Get the Most Legit Information and Guide on the Latest Jobs in Nigeria, Facebook and Education Here

NECO Chemistry Questions and Answers 2023/2024 (Essay and Objectives)
NECO Chemistry Questions and Answers 2023. I will be showing you past Chemistry objectives and theory repeated questions for free in this post. You will also understand how NECO Chemistry questions are set and how to answer them.
The National Examinations Council (NECO) is an examination body in Nigeria that conducts the Senior Secondary Certificate Examination and the General Certificate in Education in June/July and December/January respectively.
Table of Contents
NECO Chemistry Objectives and Essay Answers 2023 (Expo)
The 2023 NECO Chemistry expo will be posted here today 24th July during the NECO Chemistry examination. Keep checking and reloading this page for the answers.
NECO 2023 Chemistry Answers Loading.
OBJ Answers:
1-10: DEADADECAD
11-20: BAEDDBDBAE
21-30: CCDCABDDCD
31-40: EBEECEBCEE
41-50: BCCECDDADD
51-60: DABBDEAECA
————————————————————————————————————-
NECO Chemistry Questions and Answers For Practice
The following NECO Chemistry questions are questions to expect in the 2023 NECO examination.
1. The minimum amount of energy required for effective collisions between reacting particles is known A) Activation energy B) Bond energy C) Kinetic energy D) Potential energy
2. The bond formed between H2OH2O and H+H+ to form the hydroxonium H3O+H3O+ is A) Dative B) Covalent C) Electrovalent D) Ionic
3. An element XX forms the following oxides X2O,XOX2O,XO and XO2.XO2. This phenomenon illustrates the law of ________. A) Conservation of mass B) Definite proportion C) Mass action D) Multiple proportion
4.. How many moles of oxygen would contain 1.204×10241.204×1024 molecules? NB: Avogadro’s constant (NA) =6.02×1023=6.02×1023 A) 1 B) 2 C) 3 D) 4
See: NECO Timetable
5. Which of the following statements about solids is correct? A) Solid particles are less orderly than those of a liquid B) Solid have lower densities than liquids C) Solid particles have greater kinetic energies than those of liquids D) Solid particles cannot be easily compressed
6. Which of the following apparatus can be used to measure a specific volume of a liquid accurately? A) Beaker B) Conical flask C) Measuring cyclinder D) Pipette
7. The general gas equation PVT=KPVT=K is a combination of A) Boyle’s and Charles’ laws B) Boyle’s and Graham’s laws C) Charles’ and Graham’s laws D) Dalton’s and Graham’s laws
8. The spreading of the scent of a flower in a garden is an example of? A) Brownian motion B) Diffusion C) Osmosis D) Tynadal effect
9. Propane and carbon (IV) oxide diffuse at the same rate because [H = 1.00, C = 12.0, O = 16.0] Options A) They are both gases B) Their molecules contain carbon C) They have the same relative molecular mass D) Both are denser than air
1O. The energy which accompanies the addition of an electron to an isolated gaseous atom is A) Atomization B) Electronegativity C) Electron affinity D) Ionization
11. A sample of hard water contains some calcium sulphate and calcium hydrogen carbonate. The total hardness may therefore be removed by A. boiling the water B. adding excess calcium hydroxide C. adding a calculated amount of calcium hydroxide D. adding sodium carbonate E. adding magnesium hydroxide
12. During the electrolysis of copper II sulphate between platinum electrodes, if litmus solution is added to the anode compartment, A. the litmus turns blue but no gas is evolved B. the litmus turns blue and oxygen is evolved C. the litmus turns blue and hydrogen is evolved D. the litmus turns red and oxygen is evolved E. the litmus turns red and then becomes colourless
13. The reaction between an organic acid and an alcohol in the presence of an acid catalyst is known as; A. saponification B. dehydration C. esterification D. hydrolysis E. hydration
14. The IUPAC names of the compounds CH3COOH and CH2=CH2 are respectively; A. acetic acid and ethane B. ethanoic acid and ethene C. methanoic acid and ethylene D. ethanol and ethene E. acetic acid and ethylene
15. If 30cm3 of oxygen diffuses through a porous pot in 7 seconds, how long will it take 60cm3 of chlorine to diffuse through the same pot, if the vapour densities of oxygen and chlorine are 16 and 36 respectively? A. 9.3 sec B. 14 sec C. 21 sec D. 28 sec E. 30.3 sec
16. When heat is absorbed during a chemical reaction, the reaction is said to be A. thermodynamic B. exothermic C. isothermal D. endothermic E. thermostatic
17. When large hydrocarbon molecules are heated at high temperature in the presence of a catalyst to give smaller molecules, the process is known as A. disintegration B. polymerization C. cracking D. degradation E. distillation
18. The pH of four solutions W, X, Y, Z are 4, 6, 8, 10 respectively, therefore A. none of these solutions is acidic B. the pH of Y is made more acidic by addition of distilled water C. Z is the most acidic solution D. W is the most acidic solution E. X is neutral
19. When each of the nitrates of Potassium, Magnesium and iron is heated, A. all the nitrates decompose to their oxides B. the nitrate of magnesium gives the nitrite and oxygen C. the nitrates of iron magnesium and iron give the oxides D. the nitrate of iron gives the nitrite and oxygen E. the nitrate of the magnesium is not decomposed
2O. Which of the following metals cannot replace hydrogen from water or steam? A. Sodium B. Magnesium C. Iron D. Calcium E. Copper
21. small quantity of solid ammonium chloride (NH4Cl) was heated gently in a test tube, the solid gradually disappears to produce two gases. Later, a white cloudy deposit was observed on the cooler part of the test tube. The ammonium chloride is said to have undergone A. distillation B. sublimation C. precipitation D. evaporation E. decomposition
22. Elements P, Q, R, S have 6, 11, 15, 17 electrons respectively, therefore, A. P will form an electrovalent bond with R B. Q will form a covalent bond with S C. R will form an electrovalent bond with S D. Q will form an electrovalent bond with S E. Q will form a covalent bond with R
23. An element X forms the following compounds with chlorine; XCl4, XCl3, XCl2. This illustrates the A. law of multiple proportions B. law of chemical proportions C. law of simple proportions D. law of conservation of mass E. law of definite proportions
24. The oxidation state of chlorine in potassium chlorate is A. +1 B. +2 C. +3 D. +5 E. +7
25. 10 When air which contains the gases Oxygen, nitrogen, carbondioxide, water vapour and the rare gases, is passed through alkaline pyrogallol and then over quicklime, the only gases left are; A. nitrogen and carbondioxide B. the rare gases C. nitrogen and oxygen D. nitrogen and the rare gases E. nitrogen, carbondioxide and the rare
26. Which of the following statements is NOT correct? A. The average kinetic energy of a gas is directly proportional to its temperature B. At constant tempearture, the volume of a gas increases as the pressure increases C. The pressure of a gas is inversely proportional to its volume D. The temperature of a gas is directly proportional to its volume E. The collisions of molecules with each other are inelastic
27. Zinc Oxide is a A. Basic Oxide B. Acidic Oxide C. Amphoteric Oxide D. Neutral Oxide E. Reactive Oxide
28. When sodium chloride and metallic sodium are each dissolved in water A. both processes are exothermic B. both processes are endothermic C. the dissolution of metallic sodium is endothermic D. the dissolution of metallic sodium is exothermic E. the dissolution of sodium chloride is explosive
29. The periodic classification of elements is an arrangement of the elements in order of their A. Atomic Weights B. Isotopic Weights C. Molecular Weights D. Atomic Numbers E. Atomic Masses
3O. In the reaction between sodium hydroxide and sulphuric acid solutions, what volume of 0.5 molar sodium hydroxide would exactly neutralise 10cm3 of 1.25 molar sulphuric acid? A. 5cm3 B. 10cm3 C. 20cm3 D. 25cm3 E. 50cm3
Recommended: How to check NECO Result
NECO Chemistry Questions And Answers 2023 (Paper 2)
Don’t worry about these NECO Chemistry Questions And Answers 2023. All you need to do is to keep on refreshing this page for the 2023 NECO Chemistry Questions And Answers for this year. It will be posted here in few minutes.
Tips on How to Pass 2023 NECO Chemistry Examinations
The following guidelines will help you pass the 2023 NECO Chemistry examination with flying colours.
Have a Target and Work Towards Actualizing it
You have decided to pass NECO Chemistry 2023 and I am sure of that. Now, the next thing you should do is set targets.
You have told yourself, “I will score A in NECO Chemistry 2023”, that’s not all. You need to plan on how to make it happen. Create a timetable and master plan to achieve your goals.
Get the Recommended Textbook on Chemistry for 2023 NECO Examination
Normally, NECO recommends books for the examination. But apart from NECO Literature in English where certain novels are compulsory, you are free to use any good Chemistry textbook to prepare for NECO 2023 exam.
Some textbooks are more difficult to understand. If you have any topic you are finding difficult to understand, then get a textbook that will simplify the topics and make life better for you.
Do not Skip Chemistry Examples and Exercise you Will Come Across While Reading:
Many candidates are fond of skipping exercises and even examples while studying textbooks. In fact, we like notebooks so much that we could ask, “can I read my notebook and pass NECO Chemistry 2023?” Don’t be scared of attempting exercises in Biology. Face the challenges.
If you have any questions about the NECO Chemistry Questions and Answers 2023 , kindly drop your question in the comment box.
Last Updated on July 25, 2023 by Admin
Related posts:

122 thoughts on “NECO Chemistry Questions and Answers 2023/2024 (Essay and Objectives)”
Please when will the answer come up
I just need questions for the two
I am really greatfull for the coperation you showed to us most especially me if not for you guys thisneco would have been trouble so sincear thank you
WHERE IS THE ANSWER FOR THE OBJ QUESTIONS THAT IS UP THERE
Leave a Comment Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.

NECO Chemistry Questions and Answers 2023 Objectives and Essay
- Post author: Study Admin
- Post published: October 26, 2023
- Post category: School News
- Post comments: 0 Comments
NECO chemistry 2023 answers are now available. NECO chemistry questions and answers 2023/2024 objective and essay and other exam details for NECO 2023 are on this page. See the 2023 NECO chemistry answers for both objective and theory below. Get the NECO chemistry objective and essay answers here.
The 2023 chemistry NECO OBJ and theory questions and answers are provided here for free. All you have to do is to go through the questions and take note of the NECO chemistry answers 2023. Read on to find out.
NECO Chemistry Questions and Answers 2023 Objective and Essay
Have you been searching on Google in order to get the NECO chemistry questions and answers 2023? If so, we have got you covered!
We have the 2023 NECO chemistry questions and our team of experts will soon upload the NECO chemistry questions and their accurate answers to help you pass the 2023 NECO chemistry examination.
The 2023 NECO chemistry theory questions and OBJ will be uploaded any moment from now. So if you are searching for the NECO chemistry answers 2023 for objective and theory, then you are on the right page. See NECO chemistry objective and essay questions and answers below.
NECO Chemistry Answers 2023 Objective and Theory
The National Examination Council (NECO) is an examination body in Nigeria that conducts the Senior Secondary Certificate Examination and the General Certificate in Education in June/July and November/December respectively.
The 2023 NECO chemistry questions are set from the SS1 to SS3 chemistry syllabus. So all the questions you will encounter in this year’s examination are in the syllabus, and nearly 95% of the questions are repeated.
You don’t have to worry about the 2023 NECO chemistry questions and answers PDF (essay and objective). The NECO chemistry answers 2023 will be uploaded any moment from now. All you need to do is to keep refreshing this page so as not to miss out.
Once again, keep refreshing this page because we will upload the original NECO chemistry questions and answers for this year’s exams on this page at any moment from now. Also, to download the past questions and answers, click on this link NECO chemistry past questions .
If you have any questions about the NECO chemistry questions 2023 and answers, feel free to use the comment box below or use the Chat With Us button and we will respond immediately.
The 2023 NECO chemistry answers will be posted here. Be patient. Keep checking and reloading this page for the correct answers. NECO 2023 chemistry answers loading…….
There is nothing like NECO chemistry expo 2023 online. All students are advised to avoid all patronizing online fraudsters/vendors who claim to provide such services.
You Might Also Like
Neco electronics questions and answers 2024 objective and essay, uniuyo postgraduate admission form 2024/2025 session, fugashua post utme form 2024/2025 academic session, leave a reply cancel reply.
Save my name, email, and website in this browser for the next time I comment.

How to Pass NECO Chemistry: Objective and Essay Questions and Answers Explained (With Examples)

Chemistry is one of the core subjects in the Senior Secondary Certificate Examination (SSCE) administered by the National Examinations Council (NECO). It is a required subject for students who wish to study science-related courses at a higher institution. As a result, it is important to properly prepare for the NECO chemistry exam and score high marks.
In this article, we will provide you with some helpful information on how to prepare for the NECO chemistry exam, as well as some sample questions and answers for both the objective and essay sections. We will also give you some helpful tips on how to answer the questions correctly and avoid typical mistakes.
What is NECO Chemistry Exam?
The NECO chemistry exam is a test of your knowledge and comprehension of the fundamental ideas and principles of chemistry. It covers subjects like organic chemistry, physical chemistry, analytical chemistry, chemical reactions, the periodic table, and environmental chemistry. It also covers topics like atomic structure and chemical bonding.
The NECO chemistry exam is divided into two sections: Paper 1 consists of 60 multiple-choice questions that must be answered in one hour. Paper 2 contains two sections: Section A (Theory) and Section B (Practical). Section A contains six essay questions, four of which must be answered in two hours. Section B contains three practical questions, one of which you must answer in one hour.
The NECO chemistry exam has a total of 200 marks . Paper 1 carries 60 marks , Paper 2 Section A carries 80 marks , and Paper 2 Section B carries 60 marks . To pass the exam, you must score at least 50% on each paper.
Related Article: Lester B. Pearson Scholarship in Canada 2023-2024 [ Fully Funded]
How to Prepare for NECO Chemistry Exam?
You must do the following to properly prepare for the NECO chemistry exam:
- Thoroughly study the NECO chemistry syllabus and understand the objectives and outcomes of each topic.
- Review your class notes and textbooks on a regular basis to ensure you understand the key concepts, formulas, definitions, laws, and equations.
- Practice answering past questions and answers from previous years’ exams and mock tests. You can get them online or in bookstores.
- Revise your weak areas and clarify any uncertainties with your teachers or classmates.
- Attend revision classes or tutorials, if possible, and learn from your instructors’ feedback and corrections.
- Prepare your practical materials and equipment in advance, and become familiar with the procedures and safety rules.
- Before the exam, eat well, sleep well, and stay healthy. Avoid stressful situations and distractions that can affect your concentration and performance.
Related post: How to Apply for Student Loan in Nigeria
NECO Chemistry Objective Questions and Answers 2023
The objective section of the NECO chemistry exam tests your ability to recall facts, recognize concepts, apply principles, analyze data, and solve problems. For each question, you must select the correct option from four alternatives (A, B, C, or D).
Here are some sample objective questions and answers for the NECO chemistry exam 2023:
- Which of the following elements has the highest electronegativity?
A) Fluorine B) Chlorine C) Oxygen D) Nitrogen Answer: A) Fluorine
2. What is the name of the compound with the formula CH₃COOH?
A) Methanoic acid B) Ethanoic acid C) Propanoic acid D) Butanoic acid Answer: B) Ethanoic acid
3. What is the oxidation number of sulfur in SO₄²⁻?
A) +2 B) +4 C) +6 D) +8 Answer: C) +6
4. What type of reaction occurs when zinc reacts with hydrochloric acid?
A) Combination reaction B) Decomposition reaction C) Displacement reaction D) Neutralization reaction Answer: C) Displacement reaction
5. What is the empirical formula of benzene?
A) CH B) CH₂ C) C₂H₂ D) C₂H₄ Answer: B) CH₂
See Also: How to Borrow Money from Opay
Tips for Answering Objective Questions
To answer the objective questions correctly, you need to follow these tips:
- Carefully read the question and fully understand what it is asking.
- Remove any options that are obviously incorrect or irrelevant.
- Choose the best option that fits the question based on your knowledge of chemistry and logic.
- If you are not sure of the answer, make an educated guess based on clues from the question or the options.
- Don’t spend too much time on one question. If you get stuck, move on to the next one and come back later if you have time.
- On the answer sheet, write your answers clearly and neatly. Do not make any stray marks or erase any marks.
NECO Chemistry Essay Questions and Answers 2023
The NECO chemistry exam essay section tests your ability to explain concepts, describe processes, compare and contrast phenomena, evaluate arguments, and communicate effectively. For each question, you must write detailed and coherent answers.
Here are some sample essay questions and answers for the NECO chemistry exam 2023:
- (a) Define the following terms:
(i) Atomic number (ii) Mass number (iii) Isotopes (b) Write the electronic configuration of the following elements: (i) Sodium (ii) Magnesium (iii) Chlorine (c) Draw the dot-and-cross diagram of sodium chloride.
(a) (i) The atomic number is the number of protons in the nucleus of an element’s atom. It determines the element’s identity as well as its chemical properties.
(ii) Mass number is the sum of the number of protons and neutrons in the nucleus of an atom of an element. It determines the mass and stability of the atom.
(iii) Isotopes are atoms of the same element with different numbers of neutrons in their nuclei. They have the same atomic number but different mass numbers.
(b) (i) Sodium has an atomic number of 11 and a mass number of 23. Its electronic configuration is 2,8,1 or 1s²2s²2p⁶3s¹.
(ii) Magnesium has an atomic number of 12 and a mass number of 24. Its electronic configuration is 2,8,2 or 1s²2s²2p⁶3s².
(iii) Chlorine has an atomic number of 17 and a mass number of 35. Its electronic configuration is 2,8,7 or 1s²2s²2p⁶3s²3p⁵.
(c) Sodium chloride is formed when one electron is transferred from sodium to chlorine, resulting in the formation of sodium ion (Na+) and chloride ion (Cl).
2. (a) State two differences between metals and non-metals based on their physical properties. (b) Give two examples of metals and two examples of non-metals. (c) Explain why metals are good conductors of electricity.
Read Also: How to Apply as a Teacher in the UK from Nigeria
(a) The following are two physical property distinctions between metals and nonmetals:
- Nonmetals have low melting and boiling points, whereas metals have high melting and boiling points.
- Metals are malleable and ductile, whereas nonmetals are brittle and easily broken.
(b) Two examples of metals are iron and copper . Two examples of non-metals are oxygen and carbon .
(c) Metals are good conductors of electricity because their outermost shells contain free electrons that can easily move through the metal lattice when a potential difference is applied across it. These free electrons transfer electric charge from one end to the other, resulting in an electric current.
3. (a) Define the term acid-base titration. (b) List two types of indicators used in acid-base titrations and state their colour changes. (c) Write a balanced equation for the reaction between hydrochloric acid and sodium hydroxide.
(a) Acid-base titration is a laboratory technique for determining the concentration of an acid or a base by neutralizing it with a known-concentration standard solution of the opposite type.
(b) Two types of indicators used in acid-base titrations are:
- Methyl orange: In acidic solutions, it turns red; in alkaline solutions, it turns yellow.
- Phenolphthalein: In acidic solutions, it is colorless; in alkaline solutions, it is pink.
(c) The balanced equation for the reaction between hydrochloric acid and sodium hydroxide is:
HCl + NaOH → NaCl + H₂O
Read Also: How to Apply for an International Passport in Nigeria 2023 and How Much it Costs
In this article, we have provided you with some useful information on how to prepare for the NECO chemistry exam in 2023, as well as some sample questions and answers for both the objective and essay sections. We hope that this article will help you pass the exam and achieve your academic goals. Goodluck in your exams!
You can also check other of our related articles here
Hi Guys! I have been using DStv, GOtv, and Startimes for more than a decade now and I will be sharing some information about them here.
That's not all, I also love On-Demand streaming platforms such as Netflix and ShowMax equally.
Read more about Ibiok Samuel
Similar Posts
Lester b. pearson scholarship in canada 2023-2024 [ fully funded].
The Lester B. Pearson Scholarship in Canada is a remarkable opportunity for international students who wish to pursue their undergraduate studies at the University of Toronto. This scholarship is named after Lester Bowles Pearson, a former Canadian Prime Minister and Nobel Peace Prize laureate. The scholarship program seeks to recognize and support individuals who have…
The Chevening Africa Media Freedom Fellowship Application
Introduction Welcome to our comprehensive guide to the Chevening Africa Media Freedom Fellowship application process. As an aspiring fellow, you have embarked on an extraordinary adventure to empower African journalists and promote media freedom in Africa. In this post, we will delve into the complexities of the fellowship, emphasizing its significance, qualifying criteria, application process,…
NECO Chemistry Questions and Answers 2023 (100% Sure) Theory & Obj Solution
Get free Verified NECO Chemistry Questions and Answers 2023. NECO June/July Free Chemistry EXPO answers. National Examination Council Chemistry Theory and Objective Answers for you to have good NECO result. You will also understand how NECO Chemistry questions are set and how to answer them. The National Examination Council is an examination body in Nigeria that conducts the Senior Secondary Certificate Examination and the General Certificate in Education in June/July and November/December respectively.
Click Here Now To Chat Us on WhatsApp For Subscription Details

Click Here To See All NECO Chemistry Question Paper 2023
Please Note that the NECO 2023 Chemistry Questions and Answers and any other NECO expo is provided by us for free. We understand that a lot of website charge of collect money from student to provide NECO expo Chemistry Answers to them. NECO questions and answers are provided for free. We will do same during Other Exam like NECO GCE.
NECO 2023 Chemistry Questions will be posted in this page. Our Team are right now with the question paper. It is under verification and once the verification process complete, we will go ahead to upload it here.
Be at alert! Keep refreshing this page for NECO Chemistry Theory Questions and Answers
2023 Chemistry Essay Questions Loading 93.8%
Also Read: How To Check Your JAMB Result
Click Here To Join The 2023 NECO Direct Link answers Group
NECO Chemistry Questions and Answers 2023 OBJECTIVES (OBJ) ANSWERS Loading
2023 VERIFIED Chemistry OBJ:…Loading
CHEMISTRY-OBJ
Please Click Here To See The NECO Chemistry OBJ Answers
CHEMISTRY-Obj! 1-10 DEADADECAD 11-20 BAEDDBDBAE 21-30 CCDCABDDCD 31-40 EBEECEBCEE 41-50 BCCECDDADD 51-60 DABBDEAECA Solved By beafans.com Completed!!
NECO Chemistry Questions and Answers 2023 loading…
Please do not panic and fall into the right hand. 2023 Chemistry Answers will be posted for free here once ready.
We are right now getting things ready for you. The NECO 2023 Chemistry theory questions and answers will be posted any moment from now. All you need do is to keep refreshing this page until you see the answers.
NECO Chemistry -ESSAY-ANSWERS-joberplanet.com
Answers Loading….
CHEMISTRY-ANSWERS
(1ai) (i) Manufacturing sulfuric acid (ii) Vulcanization of rubber (iii) Formulation of Pesticides and fungicides
(1aii) (i) It is a colorless gas that has a distinct smell of rotten eggs (ii) Hydrogen sulphide is soluble in water to some extent
(1aiii) Soaps are made from natural products while detergents are made from synthetic products.
(1aiv) Detergents is for household cleaning and laundry purposes
(1bi) Number of neutrons = Mass number (A) – Atomic number (Z)
I. ²³₁₁X A = 23 (mass number) Z = 11 (atomic number)
Number of neutrons = 23 – 11 = 12
II. ³⁹₁₉Y A = 39 (mass number) Z = 19 (atomic number)
Number of neutrons = 39 – 19 = 20
(1bii) Molar mass: Na = 22.99 g/mol O₂ = 2 * 16.00 g/mol = 32.00 g/mol
Now, let’s calculate the mass of oxygen needed:
First, calculate the number of moles of sodium (Na) in 9.2g: Number of moles = Mass / Molar mass Number of moles of Na = 9.2g / 22.99 g/mol ≈ 0.4002 mol
Since the mole ratio of Na to O₂ is 4:1, the number of moles of O₂ needed is: Number of moles of O₂ = 0.4002 mol / 4 ≈ 0.1001 mol
Now, calculate the mass of oxygen needed: Mass of O₂ = Number of moles of O₂ * Molar mass of O₂ Mass of O₂ = 0.1001 mol * 32.00 g/mol ≈ 3.204 g
Therefore, approximately 3.204 grams of oxygen are needed to burn 9.2 grams of sodium.
(1biii) CaCO₃(s) + 2 HCl(aq) —> CaCl₂(aq) + CO₂(g) + H₂O(l)
From the balanced equation, 1 mole of calcium carbonate (CaCO₃) reacts with 2 moles of HCl to produce 1 mole of calcium chloride (CaCl₂).
Molar masses: CaCO₃ = Ca(40.08) + C(12.01) + 3O(16.00) = 100.09 g/mol CaCl₂ = Ca(40.08) + 2Cl(35.45) = 110.98 g/mol
Now, let’s calculate the number of moles of CaCO₃ in 50g:
Number of moles of CaCO₃ = Mass / Molar mass Number of moles of CaCO₃ = 50g / 100.09 g/mol ≈ 0.4998 mol
Since the mole ratio of CaCO₃ to CaCl₂ is 1:1, the number of moles of CaCl₂ that can be obtained is also approximately 0.4998 mol.
Thus, about 0.4998 moles of calcium chloride can be obtained from 50g of limestone in the presence of excess hydrogen chloride.
(1ci) (i) Sol: A sol is a colloidal solution in which solid particles are dispersed in a liquid medium. (ii) Aerosol: An aerosol is a colloidal solution in which liquid or solid particles are dispersed in a gas medium.
(1cii) The law of definite proportions, also known as the law of constant composition, states that a given chemical compound always contains its constituent elements in fixed and definite proportions by mass. This means that the ratio of the masses of the elements in a compound is constant, regardless of the compound’s origin or method of preparation.
(I) Sodium trioxonitrate (V) is also known as sodium nitrate, with the chemical formula NaNO₃.
The atomic masses are as follows: Na (Sodium) = 22.99 g/mol N (Nitrogen) = 14.01 g/mol O (Oxygen) = 16.00 g/mol
Relative molecular mass of NaNO₃ = (1 * Na) + (1 * N) + (3 * O) Relative molecular mass of NaNO₃ = (1 * 22.99 g/mol) + (1 * 14.01 g/mol) + (3 * 16.00 g/mol) Relative molecular mass of NaNO₃ = 22.99 g/mol + 14.01 g/mol + 48.00 g/mol Relative molecular mass of NaNO₃ = 85.00 g/mol
Therefore, the relative molecular mass of sodium nitrate (NaNO₃) is 85.00 g/mol.
(II) Copper (II) trioxosulphate (VI) pentahydrate is also known as copper (II) sulfate pentahydrate, with the chemical formula CuSO₄ · 5H₂O.
The atomic masses are as follows: Cu (Copper) = 63.55 g/mol S (Sulfur) = 32.06 g/mol O (Oxygen) = 16.00 g/mol H (Hydrogen) = 1.01 g/mol
Relative molecular mass of CuSO₄ · 5H₂O = (1 * Cu) + (1 * S) + (4 * O) + (10 * H) + (5 * O)
Relative molecular mass of CuSO₄ · 5H₂O = (1 * 63.55 g/mol) + (1 * 32.06 g/mol) + (4 * 16.00 g/mol) + (10 * 1.01 g/mol) + (5 * 16.00 g/mol)
Relative molecular mass of CuSO₄ · 5H₂O = 63.55 g/mol + 32.06 g/mol + 64.00 g/mol + 10.10 g/mol + 80.00 g/mol
Relative molecular mass of CuSO₄ · 5H₂O = 249.71 g/mol
If you have any questions about the NECO Chemistry theory & Obj 2023, kindly let us know in the comment box.
We are happy you are here for the 2023 Chemistry answers. The complete solution will be made free in some minutes before the Chemistry examination.
NECO Chemistry questions 2023, NECO 2023 Chemistry questions and answers, Chemistry NECO 2023, NECO questions 2023/2023, Chemistry 2023,
Therefore, the relative molecular mass of copper (II) sulfate pentahydrate (CuSO₄ · 5H₂O) is 249.71 g/mol.
(2ai) Mass of silver deposited (in grams) = (Current in Amperes × Time in seconds × Atomic mass of silver) / (1 Faraday)
Given: Current = 4.6 A Time = 90 minutes = 90 × 60 seconds = 5400 seconds Atomic mass of silver (Ag) = 108g/mol 1 Faraday = 96,500C
Substituting the values to calculate the mass of silver deposited:
Mass of silver deposited = (4.6 A × 5400 s × 108 g/mol) / 96,500 C
Mass of silver deposited ≈ (2,682,720 g·s/mol) / 96,500 C
Mass of silver deposited ≈ 27.8g
(2aii) (i) Electrode surface area (ii) Electrolyte temperature
(2aiii) (i) The oxidizing agent is MnO₄⁻(aq) (ii) The reducing agent is Fe²⁺(aq)
(2aiv) MnO₄⁻(aq) + 8H⁺(aq) + 5e⁻ —-> Mn²⁺ + 4H₂O(l)
(2bi) Coming
(2bii) (i) Gases have no fixed shape or volume. (ii) Gases have low density compared to solids and liquids. (iii) Gases have high kinetic energy and are in constant motion.
(2biii) Faraday’s second law of electrolysis states that the mass of a substance deposited (or liberated) during electrolysis is directly proportional to the quantity of electric charge passed through the electrolyte.
(2biv) (i) Charcoal (ii) Coal
(2bv) Na (Sodium) > Ca (Calcium) > Mg (Magnesium) > Al (Aluminum)
(3ai) (i) Butan-2-ol – Secondary alkanol (ii) 2-methylpropanol – Primary alkanol (iii) 2-methylpropan-2-ol – Tertiary alkanol
(3aii) (i) Fermentation (ii) Ethylene hydration
(3aiii) Let the relative molecular mass of gas Z be M. (Rate of diffusion of hydrogen)/(Rate of diffusion of gas Z) = √(molar mass of gas Z)/√(molar mass of hydrogen) 6/1 = (√M)/(√2) 36 = M/2 M = 2×36 M = 72
(3bi) 1s², 2s², 2p⁴
(3bii) (i) It is a colorless (ii) It is soluble in water. (iii) It is tasteless
(3biii) (i) Identify the longest chain. (ii) Name the substituents alphabetically
(3biv) C₂H₄ + O₂ —> 2CO₂ + 2H₂O
(3ci) Endothermic reaction can be defined as a form of heat reaction in which heat is absorbed from the surrounding into the reacting system.
(3cii) Zn(s) + H₂SO₄(aq) —> ZnSO₄(aq) + H₂(g)
(3ciii) Redox reaction.
(3iv) (i) For refining petrol (ii) For food processing (iii) For producing fertilizer
(4ai) A super saturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature.
(4aii) 15/345 = Solubility *25/1000
Solubility =1000*15/25*345=15000/8625
Solubility = 1.79mol/dm³
(4aiii) (i) H₃0⁺ (ii) NH₄⁺ (iii) [CN]⁻₆
(4bi) (i) It has no chemical formula (ii) It can be separated physically (iii) Freezing air slowly yields different liquids at different temperatures
(4bii) (i) Noble gases (ii) Carbon (iv) oxide
(4biii) H₂SO₄ —-> 2H+ + SO₄²⁻
1 mole of H₂SO₄ = 2 mole of H⁺
0.1 mole of H₂SO₄ = 0.2 mole of H⁺ Mole = no. of H⁺/Avogadro’s constant
No. of H⁺ = Mole * Avogadro’s constant = 0.2 * 6.0*10²³ = 1.2*10²³ ions
(4biv) (i) Dative bonding (ii) Hydrogen bonding
(4bv) (i) BRASS: Constituent: Copper and zinc. Use: Brass is used in the production of musical instruments decorative items and plumbing fixtures.
(ii) BRONZE: Constituent: Copper and tin. Use: Bronze is used in the production of statues coins and various machinery.
(5ai) A base is a substance which when disolve produce hydroxyl ion (OH⁻) as the only negative ion
(5aii) (i) K₂O (ii) MgO
(5aiii) (i) it is used in printing inks and dyes (ii) it is used in making photographic chemicals
(5aiv) Aliphatic does not have good odour while an aromatic hydrocarbon has
(5av) M.m of XCl₃=10-8+(35-5*3) =10.8+106.5 =117.3 Vapour density =117.3/2=58.65
(5bi) (i) Temperature (ii) concentration (iii) surface area
(5bii) The law states that energy can neither be created nor destroyed in and isolated system.
(5biii) (i) burning of wood (ii) neutralization reaction

Now Loading…94.5%
We want to hear from you concerning the NECO Chemistry Questions and Answers 2023 , kindly use the comment section below to get to us.
Related Posts

Emmanuel Alayande University of Education Oyo State School Fees, Hostel Accommodation, Admission Requirements and List Of Courses Offered

Covenant University Ota School Fees, Hostel Accommodation, Admission Requirements and List Of Courses Offered

Caleb University, Lagos School Fees, Hostel Accommodation, Admission Requirements and List Of Courses Offered

Bowen University, Iwo School Fees, Hostel Accommodation, Admission Requirements and List Of Courses Offered

Chrisland University School Fees, Hostel Accommodation, Admission Requirements and List Of Courses Offered

University of Ilesa, Osun State School Fees, Hostel Accommodation, Admission Requirements and List Of Courses Offered
Comments are closed.
Type above and press Enter to search. Press Esc to cancel.
NO.1 EDUCATIONAL BLOG FOR | WAEC | NECO | JAMB | NABTEB | GCE | IJMB | JUPEB | POST UTME & PDF BOOKS
2023 Neco Chemistry Essay and Objective Questions and Answers Expo
July 23, 2023 Kazeem Neco expo , Waec Daily Updates 0

Table of Contents
2023 Neco Chemistry Essay and Objective Questions and Answers
The National Examination Council (NECO) Has Scheduled The 2023 Neco Chemistry Essay and Objective Questions and Answers Paper To Kick of on Monday 24th July 2023.
This brings the attention of candidates writing the exam in to searching for 2023 Neco Chemistry Essay and Objective Questions and Answers , 2023 NECO Chemistry Essay and Objective Expo 2023, Neco chemistry essay and objective 2023 and etc.
In this section, you will read the steps and requirements needed for you to get 2023 Neco Chemistry Essay and Objective 2023 Questions And Answers before exam.
NECO Chemistry Essay and Objective 2023 Paper is Categorized in to 2 parts;
- NECO Chemistry Essay 2023
- Neco Chemistry Objective 2023
Here on zamgist, we have solved all the questions. That is 2023 Neco Chemistry Essay and Objective Questions and Answers.

CLICK HERE TO CHAT ME UP ON WHATSAPP & GET IT NOW
NECO Chemistry Essay and Objective Expo 2023
==> Direct SMS: N1000 MTN CARD Direct SMS MEANS all answers(theory & obj) will come direct to ur phone as sMs.
==> Online PIN: N500 MTN CARD Online PIN MEANS The Pin to access our answers online via https://examdubs.com.ng/ will be sent to u at least 4 hours before the exam to access our answers.
==> WhatsApp: N500 MTN CARD Whatsapp MEANS The answer will be sent to you on WhatsApp after we confirm your subscription. Add Us On WhatsApp at 08023429251.
Send The Following details:-
(i) MTN CARD Pin(s) (ii) Your Name (iii) Subject (iv) Phone number ===> 08023429251 via WhatsApp
RELATED POST:
Civic Education NECO 2023 Questions and Answers Expo
2023 NECO Essay and Objective Agric Questions and Answers Expo
IJMB Chemistry Paper 1 2023 Questions And Answers
2023 NECO Physics practical Questions and Answers Expo
2023 NECO Chemistry practical Questions and Answers Expo
2023 NECO History Questions and Answers Expo
Neco Physics Practical Specimen 2023
NECO Chemistry Specimen 2023
2023 NECO Data processing practical Questions and Answers Expo
NECO Physics Specimen 2023: Apparatus and Instructions
NECO Timetable 2023 For Art Student
NECO 2023 Timetable for Science students
NOTE:- All whatsapp messages sent to the above number are attended to. Always send us whatsapp message of your complaint, your message(s) will get to us and we will reply immediately.
Neco Chemistry Theory Questions 2023

CLICK HERE TO CHAT ME UP ON WHATSAPP TO PAY N500 & GET THE COMPLETE SOLUTIONS NOW
Be the first to comment
Leave a reply cancel reply.
Your email address will not be published.
Save my name, email, and website in this browser for the next time I comment.
Copyright © 2024 | WordPress Theme by ZG Themes
Jamb Expo Waec Answers Neco Runz Exam Runs Jupeb Ijmb

Neco Chemistry Objective & Essay Expo (24th July 2023)
Neco Chemistry 2023 Expo Runs Questions and Answers. We have created an atmosphere for excellent results in the ongoing Neco SSCE exam. Get live, legit and correct Neco Chemistry Expo Runs (Runz) Exam Questions and answers 6 hours before exam.
Today 24th July 2023 NECO Chemistry Answers will be delivered to you according to your Subscription pattern. Adhere to the instructions.
Neco exam is a normal diet exam always written after the West African Examination Council (WAEC), The exam has equal benefits to that of WAEC, Are you writing Neco SSCE June/July exam, subscribe to Mr Legit portal for sure and legit Questions and Answers, Guarantee of A’s and B’s in all the papers. This Webpage is well known for distributing Questions and answers earlier than other portals you see online. Take this opportunity and returning match will leave you behind. With our new developed strategy employed in recent times, I must say Candidates are always going home smiling because of accurate, early delivery of papers etc.
Many searches are made online regarding this Exam like, how do I get Neco Chemistry Expo, free Neco Chemistry Answers, Expo on neco Chemistry, best website for neco Chemistry Questions, is neco Chemistry answers available now.
neco Chemistry expo 2023, neco free Chemistry runs, 24th july Chemistry answers, Chemistry neco runz, 2023/2024 neco Chemistry free questions and answers, neco ssce Chemistry dubs, neco Chemistry objective and essay runs, 2023 neco Chemistry expo online, tomorrow Chemistry neco answer
Show me a candidate who does not know Mr Legit, then I will tell you that candidate that prefer failure to excellence. Last year was a legit exam and this year its going well so far, join the moving train and paint your result with A’s or B’s.
This portal is decorated to help candidates excel in various Neco Subjects with good grades, Mr Legit has been doing it and this year will be better than previous years, this year, we have added more interesting features to match with your expectations. All Languages Like Igbo, Hausa and Yoruba are available in our desk, we receive directly and supply to Websites, schools you see online.
Table of Contents
Neco Chemistry Answers Processing
We have different ideas for the upcoming Neco Chemistry Essay and Objective Exam, Mr Legit is the first person, Neco papers reaches first, other website owners are middlemen, Due to the increasing abuse of papers, our portal decided to hold the papers few hours before releasing it to the candidates and dealers. We have existed for 6 years now. Visit the page below to find out how to Subscribe for more subjects, if you are not interested, please avoid this page.
Recommend – NECO Expo 2023 / NECO Runs 2023 / NECO Questions and Answers
Due to the experiences we had during the course of this exam, Mr Legit mapped out three different means of sharing Neco Chemistry questions and answers to our Candidates, namely
Neco Chemistry WhatsApp Expo
With this method, Candidates will receive answers via Whatsapp platform online, we consider this to be the best base for sharing answers because all files are shareable in that platform.
Neco Chemistry Text Message Runs
Through this process, Candidates are expected to receive answers via SMS platform otherwise called offline method. This is best for candidates who does not have internet connection, however images will not be sent to subscribers.
Online Password Link Answers
As the name implies, Candidates are given a four or three digit code to bypass and view already prepared answers on Mr Legit portal, it requires internet connection, due to some circumstances beyond our direct control, this method is no longer in use by crew members.
How to Get Neco Chemistry Exam Answers
To get Neco Chemistry Exam Expo Runs Questions and Answers, there are lay down procedures to follow :
Price for Neco Chemistry Exam Answers is N800 each
Pay either through Mobile Transfer to our account or buy Mtn Recharge Card.
- Phone number to 09060948147
Neco Chemistry Exam Schedule
From the Neco Released exam timetable document, Neco Chemistry Objective, Essay will kick start on Monday 25th July 2022.
Monday 24th July
- Paper III & II: Objective & Essay – Chemistry › 10:00 am – 1:00 pm

WAEC Exam Questions and Answers
Following the change of normal routine on the pattern of exams, you can as well subscribe for Waec Exam that’s coming this August, do the needful and follow the link below : How to Get WAEC Expo Answers 2023
What is Neco Exam Objective
The National Examination Council (also known as NECO) is an examination body that is responsible for conducting the National Common Entrance Examination (NCEE), Basic Education Certificate Examination (BECE), Senior School Certificate Examination (SSCE) Internal and External for students. The exam body is currently chaired by Professor Charles Uwakwe.
How to Check NECO Result Online?
After most exams, candidates are required to check their results to enable know the level of their performance, also to check if if they can go further with the academic pursuit. To check NECO result, visit the official result checker portal. Click on Check Neco result to learn the steps in checking NECO result online.
Mr Legit Exam Contact Details
Mr Legit only official contact remains 09060948147. Our Number is available on Telegram, WhatsApp etc.
Must Read – Neco Expo 2023 WhatsApp Group Link
NB: No Free Neco Chemistry Expo 2023 nor Neco Chemistry Runs 2023
Share this:
I want to pass my exams
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.
Recent Topics
- IJMB Expo 2024 | 2024/2025 IJMB Runs Questions and Answers
- 2024 GCE Waec Hausa Expo Questions and Answers
- Neco Gce Alternative to Agric Science Practical Expo 2023
- Waec GCE Literature in English Expo 2023 Questions and Answers
- Economics Neco Expo 2023 / Economics Neco Runs 2023

Home » NECO » NECO Chemistry Questions & Answers 2023 (OBJ-Essay) Released
Home → NECO
Neco chemistry questions & answers 2023 (obj-essay) released.
See 2023 NECO Chemistry Answers & Questions Here.
The Neco chemistry answers for 2023 questions can now be seen here. The National Examination Council, NECO Chemistry SSCE paper is scheduled to be written on Monday, 24th July 2023 from 10:00 am to 1:00 pm.
This NECO Chemistry questions paper is for Papers III & II: Objective & Essay and will take a total of 3hrs to write.
Here, we will be posting the neco chemistry questions for candidates that will participate in the examination. Note that below is the questions from past questions and answers that we feel are likely questions for SSCE preparation.

NECO Chemistry Answers 2023.
1. a) (i) What is the structure of the atom as proposed by Rutherford? (ii) Distinguish between the atomic number and the mass number of an element. (iii) Explain briefly why the relative atomic mass of chlorine is not a whole number.
b) (i) What is meant by first ionization energy? (ii) List three properties of electrovalent compounds (iii) Consider the following pairs of elements: 9F and 17CL; 12Mg and 20Ca.
2. a) (i) Define nuclear fission. (ii) Consider the equilibrium reaction represented by the following equation: A2(g) + 3B2(g) 2AB3(g); H = + kJmol-1. Explain briefly the effect of each of the following changes on the equilibrium composition:
- increase in the concentration of B;
- decrease in pressure of the system;
- addition of catalyst.
b) The lattice energies of three sodium halides are as follows:
Explain briefly the trend.
c) State the property exhibited by nitrogen (IV) oxide in each of the following reactions: (i) 4Cu + 2NO2 4CuO + N2; (ii) H2O+ 2NO2 HNO3 + HNO2.
3. a) (i) Define saturated solution. (ii) Distinguish between dative bond and covalent bond. (iii) Explain why sugar and common salt do not conduct electricity in the solid state. (iii) State the type of intermolecular forces present in: hydrogen fluoride; argon. (iv) Consider the compounds with the following structures: S – H —-N and 0 – H —–N In which of the compounds is the hydrogen bond stronger? Give reason for your answer.
(b) (i) State Dalton’s Law of Partial Pressure. (ii) If 200cm3 of carbon(IV) oxide were collected over water at 18°C and 700 mmHg, determine the volume of the dry gas at s.t.p. [ standard vapour pressure of water at 18°C = 15 mmHg]
4. a) (i) Define ionic bond. (ii) What type of bond(s) exist(s) in: magnesium oxide; ammonium ion?
b) (i) Determine the oxidation number of sulphur in Na2S2O3. (ii) State Faraday’s first law. (iii) Give one example each of: acid salt; base salt.
c) (i) Name the type of energy change that occurs in each of the following processes; I2(s) ———> I2(g); C1(g) + e- ——> C1-(g). (ii) State the effect of each of the following aqueous solutions on litmus paper: Na2SO4(aq); AlC13(aq) (iii) Define the term efflorescence. (iv) Give two uses of activated charcoal.
d) (i) State one use of each of the following processes in the chemical industry: hydrogenation of vegetable oil; cracking; esterification. (ii) Calculate the amount of silver deposited in moles when 10920 coulombs of electricity is passed through a solution of a silver salt. [Faraday constant = 96500 C mol-1]
5. a) (i) Define in terms of electron transfer I. oxidizing agent; II. reducing agent. (ii) Write a balanced equation to show that carbon is a reducing agent. (iii) State the change in oxidation number of the specie that reacted with carbon in 5 (a)(ii).
b) A gas X has a vapour density of 32. It reacts with sodium hydroxide solution to form salt and water only. It decolourizes acidified potassium tetraoxomanganate (VII) solution and reacts with H2S to form sulphur. Using the information provided: identify gas X; state two properties exhibited by X; give two uses of X.
c) Consider the following substances: (1) sodium; (2) lead (II) iodide; (3) hydrogen; (4) magnesium; (5) oxygen. Which of the substances (i) conducts electricity? (ii) is produced at the cathode during electrolysis of H2SO4(aq)? (iii) corresponds to the molecular formula AB2 ? (iv) is an alkaline earth metal?
d) (i) Define the term salt. (ii) Mention two types of salt. (iii) Give an example of each of the salts mentioned in 5(d)(ii) above.
e) In a neutralization reaction, dilute tetraoxosulphate (VI) acid completely reacted with sodium hydroxide solution. (i) Write a balanced equation for the reaction. (ii) How many moles of sodium hydroxide would be required for the complete neutralization of 0.50 moles of tetraoxosulphate (VI) acid?
Note: There is nothing like Neco chemistry Expo online. Neco Ssce candidates are to desist from patronizing online fraudsters / vendors who says they can provide such services as they are not real.
Neco Chemistry Objective Questions 2023.
NOTE: There is nothing like Neco chemistry expo online. Do not be deceived by fraudsters posing fake NECO chemistry answers on the internet.
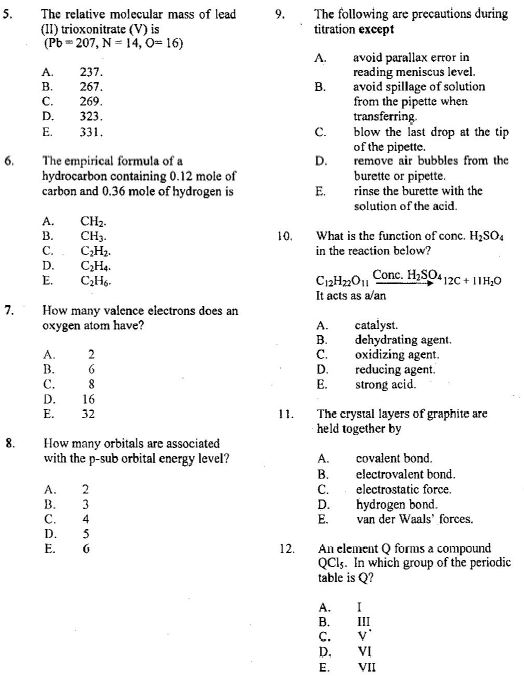
13. At a particular temperature and pressure, 15.0 g of CO2 occupy 7.16 liters. What is the volume of 12.0 g of CH4 at the same temperature and pressure? A.
Keep following this page. If you have any questions, endeavour to use the comment box below…
Industrial Action: ASUU, FG Schedule Another Meeting for November 20
Neco animal husbandry answers 2023 [essay-obj-practical] is out, other recommended posts for you, neco bece 2023 registration form & time table is now out, neco data processing answers 2023 practicals is out, comments (33).
Are the questions legitimate
It was very useful Please add me up
Can i get 2022 neco chemistry theory…
This is very good
You are in point but not at all will people read there is expo
Can I get chemistry practical question of 2023?
We should all read our books & not rely on expo
Why u con dey this site
Today chemistry answers
Yes I agree wholeheartedly agree with you.
So what are u doing here U are cross checking ur work nonsense
I hope the questions are not fake
I need to join this group so that I can excel in my exam. So lovely. Thanks.
I want to join this group
I really need to join this group
I need the Neco 2021 questions and answers
How about the answers
Obj and theory answers chemistry pls
Objective pls
How can I join please
I loved to join this group,this website is great, thanks so much
These chemistry questions above, the correct ones for neco 2021?
I wish to joined dis group
For chemistry obj n essay
please i need neco 2022 answers
I just love unn-edu.info. I wrote Agric exams neco today main paper and can you imagine that some of the questions they posted here came out today . So I advise anything that is posted here should be given utmost attention.
Oyebode precious read your books, stop looking for expo
Where are the solution for those questions above.
The answer for this
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Notify me of follow-up comments by email.
This site uses Akismet to reduce spam. Learn how your comment data is processed .

Home » PAST QUESTIONS » 2023 NECO Chemistry Essay Questions and Answers For all Candidates
2023 NECO Chemistry Essay Questions and Answers For all Candidates

2023 Neco Chemistry Questions: If you are a candidate who is in search of Neco Agricultural Science Questions then you are on the right website because we try to make sure all our audience studies hard to pass with proper information.
If you need us to help you with more updated information at the right time about the 2023 Neco Chemistry Questions, kindly subscribe or bookmark our website for us to provide you with it.
Nkedugists understand how students feel whenever it comes to closing time for an external exam such as Waec, Neco , Jamb, and Post Utme, that is why we make sure any question we are releasing/publishing is always the best option questions you are to see in your exam day because we always follow scholars and examiners. below are what we taught about before publishing it, which are;
- NECO GCE Chemistry Questions Questions
- NECO Chemistry Questions Questions
- 2020/2021 NECO Chemistry Questions
- NECO Chemistry Questions
- GCE Chemistry Questions
- NECO Chemistry Questions
- Chemistry Questions for GCE
- 2020 NECO Chemistry Questions
- 2022 Neco Chemistry Questions
- Neco Chemistry Questions 2022/2023
CHEMISTRY PAPER II Paper 2 will last for 1 hour 30 min Attempt two questions from this section
(1a) Ammonium chloride —> sublimation Ink —> chromatography Iron filling —> magnetic separation
(1aii) -Example Of physical Change (i)change of matter (ii)evaporation of sodium chloride
-Example of chemical change (i)burning of firewood (ii)rusting of iron
(1bi) (i)they are good reducing agent (ii)they form basic oxide (iii)they formed electro valent bonding
(1bii) Aluminum tetraoxosulphate(vi) is prepared by the action of hot concentrated tetraoxosulphate(vi)acid on aluminum oxide Al2O3(s) + 3H2SO4(aq)—->Al2(SO4)3(aq)+3H2O(i) Aluminum tetraoxosulphate(vi) is moderately soluble in water
(1ci) Hydrogen
(1cii) (i)it is denser than air (ii)it is very poisonous
(1ciii) Pb(NO3)2+H2S—->Pbs+2HNO3
(1civ) It turn black due to the formation of black lead (II) sulphide
(1di) Water gas contains flammable gases whereas the producer gas contains both flammable and non-flammable gases
(1dii) (I) Diamond —> Octahedral in shape (II) Graphite —> Hexagonal in shape
(1e) (i) For manufacturing of ammonia and fertilizer (ii) It is used as a refrigerant and also used to shrink metal parts (iii) It is used in grinding substances that are too tough to grind at normal temperature
(2aii) Avogadro’s law states that equal volumes of all gases under the same temperature and pressure contain the same number of molecules
(2aii) Given : 2SO2(aq) + O2(g) –> 2SO3(aq)
The volume of oxygen, O2 required = 1/2*7.50dm³ =3.75dm³
No of moles required = 3.75/22.4moles =0.1674moles
No of oxygen molecules required = 0.1674×6.02×10^23 =1.008×10^23molecules
(2bi) (I) NaCl —–> Valency of chlorine is 1 (II) NaO2 —–> Valency of oxygen is 2 (III) Na3N ——> Valency of Nitrogen is 3
(2bii) (i)Formation of black soap (ii)Formation of glycerol (iii)It helps in the precipitation of large biomolecules such as protein
(2ci) (i)Na2O (ii)MgO (iii)CaO
(2cii) Because they react with water to form acids
(2ciii) – Reaction with sulphur S(s) + O2(g) —> SO2(g)
– Reaction with metals 4Na(s) + O2(g) —-> 2NaO(s)
(2di) Ion is any atom or group of atoms which possess an electric charge
(2dii) TABULATE PLS ION : H^+, F^-, N^3+
NUMBER OF PROTONS: 1,9,13
NUMBER OF NEUTRONS : 0, 10, 14
NUMBER OF ELECTRONS : 0, 10, 10
(2ei) Monosaccharides are simple six-carbon sugar having the formula C6H12O6 eg glucose and its isomers are fructose and galactose, both obtainable from fruits and honey
(2eii) (i)fructose (ii)galactose
What’s your take on this? drop your comment box below Nkedugists urge you to use this same opportunity to share this information across to others using our Facebook, Twitter, or Google+ share button below.
You may also like

Download KANO State Compendium Past Questions and...
Kaduna state free nda past questions and answers for....

Download Akwa Ibom State NDA Compendium Past Questions...
Adamawa state nda compendium past questions and....

Download Western Delta University Postgraduate Test...

Download Christopher University Postgraduate Exam Past...
About the author.
15 Comments
Thanks for the update
2023 NECO CHEMISTRY QUESTIONS AND ANSWERS ARE NOW AVAILABLE.. TO GET IT,CALL OR MESSAGE 08024485178 ON WHATSAPP NOW…
NO TIME TO WASTE….
NECO 2023 CHEMISTRY SCIENCE QUESTION AND ANSWER NOW AVAILABLE!!!
IF YOU NEED THE ANSWERS ON WHATSAPP , IT COST 800
ADD US ON WHATSAPP WITH==>
09035742503
Please I need only the questions
Pls is this question real and correct
Please can I get the objectives and also the questions to the essay? Thank you.
Is it this year own
Is this 2023 neco question
2023 NECO CHEMISTRY QUESTIONS AND ANSWERS ARE NOW AVAILABLE.. TO GET IT,CALL OR MESSAGE 08024485178 ON WHATSAPP NOW………..
NO TIME TO WASTE………….
Number please
I want the objective and the essay answer
Leave a Comment X
Save my name, email, and website in this browser for the next time I comment.
You cannot copy content of this page
📌 Don't miss your JAMB, UTME, Post-UTME, Admission List updates! Join us on WhatsApp
NECO GCE Timetable For 2023 SSCE External Candidate
NECO GCE timetable 2023 is out. The official NECO 2023 GCE timetable for November/December examination is out. The released NECO timetable will guide all GCE candidates on the schedule of their examinations. Dates ant time of papers to be written are available on this time table.
Some websites have posted different articles on the 2023 NECO November/December Timetable, some even posted last year’s timetable. The National Examination Council, NECO has however released their official timetable for the 2023 external exams.
NECO GCE Timetable 2023 for Nov/Dec Exams [Official]
Interested persons can bookmark this page for future reference, you can also print if you want to have a hard copy. This can help and guide candidates applying for the exam in setting up a plan for your reading and general preparations for the Examination.
SEE ALSO: NECO GCE Registration (Application Procedure)
The schedule on the 2023 NECO GCE timetable has it that examination will commence on Monday, 20TH November and end on Wednesday, 20TH December, 2023 .
Monday 20th Nov.
- French Paper IV (Oral) – Actual date and time will be fixed by the Council
- Arabic Paper IV (Oral) – Actual date and time will be fixed by the Council
Tuesday 21st Nov.
- Arabic Paper III (Objective) – 2.00 pm – 3.30 pm
- Arabic Paper II (Essay & Literature) – 3.30 pm – 5.30 pm
Wednesday 22nd Nov.
- Chemistry Paper I (Alternative to Practical) – 2.00 pm – 4.00 pm
Thursday 23rd Nov.
- Christian Religious Studies Paper III & II (Objective & Essay) – 2.00 pm – 4.30 pm
- Islamic Studies Paper III & II (Objective & Essay) – 2.00 pm – 4.30 pm
Friday 24th Nov.
- Literature in English Paper II (Drama & Poetry) – 3.00 pm – 4.40 pm
Saturday 25th Nov.
- Financial Accounting Paper III (Objective) – 10.00 am – 11.20 am
- Financial Accounting Paper II (Theory & Practice) – 11.20 am – 1.50 pm
- Health Education Paper III & II (Objective & Essay) – 3.30 pm – 5.30 pm
Monday 27th Nov.
- Physics Paper I (Alternative to Practical) – 2.00 pm – 4.45 pm
Tuesday 28th Nov.
- Biology Paper I (Alternative to Practical) – 2.00 pm – 4.00 pm
Wednesday 29th Nov.
- Economics Paper III & II (Objective & Essay) – 2.00 pm – 5.00 pm
Thursday 30th Nov.
- Technical Drawing Paper I Practical – 2.00 pm – 5.00 pm
- Physical Education Paper I (Theory of Practice) – 2.00 pm – 4.00 pm
Friday 1st Dec.
- Agricultural Science Paper I (Alternative to Practical) – 3.00 pm – 4.30 pm
Saturday 2nd Dec.
- General Mathematics Paper III (Objective) – 10.00 am – 11.45 am
- General Mathematics Paper II (Essay) – 12.00 noon – 2.30 pm
- Health Education Paper I (Test of Practical) – 3.45 pm – 5.15 pm
Monday 4th Dec.
- Physics Paper III & II (Objective & Essay) – 2.00 pm – 5.00 pm
- Commerce Paper III & II (Objective & Essay) – 2.00 pm – 4.00 pm
Tuesday 5th Dec.
- Technical Drawing Paper III & IV (Objective & Drawing) – 2.00 pm – 4.30 pm
- Physical Education Paper III & II (Objective & Essay) – 2.00 pm – 4.00 pm
Wednesday 6th Dec.
- Chemistry Paper II & II (Objective & Essay) – 2.00 pm – 5.00 pm
Thursday 7th Dec.
- Data Processing Paper III & II (Objective & Essay) – 2.00 pm – 5.00 pm
- Store keeping Paper III & II (Objective & Essay) – 2.00 pm – 4.40 pm
- Marketing Paper III & II (Objective & Essay) – 2.00 pm – 4.40 pm
- Salesmanship Paper III & II (Objective & Essay) – 2.00 pm – 4.40 pm
Friday 8th Dec.
- Literature in English Paper III (Objective) – 3.00 pm – 4.00 pm
- Literature in English Paper IV (Prose) – 4.00 pm – 5.15 pm
Saturday 9th Dec.
- Further Mathematics Paper III (Objective) – 10.00 am – 12.00 noon
- Further Mathematics Paper II (Essay) – 12.10 pm – 2.40 pm
- History Paper III & II (Objective & Essay) – 10.00 am – 12.00 noon
Monday 11th Dec.
- Biology Paper III & II – 2.00 pm – 4.30 pm
Tuesday 12th Dec.
- French Paper I – 2.00 pm – 3.00 pm
- French Paper II – 3.00 pm – 4.45 pm
Wednesday 13th Dec.
- Geography Paper III & I – 2.00 pm – 4.30 pm
Thursday 14th Dec.
- Civic Education Paper III & II – 2.00 pm – 5.00 pm
Friday 15th Dec.
- Geography Paper II – 3.00 pm – 5.00 pm
Saturday 16th Dec.
- English Language Paper III – 10.00 am – 11.00 am
- English Language Paper II – 11.15 am – 1.00 pm
- English Language Paper IV – 1.15 pm – 2.00 pm
- Data Processing Paper I – 3.15 pm – 5.00 pm
Monday 18th Dec.
- Agricultural Science Paper III & II – 2.00 pm – 4.30 pm
Tuesday 19th Dec.
- Government Paper III & II – 2.00 pm – 4.40 pm
Wednesday 20th Dec.
- Hausa Paper III & II – 2.00 pm – 5.00 pm
- Igbo Paper III & II – 2.00 pm – 5.00 pm
- Yoruba Paper III & II – 2.00 pm – 5.00 pm
Candidates should confirm the specific Venues for Oral French, Arabic and Stenography I & IV from NECO State Office where they are sitting for the examination. NOTE:
- Candidates should come along with their Computers/Laptops for Stenography Paper I & IV to the specified venue.
- The Nigerian languages i.e. Hausa. Igbo and Yoruba include the Literature aspect
- Possession of GSM in Examination Hall is PROHIBITED.
- Bring of PROGRAMMABLE CALCULATOR is not allowed.
- BLIND CANDIDATES ARE TO SIT FOR THE EXAMINATION IN THEIR RESPECTIVE NECO STATE OFFICES AND MAKE USE OF THE SUPPLIED BRAILLE SHEET.
- THE ALBINOS AND BLIND CANDIDATES ARE TO BE GIVEN 30 MINUTES EXTRA TIME ACROSS ALL PAPERS.
You can also download the timetable as National Examination Council.
Share this NECO GCE time table with your friends that will also be writing the 2023 NECO Nov/Dec Examination.
You should also read...
- Federal Poly Nasarawa Admission List 2023/2024 (ND)
- BUK School Fees Schedule, Payment Procedure for 2019/2020
- Tai Solarin College of Education Post COVID-19 Resumption Date For 2019/2020
- YABATECH ND Full-Time Admission List 2023/2024 |Merit & Supplementary
- UNIOSUN Shuts Down Indefinitely As Students Protest Colleague’s Death
- Akwa Ibom State University School Fees (Tuition & Charges)
- Federal Poly Bida Post-UTME Form 2023/2024 | Cut-off Mark Out
- Nigeria Immigration Exam / Aptitude Test Venues List Nationwide
Agnes Omachi
One comment.
please I will appreciate if you could send 2015 GCE WAEC to me now or better still my email. thanks. Yemi.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
This site uses Akismet to reduce spam. Learn how your comment data is processed .

JOIN OUR WAEC & NECO WHATSAPP SAPP GROUP

Chemistry-Obj USE THIS OBJ FOR A1.. FEW CORRECTION MADE 01-10: DEADADECAD 11-20: BAEDDBDBAE 21-30: CCDCADDECD 31-40: EBEEBEBCEE 41-50: BCCECEDADD 51-60: DABBDEAECA
CHEMISTRY-ANSWERS! (1ai) (i) Manufacturing sulfuric acid (ii) Vulcanization of rubber (iii) Formulation of Pesticides and fungicides (1aii) (i) It is a colorless gas that has a distinct smell of rotten eggs (ii) Hydrogen sulphide is soluble in water to some extent (1aiii) Soaps are made from natural products while detergents are made from synthetic products. (1aiv) Detergents is for household cleaning and laundry purposes (1bi) Number of neutrons = Mass number (A) – Atomic number (Z) I. ²³₁₁X A = 23 (mass number) Z = 11 (atomic number) Number of neutrons = 23 – 11 = 12 II. ³⁹₁₉Y A = 39 (mass number) Z = 19 (atomic number) Number of neutrons = 39 – 19 = 20 (1bii) Molar mass: Na = 22.99 g/mol O₂ = 2 * 16.00 g/mol = 32.00 g/mol Now, let’s calculate the mass of oxygen needed: First, calculate the number of moles of sodium (Na) in 9.2g: Number of moles = Mass / Molar mass Number of moles of Na = 9.2g / 22.99 g/mol ≈ 0.4002 mol Since the mole ratio of Na to O₂ is 4:1, the number of moles of O₂ needed is: Number of moles of O₂ = 0.4002 mol / 4 ≈ 0.1001 mol Now, calculate the mass of oxygen needed: Mass of O₂ = Number of moles of O₂ * Molar mass of O₂ Mass of O₂ = 0.1001 mol * 32.00 g/mol ≈ 3.204 g Therefore, approximately 3.204 grams of oxygen are needed to burn 9.2 grams of sodium. (1biii) CaCO₃(s) + 2 HCl(aq) —> CaCl₂(aq) + CO₂(g) + H₂O(l) From the balanced equation, 1 mole of calcium carbonate (CaCO₃) reacts with 2 moles of HCl to produce 1 mole of calcium chloride (CaCl₂). Molar masses: CaCO₃ = Ca(40.08) + C(12.01) + 3O(16.00) = 100.09 g/mol CaCl₂ = Ca(40.08) + 2Cl(35.45) = 110.98 g/mol Now, let’s calculate the number of moles of CaCO₃ in 50g: Number of moles of CaCO₃ = Mass / Molar mass Number of moles of CaCO₃ = 50g / 100.09 g/mol ≈ 0.4998 mol Since the mole ratio of CaCO₃ to CaCl₂ is 1:1, the number of moles of CaCl₂ that can be obtained is also approximately 0.4998 mol. Thus, about 0.4998 moles of calcium chloride can be obtained from 50g of limestone in the presence of excess hydrogen chloride.
(1ci) (i) Sol: A sol is a colloidal solution in which solid particles are dispersed in a liquid medium. (ii) Aerosol: An aerosol is a colloidal solution in which liquid or solid particles are dispersed in a gas medium. (1cii) The law of definite proportions, also known as the law of constant composition, states that a given chemical compound always contains its constituent elements in fixed and definite proportions by mass. This means that the ratio of the masses of the elements in a compound is constant, regardless of the compound’s origin or method of preparation. (1ciii) (I) Sodium trioxonitrate (V) is also known as sodium nitrate, with the chemical formula NaNO₃. The atomic masses are as follows: Na (Sodium) = 22.99 g/mol N (Nitrogen) = 14.01 g/mol O (Oxygen) = 16.00 g/mol Relative molecular mass of NaNO₃ = (1 * Na) + (1 * N) + (3 * O) Relative molecular mass of NaNO₃ = (1 * 22.99 g/mol) + (1 * 14.01 g/mol) + (3 * 16.00 g/mol) Relative molecular mass of NaNO₃ = 22.99 g/mol + 14.01 g/mol + 48.00 g/mol Relative molecular mass of NaNO₃ = 85.00 g/mol Therefore, the relative molecular mass of sodium nitrate (NaNO₃) is 85.00 g/mol. (II) Copper (II) trioxosulphate (VI) pentahydrate is also known as copper (II) sulfate pentahydrate, with the chemical formula CuSO₄ · 5H₂O. The atomic masses are as follows: Cu (Copper) = 63.55 g/mol S (Sulfur) = 32.06 g/mol O (Oxygen) = 16.00 g/mol H (Hydrogen) = 1.01 g/mol Relative molecular mass of CuSO₄ · 5H₂O = (1 * Cu) + (1 * S) + (4 * O) + (10 * H) + (5 * O) Relative molecular mass of CuSO₄ · 5H₂O = (1 * 63.55 g/mol) + (1 * 32.06 g/mol) + (4 * 16.00 g/mol) + (10 * 1.01 g/mol) + (5 * 16.00 g/mol) Relative molecular mass of CuSO₄ · 5H₂O = 63.55 g/mol + 32.06 g/mol + 64.00 g/mol + 10.10 g/mol + 80.00 g/mol Relative molecular mass of CuSO₄ · 5H₂O = 249.71 g/mol Therefore, the relative molecular mass of copper (II) sulfate pentahydrate (CuSO₄ · 5H₂O) is 249.71 g/mol.
(2ai) Mass of silver deposited (in grams) = (Current in Amperes × Time in seconds × Atomic mass of silver) / (1 Faraday)
Given: Current = 4.6 A Time = 90 minutes = 90 × 60 seconds = 5400 seconds Atomic mass of silver (Ag) = 108g/mol 1 Faraday = 96,500C Substituting the values to calculate the mass of silver deposited: Mass of silver deposited = (4.6 A × 5400 s × 108 g/mol) / 96,500 C Mass of silver deposited ≈ (2,682,720 g·s/mol) / 96,500 C Mass of silver deposited ≈ 27.8g
(2aii) (i) Electrode surface area (ii) Electrolyte temperature
(2aiii) (i) The oxidizing agent is MnO₄⁻(aq) (ii) The reducing agent is Fe²⁺(aq)
(2aiv) MnO₄⁻(aq) + 8H⁺(aq) + 5e⁻ —-> Mn²⁺ + 4H₂O(l)

(2bii) (i) Gases have no fixed shape or volume. (ii) Gases have low density compared to solids and liquids. (iii) Gases have high kinetic energy and are in constant motion.
(2biii) Faraday’s second law of electrolysis states that the mass of a substance deposited (or liberated) during electrolysis is directly proportional to the quantity of electric charge passed through the electrolyte.
(2biv) (i) Charcoal (ii) Coal
(2bv) Na (Sodium) > Ca (Calcium) > Mg (Magnesium) > Al (Aluminum)
(3ai) (i) Butan-2-ol – Secondary alkanol (ii) 2-methylpropanol – Primary alkanol (iii) 2-methylpropan-2-ol – Tertiary alkanol (3aii) (i) Fermentation (ii) Ethylene hydration (3aiii) Let the relative molecular mass of gas Z be M. (Rate of diffusion of hydrogen)/(Rate of diffusion of gas Z) = √(molar mass of gas Z)/√(molar mass of hydrogen) 6/1 = (√M)/(√2) 36 = M/2 M = 2×36 M = 72 (3bi) 1s², 2s², 2p⁴ (3bii) (i) It is a colorless (ii) It is soluble in water. (iii) It is tasteless (3biii) (i) Identify the longest chain. (ii) Name the substituents alphabetically (3biv) C₂H₄ + O₂ —> 2CO₂ + 2H₂O (3ci) Endothermic reaction can be defined as a form of heat reaction in which heat is absorbed from the surrounding into the reacting system. (3cii) Zn(s) + H₂SO₄(aq) —> ZnSO₄(aq) + H₂(g) (3ciii) Redox reaction. (3iv) (i) For refining petrol (ii) For food processing (iii) For producing fertilizer
(4ai) A super saturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature. (4aii) 15/345 = Solubility *25/1000 Solubility =1000*15/25*345=15000/8625 Solubility = 1.79mol/dm³ (4aiii) (i) H₃0⁺ (ii) NH₄⁺ (iii) [CN]⁻₆ (4bi) (i) It has no chemical formula (ii) It can be separated physically (iii) Freezing air slowly yields different liquids at different temperatures (4bii) (i) Noble gases (ii) Carbon (iv) oxide (4biii) H₂SO₄ —-> 2H+ + SO₄²⁻ 1 mole of H₂SO₄ = 2 mole of H⁺ 0.1 mole of H₂SO₄ = 0.2 mole of H⁺ Mole = no. of H⁺/Avogadro’s constant No. of H⁺ = Mole * Avogadro’s constant = 0.2 * 6.0*10²³ = 1.2*10²³ ions (4biv) (i) Dative bonding (ii) Hydrogen bonding (4bv) (i) BRASS: Constituent: Copper and zinc. Use: Brass is used in the production of musical instruments decorative items and plumbing fixtures. (ii) BRONZE: Constituent: Copper and tin. Use: Bronze is used in the production of statues coins and various machinery.
(5ai) A base is a substance which when disolve produce hydroxyl ion (OH⁻) as the only negative ion
(5aii) (i) K₂O (ii) MgO
(5aiii) (i) it is used in printing inks and dyes (ii) it is used in making photographic chemicals
(5aiv) Aliphatic does not have good odour while an aromatic hydrocarbon has
(5av) M.m of XCl₃=10-8+(35-5*3) =10.8+106.5 =117.3 Vapour density =117.3/2=58.65
(5bi) (i) Temperature (ii) concentration (iii) surface area
(5bii) The law states that energy can neither be created nor destroyed in and isolated system.
(5biii) (i) burning of wood (ii) neutralization reaction

Completed!!

I’m a blogger living in Nigeria. I like to share education guides and information from various sources. I created Jambclass to serve as a platform to disseminate quality, credible and dependable information regarding various ways to excel academically. I strive to keep Nigerian youths and students informed. I started this Blog with the Vision To Inspire and Empower Young Persons; helping them Realize and Maximize their Potential.

Leave a Reply
Name (required)
Email (required, but never shared)
NOTE:- Your comment will appear after it has been approved by an admin .
Home | Recent Posts | Pages
About Jambclass
Copyright © 2022 Jambclass
itsmyschoollibrary
Your lesson notes in a blink.

WELCOME CANDIDATES
2023 NECO TIME TABLE
2023 NECO PRACTICAL SPECI MEN
NECO Biology Specimen Likely Questions with Solutions
NECO PAST QUESTIONS
NECO questions and answers join group
WE GAT YOU COVERED
Check also for 👇 👇
Join our Post UTME Study group
Waec 2023 specimens
Waec 2023 questions and answers
Collections of formal letters for WAEC, NECO, GCE
Collections of Argumentative Essay for WAEC, NECO, GCE
Collections of Narrative Essay for WAEC, NECO, GCE
👇👇👇👇👇👇👇👇👇👇👇
Contact us for further enquires
The National Examination Council of Nigeria (NECO) has released the time table for it’s June/July SSCE 2023 examination and it will run from 3rd July to 11th August, 2023.
NECO 2023 SSCE INTERNAL EXAMINATION TIMETABLE
Monday, 3rd July to Friday, 7th July 2023 (Actual date and time will be fixed by the Council.) Physical Education (Practical) Auto Mechanics (Practical) Woodwork (Practical) Home Management (Practical) Foods and Nutrition (Practical) Performance Test, Music Technology/Alternative Aural Music Oral French Oral Arabic Auto Body Repair and Spray Painting (Practical) Auto Electrical Work Auto Mechanical Work (Practical) Air Conditioning and Refrigeration (Practical) Welding and Fabrication, Engineering Craft Practice (Practical) Electrical Installation & Maintenance Work (Practical) Radio, Television and Electronics Work (Practical) Blocklaying, Bricklaying and Concrete Work (Practical) Painting and Decoration (Exhibition) Plumbing and Pipe Fitting (Practical) Machine Woodworking (Practical) Carpentry and Joinery (Practical) Furniture Making (Practical) Upholstery (Practical) Catering Craft Practice (Practical) Garment Making (Practical) Clothing and Textiles (Practical) Dyeing and Bleaching (Exhibition) Printing Craft Practice (Exhibition) Cosmetology (Practical) Photography (Practical) Leather Goods, Manufacturing and Repair (Exhibition) GSM Maintenance and Repairs (Practical) Animal Husbandry (Progressive Assessments) ===========================
Monday, 10th July 2023 Data Processing (Practical) 10:00am – 1:00pm ===========================
Tuesday, 11th July 2023 Biology (Practical) 10:00am – 12:00noon Music (Objective & Essay) 2:00pm – 4:30pm Insurance (Objective & Essay) 2:00pm – 4:40pm ===========================
Preparing to write NECO? Join group
Wednesday, 12th July 2023 History (Objective & Essay) 10:00am – 1:00pm Building Construction (Drawing) 10:00am – 1:00pm Foods & Nutrition (Objective & Essay) 2:00pm – 4:30pm Woodwork (Drawing & Design) 2:00pm – 4:00pm ===========================
Thursday, 13th July 2023 Chemistry (Practical) 10:00am – 12:00noon Government (Objective & Essay) 2:00pm – 4:40pm View All……..
NECO PRACTICAL SPECIMENS
🟣🟣🟣🟣🟣🟣🟣🟣🟣🟣🟣🟣🟣
BIOLOGY PRACTICAL SPECIMEN The information contained here is highly confidential. Therefore, efforts should be made to avoid candidates getting to know of this either directly or indirectly before the examination.
Each candidate should bring to the examination hall/laboratory: A sharpened Pencil (HB). An Eraser. A Ruler. A Sharp Razor Blade or Scalpel or Knife. The school should provide the following for the candidates: Iodine Solution.
Specimen A – Land Snail Specimen B – Toad Specimen C – Spider Specimen D – Crayfish Specimen E- Spirogyra Filaments Specimen F – mucor/Rhizopus Specimen G – Groundnut Seeding (A week old) Specimen H – Maize Seedling (A week old) Specimen I – Microscope Specimen J – Slide (plain) Specimen K – Pigeon Specimen L – Agama Lizard Specimen M – Rat Specimen N – Tilapia
Need Assistance? Whatsapp us
🔵🔵🔵🔵🔵🔵🔵🔵🔵🔵🔵🔵🔵🔵
AGRIC SCIENCE PRACTICAL SPECIMEN Great care should be taken to ensure that the information given overleaf does not reach the candidates either directly or indirectly before the examination.
The provision of specimens, materials, and equipment for the examination is your responsibility. [i] Each candidate should be provided with the following specimens labeled accordingly;
Specimen A – WATERING CAN SPECIMEN B – PLIER SPECIMEN C – KNAPSACK SPRAYER SPECIMEN D – LITMUS PAPER SPECIMEN E – CROWDING SPECIMEN F – LIMESTONE SPECIMEN G – CLAYEY SOIL (POWDERY FORM) SPECIMEN H – YAM TUBER SPECIMEN | – CASSAVA TUBER SPECIMEN J – ORANGE FRUIT SPECIMEN K – GROUNDNUT CAKE
SPECIMEN L – HIDES AND SKIN SPECIMEN M – DIGESTIVE TRACT OF A BIRD
[ii] Where a specimen is not readily available in sufficient quantity, it should be shared between small groups of candidates. REPORT FORM: The report form is provided separately on which the following information must be stated
View All Subjects….
STUDY NECO PAST QUESTIONS
NECO Civic Education Theory Questions.
Answer two questions from each section
1.(a) Explain the meaning of values, (b) Identify four ways through which the society can promote justice and selflessness.
2.(a) What is the meaning of UIV? (b) Discuss four symptoms and effects of HIV/AIDs.
3. Discuss five main functions of the legislature.
4.(a) What is cuitism? (b) Explain three reasons why students join cult, (c) Identify three preventive measures against cultism.
SECTION B 5. Explain five consequences of human trafficking, 6.(a) What is the meaning of NAPTIP? (b) Explain four functions of NAPTIP.
7.(a) Define public service, (b) Discuss four ways of improving public service in Nigeria.
8.(a) What is the meaning of NDLEA? (b) What is drug abuse? (c) Explain three ways of preventing drug abuse.
(i) What is the possible cause of the pop-up? (ii) State a possible source of the element causing the pop-up. (iii) What measure would be necessary to stop the pop-up from reoccurring?
(c) Mr. Aneke is billed to address a large audience in an auditorium. (i) What computer application package is suitable to prepare and deliver his speech? (ii) State the output device that can be used to transmit Mr. Aneke’s speech note from his computer to a large screen in the auditorium.
(d) Give two types of computer network.
NECO Chemistry Questi ons.
1. a) (i) What is the structure of the atom as proposed by Rutherford? (ii) Distinguish between the atomic number and the mass number of an element. (iii) Explain briefly why the relative atomic mass of chlorine is not a whole number.
b) (i) What is meant by first ionization energy? (ii) List three properties of electrovalent compounds (iii) Consider the following pairs of elements: 9F and 17CL; 12Mg and 20Ca.
2. a) (i) Define nuclear fission.
(ii) Consider the equilibrium reaction represented by the following equation: A2(g) + 3B2(g) 2AB3(g); H = + kJmol-1. Explain briefly the effect of each of the following changes on the equilibrium composition:
- increase in the concentration of B;
- decrease in pressure of the system;
- addition of catalyst.
b) The lattice energies of three sodium halides are as follows:
Explain briefly the trend.
c) State the property exhibited by nitrogen (IV) oxide in each of the following reactions: (i) 4Cu + 2NO2 4CuO + N2; (ii) H2O+ 2NO2 HNO3 + HNO2.
3. a) (i) Define saturated solution. (ii) Distinguish between dative bond and covalent bond. (iii) Explain why sugar and common salt do not conduct electricity in the solid state……
View All Subjects…..

NECO GCE 2023 Chemistry Objective & Theory Questions And Answers
December 5, 2023 Wakagist Admin Neco 0

(1) ATTENTION :- PLEASE DONT COME HERE FOR FREE ANSWER… BE WARNED
(2) attention:- you are advise to send your subscription details on whatsapp… add us on whatsapp with => 09054348225, subscription price, == direct sms: #1000 mtn card.
Direct SMS MEANS all answers (theory & obj) will come direct to your phone as SMS.
== WhatsApp:: #500 MTN CARD
WhatsApp MEANS all answers (theory & obj) will be sent to you on whatsapp.
== Online PIN:: #500 MTN CARD
Online PIN MEANS The Pin to access our answers online via https://wakagist.com/answers will be sent to u at least 2hours before the exam to access our answers
CLICK HERE TO ENTER YOUR PASSWORD GIVEN TO YOU
Send The Following details:-
(i) MTN CARD Pin(s) (ii) Your Name (iii) Subject (iv) Phone number
===> 09054348225 via sms
NOTE :- All SMS Sent To The Above Number Are Attended To, Our Phone Number Might Be Diverted To Avoid Distraction. Always Send Us SMS Of Your Complaint Even When Our Number Is Not Available, Your Message(s) Will Get To Us And We Will Reply You ASAP.
IF YOU NEED THE ANSWER ON WHATSAPP IS AVAILABLE, IT COST #500… ADD US ON WHATSAPP WITH => 09054348225
Be the first to comment, leave a reply cancel reply.
Your email address will not be published.
Save my name, email, and website in this browser for the next time I comment.
ADVERTISE | CONTACT US | PRIVACY POLICY | ABOUT US | DISCLAIMER
NECO Chemistry Questions and Answers 2022/2023 Theory & OBJ Expo
- March 29, 2022
Getting NECO Chemistry Questions 2021/2022 with solved NECO chemistry answers 2021 before the exam had been a big dream for candidates looking for NECO Chemistry questions and answers in 2021
That is the reason we are sharing this NECO chemistry expo 2021 with candidates so that they will not be stranded during the exam.
Proper usage of this material will guarantee you sure insights on the kind of NECO questions for chemistry 2021 that you should expect and how to draft expo answers for them.
OBJ (Paper 1)NECO Questions and Answers for Chemistry 2021.
The chemistry questions and answers for NECO 2021 will be in two papers. The first paper (paper 1) is the OBJ (objective) and the second paper is paper 2 (theory, essay, calculations and show working).
So in this aspect, I will supply you with the basic NECO chemistry questions 2021 samples with correct NECO chemistry answers 2021 to the NECO 2021 chemistry questions and answers samples.
Check out the sample solved Questions and Answers below.
- Solved Chemistry Answers
- Practice Chemistry Questions and answers
- Secret on how to pass NECO 2021
Sample OBJ Chemistry Questions and Answers for NECO 2021

Download NECO 2021 Syllabus for Chemistry
1. Who found the Periodic table?
- Period Dickson
- John Dalton
The correct answer is D. Mendeleev
2. Periodic table is arranged in ____and _____
- Lanthanide and Actinide
- Groups and periods
- Groups and electronic configuration
- Atomic number and outer shell
- Transition and atomic shell
The correct answer is B. Groups and periods
3. Which of these is not a constituent of Urine.
The answer is E. Bacteria
4. Bronze is made up of _____ and _____?
- Iron and copper
- Tin and iron
- Copper and tin
- Nickel and iron
- Zinc and copper
The answer is copper and tin
5. Which of the following is not a metalloids
The correct answer is D. Nickel
6. The most reactive non-metal is_____?
The correct answer is A. Fluorine
7. What is the I.U.P.A.C name of CH 3 CH 2 OH?
The correct answer is D. Ethanol
8. Which of these is not an aromatic compound
- Acetophenone
The correct answer is E. Tulane
9. The chemical symbol for Silver is____?
The answer is B. Ag
10. An element with an atomic number of 16 will likely belong to group_____ on the periodic table.
The answer is E.6
11. Group 7 elements are also known as _______?
- Alkali metals
- Transition elements.
- Alkali earth metals.
The correct answer is A. Halogens
Get answers for chemistry practical paper
Theory Paper 2, Chemistry Questions and answers 2021 Samples
These are NECO 2021 theory Chemistry Questions and answers 2021 Samples for paper 2. This is the second paper (paper 2) and it is an “essay, calculations and show working section”.
Practice these questions because they might come up in the exam.
1. a. State Faraday’s first law of electrolysis.
Faraday’s first law of electrolysis states that the mass (m) of a substance liberated at an electrode during electrolysis is directly proportional to the quantity of electricity (Q) passing through the electrolyte.
Mathematically;
Where quantity of electricity = current (I) x time (t).
i.e Q = It.
ii. Explain an electrolytic cell.
An electrolytic cell consists of a container of electrolytes with two electrodes connected to a suitable direct current supply.
2a. List 4 constituents of Air.
- Carbon (iv) oxide
- Rare gases.
2b. Write the IUPAC name of CH 2 OH CHOHCHO: Ans. 2, 3-Dihydroxy propanal.
3. When 4.5g of liquid water was formed by burning hydrogen gas in oxygen, -72KJ of heat was given off. Calculate the standard heat of the formation of water.
Equation of reaction.
H 2(g) + 1 / 2 O 2(g) → H 2 O(l) ∆H © f = ? KJmol -1 .
4.5g of liquid water produces -72KJ of heat
1.0g of liquid water produces – 72
= -16.0KJ of heat
Therefore, 18g of liquid water will produce: (16.0 x 18) KJ of heat = -288KJmol -1
4. Define the following terms:
- Bomb calorimeter
- Heat of fusion
- Spontaneous reaction
- Gibbs free energy.
Bomb calorimeter: A bomb calorimeter consists of a strong cylindrical steel vessel lined with enamel to prevent corrosion.
The heat of fusion: This is the heat change when one mole of solid is melted.
Entropy: Entropy is a degree of disorderliness or randomness of a system.
Spontaneous reaction: A spontaneous reaction is a reaction that occurs on its own without the assistance of an external agent.
Gibbs free energy: The free energy (G), of a chemical system is the energy that is available for doing work.
NECO Expo for Chemistry exam 2021 (Important terms)
Now that you have gotten some sample NECO chemistry theory and OBJ questions for 2021 to practice with, let me give you a quick and common expo hint for Chemistry exam 2021 (Important terms to note)
IUPAC: IUPAC means, International Union of Pure and Applied Chemistry.
Nucleus: The Nucleus is made up of Proton and neutron.
Laws of Chemical combination includes;
- Law of conservation of mass
- The law of definite proportions or constant composition.
- Law of multiple proportions.
Chemical symbol: A chemical symbol of an element represents an atom of the element.
Radicals: Radicals are groups of an atom carrying charges that keep their identity and react as a single unit.
Empirical formula: Empirical formula is the simplest possible formula giving the ratio of atoms in a molecule of the compound.
Mole: A mole is the amount of substance that contains as many elementary entities as that in a 12g of carbon.
Isotope: An isotope occurs in elements with the same atomic number (Number of protons) but different mass numbers (because of varying numbers of neutrons).
- The Avogadro’s Number (N A ) is 6.02×1023
PH scale: the PH scale is numbered from 0 – 14. From 0 to 6 indicates the degree of acidity, 7 is neutral while, above 7 to 14 indicates the degree of alkalinity.
I am sure these NECO Chemistry Questions and answers 2021 material will help you to perform better in this exam.
Please, treat the above questions as sample questions and not as the main exam questions.
Thanks a lot
you’re welcome.
Thanks for your help
Thanks so much for your help
Nice page of learning
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
NECO Accounting Questions and Answers 2022/2023 (Essay and Objectives)
Neco civic education questions and answers 2021/2022 (theory & obj), you may also like, waec gce commerce questions, answers 2020/2021 & syllabus.
- April 19, 2022
NECO CRS/CRK Questions and Answers 2022/2023 (Essay & Objectives)
- February 23, 2022
WAEC Questions and Answers 2022/2023 [Free Expo Runs] All Subjects
Neco igbo, hausa & yoruba language answers [5th august 2021].
- April 7, 2022
WAEC GCE Civic Education Questions, Answers 2020/2021 & Syllabus
- April 26, 2022
NECO English Language Questions and Answers 2022/2023 Expos
- April 4, 2022

IMAGES
VIDEO
COMMENTS
The following NECO Chemistry questions are questions to expect in the 2023 NECO examination. 1. The minimum amount of energy required for effective collisions between reacting particles is known. A) Activation energy. B) Bond energy. C) Kinetic energy. D) Potential energy. 2.
The 2023 NECO chemistry questions are set from the SS1 to SS3 chemistry syllabus. So all the questions you will encounter in this year's examination are in the syllabus, and nearly 95% of the questions are repeated. You don't have to worry about the 2023 NECO chemistry questions and answers PDF (essay and objective).
The NECO Chemistry Practical Syllabus is similar to the WAEC Syllabus, if not the same. This video, which is the detailed solution to past WAEC and NECO ques...
Here are some sample essay questions and answers for the NECO chemistry exam 2023: (a) Define the following terms: (i) Atomic number (ii) Mass number (iii) Isotopes (b) Write the electronic configuration of the following elements: (i) Sodium (ii) Magnesium (iii) Chlorine (c) Draw the dot-and-cross diagram of sodium chloride. Answer:
Relative molecular mass of CuSO₄ · 5H₂O = 63.55 g/mol + 32.06 g/mol + 64.00 g/mol + 10.10 g/mol + 80.00 g/mol. Relative molecular mass of CuSO₄ · 5H₂O = 249.71 g/mol. If you have any questions about the NECO Chemistry theory & Obj 2023, kindly let us know in the comment box. We are happy you are here for the 2023 Chemistry answers.
In this section, you will read the steps and requirements needed for you to get 2023 Neco Chemistry Essay and Objective 2023 Questions And Answers before exam. NECO Chemistry Essay and Objective 2023 Paper is Categorized in to 2 parts; NECO Chemistry Essay 2023. Neco Chemistry Objective 2023. Here on zamgist, we have solved all the questions.
Welcome to the NECO 2023 CHEMISTRY Objectives & Essay Answers to Questions for the year 2023. 2023 NECO Chemistry Objectives & Essay Questions and PREMIUM EXAM RUNZ WEBSITE Vibrant Exam Expo Website for Jamb Runz, Waec Runz, Neco Runz, Nabteb Expo, Bece Expo, Ijmb Runz, Jupeb runz, Nabteb Expo, Joint Exam Runz And All School Updates
NATIONAL EXAMINATIONS COUNCIL (NECO) ... NIGER STATE 2023 SENIOR SCHOOL CERTIFICATE EXAMINATION (SSCE) INTERNAL EXAMINATION TIMETABLE MONDAY 3RD JULY TO FRIDAY 11TH AUGUST, 2023 ... Objective & Essay - Chemistry 3hrs 10:00am - 1:00pm 4123/4122 Paper III & II: Objective & Essay - Hausa 3hrs 2:00pm - 5:00pm
The National Examination Council (NECO) has released the official time table for the November/December NECO GCE. Before proceeding to the time table, candidates concerned should take note of the instructions below Candidates should confirm the specific venues for Oral French, Arabic and Stenography I & IV from NECO State…. 2023 neco chemistry ...
National Examination Council NECO Chemistry Past Questions - Free download as PDF File (.pdf), Text File (.txt) or read online for free. Download Now! National Examination Council NECO Chemistry Past Questions; Paper 2 - Theory/Essay Questions; Paper 3 - Objective Test Questions
This video explains the chemistry of reactions behind the qualitative analysis of the 2023 NECO Chemistry Practical examination. It shows the different types...
Neco 2023 Chemistry Obj & Essay Question And Answer Now Available. July 22, 2023 Wakagist Neco 0 *NECO CHEMISTRY OBJECTIVE AND ESSAY ANSWERS/SOLUTIONS* *CHEMISTRY OBJECTIVE* 01-10: DEADADECAD 11-20: BAEDDBDBAE 21-30: CCDCABDDCD 31-40: EBEECEBCEE 41-50: BCCECDDADD 51-60: DABBDEAECA ===== *INSTRUCTION*; ANSWER FOUR (4) QUESTIONS ONLY. ...
Neco Chemistry 2023 Expo Runs Questions and Answers. We have created an atmosphere for excellent results in the ongoing Neco SSCE exam. Get live, legit and correct Neco Chemistry Expo Runs (Runz) Exam Questions and answers 6 hours before exam. Today 24th July 2023 NECO Chemistry Answers will be delivered to you according to your Subscription ...
B. 2023 NECO CHEMISTRY (ESSAY) ANSWERS: TO SUBSCRIBE FOR NECO CHEMISTRY OBJ & THEORY ANSWERS VIA LINK ONLY. JUST GO OUT AND BUY MTN CARDS OF N600 ( 200 + 200 + 200 = 600) GO TO YOUR MESSAGE, TYPE THE MTN CARD PINS CORRECTLY AND SEND TO 08107431933. DON'T CALL THE NUMBER, JUST TEXT, IF THE CARDS PINS ARE VALID, A REPLY WILL BE SENT TO YOU ...
Get Free Live 2023 NECO GCE Chemistry (CHEM) OBJ & THEORY Questions and Answers Free of Charge | NECO Nov/Dec Free CHEMISTRY (Objectives and Theory) ... 2023 Chemistry Paper III & II (Objective & Essay) 2.00pm - 5.00pm. NECO GCE CHEMISTRY OBJECTIVES (OBJ) ANSWERS 2023: 1-10: CACCDABEDB 11-20: EACADCAAAC 21-30: CCACBBCDCA 31-40: BECBDCACEE 41 ...
The National Examination Council, NECO Chemistry SSCE paper is scheduled to be written on Monday, 24th July 2023 from 10:00 am to 1:00 pm. This NECO Chemistry questions paper is for Papers III & II: Objective & Essay and will take a total of 3hrs to write. Here, we will be posting the neco chemistry questions for candidates that will ...
Neco Chemistry Questions 2022/2023. CHEMISTRY PAPER II. Paper 2 will last for 1 hour 30 min. Attempt two questions from this section. (1a) Ammonium chloride —> sublimation. Ink —> chromatography. Iron filling —> magnetic separation. (1aii)
The schedule on the 2023 NECO GCE timetable has it that examination will commence on Monday, 20TH November and end on Wednesday, 20TH December, 2023. Monday 20th Nov. French Paper IV (Oral) - Actual date and time will be fixed by the Council. Arabic Paper IV (Oral) - Actual date and time will be fixed by the Council. Tuesday 21st Nov.
51-60: DABBDEAECA. CHEMISTRY-ANSWERS! (1ai) (i) Manufacturing sulfuric acid. (ii) Vulcanization of rubber. (iii) Formulation of Pesticides and fungicides. (1aii) (i) It is a colorless gas that has a distinct smell of rotten eggs. (ii) Hydrogen sulphide is soluble in water to some extent.
Wednesday, 12th July 2023 History (Objective & Essay) 10:00am - 1:00pm Building Construction (Drawing) 10:00am - 1:00pm Foods & Nutrition (Objective & Essay) 2:00pm - 4:30pm ... NECO Chemistry Questions. 1. a) (i) What is the structure of the atom as proposed by Rutherford?
NECO GCE 2023 Chemistry Objective & Theory Questions And Answers. December 5, 2023 Wakagist Admin Neco 0 (1) ... WAEC GCE 2023 Physics Essay and Practical Questions And Answers. Be the first to comment Leave a Reply Cancel reply. Your email address will not be published. Comment. Name *
Wednesday 6th Dec. 2023&2022 Chemistry Paper III & II (Objective & Essay) 3hrs 2.00 pm - 5.00 pm 6273/6272 Data Processing Paper III & II (Objective & Essay) 3hrs 2.00 pm - 5.00 pm ... (NECO) GCE Timetable 2023 Author: www.myschoolgist.com Subject: National Examinations Council (NECO) GCE Timetable 2023
The first paper (paper 1) is the OBJ (objective) and the second paper is paper 2 (theory, essay, calculations and show working). So in this aspect, I will supply you with the basic NECO chemistry questions 2021 samples with correct NECO chemistry answers 2021 to the NECO 2021 chemistry questions and answers samples.