
Du Y, Chen C, Zhang X. Global burden and trends of rotavirus infection-associated deaths from 1990 to 2019: an observational trend study. Virol J [Internet]. 2022;19(166). https://doi.org/10.1186/s12985-022-01898-9
Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, et al. Rotavirus Vaccination and the global burden of Rotavirus Diarrhea among children younger than 5 years. JAMAPediatrics. 2018;98121(10):958–65.
Parashar UD, Kang G, Diseases R, Kong H. Diagnosis, management, and prevention of rotavirus gastroenteritis in children. BMJ. 2018;347(f7204):1–19.
Cajacob NJ, Cohen MB. Update on Diarrhea. Peds Rev. 2021;37(8):313–22.
Granado-Villar D, Cunill-De Sautu B, Granados A. Acute gastroenteritis. Pediatr Rev. 2012;33(11):487–94.
Article PubMed Google Scholar
Cárcamo-Calvo R, Muñoz C, Buesa J, Rodríguez-Díaz J, Gozalbo-Rovira R. The rotavirus vaccine landscape, an update. Pathogens. 2021;10(5):1–15.
Platts-Mills JA, Steele AD. Rotavirus vaccine impact in Africa: greater than the sum of its parts? Lancet Glob Heal. 2018;6(9):e948–9.
Arashkia A, Nejat B, Farsi M, Jalilvand S, Nateghian A, Rahbarimanesh A, et al. Epidemiology and clinical characteristics of rotavirus and norovirus infections in hospitalized children less than 5 years of age with acute gastroenteritis in Tehran, Iran. Acta Med Iran. 2019;57(11):640–4.
Nakawesi JS, Wobudeya E, Ndeezi G, Mworozi EA, Tumwine JK. Prevalence and factors associated with rotavirus infection among children admitted with acute diarrhea in Uganda. 2010.
Odiit A, Mulindwa A, Nalumansi E, Mphahlele MJ. Rotavirus prevalence and genotypes among children younger than 5 years with Acute Diarrhea at Mulago. Pediatr Infect Dis J. 2014;33(S1):41–4.
World Health Organization. Cholera. Fact Sheet 107. 2012;July.
Muendo C, Laving A, Kumar R, Osano B, Egondi T, Njuguna P. Prevalence of Rotavirus infection among children with Acute Diarrhoea after Rotavirus Vaccine introduction in Kenya. BMC Pediatr. 2018;18(323):1–9.
Sharma A. Comparision of a Rapid Immunochromatography Test with Elisa to Detect. J Med Sci Clin Res. 2017;05(07):24334–40.
Habib F, Rahman M, Haque M, Sinha S, Dey S, Tanni N. A comparative study of Rotaviral Antigen Detection by ELISA and ICT in children below five years with Acute Diarrhoea in a Tertiary Care Hospital. Bangladesh Med J. 2020;49(1):14–8.
Dhiman S, Devi B, Singh K, Devi P. Comparison of enzyme-linked immunosorbent assay and immunochromatography for rotavirus detection in children below five years with acute gastroenteritis. J Clin Diagn Res. 2015;9(9):6–9.
Kiilu C, Marete I, Apondi E, Gudu E, FACTORS ASSOCIATED WITH ROTA, VIRUS DIARRHEA IN THE POST VACCINE PERIOD AS SEEN AT MOI TEACHING AND REFERRAL HOSPITAL, KENYA. East Afr Med J. 2017;94(9):709–17.
Gikonyo J, Mbatia B, Okanya P, Obiero G, Sang C, Nyangao J. Rotavirus prevalence and seasonal distribution post vaccine introduction in Nairobi county Kenya. Pan Afr Med J. 2019;33:1–9.
McHaile DN, Philemon RN, Kabika S, Albogast E, Morijo KJ, Kifaro E, et al. Prevalence and genotypes of Rotavirus among children under 5 years presenting with diarrhoea in Moshi, Tanzania: a hospital based cross sectional study. BMC Res Notes. 2017;10(1):4–9.
Yeasmin S, Hasan SMT, Chisti MJ, Id AK. Factors associated with dehydrating rotavirus diarrhea in children under five in Bangladesh: an urban-rural comparison. 2022;1–13.
Prasetyo D, Sabaroedin IM, Ermaya YS, Soenarto Y. Association between severe dehydration in Rotavirus Diarrhea and Exclusive Breastfeeding among infants at Dr. Hasan Sadikin General Hospital, Bandung, Indonesia. J Trop Med. 2015;2015:1–4.
van der Westhuizen A, Kunneke H, Kruger M. Factors Associated with severe dehydrating diarrhoea in the Rural Western Cape, South Africa. J Trop Pediatr. 2019;65(1):29415224.
Platts-mills JA, Amour C, Gratz J, Nshama R, Walongo T, Mujaga B, et al. Impact of Rotavirus Vaccine introduction and Postintroduction Etiology of Diarrhea Requiring Hospital Admission in Haydom, Tanzania. Rural Afr Setting. 2017;65:1144–51.
CAS Google Scholar
Ahmed S, Kabir ARML, Rahman A, Hussain M, Phil M, Khatoon S. Severity of Rotavirus Diarrhea in Children: one year experience in a Children Hospital of Bangladesh. Iran J Pediatr. 2009;19(2):108–16.
Hussein RA, Al-Abbas AA, Abdullah AM, Al-Bashier NT. Prevalence of Rotavirus Infection among Diarrheal Children in Baghdad City. Int J Sci Res. 2015;4(11):978–82.
Gasparinho C, Mirante MC, Mendes C, Mayer C, Nery SV, Brito M, et al. Characterization of rotavirus infection in children with acute gastroenteritis in Bengo Province, Northwestern Angola, prior to vaccine introduction. PLoS ONE. 2017;12(4):1–19.
Tagbo BN, Mwenda JM, Eke CB, Edelu BO, Chukwubuike C, Armah G, et al. Rotavirus Diarrhoea hospitalizations among children under 5 years of age in Nigeria, 2011–2016. Vaccine. 2018;36(51):7759–64.
Article CAS PubMed Google Scholar
National Association of Resident Doctors of Nigeria. Nigerian J Med Med J. 2015;24(1):17–27.
Mshana SE, Temu A, Kamugisha E, Mwizamholya DL, Hokororo A, Seni J. Prevalence and factors associated with group a rotavirus infection among children with acute diarrhea in Mwanza, Tanzania. J Infect Dev Ctries. 2012;6(6):508–15.
PubMed Google Scholar
Omona S, Malinga GM, Opoke R, Openy G, Opiro R. Prevalence of diarrhoea and associated risk factors among children under five years old in Pader District, northern Uganda. BMC Infect Dis. 2020;20(1):1–9.
Download references
This study did not receive any specific grant from funding agencies in public, commercial, or not for profit sectors.
Author information
Authors and affiliations.
Department of Pediatrics and Child Health, Kampala International University, Kampala, Uganda
Goretty Laker, Bernis Maren Melvis, Dickson Kajoba, Martin Nduwimana, Joel Kimera & Richard Justine Odong
Department of Surgery, Kampala International University, Kampala, Uganda
Isaac Edyedu
Mulago specialised Women and Neonatal Hospital, Kampala, Uganda
Jolly Nankunda
You can also search for this author in PubMed Google Scholar
Contributions
GL was the principle investigator, conceived and designed the study, collected data, analysed data and wrote the draft of the manuscript. JN and BMM supervised the work and revised the manuscript, MN, JK, DK, RJO and IE participated in data collection, revised the manuscript and all authors approved the final paper.
Corresponding author
Correspondence to Goretty Laker .
Ethics declarations
All methods were carried out in accordance with relevant guidelines and regulations. Ethical approval was approved by the Research and Ethics Committee of Kampala International University Western Campus (Ref No: KIU-2022-188) . All parents/guardians gave written informed consent
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Laker, G., Nankunda, J., Melvis, B.M. et al. Prevalence and factors associated with rotavirus diarrhea among children aged 3–24 months after the introduction of the vaccine at a referral hospital in Uganda: a cross-sectional study. BMC Pediatr 24 , 358 (2024). https://doi.org/10.1186/s12887-024-04842-8
Download citation
Received : 25 October 2023
Accepted : 17 May 2024
Published : 23 May 2024
DOI : https://doi.org/10.1186/s12887-024-04842-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Low income country
BMC Pediatrics
ISSN: 1471-2431
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- World J Clin Cases
- v.8(20); 2020 Oct 26
Dehydrated patient without clinically evident cause: A case report
Federica palladino.
Department of Woman, Child and of General and Specialized Surgery, Università degli Studi della Campania “Luigi Vanvitelli”, Naples 80138, Italy
Maria Cristina Fedele
Marianna casertano, laura liguori, tiziana esposito, stefano guarino, emanuele miraglia del giudice, pierluigi marzuillo.
Department of Woman, Child and of General and Specialized Surgery, Università degli Studi della Campania “Luigi Vanvitelli”, Naples 80138, Italy. [email protected]
Corresponding author: Pierluigi Marzuillo, MD, PhD, Assistant Professor, Doctor, Postdoc, Postdoctoral Fellow, Department of Woman, Child and of General and Specialized Surgery, Università degli Studi della Campania “Luigi Vanvitelli”, Via Luigi De Crecchio 2, Naples 80138, Italy. [email protected]
Patients affected by cystic fibrosis can present with metabolic alkalosis such as Bartter’s syndrome. In this case report we want to underline this differential diagnosis and we aimed focusing on the suspect of cystic fibrosis, also in case of a negative newborn screening.
CASE SUMMARY
In a hot August –with a mean environmental temperature of 36 °C– an 8-mo-old female patient presented with severe dehydration complicated by hypokalemic metabolic alkalosis, in absence of fever, diarrhea and vomiting. Differential diagnosis between cystic fibrosis and tubulopathies causing metabolic alkalosis (Bartter’s Syndrome) was considered. We started intravenous rehydration with subsequent improvement of clinical conditions and serum electrolytes normalization. We diagnosed a mild form of cystic fibrosis (heterozygous mutations: G126D and F508del in the cystic fibrosis transmembrane conductance regulator gene). The trigger factor of this condition had been heat exposure.
When facing a patient with hypokalemic metabolic alkalosis, cystic fibrosis presenting with Pseudo-Bartter’s syndrome should be considered in the differential diagnosis, even if the newborn screening was negative.
Core Tip: We report a case of cystic fibrosis presenting with hypokalemic metabolic alkalosis caused by dehydration after heat exposure. We diagnosed a mild form of cystic fibrosis. In particular we wanted focusing on differential diagnosis between cystic fibrosis and Bartter’s Syndrome. We want to highlight that atypical forms of cystic fibrosis could escape to neonatal screening and prompt diagnosis is important for prognosis.
INTRODUCTION
Cystic fibrosis (CF) is a monogenic disease caused by mutations in the cystic fibrosis transmembrane conductance regulator ( CFTR ) gene on chromosome 7. CF is complex and greatly variable in clinical expression[ 1 ]. Airways, pancreas, male genital system, intestine, liver, bone, and kidney are involved. CFTR -related disorders are conditions determined by mutations in the CFTR gene but not giving the usual CF clinical picture. They are often mild form of CF and their clinical manifestations are limited to a single district and include episodes of recurrent pancreatitis or isolated bilateral bronchiectasis. Males can manifest bilateral agenesis of the vas deferens with no digestive or respiratory involvement[ 2 ]. Both CF and CFTR -related disorders can present metabolic alkalosis, such Bartter’s syndrome (BS).
In this case report we want to underline differential diagnosis between cystic fibrosis and tubulopathies causing metabolic alkalosis (such as BS). Moreover, we aimed focusing on the possibility of suspect diagnosis of cystic fibrosis, nevertheless a negative newborn screening.
CASE PRESENTATION
Chief complaints.
In a hot August –with a mean environmental temperature of 36 °C– an 8-mo-old female patient come to our observation because of somnolence and reduced response to stimuli.
History of present illness
Weight loss in the last 7 d and low-quantity micturition in the last 24 h were reported.
History of past illness
In the first months of life the patient presented three episodes of upper respiratory tract infections.
Personal and family history
Neonatal screening for cystic fibrosis, hypothyroidism, and phenylketonuria were normal. She was assuming about 400 mL/d of milk. With the exception of 400 IU/d of Vitamin D, no other medications were administered.
Physical examination
She showed slightly dry mucous membranes, tachycardia (140 beats/min) and refill time of about 2 s. Fever was absent as such as vomiting or diarrhea.
Laboratory examinations
Urinalysis did not reveal any abnormality. Serum chemistry was as follows: Venous pH 7.5, bicarbonate 35.1 mmol/L, sodium 135 mEq/L, potassium 2.6 mEq/L, chloride 86 mEq/L, creatinine 0.31 mg/dL, calcium 10.7 mg/dL, phosphorous 4.3 mg/dL, magnesium 2.2 mEq/L. Aspartate and alanine aminotransferase, γ-Glutamyl-transferase, glycaemia, bilirubin, erythrocyte sedimentation rate, C-reactive protein, procalcitonin, alkaline phosphatase and complete blood count were within normal limits. Urinary calcium/creatinine ratio was 0.02 mg/mg, fractional excretion of sodium (FeNa) 0.7%. Electrocardiography was normal.
After 36 h of treatment, clinical conditions of the patient and serum electrolytes improved. Venous pH was 7.44, HCO3- 25.5 mmol/L, Na 142 mEq/L, K 4.5 mEq/L, CL 105 mEq/L.
We submitted our patient to sweat test when she became well hydrated with normal acid-base and electrolyte balance. The sweat test showed NaCl in the sweat of 100 mmol/L (normal value < 60 mmol/L). High sweat NaCl values were confirmed at the following sweat test after 2 d (sweat NaCl 87 mmol/L). So, we performed genetics analysis for cystic fibrosis. Molecular diagnosis showed the following heterozygous mutations: G126D and F508del in the gene CFTR (Figure (Figure1). 1 ). This genotype has been described as cause of mild cases of cystic fibrosis or of atypical forms (better known as CFTR -related disorders)[ 3 ].

Molecular diagnosis showed the following heterozygous mutations: G126D and F508del in the gene CFTR . A: Locus of cystic fibrosis transmembrane conductance regulator gene on Chromosome 7q31.2. The figure was created and modified by DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans using Ensembi Resources; B: Graphic view of cystic fibrosis transmembrane conductance regulator with pathogenic variants of our patient.
Imaging examinations
Abdomen ultrasonography was normal.
FINAL DIAGNOSIS
Hypokalemic metabolic alkalosis due to cystic fibrosis presenting with Pseudo-Bartter syndrome.
When patient was admitted intravenous rehydration with a solution containing NaCl 0.9% and glucose 2.5% with the addition of KCL 40 mEq/m 2 per day was started. When dehydration status and hypokalemic metabolic alkalosis was resolved this infusion was stopped.
OUTCOME AND FOLLOW-UP
Gene mutation analysis identified two heterozygous mutations of the CFTR gene: G126D and F508del. This genotype has been described as cause of mild cases of cystic fibrosis or of atypical forms (better known as CFTR -related disorders)[ 3 ].
The variable phenotype of patient affected by CFTR- Related disorders makes very complicated the genetic counseling. A regular clinical evaluation is necessary because CF symptoms may appear later[ 1 ]. She will undergo follow up visits at 3, 6, 12 mo after the diagnosis and yearly thereafter, because the atypical form (or CFTR -related disorders) could get worse over time. Proper immunization and influenza vaccination were recommended[ 4 ].
The main causes of metabolic alkalosis are shown in the Table Table1 1 [ 5 - 8 ]. Our patient had no signs of gastro-intestinal losses neither assumed any drug or too much calcium and absorbable alkali. Therefore, possible causes were or skin (due to cystic fibrosis) or renal (due mainly to BS) losses. However, our patient recovered too promptly compared with a dehydrated patient with BS[ 9 , 10 ]. Moreover, FeNa was < 1% demonstrating extra-renal losses of sodium (Table (Table2 2 )[ 11 ] and increasing the suspect of cystic fibrosis. In the cystic fibrosis, just chloride depletion (in our case through sweating) is the main cause of electrolyte abnormalities. Physical activity, fever, and heat exposure can determinate an excessive sweat production that causes an extracellular fluid volume and chloride depletion.
Main causes of metabolic alkalosis (modified from references[ 5 - 8 ])
11β-HSDH: 11β-hydroxysteroid-dehydrogenase.
Bartter’s syndrome and cystic fibrosis: Differential diagnosis (modified from references[ 9 - 18 ])
ABE: Acid-Basic Equilibria; FeNa + u : Urinary Fractional excretion of sodium; Na + pl : Plasmatic Sodium; Cl - pl : Plasmatic Chloride; Cl - u : Urinary Chloride.
The extracellular fluid volume depletion leads to a release of antidiuretic hormone with subsequent sodium reduction, and activation of renin-aldosterone system with potassium reduction. Moreover, chloride depletion causes increased renal bicarbonate reabsorption. This mechanism is regulated by a chloride–bicarbonate exchanger (pendrin) located on the intercalated cells, sited in cortical collected duct. When chloride depletion occurs, HCO 3 - secretion is inhibited by insufficient Cl - for anion exchange. In addition, pendrin is reduced in case of potassium depletion. All these mechanisms determine hypokalemic metabolic alkalosis. According to these pathophysiological mechanisms, chloride supplementation, more than sodium and potassium supplementation, is needed to correct the metabolic alkalosis state. The presence of chloride, in fact, increases pendrin activity, with bicarbonate secretion in the lumen of collected duct[ 12 , 13 ].
This kind of clinical and laboratory presentation of CF (known as Pseudo-BS)[ 14 - 18 ], usually, involves children < 2.5 years and is the presenting clinical picture of CF. Episode of vomiting, excessive sweating, heat exposure, fever or respiratory infection could cause a Pseudo-BS, in case of an underlining CF[ 15 , 18 ]. In our case, with the exception of heat exposure, there were no other reasons justifying the dehydration (gastrointestinal fluid losses with diarrhea and/or vomiting, reduction of salt and fluid intake and/or absorption, excessive sweat production for physical activity or fever).
For this reason, we performed sweat test in the suspect of cystic fibrosis. Sweat test is the gold standard to diagnose classical or atypical forms of CF[ 19 ]. In fact, even with over 1000 mutations in the CFTR gene on chromosome 7 are known and it is possible to find children with cystic fibrosis which do not present identifiable gene mutations[ 20 ]. Nevertheless, some mutations could show atypical and very mild clinical manifestations[ 1 ]. In these cases, sweat test results can be intermediate or negative (2%) and so other diagnostic tests are indicated, if clinical manifestations persist ( i.e ., genetic CFTR tests, CFTR functional tests, such as nasal potential difference, intestinal current measurement)[ 21 , 22 ]. Moreover, it is possible that these patients passed the new-born screening because their sufficient pancreatic status[ 21 - 24 ]. So, clinicians must not exclude, a priori, the suspicious of CF in patients with 1 or more clinical manifestations of CF, despite of negative newborn screening results[ 25 - 27 ].
In conclusion, with this case presentation we would highlight that the Pseudo-Bartter’s syndrome could be an initial clinical presentation of cystic fibrosis, and that the heat exposure might be a trigger of this condition. When facing a Pseudo-Bartter’s syndrome, cystic fibrosis should not be excluded from differential diagnosis, even if the new-born screening was negative.
Informed consent statement: Informed written consent was obtained from the patient for publication of this reporting.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Invited manuscript
Peer-review started: April 10, 2020
First decision: September 14, 2020
Article in press: September 26, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pandey A, Yang L S-Editor: Zhang L L-Editor: A P-Editor: Li JH
Contributor Information
Federica Palladino, Department of Woman, Child and of General and Specialized Surgery, Università degli Studi della Campania “Luigi Vanvitelli”, Naples 80138, Italy.
Maria Cristina Fedele, Department of Woman, Child and of General and Specialized Surgery, Università degli Studi della Campania “Luigi Vanvitelli”, Naples 80138, Italy.
Marianna Casertano, Department of Woman, Child and of General and Specialized Surgery, Università degli Studi della Campania “Luigi Vanvitelli”, Naples 80138, Italy.
Laura Liguori, Department of Woman, Child and of General and Specialized Surgery, Università degli Studi della Campania “Luigi Vanvitelli”, Naples 80138, Italy.
Tiziana Esposito, Department of Woman, Child and of General and Specialized Surgery, Università degli Studi della Campania “Luigi Vanvitelli”, Naples 80138, Italy.
Stefano Guarino, Department of Woman, Child and of General and Specialized Surgery, Università degli Studi della Campania “Luigi Vanvitelli”, Naples 80138, Italy.
Emanuele Miraglia del Giudice, Department of Woman, Child and of General and Specialized Surgery, Università degli Studi della Campania “Luigi Vanvitelli”, Naples 80138, Italy.
Pierluigi Marzuillo, Department of Woman, Child and of General and Specialized Surgery, Università degli Studi della Campania “Luigi Vanvitelli”, Naples 80138, Italy. [email protected] .

IMAGES
VIDEO
COMMENTS
Study with Quizlet and memorize flashcards containing terms like A nurse is assisting with the admission of a preschooler who has diarrhea from an unknown cause. Which of the following actions should the nurse take? Provide 50 mL/kg of oral rehydration fluid over 4 hr. Administer polyethylene glycol electrolyte solution daily. Obtain a rectal temperature every 2 hr. Give the rotavirus ...
Insert the swab 2.5 cm (1 in) into the rectum for 30 seconds. Place a specimen of 2 different stools in the container for the test. Put the stool sample in a sterile container before sending it to the laboratory. Place a urinary collection bag on the child before collecting the stool specimen.
ATI pediatric dehydration Case study Test. A nurse is preparing to obtain a stool specimen for clostridium difficile from a preschooler who is wearing diaper briefs due to recent occurrences of diarrhea which of the following action should the nurse take? Click the card to flip 👆. Place a urinary collection bag on the child before collecting ...
ATI video case study video case studies rn module:fluid electrolyte balance: pediatric dehydration nurse is assessing child who has severe dehydration. which of. Skip to document. University; High School. Books; ... 4-A nurse is preparing to obtain a stool specimen from a preschooler to test for the presence of Clostridium difficile. The child ...
RN Pediatric Dehydration Case Study Test 100% Total Time Use: 6 min RN Pediatric Dehydration Case Study Test - History Date/Time Score Time Use RN Pediatric Dehydration Case Study Test 9/23/2022 9:33:00 PM 100% 6 min RN Pediatric Dehydration Case Study Test Information: Page 1 of 3 Report Created on: 9/23/2022 9:33:00 PM EDT REP_Indv_Student ...
November 10, 2020. Pediatric Case Study. 1. Background Information As asked by Brenda: Based on this information, what are your primary concerns for Jodi-Lynn at this time?. Based on the information, Jodi-Lynn (JL) - a 12-month-old infant - has a risk of becoming/being severely dehydrated.
Dehydration in Children. This podcast gives medical students an approach to identifying and correcting dehydration, plus calculating fluid requirements, in pediatric patients. It was written by Michelle Bischoff and Dr. Melanie Lewis. Michelle is a medical student at the University of Alberta and Dr. Lewis is a General Pediatrician and ...
A prospective study of the usefulness of clinical and laboratory parameters for predicting percentage of dehydration in children. Pediatr Emerg Care. 1997;13(3):179-182.
Dehydration is a major cause of morbidity and mortality in infants and young children worldwide. Each year approximately 760,000 children of diarrheal disease worldwide. Most cases of dehydration in children are the consequence of acute gastroenteritis. Acute gastroenteritis in the United States is usually infectious in etiology.
RN Gas Exchange/Oxygenation: Asthma 3.0 Case Study Test. 5 terms. Eva_Cortez_ Preview. PN Pediatric Dehydration Case Study Test. 5 terms. slkgoijkgh. Preview. Professional Nursing Exam 1 (NUR 266) 115 terms. Morgan_Mackey56. Preview. Foundations Exam 2 . 115 terms. siennamarie918. Preview. assessing abdomen ch 23 wb.
Dehydration results from. Increased fluid loss. Decreased fluid intake. Both. The most common source of increased fluid loss is the gastrointestinal tract—from vomiting, diarrhea, or both (eg, gastroenteritis).Other sources are renal (eg, diabetic ketoacidosis), cutaneous (eg, excessive sweating, burns), and 3rd-space losses (eg, into the intestinal lumen in bowel obstruction or ileus).
Individual Performance Profile PN Pediatric Dehydration Case Study Test Individual Name: ADRIANA MCCRARY Student Number: 261597 Institution: Unitek College Sacramento PN Program Type: PN Test Date: 5/30/2022 Individual Score: 100.0% Practice Time: 4 min Individual Performance in the Major Content Areas # Individual Individual Score (% Correct) Sub-Scale Points Score Fluid & Electrolytes 1 100. ...
A previously healthy 11-year-old white boy presents to the emergency department with a 3-day history of nausea, anorexia, weakness, abdominal pain, and an episode of vomiting. He has no history of fever, diarrhea, constipation, respiratory or urinary symptoms, or use of laxatives or diuretics.Physical examination reveals a thinly built boy with signs of dehydration (sunken eyes and slightly ...
Dehydration is a physiologic response to a variety of diseases and conditions that results in a negative fluid balance due to decreased intake; increased output via renal, gastrointestinal, or insensible losses; or a systemic response to the specific disease state (eg, burns or sepsis). Dehydration causes total body water and electrolyte losses ...
RN Fluid & Electrolyte Balance: Dehydration 3 Case Study Test Individual Name: MELANIE LAVIMONIERE Student Number: 008018784 Institution: New England Institute of Tech ADN Program Type: ADN Test Date: 9/10/ ... Pediatric Nursing 5 100% Ability to apply nursing knowledge to clinical problems experienced by children. Topics include basic ...
Discussion. This multidisciplinary case on dehydration can be used early in medical education to introduce students to clinical scenarios while learning fundamental science content. An integrated approach to medical content and versatility with regard to class size make this case a valuable teaching tool.
Nager AL, Wang VJ. Comparison of nasogastric and intravenous methods of rehydration in pediatric patients with acute dehydration. Pediatrics. 2002;109(4):566-572. (Prospective study; 96 subjects) Fox J, Richards S, Jenkins HR, et al. Management of gastroenteritis over 10 years: changing culture and maintaining the change. Arch Dis Child.
ATI Video Case Studies Pediatric Dehydration Quiz. The priority action for Matthew is to maintain contact precautions due to the unknown cause of diarrhea, fever and incontinence. This is to prevent spread of the unknown causative agent if it is infectious. Secondly, I would continue to run labs for possible causes and monitor intake and output ...
1. BACKGROUND. Dehydration secondary to gastroenteritis remains a major cause of morbidity and mortality ().Acute evaluation and treatment of children presenting with dehydration represent one of the most common situation in the pediatric emergency department ().Underestimating fluid deficit and not providing proper rehydration can lead to acidosis, electrolyte disturbances, acute kidney ...
Fluid & Electrolyte Balance: Pediatric Dehydration 2/6/2024 4 min 100% Case 2/6/2024 6 min 46 sec Auto-reviewed Case Information: Individual Score RN Fluid & Electrolyte Balance: Dehydration 3 Case Study Test - Score Details of Most Recent Use COMPOSITE SCORES 100% Individual Score RN Fluid & Electrolyte Balance: Dehydration 3. Case ...
Rotavirus has a significant morbidity and mortality in children under two years. The burden of rotavirus diarrhea 4 years post introduction of rotavirus vaccine in Uganda is not well established. This study aimed to determine the prevalence, severity of dehydration and factors associated with rotavirus diarrhea among children aged 3 to 24 months after the introduction of the vaccine at Fort ...
what action to take? ensure child has voided. assessing 4 yr old w severe dehydration. manifestations to expect? 10% wt loss. providing dc teaching abt oral re-hydration to parent of preschooler. indicates understanding? offer cup of oral re-hydration fluid every time he has diarrhea. Study with Quizlet and memorize flashcards containing terms ...
1 INTRODUCTION. Leukemoid reaction is one of the relatively benign conditions in children compared to the adult population, where it is associated with high morbidity and mortality. 1 It was initially described by Krumbhaar in 1926 when several patients were reported with non-leukemic conditions having leukemia-like blood film, which was named a "leukemoid reaction." 2 It has recently been ...
CASE SUMMARY. In a hot August -with a mean environmental temperature of 36 °C- an 8-mo-old female patient presented with severe dehydration complicated by hypokalemic metabolic alkalosis, in absence of fever, diarrhea and vomiting. Differential diagnosis between cystic fibrosis and tubulopathies causing metabolic alkalosis (Bartter's ...
Which intravenous fluids does the nurse anticipate Tram will receive for initial rehydration? The nurse documents Tram's weight as 14lbs (6.4kg.) The HCP prescribes an IV infusion of 5% dextrose in 1/4 normal saline at 100ml over 20 minutes, to be infused at 8mL/kg/hr. How many milliliters of the prescribed solution should the RN infuse each hour?
Severe diarrhea associated with dehydration and infection occurred in patients treated with Verzenio. Across four clinical trials in 3691 patients, diarrhea occurred in 81 to 90% of patients who received Verzenio. ... -small cell lung cancer (NSCLC) with a rearranged during transfection (RET) gene fusion, as detected by an FDA-approved test ...