
- Global Locations -
Headquarters
Future Market Insights, Inc.
Christiana Corporate, 200 Continental Drive, Suite 401, Newark, Delaware - 19713, United States
616 Corporate Way, Suite 2-9018, Valley Cottage, NY 10989, United States
Future Market Insights
1602-6 Jumeirah Bay X2 Tower, Plot No: JLT-PH2-X2A, Jumeirah Lakes Towers, Dubai, United Arab Emirates
3rd Floor, 207 Regent Street, W1B 3HH London United Kingdom
Asia Pacific
IndiaLand Global Tech Park, Unit UG-1, Behind Grand HighStreet, Phase 1, Hinjawadi, MH, Pune – 411057, India
- Consumer Product
- Food & Beverage
- Chemicals and Materials
- Travel & Tourism
- Process Automation
- Industrial Automation
- Services & Utilities
- Testing Equipment
- Thought Leadership
- Upcoming Reports
- Published Reports
- Contact FMI
Clinical Research Organization Market

Analysis and Review: Clinical Research Organization Market by Service (Drug Discovery Services, Pre-Clinical Services, Clinical Services, Post-Approval Services), by Production (In-House, Outsourced), by Indication (Oncology, CNS, Cardiovascular Diseases, Metabolic Disorders, Immunology, Respiratory, Musculoskeletal Disorders, Hematological Disorders), by End User (Pharmaceutical & Biotechnology Companies, Medical Device Companies, Governments & Private Firms, Academic Institutions, Others) and By Region- Forecast for 2023 – 2033
Market Insights on Clinical Research Organization Market covering sales outlook, demand forecast and up-to-date key trends
- Report Preview
- Request Methodology
Clinical Research Organization Market Snapshot (2023 to 2033)
According to Future Market Insights (FMI) analysis in a recent market survey, the global clinical research organization market was valued at US$ 58.0 Billion in 2022 and is expected to reach US$ 139.6 Billion by 2033.
Market Outlook:
The Clinical Research Organization (CRO) market refers to the industry segment companies and organizations providing clinical research services to pharmaceutical, biotechnology, and medical device companies. It encompasses the commercial activities and financial transactions associated with outsourcing clinical trials and research studies.
The market is driven by the increasing demand for efficient and cost-effective drug development processes. Pharmaceutical and biotech companies often outsource clinical research activities to CROs to leverage their specialized expertise, infrastructure, and resources. This allows the companies to focus on their core competencies, such as drug discovery and marketing, while relying on CROs for the execution of clinical trials.
Don't pay for what you don't need
Customize your report by selecting specific countries or regions and save 30%!
Sales Analysis of Clinical Research Organization Market from 2018 to 2022 Vs Market Outlook for 2023 to 2033
Sales of the market grew at a CAGR of 6.4% between 2018 to 2022.
With a historical forecast of stable growth, the clinical research organization (CRO) industry has seen significant growth in recent years. The rise in demand for outsourced clinical trials from pharmaceutical, biotechnology, and medical device businesses is what is causing this surge.
The clinical research organization (CRO) market is anticipated to maintain its growth trajectory in the upcoming years, according to the projection. The expansion of the CRO market is anticipated to be fueled by an increase in clinical trials, a rise in the need for personalized medication, and the prevalence of chronic diseases. Various clinical trial processes, including patient recruiting and retention, data analysis, and medication discovery, are using these technologies.
Considering this, FMI expects the global clinical research organization market to grow at a CAGR of 8.4% through the forecasted years.
What are the Key Opportunities in the Clinical Research Organization Market?
CROs can provide specialized services for carrying out clinical studies in this field as personalized medicine and targeted medicines gain popularity. This covers the identification of biomarkers, patient screening, and customized trial planning. CROs now have more potential due to the use of real-world data and virtual trials.
Companies with expertise in these fields can provide pharma/biotech firms with specialized services and assistance for real-world research as well as for virtual studies, which can shorten the trial duration and expense. It can also include telemedicine and digital health technology in clinical trials to raise study compliance, increase patient participation, and offer ongoing monitoring. CROs can be assisted in adopting these technologies and incorporating them into clinical trial designs by businesses offering specialised services.
They are now also able to provide specialized services in data analytics and machine learning due to the increased availability of real-time data in clinical trials. To do this, one can employ predictive analytics to foresee dangers, spot trends, and improve study design, which thereby helps in the growth of the global market.

Principal Consultant
Talk to Analyst
Find your sweet spots for generating winning opportunities in this market.
Which Factors Could Possibly Restrain the Growth of the Clinical Research Organization Market?
Ensuring compliance with regulatory regulations is one of the biggest issues for CRO businesses. Navigating through each nation's unique legislation can be time-consuming and expensive. To make sure that all applicable regulations are followed, CROs must have a strong regulatory staff. Companies in the pharma and biotech industries are increasingly looking for CROs that can offer services at a low cost without sacrificing quality or timeliness. To remain competitive, CROs may need to modify their pricing strategies and business models in response to margin concerns.
Large-scale multicenter trials might be difficult to finance since there is sometimes a lack of funding for clinical trials. To maximize the cost-effectiveness of clinical studies, sponsors, researchers, and CROs must collaborate due to the high cost of drug development and growing cost constraints. The capabilities of CROs may also be constrained by a lack of highly skilled workers, including researchers, data administrators, and statisticians. Lack of qualified personnel may cause inefficiencies, delays in hiring and retaining staff, and a general decline in the quality of project outputs.
Country-wise Insights
What makes the usa the dominating country in the clinical research organization market.
The USA clinical research organization market is expected to register 32.0% in the global market in 2022.
There are several life science businesses in the United States, and these businesses are embracing innovations in clinical research. CROs that offer these services are in demand as technological innovations like telemedicine and virtual trials are used more frequently. Drug development efforts by biotech and pharmaceutical firms are increasing in the USA These businesses are turning more and more to CROs for assistance, including access to facilities and programs for current patients, enrollment and recruiting information, personalized protocol design, higher trial completion rates, and lower costs.
Why is China considered a Lucrative Market for Clinical Research Organization Services?
China accounted for around 9.3% market share in 2022 globally.
The clinical research organization (CRO) market in China is expanding due to several factors. The rapidly ageing and expanding Chinese population is a significant contributing element, which has raised demand for cutting-edge medical cures and treatments. Furthermore, recent reforms and laws adopted by the Chinese government have made it simpler to conduct clinical trials and research there.
The government has put in place many steps to hasten the regulatory approval procedure for novel pharmaceuticals and therapies, luring multinational companies to locate their research and development operations in the nation. Additionally, the development of healthcare and rising income levels in China have raised the demand for advanced healthcare services, such as clinical research and triage.
What Makes India an Emerging Market for Clinical Research Organization?
India holds a 7.4% value share in the global market in 2022.
The clinical research organization (CRO) market is expanding in India for a variety of reasons. Large and diversified patient populations are a significant contributing element, which makes India a desirable location for carrying out clinical studies. Due to the country's accessible healthcare services and cheaper cost of living, clinical trials in India are also more economical than in many other nations.
The supportive regulatory environment created by the Indian government, which promotes clinical research activities by streamlining the approval procedure for clinical trials and cutting the time needed to secure regulatory approvals, is another important aspect. More foreign money has also been invested in India's clinical research sector as a result of the rise in medical tourism there.
Get the data you need at a Fraction of the cost
Personalize your report by choosing insights you need and save 40%!
Category-wise Insights
Which service is largely adopted for clinical research organizations.
The post-approval services segment held the major chunk of about 45.7% of the global market by the end of 2022.
A key factor in the expansion of the clinical research organization (CRO) market is post-approval services. Post-approval services are the actions taken after regulatory bodies or authorities have given their clearance for the use of a product or therapy. These services are essential for maintaining the product's safety and effectiveness as well as for carrying out additional studies that could increase the product or therapy's effectiveness.
In order to diversify their service portfolio and give their clients value-added services, several CROs offer post-approval services. Pharmacovigilance, medical monitoring, safety monitoring, data management, statistical analysis, quality assurance, and regulatory compliance are a few examples of the services that may be provided.
Which production type is largely preferred for the clinical research organization market?
The In-house production segment contribute 58.3% share of the global market in 2022.
The term in-house describes a method of conducting all phases of clinical research within a single organization, often comprising study design, data management, monitoring, and statistical analysis. Clinical research organizations (CRO) market development is still mostly fueled by internal clinical research conducted by pharmaceutical and biotech corporations. The in-house methodology has several benefits, including more control over studies, quicker decision-making, and reduced reliance on outside suppliers.
A pharmaceutical or biotech company's ability to quickly and efficiently react to any noteworthy events or changes in the study protocol can be facilitated by in-house clinical research, which provides better control over study data and technological competence.
Which Indication Dominates the Global Market in 2022?
The oncology by indication segment accounted for a revenue share of 23.4% in the global market at the end of 2022.
One of the markets for clinical research organizations (CROs) that is expanding the quickest is oncology, which has had a big impact on the development of the sector. Cancer research is an appealing subject for CROs to concentrate their attention on because it is a complex and fast-developing field of medicine that calls for a high degree of knowledge and resources.
Many variables, such as the rising cancer burden globally, developments in molecular biology and genomics, and the creation of more advanced and novel immuno-oncology medicines, all contribute to the expansion of oncology research and the CRO business. The need for more advanced oncology therapies that can address patients' complicated requirements is growing as cancer rates are expected to keep rising.
Which End User Segment Propels the Market Growth?
The pharmaceutical and biotechnology companies hold a share of 45.6% of the global market in 2022.
The market for clinical research organizations (CROs) is expanding mostly due to the efforts of the pharmaceutical and biotechnology industries. These businesses invest a lot of money in the discovery of novel treatments and medications, and they frequently contract out some or all of the clinical research work to CROs to increase productivity and access to funding.
A great deal of financing for clinical research studies-particularly those involving new medicinal compounds-comes from pharmaceutical and biotech corporations. These businesses frequently look to collaborate with CROs who can offer specialized knowledge in research design, data administration, and general project management. Pharmaceutical and biotech companies can shorten the time it takes to develop new drugs by outsourcing clinical trial efforts to CROs and concentrating internal resources on core research and development tasks.
Competitive Landscape
The market's key vendors are concentrating on diversifying their product offerings to strengthen their market share in clinical research organizations and to broaden their presence in developing nations. Pricing strategies, market strategies, technology improvements, regulatory compliance, and acquisition and distribution agreements with other companies to extend their business are the main tactics used by manufacturers to acquire a competitive edge in the market.
For instance:
- In January 2020, Charles River announced a scientific collaboration with Takeda Pharmaceutical Company Limited in order to focus on drug discovery in the four core therapeutic areas that Takeda works on. These four therapeutic areas include oncology, gastroenterology, neuroscience, and rare disease. This collaboration focuses on delivering preclinical candidates.
- In July 2022, Labcorp launched a new test called the neurofilament light chain (NfL) blood test, which will help in the identification and confirmation of neurodegenerative diseases like multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, and others.
Similarly, recent developments have been tracked by the team at Future Market Insights related to companies in the clinical research organization market space, which are available in the full report
Scope of the Clinical Research Organization Report
Key segments covered in clinical research organization industry research, by service:.
- Drug discovery Services
- Pre-clinical Services
- Clinical Services
- Post Approval Services
By Production:
By indication:.
- Cardiovascular Diseases
- Metabolic Disorders
- Respiratory
- Musculoskeletal Disorders
- Hematological Disorders
By End User:
- Pharmaceutical & Biotechnology Companies
- Medical Device Companies
- Governments & Private Firms
- Academic Institutions
- North America
- Latin America
- Middle East and Africa (MEA)
Frequently Asked Questions
How big is the clinical research organization market.
The clinical research organization market is slated to attain US$ 62.43 billion in 2023.
What Might be the Market’s Size by 2033?
The market is expected to attain a value of US$ 139.56 billion by 2033.
Which Opportunities are Emerging in the Market?
Increasing popularity of targeted medicines and personalized medicines are creating opportunities for key players.
Which Factors Pull Back the Market Growth?
Strict regulatory regulations and lack of funding for clinical trials are pulling back the market’s growth.
Who are the Key Players in the CRO Industry?
IQVIA Inc, Parexel International Corporation, and ICON plc. are the key players in the CRO industry.
Table of Content
List of tables, list of charts.
Explore Healthcare Insights
Talk To Analyst
Your personal details are safe with us. Privacy Policy*
- Talk To Analyst -
This report can be customized as per your unique requirement
- Get Free Brochure -
Request a free brochure packed with everything you need to know.
- Customize Now -
I need Country Specific Scope ( -30% )
I am searching for Specific Info.
- Download Report Brochure -

You will receive an email from our Business Development Manager. Please be sure to check your SPAM/JUNK folder too.
Report Description
Table of content, methodology.
- Healthcare & Pharmaceuticals
- Clinical Research Organization Market Growth & Trends [2031]
Global Clinical Research Organization (CRO) Market
Segments - By Service (Clinical Research Services, Early Phase Development Services, Laboratory Services, Consulting Services, and Others), By Molecule Type (Small molecules and Biologics), By End-user (Pharmaceutical & Biotechnology Company, Research & Academic Institute, and Others), and Regions (North America, Asia Pacific, Europe, Latin America, Middle East, and Africa) - Global Industry Analysis, Size, Share, Growth, Trends and Forecast 2023-2031
Raksha Sharma
Fact-checked by :
Vineet Pandey
Vishal Golekar
Clinical Research Organization Market Outlook
The global Clinical Research Organization (CRO) market was estimated at USD 80.6 Billion in 2022 and is anticipated to reach USD 220.3 Billion by 2031 , expanding at a CAGR of 12.2% during the forecast period. A clinical research organization (CRO) provides support to the pharmaceuticals, biotechnology, and medical devices industries in the form of research services outsourced on a contract basis. Clinical research organizations (CROs) play a crucial role in the development of pharmaceuticals, medical devices, and therapies. A sponsor (a business, organization, or institution) who wishes to run a clinical trial hires CROs to plan, coordinate, execute, and manage the lifecycle of the clinical trial, safely and efficiently.
CROs have the knowledge, capabilities, processes, and procedures that are needed to develop and run a successful clinical trial while ensuring trial quality and compliance with national and international standards. A CRO serves as the main contact between the sponsor and other stakeholders throughout the trial and communicates with ethics and compliance committees, regulatory personnel, vendors, physicians, and research coordinators.
Macro-economic Factors
Increasing healthcare expenditure.
Healthcare expenditure refers to the amount of money spent on healthcare goods and services, such as medical treatments, hospitalizations, medical equipment, and other healthcare-related expenses. It includes both public and private spending on healthcare, such as expenditure by governments, insurance companies, and individuals. Global spending on health more than doubled in real terms over the past two decades, reaching around US$ 8.5 trillion in 2019, or 9.8% of global GDP. Increasing healthcare spending raises the amount of funds for clinical research and development. This results in a large number of clinical trials and high demand for the services of CROs. High-income countries accounted for nearly 80% of global spending on health, with the US accounting for more than 40% alone.
Regulatory Environment
The regulatory environment has a significant impact on the clinical rese arch organization (CRO) market. Regulatory requirements for conducting clinical trials vary by country and region and considerably affect the demand for the services of CROs, the cost of conducting clinical trials, and the profitability of CROs. Government policies, such as regulations on quality standards, impact the market significantly. For instance, the Drugs and Cosmetics Act (1940) regulates the import, manufacture, and distribution of drugs and ensures that drugs and cosmetics sold are safe, effective, and conform to essential quality standards.
Clinical Research Organization Market Dynamics
Market drivers.
- Rise in the Number of Drug Development Activities
Rising Drug development activities are one of the positive factors for the clinical research organizations (CROs) market. The demand for CRO services increases drastically, as pharmaceuticals and biotechnology companies continue to invest in research and development to bring new drugs to market.
Drug development is a complex and expensive process that involves various steps, that includes drug discovery, preclinical studies, clinical trials, regulatory approval, and others. CROs offer significant cost savings to a large number of pharmaceutical companies by providing economies of scale. Drug development activities are increasing worldwide, which is a primary driving force of clinical research organizations. The demand for clinical trials to test the safety and efficacy of new drugs and medical devices is increasing, as the pharmaceutical industry continues to expand. For instance, in 2022 , Labcorp Drug Development helped to develop 100% of oncology drugs approved by the FDA (The United States Food and Drug Administration).
- Technology Advancement and Outsourcing of Clinical Trials
Outsourcing of clinical trials has become a common practice in the pharmaceutical and biotechnology industries. Clinical trials are complex and require significant resources, including specialized equipment, facilities, and personnel.
Many pharmaceuticals and biotech companies are outsourcing their clinical trial activities to clinical research organizations (CROs) to reduce costs, streamline operations, and improve efficiency. The demand for CROs increases, as more pharmaceutical companies outsource their clinical trials. This is boosting the market for these organizations.
Market Restraint
Regulatory Compliance
Regulatory compliance is an essential aspect of conducting clinical trials, and it is considered to be a significant restraint for the market. CROs are required to comply with strict regulations and guidelines set by regulatory authorities such as the FDA, EMA (The European Medicines Agency), and other national regulatory agencies.
Protecting the rights, safety, and welfare of people who participate in clinical trials is a critical aspect of the regulations set by the FDA. The organization oversees clinical trials to ensure they are designed, conducted, analyzed, and reported according to federal law and good clinical practice (GCP) regulations. Compliance with these regulations is challenging and time-consuming, which makes it difficult for CROs to compete in the market.
Scope of Clinical Research Organization Market Report
The report on the global Clinical Research Organization (CRO) market includes an assessment of the market, trends, segments, and regional markets. Overview and dynamics have also been included in the report.
Clinical Research Organization Market Segmental Outlook
On the basis of Service , the global clinical research organization (CRO) market is segmented into clinical research services, early-phase development services, laboratory services, consulting services, and others. The clinical research services segment is expected to expand at a CAGR of 12.4% from 2023 to 2031 . The clinical research services are further categorized into Phase I, Phase II, Phase III, and Phase IV. The growth of the segment is attributed to the increasing prevalence of chronic diseases and the increasing demand for effective medications & diagnostics products across the globe. Furthermore, various contract research organizations offer a wide range of clinical trial research services and support different areas of medical device & drug development. In 2020 , Parexel and Synairgen plc formed a strategic collaboration for conducting a Phase III study of an Interferon-beta (IFN-beta) treatment for COVID - 19 patients. Such strategic initiatives by CROs are expected to minimize the hindrance and boost the segment.
In terms of molecule type , the global clinical research organization (CRO) market is segmented into small molecules and biologics. The small molecules segment is expected to hold 38.5% share of the market during the forecast period, owing to the increasing number of chronic diseases. According to the British Heart Foundation's statistics in August 2022 , around 7.6 billion people in the UK suffered from some form of heart or cardiovascular disease in 2021 . Such an increase in the number of chronic diseases is expected to increase the demand for treatments, and ultimately the demand for small molecules.
Regional Outlook
On the basis of region , the global clinical research organization (CRO) market is divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America accounted for 40.0% share of the market in 2022 . This is attributed to the presence of major pharmaceutical companies in the region and their widespread drug development activity and excellent healthcare infrastructure. Additionally, several key pharmaceutical companies in the region are outsourcing R&D and clinical trials, due to the changes in competition and reimbursement from generic drugs. This is boosting the Clinical Research Organization (CRO) market in North America.
Key Benefits for Industry Participants & Stakeholders
- In-depth Analysis of the Global Clinical Research Organization (CRO) Market
- Historical, Current, and Projected Market Size in Terms of Value
- Potential & Niche Segments and Regions Exhibiting Promising Growth Covered
- Industry Drivers, Restraints, and Opportunities Covered in the Study
- Recent Industry Trends and Developments
- Competitive Landscape & Strategies of Key Players
- Neutral Perspective on Global Clinical Research Organization (CRO) Market Performance
- Clinical Research Services
- Early Phase Development Services
- Laboratory Services
- Consulting Services
By Molecule Type
- Small molecules
By End-user
- Pharmaceutical & Biotechnology Company
- Research & Academic Institute
- North America
- Asia Pacific
- Latin America
- Middle East & Africa
Key Market Players Profiled in the Report
- Asymchem Laboratories (Tianjin) Co., Ltd
- Charles River Laboratories.
- Dalton Pharma Services
- Eurofins Scientific
- Frontage Holdings Corporation
- Hangzhou Tigermed Consulting Co., Ltd.
- Jubilant Pharmova Limited
- Medpace, Inc.
- Parexel International Corporation
- Pharmaron Beijing Co., Ltd.
- Piramal Enterprises Ltd.
- REPROCELL Inc.
- Sun Pharmaceutical Industries Ltd.
- Syneos Health
- Thermo Fisher Scientific Inc.
- WuXi AppTec
Competitive Landscape
- Manufacturers operating in the global Clinical Research Organization (CRO) market include Asymchem Laboratories (Tianjin) Co., Ltd, Charles River Laboratories., Dalton Pharma Services, Domainex, Eurofins Scientific, Evotec, Frontage Holdings Corporation, Genscript, Hangzhou Tigermed Consulting Co., Ltd., ICON plc, Inotiv, IQVIA Inc, Jubilant Pharmova Limited, LabCorp, Medpace, Inc., Parexel International Corporation, Pharmaron Beijing Co., Ltd., Piramal Enterprises Ltd, REPROCELL Inc., SGS, Sun Pharmaceutical Industries Ltd., Syneos Health, Thermo Fisher Scientific Inc., WuXi AppTec.
- Market players are pursuing key strategies such as acquisitions, collaborations, and geographic expansion to increase their market share.
Purchase Premium Report
- Single User $4200
- Multi User $5200
- Corporate User $6600
- Online License $2999
- Excel Data Pack $2599
Customize This Report
- Ask for Research To Be Focused On Specific Regions or Segments
- Receive Data As Per Your Format and Definition
- Companies Profiled based on Your Requirements
- Breaking Down Competitive Landscape as per Your Requirements
- Any Level of Customization
Our Clients

We needed a highly accurate and precise report, which Growth Market Reports delivered promptly. The company compiled information from a wide array of reliable agencies and sourcess.It is extremely satisfactory to be working with you. Strategy Head of Major Tech Company
We were very pleased to contact Growth Market Reports as they tailored reports precisely as per our requirements. As we are dealing with the aerospace and defense industry, we need reports of high accuracy and substantial quality. Major Player in Defense Industry
Extremely delighted to have a well-crafted report on “Global Packaging Solutions Market Research Report” from your team. Thank you for providing me with all our requirements and for incorporating our suggestions. CMO of Leading Packaging Company from USA
I had a good experience working with Growth Market Reports as they were very open to all constructive changes in the report. I found that the report had its charm embedded with ample of data. Founder and Managing Partner of Major Korean Company
Our company has been working with Growth Market Reports for some years now and we are very happy with the quality of the reports provided by the company.I, on behalf of my organization, would like to thank you for offering professional reports. Global Consulting Firm
Quick Contact
+1 909 414 1393
Growth Market Report
Certified By
FAQ Section
Some frequently asked questions about this report!
1. What is the major end-user of Clinical Research Organization (CRO) that driving the market growth?
The research & academic institute segment held a market share of 33.3% in 2022 and is anticipated to expand at a CAGR of 12.2% during the forecast period. It is typically responsible for scientific research for the advancement of new drugs.
2. Who are the major players operating in the market?
Major manufacturers include IQVIA Inc, LabCorp, Thermo Fisher Scientific Inc., ICON plc, and Syneos Health are among the key players that hold a major chunk of the market.
3. Can I ask for different company profiles?
Additional company profiles are provided on request
4. What are the criteria used for selecting a company profile?
Factors such as competitive strength and market positioning are key areas considered while selecting key companies to be profiled.
5. What are the factors driving the Clinical Research Organization (CRO) market?
The rise in the number of drug development activities, technology advancement and outsourcing of clinical trials are the key driving factors for the market.
6. How big will be the Clinical Research Organization (CRO) market in 2030?
According to this Growth Market Reports report, the Clinical Research Organization (CRO) market is expected to register a CAGR of 12.3 % during the forecast period, 2015-2030, with an anticipated valuation of USD 220.3 Billion by the end of 2030.
7. Which macroeconomic factors affect the market?
Economic Condition, Increasing Healthcare Expenditure, Advancement in Technology, and Regulatory Environment are key macroeconomic factors shaping the market during the forecast period.
8. What is the impact of COVID-19 on the overall market during 2019 -2020?
The pandemic has positive impact on the global Clinical Research Organization (CRO) market. The COVID-19 outbreak spread worldwide, increase in number of clinical research activities has boost the demand of Clinical Research Organization (CRO).
9. What additional data analysis is available in a report?
In addition to market size (in USD Billion), the company market share (in % for the base year 2022), the impact of key regulations, current & future trends, and reimbursement scenario overview: private and public healthcare institutions by region have been provided.
10. What is the base year calculated in the global Clinical Research Organization (CRO) market report and what is the analysis period?
The base year considered for the global Clinical Research Organization (CRO) market report is 2021. The complete analysis period is 2016 to 2031, wherein, 2016 to 2021 are the historic years, and the forecast is provided from 2023 to 2031.
Related Reports
Some other reports from this category!
Drugs for Herpes Labialis (Oral Herpes) Market Trends [2031]
Dengue treatment market size, share & growth report | 2030, mmr vaccine market trends, growth, industry & revenue [2030], acromegaly treatment market size, share & growth report 2031, teenager myopia control market size, share & industry | 2031, cannabidiol (cbd) market share, opportunities, industry 2030, prosthetic joint infections treatment market forecast [2031], cannabis market size, share, growth & industry trends [2031], heart valve replacement market size, share & industry | 2030, ibuprofen market size, share, industry & opportunities 2031.
- Request For Sample
- Clinical Research Organization Market
Clinical Research Organization Market Size, Share, Opportunities, And Trends By Service Type (Early Phase Development Services, Clinical Research Services, Laboratory Services, Regulatory Consulting Services), By Therapeutic Area (Oncology, Clinical Pharmacology, Cardiology, Infectious Disease, Neurology, Gastroenterology & Hepatology, Ophthalmology, Others), By End-user (Pharmaceutical & Biopharmaceutical Companies, Medical Device Companies, Academic Institutes), And By Geography - Forecasts From 2024 To 2029
- Published : Mar 2024
- Report Code : KSI061616785
- Pages : 145
- Description
- Table Of Contents
- Companies Profiled
Offered with this report
Market Data and Estimates in Excel
2 Months of Post Sale Analyst Support
Requirement Specific Customization
The clinical research organization market is anticipated to grow significantly over the forecast period.
The market for clinical research organizations (CROs) has grown significantly due to factors such as the demand for specialized knowledge, growing R&D expenses, and the complexity of clinical trials. Pharmaceutical, biotechnology, and medical device firms can benefit from a variety of services provided by CROs, including data management, regulatory compliance, clinical trial management, and pharmacovigilance. The number of clinical trials that have been outsourced to CROs has increased recently because of their capacity to offer affordable solutions, access to patient populations throughout the world, and proficiency in handling regulatory requirements in various geographic areas. The CRO industry has expanded because of technological breakthroughs like artificial intelligence and electronic data capture, which have improved data analysis capabilities and streamlined trial procedures. The CRO industry is very cutthroat, with both big international companies and specialty suppliers competing for market share. CROs frequently use mergers and acquisitions as a means of growing their service offerings and geographic reach. In the upcoming years, it is anticipated that the CRO market will continue to rise because of the rising demand for novel therapeutics and strict regulatory requirements.
Market Drivers
- The increasing demand for outsourcing is expected to drive the clinical research organization market.
The demand for Clinical Research Organizations to handle clinical trial outsourcing is significantly increasing in the biotechnology and pharmaceutical industries. The need to reduce expenses, shorten the time it takes to create new drugs and get access to specialized knowledge is what is driving this trend. Businesses can reduce operational risk, maximize resource allocation, and concentrate on their core strengths by utilizing the services of CROs.
Furthermore, outsourcing makes it possible to connect with a worldwide network of researchers and patients, which makes it easier to carry out trials in a variety of geographical locations. All things considered, the growing demand for outsourcing highlights how essential it is to boost productivity and creativity within the drug development ecosystem.
Chronic diseases increase will also drive the market. According to NCBI, 2023, between 2020 and 2050, there will be a 61.11% increase in the number of Americans 50 years of age and older, from 137.25 million to 221.13 million. The number of people 50 years of age and older who have at least one chronic illness is predicted to rise from 71.522 million in 2020 to 142.66 million in 2050, a 99.5% increase. Simultaneously, it is anticipated that the number of people with multimorbidity will rise from 7.8304 million in 2020 to 14.968 million in 2050, a 91.16% increase. According to race, by 2050, non-Hispanic whites (64.6%), non-Hispanic blacks (61.47%), and Hispanics and other races (64.5%) are expected to have one or more chronic illnesses.
- Globalization of clinical trials is expected to boost the market.
Clinical trial globalization is a reflection of a major change in the pharmaceutical and biotechnology sectors toward conducting research across a variety of geographical locations. Regulatory incentives, economic benefits in emerging markets, and access to larger and more diversified patient populations are some of the elements driving this trend. Companies can ensure wider applicability of data, improve variety in research populations, and speed up patient enrollment by expanding trial locations abroad.
Globalization does, however, come with drawbacks, like complicated regulations, disparities in culture, and logistical difficulties. Notwithstanding these obstacles, clinical trials' globalization is nonetheless vital to the advancement of medical research and the enhancement of patients' access to cutting-edge therapies on a global scale.
- Advancing technology is a significant driver for the clinical research organization market.
Technological developments are transforming clinical research by improving patient involvement, data quality, and efficiency. More accurate patient selection, predictive modeling, and real-time trial progress monitoring are made possible by innovations like artificial intelligence, machine learning, and big data analytics. Wearables and remote monitoring tools are examples of digital health technology that enable decentralized trial conduct, enhancing patient convenience and cutting expenses.
Moreover, computerized data capture technologies expedite the process of developing new drugs by streamlining data collecting and processing. All things considered, these technological developments enable researchers to make data-driven choices, maximize trial results, and eventually accelerate the release of safe and efficient treatments onto the market.
IQVIA received recognition for its AI software in June 2023. In order to enable real-world data research, the program, which incorporates natural language processing technology, assists in the analysis of complicated and unstructured patient data, offering distinctive insights into patient care and disease states. What makes the AI software unique is its capacity to offer insights that can be put into practice. It opens up important perspectives on population health, such as the social determinants of health (SDoH), which determine most health outcomes. One of the biggest healthcare systems in Illinois, Northshore-Edward-Elmhurst Health, is using this technology to help doctors identify and screen 56% more at-risk patients based on SDoH, enabling patients in need to receive focused intervention.
Asia Pacific region is expected to grow significantly.
The clinical research organization (CRO) market in Asia-Pacific is expanding significantly as a result of several important factors. The growing incidence of chronic illnesses, growing investments in healthcare infrastructure, and the development of the biotechnology and pharmaceutical industries are the main drivers of this rise. In addition, the area has cheaper operating expenses than Western markets and a sizable and diversified patient base. In nations like China, India, and South Korea, the conduct of clinical trials is further facilitated by regulatory reforms and advantageous government policies.
Consequently, the CRO market has experienced significant expansion, with Asia-Pacific emerging as a major hub for outsourcing clinical research activities. For instance, In November 2021, Based on the encouraging pre-clinical outcomes of LG00034053, a novel medication candidate for the treatment of osteoarthritis, LG Chem declared on the 4th that the company has been granted approval by the Korean Ministry of Food and Drug Safety for the Phase 1b/2 clinical trials.LG Chem aims to expedite the creation of novel medications by creating clinical trials that connect Phases 1 and 2. With the help of this approval, LG Chem will carry out studies at Samsung Medical Center to determine the best dose for patients with mild to moderate knee osteoarthritis (K&L2-3) by assessing factors like safety and tolerability, pharmacokinetics (the process of drug absorption, distribution, metabolism, and excretion), and effectiveness.
Market Restraints
- Increasing competition and consolidation could constraint the market
Demand growth is causing the Clinical Research Organization market to become more competitive and consolidated. As more businesses join the market, the rivalry for talent and contracts gets fiercer, which puts pressure on prices. Consolidation of the sector is fueled by larger CROs acquiring smaller ones to increase capabilities and reach globally. Even while there are advantages like economies of scale, smaller CROs could find it difficult to compete. Players in the CRO sector face a great deal of difficulty in striking a balance between competitiveness and consolidation.
Market Developments
- January 2024- Labcorp, a global leader of innovative and comprehensive laboratory services, and Hawthorne Effect, Inc., a complete clinical trials solution integrating technology, announced a strategic collaboration to advance centralized clinical trial capabilities for sponsors seeking to increase patient diversity and inclusion, decrease site burden, and accelerate enrollment and clinical study timelines.
- October 2023- Through creative and integrated technology-enabled pharmacovigilance (PV) safety services and solutions, IQVIA, a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry, announced a strategic collaboration with argenx to advance treatment for patients with rare autoimmune diseases.
Segmentation
- Early phase development services
- Clinical research services
- Laboratory services
- Regulatory consulting services
- Clinical pharmacology
- Infectious disease
- Gastroenterology & Hepatology
- Ophthalmology
- Pharmaceutical & Biopharmaceutical Companies
- Medical Device Companies
- Academic Institutes
- United Kingdom
- Saudi Arabia
- South Korea
1. INTRODUCTION
1.1. Market Overview
1.2. Market Definition
1.3. Scope of the Study
1.4. Market Segmentation
1.5. Currency
1.6. Assumptions
1.7. Base, and Forecast Years Timeline
1.8. Key Benefits to the Stakeholder
2. RESEARCH METHODOLOGY
2.1. Research Design
2.2. Research Processes
3. EXECUTIVE SUMMARY
3.1. Key Findings
3.2. Analyst View
4. MARKET DYNAMICS
4.1. Market Drivers
4.2. Market Restraints
4.3. Porter’s Five Forces Analysis
4.3.1. Bargaining Power of Suppliers
4.3.2. Bargaining Power of Buyers
4.3.3. Threat of New Entrants
4.3.4. Threat of Substitutes
4.3.5. Competitive Rivalry in the Industry
4.4. Industry Value Chain Analysis
4.5. Analyst View
5. CLINICAL RESEARCH ORGANIZATION MARKET BY SERVICE TYPE
5.1. Introduction
5.2. Early phase development services
5.2.1. Market Trends and Opportunities
5.2.2. Growth Prospects
5.2.3. Discovery services
5.2.4. Chemistry, manufacturing & control (CMC)
5.2.5. Preclinical services
5.2.5.1. Pharmacokinetics/Pharmacodynamics (PK/PD)
5.2.5.2. Toxicology services
5.2.5.3. Others
5.3. Clinical research services
5.3.1. Market Trends and Opportunities
5.3.2. Growth Prospects
5.3.3. Phase I
5.3.4. Phase II
5.3.5. Phase III
5.3.6. Phase IV
5.4. Laboratory services
5.4.1. Market Trends and Opportunities
5.4.2. Growth Prospects
5.4.3. Bioanalytical testing services
5.4.4. Analytical testing services
5.4.5. Physical Characterization
5.4.6. Raw Material Testing
5.4.7. Batch Release Testing
5.4.8. Stability Testing
5.4.9. Others
5.5. Regulatory consulting services
5.5.1. Market Trends and Opportunities
5.5.2. Growth Prospects
6. CLINICAL RESEARCH ORGANIZATION MARKET BY THERAPEUTIC AREA
6.1. Introduction
6.2. Oncology
6.2.1. Market Trends and Opportunities
6.2.2. Growth Prospects
6.3. Clinical pharmacology
6.3.1. Market Trends and Opportunities
6.3.2. Growth Prospects
6.4. Cardiology
6.4.1. Market Trends and Opportunities
6.4.2. Growth Prospects
6.5. Infectious disease
6.5.1. Market Trends and Opportunities
6.5.2. Growth Prospects
6.6. Neurology
6.6.1. Market Trends and Opportunities
6.6.2. Growth Prospects
6.7. Gastroenterology & Hepatology
6.7.1. Market Trends and Opportunities
6.7.2. Growth Prospects
6.8. Ophthalmology
6.8.1. Market Trends and Opportunities
6.8.2. Growth Prospects
6.9. Others
6.9.1. Market Trends and Opportunities
6.9.2. Growth Prospects
7. CLINICAL RESEARCH ORGANIZATION MARKET BY END-USER
7.1. Introduction
7.2. Pharmaceutical & Biopharmaceutical Companies
7.2.1. Market Trends and Opportunities
7.2.2. Growth Prospects
7.3. Medical Device Companies
7.3.1. Market Trends and Opportunities
7.3.2. Growth Prospects
7.4. Academic Institutes
7.4.1. Market Trends and Opportunities
7.4.2. Growth Prospects
8. CLINICAL RESEARCH ORGANIZATION MARKET BY GEOGRAPHY
8.1. Introduction
8.2. North America
8.2.1. By Service Type
8.2.2. By Therapeutic Area
8.2.3. By End User
8.2.4. By Country
8.2.4.1. United States
8.2.4.1.1. Market Trends and Opportunities
8.2.4.1.2. Growth Prospects
8.2.4.2. Canada
8.2.4.2.1. Market Trends and Opportunities
8.2.4.2.2. Growth Prospects
8.2.4.3. Mexico
8.2.4.3.1. Market Trends and Opportunities
8.2.4.3.2. Growth Prospects
8.3. South America
8.3.1. By Service Type
8.3.2. By Therapeutic Area
8.3.3. By End User
8.3.4. By Country
8.3.4.1. Brazil
8.3.4.1.1. Market Trends and Opportunities
8.3.4.1.2. Growth Prospects
8.3.4.2. Argentina
8.3.4.2.1. Market Trends and Opportunities
8.3.4.2.2. Growth Prospects
8.3.4.3. Others
8.3.4.3.1. Market Trends and Opportunities
8.3.4.3.2. Growth Prospects
8.4. Europe
8.4.1. By Service Type
8.4.2. By Therapeutic Area
8.4.3. By End User
8.4.4. By Country
8.4.4.1. United Kingdom
8.4.4.1.1. Market Trends and Opportunities
8.4.4.1.2. Growth Prospects
8.4.4.2. Germany
8.4.4.2.1. Market Trends and Opportunities
8.4.4.2.2. Growth Prospects
8.4.4.3. France
8.4.4.3.1. Market Trends and Opportunities
8.4.4.3.2. Growth Prospects
8.4.4.4. Italy
8.4.4.4.1. Market Trends and Opportunities
8.4.4.4.2. Growth Prospects
8.4.4.5. Spain
8.4.4.5.1. Market Trends and Opportunities
8.4.4.5.2. Growth Prospects
8.4.4.6. Others
8.4.4.6.1. Market Trends and Opportunities
8.4.4.6.2. Growth Prospects
8.5. Middle East and Africa
8.5.1. By Service Type
8.5.2. By Therapeutic Area
8.5.3. By End User
8.5.4. By Country
8.5.4.1. Saudi Arabia
8.5.4.1.1. Market Trends and Opportunities
8.5.4.1.2. Growth Prospects
8.5.4.2. UAE
8.5.4.2.1. Market Trends and Opportunities
8.5.4.2.2. Growth Prospects
8.5.4.3. Others
8.5.4.3.1. Market Trends and Opportunities
8.5.4.3.2. Growth Prospects
8.6. Asia Pacific
8.6.1. By Service Type
8.6.2. By Therapeutic Area
8.6.3. By End User
8.6.4. By Country
8.6.4.1. Japan
8.6.4.1.1. Market Trends and Opportunities
8.6.4.1.2. Growth Prospects
8.6.4.2. China
8.6.4.2.1. Market Trends and Opportunities
8.6.4.2.2. Growth Prospects
8.6.4.3. India
8.6.4.3.1. Market Trends and Opportunities
8.6.4.3.2. Growth Prospects
8.6.4.4. South Korea
8.6.4.4.1. Market Trends and Opportunities
8.6.4.4.2. Growth Prospects
8.6.4.5. Taiwan
8.6.4.5.1. Market Trends and Opportunities
8.6.4.5.2. Growth Prospects
8.6.4.6. Thailand
8.6.4.6.1. Market Trends and Opportunities
8.6.4.6.2. Growth Prospects
8.6.4.7. Indonesia
8.6.4.7.1. Market Trends and Opportunities
8.6.4.7.2. Growth Prospects
8.6.4.8. Others
8.6.4.8.1. Market Trends and Opportunities
8.6.4.8.2. Growth Prospects
9. COMPETITIVE ENVIRONMENT AND ANALYSIS
9.1. Major Players and Strategy Analysis
9.2. Market Share Analysis
9.3. Mergers, Acquisitions, Agreements, and Collaborations
9.4. Competitive Dashboard
10. COMPANY PROFILES
10.1. Charles River Laboratories, Inc.
10.2. Laboratory Corporation of America Holdings
10.3. IQVIA
10.4. Pharmaceutical Product Development
10.5. PAREXEL International
10.6. Syneos Health
10.7. ICON plc.
10.8. Medpace
10.9. CTI Clinical Trial and Consulting Services
10.10. QPS Neuropharmacology
Charles River Laboratories, Inc.
Laboratory Corporation of America Holdings
Pharmaceutical Product Development
PAREXEL International
Syneos Health
CTI Clinical Trial and Consulting Services
QPS Neuropharmacology
Related Reports
License Type
Explore custom options available with this study.
- Customize this report
- Buy sections of the study
- Buy country specific report
Subscribe Us
Let us know the industries you are interested in, request free sample.

- Pharmaceuticals
Clinical Research Organization Market
Global Clinical Research Organization Market Size, Trends & Analysis - Forecasts to 2028 By Type (Drug Discovery, Pre-clinical, and Clinical), By Service (Project Management/Clinical Supply Management, Data Management, Regulatory/Medical Affairs, Medical Writing, Clinical Monitoring, Quality Management/ Assurance, Bio-statistics, Investigator Payments, Laboratory, Patient and site Recruitment, Technology, and Others), By Application (Oncology, Cardiovascular, Autoimmune/Inflammation, Central nervous system (CNS), Dermatology, Infectious diseases, Diabetes, Pain, and Others), By End User (Pharmaceutical & Biopharmaceutical Companies, Medical Device Companies, and Others), and By Region (North America, Asia Pacific, Central & South America, Europe, and Middle East and Africa), Competitive Landscape, Company Market Share Analysis, and End User Analysis
- Report Summary
- Table of Content
- Research Methodology
- Customize this research
Global Clinical Research Organization Market Size
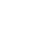
The global clinical research organization market is projected to grow at a CAGR of 7.6% from 2023 to 2028.
Clinical research organizations (CROs) offer outsourced services to the biotechnology and pharmaceutical sectors. They carry out clinical studies for novel medications and treatments. Drug firms themselves or academic institutions doing the trials can hire CROs. CROs are created to save expenses for businesses creating novel medications and pharmaceuticals for specialized markets. They want to make the medication market entrance and development simpler because it is no longer necessary for huge pharmaceutical corporations to handle everything "in-house”. These organizations offer services that can speed up the whole process of bringing a novel technology or medication to market, from concept creation through FDA marketing approval, without the requirement for the drug's sponsor to keep any people on staff to deliver these services. The pharmaceutical and drug development companies devote a lot of resources and funding to CROs supporting more globally coordinated and collaborative research activities as a result of the growing priority placed on research and development. This is the rationale behind the rising importance of strategic alliances in the value chain for novel medication development.
The increasing demand for efficient and cost-effective drug development processes, increased research and development spending in the pharmaceutical industry, and the growing demand for new clinical trial designs for cell & gene therapies are expected to support the growth of the market during the forecast period. Additionally, the market is expanding as a result of the increased incidence of chronic diseases, the high cost of in-house drug research, the focus on rare diseases, and the numerous orphan medications in development.
CROs play a crucial role in clinical trials as they have plenty of opportunities in the fast-expanding therapeutic disciplines of oncology, neurology, cardiology, and vaccines. To increase patient involvement, study compliance, and continuous monitoring, CROs can include telemedicine and digital health technologies in clinical trials, which presents an opportunity for the major players in the global market. However, CROs continue to face a various challenges, such as commoditization, intense competition, and cutting-edge patient engagement models. Due to market segmentation and larger businesses capturing a larger share of the market, smaller CROs are under pressure.
Well-established companies these areas can provide pharmaceutical and biotechnology companies with specialized services and support for both real-world research and virtual studies, which can reduce the length and cost of the trial. They may now also provide specialized services in data analytics and machine learning due to the increasing accessibility of real-time data in clinical trials. To do this, one may use predictive analytics to anticipate risks, identify trends, and enhance research design, which contributes to the expansion of the global market.
Digital therapies are software-based treatments intended to treat or manage medical diseases. CROs have become more involved in the development and assessment of these interventions. To improve patient results, these therapies are frequently utilized in conjunction with conventional pharmacological techniques. CROs were using big data and sophisticated analytics approaches to glean valuable insights from vast amounts of clinical and patient data. This support better decision-making, trend spotting, and trial protocol optimization.
Global Clinical Research Organization Market: By Type
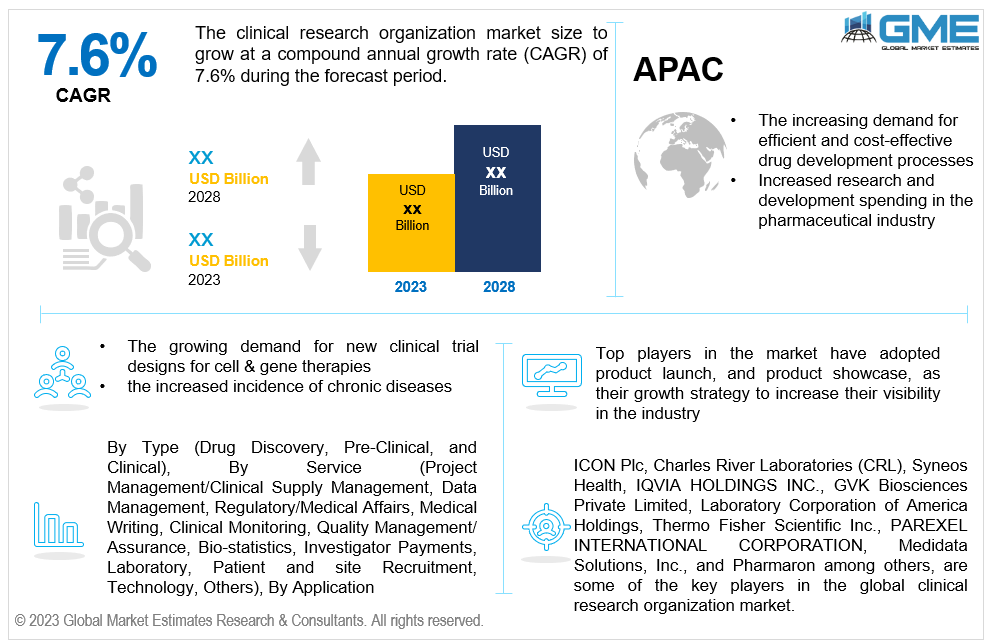
The pre-clinical is expected to be the fastest-growing segment in the market during the forecast period. Leading CROs have amassed a large amount of pre-clinical experience, and CROs are utilizing this expertise to provide highly effective and precise pre-clinical services. Additionally, CROs let small and midsize businesses participate in the difficult drug development process without having to make a substantial investment in capital equipment. Pre-clinical trials are more successful, according to an article by Anju Life Sciences Software that was published in March 2021, due to higher data quality, better safety judgments, lower trial operating expenses, and quicker study execution.
The clinical segment holds the largest share of the market during the forecast period. The segment is expanding due to the rising incidence of chronic diseases and the rising demand for rapid and precise disease diagnostics.
Global Clinical Research Organization Market: By Service
Data management is anticipated to be the fastest-growing segment in the market during the forecast period. To collect data from clinical trial locations, data management entails using electronic data capture (EDC) systems or using alternative data gathering techniques. This can entail making case report forms (CRFs) and guaranteeing that the information gathered conforms with legal requirements.
The project management/clinical supply management segment holds the largest share of the market. Clinical trial design, coordination, and execution are the main goals of these services, which also make sure that clinical supplies are available and managed appropriately. In the CRO industry, project management services entail supervising every component of a clinical study to guarantee its proper execution within specified schedules and financial restrictions. Clinical supply management services prioritize the prompt and effective provision of research medications, medical equipment, and other materials needed for the clinical trial.
Global Clinical Research Organization Market: By Application
The central nervous system (CNS) is anticipated to be the fastest-growing segment in the market during the forecast period. CROs are essential to the management of clinical trials for CNS illnesses including Parkinson's, Alzheimer's, and other similar conditions. They offer knowledge in protocol formulation, location choice, patient recruiting, and trial administration as a whole.
The oncology segment holds the largest share of the market. Preclinical research, clinical trials, and other stages of the drug development process are all supported by a variety of services offered by oncology CROs. Since there are many different medications being developed in the cancer industry, oncology CROs need to have a thorough understanding of the disease process and cutting-edge therapies. Additionally, they must be able to plan and carry out clinical studies that adhere to the exacting requirements of the FDA and other authorities.
Global Clinical Research Organization Market: By End User
The medical device companies is anticipated to be the fastest-growing segment in the market during the forecast period. Clinical trials for new or enhanced medical devices are carried out by CROs on behalf of medical device manufacturers. CROs offer competence in patient recruiting, data management, regulatory compliance, research design, and statistical analysis. They aid manufacturers of medical devices in navigating the challenging world of clinical research and support the efficient and successful conduct of clinical studies.
The pharmaceutical & biopharmaceutical companies segment holds the largest share of the market. Due to the complicated nature of the drug development process, pharmaceutical companies are increasingly depending on CROs for drug research and development services. In order to cut costs and speed up time to market, biotechnology companies are also outsourcing their R&D operations to CROs. Thus, it is anticipated that increasing investment in R&D spending would fuel this segment's growth throughout the forecast period.
Global Clinical Research Organization Market: By Region
Regionally, North America is projected to hold the leading position in the global region in the market. This is due to the region conducting a rising number of clinical trials as well as the existence of several CROs in nations like the United States and Canada. CRO companies are in great demand throughout the region as the desire to outsource R&D operations grows.
Asia Pacific is predicted to have rapid growth during the forecast period. This can be attributed to elements including rising healthcare costs, expanding government funding for clinical research, and an increase in the number of people with chronic illnesses. Clinical trials are being conducted in China at a higher rate thanks to the government's and China's Food and Drug Administration's regulatory flexibility.
Europe market is anticipated to see stable market growth throughout the forecast period due to the favorable reimbursement policies for clinical trials and the rising number of biopharmaceutical businesses.
Global Clinical Research Organization Market Share and Competitor Analysis
Some of the key players in the global clinical research organization market are ICON Plc, Charles River Laboratories (CRL), Syneos Health , IQVIA HOLDINGS INC., GVK Biosciences Private Limited, Laboratory Corporation of America Holdings, Thermo Fisher Scientific Inc., PAREXEL INTERNATIONAL CORPORATION, Medidata Solutions, Inc., and Pharmaron among others..
Please note: This is not an exhaustive list of companies profiled in the report.
1 STRATEGIC INSIGHTS ON NEW REVENUE POCKETS
1.1 Strategic Opportunity & Attractiveness Analysis
1.1.1 Hot Revenue Pockets
1.1.2 Market Attractiveness Score
1.1.3 Revenue Impacting Opportunity
1.1.4 High Growing Region/Country
1.1.5 Competitor Analysis
1.1.6 Consumer Analysis
1.2 Global Market Estimates' View
1.3 Strategic Insights across Business Functions
1.3.1 For Chief Executive Officers
1.3.2 For Chief Marketing Officers
1.3.3 For Chief Strategy Officers
1.4 Evaluate the Potential of your Existing Business Lines vs. New Lines to Enter Into
2 TECHNOLOGICAL TRENDS
2.1 Technological Adoption Rate
2.2 Current Trend Impact Analysis
2.3 Future Trend Impact Analysis
2.4 Data Metrics on Feed Stocks
3 GLOBAL MARKET OUTLOOK
3.1 Market Pyramid Analysis
3.1.1 Introduction
3.1.2 Adjacent Market Opportunities
3.1.3 Ancillary Market Opportunities
3.2 Demand Side Analysis
3.2.1 Market Drivers: Impact Analysis
3.2.2 Market Restraints: Impact Analysis
3.2.3 Market Opportunities: Impact Analysis
3.2.4 Market Challenges: Impact Analysis
3.3 Supply Side Analysis
3.3.1 Porter’s Five Forces Analysis
3.3.1.1 Threat of New Entrants
3.3.1.2 Threat of New Substitutes
3.3.1.3 Bargaining Power of Suppliers
3.3.1.4 Bargaining Power of Buyers
3.3.1.5 Intensity of Competitive Rivalry
3.3.2 SWOT Analysis; By Factor (Political & Legal, Economic, and Technological)
3.3.2.1 Political Landscape
3.3.2.2 Economic Landscape
3.3.2.3 Social Landscape
3.3.2.4 Technology Landscape
3.3.3 Value Chain Analysis
3.3.4 Trend Analysis
3.3.5 Gap Analysis
3.3.6 Cost Analysis
4 GLOBAL CLINICAL RESEARCH ORGANIZATION MARKET, BY TYPE
4.1 Introduction
4.2 Clinical Research Organization Market: Type Scope Key Takeaways
4.3 Revenue Growth Analysis, 2022 & 2028
4.4 Drug Discovery
4.4.1 Drug Discovery Market Estimates and Forecast, 2020-2028 (USD Million)
4.5 Pre-Clinical
4.5.1 Pre-Clinical Market Estimates and Forecast, 2020-2028 (USD Million)
4.6 Clinical
4.6.1 Clinical Market Estimates and Forecast, 2020-2028 (USD Million)
5 GLOBAL CLINICAL RESEARCH ORGANIZATION MARKET, BY SERVICE
5.1 Introduction
5.2 Clinical Research Organization Market: Service Scope Key Takeaways
5.3 Revenue Growth Analysis, 2022 & 2028
5.4 Project Management/Clinical Supply Management
5.4.1 Project Management/Clinical Supply Management Market Estimates and Forecast, 2020-2028 (USD Million)
5.5 Data Management
5.5.1 Data Management Market Estimates and Forecast, 2020-2028 (USD Million)
5.6 Regulatory/Medical Affairs
5.6.1 Regulatory/Medical Affairs Market Estimates and Forecast, 2020-2028 (USD Million)
5.7 Medical Writing
5.7.1 Medical Writing Market Estimates and Forecast, 2020-2028 (USD Million)
5.8 Clinical Monitoring
5.8.1 Clinical Monitoring Market Estimates and Forecast, 2020-2028 (USD Million)
5.9 Quality Management/ Assurance
5.9.1 Quality Management/ Assurance Market Estimates and Forecast, 2020-2028 (USD Million)
5.10 Bio-statistics
5.10.1 Bio-statistics Market Estimates and Forecast, 2020-2028 (USD Million)
5.11 Investigator Payments
5.11.1 Investigator Payments Market Estimates and Forecast, 2020-2028 (USD Million)
5.12 Laboratory
5.12.1 Laboratory Market Estimates and Forecast, 2020-2028 (USD Million)
5.13 Patient and site Recruitment
5.13.1 Patient and site Recruitment Market Estimates and Forecast, 2020-2028 (USD Million)
5.14 Technology
5.14.1 Technology Market Estimates and Forecast, 2020-2028 (USD Million)
5.15 Others
5.15.1 Others Market Estimates and Forecast, 2020-2028 (USD Million)
6 GLOBAL CLINICAL RESEARCH ORGANIZATION MARKET, BY APPLICATION
6.1 Introduction
6.2 Clinical Research Organization Market: Application Scope Key Takeaways
6.3 Revenue Growth Analysis, 2022 & 2028
6.4 Oncology
6.4.1 Oncology Market Estimates and Forecast, 2020-2028 (USD Million)
6.5 Cardiovascular
6.5.1 Cardiovascular Market Estimates and Forecast, 2020-2028 (USD Million)
6.6 Autoimmune/Inflammation
6.6.1 Autoimmune/Inflammation Market Estimates and Forecast, 2020-2028 (USD Million)
6.7 Central nervous system (CNS)
6.7.1 Central nervous system (CNS) Market Estimates and Forecast, 2020-2028 (USD Million)
6.8 Dermatology
6.8.1 Dermatology Market Estimates and Forecast, 2020-2028 (USD Million)
6.9 Infectious diseases
6.9.1 Infectious diseases Market Estimates and Forecast, 2020-2028 (USD Million)
6.10 Diabetes
6.10.1 Diabetes Market Estimates and Forecast, 2020-2028 (USD Million)
6.11.1 Pain Market Estimates and Forecast, 2020-2028 (USD Million)
6.12 Others
6.12.1 Others Market Estimates and Forecast, 2020-2028 (USD Million)
7 GLOBAL CLINICAL RESEARCH ORGANIZATION MARKET, BY END USER
7.1 Introduction
7.2 Clinical Research Organization Market: End User Scope Key Takeaways
7.3 Revenue Growth Analysis, 2022 & 2028
7.4 Pharmaceutical & Biopharmaceutical Companies
7.4.1 Pharmaceutical & Biopharmaceutical Companies Market Estimates and Forecast, 2020-2028 (USD Million)
7.5 Medical Device Companies
7.5.1 Medical Device Companies Market Estimates and Forecast, 2020-2028 (USD Million)
7.6 Others
7.6.1 Others Market Estimates and Forecast, 2020-2028 (USD Million)
8 GLOBAL CLINICAL RESEARCH ORGANIZATION MARKET, BY REGION
8.1 Introduction
8.2 North America Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.2.1 By Type
8.2.2 By Service
8.2.3 By Application
8.2.4 By End User
8.2.5 By Country
8.2.5.1 U.S. Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.2.5.1.1 By Type
8.2.5.1.2 By Service
8.2.5.1.3 By Application
8.2.5.1.4 By End User
8.2.5.2 Canada Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.2.5.2.1 By Type
8.2.5.2.2 By Service
8.2.5.2.3 By Application
8.2.5.2.4 By End User
8.2.5.3 Mexico Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.2.5.3.1 By Type
8.2.5.3.2 By Service
8.2.5.3.3 By Application
8.2.5.3.4 By End User
8.3 Europe Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.3.1 By Type
8.3.2 By Service
8.3.3 By Application
8.3.4 By End User
8.3.5 By Country
8.3.5.1 Germany Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.3.5.1.1 By Type
8.3.5.1.2 By Service
8.3.5.1.3 By Application
8.3.5.1.4 By End User
8.3.5.2 U.K. Presered Flowers Market Estimates and Forecast, 2020-2028 (USD Million)
8.3.5.2.1 By Type
8.3.5.2.2 By Service
8.3.5.2.3 By Application
8.3.5.2.4 By End User
8.3.5.3 France Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.3.5.3.1 By Type
8.3.5.3.2 By Service
8.3.5.3.3 By Application
8.3.5.3.4 By End User
8.3.5.4 Italy Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.3.5.4.1 By Type
8.3.5.4.2 By Service
8.3.5.4.3 By Application
8.3.5.4.4 By End User
8.3.5.5 Spain Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.3.5.5.1 By Type
8.3.5.5.2 By Service
8.3.5.5.3 By Application
8.3.5.5.4 By End User
8.3.5.6 Netherlands Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.3.5.6.1 By Type
8.3.5.6.2 By Service
8.3.5.6.3 By Application
8.3.5.6.4 By End User
8.3.5.7 Rest of Europe Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.3.5.7.1 By Type
8.3.5.7.2 By Service
8.3.5.7.3 By Application
8.3.5.7.4 By End User
8.4 Asia Pacific Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.1 By Type
8.4.2 By Service
8.4.3 By Application
8.4.4 By End User
8.4.5 By Country
8.4.5.1 China Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.1.1 By Type
8.4.5.1.2 By Service
8.4.5.1.3 By Application
8.4.5.1.4 By End User
8.4.5.2 Japan Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.2.1 By Type
8.4.5.2.2 By Service
8.4.5.2.3 By Application
8.4.5.2.4 By End User
8.4.5.3 India Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.3.1 By Type
8.4.5.3.2 By Service
8.4.5.3.3 By Application
8.4.5.3.4 By End User
8.4.5.4 South Korea Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.4.1 By Type
8.4.5.4.2 By Service
8.4.5.4.3 By Application
8.4.5.4.4 By End User
8.4.5.5 Singapore Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.5.1 By Type
8.4.5.5.2 By Service
8.4.5.5.3 By Application
8.4.5.5.4 By End User
8.4.5.6 Malaysia Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.6.1 By Type
8.4.5.6.2 By Service
8.4.5.6.3 By Application
8.4.5.6.4 By End User
8.4.5.7 Thailand Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.7.1 By Type
8.4.5.7.2 By Service
8.4.5.7.3 By Application
8.4.5.7.4 By End User
8.4.5.8 Indonesia Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.8.1 By Type
8.4.5.8.2 By Service
8.4.5.8.3 By Application
8.4.5.8.4 By End User
8.4.5.9 Vietnam Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.9.1 By Type
8.4.5.9.2 By Service
8.4.5.9.3 By Application
8.4.5.9.4 By End User
8.4.5.10 Taiwan Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.10.1 By Type
8.4.5.10.2 By Service
8.4.5.10.3 By Application
8.4.5.10.4 By End User
8.4.5.11 Rest of Asia Pacific Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.4.5.11.1 By Type
8.4.5.11.2 By Service
8.4.5.11.3 By Application
8.4.5.11.4 By End User
8.5 Middle East and Africa Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.5.1 By Type
8.5.2 By Service
8.5.3 By Application
8.5.4 By End User
8.5.5 By Country
8.5.5.1 Saudi Arabia Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.5.5.1.1 By Type
8.5.5.1.2 By Service
8.5.5.1.3 By Application
8.5.5.1.4 By End User
8.5.5.2 U.A.E. Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.5.5.2.1 By Type
8.5.5.2.2 By Service
8.5.5.2.3 By Application
8.5.5.2.4 By End User
8.5.5.3 Israel Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.5.4.3.1 By Type
8.5.4.3.2 By Service
8.5.4.3.3 By Application
8.5.5.3.4 By End User
8.5.5.4 South Africa Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.5.5.4.1 By Type
8.5.5.4.2 By Service
8.5.5.4.3 By Application
8.5.5.4.4 By End User
8.5.5.5 Rest of Middle East and Africa Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.5.5.5.1 By Type
8.5.5.5.2 By Service
8.5.5.5.2 By Application
8.5.5.5.4 By End User
8.6 Central & South America Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.6.1 By Type
8.6.2 By Service
8.6.3 By Application
8.6.4 By End User
8.6.5 By Country
8.6.5.1 Brazil Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.6.5.1.1 By Type
8.6.5.1.2 By Service
8.6.5.1.3 By Application
8.6.5.1.4 By End User
8.6.5.2 Argentina Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.6.5.2.1 By Type
8.6.5.2.2 By Service
8.6.5.2.3 By Application
8.6.5.2.4 By End User
8.6.5.3 Chile Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.6.5.3.1 By Type
8.6.5.3.2 By Service
8.6.5.3.3 By Application
8.6.5.5.4 By End User
8.6.5.4 Rest of Central & South America Clinical Research Organization Market Estimates and Forecast, 2020-2028 (USD Million)
8.6.5.4.1 By Type
8.6.5.4.2 By Service
8.6.5.4.3 By Application
8.6.5.4.4 By End User
9 COMPETITIVE LANDCAPE
9.1 Company Market Share Analysis
9.2 Four Quadrant Positioning Matrix
9.2.1 Market Leaders
9.2.2 Market Visionaries
9.2.3 Market Challengers
9.2.4 Niche Market Players
9.3 Vendor Landscape
9.3.1 North America
9.3.2 Europe
9.3.3 Asia Pacific
9.3.4 Rest of the World
9.4 Company Profiles
9.4.1 ICON Plc
9.4.1.1 Business Description & Financial Analysis
9.4.1.2 SWOT Analysis
9.4.1.3 Products & Services Offered
9.4.1.4 Strategic Alliances between Business Partners
9.4.2 Charles River Laboratories (CRL)
9.4.2.1 Business Description & Financial Analysis
9.4.2.2 SWOT Analysis
9.4.2.3 Products & Services Offered
9.4.2.4 Strategic Alliances between Business Partners
9.4.3 Syneos Health
9.4.3.1 Business Description & Financial Analysis
9.4.3.2 SWOT Analysis
9.4.3.3 Products & Services Offered
9.4.3.4 Strategic Alliances between Business Partners
9.4.4 IQVIA HOLDINGS INC.
9.4.4.1 Business Description & Financial Analysis
9.4.4.2 SWOT Analysis
9.4.4.3 Products & Services Offered
9.4.4.4 Strategic Alliances between Business Partners
9.4.5 Laboratory Corporation of America Holdings
9.4.5.1 Business Description & Financial Analysis
9.4.5.2 SWOT Analysis
9.4.5.3 Products & Services Offered
9.4.5.4 Strategic Alliances between Business Partners
9.4.6 THERMO FISHER SCIENTIFIC INC.
9.4.6.1 Business Description & Financial Analysis
9.4.6.2 SWOT Analysis
9.4.6.3 Products & Services Offered
9.4.6.4 Strategic Alliances between Business Partners
9.4.7 PAREXEL INTERNATIONAL CORPORATION
9.4.7.1 Business Description & Financial Analysis
9.4.7.2 SWOT Analysis
9.4.7.3 Products & Services Offered
9.4.8.4 Strategic Alliances between Business Partners
9.4.8 Medidata Solutions, Inc.
9.4.8.1 Business Description & Financial Analysis
9.4.8.2 SWOT Analysis
9.4.8.3 Products & Services Offered
9.4.9 Pharmaron
9.4.9.1 Business Description & Financial Analysis
9.4.9.2 SWOT Analysis
9.4.9.3 Products & Services Offered
9.4.9.4 Strategic Alliances between Business Partners
9.4.10 GVK Biosciences Private Limited
9.4.10.1 Business Description & Financial Analysis
9.4.10.2 SWOT Analysis
9.4.10.3 Products & Services Offered
9.4.10.4 Strategic Alliances between Business Partners
9.4.11 Other Companies
9.4.11.1 Business Description & Financial Analysis
9.4.11.2 SWOT Analysis
9.4.11.3 Products & Services Offered
9.4.11.4 Strategic Alliances between Business Partners
10 RESEARCH METHODOLOGY
10.1 Market Introduction
10.1.1 Market Definition
10.1.2 Market Scope & Segmentation
10.2 Information Procurement
10.2.1 Secondary Research
10.2.1.1 Purchased Databases
10.2.1.2 GMEs Internal Data Repository
10.2.1.3 Secondary Resources & Third Party Perspectives
10.2.1.4 Company Information Sources
10.2.2 Primary Research
10.2.2.1 Various Service of Respondents for Primary Interviews
10.2.2.2 Number of Interviews Conducted throughout the Research Process
10.2.2.3 Primary Stakeholders
10.2.2.4 Discussion Guide for Primary Participants
10.2.3 Expert Panels
10.2.3.1 Expert Panels Across 30+ Industry
10.2.4 Paid Local Experts
10.2.4.1 Paid Local Experts Across 30+ Industry Across each Region
10.3 Market Estimation
10.3.1 Top-Down Approach
10.3.1.1 Macro-Economic Indicators Considered
10.3.1.2 Micro-Economic Indicators Considered
10.3.2 Bottom Up Approach
10.3.2.1 Company Share Analysis Approach
10.3.2.2 Estimation of Potential Product Sales
10.4 Data Triangulation
10.4.1 Data Collection
10.4.2 Time Series, Cross Sectional & Panel Data Analysis
10.4.3 Cluster Analysis
10.5 Analysis and Output
10.5.1 Inhouse AI Based Real Time Analytics Tool
10.5.2 Output From Desk & Primary Research
10.6 Research Assumptions & Limitations
10.7.1 Research Assumptions
10.7.2 Research Limitations
LIST OF TABLES
1 Global Clinical Research Organization Market, By Type, 2020-2028 (USD Mllion)
2 Drug Discovery Market, By Region, 2020-2028 (USD Mllion)
3 Pre-Clinical Market, By Region, 2020-2028 (USD Mllion)
4 Clinical Market, By Region, 2020-2028 (USD Mllion)
5 Global Clinical Research Organization Market, By Service, 2020-2028 (USD Mllion)
6 Project Management/Clinical Supply Management Market, By Region, 2020-2028 (USD Mllion)
7 Data Management Market, By Region, 2020-2028 (USD Mllion)
8 Regulatory/Medical Affairs Market, By Region, 2020-2028 (USD Mllion)
9 Medical Writing Market, By Region, 2020-2028 (USD Mllion)
10 Clinical Monitoring Market, By Region, 2020-2028 (USD Mllion)
11 Quality Management/ Assurance Market, By Region, 2020-2028 (USD Mllion)
12 Bio-statistics Market, By Region, 2020-2028 (USD Mllion)
13 Investigator Payments Market, By Region, 2020-2028 (USD Mllion)
14 Laboratory Market, By Region, 2020-2028 (USD Mllion)
15 Patient and site Recruitment Market, By Region, 2020-2028 (USD Mllion)
16 Technology Market, By Region, 2020-2028 (USD Mllion)
17 Others Market, By Region, 2020-2028 (USD Mllion)
18 Global Clinical Research Organization Market, By Application, 2020-2028 (USD Mllion)
19 Oncology Market, By Region, 2020-2028 (USD Mllion)
20 Cardiovascular Market, By Region, 2020-2028 (USD Mllion)
21 Autoimmune/Inflammation Market, By Region, 2020-2028 (USD Mllion)
22 Central nervous system (CNS) Market, By Region, 2020-2028 (USD Mllion)
23 Dermatology Market, By Region, 2020-2028 (USD Mllion)
24 Infectious diseases Market, By Region, 2020-2028 (USD Mllion)
25 Diabetes Market, By Region, 2020-2028 (USD Mllion)
26 Pain Market, By Region, 2020-2028 (USD Mllion)
27 Others Market, By Region, 2020-2028 (USD Mllion)
28 Global Clinical Research Organization Market, By END USER, 2020-2028 (USD Mllion)
29 Pharmaceutical & Biopharmaceutical Companies Market, By Region, 2020-2028 (USD Mllion)
30 Medical Device Companies Market, By Region, 2020-2028 (USD Mllion)
31 Others Market, By Region, 2020-2028 (USD Mllion)
32 Regional Analysis, 2020-2028 (USD Mllion)
33 North America Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
34 North America Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
35 North America Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
36 North America Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
37 North America Clinical Research Organization Market, By Country, 2020-2028 (USD Million)
38 U.S Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
39 U.S Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
40 U.S Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
41 U.S Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
42 Canada Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
43 Canada Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
44 Canada Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
45 CANADA Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
46 Mexico Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
47 Mexico Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
48 Mexico Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
49 mexico Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
50 Europe Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
51 Europe Clinical Research Organization Market, By service, 2020-2028 (USD Million)
52 Europe Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
53 europe Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
54 europe Clinical Research Organization Market, By country, 2020-2028 (USD Million)
55 Germany Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
56 Germany Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
57 Germany Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
58 germany Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
59 UK Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
60 UK Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
61 UK Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
62 U.kClinical Research Organization Market, By End User, 2020-2028 (USD Million)
63 France Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
64 France Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
65 France Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
66 france Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
67 Italy Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
68 Italy Clinical Research Organization Market, By T Service Type, 2020-2028 (USD Million)
69 Italy Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
70 italy Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
71 Spain Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
72 Spain Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
73 Spain Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
74 spain Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
75 Rest Of Europe Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
76 Rest Of Europe Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
77 Rest of Europe Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
78 REST OF EUROPE Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
79 Asia Pacific Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
80 Asia Pacific Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
81 Asia Pacific Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
82 asia Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
83 Asia Pacific Clinical Research Organization Market, By Country, 2020-2028 (USD Million)
84 China Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
85 China Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
86 China Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
87 china Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
88 India Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
89 India Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
90 India Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
91 india Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
92 Japan Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
93 Japan Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
94 Japan Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
95 japan Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
96 South Korea Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
97 South Korea Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
98 South Korea Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
99 south korea Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
100 Middle East and Africa Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
101 Middle East and Africa Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
102 Middle East and Africa Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
103 MIDDLE EAST and AFRICA Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
104 Middle East and Africa Clinical Research Organization Market, By Country, 2020-2028 (USD Million)
105 Saudi Arabia Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
106 Saudi Arabia Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
107 Saudi Arabia Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
108 saudi arabia Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
109 UAE Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
110 UAE Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
111 UAE Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
112 uae Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
113 Central & South America Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
114 Central & South America Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
115 Central & South America Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
116 CENTRAL & SOUTH AMERICA Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
117 Central & South America Clinical Research Organization Market, By Country, 2020-2028 (USD Million)
118 Brazil Clinical Research Organization Market, By Type, 2020-2028 (USD Million)
119 Brazil Clinical Research Organization Market, By Service, 2020-2028 (USD Million)
120 Brazil Clinical Research Organization Market, By Application, 2020-2028 (USD Million)
121 brazil Clinical Research Organization Market, By End User, 2020-2028 (USD Million)
122 ICON Plc: Products & Services Offering
123 Charles River Laboratories (CRL): Products & Services Offering
124 Syneos Health: Products & Services Offering
125 IQVIA HOLDINGS INC.: Products & Services Offering
126 Laboratory Corporation of America Holdings: Products & Services Offering
127 THERMO FISHER SCIENTIFIC INC.: Products & Services Offering
128 PAREXEL INTERNATIONAL CORPORATION : Products & Services Offering
129 Medidata Solutions, Inc.: Products & Services Offering
130 Pharmaron, Inc: Products & Services Offering
131 GVK Biosciences Private Limited: Products & Services Offering
132 Other Companies: Products & Services Offering
LIST OF FIGURES
1 Global Clinical Research Organization Market Overview
2 Global Clinical Research Organization Market Value From 2020-2028 (USD Mllion)
3 Global Clinical Research Organization Market Share, By Type (2022)
4 Global Clinical Research Organization Market Share, By Service (2022)
5 Global Clinical Research Organization Market Share, By Application (2022)
6 Global Clinical Research Organization Market Share, By End User (2022)
7 Global Clinical Research Organization Market, By Region (Asia Pacific Market)
8 Technological Trends In Global Clinical Research Organization Market
9 Four Quadrant Competitor Positioning Matrix
10 Impact Of Macro & Micro Indicators On The Market
11 Impact Of Key Drivers On The Global Clinical Research Organization Market
12 Impact Of Challenges On The Global Clinical Research Organization Market
13 Porter’s Five Forces Analysis
14 Global Clinical Research Organization Market: By Type Scope Key Takeaways
15 Global Clinical Research Organization Market, By Type Segment: Revenue Growth Analysis
16 Drug Discovery Market, By Region, 2020-2028 (USD Mllion)
17 Pre-Clinical Market, By Region, 2020-2028 (USD Mllion)
18 Clinical Market, By Region, 2020-2028 (USD Mllion)
19 Global Clinical Research Organization Market: By Service Scope Key Takeaways
20 Global Clinical Research Organization Market, By Service Segment: Revenue Growth Analysis
21 Project Management/Clinical Supply Management Market, By Region, 2020-2028 (USD Mllion)
22 Data Management Market, By Region, 2020-2028 (USD Mllion)
23 Regulatory/Medical Affairs Market, By Region, 2020-2028 (USD Mllion)
24 Medical Writing Market, By Region, 2020-2028 (USD Mllion)
25 Clinical Monitoring Market, By Region, 2020-2028 (USD Mllion)
26 Quality Management/ Assurance Market, By Region, 2020-2028 (USD Mllion)
27 Bio-statistics Market, By Region, 2020-2028 (USD Mllion)
28 Investigator Payments Market, By Region, 2020-2028 (USD Mllion)
29 Laboratory Market, By Region, 2020-2028 (USD Mllion)
30 Patient and site Recruitment Market, By Region, 2020-2028 (USD Mllion)
31 Technology Market, By Region, 2020-2028 (USD Mllion)
32 Others Market, By Region, 2020-2028 (USD Mllion)
33 Global Clinical Research Organization Market: By Application Scope Key Takeaways
34 Global Clinical Research Organization Market, By Application Segment: Revenue Growth Analysis
35 Oncology Market, By Region, 2020-2028 (USD Mllion)
36 Cardiovascular Market, By Region, 2020-2028 (USD Mllion)
37 Autoimmune/Inflammation Market, By Region, 2020-2028 (USD Mllion)
38 Central nervous system (CNS) Market, By Region, 2020-2028 (USD Mllion)
39 Dermatology Market, By Region, 2020-2028 (USD Mllion)
40 Infectious diseases Market, By Region, 2020-2028 (USD Mllion)
41 Diabetes Market, By Region, 2020-2028 (USD Mllion)
42 Pain Market, By Region, 2020-2028 (USD Mllion)
43 Others Market, By Region, 2020-2028 (USD Mllion)
44 Global Clinical Research Organization Market: By End User Scope Key Takeaways
45 Global Clinical Research Organization Market, By End User Segment: Revenue Growth Analysis
46 Pharmaceutical & Biopharmaceutical Companies Market, By Region, 2020-2028 (USD Mllion)
47 Medical Device Companies Market, By Region, 2020-2028 (USD Mllion)
48 Others Market, By Region, 2020-2028 (USD Mllion)
49 Regional Segment: Revenue Growth Analysis
50 Global Clinical Research Organization Market: Regional Analysis
51 North America Clinical Research Organization Market Overview
52 North America Clinical Research Organization Market, By Type
53 North America Clinical Research Organization Market, By Service
54 North America Clinical Research Organization Market, By Application
55 North America Clinical Research Organization Market, By End User
56 North America Clinical Research Organization Market, By Country
57 U.S. Clinical Research Organization Market, By Type
58 U.S. Clinical Research Organization Market, By Service
59 U.S. Clinical Research Organization Market, By Application
60 U.S. Clinical Research Organization Market, By End User
61 Canada Clinical Research Organization Market, By Type
62 Canada Clinical Research Organization Market, By Service
63 Canada Clinical Research Organization Market, By Application
64 Canada Clinical Research Organization Market, By End User
65 Mexico Clinical Research Organization Market, By Type
66 Mexico Clinical Research Organization Market, By Service
67 Mexico Clinical Research Organization Market, By Application
68 Mexico Clinical Research Organization Market, By End User
69 Four Quadrant Positioning Matrix
70 Company Market Share Analysis
71 ICON Plc: Company Snapshot
72 ICON Plc: SWOT Analysis
73 ICON Plc: Geographic Presence
74 Charles River Laboratories (CRL): Company Snapshot
75 Charles River Laboratories (CRL): SWOT Analysis
76 Charles River Laboratories (CRL): Geographic Presence
77 Syneos Health: Company Snapshot
78 Syneos Health: SWOT Analysis
79 Syneos Health: Geographic Presence
80 IQVIA HOLDINGS INC.: Company Snapshot
81 IQVIA HOLDINGS INC.: Swot Analysis
82 IQVIA HOLDINGS INC.: Geographic Presence
83 Laboratory Corporation of America Holdings: Company Snapshot
84 Laboratory Corporation of America Holdings: SWOT Analysis
85 Laboratory Corporation of America Holdings: Geographic Presence
86 Thermo Fisher Scientific Inc.: Company Snapshot
87 Thermo Fisher Scientific Inc.: SWOT Analysis
88 Thermo Fisher Scientific Inc.: Geographic Presence
89 Parexel International Corporation : Company Snapshot
90 Parexel International Corporation : Swot Analysis
91 Parexel International Corporation : Geographic Presence
92 Medidata Solutions, Inc.: Company Snapshot
93 Medidata Solutions, Inc.: SWOT Analysis
94 Medidata Solutions, Inc.: Geographic Presence
95 Pharmaron, Inc.: Company Snapshot
96 Pharmaron, Inc.: SWOT Analysis
97 Pharmaron, Inc.: Geographic Presence
98 GVK Biosciences Private Limited: Company Snapshot
99 GVK Biosciences Private Limited: SWOT Analysis
100 GVK Biosciences Private Limited: Geographic Presence
101 Other Companies: Company Snapshot
102 Other Companies: SWOT Analysis
103 Other Companies: Geographic Presence
The Global Clinical Research Organization Market has been studied from the year 2019 till 2028. However, the CAGR provided in the report is from the year 2023 to 2028. The research methodology involved three stages: Desk research, Primary research, and Analysis & Output from the entire research process.

The desk research involved a robust background study which meant referring to paid and unpaid databases to understand the market dynamics; mapping contracts from press releases; identifying the key players in the market, studying their product portfolio, competition level, annual reports/SEC filings & investor presentations; and learning the demand and supply-side analysis for the Clinical Research Organization Market.

The primary research activity included telephonic conversations with more than 50 tier 1 industry consultants, distributors, and end-use product manufacturers.

Finally, based on the above thorough research process, an in-depth analysis was carried out considering the following aspects: market attractiveness, current & future market trends, market share analysis, SWOT analysis of the company and customer analytics.

1. Discounted price for multiple reports across domains
2. Complimentary 10 hours free analyst time for market review
3. Free business intelligence platform with subscription
4. Free upgrade to enterprise license (allows to share across all company locations)
5. Choose reports from a database of more than 10,000 reports
6. Free trial, before you make a purchase decision. No purchase commitment .
Frequently Asked Questions

- Base Year for Estimate 2023
- Report Id 4289
This FREE sample includes market data points, ranging from trend analyses to market estimates & forecasts. See for yourself.
Or view our licence options:
We never share your personal data. Privacy Policy
Tailor made solutions just for you
80% of our clients seek made-to-order reports. How do you want us to tailor yours?
OUR CLIENTS

- Known globally as a renowned B2B information integrator and analyser providing actionable insights with disruptive research that correlates markets, technologies, new use cases and companies
- 5500+ companies worldwide approach us every year for their revenue growth initiatives
- 80% of Fortune 2000 Companies rely on our research to identify new revenue sources
- Patent-pending in-house 'Artificial Intelligence powered Real-Time Competitive Analytics Tool‘
- 95% Renewal Rate
Clinical Trials Market Size & Share Analysis - Growth Trends & Forecasts (2024 - 2029)
The Report Covers Global Clinical Trials Market Growth Analysis and is Segmented by Phase (Phase I, Phase II, Phase III, and Phase IV), Design (Treatment Studies and Observational Studies), and Geography (North America, Europe, Asia-Pacific, Middle East and Africa, and South America).
Clinical Trial Market Size

Need a report that reflects how COVID-19 has impacted this market and its growth?
Clinical Trial Market Analysis
The Clinical Trials Market size is estimated at USD 50.66 billion in 2024, and is expected to reach USD 67.5 billion by 2029, growing at a CAGR of 5.91% during the forecast period (2024-2029).
The COVID-19 pandemic tremendously impacted the market for clinical trials, as there has been a rising focus on developing new therapeutics or vaccines to treat the disease. Also, COVID-19 brought a shift in terms of the way clinical trials are performed. There has been an increased interest in virtual/decentralized trials in the clinical trial space, and those have been featured on conference agendas and in articles for a long time. Moreover, COVID-19 forced some of the trials to move to a virtual model to keep the trials on track during the pandemic. Additionally, as of November 8, 2022, a total of 8,397 studies have been registered for COVID-19 on the ClinicalTrials.gov website, among which 2,932 studies were registered for Europe alone, followed by 2,290 studies in North America. Thus, such an increase in the number of clinical trials registered to find an effective treatment for the disease is anticipated to drive market growth. Therefore, COVID-19 is predicted to have a significant impact on the market studied.
The major factors propelling the market's growth include the high demand for clinical trials in emerging markets, increased research and development (R&D) spending in the pharmaceutical industry, an increasing prevalence of diseases; and the focus on rare diseases and multiple orphan drugs in the pipeline. For instance, Novartis AG, one of the major players in the studied market, invested USD 9,540 million in the year 2021, which increased from USD 8,980 million in 2020. In addition, another market player, Pfizer Inc., invested USD 13,829 million in 2021 on R&D as compared to USD 9,393 in FY 2020. Thus, the increased research and development expenses by the major players in the market are expected to drive the growth of the market.
Factors such as growing burden of diseases need more advanced and effective medicines for treatment and thus contributes to the growth of the market. For instance, as per IDF Atlas 2021 edition, in 2021, there were 536.6 million people aged 20-79 years living with diabetes around the world and this number is expected to reach 783.7 million by 2045. Such high burden of diseases also propel the growth of the market.
Additionally, the initiatives taken by the government in different regions also conbtribute to the growth of the market. For instance, in January 2022, the European Commission (EC), the Heads of Medicines Agencies (HMA), and the European Medicines Agency (EMA) launched an initiative to transform how clinical trials are initiated, designed, and ran, referred to as Accelerating Clinical Trials in the EU (ACT EU). The aim of ACT EU is to further develop Europe as a focal point for clinical research, to further promote the development of high-quality, safe, and effective medicines, and to better integrate clinical research into the European health system. Such, initiatives by governments across the globe are contributing to market growth.
Therefore, owing to the factors mentioned above, the studied market is anticipated to grow over the forecast period. However, the lack of a skilled workforce in clinical research and stringent regulations for patient enrolment are factors that are expected to hinder the market growth during the analysis period.
Clinical Trial Market Trends
Phase iii by phase segment is expected to grow over the forecast period.
Phase III clinical trials evaluate the comparative effect of the new medication over the previous medications available or conducted to confirm and expand on safety and effectiveness results from Phase 1 and 2 trials. This usually involves up to 3,000 participants with the condition that the new medication is meant to treat and may last for many years. Also, the number of Phase III clinical trials remains comparatively higher than Phase II and Phase I trials, owing to their greater complexity and need for a larger patient pool. Factors such as increasing research activities, the growing burden of diseases, and many investigative drugs in Phase III are propelling the growth of the market segment.
The high number of clinical trials in Phase III is driving the growth of the market segment. For instance, according to the data from clinicaltrials.gov, as of November 8, 2022, 9,137 clinical trials were in Phase III for cancer, 5,069 for cardiology, and 5,217 for respiratory studies. Thus, such a high number of clinical trials registered under phase III of clinical trials is expected to contribute to the segment's growth.
Additionally, the Phase III trials conducted by market players are also contributing to the growth of the market segment. For instance, in May 2022, Lipidor AB reported that half of the patients have been enrolled in the Phase III study of AKP02 skin spray for mild to moderate psoriasis. Also, in August 2022, Wockhardt Ltd initiated a global Phase III clinical study of its new antibiotic candidate WCK 5222. It is entirely a new class of antibiotic known as "β-lactam ENHANCER', and is targeted for the treatment of hospitalized adults with complicated urinary tract infections, including acute pyelonephritis. Such a high number of studies in Phase III depicts the growth of the segment.
Thus, the factors mentioned above are expected to propel the segment's growth over the forecast period.

North America is Expected to Dominate the Market Over The Forecast Period
The North American region is expected to contribute significantly to the market growth during the study period owing to factors such as high R&D expenditure of the pharmaceutical industry, presence of well-established players, robust regulatory framework, and rising prevalence of diseases, coupled with the significant contribution of the United States.
The American Cancer Society estimated that in the United States, around 1,918,0303 new cases are estimated to register in 2022. Thus, the high burden of cancer is expected to boost the demand for the development of drugs and devices for disease diagnosis and treatment, thereby driving the market growth.
Additionally, the support from the government of the countries in the region is also contributing to the growth of the market. For instance, in June 2022, the Government of Canada launched the Clinical Trials Fund (CTF), supported by a Budget 2021 investment of USD 250 million over three years for the Canadian Institutes of Health Research (CIHR). With this funding, the government aims to improve health outcomes for Canadians while ensuring Canada is well-positioned to respond to future pandemics and other health priorities. The CTF will strengthen the clinical trials infrastructure in Canada and support the training of new clinical researchers.
Furthermore, the major market players in the region are active in the innovation of new drugs and devices, which is another factor predicted to contribute to the market growth in the region. For instance, in September 2021, Janssen started the Phase III trial for the investigational respiratory syncytial virus (RSV) vaccine among older adults. The study will evaluate the efficacy, safety and immunogenicity of Janssen's investigational adult vaccine against lower respiratory tract disease (LRTD) throughout North America and some other countries of different regions. Such trials are expected to propel the growth of the market in the region.
Such continuous developments are expected to fuel the clinical trials market in the North American region.

Clinical Trial Industry Overview
The clinical trials market is moderately competitive. Strategic partnerships between pharmaceutical companies and CROs are expected to impact the market's growth significantly. Also, the quick adoption of advanced technology for improved healthcare contributes to the growth of the market. Some of the key players are Clinipace, Eli Lilly and Company, Laboratory Corporation of America, ICON PLC, and Novo Nordisk AS.
Clinical Trial Market Leaders
Laboratory Corporation of America
Eli Lilly and Company
Novo Nordisk AS
*Disclaimer: Major Players sorted in no particular order

Clinical Trial Market News
- July 2022: An early-stage clinical trial investigating an investigational vaccine to stave off Nipah virus infection was started by the National Institute of Allergy and Infectious Diseases (NIAID), a division of the National Institutes of Health (NIH) of the United States.
- May 2022: The International AIDS Vaccine Initiative (IAVI) and Moderna Inc. started a Phase I clinical trial of an mRNA vaccine antigen in Rwanda and South Africa.

Clinical Trial Market Report - Table of Contents
1. INTRODUCTION
1.1 Study Assumptions and Market Definition
1.2 Scope of the Study
2. RESEARCH METHODOLOGY
3. EXECUTIVE SUMMARY
4. MARKET DYNAMICS
4.1 Market Overview
4.2 Market Drivers
4.2.1 Demand for Clinical Trials in the Emerging Markets
4.2.2 High R&D Expenditure of the Pharmaceutical Industry
4.2.3 Rising Prevalence of Diseases
4.3 Market Restraints
4.3.1 Lack of Skilled Workforce in Clinical Research
4.3.2 Stringent Regulations for Patient Enrollment
4.4 Porter's Five Forces Analysis
4.4.1 Threat of New Entrants
4.4.2 Bargaining Power of Buyers/Consumers
4.4.3 Bargaining Power of Suppliers
4.4.4 Threat of Substitute Products
4.4.5 Intensity of Competitive Rivalry
5. MARKET SEGMENTATION (Market Size by Value - USD million)
5.1 By Phase
5.1.1 Phase I
5.1.2 Phase II
5.1.3 Phase III
5.1.4 Phase IV
5.2 By Design
5.2.1 Treatment Studies
5.2.1.1 Randomized Control Trial
5.2.1.2 Adaptive Clinical Trial
5.2.1.3 Non-randomized Control Trial
5.2.2 Observational Studies
5.2.2.1 Cohort Study
5.2.2.2 Case Control Study
5.2.2.3 Cross Sectional Study
5.2.2.4 Ecological Study
5.3 Geography
5.3.1 North America
5.3.1.1 United States
5.3.1.2 Canada
5.3.1.3 Mexico
5.3.2 Europe
5.3.2.1 Germany
5.3.2.2 United Kingdom
5.3.2.3 France
5.3.2.4 Italy
5.3.2.5 Spain
5.3.2.6 Rest of Europe
5.3.3 Asia-Pacific
5.3.3.1 China
5.3.3.2 Japan
5.3.3.3 India
5.3.3.4 Australia
5.3.3.5 South Korea
5.3.3.6 Rest of Asia-Pacific
5.3.4 Middle East and Africa
5.3.4.1 GCC
5.3.4.2 South Africa
5.3.4.3 Rest of Middle East and Africa
5.3.5 South America
5.3.5.1 Brazil
5.3.5.2 Argentina
5.3.5.3 Rest of South America
6. COMPETITIVE LANDSCAPE
6.1 Company Profiles
6.1.1 Clinipace
6.1.2 Laboratory Corporation of America
6.1.3 Eli Lilly and Company
6.1.4 ICON PLC
6.1.5 Novo Nordisk AS
6.1.6 PAREXEL International Corporation
6.1.7 Pfizer Inc.
6.1.8 Pharmaceutical Product Development LLC
6.1.9 IQVIA
6.1.10 F. Hoffmann-La Roche Ltd
6.1.11 Sanofi SA
6.1.12 Syneos Health
6.1.13 ClinDatrix Inc
6.1.14 Charles River Laboratory
- *List Not Exhaustive
7. MARKET OPPORTUNITIES AND FUTURE TRENDS
Clinical Trial Industry Segmentation
As per the scope of the report, clinical trials are experiments that are conducted under clinical research and follow a regulated protocol. These experiments are primarily performed to obtain data regarding the safety and efficacy of newly developed drugs. Clinical trial data is mandatory for drug approval and for it to be introduced in the market. This process is expensive and time-consuming and requires expertise at all stages. The Clinical Trials Market is Segmented by Phase (Phase I, Phase II, Phase III, and Phase IV), Design (Treatment Studies and Observational Studies), and Geography (North America, Europe, Asia-Pacific, Middle East and Africa, and South America). The market report also covers the estimated market sizes and trends for 17 different countries across major regions globally. The report offers values (in USD million) for the above segments.
Clinical Trial Market Research FAQs
How big is the clinical trials market.
The Clinical Trials Market size is expected to reach USD 50.66 billion in 2024 and grow at a CAGR of 5.91% to reach USD 67.50 billion by 2029.
What is the current Clinical Trials Market size?
In 2024, the Clinical Trials Market size is expected to reach USD 50.66 billion.
Who are the key players in Clinical Trials Market?
Clinipace, Laboratory Corporation of America, ICON PLC, Eli Lilly and Company and Novo Nordisk AS are the major companies operating in the Clinical Trials Market.
Which is the fastest growing region in Clinical Trials Market?
North America is estimated to grow at the highest CAGR over the forecast period (2024-2029).
Which region has the biggest share in Clinical Trials Market?
In 2024, the Asia Pacific accounts for the largest market share in Clinical Trials Market.
What years does this Clinical Trials Market cover, and what was the market size in 2023?
In 2023, the Clinical Trials Market size was estimated at USD 47.83 billion. The report covers the Clinical Trials Market historical market size for years: 2019, 2020, 2021, 2022 and 2023. The report also forecasts the Clinical Trials Market size for years: 2024, 2025, 2026, 2027, 2028 and 2029.
Why is North America anticipated to witness a rapid growth rate in the Clinical Trials Market?
North America is anticipated to witness a rapid growth rate in the Clinical Trials Market due to a) High R&D expenditure of the pharmaceutical industry b) Presence of well-established players c) Robust regulatory framework d) Rising prevalence of diseases.
Which is the major segment in the Clinical Trials Market by phase?
The major segment in the Clinical Trials Market by phase is Phase III. This is mainly due to the high number of clinical trials conducted in this phase compared to others.
Our Best Selling Reports
- Sports Apparel Market
- EV Charging Station Market
- Automotive Suspension Systems Market
- Electric 3 Wheeler Market
- Pharmaceutical Plastic Packaging Market
- Security Paper Market
- Japan Used Car Market
- Lens Market
- Footwear Market
- Automotive Transmission Market
Clinical Research Industry Report
This comprehensive report offers a deep dive into the clinical trials industry, providing a detailed analysis of key market drivers and market segments. Mordor Intelligence offers customization based on your specific interests, including: 1. Service - Laboratory, Analytical Testing, Bioanalytical Testing 2. Therapeutic Area - Oncology, Neurology, CNS, Autoimmune/Inflammation 3. Application - MABs, CGT 4. End-User - Hospitals, Laboratories, and Clinics
Clinical Research Market Report Snapshots
- Clinical Research Market Size
- Clinical Research Market Share
- Clinical Research Market Trends
- Clinical Research Companies
Please enter a valid email id!
Please enter a valid message!

Clinical Trials Market Get a free sample of this report
Please enter your name
Business Email
Please enter a valid email
Please enter your phone number
Get this Data in a Free Sample of the Clinical Trials Market Report
Please enter your requirement

Thank you for choosing us for your research needs! A confirmation has been sent to your email. Rest assured, your report will be delivered to your inbox within the next 72 hours. A member of our dedicated Client Success Team will proactively reach out to guide and assist you. We appreciate your trust and are committed to delivering precise and valuable research insights.
Please be sure to check your spam folder too.
Sorry! Payment Failed. Please check with your bank for further details.
Add Citation APA MLA Chicago
➜ Embed Code X
Get Embed Code
Want to use this image? X
Please copy & paste this embed code onto your site:
Images must be attributed to Mordor Intelligence. Learn more
About The Embed Code X
Mordor Intelligence's images may only be used with attribution back to Mordor Intelligence. Using the Mordor Intelligence's embed code renders the image with an attribution line that satisfies this requirement.
In addition, by using the embed code, you reduce the load on your web server, because the image will be hosted on the same worldwide content delivery network Mordor Intelligence uses instead of your web server.
Please enter your Full Name
Please enter your Email
Please enter a valid Phone Number
Please enter your Company Name
Please enter your Partnership
Please enter your Job Title
Please select one option.
Please verify captcha
Clinical Research Organizations
Outsourced Market for Patient trials.
Beroe LiVE.Ai™
AI-powered self-service platform for all your sourcing decision needs across 1,200+ categories like Clinical Research Organizations.
Market Data, Sourcing & Supplier Intelligence, and Price & Cost Benchmarking.
The World’s first Digital Market Analyst
Abi, the AI-powered digital assistant brings together data, insights, and intelligence for faster answers to sourcing questions
Abi is now supercharged with GPT4 AI engine. Enjoy the ease of ChatGPT, now on Abi
Clinical Research Organizations Suppliers
Find the right-fit clinical research organizations supplier for your specific business needs and filter by location, industry, category, revenue, certifications, and more on Beroe LiVE.Ai™.

Up to 3 months
The Supplier Evaluation Risk (SER) Rating is Dun & Bradstreet’s proprietary scoring system used to assess the probability that a business will seek relief from creditors or cease operations within the next 12 months. SER ratings range from 1 to 9, with 9 indicating the highest risk of failure. We’ve prepared an infographic to help business owners better understand what influences their SER Rating.

Related Categories
AI & Digital Ops In Patient Access
Artificial Intelligence in Patient Engagement
Cell Therapies
Clinical Trial Supply (CTS)
Connected Devices and Wearables
Drug Development Biometrics Services
Drug Development Core Lab
Early Phase Clinical Trials
Lab Animals
Lab Equipment and Supplies
Lab Equipment and Supplies -Trends, Pricing and Contract Analysis
Patient Recruitment

Use the Clinical Research Organizations market, supplier and price information for category strategy creation and Quaterly Business Reviews (QRBs)
Clinical Research Organizations market report transcript
Global market outlook on clinical research organizations.
The global CRO market was estimated at $31.6 billion in 2018, and it is expected to grow at a CAGR of 12 percent to reach $45.2 billion by 2022
This is due to the increased outsourcing (>50 percent) seen from the pharmaceutical and biotechnology companies, with 85 percent comprised by the clinical segment
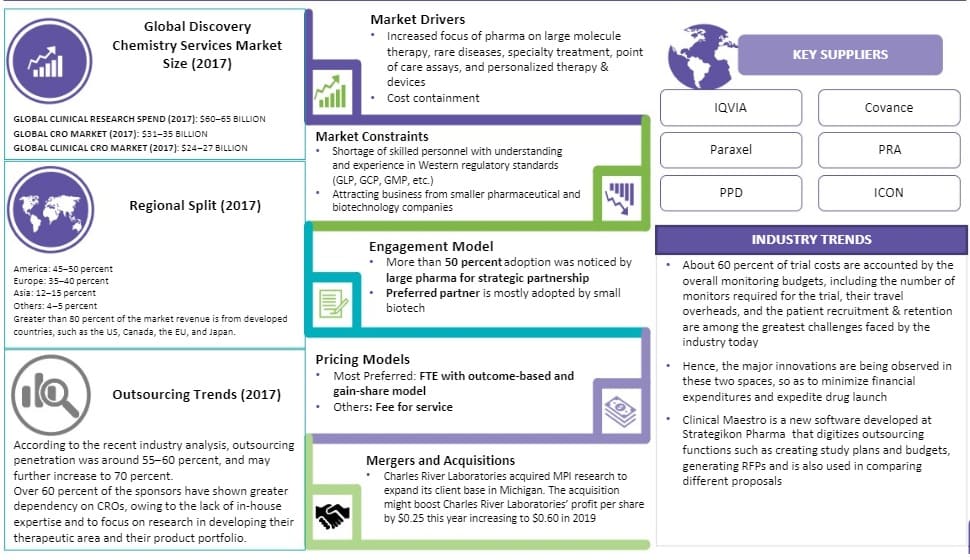
- Drug discovery is the largest segment by service type and accounts for approximately 33%,of the total CRO market
- Although spend on specialty focus, such as rare diseases, personalized medicines, and specialty oncology, has increased, the larger portfolio by spend size would continue to drive the programmatic CRO market
- The clinical segment of the CRO market is valued at $30.6 billion in 2017 and is expected to grow at a CAGR of 7 percent. Almost 85 percent of the global CRO market revenue comes from the clinical CRO market. This is directly related to about 50 percent of Phase II–IV activities already being outsourced by pharma companies
- On the other hand, the preclinical segment was valued at $2.8-3 billion in 2017 and is expected to grow at a CAGR 10 percent, as more in-house preclinical activities such as discovery and early phase studies are beginning to be outsourced by pharma companies at a range of 38-40 percent
- The CRO market is split regionally as America: 45–50 percent, Europe: 35–40 percent, Asia: 12–15 percent, Others: 4–5 percent. Greater than 80 percent of the market revenue is from developed countries, such as the US, Canada, the EU, and Japan.
- The top CROs are IQVIA, Paraxel, PPD, Covance, and PRAICON.
- Constraints in the contract research organization market include shortage of skilled personnel with understanding and experience in western regulatory standards (GLP, GCP, GMP, etc.) and challenges in attracting business from smaller pharmaceutical and biotechnology companies.
Regional Analysis
North America and Europe hold greater than 80 percent of the CRO market, as pharma spend ~70 percent and 18 percent of their clinical trial R&D spending in these regions, respectively
- Though the cost of conducting clinical trials in emerging countries will be 60 percent less than that of developed countries, greater than 80 percent of the market revenue is from developed countries - US, Canada, the EU, Japan
- APAC contributes to 10 –15 percent of the overall CRO revenue, which makes this region the third largest market share holder with increased trials being seen in China, Russia and South Korea
- Increased outsourcing of clinical services such as Clinical Data Management, Biostatistics, Pharmacovigilance, etc., by large pharma to emerging market of India is being seen. The reason being low cost labor availability, which will further drive up the revenue of Asia-Pacific region in the future
CRO Market Drivers
- Pharma companies have increased outsourcing their in-house R&D activities to CROs
- Large molecule development outsourcing has increased from ~35 percent to 45 percent, due to more supply and availability of reliable single use technology
Cost Containment
- Pharmaceutical companies one of the major factors is to reduce costs, by lowering their internal capacities in manufacturing and R&D. This leads to expenses being diverted toward outsourcing, making CROs play an integral part of pharma to increase their productivity, access new capabilities, shift fixed to variable costs, and improve their global reach
Real World –Data and Analytics
- As regulators and payers place increasing importance on RWD, pharma is seen to partner with CRO, to support with predictive modeling, analytics, etc. This is driving CROs to either acquire or partner with specialists in this area
- E.g., PPD is working to expand its expertise for RWD by joining forces with a division of insurance giant Anthem
Emerging Markets
- The emerging markets of Brazil, China, Russia are witnessing increased trials, creating demand for regional CROs, with strong regulatory knowledge. For example, OCT, Intrials, etc.
- Examples: Offshoring of HEOR, pharmacovigilance services to India offer cost saving of ~30–45 percent. The acquisition of Quantum Solutions India by Parexel is an example of this growing trend. South Korea is emerging as the next early phase destinations, with a 8.22 percent CAGR between 2012 and 2016
New Areas of Research and Regulations
- The increased focus of pharma on large molecule therapy, rare diseases, specialty treatment, point of care assays, and personalized therapy & devices will require clinical trial testing and submission of data to the relevant regulatory authorities. For example, >80 percent of CRAs of INC Research are principally focused on one therapeutic area
- Also, the depth of regulatory scrutiny has increased, such as early RWD collection and reporting every adverse events, driving increased clinical trials
Clinical Research Organizations Market Overview
Currently, EDC and CTMS are the most commonly used data sources for clinical data management market, however, with the growing adoption of RBM, majority of the companies will move to more sophisticated technologies for clinical research organization.
60 percent of trial costs are accounted by the overall monitoring budgets, including the number of monitors required for the trial, their travel overheads, and the patient recruitment & retention. Hence, the major innovations are being observed in these two spaces, so as to minimize financial expenditures and expedite drug launch.
The fragmented contract research organizations market witnessed continuous mergers and acquisitions from 2012 to 2017. This has, in turn, resulted in continued consolidation of the contract research organization market, with the top 10 suppliers, holding >55 percent of the market share.
Why You Should Buy This Report
- The Beroe analysis report gives the CRO market drivers, technology trends and innovations and outsourcing penetration analysis.
- The report lists out the market share of the top contract research organizations, key mergers and acquisitions, recent trends and innovations and best industry practices.
- It lists the major suppliers in the clinical data management market.
Interesting Reads:
Discover the world of market intelligence and how it can elevate your business strategies.
Learn more about how market intelligence can enable informed decision-making, help identify growth opportunities, manage risks, and shape your business's strategic direction.
Get Ahead with AI-Enabled Market Insights Schedule a Demo Now
Schedule a Demo Now
- Healthcare IT
- Contract Research Organization (CRO) Services Market
"Designing Growth Strategies is in our DNA"
Contract Research Organization (CRO) Services Market Size, Share & COVID-19 Impact Analysis, By Service Type (Early Phase Development Services, Clinical, Laboratory Service and Others), By Application (Oncology, CNS Disorder, Cardiology, Infectious Disease, Metabolic Disorder, and Others), By End-User (Pharmaceutical and Biotech Companies, Medical Device Companies, Academic and Research Institutes and Others), and Regional Forecast, 2023-2030
Last Updated: April 22, 2024 | Format: PDF | Report ID: FBI100864
- Segmentation
- Methodology
- Infographics
- Request Sample PDF
KEY MARKET INSIGHTS
The contract research organization (CRO) services market size was valued at USD 82.60 billion in 2023 and is projected to grow USD 188.52 billion by 2030, exhibiting a CAGR of 12.5% during 2023-2030. Rising chronic disease burden and pressure for new treatments drive the CRO market.
Contract research organization (CRO) services provides clinical management services to pharmaceutical, biotechnology, and medical device manufacturers. Clinical trial management gets complicated due to many factors, such as sponsors, manufacturers, ethical committees, competent authorities, foundations, researchers, centers, and legal departments. Moreover, it is crucial to work under the guidelines of good clinical practices and harmonization guides, ensuring the unaffected quality of the study.
CRO services sources of revenue are generally from the R&D budgets of pharmaceutical, biotechnology, medical device companies, governments, and other medical research entities. Similarly, the future revenue growth for the CROs can be attributed to overall R&D spending and growth in pharmaceutical R&D outsourcing. The increasing need for time-efficient and cost-effective drug development with high therapeutic expertise has increased the demand for CRO services. In many cases, outsourcing R&D functions provide the opportunity to optimize internal cost structures by exploiting CROs' capacity, scale, and expertise. Therefore, increased outsourced penetration is anticipated in the clinical development and post-approval stages. Certain early-stage functions like safety assessment and regulatory-related services also boost the outsourcing trends, thus propelling the market's growth. The organizations collaborate with other companies to develop innovative products that help in their trial services.
For instance, in April 2021, Pharmaceutical Product Development, LLC. (PPD) and Science 37 entered into a collaboration in which PPD would use Science 37’s Decentralized clinical trial Software as a Service (SaaS) -based technology platform to design, build, test, implement, and execute digital trials. This factor would eventually help the organization in expanding its market share.
COVID-19 IMPACT
Accelerated Drug Development Efforts Augmented Market Growth During Pandemic
COVID-19 has had both positive and negative impacts on the market. During 2020 the CRO services market growth was slowed due to lockdown restrictions. But soon, the market observed a spike in its growth. During COVID-19, many pharmaceutical and biopharmaceutical companies boosted their R&D and manufacturing processes to develop and distribute test kits, vaccines, and drugs against the SARS-CoV-2 virus. To boost R&D, many pharma and biotech companies have collaborated with CROs through long-term agreements, partnerships, and collaborations worldwide. For instance, in January 2021, ICON partnered with Pfizer and BioNTech to develop an experimental program for COVID-19 vaccines.
Moreover, for COVID-19 research, Contract research organization services provide dedicated research. After the COVID-19 outbreak, many new services, partnerships, and collaborations worldwide were recorded with pharma companies and academic institutes for COVID-19 vaccine research and development.
Amid the COVID-19 pandemic, the global market observed a significant increase in its market value due to the high demand for these services and increased revenue generated by the market players. For instance, IQVIA generated an estimated revenue of USD 13.65 billion in 2021, increasing 20.2% from the prior year.
LATEST TRENDS
Request a Free sample to learn more about this report.
Growing Investment by Pharma, Biopharma, and Medical Devices Makers to Boost Market Growth
Most biopharmaceutical, pharmaceutical, and medical devices businesses continue investing significant resources into developing new medications and technologies. Sponsors generally outsource therapeutic and other product development functions to independent services providers, which leads them to use a more flexible cost structure and avoid maintaining redundant development capabilities worldwide.
Increasing investments by many key businesses in clinical and non-clinical research activities and outsourcing to many contract research organizations services that help provide cost-effective development options fuel the global market growth. The trend shows that the key pharma companies are enhancing their R&D efficiencies and collaborating on added R&D. The pharma R&D sector is changing as companies are looking to allow more people to work remotely and focus on making trial endpoints more patient-centric and responsive. These factors will augment the market growth.
DRIVING FACTORS
Rise in Prevalence of Chronic Diseases is Fueling Contract Research Organization Services Market Growth
The global burden of chronic diseases is swiftly rising. For instance, according to the UN's Food and Agriculture Organization, chronic diseases accounted for approximately 60% of the world's 56.5 million documented deaths and 46% of the global disease burden. Based on an article from BMC Public Health in June 2020, WHO estimated that by 2020, non-communicable diseases (NCDs) would account for 80% of the global disease burden. Cardiovascular diseases account for more than half of all chronic disease deaths, while obesity and diabetes rise due to their widespread prevalence and earlier onset.
Chronic disease is a problem that affects people worldwide, not only in developed countries. Despite common belief, emerging countries are increasingly facing chronic disease-related public health problems. According to WHO, in five of the WHO's six regions, chronic diseases such as cancer, neurological disorders, immunological disorders, and others account for most mortality. Infectious diseases like HIV/AIDS, TB, malaria, and other similar disorders are still prevalent in Sub-Saharan Africa, and this trend is predicted to continue.
Although worldwide healthy life expectancy has climbed gradually (by over 6.5 years) between 1990 and 2019, it has not increased as much as overall life expectancy in 198 of the 204 nations studied, indicating that mortality has decreased even with the increasing prevalence of chronic diseases. Based on a WHO article published in April 2021, around 77% of NCD deaths happen in low and middle-income countries. Cardiovascular diseases accounted for most NCD deaths, approximately 17.9 million annually. Number of deaths due to cancer, respiratory diseases and diabetes were 9.3 million, 4.1 million and 1.5 million, respectively. The increasing prevalence of chronic diseases and rising awareness against these diseases have increased the pressure on the market players to develop efficient medications and is expected to boost the market significantly. These factors, in turn, drive the market.
Increasing Number of Clinical Trials Resulting in Increased Outsourcing to CROs
Clinical trials are essential for medication development processes all over the world. It helps discover novel treatments for diseases and new techniques for detecting, diagnosing, and preventing disease development. Clinical trials offer the scientific foundation for guiding and treating patients and evaluating novel medications and equipment. Clinical trial results help direct the right direction even though researchers may not get the expected results. Clinical trials in the United States are comparatively less than those conducted in other nations worldwide. Many clinical trials are conducted outside the United States and the European Union since it is often easier and less expensive.
Clinical trial success rates are highly dependent on the study's stage and the treatments or items being developed. Based on the article from Biotechnology Innovation Organization, only about 9% of medications make it through phase I to approval. Recently, the number of clinical trials that have been filed has risen significantly. Clinical trials have become increasingly sophisticated, yet they are still necessary to study and develop novel medications and technologies. This increase in the number of clinical trials is anticipated to boost the development of new drugs and is expected to drive the market's growth in the forecast period.
RESTRAINING FACTORS
Lack of Skilled Professionals to Restrain Growth of the Market
The surge in globalization is boosting the rate of innovation and technology. New opportunities are constantly being added in terms of occupation. Furthermore, increasing industrialization and new services have increased the demand for new skills. This trend has also increased competency in job opportunities. CROs face issues attracting and maintaining highly competent experts as they compete for qualified and experienced scientists with biotechnology, pharmaceutical, medical devices businesses, and academic and research institutions. Companies must offer better compensation and incentives to better compete, affecting the finances and outcomes of players, mainly small-scale analytical testing providers.
This scarcity of qualified specialists is a barrier to adopting novel procedures and technologies, restricting market growth. For instance, as per Labor Statistics, the U.S. market experienced a lack of experienced workforce in 2019. Skill development has not yet been successfully delivered by many countries worldwide. There are gaps between the working-age population and basic literacy. For instance, according to World Bank, around 750 million people aged 15 and above globally are not reading or writing, making it 18% of the global population.
SEGMENTATION
By service type analysis.
To know how our report can help streamline your business, Speak to Analyst
Clinical Segment Dominated Global CRO Services Market Due to Rising Number of Clinical Trials for Chronic Diseases
By service type, the market is divided into early phase development services, laboratory services, clinical, and others.
The clinical segment held a significant portion of the global contract research organization services market share in 2022. This is attributed to the high prevalence and rise in the number of chronic disease cases. Also, the growth in outsourcing R&D activities in the biotechnology & pharmaceutical sectors propels this market.
The early phase development services segment is estimated to expand at a comparatively higher CAGR during the forecast period. This is because of the increasing investment in the development of life-saving drugs.
By Application Analysis
Oncology Segment to Dominate Led by Continuous Development of Cancer Treatment Solutions
Based on application, the market can be segmented into CNS disorder, oncology, cardiology, metabolic disorder, infectious disease, and others.
Oncology has the highest market share as there is advancement in research and technology, and there is more focus on addressing broader global disease threats such as oncology. The stringent regulatory protocols and burdensome penalties for non-compliance have also increased the duration, cost, and complexity of clinical trials. It has been observed that the most active and fastest-growing therapeutic area for the CRO is oncology, representing the single most considerable focus in clinical development at present.
CNS Disorder has the second-highest CAGR due to the growing prevalence of Alzheimer's, Dementia, and Parkinson's disease.
By End User Analysis
Pharmaceutical Biotech Companies to Hold Dominant Share Backed by Increasing R&D
Based on end-users, the market can be segmented into medical device companies, academic and research institutes, pharmaceutical and biotech companies, and others.
Pharmaceutical and biotech companies have the highest market share as pharmaceutical sponsors are investing more in R&D and outsourcing a larger portion of R&D to independent service providers. According to Credit Suisse, only 41% of the clinical development was outsourced in 2016. It was anticipated to increase to 50% in 2020 because the cost and complexity of the development increase in onerous reimbursement and regulatory environment as using CRO partners, these pharmaceutical organizations seek more efficient solutions.
CROs for medical device is also growing at a faster pace. Management of medical technologies and new developments in healthcare by the CRO service providers has increased the market size share for medical devices.
REGIONAL INSIGHTS
North America Contract Research Organization (CRO) Services Market Size, 2022 (USD Billion)
To get more information on the regional analysis of this market, Request a Free sample
In terms of geography, the global market can be categorized into North America, Europe, Asia Pacific, and the Rest of the World.
The North America CRO services market was valued at USD 36.97 billion in 2022 and is expected to hold a major share of the global market in the forecast period. Factors responsible for the region’s dominance include the important pharmaceutical companies located in the region and their overall drug development activity and good healthcare infrastructure. Pharmaceutical organizations have increased their focus on outsourcing clinical trials to treat different disease conditions. Also, these organizations are investing more in R&D activities.
Europe was the second largest region in the market globally in 2022 and is estimated to hold this position during the forecast period. This is due to the surge in the incidence of diseases. Based on the article from Strategic Policy Group, the number of older people aged 65 years or more in the region would increase significantly, rising from around 90.5 million in 2019 to 129.8 million by 2050. Moreover, healthcare expenditure is surging, and pharma companies depend more on contract research organizations to offer more efficiency and productivity.
The Asia Pacific market is projected to grow at the fastest CAGR.The high growth is mainly attributed to the surge in R&D activity and growing shift towards outsourcing. Furthermore, low cost resources in the Asia Pacific region is one of the major factors driving the market growth in the region.
For instance, as per IBEF 2019 report, India’s cost of production is approximately 33.0% lower than that of the U.S.
The market in Rest of the World is expected to hold limited share in the market during the forecast period. However, increasing healthcare expenditure in the countries in rest of the world is expected to fuel market growth.
KEY INDUSTRY PLAYERS
Labcorp Adopts Merger & Acquisition Strategies to Bolster Portfolio and Strengthen Market Foothold
Labcorp Drug Development accounted for the major shares of the market 2022. The increased growth of the market was majorly due to the company’s strong focus on mergers and acquisition to upskill their services. For instance, in July 2021, Labcorp announced the expansion of its oncology portfolio by acquiring Omniseq. This acquisition upskilled the company’s contract research organization services portfolio.
Other key players, such as IQVIA, Pharmaceutical Product Development, LLC, and ICON pIc, held significant shares in the market. This was due to its strong focus on R&D to expand its product offerings and strengthen its global presence. Furthermore, companies are also focusing on incorporating new technologies, such as artificial intelligence , to increase the efficacy of their service offerings.
LIST OF KEY COMPANIES PROFILED:
- Pharmaceutical Product Development, LLC (U.S.)
- Medpace Holdings, Inc. (U.S.)
- Iqvia (U.S.)
- ICON plc (Ireland)
- KCR SA (U.S.)
- PSI (Switzerland)
- Parexel International Corporation (U.S.)
- Labcorp Drug Development (U.S.)
KEY INDUSTRY DEVELOPMENTS:
- December 2021 –Thermo Fisher Scientific Inc. completed the acquisition of Pharmaceutical Product Development, LLC. This acquisition enhanced the key services and offerings of the company.
- December 2021 – Laboratory Corporation of America Holdings completed the acquisition of Toxikon Corporation. This acquisition bolstered the company’s strong non-clinical development portfolio.
- November 2021 – Icon plc announced that its Accellacare Site Network has expanded in reach and capabilities through new partnerships with six research sites across four countries.
- November 2021 – Iqvia announced data aggregation strategy as the foundation to optimize the market insights. It helps to harness the right data and services to optimize the patient. This increased the efficiency of the company’s services.
- October 2021 – Parexel International Corporation and Kyoto University Hospital formed a strategic partnership to expand clinical research opportunities.
REPORT COVERAGE
An Infographic Representation of Contract Research Organization (CRO) Services Market

To get information on various segments, share your queries with us
The global market report provides a detailed market analysis. It focuses on key aspects such as an overview of the market, penetration of outsourcing in R&D and key countries, pricing analysis. Additionally, it includes key industry developments such as mergers, partnerships, & acquisitions, the impact of COVID-19 on the market, and brand analysis. Besides these, the report offers insights into the market trends and highlights key industry developments. In addition to the aforementioned factors, the report encompasses several factors that have contributed to the growth of the market over recent years. The report also covers a regional analysis of different segments.
Report Scope & Segmentation
Frequently asked questions.
Fortune Business Insights says that the global market size was USD 73.38 billion in 2022 and is projected to reach USD 188.52 billion by 2030.
In 2022, North America stood at USD 36.97 billion.
Growing at a CAGR of 12.5%, the market will exhibit steady growth in the forecast period (2023-2030).
The clinical segment is expected to be the leading segment in this market during the forecast period.
The increasing number of clinical trials and surge in the cases of chronic disease are some of the major factors driving the markets growth.
Labcorp and IQVIA are some of the major players in the global market.
North America dominated the market in 2022.
The surge in the outsourcing for R&D by the pharmaceutical organizations more efficiency and productivity are factors that drive the adoption of the service.
Seeking Comprehensive Intelligence on Different Markets? Get in Touch with Our Experts
- STUDY PERIOD: 2019-2030
- BASE YEAR: 2022
- HISTORICAL DATA: 2019-2021
- NO OF PAGES: 121
Personalize this Research
- Granular Research on Specified Regions or Segments
- Companies Profiled based on User Requirement
- Broader Insights Pertaining to a Specific Segment or Region
- Breaking Down Competitive Landscape as per Your Requirement
- Other Specific Requirement on Customization

Healthcare Clients

Related Reports
- Contract Development and Manufacturing Organization (CDMO) Outsourcing Market
- ASEAN Contract Development and Manufacturing Organization (CDMO) Market
- Contract Manufacturing Organization (CMO) Market
- Europe CRO Services Market
- Clinical Trials Market
Client Testimonials
“We are quite happy with the methodology you outlined. We really appreciate the time your team has spent on this project, and the efforts of your team to answer our questions.”
“Thanks a million. The report looks great!”
“Thanks for the excellent report and the insights regarding the lactose market.”
“I liked the report; would it be possible to send me the PPT version as I want to use a few slides in an internal presentation that I am preparing.”
“This report is really well done and we really appreciate it! Again, I may have questions as we dig in deeper. Thanks again for some really good work.”
“Kudos to your team. Thank you very much for your support and agility to answer our questions.”
“We appreciate you and your team taking out time to share the report and data file with us, and we are grateful for the flexibility provided to modify the document as per request. This does help us in our business decision making. We would be pleased to work with you again, and hope to continue our business relationship long into the future.”
“I want to first congratulate you on the great work done on the Medical Platforms project. Thank you so much for all your efforts.”
“Thank you very much. I really appreciate the work your team has done. I feel very comfortable recommending your services to some of the other startups that I’m working with, and will likely establish a good long partnership with you.”
“We received the below report on the U.S. market from you. We were very satisfied with the report.”
“I just finished my first pass-through of the report. Great work! Thank you!”
“Thanks again for the great work on our last partnership. We are ramping up a new project to understand the imaging and imaging service and distribution market in the U.S.”
“We feel positive about the results. Based on the presented results, we will do strategic review of this new information and might commission a detailed study on some of the modules included in the report after end of the year. Overall we are very satisfied and please pass on the praise to the team. Thank you for the co-operation!”
“Thank you very much for the very good report. I have another requirement on cutting tools, paper crafts and decorative items.”
“We are happy with the professionalism of your in-house research team as well as the quality of your research reports. Looking forward to work together on similar projects”
“We appreciate the teamwork and efficiency for such an exhaustive and comprehensive report. The data offered to us was exactly what we were looking for. Thank you!”
“I recommend Fortune Business Insights for their honesty and flexibility. Not only that they were very responsive and dealt with all my questions very quickly but they also responded honestly and flexibly to the detailed requests from us in preparing the research report. We value them as a research company worthy of building long-term relationships.”
“Well done Fortune Business Insights! The report covered all the points and was very detailed. Looking forward to work together in the future”
“It has been a delightful experience working with you guys. Thank you Fortune Business Insights for your efforts and prompt response”
“I had a great experience working with Fortune Business Insights. The report was very accurate and as per my requirements. Very satisfied with the overall report as it has helped me to build strategies for my business”
“This is regarding the recent report I bought from Fortune Business insights. Remarkable job and great efforts by your research team. I would also like to thank the back end team for offering a continuous support and stitching together a report that is so comprehensive and exhaustive”
“Please pass on our sincere thanks to the whole team at Fortune Business Insights. This is a very good piece of work and will be very helpful to us going forward. We know where we will be getting business intelligence from in the future.”
“Thank you for sending the market report and data. It looks quite comprehensive and the data is exactly what I was looking for. I appreciate the timeliness and responsiveness of you and your team.”
Get in Touch with Us
+1 424 253 0390 (US)
+44 2071 939123 (UK)
+91 744 740 1245 (APAC)
[email protected]
- Request Sample
Sharing this report over the email

The global contract research organization (CRO) services market is projected to grow from $82.60 billion in 2023 to $188.52 billion by 2030
Read More at:-
- [email protected]
- +1 909 414 1393
- Report Details
- Table Of Content
- Request Free Sample

Global Clinical Research Organisation (CRO) Market by Type (Small molecules, Biologics), By Application (Pharmaceutical Company, Research Institute, Other) and Region (North America, Latin America, Europe, Asia Pacific and Middle East & Africa), Forecast From 2022 To 2030
- Table of Content
- Free Sample
Market Overview:
The global clinical research organisation (CRO) market is expected to grow at a CAGR of 7.5% from 2018 to 2030. The growth in the CRO market can be attributed to the increasing number of clinical trials, rising demand for contract research services, and growing focus on outsourcing by pharmaceutical and biotechnology companies. The CRO market is segmented on the basis of type into small molecules and biologics. The small molecules segment is expected to account for the majority share of the CRO market in 2018. However, the biologics segment is projected to grow at a higher CAGR during the forecast period owing to increasing demand for novel therapies and rising prevalence of chronic diseases. On the basis of application,the CRO market is divided into three segments: pharmaceutical companies, research institutes, and other end users such as medical device manufacturers and academic institutions. Pharmaceutical companies are expected to account forthe largest shareof th eCR Omarketin2018 .
Product Definition:
A clinical research organisation (CRO) is a company or institution that conducts clinical trials on behalf of pharmaceutical, biotechnology, and medical device companies. The CRO industry has exploded in recent years as the number of drug and device approvals by the U.S. Food and Drug Administration (FDA) has increased.
The use of CROs allows pharmaceutical, biotechnology, and medical device companies to focus on their core competencies – developing new drugs and devices – while outsourcing much of the work associated with conducting clinical trials.
Small molecules:
Small molecules are the most commonly used drug ingredients. They account for more than 90% of all pharmaceutical drugs. Small molecules can be administered to patients in various forms such as tablets, injections, and inhalation solutions.
The global small molecule's market is expected to grow at a CAGR of XX% during the forecast period owing to increasing R&D activities by major companies.
Biologics are living organisms or products derived from living organisms and used as therapeutic agents. They are different from conventional drugs as they have a unique mechanism of action, grow in nature and can only be obtained from the original source (biological source) or through biotechnology.
The global CRO market size was valued at USD 21 billion in 2015 and is expected to grow at a CAGR of XX% over the forecast period.
Application Insights:
The pharmaceutical company segment dominated the global CRO market in 2017. This is attributed to factors such as presence of a strong pipeline and presence of key products in the pipeline that are ready for clinical trials. For instance, according to data published by WHO, around 10-20% of drugs developed complete clinical testing every year. Similarly, according to an article published by Statista in 2018, around 20% - 25% of all medicines developed have completed Phase III clinical trials and are ready for commercialization. These factors indicate that there is ample scope for growth over the forecast period for this segment.
However, other segments such as research institutes and others also exhibit significant potential growth owing to increasing government funding towards R&D activities coupled with growing awareness about advanced treatments among patients globally (through various media outlets).
Regional Analysis:
North America dominated the global market in 2017. This can be attributed to the presence of a large number of CROs, increasing outsourcing activities by North American CROs, and high healthcare expenditure in this region. Moreover, growing focus on clinical trials for new drug development is expected to drive regional growth over the forecast period.
Asia Pacific is anticipated to witness lucrative growth during the forecast period owing to rising investments by global players for expanding their commercial reach and improving their revenue margins from low-cost countries such as India and China. In addition, government initiatives encouraging domestic level R&D are expected to fuel regional demand during the forecast period.
Growth Factors:
- Increasing demand for clinical research services due to the increase in number of drug approvals by the USFDA and other regulatory agencies across the globe.
- Growing demand for contract research services from biotechnology and pharmaceutical companies due to their focus on core competencies.
- Proliferation of clinical trials owing to increasing prevalence of chronic diseases, such as cancer, diabetes, and cardiovascular diseases.
- Rising public-private investments in life sciences sector that is fuelling the growth of CROs worldwide
Scope Of The Report
Global clinical research organisation (cro) market report segments:.
The global Clinical Research Organisation (CRO) market is segmented on the basis of:
Small molecules, Biologics
The product segment provides information about the market share of each product and the respective CAGR during the forecast period. It lays out information about the product pricing parameters, trends, and profits that provides in-depth insights of the market. Furthermore, it discusses latest product developments & innovation in the market.
Applications
Pharmaceutical Company, Research Institute, Other
The application segment fragments various applications of the product and provides information on the market share and growth rate of each application segment. It discusses the potential future applications of the products and driving and restraining factors of each application segment.
Some of the companies that are profiled in this report are:
- Charles River Laboratories
- Genscript Biotech
- GVK Biosciences
- Aurobindo Pharma
- Eurofins Scientific
- Dalton Pharma
- Jubilant Biosys
- Lupin Pharmaceuticals
- Piramal Pharma
- Sun Pharmaceutical
- Sai Ganga Panakeia
- Vitas Pharma
- Pharmaron Beijing
- WuXi AppTec
- Asymchem Laboratories
- Porton Pharma
- ChemPartner
- Hangzhou Tigermed
Highlights of The Clinical Research Organisation (CRO) Market Report:
- The market structure and projections for the coming years.
- Drivers, restraints, opportunities, and current trends of market.
- Historical data and forecast.
- Estimations for the forecast period 2030.
- Developments and trends in the market.
Small molecules
- By Application:
Pharmaceutical Company
Research institute.
- Market scenario by region, sub-region, and country.
- Market share of the market players, company profiles, product specifications, SWOT analysis, and competitive landscape.
- Analysis regarding upstream raw materials, downstream demand, and current market dynamics.
- Government Policies, Macro & Micro economic factors are also included in the report.
We have studied the Clinical Research Organisation (CRO) Market in 360 degrees via. both primary & secondary research methodologies. This helped us in building an understanding of the current market dynamics, supply-demand gap, pricing trends, product preferences, consumer patterns & so on. The findings were further validated through primary research with industry experts & opinion leaders across countries. The data is further compiled & validated through various market estimation & data validation methodologies. Further, we also have our in-house data forecasting model to predict market growth up to 2030.
Regional Analysis
- North America
- Asia Pacific
- Middle East & Africa
- Latin America
Note: A country of choice can be added in the report at no extra cost. If more than one country needs to be added, the research quote will vary accordingly.
The geographical analysis part of the report provides information about the product sales in terms of volume and revenue in regions. It lays out potential opportunities for the new entrants, emerging players, and major players in the region. The regional analysis is done after considering the socio-economic factors and government regulations of the countries in the regions.
How you may use our products:
- Correctly Positioning New Products
- Market Entry Strategies
- Business Expansion Strategies
- Consumer Insights
- Understanding Competition Scenario
- Product & Brand Management
- Channel & Customer Management
- Identifying Appropriate Advertising Appeals
8 Reasons to Buy This Report
- Includes a Chapter on the Impact of COVID-19 Pandemic On the Market
- Report Prepared After Conducting Interviews with Industry Experts & Top Designates of the Companies in the Market
- Implemented Robust Methodology to Prepare the Report
- Includes Graphs, Statistics, Flowcharts, and Infographics to Save Time
- Industry Growth Insights Provides 24/5 Assistance Regarding the Doubts in the Report
- Provides Information About the Top-winning Strategies Implemented by Industry Players.
- In-depth Insights On the Market Drivers, Restraints, Opportunities, and Threats
- Customization of the Report Available
Frequently Asked Questions?
A Clinical Research Organisation (CRO) is a company or organisation that conducts clinical trials.
Some of the major players in the clinical research organisation (cro) market are Charles River Laboratories, IQVIA, LabCorp, Domainex, Evotec AG, Genscript Biotech, GVK Biosciences, Aurobindo Pharma, Eurofins Scientific, Reprocell, Dalton Pharma, NovAliX, Jubilant Biosys, Lupin Pharmaceuticals, Piramal Pharma, Sun Pharmaceutical, Sai Ganga Panakeia, Vitas Pharma, Pharmaron Beijing, WuXi AppTec, Asymchem Laboratories, Porton Pharma, ChemPartner, Hangzhou Tigermed.
The clinical research organisation (cro) market is expected to register a CAGR of 7.5%.
Report Payment
Request for sample, ask for discount, enquiry before buying, related reports.

Global Arcade Gaming Market by Type (Racing, Shooting, Sports, Action), By Application (R...

Global Online Sports Apparel Retailing Market by Type (For Women, For Men, For Kids), By ...

Global Lottery Software Market by Type (On-Premise, Cloud Based), By Application (Persona...

Global Cloud Supply Chain Management Market by Type (Training and Consulting, Support and...

Global Cybersecurity Market by Type (Enterprise Security, Endpoint Security, Cloud Securi...

Global Online Virtual Classroom Software Market by Type (On-premise, Cloud-based), By App...
Our trusted clients.

Industry Growth Insights is Product of YOAAP Media Services LLP. | COPYRIGHT © 2024 IGI | All Rights Reserved.
Get call back from us

Thank you for subscribing to our newsletter. You Should receive a confirmation email soon.

- Healthcare /
- Biotechnology /
- Contract Research Organization

Healthcare Contract Research Organization Market Size, Share & Trends Analysis Report By Type (Drug Discovery, Pre-clinical, Clinical), By Services (Clinical Monitoring, Data Management), By Region, And Segment Forecasts, 2023-2030
- Region: Global
- Grand View Research
- ID: 4538855
- Description
Table of Contents
Companies mentioned, methodology, related topics, related reports.
- Purchase Options
- Ask a Question
- Recently Viewed Products
Healthcare Contract Research Organization Market Report Highlights
- Based on type, the clinical segment has dominated the global healthcare CRO market in 2022, with the largest share of 76.2% owing to the fact that it comprises four elaborate phases, including human subjects. The significant demand for new treatments has further contributed to the growth of the segment
- Among services, the clinical monitoring segment was the largest in terms of market share at 20.2% in 2022
- North America held a significant market share of 24.8% in 2022 due to the presence of several global players who invest a major part of their revenue in research activities
- Asia Pacific is anticipated to witness lucrative growth of 9.5% over the forecast period due to the reduced cost it offers in comparison to the western economies
- China, Japan, and India are projected to witness tremendous growth in the contract research organization market owing to high disease prevalence
- Charles River Laboratories
- Syneos Health
- GVK Biosciences Private Limited
- Laboratory Corporation of America Holdings
- Thermo Fisher Scientific Inc.
- Parexel International Corporation
- Medidata Solutions, Inc
- Pharmaron GMBH.
- CTI Clinical Trial & Consulting
- Worldwide Clinical Trials
- Wuxi AppTec
- Advanced Clinical
Table Information
- Biotechnology
- Contract Research
- Drug Discovery
- Healthcare Services
- Preclinical Development

Contract Research Outsourcing - Global Strategic Business Report
- Report

Global Healthcare Contract Research Organization Market by Type (Clinical, Drug Discovery, Pre-Clinical), Service (Bio-Statistics, Clinical Monitoring, Data Management) - Forecast 2024-2030

Recombinant Proteins Manufacturing Services Market Size, Share & Trends Analysis Report, By Service Type (Pre-clinical & Clinical Services, Commercial Production), By Host Cell, By End-user, By Region, And Segment Forecasts, 2023 - 2030
- August 2023

Global Contract Research Organization Services Market by Type (Clinical Research Services, Consulting Services, Data Management Services), Trial Phase (Phase I, Phase II, Phase III), Therapeutic Area, Molecule Type, End-User - Forecast 2024-2030
ASK A QUESTION
We request your telephone number so we can contact you in the event we have difficulty reaching you via email. We aim to respond to all questions on the same business day.
Request a Quote
YOUR ADDRESS
YOUR DETAILS
PRODUCT FORMAT
DOWNLOAD SAMPLE
Please fill in the information below to download the requested sample.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Heart-Healthy Living
- High Blood Pressure
- Sickle Cell Disease
- Sleep Apnea
- Information & Resources on COVID-19
- The Heart Truth®
- Learn More Breathe Better®
- Blood Diseases and Disorders Education Program
- Publications and Resources
- Blood Disorders and Blood Safety
- Sleep Science and Sleep Disorders
- Lung Diseases
- Health Disparities and Inequities
- Heart and Vascular Diseases
- Precision Medicine Activities
- Obesity, Nutrition, and Physical Activity
- Population and Epidemiology Studies
- Women’s Health
- Research Topics
- Clinical Trials
- All Science A-Z
- Grants and Training Home
- Policies and Guidelines
- Funding Opportunities and Contacts
- Training and Career Development
- Email Alerts
- NHLBI in the Press
- Research Features
- Past Events
- Upcoming Events
- Mission and Strategic Vision
- Divisions, Offices and Centers
- Advisory Committees
- Budget and Legislative Information
- Jobs and Working at the NHLBI
- Contact and FAQs
- NIH Sleep Research Plan
- News and Events
- < Back To All News
Researchers review findings and clinical messages from the Women’s Health Initiative 30 years after launch

Data from influential study underscore the importance of personalized and shared decision-making to support the health of postmenopausal women
WHAT: A new review in JAMA highlights key findings and clinical messages from the Women’s Health Initiative (WHI), the largest women’s health study in the United States. The WHI is supported by the National Institutes of Health’s National Heart, Lung, and Blood Institute (NHLBI), and was created to study factors that may reduce risks for cardiovascular disease, cancer, hip fractures, and other conditions in postmenopausal women. More than 68,000 women enrolled in clinical trials between 1993 and 1998 and were followed for up to 20 years.
After reviewing these long-term data, the researchers explain the primary findings:
Hormone therapy and menopause. The WHI study found that estrogen or a combination of estrogen and progestin, two types of hormone replacement therapies, had varying outcomes with chronic conditions, and the evidence does not support the use of these therapies to reduce risks for chronic diseases, such as heart disease, stroke, cancer, and dementia. The study was not designed to assess the effects of FDA-approved hormone therapies for treating menopausal symptoms, the benefits of which had been established before the WHI study began.
The authors reinforce the importance of women making shared decisions with physicians about the benefits or risks of taking hormone therapy during menopause. For example, women younger than age 60 with low-to-average risk for cardiovascular disease and breast cancer who want to take hormone therapy may experience greater health benefits than risks during early menopause to treat moderate-to-severe symptoms, such as bothersome hot flashes or night sweats.
Calcium and vitamin D supplements and bone fractures. A combined calcium and vitamin D supplement was not associated with reduced risks for hip fractures among postmenopausal women at average risk for osteoporosis, according to the study. However, the authors note that supplements can help fill nutrient gaps among women who do not meet the daily recommended intake for these nutrients . Therefore, women with questions about adequate intake and levels should consult with their healthcare provider.
Low-fat diets and cancer. A low-fat dietary pattern with at least five daily servings of fruits and vegetables and increased grains did not reduce the risk of breast or colorectal cancer. However, upon subsequent analyses during the follow-up period, researchers found that this type of eating pattern was associated with a reduced risk of death from breast cancer.
Findings from the clinical trials and study observations can vary based on multiple factors, such as age and underlying cardiovascular disease risks, so women ages 50 and older should work with their clinicians to make individualized and shared medical decisions, the researchers noted.
STUDY: Manson, JE, Crandall CJ, Rossouw JE, et al. The Women’s Health Initiative randomized trials and clinical practice: A review. JAMA ; 2024. Doi: 10.1001/jama.2024.6542.
WHO: Candice A. Price, Ph.D., program director of the epidemiology branch, located within the Division of Cardiovascular Sciences at NHLBI, is available to discuss this review.
About the National Heart, Lung, and Blood Institute (NHLBI): NHLBI is the global leader in conducting and supporting research in heart, lung, and blood diseases and sleep disorders that advances scientific knowledge, improves public health, and saves lives. For more information, visit www.nhlbi.nih.gov .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
More Information
Related health topics, health education, related news.
Advancing social justice, promoting decent work ILO is a specialized agency of the United Nations
Migrated Content
Labour markets have shown surprising resilience despite deteriorating economic conditions, but recovery from the pandemic remains uneven as new vulnerabilities and multiple crises are eroding prospects for greater social justice.
Additional details
- ISBN: 9789220400418 (print)
- ISBN: 9789220400425 (web PDF)
Related content

Executive Summary
World Employment and Social Outlook: Trends 2024
WESO Trends 2024
Global unemployment rate set to increase in 2024 while growing social inequalities raise concerns, says ILO report
[ORGANIZATION OF MEDICAL CARE FOR CHILDREN WITH A NEW CORONAVIRUS INFECTION IN PATIENT CONDITIONS ON THE EXAMPLE OF THE CHILDREN'S CITY CLINICAL HOSPITAL NAMED AFTER Z. A. BASHLYAEVA]
Affiliations.
- 1 Children's City Clinical Hospital named after Z. A. Bashlyaeva of the Moscow City Health Department, 125373, Moscow, Russian Federation.
- 2 Pirogov Russian National Research Medical University, 117997, Moscow, Russian Federation.
- 3 Russian Medical Academy of Continuous Professional Education of the Ministry of Healthcare of the Russian Federation, 125993, Moscow, Russian Federation.
- 4 Pirogov Russian National Research Medical University, 117997, Moscow, Russian Federation, [email protected].
- 5 Research Institute for Healthcare Organization and Medical Management of Moscow Healthcare Department, 115088, Moscow, Russian Federation.
- PMID: 34792888
- DOI: 10.32687/0869-866X-2021-29-s2-1343-1349
The article presents an analysis of the work of the largest children's COVID-19 center in Moscow, organized on the basis of the Children's City Clinical Hospital named after Z. A. Bashlyaeva of the Moscow City Health Department. From March to November 2020 at the COVID-19 Center were hospitalized 2,837 patients with suspected/confirmed diagnosis of COVID-19, in total in 2020 1,876 children with a confirmed diagnosis of COVID-19 were treated, 58 (3%) children were in serious condition in the intensive care unit, of which children 11-18 years old were 25%. At the 2020 neonatal COVID-19 center, 215 newborns were observed with suspected COVID-19 diagnosis. The diagnosis of COVID-19 was confirmed in 18 children, while 8 newborns came from the home of COVID-19. In the Center for rehabilitation, where children aged 0 to 3 years old who were born with very low and extremely low body weight are observed, dispensary observation for children who have undergone COVID-19 is organized. 45 children who were observed fell ill with the new coronavirus infection. There were no deaths among children with COVID-19.
Keywords: COVID-19; COVID-center; children; new coronavirus infection; newborns; treatment.
- COVID-19 Testing*
- Child, Preschool
- Hospitals, Pediatric
- Infant, Newborn
- Retrospective Studies

IMAGES
VIDEO
COMMENTS
Clinical Research Organization Market Snapshot (2023 to 2033) According to Future Market Insights (FMI) analysis in a recent market survey, the global clinical research organization market was valued at US$ 58.0 Billion in 2022 and is expected to reach US$ 139.6 Billion by 2033. Market Outlook: The key players in the market are Charles River ...
The global Clinical Research Organization (CRO) market was estimated at USD 80.6 Billion in 2022 and is anticipated to reach USD 220.3 Billion by 2031, expanding at a CAGR of 12.2% during the forecast period. A clinical research organization (CRO) provides support to the pharmaceuticals, biotechnology, and medical devices industries in the form ...
The global clinical trials market size was valued at USD 80.7 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 6.49% from 2024 to 2030. The market growth spiked in 2020 owing to the COVID-19 pandemic. This growth pattern was witnessed by both virtual clinical trials and traditional ones.
The market for clinical research organizations (CROs) has grown significantly due to factors such as the demand for specialized knowledge, growing R&D expenses, and the complexity of clinical trials. Pharmaceutical, biotechnology, and medical device firms can benefit from a variety of services provided by CROs, including data management ...
Clinical Research Organizations. 9 comprehensive market analysis studies and industry reports on the Clinical Research Organizations sector, offering an industry overview with historical data since 2019 and forecasts up to 2029. This includes a detailed market research of 87 research companies, enriched with industry statistics, industry ...
talent, according to BDO's 2022/2023 Clinical Research Organization Insights Report. This summary report is a selection of high-level insights from the recently published 2022 CRO Industry Global Compensation & Turnover Survey. This report provides clinical research organizations with a unique source of comprehensive data on the global
Global Clinical Research Organization Market Size. The global clinical research organization market is projected to grow at a CAGR of 7.6% from 2023 to 2028. Clinical research organizations (CROs) offer outsourced services to the biotechnology and pharmaceutical sectors. They carry out clinical studies for novel medications and treatments.
The Clinical Trials Market size is estimated at USD 50.66 billion in 2024, and is expected to reach USD 67.5 billion by 2029, growing at a CAGR of 5.91% during the forecast period (2024-2029). The COVID-19 pandemic tremendously impacted the market for clinical trials, as there has been a rising focus on developing new therapeutics or vaccines ...
Globally, the clamp studies market for CROs is expected to grow with a CAGR of 8.0% from USD 5.3 Bn in 2021 to reach to about USD 7.78 Bn by 2026. While, in India, the glucose clamp studies market is expected to reach to about USD 0.050 Bn in 2026 from USD 0.032 Bn in 2021 at a CAGR of about 9.1%.
The global clinical trials market size was valued at USD 57.76 billion in 2023 and is projected to grow from USD 61.58 billion in 2024 to USD 106.78 billion by 2032, exhibiting a CAGR of 7.1% during the forecast period (2024-2032). Clinical trials are an important process in developing new therapeutics or medical devices.
Clinical Research Organizations market report transcript. The global CRO market was estimated at $31.6 billion in 2018, and it is expected to grow at a CAGR of 12 percent to reach $45.2 billion by 2022. This is due to the increased outsourcing (>50 percent) seen from the pharmaceutical and biotechnology companies, with 85 percent comprised by ...
KEY MARKET INSIGHTS. Listen to Audio Version. The contract research organization (CRO) services market size was valued at USD 82.60 billion in 2023 and is projected to grow USD 188.52 billion by 2030, exhibiting a CAGR of 12.5% during 2023-2030. Rising chronic disease burden and pressure for new treatments drive the CRO market.
pandemic, Clinical Research Organizations (CROs) are challenged more than ever to address high turnover levels and lackluster compensation strategies to compete for highly sought-after talent, according to BDO's 2021/2022 Clinical Research Organization Insights Report. This summary report highlights key trends from the recently published 2021 CRO
Market Overview: The global clinical research organisation (CRO) market is expected to grow at a CAGR of 7.5% from 2018 to 2030. The growth in the CRO market can be attributed to the increasing number of clinical trials, rising demand for contract research services, and growing focus on outsourcing by pharmaceutical and biotechnology companies.
Clinical Trials Market size recorded more than USD 55.5 billion in 2022 and will exhibit 5.9% CAGR from 2023-2032. Burgeoning prevalence of chronic diseases is expected to favor market dynamics. The significantly increasing number of initiatives undertaken by several government and non-government organizations will anchor clinical trials ...
PUNE, India, June 26, 2023 /PRNewswire/ -- According to a recent market study published by Growth Market Reports, titled, "Global Clinical Research Organization (CRO) Market Segments - By Service ...
What Does the Report Cover? Future Market Insights offers a unique perspective and actionable insights on the clinical research organization market in its latest study, presenting a historical ...
The clinical research organizations category is expected to grow at a CAGR of 12.2% from 2023 to 2030. Factors such as increasing healthcare expenditure, rise in the number of drug development activities and increasing globalization of clinical trials are fueling the category growth. Rising healthcare costs lead to more funding for clinical ...
Healthcare Contract Research Organization Market Report Highlights. Based on type, the clinical segment has dominated the global healthcare CRO market in 2022, with the largest share of 76.2% owing to the fact that it comprises four elaborate phases, including human subjects. ... Table 15: U.S. Healthcare Contract Research Outsourcing Market ...
X7 Research is a full-service Contract Research Organization with operation in Eurasian Economic Union (EAEU) and CIS. | X7 Research provides clinical trials and drug registration services in EAEU ...
PUNE, India, April 30, 2024 /PRNewswire/ -- The report titled "Clinical Trial Analytics Services Market by Services (Clinical Data Analytics, Clinical Trial Data Management, Clinical Trial ...
Report Overview. The global facial recognition market size was valued at USD 5.15 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 14.9% from 2023 to 2030.Technology is improving, evolving, and expanding at an explosive rate. Technologies such as biometrics are extensively used to enhance security. These are used across various applications, such as access ...
A new review in JAMA highlights key findings and clinical messages from the Women's Health Initiative (WHI), the largest women's health study in the United States. The WHI is supported by the National Institutes of Health's National Heart, Lung, and Blood Institute (NHLBI), and was created to study factors that may reduce risks for cardiovascular disease, cancer, hip fractures, and other ...
The report reveals a complex global employment scenario. It forecasts a slight increase in global unemployment in 2024, signalling emerging labour market challenges. The report highlights disparities between high and low-income countries, noting higher unemployment and poverty rates in lower-income nations.
The class of buildings is indicated according to the classification of the Moscow Research Forum 2013 Source: Knight Frank Research, 2021 Supply At the end of 2020, the total supply of offices in the Moscow market amounted to 16.99 million sq. m., including 4.59 million sq. m. of Class A and 12.40 million sq. m. of Class B.
Affiliations 1 Children's City Clinical Hospital named after Z. A. Bashlyaeva of the Moscow City Health Department, 125373, Moscow, Russian Federation.; 2 Pirogov Russian National Research Medical University, 117997, Moscow, Russian Federation.; 3 Russian Medical Academy of Continuous Professional Education of the Ministry of Healthcare of the Russian Federation, 125993, Moscow, Russian ...