
- Book Solutions
- State Boards

Case Study Questions Class 7 Science Nutrition in Plants
Case study questions class 7 science chapter 2 nutrition in plants.
CBSE Class 7 Case Study Questions Science Nutrition in Plants. Important Case Study Questions for Class 7 Board Exam Students. Here we have arranged some Important Case Base Questions for students who are searching for Paragraph Based Questions Nutrition in Plants.
At Case Study Questions there will given a Paragraph. In where some Important Questions will made on that respective Case Based Study. There will various types of marks will given 1 marks, 2 marks, 3 marks, 4 marks.
CBSE Case Study Questions Class 7 Science Nutrition in Plants
Case study 1
Carbohydrates,proteins, fats, vitamins and minerals are components of food. These components of food are called nutrients and are necessary for our body.All living organisms require food. Plants can synthesise food for themselves but animals including humans cannot. They get it from plants or animals that eat plants. Thus,humans and animals are directly or indirectly dependent on plants.Plants are the only organisms that can prepare food for themselves by using water, carbon dioxide and minerals.The raw materials are present in their surroundings.The nutrients enable living organisms to build their bodies, to grow, to repair damaged parts of their bodies and provide the energy to carry out life processes. Nutrition is the mode of taking food by an organism and its utilization by the body.
The mode of nutrition in which organisms make food themselves from simple substances is called autotrophic ( auto = self; trophos = nourishment) nutrition. Therefore,plants are called autotrophs. Animals and most other organisms take in food prepared by plants. They are called heterotrophs ( heteros = other).
Que. 1) What are the components of food?
(a) Vitamins
(b) Carbohydrates
(c) Proteins
(d) All of the above
Que. 2) Which organism are autotrophs?
(b) Animals
(c) Bacteria
Que. 3) Plants produce food by using which of the following components.
(b) Carbon dioxide
(d) Both (a) & (b)
Que. 4) Define autotrophic nutrition?
Que. 5) What are the things enabled by nutrients to living organisms?
Que. 1) (d) All of the above
Que. 2) (d) Plants
Que. 3) (d) Both (a) & (b)
Que. 4) Answer: it is the mode of nutrition in which organisms like plants make food on their own from simple substances.
Que. 5) Answer: nutrients enable the living beings to grow, to build their bodies, to repair damaged parts like tissues of the body and also provide energy to carry out metabolism.
Case study 2
Leaves are the food factories of plants. Therefore, all the raw materials must reach the leaf. Water and minerals present in the soil are absorbed by the roots and transported to the leaves. Carbon dioxide from air is taken in through the tiny pores present on the surface of leaves. These pores are surrounded by ‘guard cells’. Such pores are called stomata.Water and minerals are transported to the leaves by the vessels which run like pipes throughout the root, the stem, the branches and the leaves. They form a continuous path or passage for the nutrients to reach the leaf. They are called vessels. The leaves have a green pigment called chlorophyll. It helps leaves to capture the energy of the sunlight. This energy is used to synthesise (prepare) food from carbon dioxide and water.Since the synthesis of food occurs in the presence of sunlight, it is called photosynthesis ( Photo : light; synthesis :to combine). So we find that chlorophyll, sunlight, carbon dioxide and water are necessary to carry out the process of photosynthesis. It is a unique process on the earth . The solar energy is captured by the leaves and stored in the plant in the form of food. Thus, sun is the ultimate source of energy for all living organisms.
Que. 1) What are the food factories of plants?
(d) None of the above
Que. 2) Leaves contain ……………………………………………………………………… pigment.
(a) Chlorophyll
(b) Green pigment
(c) carotenoid
Que. 3) …………………………………………………………………… is the ultimate source of energy for all living organisms.
(c) Infrared light
(d) Visible light
Que. 4) How does the leaves capture the sunlight energy?
Que. 5) Define photosynthesis?
Que. 1) (a) Leaves
Que. 2) (d) Both (a) & (b)
Que. 3) (b) Sun
Que. 4) Answer: leaves capture the sunlight energy with the help of a green pigment called chlorophyll which is present in the mesophyll cells of leaves.
Que. 5 ) Answer: The process of synthesis of food from carbon dioxide and water in the presence of sunlight is called as photosynthesis.
Case study 3
Plants synthesise carbohydrates through the process of photosynthesis. Thecarbohydrates are made of carbon, hydrogen and oxygen. These are used to synthesise other components of food such as proteins and fats. But proteins are nitrogenous substances which contain nitrogen. Nitrogen is present inabundance in gaseous form in the air.
However, plants cannot absorb nitrogen in this form. Soil has certain bacteria that convert gaseous nitrogen into a usable form and release it into the soil. These are absorbed by the plants along with water. Also, you might have seen farmers adding fertilisers rich in nitrogen to the soil. In this way the plants fulfil their requirements of nitrogen along with the other constituents. Plants can then synthesise proteins and vitamins.
Besides leaves, photosynthesis also takes place in other green parts of the
Plant — in green stems and green branches. The desert plants have scale- orspine-like leaves to reduce loss of water by transpiration. These plants havegreen stems which carry out photosynthesis.
Que. 1) Carbohydrates are made up of which of the following components?
(a) Hydrogen
Que. 2) …………………………………………………..…… is a nitrogen containing compound.
(a) Carbohydrates
(b) Hydrogen
(d) Proteins
Que. 3) Which organisms convert gaseous nitrogen into a useable form?
(a) Earthworm
(c) Microbe
(d) Bacteria
Que. 4) How does desert plants reduce loss of water by transpiration?
Que. 5) How do plants fulfil their requirements of nitrogen?
Que. 2) (d) Proteins
Que. 3) (d) Bacteria
Que. 4) Answer: Desert plants have scaly or spiny leaves as compared to other normal plants to reduce water loss by transpiration.
Que. 5) Answer: Certain bacterias present in soil converts gaseous nitrogen into more useable form and release it into soil and also nitrogen containing fertilizers are added to soil to fulfil nitrogen requirement of plants
Case study 4
Oxygen which is essential for the survival of all organisms is produced during photosynthesis. In the absence ofphotosynthesis, life would be impossible on the earth.During photosynthesis, chlorophyll containing cells of leaves, in the presence of sunlight, use carbon dioxide and water to synthesise carbohydrates. The process can be represented in an equation: During the process oxygen is released. The presence of starch in leaves indicates the occurrence of photosynthesis. Starch is also a carbohydrate
Carbon dioxide+ water ——– sunlight——–> carbohydrate+ oxygen

Que. 1) Which of the following organism produces oxygen?
Que. 2) Photosynthesis occurs in the presence of light?
(c) Both (a)&(b)
Que. 3) ………………………………………………………………… component is essential for the survival of all the living organisms.
(c) Nitrogen
(d) Hydrogen
Que. 4) Write the equation of photosynthesis?
Que. 5) What is the indication of the occurrence of photosynthesis in a plant?
Que. 1) (b) Plants
Que. 2) (a) True
Que. 3) (a) Oxygen
Que. 4) Answer: 6CO 2 + 6H 2 O UV LIGHT C 6 H 12 0 6 + 6O 2
Que. 5) Answer : The presence of reserve food material (i.e. Starch) in the leaves of plant indicates the occurrence of photosynthesis.
Case study 5
Take two potted plants of the same kind. Keep one in the dark (or in a black box)for 72 hours and the other in sunlight. Perform iodine test with the leaves of both the plants. Record the results. Now leave the pot which was earlier kept in the dark, in the sunlight for 3 – 4 days and perform the iodine test again on its leaves. Record the observations in a notebook. The leaves other than green also have chlorophyll. The large amount of red, brown and other pigments mask the green color.

Photosynthesis takes place in these leaves also. You often see slimy, green patches in ponds or stagnant water bodies. These are generally formed by the growth of organisms called algae. Algae are green in color because they contain chlorophyll which gives them the green color. Algae can also preparetheir own food by photosynthesis.
Que. 1) For how many hours does the potted plant needs to be kept in dark.
(a) 42 hours
(b) 65 hours
(c) 2 hours
(d) 72 hours
Que. 2) Which colour of leave have the maximum chlorophyll?
Que. 3) ……………………………………………………………….. are the slimy green patches in the pond.
(b) Bacteria
Que. 4) Which organism other than plant can form its own food and through which process?
Que. 5) Give a reason. The formation of green patches form in a pond?
Que. 1) (d) 72 hours
Que. 2) (d) Green
Que. 3) (c) Algae
Que. 4) Answer: Algae is the other organism which can form its own food through a process called photosynthesis
Que. 5) Answer: green patches are formed in the pond due to the uncontrolled growth of algae and algae are green in colour.
Chapter no. 6 study case is not opening our humble request to see it and solve the problem
Chapter no.3 case is not opening .My humble request to see and solve it.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
We have a strong team of experienced Teachers who are here to solve all your exam preparation doubts
Tripura board class 6 bengali solutions chapter 1 লব-কুশের রামায়ণ গান, sikkim scert class 5 evs chapter 8 smart buyers solution, sikkim scert class 5 evs chapter 7 dhaanbari solution, 2025 solved icse specimen paper class 10 english paper 1.
Sign in to your account
Username or Email Address
Remember Me
- Nutrition in Plants Class 7 Case Study Questions Science Chapter 1
Last Updated on April 5, 2024 by XAM CONTENT
Hello students, we are providing case study questions for class 7 science. Case study questions are the new question format that is introduced in CBSE board. The resources for case study questions are very less. So, to help students we have created chapterwise case study questions for class 7 science. In this article, you will find case study questions for cbse class 7 science chapter 1 Nutrition in Plants.
Table of Contents
Case Study Questions on Nutrition in Plants
Question 1:
Read the given passage below and answer the question:
Carnivorous plant is especially adapted for capturing and digesting insects and other animals by means of ingenious pitfalls and traps. There are more than 600 known species of carnivorous plants. The apparent trapping mechanism, which is always a modified leaf is a distinctive feature to these plants. The pitcher plant is an example of a carnivorous plant. The leaf of the Pitcher plant is modified into pitcher like structure to trap the insects. The apex of the leaf acts like a lid which can open and close the mouth of the pitcher.
Q.1. Insect eating carnivorous plants are also called ________plant. (a) autotrophic (b) saprophytic (c) insectivorous (d) symbiotic
Difficulty Level: Easy
Ans. Option (c) is correct. Explanation: Carnivorous plants eat insects for their food requirements, so they are called insectivorous plants.
Q.2. One of the most important nutrients a pitcher plant need from insects is: (a) Carbon dioxide (b) Nitrogen (c) Water (d) Oxygen
Ans. Option (b) is correct. Explanation: Pitcher plants grow in soil that is deficient in nitrogen content so they feed on insects to obtain the nitrogen.
Q.3. Consider the following statements about the Pitcher plant: (A) It is a parasite. (B) The leaves are green in colour. (C) The hair present inside the lid is directed downward. (D) Digestive juice is secreted in the apex of the leaf. The correct statements are: (a) (A) and (B) (b) (B) and (C) (c) (C) and (D) (d) (A), (B) and (D)
Difficulty Level: Difficult
Ans. Option (b) is correct. Explanation: Pitcher plant is not a parasite. The plants are grouped under carnivorous plants as they feed upon insects. The digestive juices secreted in the pitcher, the modified part of leaf.
Q.4. What type of mode of nutrition is found in pitcher plants?
Ans. The pitcher plants have chlorophyll so they perform photosynthesis to produce their own food however, they grow in soil that lacks nitrogen content so, they feed on insects to obtain the nitrogen needed for their growth. Hence, they have both autotrophic as well as a partial heterotrophic mode of nutrition.
Q.5. How does a pitcher plant catch insects? (Medium)
Difficulty Level: Medium
Ans. When the insects land in the pitcher its lid closes and the insects are trapped and entangled into the hair. Digestive juices are secreted in the pitcher so the insects get digested and nutrients get absorbed.
Heat Class 7 Case Study Questions Science Chapter 3
Nutrition in animals class 7 case study questions science chapter 2, topics from which case study questions may be asked.
- Define nutrients.
- Discuss the mode of nutrition in plants.
- Describe the process of photosynthesis.
- List the things required for the process of photosynthesis.
- Discuss what are saprotrophs.
- Describe the symbiosis relationship.
- Discuss the nutrients replenishment in soil.
For further practice on case study questions related to Class 7 Science Chapter 1 Nutrition in Plants, we recommend exploring the link given below.
Frequently Asked Questions (FAQs) on Nutrition in Plants Case Study Questions
Q1: what are case study questions for cbse examinations.
A1: Case study questions in CBSE examinations typically involve scenarios or real-life examples, requiring students to apply their understanding of concepts to solve problems or analyze situations.
Q2: Why are case study questions important for understanding class 7 science chapters?
A2: Case study questions provide a practical context for students to apply theoretical knowledge to real-world situations, fostering deeper understanding and critical thinking skills.
Q3: How should students approach answering case study questions for CBSE?
A3: Students should carefully read the case study, identify the key issues or problems presented, analyze the information provided, apply relevant concepts and principles of plant nutrition, and formulate well-supported solutions or responses.
Q4: Are there any resources available online for students to practice case study questions on class 7 science chapters for CBSE exams?
A4: Yes, several educational websites offer case study questions for CBSE students preparing for science examinations. We also offer a collection of case study questions for all classes and subject on our website. Visit our website to access these questions and enhance your learning experience.
Q5: How can students effectively prepare for case study questions on nutrition in plants for CBSE exams?
A5: Effective preparation strategies include regular revision of concepts, solving practice questions, analyzing case studies from previous exams, seeking clarification on doubts, and consulting with teachers or peers for guidance and support.
Q6: How can teachers incorporate case study questions on nutrition in plants class 7 science into classroom teaching?
A6: Teachers can integrate case studies into lesson plans, group discussions, or interactive activities to engage students in active learning, promote problem-solving skills, and facilitate a deeper understanding of nutrition in plants.

Related Posts
Introduction to Plant Nutrition
- First Online: 13 July 2021
Cite this chapter

- Renato de Mello Prado 2
1161 Accesses
3 Citations
The introduction to plant nutrition addresses basic and general topics on the importance of this area to meet nutritional requirements and promote crop growth, development, and yield. We will address important topics, such as (1) concepts of plant nutrition and its relationship with related disciplines; (2) the concept of nutrient and criteria of essentiality; (3) relative composition of nutrients in plants; (4) nutrient accumulation by crops and crop formation; (5) other chemical elements of interest in plant nutrition, such as potentially toxic and beneficial elements, with emphasis on silicon; and (6) hydroponic cultivation, preparation, and use of nutritional solutions.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Alonso TAS, Barreto RF, Prado RM, et al. Silicon spraying alleviates calcium deficiency in tomato plants, but ca-EDTA is toxic. J Plant Nutr Soil Sci. 2020;183:659–64. https://doi.org/10.1002/jpln.202000055 .
Article CAS Google Scholar
Alvarez RCF, Prado RM, Felisberto G, et al. Effects of soluble silicate and nanosilica applied to oxisol on rice nutrition. Pedosphere. 2018;28:597–606. https://doi.org/10.1016/S1002-0160(18)60035-9 .
Article Google Scholar
Alves BJR, Santos JCF, Virgem Filho AC, et al. Avaliação da disponibilidade de macro e micronutrientes para arroz de sequeiro cultivado em um solo calcário da região de Irece, Bahia. Revista Universidade Rural. 2002;22:15–24.
Google Scholar
Alves AU, Prado RM, Gondim ARO, et al. Effect of macronutrient omission on beet development and nutritional status. Hortic Bras. 2008;26:282–5.
Alves RC, Nicolau MCM, Checchio MV, et al. Salt stress alleviation by seed priming with silicon in lettuce seedlings: an approach based on enhancing antioxidant responses. Bragantia. 2020;79:19–29. https://doi.org/10.1590/1678-4499.20190360 .
Amaral DW, Deficiências de macronutrientes e de boro em seringueira (Hevea brasiliensis L.). Dissertação, Escola Superior de Agricultura Luiz de Queiroz. 1983.
Avilán LR. Efectos de la deficiencia de macronutrientes sobre el crecimiento y la composicion quimica de la parcha granadina (Passiflora quadrangularis L.) cultivada en soluciones nutritivas. Agronomía Tropical. 1974;24:133–40.
Avilán LR. Efecto de la omisión de los macronutrientes en el desarollo y composición química de la guanábana (Annona muricata L.) cultivada en soluciones nutritivas. Agronomia Tropical. 1975;25:73–9.
Backes FAAL, Santos OSS, Pila FG, et al. Nutrients replacement in nutrient solution for lettuce hydroponic cultivation. Ciênc Rural. 2004;34:1407–14. https://doi.org/10.1590/S0103-84782004000500013 .
Barreto RF, Prado RM, Leal AJF, et al. Mitigation of ammonium toxicity by silicon in tomato depends on the ammonicum concentration. Acta Agric Scand B Soil Plant Sci. 2016;66:483–8. https://doi.org/10.1080/09064710.2016.1178324 .
Barreto RF, Prad RM, Schiavon Júnior PA, et al. Silicon alleviates ammonium toxicity in cauliflower and in broccoli. Sci Hortic. 2017;225:743–50. https://doi.org/10.1016/j.scienta.2017.08.014 .
Barreto RF, Prado RM, Habermann E, et al. Warming change nutritional status and improve Stylosanthes capitata Vogel growth only under well-watered conditions. J Soil Sci Plant Nutr. 2020;20:00255. https://doi.org/10.1007/s42729-020-00255-5 .
Barroso DG, Figueiredo FAMMA, Pereira RC, et al. Diagnóstico de deficiência de macronutrientes em mudas de teca. Rev Árvore. 2005;29:671–9. https://doi.org/10.1590/S0100-67622005000500002 .
Bataglia OC, Mascarenhas HAA. Absorção de nutrientes pela soja. Campinas: Instituto Agronômico; 1977.
Batista MMF, Viégas IJM, Frazão DAC, et al. Effect of macronutrient omission in growth, symptoms of nutricional deficiency and mineral composition in soursop plants ( Annona muricata ). Rev Bras Frutic. 2003;25:315–8. https://doi.org/10.1590/S0100-29452003000200033 .
Borges BMMN, Flores RA, Almeida HJ, et al. Macronutrient omission and the development and nutritional status of basil in nutritive solution. J Plant Nutr. 2016;39:1627–33. https://doi.org/10.1080/01904167.2016.1187742 .
Braccini MCL, Martinez HEP, Braccini AL, et al. Rhizosphere pH evaluation of coffee genotypes in response to soil aluminum toxicity. Bragantia. 2000;59:83–8. https://doi.org/10.1590/S0006-87052000000100013 .
Browne CA. Liebig and the law of the minimum. In: Moulton FR, editor. Liebig and after Liebig: a century of progress in agricultural chemistry. Washington, DC: American Association for the Advancement of Science; 1942. p. 71–82.
Brownell PF, Bielig LM. The role of sodium in the conversion of pyruvate to phosphoenolpyruvate in mesophyll chloroplasts of C4 plants. Aust J Plant Physiol. 1996;23:171–7. https://doi.org/10.1071/PP9960171 .
Brownell PF, Wood JG. Sodium as an essential micronutrient element for Atriplex vesicaria, Heward. Nature. 1957;179:635–6. https://doi.org/10.1038/179635a0 .
Buchelt AC, Teixeira GCM, Oliveira KS, et al. Silicon contribution via nutrient solution in forage plants to mitigate nitrogen, potassium, calcium, magnesium, and sulfur deficiency. J Soil Sci Plant Nutr. 2020;20:1532–48. https://doi.org/10.1007/s42729-020-00245-7 .
Bugbee B. Nutrient management in recirculanting hydroponic culture. In: Abstracts of the 16rd annual conference on hydroponics. Tucson: Hydroponic Society of America; 1995. p. 15.
Calero Hurtado A, Chiconato DA, Prado RM, et al. Silicon attenuates sodium toxicity by improving nutritional efficiency in sorghum and sunflower plants. Plant Physiol Biochem. 2019;142:224–33. https://doi.org/10.1016/j.plaphy.2019.07.010 .
Article CAS PubMed Google Scholar
Calero Hurtado A, Chiconato DA, Prado RM, et al. Silicon application induces changes C:N:P stoichiometry and enhances stoichiometric homeostasis of sorghum and sunflower plants under salt stress. Saudi J Biol Sci. 2020;27:3711–9. https://doi.org/10.1016/j.sjbs.2020.08.017 .
Article CAS PubMed PubMed Central Google Scholar
Carmello QAC, Rossi F. Hidroponia: solução nutritiva – Manual. Viçosa: Centro de Produções Técnicas; 1997.
Carvalho JM, Barreto RF, Prado RM, et al. Elevated CO 2 and warming change the nutrient status and use efficiency of Panicum maximum Jacq. PlosOne. 2020a;15:e0223937. https://doi.org/10.1371/journal.pone.0223937 .
Carvalho JM, Barreto RF, Prado RM, et al. Elevated [CO 2 ] and warming increase the macronutrient use efficiency and biomass of Stylosanthes capitata Vogel under field conditions. J Agron Crop Sci. 2020b;206:597–606.
Castellane PD, Araujo JAC. Cultivo sem solo - Hidroponia. Funep: Jaboticabal; 1995.
Cavalcante VS, Prado RM, Vasconcelos RL, et al. Growth and nutritional efficiency of watermelon plants grown under macronutrient deficiencies. HortScience. 2019;54:738–42. https://doi.org/10.21273/HORTSCI13807-18 .
Cobra Netto A, Acoorsi WR, Malavolta E. Studies on the mineral nutrition of the bean plant ( Phaseolus vulgaris L., var). An Esc Super Agric Luiz de Queiroz. 1971;28(257):274. https://doi.org/10.1590/S0071-12761971000100018 .
Coelho AM, França GE, Pitta GVE, et al. Cultivo do milho: diagnose foliar do estado nutricional da planta. Sete Lagoas: Embrapa; 2002.
Cometti NN, Furlani PR, Ruiz HA, et al. Soluções nutritivas: formulação e aplicações. In: Fernandes MS, editor. Nutrição mineral de plantas. Viçosa: Sociedade Brasileira de Ciência do Solo; 2006. p. 89–114.
Dantas JP, Bergamin Filho H, Malavolta E. Studies on the mineral nutrition of Vigna Sinensis. II. Effects of deficiencies of macronutrients on growth, yield and leaf composition. An Esc Super Agric Luiz de Queiroz. 1979;36:247–57. https://doi.org/10.1590/S0071-12761979000100014 .
David CHO, Paiva Neto VB, Campos CNS, et al. Nutritional disorders of macronutrients in Bletia catenulata . HortScience. 2019;54:1836–9. https://doi.org/10.21273/HORTSCI14284-19 .
Deus ACF, Prado RM, ALVAREZ RCF, et al. Role of silicon and salicylic acid in the mitigation of nitrogen deficiency stress in rice plants. SILICON. 2019;11:1–9. https://doi.org/10.1007/s12633-019-00195-5 .
Dias LE, Faria SM, Franco AA. Crescimento de mudas de Acacia mangium Willd em resposta à omissão de macronutrientes. Rev Árvore. 1994;18:123–31.
Djanaguiraman M, Durga Devi D, Shankler AK, et al. Selenium – na antioxidative protectant in soybean during senescence. Plant Soil. 2005;272:77–86. https://doi.org/10.1007/s11104-004-4039-1 .
Epstein E. Mineral nutrition of plants: principles and perspectives. New York: Wiley; 1972.
Epstein E. Nutrição mineral das plantas e perspectivas. Portuguese edition: Malavolta E. São Paulo: Universidade de São Paulo; 1975. p. 1975.
Epstein E. Photosynthesis, inorganic plant nutrition, solutions, and problems. Photosynth Res. 1995;46:37–9. https://doi.org/10.1007/BF00020413 .
Epstein E. Silicon in plant nutrition. In: abstracts of the 2rd silicon in agriculture conference. Tsuruoka: Japanese Society of Soil Science and Plant Nutrition; 2002. p. 1.
Epstein E, Bloom A. Nutrição mineral de plantas: princípios e perspectivas. In: Maria Edna Tenório Nunes, Português editors. Londrina: Planta; 2006.
Erying C, Ling Q, Yanbing Y, et al. Variability of nitrogen use efficiency by foxtail millet cultivars at the seedling stage. Pesq Agropec Bras. 2020;55:1–9. https://doi.org/10.1590/s1678-3921.pab2020.v55.00832 .
Fageria NK. Potassium use efficiency of upland rice genotypes. Pesq Agropec Bras. 2000;35:2115–20. https://doi.org/10.1590/S0100-204X2000001000025 .
Fageria NK, Baligar VC, Jones CA. Rice in. In: Fageria NK, Baligar VC, Jones CA, editors. Growth and mineral nutrition of field crops. M. New York: Dekker; 1997. p. 283–343.
Fasabi JAV, Carências de macro e micronutrientes em plantas de malva (Urena lobata), variedade BR-01. Dissertação, Faculdade de Ciências Agrárias do Pará. 1996.
Felisberto G, Prado RM, Oliveira RLL, et al. Are nanosilica, potassium silicate and new soluble sources of silicon effective for silicon foliar application to soybean and rice plants? SILICON. 2020;12:00668. https://doi.org/10.1007/s12633-020-00668-y .
Fernandes LA, Alves DS, Ramos SJ, et al. Mineral nutrition of Cyclanthera pedata . Pesq Agropec Bras. 2005;40:719–22. https://doi.org/10.1590/S0100-204X2005000700014 .
Ferreira RLC, Prado RM, Souza Júnior JP, et al. Oxidative stress, nutritional disorders, and gas exchange in lettuce plants subjected to two selenium sources. J Soil Sci Plant Nutr. 2020;20 https://doi.org/10.1007/s42729-020-00206-0 .
Flores RA, Borges BMMN, Almeida HJ, et al. Growth and nutritional disorders of eggplant cultivated in nutrients solutions with suppressed macronutrients. J Plant Nutr. 2014;38:1097–9. https://doi.org/10.1080/01904167.2014.963119 .
Flores RA, Arruda EM, Damin V, et al. Physiological quality and dry mass production of Sorghum bicolor following silicon (Si) foliar application. Aust J Crop Sci. 2018;12:631–8. https://doi.org/10.21475/ajcs.18.12.04.pne967 .
Föhse D, Claassen N, Jungk A. Phosphorus efficiency of plants. I. External and internal P requirement and P uptake efficiency of different plant species. Plant Soil. 1988;110:101–9. https://doi.org/10.1007/BF00010407 .
Franco CF, Prado RM. Nutrition solutions in the culture of guava: effect in the development and nutricional state. Acta Scient Agron. 2006;28:199–205 https://doi.org/10.4025/actasciagron.v28i2.1042 .
Frazão JJ, Prado RM, de Souza Júnior JP, et al. Silicon changes C:N:P stoichiometry of sugarcane and its consequences for photosynthesis, biomass partitioning and plant growth. Sci Rep. 2020;10:12492. https://doi.org/10.1038/s41598-020-69310-6 .
Freitas FA, Koop MM, Souza RO, et al. Nutrient absorption in aluminum stressed rice plants under hydroponic culture. Ciênc Rural. 2006;36:72–9. https://doi.org/10.1590/S0103-84782006000100011 .
Furlani PR, Clark RB. Screening sorghum for aluminium tolerance in nutrient solutions. Agron J. 1981;73:587–94.
Furlani PR. Cultivo de alface pela técnica de hidroponia – NFT. Campinas: Instituto Agronômico; 1995. 18p.
Gabelman WH, Gerloff GC. The search for and interpretation of genetic controls that enhance plant growth under deficiency levels of a macronutrient. Plant Soil. 1983;72:335–50.
Gonçalves FC, Neves OSC, Carvalho JG. Nutritional deficiency in “Umbuzeiro” seedlings caused by the omission of macronutrients. Pesq Agropec Bras. 2006;41:1053–7. https://doi.org/10.1590/S0100-204X2006000600023 .
Goussain MM, Moraes JC, Carvalho JG, et al. Effect of silicon application on corn plants upon the biological development of the fall armyworm Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Neotrop Entomol. 2002;31:305–10. https://doi.org/10.1590/S1519-566X2002000200019 .
Graham RD. Breeding for nutritional characteristics in cereals. In: Tinker PB, Lauchli A, editors. Advances in plant nutrition. New York: Praeger; 1984. p. 57–102.
Grant CA, Flaten DN, Tomasiewics DJ, et al. A importância do fósforo no desenvolvimento inicial da planta. Informações Agronômicas. 2001;95:1–5.
Guedes VHF, Prado RM, Frazão RJJ, et al. Foliar-applied silicon in sorghum ( Sorghum bicolor L.) alleviate zinc deficiency. SILICON. 2020;13:0825–3. https://doi.org/10.1007/s12633-020-00825-3 .
Haag HP, Sarruge JR, Camargo PN, et al. Studies on the mineral diet of coffee. XXVI. Effects of multiple deficiencies in mineral appearance, growth and composition. An Esc Super Agric Luiz de Queiroz. 1969;26:119–39. https://doi.org/10.1590/S0071-12761969000100011 .
Habermann E, Oliveira EAD, Contin DR, et al. Warming and water deficit impact leaf photosynthesis and decrease forage quality and digestibility of a C4 tropical grass. Physiol Plantarum. 2019;165:383–402. https://doi.org/10.1111/ppl.12891 .
Hoagland DR, Arnon DI. The water culture method for growing plants without soils. Berkeley: California Agricultural Experimental Station; 1950. 347p
Jensen MH, Collins WL. Hydroponic vegetable production. In: Janick J, editor. Horticultural reviews. New York: Willey Press; 1985. p. 483–557.
Jo J, Jang YS, Kim KY, et al. Isolation of ALU1-P gene encoding a protein with aluminum tolerance activity from arthrobacter viscosus. Biochem Bioph Res Co. 1997;239:835–9. https://doi.org/10.1006/bbrc.1997.7567 .
Jones JB Jr. Plant nutrition manual. Boca Raton: CRC Press; 1998. 147p
Kathpalia R, Bhatla SC. Plant mineral nutrition. In: Bhatla SC, Lal MA, editors. Plant physiology, development and metabolism. Singapore: Springer; 2018. p. 37–81. https://doi.org/10.1007/978-981-13-2023-1_2 .
Chapter Google Scholar
Kolesnikov M, Gins V. Forms of silicon in medicinal plants. Appl Biochem Microbiol. 2001;37:524–7.
Körndorfer GH, Snyder GH, Ulloa M, et al. Calibration of soil and plant silicon analysis for rice production. J Plant Nutr. 2001;24:1071–84. https://doi.org/10.1081/PLN-100103804 .
Körndorfer GH, Pereira HS, Camargo MS. Papel do silício na produção da cana-de-açúcar. Stab. 2002;21:6–9.
Lata-Tenesaca LF, Prado RM, Piccolo CM et al. Silicon modifies C:N:P stoichiometry, and increases nutrient use efficiency and productivity of quinoa. Sci Rep 2021;11:9893. https://doi.org/10.1038/s41598-021-89416-9 .
Lauchli A. Soil science in the next twenty five years: does a biotechnology play a role? Soil Sci Soc Am J. 1987;51:1405–9. https://doi.org/10.2136/sssaj1987.03615995005100060003x .
Lavres Junior J, Boaretto RM, Silva MLS, et al. Deficiencies of macronutrients on nutritional status of castor bean cultivar Iris. Pesq Agropec Bras. 2005;40:145–51. https://doi.org/10.1590/S0100-204X2005000200007 .
Lehr JJ. Sodium as a plant nutrition. J Sci Food Agric. 1953;4:460–1. https://doi.org/10.1002/jsfa.2740041002 .
Li B, Mckeand SE, Allen HL. Genetic variation in nitrogen use efficiency of loblolly pine seedlings. For Sci. 1991;37:613–26.
Lima Filho OF, Malavolta E. Symptoms of nutritional disorders in stevia ( Stevia rebaudiana (bert.) bertoni). Sci Agric. 1997;54:53–61. https://doi.org/10.1590/S0103-90161997000100008 .
Loué A. Oligoelements en agriculture. Paris: SCPA Nathan; 1993. 577p
Ma JF, Takahashi E. Soil, fertiliser, and plant silicon research in Japan. Amsterdam: Elsevier; 2002.
Ma JF, Yamaji N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015;20:435–42. https://doi.org/10.1016/j.tplants.2015.04.007 .
Malavolta E. Elementos de nutrição de plantas. São Paulo: Agronômica Ceres; 1980. 251p
Malavolta E. Manual de nutrição mineral de plantas. São Paulo: Agronômica Ceres; 2006.
Malavolta E, Vitti GC, Oliveira SA. Avaliação do estado nutricional das plantas: princípios e aplicações. Piracicaba: Associação Brasileira de Potassa e do Fósforo; 1997. 319p
Mantovani C, Prado RM, Pivetta KFL. Silicon foliar application on nutrition and growth of Phalaenopsis and Dendrobium orchids. Sci Hortic. 2018;18:83–92. https://doi.org/10.1016/j.scienta.2018.06.088 .
Mantovani C, Pivetta KFL, Prado RM, et al. Silicon toxicity induced by different concentrations and sources added to in vitro culture of epiphytic orchids. Sci Hortic. 2020;265:109272. https://doi.org/10.1016/j.scienta.2020.109272 .
Marin A, Santos DMM, Banzatto DA, et al. Seed germination of pigonpea ( Cajanus cajan (L.) Millsp.) under water stress and aluminum sublethal doses. Bragantia. 2004;63:13–24. https://doi.org/10.1590/S0006-87052004000100002 .
Marschner H. Mineral nutrition of higher plants. London: Academic Press; 1986.
Marschner H. Mineral nutrition of higher plants. London: Academic; 1995.
Martinez HEP, Silva Filho JB. Introdução ao cultivo hidropônico. Viçosa: Universidade Federal; 2004.
Mccray JM, Ezenwa IV, Rice RW et al. Sugarcane plant nutrient diagnosis. 2006. http://edis.ifas.ufl.edu/sc075 . Accessed 21 Sept 2006
Mendonça RJ, Cambraia J, Oliva MA, et al. Rice cultivars ability to change nutrient solution pH in the presence of aluminum. Pesq Agropec Bras. 2005;40:447–52. https://doi.org/10.1590/S0100-204X2005000500004 .
Mengel K, Kirkby EA. Principles of plant nutrition. Worblaufen-Bern: International Potash Institute; 1987.
Menosso OG, Costa JA, Anghinoni I, et al. Root growth and production of organic acids by soybean cultivars with different tolerance to aluminum. Pesq Agropec Bras. 2001;36:1339–45. https://doi.org/10.1590/S0100-204X2001001100003 .
Miyake Y, Takahashi E. Silicon deficiency of tomato plant. Soil Sci Plant Nutr. 1978;24:175–89. https://doi.org/10.1080/00380768.1978.10433094 .
Miyake Y, Takahashi E. Effect of silicon on the growth of soybean plants in solution culture. Soil Sci Plant Nutr. 1985;31:625–36. https://doi.org/10.1080/00380768.1985.10557470 .
Monteiro FA, Ramos AKB, Carvalho DD, et al. Growth of Brachiaria brizantha Stapf. cv. Marandu in nutrient solution with macronutrient omissions. Sci Agric. 1995;52:135–41. https://doi.org/10.1590/S0103-90161995000100022 .
Morgan PW (1990) Effects of abiotic stresses on plant hormone systems. In: Alscher RC, Cumming JR Stress responses in plants: adaptation and acclimation mechanisms. Willey-Liss, New York, pp. 113–146.
Müntz K. Deposition of storange proteins. Plant Mol Biol. 1998;38:77–99.
Article PubMed Google Scholar
Oliveira KR, Souza JP Jr, Bennett SJ. Exogenous silicon and salicylic acid applications improve tolerance to boron toxicity in field pea cultivars by intensifying antioxidant defence systems. Ecot Environ Safety. 2020a;201:110778. https://doi.org/10.1016/j.ecoenv.2020.110778 .
Oliveira RLL, Prado RM, Felisberto G, et al. Silicon mitigates manganese deficiency stress by regulating the physiology and activity of antioxidant enzymes in sorghum plants. J Soil Sci Plant Nutr. 2019;19:524–534. https://doi.org/10.1007/s42729-019-00051-w .
Oliveira KS, Prado RM, Guedes VHF. Leaf spraying of manganese with silicon addition is agronomically viable for corn and sorghum plants. J Soil Sci Plant Nutr. 2020b;20:00173–6. https://doi.org/10.1007/s42729-020-00173-6 .
Osório CRWS, Teixeira GCM, Barreto RF, et al. Macronutrient deficiency in snap bean considering physiological, nutritional, and growth aspects. PlosOne. 2020;15:e0234512. https://doi.org/10.1371/journal.pone.0234512 .
Peixoto MM, Flores RA, Couto CA, et al. Silicon application increases biomass yield in sunflower by improving the photosynthesizing leaf area. SILICON. 2020;13:0818–2. https://doi.org/10.1007/s12633-020-00818-2 .
Perry C, Tucker K. Biosilicification: the role of the organic matrix in the structure control. J Biol Inorg Chem. 2000;5:537–50.
Prado RM. Effect of limestone application on development, nutritional status and fruit production of guava and star fruit during three years in orchards under implantation. Jaboticabal, São Paulo State University - Doctoral thesis, 2003.
Prado RM, Leal RM. Nutritional disorders due to deficiency in sunflower var. Catissol 01. Pesq Agropec Trop. 2006;36:173–9.
Prado RM, Natale W. Effect of application of calcium silicate on growth, nutritional status and dry matter production of passion fruit seedlings. Rev Bras Eng Agríc Ambient. 2005;9:185–190. https://doi.org/10.1590/S1415-43662005000200006 .
Prado RM, Correa MCM, Cintra ACO et al. Micronutrients released from one basic slag applied a ultisol cultivated with guava plants ( Psidium guajava L.). Rev Bras Frutic. 2002a;24:536–542. https://doi.org/10.1590/S0100-29452002000200051 .
Prado RM, Coutinho ELM, Roque CG et al. Evaluation of slag and calcareous rocks as corrective of the acidity of the ground in the culture of lettuce Pesq Agrop Bras. 2002b;37:539–546. https://doi.org/10.1590/S0100-204X2002000400016 .
Prado RM, Cruz FJR, Ferreira RLC. Selenium biofortification and the problem of its safety. In: Shiomi N, editor. Superfood and functional food: an overview of their processing and utilization. Rijeka: InTech; 2017. p. 221–38.
Prado RM, Felisberto G, Barreto RF. Nova abordagem do silício na mitigação de estresse por deficiência de nutrientes. In: Prado RM, Campos CNS, editors. Nutrição e adubação de grandes culturas. Jaboticabal: FCAV; 2018. p. 17–26.
Raboy V. Seeds for a better future: ‘low phytate’ grains help to overcome malnutrition and reduce pollution. Trends Plant Sci. 2001;6:458–62. https://doi.org/10.1016/S1360-1385(01)02104-5 .
Rains DW. Mineral metabolism. In: Bonner J, Varner JE, editors. Plant biochemistry. New York: Academic; 1976. p. 561–98.
Rocha Filho JVC, Haag HP, Oliveira GD. The effects of mineral nutrient deficiencies on Eucalyptus urophylla growth in nutrient solutions. An Esc Super Agric Luiz de Queiroz. 1978;35:19–34. https://doi.org/10.1590/S0071-12761978000100002 .
Rocha JR, Prado RM, Teixeira GCM et al. Si fertigation attenuates water stress in forages by modifying carbon stoichiometry, favouring physiological aspects. J Agron Crop Sci. 2021;207:12479. https://doi.org/10.1111/jac.12479 .
Rosolem CA, Leite VM. Coffee leaf and stem anatomy under boron deficiency. Rev Bras Ciênc Solo. 2007;31:477–83. https://doi.org/10.1590/S0100-06832007000300007 .
Rozane DE, Natale W, Prado RM, et al. Size of samples for nutritional status assessment of mango trees. Rev Bras Frutic. 2007;29:371–6. https://doi.org/10.1590/S0100-29452007000200035 .
Rublo G, Liao H, Yan X, et al. Topsoil foraging and its role in plant competitiveness for phosphorus in common bean. Crop Sci. 2003;43:598–607. https://doi.org/10.2135/cropsci2003.5980 .
Salvador JO, Muraoka T, Rossetto R, et al. Symptoms of mineral deficiencies in cupuaçu plants ( Theobroma gramdiflorum ) grown in nutrient solution. Sci Agric. 1994;51:407–14. https://doi.org/10.1590/S0103-90161994000300005 .
Salvador JO, Moreira A, Muraoka T. Visual symptoms of micronutrient deficiency and of mineral content in guava young plant leaves. Pesq Agropec Bras. 1999;34:1655–62. https://doi.org/10.1590/S0100-204X1999000900016 .
Samonte SOPB, Wilson LT, Medley JC, et al. Nitrogen utilization efficiency: relationships with grain yield, grain protein, and yield-related traits in rice. Agron J. 2006;98:168–76. https://doi.org/10.2134/agronj2005.0180 .
Santi A, Camargos SL, Scaramuzza WLMP, et al. The macronutrients deficiency in sorghum. Ciênc Agrotec. 2006;30:228–33. https://doi.org/10.1590/S1413-70542006000200006 .
Santos OS. Hidroponia da alface. Santa Maria: Imprensa Universitária; 2000.
Santos LCN, Teixeira GCM, Prado RM, et al. Response of pre-sprouted sugarcane seedlings to foliar spraying of potassium silicate, sodium and potassium silicate, nanosilica and monosilicic acid. Sugar Tech. 2020;22:00833. https://doi.org/10.1007/s12355-020-00833-y .
Sarcinelli TS, Ribeiro ES Jr, Dias LE, et al. Symptoms of nutritional deficiency in seedlings of Acacia holosericea submitted to absence of macronutrients. Rev Árvore. 2004;28:173–81. https://doi.org/10.1590/S0100-67622004000200003 .
Siddiqi MY, Glass ADM. Utilisation index: a modified approach to the estimation and comparison of nutrient utilisation efficiency in plants. J Plant Nutr. 1981;4:289–302. https://doi.org/10.1080/01904168109362919 .
Silva JRS, Falcão NPS. Characterization of symptoms of nutritional deficiencies in peach palm cultivated in nutrient solution. Acta Amazon. 2002;32:529–39. https://doi.org/10.1590/1809-43922002324539 .
Silva Junior GB, Prado RM, Campos CNS, et al. Silicon mitigates ammonium toxicity in yellow passion fruit seedlings. Chil J Agric Res. 2019;79:425–34. https://doi.org/10.4067/S0718-58392019000300425 .
Silva ES, Prado RM, Soares AAVL, et al. Response of corn seedlings ( Zea mays L) to different concentrations of nitrogen in absence and presence of silicon. SILICON. 2020;12:00480–8. https://doi.org/10.1007/s12633-020-00480-8 .
Silva JLF, Prado RM. Elucidating the action mechanisms of silicon in the mitigation of phosphorus deficiency and enhancement of its response in sorghum plants. J Plant Nutr. 2021;45:8155. https://doi.org/10.1080/01904167.2021.1918155 .
Silva DL, Prado RM, Tenesaca LFL et al. Silicon attenuates calcium deficiency by increasing ascorbic acid content, growth and quality of cabbage leaves. Sci Rep. 2021;11:1770. https://doi.org/10.1038/s41598-020-80934-6 .
Souza Júnior JP, Prado RM, Sarah MMS, et al. Silicon mitigates boron deficiency and toxicity in cotton cultivated in nutrient solution. J Plant Nutr Soil Sci. 2019;182:805–14. https://doi.org/10.1002/jpln.201800398 .
Souza Junior JP, Frazão JJ, Morais TCB, et al. Foliar spraying of silicon associated with salicylic acid increases silicon absorption and peanut growth. SILICON. 2020;12:00517. https://doi.org/10.1007/s12633-020-00517-y .
Souza JZ, Prado RM, Silva SLO, et al. Silicon leaf fertilization promotes biofortification and increases dry matter, ascorbate content, and decreases post-harvest leaf water loss of chard and kale. Comm Soil Sci Plant Anal. 2018;50:164–72. https://doi.org/10.1080/00103624.2018.1556288 .
Stein AJ. Global impacts of human mineral malnutrition. Plant Soil. 2010;335:133–54. https://doi.org/10.1007/s11104-009-0228-2 .
Subbarao GV, Ito O, Berry WL, et al. Sodium – a functional plant nutrient. Crit Rev Plant Sci. 2003;22:391–416. https://doi.org/10.1080/07352680390243495 .
Svecnjak Z, Rengel Z. Canola cultivars differ in nitrogen utilization efficiency at vegetative stage. Field Crops Res. 2006;97:221–6. https://doi.org/10.1016/j.fcr.2005.10.001 .
Swiader JM, Chyan Y, Freiji FG. Genotypic differences in nitrate uptake and utilization efficiency in pumpkin hybrids. J Plant Nutr. 1994;17:1687–99. https://doi.org/10.1080/01904169409364840 .
Teixeira GCM, Prado RM, Rocha AMS, et al. Silicon in pre-sprouted sugarcane seedlings mitigates the effects of water deficit after transplanting. J Soil Sci Plant Nutr. 2020a;20:00170–4. https://doi.org/10.1007/s42729-019-00170-4 .
Teixeira GCM, Prado RM, Oliveira KS, et al. Silicon increases leaf chlorophyll content and iron nutritional efficiency and reduces iron deficiency in sorghum plants. J Soil Sci Plant Nutr. 2020b;20:1311–20. https://doi.org/10.1007/s42729-020-00214-0 .
Veloso CAC, Muraoka T. Diagnosis of macronutrient deficiency symptoms in black pepper ( Piper nigrum L.). Sci Agric. 1993;50:232–6. https://doi.org/10.1590/S0103-90161993000200010 .
Veloso CAC, Muraoka T, Malavolta E, et al. Diagnosis of macronutrient deficiencies in black pepper. Pesq Agropec Bras. 1998;33:1883–8.
Viciedo DO, Prado RM, Martinez CAH, et al. Short-term warming and water stress affect Panicum maximum Jacq. stoichiometric homeostasis and biomass production. Sci Total Environ. 2019a;681:267–74. https://doi.org/10.1016/j.scitotenv.2019.05.108 .
Viciedo DO, Prado RM, Toledo RL, et al. Silicon supplementation alleviates ammonium toxicity in sugar beet ( Beta vulgaris L.). J Soil Sci Plant Nutr. 2019b;19:413–9. https://doi.org/10.1007/s42729-019-00043-w .
Viciedo DO, Prado RM, Toledo RL, et al. Physiological role of silicon in radish seedlings under ammonium toxicity. Food Sci Tech. 2020a;100:10587. https://doi.org/10.1002/jsfa.10587 .
Viciedo DO, Prado RM, Martinez CAH, et al. Water stress and warming impact nutrient use efficiency of Mombasa grass ( Megathyrsus maximus ) in tropical conditions. J Agron Crop Sci. 2020b;206:12452. https://doi.org/10.1111/jac.12452 .
Viciedo DO, Prado RM, Martinez CAH, et al. Changes in soil water availability and air-temperature impact biomass allocation and C:N:P stoichiometry in different organs of Stylosanthes capitata Vogel. J Environ Manag. 2021;278:111540. https://doi.org/10.1016/j.jenvman.2020.111540 .
Viégas IJM, Carvalho JG, Rocha Neto OG, et al. Carência de macronutrientes em plantas de quina. Belém: Embrapa; 1998.
Viegas IJM, Frazão DAC, Thomaz MAA, et al. Nutritional limitations for Euterpe oleracea in yellow Latosol of Para state - Brazil. Rev Bras Frutic. 2004a;26:382–4. https://doi.org/10.1590/S0100-29452004000200052 .
Viegas IJM, Thomaz MAA, Silva JF, et al. Effect of omission of macronutrient and boron on growth, on symptoms of nutritional deficiency and mineral composition in camucamuzeiro plants ( Myrciaria dubia ). Rev Bras Frutic. 2004b;26:315–9. https://doi.org/10.1590/S0100-29452004000200032 .
Vitorello VA, Capaldi FR, Stefanuto VA. Recent advances in aluminum toxicity and resistance in higher plants. Braz J Plant Physiol. 2005;17:129–43. https://doi.org/10.1590/S1677-04202005000100011 .
Warington K. The effect of boric acid and borax on the broad bean and certain other plants. Ann Bot. 1923;37:629–72. https://doi.org/10.1093/oxfordjournals.aob.a089871 .
Wen TN, Li C, Chien CS. Ubiquity of selenium containing t RNA in plants. Plant Sci. 1988;57:185–93. https://doi.org/10.1016/0168-9452(88)90124-0 .
Yamamoto Y, Kobayashi Y, Devi SR et al. Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol. 2002;128:63–72. https://doi.org/10.1104/pp.010417 .
Zakir Hossain AKM, Koyama H, Hara T. Growth and cell wall properties of two wheat cultivars differing in their sensitivity to aluminum stress. J Plant Physiol. 2006;163:39–47. https://doi.org/10.1016/j.jplph.2005.02.008 .
Download references
Author information
Authors and affiliations.
São Paulo State University, Jaboticabal, São Paulo, Brazil
Renato de Mello Prado
You can also search for this author in PubMed Google Scholar
Rights and permissions
Reprints and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
de Mello Prado, R. (2021). Introduction to Plant Nutrition. In: Mineral nutrition of tropical plants. Springer, Cham. https://doi.org/10.1007/978-3-030-71262-4_1
Download citation
DOI : https://doi.org/10.1007/978-3-030-71262-4_1
Published : 13 July 2021
Publisher Name : Springer, Cham
Print ISBN : 978-3-030-71261-7
Online ISBN : 978-3-030-71262-4
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

10.3: Plant Nutrition
- Last updated
- Save as PDF
- Page ID 46192
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
- Discuss the common nutritional needs of plants
Plants obtain food in two different ways. Autotrophic plants can make their own food from inorganic raw materials, such as carbon dioxide and water, through photosynthesis in the presence of sunlight. Green plants are included in this group. Some plants, however, are heterotrophic: they are totally parasitic and lacking in chlorophyll. These plants, referred to as holo-parasitic plants, are unable to synthesize organic carbon and draw all of their nutrients from the host plant.
Plants may also enlist the help of microbial partners in nutrient acquisition. Particular species of bacteria and fungi have evolved along with certain plants to create a mutualistic symbiotic relationship with roots. This improves the nutrition of both the plant and the microbe. The formation of nodules in legume plants and mycorrhization can be considered among the nutritional adaptations of plants. However, these are not the only type of adaptations that we may find; many plants have other adaptations that allow them to thrive under specific conditions.
Nutritional Requirements
Plants are unique organisms that can absorb nutrients and water through their root system, as well as carbon dioxide from the atmosphere. Soil quality and climate are the major determinants of plant distribution and growth. The combination of soil nutrients, water, and carbon dioxide, along with sunlight, allows plants to grow.
The Chemical Composition of Plants
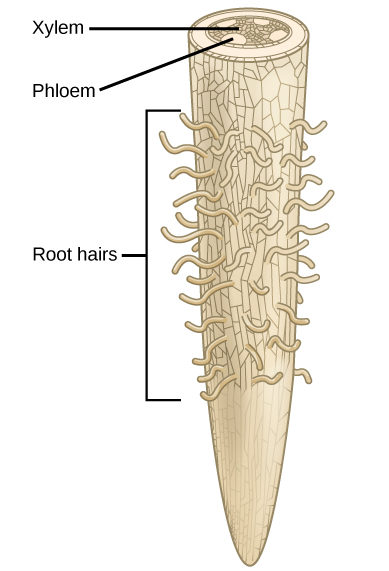
Since plants require nutrients in the form of elements such as carbon and potassium, it is important to understand the chemical composition of plants. The majority of volume in a plant cell is water; it typically comprises 80 to 90 percent of the plant’s total weight. Soil is the water source for land plants, and can be an abundant source of water, even if it appears dry. Plant roots absorb water from the soil through root hairs and transport it up to the leaves through the xylem. As water vapor is lost from the leaves, the process of transpiration and the polarity of water molecules (which enables them to form hydrogen bonds) draws more water from the roots up through the plant to the leaves (Figure 1). Plants need water to support cell structure, for metabolic functions, to carry nutrients, and for photosynthesis.
Plant cells need essential substances, collectively called nutrients, to sustain life. Plant nutrients may be composed of either organic or inorganic compounds. An organic compound is a chemical compound that contains carbon, such as carbon dioxide obtained from the atmosphere. Carbon that was obtained from atmospheric CO 2 composes the majority of the dry mass within most plants. An inorganic compound does not contain carbon and is not part of, or produced by, a living organism. Inorganic substances, which form the majority of the soil solution, are commonly called minerals: those required by plants include nitrogen (N) and potassium (K) for structure and regulation.
Essential Nutrients
Plants require only light, water and about 20 elements to support all their biochemical needs: these 20 elements are called essential nutrients (Table 1). For an element to be regarded as essential , three criteria are required: 1) a plant cannot complete its life cycle without the element; 2) no other element can perform the function of the element; and 3) the element is directly involved in plant nutrition.
Macronutrients and Micronutrients
The essential elements can be divided into two groups: macronutrients and micronutrients. Nutrients that plants require in larger amounts are called macronutrients . About half of the essential elements are considered macronutrients: carbon, hydrogen, oxygen, nitrogen, phosphorus, potassium, calcium, magnesium and sulfur. The first of these macronutrients, carbon (C), is required to form carbohydrates, proteins, nucleic acids, and many other compounds; it is therefore present in all macromolecules. On average, the dry weight (excluding water) of a cell is 50 percent carbon. As shown in Figure 2, carbon is a key part of plant biomolecules.

The next most abundant element in plant cells is nitrogen (N); it is part of proteins and nucleic acids. Nitrogen is also used in the synthesis of some vitamins. Hydrogen and oxygen are macronutrients that are part of many organic compounds, and also form water. Oxygen is necessary for cellular respiration; plants use oxygen to store energy in the form of ATP. Phosphorus (P), another macromolecule, is necessary to synthesize nucleic acids and phospholipids. As part of ATP, phosphorus enables food energy to be converted into chemical energy through oxidative phosphorylation. Likewise, light energy is converted into chemical energy during photophosphorylation in photosynthesis, and into chemical energy to be extracted during respiration. Sulfur is part of certain amino acids, such as cysteine and methionine, and is present in several coenzymes. Sulfur also plays a role in photosynthesis as part of the electron transport chain, where hydrogen gradients play a key role in the conversion of light energy into ATP. Potassium (K) is important because of its role in regulating stomatal opening and closing. As the openings for gas exchange, stomata help maintain a healthy water balance; a potassium ion pump supports this process.
Magnesium (Mg) and calcium (Ca) are also important macronutrients. The role of calcium is twofold: to regulate nutrient transport, and to support many enzyme functions. Magnesium is important to the photosynthetic process. These minerals, along with the micronutrients, which are described below, also contribute to the plant’s ionic balance.
In addition to macronutrients, organisms require various elements in small amounts. These micronutrients , or trace elements, are present in very small quantities. They include boron (B), chlorine (Cl), manganese (Mn), iron (Fe), zinc (Zn), copper (Cu), molybdenum (Mo), nickel (Ni), silicon (Si), and sodium (Na).

Deficiencies in any of these nutrients—particularly the macronutrients—can adversely affect plant growth (Figure 3). Depending on the specific nutrient, a lack can cause stunted growth, slow growth, or chlorosis (yellowing of the leaves). Extreme deficiencies may result in leaves showing signs of cell death.
Hydroponics is a method of growing plants in a water-nutrient solution instead of soil. Since its advent, hydroponics has developed into a growing process that researchers often use. Scientists who are interested in studying plant nutrient deficiencies can use hydroponics to study the effects of different nutrient combinations under strictly controlled conditions. Hydroponics has also developed as a way to grow flowers, vegetables, and other crops in greenhouse environments. You might find hydroponically grown produce at your local grocery store. Today, many lettuces and tomatoes in your market have been hydroponically grown.
Plants can absorb inorganic nutrients and water through their root system, and carbon dioxide from the environment. The combination of organic compounds, along with water, carbon dioxide, and sunlight, produce the energy that allows plants to grow. Inorganic compounds form the majority of the soil solution. Plants access water though the soil. Water is absorbed by the plant root, transports nutrients throughout the plant, and maintains the structure of the plant. Essential elements are indispensable elements for plant growth. They are divided into macronutrients and micronutrients. The macronutrients plants require are carbon, nitrogen, hydrogen, oxygen, phosphorus, potassium, calcium, magnesium, and sulfur. Important micronutrients include iron, manganese, boron, molybdenum, copper, zinc, chlorine, nickel, cobalt, silicon and sodium.
Autotrophic Plants
Nitrogen fixation: root and bacteria interactions.
Nitrogen is an important macronutrient because it is part of nucleic acids and proteins. Atmospheric nitrogen, which is the diatomic molecule N 2 , or dinitrogen, is the largest pool of nitrogen in terrestrial ecosystems. However, plants cannot take advantage of this nitrogen because they do not have the necessary enzymes to convert it into biologically useful forms. However, nitrogen can be “fixed,” which means that it can be converted to ammonia (NH 3 ) through biological, physical, or chemical processes. Biological nitrogen fixation (BNF) is the conversion of atmospheric nitrogen (N 2 ) into ammonia (NH 3 ), exclusively carried out by prokaryotes such as soil bacteria or cyanobacteria. Biological processes contribute 65 percent of the nitrogen used in agriculture.
The most important source of BNF is the symbiotic interaction between soil bacteria and legume plants, including many crops important to humans (Figure 4). The NH 3 resulting from fixation can be transported into plant tissue and incorporated into amino acids, which are then made into plant proteins. Some legume seeds, such as soybeans and peanuts, contain high levels of protein, and serve among the most important agricultural sources of protein in the world.

Practice Question
Farmers often rotate corn (a cereal crop) and soy beans (a legume), planting a field with each crop in alternate seasons. What advantage might this crop rotation confer?
[practice-area rows=”2″][/practice-area] [reveal-answer q=”890921″]Show Answer[/reveal-answer] [hidden-answer a=”890921″]Soybeans are able to fix nitrogen in their roots, which are not harvested at the end of the growing season. The belowground nitrogen can be used in the next season by the corn.[/hidden-answer]
Soil bacteria, collectively called rhizobia , symbiotically interact with legume roots to form specialized structures called nodules , in which nitrogen fixation takes place. This process entails the reduction of atmospheric nitrogen to ammonia, by means of the enzyme nitrogenase . Therefore, using rhizobia is a natural and environmentally friendly way to fertilize plants, as opposed to chemical fertilization that uses a nonrenewable resource, such as natural gas. Through symbiotic nitrogen fixation, the plant benefits from using an endless source of nitrogen from the atmosphere. The process simultaneously contributes to soil fertility because the plant root system leaves behind some of the biologically available nitrogen. As in any symbiosis, both organisms benefit from the interaction: the plant obtains ammonia, and bacteria obtain carbon compounds generated through photosynthesis, as well as a protected niche in which to grow (Figure 5).

Mycorrhizae: The Symbiotic Relationship between Fungi and Roots
A nutrient depletion zone can develop when there is rapid soil solution uptake, low nutrient concentration, low diffusion rate, or low soil moisture. These conditions are very common; therefore, most plants rely on fungi to facilitate the uptake of minerals from the soil. Fungi form symbiotic associations called mycorrhizae with plant roots, in which the fungi actually are integrated into the physical structure of the root. The fungi colonize the living root tissue during active plant growth.

Through mycorrhization, the plant obtains mainly phosphate and other minerals, such as zinc and copper, from the soil. The fungus obtains nutrients, such as sugars, from the plant root (Figure 6). Mycorrhizae help increase the surface area of the plant root system because hyphae, which are narrow, can spread beyond the nutrient depletion zone. Hyphae can grow into small soil pores that allow access to phosphorus that would otherwise be unavailable to the plant. The beneficial effect on the plant is best observed in poor soils. The benefit to fungi is that they can obtain up to 20 percent of the total carbon accessed by plants. Mycorrhizae functions as a physical barrier to pathogens. It also provides an induction of generalized host defense mechanisms, and sometimes involves production of antibiotic compounds by the fungi.
There are two types of mycorrhizae: ectomycorrhizae and endomycorrhizae. Ectomycorrhizae form an extensive dense sheath around the roots, called a mantle. Hyphae from the fungi extend from the mantle into the soil, which increases the surface area for water and mineral absorption. This type of mycorrhizae is found in forest trees, especially conifers, birches, and oaks. Endomycorrhizae, also called arbuscular mycorrhizae, do not form a dense sheath over the root. Instead, the fungal mycelium is embedded within the root tissue. Endomycorrhizae are found in the roots of more than 80 percent of terrestrial plants.
Heterotrophic Plants
Some plants cannot produce their own food and must obtain their nutrition from outside sources—these plants are heterotrophic. This may occur with plants that are parasitic or saprophytic. Some plants are mutualistic symbionts, epiphytes, or insectivorous.
Plant Parasites
A parasitic plant depends on its host for survival. Some parasitic plants have no leaves. An example of this is the dodder (Figure 7a), which has a weak, cylindrical stem that coils around the host and forms suckers. From these suckers, cells invade the host stem and grow to connect with the vascular bundles of the host. The parasitic plant obtains water and nutrients through these connections. The plant is a total parasite (a holoparasite) because it is completely dependent on its host. Other parasitic plants (hemiparasites) are fully photosynthetic and only use the host for water and minerals. There are about 4,100 species of parasitic plants.
Saprophytes
A saprophyte is a plant that does not have chlorophyll and gets its food from dead matter, similar to bacteria and fungi (note that fungi are often called saprophytes, which is incorrect, because fungi are not plants). Plants like these use enzymes to convert organic food materials into simpler forms from which they can absorb nutrients (Figure 7b). Most saprophytes do not directly digest dead matter: instead, they parasitize fungi that digest dead matter, or are mycorrhizal, ultimately obtaining photosynthate from a fungus that derived photosynthate from its host. Saprophytic plants are uncommon; only a few species are described.

A symbiont is a plant in a symbiotic relationship, with special adaptations such as mycorrhizae or nodule formation. Fungi also form symbiotic associations with cyanobacteria and green algae (called lichens). Lichens can sometimes be seen as colorful growths on the surface of rocks and trees (Figure 8a). The algal partner (phycobiont) makes food autotrophically, some of which it shares with the fungus; the fungal partner (mycobiont) absorbs water and minerals from the environment, which are made available to the green alga. If one partner was separated from the other, they would both die.
An epiphyte is a plant that grows on other plants, but is not dependent upon the other plant for nutrition (Figure 8b). Epiphytes have two types of roots: clinging aerial roots, which absorb nutrients from humus that accumulates in the crevices of trees; and aerial roots, which absorb moisture from the atmosphere.

Insectivorous Plants

An insectivorous plant has specialized leaves to attract and digest insects. The Venus flytrap is popularly known for its insectivorous mode of nutrition, and has leaves that work as traps (Figure 9).
The minerals it obtains from prey compensate for those lacking in the boggy (low pH) soil of its native North Carolina coastal plains. There are three sensitive hairs in the center of each half of each leaf. The edges of each leaf are covered with long spines. Nectar secreted by the plant attracts flies to the leaf. When a fly touches the sensory hairs, the leaf immediately closes. Next, fluids and enzymes break down the prey and minerals are absorbed by the leaf. Since this plant is popular in the horticultural trade, it is threatened in its original habitat.
Contributors and Attributions
- Introduction to Plant Nutrition. Authored by : Shelli Carter and Lumen Learning. Provided by : Lumen Learning. License : CC BY: Attribution
- Biology. Provided by : OpenStax CNX. Located at : http://cnx.org/contents/[email protected] . License : CC BY: Attribution . License Terms : Download for free at http://cnx.org/contents/[email protected]

Gurukul of Excellence
Classes for Physics, Chemistry and Mathematics by IITians
Join our Telegram Channel for Free PDF Download
Case Study Questions for Class 7 Science Chapter 1 Nutrition in Plants
- Last modified on: 10 months ago
- Reading Time: 7 Minutes
[Download] Case Study Questions for Class 7 Science Chapter 1 Nutrition in Plants
Here we are providing case study or passage-based questions for class 7 science chapter 1 Nutrition in Plants.
Case Study/Passage Based Questions
The bodies of living organisms are made of tiny units called cells. Cells can be seen only microscope. Some organisms are made of only one cell. The cell is enclosed by a thin boundary, called the cell membrane.
- The smallest structure units of a living organism is (a) a cell (b) a tissue (c) either a cell or a tissue (d) none of these
- In bigger bodies there are a large number of cells, whereas in some organisms there is only one cell. The cell can be seen (a) with naked eye (b) under the microscope (c) both the above are correct (d) cell cannot be seen by us
- Cell membrane (a) is the thin outer boundary of the cell (b) is a semi-permeable membrane (c) both the above are correct (d) none of the above is correct
Related Posts
You May Also Like
Category Lists (All Posts)
All categories of this website are listed below with number of posts in each category for better navigation. Visitors can click on a particular category to see all posts related to that category.
- Full Form (1)
- Biography of Scientists (1)
- Assertion Reason Questions in Biology (37)
- Case Study Questions for Class 12 Biology (14)
- DPP Biology for NEET (12)
- Blog Posts (35)
- Career Guidance (1)
- Assertion Reason Questions for Class 10 Maths (14)
- Case Study Questions for Class 10 Maths (15)
- Extra Questions for Class 10 Maths (12)
- Maths Formulas for Class 10 (1)
- MCQ Questions for Class 10 Maths (15)
- NCERT Solutions for Class 10 Maths (4)
- Quick Revision Notes for Class 10 Maths (14)
- Assertion Reason Questions for Class 10 Science (16)
- Case Study Questions for Class 10 Science (14)
- Evergreen Science Book Solutions for Class 10 (17)
- Extra Questions for Class 10 Science (23)
- HOTS for Class 10 Science (17)
- Important Questions for Class 10 Science (10)
- Lakhmir Singh Class 10 Biology Solutions (4)
- Lakhmir Singh Class 10 Chemistry Solutions (5)
- Lakhmir Singh Class 10 Physics Solutions (5)
- MCQ Questions for Class 10 Science (20)
- NCERT Exemplar Solutions for Class 10 Science (16)
- NCERT Solutions for Class 10 Science (15)
- Quick Revision Notes for Class 10 Science (4)
- Study Notes for Class 10 Science (17)
- Assertion Reason Questions for Class 10 Social Science (14)
- Case Study Questions for Class 10 Social Science (24)
- MCQ Questions for Class 10 Social Science (3)
- Topicwise Notes for Class 10 Social Science (4)
- CBSE CLASS 11 (1)
- Assertion Reason Questions for Class 11 Chemistry (14)
- Case Study Questions for Class 11 Chemistry (11)
- Free Assignments for Class 11 Chemistry (1)
- MCQ Questions for Class 11 Chemistry (8)
- Very Short Answer Questions for Class 11 Chemistry (7)
- Assertion Reason Questions for Class 11 Entrepreneurship (8)
- Important Questions for CBSE Class 11 Entrepreneurship (1)
- Assertion Reason Questions for Class 11 Geography (24)
- Case Study Questions for Class 11 Geography (24)
- Assertion Reason Questions for Class 11 History (12)
- Case Study Questions for Class 11 History (12)
- Assertion and Reason Questions for Class 11 Maths (16)
- Case Study Questions for Class 11 Maths (16)
- Formulas for Class 11 Maths (6)
- MCQ Questions for Class 11 Maths (17)
- NCERT Solutions for Class 11 Maths (8)
- Case Study Questions for Class 11 Physical Education (11)
- Assertion Reason Questions for Class 11 Physics (15)
- Case Study Questions for Class 11 Physics (12)
- Class 11 Physics Study Notes (5)
- Concept Based Notes for Class 11 Physics (2)
- Conceptual Questions for Class 11 Physics (10)
- Derivations for Class 11 Physics (3)
- Extra Questions for Class 11 Physics (13)
- MCQ Questions for Class 11 Physics (16)
- NCERT Solutions for Class 11 Physics (16)
- Numerical Problems for Class 11 Physics (4)
- Physics Formulas for Class 11 (7)
- Revision Notes for Class 11 Physics (11)
- Very Short Answer Questions for Class 11 Physics (11)
- Assertion Reason Questions for Class 11 Political Science (20)
- Case Study Questions for Class 11 Political Science (20)
- CBSE CLASS 12 (8)
- Extra Questions for Class 12 Biology (14)
- MCQ Questions for Class 12 Biology (13)
- Case Studies for CBSE Class 12 Business Studies (13)
- MCQ Questions for Class 12 Business Studies (1)
- Revision Notes for Class 12 Business Studies (10)
- Assertion Reason Questions for Class 12 Chemistry (15)
- Case Study Based Questions for Class 12 Chemistry (14)
- Extra Questions for Class 12 Chemistry (5)
- Important Questions for Class 12 Chemistry (15)
- MCQ Questions for Class 12 Chemistry (8)
- NCERT Solutions for Class 12 Chemistry (16)
- Revision Notes for Class 12 Chemistry (7)
- Assertion Reason Questions for Class 12 Economics (9)
- Case Study Questions for Class 12 Economics (9)
- MCQ Questions for Class 12 Economics (1)
- MCQ Questions for Class 12 English (2)
- Assertion Reason Questions for Class 12 Entrepreneurship (7)
- Case Study Questions for Class 12 Entrepreneurship (7)
- Case Study Questions for Class 12 Geography (18)
- Assertion Reason Questions for Class 12 History (8)
- Case Study Questions for Class 12 History (13)
- Assertion Reason Questions for Class 12 Informatics Practices (13)
- Case Study Questions for Class 12 Informatics Practices (11)
- MCQ Questions for Class 12 Informatics Practices (5)
- Assertion and Reason Questions for Class 12 Maths (14)
- Case Study Questions for Class 12 Maths (13)
- Maths Formulas for Class 12 (5)
- MCQ Questions for Class 12 Maths (14)
- Problems Based on Class 12 Maths (1)
- RD Sharma Solutions for Class 12 Maths (1)
- Assertion Reason Questions for Class 12 Physical Education (11)
- Case Study Questions for Class 12 Physical Education (11)
- MCQ Questions for Class 12 Physical Education (10)
- Assertion Reason Questions for Class 12 Physics (16)
- Case Study Based Questions for Class 12 Physics (14)
- Class 12 Physics Conceptual Questions (16)
- Class 12 Physics Discussion Questions (1)
- Class 12 Physics Latest Updates (2)
- Derivations for Class 12 Physics (8)
- Extra Questions for Class 12 Physics (4)
- Important Questions for Class 12 Physics (8)
- MCQ Questions for Class 12 Physics (14)
- NCERT Solutions for Class 12 Physics (18)
- Numerical Problems Based on Class 12 Physics (16)
- Physics Class 12 Viva Questions (1)
- Revision Notes for Class 12 Physics (7)
- Assertion Reason Questions for Class 12 Political Science (16)
- Case Study Questions for Class 12 Political Science (16)
- Notes for Class 12 Political Science (1)
- Assertion Reason Questions for Class 6 Maths (13)
- Case Study Questions for Class 6 Maths (13)
- Extra Questions for Class 6 Maths (1)
- Worksheets for Class 6 Maths (1)
- Assertion Reason Questions for Class 6 Science (16)
- Case Study Questions for Class 6 Science (16)
- Extra Questions for Class 6 Science (1)
- MCQ Questions for Class 6 Science (9)
- Assertion Reason Questions for Class 6 Social Science (1)
- Case Study Questions for Class 6 Social Science (26)
- NCERT Exemplar for Class 7 Maths (13)
- NCERT Exemplar for Class 7 Science (19)
- NCERT Exemplar Solutions for Class 7 Maths (12)
- NCERT Exemplar Solutions for Class 7 Science (18)
- NCERT Notes for Class 7 Science (18)
- Assertion Reason Questions for Class 7 Maths (14)
- Case Study Questions for Class 7 Maths (14)
- Extra Questions for Class 7 Maths (5)
- Assertion Reason Questions for Class 7 Science (18)
- Case Study Questions for Class 7 Science (17)
- Extra Questions for Class 7 Science (19)
- Assertion Reason Questions for Class 7 Social Science (1)
- Case Study Questions for Class 7 Social Science (30)
- Assertion Reason Questions for Class 8 Maths (7)
- Case Study Questions for Class 8 Maths (17)
- Extra Questions for Class 8 Maths (1)
- MCQ Questions for Class 8 Maths (6)
- Assertion Reason Questions for Class 8 Science (16)
- Case Study Questions for Class 8 Science (11)
- Extra Questions for Class 8 Science (2)
- MCQ Questions for Class 8 Science (4)
- Numerical Problems for Class 8 Science (1)
- Revision Notes for Class 8 Science (11)
- Assertion Reason Questions for Class 8 Social Science (27)
- Case Study Questions for Class 8 Social Science (23)
- CBSE Class 9 English Beehive Notes and Summary (2)
- Assertion Reason Questions for Class 9 Maths (14)
- Case Study Questions for Class 9 Maths (14)
- MCQ Questions for Class 9 Maths (11)
- NCERT Notes for Class 9 Maths (6)
- NCERT Solutions for Class 9 Maths (12)
- Revision Notes for Class 9 Maths (3)
- Study Notes for Class 9 Maths (10)
- Assertion Reason Questions for Class 9 Science (16)
- Case Study Questions for Class 9 Science (15)
- Evergreen Science Book Solutions for Class 9 (15)
- Extra Questions for Class 9 Science (22)
- MCQ Questions for Class 9 Science (11)
- NCERT Solutions for Class 9 Science (15)
- Revision Notes for Class 9 Science (1)
- Study Notes for Class 9 Science (15)
- Topic wise MCQ Questions for Class 9 Science (2)
- Topicwise Questions and Answers for Class 9 Science (15)
- Assertion Reason Questions for Class 9 Social Science (15)
- Case Study Questions for Class 9 Social Science (19)
- CHEMISTRY (8)
- Chemistry Articles (2)
- Daily Practice Problems (DPP) (3)
- Books for CBSE Class 9 (1)
- Books for ICSE Class 10 (3)
- Editable Study Materials (8)
- Exam Special for CBSE Class 10 (3)
- H. C. Verma (Concepts of Physics) (13)
- Study Materials for ICSE Class 10 Biology (14)
- Extra Questions for ICSE Class 10 Chemistry (1)
- Study Materials for ICSE Class 10 Chemistry (5)
- Study Materials for ICSE Class 10 Maths (16)
- Important Questions for ICSE Class 10 Physics (13)
- MCQ Questions for ICSE Class 10 Physics (4)
- Study Materials for ICSE Class 10 Physics (8)
- Study Materials for ICSE Class 9 Maths (7)
- Study Materials for ICSE Class 9 Physics (10)
- Topicwise Problems for IIT Foundation Mathematics (4)
- Challenging Physics Problems for JEE Advanced (2)
- Topicwise Problems for JEE Physics (1)
- DPP for JEE Main (1)
- Integer Type Questions for JEE Main (1)
- Integer Type Questions for JEE Chemistry (6)
- Chapterwise Questions for JEE Main Physics (1)
- Integer Type Questions for JEE Main Physics (8)
- Physics Revision Notes for JEE Main (4)
- JEE Mock Test Physics (1)
- JEE Study Material (1)
- JEE/NEET Physics (6)
- CBSE Syllabus (1)
- Maths Articles (2)
- NCERT Books for Class 12 Physics (1)
- NEET Chemistry (13)
- Important Questions for NEET Physics (17)
- Topicwise DPP for NEET Physics (5)
- Topicwise MCQs for NEET Physics (32)
- NTSE MAT Questions (1)
- Physics (1)
- Alternating Current (1)
- Electrostatics (6)
- Fluid Mechanics (2)
- PowerPoint Presentations (13)
- Previous Years Question Paper (3)
- Products for CBSE Class 10 (15)
- Products for CBSE Class 11 (10)
- Products for CBSE Class 12 (6)
- Products for CBSE Class 6 (2)
- Products for CBSE Class 7 (5)
- Products for CBSE Class 8 (1)
- Products for CBSE Class 9 (3)
- Products for Commerce (3)
- Products for Foundation Courses (2)
- Products for JEE Main & Advanced (10)
- Products for NEET (6)
- Products for ICSE Class 6 (1)
- Electrostatic Potential and Capacitance (1)
- Topic Wise Study Notes (Physics) (2)
- Topicwise MCQs for Physics (2)
- Uncategorized (138)
Test series for students preparing for Engineering & Medical Entrance Exams are available. We also provide test series for School Level Exams. Tests for students studying in CBSE, ICSE or any state board are available here. Just click on the link and start test.
What is Case Study Question for Class 7 Science?
Case study or passage-based questions in class 7 Science typically require students to read a given scenario or passage and answer questions based on the information provided. These questions assess students’ comprehension, analytical thinking, and application of scientific concepts.
Best Ways to Prepare for Case Study Questions
To develop a strong command on class 6 Science case study questions, you can follow these steps:
- Read the textbook and study materials: Familiarize yourself with the concepts and topics covered in your class 6 Science curriculum. Read the textbook thoroughly and take notes on important information.
- Practice analyzing case studies: Look for case studies or passages related to class 6 Science topics. Analyze the given information, identify key details, and understand the context of the situation.
- Develop comprehension skills: Focus on improving your reading comprehension skills. Practice reading passages or articles and try to summarize the main points or extract relevant information. Pay attention to details, vocabulary, and the overall structure of the passage.
- Understand scientific concepts: Ensure that you have a solid understanding of the scientific concepts discussed in class. Review the fundamental principles and theories related to each topic.
- Make connections: Try to connect the information provided in the case study to the concepts you have learned in class. Identify any cause-effect relationships, patterns, or relevant scientific principles that apply to the situation.
- Practice critical thinking: Develop your critical thinking skills by analyzing and evaluating the information given in the case study. Think logically, consider multiple perspectives, and draw conclusions based on the evidence provided.
- Solve practice questions: Look for practice questions or sample case study questions specifically designed for class 6 Science. Solve these questions to apply your knowledge, practice your analytical skills, and familiarize yourself with the format of case study questions.
- Seek clarification: If you come across any challenging concepts or have doubts, don’t hesitate to ask your teacher for clarification. Understanding the underlying principles will help you tackle case study questions effectively.
Download CBSE Books
Exam Special Series:
- Sample Question Paper for CBSE Class 10 Science (for 2024)
- Sample Question Paper for CBSE Class 10 Maths (for 2024)
- CBSE Most Repeated Questions for Class 10 Science Board Exams
- CBSE Important Diagram Based Questions Class 10 Physics Board Exams
- CBSE Important Numericals Class 10 Physics Board Exams
- CBSE Practical Based Questions for Class 10 Science Board Exams
- CBSE Important “Differentiate Between” Based Questions Class 10 Social Science
- Sample Question Papers for CBSE Class 12 Physics (for 2024)
- Sample Question Papers for CBSE Class 12 Chemistry (for 2024)
- Sample Question Papers for CBSE Class 12 Maths (for 2024)
- Sample Question Papers for CBSE Class 12 Biology (for 2024)
- CBSE Important Diagrams & Graphs Asked in Board Exams Class 12 Physics
- Master Organic Conversions CBSE Class 12 Chemistry Board Exams
- CBSE Important Numericals Class 12 Physics Board Exams
- CBSE Important Definitions Class 12 Physics Board Exams
- CBSE Important Laws & Principles Class 12 Physics Board Exams
- 10 Years CBSE Class 12 Chemistry Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Physics Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Maths Previous Year-Wise Solved Papers (2023-2024)
- 10 Years CBSE Class 12 Biology Previous Year-Wise Solved Papers (2023-2024)
- ICSE Important Numericals Class 10 Physics BOARD Exams (215 Numericals)
- ICSE Important Figure Based Questions Class 10 Physics BOARD Exams (230 Questions)
- ICSE Mole Concept and Stoichiometry Numericals Class 10 Chemistry (65 Numericals)
- ICSE Reasoning Based Questions Class 10 Chemistry BOARD Exams (150 Qs)
- ICSE Important Functions and Locations Based Questions Class 10 Biology
- ICSE Reasoning Based Questions Class 10 Biology BOARD Exams (100 Qs)
✨ Join our Online JEE Test Series for 499/- Only (Web + App) for 1 Year
✨ Join our Online NEET Test Series for 499/- Only for 1 Year
Leave a Reply Cancel reply
Join our Online Test Series for CBSE, ICSE, JEE, NEET and Other Exams

Editable Study Materials for Your Institute - CBSE, ICSE, State Boards (Maharashtra & Karnataka), JEE, NEET, FOUNDATION, OLYMPIADS, PPTs
Discover more from Gurukul of Excellence
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading

ORIGINAL RESEARCH article
Biological mode of action of a nitrophenolates-based biostimulant: case study.

- 1 Laboratory of Basic Research in Horticulture, Faculty of Horticulture, Biotechnology and Landscape Architecture, Warsaw University of Life Sciences – SGGW, Warsaw, Poland
- 2 Department of Vegetable and Medicinal Plants, Faculty of Horticulture, Biotechnology and Landscape Architecture, Warsaw University of Life Sciences – SGGW, Warsaw, Poland
The challenges facing modern plant production involve (i) responding to the demand for food and resources of plant origin from the world's rapidly growing population, (ii) coping with the negative impact of stressful conditions mainly due to anthropopressure, and (iii) meeting consumers' new requirements and preferences for food that is high in nutritive value, natural, and free from harmful chemical additives. Despite employing the most modern plant cultivation technologies and the progress that has been made in breeding programs, the genetically-determined crop potential is still far from being fully exploited. Consequently yield and quality are often reduced, making production less, both profitable and attractive. There is an increasing desire to reduce the chemical input in agriculture and there has been a change toward integrated plant management and sustainable, environmentally-friendly systems. Biostimulants are a category of relatively new products of diverse formulations that positively affect a plant's vital processes and whose impact is usually more evident under stressful conditions. In this paper, information is provided on the mode of action of a nitrophenolates-based biostimulant, Atonik, in model species and economically important crops grown under both field and controlled conditions in a growth chamber. The effects of Atonik on plant morphology, physiology, biochemistry (crops and model plant) and yield and yield parameters (crops) is demonstrated. Effects of other biostimulants on studied in this work processes/parameters are also presented in discussion.
Introduction
The challenge facing modern plant production nowadays is to respond to the increasing demand for food and resources of plant origin by the world's rapidly growing population. Yield is negatively affected by various adverse environmental conditions and increasing anthropopression and despite employing the most modern plant cultivation technologies and the progress being made in breeding programs, the genetically-determined crop potential is still far from being fully exploited. According to Bray et al. (2000) , stresses can reduce average productivity by 65–87%, depending on the crop. This consequently makes plant production less profitable for farmers and less attractive for consumers.
Biostimulants syn . biostimulators are a category of relatively new products of diverse formulations that positively affect a plant's vital processes ( Calvo et al., 2014 ), usually more evident under stressful conditions, by increasing a plant's tolerance to stresses and repairing damage caused by unfavorable conditions.
Biostimulants may be of natural or synthetic origin and consist of various organic and inorganic components. Among naturally derived biostimulants are preparations based on free amino acids, extracts from seaweed and fruit, effective microorganisms, humic substances, and chitosan ( Calvo et al., 2014 ). Synthetic biostimulants are composed, among others, of plant growth regulators, phenolic compounds, inorganic salts, essential elements, and other substances that have stimulating properties for plants.
Although the term “biostimulant” has been used for many years, it is still not fully defined. The European Biostimulant Industry Council (EBIC) describes biostimulants as a preparations “… containing substance(s) and/or micro-organisms whose function, when applied to plants or the rhizosphere is to stimulate natural processes to enhance/benefit nutrient uptake, nutrient efficiency, tolerance to abiotic stress, and crop quality….” Biostimulants do not replace, but rather complement plant protection products and fertilizers. They have no direct action against pests and they operate through different mechanisms than fertilizers, regardless of the occasional presence of nutrients in these products ( http://www.biostimulants.eu ).
It is impossible to suggest one common mode of action for all biostimulants, therefore this work focused on Atonik, known as Chapperone (USA) or Asahi SL (Poland). Atonik is a Japanese synthetic biostimulant composed of three phenolic compounds: sodium para-nitrophenolate PNP (0.3%), sodium ortho-nitrophenolate ONP (0.2%) and sodium 5-nitroguaiacolate 5NG (0.1%), and water. Atonik has been used successfully for many years in the cultivation of most important crops worldwide. Its positive effect on yield is already well proven ( Djanaguiraman et al., 2004a , 2009 ; Bynum et al., 2007 ; Grajkowski and Ochmian, 2007 ; Budzyński et al., 2008 ; Černý et al., 2008 ; Kositorna and Smoliński, 2008 ; Kozak et al., 2008a ; Malarz et al., 2008 ; Michalski et al., 2008 ; Sawicka and Mikos-Bielak, 2008 ), but knowledge about its mode of action has, until this study, been fragmented, not covered thoroughly in literature, and sometimes even controversial. Early works described some of the potential positive properties of Atonik. It has been shown that the nitrophenolates making up this biostimulant increase cytoplasm streaming ( Yamaki et al., 1953 ; Wilson and Kaczmarek, 1993 ). Plants treated with nitrophenolates have greater inhibition of IAA oxidase, which ensures a higher activity of naturally synthesized auxins ( Stutte and Clark, 1990 ). The phosphorylated form of para-nitrophenolate enhances IAA activity when used as a substrate for phosphatases via increased high-affinity binding sites of IAA ( Davies, 1987 ) and could be as effective as ATP ( Koizumi et al., 1990 ). According to Stutte et al. (1987) , plants exposed to nitrophenolates uptake more nutrients from the medium. Furthermore, Sharma et al. (1984) showed a significant increase in the activity of nitrate reductase, an important enzyme in nitrogen metabolism.
More recent studies prove that Atonik positively affects various processes controlling plant growth, development and productivity. Biostimulant-treated plants are more advanced in growth and development ( Djanaguiraman et al., 2005b ; Gulluoglu et al., 2006 ; Kozak et al., 2008a ; Borowski and Blamowski, 2009 ) and accumulate more biomass ( Gruszczyk and Berbeć, 2004 ; Djanaguiraman et al., 2005a , 2009 ; Kołodziej, 2008 ). Atonik increases the intensity of photosynthesis ( Borowski and Blamowski, 2009 ) and transpiration rate, but usually without a reduction in relative water content ( Wróbel and Woźniak, 2008 ; Borowski and Blamowski, 2009 ). The positive effects of Atonik are much more evident when plants are grown under adverse conditions. It has been found that biostimulants play a protective role against various abiotic stresses, such as low or high temperatures, drought, heavy metals, and salinity ( Gulluoglu et al., 2006 ; Gawrońska et al., 2008 ; Wrochna et al., 2008 ; Borowski and Blamowski, 2009 ). Moreover, some results have indicated that if plants were grown under optimal conditions, the positive effect of this preparation might not be recorded ( Budzyński et al., 2008 ; Księżak, 2008 ).
However, the works presented above individually only cover a narrow range of processes and/or parameters. This paper provides the first comprehensive study of the Atonik mode of action and demonstrates the effects of biostimulant on yield and its components, plant morphology, physiology and biochemistry in the model plant Arabidopsis thaliana L. and some crops that are economically important ( Brassica napus L. var. oleifera and Cucumis sativus L.).
Materials and Methods
The experiments were carried out on crops: oilseed rape and cucumber and A. thaliana used as model plant. Plants were grown in field conditions and growth chambers under optimal, drought or noble metal stresses. Concentrations of Atonik and the number of its applications were first determined in preliminary studies in order to ensure a stimulative/protective effect of the biostimulant in particular species and growing conditions.
Effect of Atonik on Field-Grown Oilseed Rape Plants
Plant material and growing conditions.
Oilseed rape cv . “Lisek” plants were cultivated in the 2007 and 2008 growing seasons in the experimental field in Chylice of the Warsaw University of Life Sciences—SGGW. The field is situated 105 m above sea level and located at 22°33'25” N and 52°05'71” E. The 30-year average annual temperature and rainfall are 7.8°C (12.8°C during the growing seasons) and 592 mm (448 mm during the growing seasons) respectively. The soil (black degraded, composed of loamy sand) is classified as average good, with a 0.8–1.6% content of organic matter and pH 6.0–6.2. Experiments were conducted in completely randomized blocks in four replicates (plots of 18 or 14.4 m 2 in 2007 and 2008, respectively). The seeds were sown at a spacing of 30 × 6.5 cm. For the measurements, five plants from each plot were chosen. In the 2007 growing season the experimental plants were grown in 25 L pots filled with soil taken from the particular plots, and the pots were placed (buried) on the appropriate plots, following a statistical design. Routine agricultural practices recommended for this species and location were employed. Both vegetative seasons were characterized by similar growing conditions, the only exception was a strong late spring frost in 2007. Atonik was applied in spring as a single (BBCH 29–31) or double (BBCH 29–31 and 51) foliar spray in a concentration of 0.2% v/v in 300 L ha −1 . NPK fertilizers were applied as 194 kg N ha −1 (34—autumn, 160—spring), 80 kg P ha −1 and 120 kg K ha −1 .
Measured parameters/processes
One (2007) or three (2008) weeks after the Atonik application, the following parameters were measured: (i) plant gas exchange: intensity of photosynthesis and transpiration, stomatal resistance (Photosynthesis System LICOR 6200, Lincoln, NE, USA), (ii) chlorophyll content (CCM-200, OPTI-SCIENCES, USA) and (iii) chlorophyll a fluorescence (Handy PEA, Hansatech, UK). The measurements were performed for 9 (2007) and 10 (2008) weeks. After harvest, (i) the height of the plants was measured, (ii) the number of leaves, primary laterals, pods and seeds in pods were counted, and (iii) the accumulation of biomass (after drying at 105°C for 2 h and then at 75°C for 48 h) and yield of seeds ( via weighing of air dry seeds) were recorded.
Effect of Atonik on Field-Grown Cucumber Plants
Cucumber cvs . “Octopus F1” (Syngenta Seeds), Opera F1 and Sonate F1 (both Rijk Zwaan) plants were cultivated in the 2012 growing season in the experimental field of the Department of Vegetable and Medicinal Plants at Wilanów, Poland. Plants were grown in deep medium-heavy alluvial soil (classified as good) with a 1.9–2.3% content of organic matter and pH 6.0–6.5. The experiment was arranged in a two-factor split-plot design with four replicates (plots of 6 m 2 ). Seeds were sown manually on 14 May into plastic pots of 8 cm diameter filled with peat substrate. On 24 May, when the plants had 1–2 leaves, seedlings were planted in the field at a spacing of 30 × 150 cm. There were 14 plants in the plot. Atonik was applied as a foliar spray (12 and 27 June and 27 July) in a concentration of 0.1% v/v in 500 L ha −1 . Control plants were treated with water. During the period of water shortage, plants were T-Tape irrigated. The soil content of N, P, and K was kept at the optimum level, with fertilizers applied to equal the average of 150 kg N ha −1 (60 kg N side dressing), 50 kg P ha −1 and 190 kg K ha −1 . The harvest was carried out successively, twice a week (13 times), starting from the middle of September.
At harvest, the total and marketable yield was recorded. Yield quality was evaluated by determining the content of: (i) dry matter (drying to constant weight at 105°C), (ii) sugars (Luff–Schoorl method), (iii) vitamin C (titration with Tillmans' method), (iv) nitrates (spectrophotometer Tecator Fiastar 5010 at wavelength 540 nm), (v) phosphorus (spectrophotometer Shimadzu 1700 at wavelength 460 nm), (vi) potassium, and (vii) calcium (both using flame spectrophotometer Sherwood Model 410). Marketable fruits were graded according to the Polish standard PN-85/R-75359 into two pickling grades of (i) 6–10 cm long with a diameter of 2.5–4.5 cm and (ii) 9–15 cm long with a diameter of 4.5–5.5 cm.
Effect of Atonik on A. Thaliana Plants Grown Under Optimal, Drought, and Pt Stress
A. thaliana Col 4 seeds (Lehle Seeds, Round Rock, TX, USA) were sown onto multiplates filled with substrate (Universal Kronenerde soil and sand in the proportion 2:1 v/v). Uniform, 6-week-old seedlings were transplanted to (i) pots (Ø 10 cm) containing the same substrate or (ii) hydroponics culture filled with 0.3 dm 3 of a Hoagland solution ( Arnon and Hoagland, 1940 ) modified by Siedlecka and Krupa (2002) . The nutrient solution was continuously aerated and renewed weekly. Plants were grown in growth chambers (Simez Control s.r.o. Vsetin, Czech Republic) at 22/18°C with a photoperiod 8/16 h day/night, irradiance of 250–280 μmol m −2 s −1 PAR and relative humidity of 60%.
Drought stress
Before drought treatment, the maximum water capacity (MWC) of the substrate was determined. Drought stress was imposed on the soil as a result of a daily limited water supply via pot weighing to the levels of 50, 40, 30, and 20% of MWC (three experiments) or 45 and 25% of MWC (two experiments). Depending on the experiment, the combination consisted of 6–12 plants. On the day on which the substrate attained the desired MWC, the plants were treated once with Atonik as a foliar spray at a concentration of 0.1% (with an amount of water equal to 300 L ha −1 in the field conditions) and grown for a further 4 weeks. Control plants were cultivated at 60 or 65% MWC (optimal water conditions) and sprayed with distilled water.
During the first week the nutrient solution was used at half strength and thereafter the complete composition of macro- and microelements was supplied. Two weeks after plants were transplanted to hydroponics, during the nutrient solution change, Pt and Atonik were added. Pt, in oxidation state II, was added at concentrations of 2.5, 25, and 50 μM in the form of [Pt(NH 3 ) 4 ](NO 3 ) 2 . Atonik was added at a concentration of 0.005% v/v. After treatment, the plants were grown for a further 3 weeks. In total, three experiments were carried out, with 5–6 plants per combination. Control plants were grown in Pt and Atonik-free medium.
During plant growth the following parameters were measured: (i) plant gas exchange: intensity of photosynthesis and transpiration, stomatal resistance (Photosynthesis System LICOR 6200, Lincoln, NE, USA), (ii) chlorophyll content (CCM-200, OPTI-SCIENCES, USA), (iii) chlorophyll a fluorescence (Handy PEA, Hansatech, UK), and (iv) water uptake ( via daily pot weighing). At harvest, sub-samples were collected for (i) relative water content (RWC, via weighing) and (ii) membrane injury (conductometrically, MultiLevel 1, WTW, Germany) and data recorded on (iii) the height of plants, (iv) length and number of inflorescences, (v) number of pods, (vi) number and area of leaves (Leaf Area, Root Length and Image Analyzing System, Skye, UK), and (vii) biomass accumulated by the whole plants and particular organs (after drying at 105°C for 2 h and then at 75°C for 48 h).
The number of replications, depending on the parameter, was between 3 and 36, and is indicated in the specific tables or figures. Differences between the combinations were evaluated with one or two-factor analysis of variance by LSD (Student's t -test) or HSD (Tukey test) at α = 0.05. The presented data are mean ± SE (where indicated).
Atonik-treated plants in the 2007 season were taller than the control, and produced slightly more pods (0.1–4.1%) and seeds in pods (0.9–2.8%) (Table 1 ). On the other hand these plants developed a lower number of primary laterals. In the 2008 season, the biostimulant had no effect on the plants' height. Regardless of whether Atonik was used once or twice, the number of laterals (9.5–12.5%) and seeds (0.7–2%) was greater. Only the single spray increased the number of pods (8.1%) (Table 1 ).
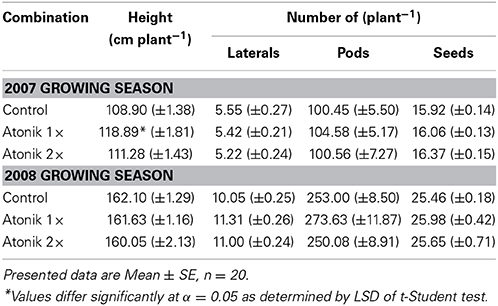
Table 1. Effect of Atonik on selected morphological parameters of oilseed rape plants .
The fresh weight of Atonik-treated plants in 2007 was 12.5% higher than that of the control and in the case of dry matter Atonik contributed to an increase of between 11.9–23.7% (Table 2 ). The fresh weight and dry matter of stem and pods with seeds were also greater. Higher values were obtained for a single spray. In the next season the positive influence of Atonik on accumulated biomass was less evident and was recorded after a single spray only. Atonik slightly increased the fresh weight and dry matter of the aboveground part, the main stem and pods with seeds. The weight of the laterals was adversely affected. The yield of plants sprayed once with Atonik exceeded the control by 35% (2007) or by just 3.6% (2008). After the double application no positive effect or even reduction was noted (Table 2 ).
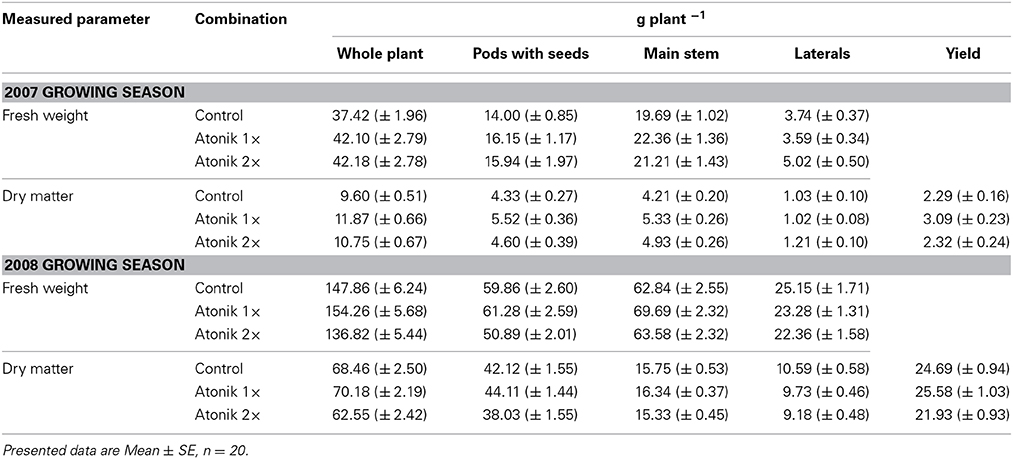
Table 2. Effect of Atonik on biomass accumulation and seed yield in oilseed rape plants .
In the 2007 season, irrespective of the number of treatments, Atonik increased photosynthesis intensity (1–22%) and this effect lasted up to 7 weeks following the first spray (Table 3 ). In the following year the positive effect on this process remained for 4 weeks (3.6–20.3%). In the 2007 season, the sprayed plants were usually characterized by a higher intensity of transpiration and lower stomatal resistance. In contrast to this, in the 2008 season the effect of Atonik on these parameters was ambiguous. The total chlorophyll content in both growing seasons was, with a few exceptions, higher in biostimulant-treated plants (Table 3 ).
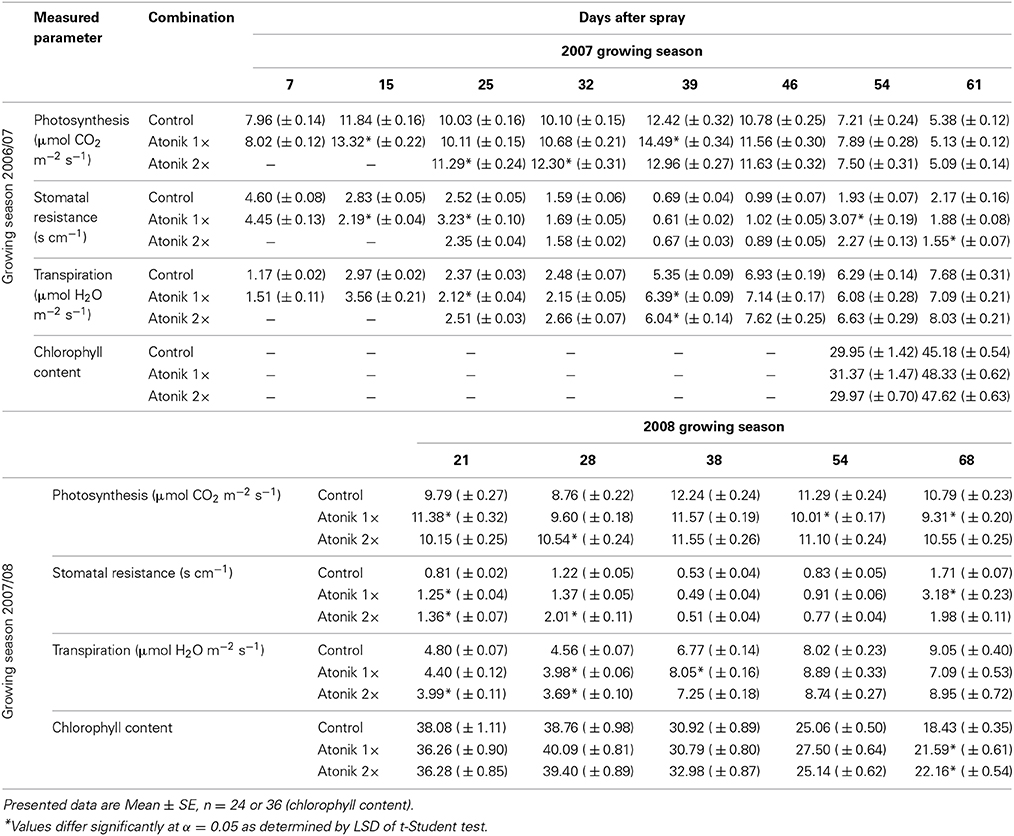
Table 3. Effect of Atonik on the intensity of photosynthesis and transpiration, stomatal conductance, transpiration and chlorophyll content in oilseed rape plants .
Measurements of chlorophyll a fluorescence showed that in the 2007 season Atonik did not affect Fv/Fm (maximum quantum efficiency of Photosystem II) and P.I. (Performance Index) up to the late spring frost (−4.2°C) that occurred between the 36 and 39th day after the first application of the biostimulant (Figure 1A ). Following the frost, a lowering in the Fv/Fm and P.I. values in the control was recorded, while in the treated plants they did not change. Moreover, the positive effect on P.I. remained for the next 22 days. The values of these parameters in the 2008 season during the 8 weeks after the first spray were similar between the treated and untreated plants. Starting from week 10, a reduction in these parameters after the application of Atonik was noted (Figure 1B ).
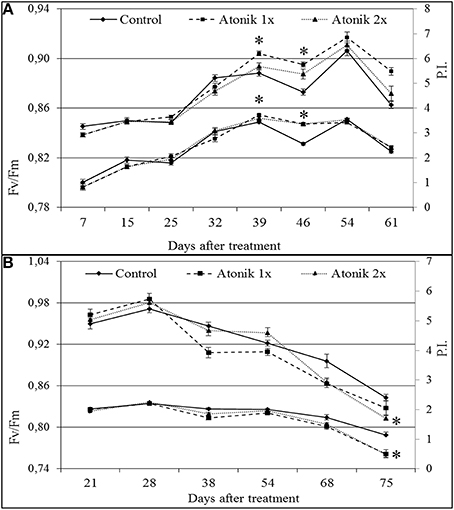
Figure 1. Effect of Atonik on selected parameters of chlorophyll a fluorescence (Fv/Fm and PI) in oilseed rape cv . Lisek plants grown under field conditions during the 2007 (A) and 2008 (B) vegetation seasons. Presented data are mean ± SE, n = 24. *Values differ significantly at α = 0.05 as determined by LSD of t -Student test.
There was no significant effect of the Atonik on total or marketable yield, or any interactions of both traits examined (Table 4 ). Slightly increased yields after biostimulant treatment were recorded only for the cultivar Octopus F 1 . Yields of fruits were significantly related to the cultivar. The highest values of fruit mass were recorded for cultivar Sonate F 1 and the lowest for Octopus F 1 . On average, for all the examined cultivars, the content of dry matter and soluble solids were significantly higher after treatment with the biostimulant. When the cultivars were examined separately, it came out that dry content increased by Atonik, except in Opera F 1 . Soluble solids were always higher in plants sprayed with the biostimulant. The content of nitrates was higher on average in plants treated with Atonik. The exception was the Sonate F 1 cultivar. In plants sprayed with the biostimulant, a higher content of phosphorus was recorded. The content of potassium was only significantly affected by the cultivar and the highest was found in Octopus F 1 , while the lowest was in Sonate F 1 . The content of calcium was affected by both the biostimulant and the cultivar. The effect of Atonik on this parameter was adverse and the Sonate F 1 cultivar was characterized as having the greatest content of calcium and Opera F 1 the lowest (Table 4 ).
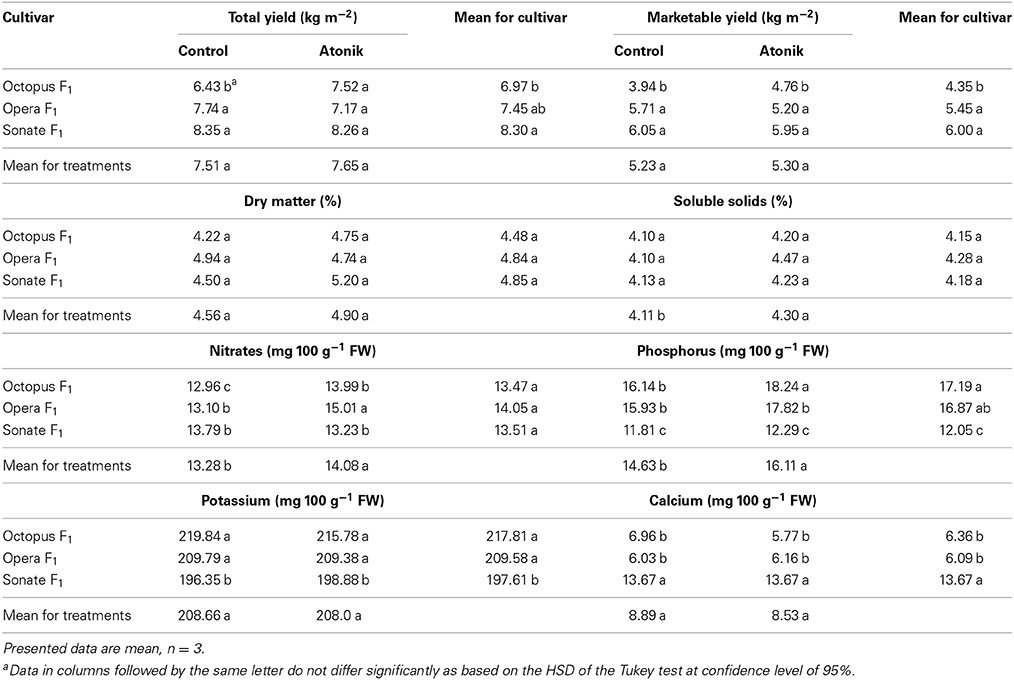
Table 4. Effect of Atonik on the total and marketable yield of cucumber fruit, content of dry matter, soluble solids, nitrates, phosphorus, potassium, and calcium .
Effect of Atonik on A. Thaliana Plants Grown Under Optimal, Drought, and Pt Stresses
Optimal and drought conditions.
Atonik had a positive effect on A. thaliana grown in optimal conditions and clearly diminished the negative impact of drought (Figure 2 ). Plants sprayed with Atonik were taller and developed more inflorescences (by 14–56%) and pods (by 93–450%) (Table 5 ). In 20 and 30% of MWC their number reduced. Leaf area was always greater in Atonik-treated plants, and this increase ranged between 3–43% (Table 5 ).

Figure 2. Effect of Atonik on growth and development of A. thaliana plants grown under drought stress conditions (40% MWC) .
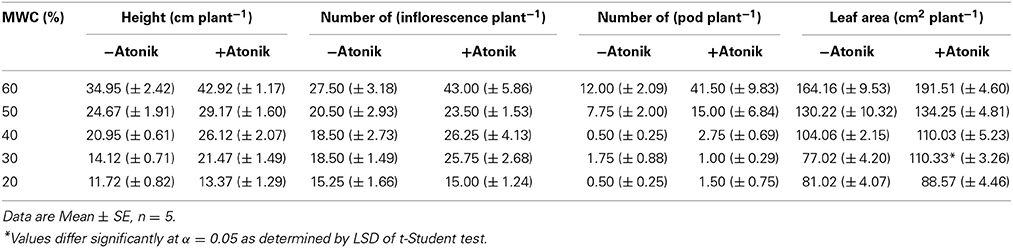
Table 5. Effect of Atonik on selected morphological parameters of A. thaliana plants grown under optimal and drought stress conditions .
A. thaliana treated with Atonik produced more biomass and this was true for optimal conditions and every level of drought stress (Table 6 ). The increase of biomass accumulation recorded ranged between 2.5–46 and 1–47%, respectively for fresh weight and dry matter. The positive effect of Atonik was more evident in the case of generative organs (Table 6 ).
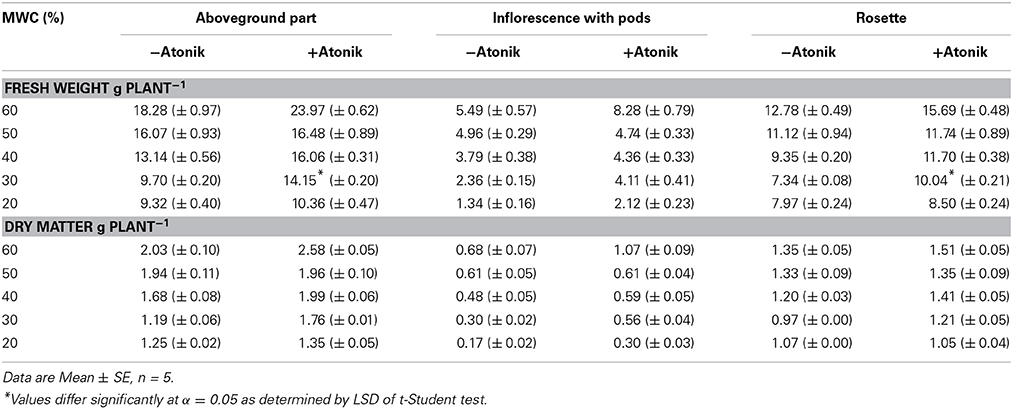
Table 6. Effect of Atonik on the fresh matter of the whole aboveground part, inflorescence and rosette of A. thaliana plants grown under optimal and drought stress conditions .
The efficiency of the photosynthetic apparatus of A. thaliana plants was positively affected by the biostimulant (Table 7 ). The intensity of photosynthesis was usually higher in Atonik-treated plants and this increase ranged from 0.5 to as high as 55.5%. The greater intensity of photosynthesis corresponded well with the significantly lowered stomatal resistance. The effect of Atonik on chlorophyll content in A. thaliana was not uniform. Measurements taken seven days after the treatment revealed the biostimulant's positive effect on this parameter, but 14 days after the Atonik application a greater chlorophyll content was recorded in 50 and 40% of MWC. Atonik also influenced parameters of chlorophyll a fluorescence, especially 14 days after its application, when the negative effects of drought stress were more evident (Table 7 ).
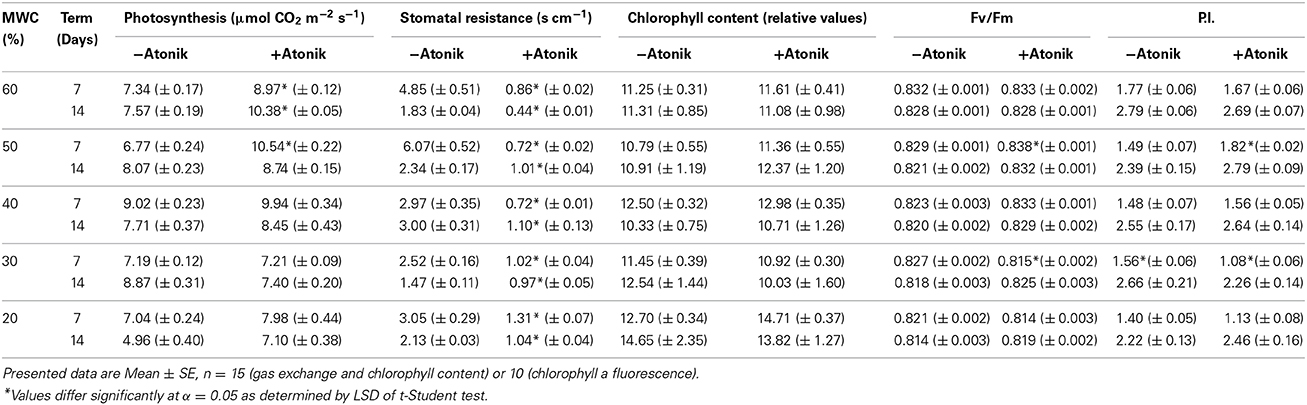
Table 7. Effect of Atonik on intensity of photosynthesis, stomatal resistance, chlorophyll content and selected parameters of chlorophyll a fluorescence of A. thaliana L. plants grown under optimal and drought stress conditions .
The intensity of transpiration increased after Atonik treatment (Table 8 ). RWC was either only lowered slightly or, at higher drought levels, even increased due to biostimulant application. Plants sprayed with Atonik uptake more water from the medium (Table 8 ).
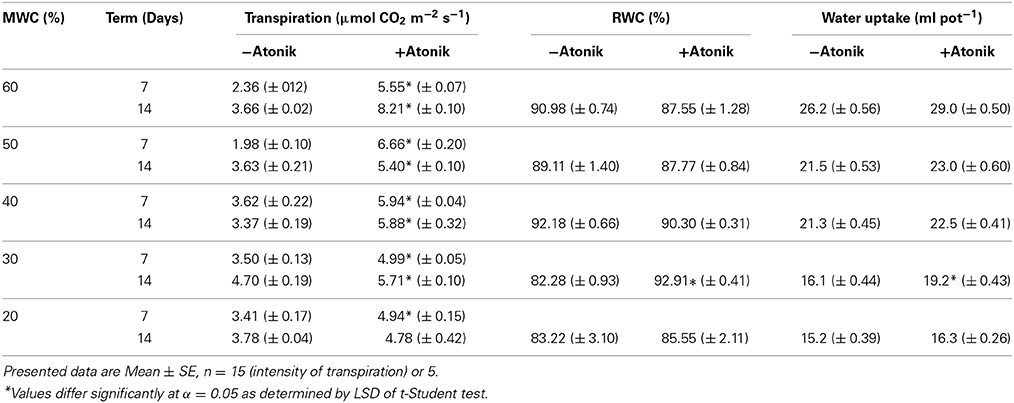
Table 8. Effect of Atonik on intensity of transpiration, RWC, and water uptake of A. thaliana plants grown under optimal and drought stress conditions .
Optimal and Pt stress conditions
Treatment with Atonik, independently from Pt concentration, had a positive effect on A. thaliana plants. The area and number of leaves were greater than in the control by 8.6–15.1 and 0.2–35.5%, respectively (Table 9 ). Only plants grown in the Pt-free medium had a decreased number of leaves after Atonik application. The biostimulant had a positive effect on biomass accumulation in the aboveground parts of the plants exposed to Pt at concentrations of 2.5 and 25 μM, and the range of this increase amounted to 13–14.5%. In the case of 50 μM, a positive effect was not recorded. Atonik always increased the fresh weight and dry matter of roots (Table 9 ).
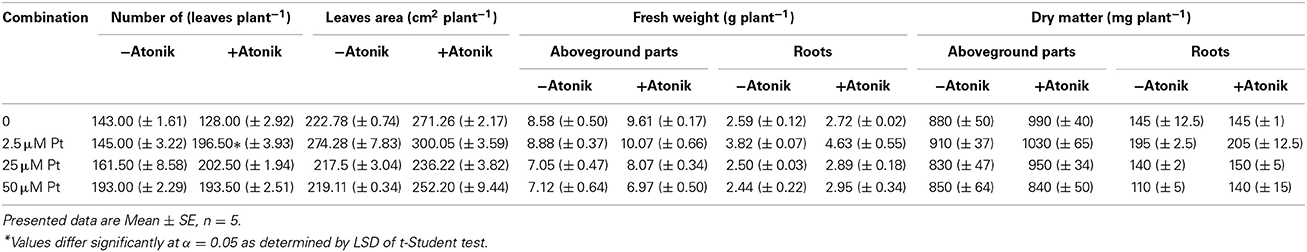
Table 9. Effect of Atonik on the number and area of leaves, and biomass accumulation in A. thaliana plants exposed to Pt ions .
Intensity of photosynthesis was greater (up to 17.5%) and stomatal resistance was lower (up to 42.5%) in Atonik-treated plants (Table 10 ). The biostimulant also had a positive effect on the chlorophyll content in leaves, which was higher by 5.1–13.0%. Treatment with Atonik raised the values of Fv/Fm and P.I. in plants exposed to Pt ions (Table 10 ).
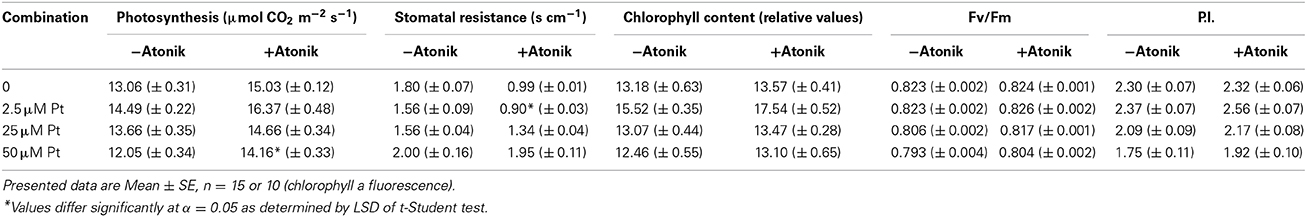
Table 10. Effect of Atonik on intensity of photosynthesis, stomatal resistance, chlorophyll content and selected parameters of chlorophyll a fluorescence (Fv/Fm and P.I.) of A. thaliana plants exposed to Pt ions .
Treatment with Atonik always increased the intensity of transpiration, which was especially evident in 2.5 μM of Pt (Table 11 ). Effect of Atonik on RWC was marginal as the biostimulant increased this parameter by 3–4% in two lower Pt concentrations, and decreased it by 2% in the highest (Table 11 ).
Table 11. Effect of Atonik on the intensity of transpiration, RWC, and membrane injuries of A. thaliana plants exposed to Pt ions .
Membrane injuries were reduced in biostimulant treated plants (Table 11 ). After application of Atonik, the level of membrane injuries decreased by 9.5–13.8% in roots and 0.5–1.7% in leaves (Table 11 ).
Effect of Atonik on Growth and Development
The results of this study have clearly demonstrated that Atonik affects all stages of plant development. Changes caused by the biostimulant application are recorded from seed germination and seedling growth (our other study on Atriplex hortensis, Lolium perenne , and Sinapis alba , data not shown) through the whole ontogenesis. The positive effect of Atonik on germination and seedling growth has been reported by Djanaguiraman et al. (2005a) and Kozak et al. (2008b) . This can be explained by the fact that phenolic compounds, which are components of Atonik, interact with gibberellins, which promote seed germination ( Taiz and Zeiger, 2002 ). Fully developed plants treated with a biostimulant are more advanced in growth and development, which has been shown in this work on A. thaliana and oilseed rape. A. thaliana plants had an increased leaf area and better-developed root system. The stimulation of elongative growth, as a result of the application of Atonik, might be attributed to the greater concentration and/or activity of auxins ( Djanaguiraman et al., 2004a , 2005b ). Plants treated with a biostimulant are characterized as having a higher inhibition of IAA oxidase, which ensures greater activity of naturally synthesized auxins ( Stutte and Clark, 1990 ) and a greater number of high-affinity binding sites of IAA ( Libbenga and Mennes, 1987 ). Feverfew ( Gruszczyk and Berbeć, 2004 ), cotton ( Djanaguiraman et al., 2005a , 2009 ), tomato ( Djanaguiraman et al., 2004b , 2005a ), maize ( Michalski et al., 2008 ), and soya ( Kozak et al., 2008a ) are all taller after the Atonik application. The biostimulant stimulates the growth of shoots in sweet pepper ( Panajotov et al., 1997 ) and roots in cotton ( Djanaguiraman et al., 2005a ) and ginseng ( Kołodziej, 2004 ). The promotion of leaf development is noted in cotton and tomato ( Djanaguiraman et al., 2005b , 2009 ) and sweet pepper ( Panajotov et al., 1997 ). Other biostimulants also stimulate plant growth. For example bio-algeen S90 increases the height of tomato plants ( Dobromilska and Gubarewicz, 2008 ). The length of shoots has been positively influenced by various biostimulants in bell pepper, raspberry, and apple ( Basak and Mikos-Bielak, 2008 ; Ochmian et al., 2008 ; Stępowska, 2008a ). Bio Jodis, Goëmar Goteo, Bio-algeen S90 and Resistim stimulate root growth in tomato ( Kossak and Dyki, 2008 ). A greater number of leaves and/or their area have been recorded in tomato treated with Bio-algeen S90 ( Dobromilska and Gubarewicz, 2008 ), apple with Kelpak ( Basak and Mikos-Bielak, 2008 ) and bell pepper with four different biostimulants ( Stępowska, 2008a ).
However, in literature there is also data indicating a lack of positive effects of biostimulants on plant growth. Malarz et al. (2008) demonstrate the marginal influence of Atonik on the height of spring rape. Atonik did not affect the growth of oilseed rape ( Budzyński et al., 2008 ), bell pepper ( Csizinszky, 2001 ), or maize ( Księżak, 2008 ) at all.
Atonik-treated plants are more advanced in generative development. In this study, the biostimulant increased the number of inflorescences, pods and seeds. This was true for A. thaliana and oilseed rape, irrespective of whether the plants were grown in the field or in growth chambers, no matter if under optimal or stress conditions. These results confirmed previous findings by Budzyński et al. (2008) and Malarz et al. (2008) , who also demonstrate the positive effect of Atonik on the generative development of oilseed rape. Atonik also increases the number of pods and seeds in soya ( Gulluoglu et al., 2006 ; Kozak et al., 2008a ), flowers and bolls in cotton ( Djanaguiraman et al., 2005b ), flowers and fruits in tomato ( Djanaguiraman et al., 2004a ), and inflorescences in feverfew ( Gruszczyk and Berbeć, 2004 ). Above corresponds well with works of Górnik and Grzesik (2002 , 2005) , who found that Atonik improves the generative development of China aster, but only when applied during flowering. A greater number of flowers and fruits has also been reported in tomato and apple plants treated with Bio-algeen S-90 and Frigocur, respectively ( Basak and Mikos-Bielak, 2008 ; Dobromilska and Gubarewicz, 2008 ). Goëmar BM 86 stimulates fruit growth in pears ( Błaszczyk, 2008 ) and ripening in raspberries ( Krok and Wieniarska, 2008 ).
In contrast, Krawiec (2008) found an ambiguous effect of simultaneous treatment with Goëmar BM 86 and Atonik on the number of fruits in chokeberries. Atonik did not affect the size and diameter of strawberry fruits ( Miranda-Stalder et al., 1990 ) or the number of grains in the cob and size of the cob in maize ( Księżak, 2008 ).
Effect of Atonik on Biomass Accumulation and Yielding
This study's results have shown, that the faster growth and development of Atonik-treated plants is associated with a greater biomass accumulation. After the application of the biostimulant, the fresh weight and dry matter of whole A. thaliana and oilseed rape plants, as well as their particular organs, were greater. In the case of oilseed rape, this effect was more pronounced in the 2007 vegetative season in which plants experienced a spring frost. It is worth mentioning that the increase of biomass accumulated in generative organs was greater than in vegetative ones, which also supported the hypothesis mentioned above concerning the promotion of generative development. A greater biomass accumulation in oilseed rape sprayed with Atonik has also been recorded by Bečka et al. (2004) . Similar results are recorded for cotton and tomato ( Djanaguiraman et al., 2004b , 2005a ), goldenrod ( Kołodziej, 2008 ), Amaranth sp. ( Wrochna et al., 2008 ), and common osier ( Harasimowicz-Hermann and Czyż, 2008 ). A stimulation of dry-matter accumulation in the roots and aboveground organs of oilseed rape treated with Route has been reported by Krawczyk and Skoczyński (2008) . Bio-algeen S-90 increases the dry matter of tomato fruits ( Dobromilska and Gubarewicz, 2008 ) and Goëmar Goteo positively affects biomass accumulation in lettuce ( Kowalczyk and Zielony, 2008 ) and nappa cabbage ( Gajewski et al., 2008 ). Stępowska (2008a) has recorded a greater weight of whole plants and separately of roots and leaves in bell pepper treated with different biostimulants. The increase in biomass accumulation resulting from biostimulant treatment is not usually very spectacular and ranges from just a little to 20%, but much higher values are also reported, as in the case of feverfew and ginseng plants in which the application of Atonik results in the increase in biomass of 54 and 51.5% (fresh weight and dry matter) and 43 and 61% (fresh weight of roots and aboveground organs) respectively ( Gruszczyk and Berbeć, 2004 ; Kołodziej, 2004 ).
The increased biomass accumulation after Atonik application usually resulted in a higher yield. In this study the biostimulant increased the yield of oilseed rape, but only when it was applied as a single spray. This has also been shown in oilseed rape by Budzyński et al. (2008) and Malarz et al. (2008) , as well as in many other species, such as beetroot ( Černý et al., 2002 ; Kositorna and Smoliński, 2008 ), potato ( Czeczko and Mikos-Bielak, 2004 ; Sawicka and Mikos-Bielak, 2008 ), cotton ( Djanaguiraman et al., 2005b , 2009 ; Bynum et al., 2007 ), maize ( Michalski et al., 2008 ), soya ( Kozak et al., 2008a ), tomato ( Djanaguiraman et al., 2004a , b ; Gajc-Wolska et al., 2010 ), apple ( Basak and Mikos-Bielak, 2008 ), common chicory ( Černý et al., 2008 ), leek and celery ( Czeczko and Mikos-Bielak, 2004 ), and raspberries ( Grajkowski and Ochmian, 2007 ). Other biostimulants also increase yield and this has been reported for a great number of crops, such as apple, bell pepper, cereals, lettuce, lupine, maize, mustard, nappa cabbage, pea, potato, raspberry, and strawberry ( Abetz and Young, 1983 ; Dobromilska and Gubarewicz, 2008 ; Gajewski et al., 2008 ; Kossak and Dyki, 2008 ; Kowalczyk and Zielony, 2008 ; Matysiak and Kaczmarek, 2008 ; Ochmian et al., 2008 ; Sas-Paszt et al., 2008 ; Stępowska, 2008b ; Wrona and Misiura, 2008 ; Khan et al., 2009 ).
However, there are reported studies showing that biostimulants have either a minor, no influence on yield or even a negative effect. The lack of a positive effect of Atonik on yield has been recorded here in cucumber and earlier reported by Miranda-Stalder et al. (1990) , Csizinszky (2001) , Krawiec (2008) , and Księżak (2008) in strawberries, bell pepper, chokeberries and maize. Basak and Mikos-Bielak (2008) showed that Frigocur, Kelpak, and Help even negatively affect the yield of apples.
The effect of biostimulants on biomass accumulation and yield may depend on a number of environmental factors. In literature the emphasis is on the influence of the cultivar, preparation concentration and term of its application, growing conditions, fertilization employed, and location ( Basak and Mikos-Bielak, 2008 ; Łyszkowska et al., 2008 ; Maciejewski et al., 2008 ; Sas-Paszt et al., 2008 ; Gajc-Wolska et al., 2009 ; 2012 ).
Effect of Atonik on Photosynthetic Apparatus
Stimulated biomass production and yield recorded for many species are attributed to a more efficient photosynthetic apparatus in plants sprayed with Atonik. This has been shown in this study for A. thaliana and oilseed rape. Plants treated with Atonik had higher (i) leaf area, (ii), chlorophyll content, (iii) intensity of photosynthesis, and (iv) values of chlorophyll a fluorescence parameters. In our preliminary studies on wheat also increase in LAI (Leaf Area Index, data not shown) was recorded.
In this work, the biostimulant increased the chlorophyll content in both Brassicaceae species examined and under all experimental conditions. It is worth mentioning that this increase in the case of oilseed rape was more evident at the end of the growing season, which may suggest that Atonik either promotes de novo chlorophyll biosynthesis or slows down its degradation, delaying the aging processes. A similar result was reported by Djanaguiraman et al. (2009) in cotton. A greater chlorophyll content in plants treated with Atonik was recorded also in common osier ( Wróbel and Woźniak, 2008 ), Amaranthus sp. ( Wrochna et al., 2008 ) and cotton ( Djanaguiraman et al., 2009 ). Four different biostimulants increased the content of chlorophyll in bell pepper ( Stępowska, 2008a ). In contrast to the above, Kowalczyk et al. (2008) did not find that Atonik and Aminoplant had a positive effect on the content of chlorophyll in lettuce, and Krajewska and Latkowska (2008) even demonstrated a reduction of chlorophyll content in hosta and bergenia treated with Siapton.
In A. thaliana and oilseed rape plants treated with Atonik, the intensity of photosynthesis was greater, which is in the line with the results of Borowski and Blamowski (2009) , Wróbel and Woźniak (2008) and Djanaguiraman et al. (2009) on basil, common osier and cotton. A new discovery from this study has been that the positive effect of Atonik on the intensity of photosynthesis may last up to 7 weeks, which is much longer than previously believed. According to the manufacturer of Atonik, its working time was estimated to be a maximum of 2–3 weeks. A higher intensity of photosynthesis could be explained, at least partially, by lowered stomatal resistance (or increased stomatal conductance), which ensures an easier and greater CO 2 flow to chloroplast. Increased stomatal conductance has been reported for basil plants treated with Atonik ( Borowski and Blamowski, 2009 ) and cotton with PGR-IV ( Zhao and Oosterhuis, 1997 ). Atonik accelerates the transport of photoassimilates within cells and between them to various tissues and organs ( Yamaki et al., 1953 ). Wilson and Kaczmarek (1993) show that the phosphorylated form of sodium para-nitrophenolate reduces the activity of cation channels (Ca 2+ , K + , and Na + ) by inhibiting the activity of the enzyme tyrosine phosphatase. A decreased activity in the cation channel causes the reduction of Ca 2+ concentration in the cells, which results in the increase of cytoplasm movement ( Roberts and Harmon, 1992 ). The above is in line with Oosterhuis and Robertson (2000) , who suggest that the increased photosynthesis in cotton treated with PGR-IV is related to a quicker transport of assimilates from its source (leaves) to various sinks.
Atonik also has a positive effect on the parameters of chlorophyll a fluorescence. The values of Fv/Fm and P.I were usually higher in Atonik-treated A. thaliana plants. The positive effect of this biostimulant on chlorophyll a fluorescence has previously been reported by Djanaguiraman et al. (2009) in cotton. In contrast to the above, biowska ( Gawlik and Gołębiowska (2008) record decreased values of Fv/Fm in pea plants sprayed with humic acids.
It should be pointed out that although the level of beneficial influence on particular/parameters of the photosynthetic apparatus is not very spectacular, it has to be taken into consideration that they “work additively.” Photosynthesis takes place over several hours a day during most of the sunny days of the vegetation season, which, together with the positive effects on other processes, substantially contributes to greater final plant productivity.
Effect of Atonik on Plant Water Status
Application of Atonik also affects a plant's water status. The lowered stomatal resistance earlier discussed leads to higher intensity of transpiration in A. thaliana and oilseed rape plants, as reported by Wróbel and Woźniak (2008) , Borowski and Blamowski (2009) , and Zhao and Oosterhuis (1997) . Increased transpiration intensity means greater water loss by plants and, as a consequence, it can be expected that RWC should be lower, especially in A. thaliana plants grown under drought stress conditions. Contrary to this expectation, RWC was almost unchanged or, in some cases, even slightly higher. This result can be explained by the improved water uptake after Atonik application, as shown here by daily pot weighing, which is related to a better-developed root system, both in terms of length and biomass. Improved RWC in biostimulant-sprayed plants has also been reported by Wrochna et al., (2008) in Amaranthus sp. and Wróbel and Woźniak (2008) in common osier.
It is worth noticing that simultaneously with more efficient water uptake from soil, plants are also taking up more nutrients, as demonstrated by Stutte et al. (1987) and Oosterhuis (2008) .
Effect of Atonik on Plant Quality
Atonik changes the chemical composition of cucumber fruits, positively in the case of soluble solids and phosphorus, but negatively in terms of nitrates and calcium. In literature there is data that the application of Atonik increases the content of carbohydrates ( Czeczko and Mikos-Bielak, 2004 ; Djanaguiraman et al., 2005a ; Kositorna and Smoliński, 2008 ), crude fat ( Malarz et al., 2008 ), amino acids ( Djanaguiraman et al., 2005a ) proteins ( Czeczko and Mikos-Bielak, 2004 ; Djanaguiraman et al., 2005a ; Oosterhuis, 2008 ), but decreases the level of nitrates ( Kowalczyk et al., 2008 ). On the other hand, Atonik may decrease the concentration of vitamin C ( Czeczko and Mikos-Bielak, 2004 ; Grajkowski and Ochmian, 2007 ).
Effect of Atonik on the Mitigation of Stress Effects
There is common opinion that Atonik mitigates effect of stress conditions. This study proved that the application of Atonik diminished the negative impact of drought and noble metal stresses in A. thaliana and enhanced the recovery from the late spring frost in oilseed rape. A. thaliana plants grown with a water deficit and Pt stresses and treated with a biostimulant had accelerated growth and development, accumulated more biomass and all studied physiological processes were stimulated. Protective effect of Atonik was especially evident in the case of photosynthetic apparatus. For example in oilseed rape grown in the 2007 season, when the late spring frost occurred, Atonik improved chlorophyll a fluorescence parameters. Chlorophyll a fluorescence is informative tool to analyze and understand plant's response to fluctuations in environmental conditions. Higher intensity of photosynthesis was recorded in A. thaliana plants grown under drought conditions. One of the first responses of plants to drought stress is the closing of stomata, a process controlled by, among other, ABA ( Blatt, 2000 ; Schroeder et al., 2001 ; Shinozaki and Yamaguchi-Schinozaki, 2007 ). A decreased level of free ABA after application of Atonik has been shown in other studies conducted by the authors in A. thaliana plants grown with water deficit ( Przybysz et al., 2010 ). Changes in ABA regulation by lowering its concentration resulted in more efficient gas exchange and stimulated growth in stress conditions recorded in this work.
More evidence that Atonik protects plants against the negative effects of stress was shown in this work in the decreased level of plasma membrane injuries caused by Pt, both in the roots and leaves of A. thaliana . Similar results were obtained in previous work on plants exposed to Cd 2+ ( Gawrońska et al., 2008 ). The reduction of membrane injuries in the case of both metals was more pronounced in roots, which were in direct contact with toxic elements. A decrease in plasma membrane injuries has also been found in Atonik-treated Amaranthus sp. ( Wrochna et al., 2008 ), basil ( Borowski and Blamowski, 2009 ), and cotton ( Djanaguiraman et al., 2009 ).
The protective role of Atonik has also been recorded in the case of heavy metals in the example of Cd 2+ ( Gawrońska et al., 2008 ), salinity ( Wrochna et al., 2008 ), spring frost ( Basak and Mikos-Bielak, 2008 ), and heat ( Gulluoglu et al., 2006 ). Górnik et al. (2007) and Górnik and Grzesik (2008) recorded an increased tolerance of grape cuttings to extreme temperatures and water deficit after treatment with a few biostimulants. Since many defense mechanisms against different unfavorable conditions, especially of abiotic origin, are very much the same, it can be assumed that Atonik probably also decreases the negative effects of other stresses not mentioned in this work.
Most of the stresses may induce the appearance of excessive amounts of reactive oxygen species (ROS) and consequently the exacerbation of oxidative stress ( Iturbe-Ormaetxe et al., 1998 ). Discussed above reduction of membrane injuries may reduce creating of ROS in plants. Moreover, it has been reported that the application of Atonik contributes to a decreased level of oxidative stress by increasing (i) the activity of anti-oxidizing system enzymes: ascorbate peroxidase, catalase, glutathione reductase, and (ii) total antioxidative capacity to a greater extent than the increase in anion-radical level ( Wrochna et al., 2008 ; Djanaguiraman et al., 2004a , 2005a , b , 2009 ). Atonik also positively affects the production of proline and polyols, two important compatible metabolites involved in anti-stress mechanisms ( Djanaguiraman et al., 2004b , 2009 ).
All changes presented above have probably their origin in modified, after Atonik treatment, profile of gene expression. In the literature some studies report that after the application of biostimulants expression of genes related to defense mechanism is upregulated. The treatment of A. thaliana plants exposed to freezing stresses with algae extract result in changes of expression in about 5% (1113) of all A. thaliana genes ( Nair et al., 2012 ). About 2% (463 genes) of the differentially expressed genes are upregulated and 3% (650 genes) downregulated. The authors report that some of these genes were involved in the plant's defense mechanisms ( Nair et al., 2012 ). The application of algal extracts prior to pathogen infection in alfalfa cause upregulation of 152 genes, mostly plant defense genes, such as those involved in phytoalexin, PR proteins, cell wall proteins, and oxylipin pathways ( Cluzet et al., 2004 ). In A. thaliana grown under salt stress and treated with Aminoplant, Cambri et al. (2008) demonstrate changes in expression of a few genes responsible for the plant's defense mechanisms.
There is a commonly held view, as also demonstrated in this work, that the positive impact of biostimulants is more evident and that the potential of these compounds can be fully exploited only when plants are grown under stressful conditions, while under optimal conditions their positive effect is sometimes marginal ( Budzyński et al., 2008 ; Krawiec, 2008 ; Maciejewski et al., 2008 ) or even not reported at all ( Csizinszky, 2001 ). Possible protective effect of biostimulants depends also on many other, not discussed here factors, mostly the level and duration of stresses and moment of Atonik application.
Conclusions
The biostimulant Atonik affects every level of a plant's biological organization in terms of structure and function, from canopy and whole plant, via particular organs and cells, to physiological and biochemical processes.
(1) Atonik stimulates plant growth and development, particularly generative.
(2) Biomass accumulation, both fresh weight and dry matter, and yield production are stimulated by Atonik due to a higher efficiency of the photosynthetic apparatus manifested by (i) a higher leaf area, (ii) a higher chlorophyll content, (iii) greater intensity of photosynthesis, and (iv) an improvement of chlorophyll a fluorescence parameters.
(3) Despite higher transpiration and lower stomatal resistance, RWC was unchanged in Atonik-treated plants due to the promotion of root development and consequently an increased water uptake.
(4) The effect of Atonik on the quality and chemical composition of fruits was diverse and depended on the parameter measured and cultivar examined.
(5) The application of Atonik played simulative role under optimal conditions and protective against spring frost, drought, and noble metal stresses.
(6) The positive effect of Atonik is much more pronounced when plants are growing under stress conditions.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study has been supported by Arysta LifeScience Poland Ltd., Asahi Chemical Mfg. Co. Ltd., Japan and Warsaw Plant Health Initiative FP7-REGPOT-2011-1-286093.
The authors would like to thank the anonymous Reviewers and Editors for their time and effort in improving the quality of the paper.
Arkadiusz Przybysz conducted the experiments, collected, and analyzed data on oilseed rape and Arabidopsis and wrote the first version of the manuscript. Helena Gawrońska designed the experiments, analyzed the data on oilseed rape and Arabidopsis and corrected the manuscript. Janina Gajc-Wolska conducted the experiments, collected, and analyzed the data on cucumber and corrected the manuscript.
Abetz, P., and Young, C. L. (1983). The effect of seaweed extract sprays derived from Ascophyllum nodosum on lettuce and cauliflower crops. Bot. Mar . 26, 487–492. doi: 10.1515/botm.1983.26.10.487
CrossRef Full Text | Google Scholar
Arnon, D. I., and Hoagland, D. R. (1940). Crop production in artificial culture solutions and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci . 50, 463–471.
Google Scholar
Basak, A., and Mikos-Bielak, M. (2008). “The use of some biostimulators on apple and pear trees,” in Monographs Series: Biostimulators in Modern Agriculture: Fruit Crops , ed A. Sadowski (Warsaw: Editorial House Wieś Jutra), 7–17.
Bečka, D., Vašák, J., Kroutil, P., and Ŝtranc, P. (2004). Autumn growth and development of different winter oilseed rape variety types at three inputs levels. Plant Soil Environ . 50, 168–174.
Błaszczyk, J. (2008). “Quality of ‘conference’ pears trees as affected by Goëmar BM 86 and fruton,” in Monographs Series: Biostimulators in Modern Agriculture: Fruit Crops , ed A. Sadowski (Warsaw: Editorial House Wieś Jutra), 18–24.
Blatt, M. R. (2000). Cellular signaling and volume control in stomatal movements in plants. Annu. Rev. Cell Dev. Biol . 16, 221–241. doi: 10.1146/annurev.cellbio.16.1.221
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Borowski, E., and Blamowski, Z. K. (2009). The effects of triacontanol ‘TRIA’ and Asahi SL on the development and metabolic activity of sweet basil ( Ocimum basilicum L.) plants treated with chilling. Folia Hort 21, 39–48. doi: 10.2478/fhort-2013-0124
Bray, E. A., Bailey-Serres, J., and Weretilnyk, E. (2000). “Response to abiotic stresses,” in Biochemistry and Molecular Biology of Plants , eds W. Gruissem, B. Buchanan and R. Jones (Rockville, MD: American Society of Plant Physiologists), 1158–1249.
Budzyński, W., Dubis, B., and Jankowski, A. (2008). “Response of winter oilseed rape to the biostymulator Asahi SL applied in spring,” in Monographs Series: Biostimulators in Modern Agriculture: Field Crop , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 47–55.
Bynum, J. B., Cothren, J. T., Lemon, R. G., Fromme, D. D., and Boman, R. K. (2007). Field evaluation of nitrophenolate plant growth regulator (Chaperone) for the effect on cotton lint yield. J. Cotton Sci . 11, 20–25.
Calvo, P., Nelson, L., and Kloepper, J. W. (2014). Agricultural uses of plant biostimulants. Plant soil 383, 3–41. doi: 10.1007/s11104-014-2131-8
Cambri, D., Filippino, L., Apone, F., Arciello, S., Colucci, G., and Portoso, D. (2008). “Effect of Amonoplant® on expression of selected genes in Arabidopsis thaliana L. plants,” in Monographs Series: Biostimulators in Modern Agriculture: General Aspects , ed H. Gawrońska (Warsaw: Editorial House Wieś Jutra), 77–82.
Černý, I., Pačuta, V., Feckova, J., and Golian, J. (2002). Effect of year and Atonik application on the selected sugar beet production and quality parameters. J. Central Eur. Agric . 3, 15–21.
Černý, I., Pačuta, V., and Kovar, M. (2008). Yield and quality of chicory ( Cichorium intybus L.) in dependence on variety and foliar application of Atonik and Polybor 150. J. Central Eur. Agric . 9, 425–430.
Cluzet, S., Torregrosa, C., Jacquet, C., Lafitte, C., Fournier, J., Mercier, L., et al. (2004). Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from the green alga Ulva spp. Plant Cell Environ . 27, 917–928. doi: 10.1111/j.1365-3040.2004.01197.x
Csizinszky, A. A. (2001). Yield Response of Bell Pepper Cultivars to Foliar-Applied ‘Atonik’ Biostimulant . Bradenton: Horticultural Sciences Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida, HS819.
Czeczko, R., and Mikos-Bielak, M. (2004). Effects of Asahi bio-stimulator application in the cultivation of different vegetable species. Annales UMCS Sec. E 59, 1073–1079.
Davies, P. J. (1987). Plant Hormones and their Role in Plant Growth and Development . Dordrecht: Martinus Nijhoff Publishers. doi: 10.1007/978-94-009-3585-3
Djanaguiraman, M., Devi, D. D., Shanker, A. K., Sheeba, J. A., and Bangarusamy, U. (2004b). The role of nitrophenol on delaying abscission of tomato flowers and fruit. Food Agric. Environ . 2, 183–186.
Djanaguiraman, M., Devi, D. D., Sheeba, J. A., Bangarusamy, U., and Babu, R. C. H. (2004a). Effect of oxidative stress on abscission of tomato fruits and its regulation by nitrophenols. Trop. Agric. Res . 16, 25–36.
Djanaguiraman, M., Sheeba, J. A., Devi, D. D., and Bangarusamy, U. (2005a). Effect of Atonik seed treatment on seedling physiology of cotton and tomato. J. Biol. Sci . 5, 163–169. doi: 10.3923/jbs.2005.163.169
Djanaguiraman, M., Sheeba, J. A., Devi, D. D., and Bangarusamy, U. (2005b). Response of cotton to Atonik and TIBA for growth, enzymes and yield. J. Biol. Sci . 5, 158–162. doi: 10.3923/jbs.2005.158.162
Djanaguiraman, M., Sheeba, J. A., Devi, D. D., and Bangarusamy, U. (2009). Cotton leaf senescence can be delayed by nitrophenolate spray through enhanced antioxidant defence system. J. Agron. Crop Sci . 195, 213–224. doi: 10.1111/j.1439-037X.2009.00360.x
Dobromilska, R., and Gubarewicz, K. (2008). “Influence of Bio-algeen S-90 on the yield and quality of small-sized tomato,” in Monographs Series: Biostimulators in Modern Agriculture: Solanaceous Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 7–12.
Gajc-Wolska, J., Kowalczyk, K., Nowecka, M., Mazur, K., and Metera, A. (2012). Effect of organic-mineral fertilizers on the field and quality of endive ( Cichorium endivia L.). Acta Sci Pol. Hortorum Cultus 11, 189–200.
Gajc-Wolska, J., Łyszkowska, M., and Zielony, T. (2010). The influence of grafting and biostimulators on the yield and fruit quality of greenhouse tomato cv. (Lycopersicon esculentum Mill.) grown in the field. Veget. Crops Res. Bull . 72, 63–70. doi: 10.2478/v10032-010-0006-y
Gajc-Wolska, J., Radzanowska, J., and Łyszkowska, M. (2009). The influence of grafting and biostimulators on physical and sensorial traits of greenhouse tomato fruit. (Lycopersicum esculentum Mill.) in field production. Acta Sci. Pol. Hortorum. Cultus 8, 37–43.
Gajewski, M., Gos, K., and Bobruk, J. (2008). “The influence of Goëmar Goteo biostimulator on yield and quality of two Chinese cabbage cultivars,” in Monographs Series: Biostimulators in Modern Agriculture: Vegetable Crops , ed Z. T. Dąbrowski (Editorial House Wieś Jutra), 21–27.
Gawlik, A., and Gołębiowska, D. (2008). “The influence of humic acids on growth of ‘Ramrod’ pea ( Pisum sativum L.) plants,” in Book of Abstracts of the Conference: Biostimulators in Modern Agriculture 7-8.02 (Warsaw).
Gawrońska, H., Przybysz, A., Szalacha, E., and Słowiński, A. (2008). “Physiological and molecular mode of action of Asahi SL biostymulator under optima and stress conditions,” in Monographs Series: Biostimulators in Modern Agriculture: General Aspects , ed H. Gawrońska (Editorial House Wieś Jutra), 54–76.
Górnik, K., and Grzesik, M. (2002). Effect of Asahi SL on China aster ‘Aleksandra’ seed yield, germination and some metabolic events. Acta Physiol. Plant 24, 379–383. doi: 10.1007/s11738-002-0033-5
Górnik, K., and Grzesik, M. (2005). China aster plant growth, seed yield and quality as influenced by Asahi SL treatment. Folia Hortic . 17, 119–127.
Górnik, K., and Grzesik, M. (2008). “Improvement of rooting and development of grapevine cuttings by Asahi SL, Biochikol 020PC, Tytanit, Citrosept and Biosept,” in Monographs Series: Biostimulators in Modern Agriculture: Fruit Crops , ed A. Sadowski (Warsaw: Editorial House Wieøe Jutra), 31–41.
Górnik, K., Grzesik, M., and Mika, A. (2007). Improvement of grapevines footing and growth of plants under stress conditions by Asahi SL. Folia Hortic . 19, 57–67.
Grajkowski, J., and Ochmian, I. (2007). Influence of three biostimulants on yielding and fruit quality of three primocane raspberry cultivars. Acta Sci. Pol. Hortorum Cultus 6, 29–36.
Gruszczyk, M., and Berbeć, S. (2004). The effect of foliar application of some preparations on yield and quality of feverfew ( Chrysanthemum parthenium L.) row material. Ann. UMCS Sec. E 59, 755–759.
Gulluoglu, L., Arioglu, H., and Arslan, M. (2006). Effects of some plant growth regulators and nutrient complexes on above-ground biomass and seed yield of soybean grown under heat-stressed environment. J. Agron . 5, 126–130. doi: 10.3923/ja.2006.126.130
Harasimowicz-Hermann, G., and Czyż, K. (2008). “Effect of Asahi SL on the initial development of willow cuttings at varied soil moisture,” in Monographs Series: Biostimulators in Modern Agriculture: Ornament and Special Plants , ed A. Łukaszewska (Warsaw: Editorial House Wieś Jutra), 40–46.
Iturbe-Ormaetxe, I., Escuredo, P. R., Arrese-Igor, C., and Becana, M. (1998). Oxidative damage in pea exposed to water deficit or paraquat. Plan Physiol . 116, 173–181. doi: 10.1104/pp.116.1.173
Khan, W., Rayirath, U. P., Subramanian, S., Jithesh, M. N., Rayorath, P., Hodges, D. M., et al. (2009). Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul . 4, 386–399. doi: 10.1007/s00344-009-9103-x
Koizumi, S., Maruyama, A., and Fulio, T. (1990). Purification on characterisation of ascorbic acid phosphorylating enzyme from Pseudomonas azotocolligans. Agric. Biol. Chem . 54, 3235–3239. doi: 10.1271/bbb1961.54.3235
Kołodziej, B. (2004). Wpływ Atoniku oraz nawożenia dolistnego na plonowanie i jakość surowca żeń-szenia amerykańskiego ( Panax quinquefolium L.). Ann. UMCS Sec. E 59, 157–162.
Kołodziej, B. (2008). The effect of plantation establishment method and Atonik application in goldenrod ( Solidago virgaurea L. ssp. virgaurea) cultivation. Acta Sci. Pol Hortorum Cultus 7, 33–39.
Kositorna, J., and Smoliński, M. (2008). “Asahi SL biostimulator in protection of sugar beet from herbicide stress,” in Monographs Series: Biostimulators in Modern Agriculture: Field Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 41–49.
Kossak, K., and Dyki, B. (2008). “Effects of biostimulators on culture of Alboney F1 greenhouse tomato,” in Monographs Series: Biostimulators in Modern Agriculture: Solanaceous Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 13–20.
Kowalczyk, K., and Zielony, T. (2008). “Effect of Goteo treatment on yield and fruit quality of tomato grown on rockwool,” in Monographs Series: Biostimulators in Modern Agriculture: Solanaceous Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 21–26.
Kowalczyk, K., Zielony, T., and Gajewski, M. (2008). “Effect of Aminoplant and Asahi SL on yield and quality of lettuce grown on rockwool,” in Monographs Series: Biostimulators in Modern Agriculture: Vegetable Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 35–43.
Kozak, M., Malarz, W., Serafin-Andrzejewska, M., and Kotecki, A. (2008a). “The effect of sowing rate and Asahi SL biostimulator on soybean growth and yield,” in Monographs Series: Biostimulators in Modern Agriculture: Field Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 77–84.
Kozak, M., Malarz, W., Serafin-Andrzejewska, M., and Kotecki, A. (2008b). “The effect of different sowing rate and Asahi SL treatment on soybean sowing value,” in Monographs Series: Biostimulators in Modern Agriculture: Field Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 85–91.
Krajewska, J., and Latkowska, M. J. (2008). “Effects of biostimulants Asahi SL and Siapton 10L on the growth of Bergenia cordifolia ((Haw.) Sternb.) ‘Rotblum’ and Hosta sp. (Tratt.) ‘Sum and Substance’ and ‘Minuteman’,” in Monographs Series: Biostimulators in Modern Agriculture: Ornament and Special Plants , ed A. Łukaszewska (Warsaw: Editorial House Wieś Jutra), 33–39.
Krawczyk, R., and Skoczyński, J. (2008). “Winter survival and yield of oilseed rape depending on sowing date and application of micronutrient preparation Route® acting as a growth stimulator,” in Monographs Series: Biostimulators in Modern Agriculture: Field Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 33–40.
Krawiec, P. (2008). “Effects of biostimulators on growth, cropping and fruit quality of chokeberry,” in Monographs Series: Biostimulators in Modern Agriculture: Fruit Crops , ed A. Sadowski (Warsaw: Editorial House Wieś Jutra), 42–48.
Krok, K., and Wieniarska, J. (2008). “Effect of Goëmar BM 86 application on development and quality of primocane raspberry fruits,” in Monographs Series: Biostimulators in Modern Agriculture: Fruit Crops , ed A. Sadowski (Warsaw: Editorial House Wieś Jutra), 49–59.
Księżak, J. (2008). “Effect of biostimulator Asahi SL on yield of maize grain,” in Monographs Series: Biostimulators in Modern Agriculture: Field Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 60–65.
Libbenga, K. R., and Mennes, A. M. (1987). “Hormone binding and its role in hormone action,” in Plant Hormones and their Role in Plant Growth and Development , ed P.J. Davies (Dordrecht: Martinus Nijhoff Publishers), 194–221. doi: 10.1007/978-94-009-3585-3_11
Łyszkowska, M., Gajc-Wolska, J., and Kubiś, K. (2008). “The influence of biostimulator on yield and quality of leaf and iceberg lettuce – grown under field conditions,” in Monographs Series: Biostimulators in Modern Agriculture: Vegetable Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 28–34.
Maciejewski, T., Michalski, T., Bartos-Spychała, M., and Cieślicki, W. (2008). “Effect of the application of the biostimulator Asahi SL on the yield of potato tubers and their quality,” in Monograph Series: Biostimulators in Modern Agriculture: Solanaceous Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 52–60.
Malarz, W., Kozak, M., and Kotecki, A. (2008). “The use of Asahi SL biostimulator in spring rape growing,” in Monographs Series: Biostimulators in Modern Agriculture: Field Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 25–32.
Matysiak, K., and Kaczmarek, S. (2008). “Potential advantages of Kelpak bioregulator applied to some field crops,” in Monographs Series: Biostimulators in Modern Agriculture: Field Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 99–108.
Michalski, T., Bartos-Sychała, M., Maciejewski, T., and Jarosz, A. (2008). “Effect of biostymulator Asahi SL on cropping of maize grown for grain,” in Monographs Series: Biostimulators in Modern Agriculture: Field Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 66–76.
Miranda-Stalder, S. H., Gloria, B. A., and Castro, P. R. C. (1990). Effect of growth regulators on morphological characteristics and productivity of strawberry “Sequoia'. An Esc Super Agric, Luiz de Queiroz 47, 317–334. doi: 10.1590/S0071-12761990000200005
Nair, P., Kandasamy, S., Zhang, J., Ji, X., Kirby, C., Benkel, B., et al. (2012). Transcriptional and metabolomic analysis of Ascophyllum nodosum mediated freezing tolerance in Arabidopsis thaliana . BMC Genomics 13:643. doi: 10.1186/1471-2164-13-643
Ochmian, I., Grajkowski, J., and Skupień, K. (2008). “Influence of three biostimulators on growth, yield and fruit chemical composition of ‘Polka’ raspberry,” in Monographs Series: Biostimulators in Modern Agriculture: Fruit Crops , ed A. Sadowski (Warsaw: Editorial House Wieś Jutra), 68–77.
Oosterhuis, D. (2008). “Atonik™ biostimulators for increased nitrogen, protein and yield of cotton,” in Book of Abstracts of the Conference Biostimulators in Modern Agriculture 7-8.02 (Warsaw), 18.
Oosterhuis, D., and Robertson, W. C. (2000). “The use plant growth regulators and other additives in cotton production,” in Arkansas Agricultural Experiment Station Special Report 198, Proceedings of the 2000 Cotton Meeting (Fayetteville, AR), 22–32.
Panajotov, N. D., Jevtic, S., and Lazic, B. (1997). Sweet pepper response to the application of the plant growth regulator Atonic. Acta Hortic . 462, 197–202.
Przybysz, A., Janowiak, F., Słowiński, A., and Gawrońska, H. (2010). Protective role of Asahi SL against drought stress. Zeszyty Problemowe Postępów Nauk Rolniczych PAN 545, 199–223.
Roberts, D. M., and Harmon, A. C. (1992). Calcium-modulated proteins: targets of intracellular calcium signals in higher plants. Annu. Rev. Plant Phys . 43, 375–414. doi: 10.1146/annurev.pp.43.060192.002111
Sas-Paszt, L., Żurawicz, E., Masny, A., Filipczak, J., Pluta, S., Lewandowski, M., et al. (2008). “The use of biostimulators in small fruit growing,” in Monograph Series: Biostimulators in Modern Agriculture: Field Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 76–90.
Sawicka, B., and Mikos-Bielak, M. (2008). “Modification of potato tuber chemical composition by applications of the Asahi SL biostimulator,” in Monograph series: Biostimulators in Modern Agriculture: Solanaceous Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 61–67.
Schroeder, J. I., Allen, G. J., Hugouvieux, V., Kwak, J. M., and Waner, D. (2001). Guard cell signal transduction. Annu. Rev. Plant Phys . 52, 627–658. doi: 10.1146/annurev.arplant.52.1.627
Sharma, R., Sharma, B., and Singh, G. (1984). Phenols as regulators of nitrate reductase activity in Cicer arietiman . Φ YTON 44, 185–188.
Shinozaki, K., and Yamaguchi-Schinozaki, K. (2007). Gene networks involved in drought stress response and tolerance. J. Exp. Bot . 58, 221–227. doi: 10.1093/jxb/erl164
Siedlecka, A., and Krupa, Z. (2002). Simple method of Arabidopsis thaliana cultivation in liquid nutrient medium. Acta Physiol. Plant 24, 163–166. doi: 10.1007/s11738-002-0007-7
Stępowska, A. (2008a). “Biostimulators in sweet pepper cultivation under covers,” in Monographs Series: Biostimulators in Modern Agriculture: Solanaceous Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 36–44.
Stępowska, A. (2008b). “Effect of GA 142 (Goëmar Goteo) and (Goëmar BM 86) extracts on sweet pepper yield in non-heated tunnels,” in Monographs Series: Biostimulators in Modern Agriculture: Solanaceous Crops , ed Z. T. Dąbrowski (Warsaw: Editorial House Wieś Jutra), 45–51.
Stutte, C. A., and Clark, T. H. (1990). Radiolabeled Studies of Atonik in Cotton using HPLC . Arysta LifeScience Report. Fayetteville, AR: Altheimer Laboratory, University of Arkansas.
Stutte, C. H. A., Urwiler, M. J., and Clark, T. H. (1987). Laboratory and Field Evaluation of Atonik on Cotton . Arysta LifeScience Report. Fayetteville, AR: University of Arkansas.
Taiz, L., and Zeiger, E. (2002). Plant Physiology . Sunderland: Sinauer Associates, Inc., Publishers.
Wilson, G. F., and Kaczmarek, L. K. (1993). Mode-switching of voltage-gated cation channel is mediated by a protein kinase A-regulated tyrosine phosphatase. Nature 366, 433–438. doi: 10.1038/366433a0
Wróbel, J., and Woźniak, A. (2008). “The effect of Atonik plant growth stimulator on physiological indicators of the basket willow ( Salix viminalis L.) cultivated in anthropogenic soil,” in Monographs Series: Biostimulators in Modern Agriculture: Ornament and Special Plants , ed A. Łukaszewska (Warsaw: Editorial House Wieś Jutra), 47–55.
Wrochna, M., Łata, B., Borkowska, B., and Gawrońska, H. (2008). “The effect Asahi SL of biostimulators on ornament amaranth ( Amaranthus sp.) plants exposed to salinity in growing medium,” in Monographs Series: Biostimulators in Modern Agriculture: Ornament and Special Plants , ed A. Łukaszewska (Warsaw: Editorial House Wieś Jutra), 15–32.
Wrona, D., and Misiura, M. (2008). “Effect of Goëmar BM 86 on yield and quality,” in Monographs Series: Biostimulators in Modern Agriculture: Fruit Crops , ed A. Sadowski (Warsaw: Editorial House Wieś Jutra), 91–96.
Yamaki, T., Nakasawa, K., Nakamura, K., Terakawa, H., and Hayashi, T. (1953). A plant physiological study of Atonik (Advance Report). Agric. Hortic . 28, 1–3.
Zhao, D., and Oosterhuis, D. (1997). Physiological response of growth chamber-grown cotton plants to the plant growth regulator PGR-IV under water-deficit stress. Environ. Exp. Bot . 38, 7–14. doi: 10.1016/S0098-8472(97)00002-6
Keywords: biomass accumulation, efficiency of photosynthetic apparatus, growth and development, nitrophenolates, water status, yield, yield parameters
Citation: Przybysz A, Gawrońska H and Gajc-Wolska J (2014) Biological mode of action of a nitrophenolates-based biostimulant: case study. Front. Plant Sci . 5 :713. doi: 10.3389/fpls.2014.00713
Received: 16 July 2014; Accepted: 27 November 2014; Published online: 16 December 2014.
Reviewed by:
Copyright © 2014 Przybysz, Gawrońska and Gajc-Wolska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arkadiusz Przybysz, Laboratory of Basic Research in Horticulture Faculty of Horticulture, Biotechnology and Landscape Architecture, Warsaw University of Life Sciences – SGGW, Nowoursynowska 159, Warsaw 02-776, Poland e-mail: [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- K-State home
- Horticulture & Natural Resources
Plant Nutrition Case Studies
The following case studies focus on plant nutrient management problems and are used in the course Plant Nutrition and Nutrient Management (HORT 815 at Kansas State University and HORT/AGRO 424/824 at the University of Nebraska-Lincoln). Contact Kimberly Williams for the solutions if you are an instructor who would like to use these case studies in your class.
Horticulture Scenarios
- The Case of the Purpling Geraniums (pdf)
- Why are the Edges of the Geranium Foliage Turning Crispy? (pdf)
- A Golf Course's Battle with Diseased Bermudagrass (pdf)
- The Case of the Scorched Cut Chrysanthemum (pdf)
- Why are the Begonia Plugs Dying? (pdf)
- The Greenhouse's Battle with Diseased Gloxinia (pdf)
Agronomy Scenarios
- Is Raptor to Blame for the Yellow Soybeans? (pdf)
- Why is the Growth of this Corn so Variable? (pdf)
- The Case of the Yellow and Twisted Maize (pdf)
- The Case of the Stunted Corn (pdf)
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
UP Class 7th Science
Course: up class 7th science > unit 6.
- Unit test Nutrition in plants
Nutrition in Plants Important Questions Class 7 Science Chapter 1
Nutrition in Plants Class 7 Science Chapter 1 Important Questions and Answers are provided here. We prepared these extra questions based on the latest NCERT Class 7 Science Book. These important questions will help you to properly understand a particular concept of the chapter. Practicing class 7 important questions before the exam will help you to get excellent marks in the exam.
Class 7 Science Chapter 1 Nutrition in Plants Important Questions
Very short answer type question.
1: Name some components of food. Answer: Carbohydrates, proteins, fats, vitamins and minerals.
2: Define nutrients. Answer: Carbohydrates, proteins, fats, vitamins and minerals are essential components of food, these components are called nutrients.
3: Give an example of autotrophs. Answer: All green plants.
4: Give an example of heterotrophs. Answer: Animals and human beings.
5: Plants prepare their food by using raw materials present in ___________. Answer: Surrounding
6: What do you mean by nutrition? Answer: Nutrition is the mode of taking food by an organism and its utilisation by body.
7: Name the food factories of plants. Answer: Leaves
8: Name the tiny pores present on the surface of leaves. Answer: Stomata
9: Name the green pigment present in leaves. Answer: Chlorophyll
10: ____________ helps leaves to capture the energy of sunlight Answer: Chlorophyll
11: Why photosynthesis is named so? Answer: Because the synthesis of food occurs in presence of sunlight.
12: Sun is the ultimate source of energy for all living organisms. True / False Answer: True
13: Where does the nucleus of cell lies? Answer: In the centre of cell.
14: State the equation for the process of photosynthesis. Answer: Carbon dioxide + water → carbohydrate + Oxygen
15: The nucleus in a cell is surrounded by a jelly like substance called ___________. Answer: Cytoplasm
16: Why algae present in stagnant water bodies are green in colour? Answer: Because they contain green colour pigment chlorophyll.
17: Name a component of food other than carbohydrate synthesize by plants. Answer: Proteins and fats
18: Name some insectivorous plants. Answer: Pitcher plants and Venus flytraps are insectivorous plants.
19: During photosynthesis plants take in ____________ and releases ____________. Answer: Carbon dioxide, oxygen
20: Some organisms live together and share shelter and nutrients, this type of relationship is called Answer: Symbiotic relationship.
21: Lichen is a symbiotic association between __________ and fungi. Answer: algae
22: Name the edible fungi. Answer: Mushroom.
23: Name the organism responsible for converting atmospheric nitrogen into soluble forms. Answer: Rhizobium bacteria.
24: Give an example of parasites. Answer: Cuscuta plants.
25: Give an example of saprotrophs.
Answer: Fungi
26: Carbon dioxide is released during photosynthesis. True/ False. Answer: False
27: During photosynthesis solar energy is converted into chemical energy. True/ False. Answer: True
28: Name a plant that has both autotrophic and heterotrophic mode of nutrition. Answer: Insectivorous plants
29: Name a parasitic plant with yellow, slender and tubular type of stem. Answer: Amarbel
30: Name the pores present in leaves through which exchange of gas takes place. Answer: Stomata
31: Animals are autotrophs. True/ False. Answer: False
Short Answer Type Questions
1. What is Nutrients?
Answer: Carbohydrates, proteins, fats, vitamins and minerals are components of food. The chemical substance present in components of food is necessary for our body and is called nutrients.
2. How humans and animals are directly or indirectly dependent on plants.
Answer: All living organisms require food. Plants can make their food themselves but animals including humans cannot. They get it from plants or animals that eat plants. Thus, humans and animals are directly or indirectly dependent on plants.
3. What is food?
Answer: Food is the source of energy and every cell of an organism gets energy by the breakdown of glucose. The cells use this energy to carry out vital activities of life.
4. Why do we need food?
Answer: Living organisms need food to build their bodies, to grow, to repair damaged parts of their bodies and provide the energy to carry out life processes.
5. How do plants obtain the raw materials from the surroundings?
Answer: Water and minerals present in the soil are absorbed by the roots and transported to the leaves.
Carbon dioxide from air is taken in through the tiny pores present on the surface of the leaves. Such pores are called stomata. These pores are surrounded by ‘guard cells’.
6. What is cell?
Answer: The bodies of living organisms are made of tiny units called cells therefore Cell are called the building blocks of living organism. Cells can be seen only under the microscope. Some organisms are made of only one cell. They are called Unicellular Ex. Amoeba, Paramecium etc. Living organism made up of many cells are called Multi cellular like man, tree etc.
7. What is the cell membrane?
Answer: The cell is enclosed by a thin outer boundary, called the cell membrane Most cells have a distinct, centrally located spherical structure called the nucleus The nucleus is surrounded by a jelly-like substance called cytoplasm.
8. What is tissue?
Answer: A tissue is a group of cells that perform specialized function in an organism. For example, the vascular tissue for the transport of water and nutrients in the plant is called the xylem.
9. What are the main requirements of photo synthesis?
Answer: Chlorophyll, sunlight, carbon dioxide and water are necessary to carry out the process of Photosynthesis.
10. Explain the process of Photosynthesis?
Answer: Carbon dioxide from air is taken in through stomata. chlorophyll helps leaves to capture the energy of the sunlight. This energy is used to synthesize (prepare) food from carbon dioxide and water. Since the synthesis of food occurs in the presence of sunlight, it is called photosynthesis.
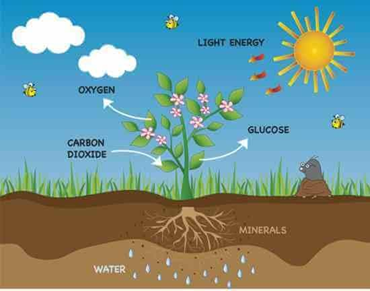
11. Why sun is called the ultimate source of energy for all living organisms?
Answer: The solar energy is captured by the leaves and stored in the plant in the form of food. and this in turn use by other organism to get food to obtain energy Thus, sun is the ultimate source of energy for all living organisms.
12. Why algae are green in colour?
Answer: Algae contain chlorophyll which gives them the green colour. It can also prepare their own food by photosynthesis.
13. What are the main components presents in carbohydrates?
Answer: The main components present in carbohydrates are carbon, hydrogen and oxygen.
14: Differentiate between nutrients and nutrition.
Answer: Carbohydrates, proteins, fats, vitamins and minerals are essential components of food, these components are called nutrients, but Nutrition is the mode of taking food by an organism and its utilisation by the body.
15: Differentiate between autotrophs and heterotrophs.
Answer: Green plants are called autotrophs as they prepare their own food from simple substances, but animals and most other organisms are called heterotrophs as they take in ready-made food prepared by the plants.
16: Explain the food factory of plants.
Answer: Leaves are called food factory of plants, as the synthesis of food takes place in leaves of plants. Water and minerals present in soil are absorbed by roots and transported to leaves via stem. Carbon dioxide from air is taken in through tiny pores on surface of leaves called stomata.
17: How water and minerals are transported to leaves from roots?
Answer: There are vessels inside a plant which run like pipes throughout the root, stem branches and leaves, by going through these vessels water and minerals are transported to leaves from roots.
18: Draw a labelled diagram of cell showing nucleus and cytoplasm. Answer:
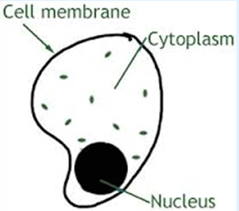
19: Define chlorophyll.
Answer: Chlorophyll is the green colour pigment which helps leaves to capture energy from sunlight to carry out the food making process of plants by the leaves.
20: Explain the role of chlorophyll in the process of photosynthesis.
Answer: Chlorophyll is the green colour pigment which helps leaves to capture energy from sunlight to carry out the food making process of plants by the leaves. It is the green photosynthesis pigment which provides energy necessary for photosynthesis.
21: Define photosynthesis along with the equation for the same.
Answer: Photosynthesis is the food manufacturing process of green plants containing chlorophyll, in presence of sunlight, with the help of carbon dioxide and water to synthesise carbohydrates. The equation for the process is as follow: Carbon dioxide + water —> carbohydrate + Oxygen
22: What is the function of stomata in leaf of a plant?
Answer: Stomata are the tiny pores present on the surface of leaves which helps in exchange of gases, the pores in stomata are surrounded by guard cells.
23: Why do we need food?
Answer: Living organisms need food to build their bodies, to grow, to repair damaged parts of their bodies and provide with energy to carry out life processes.
24: Draw a labelled diagram showing the process of photosynthesis. Answer:
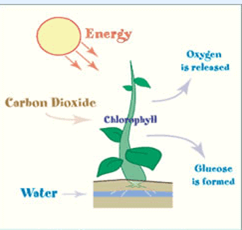
25: Draw diagram of a leaf showing chlorophyll, and stomata in it. Answer:
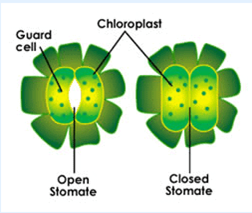
26: What is the cell membrane?
Answer: The cell is enclosed by a thin outer boundary, called the cell membrane Many cells have a distinct, centrally located spherical structure called the nucleus. The nucleus is surrounded by a jelly-like substance called cytoplasm.
27: What are the main requirements of photosynthesis?
Answer: Chlorophyll, sunlight, carbon dioxide and water are necessary to carry out the process of Photosynthesis.
28: Why colours of algae are green?
Answer: Algae contain chlorophyll which gives them green colour and because of chlorophyll it can also prepare their own food by photosynthesis.
29: What are the main components presents in carbohydrates?
Answer: The main components presents in carbohydrates are carbon, hydrogen and oxygen.
30: From where do the plants obtain nitrogen?
Answer: Soil has certain bacteria that convert gaseous nitrogen into a usable form and release it into the soil. These soluble forms are absorbed by the plants along with water. By adding fertilizers rich in nitrogen to the soil farmers also made nitrogen available for plants.
31: Define insectivorous plants along with examples.
Answer: There are few plants which can trap insects and digest them. Such plants may be green or of some other colour. Such insect-eating plants are called insectivorous plants. Example: Venus Flytrap and Pitcher plant.
32: Explain how Pitcher plants get their nutrition?
Answer: When an insect lands in the pitcher, the lid closes and the trapped insect gets entangled into the hair. The insect is digested by the digestive juices secreted in the pitcher.
Long Answer Type Questions
1: Sun is called the ultimate source of energy for all living organisms. Comments.
Answer: The solar energy is very important to carry out the process of photosynthesis, it is captured by the leaves and stored in the plant in the form of food. And this in turn use by other organism to get food to obtain energy Thus, we say that sun is the ultimate source of energy for all living organisms.
2. What is Symbiosis? What is Symbiotic relationship?
Answer: Symbiosis: It is the type of nutrition in which two different kinds of depend on each other for their nutrition. In this both the organisms are benefited by each other Example: Lichen. In this one alga and one fungus live together and remain in symbiotic relationship.
Symbiotic Relationship: Some organisms live together and share shelter and nutrients. This type of relationship is called symbiotic relationship.
3: Explain the two mode of nutrition in plants.
Answer:
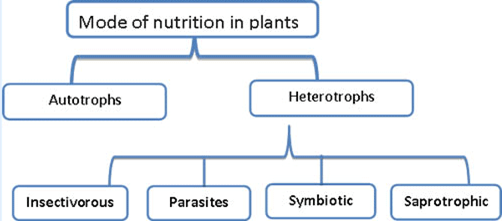
Autotrophs or Autotrophic : – In this mode of nutrition organisms make their food themselves from simple substances. All green plants are Autotrophs (Auto means self and trophs means nourishment)
Heterotrophs or heterotrophic : – Heterotrophic organisms are those who obtain food from other organisms. Since these organisms depend on other organisms for their food, they are called consumers. All animals and non-green plants like fungi come under this category.
4. What are stomata? Explain their function.
Answer: Stomata are tiny pores on the underside of the leaf surface that are surrounded by guard cells. Their functions are to exchange gases by diffusion for photosynthesis and respiration and to cause transpiration by evaporation of water from the leaf surface.
5. How is sunlight used by the plant for photosynthesis?
Answer: Sunlight is the energy source for photosynthesis. It is trapped by the green pigment chlorophyll that is present in the leaves and all green parts pf the plants. The chlorophyll is present in organelles called chloroplasts. Most of the chlorophyll is present in the leaves and therefore leaves are the major site for trapping sunlight to convert it to chemical energy.
6. Explain how photosynthesis occurs in plants.
Answer: Photosynthesis is the process by which solar energy is converted to chemical energy by the green plants. In this process simple inorganic molecules like carbon dioxide and water are used to synthesise organic food like starch. The reaction requires energy from sunlight. Sunlight is trapped by the pigment chlorophyll present in the leaves. The raw materials for photosynthesis are carbon dioxide and water. Carbon dioxide is absorbed from the atmosphere whereas water is absorbed from the soil. The energy from sunlight converts carbon dioxide and water to starch and oxygen. Starch is used as food by plants and other animals whereas the oxygen is released into the atmosphere. The overall reaction of photosynthesis can be represented as follows:
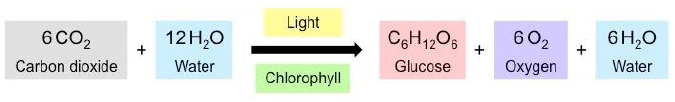
7. How do plants obtain nutrients other than carbohydrates?
Answer: Plants synthesise carbohydrates using energy from sunlight to convert carbon dioxide and water to starch. The other nutrients are however obtained directly from soil. Nitrogen is absorbed as soluble nitrogen compounds from the soil. The nitrogen compounds are present in the soil due to the action of nitrogen fixing bacteria like Rhizobium that live in symbiotic association with roots of leguminous plants. Nitrogen compounds can be replenished by the addition of fertilizers and manure to the soil. Some plants like the pitcher plant and Venus flytrap fulfill their nitrogen requirements by insectivory. In this case the insects are trapped and digested by plant parts and the nutrients are released into the plant body.
8. What is the mode of nutrition in fungi?
Answer: The mode of nutrition in fungi is heterotrophic. They cannot synthesize their own food and are dependent on other ‘organisms’ for their carbon source. They perform extracellular digestion by releasing enzymes into their environment and obtain organic and inorganic nutrients through absorption. There are three main ways of obtaining nutrition: (i) Saprotrophic: Decomposition of ‘dead organic matter’. (ii) Parasitic: Feeding from a living host. (iii) Mutualism: Living in a mutually beneficial interaction with another organism. Example: lichen is a mutualism between fungi and algae).
9. How can we demonstrate that chlorophyll is necessary for photosynthesis?
Answer: Importance of chlorophyll can be demonstrated by using a variegated leaf. The outline of the leaf is traced on a paper and the green areas are marked before the start of the experiment. The leaf is placed in sunlight for few hours to allow photosynthesis. The leaf is then decolourized by boiling in alcohol. To this iodine solution is added. It can be observed that the green areas of the leaf turn blue-black in response to iodine solution indicating the presence of starch. Thus it can be seen that photosynthesis occurs in the green areas of the variegated leaf showing that chlorophyll is important for photosynthesis.
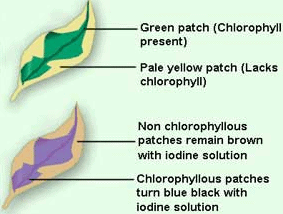
10: Why do organisms need to take food?
Answer: Food is required by all living organisms mainly for four reasons or purposes:
- Food helps a living organism to grow. If enough food is not given or if, the food given is not of right kind, the organism will not have proper growth.
- Another important function of food is to provide energy which is required for any living organism for movements and other activities.
- Food is also needed by living organisms for replacement and repairing of their damaged parts.
- Food provide us the power to fight against infections and diseases.
11: Distinguish between a parasite and a saprotroph. Answer:
12: Give a brief description of the process of synthesis of food in green plants.
Answer: Leaves have a green pigment called chlorophyll. In presence of sunlight, they use carbon dioxide and water to synthesize carbohydrate. During this process oxygen is released. The carbohydrates ultimately get converted into starch. Carbon dioxide from air is taken through stomata. Water and minerals are absorbed by roots and transported to leaves.
13. Whether food is made in all parts of a plant or only in certain parts?
Answer: Only certain part plant like leaves having green pigment chlorophyll. So Leaves are called the food factories of plants.
Besides leaves, photosynthesis also takes place in other green parts of the plant — in green stems and green branches. The desert plants have scale- or spine-like leaves to reduce loss of water by transpiration. These plants have green stems which carry out photosynthesis.
14. How do the raw materials transport them to the food factories of the plants?
Answer: Plants have pipe-like vessels to transport water and nutrients from the soil. The vessels are made of special cells, forming the vascular tissue. The vascular tissue for the transport of water and nutrients in the plant is called the xylem. The vascular tissue for the transport of water and nutrients in the plant is called the xylem. Thus, xylem and phloem transport substances in plants.
15. Why are leaves called the food factories of plants? Explain.
Answer: Leaves are called the food factories of plants due to following functions: 1. Green leaves have all the raw materials necessary to carry the process of photosynthesis. 2. They have chlorophyll (green pigment) which captures the energy of sunlight. 3. Leaves consist of tiny pores called stomata on their surface. 4. Carbon dioxide from air is taken in through stomata. 5. Water and minerals are absorbed by the roots from the soil and transported to the leaves by vessels.
16: How would you test the presence of starch in leaves?
Answer: Take a potted plant which has been exposed to sunlight and pluck a leave from the plant. Then boil it in water for 5 min to soften it and then place the leave in a test tube containing alcohol ,place the test tube in a beaker containing water gently heat the beaker till the alcohol dissolves in the chlorophyll and the leaves loses its green colour. Now wash the leaf with water and then place it on a plate and add a few drops of iodine solution the parts that turn blue black show the
17: How humans and animals are directly or indirectly dependent on plants?
Answer: All living organisms require food. Plants can make their food themselves by organic substances but animals including humans cannot make their food themselves. They get it from plants or animals that eat plants. Thus, humans and animals are directly or indirectly dependent on plants.
18: Whether food is made in all parts of a plant or only in certain parts? Explain.
Answer: Only certain parts of plant like leaves have green pigment called chlorophyll. So Leaves are called the food factories of plants. Besides leaves, photosynthesis also takes place in other green parts of the plant like in green stems and green branches. The desert plants have scale or spine like leaves to reduce loss of water by transpiration. These plants have green stems which carry out the process of photosynthesis.
19: What is cell?
Answer: The body of living organisms are made of tiny units called cells, therefore Cell are called the building blocks of living organism. Cells can be seen only under the microscope. Some organisms are made of single cell they are called Unicellular. Ex. Amoeba, Paramecium etc. While others are made of multicells and are called multicellular. Ex. man, tree etc.
20: What is saprotrophic mode of nutrition?
Answer: This mode of nutrition in which organisms take in nutrients in solution form from dead and decaying matter is called saprotrophic nutrition. Plants which use saprotrophic mode of nutrition are called saprotrophs. Example Fungi that secrete digestive juices on the dead and decaying matter and convert it into a solution. Then they absorb the nutrients from it.
21: What do you understand by symbiotic relationship present in some organism?
Answer: Some organisms live together and share shelter and nutrients. This is called symbiotic relationship. E.g. an alga, and a fungus live together fungus provides shelter, water and minerals to the alga and, in return, the alga provides food which it prepares by photosynthesis. In this kind of association both partners are benefited.
22: How nutrients are replenished in soil?
Answer: Nutrients are replenished in soil by following ways:
- By spreading manure or fertilizers that contain nutrients such as nitrogen in the fields
- By the bacterium Rhizobium that is commonly present in rot nodules of leguminous plant that can take atmospheric nitrogen and convert it into a soluble form like nitrates.
23: What do you mean by Symbiosis?
Answer: Symbiosis is the type of nutrition in which two different kinds of organisms depend on each other for their nutrition. In this both the organisms are benefited by each other e.g., lichen is a symbiotic association between algae and fungi. In this one alga and one fungus live together and remain in symbiotic relationship.
24: What is the role of leguminous plants in replenishing soil fertility?
Answer: Rhizobium is a type of bacteria that cannot make its own food and lives in the roots of gram, peas, moong beans and other legumes, it converts atmospheric nitrogen into usable form which increases the fertility of soil, and legumes provide food and shelter to the bacteria.
25: What do you mean by parasitic nutrition?
Answer: The mode of by which parasitic organism get and synthesize their food is called parasitic nutrition. Example Cuscuta. It does not have chlorophyll; it takes readymade food from the plant on which it is climbing. The plant on which it climbs is called a host. In a parasitic nutrition only one of the partners is benefited and other is not.
- NCERT Exemplar
- NCERT Exemplar Class 7
- Class 7 Science
- Chapter 1 Nutrition in Plants
NCERT Exemplar Solutions Class 7 Science Chapter 1 Nutrition in Plants
NCERT Exemplar Solutions for Class 7 Science Chapter 1 Nutrition in Plants play a crucial role in understanding the topic clearly, thereby playing a pivotal role from the perspective of your future studies. Studying this exemplar will help you gain complete knowledge on the topics, and thus, it will aid you in making your basics strong.
These Exemplar Solutions for Class 7 Science have answers to 8 MCQS, 2 very short answer questions, 6 short answer questions and 5 long answer questions from Chapter 1.
Download the PDF of NCERT Exemplar for Class 7 Science Chapter 1 – Nutrition in Plants

NCERT Exemplar for Class 7 Chapter 1 Nutrition in Plants Importance
NCERT Exemplar solutions for Class 7 Science Chapter 1 Nutrition in Plants deal with a variety of questions on the mode of nutrition in plants, how plants fulfil their nutrition requirement, the biochemical process of photosynthesis, their equation, how plants prepare their own food by photosynthesis, diagrammatic representation and pathways. This solution also covers questions on the mode of nutrition in saprophytes, which proves to be an extension of learning nutrition in different organisms.
Sub-topics of Class 7 Science Chapter 1 Nutrition in Plants
1.1 – Mode of Nutrition in Plant
1.2 – Photosynthesis – Food Making Process in Plants
1.3 – Other Modes of Nutrition in Plants
1.4 – Saprotrophs
1.5 – How Nutrients Are Replenished in the Soil
Access Answers to NCERT Exemplar Solutions for Class 7 Science Chapter 1- Nutrition in Plants
Multiple-choice questions.
1. Organisms which prepare food for themselves using simple, naturally available raw materials are referred to as
(a) heterotrophs
(b) autotrophs
(c) parasites
(d) saprophytes
The answer is (b) autotrophs
Explanation:
Organisms which prepare food for themselves using simple, naturally available raw materials are referred to as autotrophs.
Organisms which are dependent on plants for their food are known as heterotrophs.
Parasites are those organisms which live and are dependent on the host for food. They obtain food at the cost of their host.
Saprophytes are organisms which eat dead and decaying matter as food.
2. In the absence of which of the following, will photosynthesis not occur in leaves?
(a) Guard cells
(b) Chlorophyll
(c) Vacuole
(d) Space between cells
The answer is (b) Chlorophyll
The leaves have a green pigment called chlorophyll. It helps leaves to capture the energy of the sunlight. This energy is used to synthesise (prepare) food from carbon dioxide and water.
3. Which of the following statements is/are correct?
(i) All green plants can prepare their own food.
(ii) Most animals are autotrophs.
(iii) Carbon dioxide is not required for photosynthesis.
(iv) Oxygen is liberated during photosynthesis.
Choose the correct answer from the options below:
(a) (i) and (iv)
(b) (ii) only
(c) (ii) and (iii)
(d) (i) and (ii)
Answer is (a) (i) and (iv)
Statement ii) is wrong because animals are heterotrophs.
Statement iii) is wrong because CO2 is necessary for photosynthesis.
4. Pitcher plant traps insects because it
(a) is a heterotroph.
(b) grows in soils which lack nitrogen.
(c) does not have chlorophyll.
(d) has a digestive system like human beings.
The answer is (b) grows in soils which lack nitrogen.
Pitcher plant grows in the soil, which is deficient in Nitrogen. Pitcher plants carry out photosynthesis to produce carbohydrates. But for nitrogen source, it traps and digests the insects.
5. The term that is used for the mode of nutrition in yeast, mushroom and bread-mould is
(a) autotrophic
(b) insectivorous
(c) saprophytic
(d) parasitic
The answer is (c) saprophytic
These are all fungi. They absorb the nutrients from the dead and decaying matter. They secrete digestive juices, which help them to digest the organic material. This type of absorbing nutrition is called the saprophytic mode of nutrition.
6. When we observe the lower surface of a leaf through a magnifying lens, we see numerous small openings. Which of the following is the term given to such openings?
(a) Stomata
The answer is (a) Stomata
Lamina is the green expanded part of the leaf with veins and veinlets. The midrib is the middle prominent vein. Veins provide rigidity to leaves and act as channels for the transport of water, minerals and food materials. Stomata are the minute pores usually located on the underside of the leaves and take part in the exchange of gases (02 and CO2) during photosynthesis and respiration. They are surrounded by guard cells.
7. Two organisms are good friends and live together. One provides shelter, water, and nutrients, while the other prepares and provides food. Such an association of organisms is termed as
(a) saprophyte
(b) parasite
(c) autotroph
(d) symbiosis
The answer is (d) symbiosis
Saprophyte is those which feed on dead and decaying matter. Parasites are organisms which feed on other organisms. Autotrophs are organisms which prepare their own food.
8. Which of the following raw material is available in the air for photosynthesis?
(b) Carbon dioxide
(c) Nitrogen
(d) Hydrogen
The answer is (b) Carbon dioxide
Plants prepare their own food by using Carbon dioxide and water to produce carbohydrates and oxygen. Carbon-di-oxide present in the air is utilized by plants as a carbon source.
Very Short Answer Questions
9. Potato and ginger are both underground parts that store food. Where is the food prepared in these plants?
In the plants, the shoot system and leaves stay above ground. They prepare food by photosynthesis and store energy in the underground part of the plant.
10. Photosynthesis requires chlorophyll, and a few other raw materials. Add the missing raw materials to the list given below: Water, minerals, _____________, ______________.
Sunlight/light energy, (b) carbon dioxide.
Short Answer Questions
11. A goat eats away all the leaves of a small plant (balsam). However, in a few days, new leaves could be seen sprouting in the plant again. How did the plant survive without leaves?
Plants have stored food in their stems and roots. Because of this, plants live for a few days without leaves.
12. Unscramble the following to form terms related to modes of nutrition.
(i) RASPAEIT
(ii) ROPEHYTSAP
(iii) TOROPHAUT
(iv) SIBIOMSYS
13. Nitrogen is an essential nutrient for plant growth. But farmers who cultivate pulse crops like green gram, Bengal gram, black gram, etc., do not apply nitrogenous fertilizers during cultivation. Why?
Roots of pulses have a symbiotic relationship with a bacteria called Rhizobium. This bacteria fixes atmospheric nitrogen, which will be utilized by leguminous plants. Hence, farmers don’t apply nitrogen fertilizers while cultivating cereals.
14. Wheat dough is left in the open, and after a few days, it starts to emit a foul smell and becomes unfit for use. Give reason.
Carbohydrates present in the dough will provide nutrients for the growth of yeast and other fungi. These break down glucose to emit a foul smell and spoil dough.
15. Sunlight, chlorophyll, carbon dioxide, water and minerals are raw materials essential for photosynthesis. Do you know where they are available? Fill in the blanks with the appropriate raw materials.
(a) Available in the plant: _______________
(b) Available in the soil: _______________, _______________
(c) Available in the air: _______________
(d) Available during day: _______________
(a) Available in the plant: Chlorophyll
(b) Available in the soil: Water, Minerals
(c) Available in the air: Carbon-di-oxide
(d) Available during day: Sunlight
16. Observe the diagram given in Figure 1.1 and label the following terms given in the box.

Long Answer Questions
17. Match the organisms given in Column I with their mode of nutrition given in Column II.
18. Wild animals like tiger, wolf, lion and leopard do not eat plants. Does this mean that they can survive without plants? Can you provide a suitable explanation?
Wild animals like tiger, wolf, lion and leopard do not eat plants. But they feed on herbivore animals which eat plants. If plants do not exist, these animals cannot exist due to the lack of food. Further, wild animals cannot live as they will not get food. Hence, it is said that, directly or indirectly, all living organisms depend on plants for food.
19. Fill in the blanks of the paragraph given below with the words provided in the box.

Leaves have a green pigment called (a) which captures (b) from sunlight. This (c) is used in the process of (d) and along with other raw materials like (e) and (f), synthesize (g).
Leaves have a green pigment called Chlorophyll which captures energy from sunlight. This energy is used in the process of photosynthesis and, along with other raw materials like water and carbon-di-oxide synthesises food.
20. Spot as many organisms as possible in the puzzle given in Figure 1.2 by encircling them as shown. Write the names on a sheet of paper and categorise them into autotrophs and heterotrophs. Classify the heterotrophs into herbivores, carnivores, omnivores and saprophytes.
Number of organisms: 22
(Some examples are given. You may find the rest of the organisms.)

Autotrophs – Rose, Mango, Bhindi, Carrot, Banyan, Tulsi, Ginger, Yam
Heterotrophs – Elephant, Ant, Yeast, Tiger, Mushroom, Fox, Mice, Owl, Cow, Crow, Rabbit, Bee, Fish
Herbivores – Elephant, Cow, Rabbit, Bee
Carnivores – Fox, Tiger
Omnivores – Ant, Mice, Owl, Crow, Fish
Saprophytes – Mushroom, Yeast
21. Can you give me a name? Solve each of the following riddles by writing the name of the organism and its mode of nutrition. One riddle is solved to help you.
(a) I am tall, but I cannot move. I am green and can prepare my own food. tree, autotroph
(b) I live in water; people keep me in an aquarium and feed me. ,
(c) I am small, and I can fly. I disturb your sleep, bite you and suck your blood which is my food. ,
(d) I am white and soft. I grow well in the rainy season. Children pluck me from the ground and admire me. I absorb nutrients from decomposed dead parts of plants and animals in the soil. ,.
(b) fish, heterotroph
(c) mosquito, parasite
(d) mushroom, saprophyte
BYJU’S provides you with the best study materials, notes, Sample papers , important questions, NCERT textbooks and NCERT solutions for all the chapters of all the subjects and classes. Keep Visiting BYJU’S for an effective learning experience.
Frequently Asked Questions NCERT Exemplar for Class 7 Science Chapter 1 Nutrition in Plants
Potato and ginger are both underground parts that store food. where is the food prepared in these plants.
In these plants, the shoot system and leaves stay above ground. They prepare food by photosynthesis and store energy in the underground part of the plant.
A goat eats away all the leaves of a small plant (balsam). However, in a few days, new leaves could be seen sprouting in the plant again. How did the plant survive without leaves?
Nitrogen is an essential nutrient for plant growth. but farmers who cultivate pulse crops like green gram, bengal gram, black gram, etc., do not apply nitrogenous fertilisers during cultivation. why.
Roots of pulses have a symbiotic relationship with a bacteria called Rhizobium. This bacteria fixes atmospheric nitrogen, which will be utilised by leguminous plants. Hence, farmers don’t apply nitrogen fertilisers while cultivating cereals.
Wheat dough is left in the open, and after a few days, it starts to emit a foul smell and becomes unfit for use. Give a reason.
Wild animals like tiger, wolf, lion and leopard do not eat plants. does this mean that they can survive without plants can you provide a suitable explanation.
Wild animals like tiger, wolf, lion and leopard do not eat plants. But they feed on herbivore animals which eat plants. If plants do not exist, these animals will not exist due to the lack of food. Further, wild animals cannot live as they will not get food. Hence, it is said that, directly or indirectly, all living organisms depend on plants for food.
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Request OTP on Voice Call
Post My Comment
Please tell me how the plant synthesis their own food?
Learn how plants synthesis their own food from the link given below. https://byjus.com/questions/explain-the-process-of-synthesis-of-food-in-green-plants/
- Share Share
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.


NCERT Solutions for Class 7 Science Chapter 1 Nutrition in Plants

Chapter 1 Nutrition in Plants Class 7 Science NCERT Solutions
Class 7 science chapter 1 nutrition in plants ncert questions and answers - topics, chapter 1 nutrition in plants ncert solutions for class 7 science - notes, chapter 1 nutrition in plants class 8 science questions and answers - mcq questions with answers, contact form.
A to Z Classes
Cbse, ncert and icse solution online, class 7 science case study question, case study question class 7 science (cbse / ncert board).
Class 7 Science Case Study Question and Answer: CBSE / NCERT Board Class 7 Science Case Study Question prepared by expert Science Teacher. Students can learn Case Based Question / Paragraph Type Question for NCERT Class 7 Science.
There are total 18 chapter Nutrition in Plants, Nutrition in Animals, Fibre to Fabric, Heat, Acids, Bases and Salts, Physical and Chemical Changes, Weather, Climate and Adaptations of Animals to Climate, Winds, Storms and Cyclones, Soil, Respiration in Organisms, Transportation in Animals and Plants, Reproduction in Plants, Motion and Time, Electric Current and Its Effects, Light, Water: A Precious Resource, Forests: Our Lifeline, Wastewater Story
For any problem during learning any Case or any doubts please comment us. We are always ready to help You.
CBSE Class 7 Science Case Study Question
- Chapter 1 Nutrition in Plants Case Study Question
- Chapter 2 Nutrition in Animals Case Study Question
- Chapter 3 Fibre to Fabric Case Study Question
- Chapter 4 Heat Case Study Question
- Chapter 5 Acids, Bases and Salts Case Study Question
- Chapter 6 Physical and Chemical Changes Case Study Question
- Chapter 7 Weather, Climate and Adaptations of Animals to Climate Case Study Question
- Chapter 8 Winds, Storms and Cyclones Case Study Question
- Chapter 9 Soil Case Study Question
- Chapter 10 Respiration in Organisms Case Study Question
- Chapter 11 Transportation in Animals and Plants Case Study Question
- Chapter 12 Reproduction in Plants Case Study Question
- Chapter 13 Motion and Time Case Study Question
- Chapter 14 Electric Current and Its Effects
- Chapter 15 Light
- Chapter 16 Water: A Precious Resource
- Chapter 17 Forests: Our Lifeline
- Chapter 18 Wastewater Story
What is Case Study Question?
Ans. At case Study there will one paragraph and on the basis of that concept some question will made. Students have to solve that question.
How many marks will have at case based question?
Most of time 5 questions will made from each case. There will 1 or 2 marks for each question.
Important links:
- Lakhmir Singh Class 7 Solution
- NCERT Class 7 Math Solution
Copyright © 2024 | WordPress Theme by MH Themes
The Site is down as we are performing important server maintenance, during which time the server will be unavailable for approximately 24 hours. Please hold off on any critical actions until we are finished. As always your feedback is appreciated.

- Study Packages
- NCERT Solutions
- Sample Papers
- Online Test

- Questions Bank
- Nutrition in Plants
- Test Series
- Ncert Solutions
- Solved Papers
- Current Affairs
- JEE Main & Advanced
- Pre-Primary
- MP State Exams
- UP State Exams
- Rajasthan State Exams
- Jharkhand State Exams
- Chhattisgarh State Exams
- Bihar State Exams
- Haryana State Exams
- Gujarat State Exams
- MH State Exams
- Himachal State Exams
- Delhi State Exams
- Uttarakhand State Exams
- Punjab State Exams
- J&K State Exams
7th Class Science Nutrition in Plants Question Bank
Done nutrition in plants total questions - 40.
question_answer 1) Which of the following is insectivorous?
A) Mushroom done clear
B) Cuscuta done clear
C) Mucor done clear
D) Nepenthes done clear
question_answer 2) Which of the following statements is true about croton plants?
A) Croton plants do not contain chlorophyll. done clear
B) Croton plants are dark red in colour. Hence they depend on other plants for food. done clear
C) Croton plants have chlorophyll but it is hidden by dark red colour pigments. done clear
D) Croton plants are parasites done clear
A) P and S only done clear
B) Q, R and S only done clear
C) P, Q and R only done clear
D) P, Q, R and S done clear
A) To show that air is a basic need of plants. done clear
B) To show that food is a basic need of plants. done clear
C) To show that water is a basic need of plants. done clear
D) To show that sunlight is a basic need of plants. done clear
question_answer 5) The equation given below represents photosynthesis. \[\text{X+Water}\xrightarrow[\text{Chlorophyll}]{\text{Sunlight}}\text{Glucose+Y}\] Which of the following is represented by X and Y in the given equation?
A) X - Carbon dioxide, Y- Oxygen done clear
B) X-Oxygen, Y-Carbon done clear
C) X - Carbon dioxide, Y- Hydrogen done clear
D) X - Oxygen, Y - Carbon dioxide done clear
question_answer 6) How does photosynthesis help to maintain the percentage of oxygen and carbon dioxide in the atmosphere?
A) By giving off carbon dioxide and absorbing oxygen. done clear
B) By giving off oxygen and absorbing carbon dioxide. done clear
C) By releasing oxygen and carbon dioxide. done clear
D) By absorbing oxygen and carbon dioxide. done clear
question_answer 7) Which part of the leaf controls the rate of loss of water to the air?
A) Midrib done clear
B) Stomata done clear
C) Vascular bundles done clear
D) Veins done clear
question_answer 8) What role does the insect play in the insectivorous plant?
A) Fertilization process. done clear
B) Provides nutrients to the plant. done clear
C) Dispersal of seeds. done clear
D) Provides carbon dioxide to the plant. done clear
question_answer 9) What is the role of the bacteria in leguminous plants?
A) Convert oxides of nitrogen into soil nitrates. done clear
B) Convert atmospheric nitrogen gas into soil nitrates. done clear
C) Convert soil nitrates into gaseous nitrogen. done clear
D) Convert plant proteins into ammonia. done clear
A) (i) and (ii) only done clear
B) (i) and (iii) only done clear
C) (ii) and (iii) only done clear
D) (i), (ii) and (iii) done clear
question_answer 11) Symbiosis is the phenomenon in which two different kinds of organisms pool together their nutritional requirements. Which of the following options represents such association?

A) Autotrophic nutrition. done clear
B) Saprophytic nutrition. done clear
C) Parasitic nutrition. done clear
D) Symbiotic nutrition. done clear
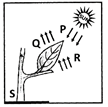
A) P done clear
B) Q done clear
C) R done clear
D) S done clear
A) P-Parasitic; Q-Mosquito; R-Algae done clear
B) P-Autotrophic; Q-Mushroom; R-Yeast done clear
C) P-Parasitic; Q-Nepenthes; R-Mould done clear
D) P-Autotrophic; Q-Mushroom; R-Yeast done clear
question_answer 15) Which of the following is the characteristic feature of organisms exhibiting symbiosis?
A) Organism feeds on dead and decaying organic matter. done clear
B) Organism traps and feeds on insects. done clear
C) Two organisms live together and get benefitted from each other. done clear
D) One organism grows as parasite on the body of other. done clear
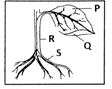
A) P done clear
B) R done clear
C) Q done clear
question_answer 17) Which structure in a green plant controls the opening and closing of stomata?
A) Guard cell done clear
B) Mesophyll done clear
C) Phloem done clear
D) Xylem done clear
question_answer 18) What is the principal source of energy input to biological systems?
A) Carbohydrates from plants. done clear
B) Light from the sun. done clear
C) Nutrients from the soil. done clear
D) Oxygen from the air. done clear
question_answer 19) How does most carbon dioxide reach into the photosynthesizing cells of a green leaf?
A) Through the hypodermis of the leaf. done clear
B) Diffusion through the stomata of the leaf. done clear
C) Movement through the phloem. done clear
D) Movement through the xylem. done clear
question_answer 20) Which of the following organisms are found as slimy, green patches on ponds or on stagnant water?
A) Fungi done clear
B) Bryophytes done clear
C) Bacteria done clear
D) Algae done clear
question_answer 21) Leaves are green due to the presence of a green coloured pigment called chlorophyll. Which of the following is the main function of chlorophyll?
A) To absorb carbon dioxide from the atmosphere. done clear
B) To absorb water and minerals from the soil. done clear
C) To give greenish colour to the leaves. done clear
D) To trap sunlight. done clear
question_answer 22) Fungi does not contain chlorophyll to synthesize their food. Then how do they get their food?
A) From dead and decaying plants. done clear
B) By photosynthesis. done clear
C) By eating small insects which come near it. done clear
D) From the water we pour near it. done clear
question_answer 23) A plant was kept in a dark room for a week. When its leaves were tested with iodine solution, it did not show the presence of starch. From this experiment we can conclude that ____ is essential for photosynthesis.
A) Sunlight done clear
B) Oxygen done clear
C) Carbondioxide done clear
D) Water done clear
question_answer 24) What is the function of root nodules in leguminous plants?
A) Store food done clear
B) Provide extra strength done clear
C) Perform photosynthesis done clear
D) Give shelter to bacteria done clear
question_answer 25) Which function is performed by the bacteria present in the root nodules of leguminous plants?
A) To store food. done clear
B) To perform photosynthesis. done clear
C) To fix the atmospheric nitrogen. done clear
D) To simplify the complex minerals present in the soil. done clear
question_answer 26) Which of the following is present in root nodules of leguminous plants that fixes the atmospheric nitrogen?
A) Rhizobium bacteria done clear
B) Blue-green algae done clear
C) Nitrifying bacteria done clear
D) Paramoecium done clear
question_answer 27) In nepenthes plant, leaves are modified into which of the following structures?
A) Leaf tendrils for mechanical support. done clear
B) Spines to reduce the loss of water. done clear
C) Pitchers to trap the insects. done clear
D) Fleshy leaves to store the food and water. done clear
question_answer 28) Which statement is true about parasitic plants?
A) Organisms which prepare food on their own. done clear
B) Organisms which break glucose into alcohol and carbon dioxide. done clear
C) Organisms which draw nutrition from living tissues of other plants. done clear
D) Plants which kill insects for their food. done clear
question_answer 29) Which of the following is an insectivorous plant?
A) Sundew done clear
B) Lichen done clear
C) Fern done clear
D) Mould done clear
question_answer 30) Which statement describes symbiosis?
A) A mutually beneficial relationship between two organisms. done clear
B) Interdependence between two organisms. done clear
C) Phenomenon in which carnivorous plants kill insects as well as prepare food through photosynthesis. done clear
D) Dependence on another organism for its habitat. done clear
question_answer 31) Carbohydrates, the most abundant biomolecules on Earth are produced by which of the following organisms?
A) Some bacteria, algae and green plant cells. done clear
B) All bacteria, fungi and algae. done clear
C) Fungi, algae and green plant cells. done clear
D) Viruses, fungi and bacteria. done clear
question_answer 32) Which process causes oxygen to enter the atmosphere?
A) Fat metabolism done clear
B) Respiration done clear
C) Photosynthesis done clear
D) All of these done clear
question_answer 33) Which of the following statements is true about the importance of photo-synthesis?
A) Source of food for all living organisms. done clear
B) It converts atmospheric carbon dioxide to glucose. done clear
C) It provides nourishment and helps in the growth and development of plants. done clear
D) All of these done clear
question_answer 34) Which structure in a green plant transports the synthesized food from leaves to all other parts of the plant body?
question_answer 35) What is the function of the bacteria present in the roots of bean plants
A) Breaks down nitrates into nitrogen. done clear
B) Converts ammonium salts into nitrogen. done clear
C) Converts nitrogen into nitrates. done clear
D) Converts nitrates into ammonium compounds. done clear
question_answer 36) Amarbel is an example of
A) autotroph. done clear
B) parasite. done clear
C) saprotroph. done clear
D) host. done clear
question_answer 37) Lichens are examples of which of the following types?
A) Symbiotic algae and fungi. done clear
B) Carnivorous plants. done clear
C) Animals which can perform photosynthesis. done clear
D) Parasitic algae. done clear
question_answer 38) Where does most of the water for photosynthesis come from?
A) From the soil through the roots of the plant. done clear
B) From air through the tiny pores in the leaf. done clear
C) As a result of respiration within the leaf. done clear
D) From water vapour in the air. done clear
question_answer 39) Which of these plants trap and feed on insects?
A) Cuscuta done clear
B) China rose done clear
C) Pitcher plant done clear
D) Rose done clear
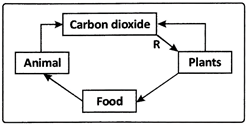
A) Decomposition done clear
B) Respiration done clear
C) Photosynthesis done clear
D) Nutrition done clear
Study Package

Question - Nutrition in Plants
Related question.

Reset Password.
OTP has been sent to your mobile number and is valid for one hour
Mobile Number Verified
Your mobile number is verified.

IMAGES
VIDEO
COMMENTS
CBSE Case Study Questions Class 7 Science Nutrition in Plants. Case study 1. Carbohydrates,proteins, fats, vitamins and minerals are components of food. These components of food are called nutrients and are necessary for our body.All living organisms require food. Plants can synthesise food for themselves but animals including humans cannot.
Case Study Questions on Nutrition in Plants. Questions. Question 1: Read the given passage below and answer the question: Carnivorous plant is especially adapted for capturing and digesting insects and other animals by means of ingenious pitfalls and traps. There are more than 600 known species of carnivorous plants.
An advance in Plant Nutrition is an essential contribution to the field of agriculture and botany, encapsulaing the latest research and breakthroughs in understanding plant nutriion. This comprehensive review delves into the intricate mechanisms governing nutrient uptake, transport, and assimilation in plants, shedding light on the complex ...
Although plant nutrition is a new science, with only 180 years old, it advanced extraordinarily from the demystication of the humus theory, in 1840, to recent discoveries regarding nutrient absorption through identication of genes that encode proteins (carriers). Thus, the study of plant nutrition establishes what are the essential elements for
CBSE Class 7 Science Chapter 1 Nutrition in Plants Case Study Questions.Website Link: https://xamcontent.com/class-7-science-chapter-1-case-study-questions/T...
For an element to be regarded as essential, three criteria are required: 1) a plant cannot complete its life cycle without the element; 2) no other element can perform the function of the element; and 3) the element is directly involved in plant nutrition. Table 1. Essential Elements for Plant Growth.
Nutrition in plants. Unit 7. Nutrition in animals. Unit 8. Respiration in organisms. Unit 9. Transport in animals and plants. Unit 10. Excretion in living organisms. Unit 11. Reproduction in plants. Unit 12. Beneficial and harmful plants and animals. Unit 13. Food, Health and Disease. Unit 14. Sound. Unit 15. Energy. Unit 16. Light. Unit 17 ...
[Download] Case Study Questions for Class 7 Science Chapter 1 Nutrition in Plants Here we are providing case study or passage-based questions for class 7 science chapter 1 Nutrition in Plants. Case Study/Passage Based Questions Passage-1 The bodies of living organisms are made of tiny units called cells. Cells can be seen only microscope. Some … Continue reading Case Study Questions for ...
Recently, several studies have reported on plant nutrition and its impact on human health, ... Plant nutrition management for human health will also be discussed, including many case studies, such as plant nutrition management under salt-affected and contaminated soils. 2. Methodology of the Review
This article is part of the Research Topic Organic-based Foliar Biostimulation and Nutrition in Plants View all 12 articles. ... : Przybysz A, Gawrońska H and Gajc-Wolska J (2014) Biological mode of action of a nitrophenolates-based biostimulant: case study. Front. Plant Sci. 5:713. doi: 10.3389/fpls.2014.00713. Received: 16 July 2014 ...
Plant Nutrition Case Studies. The following case studies focus on plant nutrient management problems and are used in the course Plant Nutrition and Nutrient Management (HORT 815 at Kansas State University and HORT/AGRO 424/824 at the University of Nebraska-Lincoln).Contact Kimberly Williams for the solutions if you are an instructor who would like to use these case studies in your class.
Case studies on Integrated Plant Nutrient Management. This guide on integrated plant nutrient management, dealing with various aspects of plant nutrition, is an attempt to provide support to the ongoing efforts directed at enhanced and sustainable agricultural production. It seeks to bridge the scientific knowledge gap, and it presents updated ...
Plants absorb mineral nutrients from the soil in order to make their own food and for other important processes. Soils need to be enriched with nutrients such as nitrogen, phosphorus, potassium etc regularly. Only then can we grow plants and keep them healthy. There are 17 most important nutrients for plants.
NCERT Solutions Nutrition in Plants Class 7. Class 7 Maths Class 7 Science. Easy to understand notes for Class 7 science chapter 1 - Nutrition in Plants. Learn about modes of nutrition, photosynthesis, parasitic, Insectivorous, saprophytic and symbiotic mode of nutrition. Also, learn about how nutrients are replenished in the soil.
Autotrophic - Plants exhibit autotrophic nutrition and are called primary producers. Plants synthesis their food by using light, carbon dioxide and water. Heterotrophic - Both animals and human beings are called heterotrophs, as they depend on plants for their food. Also Refer: Different Modes Of Nutrition in Living Organisms.
Q.7. Name the following: (i) A parasitic plant with yellow, slender and tubular stem. (ii) A plant that has both autotrophic and heterotrophic mode of nutrition. (iii) The pores through which leaves exchange gases. Ans. (i) cuscuta (ii) Insectivorous plant (iii) Stomata. Q.8. Tick the correct answer:
Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere.
Very Short Answer Type Question. 1: Name some components of food. Answer: Carbohydrates, proteins, fats, vitamins and minerals. 2: Define nutrients. Answer: Carbohydrates, proteins, fats, vitamins and minerals are essential components of food, these components are called nutrients. 3: Give an example of autotrophs.
False, chlorophyll is present in leaf of plants not in animal cells. Question 5. Fungi are green plants that can synthesise their own food. Answer: False, fungi are not green and they are not classified as plants. They are saprophytic organisms which derive nutrition from dead and decaying matter. Question 6.
This solution also covers questions on the mode of nutrition in saprophytes, which proves to be an extension of learning nutrition in different organisms. Sub-topics of Class 7 Science Chapter 1 Nutrition in Plants. 1.1 - Mode of Nutrition in Plant. 1.2 - Photosynthesis - Food Making Process in Plants. 1.3 - Other Modes of Nutrition in ...
Step 1: A fresh leaf is taken. Step 2: The leaf is boiled in water for few minutes to kill the cells in the leaf. Step 3: Now, dip this leaf in iodine solution. Step 4: The color of the leaf will changes into blue black color when iodine is added to it which shows the presence of starch in it. 4.
Chapter 1 Nutrition in Plants Case Study Question. Chapter 2 Nutrition in Animals Case Study Question. Chapter 3 Fibre to Fabric Case Study Question. Chapter 4 Heat Case Study Question. Chapter 5 Acids, Bases and Salts Case Study Question. Chapter 6 Physical and Chemical Changes Case Study Question. Chapter 7 Weather, Climate and Adaptations of ...
P - Carbon dioxide is essential for photosynthesis to take place. Q - The products of photosynthesis are simple sugars. R - Photosynthesis occurs in the green leaves of plants. S - Sunlight is not used as an energy source by plants to make food during photosynthesis. A) P and S only.