A case report of severe systemic herpes simplex virus-1 (HSV-1) infection with multi-organ involvement after a course of oral corticosteroid treatment
Affiliations.
- 1 Department of Internal Medicine, Memorial Healthcare System, 703 N. Flamingo Rd, GME 2nd Floor, 33028, Pembroke Pines, FL, USA.
- 2 Division of Infectious Disease, Memorial Healthcare System, 5647 Hollywood Blvd, 33021, Hollywood, FL, USA.
- 3 Division of Infectious Disease, Memorial Healthcare System, 5647 Hollywood Blvd, 33021, Hollywood, FL, USA. [email protected].
- PMID: 36335305
- PMCID: PMC9636695
- DOI: 10.1186/s12879-022-07815-3
Background: Herpes simplex virus (HSV) rarely causes organ-invasive infection. Diagnosis and treatment for such infections are often delayed, and mortality is high. We present the first reported case of disseminated HSV-1 infection in an adult causing liver failure, myocarditis, and encephalitis in a patient who recovered after receiving parenteral acyclovir treatment.
Case presentation: A 46-year-old female presented with fever, chills, and malaise after 2 weeks of oral corticosteroid treatment for uveitis. She was diagnosed with disseminated HSV-1 infection with multi-organ involvement causing hepatitis, encephalitis, and myocarditis. Diagnosis was made timely using serum polymerase chain reaction (PCR) for HSV DNA and the patient was given intravenous acyclovir treatment promptly, which led to her survival without significant morbidity.
Conclusions: Clinicians should have a low threshold for suspecting HSV infection and ordering HSV PCR to decrease morbidity and mortality when there is a high clinical suspicion of systemic HSV infection with multi-organ involvement. Serum PCR for HSV DNA is an excellent modality for an initial diagnostic approach. Further research is warranted to elucidate causality between a course of corticosteroid therapy and systemic HSV-1 infection without major immunosuppressive comorbidities or treatments.
Keywords: Acyclovir; Case report; Disseminated infection; Herpes simplex virus; Multi-organ failure.
© 2022. The Author(s).

Publication types
- Case Reports
- Acyclovir / therapeutic use
- Adrenal Cortex Hormones / therapeutic use
- Antiviral Agents / therapeutic use
- Encephalitis*
- Encephalitis, Herpes Simplex* / drug therapy
- Herpes Simplex* / diagnosis
- Herpes Simplex* / drug therapy
- Herpesvirus 1, Human* / genetics
- Middle Aged
- Myocarditis* / drug therapy
- Adrenal Cortex Hormones
- Antiviral Agents
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Global health
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 2018, Issue
- Herpes simplex virus type 1: an atypical presentation of primary infection
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Inês Medeiros 1 ,
- Cristiana Maximiano 1 ,
- Teresa Pereira 2 ,
- Maria Miguel Gomes 1
- 1 Paediatrics Department , Hospital de Braga , Braga , Portugal
- 2 Dermatology Department , Hospital de Braga , Portugal
- Correspondence to Dr Inês Medeiros, inesdemedeiros{at}hotmail.com
https://doi.org/10.1136/bcr-2018-224967
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
- paediatrics
- dermatology
Description
A 3-year-old female child with personal history of atopic dermatitis presented with confluent vesicular and shallow ulcers pruritic rash surrounded by an erythematous base located to both hands and wrists, with 1-week evolution. She had no fever or other symptoms. There was no personal or family history of herpetic infections. Topical antibiotic, topical corticosteroid and oral antihistaminic were tried with no improvement. Physical examination was unremarkable except for generalised dry skin and lesions in figure 1 . On suspicion of superinfected viral rash or bullous impetigo she was treated with oral amoxicillin and clavulanic acid (concentration of 250 mg/62.5 mg with the dose of 50 mg/kg/day) and topical fusidic acid. One week later, at re-evaluation, there was progression of the rash ( figure 2 ) and the mother reported herpes labialis on the father. At this time, the diagnosis of primary eczema herpetic infection was also considered. Oral acyclovir (20 mg/kg every 6 hours for 5 days) and cefuroxime (30 mg/kg every 12 hours for 7 days) were started, with complete resolution of the lesions after 10 days. PCR assay of lesion’s swab was positive for herpes simplex virus (HSV) type 1 and bacterial culture was negative.
- Download figure
- Open in new tab
- Download powerpoint
Grape-like clustered shallow ulcers in the dorsal surface of the left hand.
Confluent vesicles and shallow ulcers with bilateral localisation in both hands, grouped in the left hand and disseminated in the right hand.
The authors want to emphasise the fact that the primary HSV infection can present in atypical forms, in which the lesions may be generalised, symptomatic, severe and with bilateral involvement. Therefore, this diagnosis should be considered in the differential diagnosis of other vesiculobullous diseases 3 .
Learning points
Acute herpetic gingivostomatitis is the most common clinical presentation of herpes simplex virus (HSV) primary infection in children aged 6 months to 5 years.
Eczema herpeticum is a rapid dissemination of an HSV infection over the eczematous atopic skin, prone to superinfection with Staphylococcus aureus or Streptococcus pyogenes .
HSV PCR assay is the most sensitive method to confirm HSV infection.
- Stanberry LR
- Bolognia J ,
- Jorizzo J ,
Contributors IM collected the data, wrote the manuscript and reviewed the literature. CM collected the data and reviewed the literature. TP and MMG did the critical review of the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared
Patient consent Parental/guardian consent obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
- Case Report
- Open access
- Published: 20 May 2023
A case of herpes simplex virus induced peripheral neuropathy and encephalitis with positive GM3 and CASPR2 antibody
- Hongji Lu 1 , 2 na1 ,
- Yingdi Liao 3 na1 ,
- Changlin Zhang 2 , 5 ,
- Wanxin Wen 1 , 2 ,
- Yaming Du 2 ,
- Min Zhao 2 , 4 &
- Lixin Wang 1 , 2
BMC Neurology volume 23 , Article number: 199 ( 2023 ) Cite this article
11k Accesses
1 Citations
2 Altmetric
Metrics details
We reported on a case involving an older patient with HSV-1 encephalitis who simultaneously experienced the onset of peripheral nerve symptoms associated with the presence of anti-GM3 immunoglobulin G (IgG).
Case presentation
A 77-year-old male was admitted to hospital with high fever, weakness of both lower limbs, and an unstable gait. A CSF test revealed a strikingly increased protein level (1,002 mg/L, normative values: 150-450 mg/L) and MRI revealed hyper-signal lesions in the right temporal lobe, right hippocampus, right insula, and right cingulate gyrus. The CSF was positive for HSV PCR (HSV-1,17870). In addition, the serum samples were positive for CASPR2 antibodies (antibody titer: 1/10) and anti-GM3 immunoglobulin G (IgG) (+). The patient was diagnosed with HSV-1-induced peripheral nerve symptoms that were associated with encephalitis and the presence of anti-GM3 IgG and anti-CASPR2 antibodies. The patient had received included intravenous immunoglobulin, intravenous acyclovir, and corticosteroids therapy. At the one-year follow-up examination, he had regained the necessary skills associated with daily life.
Conclusions
Herpes simplex virus infection often induces encephalitis, and reaction to the virus may trigger an autoimmune response. Early diagnosis and treatment can avoid the progression of the disease to include autoimmune encephalitis.
Peer Review reports
The herpes virus can establish a latent infection in the host and cause recurring disease when reactivation occurs. Of the herpes viruses currently identified, the neurotropic herpes simplex virus type 1 (HSV-1) can invade the central nervous system (CNS) and the peripheral nervous system (PNS) [ 1 , 2 ]. Herpes simplex virus encephalitis (HSVE) is an infectious neurological emergency [ 3 ]. HSVE is one of the most devastating viral infections that occur in humans, and the incidence of HSVE worldwide is estimated to be 2 to 4 cases/1,000,000 [ 3 ]. 80% of patients with HSVE present with fever, headache, and an altered level of consciousness [ 4 ]. Moreover, according to a recent study, 27% of HSVE patients develop autoimmune encephalitis, which primarily results from a potential trigger of the immune response within two months after the infection occurs [ 5 ]. However, PNS symptoms resulting from an HSV-1 infection are rare; several studies have indicated that PNS complications of HSV-1 include acute peripheral facial palsy due to reactivation of the HSV, which might be involved in the pathogenesis [ 1 , 6 ]. In addition, only a series of case reports has documented that some patients with Guillain-Barre syndrome (GBS) have been infected with HSV preceding the onset of acute neurological defects that occurred via a possible mechanism involving an immunological reaction [ 7 , 8 ]. Therefore, a report was warranted that focused on HSV-mediated encephalitis and peripheral neuropathy. Here, we reported on a case involving an older patient with HSV-1 encephalitis who simultaneously experienced the onset of peripheral nerve symptoms associated with the presence of anti-GM3 immunoglobulin G (IgG). Besides, the virus might have simultaneously triggered an autoimmune response that resulted in the production of positive antibodies against contacting associated protein-like 2 receptor (CASPR2).
The patient was a 77-year-old male who had not previously experienced small blisters or “blebs” around his mouth, nose, or genitals. He did not have any other diseases. However, he did have a medical history that included intermittent treatment for hypertension. On 9 May 2019, the patient started to suffer from a fever of 38.5℃. He also experienced an aversion to cold, sore throat, painful joints, and a headache with primarily bilateral temporal pain. The patient was treated in the emergency department of the GDTCM. However, his condition continued to deteriorate.
The patient was re-admitted to our hospital on 12 May 2019 due to the presence of a high fever, weakness of both lower limbs, and an unstable gait. After admission, the patient gradually developed weakness in both upper limbs, dysphagia, irritability, loss of comprehension and mental capacity, and disorientation. The patient was transferred to the Neurological Intensive Care Unit (NICU) from the emergency department on 13 May 2019. On neurological examination, the patient exhibited drowsiness, restlessness, decreased short-term and long-term memory, and decreased mental capacity. A cranial nerve examination revealed bilateral peripheral facial palsy, dysphagia, hoarseness, weakness of both sides of the soft palate, and loss of pharyngeal and palate reflexes. His eyes movements were normal. A motor function examination revealed flaccid limb paralysis, loss of tendon reflexes, level 2 muscle strength for both lower limbs, and level 3 muscle strength for both upper limbs. A CSF test revealed a strikingly increased protein level (1,002 mg/L, normative values: 150-450 mg/L) and a red blood cell count of 630/µL (normative values: 0µL ). While the patient’s blood pressure was normal, his glucose, leukocyte, and lymphocyte counts were also normal. The second lumbar puncture revealed an increased protein level (1,745 mg/L, normative values: 150-450 mg/L), a red blood cell count of 43/µL and slight elevated white blood cell (77/µL, normative values: 0–8µL). We initially considered the possibility that the patient had GBS due to the pre-infection, peripheral symmetric damage to his limbs and cranial nerves, and albuminocytologic dissociation. Therefore, we provided intravenous immunoglobulin therapy (IVIG, 0.4 kg/d) for five days.
The patient presented a worsening condition one day later, including lethargy, with a GCS score of 7. He developed hypoxemia caused by increased sputum secretion due to bulbar paralysis, which blocked his airway. The patient underwent tracheal intubation and was placed on a ventilator. Subsequently, the patient experienced status epilepticus, and we provided anti-epilepsy treatment. We considered the patient might have experienced CNS damage due to the presence of considerable advanced neurological dysfunction. We carried out MRI imaging and an electroencephalogram (EEG) on the patient. We also performed autoimmune antibody tests on CSF and serum samples. The MRI revealed hyper-signal lesions in the right temporal lobe, right hippocampus, right insula, and right cingulate gyrus (Fig. 1 ). The EEG revealed epileptiform discharges in the right temporal region and right frontal region, which were consistent with a diagnosis of viral or autoimmune encephalitis. Therefore, the patient was treated intravenously with 10 mg/kg acyclovir every eight hours for eight days. In addition, methylprednisolone (MP, 1 g/d) pulse therapy was administered for five days, followed by a decreased MP dosage (0.5 g/d) for five days, then with oral prednisolone (60 mg/d). During this time, the second-generation sequencing of the CSF sample and the autoantibody spectrum for peripheral neuropathy were evaluated. The CSF was positive for HSV PCR (HSV-1, specific sequence 17,870). The immunologic tests were performed on the serum and CSF samples, including those for anti-ganglioside antibodies associated with peripheral neuropathy and antibodies associated with autoimmune encephalitis. The CSF samples were negative for antibodies of autoimmune encephalitis. The serum samples were positive for CASPR2 antibodies (antibody titer:1/10) and anti-GM3 immunoglobulin G (IgG) (+) using the indirect tissue immunofluorescence and cell-based assays. These tests verified the diagnosis of HSVE but were only suggestive of the presence of autoimmune encephalitis.
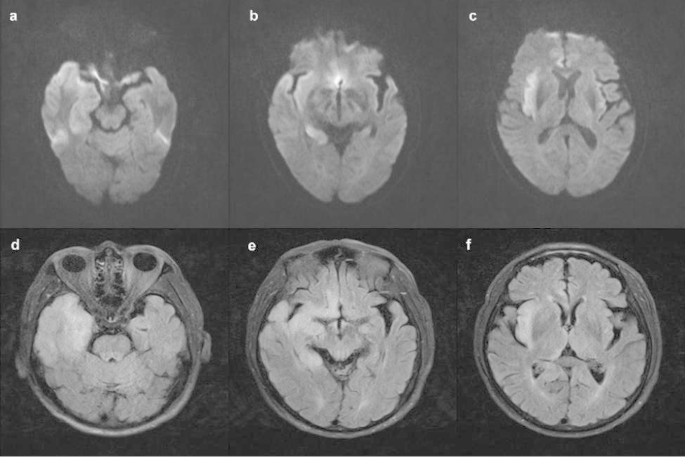
MRIs in acute phase. DWI( a-c ) and FLAIR( d-f ) show hyper-signal lesions in the right temporal lobe, right hippocampus, right insula, and right cingulate gyrus
Progressive improvement in consciousness, motor function, and respiratory function was observed during the second week of treatment. The patient was removed from the ventilator and transferred out of the NICU. When the patient was discharged from the hospital, he was completely conscious but had poor cognitive ability (a modified Rankin Scale [mRS] score of 2). The CSF autoimmune encephalitis antibodies and serum anti-GM3 IgG were negative before the patient was discharged. Six months later, this patient presented with fever and delayed reaction was admitted again in GDTCM. His peripheral white blood cells and procalcitonin(2.52 ng/ml, normative values: 0-0.05 ng/ml) were elevated. Chest X-ray showed inflammation of the left upper lingular segment and both lower lungs. There was no abnormal EEG activity. However, the patient refused to undergo a lumbar puncture and we were unable to assess the CSF. On treatment, ceftriaxone, levofloxacin and piperacillin sodium sulbactam were given. Subsequently, the patient’s temperature returned to normal and his mental status improved. At the one-year follow-up examination, despite a deficit in his short-term memory, the patient had not experienced any relapse of the HSVE or occurrence of seizures. He had regained the necessary skills associated with daily life (a mRS score of 1). The whole progress of diagnosis and treatment were shown in Fig. 2 .

The progress of diagnosis and treatment for this patient
Discussion and conclusion
According to preliminary published literature [ 1 ], HSV-1 infection can cause not only encephalitis but also peripheral neuropathy [ 3 , 8 ]. Only a few cases have reported that the clinical features of HSV infection have been identified with other peripheral neurological symptoms such as muscle weakness, except for facial palsy. Previous cases have only reported HSV invasion of the central nervous system alone or the peripheral nervous system alone. Meanwhile, there also is a growing body of literature demonstrating that HSVE may induce autoimmune encephalitis that is primarily mediated by anti-N-methyl-D-aspartate receptors (NMDAR) in the serum and CSF [ 5 ]. These results verify that autoimmune antibodies might develop approximately one month after treatment of HSVE.
Here, we report on a case in which an HSV-1 infection induced a peripheral neuropathy syndrome that included progressive limb weakness, cranial nerve defects, and the presence of anti-GM3 IgG in the patient’s serum. Unfortunately, because the patient’s condition worsened, electromyography (EMG) examination was not performed in time. When the patient’s condition improved and was transferred out of the ICU, he underwent EMG examination. The results showed that peripheral damage to the right gastrocnemius nerve (sensory fiber involvement, axonal damage) and prolonged H-reflex latency of the tibial nerve bilaterally. EMG findings do not support demyelination changes,which may be caused by disease progression or drug treatment effect.
Herpes simplex virus (HSV) is one of the common pathogens causing encephalitis. The clinical features of this patient include a history of antecedent infection, acute or subacute onset, mainly manifesting as multiple cranial nerve damage, impaired consciousness, dysphagia, slurred speech. Therefore we have to differentiate it from brainstem encephalitis. And abnormalities associated with HSVE on MRI were seen in the temporal lobe or brainstem [ 9 ]. However, this patient’s MRI examination suggested no abnormal signals in the brainstem. In addition, it should be differentiated from limbic encephalitis, which is characterized by memory loss, as well as irritability, confusion, hallucinations, partial or generalized epilepsy, drowsiness, and dementia.Limbic encephalitis refers to the involvement of limbic structures such as the hippocampus, amygdala, insula and cingulate cortex. But in our case, we reported that HSV triggered the production of autoimmune brain CASPR2 antibodies in the serum. Therefore, we assumed that autoimmune antibodies might have appeared in the serum of this older patient after an initial HSV infection. It is well recognized that post-infectious immune-mediated targeting of the nervous system can occur. Infection with HSV triggers a robust immune response that helps clear the infection from the innate immune system. Toll-like receptors generate and activate signaling pathways that result, in the early stages, in the production of pro-inflammatory cytokines such as tumor necrosis factor and various interleukins [ 10 ]. The inflammatory process recruits immune cells to induce necrosis and apoptosis of infected cells.
We determined that the patient, who had an HSV infection, experienced both PNS and CNS symptoms and additional examination findings revealed hyper-signal lesions in the right cerebral lobe. A series of complications associated with the PNS occurred that was mediated through the serum peripheral neuropathy antibodies. The question remained as to why there were anti-GM3 Ig-G and CASPR2 antibodies in the patient’s serum. It is well known that anti-ganglioside antibodies can be found in patients with acute inflammatory demyelinating polyradiculoneuropathy. These antibodies might present immunological characteristics, especially antibodies such as GM1, GM1b, or GD1b, which have been observed previously [ 11 ]. However, abundant evidence indicates that the expression level of GM3 is negatively correlated with the malignancy of certain types of tumors [ 12 ]. We also screened the tumor of this patient, performed the chest CT and abdominal CT, and detected tumor markers,but no tumor was found. Therefore, this is the first report of an older HSVE patient exhibiting anti-GM3 IgG antibodies in association with both lower limb weakness and peripheral cranial nerve damage.
CASPR2 is the primary target antigen for auto-antibodies against the neuronal voltage-gated potassium channel complex, which is expressed and distributed in the CNS and PNS. Anti-CASPR2 could be used as a target of cellular immunity to inhibit effective regeneration of the myelin sheath and serve as a marker for autoimmune encephalitis [ 13 ]. However, the CRSPR2 antibody in the CSF was negative, so the patient could not be diagnosed as having autoimmune encephalitis. However, there was a tendency that the condition could have developed into autoimmune encephalitis. The negative results did not rule out the possibility of a timely diagnosis and treatment. In general, HSV-1 might trigger an immune response and produce a series of autoimmune antibodies with peripheral neuropathy presented in HSVE after the first episode of a primary infection. Also, immunocompetent patients exhibit more severe manifestations than older immunocompromised patients [ 14 ].
This patient experienced improvement in his neurological impairment, and there was no occurrence of HSVE and epilepsy with the early diagnosis and treatment, given our awareness of the possibility of autoimmune encephalitis. Therefore, it is suggested that when patients with HSVE are admitted, an early test is carried out to look for the presence of autoantibodies, including GM3, CASPR2, and NMDR antibodies in the CSF and serum. Treatment that includes the addition of corticosteroids and immunoglobulin to aciclovir could improve the patients’ prognosis. On the other hand, delaying corticosteroid therapy might prevent the subsequent development of autoimmune antibodies. However, this case study also had shortcomings. We did not retest second-generation sequencing and antibodies associated with autoimmune encephalitis of the CSF sample during follow-up.
The authors would like to express their gratitude to EditSprings ( https://www.editsprings.cn ) for the expert linguistic services provided.
Data Availability
All data related to this case report are documented within this manuscript.
Abbreviations
Central nervous system
Contacting associated protein-like 2 receptor
Electroencephalogram
Guillain-barre syndrome
Herpes simplex virus type 1
Herpes simplex virus encephalitis
Immunoglobulin G
Neurological intensive care unit
Peripheral nervous system
Steiner I. Herpes virus infection of the peripheral nervous system. Handb Clin Neurol. 2013;115:543–58. https://doi.org/10.1016/B978-0-444-52902-2.00031-X .
Article PubMed Google Scholar
Steiner I, Kennedy PG, Pachner AR. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol. 2007;6(11):1015–28. https://doi.org/10.1016/S1474-4422(07)70267-3 .
Hjalmarsson A, Blomqvist P, Sköldenberg B.Herpes simplex encephalitis in Sweden. 1990–2001: incidence, morbidity, and mortality. Clin Infect Dis; 2007;45(7):875–80. https://doi.org/10.1086/521262 .
Conrady CD, Drevets DA, Carr DJ. Herpes simplex type I (HSV-1) infection of the nervous system : is an immune response a good thing? J Neuroimmunol. 2010;220(1–2):1–9. https://doi.org/10.1016/j.jneuroim.2009.09.013 .
Armangue T, Spatola M, Vlagea A, et al. Spanish herpes Simplex Encephalitis Study Group. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17(9):760–72. https://doi.org/10.1016/S1474-4422(18)30244-8 .
Adour KK, Bell DN, Hilsinger RL Jr.Herpes simplex virus in idiopathic facial paralysis (Bell palsy). JAMA. 1975;11(6):527–30.
Bernsen HJ, Van Loon AM, Poels RF et al. Herpes simplex virus specific antibody determined by immunoblotting in cerebrospinal fluid of a patient with the Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry 1989;52(6):788–91. https://doi.org/10.1136/jnnp.52.6.788 .
De Fine OB, Buhl M. Herpes simplex virus and Guillain-Barré polyradiculitis. Br Med J. 1975;25(5951):192–3. https://doi.org/10.1136/bmj.1.5951.192 .
Miura S, Kurita T, Noda K, et al. Symmetrical brainstem encephalitis caused by herpes simplex virus. J Clin Neurosci. 2009;16(4):589–90. https://doi.org/10.1016/j.jocn.2008.06.005 .
Zhang SY, Jouanguy E, Sancho-Shimizu V, et al. Human toll-like receptor-dependent induction of interferons in protective immunity to viruses. Immunol Rev. 2007;220(1):225–36. https://doi.org/10.1111/j.1600-065X.2007.00564.x .
Fan C, Jin H, Hao H, et al. Anti-ganglioside antibodies in Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy in chinese patients. Muscle Nerve. 2017;55(4):470–5. https://doi.org/10.1002/mus.25266 . Epub 2016 Dec 23.
Ene CD, Tampa M, Nicolae I et al. Antiganglioside Antibodies and Inflammatory Response in Cutaneous Melanoma. J Immunol Res 2020;13;2020:2491265. https://doi.org/10.1155/2020/2491265 .
Irani SR, Alexander S, Waters P et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain 2010 Sep;133(9):2734–48. https://doi.org/10.1093/brain/awq213 . Epub 2010 Jul 27.
Tan IL, McArthur JC, Venkatesan A et al. Atypical manifestations and poor outcome of herpes simplex encephalitis in the immunocompromised. Neurology. 2012 Nov 20;79(21):2125-32. https://doi.org/10.1212/WNL.0b013e3182752ceb .
Download references
Acknowledgements
Not applicable.
This study was funded and supported by Zhaoyang Talent Program of Guangdong provincial hospital of TCM (grant number:ZY2022YL19) and by Traditional Chinese Medicine Research Project of Guangdong Province(grant number:20200514103821)
Author information
Hongji Lu and Yingdi Liao contributed equally to this work and share first authorship.
Authors and Affiliations
The Neurological Intensive Care Unit of Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, 510120, China
Hongji Lu, Wanxin Wen & Lixin Wang
The Second Clinical School, Guangzhou University of Chinese Medicine, Guangzhou, 510120, China
Hongji Lu, Changlin Zhang, Wanxin Wen, Yaming Du, Min Zhao & Lixin Wang
The Rehabilitation Department, Kunming Municipal Hospital of Traditional Chinese Medicine, Kunming, 650000, China
Yingdi Liao
The Encephallopathy Department.1 of Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, 510120, China
Department of Stroke Center, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, 510120, China
Changlin Zhang
You can also search for this author in PubMed Google Scholar
Contributions
Lixin Wang and Hongji Lu proposed the case report design, verified the clinical data, and revised the manuscript. Yingdi Liao drafted the manuscript. Changlin Zhang, Wanxin Wen, Yaming Du, Min Zhao conducted the clinical management and follow-up for this patient. All authors contributed to the preparation of the manuscript and agreed on its publication.All authors reviewed the manuscript.
Corresponding author
Correspondence to Lixin Wang .
Ethics declarations
Ethics approval and consent to participate.
Ethics approval or consent to participate was not applicable.
Consent for publication
Written informed consent was obtained from the patient and guardian for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
All authors have no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lu, H., Liao, Y., Zhang, C. et al. A case of herpes simplex virus induced peripheral neuropathy and encephalitis with positive GM3 and CASPR2 antibody. BMC Neurol 23 , 199 (2023). https://doi.org/10.1186/s12883-023-03238-y
Download citation
Received : 10 November 2022
Accepted : 04 May 2023
Published : 20 May 2023
DOI : https://doi.org/10.1186/s12883-023-03238-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Herpes simplex virus
- Peripheral neuropathy
- Encephalitis
- GM3 antibody
- CASPR2 antibody
BMC Neurology
ISSN: 1471-2377
- General enquiries: [email protected]
- Search Menu
- Sign in through your institution
- Advance articles
- Editor's Choice
- Supplement Archive
- Cover Archive
- IDSA Guidelines
- IDSA Journals
- The Journal of Infectious Diseases
- Open Forum Infectious Diseases
- Photo Quizzes
- State-of-the-Art Reviews
- Voices of ID
- Author Guidelines
- Open Access
- Why Publish
- Advertising and Corporate Services
- Advertising
- Journals Career Network
- Reprints and ePrints
- Sponsored Supplements
- Branded Books
- About Clinical Infectious Diseases
- About the Infectious Diseases Society of America
- About the HIV Medicine Association
- IDSA COI Policy
- Editorial Board
- Self-Archiving Policy
- For Reviewers
- For Press Offices
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Current guidelines for neonatal herpes, conclusions, supplementary data.
- < Previous
Diagnosis and Management of Genital Herpes: Key Questions and Review of the Evidence for the 2021 Centers for Disease Control and Prevention Sexually Transmitted Infections Treatment Guidelines
- Article contents
- Figures & tables
Christine Johnston, Diagnosis and Management of Genital Herpes: Key Questions and Review of the Evidence for the 2021 Centers for Disease Control and Prevention Sexually Transmitted Infections Treatment Guidelines, Clinical Infectious Diseases , Volume 74, Issue Supplement_2, 15 April 2022, Pages S134–S143, https://doi.org/10.1093/cid/ciab1056
- Permissions Icon Permissions
Genital herpes, caused by herpes simplex virus (HSV) type 1 or type 2, is a prevalent sexually transmitted infection (STI). Given that HSV is an incurable infection, there are important concerns about appropriate use of diagnostic tools, management of infection, prevention of transmission to sexual partners, and appropriate counseling. In preparation for updating the Centers for Disease Control and Prevention (CDC) STI treatment guidelines, key questions for management of genital herpes infection were developed with a panel of experts. To answer these questions, a systematic literature review was performed, with tables of evidence including articles that would change guidance assembled. These data were used to inform recommendations in the 2021 CDC STI treatment guidelines.
Genital herpes is a chronic sexually transmitted infection (STI) characterized by recurrent, self-limited genital ulcers, caused by herpes simplex virus type 1 (HSV-1) or type 2 (HSV-2). While HSV-1 is associated with both oral and genital infection, HSV-2 nearly exclusively causes genital disease. HSV-1 and HSV-2 are prevalent infections, with 47.8% and 12.1% of the United States (US) population aged 14–49 years seropositive for HSV-1 and HSV-2, respectively, in 2015–2016 [ 1 ]. HSV-1 seroprevalence reflects oral and genital infection, but HSV-1 is an increasing cause of first-episode genital herpes, particularly in well-resourced settings [ 2–4 ]. Genital herpes is unrecognized in most people; in a National Health and Nutrition Examination Survey study, only 13% of HSV-2–seropositive persons had been diagnosed with genital herpes [ 5 ]. Accurate diagnosis of genital herpes can be realized using type-specific molecular or virologic tests when genital ulcers are present, and type-specific serologic test to detect antibody when lesions are not present. Genital herpes can be managed either by episodic antiviral therapy, in which patients take short courses of antiviral therapy at the time of a genital herpes recurrence, or suppressive antiviral therapy, in which patients take medications on a daily basis to prevent recurrences and shedding. The virus may be present in the genital tract without symptoms, leading to transmission to sex partners or neonates, when present in the genital tract during delivery. In addition, HSV-2 fuels the human immunodeficiency virus (HIV) epidemic, with a 3-fold increased risk of HIV acquisition among persons with HSV-2 infection as compared to those without HSV-2 infection [ 6 ]. Genital herpes is associated with significant stigma, which can be combatted by patient education of the natural history of infection. This evidence review was performed to update approaches to the diagnosis, treatment, and prevention of transmission of genital herpes infections to sex partners and neonates. Furthermore, extragenital manifestations of genital herpes infections, such as HSV-2 meningitis and HSV hepatitis, were reviewed. Finally, treatment of HSV-2 infection in the setting of prevention and treatment of HIV infection was also reviewed. Key questions were generated with an expert panel, followed by a literature review and summary of the published evidence. The findings were presented at the Centers for Disease Control and Prevention (CDC) STI treatment guidelines meeting in June 2019.
Seven panel members with broad expertise in genital herpes infection collaborated with CDC in generation and review of key questions. The key questions were broken down into 6 specific areas: diagnosis, treatment, prevention of sexual transmission/management of sex partners, HSV-2/HIV interactions, prevention of neonatal herpes, and counseling of adults with genital herpes.
Literature Review
Several search strategies of the literature were utilized to capture articles published between 1 January 2013 and 5 February 2019. This time encompassed the period since the prior literature review for the 2015 CDC sexually transmitted diseases (STD) treatment guidelines. The PubMed/Medline computerized database of the US National Library of Medicine was searched, and articles were filtered to include only human studies and to exclude reviews. Medical Subject Heading (MeSH) search terms included the following: HSV OR herpes simplex virus OR herpes genitalis OR genital herpes AND therapeutics OR treatment outcome OR antiviral agents. This search yielded 4760 manuscripts. An additional filter of “clinical trial” was added, yielding 143 manuscripts. Additional searches to address key questions included above herpes terms AND diagnostic, AND serology, herpes simplex virus type 1 AND genital, AND acquisition, AND transmission, AND meningitis AND treatment, AND condoms, AND circumcision, AND tenofovir, AND HIV AND treatment, AND prevention AND neonatal, AND hepatitis AND pregnancy, AND acyclovir resistant AND treatment, AND counseling. Abstracts were reviewed and those that would impact treatment recommendations were selected, reviewed, and included in the Table of Evidence ( Supplementary Table 1 ). The table included citation, study design, study population type/setting, exposure/intervention, outcome measures, reported findings, design analysis quality/biases, and subjective quality rating in the context of the modified rating system used by the US Preventive Services Task Force (USPSTF). The evidence was then reviewed and incorporated into the 2021 CDC STI treatment guidelines.
I. Diagnosis of Genital Herpes
Key Question 1: What are the optimal tests for HSV-1 or HSV-2 detection from a genital ulcer/lesion/suspected HSV outbreak?
As of 5 April 2019, 17 HSV nucleic acid amplification test (NAAT)/polymerase chain reaction (PCR) diagnostic assays were US Food and Drug Administration (FDA)–approved for detection of HSV from clinical specimens [ 7 ]. Although these tests vary in sensitivity and specificity, most available tests with published data have >90% sensitivity and specificity [ 8–10 ]. In addition, some laboratories use “in-house”–developed HSV PCR assays, for which test performance characteristics may be difficult to assess. Providers should be aware of the test characteristics of HSV NAATs that are performed in their clinical setting. Assays that differentiate between HSV-1 and HSV-2 infection should be utilized, to provide patients with information regarding expected natural history of genital herpes.
While HSV molecular assays are highly sensitive and specific, there may be situations in which false-negative results occur. For instance, the yield of HSV viral culture decreases as genital ulcers heal [ 11 ]; it is likely that healing lesions may become negative for HSV DNA as well. HSV molecular assays should not be obtained in the absence of a genital ulcer to diagnose genital herpes infection; due to the intermittent nature of genital HSV shedding, swabs obtained in the absence of genital ulcers would not be sensitive. In these situations, HSV serologic assays should be performed.
HSV culture is less sensitive than NAAT/PCR [ 12 ]. When available, NAAT/PCR assays are preferred. However, if HSV culture is the only test available due to cost or laboratory availability, it is reasonable to perform HSV culture to make the diagnosis of HSV genital ulcer disease. Given the lower sensitivity of culture, if HSV is suspected and results are negative, further investigation through the use of serology may be warranted, particularly to rule out HSV-2 infection.
HSV direct immunofluorescence assay and Tzanck smear lack sensitivity and are not recommended for diagnosis of HSV genital ulcer disease [ 13 ].
Key Question 2: What is the optimal strategy for use of serologic assays to diagnose HSV-1 and HSV-2 infection to avoid false-positive or false-negative diagnoses?
Type-specific HSV serologic assays differentiate between HSV-1 and HSV-2. FDA-approved, commercially available assays test sera for antibodies to HSV glycoprotein G-1 or HSV glycoprotein G-2 using enzyme immunoassay (EIA) or chemiluminescent immunoassay (CLIA) [ 14 ]. The gold standard for HSV serologic testing is Western blot/immunoblot assays, which target antibodies to several HSV antigens in addition to glycoprotein G [ 15 ]. Compared to the gold standard, there are serious limitations to the EIA/CLIA for both HSV-1 and HSV-2 testing. HSV-1 assays lack sensitivity, which can result in false-negative diagnoses. In one study, the sensitivity for detecting HSV-1 antibodies was 70.2% [ 16 ].
Currently available HSV-2 serologic tests lack specificity. The EIA results provide index values, which are quantitative measures of the amount of antibody present. For the first approved EIA, the HerpeSelect, an index value <0.9 is negative, 0.9–1.1 is indeterminate, and >1.1 is considered positive per the manufacturer’s label. However, compared to the Western blot, HSV-2 specificity is very low, in one recent study only 57.4% [ 16 ]. The test characteristics are highly dependent upon the index value, with index values of 1.1–2.9 having only 39.8% specificity, and index value of ≥3.0 having 78.6% specificity [ 16 ]. Persons with HSV-1 infection are more likely to have a false-positive HSV-2 test with a low index value compared to those without HSV-1 infection [ 16 ]. Multiple studies have replicated these results in a wide variety of settings, including STD clinics [ 17 ].
The sensitivity of HSV-2 serologic testing is high, estimated at 92% [ 16 ]. False-negative tests may occur after acquisition of the virus during the window period. The window period may be up to 12 weeks [ 18 ]. Therefore, in someone who tests negative for HSV-2 serology immediately after exposure, serologic testing should not be repeated until 12 weeks after exposure to determine if HSV-2 was acquired.
HSV-2 is a chronic infection that impacts long-term sexual health, and therefore accurate diagnosis is paramount. Serologic diagnosis of HSV-2 should ideally be performed only if patients and providers are aware of the assay limitations and low positive results (index value <3.0) can be confirmed with a second assay using a different gG antigen. Given the poor specificity of HerpeSelect assays, particularly among those with HSV-1 infection, these results should ideally be confirmed with a second method prior to giving results to the patient. Prior studies have shown that using the Biokit HSV-2 rapid assay as a confirmatory test improves the specificity of HerpeSelect from 93.2% to 98.7% when compared to the gold-standard Western blot [ 19 ]. A strategy combining the HerpeSelect with the Biokit assay improves the positive predictive value from 80.5% to 95.6%, and has the greatest impact among low-prevalence populations [ 19 ]. If the Biokit is not available, providers could consider using the Western blot as a confirmatory test. However, access to both of these tests may be limited in some settings. Given that specificity of these assays improves substantially with higher index values, an index value ≥3.0 may be sufficient for diagnosis of HSV-2 infection without further confirmatory testing. However, providers should be aware that false positives have been described even at index values >3.5 with HerpeSelect and other FDA-approved assays [ 20 ]. Further research and tools are needed to optimize HSV serologic testing.
Key Question 3: When should serologic diagnosis of HSV-2 be obtained?
The USPSTF recommends against screening for HSV-2 infection among asymptomatic adolescents and adults [ 21 ]. Given the current limitations of commercially available serologic tests as noted above, this approach is reasonable for asymptomatic people with low pretest probability of infection (few lifetime sexual partners, no known HSV-2 seropositive partners, no genital symptoms). In addition, screening of pregnant women is not recommended [ 22 ].
Persons who have genital symptoms that could be consistent with genital herpes infection should undergo HSV-2 serologic testing to establish the diagnosis of HSV-2 infection. These symptoms include classic or atypical genital symptoms. In addition, people who have been told that they have genital herpes without a virologic diagnosis have a high pretest probability of HSV-2 infection and should undergo HSV-2 serologic testing.
In addition, persons who are at increased risk of HSV-2 infection based on epidemiologic risk could be considered for HSV-2 serologic screening, with the goal of identifying undiagnosed symptomatic infection. Examples of increased epidemiologic risk include sexual activity with a partner with genital HSV-2 infection.
HSV-1 serologic testing does not distinguish between oral and genital infection and should not be performed to diagnose genital HSV-1 infection. Optimally, genital HSV-1 infection should be diagnosed by recovering HSV-1 from genital surfaces using PCR or culture. HSV-1 serologic screening to diagnose genital HSV-1 infection is not recommended.
II. Treatment of Adults With Genital Herpes
Key Question 1: What are the most practical treatment regimens for first clinical episode of genital herpes, episodic therapy, and suppressive therapy?
While several dosing strategies of anti-herpesvirus medications (acyclovir, famciclovir, and valacyclovir) have been studied and are FDA-approved for first clinical episode genital herpes, episodic therapy, and suppressive therapy, several regimens are less practical for use than others due to frequency of dosing. Dosing strategies that are most feasible for patient adherence should be prioritized.
Although episodic and suppressive therapy for genital HSV-1 infection have not been studied as comprehensively as for genital HSV-2, the same medication dosages and frequencies are recommended for genital HSV-1 infection.
Key Question 2: What are the preferred management approaches to the treatment of genital herpes?
There are 2 important goals for management of genital herpes: (1) prevention of symptoms/recurrences and improvement in quality of life and (2) prevention of transmission to sexual partners. Given these goals, the recommended approaches to management of genital HSV infection differ based on the viral type (HSV-1 vs HSV-2) and the presence and absence of symptoms.
HSV-2: Symptomatic Infection
Symptomatic HSV-2 infection may be managed by suppressive therapy (daily medication to suppress recurrences and prevent transmission to sexual partners) or episodic therapy (short-term therapy to treat symptomatic recurrences). All patients should be aware of both treatment approaches to chronic HSV-2 infection and should be offered suppressive therapy. Although suppressive therapy to prevent HSV-2 transmission was studied in heterosexual couples, the mechanism for prevention is through suppression of viral shedding [ 23 ], and there is no biologic rationale that shedding would not be prevented in other populations. Therefore, suppressive therapy can be considered to prevent transmission in men who have sex with men (MSM), women who have sex with women, and transgender persons. However, suppressive therapy is not effective to decrease the risk of transmission among persons with HIV/HSV-2 coinfection [ 24 ].
HSV-2: Asymptomatic Infection
Approximately 20% of persons who are HSV-2 seropositive do not note genital symptoms consistent with genital herpes, even after receiving education about typical signs and symptoms of genital herpes [ 25 ]. Episodic and suppressive antiviral therapy are used predominantly to treat recurrences, prevent recurrences, and to prevent transmission to sexual partners. For patients with serological evidence of HSV-2 infection without symptomatic recurrences, neither episodic nor suppressive therapy are indicated for prevention of recurrences. The trial of suppressive therapy to prevent HSV-2 transmission to heterosexual sexual partners was conducted in persons who had symptomatic HSV-2 infection [ 23 ]. Among persons with asymptomatic infection, the efficacy of suppressive therapy to prevent HSV-2 transmission to sexual partners has not been studied. Given the 50% reduced risk of shedding among those with asymptomatic HSV-2 infection compared to symptomatic infection [ 26 ], the benefit of suppressive therapy for prevention of transmission is unknown in this population. Many persons diagnosed with genital HSV-2 infection recognize symptoms after education about the clinical manifestations of infection, and therefore may realize that they are symptomatic [ 25 ].
Genital HSV-1 Infection
Recurrences are less frequent with genital HSV-1 infection compared to genital HSV-2 infection [ 27 , 28 ]. Given this, episodic therapy is preferred over suppressive therapy in persons with genital HSV-1 infection. For patients with frequently recurring genital HSV-1, suppressive therapy may be considered.
For persons with either genital HSV-1 infection or those with asymptomatic HSV-2 infection, suppressive therapy may be considered for those who have substantial psychosocial distress due to genital herpes and/or anxiety about transmission to sexual partners.
Table 1 summarizes the evidence base for episodic and suppressive therapy among those with symptomatic HSV-1 and HSV-2 infection or asymptomatic HSV-2 infection.
Evidence for Use of Suppressive Therapy Among Populations with HSV-1 and HSV-2 Infection, with USPSTF Grade framework [ 29 ]
Abbreviations: A, recommended, high certainty that benefit is substantial; B, recommended, high certainty of moderate benefit or moderate certainty that benefit is moderate to substantial; C, recommend selectively–moderate certainty that there is small benefit; I, insufficient evidence to recommend; level of certainty, high, moderate, low.
Only studied in heterosexual population.
Key Question 3: Are there new therapies to treat recurrent genital herpes?
a. First clinical episode and episodic therapy
No new treatment regimens are FDA-approved for first clinical episode HSV or episodic HSV therapy.
b. Suppression
No new treatment regimens are FDA-approved for suppressive therapy of genital herpes recurrences.
Key Question 4: Are there new recommendations for treatment of acyclovir-resistant genital herpes?
Case reports suggest that brincidofovir [ 30 , 31 ], imiquimod [ 32 ], and topical cidofovir [ 33 ] may be useful in the treatment of acyclovir-resistant HSV infections. Clinical trials are ongoing for helicase-primase inhibitors (see Key Question 7).
Key Question 5: Of approved antiviral therapies, are there data comparing the efficacy of antivirals for episodic or suppressive therapy?
No new data comparing the efficacy of antivirals for episodic or suppressive therapy are available.
Key Question 6: Are there any data on the effectiveness of antivirals for genital HSV-1 vs genital HSV-2 infection?
No comparative data are available for treatment of genital HSV-1 vs HSV-2 infection. Based on the known biology of the infections and in vitro susceptibilities, it is not expected that there would be a difference in efficacy for treatment between the viral types.
Key Question 7: Are there new antivirals in development for treatment of genital herpes that are approved or have entered clinical trials?
Helicase-primase inhibitors have been studied in early-phase clinical trials but have not been evaluated in phase 3 studies and are not FDA-approved for treatment at this time [ 34–36 ]. There is currently an open-label study evaluating the helicase-primase inhibitor pritelivir for use in immunocompromised persons with acyclovir-resistant HSV infections (ClinicalTrials.gov identifier NCT03073967), and an early-access program to pritelivir has been initiated ( https://www.aicuris.com/75n6/Pritelivir-AIC316-.htm ).
Tenofovir preparations (tenofovir [TFV] intravaginal gel and oral tenofovir disoproxil fumarate [TDF]) have been studied in a crossover study for prevention of genital shedding and recurrences among women with HSV-2 infection and without HIV infection [ 37 ]. There was no difference in shedding or lesions with use of oral or vaginal tenofovir as compared to placebo [ 37 ]. Tenofovir is not recommended for treatment of HSV-2 infection.
Key Question 8: What is the preferred treatment for HSV meningitis?
HSV-2 meningitis is a rare complication of genital HSV-2 infection, which more commonly affects women [ 38 ]. HSV-2 meningitis is characterized clinically by signs of meningitis (headache, photophobia, fever, meningismus) and cerebrospinal fluid (CSF) lymphocytic pleocytosis, accompanied by mildly elevated protein and normal glucose [ 39 ]. HSV PCR from CSF should be obtained in suspected cases [ 40 , 41 ]. Optimal therapies for HSV-2 meningitis have not been studied, and practice patterns are highly variable [ 38 ]. For first episode HSV-2 meningitis, acyclovir 10mg/kg intravenously (IV) every 8 hours until resolution of fever and headache, followed by valacyclovir 1g TID (3 times daily) to complete a 14-day course, is suggested. Among persons with established recurrent HSV-2 meningitis, oral therapy may be used for the entire course. It is essential to distinguish cases of HSV encephalitis from HSV meningitis. HSV encephalitis is a much more severe infection with high neurologic morbidity and mortality, and should be treated with 14–21 days of IV acyclovir [ 42 ].
Recurrent HSV-2 meningitis is a rare complication of genital HSV-2 infection. However, most cases of recurrent lymphocytic meningitis are caused by HSV-2 (84% in 1 series [ 43 ], 78% in another [ 44 ]).
A randomized clinical trial showed that suppressive therapy (valacyclovir 500mg twice daily [BID]) did not prevent recurrent HSV-2 meningitis episodes, but it is likely that the dose was not sufficient for central nervous system penetration [ 45 ]. There was a statistically significant increased risk of HSV-2 meningitis the year after valacyclovir was discontinued, concerning for rebound. Valacyclovir 500mg BID is not recommended for suppression of HSV-2 meningitis.
III. Prevention of Sexual Transmission of Genital Herpes/Management of Sex Partners
Key Question 1: Are there new approaches for prevention of HSV-1/2 transmission from persons with genital herpes infection?
No new data were identified for prevention of HSV-1/2 transmission to sexual partners. The pivotal study performed in HSV-2–discordant, HIV-seronegative heterosexual couples showed a 48% decreased risk of transmission for those HSV-2–seropositive persons on valacyclovir 500mg daily compared to placebo [ 23 ].
a. What are the optimal strategies to prevent acquisition of genital herpes among persons who do not have HSV-2 or genital HSV-1 infection?
There are no new strategies for prevention of HSV identified. Consistent use of condoms and knowledge and disclosure of HSV-2 serostatus have been shown to decrease the risk of HSV-2 transmission previously [ 46 , 47 ].
b. Are vaccines available to prevent HSV acquisition or transmission?
Several therapeutic vaccines have been tested in early-phase clinical trials, but none have reached phase 3 trials and none are FDA-approved [ 48–50 ].
c. Are there new data on the effectiveness of condoms to prevent genital herpes?
An observational study conducted among HIV/HSV-2 serodiscordant heterosexual couples in several sites in Africa showed that condoms reduced the per-act risk of transmission from men to women by 96% (relative risk [RR], 0.04 [95% confidence interval {CI}, .01–.16]), but were not significantly effective in preventing transmission from women to men (RR, 0.35 [95% CI, .12–1.04]) [ 51 ]. These data are consistent with prior studies of condoms for prevention of HSV-2 transmission, particularly showing the efficacy of male condoms for prevention of transmission to women.
Key Question 2: Are there medications that can be taken as preexposure prophylaxis (PrEP) to prevent acquisition of genital herpes?
d. There are no data to indicate that antiherpesvirus medication (acyclovir, famciclovir, or valacyclovir) can be taken as PrEP to prevent HSV-2 acquisition.
e. What is the impact of taking HIV PrEP (TDF or TDF/emtricitabine [FTC]) on acquisition or reactivation of HSV-2 among HIV-seronegative persons?
Among HIV/HSV-2–seronegative men/women in HIV/HSV-2–heterosexual discordant partnerships in Africa, daily TDF was associated with 30% reduced risk of HSV-2 seroconversion (95% CI, .49–.99) [ 52 ].
Among MSM and transgender women, daily TDF/FTC was not associated with difference in HSV-2 acquisition, but there was a lower risk of clinically graded moderate to severe ulcers among those randomized to TDF/FTC [ 53 ].
Among MSM, on-demand PrEP (TDF/FTC) was not associated with decreased risk for HSV-2 acquisition [ 54 ].
Among women at high risk of acquiring HIV in South Africa, pericoitally applied intravaginal 1% TFV gel was associated with 51% reduction in HSV-2 acquisition (95% CI, .30–.77) [ 55 ]. Daily intravaginal 1% TFV gel with use confirmed by TFV detection in plasma did not meaningfully reduce the risk of HSV-2 acquisition among sexually active women in Africa (RR, 0.59; P = .60) [ 56 ].
Based on these data, oral TDF/FTC when used for HIV prevention as daily PrEP may also decrease the risk of HSV-2 acquisition among heterosexual populations. However, there is insufficient evidence that TDF/FTC use among those who are not at risk of HIV acquisition will prevent HSV-2 infection, and it should not be used for this sole purpose.
TFV gel may also be associated with decreased risk of HSV-2 acquisition when used pericoitally or daily. However, TFV gel is not FDA-approved at this time. In addition, there is insufficient evidence that TFV gel should be used among those who are not at risk of HIV acquisition to prevent HSV-2 infection.
Key Question 3: What is the impact of taking TDF/FTC on acquisition or reactivation of HSV-2 among individuals living with HIV?
Oral TDF does not prevent HSV-2 acquisition among persons with HIV infection who are taking TDF as part of the antiretroviral regimen [ 57 ]. In a subgroup analysis, there was a reduced risk of HSV-2 seroconversion among those with a CD4 count <200 cells/µL (reduced seroincidence by 56% [95% CI, 7%–80%]) [ 57 ].
In an observational study of persons with HSV-2 and HIV coinfection on antiretroviral therapy (ART), there was no difference in HSV-2 shedding rate between those using TDF-containing regimens vs non-TDF-containing regimens [ 58 ].
Key Question 4: What is the role of medical male circumcision (MMC) for prevention of genital HSV-2 infection in men and women?
Among men, there are inconsistent results regarding the efficacy of MMC to prevent HSV-2 acquisition. In one randomized controlled trial (RCT) of MMC in heterosexual adult men in Rakai, Uganda, HSV-2 acquisition was significantly decreased among men who underwent MMC (hazard ratio [HR], 0.72 [95% CI, .56–.92]) [ 59 ]. Additionally, a single-site study in South Africa found a 30% reduction in HSV-2 acquisition (95% CI, 1%–51%) [ 60 ]. However, another single-site study in Kenya with high HSV-2 incidence in both arms found no significant difference in HSV-2 acquisition with 72 months of follow-up (HR, 0.89 [95% CI, .73–1.09]) [ 61 ].
A systematic review of trials of MMC showed high consistency for decreased risk of HSV-2 acquisition among women with a male partner who underwent MMC in studies in Africa [ 62 ].
From review of these data, we conclude that MMC may be associated with decreased risk of HSV-2 acquisition in adult, heterosexual men and may be associated with a decreased risk of HSV-2 transmission from men to women.
IV. HSV-2/HIV Interactions
Key Question 1: What are the appropriate agents and regimens for treatment of HSV in persons with HIV infection?
No new data are available to change prior recommendations for treatment of HSV in persons with HIV infection.
Key Question 2: What is the effect of antiretroviral initiation on genital HSV-2 infection?
Several studies have explored the impact of ART initiation on genital ulcer disease (GUD), which is a common manifestation of immune reconstitution inflammatory syndrome reported in several studies [ 63 , 64 ].
In an observational study comparing persons with HIV infection who were ART treated vs ART naive, there was no difference in HSV-2 shedding rate. The shedding rate in this study was low [ 65 ].
In an RCT of acyclovir 400mg BID vs placebo among women in Uganda who started ART when CD4 count decreased to 250 cells/μL, GUD increased during the first 3 months after initiating ART and returned to baseline at 6 months [ 66 ]. The risk of GUD was significantly reduced on acyclovir (prevalence risk ratio, 0.42 [95% CI, .23–.74]) [ 51 ]. Another study showed that GUD incidence increased after starting ART and shedding increased; GUD incidence was lowest in persons on acyclovir [ 67 ].
Based on these data, suppressive acyclovir should be considered for the first 6 months after starting ART among people who are HSV-2 seropositive to reduce the risk of GUD, particularly among those with CD4 count <200 cells/μL, who are at highest risk of HSV-2 reactivation. Although valacyclovir and famciclovir have not been studied in this setting, given that these medications effectively suppress HSV-2 among people living with HIV, it may be reasonable to use these as well.
Key Question 3: Should antiviral therapy for HSV be administered to reduce the risk of HIV or HSV-2 transmission to sex partners in serodiscordant partnerships or to reduce mother-to-child transmission (MTCT) of HIV?
Well-conducted, large RCTs have shown that suppressive acyclovir (400 BID) does not reduce the risk of HIV or HSV-2 transmission among ART-naive persons with HIV/HSV-2 coinfection in heterosexual discordant partnerships [ 68 ].
A systematic review showed that HSV-2 infection is associated with increased risk of MTCT of HIV (odds ratio, 1.57 [95% CI, 1.17–2.11]) [ 69 ]. Studies of valacyclovir suppression to prevent MTCT of HIV infection to infants were conducted in the era before universal ART for all women who are pregnant with HIV was recommended [ 70 ], but similar trials have not been conducted in the universal ART era.
Based on these data, suppressive antiherpesvirus therapy is not recommended to prevent HIV or HSV-2 transmission among sexual partners of people with HIV/HSV-2 coinfection. There are insufficient data to assess whether suppressive antiviral therapy for HSV is beneficial for prevention of MTCT of HIV in the ART era.
Key Question 4: What is the role of anti–HSV-2 suppressive therapy in people living with HSV-2 and HIV to prevent HIV progression?
With HIV Infection, Not on ART
Suppressive valacyclovir (1g BID) is associated with slight decrease in plasma HIV viral load compared to suppressive acyclovir (400 BID) [ 71 ]. These data are no longer relevant as ART is now recommended for all persons.
With HIV Infection, Receiving ART
Suppressive antiherpesvirus therapy (valacyclovir 1g daily) was not associated with changes in CD4 cell counts or plasma HIV viral load in a placebo-controlled study [ 72 ].
In another study, valacyclovir 500mg BID was not associated with changes in inflammatory and immune activation markers among persons with HIV infection on ART [ 73 ].
Based on these data, there is no evidence that suppressive antiherpesvirus therapy is effective for delay of HIV disease progression or associated with decrease in HIV-related inflammation in persons on ART.
V. Prevention of Neonatal Herpes
Key Question 1: Is there evidence for or against routine screening of pregnant women with HSV type-specific serologies?
The American College of Obstetrics and Gynecology recommends against routine screening for HSV serostatus during pregnancy based on a lack of evidence for cost-effectiveness [ 22 ]. It is recommended to screen pregnant women for a history of genital herpes. There are no new data to inform routine screening of pregnant women for serologic evidence of HSV infection.
Key Question 2: Should women with HSV-2 infection receive suppressive therapy during pregnancy to reduce the risk of cesarean deliveries, HSV shedding, or HSV transmission to the infant?
Prior randomized clinical trials have demonstrated that women with a history of genital herpes have decreased risk of viral shedding, recurrences, and cesarean deliveries when suppressive acyclovir (400mg TID) or valacyclovir 500mg BID is given starting at 36 weeks’ gestational age [ 74 , 75 ]. A trial of 200 HSV-2–seropositive, HIV-seronegative women in Uganda randomized to receive acyclovir 400mg BID or placebo starting at 28 weeks’ gestation showed decreased risk of preterm birth and a trend toward decreased risk of premature rupture of membranes at 36 weeks among the acyclovir group [ 76 ]. However, additional data are needed to replicate this finding among a larger sample size.
Key Question 3: Are there any new data on the safety of HSV antivirals during pregnancy?
A case-control study from the National Birth Defects Prevention Study showed a 4.7-fold increased odds (95% CI, 1.7–13.3) for gastroschisis among women who used antiherpes medications between the month prior to conception and the third month of pregnancy [ 77 ]. There was also an increased risk for gastroschisis among women who were not using antiviral therapy but had a self-reported history of genital herpes. In addition, there were significant demographic differences between cases and controls, and possible recall bias. Acyclovir remains category B.
Key Question 4: Are there strategies to prevent neonatal herpes among women who acquire genital herpes during pregnancies and are at highest risk to transmit?
Treatment with suppressive-dose acyclovir (400 TID) at week 36 has been shown to prevent HSV recurrences requiring cesarean delivery at term [ 74 , 78 ]; whether this approach reduces the risk of neonatal HSV is unknown. Infants with neonatal herpes born to women who received suppressive therapy at the end of pregnancy have been reported [ 79 ]. Providers should be aware that acquisition of genital herpes during pregnancy is associated with the highest risk of transmission [ 80 ]. Invasive procedures at the time of delivery should be avoided if possible [ 22 ]. All women who have active genital lesions or prodrome at delivery should have a cesarean delivery [ 22 ].
Key Question 5: Are there strategies to prevent, diagnose, or treat HSV hepatitis in pregnancy?
Hepatitis is a rare manifestation of disseminated HSV, thought to be acquired through the genital tract, in pregnant women, characterized by severe hepatitis and fulminant liver failure with associated high mortality (25%) [ 81 ]. Women most frequently present in the second and third trimester with fever and hepatitis, with markedly elevated aminotransferases [ 81 ]. They may not have any genital or skin lesions. A high index of suspicion for HSV is necessary, and the diagnosis is made by HSV PCR from blood [ 82 ]. HSV should be ruled out in pregnant women with fever and unexplained severe hepatitis. In a small case series, no deaths were seen in women who were treated with empiric IV acyclovir [ 81 ]. For women who are diagnosed with HSV hepatitis, IV acyclovir 10mg/kg every 8 hours should be given until resolution.
Recommendations regarding treatment of neonatal herpes are beyond the scope of these guidelines. Guidance on management of neonatal herpes is detailed in the Red Book 2021 [ 83 ]. Algorithms for management, evaluation, and treatment of neonatal herpes are provided in the Red Book [ 83 ].
Counseling of Adults With Genital Herpes
Key Question 1: Are there evidence-based strategies for counseling persons with newly diagnosed genital herpes?
There are no evidence-based strategies for counseling patients with newly diagnosed genital herpes.
Key Question 2: What information should be included when counseling patients with newly diagnosed genital herpes?
Women have been shown to use various coping strategies after learning of diagnosis of HSV-2 infection [ 84 ]. These coping strategies decreased over time, suggesting that women adjust to the diagnosis.
While there are no data available to guide best counseling practices, information regarding the natural history (symptoms, asymptomatic shedding and transmission), management (suppressive and episodic therapy), prevention (suppressive therapy, no sex with prodrome/symptoms, disclosure, male condoms, suppressive therapy), and risk of genital herpes acquisition should be discussed. Information regarding asymptomatic HSV-2 infection should also be included. A comprehensive online genital herpes counseling tool has been developed by the Government of Canada [ 85 ].
How Do Counseling Messages Differ for Those With Genital HSV-1 vs HSV-2 Infection?
Genital HSV-1 infection is associated with less shedding and fewer recurrences compared to HSV-2 [ 86 ]. Therefore, although transmission to sexual partners is possible, it is less likely than HSV-2 because of decreased risk of shedding, particularly more than a year after infection [ 87 ]. Episodic therapy is recommended for recurrences. The risk-benefit ratio of suppressive therapy is unknown because there are fewer recurrences in genital HSV-1 infection. Suppressive therapy for genital HSV-1 infection has not been shown to reduce the risk of HSV-1 transmission to sexual partners.
Genital herpes remains an important STI given the high prevalence of HSV-2, the increasing proportion of cases due to HSV-1, and morbidity associated with recurrences of GUD. HSV-2 has a key role in fueling the HIV epidemic and, although rare, HSV-1 and HSV-2 are associated with devastating outcomes when acquired during pregnancy, both among women and neonates. Review of the literature for the 2021 CDC STI treatment guidelines has revealed few substantive advances for the management of genital herpes infections. Greater availability of nucleic acid amplification tests to diagnose HSV in the setting of genital ulcers will improve diagnosis in the acute setting, but serologic assays lack diagnostic accuracy and advances in the diagnostic algorithm as well as new diagnostic tools are needed. While symptoms of genital herpes can be managed and transmission to sexual partners prevented with antiviral therapy, novel therapies with new mechanisms of action will improve our ability to care for patients. Given that genital herpes affects a substantial proportion of adults, ongoing research to advance the field is urgently needed.
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments. The following experts are thanked for their advice and input:
Charles Ebel, Carolyn Gardella, Darrell Tan, Barbara Van Der Pol, Nicholas Van Wagoner, Anna Wald.
Supplement sponsorship. This supplement is sponsored by The Centers for Disease Control and Prevention.
Potential conflicts of interest. The author receives royalties from UpToDate; has been a consultant for AbbVie and Gilead; has received funds from MedPace for serving on a data and safety monitoring board; and has received grants from the National Institutes of Health, the Centers for Disease Control and Prevention, and the Bill & Melinda Gates Foundation.
The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
McQuillan G , Kruszon-Moran D , Flagg EW , Paulose-Ram R. Prevalence of herpes simplex virus type 1 and type 2 in persons aged 14-49: United States, 2015-2016. NCHS Data Brief 2018 ; 304 : 1 – 8 .
Google Scholar
Roberts CM , Pfister JR , Spear SJ. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis 2003 ; 30 : 797 – 800 .
Durukan D , Fairley CK , Bradshaw CS , et al. . Increasing proportion of herpes simplex virus type 1 among women and men diagnosed with first-episode anogenital herpes: a retrospective observational study over 14 years in Melbourne, Australia. Sex Transm Infect 2019 ; 95 : 307 – 13 .
Dabestani N , Katz DA , Dombrowski J , Magaret A , Wald A , Johnston C. Time trends in first-episode genital herpes simplex virus infections in an urban sexually transmitted disease clinic. Sex Transm Dis 2019 ; 46 : 795 – 800 .
Fanfair RN , Zaidi A , Taylor LD , Xu F , Gottlieb S , Markowitz L. Trends in seroprevalence of herpes simplex virus type 2 among non-Hispanic blacks and non-Hispanic whites aged 14 to 49 years—United States, 1988 to 2010. Sex Transm Dis 2013 ; 40 : 860 – 4 .
Masese L , Baeten JM , Richardson BA , et al. . Changes in the contribution of genital tract infections to HIV acquisition among Kenyan high-risk women from 1993 to 2012. AIDS 2015 ; 29 : 1077 – 85 .
US Food and Drug Administration . In vitro diagnostics: nucleic acid base tests/microbial tests. 2019 . Available at: https://www.fda.gov/medical-devices/vitro-diagnostics/nucleic-acid-based-tests . Accessed 5 April 2019.
Binnicker MJ , Espy MJ , Duresko B , Irish C , Mandrekar J. Automated processing, extraction and detection of herpes simplex virus types 1 and 2: a comparative evaluation of three commercial platforms using clinical specimens. J Clin Virol 2017 ; 89 : 30 – 3 .
Van Der Pol B. Type-specific detection of herpes simplex virus type 1 and type 2 using the cobas(R) HSV 1 and 2 test on the cobas(R) 4800 platform. Expert Rev Mol Diagn 2016 ; 16 : 1145 – 54 .
Parra-Sanchez M , Marcuello Lopez A , Garcia-Rey S , et al. . Performance of the HSV OligoGen kit for the diagnosis of herpes simplex virus type 1 and 2. Diagn Microbiol Infect Dis 2016 ; 85 : 315 – 7 .
Corey L , Adams HG , Brown ZA , Holmes KK. Clinical course of genital herpes simplex virus infections in men and women. Ann Intern Med 1983 ; 48 : 973 .
Wald A , Huang ML , Carrell D , Selke S , Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis 2003 ; 188 : 1345 – 51 .
Gitman MR , Ferguson D , Landry ML. Comparison of Simplexa HSV 1 & 2 PCR with culture, immunofluorescence, and laboratory-developed TaqMan PCR for detection of herpes simplex virus in swab specimens. J Clin Microbiol 2013 ; 51 : 3765 – 9 .
Wald A , Ashley-Morrow R. Serological testing for herpes simplex virus (HSV)-1 and HSV-2 infection. Clin Infect Dis 2002 ; 35 : S173 – 82 .
Ashley RL , Militoni J , Lee F , Nahmias A , Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol 1988 ; 26 : 662 – 7 .
Agyemang E , Le QA , Warren T , et al. . Performance of commercial enzyme-linked immunoassays for diagnosis of herpes simplex virus-1 and herpes simplex virus-2 infection in a clinical setting. Sex Transm Dis 2017 ; 44 : 763 – 7 .
Golden MR , Ashley-Morrow R , Swenson P , Hogrefe WR , Handsfield HH , Wald A. Herpes simplex virus type 2 (HSV-2) Western blot confirmatory testing among men testing positive for HSV-2 using the focus enzyme-linked immunosorbent assay in a sexually transmitted disease clinic. Sex Transm Dis 2005 ; 32 : 771 – 7 .
Ashley-Morrow R , Krantz E , Wald A. Time course of seroconversion by HerpeSelect ELISA after acquisition of genital herpes simplex virus type 1 (HSV-1) or HSV-2. Sex Transm Dis 2003 ; 30 : 310 – 4 .
Morrow RA , Friedrich D , Meier A , Corey L. Use of “Biokit HSV-2 rapid assay” to improve the positive predictive value of Focus HerpeSelect HSV-2 ELISA. BMC Infect Dis 2005 ; 5 : 84 .
Prince HE , Batterman HJ , Schwab DA. Herpes simplex virus type 2 (HSV-2) IgG index values in two immunoassays in relation to HSV-2 IgG inhibition assay results. Diagn Microbiol Infect Dis 2019 ; 95 : 114864 .
Feltner C , Grodensky C , Ebel C , et al. . Serologic screening for genital herpes: an updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016 ; 316 : 2531 – 43 .
ACOG Committee on Practice Bulletins . Clinical management guidelines for obstetrician-gynecologists. No. 82 June 2007. Management of herpes in pregnancy. Obstet Gynecol 2007 ; 109 : 1489 – 98 .
Corey L , Wald A , Patel R , et al. . Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med 2004 ; 350 : 11 – 20 .
Mujugira A , Magaret AS , Celum C , et al. . Daily acyclovir to decrease herpes simplex virus type 2 (HSV-2) transmission from HSV-2/HIV-1 coinfected persons: a randomized controlled trial. J Infect Dis 2013 ; 208 : 1366 – 74 .
Wald A , Zeh J , Selke S , et al. . Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons . N Engl J Med 2000 ; 342 : 844 – 50 .
Tronstein E , Johnston C , Huang ML , et al. . Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 2011 ; 305 : 1441 – 9 .
Langenberg AG , Corey L , Ashley RL , Leong WP , Straus SE. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. N Engl J Med 1999 ; 341 : 1432 – 8 .
Benedetti JK , Corey L , Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med 1994 ; 121 : 847 – 54 .
US Preventive Services Task Force . U.S. Preventive Services task force ratings. 2021 . Available at: https://www.uspreventiveservicestaskforce.org/uspstf/us-preventive-services-task-force-ratings . Accessed 14 August 2021.
El-Haddad D , El Chaer F , Vanichanan J , et al. . Brincidofovir (CMX-001) for refractory and resistant CMV and HSV infections in immunocompromised cancer patients: a single-center experience. Antiviral Res 2016 ; 134 : 58 – 62 .
Voigt S , Hofmann J , Edelmann A , Sauerbrei A , Kuhl JS. Brincidofovir clearance of acyclovir-resistant herpes simplex virus-1 and adenovirus infection after stem cell transplantation. Transpl Infect Dis 2016 ; 18 : 791 – 4 .
Tandon S , Singh J , Sinha S , Sharma DP. Recalcitrant hypertrophic herpes genitalis in HIV-infected patient successfully treated with topical imiquimod. Dermatol Ther 2017 ; 30 : 6 .
Epstein JB , Gharapetian S , Rejali AR , Zabner R , Lill M , Tzachanis D. Complex management of resistant oral herpes simplex virus infection following hematopoietic stem cell transplantation: potential role of topical cidofovir. Support Care Cancer 2016 ; 24 : 3603 – 6 .
Wald A , Corey L , Timmler B , et al. . Helicase-primase inhibitor pritelivir for HSV-2 infection. N Engl J Med 2014 ; 370 : 201 – 10 .
Wald A , Timmler B , Magaret A , et al. . Effect of pritelivir compared with valacyclovir on genital HSV-2 shedding in patients with frequent recurrences: a randomized clinical trial. JAMA 2016 ; 316 : 2495 – 503 .
Tyring S , Wald A , Zadeikis N , Dhadda S , Takenouchi K , Rorig R. ASP2151 for the treatment of genital herpes: a randomized, double-blind, placebo- and valacyclovir-controlled, dose-finding study. J Infect Dis 2012 ; 205 : 1100 – 10 .
Bender Ignacio RA , Perti T , Magaret AS , et al. . Oral and vaginal tenofovir for genital herpes simplex virus type 2 shedding in immunocompetent women: a double-blind, randomized, cross-over trial. J Infect Dis 2015 ; 212 : 1949 – 56 .
Landry ML , Greenwold J , Vikram HR. Herpes simplex type-2 meningitis: presentation and lack of standardized therapy. Am J Med 2009 ; 122 : 688 – 91 .
Shalabi M , Whitley RJ. Recurrent benign lymphocytic meningitis. Clin Infect Dis 2006 ; 43 : 1194 – 7 .
Schlesinger Y , Tebas P , Gaudreault-Keener M , Buller RS , Storch GA. Herpes simplex virus type 2 meningitis in the absence of genital lesions: improved recognition with use of the polymerase chain reaction. Clin Infect Dis 1995 ; 20 : 842 – 8 .
Binnicker MJ , Espy MJ , Irish CL. Rapid and direct detection of herpes simplex virus in cerebrospinal fluid by use of a commercial real-time PCR assay. J Clin Microbiol 2014 ; 52 : 4361 – 2 .
Tunkel AR , Glaser CA , Bloch KC , et al. . The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2008 ; 47 : 303 – 27 .
Kallio-Laine K , Seppanen M , Kautiainen H , et al. . Recurrent lymphocytic meningitis positive for herpes simplex virus type 2. Emerg Infect Dis 2009 ; 15 : 1119 – 22 .
Kupila L , Vainionpaa R , Vuorinen T , Marttila RJ , Kotilainen P. Recurrent lymphocytic meningitis: the role of herpesviruses. Arch Neurol 2004 ; 61 : 1553 – 7 .
Aurelius E , Franzen-Rohl E , Glimaker M , et al. . Long-term valacyclovir suppressive treatment after herpes simplex virus type 2 meningitis: a double-blind, randomized controlled trial. Clin Infect Dis 2012 ; 54 : 1304 – 13 .
Martin ET , Krantz E , Gottlieb SL , et al. . A pooled analysis of the effect of condoms in preventing HSV-2 acquisition. Arch Intern Med 2009 ; 169 : 1233 – 40 .
Wald A , Krantz E , Selke S , Lairson E , Morrow RA , Zeh J. Knowledge of partners’ genital herpes protects against herpes simplex virus type 2 acquisition. J Infect Dis 2006 ; 194 : 42 – 52 .
Bernstein DI , Wald A , Warren T , et al. . Therapeutic vaccine for genital herpes simplex virus-2 infection: findings from a randomized trial. J Infect Dis 2017 ; 215 : 856 – 64 .
Dutton JL , Woo WP , Chandra J , et al. . An escalating dose study to assess the safety, tolerability and immunogenicity of a herpes simplex virus DNA vaccine, COR-1. Hum Vaccin Immunother 2016 ; 12 : 3079 – 88 .
Van Wagoner N , Fife K , Leone PA , et al. . Effects of different doses of GEN-003, a therapeutic vaccine for genital herpes simplex virus-2, on viral shedding and lesions: results of a randomized placebo-controlled trial. J Infect Dis 2018 ; 218 : 1890 – 9 .
Magaret AS , Mujugira A , Hughes JP , et al. . Effect of condom use on per-act HSV-2 transmission risk in HIV-1, HSV-2-discordant couples. Clin Infect Dis 2016 ; 62 : 456 – 61 .
Celum C , Morrow RA , Donnell D , et al. . Daily oral tenofovir and emtricitabine–tenofovir preexposure prophylaxis reduces herpes simplex virus type 2 acquisition among heterosexual HIV-1–uninfected men and women. Ann Intern Med 2014 ; 161 : 1 – 15 .
Marcus JL , Glidden DV , McMahan V , et al. . Daily oral emtricitabine/tenofovir preexposure prophylaxis and herpes simplex virus type 2 among men who have sex with men. PLoS One 2014 ; 9 : e91513 .
Chaix ML , Charreau I , Pintado C , et al. . Effect of on-demand oral pre-exposure prophylaxis with tenofovir/emtricitabine on herpes simplex virus-1/2 incidence among men who have sex with men: a substudy of the ANRS IPERGAY trial. Open Forum Infect Dis 2018 ; 5 : ofy295 .
Abdool Karim SS , Abdool Karim Q , Gengiah TN. Tenofovir gel to prevent HSV-2 infection. N Engl J Med 2015 ; 373 : 1980 – 1 .
Marrazzo JM , Rabe L , Kelly C , et al. . Tenofovir gel for prevention of herpes simplex virus type 2 acquisition: findings from the VOICE trial. J Infect Dis 2019 ; 219 : 1940 – 7 .
Celum C , Hong T , Cent A , et al. . Herpes simplex virus type 2 acquisition among HIV-1-infected adults treated with tenofovir disoproxyl fumarate as part of combination antiretroviral therapy: results from the ACTG A5175 PEARLS study. J Infect Dis 2017 ; 215 : 907 – 10 .
Tan DH , Kaul R , Raboud JM , Walmsley SL. No impact of oral tenofovir disoproxil fumarate on herpes simplex virus shedding in HIV-infected adults. AIDS 2011 ; 25 : 207 – 10 .
Tobian AA , Serwadda D , Quinn TC , et al. . Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med 2009 ; 360 : 1298 – 309 .
Sobngwi-Tambekou J , Taljaard D , Lissouba P , et al. . Effect of HSV-2 serostatus on acquisition of HIV by young men: results of a longitudinal study in Orange Farm, South Africa. J Infect Dis 2009 ; 199 : 958 – 64 .
Mehta SD , Moses S , Agot K , et al. . Medical male circumcision and herpes simplex virus 2 acquisition: posttrial surveillance in Kisumu, Kenya. J Infect Dis 2013 ; 208 : 1869 – 76 .
Grund JM , Bryant TS , Jackson I , et al. . Association between male circumcision and women’s biomedical health outcomes: a systematic review. Lancet Glob Health 2017 ; 5 : e1113 – 22 .
Ratnam I , Chiu C , Kandala NB , Easterbrook PJ. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis 2006 ; 42 : 418 – 27 .
Couppie P , Sarazin F , Clyti E , et al. . Increased incidence of genital herpes after HAART initiation: a frequent presentation of immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients. AIDS Patient Care STDS 2006 ; 20 : 143 – 5 .
Tan DH , Raboud JM , Kaul R , Walmsley SL. Antiretroviral therapy is not associated with reduced herpes simplex virus shedding in HIV coinfected adults: an observational cohort study. BMJ Open 2014 ; 4 : 2013 – 004210 .
Tobian AA , Grabowski MK , Serwadda D , et al. . Reactivation of herpes simplex virus type 2 after initiation of antiretroviral therapy. J Infect Dis 2013 ; 208 : 839 – 46 .
Fife KH , Mugwanya K , Thomas KK , et al. . Transient increase in herpes simplex virus type 2 (HSV-2)-associated genital ulcers following initiation of antiretroviral therapy in HIV/HSV-2-coinfected individuals. J Infect Dis 2016 ; 213 : 1573 – 8 .
Mujugira A , Magaret AS , Celum C , et al. ; Partners in Prevention HSV/HIV Transmission Study Team . Daily acyclovir to decrease HSV-2 transmission from HSV-2/HIV-1 co-infected persons: a randomized controlled trial. J Infect Dis 2013 ; 208 : 1366 – 74 .
Sivarajah V , Venus K , Yudin MH , Murphy KE , Morrison SA , Tan DH. Does maternal HSV-2 coinfection increase mother-to-child transmission of HIV? A systematic review. Sex Transm Infect 2017 ; 93 : 535 – 42 .
Drake AL , Roxby AC , Ongecha-Owuor F , et al. . Valacyclovir suppressive therapy reduces plasma and breast milk HIV-1 RNA levels during pregnancy and postpartum: a randomized trial. J Infect Dis 2012 ; 205 : 366 – 75 .
Perti T , Saracino M , Baeten JM , et al. . High-dose valacyclovir decreases plasma HIV-1 RNA more than standard-dose acyclovir in persons coinfected with HIV-1 and HSV-2: a randomized crossover trial. J Acquir Immune Defic Syndr 2013 ; 63 : 201 – 8 .
Van Wagoner N , Geisler WM , Bachmann LH , Hook EW. The effect of valacyclovir on HIV and HSV-2 in HIV-infected persons on antiretroviral therapy with previously unrecognised HSV-2. Int J STD AIDS 2015 ; 26 : 574 – 81 .
Yi TJ , Walmsley S , Szadkowski L , et al. . A randomized controlled pilot trial of valacyclovir for attenuating inflammation and immune activation in HIV/herpes simplex virus 2-coinfected adults on suppressive antiretroviral therapy. Clin Infect Dis 2013 ; 57 : 1331 – 8 .
Sheffield JS , Hill JB , Hollier LM , et al. . Valacyclovir prophylaxis to prevent recurrent herpes at delivery: a randomized clinical trial. Obstet Gynecol 2006 ; 108 : 141 – 7 .
Scott LL , Hollier LM , McIntire D , Sanchez PJ , Jackson GL , Wendel GD Jr . Acyclovir suppression to prevent recurrent genital herpes at delivery. Infect Dis Obstet Gynecol 2002 ; 10 : 71 – 7 .
Nakubulwa S , Kaye DK , Bwanga F , Tumwesigye NM , Nakku-Joloba E , Mirembe F. Effect of suppressive acyclovir administered to HSV-2 positive mothers from week 28 to 36 weeks of pregnancy on adverse obstetric outcomes: a double-blind randomised placebo-controlled trial. Reprod Health 2017 ; 14 : 31 .
Ahrens KA , Anderka MT , Feldkamp ML , Canfield MA , Mitchell AA , Werler MM. Antiherpetic medication use and the risk of gastroschisis: findings from the National Birth Defects Prevention Study, 1997-2007. Paediatr Perinat Epidemiol 2013 ; 27 : 340 – 5 .
Scott LL , Hollier LM , McIntire D , Sanchez PJ , Jackson GL , Wendel GD Jr . Acyclovir suppression to prevent clinical recurrences at delivery after first episode genital herpes in pregnancy: an open-label trial. Infect Dis Obstet Gynecol 2001 ; 9 : 75 – 80 .
Pinninti SG , Angara R , Feja KN , et al. . Neonatal herpes disease following maternal antenatal antiviral suppressive therapy: a multicenter case series. J Pediatr 2012 ; 161 : 134 – 8.e1 .
Brown Z , Vontver L , Benedetti J , et al. . Effects on infants of first episode genital herpes during pregnancy. N Engl J Med 1987 ; 317 : 1247 – 51 .
Magawa S , Tanaka H , Furuhashi F , et al. . A literature review of herpes simplex virus hepatitis in pregnancy. J Matern Fetal Neonatal Med 2018 ; 29 : 1 – 6 .
Masadeh M , Shen H , Lee Y , et al. . A fatal case of herpes simplex virus hepatitis in a pregnant patient. Intractable Rare Dis Res 2017 ; 6 : 124 – 7 .
American Academy of Pediatrics . Red Book 2021 . 2021 Available at: https://redbook.solutions.aap.org/book.aspx?bookid=2591 . Accessed 14 August 2021.
Davis A , Roth A , Brand JE , Zimet GD , Van Der Pol B. Coping strategies and behavioural changes following a genital herpes diagnosis among an urban sample of underserved Midwestern women. Int J STD AIDS 2016 ; 27 : 207 – 12 .
Government of Canada . Genital herpes counselling tool. 2019 . Available at: https://www.canada.ca/en/public-health/services/infectious-diseases/sexual-health-sexually-transmitted-infections/canadian-guidelines/sexually-transmitted-infections/genital-herpes-counselling-tool.html . Accessed 11 April 2021.
Langenberg A , Corey L , Ashley R , Leong W , Straus S. A prospective study of new infections with herpes simplex virus type 1 and type 2. N Engl J Med 1999 ; 341 : 1432 – 8 .
Johnston C , Magaret A , Stern M , et al. , eds. Natural history of oral and genital herpes simplex virus type 1 (HSV-1) shedding and lesions following first episode genital HSV infection. In: World STI & HIV Congress , Vancouver, Canada , 2019 .
- simplexvirus
- genital herpes
- centers for disease control and prevention (u.s.)
- human herpesvirus 1
- human herpesvirus 2
- sexually transmitted diseases
- treatment guidelines
- herpes simplex type 2 infection
Supplementary data
Email alerts, more on this topic, related articles in pubmed, citing articles via, looking for your next opportunity.
- Recommend to your Library
Affiliations
- Online ISSN 1537-6591
- Print ISSN 1058-4838
- Copyright © 2024 Infectious Diseases Society of America
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
- Open access
- Published: 03 June 2024
Clinical characteristics and outcomes of patients with Herpes Simplex Encephalitis in Vietnam: a retrospective study
- Ta Thi Dieu Ngan 1 , 2 , 3 ,
- Nguyen Thi Tuyet 4 ,
- Dinh Trong Hung 1 ,
- Nguyen Trung Cap 2 ,
- Duy Manh Nguyen 5 &
- Vu Quoc Dat 1 , 3
BMC Infectious Diseases volume 24 , Article number: 556 ( 2024 ) Cite this article
Metrics details
Herpes simplex encephalitis (HSE) is an important central nervous infection with severe neurological sequelae. The aim of this study was to describe clinical characteristic and outcomes of patients with HSE in Vietnam.
This was a retrospective study of 66 patients with herpes simplex encephalitis who admitted to the National Hospital for Tropical Diseases, Hanoi, Vietnam from 2018 to 2021. The detection of herpes simplex virus (HSV) in cerebrospinal fluid was made by the real-time PCR assay. We reported the clinical manifestation on admission and evaluated clinical outcomes at the hospital discharge by modified Rankin Scale (mRS). Multivariate logistic regression analysis was used to analyze the independent risk factors of severe outcomes.
Of the 66 patients with laboratory confirmed HSE, the median age was 53 years (IQR 38–60) and 44 patients (69.7%) were male. The most common manifestations included fever (100%), followed by the consciousness disorder (95.5%). Other neurological manifestation were seizures (36.4%), memory disorders (31.8%), language disorders (19.7%) and behavioral disorders (13.6%). Conventional magnetic resonance imaging (MRI) showed 93.8% patients with temporal lobe lesions, followed by abnormalities in insula (50%), frontal lobe (34.4%) and 48.4% of patients had bilateral lesions. At discharge, 19 patients (28.8%) completely recovered, 15 patients (22.7%) had mild sequelae, 28 patients (42.4%) had moderate to severe sequelae. Severe neurological sequelae were memory disorders (55.8%), movement disorders (53.5%), language disorders (30.2%). Multivariate logistic regression analysis showed that Glasgow score decrement at admission, seizures, and time duration from onset of symptoms to the start of Acyclovir treatment > 4 days were independent factors associated with severe outcomes in HSE patients.
Glasgow score decrement, seizures and delay treatment with Acyclovir were associated with the poor outcome of patients with HSE.
Peer Review reports
Introduction
Herpes simplex virus (HSV) encephalitis is a life-threatening central nervous system infection associated with poor outcomes [ 1 ]. It occurs sporadically with a frequency of 2–4 cases per 1,000,000 population per year in high-income countries [ 2 ]. Over 90% of HSE cases are caused by HSV-1, with the remainder attributed to HSV-2 [ 3 ].
Prior to the availability of effective antiviral treatment, the mortality rate of encephalitis caused by HSV was about 70% [ 4 ]. In the 1980s, the introduction of intravenous Acyclovir significantly improved the prognosis of HSE, reducing the mortality rate to only 6-11% [ 3 ]. In the 1990s, the use of polymerase chain reaction (PCR) for HSV detection in the cerebrospinal fluid further enhanced early prognosis and treatment [ 5 ]. However, the rate of neurological sequelae remains high, happening in 44–62% of the surviving patients [ 6 ] with the delay in antiviral initiation as the most common reason [ 7 , 8 , 9 ].
In the context of Vietnam, HSE constitutes 7% of pediatric cases with acute encephalitis [ 10 ] and around 3% of suspected central nervous system (CNS) infections in adults [ 11 ]. However, research on this severe condition, both domestically and internationally, is restricted, due to the low incidence of this disease and the prevalence of small-scale studies primarily focusing on clinical symptoms. This study aims to address this gap by providing a comprehensive analysis of the clinical characteristics and prognostic factors linked to poor outcomes in HSE patients using a substantial cohort of cases from 2018 to 2021 at the National Hospital for Tropical Diseases (NHTD), a tertiary hospital specialized in treating infectious diseases.
Materials & methods
Study design.
This retrospective study analyses medical records of all patients who were diagnosed with HSE at the National Hospital for Tropical Diseases (NHTD), which is a referral hospital for treatment of infectious diseases in the North of Vietnam, from July 1, 2018, to June 30, 2021. The inclusion criteria of patients included: (1) over 18 years of age, (2) suspected encephalitis according to the guidelines of the International Encephalitis Association (2013) [ 12 ] and (3) had positive HSV DNA in cerebrospinal fluid tests using Real-time PCR assay (Bio-Rad Iq5 Multicolor Real Time PCR Detection System and Chromo4 real-time PCR detection system). Criteria for suspected encephalitis consisted of major criterion of altered mental status (defined as decreased or altered level of consciousness, coma, or personality changes) lasting ≥ 24 h with no other identifiable cause and at least of 2 minor criteria of fever, generalized or partial convulsions, new onset of focal neurologic findings, CSF with lymphocytosis (≥ 5 white blood cells/µl) and brain parenchymal abnormalities on neuroimaging or EEG suggestive of encephalitis [ 12 ]. Patients with pre-existing brain injury, history of mental disorders, inability to communicate normally (mute or deaf), or concurrent bacterial meningitis were excluded from the study. The protocol of this study was approved by the Ethics Committee and review board of the National Hospital for Tropical Diseases (IORG0006480) with approval number 9 A/HDDD-NĐTƯ dated 22, June 2021. As the study was conducted retrospectively, the informed consent waiver was accepted by the Ethics Committee and review board of the National Hospital for Tropical Diseases.
Data collection and outcome assessment
We collected information of (1) demographics, (2) clinical symptoms and signs, (3) results of laboratory testing and cerebrospinal fluid analysis at admission and during the hospitalization, (4) reports of brain MRI and CT, and (5) treatment (Acyclovir, mechanical ventilator).
The patient’s outcomes at the time of discharge were evaluated using the modified Rankin scale (mRS) [ 13 ]. The scores of mRS were classified as follow: 0 (No sequelae), 1 (No significant sequelae despite having symptoms, capable of performing all usual tasks and activities), 2 (Slight sequelae, unable carry out all previous activities, but able to look after own affairs without assistance), 3 (Moderate sequelae, requiring some help but able to walk without assistance), 4 (Moderately severe sequelae, unable to wall or attend to own bodily needs without assistance), 5 (Severe sequelae, being bedridden, incontinent and requiring constant nursing care or attention) and 6 (Dead). The outcomes of the patients were categorized as mild if the mRs score was < 3 or as severe if the mRS score was ≥ 3.
Statistical analysis
SPSS software version 20.0 was used for statistical analysis. Descriptive analysis was performed to explore the demography, clinical manifestations, and laboratory results. As the data were not normally distributed, non-parametric tests were used for statistical analysis, specifically the Mann-Whitney U test to compare the median of the quantitative variables and χ2 test to compare the proportions of the qualitative variables. While a preliminary analysis was published on a subset of participants in Vietnam Medical Journal [ 14 ] this work was performed on the completed sample using the univariate and multivariate logistic regression analysis to explore the independent factors associated with disease severity. The statistically significant cutoff was p < 0.05.
Patient characteristics
66 eligible patients were included in the study, with a median age of 53 (IQR, 38–60 years), of whom 46 patients (69.7%) were male. There were 30.3% of patients who had at least one comorbidity, in which the most common comorbidity was cardiovascular diseases (18.2%), followed by diabetes (9.1%). Most of the patients (90.9%) were transferred from primary and secondary-level hospitals to the NHTD. The median time from the onset of symptoms to hospital admission was approximately 5.5 days (IQR 3–7 days) while the median time from the onset of symptoms to administration of Acyclovir was 6.0 days. The clinical manifestation and laboratory results were showed in the Table 1 . Fever was presented in all patients while meningeal signs were apparent in 57 patients (86.4%). 63 patients (95.5%) were admitted with disturbed consciousness, in which 24 patients (36.4%) had seizures. The median of the GCS is 13, which has been rounded up to preserve its clinical significance. 25 patients (37.9%) required admission to the intensive care unit, in which the majority of patients required mechanical ventilation and developed ventilator associated pneumonia.
64 patients (97%) had brain MRI results. The median duration from the onset of symptoms to the MRI examination was 6 (IQR, 4–9) days with the common lesions being observed in the temporal lobe (93.8%), insular lobe (50%), and frontal lobe (34.4%).
Figure 1 . illustrates patient outcomes at discharge: 28.8% achieved full recovery (mRS = 0), 22.7% had mild sequelae (0 < mRS ≤ 2), 42.4% experienced moderate to severe sequelae (3 ≤ mRS ≤ 5), and 4 deaths. Of the 43 patients with sequelae, common symptoms included memory disorders (55.8%), movement disorders (53.5%), and language disorders (30.2%).
Outcomes at discharge according to the mRS
Prognostic factors for severe outcome
The differences in the frequency of symptoms and laboratory results on admission according to mRS were showed in the Tables 2 and 3 . The patients with mRS score ≥ 3 had higher rate of seizures (56.2% vs. 17.6%), lower GCS score (12 points vs. 13 points), higher rate of hemiplegia (15.6% vs. 0%), and more frequency of multiple lobar involvements in MRI (46.7% vs. 14.7%).
Multivariate logistic regression analysis showed that lower GCS at admission (aOR: 0.25–0.76 per 1 point increment), seizure (aOR 1.65–37.14) and time from onset to the start of Acyclovir treatment > 4 days (aOR 1.48–44.31) were independent factors related to severe prognosis in patients with HSE, as shown in Table 4 .
A study between 2014 and 2017 in Vietnam reveals that the main pathogens responsible for CNS infections among 137 patients were Streptococccus suis (12%) and Neisseria meningitidis (7%), followed by HSV (3%) [ 15 ]. Similarly, another study in Southern Vietnam over the duration of 12 years (1996–2008) found that HSV ranked 2nd among viral etiologies for CNS infection (6.5%) after Japanese encephalitis virus (12%) [ 16 ]. Our study is the first to focus on HSE in the Northern Vietnamese regions with a significant number of patients. Within the scope of our research, we found that the in-hospital mortality was at 6.1%, which was similar to the those reported by studies in both France (5.5%) [ 17 ] and India (6.8%) [ 18 ]. Additionally, we also identified an association between poor outcomes in HSE patients and neurological symptoms as well as delays in antiviral initiations, serving as potential prognosis factors.
Among the symptoms, consciousness status at the time of admission determined by the GCS score is strongly associated with poor outcomes in HSE patients. According to our analysis, the lower the GCS, the higher the risk of being discharged from hospital in severe condition. The probability of hospital discharge with severe conditions decreases by 0.44 times when GCS increases by 1 point. This result is in accordance with that of the study by Kamei et al., in which the same probability decreases by 1.45 times per 1 increment of GCS score before treatment [ 19 ]. Although we did not focus on the threshold of GCS as a prognostic factor, previous reports have discussed this. A multinational study by Erdem et al. in 2015 with 438 patients showed that those with a GCS score of less than 5 experienced poor outcomes more frequently [ 20 ]. Similarly, Whitley also assessed the prognostic capability of GCS score across all age ranges and confirmed that a GCS score of less than 6 can predict a poor outcome in HSE patients, irrespective of the medicine administered or the age of the patients [ 21 ].
Along with worsening consciousness, seizure is another clinical manifestation of HSE patients, with an incidence rate ranging from 35 to 65% [ 22 ]. Our analysis indicates that seizure on admission is an independent prognosis of severity. In particular, patients with seizures are 7.84 times more likely in a severe condition than those without. Therefore, the presence of seizures should also be closely monitored in HSE patients.
In treating HSE patients, the administration of Acyclovir has been reported to be of paramount importance, especially during the early stage of the disease [ 8 , 20 , 23 , 24 ]. Correspondingly, our analysis found that patients who received Acyclovir 4 days after the onset of symptoms are 8.1 times more likely to be in severe conditions, indicating that it is more beneficial for patients to be administered Acyclovir early. However, the benefits of the empirical use of Acyclovir in patients likely diagnosed with HSE without any confirmation from molecular tests such as HSV CFS PCR tests have not been clinically proven. In the study by Benson and Swadron, 17 out of 24 patients (71%) with suspected HSE was not administered with Acyclovir in the emergency department but rather in in-patient settings, though the morbidity and mortality of the patients were not examined due to the short study length [ 25 ]. In addition, despite having high sensitivity and specificity [ 21 ], standard HSV CFS PCR test requires long time for confirmation and also poses possibility of false positives [ 26 , 27 ], leading to our proposal that the decision to treat HSE early should be based on clinical evaluation and brain imaging rather than deferring treatments until the PCR result is available.
Due to the retrospective nature of our study, we were restricted to evaluate the outcomes of the patients at the time of hospital discharge, and we could not follow up on the sequelae caused by HSE for a longer term. It is also probable that there are some restrictions in comparing the clinical characteristics because the results did not correspond to the disease progression of the patients included in the study as well as the referral system from tertiary healthcare centers might cause delays in recording such characteristics.
HSE was associated with poor outcome and high case-fatality at hospital discharge in Vietnamese patients. This study highlights the importance of early diagnosis and empirical treatment in the lower level of healthcare system in Vietnam to improve the clinical outcome.
Data availability
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Alanine aminotransferase
Aspartate aminotransferase
Central nervous system
Cerebrospinal fluid
Computed tomography
C reactive protein
- Herpes simplex encephalitis
Herpes simplex virus
Glasgow Coma Score
Magnetic resonance imaging
Modified Rankin Scale
National Hospital for Tropical Diseases
Polymerase Chain Reaction
White blood cell
Rui-Yun Zhang. Research on early diagnosis and impact prognostic factors of herpes simplex encephalitis. Int J Clin Exp Med. 2016;:9(2):4695–8.
Google Scholar
Solomon T, Hart IJ, Beeching NJ. Viral encephalitis: a clinician’s guide. Pract Neurol. 2007;7:288–305.
Article PubMed Google Scholar
Tyler KL. Herpes simplex virus infections of the central nervous system: encephalitis and meningitis, including Mollaret’s. Herpes J IHMF. 2004;11(Suppl 2):A57–64.
Whitley RJ, Soong SJ, Dolin R, Galasso GJ, Ch’ien LT, Alford CA. Adenine arabinoside therapy of biopsy-proved herpes simplex encephalitis. National Institute of Allergy and Infectious diseases collaborative antiviral study. N Engl J Med. 1977;297:289–94.
Article CAS PubMed Google Scholar
Aurelius E, Johansson B, Sköldenberg B, Staland A, Forsgren M. Rapid diagnosis of herpes simplex encephalitis by nested polymerase chain reaction assay of cerebrospinal fluid. Lancet Lond Engl. 1991;337:189–92.
Article CAS Google Scholar
Whitley RJ, Alford CA, Hirsch MS, Schooley RT, Luby JP, Aoki FY, et al. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med. 1986;314:144–9.
Dagsdóttir HM, Sigurðardóttir B, Gottfreðsson M, Kristjánsson M, Löve A, Baldvinsdóttir GE, et al. Herpes simplex encephalitis in Iceland 1987–2011. SpringerPlus. 2014;3:524.
Article PubMed PubMed Central Google Scholar
Sili U, Kaya A, Mert A. Herpes simplex virus encephalitis: clinical manifestations, diagnosis and outcome in 106 adult patients. J Clin Virol. 2014;60:112–8.
Raschilas F, Wolff M, Delatour F, Chaffaut C, De Broucker T, Chevret S, et al. Outcome of and prognostic factors for Herpes Simplex Encephalitis in Adult patients: results of a Multicenter Study. Clin Infect Dis. 2002;35:254–60.
Bich HT, Van Lam N, Hoang Thi Hoa. Complications of Encephalitis and Health Care Needs at Vietnam National Children’s hospital (2018–2019). J Pediatr Res Pract. 2020;4:41–8.
Taylor WR, Nguyen K, Nguyen D, Nguyen H, Horby P, Nguyen HL, et al. The Spectrum of Central Nervous System infections in an Adult Referral Hospital in Hanoi, Vietnam. PLoS ONE. 2012;7:e42099.
Article CAS PubMed PubMed Central Google Scholar
Venkatesan A, Tunkel AR, Bloch KC, Lauring AS, Sejvar J, Bitnun A, et al. Case definitions, Diagnostic algorithms, and priorities in Encephalitis: Consensus Statement of the International Encephalitis Consortium. Clin Infect Dis. 2013;57:1114–28.
Riera-Mestre A, Gubieras L, Martínez-Yelamos S, Cabellos C, Fernández-Viladrich P. Adult herpes simplex encephalitis: fifteen years’ experience. Enfermedades Infecc Microbiol Clínica. 2009;27:143–7.
Article Google Scholar
Ta Thi Dieu Ngan, Nguyen Thi Tuyet. Evaluation the outcomes of herpes simplex encephalitis in patients treated at National Hospital for Tropical Diseases from the year 2018 to 2022. Vietnam Med J. 2023;523:20–5.
Gabor JJ, Anh CX, Sy BT, Hoan PQ, Quyen DT, The NT, et al. Aetiologies and clinical presentation of central nervous system infections in Vietnamese patients: a prospective study. Sci Rep. 2022;12:18065.
Tan LV, Thai LH, Phu NH, Nghia HDT, Chuong LV, Sinh DX, et al. Viral aetiology of central nervous system infections in adults admitted to a tertiary referral hospital in southern Vietnam over 12 years. PLoS Negl Trop Dis. 2014;8:e3127.
Jouan Y, Grammatico-Guillon L, Espitalier F, Cazals X, François P, Guillon A. Long-term outcome of severe herpes simplex encephalitis: a population-based observational study. Crit Care. 2015;19:345.
Jain P, Jain A, Kumar A, Prakash S, Khan DN, Singh KP, et al. Epidemiology and etiology of Acute Encephalitis Syndrome in North India. Jpn J Infect Dis. 2014;67:197–203.
Kamei S, Sekizawa T, Shiota H, Mizutani T, Itoyama Y, Takasu T, et al. Evaluation of combination therapy using aciclovir and corticosteroid in adult patients with herpes simplex virus encephalitis. J Neurol Neurosurg Psychiatry. 2005;76:1544–9.
Erdem H, Cag Y, Ozturk-Engin D, Defres S, Kaya S, Larsen L, et al. Results of a multinational study Suggest the need for Rapid Diagnosis and early antiviral treatment at the Onset of Herpetic Meningoencephalitis. Antimicrob Agents Chemother. 2015;59:3084–9.
Whitley RJ. Herpes simplex encephalitis: adolescents and adults. Antiviral Res. 2006;71:141–8.
Gnann JW, Whitley RJ. Herpes Simplex Encephalitis: an update. Curr Infect Dis Rep. 2017;19:13.
Stahl JP, Mailles A, Broucker TD. Group the SC and I. herpes simplex encephalitis and management of acyclovir in encephalitis patients in France. Epidemiol Infect. 2012;140:372–81.
Sheybani F, Arabikhan HR, Naderi HR. Herpes Simplex Encephalitis (HSE) and its outcome in the patients who were admitted to a Tertiary Care Hospital in Mashhad, Iran, over a 10-year period. J Clin Diagn Res JCDR. 2013;7:1626–8.
CAS PubMed Google Scholar
Benson PC, Swadron SP. Empiric acyclovir is infrequently initiated in the Emergency Department to patients ultimately diagnosed with encephalitis. Ann Emerg Med. 2006;47:100–5.
Weil AA, Glaser CA, Amad Z, Forghani B. Patients with suspected herpes simplex encephalitis: rethinking an initial negative polymerase chain reaction result. Clin Infect Dis off Publ Infect Dis Soc Am. 2002;34:1154–7.
Adler AC, Kadimi S, Apaloo C, Marcu C. Herpes simplex encephalitis with two false-negative cerebrospinal fluid PCR tests and review of negative PCR results in the clinical setting. Case Rep Neurol. 2011;3:172–8.
Download references
Acknowledgements
Not applicable.
Author information
Authors and affiliations.
Department of Infectious Diseases, Hanoi Medical University, 1 Ton That Tung Street, Dong Da district, Hanoi, Vietnam
Ta Thi Dieu Ngan, Dinh Trong Hung & Vu Quoc Dat
National Hospital for Tropical Diseases, 78 Giai Phong Street, Dong Da District, Hanoi, Vietnam
Ta Thi Dieu Ngan & Nguyen Trung Cap
Hanoi Medical University Hospital, 1 Ton That Tung Street, Dong Da District, Hanoi, Vietnam
Ta Thi Dieu Ngan & Vu Quoc Dat
Thai Nguyen University of Medicine and Pharmacy, 284 Luong Ngoc Quyen Street, Thai Nguyen City, Thai Nguyen Province, Vietnam
Nguyen Thi Tuyet
Grinnell College, 1115 8th Avenue, Grinnell, IA, 50112, USA
Duy Manh Nguyen
You can also search for this author in PubMed Google Scholar
Contributions
T.T.D.N. designed the study and drafted the manuscript. N.T.T. collected, analyzed, and interpreted the data. D.M.N. interpreted the data and drafted the manuscript. All authors reviewed the manuscript.
Corresponding author
Correspondence to Ta Thi Dieu Ngan .
Ethics declarations
Ethics approval and consent to participate.
The protocol of research has been approved by the Ethics Committee and review board of the National Hospital for Tropical Diseases (IORG0006480), approval number 9 A/HĐĐĐ-NĐTƯ dated on June 22, 2021. The Ethics Committee and review board of the National Hospital for Tropical Disease also approved the waiver of informed consent for participation due to the retrospective nature of the study.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ngan, T.T.D., Tuyet, N.T., Hung, D.T. et al. Clinical characteristics and outcomes of patients with Herpes Simplex Encephalitis in Vietnam: a retrospective study. BMC Infect Dis 24 , 556 (2024). https://doi.org/10.1186/s12879-024-09453-3
Download citation
Received : 06 November 2023
Accepted : 30 May 2024
Published : 03 June 2024
DOI : https://doi.org/10.1186/s12879-024-09453-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Prognostic factors
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
- Education Home
- Medical Education Technology Support
- Graduate Medical Education
- Medical Scientist Training Program
- Public Health Sciences Program
- Continuing Medical Education
- Clinical Performance Education Center
- Center for Excellence in Education
- Research Home
- Biochemistry & Molecular Genetics
- Biomedical Engineering
- Cell Biology
- Microbiology, Immunology, & Cancer Biology (MIC)
- Molecular Physiology & Biological Physics
- Neuroscience
- Pharmacology
- Public Health Sciences
- Office for Research
- Clinical Research
- Clinical Trials Office
- Funding Opportunities
- Grants & Contracts
- Research Faculty Directory
- Cancer Center
- Cardiovascular Research Center
- Carter Immunology Center
- Center for Behavioral Health & Technology
- Center for Brain Immunology & Glia
- Center for Diabetes Technology
- Center for Immunity, Inflammation & Regenerative Medicine
- Center for Public Health Genomics
- Center for Membrane & Cell Physiology
- Center for Research in Reproduction
- Myles H. Thaler Center for AIDS & Human Retrovirus Research
- Child Health Research Center (Pediatrics)
- Division of Perceptual Studies
- Research News: The Making of Medicine
- Core Facilities
- Virginia Research Resources Consortium
- Center for Advanced Vision Science
- Charles O. Strickler Transplant Center
- Keck Center for Cellular Imaging
- Institute of Law, Psychiatry & Public Policy
- Translational Health Research Institute of Virginia
- Clinical Home
- Anesthesiology
- Dermatology
- Emergency Medicine
- Family Medicine
- Neurosurgery
- Obstetrics & Gynecology
- Ophthalmology
- Orthopaedic Surgery
- Otolaryngology
- Physical Medicine & Rehabilitation
- Plastic Surgery, Maxillofacial, & Oral Health
- Psychiatry & Neurobehavioral Sciences
- Radiation Oncology
- Radiology & Medical Imaging
- UVA Health: Patient Care
- Diversity Home
- Diversity Overview
- Student Resources
- GME Trainee Resources
- Faculty Resources
- Community Resources
Anna Cliffe, PhD, Awarded $2.7 Million to Study How ATRX Protein Protects Against Herpes Simplex Virus, which impacts more than 60% of the U.S. population.
May 30, 2024 by [email protected]
Anna Cliffe, PhD, an associate professor in the Department of Microbiology, Immunology, and Cancer Biology, was awarded a $2.7 million R01 grant from the National Institutes of Health for a project titled “The role of ATRX in both promoting the establishment of HSV latency and restricting reactivation.”
The Cliffe lab studies mechanisms of Herpes Simplex Virus (HSV) latent infection and reactivation in neurons. More than 60% of the U.S. population is infected with the virus, which can cause a range of disease outcomes, including oral and genital lesions, keratitis, and encephalitis, and potentially contribute to the development of Alzheimer’s disease. The virus persists for life as a latent infection of neurons and can periodically reactivate in response to a variety of stimuli to cause disease. LEARN MORE
- MIC Graduate Studies
- MIC Postdocs
- MIC Seminars
- Diversity, Equity and Inclusion
- Latest News
- Uncategorized
Harmol used for the treatment of herpes simplex virus induced keratitis
- Open access
- Published: 27 May 2024
- Volume 21 , article number 118 , ( 2024 )
Cite this article
You have full access to this open access article

- Huanhuan Xu 1 na1 ,
- Nan Zhou 4 na1 ,
- Zhenping Huang 1 ,
- Jing Wu 3 &
- Yajie Qian 2
223 Accesses
23 Altmetric
Explore all metrics
Herpes simplex virus type 1 (HSV-1) infection of the eyes results in herpes simplex keratitis (HSK), which has led to vision loss and even blindness in patients. However, the rate of drug resistance in HSV is on the rise; therefore, new antiviral agents with sufficient safety profiles must be developed. At present, we assessed the anti-HSV-1 activity of 502 natural compounds and their ability to reduce the HSV-1-induced cytopathic effect. We chose harmol for further studies because it exhibited the highest antiviral activity. We found that harmol inhibited both HSV-1 F and HSV-1/153 (a clinical drug-resistant strain) replication, with an EC 50 of 9.34 µM and 5.84 µM, respectively. Moreover, harmol reduced HSV-1 replication in corneal tissues and viral progeny production in tears, and also alleviated early corneal surface lesions related to HSK. For example, harmol treatment preserved corneal thickness and nerve density in HSK mice. Interestingly, harmol also showed a promising antiviral effect on HSV-1/153 induced HSK in mouse model. Furthermore, harmol combined with acyclovir (ACV) treatment showed a greater antiviral effect than either one alone in vitro. Therefore, harmol may be a promising therapeutic agent for managing HSK.
Similar content being viewed by others

Medicinal Plants Against Herpes Simplex Virus (HSV) Type 2 Infections: Ethnopharmacology, Chemistry, Clinical, and Preclinical Studies

Medicinal Plants Against Herpes Simplex Virus (HSV) Type 2 Infections: Ethnopharmacology, Chemistry, and Clinical and Preclinical Studies
Anti-herpes virus activity of the carnivorous botanical, Sarracenia purpurea
Introduction.
Herpes simplex keratitis (HSK) is a prevalent infectious disease in ophthalmology clinics [ 8 ] characterized by corneal stromal implication, progressive corneal opacity and visual impairment [ 34 ]. The estimated incidence of HSK in developed countries is 10–30/100,000 of the population per year, which is higher in developing nations [ 38 ]. In fact, in low-income countries, HSK is responsible for a significant number of cases of blindness compared to other infectious eye diseases [ 22 ]. If HSK is not controlled in a timely manner, it can lead to potentially fatal encephalitis [ 39 ]. Therefore, exploring HSK treatment options is essential for the clinical management of HSK.
HSK is associated with herpes simplex virus type 1 (HSV-1) infection and lesser extent HSV-2 [ 9 ]. HSV-1 is a highly prevalent enveloped double-stranded DNA (dsDNA) virus that exhibits structural complexity and is widely distributed among the global population [ 4 , 41 ]. Therefore, blocking HSV infection is critical to controlling HSK development. Currently, ACV, a nucleoside analog, has been considered a gold standard medication for HSV infections [ 43 ]. However, drug resistance to HSV is on the rise, particularly in immunocompromised patients [ 36 ]. Worryingly, current anti-herpesvirus drugs have similar pharmacologic effects [ 32 ]. Therefore, it is crucial to explore novel anti-HSV-1 agents that possess unique pharmacological properties.
Harmol, a β-carboline alkaloid, is present in several medicinal plants, including Peganum harmala, Passiflora incarnate, and Bansteriopsis caapi [ 1 ]. Chemically, harmol (C 12 H 10 N 2 O with Molecular Mass 198.225) is a natural indole alkaloid, 9 H-beta-carboline with a methyl substituent at C-1 and a hydroxy group at C-7; having similar functionality as beta-carboline [ 35 ]. Previous study showed that harmol exhibited antifungal effects because harmol suppressed the infection of Penicillium digitatum and Botrytis cinerea [ 30 ]. Furthermore, harmol also inhibited the replication of Newcastle disease virus (NDV, a negative-stranded RNA virus) [ 44 ], and dengue virus (DENV, a positive-stranded RNA virus) [ 33 ], which suggested that harmol might have potential antiviral activity. The antiviral activity of harmol in HSV (dsDNA virus) infectious diseases, especially HSV-1-related HSK, was largely unknown.
In our current study, we assessed the anti-HSV activity of 502 natural compounds in vitro and harmol was identified as the most effective anti-HSV compound. Then we evaluated the therapeutic efficacy of harmol in the HSK mouse model and found that harmol not only inhibited HSV-1 and HSV-1/153 infections in vit ro but also alleviated the early symptoms of HSK in vivo.
Materials & methods
Cells and viruses.
Vero cells were obtained from the American Type Culture Collection (ATCC) and maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) with 10% fetal bovine serum (FBS) (FND500, ExCell Bio, Shanghai, China). HSV-1 F strain (ATCC VR-733) and ACV-resistant clinical HSV-1 strain (HSV-1/153) were kindly provided by Yan Lu, Department of Ophthalmology, Jingling Hospital, Jiangsu, China [ 17 ]. HSV-1/153, a TK-mutant derived from HSV-1 (KOS)-HSV-1/Blue was first isolated by Tao Peng (Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences) [ 45 ].
HSV-1 and HSV-1/153 were propagated on Vero cells. Vero cells were inoculated with HSV-1 or HSV-1/153 at a multiplicity of infection (MOI) = 0.1 when reaching ~ 80% confluence. And then cultural supernatant containing virus particles was collected when VSV-GFP-infected Vero cells showed > 80% cytopathic effect (CPE). The cultural supernatant containing virus particles was centrifuged at 1000 ~ 1500 g for 5 min to remove cellular debris. Virus particles were preserved in DMEM basic medium at a reduced volume of 1/100 and stored in small aliquots at -80 °C until required. Viral titers were determined by tissue culture infective dose (TCID 50 ) assay. Titers were expressed as Log 10 Plaque-forming unit (PFU) per mL (PFU/mL).
The harmol used in this study was isolated from Peganum harmala Linn, and was obtained from MedChemExpress (Shanghai, China). Harmol powder was prepared by dissolving it in Dimethyl Sulphoxide (DMSO). ACV was obtained from the National Institutes for Food and Drug Control in China and dissolved in ddH 2 O.The antibodies against gD-1 (Cat# sc-69,802) (Santa Cruz, CA, USA), and GAPDH (CST, MA, USA) were stored at -20℃. Goat anti-rabbit IgG IRDyeTM 680 antibody and Goat anti-mouse IgG IRDyeTM 800 antibody were obtained from LI-COR (Lincoln, NE, USA). RIPA lysis buffer was obtained from Beyotime (Nanjing, Jiangsu, China).
The natural product library
The library was purchased from the National Center in Shanghai City, which contains 502 natural compounds. Each natural product in the library was highly purified with a known molecular weight and chemical constitution. All of the products were dissolved in 96-well plates with DMSO to 10 mM. The concentration of the compounds used for the screening assay was 10 µM.
Cytotoxicity assay
Vero cells were treated with harmol or ACV for 72 h, and then cell viability was detected by Cell Counting Kit 8 (CCK-8) (Beyotime Biotechnology, Shanghai, China) assay. Subsequently, OD values were detected at 450 nm. The formula of cell viability was (OD treatment – OD treatment-blank )/ (OD control – OD control-blank ) × 100%. The 50% cytotoxic concentration (CC 50 ), the concentration of compound that inhibited the cell viability by 50%, was calculated based on the CalcuSyn computer program [ 13 ].
Natural compounds screening assays
Screening of natural monomer compounds with anti-HSV-1 activity from a library of compounds by a cytopathic effect (CPE) inhibition assay [ 25 ]. Vero cells (4 × 10 4 cells) were seeded into 96-well plates and cultured for 24 h. The cells were treated with each compound (10 µM) in natural product library or ACV (1 µM) at 37 °C for 30 min. ACV was considered a positive control. Subsequently, the cells were infected with HSV-1 F (MOI = 0.5). For the compounds screening experiment, viral inhibition (%) was calculated by CPE. After 48 h cultured, virus-infected Vero cells were placed under an inverted microscope (Nikon, TS100) to observe CPE (%), which was recorded from 0 to 100% depending on the extent of the CPE area [ 20 ]. And then the viral inhibition (%) was calculated as follows: 100% - CPE (%). The infected cells showed 100% CPE under the microscope (Scale bar: 50 μm).
Antiviral activity of harmol
Serial dilutions of harmol were added in 100 µL total volume and infected with HSV-1 (MOI = 0.5) for 48 h. The EC 50 , which represents the concentration of the compound that provided 50% protection against virus-induced CPEs in cells, was determined using the CalcuSyn computer program [ 13 ]. Selectivity index (SI) was defined as the safe range for determining the effects of drugs, and SI = CC 50 /EC 50 . Vero cells were pretreated with harmol or ACV, and then they were infected with HSV-1 F or HSV-1/153 (MOI = 0.5 or 1.0) in the presence of the test compound. Subsequently, the in-cell Western assay was used to determine the viral replication or viral progeny by detecting the expression of HSV-1 gD-1 protein. Viral inhibition (%) of harmol was calculated using the following formula: [1 − fluorescence gD /fluorescence control ] * 100%.

Quantitative real-time PCR (qPCR)
HSV-1F or HSV-1F/153 infected Vero cells were treated with harmol or ACV for 48 h. Total RNA was extracted using Trizol assay, and then reverse transcribed RNA into cDNA with a total reaction volume of 10 µL. Subsequently, 2 × AceQ Universal SYBR qPCR master mix was used for qPCR detection. The qPCR was performed using an ABI 7300 instrument from the USA. The primer sequences of HSV-1 gD-1 were as following : Forward, 5’ AGCAGGGGTTAGGGAGTTG 3’; Reverse, 5’ CCATCTTGAGAGAGGCATC 3’.
In-cell western assay
The In-cell Western assay was performed according to our previous study [ 52 ] via using the Odyssey Imaging System from Li-COR Biosciences (NE, USA). The HSV-1 replication was quantified using Mouse gD-1 antibody (green), and the relative amount of gD protein expression was determined by normalizing it to the endogenous DRAQ5 signal (red) (Biostatus, Cat# DR05500, UK) in all experiments.
Western blot assay
The cells were lysed by adding RIPA lysis buffer supplemented with protease inhibitors and incubated in an ice bath for 10 min. The total protein concentrations were determined using the BCA protein assay kit from Pierce (Rockford, IL, USA). The western blot assay was performed according to our previous study [ 52 ]. The protein bands were scanned using the Li-COR Odyssey Infrared Imager, and then the gray levels of the blots were quantified using Image J software.
TCID 50 assay
The viral titers for HSV-1 F and HSV-1/153 were estimated by 50% tissue culture infective dose (TCID 50 ) method according to previous methods [ 23 ]. Vero cells were seeded in 96-well plates and cultured in DMEM medium supplemented with 2% FBS for 24 h. Subsequently, 10-fold serial dilutions of the supernatant was collected from HSV-1-infected, harmol-treated and ACV-treated Vero cells, as well as HSK mouse tear samples were used to infect Vero cell monolayers for 48 h. Calculations of TCID 50 were performed according to the Reed-Muench method [ 7 ], the viral titers were expressed as log 10 PFU/mL, and PFU/mL = 0.56×TCID 50 [ 47 ].
Mouse HSK model establishment
To the best of our knowledge, primary infection of the cornea of BALB/c mouse with a large dose of the HSV-1 (1 × 10 5 PFU/mL) can lead to severe HSK and persist for several months [ 46 ]. A previous study found that HSV-1-related corneal infection led to the establishment of sympathetic latency in the HSK mouse model, which was similar to that in HSK patients [ 21 ]. Moreover, the pathogenesis of the HSK mouse model was consistent with that of HSK patients [ 50 ].
BALB/c mice ( n = 24) were randomly divided into four groups including control group, HSK group, harmol treatment group, and ACV treatment group. Mice were anesthetized with an intraperitoneal injection of 1% sodium pentobarbital (80 mg/kg) for anesthesia. And then eyes were inoculated with 5 µL of HSV-1 F (1 × 10 5 PFU/mL), HSV-1/153 (1 × 10 5 PFU/mL) or PBS by 3 (horizontal) × 3 (vertical) strokes on the right cornea with a 27-gauge needle. 24 h after infection (day 1), mice were treated with harmol (0.01 mg/kg, 5 µL/mouse), ACV (0.01 mg/kg, 5 µL/mouse), or PBS (equal volume of PBS) on days 1, 2, 3, 4 and 5 post-infection via eye drops (3 times/day). Then, we chose days 3 and 5 to evaluate the antiviral activity of harmol in vivo as well as the body weight of HSK mice decreased from day 3 and reached a maximum on day 5, which was consistent with a previous study [ 49 ].
Corneal fluorescein sodium staining and scoring
The mice were treated with fluorescein staining on days 3, 4, and 5. The eyes were then rinsed with 0.9% saline and subjected to a biomicroscope for examination under blue light. The scoring of epithelial keratitis following viral infection (0 to 4) was performed by fluorescein sodium staining based on a previous study [ 9 ].
0 point: no visible damage.
1 point: diffuse punctate lesion.
2 point: dendritic lesion occupying less than 1/4 of the entire epithelial area.
3 point: severe dendritic lesion extending more than 1/4 of the entire epithelial area.
4 point: geographic lesion on the epithelial area.
Blepharitis scoring
The scoring of blepharitis (0 to 4) on day 3 and 5 post infection (d pi) were determined according to a previous study [ 28 ]. The following is a brief description of these criteria:
0 point: no swelling of eyelids;
1 point: mild swelling of the eyelids;
2 points: moderate swelling of the eyelids plus moderate crusting;
3 points: half of eyelids shut plus severe crusting;
4 points: eyelids completely shut.
RTvue OCT examination
The corneal thickness was obtained using RTvue OCT (RTvue 100-2, Optovue, Inc, USA), which was equipped with a cornea-anterior module long adaptor lens. The mice were anesthetized and the “pachymetry” scan pattern was used for image collection. To measure the corneal thickness, four points on the horizontal and vertical radial lines within central 1 mm zones of the cornea were selected respectively, and then each point thickness was measured manually. The average thickness of these eight points was calculated as corneal thickness.
In vivo confocal microscopy (IVCM) examination
BALB/c mice were anaesthetized via intraperitoneal injection with 6 mg/mL ketamine and 4 mg/mL xylazine. The corneas of the mice were positioned on the objective lens and the front surface of a poly-methyl-methacrylate (PMMA) cap (Tomo-cap; Heidelberg Engineering GmbH), which served as a coupling medium, in all IVCM (HRT III RCM, Heidelberg Engineering, Germany) tests. The operator viewed real-time video images displayed on a computer screen, manually adjusting the target area and depth. Subbasal nerve plexus images and corneal vasculature images measuring 400 × 400 microns were then captured. Based on a previous study [ 12 , 16 ], the images were processed using NeuronJ, version 1.4.3, a plug-in for ImageJ ( https://imagescience.org/meijering/software/neuronj/ ), which traces nerves and calculates the nerve density.
Statistical analysis
For in vivo experiments, 3–6 mice were included in each group and conducted once experiment in this study. Data were analyzed by GraphPad Prisms version 9.0 software (GraphPad Software, La Jolla, CA, USA). Statistical differences between groups were analyzed by One-way ANOVA, Repeated measures ANOVA, Dunnett-t test, SNK-q test, Student t test. Statistical significance was expressed as P < 0.05.
Harmol inhibits HSV-1 replication
We first screened the natural compounds with anti-HSV-1 activity by CPE inhibition assay in Vero cells. We found that 22 compounds showed the percent of viral inhibition > 75%, and harmol (the percent of viral inhibition > 90%) was chosen for further study because of its high antiviral activity (Fig. 1 A). We next investigated the cytotoxicity of harmol in Vero cells to explore the proper concentration for further study. In our study, CC 50 of harmol was 242.54 µM, and non-cytotoxic concentrations of harmol (12.5 µM) were confirmed for the following study in vitro (Fig. 1 B). A previous study has demonstrated that the EC 50 values of ACV for HSV-1 F and HSV-1/153 were 0.209 ± 0.056 µM and > 100 µM, respectively [ 9 ]. In our experiment, a working concentration of 1 µM ACV was utilized as a positive control. Both harmol (12.5 µM) and ACV (1 µM) treatments were able to reduce HSV-1-related CPEs (Fig. 1 C). In summary, harmol was the most potent anti-HSV-1 agent from the nature product library.

A primary anti-HSV-1 screen assay. A After a 2 h pretreatment with these compounds, Vero cells were infected with HSV-1 F (MOI = 0.5). The anti-HSV-1 effect was determined by measuring the CPE as a percentage. B Vero cells in 96-well plate were cultured with harmol (0, 6.25, 12.5, 25.0, 50.0, 100.0, 150.0, 200.0, 250.0 µM) for 48 h. Cell viability was measured using a CCK-8 assay. C Vero cells were infected with HSV-1 F (MOI = 0.5) and then they were treated with harmol (12.5 µM) or ACV (1 µM) for 24 h. Images were obtained by inverted microscope, scale bar: 50 μm. The experiments were duplicated three times, and corresponding results were shown ( ns : non-significant, ** P < 0.01, *** P < 0.001)
Harmol inhibited HSV-1 replication in vitro
Initially, we investigated the anti-HSV-1 effect of harmol by measuring gD-1 protein expression. ACV treatment at 1 µM was considered as a positive control in our present study. ACV (1 µM) and harmol (0, 3.125, 6.25, 12.5, 25, 50 µM) treatments both reduced HSV-1 gD-1 protein expression (Fig. 2 A and B). We further assessed the anti-HSV-1 activity of harmol. The EC 50 of harmol on HSV-1 F was 9.34 µM, which is lower than its cytotoxicity concentrations (Fig. 2 C). Harmol could also reduce the production of offspring viruses (Fig. 2 D and E). We further attempted to investigate whether harmol could suppress HSV-1/153 replication. Our present results showed that harmol, but not ACV, also reduced the protein level of HSV-1/153 gD-1 in Vero cells (Fig. 2 F and G). Furthermore, the EC 50 of harmol on HSV-1/153 was 5.84 µM (Fig. 2 H). Harmol also exhibited a reduction of 2 logs of HSV-1/153 viral titers (Fig. 2 I). The SI value of harmol on the HSV-1 F strain was 26.0, and SI value of harmol on the HSV-1/153 strain was 44.5. In summary, harmol reduced HSV-1 F and HSV-1/153 replication and viral progeny production in vitro.

Harmol reduced HSV-1 F and HSV-1/153 replication in vitro. A Vero cells were pretreated with ACV (1 µM) or harmol (0, 3.125, 6.25, 12.5, 25, 50 µM) for 2 h, subsequently, they were challenged by HSV-1 F (MOI = 0.5) for 24 h. The HSV-gD-1 (green) expression was identified by in-cell western assay, which was further normalized by DRAQ5 (red). B The expression of gD-1 mRNA was detected by qPCR analysis. C Anti-HSV-1 effect (%) was counted by in-cell western assay. D , E HSV-1-infected Vero cells were treated with different concentrations of harmol or ACV. The supernatant was collected at 48 h and the viral titers were measured by TCID 50 assay. ACV treatment at 1 µM was used as a positive control. All experiments were duplicated three times, and corresponding results were shown. F , G Vero cells were pretreated with various concentrations of harmol (0, 3.125, 6.25, 12.5, 25, 50 µM) for 2 h, and then they were infected with HSV-1/153 (MOI = 0.5) for 48 h. gD-1 protein expression (green) and DRAQ5 (red) were determined by In-cell Western assay. H Anti-HSV-1 effect (%) was shown. I The supernatant was collected at 48 h and the viral titers were measured by a TCID 50 assay. All trials were duplicated three times, and corresponding results were shown. Data expressed as Mean ± SD ( ns : non-significant, ** P < 0.01, *** P < 0.001)
Harmol, in combination with ACV, showed a better anti-HSV-1 effect
We further assessed the anti-HSV-1 effect of harmol in combination with ACV. Compared to harmol or ACV treatment groups, harmol, together with ACV, inhibited HSV-1 F gD-1 mRNA expression and the production of its progeny viruses (Fig. 3 A, B). Furthermore, dose-increased harmol treatment (0, 3.12, 6.25, 12.5 µM) significantly reduced gD protein expression, and harmol in combination with ACV showed better anti-HSV-1 F effects (Fig. 3 C, D). The same outcomes were achieved in cells infected with HSV-1/153 (Fig. 3 E-H). Altogether, our results indicated that harmol enhanced the anti-HSV-1 effect of ACV, including ACV-resistant strain.

The combination of harmol and ACV was more effective than either one alone. A HSV-1 infected Vero cells were treated with harmol (12.5 µM), ACV (1 µM) or harmol (12.5 µM) + ACV (1 µM) for 48 h in the presence of harmol and ACV. qPCR assay was used to detect the gD-1 mRNA expression level. B The cell supernatants derived from ( A ), were further evaluated by a TCID 50 assay, the viral titers were expressed as log 10 PFU/mL (PFU/mL = 0.56×TCID 50 ). C , D An MOI of 0.5 of HSV-1 F-infected Vero cells were treated with 0.02% DMSO, harmol (0, 3.12, 6.25, 12.5 µM), ACV (1.0 µM) and harmol (3.12, 6.25, 12.5 µM) + ACV (1.0 µM) for 48 h. The gD-1 protein expression was detected by Western Blot assay ( C ) and the quantification of the blots was performed by Image J software ( D ). E - G HSV-1/153 infected Vero cells were treated with 0.02% DMSO, harmol (12.5 µM), ACV (1.0 µM) and harmol (12.5 µM) together with ACV (1.0 µM). Total RNA and total proteins were harvested to detect gD-1 mRNA and protein expression, respectively. H TCID 50 assay was used to measure the viral proliferation from the cell supernatants derived from ( E ). Data expressed as Mean ± SD ( ns : non-significant, * P < 0.05, *** P < 0.001)
Harmol may exhibit anti-HSV-1 effects at various stages during HSV-1 infection
To investigate at which stage of the HSV-1 infection cycle harmol exerts its antiviral effects. We next conducted a time of addition experiment. Vero cells were infected with HSV-1 (MOI = 0.5), and then they were added with harmol (1 µM) at various times (-2 h, 0 h, 6 h, 12 h, and 24 h) post-infection. We utilized qPCR assay to detect gD-1 mRNA expression. We observed that pretreatment with harmol significantly inhibited HSV-1 replication, a phenomenon that was also observed at other time points of harmol addition, despite the diminished antiviral efficacy of harmol with prolonged addition time ( Fig. 4 ) . In conclusion, harmol may exert anti-HSV-1 effects during the early stages of viral infection.

The time of addition experiment of harmol. Vero cells were infected with HSV-1 (MOI = 0.5), and then they were added with harmol (1 µM) at various times (-2 h, 0 h, 6 h, 12 h, and 24 h) post-infection.We utilized qPCR assay to detect gD-1 mRNA expression
Safety of topical treatment of harmol on the mouse cornea
To investigate the safety of topical application of harmol on the murine corneas, the eyes were treated with 5 µL harmol (0.01 mg/kg, approximately equal to 100 µM) three times on day 1, day 3, and day 5 as compared to PBS treatment. The body weights of mice were recorded daily with harmol administration, and harmol did not affect the weight of the treated mice compared to those treated with PBS (Fig. 5 A). The fluorescein sodium staining confirmed that corneal transparency and epithelial were not damaged by harmol at 5 d (Fig. 5 B). Altogether, harmol at a concentration of 0.01 mg/kg had no toxicity to the corneas of mice.

The safety of harmol on the murine cornea. A Harmol-treated HSK mice show the weight gain as compared to PBS-treated ones without any significant differences ( n = 6). B Corneal images were collected via white light and cobalt blue light at day 5 ( n = 6). Data expressed as Mean ± SD ( ns : non-significant)
Eyedrop treatment of harmol alleviated the severity of HSK in vivo
Next, we established an HSK mouse model to assess the anti-HSV effect of harmol in vivo. Compared to the HSK group, harmol treatment relieved HSV-1 F-related corneal opacity at 3 and 5 dpi (Fig. 6 A). Blepharitis was observed in the HSK group at 3 and 5dpi, which was alleviated by harmol treatment due to a reduction in blepharitis scores (Fig. 6 B). Corneal fluorescein staining with cobalt blue light was used to assess corneal epithelial injury, and harmol treatment also alleviated HSV-1 F-induced early corneal injury (Fig. 6 C). In addition, harmol-treated mice showed less fluorescein staining and there was no epithelial defect in uninfected corneas (Fig. 6 D). Furthermore, harmol treatment significantly reduced the body weight loss caused by HSV-1 F infection (Fig. 6 E). All together, these results demonstrated that topical application of harmol efficiently ameliorated the ocular diseases in mice with HSV-1 infection.

Harmol alleviated the scores of HSK early corneal diseases in vivo. A Corneal images at 3 dpi and 5 dpi were obtained following HSV-1 infection. B Blepharitis scores were calculated at 3 dpi and 5 dpi ( n = 6). C Photographs of sodium fluorescein staining were recorded in mice ( n = 6). D The relative defect (%) of corneal lesions was calculated as percentage of fluorescein staining areas to the whole corneal. E Rate of weight loss in mice infected with HSV-1. F Representative images of subbasal nerve plexus, corneal vasculature, and keratocytes at 5 dpi using IVCM. Traced nerve fibers are observed in purple. G Corneal nerve density was quantified by Image J ( n = 6). H Corneal thickness was obtained by RTvue OCT at 5 dpi. I Corneal thickness was measured. J The tear swabs of HSK and harmol treatment groups were calculated via TCID 50 assay ( n = 6). Data expressed as Mean ± SD ( ns : non-significant, * P < 0.05, ** P < 0.01, *** P < 0.001)
SNP density, corneal vasculature, and keratocytes of HSK mice were observed by IVCM after 5 days of infection. The central nerve density in HSK group was notably reduced as compared to the control group (3.26 ± 0.36 vs. 7.85 ± 0.78 mm/mm 2 ); HSV infection reduced the central nerve density was significantly alleviated in the harmol treatment group (4.96 ± 0.79 mm/mm 2 ) (Fig. 6 F and G). We were not able to evaluate the effect on corneal neovascularization because we only scored the disease process on day 5 post-infection as well as the acute HSK model was well performed as long as on day 7 post-infection. Furthermore, we observed that harmol treatment significantly alleviated HSK-related early corneal injury, such as corneal vascular damage, disruption of stromal architecture, disorganized collagen lamellae, scattered microdeposits, and reduced keratocytes (Fig. 6 F). Moreover, RTvue OCT was used to examine the corneal thickness of mice at 5 dpi. As shown in Fig. 6 H and I, harmol-treated mice retained ocular structural integrity as compared with HSK mice; however, the limbal epithelium and surrounding conjunctiva of HSK mice were significantly thickened (179.80 ± 47.70 vs. 130.30 ± 6.17 μm). The tear swabs from harmol and PBS-treated groups were collected at 3 dpi and 5 dpi and the viral titers were calculated by TCID 50 . As expected, harmol treatment decreased 2 logs of HSV-1 F viral titers in tears at 5 dpi (Fig. 6 J). Our results demonstrated that topical application of harmol not only inhibition HSV-1 replication in vivo but also alleviated HSV-1 F-induced early ocular pathology.
Harmol alleviated HSV-1/153 induced HSK early corneal diseases
In the present decades, the emergence of HSV-1 resistance to ACV has been increasingly reported [ 5 ]. HSV-1/153, an ACV resistance strain, was used for further in vivo study. We found that harmol not ACV treatment significantly reduced the blepharitis scores at 5 dpi in mice with HSV-1/153 infection (Fig. 7 A and B). Furthermore, harmol treatment notably alleviated the reduction of the early defect of corneal lesions caused by HSV-1/153 infection at 5 dpi (Fig. 7 C and D). In addition, we also observed that harmol treatment protected mice from weight loss caused by HSV-1/153 infection; however, ACV treatment could not protect HSV-1/153-infected mice from weight loss (Fig. 7 E). Collectively, harmol could fight against ACV-resistant HSV infections in vivo.

Harmol not ACV alleviated HSV-1/153-induced early HSK corneal diseases. A Corneal images at 3 and 5 dpi were obtained following mice with HSV-1/153 infection. B Blepharitis scores were calculated at 3 and 5 dpi ( n = 6). C Photographs of sodium fluorescein staining were recorded in mice ( n = 6). D The relative defect (%) of corneal lesions were calculated as percentage of fluorescein staining areas to the whole corneal areas. E The relative weight of mice for 5 days after infection ( n = 6). Data expressed as Mean ± SD ( ns : non-significant, * P < 0.05, ** P < 0.01, *** P < 0.001)
The development of HSK was associated with HSV-1 infection, which has led to vision loss and even blindness in patients worldwide. As a result of the overuse of medications, drug resistance in HSV infectious disease has significantly risen, presenting a major public health challenge [ 42 ], which is one of the key factors in the protracted course of HSK. Thus, new drugs with different mechanisms of action and minimal toxicity are needed for the treatment of HSV infectious diseases [ 27 ]. At present, harmol, a β-carboline alkaloid, not only inhibited HSV-1 F and HSV-1/153 strain replication in corneal tissues of mice but also alleviated viral early infection-induced corneal lesions.
Harmol has also been identified as an antiviral agent for the management of RNA viruses, including NDV and DENV [ 33 , 44 ]. At present, harmol treatment notably suppressed HSV-1 F infection in vitro and in vivo. Interestingly, the replication of HSV-1/153, the ACV-resistant strain, was also suppressed by harmol but not ACV treatment in vitro and in vivo. Nucleoside analogs represented by ACV are the main drugs for HSV-1 infectious disease management [ 27 ]. ACV inhibited viral replication by interfering with viral DNA polymerase activity and incorporating viral DNA [ 36 ]. A previous study reported that the resistance of HSV to ACV was linked to mutations in viral DNA [ 37 ]. At present, harmol, in combination with ACV, showed a better anti-HSV-1 effect, which indicated that harmol exhibited anti-HSV-1 activity was different from ACV. Interestingly, this study found that the time addition of harmol significantly inhibited the expression of HSV-1 gD-1 mRNA at 24 h post-infection, suggesting that harmol may inhibit HSV-1 replication at various stages of the replication process ( Fig. 4 ) . A previous study reported that β-Carbolines, including 9-methyl-norharmane, 9-methyl-harmane, and 6-methoxy-harmane, whose structures were similar to harmol, were identified as novel antiviral agents against HSV-1 and HSV-2 [ 15 ]. Furthermore, Bag et al., found that harmaline, one of the β-Carbolines, interfered with the binding of the immediate-early complex to the ICP0 promoter during the immediate-early stage of viral replication [ 6 ]. Additionally, Gonzalez et al., also reported that β-Carbolines were found to interfere with the late proteins expression [ 15 ]. Our study also suggested that harmol, a β-Carbolines compound, may affect HSV-1 replication at various stages of the viral replication process, since it exhibited significant antiviral activity from 0 to 24 h post-infection. In summary, we induced that the antiviral mechanisms of harmol may be different from ACV treatment because harmol blocked the HSV-1 replication even when it was added at late time during HSV-1 infection.
In addition to the possible antiviral mechanisms of β-Carbolines, there are multiple potential antiviral mechanisms of harmol based on previous studies. A previous study have identified targeting the autophagy process as an antiviral pathway to affect the viral replication [ 19 ]. The autophagy activation inhibited HSV-1 replication [ 24 , 51 ], which was associated with the activation of antiviral innate immune response [ 26 , 51 ]. Harmol has been reported to be an autophagy activator in a variety of disease models. For instance, harmol can activate the autophagy process by triggering extracellular signal-regulated kinase 1/2 (ERK1/2) and AMP-activated protein kinase [ 3 , 48 ]. Previous studies have reported that the activation of ERK or AMPK pathways were important for the host fighting against HSV infection [ 14 , 18 ]. Furthermore, Deyan Chen et al., reported that harmine, also known as β-carboline alkaloid, blocked HSV-1 infection through inhibiting NF-κB and MAPK pathways in vitro [ 10 ]. Subsequently, they also found that harmine suppressed EV71 replication via inhibiting the NF-κB pathway in vitro [ 11 ]. The above evidence implied that harmol might also suppress HSV-1 F or HSV-1/153 replication and alleviate pathologic damage of HSK through targeting NF-κB and MAPK pathways. Harmol has been identified as one of the metabolites of harmine [ 2 ], and harmol has been demonstrated as a better therapeutic window as compared to harmine in multiple disease models [ 31 , 40 ]. Furthermore, the toxic effects of harmol were lower than harmine [ 29 ]. Therefore, we speculated that harmol also probably exerted an antiviral effect via modulating the autophagy pathways or NF-κB pathways, which has the potential to address the problem of ACV resistance in HSV-1 in the future, and further studies will be required to identify the exact mechanism of the antiviral effect of harmol.
However, there are some limitations in this study. The primary objective of this study was to assess the antiviral effect of harmol. As a result, we only examined the pathological alterations in HSK at 3 and 5 dpi. It should be noted that this limited timeframe of HSK acute model may not provide a comprehensive evaluation of corneal clouding or corneal neovascularization. Furthermore, the evaluation of harmol’s anti-HSV-1 efficacy in this study is preliminary, therefore, these results should be interpreted with caution.
Conclusions
These findings demonstrated that harmol induced a significant resistance to HSV-1 F and HSV-1/153 replication in vitro and in vivo and alleviated early symptoms of HSK. Thus, harmol was a promising new anti-HSV-1 agent with a different action from ACV for the new anti-HSV-1 therapy.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abe A, Kokuba H. Harmol induces autophagy and subsequent apoptosis in U251MG human glioma cells through the downregulation of survivin. Oncol Rep. 2013;29(4):1333–42. https://doi.org/10.3892/or.2013.2242 .
Article CAS PubMed Google Scholar
Abe A, Yamada H. Harmol induces apoptosis by caspase-8 activation independently of Fas/Fas ligand interaction in human lung carcinoma H596 cells. Anticancer Drugs. 2009;20(5):373–81. https://doi.org/10.1097/CAD.0b013e32832a2dd9 .
Abulimiti G, Zeng J, Aimaiti M, Lei X, Mi N. Harmol hydrochloride dihydrate induces autophagy in neuro cells and promotes the degradation of α-Syn by Atg5/Atg12-dependent pathway. Food Sci Nutr. 2022;10(12):4371–9. https://doi.org/10.1002/fsn3.3031 .
Article CAS PubMed PubMed Central Google Scholar
Ahmad I, Wilson DW. HSV-1 cytoplasmic envelopment and egress. Int J Mol Sci. 2020;21(17). https://doi.org/10.3390/ijms21175969 .
Anton-Vazquez V, Mehra V, Mbisa JL, Bradshaw D, Basu TN, Daly ML, Zuckerman M. Challenges of aciclovir-resistant HSV infection in allogeneic bone marrow transplant recipients. J Clin Virol. 2020;128:104421. https://doi.org/10.1016/j.jcv.2020.104421 .
Bag P, Ojha D, Mukherjee H, Halder UC, Mondal S, Biswas A, Chattopadhyay D. A dihydro-pyrido-indole potently inhibits HSV-1 infection by interfering the viral immediate early transcriptional events. Antiviral Res. 2014;105:126–34. https://doi.org/10.1016/j.antiviral.2014.02.007 .
Capelli N, Dubois M, Pucelle M, Da Silva I, Lhomme S, Abravanel F, Izopet J. Optimized Hepatitis E virus (HEV) culture and its application to measurements of HEV infectivity. Viruses. 2020;12(2). https://doi.org/10.3390/v12020139 .
Chapellier B, Guindolet D, Pereira D, Galetto R, Sahel JA, Labetoulle M, Gabison EE. Meganuclease targeting HSV-1 protects against herpetic keratitis: application to corneal transplants. Mol Ther Nucleic Acids. 2022;30:511–21. https://doi.org/10.1016/j.omtn.2022.11.006 .
Chen D, Liu Y, Zhang F, You Q, Ma W, Wu J, Wu Z. 6-Thioguanine inhibits herpes Simplex Virus 1 infection of eyes. Microbiol Spectr. 2021;9(3):e0064621. https://doi.org/10.1128/Spectrum.00646-21 .
Article PubMed Google Scholar
Chen D, Su A, Fu Y, Wang X, Lv X, Xu W, Wu Z. Harmine blocks herpes simplex virus infection through downregulating cellular NF-κB and MAPK pathways induced by oxidative stress. Antiviral Res. 2015;123:27–38. https://doi.org/10.1016/j.antiviral.2015.09.003 .
Chen D, Tian X, Zou X, Xu S, Wang H, Zheng N, Wu Z. Harmine, a small molecule derived from natural sources, inhibits enterovirus 71 replication by targeting NF-κB pathway. Int Immunopharmacol. 2018;60:111–20. https://doi.org/10.1016/j.intimp.2018.04.050 .
Chirapapaisan C, Thongsuwan S, Chirapapaisan N, Chonpimai P, Veeraburinon A. Characteristics of corneal subbasal nerves in different age groups: an in vivo Confocal Microscopic Analysis. Clin Ophthalmol. 2021;15:3563–72. https://doi.org/10.2147/opth.S324169 .
Article PubMed PubMed Central Google Scholar
Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–81. https://doi.org/10.1124/pr.58.3.10 .
Doshi H, Spengler K, Godbole A, Gee YS, Baell J, Oakhill JS, Heller R. AMPK protects endothelial cells against HSV-1 replication via inhibition of mTORC1 and ACC1. Microbiol Spectr. 2023;11(5):e0041723. https://doi.org/10.1128/spectrum.00417-23 .
Gonzalez MM, Cabrerizo FM, Baiker A, Erra-Balsells R, Osterman A, Nitschko H, Vizoso-Pinto MG. β-Carboline derivatives as novel antivirals for herpes simplex virus. Int J Antimicrob Agents. 2018;52(4):459–68. https://doi.org/10.1016/j.ijantimicag.2018.06.019 .
Harrison WW, Putnam NM, Shukis C, Nguyen E, Reinard K, Hundelt E, Yevseyenkov V. The corneal nerve density in the sub-basal plexus decreases with increasing myopia: a pilot study. Ophthalmic Physiol Opt. 2017;37(4):482–8. https://doi.org/10.1111/opo.12366 .
Ireland PJ, Tavis JE, D’Erasmo MP, Hirsch DR, Murelli RP, Cadiz MM, Morrison LA. Synthetic α-Hydroxytropolones inhibit replication of wild-type and acyclovir-resistant herpes simplex viruses. Antimicrob Agents Chemother. 2016;60(4):2140–9. https://doi.org/10.1128/aac.02675-15 .
Ishimaru H, Hosokawa K, Sugimoto A, Tanaka R, Watanabe T, Fujimuro M. MG132 exerts anti-viral activity against HSV-1 by overcoming virus-mediated suppression of the ERK signaling pathway. Sci Rep. 2020;10(1):6671. https://doi.org/10.1038/s41598-020-63438-1 .
Ke PY. Autophagy and antiviral defense. IUBMB Life. 2022;74(4):317–38. https://doi.org/10.1002/iub.2582 .
LaBarre DD, Lowy RJ. Improvements in methods for calculating virus titer estimates from TCID50 and plaque assays. J Virol Methods. 2001;96(2):107–26. https://doi.org/10.1016/s0166-0934(01)00316-0 .
Lee S, Ives AM, Bertke AS. Herpes Simplex Virus 1 reactivates from autonomic ciliary ganglia independently from sensory trigeminal ganglia to cause recurrent ocular disease. J Virol. 2015;89(16):8383–91. https://doi.org/10.1128/jvi.00468-15 .
Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20(1):1–13. https://doi.org/10.1097/00003226-200101000-00001 .
Liu S, Li L, Tan L, Liang X. Inhibition of herpes simplex Virus-1 replication by natural compound honokiol. Virol Sin. 2019;34(3):315–23. https://doi.org/10.1007/s12250-019-00104-5 .
Liu Y, Tang Q, Rao Z, Fang Y, Jiang X, Liu W, Zeng N. Inhibition of herpes simplex virus 1 by cepharanthine via promoting cellular autophagy through up-regulation of STING/TBK1/P62 pathway. Antiviral Res. 2021;193:105143. https://doi.org/10.1016/j.antiviral.2021.105143 .
Liu YY, Deng HY, Yang G, Jiang WL, Grossin L, Yang ZQ. Short hairpin RNA-mediated inhibition of HSV-1 gene expression and function during HSV-1 infection in Vero cells. Acta Pharmacol Sin. 2008;29(8):975–82. https://doi.org/10.1111/j.1745-7254.2008.00828.x .
Ma Z, Bai J, Jiang C, Zhu H, Liu D, Pan M, Liu X. Tegument protein UL21 of alpha-herpesvirus inhibits the innate immunity by triggering CGAS degradation through TOLLIP-mediated selective autophagy. Autophagy. 2023;19(5):1512–32. https://doi.org/10.1080/15548627.2022.2139921 .
Majewska A, Mlynarczyk-Bonikowska B. 40 years after the Registration of Acyclovir: do we need new anti-herpetic drugs? Int J Mol Sci. 2022;23(7). https://doi.org/10.3390/ijms23073431 .
Moein HR, Sendra VG, Jamali A, Kheirkhah A, Harris DL, Hamrah P. Herpes simplex virus-1 KOS-63 strain is virulent and causes titer-dependent corneal nerve damage and keratitis. Sci Rep. 2021;11(1):4267. https://doi.org/10.1038/s41598-021-83412-9 .
Nakagawa Y, Suzuki T, Ishii H, Ogata A, Nakae D. Mitochondrial dysfunction and biotransformation of β-carboline alkaloids, harmine and harmaline, on isolated rat hepatocytes. Chem Biol Interact. 2010;188(3):393–403. https://doi.org/10.1016/j.cbi.2010.09.004 .
Olmedo GM, Cerioni L, González MM, Cabrerizo FM, Rapisarda VA, Volentini SI. Antifungal activity of β-carbolines on Penicillium digitatum and Botrytis Cinerea. Food Microbiol. 2017;62:9–14. https://doi.org/10.1016/j.fm.2016.09.011 .
Olmedo GM, Cerioni L, González MM, Cabrerizo FM, Volentini SI, Rapisarda VA. UVA photoactivation of Harmol enhances its antifungal activity against the Phytopathogens Penicillium digitatum and Botrytis Cinerea. Front Microbiol. 2017;8:347. https://doi.org/10.3389/fmicb.2017.00347 .
Piret J, Boivin G. Antiviral resistance in herpes simplex virus and varicella-zoster virus infections: diagnosis and management. Curr Opin Infect Dis. 2016;29(6):654–62. https://doi.org/10.1097/qco.0000000000000288 .
Quintana VM, Piccini LE, Panozzo Zénere JD, Damonte EB, Ponce MA, Castilla V. Antiviral activity of natural and synthetic β-carbolines against dengue virus. Antiviral Res. 2016;134:26–33. https://doi.org/10.1016/j.antiviral.2016.08.018 .
Ren J, Antony F, Rouse BT, Suryawanshi A. Role of Innate Interferon responses at the ocular surface in herpes Simplex Virus-1-Induced Herpetic Stromal Keratitis. Pathogens. 2023;12(3). https://doi.org/10.3390/pathogens12030437 .
Rodríguez L, López A, Moyna G, Seoane GA, Davyt D, Vázquez Á, Carrera I. New insights into the Chemical composition of Ayahuasca. ACS Omega. 2022;7(14):12307–17. https://doi.org/10.1021/acsomega.2c00795 .
Rousseau A, Pharm SB, Gueudry J, Deback C, Haigh O, Schweitzer C, Labetoulle M. Acyclovir-resistant herpes Simplex Virus 1 Keratitis: a concerning and emerging clinical challenge. Am J Ophthalmol. 2022;238:110–9. https://doi.org/10.1016/j.ajo.2022.01.010 .
Sauerbrei A, Bohn K, Heim A, Hofmann J, Weissbrich B, Schnitzler P, Hamprecht K. Novel resistance-associated mutations of thymidine kinase and DNA polymerase genes of herpes simplex virus type 1 and type 2. Antivir Ther. 2011;16(8):1297–308. https://doi.org/10.3851/imp1870 .
Sibley D, Larkin DFP. Update on herpes simplex keratitis management. Eye (Lond). 2020;34(12):2219–26. https://doi.org/10.1038/s41433-020-01153-x .
Stepp MA, Menko AS. Immune responses to injury and their links to eye disease. Transl Res. 2021;236:52–71. https://doi.org/10.1016/j.trsl.2021.05.005 .
Tarpley M, Oladapo HO, Strepay D, Caligan TB, Chdid L, Shehata H, Williams KP. Identification of harmine and β-carboline analogs from a high-throughput screen of an approved drug collection; profiling as differential inhibitors of DYRK1A and monoamine oxidase A and for in vitro and in vivo anti-cancer studies. Eur J Pharm Sci. 2021;162:105821. https://doi.org/10.1016/j.ejps.2021.105821 .
Tognarelli EI, Palomino TF, Corrales N, Bueno SM, Kalergis AM, González PA. Herpes Simplex Virus Evasion of early host antiviral responses. Front Cell Infect Microbiol. 2019;9:127. https://doi.org/10.3389/fcimb.2019.00127 .
Treml J, Gazdová M, Šmejkal K, Šudomová M, Kubatka P, Hassan STS. Natural products-Derived chemicals: breaking barriers to Novel Anti-HSV Drug Development. Viruses. 2020;12(2). https://doi.org/10.3390/v12020154 .
Vere Hodge RA, Field HJ. Antiviral agents for herpes simplex virus. Adv Pharmacol. 2013;67:1–38. https://doi.org/10.1016/b978-0-12-405880-4.00001-9 .
Wang C, Wang T, Hu R, Duan L, Hou Q, Han Y, Yang Z. 9-Butyl-harmol exerts antiviral activity against Newcastle Disease Virus through Targeting GSK-3β and HSP90β. J Virol. 2023;97(3):e0198422. https://doi.org/10.1128/jvi.01984-22 .
Wang Y, Wang Q, Zhu Q, Zhou R, Liu J, Peng T. Identification and characterization of acyclovir-resistant clinical HSV-1 isolates from children. J Clin Virol. 2011;52(2):107–12. https://doi.org/10.1016/j.jcv.2011.06.009 .
West DM, Rosso D, Yin CR, X. T., Stuart PM. CXCL1 but not IL-6 is required for recurrent herpetic stromal keratitis. J Immunol. 2014;192(4):1762–7. https://doi.org/10.4049/jimmunol.1302957 .
Wulff NH, Tzatzaris M, Young PJ. Monte Carlo simulation of the Spearman-Kaerber TCID50. J Clin Bioinforma. 2012;2(1):5. https://doi.org/10.1186/2043-9113-2-5 .
Xu J, Ao YL, Huang C, Song X, Zhang G, Cui W, Zhang Z. Harmol promotes α-synuclein degradation and improves motor impairment in Parkinson’s models via regulating autophagy-lysosome pathway. NPJ Parkinsons Dis. 2022;8(1):100. https://doi.org/10.1038/s41531-022-00361-4 .
Yin D, Ling S, Wang D, Dai Y, Jiang H, Zhou X, Cai Y. Targeting herpes simplex virus with CRISPR-Cas9 cures herpetic stromal keratitis in mice. Nat Biotechnol. 2021;39(5):567–77. https://doi.org/10.1038/s41587-020-00781-8 .
Yun H, Lathrop KL, Hendricks RL. A Central Role for sympathetic nerves in herpes stromal keratitis in mice. Invest Ophthalmol Vis Sci. 2016;57(4):1749–56. https://doi.org/10.1167/iovs.16-19183 .
Zheng Z, Zhao M, Shan H, Fang D, Jin Z, Tang J, Li M. Noncanonical autophagy is a new strategy to inhibit HSV-1 through STING1 activation. Autophagy. 2023;1–17. https://doi.org/10.1080/15548627.2023.2237794 .
Zhou N, Zheng D, You Q, Chen T, Jiang J, Shen W, Hu K. Therapeutic potential of biochanin A in Herpes Simplex Keratitis. Pharmaceuticals (Basel). 2023;16(9). https://doi.org/10.3390/ph16091240 .
Download references
Acknowledgements
We thank Professor Yan Lu, Jinling hospital of Nanjing University, Jiangsu, China for providing the acyclovir–resistant clinical HSV-1 strain HSV-1/153. We also thank Deyan Chen Ph.D., Nanjing University, Jiangsu, China for providing the natural compounds used in this study.
The study was supported by the Key Basic Research Project of Jiangsu Province (No. BK20201119 to Q.Y.J.).
Author information
Huanhuan Xu and Nan Zhou contributed equally to this work.
Authors and Affiliations
Department of Ophthalmology, Jinling Hospital, Nanjing Medical University, Nanjing, Jiangsu, China
Huanhuan Xu & Zhenping Huang
Department of Caries and Endodontics, Nanjing Stomatological Hospital, Medical School of Nanjing University, 30# Zhongyang Road, Xuanwu District, Nanjing, Jiangsu, 210008, China
Medical School of Nanjing University, 22# Hankou Road, Nanjing, 210093, Jiangsu Province, China
Department of Ophthalmology, Affiliated Drum Tower Hospital, Medical School of Nanjing University, 22# Hankou Road, Nanjing, Jiangsu, 210093, China
You can also search for this author in PubMed Google Scholar
Contributions
H.X., Y.Q. and N.Z. designed the project. H.X., Y.Q. and J.W. prepared the manuscript. H.X. and N.Z. performed most of the experiments. H.X., N.Z. and Y.Q. analyzed the data. J.W., Z.H., and Y.Q. reviewed and revised the manuscript. Z.H., and Y.Q. supervised the study. All authors reviewed the manuscript.
Corresponding authors
Correspondence to Zhenping Huang , Jing Wu or Yajie Qian .
Ethics declarations
Ethics approval and consent to participate.
Animal experiments were conducted in accordance with the guidelines and regulations set by the Laboratory Animal Ethics Committee of JingLing Hospital (Approval No.2022DZGKJDWLS-0033) and the Laboratory Animal Management Committee of Jiangsu Province. BALB/c mice, aged 6 weeks and with normal eye development, were obtained from the Animal Core Facility of Nanjing Medical University and maintained under specific pathogen-free conditions. The eyes of the mice were visually inspected, and tear samples were collected using eye swabs for viral titers measurement.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Xu, H., Zhou, N., Huang, Z. et al. Harmol used for the treatment of herpes simplex virus induced keratitis. Virol J 21 , 118 (2024). https://doi.org/10.1186/s12985-024-02384-0
Download citation
Received : 30 January 2024
Accepted : 06 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1186/s12985-024-02384-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Herpes simplex virus type 1
- Herpes simplex keratitis
- ACV-resistance
- Find a journal
- Publish with us
- Track your research
- Open access
- Published: 28 May 2024
Development of a highly effective combination monoclonal antibody therapy against Herpes simplex virus
- Narges Seyfizadeh 1 ,
- David Kalbermatter 2 , 6 ,
- Thomas Imhof 1 ,
- Moritz Ries 1 ,
- Christian Müller 1 ,
- Leonie Jenner 1 ,
- Elisabeth Blumenschein 1 ,
- Alexandra Yendrzheyevskiy 1 ,
- Frank Grün 3 ,
- Kevin Moog 1 ,
- Daniel Eckert 1 ,
- Ronja Engel 1 ,
- Philipp Diebolder 4 , 5 ,
- Mohamed Chami 2 ,
- Jürgen Krauss 1 ,
- Torsten Schaller 1 na1 &
- Michaela Arndt ORCID: orcid.org/0000-0001-9597-4112 1 na1
Journal of Biomedical Science volume 31 , Article number: 56 ( 2024 ) Cite this article
268 Accesses
Metrics details
Infections with Herpes simplex virus (HSV)-1 or -2 usually present as mild chronic recurrent disease, however in rare cases can result in life-threatening conditions with a large spectrum of pathology. Monoclonal antibody therapy has great potential especially to treat infections with virus resistant to standard therapies. HDIT101, a humanized IgG targeting HSV-1/2 gB was previously investigated in phase 2 clinical trials. The aim of this study was to develop a next-generation therapy by combining different antiviral monoclonal antibodies.
A lymph-node derived phage display library (LYNDAL) was screened against recombinant gB from Herpes simplex virus (HSV) -1 and HDIT102 scFv was selected for its binding characteristics using bio-layer interferometry. HDIT102 was further developed as fully human IgG and tested alone or in combination with HDIT101, a clinically tested humanized anti-HSV IgG, in vitro and in vivo. T-cell stimulating activities by antigen-presenting cells treated with IgG-HSV immune complexes were analyzed using primary human cells. To determine the epitopes, the cryo-EM structures of HDIT101 or HDIT102 Fab bound to HSV-1F as well as HSV-2G gB protein were solved at resolutions < 3.5 Å.
HDIT102 Fab showed strong binding to HSV-1F gB with Kd of 8.95 × 10 –11 M and to HSV-2G gB with Kd of 3.29 × 10 –11 M. Neutralization of cell-free virus and inhibition of cell-to-cell spread were comparable between HDIT101 and HDIT102. Both antibodies induced internalization of gB from the cell surface into acidic endosomes by binding distinct epitopes in domain I of gB and compete for binding. CryoEM analyses revealed the ability to form heterogenic immune complexes consisting of two HDIT102 and one HDIT101 Fab bound to one gB trimeric molecule. Both antibodies mediated antibody-dependent phagocytosis by antigen presenting cells which stimulated autologous T-cell activation. In vivo, the combination of HDIT101 and HDIT102 demonstrated synergistic effects on survival and clinical outcome in immunocompetent BALB/cOlaHsd mice.
This biochemical and immunological study showcases the potential of an effective combination therapy with two monoclonal anti-gB IgGs for the treatment of HSV-1/2 induced disease conditions.
Herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) constitute a significant global health concern due to their wide range of clinical manifestations. Infections can affect the skin and mucous membranes, the eyes, the nervous system, or they can lead to disseminated viral spread throughout the body. Clinically, these conditions may manifest as oral and genital herpes, herpes keratitis, herpes encephalitis, and neonatal herpes, respectively [ 1 ]. Primary HSV infection occurs through direct contact with a person who is actively shedding the virus, often unknowingly, from their mucosa or skin. The virus persists in nerve ganglia within the dermatome of the primary infection site for a lifetime, potentially causing chronic recurrent mucosal and skin lesions through anterograde axonal transport. HSV-1 typically causes non-sexually transmitted oral herpes infection, while HSV-2 is the most common cause of genital ulcers across the globe [ 2 ]. Frequent HSV recurrences often cause significant emotional, psychological and psychosocial distress [ 3 ]. Newborns and immunocompromised individuals have a particularly increased risk of morbidity and mortality from HSV infections [ 4 , 5 , 6 ]. The significance of developing novel therapies for HSV mediated infectious disease conditions has widely been acknowledged, leading the National Institutes of Health to recently launch a strategic plan aimed at advancing research to improve knowledge on biology and diagnostic possibilities for HSV mediated disease as well as to develop interventions to mitigate its health consequences (nih.gov).
Since many decades virostatic agents (e.g. acyclovir and analogues and derivatives) represent the standard of care treatment of for HSV associated disease. Despite several attempts the development of effective vaccines against HSV has thus far failed [ 7 , 8 , 9 ]. Antibody therapeutics have revolutionized treatment options in a vast variety of diseases resulting in the approval of more than 100 products by the US Food and Drug Administration (FDA) [ 10 ]. Despite this progress, fewer than 10 monoclonal antibodies (mAbs) targeting pathogens have been FDA approved, and none of those for the treatment of HSV1/2 associated disease conditions. HSV disease animal model studies have shown that UB-621 and HSV8, two fully human IgG1 mAbs, to reduce mortality in an intraperitoneal HSV-1 challenge model of adult mice [ 11 , 12 ] and to exhibit prophylactic activities in a neonatal mouse infection model [ 13 ]. Since topical application of HSV8 protected mice from vaginal transmission of HSV-2 [ 14 ], a randomized phase 1 clinical trial of HSV8 in combination with a broadly neutralizing anti-HIV antibody was conducted showing that single and repeated application as a vaginal film (MB66) was safe and well tolerated. Furthermore, an ex vivo bioactivity for both mAbs in vaginal secretions could be demonstrated [ 15 ]. A phase 1 dose-escalation clinical trial for subcutaneous administration of UB-621 completed in 2017 proved safety and tolerability in healthy volunteers. In 2023, three phase 2 trials for UB-621 in patients with recurrent genital HSV-2 infection have been started, but are not yet recruiting (NCT03595995, NCT04714060, NCT04979975).
We have developed the humanized monoclonal antibody HDIT101, whose ancestor is the murine monoclonal antibody 2c, which has been generated through hybridoma technology from mice hyperimmunized with HSV-1, binding to glycoprotein B (gB) of HSV-1 and HSV-2, a key component of the cell-entry machinery of HSV. This mechanism allows HDIT101 to neutralize virus particles and effectively inhibit cell-to-cell spread of both HSV-1 and HSV-2. As a result, the antibody has been shown to prevent death in immunodeficient mice challenged with lethal doses of both wild type and multi-resistant HSV strains [ 16 , 17 ].
After demonstrating excellent tolerability in an intravenous clinical Phase 1 dose escalation trial in healthy volunteers [ 16 ], HDIT101 has advanced to a recently completed randomized Phase 2 clinical trial involving 122 patients suffering from chronic recurrent genital herpes. Aim of the trial was to compare the safety and efficacy of a single infusion of HDIT101 with episodic standard-of-care Valaciclovir (VAL) treatment in highly affected patients who have reported at least 4 anogenital HSV-2 recurrences within the last 12 months prior to study enrolment. Based on the highly potent neutralization capacity of HDIT101 in preclinical studies, the ‘percentage of days with lesion recurrences relative to the days on study after initial treatment’ was selected as the primary endpoint of the MATCH-2 trial. While this primary endpoint failed to demonstrate superiority of HDIT101 over standard-of-care treatment improved HDIT101 activity over VAL was observed for the key secondary endpoints ‘mean time to first recurrence’ and ‘mean recurrence rate’ . Most notably, the delay in mean time to first recurrence in favour of HDIT101 started to become apparent in the Kaplan–Meier plot only from day 35 post infusion, i.e., at a time when the antibody concentration in the circulation was depleted by approximately two half-lives. One possible explanation for the observed long-term effects of HDIT101 could be its role in promoting A ntibody D ependent C ellular P hagocytosis (ADCP), involving the uptake of antibody-coated HSV particles by antigen presenting cells, and subsequent T cell activation. Consequently, the observed clinical benefits of HDIT101 in the MATCH-2 trial could likely be the result of the induction of T cell immunity translating into improved recurrence control in chronically infected patients.
Based on these results we were interested to identify more antibodies with similar properties for further potential development into clinical vaccine candidates as either successor of HDIT01 or to be developed in combination with HDIT101. For selection of such antibodies we employed a human antibody phage display library previously been generated from B cell repertoires of tumour draining lymph nodes from head and neck cancer patients, referred to as ‘lymph node-derived antibody libraries’ (LYNDAL) [ 18 ]. One of the selected antibodies revealed very similar properties as HDIT01 and was thus formatted into the fully human IgG1 antibody HDIT102 for further characterization.
We here describe the preclinical characterization of HDIT102 and provide a rationale for further developing this antibody as a fully human successor of HDIT101 or as a combinatorial therapeutic. In summary, we have demonstrated in the present study that HDIT102 potently blocked HSV-1 and HSV-2 replication in vitro and in vivo and also exerted synergistical effects with HDIT101 IgG in vivo. We further succeeded in generating cryo-electron microscopic (cryo-EM) co-structures of HSV-1 and HSV-2 gB proteins with HDIT101 and HDIT102 Fabs at resolutions < 3.5 Å so that the exact binding sites of the antibodies could be determined. To our knowledge this is the first report of resolving HSV-2-gB complex. The formation of heterogeneous gB immune complexes observed in the structure may elucidate the synergistic in vivo effects seen in the combination therapy of HDIT101 and HDIT102, as opposed to their monotherapies, presumably by inducing a more robust immune response.
Material and methods
Cell lines, viruses and animals.
HEK293-6E cell line established from embryonic kidney cells (National Research Council Canada) was used for recombinant antibodies and protein production. HEK293T cell line established from embryonic kidney cells, expressing the SV40 large T antigen (American Type Culture Collection (CRL-3216)) was transiently transfected with gB mutants and was used in fusion and binding assay. Raji cell line established from Burkitt's lymphoma B lymphocytes (American Type Culture Collection) used in ADCC assay. Vero cell line established from normal epithelial kidney cell (American Type Culture Collection) was used for as a permissive cell line for HSV virus in different assays and virus production. Modified Jurkat cells stably expressing the FcγRIIIa receptor, V158 (high affinity) variant, and an NFAT response element driving expression of firefly luciferase provided in ADCC Promega Kit. Clonal HEK293T ectopically and stably expressing HSV-1F gB or gB-GFP were generated by VSV-G pseudotyped lentiviral vector transduction using a transfer vector encoding for codon-optimized HSV-1F gB and subsequent clone selection by limiting dilution. All cell lines were grown in medium recommended by the provider. Primary blood mononuclear cells (PBMCs) were isolated from buffy coats by Ficoll separation and washed in PBS before freezing in freezing media (fetal calf serum (FCS) with 10% dimethyl sulfoxid). Primary CD14 + monocyte-derived macrophages or dendritic cells and CD14- cell fractions containing T-cells were grown in RPMI supplemented with penicillin/streptomycin and 10% fetal calf serum. For all in vivo experiments, 6-week-old female mice (BALB/cOlaHsd) (16–19 g) were purchased from ENVIGO and housed according to regulatory guidelines.
Virus propagation and titration
HSV-1F and HSV-2G virus stocks were purchased from ATCC and expanded in Vero and used for in vitro and in vivo experiments. Vero cells were cultured until reaching 100% confluency in T175 flasks. The culture medium was removed and after one wash with PBS infected with HSV-1F/HSV-2G at MOI of 0.01 in DMEM without FCS for 2h at 37°C. 30mL medium with 10% heat inactivated FCS was added and the infected cells were incubated 2–3 days. The flasks were subjected to three freeze–thaw cycles (-80°C, room temperature (RT)), to lyse the cells and release virus into the supernatant. Cell debris was removed by centrifugation (15min, 300g) and the virus-containing supernatant filtered using a 0.45μm filter, followed by ultracentrifugation at 20k rpm for 1 h (SW32Ti swinging rotor, Beckman Coulter). The virus pellets from nine infected T175 flasks were pooled in 1 mL PBS and aliquots were stored at -80°C. The titers of HSV stocks were determined by TCID50 assay (tissue culture infectious dose 50%). For this purpose, 1 × 10 4 Vero cells were plated per well in 100μl in a 96-well plate. The following day, a 1:10 dilution series of virus, starting with 1:1000 dilution of stock was prepared. The medium in the 96-well plate was replaced by 100 μL virus dilution per well, for each dilution step 10 wells were used. After 2 h incubation at 37°C, supernatant was replaced with fresh DMEM supplemented with 10% heat inactivated FCS and the plates were incubated at 37°C. After 3 days, the plates were examined under the light microscope for cytopathic effect and the TCID50/ml of virus stock was calculated.
Selection, cloning, expression, and purification of IgG
To select human Herpes Simplex Virus Type 1 (HSV-1) specific single chain fragment variables (scFvs), a human lymph node-derived antibody phage display library was screened against recombinantly expressed glycoprotein B ectodomain from HSV-1 (strain KOS) [ 18 ]. According to the binding profile and HSV neutralization capacity, the most promising candidate, HDIT102, was chosen for further investigation. To generate an IgG with the same constant domains as HDIT101, codon-optimized variable heavy chain (VH) and variable light chain (VL) cDNAs of the selected scFv were synthesized appropriate restriction sites for cloning into pConPlus vectors containing IgG1 heavy/light-chain constant regions cDNAs. The synthesized VH coding sequences were cloned into pConPlus containing heavy chain constant region using Hind III and Apa I. VL coding cDNA was cloned into pConPlus Lambda encoding light-chain constant region using Hind III and Avr II. Afterwards, pConPlus vectors encoding light chain and heavy chains were cut with Not I and Pvu I and ligated to generate a double-gene vector. HDIT101 was produced at GMP-grade and HDIT102 at research grade quality by contract manufacturers.
HSV antibody neutralization assay
An end point titration assay was used to assess the antiviral activity of HDIT101, HDIT102, and a combination of both antibodies against cell-free virus. Briefly, different antibody dilutions were incubated with a constant viral dose (100 TCID50 HSV-1F or HSV-2G) for 1 h at 37°C. The antibody-virus mixtures were applied to 80–90% confluent Vero cells in 96-well plates (2.0 × 10 4 cells per well) in a volume of 100μL per well. As a control, Vero cells were infected with a viral dose of 100 TCID50 without prior incubation with antibody. The extent of the cytopathic effect was examined by light microscopy three days after infection. The neutralization titre was determined to be the highest antibody dilution at which the virus was completely neutralized and the formation of a CPE in the inoculated cell cultures was completely prevented. In addition, the neutralizing antibody concentration at which 50% of the cell culture wells are protected from infection (IC50) were calculated as described before [ 19 ].
Cell to cell spread inhibition assay
2.0 × 10 5 Vero cells were seeded in each well of a 4-well chamber slide. The following day, the culture medium was discarded, and the cells were inoculated with a viral load of 200 TCID50 HSV-1F or HSV-2G in a volume of 500 μL DMEM per well. Four hours after infection, the supernatant containing virus was discarded, unbound viral particles were removed by washing one time with 500 μL PBS, and the cells were incubated with 500 μL culture medium containing 10% FCS and 500 nM of HDIT102. As a control for inhibition of cell to cell spread, human polyclonal anti-HSV antibodies (Enzygnost) was used with a dilution of 1:20 in culture medium. After two days of incubation, the medium was discarded. The cells were washed once with PBS and then fixed with 5% paraformaldehyde solution and washed again with PBS. Subsequently, HSV-infected cells were stained using a FITC-conjugated anti-HSV antibody. After one hour, the supernatant was discarded, and cells were washed with PBS to remove excess antibodies. Afterwards, the cells were subjected to staining with Hoechst to visualise nuclei and then fixed with 5% paraformaldehyde for 15 min. The evaluation was carried out by 20 × magnification fluorescence microscopy using an inverted microscope (Leica).
Mouse HSV infection model
In vivo efficacy of the HDIT102 or Fc mutant N297A was investigated in an HSV-2 infection BALB/cOlaHsd mouse model. One week prior to the experiment, 6-week-old female mice BALB/c (weight: 16–19 g) were purchased from ENVIGO and one week prior to virus inoculation pre-treated subcutaneously with medroxyprogesteron (longacting progestin Depo-Clinovir, prepared at 25 mg/mL in PBS and 100 μL per mouse). On the day of intravaginal virus inoculation, the experimental animals were anesthetized by isoflurane. During the short anaesthesia, the vaginal mucosa was cleaned from the vaginal secretions by using a sterile ESwab and the experimental animals were infected by intravaginal inoculation of 10 μL of virus stock (containing 5.0 × 10 4 TCID50 HSV-2G) to the vaginal mucosa using a pipette. Afterwards, a small amount of Epiglu tissue adhesive was applied on the surface to temporarily glue the vagina (avoids inoculum to flow out). The glue was lost within 1 day after inoculation. The efficiency of antibodies in protecting mice from a lethal HSV-2G infection was assessed by the intraperitoneal administration after infection. The experimental animals were regularly inspected for weight loss and the occurrence of perineal hair loss (HL), redness (R) and swelling (S) and neurologic damage (e.g. hind limb paralysis, gastrointestinal track blockage) over an observation period of 60 days. Visible inspection was graded from slight to severe symptoms accordingly + / + + / + + + . Experimental animals were sacrificed in case of severe signs of herpes encephalitis, or paralysis or occurrence of severe lesions to prevent undue suffering. All experiments were done in line with ethical approval. To demonstrate antiviral activity doses of 300 μg or 600 μg HDIT102 (each n = 10) and 600 μg HDIT102-N297A ( n = 5) in 100 μL PBS were tested. Control groups were treated with PBS. For combination experiments HDIT102 IgG was pre-mixed with HDIT101 IgG at equimolar ratio (combination therapy) and injected at a final total IgG dose of 300 μg intraperitoneally. HDIT101 or HDIT102 alone (monotherapy) were injected intraperitoneally at the same dose. 20 mice per treatment group and 15 mice for the control arm were used in total. The statistical differences between survival curves were calculated using Logrank Mantel Cox test. The differences between cumulative combined clinical scores were analyzed using Kolmogorov-Smirnow test.
Competition binding assay
Competitive binding assays with HDIT101 and HDIT102 were done by flow cytometry and enzyme-linked immunosorbent assay (ELISA). HEK293T cells expressing HSV-1F gB were incubated with 10 μg/mL of the murine ancestor of HDIT101 (MAb2c) in combination with a serial dilution of either the humanized IgG HDIT101, the fully human IgG HDIT102, or a none competing control IgG (huRFB4). Subsequently, the cells were stained with anti-murine IgG-APC and analyzed in flow cytometry. For ELISA His-tagged Fab102 was added in twofold serial dilutions to either HSV-1 gB or HSV-2 gB coated microtiter plates. After 1.5 h at room temperature wells were washed and subsequently incubated for 1 h with Penta His-HRP conjugate (1:20,000). Wells were washed again prior to incubation with a 100-fold molar excess Fab101 or blocking buffer for 1.5 h. After a final wash, bound Fab102 was detected using TMB chromogenic substrate and signal was detected using a Tecan plate reader.
Cross-reactivity binding assay to other herpesviridae members
To check for HDIT102 cross-reactivity with other common members of the herpesviridae family, commercial Enzygnost anti-HSV/VZV/CMV/EBV IgG kits were used according to the manufacturer’s instructions. Bound antibodies were detected with rabbit anti-human Fcγ IgG-HRP conjugated polyclonal antibody (Jackson ImmunoResearch).
The test antibodies were incubated with HSV-infected Vero cells or HEK293T cells stably expressing gB and engineered Jurkat reporter cells stably expressing the FcγRIIIa receptor, V158 (high affinity) variant, and an NFAT response element driving expression of firefly luciferase (Promega). Vero cells were infected with HSV-1F/2G at MOI 1. Twenty hours after infection, cells were harvested and distributed in white flat bottom 96 well plates (1.25 × 10 4 cells per well) and incubated 6 h together with Jurkat effector cells at an effector:target ratio of 6:1 and serial dilutions of test antibodies. Noninfected Vero cells and Raji cells incubated with Rituximab served as negative and positive controls, respectively. Luciferase substrate was added and after 15 min incubation luminescence intensity was measured using a plate reader. Triplicate reads were performed, and means were calculated. Plate Background from control wells was subtracted. Fold of ADCC induction was calculated.
To measure and compare complement activation in the presence or absence of neutralizing antibodies, Vero cells were infected with HSV-1F or HSV-2G at an MOI of 1 for 20 h in T75 flasks (containing 4 × 10 5 cells). After the incubation time the infected Vero cells were harvested and washed once with PBS. Afterwards, 75 μg of neutralizing antibodies were prepared in DMEM with 20% heat-inactivated or not heat-inactivated human IgG-depleted serum and added to 5 × 10 5 infected Vero cells and incubation was carried out for 4 h at 37°C. Then, the supernatant was subjected for the quantitative determination of complement activity using a commercial ELISA kit designed to measure human terminal complement complex C5b-9 (TCC C5b-9) concentration by following the protocol of the kit (Human terminal complement complex C5b-9 ELISA, Creative Biolabs). The assay is based on a sandwich enzyme immunoassay technique. In addition, infected Vero cells were washed with PBS once and stained with SYTOX Blue (Dead Cell Stain) for 15 Minutes at RT to discriminate dead and viable cells. subjected.
Alanine scanning mutagenesis and fusion inhibition assay
Specific HSV-1F gB amino acid residues in close proximity to HDIT101 or HDIT102 CDR residues in the cryo-EM co-structure were interrogated for their contribution to IgG-mediated inhibition of gB-induced cell–cell fusion by substitution to alanine. Mammalian expression plasmids encoding wild type HSV-1F gB or single amino acid point mutants were co-transfected into a 1:1 mix of HEK293T-GFP and HEK293T-E2C fluorescent reporter cells, together with mammalian expression plasmids encoding HSV-1F gD, gH and gL protein. Transfected cells were incubated at 37°C and 5% CO 2 for 5 h before HDIT101 or HDIT102 was added to final concentration of 75μg/mL. In control samples no antibody was added. Fusion of cells was judged by the presence of GFP + E2C + double positive cells by flow cytometry two days later.
Generation of HDIT101/HDIT102-resistant mutant viruses in vitro
Vero cells (8 × 10 6 cells in T75 flask) were infected with HSV-1/2 at MOI 0.01 and passaged in the presence of increasing concentrations of neutralizing mAbs in multiple rounds. After each round, viral supernatant was harvested, purified through 0.45 μm filter before inoculating fresh Vero cells. After 4 rounds of passaging, harvested virus was characterized for its neutralizing potency in the absence or presence of neutralizing antibody concentrations and an aliquot of infected cells was used for DNA extraction (QiaAmp, Qiagen). Partial gB coding regions were amplified by PCR and products were Sanger-sequenced (Eurofins MWG) to determine resistance mutations. Clonal resistant viruses were grown by limiting dilution of resistant virus pool and expansion of single plaques under antibody pressure.
CD14 + monocyte selection and macrophage/dendritic cell differentiation and phagocytosis assay
Monocyte-derived macrophages (MDMs) were generated from buffy-coat primary blood mononuclear cells (PBMCs) of three independent healthy donors by isolation of CD14 + monocytes by magnetic separation using MACS microbeads (Miltenyi) and differentiation using 80 ng/mL granulocyte–macrophage colony stimulating factor (GM-CSF) to generate type 1 MDMs or 50 ng/mL M-CSF to generate type 2 MDMs or 80 ng/mL GM-CSF + 20 ng/mL interleukin 4 to generate monocyte-derived dendritic cells (MDDC) for 7 days. The differentiated cells were then exposed to HSV-1 labelled with a pH-sensitive dye (IncuCyte pHrodo Orange Cell Labeling Dye, Sartorius) at an MOI of 10 in the presence or absence of 150 μg/mL HDIT101 and/or HDIT102 antibody or 50 μg/mL Acyclovir. Three technical replicates were then monitored using an Incucyte system (Sartorius) at intervals of one hour. The amount of virus taken up as measured by dye fluorescence was normalized to the cell count in the imaged area.
Macrophage-dependent activation of T cells
Autologous T-cell activation was measured after exposure of HSV-HDIT101 and/or HDIT102 immune complexes to MDMs from two independent HSV-seropositive healthy donors. MDMs were pre-incubated for 24 h with HSV-1F (MOI 10) in the presence or absence of neutralizing amounts of HDIT102 and/or HDIT101 before addition of autologous T-cells. Analysis was done by flow cytometry using BV785-labeled anti-CD69 IgG (Biolegend) as T-cell activation marker, APC-labelled anti-CD4 (Biolegend) and BV605-labeled anti-CD8 (Biolegend) 24 h after addition of the autologous CD14- fraction to the pre-incubated MDMs. Percentage of CD69-positive cells as well as mean fluorescent intensity (CD69 MFI) were measured and analyzed separately for CD4 + as well as CD8 + cells.
Protein production
GMP-grade HDIT101 IgG1 was produced for clinical trials by Celonic Germany GmbH. The Fab proteins were prepared by Papain digestion of HDIT101. For this, we used a Pierce Fab Preparation Kit (ThermoFisher) and dialyzed the obtained protein 3 times against 50 mM Tris, 150 mM NaCl, pH 8 buffer. HDIT102 Fab was generated recombinantly by transient expression in HEK293-E6 cells. For this the HDIT102 VH-CH1 domain fused to a double Strep-tag and the HDIT102 light chain were cloned into a mammalian expression vector.
The codon-optimized sequence of HSV-1F gB (aa 30–729; UniProtKB P06436.1) and HSV-2G gB (aa 22–724; UniProtKB: A0A410TI43) ectodomain proteins including a BM40 signal peptide and a C-terminal double Strep-tag were cloned into a mammalian expression vector. The proteins were then transiently expressed in HEK293-E6 suspension cells cultured in F17 medium (ThermoFisher) supplemented with 0.1% Kolliphor (Sigma) and 4 mM Glutamine. HEK293 cells were transfected with PeiMax (Polysciences) at a cell density of 1.5–2 × 10 6 cells/mL with 1 μg plasmid and 2 μg PeiMax/mL culture media. 24 h after the transfection Tryptone N1 feeder (Organi Technie) was added to the cultures. On day 5 after the transfection the supernatant was harvested by two centrifugation steps, first 1200 rpm to remove the cells and then 3600 rpm to remove cell debris. Next, the pH of the supernatant was adjusted by the addition of 1 mL 2 M Tris buffer pH 9 / 100 mL supernatant. We then purified both proteins by Strep-Tactin XT (IBA, Germany) gravity flow purification according to the manufacturer protocol.
The Strep-tag of both gB proteins was then removed by thrombin digestion (Serva) and dialysis against 50 mM Tris, 150 mM NaCl, pH 8. The thrombin digested gB proteins were then purified 3 × times by Strep-Tactin XT affinity chromatography to deplete the sample from any remaining Strep-tagged protein. Next, we concentrated the proteins with Amicon spin columns (cut-off 30kD) and the proteins were further purified with a Superdex 200 10/300 GL SEC column and an Äkta Pure FPLC system. The peek fractions were pooled and concentrated with Amicon spin columns.
Determining co-structures by cryo-EM
For cryo-EM grid preparation and data collection, the following steps exemplified for HSV-2 gB and HDIT101 Fab were performed. Procedures to solve cryo-EM structures HSV-2 gB/HDIT102 Fab, HSV-1 gB/HDIT101 Fab and HSV-1 gB/HDIT102 Fab were similar, and details are shown in Tables 1 and 2 , respectively. The procedure for one of the complexes is described in more detail here: Recombinant HSV-2G gB ectodomain and HDIT101 Fab were mixed in a ratio of 1 to 3.5. A 4 μl aliquot of the mixture was adsorbed onto glow-discharged Quantifoil Au-R2/1-300mesh holey carbon-coated grids (Quantifoil, Germany), blotted with Whatman 1 filter paper and vitrified into liquid ethane at -180°C using a Leica EM GP2 plunger (Leica microsystems, Austria) operated at 10°C and 85% humidity. Data was acquired on a Glacios TEM (ThermoFisher) operated at 200 kV and equipped with a Quantum K3 direct electron detector (Gatan). Micrograph movies of 40 frames were recorded in counting mode at a magnification of 45,000 × (pixel size 0.878 Å) with a dose of 1.325 e − /Å 2 /frame, resulting in a total accumulated dose on the specimen level of approximately 53 e − /Å 2 per exposure. All image processing steps were performed with Relion v4.0 [ 20 ]. Dose weighting and motion correction of dose-fractionated and gain-corrected movies were performed using Relion’s implementation of the UCSF motioncor2 program. Contrast transfer function (CTF) parameters were estimated using ctffind 4.1.14 [ 21 ]. Micrographs displaying strong drift, astigmatism greater than 1000 Å and maximum CTF resolution worse than 8 Å were excluded from further processing. A total of 6 million particles were picked using the Laplacian-of-Gaussian (LoG) filter in Relion 4.0 [ 20 ]. The particle dataset was cleaned through five rounds of reference-free 2D classification resulting in 915′372 particles. Relion’s Stochastic Gradient Desecnt (SGD) algorithm was used to generate a de novo 3D initial model from the 2D particles. The particle dataset was further cleaned through three rounds of unsupervised 3D classification. The remaining 234′096 particles were subjected to Bayesian particle polishing, CTF and aberration refinement, and a final high-resolution 3D refinement, which resulted in a final map with an overall resolution of 3.45 Å according to the gold standard Fourier shell correlation (FSC) at FSC = 0.143 (Fig. S3). The HSV-1 gB X-ray structure (PDB-ID: 2GUM) was manually mutated according to a sequence alignment with sequence QAU10436.1 (UniProt entry A0A410TI43) and placed into the final map using coot [ 22 ]. For the HDIT101 Fab, the crystal structure of a humanized recombinant Fab fragment of a murine antibody (PDB-ID 3AAZ) was mutated in coot [ 22 ] based on a sequence alignment generated by Needle EMBOSS [ 23 ]. Three HDIT101 Fabs were placed into the final map using coot [ 22 ]. Molrep of the CCP-EM software suite v1.6 [ 24 ] was used for the initial fitting of gB and the three HDIT101 Fabs into the final map. The final protein model was obtained by several iterations of manual model building in coot [ 22 ], Refmac-Servalcat refinement and model validation in the CCP-EM software suite v1.6 [ 24 ].
Bio-layer interferometry (BLI)
First, HSV-1/2 gB ectodomain proteins were dialyzed 3 times against PBS buffer, then the proteins were biotinylated with EZ-Link NHS-PEG4-Biotin reagent (ThermoFisher) at a molar ratio of gB protein as monomer to biotin equals 1:3. Next, the proteins were dialyzed to PBS buffer once and stored at 4°C. For BLI measurements we used a Sartorius Octet R8 machine, Octet Streptavidin biosensors and Octet BLI Discovery 12.2.2.20 software. In the beginning we performed ligand loading optimization experiments with different ligand concentrations and a fixed analyte concentration. All samples were diluted in kinetic buffer (PBS pH 7.4 with 0.02% Tween-20, 0.1% albumin (Sigma) and 0.05% sodium azide).
For the kinetic measurement we used the determined optimal ligand concentration of HSV-1/2 biotinylated gB protein and serial dilutions of the analyte HDIT101 or HDIT102 IgG or Fab. All the kinetic measurements were then performed in triplicate. The binding data were then analysed with Octet Analysis Studio 12.2.2.26 software (Sartorius).
Positive selection analysis
HSV-1 and HSV-2 gB DNA sequences were retrieved by nucleotide BLAST (NCBI) against HSV-1F gB or HSV-2G gB sequences. Sequences were aligned using DNADynamo (Bluetractor software). Only full lengths sequences without any non-assignable nucleotides or frame shifts were considered in the alignment. In total 451 HSV-1 gB and 368 HSV-2 gB cleaned DNA sequences were retrieved. Positive selection analysis was performed using single-likelihood ancestor counting (SLAC) ( www.datamonkey.org ) for 185 non-identical HSV-1 and 201 non-identical HSV-2 gB sequences [ 25 ]. Phylogenetic trees were visualized using FigTree.
Binding characteristics of the fully human IgG HDIT102
Glycoprotein B plays a crucial role in the viral entry process and is indispensable for HSV replication and pathogenesis. We generated HDIT102, a fully human IgG1 antibody, whose variable domains were isolated from an scFv phage display library utilizing HSV-experienced B cell repertoires, through targeted selection against gB of HSV-1. Glycoprotein B is a highly conserved viral protein found among herpesviruses. To exclude potential cross-reactivity of HDIT102 to other members of the herpes virus family, it was demonstrated that the IgG binds specifically to HSV and not to VZV, HCMV and EBV using routine immunodiagnostics ELISAs for virus specific serology (Fig. S1A). We then compared the binding affinity of HDIT102 with that of the antibody HDIT101, a humanized IgG1 currently in clinical development, using biolayer-interferometry (Octet, Sartorius). The association rates (ka) for HDIT101 and HDIT102 IgG were comparable (5.0 × 10 5 and 7.0 × 10 5 M −1 s −1 ). However, in contrast to HDIT101, HDIT102 IgG exhibited an exceptionally slow dissociation rate (kdis), rendering it non measurable (Fig. S1B, C). Due to the absence of measurable kdis for HDIT102, the Kd binding constant for its IgG format could not be determined. Therefore, Fab fragments of both antibodies, Fab101 and Fab102, were generated for investigating the monovalent binding kinetics. Fab102 had slightly increased association rates (ka) to HSV-1F gB (9.92 × 10 5 M −1 s −1 ) as well as to HSV-2G gB (8.65 × 10 5 M −1 s −1 ) as compared to Fab101 (HSV-1F gB, 4.35 × 10 5 M −1 s −1 and HSV-2G gB, 2.12 × 10 5 M −1 s −1 ) (Fig. 1 A, B and C). The dissociation rate for Fab102 was dramatically decreased compared to Fab101 for both HSV-1F (Fab102 vs. Fab101, 8.85 × 10 –5 s −1 vs. 3.13 × 10 –3 s −1 ) and HSV-2G (Fab102 vs. Fab101, 2.86 × 10 –5 s −1 vs. 3.79 × 10 –3 s −1 ), mirroring the pattern observed with HDIT102 IgG. This leads to a significantly lower Kd for Fab102 when binding to HSV-1F gB (Fab102 vs. Fab101, 8.95 × 10 –11 M vs. 7.26 × 10 –9 M) and HSV-2G gB (Fab102 vs. Fab101, 3.29 × 10 –11 M vs. 1.81 × 10 –8 M).

Bio-layer interferometry (BLI) analysis of recombinant HSV-1 or HSV-2 gB interaction with HDIT101 or HDIT102 Fab. Biotinylated gB of HSV-1F ( A ) or HSV-2G ( B ) was immobilized on streptavidin biosensor tips and incubated with a serial dilution of HDIT102 (100, 33.3, 11.1, 3.7, 1.24 or 0.41 nM). Octet sensorgrams were recorded. C Measurements of association (ka) and dissociation (kdis) rates and calculation of binding affinities using a dilution series of HDIT101 or HDIT102 Fab binding to recombinant HSV-1F or HSV-2G gB
HDIT102 exhibits potent neutralization capacity in vitro and prevents disease in vivo
To examine the antiviral activity of HDIT102 towards cell-free virus, neutralization capacities of different antibody dilutions were determined for HSV-1F or HSV-2G. As control, Vero cells were infected without prior incubation of virus with antibody. The highest antibody concentration preventing the viral cytopathic effect (CPE) to 50% and 100% relative to the control were determined three days after infection and considered the endpoint. Although HDIT102 exhibited a significantly higher affinity than HDIT101, its in vitro neutralization capacity for HSV-2 was comparable to that of HDIT101, whereas for HSV-1, concentrations twice as high as those of HDIT101 were required for 50% and 100% neutralization. Inhibitory concentrations for neutralizing HSV-1F by HDIT102 were 25.5 nM and 62.5 nM, while inhibitory concentrations for HSV-2G were 12.5 nM and 31.25 nM, for 50% and 100% neutralization, respectively (Fig. 2 ). Furthermore, the combination of both antibodies in a 1:1 ratio maintained neutralization efficiency comparable to that of a single antibody treatment. The combination prevented virus-induced CPE of HSV-1 by 50% at a concentration of 18 nM and by 100% at 31.25 nM. For HSV-2, the concentrations required were 9.3 nM to achieve 50% and 23.44 nM for 100% neutralization (Fig. 2 ).
In vitro antiviral activities and in vivo efficacy of HDIT102. A The antibody concentration required for reducing the virus-induced cytopathic effect (CPE) by 50% and 100% was determined by an endpoint dilution assay. Serial dilutions of antibodies were incubated with 100 TCID50 of HSV-1F or HSV-2G. The antibody virus inoculum was applied to Vero cell monolayers grown in microtiter plates and CPE was scored after 72 h. Means and error bars, showing standard deviation of mean, were calculated based on three independent experiments. B Inhibition of HSV-2G cell-to-cell spread by HDIT101 and HDIT102. Fluorescence microscopy images of Vero cells infected with HSV-2G and subsequently treated with either HDIT101 (75 μg/ml), HDIT102 (75 μg/ml), human polyclonal anti-HSV antibody (1:20) or left untreated. Plaque formation was visualised by anti-HSV immuno- and Hoechst staining. Representative images are shown. Arrows show plaques or initially infected cells. C Fluorescence microscopy images of HEK293T cells ectopically expressing HSV-1 gB-GFP and treated with either 5 μg/mL HDIT101 or HDIT102 (IgG vs. Fab) or with an irrelevant isotype control antibody. D Incucyte data of HSV-1F-infected Vero cells incubated with either HDIT101, HDIT102 or isotype control IgG labelled with a pH-sensitive dye. Uptake of IgGs into the endosomal pathway was measured over time by tracking fluorescence. E HDIT102 treatment of immunocompetent BALB/cOlaHsd mice after a lethal intravaginal HSV-2G infection. The mice were infected with HSV-2G (5 × 10 4 TCID50) intravaginally and four hours later 600 μg or 300 μg of HDIT102 were injected intraperitoneally, while the control group received PBS (each group, n = 10). The statistical differences between survival curves were calculated using Logrank Mantel Cox test, ** p < 0.05, *** p < 0.001
Cell-to-cell spread has been suggested as the predominant way of transmission in vivo and titres of antibodies preventing cell-to-cell spread have been proposed to correlate with reduced number of recurrences for HSV-1 [ 26 ]. HDIT101 has been demonstrated to block cell-to-cell transmission in Vero cells [ 17 ]. Similarly, HDIT102 blocked cell-to-cell transmission of HSV-1F or HSV-2G in Vero cells (Fig. 2 B and Fig. S2B). We speculated that both HDIT101 and HDIT102 may block cell-to-cell spread by binding to gB on the cell surface before gB becomes internalized and incorporated into membranes of newly produced viruses. When HEK293T cells expressing HSV-1 gB carboxyterminally fused to GFP were treated with HDIT101 or HDIT102, large aggregates of gB-GFP could be detected that were dependent on the presence of bivalently binding IgGs since aggregates were absent for monovalently binding Fab fragments (Fig. 2 C). To demonstrate this for gB expressed on infected cells, Vero cells infected with HSV-1 were treated with either HDIT101 or HDIT102 IgG being labelled with a pH-sensitive dye and uptake into endosomes was measured over time. HDIT101 as well as HDIT102 induced endosomal internalization of gB in infected Vero cells, suggesting that cell-to-cell spread is blocked by IgG recruitment of cell-surface exposed gB to endosomes trapping and inactivating progeny viruses before or at the stage of gB incorporation into the viral membrane (Fig. 2 D). To demonstrate antiviral activity of HDIT102 in vivo, immunocompetent BALB/c mice were intravaginally infected with a lethal dose of HSV-2G and treated afterwards with 300μg or 600μg HDIT102 intraperitoneally. Significantly more mice survived in the HDIT102 treatment groups as compared to the control group and a dose-dependent effect was observed (Fig. 2 E).
HDIT101 and HDIT102 bind to overlapping epitopes in gB domain I and can form heterogenic immune complexes with trimeric gB
To determine the exact epitopes of HDIT101 and HDIT102 in gB, we solved the cryo-EM co-structures of Fab bound to HSV-1F or HSV-2G recombinant gB ectodomain in post-fusion conformation at resolutions of 3.12Å to 3.45Å (Fig. S3). The Fab fragments of HDIT101 and HDIT102 both bound to domain I of gB (HSV-1F/HSV-2G amino acids 154–364/146–356), indicating a shared epitope region (Fig. 3 A, B, C and Fig. S4A, B, C). When we compared the cryoEM structure of HSV-1 gB solved in complex with HDIT101 Fab or HDIT102 Fab with the published x-ray crystal structure PDB: 2GUM [ 27 ] using the pairwise structure alignment tool TM-align [ 28 ], we observed a TM-score of 0.96 and 0.99, respectively, demonstrating almost identical structures (Fig. S4D, E, F). Our cryo-EM structure revealed the structural details of the HSV-1 gB amino acid residues T331-T337, which were not resolved in the X-ray crystal structure or by two other cryoEM structures (PDB: 7UI0 and PDB:7UHZ) [ 29 ]. The region L460-A490 which is bearing the most differences between HSV-1 and HSV-2 gB and was not resolved by x-ray crystallography could also not be resolved by cryoEM, suggesting high flexibility. We next compared the HSV-1F gB structure with HSV-2G gB when bound either by HDIT101 Fab or HDIT102 Fab (Fig. S4 G, H). The TM-score of HSV-1F gB vs. HSV-2G gB structures when HDIT101 Fab or HDIT102 Fab bound was 0.98 and 1.00, respectively, demonstrating that HSV-1F and HSV-2G gB ectodomains obtained almost identical protein structures in the post-fusion conformation, despite 24 amino acids are different between HSV-1 and HSV-2 gB sequences (Fig. S4I).
Determination of the cryo-EM structure of HDIT101 or HDIT102 Fab bound to trimeric HSV-2G gB at a resolution < 3.5 Å. A Side, bottom and top view of co-structure of trimeric HSV-2G gB in post-fusion conformation (brown) with three HDIT101 Fab molecules (blue) solved by cryo-EM at a resolution of 3.45 Å are shown in cartoon representation. HSV-2 gBG domain I (amino acid residues I146-C356) is highlighted in cyan. B The co-structure of HDIT102 Fab (magenta) bound to trimeric HSV-2G gB in post-fusion conformation (brown) was determined at a resolution of 3.12 Å and side, bottom and top views are shown in cartoon representation. C Overlay of the co-structures represented as surface models indicating overlapping epitopes of HDIT101 and HDIT102 Fabs on HSV-2G gB indicating perpendicular orientation of both Fabs to another. D Critical HSV-2G gB residues in close contact with HDIT101 HC (light blue) and LC (violet) CDR residues are are shown as sticks and highlighted in different colours. E Critical HSV-2G gB residues in close contact with HDIT102 HC (pink) and LC (light pink) CDR residues are shown as sticks and highlighted in different colours. F Competitive binding towards HSV-1 gB and HSV-2 gB of HDIT101 and HDIT102 was performed by ELISA using their respective Fab fragments. Binding of Fab102-His at increasing concentrations was detected with a Penta His-HRP conjugate and chromogenic substrate TMB in the presence or absence of Fab101 at 100-fold molar excess. Competition assay of the humanized IgG HDIT101 and human IgG HDIT102 to clonal HEK293T cells ectopically expressing HSV-1 gB. HEK293T HSV-1gB cells were incubated with a fixed amount of fluorochrome-labelled murine MAb2c IgG2 containing identical CDRs to HDIT101 in combination with increasing amounts of either HDIT101, HDIT102 or an irrelevant human IgG (anti-CD22). Mean fluorescent intensities (MFI) were measured by flow cytometry. G 2D-class averages from cryo-EM analyses of recombinant trimeric HSV-1F gB mixed with 1:1 molar ratio of HDIT101 and HDIT102 Fabs. gB trimers with heterogenic binding of two HDIT102 Fab molecules and one HDIT101 Fab molecule to the gB trimer (left) could be easily separated from homogenic HDIT102 binding to gB trimers by the perpendicular orientation of HDIT101 and HDIT102 Fabs when bound to gB
We next investigated which gB residues were in close proximity of the HDIT101 and HDIT102 complementary determining regions (CDRs). HDIT101 heavy and light chain CDR residues were in close proximity with HSV-1F/HSV-2G gB residues Y303/Y295, R304/R296, E305/E297, H308/H300 and D323/D315, while HDIT102 CDR residues were near HSV-1F/HSV-2G gB residues D199/D191, K204/K196, R304/R296, K320/K312, Y326/Y318 and R335/R327 (Fig. 3 D, E and Fig. S4J, K).
HSV-2 gB Y295 made polar contact with a distance of 2.8Å with HDIT101 HC CDR2 N54 and D56 (Fig. S5A). HSV-2 gB E297 made polar contact with HDIT101 LC CDR1 Y32 and gB D315 with gB R296 and HDIT101 LC CDR1 H30a, thereby positioning R296 in proximity to HDIT101 LC CDR1 H30a, LC CDR1 Y32, LC CDR3 W96 and HC CDR3 Y97 (Fig. S5B). HSV-2 gB H300 formed non-polar bonds with the peptide-backbone of HC CDR1 S31a and CDR3 G98 (Fig. S5C). In the HDIT102 co-structure HSV-2 gB D191 made polar contact at 2.8Å and 2.7Å distance with HC CDR3 T100a and T100b, respectively (Fig. S5D). HSV-2 gB K196 made polar contact at 2.5Å distance with LC CDR2 D51 (Fig. S5E) and gB R327 formed polar contact with LC CDR2 D53 and non-polar contact with LC CDR2 Y50 (Fig. S5F). HSV-2 gB Y318 made polar contact with HC CDR3 D101 (Fig. S5G). The distance between HSV-2 gB R296 to HDIT102 CDR residues was > 3.5Å and no polar bonds were observed.
As the cryoEM structure showed that the epitopes of HDIT101 and HDIT102 have one shared amino acid at position (HSV1/HSV2 R304/R296) we investigated if the epitopes of HDIT101 and HDIT102 allow simultaneous binding of both IgGs and performed competition assays. Using the Fab fragments of HDIT101 and HDIT102 in an ELISA based assay, HDIT101, even when present at a 100-fold molar excess, did not displace HDIT102 from binding to either HSV-1 gB or HSV-2 gB, which is very likely due to the extremely slow dissociation rate of HDIT102. Using HEK293T cells ectopically expressing HSV-1F gB increasing concentrations of either HDIT101 IgG or HDIT102 IgG but not an irrelevant control IgG were able to reduce the binding signal of the murine MAb2c containing identical CDRs to HDIT101. These results indicate that steric hindrance prevents HDIT101 and HDIT102 from simultaneous binding to their epitopes (Fig. 3 F). Interestingly, structural analyses showed that both Fabs bound in a perpendicular way, suggesting that inter-gB-trimer crosslinking by HDIT101 and HDIT102 IgG may result in differently oriented Fc domains and that heterogenic immune complexes may be formed by mixed binding of HDIT101 and HDIT102 to individual protomers within a trimer. To analyze this, a 1:1 molar mixture of HDIT101 and HDIT102 Fabs was incubated with recombinant HSV-1 gB trimers and 2D classes were calculated from cryo-EM micrographs. While the majority of classes showed homogenic immune complexes of three HDIT102 Fab molecules bound per gB trimer, heterogenic complexes with two HDIT102 and one HDIT101 Fab molecule could be easily identified based on the perpendicular orientation of HDIT101 and HDIT102 Fabs when bound to gB (Fig. 3 G). We detected only heterogenic trimers consisting of two HDIT102 Fabs and one HDIT101 Fab. Neither trimers with two HDIT101 Fabs and one HDIT102 Fab nor homogenic trimers consisting of three HDIT101 Fab molecules were observed. We did not observe more than three Fab molecules bound per gB trimer which is in line with overlapping epitopes and the competition of binding to a single gB protomer. The data suggests that each protomer within the trimeric gB may be bound by either HDIT101 or HDIT102 and in the presence of both, heterogenic immune complexes may be formed.
Signatures of evolutionary pressure by HDIT102-like endogenous IgG on HSV-2, but not HSV-1
To verify the structural data that was revealed using cryo-EM, HSV-1F or HSV-2G were propagated in Vero cells for several rounds with increasing concentrations of either HDIT101 or HDIT102. After five rounds of replication, presence of suboptimal concentrations of HDIT101 generated resistance mutant HSV-1F R304Q and HSV-2G R296Q, respectively, while propagation of the viruses under increasing doses of HDIT102 generated resistance escape mutants HSV-1F R335Q and HSV-2G R327W, respectively (Fig. S6A). We confirmed the importance of the identified gB residues in HDIT101 or HDIT102 induced inhibition of fusion using a flow cytometry-based fusion assay. gB amino acids conferring resistance and amino acids identified to be in close proximity to Fab residues in the cryo-EM structures were analyzed by alanine-substitution in the fusion assay, confirming the importance of HSV-1F gB D199, K204, Y326 and R335 as interface residues for HDIT102- and Y303, R304, E305, H308 and D323 as interface residues for HDIT101-induced fusion inhibition (Fig. S6B-E).
We next analyzed 451 HSV-1 and 368 HSV-2 full-length gB DNA sequences retrieved by nucleotide BLAST (NCBI) and found that HSV-1/2 R304/R296, Y303/Y295, E305/E297, H308/H300 and D323/D315 were conserved to 100%, suggesting that the HDIT101 targeted gB region is not under natural selection pressure neither in HSV-1 nor in HSV-2. In contrast, 451/451 (100%) of gB sequences from HSV-1 isolates had R335, however 55/368 (14.9%) of gB sequences from HSV-2 isolates possessed Q327, the same site that changed and developed resistance in vitro when HSV-2G was propagated in the presence of increasing doses of HDIT102 (Fig. S6F, G). Analysis for evolutionary positive selection using single-likelihood ancestor counting (SLAC) with n = 185 non-identical HSV-1 gB sequences and n = 201 non-identical HSV-2 gB sequences resulted for HSV-1 in 1 positively and 38 negatively selected sites and for HSV-2 gB in 6 positively and 13 negatively selected sites, suggesting that HSV-2 gB is under stronger selective pressure as compared to HSV-1 gB (Fig. S6H). When analyzing inferred non-synonymous and synonymous substitution rates for HSV-2 gB position 327 we found evidence for positive selection with dN-dS = 36.1 and p (dN/dS > 1) = 0.0375, suggesting that this site is indeed under positive selective pressure [ 25 ]. Mapping Q327 in a phylogenetic tree suggests a possible fixation of Q327 in branches (Fig. S6I), arguing that Q327 is evolving from R327. The data suggest that natural HDIT102-like antibodies exert immunologic pressure towards HSV-2, but not HSV-1, demonstrating suitability of HDIT102 as therapeutic candidate. Of note, we were unable to generate double resistant mutants of HSV-1F or HSV-2G when the viruses were propagated under suboptimal concentrations of a 1:1 molar mixture of HDIT101 and HDIT102 IgG in Vero cells. We also tried to generate double resistant mutants by starting with either HDIT101- or HDIT102-resistant viruses and propagation in suboptimal increasing concentrations of the respective other antibody, without success. Cloning of an HSV-1 gB mutant containing both, resistance change R304A (HDIT101) in combination with resistance change R335A (HDIT102), resulted in a functionally active gB protein showing fusion activity in the cell-to-cell fusion assay, which was resistant to both HDIT101 as well as HDIT102 treatment (data not shown). Together these data suggest that a double resistant mutant gB could be functionally active, however may not evolve readily in the context of virus replication.
The Fc effector function of HDIT101 and HDIT102 to mediate HSV-1 phagocytosis by monocyte-derived macrophages and dendritic cells likely contributes to in vivo efficacy
The efficacy of antibodies in therapeutic application often not solely depends on their binding capacity but also on the Fc-mediated effector function. To better understand the effects of how HDIT101 and HDIT102 work, we first tested whether both antibodies would induce antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC). We found that neither HDIT101, nor HDIT102, induced ADCC or CDC (Fig. S7 and data not shown). We next tested whether the antibodies would mediate phagocytosis of virus particles (ADCP) by antigen presenting cells (APCs). Human monocyte-derived macrophages (MDM) of type 1, type 2, or dendritic cells (MDDC) were generated from PBMCs and present a suitable primary model to test Fc-effector functions in cell culture. To trace the virus, we labelled the virus with a pH-sensitive dye. The cells were incubated with labelled virus, either without antibodies or in the presence of either HDIT101, HDIT102, or a 1:1 molar mix of both antibodies. The fluorescent signal indicates uptake into acidic endosomes and the impact of the investigated antibody on cellular uptake could be investigated. HDIT101 as well as HDIT102 exerted an enhanced uptake of virus particles into acidic endosomes within ten hours as compared to virus alone or virus in combination with acyclovir. The combination of both antibodies showed no substantial enhancement over using the individual antibodies (Fig. 4 A). The data suggest that the HDIT101 and HDIT102 Fc domain contributes to promoting ADCP of viral particles. Fc-dependent uptake of viral immune complexes may hence impact also the in vivo efficacy of the investigated antibodies.

HDIT102 induces phagocytosis by myeloid cell types and the Fc effector function likely contributes to in vivo efficacy. A CD14 + monocytes were isolated from PBMCs and differentiated for one week to monocyte-derived macrophages (MDM) or monocyte-derived dendritic cells (MDDC) by adding GM-CSF (MDM type 1), M-CSF (MDM type 2), or GM-CSF + IL-4 (MDDC). The differentiated cells were then exposed to HSV-1 labelled with a pH-sensitive dye with or without the addition of HDIT101 and/or HDIT102 antibody or acyclovir. Three technical replicates each were then monitored using an Incucyte system (Sartorius) at intervals of 1h. The amount of virus taken up as measured by dye fluorescence was normalized to the cell count in the imaged area. Representative results for one of three independent PBMC donors are shown. B Eight-week-old immunocompetent female BALB/cOlaHsd mice ( n = 5, each group) were infected intravaginally with HSV-2G using a lethal dose of 5 × 10 4 TCID50. Subsequently, 600 μg of HDIT102 or HDIT102-N297A mutant were injected intraperitoneally, while the control group received no treatment. The statistical differences between survival curves were calculated using Logrank Mantel Cox test. p = 0.15 (HDIT102 vs. HDIT102-N297A). p = 0.0016 (HDIT102 vs. untreated). p = 0.03 (HDIT102-N297A vs. untreated)
To elaborate on this further, we generated a commonly used IgG Fc domain mutant HDIT102 version containing the N297A substitution in the Fc domain, a change which substantially decreases Fc-gamma dependent activity and analyzed the requirement for a functional Fc domain for efficient therapeutic treatment in vivo. Immunocompetent female BALB/cOlaHsd mice were infected intravaginally with HSV-2G and treated with the same dose of wild type HDIT102, or HDIT102-N297A mutant antibodies, and disease development and survival was monitored. The results show a substantially longer survival of HDIT102 wild type as compared to HDIT102-N297A treated mice, suggesting that the Fc domain and interaction with Fc-receptors may be implicated in the in vivo efficacy (Fig. 4 B). Similar data was obtained for HDIT101 (data not shown). Of note, HDIT102-N297A treated mice were still protected to some degree as compared to control mice, potentially explained by some remaining ADCP activity of the N297A mutant, as has been described in earlier studies [ 30 , 31 , 32 ].
HDIT101 as well as HDIT102 increase T-cell activation after ADCP by macrophages
Data from the clinical program for HDIT101 suggested that modulation of the immune responses could be involved in the HDIT101-mediated effects. Since HDIT102, as well as HDIT101, were able to enhance uptake of viral particles into acidic endosomes of APCs in vitro and the Fc domain contributed to efficacy in mice, we speculated that this could enhance also T-cell responses. T-cells are binding to APC-processed peptides presented via MHC-I and MHC-II. We tested the hypothesis that enhanced phagocytosis of HSV particles by HDIT101 and/or HDIT102 may lead to an enhanced antiviral T-cell response. To do that MDM type 1 was generated from HSV-1 seropositive donors and incubated with either HSV-1F alone or in combination with HDIT101, HDIT102 or both antibodies together. As negative control, the cells were incubated with both antibodies in the absence of virus or left untreated. Twenty-four hours after stimulation, autologous T-cells (CD14- fraction) were added and after further 24 h the CD4 + and CD8 + cell populations were analysed for activation by measuring CD69. In two independent donors the stimulation of MDMs with immune complexes of HSV-1F with either HDIT101, HDIT102 or HDIT101 + HDIT102 induced CD69 expression on the surface of both, CD4 + and CD8 + cells (Fig. 5 A, B). While the percentage of CD69 + cells was higher in the CD4 + cell population, the MFI of CD69 was higher in the CD69 + CD8 + cell population, suggesting that a higher fraction of CD4 + than CD8 + T-cells becomes activated, but that activation strength as measured by CD69 quantity per cell is higher in the responsive CD8 + T-cells. Together the data demonstrate that both antibodies alone or in combination are capable of mediating phagocytosis that may result in an enhanced activation of T-cells in vitro.

HDIT102-induced phagocytosis of HSV-1 by MDMs activates autologous T-cells. Autologous T-cell activation in the CD14- fraction of PBMCs was measured after exposure to CD14 + monocyte derived macrophages (type 1) from two independent HSV-seropositive healthy donors, ( A ) donor 1, ( B ) donor 2, that were pre-stimulated for 24 h with HSV-1F (MOI 10) in the presence or absence of neutralizing amounts of HDIT102 or HDIT101 or a 1:1 combination of both. Analysis of activation was done by measuring CD69 expression on CD4 + and CD8 + cell populations using flow cytometry. Percentage of CD69 + cells as well as mean fluorescent intensity were measured and analyzed separately
The combination of HDIT101 and HDIT102 exerts synergistic therapeutic effects in vivo
We did not observe general synergistic effects when comparing the HDIT101 and HDIT102 combination treatment with the individual antibodies at equal IgG quantities in any of the in vitro assays tested, including neutralization, cell-to-cell spread, ADCP and T-cell activation by stimulated APCs. To analyze the combination and possible synergy of both IgGs in vivo immunocompetent BALB/cOlaHsd mice were infected intravaginally with HSV-2G and treated four hours later with either 300 μg HDIT101, or 300 μg HDIT102 or with a combination of 150 μg HDIT101 + 150 μg HDIT102 and survival as well as clinical scores were monitored over time. Surprisingly, while for both HDIT101 or HDIT102 treatment groups half of the mice did not survive by day 60, the combination of both IgGs resulted in a significantly longer survival at the same total IgG dose, rescuing 90% of the animals, indicating that both antibodies induce a synergistic therapeutic effect in vivo (Fig. 6 A). When comparing the cumulative combined clinical scores for the treated animals the benefits of the combination of HDIT101 and HDIT102 on symptom development are already visible in the early days after treatment, demonstrating also synergistic effects on prevention of symptom development (Fig. 6 B). Together the data show, that the combination of HDIT101 and HDIT102 IgG exerted synergistic effects in the acute intravaginal HSV-2 infection of immunocompetent BALB/cOlaHsd mice, suggesting that the combination may be an attractive new drug product.

Combination therapy with HDIT101 and HDIT102 exerts synergistic effects in vivo. Eight-week-old BALB/c mice were infected with a lethal dose of HSV-2G (5 × 10. 4 TCID50). HDIT102 was mixed with HDIT101 at equimolar ratio (combination therapy) and injected at a final total IgG dose of 300 μg intraperitoneally. HDIT101 or HDIT102 alone (monotherapy) were injected intraperitoneally at the same dose (300 μg). The graphs show combined results from three independent experiments. In total, 20 mice per treatment group and 15 mice for the control arm were used. Survival ( A ) and clinical symptoms ( B ) were scored for a period of 60 days. The statistical differences between survival curves were calculated using Logrank Mantel Cox test. ** p = 0.0069. The differences between cumulative combined clinical scores were analyzed using Kolmogorov-Smirnow test. **** p < 0.0001
Monoclonal antibodies show potential as emerging therapeutics for managing viral infections. Based on the high medical need for novel viable therapeutics in HSV associated disease conditions, we have developed the fully human antibody HDIT102. Similar to the humanized antibody HDIT101, which we have previously employed in clinical phase I and II trials, HDIT102 targets domain I of the fusion protein gB of HSV-1 and HSV-2. Domain I is part of the outermost exposure on the gB surface in the prefusion conformation and contributes to the structural rearrangements that gB undergoes during the fusion process, making it an attractive target for antibody therapy. HDIT102 demonstrated superior binding characteristics to HSV-2 gB as compared to the humanized antibody HDIT101, while showing similar efficiency in neutralizing cell-free virus and blocking cell-to-cell spread in vitro. Cell-to-cell spread inhibition is likely the main way of viral spread in vivo and it was proposed for HSV-1 that individuals with higher levels of cell-to-cell spread inhibiting antibodies may have fewer orolabial recurrences [ 26 ], suggesting that this characteristic may be advantageous when developing a monoclonal antibody therapy against HSV. Cell-to-cell spread of HSV has been considered as viral immune evasion mechanism [ 33 ]. In fact, also for other viruses, e.g. SARS-CoV-2 it was shown that cell-to-cell transmission is more refractory to neutralizing antibodies and convalescent plasma [ 34 ]. HSV gB and other HSV glycoproteins have been proposed to be transported to the cell membrane and re-imported via endosomes in a Rab-dependent way before becoming incorporated into the viral membrane within the trans-Golgi network [ 35 ]. The data provided here suggest that both HDIT101 and HDIT102 bind to gB exposed at the cell-surface of infected cells and are co-internalized and transported on the natural gB trafficking way, hence interact with gB before becoming incorporated into the viral membrane, blocking spreading of newly produced progeny viruses.
For structural analysis of gB as druggable target and to identify the epitopes of both antibodies we utilized cryoEM. We have solved here for the first time the cryoEM structure of the HSV-2 gB ectodomain in complex with two different Fabs binding to domain I at a resolution between 3.1–3.5Å. We also solved the HSV-1 gB ectodomain cryoEM structure in complex with the two different Fabs which revealed very high structural similarity to the published x-ray crystal structure (PDB: 2GUM, [ 27 ]) or cryoEM structures (PDB: 7UI0 and PDB:7UHZ) [ 29 ]. We observed very high similarity in the solved HSV-2 gB ectodomain structure to HSV-1 gB, despite 24 amino acid differences, however, this may not be surprising given the overall strong conservation of gB postfusion structural organization within the Herpesviridae [ 36 , 37 , 38 , 39 ]. There are major structural rearrangements between the pre- and postfusion conformation of HSV1 gB [ 40 , 41 ]. However, the amino acid residues near the HDIT101 and HDIT102 CDRs in the post-fusion conformation are all solvent exposed in the pseudoatomic model of the prefusion conformation of glycoprotein B of Herpes simplex virus 1 (PDB-ID: 6Z9M), suggesting that their accessibility does not preclude the potential binding of one of the two Fabs, HDIT101 and HDIT102.
As both antibodies target conserved regions of gB, we investigated occurrence of mutations within the antibody epitopes. While for each antibody treatment alone, resistant viruses with single amino acid substitutions HSV-1 gB R304Q (HDIT101), HSV-2 gB R296Q (HDIT101), HSV-1 gB R335Q (HDIT102) and HSV-2 gB R327W (HDIT102) grew out in a small number of rounds of replication under suboptimal antibody concentrations in vitro, this was not the case when using an antibody mix containing a 1:1 molar ratio of both IgGs, suggesting that targeting overlapping but not identical epitopes with two different monoclonal antibodies may confer a stronger pressure on the virus, possibly due to structural constraints in the targeted gB region. Quite surprisingly, when analyzing public gB sequences of clinical isolates, we found signs for evolutionary pressure of HDIT102-like endogenous antibodies on HSV-2 isolates, but not on HSV-1. To our knowledge this is the first sign for differences in the immunologic response against gB and evolutionary immune escape between HSV-1 and HSV-2. Nearly 15% of HSV-2 gB sequences had glutamine at gB amino acid position 327, a position that conferred HDIT102-resistance in vitro when changed from R327 in an alanine scan, while 100% of HSV-1 isolate gB sequences had arginine at this position (R335) suggesting that naturally HDIT102-like antibodies exert immunological pressure on HSV-2, but surprisingly not on HSV-1. Given that HDIT102 was derived from an scFv library in which VH and VL are artificially shuffled, it is possible that natural HDIT102-like antibodies target the same residue in gB, but may bind slightly differently, leading to the natural change R327Q as compared to HDIT102-induced in vitro substitution R327W.
Therapeutic effects by HDIT101 in vivo have been shown before [ 17 ]. In this previous work the efficacy of HDIT101 was tested only in an immunodeficient HSV-1 NOD/SCID mouse model and administered at 3 single dose levels between 2,5mg/kg and 15mg/kg, resulting in an 80% cure rate at the highest dose level. The murine ancestor of HDIT101, mAb 2c, has, however, been shown to neutralize HSV-2 less efficiently than HSV-1 [ 42 ]. We have shown here that a combination therapy with HDIT101 and HDIT102 exerts potent synergistic antiviral effects in an acute HSV-2 intravaginal infection mouse model. Combinations with therapeutic antibodies showing no signs of synergism in vitro but exerting potent therapeutic synergy in vivo have been investigated for other viral diseases before, e.g. Chikungunya virus [ 43 ]. Of note however, we have shown here that the combination of two antibodies binding to neighbouring epitopes in the same structurally confined region of the trimer-forming glycoprotein B of herpes simplex virus, can lead to synergistic effects in vivo. In Cryo-EM analyses we did not detect more than three Fab molecules binding to a single gB ectodomain trimer when incubation with a 1:1 HDIT101 + HDIT102 Fab mix, strongly arguing for steric hindrance for binding of HDIT101 to its epitope if HDIT102 has occupied its epitope on the same gB protomer and vice versa. The majority of trimeric gB ectodomain in post-fusion conformation was bound homogenically by three HDIT102 Fab molecules, as shown in cryo-EM 2D images. However also heterogenic structures with two HDIT102 and one HDIT101 Fab molecules could be identified. When considering different binding kinetics of the antibodies (for HDIT102 an extraordinarily low off rate after gB binding) it is conceivable that heterogenic interaction of two different antibodies with trimeric HSV gB could lead to irregularly cross-linked immune complexes that after processing may be more immunogenic in vivo and may well explain the synergistic effects observed in the combination of HDIT101 and HDIT102 in vivo. In this regard, the perpendicular orientation of both Fabs when bound to trimeric gB may play a role. Our findings could potentially be relevant for other multivalent proteins, such as trimeric fusion proteins with multiple epitopes available, the concept behind this idea, however, relies on multimeric protein targets that can form heterogenic immune complexes when bound by different antibodies at each protomer. Despite our attempts we were unable to recapitulate the synergistic effects seen in vivo by any tested in vitro assay. It is hence conceivable that the presence of important immunological components, which are lacking in the in vitro assay, may explain the synergistic effects observed in vivo.
Most interestingly, despite both HDIT101 and HDIT102 are potently inducing antibody-dependent phagocytosis (ADCP), they are not capable of mediating ADCC or CDC, it is imperative to acknowledge that this activity was observed in vitro using human effector cells. Nonetheless, our interpretation remains valid, as demonstrated by the mouse in vivo model. While it is crucial to recognize interspecies differences, it is also noteworthy to acknowledge the substantial functional homology of ADCP between humans and mice, supported by similarities in Fc receptors and Fc affinities. Of note, anti-HSV antibodies mediating antibody-dependent cellular cytotoxicity (ADCC) have been connected to superior protection [ 44 , 45 , 46 ]. Low levels of ADCC-mediating antibodies were suggested to play an important role for the absent protection by vaccines against HSV-2 [ 47 , 48 ]. The role of antibody-mediated cellular phagocytosis (ADCP) and subsequent immune cell activation in the immune response to HSV-1/2 infection, in particular T-cell activation, has not been investigated in depth [ 47 , 49 ]. ADCP is a known Fc-effector function and has been proposed to be implicated in anti-HSV effects observed in vaccine trials [ 47 ]. Indeed, ADCP has also been recently proposed as possible main function of anti-cancer monoclonal antibody therapies, suggesting a more widespread and understudied part of monoclonal antibody effector functions [ 50 ]. As consequence of induced ADCP by HDIT101 and HDIT102, antigen-presenting cells may conceivably process antibody-HSV complexed particles and subsequently recruit CD4 + and CD8 + T-cells to MHC presented viral peptides. Changes from the conventional proteasome to the immunoproteasome and alterations in the MHC-I and MHC-II immunopeptidome during proinflammatory conditions have been described [ 51 , 52 ]. Likewise, MHC-independent activation of T-cells may also play a role. While we did not observe quantitative differences in autologous T-cells activated in the presence of HDIT101, HDIT102 or a combination of both in vitro, a combination of different monoclonal antibodies may induce quantitative or qualitative differences in vivo, which may account for the synergistic antiviral effects of a combination therapy with HDIT101 and HDIT102 observed in vivo. Future studies should determine the APC-presented MHC-immunopeptidome after ADCP in absence or presence of different monoclonal antibodies and combinations or differences in TCR-clonalities of activated T-cell subsets.
In conclusion, we have shown that the fully human antibody HDIT102 has great potential for further clinical development as a potent novel HSV therapeutic particularly in combination with its clinical humanized ancestor antibody HDIT101.
The combination of two monoclonal antibodies for the treatment of chronic HSV-2 may provide a novel therapeutic option. Antibody characteristics to inhibit cell-to-cell spread, to mediate uptake of cell free-viruses by phagocytic cells and concomitantly stimulate T-cell responses may promote cellular immunity and may have benefits in preventing recurrences.
Availability of data and materials
The structures and EM-maps have been deposited in the Protein Data Bank and Electron Microscopy Data Bank, respectively (PDB ID codes: 8RGZ, 8RH0, 8RH1, 8RH2; EMBD ID codes: 19163, 19164, 19165, 19166).
James C, Harfouche M, Welton NJ, Turner KM, Abu-Raddad LJ, Gottlieb SL, Looker KJ. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull World Health Organ. 2020;98(5):315–29.
Article PubMed PubMed Central Google Scholar
Looker KJ, Johnston C, Welton NJ, James C, Vickerman P, Turner KME, Boily MC, Gottlieb SL. The global and regional burden of genital ulcer disease due to herpes simplex virus: a natural history modelling study. BMJ Glob Health. 2020;5(3):e001875.
Merin A, Pachankis JE. The psychological impact of genital herpes stigma. J Health Psychol. 2011;16(1):80–90.
Article PubMed Google Scholar
Kimberlin DW, Lin CY, Jacobs RF, Powell DA, Frenkel LM, Gruber WC, Rathore M, Bradley JS, Diaz PS, Kumar M, Arvin AM, Gutierrez K, Shelton M, Weiner LB, Sleasman JW, de Sierra TM, Soong SJ, Kiell J, Lakeman FD, Whitley RJ. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001;108(2):223–9.
Article CAS PubMed Google Scholar
Berrington WR, Jerome KR, Cook L, Wald A, Corey L, Casper C. Clinical correlates of herpes simplex virus viremia among hospitalized adults. Clin Infect Dis. 2009;49(9):1295–301.
Jellinge ME, Hansen F, Coia JE, Song Z. Herpes simplex virus type 1 pneumonia-A review. J Intensive Care Med. 2021;36(12):1398–402.
Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM Jr, Handsfield HH, Warren T, Marr L, Tyring S, DiCarlo R, Adimora AA, Leone P, Dekker CL, Burke RL, Leong WP, Straus SE. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA. 1999;282(4):331–40.
Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD, Herpevac Trial for W. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2012;366(1):34–43.
Article CAS PubMed PubMed Central Google Scholar
Belshe RB, Blevins TP, Yu Y, Nethington AE, Bellamy A, Bryant C, Morrison LA. Neutralizing antibody kinetics and immune protection against herpes simplex virus 1 genital disease in vaccinated women. J Infect Dis. 2023;227(4):522–7.
Mullard A. FDA approves 100th monoclonal antibody product. Nat Rev Drug Discov. 2021;20(7):491–5.
Lee CC, Lin LL, Chan WE, Ko TP, Lai JS, Wang AH. Structural basis for the antibody neutralization of herpes simplex virus. Acta Crystallogr D Biol Crystallogr. 2013;69(Pt 10):1935–45.
Sanna PP, De Logu A, Williamson RA, Hom YL, Straus SE, Bloom FE, Burton DR. Protection of nude mice by passive immunization with a type-common human recombinant monoclonal antibody against HSV. Virology. 1996;215(1):101–6.
Backes IM, Byrd BK, Slein MD, Patel CD, Taylor SA, Garland CR, MacDonald SW, Balazs AB, Davis SC, Ackerman ME, Leib DA. Maternally transferred mAbs protect neonatal mice from HSV-induced mortality and morbidity. J Exp Med. 2022;219(12):e20220110.
Zeitlin L, Whaley KJ, Sanna PP, Moench TR, Bastidas R, De Logu A, Williamson RA, Burton DR, Cone RA. Topically applied human recombinant monoclonal IgG1 antibody and its Fab and F (ab’)2 fragments protect mice from vaginal transmission of HSV-2. Virology. 1996;225(1):213–5.
Politch JA, Cu-Uvin S, Moench TR, Tashima KT, Marathe JG, Guthrie KM, Cabral H, Nyhuis T, Brennan M, Zeitlin L, Spiegel HML, Mayer KH, Whaley KJ, Anderson DJ. Safety, acceptability, and pharmacokinetics of a monoclonal antibody-based vaginal multipurpose prevention film (MB66): a phase I randomized trial. PLoS Med. 2021;18(2):e1003495.
Blank A, Hohmann N, Dettmer M, Manka-Stuhlik A, Mikus G, Stoll F, Stutzle-Schnetz M, Thomas D, Exner E, Schmitt-Bormann B, Schaller T, Laage R, Schonborn-Kellenberger O, Arndt M, Haefeli WE, Krauss J. First-in-human, randomized, double-blind, placebo-controlled, dose escalation trial of the anti-herpes simplex virus monoclonal antibody HDIT101 in healthy volunteers. Clin Transl Sci. 2022;15(10):2366–77.
Krawczyk A, Arndt MA, Grosse-Hovest L, Weichert W, Giebel B, Dittmer U, Hengel H, Jager D, Schneweis KE, Eis-Hubinger AM, Roggendorf M, Krauss J. Overcoming drug-resistant herpes simplex virus (HSV) infection by a humanized antibody. Proc Natl Acad Sci U S A. 2013;110(17):6760–5.
Diebolder P, Keller A, Haase S, Schlegelmilch A, Kiefer JD, Karimi T, Weber T, Moldenhauer G, Kehm R, Eis-Hubinger AM, Jager D, Federspil PA, Herold-Mende C, Dyckhoff G, Kontermann RE, Arndt MA, Krauss J. Generation of “LYmph Node Derived Antibody Libraries” (LYNDAL) for selecting fully human antibody fragments with therapeutic potential. MAbs. 2014;6(1):130–42.
Krawczyk A, Krauss J, Eis-Hubinger AM, Daumer MP, Schwarzenbacher R, Dittmer U, Schneweis KE, Jager D, Roggendorf M, Arndt MA. Impact of valency of a glycoprotein B-specific monoclonal antibody on neutralization of herpes simplex virus. J Virol. 2011;85(4):1793–803.
Kimanius D, Dong L, Sharov G, Nakane T, Scheres SHW. New tools for automated cryo-EM single-particle analysis in RELION-4.0. Biochem J. 2021;478(24):4169–85.
Rohou A, Grigorieff N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J Struct Biol. 2015;192(2):216–21.
Casanal A, Lohkamp B, Emsley P. Current developments in coot for macromolecular model building of electron cryo-microscopy and crystallographic data. Protein Sci. 2020;29(4):1069–78.
Rice P, Longden I, Bleasby A. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 2000;16(6):276–7.
Nicholls RA, Tykac M, Kovalevskiy O, Murshudov GN. Current approaches for the fitting and refinement of atomic models into cryo-EM maps using CCP-EM. Acta Crystallogr D Struct Biol. 2018;74(Pt 6):492–505.
Kosakovsky Pond SL, Frost SD. Not so different after all: a comparison of methods for detecting amino acid sites under selection. Mol Biol Evol. 2005;22(5):1208–22.
Alt M, Wolf S, van de Sand L, Dittrich R, Tertel T, Brochhagen L, Dirks M, Aufderhorst UW, Thummler L, Otte M, Rainer K, Dittmer U, Giebel B, Trilling M, Silke HC, Lotfi R, Roggendorf M, Witzke O, Krawczyk A. Cell-to-cell spread inhibiting antibodies constitute a correlate of protection against herpes simplex virus type 1 reactivations: a retrospective study. Front Immunol. 2023;14:1143870.
Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. Crystal structure of glycoprotein B from herpes simplex virus 1. Science. 2006;313(5784):217–20.
Zhang Y, Skolnick J. TM-align: a protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 2005;33(7):2302–9.
Kuraoka M, Aschner CB, Windsor IW, Mahant AM, Garforth SJ, Kong SL, Achkar JM, Almo SC, Kelsoe G, Herold BC. A non-neutralizing glycoprotein B monoclonal antibody protects against herpes simplex virus disease in mice. J Clin Invest. 2023;133(3):e161968.
Lo M, Kim HS, Tong RK, Bainbridge TW, Vernes JM, Zhang Y, Lin YL, Chung S, Dennis MS, Zuchero YJ, Watts RJ, Couch JA, Meng YG, Atwal JK, Brezski RJ, Spiess C, Ernst JA. Effector-attenuating substitutions that maintain antibody stability and reduce toxicity in mice. J Biol Chem. 2017;292(9):3900–8.
Wang X, Mathieu M, Brezski RJ. IgG Fc engineering to modulate antibody effector functions. Protein Cell. 2018;9(1):63–73.
Slein MD, Backes IM, Garland CR, Kelkar NS, Leib DA, Ackerman ME. Effector functions are required for broad and potent protection of neonatal mice with antibodies targeting HSV glycoprotein D. Cell Rep Med. 2024;5(2):101417.
Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol. 2008;6(11):815–26.
Zeng C, Evans JP, King T, Zheng YM, Oltz EM, Whelan SPJ, Saif LJ, Peeples ME, Liu SL. SARS-CoV-2 spreads through cell-to-cell transmission. Proc Natl Acad Sci U S A. 2022;119(1):e2111400119.
Spearman P. Viral interactions with host cell Rab GTPases. Small GTPases. 2018;9(1–2):192–201.
Backovic M, Longnecker R, Jardetzky TS. Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B. Proc Natl Acad Sci U S A. 2009;106(8):2880–5.
Cooper RS, Heldwein EE. Herpesvirus gB: a finely tuned fusion machine. Viruses. 2015;7(12):6552–69.
Connolly SA, Jardetzky TS, Longnecker R. The structural basis of herpesvirus entry. Nat Rev Microbiol. 2021;19(2):110–21.
Burke HG, Heldwein EE. Crystal structure of the human cytomegalovirus glycoprotein B. PLoS Pathog. 2015;11(10):e1005227.
Vollmer B, Pražák V, Vasishtan D, Jefferys EE, Hernandez-Duran A, Vallbracht M, et al. The prefusion structure of herpes simplex virus glycoprotein B. Sci Adv. 2020;6(39):eabc1726.
Baquero E, Albertini AA, Gaudin Y. Recent mechanistic and structural insights on class III viral fusion glycoproteins. Curr Opin Struct Biol. 2015;33:52–60.
Silke Heilingloh C, Lull C, Kleiser E, Alt M, Schipper L, Witzke O, Trilling M, Eis-Hübinger AM, Dittmer U, Krawczyk A. Herpes simplex virus type 2 is more difficult to neutralize by antibodies than herpes simplex virus type 1. Vaccines. 2020;8(3):478.
Article Google Scholar
Pal P, Dowd KA, Brien JD, Edeling MA, Gorlatov S, Johnson S, Lee I, Akahata W, Nabel GJ, Richter MK, Smit JM, Fremont DH, Pierson TC, Heise MT, Diamond MS. Development of a highly protective combination monoclonal antibody therapy against Chikungunya virus. PLoS Pathog. 2013;9(4):e1003312.
Balachandran N, Harnish D, Rawls WE, Bacchetti S. Glycoproteins of herpes simplex virus type 2 as defined by monoclonal antibodies. J Virol. 1982;44(1):344–55.
Kohl S, West MS, Prober CG, Sullender WM, Loo LS, Arvin AM. Neonatal antibody-dependent cellular cytotoxic antibody levels are associated with the clinical presentation of neonatal herpes simplex virus infection. J Infect Dis. 1989;160(5):770–6.
Kohl S, Strynadka NC, Hodges RS, Pereira L. Analysis of the role of antibody-dependent cellular cytotoxic antibody activity in murine neonatal herpes simplex virus infection with antibodies to synthetic peptides of glycoprotein D and monoclonal antibodies to glycoprotein B. J Clin Invest. 1990;86(1):273–8.
Petro CD, Weinrick B, Khajoueinejad N, Burn C, Sellers R, Jacobs WR Jr, Herold BC. HSV-2 DeltagD elicits FcgammaR-effector antibodies that protect against clinical isolates. JCI Insight. 2016;1(12):e88529.
Burn Aschner C, Loh LN, Galen B, Delwel I, Jangra RK, Garforth SJ, Chandran K, Almo S, Jacobs WR Jr, Ware CF, Herold BC. HVEM signaling promotes protective antibody-dependent cellular cytotoxicity (ADCC) vaccine responses to herpes simplex viruses. Sci Immunol. 2020;5(50):eaax2454.
Backes IM, Leib DA, Ackerman ME. Monoclonal antibody therapy of herpes simplex virus: an opportunity to decrease congenital and perinatal infections. Front Immunol. 2022;13:959603.
Cao X, Chen J, Li B, Dang J, Zhang W, Zhong X, Wang C, Raoof M, Sun Z, Yu J, Fakih MG, Feng M. Promoting antibody-dependent cellular phagocytosis for effective macrophage-based cancer immunotherapy. Sci Adv. 2022;8(11):eabl9171.
Groettrup M, Kirk CJ, Basler M. Proteasomes in immune cells: more than peptide producers? Nat Rev Immunol. 2010;10(1):73–8.
Jurewicz MM, Stern LJ. Class II MHC antigen processing in immune tolerance and inflammation. Immunogenetics. 2019;71(3):171–87.
Kucukelbir A, Sigworth FJ, Tagare HD. Quantifying the local resolution of cryo-EM density maps. Nat Methods. 2014;11(1):63–5.
Download references
Acknowledgements
We thank all blood donors for contributing primary cells for this study. We thank members of Heidelberg ImmunoTherapeutics for critical discussions.
Heidelberg ImmunoTherapeutics GmbH.
Author information
Torsten Schaller and Michaela Arndt contributed equally to this work.
Authors and Affiliations
Heidelberg ImmunoTherapeutics GmbH, Max-Jarecki Str. 21, Heidelberg, 69115, Germany
Narges Seyfizadeh, Thomas Imhof, Moritz Ries, Christian Müller, Leonie Jenner, Elisabeth Blumenschein, Alexandra Yendrzheyevskiy, Kevin Moog, Daniel Eckert, Ronja Engel, Jürgen Krauss, Torsten Schaller & Michaela Arndt
Biozentrum, University of Basel, Spitalstrasse 41, Basel, CH – 4056, Switzerland
David Kalbermatter & Mohamed Chami
Vanudis GmbH, Max-Jarecki Str. 21, Heidelberg, 69115, Germany
National Center for Tumor Diseases (NCT), Im Neuenheimer Feld 460, Heidelberg, 69120, Germany
Philipp Diebolder
Present address: Bio-Rad AbD Serotec GmbH, Anna-Sigmund-Str. 5, Neuried, 82061, Germany
Present address: University of Bern, Institute of Anatomy, Balzerstrasse 2, Bern, 3012, Switzerland
David Kalbermatter
You can also search for this author in PubMed Google Scholar
Contributions
N.S., T.S., M.C., J.K., T.I., C.M. and M.A. conceived and designed the experiments. N.S., T.S., M. A, J.K. wrote the manuscript. N.S., C.M., M.R., K.M. performed animal experiments (with the help of R.E., D.E. and T.S.). P.D. performed phage display library screens. T.I., M.R., F.G. performed biochemical experiments and protein productions. D.K. and M.C. revealed cryo-EM structures. L.J., E.B. and C.M. performed primary cell experiments. A.Y. and N.S. generated virus stocks and performed viral assays. A.Y., K.M., D.E. and R.E. generated gB mutants and performed binding and fusion assays. All the authors have reviewed the final version of the manuscript.
Corresponding author
Correspondence to Michaela Arndt .
Ethics declarations
Ethics approval and consent to participate.
PBMC were derived from anonymous buffy coats from the DRK Blutspendedienst, Mannheim, Germany. All in vivo animal studies were in line with ethically and regulatory requirements and approvals.
Consent of publication
Not applicable.
Competing of interests
N.S., T.S., T.I., M.R., L.J., E.B., C.M., A.Y., K.M., D.E., R.E. are or were employed by Heidelberg ImmunoTherapeutics GmbH. M.A. is Chief Executive Officer of Heidelberg ImmunoTherapeutics GmbH. All other authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12929_2024_1045_moesm1_esm.pdf.
Supplementary Material 1. Fig. S1. HDIT102 IgG does not cross-react with other herpesviruses than HSV-1/2 and has a very low dissociation rate (kdis). (A) Binding of a dilution series of HDIT102 at several concentrations to HSV-1/2, VZV, HCMV and EBV antigens was analyzed by ELISA using microplates coated with respective viral antigens (Enzygnost, Siemens). Absorbance at 450 nm was measured. Anti-HSV-ELISA does not discriminate between detection of anti-HSV-1 and anti-HSV-2 IgGs. Binding was detected with an HRP-conjugated anti-human gamma Fc-specific IgG. Cytotect (anti-CMV polyclonal antibody preparation) was used as a positive control for all. (B) HDIT101 IgG was tested in biolayer-interferometry against immobilized HSV-1F gB. (C) HDIT102 IgG was tested in biolayer-interferometry against immobilized HSV-1F gB. Fig. S2. HDIT102 efficiently inhibits cell-to-cell spread of HSV-1F in Vero cells. Inhibition of HSV-1F cell-to-cell spread by HDIT102. Fluorescence microscopy images of Vero cells infected with HSV-1F and subsequently treated with either HDIT102, HDIT101, human polyclonal anti-HSV antibody or left untreated. Plaque formation was visualised by anti-HSV immuno- and Hoechst staining. Representative images are shown. Arrows show plaques or initially infected cells. Fig. S3. Cryo-EM data analysis of co-structures of trimeric HSV-1F and HSV-2G gB. (A) - (D) The plots show the Fourier shell correlation (FSC) curves of the final calculated density map (black) and the FSC curve calculated between the final map and the atomic model (grey). The reported resolutions for the maps is based on the “gold-standard” FSC = 0.143 criterion and FSC = 0.5 for the FSC between map and model. (E) - (H) The final 3D reconstructions are shown in three different views (top, side and bottom) and colored according to the local resolution calculated using the ResMap [ 53 ] implementation in RELION 4.0 [ 20 ]. Fig. S4. HSV-1 gB cryo-EM co-structure with HDIT101 or HDIT102 Fab. (A) Side, bottom and top view of co-structure of trimeric HSV-1F gB in post-fusion conformation (green) with three HDIT101 Fab molecules (blue) solved by cryo-EM at a resolution of 3.27 Å are shown. (B) The co-structure of HDIT102 Fab (magenta) bound to trimeric HSV-1F gB in post-fusion conformation (green) was determined at a resolution of 3.44 Å and side, bottom and top views are shown. (C) Overlay of the co-structures indicating overlapping epitopes of HDIT101 and HDIT102 Fabs on HSV-1F gB indicating perpendicular orientation of both Fabs to another. (D) Overlay of published x-ray crystal structure (PDB:2GUM) with HSV-1 cryoEM gB structure derived in complex with HDIT101 Fab. (E) Overlay of published x-ray crystal structure (PDB:2GUM) with HSV-1 cryoEM gB structure derived in complex with HDIT102 Fab. (F) Overlay of HSV-1 cryoEM gB structure derived in complex with HDIT101 Fab and HDIT102 Fab. (G) Overlay of HSV-1 and HSV-2 cryoEM gB structures derived in complex with HDIT101 Fab. (H) Overlay of HSV-1 and HSV-2 cryoEM gB structures derived in complex with HDIT102 Fab. (I) Overlay of HSV-1 and HSV-2 cryoEM gB structures derived in complex with HDIT102 Fab with differing amino acid residues marked in magenta. (J) Detailed image of the HSV-1 gB residues interacting with HDIT101 (compare with Fig. 3D). (K) Detailed image of the HSV-1 gB residues interacting with HDIT102 (compare with Fig. 3E). Fig. S5. HSV-2 gB interactions with HDIT101 or HDIT102 CDR amino acid residues. HSV-2 gB residues within 4Å distance of residues in the HDIT101 or HDIT102 CDRs were determined by Pymol and subsequently analyzed individually for polar (red) or non-polar (yellow) interactions. Oxygen groups are highlighted in red, nitrogen groups in blue. Carbon atoms involved in non-polar interactions were not coloured. (A)-(C) Interaction of HDIT101 CDR residues with HSV-2 gB residues in the binding interface. (D)-(G) Interaction of HDIT102 CDR residues with HSV-2 gB residues in the binding interface. Fig. S6. Analysis of epitope residues conferring resistance to HDIT101 or HDIT102 induced fusion inhibition. (A) Amino acid alignment of HSV-1F and HSV-2G gB with indicated key residues conferring resistance to HDIT101 (HSV-1 gB R304Q/ HSV-2 gB R296Q) or HDIT102 (HSV-1 gB R335Q/ HSV-2 gB R327Q) evolving in vitro . (B) Cell-cell fusion assay and inhibition of selected HSV-1 gB mutants by HDIT102 IgG. Changes in fusion inhibition by HDIT102 were calculated comparing HDIT102 treated with untreated cells for each mutant and normalized to wild type gB (WT gB) mean values are shown for at least three independent biological replicates ( n =3). Statistical analyses were performed using unpaired t-tests. (C) Relative fusion activity of selected gB mutants compared to wild type protein. Fusion activity as a combined indicator of cell surface expression and fusogenicity of gB mutants was analyzed by comparison to wild type gB (WT gB) in the absence of any antibody. Mean values of three independent biological replicates are shown with error bars indicating standard deviation ( n =3). Statistical analyses were performed using unpaired t-tests. (D) Cell-cell fusion assay and inhibition of selected HSV-1 gB mutants by HDIT101. Changes in fusion inhibition by HDIT102 were calculated comparing HDIT101 treated with untreated cells for each mutant and normalized to wild type gB (WT gB) mean values are shown for at least three independent biological replicates ( n =3). Statistical analyses were performed using unpaired t-tests. (E) Relative fusion activity of selected gB mutants compare to wild type protein. Fusion activity as combined indicator of cell surface expression and fusogenicity of gB mutants was analyzed by comparison to wild type gB (WT gB) in the absence of any antibody. Mean values of three independent biological replicates are shown with error bars indicating standard deviation ( n =3). Statistical analyses were performed using unpaired t-tests. 451 HSV-1 (F) and 368 HSV-2 (G) gB DNA sequences were retrieved from the NCBI sequence database by using nucleotide BLAST against HSV-1F or HSV-2G gB DNA sequences, aligned and analyzed for variations in the HDIT101 or HDIT102 epitope regions to study signatures of evolutionary pressure from natural HDIT102-like antibodies. The graphs illustrate the conservation at each epitope amino acid position in the gB proteins. The residue number is indicated in the x-axis and the percentage of conservation is indicated in the y-axis. (H) Single-likelihood ancestor counting analysis was performed on alignments of 185 full-length HSV-1 gB and 201 full-length HSV-2 gB sequences to identify positively selected residues. The difference in non-synonymous and synonymous substitution rates are shown on the y-axis and gB codon position in the alignment on the x-axis. (I) Phylogentic tree demonstrating HSV-2 gB isolates containing R327 or Q327. Fig. S7. HDIT102 does not elicit ADCC on HSV-1F or HSV-2G infected Vero cells or HEK293T cells ectopically expressing gB. Different dilutions of HDIT102 IgG or human polyclonal serum containing anti-HSV IgGs were added to HSV-1F or HSV-2G infected target cells, followed by incubation with effector Jurkat cells (Promega #G7010) stably expressing FcγRIIIA receptor, V158 (high affinity) variant and the NFAT-luciferase reporter at an effector-to-target cell ratio of 6:1. Infected Vero cells or HEK293T cells expressing either HSV-1 gB or HSV-2 gB were used as target cells. ADCC was quantified by luminescence readout from luciferase activity upon NFAT pathway activation. (A) ADCC activity of HDIT102 IgG or isotype control on Vero cells infected with either HSV-1F or HSV-2G or uninfected. Mean of three technical replicates is shown. (B) Same as in A, however using human polyclonal antibody as control. (C) Summary of A and B showing fold ADCC induction at highest antibody concentration. (D) ADCC activity of HDIT102 IgG on parental HEK293T or HEK293T stably expressing either HSV-1F gB or HSV-2G gB. Mean of three technical replicates is shown. (E) Same as in D, however using human polyclonal antibody as control. (F) Summary of D and E showing fold ADCC induction at highest antibody concentration. (G) and (H) Analysis of HDIT102-mediated complement dependent cytotoxicity by quantitative evaluation of the terminal C5b-9 complement complex. An ELISA was employed to measure supernatant levels of C5b-9 complex to quantify complement activation by HDIT102. HSV-1F or HSV-2G infected or uninfected Vero cells were incubated with heat-inactivated or with not heat-inactivated IgG-depleted human serum in the presence or absence of test antibodies (either HDIT102, human polyclonal IgG, or Cytotect as positive controls, or unrelated isotype IgG as negative control). Statistical analysis was done using two-way ANOVA, error bars represent standard deviation of the mean for two technical replicates, **** p < 0.0001)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Seyfizadeh, N., Kalbermatter, D., Imhof, T. et al. Development of a highly effective combination monoclonal antibody therapy against Herpes simplex virus. J Biomed Sci 31 , 56 (2024). https://doi.org/10.1186/s12929-024-01045-2
Download citation
Received : 20 December 2023
Accepted : 21 May 2024
Published : 28 May 2024
DOI : https://doi.org/10.1186/s12929-024-01045-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Herpes simplex virus (HSV)
- Glycoprotein B (gB)
- Therapeutic monoclonal antibody
- Combination therapy
Journal of Biomedical Science
ISSN: 1423-0127
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Clin Diagn Res
- v.9(5); 2015 May
Case Presentation of a 23-Month-old Herpes Simplex Virus-infected Girl with Brain and Oesophageal Involvement
Karam-ali kasiri.
1 Assistant Professor, Department of Pediatrics, Shahrekord University of Medical Sciences, Shahrekord, Iran.
Noushin Rostampour
2 Assistant Professor, Department of Pediatrics, Shahrekord University of Medical Sciences, Shahrekord, Iran.
Abolfazl Khoshdel
3 Associate Professor, Department of Pediatrics, Shahrekord University of Medical Sciences, Shahrekord, Iran.
Herpes simplex virus (HSV) is the most common identifiable cause of serious or life threatening sporadic, endemic encephalitis. Typical HSV encephalitis in patients outside neonatal age is caused by HSV-1. A 23-month-old girl was referred to our hospital with a three-day history of fever, listlessness, slurred speech, and suspicious oesophageal foreign body impaction. Laboratory evaluations showed white blood cell count of 10900 /mm3 with 65% neutrophils. Upper endoscopy revealed diffuse severe ulceration in middle to distal third of oesophagus and no foreign body was found in oesophagus or stomach. Parenteral acyclovir was prescribed for herpes encephalitis in addition to antibiotics for central nervous system infection. Chest X-ray and brain MRI was unremarkable. Lumbar puncture revealed normal protein and glucose with 10 white cell count. She developed a raising liver enzyme tests. Total and direct bilirubin was 1.2 mg/dc and 0.2 mg/dc respectively. Because of neurological symptoms, acyclovir was adopted for our patient from the beginning. The girl did not respond to medication and died after 28 days. Progression of her disease prior to referral appears to contribute to the administered treatment inefficacy. Severe rapid progression of disease prior to referral and potential resistance to acyclovir could cause treatment failure.
Case Report
A previously well, 23-month-old girl was referred to our hospital with a three-day history of fever, listlessness, slurred speech, and suspicious oesophageal foreign body impaction. She had difficulty swallowing liquids and solids as well as drooling. There was no significant medical history. Vital signs: heart rate: 120/min, temperature: 39.1°C and other physical findings were normal. In physical examinations, there was no lesion, ulceration, or erythema of the gingiva, buccal mucosa, and tongue. The height was 87 cm (50-75th) and the weight was 11 kg (10-25th).
Laboratory evaluations showed white blood cell count of 10900 /mm3 with 65% neutrophils. Liver function was normal. HIV test was negative. Upper endoscopy revealed diffuse severe ulceration in lower to middle third of oesophagus and no foreign body was found in oesophagus or stomach [ Table/Fig-1 , ,2]. 2 ]. Since endoscopy was done to find foreign body, no biopsy was taken. Then, as cerebral symptoms progressed, HSV was raised.

Middle oesophageal one-third

Lower oesophageal one-third
On the night of admission, she became lethargic and experienced status epilepticus and her seizures were resistant to drugs. As a result, she was intubated and thiopental started. After observation of neurologic signs, parenteral acyclovir was prescribed for herpes encephalitis in addition to antibiotics for CNS infection.
Other investigations including chest X-ray and brain MRI, which was run prior to intubation, was unremarkable. Lumbar puncture revealed normal protein and glucose with 10 white cell count (PMN=75%). Cerebrospinal fluid (CSF) viral polymerase chain thirdreaction (PCR) confirmed herpes simplex virus type 1 (HSV-1). In follow-up she developed a raising liver enzyme tests (AST=2700, ALT=2300). Total and direct bilirubin was 1.2 mg/dc and 0.2 mg/dc respectively. A repeated lumbar puncture on day 14 showed negative result for HSV.
Primary HSV infection is commonly seen in children. The maximum incidence of the disease is at two years of life with gingivostomatitis [ 1 ] . Herpes oesophagitis in the immunocompetent host is a self-limited condition and usually a primary infection, but it may be reactivated occasionally [ 2 ] ; it has been associated with gastric involvement in a few cases [ 3 ] . The trauma to the oesophageal tissue could predispose to herpes oesophagitis in an immunocompetent host [ 4 ] . Other important observations have been bloody vomiting and ooesophageal ulceration [ 5 ] .
HSV-1 may lead to chronic ulcerative infection in immunosuppressed children. It is also a common reason for fatal sporadic encephalitis [ 6 ] . Central nervous system (CNS) infected with HSV has various manifestations. Recently, a great deal of knowledge has been gathered about the pathogenicity, diagnosis, and treatment of HSV [ 7 ] . As with other reports, dysphagia and fever were noted in this report. In the young child refusal of oral intake may be due to both odynophagia and oral discomfort [ 8 ]. Although mucosal lesions and ulcers in the mouth and tongue were not noted in physical examinations of our patient, severe lesions caused by inflammation of the oesophagus were seen by endoscopy, which is consistent with other reported HSV-infected cases [ 8 ].
HSV is also a common cause of ulcerative oesophagitis in the immunocompromised or debilitated host [ 9 ]. The presence of HSV-1 was confirmed in our patient’s CSF. In the studies where the patients had symptoms similar to our patient, the isolated HSV was mostly HSV-1 [ 8 , 10 ], which is in agreement with the present report.
Antiviral therapy with acyclovir has decreased mortality. However, morbidity is still high. The clinicians must be highly suspicious because HSV could mimic other CNS diseases and speedy initiation of treatment is essential [ 7 ] .
The management of hospitalized patient includes intravenous (IV) acyclovir, IV therapy and supportive care for nutrition and acid suppression [ 8 ].
In a study, the most interesting finding in 21 children with confirmed herpes simplex encephalitis was negative HSV CSF PCR test in 29% of the patients and extratemporal lobe involvement in 59% of the patients [ 10 ]. In a study on Nigerian children, children of 9-16 weeks age were more susceptible to HSV-1 infection and HSV-1 infection was significantly associated with age. A higher prevalence was obtained in female children (61.5%) than male (54.5%), as well [ 6 ] . In a study in Australia from 1999 to 2011, totally 29 mortalities due to HSV occurred in children under 15 years, of whom nine were female and 15 died within the first month of life. 69% of the children died prior to the age of 12-month-old [ 11 ].
Al-Hussaini and Fagih reported three cases of oesophagus inflammation due to HSV referred with fever, dysphagia, and odynophagia. One of them was diagnosed as suspicious herpes oesophagitis. Another child, a 3-year-old girl, was administered with IV acyclovir, and the third case was treated as self-restricted. All three were healthy in follow-up [ 8 ]. Lack of similar research could be due to rarity of the patients similar to our reported case. According to previous works, early onset of acyclovir, particularly in patients with odynophagia, may assist in recovery [ 8 ].
Ali et al., confirmed HSV-1 infection in an 11-month-old infant’s CSF and administered parenteral acyclovir. The infant responded to treatment and was discharged after 16 days without neurological complications [ 12 ]. Wang et al., study indicated that the prevalence rate of acyclovir-resistant HSV-1 in children was higher than predicted. Moreover, various mechanisms resulting in the resistance were identified. These results suggest that new anti-herpetics with various working mechanisms should be seriously considered [ 13 ].
HSV infections are prevalent. Children are infected with HSV, which may cause asymptomatic acquisition of life-threatening disease. HSV treatment in children can be addressed per severity and time of acquisition [ 14 ].
MRI findings are more likely to be abnormal at initial evaluation for HSV encephalitis because of its high sensitivity to changes in brain water content. Findings in MRI include hyperintensity of temporal and brain stem areas on T2 weighted images, and imaging results may be normal if performed in the early stages of disease [ 15 ]. In the present patient, MRI was performed in early stage of the disease and normal MRI result, which has also been reported in the setting of the disease, does not rule out herpes meningoencephalitis [ 16 ].
Because of neurological symptoms, acyclovir was adopted for our patient from the beginning. Progression of her disease prior to referral appears to contribute to the administered treatment inefficacy. It seems that severe rapid progression of disease prior to referral and potential resistance to acyclovir caused treatment failure.
The most obvious finding from this case is herpes oesophagitis, which must be considered in the patients with drooling and dysphagia.
Acknowledgment
Hereby, we gratefully thank Research and Technology Deputy of Shahrekord University of Medical Sciences.
Financial or Other Competing Interests

IMAGES
VIDEO
COMMENTS
Background Herpes simplex virus (HSV) rarely causes organ-invasive infection. Diagnosis and treatment for such infections are often delayed, and mortality is high. We present the first reported case of disseminated HSV-1 infection in an adult causing liver failure, myocarditis, and encephalitis in a patient who recovered after receiving parenteral acyclovir treatment. Case presentation A 46 ...
Case Study: Nasal Herpes Simplex Virus Infection. Stress and immunosuppression can trigger reactivation of latent virus. Above: Herpes simplex virus infection of the nose. A shave biopsy of the lateral border of the ulcerated plaque (arrow) revealed ulceration with spongiosis, serous crust and a lymphohistiocytic infiltrate.
Keywords: Herpetic gingivostomatitis, Oral lesions, Herpes simplex virus, Perioral lesions, Infants, Case report, Herpes simplex virus type 1. Core Tip: ... However, in this case study, we report the case of a five-month-old healthy girl who presented with herpetic gingivostomatitis and perioral vesicles. Primary herpetic infection is very rare ...
Two previous studies of a herpes simplex virus type 2 (HSV-2) subunit vaccine containing glycoprotein D in HSV-discordant couples revealed 73% and 74% efficacy against genital disease in women who ...
1. Introduction. In 2017, I emphasised the steady increase in the number of publications supporting directly or indirectly the involvement of herpes simplex virus type 1 (HSV1) in Alzheimer's disease (AD) [].Since then, the number has increased further and so greatly that it is not possible in a review of reasonable and digestible length to discuss all or even many of the more recently ...
Background: Herpes simplex virus (HSV) rarely causes organ-invasive infection. Diagnosis and treatment for such infections are often delayed, and mortality is high. We present the first reported case of disseminated HSV-1 infection in an adult causing liver failure, myocarditis, and encephalitis in a patient who recovered after receiving parenteral acyclovir treatment.
Herpes simplex virus type 1 and type 2 cause genital herpes. Antiviral therapy is used for symptomatic outbreaks, and as daily suppressive therapy, it reduces recurrences of symptoms, asymptomatic ...
Figure 1. Pathogenesis of Neonatal Herpes Simplex Virus (HSV) Infection. Table 2. Common Misperceptions about Neonatal Herpes. HSV-2 is detected in genital secretions at term by culture in ...
Neonatal herpes simplex virus (HSV) infections are often life-threatening. Although sometimes difficult to diagnose, most infections can be treatable when found early. Infection with HSV should be kept high on the differential diagnosis of a febrile newborn younger than 1 month old, and treatment should be strongly considered for infants with certain risk factors, even before definitive ...
Infections tend to be unilateral, mild and self-limiting, except in the immunocompromised patient and newborns. 1 2. The authors want to emphasise the fact that the primary HSV infection can present in atypical forms, in which the lesions may be generalised, symptomatic, severe and with bilateral involvement. Therefore, this diagnosis should be ...
The herpes virus can establish a latent infection in the host and cause recurring disease when reactivation occurs. Of the herpes viruses currently identified, the neurotropic herpes simplex virus type 1 (HSV-1) can invade the central nervous system (CNS) and the peripheral nervous system (PNS) [1, 2].Herpes simplex virus encephalitis (HSVE) is an infectious neurological emergency [].
Overview. Herpes simplex virus (HSV), known as herpes, is a common infection that can cause painful blisters or ulcers. It primarily spreads by skin-to-skin contact. It is treatable but not curable. There are two types of herpes simplex virus. Type 1 (HSV-1) mostly spreads by oral contact and causes infections in or around the mouth (oral ...
Introduction. Viral infections in a patient with systemic lupus erythematosus (SLE) may complicate the approach to management. Viral etiologies are considered by some to be under diagnosed given the lack of clinical recommendations for diagnosing and managing viral infections in SLE patients1. Disseminated herpes simplex virus (HSV) infections ...
Abstract. Genital herpes, caused by herpes simplex virus (HSV) type 1 or type 2, is a prevalent sexually transmitted infection (STI). Given that HSV is an incurable infection, there are important concerns about appropriate use of diagnostic tools, management of infection, prevention of transmission to sexual partners, and appropriate counseling.
Systemic or pulmonary reactivations of herpes simplex virus 1 (HSV-1) have been reported in critically ill patients with COVID-19, posing a dilemma for clinicians in terms of their diagnostic and clinical relevance. Prevalence of HSV-1 reactivation may be as high as > 40% in this population, but with large heterogeneity across studies, likely reflecting the different samples and/or cut-offs ...
Herpes simplex encephalitis (HSE) is an important central nervous infection with severe neurological sequelae. The aim of this study was to describe clinical characteristic and outcomes of patients with HSE in Vietnam. This was a retrospective study of 66 patients with herpes simplex encephalitis who admitted to the National Hospital for Tropical Diseases, Hanoi, Vietnam from 2018 to 2021.
Herpes simplex virus (HSV) rarely causes organ-invasive infection. Diagnosis and treatment for such infections are often delayed, and mortality is high. We present the first reported case of disseminated HSV-1 infection in an adult causing liver failure, myocarditis, and encephalitis in a patient who recovered after receiving parenteral ...
3 41 Abstract Background:42 Genital herpes simplex virus (HSV) type 1 and 2 infections are lifelong and can 43 cause symptomatic genital ulcer disease (GUD). HSV-2 almost always causes sexually 44 transmitted genital infection, while HSV-1 mainly causes oral infection but can be sexually transmitted to cause genital infection. This study estimated45 genital infection with both HSV types
11571 Background: OH2 is an attenuated oncolytic Herpes Simplex-2 Virus expressing GM-CSF. In a phase I/II open-label clinical trial OH2 was safe and efficacious as monotherapy. When combined with HX008, a humanized anti-PD-1 antibody, OH2 demonstrated a disease control rate of 50% in solid tumors (1). Here, we present the efficacy results observed in a sarcoma-specific expansion cohort of the ...
Herpes simplex virus type 1 (HSV-1) is a member of the Herpesviridae family of viruses and commonly infects humans, including the lips, as herpes labialis ('cold sore'), also causes herpetic skin lesions of the finger or thumb (whitlow), and herpetic keratitis. Due to its affinity for nerve cells, HSV-1 can cause meningitis or encephalitis.
The Cliffe lab studies mechanisms of Herpes Simplex Virus (HSV) latent infection and reactivation in neurons. More than 60% of the U.S. population is infected with the virus, which can cause a range of disease outcomes, including oral and genital lesions, keratitis, and encephalitis, and potentially contribute to the development of Alzheimer ...
Tingling, burning, or itching at the site the lesions will eventually appear. Which instruction should the nurse include about condom application? Ensure no air is trapped in the tip of the condom. How should the nurse reply? Delivery is by cesarean section if an active outbreak of HSV is present.
BackgroundHerpes simplex virus type 2 (HSV-2) is the most common cause of genital ulcers in industrialized countries. ... Here, we report an unusual case of co-reactivation of herpes zoster and genitalis in an immunocompetent male. We recommend the use of molecular testing to confirm the diagnosis of VZV or HSV infection in all cases of genital ...
Herpes simplex virus 1, or HSV 1, mostly shows up as painful blisters around the mouth, commonly referred to as "cold sores," but it can also infect the genital region, according to Jerome. "These ...
In this study, we showed that a cellular nuclear protein, when induced by IFN, can reduce wild-type herpes simplex virus infection through effects on viral chromatin. This study defines the impact of this nuclear IFN-stimulated gene and provides opportunities for future antiviral strategies relating to epigenetic silencing within the nucleus.
Herpes simplex virus type 1 (HSV-1) infection of the eyes results in herpes simplex keratitis (HSK), which has led to vision loss and even blindness in patients. However, the rate of drug resistance in HSV is on the rise; therefore, new antiviral agents with sufficient safety profiles must be developed. At present, we assessed the anti-HSV-1 activity of 502 natural compounds and their ability ...
Of the cases with a known cause, 20 to 50% are attributed to viruses. 2,3 Herpes simplex virus (HSV) accounts for 50 to 75% of identified viral cases, with varicella-zoster virus (VZV ...
Recent multi-omics analyses reported the herpes simplex virus type 1 (HSV-1) genome encodes an additional number of potential coding sequences (CDSs). However, the expressions of these CDSs at the peptide or protein levels and the biological effects of ...
Background Infections with Herpes simplex virus (HSV)-1 or -2 usually present as mild chronic recurrent disease, however in rare cases can result in life-threatening conditions with a large spectrum of pathology. Monoclonal antibody therapy has great potential especially to treat infections with virus resistant to standard therapies. HDIT101, a humanized IgG targeting HSV-1/2 gB was previously ...
Herpes simplex virus (HSV) is the most common identifiable cause of serious or life threatening sporadic, endemic encephalitis. ... In a study in Australia from 1999 to 2011, totally 29 mortalities due to HSV occurred in children under 15 years, ... The most obvious finding from this case is herpes oesophagitis, which must be considered in the ...