An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.


StatPearls [Internet].
Neonatal hiv.
Malak Abbas ; Arsala Bakhtyar ; Rima Bazzi .
Affiliations
Last Update: September 20, 2022 .
- Continuing Education Activity
Over 95% of HIV-infected pediatric cases are a result of vertical transmission. Neonatal HIV is the concern for HIV in an infant born to a mother with known or suspected HIV disease. It is important to identify those patients early so that appropriate treatment and monitoring can be implemented. This activity outlines the evaluation and management of neonatal HIV and reviews the interprofessional team's role in managing these patients.
- Identify the etiology and epidemiology of neonatal HIV.
- Assess the common presentation, history, and diagnosis of neonatal HIV.
- Determine the appropriate treatment and management options available for neonatal HIV.
- Determine interprofessional team strategies for improving care coordination and communication to advance the identification of those at risk for neonatal HIV and improve outcomes.
- Introduction
Over 95% of HIV-infected pediatric cases are a result of vertical transmission. The pathophysiology of the HIV disease state in the pediatric population is similar to adults. However, differences occur in the clinical presentation, mode of infection, and therapeutic options. The pediatric and neonatal populations have a weaker immune system than adults; therefore, if infected with HIV, they are at a greater risk of opportunistic infections. As such, the delay of treatment may result in a rapid progression of the disease.
One of the greatest advancements in medicine has been the prevention of mother-to-child transmission (MTCT) of HIV type 1 (HIV-1). The rate of transmission of HIV to neonates has been reduced to less than 1% with the implementation of appropriate strategies and careful planning. The increase in comprehensive serologic screening and the treatment of HIV-infected pregnant females has resulted in the reduction of vertical transmission. There are evidence-based prevention modalities that can be utilized at different stages of pregnancy and postpartum to improve outcomes. Antiretroviral therapies (ART) can be prescribed during gestation, antepartum during vaginal or elective cesarean delivery, postnatally to the neonate, or when breastfeeding. [1] [2]
HIV is a ribonucleic acid (RNA) viral pathogen with 2 subtypes: HIV-1 and HIV-2. HIV-1 is the most common type worldwide and is more transmissible and progresses faster than HIV-2. Presumably, this Retroviridae family originated from wild chimpanzees in Central Africa. [3] The virus is transmitted across mucous membranes via penetrative unprotected sexual intercourse or intravenous drug use, blood transfusions in developing countries, vertical transmission, or through breastfeeding. [4] [5] The risk of transmission via lactation is about 12-14%, with the risk increasing in high viral load states. [6] [7] Overall, the probability of vertical transmission is about 25% without the utilization of appropriate ART therapy during pregnancy. Several risk factors that increase the chance of this transmission were observed in clinical trials. The risk factors include elevated maternal plasma viral RNA concentrations, maternal breast milk viral load, acute maternal seroconversion, advanced maternal disease, and decreased CD4+ T-cell count of the mother. [8] [9] [10] [11] [12]
- Epidemiology
The burden of MTCT is a worldwide epidemic, with an estimated 160,000 infants infected annually with HIV as of 2018. The majority of mothers and neonates infected with HIV are located in sub-Saharan Africa. [13] Overall, the rate of perinatal transmission of HIV has decreased substantially over the past 20 years to less than 1% in the United States and Europe. [14] [15] In the United States, approximately more than 5,000 pregnant females are HIV positive. [16] In the year 2013, nationwide in the United States, there were only 69 infants born with HIV infection, leading to an estimated incidence of 1.8 out of 100,000 live births for perinatally-acquired HIV infection. [14] The Centers for Disease Control and Prevention (CDC) in the United States (US) has set goals to eliminate perinatal HIV spread, which has caused a significant decline in MTCT transmission. The goal is to reduce the incidence of perinatal HIV to less than 1 in 100,000 births. [14] During the peak of HIV transmission in 1991, the reported incidence of neonates born with HIV was 42.8 per 100,000 births, with a substantial decline to 1.3 per 100,000 live-born infants in 2015. [17] Due to racial disparities in healthcare, the incidence of perinatal HIV is 5 times greater in Black versus White infants. [18]
- Pathophysiology
The main target for HIV entry into the cells is through infection of cells expressing the CD4 receptor and chemokine receptors CCR5 and CXCR4. [19] Additionally, the HIV virus infects dendritic cells, activated CD4 T-lymphocytes, monocytes, and macrophages. [20] The result is increased host susceptibility to diseases due to decreased immune-protective functions.
Infants with HIV-1 infections have higher viral loads and a faster progression to AIDS than adults with HIV. [21] [22] The most common mode of transmission in a neonate with HIV is mother-to-child transmission (MTCT). The virus may be transmitted during different stages of pregnancy and postpartum, with the perinatal period as the most common transmission time. [23]
In-utero Transmission
The mechanism of in-utero transmission is predicted to be by transcytosis across placental cells. The placenta may also host the virus to replicate before moving to the fetus. [24] The HIV-1 virus may also traverse the trophoblastic placental barrier via endocytosis, specifically crossing cytotrophoblasts or syncytiotrophoblasts within the uterine wall. HIV-1 may also spread to the fetus via villous capillaries. The risk of in-utero transmission increases with inflammation and infection of the placenta and amniotic membranes. [24] [25] [26] [27] [28]
Intrapartum Transmission
Intrapartum transmission is predicted to be the greatest risk of vertical infections. The risk increases with longer exposure to maternal cervicovaginal secretions and blood. Research also demonstrates that the chance of infection is greater with membrane rupture of more than 4 hours. [29] Moreover, data also demonstrates that neonates with low birth weights and those born prematurely have an increased rate of transmission due to their reduced immunologic defenses and weaker skin barrier. [30]
Postnatal MTCT
Postnatal MTCT occurs during breastfeeding. The mechanism of transmission through breast milk is not fully understood. However, multiple large prospective cohort trials have demonstrated a greater risk of spread of the HIV virus with breast-feeding. In addition to breast milk, studies have also confirmed that HIV RNA can also be found in colostrum. [31] [32] Potential entry of the HIV virus from breast milk to the infant is through their intestines or tonsillar tissues. [33] [34] [35] [36]
- Histopathology
Due to the nature of the disease state, early identification of HIV may be difficult due to subtle clinical symptoms. As such, the use of histopathology of tissue samples may help identify HIV in patients. The capsid size of the HIV-1 virus varies between 110 and 146 nm. [37] It can be visualized with structured illumination microscopy (SIM). It is almost impossible to visualize individual virions using confocal microscopy. Assessing the histopathology of the placenta may identify the presence of intrauterine HIV infection. Multiple studies have demonstrated that full-term placenta from HIV-1–positive females contained infection in syncytiotrophoblasts, cytotrophoblasts, and villous-endothelial cells. [38] In vitro, studies of trophoblast barriers have demonstrated that the direct interaction between the trophoblast barrier and HIV-1 infected cells resulted in viral transcytosis. [24]
- History and Physical
Neonates may not display any symptoms for the initial few months of life, as such complicating the diagnosis of HIV. Studies have suggested that children may remain asymptomatic until 3-5 years of age. In untreated children, the most commonly exhibited manifestations of HIV infection include but are not limited to recurrent bacteremia, increased opportunistic infections, frequent diarrhea, cardiomyopathy, hepatitis, generalized lymphadenopathy, splenomegaly, hepatomegaly, oral candidiasis, cancers, and central nervous system manifestations, such as growth delay, delayed cognition, low IQ, and frequently global developmental delay.
The CDC strongly recommends testing all pregnant females for HIV as part of the standard prenatal care. This testing proves to have a better prognosis for the neonate. However, due to a lack of adequate healthcare access in certain geographical areas of the world, the unknown HIV status of pregnant females leads to inadequate treatment and poor outcomes for the neonate. [39] [40] Females who have an unknown HIV status should be offered a rapid diagnostic test at the time of delivery. A definitive HIV diagnosis can be made in infants by the age of 4 to 6 months using virologic testing.
Neonatal HIV diagnostics differs from that of adults and older children. It is not appropriate to test for HIV antibodies. The utilization of novel combination antigen/antibody immunoassays to confirm the diagnosis of HIV in neonates is not recommended as positive results confer passive transfer of maternal antibodies. Maternal HIV antibodies persist until 18 months. [5] [40] Using viral load assays or nucleic acid tests (NATS), which include qualitative RNA assays, quantitative HIV RNA assay, or DNA polymerase chain reaction (PCR) assays, is more appropriate to confirm the diagnosis of HIV in neonates. The only FDA-approved qualitative RNA test is the APTIMA HIV-1 RNA Qualitative Assay. [41] These assays are able to detect the virus in at least 30% to 50% of cases at birth and an almost 100% confirmation by the age of 4 to 6 months. HIV quantitative RNA assay has been found to be just as comparable to HIV DNA PCR, with 100% specificity at birth, 1 month, 3 months, and 6 months. [42] Two negative virologic tests completed at 1 month and before 6 months of age are required to definitely exclude the diagnosis of HIV. Additionally, the infant must have negative clinical evidence and other laboratory markers of HIV, including normal to high CD4 T-lymphocyte count. [5] [43]
Infants are categorized as high or low risk for HIV infection. Neonates born to mothers who received adequate prenatal care and were adherent to their ART, and who had undetectable viral loads are considered low risk. On the contrary, neonates are considered high risk if they were born to mothers who lacked prenatal care, had elevated HIV viral loads, and had a new diagnosis of HIV infection while pregnant. [5]
The table below indicates (X) the proposed recommended testing schedule for HIV perinatal exposure. [43] [40]
Birth 14-21 Days
After a confirmed diagnosis of HIV, additional labs should be ordered, including CD4+ T-cell count, CD8+ T-cell count, plasma viral load of RNA, growth or development factors, and HIV-associated conditions, such as anemia, leukopenia, thrombocytopenia, hepatic transaminitis, etc. Before initiating ART, obtain genetic testing, a baseline CD4 count, plasma viral load, complete blood count (CBC), hepatic function, renal function, comprehensive metabolic panel, urinalysis, serum lipids, and blood glucose.
- Treatment / Management
The Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV strongly recommends the initiation of ART in all pediatrics with HIV. [44] There has been a significant 80% to 90% decrease in morbidity and mortality since the introduction of ART initiation in neonates. [45] [46] As confirmed by the CHER trial and other studies, there is a decrease in viral reservoirs, opportunistic infections, and disease progression to AIDS with early initiation of effective and early ART. [47] [48] [49] [50] [51] [52] Infants with any level of risk of exposure to HIV should be started on the appropriate ART within 6 hours of birth. The goals of treatment for HIV-exposed neonates include a reduction in morbidity and mortality, suppression of viral replication, facilitation of HIV remission, viral control, prevention of disease progression, maintenance of immunologic function, reduction of opportunistic infections, and prevention of drug resistance. [53] [54]
There aren’t many randomized control trials that compare different regimens in pediatrics and neonates, and the available literature is variable. Most of the data is extracted from non-randomized studies, pharmacokinetic trials, and phase 1 or 2 of drug trials. In general, the initiation of an antiretroviral regimen in pediatrics should include 2 nucleoside reverse transcriptase inhibitors (NRTIs) with an additional drug from another class, including an integrase strand transfer inhibitor (INSTI), a protease inhibitor (PI) with a booster, or a non-nucleoside reverse transcriptase inhibitor (NNRTI). Before initiating a regimen, Factors to consider include the patient’s age, weight, family preference, drug resistance, genetic testing, mutation testing, and sexual maturity rating (SMR). In children with other confections, such as hepatitis B virus (HBV), the choice of agent should include coverage for HIV and the co-contagion.
There are 3 studies that compared the addition of a PI-boosted versus NNRTI to the 2 NRTI backbones. In the P1060 trial, a total of 288 children from 6 African countries and India with ages from 2 to 36 months were enrolled in a randomized trial. The children received zidovudine (ZDV) plus lamivudine as the NRTI backbone and were randomized to either the PI with booster group (ritonavir booster [LPV/r]) or NNRTI group (nevirapine [NVP]). The data demonstrated that LPV/r is superior to NVP in NVP-naive children; however, there were limitations. [55] Whereas the PROMOTE trial did not find any differences between the 2 groups. [56] Of note, LPV/r should be avoided in neonates before 42 weeks of age and those who are younger than 14 days.
Data for utilizing an INSTI-based regimen are extracted from safety trials and adult comparative trials. Four INSTIs are approved for the treatment of ART-naïve children with HIV, which include: bictegravir (BID), dolutegrevir (DTG), Elvitegravir/cobicistat (EVG/c), and raltegravir (RAL). [57] INSTI regimens are attractive due to their lack of drug interactions, low toxicity, and virologic efficacy. RAL is FDA-approved for neonates and infants weighing 2 kg or more. DTG is FDA-approved for children 30 kg or more, and BIC is approved for children weighing 25 kg or more. [58] [59]
Zidovudine (ZDV) plus lamivudine (3TC) or emtricitabine (FTC) are the preferred dual NRTI backbone in neonates and infants under 3 months. ZDV is FDA-approved for prophylaxis and for HIV treatment initiation in infants ≥ 4 weeks of age. [60] [61] [62] [63] The preferred regimen for infants 3 months and older is abacavir (ABC) plus 3TC or FTC. [64] [65] [66] [67] Alternatively, ZDV plus ABC can be used in infants 3 months and older; however, European studies have demonstrated lower rates of viral suppression and increased toxicity with this combination. [65] [68] In addition to the 2 NRTI backbones, the following combination regimens are preferred in each age group:
- NVP: Age under 14 days
- RAL: Age under 14 days and a weight of 2 kg or more
- LPV/r or RAL (alternative: NVP): Age 14 days or older to 3 years
- Differential Diagnosis
There are other diseases that need exclusion when diagnosing HIV. These include malnutrition, lymphadenopathy, pediatric chronic anemia, malabsorption syndrome, constitutional growth delay, autoimmune and chronic benign neutropenia, and other immunodeficiencies. Furthermore, the clinician should also look for other congenital co-infections, including syphilis, TORCH infections (Toxoplasmosis, Rubella, Cytomegalovirus, herpes simplex virus), hepatitis B, hepatitis C, or tuberculosis infection.
- Toxicity and Adverse Effect Management
Any ART is associated with a variety of side effects. Many ARTs result in increased levels of hepatic transaminases as a result of hepatitis. Baseline labs are recommended before initiation of any regimen.
- Zidovudine: Can induce leukopenia, anemia, and macrocytosis
- Protease inhibitors: May lead to hyperglycemia
- Atazanavir: Can cause hyperbilirubinemia.
If untreated, HIV can increase the rate of morbidity and mortality. However, due to the advancement of ART, increased monitoring, and data from clinical trials, pediatric and adult patients have better prognoses and outcomes. The average survival rate is about 10 years of age, with approximately 15% of children having a rapid progression of the disease. The clinician should collaborate with the patient to optimize their nutrition, control viral replication, initiate aggressive treatment for opportunistic infections, and decrease social stressors. The risk of complications is greater with co-infections and hematological disturbances, such as anemia, thrombocytopenia, and neutropenia.
- Complications
Complications of HIV infection in neonates and pediatric populations occur as a result of their immunocompromised status. They are at greater risk for opportunistic infections, candida esophagitis, Pneumocystis jirovecii pneumonia, and cancers. Furthermore, complications are more likely to occur with antiretroviral drug resistance. However, with careful monitoring and drug-resistance testing, the ability to select more optimized and effective regimens is possible.
- Consultations
Consultation with a perinatologist and a pediatric infectious disease consultant is strongly encouraged to help provide a more comprehensive workup, diagnosis, and ongoing monitoring and management.
- Deterrence and Patient Education
Before initiating or altering ART, the clinician should identify potential barriers and compliance issues. Developing novel drugs and enhanced formulations has led to better medication tolerability, less toxicity, and increased adherence.
HIV-positive mothers should be discouraged from breastfeeding neonates who do not have a confirmed HIV-positive status. If a female continues to breastfeed, the infant should be monitored and tested every 3 months throughout breastfeeding and postdiscontinuation of breastfeeding at the interval of 4 to 6 weeks, 3 months, and at 6 months. [69] [70] Mothers should also be warned about the risks of feeding premasticated food to the infant. [71] [72] [73]
- Pearls and Other Issues
Key facts to keep in mind about neonatal HIV are as follows:
- When making a selection for appropriate ART to initiate in a pregnant female, it is important to consider tolerability, neonatal risk of exposure, pharmacokinetic differences, and overall risk-benefit of each regimen.
- The monitoring of infants with HIV is challenging as there is variability with viral loads and CD4 counts depending on the age.
- Studies have demonstrated that administering zidovudine (ZDV) monotherapy to both the mother and neonate reduces MTCT from 25% to 8%. The MTCT rate is reduced to less than 1% when combined with other ART. [7] ZDV exhibits its actions by metabolizing into its active form in the placenta, thus inhibiting the replication of HIV within the placental cells.
- Repeated negative HIV test results are needed postpartum due to the increased risk of transmission of HIV during labor and delivery. [5]
- Enhancing Healthcare Team Outcomes
The treatment of perinatal HIV exposure involves a team approach involving an infectious disease specialist, perinatalist, pediatrician, neonatologist, obstetrician, HIV pharmacist, and nursing staff. Prompt and early communication between all team members assures comprehensive and optimized care for the neonate. Infectious disease specialists and neonatologists are usually involved in acute management during the neonatal period. Infectious disease specialists are responsible for monitoring disease progression and drug regimens.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Malak Abbas declares no relevant financial relationships with ineligible companies.
Disclosure: Arsala Bakhtyar declares no relevant financial relationships with ineligible companies.
Disclosure: Rima Bazzi declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Abbas M, Bakhtyar A, Bazzi R. Neonatal HIV. [Updated 2022 Sep 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. [Cochrane Database Syst Rev. 2007] Review Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Volmink J, Siegfried NL, van der Merwe L, Brocklehurst P. Cochrane Database Syst Rev. 2007 Jan 24; (1):CD003510. Epub 2007 Jan 24.
- Review Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. [Cochrane Database Syst Rev. 2011] Review Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Siegfried N, van der Merwe L, Brocklehurst P, Sint TT. Cochrane Database Syst Rev. 2011 Jul 6; (7):CD003510. Epub 2011 Jul 6.
- Review Antiretroviral therapy (ART) for treating HIV infection in ART-eligible pregnant women. [Cochrane Database Syst Rev. 2010] Review Antiretroviral therapy (ART) for treating HIV infection in ART-eligible pregnant women. Sturt AS, Dokubo EK, Sint TT. Cochrane Database Syst Rev. 2010 Mar 17; (3):CD008440. Epub 2010 Mar 17.
- Review Operational issues and barriers to implementation of prevention of mother-to-child transmission of HIV (PMTCT) interventions in Sub-Saharan Africa. [Curr HIV Res. 2013] Review Operational issues and barriers to implementation of prevention of mother-to-child transmission of HIV (PMTCT) interventions in Sub-Saharan Africa. Aizire J, Fowler MG, Coovadia HM. Curr HIV Res. 2013 Mar; 11(2):144-59.
- Review Cesarean section delivery to prevent vertical transmission of human immunodeficiency virus type 1. Associated risks and other considerations. [Ann N Y Acad Sci. 2000] Review Cesarean section delivery to prevent vertical transmission of human immunodeficiency virus type 1. Associated risks and other considerations. Read JS. Ann N Y Acad Sci. 2000 Nov; 918:115-21.
Recent Activity
- Neonatal HIV - StatPearls Neonatal HIV - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
Human Immunodeficiency Virus (HIV) Infection in Infants and Children
- Epidemiology |
- Transmission of HIV |
- Classification |
- Symptoms and Signs |
- Diagnosis |
- Treatment |
- Transition to Adult Care |
- Prognosis |
- Prevention |
- Key Points |
- More Information |
Human immunodeficiency virus (HIV) infection is caused by the retrovirus HIV-1 (and less commonly by the related retrovirus HIV-2). Infection leads to progressive immunologic deterioration and opportunistic infections and cancers. The end stage is acquired immunodeficiency syndrome (AIDS). Diagnosis is by viral antibodies in children 18 months and virologic nucleic acid amplification tests (such as polymerase chain reaction testing) in children 18 months. Treatment is with combinations of antiretroviral medications.
(See also Human Immunodeficiency Virus (HIV) Infection in adults.)
The general natural history and pathophysiology of pediatric HIV infection is similar to that in adults; however, the method of infection, clinical presentations, and treatments often differ.
Children with HIV infection may also have unique social integration issues .
General references
ClinicalInfo.HIV.gov/Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV: Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection
Weinberg GA, Siberry GK: Pediatric human immunodeficiency virus infection. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases , 9th ed., edited by JE Bennett, R Dolin, and MJ Blaser. Philadelphia, Elsevier, 2020, pp. 1732–1738.
Epidemiology of HIV Infection in Infants and Children
In the United States, since HIV infection was first recognized, more than 10,000 cases have been reported in children and young adolescents, but this number represents only 1% of total cases. In 2019, 1 ).
More than 95% of US children with HIV infection acquired the infection from their mother, through either antenatal or perinatal transmission (also called vertical transmission or mother-to-child transmission [MTCT]). Most of the remainder (including children with hemophilia or other coagulation disorders) received contaminated blood or blood products. Some cases were the result of sexual abuse.
MTCT has declined significantly in the United States from approximately 25% in 1991 (resulting in > 1600 infected children annually) to ≤ 1% in 2019 (resulting in approximately 50 children infected annually). MTCT has been reduced by using comprehensive serologic screening and treating of infected pregnant women during both pregnancy and delivery and by providing short-term antiretroviral prophylaxis to exposed newborns. Approximately 3000 to 5000 pregnant women with HIV infection give birth annually in the United States, so attention to preventing MTCT remains critical in preventing HIV infection in infants and children.
Although the number of children infected annually has decreased, the total number of US adolescents and young adults (13 to 24 years of age) with HIV infection continues to increase despite the marked success in decreasing perinatal HIV infection. In 2019, about 36,000 new cases of HIV infection in the United States were diagnosed; 20% of these were among adolescents and young adults 13 to 24 years of age (the majority of whom were 18 years of age or older) ( 1 ). This paradoxical increase in the number of children and adolescents with HIV infection is a result of both greater survival rates among perinatally infected children and new cases of HIV infection acquired via sexual transmission among other adolescents and young adults (in particular, among young men who have sex with men). Reducing transmission of HIV among young men who have sex with men continues to be an important focus of domestic HIV control efforts as is continuing the reduction of MTCT.
Worldwide, in 2021, about 1.7 million children 2 ). Each year, about 160,000 more children are infected (10% of all new infections), and about 100,000 children die.
Although these numbers represent a daunting amount of illness, new programs created to deliver antiretroviral therapy (ART) to pregnant women and children have reduced the annual number of new childhood infections and childhood deaths by 33 to 50% in the past few years ( 1 ). However, infected children still do not receive ART nearly as often as adults, and interrupting vertical transmission (MTCT) and providing treatment to children with HIV infection remain the two most important goals of global pediatric HIV medicine.
Epidemiology references
1. Centers for Disease Control and Prevention : HIV Surveillance Report, 2020. Vol. 33. Published May 2022. Accessed 11/29/2022.
2. UNAIDS : Global HIV & AIDS statistics—Fact sheet. Accessed 12/19/2022.
Transmission of HIV Infection in Infants and Children
The infection risk for an infant born to a mother with HIV infection who did not receive ART during pregnancy is estimated at 25%.
Risk factors for MTCT include
Seroconversion during pregnancy or breastfeeding (major risk)
High plasma viral RNA concentrations (major risk)
Advanced maternal disease
Low maternal peripheral CD4+ T-cell counts
Prolonged rupture of membranes is no longer thought to be an important risk factor.
Prevention of HIV Infection in Infants and Children ). ZDV monotherapy reduces MTCT from 25% to about 8%, and current combination ART reduces it to ≤ 1%.
HIV has been detected in both the cellular and cell-free fractions of human breast milk. Estimates of the overall risk of transmission through breastfeeding are 12 to 14%, reflecting varying durations of breastfeeding. Transmission by breastfeeding is greatest in mothers with high plasma viral RNA concentrations (eg, women who become infected during pregnancy or during the period of breastfeeding).
Early in the HIV pandemic, HIV was transmitted to young children via contaminated blood products (eg, whole blood or cellular or plasma blood components such as packed red blood cells, intravenous immune globulin); however, transmission via this route no longer occurs when blood products are screened for HIV (and, in the case of immune globulin, also prepared with viral inactivation steps).
Transmission of HIV via sexual activity of adolescents is similar to that of adults (see Transmission of HIV Infection in adults).
Classification of HIV Infection in Infants and Children
HIV infection causes a broad spectrum of disease, of which AIDS is the most severe. Past classification schemes established by the Centers for Disease Control and Prevention (CDC) defined the progression of clinical and immunologic decline. These clinical and immunologic categories have become much less relevant in the era of combination ART, because when ART is taken as prescribed, it almost invariably decreases symptoms and increases CD4+ T-cell counts. However, immunologic staging based on CD4+ T-cell counts remains valuable for planning opportunistic pathogen prophylaxis.
The clinical categories in children < 13 years are available from ClinicalInfo.HIV.gov's Appendix C: CDC Pediatric HIV CD4 Cell Count/Percentage and HIV-Related Diseases Categorization table and are shown in table Immunologic Categories (HIV Infection Stages) for Children < 13 Years With HIV Infection Based on Age-Specific CD4+ T-Cell Count or Percentage . In infants and children, HIV infection and disease may progress more rapidly than in adolescents and adults.
Symptoms and Signs of HIV Infection in Infants and Children
Children receiving combination antiretroviral therapy (art).
Combination ART has significantly changed the clinical manifestations of pediatric HIV infection. Although bacterial pneumonia and other bacterial infections (eg, bacteremia, recurrent otitis media) still occur more often in children with HIV infection, opportunistic infections and growth failure are much less frequent than in the pre-ART era. New problems, such as alterations in serum lipids, hyperglycemia, fat maldistribution (lipodystrophy and lipoatrophy), nephropathy, and osteonecrosis, are reported; however, the incidence is lower in children than in adults with HIV infection.
Although combination ART clearly improves neurodevelopmental outcome, there seems to be an increased rate of behavioral, developmental, and cognitive problems in treated children with HIV infection. It is unclear whether these problems are caused by HIV infection itself, medications, or other biopsychosocial factors that occur among children with HIV infection. It is unknown whether any additional effects of HIV infection or ART during critical periods of growth and development will manifest later in life. However, no such effects have been noted in perinatally infected children who were treated with ART and are now young adults. To detect such adverse effects, providers will need to monitor children with HIV infection over time.
Natural history in untreated children
Infants infected perinatally usually are asymptomatic during the first few months of life, even if no combination ART is begun. Although the median age at symptom onset is about 3 years, some children remain asymptomatic for > 5 years and, with appropriate ART, are expected to survive to adulthood.
In the pre-ART era, about 10 to 15% of children had rapid disease progression, with symptoms occurring in the first year of life and death occurring by 18 to 36 months; these children were thought to have acquired HIV infection earlier in utero. However, most children likely acquire infection at or near birth and have slower disease progression (surviving beyond 5 years even before ART was used routinely).
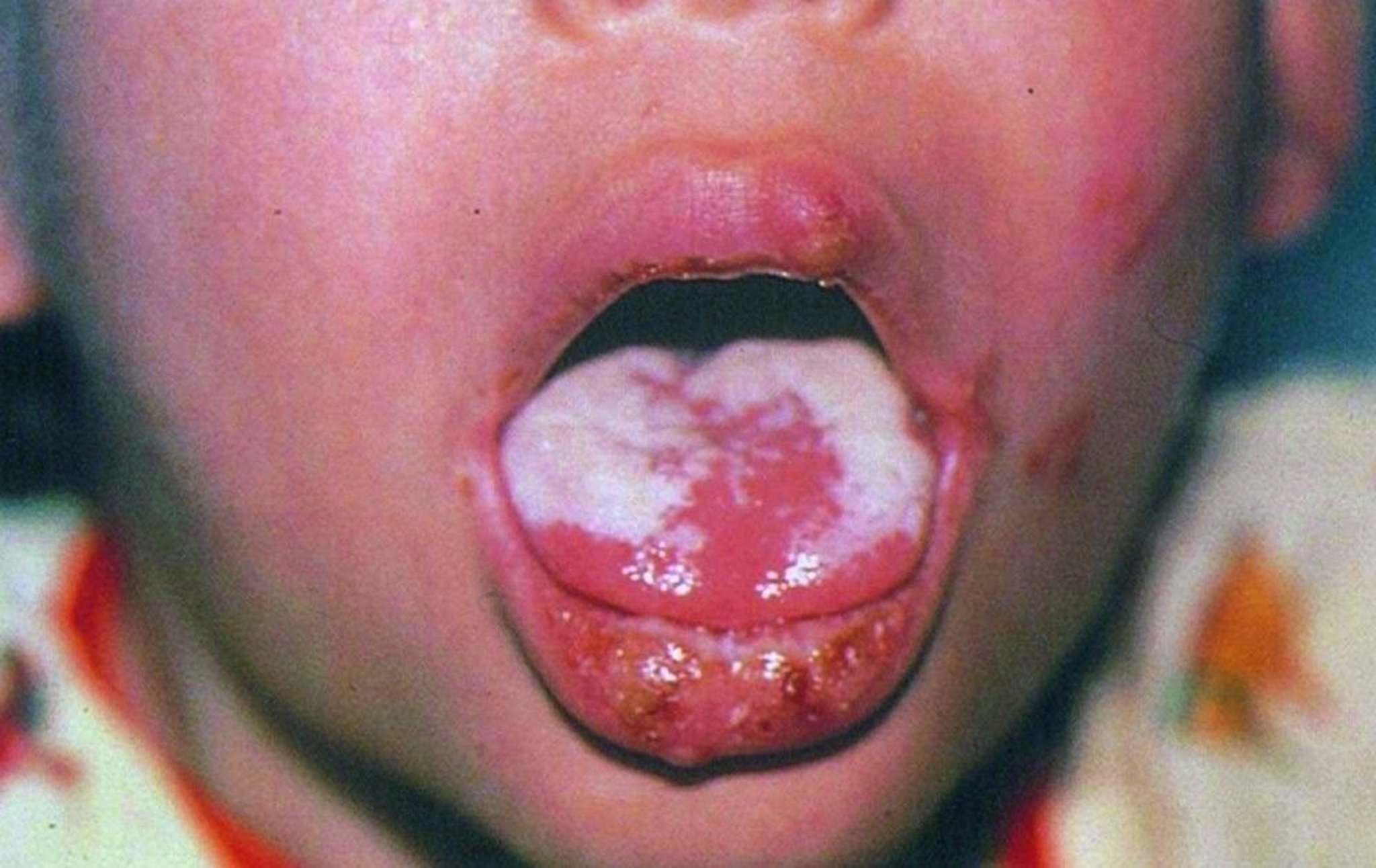
© Springer Science+Business Media
In infants who are not receiving ART, disease manifestations include failure to thrive, neurologic problems (eg, loss or delay in motor skills, irritability, poor head growth), failure to thrive, and Pneumocystis pneumonia.
Older children who are not receiving ART frequently have recurrent otitis media, sinusitis, bacterial pneumonia, bacteremia, herpes zoster, and lymphoid interstitial pneumonitis. Older children and adolescents whose disease manifests late in childhood (called slow progressors or nonprogressors) may present with persistent generalized lymphadenopathy, esophageal candidiasis, and lymphoma of the brain or other sites, which is similar to manifestations in adults who are not receiving ART.
All of these manifestations, including opportunistic infections, occur only rarely in people who are receiving combination ART.
Complications of HIV in children
When complications occur, they typically involve opportunistic infections (and rarely cancer). Combination ART has made such infections uncommon, and they now occur mainly in undiagnosed children who have not yet received ART or in children who are not adherent to ART.
When opportunistic infections occur, Pneumocystis jirovecii pneumonia is the most common and serious and has high mortality. Pneumocystis pneumonia can occur as early as age 4 to 6 weeks but occurs mostly in infants aged 3 to 6 months who acquired infection before or at birth. Infants and older children with Pneumocystis pneumonia characteristically develop a subacute, diffuse pneumonitis with dyspnea at rest, tachypnea, oxygen desaturation, nonproductive cough, and fever (in contrast to non–HIV-infected immunocompromised children and adults, in whom onset is often more acute and fulminant).
Other opportunistic infections in immunosuppressed children include Candida esophagitis , disseminated cytomegalovirus infection , chronic or disseminated herpes simplex virus infection and varicella-zoster virus infection , and, less commonly, Mycobacterium tuberculosis and M. avium complex infections, chronic enteritis caused by Cryptosporidium or other organisms, and disseminated or CNS cryptococcal or Toxoplasma gondii infection .
Cancers in immunocompromised children with HIV infection are relatively uncommon, but leiomyosarcomas and certain lymphomas, including CNS lymphomas and non-Hodgkin B-cell lymphomas (Burkitt type), occur much more often than in immunocompetent children. Kaposi sarcoma is very rare in children with HIV infection. (See Cancers Common Among Patients with HIV Infection .)
Diagnosis of HIV Infection in Infants and Children
Serum antibody tests
Virologic nucleic acid tests (includes HIV RNA/DNA or HIV RNA assays)
HIV-specific tests
Children 18 months retain maternal antibody, causing false-positive results even with the 4th-generation HIV-1/2 antigen/antibody combination immunoassay. Therefore, in these children, the diagnosis must be made by HIV virologic assays, or nucleic acid tests (NATs) as they are known collectively, such as qualitative RNA or RNA/DNA assays . Newer real-time RNA or RNA/DNA assays can be used to diagnose about 30 to 50% of cases at birth and nearly 100% of cases by 4 to 6 months of age, including children with non-subtype B and group O strains of HIV more commonly found outside of the United States. HIV viral culture has acceptable sensitivity and specificity but has been replaced by NATs because it is technically more demanding and hazardous. (See also ClinicalInfo.HIV.gov's Diagnosis of HIV Infection in Infants and Children .)
In children > 18 months, the diagnosis of HIV infection is made using a series of tests: a serum 4th-generation HIV-1/2 antigen/antibody combination immunoassay , followed by a 2nd-generation HIV-1/2 antibody differentiation assay , and, if required, an HIV-1 qualitative RNA assay . This diagnostic testing algorithm has supplanted the previous sequential testing by serum immunoassay and Western blot confirmation. Only very rarely does an older child with HIV infection lack HIV antibody because of significant hypogammaglobulinemia.
The quantitative HIV RNA assay is most commonly used to determine HIV plasma viral load for monitoring efficacy of treatment. It may also be used for infant diagnostic testing; however, care must be taken because test specificity is uncertain at very low RNA concentrations ( < 5000 copies/mL) and sensitivity is unknown in infants of mothers with complete treatment-mediated viral suppression at the time of delivery.
Rapid point-of-care tests may be done using rapid immunoassay tests for HIV antibody because these tests may provide results in minutes to hours using oral secretions, whole blood, or serum. In the United States, these tests are most useful in labor and delivery units to test women of unknown HIV serostatus, thus allowing perinatal counseling, commencement of ART to prevent MTCT, and testing of the infant by virologic NATs to be arranged during the birth visit. These tests provide similar advantages in other episodic care settings (eg, emergency departments, adolescent medicine clinics, sexually transmitted infection clinics) and in medically underserved areas of the world.
However, rapid assays typically require confirmatory tests, such as a second antigen/antibody assay, an HIV-1/2 antibody differentiation assay, or a NAT. These confirmatory tests are especially important because in areas where the expected HIV prevalence is low, even a specific rapid assay yields mostly false-positive results (low positive predictive value by Bayes theorem ). The higher the pre-test probability of HIV (ie, seroprevalence), the higher the positive predictive value of the test.
As more laboratories are able to do same-day testing using 4th-generation HIV-1/2 antigen/antibody combination immunoassays, there will be less need for the comparatively less sensitive and less specific rapid immunoassays. Again, neither rapid immunoassays nor 4th generation HIV1/2 antigen/antibody assays are sensitive enough for HIV diagnosis in a child
Pre-test counseling before HIV testing of a child involves discussing the possible psychosocial risks and benefits of testing with the mother or primary caregiver (and the child, if old enough). Most US jurisdictions (and CDC recommendations) now follow an opt-out, oral discussion rather than requiring formal oral (or written) consent. Providers should act in accordance with their state, local, and hospital laws and regulations. Counseling and consent requirements should not deter testing if it is medically indicated; refusal of a patient or guardian to give consent does not relieve providers of their professional and legal responsibilities, and sometimes authorization for testing must be obtained by other means (eg, court order).
Test results should be discussed in person with the family, the primary caregiver, and, if old enough, the child. If the child is HIV-positive, appropriate counseling and subsequent follow-up care must be provided. In all cases, maintaining confidentiality is essential.
Children and adolescents with HIV infection or AIDS must be reported to the appropriate public health department in accordance with state, local, and hospital laws.
(For questions regarding neonatal diagnosis, clinicians can call the Perinatal HIV Consultation and Referral Services Hotline: 1-888-HIV-8765 [1-888-448-8765].)
HIV testing schedules for pregnant women and newborns
(See also the Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV's Recommendations for the Use of Antiretroviral Drugs During Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States and Maternal HIV Testing and Identification of Perinatal HIV Exposure and the U.S. Preventive Services Task Force's 2019 Human Immunodeficiency Virus (HIV) Infection: Screening recommendation statement.)
HIV infection testing for all pregnant women should be done before pregnancy or early in pregnancy so that combination antiretroviral (ARV) medications may be given for their own health and to prevent MTCT. Current recommendations suggest repeat testing in the third trimester to detect newly acquired HIV infection—the treatment of which even late in pregnancy will still improve the woman's health and help lessen MTCT ( 1 ).
HIV infection testing for newborns is done on varying schedules, depending on whether an infant perinatally exposed to HIV by a mother living with HIV infection is considered at low or higher risk of transmission; higher-risk infants are tested more frequently.
Low risk of perinatal HIV transmission is defined by the following:
The mother received antiretroviral therapy (ART) during pregnancy.
The mother had sustained virologic suppression as shown by plasma HIV viral RNA of
There were no concerns about the mother's adherence to ART.
Testing of infants at low risk is recommended at the following ages:
14 to 21 days
1 to 2 months (at least 2 weeks after cessation of ARV prophylaxis )
4 to 6 months
Higher risk of perinatal HIV transmission is defined as a mother living with HIV infection who has one or more of the following factors:
Did not receive prenatal care
Did not receive ART during pregnancy, or received only intrapartum ART
Initiated ART late in pregnancy (during the late second or third trimester)
Diagnosed with acute HIV infection during pregnancy
Had an unknown or a detectable (≥ 50 copies/mL) HIV plasma viral load near delivery (particularly when delivery was vaginal)
Had acute or primary HIV infection during pregnancy or is breastfeeding (in which case breastfeeding should be stopped)
Testing of infants at higher risk is recommended at the following ages:
Birth (blood sample should be from newborn, not from umbilical cord blood)
1 to 2 months
2 to 3 months (2 to 6 weeks after cessation of ARV prophylaxis )
A positive test should be confirmed immediately using the same or another virologic test; two positive tests confirm HIV infection.
If the serial HIV virologic tests are negative at ≥ 2 weeks and at ≥ 4 weeks and in the absence of any AIDS-defining illness, the infant is considered presumptively uninfected (ie, with > 95% accuracy). If HIV virologic tests are also negative at ≥ 4 weeks and at ≥ 4 months, and again in the absence of any AIDS-defining illness, the infant is considered definitively uninfected.
Some experts continue to recommend follow-up antibody tests (1 antigen/antibody combination assay at > 18 months or, alternatively, 2 such assays done between 6 months and 18 months) to definitively exclude HIV infection and confirm seroreversion (loss of passively acquired HIV antibodies), especially if the infant was not in the low-risk category or was suspected to have exposure after birth (eg, from breast milk, percutaneous exposure, or sexual abuse). Seroreversion occurs at a median of 14 months of age; late seroreversion occasionally occurs up to 18 to 24 months of age, complicating the interpretation of antibodies in perinatally exposed infants. Expert consultation should be sought, and repeated testing (along with virologic NATs) is indicated for the perinatally exposed toddler with positive antibodies.
If an infant < 18 months with a positive antibody test but negative virologic tests develops an AIDS-defining illness (see ClinicalInfo.HIV.gov's Appendix C: CDC Pediatric HIV CD4 Cell Count/Percentage and HIV-Related Diseases Categorization table), HIV infection is diagnosed.
Additional tests after HIV diagnosis
Once infection is diagnosed, other tests are done:
CD4+ T-cell count
CD8+ T-cell count
Plasma viral RNA concentration
Infected children require measurement of CD4+ and CD8+ T-cell counts and plasma viral RNA concentration (viral load) to help determine their degree of illness, prognosis, and the effects of therapy. CD4+ counts may be normal (eg, above the age-specific cutoffs of category 1 in table Immunologic Categories (HIV Infection Stages) for Children < 13 Years With HIV Infection Based on Age-Specific CD4+ T-Cell Count or Percentage ) initially but fall eventually. CD8+ counts usually increase initially and do not fall until late in the infection. These changes in cell populations result in a decrease in the CD4+:CD8+ cell ratio, a characteristic of HIV infection (although sometimes occurring in other infections). Plasma viral RNA concentrations in untreated children < 12 months are typically very high (mean of about 200,000 RNA copies/mL). By 24 months, viral concentrations in untreated children decrease (to a mean of about 40,000 RNA copies/mL).
Although not routinely measured, serum immunoglobulin concentrations, particularly IgG and IgA, often are markedly elevated, but occasionally some children develop panhypogammaglobulinemia. Patients may be anergic to skin test antigens.
Diagnosis reference
1. Pollock L, Cohan D, Pecci CC, Mittal P : ACOG Committee opinion no. 752: Prenatal and perinatal human immunodeficiency virus testing. Obstet Gynecol 133(1):187, 2019. doi: 10.1097/AOG.0000000000003048
Treatment of HIV Infection in Infants and Children
Combinations of antiretroviral (ARV) medications (antiretroviral therapy [ART])
Supportive care
Combination ART is individualized to the child but most commonly includes 3 medications:
Two nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) plus
One integrase strand transfer inhibitor (INSTI) or one protease inhibitor
Sometimes a non-nucleoside reverse transcriptase inhibitor (NNRTI) is given with 2 NRTIs.
Because of the success of combination ART, much of the current focus is on the management of HIV infection as a chronic disease, addressing both medical and social issues. Important long-term medical issues include the need to manage HIV-related and drug-related metabolic complications and to account for age-related changes in drug pharmacokinetics and pharmacodynamics. Social issues include the need to cope with peer pressure, ensure school success and appropriate career choice, and educate children about transmission risk. Adolescents often have difficulty seeking and following health care advice and need particular help with treatment adherence.
Challenges for infants and younger children include lack of pediatric pharmacokinetic data for newer compounds, palatability and tolerability of liquid formulations, and lack of fixed-dosed combination tablets.
Children and adolescents should be managed in collaboration with experts who have experience in the management of pediatric HIV infection.
Indications for ART in children
(For a discussion of some ARV medications and dosages, see Antiretroviral Therapy in Children and see the Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV's Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection and see Appendix A: Pediatric Antiretroviral Drug Information .)
Initiation of ART for children is similar to that in adults; essentially, all children with HIV infection should be given ART as soon as possible (rapid initiation, within 1 to 2 weeks of diagnosis). There is both strong consensus and clinical trial evidence for early initiation of ART in infants with HIV infection.
The goal of therapy at all ages is similar to that in adults:
Suppress HIV replication (as measured by HIV plasma viral load).
Maintain or achieve age-normal CD4+ counts and percentages with the least amount of drug toxicity.
Before making the decision to initiate therapy, the practitioner should fully assess the readiness of the caregiver and child to adhere with ARV medication administration and discuss the potential benefits and risks of therapy. Because expert opinions on therapeutic strategies change rapidly, consultation with experts is strongly advised.
Adherence to ART
ART is successful only if the family and child are able to adhere to a possibly complex medical regimen. Nonadherence not only leads to failure to control HIV but also selects drug-resistant HIV strains, which reduces future therapeutic choices.
Barriers to adherence should be addressed before starting treatment. Barriers include availability and palatability of pills or suspensions, adverse effects (including those due to drug interactions with current therapy), pharmacokinetic factors such as the need to take some medications with food or in a fasted state, and a child’s dependence on others to give medications (and parents with HIV infection may have problems with remembering to take their own medications). Newer once- or twice-daily combination regimens and more palatable pediatric formulations help improve adherence, and the growing availability of once-daily fixed-dose combination tablets for older children and adults has helped many youth living with HIV infection.
Adherence may be especially problematic in adolescents regardless of whether they have been infected perinatally or have acquired HIV infection later on through sexual activity or injection drug use. Adolescents have complex biopsychosocial issues, such as low self-esteem, chaotic and unstructured lifestyles, fear of being singled out because of illness, and sometimes a lack of family support, all of which may reduce ART adherence. In addition, adolescents may not be developmentally able to understand why ARV medications are necessary during periods of asymptomatic infection and they may worry greatly about adverse effects.
Despite frequent contact with the medical system, perinatally infected adolescents may fear or deny their HIV infection, distrust information provided by the health care team, and poorly make the transition to the adult health care system (see Transition to Adult Care ). Treatment regimens for adolescents must be made in consideration of these issues. Although the goal is to have the adolescent adhere to a maximally potent regimen of ARV medications, a realistic assessment of the adolescent's maturity and support systems may suggest that the treatment plan begin by focusing on avoidance of opportunistic illness and providing information about reproductive health services, housing, and how to succeed in school. Once care team members are confident the adolescent is receiving proper support, they can decide exactly which ARV medications are best.
Clinical and laboratory monitoring are important for identifying drug toxicity and therapeutic failure.
At entry into care and at initiation of ART (and if changing ART regimen): Physical examination, adherence evaluation, complete blood count, serum chemistry values including electrolytes, liver and kidney tests, HIV plasma viral load, CD4+ lymphocyte counts, and, for adolescent girls, a pregnancy test
Every 3 to 4 months: Physical examination, adherence evaluation, complete blood count, serum chemistry values, including electrolytes, liver and kidney tests, HIV plasma viral load, and CD4+ lymphocyte counts
Every 6 to 12 months: Lipid profiles and urinalysis; complete blood count and serum chemistry values including electrolytes, and liver and kidney tests if not done already in those with a stable clinical status; adherence evaluation
HIV genotypic resistance testing should be done at entry into care and upon ART changes due to presumed virologic failure.
abacavir should be given only to patients who are HLA-B*5701–negative. This testing is most often done at entry into care so that safety of possible future use of abacavir is known.
If children have a stable treatment status, ie, nondetectable HIV RNA and normal age-adjusted CD4+ lymphocyte counts without clinical signs of toxicity for at least 12 months, and a stable family support system, many clinicians will extend the interval of laboratory evaluations to every 6 to 12 months. However, clinical care visits every 3 months with measurement of HIV plasma viral load are valuable because clinicians have the opportunity to review adherence, monitor growth and clinical symptoms, and update weight-based dosing of ARV medications as needed.
Prevention of opportunistic infections
Prophylactic treatment is recommended in certain children with HIV infection for prevention of Pneumocystis pneumonia and M. avium complex infections. Data are limited on the use of prophylaxis for opportunistic infection by other organisms, such as cytomegalovirus, fungi, and Toxoplasma . Guidance on prophylaxis of these and other opportunistic infections is also available at ClinicalInfo.HIV.gov .
Prophylaxis against Pneumocystis pneumonia is indicated for
Children with HIV infection who are ≥ 6 years of age with CD4+ count < 200 cells/mcL or CD4+ percentage < 14%
Children with HIV infection who are 1 to < 500 cells/mcL or CD4+ percentage < 22%
Infants with HIV infection who are < 12 months of age regardless of CD4+ count or percentage
Infants born to women with HIV infection (beginning at 4 to 6 weeks of age) until HIV infection is either presumptively excluded by documentation of 2 negative virologic test results (1 at ≥ 2 weeks of age and 1 at ≥ 4 weeks of age) or definitively excluded by documentation of 2 negative virologic test results (1 at ≥ 1 month of age and 1 at ≥ 4 months of age) (NOTE: For these definitions of HIV exclusion to be valid, the infant must not be breastfeeding.)
Once immune reconstitution with combination ART occurs, discontinuation of Pneumocystis pneumonia prophylaxis may be considered for children with HIV infection who have received combination ART for > 6 months and whose CD4+ percentage and CD4+ count have remained higher than the previously described treatment thresholds for > 3 consecutive months. Subsequently, the CD4+ percentage and count should be reevaluated at least every 3 months, and prophylaxis should be reinstituted if the original criteria are reached.
The medication of choice for Pneumocystis 2 orally 2 times a day on 3 consecutive days/week (eg, Monday-Tuesday-Wednesday); alternative schedules include the same dose 2 times a day every day, the same dose 2 times a day on alternate days, or twice the dose (TMP 150 mg/SMX 750 mg/m 2 ) once a day for 3 consecutive days/week. Some experts find it easier to use weight-based dosing (TMP 2.5 to 5 mg/SMX 12.5 to 25 mg/kg orally 2 times a day).
Prophylaxis against complex infection is indicated in
Children ≥ 6 years with CD4+ count < 50 cells/mcL
Children 2 to 6 years with CD4+ count < 75 cells/mcL
Children 1 to 2 years with CD4+ count < 500 cells/mcL
Children < 1 year with CD4+ count < 750 cells/mcL
Psychosocial approach to children with HIV infection
HIV infection in a child affects the entire family. Serologic testing of siblings and parents is recommended for those families with a child with perinatally acquired infection. This may not be necessary for those families without known HIV infection who adopt a child with HIV infection. The physician must provide education and ongoing counseling.
Children with HIV infection should be taught good hygiene and behavior to reduce risk to others. How much and when children are told about the illness depends on age and maturity. Older children and adolescents should be made aware of their diagnosis and the possibility of sexual transmission and should be counseled appropriately. Families may be unwilling to share the diagnosis with people outside the immediate family because it can create social isolation. Feelings of guilt are common. Family members, including children, can become clinically depressed and require counseling.
Because HIV infection is not acquired through the typical types of contact that occur among children (eg, through saliva or tears), children with HIV infection should be allowed to attend school without restrictions. Similarly, there are no inherent reasons to restrict foster care, adoptive placement, or child care of children with HIV infection. Conditions that may pose an increased risk to others (eg, aggressive biting or the presence of exudative, weeping skin lesions that cannot be covered) may require special precautions.
The number of school personnel aware of the child’s condition should be kept to the minimum needed to ensure proper care. The family has the right to inform the school, but people involved in the care and education of a child with HIV infection must respect the child’s right to privacy. Disclosures of information should be made only with the informed consent of the parents or legal guardians and age-appropriate assent of the child.
Routine vaccinations
Routine pediatric vaccination protocols (including for COVID-19 ) are recommended for children with HIV infection, with several exceptions.
The main exception is that live-virus vaccines and live-bacteria vaccines (eg, bacille Calmette–Guérin [BCG]) should be avoided or used only in certain circumstances (see table Considerations for Use of Live Vaccines in Children With HIV Infection ).
Live oral poliovirus vaccine (which is not available in the United States but is still used in other parts of the world) and live-attenuated influenza vaccine are not recommended; however, inactivated polio vaccine should be given according to the routine schedule, and inactivated influenza vaccination should be given yearly.
The live measles-mumps-rubella (MMR) vaccine and varicella vaccine should not be given to children with manifestations of severe immunosuppression. However, the MMR and varicella-zoster virus (VZV) vaccines (separately; not combined as MMRV vaccine, which has a higher titer of attenuated varicella virus, the safety of which has not been shown in this population) can be given to asymptomatic patients following the routine schedule and to patients who have had HIV symptoms but who are not severely immunocompromised (ie, not in category 3 [see table Immunologic Categories (HIV Infection Stages) for Children < 13 Years With HIV Infection Based on Age-Specific CD4+ T-Cell Count or Percentage ], including having a CD4+ T-cell percentage of ≥ 15%). If possible, the MMR and VZV vaccines should be given starting at age 12 months in symptomatic patients to enhance the likelihood of an immune response, ie, before the immune system deteriorates. The second dose of each may be given as soon as 4 weeks later in an attempt to induce seroconversion as early as possible, although typically a 3-month interval between varicella vaccine doses is preferred in noninfected children < 13 years. If the risk of exposure to measles is increased, such as during an outbreak, the measles vaccine should be given at an earlier age, such as 6 to 9 months.
routine schedule . Safety and efficacy data are limited in symptomatic infants but there very likely is overall benefit to immunization, particularly in areas where rotavirus causes significant mortality.
The BCG vaccine is not recommended in the United States because it is an area of low tuberculosis (TB) prevalence. However, elsewhere in the world, especially in countries where TB prevalence is high, BCG is routinely used; many of these countries also have high HIV prevalence among childbearing women. BCG as a live bacterial vaccine has caused some harm in children with HIV infection but likely protects children who do not have HIV infection and even some children who do have HIV infection from acquiring TB. The World Health Organization (WHO) now recommends that children who are known to be HIV-infected, even if asymptomatic, should no longer be immunized with BCG vaccine. However, BCG may be given to asymptomatic infants of unknown HIV infection status born to women with HIV infection, depending on the relative incidence of TB and HIV in the particular area. BCG also may be given to asymptomatic infants born to women of unknown HIV infection status.
In some areas of the world, children are routinely given the or the dengue virus vaccine ; these live virus vaccines should be given only to those without severe immunosuppression.
Because children with symptomatic HIV infection generally have poor immunologic responses to vaccines, they should be considered susceptible when they are exposed to a vaccine-preventable disease (eg, measles, tetanus, varicella) regardless of their vaccination history. Such children should receive passive immunization with IV immune globulin. IV immune globulin also should be given to any nonimmunized household member who is exposed to measles.
Seronegative children living with a person with symptomatic HIV infection should receive inactivated poliovirus vaccine rather than oral polio vaccine. Influenza (inactivated or live), MMR, varicella, and rotavirus vaccines may be given normally because these vaccine viruses are not commonly transmitted by the vaccinee. Adult household contacts should receive annual influenza vaccination (inactivated or live) to reduce the risk of transmitting influenza to the person with HIV infection.
Additional recommendations for children with HIV infection are
1 to 2 months after the last dose of the series, children with HIV infection should be tested to determine whether the level of antibodies to hepatitis B surface antigen (anti-HBs) is protective ( ≥ 10 mIU/mL).
Children and adolescents with HIV infection who are pneumococcal conjugate vaccine . (See also Pneumococcal Advisory Committee on Immunization Practices [ACIP] Vaccine Recommendations .)
Certain postexposure treatment recommendations also differ. Quadrivalent meningococcal conjugate immunization has been recommended for routine and catch-up use in children, adolescents, and adults with HIV infection (see also ACIP recommendations for the use of meningococcal conjugate vaccines in people who have HIV ).
Treatment reference
1. Kobayashi M, Farrar JL, Gierke R, et al : Use of 15-valent pneumococcal conjugate vaccine among U.S. children: Updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep 71(37):1174–1181, 2022. doi: 10.15585/mmwr.mm7137a3
Transition to Adult Care
Transition of youth with HIV infection from the pediatric health care model to the adult health care model takes time and advance planning. This process is active and ongoing and does not simply involve a one-time referral to an adult care clinic or office. The pediatric health care model tends to be family-centered, and the care team includes a multidisciplinary team of physicians, nurses, social workers, and mental health professionals; perinatally infected youth may have been cared for by such a team for their entire life.
In contrast, the typical adult health care model tends to be individual-centered, and the health care practitioners involved may be located in separate offices requiring multiple visits. Health care practitioners at adult care clinics and offices are often managing high patient volumes, and the consequences of lateness or missed appointments (which may be more common among adolescents) are stricter. Finally, changes in insurance coverage in adolescence or young adulthood can complicate transition of medical care as well.
Planning transition over several months and having adolescents have discussions or joint visits with the pediatric and adult health care practitioners can lead to a smoother and more successful transition. A resource for transition of youth with HIV infection into adult health care is available from the American Academy of Pediatrics (see Transitioning HIV-Infected Youth Into Adult Health Care ).
Prognosis for HIV Infection in Infants and Children
In the pre-ART era, 10 to 15% of children from high-resource countries and perhaps 50 to 80% of children from low-resource countries died before age 4 years; however, with appropriate combination ART regimens, most perinatally infected children survive well into adulthood. Increasing numbers of these perinatally infected young adults have given birth to or fathered their own children.
Nevertheless, if opportunistic infections occur, particularly Pneumocystis pneumonia, progressive neurologic disease, or severe wasting, the prognosis is poor unless virologic and immunologic control is regained with combination ART. Mortality due to Pneumocystis pneumonia ranges from 5 to 40% if treated and is almost 100% if untreated. Prognosis is also poor for children in whom virus is detected early (ie, by 7 days of life) or who develop symptoms in the first year of life.
There have been several reported cases of adults in whom replication-competent HIV was eradicated (ie, these people were "cured" for > 5 years). These adults each required a hematopoietic stem cell transplant for leukemia. The donor cells were homozygous for the CCR5-delta 32 mutation, which made the engrafted lymphocytes resistant to infection with CCR5-tropic HIV; subsequently, HIV has remained undetectable. It is likely that ART, bone marrow ablation, and graft-vs-host disease also contributed to these cures.
At least one infant born to a mother with HIV infection who had not received prenatal care or prenatal (or intrapartum) ART was preliminarily thought to have been cured but upon further clinical follow-up was found to have persistent HIV infection. This infant was given combination ART at high doses (not yet known to be safe and effective for general use) beginning on day 2 of life through 15 months of age, after which time it was inadvertently interrupted. Nevertheless, at 24 months of age the infant had no detectable replicating virus RNA (a "functional cure") but did have detectable proviral DNA. Subsequently, however, HIV replication ensued. No infants or children have been permanently cured of their HIV infection, and it is not yet known if cure is possible.
What is known, however, is that HIV infection is a treatable infection that is already compatible with long-term survival if effective ART is given. Future research will undoubtedly uncover ways to improve ART tolerance and efficacy and perhaps help achieve the goal of curative therapy. At present, interruption of ART in either infancy, childhood, or adulthood is not recommended .
Prevention of HIV Infection in Infants and Children
For pre-exposure prevention, see Pre-exposure prophylaxis (PrEP) .
For postexposure prevention, see Postexposure prophylaxis (PEP) .
Prevention of perinatal transmission
Appropriate prenatal ART attempts to optimize maternal health, interrupt MTCT, and minimize in utero drug toxicity. In the United States and other countries where ARV medications and HIV testing are readily available, treatment with ARV medications is standard for all pregnant women with HIV infection (see treatment of HIV infection in adults ). Rapid HIV testing of pregnant women who present in labor without documentation of their HIV serostatus may allow immediate institution of such measures.
All pregnant women with HIV infection dolutegravir and a small increase in infant neural tube defects, the apparent increase was not present after further study, and it is unknown whether this increase was truly due to dolutegravir or to another factor, such as folate deficiency. The majority of experts believe that women with HIV infection already receiving combination ART who become pregnant should continue that therapy, even early during the first trimester.
Elective cesarean delivery before onset of labor is recommended if the maternal HIV plasma viral load is > 1000 copies/mL. If labor has already begun, it is less certain whether cesarean delivery reduces MTCT.
When patients present in labor,
Recent HIV plasma viral load > 1000 copies/mL
Unknown HIV plasma viral load near delivery
Are thought to have had incomplete adherence to ART
Many experts now believe that IV ZDV is not required during labor for women receiving combination ART who have achieved HIV plasma viral loads
After delivery, combination ART is continued for all women, even those who had not previously received ART.
All newborns exposed to HIV should receive a postpartum ARV regimen to reduce the risk of HIV infection. Treatment should begin as soon as possible, preferably within 6 to 12 hours of delivery. The ARV regimen is determined by maternal and infant risk factors for perinatal HIV transmission (see the Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV's Maternal HIV Testing and Identification of Perinatal HIV Exposure recommendations).
Preventive regimens are categorized as
ARV prophylaxis
Presumptive HIV therapy
Low-risk infants are candidates for ARV prophylaxis. They include full-term neonates born to women who have had sustained virologic suppression with ART (as shown by an HIV plasma viral load
Low-risk infants should be given ARV prophylaxis with ZDV 4 mg/kg orally twice daily for the first 4 weeks of life. ZDV is the backbone of infant prophylaxis and is used for all infants born to women with HIV infection regardless of the risk factors.
Some experts advise ZDV may be given for 2 weeks to select infants born at ≥ 37 weeks gestation to women who meet low-risk criteria, who have been given ART for more than 10 consecutive weeks, and who have maintained viral suppression for the duration of the pregnancy (see the Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV's Management of Infants Born to People with HIV Infection ).
High-risk infants are given presumptive HIV therapy (see table Neonatal Antiretroviral Management According to Risk of HIV Infection Antiretroviral Dosing for Neonates with Perinatal HIV Exposure ) for up to 6 weeks or, rarely, longer. This therapy initially serves as prophylaxis but also as preliminary treatment for those later confirmed to have HIV.
Very few ARV medications (notably ZDV, nevirapine , lamivudine , abacavir , and raltegravir ) are considered safe and effective for infants zidovudine , lamivudine , nevirapine , and, for late preterm infants, raltegravir ) have dosing data available for preterm infants. The optimal ARV regimen for neonates born to women with ARV drug-resistant virus is unknown.
Infants who subsequently have a positive HIV virologic test are given ART with three medications as appropriate for treatment of known HIV infection. An expert in pediatric or maternal HIV infection should be immediately consulted (see information at ClinicalInfo.HIV.gov or at the National Clinician Consultation Center ). Clinicians also can call the Perinatal HIV Consultation and Referral Services Hotline at 1-888-HIV-8765 (1-888-448-8765) for questions regarding interventions to decrease vertical HIV transmission and neonatal diagnosis.
Some mothers with HIV infection who live in the United States or in other countries where safe, affordable, and alternative sources of feeding are available may choose to breastfeed if they are receiving ART and have a sustained, undetectable viral load. The decision to breastfeed should be made only after counseling and shared decision-making discussions. Some recommendations for continuing neonatal ARV prophylaxis and using an increased diagnostic testing frequency in this situation have been suggested, but a consensus has not yet been reached because data are incomplete. An expert in pediatric HIV infection should be consulted (see Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV's Infant Feeding for Individuals with HIV in the United States ).
Additionally, in countries where infectious diseases and undernutrition are major causes of early childhood mortality and safe, affordable infant formula is not available, the protection breastfeeding offers against the mortality risks of respiratory and gastrointestinal infections may counterbalance the risk of HIV transmission. In these countries, the World Health Organization (WHO) recommends mothers with HIV infection continue to breastfeed for at least 12 months of the infant's life (see the WHO's Guideline: Updates on HIV and Infant Feeding ).
Donating to milk banks is contraindicated for women with HIV infection in the United States and in other countries where safe and affordable alternative sources of feeding are readily available.
Premastication (prechewing) of food, practiced by some mothers of young infants, is also contraindicated for women with HIV infection.
Prevention of adolescent transmission
Because adolescents are at special risk of HIV infection, they should receive education, have access to HIV testing, and know their serostatus. Education should include information about transmission, implications of infection, and strategies for prevention, including abstaining from high-risk behaviors and engaging in safe sex practices (eg, correct and consistent use of condoms ) for those who are sexually active. Efforts should especially target adolescents at high risk of HIV infection, in particular, Black and Hispanic adolescent men who have sex with other men because these are the fastest-growing US demographics of new HIV infections among youth; however, all adolescents should receive risk-reduction education.
In most US states, informed consent is necessary for testing and the release of information regarding HIV serostatus. Decisions regarding disclosure of HIV status to a sex partner without the patient’s consent should be based on the following:
Possibility of intimate partner violence to the patient after disclosure to the partner
Likelihood that the partner is at risk
Whether the partner has reasonable cause to suspect the risk and to take precautions
Presence of a legal requirement to withhold or disclose such information
Pre-exposure prophylaxis (PrEP)
Data regarding infants of HIV-negative mothers taking TDF/FTC PrEP during pregnancy are incomplete, but, currently, no adverse effects have been reported in children born to women with HIV infection treated with TDF/FTC. Use of PrEP to reduce the risk of HIV infection in injection drug users is being studied.
Adolescents in the United States often face a barrier to seeking sexually transmitted infection and HIV services partly because they fear breach of confidentiality (that is, that their parents or guardians will be told). This has been a barrier to administration of PrEP to adolescents as well. Issues of cost (with possible lack of insurance reimbursement) also may be more complex for adolescents receiving PrEP than for adults receiving PrEP. Despite these potential barriers, PrEP for sexually active adolescents, particularly those with high-risk sexual behavior, should be strongly considered. A recent compendium of minor consent laws for sexually transmitted infections and HIV services is available to help guide clinicians ( 1 ).
Pre-Exposure Prophylaxis (PrEP) . For further discussion, see PrEP to Prevent HIV and Promote Sexual Health from the New York State Department of Health AIDS Institute.
PrEP reference
1. Nelson KM, Skinner A, Underhill K : Minor consent laws for sexually transmitted infection and HIV services. JAMA 328(7):674–676, 2022. doi: 10.1001/jama.2022.10777
Most HIV cases in infants and children result from mother-to-child transmission (MTCT) before or during birth, or from breastfeeding in countries where safe and affordable infant formula is not available.
Maternal antiretroviral therapy (ART) can reduce incidence of MTCT from about 25% to
Neonates born to women living with HIV infection are treated for a short time with antiretroviral (ARV) medications to interrupt MTCT.
Diagnose children
Diagnose children > 18 months using a 4th-generation HIV-1/2 antigen/antibody combination immunoassay followed by a 2nd-generation HIV-1/2 antibody differentiation assay and, if required, an HIV-1 qualitative RNA assay.
Urgently treat (using rapid initiation) all infants with HIV infection
Treat all other children and adolescents with HIV infection as soon as issues of adherence are more fully assessed and addressed with the children and their caretakers.
Combination ART is given, preferably using a fixed-dose combination product if feasible, for increased adherence.
Adolescents who do not have HIV infection may be given PrEP to prevent acquisition of HIV infection, but issues of confidentiality and cost may be more problematic than for adults receiving PrEP.
Give prophylaxis for opportunistic infections based on age and CD4+ count.
More Information
The following English-language resources may be useful. Please note that THE MANUAL is not responsible for the content of these resources.
See the following US government sites for information on drug treatment, including adverse effects, dosing (especially for information on fixed-dose combination products), and drug interactions, educational materials, and quick links to related topics:
ClinicalInfo.HIV.gov: Recommendations for the Use of Antiretroviral Drugs During Pregnancy and Interventions to Reduce Perinatal HIV Transmission in the United States
ClinicalInfo.HIV.gov: Appendix C: CDC Pediatric HIV CD4 Cell Count/Percentage and HIV-Related Diseases Categorization
ClinicalInfo.HIV.gov: Diagnosis of HIV Infection in Infants and Children
ClinicalInfo.HIV.gov: Maternal HIV Testing and Identification of Perinatal HIV Exposure
ClinicalInfo.HIV.gov: Appendix A: Pediatric Antiretroviral Drug Information
ClinicalInfo.HIV.gov: Management of Infants Born to People with HIV Infection
ClinicalInfo.HIV.gov: Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV
ClinicalInfo.HIV.gov: Guidelines for the Prevention and Treatment of Opportunistic Infections in Children with and Exposed to HIV
ClinicalInfo.HIV.gov: Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV
ClinicalInfo.HIV.gov: Infant Feeding for Individuals with HIV in the United States
World Health Organization: Guideline: Updates on HIV and Infant Feeding
Centers for Disease Control and Prevention (CDC): Pre-Exposure Prophylaxis (PrEP)
U.S. Preventive Services Task Force (USPSTF): Human Immunodeficiency Virus (HIV) Infection: Screening recommendation statement (2019)
The following resources provide information about various other prevention, treatment, and education aspects of HIV/AIDS:
New York State Department of Health AIDS Institute HIV Clinical Guidelines Program: Disseminates practical, evidence-based clinical guidelines that promote quality medical care for people in New York who are living with and/or are at risk of acquiring HIV and certain other illnesses
New York State Department of Health AIDS Institute: Pre-exposure prophylaxis (PrEP) guidelines, education, and training for HIV prevention
New York State Department of Health AIDS Institute: Comprehensive information regarding all aspects of HIV/AIDS, including treatment, social awareness, resources for consumers, and training for professionals
UNAIDS: Comprehensive information on how the organization directs, advocates, coordinates, and provides technical support needed to connect leadership from governments, the private sector, and communities to deliver life-saving HIV services
National Clinician Consultation Center: Up-to-date HIV/AIDS guidelines and key treatment protocols for HIV/AIDS treatment, prevention, and exposure
American Academy of Pediatrics: Transitioning HIV-Infected Youth Into Adult Health Care
Perinatal HIV Consultation and Referral Services Hotline 1-888-HIV-8765 (1-888-448-8765): Free 24-hour clinical consultation and advice on treating pregnant women with HIV infection and their infants

- Cookie Preferences

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
Neonatal HIV
Affiliation.
- 1 Beaumont Health
- PMID: 33351437
- Bookshelf ID: NBK565879
Over 95% of HIV-infected pediatric cases are a result of vertical transmission. The pathophysiology of the HIV disease state in the pediatric population is similar to adults. However, differences occur in the clinical presentation, mode of infection, and therapeutic options. The pediatric and neonatal populations have a weaker immune system than adults; therefore, if infected with HIV, they are at a greater risk of opportunistic infections. As such, the delay of treatment may result in a rapid progression of the disease.
One of the greatest advancements in medicine has been the prevention of mother-to-child transmission (MTCT) of HIV type 1 (HIV-1). The rate of transmission of HIV to neonates has been reduced to less than 1% with the implementation of appropriate strategies and careful planning. The increase in comprehensive serologic screening and the treatment of HIV-infected pregnant females has resulted in the reduction of vertical transmission. There are evidence-based prevention modalities that can be utilized at different stages of pregnancy and postpartum to improve outcomes. Antiretroviral therapies (ART) can be prescribed during gestation, antepartum during vaginal or elective cesarean delivery, postnatally to the neonate, or when breastfeeding.
Copyright © 2024, StatPearls Publishing LLC.
- Continuing Education Activity
- Introduction
- Epidemiology
- Pathophysiology
- Histopathology
- History and Physical
- Treatment / Management
- Differential Diagnosis
- Toxicity and Adverse Effect Management
- Complications
- Consultations
- Deterrence and Patient Education
- Pearls and Other Issues
- Enhancing Healthcare Team Outcomes
- Review Questions
Publication types
- Study Guide
Disclaimer » Advertising
- HealthyChildren.org

- Previous Article
- Next Article
Practice Gap
Epidemiology of pediatric hiv infection, hiv: pathogen and pathogenesis, preventing hiv infection in infants, children, and youth, recognizing hiv infection in infants, children, and adolescents: routine testing and clinical presentations, indications for routine hiv testing, adolescents, clinical presentations that warrant hiv testing, pretest counseling and consent for testing, hiv testing assays in infants, children, and adolescents, management of hiv-exposed infants, neonatal arv prophylaxis, routine hiv virologic testing schedule for hiv-exposed infants, pcp prophylaxis and immunizations, importance of primary care visits, long-term concerns for hiv-exposed uninfected infants, management of hiv infection in infants, children, and adolescents, baseline evaluation, art: goals and principles, prophylaxis and immunizations for hiv-infected infants, children, and adolescents, complications of hiv infection in the cart era, counseling and support, coping with the diagnosis and prognosis, disclosure of hiv infection status, adherence to care and treatment, school and sports participation, transition to adult health care, preventing and managing hiv infection in infants, children, and adolescents in the united states.
AUTHOR DISCLOSURE
Dr Siberry has disclosed no financial relationships relevant to this article. This commentary does not contain a discussion of an unapproved/investigative use of a commercial product/device.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
George K. Siberry; Preventing and Managing HIV Infection in Infants, Children, and Adolescents in the United States. Pediatr Rev July 2014; 35 (7): 268–286. https://doi.org/10.1542/pir.35-7-268
Download citation file:
- Ris (Zotero)
- Reference Manager
Effective prevention strategies have reduced the risk of perinatal transmission of human immunodeficiency virus (HIV) infection to less than 1% to 2% in the United States, but failures to fully implement these strategies result in continued preventable infant HIV infections. In addition, the increasing number of sexually acquired HIV infections in adolescents underscores the important role of the pediatrician in preventing and diagnosing HIV infection in youth.
After completing this article, readers should be able to:
Recognize the important role that the pediatrician plays in the prevention, detection, and care of patients infected with and affected by human immunodeficiency virus (HIV).
Understand the epidemiology of HIV infection in infants, children, and adolescents.
Select the proper HIV diagnostic testing plan for infants, children, and adolescents.
Plan the comprehensive management of HIV-exposed infants.
Recognize the clinical conditions suggestive of HIV infection, including the major opportunistic infections seen in patients with HIV/AIDS.
Understand the principles, monitoring, and complications of HIV treatment in infants, children, and adolescents.
Since the first description of infants with human immunodeficiency virus (HIV) infection in the early 1980s, ( 1 )( 2 ) tremendous advances have been made in the understanding, prevention, and treatment of HIV infection. Effective prevention strategies have reduced the risk of perinatal transmission, or maternal-to-child transmission (MTCT), of HIV infection to less than 1% to 2% in the United States, and the World Health Organization has made global elimination of new infant HIV infections a realistic target by 2015. ( 3 ) For those children who have HIV infection, the development of potent antiretroviral (ARV) drugs has transformed a once progressive and often fatal infection for children into a chronic condition with markedly reduced morbidity and expectations for long and productive lives.
Worldwide, an estimated 34 million people are living with HIV infection; 3.4 million (approximately 10%) are younger than 15 years. ( 4 ) Nearly all (95%) children younger than 15 years acquired HIV infection perinatally; in fact, a substantial number of the 2 million adolescents (ages 10-19 years) with HIV infection worldwide are thought to be long-term survivors of perinatal HIV infection, but data have not been collected in a way to distinguish perinatal (vertical) and behavioral (horizontal) routes of HIV transmission in this age group. ( 5 )
As of 2011, in the United States, 4500 children (ages <15 years) had perinatal HIV infection, but this number represents approximately half of all perinatally infected HIV-infected people in the United States because diminishing numbers of new infant infections and markedly improved long-term survival of children with perinatal HIV infection have meant that most perinatally infected children are now adolescents and young adults. ( 5 )( 6 )( 7 )
The predominant route of HIV infection in children is MTCT, including intrauterine, intrapartum, and postnatal (through breastfeeding) transmission. In the absence of ARV preventive interventions, in nonbreastfeeding populations, 25% to 30% of infants born to HIV-infected women will become infected; the risk increases to as high as 50% for infants with prolonged breastfeeding. Sexual transmission is an important mode of transmission for adolescents, especially for adolescent girls in settings with generalized HIV epidemics and for young men who have sex with men (MSM). Less common routes of transmission include transfusion with blood products tainted with HIV (before routine screening of blood products for HIV was established), percutaneous exposure, and, rarely, HIV-infected caretakers chewing or warming food in their mouths and then feeding it to infants and children. ( 8 )
HIV-1 and HIV-2 are enveloped, single-strand RNA retroviruses. HIV-1 is overwhelmingly responsible for HIV infections worldwide, including the United States. HIV-2 causes infection predominantly in people from parts of West Africa, but it is less transmissible and generally associated with lower levels of viral replication and less severe disease. ( 9 )
The principal targets of HIV are cells expressing the CD4 + molecule: CD4 + T lymphocytes (CD4 T cells) and monocytes or macrophages. HIV binds the CD4 target with a cellular coreceptor (CCR5 or CXCR4), resulting in virus envelope fusion with the host cell wall that permits viral entry into the cell. CD4 + cells in the gut are a major target, and the virus disseminates widely soon after infection, including to the central nervous system. CD4 T-cell infection is followed by viral replication, release of HIV virions, and CD4 T-cell death, leading over time to progressive CD4 T-cell depletion and impairment of cellular immunity, the hallmark of HIV-related immunodeficiency.
In a small proportion of CD4 T cells, HIV entry instead leads to integration of the HIV genome (HIV RNA reverse transcribed to a DNA sequence) into the cellular genome of a CD4 T cell that enters a quiescent phase as a memory CD4 T cell, harboring its latent HIV infection for activation months or years later. Such latent infection of long-lived memory T cells underlies a main barrier to sterilizing cure of HIV infection. ( 10 )
The most important strategies to prevent MTCT in the United States have been administering ARV drugs to HIV-infected mothers and their infants, elective caesarean section for HIV-infected women who reach term without achieving plasma HIV virologic suppression, and providing replacement feeding instead of breast milk to infants of HIV-infected mothers. In settings outside the United States where infant replacement feeding confers an unacceptably high risk of HIV-unrelated morbidity and mortality (including in many sub-Saharan African countries), the additional strategy of administering ARV drugs to mothers or infants during breastfeeding has become an effective means to allow breastfeeding while reducing the risk of HIV transmission. In the United States, however, all HIV-infected women are still advised against breastfeeding, regardless of ARV use and maternal plasma HIV suppression, because neither maternal nor infant ARV prophylaxis completely eliminates breast milk HIV transmission (residual transmission can be as high as 5%) and safe and affordable replacement feeding is available. ( 11 )( 12 )
The ability of ARV drugs to prevent MTCT was first found in the landmark AIDS Clinical Trials Group 076 trial, which found that zidovudine administered during pregnancy, intrapartum, and (to the infant) after birth could cut transmission from 26% to 8% in the absence of breastfeeding. ( 13 ) Subsequent studies found even greater efficacy for combination ARV therapy (cART), which was generally composed of at least 3 ARV drugs from at least 2 different classes. Routine use of cART for pregnant women in the United States has resulted in MTCT risk less than 1% to 2% and estimated annual new infant infections numbering about 200 nationwide. ( 14 ) In fact, pregnant women who achieve consistent plasma HIV virologic suppression during pregnancy are at such low risk of MTCT that neither elective caesarean section nor intrapartum ARV therapy (intravenous zidovudine) is recommended to further reduce transmission risk. ( 11 ) However, failure to identify HIV infection in pregnant women, barriers that prevent HIV-infected women from receiving cART during pregnancy, and incident HIV infection in pregnant and breastfeeding women remain important problems that contribute to residual infant infections in the United States. ( 14 )( 15 )
The latest recommendations by the US Department of Health and Human Services for preventing MTCT in the United States can be found at http://aidsinfo.nih.gov/guidelines/html/3/perinatal-guidelines . The comprehensive prevention approach is multipronged: routine testing for HIV in pregnant women, administering ARV drugs to HIV-infected pregnant women and their infants, supporting women’s retention in care and adherence to cART, offering elective cesarean section to women who have not achieved HIV plasma viral RNA concentration (viral load) less than 1000 copies/mL by the end of pregnancy, minimizing invasive obstetric procedures (eg, fetal scalp electrode), avoidance of breastfeeding, primary prevention of HIV infection in women of reproductive age, and ensuring access to family planning services for HIV-infected women.
HIV testing is recommended as early as possible in each pregnancy, including for women who tested HIV negative during a prior pregnancy. Retesting in late pregnancy should be considered for all HIV-seronegative women and is recommended for pregnant women who are at high risk of HIV infection, such as women with a known HIV-infected partner, history of injection drug use, or sexually transmitted infection (STI) diagnosis signs or symptoms of acute HIV infection; women who reside in jurisdictions with elevated HIV incidence among women of childbearing age (≥17 HIV cases per 100,000 person-years); and women receiving health care in facilities with at least 1 diagnosed HIV case per 1,000 pregnant women per year. ( 16 )( 17 ) Women presenting in labor who have not received appropriate HIV testing in pregnancy should undergo rapid HIV antibody testing. If the results are positive, a confirmatory HIV test should be performed as soon as possible, and maternal and infant ARV therapy should be initiated, pending the confirmatory test result. If the confirmatory HIV test result is positive, infant ARV therapy should be continued; if the test result is negative, then infant ARV therapy should be stopped.
Newborn nurseries should have procedures in place to alert nursery staff when an HIV-exposed infant is born because neonatal ARV prophylaxis should be initiated as soon after birth as possible, ideally within 6 to 12 hours (see the Management of HIV-Exposed Infant section). On admission to the newborn nursery and at the first newborn outpatient visit, documented maternal HIV testing results should be confirmed. For a newborn whose mother did not receive appropriate HIV testing in pregnancy, HIV exposure status should be confirmed by performing rapid HIV antibody testing on the mother; if maternal testing cannot be performed, infant antibody testing should be performed to assess potential HIV exposure. If a rapid maternal or infant antibody test result is positive, the infant should initiate ARV therapy immediately, pending confirmatory testing, as for women with positive rapid antibody test results during labor and delivery (see above).
HIV infection should be suspected in patients who present with typical clinical findings, but many children and youth have their conditions diagnosed before clinical manifestations develop because of several routine indications for HIV testing.
All infants born to women with HIV infection should undergo a scheduled series of HIV virologic tests that will lead to confirmation or exclusion of perinatal HIV infection (see the Management of HIV-Exposed Infant section). If the maternal HIV status has not been determined, maternal HIV antibody testing (or, if the mother is not available, infant HIV antibody testing) should be requested to determine whether the infant is HIV exposed.
Many new US cases of HIV infection are diagnosed in infants and children born outside the United States, especially in high HIV prevalence settings, such as sub-Saharan Africa. ( 18 ) Some of these children have come to the United States with their mothers or families, whereas others have been adopted. HIV antibody testing should be offered to foreign-born children (particularly from settings of moderate or high HIV prevalence) to evaluate for infection (in those age ≥18 months) or for perinatal exposure (in those age <18 months); those younger than 18 months who are HIV antibody positive will require additional HIV virologic testing, as for HIV-exposed newborns, to assess whether they are HIV infected (see the HIV Testing in Infants, Children, and Adolescents section).
Clinicians caring for infants and children with HIV exposure or infection should ensure that the siblings of these patients have also been evaluated for HIV infection. For instance, when managing an HIV-exposed infant, the clinician should recommend to the mother that she have her other children tested for HIV infection, even if they appear healthy, unless there is documentation that she did not have HIV infection at the time she was pregnant with or breastfeeding those older children. Although unusual, some undiagnosed (and thus untreated) perinatally HIV-infected children have survived into their teens without serious illness, so there is no upper age limit for siblings to be considered for HIV testing.
The Centers for Disease Control and Prevention (CDC) recommends performing an HIV test routinely beginning at age 13 years, the US Preventive Services Task Force guidelines recommend routine screening beginning at age 15 years, and the American Academy of Pediatrics recommends HIV testing at least once by age 16 to 18 years in patient populations with HIV prevalence of 0.1% or higher. ( 16 )( 19 )( 20 ) Thus, routine HIV screening is recommended for nearly all adolescents at least once, even in the absence of specific risk factors. The indications for and intervals between subsequent HIV testing are determined by level of risk. Those at very high risk should be offered HIV testing at least annually, whereas an interval of 3 to 5 years is reasonable for those at elevated but lesser degree of risk. ( 16 ) Important indicators of very high risk include young MSM and intravenous drug users. Other adolescents at increased risk of HIV infection include those whose sexual partners are MSM, intravenous drug users, or HIV infected; those who report unprotected anal or vaginal sexual intercourse; those who have STIs; and sexually active youth who live in an area of increased HIV prevalence (defined by the CDC as a community with an HIV seroprevalence of at least 1%).
Many infants and children with HIV infection may have prolonged periods without severe illnesses or clinical manifestations of HIV infection. However, perinatally infected infants (especially those infected in utero) are at high risk of rapid progression to severe illness and death. Although full implementation of prevention of MTCT policies should prevent nearly all infant HIV infections and identify early those few infections that occur despite appropriate interventions, there are still infants and children whose perinatal HIV exposure and resulting infection have escaped detection; it is therefore important for clinicians to recognize specific infections and clinical presentations that may be a sign of unrecognized HIV infection (or other immunodeficiency). Table 1 summarizes the clinical manifestations of untreated HIV infection.
Relative Frequency of Clinical Conditions in Untreated Human Immunodeficiency Virus Infection
HPV=human papillomavirus, MAC= Mycobacterium avium complex, PCP =Pneumocystis jiroveci pneumonia.
Some conditions belong to more than one category.
Adapted from Simpkins et al. ( 21 )
Pneumocystis pneumonia (PCP), caused by the fungus Pneumocystis jirovecii (formerly carinii) , was among the most common and deadly presentations of HIV infection in infants early in the epidemic. ( 22 ) Rare in the first month of life, this condition peaks at ages 3 to 6 months. Infants present with progressive cough, poor feeding, dyspnea, and often fever. Onset can be gradual or abrupt, but progression to hypoxia, respiratory failure, and death will occur without prompt recognition and treatment. ( 22 )
Mucosal candidiasis is a common and early sign of HIV infection in untreated infants. Although an episode of oral thrush can occur in infants without immunodeficiency, oral candidiasis that is severe, persistent, or recurrent is an important manifestation of the cellular immune dysfunction caused by HIV infection and other diseases that cause immunodeficiency.
Recurrent bacterial pneumonia and other bacterial infections (sinusitis and otitis) were common in HIV-infected infants and children before cART and may be the first clue to unrecognized HIV infection. Although these infections generally have similar presentations and pathogens (especially pneumococcus) in HIV-infected and uninfected children, their increased frequency and recurrence are typical in children with HIV infection.
The constellation of persistent parotid gland swelling, lymphadenopathy, and chronic interstitial lung disease (lymphocytic interstitial pneumonitis) was a typical pattern in pediatric HIV infection, often in those untreated children who were spared more serious infections in the first few years of life.
Growth monitoring and neurodevelopmental screening, essential aspects of standard pediatric primary care, may reveal failure to thrive, stunting, or abnormal motor and cognitive development that may be important clues to untreated HIV infection in infants and children.
All children with tuberculosis disease should be tested for HIV infection. Adults with HIV infection are more likely to develop contagious tuberculosis disease, increasing the risk of tuberculosis infection in children in their households. HIV-infected children who acquire tuberculosis infection are then more likely to develop tuberculosis disease because of their HIV-related immunologic impairments.
Herpes zoster (shingles) is uncommon in children, but most children who develop it probably do not have an immunodeficiency disorder. Children with untreated HIV infection, however, frequently develop zoster. In the United States, where unrecognized HIV infection in children is fortunately rare, an episode of zoster may not warrant automatic HIV testing. However, an episode of zoster in a child merits thorough review of the child’s history of other illnesses, careful physical examination, and ascertainment of health status of mother and siblings to determine whether HIV testing is warranted. Clinicians should have a low threshold for performing HIV testing in children with zoster that is severe or recurrent.
In adolescents, the diagnosis of a new STI should prompt HIV testing. HIV infection does not directly increase the risk of contracting STIs, but an incident STI is a marker of the same sexual risk behavior that increases the risk of HIV transmission.
Those who care for adolescents, including pregnant and breastfeeding women, should be able to recognize presentations of primary HIV infection several days to weeks after incident HIV infection when HIV viremia (and potential for transmission to others) is high and HIV antibodies have not yet appeared. Most episodes (50%-90%) of primary HIV infection are symptomatic, but the variable severity and nonspecific flulike or mononucleosis-like nature of acute retroviral syndrome often result in patients not seeking medical care for their illness and/or clinicians not recognizing it as possible primary HIV infection. ( 23 ) The most common features include fever, vomiting, diarrhea, headache, myalgias, lymphadenopathy, and rash ( Table 2 ). Sexually active patients with lymphadenopathy, maculopapular rash, and shallow, sharp ulcers of the oral and/or anogenital mucosae should be evaluated for acute HIV infection. Because this early phase of HIV infection precedes the full antibody response, patients with suspected acute HIV infection should be tested with a fourth-generation combined antigen-antibody test or a plasma HIV RNA test (see the HIV Testing Assays section). Patients diagnosed as having acute HIV infection should be counseled about their high risk of transmitting HIV through unprotected sexual contact with others; HIV specialists should be consulted promptly for recommendations regarding HIV treatment. Because of the high risk of MTCT after acute HIV infection in pregnancy or during breastfeeding, pregnant women with acute HIV infection should be urgently referred for comprehensive care and ARV therapy initiation, and breastfeeding women with acute infection should be counseled to stop breastfeeding immediately, be referred for their own care, and have their infants undergo evaluation for HIV infection.
Presentation of Acute Human Immunodeficiency Virus Infection
Adapted with permission from Lippincott Williams and Wilkins/Wolters Kluwer Health: Richey LE, Alperin J. Acute human immunodeficiency virus infection. Am J Med Sci. 2013:345(2):136–142. ( 24 )
Promotional and commercial use of the material in print, digital or mobile device format is prohibited without permission from the publisher, Lippincott Williams & Wilkins. Please contact [email protected] for further information.
Patients who meet criteria for routine HIV screening (eg, pregnant women and initial testing for adolescents) should be notified that HIV testing is recommended but given the option to decline testing. ( 16 ) HIV testing provides an important opportunity to educate patients about HIV and to counsel them about sexual practices and other behaviors that may elevate their risk of HIV infection. However, mandatory HIV prevention counseling and separate written consent for HIV testing can be barriers to HIV testing and are not recommended by the CDC. Clinicians should be familiar with their local laws and regulations because some jurisdictions and institutions continue to require such counseling and/or written consent to proceed with testing. ( 25 ) The clinician should make a clear plan with the patient for delivering the test results, including timing (if not using rapid testing), location (usually best to discuss results in person), who else (eg, parents, partners, friends) should or should not be present for discussion of results, and what additional confirmatory testing will be needed if initial test results are positive.
In most cases, the plan for HIV testing of perinatally exposed newborns has been discussed with the mother (and often other caretakers) even before the infant’s birth. However, each clinical encounter with the infant and family is an opportunity to review the HIV testing plans and the interpretation of available results.
In older children for whom HIV testing is indicated (usually those who escaped detection in infancy), the discussion of the purpose and plan for testing will depend on the age of the child and must take into account how to respect the (HIV-infected) mother’s feelings about discussing her own HIV diagnosis with the child and other family members. This process is best handled by an interdisciplinary team (eg, physician, nurse, and social worker) experienced with such situations.
Several assays are available for diagnostic HIV testing ( Table 3 ); assay selection depends on availability, desired turnaround time, age, and suspicion of acute HIV infection.
Diagnostic Assays Used for HIV Testing
EIA=enzyme immunoassay; HIV=human immunodeficiency virus; IFA=immunofluorescent assay; PCR=polymerase chain reaction; WB=Western blot.
See Centers for Disease Control and Prevention ( 26 ) for additional information.
The current standard HIV diagnostic testing for children (age ≥18 months), adolescents, and adults (including pregnant women) relies on detection of HIV antibody in a blood specimen in 2 steps: a screening enzyme-linked immunoassay (EIA) is performed first, and if the result is reactive, a confirmatory HIV antibody test, such as a Western blot, is then performed. Both tests must be positive to meet the criteria for HIV infection. Rapid, point-of-care EIAs are available for detecting HIV antibodies in blood and saliva specimens; negative results reliably exclude established HIV infection and standard EIA tests (none is sensitive for detecting acute HIV infection), but positive test results need to be confirmed with standard HIV diagnostic assays.
Newer fourth-generation HIV testing assays that detect the HIV p24 antigen and both IgM and IgG antibodies to p24 antigen have higher sensitivity, especially for identification of recent HIV infections. ( 26 ) The CDC is anticipated to update their guidelines to recommend use of these fourth-generation assays as the preferred initial test to screen for HIV infection (for those at least age 18 months), and most experts prefer this assay to traditional antibody assays when acute HIV infection is suspected.
Virologic testing includes assays that detect HIV antigens (including the fourth-generation combined antigen-antibody assays discussed above) and those that detect HIV DNA (by polymerase chain reaction [PCR]) or HIV RNA (by PCR and other methods). These assays are important diagnostically for recent or primary HIV infection, when viremia is present but antibody is not, and in infants (up to age 18 months), in whom the presence of passively transferred maternal HIV antibodies requires virologic detection to identify those infants who are infected. The DNA PCR detects intracellular proviral DNA, the result of viral reverse transcriptase transcribing HIV RNA to DNA in the host cell. The HIV DNA PCR test is used almost exclusively for infant diagnosis, although the DNA PCR or RNA assays are equally acceptable for this purpose. Combined antigen-antibody assays should not be used for infant diagnosis. HIV RNA assays and combined antigen-antibody assays are both appropriate for detecting primary HIV infection (beyond age 18 months). Quantitative HIV RNA assays are also routinely used for monitoring of response to ART in HIV-infected people and may be more widely available than the DNA PCR assays.
Primary medical care practitioners for infants should know how to manage the HIV-exposed infant. The components of special care for such infants include ARV prophylaxis, HIV diagnostic testing, evaluation for the need for PCP prophylaxis, routine immunizations, monitoring for manifestations of HIV infection, and reinforcing education and counseling for the mother and family. Detailed, regularly updated guidelines for managing HIV-exposed infants are available at http://www.aidsinfo.nih.gov/guidelines/html/3/perinatal-guidelines/0 .
All HIV-exposed infants should receive zidovudine prophylaxis, started as soon after birth as possible and preferably within 6 to 12 hours of delivery. The usual dose (gestational age ≥35 weeks) is 4 mg/kg orally twice daily and should be given for 6 weeks. Zidovudine dosing is different for preterm infants (gestational age <35 weeks) ( Table 4 ). Zidovudine can also be given intravenously (at a different dose) for newborns initially unable to tolerate oral medications. In addition to zidovudine, newborns at high risk of perinatal infection (those whose mothers did not receive ARV drugs during pregnancy) should be given 3 oral doses of nevirapine beginning as soon as possible after birth (within 48 hours) ( Table 4 ). These drugs are generally well tolerated by infants; anemia and neutropenia related to zidovudine are the most common adverse effects, and these conditions usually resolve within several weeks after discontinuation of zidovudine therapy. Complete blood cell count with differential should be measured at baseline; hematologic parameters should be reassessed at approximately age 4 weeks in infants with hematologic risk factors (such as prematurity or anemia at baseline) or clinical suspicion of anemia. Early discontinuation of infant ARV prophylaxis because of hematologic toxic effects should be undertaken in consultation with a pediatric HIV expert.
Neonatal ARV Drug Dosing for Prevention of Mother-to-Child Transmission of HIV
ARV=antiretroviral; HIV=human immunodeficiency virus.
Adapted from AIDS Info. ( 11 )
Nevirapine dosing given as actual doses not as milligram per kilogram dosing.
In 2013, researchers described an infant with well-documented perinatal HIV infection who received an ART treatment (not prophylaxis) regimen beginning at age 30 hours until shortly after age 1 year who had no evidence of HIV infection after the family discontinued her treatment. This case of apparent resolution of infection after early, intensive ARV treatment has sparked a great deal of interest in the potential for early, multidrug therapy for high-risk newborns to result in functional cure. Until more evidence is available, this approach should be considered experimental, and deviation from standard neonatal prophylaxis regimens should only be undertaken under the guidance of a pediatric HIV expert. The Perinatal HIV Hotline can also be helpful for providing guidance ( http://www.nccc.ucsf.edu/ ).
Virologic testing (HIV DNA PCR or HIV RNA assays) should be performed within the first 14 to 21 days of life, at age 1 to 2 months, and then at age 4 to 6 months for all HIV-exposed infants. Many experts also perform a test in the newborn nursery, especially for high-risk infants (eg, whose mothers did not achieve virologic suppression by the time of delivery). Testing should never be performed on cord blood. HIV can be presumptively excluded (in nonbreastfed infants) on the basis of 2 negative results on virologic tests performed no earlier than age 14 days and at least 1 performed no earlier than age 1 month or based on 1 negative result on a virologic test performed no earlier than age 8 weeks. HIV can be definitively excluded on the basis of 2 negative results on virologic tests both performed no earlier than age 1 month and at least 1 performed no earlier than age 4 months.
For infants whose mothers were diagnosed as having HIV infection during breastfeeding, the recommended infant virologic testing schedule is baseline and then intervals of 4 to 6 weeks, 3 months, and 6 months after recognition of maternal infection and interruption of breastfeeding.
If, at any time, a virologic test result is positive, the infant should be promptly recalled for confirmatory testing and assessment.
All infants with known or possible HIV infection, regardless of CD4 cell count or percentage, should be prescribed PCP prophylaxis beginning at age 6 weeks. The preferred agent for prophylaxis is 2.5 to 5 mg/kg of cotrimoxazole (based on trimethoprim component) per dose given twice daily, usually on 3 days (consecutive or alternating) per week. Infants in whom HIV has been presumptively or definitively excluded by age 6 weeks do not need to start PCP prophylaxis; for infants who do not have virologic test results available by age 6 weeks, PCP prophylaxis should be started and then can be discontinued as soon as virologic testing results demonstrate presumptive or definitive absence of HIV infection.
HIV-exposed infants should receive all of standard immunizations in the first few months of life. ( 23 ) In fact, these infants may have lower levels of protective antibodies passively transferred from their HIV-infected mothers, putting them at higher risk of pneumococcal and other vaccine preventable diseases. ( 27 )( 28 ) Although there is some theoretical concern about administering live rotavirus vaccine to infants with possible HIV infection, this vaccine is still generally recommended based on low likelihood that infants in the United States will be HIV infected and limited data demonstrating it is well tolerated in HIV-infected infants. ( 29 )
Routine health maintenance visits for HIV-exposed infants offer the opportunity to detect growth faltering, abnormal neurodevelopment, and physical examination findings (eg, candidiasis) that may signal the presence of HIV infection. These visits also permit clinicians to assess social support needs for mothers and families, review with parents and caretakers the testing and prophylaxis results and plans for the infant, and reinforce safe infant feeding recommendations. Feeding counseling messages should include complete avoidance of breastfeeding and advice that all HIV-infected adult caretakers should avoid prewarming or prechewing food in their mouths before feeding it to their infants. ( 30 )
As a result of the use of ARV drugs and other prevention strategies, most of the approximately 9,000 infants born annually in the United States to HIV-infected mothers ( 31 ) will escape HIV infection. Most ARV drugs have been used extensively enough in pregnancy to conclude there is little or no increased risk of congenital defects (although the potential teratogenicity of efavirenz continues to be debated). ( 32 ) However, surveillance remains important because new ARV drugs and combinations will be used by pregnant women. ( 33 ) There is also evidence that use of combination ARV regimens in pregnancy, especially those that include protease inhibitors, may increase the risk of preterm birth and lower birth weight. ( 34 ) Longitudinal studies of perinatally ARV-exposed, HIV-uninfected children have been largely reassuring but have raised some concerns about subtle ARV effects on hematologic measures, immune function, growth, language, and neurocognitive outcomes, and effects in many organ systems. ( 35 )( 36 )( 37 )( 38 ) In addition, children may experience long-term adverse effects of problems that are more common in HIV-affected families in the United States (eg, poverty, mental illness, and substance abuse) and of their mothers managing their HIV infection. Clinicians can assist mothers in deciding how and when to disclose their HIV infection to their older children and can encourage mothers to have advance care plans in place in case of sudden, severe illness.
Patients who have positive HIV test results should be referred promptly to an HIV specialist for comprehensive evaluation ( Table 5 ) so the clinical and immunologic stage of disease can be assessed and treatment recommended. The specialist group should be contacted as soon as the positive result is known because immediate initiation of ART may be indicated, especially for infected infants and patients with advanced disease.
Clinical and Laboratory Monitoring of Children Before and After Initiation of ART
ALT=alanine aminotransferase; ART=antiretroviral therapy; AST=aspartate aminotransferase; CBC=complete blood cell; HIV=human immunodeficiency virus.
Adapted from Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children . ( 17 )
For children who are on stable ART, many clinicians consider 6-month intervals between monitoring laboratory tests.
In children receiving nevirapine, serum transaminase levels should be measured every 2 weeks for the first 4 weeks of therapy, then monthly for 3 months, and every 3 to 4 months thereafter.
Some clinicians do not recommend a CD4 cell count or percentage at this time, considering it too early to expect an immunologic response.
Initial evaluation of an HIV-infected infant or child should include the mother’s medical history, child’s medical history, family history, and social history. A comprehensive physical examination should be performed and documented, including a developmental evaluation. Assessment of HIV-infected adolescent patients, as for all adolescents, should include a sexual history, substance use history, and sexual maturity staging.
Initial laboratory testing in an HIV-infected patient should include CD4 percentage and absolute cell counts, plasma quantitative HIV RNA concentration (viral load), HIV genotype to assess for baseline drug resistance mutations, complete blood cell count with differential, serum chemical analyses with liver and renal function tests, a lipid profile, and urinalysis ( Table 5 ). For children younger than 5 years old, CD4 percentage is often used for monitoring immune status because the absolute CD4 cell count in this age group varies with age-related changes in absolute lymphocyte count. Screening for hepatitis B and C infection and tuberculosis is recommended for all HIV-infected patients. In addition, sexually active adolescents should be screened for Chlamydia infection, gonorrhea, syphilis, and human papillomavirus infections. In contrast to the guidelines for cervical cancer screening in healthy women, cervical Papanicolaou smears are indicated routinely in all sexually active, HIV-infected adolescent girls, with colposcopy recommended for evaluation of abnormal results. Similarly, most experts perform anal Papanicolaou smears in HIV-infected adolescent MSM and HIV-infected sexually active women; anoscopy is recommended for evaluation of abnormal results.
HIV infection is a multisystem disease; clinical manifestations range from asymptomatic to complications that affect virtually every organ system ( Table 1 ). The CDC classification system designates clinical stages based on the patient’s medical history and degree of immunosuppression based on CD4 cell count or percentage ( Table 6 and Table 7 ). This information permits an estimated risk of future morbidity and mortality and provides a rationale for instituting specific opportunistic infection (OI) prophylaxis and initiating or deferring ART.
CD4 T-Lymphocyte–Based Assessment of Degree of Immunosuppression in Human Immunodeficiency Virus Infection ( 39 )
Clinical Staging of HIV Infection ( 39 ) a
HIV=human immunodeficiency virus.
Until 2014, CD4-based immunosuppression (none, moderate, severe) was categorized separately from a clinical staging in children (<13 years old): N, no signs or symptoms; A, mild signs or symptoms; B, moderate signs or symptoms; C, severe, AIDS-defining illness.( 40 )
The goals of ART are to maximize the quality and longevity of life through complete suppression of viral replication (goal of nondetectable viral load), preservation or restoration of immunologic function (goal of normal CD4 cell count or percentage), and prevention of or improvement in clinical disease status (goal of asymptomatic state). Additional prevention goals for ART include prevention of MTCT in pregnant women and reduction in sexual transmission for HIV-infected youth who have uninfected sexual partners.
The decision to start ART requires balancing of health benefits of HIV treatment with the potential adverse effects of ART and patient readiness to take daily medications. On the basis of clinical trial evidence indicating that prompt ART initiation in HIV-infected infants markedly reduces risk of death and morbidity, ( 41 ) ART is routinely recommended for all infants (age <12 months). For both prevention of MTCT and maternal health reasons, ART is also routinely recommended for all pregnant women. ART has generally been recommended for children (beyond infancy), adolescents, and adults based on clinical stage of their HIV infection, level of CD4-defined immunodeficiency, and, to a lesser extent, plasma viral load ( Table 8 ). ( 17 )( 42 ) Current US guidelines have moved to recommend ART for all adolescents and adults based on several factors: currently available ARV regimens are simpler, safer, and highly potent; cohort studies suggest clinical benefits even at higher CD4 levels; and treating HIV-infected people markedly reduces HIV transmission to sexual partners. ( 43 ) In fact, some experts advocate for intensive testing accompanied by immediate ART for those who test positive (the test-and-treat approach) as a way to contain the spread of HIV in communities and populations. ( 44 ) Because preadolescent children are not at risk of sexual transmission and have not been found to benefit from ART at higher CD4 cell counts, current guidelines permit but do not strongly recommend ART for children who do not meet clinical and laboratory criteria.
Summary of Recommendations for Starting Antiretroviral Therapy ( 17 )( 38 )
HIV=human immunodeficiency virus; VL=viral load.
The most common ART regimens include 2 nucleoside (or nucleotide) reverse transcriptase inhibitors and one of the following: nonnucleoside reverse transcriptase inhibitor, protease inhibitor, or integrase inhibitor. The preferred and alternative initial ARV drug regimens vary by age, will be altered if baseline ARV drug resistance is detected, are updated frequently (see www.aidsinfo.gov ), and should generally be prescribed by or in collaboration with an HIV specialist, so they will not be detailed here.
ART is generally composed of at least 3 ARV drugs from at least 2 different ARV drug classes. For older children and adults starting ART for the first time, most ARV regimen options can be given once daily, often as a single pill that is a coformulation of 3 ARV drugs. For infants and younger children, there are fewer ARV options, the regimen is given as separate ARV drugs, administration is at least twice daily, and some of the liquid formulations (especially lopinavir-ritonavir) have poor palatability. For patients of all ages, ART efficacy depends on high levels of adherence to the regimen; as frequency of missed ARV doses increases, achieving ART goals becomes less likely and the emergence of drug resistance increases. For ARV drugs (such as efavirenz) in which a single point mutation in the viral genome results in complete drug resistance, resistance emerges quickly with poor adherence; for other drugs (such as most protease inhibitors) that require multiple viral mutations to make the virus resistant, resistance emerges only after longer periods of nonadherence.
Planning treatment collaboratively with the patient and family strengthens the therapeutic relationship and promotes successful adherence and HIV control. Enlisting adult support in the home is beneficial regardless of the patient’s age. Frequent clinical follow-up with viral load testing allows the clinician to identify problems early and help patients and families find successful solutions. Children starting a new ARV regimen should be evaluated in person or by telephone within 1 to 2 weeks of starting ART to screen for adverse effects and to assess adherence. Many clinicians will plan additional contacts (in person or by telephone) with children and caregivers to support adherence during the first few weeks of therapy. Some clinicians also recommend an HIV RNA measurement within the initial weeks of therapy for early assessment of response and adherence to therapy. Patients generally achieve a undetectable viral load within 6 months, although suppression of extremely high viral loads in some infants may take several weeks longer. Failure to achieve undetectable viral load in this time frame strongly suggests suboptimal adherence rather than viral resistance to the ARV regimen. Immediate and intensive adherence counseling and support are warranted because continued nonadherence can allow for development of drug resistance.
Once HIV infection is controlled on a stable regimen, most patients are seen every 3 to 4 months for routine monitoring of viral load, CD4 cell response, and clinical status, including evaluation for potential medication adverse effects or toxic effects ( Table 5 ). For patients having difficulty taking (eg, because of poor palatability) or tolerating (eg, because of adverse effects, such as nausea or diarrhea) one ARV drug in the regimen, substitution of one new ARV drug can be effective. Patients who experience treatment failure with drug resistance will usually be offered a new regimen (change at least 2 of the ARV drugs) based on the resistance patterns as well as robust adherence counseling and support.
Drug-drug interactions among different ARV drugs and between ARV and non-ARV drugs (including nonprescription and herbal medicines) are common, complicated, and potentially dangerous. Interactions can result in excessive toxic effects or loss of efficacy. As part of every clinical encounter, and especially when a new drug will be prescribed, the patient’s complete medication list should be reviewed and confirmed with the patient (and family); potential adverse drug interactions should be evaluated in collaboration with a pharmacist and/or through use of other available resources. ( 45 )( 46 )( 47 )
Effective ART markedly reduces the risk of OIs and improves the protective response elicited by many immunizations. However, regular assessment of need for OI prophylaxis and attention to recommended immunizations remain essential; guidelines for preventing and treating OIs, including immunization recommendations, are updated regularly ( http://aidsinfo.nih.gov/contentfiles/lvguidelines/oi_guidelines_pediatrics.pdf ).
As part of every HIV monitoring visit, patients should be evaluated for indications for OI prophylaxis based on their immunologic (CD4) status, illness history, and exposures. This is especially important for infants, all patients who have not yet started ART, and patients in whom ART fails to result in virologic suppression (most commonly due to nonadherence).
PCP is one of the most common and deadly OIs. Cotrimoxazole is recommended for all HIV-exposed infants until HIV infection is presumptively or definitively excluded, for all HIV-infected infants until age 12 months, and for HIV-infected children and adolescents older than 1 year with CD4 values in the severe immune suppression category. In addition, children who have had PCP prophylaxis should receive cotrimoxazole prophylaxis after PCP treatment, at least until they have sustained improvement of immunologic status on ART.
Mycobacterium avium complex causes disease in patients with even more advanced immunosuppression than the threshold at which PCP occurs. Primary prevention of M avium complex with azithromycin or clarithromycin is thus recommended at lower CD4 values (age ≥6 years with CD4 cell count <50/μL; ages 2 to <6 years with CD4 cell count <75/μL; ages 1 to <2 years with CD4 cell count <500/μL; ages <1 year old with CD4 cell count <750/μL).
As part of every HIV monitoring visit, patients should be evaluated for indicated vaccines. Although this recommendation seems common sense, studies have found that HIV-infected children are at increased risk of not receiving recommended vaccines. ( 48 )( 49 ) The recommended immunization schedule for HIV-infected children and youth is mostly the same as that for HIV-uninfected peers and is presented in Figure 1 and Figure 2 of the OI guidelines. ( 23 ) There are, however, several important exceptions ( Table 9 ).
Summary of How Immunization Recommendations for HIV-Infected Children Differ From Standard Immunization Schedule
ART=antiretroviral therapy; HBsAb=hepatitis B surface antibody; HBV=hepatitis B virus; Hib= Haemophilus influenzae type b; HIV=human immunodeficiency virus; MCV=meningococcal conjugate vaccine; MMR=measles-mumps-rubella; MMR-V=measles-mumps-rubella-varicella; PCV13=13-valent pneumococcal conjugate vaccine; PPSV23=23-valent pneumococcal polysaccharide vaccine.
Although ART markedly reduces the risk of infections due to pneumococcus and other encapsulated bacteria, these infections continue to occur at higher rates in HIV-infected children. As a result, in addition to receiving the standard series of pneumococcal conjugate vaccine in the first 2 years of life, HIV-infected children should also receive the 23-valent pneumococcal polysaccharide vaccine at age 2 years and then 3 to 5 years later. Furthermore, older children (ages 6-18 years) who never received the 13-valent pneumococcal conjugate vaccine should receive one dose. Finally, children who are not fully vaccinated against Haemophilus influenzae type b by age 5 years should receive a single dose of H influenzae type b conjugate vaccine.
HIV-infected children have been found to be less likely to respond to some vaccines. Thus, they should receive a 2-dose primary series of meningococcal conjugate vaccine instead of a single dose. They should also have anti–hepatitis B surface antibody measured 1 to 2 months after completing the HBV vaccine series to confirm a protective response.
Because of the potential for attenuated live vaccines to cause disease in immunocompromised hosts, use of live vaccines is either not recommended or limited to children without severe immune suppression. Limited data demonstrate that live-attenuated intranasal influenza vaccine is safe and immunogenic in HIV-infected children without severe immunosuppression, ( 50 ) but, until more data are available, injectable influenza vaccine rather than live-attenuated intranasal influenza vaccine is recommended for all HIV-infected children. Measles-mumps-rubella (MMR) and varicella vaccines are recommended for HIV-infected children who do not have evidence of severe immunosuppression. The MMR and varicella combination vaccine, however, should not be used because it contains a higher titer of varicella vaccine (than the monovalent varicella vaccine) and has not been studied in HIV-infected children.
Many people in the United States with perinatal HIV infection received their MMR (and most other) vaccines as infants and young children in an era before ART was available. Pre-ART responses to MMR are not as reliable or durable as responses in HIV-infected children receiving ART. On the basis of evidence that high proportions of US youth with perinatal HIV infection lack immunity to MMR and evidence that reimmunization is effective in those who were not immune when MMR vaccination preceded ART, it is recommended that individuals with perinatal HIV infection who were vaccinated before effective ART should receive 2 appropriately spaced doses of MMR vaccine doses once effective cART has been established, unless they have other acceptable current evidence of MMR immunity.
With effective cART, HIV-infected children experience much lower rates of serious illness, can participate in the same activities as their peers, and expect to live long and relatively healthy lives. Whether life expectancy with treated perinatal HIV infection will be the same as that for people without perinatal HIV infection is unknown because the oldest people with perinatal infection are just reaching their 30s.
The pattern of OIs and other illnesses that were typical of untreated HIV infection ( Table 1 ) is uncommon today, although these problems continue to occur in children with unrecognized HIV infection and in children (especially adolescents) who are not able to receive cART reliably. The spectrum of problems seen in children and youth receiving effective cART includes complications of chronic HIV infection itself and residual effects of problems that occurred before cART was initiated, adverse effects of ARV drugs, and comorbidities that are more common in US communities burdened by poverty, mental illness, and substance abuse where perinatal HIV infection is most likely to occur ( Table 10 ).
Complications and Problems in Perinatally Infected Children and Youth Receiving Effective cART
ARV=antiretroviral; cART=combination antiretroviral therapy; HIV=human immunodeficiency virus; NRTI=nucleoside reverse transcriptase inhibitor.
See Chapter 113, Siberry GK and Hazra R. Management of HIV Infection, in Principles and Practice of Pediatric Infectious Diseases , 4 th ed., Long SS, Pickering LK and Prober CG, eds. Elsevier Saunders, 2011, Philadelphia.( 51 )
Learning of a new diagnosis of HIV infection for oneself or one’s child is emotionally devastating for most people. While providing a listening ear and emotional support, clinicians also can offer hope and reassurance about the availability of effective treatment that can result in improved quality of life and survival for people living with HIV infection in the United States.
HIV infection remains a stigmatizing diagnosis. Ignorance, misinformation, and fear in families and communities cause people living with HIV infection to keep their status a secret. However, this practice has negative consequences, such as isolating the HIV-positive individual from social support and risking additional spread of HIV to sexual partners. Planned disclosure to family members and friends can increase practical and emotional support for the HIV-positive person. Sexual partners can make informed decisions about how to protect themselves from exposure to HIV.
In contrast to adolescents and adults, disclosure of HIV status to children should be undertaken over time, providing sequential pieces of practical health information that match the developmental capacity of the child. This process builds a strong foundation for children to participate meaningfully in their HIV care. Most perinatally infected children learn of their HIV diagnosis by name between ages 8 and 10 years.
Most people do not adhere to the treatment recommendations of their health care practitioners all of the time. Infants and young children depend on their adult caretakers for adherence. Developmentally normal behaviors and stages (eg, toddlers and adolescents) can make adherence to medications especially difficult.
Poor adherence leads to poor health outcomes in many diseases, such as asthma and diabetes. However, HIV treatment demands very high levels of adherence to drug regimens to avoid the development of viral resistance and the loss of future efficacy of anti-HIV drugs. The need for intensive education and support for children and adolescents living with HIV infection cannot be overstated.
Children and adolescents who have HIV infection can participate fully in the educational and extracurricular activities in school. There is no obligation to notify school personnel of a student’s HIV infection status. Any sport may be played if the student’s health status allows. For all athletes, regardless of HIV infection status, skin lesions should be covered properly, and athletic personnel should use standard precautions when handling blood or body fluids that have visible blood. Certain high-contact sports (such as wrestling and boxing) may create a situation that favors viral transmission (likely bleeding plus skin breaks). Some experts advise athletes who have a detectable viral load to avoid such high-contact sports.
Children born with HIV infection in the United States during the 1980s are now young adults. They continue to be the pioneers who challenge our assumptions and identify unmet needs for care and support services. There is a pressing need to develop and implement programs to transition youth successfully to adult HIV health care clinicians. ( 52 ) Practical concerns, such as transmitting a complete and coherent medical record, and psychological concerns, such as the loss of long-term supportive relationships, must be addressed.
NOTE: The content of this article is solely the responsibility of the author and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health (NIH). The author is a U.S. government employee who must comply with the NIH Public Access Policy, and the author or NIH will deposit, or have deposited, in the NIH PubMed Central archive, an electronic version of the final, peer-reviewed manuscript to be made publicly available no later than 12 months after the official date of publication.
English: http://www.healthychildren.org/English/health-issues/conditions/sexually-transmitted/Pages/Where-We-Stand-Preventing-Prenatal-Transmission-of-HIV-.aspx
Spanish: http://www.healthychildren.org/spanish/health-issues/conditions/sexually-transmitted/paginas/where-we-stand-preventing-prenatal-transmission-of-hiv-.aspx
English: http://www.healthychildren.org/English/health-issues/conditions/sexually-transmitted/Pages/HIV-Human-Immunodeficiency-Virus.aspx
Spanish: http://www.healthychildren.org/spanish/health-issues/conditions/sexually-transmitted/paginas/hiv-human-immunodeficiency-virus.aspx
antiretroviral therapy
antiretroviral
combination antiretroviral therapy
Centers for Disease Control and Prevention
enzyme-linked immunoassay
human immunodeficiency virus
Mycobacterium avium complex
measles-mumps-rubella
men who have sex with men
maternal-to-child transmission
opportunistic infection
Pneumocystis pneumonia
polymerase chain reaction
sexually transmitted infection
Competing Interests
Advertising Disclaimer »
Citing articles via
Email alerts.


Affiliations
- Editorial Board
- ABP Content Spec Map
- Pediatrics On Call
- Online ISSN 1526-3347
- Print ISSN 0191-9601
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
INTRODUCTION
The case definition and classification of pediatric HIV infection, clinical manifestations of some of the AIDS-defining conditions ( table 1 ), and outcomes of HIV infection in children are reviewed here. The epidemiology of pediatric HIV, prophylactic treatment of infants born to mothers with HIV, diagnostic testing for HIV in young children, approach to febrile HIV-infected infants and children, and issues related to HIV infection in adolescents are discussed separately:
● (See "Epidemiology of pediatric HIV infection" .)
● (See "Intrapartum and postpartum management of pregnant women with HIV and infant prophylaxis in resource-rich settings", section on 'Infant prophylaxis' .)
● (See "Diagnostic testing for HIV infection in infants and children younger than 18 months" .)
- Physician Physician Board Reviews Physician Associate Board Reviews CME Lifetime CME Free CME
- Student USMLE Step 1 USMLE Step 2 USMLE Step 3 COMLEX Level 1 COMLEX Level 2 COMLEX Level 3 96 Medical School Exams Student Resource Center NCLEX - RN NCLEX - LPN/LVN/PN 24 Nursing Exams
- Nurse Practitioner APRN/NP Board Reviews CNS Certification Reviews CE - Nurse Practitioner FREE CE
- Nurse RN Certification Reviews CE - Nurse FREE CE
- Pharmacist Pharmacy Board Exam Prep CE - Pharmacist
- Allied Allied Health Exam Prep Dentist Exams CE - Social Worker CE - Dentist
- Point of Care
- Free CME/CE
Neonatal HIV
Introduction.
Over 95% of HIV-infected pediatric cases are a result of vertical transmission. The pathophysiology of the HIV disease state in the pediatric population is similar to adults. However, differences occur in the clinical presentation, mode of infection, and therapeutic options. The pediatric and neonatal populations have a weaker immune system than adults; therefore, if infected with HIV, they are at a greater risk of opportunistic infections. As such, the delay of treatment may result in a rapid progression of the disease.
One of the greatest advancements in medicine has been the prevention of mother-to-child transmission (MTCT) of HIV type 1 (HIV-1). The rate of transmission of HIV to neonates has been reduced to less than 1% with the implementation of appropriate strategies and careful planning. The increase in comprehensive serologic screening and the treatment of HIV-infected pregnant females has resulted in the reduction of vertical transmission. There are evidence-based prevention modalities that can be utilized at different stages of pregnancy and postpartum to improve outcomes. Antiretroviral therapies (ART) can be prescribed during gestation, antepartum during vaginal or elective cesarean delivery, postnatally to the neonate, or when breastfeeding. [1] [2]
Register For Free And Read The Full Article
Learn more about a subscription to statpearls point-of-care.
HIV is a ribonucleic acid (RNA) viral pathogen with 2 subtypes: HIV-1 and HIV-2. HIV-1 is the most common type worldwide and is more transmissible and progresses faster than HIV-2. Presumably, this Retroviridae family originated from wild chimpanzees in Central Africa. [3] The virus is transmitted across mucous membranes via penetrative unprotected sexual intercourse or intravenous drug use, blood transfusions in developing countries, vertical transmission, or through breastfeeding. [4] [5] The risk of transmission via lactation is about 12-14%, with the risk increasing in high viral load states. [6] [7] Overall, the probability of vertical transmission is about 25% without the utilization of appropriate ART therapy during pregnancy. Several risk factors that increase the chance of this transmission were observed in clinical trials. The risk factors include elevated maternal plasma viral RNA concentrations, maternal breast milk viral load, acute maternal seroconversion, advanced maternal disease, and decreased CD4+ T-cell count of the mother. [8] [9] [10] [11] [12]
Epidemiology
The burden of MTCT is a worldwide epidemic, with an estimated 160,000 infants infected annually with HIV as of 2018. The majority of mothers and neonates infected with HIV are located in sub-Saharan Africa. [13] Overall, the rate of perinatal transmission of HIV has decreased substantially over the past 20 years to less than 1% in the United States and Europe. [14] [15] In the United States, approximately more than 5,000 pregnant females are HIV positive. [16] In the year 2013, nationwide in the United States, there were only 69 infants born with HIV infection, leading to an estimated incidence of 1.8 out of 100,000 live births for perinatally-acquired HIV infection. [14] The Centers for Disease Control and Prevention (CDC) in the United States (US) has set goals to eliminate perinatal HIV spread, which has caused a significant decline in MTCT transmission. The goal is to reduce the incidence of perinatal HIV to less than 1 in 100,000 births. [14] During the peak of HIV transmission in 1991, the reported incidence of neonates born with HIV was 42.8 per 100,000 births, with a substantial decline to 1.3 per 100,000 live-born infants in 2015. [17] Due to racial disparities in healthcare, the incidence of perinatal HIV is 5 times greater in Black versus White infants. [18]
Pathophysiology
The main target for HIV entry into the cells is through infection of cells expressing the CD4 receptor and chemokine receptors CCR5 and CXCR4. [19] Additionally, the HIV virus infects dendritic cells, activated CD4 T-lymphocytes, monocytes, and macrophages. [20] The result is increased host susceptibility to diseases due to decreased immune-protective functions.
Infants with HIV-1 infections have higher viral loads and a faster progression to AIDS than adults with HIV. [21] [22] The most common mode of transmission in a neonate with HIV is mother-to-child transmission (MTCT). The virus may be transmitted during different stages of pregnancy and postpartum, with the perinatal period as the most common transmission time. [23]
In-utero Transmission
The mechanism of in-utero transmission is predicted to be by transcytosis across placental cells. The placenta may also host the virus to replicate before moving to the fetus. [24] The HIV-1 virus may also traverse the trophoblastic placental barrier via endocytosis, specifically crossing cytotrophoblasts or syncytiotrophoblasts within the uterine wall. HIV-1 may also spread to the fetus via villous capillaries. The risk of in-utero transmission increases with inflammation and infection of the placenta and amniotic membranes. [24] [25] [26] [27] [28]
Intrapartum Transmission
Intrapartum transmission is predicted to be the greatest risk of vertical infections. The risk increases with longer exposure to maternal cervicovaginal secretions and blood. Research also demonstrates that the chance of infection is greater with membrane rupture of more than 4 hours. [29] Moreover, data also demonstrates that neonates with low birth weights and those born prematurely have an increased rate of transmission due to their reduced immunologic defenses and weaker skin barrier. [30]
Postnatal MTCT
Postnatal MTCT occurs during breastfeeding. The mechanism of transmission through breast milk is not fully understood. However, multiple large prospective cohort trials have demonstrated a greater risk of spread of the HIV virus with breast-feeding. In addition to breast milk, studies have also confirmed that HIV RNA can also be found in colostrum. [31] [32] Potential entry of the HIV virus from breast milk to the infant is through their intestines or tonsillar tissues. [33] [34] [35] [36]
Histopathology
Due to the nature of the disease state, early identification of HIV may be difficult due to subtle clinical symptoms. As such, the use of histopathology of tissue samples may help identify HIV in patients. The capsid size of the HIV-1 virus varies between 110 and 146 nm. [37] It can be visualized with structured illumination microscopy (SIM). It is almost impossible to visualize individual virions using confocal microscopy. Assessing the histopathology of the placenta may identify the presence of intrauterine HIV infection. Multiple studies have demonstrated that full-term placenta from HIV-1–positive females contained infection in syncytiotrophoblasts, cytotrophoblasts, and villous-endothelial cells. [38] In vitro, studies of trophoblast barriers have demonstrated that the direct interaction between the trophoblast barrier and HIV-1 infected cells resulted in viral transcytosis. [24]
History and Physical
Neonates may not display any symptoms for the initial few months of life, as such complicating the diagnosis of HIV. Studies have suggested that children may remain asymptomatic until 3-5 years of age. In untreated children, the most commonly exhibited manifestations of HIV infection include but are not limited to recurrent bacteremia, increased opportunistic infections, frequent diarrhea, cardiomyopathy, hepatitis, generalized lymphadenopathy, splenomegaly, hepatomegaly, oral candidiasis, cancers, and central nervous system manifestations, such as growth delay, delayed cognition, low IQ, and frequently global developmental delay.
The CDC strongly recommends testing all pregnant females for HIV as part of the standard prenatal care. This testing proves to have a better prognosis for the neonate. However, due to a lack of adequate healthcare access in certain geographical areas of the world, the unknown HIV status of pregnant females leads to inadequate treatment and poor outcomes for the neonate. [39] [40] Females who have an unknown HIV status should be offered a rapid diagnostic test at the time of delivery. A definitive HIV diagnosis can be made in infants by the age of 4 to 6 months using virologic testing.
Neonatal HIV diagnostics differs from that of adults and older children. It is not appropriate to test for HIV antibodies. The utilization of novel combination antigen/antibody immunoassays to confirm the diagnosis of HIV in neonates is not recommended as positive results confer passive transfer of maternal antibodies. Maternal HIV antibodies persist until 18 months. [5] [40] Using viral load assays or nucleic acid tests (NATS), which include qualitative RNA assays, quantitative HIV RNA assay, or DNA polymerase chain reaction (PCR) assays, is more appropriate to confirm the diagnosis of HIV in neonates. The only FDA-approved qualitative RNA test is the APTIMA HIV-1 RNA Qualitative Assay. [41] These assays are able to detect the virus in at least 30% to 50% of cases at birth and an almost 100% confirmation by the age of 4 to 6 months. HIV quantitative RNA assay has been found to be just as comparable to HIV DNA PCR, with 100% specificity at birth, 1 month, 3 months, and 6 months. [42] Two negative virologic tests completed at 1 month and before 6 months of age are required to definitely exclude the diagnosis of HIV. Additionally, the infant must have negative clinical evidence and other laboratory markers of HIV, including normal to high CD4 T-lymphocyte count. [5] [43]
Infants are categorized as high or low risk for HIV infection. Neonates born to mothers who received adequate prenatal care and were adherent to their ART, and who had undetectable viral loads are considered low risk. On the contrary, neonates are considered high risk if they were born to mothers who lacked prenatal care, had elevated HIV viral loads, and had a new diagnosis of HIV infection while pregnant. [5]
The table below indicates (X) the proposed recommended testing schedule for HIV perinatal exposure. [43] [40]
After a confirmed diagnosis of HIV, additional labs should be ordered, including CD4+ T-cell count, CD8+ T-cell count, plasma viral load of RNA, growth or development factors, and HIV-associated conditions, such as anemia, leukopenia, thrombocytopenia, hepatic transaminitis, etc. Before initiating ART, obtain genetic testing, a baseline CD4 count, plasma viral load, complete blood count (CBC), hepatic function, renal function, comprehensive metabolic panel, urinalysis, serum lipids, and blood glucose.
Treatment / Management
The Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV strongly recommends the initiation of ART in all pediatrics with HIV. [44] There has been a significant 80% to 90% decrease in morbidity and mortality since the introduction of ART initiation in neonates. [45] [46] As confirmed by the CHER trial and other studies, there is a decrease in viral reservoirs, opportunistic infections, and disease progression to AIDS with early initiation of effective and early ART. [47] [48] [49] [50] [51] [52] Infants with any level of risk of exposure to HIV should be started on the appropriate ART within 6 hours of birth. The goals of treatment for HIV-exposed neonates include a reduction in morbidity and mortality, suppression of viral replication, facilitation of HIV remission, viral control, prevention of disease progression, maintenance of immunologic function, reduction of opportunistic infections, and prevention of drug resistance. [53] [54] (A1)
There aren’t many randomized control trials that compare different regimens in pediatrics and neonates, and the available literature is variable. Most of the data is extracted from non-randomized studies, pharmacokinetic trials, and phase 1 or 2 of drug trials. In general, the initiation of an antiretroviral regimen in pediatrics should include 2 nucleoside reverse transcriptase inhibitors (NRTIs) with an additional drug from another class, including an integrase strand transfer inhibitor (INSTI), a protease inhibitor (PI) with a booster, or a non-nucleoside reverse transcriptase inhibitor (NNRTI). Before initiating a regimen, Factors to consider include the patient’s age, weight, family preference, drug resistance, genetic testing, mutation testing, and sexual maturity rating (SMR). In children with other confections, such as hepatitis B virus (HBV), the choice of agent should include coverage for HIV and the co-contagion.
There are 3 studies that compared the addition of a PI-boosted versus NNRTI to the 2 NRTI backbones. In the P1060 trial, a total of 288 children from 6 African countries and India with ages from 2 to 36 months were enrolled in a randomized trial. The children received zidovudine (ZDV) plus lamivudine as the NRTI backbone and were randomized to either the PI with booster group (ritonavir booster [LPV/r]) or NNRTI group (nevirapine [NVP]). The data demonstrated that LPV/r is superior to NVP in NVP-naive children; however, there were limitations. [55] Whereas the PROMOTE trial did not find any differences between the 2 groups. [56] Of note, LPV/r should be avoided in neonates before 42 weeks of age and those who are younger than 14 days. (A1)
Data for utilizing an INSTI-based regimen are extracted from safety trials and adult comparative trials. Four INSTIs are approved for the treatment of ART-naïve children with HIV, which include: bictegravir (BID), dolutegrevir (DTG), Elvitegravir/cobicistat (EVG/c), and raltegravir (RAL). [57] INSTI regimens are attractive due to their lack of drug interactions, low toxicity, and virologic efficacy. RAL is FDA-approved for neonates and infants weighing 2 kg or more. DTG is FDA-approved for children 30 kg or more, and BIC is approved for children weighing 25 kg or more. [58] [59]
Zidovudine (ZDV) plus lamivudine (3TC) or emtricitabine (FTC) are the preferred dual NRTI backbone in neonates and infants under 3 months. ZDV is FDA-approved for prophylaxis and for HIV treatment initiation in infants ≥ 4 weeks of age. [60] [61] [62] [63] The preferred regimen for infants 3 months and older is abacavir (ABC) plus 3TC or FTC. [64] [65] [66] [67] Alternatively, ZDV plus ABC can be used in infants 3 months and older; however, European studies have demonstrated lower rates of viral suppression and increased toxicity with this combination. [65] [68] In addition to the 2 NRTI backbones, the following combination regimens are preferred in each age group: (A1)
- NVP: Age under 14 days
- RAL: Age under 14 days and a weight of 2 kg or more
- LPV/r or RAL (alternative: NVP): Age 14 days or older to 3 years
Differential Diagnosis
There are other diseases that need exclusion when diagnosing HIV. These include malnutrition, lymphadenopathy, pediatric chronic anemia, malabsorption syndrome, constitutional growth delay, autoimmune and chronic benign neutropenia, and other immunodeficiencies. Furthermore, the clinician should also look for other congenital co-infections, including syphilis, TORCH infections (Toxoplasmosis, Rubella, Cytomegalovirus, herpes simplex virus), hepatitis B, hepatitis C, or tuberculosis infection.
Toxicity and Adverse Effect Management
Any ART is associated with a variety of side effects. Many ARTs result in increased levels of hepatic transaminases as a result of hepatitis. Baseline labs are recommended before initiation of any regimen.
- Zidovudine: Can induce leukopenia, anemia, and macrocytosis
- Protease inhibitors: May lead to hyperglycemia
- Atazanavir: Can cause hyperbilirubinemia.
If untreated, HIV can increase the rate of morbidity and mortality. However, due to the advancement of ART, increased monitoring, and data from clinical trials, pediatric and adult patients have better prognoses and outcomes. The average survival rate is about 10 years of age, with approximately 15% of children having a rapid progression of the disease. The clinician should collaborate with the patient to optimize their nutrition, control viral replication, initiate aggressive treatment for opportunistic infections, and decrease social stressors. The risk of complications is greater with co-infections and hematological disturbances, such as anemia, thrombocytopenia, and neutropenia.
Complications
Complications of HIV infection in neonates and pediatric populations occur as a result of their immunocompromised status. They are at greater risk for opportunistic infections, candida esophagitis, Pneumocystis jirovecii pneumonia, and cancers. Furthermore, complications are more likely to occur with antiretroviral drug resistance. However, with careful monitoring and drug-resistance testing, the ability to select more optimized and effective regimens is possible.
Consultations
Consultation with a perinatologist and a pediatric infectious disease consultant is strongly encouraged to help provide a more comprehensive workup, diagnosis, and ongoing monitoring and management.
Deterrence and Patient Education
Before initiating or altering ART, the clinician should identify potential barriers and compliance issues. Developing novel drugs and enhanced formulations has led to better medication tolerability, less toxicity, and increased adherence.
HIV-positive mothers should be discouraged from breastfeeding neonates who do not have a confirmed HIV-positive status. If a female continues to breastfeed, the infant should be monitored and tested every 3 months throughout breastfeeding and postdiscontinuation of breastfeeding at the interval of 4 to 6 weeks, 3 months, and at 6 months. [69] [70] Mothers should also be warned about the risks of feeding premasticated food to the infant. [71] [72] [73]
Pearls and Other Issues
Key facts to keep in mind about neonatal HIV are as follows:
- When making a selection for appropriate ART to initiate in a pregnant female, it is important to consider tolerability, neonatal risk of exposure, pharmacokinetic differences, and overall risk-benefit of each regimen.
- The monitoring of infants with HIV is challenging as there is variability with viral loads and CD4 counts depending on the age.
- Studies have demonstrated that administering zidovudine (ZDV) monotherapy to both the mother and neonate reduces MTCT from 25% to 8%. The MTCT rate is reduced to less than 1% when combined with other ART. [7] ZDV exhibits its actions by metabolizing into its active form in the placenta, thus inhibiting the replication of HIV within the placental cells.
- Repeated negative HIV test results are needed postpartum due to the increased risk of transmission of HIV during labor and delivery. [5]
Enhancing Healthcare Team Outcomes
The treatment of perinatal HIV exposure involves a team approach involving an infectious disease specialist, perinatalist, pediatrician, neonatologist, obstetrician, HIV pharmacist, and nursing staff. Prompt and early communication between all team members assures comprehensive and optimized care for the neonate. Infectious disease specialists and neonatologists are usually involved in acute management during the neonatal period. Infectious disease specialists are responsible for monitoring disease progression and drug regimens.
. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2013 Jun:(): [PubMed PMID: 24716260]
Hurst SA, Appelgren KE, Kourtis AP. Prevention of mother-to-child transmission of HIV type 1: the role of neonatal and infant prophylaxis. Expert review of anti-infective therapy. 2015 Feb:13(2):169-81. doi: 10.1586/14787210.2015.999667. Epub [PubMed PMID: 25578882]
Worobey M, Gemmel M, Teuwen DE, Haselkorn T, Kunstman K, Bunce M, Muyembe JJ, Kabongo JM, Kalengayi RM, Van Marck E, Gilbert MT, Wolinsky SM. Direct evidence of extensive diversity of HIV-1 in Kinshasa by 1960. Nature. 2008 Oct 2:455(7213):661-4. doi: 10.1038/nature07390. Epub [PubMed PMID: 18833279]
Mofenson LM, Mother-child HIV-1 transmission: Timing and determinants. Obstetrics and gynecology clinics of North America. 1997 Dec; [PubMed PMID: 9430166]
Siberry GK. Preventing and managing HIV infection in infants, children, and adolescents in the United States. Pediatrics in review. 2014 Jul:35(7):268-86. doi: 10.1542/pir.35-7-268. Epub [PubMed PMID: 24986927]
Bispo S, Chikhungu L, Rollins N, Siegfried N, Newell ML. Postnatal HIV transmission in breastfed infants of HIV-infected women on ART: a systematic review and meta-analysis. Journal of the International AIDS Society. 2017 Feb 22:20(1):21251. doi: 10.7448/IAS.20.1.21251. Epub [PubMed PMID: 28362072]
Bansaccal N, Van der Linden D, Marot JC, Belkhir L. HIV-Infected Mothers Who Decide to Breastfeed Their Infants Under Close Supervision in Belgium: About Two Cases. Frontiers in pediatrics. 2020:8():248. doi: 10.3389/fped.2020.00248. Epub 2020 May 27 [PubMed PMID: 32537442]
Connor EM,Sperling RS,Gelber R,Kiselev P,Scott G,O'Sullivan MJ,VanDyke R,Bey M,Shearer W,Jacobson RL, Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. The New England journal of medicine. 1994 Nov 3; [PubMed PMID: 7935654]
Shaffer N, Chuachoowong R, Mock PA, Bhadrakom C, Siriwasin W, Young NL, Chotpitayasunondh T, Chearskul S, Roongpisuthipong A, Chinayon P, Karon J, Mastro TD, Simonds RJ. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet (London, England). 1999 Mar 6:353(9155):773-80 [PubMed PMID: 10459957]
Jamieson DJ, Sibailly TS, Sadek R, Roels TH, Ekpini ER, Boni-Ouattara E, Karon JM, Nkengasong J, Greenberg AE, Wiktor SZ. HIV-1 viral load and other risk factors for mother-to-child transmission of HIV-1 in a breast-feeding population in Cote d'Ivoire. Journal of acquired immune deficiency syndromes (1999). 2003 Dec 1:34(4):430-6 [PubMed PMID: 14615662]
Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, Allen M, Nakabiito C, Sherman J, Bakaki P, Owor M, Ducar C, Deseyve M, Mwatha A, Emel L, Duefield C, Mirochnick M, Fowler MG, Mofenson L, Miotti P, Gigliotti M, Bray D, Mmiro F. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet (London, England). 2003 Sep 13:362(9387):859-68 [PubMed PMID: 13678973]
John GC, Nduati RW, Mbori-Ngacha DA, Richardson BA, Panteleeff D, Mwatha A, Overbaugh J, Bwayo J, Ndinya-Achola JO, Kreiss JK. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. The Journal of infectious diseases. 2001 Jan 15:183(2):206-212 [PubMed PMID: 11120927]
Kempton J, Hill A, Levi JA, Heath K, Pozniak A. Most new HIV infections, vertical transmissions and AIDS-related deaths occur in lower-prevalence countries. Journal of virus eradication. 2019 Apr 1:5(2):92-101 [PubMed PMID: 31191912]
Nesheim SR, Wiener J, Fitz Harris LF, Lampe MA, Weidle PJ. Brief Report: Estimated Incidence of Perinatally Acquired HIV Infection in the United States, 1978-2013. Journal of acquired immune deficiency syndromes (1999). 2017 Dec 15:76(5):461-464. doi: 10.1097/QAI.0000000000001552. Epub [PubMed PMID: 28991886]
Peters H, Francis K, Sconza R, Horn A, S Peckham C, Tookey PA, Thorne C. UK Mother-to-Child HIV Transmission Rates Continue to Decline: 2012-2014. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Feb 15:64(4):527-528. doi: 10.1093/cid/ciw791. Epub [PubMed PMID: 28174911]
Nesheim SR,FitzHarris LF,Lampe MA,Gray KM, Reconsidering the Number of Women With HIV Infection Who Give Birth Annually in the United States. Public health reports (Washington, D.C. : 1974). 2018 Nov; [PubMed PMID: 30265616]
Peters H, Thorne C, Tookey PA, Byrne L. National audit of perinatal HIV infections in the UK, 2006-2013: what lessons can be learnt? HIV medicine. 2018 Apr:19(4):280-289. doi: 10.1111/hiv.12577. Epub 2018 Jan 16 [PubMed PMID: 29336508]
Nesheim SR, FitzHarris LF, Mahle Gray K, Lampe MA. Epidemiology of Perinatal HIV Transmission in the United States in the Era of Its Elimination. The Pediatric infectious disease journal. 2019 Jun:38(6):611-616. doi: 10.1097/INF.0000000000002290. Epub [PubMed PMID: 30724833]
Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet (London, England). 2014 Jul 19:384(9939):258-71. doi: 10.1016/S0140-6736(14)60164-1. Epub 2014 Jun 5 [PubMed PMID: 24907868]
Lucas S,Nelson AM, HIV and the spectrum of human disease. The Journal of pathology. 2015 Jan; [PubMed PMID: 25251832]
Ahmad N. Molecular mechanisms of HIV-1 mother-to-child transmission and infection in neonatal target cells. Life sciences. 2011 May 23:88(21-22):980-6. doi: 10.1016/j.lfs.2010.09.023. Epub 2010 Oct 1 [PubMed PMID: 20888841]
Little K, Thorne C, Luo C, Bunders M, Ngongo N, McDermott P, Newell ML. Disease progression in children with vertically-acquired HIV infection in sub-Saharan Africa: reviewing the need for HIV treatment. Current HIV research. 2007 Mar:5(2):139-53 [PubMed PMID: 17346131]
Saharan S, Lodha R, Agarwal R, Deorari AK, Paul VK. Perinatal HIV. Indian journal of pediatrics. 2008 Apr:75(4):359-62. doi: 10.1007/s12098-008-0039-0. Epub 2008 May 18 [PubMed PMID: 18536891]
Al-Husaini AM, Role of placenta in the vertical transmission of human immunodeficiency virus. Journal of perinatology : official journal of the California Perinatal Association. 2009 May; [PubMed PMID: 19020526]
Ehrnst A, Lindgren S, Dictor M, Johansson B, Sönnerborg A, Czajkowski J, Sundin G, Bohlin AB. HIV in pregnant women and their offspring: evidence for late transmission. Lancet (London, England). 1991 Jul 27:338(8761):203-7 [PubMed PMID: 1676777]
Rouzioux C, Costagliola D, Burgard M, Blanche S, Mayaux MJ, Griscelli C, Valleron AJ. Estimated timing of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission by use of a Markov model. The HIV Infection in Newborns French Collaborative Study Group. American journal of epidemiology. 1995 Dec 15:142(12):1330-7 [PubMed PMID: 7503054]
Bornstein MH. Qualities of color vision in infancy. Journal of experimental child psychology. 1975 Jun:19(3):401-19 [PubMed PMID: 1176886]
King CC,Ellington SR,Kourtis AP, The role of co-infections in mother-to-child transmission of HIV. Current HIV research. 2013 Jan [PubMed PMID: 23305198]
Landesman SH, Kalish LA, Burns DN, Minkoff H, Fox HE, Zorrilla C, Garcia P, Fowler MG, Mofenson L, Tuomala R. Obstetrical factors and the transmission of human immunodeficiency virus type 1 from mother to child. The Women and Infants Transmission Study. The New England journal of medicine. 1996 Jun 20:334(25):1617-23 [PubMed PMID: 8628356]
Kourtis AP, Bulterys M, Nesheim SR, Lee FK. Understanding the timing of HIV transmission from mother to infant. JAMA. 2001 Feb 14:285(6):709-12 [PubMed PMID: 11176886]
Van de Perre P, Simonon A, Msellati P, Hitimana DG, Vaira D, Bazubagira A, Van Goethem C, Stevens AM, Karita E, Sondag-Thull D. Postnatal transmission of human immunodeficiency virus type 1 from mother to infant. A prospective cohort study in Kigali, Rwanda. The New England journal of medicine. 1991 Aug 29:325(9):593-8 [PubMed PMID: 1812850]
Van de Perre P,Lepage P,Homsy J,Dabis F, Mother-to-infant transmission of human immunodeficiency virus by breast milk: presumed innocent or presumed guilty? Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1992 Sep [PubMed PMID: 1445596]
Lehman DA, Farquhar C. Biological mechanisms of vertical human immunodeficiency virus (HIV-1) transmission. Reviews in medical virology. 2007 Nov-Dec:17(6):381-403 [PubMed PMID: 17542053]
Shen R, Smythies LE, Clements RH, Novak L, Smith PD. Dendritic cells transmit HIV-1 through human small intestinal mucosa. Journal of leukocyte biology. 2010 Apr:87(4):663-70. doi: 10.1189/jlb.0909605. Epub 2009 Dec 9 [PubMed PMID: 20007245]
Veazey R, Lackner A. The mucosal immune system and HIV-1 infection. AIDS reviews. 2003 Oct-Dec:5(4):245-52 [PubMed PMID: 15012003]
Lapenta C,Boirivant M,Marini M,Santini SM,Logozzi M,Viora M,Belardelli F,Fais S, Human intestinal lamina propria lymphocytes are naturally permissive to HIV-1 infection. European journal of immunology. 1999 Apr [PubMed PMID: 10229087]
Marno K, Al'Zoubi L, Pearson M, Posch M, McKnight Á, Wheeler AP. The evolution of structured illumination microscopy in studies of HIV. Methods (San Diego, Calif.). 2015 Oct 15:88():20-7. doi: 10.1016/j.ymeth.2015.06.007. Epub 2015 Jun 10 [PubMed PMID: 26071977]
Moonim MT, Alarcon L, Freeman J, Mahadeva U, van der Walt JD, Lucas SB. Identifying HIV infection in diagnostic histopathology tissue samples--the role of HIV-1 p24 immunohistochemistry in identifying clinically unsuspected HIV infection: a 3-year analysis. Histopathology. 2010 Mar:56(4):530-41. doi: 10.1111/j.1365-2559.2010.03513.x. Epub [PubMed PMID: 20459560]
Gilleece DY, Tariq DS, Bamford DA, Bhagani DS, Byrne DL, Clarke DE, Clayden MP, Lyall DH, Metcalfe DR, Palfreeman DA, Rubinstein DL, Sonecha MS, Thorley DL, Tookey DP, Tosswill MJ, Utting MD, Welch DS, Wright MA. British HIV Association guidelines for the management of HIV in pregnancy and postpartum 2018. HIV medicine. 2019 Mar:20 Suppl 3():s2-s85. doi: 10.1111/hiv.12720. Epub [PubMed PMID: 30869192]
Abdollahi A,Saffar H, The Diagnosis of HIV Infection in Infants and Children. Iranian journal of pathology. 2016 Spring [PubMed PMID: 27499768]
Lilian RR, Kalk E, Bhowan K, Berrie L, Carmona S, Technau K, Sherman GG. Early diagnosis of in utero and intrapartum HIV infection in infants prior to 6 weeks of age. Journal of clinical microbiology. 2012 Jul:50(7):2373-7. doi: 10.1128/JCM.00431-12. Epub 2012 Apr 18 [PubMed PMID: 22518871]
Burgard M, Blanche S, Jasseron C, Descamps P, Allemon MC, Ciraru-Vigneron N, Floch C, Heller-Roussin B, Lachassinne E, Mazy F, Warszawski J, Rouzioux C, Agence Nationale de Recherche sur le SIDA et les Hepatites virales French Perinatal Cohort. Performance of HIV-1 DNA or HIV-1 RNA tests for early diagnosis of perinatal HIV-1 infection during anti-retroviral prophylaxis. The Journal of pediatrics. 2012 Jan:160(1):60-6.e1. doi: 10.1016/j.jpeds.2011.06.053. Epub 2011 Aug 24 [PubMed PMID: 21868029]
Havens PL, Mofenson LM, American Academy of Pediatrics Committee on Pediatric AIDS. Evaluation and management of the infant exposed to HIV-1 in the United States. Pediatrics. 2009 Jan:123(1):175-87. doi: 10.1542/peds.2008-3076. Epub [PubMed PMID: 19117880]
Foster C,Bamford A,Turkova A,Welch S,Klein N, Paediatric European Network for Treatment of AIDS Treatment Guideline 2016 update: antiretroviral therapy recommended for all children living with HIV. HIV medicine. 2017 Feb [PubMed PMID: 27385585]
Kapogiannis BG, Soe MM, Nesheim SR, Abrams EJ, Carter RJ, Farley J, Palumbo P, Koenig LJ, Bulterys M. Mortality trends in the US Perinatal AIDS Collaborative Transmission Study (1986-2004). Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011 Nov:53(10):1024-34. doi: 10.1093/cid/cir641. Epub [PubMed PMID: 22002982]
Mirani G, Williams PL, Chernoff M, Abzug MJ, Levin MJ, Seage GR 3rd, Oleske JM, Purswani MU, Hazra R, Traite S, Zimmer B, Van Dyke RB, IMPAACT P1074 Study Team. Changing Trends in Complications and Mortality Rates Among US Youth and Young Adults With HIV Infection in the Era of Combination Antiretroviral Therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015 Dec 15:61(12):1850-61. doi: 10.1093/cid/civ687. Epub 2015 Aug 12 [PubMed PMID: 26270680]
Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, Jean-Philippe P, McIntyre JA, CHER Study Team. Early antiretroviral therapy and mortality among HIV-infected infants. The New England journal of medicine. 2008 Nov 20:359(21):2233-44. doi: 10.1056/NEJMoa0800971. Epub [PubMed PMID: 19020325]
Kuhn L,Paximadis M,Da Costa Dias B,Loubser S,Strehlau R,Patel F,Shiau S,Coovadia A,Abrams EJ,Tiemessen CT, Age at antiretroviral therapy initiation and cell-associated HIV-1 DNA levels in HIV-1-infected children. PloS one. 2018 [PubMed PMID: 29649264]
Tagarro A, Chan M, Zangari P, Ferns B, Foster C, De Rossi A, Nastouli E, Muñoz-Fernández MA, Gibb D, Rossi P, Giaquinto C, Babiker A, Fortuny C, Freguja R, Cotugno N, Judd A, Noguera-Julian A, Navarro ML, Mellado MJ, Klein N, Palma P, Rojo P. Early and Highly Suppressive Antiretroviral Therapy Are Main Factors Associated With Low Viral Reservoir in European Perinatally HIV-Infected Children. Journal of acquired immune deficiency syndromes (1999). 2018 Oct 1:79(2):269-276. doi: 10.1097/QAI.0000000000001789. Epub [PubMed PMID: 30211778]
Nesheim S, Taylor A, Lampe MA, Kilmarx PH, Fitz Harris L, Whitmore S, Griffith J, Thomas-Proctor M, Fenton K, Mermin J. A framework for elimination of perinatal transmission of HIV in the United States. Pediatrics. 2012 Oct:130(4):738-44. doi: 10.1542/peds.2012-0194. Epub 2012 Sep 3 [PubMed PMID: 22945404]
Centers for Disease Control and Prevention (CDC). Revised surveillance case definition for HIV infection--United States, 2014. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2014 Apr 11:63(RR-03):1-10 [PubMed PMID: 24717910]
Flynn PM,Abrams EJ, Growing up with perinatal HIV. AIDS (London, England). 2019 Mar 15 [PubMed PMID: 30531318]
Kuhn L, Shiau S. The pharmacological treatment of acute HIV infections in neonates. Expert review of clinical pharmacology. 2017 Dec:10(12):1353-1361. doi: 10.1080/17512433.2017.1398645. Epub 2017 Nov 3 [PubMed PMID: 29098898]
Mofenson LM, Brady MT, Danner SP, Dominguez KL, Hazra R, Handelsman E, Havens P, Nesheim S, Read JS, Serchuck L, Van Dyke R, Centers for Disease Control and Prevention, National Institutes of Health, HIV Medicine Association of the Infectious Diseases Society of America, Pediatric Infectious Diseases Society, American Academy of Pediatrics. Guidelines for the Prevention and Treatment of Opportunistic Infections among HIV-exposed and HIV-infected children: recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2009 Sep 4:58(RR-11):1-166 [PubMed PMID: 19730409]
Violari A, Lindsey JC, Hughes MD, Mujuru HA, Barlow-Mosha L, Kamthunzi P, Chi BH, Cotton MF, Moultrie H, Khadse S, Schimana W, Bobat R, Purdue L, Eshleman SH, Abrams EJ, Millar L, Petzold E, Mofenson LM, Jean-Philippe P, Palumbo P. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. The New England journal of medicine. 2012 Jun 21:366(25):2380-9. doi: 10.1056/NEJMoa1113249. Epub [PubMed PMID: 22716976]
Ruel TD, Kakuru A, Ikilezi G, Mwangwa F, Dorsey G, Rosenthal PJ, Charlebois E, Havlir D, Kamya M, Achan J. Virologic and immunologic outcomes of HIV-infected Ugandan children randomized to lopinavir/ritonavir or nonnucleoside reverse transcriptase inhibitor therapy. Journal of acquired immune deficiency syndromes (1999). 2014 Apr 15:65(5):535-41. doi: 10.1097/QAI.0000000000000071. Epub [PubMed PMID: 24326597]
Tsiang M,Jones GS,Goldsmith J,Mulato A,Hansen D,Kan E,Tsai L,Bam RA,Stepan G,Stray KM,Niedziela-Majka A,Yant SR,Yu H,Kukolj G,Cihlar T,Lazerwith SE,White KL,Jin H, Antiviral Activity of Bictegravir (GS-9883), a Novel Potent HIV-1 Integrase Strand Transfer Inhibitor with an Improved Resistance Profile. Antimicrobial agents and chemotherapy. 2016 Dec [PubMed PMID: 27645238]
Oliveira M, Ibanescu RI, Anstett K, Mésplède T, Routy JP, Robbins MA, Brenner BG, Montreal Primary HIV (PHI) Cohort Study Group. Selective resistance profiles emerging in patient-derived clinical isolates with cabotegravir, bictegravir, dolutegravir, and elvitegravir. Retrovirology. 2018 Aug 17:15(1):56. doi: 10.1186/s12977-018-0440-3. Epub 2018 Aug 17 [PubMed PMID: 30119633]
Neogi U, Singh K, Aralaguppe SG, Rogers LC, Njenda DT, Sarafianos SG, Hejdeman B, Sönnerborg A. Ex-vivo antiretroviral potency of newer integrase strand transfer inhibitors cabotegravir and bictegravir in HIV type 1 non-B subtypes. AIDS (London, England). 2018 Feb 20:32(4):469-476. doi: 10.1097/QAD.0000000000001726. Epub [PubMed PMID: 29239896]
Mulenga V, Musiime V, Kekitiinwa A, Cook AD, Abongomera G, Kenny J, Chabala C, Mirembe G, Asiimwe A, Owen-Powell E, Burger D, McIlleron H, Klein N, Chintu C, Thomason MJ, Kityo C, Walker AS, Gibb DM, CHAPAS-3 trial team. Abacavir, zidovudine, or stavudine as paediatric tablets for African HIV-infected children (CHAPAS-3): an open-label, parallel-group, randomised controlled trial. The Lancet. Infectious diseases. 2016 Feb:16(2):169-79. doi: 10.1016/S1473-3099(15)00319-9. Epub 2015 Oct 5 [PubMed PMID: 26481928]
Van Dyke RB,Wang L,Williams PL, Toxicities associated with dual nucleoside reverse-transcriptase inhibitor regimens in HIV-infected children. The Journal of infectious diseases. 2008 Dec 1 [PubMed PMID: 19000014]
Moyle GJ, Sabin CA, Cartledge J, Johnson M, Wilkins E, Churchill D, Hay P, Fakoya A, Murphy M, Scullard G, Leen C, Reilly G, RAVE (Randomized Abacavir versus Viread Evaluation) Group UK. A randomized comparative trial of tenofovir DF or abacavir as replacement for a thymidine analogue in persons with lipoatrophy. AIDS (London, England). 2006 Oct 24:20(16):2043-50 [PubMed PMID: 17053350]
Carr A, Workman C, Smith DE, Hoy J, Hudson J, Doong N, Martin A, Amin J, Freund J, Law M, Cooper DA, Mitochondrial Toxicity (MITOX) Study Group. Abacavir substitution for nucleoside analogs in patients with HIV lipoatrophy: a randomized trial. JAMA. 2002 Jul 10:288(2):207-15 [PubMed PMID: 12095385]
DeJesus E, Rockstroh JK, Lennox JL, Saag MS, Lazzarin A, Zhao J, Wan H, Rodgers AJ, Walker ML, Miller M, DiNubile MJ, Nguyen BY, Teppler H, Leavitt R, Sklar P, STARTMRK Investigators. Efficacy of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naïve HIV-1-infected patients: week-192 overall and subgroup analyses from STARTMRK. HIV clinical trials. 2012 Jul-Aug:13(4):228-32. doi: 10.1310/hct1304-228. Epub [PubMed PMID: 22849964]
Green H, Gibb DM, Walker AS, Pillay D, Butler K, Candeias F, Castelli-Gattinara G, Compagnucci A, Della Negra M, de Rossi A, Feiterna-Sperling C, Giaquinto C, Harper L, Levy J, Saidi Y, Wintergerst U, Paediatric European Network for the Treatment of AIDS (PENTA). Lamivudine/abacavir maintains virological superiority over zidovudine/lamivudine and zidovudine/abacavir beyond 5 years in children. AIDS (London, England). 2007 May 11:21(8):947-55 [PubMed PMID: 17457088]
Sax PE, Tierney C, Collier AC, Fischl MA, Mollan K, Peeples L, Godfrey C, Jahed NC, Myers L, Katzenstein D, Farajallah A, Rooney JF, Ha B, Woodward WC, Koletar SL, Johnson VA, Geiseler PJ, Daar ES, AIDS Clinical Trials Group Study A5202 Team. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. The New England journal of medicine. 2009 Dec 3:361(23):2230-40. doi: 10.1056/NEJMoa0906768. Epub 2009 Dec 1 [PubMed PMID: 19952143]
Smith KY,Patel P,Fine D,Bellos N,Sloan L,Lackey P,Kumar PN,Sutherland-Phillips DH,Vavro C,Yau L,Wannamaker P,Shaefer MS, Randomized, double-blind, placebo-matched, multicenter trial of abacavir/lamivudine or tenofovir/emtricitabine with lopinavir/ritonavir for initial HIV treatment. AIDS (London, England). 2009 Jul 31 [PubMed PMID: 19542866]
Paediatric European Network for Treatment of AIDS (PENTA). Comparison of dual nucleoside-analogue reverse-transcriptase inhibitor regimens with and without nelfinavir in children with HIV-1 who have not previously been treated: the PENTA 5 randomised trial. Lancet (London, England). 2002 Mar 2:359(9308):733-40 [PubMed PMID: 11888583]
Committee on Pediatric Aids. Infant feeding and transmission of human immunodeficiency virus in the United States. Pediatrics. 2013 Feb:131(2):391-6. doi: 10.1542/peds.2012-3543. Epub 2013 Jan 28 [PubMed PMID: 23359577]
King CC, Kourtis AP, Persaud D, Nelson JA, Ziemniak C, Hudgens MG, Tegha G, Chasela CS, Jamieson DJ, van der Horst CM. Delayed HIV detection among infants exposed to postnatal antiretroviral prophylaxis during breastfeeding. AIDS (London, England). 2015 Sep 24:29(15):1953-61. doi: 10.1097/QAD.0000000000000794. Epub [PubMed PMID: 26153671]
Gaur AH,Freimanis-Hance L,Dominguez K,Mitchell C,Menezes J,Mussi-Pinhata MM,Peixoto MF,Alarcon J,Coelho DF,Read JS, Knowledge and practice of prechewing/prewarming food by HIV-infected women. Pediatrics. 2011 May [PubMed PMID: 21482608]
Centers for Disease Control and Prevention (CDC). Premastication of food by caregivers of HIV-exposed children--nine U.S. sites, 2009-2010. MMWR. Morbidity and mortality weekly report. 2011 Mar 11:60(9):273-5 [PubMed PMID: 21389930]
Hafeez S, Salami O, Alvarado M, Maldonado M, Purswani M, Hagmann S. Infant feeding practice of premastication: an anonymous survey among human immunodeficiency virus-infected mothers. Archives of pediatrics & adolescent medicine. 2011 Jan:165(1):92-3. doi: 10.1001/archpediatrics.2010.264. Epub [PubMed PMID: 21199989]
Use the mouse wheel to zoom in and out, click and drag to pan the image
- Open access
- Published: 03 November 2021
The effects of exposure to HIV in neonates at a referral hospital in South Africa
- Helena Mellqvist 1 , 2 ,
- Robin T. Saggers 3 , 4 ,
- Anders Elfvin 2 , 5 ,
- Elisabet Hentz 2 , 5 &
- Daynia E. Ballot 3
BMC Pediatrics volume 21 , Article number: 485 ( 2021 ) Cite this article
1512 Accesses
1 Citations
2 Altmetric
Metrics details
Fewer infants are infected with HIV through mother-to-child transmission, making HIV-exposed but uninfected (HEU) infants a growing population. HIV-exposure seems to affect immunology, early growth and development, and is associated with higher morbidity and mortality rates. Currently, there is a lack of information regarding the clinical effects of HIV-exposure during the neonatal period.
To identify a possible difference in mortality and common neonatal morbidities in HEU neonates compared to HIV-unexposed neonates.
This was a retrospective, descriptive study of all neonates admitted to the neonatal unit at Charlotte Maxeke Johannesburg Academic Hospital between 1 January 2017 and 31 December 2018. HEU neonates were compared to HIV-unexposed neonates.
There were 3236 neonates included, where 855 neonates were HEU. The HEU neonates had significantly lower birth weight and gestational age. The HEU neonates had higher rates of neonatal sepsis (19.8% vs 14.2%, OR 1.49, p < 0.001), specifically for late onset sepsis, and required more respiratory support. NCPAP and invasive ventilation was more common in the HEU group (36.3% vs 31.3% required NCPAP, p = 0.008, and 20.1% vs 15,0% required invasive ventilation, p < 0.001). Chronic lung disease was more common among HIV-exposed neonates (12.2% vs 8.7%, OR 1.46, p = 0.003). The difference in mortality rates between the study groups was not significant (10.8% of HEU neonates and 13.3% of HIV-unexposed).
Conclusions
HEU neonates had higher rates of neonatal sepsis, particularly late-onset sepsis, required more respiratory support and had higher rates of chronic lung disease. Mortality of HEU neonates was not different HIV-unexposed neonates.
Peer Review reports
Human Immunodeficiency Virus (HIV) is one of the world’s biggest health threats. In 2018, the Republic of South Africa had the third highest prevalence of HIV in the world, with 20.4% of adults infected according to the Joint United Nations Programme on HIV/AIDS (UNAIDS) estimations, which equals 7.5 million people. [ 1 ] In addition, 260,000 children between the ages of 0–14 years of age were infected. One of the biggest tragedies of the epidemic, is the transmission of the virus from mother to child, which can occur in utero, intrapartum or postnatally (predominantly through breastfeeding). [ 2 , 3 ] HIV-infection during infancy has a high risk of mortality, with a net survival of 52% at 1 year if infected perinatally and 78% at 1 year post infection if infected through breastfeeding. [ 4 ]
In an effort to stop the HIV/AIDS-epidemic, South Africa has implemented the largest treatment programme in the world, with nearly 4.8 million people on treatment. Treatment for prevention of mother-to-child transmission (PMTCT) was given to 87% of HIV-positive, pregnant women in South Africa during 2018. [ 1 ] The nation has adapted the Option B+ by World Health Organisation, which is that all pregnant and breastfeeding women should receive lifelong anti-retroviral therapy (ART), regardless of CD4 count or clinical stage. [ 5 ] The therapy provided is a fixed-dose combination (FDC) of Tenofovir, Lamivudine or Emtricitabine and Efavirenz. This FDC-therapy should be initiated at the first antenatal care visit, or at least 12 weeks prior to labour. If the mother is consistently on ART, her HIV-exposed infant will receive HIV-prophylaxis of Nevirapine immediately after birth. The infant will be tested for HIV using polymerase chain reaction (PCR) at birth as well as at 10 weeks of age and 6 weeks after cessation of breastfeeding, as early infant diagnosis and treatment has shown to reduce mortality and prevent disease progression [ 6 , 7 ]. An uninfected infant is given HIV-prophylaxis with Nevirapine daily until at least 6 weeks of age. The mother will then continue with lifelong anti-retroviral therapy. [ 8 ] Annually, approximately 53,000 HIV-infections in infants are averted due to PMTCT in South Africa. Still, 4.89% of children with HIV-positive mothers born during 2018 in South Africa, were infected with the virus. [ 1 ] The averted infections result in a growing population of HIV-exposed uninfected (HEU) infants.
Several studies have been conducted to specify the health effects of HIV-exposure during early life, with many showing heterogenous results. [ 9 ] Some studies have shown increased mortality in HEU children compared to unexposed children [ 10 , 11 , 12 ], while others have shown no difference in mortality rates [ 13 , 14 ]. Another example of this heterogenicity is seen when observing neonatal sepsis, where one large meta-analysis showed that HEU neonates were more than twice as likely to have neonatal Group B Streptococcus (GBS) disease compared to HIV-unexposed neonates. Specifically, no significant difference was found in the rate of early-onset disease but HEU’s were 4.43 times more likely to have late-onset neonatal GBS disease (95% CI: 1.81–10.85; p = 0.001). [ 15 ] At the same time, a South African study from 2012 showed HEU neonates to have a lower risk of early-onset sepsis and a similar rate of late-onset sepsis compared to HIV-unexposed neonates. [ 16 ]
Other research has shown more definitive results. In the HEU population, infectious diseases, such as tuberculosis, pneumonia and lower respiratory tract infection are more frequent and occasionally also more severe; for example, HEU infants with pneumonia have higher rates of treatment failure and in-hospital mortality. [ 17 , 18 , 19 , 20 ] The disease-causing pathogens amongst HEU children with pneumonia has also been shown to be unusually diverse, with infections caused by Pseudomonas aeruginosa, Escherichia coli, Pneumocystis jirovecii and Aspergillus spp. [ 21 ]
There are several possible reasons for increased mortality and morbidity in HIV exposed infants: differences in environmental factors, including socioeconomic factors, co-infections and ART-exposure, lower birth weight and gestational age and altered immune factors. [ 3 ] HIV-exposure is associated with low birth weight (defined as birth weight < 2500 g) and prematurity (defined as gestational age < 37 weeks), and higher proportions of infants are small for gestational age (defined as birth weight for gestational age below the tenth percentile of a standard population). [ 22 , 23 ] The exposure to a chronic immune activation that HIV causes in the mother has also been shown to affect the infant’s immunology, with lower absolute levels of CD4+ T cells and naive CD8+ T cells, but increased levels of immature double-negative (CD4–CD8–) T cells, which may indicate disturbed thymic function. [ 24 ] In addition, HEU infant have lower levels of maternal transferred antibodies at birth. [ 25 ]
Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) has developed the Project to Improve Neonatal Care (PRINCE), an on-going clinical audit in order to provide real-time data to identify areas for improvement. The prevalence of HIV amongst antenatal women in the city of Johannesburg in 2015 was 29.6%, in relation to the national point estimate of HIV-prevalence among antenatal women of 30.8%. [ 26 ] With the growing population of HEU neonates, more studies are needed investigating this group of patients in order to improve their care.
The aim of the study was to identify a possible difference in the mortality and the characteristics of the hospital stay in HIV-exposed uninfected neonates compared to HIV-unexposed neonates.
Study design
This was a secondary analysis of an existing database of neonates admitted to the neonatal unit at CMJAH between 1 January 2017 and 31 December 2018. The inclusion criterion was known maternal HIV-status. Neonates with a birth weight below 500 g were considered non-viable and excluded. Neonates with positive or intermediate birth HIV-PCR were excluded, as well as neonates on whom no HIV-PCR was conducted.
Definitions
Maternal and neonatal information was collected. Clinical outcome included duration of hospital stay and outcome (death or survival to discharge.) Place of birth was either ‘inborn’ if born at the study hospital or ‘outborn’. Resuscitation in delivery room was defined as the use of face mask ventilation. Diseases were defined according to the Vermont-Oxford Network (VON) definitions. [ 27 ] Grades 2 and 3 necrotizing enterocolitis (NEC), diagnosed both clinically and radiologically according to the modified Bell’s staging criteria, were included in the NEC variable. Retinopathy of prematurity included grades 3 and 4, whereas intraventricular haemorrhage included grade 3 and 4. Chronic lung disease was defined as requiring oxygen on day 28 of life. Sepsis was classified as culture proven only, with onset within 72 h of birth being classified as early-onset and after 72 h as late-onset sepsis.
The neonatal records at CMJAH are stored on the REDCap (Research Electronic Data Capture) database, hosted by University of Witwatersrand. [ 28 ] REDCap is a secure web-based programme that aids data capture for the purposes of clinical audit and quality improvement. Every week clinical staff collected data from records of patients discharged from the unit. This information was verified and entered onto the REDCap database. Maternal and infant characteristics, as well as information regarding clinical outcome and hospital stay was retrieved from the database.
Statistical analysis
Data was analysed using IBM SPSS (Version 26). We described normally distributed continuous variables using mean and standard deviation and categorical variables using frequency and percentages. Skewed data was described using median and interquartile range. We divided the study sample into HIV-exposed uninfected and HIV-unexposed groups. Continuous variables were compared using unpaired t-test or Mann-Whitney U test as appropriate, while categorical variables were compared using the Chi-square test. A 2-sided p -value < 0.05 was considered significant.
Ethical considerations
All methods were carried out in accordance with relevant guidelines and regulations. Since this was a retrospective review of an existing database, informed consent was waived, and ethics approval obtained from the Human Research Ethics Committee (HREC) of the University of the Witwatersrand (clearance certificate number M190873).
A total of 3370 neonates were admitted to the neonatal unit between 1 January 2017 and 31 December 2018. The inclusion process is described in Fig. 1 . Of 3350 neonates with known maternal HIV-status, 962 neonates were born to mothers with HIV and consequently were HIV-exposed (28.7%). Of the HIV-exposed neonates, 879/895 (98.2%) received HIV-prophylaxis and 873 (90.7%) had successful HIV-PCR done at birth. Eighteen of these neonates tested HIV-positive, which resulted in a transmission rate of 2.1% of the tested neonates. Therefore, 855 neonates can be considered as HIV-exposed but uninfected (HEU) at birth and were included in the analysis. The control group consisted of 2381 HIV-unexposed neonates.
Flowchart of the study population of HIV-exposed and HIV-unexposed neonates
The maternal characteristics of the study population are described in Table 1 . In summary, the HIV-positive mothers had on average higher age and more pregnancies, but fewer parities (all significant differences with p -values < 0.001). The attendance at antenatal care was lower in the HIV-positive group. Other infectious diseases such as tuberculosis and syphilis were more common amongst HIV-positive mothers. The majority of HIV positive mothers (98.5% 801/815) were on ART.
The majority of the neonates in both groups were delivered at the study hospital, but more HEU neonates were outborn (203/847, 24.0% compared to 435/2358, 18.4%; p < 0.001). HEU neonates also had higher occurrence of vaginal delivery than HIV-unexposed (395/817, 48.3% vs 947/2313, 40.9%; p < 0.001). Caesarean section occurred in a total of 1788 cases, with 1459 (82.2%) being emergency surgeries. There was no significant association with HIV-exposure and emergency or elective caesarean section. The characteristics of the included neonates are described in Table 2 . The HEU neonates had significantly lower birth weight and gestational age.
A total of 408 deaths occurred during the study period: 92/855 (10.8%) were HEU compared to 13.3% (316/2381) of the HIV-unexposed neonates. The difference in mortality between the study groups was not significant ( p = 0.058). The median age on outcome (discharge or death) was 6 days in the HIV-unexposed group and 8 days in the HEU group ( p = 0.005). The median length of stay at the neonatal unit was 6 days in the HIV-unexposed group and seven in the HEU group ( p = 0.015). When defined as early neonatal mortality (death within the first 7 days of life) to take days at risk into consideration, the difference in mortality was still not significant.
The difference in respiratory support and diagnoses are shown in Fig. 2 . Initial resuscitation in the delivery room and oxygen after initial resuscitation was required equally in both groups, but HIV-exposed neonates had higher frequency of respiratory support requirements after initial resuscitation. NCPAP was required by 36.3% of HEU, compared to 31.3% of HIV-unexposed ( p = 0.008). Invasive ventilation was also required more by HEU ( p < 0.001), yet with no significant difference in the duration of respiratory support between the study groups.
Use of respiratory support and respiratory morbidities between HIV-exposed uninfected and HIV-unexposed neonates. * significant differences with 2-sided p -values below α = 0,05. HEU = HIV-exposed uninfected; NCPAP = nasal continuous positive airway pressure
Chronic lung disease was more common amongst HEU neonates ( p = 0.003) with an OR of 1.46 (95% CI 1.13–1.88). Twenty-eight neonates had pneumothorax (nine HEU and 19 HIV-unexposed), with no significant difference in frequency between the study groups.
A total of 506 neonates had neonatal sepsis during the time-period studied. Thirty-eight of these neonates had both early- and late-onset sepsis. The distribution between the HIV-exposure groups is shown in Table 3 . Any neonatal sepsis (early and/or late) was associated with HIV-exposure. LOS was significantly more common amongst HEU (16.5% compared to 12.1%), but EOS did not have a significant association with HIV-exposure on its own.
Other neonatal diseases such as necrotising enterocolitis, retinopathy of prematurity, intraventricular haemorrhage and patent ductus arteriosus were not significantly different between the two groups. Clinical features at birth such as hypothermia and low 5-min Apgar score was also not significantly different between the two groups.
This study found an association between HIV exposure and higher rates of neonatal sepsis, specifically LOS, and the requirement of more respiratory support. The characteristics of the included neonates and mothers in this study are potentially not representable for all babies born at the study site, as the study population only included neonates admitted to the neonatal unit. Realistically, the mean birth weight and gestational age in the study group are lower in the study population than in the general population of neonates born at the study site, including full-term, healthy neonates. HIV-infected children have more hospital admissions, making it possible for the HIV-exposure rate to be falsely high. [ 11 ] This argument is also strengthened by a study from the paediatric intensive care unit at CMJAH from 2013 to 2014 which showed a HIV-exposure rate of 34.1%, potentially caused by more severe illnesses amongst HIV-exposed children. [ 29 ] Nationally in 2017, 28.7% of women receiving antenatal care were HIV-positive, but in another urban hospital in Johannesburg the rate was lower at 23.2%. [ 30 , 31 ]
A study conducted at the neonatal unit at CMJAH between 2015 and 2017 (overlapping the study period of this report, and therefore including some of the same population) showed comparable HIV-exposure rate of 28.6%, infection rate of 2.52% and a birth HIV-PCR rate of 88.1%. [ 32 ] Notable is a trend towards lower infection rate and higher rates of PCR done at birth in this study, but since the years are not separated in either of the studies a statistical comparison cannot be made fully. The 90.7% birth HIV-PCR rate found in this study shows great potential for improvement as ideally it should be 100%. Neonates with indeterminate or invalid PCR result contribute to lowering this rate. With the current laboratory testing system, there is a lag of approximately three days between taking a PCR test and obtaining the result. As point-of-care testing becomes more widely accessible, birth HIV-PCR results may become more readily available.
The findings of this study also strengthen previous reports on HIV-exposure’s association with birth at lower gestational age and lower birth weight. The reason as to why this difference exists is not yet established. Many have discussed that the main factor is unhealthier mothers, not only from the chronic disease but also since HIV-infected women in South Africa often have lower socioeconomic status. In a large meta-analysis, HIV-exposed neonates were more likely to have low birth weight in developing countries compared with women in developed countries showing maternal health to be a strong contributing factor. [ 22 ] Keeping this in mind, this association with low birth weight was demonstrated in developed countries, although not as strongly linked, making the association not fully explained.
The mortality rates in this study were not significantly different between the study groups, despite the differences in characteristics and illness. The increased requirement of respiratory support was also found in the study from 2013 to 2014; HIV exposure may be associated with more severe illness. [ 29 ] Another possible reason is more prematurity amongst HIV-exposed neonates. Interestingly, neonatal sepsis and specifically LOS was found to be significantly more common amongst HEU neonates, which previously had shown heterogenous associations. Other neonatal morbidities were not found to be significant.
Possible confounders in this study are maternal factors other than HIV-infection, such as comorbidities like maternal tuberculosis and/or syphilis and higher maternal age. Additionally, HIV-positive women were found to have more pregnancies and lower number of parities. A possible reason for this is due to more miscarriages, as other studies have shown, but that information was not collected in this study. [ 33 ] This finding could be a possible new field of research.
A question remaining is the causative relationship, as HIV-exposure is not only exposure to the virus and chronic maternal immune activation – it is also exposure to ART-drugs, vertical transmission of other pathogens and poorer maternal health. Other studies have shown that these environmental factors cannot explain the association fully, since HIV-exposure seems to affect neonatal immunology. [ 24 , 25 ] Further studies establishing the causes are needed in order to identify preventable reasons and to bridge the health gap between HIV-exposed and unexposed neonates.
Methodological considerations
As this is a retrospective study, there are some methodological aspects to consider. The analysis was limited by unavailable information, where maternal disease status, CD4 count, duration of ART, and high or low risk of HIV-transmission was unknown. The study also did not take multiple births into account. The benefit of the chosen method is that a large study population was available for analysis.
The aim of the study was to compare the rates of mortality and morbidity between HEU and HU neonates, only associations are described rather than causative relationships. Since the study was based on an admitted population, there are limitations as to the applicability of the results. These results are not applicable to all neonates but may be applicable to other admitted populations in similar settings.
Just less than a third (28,7%) of neonates admitted during the study period were HIV-exposed uninfected. This study suggests that HIV-exposure amongst neonates is associated with birth at a lower gestational age and lower birth weight. HIV-exposure was also associated with increased rates of neonatal sepsis, respiratory support, and chronic lung disease. The clinical consequences that should follow affects the care of both mothers and neonate. HIV-infected women might benefit from more nutritional education and support during pregnancy in antenatal care facilities, to help close the gap in birth weight and gestational age. Health care providers caring for HIV-exposed neonates should be mindful of the increased risk in neonatal sepsis and respiratory disease, in order to identify and if possible, treat these severe illnesses at an early stage.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author [RTS]. The data are not publicly available due to them containing information that could compromise research participant privacy.
Abbreviations
Acquired Immunodeficiency Syndrome
Antiretroviral therapy
Confidence interval
Charlotte Maxeke Johannesburg Academic Hospital
Early-onset sepsis
Fixed dose combination
Group B Streptococcus
- HIV-exposed uninfected
Human Immunodeficiency Virus
Interquartile range
Late-onset sepsis
Necrotizing enterocolitis
Nasal continuous positive airway pressure
Polymerase chain reaction
Prevention of mother to child transmission
Research Electronic Data Capture
Standard deviation
Vermont Oxford Network
(UNAIDS) UNPoHA. AIDS Info [2019-09-11]. Country Factsheet, South Africa - 2018]. Available from: http://aidsinfo.unaids.org/?did=5b4e7dc0dddb54192bb396e4&r=world&t=null&tb=q&bt=undefined&ts=0,0&qla=C&qls=ZAF .
Kourtis AP, Bulterys M. Mother-to-child transmission of HIV: pathogenesis, mechanisms and pathways. Clin Perinatol. 2010;37(4):721–37 vii.
Article Google Scholar
Auriti C, De Rose DU, Santisi A, Martini L, Piersigilli F, Bersani I, et al. Pregnancy and viral infections: mechanisms of fetal damage, diagnosis and prevention of neonatal adverse outcomes from cytomegalovirus to SARS-CoV-2 and Zika virus. Biochim Biophys Acta Mol basis Dis. 1867;2021(10):166198.
Marston M, Becquet R, Zaba B, Moulton LH, Gray G, Coovadia H, et al. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol. 2011;40(2):385–96.
(WHO) WHO. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015.
National department of health SA. National consolidated guidelines for the prevention och mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. In: Department of Health SA, editor. www.doh.gov.za2015 .
Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–44.
Article CAS Google Scholar
Provincial Government of the Western Cape- Department of Health HASTHD. The Western Cape Consolidated Guidelines for HIV Treatment: Prevention of Mother-to- Child Transmission of HIV (PMTCT), Children, Adolescents and Adults. 2018.
Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland-Jones SL. HIV-exposed uninfected children: a growing population with a vulnerable immune system? Clin Exp Immunol. 2014;176(1):11–22.
Chilongozi D, Wang L, Brown L, Taha T, Valentine M, Emel L, et al. Morbidity and mortality among a cohort of human immunodeficiency virus type 1-infected and uninfected pregnant women and their infants from Malawi, Zambia, and Tanzania. Pediatr Infect Dis J. 2008;27(9):808–14.
Shapiro RL, Lockman S, Kim S, Smeaton L, Rahkola JT, Thior I, et al. Infant morbidity, mortality, and breast milk immunologic profiles among breast-feeding HIV-infected and HIV-uninfected women in Botswana. J Infect Dis. 2007;196(4):562–9.
Ruperez M, Gonzalez R, Maculuve S, Quinto L, Lopez-Varela E, Augusto O, et al. Maternal HIV infection is an important health determinant in non-HIV-infected infants. Aids. 2017;31(11):1545–53.
Rollins NC, Ndirangu J, Bland RM, Coutsoudis A, Coovadia HM, Newell ML. Exclusive breastfeeding, diarrhoeal morbidity and all-cause mortality in infants of HIV-infected and HIV uninfected mothers: an intervention cohort study in KwaZulu Natal, South Africa. PLoS One. 2013;8(12):e81307.
Chopra M, Doherty T, Goga A, Jackson D, Persson LA. Survival of infants in the context of prevention of mother to child HIV transmission in South Africa. Acta Paediatr. 2010;99(5):694–8.
Cools P, van de Wijgert J, Jespers V, Crucitti T, Sanders EJ, Verstraelen H, et al. Role of HIV exposure and infection in relation to neonatal GBS disease and rectovaginal GBS carriage: a systematic review and meta-analysis. Sci Rep. 2017;7(1):13820.
Cutland CL, Schrag SJ, Zell ER, Kuwanda L, Buchmann E, Velaphi SC, et al. Maternal HIV infection and vertical transmission of pathogenic bacteria. Pediatrics. 2012;130(3):e581–90.
Marquez C, Chamie G, Achan J, Luetkemeyer AF, Kyohere M, Okiring J, et al. Tuberculosis infection in early childhood and the association with HIV-exposure in HIV-uninfected children in rural Uganda. Pediatr Infect Dis J. 2016;35(5):524–9.
Koyanagi A, Humphrey JH, Ntozini R, Nathoo K, Moulton LH, Iliff P, et al. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pediatr Infect Dis J. 2011;30(1):45–51.
Cohen C, Moyes J, Tempia S, Groome M, Walaza S, Pretorius M, et al. Epidemiology of acute lower respiratory tract infection in HIV-exposed uninfected infants. Pediatrics. 2016;137(4):e20153272.
Kelly MS, Wirth KE, Steenhoff AP, Cunningham CK, Arscott-Mills T, Boiditswe SC, et al. Treatment failures and excess mortality among HIV-exposed, uninfected children with pneumonia. J Pediatric Infect Dis Soc. 2015;4(4):e117–26.
McNally LM, Jeena PM, Gajee K, Thula SA, Sturm AW, Cassol S, et al. Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in south African children: a prospective descriptive study. Lancet. 2007;369(9571):1440–51.
Xiao PL, Zhou YB, Chen Y, Yang MX, Song XX, Shi Y, et al. Association between maternal HIV infection and low birth weight and prematurity: a meta-analysis of cohort studies. BMC Pregnancy Childbirth. 2015;15:246.
Sania A, Smith ER, Manji K, Duggan C, Masanja H, Kisenge R, et al. Neonatal and Infant Mortality Risk Associated with Preterm and Small for Gestational Age Births in Tanzania: Individual Level Pooled Analysis Using the Intergrowth Standard. J Pediatr. 2018;192:66–72 e4.
Clerici M, Saresella M, Colombo F, Fossati S, Sala N, Bricalli D, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96(12):3866–71.
de Moraes-Pinto MI, Almeida AC, Kenj G, Filgueiras TE, Tobias W, Santos AM, et al. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. J Infect Dis. 1996;173(5):1077–84.
Department of Health RoSA. The 2015 National Antenatal Sentinel HIV & Syphilis Survey 2017.
Vermont Oxford Network [Available from: https://public.vtoxford.org/ ].
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Mopeli RKB, D.E.; White, D.A. An audit of primary medical conditions in children admitted to the paediatric intensive care unit of Charlotte Maxeke Johannesburg academic hospital. S Afr J Child Health. 2016;10(4):221226.
Google Scholar
Woldesenbet SA, Kufa T, Barron P, Chirombo BC, Cheyip M, Ayalew K, et al. Viral suppression and factors associated with failure to achieve viral suppression among pregnant women in South Africa: a national cross-sectional survey. Aids. 2019.
Technau KG, Kuhn L, Coovadia A, Carmona S, Sherman G. Improving early identification of HIV-infected neonates with birth PCR testing in a large urban hospital in Johannesburg, South Africa: successes and challenges. J Int AIDS Soc. 2017;20(1):21436.
Benali G, Ramdin T, Ballot D. An audit of mother to child HIV transmission rates and neonatal outcomes at a tertiary hospital in South Africa. BMC Research Notes. 2019;12(1):586.
Malaba TR, Phillips T, Le Roux S, Brittain K, Zerbe A, Petro G, et al. Antiretroviral therapy use during pregnancy and adverse birth outcomes in south African women. Int J Epidemiol. 2017;46(5):1678–89.
Download references
Acknowledgements
Thank you to Rose Bandini (Project to Improve Neonatal Care, CMJAH) who was a great source of knowledge and support.
This study was financially supported by Stena A Ohlssons Stiftelse för Forskning and Landenska Donationsfonden with their scholarships awarded to HM. Further, this work was supported by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG- 117661).
Author information
Authors and affiliations.
Futurum, County Hospital Ryhov, Jonkoping, Sweden
Helena Mellqvist
Department of Pediatrics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
Helena Mellqvist, Anders Elfvin & Elisabet Hentz
School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
Robin T. Saggers & Daynia E. Ballot
Department of Paediatrics and Child Health, Charlotte Maxeke Johannesburg Academic Hospital, Jubilee Road, Parktown, Johannesburg, South Africa
Robin T. Saggers
Region Västra Götaland, Department of Pediatrics, The Queen Silvia Children’s Hospital, Sahlgrenska University Hospital, Gothenburg, Sweden
Anders Elfvin & Elisabet Hentz
You can also search for this author in PubMed Google Scholar
Contributions
HM conceptualized the study, performed data collection, analyzed and interpreted the results, drafted the initial manuscript, and revised the manuscript. RTS, AE, EH and DEB supervised the study, reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Robin T. Saggers .
Ethics declarations
Ethics approval and consent to participate.
All methods were carried out in accordance with relevant guidelines and regulations. Since this was a retrospective review of an existing database, informed consent was waived by the Institutional Review Board. The study was approved by the Human Research Ethics Committee (HREC) of the University of the Witwatersrand (clearance certificate number M190873).
Consent for publication
Not applicable.
Competing interests
The research for this study was done in partial fulfillment of HM’s undergraduate degree at the University of Gothenburg, Gothenburg, Sweden. This was performed during a research elective to the University of the Witwatersrand, Johannesburg, South Africa.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Mellqvist, H., Saggers, R.T., Elfvin, A. et al. The effects of exposure to HIV in neonates at a referral hospital in South Africa. BMC Pediatr 21 , 485 (2021). https://doi.org/10.1186/s12887-021-02969-6
Download citation
Received : 09 June 2021
Accepted : 21 October 2021
Published : 03 November 2021
DOI : https://doi.org/10.1186/s12887-021-02969-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- HIV-exposed neonate
- HIV-unexposed neonate
- HIV positive mother
- Neonatal mortality
- Neonatal morbidity
- Low and middle-income countries; South Africa
BMC Pediatrics
ISSN: 1471-2431
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Over 95% of HIV-infected pediatric cases are a result of vertical transmission. The pathophysiology of the HIV disease state in the pediatric population is similar to adults. However, differences occur in the clinical presentation, mode of infection, and therapeutic options. The pediatric and neonatal populations have a weaker immune system than adults; therefore, if infected with HIV, they ...
Each year approximately 8500 women with HIV infection give birth in the United States. 1 Through the implementation of effective, cost-saving 2 preventive strategies during pregnancy, the rate of perinatal transmission of HIV has remained low at <1% to 2%. 1 These preventive strategies include (1) the provision of universal opt-out HIV testing for all pregnant women and for those who have HIV ...
aspects of perinatal HIV, including infant care, and may also be a helpful resource in this situation. Infant Feeding • HIV-infected mothers should be counseled not to breastfeed their infants. • Mothers with unknown HIV status that test positive on rapid HIV screening should not breastfeed until infection is ruled out with additional testing.
All newborns with perinatal exposure to HIV should receive antiretroviral (ARV) drugs during the neonatal period to reduce the risk of perinatal HIV transmission, with selection of the appropriate ARV regimen guided by the level of transmission risk. HIV transmission can occur in utero, intrapartum, or during breastfeeding.
Infants with perinatal HIV exposure who are being breastfed should have virologic diagnostic testing at the standard time points: 14 to 21 days, 1 to 2 months, and 4 to. 6 months (see Table 13 below). In addition, a virologic test at birth is recommended.
Postnatal Management of the Neonate Exposed to HIV. Following birth, infants exposed to HIV should have a detailed physical examination, and a thorough birthing parent health history should be obtained. Pregnant people with HIV may have coinfections with other pathogens that can be transmitted during pregnancy and the birthing process, such as ...
High-risk infants are given presumptive HIV therapy (see table Neonatal Antiretroviral Management According to Risk of HIV Infection Antiretroviral Dosing for Neonates with Perinatal HIV Exposure) for up to 6 weeks or, rarely, longer. This therapy initially serves as prophylaxis but also as preliminary treatment for those later confirmed to ...
However, differences occur in the clinical presentation, mode of infection, and therapeutic options. The pediatric and neonatal populations have a weaker immune system than adults, therefore, if infected with HIV, they are at a greater risk of opportunistic infections. As such, the delay of treatment may result in a rapid progression of the ...
Since the first description of infants with human immunodeficiency virus (HIV) infection in the early 1980s, ()() tremendous advances have been made in the understanding, prevention, and treatment of HIV infection.Effective prevention strategies have reduced the risk of perinatal transmission, or maternal-to-child transmission (MTCT), of HIV infection to less than 1% to 2% in the United States ...
Advances in perinatal HIV management have averted a significant number of infections in neonates and have made the possibility of elimination of mother-to-child transmission a reality; however, significant gaps in implementation of early testing programs as well as the expansion of therapeutic strategies to neonates are hindering prevention efforts and access to safer, more effective and ...
INTRODUCTION. Considerable progress has been made towards eliminating HIV among children; however, the global burden of pediatric HIV and acquired immune deficiency syndrome (AIDS) remains a challenge for health care workers around the world, particularly in resource-limited settings [].The case definition and classification of pediatric HIV infection, clinical manifestations of some of the ...
Perinatal transmission of HIV can occur in pregnancy, labor and delivery, and breastfeeding, with the greatest risk during labor and delivery. 11 Strategies to prevent mother-to-child transmission ...
March 2021: These guidelines provide new and updated recommendations on the use of point-of-care testing in children under 18 months of age and point-of-care tests to monitor treatment in people living with HIV; the treatment monitoring algorithm; and timing of antiretroviral therapy (ART) among people living with HIV who are being treated for tuberculosis.
However, differences occur in the clinical presentation, mode of infection, and therapeutic options. The pediatric and neonatal populations have a weaker immune system than adults; therefore, if infected with HIV, they are at a greater risk of opportunistic infections. As such, the delay of treatment may result in a rapid progression of the ...
1. Human immunodeficiency virus (HIV) screening is strongly recommended for all pregnant people and should be offered as a routine component of initial prenatal care. 2. Optimal management of HIV in pregnancy has been demonstrated to reduce perinatal transmission to <1%. 3. Management of HIV positive pregnant people requires co-ordination of care
Fewer infants are infected with HIV through mother-to-child transmission, making HIV-exposed but uninfected (HEU) infants a growing population. HIV-exposure seems to affect immunology, early growth and development, and is associated with higher morbidity and mortality rates. Currently, there is a lack of information regarding the clinical effects of HIV-exposure during the neonatal period.
1. Purpose. This guideline is to provide a pathway for the care of obstetric women with human immunodeficiency virus (HIV) infection and the prevention of mother-to-child transmission of HIV at the Women's. Key elements include: Communication. Antenatal management. Intrapartum management. Post-delivery management. Infant management.
Panel's Recommendations. All newborns perinatally exposed to HIV should receive appropriate antiretroviral (ARV) drugs as soon as possible, preferably within 6 hours, after delivery (see Antiretroviral Management of Infants with Perinatal HIV Exposure or HIV Infection) (AI). For infants in whom presumptive HIV therapy is initiated, hemoglobin ...
Signs and symptoms of pediatric HIV infection found during physical examination include the following: Candidiasis: Most common oral and mucocutaneous presentation of HIV infection. Thrush in the oral cavity and posterior pharynx: Observed in approximately 30% of HIV-infected children. Linear gingival erythema and median rhomboid glossitis.
Presentation during labor. For women who present in labor and have not had prenatal testing, rapid testing should be offered. Unlike the ELISA, the rapid HIV test is a blood or saliva antibody test and results are usually available within an hour. ... Swiss Neonatal HIV Study Group. AIDS. 1998 Jan 22. 12(2):205-10.
Thus, for example, the diagnosis of HIV infection may follow an investigation of a prolonged or unusual presentation of an infection or a malignancy. Some studies suggest that children vertically infected with HIV become symptomatic from the neonatal period up to age 8 years and that 57% of this group have associated disease within the first year.
Introduction. With appropriate anti-retroviral treatment of mothers during pregnancy and labour and avoidance of breast feeding as appropriate (see below), the risk of transmission of HIV from mother to baby is reduced from 40% to <0.1%. The risk of perinatal transmission depends primarily upon the maternal viral load at delivery; where this is ...