- Technical Support
- Find My Rep

You are here
Clinical Nursing Research
Preview this book.
- Description
- Aims and Scope
- Editorial Board
- Abstracting / Indexing
- Submission Guidelines
Clinical Nursing Research (CNR) is a leading international nursing journal, published eight times a year. CNR aims to publish the best available evidence from multidisciplinary teams, with the goal of reporting clinically applicable nursing science and phenomena of interest to nursing. Part of CNR’s mission is to bring to light clinically applicable solutions to some of the most complex problems important to nursing. This requires the efforts of diverse investigative teams. CNR publishes articles that are of interest to all the following disciplines: nursing, psychology, social work, medicine, exercise science, public health, physical therapy, occupational therapy, respiratory therapy, pharmacy, engineering, sociology, anthropology, health communication, and computer science. CNR invites submissions of original research, literature reviews, methodologies/methods, applied theoretical papers, and brief reports on a variety of health issues. Editorial and commentary manuscripts are typically invited. However, unsolicited editorial and commentary manuscripts will be considered on a case-by-case basis. Authors should query the Editor-In-Chief prior to submission of editorials and commentaries. Papers on any and all populations and settings in which nurses practice at any level are especially invited.
CNR does not accept papers on the study of nurses, nursing students, or students in the health professions unless clinically relevant patient outcomes are key outcomes and measured accordingly.
Clinical Nursing Research is a refereed journal publishing research articles that focus on nursing practice. It disseminates research findings of particular interest to practicing nurses, provides an international forum for discussion among clinical nurse researchers and by identifying practical applications of research, enhances practice. Manuscripts of interest to CNR are those that focus on assessment and/or measures of intervention effectiveness for application in practice settings. CNR does not publish research on nurses. This journal is a member of the Committee on Publication Ethics (COPE).
- Academic Search - Premier
- Academic Search Elite
- Applied Social Sciences Index & Abstracts (ASSIA)
- Clarivate Analytics: Current Contents - Physical, Chemical & Earth Sciences
- Comprehensive MEDLINE with FullTEXT
- Corporate ResourceNET - Ebsco
- Current Citations Express
- EBSCO: Business Source - Main Edition
- EBSCO: Family Studies Abstracts
- EBSCO: Health Source - Nursing/Academic Edition
- Family Index Database
- Gale: Diversity Studies Collection
- Health Business FullTEXT
- Health Care Database
- Health Service Abstracts
- Health Source Plus
- International Nursing Index
- Journal Citation Report – Science edition
- Journal Citation Reports/Social Sciences Edition
- MasterFILE - Ebsco
- ProQuest: Applied Social Science Index & Abstracts (ASSIA)
- Psychological Abstracts
- STM Abstracts
- Science Citation Index Expanded (Web of Science)
- Social SciSearch
- Standard Periodical Directory (SPD)
- TOPICsearch - Ebsco
- Vocational Search
This Journal is a member of the Committee on Publication Ethics .
This Journal recommends that authors follow the Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals formulated by the International Committee of Medical Journal Editors (ICMJE). Please review the ICMJE 4 Criteria for Authorship to determine who meets criteria for authorship versus who should be placed in the Acknowledgments.
Please read the guidelines below then visit the Journal’s submission site https://mc.manuscriptcentral.com/cnr to upload your manuscript. Please note that manuscripts not conforming to these guidelines may be returned. Remember you can log in to the submission site at any time to check on the progress of your paper through the peer review process.
Sage disseminates high-quality research and engaged scholarship globally, and we are committed to diversity and inclusion in publishing. We encourage submissions from a diverse range of authors from across all countries and backgrounds. Only manuscripts of sufficient quality that meet CNR Aims and Scope will be reviewed.
There are no fees payable to submit or publish in this Journal. Open Access options are available - see section 3.3 below.
As part of the submission process you will be required to confirm the following: (1) you are submitting your original work and that you have the rights in the work; (2) you are submitting the work for first publication in CNR. The manuscript has not been submitted elsewhere, is currently under consideration for publication elsewhere, and the manuscript has not been published elsewhere; (3) You have obtained and can supply all necessary permissions for the reproduction of any copyright works not owned by you. Please include statements #1-3 in your cover letter.
- What do we publish? 1.1 Aims & scope 1.2 Presubmission inquiry 1.3 Article types 1.4 General guidelines 1.5 Writing your paper 1.6 Diversity, equity, inclusion, and belonging
- Editorial policies 2.1 Peer review policy 2.2 Authorship 2.3 Acknowledgements 2.3.1 Third party submissions 2.3.2 Writing assistance 2.3.3 Artificial intelligence-assisted technologies 2.4 Funding 2.5 Declaration of conflicting interests 2.6 Research ethics and patient consent 2.7 Clinical trials 2.8 Reporting guidelines 2.9 Research data
- Publishing policies 3.1 Publication ethics 3.1.1 Plagiarism 3.1.2 Prior publication 3.2 Contributor’s publishing agreement 3.3 Open access and author archiving
- Preparing your manuscript 4.1 Formatting 4.2 Artwork, figures, and other graphics 4.3 Supplemental material 4.4 Reference style 4.5 English language editing services
- Submitting your manuscript 5.1 ORCID 5.2 Information required for completing your submission 5.3 Permissions
- On acceptance and publication 6.1 Sage Production 6.2 Online First publication 6.3 Access to your published article 6.4 Promoting your article
- Further information 7.1 Appealing the publication decision
1. What do we publish?
1.1 Aims & scope
Before submitting your manuscript to CNR, please ensure you have read the Aims & Scope ( CNR Aims and Scope ).
1.2 Presubmission inquiry
Pre-Submission inquiry is not required, but is encouraged. Pre-submission inquiries should be addressed to the CNR Editor-In-Chief, Dr. Melissa Pinto, at [email protected] . Please place “CNR Inquiry” in the email title line.
1.3 Article types
CNR accepts the following article types: Research Article, Literature Reviews, Brief Reports, Applied Theoretical and Conceptual Frameworks, Methods, Commentaries, Editorials, and Registered Reports. The section below summarizes the article types and length (word limit).
Research Article
Qualitative, Quantitative, and Mixed Methods
Maximum word limit/tables and figures:
Non-intervention study: 3,500 words
Intervention Study: 4,500 words**
No more than 8 figures and tables total
Literature Reviews
All types, but must use PRISMA or conventional guidelines for the conduct of the review. Systematic, umbrella, and rapid reviews must be registered in PROSPERO.
3,500 words
Brief Reports
Pilot, feasibility, and developmental research. (Sample sizes for short reports that use quantitative methods are usually less than 100.)
Non-interventional study: 2,500 words
Interventional study: 3,500 words**
Applied Theoretical and Conceptual Frameworks
Development, testing, and/or critique theoretical constructs or conceptual frameworks that are actionable and can be used for clinical research.
No more than 4 figures and tables total
Instrument development and psychometric studies, new analytic approaches, sampling, recruitment and retention of participants, and implications for the conduct of research in novel settings. CNR is less interested in studies of instrument translation into different languages.
Commentaries***
Short communication that is narrowly focused. The paper should highlight one or more research articles published in CNR within the last year. Commentaries most often focus on a contained segment of knowledge within a larger field. Commentaries should not focus on the overall field. Authors may wish to explain how findings can be placed into new or additional contexts. Opinions that are factually based are acceptable.
1,200 words
No more than 2 tables and figures total
Editorials***
Short communication that covers an aspect of a topic relevant to CNR’s scope and aims. Editorials are not typically completed in response to specific CNR articles.
No more than 2 tables or figures total
Registered Reports, Pre-Data or Post-Data:
There are two types of Registered Reports:
Registered Reports – Pre-Data, i.e., before any data have been gathered
Registered Reports – Post-Data, i.e., before already existing data have been examined and analyzed.
These submissions are reviewed in two stages. In Stage 1, a study proposal is considered for publication prior to data collection and/or analysis. Stage 1 submissions should include a complete Introduction, Methods, and Proposed Analyses. High-quality proposals will be accepted in principle before data collection and/or data analysis commences. Once the study is completed, the author will finish the article including Results and Discussion sections (Stage 2). Publication of the Stage 2 submission is guaranteed as long as the approved Stage 1 protocol is followed and the conclusions are appropriate. Full details can be found here . The Journal’s manuscript requirements should be adhered to for the stage 2 submission.
Please see Conn, Algase, Rawl, Zerwic, & Wyman, 2010) for guidance.
Word limits do not include the abstract, references, figures or tables.
**Please review the Conn, Algase, Rawl, Zerwic, & Wyman, (2010) for components to be included in intervention studies. Conn, V.S., Algase, D.L., Rawl, S.M., Zerwic, J.J. & Wyman, J.F. (2010). Publishing pilot intervention work. Western Journal of Nursing Scholarship, 32(8), 994-1010. Direct link is here: Conn, Algase, Rawl, Zerwic, & Wyman (2010).
***Commentaries and Editorials are typically by invitation only. Authors are expected to possess sufficient topical and field expertise. Occasionally, CNR will consider unsolicited commentaries and editorials. Authors interested in submitting an unsolicited commentary or editorial are highly encouraged to send a pre-submission inquiry to the Editor-In-Chief, Melissa D. Pinto, PhD, RN, FAAN ( [email protected] ). Please place “CNR Inquiry” in the email title line so your email will be easily located by the Editor.
Please note, medical research involving human subjects must be conducted according to the World Medical Association Declaration of Helsinki . Every manuscript submission of original research must have a statement of local IRB approval or adherence to the Declaration of Helsinki standards for protection of human subjects noted in the Methods section.
Manuscript Types CNR Does NOT Accept:
- Studies that focus on nurses/healthcare providers, students (nursing, medical, allied health, etc.) and are devoid of primary outcomes that are patient focused and measured accordingly.
- Case reports
- Case studies
- Single subject studies
- Study protocols
1.4 General guidelines
Manuscript Style and Formatting: Manuscripts should be submitted in APA Style. Please add continuous line numbering on the left side throughout the document. Authors’ names must not appear anywhere in the manuscript other than on the title page.
Manuscript Length: Please see 1.3 Article Type for specific instructions on manuscript length. Please note: the word limit does not include abstract, references, figures or tables.
Title Page: A separate title page must include: (1) the title, (2) all authors’ names, credentials, institutions (with school/college and department), addresses (including street, city, state, zip code, and country), and emails. Corresponding author(s) be denoted in the “Author Notes” on the title page. The Corresponding author should include all the above information, plus telephone number. Any Acknowledgements should also be listed on the title page as an Author Note.
The manuscript titles should not exceed 13 words. And please refrain from including the name of the country or location in the manuscript title or keywords, unless it essential to identification of the topic. Also, please refrain from using hyphens in the manuscript title unless it is absolutely necessary, such case may include name of an intervention or a specific method that is hyphenated.
Please use a colon in the manuscript title when applicable. Specifically, for:
- Randomized Controlled Trials, Crossover Trials, Parallel-Design Double-Blind Trial, Equivalence and Noninferiority Trial, Cluster Trial, please use the subheading in the title: A Randomized Controlled/Clinical Trial.”Example: Artificial Intelligence Based Digital Therapeutic to Treat Depression: A Randomized Controlled Trial (12 words).
- Nonrandomized Controlled Trial, please use the subtitle: A Nonrandomized Controlled Trial.” Example: Mindfulness Intervention to Reduce Stress Among Adolescents: A Nonrandomized Controlled Trial (11 words)
- Meta-Analysis, please include the subtitle, “A Meta-Analysis.” Example: Mindfulness Based Interventions to Treat Anorexia Nervosa: A Meta-Analysis. (10 words)
- Systematic Review (without meta-analysis), please use the subtitle: “A Systematic Review” Example: New Biomarkers for Fibromyalgia: A Meta-Analysis (6 words)
For all other article types, please use a succinct and descriptive title. While not required, authors may want to consider using a colon in the title.
Abstract : Abstracts should be structured, 350 words or less, for all paper types except Commentaries and Editorials.
The structured abstract headings should include the following headings (as appropriate for the article type): Background, Purpose/Objective, Design, Setting, Sample (Include sample size, n=XXX), Intervention (if applicable), Measures, Analysis (statistical tests used), Results (Primary result in quantitative form and statistical values, as appropriate), and Conclusions.
Copyright and Releases: Include a typed cover letter with your submission which states “ the authors are submitting the manuscript to Clinical Nursing Research (CNR) exclusively and do so with the understanding that if the paper is accepted for publication, copyright belongs to the publisher.” If the manuscript is accepted for publication, the Corresponding Author will be required to sign a Journal Contributor's Publishing Agreement.
Tables and Figures: All figures and tables with 17 or more columns must be camera-ready. Please submit black and white photographs of your figures or original line drawings. Please group tables and figures at the end of the manuscript; do not embed them within the body of the text itself. Please do not include more than one table on each page.
Permission: Include proof of written permission for all quotations which require permission or exceed 300 words in length and for all tables and figures from sources for which the authors do not hold the copyright.
Submission: Please submit your manuscript to the Sage Track website at Clinical Nursing Research Submission
Please Note: Manuscripts that do not conform to the author guidelines may be automatically returned without review or comment.
1.5 Writing your paper
The Sage Author Gateway provides general advice on how to get published, plus links to further resources.
1.6 Diversity, equity, inclusion, and belonging
CNR has Associate Editors for Diversity, Equity, and Inclusion that oversee the journal’s overarching aim for inclusivity. Authors are encouraged to use inclusive language in the Supplementary Table 1. Examples of Inclusive Language From Centers of Disease Control and Prevention’s Health Equity Guiding Principles for Inclusive Communication published in: Doubeni, C.A. & Corley, D.A. . (2021). Advancing diversity, equity, and inclusion in scientific publishing. Gastroenterology, 162 , 59-62.
Authors will be required to complete a supplementary table on representativeness of study participants and a lay person impact statement. Authors will be prompted to complete this and be provided with further instructions as the paper nears acceptance.
2. Editorial policies
2.1 Peer review policy
CNR is committed to delivering high quality, fast peer-review for your paper, and as such, has partnered with Web of Science. Web of Science is a third party service that seeks to track, verify, and give credit for peer review. Review for CNR can opt into Web of Science (or any other peer-review tracking profile offered by Sage) in order to claim their reviews or have their reviews automatically verified, and added to their review profile. Reviewers claiming credit for reviews will be associated with the relevant journal, but the article name, reviewer’s decision, and the content of the review is not published. CNR does not issue certificates for peer-review. If a reviewer would like evidence for peer-review, CNR encourages the reviewer to please use Web of Science. For more information, please visit the Web of Science website.
The Editor-In-Chief, Associate Editors or members of the Editorial Board may occasionally submit their own manuscripts for possible publication to CNR. In these cases, the peer-review process is managed by alternative members of the CNR Editorial Team (Associate Editors, etc.); the submitting authors cannot access and do not have any involvement in the manuscript decision.
2.2 Authorship
Papers should only be submitted for consideration once consent is provided by all authors. The author who submits the manuscript should carefully check to ensure all those whose work contributed to the paper are acknowledged as co-authors in accordance with ICMJE 4 Criteria for Authorship . . The corresponding author(s) are required to attest that all authors meet the criteria.
When a large multicentre group has conducted the work, the group should identify the individuals who accept direct responsibility for the manuscript. These individuals should fully meet criteria for authorship.
Please note that AI chatbots, for example ChatGPT, should not be listed as authors. For more information see the policy on Use of ChatGPT and generative AI tools .
2.3 Acknowledgements
All contributors who do not meet the criteria for authorship should be listed in the acknowledgment section (see Title Page, Section 1.4), under Acknowledgements .
2.3.1 Third party submissions
Where an individual who is not listed as an author submits a manuscript on behalf of the author(s), a statement must be included in the Acknowledgements section of the manuscript and in the accompanying cover letter. The statements must: (1) Disclose this type of editorial assistance – including the individual’s name, company and level of input , (2) Identify any entities that paid for this assistance, and (3) Confirm that the listed authors have authorized the submission of their manuscript via third party and approved any statements or declarations, e.g. conflicting interests, funding, etc. Where appropriate, Sage reserves the right to deny consideration to manuscripts submitted by a third party rather than by the authors themselves.
2.3.2 Writing assistance
Individuals who provided writing assistance, e.g. from a specialist communications company, do not qualify as authors and so should be included in the Acknowledgements section. Authors must disclose any writing assistance – including the individual’s name, company and level of input – and identify the entity that paid for this assistance. It is not necessary to disclose use of language publishing services.
2.3.3 Artificial intelligence-assisted technologies
CNR follows best practices as outlined by ICMJE. Specifically, CNR requires that authors adhere to their ICMJE recommendations (See #4 of May 2023 ICMJE AI Recommendations ). ChatGPT, large language models, and chatbots require disclosure in the cover letter to the Editor, in the submitted work and how it was used. Authors are responsible for the accuracy of any information, and they must assert that there is no plagiarism in the document. This includes any material that should be appropriately referenced.
2.4 Funding
Clinical Nursing Research requires all authors to acknowledge their funding in a consistent fashion under a separate heading. Please visit the Funding Acknowledgements page on the Sage Journal Author Gateway to confirm the format of the acknowledgment text in the event of funding, or state that: T his research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
2.5 Declaration of conflicting interests
It is the policy of CNR to require a declaration of conflicting interests from all authors enabling a statement to be carried within the paginated pages of all published articles.
Please ensure that a ‘Declaration of Conflicting Interests’ statement is included at the end of your manuscript, after any acknowledgements and prior to the references. If no conflict exists, please state that ‘The Author(s) declare(s) that there is no conflict of interest’. For guidance on conflict of interest statements, please see the ICMJE recommendations here .
2.6 Research ethics and patient consent
Medical research involving human subjects must be conducted according to the World Medical Association Declaration of Helsinki . Every manuscript submission must have a statement of local IRB approval or adherence to the Declaration of Helsinki standards for protection of human subjects.
Submitted manuscripts should conform to the ICMJE Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals , and all papers reporting animal and/or human studies must state in the methods section that the relevant Ethics Committee or Institutional Review Board provided (or waived) approval. Please ensure that you have provided the full name and institution of the review committee, in addition to the approval number.
For research articles, authors are also required to state in the methods section whether participants provided informed consent and whether the consent was written or verbal.
Information on informed consent to report individual cases or case series should be included in the manuscript text. A statement is required regarding whether written informed consent for patient information and images to be published was provided by the patient(s) or a legally authorized representative. Please do not submit the patient’s actual written informed consent with your article, as this in itself breaches the patient’s confidentiality. CNR requests that you confirm to us, in writing, that you have obtained written informed consent, but the written consent itself should be held by the authors/investigators themselves, for example in a patient’s hospital record. The confirmatory letter may be uploaded with your submission as a separate file.
Please also refer to the ICMJE Recommendations for the Protection of Research Participants .
2.7 Clinical trials
Clinical Nursing Research conforms to the ICMJE requirement that clinical trials are registered in a WHO-approved public trials registry at or before the time of first patient enrolment as a condition of consideration for publication. The trial registry name and URL, and registration number must be included at the end of the abstract.
2.8 Reporting guidelines
The relevant EQUATOR Network reporting guidelines should be followed depending on the type of study. For example, all randomized controlled trials submitted for publication should include a completed CONSORT flow chart as a cited figure and the completed CONSORT checklist should be uploaded with your submission as a supplementary file. Systematic reviews and meta-analyses should include the completed PRISMA flow chart as a cited figure and the completed PRISMA checklist should be uploaded with your submission as a supplementary file. The EQUATOR wizard can help you identify the appropriate guideline.
Other resources can be found at NLM’s Research Reporting Guidelines and Initiatives
2.9. Research Data
The journal is committed to facilitating openness, transparency and reproducibility of research, and has the following research data sharing policy. For more information, including FAQs please visit the Sage Research Data policy pages .
Subject to appropriate ethical and legal considerations, authors are encouraged to:
- share your research data in a relevant public data repository
- include a data availability statement linking to your data. If it is not possible to share your data, we encourage you to consider using the statement to explain why it cannot be shared.
- cite these data in your research
3. Publishing policies
3.1 Publication ethics
Sageis committed to upholding the integrity of the academic record. We encourage authors to refer to the Committee on Publication Ethics’ International Standards for Authors and view the Publication Ethics page on the Sage Author Gateway .
3.1.1 Plagiarism
Clinical Nursing Research and Sage take issues of copyright infringement, plagiarism or other breaches of best practice in publication very seriously. We seek to protect the rights of our authors and we always investigate claims of plagiarism or misuse of published articles. Equally, we seek to protect the reputation of the journal against malpractice. Submitted articles may be checked with duplication-checking software. Where an article, for example, is found to have plagiarized other work or included third-party copyright material without permission or with insufficient acknowledgement, or where the authorship of the article is contested, we reserve the right to take action including, but not limited to: publishing an erratum or corrigendum (correction); retracting the article; taking up the matter with the head of department or dean of the author's institution and/or relevant academic bodies or societies; or taking appropriate legal action.
Authors are responsible for use of AI-Assisted Technologies as outlined in the May 2023 ICMJE Recommendations #4 on AI-Assisted Technologies. Prevention of plagiarism is the sole responsibility of the author. Plagiarism from AI-Assisted Technologies will be treated the same as other cases of plagiarism, and will be subject to investigation and action including, but not limited to: publishing an erratum or corrigendum (correction); retracting the article; taking up the matter with the head of department or dean of the author's institution and/or relevant academic bodies or societies; or taking appropriate legal action by Sage.
3.1.2 Prior publication
If material has been previously published it is not generally acceptable for publication in a Sage journal. However, there are certain circumstances where previously published material can be considered for publication. Please refer to the guidance on the Sage Author Gateway or if in doubt, contact the Editor at the address given below.
3.2 Contributor’s publishing agreement Before publication, Sage requires the author as the rights holder to sign a Journal Contributor’s Publishing Agreement. Sage’s Journal Contributor’s Publishing Agreement is an exclusive licence agreement which means that the author retains copyright in the work but grants Sage the sole and exclusive right and licence to publish for the full legal term of copyright. Exceptions may exist where an assignment of copyright is required or preferred by a proprietor other than Sage. In this case copyright in the work will be assigned from the author to the society. For more information please visit the Sage Author Gateway
3.3 Open access and author archiving
Clinical Nursing Research offers optional open access publishing via the Sage Choice programme and Open Access agreements, where authors can publish open access either discounted or free of charge depending on the agreement with Sage. Find out if your institution is participating by visiting Open Access Agreements at Sage . For more information on Open Access publishing options at Sage please visit Sage Open Access . For information on funding body compliance, and depositing your article in repositories, please visit Sage’s Author Archiving and Re-Use Guidelines and Publishing Policies .
4. Preparing your manuscript for submission
4.1 Formatting
The preferred format for your manuscript is Word (.doc, .doxc, .rtf., etc.). LaTeX files are also accepted. Word and (La)Tex templates are available on the Manuscript Submission Guidelines page of our Author Gateway. Please use APA Style for spacing and margins.
4.2 Artwork, figures and other graphics
For guidance on the preparation of illustrations, pictures and graphs in electronic format, please visit Sage’s Manuscript Submission Guidelines .
4.3 Supplemental material
4.4 Reference style
CNR uses APA reference style. View the APA guidelines to ensure your manuscript conforms to this reference style.
4.5 English language editing services
Authors seeking assistance with English language editing, translation, or figure and manuscript formatting to fit the journal’s specifications should consider using Sage Language Services. Visit Sage Language Services on our Journal Author Gateway for further information.
5. Submitting your manuscript
Clinical nursing research is hosted on sage track, a web based online submission and peer review system powered by scholarone™ manuscripts. visit https://mc.manuscriptcentral.com/cnr to login and submit your article online..
IMPORTANT: Please check whether you already have an account in the system before trying to create a new one. If you have reviewed or authored for the journal in the past year it is likely that you will have had an account created. For further guidance on submitting your manuscript online please visit ScholarOne Online Help .
As part of our commitment to ensuring an ethical, transparent and fair peer review process Sage is a supporting member of ORCID, the Open Researcher and Contributor ID . ORCID provides a unique and persistent digital identifier that distinguishes researchers from every other researcher, even those who share the same name, and, through integration in key research workflows such as manuscript and grant submission, supports automated linkages between researchers and their professional activities, ensuring that their work is recognized.
The collection of ORCID IDs from corresponding authors is now part of the submission process of this journal. If you already have an ORCID ID you will be asked to associate that to your submission during the online submission process. We also strongly encourage all co-authors to link their ORCID ID to their accounts in our online peer review platforms. It takes seconds to do: click the link when prompted, sign into your ORCID account and our systems are automatically updated. Your ORCID ID will become part of your accepted publication’s metadata, making your work attributable to you and only you. Your ORCID ID is published with your article so that fellow researchers reading your work can link to your ORCID profile and from there link to your other publications.
If you do not already have an ORCID ID please follow this link to create one or visit our ORCID homepage to learn more.
5.2 Information required for completing your submission
You will be asked to provide contact details and academic affiliations for all co-authors via the submission system and identify who is to be the corresponding author. These details must match what appears on your manuscript. The affiliation listed in the manuscript should be the institution where the research was conducted. If an author has moved to a new institution since completing the research, the new affiliation can be included in a manuscript note at the end of the paper. At this stage please ensure you have included all the required statements and declarations and uploaded any additional supplementary files (including reporting guidelines where relevant).
5.3 Permissions
Please also ensure that you have obtained any necessary permission from copyright holders for reproducing any illustrations, tables, figures or lengthy quotations previously published elsewhere. For further information including guidance on fair dealing for criticism and review, please see the Copyright and Permissions page on the Sage Author Gateway .
6. On acceptance and publication
6.1 Sage Production
Your Sage Production Editor will keep you informed as to your article’s progress throughout the production process. Proofs will be made available to the corresponding author via our editing portal Sage Edit or by email, and corrections should be made directly or notified to us promptly. Authors are reminded to check their proofs carefully to confirm that all author information, including names, affiliations, sequence and contact details are correct, and that Funding and Conflict of Interest statements, if any, are accurate. Please note that if there are any changes to the author list at this stage all authors will be required to complete and sign a form authorizing the change.
6.2 Online First publication
Online First allows final articles (completed and approved articles awaiting assignment to a future issue) to be published online prior to their inclusion in a journal issue, which significantly reduces the lead time between submission and publication. Visit the Sage Journals help page for more details, including how to cite Online First articles.
6.3 Access to your published article
Sage provides authors with online access to their final article.
6.4 Promoting your article
Publication is not the end of the process! You can help disseminate your paper and ensure it is as widely read and cited as possible. The Sage Author Gateway has numerous resources to help you promote your work. Visit the Promote Your Article page on the Gateway for tips and advice.
7. Further information
Any correspondence, queries or additional requests for information on the manuscript submission process should be sent to the Clinical Nursing Research editorial office as follows: [email protected] .
7.1 Appealing the publication decision
Any correspondence, queries or additional requests for information on the manuscript submission process should be sent to the CNR editorial office.
- Read Online
- Sample Issues
- Current Issue
- Email Alert
- Permissions
- Foreign rights
- Reprints and sponsorship
- Advertising
Individual Subscription, E-access
Individual Subscription, Print Only
Individual Subscription, Combined (Print & E-access)
Institutional Backfile Purchase, E-access (Content through 1998)
Institutional Subscription, E-access
Institutional Subscription & Backfile Lease, E-access Plus Backfile (All Online Content)
Institutional Subscription, Print Only
Institutional Subscription, Combined (Print & E-access)
Institutional Subscription & Backfile Lease, Combined Plus Backfile (Current Volume Print & All Online Content)
Individual, Single Print Issue
Institutional, Single Print Issue
To order single issues of this journal, please contact SAGE Customer Services at 1-800-818-7243 / 1-805-583-9774 with details of the volume and issue you would like to purchase.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Study Protocol
A global perspective of advanced practice nursing research: A review of systematic reviews protocol
Roles Conceptualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations Susan E. French Chair in Nursing Research and Innovative Practice, Ingram School of Nursing, Faculty of Medicine and Health Sciences, McGill University, Montréal, Québec, Canada, Centre intégré universitaire de santé et de services sociaux de l’Est-de-l’Île-de-Montréal (CIUSSS-EMTL), Montréal, Québec, Canada
Contributed equally to this work with: Isabelle Savard, Li-Anne Audet, Abby Kra-Friedman
Affiliation Ingram School of Nursing, Faculty of Medicine and Health Sciences, McGill University, Montréal, Québec, Canada
Affiliations Henrietta Szold School of Nursing, Faculty of Medicine, Hebrew University of Jerusalem, Hadassah Ein Kerem, Jerusalem, Israel, School of Nursing, Duquesne University, Pittsburgh, Pennsylvania, United States of America
Affiliation Centre intégré universitaire de santé et de services sociaux de l’Est-de-l’Île-de-Montréal (CIUSSS-EMTL), Montréal, Québec, Canada
Affiliation Alice Lee Centre for Nursing Studies, Yong Loo Lin School of Medicine, National University of Singapore, National University Health System, Singapore, Singapore
Affiliation College of Nursing, University of Kentucky, Lexington, Kentucky, United States of America
Affiliation School of Nursing, MGH Institute of Health Professions, Boston, Massachusetts, United States of America
Affiliation School of Nursing, Old Dominion University, Virginia Beach, Virginia, United States of America
Affiliation School of Health Sciences, University of Dundee, Dundee, Scotland, United Kingdom
Affiliation Louise Herrington School of Nursing, Baylor University, Dallas, Texas, United States of America
Affiliation St James Public Health Services, Montego Bay, St James, Jamaica
Affiliation Department of Nursing and Midwifery, University of Huddersfield, Queensgate, Huddersfield, United Kingdom
- Kelley Kilpatrick,
- Isabelle Savard,
- Li-Anne Audet,
- Abby Kra-Friedman,
- Renée Atallah,
- Mira Jabbour,
- Wentao Zhou,
- Kathy Wheeler,
- Elissa Ladd,

- Published: January 24, 2023
- https://doi.org/10.1371/journal.pone.0280726
- Reader Comments
Introduction
In 2020, the World Health Organization called for the expansion and greater recognition of all nursing roles, including advanced practice nurses (APNs), to better meet patient care needs. As defined by the International Council of Nurses (ICN), the two most common APN roles include nurse practitioners (NPs) and clinical nurse specialists (CNSs). They help ensure care to communities as well as patients and families with acute, chronic or complex conditions. Moreover, APNs support providers to deliver high quality care and improve access to services. Currently, there is much variability in the use of advanced practice nursing roles globally. A clearer understanding of the roles that are in place across the globe, and how they are being used will support greater role harmonization, and inform global priorities for advanced practice nursing education, research, and policy reform.
To identify current gaps in advanced practice nursing research globally.
Materials and methods
This review of systematic reviews will provide a description of the current state of the research, including gaps, on advanced practice nursing globally. We will include reviews that examine APNs, NPs or CNSs using recognized role definitions. We will search the CINAHL, EMBASE, Global Health, HealthStar, PubMed, Medline, Cochrane Library Database of Systematic Reviews and Controlled Trials Register, Database of Abstracts of Reviews of Effects, Joanna Briggs Institute, and Web of Science electronic databases for reviews published from January 2011 onwards, with no restrictions on jurisdiction or language. We will search the grey literature and hand search the reference lists of all relevant reviews to identify additional studies. We will extract country, patient, provider, health system, educational, and policy/scope of practice data. We will assess the quality of each included review using the CASP criteria, and summarize their findings. This review of systematic reviews protocol was developed following the PRISMA-P recommendations.
PROSPERO registration number
CRD42021278532.
Citation: Kilpatrick K, Savard I, Audet L-A, Kra-Friedman A, Atallah R, Jabbour M, et al. (2023) A global perspective of advanced practice nursing research: A review of systematic reviews protocol. PLoS ONE 18(1): e0280726. https://doi.org/10.1371/journal.pone.0280726
Editor: Xian-liang Liu, Charles Darwin University, AUSTRALIA
Received: October 1, 2021; Accepted: January 8, 2023; Published: January 24, 2023
Copyright: © 2023 Kilpatrick et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All data is included in the paper and/or Supporting information .
Funding: This work is supported by the McGill University Faculty of Medicine and Health Sciences and the Newton Foundation via the Susan E. French Chair in Nursing Research and Innovative Practice held by KK. KK is also supported by a Fonds de recherche du Québec-Santé ( https://frq.gouv.qc.ca/en/health/ ) Research Scholar Senior (Award Number 298573) salary award. There was no additional external funding received for this study, and the authors received no specific funding for this work. All the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
In 2020, the World Health Organization [ 1 ] called for the expansion and greater recognition of all nursing roles, including nurses in advanced practice, to better meet patient care needs. Nurses in advanced practice roles, as defined by the International Council of Nurses (ICN), are most often identified as advanced practice nurses (APNs), with the two most common APN roles being nurse practitioners (NPs) and clinical nurse specialists (CNSs) [ 2 ]. They help ensure care to communities as well as patients and families with acute, chronic or complex conditions [ 2 ]. In addition to providing direct care, NPs and CNSs support care providers to deliver high quality care and improve access to services [ 3 – 5 ]. Nurses in these roles have educational preparation at the Master’s level or above in addition to in-depth clinical expertise and complex decision-making skills [ 6 ]. A global analysis of advanced practice nursing policy, regulation and practice by Ladd et al. [ 7 ] highlighted that advanced practice nursing roles are growing at an accelerated rate. However, these authors argue that advanced practice nursing roles have emerged unequally across the globe in response to local care needs without clear supports to develop consistent expanded roles for nurses. A recent review of systematic reviews of primary healthcare NP roles identified 396 primary studies included in the 40 systematic reviews representing on average 3 countries (range not reported to 9) [ 8 ]. Although there are several systematic reviews of APN and CNS roles in other clinical settings [ 4 , 5 , 9 ], no synthesis of this body of evidence is available for other recognized advanced practice roles, making it challenging to compare advanced practice nursing roles across jurisdictions.
Currently, there is much variability in the use of advanced practice nursing roles globally [ 1 , 7 , 10 , 11 ]. A clearer understanding of the roles that are in place across the globe, how they are being used and the outcomes that are being assessed would support greater role harmonization, and inform global priorities for advanced practice nursing education, research, and policy reform.
To identify current gaps in advanced practice nursing research globally, we propose to conduct of review of systematic reviews of studies examining APNs, NPs or CNSs using recognized advanced practice nursing role definitions [ 2 ]. We will seek to answer the question: Do current systematic reviews that include APNs, NPs or CNSs represent countries where these roles are found globally? To do so, we will address the following three aims:
- Identify the countries included in systematic reviews of APNs, NPs or CNSs;
- Describe the types of included studies, study population, role definitions, and context of care identified in the systematic reviews; and
- Examine the types of outcomes of APN, NP or CNS roles included in systematic reviews globally.
This review of systematic reviews will provide a description of the current state of the research, including gaps, on advanced practice nursing globally. We adapted methods used in an umbrella review that sought to identify indicators sensitive to the practice of primary healthcare NP practice [ 12 ]. The protocol for the review of reviews was developed following the PRISMA-P recommendations by Shamseer et al. [ 13 ]. The review of reviews is registered with the PROSPERO International Prospective Register of Systematic Reviews (Prospero ID CRD42021278532).
Inclusion criteria
Types of studies..
We will include all relevant published and unpublished systematic reviews reported from January 2011 onwards, with no restrictions on jurisdiction or language. For a review to be identified as systematic, a specific research question must be present or sufficient information must be provided so reviewers can identify the components of a research question (i.e., PICOS) related to advanced practice nursing. Additionally, the review must use prespecified inclusion and exclusion criteria, as well as systematic methods to identify relevant published and unpublished evidence to minimize the risk of bias in the retained studies [ 14 ]. Systematic reviews will be included provided the advanced practice nursing role is clearly defined and the APN, NP or CNS has decision-making autonomy [ 2 ].
Types of participants.
Participants will include patients and providers. Patients of any age, health condition, groups or communities receiving care from an APN, NP or CNS in all types (e.g., public/private; teaching/non-teaching,), sizes (e.g., small/medium/large) and locations (e.g., urban/rural) of community or care agencies (e.g., acute, long-term care, primary care, home care) will be retained. Providers will include all members of the healthcare team in all types, sizes, and locations of organizations. We will extract data to describe the country, number of participants, patient health conditions (e.g., diabetes, mental health), type of care (e.g., post-operative care), organizational characteristics, provider roles in the team, reason of APN, NP or CNS intervention (e.g., educational offering), and type of outcome.
Types of interventions.
We will include studies of APNs, NPs or CNSs in all sectors. To capture the countries where the roles that are implemented, we will identify studies in acute care and primary healthcare settings. Acute care will be defined as in-hospital or specialized ambulatory care to address specific health conditions [ 15 ]. Primary care will refer to the entry point of the healthcare system where patients receive comprehensive healthcare services for common health concerns [ 16 ].
Advanced practice nursing includes clinical and non-clinical activities related to education, research, and administration [ 17 , 18 ]. According to the International Council of Nurses, APNs are nurses prepared at the graduate level who have acquired in-depth expertise, complex decision-making skills and advanced clinical competencies [ 2 ]. Master’s or doctoral educational preparation is recommended and in many countries is required with national board certification for licensure and entry-level practice [ 2 ]. Given the diversity of terms used globally to identify APNs, NPs, and CNSs, members of the research team will help identify role titles specific to their region. For example, CNSs may be identified as nurse consultants in some regions in the United Kingdom. We will be attentive to the countries and geographical distribution of the systematic reviews that are identified and adjust our search strategy as needed.
NPs are autonomous clinicians who practice in ambulatory, acute and long-term care as primary and/or specialty care providers, both independently and in coordination with healthcare professionals and others. NPs assess, diagnose, treat, and manage acute episodic and chronic illnesses. NPs are experts in health promotion and disease prevention. They order, conduct, supervise, and interpret diagnostic and laboratory tests, prescribe pharmacological agents and non-pharmacologic therapies, as well as teach and counsel patients, among other services. In addition to clinical practice, they may serve as healthcare researchers, interdisciplinary consultants, and patient advocates. NPs provide a wide range of services to individuals, families, groups, and communities [ 3 ]. For nurses to be considered as NPs in our review of reviews, the review must specify that they completed a formal post-baccalaureate or graduate NP education program.
CNSs have expertise in a nursing specialty and perform a role that includes practice, consultation, collaboration, education, research and leadership. CNSs assist in providing solutions for complex healthcare issues and are leaders in the development of clinical practice guidelines, promoting the use of evidence, and facilitating system change [ 2 ]. CNSs specialize in a specific area of practice that may be defined in terms of a population, setting, disease or medical subspecialty, type of care or type of problem. For nurses to be considered as CNSs, the review has to specify that they completed a graduate degree and the role described must be reflective of the CNS role definition.
Types of comparators.
We will extract data related to the comparator (i.e., control) group to provide a brief description of the group to which care is being compared. Comparator groups can include the following, among others: usual care, best care, care provided by other healthcare professionals (e.g., physicians), or adherence to clinical practice guidelines.
Types of outcomes.
The outcomes of interest for this review of reviews will include any outcome of an advanced practice nursing role. We will document measures at the levels of the patient (e.g., health status, patient satisfaction, quality of life), the provider (e.g., job satisfaction, quality of care), the health system (e.g., costs, length of hospital stay, rehospitalisation, resource utilisation), education, or policy/scope of practice. Outcomes will be categorized as clinical, provider, health system, educational, policy/scope of practice.
Exclusion criteria
We will exclude reviews developed to address broad research questions (e.g., integrative reviews, literature reviews, scoping reviews).
We will exclude from the review of reviews studies related to physician assistants. Certified registered nurse anesthetists are excluded because, as of yet, they do not have global APN presence in the majority of countries with APN roles. We will also exclude nurse midwives since, across the different countries, not all regulatory requirements require these roles to be filled by nurses and nor are these roles consistently identified as advanced practice nursing roles. In reviews that include a mix of APN, NP and CNS roles and other provider roles, we will extract only data related to APNs, NPs and CNSs.
Moreover, we will exclude reviews where the impact of the APNs, NPs or CNSs cannot be teased out and is not reported separately from that of other types of nurses or healthcare team members. We will develop a list of all excluded reviews, along with the reasons justifying their exclusion.
Database search
We will limit our search to January 2011 onwards to capture the most up-to-date trends, as evidence is outdated after five years in about half of published reviews [ 19 ]. We will search the following electronic databases: CINAHL, EMBASE, Global Health, HealthStar, PubMed, Medline, Cochrane Library Database of Systematic Reviews and Controlled Trials Register, Database of Abstracts of Reviews of Effects (DARE), Joanna Briggs Institute, and Web of Science. We will combine subject headings and keywords related to advanced practice nursing (e.g.: advanced practice nursing, nurse-led), APN (e.g., advanced practice nurse, advanced practice clinician, advanced practitioner, nurse prescriber), NP (e.g., nurse practitioner, advanced practice registered nurse, family nurse practitioner, primary healthcare nurse practitioner, adult gerontology nurse practitioner, pediatric nurse practitioner, oncology nurse practitioner, emergency nurse practitioner, mental health nurse practitioner, neonatal nurse practitioner), and CNS (e.g., nurse specialists, clinical nurse specialist, infection control practitioner, nurse consultant, specialist nurse) roles/titles, along with a search filter based on the CADTH systematic reviews and meta-analyses search filter and that developed by Lunny et al. for reviews of systematic reviews to capture a broad range of roles across settings [ 20 , 21 ]. Subject headings and keywords will also include more general roles/titles, as well as those specific to primary and acute care settings, and corresponding acronyms where applicable. The full preliminary search strategy developed for the PubMed database, which will subsequently be adapted to each electronic database, is presented in S1 Appendix . We will adapt strategies reviewed by an academic librarian that have been used successfully in previous reviews [ 21 ]. In addition, we will hand search the reference lists of all relevant reviews to identify additional studies.
Moreover, we will search the grey literature will for the period of January 2011 onwards using the following websites and tools: World Health Organization, Organization for Economic Co-operation and Development (OECD), International Council of Nurses, CADTH Information Services, CADTH Grey Matters Tool, and ProQuest Dissertation and Theses. We will search the PROSPERO International Prospective Register of Systematic Reviews to identify registered review protocols, and will contact authors of registered PROSPERO reviews to ascertain study status. For each website, the content will be searched using the same search terms as those used for the published literature, e.g.: (Advanced practice nurs* OR Nurse practitioner* OR Clinical nurse specialist*) AND (Primary care OR Acute care) AND Systematic review*. If there is not an inherent search function on the website, a search will be conducted of all webpages and weblinks. The preliminary search strategy for the grey literature is presented in S2 Appendix .
Study selection
To enhance inter-rater agreement, all reviewers will be trained to use the screening instrument and inclusion/exclusion criteria. We will upload the retained studies into the EndNote and RAYYAN software [ 22 ], after which duplicates will be removed. Two reviewers will independently screen titles and abstracts using the predefined inclusion/exclusion criteria, and recommend exclusion or further full-text review. Any discrepancies will be discussed among the reviewers. Inter-rater agreement will be estimated using the kappa statistic. Additional training sessions will be planned if inter-rater agreement is low and Cohen’s kappa is below 60% [ 23 ].
To be included in our review of reviews, each paper must be identified as a systematic review, and focus on an advanced practice nursing role or intervention. If the abstract contains insufficient information or there is no abstract available, we will complete a full-text review. We will complete a full-text review for all the reviews retained after the initial screening, again using the predefined inclusion/exclusion criteria. Any coding discrepancies will be discussed among the reviewers until agreement is reached on the inclusion or exclusion of the review. In the event they are unable to reach a consensus, a third reviewer will act as tie-breaker.
Data extraction
Data from included full-text papers will be extracted by one coder and subsequently reviewed by a second coder. Any discrepancies will be resolved by consensus. A structured tool developed for a previous review of reviews will be adapted and pilot-tested by the investigators [ 12 ]. We will extract data from the methods and results section of each full-text paper. The data we will extract will include: review aim or focus; review characteristics (e.g., publication year); name and number of electronic databases searched; participant and intervention characteristics; number and types of studies included in the review; countries where studies were conducted; specification of patient, provider, health system, educational, policy, and scope of practice outcomes; and funding source [ 24 ]. Additionally, we will document APN, NP or CNS and non-APN involvement in the research team who conducted the review by extracting data related to the professional designation of the research team members.
Design of included studies
Because the addition of APNs, NPs and CNSs is a complex healthcare system intervention, different types of information are needed to inform research about advanced practice nurses [ 25 ]. Systematic reviews included in our review of systematic reviews may include the results of randomized controlled trials, prospective controlled observational studies and cohort studies, retrospective controlled observational and cohort studies, and surveys. We will develop a summary table to present key findings.
Assessment of review quality
Two reviewers will independently rate each systematic review using the 10-item Critical Appraisal Skills Programme (CASP) criteria [ 26 ] to assess the systematic review’s methodologic quality. As described above, inter-rater agreement will be assessed using Cohen’s kappa, and any disagreements will be discussed among the reviewers until they come to a consensus. We will generate a summary table with the CASP ratings.
The primary outcome of the review of reviews is to document APNs, NPs or CNSs research globally to identify gaps in current research. We will examine each advanced practice nursing role separately.
Data synthesis
A narrative synthesis of the findings will be compiled. We will use an iterative process to identify patterns and relationships emerging across the different reviews and years when they were conducted [ 27 ]. We will develop summary tables outlining the key review characteristics (e.g., publication year, countries where primary studies were conducted), outcomes (i.e., patient, provider, health system, educational, policy/scope of practice), type of advanced practice nursing role, and quality assessment. We will keep a record of all review-related decisions. No additional quantitative analyses are planned as this is not recommended for overviews because of the potential risk of overlap in studies that appear in more than one review [ 28 ].
The identification of advanced practice nursing roles that are currently in place, the countries where these nurses practice and the outcomes being used to examine practice will shed light on current gaps in the literature, and identify stronger and weaker areas of evidence related to advanced practice nursing globally. The review of systematic reviews builds on a recently completed umbrella review of NPs in primary healthcare. The current review of reviews will synthesize the characteristics of advanced practice nursing roles, study populations, contexts and outcomes to determine how closely these roles align with ICN definitions. In contexts where the roles are not optimally implemented or utilized, the findings will support the development of recommendations at the clinical, educational, and regulatory levels to improve role clarity, role implementation and access to high quality care. In addition, the development of an international strategic plan for APN role development will aid countries hoping to further expand APN practice.
Supporting information
S1 checklist. prisma-p 2015 checklist..
https://doi.org/10.1371/journal.pone.0280726.s001
S1 Appendix. Preliminary search strategies (PubMed) for the published literature.
https://doi.org/10.1371/journal.pone.0280726.s002
S2 Appendix. Preliminary search strategies for the grey literature.
https://doi.org/10.1371/journal.pone.0280726.s003
- 1. World Health Organization. State of the World’s Nursing Report 2020- Investing in education, jobs and leadership. Health Workforce. 6 April 2020. Available from: https://www.who.int/publications/i/item/9789240003279 .
- 2. International Council of Nurses, Schober M, Lehwaldt D, Rogers M, Steinke M, Turale S, et al. Guidelines on Advanced Practice Nursing. 2020. Available from: https://www.icn.ch/system/files/documents/2020-04/ICN_APN%20Report_EN_WEB.pdf .
- 3. American Association of Nurse Practitioners (ANNP). Discussion paper: Quality of nurse practitioner practice. 2020. Available from: https://storage.aanp.org/www/documents/advocacy/position-papers/Quality-of-NP-Practice-Bib_11.2020.pdf .
- View Article
- PubMed/NCBI
- Google Scholar
- 6. Canadian Nurses Association, Almost J. Regulated Nursing in Canada: The Landscape in 2021. February 2021. Available from: https://www.cna-aiic.ca/en/nursing-practice/the-practice-of-nursing/regulated-nursing-in-canada .
- 11. Schumann L, Bird B, Pilane C, Duff E, Geese F, Jelic M, et al. Mapping of advanced nursing competencies from nineteen respondent countries against the strong model of advanced practice nursing (2000) and the International Council of Nurses (2008) Advanced Practice Nursing Competencies (2013–2017). International Council of Nurses NP/APN Network Research Subgroup Publication. March 2019. Available from: https://internationalaanporg/Research/SG .
- 21. CADTH. CADTH search filters database. Ottawa. 2022. Available from: https://searchfilters.cadth.ca/list?q=&p=1&ps=20&topic_facet=systematic%20reviews%20000000%7CSystematic%20reviews .
- 26. Critical Appraisal Skills Programme (CASP). CASP Checklist: 10 questions to help you make sense of a systematic review. 2018. Available from: https://casp-uk.net/casp-tools-checklists/ .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Perspective
- Open access
- Published: 16 May 2024
Patient-centricity in digital measure development: co-evolution of best practice and regulatory guidance
- Suvekshya Aryal ORCID: orcid.org/0000-0002-6077-4919 1 ,
- Jennifer M. Blankenship 1 ,
- Shelby L. Bachman ORCID: orcid.org/0000-0002-4460-4661 1 ,
- Soohyun Hwang 1 ,
- Yaya Zhai 1 ,
- Jennifer C. Richards 1 ,
- Ieuan Clay ORCID: orcid.org/0000-0001-9722-8834 1 &
- Kate Lyden 1
npj Digital Medicine volume 7 , Article number: 128 ( 2024 ) Cite this article
4 Altmetric
Metrics details
- Drug development
- Drug regulation
Digital health technologies (DHTs) have the potential to modernize drug development and clinical trial operations by remotely, passively, and continuously collecting ecologically valid evidence that is meaningful to patients’ lived experiences. Such evidence holds potential for all drug development stakeholders, including regulatory agencies, as it will help create a stronger evidentiary link between approval of new therapeutics and the ultimate aim of improving patient lives. However, only a very small number of novel digital measures have matured from exploratory usage into regulatory qualification or efficacy endpoints. This shows that despite the clear potential, actually gaining regulatory agreement that a new measure is both fit-for-purpose and delivers value remains a serious challenge. One of the key stumbling blocks for developers has been the requirement to demonstrate that a digital measure is meaningful to patients. This viewpoint aims to examine the co-evolution of regulatory guidance in the United States (U.S.) and best practice for integration of DHTs into the development of clinical outcome assessments. Contextualizing guidance on meaningfulness within the larger shift towards a patient-centric drug development approach, this paper reviews the U.S. Food and Drug Administration (FDA) guidance and existing literature surrounding the development of meaningful digital measures and patient engagement, including the recent examples of rejections by the FDA that further emphasize patient-centricity in digital measures. Finally, this paper highlights remaining hurdles and provides insights into the established frameworks for development and adoption of digital measures in clinical research.
Similar content being viewed by others

Digitizing clinical trials
Evolving regulatory perspectives on digital health technologies for medicinal product development
Overcoming barriers to patient adherence: the case for developing innovative drug delivery systems
Introduction.
Digital health technologies (DHTs) offer the ability to capture data remotely, continuously, and with low patient burden 1 , 2 , 3 . As such, DHTs are paving the way for the development of a new class of clinical outcome assessments (COAs) that reflect how patients feel, function, and survive in real-world environments 4 . Many believe the evidence generated from DHTs provides an opportunity for a more holistic and direct understanding of the patient experience 2 , 5 , and consequently, there has been a significant investment into developing new DHT-derived outcome measures 6 . Initial efforts were technology-driven and centered around expanding the limits of what can be measured 1 , 7 . However, following the overall trend in the industry for a more patient-centric approach to drug development, there is an increased focus on understanding what should be measured with DHTs 1 , 2 , 8 , 9 , 10 , 11 , 12 .
The US Food and Drug Administration (FDA) has progressively evolved their guidance and exerted pressure on drug developers to move towards a more patient-focused approach, encouraging the systematic incorporation of the patient voice into all aspects of drug development and evaluation 13 . Over the past decade, there has been an increasing emphasis on engaging patients in protocol development, endpoint selection, and the development of fit-for-purpose evidence generation tools 12 . Both the 2009 Patient-reported Outcome (PRO) guidance for industry 14 and the 2012 Patient-Focused Drug Development (PFDD) initiative 12 provide roadmaps on how to incorporate the patient experience into medical product development. The most recently released four-part guidance series further emphasizes this by providing stepwise recommendations on how to collect and submit patient experience data for regulatory decision making. Collectively, the goal of these initiatives is to develop interventions that induce a treatment benefit that is considered meaningful to patients.
This viewpoint aims to illustrate how the recent FDA PFDD guidance has shaped digital measure development with a particular emphasis on generating evidence that DHT-derived COAs are meaningful to patients. This paper discusses various methodologies available to establish the meaningfulness of digital measures required to facilitate regulatory endorsement, and highlights the progress made, hurdles that remain, and future directions needed for the successful adoption and implementation of digital measures.
Shaping digital measure development: the role of regulatory guidance
The scientific, financial, and operational advantages of digital measures in drug development have been extensively discussed 6 , 15 , 16 , however, despite this, adoption has been limited to date. Some of the resistance from sponsors is due to the uncertainty that endpoints derived from DHTs will be of value in discussions with regulators. Given the importance of PFDD at the FDA, establishing clear rationales of meaningfulness of digital measures provides the best opportunity for regulatory qualification, which in turn paves the way for broad utilization in future drug development trials. Digital measures capture aspects of life in real-world environments, and compelling cases can be made that outcomes derived from DHTs will be inherently meaningful to patients, yet robust evidence is required to support those arguments. Recent guidance describes what is required to justify the seemingly simple statement that a new digital measure is “patient-centric”.
Understanding the FDA guidance on driving patient-centric digital measure development
The FDA’s recent four-part guidance on PFDD significantly expands their position on patient engagement in drug development 17 . This guidance series is applicable to all COA categories (i.e., PROs, clinician-reported outcomes, observer-reported outcomes, and performance outcomes), and outcomes derived from DHTs. Part 1 18 and part 4 19 of the series primarily address comprehensive and representative input from patients and caregivers for regulatory decision making. Part 2 20 focuses on the methods and best practices for gathering patient input on what is important to them in disease and treatment, including a focus on psychometric testing. Part 3 21 provides a general roadmap for developing patient-focused outcome measurement and supporting a COA as fit-for-purpose. It also provides recommendations for evaluating COAs based on their construct and content validity, as well as reliability and responsiveness to change. While the FDA has consistently required evidence to support the claim that COAs are selected based on patient-centric concepts 22 , this recent guidance series emphasizes the importance of adopting robust methodologies to generate such evidence.
Digital measure developers have started adopting this guidance to initiate regulatory submissions amidst discussion of a dedicated pathway for deploying digital measures as drug development tools (DDTs) 23 .
Evolving FDA expectations on incorporating meaningfulness in digital measure development
In the history of the FDA DDT-COA qualification program 4 , which evaluates whether COAs are a well-defined and meaningful assessment of how patients feel, function, and survive in specified contexts of use, 9 letter of intents (LOIs) for DHT-derived COAs have been submitted for qualification 24 . The earliest submission of an LOI accepted into the qualification program was reported in 2018 for the assessment of physical activity via accelerometry 25 . In total, 6 LOIs for DHT-derived COAs have been accepted into the program; however, it has been 4 years since the last LOI was accepted by the FDA. The most common reason for LOI rejection is a lack of meaningfulness of the measure to patients, which is pushing developers to align with FDA’s evolving expectations, particularly for the projects that were planned or submitted years before the recent guidance was released.
For example, the FDA rejected an LOI for a DHT-derived COA assessing motor symptom severity using a finger tapping test on a smartphone, for use in Parkinson’s disease 26 . While a case could be made that maintaining fine motor skills is important for patients with Parkinson’s disease, in the rejection letter FDA was concerned about the meaningfulness of the act of tapping repeatedly on a screen to patients 26 .
Similarly, the FDA rejected an LOI for a COA reflecting aspects of gait abnormality in Huntington’s Disease because the advanced gait assessment proposed was not inherently easy to understand. Further, as indicated in the decision letter, there was a general lack of clarity on whether changes in the proposed gait parameters were meaningful to patients and if changes in gait parameters would reflect changes in everyday functioning 27 . The FDA’s response was consistent in its most recent rejection of an LOI for an actigraphy-derived COA that reflected aspects of functional mobility such as gait, balance and coordination for use in neurodegenerative diseases 28 . The reviewers expressed concerns as to whether the measures are relevant and important to functional mobility and whether the gait kinematic parameters suggested in the conceptual model would be meaningful to patients. To note, there has been an uptake of DHTs in the neurological field, signaling early integration in clinical research, particularly in Parkinson’s disease 29 . Evidence of patient-centric development will inform success in regulatory approval and wider adoption, but the approach needs to be tailored to the patient population. For example, in Alzheimer’s disease, patients find difficulties articulating their lived experiences due to cognitive decline, and so the development effort may require more nuance.
The rejections in the FDA COA qualification program indicate a shift in the rigor of evidence required to demonstrate that digital measures are meaningful to patients 30 . A separate guidance, issued for remote data acquisition using DHTs in clinical trials 31 , outlines that evidence is needed to demonstrate that a DHT is usable and acceptable by patients, accurately measures the outcomes it claims to measure, and that the derived outcomes are clinically relevant to the specific population (i.e. conforms to evidentiary standards detailed in the V3 framework 32 ). Although evidence collection along this continuum is critical for DHT-derived COAs and biomarkers to be accepted or qualified by regulators, FDA’s recent guidance series related to meaningfulness of the measures captured by the DHTs is at the forefront. Alignment across stakeholders around the V3 framework has catalyzed innovation, leading to adaptations of established methodologies and concepts from the “traditional” biomarker field 33 . Current regulatory guidance is now prompting a similar cross-pollination, with digital measure developers increasingly turning to established practices from fields like psychometrics research, product development and user experience, in order to robustly demonstrate that the chosen measurement concepts are meaningful to patients. The FDA also offers various channels of early engagement for submissions, for example, through the Critical Path Innovation Meeting (CPIM) for COAs 34 and pre-LOI program for biomarkers 35 . This encourages a dialog between researchers, developers and regulators in the pre-competitive or pre-submission stage.
Establishing meaningful aspects of health in practice
Engaging with patients to determine meaningful aspects of health.
The Digital Measures that Matter framework 1 provides a hierarchical model for establishing meaningfulness. First, patients’ experiences are mapped to high-level Meaningful Aspects of Health (MAH). MAH are then linked to broader and specific measurable concepts of interest, and finally to specific outcomes and endpoints. The framework helps to understand meaningful evidence as something rooted in MAH and avoid “technological determinism”, i.e. defining a patient’s lived experience by what can be measured with a technology instead of first considering what should be measured. Similarly, the PFDD guidance emphasizes using robust methods and psychometric analysis to gather patients’ experience and input on important aspects of life that should be assessed in the disease and its treatment 20 , 21 . The following sections present a brief recap of the key methodologies available to determine meaningful measurement concepts. The best method to gather patient input must be determined on a case-by-case basis and is dependent on the specific needs and context of the research in question.
The FDA encourages facilitated discussions with patients and caregivers to incorporate patient voice through the established model of FDA-, or externally-led PFDD meetings that organizations can adopt for collaborative meetings with patients and key stakeholders in any specific disease area 36 . Consensus on high-level MAH is often gathered in these meetings, catalyzing further research that can then focus on determining specific measurable concepts or outcomes linked to identified MAH. Qualitative research methods, such as one-on-one interviews and focus group discussions, are recommended to gain a deeper understanding of individual or group experiences. Quantitative methods such as surveys can also be useful for gathering patient input, but considerations should be taken to develop clear, concise and relevant questions and pilot with small groups of participants before launching larger studies. Mixed-methods approaches that integrate quantitative and qualitative approaches can provide a comprehensive understanding of patient perspectives and can help understand the generalizability of any observations.
These methods can provide foundational evidence to support the meaningfulness of novel DHT-derived COAs. The examples discussed below and in Table 1 showcase how sponsors and researchers are using the Measures that Matter framework and the roadmap developed by the FDA to gather patient input for COA development.
Obtaining diverse patient input to establish meaningfulness: key examples
In an example of patient-centric development, a mixed-methods study was conducted to understand MAH in atopic dermatitis 37 . The results found that scratch was the most burdensome and the most bothersome symptom that kept patients awake at night. The patients’ treatment considerations included relief from nocturnal scratch and adopting technologies that would measure scratch. This evidence is key for development and discovery of novel products and interventions that improve the lives of many individuals living with atopic dermatitis.
In another example, findings from an FDA-led PFDD meeting were used to identify MAH 16 in idiopathic pulmonary fibrosis (IPF). The Voice of the Patient report provided the justification that higher-intensity physical activity is meaningful to patients with IPF. As a result, moderate-to-vigorous physical activity derived from a wrist actigraphy sensor was incorporated as a primary endpoint in a Phase 3 IPF trial 38 . This serves as a key example of how existing sources of evidence should be leveraged to gather patient input whenever possible.
Additionally, the development of stride velocity 95 th centile (SV95C) in Duchenne Muscular Dystrophy (DMD) 39 , with an accepted LOI and endpoint qualified by EMA, established MAH through qualitative investigation in clinical experts, and patients and caregivers 40 . Evidence from these efforts indicated that patients and their caregivers view ambulatory function as key to their independence, want to maintain or improve it through treatment, and consider its real-world measurement important. ( See Table 1 ).
Co-creating a conceptual framework with patients
Patient involvement in digital measure development is crucial beyond the initial step of establishing MAH. In the subsequent step of developing a conceptual framework, concepts identified as MAH are linked to measurable outcomes and then mapped to specific endpoints 21 . At this stage, patient input is important for ensuring that identified concepts and outcomes resonate with their lived experiences.
Such co-creation is the hallmark of Mobilise-D’s development effort. The consortium is developing digital measures of mobility in various conditions such as Parkinson’s disease, multiple sclerosis, chronic obstructive pulmonary disease, hip fracture, heart failure, frailty and sarcopenia 41 . Their work includes an exhaustive literature review of qualitative evidence of meaningfulness of walking. Patients were directly involved in the interpretation of the findings, where they helped identify several concepts of walking experience that are universally meaningful 42 , 43 . This level of patient engagement confirms that development efforts are rooted in patient experience.
Engaging with patients to evaluate user experiences and deliver value with DHTs
Meaningfulness in defining the measured concept is, of course, a foundational step, but this work is inconsequential if the implementation of the digital measure is not thoughtful 6 . Engaging patients provides a path to inform what evidence needs to be considered, how a measure will be implemented, and whether direct value for patients will be delivered. Therefore, once the conceptual framework supporting a digital measure is established, evidence of usability and acceptability of the relevant technology should be generated, beyond the required verification and validation 32 . As such, meaningfulness can be enhanced by ensuring that a measure that is rooted in patient experience is also acceptably captured by DHTs based on the user experience 31 . Equally, thinking of the value that can be directly delivered to patients, for example through the return of a summary of health data or trial results, opens further opportunities for engagement.
Establishing DHT acceptability and feasibility through real-world testing
There are various factors to consider while user testing DHTs, such as technical features, ease and comfort of use, interference with daily life, and the perceived benefit of use in the real-world 43 , 44 . Engaging with patients who have used and tested a given DHT in their lived environment is critical to generating such evidence. In a notable example, IDEA-FAST, another IMI-funded research consortium aiming to develop digital measures of sleep, fatigue, and activities of daily living in neurodegenerative and immune-mediated inflammatory diseases, has ongoing work to directly evaluate feasibility of using multiple sensing devices 45 . Researchers are using mixed-methods approaches to evaluate user experience, acceptability, and compliance. Patients’ daily reports are collected through questionnaires and daily diary, and objective measures of sensor wear time and compliance are collected directly from the DHTs 46 . Such data are also critical to evaluate whether DHTs are feasible for continuous and reliable data collection in the real world 44 .
To ensure representativeness of a DHT’s acceptability and feasibility, it is crucial that real-world device testing is performed in individuals from diverse socio-demographic backgrounds and disease severities. As DHTs pave the path for decentralized trials, gathering a broad spectrum of experiences becomes a priority in order to achieve inclusivity and representation in clinical trials 47 . Moreover, understanding the patient’s journey requires recognizing that distinct “personas” and preferences are dynamic and differ significantly among patient groups. For example, while a given wearable technology might be well received by patients with mild Alzheimer’s disease, its acceptability may be different for those with moderate and severe forms of the disease. Similarly, for cancer patients undergoing intensive treatment, the tolerance for wearable sensors may be diminished during treatment compared to other times.
Returning DHT-derived data back to the patients
Return of summarized health data to participants, especially those collected using DHTs, has recently been recognized as preferred by patients 48 and known to help motivate patients and encourage communications with clinicians 49 . This serves as an opportunity for continuous patient engagement during the digital measure development process. It is also one way to ensure that meaningfulness is retained when progressing from the establishment of MAH and conceptual framework to digital measure deployment. In IDEA-FAST’s feasibility study utilizing multiple sensing devices (measuring physical activity, cognition and other health metrics), a summary of health data was provided back to patients at different time points during the study. To understand the importance of returning data and the best mechanisms to do so, the study explored patients’ perceived benefits of access to data, as well as use and interpretability of the data 50 . The preliminary findings suggested that patients reported using the information to monitor their health and performance, and felt confident about their adherence to the devices, which also fostered communications with clinicians. Some patients had difficulties interpreting the data, and some were confused about missing data. While the research on this front is still new, providing patients with access to their post-study data can be valuable to continue engagement at the final stage of digital measure deployment. As such, innovations on this front, for returning accessible and interpretable data, such as through mobile app features or summary reports, may be a worthwhile investment.
Traditionally, in clinical trials such return of data or results is uncommon. The return of DHT-derived data as discussed here is in line with the patient engagement efforts crucial for generating the evidence required for identifying and developing digital measures that are ultimately integrated in the clinical trials. Nevertheless, this must be approached with caution, and depending on the type of study there may be ethical and legal implications as well as errors or biases impacting patient behavior and the data thereafter 51 . Key considerations here include clear communication with patients (in informed consent), and proper documentation of plans for handling incidental findings and ways of mitigating risks (in IRB application) 52 .
Remaining hurdles for digital measure development and adoption
The past 10 years of FDA PFDD guidance have culminated in a deepening focus on patient engagement throughout the process of DDT-COA development. The patterns observed in the FDA COA qualification submissions reveal an increased emphasis on meaningfulness to patients, underscoring the importance, for acceptance and qualification by regulators, of developing digital measures that resonate with patients’ lived experiences in disease or treatment. As such, the regulatory guidance requires a continuous co-evolution with the digital measure field.
The FDA showcases commitment to building internal infrastructure and expertise to continue to advance the field. The future looks promising as both developers and regulators realize the significant benefits of PFDD in improving patient outcomes with increased efficiency, expedited regulatory decisions, and enhanced stakeholder engagement 6 , 15 , 41 . Figure 1 summarizes a roadmap for frequent patient engagement throughout the process of digital measure development. The roadmap also suggests an iterative process of communicating the results back to the patients and refining the development approach so that the meaningfulness is retained throughout.
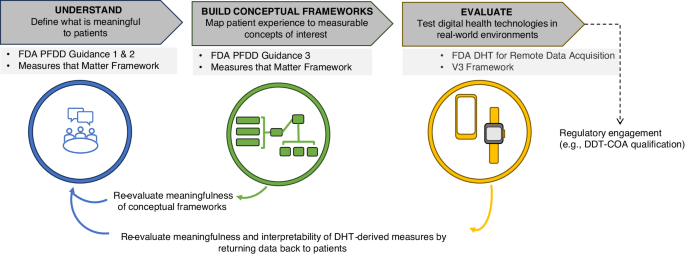
Guidance from FDA 17 , 31 and others 1 , 32 emphasizes the importance of developing measures that matter to patients. The established process of developing digital measures that matter to patients (upper dark gray boxes) involves intermittent engagement with patients to define what is meaningful and determine the feasibility of capturing measures with DHTs (applicable guidance noted in light sgrey boxes). Several consortium projects have put this approach into practice; these include DiMe 37 , Mobilise-D 60 , and IDEA-FAST 61 . To move towards co-creating digital measures with patients, providing value to patients throughout the process of development is a necessary next step. As depicted in the lower section of the figure, this approach ensures the measures developed remain rooted in the patient voice and offer opportunities to iteratively refine the foundational evidence supporting such measures. DDT-COA Drug Development Tool-Clinical Outcome Assessment, DHT Digital Health Technologies, DiMe The Digital Medicine society, FDA Food and Drug Administration, PFDD Patient-Focused Drug Development.
Despite progress, challenges persist. First, patient experiences can be highly heterogeneous making it difficult to identify a single aspect of health that is meaningful and generalizable across all patients. Further, the ways in which a single aspect of health manifests across patients may also differ. For example, the fear of losing independence, while common across different phases of a patient journey, may translate to different measurement concepts depending on multiple factors including disease severity, culture, or other socioeconomic variables.
Secondly, even with detailed regulatory guidance on developing meaningful measures, there is no straightforward or standard path to successful development of a conceptual model. Without a clear roadmap for generating a specific concept of interest from the established MAH, qualification of DHT-derived COAs is challenging. Further, challenges in defining clinical significance remain due to the nature of digital measures and variability across patients and to lack or absence of associated anchors. There are a few examples of successful development in this case, such as the SV95C in DMD 40 , and the FDA’s guidance on PRO (and other COA) development can be utilized to understand methodologies in this context 19 . Still, there is a need for further guidance on these issues.
Furthermore, challenges lie in the classification of digital measures as COAs vs biomarkers in drug development 23 . As many behavioral digital measures are DHT-derived, they occupy a gray area between directly measuring “feel and function” (which would be characteristic of a COA), and a precisely defined, yet relatively abstract, measurement concept (which would be characteristic of a biomarker) 23 . Clarity from regulators will be required to understand whether individual measures are more suitable as COAs or biomarkers going forward.
Finally, there is a need to facilitate adoption internationally. In Europe, EMA has also released its own guidance on DHT-based methodologies to support medical products 53 , 54 . The EMA database points to various developments in this space. At the forefront is the recent qualification of SV95C as a primary endpoint in DMD clinical trials 15 , 40 . Additionally, Mobilise-D and IDEA-FAST have engaged the EMA for qualification advice on their digital measure development studies 55 , 56 , 57 . The forthcoming regulatory decisions may also provide valuable insights into alignment or divergence in approval mechanisms and approaches of EMA and FDA. Outside of EMA and FDA, countries such as the UK, Canada, and Japan, as well as international organizations such as WHO, have released guidance for DHTs and real-world data, but specific developments around qualification of measures have yet to be reported.
The co-evolution of best practice, regulatory expectations, and guidance will progress as more examples of DHT-derived COAs emerge. Furthermore, key frameworks will be implemented and refined, and methodologies from other fields will be adapted in order to fulfill evidentiary requirements. More active engagement and dialog between developers and regulators will be essential to fuel the co-evolution. Thus, there is a need for more developers to take the step of engaging with regulators for qualification and acceptance, and regulators to further clarify and enable digital measure LOIs to be submitted. Engaging interdisciplinary stakeholders is also critical. Scientific and clinical experts play an important role as subject matter experts or principal investigators and support all aspects of the design and implementation. Along with other key stakeholders like the payers, they are vital for advocating for the utility of DHTs. Further, payers are also crucially positioned to improve patient care by establishing reimbursement policies around DHTs and providing wealth of data to facilitate real-world evidence and health economics and outcomes research.
In conclusion, while there are remaining hurdles to the successful development, qualification, and widespread adoption of digital measures, the path forward, driven by co-creation and stakeholder collaboration, holds the promise of a transformed and more patient-centric future in drug development. Ultimately, digital measures that matter to patients and that deliver value to them will prove to be beneficial to all stakeholders.
Manta, C., Patrick-Lake, B. & Goldsack, J. C. Digital measures that matter to patients: a framework to guide the selection and development of digital measures of health. Digit. Biomark. 4 , 69–77 (2020).
Article PubMed PubMed Central Google Scholar
Clay, I. et al. Reverse engineering of digital measures: inviting patients to the conversation. Digit. Biomark. 7 , 28–44 (2023).
Goldsack, J. C., Dowling, A. V., Samuelson, D., Patrick-Lake, B. & Clay, I. Evaluation, acceptance, and qualification of digital measures: from proof of concept to endpoint. Digit. Biomark. 5 , 53–64 (2021).
Center for Drug Evaluation and Research. Clinical Outcome Assessment (COA) Qualification Program. FDA https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/clinical-outcome-assessment-coa-qualification-program (2023).
Clay, I. Impact of digital technologies on novel endpoint capture in clinical trials. Clin. Pharmacol. Ther. 102 , 912–913 (2017).
Article PubMed Google Scholar
Marra, C., Chen, J. L., Coravos, A. & Stern, A. D. Quantifying the use of connected digital products in clinical research. NPJ Digit. Med. 3 , 50 (2020).
Burq, M. et al. Virtual exam for Parkinson’s disease enables frequent and reliable remote measurements of motor function. Npj Digit. Med. 5 , 1–9 (2022).
Google Scholar
Lacombe, D. et al. Moving forward from drug-centred to patient-centred research: A white paper initiated by EORTC and developed together with the BioMed Alliance members. Eur. Respir. J. 53 , 1801870 (2019).
Timpe, C., Stegemann, S., Barrett, A. & Mujumdar, S. Challenges and opportunities to include patient-centric product design in industrial medicines development to improve therapeutic goals. Br. J. Clin. Pharmacol. 86 , 2020–2027 (2020).
Stegemann, S., Ternik, R. L., Onder, G., Khan, M. A. & van Riet-Nales, D. A. Defining patient centric pharmaceutical drug product design. AAPS J. 18 , 1047–1055 (2016).
Article CAS PubMed Google Scholar
Taber, D. J. et al. A viewpoint describing the American Society of Transplantation rationale to conduct a comprehensive patient survey assessing unmet immunosuppressive therapy needs. Clin. Transplant. 37 , e14876 (2023).
Perfetto, E. M., Burke, L., Oehrlein, E. M. & Epstein, R. S. Patient-focused drug development: a new direction for collaboration. Med. Care 53 , 9 (2015).
Center for Drug Evaluation and Research. FDA Patient-Focused Drug Development Guidance Series for Enhancing the Incorporation of the Patient’s Voice in Medical Product Development and Regulatory Decision Making. FDA (2020).
Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual. Life Outcomes 4 , 79 (2006).
Servais, L. et al. First regulatory qualification of a novel digital endpoint in duchenne muscular dystrophy: a multi-stakeholder perspective on the impact for patients and for drug development in neuromuscular diseases. Digit. Biomark. 5 , 183–190 (2021).
King, C. S. et al. A phase-2 exploratory randomized controlled trial of inopulse in patients with fibrotic interstitial lung disease requiring oxygen. Ann. Am. Thorac. Soc. 19 , 594–602 (2022).
Center for Drug Evaluation and Research. FDA Patient-Focused Drug Development Guidance Series for Enhancing the Incorporation of the Patient’s Voice in Medical Product Development and Regulatory Decision Making. https://www.fda.gov/drugs/development-approval-process-drugs/fda-patient-focused-drug-development-guidance-series-enhancing-incorporation-patients-voice-medical (2023).
Patient-Focused Drug Development: Collecting Comprehensive and Representative Input; Guidance for Industry, Food and Drug Administration Staff, and Other Stakeholders; Availability. Federal Register https://www.federalregister.gov/documents/2020/06/17/2020-13046/patient-focused-drug-development-collecting-comprehensive-and-representative-input-guidance-for (2020).
Center for Drug Evaluation and Research. Patient-Focused Drug Development: Incorporating Clinical Outcome Assessments Into Endpoints for Regulatory Decision-Making. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-incorporating-clinical-outcome-assessments-endpoints-regulatory (2023).
Center for Drug Evaluation and Research. Patient-Focused Drug Development: Methods to Identify What Is Important to Patients. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-methods-identify-what-important-patients (2022).
Center for Drug Evaluation and Research. Patient-Focused Drug Development: Selecting, Developing, or Modifying Fit-for-Purpose Clinical Outcome Assessments. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-selecting-developing-or-modifying-fit-purpose-clinical-outcome (2022).
Gnanasakthy, A., Qin, S. & Norcross, L. FDA guidance on selecting, developing, or modifying fit-for-purpose clinical outcome assessments: old wine in a new bottle? Patient - Patient-Centered Outcomes Res 16 , 3–5 (2023).
Article Google Scholar
Izmailova, E. S., Demanuele, C. & McCarthy, M. Digital health technology derived measures: Biomarkers or clinical outcome assessments? Clin. Transl. Sci. 16 , 1113–1120 (2023).
CDER & CBER Drug Development Tool Qualification Project Search. FDA https://force-dsc.my.site.com/ddt/s/ .
Center for Drug Evaluation and Research. DDT COA #000102: Physical Activity Accelerometry Assessment for Analgesic Clinical Trials (PAACT). FDA https://www.fda.gov/drugs/clinical-outcome-assessment-coa-qualification-program/ddt-coa-000102-physical-activity-accelerometry-assessment-analgesic-clinical-trials-paact (2021).
Center for Drug Evaluation and Research. DDT COA #000142: Virtual Motor Exam for Parkinson’s Disease, Part III Estimator (VME Part III). FDA https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/ddt-coa-000142-virtual-motor-exam-parkinsons-disease-part-iii-estimator-vme-part-iii (2021).
Center for Drug Evaluation and Research. DDT COA #000129: Advanced Gait Analysis. FDA https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/ddt-coa-000129-advanced-gait-analysis (2020).
Center for Drug Evaluation and Research. DDT COA #000146: Instrumented Score of Ataxia Mobility (I-SAM). FDA https://force-dsc.my.site.com/ddt/s/ddt-project?ddtprojectid=143 (2021).
Masanneck, L., Gieseler, P., Gordon, W. J., Meuth, S. G. & Stern, A. D. Evidence from ClinicalTrials.gov on the growth of Digital Health Technologies in neurology trials. Npj Digit. Med. 6 , 1–5 (2023).
Diao, J. A., Raza, M. M., Venkatesh, K. P. & Kvedar, J. C. Watching Parkinson’s disease with wrist-based sensors. Npj Digit. Med. 5 , 1–2 (2022).
Conway, A. Digital Health Technologies for Remote Data Acquisition in Clinical Investigations: Guidance for Industry, Investigators, and Other Stakeholders. FDA Draft Guidance. https://www.fda.gov/media/155022/download (2021).
Goldsack, J. C. et al. Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs). Npj Digit. Med. 3 , 55 (2020).
Babrak, L. M. et al. Traditional and Digital Biomarkers: Two Worlds Apart? Digit. Biomark. 3 , 92–102 (2019).
Center for Drug Evaluation andResearch. Critical Path Innovation Meetings (CPIM). FDA https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/critical-path-innovation-meetings-cpim (2023).
Center for Drug Evaluation and Research. Resources for Biomarker Requestors. FDA https://www.fda.gov/drugs/biomarker-qualification-program/resources-biomarker-requestors (2021).
Center for Drug Evaluation and Research. FDA-led Patient-Focused Drug Development (PFDD) Public Meetings. FDA https://www.fda.gov/industry/prescription-drug-user-fee-amendments/fda-led-patient-focused-drug-development-pfdd-public-meetings (2023).
Cesnakova, L. et al. A patient-centred conceptual model of nocturnal scratch and its impact in atopic dermatitis: A mixed-methods study supporting the development of novel digital measurements. Ski. Health Dis. 3 , e262 (2023).
Bellerophon Announces FDA Acceptance of Change to Ongoing Phase 3 REBUILD Study of INOpulse® for Treatment of Fibrotic Interstitial Lung Disease | Bellerophon Therapeutics, Inc. https://bellerophon.gcs-web.com/news-releases/news-release-details/bellerophon-announces-fda-acceptance-change-ongoing-phase-3 .
Center for Drug Evaluation and Research. DDT-COA-000103, ActiMyo®. FDA https://force-dsc.my.site.com/ddt/s/ddt-project?ddtprojectid=19 .
European Medicines Agency. Final Qualification Opinion for Stride Velocity 95th Centile as Primary Endpoint in Studies in Ambulatory Duchenne Muscular Dystrophy Studies https://www.ema.europa.eu/en/documents/scientific-guideline/qualification-opinion-stride-velocity-95th-centile-primary-endpoint-studies-ambulatory-duchenne_en.pdf (2023).
Mikolaizak, A. S. et al. Connecting real-world digital mobility assessment to clinical outcomes for regulatory and clinical endorsement-the Mobilise-D study protocol. PLOS ONE 17 , e0269615 (2022).
Article CAS PubMed PubMed Central Google Scholar
Delgado-Ortiz, L. et al. Listening to the patients’ voice: a conceptual framework of the walking experience. Age Ageing 52 , afac233 (2023).
Keogh, A. et al. Acceptability of wearable devices for measuring mobility remotely: Observations from the Mobilise-D technical validation study. Digit. Health 9 , 20552076221150745 (2023).
PubMed PubMed Central Google Scholar
Moore, K. et al. Older Adults’ Experiences With Using Wearable Devices: Qualitative Systematic Review and Meta-synthesis. JMIR MHealth UHealth 9 , e23832 (2021).
IDEA-FAST. https://idea-fast.eu/ .
IDEA-FAST_D2.1_FS-Approvals_v1.0_IMI.pdf.
Peters, U. et al. Considerations for Embedding Inclusive Research Principles in the Design and Execution of Clinical Trials. Ther. Innov. Regul. Sci. 57 , 186–195 (2023).
Branco, D., Bouça, R., Ferreira, J. & Guerreiro, T. Designing Free-Living Reports for Parkinson’s Disease. in Extended Abstracts of the 2019 CHI Conference on Human Factors in Computing Systems 1–6 (Association for Computing Machinery, New York, NY, USA, 2019). https://doi.org/10.1145/3290607.3313032 .
Riggare, S., Stamford, J. & Hägglund, M. A long way to go: patient perspectives on digital health for Parkinson’s disease. J. Park. Dis. 11 , S5–S10 (2021).
Rainey, J. et al. Data Contribution Summaries for Patient Engagement in Multi-Device Health Monitoring Research. in Adjunct Proceedings of the 2021 ACM International Joint Conference on Pervasive and Ubiquitous Computing and Proceedings of the 2021 ACM International Symposium on Wearable Computers 536–541 (ACM, Virtual USA, 2021). https://doi.org/10.1145/3460418.3479371 .
Shen, F. X. et al. Returning Individual Research Results from Digital Phenotyping in Psychiatry. Am. J. Bioeth. 24 , 69–90 (2024).
Gelinas, L., Morrell, W., White, S. A. & Bierer, B. E. Navigating the ethics of remote research data collection. Clin. Trials 18 , 606–614 (2021).
European Medicines Agency. Questions and answers: Qualification of digital technology-based methodologies to support approval of medicinal products. https://www.ema.europa.eu/en/documents/other/questions-and-answers-qualification-digital-technology-based-methodologies-support-approval-medicinal-products_en.pdf .
Qualification of novel methodologies for medicine development | European Medicines Agency. https://www.ema.europa.eu/en/qualification-novel-methodologies-medicine-development .
European Medicines Agency. Letter of support for Mobilise-D digital mobility outcomes as monitoring biomarkers. EMA https://www.ema.europa.eu/en/documents/other/letter-support-mobilise-d-digital-mobility-outcomes-monitoring-biomarkers-follow_en.pdf .
IDEA-FAST qualification advice request submitted to EMA! – IDEA-FAST. https://idea-fast.eu/idea-fast-qualification-advice-request-submitted-to-ema/ .
Office of the Commissioner. Focus Area: Biomarkers. FDA https://www.fda.gov/science-research/focus-areas-regulatory-science-report/focus-area-biomarkers (2022).
Center for Drug Evaluation and Research. DDT-COA-000120, Scratch Sensor. FDA https://force-dsc.my.site.com/ddt/s/ddt-project?ddtprojectid=56 .
Johri, S. et al. MVPA Reflects Life Participation In Patients Using INOpulse Therapy.
Rochester, L. et al. A roadmap to inform development, validation and approval of digital mobility outcomes: the mobilise-D approach. Digit. Biomark. 4 , 13–27 (2020).
Nobbs, D. et al. Regulatory qualification of a cross-disease digital measure: benefits and challenges from the perspective of IMI Consortium IDEA-FAST. Digit. Biomark. 7 , 132–138 (2023).
Download references
Acknowledgements
The authors thank Robert Wright for helpful input in developing the initial outline of this work. The study received no external funding.
Author information
Authors and affiliations.
VivoSense Inc, Newport Coast, CA, USA
Suvekshya Aryal, Jennifer M. Blankenship, Shelby L. Bachman, Soohyun Hwang, Yaya Zhai, Jennifer C. Richards, Ieuan Clay & Kate Lyden
You can also search for this author in PubMed Google Scholar
Contributions
S.A., J.M.B. and I.C. were involved in conception, design and first draft of this manuscript. S.A., J.M.B., S.L.B., S.H., Y.Z., J.C.R., I.C., and K.L. contributed to subsequent drafts and revisions of the final manuscript.
Corresponding author
Correspondence to Suvekshya Aryal .
Ethics declarations
Competing interests.
SA, JMB, SLB, SH, YZ, JCR, IC, and KL are employees of VivoSense, Inc. IC is on the Editorial Board of Karger Digital Biomarkers and the Scientific Advisory Board for IMI IDEA-FAST and has received fees for lectures and consulting on digital health at ETH Zürich and FHNW Muttenz.
Not relevant due to the nature of this manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Aryal, S., Blankenship, J.M., Bachman, S.L. et al. Patient-centricity in digital measure development: co-evolution of best practice and regulatory guidance. npj Digit. Med. 7 , 128 (2024). https://doi.org/10.1038/s41746-024-01110-y
Download citation
Received : 09 November 2023
Accepted : 15 April 2024
Published : 16 May 2024
DOI : https://doi.org/10.1038/s41746-024-01110-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.9(8); 2023 Aug
- PMC10432995

Clinical competency and associated factors among undergraduate nursing students studying in universities of Southern regional state of Ethiopia, 2021
Tamene fetene terefe.
a Department of Pediatric and Child Health Nursing, Injibara University, Injibara, Ethiopia
Haimanot Abebe Geletie
b Department of Nursing, Wolkite University, Wolkite, Ethiopia
Fisha Alebel GebreEyesus
Tadesse tsehay tarekegn, baye tsegaye amlak.
c Department of Nursing, Debre Markos University, Debre Markos, Ethiopia
Kassa Kindie
d Department of Nursing, Mizan Tepi University, Mizan, Ethiopia
Omega Tolessa Geleta
Agerie aynalem mewahegn, bogale chekole temere, shegaw tesfa mengist, masino tessu beshir.
g Department of Nursing, Arsi University, Arsi, Ethiopia
Alemayehu Wondie
e Department of Biomedical Science, Wolkite University, Wolkite, Ethiopia
Belayneh Mengist
f Department of Public Health, Debremarkos University, Debremarkos, Ethiopia
Associated Data
Data included in article/supp. material/referenced in article.
Clinical practice is the means by which nursing students learn to apply the theory, facilitating integration of theoretical knowledge and practical skill in the clinical setting which becomes arts and science of profession. This correlation of theory and practice, and the building of meaningful experience, take place during clinical practice in the health care service. Even though, nursing students need to have clinical competency during practical setting, there were little available evidences regarding to their competency status in Ethiopia. Therefore, this study was aimed to assess magnitude of clinical competency and its predictors among undergraduate nursing students studying in universities of Southern regional state of Ethiopia in 2021 G C.
Multi-centered institutional based cross-sectional study was conducted among 414 undergraduate nursing students studying in eight universities of Southern regional state of Ethiopia in 2021 academic year. Systematic random sampling technique after proportional allocation to each selected university was used to select the study participants. Data were collected using pretested structured questionnaire by face to face interview after written informed consent was obtained from each participant. Data were cleaned, coded and entered into Epidata version 3.01 and analyzed using statistical package for social science (SPSS) software version 26. Descriptive statistic for all variables and bi-variable and multi-variables logistic regression analysis to identify factors associated with clinical competency was computed and expressed in odds ratio. The result was presented in the form of text, tables and figures and those variables with P -value of <0.05 in multivariable analysis were declared as statistically significant.
From 423 total calculated sample sizes, 414 of them were participated in this study giving a response rate of 97.8%. From those participants, 248 (59.9%) of them has clinical competency [95% CI: (55.18%, 64.62%)]. In multivariable analysis, studying in post basic program [AOR: 5.58], conducive clinical learning environment [AOR: 4.10], good staff-student interaction [AOR: 7.44], satisfaction [AOR: 20.66] and positive attitude towards clinical practice [AOR: 2.49] were factors significantly associated with clinical competency.
In this study, the overall magnitude of clinical competency was found to be unsatisfactory (59.9%). Studying in private program, non-conducive clinical learning environment, poor staff-student interaction, low satisfaction and negative attitude towards clinical practice were identified as factors associated with clinical incompetency. Policy makers, universities and teaching health facilities need to work collaboratively to create nurses with clinical competency by focusing on proper screening to select candidates for studying in private program, creating conducive clinical learning environment, integrating students with clinical staffs to facilitate learning and positive attitude change of students towards their profession to increase level of satisfaction.

1. Background
Nursing is both an art and a science of giving care and helping a patient by providing smooth relationship with him/her to achieve clients' optimal health [ 1 ]. Nursing students are expected to achieve the maximum level of clinical competency during the study time [ 2 ]. Since it is a practice-based profession, learning in the clinical environment is an important component of health sciences education [ 3 ]. Clinical practice helps nursing students to improve their skills and adapt to professional roles [ 4 ]. The outcome of effective learning in a clinical setting is achieving clinical competency, which is the ability to successfully apply professional knowledge, attitudes and skills to new situation [ 5 ]. Competency can also be described as a person's major and essential skills related to job performance [ 6 ]. Clinical competency is the ability to effectively integrate cognitive, affective and psychomotor skills while providing health care [ 7 ]. It is a basic requirement that nurses should have in clinical settings to improve quality of health care services [ 8 ]. Clinical learning experience as an integral aspect of skill development is essential for health science students to acquire clinical competency and abilities to learn independently, make decisions and express ethical commitments about the condition of the client while joining health care institutions [ 9 ].
Researcher evidences have shown that many graduate nurses' have clinical incompetency. The study done among 10 European countries showed that there were differences between the levels of clinical incompetency in each country and ranging from 39.9 to 50 [ 10 ]. Another study conducted in Annals’ University of Finland among graduating nursing students showed the levels of clinical incompetency was 32.3% [ 11 ]. Similarly, the study conducted in Iran showed that 50% of nursing and midwifery students had clinical incompetency [ 12 ]. In Ethiopia, the magnitude of clinical incompetency was high ranged from 51.3 to 74.8% [ 3 , [13] , [14] , [15] , [16] , [17] ].
Currently, the poor quality of skills of health professionals is a great concern of Ethiopian government and public sectors. It is also an important cause of mal-practice and low client satisfaction as reported in many health facilities. Even if nursing faculties are responsible to train nurses who have high level of clinical competency to satisfy the needs of all concerned bodies, shortage of nurses having clinical competency is still the major challenge and serious issue particularly in sub Saharan Africa countries including Ethiopia [ 18 ]. Clinical practice problems of health professionals can negatively affect client care, other staff, team work and the work place in general. In Ethiopia, lack of nurse's clinical competency is one of the major reasons for changing of educational curriculum from three-year program to a four year program [ 19 ]. The following factors were identified in previously conducted researches as inhibiting clinical competency; high levels of stress and anxiety [ 16 ], poor interpersonal relationship [ 20 ], inadequate demonstration room in the university [ 21 ], theory-practice gap [ 22 ], inadequate clinical time [ 3 ], overcrowded clinical facilities [ 21 ], shortage of equipment and staff [ 21 ], and lack of feedback [ 23 ]. Even if efforts has been made to increase clinical competency of health science students, still it is a problem and hindering factors are poorly understood in Ethiopia. Therefore, the main aim of this study was to identify determinant factors of clinical competency among undergraduate nursing students studying in universities of Southern regional state of Ethiopia based on Benner's skill acquisition theory.
1.1. Conceptual framework
This study was based on Benner's Novice to Expert skill acquisition theory. Benner's skill acquisition theory introduced by Dr Patricia Benner in 1982 is generated from the Dreyfus Model of Skill Acquisition and essentially discusses how an individual gains new skills and knowledge from novice stage to expert stage [ 24 ]. Patricia Benner's model stands on how a nurse develop nursing knowledge, skill, clinical competence and comprehension of patient care through complete theoretical training and experiential learning from novice stage to expert stage [ 24 ]. Development through these phases is affected from clinical experience, length of working time in profession [ 24 ].
2.1. Study design, setting and sampling
Multi-centered institutional based cross-sectional study was conducted among under graduate nursing students of south region universities of Ethiopia from July July 15, 2021 to September September 30, 2021. Ethiopia has currently 10 regional administrative states and 2 city administrative states. There were a total of 51 higher educational institutions in Ethiopia and 12 of them were found in south regional state of Ethiopia. From those higher institutions only 8 of them have nursing schools in regular, private (the students in this program are self-sponsored and either generic or post diploma with minimum of 2 years work experience), and post-basic (the students in this program are government sponsored and must be post diploma with minimum of 2 years working experience) programs, namely; Wolkite University, Hawassa University, Wachamo University, Wolayita Sodo University, Arbaminchi University, Dilla University, Metu University and Mizan-Tepi University. There were a total of 1140 under graduate nursing students on 2021 academic year in those universities. Undergraduate nursing students studying at eight universities of southern regional state of Ethiopia in 2021 academic year were included in this study.
This study has two specific objectives; firstly to assess the magnitude of clinical competency and secondly to identify factors associated with clinical competency among undergraduate nursing students studying at universities in the Southern regional state of Ethiopia.
The required sample size for the first objective was calculated by single population proportion formula by using the prevalence of clinical competency as 48.7% based on study conducted at University of Gonder and Bahir Dar University [ 3 ], 95% confidence interval, 5% (0.05) margin of error and adding 10% for possible non response rate which becomes 423. Sample size for the second objective was computed by double population proportion formula using Epi-info 7 software, 95% confidence interval, 5% (0.05) margin of error, 90% power, 1:1 ratio of exposed to non-exposed outcomes and adding 10% for possible non response rate based on the study conducted at University of Gonder and Bahir Dar University; Ethiopia. Comparison was made on the calculated values and the maximum value of 423 was taken as the final sample for this study.
From a total of 12 universities in southern regional state of Ethiopia 8 of them were purposively selected for having nursing schools in regular, private and post-basic programs and systematic random sampling technique after proportional allocation to those university, using students identity number as a sampling frame were used to select study participants every K (K = 2) value. ( Figure1 ).

Schematic presentation of sampling procedure to select the study participants of undergraduate nursing students studying at universities in Sothern region; Ethiopia, 2021.
2.2. Operational definitions
Clinical competency: Those who scored 50% and above of all competency domain assessment questions [ 7 ]. Clinical incompetency: those who scored below 50% of all competency domain assessment questions [ 15 ]. Good staff and student interaction: those who have 2/3 and above of good staff and student interaction characters [ 15 ]. Satisfaction to clinical practice: Students who scored above the mean score of all satisfaction assessment scales [ 25 ]. Favorable attitude towards clinical practice: Those who scored above the mean score of all attitude assessment scales [ 26 ].
2.3. Data collection tool and procedure
Structured questionnaire developed after the review of different literatures was used as a data collection tool [ [14] , [15] , [16] , 26 ]. Data collection instrument was first developed after intensive review of different literatures and then it was pretested and adapted into local context. The content validity of the tool was ensured by expert evaluation, in addition to using a questionnaire validated in another similar studies [ 15 ] and also its reliability was tested by using cronbach's alpha value, which revealed high reliability (0.85). The data were collected using self-administered structured questionnaires. The data collection instrument contains 7 parts: Socio-demographics related questions, which includes; sex, age, religion, ethnicity, residence, substance use and cumulative grade point average (CGPA) of students. Clinical competency was measured using five domains; domain 1, ethical practice (8 items), and domain 2, holistic approaches to care and the integration of knowledge (21 items), domain 3, interpersonal relationships (8 items), domain 4, organization and management of care (3 items) and domain 5, personal and professional development (2 items) and total of 42 items scored on 1 to 5 based on the students perception of their performance. If the student perceives that he/she cannot perform activities satisfactorily to the level required in clinical environment scores 1, if perform activities, but require supervision and assistance scores 2, if can perform activities without assistance scores 3, if can perform activities without assistance and adhering to evidence based practice scores 4 and if can perform activities without assistance and adhering to evidence based practice plus demonstrate initiative to special problem situations, and can lead others scores 5. The rest five parts of the measuring instrument (instructor related (14 items), environmental related (7 items), staff-student interaction (3 items), assessment related (6 items), student's attitude related (14 items), and student's satisfaction (16 items)) related questions were measured using items rated on a five -point Likert scale as 1 = strongly disagree, 2 = disagree, 3 = neutral, 4 = agree and 5 = strongly agree. Eight BSc nurses and two supervisors were employed to collect data by using self-administered questionnaire after three day training by the principal investigator.
2.4. Data quality control
Data were collected by using pretested and structured questionnaire after experts’ evaluation. Pre-test on 5% of the sample size was done at Addis Ababa Universities, Ethiopia and necessary correction was made before the actual data collection was proceeding. Data were entered in to Epi-data software was used for data entry and data were cleaned, coded and checked for completeness and accuracy before analysis. Three day training was given for all data collectors and supervisors for mutual consensus about data collection tool and procedure. The collected data were cross checked daily for completeness and consistency by the principal investigator.
2.5. Data analysis
After data were checked for completeness and consistency, it was entered in to EPI data version 3.1, coded and exported to SPSS version 25 for analyses. Data were analyzed for descriptive summery statistics and presented using text, frequency tables, graphs, percentage, mean and standard deviation.
Binary logistic regression analysis was computed, and all variables with p-value <0.05 in bi-variable logistic regression analysis were included in the multivariable logistic regression analysis. Association between variables was tested and expressed as an adjusted odds ratio (AOR) with 95% CI. Variables with a two-tailed t -test P -value of <0.05 were considered as statistically significant. In addition, variance inflation factor (VIF) and tolerance to check for multicollinarity and Hosmer and Lemeshow goodness of fit test to ensure for general model fitness was used.
3.1. Socio-demographic characteristics
A total of 414 students were participated in this study giving a response rate of 97.8%. Out of 414 respondents almost half of respondents 210 (50.7%) were females and 204 (49.3%) were males. Regarding to age majority of the respondent 316 (76.3%) were ≤24 years. Regarding religions most of the participants 175 (42.3%) were orthodox Christians ( Table 1 ). Less than half of participants 184 (44.4%) have cumulative grade point average (CGPA) ranging from 3.00 to 3.50 ( Fig. 2 ).
Socio demographic characteristics of the study participant among undergraduate nursing students in Universities in Southern Ethiopia, 2021 (n = 414).

Cumulative grade point average (CGPA) of undergraduate nursing students studying at universities in Sothern regional state of Ethiopia, 2021.
3.2. Students and staffs related factors
Nearly half of the participants [202 (48.8%)] had poor interaction with clinical staffs and poor satisfaction with their clinical practice [203 (49%). From those study participants 169 (40.8%) of them had negative attitude towards clinical practice.
3.3. Clinical-instructor related factors
Among a total of 414 study subjects, 226 (54.6%) of them perceive their clinical instructors as poor and 244 (58.9%) of students perceived their clinical instructors way of assessment and their quality as poor.
3.4. Clinical learning environment related factor
Above half of the study participants [225 (54.3%)] perceived their clinical learning environment as non-conducive.
3.5. Magnitude of clinical competency
From those study participants, 248 (59.9%) of them has clinical competency [95% CI: (55.18%, 64.62%)].
3.6. Factors associated with clinical competency
Logistic regression analysis was computed to identify factors associated with clinical competency. In bivariate analysis, substance use, study program, staff-student interaction, clinical learning environment, instructor's quality, assessment quality, student's satisfaction toward the clinical practice and student's attitude were significantly associated with students' clinical competency at p < 0.05. However, in multivariate analysis, study program, staff-student interaction, environmental condition, student's satisfaction toward the clinical practice and student's attitude were significantly associated with clinical competency.
Final regression equation: Logit of clinical competency = 5.58*(study program) + 7.44*(student and staff interaction) + 4.10*(clinical environment) + 20.66*(satisfaction) + 2.49*(attitude) + 3.566.
Those students who studied in post-basic program had 5.6 times more clinical competency as compared to those studying in private program. Students having good interaction with staffs had 7 times more likely to had clinical competency as compared to those having poor interaction. Students with perceived conducive clinical environment were nearly 4 times more clinical competency than the opposite one. Satisfaction with the overall clinical practice increases the odds of students to had clinical competency by 20.7 times and those students having positive attitude towards clinical practice were almost 2.5 times more clinical competency than those students with negative attitude ( Table 2 ).
Multivariate analysis of factors affecting clinical competency of nursing students studying in universities of Southern regional state of Ethiopia, 2021 (n = 414).
4. Discussion
This was a study on clinical competency among undergraduate nursing students studying in universities of Southern regional state of Ethiopia. In this study the overall magnitude of clinical competency was 59.9% [95% CI: (55.18%, 64.62%)] and clinical incompetency was 40.1% [95% CI: (35.38%, 44.82%)]. The magnitude of clinical competency in this study was higher than studies conducted in Iran (50%) [ 12 ] in Dilla University, Ethiopia (39.3%) [ 14 ], Mettu University, Ethiopia (24.5%) [ 16 ], University of Gondar and Bahir Dar University (48.7.%) [ 3 ], universities in Northern Ethiopia (33.6%) [ 15 ]. The possible justification for this variation might be due to the fact that, others include all nursing students attending at least one clinical practice (second year and above), in contrast to the current study which includes only 4th year undergraduate nursing students, who were frequently exposed to clinical practice environment with better clinical experience and competency. In addition to these others were single centered studies, addressing only students studying in the regular program.
The magnitude of clinical competency in this study was lower than studies conducted Annals' University of Finland (67.7%) [ 11 ]. The possible justification for this variation might be due to the fact that Finland's study includes midwifes and also uses different measuring tool. Another possible justification might be due to the socio demographic variation among the participants.
In this study, students studying in post-basic program had 5.6 times more clinical competency as compared to those studying in private program, similar with a studies conducted in Hawassa, Ethiopia [ 21 ]. This might be due to; students studying in private program are usually recruited when they fail to score pass-mark for regular program and most of them have engaged in other areas of work, giving less emphasis for their clinical practice. In contrast to this, students studying in post-basic program were recruited from health care institutions to upgrade their professional level and easily adapt clinical learning environment with ability to achieve the required clinical competency [ 27 ]. Students having good interaction with clinical staffs were 7 times more clinical competency as compared to those having poor interaction with working staffs, which was consistent with studies in University of Gondar and Bahir Dar [ 3 ]. Working staffs have a responsibility to facilitate students learning during clinical practice by providing necessary information, guiding during nursing procedures, supporting in patient cares and integrating students with the clinical environment. To accomplish all those responsibilities students need to have good and professional relationship with clinical staffs that helps them to have clinical competency [ 28 ]. Also in this study, students with perceived conducive clinical environment were nearly 4 times more clinical competency as compared to those with perception of clinical environment as non-conducive for learning, consistent with study in Tanzania [ 4 ]. This is due to the fact that clinical environment with poor facilities, overcrowding, and distance from student's residency, hazardous and inadequate class rooms and libraries possess a great challenge for students during clinical practice and students practicing in those areas may have clinical incompetency than those studying in conducive environment [ 29 ]. In this study, students with good satisfaction with the overall clinical practice were 20.7 times more clinical competency than the counterpart and having positive attitude towards clinical practice increases the odds of clinical competency by 2.5 times as compared to those students with negative attitude. Students usually feel dissatisfied when they perceived that they don't achieve clinical learning objectives and required competency. Dissatisfaction with clinical practice may be related to less supportive environment, poor facilities and negative attitude towards their profession. Lack of motivation, seeking less attention for clinical practice, lack of reward for their best performance, discrimination, incongruence between theory and real practice and discouraging environment may leads to negative attitude for clinical practice and associated clinical incompetency [ 30 ].
5. Conclusion
In this study, the magnitude of clinical competency was found to be unsatisfactory (59.9%). Factors associated clinical competency was studying in post-basic program, perceived conducive clinical learning environment, good staff-student interaction, satisfaction and positive attitude towards clinical practice. These, policy makers, universities, teaching health institutions and instructors should work collaboratively focusing on private program studies, creating enabling clinical teaching environment, smooth relationship with clinical staffs and maintaining positive attitude of nursing students towards their profession.
5.1. Limitation of the study
The study was not supported by qualitative methods (the actual clinical practice may not be observed) and it may introduce desirability bias. It may also vulnerable for all drawbacks of cross sectional study design.
Consent for publication
Not applicable.
Authors received no any financial support for conducting and publication of this article.
Ethical considerations
Ethical clearance and support letter was obtained from Wolkite University ethical review committee with approval number RCS/127/46 and signed informed consent was obtained from each participants. To protect and respect the privacy of participants, we avoid personal identifiers; use coded data, keeping data in confidential place and using the data for research purpose only. COVID-19 preventions measures were applied throughout the data collection process and all methods were performed in accordance with the regulations and guidelines of Wolkite University.
Author contribution statement
Tamene Fetene Terefe and Haimanot Abebe Geletie: Conceived and designed the experiments; Analyzed and interpreted the data.
Fisha Alebel GebreEyesus, Tadesse Tsehay Tarekegn and Baye Tsegaye Amlak: Analyzed and interpreted the data; Wrote the paper.
Kassa Kindie, Omega Tolessa Geleta and Agerie Aynalem Mewahegn: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Bogale Chekole Temere and Shegaw Tesfa Mengist: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Masino Tessu, Alemayehu Wondie and Belayneh Mengist: Analyzed and interpreted the data; Wrote the paper.
Data availability statement
Declaration of competing interest.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
First of all we would like to acknowledge Wolkite University for providing ethical clearance to conduct this research. Our grateful thanks also forwarded to universities in southern regional state of Ethiopia for their permission to conduct this research and cooperation throughout the process. Finally we would like to thank data collectors and all study participants for their consent to provide baseline data.
Appendix A Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e18677 .
Appendix A. Supplementary data
The following is the supplementary data related to this article:
College of Nursing
Easing the pressure: supporting icu nurse decision making through digital innovation.

College of Nursing Assistant Professor Anna Krupp , PhD, MSHP, RN and Associate Professor Karen Dunn Lopez , PhD, MPH, RN, FAAN understand that intensive care unit (ICU) patients have a greater chance of developing functional decline, which may include new limitations in walking or a decreased ability to manage basic physical needs after hospital discharge. One common contributing factor for this is long periods of immobility, or remaining in bed, during ICU hospitalization.
With funding from the Agency for Healthcare Research and Quality , Krupp and Dunn Lopez are proposing to develop a decision support tool in the electronic health record. The goal is to make complex decisions about when it is safe to assist ICU patients out of bed more efficient for nurses. Currently, nurses look in multiple locations in the EHR for this information. The tool will summarize key patient information on one screen.
“ICU nurses make hundreds of decisions during a shift and the decision to assist a patient to sit on the edge of the bed or walk in the room requires that nurses know a lot about the patient and their stability over the previous shift,” said Clinical Assistant Professor and Co-Investigator Heather Dunn , PhD, ACNP-BC, ARNP. “Enhancing mobility in the ICU is crucial for positive patient outcomes. However, assessing readiness for activities like walking is challenging when data needs to be gathered from multiple sections of the medical record.”

The project will be conducted in two phases. First, they’ll develop the decision support tool with input from practicing ICU nurses. Next, the tool will be studied in two environments—a simulated EHR with nurses from across the nation and a real-world trial in the ICU.
Both Krupp and Dunn Lopez bring differing expertise. Dunn Lopez will use her knowledge with usability science and focus her time in a simulated setting identifying the ease, use, and effectiveness of the tool.
“One thing we know is that if something is not easy to use, it isn’t going to get used. But there are methods that can make sure that what you are developing is useful to the people who use it,” Dunn Lopez said.
Krupp will apply her ICU-based clinical expertise with her implementation science training to plan and study how decision support is used in everyday clinical practice.

“The best-designed tool does not guarantee routine use in complex healthcare settings. Implementation science identifies and addresses contextual factors to help promote its use,” said Krupp.
Krupp and Dunn Lopez suspect the results of the study will influence a “pragmatic way of accelerating the use of patient data with guideline recommendations at the point of care to support ICU clinicians in delivering evidence-based care, decreasing the duration of bed rest, and reducing hospital-acquired functional decline.”
Read more from our spring 2024 alumni newsletter .
- Open access
- Published: 17 May 2024
Nursing practice of routine gastric aspiration in preterm infants and its link to necrotizing enterocolitis: is the practice still clinically relevant?
- Osama Mohamed Elsayed Ramadan ORCID: orcid.org/0000-0002-9616-8590 1 ,
- Majed Mowanes Alruwaili ORCID: orcid.org/0000-0001-8491-8190 1 ,
- Abeer Nuwayfi Alruwaili 1 ,
- Nadia Bassuoni Elsharkawy 1 , 2 ,
- Enas Mahrous Abdelaziz 1 , 3 ,
- Mohammed Elsayed Zaky 1 , 4 ,
- Marwa Mamdouh shaban 5 &
- Mostafa Shaban 1 , 6
BMC Nursing volume 23 , Article number: 333 ( 2024 ) Cite this article
Metrics details
The practice of routine gastric residual aspiration in preterm infants remains controversial, with conflicting evidence regarding its impact on necrotizing enterocolitis (NEC). As front-line caregivers, nurses play a vital role in gastric aspiration procedures and must be informed by evidence. This quasi-experimental nursing study aimed to assess whether gastric aspiration is clinically relevant in reducing the risk of NEC in preterm infants.
A total of 250 preterm infants from two NICUs in Egypt were allocated to the gastric aspiration ( n = 125) and non-aspiration ( n = 125) groups. Feeding practices, gastric residuals, and incidence/severity of NEC were compared between groups according to modified Bell’s criteria. Risk factors were analyzed using multivariate regression. There were no significant baseline differences between the groups. The gastric residual attributes and feeding outcomes did not differ substantially from aspiration. The overall incidence of NEC was 14–15%, with no significant differences in the odds of onset or progression of NEC by stage between the groups. Lower gestational age and birth weight emerged as stronger predictors of NEC. Routine gastric aspiration does not appear to directly prevent or reduce the severity of NEC in this population. Although gastric residuals retain clinical importance, study findings question assumptions that aspiration protects against NEC and informs nursing practice. Evidence-based feeding protocols must continually evolve through ongoing research on modifiable risk factors for this devastating intestinal disease in preterm infants.
Peer Review reports
Introduction
Preterm birth, defined as delivery before 37 weeks of gestation, poses a significant challenge in neonatal nursing due to underdeveloped organ systems and increased susceptibility to complications [ 1 , 2 , 3 , 4 ]. The care of preterm infants requires a collaborative, interdisciplinary approach involving neonatal nurses, physicians, and other healthcare professionals to optimize outcomes and mitigate the risk of complications. The immature organ systems of preterm infants leave them vulnerable to a myriad of complications, necessitating specialized care in neonatal intensive care units (NICUs) [ 5 , 6 , 7 , 8 ].
Among the many challenges faced by preterm infants, achieving optimal nutrition is recognized as a critical factor in ensuring their growth, development, and general health [ 9 , 10 , 11 , 12 , 13 , 14 ]. However, providing adequate nutrition is complicated by the prevalence of feeding difficulties in this population [ 15 , 16 ], such as poorly coordinated sucking and swallowing reflexes [ 17 ], as well as the looming risk of serious complications, particularly necrotizing enterocolitis (NEC) [ 5 , 18 , 19 , 20 , 21 , 22 ]. In addition to poorly coordinated sucking and swallowing reflexes, preterm infants often experience feeding intolerance, which can manifest as increased gastric residuals and may prompt the use of gastric residual aspiration to assess feeding readiness and prevent complications [ 23 ].
NEC is a life-threatening gastrointestinal emergency that disproportionately affects preterm infants, with potentially devastating consequences [ 15 , 24 ]. The condition is characterized by inflammation and necrosis of the intestinal tissue, leading to high rates of morbidity and mortality [ 25 , 26 , 27 ]. Despite advances in neonatal care, the precise etiology of NEC remains elusive [ 28 , 29 , 30 , 31 ], although several risk factors have been identified, including prematurity, formula feeding, and aberrant gut microbial colonization [ 23 , 32 ]. The complex multifactorial nature of NEC underscores the importance of early detection and prompt intervention to mitigate its impact on preterm infants [ 33 , 34 ]. In this context, neonatal nurses play a crucial role in preventing and managing NEC through meticulous monitoring, clinical evaluation, and implementing evidence-based feeding protocols [ 35 , 36 , 37 ].
Nurses in NICUs are familiar with the prevalence of NEC and its significant effects on the care and results of premature infants [ 38 , 39 , 40 ]. Routine practices such as measuring the residual volume of the stomach (GRV) for the diagnosis and prevention of complications associated with NEC highlight the crucial and practical role of nursing in neonatal care [ 28 , 29 , 30 , 31 ]. One of the most common practices in NICUs around the world is the routine aspiration of gastric residuals prior to feeding as a means of assessing feeding tolerance, securing feeding tube placement, and preventing potential complications [ 1 , 41 , 42 , 43 , 44 , 45 ]. This procedure involves aspiration of the contents of the stomach through a feeding tube to assess the volume and characteristics of the residuals, which have been traditionally used to guide feeding decisions, despite ongoing debate about their clinical significance and reliability as indicators of digestive function and feeding readiness [ 42 , 46 , 47 ].
A growing body of research has sought to elucidate the relationship between gastric residual aspiration and the development of NEC, producing contradictory and inconclusive results [ 48 , 49 ]. Some studies suggest that routine aspiration of gastric residuals can disrupt the delicate balance of the developing gut microbiome, potentially increasing the risk of NEC [ 15 , 50 , 51 , 52 , 53 , 54 , 55 ]. On the contrary, other investigations have failed to demonstrate a significant association between gastric residual aspiration and the incidence of NEC [ 54 , 56 ]. This lack of consensus within the scientific community highlights the urgent need for more research to clarify the role of gastric residual aspiration in the pathogenesis and prevention of NEC [ 57 , 58 , 59 ].
Beyond clinical practice, this research has major implications for preterm infant nursing education and policy. This study’s contribution to stomach residual aspiration and NEC understanding helps change the curriculum of neonatal nursing programs, ensuring future nurses have the latest and most evidence-based procedures. This research can also influence clinical recommendations and methods in NICUs worldwide to standardize NEC prevention and care. In summary, this study is crucial to understanding the complex link between residual gastric aspiration and NEC in preterm infants. This research fills a gap in the literature to clarify how this frequent practice contributes to a potentially fatal condition. Our objective is to improve evidence-based neonatal care and provide preterm infants with the best treatment to support their growth, development, and well-being through a comprehensive and rigorous approach. Neonatal nurses must endeavor to understand the problems faced by our most fragile preterm infants, and this study is an essential step toward knowledge and excellence in care.
Materials and methods
Research question.
Is there a difference in the incidence and severity of necrotizing enterocolitis (NEC) between preterm infants who undergo routine gastric residual aspiration and those who do not?
Our central hypothesis is that gastric residual aspiration in preterm infants could influence the incidence of NEC. Specifically, we postulate that:
H1. Preterm Infants who undergo routine gastric residual aspiration have a reduced risk of developing NEC compared to those who do not undergo aspiration. H2. Routine assessment of gastric residuals may not provide significant clinical benefits in predicting or preventing complications such as NEC.
A quasi-experimental design was used to achieve the objective of the study. Quasi-experiments aim to estimate the causal effects of an intervention on the target population without randomly assigning subjects to a group [ 60 ].
The first NICU is located at Cairo University Children’s Hospital (El Monira). With a capacity of 40 incubators, it offers complimentary advanced neonatal care to infants throughout Egypt. This unit is segmented into an intermediate care area that houses 15 incubators for secondary treatment and an intensive care section with 25 incubators dedicated to tertiary care. Additional amenities include isolation chambers, breastfeeding support, and outpatient clinics.
On the contrary, the second NICU is located on the fourth floor of the maternity wing of El Manial University Hospital. This unit, equipped with 35 incubators, also provides state-of-the-art neonatal care. It features an intermediate care section with 15 incubators, two intensive care zones (each containing 5 incubators) designed for diverse and infected neonates, immediate postnatal care with 10 incubators, a designated breastfeeding area, and a medication preparation facility.
A priori power analysis was performed to determine the target sample size needed to detect a significant difference in the incidence of NEC between the gastric aspiration and non-aspiration groups. Based on previous studies, the incidence of NEC in preterm infants is approximately 10% [ 61 , 62 ]. We hypothesized that the gastric aspiration intervention could reduce this incidence by 3%, which would be clinically significant given the available sample size. With a power of 80%, an alpha of 0.05, and the recruited sample size of 125 infants per group (250 infants in total), the study is sufficiently powered to detect a 3% reduction in the incidence of NEC between the groups. Power analysis was conducted using G*Power software (version 3.1.9.7) [ 63 ].
The software calculated a total sample size of 236 infants, rounded to 250 to account for potential attrition. Therefore, this study aimed to recruit a convenience sample of 250 preterm infants admitted to the NICU of El Manial University Hospital and Elmonira Pediatric Hospital, with a target of 125 infants assigned to each study group. This sample size provides adequate statistical power to detect a clinically significant difference of 3% in the incidence of NEC between the gastric residual aspiration and non-aspiration groups.
- The allocation procedure was as follows:
As infants were admitted to the NICU, they were screened for eligibility based on the predefined inclusion and exclusion criteria. Eligible infants underwent a 48-hour observation period before enrollment. After obtaining informed parental consent, the enrolled infants were allocated to either the gastric aspiration or non-aspiration group as they were recruited. The allocation was quasi-random based on the order of admission to the NICU, assigning approximately half to each study group in an alternating fashion throughout the recruitment period. For example, the first eligible enrolled infant was allocated to the aspiration group, the second to the non-aspiration group, the third to the aspiration group, and so on until the target sample size was achieved in both groups. This allocation order was not completely random but intended to distribute interventions evenly across the recruitment timeframe. The final group allocation was 125 infants in the gastric residual aspiration group and 125 infants in the non-aspiration group.
Eligibility criteria
Inclusion criteria.
Subjects were considered if they were preterm infants born at less than 37 weeks of gestation receiving tube feeding (orogastric or nasogastric).
While the inclusion criteria encompassed all preterm infants born before 37 weeks, it is important to note that the study population primarily consisted of infants with lower gestational ages, as evidenced by the reported mean gestational age of 28.5 weeks. This is likely due to the fact that infants with lower gestational ages are more likely to require nasogastric feeding and are at a higher risk of developing feeding-related complications, such as necrotizing enterocolitis. It is important to note that the risk of developing NEC is inversely related to gestational age, with infants born at lower gestational ages being at a higher risk compared to those born at later gestational ages [ 64 , 65 , 66 ]. This increased risk is likely due to the greater immaturity of the gastrointestinal tract, immune system, and other organ systems in infants born at earlier gestational ages [ 66 ].
Exclusion criteria
Subjects with intrauterine growth retardation (birth weight below the 10th percentile for gestational age and sex) or preterm infants with respiratory distress (> 80 breaths/min) are excluded. Other factors could include circulatory instability that requires inotropic treatment, highly suspected early-onset sepsis with a change in clinical general condition, worse peripheral perfusion, and circulatory decompensation before the start of the study (within the first 6 h after admission to the neonatal unit); and gastrointestinal tract malformations such as congenital diaphragmatic hernia and other life-limiting congenital severe malformations, since any of these health problems can affect preterm infant feeding.
Data collection tools
Detailed clinical information was extracted from infant medical and nursing records using a standardized data collection form. The information collected included:
- Neonatal characteristics: gestational age, birth weight, sex, maternal complications.
- Medical history: antenatal steroids, multiple births.
- Vital signs: heart rate, respiratory rate, oxygen saturation.
- Feeding details: route of enteral feeding (oral vs. nasal tube), type of milk (breast milk or formula) and time to reach full feeds.
- Gastric residuals: volume, consistency, frequency of measurement.
- Complications: suspected or confirmed necrotizing enterocolitis and staging according to modified Bell’s criteria.
The validity and reliability of medical records as a data source have been well-established in previous studies [ 15 , 24 ]. Data was extracted by trained nurses using an extraction manual to ensure standardized collection. Inter-rater reliability testing between data extractors showed excellent agreement.
Modified Bell’s Staging Criteria [ 67 ] was used to diagnose and classify NEC based on clinical, laboratory, and radiographic findings. This classification system has been extensively validated with high sensitivity (95%) and specificity (98%) for identifying NEC infants [ 67 ]. Stage progression was also documented. All stages were overread by an independent pediatric gastroenterologist to confirm NEC diagnosis and staging. Inter-rater reliability between nurses and the gastroenterologist was substantial (kappa = 0.82) [ 68 ].
A detailed feeding tolerance form was used to record gastric residual volumes, characteristics, and frequency. This form demonstrated excellent validity based on comparisons with actual measured residual volumes ( r = 0.91). Test-retest reliability over multiple feeds was also high ( r = 0.88), confirming consistency.
Using validated data collection tools and standardized extraction procedures, we aimed to ensure high-quality data capture to reliably test our study hypotheses related to gastric residuals, feeding, and NEC outcomes. The validity testing and inter-rater reliability help minimize information bias and standardize clinical data interpretation between extractors.
Ethics approval
Ethical approval was obtained from the Cairo University Faculty of Nursing, before the start of the study (IRB20194041701). Subsequently, written informed consent was obtained from the parents of preterm infants within 48 h and 24 h after the start of feeding. After the parents of eligible premature infants have been informed of the nature of the investigation. They were also assured of the confidentiality of their wards and informed that their participation was voluntary and that they had the right to withdraw at any time.
A systematic recruitment procedure was crucial to successfully conducting our research. The following steps represent the approach we adopted:
Participant recruitment
The study population included preterm infants admitted to the El Manial University Hospital NICU and Elmonira Pediatric Hospital in Egypt between January 2022 and April 2023. Infants were assessed for eligibility within 48 h of NICU admission. The inclusion criteria were gestational age < 37 weeks and enteral feeding (oral or nasal tube). Exclusion criteria included intrauterine growth restriction, respiratory distress > 80 breaths/min, circulatory instability requiring inotropes, suspected early-onset sepsis, and significant congenital anomalies. Eligible infants underwent a 48-hour observation period before enrollment. Parents/caregivers of eligible infants were provided with study information and gave written informed consent for participation.
Group allocation
Infants enrolled were assigned 1:1 to the gastric residual aspiration or non-aspiration groups when admitted to the NICU. Allocation order was quasi-random based on admission date, with the goal of approximately equal group sizes. The final group sizes were 125 infants in the aspiration group and 125 infants in the non-aspiration group.
Feeding interventions
In the aspiration group, gastric residuals were aspirated through an orogastric or nasogastric tube and measured in milliliters before each feeding. The appearance of the aspirate was also noted. Feeding decisions, such as continuing, reducing, or withholding feeds, were based on the volume and characteristics of the aspirated gastric residuals, as per standard NICU protocols.
In the non-aspiration group, gastric residuals were not actively aspirated using a syringe. Instead, the orogastric or nasogastric tube was left open, allowing passive drainage of the gastric contents into a collection bag. This passive drainage was allowed to occur for a short period, typically a few minutes, before each feeding to prevent any build-up of gastric contents that could impede feeding. The volume and appearance of passively drained gastric residuals were not routinely measured or used to guide feeding decisions in this group. Instead, feeding continuation or modification was based on the clinical presentation of the infants and signs of feeding intolerance, such as abdominal distension or emesis.
Nurses in the non-aspiration group were provided with clear guidelines and training to ensure adherence to the study protocol. Regular audits and staff meetings were conducted to reinforce the importance of following the non-aspiration protocol and to address any concerns or questions raised by the nursing staff. In the event of any clinical concerns, such as signs of feeding intolerance or suspected NEC, nurses were advised to consult with the medical team and follow standard NICU protocols for managing these situations. If, in the clinical judgment of the medical team, aspiration of gastric residuals was deemed necessary for diagnostic or therapeutic purposes, this was documented as a protocol deviation, and the reasons for the deviation were recorded.
Data collection
Demographic data, medical history, feeding details, and clinical outcomes were extracted from medical records using a standardized data collection form. Gastric residual characteristics were recorded at each feeding. The diagnosis and staging of NEC were performed using Bell criteria, supplemented by laboratory tests, imaging, and clinical judgment. Data collection spanned only the initial hospitalization in the NICU. Long-term follow-up was not performed. All data were deidentified before analysis. The statisticians performing the data analysis were blinded to group allocation.
Statistical analysis
All statistical analyses were performed with SPSS© version 26 (IBM Corp©, Armonk, NY, 2023). The level of significance was established at 0.05 for all analyses. Descriptive statistics, including frequencies, percentages, means, and standard deviations, were used to summarize the characteristics of the study population. Differences in baseline characteristics between the gastric residual aspiration and non-aspiration groups were compared using independent samples t-tests for continuous variables and the chi-square test for categorical variables. Differences in primary and secondary outcomes, such as incidence of NEC, feeding intolerance, time to full feed, etc., between the two groups were compared using independent samples t-tests for continuous variables and chi-square or Fisher’s exact test for categorical variables. Bivariate correlations between neonatal risk factors and NEC outcomes were examined using Pearson’s correlation coefficient. A multivariate binary logistic regression analysis was performed to determine risk factors independently associated with NEC. Variables with p < 0.25 in bivariate analyses were included in the multivariate model. Odd ratios and 95% confidence intervals were calculated. Model diagnostics were performed to check for multicollinearity and outliers. The general fit of the model was assessed using the Hosmer-Lemeshow goodness-of-fit test.
Figure 1 presents the study flow chart, which summarizes the participant recruitment, allocation, follow-up, and analysis process. A total of 300 preterm infants were admitted to the NICUs during the study period. Of these, 20 infants were not assessed for eligibility due to not meeting the basic criteria for consideration ( n = 12), being transferred or discharged before the eligibility assessment ( n = 6), and parents declining participation before the eligibility assessment ( n = 2). Of the 280 preterm infants assessed for eligibility, 30 were excluded. Among the excluded infants, 15 did not meet the inclusion criteria, and 15 met the exclusion criteria. The reasons for exclusion were intrauterine growth retardation ( n = 5), respiratory distress ( n = 4), circulatory instability ( n = 3), suspected early-onset sepsis ( n = 2), gastrointestinal tract malformations ( n = 1), and other severe congenital malformations ( n = 0). A total of 250 preterm infants were found eligible for the study and were allocated to either the gastric residual aspiration group ( n = 125) or the non-aspiration group ( n = 125). No infants were lost to follow-up in either group, resulting in a final sample size of 125 infants analyzed in each group.
Study flow chart
Table 1 shows that the two study groups were well-matched at enrollment, with no significant differences in key baseline characteristics. The mean gestational age (28.5 ± 2.3 vs. 28.7 ± 2.1 weeks) and birth weight (1100 ± 150 vs. 1125 ± 140 g) were similar between the gastric residual aspiration and non-aspiration groups. The distribution of male sex (60% vs. 58%), maternal complications (30% vs. 28%), antenatal steroid use (55% vs. 53%), multiple births (25% vs. 24%), and location of the NICU (El Monira: 50% vs. 49%; El Manial: 50% vs. 51%) were also comparable between the groups. The incidence of common morbidities, including respiratory distress syndrome, patent ductus arteriosus, intraventricular haemorrhage, late-onset sepsis, and retinopathy of prematurity, was comparable between the gastric residual aspiration and non-aspiration groups. The similarity in baseline characteristics suggests that any differences in outcomes observed between the groups are less likely to be attributed to pre-existing differences in the study population, strengthening the internal validity of the study.
Table 2 offers a focused view of feeding practices and results between the gastric residual aspiration and non-aspiration groups. Initial observations reveal that both groups follow comparable practices: a slight preference for oral feeding over nasal feeding and a moderate lean toward formula milk over breast milk. The average duration for the infants to reach full feed is almost similar between the groups, with only a minor difference of 0.2 days. Furthermore, the incidence of feeding problems is closely correlated between the two cohorts. Overall, this table underscores the consistency in feeding practices and outcomes across the two groups, further strengthening the study’s internal validity by ensuring that any outcomes can be attributed more confidently to the intervention (aspiration vs. non-aspiration) rather than differences in feeding practices.
Table 3 compares the characteristics of gastric residuals between infants who underwent active gastric residual aspiration and those who did not. In the non-aspiration group, gastric residuals were not actively aspirated but were allowed to drain passively into a collection bag. The data presented for the non-aspiration group represent the volume and characteristics of these passively drained residuals. The mean volume of gastric residuals was slightly higher in the aspiration group (5 ± 5 ml) compared to the non-aspiration group (4 ± 4.5 ml), but the difference was not statistically significant ( p = 0.12). Similarly, the percentage of infants with residuals stained with bile (40% vs. 38%) and the mean frequency of residuals (2.5 ± 1 vs. 2.4 ± 0.9) were comparable between the groups, without statistically significant differences ( p = 0.15 and p = 0.10, respectively). These findings suggest that the practice of gastric residual aspiration did not significantly alter the volume, appearance, or frequency of gastric residuals in preterm infants.
Table 4 presents the clinical outcomes and complications in preterm infants according to whether they underwent gastric residual aspiration or not. The duration of stay in the NICU was similar between the two groups, with a mean duration of 28 ± 5 days in the aspiration group and 27 ± 4.5 days in the non-aspiration group. The rates of hospital readmissions within 30 days (8% vs. 8.8%), mortality (4% vs. 4.8%), and other complications such as sepsis and respiratory issues (16% vs. 16.8%) were also comparable between the groups. These findings suggest that the practice of gastric residual aspiration did not significantly influence clinical outcomes or the incidence of complications in preterm infants.
Table 5 presents a multivariate regression analysis identifying potential risk factors for NEC in preterm infants. Gestational age and birth weight emerge as significant determinants, with earlier gestation and lower weight presenting an increased risk. Although not definitively significant, the practice of gastric residual aspiration suggests a possible association that deserves closer inspection. In contrast, other variables, such as maternal complications, feeding type, and location of the NICU, do not show a strong association with the development of NEC. The table underscores the intricate etiology of NEC and emphasizes the need to consider multiple factors that influence it holistically.
Table 6 compares the incidence and severity of necrotizing enterocolitis (NEC) between infants who underwent residual gastric aspiration and those who did not. Most of the infants in both groups did not develop NEC (85.6% in the aspiration group vs. 84.8% in the non-aspiration group). The incidence of suspicious NEC (Stage 1), definite NEC (stage 2) and advanced NEC (Stage 3) was similar between the groups, with no statistically significant differences, as evident from odds ratios close to 1 and p-values > 0.05. The overall risk of developing NEC was also comparable between the groups (14.4% vs. 15.2%, OR = 0.94, 95% CI: 0.48–1.84, p = 0.8). These findings suggest that the practice of gastric residual aspiration did not significantly influence the incidence or severity of NEC in preterm infants.
Table 7 explores the association between feeding practices and the development of necrotizing enterocolitis (NEC) in preterm infants who underwent residual gastric aspiration compared to those who did not. The percentage of infants with oral initial feeding (60% vs. 58%), time to full feed > 5 days (35% vs. 33%), use of formula milk use (60% vs. 58%), and feeding problems (30% vs. 28%) were similar between the two groups. The odds ratios for these feeding parameters were close to 1, and the p-values were > 0.05, indicating that there were no statistically significant differences in the association between feeding practices and the development of NEC based on the practice of gastric residual aspiration. These findings suggest that the feeding practices examined in this study did not significantly influence the relationship between gastric residual aspiration and NEC in preterm infants.
This quasi-experimental study offered valuable information on the relationship between routine gastric residual aspiration and necrotizing enterocolitis (NEC) development in preterm infants. The findings suggest that aspiration of gastric residuals before feeding may not directly alter the risk or severity of NEC.
Feeding practices and residual characteristics
A thorough analysis of the core of the study revealed fascinating insights into food practices and residual gastric properties. The mere act of gastric residual aspiration does not appear to profoundly affect feeding behavior, residual volume, or consistency. Our findings are in contrast to those of [ 69 ], who found that routine gastric residual aspiration was associated with a delay in the time to reach full enteral feeding in preterm infants. In their randomized controlled trial, infants in the aspiration group took longer to achieve full feeds compared to those in the no-aspiration group (median 11 days vs. 9 days, p = 0.01). The authors suggested that the practice of routine gastric residual aspiration may disrupt the normal gastrointestinal tract development and lead to feeding intolerance.
Residual gastric aspiration could affect the progression of feeding in preterm infants. On the other hand, our findings are consistent with those of [ 50 ], who found no significant differences in feeding practices between infants undergoing gastric residual aspiration and those who did not. Although it is tempting to view gastric residuals as mere indicators of food intolerance, one might wonder if their role extends beyond this. They may be early signs or precursors to more severe conditions such as NEC.
No difference in NEC incidence with aspiration
Our central finding was that the incidence of NEC did not differ significantly between the gastric aspiration and non-aspiration groups. Approximately 15% of the infants developed some stage of NEC in both arms. Previous studies have been inconsistent, with some noting a higher NEC with aspiration [ 61 , 65 ] while others did not find any difference [ 50 , 51 , 52 , 53 ]. Although the multivariate analysis (Table 5 ) suggested a possible association between gastric residual aspiration and NEC, with an odds ratio of 1.20 (95% CI: 0.98–1.47), the relationship did not reach statistical significance at the conventional 0.05 level ( p = 0.08). It is important to note that the confidence interval for the odds ratio was wide and included 1, indicating that the true effect size could be smaller or larger than the point estimate. Furthermore, the interpretation of our results should be made with caution, given the limitations of our study design and sample size. Larger, well-controlled studies are needed to more definitively establish the relationship between gastric residual aspiration and NEC in preterm infants. While our findings suggest a possible association, they do not provide conclusive evidence that routine aspiration intrinsically increases the risk of NEC.
However, the etiology of NEC is complex. Although aspiration did not emerge as an independent risk factor in our regression analysis, elements such as lower gestational age and birth weight did. This agrees with [ 70 ], confirming the multifactorial nature of NEC. Our findings add to the evidence that routine gastric aspiration does not appear to directly precipitate the onset of NEC in preterm infants, but many factors are at play.
No difference in NEC severity with aspiration
Furthermore, we analyzed the outcomes of NEC by stage severity. The percentages of infants with mild, moderate, or advanced NEC were comparable. Previous studies have not examined the severity in detail [ 51 , 69 ]. This suggests that routine aspiration practice may not worsen progression or exacerbate NEC once present. However, larger samples are needed to confirm this due to the low frequency of advanced NEC.
The lack of difference in NEC severity between the aspiration and non-aspiration groups suggests that routine gastric residual aspiration may not be an effective strategy for mitigating the severity of NEC once it develops. This finding may have implications for the management of preterm infants with suspected or confirmed NEC.
No major impact of clinical outcomes on aspiration
Longitudinal stay, readmission, mortality, and other complications were similar regardless of gastric aspiration. Some researchers have proposed that aspiration can delay feeding progress and prolong hospitalization [ 71 ]. However, we found minimal differences in clinical outcomes. However, no long-term follow-up was conducted; potential outcomes remain unknown.
The mean length of stay in the NICU of approximately 27–28 days for infants with a mean gestational age of 28.5 weeks in our study is shorter than what has been reported in some other studies. This difference may be attributed to variations in clinical practices, discharge criteria, and the availability of local resources and support services. It is important to note that the length of stay reported in our study represents only the initial hospital stay and does not include any subsequent home care or readmissions. Future studies should consider exploring factors that influence the duration of hospital stay for preterm infants in different settings and the potential impact of these variations on long-term outcomes. While shorter hospital stays may be beneficial for infants and their families, it is crucial to ensure that early discharge does not compromise the provision of appropriate care and support for preterm infants at risk of developing NEC or other complications.
No difference in residual volume/frequency with aspiration
Interestingly, residual volume and frequency did not differ significantly between the aspiration and non-aspiration groups. Some have hypothesized that aspiration could alter gastric motility and residuals [ 72 ], but we did not find significant effects. A minimal comparison of residuals between groups has been studied. While aspiration did not appear to impact residual characteristics, the clinical utility of measuring volumes warrants further scrutiny.
Limitations, future directions, and contrasting perspectives
Our study has several limitations that should be considered when interpreting the results. The quasi-experimental design, which lacks randomization, may have introduced selection bias and limited the generalizability of our findings. Furthermore, our study may have been subject to other biases, such as patient variability and nurses’ expertise in performing routine gastric aspiration. To address these limitations, future studies should employ randomized patient selection, standardized protocols for gastric residual aspiration, and stratification of patients based on key characteristics.
Although our study sheds light on the potential non-significance of gastric residual aspiration in the context of NEC, it is paramount to approach this insight with caution. The multifaceted nature of NEC underscores the likelihood that a confluence of factors, rather than a singular determinant, dictates its onset and progression. Moreover, while our findings are comprehensive, they do not provide a conclusive argument for the clinical relevance of gastric residual aspiration. Some might argue that, while aspiration may not directly influence NEC, its indirect implications, such as affecting the gut microbiota, cannot be overlooked. Future studies should also explore the potential indirect effects of gastric residual aspiration on the development of NEC, such as its impact on the gut microbiome and immune function, as these factors may play a crucial role in the pathogenesis of NEC.
The importance of evidence-based feeding protocols in reducing the incidence of NEC in preterm infants has been highlighted in a recent systematic review by Jasani and Patole [ 73 ]. The authors found that standardized feeding regimens, which include strategies such as slow advancement of enteral feeds and the use of human milk, can significantly reduce the risk of NEC in this vulnerable population. Our findings and the evidence presented in this systematic review underscore the need for neonatal healthcare providers to adopt and adhere to evidence-based feeding protocols to improve outcomes for preterm infants.
In conclusion, while our study suggests that routine gastric aspiration may not alter NEC or clinical outcomes in preterm infants, we caution against generalized conclusions given the multifaceted nature of NEC etiology. More data are needed to shape evidence-based feeding guidelines and improve neonatal care.
Conclusions
This quasi-experimental study provides valuable information on the relationship between gastric residual aspiration and necrotizing enterocolitis (NEC) among preterm infants. Our findings suggest that aspirating gastric residuals before feeding may not substantially alter the risk or severity of developing NEC. Several crucial observations support this conclusion. First, the gastric residual aspiration and non-aspiration groups in this study exhibited remarkably similar baseline characteristics. This homogeneity in gestational age, birth weight, feeding practices, and other variables lends credibility by minimizing potential confounders. Furthermore, the mere act of aspirating gastric residuals did not appear to profoundly affect residual volume, consistency, feeding progression, or other results. More importantly, the overall incidence and severity of the staging of NEC were comparable between the two groups.
Our regression analysis revealed that lower gestational age and birth weight played a more pronounced role in NEC development than gastric aspiration. This is consistent with other recent research demonstrating the complex and multifactorial nature of the etiology of NEC. While our study provides evidence to downplay the importance of routine gastric aspiration, it is only one piece of the complex puzzle behind the pathogenesis of NEC. Based solely on these findings, we cannot conclusively determine that gastric residuals lack clinical utility. However, this research highlights the need to holistically examine the multiple risk factors contributing to NEC using robust multicenter studies. In summary, although gastric residuals remain important clinical markers, our findings call into question the premise that routine aspiration directly reduces the risk of NEC in preterm infants. This has implications for the development of evidence-based nutrition guidelines. More research is needed incorporating advances in neonatal care to deepen our understanding of the etiology and prevention of NEC.
Data availability
Data will be available upon the corresponding author’s request.
Abbreviations
Necrotizing Enterocolitis
Neonatal Intensive Care Unit
Institutional Review Board
Gastric Residual Volume
Standard Deviation
Confidence Interva
Statistical Package for the Social Sciences
Ramadan O, Rashad H, Darwish M, Dabash S. Effect of non-aspirated gastric residual content on preterm infants’ health status. Int J Health Sci (Qassim). 2022;6:6875–87.
Google Scholar
Hilbrands J, Feuling MB, Szabo A, Teng BQ, Fabus N, Froh M et al. Nutrition Screening in the Pediatric Intensive Care Unit: evaluation of an Electronic Medical Record-based Tool. Nutrients. 2023;15.
Jenkins B, Calder PC, Marino LV. Gastric residual volume monitoring practices in UK intensive care units: a web-based survey. J Intensive Care Soc. 2023. https://doi.org/10.1177/17511437231210483/ASSET/IMAGES/LARGE/10.1177_17511437231210483-FIG3.JPEG .
Article PubMed PubMed Central Google Scholar
Metheny NA, Mills AC, Stewart BJ. Monitoring for intolerance to gastric tube feedings: a national survey. Am J Crit Care. 2012;21.
Access O, De Luca D, Raymakers P, Van Dijk M, Tibboel D. Intensive Care Medicine – Paediatric and Neonatal (ICM-PN): creating a journal to fill a gap and strengthen our domain. Intensive Care Medicine – Paediatric and Neonatal 2023 1:1. 2023;1:1–2.
Sundquist Beauman S, Eklund WM, Short MA, Kenner C. Nurses’ knowledge, communication needs, and future directions in neonatal research: results of an International Survey. Adv Neonatal Care. 2023;23:338.
Abukari AS, Schmollgruber S. Concepts of family-centered care at the neonatal and paediatric intensive care unit: a scoping review. J Pediatr Nursing: Nurs Care Child Families. 2023;71:e1–10.
Article Google Scholar
Campbell R, Goodman-Williams R, Feeney H, Fehler-Cabral G. Assessing Triangulation Across methodologies, methods, and Stakeholder groups: the joys, woes, and politics of interpreting Convergent and Divergent Data. Am J Evaluation. 2020;41:125–44.
Davis BE, Leppert MOc, German K, Lehmann CU, Adams-Chapman I. Primary care Framework to monitor Preterm infants for neurodevelopmental outcomes in early childhood. Pediatrics. 2023;152.
Ahmed B, Abushama M, Konje JC. Prevention of spontaneous preterm delivery – an update on where we are today. J Maternal-Fetal Neonatal Med. 2023;36.
Morniroli D, Tiraferri V, Maiocco G, De Rose DU, Cresi F, Coscia A et al. Beyond survival: the lasting effects of premature birth. Front Pediatr. 2023;11.
Lawn JE, Ohuma EO, Bradley E, Okwaraji YB, Yargawa J, Blencowe H, et al. Small babies, big risks: global estimates of prevalence and mortality for vulnerable newborns to accelerate change and improve counting. Lancet. 2023;401:1707–19.
Article PubMed Google Scholar
Hunter PJ, Awoyemi T, Ayede AI, Chico RM, David AL, Dewey KG, et al. Biological and pathological mechanisms leading to the birth of a small vulnerable newborn. Lancet. 2023;401:1720–32.
Ashorn P, Ashorn U, Muthiani Y, Aboubaker S, Askari S, Bahl R, et al. Small vulnerable newborns—big potential for impact. Lancet. 2023;401:1692–706.
Kumar J, Meena J, Mittal P, Shankar J, Kumar P, Shenoi A. Routine prefeed gastric aspiration in preterm infants: a systematic review and meta-analysis. Eur J Pediatr. 2021;180:2367–77.
Abiramalatha T, Thanigainathan S, Ninan B. Routine monitoring of gastric residual for prevention of necrotising enterocolitis in preterm infants. Cochrane Database Syst Rev. 2019;7:CD012937.
PubMed Google Scholar
Djamnezhad D, Koltcheva N, Dizdarevic A, Mujezinovic A, Peixoto C, Coelho V, et al. Social and Emotional Learning in Preschool settings: a systematic map of systematic reviews. Front Educ (Lausanne). 2021;6:691670.
Welborn AC, Nichols T, Gringle M, Lewallen L. Neonatal intensive care nurses’ accounts of care for mothers/families with substance-exposed pregnancies: a critical discourse analysis. J Adv Nurs. 2024;80:566–79.
Alsadaan N, Ramadan OME, Alqahtani M, Shaban M, Elsharkawy NB, Abdelaziz EM et al. Impacts of Integrating Family-Centered Care and Developmental Care Principles on Neonatal Neurodevelopmental Outcomes among High-Risk Neonates. Children. 2023, Vol 10, Page 1751. 2023;10:1751.
McDonald R, Moloney W. Improving the implementation of family-centered care within the neonatal care unit: empowering parents to participate in Infant Care. J Perinat Neonatal Nurs. 2023;37:242–51.
Sibdow A, Phd A, Schmollgruber S. Perceived barriers of family-centred care in neonatal intensive care units: a qualitative study. Nurs Crit Care. 2024. https://doi.org/10.1111/NICC.13031 .
Vetcho S, Ullman AJ, Petsky H, Wiroonpanich W, Cooke M. Parent and interdisciplinary professional perceptions of family-centered care in Thai NICU: a qualitative study. Nurs Crit Care. 2023;28:47–55.
Kamity R, Kapavarapu PK, Chandel A. Feeding problems and long-term outcomes in Preterm Infants—A systematic Approach to evaluation and management. Children. 2021;8.
Akar S, Turgut M. Do we control gastric residuals unnecessarily in premature newborns? AGRA study: avoidance of gastric residual aspiration. World J Pediatr Surg. 2020;3:e000056.
Perin J, Mulick A, Yeung D, Villavicencio F, Lopez G, Strong KL, et al. Global, regional, and national causes of under-5 mortality in 2000–19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc Health. 2022;6:106–15.
Ohuma EO, Moller A-B, Bradley E, Chakwera S, Hussain-Alkhateeb L, Lewin A, et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet. 2023;402:1261–71.
Miller L, Schmidt CN, Wanduru P, Wanyoro A, Santos N, Butrick E et al. Adapting the preterm birth phenotyping framework to a low-resource, rural setting and applying it to births from Migori County in western Kenya. BMC Pregnancy and Childbirth 2023 23:1. 2023;23:1–12.
Hal NJ, Pierro A. Necrotizing Enterocolitis. Operative Pediatric Surgery: Seventh edition. 2022;:487–97.
Alsaied A, Islam N, Thalib L. Global incidence of necrotizing enterocolitis: a systematic review and Meta-analysis. BMC Pediatr. 2020;20:1–15.
Klerk DH, van Avezaath LK, Loeffen EAH, Hulscher JBF, Kooi EMW. Fetal–neonatal exposure to antibiotics and NEC development: a systematic review and meta-analysis. Front Pediatr. 2023;10.
Lopes RB, Moreira MEL, Hermeto F. Necrotizing Enterocolitis. Perinatology: Evidence-Based Best Practices in Perinatal Medicine. 2023;:1215–28.
Li L, Liu L, Chen F, Huang L. Clinical effects of oral motor intervention combined with non-nutritive sucking on oral feeding in preterm infants with dysphagia. J Pediatr (Rio J). 2022;98:635–40.
Dorling J, Tume L, Arch B, Woolfall K, Latten L, Roper L, et al. Original research: gastric residual volume measurement in British neonatal intensive care units: a survey of practice. BMJ Paediatr Open. 2020;4:601.
Abiramalatha T, Thanigainathan S, Ramaswamy VV, Rajaiah B, Ramakrishnan S. Routine monitoring of gastric residual for prevention of necrotising enterocolitis in preterm infants. Cochrane Database Syst Rev. 2023;6:CD012937–012937.
Skinner AM, Narchi H. Preterm nutrition and neurodevelopmental outcomes. World J Methodol. 2021;11:278.
Embleton ND, Jennifer Moltu S, Lapillonne A, Van Den Akker CHP, Carnielli V, Fusch C, et al. Enteral Nutrition in Preterm infants (2022): a position paper from the ESPGHAN Committee on Nutrition and Invited experts. J Pediatr Gastroenterol Nutr. 2023;76:248–68.
Picaud JC. Reply to commenting letter for postnatal growth and body composition in extremely low birth weight infants fed with individually adjusted fortified human milk: a cohort study by Perrin et al. Eur J Pediatr. 2023;182:3801–2.
Shulhan J, Dicken B, Hartling L, Larsen BMK. Current knowledge of necrotizing enterocolitis in Preterm infants and the impact of different types of Enteral Nutrition products. Adv Nutr. 2017;8:80.
Singh DK, Miller CM, Orgel KA, Dave M, Mackay S, Good M. Necrotizing enterocolitis: bench to bedside approaches and advancing our understanding of disease pathogenesis. Front Pediatr. 2023;10.
Cuna A, Morowitz MJ, Sampath V. Early antibiotics and risk for necrotizing enterocolitis in premature infants: a narrative review. Front Pediatr. 2023;11:1112812.
Lagerquist E, al-Haddad BJS, Irvine J, Muskthel L, Rios A, Upadhyay K. Feeding volume advancement in preterm neonates: a level 4 neonatal intensive care unit quality improvement initiative. Nutr Clin Pract. 2023;38:1175–80.
Bautista GM, Cera AJ, Chaaban H, McElroy SJ. State-of-the-art review and update of in vivo models of necrotizing enterocolitis. Front Pediatr. 2023;11.
Wang N, Yu Z, Editorial. Topic opportunities, barriers and pitfall of current nutritional practice in preterm infants. Front Pediatr. 2023;11:1172361.
Assad M, Jerome M, Olyaei A, Nizich S, Hedges M, Gosselin K, et al. Dilemmas in establishing preterm enteral feeding: where do we start and how fast do we go? J Perinatol 2023. 2023;43:9.
Kappel SS, Maastrup R, Sangild PT, Jakobsen KT, Christensen VB, Aunsholt L. Nurses’ and Physicians’ Rationale behind Clinical Performance and Interpretation of Routine Prefeed gastric aspiration in Preterm infants: a cross-sectional study. J Perinat Neonatal Nurs. 2023;37:77–83.
Bethell GS, Hall NJ. Recent advances in our understanding of NEC diagnosis, prognosis and surgical approach. Front Pediatr. 2023;11.
Liu K, Guo J, Yang J, Su Y. The Association of Human Milk Proportion with the Clinical Outcomes of Necrotizing Enterocolitis in Preterm Infants: A Retrospective Study. Nutrients 2023, Vol 15, Page 3796. 2023;15:3796.
Duess JW, Sampah ME, Lopez CM, Tsuboi K, Scheese DJ, Sodhi CP et al. Necrotizing enterocolitis, gut microbes, and sepsis. Gut Microbes. 2023;15.
Ganji N, Li B, Lee C, Pierro A. Necrotizing enterocolitis: recent advances in treatment with translational potential. Pediatr Surg Int. 2023;39:1–15.
Elia S, Ciarcià M, Miselli F, Bertini G, Dani C. Effect of selective gastric residual monitoring on enteral intake in preterm infants. Ital J Pediatr. 2022;48:1–6.
Riskin A, Cohen K, Kugelman A, Toropine A, Said W, Bader D. The impact of routine evaluation of gastricr residual volumes on the time to achieve full enteral feeding in preterm infants. J Pediatr. 2017;189:128–34.
Purohit G, Mehkarkar P, Athalye-Jape G, Nathan E, Patole S. Association of gastric residual volumes with necrotising enterocolitis in extremely preterm infants—a case–control study. Eur J Pediatr. 2022;181:253.
Abiramalatha T, Thanigainathan S, Ramaswamy VV, Rajaiah B, Ramakrishnan S. Re-feeding versus discarding gastric residuals to improve growth in preterm infants. Cochrane Database Syst Rev. 2023;6:CD012940.
Parker LA, Weaver M, Murgas Torrazza RJ, Shuster J, Li N, Krueger C, et al. Effect of aspiration and evaluation of gastric residuals on intestinal inflammation, bleeding, and gastrointestinal peptide level. J Pediatr. 2020;217:165–e1712.
Article CAS PubMed Google Scholar
Parker LA, Weaver M, Murgas Torrazza RJ, Shuster J, Li N, Krueger C, et al. Effect of gastric residual evaluation on enteral intake in extremely preterm infants: a randomized clinical trial. JAMA Pediatr. 2019;173:534–43.
Dorling J, Tume L, Arch B, Woolfall K, Latten L, Roper L, et al. Gastric residual volume measurement in British neonatal intensive care units: a survey of practice. BMJ Paediatr Open. 2020;4:e000601.
Austin T. The development of neonatal neurointensive care. Pediatr Res. 2019. https://doi.org/10.1038/s41390-019-0729-5 .
Batey N, Henry C, Garg S, Wagner M, Malhotra A, Valstar M et al. The newborn delivery room of tomorrow: emerging and future technologies. Pediatric Research. 2022. 2022;:1–9.
Wagner M, Gröpel P, Eibensteiner F, Kessler L, Bibl K, Gross IT, et al. Visual attention during pediatric resuscitation with feedback devices: a randomized simulation study. Pediatr Res. 2022;91:1762–8.
Maciejewski ML. Quasi-experimental design. Biostat Epidemiol. 2020;4:38–47.
Savarino G, Carta M, Cimador M, Corsello A, Giuffrè M, Schierz IAM, et al. Necrotizing enterocolitis in the preterm: newborns medical and nutritional management in a single-center study. Ital J Pediatr. 2021;47:1–9.
Williams S, Bostain R, Couch N, Kamdar T, Oh W, Thompson L et al. Routine versus no assessment of gastric residual volumes in preterm infants receiving enteral feeding via intermittent feeding tubes: a randomized controlled trial. J Maternal-Fetal Neonatal Med. 2023;36.
Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91.
Lamireau N, Greiner E, Hascoët JM. Risk factors associated with necrotizing enterocolitis in preterm infants: a case-control study. Arch Pediatr. 2023;30:477–82.
Campos-Martinez AM, Expósito-Herrera J, Gonzalez-Bolívar M, Fernández-Marin E, Uberos J. Evaluation of risk and preventive factors for necrotizing enterocolitis in premature newborns. A systematic review of the literature. Front Pediatr. 2022;10:874976.
Xie YL, Lai SH, Liu SJ, Xiu WL. Risk factors of necrotizing enterocolitis in twin preterm infants. BMC Pediatr. 2024;24:1–5.
Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33:179–201.
Article CAS PubMed PubMed Central Google Scholar
Caplan MS, Jilling T. New concepts in necrotizing enterocolitis. Curr Opin Pediatr. 2001;13:111–5.
Abiramalatha T, Thanigainathan S, Ramaswamy VV, Rajaiah B, Ramakrishnan S. Routine monitoring of gastric residual for prevention of necrotising enterocolitis in preterm infants. Cochrane Database of Systematic Reviews. 2023;2023.
Wang ZL, An Y, He Y, Hu XY, Guo L, Li QY et al. Risk factors of necrotizing enterocolitis in neonates with sepsis: A retrospective case-control study. https://doi.org/101177/2058738420963818. 2020;34.
Parker LA, Weaver M, Murgas Torrazza RJ, Shuster J, Li N, Krueger C, et al. Effect of gastric residual evaluation on Enteral Intake in extremely Preterm infants: a Randomized Clinical Trial. JAMA Pediatr. 2019;173:534.
Chandran S, Anand AJ, Rajadurai VS, Seyed ES, Khoo PC, Chua MC. Evidence-based practices reduce necrotizing enterocolitis and improve Nutrition outcomes in very low-birth-weight infants. JPEN J Parenter Enter Nutr. 2021;45:1408–16.
Jasani B, Patole S. Standardized feeding regimen for reducing necrotizing enterocolitis in preterm infants: an updated systematic review. J Perinatol. 2017;37:827–33.
Download references
Acknowledgements
We sincerely thank the neonatal intensive care unit (NICU) staff, especially the nurses, at El Manial University Hospital and Elmonira Pediatric Hospital. This research would not have been possible without their dedication to caring for premature infants and families. We are grateful for the opportunity to collaborate with these nursing professionals to advance knowledge and improve outcomes.
No financial or non-financial benefits have been received from any party related directly or indirectly to the subject of this article.
Author information
Authors and affiliations.
College of Nursing, Jouf University, Sakaka, Saudi Arabia, 72388
Osama Mohamed Elsayed Ramadan, Majed Mowanes Alruwaili, Abeer Nuwayfi Alruwaili, Nadia Bassuoni Elsharkawy, Enas Mahrous Abdelaziz, Mohammed Elsayed Zaky & Mostafa Shaban
Maternal and Newborn Health Nursing Department, Faculty of Nursing, Cairo University, Cairo, Egypt
Nadia Bassuoni Elsharkawy
Psychiatric Mental Health Nursing Department, Faculty of Nursing, Cairo University, Cairo, Egypt
Enas Mahrous Abdelaziz
Medical Surgical Nursing Department, Faculty of Nursing, Cairo University, Cairo, Egypt
Mohammed Elsayed Zaky
Lecturer of Community Health Nursing, Faculty of Nursing, Cairo University, Cairo, Egypt
Marwa Mamdouh shaban
Geriatric Nursing Department, Faculty of Nursing, Cairo University, Cairo, Egypt
Mostafa Shaban
You can also search for this author in PubMed Google Scholar
Contributions
O.M.E.S.R. conceptualized the study idea, designed the study methodology, conducted the literature review and analysis, drafted the initial manuscript, and revised the manuscript for intellectual content. M.M.A. contributed to study conceptualization, methodology design, data analysis and interpretation, and critically revised the manuscript. A.N.A. assisted with study design, literature analysis, data interpretation, and provided critical revisions on manuscript drafts. N.B.E. participated in developing the study methodology, analyzing and interpreting results, and reviewing manuscript drafts. E.M.A. was involved with study design, data analysis, drafting parts of the manuscript, and reviewing the final manuscript. M.E.Z. contributed to conceiving the study foundations, interpreting findings, drafting sections of the paper, and revising intellectual content. M.M.S. aided in conceptualizing the study, analyzing literatures, drafting manuscript subsections, and critically revising the complete draft. M.S. made substantial contributions to study origins, data evaluations, drafting portions of the original manuscript, and critically editing revisions. All authors provided final approval of the version to be published and agree to be accountable for all aspects of the work.
Corresponding authors
Correspondence to Osama Mohamed Elsayed Ramadan or Majed Mowanes Alruwaili .
Ethics declarations
Ethical approval.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board obtained from the Faculty of Nursing, Cairo University, before the start of the study (IRB20194041701).
Informed consent
Informed consent was obtained from all parents of the preterm infants involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Elsayed Ramadan, O.M., Alruwaili, M.M., Alruwaili, A.N. et al. Nursing practice of routine gastric aspiration in preterm infants and its link to necrotizing enterocolitis: is the practice still clinically relevant?. BMC Nurs 23 , 333 (2024). https://doi.org/10.1186/s12912-024-01994-x
Download citation
Received : 28 January 2024
Accepted : 06 May 2024
Published : 17 May 2024
DOI : https://doi.org/10.1186/s12912-024-01994-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Necrotizing enterocolitis
- Preterm infants
- Gastric aspiration
- Gastric residuals
- Feed tolerance
- Evidence-based practice
BMC Nursing
ISSN: 1472-6955
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
The purpose of this paper is to describe a model to guide nursing science in a clinical practice‐based setting. Exemplars are provided to highlight the application of this nursing research model, which can be applied to other clinical settings that aim to fill evidence gaps in the literature. ... Patients' healthcare needs are becoming ...
Evidence-based nursing practice is a process through which nurses execute their clinical duties while utilizing the current and valid research findings, their clinical expertise, the client values and preferences, and the available resources to make a practice decision (International Council for Nurses, 2012; Registered Nurses' Association of ...
The Journal of Clinical Nursing publishes research and developments relevant to all areas of nursing practice- community, geriatric, mental health, pediatric & more. ... The paper by Zhon and Hovis (2024) is a thorough review and critique of the empirical work on the impact of the COVID-19 pandemic on suicide amongst health care workers. ...
Clinical Nursing Research (CNR) is a leading international nursing journal, published eight times a year.CNR aims to publish the best available evidence from multidisciplinary teams, with the goal of reporting clinically applicable nursing science and phenomena of interest to nursing. Part of CNR's mission is to bring to light clinically applicable solutions to some of the most complex ...
Nursing students' integration of theoretical knowledge and practical abilities is facilitated by their practice of nursing skills in a clinical environment. A key role of preceptors is to assess the learning goals that nursing students must meet while participating in clinical practice. Consequently, the purpose of this study was to explore the current evidence in relation to competency ...
It is a systematic approach that integrates the best available evidence, clinical expertise, and patient preferences to inform and guide nursing practice. This research paper provides a ...
Journal of Research in Nursing publishes quality research papers on healthcare issues that inform nurses and other healthcare professionals globally through linking policy, research and development initiatives to clinical and academic excellence. View full journal description. This journal is a member of the Committee on Publication Ethics (COPE).
Lastly, we show that critical thinking constitutes a fundamental component in the research process, and can improve research competencies in nursing. We conclude that future research and actions must go further in the search for new evidence and open new horizons, to ensure a positive effect on clinical practice, patient health, student ...
Clinical Nursing Research is a refereed journal publishing research articles that focus on nursing practice.It disseminates research findings of particular interest to practicing nurses, provides an international forum for discussion among clinical nurse researchers and by identifying practical applications of research, enhances practice.
1 INTRODUCTION. When trying to improve healthcare delivery, the focus is often on reversing the shortcomings of systems and services, with a view to improving clinical patient outcomes (Hill et al., 2020).This deficit-based approach is prevalent in nursing, where practice improvement strategies are often initiated by a focus on problems, mistakes or errors.
Introduction In 2020, the World Health Organization called for the expansion and greater recognition of all nursing roles, including advanced practice nurses (APNs), to better meet patient care needs. As defined by the International Council of Nurses (ICN), the two most common APN roles include nurse practitioners (NPs) and clinical nurse specialists (CNSs). They help ensure care to ...
The topics for the project were all related to clinical nursing practice in medical, surgical, or palliative units in the hospital, or in nursing homes and home care. ... During the process of searching for research papers and conducting their own studies, the students thought there was too little research on nursing and nursing interventions ...
A 2007 study by Woodward and colleagues in the Journal of Research in Nursing found that nurse clinicians engaged in research often perceive a lack of support from nurse managers and resentment from colleagues who see the research as taking them away from clinical practice. The distinction often drawn between nursing research and clinical ...
19 Jan 2024. 23 Nov 2023. 17 Oct 2023. 06 Oct 2023. Nursing Research and Practice focuses on all areas of nursing and midwifery. The journal focuses on sharing data and information to support evidence-based practice.
Background Transitions in healthcare delivery, such as the rapidly growing numbers of older people and increasing social and healthcare needs, combined with nursing shortages has sparked renewed interest in differentiations in nursing staff and skill mix. Policy attempts to implement new competency frameworks and job profiles often fails for not serving existing nursing practices. This study ...
Hamric proposed a definition of advanced nursing practice that includes several defining characteristics. The conceptual model covers most of the competency pillars that are currently used in the UK, such as education, clinical practice, guidance and coaching, evidence-based practice, ethical decision-making and leadership.
In study done by Hart and Rotem stressful events for nursing students during clinical practice have been studied. They found that the initial clinical experience was the most anxiety producing part of their clinical experience [ 4 ]. The sources of stress during clinical practice have been studied by many researchers [ 5 - 10] and [ 11 ].
Digital health technologies (DHTs) have the potential to modernize drug development and clinical trial operations by remotely, passively, and continuously collecting ecologically valid evidence ...
Understanding the stressors and coping strategies of nursing students in their first clinical training is important for improving student performance, helping students develop a professional identity and problem-solving skills, and improving the clinical teaching aspects of the curriculum in nursing programmes. While previous research have examined nurses' sources of stress and coping styles ...
Clinical practice guidelines (CPGs) are systematically developed statements intended to affect decisions made by health care providers, policy makers, and patients. 1 They are based on a rigorous review of the best available evidence and provide recommendations on how to optimize patient management. 2 Today, CPGs are an important component in ...
Favorable attitude towards clinical practice: Those who scored above the mean score of all attitude assessment scales [ 26 ]. 2.3. Data collection tool and procedure. Structured questionnaire developed after the review of different literatures was used as a data collection tool [ [14], [15], [16], 26 ].
The MSN clinical practice project provides opportunities for meeting competencies identified within the AACN's (2021), Essentials: Core Competencies for Professional Nursing Education. The following domains met by the clinical practice project are listed below: Knowledge for Nursing Practice. Person-Centered Care.
This paper contributes to the performance of personal hygiene. It is necessary to closely monitor the hemodynamic parameters of intensive care patients when they are being given bed baths. This study has direct effects in awakening nurses to the potential risk of bed baths causing a change in hemodynamic parameters in intensive care patients ...
Krupp will apply her ICU-based clinical expertise with her implementation science training to plan and study how decision support is used in everyday clinical practice. Anna Krupp (left-right), Assistant Professor Heather Dunn, doctoral student Nikta Kia, and Karen Dunn Lopez discuss the results of a focus group they conducted on clinical ...
Beyond clinical practice, this research has major implications for preterm infant nursing education and policy. This study's contribution to stomach residual aspiration and NEC understanding helps change the curriculum of neonatal nursing programs, ensuring future nurses have the latest and most evidence-based procedures.