- Resume Builder
- Resume Templates
- Resume Formats
- Resume Examples
- Cover Letter Builder
- Cover Letter Templates
- Cover Letter Formats
- Cover Letter Examples
- Career Advice
- Interview Questions
- Resume Skills
- Resume Objectives
- Job Description
- Job Responsibilities
- FAQ’s

Quality Manager Resume Examples
Writing a resume for a position in quality management requires a specific set of skills and qualifications. It is important to highlight your experience, accomplishments, and knowledge in managing quality assurance, process and product improvement, and regulatory compliance. This guide provides tips on how to write an effective resume for a quality manager position. It also provides sample resumes for the position, to help inspire and give you an idea of what a successful quality manager resume should look like. With these tips and examples, you can craft a resume that will get you noticed and give you a great chance of landing the job.
If you didn’t find what you were looking for, be sure to check out our complete library of resume examples .

Start building your dream career today!
Create your professional resume in just 5 minutes with our easy-to-use resume builder!
Quality Manager
123 Main Street | Anytown, USA 99999 | Phone: (123) 456-7890 | Email: [email protected]
I am a highly organized and skilled Quality Manager with 5+ years of experience in the manufacturing industry. I have a incredible track record in driving customer satisfaction and loyalty through the implementation of effective quality management systems and procedures. I am proficient in leading teams of quality assurance professionals to ensure compliance with the relevant industry standards and customer requirements. My expertise in root- cause analysis and problem solving has enabled me to identify and address areas of non- compliance proactively. I am confident that I can help any organization achieve its quality objectives.
Core Skills :
- Quality Management System (QMS)
- Quality Assurance (QA)
- Regulatory Affairs
- Root- Cause Analysis
- Problem Solving
- Documentation
- Team Leadership
Professional Experience : Quality Manager, XYZ Manufacturing Corporation, May 2015 – Present
- Develop, implement and maintain a comprehensive Quality Management System (QMS) to ensure product compliance with customer and regulatory standards.
- Monitor and review product quality standards and process performance to identify areas of non- compliance.
- Lead monthly quality audit activities to ensure compliance with QMS requirements.
- Develop and maintain key process indicators and other quality metrics to track performance and identify areas of improvement.
- Monitor and analyze product performance data to identify potential issues and areas of risk.
- Create and implement corrective and preventive action plans to address areas of non- compliance.
- Lead cross- functional teams of engineers and technicians to develop and deploy quality control protocols and procedures.
Education : Bachelor of Science in Quality Management, ABC University, 2012 Certified Quality Manager, International Institute of Quality Assurance, 2014
Create My Resume
Build a professional resume in just minutes for free.
Quality Manager Resume with No Experience
Dynamic, organized, and motivated Quality Manager with proven ability to motivate teams to meet organizational objectives. Proven track record of success in streamlining processes and procedures to improve efficiency. Experienced in leading and managing cross- functional teams and ensuring customer satisfaction.
- Expertise in developing and implementing quality management systems
- Strong knowledge of regulatory and compliance standards
- Highly organized with excellent problem- solving skills
- Excellent verbal and written communication skills
- Proficient with Microsoft Office Suite
- Proficient in statistical quality control methods
- Ability to interpret customer feedback and design solutions
Responsibilities
- Develop and implement quality management systems to ensure customer satisfaction
- Monitor and review quality performance metrics to identify areas for improvement
- Coordinate and oversee quality inspections and audits
- Analyze customer feedback for insights and improvement opportunities
- Develop and implement corrective and preventive actions to address quality issues
- Train and coach teams on quality systems and processes
- Monitor and report on compliance to regulatory and compliance standards
- Develop and maintain relationships with suppliers and customers
Experience 0 Years
Level Junior
Education Bachelor’s
Quality Manager Resume with 2 Years of Experience
A highly organized and motivated Quality Manager with two years of experience leading and managing quality assurance teams. Experienced in developing and executing quality management systems, ensuring quality assurance operations are compliant with customer requirements, and cultivating and maintaining relationships with customers and suppliers. Proven record of successful and efficient operations, customer satisfaction, and continuous improvement. Skilled in problem- solving and data analysis, with a track record of success in the development of successful and efficient operations and customer satisfaction.
- Quality Assurance
- Quality Control
- ISO Standards
- Data Analysis
- Quality Systems Management
- Relationship Management
Responsibilities :
- Develop and implement quality assurance systems and processes, in accordance with customer requirements and ISO standards.
- Lead and manage quality assurance teams, while developing and executing training programs.
- Monitor quality assurance operations and customer requirements to ensure compliance.
- Identify and resolve quality- related issues and implement corrective actions.
- Analyze quality data to identify trends and assess performance.
- Coordinate with suppliers and customers to ensure quality standards are met.
- Develop and implement continuous improvement initiatives.
- Create and maintain comprehensive quality assurance documentation.
Experience 2+ Years
Quality Manager Resume with 5 Years of Experience
Innovative and results- oriented Quality Manager with 5 years of experience in driving continuous improvement initiatives, leading cross- functional teams, and building relationships with key stakeholders. Proven track record of success in designing and implementing quality management systems, developing quality protocols, and reducing defects in production. Possesses excellent communication, problem- solving, and organizational skills.
- Quality System Design
- Process Improvement
- Quality Standards Development
- Root Cause Analysis
- Document Control
- Trend Analysis
- Cross- Functional Team Leadership
- Developed and implemented quality management system based on ISO 9001 standards.
- Developed, updated, and enforced quality standards, policies, and processes.
- Conducted root cause analysis of existing processes and identified areas for improvement.
- Monitored production processes and identified defect trends to reduce production costs.
- Implemented corrective action plans to improve quality and efficiency while reducing defects.
- Planned, organized, and led audits to ensure compliance with quality and safety standards.
- Developed and maintained document control systems to ensure accuracy and reliability.
- Analyzed quality performance metrics to identify process trends and areas for improvement.
- Led cross- functional teams to develop and implement process changes in order to improve quality and customer satisfaction.
Experience 5+ Years
Level Senior
Quality Manager Resume with 7 Years of Experience
Highly experienced Quality Manager with 7 years of experience in the field of quality management and improvement. Adept at leading and managing a wide range of quality improvement projects, implementing solutions to improve quality, and developing and maintaining a quality management system. Possesses excellent knowledge of quality assurance processes and techniques, and a strong understanding of quality metrics and system analysis.
- System Analysis
- Quality Metrics
- Quality Management Systems
- Quality Improvement
- Project Management
- Develop and maintain quality management system and ensure quality improvement across the organization.
- Provide guidance and assistance to staff to support quality assurance.
- Monitor performance and analyze quality data to identify improvement opportunities.
- Develop and implement processes and policies for quality management and improvement.
- Design and implement quality control plans and processes.
- Audit quality systems and evaluate process effectiveness.
- Monitor production activities to ensure compliance with quality standards.
- Liaise with clients and vendors to ensure quality standards are met.
- Investigate and resolve customer complaints regarding quality.
- Train and mentor employees on quality assurance principles and processes.
- Create and maintain documentation of quality standards and procedures.
Experience 7+ Years
Quality Manager Resume with 10 Years of Experience
Experienced Quality Manager with 10 years of experience in optimizing processes and developing procedures to maintain and improve quality standards across all operations. Possess expertise in conducting audits and providing training to ensure that quality control processes are followed and corrective actions are taken as necessary. Adept at developing and implementing quality management systems, ensuring customer satisfaction, and maximizing productivity.
- Training & Development
- Regulatory Compliance
- Reporting & Documentation
- Communication & Interpersonal
- Develop and implement quality control processes to ensure product quality and customer satisfaction
- Conduct audits to identify potential issues in production process and take corrective action
- Monitor and report on quality metrics and performance indicators to ensure compliance to standards
- Develop and maintain quality management systems to ensure regulatory compliance
- Train team members on quality standards and ensure they are implemented
- Investigate customer complaints and take corrective action to improve customer satisfaction
- Liaise with suppliers to ensure quality of incoming materials
- Provide advice, guidance and support on quality related issues
Experience 10+ Years
Level Senior Manager
Education Master’s
Quality Manager Resume with 15 Years of Experience
An experienced and results- oriented Quality Manager with 15 years of professional experience in developing and implementing quality assurance systems, driving continuous improvement, and ensuring customer satisfaction. Possessing a strong understanding of quality systems, auditing, and industry standards. Eager to contribute to a successful organization and ensure quality control and customer satisfaction.
- Quality Assurance and Control
- Quality System Development
- Quality Management
- Continuous Improvement
- Regulations Compliance
- Training and Development
- Budgeting & Cost Control
- Risk Management
- Supplier Evaluation & Management
- Develop and implement quality assurance standards and procedures to ensure product compliance with applicable standards and regulations.
- Monitor quality control procedures to identify and resolve issues that could lead to costly problems.
- Perform quality control audits on a regular basis and document findings.
- Develop and implement corrective and preventive action plans in response to audit findings.
- Develop and maintain quality metrics and provide performance feedback to management.
- Develop training materials and conduct training on quality assurance procedures.
- Work with suppliers to ensure compliance with quality requirements.
- Monitor and analyze production processes to identify areas for improvement.
- Conduct root cause analysis to investigate non- conformities and design corrective actions.
- Ensure all stakeholders are aware of relevant quality and safety requirements.
Experience 15+ Years
Level Director
In addition to this, be sure to check out our resume templates , resume formats , cover letter examples , job description , and career advice pages for more helpful tips and advice.
What should be included in a Quality Manager resume?
A quality manager is responsible for ensuring that the products and services offered by a business meet the highest standards of quality. If you’re looking to break into the quality management field, having a great resume is essential. Your resume should outline your knowledge and expertise, as well as your relevant experience and accomplishments. Here are some key components to include in your quality manager resume:
- Professional Summary: Include a brief statement that outlines your experience and qualifications for the position.
- Qualifications and Certifications: List any relevant qualifications or certifications that are necessary for the role.
- Work Experience: Outline your relevant work experience, including where you worked, what duties you performed, and any accomplishments.
- Education and Training: List any degrees or certifications that you have obtained that are related to quality management.
- Technical Skills: List any technical skills that are necessary for the role, such as familiarity with specific software or working knowledge of industry standards.
- Leadership Experience: If you have any previous leadership experience, outline it here.
- Professional Associations: If you belong to any professional organizations, list them here.
- Awards and Achievements: Include any awards or accomplishments that you have received in the field of quality management.
By including all of these elements in your quality manager resume, you can ensure that you have a comprehensive and effective document that will help you land the job of your dreams.
What is a good summary for a Quality Manager resume?
A good summary for a Quality Manager resume should include:
- Proactive and results-oriented professional with 10+ years of experience in Quality Management in various industries.
- Expertise in quality assurance processes and procedures, root cause analysis and corrective action implementation.
- Hands-on experience in leading quality audits, developing quality management systems, training staff and creating process documentation.
- Skilled in identifying improvement opportunities and developing strategies to ensure product and service quality.
- Recognized for a commitment to customer satisfaction and the ability to work in fast-paced environments.
- Certified Quality Manager (CQM) from the American Society for Quality.
The summary should be tailored to the individual and should emphasize the candidate’s strongest qualifications, skills and accomplishments. The summary should be concise and serve as an effective introduction to the employer.
What is a good objective for a Quality Manager resume?
A Quality Manager is responsible for ensuring that a business meets the set standards for quality in its products and services, and a good objective for a Quality Manager resume should reflect this. A good objective for a Quality Manager resume should demonstrate an understanding of the key tasks, qualities, and responsibilities required for the role.
- Show a commitment to quality standards: Quality Managers need to be able to ensure that a business meets the set standards for quality in its products and services.
- Demonstrate the ability to efficiently manage processes: Quality Managers need to be able to manage processes and resources in order to meet desired quality goals.
- Highlight problem-solving skills: Quality Managers need to be able to identify problems and develop solutions that will improve the quality of products and services.
- Stress the importance of customer satisfaction: Quality Managers must prioritize customer satisfaction and provide solutions that meet customer needs.
- Display a commitment to continuous improvement: Quality Managers must be able to identify areas for improvement and develop strategies for improving performance.
By emphasizing these qualities in a Quality Manager resume, you can ensure that you stand out from the competition and demonstrate your suitability for the role.
How do you list Quality Manager skills on a resume?
When applying to a position as a Quality Manager, it is important to list the skills and qualifications necessary for the job on your resume. Having a comprehensive list of quality manager skills on your resume will make it stand out above other applicants. Here are a few skills to consider including:
- Quality Assurance: Quality Managers are responsible for ensuring that all products and services meet customer standards and expectations. This includes developing and implementing quality control systems, maintaining records of defects, and conducting regular inspections.
- Problem-Solving: Quality Managers must be able to identify and resolve any issues that arise in order to keep production running smoothly. This includes being able to think critically and analyze complex situations.
- Leadership: Quality Managers are expected to lead a team of quality assurance specialists and make decisions to ensure the success of the project. This requires strong communication, delegation, and conflict resolution skills.
- Communication: Quality Managers must establish and maintain positive relationships with customers and suppliers. This requires clear and effective communication to provide updates, answer questions, and provide guidance.
- Technical Knowledge: Quality Managers must understand the technical aspects of their products and services in order to identify problems and develop solutions. This includes knowledge of industry standards, regulations, and safety concerns.
What skills should I put on my resume for Quality Manager?
When writing a quality manager resume, you must make sure to emphasize the important skills that are necessary for the job. Quality managers must be highly organized, detail-oriented, and have strong problem solving and communication skills. Here are some of the key skills that every quality manager should highlight on their resume:
- Problem-Solving: Quality managers must have the ability to quickly identify and solve problems that arise during the production process. They must be able to work effectively with a wide range of stakeholders, investigate problems and develop efficient solutions.
- Quality Assurance: Quality managers are responsible for ensuring that all products and services meet the organization’s standards and requirements. They must be able to design and implement quality assurance procedures, analyze quality data, and develop corrective action plans.
- Regulatory Compliance: Quality managers must have a thorough understanding of applicable regulations and guidelines and must ensure that their organization is compliant. They must be knowledgeable about the relevant industry standards and have the ability to stay up-to-date on any changes.
- Communication: Quality managers must be able to communicate effectively with a wide range of stakeholders, including production staff, customers, vendors, and other departments. They should be able to explain complex quality processes in simple terms and have the ability to convey information clearly.
- Leadership: Quality managers must be able to lead teams and direct their efforts in order to ensure the efficient and successful completion of projects. They must be able to motivate, train, and mentor staff, and they must have the ability to delegate tasks and resolve conflicts.
By including these key skills on your resume, you will be able to demonstrate your qualifications and experience to potential employers. Quality managers must have a wide range of skills, but these are the most important ones to emphasize when creating your resume.
Key takeaways for an Quality Manager resume
For a quality manager, having a well-crafted resume is critical for standing out in the crowded job market. In addition to showcasing your experience and accomplishments, a good quality manager resume should also highlight your key skills and qualifications. Here are four key takeaways for creating an effective quality manager resume:
- Highlight Your Experience: A quality manager resume should focus on your relevant experience in quality assurance and management. Be sure to list your qualifications, certifications, and any awards or recognitions you have received. If you have managed multiple projects or initiatives, include this information as well.
- Showcase Your Skills: Quality managers need to have strong problem-solving and analytical skills, so be sure to highlight these abilities in your resume. You should also mention any software or systems you are familiar with and list any relevant technical skills you possess.
- Demonstrate Your Leadership: Quality managers need to be able to lead teams, set goals, and make decisions. Include any examples of successful projects you have worked on or challenges that you have faced and overcome.
- Detail Your Accomplishments: Quality managers need to be able to showcase their successes. Include any positive feedback you have received from colleagues or customers, as well as any goals you have achieved.
By showcasing your experience and successes, you can help make your resume stand out to potential employers. Following these tips can help you create an effective quality manager resume that will help you land the job.
Let us help you build your Resume!
Make your resume more organized and attractive with our Resume Builder

- Career Blog
Quality Manager Resume: Examples and Writing Guide

In this article, we will explore the world of Quality Manager Resumes. A Quality Manager Resume is a document that showcases the skills, experiences, and achievements of a Quality Manager. Employers need Quality Managers to ensure that their products or services meet a high standard of quality. Crafting an effective Quality Manager Resume is essential for standing out in a competitive job market. In this article, we will discuss the elements that make a great Quality Manager Resume and provide examples and tips to help you write your own. So, let’s dive in!
Understanding the Role of a Quality Manager
A Quality Manager is a professional who is responsible for ensuring that products, services, and processes of an organization meet the required standards and specifications. They oversee quality control measures, testing, and inspection, and are responsible for making sure that products meet regulatory and industry requirements.
What does a Quality Manager do?
A Quality Manager plays a critical role in ensuring the quality and consistency of products, services, and processes within an organization. Their duties may include:
- Establishing and implementing quality control procedures
- Analyzing and interpreting data
- Identifying areas for improvement and implementing solutions
- Developing quality standards and specifications
- Conducting quality audits and inspections
- Training, coaching, and mentoring team members on quality processes and procedures
What skills are required to be a Quality Manager?
To be a successful Quality Manager, you will need to possess a combination of technical and soft skills. These may include:
- Strong analytical and problem-solving skills
- Excellent communication and interpersonal abilities
- Knowledge of quality control procedures and methodologies
- Attention to detail and accuracy
- Proficiency in data analysis and interpretation
- Experience with production processes and industry-specific regulations
- Strong leadership and organizational skills
- Ability to work effectively in a team and independently
- Continuous improvement mindset
What qualities are employers looking for in a Quality Manager?
Employers are typically looking for Quality Managers who have a strong track record of success in creating and implementing quality control procedures and methodologies. Additionally, they often look for candidates who possess:
- Professional certifications in quality management or a related field
- Experience in their specific industry or sector
- Strong leadership and management skills
- A customer-oriented approach
- The ability to manage multiple projects simultaneously
- A commitment to continuous improvement and learning
- A positive attitude and strong work ethic
A successful Quality Manager will possess a combination of technical and soft skills, as well as a commitment to continuous improvement and a customer-oriented approach. By demonstrating these qualities and competencies, they stand to become an invaluable asset to any organization.
Tips for Writing an Attention-Grabbing Quality Manager Resume
When crafting your Quality Manager resume, it’s important to keep in mind that your goal is to make an impactful first impression on your potential employer. This can be achieved by following these tips:
Understanding the importance of a targeted and relevant resume
The first step in writing an attention-grabbing resume is to understand the importance of tailoring your resume to the job you’re applying for. Your resume should reflect the requirements of the job posting, and highlight how your specific skill set and experience can meet those requirements.
Highlighting core skills and achievements
Be sure to include a section in your resume that highlights your core skills and achievements. This is your opportunity to showcase your unique strengths and what sets you apart from other applicants.
Using a professional format and structure
A hiring manager will appreciate a well-organized and easy-to-read resume. Ensure that your resume is structured in a professional manner, and that all sections are clearly labeled. Be consistent with font size and formatting throughout the document.
Making sure your resume is easy to read and visually appealing
In addition to a professional structure, it’s also important to make sure your resume is visually appealing. Avoid using too many graphics or fancy fonts, as this can be distracting. Instead, use white space to break up sections and make the resume easy to read.
By following these tips, you can create an attention-grabbing Quality Manager resume. Remember, your goal is to stand out from the competition and land an interview. A well-crafted, targeted resume can help you achieve that goal.
Key Components of a Quality Manager Resume
When it comes to crafting a quality manager resume, there are several essential components that should be included in order to impress potential employers and stand out from the competition.
The Necessary Sections to Include
The following sections are a must-have in any quality manager resume:
Contact Information: Your full name, phone number, email address, and LinkedIn profile URL should be clearly visible at the top of your resume.
Professional Summary/Objective Statement: This section should be a brief, three to five sentence summary of your qualifications and career goals.
Skills: When drafting your Skills section, try to focus on relevant quality management skills such as ISO certification, Six Sigma, root cause analysis, and statistical process control.
Work Experience: This section should list your work experience in reverse chronological order, along with your job title, employer, dates of employment, and key accomplishments.
Education: List your highest degree first, including the name of the institution, your major, and the date of graduation.
How to Showcase Relevant Education and Experience
When it comes to featuring your education and experience in a quality manager resume, it’s essential to highlight the aspects that are most relevant to the job you’re applying for. You can do this by using specific keywords and phrases from the job posting in your resume.
Be sure to include any relevant certifications or training programs as well. Quality managers with certifications in ISO or Six Sigma are particularly valuable to employers.
Writing a Compelling Summary or Objective Statement
Your professional summary or objective statement should be concise but impactful. Focus on your most significant accomplishments and unique skills, and demonstrate how these factors make you the ideal candidate for the job.
For example:
“Results-driven quality manager with over 10 years of experience in improving product quality and reducing defects. Skilled in leading cross-functional teams, implementing process improvements, and developing robust quality management systems. Seeking a challenging role in a fast-paced manufacturing environment that values quality and continuous improvement.”
By following these guidelines, you will be well on your way to crafting a standout quality manager resume that will land you your dream job.
Example Quality Manager Resumes
Finding the right job as a quality manager can be tough, especially if you’re not sure where to start. That’s why we’ve created a collection of example resumes to help you showcase your skills and experience effectively. Whether you’re a seasoned pro or just starting out, we’ve got you covered.
Examples and Templates for Different Levels of Experience
On our website, you can find quality manager resume examples at different levels of experience, so you can choose the one that best suits your needs. For example, if you’re a recent graduate or just starting out, we have entry-level resumes that showcase your education and skills. And for those with more experience, we have resumes that highlight your accomplishments and leadership skills.
In addition, we also offer different templates that will help you create a professional and well-organized resume in no time.
Quality Manager Resume (Entry-Level)
Emily Johnson
Quality Manager
Dedicated and detail-oriented Quality Manager with a strong academic background and a passion for ensuring product quality. Seeking an entry-level role to apply my knowledge of quality management principles and gain hands-on experience in implementing quality control processes. Strong analytical and problem-solving skills, with a focus on continuous improvement and customer satisfaction.
- Bachelor’s Degree in Industrial Engineering, University of XYZ
Academic Quality Improvement Project
- Led a team project to analyze and improve the quality of a manufacturing process in a simulated environment.
- Conducted data analysis to identify process variations and implemented corrective actions to improve product quality.
- Developed quality control charts to monitor process performance and ensure adherence to specifications.
Internship – Quality Assurance, ABC Manufacturing Company
- Assisted in implementing quality control measures to ensure compliance with industry standards.
- Conducted inspections and tests to verify product quality and identify non-conformities.
- Participated in root cause analysis and corrective action implementation to address quality issues.
- Quality Management Principles
- Data Analysis
- Root Cause Analysis
- Quality Control
- Continuous Improvement
- Problem-solving
- Communication
Certifications
- Six Sigma Yellow Belt (In Progress)
Quality Manager Resume (Experienced)
Robert Thompson
Quality Manager | Continuous Improvement Expert
Results-driven Quality Manager with over 8 years of experience in implementing and managing quality control processes. Proven track record in leading cross-functional teams, driving process improvements, and ensuring product quality. Skilled in quality management systems, root cause analysis, and implementing corrective actions. Strong leadership, problem-solving, and communication skills.
Professional Experience
Quality Manager, XYZ Manufacturing Company
- Led a team of quality professionals to implement and maintain a quality management system based on ISO 9001 standards.
- Developed and implemented quality control processes and procedures to ensure product conformity and customer satisfaction.
- Conducted root cause analysis and implemented corrective and preventive actions to address quality issues and improve process performance.
- Led continuous improvement initiatives, resulting in a reduction in non-conformities and improved process efficiency.
Senior Quality Engineer, ABC Automotive Company
- Implemented statistical process control (SPC) techniques to monitor and control manufacturing processes.
- Led cross-functional teams in problem-solving and root cause analysis to address quality issues and improve product quality.
- Developed and delivered training programs on quality control and process improvement methodologies to enhance employee knowledge and skills.
- Quality Management Systems (ISO 9001)
- Corrective and Preventive Actions
- Statistical Process Control (SPC)
- Leadership and Team Management
- Problem-solving and Decision-making
- Six Sigma Black Belt
- Certified Quality Manager (CQM)
How to Personalize Each Resume to Meet Specific Job Requirements
At the heart of an effective quality manager resume is the ability to tailor it to the specific job requirements. This means that each resume should be personalized to fit the company and position you’re applying for.
We recommend that you read the job description carefully and identify the key skills and experience that the employer is looking for. Then, you can highlight and emphasize those skills in your resume. For instance, if the job requires experience in ISO 9001:2015 or Lean Six Sigma methodologies, make sure to include your related experience in your resume.
It’s also important to include examples of your accomplishments and how they relate to the job you’re applying for. This will give the employer a better understanding of your capabilities and how you can help their organization.
Using our quality manager resume examples and templates, you can create an effective resume that showcases your skills and helps you stand out from other candidates. And by personalizing each resume to fit the specific job requirements, you can increase your chances of landing your dream job.
Tailoring Your Quality Manager Resume to Specific Industries
Quality Managers work across many different industries, and each industry has its own unique quality standards and requirements. Therefore, it is important to tailor your Quality Manager resume to the specific industry in which you are seeking employment. This can increase your chances of being noticed by potential employers and ultimately securing the job you desire.
Understanding the different industries in which Quality Managers work
Quality Managers can be found in a variety of industries such as manufacturing, healthcare, construction, food and beverage, and pharmaceuticals, to name a few. Each of these industries has their own quality standards and compliance requirements that must be met to ensure the delivery of safe and high-quality products or services to customers. For instance, a Quality Manager in the pharmaceutical industry is responsible for ensuring that drugs are manufactured in compliance with regulations set by the FDA, whereas a Quality Manager in healthcare is responsible for maintaining patient safety and regulatory compliance.
Adapting your resume to highlight relevant experiences and skills based on industry
When tailoring your resume, it is important to highlight relevant experiences and skills that are specific to the industry in which you are applying. For instance, if you are applying for a Quality Manager position in the food and beverage industry, highlight your experience with HACCP (Hazard Analysis and Critical Control Points) as well as your knowledge of food safety regulations.
Similarly, if you are applying for a Quality Manager position in the construction industry, highlight your experience with ISO 9001 (Quality Management System) and your knowledge of OSHA (Occupational Safety and Health Administration) regulations.
Tailoring your Quality Manager resume to specific industries can help you stand out in a competitive job market. Be sure to research the industry and the company you are applying to, and customize your resume to highlight your relevant experiences and skills that will make you an ideal candidate for the position.
Writing a Cover Letter for a Quality Manager Position
In the job application process, a cover letter serves as an introduction of yourself to the hiring manager. It is your chance to grab their attention and make a great first impression. As a Quality Manager, your cover letter should not only showcase your qualifications but also demonstrate your ability to communicate clearly and effectively.
Here are some tips for writing a targeted and engaging cover letter:
Understanding the role of a cover letter in the job application process
A cover letter is your chance to set yourself apart from other applicants. It should be tailored to the specific company and position you are applying for. It is an opportunity to highlight your most relevant skills and experiences, and explain how they make you the best candidate for the position. A well-written cover letter can also demonstrate your attention to detail and professional writing skills.
Tips for writing a targeted and engaging cover letter
Research the company: Before writing your cover letter, research the company you are applying to. Learn about their mission, values, and culture. Understanding the company’s goals and needs can help you tailor your cover letter to the position.
Highlight your qualifications: Use your cover letter to highlight your relevant experience and qualifications. Explain how your skills and experiences match the job requirements. Be specific and use examples to support your claims.
Use a professional tone: Your cover letter should be professional and polished. Avoid using slang, abbreviations, or casual language. Use a formal tone and make sure to proofread for errors.
Personalize your letter: Address your cover letter to the hiring manager by name if possible. If you are unable to find their name, use a professional greeting such as “Dear Hiring Manager.”
Keep it concise: Your cover letter should be no longer than one page. Keep your writing clear and to the point. Use bullet points or short paragraphs to make it easy to read.
Example cover letters for Quality Manager positions
Dear Hiring Manager,
I am excited to apply for the Quality Manager position at XYZ Company. With six years of experience in the industry, I have developed a deep understanding of quality control processes and the ability to lead a team to success.
At my past company, I reduced product defects by 10% through process improvement initiatives. My experience in Lean and Six Sigma methodologies has also been valuable in identifying areas of improvement and implementing changes that have resulted in cost savings.
I am confident that my skills and experiences make me an ideal candidate for the Quality Manager position at XYZ Company. Thank you for considering my application.
Sincerely, [Your name]
I am writing to express my interest in the Quality Manager position at ABC Corporation. As a certified Quality Engineer with over eight years of experience in the field, I have a proven track record of success in managing quality control processes.
My experience leading cross-functional teams has been instrumental in driving process improvements and increasing product quality.
Best Practices for Optimizing Your Quality Manager Resume for Applicant Tracking Systems (ATS)
When applying for a quality manager position, it is essential to understand how applicant tracking systems (ATS) screen resumes. ATS is software used by recruiters to manage and sort resumes based on specific keywords, phrases, and job-related criteria.
To optimize your resume for ATS, follow the tips below:
Use relevant keywords: Read the job posting carefully and pick out relevant keywords and phrases. Include them in your resume to increase the chances of being selected by the ATS.
Keep it simple: Use a simple and clear resume format. Avoid using fancy fonts, graphics, and formatting that can confuse the ATS.
Use relevant headings: Use headings such as “work experience,” “skills,” and “education” that are easy for ATS to recognize.
Use bullet points: Use bullet points to highlight your relevant experience, skills, and achievements. This makes it easier for the ATS to scan and identify the information.
Always use a text-based document: Use a simple and common file format such as .docx or .pdf (text-based) to ensure that the ATS can easily scan your resume.
Common mistakes when preparing your resume for ATS include:
Adding unnecessary information and graphics that can confuse the ATS.
Using headers, footers, and tables that the ATS may not recognize.
Using abbreviations, slang, or technical jargon that the ATS may not identify.
When preparing your quality manager resume for ATS, focus on using relevant keywords, a clear format, relevant headings, bullet points and avoid using unnecessary information and graphics. This will help increase your chances of getting shortlisted and landing a quality manager job.
Proofreading and Editing your Quality Manager Resume
One of the most crucial stages in creating a quality manager resume is the proofreading and editing process. It’s important to ensure that your resume is free from errors and inconsistencies so that it presents you in the best light possible.
The importance of proofreading and editing your resume
Proofreading and editing your quality manager resume is essential because it can make the difference between getting an interview for your dream job or being dismissed by potential employers. Even the tiniest error or inconsistency can reflect poorly on you and your attention to detail. In a world where employers receive hundreds of resumes for a single role, a resume that contains mistakes is unlikely to stand out.
Tips for spotting errors and inconsistencies
To ensure the quality of your manager resume, here are some tips for spotting errors and inconsistencies:
- Read your resume out loud – this helps you to identify awkward phrasing and grammatical errors.
- Take a break before proofreading – give yourself some space before you proofread, as this can help you approach your resume with fresh eyes.
- Use proofreading software – programs like Grammarly and Hemingway can help you identify errors and suggest corrections.
- Enlist a friend or family member to help – sometimes you need outside help to catch mistakes that you might have missed.
Tools and resources for editing and proofreading
Several tools and resources can help you in your editing and proofreading process. Here are some of the most popular:
- Grammarly – A widely used grammar-checking tool that helps detect grammar, spelling, and punctuation errors.
- Hemingway – A writing app that helps you simplify your writing, making it clear and concise.
- Microsoft Word – Microsoft Word has an in-built grammar-checking feature that can help you detect errors and offer suggestions.
- Online proofreading services – Services like Scribendi and ProWritingAid offer professional proofreading services that can help you take your resume to the next level.
Proofreading and editing your quality manager resume is an essential part of the job-seeking process. By following our tips and using the tools and resources provided, you can ensure that your resume is free from errors and inconsistencies and presents you as the best candidate for the job.
X. Frequently Asked Questions about Quality Manager Resumes
Quality Manager positions require a certain level of expertise and specialized knowledge. When crafting a resume for such a role, it’s important to highlight the specific skills and experiences that make you a qualified candidate. Here are some commonly asked questions about writing resumes for Quality Manager positions, along with answers and advice to help job seekers stand out from the competition.
1. What skills should I include on my Quality Manager resume?
Skills are a vital component of any Quality Manager resume. A few key skills to consider including are quality control, process improvement, data analysis, problem-solving, and leadership. Additionally, soft skills such as communication, collaboration, and attention to detail are important to emphasize. Review the job description and industry standards to tailor your skills to the position specifically.
2. How can I showcase my accomplishments on my resume?
It’s important to quantify your accomplishments on your resume, such as improving product quality, increasing efficiency, or reducing costs. Consider using bullet points to highlight specific achievements and numbers that indicate your impact on the organization. Use strong action verbs and avoid generic descriptions that do not convey the scope of your responsibilities.
3. Should I include certifications on my Quality Manager resume?
Adding certifications that reflect your expertise to your Quality Manager resume is beneficial, especially if the certification is relevant to the position you are applying for. Examples of certifications that would be valuable for Quality Managers are ISO 9001 Quality Management System certifications, Six Sigma certifications or industry-specific certifications.
4. How should I format my Quality Manager resume?
When searching for Quality Manager positions, recruiters and hiring managers look for resumes that are easy to read and visually pleasing. Use a clean, organized format that is easy to follow, with consistent formatting and clear section headings. Include a summary statement at the beginning of your resume that highlights your key achievements, job experience, and relevant skills.
5. What common mistakes should I avoid on my Quality Manager resume?
Mistakes on a Quality Manager resume can be costly for candidates, as it portrays a lack of attention to detail, a crucial trait for Quality Manager roles. Avoid grammatical errors, typos or inconsistency in formatting, education details, or job descriptions. Do not oversell or use empty phrases, as this may backfire during the hiring process.
By answering these frequently asked questions and heeding these recommendations, job seekers can make a strong impression on potential employers, increasing their chances of landing a Quality Manager position. The key is to maintain a balance between highlighting your skills and experiences while keeping the focus on how the organization benefits from your expertise.
Related Articles
- QA Team Lead Job Description: Complete Guide for 2023
- 25 Fast Tips for Finding a New Job in 2023
- Acing Interviews with the STAR Method: Best Examples
- 25 Restaurant Manager Resume Examples with Helpful Tips
- 30 Common Situational Interview Questions and Expert Answers
Rate this article
0 / 5. Reviews: 0
More from ResumeHead

- • Designed and implemented a new automated testing framework, increasing test coverage by 40%.
- • Spearheaded the QA process for a major software release, resulting in a 30% decrease in post-launch critical issues.
- • Led a cross-functional team of 10 to establish formal QA processes in line with industry best practices, cutting down the overall testing cycle time by 25%.
- • Coordinated with clients, providing insightful QA services for new technologies, resulting in the successful onboarding of 4 key customers.
- • Delivered comprehensive training materials for QA practices, significantly enhancing the team's efficiency and expertise.
- • Successfully managed multiple product deployments into production, ensuring a 99.9% success rate on first attempt.
- • Developed and optimized test strategies for Amazon's e-commerce platform, contributing to a 20% reduction in critical bugs.
- • Managed the creation and execution of test documents, improving defect identification by 15%.
- • Implemented quality standards that improved product release efficiency by 18%.
- • Played a pivotal role in the rollout of a major feature update, which led to a 10% increase in user satisfaction.
- • Worked closely with development teams to align technical solutions with business needs, improving project outcomes.
- • Conducted in-depth analytics and product specification reviews, improving testing accuracy by 10%.
- • Led the testing efforts for new travel search features, directly contributing to a 5% growth in market share.
- • Collaborated with other departments to troubleshoot and resolve quality issues swiftly.
- • Authored detailed audit reports and quality documentation to enhance transparency and accountability within the QA team.
5 QA Manager Resume Examples & Guide for 2024
Your QA manager resume must highlight your expertise in quality assurance methodologies. Demonstrate an impeccable track record of implementing effective QA systems and processes. Showcase your leadership skills by detailing how you've successfully managed teams. Include metrics that reflect the positive impact of your QA initiatives on product quality and customer satisfaction.
All resume examples in this guide

Resume Guide
Resume Format Tips
Resume Experience
Skills on Resume
Education & Certifications
Resume Summary Tips
Additional Resume Sections
Key Takeaways

As a QA manager, articulating your extensive experience in quality assurance while keeping your resume concise can be a significant challenge. Our guide will provide you with the techniques to efficiently present your achievements and skills, ensuring your resume stands out to potential employers.
- Defining the highlights of your qa manager career through your resume summary, objective, and experience.
- Real-world qa manager resume samples with best practices on how to stand out amongst the endless pile of candidate resumes.
- Most in-demand qa manager resume skills and certifications across the industry.
- Standardizing your resume layout, while maintaining your creativity and individuality.
If the qa manager resume isn't the right one for you, take a look at other related guides we have:
- Quality Control Inspector Resume Example
- Quality Assurance Specialist Resume Example
- Quality Assurance Technician Resume Example
- Quality Assurance Manager Resume Example
- Quality Supervisor Resume Example
- Quality Manager Resume Example
- Quality Consultant Resume Example
- Quality Control Specialist Resume Example
- Quality Auditor Resume Example
- Release Manager Resume Example
Creating the best qa manager resume format: four simple steps
The most appropriate qa manager resume format is defined by precision and a systematic approach. What is more, it should reflect upon how your application will be assessed by recruiters. That is why we've gathered four of the most vital elements to keep in mind when designing your resume:
- It's all about presenting how your experience or skills align with the job. Use the reverse-chronological resume format , if your expertise is relevant to the qa manager role. Otherwise, select the functional skill-based resume format or the hybrid resume format to shift the focus to your skill set.
- Resume header - make sure you've filled out all relevant (and correct) information, like your contact details and link to your portfolio.
- Resume length - unless you've over a decade of applicable expertise in the field, stick with a one-page resume format. If you'd like to present more of your professional experience, go up to two pages.
- Resume file - submit your qa manager resume in a PDF format to ensure all information stays in the same place.
Upload & Check Your Resume
Drop your resume here or choose a file . PDF & DOCX only. Max 2MB file size.
Mention specific courses or projects that are pertinent to the job you're applying for.
QA Manager resume sections to answer recruiters' checklists:
- Header to help recruiters quickly allocate your contact details and have a glimpse over your most recent portfolio of work
- Summary or objective to provide an overview of your career highlights, dreams, and goals
- Experience to align with job requirements and showcase your measurable impact and accomplishments
- Skills section/-s to pinpoint your full breadth of expertise and talents as a candidate for the QA Manager role
- Education and certifications sections to potentially fill in any gaps in your experience and show your commitment to the industry
What recruiters want to see on your resume:
- Demonstrated experience in developing and implementing comprehensive quality assurance processes and strategies
- Strong knowledge of QA methodologies, tools, and software testing lifecycle
- Proven ability to lead and manage QA teams effectively, including hiring, training, and performance management
- Experience with automated testing frameworks and continuous integration/deployment (CI/CD) pipelines
- Expertise in regulatory compliance and standards within the industry, such as ISO 9001 or industry-specific regulations
Defining your professional expertise in your qa manager resume work experience section
The work experience section, often the most detailed part of your resume , is where you discuss your past roles and achievements. To effectively list your experience, consider these four key tips:
- Align your expertise with the job requirements. It's vital to integrate keywords matching the job criteria to pass initial assessments;
- Show, don’t just tell. Quantify your responsibilities by stating your actual achievements in previous roles;
- Include measurable metrics. For instance, how did your performance impact the annual ROI?
- Highlight crucial industry skills. Mention both technological knowledge and interpersonal skills in this section.
These guidelines will help you craft an impressive qa manager resume work experience section that is bound to catch recruiters' attention.
- Led a team of 15 QA analysts in developing and implementing a comprehensive testing strategy for a new software release, resulting in a 30% decrease in post-launch bug reports.
- Orchestrated the successful transition to automated testing tools, ultimately increasing testing efficiency by 45% while maintaining a 99% test accuracy rate.
- Collaborated with cross-functional teams to integrate quality assurance processes into the early stages of product development, which slashed time-to-market by 20%.
- Revitalized underperforming QA department, increasing defect detection rates by 55% within the first year through the implementation of agile testing methodologies.
- Managed and mentored a team of 20 QA professionals, fostering a culture of continuous improvement that led to a sustained performance increase.
- Negotiated and managed software quality control contracts, saving the company over $200,000 annually while improving service quality.
- Initiated a Quality Management System overhaul that aligned with ISO 9001:2015 standards, drastically improving audit performance.
- Drove the integration of user experience testing into the QA process, which increased customer satisfaction scores by 40%.
- Pioneered a risk-based prioritization framework which reduced critical bug occurrences by 60% during high-volume production phases.
- Spearheaded a multinational QA team in the rollout of a global ERP system, ensuring compliance across 4 continents and 12 time zones.
- Executed a department-wide upskilling initiative to improve QA methodologies, ultimately lifting team performance metrics by an average of 35%.
- Championed a cross-departmental bug tracking system that led to the early detection and resolution of defects.
- Developed a defect containment strategy that saw a 25% reduction in production defects, enhancing overall product reliability.
- Designed and implemented a custom automated testing framework tailored to the company's products, yielding a 100% coverage rate for critical functionalities.
- Conducted comprehensive market research to keep the QA strategies aligned with industry best practices, positioning the company ahead of competitors.
- Managed a distributed QA team through a major version release which doubled the user base from 500,000 to over 1 million.
- Implemented a continuous integration and testing pipeline that cut down release cycles from 4 weeks to 1 week.
- Conducted quarterly QA workshops and seminars, empowering the team with the latest techniques in automated and manual testing procedures.
- Piloted a specialized QA task force addressing high-impact defects, which bolstered system stability by 70%.
- Orchestrated the QA testing efforts for a high-profile merger, ensuring seamless integration of complex systems from both companies.
- Innovated a company-wide quality KPI dashboard providing real-time data to stakeholders, honing the decision-making process.
- Led the quality assurance team in adopting DevOps practices, reducing the software deployment time by 50% while increasing deployment frequency.
- Served as the critical communication liaison between the QA team and senior management, delivering monthly performance reports that highlighted key successes and areas for improvement.
- Negotiated with third-party vendors to incorporate their testing tools into our processes, which enhanced the team's testing capabilities.
Quantifying impact on your resume
- Included percentage improvements in product quality metrics over a specific period.
- Detailed the number of product releases successfully managed and any reduction in release cycle times.
- Quantified the scale of testing efforts by noting the total number of test cases developed and executed.
- Highlighted cost savings achieved through process improvements or tool implementations in dollar figures.
- Described the increase in team productivity or efficiency with a percentage to showcase leadership impact.
- Outlined the growth in the QA team size or skills under management to demonstrate leadership and expansion capability.
- Reported the decrease in customer-reported issues post-release by providing comparative figures.
- Mentioned specific tools or technologies implemented that led to measurable process enhancements.
Action verbs for your qa manager resume
QA Manager resume without experience: a walk-through guide
If you don't happen to have any relevant experience yet, you can substitute this with:
- Short-term gigs and stunts - like month-long internships, that you have done during your university days
- Contract work - be specific about the relevance and outcomes of each role you include
- Resume format that prioritizes your skills - the functional-skill-based format or hybrid format could work
- Research roles - feature those especially prominently if you've participated in a noteworthy project or your role was of utmost importance to the project's success.
Recommended reads:
- How To List Certifications On A Resume (Examples Included)
- How to List Expected Graduation Date on Your Resume
Highlight any significant extracurricular activities that demonstrate valuable skills or leadership.
Shining a light on your qa manager hard skills and soft skills
To win recruiters over, you must really have a breadth of skill set presented and supported within your qa manager resume.
On hiring managers' checklists, you'd initially discover hard or technical skills. Those are the technology (and software) that help you perform on the job. Hard skills are easy to quantify via your education, certificates, and on-the-job success.
Another main criterion recruiters are always assessing your qa manager resume on is soft skills. That is your ability to communicate, adapt, and grow in new environments. Soft skills are a bit harder to measure, as they are gained both thanks to your personal and professional experience.
Showcase you have the ideal skill set for the role by:
- Dedicating both a skills box (for your technical capabilities) and an achievements or strengths section (to detail your personal skills).
- When listing your skills, be specific about your hard skills (name the precise technology you're able to use) and soft skills (aim to always demonstrate what the outcomes were).
- Avoid listing overused cliches in the skills section (e.g. Microsoft Office and Communication), unless they're otherwise specified as prominent for the role.
- Select up to ten skills which should be defined via various sections in your resume skills sidebar (e.g. a technical skills box, industry expertise box with sliders, strengths section with bullets).
Spice up your resume with leading technical and people skills, that'd help you get noticed by recruiters.
Top skills for your qa manager resume:
Test Automation
Software Development Life Cycle (SDLC)
Quality Assurance Methodologies
Test Plan Development
Defect Tracking
Performance Testing
Security Testing
Continuous Integration/Continuous Deployment (CI/CD)
Agile and Scrum Methodologies
Test Case Management
Communication
Problem Solving
Critical Thinking
Team Management
Decision Making
Adaptability
Time Management
Attention to Detail
Influencing Skills
If you happen to have plenty of certificates, select the ones that are most applicable and sought-after across the industry. Organize them by relevance to the role you're applying for.
Listing your education and certifications on your qa manager resume
Don't underestimate the importance of your resume education section . As it may hint at various skills (and experience) that are relevant to the job. When writing your education section:
- Include only higher education degrees with information about the institution and start/end dates
- If you're in the process of obtaining your degree, include your expected graduation date
- Consider leaving off degrees that aren't relevant to the job or industry
- Write a description of your education if it presents you with an opportunity to further showcase your achievements in a more research-focused environment
When describing your certifications on your resume, always consider their relevancy to the role. Use the same format to describe them as you would for your education. If you're wondering what the best certificates out there are for qa manager roles, check out the list below.
The top 5 certifications for your qa manager resume:
- Certified Manager of Quality/Organizational Excellence (CMQ/OE) - American Society for Quality (ASQ)
- Certified Software Quality Engineer (CSQE) - American Society for Quality (ASQ)
- Professional Scrum Master (PSM) - Scrum.org
- Certified Quality Improvement Associate (CQIA) - American Society for Quality (ASQ)
- ISO 9001:2015 Certified Lead Auditor - Exemplar Global or other accredited certification bodies
If you failed to obtain one of the certificates, as listed in the requirements, but decide to include it on your resume, make sure to include a note somewhere that you have the "relevant training, but are planning to re-take the exams". Support this statement with the actual date you're planning to be re-examined. Always be honest on your resume.
- How to List Continuing Education on Your Resume
Should you write a resume summary or an objective?
No need to research social media or ask ChatGPT to find out if the summary or objective is right for your qa manager resume.
- Experienced candidates always tend to go for resume summaries. The summary is a three to five sentence long paragraph that narrates your career highlights and aligns your experience to the role. In it you can add your top skills and career achievements that are most impressive.
- Junior professionals or those making a career change, should write a resume objective. These shouldn't be longer than five sentences and should detail your career goals . Basically, how you see yourself growing in the current position and how would your experience or skill set could help out your potential employers.
Think of both the resume summary and objective as your opportunity to put your best foot forward - from the get go - answering job requirements with skills.
Use the below real-world qa manager professional statements as inspiration for writing your resume summary or objective.
Resume summaries for a qa manager job
- Seasoned QA Manager with over a decade of experience leading cross-functional teams in the fast-paced tech industry. Proficient in Agile methodologies and wield an excellent record of reducing defects by 45% in the previous role. Spearheaded the successful deployment of a quality-driven development strategy in a multinational software firm.
- Veteran quality assurance specialist with 15 years of expertise managing rigorous testing protocols within the pharmaceutical sector. Exceptional command of FDA regulations, ISO standards, and lean manufacturing practices, having driven a 30% increase in compliance adherence for a top-tier pharmaceutical company.
- A dynamic professional poised to transfer 7 years of project management experience in construction into a quality-centric career. Exhibiting an adept understanding of quality control standards and project lifecycle management, compelled by a drive to excel in implementing meticulous QA processes in a technology-focused environment.
- Accomplished financial auditor aiming to pivot to a QA Manager role, armed with 8 years of comprehensive experience in risk analysis and mitigation strategies. With a keen eye for detail and a strong grasp of statistical quality control techniques, ready to ensure superior software product quality and integrity.
- Avid, results-oriented individual eager to dive into a quality assurance management career, bringing forth a robust academic background in software engineering coupled with an unyielding dedication to mastering testing methodologies and quality systems in a challenging new industry.
- Highly motivated graduate with a Bachelor's in Information Technology, enthusiastic about beginning a career journey in QA management. Ready to nurture a profound understanding of automated testing tools and quality improvement processes, aiming to contribute a fresh perspective and vigor in achieving excellence in quality standards.
Other qa manager resume sections to support your expertise and skills
Recruiters are always on the lookout for that qa manager candidate who brings about even more value to the role.
This can be either via their personality or additional accreditations they have across the industry.
Add to your resume any of the four sections that fit your profile:
- Projects for your most impressive, cutting-edge work;
- Awards or recognitions that matter the most;
- Publications further building up your professional portfolio and accreditations;
- Hobbies and interests to feature the literature you read, how you spend your time outside of work, and other personality traits you deem may help you stand out .
Key takeaways
- The layout of your resume should take into consideration your professional background while integrating vital sections and design elements;
- Highlight your most pertinent achievements for the role all through different sections;
- Be very specific when selecting your certifications, hard skills, and soft skills to showcase the best of your talents;
- Include within the top one-third of your qa manager resume a header and summary to help recruiters understand your experience and allocate your contact details. A skills box is optional, but it will help you align your expertise with the role;
- Detail the full extent of your professional experience with specific bullets that focus on tasks, actions, and outcomes.

Looking to build your own QA Manager resume?

- Resume Examples
The 11 tools you should use to create your personal brand
Curating github links on your resume: projects, seniority, and how to guide, how to send a career fair follow-up email [samples included], how to make a resume that stands out in 2024: a guide that stands out, megan's project manager resume got her 2 job offers in 3 months, sam landed a job at spotify with enhancv.
- Create Resume
- Terms of Service
- Privacy Policy
- Cookie Preferences
- Resume Templates
- AI Resume Builder
- Resume Summary Generator
- Resume Formats
- Resume Checker
- Resume Skills
- How to Write a Resume
- Modern Resume Templates
- Simple Resume Templates
- Cover Letter Builder
- Cover Letter Examples
- Cover Letter Templates
- Cover Letter Formats
- How to Write a Cover Letter
- Resume Guides
- Cover Letter Guides
- Job Interview Guides
- Job Interview Questions
- Career Resources
- Meet our customers
- Career resources
- English (UK)
- French (FR)
- German (DE)
- Spanish (ES)
- Swedish (SE)
© 2024 . All rights reserved.
Made with love by people who care.
Resume Builder
- Resume Experts
- Search Jobs
- Search for Talent
- Employer Branding
- Outplacement
- Resume Samples
- Quality Assurance
Manager, Quality Assurance Resume Samples
The guide to resume tailoring.
Guide the recruiter to the conclusion that you are the best candidate for the manager, quality assurance job. It’s actually very simple. Tailor your resume by picking relevant responsibilities from the examples below and then add your accomplishments. This way, you can position yourself in the best way to get hired.
Craft your perfect resume by picking job responsibilities written by professional recruiters
Pick from the thousands of curated job responsibilities used by the leading companies, tailor your resume & cover letter with wording that best fits for each job you apply.
Create a Resume in Minutes with Professional Resume Templates
- Enhancing test quality and effectiveness by working with the Development and Program Management to prevent problems and ensure that products can be tested effectively. It is strongly recommended that the TM ensures Defect Management life cycle adherence and preparation of “fix packages” to development as it allows the TM to have some form of control over development
- Develops tools, implements, manages and monitors performance of the Quality Assurance program to assure that food safety, plant cleanliness and plant conditions are maintained to Starbucks standards. Reviews performance to standards utilizing audits, pests control reports and health department inspection reports to track progress and to identify improvement opportunities and priorities. Provides summary assessments and recommendations to Operations leadership teams regarding compliance with food safety, cleanliness and store condition standards. Works with operations leaders to focus on key issues
- Develops tools, implements, manages and monitors performance of the Retail Quality Assurance program to assure that food safety, store cleanliness and store conditions are maintained to Starbucks standards. Reviews performance to standards utilizing audits, pests control reports and health department inspection reports to track progress and to identify improvement opportunities and priorities. Provides summary assessments and recommendations to Operations leadership teams regarding compliance with food safety, cleanliness and store condition standards. Works with Operations leaders to focus on key issues
- Provides technical support and acts in a liaison role with the Quality Control function at Blue Line Food Service Distribution to develop and insure controls are developed, executed and maintained. Ensures all USDA and FDA regulations are communicated and implemented
- Serves as an internal Performance Improvement consultant to the medical center departments and the medical staff by staying appraised of the most current PI practices and standards. Provide consultation to departments and multidisciplinary teams; facilitate PI teams including the Failure Mode and Effects Analysis Team. Work closely with teams and physician leaders to ensure quality processes are followed and quality tools are utilized in activities
- Coordinates the receiving and testing of product samples from all distribution centers as per set schedule. Coordinates sanitation audits of distribution centers and follows up on corrective actions for deficiencies
- Support the Medical Staff Quality Committee and peer review activities. Collaborate with chair for meeting agenda and follow-up. Perform chart review activities to support peer review and other Committee activities. Identifies issues and develop action plans
- Provides work leadership to more junior level staff doing the same type of work; may assist in training and supervising work of more junior level staff
- Provides day-to-day supervision and direction to the QA team, including performance management and career development
- Perform annual performance appraisals for all direct reports and develop key personnel through coaching, training, and organizational development
- Work with company management as well as the CAPA program manager to resolve incidents, develop corrective actions and monitor the effectiveness of resolutions
- Develops reports and provides more senior management with metric data for assigned area(s) of responsibility processing and service performance
- Perform managerial responsibilities, for example, hire/fire, disciplinary actions, performance reviews, and professional development
- Prepare presentations for management review and routinely monitor performance to drive improvements
- Coordinate and/or perform training for plant personnel, including new hire and annual refresher training
- Maintain safe working conditions for employees and compliance with all state and federal regulations regarding health and safety issues
- Develops, organizes, and administers training for plant employees on GMP's, SQF, and food safety preventive control related topics
- Work with plant employees on Good Manufacturing, Sanitation Practices, and Safety
- Lead plant sanitation program in effectiveness with rigorous adherence, generation of SOP's and standard work
- Coordinate plant compliance with company Records Management policies
- Coaches and partners with direct reports to effectively manage performance. Communicates company and cascading goals and assists in establishing goals that align with department and company goals. Provides on-going performance feedback following DFA FMIC Performance Management process. Actively works with direct reports to cultivate capabilities and utilize strengths
- An accountable leader who is detail and quality oriented with a solid understanding of quality principles, methods, and procedures
- An accountable team player who is detail and quality oriented with an in-depth understanding of quality assurance principles, systems and procedures
- Strong knowledge of quality assurance methodologies and strategies
- Experience in solid dose manufacturing is strongly desirable
- Strong attention to detail and proven ability to meet deadlines
- Ability to gain the respect of the development teams on the basis of technical knowledge, hands-on style and decision-making ability on technical issues
- Ability to project and maintain a professional and positive attitude
- Ability to utilize knowledge and interpersonal skills to provide leadership, direction and development of others
- Good organizational skills and the ability to handle multiple activities with changing priorities simultaneously
- Ability to utilize knowledge and interpersonal skills to provide leadership, direction and development cross-functionally
15 Manager, Quality Assurance resume templates

Read our complete resume writing guides
How to tailor your resume, how to make a resume, how to mention achievements, work experience in resume, 50+ skills to put on a resume, how and why put hobbies, top 22 fonts for your resume, 50 best resume tips, 200+ action words to use, internship resume, killer resume summary, write a resume objective, what to put on a resume, how long should a resume be, the best resume format, how to list education, cv vs. resume: the difference, include contact information, resume format pdf vs word, how to write a student resume, manager, quality assurance & control resume examples & samples.
- Lead a team of risk specialists to execute with excellence in alignment with the standards of the Internal Control guiding principle: to be a valued business partner, executing on effective risk mitigation controls while earning and maintaining the confidence of our business partners, customers and regulators
- Ongoing development of self and team to ensure strong understanding of TDAF business and associated inherent risks
- Design, implement and maintain risk management and risk assessment programs such as Quality Assurance / Quality Control (QA/QC) key control test plans, Quality Monitoring scorecards and protocols, and Manager’s Handbook
- Facilitate execution of annual process Risk and Control Self Assessment (pRCSA) exercises across all TDAF business lines
- Assist with the development of processes to challenge control design effectiveness and execute re-performance testing
- Act as a subject matter expert for the identification and assessment of risks and corresponding controls
- Draw conclusions from business results and identify recommendations which will contribute to the overall management of Operational Risk within TDAF
- Develop and deliver programs to raise awareness and understanding of operational risk, key risk issues and management processes and work with operating business management to enhance risk management practices and processes
- Execute operational risk measurement methodology and risk-reporting consistent with firm-wide standards, including Key Risk Indicators (KRI), control gap / remediation plan tracking, and associated reporting
- Act as TDAF Privacy Designate
- Participate in review of policies and procedures to ensure consistency with standard firm-wide operational risk policies, harmonized standards and procedures
- Support operating business’s resolution of internal and external Auditor’s findings including GAML, OSFI, FCO, LCM, FCAC and all others as necessary
- Proven strong leadership skills in alignment with TD Leadership Profile
- Resourceful, self-starter; ability to execute with minimal supervision
- Strong knowledge of TD organizational structure and proven ability to leverage internal stakeholder groups
- Strong presentation skills, both verbal and written
- Strong influencing skills and the ability to work in teams
- Robust organizational skills with the ability to work in a fast paced environment and manage multiple deadlines and priorities, and proven ability to manage through change
Manager Quality Assurance, Rise Program Resume Examples & Samples
- Accountable for planning, co-ordination and execution of Testing for a large/complex business driven initiative
- Accountable to build and maintain detailed project plans for Testing deliverables
- Prioritize and manage workload while ensuring high quality results that confirm to overall project timelines. Where needed you are able to work with Project Managers to identify changes in plan
- Regularly document progress and communicate progress across the program team to ensure stakeholders are informed of any dependency changes. Ensure both partners and yourself are aware of other items that impact deliverables
- Work closely with internal sources of credit risk data while leveraging widely accepted data mining languages/tools
- Familiar with PMLC and SDLC methodologies, in particular the leadership and execution of Test phases
- Develop a sufficient understanding of ERM and the business aspects of the RISE Program to effectively identify issues and dependencies
- Experience using relational databases within various data storage environments (ie. SQL, PC/SAS, Mainframe)
- Strength and breadth in utilizing industry accepted business intelligence and reporting tools (ie. Cognos, Business Objects etc)
- Familiar with industry practices related to the monitoring of key risk indicators, adjudication and credit portfolio management would be beneficial
- Experience documenting process maps, ERD and DFD while leveraging six sigma practices to redesign business oriented processes
- Ability to self-regulate conflicting priorities and schedules to ensure expectations are managed accordingly
- Excellent facilitation, verbal and written communication skills with attention to detail and the ability to communicate with wide variety of audiences and levels
- Demonstrates ownership, responsibility and personal commitment to meet challenging objectives
- An enthusiastic team player who enjoys sharing their knowledge, experience and ideas, and is open to the ideas of others
- Adept at reaching out to new teams/contacts and building strong mutually beneficial relationships
- Strong problem-solving skills and the ability to proactively identify risks/issues and recommend solutions
- The ability to challenge and to influence change
- Specialized technical knowledge of data governance concepts / theories and metrics
- Knowledge of management and/or credit risk reporting
- Superior multi-tasking skills and the ability to work in a fast-paced, often deadline-oriented and dynamic environment
- High degree of personal initiative
Project Manager, Quality Assurance Resume Examples & Samples
- Lead a team of professional auditors throughout all audit phases. Execute audit activities
- Identify competent and sufficient resources, with relevant skill sets required to staff each assignment
- Lead and manage staff assigned to the engagement; manage all key milestones including reporting to IAS management and key stakeholders; review audit work for sufficient coverage and adherence to Internal Audit standards; provide performance feedback to the assigned staff
- Deliver in-house training in the areas of specific expertise within the Project Management discipline, Emerging areas, as well as the related risk management frameworks, compliance and audit
- Works Strategically – seek opportunities for sustainable growth beyond existing boundaries through innovation and collaboration
- Support a High Performance Culture – support change and continuous renewal
- Lead by example in promoting teamwork across IAS
Manager, Quality Assurance Resume Examples & Samples
- Lead a team of QA analysts to deliver high-quality video playback software to multiple platforms across a wide array of languages and environments, including web, iOS, Android, and connected devices
- Effectively prioritize and delivery of quality testing on time, achieving department and business goals
- Explore, recommend and implement automation solutions to optimize testing efforts
- Work with other internal QA teams to define clear responsibilities and hand-off
- Develop team members, guiding their work efforts as well as growing their skillsets and helping them achieve their career goals
- Design and implement best practices and procedures for quality assurance, including project structure, process improvements, documentation, code management, and deployment methodologies
- Work with internal product leaders, business leaders, partners and technology stakeholders to ensure QA goals and procedures meet business requirements
- Take ownership of QA for third-party vendor products delivered into the group
- Bachelor’s degree in Software Development, Quality Assurance, Technology Management, or equivalent experience required
- Expertise in software Quality Assurance practices, including test script generation, client-side and server-side automation, load testing, and deployment management
- 2-3 years experience managing a Quality Assurance team
- Exposure to multiple delivery platforms, including web, iOS, Android, and connected devices
- Expertise in the SDLC process, versioning, code management, and deployment methodologies
- Interested candidates must submit a resume/CV through nbcunicareers.com to be considered
- Must be willing to work at Englewood Cliffs, NJ
- Must be willing submit to a background investigation
- Must have unrestricted work authorization to work in the United States
- Excellent written and oral communication skills and effective interpersonal skills
- Proven leadership abilities
- Experience in Television/Broadcast, Media, or related field
- Experience with interactive media, mobile design & app development
Assistant Manager, Quality Assurance Resume Examples & Samples
- Work with Blizzard executive, development, international, and QA teams
- Work with department leaders to ensure QA teams have staffing, training, structure, and processes to meet product testing demands
- Keep current with product risks, releases, and business goals while strategically managing project risks for the test team
- Mentor QA personnel to facilitate their growth within the department and company– provide coaching, actionable feedback, development plan guidance, and disciplinary action as necessary
- Interview, hire, and train world-class QA personnel
- Assist in the development and implementation of procedures for more effective and efficient testing of company products
- Contribute to a positive work environment
- Complete all tasks and duties as assigned, on time and with high quality
Senior Test Manager, Quality Assurance Resume Examples & Samples
- Manage and coach experienced test leadership
- Review test effectiveness across the test organization and drive improvements to Blizzard’s test policy and strategy
- An influential leader that drives collaborative commitment to quality across Blizzard
- Effective management of the cost of quality while maintaining or increasing quality output
- Oversee and coordinate all cross team test planning, execution, control, and closure phases for the global release
- Aggregate global test plans into a cohesive test schedule and maintain it through all test phases
- Work with external vendors to enhance test coverage and capability
- Manage the consistency and effectiveness of the test leadership team’s KPIs
- Justify and assist in acquisition of key resource allocation including test capability and environments
- Develop and maintain outstanding business relationships with all test stakeholders
- Create and control ROI metrics on automation tools and associated testware
- Keep all teams tools and testware up to date and within service standards
- A description of a recent project that you worked on; it should be
Test Manager, Quality Assurance Resume Examples & Samples
- An influential leader that drives collaborative commitment to quality across Blizzard Entertainment
- An experienced expert and coach in both established and developing test techniques
- Deliver executive-worthy “State of the product” presentations at each test phase
- Utilize department process improvement models to improve the test team’s capabilities and standards
- Manage and lead a team of Software Quality Engineers. Management includes resource management for personnel & hardware, project scoping, tracking
- Participate in creating master test plan and specifications
- Experience with agile development methodologies
- Familiarity with tools such as JIRA , Maven/Ant, Jenkins/Bamboo/TeamCity
Manager Quality Assurance / Post Loan Review Resume Examples & Samples
- Completion of approximately 2700 post loan reviews/quality assurance reviews annually
- Work with CAG team and Enhanced Performance Management process to provide feedback to Directors/ EPM group as this feedback is the check on productivity and may impact STI per CAG employee each quarter
- This role will form part of an arms-length quality assurance team that will be designed to be separate from CAG and independent of the team
- Define, develop and maintain quality assurance/control processes, guidelines and SLAs to ensure standards are met across all applications and services. Drives best practices throughout the QA team and ensures consistency across projects. Ensures all team members are following the defined SDLC process. Understand business model and customer needs and apply/modify QA techniques accordingly
- Identify, develop and execute upon opportunities for process and strategic improvements. Participate in MHE-wide initiatives to bring consistency to the company's QA efforts. Ensure focus on both Quality Assurance and Quality Control improvements. Ensure team members are aligned and executing upon strategic QA efforts
- Manage internal and/or external QA Analysts team members and ensure they maintain quality standards. Mentor new or less seasoned Quality Analysts. Hire necessary staff to support department efforts. Applies continues improvement strategies to ensure team competency growth
- Ensure all projects teams are appropriately staffed to meet commitments and delivery deadlines. Work in conjunction with Product Manager, PM, Engineering Lead to review project status on a regular basis and ensure teams are operating optimally. Identify, develop and execute upon operational changes to make teams more effective. Encourages team agility, improves time to market while increasing customer satisfaction
- Stay current on industry trends and standards; conceive and present to senior management and peers ways to improve current practices to stay competitive in the marketplace and on the cutting edge while maintaining strict quality standards
- Ability to communicate directly with customers to assist with implementation issues or to develop custom integration of applications
- Provides effective communication regarding issues, objectives, initiatives and performance to plan
- Gain and maintain awareness of DPG product development cycles
- Maintain high level of quality in all duties assigned
- Develop and manage a working relationship with several outside quality assurance vendors
- Assists with trade-off analysis on technical decisions and advises on the appropriate selection of optimal standards, methods, tools and applications, and helps to make correct choices from alternatives
- The position will analyze metrics for recurring issues and make recommendation for solutions
- The position will be able to analyze software gaps and make recommendations to improve overall software quality
- Prioritize own work and that of direct reports to accommodate ambitious timelines. Also requires coordination with other functional leads to resolve project interdependencies and prioritize deliverables to meet the overall timeline
- Bachelor’s degree in Computer Science or equivalent experience in software engineering or related field
- Minimum of six years of software quality assurance experience in all phases of life cycle, including requirements/user story analysis, test planning, estimation, scheduling, testing, defect tracking, and reporting
- Minimum of three years in lead or management position
- Programming background strongly preferred
- Strong understanding of different software development life cycles (agile, waterfall) and contemporary software quality assurance processes and automated tools
- Expert understanding of web-delivered applications, web services and n-tier software testing
- Strong experience testing web-delivered applications across multiple browsers and platforms
- Experience with responsive design testing and mobile testing strategies
- Must have demonstrated experience in the following technologies: HTML, CSS, JavaScript, AJAX, JSON and Java. Ruby, PHP, Tomcat and Apache will be considered a strong plus
- Must have demonstrated experience in the following or similar testing tools: JIRA, Quality Center
- Strong experience with automation solutions such as Selenium, Cucumber, Gherkin or similar automated testing tools/frameworks
- Experience with DynaTrace, SumoLogic or similar tools will be a strong plus
- Excellent communication and inter personal skills with ability to communicate with both technical and non-technical audiences. Able to present and communicate own ideas, debate and defend under scrutiny
Associate Manager, Quality Assurance Resume Examples & Samples
- Lead the Multi-Platform engineering QA team
- Responsible for the strategy, management, and direction of the Quality Assurance organization which includes Functional, Non-Functional, System and Security Test
- Directs all aspects of Quality Assurance, using a well-grounded understanding of the Software Development Lifecycle (SDLC) and proven Software Quality Assurance processes and metrics
- Functions as an integral member of the Development team
- Lead the development of QA test plans and testing schedules
- Lead acceptance testing of new or existing products
- Ensure departmental financial and operational goals are met
- Manage and communicate QA schedules at regular intervals, highlight risks to the executive team, and alert leadership of any QA scheduling conflicts
- Ensures final products meet quality and performance standards
- Participate in sprint planning sessions and ensure team has adequate tasks and insists team to track time to perform data analysis such as planned vs actuals
- A minimum of 5 years of Quality and product release experience
- Demonstrated strong leadership experience
- A minimum of 1 year of managing QA staff
- Metrics and leading indicators experience, collection, analysis, and decision making
- Demonstrated thought leadership, continually striving to improve, become more efficient, and more effective
- Proven ability to work in a dynamic environment interacting with multiple dependent QA efforts across multiple work streams where priorities shift to respond to changing business needs
Manager Quality Assurance & Support Resume Examples & Samples
- Manage the UAT process by analyzing user requirements, participate in design sessions, preparing UAT strategies, plans and completion reports. Preparing, documenting and executing detailed UAT functions/cases and ensuring anticipated results are achieved. Raise and manage defects, working with business users, project team and developers to ensure that defects are investigated, resolved and retested prior to implementation
- Manage the coordination of day to day testing efforts for assigned application systems and project testing efforts with other testing personnel, end users, technical support and development areas
- Participate in work sessions to develop external design documents to gain a full understanding of a project and to ensure requirements are adequately documented to allow for their efficient translation to test materials
- Provide ongoing direction, human resources administration, coaching and support to motivate individual and team performance in a stable, professional environment while ensuring department objectives are met. Evaluate the performance of immediate subordinates through daily monitoring, regular staff meetings, quarterly reviews of objectives vs. accomplishments and annual performance appraisals
- A high level understanding of the core HR systems and their interfaces to HRIS
- Agile knowledge and test management experience in a SAS environment
- The ability to manage large volumes of work and deliver against timelines
- Hands on testing experience with data conversion projects, HR applications experience is an asset
- Excellent communication skills with a natural ability to articulate business need to technical solutions
- Complete understanding of the relationship between complex I.T. banking systems, their interfacing applications, environmental resources and code layering across multiple platforms; this seasoned and specialized knowledge is acquired through hands-on experience with the business and technical dimensions of multifaceted projects
- Excellent communication and leadership skills to address issues with QA Testing and other department staff who have varying degrees of experience and technological knowledge
- Expert understanding of UAT standards and methodology
- Strong analytical, forward thinking, decision making and organizational skills to coordinate both complex code delivery schedules and the allocation of limited resources and to resolve conflicts for multiple projects
- 5 years in Financial Institution or other sectors heavily regulated
- 5 years Quality Assurance testing experience preferably in HR role
AML Manager, Quality Assurance Resume Examples & Samples
- Assume primary accountability for all Quality Assurance strategy planning
- People manage direct reports responsible for quality assurance and/or assist with production defect tracking, monitoring and defect resolution testing
- Maintain test environments, and assist Business analysts and production support analysts with scheduling, defect tracking, documentation, metrics, end-user reporting etc
- Assist business analysts with test case quality; ensure appropriate level of test coverage and requirements/test case coverage
- Assist with production support issues by acting as an escalation point for Production support analysts
- Liaise with technology to ensure close partnership for end to end testing, exit criteria oversight, and production acceptance testing standards are maintained
- Assist with production support issue/defect tracking severity, triage and stakeholder communication
Manager Quality Assurance Resume Examples & Samples
- Implement the Enterprise Information Management Quality Assurance program
- Coordinate and implement complex code delivery schedules to test environments and identify conflicts between requirements for several projects
- Interpret complex environmental requirements and technical jargon in discussions with technical support staff and convey recommendation for optimum strategic approaches to code delivery
- Ensure the sequential promotion of code does not adversely impact testing/Production environments by providing recommendation for code delivery at meetings of code distribution
- Contribute to the efficient and effectiveness of code management by ensuring documented procedures and standards for code delivery are followed by all affected departments
- Develops quality assurance plans by conducting risk analyses; identifying critical control points and preventive measures; establishing critical limits, monitoring procedures, corrective actions, and verification procedures; monitoring
- Operational Management
- Liaise with other departments involved in the code management/control process to provide detailed technical information about code configuration requirements, communication technical instructions relating to code and data movement and problem escalation/resolution
- Accomplishes quality assurance human resource objectives by recruiting, selecting, orienting, training, assigning, scheduling, coaching, counseling, and disciplining QA employees; communicating job expectations; planning, monitoring, appraising, and reviewing job contributions; planning and reviewing compensation actions; enforcing policies and procedures
- Understanding of a data warehouse environment
- Working knowledge of DB2, Informatica, Business Objects
- Working knowledge of testing methodology and process
- Experienced in managing a distributed Quality Assurance Team
- Strong desire to support people grow, to build a reliable team through mentoring and coaching, believer in long-term career and personal development of the QA Engineers as the key to success
- Very experienced with testing approaches – black, white, grey box, regression, scenario-based, model-based, and more
- Ability to work cross functionally with people to understand and propose quality needs, to capture information in quality requirements, and to help people build a product with that understanding
- Professional certification is a plus in the fields of QA, Project Management or Business
- Establish and sustain the newly established testing centre of excellence, including governance, tools, processes and methodologies
- Lead the work of the in-house QA team - full-time and contract
- Oversee outsourcing engagements with testing partners used to cover project peaks and specialized test work
- Participate in the discovery phase of projects to define test effort
- Prepare test strategy and planning deliverables
- Develop detailed test plans and scripts; report on test execution
- Provide estimates for testing streams of projects
- Schedule QA resources across projects
- Manage QA budgets across multiple projects
- Participate in test execution as needed
- Manage test tool set and environment usage
- Model leadership behaviours that promote a culture of open communication, employee development and trust
- Maintain all Health & Safety practices to code
- Manage expenses within approved budget
- Five years’ experience testing IT applications
- Experience in leading test activities, including test planning and reporting
- Experience with testing in both waterfall and agile development environments preferred
- Experience with test tools such as Soap UI, XML Spy, Jira, Test Rail, MS project, SQL Server Queries
- Experience with testing commercial-off-the-shelf (COTS) software as well as custom builds strongly preferred
- Experience of working with and managing the relationship with 3rd party vendors and managing outsourced resources
- Experience providing support and guidance to UAT test efforts
- Retail or supply chain experience strongly preferred; solid understanding of the retail value chain
- Inspire with Service
- Lead with Passion
- Own it with Pride
- Earn lasting Relationships
- 5 or more years of management expereince
- A proven track record of launching quality consumer products
- Load and volume testing experience with mobile devices and multi-tier web application architecture
- Strong analytical skills in assessing user, functional, and technical requirements and identifying high risk key test areas
- Strong technical competency and experience with web applications and platforms, web services, .NET, XML, Ajax, JavaScript, TCP/IP, HTTP, HTML, REST, SOAP, SOA, Eclipse, SQL, and RDBMS technology
- Proven track record delivering software using methodologies, ideally in a continuous integration and delivery environment (Agile, iterative and waterfall a plus, not a requirement)
- Solid experience with testing global, high scale, customer centric web sites, online retail or other transactional website experience
- Excellent influencing skills, organizational skills and the ability to achieve results within tight deadlines
- Plan, schedule, organize, design, and execute multiple concurrent projects
- Must have strong MS Project and Excel skills (charts, pivot tables, formulas)
- Drive a quality strategy focused not only on defect detection but also prevention
- Work with offshore professional service providers to optimizes the use of offshore resources for achieving project goals
- Demonstrate test management processes and technical skills, including an understanding of manual, automated and performance test planning and execution related tools (8 years)
- Knowledge of application software development lifecycle concepts, lead and agile best practices, environment and configuration management, as well as test management methodologies and practice (8 years)
- Ability to communicate clearly and concisely, both orally and in writing
- Exceptional ability to manage and delegate numerous concurrent projects, activities, and tasks under time constraints
- Implement process quality control systems throughout supply chain
- Implement statistical sampling measures as required for all accessories and raw materials
- Implement and manage performance KPIs with suppliers
- Manage team of QC personnel (Training, development, day to day assignments)
- Coordinate new product sampling process with suppliers & development team
- B.S./B.A. in relevant business area
- Minimum 7 to 10 years’ experience managing quality (Same or similar industry and manufacturing environment)
- 3-5 years in a supervisory-manager capacity
- Experience working with Statistical Process Controls tools
- Experience working with quality sampling processes (AQL)
- Basic understanding of Lean Manufacturing principles
- Ability to communicate in English and Chinese
- Above average data management skills (Excel- Power point)
- Basic computer skills (MS Windows or equivalent)
- Knowledge of golf bag, headwear fabrication and processing is desired
- Ability to play leadership role in a cross functional team environment (communicate clearly and hold others accountable for deliverables)
- Conflict Management
- Ability to effectively present data/ proposals to various levels of Management
- Ability to collaborate with suppliers drafting win-win systems that yield target quality levels while helping suppliers maintain a cost effective operation
- Bachelor’s degree in Computer Science or equivalent experience in software engineering, quality engineering or related field
- Programming background preferred
- Experience in the following technologies: HTML, CSS, JavaScript, JSON, Java, Ruby, PHP, Tomcat and/or Apache will be considered a strong plus
Manager Quality Assurance & Process Resume Examples & Samples
- Assists with data collection and analysis to support projects
- Develops quality assurance plans by conducting hazard analyses; identifying critical control points and preventive
- Bachelor’s degree from a four year college or university in Engineering, Business Administration or related field
- 5-7 years of day-to-day operations of a theme park, manufacturing or related business required
- 3-5 years of operations management/team leadership experience; or equivalent combination of education and
- Have a good working knowledge of all aspects pertaining to mechanical, schematics, technical writing skills, and
- Demonstrated ability to work with all levels of employees and management, and willingness to give assistance to coworkers
- Stay current on industry trends and standards; find ways to improve current practices to stay competitive in the marketplace and on the cutting edge while maintaining strict quality standards
- Hire, train and manage a team of skilled Quality Engineers
- Architect and implement an automated testing strategy
- Organize and record detailed test results, provide reports and general visibility of results and project/task status, work with development teams through the design and test stages to ensure strict adherence to business requirements and to improve upon new and existing features where applicable, and log detailed and accurate defect reports and follow the defects through to resolution and closure
- Ten or more years of experience with software quality engineering required
- Four or more years of QA management experience
- Prior Behavioral Driven Development (BDD) or Test Driven Development (TDD) background that is competent in test script/framework automation using open source tools and languages (Java, Perl, Ruby, Python, etc.)
General Manager Quality Assurance Resume Examples & Samples
- Manage and enhance the quality function in full responsiveness to business needs of CPS while striving to ensure the company’s policies and procedures are enforced and adhered to, for concentrates and beverage bases manufacturing to achieve highest quality products
- Ensure full compliances with TCCC safety and environment and food safety policies as well as with central and local government regulatory requirements pertaining to safety, food safety and environment
- Lead CPS India in implementation and compliance of QSE/ ISO systems and ensure associates capability enhancement to adapt to the complexity in operation
- Set the BP targets and track, measure and analyse plant KPIs for QSE and ensure corrective and preventive actions are taken to meet the BP targets
- Strategy : Set the strategic direction and annual BP for QSE function within CPS India aligning with CPS Asia/Global QSE
- Develop QSE Goals with business needs. Design and develop plant strategies to achieve BP targets for QSE goals and objectives for CPS India, working closely with CPS Asia QSE
- Lead the development and execution of the strategy to create and lead the processes, operating models, designs to enable our System for effective implementation of the quality, food safety and EOSH objectives. Review and analyze the effectiveness of the QSE system through tracking of the key performance indicators and develop plans for improvement. Ensure TDQ, PDR, LTSIR, TIR, water usage ratio, energy usage ratio BP targets are achieved
- Provide support and leadership to achieve the BP targets for MUV, OH per part, parts per FTE, value creation targets and new product on time introduction
- Governance & Metrics for process, productivity and quality improvement
- Guide and direct development of programs and procedures for defect free manufacturing and governance on adherence. Implement consistent value and quality driven management routines in the plant, assess and increase processes quality capability to meet TDQ/PDR targets
- People & Infrastructure Capability Management
- Participate in PDF process for CPS India associates, especially for QSE associates, as a member of Plant LT
- Support to people in QSE team to develop and improve self in their capabilities, leadership and competences, working closely with CPS Asia QSE
- Review and develop Lab capability and Infrastructure to align with Global Lab strategy
- Review and develop CPS India QSE CAPEX plan aligning with Asia & Global QSE strategies
- Associates capability development through planning, scheduling of training and regular updates on new policies and changing requirements
- Implementation of Governance and Program Management
- Implements new test procedure, test equipment’s by proper investigations and validating alternate proposals and recommendations
- Review of critical control points and ensuring these are effectively controlled in manufacturing process
- Lead a cross functional teams which includes Production, supply chain, procurement, QA,HR, Finance, Admin to implement the system and EOSH requirements (ISO 9001, ISO 14001,ISO 18001,FSSC 22000,ISO/IEC 17025 and KORE). Ensuring all the certifications/audits planned for the year are achieved
- Should be well versed with SAP-QM module and Laboratory management systems. Maintain Master data and BOM with 100% accuracy. Ensure usage of active formulation & COSD as per database
- Design development of program for protecting the confidential information in the Plant. Accountable for protecting the master formula, formula notes and bill of material as per corporate policies
- Act as custodian of safety Heath and Environment management system and implement and maintain all the related policies and systems
- Obtaining consents as required by regulatory authorities and ensure compliance to consent conditions as required by regulations
- Brainstorm ideas and collaborate with CPS Asia/other CPS plants/certification agencies on implementation of requirements effectively. Share best practices for implementation of SMS/EMS across the system
- Responsible for developing protocol for validation new methods, technology and process in manufacturing. Should have knowledge of chemistry, food science and their application to the beverage industry, advanced analytical instruments, statistical process control, method validations, measurement of uncertainty and data interpretation etc
- Responsible for ensuring products manufactured and shipped is “fit for use” and meeting company’s and FSSAI requirements. Continuously monitor requirements with FSSAI regulations related to quality and food safety and ensure that plant is in compliance with all requirements. Ensuring compliance of the operation with the requirement of FSSAI, by filing all the returns, license renewal as well as compliance of the ingredients and manufacturing process with regulation
- Leading and motivating the team in exhibiting best in class GMP. Brainstorm ideas and collaborate with Corporate/other CPS plants/certification agencies on implementation of Best in class practices
- Continue to implement always-audit-ready-culture and practices. Implement standardized CAPA program and internal audit program
- Ensure authentic data and on time submission of returns, reports and other required information
- Ensure Compliances and drive Continuous Improvement
- Conduct review of scientific or other literature in order to maintain and improve QA function knowledge/skills
- Ensure ingredients, merchandises and packaging materials are in compliance to the company and local regulatory requirements
- Ensure compliance against all the safety , Health and Environment management system and related local regulatory compliances
- Review of new laws and regulations, conduct gap analysis and comply with new requirements
- Monitor to ensure 100% compliance to existing regulatory requirements
- Responsible for communications between Central and State pollution control board, Corporate/Asia, Division, Certification bodies related to systems
- Facilitate replication of best practices and adoption of standard through a defined communication programs with outside stakeholders
- Lead and implement BBS and Zero Accident Culture in the operation
- Monitor trends of accident frequency and severity rates
- Develop and implement permanent fix through improvement in documentation, processes and equipment
- Promote EMS/SMS Culture through competition, campaign, health programs, factory visit, environment day/safety week celebrations, etc
- Others as Risk Management
- Pro-actively work with stakeholders to identify and mitigate risk through process definition and various risk reduction methodologies
- Manage significant India supply (IMCR) issues, as required, thru Risk Management & Business Continuity Plan
- 3 direct reports – QA Lab Control Head, Process Control Head & System/Audit Head
- Degree in a scientific discipline is required
- Postgraduate qualification in Science (Chemistry, Food Science, and Pharmaceutical Science) is preferred. May be substituted with relevant wok experience
- Manage QC team to ensure right the first time product
- Execution of assigned tasks related to management initiatives
- Analyze risk/opportunity of QC improvements
- Develop and manage internal auto QC solutions
- Foster cooperation with business units including DCVI, LTS, MpOps, TV, Media Distribution to reduce spend on QC through quality planning
- Remove redundant QC steps while seeking out automated solutions
- Onboard/consolidate Technical specifications/requirements and QC checklists
- Optimize workflows and devise and analyze key performance indicators for managed activities (to be reported on in monthly Business Management Meetings – ISO9001)
- Prepare data supported reports and business process recommendations to senior management
- Perform ongoing root-cause analysis
- Make presentations that influence, persuade and educate senior management in worldwide customer groups in order to define, introduce and uphold business process improvements
- Drive improvements with vendor partners to uphold quality while driving down costs
- Seek opportunities across the Walt Disney Company to further leverage QA practices in order to decrease duplicate efforts and associated costs and discrepancies
- 3-4 years in management film, audio, and video post production
- Previous experience interacting closely with creative marketing groups
- Determines through investigation a detailed working knowledge of the business’ operation
- Demonstrates a thorough understanding of project management tools and techniques
- Establishes deadlines and delegates tasks for a project
- Monitors tasks and assignments to ensure accurate and timely completion
- Coordinated the work of all resources to achieve maximum productivity
- Identifies and shares ideas, innovations and best practices that can benefit others
- Acknowledges and builds on the ideas of others
- Supports team decisions to people outside of the team
- Plays multiple roles on a team as needed
- Ensure that all testing is planned, executed and delivered in accordance with industry best practices, Pramerica and Fixed Income Systems standards
- Manage an international team
- Understand and follow the complete software development life cycle methodology (.ie Waterfall, Agile)
- Build and maintain positive relationships with customers and co-workers
- Possess excellent oral and written communication skills
- Have the ability to manage multiple tasks and projects simultaneously
- Possess the ability to associate past experience with current events in order to resolve problems or generate new ideas
- Develop and execute test scenarios to ensure the stability and performance of applications
- Minimum of 7 years of experience
- Define and co-ordinate the overall QA strategy
- Understand and follow the complete software development lifecycle methodology (.ie Agile/Waterfall)Manage resources and environments needed to support the testing effort
- Ability to analyze complex customer requirements and design and execute appropriate test solutions
- Communicate QA testing status on a regular basis to relevant stakeholders
- Ensure that all stakeholder queries or issues are followed up and responded to in a timely manner
- Ensure that all defects raised during testing are appropriately tracked to closure
- Ensure that all QA risks are escalated and mitigated appropriately
- Work with the U.S and Ireland teams to ensure QA projects are delivered in line with agreed scope, quality, budget and timelines
- Be aware of all production issues and work with the relevant stakeholders to ensure that production issues are analyzed and appropriate action is taken
- Provides guidance to the Quality Assurance and Product and Development teams in identifying product and technical requirements. Serves as primary point of contact between the Quality Assurance team and Product and Development teams. -Works with Program management team as well
- Collaborates with various departments to ensure timely delivery of all documents required to support testing
- Ensures product problems are properly identified and prioritized in the formal defect tracking system and change control processes to determine release-readiness
- Coordinates internal and external software testers and facilities. Works with vendors to troubleshoot problems when necessary
- Participates in product teams and the software development life cycle process
- Consistent exercise of independent judgment and discretion in matters of significance
Consulting Manager Quality Assurance Resume Examples & Samples
- Responsible for developing, maintaining, and enhancing QA standards and procedures within the organization
- Providing technical leadership, support and training to the QA staff
- Assisting the head of Operations in reviewing and evaluating the work of the staff
- Working with the development team to define QA timelines and project schedules
- Providing detailed documentation around issues encountered and working closely with development staff to determine and document appropriate resolutions
- Working with the other members of the quality assurance team to review and improve existing systems/quality processes
- Providing comprehensive consulting to business partners and IT management with respect to QA and implementation issues
- Strong analytical and problem solving skills with 10+ years proven experience in quality assurance and testing function
- Looks at work strategically not task oriented
- Applies leadership skills beyond defined role - across the organization
- Familiarity and experience with SQL, relational databases, and defect management
- Knowledge of, or experience with automated testing tools and financial concepts (equity markets, trading concepts, financial instruments, derivatives, hedge funds) a plus
- Manage, train and develop test engineering staff members under the selected candidate's direct supervision
- Owner of testing efforts for the delivery of new products into sales, provisioning, service delivery, and billing applications
- Create, execute, and manage test strategies, test plans, re-usable test scenarios and champion automation efforts
- Direct defect management and triage functions, working cross-functionally to resolve issues and escalating to leadership as necessary
- Work within the global delivery model, leading a team of on-shore, near-shore and off-shore resources
- Produce and maintain Key Performance Indicators (KPIs) around the testing performed within the team
- Implement best-in-class testing processes to transform the organization
- Develop and implement process improvement plans in conjunction with our strategic partners
- Assist in the availability management of multiple QA environments that are used to test new software and the software deployments for Comcast's customer support application
- Prepare presentations and communicate with all levels inside Comcast and with external vendors
- Manage purchase request process for contract resources and hardware resources
- Lead Agile teams
Audit Manager, Quality Assurance Resume Examples & Samples
- Operate independently to assist the QA Director across Risk
- Work closely with the QA Director for Risk in fostering a strong QA partnership with the Chief Auditor, Risk, Chief Auditor, Capital Planning and the Audit Directors across the Risk Centre of Excellence (CoE)
- A key component of the role is to assist Chief Auditor for Capital Planning with ongoing coverage of key capital planning processes, specifically IA’s assurance over CCAR and DFAST
- Provide support to the QA Director for Risk to perform a variety of QA reviews across Risk and Capital Planning
- Facilitate pro-active interactions, including communication and clearance with Audit Owners and Chief Auditors in Risk during QA reviews
- Conduct regular scorecard reviews, share best practice and common pitfalls with relevant teams within Risk and Capital Planning and support with the provision of focused training as and when needed
- Produce effective communication tools, for example briefing notes, newsletters, QA & Methodology updates
- Complete reviews of Risk Assessments, Quarterly Plan Refresh papers, and Quarterly Business Monitoring outputs for Risk and Capital Planning
- Pro-actively identify development areas, training needs and develop and deliver required training sessions for the IA teams covering Risk and Capital Planning
- Candidates should have between 5 – 7 years of diversified experience, ideally including experience in audit or a related field
- Must have first-hand audit experience of risk management in a top tier investment bank covering one or more of credit, market, operational risk management, treasury, or trading
- Must have had first-hand audit experience of capital planning. Primarily CCAR and DFAST but exposure to ICAAP in an international context would be valuable
- Must be proficient in risk and control analysis and audit concepts for a full range of products and specialized areas and will be skilled at analyzing root causes of problems and anticipating ‘horizon’ issues
- Ideally the candidate should be qualified in a risk-related discipline e.g. FRM, CISI, PRM, ACT, CFA
- Strong presentation, relationship management and partnering skills are essential
- This individual must be an articulate and effective communicator, both orally and in writing, with an energetic, charismatic and approachable style
- Candidates must have effective persuasion skills, the ability to work effectively at the highest levels of the organization, and will display highly effective networking and influencing skills. This person will be comfortable acting as an agent for positive change with agility and flexibility
- The candidate must be a strong, energetic individual with a proven track record in managing high-performance teams
- Experience in use Metric Stream is a plus
- English language skills essential and any other regional language skills advantageous
- Reports to Quality Assurance Director, Risk
- Help define, implement, and maintain quality assurance methodologies and processes that all stakeholders can rally behind, and that are in line with the larger Quality Assurance organization
- Challenge established Development and Quality Assurance processes and standards and be a champion in driving further efficiencies
- Experience with remote and offshore test teams across multiple time zones
- Working knowledge of industry leading Quality Assurance tools for Test Case Management (TCM) and Test Automation
- Previous experience in the field of education technology
- Experience in parenteral biotechnology manufacturing
- Experience with gene therapy technologies
- Experience with packaging and labelling processes
- Experience using TrackWise, LIMS, SAP
- Experience with Medical device and other pharmaceutical technologies
- Advocates for the role of Quality Assurance and the importance of Product Safety, Quality and Regulatory compliance with colleagues across the business
- Collaborates with operations and facilities teams to implement solutions to assure that store practices and store designs meet local health regulations governing retail food establishments
- Contributes to development of department mission, strategy, programs, and operating budget
- Leads and manages investigations and troubleshooting of product concerns from internal production, retail stores and customers. Implements corrective action as needed
- Manages quality assurance, regulatory and food safety project teams in selection and evaluation of suppliers and in commercializing food, beverages, ingredients and supplies at supplier facilities. Works with Operations to ensure effective implementation of new products or new practices
- Manages the development, implementation, and monitoring of an integrated program to assure that quality, product safety and regulatory compliance standards are met for food, beverage, ingredients, merchandise and supply items manufactured, distributed and sold by Starbucks
- Organizes and directs work activities of technical individual contributors. Develops work plans and cross-functional responsibilities. Establishes key technical performance indicators and measures to ensure progress to agreed-up deliverables and implementation consistent with project timelines. Manages the project information flow and updates cross-functional team, management and senior management
- Executing total quality management program for a food manufacturer (7 years)
- Interpreting and application of regulatory requirements in food or merchandise (5 years)
- Managing technical projects (5 years)
- Public environmental health professional with experience in food service establishment inspections, retail food service establishment plan review, retail food establishment pre-operational inspection and code enforcement hearings (5 years)
- Ability to articulate and represent business unit/department's needs to relevant groups
- Strong verbal and written presentation skills
- Ability to articulate and sell quality assurance programs and continuous improvement goals, and to influence indirect resources in achieving results
- Knowledge of product regulations, food processing technology, ingredient functionality, microbiology and food safety
- Knowledge of quality assurance program methodologies and implementation within manufacturing and retail operating environments
Manager, Quality Assurance Engineering Resume Examples & Samples
- Introduce and implement new and improved tools and process to better support execution of assigned projects
- Help define, implement, and maintain automation strategies that enable the team to perform more thorough regression testing of existing functionality, earlier in the release cycle
- Gather and communicate relevant quality assurance metrics and information, and provide periodic status reports on sprint and release testing efforts
- Challenge established Development and Quality Assurance processes and standards and be a champion in driving further efficiency
- The following are the key qualifications of a candidate that would meet the QA Manager requirements
- Expert understanding of industry standard quality assurance and testing methodologies
- Demonstrate passion for the continuous improvement of processes and methodologies used in the development of software solutions
- Demonstrated experience building teams and managing multiple projects in parallel
- Bachelor of Sciences degree in Computer Science, or equivalent/relevant education
- Minimum of 4 years of prior experience as a Quality Assurance Test Engineer
- Finance and treasury risks are accurately, consistently and timely assessed for all relevant auditable entities
- The audit plan includes adequate coverage of key risks by product, function, region and/or individual country
- Finance and treasury related audit work is consistently completed; and
- Provide the IA Finance and Treasury CoE teams with appropriate training, testing strategies and guidance
- The guardian and communicator of key financial information and controls for Citi, serving a variety of internal and external “clients”
- The driver of franchise and legal entity decisions on capital planning, liquidity, budgeting, tax, strategic direction and policy management
- A critical business partner to each Global Product, Region and Function, providing the data and analysis to enable Citi's leaders to make sound decisions in support of the business' objectives
- The developer of a quality organization through recruiting, mentoring, mobility, performance assessment, training and developmental assignments, setting the standard for leadership
- QA partner to the Finance and Treasury Audit Directors and their teams. All scorecard work streams in QA as part of the QA product set and where opportunities allow, support a scorecard round
- Support and input to the maintenance and update of the scorecard strategy for IA
- Where required complete thematic analysis, best practice papers etc to be prepared for the Chief Auditor Strategy, QA, Methodology and Communications and the C15 QA Director for Global Consumer Banking and Finance
- Assisting in the review and improvement of Global Internal Audit methodology and practices through
- Candidates should have at least 8 - 10 years of diversified financial management experience including significant experience and exposure to working in and/or auditing large complex Finance and Treasury departments in large financial services institutions
- Must have extensive experience of working at top tier complex Financial Institutions/Investment Banking / Capital Markets (products and customers) and must have first-hand experience of Internal Audit
- H/She should have a thorough knowledge of operations and control systems as well as an understanding of best-in-class audit methodologies. H/She must be proficient in risk and control analysis and audit concepts for Finance and Treasury. Experience of auditing treasury, liquidity, funding, and regulatory reporting processes is a plus
- The Quality Assurance Audit Manager must be self-motivated and capable of working independently with a proven track record of delivering high-quality work
- This individual must be an articulate and effective communicator, both orally and in writing, with an energetic, charismatic and approachable style. Must have effective persuasion skills, the ability to work effectively at the highest levels of the organization, and display highly effective networking and influencing skills. This person will be comfortable acting as an agent for positive change with agility and flexibility
- This role requires an individual with good leadership skills, strong customer focus and ability to drive quality in a predictable manner. The successful candidate will have a passion and commitment to create on overarching test strategy for the publishing, delivery and installation of complex desktop software and cloud services that cater to a highly specialized market across multiple industries
- Develop a comprehensive testing plan that is customer-centric as well as scalable and metrics based
- Drive the development of test automation and tools for regression, functional, performance and load testing
- Develop and drive a world class testing strategy for our software delivery pipeline
- Actively engage with customers, both internal and external, to understand their workflows, issues, hardware and where applicable any IT policies or compliancy requirements to make sure QA testing is representative of customer experience
- Actively engage in technical issues from customers and drive them to resolution
- Work with teams within the Global Quality Engineering organization to drive future-state vision and efficiencies with respect to test planning and execution, test processes, and reporting in the mobile domain. Review and signoff on mobile test plans and strategy deliverables in conjunction with test architecture
- Ensure quality processes are being appropriately leveraged across GQE and be a change agent for improved quality processes for test data management, governance, metrics and more
- Lead and develop GQE Cast Members
- Support defect management
- Research and recommend new tools and technologies for possible use within the team
- Budget and cashflow projections and analysis
- 3-5 years of experience (minimum) in overall test management
- Working knowledge of Continuous Integration and Continuous Delivery (CI/CD) solutions and practices (experience with Jenkins is preferred)
- Working knowledge of Amazon Web Services (AWS) and other cloud services providers in support of CI/CD solutions and other GQE initiatives
- Working knowledge of code management tools such as Git and or other source control tools
- Experience with leading mobile automated testing tools, including Selenium, Appium
Fraud Risk Manager, Quality Assurance Resume Examples & Samples
- Support and/or lead fraud thematic (e.g. Channel) and portfolio (Product) reviews across the 6 Consumer operating regions, with primary accountability for the Americas
- Monthly reporting of Quality Assurance (QA) Observation statuses (globally)
- Leading a bi-annual review of the Fraud Quality Assurance Library to modify tests to include new/emerging threats and risks
- Oversee compliance to all policies and procedures established at the group level including connected Financial Crimes disciplines
- Work with the broader Independent Consumer Fraud Risk organization to ensure that the established oversight program remains effective against the back drop of the developing business environment and emerging threats
- Provide written and verbal support via job aides, white paper development and report generation for Quality Assurance Reviews
- Work collaboratively with other members of Consumer Fraud Risk leadership team to ensure maximum cohesion across Policy, Strategy/Analytics and Oversight. Assist and establish/maintain proactive fraud prevention mechanisms and fraud detection/investigation processes in the region
- 8+ years’ experience in a fraud operational or risk experience in Retail/Commercial Banking
- Proficiency in consumer banking products including Cards, Small Business Banking, Retail Commercial Banking and Wealth Management
- In depth understanding of credit and fraud risk management lifecycle
- Knowledge of key fraud prevention technologies, processes and people required to manage fraud across customer lifecycle:- Identity Verification, Application Fraud, Authentication, Session Profiling, Transactional Profiling, Link Analysis and Fraud Case management
- Understanding of audit/review methodologies and data collection techniques
- Appreciation of Operations Management techniques as applied to the end to end fraud management process
- Exceptional written communication skills to support report writing, job aide and guidance documentation
- Understanding of Consumer Banking Fraud systems and processes
- Deep understanding of Consumer Risk management and Fraud lifecycle management
- Thorough understanding of global Fraud practices and industry trends
- Undergrad in Finance, Business Administration, Economics or Engineering
Financial Intelligence Unit Manager Quality Assurance Resume Examples & Samples
- Establish procedures to design, develop and
- Interfacing with internal audit and external examiners
- Strong understanding of the
- Experience in working on dynamic
- Working knowledge of Excel, including data analysis
- Develop high quality automation test engineering best practices, test strategy and principles
- Transition from mostly manual testing with many releases to a structured release model leveraging CI principles, automation, and robust test data management
- Build robust and highly automated release level automated regression focused on ensuring a high quality end to end customer experience
- Develop a strategy and plan for robust test data provisioning framework
- Coordinate the design, implementation, and maintenance of common and product-specific test data solutions; create repeatable processes for provisioning high quality test data for multiple testing phases and testing teams; manage test data refresh on a regular basis; address test data issues and provide data accuracy and completeness to all testing resources
- Remain an active learner in all automation technologies related to application testing
- Collaborate to improve application testing processes, tools, and methodology
- Direct defect tracking processes; including software defect documentation, defect tracking tool (JIRA, ALM) utilization, ensuring open defects are monitored and escalated as needed based on aging and priority
- Cultivate proactive, technically strong service-oriented staff
- Identify career growth opportunities for your employees and manage their development
- Devise and execute training to develop and elevate the automation skills within the team
- Closely monitor industry trends and progress in the automation tool space, develop proof of concepts as needed to validate the potential impact to our test delivery process
- Ensure adherence to all Quality Assurance and Software Development Lifecycle processes and standards
- Measure success against customer expectations and business defined outcomes
- Develop strong relationships with all partners based on mutual respect, accountability, and delivering on customer needs
- Be personally accountable for projects being on-time, on-budget, with high quality, and expect the same from others
- Participate in and cultivate a diverse and inclusive culture
- 3-5 years QA technology leadership experience with a focus on Automation and Test Data Management
- Strong knowledge of automation framework concepts and automation best practices in order to be able to develop an automated testing strategy using multiple tools
- Experience with a variety of test tools and software testing practices, including developing, implementing, maintaining and managing manual/automated testing systems and environments
- Experience with testing complex multi-tiered web based systems, ecommerce or mobile and complex data-driven applications
- Solid hands-on experience using front-end UI and back-end technologies and frameworks to deliver test automation solutions on back-end, Services and UI layer, supporting a multi-browser, multi-OS and multi-device environment
- Hands-on experience with some or all of the following: Selenium WebDriver, TestNG, JUnit, JMeter, SoapUI, Maven, Jenkins, Git, TeamCity or CI/CD tools, SQL, UFT, XML, Python, Ruby, Perl, Cucumber, Gherkin
- Demonstrated knowledge of Quality Center, ALM, Jira, Rally or related tools
- Experience with testing and automating APIs, including but not limited to REST-based APIs and Services
- Experience with Agile, V-Model, and Waterfall methodologies and SDLC
- Experience with Continuous Integration/Continuous Delivery (CI/CD)
- Strong leader with deep technical and analytical abilities, insightful and logical problem isolation and solving skills, strong collaboration, negotiation and influencing skills
- Team leadership experience with excellent oral and written communication skills in a medium-large IT environment
- Results driven and goals-oriented
- Extensive analytical, problem-solving and decision-making skills
- Able to perform in a very focused and methodical manner with good time management skills
- Experience managing relationships with internal and external customers, vendors, and outsourced service providers as well as diverse technologies ranging from mainframe, distributed and digital platforms
- Proven ability to influence others not within their own management structure
- Experience managing teams of 5+ staff strongly preferred
- Experience with an Onshore/Off Shore staffing model preferred
- Bachelors degree or equivalent relevant experience
- Manage and/or support the delivery of advisory services to clients related to business integration and testing
- Lead and support business development activities at clients, such as identification, proposal development, and other pursuit activities, while working with the appropriate KPMG resources from other service lines and industries
- Guide client development and realization of testing strategy and test cycle planning
- Develop and maintain/manage client relationships
- Develop test governance processes and manage testing
- Ten years of experience in software development, testing, program management and analysis
- Knowledge of software quality principles: risk based testing, requirements testing, and management
- Experience running large testing organizations, selling, delivering, building teams, and managing a practice
- Lead the Grand Haven Quality Process and implementation of the Quality Plan and Total Quality Management (TQM) philosophy
- Coordinate quality assurance activities between the Industrial Coatings – Grand Haven operations and Coatings facilities and inter-plant functions. Provides training for the facility to ensure knowledge of Quality Systems
- Minimize variation in processes through measurement system variation reduction, standardization of procedures, implementation of statistical techniques, and training. Serve as a leader and resource on these issues. Examples of these would be Cycle Time Reduction and Waste Minimization
- Conducts internal quality audits and coordinates external audits, represents Grand Haven in such quality audits, and any other customer interactions which pertain to the Quality Process at Grand Haven
- Responsible for assuring compliance to all customer quality system requirements and direct role in meeting customer specifications and responsiveness
- Responsible for the proper maintenance of a number of activities related to quality requirements of our customers, quality requirements of the Technical groups, and those required by PPG and Coatings facility guidelines. These include detailed procedure documentation, record retention, raw material sample and record retention, analysis and communication of data, adjustment efficiency, etc., and maintenance and training in PPG computer systems
- Represents the Grand Haven Plant in interplant and PPG communications regarding Quality Services policies and projects
- Has the authority and responsibility to stop any Grand Haven process or activity that, in the incumbent’s judgment, is not consistent with the quality system goals and requirements
- Work directly with Manufacturing to align teams and efforts to support continuous improvement in Color Adjustment and reduction in QC Downtime
- Bachelor’s Degree in engineering, chemistry or other science discipline
- At least five (5) years of Quality Assurance experience in a leadership or management capacity within manufacturing
- Quality Management experience within the coatings industry
- Excellent leadership skills with the ability to mentor, coach and effectively develop others
- Strong ability to influence cross-functional teams and all levels of employees
- Strong oral and written communication skills with the ability to work well with customers and internal employees/customers
- Strong ability to problem solve to root-cause and recommend corrective actions
- Ability to work independently, be self-driven and to also work collaboratively with teams
Associate Manager Quality Assurance Resume Examples & Samples
- This Assoc. Mgr. QA will lead a team that ensures effective implementation and execution of the Kraft Heinz Supplier Quality Management System across an assigned product category. The position provides visible quality leadership ensuring that KFG supplier quality standards are understood and met through on-site assessments. This position also ensures that best practices are replicated across the category. This position requires travel up to 70%
- Lead a team that develops and implements an integrated supplier quality management strategy
- 5-7 years of experience in food or CPG industry is required
- 1-2 years of leadership/supervisory experience is required
- Food safety and regulatory requirements
- Supplier expectations in Food Safety, Regulatory and Quality
Manager, Quality Assurance & Compliance Resume Examples & Samples
- A minimum of a Bachelor’s degree (or equivalent degree) is required
- A minimum of 8 years’ experience in a FDA (or Health Canada) regulated industry is required
- Experience and understanding of the following regulations is required: 21 CFR 210, 21 CFR 211, 21 CFR Part 11, 21 CFR 820, and current Good Manufacturing Practices (cGMPs)
- Experience in Quality Assurance, Quality Systems, or New Product Development in a regulated environment is required
- Experience in technical transfer, process development, analytical chemistry, clinical research, microbiology, product safety, and process validation is preferred
- Experience in complaint vigilance and complaint reporting is preferred
- ASQ and/or Project Management certifications are a plus
- This position may require up to 10% travel as business needs dictate and will be based out of one of the following locations: Skillman, NJ; New Brunswick, NJ; Fort Washington, PA; Los Angeles, CA; Guelph, ON (Canada); Markham, ON (Canada).Quality Assurance
Manager, Quality Assurance & Training Resume Examples & Samples
- Responsible for developing, managing, and coordinating all training needs for CCM and research staff including: weekly animal care staff training, weekly American Association for Laboratory Animal Science (AALAS) certification classes, orientation programs, species-specific hands-on training courses, non-human primate orientation, facility training, and any re-training issues that may occur
- Successful completion of a full 4-year course of study in an accredited college or university leading to a bachelor's or higher degree in Animal Science; OR appropriate combination of education and experience
- Master's degree in Animal Science or related field
- Hires, trains, and coaches Quality Analyst team members on call monitoring and evaluation, assuring that they address all relevant issues within the specified standards
- Motivates and inspires team members, maintains healthy group dynamics, facilitates problem solving and collaboration, and helps drive appropriate workplace behaviors
- Willing to listen and build professional rapport with team members as well as the leadership team
- Ensures key service metrics and other goals are met
- Manages team productivity, work assignments, schedules, and time off
- Provides input on the design of quality monitoring formats and standards
- Produces and analyzes reports for trends, patterns, and root causes of issues
- Represents Quality Assurance with operations, training, recruiting, facilities and other departments
- Makes decisions and has the ability to recognize the impact of decisions on teams, departments, and company
- Resolves both simple and complicated problems in the day to day operations of the QA team
- Coordinates frequently with Knoahsoft (or third party vendor/provider of interaction monitoring and evaluation application)
- Bachelor’s degree or equivalent combination of education, training and experience
- Series 7, 63 and either 8 or 9/10 or 24 licenses required
- 3-5 years of call center experience with at least 2 of those years working specifically in a call center QA function
- An additional 3+ years of proven leadership and management skills to include hiring, training, coaching, conflict resolution, performance management, time management
- Experience developing a culture of high standards, cohesive teamwork, and customer focus
- Experience with managing work flow, processes, and schedules to accomplish goals
- Effective oral & written communication skills, excellent interpersonal skills, able to present with credibility and authority. Must be able to effectively communicate with all levels of management including both internal and external partners
- Proactive, innovative, able to develop new concepts and to deliver creative solutions
- Responsible, reliable, and accountable. Ethical and able to instill a clear sense of purpose in others
- Flexible in terms of scheduling and work load
- Previous experience with quality metrics reporting
- Knowledge of Knoahsoft quality application
- Knowledge of MS Office suite, with strong Excel skills
- Leads Quality Engineering Team to provide support and technical guidance / direction to the operational and functional areas on site, fostering an environment of proactive and continuous improvement approach to Quality
- Supports and aligns with the site commitment to ensure the organisation’s conformance to producing products meets or exceeds customer requirements and the relevant regulatory standards
- Fosters a positive employee relations environment by promoting open communication, engagement and development of team members. Promotes a culture of inclusiveness, trust, flexibility and teamwork
- Create a highly talented team by sourcing, hiring, and placing individuals in positions that play to their strengths and future potential
- Drive an environment of compliance within Stryker by working with all function managers and their support staff to define expected quality standards and the roles/responsibilities in the maintenance of these standards
- Participates in ensuring the site(s) maintains continued certification to all regulatory bodies. Plays a lead role in all internal and external audit programmes such as Corporate, notified body & FDA audits
- Ensures effective control of product/process deviations for all released product and for the implementation of effective corrective action when non-conformances arise. Assesses product & patient risk
- Supports the planning pipeline of quality improvement activities including initiatives to increase product quality, NCR & CAPA resolution, reduce cost, decrease in-efficiencies and improve risk management
- Oversight and responsibility to ensure site maintains adherence to Quality KPIs performance indicators, and takes timely action to address adverse trends and deviations. Continuously monitors and partakes in global forums to assess opportunities for improvement
- Assist in developing and supports local and global strategies for the Quality Operations function
- Technical competence on quality, compliance and governance linked to market and organization
- Be able to work well remotely and represent the global team’s purpose and agenda while collaborating and communicating with affiliates to ensure their best interest is well understood by the global team
- Capacity to influence without direct authority
- Independently able to make appropriate decisions
- Flexible when priorities change
- Capacity to manage conflicts and problem solving
- Languages: Danish or Swedish and English: fluent
- Travel: up to 40%
- Provide guidance to employees within function or department
- Write, revise, and/or review SOPs for approval
- Recommends modification to operating policies
- Reviews resumes, conducts phone screens, conducts interviews and makes recommendations regarding hiring decisions
- Make recommendations regarding promotions
- Conduct performance reviews, recommends salary increases, and career planning for all direct reports
- Serve as resource for technical/administrative development problems
- Reviews and evaluates performance issues with the employee
- Staffing responsibilities for a single functional area or department of Catalent with moderate financial responsibilities
- Provides guidance to supervisors within function or department
- May make formal presentations to small groups
- Ability to organize time for multiple tasks (major projects and daily functions)
- Schedules and executes work
- Function and problem solve independently without supervision
- Good communication and organizational skills
- Formal presentation skills to co-workers or customers
- Usually supplies and seeks information where exceptional tact and courtesy is needed
- Ability to bring projects to a timely completion using Catalent systems
- Good working knowledge of the subject area
- Knowledge of cGMP, GLP, ICH, and USP regulations or guidelines
- Supply and seek information where exceptional tact and courtesy is needed
- Lead a team of QA specialists and contractors in the areas of continuous improvement, inspection support, training, EET/CAPA tracking, metric reporting, and system design, implementation, and optimization
- Lead and provide strategic oversight for Clinical QA improvement core team and voice of the customer initiatives and monitor employee and stakeholder needs to drive continuous improvement efforts
- Lead efforts to update and streamline Clinical QA processes and SOPs by reviewing existing workflows and assist in identifying areas for improvement, design flowcharts for identified areas and write/revise SOPs to drive improvement
- Lead RACI (Responsible, Accountable, Consulted, and Informed) charting analysis where needed
- Manage and provide strategic oversight for the design, implementation, integration, and continuous improvement of key software solutions (TrackWise, LIMS, EBS, and Gilead’s batch disposition, business analytics, and CXO interface tools)
- Design, implement, and manage Clinical QA Quality Plan
- Design and maintain resource forecasting models for Clinical QA
- Manage internal and CXO investigations ensuring quality issues are thoroughly investigated with appropriate root cause analysis and corrective and preventive actions identified
- Manage internal audits for Clinical QA ensuring appropriate system improvements are identified and implemented and audit observations are responded to and closed in a timely manner
- Coordinate inspection and audit support for Clinical QA inspections and audits
- Manage Clinical QA input and Metrics output for Gilead’s Business Analytics System
- Maintain systems for organizing, tracking, trending and reporting Deviation, CAPA, batch disposition and review metrics
- In depth knowledge and experience with Operational Excellence Principles
- Critical and strategic thinking skills
- Experience generating key metrics
- Manages QA personnel, including organizing and prioritizing daily tasks, performing training, and writing performance reviews
- May participate in the writing of annual product reviews and the development of training programs regarding all aspects of producing quality products
- Interfaces with contract manufacturers to address and resolve more complex product/process performance issues
- Demonstrates keen understanding of international quality systems regulations to adopt best in class systems/processes, and drive continuous improvement initiatives
- 8+ years of experience in a GMP environment related field and a BS or BA
- Provide Quality Assurance oversight to Clinical and Pharmacovigilance functions and demonstrate a customer driven approach to ensure internal customer expectations are met and exceeded
- Perform a wide variety of activities to ensure compliance with applicable regulatory requirements associated with Clinical development and Pharmacovigilance programs
- Create and author Standard Operating Procedures (SOPs) as needed to support compliance
- Conduct external GCP audits and follow up of clinical/pharmacovigilance vendors, CROs, and suppliers
- Conduct Investigator site audits and follow up for applicable clinical trials
- Conduct internal process audits and follow up for clinical and pharmacovigilance functions
- Works directly with operating entities to ensure that inspections and audits are conducted on a continuing basis as specified to enforce requirements and meet specifications
- Ensures compliance with current global regulatory standards as well as internal standards, processes and procedures
- Interfaces with Pacira’s Clinical and Pharmacovigilance departments to address documentation and compliance issues
- Writes and/or implements changes to controlled documents (e.g., SOPs, Specifications, Methods, etc.) as needed
- Assist with regulatory inspections and partner audits
- Ensure corporate compliance with pharmacovigilance regulations including training and periodic audits
- Ensure timely completion of commitments from regulatory agency inspections and partner audits
- Ensure timely completion of Corrective and Preventive Actions (CAPAs) as needed
- 8 to 10 years of relevant Pharmaceutical Industry experience with a minimum of 4 years of GCP Quality Assurance experience
- BS/BA degree in scientific discipline from an accredited college or university or equivalent experience
- Ability to manage projects and responsibilities independently
- Strong knowledge of GCPs and their application
- Ability to use Microsoft Word and Excel applications
- Ability to work proactively and cooperatively with managers and operational staff to solve quality problems
- Good written, oral and interpersonal communication skills
- Understanding of Clinical programs
- Support Quality Management in the development and infrastructure of quality function to support company’s eventual product commercialization
- Perform QA contract manufacturing oversight and/or contract research organizations activities to ensure required quality standards are maintained in GMP, GCP and ICH regulations and guidelines
- Collaborate with Manufacturing, Quality Control and Research and Development to continually improve compliance with quality systems, internal SOPs and regulatory requirements and to resolve minor and major deviations
- Lead or participate as required in investigation teams to resolve quality issues, as necessary
- Lead or participate in cGMP compliance audits of suppliers, contract manufacturers, contract research organizations and contract laboratories
- Lead or participate in cGMP compliance audits of quality systems and support functions as required
- Lead or support Document Control related processes
- Prepare trend reports related to in-process monitoring, deviation reports, investigation reports. Follow up with functional department for timely completion of corrective and preventive actions recommended, as necessary
- Serve as the QA representative on project teams
- Support the qualification/validation of equipment and computer based systems; support process validation studies and tech transfer as needed
- Perform other related duties as assigned from time to time based on company needs
Project Manager / Quality Assurance Manager Resume Examples & Samples
- Ensure that projects are planned and executed by Project Managers according to methods, standards and good practice
- Perform reviews and readiness of projects to pass milestones defined by control model
- Ensure that needed support is provided for project manager and project organization (team and steering committee)
- Share and reuse experience and knowledge, ensure continuous improvements for project deliveries
- Capture competence development needs from Projects and lessons learnt for organization
- Follow-up findings from Project reviews through clear action plans
- Broad Process and IT knowledge
- Good knowledge of the customer business and needs
- Quality Assurance skills and experience
- Structured and organized for efficient follow-up
- Good knowledge about development and maintenance business
- Mentorship skills and mind-set
- Motivated by developing networks and proactively managing challenges
- Bachelor or Master’s degree
- At least 5 years of successful experience in project managing
- Strong business mindset and holistic approach
- Proficient in English, both written and spoken
- Excellent usage of MS Office (Power point, Excel, SharePoint)
- Manage a team of up to 30 QA/Integration Engineers in a matrix like organization structure
- Influence the implementation and growth of Agile in the QA teams
- Implement a production incident/problem feedback tracking system to reduce repeat issues in production
- Bachelor of Science/Engineering with experience of 10+ years in Software Testing
- Conversant with devising test cases from Software Requirements Specification is a must
- Exposure to different testing methodologies is essential
- Experience in both white box and black box testing
- Experience in using testing tools – specifically Jira
- API Testing with Java/C++ using any tools or frameworks
- Knowledge of web services and usage of the same
- Performance testing and analysis will be an added advantage
- Familiarity with test automation tool Selenium
- Knowledge of code profiling, memory leak testing, code coverage analysis will be an added plus
- Knowledge of the integrated development environment
- Ability to trace variables through a module and track changes to its value and currency
- Experience in checking out code from version control
- Ability to debug code
- Conditional statements, loops
- File handling
- Knowledge of coding best practices such as exception handling, variable initialization, proper loop definition, etc. including tools to help evaluate code
- Writing tests for and calculating code coverage metrics
- Code complexity metrics
- Ability to write stubs and drivers independently, to test each module to breakage."
- Strong test management experience
- Solid software testing background
- Experience in an agile environment
- Experience working within Web Technology, APIs and micro-services
- Experience of recruiting the right talent
- The ability to keep himself/ herself aware on testing industry/ good practices
- The ability to manage many threads of work at once
- Experience with the latest testing methodologies like: Exploratory Testing, Context Driven Testing, Rapid Software Testing, and Session Based Test Management
- Strong experience in writing test strategies and assessing risk, both technical and to schedule
- Proven success in both fostering and contributing to a team-oriented environment
- Clinical industry or regulated environment experience is a plus
- Ensures the performance of required inspection activities and the associated creation of inspection reports stating the conditions of a work area to ensure requirements are met
- Makes recommendations for corrective action, including participation in the project’s NCR process
- Implements principles of performance evaluation and prediction methods are used to improve project execution
- Responsible for maintaining quality standard of products and the procedures and materials that go into work scope
- Aligns quality management function with the performance needs of project, including the performance of required project surveillance’s/audits
- Manages the delivery of functional quality objectives by providing leadership and direction to team members
- Participates in the development of functional strategy and may support development of project-specific processes and procedures
Manager Quality Assurance Engineering Resume Examples & Samples
- Develop, manage, and provide leadership for a distributed Quality Assurance program
- Provide direction of QA staff members responsible for defining test strategies and executing manual and automated test cases for multiple projects
- Review, identify, and mitigate deficiencies in business requirements, technical specifications, and testing documentation to ensure completeness and testability
- Track and report on QA testing progress and risks for multiple initiatives & releases
- Define and lead relevant cross-departmental sessions focused on increasing project quality, such as test case review sessions, defect reviews, post release sessions, etc
- Establish strategy for effective QA test case, automation & defect management
- Define requirements for testing environments and collaborate with technology team on implementation procedures to manage testing environments
- Collaborate with business and technology team members on increasing quality
- Assist with the design, development, execution, and maintenance of automated and manual test scripts for enterprise applications
- Participate as a member of an Agile Team
- Technical knowledge with UFT and Java based frameworks
- Excellent written and verbal communication skills, with the ability to communicate effectively with technical and non-technical staff members
- Be comfortable working in a dynamic fast paced environment
- Demonstrate a high standard for excellence
Manager Quality Assurance Delivery GT Resume Examples & Samples
- Develop and maintain a strategy for Group Technology Cluster Operations Services aligned to IT and Business
- Implementation strategies by implementing a customized test Methodology
- Standardize the implementation of the test strategy by auditing alignment between the test process and test effort design and enforcing signed-off standards escalating to the senior management decision making level for non-conformance as a result of the audit process
- Standardize the implementation of a test process by auditing and enforcing signed-off standards escalating to the senior management decision making level
- Manage a team of Test Analysts and Testers to ensure effective daily operations
- Align IT and Business requirements as a single point of contact liaising with both stream leads
- Align expectations between vendors e.g. Viveo, internal teams e.g. development teams, testing teams and business teams and relevant external parties e.g. government, Reserve Bank liaising with both stream leads
- Communicating job expectations
- Planning, monitoring and appraising job results
- Coaching, counseling and disciplining staff
- Initiating, coordinating and enforcing systems, policies and procedures
- Approving direct reports time sheets, requests for time off and/or overtime
- Performing timely performance evaluations of direct reports
- Coordinating and conducting new hire interviews; facilitating hiring decision
- Training new hires on departmental processes and responsibilities
- Assisting Supervisor of QA in assessing deficiencies in the performance of direct reports and making recommendations for a training program
- Benchmarking state-of-the-art practices
- Strong documentation and organizational skills
- 5+ years experience in areas of Global Quality Management, Inspection, Auditing
- Working knowledge of GCP and Quality/Regulatory Guidelines and understanding of quality control procedures and acceptance criteria
- Must be computer literate and familiar with most common software applications like Word, Excel etc
- Understanding of medical and/or clinical trial terminology is desirable
- Proven ability in problem solving
- 4+ years previous supervisory or management experience in a related industry required
- Hot / cold/wet environments with loud machinery, fumes and moving vehicles
- Good Laboratory Practices
- Proficiency in MS Office Suite applications
- Work with other teams within the Global Quality Engineering (GQE) organization to drive future-state vision and efficiencies with respect to test planning and execution, test processes, and reporting
- Review and signoff on master test plans and strategy deliverables in conjunction with test architecture
- Ensure quality assurance processes are being appropriate leveraged at all levels in the organization
- Maintain dedicated team of test leads, analysts, and architects
- Provide analysis and review of strategic test plans
- Support defect triage and management
- Budget and cash flow projections and analysis
- Working knowledge of functional testing tools including HP ALM, Selenium, JIRA, Appium and HP QuickTest Professional is preferred
- Working knowledge of test data management tools and strategies is preferred
- Experience in managing embedded studio testing methods and Software Developers in Test (SDET) teams
Snr Manager, Quality Assurance Resume Examples & Samples
- Recruit, develop and lead the QA team in areas of expertise and high performance team behaviors
- Provide technical expertise and leadership to the QA group with regard Compliance and Quality Systems
- Oversee the Batch Release process
- Support Technology Transfers through Process Validation to Regulatory Approval
- Drive implementation of a science and risk based Quality Culture throughout the facility
- Lead the preparation and facilitation of Regulatory Inspections
- Oversee activities rAnchorelated to core quality systems including Deviations, Change Controls and CAPA systems
- Oversee relevant metrics for each quality system
- 8+ years experience in a cGMP regulated manufacturing environment, with exhibited knowledge or proficiency in Quality Assurance and Compliance
- Understanding and familiarity with FDA & European regulatory requirements, guidelines, and expectations
- Manage a team of QAEs / SDETs
- 8+ years of industry experience, including at least 2 years of hands-on testing and 1 years as a manager
- Strong knowledge of Testing tools and Test Automation
- 7+ years of experience with at least 1 year in a QA Manager role
- Bachelor's degree in Computer Science, Computer Engineering or equivalent combination of technical education and experience
Senior Audit Manager, Quality Assurance Resume Examples & Samples
- Providing support to the Head of Professional Practices to manage the agreed Quality Assurance programme and delivering Quality Assurance reviews on a timely basis
- Supporting the development and implementation of Internal Audit’s methodology and quality assurance strategy by engaging with key internal stakeholders
- Driving continuous improvement and championing change
Construction Manager / Quality Assurance Resume Examples & Samples
- Work hours: 8:00am – 5:00pm
- Immediate start
- 4 year accredited degree in construction management, architecture, or civil engineering
- 5 years minimum practical field experience involving vertical construction in a role of either construction administration, Quality Control or Assurance, owner’s representative, project manager, or superintendent. The candidate may substitute an additional five (5) years related experience for a professional degree
- The candidate should be assertive, demonstrate leadership, possess the ability to problem solve; be technically competent in aforementioned responsibilities, be fluent in Microsoft Office applications; and understand the construction management process
- Individuals should be highly motivated and possess excellent written and oral communication skills
Project Manager Quality Assurance Resume Examples & Samples
- This role provides project support through: ownership and management of project documentation, facilitation of project team communications
- Sets up the project control book, enters data, tracks issues and changes
- Produces status reports
- Handles financial and procurement transactions
- Supports general contract management through asset a management, audit readiness, financial process support, issues management, measurements reporting, project plan execution and, where applicable, request for service
- Works under the direction of the Project Manager to assist in driving project to successful completion
- Quality Assurance skills
- Provides Quality Assurance to the fielding process
- Provides safety and compliance oversight
- Interfaces with customer to conduct customer satisfaction surveys
- Army Intelligence Surveillance & Reconnaissance Systems operations
- Fielding and/or training
- Must possess an active SECRET security clearance with the ability to obtain and maintain a TOP SECRET security clearance with Special Compartmented Information access
- Must be able to travel to locations CONUS & OCONUS
- Applicants selected will be subject to a government security investigation and must meet eligibility requirements for access to **classified information
- Must possess superior oral and written communications skills
- Must be an expert in Army C4ISR Systems
- Must be an expert in Total Package Fielding (AR 700-142 & DA PAM 700-142)
- Experience with Windows Office Products
- Understanding of site support operations
- Development, implementation, and maintenance of quality systems to assure FDA and ISO compliance
- Optimize quality systems to effectively identify, measure, and reduce the cost of quality activities
- Identify, support, and manage departmental resources to achieve facility business and quality objectives
- Leads all facility audits (FDA, ISO, Corporate, Division, etc.)
- Plan, establish and implement an internal audit program
- Review and approve all Engineering changes to product, processes, and materials
- Promote and support multi-discipline teamwork, i.e., activities that support business objectives and quality system enhancement
- Design reporting systems which provide visibility of key quality metrics and provide leadership in establishing, reporting, implementation, trend analysis and corrective action to improve quality metric trends (i.e., rejections, scrap, complaints, etc.)
- Plan, establish, and implement a facility environmental compliance program
- Plan, establish, and implement quality control plans for products manufactured within the facility
- Plan, establish, and implement a supplier assurance program
- Responsibility for Facility Process Water System and Environmental compliance
- Identify, schedule, plan, and obtain approvals from FDA and other Regulatory agencies
- Provide technical engineering leadership to Quality Engineers
- Ability to develop leadership characteristics in direct reports
- Provide training, development, and supervision of quality assurance personnel
- Provide high level Process Control expertise to manufacturing floor
- Serve as Management Representative with authority and responsibility for assuring an effective quality system and periodically reporting on performance to management
- Bachelors degree in Engineering or related technical field
- Advanced data analysis computer skills to include Minitab and SPC systems
- Five or more years previous managerial experience
- Ten or more years experience in a federally regulated industry to include QA, compliance, and/or Regulatory Affairs
- Strong skills in root cause analysis and problem solving
- Established proficiency in leadership, communication, and project management skills
- Experience with new product development, validation and transfer to manufacturing facilities
- Must have excellent verbal, written, and interpersonal skills
- Strong working knowledge of GMP and ISO requirements for medical devices, statistical quality tools, SQC, Process Validation, and FMEA
- National certification in CQE, CRE, or CQA preferred
Manager, Quality Assurance & Testing Resume Examples & Samples
- Estimation and planning, risk assessment, contingency planning, tracking and management of progress, issues, budget, risk and resources
- Accountability for project budget and time lines
- Proven leadership skills and experience managing delivery of TESTING projects
- Deep experience in Quality Assurance and Testing
- 5+ years experience as test manager, and/or test analyst
- Experience with proven project methodology tools
- As a new employee you will bring your prior “best in class” experience to TransUnion to help drive our efforts to continuously improve
- Lead a dynamic team of Quality Engineers using modern application development technologies
- Mentor technical resources to grow their capabilities
- Play a key role in determining continuous process improvement, tooling and technology roadmap
- Manage customer intake/ requirements; organize teams around the delivery of business value and quality
- Participate in the development of TransUnion’s continuous integration, continuous deployment and automated testing strategies
- Ensures regulatory compliance with applicable regulations and internal procedures for the manufacturing, service and distribution area
- Identifies areas requiring quality improvement and initiatives and follows through on action plans necessary for implementation
- Supports Operations Engineering and Manufacturing to resolve technical issues by implementing corrective and preventive actions and reporting on status, as applicable
- Provides input on quality metrics for areas assigned as presented for Management Review
- Accomplishes results through subordinates
- Participates in internal and external audits representing Quality for the areas assigned
- Drives continuous improvement efforts, through facilitating, leading, and collaborating with cross functional teams
- Responsible for team building, identifying resource gaps, and working in collaboration with personnel to create personal development plans to enhance the capabilities of the department
- Develops, manages and executes Quality Plans
- Maintains compliance with Human Resource policies
- Managerial experience in one or more of the following: quality, engineering, manufacturing operations or technical customer service and support
- Direct experience with medical devices, and regulatory environments (i.e. QSRs, ISO9001, ISO13485, etc.) required
- Must be able to apply required regulatory principles to the production and process controls sub-systems
- Must have knowledge of Quality Engineering discipline, including statistics. ASQ Certified Quality Engineer preferred
- Strong working knowledge of DMAIC \Six Sigma problem solving process. Belt certified a big plus
- Proven track record of initiating and driving continuous improvements
- Teamwork oriented and ability to lead and influence without authority
- Maintain quality systems and assure compliance with, including but not limited to, FDA’s quality system regulations (21CFR820), Canadian CMDCAS, and ISO 13485
- Lead the Cardiovascular Quality Review Board (QRB). Plan, implement, and manage systems for the tracking and review of complaints, investigations and corrective actions. Prepare Medical Device Reports (MDR) as required
- Support regulatory submissions of all new and modified devices for fulfillment of FDA, EU and Canadian premarket obligations. Identify appropriate electrical and mechanical safety standards
- Lead internal environmental, health and safety activities at the Morrisville, NC site
- Perform internal and vendor compliance audits
- BS in Computer or Physical Science. Minimum of 5 years’ experience medical device or pharmaceutical industry; minimum of 2 years as supervisor
- Minimum 5 years Quality experience within the medical device industry (ISO 9001, ISO13485); other regulated industries may be considered
- Experience coaching/mentoring and developing quality assurance groups/organizations
- Demonstrated capability to lead and influence multi-disciplined teams of people to achieve goals
- Excellent interpersonal, communication and people management skills
- Ability to work on one’s own initiative
- Ability to prioritize the workload and effective time management in a fast paced environment
- Ability to travel (domestic or international) @10%
- Bachelor’s degree in a life sciences / allied health field required
- Six (6) – ten (10) years of Quality Assurance experience in a pharmaceutical or biotech company
- Experience in development, implementation of Quality Systems is preferred
- Strong understanding and practical experience with contractor management
- Experience with FDA submissions
- Knowledge of global regulatory and cGxP compliance standards
- Experience with regulatory inspections
- Experience in Quality aspects of preparation efforts for product approval and commercialization
- Ability to understand and execute on the company’s mission and values
- Strong analytical and decision making skills with attention to detail and quality
- Exhibits impeccable confidentiality on sensitive, supporting tasks
- Ability to anticipate and solve problems while analyzing complex issues and environments
- Ability to communicate and work effectively with all levels of employees in various communication mediums
- Possess a high degree of personal responsibility
- Ability to adapt and succeed in a milestone-driven, rapidly changing product development and commercialization environment
- Ability to prioritize and re-prioritize activities as needed to accomplish unanticipated requests or initiate new projects requiring immediate attention
- Exceptional planning, organization, and execution skills
- Demonstrates the highest ethical standards and trustworthiness
- Strong verbal and written communication and interpersonal skills
- Willingness to travel to investigator, vendor, or regulatory agency meetings and audits
- Ability to interpret a variety of instructions furnished in written, oral, diagrammatic, or spreadsheet formats
- Generate contractor change controls in accordance with quality agreement requirements
- Perform assessments of raw material changes, process changes, and manufacturing support utility changes with relevance to regulatory filings and quality agreements
- Provide notifications of change controls to partners where required
- Responsible for bringing out of specification results and deviations to closure and the timely implementation of corrective actions
- Provide support for clinical product stability studies
- Prepare and revise chemistry, manufacturing and controls sections and summary sections of IMPD/IND/CTD, supplements, amendments and other submissions as needed
- Drafting Quality Agreements and ensuring adherence to agreement requirements
- Analytical Support
- Contract Manufacturing Organization QA Liaison
- Clinical Operations Support
- Assist with maintaining file organization within the QA Dept
- Minimum BS Degree preferably in life sciences, or equivalent work experience. Advanced degrees can reduce experience requirements for advance levels
- Minimum of 8 years experience in the pharmaceutical, device, diagnostic or medical field and appropriate education
- Attention to detail with an ability to detect and correct errors/inconsistencies in various types of documents
- Thorough understanding of GMPs and familiarity of GLPs and GCPs is essential
- Good writing and verbal communication skills
- Ability to multitask and work within a fast-paced dynamic team environment
Manager, Quality Assurance Raw Materials Resume Examples & Samples
- Change controls
- Organizes QA resource assigned to functional area to meet goals and timings; coordinates prioritization of activities with area management
- Ability to interpret and communicate information at all levels of organization
- Excellent written and oral comminucation skills
- Ability to organize and maintain data
- BS in life sciences discipline or related field (chemistry, biology, or pharmacy preferred) with experience in a pharmaceutical/biologics manufacturing environment desired. Minimum 7 years experience in pharmaceutical industry; biologics experience preferred. Minimum 2 years experience in supervisory position
- Leading and managing a team and responsible for
- Managing and mentoring a team
- Excellent written, oral, and interpersonal communication skills with ability to effectively interact with a broad spectrum of audiences
- Excellent time and project management skills with the ability to manage many diverse issues simultaneously
- Skilled in peer relationships, drive for results, dealing with ambiguity, learning on the fly, and individual courage
- Ability to arrange schedules and own activities to accomplish objectives and timelines
- Effectively organize and manage work responsibilities under general guidance
- BA/BS degree and a minimum of 7 years of industry/relevant experience required
Manager, Quality Assurance MD Resume Examples & Samples
- Lead the Quality Assurance (QA) efforts and team to ensure we deliver the highest quality solutions
- Assess and ensure quality of design, development, and delivery across the product development cycle for our software solutions
- Work closely with Engineering and Product Management to understand user needs and to develop and adhere to QA processes and guidelines
- Build and manage the software, process, and product QA staff
- Coordinate with the QA staff embedded in the development teams
- Maintain quality systems and assure compliance with, including but not limited to, FDA’s quality system regulations (21CFR820), Canadian CMDCAS, and 93/42/EEC
- Investigate and implement continuous quality improvementtools and techniques to ensure quality system performance is always challenged
- Working knowledge of the principles of manufacturing management
- Working knowledge of basic elements of Good Manufacturing Practices in a manufacturing environment
- HACCP, Statistical Process Control
- Good Laboratory Practices, Good Manufacturing Practices
- Lead the Medical Informatics Quality Assurance (QA) efforts to ensure we deliver the highest quality solutions
- Bachelors Degree and 5+ years experience as a QA or RA professionalwithin a regulated industry
- 2+ years managerial experience
- Proven ability to apply regulatory knowledge to solve real world problems in an effective and efficient manner
- Strong understanding of FDA and EU medical device and privacy regulations
- Independent self starter, capable of setting, maintaining and achieving goals within a fast paced environment
- Manages a wide variety of compliance oversight activities expanding several quality subsystems to ensure the safety, efficacy and purity of the products manufactured by Biogen
- Plans, implements and directs core functions of direct reports as they relate to Quality Engineering functions
- Proactive identification and resolution of both technical and compliance issues/gaps; Quality system evaluation and improvement. Develops and implements aspects of various Quality Systems/System Improvements
- Analysis of departmental and site metrics for compliance. Tracks and trends key performance indicators and evaluates site performance against these KPIs
- Participates effectively in a leadership or membership role for both corporate, global and site cross-functional teams as a representative of Quality Assurance. Ensures optimum team dynamics and results
- Provides mentorship and training within and across functions
- This role is viewed as expert within a particular aspect of a given Quality system. Maintain knowledge of relevant FDA/EMA regulations and compliance
- Understanding and application of and ability to interpret and apply cGMP’s, relevant laws, guidance’s, and directives to bio/pharmaceutical situations
- Advanced leadership abilities with respect to operations and personnel management
- This role requires the ability to develop advanced concepts, techniques, and standards, as well as development of new applications based on professional principles and theories
- Excellent oral and written communication skills, excellent organizational skills with the ability to multi-task and coordinate multiple activities in parallel
- Advanced knowledge of quality systems particularly lot dispositioning, document lifecycle management, change control and exception management
- Must be able to process large volumes of information, make decisions under pressure, and provide leadership in difficult situations
- Ability to manage and prioritize multiple and varied tasks efficiently and accurately
- Leads and manages investigations and troubleshooting of product concerns from retail stores and customers. Implements corrective action as needed
- Manages quality assurance, regulatory and food safety project teams in selection and evaluation of suppliers and in commercializing food, beverages, ingredients and supplies at supplier facilities. Works with Operations to ensure effective implementation of new products or new practices in stores
- Organizes and directs work activites of technical individual contributors. Develops work plans and cross-functional responsibilities. Establishes key technical performance indicators and measures to ensure progress to agreed-up deliverables and implementation consistent with project timelines. Manages the project information flow and updates cross-functional team, management and senior management
- Review multi-product Batch Production Records
- Perform manufacturing line clearance
- Maintain material lot files, databases and spreadsheets
- Review deviations, investigations and corrective actions to ensure compliance and completion within a specific time frame
- Review analytical data packages in support of raw materials, starting materials, intermediates and API release
- Author, revise, review and implement Standard Operating Procedures
- Assist with/Perform internal/external audits
- Assist with Material Transfers
- Collaborate with other functional groups and accept other duties and responsibilities as needed
- Demonstrated in-depth understanding of cGMP manufacturing for biopharmaceutical products
- Experience in supporting all phases of API development, ideally spanning early development to registration
- Demonstrated success working effectively outside defined scope of responsibility, when required
- Have a bias for action and display a sense of urgency
- Possess strong organizational and interpersonal skills while maintaining high quality and efficiency standards
- Establish a good working relationship with clients and support the delivery of intermediates/API in a timely fashion
- Exhibit excellent written communication skills with the ability to write SOP’s concisely and accurately
- Must be skilled in using multiple computer programs (MS Office Suite, MS Project, MS Visio and Adobe Acrobat)
- Lead and manage a QA Team to successfully deliver the project to the required quality standards
- Establish the Project Management System
- Develop, implement and monitor the Project Quality Plan
- Communication of the quality plan and its objectives to staff, consultants and contractors
- Support all project process owners in the development and implementation of their plans and procedures and the monitoring of their performance on all quality matters
- Develop and implement audit schedules and plans, including unannounced visits
- Carry out and/or lead internal and external audits
- Develop statistical reports on Quality Performance
- Identify and communicate lessons learned
- Coordinate and manage any 3rd Party Audits as required
- Minimum of 15 years of experience working in an environment where multi-projects, e.g. 20+ are underway in parallel, i.e. Portfolio of engineering
- Experienced in the certification process, and implementation and compliance to ISO 9001, ISO 14001 and OHSAS 18001
- Minimum of 5+ years in a management role
- Undergraduate Degree
- Relevant QA Qualification
- Excellent report writing and communication skills
- Preferably previous experience working in the Middle East for global engineering company with contract(s) with National Oil Companies (NOC)
- Previous work with Saudi Aramco
- Accepts responsibility
- Manage Change
- Planning & Organizing
- Business Process Thinking
Manager Quality Assurance IT Human Capital Management & Workforce Management Resume Examples & Samples
- Identifies and communicates key responsibilities and practices to ensure the immediate team of direct reports promotes a successful attitude, confidence in leadership, and
- Teamwork to achieve business results
- Works with business and information technology leaders to properly set priorities, to manage incidents and escalations, and to predict and resolve problems that may occur
- Initiates corrective actions and preventative measures
- Negotiates business commitments and resource requirements. Works with business and technology leadership to evaluate performance of software quality assurance and ensure
- Knowledge of and satisfaction of support and services delivery
- Functions as a consultant regarding software quality assurance products and services provided by suppliers and vendors. Selects, negotiates, and manages software products and services, as well as professional contract resources in order to meet demands
- Promotes advancements in technology capabilities through strategic planning initiatives. Develops technology plans and roadmaps for specific technical and functional areas, including resource utilization, capacity, budgets and capabilities. Manages the allocation of resources to both development projects and sustainment services
- Prepares, communicates and educates client groups and teams on changes in policies and practices within the organization
- Leads team in implementation of test environment and test data management, test and quality assurance processes and standards, release management and deployment standards
- Oversees testing related concerns in all phases of software development and sustainment projects, including pre- initiation, initiation, definition, specification, development and testing
- Experience in software development and quality assurance testing (9 years)
- Experience instituting quality assurance, release management and test process, practices, methods and tools including test automation and performance test capabilities (9
- Managing teams of Software Quality Assurance professionals including test analysts and test engineers both onsite and offshore (9 years)
- Financial & budget management, sourcing and procurement, vendor and contract management (9 years)
- Bachelor's degree with coursework in Computer Science, Information Systems, Informatics, or related field or degree equivalent; and/or work experience in Software
- Development Quality Assurance (9 years)
- Exceptional oral and written communication skills with the ability to communicate clearly and concisely at various organizational levels
- Ability to create and deliver results in a highly collaborative environment
- Team player who exhibits effective interpersonal skills with a collaborative style
- Knowledge of testing methodologies and software development lifecycle concepts
- Strong technical skills including an understanding of automated test script execution and related tools
- Ability to work with one or more programming languages used for test script development
Configuration Manager / Quality Assurance Resume Examples & Samples
- Will work with the technical team to define the required documents and assist with the government required formats
- Manage the CM of all required documents IAW with company policies
- Act as the QA Manager for all assigned programs IAW ISO 9001 and company policies
- Experienced in building technical documents from scratch with input from VIBES technical team members
- Proficient in editing and formatting existing documents to meet standard government requirements and formatting
- Proficient with Microsoft office suite of tools
- Bachelor's degree from an accredited college/university plus 8 years’ overall experience or equivalent years of experience
- Minimum 5 years’ relevant experience working with technical documentation writing or editing
- Strong working knowledge of the systems engineering process and the system engineering lifecycle
- Previous work within the intelligence systems in a DoD environment is a plus
- Review and approve deviations, CAPAs, Change Controls procedures
- Review documents and processes to ensure compliance
- Training and coaching others
- Interpret complex, explicit documents to ensure quality standards and compliance
- Following accurate oral & written procedures in performing QA tasks
- Bachelor of Science in Engineering or Life Sciences with 7 years of experience in a science related field
- Manages QA activities, including organizing and prioritizing daily tasks, related to new product launches, technology transfers and validation for commercial drug product (solid dosage, steriles), packaging and labeling
- Serve as project lead within the QA function
- Determines corrective actions based on investigative findings and in consideration of the long-term impact of decisions
- Works on problems that are moderately complex in nature where analysis of situations involved in-depth and detailed evaluation of various factors
- Provide batch record review and final disposition for batches manufactured to support validation activities
- Interface extensively with internal stakeholders and contract manufacturers to address and resolve complex product and process issues
- Participates in developing Standard Operating Procedures to ensure quality
- Requires a thorough understanding of the relationship between quality, technical, regulatory and business functions in the pharmaceutical industry
- Previous CMC development experience is helpful. Must be able to work independently to resolve complex issues and negotiate best solutions with some direct supervision
- Exercises judgment within defined practices and policies in selecting methods, techniques and evaluation criteria for obtaining results
Laboratory Manager, Quality Assurance Resume Examples & Samples
- Management and Leadership of the laboratory in a complex manufacturing environment
- Managing laboratory resources to ensure the appropriate level of support and prioritization for manufacturing activities and timely delivery of products to customers
- Ensuring all laboratory activities are conducted in accordance with all applicable government quality regulations, safety requirements, and J&J policies
- Ensuring the appropriate oversight and guidance for laboratory investigations including reviewing investigations to ensure that reports are consistent, complete and in alignment with applicable standards and procedures with appropriate content and references
- Ensuring that all laboratory personnel are appropriately trained and records maintained
- Ensuring that laboratory equipment and systems are maintained in accordance with procedures and standards
- Oversight of Instrument Lifecycle, Laboratory Software Lifecycle systems, Laboratory Quality Systems including Laboratory Change Management activities
- Review and approve Quality Records (e.g. non-conformances, CAPA) related to laboratory events
- Provide support to internal and external audits. Reviewing and approving technical protocols and reports to support validation/verification and qualification activities
- Oversight of interactions with Contract Laboratories to ensure effective communication and project management. Additionally, working with internal business partners to develop Quality Agreements and work plans with Contract Laboratories
- Monitoring laboratory metric trends on a regular basis and ensuring that appropriate investigation, corrective actions and/or escalation is conducted as required for recurring trends involving products, methodologies, instrumentation and scientists
- Leading/sponsoring the implementation of improvement initiatives to address recurring laboratory issues
- Assessing new technology and laboratory automation in collaboration with colleagues throughout Johnson & Johnson to increase laboratory efficiency, reduce testing time, reduce data variability, minimize chemical consumption and reduce waste
- Maintaining up to date knowledge of the company’s products and manufacturing processes as wells as industry and regulatory trends related to relevant laboratory testing
- Communicating and elevating critical business related issues as well as opportunities to senior management
- Planning, promoting and organizing required training activities related to different laboratory testing areas
- Establishing and maintaining an annual operational budget
- Monitoring departmental activities to ensure that laboratory personnel follow all company guidelines related to Health, Safety and Environmental practices
- Serving as a departmental representative, as needed
- A minimum of a Bachelor’s Degree in Chemistry or related science (such as Materials Science or Pharma Science)
- A minimum of 7 years’ laboratory experience in a GMP Laboratory environment
- Experience with the following lab instruments: Lab Instruments: HPLC, UPLC, GC, UV-vis, FTIR, AA, ICP, XRF, Instron, and wet chemical techniques (Karl Fischer, titrations, etc)
- The ability to develop strategies that deliver successful results to the business
- Ability to interact with key internal and external stakeholders
- Prior experience in a supervisory or people management capacity
- Good interpersonal, organizational and communication skills
- Ability to perform in a leadership role and to effectively manage people
- Develops and communicates the vision for ensuring high quality delivery of product solutions in alignment with the product portfolio roadmap
- Collaborate with product owners and other technologists to ensure quality assurance is integrated into all aspects of a project and/or product
- Creates test strategies and plans including scheduling, risk mitigation strategies and resource leveling to support the needs of the Product team
- Defines test case writing procedures and standards. Reviews test cases to ensure clarity, accuracy, and efficacy. Responsible for ensuring Quality Assurance best practices are met
- Drives the implementation of automated software solutions to increase the speed and efficiency of QA. Will play a key role in the development of the automated tests
- Provide QA leadership by quickly identifying complex problems and choosing appropriate test solutions. Involved with test case preparation
- Define and implement processes and procedures for functional, load, performance and stress tests
- Development and communication of key metrics
- Champion QA initiatives to improve the efficiency and effectiveness of the QA team and contribute improvements to the complete software release cycle
- Ensures the QA team adapts to changing technologies and development approaches, including training, as necessary
- Lead the QA team in establishing processes that support Agile development frameworks such as Scrum
- Experience in building a quality assurance team of 3+ people, providing both leadership and tactical execution
- Core understanding of Agile development processes
- Experienced with effective automated testing approaches and various implementation techniques
- Proven experience in creating and executing detailed test cases and test plans
- Knows how to organize people and activities. Understands how to separate and combine tasks into efficient work flow. Knows what to measure and how to measure it
- Demonstrated understanding of metric reports and how they are used
- Manage a team with diverse backgrounds
- Strong problem solving skills. Looks beyond the obvious and doesn't stop at the first answers, uses rigorous logic and methods to solve difficult problems with effective solutions, and probes all appropriate sources for potential answers
- Highly collaborative and a team player
- NoSQL experience
- Bachelor's degree or higher in Computer Science, Math, or related field
- 3+ years of clearly demonstrated experience as a QA Lead and/or Manager, providing both leadership and tactical execution
SMB Program Manager, Quality Assurance Resume Examples & Samples
- Ensure that the organization’s Quality Management System conforms to customer, internal, CPOC/ISO and regulatory requirements
- Determining, negotiating and agreeing on in-house quality procedures, standards and specification
- Ensure evaluation of, and reporting on, vendor quality systems
- Report to stakeholders on the performance of the QMS (e.g., results of quality audits, corrective actions), including the need for improvement
- Oversee inspection (examination) of incoming cases, ensuring that they meet requirements
- Manage the monitoring, measurement, and review of internal processes, especially those that affect the quality of the organization’s output
- Work with internal teams, contractors, and outsourcing partners to develop improvement and sustenance strategies
- Validates quality processes by establishing product specifications and quality attributes, measuring production, documenting evidence, determining operational and performance qualification, writing and updating quality assurance procedures
- Prepare quality documentation and reports by collecting, analyzing and summarizing information and trends including failed processes, stability studies, attribution, corrective actions, and re-validations
- Responsible for accuracy and timely inspection/calibration of monitoring and measuring devices
- Planning and executing on improvement project with cross functional and global teams to drive uniformity and high service standards
- 5+ years of relevant experience in a dynamic Sales or Customer Service organization, in a similar role
- BA/BS degree from a top-university. MBA/Diploma in management is preferred
- Ability to establish shared goals and drive alignment across multiple stakeholders
- Experience leading cross-functional projects in a dynamic environment with multiple dependencies and constraints
- Good numerical skills and an understanding of basic statistics
- Understanding of requirements to develop QA processes from scratch, through to implementation and post-training assessment
- Experience in driving quality improvement projects industry standards in a must
- Native proficiency in one of these Asian languages - Japanese, Korean or Bahasa Indonesia, is an added advantage
- Six sigma certification is preferable
- Provide operational support to manufacturing operations at CMO sites including formulation, fill/finish, assembly, packaging and labeling activities
- Primary interface with External Manufacturing Project Managers for all quality related issues at CMOs
- Resolving problems, identifying trends and determining system improvements
- Ensuring compliance with internal procedures and regulatory expectations by proactively working with quality organizations at CMOs
- Preparing and completing action plans; implementing productivity, quality, and customer-service standards
- Implementing change when necessary within the guidelines of GMP manufacturing operations
- Requires BS/BA in a scientific discipline with 5+ years of related experience in a cGMP environment, preferably in a project management capacity for pharmaceutical or biotechnology industries. QA/QC experience preferred
Team Manager Quality Assurance Client Onboarding Resume Examples & Samples
- Embracement, implementation and operational management of the strategy of Client Onboarding (COB) including Know Your Customer (KYC/CDD) and Client Data Management based on input from internal and external stakeholders
- Direct responsibility for the operational performance of your team in the Netherlands. (+/- 12 FTE QA experts fg 9-10)
- Set up and coaching of your team to establish the right mix of people and required level of professionalism and motivation within the team
- Building a strong team and coaches them to turn in an exceptional performance
- Monitoring operational output (both on qualitative and quantitative standards) of your team
- Actively developing and maintaining an internal network (e.g. Regions, Markets, Compliance, Legal)
- As Team Manager Client Onboarding you work in a high demanding, dynamic and international environment
- Partnership
- Deliver results
- Planning, establishing, directing and coordinating the local quality assurance procedures required to maintain an ISO 9001 quality management system to meet corporate quality policies
- Assist in development of short and long term quality goals and objectives of the Medium Voltage business unit as a member of the business leadership team
- Develop strategies to move the quality improvement from reactive to pro-active prediction model. Improve processes via influencing cross functional teams to reach quality improvement goals
- Managing the Customer Quality organization on matters related to customer satisfaction and overall product quality
- Develop Reliability programs that improve the design and performance of Rockwell Automation, Allen-Bradley products and services. Activities to begin with new product design and continue reliability growth through the life of the product
- Bachelor's Degree in Electrical, Mechanical, Manufacturing or Industrial Engineering. Equivalent experience of at least 10 years in the low/medium voltage industry will be considered
- Strong background in the quality disciplines, including statistics
- 8 or more years in quality engineering, engineering or manufacturing
- Knowledge on Low Voltage (LV) and Medium Voltage (MV) distribution system, LV/MV Drive products, and solutions
- 8 or more year additional experience in manufacturing engineering, field commissioning engineering, application engineering, configuration and quotation engineering and/or product design and development in LV/MV power distribution systems is an asset
- Legal authorization to work in Canada is required. We will not sponsor individuals for employment visas for this job opening
- Any offer of employment is conditional upon the successful completion of a background investigation and drug screen
- Apply risk management tools and techniques
- Ensure Compliance with global directives, standards and guidance documents
- Participate in efforts to implement ongoing process management mechanisms (process mapping, documentation, metrics, monitoring systems and process control techniques)
- Participate on peer teams to meet desired project outcomes
- · Build strong and positive cross functional working relationships by working to include them in the improvement activities/training, making sure their needs and requirements are carefully considered
- May participate on the floor inspection readiness
- Staying up-to-date on training of all procedures
- Adhering to Sanofi Pasteur Biologics safety procedures and guidelines
Program Manager Quality Assurance Resume Examples & Samples
- Oversees tactical planning and day-to-day management of one or more programs in the ST portfolio
- Collaborates with ST and business leadership on annual strategic planning for the CRM and BI programs
- Develops a deep understanding of projects within the CRM and BI programs and the broader ST portfolio; manages assigned projects with a clear understanding of business and ST context, and develops a deep understanding of cross-program portfolio synergies and dependencies as they apply to QA
- Drives complex, cross-functional enterprise projects, leading multiple efforts within a specific program simultaneously, with complexity as a limiting factor
- Leads high performing, cross-functional, often globally distributed QA teams
- Works independently, without the need for day-to-day guidance
- Serves as program manager for highly complex QA initiatives within the program spanning multiple platforms
- Manages QA project execution from analysis/design through build, test, and deployment
- When leading an Agile projects, partners closely with the scrum master and product owner on all activities
- Ensures that QA program and project status reports and quarterly updates are relevant, transparent, and add value
- Partners with relevant QA leadership, ST, and business hiring managers to ensure that projects have the appropriate QA skillsets and the resources required for delivery
- Minimum of three years’ experience as a senior project manager or QA project lead in IT or a software development setting; must have at least five years overall experience working in IT or a software development setting
- Experience using Agile methods and working on complex, integrated platforms required
- Experience managing QA projects spanning enterprise wide initiatives leading test efforts of various sizes and
- Ability to apply knowledge of multidisciplinary business principles and practices to achieve successful
- Promote and instill a safety first culture in the Laboratory as well as support the Honeywell Structured Safety Program across the entire site
- Conduct daily laboratory safety assessments
- Lead the laboratory by coordinating and optimizing resources (technology, people, and equipment)
- Responsible for the learning, the development, and the performance management in Honeywell Product Development (HPD) of direct reports (12 Lab Analysts & 2 Chemists)
- Drive continuous improvement in the Shreveport Laboratory including the integration of the Honeywell Operating System (HOS), Lead the Built in Quality Element, lead compliance with the ISO 9001 Standard and RC14001
- Maintaining the policy and procedural manuals for the laboratory
- Assure that all laboratory quality problems are satisfactorily resolved through root cause analysis
- Establish and align performance goals and improvement objectives for the Shreveport Laboratory with the Site goals & objectives
- Preparing reports, records, and other required documents and paperwork
- Coordinate capital planning for the Laboratory and serve as a focal point for the procurement of analytical equipment
- Primary liaison between the Laboratory and Operations in the event of quality issues and / or additional resource requirements. Facilitate an effective investigation and root cause analysis with the collaboration of the operations teams
- Respond to laboratory "off hour" emergency notifications providing guidance and direction 24/7 either directly or through designee
- Lead the daily Laboratory Tier Meeting, attend the daily Operations Tier 3 Meeting, and attend the daily Leadership Tier 4 Meeting
- Represent the Laboratory on the site's management teams including the Leadership Team, the HOS Steering Team, the Health, Safety & Environment (HS&E) Council, and the Quality Improvement Council
- Develop and adhere to the annual budget for the Laboratory and monitoring department spending
- Bachelor's degree in Chemistry, Chemical Engineering, Biology, or a related discipline
- Minimum 10 years of experience in an industrial quality control laboratory or commercial analytical laboratory setting
- Minimum 3 years supervisory experience
- Minimum 5 years of experience in a GxP regulated environment
- Leading projects and teams with measurable deliverable outcomes
- Experience in representing the QA Department at upper level management meetings and/or outside of the organization
- Making QA decisions independently
- Experience in representing department in audits and inspections
- Managing team related projects
- Experience in monitoring the overall effectiveness of the in place quality systems and gap analysis
- Expert knowledge of GxP
- Must possess strong communicating skills with the ability to communicate effectively up and down, at all levels of the organization
- Demonstrates comprehensive decision making skill by taking independent and timely decisions; communicates, influences and escalates issues, and serves as final authority, as appropriate
- Strong partnering and negotiating with multiple internal, and frequently also external stakeholders
- In conjunction with the Director, be a leader with a clear understanding of regulatory expectations
- Ability to hire, develop and retain top talent for the QA department
- Capability of leading multiple projects within multidisciplinary environments at the same time
- Bachelor’s degree in Chemistry, Biology, or Biochemical Engineering with 9+ years exp. OR
- Master’s degree in Chemistry, Biology, or Biochemical Engineering with 7+ years exp. OR
- Doctoral degree in Chemistry, Biology, or Biochemical Engineering with 4+ years exp
- Minimum of 4 years experience in Quality Assurance role in quality assurance and/or validation
- Minimum of 2 years of management, project management or leadership experience
- Experience with collaborative cross-site projects
- Strong understanding of medical device QSR (FDA 21 CFR part 820), ISO 13485 and ISO 9001 regulations for diagnostics and life science industry
- Good understanding of risk management, FMEA, validation, and design controls
- Good understanding of validation best practices and regulatory requirements
- Strong problem solving and conflict resolution skills
- Extensive knowledge of US and European Drug Product GMP requirements and associated guidelines
- Experience in participating in pharmaceutical technology transfer teams
- Experience in the qualification of facilities, utilities, equipment and processes
- Ability to increase others knowledge of US end European GMP regulations and guidance
- Has a thorough understanding of the technology, processes, people and equipment of the plant site
- Has a thorough understanding of the regulatory process from GLP through commercial manufacturing
- Requires a Bachelor’s degree in a scientific discipline or equivalent
- Minimum of 8 years’ experience in a Quality Assurance role in the pharmaceutical industry, preferably with parenteral dosage forms
- Evaluate adequacy of quality assurance standards
- Document internal audits and other quality assurance activities
- Minimum 9 years of industry experience in Quality Assurance
- Skills typically required for this job are typically acquired through the completion of an undergraduate degree
- KBR experience preferred
- Work closely with product managers, development teams and data services to develop appropriate test plans, cases and steps from user stories and acceptance criteria
- Conduct manual and automated testing of application suite
- Drive resolution of bugs and issues to ensure timelines are kept in a dynamic and fast paced environment
- Manage a small dedicated team as well as lead others without direct authority
- Provide continuous improvement in the quality management process
- Research and provide proof of concept for new tools and methodologies
- Work independently in the absence of supervision
- Assume and perform other duties and responsibilities not specifically outlined herein, but which are logically and properly inherent to the position
- High attention to detail and strong follow-up and resolution skills
- Experience in automated testing technologies
- Experience in web, mobile and device testing
- Experience working with AWS, Rackspace or Azure
- Meetings or Pharma Industry experience a plus
- Manage a Quality Assurance team of 6 or more individual contributors
- Manage the Quality function of the overall LoanServ product
- Strong process and project management skills, allowing focus and successful multi-tasking in a dynamic environment on multiple testing projects. (demonstrated abilities)
- Utilize leadership skills within the team while setting high expectations for quality results, and provide the vision for the next level of quality initiatives
- Promote business and technical education to train across products
- 8+ years of software industry experience
- 5+ years of hands-on testing experience and 3+ years as a QA team lead or manager (preferred)
- Experience managing or working with remote teams
- Ability to document and track defects, as well as produce detailed reports and quality metrics
- Experience creating and implementing quality strategies and plans
- Experience dealing with 3rd party integration partner testing
Manager, Quality Assurance Validation Resume Examples & Samples
- Manages the Validation department daily work activities including: workload, general guidance to direct reports and across functional groups, and serves as resource for technical / administrative problems
- Hires, trains, motivates, leads, develops and evaluates staff. Takes corrective action as necessary on a timely basis and in accordance with company policy. Ensures compliance with current federal, state and local regulations. Consults with Human Resources Department as appropriate
- Informs personnel of communications, decisions, policies and all matters that affect their performance, attitudes and results
- Leads the commissioning and qualification of new equipment, instruments, including major capital projects and computer systems
- Maintains and enhances validation programs for existing equipment, utility commissioning and qualification, and computer systems
- Assists in the development of a process validation program including assessments of Critical Quality Attributes and Critical Process Parameters
- Drafts, reviews, executes and approves protocols and reports related to equipment, facility, method, computer, and process validation. This includes deviation reporting during execution
- Acts as Validation contact and primary Subject Matter Expert (SME) for Validation and Quality Risk Management
- Ensures the site Validation Master Plan remains current and aligned with corporate policies and industry standards/expectations
- Collaborates significantly with cross functional groups, including Quality, Manufacturing, Process Development, Facilities, and Regulatory Affairs
- Thorough knowledge of cGMP regulations related to validation including ISPE and ASTM standards
- Experience with 21 CFR Part 11 Compliance is preferred
- Experience in process validation is preferred
- Proven track record in validation and qualification of complex equipment / systems
- Ability to lead and partner successfully with staff and chain of command
- High customer service orientation
- Action Planning
- Knowledge of call center business
- Call center experience
- Manage the day to day operation of the Manufacturing Quality Control laboratories ensuring
- Hire, train, and supervise all quality control associates assigned to the manufacturing facilities
- Stay up to date on all existing and new regulatory requirements to ensure Weis Markets, Inc. is in compliance
- Develop a thorough understanding of the Appendix N program and applicable FDA 2400 series forms and maintain as changes occur
- Act as a project manager/lead in the implementation of new regulations
- Manage the Weis Markets product complaint program to ensure complaints are properly investigated and our customers are satisfied
- Act as the direct point of contact with suppliers and category managers with product complaint investigations
- Communicate and update policies, procedures, and programs as dictated by the FDA, USDA, and various health departments’ regulations
- Monitor and assess the effectiveness of current quality control programs, revise and update
- Monitor and maintain the HACCP and SQF programs as applicable to each manufacturing facility
- Ensure Weis Markets is in compliance as it relates to proper labeling on products manufactured at Weis Markets, Inc. manufacturing facilities
- Develop measurable goals and objectives for staff and properly evaluate
- Develop and Maintain relationships with State and Federal Agency Inspectors
- 10 years of related IT job experience
- 5 years of management experience
- Bachelors degree in Information Systems, Computer Science, a related field, or related job experience
- Working knowledge or experience in automated and performance testing tools and strategies
- Ability to effectively collaborate and communicate with internal and external stakeholders, including
- Senior Leadership
- Demonstrated ability to define problems, collect data, establishes facts, and draw valid conclusions
- Demonstrated ability to interpret an extensive variety of technical instructions in mathematical or diagram form and deal with several abstract and concrete variables
- Excellent written and verbal communication skills
- Ability to write business correspondence and reports, systems documentation, and procedure manuals
- Manages and supports the process of complaint investigation, identifying root cause and corrective action with vendors
- Manages specification program, maintaining current supplier information, creating and maintaining food and packaging specifications
- Monitors audit plans to ensure supplier/product compliance to specification and Good Manufacturing Practice
- Provides technical expertise and support to new product development, on projects designed to ensure food safety, improve food quality, optimize products
- Examines, reviews and investigates complaint data to reduce compliance issues and to facilitate continuous improvement
- Manages suppliers to ensure that quality and food safety initiatives are met on a continuous basis
- Supports approval of new and/or alternate suppliers of food products with respect to food safety, quality attributes and product consistency
- 3-5 years of work related experience in food industry, food manufacturing experience desired
- B.S. in Food Science or related science field
- Strong experience in a variety of food manufacturing categories
- Demonstrated problem-solver
- HACCP certification, knowledge of Global Food Safety Certification standards
- Ability to travel as much as 20% of the time
- Ability to work effectively with associates throughout the organization
- Efficient, self-starter with a positive attitude
- Proficient in Microsoft Office including Word, Excel and PowerPoint, and Oracle
- Responsible in full for adherence with the quality agreement provisions
- Responsible to ensure all deviations are appropriately investigated and recorded
- Responsible to drive CAPA items to complete and timely completion
- Directs the investigations of customer product complaints and assures the completion of the appropriate documentation
- Performs assessments for all product-related changes, assess relevance to regulatory filings, decide to implement and provide change controls for approval to customers where required
- Ensures an efficient cGMP compliant life cycle management of all products manufactured
- Has the authority to make quality decisions for the project in internal and external meetings
- Auditing experience, auditor certification is an asset, Competent level of general IT skill is required, General knowledge of manufacturing processes and analytical methods, Oversee project execution to identify non-compliance from quality standards, Subject matter expertise in cGMP compliant manufacturing, Understanding of the applicable cGMP regulations
- Project tracking and reporting
- 5+ years in software/video game QA
- 3+ year’s leadership experience
- Recent experience managing large test teams on multiple software projects
- BA/BS or equivalent combination of technical training and work experience
Asst Manager Quality Assurance Resume Examples & Samples
- 3 Lead and develop direct reports ensuring they are competently skilled to perform their QA roles. Lead by example; consistently motivate staff through education to high levels of competency that drive success in a dynamic and regulated environment. Upon proper review with center and quality management, recruit, hire, conduct performance reviews and enact corrective actions, up to and including termination
- 8 Adhere to all HR policies and practices through fair and equitable treatment of all employees. Communicate effectively with HR to ensure HR compliance and ensure center management is doing the same
- 9 Comply with all federal, state and local regulatory and company policies and procedures
- 1 year Quality experience (CSA/CSAb) required OR
- 15% - Manages direct and overhead budgets within agreed goals or limits. Responsible for hiring employees, setting goals and objectives, providing performance feedback, coaching, evaluations, and taking appropriate action as necessary based on results
- 10% - Provides daily job assignment and direction to direct reports
- 15% - Provides assistance in creating, implementing, and attaining established site, program, and organizational goals, and provide the required resources in resolving complex technical or operational challenges
- 15% - Develops, communicates and implements program quality plans that comply with contract requirements, quality management standards and command media
- 15% - Trains and ensures full compliance with policies and procedures related to drawing requirements, controlling of nonconforming material, cause and corrective action, first article inspection, engineering milestone reviews, risk management, AS9100 requirements, as well as other responsibilities in quality engineering and inspection
- 15% - Manages effectiveness along with participation and leadership in cause and corrective activities, failure investigations, metric creation & implementation and drives actions and accountability, and special projects to support program Corrective/Preventive Action Committees (CACs/PACs) and other management boards
- 15% - Works with customers in coordinating hardware acceptance reviews, customer participation in CACs/PACs and in developing regular meetings to communicate and resolve quality issues and concerns
- Quality Manufacturing background
- Understanding and experience with AS9100 Standards
- Lead site Change Control Management to comply with Global Quality System requirement and verify the effectiveness of approved change requests. Liaise with Global / Regional Change Control community as required
- Manage document control system for use by functions within the Singapore Supply Center site. Review and approve local SOP in the GPDMS (Global Procedural Documentation Management System) to ensure they met MJN quality system requirements, and have incorporated relevant new learning / processes and regulatory changes
- Ensure timely review of materials and products quality and perform disposition to meet production schedule and delivery deadlines
- Coordinate and facilitate non-conformance (NC) investigations, and ensure that root-cause analysis by the investigation team is thorough and complete, and appropriate corrective and preventive actions (CAPA) are put in place to minimize product loss
- Prepare Potential Write-Off Alert (PWA) when required, and follow-up on sharing of key learning
- Implement an effective internal audit program and ensure compliance to requirements of
- GMP / HACCP pre-requisite programs
- Quality systems
- Halal assurance system
- Lead site team to prepare for audits (e.g. Global/Regional Quality Audit, External Certification Bodies), and ensure agreed audit findings are corrected and preventive measures are put in place
- Organize Annual Product Review to drive product quality improvement
- Support innovation projects and timely launch of new production introduction
- Manage the customer complaint handling system – evaluation, investigation and corrective/preventive action planning, implementation and monitoring
- Manage the local Mock Recall exercise as per MJN procedures
- Assist Senior Manager, Quality in liaison with Global / Regional Quality in crisis situations, such as a potential product recall, or high profile customer complaint
- Represent the Singapore Supply Center in various quality professional communities (e.g. GMC) to create a network for teaching and learning and identification of best practices
- Support OE/Engineering improvement projects to ensure compliance to MJN quality systems requirements
- Support Procurement team to help existing material suppliers to meet MJN Quality standards through sharing / training. Support the RMAQ (Raw material attribute questionnaire) review process for new material suppliers
- Develop succession and development plans for direct reports, ensuring for their career growth and succession
- Perform other tasks, roles or projects that may be assigned by the Plant Director or Senior Manager, Quality from time to time
- Minimum Bachelor degree in a scientific or technical discipline (Chemistry, Microbiology, Food Science or related)
- Practiced minimum 5 years of Quality Assurance / Quality Systems in a regulated food manufacturing industry. Experience in manufacturing of infant/follow up formula will be of advantage
- Performed supervisory role for min 3 years
- Experienced in establishing/implementing Quality Management Systems, Food Safety Management System, Halal Assurance System
- Preferably with extensive quality system audit experience
- Experienced in collaborating with various functional roles within the organization to develop strategies and execute action plans to resolve critical issues
- Product knowledge and ability to learn new markets such as Cell Therapy, Water Filtration
- Compliance with (global) legal requirements by adherence to requirements of existing and new regulations and legislation. Investigate and provide appropriate regulatory intelligence when necessary and ensure compliance
- Participates and contributes and provides deliverables to new product project meetings with regards to activities related to product labeling and product registration for on-time product commercialization
- Negotiates solutions as they relate to labeling within interdisciplinary departments
- Initiated benchmarking meeting with leading pharmaceutical manufacturer to help to eliminate labeling errors
- Work directly with in-country representatives to facilitate product commercialization as it pertains to product labeling and registration
- Provide guidance and direction pertaining to strategies necessary for product registration
- Anticipate and/or identify problems which could potentially delay product registration and provide solutions
- RA Reviewer for QARAC, QIRP and LRC processes
- Provides technical support and/or RA support to filed sales representative and Pall Medical customers
- Comply with all company policies and procedures
- Works in a manner that supports team work
- Completes work to assure commercial, regulatory and customer requirements and is results oriented
- Ensures a safe and healthy environment by complying with all company safety policies and procedures
- Carries out supervisory responsibilities in accordance with the organization’s policies and applicable laws
- Responsibilities include interviewing, hiring and training employees; planning, assigning and directing work; appraising performance; addressing complaints and resolving problems
- 7+ years of experience in the medical industry with strong knowledge of ISO9001/13485 requirements
- 7 years or more of experience interfacing directly with FDA
- 7+ years of experience demonstrating knowledge and application of medical device adverse event reporting requirements including 21 CFR parts 820 & 803, ISO13485, and Canadian Medical Device regulations
- Masters Degree, MSc or MBA preferred
- Ability to read and comprehend simple instructions, short correspondence and memo’s. Ability to write simple correspondence. Ability to effectively present information in one-on-one and small group situations to customers, clients, and other employees in the organization
- Ability to read and interpret documents such as safety rules, operating and maintenance instructions and procedure manuals. Ability to write routine reports and correspondence. Ability to speak effectively before groups of customers or employees of organization
- Ability to read, analyze and interpret general business periodicals, professional journals, technical procedures or governmental regulations. Ability to write reports, business correspondence and procedure manuals. Ability to effectively present information and respond to questions from groups of managers, clients, customers and the general public
- Ability to read, analyze and interpret common scientific and technical journals, financial reports and legal documents. Ability to respond to common inquiries or complaints from customers, regulatory agencies or members of the business community. Ability to write speeches and articles for publication that conform the prescribed style and format. Ability to effectively present information to top management, public groups and or boards of directors
- Ability to read, analyze and interpret the most complex documents. Ability to respond effectively to the most sensitive inquiries or complaints. Ability to write speeches and articles using original or innovative techniques or style. Ability to make effective and persuasive speeches and presentations on controversial or complex topics to top management, public groups and or board of directors
- Continuously improves food safety and food quality performance at the plant. Manages the internal audit program of the facility to verify that procedures mandated by the company and State and Federal governmental agencies are being adhered to. This includes GMP’s, Sanitation, Food Safety Plans Preventative Maintenance, and Safety
- Installs appropriate controls, hold/release system modifications, code rotation, batching and processing controls and other production systems where problems might exist. Troubleshoot quality problems associated with production; investigate root cause, develop and implement corrective actions, prevent reoccurrence through development of long term solutions
- Lead and manage the WWF QMS program including the implementation and maintenance of core and operational standards to prevent food quality and/or food safety issues to include environmental monitoring, pest control, sanitation, GMPs, etc
- Coordinate and manage sampling plans as per the corporate standards to monitor finished product quality, raw materials, and compositional results to ensure standards of identity are maintained
- Manages within the WhiteWave Integrated Management System (WIMS), including the leadership and oversight of the Quality Pillar
- Oversees the management of all aspects of the Quality Assurance Laboratory (raw and finished goods), including microbiological, chemical, physical and sensory aspects of all food products manufactured at this facility. This also includes coordination of the sampling and sensory testing
- Participates in troubleshooting events associated with production, ensuring root cause/corrective actions are appropriate and are fully implemented
- Manages the development, updates, and implementation the plant Food Safety, and GFSI certification programs
- Manages the reviewing and maintenance of all required processing records, regulatory inspections and reports, and consumer complaints. Develops tools to monitor deficiencies and quickly implement corrective actions when necessary
- Acts as plant liaison with regulatory agencies and client company representatives and auditors. Manages regulatory issues to include Federal and State inspections, kosher inspections, organic inspections, etc. Coordinate corrective actions with Corporate QA, plant and operations management
- Helps develop and maintain a QA department budget
- Assures that plant is audit- and inspection-ready at all times
- Coordinates QA efforts with R&D, Technical Services, and plant involving new product launches, trials, and equipment installations. Coordinates all research and development testing on associated projects
- Stays current with all key regulatory and certifying agency guidelines. Manages regulatory issues within assigned region. Ensures annual permits and certifications are renewed and kept up to date
- Improves the quality metric tracking program and develops site-specific metrics
- Delivers company and facility performance targets in areas of food quality attributes, food safety metrics, and cost
- Manages release of product following verification of conformance to specifications
- Provides direct supervision to subordinates, including making employment decisions, providing training and development opportunities, and conducting performance reviews and coaching sessions
- Minimum of BS in Science / Food Science / Technical Discipline
- 5-10 years previous Quality Assurance experience in food/beverage manufacturing environments with emphasis on ESL, HTST, and/or Dairy equipment, including 3 years as a manager
- Knowledge of plant production systems, FSMA, GFSI schemes, GMP, HACCP, sanitation, plant safety, quality management systems, Non-GMO Verified Project, kosher regulations, NOP regulations
- Must possess exceptional spreadsheet, word processing and database computer skills
- Must have excellent communication and presentation skills
- Must have excellent recordkeeping and documentation skills
- GFSI certification training and experience is preferred
- Self-driven, results oriented, able to work independently
- Ability to work with confidential materials
- Ability to lead and partner successfully with teams, management and client
- Ability to manage multiple, complex, on‐going tasks and projects
- High level of integrity, judgment and follow through
- Strong coaching, people, and leadership skills
- Strong analytical, verbal and written communications skills
- Technology acumen and reporting
- Assure compliance to all company quality and food safety policies, and all Federal, State, and local regulatory requirements
- Assure compliance to company quality procedures, ingredient specifications, process control specifications and finished product specifications
- Lead the Quality Pillar for the plant: setting direction, building capability, maintaining 90 day plans
- Build skills and capabilities in Quality Key Element Owners and Functional Leaders. Ensure effectiveness of site QA training program (new hire and ongoing QA training.)
- Drive continual quality improvement as measured by Quality key performance indicators including internal and external audits, and company quality metrics
- Provide technical support to the manufacturing facility in relation to quality and food safety requirements
- Perform quality audits at supplier facilities in the U.S., Canada and internationally, including working with each facility to implement corrective actions
- Participate in JMS initiatives and projects for supply chain changes and new product introductions
- Accountable for leading and maintaining site Food Safety and Food Defense programs
- Responsible for site Food Safety Plan
- Lead GFSI Audit program
- Communicate JMS quality, food safety and food defense guidelines and present training as applicable to all employees
- Manage Quality Incidents for each facility with the goal of investigating to root cause and implementing corrective actions to prevent incident reoccurrence
- Coordinate holds, product disposition, product withdrawals and recalls
- Monitor quality of manufactured products using consumer complaint data, including complaint investigation, root cause analysis, complaint response and corrective action
- Manage Quality Assurance budget
- Prior QA/QC leadership experience strongly preferred
- At least 5 years of experience in food manufacturing operation is required
- 2 years of experience managing or leading personnel
- Proficiency in Microsoft applications
- Experience with Quality processes and implementation (e.g. Nonconformance processing, Audits, Safety, VRICS, SCARS,
- Risk Management, Root Cause & Corrective Action, Engineering
- Review Board decisions. Current MRB certification a plus Working knowledge of AS9100, ISO9000, AS9003
- Working through individual contributors or supervisors, ensures the effective implementation and maintenance for Quality Systems requirements including but not limited to, MDD 93/42/EEC, ISO13485, CMDR, and QSRs. Ensures organizational compliance with Quality Systems procedures and regulations
- Establishes and manages inspection, sampling and testing to assure batches/products meet specification from raw material to finished goods
- Partners with Plant Management to pro-actively identify and resolve quality issues and ensures products are manufactured in compliance with written specifications. Regularly monitors the status of corrective actions/preventive actions to ensure quality issues are resolved and products are manufactured in compliance with written specifications
- Oversees the quality system compliance of the site through internal audit program, local management review, quality meetings, and routine trend analysis of key performance indicators. Initiates corrective and preventive actions as required
- As local Management Representative having responsibility and authority at the local level that includes
- Ensuring that processes needed for the quality management system are established, implemented, and maintained,
- Reporting to top management on the performance of the quality management system and any need for improvement, and
- Ensuring the promotion of awareness of regulatory and customer requirements throughout the organization
- Represents Haemonetics in 2nd (e.g. customer, contractor) and 3rd (e.g. FDA, ISO) party quality system audits
- Partners with plant management to integrate design and engineering change to processes and product through a quality plan including verification and validation
- Promotes and educates personnel on quality system requirements, responsibility, and accountability. Interacts with senior management and collaborates across functions, and Is subject matter expert (Haemonetics QMS)
- Participate in the development of short- and long- term goals and plans for the work group
- Provide effective leadership and management to Quality Assurance Analysts
- Provide technical project management
- Manage staff in globally distributed locations
- Partner with development and other areas of the organization on technical projects
- Promote and drive metrics into all areas of the organization
- Develop strategy around process improvements, staffing, reporting, and overall QA strategy
- Develop and execute testing automation strategy
- Mentor senior engineers to ensure skills are adequate to perform in their assigned roles
- Demonstrate awareness of business objectives, impacts to the company and accountability for decisions made within the team
- Coordinate the development, implementation and recording of suitable training programs to ensure awareness of best practices and compliance requirements
- Assist in the coordination of departmental administrative procedures, including: performance planning and review, promotion planning, hiring etc
- Facilitate regulatory inspections and report results of such inspections periodically to management
- Advise functional staff regarding regulatory compliance and industry expectations
- Provides leadership in the execution of effective testing of code functionality, as well as the technical leadership in regard to testing techniques
- Plan, write, execute and evaluate software quality assurance (SQA) tests of code releases
- Document, track and report software defects following department standards and procedures
- Analyze and resolve problems
- Responsible for the development and maintenance of an effective organization for areas of responsibility, including: a) evaluation of staff in accordance with project deliverables; b) efficient work flow patterns; c) coordination of training in software/hardware, and departmental procedures; d) effective delineation of duties and responsibilities; e) suitable staffing commitments; f) appropriate supervision
- Responsible for the development, implementation, documentation, and monitoring of an ongoing quality assurance program. Ensures compliance with department standards, and regulatory agency guidelines and standards
- Serves as liaison with other organizational components; establishes and maintains the working rapport necessary to develop cooperation and effectively integrate area services into the company’s overall business
- Must have a minimum 10 years of professional experience in related field
- 3+ years of experience with automation testing
- Level of knowledge ordinarily acquired through the completion of Associate Degree or Bachelor's degree and/or equivalent work experience
- Three or more years of progressively more responsible experience in QA testing industry standards, and project management
- Knowledge in human resource policies and procedures; organization, management and business planning; understanding of software/hardware technology
- Intermediate working knowledge of Microsoft Project, Word and Excel required
- Knowledge in HP Quality Center, JIRA, TFS
- Knowledge of at least one automation testing tool
- Ability to learn new applications
- Ability to prioritize, organize and work independently or as part of a project team to complete multiple tasks
- 10+ years of software product quality assurance experience including test planning, implementation and execution
- Experience in a management position for test engineering or QA teams supporting web applications
- Advanced working knowledge of test engineering processes and methodologies; Expertise in test planning, test case management, code coverage and defect analysis
- Significant hands-on experience in project management and Agile/Scrum product development life cycles
- Strong Java and Javascript knowledge , hands-on coding experience will be asked to write code in interview
- Thorough understanding of Design Patterns and SOA
- Thorough understanding of performance testing and automation testing. Hands on experience in JMeter, Perfmon,Selenium
- Skilled in CI processes, thorough understanding of Jenkins CI and GIT
- Strong data structure and algorithm knowledge
- Thorough understanding of Operating System Concepts
- Experience testing software and services using common API techniques (SOAP, REST, XML, etc.)
- Planning, designing, implementation and use of automated test suites including open source and commercial tools
- Demonstrated leadership, coaching and mentoring skills combined with flexibility working in a dynamic, fast moving and fast growing product development environment
- Manage multiple competing priorities while driving resolution and getting results
- Act as the clinical quality system manager, ensure the Quality Systems are established, implemented, and maintained in compliance with all appropriate regulatory standards, including federal, state and local; acts as the main point of contact for all external audits/assessments
- Oversee and assist in the development of the Internal Audit program, external audit program, proficiency testing, and management of audit corrective actions
- Coordinate regular Quality Systems meetings reviewing quality metrics
- Oversee the CAPA as well as non-conformance processes; assists with managing the document control system as well as the archiving of records according to the San Diego clinical lab site’s retention schedule
- Manage the updating and creation of QSEs and WIs for the quality department including annual review; ensure documentation of lab procedures and completion of DHF
- Ensure changes in products, procedures, and training are implemented correctly
- Manage the creation or purchase of training materials for the implementation of Quality Systems/Quality tools, ensures there is training for end-users and perspective trainers; works with training coordinator to ensure appropriate training on all procedures; including new and revised procedures
- Participate in Product Quality reviews, management reviews and preparation of Quality reports
- Coordinate site Quality System assessments and provide recommended roadmaps for improvement
- Develop and review validation and verification reports while ensuring a successful implementation of newly developed assays
- Bachelor’s degree in a life science, preferably Biology or equivalent; advanced degree in molecular science desirable, Regulatory Affairs Certification desirable
- 10+ years’ experience in Quality Assurance and/or laboratory experience in the molecular diagnostic industry, with general knowledge of statistical analysis
- Minimum of 4 years’ people management experience
- Experience as the team lead for client and regulatory audits including FDA , CAP, NY, CLEP and any additional agencies as required
- Prior experience in a clinical lab (CAP, NYS, CLIA) highly preferred
- Must possess an understanding of the fundamental principles and concepts of molecular biology, genetics, and how these relate in a clinical setting along with work flow through all phases of laboratory operations
- Careful and accurate documentation of work
- Effective interpersonal and communication skills and capable of supporting cross-functional project goals
- An understanding of Quality Systems, HIPPA, CAP, CA, NY CLEP and CLIA policies and safety procedures is required
- Working with business and development teams to understand product vision and requirements
- Understanding how all elements of the system software ecosystem work together and developing QA approaches that fit the overall strategy
- Overseeing the development and execution of test plans and monitoring and reporting on test execution
- Will work with a team of quality engineering professions to ensure the highest quality product delivery
- 7 - 10+ years of industry experience, including at least three years of hands-on testing and five years as a manager
- Experience working closely with development and business teams to communicate problem impacts and to understand business requirements
- Strong organizational skills, tracking multiple test executions simultaneously and are able to synthesize the results
- Embedded systems experience required
- Experience with systems automating client / server testing with a heterogeneous mix of client applications and embedded devices is required
- Owns service design, implementation and operation for the quality assurance services delivered to the rest of IT
- Manages the processes and people to ensure quality products meets defined business requirements and design specifications
- Manages quality assurance teams to ensure the development of systems and processes meet REIs quality assurance standards across the software development lifecycle
- Creates, analyzes, and publishes relevant quality metrics and measures across teams to communicate application and release quality, and to address technical debt, risks, and other quality issues
- Guides the development of test strategies and front end automation plans within Agile and Waterfall development methodologies utilizing open source or commercial automation tools, packages as applicable
- Ensures that the quality engineering discipline builds and utilizes robust, scalable test frameworks
- Provides quality assurance strategy for enterprise package solutions. Works closely with the vendor, development services manager, systems manager, and project manager to incorporate sound test methodologies and best practices into the quality assurance environment. Drives the discovery and adoption of new methodologies using effective communications and training of staff
- Provides leadership to validate technical requirements; ensure testability and traceability of new systems; and ensure successful generation and utilization of automation/performance test cases, scripts and plans
- Collaborates with Project Management Office to manage the progress of development projects in process. Monitors performance of assigned quality assurance assignments to ensure they are completed on time and within budget
- Collaborates and coordinates with other Information Technology (IT) departments regarding quality assurance deliverables, disciplines on overall test plans, including user acceptance, performance and regression testing, to ensure successful transition and system reliability of developed systems into the enterprise
- Ensures delivery of documentation of test results and corrective actions, including recommendations to improve quality and reliability of systems. Ensures defect management using quality assurance tools
- Provides strategic direction for quality assurance. Researches, evaluates and recommends selection of software testing products, automation and performance processes and methodologies to improve the quality and efficiencies of the quality assurance deliverables, staff, and systems
- Contributes to division strategic planning, applying a current knowledge and future vision of technology and systems which significantly impact the effective execution of business processes
- Leadership and management of the Quality Assurance Engineering team for medical device product releases
- Management of associated lab environment and equipment
- Management of the formal product release process to ensure product releases are ready for human use
- Work on a cross-functional team in the development da Vinci™ Surgical System products. Lead the Quality Assurance Engineering team to
- BS in engineering sciences or equivalent, or commensurate experience
- Minimum of 5 years experience testing complex electro-mechanical systems with embedded software and firmware
- Minimum 2 years leadership experience
- Hands-on servant leader, able to effectively plan and direct engineers in sometimes demanding circumstances to meet company goals
- Proven experience building a team and leading a test function
- Experience working on product-focused designs
- Demonstrated experience in and comfortable with all phases of the product development lifecycle including design, implementation, debug, verification, validation, and transfer to Manufacturing
- Experience with product development in a FDA or other regulated industry or for mission critical applications is desired; comfort with concepts of design input, design output, traceability, and risk analysis
- Good command of source control management and SW build development
- Hands-on engineering experience with proven ability to work well in a team environment
- Interest in the medical applications of robotics
- A real excitement to learn and get to the bottom of tough technical problems
- A desire to deliver a quality and innovative product that improves many peoples lives
- Strong track record of continuous improvement
- Assist in management and enhancement of supplier qualification process that will use risk-based tools for qualification, classification and monitoring
- Perform audits of and manage audit closure for suppliers of raw materials, components, contract labs, CMO sites, service providers and warehouses
- Develop and review supplier QTAs (in collaboration with legal and supply chain) and establish a process to assure key quality expectations are reflected in supplier’s operations and updates for changes are managed
- Collaborate with spec development team to execute specification development process to create/change GMP part numbers, specifications and associated Item Master in Oracle EBS
- Assist in the identification and development of raw material and component specification criteria, ranges and limits using CQAs as inputs
- Assist in the management and execution of supplier change management system for raw material/component suppliers, contract labs, CMOs and warehouses
- Assist in maintaining the audit schedule and approved supplier list (Oracle Agile and EBS)
- Support inspection readiness plans and interact with regulatory agencies during inspections on SQM-related matters, as needed
- Assure compliance to affiliate quality and food safety policies and standards, and all Federal, State, and local regulatory requirements pertaining to the manufacture of safe and wholesome pet food product; this includes the affiliate’s quality policies manual, ingredient specifications, process control specifications, and finish product specifications
- Monitor and assure conformance of ingredients to affiliate standards and plant expectations including supplier auditing, supplier approval, and analytical and sensory testing
- Drive continual quality improvement as measured by key indicators including internal and external audits, and achievement of factory goals such as RFT and consumer complaint reduction
- Drive continual improvement in food safety and sanitation as measured by key indicators including AIB audit performance, and other internal and external audits
- Manage and maintain effective food safety and sanitation systems such as HACCP, pest control, and good housekeeping
- Support and contribute to the achievement of factory goals
- Coordinate and support all commissioning of new products and processes and issue final sign off for the factory
- Provide technical support to factory operations including trouble shooting and solution generation, Plant Run Request, and plant projects
- Perform annual performance appraisals for all direct reports and develop key personnel through coaching, training, and employee and organizational development means
- Manage the Quality Assurance budgets
- At least 5 years of related QA experience is required
- Prior experience in the CPG industry is preferred
- A working knowledge of Good Manufacturing Practices, FDA and USDA regulations, food plant production and best practices, environmental regulations, food safety and sanitation methods and best practices, HACCP, and SPC is preferred
Manager, Quality Assurance Projects Resume Examples & Samples
- Lead the planning and implementation of the LIMS project
- Facilitate the definition of project scope, goals, and deliverables
- Ensure on-time, on-budget and quality delivery of application-based projects in collaboration with project sponsors, project champions, project teams, and other stakeholders
- Assemble project plans and teamwork assignments, direct and monitor work efforts on a daily basis, identify resource needs, perform quality reviews, and escalate functional, quality, and timeline issues appropriately
- Ensure project documentation is complete and communicated (e.g. project charter, scope statement, project schedule, project budget, project calendar, issues matrix, weekly status reports, etc.)
- Track, manage, and control project milestones, activities, and resources
- Use, develop and improve upon the existing PMO project management methodology
- Provide Leadership and project teams with advice and input on tasks throughout the project, including documentation, issues management, risk management, and the creation of plans, schedules and reports
- Resolve conflicts within the project and across dependent projects between resources, schedules, budgets, priorities, etc
- Influence stakeholders and team members in order to get buy-in on decisions that will lead to the success of critical projects
- Manage 3rd party vendors as an integral part of the project team’s activities
- Effectively share knowledge and lessons learned with other PMO project managers and leadership
- Present project status and issues at project’s Executive Steering Committee
- Excellent verbal and written communications, effective time management, and organizational skills
- Must be independent, detailed oriented, and have the ability to manage time
- Proven success with on-time, on-budget delivery of multiple large scale, enterprise wide projects
- Experience managing 3rd party vendors, including system integration firms
- Consultative skills, such as workshop facilitation
- Strong change management and negotiation skills with ability to work within a matrix-run organization
- Desire and ability to mentor staff and project teams
- Possess a high degree of personal integrity and strong work ethic and be proficient in delivering sustained results in a dynamic environment
- Able to work in a team setting and independently under minimum supervision
- Ability to work in fast paced environment
- Creative individual with excellent trouble shooting skills
- Ensure Compliance with global directives, standards and guidances
- Build strong and positive cross functional working relationships by working to include them in the improvement activities/training, making sure their needs and requirements are carefully considered
- Master’s Degree and 5 year of relevant industry experience
- Experience presenting to large groups in areas of subject matter expertise
- 5 years in the Biotechnology industry with 3 years of experience in a Quality role in a GMP environment
- Experience with reviewing deviations and CAPAs
- Experience in reviewing change controls
- Experience with an electronic Quality/Document Management System
- Strong written and verbal communication
- Experience serving as Subject Matter Expert (SME) in regulatory inspections
- Experience performing work that consistently requires independent decision making and the exercise of independent judgment and discretion
- Experience influencing others in a matrix environment
- Experience with technical writing or review
- Experience with project management or team leadership
- Understanding of industry standards
- Experience generating process metrics and reports
- Experience operating in an environment with a strict timeline
- Experience in continuous improvement methodologies
Associate Manager / Manager Quality Assurance Resume Examples & Samples
- Executed records
- Failure investigations
- Change Controls
- Reviews customer requirements and implements required processes, as needed, to meet customer expectations regarding Quality
- BS in life sciences discipline or related field (chemistry, biology, or pharmacy preferred) with experience in a pharmaceutical/biologics manufacturing environment desired. Minimum 6 years experience in pharmaceutical industry with 2+ of those years to include leadership and/or supervisory experience
- Level (Associate Manager OR Manager) will be determined based on skills and related experience*
- At least 5 years of experience in food manufacturing operations is required
- Prior QA/QC leadership experience is required
- A working knowledge of FDA regulations, including cGMPs and FSMA is preferred
- Experience with GFSI audits is preferred
- Assist the Head of Quality Assurance with the development, implementation, optimization and strict adherence to a quality assurance program which meets the most up-to-date regulatory requirements and the AMRI global quality directives. Develop and/or review and/or approve standards, policies, and procedures for all functions and departments involved with or related to the production and testing of all materials, as assigned
- Develop and motivate Quality Assurance staff to effectively carry out department functions; provide leadership, guidance and direction of staff consistent with cGMP and company corporate quality objectives. Coach and mentor staff in all aspects of their job performance and career development including training, feedback and disciplinary action. Ensure that the staff has a full understanding of department processes and procedures
- Manage the disposition of all raw materials, intermediates, packaging and factory supplies, and all finished goods for commercial and clinical operations
- Manage the Quality Events Management System for deviations, failure investigations, out of specification/out of trend investigations, calibration out of tolerance events, CAPAs and CAPA effectiveness monitoring. Ensure that all Quality Events are completed in the time required by company procedure and that all investigations are completed in a thorough manner to appropriately identify root cause and corrective/preventative actions. Authorize critical deviations and provide guidance and troubleshooting during investigation
- Manage all customer complaints and recalls. Authorize material returns and destruction, including recommendation of corrective action plans/programs for overall defect reduction
- Report serious or repeated failures or unreliability in quality of products to Head of Quality Assurance
- Manage the preparation and approval of master batch records, cleaning batch records, monographs, and test methods. Review and oversee implementation of change notices and related document change control
- Manage the issuance of production batch records, equipment log sheets, and log books
- Manage the review of all manufacturing and test documentation to assure conformance to quality standards including the review of production and cleaning batch records, shipping records, stability protocols, pest control activities, testing and inspection records, and customer contract specifications
- Approve master item number data and changes, as well as finished good labels
- Authorize reprocess, rework, disposal or return to vendor decisions. Approve inventory adjustments
- Participate on cross functional teams and quality global inter-site initiatives representing the site Quality department, as assigned
- Manage and participate in the QA on-call rotation
- Prepare, review reports and other documentation required by regulatory agencies to support the quality assurance function
- Provide support during regulatory inspections and customer audits. Assist in performing inspection readiness activities and supplier audits
- Minimum Bachelor of Science in Chemistry or a related field
- Minimum five years’ QA, RA experience in pharmaceuticals or a related cGMP regulated industry
- Minimum two years supervisory experience or coach/mentor role
- Broad knowledge of cGMP, FDA and international regulations (e.g., 21 CFR 11, EU GMP, ICH Q7), Quality Systems for active pharmaceutical ingredients (APIs), and familiarity with guidelines (e.g., FDA, ICH)
- Knowledge of pharmaceutical processes to be able to make appropriate decisions
- Strong interpersonal, written and oral communication skills
- Excellent communication and collaboration skills and demonstrated ability to work cross functionally across internal stakeholders
- Scopes and leads complex process audits, systems audits and other types of audits
- Expertise in CAPA management
- Subject matter expert in ICH GCP and regulations
- Experience in leading process improvements, SOP creation and revision
- Involvement in supporting client audits and regulatory inspections
- Experience training and mentoring junior staff members
- Travel up to 30%
- Fluent in Spanish, preferred but optional
- Intermediate Excel skills, preferred but optional
- Minimum 7 years CRO/Pharmaceutical Experience
- 6 years in GXP quality role, including at least 4 years n a GCP quality role
- Create, implement, maintain and update policies, procedures, specifications and programs that ensure Little Caesar Enterprises product safety and quality
- Identifies and resolves supplier food safety and quality issues through continuous product testing and resolution of field and customer complaints
- Manages assigned product categories and implements relevant laboratory procedures to ensure supplier specification compliance
- Works with assigned category suppliers and appropriate Little Caesars staff to ensure resolution and root cause analysis with appropriate corrective actions are taken for all product issues
- Handles all communication of product specification changes through appropriate channels to ensure change management success
- Responsible for corporate product recalls and withdrawals and maintains required documentation as required by law. Administers mock recalls to distribution centers
- Performs other duties as assigned by the Director of Quality Assurance
- Bachelor degree in Food Science, Biology, Chemistry or related discipline or equivalent experience
- Minimum of five (5) years quality assurance experience in the food industry
- Knowledge of food industry manufacturing practices and familiarity with FDA and USDA regulations as they pertain to assigned product categories and other areas of responsibility (distribution centers). This will include OSHA lab standards
- Ability to communicate effectively with franchisees, vendors and other departments. Ability to communicate complex technical data in an understanding way to a variety of audiences
- Ability to establish, document, update and track quality metrics through standardized and accepted process control procedures
- Managers must be accurate and have attention to detail in the documentation of issues, resolutions, root cause, preventive controls and policy changes
- Excellent problem-solving and decision making skills. Good interpersonal skills and ability to resolve conflicting points of view
- Demonstrated ability to manage multiple, complex projects and initiatives simultaneously with a results-oriented approach
- Computer proficiency (Microsoft Office, database, Internet and documentation software required
- Sensory abilities for product evaluation
- Ability to travel up to 30% of the time and to adhere to the LCE Corporate Travel Policy
- HACCP certification and foodservice sanitation certification
- Knowledge of SQF and BRC
- Knowledge of FSMA and how it affects suppliers, warehouses and product transport
- Experience with lab analysis and testing on products used in the pizza business
- Education or experience equivalent to a 4-year college degree is required
- At least five years related experience is required, Revenue Management processes, QA Management/experience preferred
- Demonstrated success in customer service and staff management
- Manages more than one function or area on a regular basis. Additional complexity and breadth contribute to the overall accountability of the job
- Leadership of a diverse team, including all aspects of people management, selection, training, resource capacity, job design and performance management that creates a high-performance operating environment
- Ensures quality, audit and control standards through the development of effective standards, policies, education and creative training programs
- Develops workflow to meet specialized needs to ensure that appropriate service and financial levels are met at all times
- Manages internal and external customer expectations through project planning and partnering efforts throughout the corporation
- Provides leadership and direction to project teams who have are involved with projects specific to the operational area
- Communicates project strategies and status to all impacted parties
- Meets challenges head-on by the efficient and timely development, identification, and implementation of new strategies, solutions and opportunities for improved service standards
- Coordinates training, staffing and system changes as dictated by project plans
- College degree preferred plus 5-7 years of increasingly responsible management experience
- Proven ability to make sound business decisions and deliver results
- Solid understanding of the service needs and financial impact to service levels
- Demonstrated experience in motivation, team building and collaboration
- Strong interpersonal, communication and negotiation skills - both written and verbal
- Strong project management, leadership and organization skills
- Solid understanding of the budget and salary administration processes, and the impact of each to productivity, morale and service levels
- Overall knowledge of business workflow within Unum in general and functional area(s) in particular
- Meets the standards for this position, as defined in the Talent Management framework
- Lead a team of salaried and hourly quality experts, providing mentorship, guidance and accountability for team performance
- Deploy operations related best practices to measure and improve team safety, quality, and productivity
- Build relationships with cross-functional groups to identify, reduce and ultimately eliminate impacts from vendor defects
- Develop existing and future state process maps, expanding the scope to cover vendor defect management across SpaceX
- Present details and status to executive management
- Develop policies, standards, processes, and documentation as necessary to ensure continued success
- Bachelor of Science degree in an engineering field
- 5+ years of experience in an engineering, or quality related field
- Master’s degree engineering or supply chain
- Good written, oral, and presentation skills
- Ability to operate autonomously and self-direct
- Experience in quality engineering, auditing, quality management system development
- Preferred skills or experience include: SQL, software development & project management, process flow mapping, material planning systems, warehouse management systems, lean production environments, six sigma training
- Take responsibility for validation of conformance and precision measurement of product received from SpaceX suppliers with certification reviews, a precision metrology lab, and supplier inspection programs
- Develop world-class metrology capabilities to service both one-off and ongoing needs
- Drive vision, direction, and efficiency gains to meet scale and quality objectives
- Develop statistical models to guide inspection levels necessary based on product maturity, supplier capability, and other related factors
- Lead quality, efficiency and cost improvements on supplier inspection programs
- Assist in root cause investigations when quality issues do arise. Communicate findings and train staff on improvements or preventative measures
- Ensure a high level of internal and external customer service. Investigate and correct customer issues and complaints relating to quality
- Interface with other organizations within SpaceX to ensure all quality requirements are understood and achieved
- Be a crucial voice in the refinement, interpretation, documentation, and communication of SpaceX’s quality control philosophy to key personnel within company
- 5+ years of experience in supply chain and/or quality
- 2+ years in a management capacity or Master’s Degree in engineering or business
- High level of expertise in GD&T symbols and their intent and the selection of the correct inspection technique and equipment
- Metrology expertise, CMM techniques and language (PCDMIS), Romer Arm, etc
- Project management, lean six sigma, and leadership skills
- ISO9001/AS9100/9102 (First Article Inspection) experience preferred
- High computer literacy – ability to use Word, Excel, PowerPoint and Outlook
- Review, collect and analyze data to track and identify opportunities for clinical performance improvement including Hospital Acquired Conditions and AHRQ Quality and Patient Safety Indicators. Share information with appropriate committees and Medical Center Departments for action plan development
- Serve as a resource for the Medical Staff, Nursing and Ancillary department for developing and implementing continuous quality improvement monitors
- Participate in RCA/Sentinel event teams and process review teams by assisting with development of timelines, frameworks and action planning
- RN required. Education and experience equivalent to a Bachelor’s Degree in Nursing, Hospital Administration or related healthcare field. Master’s Degree preferred
- Three to five progressively responsible experience in a hospital environment to ensure thorough working knowledge of hospital operations and regulatory requirements. Previous experience in hospital based quality program preferred
- Demonstrated proficiency in principles and tools of performance improvement including team facilitation, techniques for idea generation, problem solving and statistical process control
- Ability to maintain confidentiality according to Medical Center policies and procedures
- Knowledge of Microsoft Office software; Meditech Order processing systems; and Kronos Payroll systems
- Proficiency in various software programs including Midas + CPMS and Midas +Datavision
- Responsible for Stryker Canada’s Quality Management System and overall compliance with the ISO 9001 standard for Canada
- Liaise with the external assessment body on all matters related to the external certification process
- Lead annual Management Reviews of the Quality System to ensure and monitor its suitability, adequacy and effectiveness
- Lead the QMS and documentation system to ensure compliance with approved process and procedures
- Implement an Internal Quality Audit program covering all processes
- Ensure that suppliers used by the organization are selected, evaluated and re-evaluated and that records of this assessment are maintained
- Define and implement a QMS training program. Ensure that all new staff are inducted into the requirements of the QMS related to their own roles and responsibilities
- Partner with Regulatory Affairs to establish best practice and standardize processes where possible
- Strong knowledge of ISO9001 Requirements
- Project management and report writing; preferable
- Post-graduate diploma in Regulatory Affairs or Quality Assurance
- ISO 9001 Auditor Certification from an accredited body
- At least five years’ experience leading Quality Management Systems
- At least three years’ experience in a people management role
- Responsible for overseeing the QA staff and ensures that all QA and Regulatory programs are followed, makes sound decisions and executes quality oversight
- Executes quality objectives as directed by the Corporate Quality Assurance Manager, as well as the adherence and execution of all company policies and programs associated with Foods Safety, Quality, and Regulatory Compliance
- Works with QA staff members to prevent process or product failures. Focuses on continuous improvement of product quality through the development of quality plans, process controls, attention to process details and specifications and quality training
- Responsible for managing performance plans, work schedules, vacations, assignments, and special projects as well as training and personnel development for direct reports
- Ensures that operational and pre-operational sanitation is acceptable. Implements and develops necessary verification activities including micro sampling to verify the effectiveness of sanitation activities. Ensures GMP and Sanitation audits are conducted by the department as required with documented corrective actions and follow-up as necessary. Takes action in response to negative micro and Shelf Life data to drive improvement
- Completes reassessments of SQF Plan if
- Bachelor’s degree (B.A.) in Food Science or related field and 5-8 years’ experience in the food industry; or equivalent combination of education and experience
- Comprehensive knowledge of USDA, FSIS, HACCP, and SSOP requirements
- Knowledge of Database software; Inventory software; Manufacturing software; Spreadsheet and Word Processing software; QMS system and SAP
- May be required to work long hours and weekends
- Manage and lead Quality Assurance engineers; create a positive and dynamic environment in Quality Assurance to engage and grow staff and to deliver outcomes
- Collaborate closely with Software Engineers, Product Managers, and QA engineers to identify, prioritize, and resolve issues
- Develop test tools, environments, and frameworks to best meet the business needs
- Perform exploratory testing within an agile environment
- Accurately estimate work and resources required to perform tasks
- Become an expert at understanding our business and our customers
- Champion quality assurance across all levels of the organization including Product, Development and within the Quality Assurance team
- Effectively utilize manual and automated testing as appropriate
- Be proactive in identifying problems and providing solutions
- Continuously review processes and implement change when necessary
- To perform this job successfully, an individual must be able to perform each essential duty satisfactorily. The requirements listed below are representative of the knowledge, skill, and/or ability required
- Passionate about leading and writing great software
- High degree of efficiency and motivation in work habits in a challenging, fast-paced environment
- Committed to meeting testing deadlines
- Ability to develop and direct the career growth of direct reports
- Bachelors degree in Computer Science, Computer Engineering, or equivalent experience/knowledge
- Minimum of 5 years’ experience in a Quality Assurance environment required
- Minimum of 2 years’ experience managing a high-performing Quality Assurance team required
- Demonstrated track record of delivering high quality, successful products
- Experience implementing change and process improvements in Quality Assurance
- Experience in delivering engineering applications using an agile methodology
- Experience with issue reporting and tracking tools such as JIRA preferred
- Demonstrated experience working across departments in an organization to build awareness and value of Quality Assurance
- Experience with large data sets (big data) across multiple products desired
- Experience in the media measurement industry desired
- Advises more senior management of staffing requirements for short and long-term staffing requirements of processing volumes
- Develops and implements assigned teams training plans based on assessment of team needs
- Ensures team is appropriately trained to process and respond to members questions, concerns and/or requests
- Ensures that all service, quality and production levels for his/her assigned team(s) are successfully met and/or exceeded
- Ensures that processes are followed in accordance with internal controls and that data is appropriately managed per LFG corporate data privacy standards
- Manages the processing and administration of one or more teams relating to more complex eligibility management customer data requests, enrollment activities and member updates in an accurate, timely and efficient manner
- Monitors and controls expenses within defined budget limits
- Oversees teams activities on quality assurance checks/audits for processing of data, enrollment activity, and updates to member records for assigned area(s) of responsibility
- Provides information to more senior management to support the creation of the budget
- Reviews and resolves escalated issues and concerns with a sense of urgency; escalates issues to more senior management as needed
- 5+ Years of experience in customer service environment directly aligned to the specific responsibilities of this role, incl. 2+ years of managerial, (Required)
CO Manager, Quality Assurance Resume Examples & Samples
- 8 plus years of work experience to include
- Bachelor’s degree in Food Safety, Microbiology, Food Science, Biology, Chemistry or related field
- Certified SQF Practitioner
- Must be self-starter who can work under limited direction day to day and meet goals and objectives as set by managers
- Must be willing to take the initiative without specific direction; ability to work independently
- Proven accuracy, reliability and completeness in performance of duties
- Ability to assume additional responsibilities
- Developing, monitoring and enhancing Quality standards to meet the needs of both internal and external customers
- Review data to recommendations by individual, team, or function regarding the Quality Process to gain efficiency and/or add value
- Measures, reports and calibrates call center performance on a daily, weekly, and monthly basis
- Follows-up on established departmental goals and objectives that support divisional and enterprise missions and goals and ensures team goals support department goals
- Ability to communicate both written and orally, accurately and effectively across different levels
- Provides feedback to QA Director, suggesting methods to increase efficiencies and identify areas of opportunity to improve customer satisfaction
- Conduct random quality audits for intake channels (i.e. Calls, Chats, etc.)
- Review and document inbound/outbound calls utilizing departmental quality monitoring standards and guidelines in order to ensure that quality/adherence policies and procedures are being met
- Analyze quality reports for management that identify and communicate trends and areas of opportunity within Employee Services
- Adjust quality auditing tool in a dynamic manner based on achievement of desired performance outcomes and the need to more closely monitor areas where performance is underperforming to expectations
- Coordinate and facilitate calibration sessions to ensure all QPAs are administering standard best practices and scoring is consistent
- Participate as a Core Team member in process improvement sessions to advance the design of the quality performance systems, training, tools, and processes
- Provide ad hoc data as requested that is presented in an actionable format
- Manages team day-to-day on project-related activities. Ensures team produces, drives and manages project schedules status, resources, deliverables and timelines throughout the project with input and feedback from the business team
- Communicates well and in a timely fashion with Sr. Management regarding the status of specific projects. Manage quality assessment and improvement projects: manages metrics and processes
- Excellent working knowledge of Oracle HCM and Salesforce.com preferred
- Experience in data management in a Shared Services business environment preferred
- Must possess technical knowledge and be proficient in Microsoft Office applications such as Word, Excel, and PowerPoint
- Excellent oral and written communication skills, including the ability to organize and present information in a clear and concise way to executive leaders
- Must have above average mathematical skills, including the ability to compute rates, ratios, and percentages, and the ability to work with mathematical concepts such as probability and statistical inference
- Must be self-motivated and able to work independently with minimal supervision
- Prior Human Resources experience a plus but not requirement
- Demonstrated people and project management skills, including ability to prioritize, meet deadlines, and follow through on completion of projects
- Ability to prioritize problems in terms of strategic and possible impacts
- Problem Solving and Delegation Skills
- Analytical & Results Oriented
- Process Oriented and Operationally Focused
- 8+ years of audit experience in financial services or insurance
- A Bachelor's Degree is required
- Accounting, Finance, Economics and/or Quantitative major is desired, but not required, and a relevant certification (e.g. CPA, CFA, CIA) is a plus
- Experience in life insurance, group insurance, broker/dealers, retirement/annuities products is desired, but not required
- Strong project management and analytical skills with an ability to multi-task and manage competing priorities
- Outstanding communication skills, both oral and written
- This position is based in Newark, New Jersey. No travel required
Operations Manager, Quality Assurance Resume Examples & Samples
- Requires excellent interpersonal, written and verbal communication skills with the ability to interact with various levels of personnel
- Encourage and build positive relationships with team members
- Effective communication with all co-workers and outside sponsors
- Must possess the ability to work independently, organize work effectively and take initiative in fast-paced work environment
- Must be able to work strategically
- Strong analytical and problem solving skills, with demonstrated leadership ability
- Individual is self-motivated, self-directed, team oriented, responsible, and reliable, with a positive attitude and a proactive style
- Gains industry knowledge and understanding of the business to enable root cause analysis and offers recommendations for quality control process standards and procedures
- Ability to create and deliver presentations to numerous personnel; such as training to internal and external departments
- Proficient in PC based software programs including MS Word, Excel, PowerPoint, Access and Visio
- Intimate understanding of our business processes and work flows
- Five years’ experience working within Quality Control, Quality Assurance or Compliance in the financial industry
- Three years supervisory or managerial experience
- Direct compliance with Food Safety requirements at the facility
- Direct activities of plant laboratory
- Know and understand federal, state, and local food industry regulations and company policies as applicable to operations of facility (CFR, GMPs, SSOPs, HACCP, etc.)
- Demonstrate general understanding of Pasteurized Milk Ordinance (PMO) and compliance to regulatory requirements
- Conduct audits of facility including quality systems and physical inspection of equipment, buildings, and facility grounds for internal, regulatory, and customer requirements and generate effective reports
- Assist production management with development of corrective actions and appropriate documentation in response to audit and inspection findings
- Conduct investigations into incidents and complaints, document findings
- Direct HACCP (Hazard Analysis and Critical Control Point) program
- Direct preparations for Product Recovery and Traceability
- Conduct practice exercises with Recall Team members at required frequency
- Develop and maintain customer documents and specifications
- Coordinate product labeling requirements
- Coordinate Vendor Approval program with DFA Vendor and Specification Program Coordinator for plant suppliers
- Direct Pest Control program for facility
- Develop, implement, and maintain Food Security program
- Convey company, facility, and department goals to employees
- Dedicate effort to safety as we expect every employee to leave work in the same condition as they arrive
- Follow all DFA good manufacturing practices (GMP) and work in a manner consistent with all corporate regulatory, food safety, quality, and sanitation requirements
- Exhibit the values and ethics of Dairy Farmers of America through honesty, discretion, and sound judgment
- Attend required training, including but not limited to, Safety, GMP, Hazard Analysis and Critical Control Point (HACCP), and Safe Quality Foods (SQF)
- Lead plant sanitation program in effectiveness with rigorous adherence, generation of SOP's and standard work
- The requirements herein are intended to describe the general nature and level of work performed by employee, but is not a complete list of responsibilities, duties and skills required. Other duties may be assigned as required
- Adhere to all DFA Quality, Safety and GMP policies and procedures and report any nonconformity
- BS/MS degree and 10 years of experience in SW Quality
- Solid hand-on experience on Six Sigma DMAIC (Define, Measure, Analyze, Improve, Control) methodology for problem solving and product/process improvement
- Strong influence with cross functional teams; engineering, manufacturing, supply chain, and Airline Technical Operations – Installation & Maintenance
- Must possess total System Integration knowledge
- Practical problem solving and strategic thinking skills, critical thinker, and self-starter
- Superior communications skills with all levels of employees and both internal and external customers-including presentations, negotiations, and written reports
- Strong Quality organization and project management skills required
- A desire in Continuous Improvements
- An analytical, detail-oriented mind, as well as strong verbal / written skills
- Experience working in JIRA, JAMA, environments
- Familiar with AS9100/9115 and Airbus line fit processes and Agile SAFe practices
- Leads test activities in accordance with policies and standards
- Drives test deliverables on a project basis being responsive to schedule and quality requirements
- Implements test verification (e.g., Technical Reviews) and validation (e.g., Function Test or System Test) practices, standards, metrics, training, and processes)
- Ensures that all direct reports and their teams are trained in the skills and tools of test, project management and other required competencies of the test function
- Drives product readiness
- Leads the test lab, configuration of lab environment and test facilities, including asset scheduling and application/patch installation
- Establishes fact-based measures of test efficiency, effectiveness and product readiness
- Partners with client groups to ensure that the testing strategy is understood; in use; test prerequisites are completed, and that test outputs are analyzed
- Manages personnel issues such as hiring, training, assessment, career and succession planning
- At least 5 years’ experience in technology mostly focused in application development and/or quality assurance testing
- Experience in testing for n-tiered and web development and automation frameworks
- Have interpersonal skills to deal effectively with all business contacts
- Professional appearance and demeanor
Manager Quality Assurance Audit ERA Resume Examples & Samples
- Ensure appropriate risk adjustment coding for all risk adjusted segments (i.e., Medicaid, Medicare, and the Affordable Care Act Commercial segments)
- Enable improved accuracy in understanding the illness burden of our membership to better manage their risk, care, and health
- This position leads a team to review patient records to capture and code for accurate risk adjustment revenue, conducts audits of provider and vendor records to ensure accuracy and completeness, and ensure operational readiness for government audits of our risk adjustment practices. The incumbent will manage employees, including skill and career development, policy administration, discipline, employee relations, goal setting, and performance reviews
- Lead a team responsible for all quality assurance, provider, vendor, and government audit functions to support enterprise risk adjustment optimization across Medicare, Medicaid, and ACA populations in the following areas
- Perform all administrative duties related to the planning, scheduling, and conducting audits and maintaining records associated with audits of medical records for risk adjustment reporting
- Review patient records in accordance to published standards to analyze provider documentation to ensure that it meets standards and supports the diagnosis and procedure codes selected,
- Conduct audits on abstracted files to ensure accuracy and completeness of coding by identifying accurate coding opportunities and rechecking all diagnoses and procedures using ICD-CM (ICD-9 and ICD-10) and CPT-4 codes, and mapping to relevant HCC and/or CDPS categories
- Prepare and implement necessary internal controls for related entities consistent with CMS and State requirements to support RADV or other regulatory audits
- Manage vendor and provider audits with regard to risk adjustment and prepare a summary of findings to leadership
- Prepare reports for the Regional Provider Coach team to address provider specific opportunities for improvement based on audit findings
- Perform quality assurance reviews of audit findings and provide guidance on medical record review issues
- Conduct periodic risk adjustment workgroups, especially when changes have been made to the risk adjustment models(s), CMS and other regulatory guidance, coding guidelines, etc
- Develop implement, and/or maintain documentation and chart reviews consistent with CMS and other governmental regulations, company goals and policies
- Oversee chart abstraction projects and other chart audit activities as appropriate
- Manages the ERA Audit team, including building a successful team, developing strategic partnerships
- Working with the Director, develop the audit policies and procedures to accomplish the measurement objectives established by BCBSA, FEP Directors Office, contractual arrangements and BCBSM leadership. Manages audit work in accordance with accepted industry practices, and contractual and licensure agreements
- Prepares and reviews audit reports; reaches concurrence with respect to facts and needed actions; advise management of findings; collaborates with business areas to prepare responses and define solutions for items needing action
- Directs the team, including interviewing and hiring employees following required EEO and Affirmative Action guidelines and ensuring employees receive the proper training. Conducts performance evaluation, and is responsible for managing employees, including skill and career development, policy administration, coaching on performance management and behavior, employee relations, and cost control
- Facilitate the NCR Process at their respective sites
- Authorize and perform invalidation of NCRs, as applicable
- Perform site/area Nonconformance data analysis
- Present data analysis to the CRB as appropriate
- Submit data for Management Review
- Ensure training of all NCR users
- Facilitate the CAPA Process at their respective sites
- Originate CAPA’s in the CAPA Application
- Facilitate the CAPA Review Board and maintain objective evidence of the review
- Assure all Team Leaders, Team Members, Approvers, and CAPA Review Board members are trained
- Assure that data analyses from quality sub-systems are documented and retrievable
- Analyze CAPA data for the respective sites
- Review of test reports and other laboratory reports
- Ensure timely completion of batch cards and its review adequately
- Approval / Rejection/invalidation, of NCR, CAPA and Quality System documents as QA representative
- Authorize to train personnel on NCR/CAPA process
- Authorized to release of finished goods
- Working knowledge related to NC / CAPA in EtQ
- High degree of professional/domain knowledge
- Excellent analyzing / decision making ability
- Expert knowledge of the India Drug and Pharmaceutical Regulations (Including Schedule M), GMP’s, ISO Standards and validation methods
- Demonstrated knowledge and application of, Indian Drug and Pharmaceutical
- Regulations and MDD, ISO 9001, ISO 13485. ISO 11137, ISO 11138, ISO 11135 international standards
- Computer skills (MS Office, Visio, MS Project etc.)
- Knowledge of applicable laws and regulations
- Knowledge of manufacturing environment
- Quality systems, quality control, quality assurance experience, including hands on advanced quality systems compliance
- Strong technical, planning, and execution capabilities
- Ability to analyze, balance and prioritize risk
- Working knowledge of Microsoft Suite applications
- Working knowledge of Minitab, preferred
- Develop and sustain value-adding relationships with key customers
- Lead teams to provide Quality input and guidance to customers. Ability to use in- depth knowledge of customer requirements and expectations, as well as relevant industry standards, to recommend changes to and / or validate Quality of products
- Demonstrates ability to leverage understanding of customer management techniques for developing strong customer relationships
- Demonstrates deep end-to-end knowledge of closely related functional units and Interface between quality and other functions. Demonstrates ability to educate Quality organization on key interdependencies. Influencing business unit leaders in critical Quality and compliance areas
- Demonstrates ability to maintain strong relationships with leaders across production, planning & warehouse, engineering and supply chain functions
- Utilize understanding of the typical Quality and compliance challenges faced to Identify unusual circumstances and assess compliance at these phases and Apply appropriately J&J and applicable Health Authority standards requirements to ensure compliance of the product
- Demonstrates deep knowledge and appropriate use of all key Quality and compliance Processes and make recommendations for refinement of processes to achieve regional/global standardization
- Ability to determine necessary adjustments to Quality and compliance processes and lead the process and associated communications required to update Quality and compliance process and Demonstrates ability to appropriately evaluate and interpret process inputs and outputs. Demonstrates ability to explain and/or train others and Ensure all systems are maintained in a compliant state of control
- Ability to develop, validate, and implement metrics and J&J Quality Standards for various functions and for the Quality and compliance organization and Demonstrates ability to develop and implement processes for monitoring and reporting metrics and Leverages understanding of KPI usage to develop achievable improvement goals
- Demonstrates ability to assess and approve Quality and compliance issues and bring appropriate issues to the board, prepare and present applicable data and issues in a clear and concise manner and Demonstrates ability to lead the meeting when called upon and Identify appropriate resources and materials and provide to executive management board for review
- Coach others on proper etiquette and procedures for leading investigations
- Understand investigation results and oversee implementation of corrective actions and solutions
- Demonstrates ability to identify corrective actions that should be applied internally to other areas of the business / sectors, and escalates them appropriately for use
- Demonstrates deep knowledge of GMP/GDP/GAMP rules and applicable international regulations
- Understand how GxP application impacts production quality and consequences of non- compliance
- Develop appropriate remediation plans and activities to support product compliance
- Develop appropriate SOPs and procedures in response to new regulations
- Knowledge of Operational Excellence that includes Analytics & Problem Solving, Technology & Data Management, Decision Making, Knowledge Management
- Knowledge of Quality & Compliance which includes Quality Mindset, Compliance Orientation, Environment Health & Safety
- Knowledge of Business Fundamentals which includes Financial Management & Budgeting, Project Management, Business Case Development, Business Orientation
- Knowledge of Business Partnering which includes influencing, Change Management, Time Management, Global Mindset & Management
- 3+ years of professional experience in Regulatory Affairs, Quality Assurance, Quality Engineering or related areas in medical device or other healthcare industry
- Ability to provide effective leadership, remove obstacles, develop team members professionally, and improve team efficiency
- Familiarity with design controls, quality management systems and regulated environments
- Supervisory/managerial experience
- Possess subject matter expertise in areas such as process validation, test method validation, statistical techniques, and/or root cause analysis
Manager, Quality Assurance, Alexa Skills Resume Examples & Samples
- Bachelor’s degree in Computer Science or equivalent combination of technical education and experience
- Six or more years of industry experience, including at least three years of hands-on testing and two years as a QA manager or lead
- Fluency in SQA methodology and experience with user-level automation tools, such as Quick Test Pro, Selenium, or LoadRunner
- Experience in developing and implementing QA strategy
- Excellent written and verbal communication skills, with a track record of working effectively with Development Engineering and US-based SQA counterparts
- Strong people management skills, with a track record of hiring and growing talented SQA Engineers
- Experience in IoT (Internet of Things) or VUI design
- Proven ability to define effective, efficient, and scalable test processes for digital products and services
- Strong organizational design and recruiting skills; proven ability to build high-performance teams
- Excellent customer experience intuition; demonstrated success in delivering a world-class customer experience (ideally in the digital content industry)
- Excellent communication and persuasion skills; demonstrated success building buy-in for an innovative and bold vision
- Technical fluency; comfort understanding and discussing architectural concepts, schedule tradeoffs and new opportunities with technical team members
- Nimbleness and comfort with ambiguity; comfort responding quickly to rapidly evolving threats and opportunities
- Strong bias for action; ability to juggle multiple priorities and create a sense of urgency in a fast-paced, dynamic environment
- Proven ability to work with multiple stakeholder teams across geographies through influence versus direct management; excellent interpersonal skills
- Establishes test priorities and coordinates test activities ensuring deadlines are met, and business requirements and objectives are satisfied
- Negotiates business commitments and resource requirements. Works with business and technology leadership to evaluate performance of software quality assurance and ensure knowledge of and satisfaction of support and services delivery
- Provide direction for software quality assurance professionals responsible for software testing ensuring that software quality acceptance criteria and production release timeframes are met while delivering reliable and innovative technology solutions for our business
- Demonstrated leadership brilliance, intellectual curiosity and operational excellence
- Knowledge of and ability to drive process development, quality assurance practices, and release management
- Understanding of Infrastructure technologies including networks, servers, and databases
- Demonstrated project management skills including an understanding of project management processes and related tools
Manager, Quality Assurance Automation Resume Examples & Samples
- Directs a technically skilled staff in automated application test development to create a library of automated test scripts to reduce test time. This automation of applications testing will improve the time to market for Payroll applications on Web & Mobile
- Maintains an industry best of class knowledge of automation test technologies in order to complete test script code reviews, write test scripts, and manage the automation test library which will provide a repository of reusable test scripts used by the testing organization
- Identifies areas within or across business applications that are ideal for automated test scripting to ensure effective use of resources
- Designs, maintains, and ensures the integrity and completeness of the integrated test environment to allow meaningful results to be extracted
- Recruits, selects, hires, evaluates and ensures training of staff personnel to meet the needs of the automated testing development function. Coaches and develops staff for personal and corporate advancement through training programs, workshops and other educational resources
- Initiates novel reporting techniques on automated test results to determine a Go/No Go decision to production
- Assists in defining and executing Quality Management (Policy, Procedures, Objectives, Assurance, Training, Control, Audit, & Planning) for IT to improve the quality of the business solutions delivered to the business units
- Manages the software testing schedule, coordinating deadlines with business stakeholders and assigning resources to meet project deadlines
- Assists in developing an Enterprise automated test strategy across multiple platforms that is cost effective and reduces time to perform regression test cycles while maintaining or improving the application quality to production
- Assists in maintaining positive relationships with vendors who provide the following: automation test tools, testing services, and test training/certification for the purpose of providing a cost effective, high quality test environment for Information Technology
- Experience with six sigma and operational excellence initiatives
- Minimum of 8 years’ experience in a Quality Assurance role in the pharmaceutical industry, preferably with parenteral dosage forms. OSD and Combination Product related experience desired
- Previous experience supervising personnel required, managerial experience preferred
- Knowledge of SAP, LIMS, Track Wise preferred
Manager Quality Assurance TPU Resume Examples & Samples
- Cost effectively manage a group delivering quality control/ assurance to the Ringwood manufacturing organization
- Be responsible for, and champion, the maintenance and further development of site-wide ISO9001:2015 registration
- Coordinate the current and future testing of all raw materials, intermediates, and finished goods, including the downstream logistics
- Deliver targets on all KPI's related to the operations within the QA/QC teams: EHS, manufacturing cost optimization, product quality optimization, production efficiency optimization, and required testing processing times, and assist with the blocked stock reduction
- Issue monthly reports including relevant conclusions, and recommendations for investment/ process improvement
- Develop, implement, and maintain an effective, timely complaints handling process which will deliver the agreed KPIs. Establish and manage a strategic program to improve the Complaint Handling on a continuous basis
- Further develop and manage a quality system that provides documented business processes to support development, manufacturing, sales, and technical service which will enable customer responsiveness. Ensure that the IMS database is maintained and that all documents are appropriate, effective, and up to date
- Drive a culture of safety, quality assurance, and continuous improvement
- Be the TPU business process owner (SAP expert) for the US TPU Quality Module and as such be involved in engineering projects. Maintain QC inspection plans, and specifications in SAP system for new raw materials and finished products according to NPI procedures
- Support the introduction of best practices in the areas of process analysis through the use of statistical analysis to constantly refine the capabilities of the organization
- With the Plant Manager, develop and implement a strategic plan for the development of capabilities of the QA Organization, including career development of the Quality Team members, training, a quality assurance focus, and capital expense planning including analytical instrumentation in QC, at line, and in line. Set clear and challenging goals for Quality. Provide challenging tasks and assignments that relate to planned development areas for Individuals
- Manage the lab scale Raw Material second sourcing opportunities, led by the Quality Technologist
- Manage the Non-Conforming Sales Product investigations, root cause analysis, and Corrective/Preventative Actions to completion and Maintain the Quality Disposition data in SAP
- Work with Requisitioning, Purchasing, QC, and Maintenance to ensure calibrations and preventive maintenance are scheduled and completed on time
- Provide training to the quality team, engineers, or site that ensures the ongoing development of a quality mindset, such as: lab analyses and equipment maintenance, GMP documentation, continuous improvement, basic statistics and control charting, FTIR basics, incoming raw material testing, revising ISO documents, etc
- Develop a system for everybody at the site to share and document knowledge about manufacturing process best practices for each product family to ensure sharing of knowledge and continuous quality improvement
- Ensure all activities within the area of responsibility are compliant with regulatory and company standards in close cooperation with the Plant Manager and EHS Manager
- Ensure proper record retention as per corporate and legal required standards
- Ensure HR activities within the area of responsibility are compliant with regulatory and company standards. As part of the Corporate Guidelines, this entails the use of Performance Management and a comprehensive training program meeting the legal requirements of EHS, quality, and efficiency for all associates. In close cooperation with the HR Manager, ensure that organizational or individual problems/ requests are being dealt with professionally and, if possible, proactively
- Manage the Ringwood Quality Systems to overall effectiveness and customer satisfaction
- Participate in Environmental, Health, & Safety initiatives as set forth by the company
- Bachelor’s Degree required, Master’s Degree preferred, in Chemistry, Engineering, or related field
- Five or more years’ experience
- Six Sigma Green Belt or Black Belt certification strongly preferred
- Must have a proficiency with Microsoft Excel, PowerPoint, and Word, as well as E-mail and Internet
- Strong EHS Awareness
- SAP or ERP Experience
- ISO 9001 Auditing and Documentation Experience
- Experience with statistical analysis and sharing data to various audiences, Minitab preferred
- Work collaboratively with cross-functional team members from different levels with different backgrounds and perspectives
- Deal comfortably with more senior managers, understanding how they think and work
- Remain flexible and adapt easily to changing situations
- Share expertise and knowledge with others
- Find common ground to solve problems for the good of all
- Listen and incorporate ideas from others
- Give individuals freedom to meet goals in their own way
- Embrace change as an opportunity
Manager, Quality Assurance & Safety Resume Examples & Samples
- Bachelor’s degree with 5 years of experience. An additional 4 years of experience may be substituted in lieu of degree
- Ability to obtain and maintain a Secret Security Clearance
- Experience directly managing personnel
- Aerospace and Defense quality management experience
- Experience working under a contract containing the Ground and Flight Risk Clause
- Experience in Root Cause Analysis, Corrective and Preventive Actions
- Quality Assurance experience with US Army or DCMA
- FAA Airframe & Powerplant certificate
- AS9100 / 9110 Lead Auditor training
- OSHA 30 Hour Compliance Training
Engineering Manager, Quality Assurance Resume Examples & Samples
- You will oversee, manage and develop the test teams
- You will ensure the quality of test cases and test executions through review, training and tooling
- You will be coming up with, and implementing Software Testing strategies, driving process improvement and raising the bar of test activity across platforms
- You will provide estimation and handle resource management, preparing the plans and roadmaps for the quality team
- You will train and grow your teams by and use your eye for talent to hire a talented team of QA Engineers to ensure capabilities are developed and resources are aligned to support the growth
- Assure full compliance with all customer’s specs and requirements as per contract for every job
- Staff, organize and develop the Quality Team to respond to product or process development needs
- Act as a quality liaison with customers ensure quality support to all functional processes needed from the point of order through delivery, including order entry, design, product development, purchasing, manufacturing, test, and ship
- Establish, implement and maintain the Quality Management System. Maintain position on site leadership team as ISO management representative, or as defined in Site Quality Standard
- Define quality systems, continuously refine and improve systems to achieve desired results
- Monitor auditing function to assure compliance to systems and procedures
- Monitor and report quality performance (internal, external, supplier) and help identify areas of needed improvement
- Provide development opportunities, guidance, training and assistance to quality assurance and quality control personnel
- Monitor corrective actions and report deficiencies in responses and results
- Facilitate and support audits by external agencies and Customers. Develop responses to findings and ensure implementation and closure of all findings
- Oversee activities of staff to insure site and division level goals are achieved
- Manage PMP process for site quality organization
- Manage quality function within established budgetary guidelines
- Assure the quality assurance team operates in full compliance with all federal, state and country regulations, equal employment and OSHA laws (or specific country laws) and other safety and operational guidelines
- Interact with the functional areas including manufacturing, purchasing, engineering, production and field service/repair to evaluate failure trends, product quality problems, and quality issues
- Interact with customers and suppliers on quality issues plus resolution of any problems within process and final product acceptance
- Provide input, guidance and coaching to site leadership team to ensure continuous improvement
- Provide quality support for in-process and final inspection to establish quality standards and customers’ requirements
- Recruit, retain, manage and develop subordinate associates to ensure a well-qualified and high motivated department
- Communicate effectively both verbally and in writing with a wide variety of customer from internal and quality team members to outside final customers
- Be able to perform and coach others in data analysis and perform root cause analysis using Flowserve’s DMAIC Lite toolset
- Show continual performance improvement of site metrics through the use of data
- Review site quality system on an annual basis and revise system to improve performance. Incorporate site quality system changes as needed to ensure performance
- Perform quarterly reviews of audit findings, proposed root cause and insure corrective action is implemented and delivering expected results
- Report site quality data, in compliance with division or corporate definitions, on a monthly basis
- Develop root cause analysis and corrective actions based on quality data
- Maintain a skill and development plan for all site quality personnel
- Provide monthly report to site leadership on deficiencies in processes and actions to correct
- Manage the supplier improvement process at the site level
- Bachelor’s degree in Mechanical Engineering or equivalent
- The individual should have a minimum of 8-10 years of quality assurance experience in a highly engineered environment which is recognized for quality assurance and continuous improvement
- Experience with industries where product development and customer service satisfaction are critical measures of success would be ideal
- Organizational and planning skills
- Lead Auditor in Quality Systems Course or equivalent
- Quality Management Systems and/or ISO 9000 Training or equivalent
- Certified Lean Six Sigma Black Belt or Green Belt
- A change agent that possesses the ability to influence decision making and build critical relationships in a manufacturing organization
- Strong business and financial acumen and possesses a good working knowledge and understanding of business operations
- Results-oriented with a passion for quality, service and customer satisfaction
- Strong analytical skills and timely decision making ability
- Proven leadership skills – team building, collaborating, presentation skills, strategic and innovative thinker, and self-starter with a high energy level
- Required to travel domestically and internationally occasionally
- Fluent in both Spanish and English
- Experience in an industry utilizing design, machining, assembly, and testing processes
- Experience in both build-to-order and volume production environments
- Independently determine approach to managing daily operations; guided by general policies and management guidance
- Develop or contribute to the development of procedures and standards by which others will operate
- Interpret and execute standards and procedures directly affecting work activities
- Develops and communicates goals and objectives to staff and key partners
- Monitor goal performance and coordinate action for improvement of shift and overall PQA performance by championing department OPEX efforts. Assist project teams in establishing priorities, project timelines and in securing resources
- Actively represent Quality Assurance on incident triage teams or support efforts by providing guidance and/or technical leadership
- Partner with internal personnel at various management levels to resolve issues, establish direction, obtain resources, and drive change
- Reviewing and approving operational procedures and product manufacturing procedures, approving process validation protocols and reports for manufacturing processes, and approving environmental characterization reports and change control documents
- Approving non-conformances, representing QA on project teams and approving change-over completion controls
- Leading investigations, as well as leading plant and site audits
- Master’s degree and 3 years of Quality experience OR
- Bachelor’s degree or and 5 years of Quality experience OR
- High school diploma / GED and 12 years of Quality experience AND 2 years of managerial experience directly managing people and/or leadership experience leading teams, projects, programs or directing the allocation of resources
- Bachelor’s Degree in Life Sciences, Engineering or related field
- 6 + years of quality assurance or combination of quality and manufacturing experience in the biotechnology/pharmaceutical industry
- 3 + years of supervisory/management experience
- Experience in areas of manufacturing, investigations, CAPA, product release, validation and/or change control
- Experience in regulations, standards and guidelines that apply to biotech manufacturing in a multi-product environment
- Ability to develop meaningful team and staff goals, manage performance and coach and develop staff
- Ability to manage to established timelines and deliver results to meet or exceed department and company goals and objectives
- Thorough knowledge of monoclonal antibody manufacturing or ability to apply previous biotech manufacturing knowledge to understand manufacturing deviations
- Ability to communicate clearly, facilitate discussions and present to audiences at all levels of a site organization
- Decisive and independent risk-based decision-making ability on the floor to support manufacturing needs
- Documents performance and adherence to action plans and timelines for all QA-related activities such as
- Bachelor's Degree and 3-4 years of experience in the Textiles and/or Apparel industries is required; Textiles- and Apparel-related degrees and courses are preferred
- Apparel industry knowledge is essential
- Strong interpersonal and communications skills (Verbal and Written) to develop and maintain productive working relationships with external and internal customers and suppliers (ex.: Mexico Manufacturing)
- Possess a sound understanding of business priorities
- Ability to independently work, prioritize activities, and follow and execute plans/instructions with minimal supervision
- Self-starter, multi-task oriented and able to resolve issues in a timely fashion
- Strong computer skills including Microsoft Office, MS Access and above-average, MS Excel skills
- The preferred candidate will also have
- Review requirement specifications and technical design documents to create measurable test metrics
- Promote and implement Quality Assurance best practices
- Develop and manage QA project schedules
- Report and maintain software defects of defect management system
- Coach and mentor QA Engineers in a team oriented environment
- Assist with training where needed
- Work with Human Resources to interview and hire top engineering talent
- Bachelors degree in Computer Science or related field; equivalent experience of 5 years may be considered
- Minimum of 6 years software quality assurance testing as part of a formal QA test organization
- Experience testing applications under Windows and Internet operating environments
- Experience writing basic SQL Queries
- Experience with Mercury Interactive Product Suite
- Excellent proofreading, written and verbal communication skills
- Demonstrated ability to change direction and reprioritize abruptly
- Demonstrated ability to work in a focused, fast-paced team environment
- Experience with SaaS highly desired
- Implement testing strategies and procedures within our development environments, performing regression testing for upgraded instances in test and production environments, and developing quality control measures for production output
- Plan appropriate testing schedules with the product delivery team throughout the development cycle
- Collaborate with Development, Implementation, and Operations teams to establish quality throughout the organization
- Assist with the review of software documentation and release notes to ensure technical accuracy, compliance, and completeness
- Analyze trends, track key measures, and provide technical Quality direction to employees, management, and clients
- Develop and implement project plans for quality programs and initiatives (e.g., MPTQM, ISO 9001, Lean Six Sigma, Baldridge Self-Assessment, etc.)
- Make recommendations for capital expenditures and headcount for the Quality Assurance area
- Select, develop, and evaluate personnel to ensure efficient operation of their area
- 5 years' experience in developing and executing test strategies for web-based applications
- 4 years' working with Java-based development teams in a dynamic environment
- Bachelor's degree in Computer Science, MIS, CIS, or an equivalent
- Experience managing technical personnel or being in a lead role
- Ability to communicate and collaborate effectively
- Significant experience with automated testing platforms
- Experience with Microsoft SQL Server
- Analyze current state and design work flow within Procurement Operations to create the most efficient processes and continuous improvement. Partner with business lines both domestic and off-shore to establish quality measure metrics related to the process and design. Will make recommendations to realign work as needed for process improvement
- Lead a team of quality analysts responsible for documenting process and policy standards and conducting quality assessments and data analysis related to various work flow designs/processes. As the leader, works with team to prioritize objectives and set consistent methodology to approach the work that needs to be completed. Ensures that all risk and compliance factors are considered before implementation
- Acts as an escalation point to the analyst team on issues arising out of analysis and process standards. Provides coaching and performance management to direct reports, including annual performance reviews
- Administers the governance process over process standards, data and documentation
- Responsible for interpreting data into consumable information for procurement leaders and ensures a robust feedback loop and reporting process is in place
- Establishes best practices to continually mature the quality assurance program
- Ability to communicate across all levels of leadership within Procurement
- Effective team leadership skills
- Experience implementing and managing a quality assurance program
- Strong analytical skills and ability to transform data into meaningful information
- Attention to detail and a results driven approach
- CQPA certification preferred
- Successful application of various process improvement methodologies (i.e. Six Sigma, Kaizen, Lean, Kanban, etc.)
- Demonstrated experience in Procurement (e.g. Sourcing, Purchasing, Vendor Management or Operations)
- Strong leadership skills with the ability to work across multiple agile teams
- Solid understanding of agile software quality assurance and processes
- Strong desire to join and influence an agile development team and build quality software
- Provides leadership to team by communicating vision, strategies and objectives to employees
- Provides career counseling and professional development support
- Participating in the planning, development and testing of key features as a member of an agile team
- Investigate and implement new agile testing methodologies for robustness and efficiency
- Administers change control process for zero defect software development
- Ensures adequate product testing prior to implementation
- Administers problem management process including monitoring and reporting on problem resolution
- Makes recommendations to superiors regarding the acquisition and/or implementation of software to increase information systems efficiency
- Assigns work to subordinates, monitors performance, and conducts performance appraisals
- Interviews and makes recommendations for additional staff
- Requires experience in programming test cases (writing automated test case based on defined use case/requirements)
- Prepares and monitors budgets for each area of functional responsibility
- Provides guidance for implementation of automation
- Responsible for managing the quality functions, personnel, related training, and deployment of Standard Practices for Service Center Repair Station(s) and Quality Assurance Department of Business/Regional Aviation Services. Position has final authority over airworthiness and quality and is responsible for facility compliance with Federal Aviation Regulations
- Act as the focal point and liaison for all interactions with the FAA, for example, answer LOI, complete submittals, and ensure compliance with regulations
- Oversee applicable inspections of aircraft maintenance for service and/or overhaul and repair activities to ensure airworthiness and compliance with Repair Station manual, Quality Control manual, and regulations
- Develop and maintain the FAA Repair Station/Quality Control Manual in accordance with Federal Aviation Regulation 145
- Develop and administer the FAA Parts Manufacturing Approval as required by assigned site
- Review and interpret Federal Aviation Regulations to realign current policies, procedures, and manuals as necessary
- Develop procedures to administer company quality policy
- Prepare department budget for fiscal year operations
- Oversee and administer the Internal Quality Audit Program
- Document and communicate requirements of Technician Training Program including record keeping
- Establish and maintain quality and reliability standards by studying product and customer requirements in conjunction with management, engineers and technicians
- Establish and maintain product quality documentation system by writing and updating quality assurance procedures
- Collect, analyze, and summarize quality-related information and trends to prepare reports on product and process quality
- Review and implement, as appropriate, current best practices within Quality field to support achievement of organizational objectives
- Maintain current knowledge of new trends in quality including the while implementing appropriate best practices
- Communicate with appropriate customer representatives as required, to ensure all quality issues and inquiries are satisfied. Also provide performance feedback to customers as required
- Must have at least 7 years of experience in quality management with 5 years leadership/ supervisory/management experience
- A&P licensed
- Must have previous experience as a Chief Inspector for a 145 repair station
- Working knowledge of FAA and Federal Aviation Regulations pertaining to maintenance programs and procedures
- Computer skills necessary to learn and/or operate word processing, spreadsheet, database, email, and web-based applications
- Bachelor degree in Business, Aviation, Engineering or related field (Degree may be offset by additional experience)
- Previous experience in ISO-certified environment
- Previous experience with the FAA
- FAA Inspection Authorization
- Six Sigma background
- CRJ or Q-Series experience
- Trains and coaches Program Managers and supervisors on group observations, coaching, quality assurance processes, procedures, and completing all necessary forms for fidelity purposes
- Responsible for training, implementing, and coaching program staff on behavior management systems
- Provides observation and coaching on interactions between staff and participants
- Assists with the evaluation of training materials in response to manager requests, audit reviews, contract changes to ensure that training materials are aligned with Evidence Based Practices and support contractual obligations
- Assists with developing quality assurance assessment tools used to measure program fidelity at various programs
- Assists with reviewing and developing evidence based practice policies and procedures needed to align programming with evidence based practices
- Assists with creating fidelity surveys to measure ongoing program sustainability
- Works in partnership with Continuum of Care research department to develop intermediate outcomes and monitor trends for program effectiveness
- May be assigned special projects as needed to support new Company initiatives
Program Manager, / Quality Assurance Resume Examples & Samples
- Manages the development, implementation and continual evaluation of the CIBMTR quality management system
- Manages a bi-campus program to monitor, report and resolve quality-related incidents. Collaborating with functional area leadership, ensure incident investigation, risk assessment and relevant corrective and preventive actions (CAPA) with effectiveness checks
- Manage supplier qualification processes when appropriate to assess compliance with quality standards and pertinent regulations
- Manages a bi-campus Document Control system to assure essential documents are current, appropriately reviewed and approved, consistently titled, archived when obsolete, and kept secure, confidential and traceable
- Oversees the administration of a quality management electronic system
- Manages development of quality and process improvement training
- Facilitates cross-functional quality improvement teams and process improvement initiatives
- Develops and implements an quality assurance process
- Participates in internal and external audit processes
- Works closely with MCW and NMDP Regulatory Compliance departments to maintain operational compliance with national and international regulations and accrediations requirements of professional organizations
- Works collaboratively with Quality Assurance departments at both MCW and NMDP to support alignment of QA initiatives and responsibilities
- Conducts trend analysis and generation of quality reports
- Ensure compliance with regulatory and institutional policies
- Advises and informs Senior Leadership regarding quality issues that impact the CIBMTR
- Serves as lead content expert fro CIBMTR strategic quality intitiatives and practices, and in support of building and sustaining a culture of quality and excellence
- Participate in the development of program resources through literature searches, contact with outside programs, and review of funding opportunities
- Participate on appropriate committees relevant to the advancement of the program and the profession or area of research
- Experience in qualifying consumer software; preferably in consumer electronics
- Experience in automation, technologies and tools. Ability to drive automation initiatives
- Strong understanding on various SDLC models; ability to handle agile/waterfall process in a fast paced development environment
- Comfortable in working with large, cross-functional, multi-departmental teams across management levels in formal and informal settings
- Candidates must have current coding skills in at least one high level progamming language (Python/C++/Java/JavaScript)
- People management experience
- Ability to propose and provide good QA analysis and risk assessment to stakeholders
- Ability to coodinate and plan for multiple releases with multiple dependencies across multiple platforms, for global territories
- Bachelor’s degree in computer science, computer engineering or relevant technical discipline
- Has a natural talent for problem solving and a passion for driving improvements for upward and downward processes to raise the overall quality level of software and development approaches/environments/tools
- Has experience with software build and deploy/release systems and continuous integration/tools
Manager, Quality Assurance & Process Resume Examples & Samples
- Working with a broad range of stakeholders to ensure development of practical ideas and leading the implementation and delivery of these projects
- Interact with technical and non-technical contacts
- Analyze and process large amounts of data to help design and configure go forward solutions
- Demonstrate advanced understanding of Order to Cash, with a focus on Client billing, accounts receivable, invoicing and payment processing
- Develops simple solutions to business problems through in depth analysis, coordination and negotiation with key decision makers
- Understand complex business and information technology management processes
- Assist in the selection and tailoring of tools to support department processes and projects
- Build and nurture positive working relationships with co-workers and management with the intention to exceed their expectations
- Enable future growth and increases in complexity and scale
- Initiates new or revised departmental procedures, programs and initiatives
- Work with a software development team and Service providers in a geographically distributed structure
- Ensure the implementation of high-end, business-wide, critical strategy set by senior management
- Provide direct oversight of quality assurance team, adhering to QA standards and SLAs, and escalating risks and issues to the Engineering Delivery Head
- Invest time on a daily basis on all projects to remove impediments and provide solutions to technical challenges and make escalations as necessary
- Define and revise (if needed) metric/performance reporting under directions from Engineering Delivery Head to ensure viability of service levels
- Participate in capacity and program planning exercises
- Hire, manage and mentor 2-4 development leads who will manage a team of 6-10 engineers
- Provide input, guidance and coaching to site leadership team to insure continuous improvement
- Provide quality support for in-process and final inspection to establish quality standards and customer’s requirements Recruit, retain, manage and develop subordinate associates to ensure a well qualified and high motivated department Communicate effectively both verbally and in writing with a wide variety of customer from internal and quality team members to outside final customers
- Be able to perform and coach others in data analysis and perform root cause analysis
- Show continual performance improvement of site metrics through the use of data and CIP methodologies
- Review site quality system on an annual basis and revise system to improve performance
- Incorporate site quality system changes as needed to insure performance
- Develop Root Cause Analysis and Corrective Actions based on quality data
- Manage implementation and compliance of Quality Management program, including record keeping and training of department-level Quality representatives
- Provide monthly report to site and division leadership on deficiencies in processes and actions to correct
- Manage the supplier improvement process as the site level and participate in divisional supply management activities
- Achieve quality goals, ensure the achievement quality objectives, implement corrective actions if needed, to adjust the level and to improve quality
- Manage day-to-day quality operations and deliverables, produce and update quality reports, accompanied by detailed analysis, participate to strategic planning, related to the quality aspect
- Improve key success metrics, proceed to analysis in each Department in order to make an assessment of the quality and to devise ways for improvements
- Actively manage the staff, support, motivate and retain an outstanding team, develop and supervise the quality analysts among others, by calibration sessions, observations and internal audits
- Manage the communications; Implement and maintain a continuous feedback process with your team and the different stakeholders
- Escalate system level issues to the appropriate team, carry out regular audits to verify the quality of execution of processes in place, and make reports to the concerned department
- Ensure compliance with the company policies, processes, tools and system changes
- Perform other cognate duties
- Minimum of 3 years experience in customer service in a customer contact center
- Implements and maintains location specific food safety & quality assurance programs. Develops and leads implementation of technical plans, clearly defining the critical issues and the approach to be used in resolving food safety issues
- Ensures that facility and products meet and maintain all required company, customer and regulatory guidelines
- Leads and participates in all regulatory, third party and customer audits and inspections. Seeks to establish constructive working relationships with inspectors and auditors. Responsible for overseeing corrective action plans that may result from such inspections and audits
- Monitors plant sanitation and good manufacturing practices daily to maintain high standards of sanitation. Works with Plant Manager or designate to resolve issues
- Evaluates daily production samples to ensure that products meet DFA FMIC food safety & quality standards
- Leads consumer and customer complaint follow-up process for the facility
- Monitors incoming raw materials to ensure that they meet DFA FMIC specifications and works with suppliers and corporate purchasing department for issue resolution
- Conducts monthly plant and warehouse inspections. Responsible for annual internal SQF and FSMA compliance inspections
- Trains and supervises laboratory personnel and laboratory test results to ensure quality standards are maintained
- Develops, organizes, and administers training for plant employees on GMP's, SQF, and food safety preventive control related topics
- Consistently provides an active presence on the production floor to support operations and business needs within the facility
- Responsible for completing KPI and plant quality performance reporting on an ongoing basis. Must identify trends, complete necessary investigation and respond with corrective and preventive action plans
- Keeps Director of Quality Assurance and General Manager informed of activities that may require his/her attention
- Performs other responsibilities as determined by business needs
- Bachelor of Science in Food Science, Dairy Science or related curriculum and 5-6 years of quality assurance and manufacturing experience in the food or dairy industry, with at least 2 of those years in a management role
- Or 9-10 years of quality assurance and manufacturing experience in the food or dairy industry, with at least 2 of those years in a management role
- Dairy manufacturing experience preferred
- Advanced knowledge of quality assurance processes and methods, preferably in a dairy or food industry setting
- Familiarity with dairy manufacturing and microbiology
- Analytical ability and problem solving skills
- Working knowledge of fundamental quantitative analysis and statistical methods
- Technical writing skills to create policy documents and audit reports
- The candidate shall have, as a minimum, at least one of the following
- Five (5) years’ experience as a Construction Superintendent; or,
- Three (3) years’ experience as a Contractor Quality Control Supervisor; or,
- Two (2) year Associate’s Degree in Construction Technology or Construction Management, or,
- Four (4) year Bachelor’s Degree in Civil Engineering, Mechanical Engineering, or Electrical; or,
- Engineering degree with two (2) years’ field inspection experience
- Ability to obtain access onto a U.S. Military Installation
- Under direction of the Executive Director, Global QA and Compliance, and in coordination with the Manager, EU/Asia Pacific CTQA, execute quality standards and quality management program for global clinical trials operational procedures and activities originating in the US. Specifically, the Manager, Quality will be responsible for management of all Quality tasks related to business operations at the US locations in alignment with global policies and procedures
- Ensure quality input to and approval of all clinical trials policies and procedures
- Provide GCP input to laboratory and other ACM global operations as needed
- Project and people management, KPI tracking, process improvements, etc
- Determines test cycles, phases and conditions; sets up testing environment and equipment. Enforces QA standards for all phases of testing
- Performs technical analysis to identify the scope and schedule for upcoming projects and individual work items
- Performs installation, functional testing, regression testing and support for all phases of the SDLC
- Independently analyzes issues, recommends software changes and writes detailed bug reports. Creates and maintains ownership of test cases and test results throughout SDLC
- 5 years’ experience in testing leadership. Transportation, Government agency support would be preferred
- Microsoft Office, Jira, Jama
- Skills are leadership, Testing, QA skills, SDLC, Agile, Waterfall, presentation skills, business acumen
- These specifications are general guidelines based upon the minimum ordinarily considered essential to satisfactory performance in this position
- Individual skills and abilities may result in some deviation from these guidelines
- Demonstrated ability to integrate the whole picture through creation of end-to-end test scenarios along with functional, regression, and performance (stress/load) test plans,
- Competency to utilize Company software and proficiency in Microsoft software applications and a willingness to learn new systems as necessary
Manager, Quality Assurance Loan Management Resume Examples & Samples
- Provides third party oversight and segregation of duties of loan management by performing quality assurance review on loan files for completeness and accuracy in Loan Module; ensures specifications of loan documents are entered accurately into the system and that the system is being used properly by Department of Neighborhood Development (DND) personnel. Corrects errors and discrepancies. Identifies issues in data quality, sourcing, or documentation by reviewing existing data and processes
- Ensures data integrity in the loan module as the system of record (sub ledger for loan receivables); i.e., verifies and tests data; cross-references data against source documents to verify accuracy and completeness
- Audits loans entered into the Loan Module and interfaces with PeopleSoft Receivable and General Ledger; does sampling of loan information for testing purposes monthly or quarterly or as required. Verifies that loan balances on General Ledger are equal to that listed in the Loan Module and in the loan documents. Corrects errors preventing interfacing of loan information between AR and Accounts Payable (AP)
- Manages accounts receivables for loans; i.e., reviews invoices using AR Billing; verifies amounts and dates of payments received match that specified in loan documents; researches current invoices of loan payments in order to determine status of loans, payment histories, and if in arrears
- Manages loan collections; i.e., informs DND personnel of loans in arrears so that borrower may be contacted; enters information received from DND into loan module, records when invoices are sent out and money received; etc
- Monitors aging and write offs; reviews dunning report to ascertain age of loan; researches action taken by DND in regards to collections and reviews documentation; prepares recommendations on write offs for approval by Manager. Works with DND personnel to perform needed AR functions (dunning, write offs, etc.) and to remedy identified errors or areas of concern
- Prepares recommendations for training, new policies, data scrubbing, or other methods to resolve data quality concerns as required. Assists in development of business processes and training; updates user manuals as needed
- Provides reports on loan module to management; i.e., outstanding balances, accrued interest, number of days loans in arrears, etc
- Produces necessary statements to conform to financial reporting requirements for tax purposes, etc
- Works on projects relating to loan management as assigned
- Performs related work as required
- At least three (3) years of full-time, or equivalent part-time, professional experience in financial analysis, fiscal analysis, budget analysis, management analysis, or program administration or management or related field, in work that included loan portfolio management
- A bachelor’s degree in accounting, finance, or a related field may be substituted for two years of the required experience
- Knowledge of the methods, techniques, principles and practices of management, accounting and fiscal management relating to loan portfolio management
- Intermediate level of understanding of Excel required
- Competency in the use of related software such as Microsoft Windows, Office Suite preferred
- Experience with software such as PeopleSoft Financials preferred
- Strong interpersonal skills and the ability to interact with varying levels of staff and management
- Ability to communicate effectively orally and in writing; to make presentations; to follow oral and written instructions and procedures; to manage and audit loans; to perform quality assurance review and analyze information and data; to analyze methods and procedures; to define or identify problems and recommend solutions; to maintain confidentiality; to prepare clear and concise statistical and other reports; to make presentations; to establish and maintain effective working relationships; and to work independently and as a member of diverse teams
- Ability to exercise good judgment and focus on detail as required by the job
Related Job Titles
Quality Manager resume examples for 2024
Quality managers need to have an eye for detail and a knack for problem-solving. According to Dr. Cindy O'Reilly, Director of Quality, "You have to be able to analyze data, to understand what it's telling you, and to do something with that information. That's a very important quality for a quality manager." quality managers also need strong interpersonal skills to effectively communicate with their team, customers, and suppliers. They must be able to "work closely and advised production supervisors and production staff to ensure all quality procedures were followed and the disposition of material." They must also be able to "investigate customer complaints associated with product quality and coordinate the development of root cause analyses and the development of corrective actions."

Quality Manager resume example
How to format your quality manager resume:.
- Use the same job title on your resume as the job you're applying for: Quality Manager
- Highlight achievements rather than responsibilities in your work experience section
- Aim to fit your resume on one page, focusing on relevant information for the Quality Manager role
Choose from 10+ customizable quality manager resume templates
Choose from a variety of easy-to-use quality manager resume templates and get expert advice from Zippia’s AI resume writer along the way. Using pre-approved templates, you can rest assured that the structure and format of your quality manager resume is top notch. Choose a template with the colors, fonts & text sizes that are appropriate for your industry.

Quality Manager resume format and sections
1. add contact information to your quality manager resume.
Quality Manager Resume Contact Information Example # 1
Hank Rutherford Hill
St. Arlen, Texas | 333-111-2222 | [email protected]
2. Add relevant education to your quality manager resume
Your resume's education section should include:
- The name of your school
- The date you graduated ( Month, Year or Year are both appropriate)
- The name of your degree
If you graduated more than 15 years ago, you should consider dropping your graduation date to avoid age discrimination.
Optional subsections for your education section include:
- Academic awards (Dean's List, Latin honors, etc. )
- GPA (if you're a recent graduate and your GPA was 3.5+)
- Extra certifications
- Academic projects (thesis, dissertation, etc. )
Other tips to consider when writing your education section include:
- If you're a recent graduate, you might opt to place your education section above your experience section
- The more work experience you get, the shorter your education section should be
- List your education in reverse chronological order, with your most recent and high-ranking degrees first
- If you haven't graduated yet, you can include "Expected graduation date" to the entry for that school
Check More About Quality Manager Education
Quality Manager Resume Relevant Education Example # 1
Bachelor's Degree In Business 1996 - 1999
Arizona State University Phoenix, AZ
Quality Manager Resume Relevant Education Example # 2
Bachelor's Degree In Business 2010 - 2013
Strayer University Washington, DC
3. Next, create a quality manager skills section on your resume
Your resume's skills section should include the most important keywords from the job description, as long as you actually have those skills. If you haven't started your job search yet, you can look over resumes to get an idea of what skills are the most important.
Here are some tips to keep in mind when writing your resume's skills section:
- Include 6-12 skills, in bullet point form
- List mostly hard skills ; soft skills are hard to test
- Emphasize the skills that are most important for the job
Hard skills are generally more important to hiring managers because they relate to on-the-job knowledge and specific experience with a certain technology or process.
Soft skills are also valuable, as they're highly transferable and make you a great person to work alongside, but they're impossible to prove on a resume.
Example of skills to include on an quality manager resume
Continuous improvement is an ongoing process of improvement of products, services, and processes with the help of innovative ideas. It is an organized approach that helps an organization to find its weaknesses and improve them.
Quality standards are a specific level of standards of products that are set by the companies for the customers that have to be met and maintained throughout the process until the time of delivery. Quality standards are information that includes the customer's requirements, guidelines, and characteristics for the needed final product or service.
Product quality is the basic element of a business. It means to add features in a product or service in such a way that it meets the needs and wants of the customers. Enhancing the product quality also means improve the goods from any existing defects to ensure customer satisfaction.
In Greek alphabets, sigma is the 18th letter that means "to sum up". In statistics, the lower case symbol of sigma is the unit of measurement for standard deviation which is used to assess the variability in a given set of data. While the upper case symbol is for summation notation means to add up all the given numbers in the data set.
A quality management system is a set of techniques that deals with the procedure, process, and duties to achieve a quality goal and purpose. This particular skill focuses on the coordination and management of the company's policy. With this system, they can determine what needs improvement and understand the level of awareness and efficiency.
Customer service is the process of offering assistance to all the current and potential customers -- answering questions, fixing problems, and providing excellent service. The main goal of customer service is to build a strong relationship with the customers so that they keep coming back for more business.
Quality control is a set of instructions or procedures to ensure a manufactured product or a service is up to the highest quality standards. This set of quality control criteria are either defined by the clients or the company itself.
Top Skills for a Quality Manager
- Continuous Improvement , 7.7%
- Corrective Action , 5.5%
- Quality Standards , 5.1%
- Product Quality , 4.7%
- Other Skills , 77.0%
4. List your quality manager experience
The most important part of any resume for a quality manager is the experience section. Recruiters and hiring managers expect to see your experience listed in reverse chronological order, meaning that you should begin with your most recent experience and then work backwards.
Don't just list your job duties below each job entry. Instead, make sure most of your bullet points discuss impressive achievements from your past positions. Whenever you can, use numbers to contextualize your accomplishments for the hiring manager reading your resume.
It's okay if you can't include exact percentages or dollar figures. There's a big difference even between saying "Managed a team of quality managers" and "Managed a team of 6 quality managers over a 9-month project. "
Most importantly, make sure that the experience you include is relevant to the job you're applying for. Use the job description to ensure that each bullet point on your resume is appropriate and helpful.
- Rated as Top Performer for SMB Sales FORMULATED IT SVCS 2001 - 2002 Sr.
- Worked with CAD engineers on PCB layout.
- Coordinated all Pro/Engineering hardware, software, and training requirements for the Commercial Engineering group and Presented team status to management.
- Designed high speed data cable assemblies and developed cable routing workplans for high-performance computational systems.
- Implemented virtual NIC driver on Linux Platform utilizing the APIs exported by the NTB PCI driver.
- Performed data validations on XML Service calls between the Clients and Vendors.
- Extracted data from flat files using UNIX shell scripts.
- Provided support to the Business QA team during the Business Acceptance Testing (BAT) phase of the project.
- Utilized tools and methodologies to improve individual effectiveness and increases efficiency in the QA process.
- Coordinated the test efforts of on-site and off-shore teams and reported directly to the QA Manager.
- Trained on the latest HDP-CVD process technology, documenting new process and maintenance procedures for the Applied Materials 300mm Ultima Systems.
- Supported DUT windows OS host systems, setup, operation, updates and hardware repair.
- Implemented a Perl based web page for ETEST critical success indicators saving $500K daily and reduced factory downtime.
- Completed static analysis of McAfee Central windows 8 application using sharplinter command line tool and python script.
- Programmed motion controls systems and the associated interfacing with PLCs and HMI, simulation, testing and start-up.
- Executed the LEAN-Sigma optimization and downtime avoidance of 77% ETO sterilization process.
- Coordinated management reviews and third party audits (FDA, ISO, etc.)
- Reviewed and audited installation (IQ), operational (OQ), and procedural qualifications (PQ).
- Led Six Sigma projects to improve business processes.
- Performed first articles and implemented inspection procedures for new products/transfer components to MPROC, equipments and tool calibrations.
5. Highlight quality manager certifications on your resume
Specific quality manager certifications can be a powerful tool to show employers you've developed the appropriate skills.
If you have any of these certifications, make sure to put them on your quality manager resume:
- Certified Quality Engineer (CQE)
- Six Sigma Green Belt
- Certified Quality Auditor (CQA)
- Master Quality Manager (MQM)
- Lead Auditor
- Certified Manager of Quality/Organizational Excellence (CMQ/OE)
- NSF-IFSEA HACCP Manager (HACCP)
- Certified Manager Certification (CM)
- Certified Quality Technician (CQT)
- Certified Welding Inspector (CWI)
6. Finally, add an quality manager resume summary or objective statement
A resume summary statement consists of 1-3 sentences at the top of your quality manager resume that quickly summarizes who you are and what you have to offer. The summary statement should include your job title, years of experience (if it's 3+), and an impressive accomplishment, if you have space for it.
Remember to emphasize skills and experiences that feature in the job description.
Common quality manager resume skills
- Continuous Improvement
- Corrective Action
- Quality Standards
- Product Quality
- Quality Management System
- Customer Service
- Quality Issues
- Lean Six Sigma
- Process Improvement
- Quality Procedures
- Customer Complaints
- Supplier Quality
- Process Control
- Customer Satisfaction
- Lean Manufacturing
- Quality Audits
- Quality Metrics
- Internal Audit
- Data Analysis
- External Audits
- Quality Program
- Excellent Interpersonal
- Regulatory Compliance
- Direct Reports
- Preventive Actions
- Pharmaceutical Industry
- Customer Audits
- Product Specifications
- Various Training
Quality Manager Jobs
Links to help optimize your quality manager resume.
- How To Write A Resume
- List Of Skills For Your Resume
- How To Write A Resume Summary Statement
- Action Words For Your Resume
- How To List References On Your Resume
Quality Manager resume FAQs
How do you write a quality manager resume, what is the role of a qa manager, what should be on a manager's resume, search for quality manager jobs.
Updated April 25, 2024
Editorial Staff
The Zippia Research Team has spent countless hours reviewing resumes, job postings, and government data to determine what goes into getting a job in each phase of life. Professional writers and data scientists comprise the Zippia Research Team.
Quality Manager Related Resumes
- Auditor/Quality Resume
- Director Of Quality Resume
- Laboratory Manager Resume
- Manager, Quality Engineer Resume
- Quality Assurance Manager Resume
- Quality Assurance Supervisor Resume
- Quality Consultant Resume
- Quality Control Engineer Resume
- Quality Control Manager Resume
- Quality Coordinator Resume
- Quality Engineer Resume
- Quality Lead Resume
- Quality Specialist Resume
- Quality Supervisor Resume
- Quality Technician Resume
Quality Manager Related Careers
- Auditor/Quality
- Director Of Quality
- Laboratory Manager
- Lead Quality Control
- Manager, Quality Engineer
- Quality Assurance Director
- Quality Assurance Manager
- Quality Assurance Supervisor
- Quality Consultant
- Quality Control Engineer
- Quality Control Manager
- Quality Control Supervisor
- Quality Coordinator
- Quality Engineer
- Quality Lead
Quality Manager Related Jobs
Quality manager jobs by location.
- Quality Manager Aliso Viejo
- Quality Manager Chicago
- Quality Manager Colorado Springs
- Quality Manager Cumberland
- Quality Manager Elizabethtown
- Quality Manager Fairfield
- Quality Manager Glendale
- Quality Manager Kettering
- Quality Manager Milwaukee
- Quality Manager Norwalk
- Quality Manager Philadelphia
- Quality Manager San Gabriel
- Quality Manager Spanish Lake
- Quality Manager Sunrise
- Quality Manager Upper Chichester
- Zippia Careers
- Executive Management Industry
- Quality Manager
- Quality Manager Resume
Browse executive management jobs
Quality Manager Resume Sample
The resume builder.
Create a Resume in Minutes with Professional Resume Templates
Work Experience
- Experience in Lean and Continuous improvement projects
- Experience working in an operations capacity
- Experience driving quality improvements across in cross functional teams, e.g. design and manufacturing
- Experience in International Quality System Standards
- Strong systems and process knowledge
- What is this equation used to calculate Cpk LSL = (x̄ – LSL) / 3s ?
- What is the final phase of the APQP development process?
- What is the primary difference between Cpk and Ppk?
- Can lead Quality Engineers in a strong way & drive the quality culture in a fast paced manufacturing environment
- A person that can support the Quality Director in overall activity and setting stretch goals with the team (become a second in command for quality in the optics manufacturing business)
- Actively participate as a member of the leadership team in the deployment of the Eaton Business System (EBS) tools process to integrate initiatives into an overall comprehensive site strategy.
- Provide support to the Supply Chain Management organization relative to Supplier Quality activities of the operation as well as provide Quality interface, expertise and support to the organization’s suppliers and partners. This includes the feedback of supplier performance to suppliers and the Division
- Apply principals and initiatives of the Eaton Quality System and Eaton Business System to the plant’s business and manufacturing processes for continuous improvement
- Coordinate and manage continuous improvement activities related to Quality including 8D’s, Kaizen events, BPI’s, Six Sigma projects, and other problem solving activities
Professional Skills
- Specific skills include: basic computer skills, good feedback skills, and excellent communication skills (listening, verbal, written)
- Strong planning and organizational skills. Strong time management and leadership skills. Excellent analytical skills
- Proven leadership and managerial skills, interpersonal skills and personal development skills
- Excellent written communication and interpersonal skills along with strong time management and organizational skills
- Results oriented, strong analytical and problem solving skills, and excellent communication skills (written, verbal, presentation)
- Accomplished problem solving and analytical skills; as well as excellent verbal and written communicating skills
- Strong leadership and coaching skills and the proven experience to motivate a team
How to write Quality Manager Resume
Quality Manager role is responsible for leadership, interpersonal, computer, organizational, analytical, organization, presentation, microsoft, technical, english. To write great resume for quality manager job, your resume must include:
- Your contact information
- Work experience
- Skill listing
Contact Information For Quality Manager Resume
The section contact information is important in your quality manager resume. The recruiter has to be able to contact you ASAP if they like to offer you the job. This is why you need to provide your:
- First and last name
- Telephone number
Work Experience in Your Quality Manager Resume
The section work experience is an essential part of your quality manager resume. It’s the one thing the recruiter really cares about and pays the most attention to. This section, however, is not just a list of your previous quality manager responsibilities. It's meant to present you as a wholesome candidate by showcasing your relevant accomplishments and should be tailored specifically to the particular quality manager position you're applying to. The work experience section should be the detailed summary of your latest 3 or 4 positions.
Representative Quality Manager resume experience can include:
- Good Communication skills and coordinate ability. Strong Conflict resolution skills
- Have strong interpersonal skills and problem solving skills
- Excellent communication skills (presentation skills, oral, and written communications)
- Excellent computer, interpersonal, oral and written communication skills required. Computer skills
- Excellent problem solving skills are required, including experience preparing problem solving documents such as 8D’s
- Effective organizational and planning skills; managing near term priorities & strategic aspirations
Education on a Quality Manager Resume
Make sure to make education a priority on your quality manager resume. If you’ve been working for a few years and have a few solid positions to show, put your education after your quality manager experience. For example, if you have a Ph.D in Neuroscience and a Master's in the same sphere, just list your Ph.D. Besides the doctorate, Master’s degrees go next, followed by Bachelor’s and finally, Associate’s degree.
Additional details to include:
- School you graduated from
- Major/ minor
- Year of graduation
- Location of school
These are the four additional pieces of information you should mention when listing your education on your resume.
Professional Skills in Quality Manager Resume
When listing skills on your quality manager resume, remember always to be honest about your level of ability. Include the Skills section after experience.
Present the most important skills in your resume, there's a list of typical quality manager skills:
- Demonstrates experience utilizing effective interpersonal communication, supervisory, organizational and priority-setting skills
- Effective problem solving capabilities and interpersonal skills essential. Ability to effectively lead and develop a team with various experience levels
- Demonstrated experience managing a team of direct/indirect reports and strong leadership skills.
- Strong customer service, analytical, and reasoning skills. Better than average written and communication skills required
- Strong analytical skills and practical problem solving skills
- Experience managing others, strong leadership skills, at least 3 years’ experience in leaders role
List of Typical Experience For a Quality Manager Resume
Experience for quality manager resume.
- Experience in facilitation of meetings both internally and externally and excellent presentation skills
- Strong project or technical leadership and good presentation skills
- Leadership and managerial skills required to effectively communicate the utilization of the statistical tools to others throughout the factory
- Excellent interpersonal, verbal and written communication, analytical and presentation skills are also required. Strong attention to detail required
- Thorough understanding of manufacturing process with excellent systemic thinking abilities, strong root cause analysis,General Manufacturing Experience
- Solid planning, organizing, communication, conflict management and teamwork skills
- Strong leadership, decision making and coaching skills
- Have good customer and sub-contractor relations skills commensurate with working in public areas
- Experience required, 7+ years of progressive quality experience in an medium to high volume durable goods manufacturing environment required
- Strong leadership skills to motivate, influence, coach, and mentor others
- Effective computer skills including database maintenance, spreadsheets and word processors
- Possess strong written, verbal and interpersonal communication skills
- Strong verbal and written communication, collaboration, and time management skills
- Strong demonstrated CI- LEAN experience required
- Strong leadership skills, comfortable training Internal Audit Teams and holding Management Reviews with the Leadership Team
- Strong organizational skills, detailed documentation habits and Agile project management capabilities are essential
- Good people skills and ability to deal with all level of personnel within the business from MD to shop floor
- O Demonstrates time management skills
- Effective communications (written/verbal), presentation and facilitation skills
- Demonstrated analytical & investigational skills
- Work well with a team and excellent communication skills
- Demonstrated knowledge of quality engineering skills such as drawing interpretation, inspection planning, operator/inspector certification, gage design, etc
- Good communication skills including fluent written and verbal Dutch and English
- Leading and developing the team effectively and giving them feedbacks etc
- Auditing skills (internal/external)
- Previous experience with ISO (including auditing and developing SOP(s), knowledge of GMP/GLP and quality tracking software experience is desired
- Demonstrated success building and leading effective small and large scale solution delivery projects teams
- Effective management of multiple competing priorities in a fast paced changing environment
- More than 8 years auto mobile industry working experience including 3 years quality management experience
- Establish and maintain good working relationships resulting in excellent customer satisfaction
- Manufacturing experience with a strong understanding of quality control
- Experience working and developing strong relationships with multiple customers and suppliers in a low volume high mix industry
- Strong knowledge and practical experience of quality tools including, but not limited to, APQP, FMEA, SPC, 8D, 5W problem solving tools, PPAP and FAI’s
- Working experience with 2+ years Quality Engineer Supervisor or Manager experience in Medical Device/ Mechanical manufacturing
- Good skill in English, speaking and writing
- Effectively conducting quality control oversight of a number of projects simultaneously
- Ensure sufficient resources, skills and competences are in place to meet functional demands (Training Needs Analysis)
- Good communication skill. Fluent English speaking
- Strong experience in a functional area that is supported by a Six Sigma, Quality or Engineering position
- Experience in a manufacturing or production environment, 2-5 years' experience in Quality
- Prior quality systems experience in manufacturing environment
- Variation Reduction and Problem Solving skills
- Demonstrated experience working with customers, suppliers, or distributors
- A strong knowledge of mechanical engineering/Transmission experience
- Assess skills and proficiency of quality team; identify gaps and training opportunities
- Lead the expansion and sustainment of a committed quality culture throughout the entire value chain through influence and managing skills
- Work effectively as part of a goal-oriented team and communicating frequently with the team and other departments within the company
- Strong understanding and experience of TS19649 Quality Standards
- Prior ISO (9001) experience required
- Establish/maintain a QMS to efficiently/effectively identify and correct problems throughout the entire scope of operations
- Proven track record of good customer relations
- Construction Skills Certification Scheme (CSCS) card
- Engage in continuing education, both formal and self-study to improve needed skills
- Own validation methodology within the project leadership, including establishment of procedures to support validation
- Monitors program effectiveness and develops processes to increase the effectiveness of activities
- Progressively responsible technical experience; or a combination of education and experience
- Demonstrate ability to operate at both a strategic and operational level
- Develop auditor skills and standardise audit practices
- Have proficient interpretation skills for figures, statistics, regulations, and policies
- Effectively collaborate with leaders across the business
- Effectively partner with affiliate leaders to ensure proactive identification and resolution of issues
- Demonstrate initiative and a strong sense of urgency in the daily conduct of business
- Maintain effective department communications through prioritized work list, plant meetings, daily Supervisor and Lead hand
- Communicate the status and progress of programs to the Site Director and Corporate leadership as required, using reports, verbal skills and visual display
- Demonstrated analysis experience
- Effectively train essential plant employees in excipient principles and ISO initiatives
- Prior experience with the implementation and use of Lean Tools, Statistical Process Control, and Advanced Analytical Techniques are required
- Computer technical skills(e.g. Microsoft Office, GQTS, Team Center, Pro-7)
- English written and verbal communication and presentation skills
- Prior experience with document management software, e.g., SAP, Documentum, Agile, etc
- Prior experience with facility start-ups, or other major projects, e.g. documentation system implementation
- Good knowledge about ISO/TS lead audit and Six Sigma Black belt / Good knowledge of Statistical tools
- Good team player, active decision maker, excellent at conflict management and crisis management
- Strong English skill on both oral and written
- Knowledge and experience of quality management and the technical quality measurement of refinishes; ideally experience as Quality manager
List of Typical Skills For a Quality Manager Resume
Skills for quality manager resume.
- Strong leadership skills with demonstrated success simultaneously directing multiple projects in multiple locations
- Strong project management skills with an emphasis on critical thinking and problem solving
- Outstanding written and verbal communication skills including presentation effectiveness
- Demonstrated analytic capability and problem solving skills
- Experience leading projects, and the ability to effectively lead and influence others
- Strong Sensory skills - ability to detect Quality issues and identify differences
- Demonstrated influence leadership skills
- Monitoring & Test equipment calibration skills
- Auditing skills Lead Auditor ISO 9001:2008
- Directs and leads Quality team to effectively perform required sampling, testing, and reporting of quality data
- Direct and lead Quality Assurance associates to effectively perform required sampling, testing, and reporting of quality data
- Proven experience implementing and/or improving quality systems
- Leadership, presentation, project management, coaching, influencing, and verbal/written communication skills required
- Quality field experience including management experience in a manufacturing environment
- Extensive experience in Quality or related fields; experience in product or manufacturing engineering
- Experience in B/P reading/GD&T and experience with engineering model interpretations
- Demonstrate an understanding of quality concepts such a: cost of Quality, analytical metrics, statistics, trending and validations
- General computing skills and systems knowledge
- Computer skills including spreadsheet applications, Microsoft Access, and/or Minitab
- Over five years technical experience in engineering work, experience in supervisory role
- Word Processing, Database, ERP (SAP) and Spreadsheet PC skills
- Experience: Requires experience and practical knowledge of commercial heat treatment of alloys including steel, aluminum, titanium, nickel, brass, copper, etc
- Develops problem solving and leadership skills of subordinates
- Validates quality of incoming raw materials and finished goods in excess of $30,000,000 annually
- Strong problem solving ability and experience
- Demonstrated experience with statistical techniques and advanced quality planning
- Demonstrated quality and reliability focus and experience in previous work assignments
- Communicates effectively (written and verbal) to interface with customers and suppliers
- Six Sigma Green Belt or Black Belt Certification or demonstrated statistical analysis/control experience
- Effectively lead the QA function and engage senior management on quality issues
- Making sure the company is working as effectively as possible to keep up with competitors
- Raising corrective actions when non-conforming conditions are detected and ensuring effective corrective / preventative actions are implemented
- Working experience with ISO9001 or TS16949 standards
- Managing non-conformance through to effective conclusions to internal and external requirements
- Working knowledge of GMP, Microbiology, GLP, Hygiene controls, Validation and HACCP principals
- Assure consistent quality of production by developing and enforcing manufacturing systems, validating processes, and managing staff
- Detailed understanding of manufacturing processes and a proven track record in identifying and delivering successful continuous improvement
- Experience of the Inspection, Measuring and Testing equipment and tools (IMTE) oilfield equipment, steel stock, manufacturing and machine shops related jobs
- Ensures plant is achieving prescribed quality standards by setting clear direction and priorities, and by providing directional guidance to other departments
- Experience in powder metal (P/M) processing in addition to conventional metalworking manufacturing methods
- Expertise in designing, developing and delivering effective presentations/trainings
- Expertise in Manufacturing Quality Management with proven auditing track record
- Proven ability to lead workshops engaging diverse teams in problem solving
- Responsible for developing and communicating audit agendas in a timely manner prior to SMRAC audits
- Demonstrate courage in identifying and addressing issues. Asks for help or assistance when needed
- Ensures all plant personnel adhere to good manufacturing practices (GMP) including proper waste disposal, personal hygiene and personal practices
- Experience working in the printing industry
- Experience in metals manufacturing, engineering or quality control
- Provide pass-through characteristics to our sustaining quality team to support PFMEA development and effective control planning on the campus
- Related experience in quality assurance, including work in an injection molding production environment
- Experience of developing and maintaining a Business Management System to ISO9001 standard
- Lead and develop effective corrective actions using a suite of problem solving tools and analysis to prevent reoccurrence
- Experience with performance testing best practices for high-volume and high-performing web applications
- Seek to increase the effectiveness of the Quality Management System through benchmarking and sharing of best practices
- Juggle overlapping projects and priorities in a fast-paced environment
- Ensures timely, accurate and complete communication to create and maintain excellent working relations
- Demonstrates initiative when interfacing with constituents
- Experience leading a ISO 9001, QS9000, AS9100 or ISO/TS 16949 based Quality Management System (QMS) in a high-mix, assembly operation
- Demonstrate customer vision and advocacy. Be willing to take ownership and resolve the issues
- Working experience in Q & HSE
- Experience from working in an international evironment
- S in Engineering or related technical field with additional courses in business, etc. or equivalent experience
- Work experience of a statistical, engineering, or technical background
- Previous manufacturing related experience
- Mandatory Tubular Running Services - Oil and Gas experience with quality assurance and quality control as per API
- Practice and promotes safety and good housekeeping
- Ensures adequate corrective measures are implemented in cases of noncompliance / non-conformance and checks for their effectiveness, monitoring and closure
- Manage customer NCR process for effective investigation, Root Cause analysis and final reporting. Implement change and best practices where applicable
- Verify, by auditing, the effectiveness of corrective actions
List of Typical Responsibilities For a Quality Manager Resume
Responsibilities for quality manager resume.
- Strong written/ oral communication skills, Human relations, team building skills, and Project Management skills
- Strong leadership and demonstrated change management skills
- Be recognized as champion of behavioral/GMP and cultural initiatives with strong quality related decision making skills demonstrated
- Good facilitator with excellent interpersonal, communication and organizational skills
- Strong skills in communicating with all levels of technical and manufacturing personnel, and operating management
- Proven leadership / mentoring skills
- Excellent organizational skills while assuring a high quality work process
- Strong verbal and written communication skills to a wide audience
- Good command of both Chinese and English communication skills
- Stable and reliable, with excellent strategic skills to deal with emergencies and solve the problems
- Creates solutions for complex and multi-faceted problems, which require strong analytical skills
- Experience with MS Office suite and other computer skills
- Excellent verbal and written/interpersonal communication skills
- Demonstrate strong, effective leadership of Quality and continuous improvement principles
- Strong working knowledge and leadership skills using Six Sigma and Lean tools
- One year experience in role requiring analytical and problem solving skills
- One year experience in role requiring communication skills, ability to influence others and being a team player
- Three years experience in role requiring analytical and problem solving skills
- Experience working OCONUS with supply chains, schedules, logistics Management of materials, QA/QC and safety Skills
- Demonstrated leadership / influencing skills in a cGxP environment; preferably within a Quality role
- Excellent communication skills, tact and diplomacy to interface with various internal and external customers
- Demonstrated skills with PC office applications such as MS Word, Excel and Power Point
- Good skills in project management – cost, quality, and resources
- Demonstrated ability to effectively lead, motivate, coach and train people in varying production disciplines to achieve improved overall performance
- Strong planning, partnering and organizational skills
- Excellent communication (written and verbal) skills in German (native level) and English
- Native level of English with excellent written and verbal communication skills in Italian
- Excellent communication (written and verbal) skills in a Spanish and English
- Strong leadership skills with the ability to facilitate and influence direction
- Quality team management with highly effective communication skills and assertiveness to be the leader and advocate of Breckenridge Quality
- Solid interpersonal, teamwork, organization and time management skills
- Ensuring that management review is implemented effectively and meets the requirements of the management system
- Consulting skills to translate customer needs to internal process improvements
- Demonstrated experience in managing a team of quality control / quality assurance staff
- Develop the facility quality team to meet the requirements of the Skills Assessment matrix
- Computer skills – MS Office (SAP – an advantage)
- Supervise and develop the skills and knowledge of the Quality Department
- Leadership and team player skills
- Lead and implement change initiatives. Project Management and leadership skills in order to lead projects and team members to successful completion
- Inter-personal, negotiation and customer service skills in order to interface with internal and external customers discuss technical issues and find solutions
- Demonstrated quality experience ideally in infrastructure maintenance or construction
- Ideally 8 years experience within the medical device or pharmaceutical manufacturing industry, with at least 4 years’ experience in a leadership role
- Demonstrated experience in managing quality processes for traditional, design build or P3 projects
- Demonstrated knowledge of experience with ISO 9001:2008 and / or 9001:2015 quality management system requirements
- Prior experience with hard metals, complex machining concepts and castings
- Interact effectively with other teams, including Product Management and Development
- Expert skills in using and training quality tools (i.e. Fishbone Diagrams, 5-Whys, 8D, Gage R&R, FMEA, SPC, etc.)
- Management Skills in relation to being able to manage an entire function within a manufacturing environment
- Manage & motivate people and problem solving skills
- Lead and manage a team of Quality Engineers and Technicians, ensuring they have the relevant skills, capabilities, tools and support to perform in their roles
- Experience leading effective business change programs
- Microsoft Office skills requiring (Excel, Word and Powerpoint)
- Function effectively in a global and matrix organization
- Assesses priorities and assigns work to reports to assure the project and company priorities are met
- Manages and mentors staff including conducting performance reviews and other performance measures. Ensures staff skillset meets needs for department
- Analytical and mathematical skills; proficiency in Design of Experiments, Statistics, Reliability and Quality Engineering methodology
- Collaborative leader and effective problem solver with experience leading all levels of employees to improve the QS
- Prior experience in quality management systems, processes and certifications
- Strong understanding of quality principles, tools and methodologies. Proven track record in driving continuous improvement via quality tools
- Other Technical Skills – SAP, Minitab, Microsoft Office, packaging for LTL transportation
- Superb problem solving skills and the ability to teach
- Experience of relevant work experience in QA systems implementation and management in a medium size manufacturing environment
- Strong understanding of and experience in quality assurance systems particularly in the areas
- Several years of professional experience in quality, production and / or R&D area, including experience in the management of interdisciplinary teams
- Proven experience collaborating with multiple sites and functions to drive common solutions
- The ability to work in teams and communication skills
- Prior experience in quality management programs
- Expert interpersonal communication skills to facilitate the team process and coordinate with numerous healthcare discipline
- Communication skills to all levels of the organization and with customers
- Effectively resolve conflict with both internal and external customers
- Effectively collaborate with other organizational groups
- Able to demonstrate experience with strategic process improvement (software upgrades, procedural changes, etc)
- Superb English communication skills, both written and verbal

Related to Quality Manager Resume Samples
Global quality manager resume sample, plant quality manager resume sample, technical quality manager resume sample, senior testing resume sample, lead, quality control resume sample, software quality engineering resume sample, resume builder.
Resume Worded | Proven Resume Examples
- Resume Examples
- Research & Science Resumes
- Quality Control Resume Guide & Examples
Quality Control Manager Resume Examples: Proven To Get You Hired In 2024

Jump to a template:
- Quality Control Manager
- QA/QC Manager
- Quality Operations Manager
- Quality Systems Manager
Get advice on each section of your resume:
Jump to a resource:
- Quality Control Manager Resume Tips
Quality Control Manager Resume Template
Download in google doc, word or pdf for free. designed to pass resume screening software in 2022., quality control manager resume sample.
Quality control managers are like the gatekeepers of a product's journey from inception to the hands of the consumer. They ensure every product meets the required standards and regulations. In today's market, many companies are embracing lean manufacturing and Six Sigma methodologies, so it's key to demonstrate on your resume your knowledge and experience with these trends. Additionally, the role isn't solely about technical know-how; it requires excellent communication skills to liaise with various departments and stakeholders. Therefore, anyone drafting a resume for this role should aim to combine these dimensions seamlessly.

We're just getting the template ready for you, just a second left.
Recruiter Insight: Why this resume works in 2022
Tips to help you write your quality control manager resume in 2024, highlight relevant certifications.
Quality control managers need to be well-versed in a myriad of regulations and standards. Therefore, you should showcase any relevant industry certifications (like a Certified Quality Engineer or Six Sigma belt) you've earned on your resume to reflect your competence.

Showcase Your Communication Skills
As a quality control manager, you'll be dealing with diverse teams and stakeholders across the organization. Highlight instances when you've effectively coordinated with different departments, managed client feedback, or led a team to achieve quality objectives. This can be proof of your communication and leadership abilities.

A quality control manager is a senior-level position in which they create quality standards and manage the quality control team. They will provide directions to quality control technicians and ensure everyone follows quality benchmarks. They are involved in every phase of the manufacturing process to make sure products are ready for distribution. To become a quality control manager, you should have a bachelor’s degree in Business, Administration, or a related field. You should also have proven experience in the manufacturing industry and showcase communication and problem-solving skills. A quality control manager must have significant attention to detail.

Indicate your ability to manage a quality control team.
As a quality control manager, you must be able to deal with large teams. You should have leadership, communication, and problem-solving skills to be in this role. That’s why it is crucial to mention this trait in your quality control manager resume.

Show your evolution in the quality control industry.
You can also emphasize your value and transparency by showing how you escalated in the industry. Feel free to mention your success journey, including promotions, successful projects, and achievements in the quality control industry.

QA/QC Manager Resume Sample
Quality operations manager resume sample, quality systems manager resume sample.
We spoke with hiring managers at companies like Procter & Gamble, Johnson & Johnson, and 3M to gather their top tips for creating a Quality Control Manager resume that will get you hired. They've seen thousands of resumes throughout their careers and know exactly what they like to see on a resume, as well as what makes them quickly reject a candidate. We've compiled their advice below.
Highlight your experience with quality management systems
Employers want to see that you have hands-on experience working with quality management systems such as ISO 9001, Six Sigma, or Lean manufacturing. Provide specific examples of how you've used these systems to improve quality control processes.
- Implemented ISO 9001 quality management system, reducing defects by 25% and increasing customer satisfaction scores by 15%
- Led Six Sigma project that streamlined production processes, resulting in a 20% reduction in cycle time and $500K in annual cost savings

Quantify your impact with metrics and data
When describing your quality control experience, use hard numbers and data to quantify the impact of your work. This helps employers understand the scope and significance of your contributions.
Weak, vague descriptions of your impact look like this:
- Improved quality control processes
- Reduced defects and increased efficiency
Instead, provide specific metrics that demonstrate your value:
- Implemented quality control initiatives that reduced defect rate from 3.2% to 0.5%, saving the company $2.5M annually
- Developed and executed quality assurance plan for new product launch, resulting in 99.7% first-pass yield and zero customer complaints
Showcase your leadership and project management skills
Quality Control Managers often lead teams and oversee complex projects. Highlight any experience you have managing people, coordinating cross-functional initiatives, or driving process improvements.
Include examples like:
- Led a team of 15 quality assurance inspectors to ensure consistent product quality across three manufacturing plants
- Spearheaded a cross-functional project to identify and eliminate sources of defects, involving collaboration with engineering, production, and supply chain teams
- Developed and delivered training programs on quality control best practices to over 200 employees, improving overall quality metrics by 30%
Tailor your resume to the specific job requirements
Quality control roles can vary significantly depending on the industry and company. Carefully review the job description and tailor your resume to highlight the specific skills and experience that the employer is seeking.
For example, if the job emphasizes experience with certain quality control tools or methodologies, make sure to call out your proficiency with those specific tools:
- Expertise in using statistical process control (SPC) software to monitor and analyze production processes
- Certified Lean Six Sigma Black Belt with experience leading DMAIC projects to drive continuous improvement
Include relevant certifications and training
Quality control is a field where certifications and specialized training are highly valued. If you have any relevant certifications or have completed training programs, make sure to include them on your resume.
Some examples of valuable certifications for Quality Control Managers include:
- ASQ Certified Quality Engineer (CQE)
- ASQ Certified Manager of Quality/Organizational Excellence (CMQ/OE)
- Lean Six Sigma Green Belt or Black Belt
- ISO 9001 Lead Auditor certification
Listing these certifications demonstrates your commitment to professional development and shows employers that you have the knowledge and skills needed to excel in a quality control role.
Provide examples of your problem-solving skills
Quality Control Managers are often responsible for identifying and resolving complex quality issues. Use your resume to showcase your problem-solving abilities by providing concrete examples of how you've tackled challenges in previous roles.
Investigated and resolved a persistent issue with product defects that had caused a 20% increase in customer returns. Traced the problem to a faulty machine sensor and implemented a recalibration procedure, reducing the defect rate to less than 1% and saving the company $1.2M in potential recall costs.
Examples like this demonstrate your ability to analyze problems, find root causes, and implement effective solutions - all critical skills for a successful Quality Control Manager.
Writing Your Quality Control Manager Resume: Section By Section
summary.
A resume summary for a Quality Control Manager is an optional section that can be beneficial if you want to provide additional context about your experience and qualifications that may not be immediately evident from the rest of your resume. It's a brief overview of your most relevant skills, experience, and achievements, tailored to the specific Quality Control Manager position you're applying for.
However, it's important to note that a summary should never be used in place of a well-crafted resume that highlights your relevant experience and accomplishments. If you do choose to include a summary, make sure it adds value and doesn't simply repeat information that can be found elsewhere in your resume.

To learn how to write an effective resume summary for your Quality Control Manager resume, or figure out if you need one, please read Quality Control Manager Resume Summary Examples , or Quality Control Manager Resume Objective Examples .
1. Highlight relevant quality control experience
When writing your summary, focus on your most relevant quality control experience and how it aligns with the requirements of the job you're applying for. Consider including:
- Years of experience in quality control roles
- Specific industries or products you've worked with
- Key responsibilities and achievements
Here's an example of a summary that effectively highlights relevant experience:
Quality Control Manager with 8+ years of experience in the automotive industry. Proven track record of implementing effective quality control processes that have reduced defects by 30% and increased customer satisfaction ratings by 20%. Skilled in leading cross-functional teams and collaborating with suppliers to ensure consistent quality standards.
2. Showcase leadership and problem-solving skills
As a Quality Control Manager, your ability to lead teams and solve complex problems is crucial. Use your summary to highlight these skills and provide specific examples of how you've applied them in past roles.
Avoid generic statements like:
- Strong leadership skills
- Excellent problem-solver
Instead, provide concrete examples:
- Led a team of 15 quality control inspectors to achieve a 98% on-time delivery rate
- Developed and implemented a new testing protocol that reduced product defects by 25%
Experience
Your work experience section is the heart of your resume. It's where you show hiring managers that you have the right experience to excel in the quality control manager role.
In this section, we'll break down each critical step to writing an effective work experience section, from using the right action verbs to quantifying your impact.
1. Lead with strong action verbs
When describing your quality control experience, choose powerful action verbs that showcase your contributions and achievements. Consider verbs like:
- Implemented new quality control processes that reduced defect rate by 15%
- Spearheaded root cause analysis to identify and resolve recurring quality issues
- Collaborated with cross-functional teams to develop and execute quality improvement initiatives
- Monitored key quality metrics and generated reports for senior leadership
Avoid starting bullets with weak, passive phrases like "Responsible for" or "Tasked with". Instead, use strong verbs that emphasize your active role and ownership.
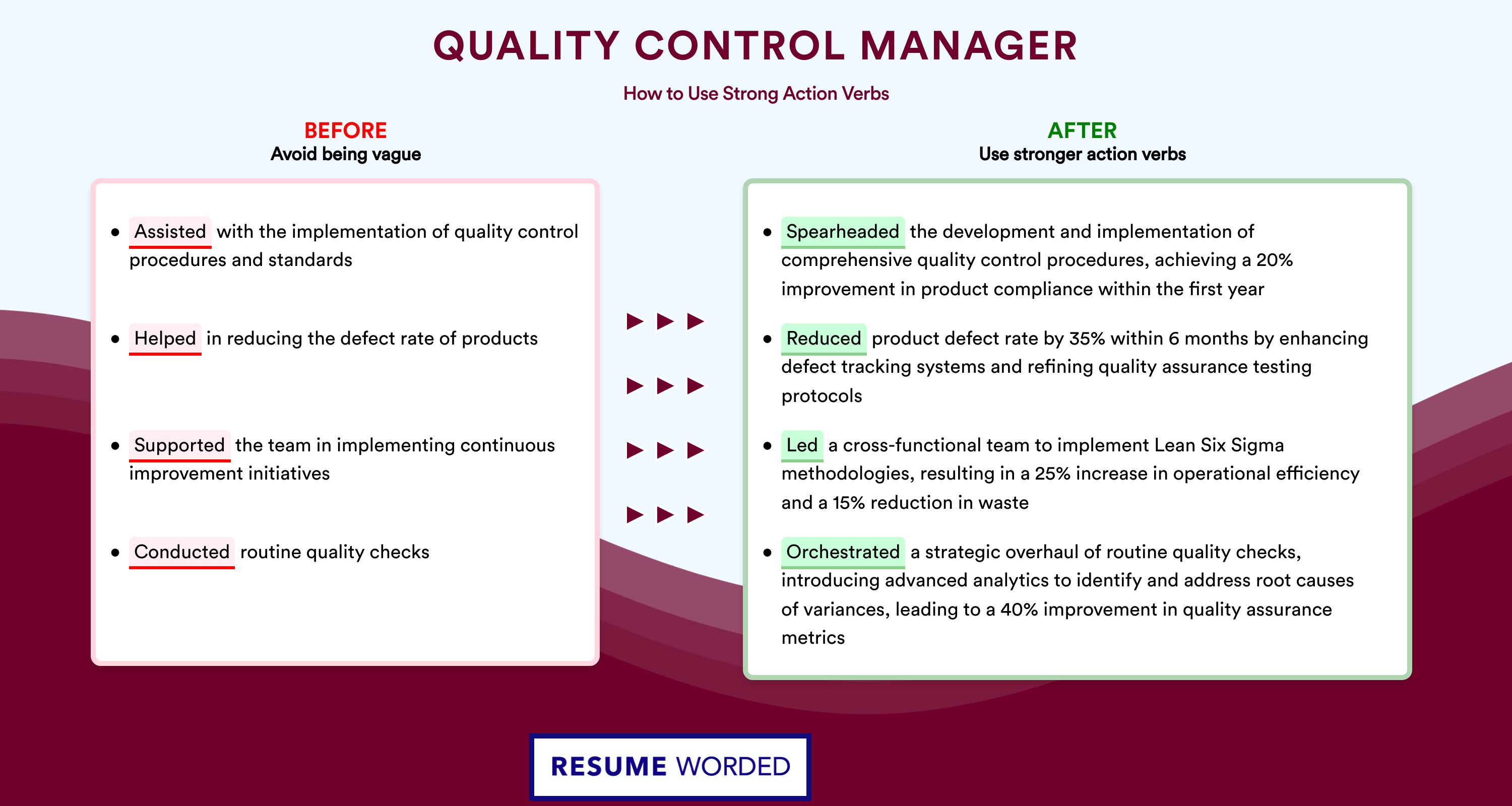
2. Quantify your quality control impact
Numbers speak louder than words. Whenever possible, quantify the results and impact of your quality control efforts. Here are some examples:
- Reduced customer complaints by 30% through implementing enhanced quality inspection procedures
- Achieved 99.7% product quality rating by leading a Lean Six Sigma DMAIC project
- Saved $500K annually by identifying and eliminating sources of waste in the QC process
If you don't have exact metrics, you can still provide numerical context:
- Led a team of 12 quality control inspectors across 3 manufacturing plants
- Managed quality control for product lines generating $10M+ in annual revenue
3. Showcase your QC skills and expertise
Quality control managers need a robust skill set spanning technical knowledge, leadership, data analysis, and problem-solving. Highlight your most relevant and impressive skills through your work experience bullets. For example:
- Expertise in quality control methods such as SPC, GD&T, and FMEA
- Proficiency in quality management systems including ISO 9001, AS9100, and Six Sigma
- Strong data analysis skills leveraging tools like Minitab and Tableau to identify trends and drive improvements
Avoid simply listing skills without context, like this:
- Skills include quality control, Six Sigma, root cause analysis
Instead, weave your skills into your accomplishments to provide a specific context and make a stronger impact.
4. Highlight promotions and career growth
If you've advanced in your quality control career, make that trajectory obvious to hiring managers. Here are some ways to highlight your career growth:
Quality Control Manager, XYZ Manufacturing (2018-Present) Quality Control Engineer, XYZ Manufacturing (2015-2018) - Promoted to Quality Control Manager to oversee quality operations across multiple production lines - Implemented a Lean QC program as Quality Engineer, resulting in promotion to QC Manager role
By showcasing your promotions and expanded responsibilities, you demonstrate your ability to succeed and grow in the quality control function. This helps position you as a strong candidate for senior-level quality control manager roles.
Education
Your education section is a vital part of your quality control manager resume. It shows hiring managers that you have the necessary knowledge and training to succeed in the role. Here are some tips to make your education section stand out:
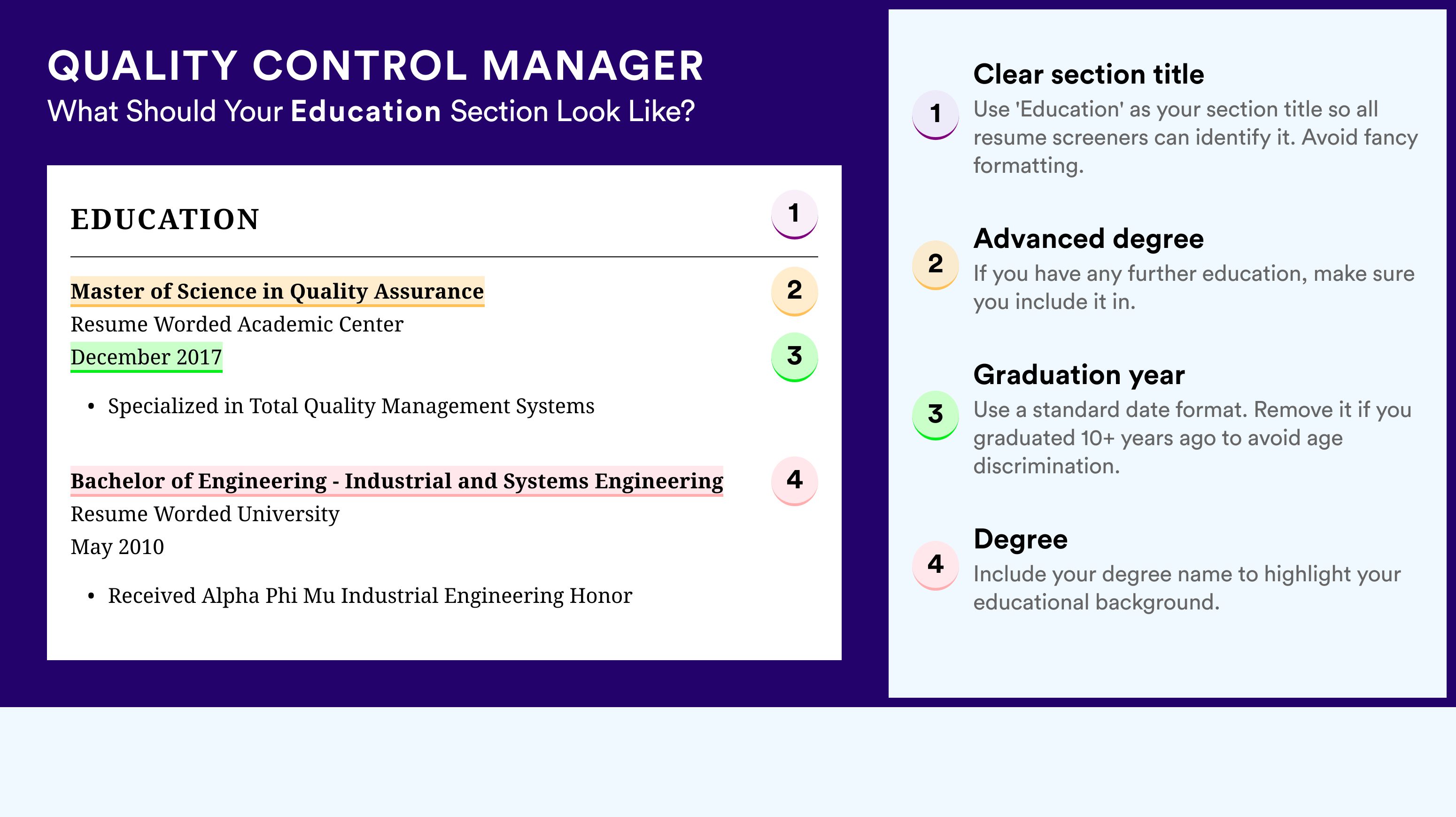
1. List your degrees in reverse chronological order
Start with your most recent degree and work backwards. Include the name of the institution, the degree you earned, and the year you graduated. If you have multiple degrees, list them separately.
Here's an example of how to format your education section:
Master of Science in Quality Management, ABC University, 2019 Bachelor of Science in Industrial Engineering, XYZ College, 2015
2. Highlight relevant coursework and projects
If you're a recent graduate or have limited work experience, you can use your education section to showcase relevant coursework and projects. This helps demonstrate your knowledge and skills to potential employers.
Some examples of relevant coursework for a quality control manager might include:
- Statistical Process Control
- Six Sigma Methodology
- Quality Management Systems
- Lean Manufacturing Principles
Avoid listing basic or introductory courses that aren't directly related to quality control, such as:
- English Composition 101
- College Algebra
- Introduction to Psychology
3. Include relevant certifications
If you have any certifications related to quality control or management, include them in your education section. This can help you stand out from other candidates and demonstrate your expertise.
Some examples of relevant certifications for a quality control manager might include:
- Certified Quality Engineer (CQE)
- Certified Six Sigma Black Belt (CSSBB)
- Certified Manager of Quality/Organizational Excellence (CMQ/OE)
Avoid listing certifications that aren't directly related to the role or industry, such as:
- CPR/First Aid Certification
- Microsoft Office Specialist
4. Keep it concise for senior-level positions
If you're a senior-level quality control manager with extensive work experience, you don't need to include as much detail in your education section. Instead, focus on your most recent and relevant degrees.
Here's an example of how a senior-level education section might look:
M.S. Quality Management, ABC University B.S. Industrial Engineering, XYZ College
Avoid listing graduation dates if you've been out of school for more than 10-15 years, as this can sometimes lead to age discrimination:
Master of Science in Quality Management, ABC University, 1985 Bachelor of Science in Industrial Engineering, XYZ College, 1980
Skills
Your skills section is one of the most important parts of your resume as a quality control manager. It's where you showcase your expertise and qualifications that make you the best fit for the job. In this section, you'll want to highlight both your technical skills and your soft skills that are relevant to quality control.
Here are some tips to help you write a strong skills section on your quality control manager resume:
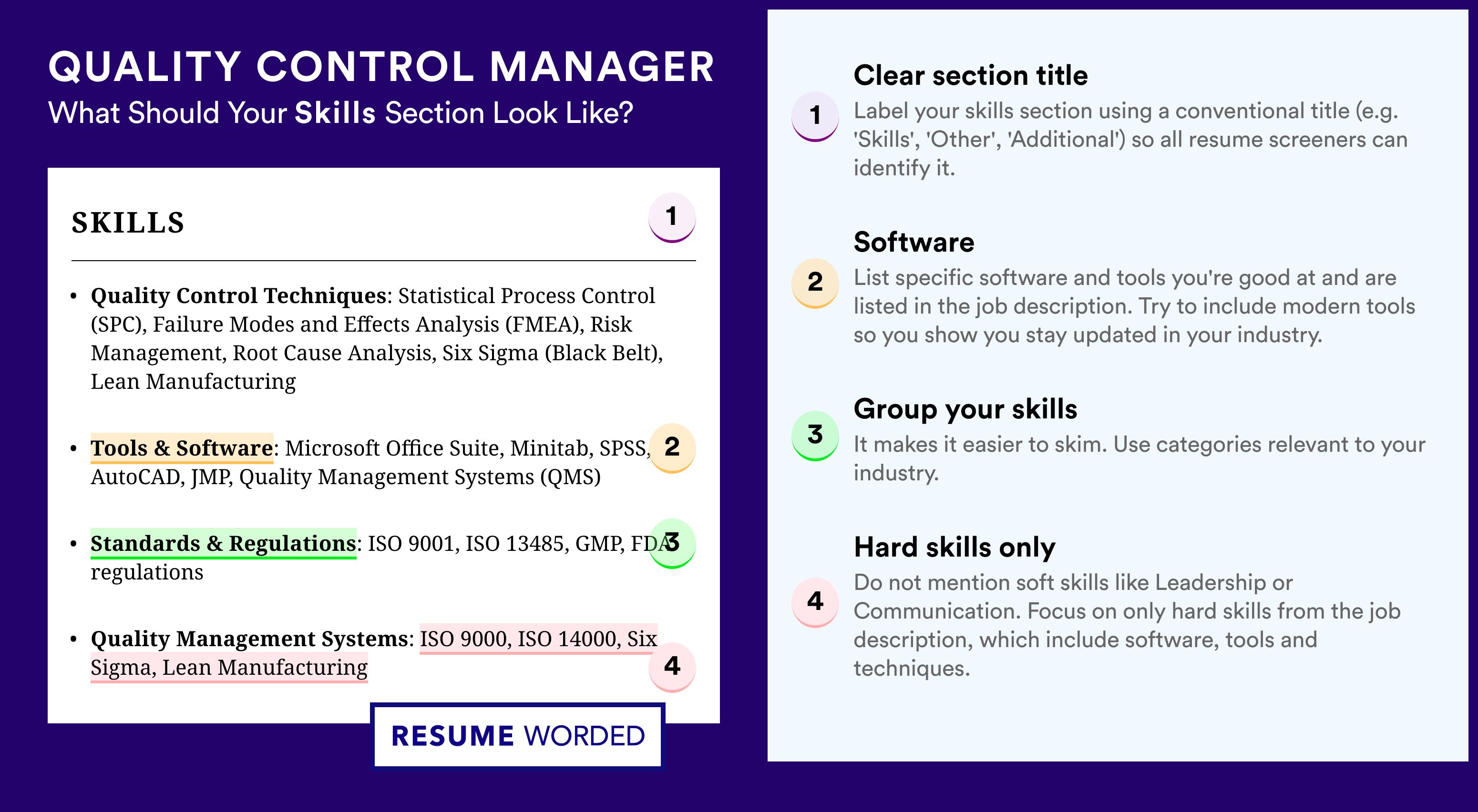
1. Tailor your skills to the job description
When writing your skills section, it's important to tailor it to the specific job you're applying for. Read through the job description carefully and make note of any skills or qualifications that are mentioned. Then, make sure to include those skills on your resume if you have them.
For example, if the job description mentions experience with Six Sigma or ISO 9001, make sure to include those specific skills. This will help your resume get past Applicant Tracking Systems (ATS) that many companies use to filter out candidates who don't have the required skills.
Six Sigma Black Belt certified Experience with ISO 9001 quality management systems Proficient in statistical process control (SPC)
2. Focus on technical skills and tools
As a quality control manager, your technical skills and knowledge of quality control tools and techniques are critical. Make sure to highlight these skills prominently in your skills section.
Some examples of technical skills to include:
- Quality Management Systems : Six Sigma, Lean, Kaizen, ISO 9001, ISO 14001
- Tools : Statistical Process Control (SPC), Failure Mode and Effects Analysis (FMEA), Root Cause Analysis, Pareto Charts, Control Charts
- Software : Minitab, SAP QM, IQS, ETQ Reliance
By including specific technical skills and tools, you'll show employers that you have the knowledge and expertise needed to succeed in a quality control manager role.
3. Avoid outdated or irrelevant skills
While it's important to highlight your skills and experience, you also want to avoid including outdated or irrelevant skills on your resume. Stick to skills that are current and in-demand in the quality control field.
Microsoft Office Data Entry Customer Service
Instead, focus on highlighting skills that are specific to quality control and relevant to the job you're applying for, like:
Auditing Continuous Improvement Problem Solving Data Analysis
4. Quantify your skills and achievements
Whenever possible, quantify your skills and achievements to make them more impactful. For example, instead of just listing 'Six Sigma' as a skill, specify your level of expertise or any relevant projects you've completed.
Six Sigma Black Belt with 5+ years of experience leading process improvement projects Reduced defects by 35% and increased productivity by 20% through Lean Six Sigma initiatives
By quantifying your skills and achievements, you'll give employers a better understanding of the value you can bring to their organization and help your resume stand out from other candidates.
Skills For Quality Control Manager Resumes
Here are examples of popular skills from Quality Control Manager job descriptions that you can include on your resume.
- Hazard Analysis and Critical Control Points (HACCP)
- Food Safety
- Quality System
- Microbiology
- Laboratory Information Management System (LIMS)
Quality Assurance
- Good Laboratory Practice (GLP)
- Standard Operating Procedure (SOP)
Skills Word Cloud For Quality Control Manager Resumes
This word cloud highlights the important keywords that appear on Quality Control Manager job descriptions and resumes. The bigger the word, the more frequently it appears on job postings, and the more likely you should include it in your resume.

How to use these skills?
Similar resume templates, inventory manager.
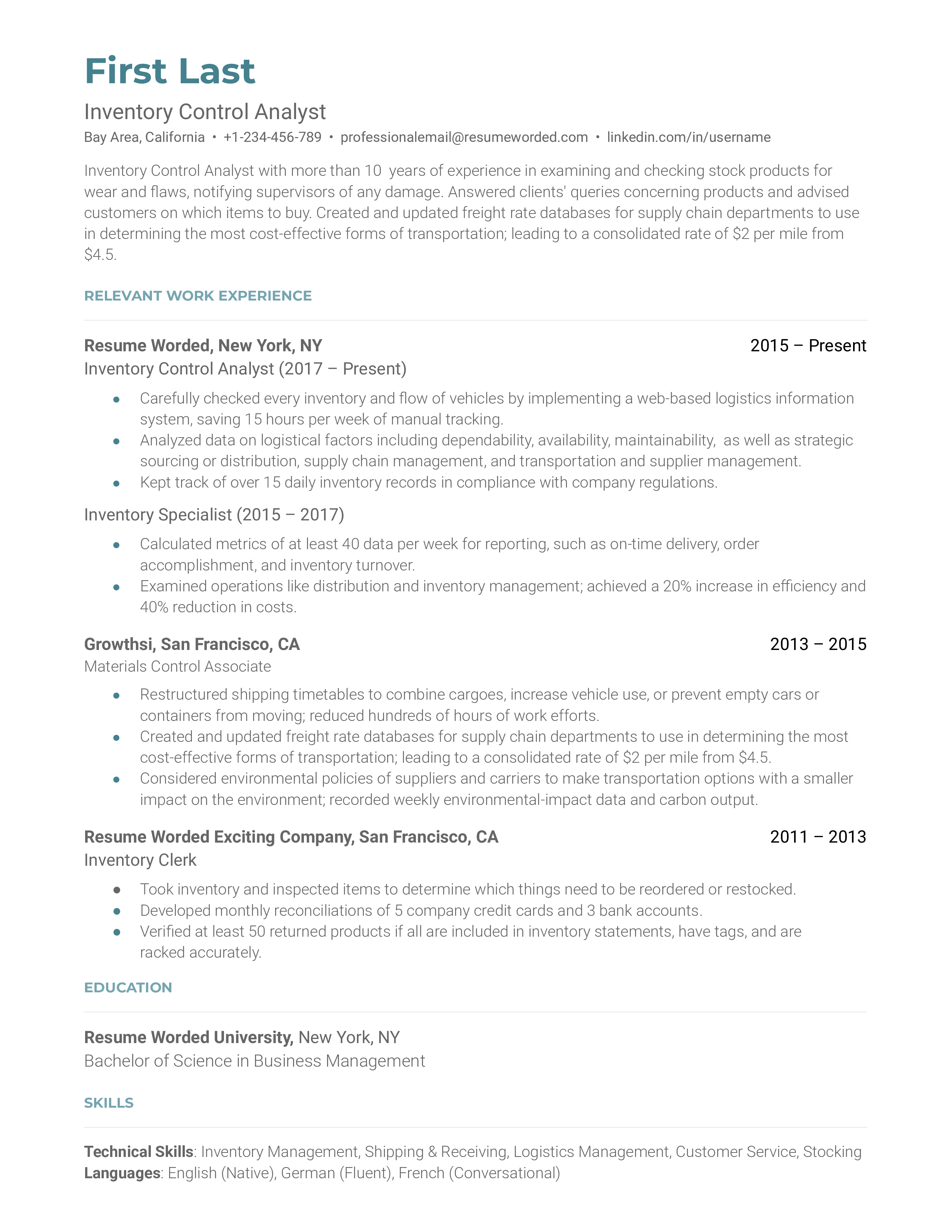
Manufacturing Engineer

- Research Assistant Resume Guide
- Health and Safety Resume Guide
- Clinical Research Resume Guide
- Environmental Scientist Resume Guide
- Chemistry Resume Guide
Resume Guide: Detailed Insights From Recruiters
- Quality Control Resume Guide & Examples for 2022
Improve your Quality Control Manager resume, instantly.
Use our free resume checker to get expert feedback on your resume. You will:
• Get a resume score compared to other Quality Control Manager resumes in your industry.
• Fix all your resume's mistakes.
• Find the Quality Control Manager skills your resume is missing.
• Get rid of hidden red flags the hiring managers and resume screeners look for.
It's instant, free and trusted by 1+ million job seekers globally. Get a better resume, guaranteed .
Quality Control Manager Resumes
- Template #1: Quality Control Manager
- Template #2: Quality Control Manager
- Template #3: Quality Control Manager
- Template #4: QA/QC Manager
- Template #5: Quality Operations Manager
- Template #6: Quality Systems Manager
- Skills for Quality Control Manager Resumes
- Free Quality Control Manager Resume Review
- Other Research & Science Resumes
- Quality Control Manager Interview Guide
- Quality Control Manager Sample Cover Letters
- Alternative Careers to a Quality Control Technician
- All Resumes
- Resume Action Verbs
Download this PDF template.
Creating an account is free and takes five seconds. you'll get access to the pdf version of this resume template., choose an option..
- Have an account? Sign in
E-mail Please enter a valid email address This email address hasn't been signed up yet, or it has already been signed up with Facebook or Google login.
Password Show Your password needs to be between 6 and 50 characters long, and must contain at least 1 letter and 1 number. It looks like your password is incorrect.
Remember me
Forgot your password?
Sign up to get access to Resume Worded's Career Coaching platform in less than 2 minutes
Name Please enter your name correctly
E-mail Remember to use a real email address that you have access to. You will need to confirm your email address before you get access to our features, so please enter it correctly. Please enter a valid email address, or another email address to sign up. We unfortunately can't accept that email domain right now. This email address has already been taken, or you've already signed up via Google or Facebook login. We currently are experiencing a very high server load so Email signup is currently disabled for the next 24 hours. Please sign up with Google or Facebook to continue! We apologize for the inconvenience!
Password Show Your password needs to be between 6 and 50 characters long, and must contain at least 1 letter and 1 number.
Receive resume templates, real resume samples, and updates monthly via email
By continuing, you agree to our Terms and Conditions and Privacy Policy .
Lost your password? Please enter the email address you used when you signed up. We'll send you a link to create a new password.
E-mail This email address either hasn't been signed up yet, or you signed up with Facebook or Google. This email address doesn't look valid.
Back to log-in
These professional templates are optimized to beat resume screeners (i.e. the Applicant Tracking System). You can download the templates in Word, Google Docs, or PDF. For free (limited time).
access samples from top resumes, get inspired by real bullet points that helped candidates get into top companies., get a resume score., find out how effective your resume really is. you'll get access to our confidential resume review tool which will tell you how recruiters see your resume..

Writing an effective resume has never been easier .
Upgrade to resume worded pro to unlock your full resume review., get this resume template (+ 4 others), plus proven bullet points., for a small one-time fee, you'll get everything you need to write a winning resume in your industry., here's what you'll get:.
- 📄 Get the editable resume template in Google Docs + Word . Plus, you'll also get all 4 other templates .
- ✍️ Get sample bullet points that worked for others in your industry . Copy proven lines and tailor them to your resume.
- 🎯 Optimized to pass all resume screeners (i.e. ATS) . All templates have been professionally designed by recruiters and 100% readable by ATS.
Buy now. Instant delivery via email.
instant access. one-time only., what's your email address.

I had a clear uptick in responses after using your template. I got many compliments on it from senior hiring staff, and my resume scored way higher when I ran it through ATS resume scanners because it was more readable. Thank you!

Thank you for the checklist! I realized I was making so many mistakes on my resume that I've now fixed. I'm much more confident in my resume now.

Quality Manager Resume Samples
The role of a quality manager is concerned with monitoring and supervising the performance of the quality management systems , measuring against set standards and producing data on performance. A well-crafted Quality Manager Resume includes the following tasks – devising and establishing quality procedures, reviewing customer requirements, establishing quality requirements, setting standards for quality, defining quality procedures , monitoring performance, supervising employees use relevant quality tools and making suggestions for improvement.
To successfully execute these tasks, the quality managers are supposed to exhibit a keen eye for details, have outstanding communication skills and be an excellent leader. These experts should have a deep understanding of quality control procedures and be aware of the latest legal standards. While a degree in the field of science may be considered sufficient to enter into this profession, holding a QC certification would prove to be advantageous.

- Resume Samples
- Quality Manager
Quality Manager Resume
Summary : Tested and proven Assistant Quality Manager, with multiple certifications and vast knowledge of process improvement possibilities, ranging from Lean, Continuous Improvement, and Six Sigma. Well versed in Quality systems and auditing from ISO to AS9100. To obtain employment with a growth-oriented business, which will engage my past and present capabilities and for the development of additional skills leading to further advancement opportunities.
Skills : Coaching, Quality Management, Quality Control, Organizational Development, Leadership Training, Auditing

Description :
- Responsible for planning, directing, and coordinating quality activities for all inspection personnel.
- Ensuring that the repair and overhaul of all articles are being conducted in accordance with all regulatory and customer requirements and that the work is inspected and returned to service by a qualified inspector.
- Ensuring that current versions of the required technical data are always available and used for the overhaul or repair of every article where maintenance has been performed by maintenance personnel.
- Administering the precision tool and test equipment calibration program which requires the performance of periodic checks and calibrations on all new and existing precision tools and equipment as well as the maintenance of current records detailing these checks/calibrations.
- Evaluating and maintaining the annual internal and external evaluation and surveillance programs and the subsequent evaluation plan and checklists necessary for assessing the implemented policies.
- Maintaining a current list of approved suppliers through which the repair station and PMA manufacturing departments may purchase aeronautical products and/or direct use material.
- Managing a list of maintenance providers (both faa certified and noncertified), for the performance of repairs, maintenance, modification, and/or overhaul of aviation articles.
- Managing existing and ongoing employee training while maintaining current records that verify individual participation.
Software Quality Manager Resume
Objective : Motivated Software Quality Manager, seeking an opportunity to utilize my skills and experience to benefit the right organization. Proven leader with the ability to motivate people to meet objectives, exceed expectations and deliver results. To obtain a continuous improvement management position focused on quality and continuous improvement initiatives that will utilize my engineering education, supervisory experience, Six Sigma knowledge, and diverse technical background.
Skills : Word, Access, Excel, PowerPoint, Outlook, Instructor, Trainer, Microsoft Office, QI Macros And Minitab

- Supporting customer and end item users with technical issues and concerns and promptly performing preventative and corrective action.
- Designing, administering and auditing conformance of the QA system to AS9100/AS9102FAI/AS9110 and Nadcap requirements.
- Leading the implementation of proactive quality management practices in all functional areas, spanning marketing and product development, manufacturing, and test.
- Teaching and facilitating the implementation of the tools of quality to the broader organization and supply chain.
- Possessing working knowledge of 8d and root cause problem-solving methodology.
- Providing reports depicting the quality levels, quality problems, and cost of quality.
- Driving quality improvement with through more effective launch of new programs.
- Providing quality reports to customers as required, and ensures frequent, effective communication with customers on all corrective action plans.
Data And Quality Manager Resume
Summary : Analytical, business-minded Data And Quality Manager with extensive experience planning and improving manufacturing operations within fast-paced automotive and aerospace industries. Innovative strategist, skilled in developing and implementing continuous improvement initiatives aimed at cutting costs, bolstering productivity and supporting the goal of operational excellence. Highly-skilled quality assurance supervisor with a proven track record of consistently improving manufacturing processes.
Skills : Microsoft Office Applications, Mini-tab, Auto-cad, Uni Graphics, Lean Manufacturing, Automotive Quality Experience, Automotive OEM, And Tier 1 Experience.

- Created a corrective action policy to reduce the number of technical support cases resulting from internal issues.
- Led teams through the process of root-cause analysis and corrective action methods and reduced the number of tech support cases by 20% in a 3 month period.
- Developed a system to address the alarming number of parts replaced under warranty, many of which were never returned and the cause of the failures was not investigated.
- The system included failure analysis reports that enabled a feedback loop to all relevant internal and external stakeholders.
- As a result, returns were reduced by greater than 30% in less than 4 months which, in turn, significantly reduced man-hours and warranty costs.
- Focused on a modular design in software and hardware for strategic reuse and scalability.
- Also, led continuous improvement efforts which improved test accuracy by 20% and reduced test time by 80%.
- Created and executed schedule, cost, capacity, scalability, risk analysis, and product qualification plan to successfully transfer more 10 + new products into full-scale production, on schedule, complete with documentation, test hardware/software.
Operations Manager/Quality Manager Resume
Summary : Motivated, personable Operations Manager/ Quality Manager - with extensive experience in a number of different high-tech manufacturing environments (machining, EDM, metal stamping, welding, and assembly). Talent for being able to think out-of-the-box to develop solutions to complex problems. Diplomatic and tactful with professionals and manufacturing personnel at all levels of the organization. Flexible and versatile - able to maintain control and focus under pressure. Poised and competent, with demonstrated ability to easily transcend cultural differences.
Skills : MS Office, Leadership, Problem Solving, Lean Manufacturing, Quality Circle Leader, Six Sigma Black Belt

- Driving, leading and troubleshooting quality investigations to ensure issues are thoroughly investigated with appropriate caps.
- Effectively interacting with production and development teams to maintain product supply and help introduce new products.
- Working with purchasing staff to establish quality requirements from external suppliers.
- Bringing together the staff of different disciplines and driving the group to plan, formulate and agree on comprehensive quality procedures.
- Persuading reluctant staff to change their way of working to incorporate quality methods.
- Managing raw material testing, in-process testing and finished producing and release procedures.
- Responsible for the creation, issuance, tracking, review, approval and/or control of labeling materials as per 21 CFR 101 regulations.
- Responsible for the overall health of the quality department through technical guidance, employee development, and actively collaborating with production.
- Upgrading and/creation of quality plans for legacy and new products.
Regional Quality Manager Resume
Summary : Accomplished Regional Quality Manager with extensive experience leading results-driven training, project management, product testing, and quality assurance. Proven ability to enhance workflow and standardize effective project management tools and systems. To bring to an established corporation: enthusiasm, dedication, responsibility, and excellent work ethic, combined with a desire to utilize the skills obtained through my experience and education.
Skills : Microsoft Office, Six Sigma Green Belt, AS9100 Internal Auditor, SAP User, Lean Improvements, Cycle Time Reduction

- Managing and maintaining the quality management system in relation to AS9100C and ISO 9000/2008.
- Working one-on-one with AS9100C lead certification auditors to supply any quality documentation needed during recertification and surveillance audits.
- Writing non-conformance documents, corrective action reports, and preventive action report to continually improve companywide quality.
- Utilizing lean six sigma principles to investigate non-conformances/ identify root causes.
- Revising and maintaining process maps, procedures, and any other quality documents to reflect processes as they are carried out, as well as in relation to AS9100C's standard for quality management systems and any other customer specific requirements.
- Writing a new company employee safety manual and forklift safety manual create a FOD training program and an AS9100C training program for familiarizing and training employees with these standards and regulations.
- Tracking shipment summary and jobs returned data to create reports to detect potential problem areas and better improve delivery times.
- Responsible for forklift safety training and ensuring all facility safety regulations are observed.
Corporate Quality Manager Resume
Headline : Corporate Quality Manager with 5 years of progressive experience in a broad range of technical precision, manufacturing, and developmental growth system environments. Proven ability to combine vision creativity and strong business acumen in order to develop lean, well-documented quality management leadership programs compliant with Sigma Six Lean, ISO 9001 and API Q1.
Skills : HACCP, FDA Food Defense, ISO, As400, Microsoft Office, Forklift Operator, SQF Implementation

- Brought on board to develop and implement a corporate-wide API spec Q1, spec 11AX & 5B program.
- Transitioned all existing recordkeeping systems from manual tracking to a digital record keeping information system.
- Represented management during all QSI preliminary setup reviews and compliance audits.
- Streamlined operational processes management including ordering, inventory, manufacturing, calibration tracking, and quality assurance methodologies reducing loss and NCP production to cycling target guidelines.
- Integrated automated digital barcode system for our calibrated precision instrument distribution.
- By connecting tooling to the employee and the workstation, shrinkage tracking and timely calibration processes were implemented.
- Developed innovative product line management processes that expedited barrel honing and quality staging prior to coating.
- Performed corporate acquisition conversion to concurrent API practices including machine maintenance scheduling, precision tooling calibration, product line sorting, critical designator print review and floor plan layout.
- Reviewed and oversaw internship program to correct print layout including the addition of API spec callouts, critical designators, non-compliant tolerances and inserting proper logo, revision creation, review, and approval formatting to meet Q1 requirement.
Industrial Quality Manager Resume
Summary : Proactive and innovative Industrial Quality Manager and problem-solver with over 10 years of experience in manufacturing, operations and quality management. Accomplished communicator with proven experience in dispute resolution and maintaining peaceful, productive and dynamic business environments.
Skills : leadership And Development, Creative Problem Solving / Decision-making, Process Improvement / Organizational Development, Resource Allocation And Management, Client Education / Training And Presentation Efficiency

- Ensured an effective ISO 9001:2008 Quality Management System (QMS) process was established and implemented, to recommend corrective and preventative action where necessary to ensure its effective implementation and improvement.
- Effectively managed and coordinated all quality management activities and staff to deliver the scope of work in line with all project expectations.
- Liaised with clients on all quality related issues during design, procurement, fabrication, construction and commissioning matters.
- Monitored and controlled project quality performance against quality requirements using appropriate control tools and report same to management, clients and project management team.
- Prepared, issued and revised the contract quality plan, inspection/verification strategy, and compliance strategies.
- Established a team of competent and experienced Quality Management (QM) discipline engineers to meet the scope of work and specify contract quality requirements.
- Ensured that contract personnel was provided with the tools and knowledge required to execute the contract/project with legislation, procedures, and compliance.
- Convened and attended meetings concerning project quality management matters as appropriate.
Operations And Quality Manager Resume
Objective : High-energy Operations And Quality Manager successful in building and motivating dynamic teams. Cultivates a company culture in which staff members feel comfortable voicing questions and concerns, as well as contributing new ideas that drive company growth. A full-time position, using my vast knowledge of Quality and Technical systems gained over many years of work in the automotive and technical industries.
Skills : Microsoft Office, Develop Quality Systems, Start-Ups, Document Control, Continuous Improvement, Customer Service, Statistical Process Control, Root Cause Analysis

- Responsible for developing, maintaining, and reporting departmental goals for quality and continual improvement.
- Participate in group, team, and departmental meetings to collect, analyze, and act on issues to improve facility performance.
- Planned, coordinated, and directed the quality management system designed to ensure continuous production consistent with established standards.
- Built, maintained, and promoted a quality driven culture within the QMS and through regularly scheduled meetings held with plant employees.
- Developed, initiated, and performed corrective actions ranging from external complaints, rejects from customers.
- Using tools such as 6 panel, fishbone, deep dive root cause analysis, 8d corrective actions, as well as executing and training on these areas.
- Performed and managed process audit schedule for the facility utilizing the layered audit process.
- Maintained MSA data and calibration system of all inspection tools used by the facility.
Maintenance Quality Manager Resume
Summary : Senior Quality Engineer position supporting a manufacturing organization through process analysis and system implementation to solve problems resulting in significant cost savings. Energetic and hands-on Quality Management and Operations leader with over ten years of experience in manufacturing, quality, software programs, and supplier management. Proven ability to lead, mentor and motivate team members in order to maximize performance.
Skills : Quality Engineering, Team Problem Solving, Training & Facilitation, Reliability Engineering, Customer Service, Advanced Quality Planning, Quality Auditing, Continuous Improvement, Lead Time Reduction, System Development, Supplier Development, New Product Launches

- Responsible for implementing and maintaining the Alcoa business system (modeled after the Toyota business system) continuous improvement process for this location of alcoa's building and construction division.
- Training and coaching all levels of employees in problem-solving and corrective action reporting.
- Facilitating quality Kaizen events quarterly to identify opportunities for improving current processes and soliciting all levels of team member involvement in brainstorming, analysis and improvement execution.
- Facilitating "take time out for problem-solving" events weekly using 5-why and 8-discipline methods to assist team members in determining root cause, developing corrective & preventive action plans and ensuring execution/follow-through.
- Initiated level 3 "flash" communication meetings combining verbal & visual tracking daily management where all departments come together & report out the key measurable status, identify daily constraints & present problem-solving results all in 15 minutes total.
- Managing quality control department with 4 direct salaried reports and 8 hourly technicians.
- Resolving customer claim issues, vendor claim issues, manage change control process and oversee operational excellence process auditing to ensure quality system compliance.
- Developing & executing annual departmental operational plans in concert with business plan & track performance objectives.
- Coordinatiing development of process FMEAs & control plans with cross-functional teams for existing processes without and proposed new processes.
Safety Manager-Quality Manager Resume
Summary : A proven Safety Manager-Quality Manager who is rich with diverse experience. Enjoys challenges, teamwork, and continuous improvement. Results-oriented Quality Assurance Manager with expertise in root cause analysis and implementation of targeted process and personnel improvements to reduce the overall cost of quality while maintaining or improving production efficiency. Seeking a challenging and rewarding position in quality assurance, engineering, or process improvement utilizing proven technical expertise, quality process knowledge, and procedure analysis.
Skills : Quality Management, Project Management, CAD Engineering Tools, Electrical System Design, Continual Improvements, Metrics Reporting, Process Engineering

- Developing, implementing and managing quality management system to ISO 9001:2008 certification.
- Responsible for continuous improvements, value mapping, inspection & testing, preventative and corrective actions, root cause analysis, solutions implementation & follow up.
- Providing excellent customer service handling customer concerns and quality action plans.
- Conducting management reviews, internal audits, process audits, and product audits.
- Authoring and standardizing checklists, process documents, and leading continuous improvement activities for engineering and manufacturing.
- Managing subcontractor quality and work processes and quality via audits, data analysis, communications, & metrics reporting.
- Leading diverse workgroups and teams through process and product improvement initiatives.
- Designing technical project overviews, system designs, and control charts.
- Constructing an intranet website providing accessibility to reports, tools, processes, and reference documents.
- Programming PLC equipment, application design verification testing, and control panel testing.
Table of Contents
Recent posts, download this pdf template., creating an account is free and takes five seconds. you'll get access to the pdf version of this resume template., choose an option., unlock the power of over 10,000 resume samples., take your job search to the next level with our extensive collection of 10,000+ resume samples. find inspiration for your own resume and gain a competitive edge in your job search., get hired faster with resume assistant., make your resume shine with our resume assistant. you'll receive a real-time score as you edit, helping you to optimize your skills, experience, and achievements for the role you want., get noticed with resume templates that beat the ats., get past the resume screeners with ease using our optimized templates. our professional designs are tailored to beat the ats and help you land your dream job..
You control your data
We and our partners use cookies to provide you with our services and, depending on your settings, gather analytics and marketing data. Find more information on our Cookie Policy . Tap "Settings” to set preferences. To accept all cookies, click “Accept”.
Cookie settings
Click on the types of cookies below to learn more about them and customize your experience on our Site. You may freely give, refuse or withdraw your consent. Keep in mind that disabling cookies may affect your experience on the Site. For more information, please visit our Cookies Policy and Privacy Policy .
Choose type of cookies to accept
These cookies allow us to analyze our performance to offer you a better experience of creating resumes and cover letters. Analytics related cookies used on our Site are not used by Us for the purpose of identifying who you are or to send you targeted advertising. For example, we may use cookies/tracking technologies for analytics related purposes to determine the number of visitors to our Site, identify how visitors move around the Site and, in particular, which pages they visit. This allows us to improve our Site and our services.
These cookies give you access to a customized experience of our products. Personalization cookies are also used to deliver content, including ads, relevant to your interests on our Site and third-party sites based on how you interact with our advertisements or content as well as track the content you access (including video viewing). We may also collect password information from you when you log in, as well as computer and/or connection information. During some visits, we may use software tools to measure and collect session information, including page response times, download errors, time spent on certain pages and page interaction information.
These cookies are placed by third-party companies to deliver targeted content based on relevant topics that are of interest to you. And allow you to better interact with social media platforms such as Facebook.
These cookies are essential for the Site's performance and for you to be able to use its features. For example, essential cookies include: cookies dropped to provide the service, maintain your account, provide builder access, payment pages, create IDs for your documents and store your consents.
To see a detailed list of cookies, click here .
This site uses cookies to ensure you get the best experience on our website. To learn more visit our Privacy Policy
- Resume Examples
- Quality Control Resume Examples (Job Description & Skills)
Quality Control Resume Examples (Job Description & Skills)

Our customers have been hired by:
You're a quality control expert, the Sherlock Holmes of product perfection. But is your resume solving the case of landing that dream job? Our guide is here to help you craft a quality control resume that's as flawless as your keen eye for detail.
With our guidance, you'll create a resume that showcases your detective-like abilities, making you the top choice for any organization. Time to investigate your career potential and crack the code to success!
This guide will show you:
- A quality control resume example better than 9 out of 10 other resumes.
- How to write a quality control resume that will land you more interviews.
- Tips and examples of how to put skills and achievements on a quality control resume.
- How to describe your experience on a resume for a quality control specialist to get any job you want.
Want to save time and have your resume ready in 5 minutes? Try our resume builder. It’s fast and easy to use. Plus, you’ll get ready-made content to add with one click. See 20+ resume templates and create your resume here .
Create your resume now

Sample resume made with our builder— See more resume examples here .
Not what you're looking for? Check other resume examples for similar jobs:
- Quality Assurance Resume Sample
- QA Manager Resume Sample
- Quality Engineer Resume Example
- Quality Tester Resume Sample
- Manual Tester Resume Sample
- ELT Tester Resume Sample
- Manufacturing Engineer Resume Sample
- Production Supervisor Resume Example
- Industrial Engineer Resume Example
- Mechanical Engineer Resume Sample
- Best Resume Samples for All Jobs
Quality Controller Resume Sample
Walter J. Brown
Quality Control Specialist
(646) 888-5555
linkedin.com/in/walterbrown
Summary of Qualifications
Detail-oriented quality control specialist with 2+ years of experience in quality inspection and issue detection. Identified and implemented new QC guidelines which cut inspection time by 30%. Seeking to use skilled quality control inspection background to become the next QC supervisor at Queens Industrial.
Work Experience
Quality Control Specialist May 2017–May 2019 ConnFox Production Center, Jamaica, NY
Key Qualifications & Responsibilities
- Made quality decisions (accepted or rejected) based on provided control outlines.
- Reviewed production records for accuracy and compliance.
- Performed routine quality inspection operations on industrial and commercial items.
- Maintained and organized all records, documentation, and other files associated with quality engineering and inspection tasks.
Key Achievements
- Identified and implemented new QC guidelines which cut inspection time by 30%.
- Earned the “NYC Quality Award for Top Performance” in 2018.
Quality Control Inspector September 2016–May 2017 Three-Headed Snake, Inc., Jackson Heights, NY
- Monitored operations output and associated production standards.
- Accepted or rejected product samples after full quality engineering inspection.
- Removed and documented all rejected product samples in the appropriate log books.
Associate Degree in Quality Control
City University of New York, Staten Island, NY
Graduation : 2016
Relevant Coursework : Statistical Process Control, Supplier Quality Control, Quality Management Systems, Calibration Technologies, Material Review.
- Measurement Gauges (Calipers, Micrometers, Microscopes, Comparators)
- Acceptance Sampling & Control Charts
- Process Protocol & Process Control
- Quality Control Inspection
- Lean Six Sigma Collaboration
- Attention to Detail
- 5S Methodology
Certifications
- Certified Production Technician (CPT) from the Manufacturing Skill Standards Council
- Certified Quality Inspector Certification from the American Society for Quality
- 2018 NYC Quality Award for Top Performance
Memberships
- American Society for Quality (ASQ)
- Spanish (Latin American): Basic Conversational Proficiency
That was our take on the perfect quality controller resume sample.
Let’s learn how to write a QC resume that won’t get rejected:
1. Choose the Best Format for Your Quality Control Resume
A quality control (QC) specialist is a worker who manages the process by which software, hardware, consumer goods, and other items are checked after various stages of production to ensure standards are met before released to the end user and consumer. QC specialists often troubleshoot and fix problems as they arise.
A quality control inspector is a management-level employee who oversees the quality checking process, the safety of manufactured products, or the readiness of software, in addition to supervising the tasks of other QC employees.
ISO 9000 is pretty straightforward when it comes to quality control guidelines.
Follow their lead on your resume—
Use a great resume format to keep everything organized and ensure it passes the hiring manager’s quality inspection.
Follow these QC resume format rules :
- Use the heading of a resume to add your resume contact information .
- Use titles which stand out to separate the sections on a resume .
- The best format for a resume for QC jobs is the chronological resume template .
- The best fonts for resumes are those that are easy for a hiring manager to read.
Pro Tip : The PDF resume format is almost always the best, unless the job description specifically asks for a Word doc to be sent in.
2. Write a Quality Control Resume Objective or Summary
A resume objective or summary is your elevator pitch for the QC job.
Also called a resume profile , this 3–4 line paragraph introduces you to the factory or company manager.
Pick the resume summary when you have loads of QC experience . The summary statement is perfect for “summing up” your past quality control responsibilities and skills.
Pick the resume objective when you’re new to quality control . The objective statement replaces your lack of experience with your future QC career goals.
Both options include a key accomplishment to prove to them you’re as great as you say.
Pro Tip : The resume heading statement goes at the top, but it doesn’t mean you have to write it first. Save it for the end, instead, so you have a good idea of what to write from the rest of your resume.
3. Create the Perfect Quality Control Job Description for a Resume
Whether inspecting pills on a pharmaceutical line or parts in an automotive factory, the experience on a resume is the core.
Ace your quality control job description like this:
- Place your most recent employment first, and go back in time from there.
- Put the job title first, then dates worked, the factory or company name, and finally 5 or 6 job responsibilities.
- Use an action word (e.g., inspected , rejected , etc.) to start each bullet point.
- Add a measurable achievement or two to back up your statement of awesomeness.
- Learn how to tailor a resume to a specific job . Factory managers throw generic resumes into the compactor.
Pro Tip: How to write a resume with no experience in quality control? It’s the same thing. However, since you don’t have QC experience, think up the most QC-relevant bullet points when writing your work history entries.
4. Make Your Quality Control Resume Education Section Shine
Many quality control job applicants neglect their educational background on a resume .
Later, they’ll wonder why they never got a call back.
Here’s how to nail your resume education section for QC jobs:
- Start with the most advanced education you have, and go back from there.
- Add the degree or certificate name, graduation dates, school name and location, and relevant coursework.
- If relevant or able to help your cause, give additional details, such as Latin honors and favorite classes.
- If you have completed a higher degree (e.g., bachelor’s degree), don’t add high school.
Pro Tip : Relevant coursework for quality control jobs don’t just mean QC-related topics. Include any classes you’ve taken related to the specific industry of the job you’re applying for, as well (e.g., automotive, software).
5. Highlight Your Quality Control Skills for Resumes
In the United States, quality control jobs will decline by 11% (more than 55,000 jobs) between 2016 and 2026.
Less demand means it’s harder for you.
To ensure you’re not a candidate they’ll pass over, show the hiring manager your QC skills are second to none with the best quality control skills list.
Key Quality Control Skills for Resume—Examples
- Detail-Oriented
- Mathematics & Statistics
- Multi-tasking Skills
- Accountability
- Microsoft Word
- Google Docs
- Acceptance Sampling
- Control Charts
- Process Protocol
- Process Control
- FDA Compliance & Regulations
- GMP Pharmaceutical Quality Control
- Current Good Manufacturing Practices (cGMP)
- First Article Inspection (FAI) Practice
- OSHA Information Quality Guidelines
- Technical Skills
- Good Communication Skills
- Decision Making Skills
- Critical Thinking Skills
- Interpersonal Skills
- Time Management Skills
- Problem Solving Skills
- Teamwork Skills
- Project Management Skills
Taking this list instead of coming up with your own doesn’t show quality or control .
Here’s what to do instead:
- Think of all the professional skills you’ve got related to quality control, and write those down.
- Don’t forget to add both hard skills and soft skills .
- Pull out the job description and keep it out in front of you.
- Finding the best resume keywords to use means just a thorough look at their job requirements.
Pro Tip: QC job hiring managers know that every candidate adds soft skills, like attention to detail, though there’s no way to quantify it. For your quality control resume, go hard on the hard skills they ask for in the job description, first and foremost.
When making a resume in our builder, drag & drop bullet points, skills, and auto-fill the boring stuff. Spell check? Check . Start building a professional resume template here for free .
When you’re done, our easy resume builder will score your resume and our resume checker will tell you exactly how to make it better.
6. Add Other Sections to Your QC Resume
You’ve so far managed to write a solid resume.
But, will it pass quality control?
See, the thing is, all the other QC job applicants have those above core sections.
So, to make yours more unique, add some choice extras.
Here are a few great additions to a resume for quality control jobs:
- QC association memberships
- Personal interests
- Resume language skills section
- Professional achievements
- Certifications for a resume
- Volunteer work on a resume
7. Attach a Cover Letter for Quality Control Resumes
How important is a cover letter?
Skip the covering letter to your own peril, as more than half of employers want one with your resume.
Here’s how to write a QC cover letter that’ll get you the interview:
- Learn how to format a cover letter before you start.
- Write an intriguing cover letter introduction .
- Excite them with your QC skills and knowledge.
- Include a numbered accomplishment to really impress them.
- A call to action is the best way of ending a cover letter .
Read up some more on what to include in a cover letter , how long is too long for a cover letter , and more great cover letter tips .
Pro Tip : Don’t forget to follow up on your job application . And, while your waiting for their reply, why not start practicing for the interview ?
Plus, a great cover letter that matches your resume will give you an advantage over other candidates. You can write it in our cover letter builder here. Here's what it may look like:

See more cover letter templates and start writing.
Got any questions on how to write a quality control resume? Not sure how to talk about QC skills and inspection abilities? Get at us in the comments below, and thanks for reading!
About Zety’s Editorial Process
This article has been reviewed by our editorial team to make sure it follows Zety's editorial guidelines . We’re committed to sharing our expertise and giving you trustworthy career advice tailored to your needs. High-quality content is what brings over 40 million readers to our site every year. But we don't stop there. Our team conducts original research to understand the job market better, and we pride ourselves on being quoted by top universities and prime media outlets from around the world.
- https://asq.org/quality-resources/iso-9000
- https://www.bls.gov/ooh/production/quality-control-inspectors.htm

Don't miss out on exclusive stories that will supercharge your career!
Get a weekly dose of inspiration delivered to your inbox
Similar articles

What Are the Best Resume Layout Examples for 2024?
Learn why the resume layout matters as much as the contents. See examples of great resume layouts and use the tips to create a professional layout for a resume all by yourself.

What Are You Passionate About: Interview Question & Answers
A complete guide on how to answer “What are you passionate about?“ in a job interview. Why do recruiters ask this question? How to prepare? What to answer?

Transferable Skills: Definition & 50+ Examples for a Resume
Learn everything about transferable skills. See 50+ examples of transferable skills. Check how to identify transferable job skills and nail them in your job-hunt.
Quality Control Manager Resume Examples
Want to use this resume?
City, State, Zip Code
Home: 000-000-0000 | Cell: 000-000-0000
Quality Control Manager with 7+ years of management and engineering work experience. Knowledge of quality assurance activities including audits recommending and implementing corrective actions providing accurate documentation of statistical reports and ensuring overall compliance. Demonstrated excellence in communication skills with various levels of management state and federal regulators stakeholders and suppliers.
- Manage training Document Management System and Quality Management system.
- Planned and executed multiple tasks to ensure controlled documents were managed per procedures.
- Provided coaching to personnel on Quality systems (QS) to include document control and training. Acted as liaison with electronic quality systems vendor(s).
- Developed and analyzed statistical data and product specifications to determine present standards and establish proposed quality and reliability expectancy of finished product.
- Formulated and maintained ASME Code Quality Control Manual in accordance with ASME requirements.
- Applied total quality management tools and approaches to analytical and reporting processes within each department.
- Oversee quality control functions for company products assisting with RMC products when needed.
- Perform solutions designs support field efforts processes submittals and review stats.
- Track and maintains data performing statistical calculations to maximize the product quality and performance.
Six Sigma Certified (ASQ SSGB/SSBB) Certified Manager of Quality/Organizational Excellence (ASQ CMQ/OE)
There are plenty of opportunities to land a Quality Control Manager job position, but it won’t just be handed to you. Crafting a Quality Control Manager resume that catches the attention of hiring managers is paramount to getting the job, and LiveCareer is here to help you stand out from the competition.
View All Quality Control Resumes
Related Skills
- Compliance Understanding
- Quality processes
- Operational improvements
- Performance evaluation
- Recruiting and Hiring
- Budget preparation
- Employee training
- Statistical analysis skills
- Problem-solving abilities
- Market research experience
Similar Resumes
Create My Resume
Quality Control Manager @ Ultradent
I am a highly driven, deeply fervent self-starter. My three strongest skills are problem solving, leading a successful team and delivering on any goal set before me. I enjoy opportunities which challe...
Experience: 13 yrs 5 mo
Quality Control Manager @ PetIQ
Responsible and analytical Manager with more than 15 years of expertise developing top-notch skills in [Type of Skill] and [Type of Skill]. A true leader experienced in the [Type of Industry] environm...
Experience: 19 yrs 8 mo
Quality Control Manager @ Electronics For Imaging Inc
Overall I'm an individual who is skilled in team management/development, conforming to business needs like GMP/5S, process development, cost reduction, and data analysis. I work well under pressure an...
Experience: 6 yrs 10 mo
Quality Control Manager @ ThyssenKrupp Materials NA
Always applying best practices when overseeing projects. Determine, honest, thrive to be the best at any career I have. My goal in life is to be the best me I can be, regardless of the mistakes I have...
Experience: 9 yrs 9 mo
Quality Control Manager @ National Pipe & Plastics
Adaptable and well-rounded Professional with a 10-year background in implementing and developing improvement processes through collaboration with cross-functional teams, regardless of position or trad...
Experience: 4 yrs 1 mo
What to read next
RESUME TIPS
*As seen in :
Quality Manager CV example
As a quality manager, you need to be an organised leader, have an eye for detail and demonstrate all of this with a top-quality application.
In fact, your CV will need to pass the strictest quality testing.
So, to help you increase your chances of landing an interview, we’ve created a quality manager CV example to guide you, along with some of our top CV writing advice.
CV templates
Quality Manager CV example

This is a good example of a Quality Manager CV which contains all of the information that a hiring manager will need to know, and presents it in a well- structured, easy-to-read manner.
Take some time to study and understand this CV, and refer to it throughout the writing of your own CV for best results.

Quality Manager CV layout and format
First impressions count, so a sloppy, disorganised CV may cause your CV to be overlooked..
Instead, perfect the format and structure of your CV by working to a clear logical structure and applying some simple formatting tricks to ease readability.
Don’t underestimate the importance of this step; if your CV lacks readability, your written content won’t even be seen.
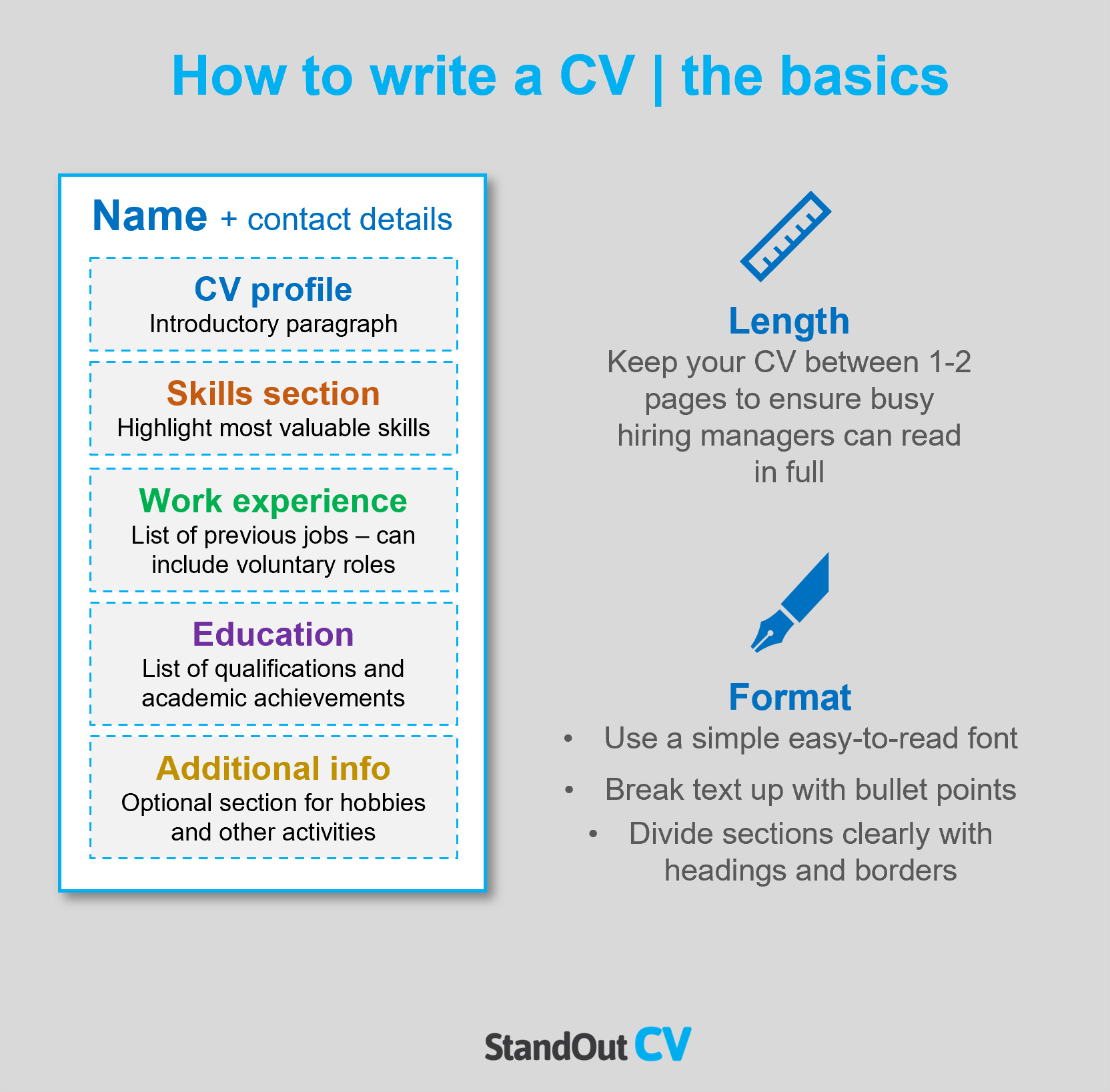
CV formatting tips
- Length: While there’s no ‘official’ CV length rule, the majority of recruiters agree that less is more. Aim for two pages of A4 or less. This is just enough room to showcase your suitability to the role, without overwhelming recruiters with irrelevant or excessive content.
- Readability : Columns, lists, bullet points, bold text and subtle colour can all help to aid the readability of your CV. Your overarching goal should be to make the content as easy to read and navigate as possible, whilst also aiming to make your key skills and achievements stand out.
- Design: When it comes to CV design, it’s best to keep things simple and sleek. While elaborate designs certainly command attention, it’s not always for the right reasons! Readability is key, so whatever you choose to do, make sure you prioritise readability above everything.
- Avoid photos: Ditch logos, images or profile photos . Not only do they take up valuable space, but they may even distract recruiters from your important written content.
Quick tip: Formatting your CV to look professional can be difficult and time-consuming when using Microsoft Word or Google Docs. If you want to create an attractive CV quickly, try our quick-and-easy CV Builder and use one of their eye-catching professional CV templates.

CV structure
For easy reading, write your CV to the following CV structure:
- Contact details – Make it easy for recruiters to get in touch with you by listing your contact details at the top of your CV.
- Profile – A short and snappy summary of your experience and skills, showcasing what makes you a good fit for the position.
- Work experience / career history – Note down all your work history, with your current position first, then working backwards.
- Education – A short list of your academic background and professional/vocational qualifications.
- Interest and hobbies – This is an optional section, which you can use to highlight any relevant hobbies or interests.
Now I’ll tell you exactly what you should include in each CV section.
CV Contact Details

Start off your CV with a basic list of your contact details. Here’s what you should include:
- Mobile number
- Email address – It’s often helpful to make a new email address, specifically for your job applications.
- Location – Share your town or city; there’s no need for a full address.
- LinkedIn profile or portfolio URL – Make sure the information on them is coherent with your CV, and that they’re up-to-date
Quick tip: Delete excessive details, such as your date of birth or marital status. Recruiters don’t need to know this much about you, so it’s best to save the space for your other CV sections.
Quality Manager CV Profile
Recruiters read through countless applications every day.
If they don’t find what they’re looking for quickly, they’ll simply move onto the next one.
That’s what makes your CV profile (or personal statement , if you’re an entry-level/graduate candidate) so important.
This short and snappy summary sits at the top of your CV, and should give a high-level overview of why you’re a good match for the job.
This way, you can ensure that busy recruiters see your suitability from the outset, and so, feel your CV is worth their time.

Tips for creating an strong CV profile:
- Keep it concise: The best CV profiles are short, sharp and highly relevant to the target role. For this reason, it’s best to write 3-4 lines of high-level information, as anything over might be missed.
- Tailor it: Not tailoring your profile (and the rest of your CV) to the role you’re applying for, is the worst CV mistake you could make. Before setting pen to paper, look over the job ad and make a note of the skills and experience required. Then, incorporate your findings throughout.
- Don’t add an objective: Career goals and objectives are best suited to your cover letter , so don’t waste space with them in your CV profile.
- Avoid cliches: Clichés like “ blue-sky thinker with a go-getter attitude” might sound impressive to you, but they don’t actually tell the recruiter much about you. Concentrate on highlighting hard facts and skills, as recruiters are more likely to take these on board.
Example CV profile for Quality Manager
What to include in your quality manager cv profile.
- Summary of experience: Demonstrate your suitability for your target jobs by giving a high level summary of your previous work work experience , including the industries you have worked in, types of employer, and the type of roles you have previous experience of.
- Relevant skills: Make your most relevant Quality Manager key skills clear in your profile. These should be tailored to the specific role you’re applying for – so make sure to check the job description first, and aim to match their requirements as closely as you can.
- Essential qualifications: If you have any qualifications which are highly relevant to Quality Manager jobs, then highlight them in your profile so that employers do not miss them.
Quick tip: Struggling to write a powerful profile? Choose from hundreds of pre-written profiles across all industries, and add one to your CV with one click in our quick-and-easy CV Builder . All written by recruitment experts and easily tailored to suit your unique skillset.
Core skills section
Underneath your profile, write a core skills section to make your most relevant skills jump off the page at readers.
It should be made up of 2-3 columns of bullet points of your relevant skills.
Before you do this, look over the job description and make a list of any specific skills, specialisms or knowledge required.
Then, make sure to use your findings in your list. This will paint you as the perfect match for the role.
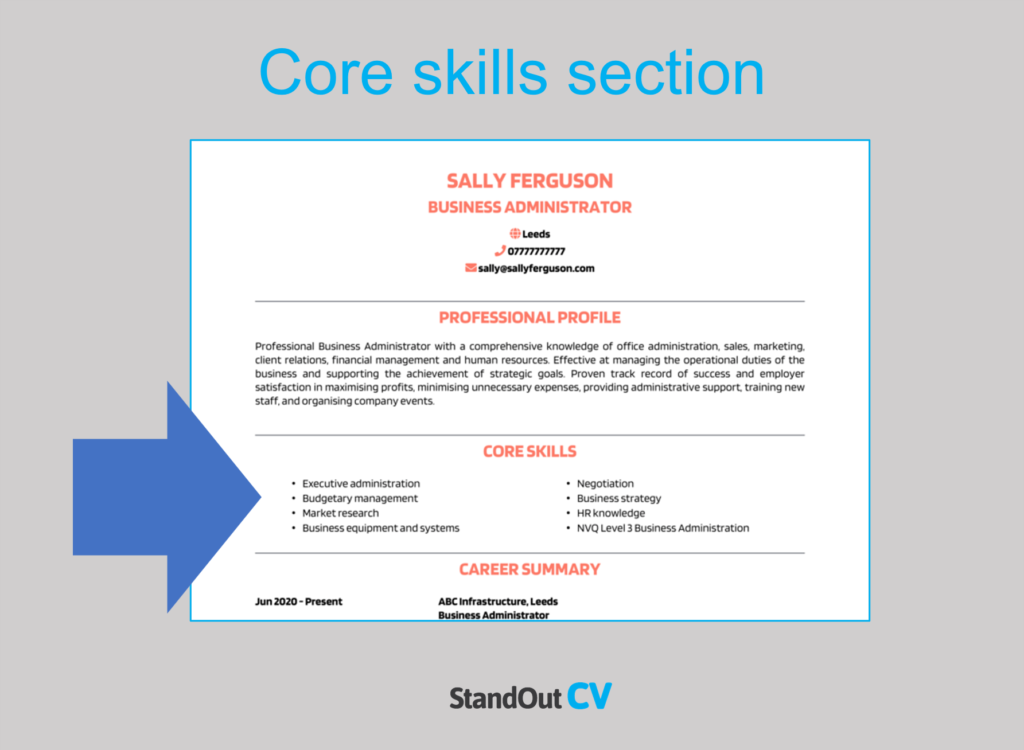
Top skills for your Quality Manager CV
ISO and BMO compliance – ensuring the organisation adheres to the standards relevant to their enterprise.
KPI setting – setting appropriate key performance indicators to demonstrate how effectively key business objectives are being achieved and to evaluate progress and success at reaching targets.
Sustainability improvement – implementing initiatives to improve company/department sustainability, meeting the needs of the present without compromising the ability of future generations to meet their needs.
Quality control plans & ITPs – implementing plans to manage the quality control and assurance of a particular element of a project.
ILM Level 4 Business Management – evidencing personal development and certifications that evidence the ability to lead a team at this level.
Quick tip: Our quick-and-easy CV Builder contains thousands of in-demand skills for every profession that can be added to your CV in seconds – saving you time and greatly improving your chances of landing job interviews.
Work experience/Career history
Recruiters will be itching to know more about your relevant experience by now.
Kick-start this section with your most recent (or current) position, and work your way backwards through your history.
You can include voluntary and freelance work, too – as long as you’re honest about the nature of the work.

Structuring your roles
Lengthy, unbroken chunks of text is a recruiters worst nightmare, but your work experience section can easily end up looking like that if you are not careful.
To avoid this, use my tried-and-tested 3-step structure, as illustrated below:

Provide a brief overview of the job as a whole, such as what the overriding purpose of your job was and what type of company you worked for.
Key responsibilities
Next up, you should write a short list of your day-to-day duties within the job.
Recruiters are most interested in your sector-specific skills and knowledge, so highlight these wherever possible.
Key achievements
Round up each role by listing 1-3 key achievements , accomplishments or results.
Wherever possible, quantify them using hard facts and figures, as this really helps to prove your value.
Example job for Quality Manager CV
Work as a quality manager for an industry-leading construction and engineering contractor, overseeing and auditing the execution of quality systems for the Greater Manchester region and working with a client base that includes the MoD & NHS with build values of up to £100m.
Key Responsibilities
- Devise and regularly update company-wide quality procedures and apply them to current projects
- Ensure compliance with ISO and company Business Management Operations
- Plan, manage and conduct regional internal and external audits, contracting and overseeing trained auditors when needed
- Amalgamate reports from regional quality assurance engineers, compiling reviews for clients and internal management
Quick tip: Create impressive job descriptions easily in our quick-and-easy CV Builder by adding pre-written job phrases for every industry and career stage.
Education section
Although there should be mentions of your highest and most relevant qualifications earlier on in your CV, save your exhaustive list of qualifications for the bottom.
If you’re an experienced candidate, simply include the qualifications that are highly relevant to Quality Manager roles.
However, less experienced candidates can provide a more thorough list of qualifications, including A-Levels and GCSEs.
You can also dedicate more space to your degree, discussing relevant exams, assignments and modules in more detail, if your target employers consider them to be important.
Interests and hobbies
This section is entirely optional, so you’ll have to use your own judgement to figure out if it’s worth including.
If your hobbies and interests could make you appear more suitable for your dream job, then they are definitely worth adding.
Interests which are related to the industry, or hobbies like sports teams or volunteering, which display valuable transferable skills might be worth including.
Writing your Quality Manager CV
An interview-winning CV for a Quality Manager role, needs to be both visually pleasing and packed with targeted content.
Whilst it needs to detail your experience, accomplishments and relevant skills, it also needs to be as clear and easy to read as possible.
Remember to research the role and review the job ad before applying, so you’re able to match yourself up to the requirements.
If you follow these guidelines and keep motivated in your job search, you should land an interview in no time.
Best of luck with your next application!

Quality Manager Resume Example
This is a resume example for an Operations Manager and Quality Manager transitioning from Operations positions in the Air Force. The resume is a useful document for anyone coming from federal or military positions.
The resume begins by using a headline to target key areas of experience in production, operations and quality management. The paragraphs in executive summary format explain more details of their experience.
Notice how the writer identifies the ability to oversee 650 personnel and over $5.5 billion in assets. The Areas of Expertise section provide bullet point references to other key areas such as Six Sigma and KPI Analysis. The entire summary focuses on high level skill sets as opposed to putting emphasis on military history.
The Air Force experience was written like private sector jobs. The job title, employer (Air Force) and dates provide a heading. The paragraphs document specific duties and responsibilities. The bullet points identify key projects, achievements and quantified results.
The resume ends with an education section that features the MBA degree along with a B. S. and A. A. S. in Aeronautics. Additional education includes Six Sigma training, Risk Management, Conflict Management and Communications. The Certifications section documents the Professional Management Certification and Secret Security Clearance.

Quality Manager Resume Example – Page 1

Quality Manager Resume Example – Page 2
Resume Statements
- Develop and implement new policies and procedures and quality processes for expansion in all areas of the business.
- Coordinate quality assurance operations to ensure effective manufacturing quality and inspection procedures are executed.
- Oversee of production, file preparation, document categorization, file quality control and digital scanning.
- Conduct customer meetings to provide status of program operations, quality standards and production metrics.
- Collaborate with Operations department to support new product development, sustain engineering projects, and manage quality control programs.
- Design new processes, statistical analysis, quality concepts and system standards.
- Create strategies and plans for product approvals including inspections.
- Maintain device and equipment quality requirements.
Quality Manager Resume Writing Tips
Are you looking to land your dream job as a quality manager in 2023? Well, they say, ‘quality is not an act. It is a habit.’ And your habit of delivering exceptional results can shine through with a well-crafted quality manager resume.
As a quality manager, you play a crucial role in ensuring that products meet the required quality and safety standards. From coordinating teams of quality control technicians to analyzing products and assigning tasks, your leadership skills are tested.
To make your resume stand out, showcasing your attention to detail, communication, time management, and teamwork skills is essential. A Bachelors Degree in a relevant engineering field, as well as previous managerial and quality control experience, are highly valued by employers.
So, whether you’re an experienced quality manager or just starting, let’s dive into the essential elements of a winning quality manager resume.
What Are the Main Quality Manager Duties and Responsibilities?
Your primary role is to coordinate teams of Quality Control Technicians, ensuring that clients receive functional and safe products.
You’ll run tests, analyze products, assign tasks, and organize work schedules. Plus, you’ll also be documenting defects and returning defective items. It’s an important and challenging position, but with your leadership skills and attention to detail, you’ll excel at it!
You’ll be crucial in maintaining and improving quality control and quality assurance processes. Your quality manager resume will reflect your experience and expertise, highlighting your ability to meet and exceed quality standards. Your resume example should showcase your work experience in managing quality control and quality assurance activities, demonstrating your ability to implement and maintain quality management systems.
You must have a strong understanding of quality standards and regulations. You will be responsible for ensuring that products meet these standards and that quality management systems are in place to support this. Your resume should highlight your experience in developing and implementing quality assurance processes and your ability to analyze data and make data-driven decisions to improve quality.
As a Quality Manager, you will be a key player in ensuring that your products meet the highest quality standards. Your attention to detail, leadership skills, and ability to work collaboratively with cross-functional teams will be essential in achieving this goal.
How to Properly Format a Quality Manager Resume
To properly format your resume, it’s essential to consider the style and organization that will effectively showcase your skills and experience.
Did you know that resumes for Quality Manager jobs typically contain an average of 2.3 pages and include 12.7 bullet points per job title?
When creating your resume format, you can use a resume builder, resume samples, or resume templates to help you write a resume to ensure you include all the necessary information.
Start with a professional resume summary highlighting your qualifications and quality management experience. Include any relevant certifications you have, such as ISO 9001 or AS9100.
Use bullet points to list your responsibilities and achievements in previous roles, focusing on areas such as audit management, product quality, and implementation of a quality management system.
When describing your experience, use action verbs and quantify your achievements whenever possible. This will help the recruiter understand your impact in previous roles.
Include any relevant education and training in the education section, such as a Bachelor’s degree in a relevant field or Six Sigma certification.
By following these tips and utilizing quality manager resume examples and samples, you can create a resume that effectively highlights your skills and experience in the field of quality management.
What Skills Should I Put On My Quality Manager Resume?
When writing your resume, you must showcase your expertise through a comprehensive list of skills demonstrating your capabilities as a quality management professional.
Start by including a bullet point list of your skills, highlighting your ability to implement corrective action and drive continuous improvement.
Mention your experience in qualification processes and proficiency in control processes and quality systems.
Highlight your knowledge of document control and your ability to lead and manage a quality assurance team.
Lastly, emphasize your expertise in conducting root cause analysis to identify and address quality issues.
These skills are crucial in quality management and will help you stand out as a qualified candidate. Remember to tailor your skills section to the job you’re applying for, highlighting the skills most relevant to the position.
What Is a Good Objective for a Quality Manager Resume?
Craft a captivating career goal for your Quality Management journey by highlighting your passion for process perfection and your commitment to continuous improvement. When writing the objective for your Quality Manager resume, it’s crucial to convey your dedication to ensuring product quality and customer satisfaction.
Start with a concise summary showcasing your field experience and expertise. Use strong action verbs and specific examples to demonstrate your ability to lead teams, implement quality control plans, and improve inspection methods. Emphasize your track record of reducing customer claims, implementing quality training programs, and coordinating APQP activities.
Highlight your certifications and technical skills, such as ICC or OSHPD inspector certification and knowledge of ISO 9000/14000 certifications. Also, mention your proficiency in Microsoft Office and excellent interpersonal, written, and verbal communication skills.
Include a Cover Letter To Accompany Your Resume
Including a well-crafted cover letter alongside your application can significantly enhance your chances of securing a quality management position. A cover letter allows you to provide more context and give a personal touch to your quality manager resume. It’s an opportunity for you to showcase your communication skills, attention to detail, and enthusiasm for the role.
When writing your cover letter, tailor it to the job you’re applying for and highlight relevant experiences and qualifications.
In your cover letter, briefly introduce yourself and mention the position you’re applying for. Then, discuss why you’re interested in the role and how your skills and experiences align with the job requirements. Choose keywords from the job description and incorporate them naturally into your letter. This shows the hiring manager that you’ve taken the time to understand the role and are a good fit for the position.
Great resume keywords include quality assurance manager, manufacturing processes, qa manager, knowledge of quality procedures, quality processes, quality engineering, quality planning, and quality assurance methodologies.
Additionally, mention any specific achievements or accomplishments demonstrating your ability to manage quality control processes effectively.
Overall, a well-written cover letter can help you stand out from other applicants and make a strong impression on the hiring manager. It’s a valuable tool in your job search and shouldn’t be overlooked when applying for a quality management position.
In conclusion, crafting a quality manager resume is essential in landing your dream job. By highlighting your skills, experience, and qualifications, you can showcase your ability to ensure products meet the required quality and safety standards. This includes demonstrating your leadership abilities, attention to detail, and strong communication and teamwork skills.
Remember to format your resume appropriately, whether it’s chronological or functional. Also, include a well-written cover letter to accompany your application.
With a compelling resume, you can vividly picture your capabilities and stand out from the competition.
Resume By Get Results Career Services
Related Resume Examples

IT Help Desk

Middle School Teacher

Compliance Officer

Plant Manager

Technology Sales

Retail Sales

Union Supervisor

Manufacturing Manager

Medical Office Manager

Human Resources Manager
Sales Account Executive

Legal Assistant

Benefits Administrator

Insurance Underwriter

Production Assistant

Marketing Executive

Civic Leader – Political
Principal attorney.

Education Consultant
Packaging Manager
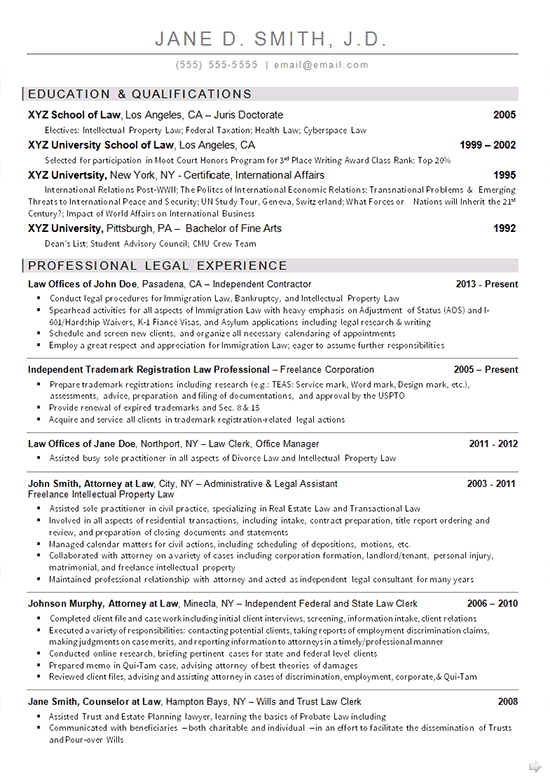
Freelance Property Lawyer

Chief Operating Officer

Hotel Manager

Product Manager

Web Developer

Executive Project Consultant

University Dean

College – International

Insurance Executive
- Next »
Latest Resumes (PDF & DOCX)

Box Office Manager

Healthcare Administrator

Vice President of Sales

Hotel Management

Sales Professional

Director of Business Development

Information Systems

Sales & Marketing Assistant
Logistics Coordinator
Latest resume writing & career articles.

How To Add Github On Your Resume

How To Put Fraternity On a Resume

8 Of The Best Careers To Go Back To School For

How To Put Eit On Resume

How To Add Military Experience To A Resume

How To Write A Double Major On A Resume – Pro Tips

How To List Temp Work On a Resume

How To Include Work Projects In Your Resume

How to Mention a Referral in a Cover Letter to Increase Your Hiring Chances

How To Name-Drop A Referral In A Cover Letter (6 Tips For Success)

How To List Your Nanny Experience On A Professional Resume

How To Say Quick Learner on a Resume + 5 of the Best Synonyms To Use

COMMENTS
Here are some key components to include in your quality manager resume: Professional Summary: Include a brief statement that outlines your experience and qualifications for the position. Qualifications and Certifications: List any relevant qualifications or certifications that are necessary for the role. Work Experience: Outline your relevant ...
Common duties listed on a Quality Control Technician resume example are running tests, analyzing products, assigning tasks, organizing work schedules, documenting defects, and returning defect items. Based on our collection of resume samples, a successful Quality Manager should demonstrate leadership, attention to details, communication, time ...
How to write a quality assurance manager resume. Here's a list of steps you can follow to write a quality assurance manager resume: 1. Add your contact information. Begin your resume by adding a header with your contact information to the top right, left or center of the document. Include your full name, phone number, email address and the city ...
Quality Manager Resume Examples & Samples. Directs, implements, and maintains operational-level strategies and objectives. Develops short to moderate-term goals and translates them into short-term plans in support of operational goals and objectives. Monitors performance against plan and ensures that goals are met.
Your quality manager resume must clearly demonstrate your attention to detail. It should reflect your ability to meticulously uphold standards. Showcase your expertise in developing and implementing quality assurance protocols. Your resume needs to convey your track record of improving processes effectively. Use This Example.
In this article, we will explore the world of Quality Manager Resumes. A Quality Manager Resume is a document that showcases the skills, experiences, and achievements of a Quality Manager. Employers need Quality Managers to ensure that their products or services meet a high standard of quality. Crafting an effective Quality Manager Resume is essential for standing out in a competitive job ...
A QA manager resume example better than 9 out of 10 other resumes. How to write a QA manager resume that will land you more interviews. Tips and examples of how to put skills and achievements on a quality assurance manager resume. How to describe your work experience on a QA manager resume to get any job your heart desires.
Quality Management Manager Resume Examples & Samples. Pediatric health and wellbeing, Maternal and Child development, QM practices, service authorizations, utilization review and coordination of healthcare services. Health care administration related to children in Out-Of-Home care. AHCCCS/Medicaid rules and policies.
The top 5 certifications for your qa manager resume: Certified Manager of Quality/Organizational Excellence (CMQ/OE) - American Society for Quality (ASQ) Certified Software Quality Engineer (CSQE) - American Society for Quality (ASQ) Professional Scrum Master (PSM) - Scrum.org.
Quality Assurance Manager Resume Examples. Quality Assurance Managers make sure products meet certain industry and legal standards. Some of their responsibilities are coordinating quality assurance programs, developing quality control procedures, making sure customer demands are respected, and training other employees with regard to quality ...
For a Quality Manager, look for clues that align with your expertise in ISO 9001:2015, problem-solving, and team leadership, among others. 2. Highlight Role-Specific Skills. Prioritize skills that have direct relevance to the position. Hard skills like "ISO 9001:2015 standards" and soft skills such as "excellent interpersonal and communication ...
Montgomery, AL | 334-555-0196 | [email protected]. Summary. Data-driven quality professional with four years of experience reducing costs and optimizing testing processes in the tech industry. Seeking a Quality Control Manager position to expand my leadership skills and precise approach to continuous improvement.
Tips to help you write your QA (Quality Assurance) Manager resume in 2024. Highlight relevant certifications. As a QA manager, having certifications such as ISTQB, CSTE, or CSQA, can be a significant advantage to set you apart from other candidates. Include any relevant certifications in your resume to showcase your dedication to the field and ...
Manager, Quality Assurance Resume Examples & Samples. Manage a team of up to 30 QA/Integration Engineers in a matrix like organization structure. Influence the implementation and growth of Agile in the QA teams. Implement a production incident/problem feedback tracking system to reduce repeat issues in production.
Quality Manager resume format and sections. 1. 1. Add contact information to your quality manager resume. Your nameshould be the biggest text on the page and be at or near the top of the document. Your addressdoesn't need to include your street name or house number - listing your city and state works just fine.
Professional Skills. Strong planning and organizational skills. Strong time management and leadership skills. Excellent analytical skills. Find and customize career-winning Quality Manager resume samples and accelerate your job search. All quality manager resume samples have been written by expert recruiters.
As a quality control manager, your technical skills and knowledge of quality control tools and techniques are critical. Make sure to highlight these skills prominently in your skills section. Some examples of technical skills to include: Quality Management Systems: Six Sigma, Lean, Kaizen, ISO 9001, ISO 14001
Quality Manager Resume. Summary : Tested and proven Assistant Quality Manager, with multiple certifications and vast knowledge of process improvement possibilities, ranging from Lean, Continuous Improvement, and Six Sigma.Well versed in Quality systems and auditing from ISO to AS9100. To obtain employment with a growth-oriented business, which will engage my past and present capabilities and ...
So—. Ace your quality control job description like this: Place your most recent employment first, and go back in time from there. Put the job title first, then dates worked, the factory or company name, and finally 5 or 6 job responsibilities. Use an action word (e.g., inspected, rejected, etc.) to start each bullet point.
Quality Control Manager Resume Examples. Quality Control Managers are in charge of coordinating quality control departments and making sure product defects are detected before delivery. Their responsibilities include implementing quality control programs, documenting defects, updating databases, training employees, and organizing work schedules.
Experience. Roscoe Technologies. 9/1/2009 - 10/1/2010. Company Name. City, State. Manage training Document Management System and Quality Management system. Planned and executed multiple tasks to ensure controlled documents were managed per procedures. Provided coaching to personnel on Quality systems (QS) to include document control and training.
Example job for Quality Manager CV. Outline. Work as a quality manager for an industry-leading construction and engineering contractor, overseeing and auditing the execution of quality systems for the Greater Manchester region and working with a client base that includes the MoD & NHS with build values of up to £100m.
How to Properly Format a Quality Manager Resume. To properly format your resume, it's essential to consider the style and organization that will effectively showcase your skills and experience. Did you know that resumes for Quality Manager jobs typically contain an average of 2.3 pages and include 12.7 bullet points per job title?