- Open access
- Published: 01 November 2022

Potential functions and applications of diverse microbial exopolysaccharides in marine environments
- Hassan A. H. Ibrahim 1 ,
- Hala E. Abou Elhassayeb 1 &
- Waleed M. M. El-Sayed ORCID: orcid.org/0000-0001-5978-2260 1
Journal of Genetic Engineering and Biotechnology volume 20 , Article number: 151 ( 2022 ) Cite this article
3723 Accesses
19 Citations
Metrics details
Exopolysaccharides (EPSs) from microorganisms are essential harmless natural biopolymers used in applications including medications, nutraceuticals and functional foods, cosmetics, and insecticides. Several microbes can synthesize and excrete EPSs with chemical properties and structures that make them suitable for several important applications. Microbes secrete EPSs outside their cell walls, as slime or as a “jelly” into the extracellular medium. These EPS-producing microbes are ubiquitous and can be isolated from aquatic and terrestrial environments, such as freshwater, marine water, wastewater, and soils. They have also been isolated from extreme niches like hot springs, cold waters, halophilic environments, and salt marshes. Recently, microbial EPSs have attracted interest for their applications such as environmental bio-flocculants because they are degradable and nontoxic. However, further efforts are required for the cost-effective and industrial-scale commercial production of microbial EPSs. This review focuses on the exopolysaccharides obtained from several extremophilic microorganisms, their synthesis, and manufacturing optimization for better cost and productivity. We also explored their role and applications in interactions between several organisms.
The marine biosphere is a heterogeneous mix of several ecosystems such as microbial mats, Antarctic Sea ice, hyper-saline marine environments, and shallow and deep-sea hydrothermal vents. Within the deep-sea hydrothermal vents, large physicochemical gradients exist. For example, the temperature of the surrounding seawater varies from 2°C to that of the hydrothermal plume, which can reach 350°C. Due to their microbial diversity, these ecosystems provide a wealth of novel biomolecules as several new microorganisms with highly diverse metabolisms have been isolated from these environments [ 1 ]. They offer vast natural resources for essential and functional commercial grade products such as EPSs [ 2 ].
Among the marine microbes, bacteria, and phytoplanktons, such as diatoms, cyanobacteria, and dinoflagellates, are the most significant sources of EPSs. Numerous EPS-producing microbes have been isolated from marine environments, such as seawater, sediment, deep-sea hydrothermal vents, and sea ice [ 3 ]. Marine microorganisms such as Acinetobacter , Arthrobacter , Pseudomonas , Halomonas , Myroides , Corynebacteria , Bacillus , and Alteromonas sp. have been studied for EPS production [ 2 ].
Commonly, EPSs are defined as natural weight polymers that are synthesized and secreted by microorganisms into their surroundings to establish the functional and structural integrity of biofilms. Hence, they are essential for determining the physicochemical properties of biofilms [ 4 ] and constitute 50–90% of a biofilm’s total organic matter [ 5 ]. Moreover, EPSs are mainly composed of polysaccharides and proteins, DNA, lipids, and humic substances [ 6 , 7 ].
Microbial polysaccharides are principally classified into several groups based on (i) their cellular location (cell wall PSs, exoPSs, and endoPSs), (ii) structure (linear and branched), (iii) sugar composition (homo- and heteropolysaccharides), and (iv) type of linkages between monomers {b-(1→3), b-(1→6), and α-(1→3)} [ 8 ]. Based on their monomeric composition, the microbial EPSs are either homopolysaccharides, consisting of a single monomer linked by glycosidic bonds, or heteropolysaccharides, which have more than two monomeric units connected by glycosidic bonds. They also contain several different organic moieties, such as organic and amino acids, along with inorganic constituents such as sulfates and phosphates [ 9 ]. The polymers that belong to the homopolysaccharides group include cellulose, curdlan, dextran, pullulan, and scleroglucan [ 10 ]. Microbial EPSs can be further grouped into four major classes; polysaccharides, slime, and microcapsular polysaccharides, inorganic polyanhydrides (polyphosphates), polyamides, and polyesters [ 11 ].
Furthermore, EPSs are ideal for several applications due to their recently discovered chemical properties and structures [ 12 ]. Latest studies have shown antioxidant, immune-modulation, anti-tumor, and antimicrobial properties of EPSs [ 13 ].
Microbial EPS is an important source of dissolved organic carbon in marine ecosystems. Bacterial EPS are rich in uronic acid, which makes them resistant to mineralization by microbes and thus, can exist for a long time in oceans. Therefore, they are prevalent in extreme marine environments and are essential for microbial survival [ 14 ]. While EPSs mainly have protective functions, their exact roles depend on the microorganisms’ surrounding environment. They can protect the microbial communities against extreme temperature and salinity and lack of nutrient accessibility by forming a barrier between the microbe and its environment [ 13 ].
EPSs have different functions in bacteria, such as forming a favorable microenvironment to facilitate attachment, exoenzyme activity, sequestration of nutrients, and protection against toxins in the surrounding medium [ 3 ]. Additionally, they are essential for aggregate formation, surface adhesion, forming biofilms and biofouling, absorption of nutrients, and so on [ 15 ].
Due to their degradability and nontoxicity, microbial EPSs have attracted interest for their applications in the marine environment, especially as bio-flocculants [ 16 ]. Furthermore, they can be used as antifouling agents in wastewater treatment, bioremediation, and petroleum industries [ 17 ]. Therefore, this review aims to present comprehensive information on marine microbial EPSs, their sources, and potential prospective applications.
Microbiology of EPS-producing marine organisms
EPSs have been primarily observed in terrestrial and marine bacteria and fungi [ 17 ], and occasionally, in yeasts [ 18 ], cyanobacteria [ 19 ], microalgae [ 20 ], and medicinal mushrooms [ 21 ]. The following sections will briefly summarize the common microbes that produce EPSs, starting with extremophilic microorganisms.
EPSs from archaea and bacteria
Different types of EPS have been isolated from different groups of archaea, especially thermophilic and halophilic groups. Thermophilic archaea have been isolated from extreme environments, including deep and shallow marine hot springs and terrestrial hot springs [ 22 ]. Various thermoacidophilic archaea, including members of the genera Thermococcus and Sulfolobus , have been reported to store polysaccharides, such as glycogen, and secrete mannan and sulfated heteropolysaccharides [ 23 ]. Significant accumulation of EPSs was observed in Archaeoglobus fulgidus and Archaeoglobus profundus in the form of biofilms [ 24 ]. Different strains of thermoacidophilic archaeon, Sulfolobus solfataricus , were used to produce sulfated EPSs [ 25 ]. Moreover, two closely related hyperthermophilic crenarchaea, Sulfolobus acidocaldarius and S. tokodaii , were studied by Koerdt et al. [ 26 ] for biofilm formation.
The EPSs synthesized by Halomonas strains had high sulfate content and a considerable amount of uronic acids showing high gelation capability [ 27 ]. However, Anton et al. [ 28 ] produced a heteropolysaccharide EPS using an archaebacterium, Haloferax mediterranei . Paramonov et al. [ 29 ] elucidated the neutral structure of EPS isolated from Haloferax gibbonsii . Furthermore, Parolis et al. [ 30 ] separated an acidic EPS from a halophilic archaeon, H. denitrificans . Moreover, Nicolaus et al. [ 31 ] isolated an obligate halophilic archaeon, Haloarcula japonica T5, that produces a sulfated EPS (Fig. 1 ). according to the previous literature, many microorganisms produce exopolysaccharides as a strategy for growing, adhering to solid surfaces, and surviving adverse conditions. The physiological role of EPS depends on the ecological niches and the natural environment in which microorganisms have been isolated.

Biofilm production by H. japonica T5 [ 31 ]
Interestingly, most bacterial species grown under appropriate culture conditions secrete mucoid polysaccharides outside the rigid cell wall structures [ 32 ]. However, the presence of EPS in bacterial cells can easily be identified by the appearance of the mucoid colony, as shown in Fig. 2 [ 33 ].
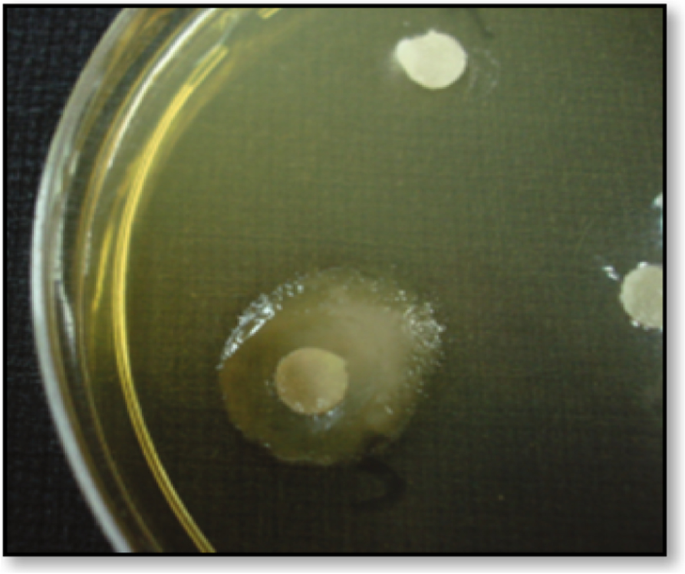
Mucoid colony of an exopolysaccharide-producing microbe on solid media [ 34 ]
Studies on EPS focus mainly on the polysaccharides produced by Gram-negative and some Gram-positive bacteria [ 35 ], such as Pseudomonas spp., Acetobacter spp., Aureobasidium spp., Sinorhizobium spp., Escherichia spp., Acetobacter spp., Bacillus spp., etc. [ 36 ]. Enos-Berlage and McCarter [ 37 ] showed that Vibrio parahaemolyticus secrete EPS.
Ravaioli et al. [ 38 ] screened 55 S. epidermidis biofilm-forming clinical isolates using a simple fluorescence-based microtiter-plate assay. Several species from the genus Enterobacter secrete EPS-containing fucose, such as Enterobacter sp. CNCM 12744, that produces EPS-containing fucose, galactose, glucose, and glucuronic acid monomers [ 39 ]. Freitas et al. [ 40 ] found that Enterobacter strain A47 (DSM 23139) produced a fucose-containing EPS. Lactic acid bacteria (LAB) are mesophiles that have been long known to produce EPS. Among their genera, Lactobacillus bulgaricus, L. helveticus , L. brevi , L. lactis , Leuconostoc mesenteroides , and Streptococcus spp. are the potent EPS producers. Also, more than 30 LAB species are polysaccharide producers, of which Leuconostoc mesenteroides is a commercially used dextran producer [ 41 ].
Marine bacteria have been reported to produce a wide range of EPSs. Isolating new EPS-producing bacteria from marine environments, particularly extreme ones, has been of interest [ 42 ]. Jayaraman & Seetharaman [ 43 ] isolated EPS from the marine bacterium, Vibrio alginolytics , which acted as a potential marine biofouling material. Similarly, the marine bacteria, Vibrio diabolicus produces hyaluronic acid-like EPS that has been commercialized with the trade name, “Hyalurift.” Amazingly, this EPS can improve bone integrity [ 44 ]. Gutierrez et al. [ 45 ] isolated a type of EPS, also known as PE12, with emulsifying activity from Pseudoalteromonas that can adsorb metal ions. Urai et al. [ 46 ] isolated marine Rhodococcus erythropolis , PR4 that produces many acidic EPSs including FR2. Additionally, the EPS-producing Pseudoalteromonas sp. strains, CAM025 and CAM036 were isolated from seawater and sea ice in the Southern Ocean [ 47 ]. CAM025 showed 30 times higher yield when grown at −2°C and 10°C than at 20°C. Al-Nahas et al. [ 48 ] isolated a marine EPS-producing Pseudoalteromonas sp from the Red Sea sponge found in Egypt.
Selim et al. [ 49 ] recently examined 83 marine isolates (from the Mediterranean Sea and the Red Sea) for EPS production. Of these, nine isolates showed the highest antioxidant activities; Bacillus circulans , B. licheniformis , B. alvei , B. insolitus , B. polymyxa , B. marinus , B. anthracis, Staphylococcus sp., and B. brevis . El Essawy et al. [ 50 ] extracted an EPS from marine Klebsiella sp. Abdelnasser et al. [ 51 ] isolated EPS-6 from the bacterial strain, Bacillus flexus from the Mediterranean Sea. Wang et al. [ 14 ] produced EPS-A from the marine bacteria, Aerococcus uriaeequi . The EPS extracted by Abdrabo et al. [ 52 ] from marine Halomonas saccharevitans AB2 isolated from the Suez Gulf, Egypt, showed promising antimicrobial and anti-tumor activities. Selim et al. [ 53 ] isolated 20 streptomycetal strains from marine sediment samples collected from the Nabq area, Red Sea, Egypt. EPS exhibiting potent anti-tumor activities were produced in vitro using four strains, particularly Streptomyces carpaticus . Ali et al. [ 54 ] optimized EPS production from marine Pseudomonas mendocina AB1 , emphasizing valuable applications such as antioxidant and antibacterial agents .
Amazingly, thermophilic bacteria, derived mainly from hydrothermal vents and hot springs, including Archaeoglobus fulgidu , Thermococcus litoralis , B. thermantarcticus , Geobacillus thermodenitrificans , B. licheniformis , Thermotoga maritima , Thermoto gamaritima , Methanococcus jannaschii , and Geobacillus tepidamans V264 hare well-documented EPSs producers [ 36 ].
Marine bacteria produce many EPSs in numerous ways. The genes for EPS synthesis are frequently found in clusters inside the genomes of the EPS-producing organisms. Further development of genetic, metabolic, and protein-engineering techniques is required to understand the underlying mechanisms involved in EPS production. This will also enable tailor-making EPS-based polymers with improved qualities for medicinal and industrial use. Novel applications can be developed by exploiting the natural design space for biopolymer synthesis [ 17 ].
EPSs from marine fungi and yeast
Ascomycota and Basidiomycota fungi can be used to produce several synthesized EPSs with unique biochemical and biological properties [ 55 ]. These EPSs are mainly heteropolysaccharides, but in the case of homopolysaccharides, glucose is their only monomer [ 22 ]. Also, there are several EPSs produced by filamentous fungi such as Botryosphaeria rhodina MMGR [ 56 ], Aspergillus versicolor LCJ-5-4 [ 57 ], Fusarium solani SD5 [ 58 ], F. oxysporum Y24-2 [ 59 ], and Penicillium griseofulvum [ 60 ]. Moreover, a coral-associated fungus, Penicillium commune produces EPS, FP2-1, when grown on potato dextrose agar medium [ 61 ].
Actually, EPSs are produced by several yeasts such as Candida , Candida famata and Candida guilliermondii [ 62 ]; Cryptococcus , Cryptococcus flavus and Cryptococcus humicolus [ 63 , 64 ]; Lipomyces ; Pichia [ 30 ]; Rhodotorula , Rhodotorula acheniorum MC [ 65 ]; Issatchenkia orientalis [ 63 ]; Kazachstania unispora [ 66 ]; and Sporobolomyces genera such as Sporobolomyces salmonicolor AL1 [ 67 ]. Additionally, Kuncheva et al. [ 65 ] produced mannan from the yeast strain, R. acheniorum MC, and glucomannan from S. salmonicolor AL 1 . Pavlova et al. [ 64 ] applied psychrophilic Antarctic yeast, C. flavus , to produce the heteropolysaccharide EPS, composed of mannose, glucose, xylose, and galactose. Rusinova-Videva et al. [ 68 ] also isolated an EPS-producing psychrophilic yeast isolate.
EPSs from marine cyanobacteria and algae
Cyanobacteria and green algae are phototrophic microorganisms with diverse cellular characteristics that change in response to the environmental conditions, such as producing EPS in response to harsh conditions. EPS is primarily found in the enclosed layer surrounding their cells/filaments and is then released into the environment. Generally, EPSs are vital for their survival under stress conditions like radiation, desiccation, and high temperatures. Microalgae and cyanobacteria EPSs are visible as a mucosal mass surrounding the cells [ 69 ]. They can closely adhere to the cells and be released into the surrounding medium [ 70 ]. They can be seen in a thin layer, known as a sheath (Fig. 3 ), which is formed adjacent to the outer cell membrane in the form of a capsule [ 22 ]. It is associated with the cell surface and may be covalently bound to the cell wall. When they are loosely associated with the cell surface and not within envelopes, they are considered as slime (Fig. 4 ).
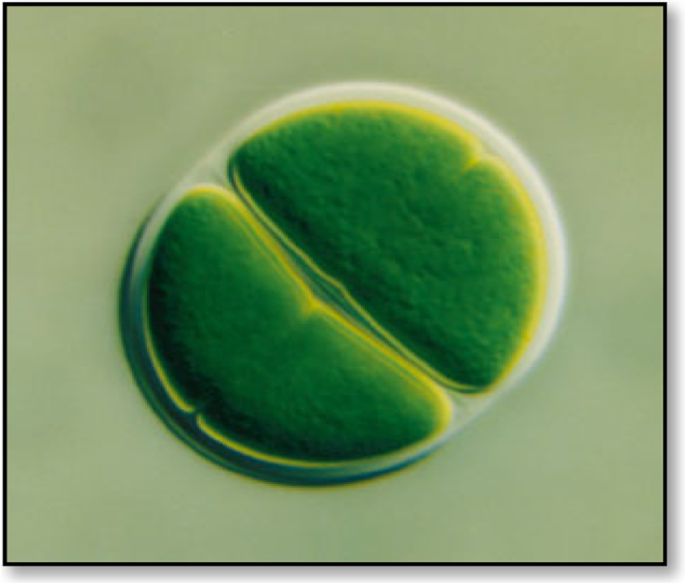
The structure of a “sheath” of the unicellular Chroococcus sp. [ 71 ]
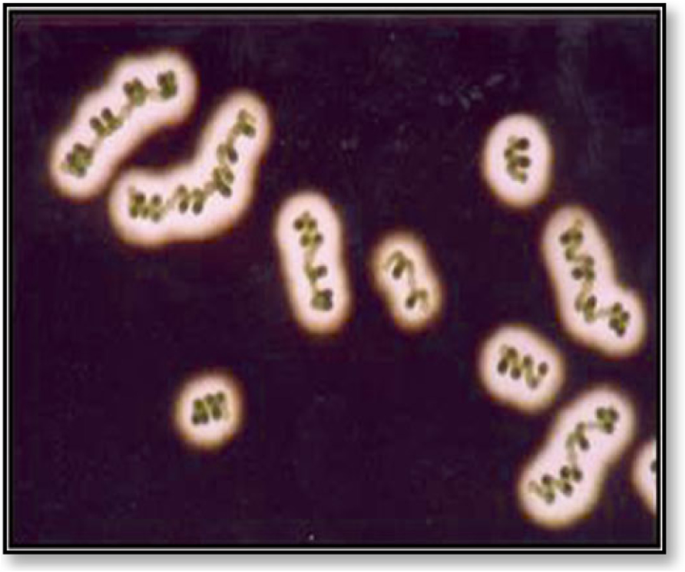
The exopolysaccharides of Cyanospira capsulata [ 22 ]
De Philippis and Vincenzini [ 71 ] characterized several EPS-producing Cyanothece strains isolated from saline environments. Additional acetyl, pyruvyl, and sulfate groups have also been detected in some EPS samples. They facilitate cell adhesion by assembling as a stalk aside from providing structural support as capsules or sheaths [ 72 ].
In cyanobacteria, EPS and slime represent most of the cell’s dry weight, while sheaths represent a relatively smaller portion [ 73 ]. Although many cyanobacteria have been shown to produce EPSs, most of them were isolated from the terrestrial environment. Only a few marine cyanobacterial strains, such as Schizothrix sp., Gscillatoria sp., Cyanothece sp. [ 74 ], and Oscillatoria sp., isolated from marine stromatolites, have been documented for EPS production [ 75 ]. Additionally, studies suggest that Spirulina sp. produces several compounds containing polysaccharides and EPSs with therapeutic functions such as anti-inflammatory properties [ 76 ]. For example, spirulan is a sulfated EPS produced by Arthrospira platensis [ 44 ].
In red microalgae, the EPS is partly dissolved in the growth medium and partly released into the medium, increasing its viscosity. The soluble EPSs produced by red microalgae are either released from the bulk fraction or are transferred directly from the cell to the growth media [ 77 ].
Production and characterization of EPSs
Microbes are more potent and cheaper sources of EPSs than plants because of their high growth rate, ability to grow in relatively affordable media, lower space requirement, and ease of manipulation. Thus, there is increasing interest in isolating and identifying novel microbial EPS that can compete with traditional EPS [ 78 ]. The research on EPSs primarily focuses on their synthesis, optimization of production to make it cost-effective, and finally, understanding their role and application in interactions between numerous organisms. Using biotechnological techniques, it is possible to obtain substantial amounts of EPSs from numerous microbes by controlling their growth conditions in a bioreactor [ 79 ].
First, a specific bacterial strain is examined for its possible EPS-producing ability to evaluate EPS production by observing if it is sticky or ropy. When liquid cultures of EPS-producing bacteria demonstrate high resistance to flow through serological pipettes and form viscous strands during free-fall from the pipette tip, they are considered “ropy” [ 80 ]. EPS production can be improved by developing novel strategies such as fermentation using genetically engineered microbes and methodologies resulting in high yield and cost-effective production. The downstream process to recover the EPS is conventionally done by removing the cells from the fermentation broth by centrifugation, followed by isolation and purification steps. Subsequently, the EPS present in the cell-free medium is precipitated using ethanol, methanol, or acetone. Then, the pellets that are recovered using centrifugation, dialyzed using the appropriate method in distilled water, are freeze-dried to obtain crude EPS [ 81 ]. The general steps of production, extraction, and characterization of EPSs are illustrated in Fig. 5 .
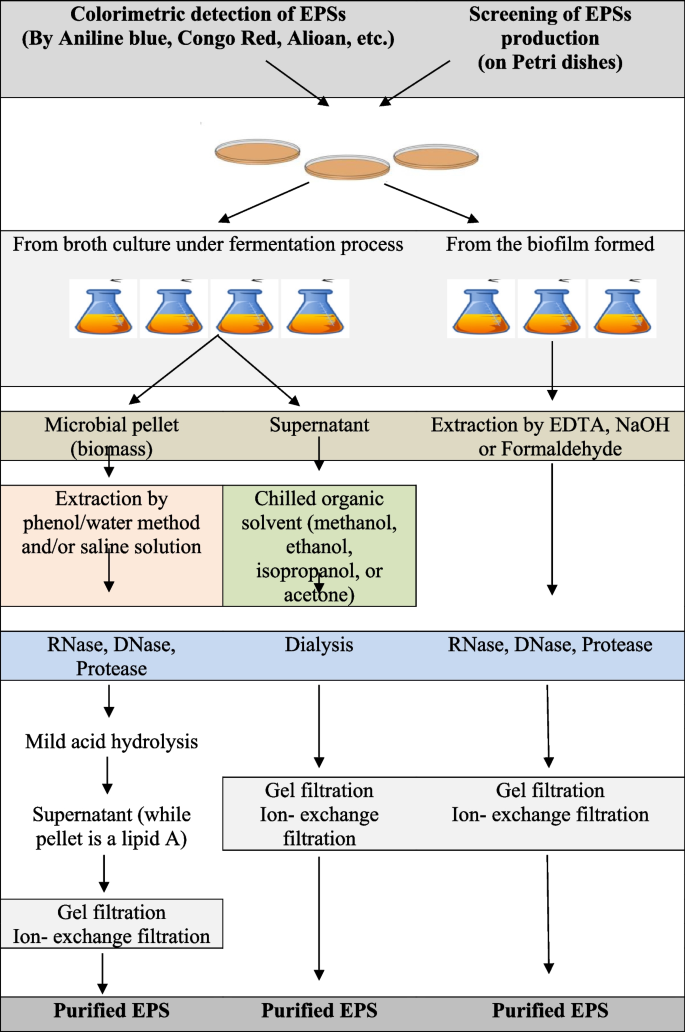
The general steps for production, extraction, and characterization of exopolysaccharides
Overall, EPS production involves selecting suitable microbes, the cultural media, and the practical method for EPS preparation and extraction. The isolation method employed can also significantly affect the EPS yield. Several physical and chemical methods have been applied to extract EPS from different sources, such as cell suspensions, sludge, biofilm, solid surfaces, and various types of water. The physical methods include centrifugation, sonication, heating, and freeze-thawing, while the chemical methods involve different chemical agents, such as organic solvents, NaOH, ethylenediamine tetraacetic acid (EDTA), and formaldehyde [ 61 ].
To isolate crude EPS, the supernatant is usually precipitated using alcohols such as ethanol (95%) or methanol and, occasionally, isopropanol or acetone at 4 °C for 12–24 h. Similarly, fungal EPSs are derived via ethanol precipitation using different ratios of the culture/water suspension and alcohol [ 82 ] . Occasionally, in strains like Ascomycota strains, the supernatant containing the EPS is treated with 5% trichloroacetic acid [ 83 ] during primary purification. The crude EPS is dialyzed against water to remove excess salts and then stored as a vacuum-dried or lyophilized powder. The next step involves using Sevage reagent to deproteinize the EPS for further purification [ 57 ]. Moreover, there are other potent methods to purify EPS from Ascomycota and Basidiomycota EPSs, such as ion exchange chromatography and gel permeation chromatography [ 82 ]. Cyanobacterial EPSs can be more easily recovered by simply precipitating the cell-free supernatants with cold ethanol [ 84 ]. Other methods include sheath extraction from Chroococcus minutus SAGB.41.79 with differential sucrose gradient centrifugation using homogenized cells. Some studies used hot water treatment of the pelleted cells, while others performed deionized water extractions to extract EPS. In other cases, cyanobacterial EPSs were isolated by treating pelleted cells with 1.5% NaCl at 60°C [ 85 ].
Furthermore, Freire-Nordi et al. [ 86 ] extracted EPSs from Staurastrum inversenii by fixing medium-starved cells with 0.5% formalin followed by progressive 4% Dakin liquid washes followed by stirring for 30 min at 40°C. Di Pippo et al. [ 84 ] recovered cyanobacterial EPSs by extracting with 0.1 M H 2 SO 4 at 95°C for 1 h. Abdullahi et al. [ 87 ] used part water at 90°C with 0.5 M NaHCO 3 at 95°C and part 1 M NaOH containing 0.2 M NaBH 4 at 95°C to extract the bulk mucilage from the fungal diatom, Phaeodactylum tricornutum .
For characterizing microbial EPS, the basic parameters that should be analyzed include the total content of carbohydrates, uronic acids, sulfated sugars, and protein that can be determined by standard methods [ 88 ]. Additionally, EPS hydrolysis using acids or other agents, including sulfuric acid, hydrochloric acid, trichloroacetic acid, and trifluoroacetic acid, should be done to break down the glycosidic linkages of the polymer and subsequently expose the monosaccharide constituents. These monomers are reduced to form sugar alditols and further derivatized by acetylation with acetic anhydride in the presence of pyridine. These volatile sugar derivatives are then subjected to gas chromatography-mass spectrometry (GC-MS) analysis and compared with the sugar standards [ 22 ]. Furthermore, several advanced methods have been approved for the qualitative analysis of EPSs, including high-performance liquid chromatography, Fourier transform infrared spectroscopy (FTIR), and nuclear magnetic resonance [ 89 ]. Recently, matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (TOF), atomic force microscopy, and X-ray diffraction have also been used for the detailed qualitative analysis of EPSs [ 90 ].
Ecological roles of microbial EPS in the marine environment
EPS constitutes most of the ocean’s reduced carbon storage and supports the survival of marine bacteria by changing the physicochemical environment around the bacterial cell. Furthermore, they protect microbial communities against extreme temperature, salinity, and nutrient availability extremes [ 10 ]. The physiological role of EPS is dependent on the ecological niches and the natural environment in which microbes have been grown. EPS are mainly essential for aggregate formation and adhesion to surfaces. In certain organisms, they are required for biofilm formation and biofouling, along with the absorption of nutrients [ 15 ]. The critical roles of EPSs are discussed in the following sections.
Aggregation of microbes
In their natural environment, most bacteria occur in microbial aggregates, and their structural and functional integrity depends on the presence of a matrix made of extracellular polymeric substances including EPS. Hence, EPS production is essential for their survival. Particularly, the organic matrix present in the intracellular space of microbial biofilms, which represents a significant store of reduced carbon on Earth, is made up of marine polysaccharides and other macromolecules such as proteins, lipids, and nucleic acids. Furthermore, the latest focus on extreme marine habitats has increased awareness for the bacteria surviving in these environments, known as extremophiles. These species serve as a model for studying the stability and potential function of their biomolecules due to their unique metabolic pathways and defense systems [ 10 ].
Undoubtedly, they have protective functions by forming a layer around the cell and providing adequate protection against high or low temperatures, salinity, and possible predators. They are essential for aggregate formation, adhesion to surfaces and other organisms, the formation of biofilm, and uptake of nutrients [ 91 ]. Notably, studies involving the microbial communities in sea ice have found strong associations between bacteria and particles, indicating the importance of EPSs in cryoprotection [ 92 ].
Formation of bacterial biofilm
The ability to build and maintain an organized multicellular bacterial population highly depends on the production of extracellular matrix components [ 93 ]. Although the biofilm matrix might consist of several molecules, we have focused on EPSs critical for biofilm development in this section. The biofilm-forming bacteria are more protected than the planktonic bacteria as the EPS matrix acts as a protective diffusion barrier [ 94 ]. Furthermore, due to its gluey nature, the EPS layer serves as a nutrient trap, facilitating bacterial growth [ 95 ]. Thus, these polymers are the primary components of the biofilms formed on solid substrates [ 96 ]. Furthermore, reports have shown that the biofilm-forming microbes are more than 1000 times resistant to antibacterial compounds such as antibiotics, toxins, surface active agents, and sanitizers than free planktonic cells. Therefore, EPS formation is crucial for the survival of these microbes [ 97 ].
A bacterial biofilm is “a structured community of bacteria encapsulated within a self-developed polymeric matrix and adherent to a living or inert surface.” It is often characterized by surface attachment, structural heterogeneity, genetic diversity, complex community interactions, and an extracellular matrix of polymeric substances [ 98 ]. In nature, bacteria colonize at various interfaces to form poly-bacterial aggregates such as mats, flocs, sludge, or biofilms, unlike planktons that are dispersed, single cells as seen in pure laboratory cultures [ 99 ].
Several essential elements influence the process of bacterial biofilm formation. Water quality, including temperature, pH, dissolved oxygen level, and the presence of organic and inorganic nutrients, is highly significant. After finding a suitable environment, the bacterium will continue to develop unless the system’s conditions become unsuitable [ 100 ]. EPSs is essential for the biofilm matrix-mediated biochemical interactions between the bacteria and its surrounding cells. Hydrated biofilms offer a stable microenvironment for storing extracellular enzymes and for the cellular uptake of small molecules [ 101 ] (Fig. 6 ).
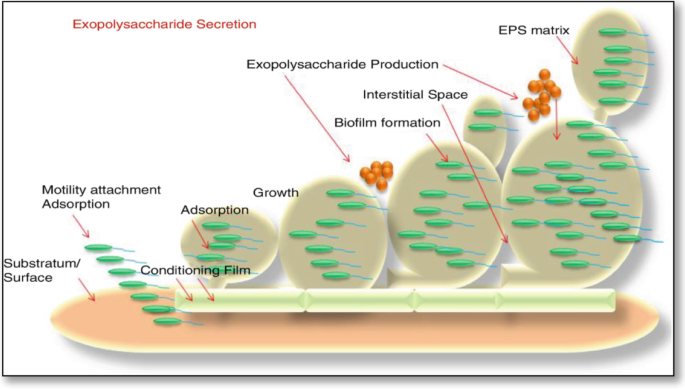
General steps involved during the formation of the exopolysaccharide matrix of a biofilm
Unlike the adhesion seen in biofilms, EPS are integral to interactions such as cell-cell cohesion and cell-solid substratum cohesion [ 102 ], which allow the bacteria to colonize densely in their hosts and microenvironment along with protecting them from harm. Therefore, understanding biofilm dynamics is crucial for developing novel and effective biofilm suppression control measures to improve desalination management [ 103 ].
The rate of synthesis and the number of EPS accumulated in the capsules in pathogenic bacteria influence their pathogenicity. Besides, EPSs plays an important role in the biofilm matrix by enabling biochemical interactions between the bacteria and its surrounding cells [ 104 ]. Hydrated biofilms offer a stable microenvironment for storing extracellular enzymes and for the cellular uptake of small molecules [ 67 ].
However, there are several critical drawbacks of EPSs as follows:
They act as starters for biofilm growth inside water pipes, affecting water quality by changing the bacterial levels (increasing coliform bacteria), reducing dissolved oxygen, and changing the taste and odor.
It provides a platform for biofouling in aquatic systems, such as organisms, ship hulls, pipelines, and reservoirs [ 17 ]. However, a collective summary of the potential roles of EPSs in bacterial biofilms is presented in Table 1 .
Architecture and featuring of the marine environment
EPS matrix molecules provide a three-dimensional framework that allows cells to localize extracellular activities and perform cooperative/antagonistic interactions that are unachievable in free-living cells [ 106 ]. In a geomicrobiological environment, EPSs influence precipitation of minerals, mainly carbonates. They might also be able to trap various particles in biofilm suspensions, limiting dispersion and element cycling [ 107 ]. Furthermore, EPSs improve sediment stability by affecting sediment cohesion, permeability, and erosion (Table 1 ). The adhesion and metal-binding ability of EPS affect mineral leaching rates in both environmental and industrial contexts. These interactions between EPSs and the abiotic environment allow them to primarily affect the biogeochemical cycling [ 107 ]. EPS alters the optical fingerprints of sediments and saltwater and is involved in biogeomineral precipitation, microbial macrostructure creation, and horizontal genetic information transfer [ 106 ].
Formation of marine snow and biological bump
EPSs enable the production of organic colloids and large cell aggregates known as marine snow. These include transparent exopolymer particles, sea-surface microlayer biofilm, and marine oil snow [ 106 ]. Excessive EPS formation occurs as a metabolic byproduct during the late phases of phytoplankton blooms, releasing a carbon pool that alternates between dissolved, colloidal, and gel phases. However, the coagulation of single particles into rapidly settling aggregates is known as a “biological bump” [ 108 ] (Fig. 7 ).
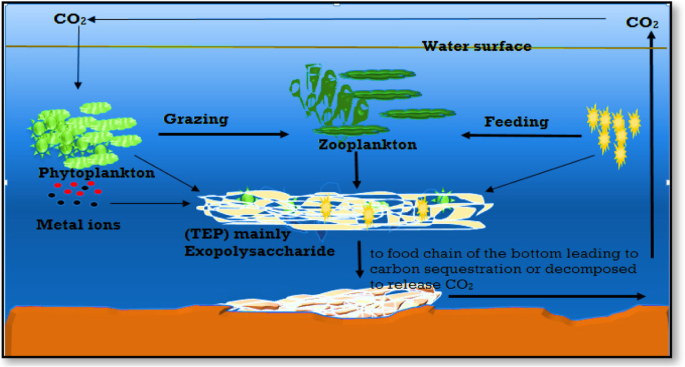
Aggregation of organic carbon by exopolysaccharides and scavenge some metal ions then sink to the bottom [ 108 ]
In addition, Krembs et al. [ 109 ] demonstrated that prevailing under-ice currents and ice drifts carry the neutrally buoyant polymeric materials over long distances. Further evidence has indicated the importance of marine bacterial exopolymer synthesis in the process of aggregate formation [ 104 ]. When this colloidal material was released into the water column, a combination of biological, chemical, and physical pressures enables its congregation and formation of microbiological heterotrophic activity centers [ 110 ].
Formation of biofouling
Biofouling is a natural phenomenon that occurs when biofilms form on water-immersed surfaces. Biofilm formation is the first and most crucial step in the biofouling process. Biofilms undergo several stages, including substratum conditioning, pioneer bacterial adherence, extracellular polymeric material release, soft macrofouling, and hard macrofouling. Biofouling has a financial impact on industries. Biosurfactants have antibacterial and biocompatible qualities, making them attractive antifouling solutions for biofouling processes. EPSs are integral for the biofouling formation in the aquatic systems and on the marine vehicles affecting vessels and other marine structures. However, biofouling starts with a layer of adsorbed organic and inorganic matter, through microbial film formation, to a community of macroscopic plants and animals (see Table 1 ) [ 17 ].
Studies showed that the addition of EPS-producing Nostoc muscorum into the soil increased the number of water-stable aggregates, either by gluing the soil particles or by stimulating the soil community to produce more EPSs [ 111 ]. The function of the hydrophobic EPSs in the adhesion process was also supported by the observation that treatment with the sulfated polysaccharide, emulcyan excreted from Phormidium sp. that masks the hydrophobicity of EPSs, caused cell detachment from solid surfaces. Benthic cyanobacteria Phormidium J-1 and Anabaenopsis circularis 6720, which could co-flocculate with suspended clay particles and attach to the benthos due to the hydrophobic interactions. In marine environments, cyanobacterial and diatom-produced EPSs form a matrix that enhance mudflat sediments by stabilizing them to against erosion and enriching them with organic matter and nutrients [ 112 ].
Biological fouling is a serious issue for constructed structures in marine and freshwater environments as microbial biofilm formation are frequently followed by colonization by several macro fouling organisms. Use of antimicrobial chemicals, usually poisonous to non-target species, is a common and effective preventative strategy. While various nontoxic surface modification approaches have been used, their effectiveness in in situ situations has been limited.
Marine microorganisms are important potential sources of antifouling chemicals due to their diverse metabolic activities and specific structural moieties. Chemical substances can disrupt the biofilm’s bacterial structure and interfere with higher organisms’ larval settling [ 113 ]. As only a small percentage of microorganisms have been found to contain bioactive substances, further research should be done to isolate and grow bacteria, especially those from harsh habitats. Aerobes, mesophiles, and heterotrophs have been found to be non-picky EPS producers isolated from hydrothermal vents [ 114 ].
Conversely, microbial EPSs used as biosurfactants and bioemulsifiers have attracted attention because of their biodegradability [ 115 ]. Therefore, a promising technology, known as microbial enhanced oil recovery (MEOR), has been developed for manipulating the function and structure of microbial environments in oil reservoirs. It is a biotechnology method in which microbes could be used to recover the additional oil from existing wells, thereby enhancing the petroleum production from an oil reservoir [ 115 ]. However, selected natural microbes producing biosurfactants and/or specific EPSs are introduced into oil wells to produce harmless by-products, such as slippery natural substances or gases, all of which aid the oil expulsion out of the well, allowing higher quantities to be recovered from the well. Genetically engineered Enterobacter cloacae are successfully used in MEOR [ 115 ].
Tolerance to water stress and UV radiation
UV-B/A irradiation damages live cells by producing reactive oxygen species (ROS), which mostly include superoxide anion (Radical dot-O 2 ) and the hydroxyl radical (Radical dot-OH) [ 116 ]. Hydroxyl and superoxide radicals are two major free radicals that can directly cause a variety of oxidations. Antioxidation may be disrupted if hydroxyl or superoxide radical scavengers are present, allowing free radical scavengers to protect living cells from UV radiation. Free radical scavenging properties have been discovered in a variety of polysaccharides [ 117 , 118 ].
The protection against water stress and UV radiation is one of the main studied roles of the EPSs in constrained environments. It is known that the cyanobacteria isolated from very dry environments, such as desert soils or the lithic surfaces of monuments, display the capacity of excreting large amounts of EPSs [ 119 , 120 ], a trait underlining adaptation to drought. Dehydration effects have been thoroughly studied. Essentially, water stress leads to the loss of membrane structural integrity and the loss of macromolecule functioning [ 121 ], so some authors associate cell death under drought conditions just with the loss of membrane integrity [ 122 ].
Although the role of EPSs in water stress has not been fully clarified, they are reportedly involved in maintaining hydration thanks to their hydrophilic/hydrophobic characteristics, which determine a gelatinous envelope around the cells that regulates water uptake and water loss processes [ 123 ]. Furthermore, they stabilize cell membranes along with non-reducing sugars sucrose and trehalose. Cyanobacteria can absorb water many times their dry weight. For example, colonies of Nostoc reportedly increase their mean diameter from 50–100 μm to 150–250 μm after wetting. At the same time, cyanobacterial filaments are extruded out of the sheaths, to be retracted inside when the general moisture level decreases [ 124 ].
One of the strongest pieces of evidence for the role of EPSs in water stress tolerance was provided by N. commune by Tamaru et al. [ 125 ]. EPS-deprived cells were significantly harmed in their capability to evolve O 2 , and a decrease in cell viability was observed. In addition, EPSs are also thought to confer an increase in freeze tolerance. Cyanobacterial EPSs provide for the structuring of the biofilms, creating preferential flows of water and nutrients. In addition, EPM creates hydrated microenvironments in which the cells are protected from harmful solar radiation and physical harm and represent a source of carbon for heterotrophs. Under laboratory conditions, Knowles and Castenholz [ 121 ] proved that EPSs produced by Nostoc sp. CCMEE 6160 improved water stress tolerance of the naturally co-habiting microalga, Chlorella sp. CCMEE 6038, which does not produce EPSs.
In biological soil crusts (BSCs), Because EPSs are involved in water capture from both rainfall and non-rainfall sources, crust-covered soils have higher water content than their naked nearby equivalents. The abundance of EPSs was proven to be positively correlated with the water capture capability of the biological crusts. In addition, a significant difference in water-retaining capability after treating soil crust samples for EPM removal was detected. Following a significant water introduction, the swelling of the EPSs is reported to cause soil pore clogging, possibly leading to water run-off [ 126 ].
The EPSs intervene in preserving the stability of the membrane vesicles during cycles of drying and swelling, as well as stabilizing desiccation-related enzymes and molecules [ 99 ]. As an example, the addition in vitro of the EPSs of Nostoc commune CHEN to membrane vesicles prevented them from fusing, counteracting one of the unwanted outcomes of the rehydration process [ 17 ].
Bio-weathering processes
The excretion of EPSs is also key in lithic substrate colonization by epilithic and endolithic cyanobacteria and in the following bio-weathering processes. Surface-dwelling populations endure more UV, temperature, and water stress, which can be mitigated by colonizing subsurface niches. The capacity to modify stone surfaces is owing to their ability to adhere and penetrate within the rock pore spaces, causing exfoliation of the upper substrate layers and irreversible unaesthetic discoloration owing to pigment release. Several investigations aimed at defining the role of EPSs in the fouling caused by cyanobacterial colonization of stone artwork, to elaborate potential control strategies [ 127 ].
About 20–30% of stone deterioration has reportedly a biological origin. Stone weathering is carried out by microorganisms by penetrating and pushing apart the cracks in the mineral substrate through cycles of drying/swelling and warming/cooling. By swelling when wetted, the mucous secretions exert great pressure from within. At the same time, mineral dissolution takes place following the release of acidic compounds, Ca 2+ , OH − , and organic ligands [ 128 ].
In the first rock layers, EPM can concentrate metal cations and nutrients present at low concentrations, sequestering them directly from the substrate. Welch and Vandevivere [ 129 ] showed how microbial EPSs enhance the dissolution of feldspathic substrates while forming complexes with framework ions in solution. Indeed, in biofilms, electrostatic interactions produced by cations provide cohesion. Cations serve both as cross-linkers in the biofilm matrix and stimulate physiology-dependent attachment processes in microbial cells by acting as cellular cations and enzyme cofactors [ 17 ].
In a recent study, Plectonema , Gloeocapsa , Gloeocapsosis, and Leptolyngbya strains isolated from epilithic biofilms showed a good affinity for Ca, Mg, and Fe cations, although to different extents [ 120 ]. Divalent cations Ca and Mg cations form cross-bridges with the charged fractions of the EPS strands, increasing the cohesion of the secretions [ 127 ]. Additionally, the capability of selectively-immobilize toxic heavy metals could represent a defensive strategy to prevent them from reaching the cells [ 73 , 130 ].
Generally, marine EPS can play a variety of functions such as adhesive, structural, protection against abiotic stress, bio weathering processes, gliding motility, and nutrient repositories in phototrophic biofilms or biological soil crusts
Gliding motility of cyanobacteria
Gliding motility requires contact with a solid surface and occurs in a direction parallel to the long axis of the cell or filament. Although the mechanistic basis for gliding motility in cyanobacteria has not been established, recent ultrastructural work has helped to identify characteristic structural features that may play a role in this type of locomotion. Among these features are the distinct cell surfaces formed by specifically arranged protein fibrils and organelle-like structures, which may be involved in the secretion of mucilage during locomotion [ 17 ].
Cyanobacteria secrete slime while gliding. That was observed that the EPSs are extruded through junction pore complexes (NPCs), which are prokaryotic organelles with diameters ranging from 70 to 80 nm and 32 nm long, spanning the cell wall. A linked channel, 13 nm in diameter, spans the peptidoglycan layer. In Phormidium uncinatum and Anabaena variabilis , JPCs are located near the cell septa, at angles of 30-40°, related to the cell axis. Slime extrusion likely propels the cell forward [ 131 ]. Oscillin, a Ca-containing protein on the surface of Phormidium sp., possibly determines the channels that direct the EPS flow. If oscillin is arranged elliptically, the cell will rotate; if the filaments are arranged radially, the cell will not rotate [ 132 ]. Thirty to 40° related to the cell axis. Slime extrusion likely propels the cell forward [ 131 ]. Oscillin, a Ca-containing protein on the surface of Phormidium sp., possibly determines the channels that direct the EPS flow. If oscillin is arranged elliptically, the cell will rotate; if the filaments are arranged radially, the cell will not rotate [ 132 ].
As effective carbon sources
The composition of the producing community fraction, environmental conditions, and biochemical processes at the community level influence the chemical and physical characteristics of the EPSs. In oligotrophic conditions, EPSs represents a notable source of organic C available for cross-feeding processes. By these means, the activity of the producing organisms is balanced by the activity of the consumers, whereas C from EPSs is the primary substrate respired after rainfall events in deserts. In the aquatic environment, the nutrients can interact with EPSs in order to increase the rate of substance uptake concentrating the dissolved organic compounds to become available to support the microbial growth. In the aquatic environment, the nutrients can interact with EPSs to increase the rate of substance uptake concentrating the dissolved organic compounds to become available to support microbial growth. In the aquatic environment, the nutrients can interact with EPSs to increase the rate of substance uptake concentrating the dissolved organic compounds to become available to support microbial growth. The secretion of EPSs affects many ocean processes [ 106 ].
As cryoprotectant in Arctic areas
Bacteria are found in abundance in the bottom layers of the ice or brine channels and are often attached to detrital particles or to living microalgal cells. In addition, the high numbers of particle-associated bacteria found in sea ice may explain observations of underlying seawater being enriched in bacterial biomass relative to the open ocean. In particular, the EPS may have a cryoprotective role in brine channels of sea ice, where extremes of high salinity and low temperature impose pressures on microbial growth and survival [ 17 ].
Particularly, some investigations of sea ice microbial communities have found bacteria strongly associated with particles and have pointed out, as mentioned before, that microbial EPSs played an important role in cryoprotection [ 92 ].
EPSs from extremophiles, such as sea ice-microbial communities, ensure their function in strong particle attachment and, more significantly, cryoprotection [ 92 ]. This EPSs defend cells from harsh external environmental conditions such as high and low temperature, salinity, radiation, high and low pH, and so on. Extremophiles can thus endure the harmful effects of severe conditions thanks to their EPS coating [ 10 ]. EPS produced at 2°C and 10°C had a higher uronic acid content than that produced at 20°C. The availability of iron as a trace metal is of critical importance in the Southern Ocean, where it is known to limit primary production. Exopolymer in the brine channels might have provided buffering against harsh winter conditions and high salinity as well as cryoprotecting the microbes living there against ice crystal formation by depressing the ice nucleation temperature of water [ 109 ]. The availability of iron as a trace metal is of critical importance in the Southern Ocean, where it is known to limit primary production. Exopolymer in the brine channels might have provided buffering against harsh winter conditions and high salinity as well as cryoprotecting the microbes living there against ice crystal formation by depressing the ice nucleation temperature of water [ 109 ].
Large amounts of microbially generated EPSs have been found in sea ice and along the ice-water contact in Arctic locations [ 109 ]. Although diatoms were considered to dominate EPS generation in this system, this material was favorably associated with bacterial abundances. With its high polyhydroxy content, high concentrations of EPSs would lower the freezing point of water in low-temperature, high-salinity brine channels, especially near the cell, where exopolymer concentrations are highest [ 109 ]. Arctic sea ice in winter showed that even at temperatures as low as 20°C and salinity of 209 parts per thousand, active bacteria were found in the brine channels and were particle-associated [ 92 ]. As well, Mancuso-Nichols et al. [ 133 ] studied EPSs produced by sea-ice isolates that were shown, by molecular weight analysis, to be between 5 and 50 times larger than the average observed for other marine EPSs. 2004). As well as, Mancuso-Nichols et al. [ 133 ] studied EPSs produced by sea-ice isolates were shown, by molecular weight analysis, to be between 5 and 50 times larger than the average observed for other marine EPSs.
Mancuso-Nichols et al. [ 47 ] isolated a strain of Antarctic Pseudoalteromonas from sea ice that produced 30 times as much EPS at 2 and 10 °C compared with 20°C in liquid culture. Generally, members of this genus are among the dominant bacteria found in this environment as determined by cultivation-dependent and independent techniques [ 134 ]. The finding of Mancuso-Nichols et al. [ 47 ] supports the proposed hypothesis that EPS production by psychrotolerant bacteria may play an important role in the sea-ice microbial community. Whether this increased EPS production at low temperature is a specific cold adaptation mechanism for this strain requires further investigation. In addition, the EPS from Pseudoalteromonas strain CAM025 is polyanionic and may bind dissolved cations such as trace metals, and therefore the presence of bacterial EPS in the Antarctic marine environment may have important ecological implications [ 133 ]. Furthermore, EPS produced by some Antarctic bacterial isolates contain uronic acids and sulfate groups and may act as ligands for cations present as trace metals in the Southern Ocean environment, enhancing the primary production of microbial communities usually limited by poor availability of trace metals such as iron (Fe +3 ) [ 15 , 135 ]).
Further studies focusing on the biotechnological potential of EPSs produced by bacteria from the Antarctic marine environment have been reported in the literature to date. Pseudoalteromonas antarctica NF3 produces an exopolymeric compound of glycoprotein character that displays the ability to coat liposomes and provides protection against surfactants. Even among closely related strains, EPSs produced by Antarctic bacteria commonly found in the marine environment were diverse [ 133 ].
Role in deep sea
Deep-sea hydrothermal vents result from oceanic plate tectonic and submarine volcanic activities. At depths of 500 to 4000 m, they can be found at sea ridges or on subduction back-arc zones. Seawater is charged with metals and other substances such as hydrogen sulfide, hydrogen, ammonia, and carbon dioxide pouring out of chimneys made of precipitates at high temperatures (up to 350°C). The plume appears in varied intensities of white or black hue (white or black smokers) according to the fluid composition [ 136 ]. These habitats are transient due to crustal volcanic activity [ 137 ]. Some other active areas with a diffuse emission of warm or cold water also exist. Deep-sea ecosystems also include cold seeps and sediments or microbial mats [ 138 ].
Until subsurface hydrothermal systems were identified along mid-ocean ridges at depths greater than 2200 m, the deep sea (>1000 m) was assumed to be a biological desert [ 139 ]. Hot fumaroles, springs and sediments, and deep-sea vents are examples of geologic formations. Temperatures can range from 380°C within the fumarole to 2°C in the surrounding seawater in these conditions, where hydrostatic pressure averages 25×106 P and temperatures can range from 380°C within the fumarole to 2°C in the surrounding seawater [ 140 ]. The vents allow hot anaerobic waters rich in hydrogen sulfide and heavy metals to escape and mix with cold oxygenated seawater. The presence of heavy metals is a characteristic of the hydrothermal vent environment. Despite these environmental extremes, a complex food web based on chemosynthesis, including dense invertebrate populations supported by a rich microbial community of heterotrophic and autotrophic bacteria, was found near the vents [ 17 ].
Over the last few decades, these vent communities have yielded an increasing number of new genera and species of both deep-sea hyperthermophilic and mesophilic bacteria. Bacteria associated with deep-sea hydrothermal vent communities have demonstrated their ability to produce unusual extracellular polymers in an aerobic carbohydrate-based medium, and so far, 3 main EPS-producing genera have been identified: Pseudoalteromonas , Alteromonas , and Vibrio [ 114 ]. Surprisingly, strains isolated from deep-sea hydrothermal vents showed resistance to heavy metals. Their purified EPSs presented the capacity to bind metals and toxic substances [ 135 ].
Applications of microbial EPSs related to the marine environment
As previously mentioned, many EPSs have revealed interesting chemical compositions and so they are widely used in biotechnological applications and several industries in the different fields of medicine, foods, cosmetics, etc. [ 141 ]. As shown in Table 2 , there are several examples of commercial microbial EPSs that entered the market such as dextran (produced by labs such as Leuconostoc mesenteroides , xanthan gum (the EPS from Xanthomonas campestris , and curdlan (produced by Alcaligenes faecalis ) [ 27 ]. Only, this context will offer applications related to the marine environment and aquatic resources. Natural products have long been regarded as important sources of possible chemotherapeutic medicines. Examinations were expanded to include maritime territories in the search for new bioactive chemicals, mesenteroides , xanthan gum (the EPS from Xanthomonas campestris , gellan (produced by Pseudomonas elodea ), and curdlan (produced by Alcaligenes faecalis ) [ 27 ]. Only, this context will offer the applications related to marine environment and aquatic resources. Natural products have long been regarded as important sources of possible chemotherapeutic medicines. Examinations were expanded to include maritime territories in the search for new bioactive chemicals.
As anti-fouling agents
Biofouling is a special class of organic fouling and is the result of complex interactions between the substrate, dissolved substances, and microorganisms [ 143 ]. Usually, it is ascribed to the accumulation of microorganisms such as bacteria, algae, and fungi on hard surfaces forming the harmful biofilms, via a multi-step and complex formation process [ 100 ]. Indeed, the biofouling is one of the most serious problems in marine as a whole and specifically in seawater desalination because it is a very costly problem, keeping busy a billion-dollar industry providing biocides, cleaners, and anti-fouling materials worldwide [ 100 , 144 ].
Marine biopolymers including EPSs and chitosan EPS may be an effective inhibitor of the initial stages of biofilm formation and subsequent biofouling activity [ 145 ]. Under their wide range of metabolic activities and unique structural moieties, marine microbes are an important potential source of anti-fouling compounds. Chemical compounds can affect the bacterial structure of the biofilm and interfere with the larval settlement of higher organisms [ 143 ].
The EPS producers isolated from hydrothermal vents are relatively non-fastidious (aerobes, mesophiles, heterotrophs) [ 114 ]. Under laboratory conditions, some bacteria from these environments produce large amounts of EPSs, which offer massive potential for the exploitation of antifoulants. They showed strong anti-microbial and anti-fouling activities [ 143 ]. However, the EPSs do not contain toxic heavy metals or other molecules that adversely affect the local ecology. In addition, they can easily be produced using relatively simple bacterial cultivation protocols and commercially available fermentation equipment [ 106 ].
These polymers may be able to inhibit the larval settlement of marine macrofoulers in a non-toxic version to some extent. As a result, EPSs used as a permanent coating on other organic films may affect biofilm formation by preventing bacterial adhesion in naturally flowing seawater. Recent research has found that some EPSs when used at very low concentrations, can prevent bacterial adhesion and the formation of an active biofilm. The possibility of EPS inhibiting microfouling via steric hindrance mechanisms should be investigated further [ 146 ].
In wastewater treatment
Microbial EPSs can adsorb metal cations, as well as other dissolved substances, which can aid in heavy metal bioremediation. This could be useful in wastewater treatment systems. Biofilms can bind to and remove metals like copper, lead, nickel, and cadmium, for example. The metal specificity and binding affinity of EPS vary depending on polymer composition and environmental factors [ 147 ].
On the other hand, the flocculation step is considered a vital stage during the treatment of raw water from pollutants. It helps in the removal of dissolved organic substances and turbidity from water through the addition of chemical coagulants such as alum, ferric chloride, and synthetic organic polymers [ 148 ]. These coagulants have some drawbacks, including ineffectiveness in cold water, high procurement costs, complete or partial non-biodegradability, human health effects, the production of large amounts of sludge, and a significant impact on the pH of treated water. Furthermore, a direct link between the use of these chemical coagulants and the development of Alzheimer’s disease has been established [ 149 ]. In addition, partial degradation of synthetic coagulant polymers produces intermediate substances, which have some neurotoxic and carcinogenic effects [ 150 ]. Therefore, searching for alternative natural-based coagulants to avoid these disadvantages becomes an insistent issue. Natural coagulants were applied in water treatment and showed many advantages such as low cost, low toxicity, biodegradability, and small volumes of sludge [ 150 ].ts were applied in water treatment and showed many advantages such as low cost, low toxicity, biodegradability and small volumes of sludge [ 150 ].
Many natural coagulants are produced from microorganisms are composed of bio-macromolecules such as polysaccharides, proteins, lipids, and nucleic acids [ 151 ]. Most studies focused on the removal of only one type of pollutants using microbial coagulants such as heavy metals or dyes [ 152 ], while no more reports about multiple pollutants removal [ 153 ]. Many EPS-producing bacteria have been discovered in extreme marine environments with high levels of toxic elements such as sulfur and heavy metals. As a result, the EPSs they produce have a strong affinity for heavy metals and could be widely used in the bio-detoxification and wastewater industries to remove heavy metals. Additional rheological studies showed the uronic-rich EPS could be expected to have the ability for some heavy metal-binding and therefore applied in the bio-detoxification and wastewater treatment [ 17 ] or dyes [ 152 ], while no more reports about multiple pollutants removal [ 153 ]. Many EPS-producing bacteria have been discovered in extreme marine environments with high levels of toxic elements such as sulfur and heavy metals. As a result, the EPSs they produce have a strong affinity for heavy metals and could be widely used in the bio-detoxification and wastewater industries to remove heavy metals. Additional rheological studies showed the uronic-rich EPS could be expected to have ability for some heavy metal-binding and therefore applied in the bio-detoxification and wastewater treatment [ 17 ].
For example, the EPS formed by Alteromonas macleodii sub. sp, fijiensis also has this property. The viscosity of this EPS had the same order of magnitude of a commercial xanthan [ 154 ]. The native EPS, produced by A. infernus, shows a very strong affinity for heavy metals such as; Pb, Cd, and Zn. In addition, the EPS secreted by Cyanothece sp. ATCC 51142 is highly effective for metal removal from solutions and can remove different metals from industrial wastes [ 155 ]. The EPS produced by Alteromonas sp. strain 1644 showed strong selectivity between monovalent and divalent ions and exhibited a great affinity for divalent ions, such as Mg cations [ 156 ].
On the other side, synthetic flocculants used in wastewater treatment plants, such as Al 2 SO 4 and poly-AlCl 2 and organic synthetic polymers of polyacrylamide derivatives and polyethylene imine, have been known to possess adverse health effects such as; carcinogenicity, neurotoxicity and Alzheimer’s disease [ 157 ]. So, the microbial EPSs as flocculants with various properties were effectively applied as safe alternatives for chemical flocculants. Bioflocculants have been expected to be harmless to the environment because of their biodegradability [ 158 ]. Several workers have reported high flocculation efficiency mediated by the EPSs produced by Sorangium cellulosum NUST06, Virgibacillus sp., Bacillus sp., and Artrobacter sp., which were isolated from fresh and marine waters [ 159 , 160 ].
In addition, heavy metals adsorption by microbial EPSs is widely reported by other strains such as Bacillus firmus [ 161 ] and Paenibacillus validus MP5 [ 152 ]. Al-Wasify et al. [ 162 ] used EPSs from Bacillus licheniformis , B. insolitus, and B. alvei as natural coagulants during the coagulation-flocculation process. They discovered that when extracted EPSs were used as sole coagulant materials, they had a high removal efficiency and that when alum was added to bacterial EPSs, the removal efficiency increased. Recently, Szewczuk-Karpisz & Wiśniewska [ 163 ] studied the Sinorhizobium meliloti 1021 EPS flocculation efficiency relative to mineral oxide suspensions (Cr 2 O 3 , SiO 2 , and ZrO 2 ). Their data verified the application of S. meliloti EPS in wastewater treatment as a potential flocculant related to these solids.
The high removal efficiencies of the studied microbial EPSs as natural coagulants, on the other hand, may be attributed to strong adsorption with positive charges carrying metals such as heavy metals, debris, oily particles, organics, and mud, resulting in the formation of large-sized and heavy-weight flocs. During rapid and slow water mixing, these new flocs grew in size. This phenomenon allows rapid degradation of organics in water which decreases levels of organic pollution in water, turbidity level, and other related physicochemical parameters [ 17 ].
In bioremediation field
In the beginning, bioremediation is considered one of the most common applications for EPSs in many fields related to the marine environment [ 164 ]. This occurs because EPSs contain many functional groups, such as amine, phosphate, hydroxyl, carboxyl, and urinate, which increase the negative charge of EPSs and their ion exchange properties and flocculation activities, as well as the ability to coordinate with metal ions and form organic precipitation [ 165 ]. Furthermore, due to the labile nature of microbial EPSs and their ability to bind heavy metals, the bound metals are routed through the marine food chain, assisting in the bioaccumulation of metal pollutants in higher trophic animals [ 166 ]. Furthermore, due to the labile nature of microbial EPSs and their ability to bind heavy metals, the bound metals are routed through the marine food chain, assisting in the bioaccumulation of metal pollutants in higher trophic animals [ 166 ].
Therefore, one of the most essential applications of EPSs is the bioremediation of targeted pollutants such as heavy metals, polycyclic aromatic hydrocarbons, petroleum, nitroaromatics, polycyclic aromatic hydrocarbons, polychlorinated biphenyls, chlorinated phenols, and aliphatics [ 167 ].
One of the mechanisms by which organisms remove or accumulate heavy metals is biosorption. It is a fast and passive metal uptake process where the cells do not need to be alive. Adsorption, absorption, intracellular or extracellular accumulation, redox reaction, ion exchange, surface complexation, and precipitation are some of the mechanisms involved in biosorption [ 168 ]. Microbial EPS can bind with anion and cations, resulting in a candidate of choice for the bioremediation process [ 169 ]. In some remediation processes, EPS modified by chemical processes such as acetylation, methylation, phosphorylation, and sulfonylation are used [ 170 ]. Acetylation of EPS decides the selectivity of metal-binding [ 171 ]. The metal binding property of the EPS plays a significant role in metal remediation from wastewater [ 172 ].
The reports of Gupta & Diwan [ 173 ] demonstrated almost 85–95% of zinc, copper, and chromium removal using a consortium developed from activated sludge. They also reported that many Gram-negative bacterial consortia could remove 75–78% of zinc, lead, chromium, nickel, copper, cadmium, and cobalt within two hours. Immobilized EPS of Chryseomonas and Paenibacillus polymyxa showed the removal of cadmium, cobalt, copper, and lead [ 174 , 175 ]. Dead cell-bound EPS of Bacillus cereus , Bacillus pumilus , and Pentoea agglomerans showed 85.5–89% of chromium removal [ 176 ]. EPS of Acidithiobacillus ferrooxidans helps the organisms to bind with the mineral and thus extract metals from the sulfide ores [ 177 ]. Salehizadeh and Shojaosadati [ 178 ] reported the biosorption of copper (74.9%), lead (98.3%), and zinc (61.8%) by the EPS of Bacillus firmus . The EPS produced by Azotobacter chroococcum XU1 showed the sorption of lead (40.48%) and mercury (47.87%) [ 179 ]. The EPS of Ensifer meliloti , showed 89, 85, and 66% of lead, nickel, and zinc ion reduction, respectively [ 180 ]. Various marine bacteria are also reported for their metal removal ability. The specific structure and high uronic acid content impart an enhanced anionic property to marine bacterial EPS, which may be responsible for metal removal. EPS of Marinobacter sp. showed sorption of metals like lead and copper [ 166 ]. EPS from marine Enterobacter cloacae demonstrated the sorption of cadmium (65%), copper (20%), and hexavalent chromium (75%) [ 181 ]. Halomonas sp. associated with marine microalga was also reported to chelate metals such as calcium, aluminum, iron, and magnesium [ 45 ]. The EPS secreted by the Pseudoalteromonas sp. SM9913 showed the adsorption of Fe 2+ (85.00%), Zn 2+ (58.15%), Cu 2+ (52.77%), Co 2+ (48.88%), Mg 2+ (30.69%), Mn 2+ (25.67%), and Cr 6+ (5.15%) [ 182 ].
Because biofilm-mediated bioremediation is an effective and safe method for removing pollutants from water [ 183 ], apart from this, it is also used to enhance oil recovery [ 115 ]. As well as, some special applications like sludge settling and dewatering were demonstrated with EPSs [ 184 ]. However, their amazing examples that support the application of microbial EPSs in the bioremediation field, are as follows:
EPSs of Hansenula anomala CCY 38-1-22 bound 90% of the total amount of Cd ions absorbed by this resistant strain, while the sensitive strain of Saccharomyces cerevisiae CCY 21-4-100 accumulated this metal predominantly in the cellular compartments [ 185 ].
Fungal EPS from Flavodon flavus may serve in the degradation of toxic organic compounds by breaking down polycyclic aromatic hydrocarbons [ 186 ], Kumar et al., [ 97 ].
Each gram of pestan, a specific EPS produced by Pestalotiopsis sp. KCTC 8637, can absorb 120 mg of lead or 60 mg of Zn [ 187 ].
Pullulan extracted from Aureobasidium pullulans CH1 strain, was reported to bioadsorb metal (Cu, Fe, Zn, Mn, Pb, Cd, Ni, and Cr) [ 188 ].
Sulfated EPS secreted by a bacterium isolated from marine microbial mats has a very high affinity for binding to Cu and Fe [ 189 ].
EPS produced by the fungus; Colletotrichum sp. contributed to the removal of Cd and Pb ions by biosorption [ 167 ].
In petroleum industry
The petroleum industry, amazingly, uses bacterial xanthan gum in oil drilling, fracturing, and pipeline cleaning, and due to its excellent compatibility with salt and resistance to thermal degradation, it is advantageous as an additive in drilling fluids [ 102 ]. Xanthan gum outperforms other polymers in terms of viscosity, thickening, salt resistance, and contamination resistance; especially in the good drilling of sea, beach, high halide layer, and permafrost layer, xanthan gum has a remarkable effect in sludge treatment, completion fluid, and tertiary oil recovery, as well as a significant function for accelerating drilling speed and preventing thawing. This product, as a kind of ideal additive, has a bright future ahead of it [ 17 ].
The rheological characteristics of xanthan gum were measured in linear core flow tests. This constitutive flow behavior was used in a radial flow simulator to predict the invasion profile of xanthan gum in the formation. Radial flow tests were performed to validate the predictions from the simulator and to observe the effect of fluid loss additives such as starch and ground Berea. Therefore, xanthan gum has already been used in the different stages of the oil industry such as; the drilling industry; because its functions are adding viscosity and shearing force, improving the suspending power of drilling fluid which is essential in using functions of the drilling fluid, oil exploitation industry; due to it contains many essential conditions required for improving oil recovery rate. Xanthan gum is an excellent additive for oilfield drilling mud, and in the oil industry compared with polyacrylamide, carboxymethylcellulose, modified starch, and some plant polysaccharides, etc. has a clear technological advantage in oilfield development for its high ability to increase the viscosity, thickening, anti-salt, and anti-pollution [ 17 ].
Further, the rheological properties of the EPS secreted by the halophilic archaebacterium; Haloferax mediterranei showed a pseudoplastic behavior and a high apparent viscosity at relatively low concentrations and this viscosity is remarkably resistant to extremes of pH, temperature, or salinity. These characteristics make this EPS to be used for enhanced oil recovery and other applications, which require a very resistant thickening agent [ 190 ].
On the other side, microbial EPSs are used as biosurfactants and bioemulsifiers that attracted great attention because of their biodegradability [ 115 ]. Therefore, there is a promising technology for manipulating the function and structure of microbial environments existing in oil reservoirs known as; microbial enhanced oil recovery (MEOR). It is a biotechnology branch in which microbes are found to recover the additional oil from existing wells, thereby enhancing the petroleum production of an oil reservoir [ 115 ]. However, selected natural microbes producing bio-surfactants and/or specific EPSs are introduced into oil wells to produce harmless by-products, such as slippery natural substances or gases, all of which aid propel oil out of the well allowing a more amount to be recovered from the well. Genetically engineered Enterobacter cloacae are successfully used in MEOR [ 115 ].
The information provided in this review supports some general conclusion points regarding the characteristics of the EPSs produced by marine microbes and their roles, functions, and applications in the marine environment:
Increasing awareness of the environment and green technology might enable the use of microbes as a renewable and alternative resource of EPSs instead of synthetic and other EPSs.
Many marine microbes are promising resources for producing EPSs that can provide significant opportunities for newer roles, functions, and applications.
Cyanobacteria and microalgae can produce more complex EPSs than other EPS-producing microorganisms.
Different types of marine and extremophilic microbes should be further explored to harness their superior characteristics for creating novel EPSs with higher productivity and unique applications.
Availability of data and materials
All data of the current study are included in this article.
Miroshnichenko M, Bonch-Osmolovskaya E (2006) Recent developments in the thermophilic microbiology of deep-sea hydrothermal vents. Extremophiles 10:85–96
Article Google Scholar
Satpute SK, Banat IM, Dhakephalkar PK, Banpurkar AG, Chopade BA (2010a) Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol Adv 28(4):436–450
Zhang Z, Chen Y, Wang R, Cai R, Fu Y, Jiao N (2015) The fate of marine bacterial exopolysaccharide in natural marine microbial communities. PLoS One 10(11):e0142690
Staudt C, Horn H, Hempel DC, Neu TR (2004) Volumetric measurements of bacterial cells and extracellular polymeric substance glycoconjugates in biofilms. Biotechnol Bioeng 88(5):585–592
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8(9):881–890
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15(2):167–193
Suresh S, Mody P (2009) Microbial Exopolysaccharides: Variety and Potential Applications. In: Microbial Production of Biopolymers and Polymer Precursors. Caister Academic Press, Norfolk
Google Scholar
Mahapatra S, Banerjee D (2013a) Optimization of a bioactive exopolysaccharide production from endophytic Fusarium solani SD5. Carbohydrate Polym 97:627–634
Nanjani SG, Soni HP (2012) Diversity and EPS production potential of halotolerant bacteria from veraval and dwarka. IOSR J Pharm Biol Sci 2(2):20–25
Poli A, Anzelmo G, Nicolaus B (2010a) Bacterial exopolysaccharides from extreme marine habitats: production, characterization and biological activities. Marine Drugs 8:1779–1802
Nwodo UU, Green E, Okoh AI (2012) Bacterial exopolysaccharides: functionality and prospects. Int J Mol Sci 13(11):14002–14015
Wefky SM, Ibrahim HAH (2017) Production, characterization and valuable applications of exoploysaccharides from marine Bacillus subtilis SH1. Polish J Microbiol 66(4):449–461
Mohamed SS, Amer SK, Selim MS, Rifaat HM (2018) Characterization and applications of exopolysaccharide produced by marine Bacillus altitudinis MSH2014 from Ras Mohamed, Sinai, Egypt. Egypt J Basic Appl Sci 5(3):204–209
Wang C, Fan Q, Zhang X, Lu X, Xu Y, Zhu W et al (2018) Isolation, characterization, and pharmaceutical applications of an exopolysaccharide from Aerococcus uriaeequi. Marine Drugs 16(9):337–350
Mancuso-Nichols C, Bowman JP, Guezennec J (2005a) Effects of incubation temperature on growth and production of exopolysaccharides by an antarctic sea ice bacterium grown in batch culture. Appl Environ Microbiol 71(7):3519–3523
Moghannem SAM, Farag MMS, Shehab AM, Azab MS (2018) Environmental microbiology exopolysaccharide production from Bacillus velezensis KY471306 using statistical experimental design. Braz J Microbiol 49(3):452–462
Ibrahim HAH, Abo El Elaa GM, Hassan SW, Abdelatif HH, Abd Rabou MAA (2020) Microbial Exopolysaccharides: from Synthesis to Valuable Applications. LAMBERT Academic Publishing, p 149 ISBN: 978-3-620-2-51454-5
Duan X, Chi Z, Wang L, Wang X (2008) Influence of different sugars on pullulan production and activities of a -phosphoglucose mutase, UDPG- pyrophosphorylase and glucosyltransferase involved in pullulan synthesis in Aureobasidium pullulans Y68. Carbohydr Polym 73:587–593
Satpute S, Banpurkar A, Dhakephalkar P, Banat IM, Chopade BA (2010b) Methods for investigating biosurfactants and bioemulsifiers: A review. Crit Rev Biotechnol 30(2):127–144
Mishra A, Jha B (2009) Isolation and characterization of extracellular polymeric substances from microalgae Dunaliella salina under salt stress. Bioresour Technol 100:3382–3386
Zou X, Sun M, Guo X (2006) Quantitative response of cell growth and polysaccharide biosynthesis by the medicinal mushroom Phellinus linteus to NaCl in the medium. World J Microbiol Biotechnol 22:1129–1133
Poli A, Di Donato P, Abbamondi GR, Nicolaus B (2011) Synthesis, production, and biotechnological applications of exopolysaccharides and polyhydroxyalkanoates by archaea. Archaea 2011:1–13. https://doi.org/10.1155/2011/693253
Rinker KD, Kelly RM (1996) Growth physiology of the hyperthermophilic archaeon Thermococcus litoralis: development of a sulfur-free defined medium, characterization of an exopolysaccharide, and evidence of biofilm formation. Appl Environ Microbiol 62(12):4478–4485
Hartzell PL, Millstein J, Lapaglia C (1999) Biofilm formation in hyperthermophilic archaea. Methods Enzymol 310:335–349
Nicolaus B, Manca MC, Romano I, Lama L (1993) Production of an exopolysaccharide from two thermophilic archaea belonging to the genus Sulfolobus. FEMS Microbiol Lett 109(2-3):203–206
Koerdt A, Gödeke J, Berger J, Thormann KM, Albers S-V (2010) Crenarchaeal biofilm formation under extreme conditions. PLoS ONE 5(11):e14104. https://doi.org/10.1371/journal.pone.0014104
Nicolaus B, Kambourova M, Oner ET (2010) Exopolysaccharides from extremophiles: from fundamentals to biotechnology. Environm Technol 31(10):1145–1158
Anton J, Meseguer I, Rodriguez-Valera F (1988) Production of an extracellular polysaccharide by Haloferax mediterranei. Appl Environ Microbiol 54(10):2381–2386
Paramonov NA, Parolis LA, Parolis H, Boán IF, Antón J, Rodriguez Valera F (1998) The structure of the exocellular polysaccharide produced by the Archaeon Haloferax gibbonsii ATCC 33959. Carbohydr Res 309(1):89–94
Parolis H, Parolis LAS, Boan IF, Rodríguez-Valera F, Widmalm G, Mancac MC et al (1996) The structure of the exopolysaccharide produced by the halophilic Archaeon Haloferax mediterranei strain R4 (ATCC 33500). Carbohydr Res 295:147–156
Nicolaus B, Lama L, Esposito E (1999) Haloarcula spp able to biosynthesize exo- and endopolymers. J Ind Microbiol Biotechnol 23(6):489–496
Glazer AN, Nikaidō H (2007) Microbial Biotechnology: Fundamentals of Applied Microbiology, 2nd edn. Yashasvi Export, Cambridge, New Delhi
Book Google Scholar
Sutherland IW (1990) Biotechnology of microbial exopolysaccharide. Cambridge Universily Press, New York, p 1
Paulo EM, Vasconcelos MP, Oliveira IS, Affe HMJ, Ascimento RN, Melo IS et al (2012) An alternative method for screening lactic acid bacteria for the production of exopolysaccharides with rapid confirmation. Ciência e Tecnologia de Alimentos, Campinas 32(4):710–714
Laws A, Gu Y, Marshall V (2001) Biosynthesis, characterization, and design of bacterial exopolysaccharides from lactic acid bacteria. Biotechnol Adv 19:597–625
Singha TK (2012) Microbial extracellular polymeric substances: production, isolation and applications. IOSR J Pharmacy 2(2):276–281
Enos-Berlage JL, McCarter L (2000) Relation of capsular polysaccharide production and colonial cell organization to colony morphology in Vibrio parahaemolyticus. J Bacteriol 182(19):5513–5520
Ravaioli S, Campoccia D, Visai L, Pirini V, Cangini I, Corazzari T, Maso A, Poggio C, Pegreffi F, Montanaro L, Arciola CR (2011) Biofilm extracellular-DNA in 55 Staphylococcus epidermidis clinical isolates from implant infections. Int J Artific Organs 34(9):840–846
Philbe JL (2002) Nouveau microorganisme de la famille des Enterobacteriaceae. French National Patent FR 2840920
Freitas F, Alves VD, Reis MAM (2011) Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trend Biotechnol 29(8):388–398
Badel S, Bernardi T, Michaud P (2011) New perspectives for Lactobacilli exopolysaccharides. Biotechnol Adv 29:54–66
Zhenming C, Yan F (2005) Exopolysaccharides from marine bacteria. J Ocean Univ China 4(1):67–74
Jayaraman M, Seetharaman J (2003) Phsicochemical analysis of the exopolysaccharides produced by a marine biofouling bacterium, Vibrio alginolytics. Process Biochemistry 38(6):841–847
De Morais MG, Stillings C, Dersch R, Rudisile M, Pranke P, Costa JAV et al (2010) Preparation of nanofibers containing the microalga Spirulina (Arthrospira). Bioresour Technol 101:2872–2876
Gutierrez T, Biller DV, Shimmield T, Green DH (2012) Metal binding properties of the EPS produced by Halomonassp: TG39 and its potential in enhancing trace element bioavailability to eukaryotic phytoplankton. Biometals 25(6):1185–1194
Urai M, Yoshizaki H, Anzai H, Ogihara J, Iwabuchi N, Harayama S et al (2007) Structural analysis of mucoidan, an acidic extracellular polysaccharide produced by a pristane-assimilating marine bacterium, Rhodococcus erythropolis PR4. Carbohydr Res 342:927–932
Mancuso-Nichols CA, Garon S, Bowman JP, Raguénès G, Guézennec J (2004) Production of exopolysaccharides by Antarctic marine bacterial isolates. J Appl Microbiol 96(5):1057–1066
Al-Nahas MO, Darwish MM, Ali AE, Amin MA (2011) Characterization of an exopolysaccharide-producing marine bacterium, isolate Pseudoalteromonas sp. AM. Afr J Microbiol Res 5(22):3823–3831
Selim MS, Mohamed SS, Shimaa RH, El Awady ME, El Sayed OH (2015) Screening of bacterial antioxidant exopolysaccharides isolated from Egyptian habitats. J Chem Pharmaceutical Res 7(4):980–986
El Essawy A, Abu Shady HK, Abu El Kher AM, Mahmoud MHM (2016) Antimicrobial, anticoagulant on fibrinolytic and prebiotic activities of exopolysaccharide produced by marine Klebsiella sp. Egypt. J Exper Biol 12(2):267–274
Abdelnasser SM, Yahya SM, Mohamed WF, Asker MM, Abu Shady HM, Mahmoud MG et al (2017) Antitumor exopolysaccharides derived from novel marine Bacillus: isolation, characterization sspect and biological activity. Asian Pac J Cancer Prev 18(7):1847–1854
Abdrabo MAA, Ibrahim HAH, Hassan SWM, Abdul-Raouf UM (2018) Antimicrobial and anti-tumor activities of exopolysaccharides produced by the biofilm of marine Halomonas saccharevitans AB2 isolated from Suez Gulf, Egypt. Egypt J Aquatic Biol Fisheries 22(5):99–119
Selim MS, Amer SK, Mohamed SS, Mounier MM, Rifaat HM (2018) Production and characterization of exopolysaccharide from Streptomyces carpaticus isolated from marine sediments in Egypt and its effect on breast and colon cell lines. J Genet Eng Biotechnol 16:23–28
Ali MA, Hassan SWM, Ibrahim HAH, Abdul-Raouf UM (2019) Optimization of exoploysaccharides production from marine Pseudomonas mendocina AB1with emphasis on different valuable applications. J Ecol Health Environ 7(1):7–20
Mahapatra S, Banerjee D (2013b) Fungal exopolisaccharide: production, composition and applications. Microbiol Insights 6:1–16
Vasconcelos AFD, Nilson KNK, Dekker RFH, Barbosa AM, Carbonero ER, Silveira JLM et al (2008) Three exopolysaccharides of the β-(1-6)-D-glucan type and a β-(1-3;1-6)-D-glucan produced by strains of Botryosphaeria rhodina isolated from rotting tropical fruit. Carbohyd Res 343:2481–2485
Chen Y, Mao W, Gao Y, Teng X, Zhu W, Chen Y et al (2013a) Structural elucidation of an extracellular polysaccharide produced by the marine fungus Aspergillus versicolor. Carbohydrate Polymer 93(2):478–483
Mahapatra S, Banerjee D (2012) Structural elucidation and bioactivity of a novel exopolysaccharide from endophytic Fusarium solani SD5. Carbohydrate Polym 90:683–689
Guo S, Mao W, Li Y, Tian J, Xu J (2013) Structural elucidation of the exopolysaccharide produced by fungus Fusarium oxysporum Y24-2. Carbohydr Res 365:9–13
Chen Y, Mao W, Wang B, Zhou L, Gu Q, Chen Y et al (2013b) Preparation and characterization of an extracellular polysaccharide produced by the deep-sea fungus Penicillium griseofulvum. Bioresour Technol 132:178–181
Osinska-Jaroszuk M, Jarosz-Wilkołazka A, Jaroszuk-Sciseł J, Szałapata K, Nowak A, Jaszek M et al (2015) Extracellular polysaccharides from Ascomycota and Basidiomycota: production conditions, biochemical characteristics, and biological properties. World J Microbial Biotechnol 31:1823–1844
Gientka I, Bzducha-Wróbel A, Stasiak-Różańska L, Agnieszka A, Błażejak BS (2016) The exopolysaccharides biosynthesis by Candida yeast depends on carbon sources. Electron J Biotechnol 22:31–37
Bao Y, Liang X, Li R, Qin L, Sheng W, Bao BX (2010) Screening and identification of exopolysaccharide-producing yeasts. Acta Microbiol Sinica 50(2):278–283
Pavlova K, Panchev I, Kuncheva M, Nikolova M (2009) Production of an exopolysaccharide by Antarctic yeast. Folia Microbiol 54(4):343–348
Kuncheva M, Pavlova K, Panchev I, Dobreva S (2007) Emulsifying power of mannan and glucomannan produced by yeasts. Int J Cosmet Sci 29(5):377–384
Chen Z, Shi J, Yang X, Liu Y, Nan B, Wang Z (2016) Isolation of exopolysaccharide-producing bacteria and yeasts from Tibetan kefir and characterisation of the exopolysaccharides. Int J Dairy Technol 69(3):410–417
Poli A, Anzelmo G, Tommonaro G, Pavlova K, Casaburi A, Nicolaus B (2010b) Production and chemical characterization of an exopolysaccharide synthesized by psychrophilic yeast strain Sporobolomyces salmonicolor AL 1 isolated from Livingston Island, Antarctica. Folia Microbiol 55(6):576–581
Rusinova-Videva S, Pavlova K, Panchev I, Kuncheva M (2014) Effect of different factors on biosynthesis of exopolysaccharide from Antarctic yeast. Biotechnol Biotechnological Equipment 24(2):507–511
de Jesus Raposo MF, de Morais RMSC, de Morais AMB (2013) Bioactivity and applications of sulphated polysaccharides from marine microalgae. Marine Drugs 11:233–252
Wotton RS (2004) The ubiquity and many roles of exopolymers (EPS) in aquatic systems. Scientia Marina 68:13–21
De Philippis R, Vincenzini M (1998) Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol Rev 22:151–175
Aboal M, Marco S, Chaves E, Mulero I, Garcia-Ayala A (2012) Ultrastructure and function of stalks of the diatom Didymosphenia geminata. Hydrobiologia 695:17–24
De Philippis R, Sili C, Paperi R, Vincenzini M (2001) Exopolysaccharide-producing cyanobacteria and their possible exploitation. J Appl Phycol 13:293–299
Kawaguchi T, Decho AW (2001) Potential roles of extracellular polymeric secretions (EPS) in regulating calcication - A study of marine stromatolites, Bahamas. Thalassas 17(2):11–19
Kawaguchi T, Decho AW (2000) Biochemical characterization of cyanobacterial extracellular polymers (EPS) from modern marine stromatolites (Bahamas). Prep Biochem Biotechnol 30(4):321–330
Matsui MS, Muizzuddin N, Arad S, Marenus K (2003) Sulfated polysaccharides from red microalgae have anti-inflammatory properties in vitro and in vivo. Appl Biochem Biotechnol 104:13–22
Arad S, Levy-Ontman O (2010) Red microalgal cell wall polysaccharides: biotechnological aspects. Curr Opin Biotechnol 21:358–364
Raza W, Makeen K, Wang Y, Xu Y, Qirong S (2011) Optimization, purification, characterization and antioxidant activity of an extracellular polysaccharide produced by Paenibacillus polymyxa SQR-21. Bioresour Technol 102:6095–6103
Laurienzo P (2010) Marine polysaccharides in pharmaceutical applications: an overview. Marine Drugs 8:2435–2465
Ruas-Madiedo P, de los Reyes-Gavilan CG (2005) Invited Review: Methods for the screening, isolation, and characterization of exopolysaccharides produced by lactic acid bacteria. J Wood Sci 88(3):843–856
Sameera V (2011) Novel techniques in the production of industrially imperative products. J Microbial Biochem Technol. 1. https://doi.org/10.4172/1948-5948.R1-003
Ma Z, Cui F, Gao X, Zhang J, Zheng L, Jia L (2015) Purification, characterization, antioxidant activity and anti-aging of exopolysaccharides by Flammulina velutipes SF-06. Antonie Van Leeuwenhoek 107(1):73–82
Yadava KL, Rahi DK, Soni SK, Rahib S (2012) Diversity of exopolysaccharide producing fungi from foot hills of shivalik ranges of Chandigarh capital region. Res Biotechol 3(4):11–18
Di Pippo F, Ellwood NTW, Gismondi A, Bruno L, Rossi F, Magni P et al (2013) Characterization of exopolysaccharides produced by seven biofilm-forming cyanobacterial strains for biotechnological applications. J Appl Phycol 25:1697–1708
De Philippis R, Margheri MC, Pelosi E, Ventura S (1993) Exopolysaccharide production by an unicellular cyanobacterium isolated from a hypersaline habitat. J Appl Phycol 5:387–394
Freire-Nordi CS, Vieira AAH, Nakaie CR, Nascimento OR (2006) Effect of polysaccharide capsule of the microalgae Staurastrum iversenii var. americanum on diffusion of charged and uncharged molecules, using EPS technique. Braz J Phys 36(1A):75–82
Abdullahi AS, Underwood GCJ, Gretz MR (2006) Extracellular matrix assembly in diatoms (Bacillariophyceae). V. Environmental effects on polysaccharide synthesis in the model diatom, Phaeodactylum tricornutum. J Phycol 42:363–378
WHO (2003) Diet, nutrition and the prevention of chronic diseases. Report of a Joint WHO/FAO Expert Consultation. Technical Report Series No. 916, Geneva
Silveira ML, Smiderle FR, Agostini F, Pereira EM, Bonatti-Chaves M, Wisbeck E et al (2015) Exopolysaccharide produced by Pleurotus sajorcaju: its chemical structure and anti-inflammatory activity. Int J Biol Macromol 75:90–96
Mishra A, Kavita K, Jha B (2011) Characterization of extracellular polymeric substances produced by micro-algae Dunaliella salina. Carbohydrate Polym 83:852–857
Sutherland IW (2001) Microbial polysaccharides from Gram-negative bacteria. Int Dairy J 11:663–674
Junge K, Eicken H, Deming JW (2004) Bacterial activity at -2 to -20°C in Arctic wintertime sea ice. Appl Environ Microbiol 70(1):550–557
Branda SS, Vik Å, Friedman L, Kolter R (2005) Biofilms: the matrix revisited. Trends Microbiol 13:20–26
Ghosh PK, Maiti TK (2016) Structure of extracellular polysaccharides (EPS) produced by Rhizobia and their functions in legume bacteria symbiosis. Achiev Life Sci 10(2):136–143
Harimawan A, Ting Y-P (2016) Investigation of extracellular polymeric substances (EPS) properties of P. aeruginosa and B. subtilis and their role in bacterial adhesion. Colloids Surf B Biointerfaces 146:459–467
Czaczyk K, Myszka K (2007) Biosynthesis of extrace llul ar polymeric substances (EPS) and its role in microbial biofilm formation. Polish J Environ Stud 16(6):799–806
Kumar MA, Anandapandian KTK, Parthiban K (2011) Production and characterization of exopolysaccharides (EPS) from biofilm forming marine bacterium. Braz Arch Biol Technol 54(2):259–265
Lin JC-T, Lee D-J, Huang C (2010) Membrane fouling mitigation: Membrane cleaning. Science 45:858–872
Flemming HC, Wingender J (2002) Extracellular polymeric substances: structure, ecological functions, technical relevance. In: Bitton G (ed) Encyclopedia of Environmental Microbiology. Wiley, New York, pp 1223–1231
Zondervan E, Roffel B (2007) Evaluation of different cleaning agents used for cleaning ultra-filtration membranes fouled by surface water. J Membrane Sci 216:67–79
Kim S-K (2014) Marine Carbohydrates: Fundamentals and Applications, Part 1, Volume 72 of Advances in Food and Nutrition Research. Academic Press, p 246 ISBN: 0128003669, 9780128003664
Chen X, Stewart PS (2002) Role of electrostatic interactions in cohesion of bacterial biofilms. Appl Microbiol Biotechnol 59(6):718–720
Algburi A, Comito N, Kashtanov D, Dicks LMT, Chikindas ML (2017) Control of biofilm formation: antibiotics and beyond. Appl Environ Microbiol 83(1-15):e02508–e02516
Decho AW (1990) Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes. In: Barnes M (ed) Oceanography and Marine Biology: an Annual Review. Aberdeen Univ Press, Aberdeen, pp 73–153
Daegelen P, Studier FW, Lenski RE, Kim JF (2009) Tracing ancestors and relatives of Escherichia coli B, and the derivation of B strains REL606 and BL21 (DE3). J Mol Biol 394:634–643
Decho AW, Gutierrez T (2017) Microbial Extracellular Polymeric Substances (EPSs) in Ocean Systems. Front Microbiol 8:922–934
Tourney J, Ngwenya BT (2014) The role of bacterial extracellular polymeric substances in geomicrobiology. Chem Geol 386:115–132
Elsakhawy TA, Sherief FA, Abd-El-Kodoos RY (2018) Marine microbial polysaccharides: environmental role and applications. Environ Biodivers 1:61–70
Krembs C, Eicken H, Junge K, Deming JW (2002) High concentrations of exopolymeric substances in Arctic winter sea ice: implications for polar ocean carbon cycle and cryoprotection of diatoms. Deep-Sea Res I 49:2163–2181
Passow U (2002) Transparent exopolymer particles (TEP) in aquatic environments. Prog Oceanogr. 55:287–333
de Caire GZ, de Cano MS, de Mule MCZ, Palma RM, Colombo K (1997) Exopolysaccharide of Nostoc muscorum (cyanobacteria) in the aggregation of soil particles. J Appl Phycol 9:249–253
Stal LJ (2003) Microphytobenthos, their Extracellular Polymeric Substances, and the Morphogenesis of Intertidal Sediments. Geomicrobiol J 20:463–478
Dobretsov S, Dahms H-U, Qian P-Y (2006) Inhibition of biofouling by marine microorganisms and their metabolites. Biofouling 22:43–54
Guezennec J (2002) Deep-sea hydrothermal vents: A new source of innovative bacterial exopolysaccharides of biotechnological interest? J Ind Microbiol Biotechnol 29:204–208
Sun S, Zhang Z, Luo Y, Zhong W, Xiao M, Yi W et al (2011) Exopolysaccharide production by a genetically engineered Enterobacter cloacae strain for microbial enhanced oil recovery. Bioresour Technol 102:6153–6158
Dissett DL, Chatterjee R, Hannon DP (1990) Photoprotective effect of superoxide scavenging antioxidants against ultraviolet radiation-induced chronic skin damage in the hairless mouse. Photodermatol Photoimmunol Photomed 7:56–62
Kim KC, Kim IG (1999) Ganoderma lucidum extract protects DNA from strand breakage caused by hydroxyl radical and UV irradiation. Int J Mol Med 4:273–277
Lee BC, Bae JT, Pyo HB, Choe TB, Kim SW, Hwang HJ, Yun JW (2003) Biological activities of the polysaccharides produced from submerged culture of the edible Basidiomycete Grifola frondosa. Enzyme Microb Technol 32:574–581
Roeselers G, Norris TB, Castenholz RW, Rysgaard S, Glud RN, Kühl M, Muyzer G (2007) Diversity of phototrophic bacteria in microbial mats from Arctic hot springs (Greenland). Environ Microbiol 9:26–38
Rossi F, Micheletti E, Bruno L, Adhikary SP, Albertano P, De Philippis R (2012) Characteristics and role of the exocellular polysaccharides produced by five cyanobacteria isolated from phototrophic biofilms growing on Indian stone monuments. Biofouling 28:215–224
Knowles EJ, Castenholz RW (2008) Effect of exogenous extracellular polysaccharides on the desiccation and freezing tolerance of rock-inhabiting phototrophic microorganisms. FEMS Microbiol Ecol 66:261–270
Wierzchos J, Davila AF, Sánchez-Almazo IM, Hajnos M, Swieboda R, Ascaso C (2012) Novel water source for endolithic life in the hyperarid core of the Atacama Desert. Biogeosciences 9:2275–2286
Pereira S, Zille A, Micheletti E, Moradas-Ferreira P, De Philippis R, Tamagnini P (2009) Complexity of cyanobacterial exopolysaccharides: composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol Rev 33:917–941
Satoh K, Hirai M, Nishio J, Yamaji T, Kashino Y, Koike H (2002) Recovery of photosynthetic systems during rewetting is quite rapid in a terrestrial cyanobacterium, Nostoc commune. Plant Cell Physiol 43:170–176. https://doi.org/10.1093/pcp/pcf020
Tamaru Y, Takani Y, Yoshida T, Sakamoto T (2005) Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc commune. Appl Environ Microbiol 71(11):7327–7333
Colica G, De Philippis R (2013) Exopolysaccharides from cyanobacteria and their possible industrial applications. In: Sharma NK, Rai AK, Stal LJ (eds) Cyanobacteria an economic perspective. Wiley, Chichester, pp 197–208
Chapter Google Scholar
Bellezza S, Albertano P, De Philippis R, Paradossi G (2006) Exopolysaccharides of two cyanobacterial strains from Roman hypogea. Geomicrobiol J 23:301–310
Pointing SB, Jayne Belnap J (2012) Microbial colonization and controls in dryland systems. Nat Rev Microbiol 10:551–562
Welch SA, Vandevivere P (1994) Effect of microbial and other naturally occurring polymers on mineral dissolution. J Geomicrobiol J 12(4):227–238
Micheletti E, Pereira S, Mannelli F, Moradas-Ferreira P, Tamagnini P, De Philippis R (2008) Sheathless mutant of cyanobacterium Gloeothece sp. Strain PCC 6909 with increased capacity to remove copper ions from aqueous solutions. Appl Environ Microbiol 74:2797–2804
Wolgemuth C, Oster G (2004) The Junctional Pore Complex and the Propulsion of Bacterial Cells. J Mol Microbiol Biotechnol 7(1-2):72–77
Hoiczyk E, Baumeister W (1998) The junctional pore complex, a prokaryotic secretion organelle, is the molecular motor underlying gliding motility in cyanobacteria. Curr Biol 8(21):1161–1168
Mancuso-Nichols C, Lardiere SD, Bowman JP, Nichols PD, Gibson JAE, Guézennec J (2005c) Chemical characterization of exopolysaccharides from Antarctic marine bacteria. Microb Ecol 49:578–589
Brinkmeyer R, Knittel K, Jürgens J, Weyland H, Amann R, Helmke E (2003) Diversity and structure of bacterial communities in Arctic versus Antarctic pack ice. Appl Environ Microbiol 69:6610–6619
Mancuso-Nichols C, Guezennec J, Bowman JP (2005b) Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents. Marine Biotechnol 7:253–271
Burgaud G, Meslet-Cladière L, Barbier G, Edgcomb VP (2014) Astonishing fungal diversity in deep-sea hydrothermal ecosystems: an untapped resource of biotechnological potential? In: La Barre S, Kornprobst JM (eds) Outstanding Marine Molecules. Wiley-VCH, Weinheim, pp 85–98
Van Dover CL, German CR, Speer KG, Parson LM, Vrijenhoek RC (2002) Evolution and biogeography of deep-sea vent and seep invertebrates. Science 295:1253–1257
Jannasch HW, Taylor CD (1984) Deep-sea microbiology. Ann Rev Microbiol 38:487–487
Snelgrove PVR, Butman CA, Grassle JF (1995) Potential flow artifacts associated with benthic experimental gear: Deep-sea mudbox examples. J Marine Res 53(5):821–845
Yayanos AA (2001) Deep-sea piezophilic bacteria. Methods Microbiol 30:615–637
Di Donato P, Poli A, Taurisano V, Abbamondi GR, Nicolaus B, Tommonaro G (2016) Recent advances in the study of marine microbial biofilm: from the involvement of quorum sensing in its production up to biotechnological application of the polysaccharide fractions. J Marine Sci Eng 4(34):1–14
Mishra A, Jha B (2013) Microbial Exopolysacchrides. In: Rosenberg E, DeLong EF, Thompson F, Lory S, Stackebrandt E (eds) The Prokaryotes: Applied Bacteriology and Biotechnology, 4th edn. Springer, Berlin, Heidelberg, pp 179–192
Satheesh S, Ba-akdah MA, Al-Sofyani AA (2016) Natural antifouling compound production by microbes associated with marine macroorganisms. Electron J Biotechnol 21:26–35
Liua CX, Zhanga DR, Hea Y, Zhaob XS, Baia R (2010) Modification of membrane surface for anti-biofouling performance: Effect of anti-adhesion and anti-bacteria approaches. J Membrane Sci 346(1):121–130
Huang R, Du Y, Zheng L, Liu H, Fan L (2004) A new approach to chemically modified chitosan sulfates and study of their influences on the inhibition of Escherichia coli and Staphylococcus aureus growth. Reactive Funct Polym 59:41–51
Watson MG, Scardino AJ, Zalizniak L, Shimeta J (2017) Inhibition of invertebrate larval settlement by biofilm ciliates. Marine Ecol Progr Series 557:77–90
Pal A, Paul AK (2008) Microbial extracellular polymeric substances: central elements in heavy metal bioremediation. Indian J Microbiol 48:49–64
Kang M, Kamei T, Magara Y (2003) Comparing polyaluminium chloride and ferric chloride for antimony removal. Water Res 37:4171–4179
Flaten TP (2001) Aluminum as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res Bull J 55:187–196
Oladoja NA (2015) Headway on natural polymeric coagulants in water and wastewater treatment operations. J Water Process Eng 6:174–192
He B, Ye J, Yin H, Qin H, Yu L, Zhang N, Peng H (2011) Production and Characteristics of Bioflocculant from Azotobacter. In: 5th International Conference on Bioinformatics and Biomedical Engineering (iCBBE), May 2011, pp 1–6
Rawat M, Rai JPN (2012) Adsorption of heavy metals by Paenibacillus validus strain MP5 isolated from industrial effluent-polluted soil. Bioremed J 16(2):66–73
Li N, Wang Y, Zhu P, Liu., Z., Guo, B., & Ren, J. (2015) Improvement of exopolysaccharide production in Lactobacillus casei LC2W by overexpression of NADH oxidase gene. Microbiol Res 171:73–77
Raguenes G, Pignet P, Gauthier G, Peres A, Christen R (1996) Description of a new polymer-secreting bacterium from a deep-sea hydrothermal vent, Alteromonas macleodii subsp fijiensis, and preliminary characterization of the polymer. Appl Environ Microbiol 62:67–73
Shah V, Ray A, Ray N, Madarnwar D (2000) Characterization of the extracellular polysaccharide produced by a marine cyanobacterium; Cyanothece sp. ATCC 51142, and its exploitation toward metal removal from solutions. Curr Microbiol 40:274–278
Bozzi L, Milas M, Tinaudo M (1996) Solution and gel rheology of a new exopolysaccharide excreted by the bacterium Aeromonas sp. strain 1644. Int J Biolog Macromol 18:83–91
Zhang M, Cui SW, Cheung PCK, Wang Q (2007) Antitumor polysaccharides from mushrooms: a review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci Technol 18:4–19
Zhang J, Wang R, Jiang P, Liu Z (2002) Production of an exopolysaccharide bioflocculant by Sorangium cellulosum. Lett Appl Microbiol 34(3):178–181
Cosa S, Mabinya LV, Olaniran OA, Okoh OO, Bernard K, Deyzel S et al (2011) Bioflocculant production by Virgibacillus sp. Rob isolated from the bottom sediment of Algoa Bay in the Eastern Cape South Africa. Molecules 16:2431–2442
Mabinya VL, Cosa S, Nwodo UU, Okoh AI (2012) Studies on bioflocculant production by Arthrobacter sp. Raats, a fresh water bacterium isolated from Tyume River, South Africa. Int J Mol Sci 13:1054–1065
Salehizadeh H, Shojaosadati SA (2003a) Removal of metal ions from aqueous solution by polysaccharide produced from Bacillus firimus. Water Resourc 37(17):4231–4235
Al-Wasify RS, Al-Sayed AA, Saleh SM, Aboelwafa AM (2015) Bacterial exopolysaccharides as new natural coagulants for surface water treatment. Int J Pharm Tech Res 8(9):198–207
Szewczuk-Karpisz K, Wiśniewska M (2018) Flocculation efficiency of the Sinorhizobium meliloti 1021 exopolysaccharide relative to mineral oxide suspensions-a preliminary study for wastewater treatment. Separation Purific Technol 201:51–59
Casillo A, Lanzetta R, Parrilli M, Corsaro MM (2018) Exopolysaccharides from marine and marine extremophilic bacteria: structures, properties, ecological roles and applications. Marine Drugs 16(69):1–34
Abdel-Aziz SM, Hamed HA, Mouafi FE, Gad AS (2012) Acidic pH-shock induces the production of an exopolysaccharide by the fungus Mucor rouxii: utilization of Beet Molasses. New York Sci J 5(2):52–61
Bhaskar PV, Bhosle NB (2006) Bacterial extracellular polymeric substance (EPS): a carrier of heavy metals in the marine food-chain. Environ Int 32(2):191–198
Da Silva, L.J., de Rezende P.F., do Amaral LA, Garcia-Cruz CH (2014). Biosorption of cadmium(II) and lead(II) from aqueous solution using exopolysaccharide and biomass produced by Colletotrichum sp. Desalination Water Treatment, 52, 7878-7886.
Dave SR, Upadhyay KH, Vaishnav AM, Tipre DR (2020) Exopolysaccharides from marine bacteria: production, recovery and applications. Environ Sustainabil 3:139–154
Saikia U, Bharanidharan R, Vendhan E, Yadav S, Shankar S (2013) A brief review on the science, mechanism and environmental constraints of microbial enhanced oil recovery (MEOR). Int J Chem Technol Res 5(3):1205–1212
Desbrieres J, Peptu CA, Savin CL, Popa M (2018) Chemically modified polysaccharides with applications in nanomedicine. In: Popa V
Sutherland IW (1983) Extracellular polysaccharides. In: Rehm H, Reed G, Dellwag H (eds) Biotechnology: biomass, microorganisms for special applications, microbialproducts I, energy from renewable resources. Verlag Chemie Gmbh, Wienheim, pp 531–574
Choi SB, Yun YS (2006) Biosorption of cadmium by various types of dried sludge: an equilibrium study and investigation of mechanisms. J Hazard Mater 138(2):378–383
Gupta P, Diwan B (2017) Bacterial Exopolysaccharide mediated heavy metal removal: a review on biosynthesis, mechanism and remediation strategies. Biotechnol Rep 13:58–71
Acosta MP, Valdman E, Leite SG, Battaglini F, Ruzal SM (2005) Biosorption of copper by Paenibacillus polymyxa cells and their exopolysaccharide. World J Microbiol Biotechnol 21(6-7):1157–1163
Ozdemir G, Ceyhan N, Manav E (2005) Utilization of an exopolysaccharide produced by Chryseomonas luteola TEM05 in alginate beads for adsorption of cadmium and cobalt ions. Bioresour Technol 96(15):1677–1682
Sultan S, Mubashar K, Faisal M (2012) Uptake of toxic Cr (VI) by biomass of exopolysaccharides producing bacterial strains. Afr J Microbiol Res 6(13):3329–3336
Yu RL, Yang OU, Tan JX, Wu FD, Jing SU, Lei MI, Zhong DL (2011) Effect of EPS on adhesion of Acidithiobacillus ferrooxidanson chalcopyrite and pyrite mineral surfaces. Trans Nonferrous Metal Socity 21(2):407–412
Salehizadeh H, Shojaosadati SA (2003b) Removal of metal ions from aqueous solution by polysaccharide produced from Bacillus firmus. Water Res 37(17):4231–4235
Rasulov BA, Yili A, Aisa HA (2013) Biosorption of metal ions by exopolysaccharide produced by Azotobacter chroococcum XU1. J Environ Protect 4(9):989
Lakzian A, Berenji AR, Karimi E, Razavi S (2008) Adsorption capability of lead, nickel and zinc by exopolysaccharide and dried cell of Ensifermeliloti. Asian J Chem 20(8):6075
Iyer A, Mody K, Jha B (2005) Biosorption of heavy metals by a marine bacterium. Marine Poll Bull 50(3):340–343
Qin G, Zhu L, Chen X, Wang PG, Zhang Y (2007) Structural characterization and ecological roles of a novel exopolysaccharide from the deep-sea psychrotolerant bacterium Pseudoalteromonas sp. SM9913. Microbiology 153:1566–1572
Singh R, Paul D, Jain RK (2006) Biofilms: implications in bioremediation. Trends Microbiol 14:389–397
Subramanian BS, Yan S, Tyagi RD, Surampalli RY (2010) Extracellular polymeric substances (EPS) producing bacterial strains of municipal wastewater sludge: isolation, molecular identification, EPS characterization and performance for sludge settling and dewatering. Water Res 44:2253–2266
Breierová E, Vajcziková I, Sasinková V, Stratilová E, Fišera M, Gregor T et al (2002) Biosorption of cadmium ions by different yeast species. Z Naturforsch 57c:634–639
Raghukumar C, Shailaja MS, Parameswaran PS, Singh SK (2006) Removal of polycyclic aromatic hydrocarbons from aqueous media by the marine fungus NIOCC #312: involvement if lignin-degrading enzymes and exopolysaccharides. Indian J Marine Sci 35(4):373–379
Moon SH, Park CS, Kim YJ, Park YI (2006) Biosorption isotherms of Pb(II) and Zn (II) on Pestan, an extracellular polysaccharide, of Pestalotiopsis sp. KCTC 8637P. Process Biochem 41(2):312–316
Radulović MĐ, Cvetković OG, Nikolić SD, Đorđević DS, Jakovljević DM, Vrvić MM (2008) Simultaneous production of pullulan and biosorption of metals by Aureobasidium pullulans strain CH-1 on peat hydrolysate. Bioresour Technol 99:6673–6677
Mironescu ID, Mironescu M, Georgescu C, Ranga IN, Iancu ML, Oprean LN (2011) Analysis of the extracellular polysaccharide-based structures produced by a halophylic archaeon. Bull UASVM Anim Sci Biotechnol 68(1-2):346–351
Moppert X, Le Costaouec T, Raguenes G, Courtois A, Simon-Colin C, Crassous P, Costa B et al (2009) Investigations into the uptake of copper, iron and selenium by a highly sulphated bacterial exopolysaccharide isolated from microbial mats. J Ind Microbiol Biotechnol 36(4):599–604
Download references
Acknowledgements
We would like to thank the members of Marine Microbiology Department, National Institute of Oceanography and Fisheries, Egypt for assistance and feedback on this review. All authors agree to authorship and submission of the manuscript for review.
No Funding has been received.
Author information
Authors and affiliations.
Marine Microbiology Department, National Institute of Oceanography and Fisheries (NIOF), Cairo, 11516, Egypt
Hassan A. H. Ibrahim, Hala E. Abou Elhassayeb & Waleed M. M. El-Sayed
You can also search for this author in PubMed Google Scholar
Contributions
W.M.M.E., and H.A.H.I. conceived the project and designed review. W.M.M.E. and H.A.H.I., wrote the manuscript with help from H.E.A.E. All authors commented on and approved the manuscript.
Corresponding author
Correspondence to Waleed M. M. El-Sayed .
Ethics declarations
Ethics approval and consent to participate.
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests. No conflicts, informed consent, human, or animal rights are applicable.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ibrahim, H.A.H., Abou Elhassayeb, H.E. & El-Sayed, W.M.M. Potential functions and applications of diverse microbial exopolysaccharides in marine environments. J Genet Eng Biotechnol 20 , 151 (2022). https://doi.org/10.1186/s43141-022-00432-2
Download citation
Received : 30 January 2022
Accepted : 08 October 2022
Published : 01 November 2022
DOI : https://doi.org/10.1186/s43141-022-00432-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Microbial exopolysaccharides
- Marine environment
- Antifouling agents
- Bioremediation
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Exopolysaccharides from marine bacteria: production, recovery and applications

2020, Environmental Sustainability
Related Papers
Journal of Genetic Engineering and Biotechnology
Waleed M . M . El-Sayed
Exopolysaccharides (EPSs) from microorganisms are essential harmless natural biopolymers used in applications including medications, nutraceuticals and functional foods, cosmetics, and insecticides. Several microbes can synthesize and excrete EPSs with chemical properties and structures that make them suitable for several important applications. Microbes secrete EPSs outside their cell walls, as slime or as a “jelly” into the extracellular medium. These EPS-producing microbes are ubiquitous and can be isolated from aquatic and terrestrial environments, such as freshwater, marine water, wastewater, and soils. They have also been isolated from extreme niches like hot springs, cold waters, halophilic environments, and salt marshes. Recently, microbial EPSs have attracted interest for their applications such as environmental bio-flocculants because they are degradable and nontoxic. However, further efforts are required for the cost-effective and industrial-scale commercial production of...
Marine Drugs
Annarita Poli
barbara nicolaus , G. Anzelmo
Michelangelo Parrilli
The marine environment is the largest aquatic ecosystem on Earth and it harbours microorganisms responsible for more than 50% of total biomass of prokaryotes in the world. All these microorganisms produce extracellular polymers that constitute a substantial part of the dissolved organic carbon, often in the form of exopolysaccharides (EPS). In addition, the production of these polymers is often correlated to the establishment of the biofilm growth mode, during which they are important matrix components. Their functions include adhesion and colonization of surfaces, protection of the bacterial cells and support for biochemical interactions between the bacteria and the surrounding environment. The aim of this review is to present a summary of the status of the research about the structures of exopolysaccharides from marine bacteria, including capsular, medium released and biofilm embedded polysaccharides. Moreover, ecological roles of these polymers, especially for those isolated from...
Annals of Tropical Medicine and Public Health
Lekshmi Gangadhar
World Journal of Pharmaceutical Sciences
Bioscience Discovery
Dr. Murtaza Hajoori
Exopolysaccharides are polymers of carbohydrates secreted by some bacteria and fungi outside their cell walls. Marine microorganisms have significant osmotic tolerance and leading to produce exopolysaccharide even at higher salt concentration. The exopolysaccharide producing bacteria were isolated from marine environment. The marine soil samples and marine water samples were collected from sea coast of Dandi, Navsari and Tithal, Valsad. Total 13 morphological distinct colonies were obtained and 05 isolate were selected having mucoid colony characterization. All the 05 isolate were screened using string test and congo red agar test. Isolate VS2B was screened and evaluated to be more potent for exopolysaccharide production of 5.60 mg/ml in Zobell marine broth on 4th day of incubation. The molecular identification of VS2B isolate was carried out by 16S rRNA sequencing and identified as Bacillus subtilis. Thus, this study demonstrates VS2B isolate may prove to be potential strain for commercial production of exopolysaccharide.
The pharma Innovation
Dhanya BE , chandra m
The exopolysaccharides (EPS) derived from marine bacteria have shown to exhibit versatile applications in diverse biotechnological segments. Therefore a study was done to bio prospect marine bacteria from surface sea water, associated with algae and sand obtained from the coastal area of Someshwar, Mangalore and was screened for exopolysaccharide production. Out of the twenty colonies isolated; two colonies that produced prominent mucoid colonies were selected, designated as YUI6-DR3A and YU17-DR6A, identified using 16S rRNA gene sequencing and the EPS produced by them in Zobell marine broth and MY broth was evaluated using standard spectrophotometric methods. The phylogenetic analyses of the isolates done on the basis of 16S rRNA gene sequence revealed the isolates YU16-DR6A and YU16-DR3A shared 97.54% and 100% similarity respectively to Pseudoalteromonas shioyasakiensis sp. SE3 T. The strains produced appreciable amount of EPS in MY media. The expolysaccharides of these newly isolated strains can be further explored for its applicability in biotechnological industries. Introduction Marine environs cover almost three quarters of earth and contain a vast biological diversity. Marine habitats are highly complex and are the largest continuous ecosystem that consists of extremely variable temperature, salinity, pressure and nutritional conditions. In the oceans, bacteria constitute the most abundant and diverse members of the microbial world. Many reports describe the efficiency of marine bacterial strains in biotechnological applications including human and environmental benefits. The bacteria isolated from these environments are potent sources of various industrially important bioactive compounds such as enzymes, exopolysaccharide (EPS), biosurfactants, antibacterial, antiviral, anticancer compounds with distinctive and diverse compositions. Marine bacteria secrete different compounds based on their habitat and their ecological functions. Many marine bacteria produce exopolysaccharides (EPS) as a strategy for growth, binding to the substratum, to survive unfavorable conditions and also to intra-and inter-specific communication and competition (Corinaldesi et al., 2017)
Nicola Fuzzati
Bacteria are well-known to synthesize high molecular weight polysaccharides excreted in extracellular domain, which constitute their protective microenvironment. Several bacterial exopolysaccharides (EPS) are commercially available for skincare applications in cosmetic products due to their unique structural features, conferring valuable biological and/or textural properties. This review aims to give an overview of bacterial EPS, an important group of macromolecules used in cosmetics as actives and functional ingredients. For this purpose, the main chemical characteristics of EPS are firstly described, followed by the basics of the development of cosmetic ingredients. Then, a focus on EPS production, including upstream and downstream processes, is provided. The diversity of EPS used in the cosmetic industry, and more specifically of marine-derived EPS is highlighted. Marine bacteria isolated from extreme environments are known to produce EPS. However, their production processes are ...
International Journal of Pharma and Bio Sciences
Dr. Reena G Desai
Exopolysaccharides (EPS) are high molecular weight polymers which are long chain sugar residues and secreted by microorganisms into the surrounding environment. EPS shows diverse applications such as in food formulations and pharmaceutical industry, etc. Five different bacteria were isolated on Zobell marine agar medium from sea water of Valsad district, Gujarat, India and screened by using YEM (Yeast Extract Mannitol) congo red agar medium. Bacteria were identified by their morphological and biochemical characterization. Analytical method such as phenol sulfuric acid method, biuret assay, specific viscosity and molecular weight of EPS producing bacteria were carried out. The EPS was characterized by thin layer chromatography and result shown the presents of L-rhamnose, L-GlcNAC, D-ribose and D-xylose combination. Isolates of EPS producing were also studied for biofilm production in test tubes and found the potential biofilm former bacteria. Among 5 isolates studied, EPS production was highiest in colony 2.
RELATED PAPERS
Oscar Cardona
Journal of Turkish Studies
Fazilet Karakuş
Prilozi nastavi srpskog jezika i književnosti
Saša Knežević
Journal of sports science & medicine
Gediminas Mamkus
H. Karttunen
Biochemical Journal
Reto Crameri
Family Process
Surgical Endoscopy and Other Interventional Techniques
Reza Zahiri
arXiv (Cornell University)
Jurnal Pengabdian Masyarakat
muhammad rivo
Giornale Italiano di Dermatologia e Venereologia
Stefania Barruscotti
Caderno Virtual de Turismo
Aguinaldo Cesar Fratucci
Conceição Coelho
Inorganica Chimica Acta
Mireille Perec
Journal of Intensive Care
Jorge Lascano
Diether Schürr
PLOS Computational Biology
Antonietta Mira
Europaisches Journal Fur Minderheitenfragen
Christian Behrendt
Marco Radici
Vela Yulia Vela Yulia
Neuroscience Letters
Alireza Komaki
Pak J Med Sci July-September
Abdul Razaque Shaikh (Registrar CPSP)
RELATED TOPICS
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- Open access
- Published: 28 November 2023
Structural characteristics of microbial exopolysaccharides in association with their biological activities: a review
- Wei Wang 1 ,
- Yuhao Ju 3 ,
- Nan Liu 1 ,
- Shengbo Shi 1 &
- Lujiang Hao 1 , 2
Chemical and Biological Technologies in Agriculture volume 10 , Article number: 137 ( 2023 ) Cite this article
1702 Accesses
2 Citations
Metrics details
Many microbial exopolysaccharides (EPS) have been reported in the last decade, and their fermentation processes, functional properties and applications, structural characterization, and biological activities have been extensively studied. Despite the great diversity of biological activities already described for EPS, only a few have been exploited industrially. The main reason for this is that the structure–activity relationship of EPS has not been clearly defined. In this review, we collected EPS-related publications from two databases, the Web of Science and China National Knowledge Infrastructure, and reviewed the correlation between the structural characteristics of EPS and observed biological activity, as reported in studies over the last decade. This review focused on the antioxidant, antitumor, immunomodulatory, hypoglycemic, antibacterial, and gut microbial-modulating activities of EPS. This review aimed to lay a foundation for researching the structure–activity relationship of EPS and provide a theoretical basis for important scientific studies and applications of EPS.
Graphical Abstract
Introduction
Microorganisms are the most abundant source of biological diversity on Earth. They exhibit novel functions and extensive biological characteristics, and are special sources of various metabolites [ 1 , 2 ]. Exopolysaccharides (EPS) are extracellular carbohydrate polymers produced by microorganisms, including bacteria, fungi, and microalgae [ 3 , 4 , 5 ]. In the natural environment, EPS usually participate in protecting microorganisms; they resist adverse conditions (e.g., desiccation, cold, and hypertonic conditions), enhance resistance, and promote nutrient uptake [ 6 , 7 , 8 ].
EPS have anticancer [ 9 ], antitumor [ 10 ], anti-inflammatory [ 11 , 12 ], antidiabetic [ 13 ], antiviral [ 14 ], antioxidant [ 15 ], cholesterol-lowering [ 16 ], hypoglycemic/hypolipidemic [ 17 , 18 ], immunomodulatory [ 19 ], and probiotic activities [ 20 , 21 ]. Owing to their novel physiological functions and extensive biological activities [ 22 ], their formability is advantageous in terms of chemical composition and structure. EPS have been widely used in the fields of food, chemicals, and cosmetics [ 23 , 24 ], and have also shown great potential for medical applications [ 25 , 26 ]. Currently, the applications of microbial EPS in medicine include drug targeting [ 27 ], delivery [ 28 , 29 ], vaccine preparation [ 30 ], tissue engineering [ 31 ], wound healing [ 32 ], anti-proliferation [ 33 ], cell carriers, and diagnostic tool manufacturing [ 3 ]. A number of carbohydrate-based drugs are also clinically used, including carragelose [ 34 , 35 ] (Fig. 1 A), cethromycin [ 36 ] (Fig. 1 B), sodium oligomannate [ 37 ] (Fig. 1 C), and lactitol [ 38 ] (Fig. 1 D).
Chemical structures of four carbohydrate-based drugs
The biological activities of EPS are closely related to their structure [ 39 ], including monosaccharide composition, molecular weight, glycosidic linkage type and position, and chain conformation [ 40 ]. Therefore, in this review, we summarized the structural characteristics and biological activities of microbial EPS and explored their structure–activity relationship provide a reference and theoretical basis for the research and application of microbial EPS.
Research status of EPS
With the mining of EPS bioactivities and their wide range of applications in numerous research areas [ 41 , 42 ], EPS research is gradually becoming an internationally cutting-edge topic with a large number of literature reports [ 3 , 43 , 44 ]. The EPS-related publications from 2012 to 2022 were statistically analyzed using the Web of Science (WOS) and China National Knowledge Infrastructure (CNKI) series databases with "exopolysaccharides" as the subject term (Fig. 2 ). The plot in Fig. 2 is similar to the global reasoning approach proposed by Chen et al. [ 45 ]. We projected the data of the two databases from the coordinate space to the nodes in an interaction space graph, which allowed us to directly analyze the information of the two databases from a global perspective.
Statistics on the number of publications on EPS in WOS/CNKI per year from 2012 to 2022. EPS Exopolysaccharides, WOS Web of Science, CNKI China National Knowledge Infrastructure
The number of EPS-related publications on the WOS has been increasing annually since the past decade; the number of EPS-related publications was 239 in 2012, increasing to 646 in 2022—an increase of 170%. Over the past five years, the number of publications on EPS has increased, with an average annual increase of approximately 70 articles. As of March 9, 2023, the total number of EPS research publications reached 4,522 in the last decade. An annual analysis of the CNKI database shows that the number of EPS-related publications has increased annually over the past decade. As of March 9, 2023, the total number of EPS research papers published in the last decade was 3,885. An increase in the number of EPS-related studies in recent years has shown that EPS have gradually become a focus of attention and a research hotspot.
Currently, research on EPS focuses on four aspects: preparation process, functional properties and applications, structural characterization, and biological activities. The classification results of the studies on EPS in the two databases over the past decade are shown in Fig. 3 , which shows that the focus of EPS research on CNKI differs significantly from that on the WOS.
Comparative statistical analysis of CNKI/WOS published literature from 2012 to 2022. Comparative general data of the number of CNKI/WOS published papers using different keywords/phrases to search: 1 fermentation and extraction; 2 functional properties and applications; 3 structural characterization; and 4 biological activity. WOS Web of Science, CNKI China National Knowledge Infrastructure
In recent years, many studies on the EPS preparation processes for CNKI have been reported and are increasing annually. Research on the biological activity of EPS has been steadily increasing annually, with a slower increase than that of the preparation process studies. However, the number of studies on the structural characterization of EPS is relatively small, with approximately 20 papers published each year (Fig. 3 A). In contrast, the number of research publications on the structural characteristics of EPS in WOS remained above 60, but the overall number accounted for a small percentage of the publications (Fig. 3 B). In summary, basic research on the structural characteristics of EPS is still very limited; therefore, conducting relevant research on these aspects is a key direction for researchers focusing on EPS.
Structural features of EPS
EPS have two different extracellular secretion states: capsular polysaccharides that adhere to the microbial cell wall to form a capsule, or slime polysaccharides that are loosely attached or even completely released into the surrounding environment to form slime [ 46 , 47 ]. EPS can be homopolysaccharides composed of the same monosaccharide, such as curdlan, or heteropolysaccharides composed of different monosaccharides[ 48 ] (Fig. 4 A). Heteropolysaccharides consist of different monosaccharides, including not only commonly observed sugars (such as glucose, galactose, and fructose), but also some rare monosaccharides (such as rhamnose, xylose, fucose, and mannose), uronic acids and amino sugars [ 20 , 49 ] (such as xanthan) [ 50 ] (Fig. 4 B). EPS with straight chains of monosaccharides, such as pullulan, are called linear polysaccharides [ 8 , 51 ] (Fig. 4 C). EPS with arms and bends, such as EPS-W1 extracted from Lactobacillus plantarum W1, are called branched polysaccharides [ 52 , 53 , 54 ] (Fig. 4 D).

Structural formulae of EPS, including homopolysaccharides [e.g., curdlan ( A )]; heteropolysaccharides [e.g., xanthan ( B )]; linear polysaccharides [e.g., pullulan ( C )]; and branched polysaccharides [e.g., EPS-W1 ( D )]. EPS Exopolysaccharides
The structural description of EPS usually includes monosaccharide composition and conformation, molecular weight range, glycosidic bond conformation, repeating units, linkage sites, and spatial structures [ 55 ]. Table 1 summarizes the various EPS obtained from different microorganisms from 2018 to 2022. According to Table 1 , we can speculate that EPS with a porous structure is more likely to have antioxidant activity, and that EPS with immunomodulatory activity are all triple-helical structures. Analyses of the monosaccharide composition, molecular weight, conformation, and biological activity of these EPS can provide useful information about their structure–activity relationships.
Structure–activity relationship of EPS
The composition and structure of EPS determine their microstructure and surface morphology, which affect their biological activity [ 77 ]. In this section, we focus on the antioxidant, antitumor, immunomodulatory, hypoglycemic, antibacterial, and gut microbial-modulating activities of microbial EPS (Fig. 5 ).
Main biological activities of microbial EPS, including antioxidant ( A ), antitumor ( B ), immunomodulatory ( C ), hypolipidemic ( D ), antibacterial ( E ), and regulation of gut microbiota ( F ). Figure modified according to [ 78 , 79 ]. EPS Exopolysaccharides, TG Triglycerides, TC Total cholesterol
Antioxidant activity
Studies have shown that EPS have significant antioxidant activity. Similar to the mechanism of other sources of polysaccharides, the hydrogen-donating capacity of bacteria-derived EPS is considered the main property of its antioxidant function, but the underlying mechanism is not clear [ 80 ]. The antioxidant activity of EPS is affected by several factors, including monosaccharide composition, glycosidic bond type, and branching patterns.
The monosaccharide composition and composition ratio of EPS significantly a effected the antioxidant activity of EPS. As an example, EPS consisting of glucose-repeating units exhibit strong superoxide and 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) scavenging activities and low hydroxyl radical scavenging activity, similar to that of the ascorbic acid standard [ 81 ]. The EPS obtained from Weissella cibaria SJ14, purified with a mannose content of 75.9%, exhibited excellent hydroxyl radical scavenging activity. This may be due to the higher mannose content [ 82 ]. In addition, high galactose-containing heteropolysaccharides obtained from W. confusa show strong scavenging ability and effective reduction of DPPH and hydrogen peroxide radicals [ 83 ]. Similarly, the two EPS components produced by Lactobacillus delbrueckii ssp. bulgaricus SRFM-1, r-EPS1 and r-EPS2, had higher proportions of galactose and exhibited stronger antioxidant activities [ 84 ]. Moreover, EPS, which consist of galactose, glucose, and rhamnose as the main monosaccharides exhibit antioxidant activity. This has been verified in EPS studies on Porphyridium cruentum and Bacillus sp. S-1, and Enterobacter ludwigeii [ 85 , 86 ].
These results suggest that the glycosidic bond type and branching pattern can affect the antioxidant activity of EPS [ 84 ]. It has been suggested that α-1,2 and 1,6 glycosidic bonds are more flexible than β-1,3 and 1,4 glycosidic bonds [ 87 , 88 , 89 ]. The EPS produced by B. amyloliquefaciens is an α-glucan composed of glucose with two α-(1 → 3) and one α-(1 → 6) glycosidic bonds, which has a superoxide anion-scavenging ability [ 75 ]. However, the role of glycosidic bonds in antioxidant activity remains unclear and requires further investigation. It has been found that EPS with a high degree of branching also has good antioxidant activity. Yang et al. isolated and purified two fractions, THPS-1 and THPS-2, from the Tetragenococcus halophilus SNTH-8, both of which were highly branched polysaccharides with high antioxidant and emulsifying abilities [ 90 ].
Furthermore, changes in bacterial fermentation conditions (e.g., pH) can alter the structure of EPS, thereby affecting its antioxidant activity. For example, the EPS produced by Alteromonas australica QD under different pH conditions. The results revealed that acidic pH EPS (AC-EPS) and alkaline pH EPS (AL-EPS) contained similar types of monosaccharides with different proportions of Man, Gal, and GlcA. AL-EPS has been found to have high antioxidant activity [ 91 ]. Similar results have been reported by Ju [ 92 ].
Antitumor activity
According to a study on EPS antitumor activity regarding its structure, the high-order structure of EPS is more important than the primary structure for EPS antitumor activity [ 93 , 94 ]. It includes the main chain composition, flexibility, molecular chain conformation, degree of branching, helical conformation, and spatial structure.
Antitumor EPS structural studies have shown that β-1,3 glycosidic bonds on glucose chains and β-1,6 glycosidic bonds on branched chains are required for their activities [ 95 ]. For instance, a variety of polysaccharide were isolated from Porphyra mushroom , whose antitumor active fraction is β-(1,3)-D-glucan with (1 → 6) branched chains [ 96 ]; the antitumor polysaccharide extracted from Auricularia auricula-judae was also composed of β-1,3-bound straight-chain glucan [ 97 ].
The flexibility of the polysaccharide backbone determines the antitumor activity of EPS to a certain extent [ 98 ]. Flexibility consists of a combination of hydrogen bonding and electrostatic repulsion of substituents within the polysaccharide molecule. High flexibility facilitates the interaction between the polysaccharide and the immune system, thus enhancing the antitumor activity of EPS [ 41 , 99 ]. It has also been reported that polysaccharide branches can weaken intramolecular interactions and disrupt intermolecular binding, thus affecting antitumor activity [ 100 , 101 ]. Bohn suggested that EPS with branching degrees of 0.2–0.33 have higher antitumor activity [ 102 ].
Morphological characteristics and chain conformation may also influence EPS antitumor activity [ 103 ]. Polysaccharides with a triple-helical conformation exhibit antitumor activity [ 104 ]. For instance, Misaki et al. found that lentinan and Auricularia auricula-judae polysaccharides with antitumor activity have β-triple helix conformation [ 97 ]. It has been found that chain conformation facilitates the interaction of polysaccharides with the immune system and enhances the antitumor activity of EPS [ 105 ]. The in vivo antitumor activity of different chain conformations of lentinan showed that the triple-helix conformation plays an important role in the antitumor activity of lentinan. Once the helical chain is disrupted, the antitumor activity decreases significantly or even disappears [ 106 ]. Poria polysaccharide is similar to lentinan; both have β-1,6 side chain glucan and no tumor activity, but through the oxidation of periodate and by Smith degradation after the removal of β-1,6 chain, the antitumor activity of polysaccharides was observed. X-ray diffraction analysis revealed that the polysaccharides formed a triple helical configuration [ 107 ]. Similarly, several other polysaccharides with antitumor activity extracted from mushrooms exhibit a triple-helical conformation in solution [ 108 , 109 ]. Furthermore, characteristic viscosity is a key factor. An appropriate characteristic viscosity is conducive to the adhesion of polysaccharides to tumor cells [ 110 ].
The antitumor activity of sulfated polysaccharides was higher than that of non-sulfated polysaccharides. EPS from Lactobacillus plantarum 70810 (e.g., r-EPS1 and r-EPS2) inhibited tumor cell growth at a higher rate than the inhibition associated with r-EPS1; the authors hypothesized that the significant antitumor activity of r-EPS2 may be closely related to the composition of the sulfate group and β-glycosidic bond in r-EPS2 [ 111 ]. Sulfated galactans isolated from Halomonas aquamarina EG27S8QL also exhibit antitumor activity [ 112 , 113 ]. However, EPSR3 from Bacillus cereus is a sulfate-free EPS, and its main component is uronic acid (28.7%). The results of this study showed that EPSR3 exhibited antitumor activity. The authors suggested that the antitumor activity of EPSR3 may be due to its uronic acid content [ 114 ]. Therefore, the relationship between acidic polysaccharides and their antitumor activities requires further investigation.
Immunomodulatory activity
Many studies have reported that EPS with certain compositions and molecular weights may be involved in immune responses [ 39 , 115 ]. Results based on the structural features and immunomodulatory activity revealed that the presence of galactose is closely related to the immunomodulatory activity of EPS [ 115 ]. Reactions between polysaccharide antigens and antibodies produced in rabbits for galactose were reported as early as 1988 [ 116 ]. EPS from probiotic Enterococcus hirae WEHI01 are composed only of galactose, with a molecular weight of 2.59 × 10 3 Da, and it effectively improves macrophage-mediated immune responses [ 117 ].
Structure–activity relationship analysis showed that the molecular weight was significantly correlated with the immunomodulatory activity of EPS. EPS with higher molecular weights may inhibit immune responses [ 118 ]. In general, the degradation of higher-to lower-molecular-weight EPS significantly improves their biological activity [ 119 ]. Surayot et al. investigated the effect of EPS produced by Lactobacillus confusus TISTR 1498 on immunomodulatory activity, which consisted only of glucose with a high molecular weight of 65 000–506 000 kDa and was unable to stimulate the production of the pro-inflammatory factors nitric oxide and cytokines by RAW264.7 cells. After partial acid hydrolysis, its molecular weight was less than 70 kDa, and it was able to significantly stimulate macrophages and induce the production of nitric oxide as well as cytokines such as TNF-α, IL-1β, IL-6, and IL-10 [ 120 ]. The mechanism may be that lower-molecular-weight EPS and cell receptors bind more strongly and are more conducive to stimulating the production of pro-inflammatory factors in RAW264.7 cells. However, another study purified two homogeneous EPS, EPS53 (high molecular weight) and EPS53d (low molecular weight), from skimmed milk fermented by S. thermophilus XJ53; EPS53 showed stronger immune activity by promoting phagocytic ability and NO release from macrophages [ 121 ]. Therefore, the relationship between the molecular weight and immune activity of EPS needs to be further studied.
Acidic heteropolysaccharides are better at inducing immune responses [ 118 ]. For example, high-molecular-weight sulfated heteropolysaccharides from Lactobacillus paracasei VL8 are mainly composed of glucose and galactose, which have strong immunomodulatory activities [ 122 ]. Nishimura-Uemura studied a heteropolysaccharide produced by Lactobacillus delbrueckii subsp. Bulgaricus OLL1073R-1, consisting of both neutral and acidic polysaccharides in a 3:2 ratio of glucose to galactose. The acidic polysaccharides contained a small amount (0.1%) of phosphate, which was able to strongly induce the proliferation of various types of macrophages, whereas the neutral polysaccharides were unable to function, and dephosphorylation of this heteropolysaccharide caused a significant reduction in the stimulatory effect [ 123 ].
Furthermore, EPS with a triple-helical conformation may have immunomodulatory activity. For instance, EPS have been isolated from Aureobasidium pullulans CGMCC 23063, which has a triple-helical conformation linked to chains and round spheres. In an in vitro cellular assay, EPS showed immunoreactivity in RAW264.7 cells [ 56 ]. A novel crude EPS with a triple helical structure produced by Lactobacillus plantarum KX041 possesses prominent immune activity, promoting the proliferation and phagocytosis of Raw264.7 [ 73 ].
Hypoglycemic activity
Current studies on the hypoglycemic mechanism of EPS mainly focus on the regulation of related enzyme activities [ 124 ] and the improvement of insulin sensitivity [ 125 ]. EPS can reduce blood sugar by inhibiting digestive enzymes [ 126 ]. The hypoglycemic activity of EPS is closely related to its molecular weight, branched structure, and high-order structure [ 127 , 128 ].
The hypoglycemic activity of EPS is closely related to its molecular weight [ 129 , 130 , 131 ]. The optimal activity of EPS can only be achieved at the appropriate molecular weight. Generally, EPS with low molecular weights exhibit better hypoglycemic activity [ 128 ]. A novel Codyceps polysaccharide with low molecular weight of 28 kDa was obtained by acid hydrolysis, and its inhibition rate on α-d-glucosidase was calculated as 40.01% [ 132 ]. Wang et al. used birch mushroom polysaccharides to simulate digestion in the intestine; the digested polysaccharide (UIOPS-1I) had a reduced molecular mass and significantly higher inhibitory activity against glucosidase [ 127 ].
Glycosidic bonds play an important role in the hypoglycemic activity of EPS [ 133 ]. It was found that most of the EPS with hypoglycemic activity have 1 → 3, 1 → 4, and 1 → 6 glycosidic bonds [ 128 ]. For example, the EPS backbone of Cordyceps militaris is dominated by a galactose 1 → 4 linkage, which effectively inhibits-glucosidase activity and restores glucose tolerance in mice [ 134 ].
It has also been suggested that EPS with a helical structure are more likely to have hypoglycemic activity, which has been verified in studies on Cordyceps militaris and Pleurotus citrinopileatus [ 17 , 65 ]. Furthermore, sulfated EPS exhibited better hypoglycemic ability than that of natural EPS. For example, the EPS isolated from the fermentation broth of Lachnum sp. YM240 is sulfated, and sulfated EPS have a higher ability to inhibit glucosidase and amylase activities than that of unsulfated EPS [ 135 ].
Antibacterial activity
EPS contain various functional groups, such as carbonyl, phosphate, and hydroxyl groups. To some extent, these functional groups interact with bacterial cell membranes to exert antimicrobial activity [ 136 , 137 ]. Novel Aspergillus spp. DHE6 produces EPS with the main functional groups -OH,–CH,–C = C, and C–O–C, which exhibit strong antibacterial activity against harmful human pathogens ( Staphylococcus aureus , Bacillus subtilis, Bacillus pertussis , and Pseudomonas aeruginosa ) [ 138 ].
EPS composed of glucose and rhamnose are more likely to exhibit antimicrobial activity, as verified in studies on EPS obtained from Lactobacillus gasseri FR4, Streptococcus thermophilus GST-6, and Lactococcus garvieae C47 [ 139 , 140 , 141 ]. Similarly, EPS are produced by Pediococcus pentosaceus SSC-12, with monosaccharide fractions of mainly glucose and rhamnose; they exhibit a strong antibacterial capacity [ 142 ]. However, an EPS with a molecular weight of 53,887 Da is produced by Lactobacillus crispatus , consisting mainly of mannose and glucose. It also has excellent antibacterial activity, which can effectively limit bacterial translocation and increase the abundance of Lactobacillus and Bifidobacterium [ 143 ]. Enterobacter sp. ACD2 EPS, with monosaccharide fractions of glucose, galactose, and fucose, containing 10% uronic acid and a small amount of fructose, showed high antibacterial activity against S. aureus and Eschericia coli [ 144 ]. In another study, dextran formed by Lactobacillus inhibited biofilm formation by C. albicans [ 145 ]. Moreover, negatively-charged EPS can interact better with pathogens through their sulfate groups, exhibiting antifungal activity [ 70 ].
Regulation of gut microbiota
EPS can also regulate the composition and function of the gut microbiota [ 20 , 146 ]. Cordyceps sinensis polysaccharides (CSPs) are composed mainly of glucose, galactose, mannose, galacturonic acid, arabinose, trace proteins, and phenolic compounds. The backbone of CSPs consist of 1,4-glucose and 1,4-galactose, with a molecular weight of approximately 28 kDa [ 147 ]. CSPs increase the abundance of probiotics ( Lactobacillus , Bifidobacterium , Bacteroides ) and decrease that of pathogenic bacteria ( Clostridium and Flexispira ) [ 148 ]. E. coli EPS (EPS-m2) are composed of glucuronic acid, glucose, fucose, galactose/N-acetyl glucosamine, arabinose, xylose, and ribose in a molar ratio of approximately 77:44:29:28:2:1:1. EPS-m2 increases the abundance of Alistipes , Acinetobacter , Alloprevotella , Howardella , and Oxalobacter , and GC detection illustrates that EPS-m2 enhances the production of SCFAs [ 149 ]. The dextran (LM742) produced by Leuconostoc mesenteroides SPCL742, with a molecular weight of 1.3 × 10 6 Da, contains α-1,6 and α-1,3 glycosidic bonds in a ratio of 26.11:1. The LM742 glucan is resistant to digestive enzymes in the human gastrointestinal conditions [ 150 ]. Additionally, EPS from Paecilomyces cicadae TJJ1213 regulates the gut microbiota and metabolism and increases the abundance of probiotics [ 151 ].
However, few studies have reported on the role of EPS in the regulation of gut microbiota, and current studies have some shortcomings; thus, the mechanism of how EPS regulate gut microbiota is yet to be further elucidated. Therefore, researchers should strengthen the study of EPS structure and its relationship with the regulation of gut microbiota in the future, and reveal the intrinsic mechanism of EPS regulation of the gut microbiota.
Other biological activities
In addition to the aforementioned biological activities, EPS exhibit other biological activities. These include emulsifying, anti-inflammatory, and antimetastatic properties. EPS containing galactose generally have emulsifying properties, as demonstrated by the EPS generated by Lysinibacillus fusiformis and Streptococcus thermophilus [ 59 , 76 ]. Sulfated heteropolysaccharides with branched and multichain structures may exhibit anti-inflammatory activities [ 152 , 153 ]. Similarly, EPS isolated from Lactobacillus crispatus with a molecular weight of 53,887 Da, consisting mainly of mannose and glucose, possesses excellent anti-inflammatory activity [ 143 ]. Acidic EPS can degrade cholesterol more effectively than neutral polysaccharides can [ 154 ]. Moreover, EPS with irregularly curled conformations may have antimetastatic properties [ 61 ].
Chemical modification of EPS
The modification of EPS by group substitution to alter the structure of polysaccharides and enhance targeted biological activity has been reported as an emerging trend [ 138 , 155 , 156 ]. Current polysaccharide modification methods include carboxymethylation, acetylation, phosphorylation, and sulfonation. Lasiodiplodan, an exocellular fungal (1 → 6)-β-D-glucan, was used to illustrate the linkage of functional group to the glucan chain [ 157 , 158 ] (Fig. 6 ).
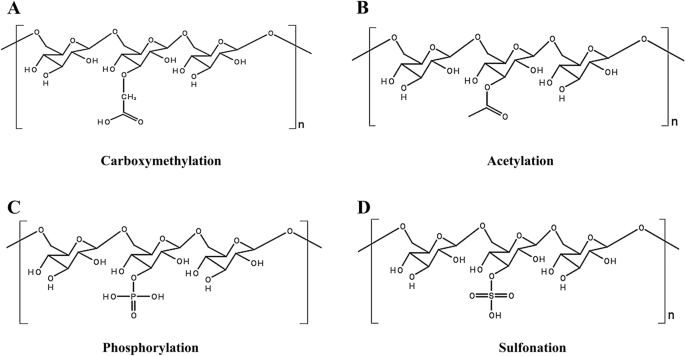
Structural representation of D-glucan modification, including carboxymethylation ( A ); acetylation ( B ); phosphorylation ( C ); and sulfonation ( D ), where a group is added to the hydroxyl group on the monosaccharide
Carboxymethylation modifications
Carboxymethylation involves the introduction of carboxymethyl groups to polysaccharide chains via etherification reactions of polysaccharides with acids or carboxylic acid derivatives to achieve changes in the spatial structure and water solubility of the polysaccharides; thus, affecting their biological activities [ 13 , 159 ]. This modification has been shown to have a significant role in enhancing EPS bioactivity [ 160 , 161 ]. For example, polysaccharides obtained from Lachnum YM240 fermentation broth were carboxymethyl-modified, and the results showed that diabetic mice fed carboxymethylated Lachnum polysaccharides had significantly lower fasting glucose and serum triglyceride levels and significantly higher insulin sensitivity [ 162 ]. Similarly, the EPS extracted from Lachnum YM281 were modified by carboxymethylation and exhibited enhanced biological activity [ 163 ].
Acetylation modifications
Acetylation alters the spatial structure of polysaccharides, thereby affecting their biological activities [ 159 , 164 ]. EPS derived from Paenibacillus polymyxa EJS-3 have a higher reducing power than that of native EPS after various chemical modifications, including acetylation and phosphorylation [ 165 ]. Similarly, the EPS produced by Lactobacillus plantarum 70810 exhibited antioxidant activity after the introduction of a new acetylation moiety [ 166 ].
Phosphorylation modifications
Phosphorylation is a reliable method for enhancing the bioactivity of EPS [ 167 ]. EPS obtained from Lactococcus lactis subsp. lactis were phosphorylated and showed antioxidant effects in vivo and in vitro [ 168 ]. Similarly, EPS produced by Lachnum YM405 were subjected to sulfonation and phosphorylation treatments. The antioxidant activity of the modified derivatives was significantly enhanced [ 169 ].
Sulfonation modifications
EPS are sulfonated to achieve the desired chain length and water solubility of the polysaccharide, which affects its biological activity. The sulfonation EPS extracted from Enterobacter cloacae Z0206 protected RAW264.7 mouse macrophages from H 2 O 2 -induced oxidative damage and inhibited DNA breakage. These results suggest that sulfonation enhances antioxidant activity by modulating water solubility and chain length and protects cells by exchanging more hydrogen atoms [ 170 ]. Similarly, sulfonated EPS produced by Streptococcus thermophilus GST-6 and S. thermophilus ASCC1275 showed stronger antimicrobial efficacy against various Gram-positive and harmful pathogens than the efficacy of non-sulfonated EPS [ 141 , 171 ].
Conclusion and future perspectives
EPS produced by microorganisms have attracted attention worldwide owing to their safety, diverse potent biological activities, and favorable advantages over other natural agents for industrial and therapeutic applications. However, the structure of EPS is complex and difficult to analyze, resulting in difficulties in investigating their structure–activity relationship, and its specific mechanism of action has not yet been revealed. In this review, we suggest that the structural characteristics of EPS, such as molecular weight, monosaccharide composition, glycosidic bond type, branching pattern, spatial structure, and chemical modifications may affect their biological activity. We believe that more advanced technology should to be used to analyze the structure of EPS. On this basis, the mechanism of action of the structure–activity relationship should be revealed from a new perspective to lay the foundation for the targeted synthesis and design of glycans. Moreover, many studies have shown that incubation conditions (e.g., time, temperature, and pH) can also affect the structure and biological activity of EPS. Therefore, changing the composition and structure of EPS through the influence of external factors can broaden its applications to some extent.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Demain AL. Importance of microbial natural products and the need to revitalize their discovery. J Ind Microbiol Biotechnol. 2014;41:185–201.
Article CAS PubMed Google Scholar
Poli A, Finore I, Romano I, Gioiello A, Lama L, Nicolaus B. Microbial diversity in extreme marine habitats and their biomolecules. Microorganisms. 2017;5:25.
Article PubMed PubMed Central Google Scholar
Moscovici M. Present and future medical applications of microbial exopolysaccharides. Front Microbiol. 2015;6:1012.
Freitas F, Torres CAV, Reis MAM. Engineering aspects of microbial exopolysaccharide production. Bioresour Technol. 2017;245:1674–83.
Xiao M, Fu X, Wei X, Chi Y, Gao W, Yu Y, et al. Structural characterization of fucose-containing disaccharides prepared from exopolysaccharides of Enterobacter sakazakii . Carbohydr Polym. 2021;252:117139.
Singha T. Microbial extracellular polymeric substances: production, isolation and applications. IOSR J Pharm (IOSRPHR). 2012;2:271–81.
Google Scholar
Flemming H-C. EPS—then and now. Microorganisms. 2016;4:41.
Decho AW, Gutierrez T. Microbial extracellular polymeric substances (EPSs) in ocean systems. Front Microbiol. 2017. https://doi.org/10.3389/fmicb.2017.00922/full .
Gargouch N, Elleuch F, Karkouch I, Tabbene O, Pichon C, Gardarin C, et al. Potential of exopolysaccharide from Porphyridium marinum to contend with bacterial proliferation, biofilm formation, and breast cancer. Mar Drugs. 2021;19:66.
Article CAS PubMed PubMed Central Google Scholar
Angelin J, Kavitha M. Exopolysaccharides from probiotic bacteria and their health potential. Int J Biol Macromol. 2020;162:853–65.
Hou C, Chen L, Yang L, Ji X. An insight into anti-inflammatory effects of natural polysaccharides. Int J Biol Macromol. 2020;153:248–55.
Stellavato A, Dabous A, D’Ambrosio S, Cimini D, Schiraldi C. Anti-inflammatory effect of exopolysaccharides from Lactobacillus brevis on co-culture models of macrophage-Like and enterocyte. FEBS Open Bio. 2022;12:186–186.
Al-Nabulsi AA, Jaradat ZW, Al Qudsi FR, Elsalem L, Osaili TM, Olaimat AN, et al. Characterization and bioactive properties of exopolysaccharides produced by Streptococcus thermophilus and Lactobacillus bulgaricus isolated from labaneh. LWT-Food Sci Technol. 2022;167:113817.
Article CAS Google Scholar
Chen L, Huang G. The antiviral activity of polysaccharides and their derivatives. Int J Biol Macromol. 2018;115:77–82.
Kodali VP, Sen R. Antioxidant and free radical scavenging activities of an exopolysaccharide from a probiotic bacterium. Biotechnol J. 2008;3:245–51.
Zou Y, Xue W, Lin X, Hu T, Liu S-W, Sun C-H, et al. Taxonomic description and genome sequence of Christensenella intestinihominis sp. Nov., a novel cholesterol-lowering bacterium isolated from human gut. Front Microbiol. 2021;12:632361.
Sun H, Yu X, Li T, Zhu Z. Structure and hypoglycemic activity of a novel exopolysaccharide of Cordyceps militaris . Int J Biol Macromol. 2021;166:496–508.
Qi Y, Wang D, Fang L, Liu X, Liu C, Zhao F, et al. Hypoglycemic effect of exopolysaccharide from Lactiplantibacillus plantarum JLAU103 on streptozotocin and high-fat diet-induced type 2 diabetic mice. Foods. 2022;11:3571.
Chen F, Huang G. Preparation and immunological activity of polysaccharides and their derivatives. Int J Biol Macromol. 2018;112:211–6.
Oerlemans MMP, Akkerman R, Ferrari M, Walvoort MTC, de Vos P. Benefits of bacteria-derived exopolysaccharides on gastrointestinal microbiota, immunity and health. J Funct Food. 2021;76:104289.
Xu M, Li Z, Zhao X, Li W. Prebiotic properties of exopolysaccharides from Lactobacillus helveticus LZ-R-5 and L. pentosus LZ-R-17 evaluated by in vitro simulated digestion and fermentation. Foods. 2022;11:2501.
Wu J-Y, Siu K-C, Geng P. Bioactive ingredients and medicinal values of Grifola frondosa (Maitake). Foods. 2021;10:95.
Freitas F, Alves VD, Reis MAM. Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol. 2011;29:388–98.
Hao L, Liu W, Liu K, Shan K, Wang C, Xi C, et al. Isolation, optimization of fermentation conditions, and characterization of an exopolysaccharide from Pseudoalteromonas agarivorans Hao 2018. Mar Drugs. 2019;17:703.
Wang C, Fan Q, Zhang X, Lu X, Xu Y, Zhu W, et al. Isolation, characterization, and pharmaceutical applications of an exopolysaccharide from Aerococcus Uriaeequi . Mar Drugs. 2018;16:337.
Xu L, Qiu Z, Gong H, Zhu C, Sang Q, Li Y, et al. Synergy of microbial polysaccharides and branched-preformed particle gel on thickening and enhanced oil recovery. Chem Eng Sci. 2019;208:115138.
Huang G, Huang H. Hyaluronic acid-based biopharmaceutical delivery and tumor-targeted drug delivery system. J Control Release. 2018;278:122–6.
Huang G, Liu Y, Chen L. Chitosan and its derivatives as vehicles for drug delivery. Drug Delivery. 2017;24:108–13.
Huang G, Huang H. Application of hyaluronic acid as carriers in drug delivery. Drug Delivery. 2018;25:766–72.
Tang Q, Huang G. Preparation and applications of glyconanoparticles. Int J Biol Macromol. 2018;116:927–30.
Di W, Zhang L, Yi H, Han X, Zhang Y, Xin L. Exopolysaccharides produced by Lactobacillus strains suppress HT-29 cell growth via induction of G0/G1 cell cycle arrest and apoptosis. Oncol Lett. 2018;16:3577–86.
PubMed PubMed Central Google Scholar
Gugliandolo C, Spano A, Maugeri TL, Poli A, Arena A, Nicolaus B. Role of bacterial exopolysaccharides as agents in counteracting immune disorders induced by herpes virus. Microorganisms. 2015;3:464–83.
Gao X, Li X, Mu J, Ho C-T, Su J, Zhang Y, et al. Preparation, physicochemical characterization, and anti-proliferation of selenium nanoparticles stabilized by Polyporus umbellatus polysaccharide. Int J Biol Macromol. 2020;152:605–15.
Nagae M, Yamaguchi Y. Sugar recognition and protein–protein interaction of mammalian lectins conferring diverse functions. Curr Opin Struct Biol. 2015;34:108–15.
Cao X, Du X, Jiao H, An Q, Chen R, Fang P, et al. Carbohydrate-based drugs launched during 2000–2021. Acta Pharmaceutica Sinica B. 2022;12:3783–821.
He XM, Liu H. Formation of unusual sugars: mechanistic studies and biosynthetic applications. Annu Rev Biochem. 2002;71:701–54.
Wang X, Sun G, Feng T, Zhang J, Huang X, Wang T, et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019;29:787–803.
Livesey G. Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutr Res Rev. 2003;16:163–91.
Chaisuwan W, Jantanasakulwong K, Wangtueai S, Phimolsiripol Y, Chaiyaso T, Techapun C, et al. Microbial exopolysaccharides for immune enhancement: fermentation, modifications and bioactivities. Food Biosci. 2020;35:100564.
Hou C, Yin M, Lan P, Wang H, Nie H, Ji X. Recent progress in the research of Angelica sinensis (Oliv.) diels polysaccharides: extraction, purification, structure and bioactivities. Chem Biol Technol Agric. 2021;8:13.
Ji X, Peng B, Ding H, Cui B, Nie H, Yan Y. Purification, structure and biological activity of pumpkin polysaccharides: a review. Food Rev Intl. 2023;39:307–19.
Ji X, Hou C, Shi M, Yan Y, Liu Y. an insight into the research concerning panax ginseng C. A. Meyer polysaccharides: a review. Food Rev Int. 2022;38:1149–65.
Article Google Scholar
Shukla S. Secondary metabolites from marine microorganisms and therapeutic efficacy: a mini review. IJMS Vol45(10) [October 2016]. 2016. http://nopr.niscpr.res.in/handle/123456789/35709 . Accessed 12 Apr 2023.
Singh R, Kumar M, Mittal A, Mehta PK. Microbial metabolites in nutrition, healthcare and agriculture. 3 Biotech. 2017;7:1–14.
Chen Y, Rohrbach M, Yan Z, Shuicheng Y, Feng J, Kalantidis Y. Graph-Based Global Reasoning Networks. 2019. p. 433–42. https://openaccess.thecvf.com/content_CVPR_2019/html/Chen_Graph-Based_Global_Reasoning_Networks_CVPR_2019_paper.html . Accessed 16 Aug 2023.
Lynch KM, Zannini E, Coffey A, Arendt EK. Lactic acid bacteria exopolysaccharides in foods and beverages: isolation, properties, characterization, and health benefits. Annu Rev Food Sci Technol. 2018;9:155–76.
Barcelos MCS, Vespermann KAC, Pelissari FM, Molina G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit Rev Food Sci Nutr. 2020;60:1475–95.
Zhang R, Edgar KJ. Properties, chemistry, and applications of the bioactive polysaccharide curdlan. Biomacromol. 2014;15:1079–96.
Jiang Y, Yang Z. A functional and genetic overview of exopolysaccharides produced by Lactobacillus plantarum. J Funct Food. 2018;47:229–40.
Sharma V, Harjai K, Shukla G. Effect of bacteriocin and exopolysaccharides isolated from probiotic on P-aeruginosa PAO1 biofilm. Folia Microbiol. 2018;63:181–90.
Cheng K-C, Demirci A, Catchmark JM. Pullulan: biosynthesis, production, and applications. Appl Microbiol Biotechnol. 2011;92:29–44.
Jaiswal P, Sharma R, Sanodiya BS, Bisen PS. Microbial exopolysaccharides: natural modulators of dairy products. J app pharm sci. 2014;4:105–9.
Schmid J, Farina J, Fiehm B, Sieber V. Editorial: microbial exopolysaccharides: from genes to applications. Front Microbiol. 2016;7:308.
Do TBT, Tran BK, Tran TVT, Le TH, Cnockaert M, Vandamme P, et al. Decoding the capability of Lactobacillus plantarum W1 isolated from soybean whey in producing an exopolysaccharide. ACS Omega. 2020;5:33387–94.
Wang Z. Analysis of monosaccharide composition of extracellular polysaccharides from Lactobacillus casei . Food Industry. 2017;10:88–92.
Liao Y, Gao M, Wang Y, Liu X, Zhong C, Jia S. Structural characterization and immunomodulatory activity of exopolysaccharide from Aureobasidium pullulans CGMCC 23063. Carbohydr Polym. 2022;288:119366.
Sun L, Cheng L, Ma Y, Lei P, Wang R, Gu Y, et al. Exopolysaccharides from Pantoea alhagi NX-11 specifically improve its root colonization and rice salt resistance. Int J Biol Macromol. 2022;209:396–404.
Li J, Wu H, Liu Y, Nan J, Park HJ, Chen Y, et al. The chemical structure and immunomodulatory activity of an exopolysaccharide produced by Morchella esculenta under submerged fermentation. Food Funct. 2021;12:9327–38.
Mathivanan K, Chandirika JU, Vinothkanna A, Govindarajan RK, Meng D, Yin H. Characterization and biotechnological functional activities of exopolysaccharides produced by Lysinibacillus fusiformis KMNTT-10. J Polym Environ. 2021;29:1742–51.
Roger O, Kervarec N, Ratiskol J, Colliec-Jouault S, Chevolot L. Structural studies of the main exopolysaccharide produced by the deep-sea bacterium Alteromonas infernus . Carbohyd Res. 2004;339:2371–80.
Mazza M, Alliot C, Sinquin C, Colliec-Jouault S, Reiller PE, Huclier-Markai S. Marine exopolysaccharide complexed with scandium aimed as theranostic agents. Molecules. 2021;26:1143.
Qing Z, Jie W, Qing S, Shu-Ming Z, Xiang-Yang S, Chan-Yuan L, et al. Characterization and antioxidant activity of released exopolysaccharide from potential probiotic Leuconostoc mesenteroides LM187. J Microbiol Biotechnol. 2021;31:1144–53.
Zhao D, Jiang J, Liu L, Wang S, Ping W, Ge J. Characterization of exopolysaccharides produced by Weissella confusa XG-3 and their potential biotechnological applications. Int J Biol Macromol. 2021;178:306–15.
Aburas H, Ispirli H, Taylan O, Yilmaz MT, Dertli E. Structural and physicochemical characterisation and antioxidant activity of an alpha-D-glucan produced by sourdough isolate Weissella cibaria MED17. Int J Biol Macromol. 2020;161:648–55.
Hao Y, Sun H, Zhang X, Wu L, Zhu Z. A novel acid polysaccharide from fermented broth of Pleurotus citrinopileatus : Hypoglycemic activity in vitro and chemical structure. J Mol Struct. 2020;1220:128717.
Chen L, Wang Z, Zhang B, Ge M, Ng H, Niu Y, et al. Production, structure and morphology of exopolysaccharides yielded by submerged fermentation of Antrodia cinnamomea . Carbohydr Polym. 2019;205:271–8.
Liu T, Zhou K, Yin S, Liu S, Zhu Y, Yang Y, et al. Purification and characterization of an exopolysaccharide produced by Lactobacillus plantarum HY isolated from home-made Sichuan Pickle. Int J Biol Macromol. 2019;134:516–26.
Long Z, Liu H, Li J, Sun J, Xue Y, Hu Z, et al. Preliminary characterization of exopolysaccharides produced by Abortiporus biennis in submerged fermentation. Sains Malays. 2019;48:2633–40.
Min W-H, Fang X-B, Wu T, Fang L, Liu C-L, Wang J. Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J Biosci Bioeng. 2019;127:758–66.
Nehal F, Sahnoun M, Smaoui S, Jaouadi B, Bejar S, Mohammed S. Characterization, high production and antimicrobial activity of exopolysaccharides from Lactococcus lactis F-mou. Microb Pathog. 2019;132:10–9.
Sahana TG, Rekha PD. A bioactive exopolysaccharide from marine bacteria Alteromonas sp. PRIM-28 and its role in cell proliferation and wound healing in vitro. Int J Biol Macromol. 2019;131:10–8.
Wang K, Niu M, Yao D, Zhao J, Wu Y, Lu B, et al. Physicochemical characteristics and in vitro and in vivo antioxidant activity of a cell-bound exopolysaccharide produced by Lactobacillus fermentum S1. Int J Biol Macromol. 2019;139:252–61.
Xu Y, Cui Y, Wang X, Yue F, Shan Y, Liu B, et al. Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041. Int J Biol Macromol. 2019;128:480–92.
Jia X, Qu L, Panpan R, Liu S, Wu Y, Xu C. Characterization and antioxidant activity of an exopolysaccharide produced by Rigidoporus microporus (Agaricomycetes). Int J Med Mushrooms. 2018;20:311–20.
Article PubMed Google Scholar
Zhao W, Zhang J, Jiang Y-Y, Zhao X, Hao X-N, Li L, et al. Characterization and antioxidant activity of the exopolysaccharide produced by Bacillus amyloliquefaciens GSBa-1. J Microbiol Biotechnol. 2018;28:1282–92.
Zhang H, Ren W, Guo Q, Xiong Z, Wang G, Xia Y, et al. Characterization of a yogurt-quality improving exopolysaccharide from Streptococcus thermophilus AR333. Food Hydrocolloids. 2018;81:220–8.
Kanamarlapudi SLRK, Muddada S. Characterization of exopolysaccharide produced by Streptococcus thermophilus CC30. Biomed Res Int. 2017;2017:e4201809.
Xiao M, Ren X, Yu Y, Gao W, Zhu C, Sun H, et al. Fucose-containing bacterial exopolysaccharides: sources, biological activities, and food applications. Food Chem X. 2022;13:100233.
Yang M, Zhou D, Xiao H, Fu X, Kong Q, Zhu C, et al. Marine-derived uronic acid-containing polysaccharides: structures, sources, production, and nutritional functions. Trends Food Sci Technol. 2022;122:1–12.
Andrew M, Jayaraman G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr Res. 2020;487:107881.
Abdhul K, Ganesh M, Shanmughapriya S, Kanagavel M, Anbarasu K, Natarajaseenivasan K. Antioxidant activity of exopolysaccharide from probiotic strain Enterococcus faecium (BDU7) from Ngari. Int J Biol Macromol. 2014;70:450–4.
Zhu Y, Wang C, Jia S, Wang B, Zhou K, Chen S, et al. Purification, characterization and antioxidant activity of the exopolysaccharide from Weissella cibaria SJ14 isolated from Sichuan paocai. Int J Biol Macromol. 2018;115:820–8.
Adebayo-Tayo B, Ishola R, Oyewunmi T. Characterization, antioxidant and immunomodulatory potential on exopolysaccharide produced by wild type and mutant Weissella confusa strains. Biotechnol Rep. 2018;19:e00271.
Tang W, Dong M, Wang W, Han S, Rui X, Chen X, et al. Structural characterization and antioxidant property of released exopolysaccharides from Lactobacillus delbrueckii ssp bulgaricus SRFM-1. Carbohydr Polym. 2017;173:654–64.
Hu X, Pan X, Wang PG, Chen M. Isolation and characterization of an antioxidant exopolysaccharide produced by Bacillus sp. S-1 from Sichuan Pickles. Carbohydr Polym. 2019;204:9–16.
Paikra SK, Panda J, Sahoo G, Mishra M. Characterization of exopolysaccharide derived from Enterobacter ludwigii and its possible role as an emulsifier. 3 Biotech. 2022;12:212.
Delbarre-Ladrat C, Sinquin C, Lebellenger L, Zykwinska A, Colliec-Jouault S. Exopolysaccharides produced by marine bacteria and their applications as glycosaminoglycan-like molecules. Front Chem. 2014;2:85.
Plazinski W, Drach M, Plazinska A. Ring inversion properties of 1→2, 1→3 and 1→6-linked hexopyranoses and their correlation with the conformation of glycosidic linkages. Carbohyd Res. 2016;423:43–8.
Peesapati S, Sajeevan KA, Patel SK, Roy D. Relation between glycosidic linkage, structure and dynamics of α- and β-glucans in water. Biopolymers. 2021;112:e23423.
Yang X, Wu J, An F, Xu J, Bat-Ochir M, Wei L, et al. Structure characterization, antioxidant and emulsifying capacities of exopolysaccharide derived from Tetragenococcus halophilus SNTH-8. Int J Biol Macromol. 2022;208:288–98.
Li F, Hu X, Sun X, Li H, Lu J, Li Y, et al. Effect of fermentation pH on the structure, rheological properties, and antioxidant activities of exopolysaccharides produced by Alteromonas australica QD. Glycoconjugate J. 2022;39:773–87.
Ju Y, Shan K, Liu W, Xi C, Zhang Y, Wang W, et al. Effect of different initial fermentation pH on exopolysaccharides produced by Pseudoalteromonas agarivorans Hao 2018 and identification of key genes involved in exopolysaccharide synthesis via transcriptome analysis. Mar Drugs. 2022;20:89.
Ohno N, Miura T, Miura NN, Adachi Y, Yadomae T. Structure and biological activities of hypochlorite oxidized zymosan. Carbohyd Polym. 2001;44:339–49.
Zhang H, Wang Z, Wang X, Yao L, Wu Z, Yang X. Research progress in chemical modification methods and biological activities of polysaccharides. Food Ferment Ind. 2010;36:102–7.
Huang F, Meng Y. Research progress in active polysaccharides. Natural Product Research and Development. 1999;5:90–8.
Ramamurthy D, Nundalall T, Cingo S, Mungra N, Karaan M, Naran K, et al. Recent advances in immunotherapies against infectious diseases. Immunother Adv. 2021;1:Itaa007.
Misaki A, Kakuta M, Sasaki T, Tanaka M, Miyaji H. Studies on interrelation of structure and antitumor effects of polysaccharides: Antitumor action of periodate-modified, branched (1 → 3)-β-d-glucan of auricularia auricula-judae, and other polysaccharides containing (1 → 3)-glycosidic linkages. Carbohyd Res. 1981;92:115–29.
Demleitner S, Kraus J, Franz G. Synthesis and antitumour activity of derivatives of curdlan and lichenan branched at C-6. Carbohyd Res. 1992;226:239–46.
Öner ET, Hernández L, Combie J. Review of Levan polysaccharide: from a century of past experiences to future prospects. Biotechnol Adv. 2016;34:827–44.
Kasaai MR. A comparative study of molecular structure, solution properties and food application for three branched polysaccharides: amylopectin, glycogen, and dextran. Curr Trends Polym Sci. 2012;16:49–63.
CAS Google Scholar
Guo MQ, Hu X, Wang C, Ai L. Polysaccharides: structure and solubility. In: Zhenbo Xu, editor. Solubility of polysaccharides. Rijeka: IntechOpen; 2017.
Chapter Google Scholar
Bohn JA, BeMiller JN. (1→3)-β-d-Glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohyd Polym. 1995;28:3–14.
Tang W, Han S, Zhou J, Xu Q, Dong M, Fan X, et al. Selective fermentation of Lactobacillus delbrueckii ssp. Bulgaricus SRFM-1 derived exopolysaccharide by Lactobacillus and Streptococcus strains revealed prebiotic properties. J Funct Foods. 2020;69:103952.
Linghong N, Ning Z. Relationship between structure and activity of active polysaccharides. Chem Ind For Prod. 2003;23:89–94.
Chen X, Xu X, Zhang L, Zeng F. Chain conformation and anti-tumor activities of phosphorylated (1→3)-β-d-glucan from Poria cocos . Carbohyd Polym. 2009;78:581–7.
Zhang L, Li X, Xu X, Zeng F. Correlation between antitumor activity, molecular weight, and conformation of lentinan. Carbohyd Res. 2005;340:1515–21.
Maeda YY, Hamuro J, Chihara G. The mechanisms of action of anti-tumour polysaccharides. I. The effects of antilymphocyte serum on the anti-tumour activity of lentinan. Int J Cancer. 1971;8:41–6.
Saitô H, Yoshioka Y, Yokoi M, Yamada J. Distinct gelation mechanism between linear and branched (1 → 3)-β-D-glucans as revealed by high-resolution solid-state 13C NMR. Biopolymers. 1990;29:1689–98.
Yu Z, Ming G, Kaiping W, Zhixiang C, Liquan D, Jingyu L, et al. Structure, chain conformation and antitumor activity of a novel polysaccharide from Lentinus edodes . Fitoterapia. 2010;81:1163–70.
Zhu Z-Y, Dong F, Liu X, Lv Q, YingYang LF, et al. Effects of extraction methods on the yield, chemical structure and anti-tumor activity of polysaccharides from Cordyceps gunnii mycelia. Carbohydr Polym. 2016;140:461–71.
Wang K, Li W, Rui X, Chen X, Jiang M, Dong M. Structural characterization and bioactivity of released exopolysaccharides from Lactobacillus plantarum 70810. Int J Biol Macromol. 2014;67:71–8.
Kokoulin MS, Kuzmich AS, Romanenko LA, Chikalovets IV. Sulfated capsular polysaccharide from the marine bacterium Kangiella japonica inhibits T-47D cells growth in vitro . Carbohydr Polym. 2022;290:119477.
Kokoulin MS, Sigida EN, Kuzmich AS, Ibrahim IM, Fedonenko YP, Konnova SA. Structure and antiproliferative activity of the polysaccharide from Halomonas aquamarina related to Cobetia pacifica. Carbohydr Polym. 2022;298:120125.
Selim S, Almuhayawi MS, Alharbi MT, Nagshabandi MK, Alanazi A, Warrad M, et al. In vitro assessment of antistaphylococci, antitumor, immunological and structural characterization of acidic bioactive exopolysaccharides from marine Bacillus cereus isolated from Saudi Arabia. Metabolites. 2022;12:132.
Wang N, Shan Z, Jia X, Wang Y, Song S, Xiao D, et al. Galf-containing polysaccharides from medicinal molds: sources, structures and bioactive properties. Trends Food Sci Technol. 2023;131:244–63.
Notermans S, Veeneman GH, Van Zuylen CWEM, Hoogerhout P, Van Boom JH. (15)-linked β-D-galactofuranosides are immunodominant in extracellular polysaccharides of Penicillium and Aspergillus species. Mol immunol. 1988;25:975–9.
Jia K, Wei M, He Y, Wang Y, Wei H, Tao X. Characterization of novel exopolysaccharides from Enterococcus hirae WEHI01 and its immunomodulatory activity. Foods. 2022;11:3538.
Hidalgo-Cantabrana C, Lopez P, Gueimonde M, de Reyes-Gavilan CG, Suarez A, Margolles A, et al. Immune modulation capability of exopolysaccharides synthesised by lactic acid bacteria and bifidobacteria. Probiotics Antimicrob Proteins. 2012;4:227–37.
Liu S, Zhou W, Ye W, Chen J, Wu C, Chen D, et al. Research advance on biological activity and structure-activity relationships of bioactive polysaccharide. Food Res Dev. 2017;38:211–8.
Surayot U, Wang J, Seesuriyachan P, Kuntiya A, Tabarsa M, Lee Y, et al. Exopolysaccharides from lactic acid bacteria: structural analysis, molecular weight effect on immunomodulation. Int J Biol Macromol. 2014;68:233–40.
Xia W, Han J, Zhu S, Wang Y, Zhang W, Wu Z. Structural elucidation of the exopolysaccharide from Streptococcus thermophilus XJ53 and the effect of its molecular weight on immune activity. Int J Biol Macromol. 2023;230:123177.
Liu Y, Mao K, Zhang N, Chitrakar B, Huang P, Wang X, et al. Structural characterization and immunomodulatory effects of extracellular polysaccharide from Lactobacillus paracasei VL8 obtained by gradient ethanol precipitation. J Food Sci. 2022;87:2034–47.
Nishimura-Uemura J, Kitazawa H, Kawai Y, Itoh T, Oda M, Saito T. Functional alteration of murine macrophages stimulated with extracellular polysaccharides from Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Food Microbiol. 2003;20:267–73.
Ji X, Guo J, Cao T, Zhang T, Liu Y, Yan Y. Review on mechanisms and structure-activity relationship of hypoglycemic effects of polysaccharides from natural resources. Food Sci Human Wellness. 2023;12:1969–80.
Yang S, Qu Y, Zhang H, Xue Z, Liu T, Yang L, et al. Hypoglycemic effects of polysaccharides from Gomphidiaceae rutilus fruiting bodies and their mechanisms. Food Funct. 2020;11:424–34.
Huang Z, Lin F, Zhu X, Zhang C, Jiang M, Lu Z. An exopolysaccharide from Lactobacillus plantarum H31 in pickled cabbage inhibits pancreas α-amylase and regulating metabolic markers in HepG2 cells by AMPK/PI3K/Akt pathway. Int J Biol Macromol. 2020;143:775–84.
Wang C, Li W, Chen Z, Gao X, Yuan G, Pan Y, et al. Effects of simulated gastrointestinal digestion in vitro on the chemical properties, antioxidant activity, α-amylase and α-glucosidase inhibitory activity of polysaccharides from Inonotus obliquus . Food Res Int. 2018;103:280–8.
Yang Y, Liu J, Tan Y, Wang S, Chen H, Zhou A. Progress in understanding the structure-activity relationship and hypoglycemic mechanism of polysaccharides. Food Science. 2021;42:355–63.
Ma Y, Mao D, Geng L, Wang Z, Xu C. Production, fractionation, characterization of extracellular polysaccharide from a newly isolated Trametes gibbosa and its hypoglycemic activity. Carbohydr Polym. 2013;96:460–5.
Ayyash M, Abu-Jdayil B, Itsaranuwat P, Galiwango E, Tamiello-Rosa C, Abdullah H, et al. Characterization, bioactivities, and rheological properties of exopolysaccharide produced by novel probiotic Lactobacillus plantarum C70 isolated from camel milk. Int J Biol Macromol. 2020;144:938–46.
Ahmad W, Boyajian JL, Abosalha A, Nasir A, Ashfaq I, Islam P, et al. High-molecular-weight dextran-type exopolysaccharide produced by the novel Apilactobacillus waqarii improves metabolic syndrome: in vitro and in vivo analyses. Int J Mol Sci. 2022;23:12692.
Zhu Z-Y, Guo M-Z, Liu F, Luo Y, Chen L, Meng M, et al. Preparation and inhibition on α-d-glucosidase of low molecular weight polysaccharide from Cordyceps militaris . Int J Biol Macromol. 2016;93:27–33.
Mansel BW, Ryan TM, Chen H-L, Lundin L, Williams MAK. Polysaccharide conformations measured by solution state X-ray scattering. Chem Phys Lett. 2020;739:136951.
Yu X. Study on Structure, Synthesis Related Enymes and Hypoglycemic Activity of Exopolysaccharide from Cordyceps Militaris Mycelium. Tianjin University of Science & Technology. 2019. https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C475KOm_zrgu4lQARvep2SAkHr3ADhkADnVu66WViDP_3OSMGO5HucmElnGcdQNvlVqxIp8JuV0GwGQzlMaw8gao&uniplatform=NZKPT . Accessed 8 Apr 2023.
Wang Y, Hou G, Li J, Surhio MM, Ye M. Structure characterization, modification through carboxymethylation and sulfation, and in vitro antioxidant and hypoglycemic activities of a polysaccharide from Lachnum sp. Process Biochem. 2018;72:177–87.
Zhou Y, Cui Y, Qu X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: a review. Carbohydr Polym. 2019;207:317–32.
Rajoka MSR, Wu Y, Mehwish HM, Bansal M, Zhao L. Lactobacillus exopolysaccharides: new perspectives on engineering strategies, physiochemical functions, and immunomodulatory effects on host health. Trends Food Sci Technol. 2020;103:36–48.
El-Ghonemy DH. Antioxidant and antimicrobial activities of exopolysaccharides produced by a novel Aspergillus sp. DHE6 under optimized submerged fermentation conditions. Biocatal Agric Biotechnol. 2021;36:102150.
Zhang J, Cao Y, Wang J, Guo X, Zheng Y, Zhao W, et al. Physicochemical characteristics and bioactivities of the exopolysaccharide and its sulphated polymer from Streptococcus thermophilus GST-6. Carbohyd Polym. 2016;146:368–75.
Rani RP, Anandharaj M, David RA. Characterization of a novel exopolysaccharide produced by Lactobacillus gasseri FR4 and demonstration of its in vitro biological properties. Int J Biol Macromol. 2018;109:772–83.
Ayyash M, Abu-Jdayil B, Itsaranuwat P, Almazrouei N, Galiwango E, Esposito G, et al. Exopolysaccharide produced by the potential probiotic Lactococcus garvieae C47: structural characteristics, rheological properties, bioactivities and impact on fermented camel milk. Food Chem. 2020;333:127418.
Fan Y, Li X, Tian R, Tang R, Zhang J. Characterization and biological activity of a novel exopolysaccharide produced by Pediococcus pentosaceus SSC-12 from silage. Microorganisms. 2022;10:18.
Ding C, Wu H, Cao X, Gao Z, Tang Z, Fan W, et al. Lactobacillus crispatus -derived exopolysaccharides with antibacterial activity limit Salmonella typhimurium invasion by inhibiting inflammasome-mediated pyroptosis. Food Funct. 2022;13:10501–15.
Almutairi MH, Helal MM. Biological and microbiological activities of isolated Enterobacter sp. ACD2 exopolysaccharides from Tabuk region of Saudi Arabia. J King Saud Univ Sci. 2021;33:101328.
Matsubara VH, Wang Y, Bandara HMHN, Mayer MPA, Samaranayake LP. Probiotic lactobacilli inhibit early stages of Candida albicans biofilm development by reducing their growth, cell adhesion, and filamentation. Appl Microbiol Biotechnol. 2016;100:6415–26.
Wan C, Qian W-W, Liu W, Pi X, Tang M-T, Wang X-L, et al. Exopolysaccharide from Lactobacillus rhamnosus ZFM231 alleviates DSS-induced colitis in mice by regulating gut microbiota. J Sci Food Agric. 2022. https://www.webofscience.com/wos/alldb/full-record/MEDLINE:35707876 . Accessed 29 Jun 2022.
Wang J, Nie S, Kan L, Chen H, Cui SW, Phillips AO, et al. Comparison of structural features and antioxidant activity of polysaccharides from natural and cultured Cordyceps sinensis . Food Sci Biotechnol. 2017;26:55–62.
Ying M, Yu Q, Zheng B, Wang H, Wang J, Chen S, et al. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohyd Polym. 2020;235:115957.
Li B, Chen H, Cao L, Hu Y, Chen D, Yin Y. Escherichia coli exopolysaccharides induced by ceftriaxone regulated human gut microbiota in vitro . Front Microbiol. 2021;12:634204.
Kim G, Bae J-H, Cheon S, Lee DH, Kim DH, Lee D, et al. Prebiotic activities of dextran from Leuconostoc mesenteroides SPCL742 analyzed in the aspect of the human gut microbial ecosystem. Food Funct. 2022;13:1256–67.
Tian J, Zhao X, Tang C, Wang X, Zhang X, Xiao L, et al. Protective effect of Paecilomyces cicadae TJJ11213 exopolysaccharide on intestinal mucosa and regulation of gut microbiota in immunosuppressed mice. Food Res Int. 2023;165:112477.
Abdel-Wahab BA, Abd El-Kareem HF, Alzamami A, Fahmy CA, Elesawy BH, Mahmoud MM, et al. Novel exopolysaccharide from marine Bacillus subtilis with broad potential biological activities: insights into antioxidant, anti-inflammatory, cytotoxicity, and anti-alzheimer activity. Metabolites. 2022;12:715.
Su Y, Zhang Y, Fu H, Yao F, Liu P, Mo Q, et al. Physicochemical and anti-uvb-induced skin inflammatory properties of Lacticaseibacillus paracasei Subsp. paracasei SS-01 strain exopolysaccharide. Fermentation. 2022;8:198.
Dilna SV, Surya H, Aswathy RG, Varsha KK, Sakthikumar DN, Pandey A, et al. Characterization of an exopolysaccharide with potential health-benefit properties from a probiotic Lactobacillus plantarum RJF(4). LWT-Food Sci Technol. 2015;64:1179–86.
Morris G, Harding SE. Polysaccharides, microbial. In: Schaechter M, editor. Encyclopedia of microbiology (Third Edition). Amsterdam: Elsevier; 2009. p. 482–94.
Larsen FH, Engelsen SB. Insight into the functionality of microbial exopolysaccharides by NMR spectroscopy and molecular modeling. Front Microbiol. 2015;6:1374.
Kagimura FY, da Cunha MAA, Barbosa AM, Dekker RFH, Maneck Malfatti CR. Biological activities of derivatized D-glucans: a review. Int J Biol Macromol. 2015;72:588–98.
Wang Z, Xie J, Shen M, Nie S, Xie M. Sulfated modification of polysaccharides: synthesis, characterization and bioactivities. Trends Food Sci Technol. 2018;74:147–57.
Ren Y-Y, Sun P-P, Ji Y-P, Wang X-T, Dai S-H, Zhu Z-Y. Carboxymethylation and acetylation of the polysaccharide from Cordyceps militaris and their α-glucosidase inhibitory activities. Nat Prod Res. 2019. https://doi.org/10.1080/14786419.2018.1533830 .
Kagimura FY, da Cunha MAA, Theis TV, Malfatti CRM, Dekker RFH, Barbosa AM, et al. Carboxymethylation of (1→6)-β-glucan (lasiodiplodan): preparation, characterization and antioxidant evaluation. Carbohyd Polym. 2015;127:390–9.
Wang X, Zhang Z, Zhao M. Carboxymethylation of polysaccharides from Tremella fuciformis for antioxidant and moisture-preserving activities. Int J Biol Macromol. 2015;72:526–30.
Wang Y, Su N, Hou G, Li J, Ye M. Hypoglycemic and hypolipidemic effects of a polysaccharide from Lachnum YM240 and its derivatives in mice, induced by a high fat diet and low dose STZ. Med Chem Commun. 2017;8:964–74.
Wu Y, Ye M, Du Z, Jing L, Surahio M, Yang L. Carboxymethylation of an exopolysaccharide from Lachnum and effect of its derivatives on experimental chronic renal failure. Carbohydr Polym. 2014;114:190–5.
Li S, Xiong Q, Lai X, Li X, Wan M, Zhang J, et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr Rev Food Sci Food Saf. 2016;15:237–50.
Liu J, Luo J, Ye H, Zeng X. Preparation, antioxidant and antitumor activities in vitro of different derivatives of levan from endophytic bacterium Paenibacillus polymyxa EJS-3. Food Chem Toxicol. 2012;50:767–72.
Wang K, Li W, Rui X, Li T, Chen X, Jiang M, et al. Chemical modification, characterization and bioactivity of a released exopolysaccharide (r-EPS1) from Lactobacillus plantarum 70810. Glycoconj J. 2015;32:17–27.
Zhang M, Su N, Huang Q, Zhang Q, Wang Y, Li J, et al. Phosphorylation and antiaging activity of polysaccharide from Trichosanthes peel. J Food Drug Anal. 2017;25:976–83.
Guo Y, Pan D, Sun Y, Xin L, Li H, Zeng X. Antioxidant activity of phosphorylated exopolysaccharide produced by Lactococcus lactis subsp. lactis. Carbohydr Polym. 2013;97:849–54.
He Y, Ye M, Jing L, Du Z, Surhio MM, Xu H, et al. Preparation, characterization and bioactivities of derivatives of an exopolysaccharide from Lachnum . Carbohyd Polym. 2015;117:788–96.
Jin M, Wang Y, Huang M, Lu Z, Wang Y. Sulphation can enhance the antioxidant activity of polysaccharides produced by Enterobacter cloacae Z0206. Carbohyd Polym. 2014;99:624–9.
Li S, Shah NP. Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC 1275. Food Chem. 2014;165:262–70.
Download references
Acknowledgements
We would like to thank Editage ( www.editage.cn ) for English language editing.
This research was supported by the Natural Science Foundation of Shandong Province (ZR2022MD097, ZR2012CM019), Education and Industry Integration Innovation Pilot Project of Qilu University of Technology (Shandong Academy of Sciences) (2022JBZ01-06), the Foundation of State Key Laboratory of Biobased Material and Green Papermaking (No. ZZ20190302), the Foundation of Shandong Provincial Key Laboratory of Biosensors (SWCG 2018–01), and the Foundation (No. 202002) of Qilu University of Technology of Cultivating Subject for Biology and Biochemistry, Science, Education and Industry Integration Innovation Pilot Project of Qilu University of Technology (Shandong Academy of Sciences) (2020KJC-ZD08).
Author information
Authors and affiliations.
School of Bioengineering, Qilu University of Technology (Shandong Academy of Sciences), Jinan, 250353, China
Wei Wang, Nan Liu, Shengbo Shi & Lujiang Hao
State Key Laboratory of Biobased Material and Green Papermaking, Qilu University of Technology (Shandong Academy of Sciences), Jinan, China
Lujiang Hao
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, and Chemical Biology Center, Peking University, Beijing, China
You can also search for this author in PubMed Google Scholar
Contributions
WW writing-review & editing. LH supervision, writing review & editing. YJ and SS writing-review & editing. YJ writing-review & editing, visualization. NL project administration, funding acquisition. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Lujiang Hao .
Ethics declarations
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Wang, W., Ju, Y., Liu, N. et al. Structural characteristics of microbial exopolysaccharides in association with their biological activities: a review. Chem. Biol. Technol. Agric. 10 , 137 (2023). https://doi.org/10.1186/s40538-023-00515-3
Download citation
Received : 24 October 2023
Accepted : 22 November 2023
Published : 28 November 2023
DOI : https://doi.org/10.1186/s40538-023-00515-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Exopolysaccharides
- Structure–activity relationship
- Biological activity
- Modification
ORIGINAL RESEARCH article
Production and characterization of exopolysaccharide from newly isolated marine probiotic lactiplantibacillus plantarum ei6 with in vitro wound healing activity.

- National Institute of Oceanography and Fisheries, NIOF, Egypt
Because of its safety, biological activities, and unique properties, exopolysaccharide (EPS) from lactic acid bacteria (LAB) has been developed as a potential biopolymer. A few studies have investigated the EPS produced by marine LAB. This study reports the wound healing activity of an EPS produced by a marine isolate identified as Lactiplantibacillus plantarum EI6, in addition to assessing L. plantarum EI6's probiotic properties. EI6 demonstrated promising antimicrobial activity against different pathogenic bacteria, as well as the ability to withstand stomach pH 3, tolerate 0.3% bile salt concentration, and exhibit no signs of hemolysis. Furthermore, EI6 was able to produce 270 mg/L of EPS upon growth for 48 h at 37°C in an MRS medium enriched with 1.0% of sucrose. The chemical features of the novel EI6-EPS were investigated: the UV-vis estimated a high carbohydrate content of ~91.5%, and the FTIR emphasized its polysaccharide nature by the characteristic hydroxyl, amide I, II, & III, and glycosidic linkage regions. The GC-MS and NMR analyses revealed the existence of five monosaccharides, namely, rhamnose, galactose, mannose, glucose, and arabinose, existing mainly in the pyranose form and linked together by α- and β-glycosidic linkages. EI6-EPS was found to be safe (IC50 > 100 μg/ml) and induced human skin fibroblasts (HSF) proliferation and migration. These findings imply that EI6 can be used as a safe source of bioactive polymer in wound care.
Introduction
The skin is the body's largest organ. It serves as a barrier and is the first line of defense against pathogens entering the body. The skin's microbiota maintains its health and homeostasis. Undesirable alternations of this homeostasis occur daily due to accidental burns, inflammation, wounds, and surgeries. In healthy individuals, wounds heal normally in a few days, but in some cases, such as in diabetic patients, some wounds take longer to heal or do not heal at all ( Mohammed et al., 2016 ). Infection of the wound is a severe complication in such cases, further delaying natural wound healing ( Wei et al., 2019 ). These patients benefit significantly from the use of topical treatments to accelerate wound healing ( Yates et al., 2012 ). Many of the available wound healing drugs are costly and induce several side effects. Therefore, there is a constant demand for effective wound healing bioactive compounds of natural origins that are safe, effective in cost, and can be better tolerated by patients ( Demirci et al., 2014 ; Okur et al., 2020 ). Curcumin, quercetin, essential oils, lawsone, resveratrol, aloe vera, andrographolide, bilirubin, and astragaloside are some bioactive compounds that have demonstrated a significant wound healing potential ( Kant et al., 2021 ).
Since the ocean covers more than 70% of the earth's surface, a diverse range of marine species provides a plentiful supply of natural products, and the relevance of marine organisms as a source of new bioactive compounds is proliferating ( Lindequist, 2016 ). The sea provides a tremendous resource for new chemicals, with marine organisms accounting for almost half of the world's biodiversity ( López-Abarrategui et al., 2012 ). Furthermore, marine organisms have produced different types of substances for a variety of reasons, including the fact that they live in a very demanding, competitive, and aggressive environment, which is very different in many ways from the terrestrial environment and necessitates the production of very specific and potent active molecules ( Demain et al., 2017 , Pham et al., 2019 ).
Exopolysaccharides (EPS) is a group of high molecular weight biopolymers produced during the metabolic process of some microorganisms such as bacteria and fungi ( Guo et al., 2013 ). Since EPS can be composed of one or more types of monosaccharides, they are classified as homopolysaccharides or heteropolysaccharides ( Min et al., 2019 ). They can also be produced, either attached to the microorganism cell surface, forming a capsule, or released into the surrounding environment ( Mazzoli et al., 2014 ). Bacteria are the most commonly used source for EPS production as they replicate rapidly, and the EPS forms loosely attached mucoid layers that can be easily separated from cells by any EPS isolation methods. They are nontoxic, biocompatible, and biodegradable ( Angelin and Kavitha, 2020 ).
Moreover, each bacterial strain produces distinct EPS with different biological activities, and bacterial EPS can be utilized alone or in combination with other materials for a wide range of applications in the biomedical and pharmaceutical fields ( Abdelhamid et al., 2020 ). The vast applications of bacterial EPS can be explained by the large number of derivatives obtained by controlling production parameters. The producing strain, the culture media composition, and culture conditions can affect the quantity, chemical structure, and bioactivity of the produced EPS ( Yilmaz et al., 2015 ). Therefore, careful selection of the EPS-producing strains, optimization of production conditions, and detailed information about the EPS structure are significant for novel EPS production with prominent biological activities ( Bachtarzi et al., 2020 ).
One of the main problems that prevents the large-scale manufacturing and commercialization of certain EPS is the pathogenicity of some of the producing strains ( Costa et al., 2018 ). On the contrary, probiotic bacteria such as Lactobacillus, Bifidobacterium, Lactococcus , and Streptococcus are generally regarded as safe, so they have been widely used for EPS production. Moreover, they can survive in gastric conditions such as high bile salt concentrations and low pH, in addition to managing to colonize the intestine. Recent studies have suggested that applying lactic acid bacteria (LAB) to the skin enhances skin health and contributes to fighting diseases. Lactobacilli strains have been proven to aid in wound healing, resistance to pathogens, and the recovery of skin inflammations. Lactobacillus sp. represents a large group of potential probiotic bacteria, and they are found in a variety of nutrition-rich habitats, including food and feed, and the gastrointestinal tracts of humans, fish, and animals ( Duar et al., 2017 ; Brandi et al., 2020 ).
The probiotic EPS has been shown to act as a prebiotic that enhances the growth and colonization of favorable bacteria in the human gastrointestinal tract ( Welman and Maddox, 2003 ). Some probiotic species have been proven to enhance wound repair in the gastrointestinal tract in different in vitro and in vivo studies ( Lukic et al., 2017 ). For instance, in the presence of L. rhamnosus GG and L. gasseri , the healing of stomach ulcers in rats is thought to be hastened. As probiotics have been shown to aid in the healing of gastric wounds, researchers have begun to investigate if they might aid in the healing of cutaneous wounds as well ( Sultana et al., 2013 ). At the same time, probiotic derivatives may be more suitable for wound healing applications, as there is no need to overcome the obstacles concerning maintaining the viability of living cells.
Therefore, this study aims to isolate EPS-producing marine LAB, assess its probiotic potential, produce a novel safe EPS with wound healing activity, and characterize its structural properties using various techniques.
Materials and Methods
Isolation and identification of eps-producing marine lab strain.
Marine shrimp samples (5 samples) were used to isolate an EPS-producing LAB. The samples were dissected, and serial dilutions of the fragmented guts were prepared using sterile saline. Subsequently, 1 ml of each dilution was inoculated into De Man, Rogosa, and Sharpe (MRS) medium (Lab M, UK) agar plates and incubated at 37°C for 48 h under anaerobic conditions (candle jar) ( Amiza et al., 2006 ). Mucoid colonies were picked up for purification by successive streaking on MRS agar plates ( Abid et al., 2018 ). Gram-positive, catalase-negative, non-motile, and non-spore-forming isolates were stored in MRS broth with glycerol at 20% (v/v) at −20°C for further studies.
Of the obtained isolates, four, namely, EI6, EI7, EI8, and EI9, were grown in MRS broth (200 ml) supplemented with 1.0% sucrose sugar for 48 h at 37°C. The cultures were then filtered through a bacterial filter to remove bacterial cells. For protein degradation, trichloroacetic acid (TCA, 10% w/v) was added to the supernatant for 30 min. Then, the culture supernatants were centrifuged at 5,000 rpm for 15 min, and the pellets were discarded. Next, cold absolute ethanol was added to the culture supernatants (3:1 v/v) and stored for 48 h at 4°C. The supernatants were centrifuged at 5,000 rpm for 15 min, and the precipitates were recovered and dialyzed against distilled water overnight through bags with a pore size of 12 kDa (Sigma, USA). Afterward, the obtained EPS was dried overnight at 30°C, and the dry weight was determined as the mean ± SD of three independent experiments ( Amer et al., 2020 ).
The isolate EI6 with the highest EPS yield was investigated under a scanning electron microscope (SEM) and characterized biochemically by the VITEK 2 system version 07.01 (BioMerieux, France) at Mabaret El Asafra Laboratories, Egypt. Moreover, the bacterial isolate was identified by 16s rRNA gene sequencing analysis. Total DNA was isolated by the DNA isolation kit (Qiagen, Germany) as described by the manufacturer. The isolated DNA was visualized by ethidium bromide staining after electrophoresis in a 1.0% agarose gel, and it was amplified by PCR using the primer pairs (Uni27F, 5′-AGAGTTTGATCCTGGCTCAG-3′ and Uni1492R, 5′-GGTTACCTTGTTACGACTT-3′) under the following conditions: 3 min of denaturation at 94°C, followed by 35 cycles of amplification at 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min. Final extension was allowed at 72°C for 10 min. The PCR product was sequenced by the Applied Biotechnology Company, Egypt. The obtained sequence was analyzed using BLAST, and the phylogenetic analysis was performed using the facilities provided by the website http://www.phylogeny.fr/ .
Assessment of the Probiotic Potential of EI6
Antimicrobial activity.
The antimicrobial activity of EI6 was evaluated using an agar well-cut diffusion test against the indicator pathogens ( Pseudomonas fluorescens ATCC 13525, Streptococcus agalactiae ATCC 13813, Aeromonas hydrophila ATCC 13037, Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 8739, Enterococcus faecalis ATCC 29212, and Klebsiella pneumonia ATCC 13883). In brief, 50 μl of 10 6 CFU/ml test pathogen was inoculated into nutrient agar plates, wells of 8 mm in diameter were cut into the agar, and 100 μl of cell-free culture supernatant of EI6 (adjusted to pH 6.5 with 1 M NaOH) was added and stored at 4°C for 1 h to allow diffusion, and then the plates were incubated at 37°C for 24 h. Then, the diameter of the inhibition zones around the wells was measured ( Zaghloul and Ibrahim, 2019 ).
Blood Hemolysis
Blood hemolytic activity of isolate EI6 was evaluated by streaking on blood agar plates (5.0% sheep blood) and observation of any signs of hemolysis (darkening: α-hemolysis, clear zone: β-hemolysis, and no change: γ-hemolysis) after 24 h of incubation at 37°C ( Guttmann and Ellar, 2000 ).

Antibiotic Susceptibility
Antibiotic susceptibility was evaluated as described by Abid et al. (2018) . Briefly, overnight culture of EI6 (10 6 CFU/mL) was inoculated on MRS agar plates. Antibiotic disks (Oxoid, UK) were added to the agar surface and incubated for 24 h at 37°C. Susceptibility was detected by the presence of an inhibition zone around the disk.
Low pH Resistance
The isolate EI6 was tested for its potential to withstand low pH values following the method described by Balamurugan et al. (2014) . Briefly, different MRS broths adjusted to pH 6.4, 4.0, 3.0, and 2.0 were inoculated with 1 ml of 10 6 CFU/ml of an overnight culture of EI6 and incubated for 24 h at 37°C. Bacterial growth was determined by measuring the optical density at 620 nm using a spectrophotometer (Unico, USA) at 1, 2, 3, 4, 5, 6, and 24 h.
Bile Salts Resistance
The ability of isolate EI6 to resist high bile salt concentrations was evaluated. For this purpose, an aliquot of 25 ml of MRS broth supplemented with varying concentrations of bile salts (0, 0.1, and 0.3%) was inoculated with 1.0 ml of 10 6 CFU/ml overnight culture of EI6 and incubated for 24 h at 37°C. Bacterial growth was monitored by measuring the optical density at 620 nm using a spectrophotometer (Unico, USA) at 1, 2, 3, 4, 5, 6, and 24 h ( Patel et al., 2014 ).
Mass Production of EPS
The marine bacterial isolate EI6 was used for the mass production of EPS. Isolate EI6 was cultured in 2,000 ml of MRS broth medium supplemented with 1.0% sucrose sugar (w/v) and incubated at 37°C for 48 h. Subsequently, the EPS was recovered, as previously stated.
Physicochemical Characterization of EI6-EPS
Total sugar content.
The total carbohydrate content of the purified EI6-EPS was determined using the recommended method by Dubois et al. (1956) . A 500 μl of EI6-EPS solution (5 mg/ml) was treated successively with 500 μl of phenol (2.5%; w/v), and 2.5 ml of sulfuric acid (>99.0%). The reaction mixture was then incubated at ambient temperature for 15 min before measuring the absorbance at 490 nm using a UV-vis spectrophotometer (JANEWAY 6800, UK). The carbohydrate content was expressed as glucose%, compared with the glucose standard curve (10–100 μg/ml) ( Sran et al., 2019 ).
Structural Functionalities
The purified EI6-EPS powder was subjected to FTIR spectroscopy analysis to identify the major and characteristic functional groups. The purified EI6-EPS powder was practically loaded over the single crystal germanium of the FTIR spectrometer (Bruker, ALPHA, Germany) equipped with the attenuated total reflectance (ATR) technique. After background subtraction, the spectrum was recorded between 4,000 and 400 cm −1 with a resolution of 4.0 cm −1 and 64 scans ( Amer et al., 2020 ; Ji et al., 2021 , 2022 ).
Structural Studies
1 H NMR experiment was operated on a JEOL-Ltd spectrometer (Japan, 500 MHz) for the structural studies of the EI6-EPS. Approximately 20 mg of the lyophilized powder was completely dissolved in 0.75 ml DMSO- d 6 under gentle warming. The 1 H NMR spectrum was recorded at 323 K, and the chemical shifts were expressed in ppm (δ). The data were analyzed using the MestReNova version 6.0.2-5475 (©2009 Mestrelab Research S.L.) software ( Trabelsi et al., 2017 ).
Monosaccharide Composition
The sugar moieties of the purified EI6-EPS were identified as silylated glycosides through the following three consecutive steps: acid hydrolysis, silylation, and then identification using gas chromatography-mass spectrometry (GC-MS) ( Chaplin, 1982 ; Dueñas-Chasco et al., 1997 ; Mao et al., 2006 ; Ruiz-Matute et al., 2011 ). About 20 mg of the EI6-EPS powder was hydrolyzed with 3 ml of sulfuric acid (2 M), heated at 105°C for 10 h, and then the tube was cooled at ambient temperature before neutralization with barium carbonate. The formed precipitates were removed by centrifugation, while the supernatant was filtered through a 20-μm syringe before lyophilization. The dried hydrolysates were silylated by adding 1:1 pyridine-BSTFA ( N,O -bis(trimethylsilyl) trifluoroacetamide) for 16 h at 80°C (50 μl/mg dried sample) ( Chaplin, 1982 ; Ruiz-Matute et al., 2011 ). A 2 μl of the derivatized sugars were injected into GC-MS (MassHunter GC-MS 1989-2014, Agilent Technologies, Inc.) following a previous separation method. The detector and injector temperatures were maintained at 320°C, and the column HP5MS (30 m × 0.25 mm × 0.25 μm) was first set at a temperature 100°C for 1 min and then ramped from 100 to 260°C at 4°C for 1 min, then held at 260°C for 10 min. The carrier helium gas was set at a flow rate of 1 ml/min ( Ben Gara et al., 2017 ). Finally, the identification of the monosaccharides was based on the NIST library ( Olasehinde et al., 2019 ).
Morphological and Elemental Studies
The SEM images of the EI6-EPS were captured by a scanning electron microscopy spectrometer (SEM, JSM-IT 200, Jeol, Japan). First, the sample was coated with gold (15 Å) for 2 min by physical vapor deposition before visualization at an accelerating voltage of 20.0 kV ( Ibrahim et al., 2020 ). The elemental composition analysis of EI6-EPS was then performed without any pretreatment using a scanning electron microscope-energy dispersive X-ray (SEM-EDX) spectrometer. The emitted X-rays were utilized to calculate the weight and atomic percentages of the recorded elements ( Kavita et al., 2014 ).
Cytotoxicity Assay
Human dermal fibroblast (HDF) was obtained from Nawah Scientific Inc. (Mokatam, Cairo, Egypt). Cells were maintained in Dulbecco's Modified Eagle Medium (DMEM; purchased from Sigma-Aldrich, USA) supplemented with 100 mg/ml of streptomycin, 100 units/ml of penicillin, and 10% of heat-inactivated fetal bovine serum in a humidified, 5.0% (v/v) CO 2 atmosphere at 37°C.
The sulforhodamine B (SRB) assay was used to determine cell viability. Aliquots of 100 μl cell suspension (5 × 10 3 cells) were added to 96-well plates and incubated in complete media for 24 h. Cells were treated with another aliquot of 100 μl media containing varying concentrations of EI6-EPS. After 72 h of EI6-EPS exposure, cells were fixed by replacing media with 150 μl of 10% TCA and incubated at 4°C for 1 h. The TCA solution was removed, and the cells were washed five times with distilled water. Aliquots of 70 μl SRB solution (0.4% w/v) were added and incubated in a dark place at room temperature for 10 min. Plates were washed three times with 1.0% acetic acid and allowed to air-dry overnight. Then, 150 μl of TRIS (10 mM) was added to dissolve protein-bound SRB stain; the absorbance was measured at 540 nm using a BMG LABTECH®-FLUOstar Omega microplate reader (Ortenberg, Germany) ( Skehan et al., 1990 ; Allam et al., 2018 ).
In vitro Wound Healing Activity
For the scratch wound assay, HSF cells were plated at a density of 3 × 10 5 CFU/well onto a coated 6-well plate and cultured overnight in 5.0% FBS-DMEM at 37°C and 5.0% CO 2 . The following day, horizontal scratches were introduced into the confluent monolayer. The plate was washed thoroughly with PBS, control wells were replenished with a fresh medium, and the second set of wells was treated with fresh media containing EI6-EPS. Images were taken using an inverted microscope at 0, 24, and 48 h time intervals. The plate was incubated at 37°C with 5.0% CO 2 in-between time points. The acquired images were analyzed by the MII ImageView software version 3.7. The wound closure % was calculated according to the formula:
where A 0 and A t are the average areas of the wound measured immediately after scratching (time = zero) and after time = t in hours, respectively ( Li et al., 2019 ).
Statistical Analysis
All experiments were conducted in triplicate, and the results were represented as mean ± standard deviation (SD). Statistical analysis was performed using Microsoft Excel 2010, and the statistical difference was assessed using one-way analysis of variance (ANOVA). Differences at P < 0.05 were considered statistically significant.
Results and Discussion
Isolation, biochemical characterization, and identification of eps-producing marine lab.
A total of four EPS-producing LAB strains (EI6, EI7, EI8, and EI9) were isolated from the guts of marine shrimp samples as they showed mucoid colonies on MRS media ( Guérin et al., 2020 ). These four isolates were examined for EPS production yield by growing in MRS media supplemented with 1.0% sucrose; they gave EPS yields of ~230, 171, 152, and 205 mg/L, respectively. Accordingly, isolate EI6 was selected for further studies as it gave the highest EPS yield of about 230 ± 1.55 mg/L.
Isolate EI6 ( Figure 1A ) appeared as gram-positive, non-spore-forming rods, and the examination under the SEM revealed its characteristic rod cell shape. Moreover, it was further characterized biochemically using the VITEK 2 system (BioMérieux, France), as described in Table 1 . The VITEK 2 characterization of the isolate EI6 implied certain biochemical properties, including resistance to four antibiotics, namely, Bacitracin, Novobiocin, Polymixin, and Optochin, and the ability to grow in 6.5% NaCl. The growth in salty media is a desired feature for starter cultures, as NaCl is one of the most significant additions for food preservation ( Abid et al., 2018 ).
Figure 1 . Scanning electron microscope of isolate EI6 (A) , and phylogenetic analysis of the marine isolate Lactiplantibacillus plantarum EI6 based on 16s rRNA gene sequence (B) .
Table 1 . Isolate EI6 biochemical characterization using VITEK ® 2 system version 07.01 (BioMerieux, France).
The molecular identification of isolate EI6 was carried out through 16s rRNA gene sequencing. EI6 was identified as Lactiplantibacillus plantarum with an identity percentage of 99%. The obtained sequence was submitted to the GenBank under the accession number MW413308, and the phylogenetic relationship of the marine isolate EI6 and its close relatives in the NCBI database is demonstrated in Figure 1B .
The growth of isolate L. plantarum EI6 was examined on blood agar, and it gave no signs of hemolysis, which implies the safety of isolate EI6 to be used as a potential probiotic and its suitability for biotechnological and industrial applications ( Nakajima et al., 2003 ).
Lactic acid bacteria produce several antimicrobial agents that give it considerable advantages in competition with the other harmful and pathogenic bacteria in the gut. For probiotic bacteria to be able to colonize the gastrointestinal tract, it is essential to have the capability to eliminate the competitors. Therefore, the antimicrobial activity of the marine L. plantarum EI6 supernatant was evaluated using an agar well-cut diffusion test against seven indicator pathogens, and the results are represented in Table 2 . The data showed that L. plantarum EI6 has potent antimicrobial activity with different inhibitory potential, as Pseudomonas fluorescens was the most affected pathogen with an inhibition zone diameter of 2.4 cm, and the least activity was detected against Staphylococcus aureus with an inhibition zone diameter of 1.2 cm, while no any activity was detected against Klebsiella pneumonia or Escherichia coli . This activity can be attributed to the antimicrobial substances produced by these LAB strains. These antimicrobial agents include organic acids (e.g., lactic acid and acetic acid), fatty acids, hydrogen peroxide, acetoin, diacetyl, and, most importantly, inhibitory peptides known as bacteriocins ( Leblanc and Todorov, 2011 ). Similarly, the broad spectrum of antimicrobial activity of Lactiplantibacillus strains has been reported ( Lavilla-Lerma et al., 2013 ).
Table 2 . Antimicrobial activity of L. plantarum EI6 cell-free supernatant against indicator pathogens.
Antibiotic susceptibility is a significant criterion for probiotic selection, since their ability to resist some antibiotics may be beneficial ( Onyibe et al., 2013 ). Antibiotic resistance may help them survive in the gut, especially when employed to reestablish intestinal bacteria balance and a healthy environment following antibiotic therapy. LAB in the gut or food products can also serve as a reservoir for antibiotic resistance genes because they may carry plasmids and transposons that encode antibiotic resistance genes. These genes could be transferred to other harmful microbes in the gut or the food chain ( Huys et al., 2013 ). Therefore, before the use of a strain of LAB as a potential probiotic, antibiotic resistance screening must be done to ensure its safety for application.
The disc diffusion method was used to assess the antibiotic susceptibility of isolate EI6 to 11 different antibiotics. According to the data, it is susceptible to seven types and resistant to four ( Table 3 ). The antibiotic resistance may be attributed to the cell wall structure, membrane permeability, and efflux mechanisms. EI6 showed susceptibility to vancomycin, and this is a fundamental property because vancomycin is the antibiotic of last resort in many cases, and it is one of the most effective antibiotics against multidrug-resistant pathogens causing clinical infections ( Nami et al., 2014 ).
Table 3 . Antibiotic susceptibility of L. plantarum EI6.
One of the major stress factors facing the ingested probiotics is the passage through the stomach and being subjected to gastric acids with a low pH value ( Berrada et al., 1991 ). Therefore, one of the selection properties of new probiotic strains is the ability to withstand low pH ( Cakir, 2003 ). The results demonstrated that L. plantarum EI6 is acid-tolerant as it was able to resist pH 4.0 and 3.0 for 4 h, which was indicated by increasing the O.D. values with survival rates of 100% and 77%, respectively, compared to the control (pH 6.2) ( Figure 2A ). Moreover, it managed to survive at pH 2.0 and increase the O.D. from 0.26 to 0.39 with a survival rate of 61.8% after 4 h. Further measurements at 6 and 24 h confirmed the acid-tolerance ability of isolate EI6 as it continued to grow and managed to increase the O.D. The ability of L. plantarum strains YO175 and OF101 to withstand low pH 2.5 and 2.0 for 4 h has been reported by Adesulu-Dahunsi (2018) , as they did not show a substantial decrease in the viable cell count and their survival rates were over 97% ( Adesulu-Dahunsi, 2018 ).
Figure 2 . Effect of different pH values (A) , and effect of different bile salts concentrations (0.0, 0.1, and 0.3%) (B) on the growth of L. plantarum EI6 at 37°C for 24 h.
The potential of L. plantarum EI6 to survive and proliferate in the presence of bile salts was investigated. For probiotics to survive in the intestine, they have to resist the antimicrobial conditions in the intestine, such as antimicrobial peptides, proteolytic enzymes, and bile salts that can destroy the bacterial cell membrane ( Giles-Gómez et al., 2016 ). Although the bile salts concentration varies in the human digestive tract, the average concentration is thought to be 0.3%, and the staying time is believed to be 4 h ( Gilliland et al., 1984 ). The influence of bile concentrations (0, 0.1, and 0.3% w/v) on the growth of the isolate EI6 is demonstrated in Figure 2B . L. plantarum EI6 was able to survive at 0.1% bile concentration, as detected by an increase in the O.D. from 0.3 to 0.68 (survival rate of 82.9%) after 4 h of incubation, and the growth was comparable to that of the control (MRS broth without bile salts). The isolate also showed good stability at 0.3% bile concentration, as the O.D. increased from 0.3 to 0.57 (survival rate of 69.5%) after 4 h of incubation. Subsequent measurements at 6 and 24 h validated isolate EI6's capacity to survive bile salts as it continued to grow and raise its O.D. The ability of LAB strains to survive high bile salt concentrations is due to the presence of a specific enzyme called bile salt hydrolase (BSH), which helps to hydrolyze conjugated bile salts and minimize their toxicity ( Du Toit et al., 1998 ).
Total Carbohydrate Content (%)
The determination of the carbohydrate amount plays a crucial role not only to signify the purity of the produced exopolysaccharide but also to determine the EPS's functional properties and define its potential applications. In this study, the carbohydrate content was estimated as glucose, accounting for ~91.5% of the EI6-EPS. The sugar content was higher than that reported for the marine strain of Rhodobacter johrii CDR-SL 7Cii (86.82%) ( Sran et al., 2019 ) but lower than the carbohydrate content of the exopolysaccharide from Enterococcus faecalis (94.7%) ( Choudhuri et al., 2020 ).
Structural Functionalization by FTIR
Chemical functionalities of the polymeric EI6-EPS structure were identified through FTIR analysis. The IR spectrum revealed characteristic signatures and distinctive functional groups of carbohydrates ( Figure 3A ). Therefore, the assignments of the IR bands were mainly based on the previously reported polysaccharide spectra ( Amer et al., 2020 ; Choudhuri et al., 2020 ). The broadband at 3,289 cm −1 in the region (3,150–3,490 cm −1 ) denoted the stretching vibration of a large number of hydroxyl (O-H) groups belonging to the sugar moieties of carbohydrates ( Abid et al., 2018 ; Amer et al., 2020 ; Choudhuri et al., 2020 ; Sirin and Aslim, 2020 ). The absorption band at 2,970–2,850 cm −1 was assigned to the stretching vibration of the aliphatic C-H of the methyl or methylene groups in hexoses (e.g., galactose and glucose) and deoxyhexoses (e.g., rhamnose and fucose), which is characteristic of polysaccharides ( Abid et al., 2018 ; Choudhuri et al., 2020 ; Sirin and Aslim, 2020 ). The absorption peaks at 1,724 and 1,656 cm −1 may relate to the stretching vibration of the C=O group in free and bonded states, respectively ( Zhou et al., 2016 ; Abid et al., 2018 ; Sirin and Aslim, 2020 ). In contrast, the stretching vibration of C=O of the carboxyl (COO − ) group was assigned to the region of 1,200–1,400 cm −1 ( Sirin and Aslim, 2020 ).
Figure 3 . FTIR spectrum (A) , GC-MS chromatogram (B) , 1 H NMR spectrum recorded at 323 K (2.0% w/v; DMSO- d 6 , 500 MHz) (C) , and SEM-EDX data of the produced exopolysaccharide (EI6-EPS) (D) .
The characteristic fingerprint region of the polysaccharide was ascertained from the bands in the area of 1,200–800 cm −1 ( Shang et al., 2013 ; Abid et al., 2018 ; Sirin and Aslim, 2020 ). The intense band at 1,017 cm −1 and the shoulder at 1,076 cm −1 were distinctive and could be assigned to the stretching vibration of the C-O and C-O-C groups, respectively ( Abid et al., 2018 ; Sirin and Aslim, 2020 ). Moreover, the small peak at 1,125 cm −1 indicated the existence of the sugar in a pyranose form ( Abid et al., 2018 ). Finally, the peaks in the region of 830–780 cm −1 revealed the α- and β-glycoside linkages between sugar moieties ( Coimbra et al., 1999 ).
Structural Study by 1 H NMR
The NMR spectroscopic technique was used to get structural information on the EI6-EPS, such as monosaccharide composition, configurations, and linkage types ( Li et al., 2011 ). The acquired 1 H NMR spectrum of EI6-EPS ( Figure 3C ) revealed a similar signature to other reported polysaccharides ( Amer et al., 2020 ; Choudhuri et al., 2020 ). The ring protons at the positions C2–C6 belong to the sugar moieties and appeared in the range of δ = 2.96–4.19 ppm, while the anomeric protons displayed in the range of δ = 4.45–5.16 ppm. The broadening of the H 2 O signal at 3.3 ppm hindered the resolution and assignment of the monosaccharide ring protons. The existence of β- and α-glycosidic linkages between sugar moieties was investigated from the resonance peaks at δ = 4.45–4.75 and δ = 4.84–5.16, respectively ( Agrawal, 1992 ), which agreed with the FTIR data. The configuration and mode of the glycosidic bonds between sugars could not be identified due to the broadness of the anomeric protons. As determined by GC-MS analysis, the shoulder peaks within the range of 3.43–4.24 ppm suggested the presence of glucose and galactose in the EPS structure ( Sivasankar et al., 2018 ).
The existence of β- and α-glycosidic linkages between sugar moieties was investigated from the resonance peaks at δ = 4.45–4.75 and δ = 4.84–5.16, respectively ( Agrawal, 1992 ), which agreed with the FTIR data. The configuration and mode of the glycosidic bonds between sugars could not be identified due to the broadness of the anomeric protons. The shoulder peaks within the range of δ = 3.43–4.24 ppm proposed the existence of glucose and galactose in the EPS structure ( Sivasankar et al., 2018 ) as determined by GC-MS analysis. It has been reported that the alkyl group region lies in the range of δ =1.2–2.3 ppm ( Wang et al., 2014 ). The presence of signals at δ = 1.13–1.21 ppm denoted the methyl (–CH 3 ) protons of the fucose and rhamnose moieties ( Agrawal, 1992 ; Choudhuri et al., 2020 ).
Monosaccharide Composition by GC-MS
The monosaccharide units that construct the structure of EI6-EPS were identified using GC-MS analysis with the help of the NIST library ( Olasehinde et al., 2019 ). The analysis indicated that EI6-EPS is composed of different sugar moieties, suggesting that it is a heterogeneous polysaccharide ( Sirin and Aslim, 2020 ). Most of the peaks in the GC chromatogram were identified as monosaccharide derivatives by the MS detector with the help of the NIST library. The GC-MS data analysis demonstrated that the EI6-EPS comprises at least five monosaccharides investigated consecutively (time = 19–24 min), denoting the most intense region in the GC chromatogram. The comparable area% was calculated based on the relative abundances of these moieties, giving the order rhamnose > galactose > mannose > glucose > arabinose with a relative area% of 26.43, 25.07, 21.24, 14.42, and 12.84, respectively ( Figure 3B ). The sugar moieties exist mainly in the pyranose with less existence of the furanose form. EI6-EPS was very similar to the sugar composition of the EPS produced by Rhodobacter johrii CDR-SL 7Cii ( Sran et al., 2019 ), Enterococcus faecium WEFA23-2 ( Jia et al., 2019 ), L. delbrueckii ssp. bulgaricus B3, and L. plantarum GD2 strains ( Sirin and Aslim, 2020 ), but with different molar ratios.
Morphological and Elemental Studies by SEM and EDX Spectroscopy
A scanning electron microscope is a valuable tool to visualize the surface topography of materials such as polymers ( Ahmed et al., 2013 ). The SEM micrographs of the EI6-EPS revealed a rough and irregular surface ( Figure 4 ) that resembled the reported morphology for the exopolysaccharides from L. delbrueckii ssp. bulgaricus B3 and L. plantarum GD2 strains ( Sirin and Aslim, 2020 ).

Figure 4 . SEM images of the produced exopolysaccharide (EI6-EPS) at 2,000× (A) , 9,000× (B) , and 15,000× (C) .
The elemental analysis utilizing EDX analysis revealed the predominance of oxygen and carbon with a mass ratio of 55.44 and 40.06 (w/w%), respectively. The high mass% of those two elements emphasized that the purified EI6-EPS is comprised mainly of carbohydrates ( Sirin and Aslim, 2020 ) ( Figure 3D ). This result is consistent with the high carbohydrate content (91.5%) as determined by UV-vis analysis. The presence of nitrogen (2.15 w/w%) and phosphorus (0.99 w/w%) proposed the lack of protein and phospholipids accompanied by the purified EI6-EPS. Other elements including chloride (1.03%), sodium (0.12%), calcium (0.19%), and magnesium (0.02%) were also detected; however, the sulfur was null. The elemental composition of the EI6-EPS was similar to the EPS produced by Rhodobacter johrii CDR-SL 7Cii ( Sran et al., 2019 ), L. delbrueckii subsp. bulgaricus NCFB 2483 ( Goh et al., 2005 ), L. delbrueckii ssp. bulgaricus B3, and L. plantarum GD2 ( Sirin and Aslim, 2020 ).
The cytotoxicity of EI6-EPS was evaluated against human dermal fibroblast cell lines. The EPS had no cytotoxic effects against HDF cell lines at low concentrations and the IC50 was determined to be > 100 μg/ml as the cells showed only a very minimal decrease in the viability at concentrations as high as 100 μg/ml, which indicates the safety of EI6-EPS toward the skin cells.
The proliferative effect of EI6-EPS on the HSF cell line was assessed using the wound scratch assay ( Figure 5 ). Wound width was calculated as the average distance between the edges of the scratches. The wound width decreases as cell migration is induced.
Figure 5 . In vitro cell migration of skin fibroblasts by EI6-EPS scratch was created in monolayer of HSF cells and was treated with EI6-EPS. Control group was without any treatment. (A) Photographs of wound area treated with EI6-EPS at different time intervals were taken using an inverted microscope. (B) Percentage wound closure in control and treated cells at different time intervals.
The in vitro HSF cell line scratch assay revealed that EI6-EPS significantly induced cell migration. After 24 h, EI6-EPS–treated cells showed 50% wound closure compared to 41% in the control group, while at 48 h, EI6-EPS–treated cells showed 77% wound closure compared to 60% in the control group.
These findings indicate that the EI6-EPS has wound healing and cell migration bioactivity, making it suitable for a variety of therapeutic and pharmacological applications.
The role of EI6-EPS in the healing process may be attributed to the thought that various cell surface receptors can identify and bind to β-glucan because of its triple-helical conformation, causing inflammatory cytokines or other modulators to be activated, as well as its antioxidant activity ( Weber et al., 2016 ; Trabelsi et al., 2017 ; Sahana and Rekha, 2019 ). However, the exact mechanism of action needs to be investigated further utilizing in vivo models.
The idealistic compound for wound healing should fulfill a variety of current global needs while also allowing for uneventful and scar-free accelerated healing. The EI6-EPS is a safe, natural, and biocompatible polymer with a cell migration improvement activity, and its structural properties are suitable for therapeutic and pharmacological applications, making it an excellent bioactive compound for use in wound care. The wound healing activity of Lactiplantibacillus sp. has been documented by Trabelsi et al. (2017) .
The chemical analysis confirmed the high purity of the EI6-EPS produced by L. plantarum EI6, as noticed by the high carbohydrate content (>90%) using the sulfuric acid method. FTIR and 1D NMR emphasized the polysaccharide nature of EI6-EPS through the characteristic curve and peaks of the recorded spectra. GC-MS results proposed that EI6-EPS is a heterogenous polysaccharide composed mainly of five monosaccharides. The outcomes of this study suggest that the polysaccharides, specifically those rich in galactose, mannose, and glucose, significantly improve the wound-healing properties of EI6-EPS ( Mapoung et al., 2021 ) and that EI6-EPS enhances keratinocyte and fibroblast cell proliferation ( Krupodorova et al., 2015 ; Veeraperumal et al., 2020 ). These findings are also in agreement with previous studies concerned with the wound-healing potential of polysaccharides derived from different sources such as seaweed ( Gracilaria lemaneiformis ) ( Veeraperumal et al., 2020 ), plant ( Bletilla striata and Astragalus membranaceus ) ( Zhao et al., 2017 ; Zhang et al., 2019 ), or mushrooms ( Ganoderma lucidum, Agaricus blazei and Phellinus gilvus ) ( Hu et al., 2019 ).
In this study, a new marine LAB isolate, L. plantarum EI6, was evaluated for its probiotic potential besides production and primary characterization of its EPS. The obtained data revealed that this isolate exhibits promising probiotic and technological properties by their ability to withstand low pH, high bile salt concentrations, and broad antibacterial activity. Furthermore, the purified EI6-EPS is a heteropolysaccharide, with a high carbohydrate content (~91.5%) that associates with the predominance of oxygen (55.44 w/w%) and carbon (40.06 w/w%) in EDX analysis, indicating efficient purification of the polysaccharides. FTIR and NMR emphasized the polysaccharide nature of the EI6-EPS, as illustrated by the recorded characteristic peaks. GC-MS identified five abundant monosaccharides in the pyranose form (i.e., rhamnose, galactose, mannose, glucose, and arabinose) connected by α- and β-glycosidic linkages. EI6-EPS has been proven to be safe on HDF cell lines and stimulates the proliferation and migration of HSF. These findings suggest that L. plantarum EI6 may be employed as a safe source for bioactive compounds for pharmacological applications. However, more in vivo research is needed to prove the beneficial effects in the wound care sector.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
EZ and MI are equally contributed in concept, design, analysis of data, and writing the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdelhamid, S. A., Mohamed, S. S., and Selim, M. S. (2020). Medical application of exopolymers produced by marine bacteria. Bull. Natl. Res. Centre 44, 1–14. doi: 10.1186/s42269-020-00323-x
CrossRef Full Text | Google Scholar
Abid, Y., Casillo, A., Gharsallah, H., Joulak, I., Lanzetta, R., Corsaro, M. M., et al. (2018). Production and structural characterization of exopolysaccharides from newly isolated probiotic lactic acid bacteria. Int. J. Biol. Macromol. 108, 719–728. doi: 10.1016/j.ijbiomac.2017.10.155
PubMed Abstract | CrossRef Full Text | Google Scholar
Adesulu-Dahunsi, A. T., Jeyaram, K., and Sanni, A. I. (2018). Probiotic and technological properties of exopolysaccharide producing lactic acid bacteria isolated from cereal-based nigerian fermented food products. Food Control 92, 225–231.
Google Scholar
Agrawal, P. K. (1992). NMR spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 31, 3307–3330. doi: 10.1016/0031-9422(92)83678-R
Ahmed, Z., Wang, Y., Anjum, N., Ahmad, A., and Khan, S. T. (2013). Characterization of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir – Part II. Food Hydrocoll. 30, 343–350. doi: 10.1016/j.foodhyd.2012.06.009
Allam, R., Al-Abd, A., Sharaf, O., Nofal, S., Khalifa, A., Mosli, H., et al. (2018). Fingolimod interrupts the cross talk between estrogen metabolism and sphingolipid metabolism within prostate cancer cells. Toxicol. Lett. 291, 77–85. doi: 10.1016/j.toxlet.2018.04.008
Amer, M. S., Zaghloul, E. H., and Ibrahim, M. I. A. (2020). Characterization of exopolysaccharide produced from marine-derived Aspergillus terreus SEI with prominent biological activities. Egypt. J. Aquatic Res. 46, 363–369. doi: 10.1016/j.ejar.2020.08.008
Amiza, M. A., Zakiah, J., Ng, L. K., and Lai, K. W. (2006). Fermentation of tempoyak using isolated tempoyak culture. Res. J. Microbiol. 1, 243–254. doi: 10.3923/jm.2006.243.254
Angelin, J., and Kavitha, M. (2020). Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 162, 853–865. doi: 10.1016/j.ijbiomac.2020.06.190
Bachtarzi, N., Speciale, I., Kharroub, K., De Castro, C., Ruiz, L., and Ruas-Madiedo, P. (2020). Selection of exopolysaccharide-producing Lactobacillus Plantarum ( Lactiplantibacillus Plantarum ) isolated from algerian fermented foods for the manufacture of skim-milk fermented products. Microorganisms 8, 1101. doi: 10.3390/microorganisms8081101
Balamurugan, R., Chandragunasekaran, A. S., Chellappan, G., Rajaram, K., Ramamoorthi, G., and Ramakrishna, B. S. (2014). Probiotic potential of lactic acid bacteria present in home made curd in southern India. Indian J. Med. Res. 140, 345–355.
PubMed Abstract | Google Scholar
Ben Gara, A., Ben Abdallah Kolsi, R., Jardak, N., Chaaben, R., El-Feki, A., Fki, L., et al. (2017). Inhibitory activities of Cystoseira crinita sulfated polysaccharide on key enzymes related to diabetes and hypertension: in vitro and animal study. Arch. Physiol. Biochem. 123, 31–42. doi: 10.1080/13813455.2016.1232737
Berrada, N., Lemeland, J. F., Laroche, G., Thouvenot, P., and Piaia, M. (1991). Bifidobacterium from fermented milks: survival during gastric transit. J. Dairy Sci. 74, 409–413. doi: 10.3168/jds.S0022-0302(91)78183-6
Brandi, J., Cheri, S., Manfredi, M., Di Carlo, C., Vita Vanella, V., Federici, F., et al. (2020). Exploring the wound healing, anti-inflammatory, anti-pathogenic and proteomic effects of lactic acid bacteria on keratinocytes. Sci. Rep. 10, 8052. doi: 10.1038/s41598-020-68483-4
Cakir, I. (2003). Determination of Some Probiotic Properties on Lactobacilli and Bifidobacteria . PhD, Ankara University, Ankara.
Chaplin, M. F. (1982). A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography. Anal. Biochem. 123, 336–341. doi: 10.1016/0003-2697(82)90455-9
Choudhuri, I., Khanra, K., Pariya, P., Maity, G. N., Mondal, S., Pati, B. R., et al. (2020). Structural characterization of an exopolysaccharide isolated from Enterococcus faecalis , and study on its antioxidant activity, and cytotoxicity against HeLa cells. Curr. Microbiol. 77, 3125–3135. doi: 10.1007/s00284-020-02130-z
Coimbra, M. A., Barros, A., Rutledge, D. N., and Delgadillo, I. (1999). FTIR spectroscopy as a tool for the analysis of olive pulp cell-wall polysaccharide extracts. Carbohydr. Res. 317, 145–154. doi: 10.1016/S0008-6215(99)00071-3
Costa, O. Y. A., Raaijmakers, J. M., and Kuramae, E. E. (2018). Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front Microbiol . 9, 1636. doi: 10.3389/fmicb.2018.01636
Demain, A. L., Vandamme, E. J., Collins, J., and Buchholz, K. (2017). “History of industrial biotechnology,” in Industrial Biotechnology , eds C. Wittmann, and J. C. Liao (KGaA; Weinheim: Wiley-VCH Verlag GmbH & Co.), 1–84. doi: 10.1002/9783527807796.ch1
Demirci, S., Ustaoglu, Z., Yilmazer, G. A., Sahin, F., and Ba,ç, N. (2014). Antimicrobial properties of zeolite-X and zeolite-A ion-exchanged with silver, copper, and zinc against a broad range of microorganisms. Appl. Biochem. Biotechnol. 172, 1652–1662. doi: 10.1007/s12010-013-0647-7
Du Toit, M., Franz, C. M., Dicks, L. M., Schillinger, U., Haberer, P., Warlies, B., et al. (1998). Characterisation and selection of probiotic lactobacilli for a preliminary minipig feeding trial and their effect on serum cholesterol levels, faeces pH and faeces moisture content. Int. J. Food Microbiol. 40, 93–104. doi: 10.1016/S0168-1605(98)00024-5
Duar, R. M., Lin, X. B., Zheng, J., Martino, M. E., Grenier, T., Pérez-Muñoz, M. E., et al. (2017). Lifestyles in transition: evolution and natural history of the genus Lactobacillus . FEMS Microbiol. Rev. 41, S27–s48. doi: 10.1093/femsre/fux030
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., and Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356. doi: 10.1021/ac60111a017
Dueñas-Chasco, M. T., Rodríguez-Carvajal, M. A., Tejero Mateo, P., Franco-Rodríguez, G., Espartero, J. L., Irastorza-Iribas, A., et al. (1997). Structural analysis of the exopolysaccharide produced by Pediococcus damnosus 2.6. Carbohydr. Res. 303, 453–458. doi: 10.1016/S0008-6215(97)00192-4
Giles-Gómez, M., Sandoval García, J. G., Matus, V., Campos Quintana, I., Bolívar, F., and Escalante, A. (2016). In vitro and in vivo probiotic assessment of Leuconostoc mesenteroides P45 isolated from pulque, a Mexican traditional alcoholic beverage. Springerplus 5, 708. doi: 10.1186/s40064-016-2370-7
PubMed Abstract | CrossRef Full Text
Gilliland, S. E., Staley, T. E., and Bush, L. J. (1984). Importance of bile tolerance of Lactobacillus acidophilus used as a dietary adjunct1. J. Dairy Sci. 67, 3045–3051. doi: 10.3168/jds.S0022-0302(84)81670-7
Goh, K. K., Haisman, D. R., and Singh, H. (2005). Development of an improved procedure for isolation and purification of exopolysaccharides produced by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2483. Appl. Microbiol. Biotechnol. 67, 202–208. doi: 10.1007/s00253-004-1739-7
Guérin, M., Silva, C. R., Garcia, C., and Remize, F. (2020). Lactic acid bacterial production of exopolysaccharides from fruit and vegetables and associated benefits. Fermentation 6, 115. doi: 10.3390/fermentation6040115
Guo, Y., Pan, D., Sun, Y., Xin, L., Li, H., and Zeng, X. (2013). Antioxidant activity of phosphorylated exopolysaccharide produced by Lactococcus lactis subsp. lactis. Carbohydr Polym 97, 849–854. doi: 10.1016/j.carbpol.2013.06.024
Guttmann, D. M., and Ellar, D. J. (2000). Phenotypic and genotypic comparisons of 23 strains from the Bacillus cereus complex for a selection of known and putative B. thuringiensis virulence factors. FEMS Microbiol. Lett. 188, 7–13. doi: 10.1111/j.1574-6968.2000.tb09160.x
Hu, F., Yan, Y., Wang, C. W., Liu, Y., Wang, J. J., Zhou, F., et al. (2019). Article effect and mechanism of Ganoderma lucidum polysaccharides on human fibroblasts and skin wound healing in mice. Chinese J. Integrat. Med. 25, 203–209. doi: 10.1007/s11655-018-3060-9
Huys, G., Botteldoorn, N., Delvigne, F., De Vuyst, L., Heyndrickx, M., Pot, B., et al. (2013). Microbial characterization of probiotics–Advisory report of the Working Group “8651 Probiotics” of the Belgian Superior Health Council (SHC). Mol. Nutr. Food Res. 57, 1479–1504. doi: 10.1002/mnfr.201300065
Ibrahim, M. I. A., Pickaerta, G., Stefan, L., Jamart-Grégoirea, B., Bodiguel, J., and Averlant-Petit, M.-C. (2020). Cyclohexamer [-(D-Phe-azaPhe-Ala)2-]: good candidate to formulate supramolecular organogels. RSC Adv. 10, 43859–43869. doi: 10.1039/D0RA07775E
Ji, X., Cheng, Y., Tian, J., Zhang, S., Jing, Y., and Shi, M. (2021). Structural characterization of polysaccharide from jujube ( Ziziphus jujuba Mill .) fruit. Chem. Biol. Technol. Agric. 8, 1–7. doi: 10.1186/s40538-021-00255-2
Ji, X., Guo, J., Ding, D., Gao, J., Hao, L., Guo, X., et al. (2022). Structural characterization and antioxidant activity of a novel high-molecular-weight polysaccharide from Ziziphus Jujuba cv. Muzao . Food Measure 16, 2191–2200. doi: 10.1007/s11694-022-01288-3
Jia, K., Tao, X., Liu, Z., Zhan, H., He, W., Zhang, Z., et al. (2019). Characterization of novel exopolysaccharide of Enterococcus faecium WEFA23 from infant and demonstration of its in vitro biological properties. Int. J. Biol. Macromol. 128, 710–717. doi: 10.1016/j.ijbiomac.2018.12.245
Kant, V., Kumari, P., Jitendra, D. K., Ahuja, M., and Kumar, V. (2021). Nanomaterials of natural bioactive compounds for wound healing: novel drug delivery approach. Curr. Drug Deliv. 18, 1406–1425. doi: 10.2174/1567201818666210729103712
Kavita, K., Singh, V. K., Mishra, A., and Jha, B. (2014). Characterisation and anti-biofilm activity of extracellular polymeric substances from Oceanobacillus iheyensis . Carbohydr. Polym. 101, 29–35. doi: 10.1016/j.carbpol.2013.08.099
Krupodorova, T. A., Klymenko, P. P., Barshteyn, V. Y., Leonov, Y. I., Shytikov, D. W., and Orlova, T. N. (2015). Effects of Ganoderma lucidum (Curtis) P. Karst and Crinipellis schevczenkovi Buchalo aqueous extracts on skin wound healing. J. Phytopharmacol . 4, 197–201. doi: 10.31254/phyto.2015.4401
Lavilla-Lerma, L., Pérez-Pulido, R., Martínez-Bueno, M., Maqueda, M., and Valdivia, E. (2013). Characterization of functional, safety, and gut survival related characteristics of Lactobacillus strains isolated from farmhouse goat's milk cheeses. Int. J. Food Microbiol. 163, 136–145. doi: 10.1016/j.ijfoodmicro.2013.02.015
Leblanc, J. G., and Todorov, S. D. (2011). Bacteriocin producing lactic acid bacteria isolated from boza, a traditional fermented beverage from balkan peninsula - from isolation to application. science against microbial pathogens. Commun. Current Res. Technol. Adv. 1, 1311–1320.
Li, J., Fan, L., and Ding, S. (2011). Isolation, purification and structure of a new water-soluble polysaccharide from Zizyphus jujuba cv. Jinsixiaozao. Carbohydrate Polym. 83, 477–482. doi: 10.1016/j.carbpol.2010.08.014
Li, L.-J., Wang, M.-Z., Yuan, T.-J., Xu, X.-H., Dad, H. A., Yu, C.-L., et al. (2019). The crude ethanol extract of Periplaneta americana L . stimulates wound healing in vitro & in vivo. Chinese Med. 14, 33. doi: 10.1186/s13020-019-0259-4
Lindequist, U. (2016). Marine-derived pharmaceuticals–challenges and opportunities. Biomol. Therap. 24, 561. doi: 10.4062/biomolther.2016.181
López-Abarrategui, C., Alba, A., Lima, L. A., Maria-Neto, S., Vasconcelos, I. M., Oliveira, J. T., et al. (2012). Screening of antimicrobials from Caribbean Sea animals and isolation of bactericidal proteins from the littoral mollusk Cenchritis muricatus . Curr. Microbiol. 64, 501–505. doi: 10.1007/s00284-012-0096-5
Lukic, J., Chen, V., Strahinic, I., Begovic, J., Lev-Tov, H., Davis, S. C., et al. (2017). Probiotics or pro-healers: the role of beneficial bacteria in tissue repair. Wound Repair Regener. 25, 912–922. doi: 10.1111/wrr.12607
Mao, W., Zang, X., Li, Y., and Zhang, H. (2006). Sulfated polysaccharides from marine green algae Ulva conglobata and their anticoagulant activity. J. Appl. Phycol. 18, 9–14. doi: 10.1007/s10811-005-9008-4
Mapoung, S., Umsumarng, S., Semmarath, W., Arjsri, P., Thippraphan, P., Yodkeeree, S., et al. (2021). Skin wound-healing potential of polysaccharides from medicinal mushroom Auricularia auricula-judae (Bull.). J. Fungi 7, 247. doi: 10.3390/jof7040247
Mazzoli, R., Bosco, F., Mizrahi, I., Bayer, E. A., and Pessione, E. (2014). Towards lactic acid bacteria-based biorefineries. Biotechnol. Adv. 32, 1216–1236. doi: 10.1016/j.biotechadv.2014.07.005
Min, W. H., Fang, X. B., Wu, T., Fang, L., Liu, C. L., and Wang, J. (2019). Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J. Biosci. Bioeng. 127, 758–766. doi: 10.1016/j.jbiosc.2018.12.004
Mohammed, B. M., Fisher, B. J., Kraskauskas, D., Ward, S., Wayne, J. S., Brophy, D. F., et al. (2016). Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int. Wound J. 13, 572–584. doi: 10.1111/iwj.12484
Nakajima, Y., Ishibashi, J., Yukuhiro, F., Asaoka, A., Taylor, D., and Yamakawa, M. (2003). Antibacterial activity and mechanism of action of tick defensin against Gram-positive bacteria. Biochim. Biophys. Acta 1624, 125–130. doi: 10.1016/j.bbagen.2003.10.004
Nami, Y., Abdullah, N., Haghshenas, B., Radiah, D., Rosli, R., and Khosroushahi, A. Y. (2014). Probiotic assessment of Enterococcus durans 6HL and Lactococcus lactis 2HL isolated from vaginal microflora. J. Med. Microbiol. 63, 1044–1051. doi: 10.1099/jmm.0.074161-0
Okur, M. E., Karantas, I. D., Senyigit, Z., Okur, N. Ü., and Siafaka, P. I. (2020). Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian J. Pharm. Sci. 15, 661–684. doi: 10.1016/j.ajps.2019.11.008
Olasehinde, T. A., Mabinya, L. V., Olaniran, A. O., and Okoh, A. I. (2019). Chemical characterization of sulfated polysaccharides from Gracilaria gracilis and Ulva lactuca and their radical scavenging, metal chelating, and cholinesterase inhibitory activities. Int. J. Food Propert. 22, 100–110. doi: 10.1080/10942912.2019.1573831
Onyibe, J. E., Oluwole, O. B., Ogunbanwo, S. T., and Sanni, A. I. (2013). Antibiotic susceptibility profile and survival of Bifidobacterium adolescentis and Bifidobacterium catenulatum of human and avian origin in stored yoghurt. Nigerian Food J. 31, 73–83. doi: 10.1016/S0189-7241(15)30079-5
Patel, A., Prajapati, J. B., Holst, O., and Ljungh, A. (2014). Determining probiotic potential of exopolysaccharide producing lactic acid bacteria isolated from vegetables and traditional Indian fermented food products. Food Biosci. 5, 27–33. doi: 10.1016/j.fbio.2013.10.002
Pham, J. V., Yilma, M. A., Feliz, A., Majid, M. T., Maffetone, N., Walker, J. R., et al. (2019). A review of the microbial production of bioactive natural products and biologics. Front. Microbiol . 10, 1404. doi: 10.3389/fmicb.2019.01404
Ruiz-Matute, A. I., Hernández-Hernández, O., Rodríguez-Sánchez, S., Sanz, M. L., and Martínez-Castro, I. (2011). Derivatization of carbohydrates for GC and GC-MS analyses. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879, 1226–1240. doi: 10.1016/j.jchromb.2010.11.013
Sahana, T. G., and Rekha, P. D. (2019). A bioactive exopolysaccharide from marine bacteria Alteromonas sp. PRIM-28 and its role in cell proliferation and wound healing in vitro . Int. J. Biol. Macromol. 131, 10–18. doi: 10.1016/j.ijbiomac.2019.03.048
Shang, N., Xu, R., and Li, P. (2013). Structure characterization of an exopolysaccharide produced by Bifidobacterium animalis RH. Carbohydr. Polym. 91, 128–134. doi: 10.1016/j.carbpol.2012.08.012
Sirin, S., and Aslim, B. (2020). Characterization of lactic acid bacteria derived exopolysaccharides for use as a defined neuroprotective agent against amyloid beta1–42-induced apoptosis in SH-SY5Y cells. Sci. Rep. 10, 8124. doi: 10.1038/s41598-020-65147-1
Sivasankar, P., Seedevi, P., Poongodi, S., Sivakumar, M., Murugan, T., Sivakumar, L., et al. (2018). Characterization, antimicrobial and antioxidant property of exopolysaccharide mediated silver nanoparticles synthesized by Streptomyces violaceus MM72. Carbohydr. Polym. 181, 752–759. doi: 10.1016/j.carbpol.2017.11.082
Skehan, P., Storeng, R., Scudiero, D., Monks, A., Mcmahon, J., Vistica, D., et al. (1990). New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 82, 1107–1112. doi: 10.1093/jnci/82.13.1107
Sran, K. S., Sundharam, S. S., Krishnamurthi, S., and Roy Choudhury, A. (2019). Production, characterization and bio-emulsifying activity of a novel thermostable exopolysaccharide produced by a marine strain of Rhodobacter johrii CDR-SL 7Cii. Int. J. Biol. Macromol. 127, 240–249. doi: 10.1016/j.ijbiomac.2019.01.045
Sultana, R., Mcbain, A. J., and O'neill, C. A. (2013). Strain-dependent augmentation of tight-junction barrier function in human primary epidermal keratinocytes by Lactobacillus and Bifidobacterium lysates. Appl. Environ. Microbiol. 79, 4887–4894. doi: 10.1128/AEM.00982-13
Trabelsi, I., Ktari, N., Ben Slima, S., Triki, M., Bardaa, S., Mnif, H., et al. (2017). Evaluation of dermal wound healing activity and in vitro antibacterial and antioxidant activities of a new exopolysaccharide produced by Lactobacillus sp.Ca6. Int. J. Biol. Macromol. 103, 194–201. doi: 10.1016/j.ijbiomac.2017.05.017
Veeraperumal, S., Qiu, H. M., Zeng, S. S., Yao, W. Z., Wang, B. P., Liu, Y., et al. (2020). Polysaccharides from Gracilaria lemaneiformis promote the HaCaT keratinocytes wound healing by polarised and directional cell migration. Carbohydr. Polym. 241, 116310. doi: 10.1016/j.carbpol.2020.116310
Wang, K., Li, W., Rui, X., Chen, X., Jiang, M., and Dong, M. (2014). Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 63, 133–139. doi: 10.1016/j.ijbiomac.2013.10.036
Weber, C., Telerman, S. B., Reimer, A. S., Sequeira, I., Liakath-Ali, K., Arwert, E. N., et al. (2016). Macrophage infiltration and alternative activation during wound healing promote MEK1-induced skin carcinogenesis. Cancer Res. 76, 805–817. doi: 10.1158/0008-5472.CAN-14-3676
Wei, Q., Zhang, Z., Luo, J., Kong, J., Ding, Y., Chen, Y., et al. (2019). Insulin treatment enhances pseudomonas aeruginosa biofilm formation by increasing intracellular cyclic di-GMP levels, leading to chronic wound infection and delayed wound healing. Am. J. Transl. Res. 11, 3261–3279.
Welman, A. D., and Maddox, I. S. (2003). Exopolysaccharides from lactic acid bacteria: perspectives and challenges. Trends Biotechnol. 21, 269–274. doi: 10.1016/S0167-7799(03)00107-0
Yates, C. C., Hebda, P., and Wells, A. (2012). Skin wound healing and scarring: fetal wounds and regenerative restitution. Birth Defects Res. C Embryo Today 96, 325–333. doi: 10.1002/bdrc.21024
Yilmaz, M. T., Dertli, E., Toker, O. S., Tatlisu, N. B., Sagdic, O., and Arici, M. (2015). Effect of in situ exopolysaccharide production on physicochemical, rheological, sensory, and microstructural properties of the yogurt drink ayran: an optimization study based on fermentation kinetics. J. Dairy Sci. 98, 1604–1624. doi: 10.3168/jds.2014-8936
Zaghloul, E., and Ibrahim, H. (2019). Comparative study on antimicrobial activity of commercial and extracted chitin and chitosan from Marsupenaeus japonicus shells. Egyptian J. Aquatic Biol. Fisheries 23, 291–302. doi: 10.21608/ejabf.2019.31536
Zhang, C., He, Y., Chen, Z., Shi, J., Qu, Y., and Zhang, J. (2019). Effect of polysaccharides from Bletilla striata on the healing of dermal wounds in mice. Evidence-Based Complement. Alternative Med. 2019:9212314. doi: 10.1155/2019/9212314
Zhao, B., Zhang, X., Han, W., Cheng, J., and Qin, Y. (2017). Wound healing effect of an Astragalus membranaceus polysaccharide and its mechanism. Mol. Med. Rep. 15, 4077–4083. doi: 10.3892/mmr.2017.6488
Zhou, K., Zeng, Y., Yang, M., Chen, S., He, L., Ao, X., et al. (2016). Production, purification and structural study of an exopolysaccharide from Lactobacillus plantarum BC-25. Carbohydr. Polym. 144, 205–214. doi: 10.1016/j.carbpol.2016.02.067
Keywords: exopolysaccharide, Lactiplantibacillus, wound healing, probiotics, chemical characterization
Citation: Zaghloul EH and Ibrahim MIA (2022) Production and Characterization of Exopolysaccharide From Newly Isolated Marine Probiotic Lactiplantibacillus plantarum EI6 With in vitro Wound Healing Activity. Front. Microbiol. 13:903363. doi: 10.3389/fmicb.2022.903363
Received: 24 March 2022; Accepted: 14 April 2022; Published: 13 May 2022.
Reviewed by:
Copyright © 2022 Zaghloul and Ibrahim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eman H. Zaghloul, eman_hamed88@yahoo.com
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Polymers (Basel)
- PMC10098801

Microbial Exopolysaccharide Composites in Biomedicine and Healthcare: Trends and Advances
Vishal ahuja.
1 University Institute of Biotechnology, Chandigarh University, Mohali 140413, Punjab, India
2 University Centre for Research & Development, Chandigarh University, Mohali 140413, Punjab, India
Arvind Kumar Bhatt
3 Department of Biotechnology, Himachal Pradesh University, Shimla 171005, Himachal Pradesh, India
J. Rajesh Banu
4 Department of Life Sciences, Central University of Tamil Nadu, Thiruvarur 610005, Tamil Nadu, India
Vinod Kumar
5 Centre for Climate and Environmental Protection, School of Water, Energy and Environment, Cranfield University, Cranfield MK43 0AL, UK
Gopalakrishnan Kumar
6 Institute of Chemistry, Bioscience and Environmental Engineering, Faculty of Science and Technology, University of Stavanger, P.O. Box 8600 Forus, 4036 Stavanger, Norway
Yung-Hun Yang
7 Department of Biological Engineering, College of Engineering, Konkuk University, Seoul 05029, Republic of Korea
8 Institute for Ubiquitous Information Technology and Applications, Seoul 05029, Republic of Korea
Shashi Kant Bhatia
Microbial exopolysaccharides (EPSs), e.g., xanthan, dextran, gellan, curdlan, etc., have significant applications in several industries (pharma, food, textiles, petroleum, etc.) due to their biocompatibility, nontoxicity, and functional characteristics. However, biodegradability, poor cell adhesion, mineralization, and lower enzyme activity are some other factors that might hinder commercial applications in healthcare practices. Some EPSs lack biological activities that make them prone to degradation in ex vivo, as well as in vivo environments. The blending of EPSs with other natural and synthetic polymers can improve the structural, functional, and physiological characteristics, and make the composites suitable for a diverse range of applications. In comparison to EPS, composites have more mechanical strength, porosity, and stress-bearing capacity, along with a higher cell adhesion rate, and mineralization that is required for tissue engineering. Composites have a better possibility for biomedical and healthcare applications and are used for 2D and 3D scaffold fabrication, drug carrying and delivery, wound healing, tissue regeneration, and engineering. However, the commercialization of these products still needs in-depth research, considering commercial aspects such as stability within ex vivo and in vivo environments, the presence of biological fluids and enzymes, degradation profile, and interaction within living systems. The opportunities and potential applications are diverse, but more elaborative research is needed to address the challenges. In the current article, efforts have been made to summarize the recent advancements in applications of exopolysaccharide composites with natural and synthetic components, with special consideration of pharma and healthcare applications.
1. Introduction
Exopolysaccharides (EPSs) are natural biopolymers synthesized by microorganisms and are secreted for various respective functions, such as defense, biofilm formation, pathogenicity, structure, adhesion, etc. [ 1 ]. EPSs are long-chain biomolecules with molecular weights ranging from 10 to 30 kD, and are produced during the late exponential and or stationary phase of microbial growth. EPSs are produced in response to environmental stress conditions, such as pH and temperature, and exposure to heavy metals or inhibitors, etc. [ 2 , 3 ]. Biochemically, EPSs are carbohydrate polymers composed of glucose, galactose, and rhamnose, accompanied by non-carbohydrate moieties such as proteins, enzymes, nucleic acid, etc. The exact composition may vary with microorganisms and growth conditions.
Based on the biochemical structure and composition, EPSs can be categorized into homo-exopolysaccharides (HOEPSs) and hetero-exopolysaccharides (HEEPSs) [ 4 ]. HOEPSs are composed of one type of monosaccharide, such as α-D-glucans, β-D-glucans, fructans, and polygalactans, interlinked with α-1-6, α-1-3, β-1-2, β-1-3, β-2-6, and β-2-1 linkage among the subunits, depending upon the monomeric units. Dextran is one of the well-known examples of HOEPSs, which is made of glucose interlinked with α-1-6 glucoside linkage. In contrast, HEEPSs are comprised of different sugar monomers, along with their respective derivatives and non-carbohydrate moieties. Succinoglycan is one of HEEPSs present in bacterial biofilm. It is comprised of saccharide-based oligomers in which sugar molecules are derivatized with acyl, pyruvate, and succinic acid [ 4 , 5 , 6 ]. Both classes are further recognized as subgroups based on the dominant sugar residues [ 4 ]. In recent years, EPSs have gained wide attention for their cost-effective production and diverse applications as pharma and healthcare products, cosmeceutical, nutraceutical, functional food, and biocontrol agents in agriculture [ 7 ], oil recovery in petroleum industries [ 8 ], heavy metal removal [ 9 ], drug delivery, and tissue regeneration and repair [ 10 ]. Microbial exopolysaccharides have applications in various sectors, as depicted in Figure 1 .

Exopolysaccharide composites with natural and synthetic polymers and their application in the health sector.
Most exopolysaccharides have gained attention in healthcare due to their nontoxicity, and biocompatibility, but dextran is the only known exopolysaccharide that has been commercialized in healthcare as a plasma volume compensator [ 6 , 11 ]. Alongside that, the majority of applications in healthcare are still under trial. Prasher et al. [ 12 ] used a dextran derivative, i.e., acetylated dextran, as a drug delivery vehicle for the treatment of respiratory disease due to biodegradability, pH sensitivity, high encapsulation efficacy, and the ability to cross the mucosal layer. Yahoum et al. [ 13 ] encapsulated metformin hydrochloride in xanthan gum microspheres and found a sustainable release of metformin hydrochloride from the microsphere. The eyes are one of the most sensitive parts of the body that need special care. EPSs have proven safe and biocompatible for biological systems, which allows their application in ophthalmic formulations. Khare et al. [ 14 ] evaluated an ophthalmic solution comprising gellan-gum-based nanosuspension with posaconazole for fungal keratitis. The main bottleneck of using native EPS molecules in commercial products is solubility in different media, bioavailability, degradation, etc. Xanthan, a HEEPS comprising a glucose backbone along with trisaccharide side chains, has poor thermal stability and electrical conductivity and is prone to microbial contamination [ 15 , 16 ]. Curdlan has high immunomodulatory potential, good gelling ability, and thermal stability, but is disadvantaged by the issue of solubility in water [ 17 ]. Hyaluronic acid has high water retention, but poor mechanical stability [ 18 , 19 ]. EPS has been blended with other natural biomolecules or synthetic polymers to improve and control the functional features of base EPS molecules, such as solubility, antimicrobial potential, mechanical strength, water retention, etc. The approach has proven beneficial, as a hyaluronic acid (HA) composite has higher mechanical strength and stability. HA composites with poly(Ne-acryloyl L-lysine) have shown a double network structure and is used for the fabrication of a more physiologically relevant 3D in vitro model for breast cancer [ 20 , 21 ]. Similarly, a HA composite with a self-assembling peptide carrying an IKVAV adhesive motif was used for the fabrication of a scaffold for breast cancer [ 22 ], and composites with silk fibroin–gelatin and heparan sulfate was used for the scaffold fabrication for cholangiocarcinoma [ 23 ]. A chitosan–curdlan composite is characterized by important features of both EPS components, as chitosan forms a fibrillary scaffold, and curdlan provides support to mesenchymal cell adhesion and promotes bone growth [ 24 ].
EPS composites have shown a way to overcome the bottlenecks of native EPS molecules by offering 3D-stable and porous architecture, offering more support for cell adhesion and proliferation, and an overexpression of enzymes governing wound healing and mineralization. However, there are a lot of factors impeding the commercialization of composite materials. Among the major challenges, stability and activity after prolonged storage must be addressed for commercialization apart from the cost of the product. The current review summarizes the major applications of EPS composites in healthcare, as well as research challenges.
2. Microbes Producing Exopolysaccharides
Exopolysaccharides are produced by diverse microorganisms, including yeasts, fungi, and bacteria, utilizing various raw materials ( Figure 2 ). Besides the native physiological role, microbial EPSs also have diverse applications in many industries. Several efforts have been made by various researchers for the production of EPSs ( Table 1 ). In comparison to yeasts and other fungi, probiotic bacteria are commonly used due to non-pathogenicity and categorization of generally regarded as safe (GRAS) [ 25 ]. It has been found that carbon source has a direct relation with EPS production and composition. A study on 20 strains of Lactobacillus paracasei revealed that with a change in carbon source, not only does EPS yield, but it also influences the monosaccharide composition. The yield of EPS was increased by 115% with optimized carbon sources, including fructose, glucose, galactose, lactose, mannose, and trehalose [ 26 ]. Among different carbon sources, including sucrose, maltose, lactose, glycerol, and sorbitol, maximum EPS production has been achieved with maltose by Candida guilliermondii and Candida famata (0.505 and 0.321, respectively) [ 27 ]. Among 156 lactic acid bacteria isolated from healthy young children’s feces, the maximum EPS production, i.e., 59.99 g/L, was reported from Weissella confusa VP30 after 48 h in growth media containing 10% sucrose [ 28 ].

Microbial production of EPS using waste, and its recovery.
The use of commercial-grade sugars/substrates has a direct impact on product cost, and is one of the factors responsible for high production costs. Hence low-cost waste materials, such as lignocellulosic residues and wastewater, are preferred as raw materials for EPS production. The carbohydrate and organic fractions present in the waste can be used by microorganisms. It opens up the opportunity to reduce product costs, along with waste management. Da Silva et al. [ 29 ] have compared coconut shells, cocoa husks, and sucrose for xanthan gum production by Xanthomonas campestris pv. campestris IBSBF 1866 and 1867. The study revealed that xanthan gum yields were higher, i.e., 4.48 g/L and 3.89 g/L, in the case of cocoa husk by Xanthomonas strains 1866 and 1867, respectively, but the apparent viscosity was higher than sucrose, i.e., 181.88 mPas over cocoa husk, with a viscosity of 112.06 mPas.
Choi et al. [ 30 ] used spent media wastewater (originated from kimchi fermentation) for EPS production using Leuconostoc mesenteroides WiKim32. Under optimal conditions, the maximum EPS productivity was 7.7–9.0 g/L, with a conversion of 38.6–45.1%. The EPSs were nontoxic and exhibited thermal tolerance and antioxidant activity. Pan et al. [ 31 ] optimized dextran production from Leuconostoc pseudomesenteroides XG5 using an L9 (33) orthogonal test. Under optimal conditions (sucrose 100 g/L, pH 7.0, 25 °C, at 100 rpm for 36 h), the maximum dextran yield of 26.02 g/L and 40.07 g/L were recorded at a laboratory scale and fed-batch fermentation, respectively. The EPS was also exhibiting water-holding capacity and antioxidant activity. It reduced the chewiness and hardness of yogurt, but the resilience increased during the 14 days of storage. Product cost is one of the main obstacles in commercialization, hence process economics is one of the major factors that must be assessed. Integration of multiple processes might improve process economics, as well as environmental adaptability. Sphingobium yanoikuyae was evaluated for the coproduction of EPS and polyhydroxyalkanoates (PHAs) using lignocellulosic hydrolysate. Hydroxymethyl furfural (HMF), one of the hydrolysis byproducts, improved the consumption of glucose and xylose during fermentation. The optimum C/N ratio of 5 resulted in the maximum EPS production of 3.24 ± 0.05 g/L, however, a further increase in the C/N ratio (30) favored PHB accumulation (38.7 ± 0.08% w / w ) [ 32 ]. Biomass hydrolysate is usually accompanied by phenolics, furfurals, and HMF, which also act as fermentation inhibitors and hinder microbial action. Some of the methods, including activated carbon-based adsorption and membrane filtration, have been proposed for hydrolysate detoxification [ 33 , 34 ]. Removal of inhibitors might improve microbial action and product yield. Bhatia et al. [ 32 ] have compared the potential of various raw and detoxified hydrolysates for EPS production. In comparison to raw hydrolysate, detoxified biomass hydrolysate showed increased EPS production and maximum EPS production was reported with detoxified pine biomass hydrolysate, i.e., 2.83 ± 0.03 g/L. Table 1 summarizes the recent efforts for EPS production using various microbes (yeasts, bacteria, and fungi), utilizing various pure carbon and organic waste materials.
Microbial production of EPS from various substrates.
Preparation of composites for healthcare applications needs high purity, therefore the downstream processing becomes an inseparable part of processing after fermentation. After production recovery, the identification of structural and chemical characteristics is necessary for further applications. The characterization of EPSs is quantitative, as well as qualitative. EPSs are mainly comprised of carbohydrates conjugated with other biomolecules, hence the basic characterization techniques employed are a colorimetric estimation and use spectrophotometry [ 45 ]. For carbohydrate estimation ‘Dinitrosalicylic Acid Reagent’ is one of the common methods which quantify the reducing sugars [ 46 ]. Similarly, for protein, Bradford’s dye-binding method [ 47 ] and Lowry’s method [ 48 ] are used. Besides basic characterization with colorimetric methods, Fourier transform infrared spectroscopy (FTIR) is employed to detect the available functional groups and structural functionalities of EPSs. For the detailed structures of EPSs, nuclear magnetic resonance (NMR) and mass spectra are used. In NMR, the sample is dissolved in deuterated solvents for quantification with respect to internal standards [ 49 , 50 ]. The mass spectrum of EPS provides a monosaccharide composition. For analysis, EPSs are hydrolyzed with acid hydrolysis, followed by silylation derivatization. The derivatives are detected and identified by gas chromatography-mass spectrometry [ 49 ]. The biological potential of EPS has followed the general procedure for antimicrobial, antioxidant, anti-inflammatory and other activities [ 45 ].
3. Microbial Exopolysaccharide Composites and Their Applications
Exopolysaccharides are natural biopolymers exhibiting diverse applications, variation in structure, adaptability, and the presence of different functional groups. These polymers have shown their suitability as drug carriers and medical sealants. Even with polymeric nature and biocompatibility, these polymers have also shown some weak points. Rapid degradation, hydrophilic nature, low mechanical and tensile strength, and stress tolerance, particularly for scaffold preparation, restrict their application. Some EPSs lacks bioactivity itself, which suggests the addition of multiple drug compounds to add bioactivity for in vivo application. Exopolysaccharide composites have shown a higher potential than native EPSs due to the presence of secondary polymer molecules that offer hybrid characteristics of both ( Table 2 ). The higher mechanical strength with different functional characteristics contributes to diverse functional groups, and is responsible for physiological and chemical properties, contributed to by the members of the composite. To date, EPS composites have been prepared with natural, as well as synthetic, polymers. The selection of secondary polymers relies upon the type of application such as drug loading and release, tissue support and engineering, or ex vivo applications such as a sealant ( Figure 3 ).

Composite scaffold prepared from different exopolysaccharides ( A ). Xanthan gum–gelatin [ 51 ]; ( B ). Xanthan gum–Konjac glucomannan [ 52 ]; ( C ). Alginate–gellan gum composites [ 53 ] and ( D ). Composite methacrylated gellan gum [ 54 ].
Piola et al. [ 51 ] prepared a composite hydrogel with gelatin and xanthan gum to support the growth of human skin cells. The composite was printed with CellInk Inkredible 3D printer, using glutaraldehyde solution as a crosslinker. The printed hydrogel was compatible and suitable for the growth of human keratinocytes, as well as fibroblast. Alvel et al. [ 52 ] also prepared a composite hydrogel of xanthan with Konjac glucomannan, which also focused on wound healing. On the other hand, a 3D scaffold for tissue engineering was prepared with alginate–gellan gum [ 53 ] and methacrylated gellan gum [ 54 ].
The applications of EPS composites with various polymers have been summarized below:
3.1. Exopolysaccharide Composites with Natural Materials
EPS itself is a natural biopolymer that is combined with other natural biomolecules, including proteins, enzymes, lipids, carbohydrates, etc., and thus in most cases, the composite has healthcare-associated applications, including medical sealants and scaffolds in tissue recovery and repair. The major advantages of EPS composites with natural polymers are bioactivity, biodegradability, and biocompatibility. As both the components are natural in origin, there will be a negligible possibility for inflammation and rejection. Alongside that, these composites will not have any toxic effect on the host, but may also act as an antimicrobial formulation for targeting microorganisms. Most biological materials can also be produced in bulk amounts using microorganisms, hence the cost of the products is also reduced along with the higher availability. The addition of other polymers may also add a specific type of bioactivity, such as antimicrobial, anti-inflammatory, and even catalytic activity to the composite, which widens the possible application in different fields [ 55 , 56 , 57 ].
3.1.1. Cellulose Composites with Natural Polymers
Cellulose is a linear structural polysaccharide comprised of glucan chains connected with cellobiose residues via β-1,4-glycosidic linkage. These structures are packed in forms of microfibrils, kept together with hydrogen bonds and Van der Waals interactions. Depending upon the packaging, it exhibits different degrees of polymerization. It is usually present in the secondary cell wall of plants, but is also present in some bacteria, including members of the Acetobacter, Agrobacterium, Azotobacter, Alcaligenes, Pseudomonas, Rhizobium, and Sarcina genera [ 58 ]. The main function of cellulose is to provide strength to structure [ 59 ], however, cellulose does not exhibit antimicrobial or similar bioactivity in itself [ 60 ], but its high mechanical strength and stress tolerance nature make it suitable for the fabrication of scaffold and dressing material when used together with some bioactive material. Various polymers have been exploited to form composites with EPSs, which improve their strength and water retention capacity and also aid in antimicrobial properties.
Ojagh et al. [ 61 ] produced cellulose with Gluconacetobacter xylinus by static fermentation. The cellulose and diethylaminoethyl cellulose were derivatized to carboxymethyl cellulose and carboxymethylated diethylaminoethyl cellulose, respectively, and processed for composite preparation. Briefly, cellulose is a carbohydrate that is rich in hydroxyl groups, which makes it negatively charged. However, similar charges on both components make interactions repulsive. Derivatization of cellulose to carboxymethylated diethylaminoethyl cellulose (CMDEAEC) has both hydroxyls, as well as amine groups in its structure. In contrast to cellulose, CMDEAEC has a net positive charge, and thus the higher amount of CMDEAEC can be loaded on BC in composite formation. The composite of bacterial cellulose–carboxymethyl cellulose has a higher drug loading capacity and swelling ratio than the composite of bacterial cellulose–carboxymethylated diethylaminoethyl cellulose, and even native bacterial cellulose itself. The drug release follows the Higuchi and Korsmeyer–Peppas models. The model is most suitable to describe the release of drugs from the polymer matrix hydrogel. The model suggests that drug release increases from the carrier with time. Methylene blue is a positively charged molecule and the presence of cellulose supports its binding to the composite. The work has also shown that chemical modification adds unique properties to EPSs, improves functionality, and enhances the interaction with another polymer to form a stable composite. Bacterial cellulose was functionalized by derivatization with two active agents, i.e., glycidyl trimethylammonium chloride and glycidyl hexadecyl ether. These agents act upon hydroxyl functional groups of glucose by a heterogeneous reaction, which simultaneously deprotonates the hydroxyl group, as well as the addition of epoxides. It was observed that mere derivatization reduced the bacterial population of Staphylococcus aureus 6538PTM and Escherichia coli (Migula) ATCC ® 8739TM almost by half (53% and 43%, respectively) within 24 h upon direct contact. However, the derivative did not have any cytotoxic effect in terms of morphology and viability upon keratinocytes (HaCaT cell line) and almost 90–100% viability was recorded after 6 days of direct contact. Modified hydrogel has shown an equitant wound closure rate in an in vitro scratch assay, with complete coverage of the wound area after 5 days [ 60 ]. It was suggested that the addition of epoxide to cellulose adds antimicrobial properties to cellulose that are also exhibited by the composite.
Composite hydrogels are made by attaching TEMPO-modified nanocrystalline cellulose to the methacrylated gelatin backbone. The human adipose-derived mesenchymal stem cells were encapsulated within the composite and cultured in normal and osteogenic media for 14 days, and the expression of valve interstitial cell phenotypes was observed. The encapsulated cells have lowered alpha-smooth muscle actin expression, while the expression of vimentin and aggrecan increased. Cells cultured in osteogenic media have a reduced expression of osteogenic genes (Runx2 and osteocalcin) that support resistance to calcification. With the composite, a tall and self-standing tubular structure was constructed with a composite hydrogel that also sustained cell viability for possible application in cardiovascular systems [ 62 ]. The addition of cellulose to chitosan has improved the mechanical strength, porosity, cytocompatibility, and drug release rate for use in bone tissue engineering. The ternary complex of alginate, chitosan, and bacterial cellulose was used for the preparation of scaffolds using a hydroxyapatite/D-glucono-δ-lactone complex-based gelling system. The composite-based scaffold can adapt to 3D morphology and is stabilized with extensive cross-linking. The smaller size of pores supports the attachment and growth of tissue, along with the required mechanical integrity. Alginate controls the swelling behaviors of the scaffold by intermolecular hydrogen bonds and prevents the degradation of the composite. It has shown high protein adsorption and release potential, along with commendable cytocompatibility, that supported its application in tissue engineering [ 63 ]. The polymer supported the 3D structure and allowed for the proliferation of cells, increasing the possible application opportunities in the reconstruction of complex and large organ structures.
3.1.2. Dextran Composites with Natural Polymers
Dextran is a non-toxic homoexopolysaccharide, mostly produced by lactic acid bacteria. It is made up of glucose subunits interconnected with α-(1→6) bonds, and forms a linear backbone. However, liner chains also have D-glucose side chains as branches attached with α-(1→4), α-(1→3), or α-(1→2) linkage. Dextran exhibited diversity in molecular weights that range from 40–70 kDa. Dextran is water soluble, increases viscosity, and has a tendency to expand in the presence of water, contributing to the volume expansion in the case of plasma volume compensation, gelling agent in foods and pharmaceuticals [ 6 , 11 ]. It is also used in the preparation of molecular sieves while purifying macromolecules [ 20 ]. The solubility and nontoxicity of dextran have a big role in its application for drug delivery and preparation of wound dressing materials for biological systems.
Dextran was oxidized with periodate-assisted oxidation, followed by a reaction with chitosan hydrochloride. The composite did not have any cytotoxicity and offered minimal swelling in phosphate-buffered saline, along with good adhesiveness. The optimum adhesive strength of the composite, i.e., 200–400 gf/cm 2 , was 4–5-fold higher than commercial fibrin glue. The burst strength of the composite was 400–410 mm of Hg, which makes it suitable to be used as a medical sealant to control bleeding during surgical procedures. It also works under high blood pressure conditions. Both, adhesiveness and hemostat were assessed and found to be suitable in a rabbit liver injury model [ 64 ]. Besides medical adhesiveness, dextran has shown commendable suitability for in vivo applications, such as drug delivery and wound healing. Dextran composites alone can contribute to both adhesiveness and drug-carrying capacity. Even though a number of drugs are available for wound dressing and tissue repair, in some cases, injury worsens due to failure of wound dressing, mainly attributed to poor bioavailability, hydrophobic nature of drugs, and high level of reactive oxygen species. Dextran-based composites have offered the solution for all these challenges of rapid tissue repair. The drug delivery and bioavailability can be improved further when used in the nano form due to increased surface area. Andrabi et al. [ 65 ] have fabricated a composite nano-hydrogel with gelatin, oxidized dextran, and curcumin and cerium oxide-based nano-formulation. Two types of dextran derivatives have been used, i.e., alkylated dextran with 1-bromohexadecane (O-hexadecyl-dextran) and oxidized dextran. Curcumin nanoparticles were synthesized with alkylated dextran, while, on the other hand, cerium oxide nanoparticles were loaded onto the gelatin hydrogel with oxidized dextran. Altogether, these formulations form a hybrid nano-hydrogel that is used for wound healing. Here, alkylated dextran was amphiphilic in nature, which maintains the bioavailability of the drug (curcumin). Cerium oxide acts as an antioxidant and controls the reactive oxygen species and other free radicals. Hydrogel has shown a prolonged and consistent release of the drug, with ~63% release in 108 h. It also promotes cell migration to induce wound healing, along with antioxidant and in vivo anti-inflammatory activity (~39%).
Poor wound healing raises concerns in cases of noncompressible injuries due to a higher rate of blood loss. These injuries mostly occur from gunshots or injuries with sharp objects, such as knives, etc. In the absence of timely repair of the injury, higher blood loss and trauma-assisted mortality have been observed. Cryogels were prepared from oxidized dextran, chitosan, collagen, and polydopamine nanoparticles. In the formulation, dextran was used as a crosslinker, while chitosan was a hemostatic agent. The cryogels have an intensively branched and interconnected macroporous structure, with a high capacity to absorb blood or water. In vitro assessment suggested a very high coagulation potential due to strong procoagulant ability, and high adhesion potential for fibrinogens and blood cells. Moreover, it can activate platelets and associate intrinsic pathways. It reduced bleeding time and blood loss [ 66 ]. The dextran–thyme magnesium-doped hydroxyapatite composite is prepared by mixing salt precipitate with dextran and essential oil solution ( Figure 4 ). The composites have shown antimicrobial activity and have considerable results against Staphylococcus aureus , Enterococcus faecalis , E. coli , Pseudomonas aeruginosa , and Candida albicans. The composites have the potential for application as antimicrobial coating materials [ 67 ].

Preparation of antimicrobial coatings with dextran, thyme essential oil, magnesium salt, and hydroxyapatite, as suggested by Iconaru et al. [ 67 ].
3.1.3. Xanthan Composites with Natural Polymers
Xanthan is a hetero-exopolysaccharide, comprising glucose, mannose, and glucuronic acid. It is produced and secreted by Xanthomonas campestris . Biochemically, it is made of repeated units of pentasaccharides interconnected with β 1, 4-linked d-glucose backbone. The chain also has a substitution with trisaccharide side-chain linkage. The monosaccharide composition represents β-D-glucose, α-D-mannose, and α-D-glucuronic acid in a 2:2:1 ratio. Xanthan is known for its high viscosity, emulsion stabilization, and shear-thinning activity. Xanthan is non-toxic and biocompatible, but it is disadvantaged by poor electrical conductivity and stability of heat [ 20 ]. In comparison to macrostructures, the application of nanomaterials increases the interactions at a molecular level, has a higher surface area, and reduces the drug dosage required due to the higher efficiency of drug delivery in the nano form [ 68 , 69 ]. The application of EPS composites as nanomaterials might improve the performance, hence efforts have been made to incorporate the nanomaterials into the composite.
Nanocomposites comprising sodium alginate and xanthan gum, reinforced with cellulose nanocrystals and/or halloysites, are highly porous, with an extensive pore network. Nanotubes and nanocrystals have uniform dispersion and partial orientation within a composite. Composites with nanocrystals and nanotubes have porosity ranging from 91.7 ± 0.81% to 88.5 ± 0.64%, and water uptake capacity ranging from 14.73.7 ± 0.46 g/g to 11.34 ± 0.32 g/g. The composite is thermally stable and has high compressive strengths of 91.1 ± 1.2 kPa to 114.4 ± 0.6 kPa in dry form, and 9.0 ± 0.8 kPa to 10.6 ± 0.8 kPa in wet form. The composite has high cytocompatibility for MC3T3-E1 osteoblastic cells, and the viability of the cells increased with nanotube components in the composite. Both nitrocellulose and nanotubes increased the mechanical stability of the composite, along with conducive bioactivity, including higher cell adhesion and proliferation. The compressive strength of the composites was higher than alginate alone, as well as the alginate-xanthan gum blend, i.e., 91 kPa and 80.7 kPa. The blending of xanthan gum lowered the stiffness, which was further improved by the addition of nanocrystals and nanotubes, with the maximum strength reaching 114.4 kPa [ 70 ]. Mechanical strength and adhesion are important parameters for wound dressing material. Hydrogels of xanthan gum and Konjac glucomannan blend have shown significant firmness and cohesiveness when both polymers are used in an equal ratio, i.e., 1:1. All the blends prepared with components of 1:1 and 2:3 ratios (glucomannan: xanthan gum) have a water content of more than 99% and water contact angle of less than 90°. This feature is also important, as it prevents dehydration in wounds and supports healing by improving cell adhesion. In vitro analysis suggested that even after 72 h of contact, the hydrogel did not affect the morphology and viability, and supported the proliferation of fibroblasts. The hydrogel also provides cell migration during the healing phase that aided in rapid wound healing, which led to the full coverage of the wound site after 12 h, while in the control, it took 24 h [ 52 ].
Medical adhesives have been used in device and tape manufacturing. Biocompatible adhesive might offer a durable and user-friendly alternative for application. Feng et al. [ 71 ] have developed medical-grade adhesives with soybean protein sources at different concentrations of xanthan gum. The composite comprising 0.5% xanthan and 0.5% soybean proteins have shown a maximal adhesion strength of 321 kPa, which was 2.6-fold higher than the control, i.e., SP alone. The addition of xanthan increased the viscosity of the composite and improved the hydrogen bond. On a molecular basis, the addition of xanthan reduced the α-helix content and increased the β-sheet content in the protein secondary structure. The composite has shown a compact and viscous surface that supports adhesion. Sometimes, bone injuries need support and artificial implants for regeneration and recovery. Zia et al. [ 72 ] have prepared a polymer composite with xanthan gum, chitosan, and nanohydroxyapatite and polyelectrolyte complex. Osteo-conductive tri-composite scaffolds were prepared for osteo-regeneration. The composite scaffold, containing polymer electrolyte and hydroxyapatite in the ratio of 1:1, exhibited a commendable porous structure with a compressive strength swelling ability with slower degradation rates. Analysis in simulated body fluid, xanthan gum, chitosan, and nano-hydroxy apatite have apatite-like surface structures. In vitro interaction studies revealed that the nanocomposite scaffold with chitosan and nanohydroxyapatite supported cellular viability, attachment, and proliferation of MG-63 cells.
Polymeric porous scaffolds were made from chitosan and xanthan, along with 5% hydroxyapatite and brushite, for advanced mesenchymal stem cells. Composite scaffolds have an amorphous structure phase, having major bands of amide for chitosan and xanthan. Scaffolds have porous structures with calcium phosphate fillers. The elasticity modulus was higher for composite scaffolds with brushite than with hydroxyapatite and composite alone. Composites alone have a higher cell viability than scaffold–calcium phosphate, having acceptable cell viability. The composite with any calcium phosphate forms had a higher inflammatory response after 48 h, while scaffold + hydroxyapatite with mesenchymal stem cells had the lowest inflammatory cell number. It was clear from the work that calcium phosphate improved mechanical strength, but lowered cell viability. The toxic effect was countered by the addition of mesenchymal stem cells [ 73 ]. Now, the era of 3D printing has started, but the fabrication of biological structures needs supportive and efficient materials with a high mechanical strength and elasticity modulus. Piola et al. [ 51 ] developed crosslinked 3D-printable hydrogel with gelatin and xanthan gum, especially for wound dressing. The results have suggested that both gelatin and xanthan gum are important for composite formation, but xanthan concentration is crucial for the printability of the composite, as 1–1.2% of xanthan is required to attain printability, irrespective of the concentration of gelatin; however, 1.2% concentration was optimum.
3.1.4. Pullulan Composites with Natural Polymers
Pullulan, an unbranched homopolysaccharide, is composed of triose units made from three glucose units interconnected by α-1,4 glycosidic bonds. These maltotriose units are linked with each other by α-1,6 glycosidic bonds. It is produced by Aureobasidium pullulans from starch, and secreted out [ 74 , 75 ]. The α-bond contributes to its aqueous solubility and flexibility. Overall, it is a biodegradable, nontoxic, and biocompatible polymer [ 76 ]. The characteristics are suitable for hydrogels and hybrid polymer fabrication, but the main drawbacks are its high-water solubility and hygroscopic nature. In addition, it has poor support for cell adhesion and proliferation, and is poorly osteogenic, mainly attributed to its hydrophilicity [ 77 , 78 ].
For making bone grafts, mechanical strength and degradation profile are important parameters. Usually, a hydrogel has a porous structure with intense networking. Pullulan is one of the non-immunogenic biopolymers that can be used for scaffold fabrication. Pullulan–dextran composite scaffolds, together with interfacial polyelectrolyte complexation fibers, have an improved adhesion for cells in comparison to pullulan. Further addition of extracellular proteins proved beneficial for cell adhesion and growth. The composite scaffold induced endothelial cell growth and followed the zero-order release kinetics for bovine serum albumin, as well as vascular endothelial growth factor [ 79 ]. Hydrogels are made from pullulan and covered with 5% hydroxyapatite nano-crystals and 3% poly(3-hydroxybutyrate). Making composites with fillers, such as butyrate, increased the compressive modulus of the composite scaffold by 10 times. The adhesion support for cells was improved by the presence of hydroxylapatite nanocrystals [ 78 ].
Posterior segment eye diseases need invasive intravitreal-injection-assisted treatment. However, prolonged injections become painful and can be countered with the use of an efficient drug delivery system. Kicková et al. [ 80 ] prepared pullulan–dexamethasone conjugates for sustainable drug delivery. Dexamethasone was loaded onto pullulan in a 1:20 ratio, and attached with pullulan via hydrogen bonds. The composite particles were stable at a wider range of temperatures, from 4 to 37 °C, at a physiological pH. At pH 5.0, these composites were acting like lysosomes and released the drug slowly, as 50% of the drug was released in the vitreous, while at pH 5.0, this occurred across a 2-day period. In vitro evaluation of biocompatibility showed no signs of toxicity on the retinal pigment epithelial cell line (ARPE-19).
Sustainable drug release from the composites has also opened the path for designing matrices and scaffolds for wound healing. The research work of Chen et al. [ 81 ] has proved that dressing materials have a critical role in wound healing time, pattern, and cellular response on wound healing, from in-depth histologic and histopathologic analysis in mice models. The dressing materials made from collagen hydrogel followed a human-like wound-healing model in mice. In comparison to the control, the hydrogel of pullulan–collagen induced rapid wound closure and healing, healing with less dense and shorter collagen fibers with random alignment.
Baron et al. [ 82 ] also used a pullulan composite for wound healing and drug delivery. Hydrogels made from oxidized pullulan and dopamine have possible applications in hemostatic wound dressing. Cryogels were prepared by (hemi)acetal and Schiff base bonds between dialdehyde pullulan and dopamine. Two types of formulation were prepared, i.e., PD1 by pullulan derivatives attached with dopamine derivatives and PD2 by adsorbed dopamine on the scaffold. PD1 is loaded with 20% dopamine, while PD2 has only 1.14% carried within the polysaccharide network. The porosity was maximum in the scaffold alone (80.41%) and reduced after loading; however, the network density was maximal in PD1 (47.1%). The water adsorption capacity was minimum for the crude scaffold (31.41%) due to the smooth texture and the maximum PD1 (59.01%). The PD1 hydrogel exhibited fast swelling initially, followed by stabilization. In comparison to the crude scaffold and PD2, PD1 has the highest mechanical stability of 558 N. The in vitro hemolytic analysis suggested a high rate of hemolysis in PD2, i.e., 99.04%, followed by 7.12% in PD1 and a minimum for the crude scaffold (0.15%).
3.1.5. Levan Composites with Natural Polymers
Levan and gellan are exopolysaccharides extensively used in the food industry. Levan is a bioactive, user-friendly, non-toxic homo-exopolysaccharide produced by microorganisms, as well as plants. Among microorganisms, Aerobacter levanicum , Bacillus subtilis , B. polymyxa , Corynebacterium laevaniformans , Pseudomonas sp., and Streptococcus sp. are among some common producers of levan. It is one of the natural fructans, made of fructose subunits interlinked by β-(2→6) glycosidic linkages, along with side chains attachment via β-(2→1) linkage to the main backbone. It is commonly used as a thickening/gelling agent, encapsulating material, and alternative to petrochemicals in medical applications [ 83 ]. Levan is soluble in water, as well as in oil. In industries, it is used as gum, sweetener, flavor carrier, surface finishing additive, gelling agent, emulsifier, cryoprotector, and osmoregulator. Its bioactivity includes antitumor and antihyperlipidemic and radioprotector activities [ 84 ].
Levan has an immunomodulatory effect on the host, hence it is commonly used as a drug carrier, as well as drug coating material. Bovine serum albumin was used as a model compound, and was encapsulated in levan nanoparticles to assess carrying and release behavior. The nanoparticle has surface charges ranging from +4.3 mV to +7.6 mV, varying in particle size, from 200 nm to 537 nm. With the increase in size, the encapsulation capacity of nanoparticles also increased from 49.3% to 71.3%. Both types of particles have shown controlled release of BSA during in vitro analysis [ 85 ]. Besides drug delivery, levan has also been used as tissue filler. Due to its flexibility, an injectable biofiller was designed with levan, together with carboxymethyl cellulose and Pluronic F127, for the regeneration of soft tissue. The hydrogel offered an elastic modulus higher than hyaluronic acid hydrogel. The presence of interconnected pores also makes it suitable to be used as a filler. In vitro analysis has confirmed that levan improved cell proliferation and collagen synthesis in human dermal fibroblast cells without any cytotoxicity. The levan hydrogel was stable over 2 weeks in vivo, which was higher than the Pluronic F127 hydrogel or hyaluronic acid hydrogel alone. Apart from this, the levan hydrogel has also shown anti-wrinkle activity in wrinkle model mice, which was also higher in comparison to the hyaluronic acid hydrogel [ 86 ]. The hydrolysed derivative of the levan polysaccharide is prepared for nanoparticle synthesis by the electro-hydrodynamic atomization (EHDA) technique, and loaded with resveratrol (encapsulation efficiency 13.8 ± 1.3%). The drug-loaded nanoparticles have followed first-order kinetics in resveratrol release at different pHs, as higher loading is accompanied by a more gradual release. It showed a burst release mechanism, as 65–70% of the drug was released initially, followed by slow release. There was no sign of toxicity during in vitro assessment with human dermal fibroblast cell lines (PCS-201-012) [ 87 ]. Activation of metalloproteinase supports the healing of injured tissue. The application of levan in biomedical dressing sealants is limited, as it gets removed in a wet environment. The addition of catechol improved the adhesion strength of the levan composite to 42.17 ± 0.24 kPa (>3 times of fibrin glue), that persists even in a wet environment, and allowed for its application in hemostatic surgeries and wound healing. Besides adhesion, it also induced rapid blood clotting and healing of rat skin incisions. In comparison to levan, the composite has a lower endotoxin level [ 88 ]. The major challenge with levan is its cost and lower yield, thus the cost of composites and prepared products will also be high. For better market reach, bulk and cost-effective production is necessary.
3.1.6. Gellan Composites with Natural Polymers
On the other hand, gellan gum is a hetero exopolysaccharide composed of two β-D-glucose, β-D glucuronic acid, and α L-rhamnose subunits. It is an anionic, water-soluble polysaccharide, secreted by Sphingomonas elodea . Gellan gum is a strong gelling agent, able to form a gel at very even low concentrations. Based on the gel-forming ability, two types of gellan gums are available, i.e., low-acyl form that makes hard and brittle gel, and the second high-acyl form, that produces soft and elastic gels. It can be used to make low-viscosity suspensions, and as a gelling agent in industries [ 74 ]. It can offer diverse forms in gelling ability, but it lacks stability against stress and shear tolerance for tissue engineering. Zheng et al. [ 89 ] have blended gelatin with gellan gum to prepare injectable scaffolds applicable for skin regeneration. The aim was to cover irregularly shaped wounds followed by recovery. Gelatin and gellan gum had a synergetic effect on the composite hydrogel and exhibited both shear-thinning, as well as self-recovering abilities. Further addition of tannic acid induced rapid wound healing in mice model.
Some of the studies have also shown the application of biopolymer composites as a cell carrier in addition to drug compounds, as suggested in the case of retinal pigment epithelium cells. The deterioration and damage in retinal pigment epithelium cells lead to blindness that can be cured with the replacement of damaged cells with healthy ones. Kim et al. [ 90 ] have shown that hydrogels prepared from gellan gum and silk sericin can be used as a cell carrier also. Sericin improved the compressive strength of composite over gellan gum to support the growth and proliferation of cells. A composite of gellan gum with 0.5% sericin has shown compressive strength to 10 kPa and improved the gene expression associated with ARPE-19 cell proliferation.
3.2. Exopolysaccharide Composites with Synthetic Polymers
Similar to natural polymers and biomolecules, synthetic polymers have also been used to make a composite with exopolysaccharides, which improves the binding ability and woven strength of any fiber. The section summarized some of the composites of exopolysaccharides and synthetic polymers.
3.2.1. Dextran Composites with Synthetic Polymers
Dextran composites are prepared with synthetic polymers mainly to improve the water/solvent interaction behaviors, biodegradability, and mechanical strength. In the context of medical/healthcare-associated operations, polymers have higher strength or compounds with additional health benefits have a critical contribution. The fabrication of advanced fabric with biodegradability and inbuilt healing power might improve the treatment and revolutionize healthcare practices. A biocompatible dressing material fiber was prepared with electrospun poly(vinyl alcohol)–dextran, prepared from dextran and poly(vinyl alcohol) by using citric acid as a cross-linker, followed by loading with sodium ampicillin. The mechanical strength was mainly governed by the concentration of citric acid, as it determines the degree of cross-linking. However, swelling, adsorption of protein, and drug release were decreased, as the CA concentration increased, while high concentrations of dextran induced the proliferation of HFB-4 cells and offered higher antimicrobial activity. After 24–48 h of treatment, all the fabrics had the potential to accelerate the wound gap closure [ 91 ]. Kenawy et al. [ 91 ] used a composite of poly(vinyl alcohol)–dextran nanofibers for injury dressing materials. The composite was prepared by poly(vinyl alcohol) and dextran cross-linked with sodium ampicillin-loaded citric acid. The composite was electrospun for the fabrication of dressing material. The composite made with 10% PVA–10% dextran and 5% citric acid offered the best nanofiber suitable for dressing materials. The concentration of the cross-linker, i.e., citric acid, greatly influenced the characteristics, including mechanical stability, thermal stability, and water uptake. The composite nanofibers with a high concentration of dextran have encouraged the proliferation of HFB-4 cells.
3.2.2. Cellulose Composite with Synthetic Polymers
Bacterial cellulose is suitable for the fabrication of biopolymers, especially for healthcare-related applications, manufacturing scaffolds, implants, artificial blood vessels, and wound dressing materials, for wound and burn cases. The composite of bacterial cellulose with poly(vinyl alcohol) and hexagonal boron nitride was used for the preparation of a 3D scaffold for bone tissue engineering, using 3D printing technology. In composite bacterial cellulose, the major characteristics of the scaffold were determined. The addition of cellulose reduced the pore size in the composite and increased the viscosity to 81.3 mPa.s. Composites with bacterial cellulose have a lower tensile strength and the highest break in elongation, i.e., 93% was observed in the case of the composite prepared with 0.5% cellulose, 12% poly(vinyl alcohol), and 0.25% boron nitride. The biocompatibility assessment with human osteoblast cells suggested that at a lower concentration of cellulose, the viability of cells reduced by it further increased when the concentration of cellulose increased from 0.1/0.2% to 0.25 and 0.5%. The composite also offered a surface for cell adhesion and proliferation [ 92 ].
In a similar line, Zhang et al. [ 93 ] have fabricated macroporous hydrogels with dextran and polydopamine to be used as a carrier for antibiotics. Polydopamine affected the structure, as well as the functionality of the hydrogel, as with the increase in the concentration of dopamine, the pore size decreased and the surface area increased. The hydrogel has shown an increase in negative charge with the increase in the concentration of polydopamine in the composite, but the storage modulus and mechanical strength was increased. With the increase in dopamine content, the swelling ratio of hydrogel reduced and induced deswelling, which was due to the reduced pore size and higher interaction of dopamine with the internal structure of the hydrogel. The hydrogel lowered the viability of NIH3T3 cells slightly on the first day, but then no further cytotoxicity effect was reported. Hydrogels with a higher concentration of polydopamine were found to be suitable for drug loading and release. Hydrogels have shown up to 71% chlorhexidine acetate loading in 4.5 h, followed by its release of 12.58%, 16.06%, and 22.03% after 12, 24, and 48 h, respectively. The trend suggested that drug release was reduced with an increase in dopamine concentration. The work of Wang et al. [ 94 ] also emphasized the application of a cellulose composite for the preparation of artificial blood vessels with small diameters for thrombosis patients. A composite of poly(-caprolactone) and cellulose acetate was used to prepare nanofiber membranes for its further application in tubular scaffold fabrication, using different types of stainless-steel collectors. The composite scaffolds have water contact angles of 126.5° and 105.5°, which was increased by constructing a square-groove. In comparison to other collector mesh, scaffolds with a large mesh have 30% and 148% higher tensile strength over random-flat and tubular scaffolds, respectively. Similarly, the long mesh also offered 103% higher suture retention strength. Biocompatibility assessment revealed that the long mesh scaffold has 88% cell viability, and the blood coagulation index (BCI) was 5 min, which was around 89% of the standard value.
3.2.3. Xanthan Composites with Synthetic Polymers
Extracellular polysaccharides are one of the most suitable materials for the preparation of scaffolds, grafts, and dressing materials, as they have water retention capacity, significant mechanical and tensile strength, and adaptability similar to the natural organs and tissues. In addition, it also supports cell adhesion, growth, and proliferation.
Cell growth has a direct influence on the conductivity and impedance of the medium, which may be used as an indirect method to assess and monitor cell growth within polymeric scaffolds. A conducting polymer composite was designed for the preparation of porous scaffolds with poly(3,4-ethylenedioxythiophene) and xanthan gum and compared with PEDOT: polystyrene sulfonate scaffolds ( Figure 5 ). The semisynthetic composite scaffold carries important characteristics, such as the conductivity of poly(3,4-ethylenedioxythiophene), along with the biocompatibility and mechanical strength of xanthan gum. Composite scaffolds have interconnected pores, with a size range of 10–150 μm, that can be tuned as required, and Young’s module range was 10–45 kPa. The composite scaffold supports the cell growth of MDCK II eGFP and MDCK II LifeAct epithelial cells [ 95 ].

3D scaffold prepared with xanthan gum and poly(3,4-ethylenedioxythiophene), as described by del Agua et al. [ 95 ].
A series of composites were prepared with xanthan gum, citric acid, gelatin, glutaraldehyde, and HPLC-grade water, and evaluated for wound healing potential in rat skin wound models. All the composites have >90% of water-holding capacity. The presence of free and bound water was confirmed with FTIR studies, with inter- as well as intramolecular hydrogen bonds. Similar to water holding capacity, all the hydrogels have also shown significant wound healing capacity in deep second-degree skin burns in rats. After 20 days of application, a composite of xanthan gum–gelatin–glutaraldehyde and xanthan–citric acid and glutaraldehyde had a higher wound healing rate over others, as well as the control [ 96 ].
Malik and et al. [ 97 ] prepared an oral, drug carrier composite with chitosan, xanthan gum, monomer 2-acrylamido-2-methylpropane sulfonic acid, and potassium persulfate by free radicle polymerization technique. The components were crosslinked with N′ N′-methylene bis-acrylamide. The composite was loaded with acyclovir as a model antiviral drug commonly prescribed in herpes simplex virus infections. The composite had a higher thermal stability and a porous structure that encapsulated the drug compound. The composite hydrogel has shown pH-dependent drug releasing behavior. Ilomuanya et al. [ 98 ] prepared a hybrid composite with antibacterial, antioxidative, and anti-inflammatory behavior for wound dressing. The composite was prepared with poly(vinyl alcohol) and xanthan gum/hypromellose/sodium carboxymethyl cellulose. Composites with silver nanoparticles have higher antimicrobial potential and have inhibition of >99% against wound pathogens such as Acinetobacter baumannii , E. coli , K. pneumoniae , P. aeruginosa , S. aureus , S. epidermidis , and Candida albicans . Maximum bactericidal effect was observed from hypromellose nanocomposite, with 99.9% growth reduction, within 1 h of application. Dressing material prepared with composites also has free radical scavenging behavior, along with a reduction in the inflammatory response in RAW 264.7 macrophages. Animal model studies have confirmed that the hypromellose-based nanocomposite has a higher wound-healing process.
3.2.4. Gellan Gum/Levan Composite with Synthetic Polymers
Composites with synthetic and natural components may have a higher applicability rate as natural polymers are biocompatible and biodegradable, but the addition of synthetic material may prolong the lifespan and modify the surface texture for the attachment of polymer. A levan-based composite was prepared with two different forms of levan, i.e., hydrolyzed and sulphated. Both hydrolyzed and sulfated levans were synthesized by microwave-assisted acid hydrolysis, with 5% acetic acid at 60% operating power for 60 s and mixing with chlorosulfonic acid for 24 h, respectively. The composite blend of 10% polycaprolactone (THF:DMF) and the aqueous solution of sulfated levan and hydrolysed levan in polyethylene oxide was used for coaxial electrospinning. The composite fiber has higher ultimate tensile strength and it increased with ShHL concentration. The composite increased the viability of L929 fibroblasts and HUVECs [ 99 ]. Adrover et al. [ 100 ] have prepared the composite beads for drug delivery with gellan gum, calcium ions (CaCl 2 ), and with/without synthetic clay laponite ionotropic gelation technique. The addition of laponite reduced the swelling degree of polymeric beads. Gellan gum and gellan-silicate composite beads were loaded with theophylline and cyanocobalamin for in vitro release behavior. Laponite controlled the drug entrapment efficiency, as well as the slower drug release into the gastric environment. Theophylline followed Fickian behavior for drug release, while cyanocobalamin release behavior was greatly influenced by the physical/chemical interaction of the composite and drug.
For bone regeneration, an ideal membrane is of utmost importance that not only induces cell proliferation followed by mineralization, but is also able to withstand stress. A composite of calcium–poly-γ-glutamic acid and glycerol with gellan gum was studied for bone regeneration. In composite membranes, calcium aggregates are distributed uniformly and act as commendable performers in protein adsorption, and bone cell proliferation with MG63 cells. The hydrogel has promising results for bone repair, and γ-PGA acts critically. The exopolysaccharide member acts as biocompatible material, and γ-PGA improved cell adhesion and proliferation. It also increased the secretion of alkaline phosphatase and induced mineralization. The third member, ‘glycerol’, enhanced mechanical strength, elongation at break, as well as diffusion rate. Both glycerol and γ-PGA delayed the degradation of the composite [ 101 ]. The main contribution of synthetic polymers in the composite is to improve the tensile and mechanical strength and delay the degradation, an important feature that is required to provide sufficient time for tissue repair and recovery.
3.2.5. Pullulan Composites with Synthetic Polymers
Drug-delivery-related applications required hydrophobic carriers and functional groups that can offer a site for attachments. Exopolysaccharides such as pullulan are soluble in an aqueous environment. However, these are sensitive to modification by hydrophobic functional groups, including the cholesterol functional group, which make them amphiphilic and ensure the drug release.
Cholesteryl-modified aminated pullulan polymers were prepared with cholesterol succinate and pullulan. Succinic anhydride cholesterol (0.2–0.6 g), 0.18 g dimethylaminoaniline, 0.35 g 1-ethyl-(3-dimethylaminopropyl) carbodiimide salt, and acid salt were dissolved in DMSO at room temperature, which activated succinic anhydride cholesterol. The activated solution was added to 5.6% amino pullulan solution in DMSO and mixed at 50 °C for 48 h, followed by cooling to room temperature. Anhydrous ethanol was added to the reaction liquid that precipitated the composite. The composition of different components affects the properties of the composite. With respect to different concentrations of cholesteryl substitution, particle size reduced from 178.0, 144.4, and 97.8 nm, with an increase in the extent of substitution. With an increase in substitution, the hydrophobicity of the pullulan derivative increased and the particle size reduced. Hydrophobicity also influenced the drug release, as derivatives with maximum hydrophobicity have the slowest drug release, i.e., 57.8%; and the lowest hydrophobicity have maximum drug release, i.e., 72.7% after 48 h. In contrast, the efficacy against lung cancer cells increased with a reduction in hydrophobicity [ 102 ]. Mommer et al. [ 103 ] have functionalized the pullulan by thiolactone-based activation that adds amines. Modified pullulan offered the possibility of forming a composite with amine-containing biological substrates. With respect to thiolactone substitution (2.5/5.0 mol%), hydrogels have different mesh sizes, i.e., 27.8 and 49.1 nm respectively. Cell proliferation studies were conducted with different cell lines (normal human dermal fibroblasts and hepatocytes) and it was reported that gelatin and H-Gly-Arg-Gly-Asp-Ser-OH (GRGDS) support cell proliferation, while H-Gly-Arg-Gly-Asp-Ser-OH as well as H-Gly-His-Lys-OH acetate salt improved the proliferation of hepatocytes (HepG2) by up to 10 folds over gelatin. A thermosensitive composite was prepared with pullulan, carrying pendant carboxymethyl groups and amphiphilic triblock copolymer, poloxamer 407, by grafting. The final composite has about 83.8% poloxamer grafted on the copolymer. The composite was highly flexible and elastic, which allowed for the copolymer to regain and recover the native structure after the removal of external force/stimuli. The gel sustained amoxicillin release over a period of 168 h. The composite can be used as a hydrogel for scaffold fabrication, as well as for drug delivery [ 104 ].
High water holding and moisture retention with mechanical properties are the main strengths of bacterial nanocellulose suitable for the preparation of wound dressing and fabrication of biomedical device production, but cellulose lacks antimicrobial and wound-healing capacity. On the other hand, pullulan can contribute to wound healing, and zinc oxide nanoparticles offered antibacterial properties. Aminoalkylsilane grafted bacterial cellulose membrane and established an interconnection between cellulose with pullulan–ZnO nanoparticle hybrid electrospun nanofibers. Dressing materials prepared from composites have better blood clotting performance over the control, i.e., BNC. The composite released ZnO and offered 5 logs of higher antibacterial activity than cellulose. Cytotoxicity analysis with L929 fibroblast cells suggested that the composite was safe for fibroblast cell proliferation. In the animal (rat) model, the composite material offered rapid healing and re-epithelialization rate with the formation of a small blood vessel and synthesis of collagen [ 105 ].
Exopolysaccharides composites in biomedical applications.
The composite preparation and applications in different fields are still not very well developed. Some of them are under screening and others have crossed trials in cell lines. The efforts have received wide attention, and for outstanding findings, patents have been awarded ( Table 3 ).
Patents awarded in microbial exopolysaccharide-related research.
Microbe-based polysaccharides are biocompatible, nonimmunogenic, biodegradable and most of these are USA FDA-approved and regarded as safe for human consumption [ 135 ]. According to GRAS notice 000099, the use of pullulan is allowed as an ingredient in tablets and capsules for dietary supplements [ 136 ]. The commercialization of such products still needs a long way to cover especially in the context of their behavior in various conditions to support their candidature.
4. Challenges and Future Prospective
Microbial EPS and associated composites have offered several advantages such as biocompatibility, biodegradability, and nontoxicity; however, there is still no product available at the commercial level. The commercialization of products prepared from EPS composites needs to address some of the common challenges, summarized below.
4.1. Strain Selection
The major fraction of EPS composites is microbial exopolysaccharides, which are produced by fermentation at various scales. Strain selection is one of the major problems associated with the large-scale production and commercialization of EPS and associated products. Several microorganisms have been screened for the production of EPS and some of them are either pathogenic or have low yield [ 137 ]. However, some of the microorganisms have offered compatible productivity without any pathogenicity, such as probiotic bacteria, including Lactobacillus , Bifidobacterium , and Lactococcus . In addition to yield, the tolerance to high salt concentrations (bile salt) and extremely acidic pH are other advantages that aided in the commercialization of products. Recent research has also proven that lactic acid bacteria also improved immunity and wound healing [ 49 , 138 ].
Genetic engineering of microorganisms is another possible strategy to improve productivity. However, the production of polysaccharides needs a combinatorial synthesis approach, as it is governed by multiple pathways. Cumulatively the pathways have the following three stages: nucleotide sugar precursors synthesis, extension, and synthesis of oligosaccharide units by glycosyltransferases and assembly of structural units to the final EPS, followed by export [ 139 ]. Some of the strategies have been summarized by Schmid et al. [ 140 ], but no report is available on the large-scale production of EPS with genetically engineered microorganisms. This may be due to the complex biosynthetic pathway governed by multiple gene systems. There is a need to work on this issue to increase EPS productivity with unique properties.
Production of exopolysaccharides, yield, and cost: Any microorganism can offer a higher possible production under optimal growth and production conditions. Before moving to higher-scale production, the identification of exopolysaccharides, their nature, and optimal conditions for maximum productivity are required [ 141 ]. As mentioned above, EPS production relies upon commercial-grade substrates with a higher cost, which also affects the product cost. A possible way out for the substrate is the utilization of low-cost lignocellulosic biomass from agricultural, as well as industrial sources, such as raw material. Previous research has used various agricultural biomass for pullulan production by Aureobasidium pullulans , i.e., hazelnut husk [ 142 ], sesame seed oil cake [ 143 ], corn cobs, and straw [ 144 ]. The process can be optimized via one factor or a statistical optimization approach. However, lignocellulosic materials, such as raw materials, need preprocessing such as hydrolysis, followed by detoxification. Acid hydrolysis is one of the most common methods used for sugar recovery in hydrolysate, but some byproducts, including furfurals and hydroxymethyl furfurals (from pentose and hexose), were also produced along with phenolics from lignin. These byproducts have shown an inhibitory effect on microbial growth and hence hydrolysate was detoxified by activated charcoal and membrane separation [ 33 , 145 , 146 ]. These additional steps for biomass processing and hydrolysate preparation increased the process, as well as product cost. The use of tolerant microorganisms might help overcome the challenges associated with inhibitors.
4.2. Downstream Processing
Downstream processing and product recovery is one of the major challenges, as it contributes to the majority of the product cost. Therefore, an efficient and low-cost downstream process is a prerequisite for the commercialization of exopolysaccharides and associated products [ 141 ]. The chemical nature and characteristics of exopolysaccharides, such as high viscosity and gel-forming ability, hinder their extraction. Some of the recovery approaches are trichloroacetic acid and proteases-assisted protein removal, dialysis, chromatography, and solvent-based precipitation used for EPS recovery. The ultra-grade of purity of a product is necessary for biomedical applications, hence the selection of the recovery process should be done as per the target product, as well as the physicochemical properties of EPS. Proteins are removed by protease/trichloroacetic acid to prevent contamination and cross-reaction. However, heat or use of concentrated acid damage the native EPS structure and change the integrity of bonds, branching, and sugar monomers. [ 147 ].
4.3. Composite-Forming Ability of EPS
Usually, EPSs are water-soluble (except a few, such as cellulose) and hydrophilic, which makes EPS prone to degradation in storage, as well as in the cellular environment. This also influences the selection of drug and secondary polymers, as components with similar charges usually make an unstable system due to repulsion. Due to the presence of hydroxyl and carboxylic functional groups, most of the EPS molecules are negatively charged, and thus loading of negatively charged groups and blending with negatively charged polymers is not possible due to electrostatic repulsion [ 137 , 148 , 149 ].
Derivatization of exopolysaccharides is one of the most common approaches to change the net charge, as well as improve interaction with other molecules, as observed in the case of cellulose, which is insoluble in water as well as organic solvents. The availability of the hydroxyl functional group makes it possible to modulate its chemical nature, including solubility, hydrophilicity/hydrophobicity, and mechanical strength either, by degradation or derivatization. Cellulose ether is the derivative prepared by replacing the hydroxyl group with the hydrocarbon group. It includes carboxymethyl, methyl, hydroxypropyl methyl, and hydroxyethyl derivatives of cellulose. Derivatization of cellulose has improved thermo-plasticity, apart from hydrophilicity of derivative, in comparison to native cellulose [ 150 ]. Similar changes were also observed in gellan gum hydrogel used for muscular injury. The addition of laminin protein improved the muscular tissue auto-healing capacity, but 3D hydrogel needs extensive crosslinking and porous structure. To attain a stable porous interconnected structure, gellan gum was derivatized with divinyl sulfone. The composite of gellan gum–divinyl sulfone derivative and gellan gum was used to prepare 3D hydrogel and functionalized with laminin-derived peptide. The composite was encapsulated with skeletal muscle cells. The modification of gellan gum with divinyl sulfone improved cross-linking that stabilized the 3D framework of hydrogel [ 151 ].
4.4. Stability and Degradation Products of EPS Composites
Exopolysaccharide composites are comprised of EPS and natural or synthetic polymers. EPS mainly has polysaccharides as a major component, while natural polymers may also have proteins and lipids, etc. Each biopolymer, including sugar/carbohydrate, proteins, lipids, and nucleic acids, is prone to degradation and may endure only a few hours to a few weeks (depending upon the environment or type of polymer). The study conducted by McClatchy et al. [ 152 ] also proved that the stability of proteins depends upon the cellular environment. One of the advantages of the use of composite scaffolds made with natural polymers is the complete degradation of EPSs such as pullulan, dextran, etc., as well as secondary polymers, into CO 2 and H 2 O. Some nitrogen-containing polymers, such as chitin and chitosan, release amino sugars which can be utilized by microorganisms and living cells [ 153 , 154 ]. To overcome the challenges associated with stability, synthetic polymers can be used instead of natural polymers. Some of the common synthetic polymers used in biomedical applications are polyarylsulfones, polysulfone, polyvinylpyrrolidone, polyamide, polycarbonate, polyacrylonitrile, PMMA, polyester polymer alloy, ethylene vinyl alcohol copolymers; and molecular-thin nanoporous silicon membranes are the common synthetic polymers used in hemodialysis membranes; polymethyl pentene in extracorporeal membrane oxygenation; polyester, polyether, and polycarbonate-based polyurethanes in catheters; nylon, polyurethanes with acrylate in wound dressing; fibrin glue in sealants and adhesive; expanded polypropylene, polytetrafluoroethylene, polyethylene terephthalate, polyvinylidene difluoride membrane in surgical meshes; and poly-tetrafluoroethylene, polyethylene terephthalate, dacron, nylon in scaffold and ligament repair [ 155 ]. It has been proven that the addition of synthetic polymer has improved the mechanical and woven strength of natural exopolysaccharides. Kenawy et al. [ 91 ] have shown that the addition of poly(vinyl alcohol) (PVA)to dextran has improved the mechanical stability of the composite for electrospinning. Further addition of citric acid as a cross-link had a conducive effect on the mechanical, as well as tensile strength and stability of the composite due to extensive cross-linking.
4.5. Side Effects of Synthetic Polymers
As discussed in the above section, synthetic polymers have positive results when blended with exopolysaccharides, but these synthetic polymers have shown side effects in a living system. Much literature has emphasized side effects and immunogenic responses with synthetic polymers. Acrylates are used in baby diapers, and prostheses [ 156 ], but acrylates may lead to serious allergic responses and dermatitis [ 157 ]. Similar kinds of side effects have also been observed with other synthetic polymers and adhesives, including anaphylaxis allergic reaction and arachnoid plasty in the case of fibrin glue [ 158 , 159 ], volitional swallowing, gastrointestinal obstruction, asthma, and allergy with polyethylene terephthalate [ 160 ] and oxidative stress from polypropylene used in mesh surgery [ 161 ]. On the other hand, some polymers have offered advantages over others, as their degradation product can be utilized in cells as in the case of poly-lactides. These kinds of polymers release lactic acids and respective oligomers upon degradation, which can be utilized by the cell during normal metabolism [ 162 ].
The major challenges associated with the commercialization of exopolysaccharide-based composites are its bulk-scale production, cost-effective recovery, and detailed analysis for optimum storage and transit conditions. However, there is no literature available that provides any information about the degradation of EPS composites, suitability of application, commercial cost of the products, and appropriate stability profile.
5. Conclusions
Exopolysaccharides have proven their candidature in the biomedical and healthcare sector mainly attributed to their biocompatibility, nontoxicity, and degradability. However, their limited mechanical and tensile strength, along with solubility in different solvents, obstructs their commercialization. Synthetic polymers have higher stability and strength, but are disadvantaged by side effects and compatibility issues. In order to achieve both compatibilities as well as tunability in strength, adaptability, and stability, composites are preferred over EPSs. The blending of natural and synthetic polymers might improve the physical and chemical characteristics. The composites have offered higher tensile and mechanical strength, along with water retention, slower degradation, drug-carrying capacity, and compatibility for biological applications, including 3D scaffold and wound dressing material fabrication, drug carrier properties, and biomedical sealant potential. Composites can support biological tissue and support healing due to improved adhesion and cell proliferation. The commercialization of composites needs in-depth study regarding stability in storage and transport, degradation behavior, and cost of the final product.
Acknowledgments
The authors acknowledge KU, the research professor the program at Konkuk University, Seoul, South Korea and Chandigarh University, Punjab India.
Funding Statement
This study was supported by the Research Program to Solve Social Issues with the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT [Grant No. 2017M3A9E4077234], and by the National Research Foundation of Korea (NRF) (NRF-2022M3I3A1082545, NRF-2022R1A2C2003138 and NRF-2021R1F1A1050325). This study was also supported by the R&D Program of MOTIE/KEIT [grant number 20016324].
Author Contributions
V.A.: Conceptualization, methodology, writing—review and editing; A.K.B.: investigation, resources; J.R.B.: writing-review and editing; V.K.: writing—review and editing; G.K., G.K. and Y.-H.Y.: supervision, writing—original draft preparation; S.K.B.: Conceptualization, methodology, writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Conflicts of interest.
The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
Exopolysaccharides from microalgae: production, characterization, optimization and techno-economic assessment
- Biotechnology and Industrial Microbiology - Research Paper
- Published: 11 September 2021
- Volume 52 , pages 1779–1790, ( 2021 )
Cite this article

- Anıl Tevfik Koçer ORCID: orcid.org/0000-0003-1519-1711 1 ,
- Benan İnan ORCID: orcid.org/0000-0002-2315-3099 1 ,
- Sedef Kaptan Usul ORCID: orcid.org/0000-0002-8178-9343 2 ,
- Didem Özçimen ORCID: orcid.org/0000-0003-2483-7617 1 ,
- Mustafa Tahsin Yılmaz ORCID: orcid.org/0000-0002-5385-8858 3 &
- İbrahim Işıldak ORCID: orcid.org/0000-0001-9654-1386 1
1866 Accesses
19 Citations
Explore all metrics
Microalgae cultivation for exopolysaccharide production has getting more attention as a result of their high hydrocarbon biosynthesis skill. The aim of this study is to examine the exopolysaccharide production potential of different species of microalgae. In this context, exopolysaccharides were produced from Chlorella minutissima , Chlorella sorokiniana and Botryococcus braunii microalgae and the effects of carbon and nitrogen content in the growth medium and illumination time on exopolysaccharide production were analyzed statistically using Box-Behnken experimental design. In addition, techno-economic assessment of exopolysaccharide production were also performed by using the most productive microalgae and optimum conditions determined in this study. As a result of the experiments, it was seen that C. minutissima , C. sorokiniana and B. braunii produced 0.245 ± 0.0025 g/L, 0.163 ± 0.0016 g/L and 0.117 ± 0.0007 g/L exopolysaccharide, respectively. Statistically, it was observed that there was an inverse relationship between the exopolysaccharide production and investigated parameters such as illumination period and carbon and nitrogen amounts of culture mediums. The techno-economic assessment comprising microalgal exopolysaccharide (EPS) bioprocess was carried out, and it showed that the system can be considered economically viable, yet can be improved with biorefinery approach.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Bioprospecting for industrially relevant exopolysaccharide-producing cyanobacteria under Portuguese simulated climate

Exopolysaccharide production by Anabaena sp. PCC 7120: physicochemical parameter optimization and two-stage cultivation strategy to maximize the product yield
Adaptation of autotrophic to heterotrophic culture of Porphyridium purpureum (Bory) K.M. Drew & R.Ross: characterization of biomass and production of exopolysaccharides
Data availability.
Not applicable.
Code availability
Ruas-Madiedo P, Hugenholtz J, Zoon P (2002) An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int Dairy J 12(2–3):163–171. https://doi.org/10.1016/S0958-6946(01)00160-1
Article CAS Google Scholar
Kumar Singha T (2012) Microbial extracellular polymeric substances: production, ısolation and applications. IOSR J Pharm 2:276–281. https://doi.org/10.9790/3013-0220276281
Article Google Scholar
Lee IY, Seo WT, Kim GJ et al (1997) Optimization of fermentation conditions for production of exopolysaccharide by Bacillus polymyxa . Bioprocess Eng 16:71–75. https://doi.org/10.1007/s004490050290
Mahapatra S, Banerjee D (2013) Fungal exopolysaccharide: production, composition and applications. Microbiol Insights 6:1–16. https://doi.org/10.4137/mbi.s10957
Xiao R, Zheng Y (2016) Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol Adv 34:1225–1244. https://doi.org/10.1016/j.biotechadv.2016.08.004
Article CAS PubMed Google Scholar
Özçimen D, İnan B, Koçer AT, Vehapi M (2018) Bioeconomic assessment of microalgal production. In: Jacob-Lopes E (ed) Microalgal Biotechnology. InTech, Rijeka, Croatia, pp 195–213. https://doi.org/10.5772/intechopen.73702
Singh NK, Dhar DW (2011) Microalgae as second generation biofuel. A review Agron Sustain Dev 31:605–629. https://doi.org/10.1007/s13593-011-0018-0
Karakaş CY, Şahin HT, İnan B, et al (2019) In-vitro cytotoxic activity of microalgal extracts loaded nano-micro particles produced via electrospraying and microemulsion methods. Biotechnol Prog 35(6):1–8. https://doi.org/10.1002/btpr.2876
Geun Goo B, Baek G, Jin Choi D et al (2013) Characterization of a renewable extracellular polysaccharide from defatted microalgae Dunaliella tertiolecta . Bioresour Technol 129:343–350. https://doi.org/10.1016/j.biortech.2012.11.077
Frengova GI, Simova ED, Beshkova DM, Simov ZI (2002) Exopolysaccharides produced by lactic acid bacteria of kefir grains. Zeitschrift fur Naturforsch - Sect C J Biosci 57:805–810. https://doi.org/10.1515/znc-2002-9-1009
Tsuda H, Hara K, Miyamoto T (2008) Binding of mutagens to exopolysaccharide produced by Lactobacillus plantarum mutant strain 301102S. J Dairy Sci 91:2960–2966. https://doi.org/10.3168/jds.2007-0538
Li M, Zhu W, Gao L, Lu L (2013) Changes in extracellular polysaccharide content and morphology of Microcystis aeruginosa at different specific growth rates. J Appl Phycol 25:1023–1030. https://doi.org/10.1007/s10811-012-9937-7
Bafana A (2013) Characterization and optimization of production of exopolysaccharide from Chlamydomonas reinhardtii . Carbohydr Polym 95:746–752. https://doi.org/10.1016/j.carbpol.2013.02.016
Gaignard C, Macao V, Gardarin C et al (2018) The red microalga Flintiella sanguinaria as a new exopolysaccharide producer. J Appl Phycol 30:2803–2814. https://doi.org/10.1007/s10811-018-1389-2
Halaj M, Paulovičová E, Paulovičová L et al (2019) Extracellular biopolymers produced by Dictyosphaerium family - chemical and immunomodulative properties. Int J Biol Macromol 121:1254–1263. https://doi.org/10.1016/j.ijbiomac.2018.10.116
DeSantis D, Mason JA, James BD et al (2017) Techno-economic analysis of metal-organic frameworks for hydrogen and natural gas storage. Energy Fuels 31:2024–2032. https://doi.org/10.1021/acs.energyfuels.6b02510
Thomassen G, Egiguren Vila U, Van Dael M et al (2016) A techno-economic assessment of an algal-based biorefinery. Clean Technol Environ Policy 18:1849–1862. https://doi.org/10.1007/s10098-016-1159-2
Davis R, Aden A, Pienkos PT (2011) Techno-economic analysis of autotrophic microalgae for fuel production. Appl Energy 88:3524–3531. https://doi.org/10.1016/j.apenergy.2011.04.018
Hoffman J, Pate RC, Drennen T, Quinn JC (2017) Techno-economic assessment of open microalgae production systems. Algal Res 23:51–57. https://doi.org/10.1016/j.algal.2017.01.005
Juneja A, Murthy GS (2017) Evaluating the potential of renewable diesel production from algae cultured on wastewater: Techno-economic analysis and life cycle assessment. AIMS Energy 5:239–257. https://doi.org/10.3934/energy.2017.2.239
Zamalloa C, Vulsteke E, Albrecht J, Verstraete W (2011) The techno-economic potential of renewable energy through the anaerobic digestion of microalgae. Bioresour Technol 102:1149–1158. https://doi.org/10.1016/j.biortech.2010.09.017
Hanrahan G, Lu K (2006) Application of factorial and response surface methodology in modern experimental design and optimization. Crit Rev Anal Chem 36:141–151. https://doi.org/10.1080/10408340600969478
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205. https://doi.org/10.1128/mmbr.35.2.171-205.1971
Article CAS PubMed PubMed Central Google Scholar
Díaz Bayona KC, Garcés LA (2014) Effect of different media on exopolysaccharide and biomass production by the green microalga Botryococcus braunii . J Appl Phycol 26:2087–2095. https://doi.org/10.1007/s10811-014-0242-5
Wang L, Min M, Li Y et al (2010) Cultivation of green algae Chlorella sp. in different wastewaters from municipal wastewater treatment plant. Appl Biochem Biotechnol 162:1174–1186. https://doi.org/10.1007/s12010-009-8866-7
Zhang J, Liu L, Chen F (2019) Production and characterization of exopolysaccharides from Chlorella zofingiensis and Chlorella vulgaris with anti-colorectal cancer activity. Int J Biol Macromol 134:976–983. https://doi.org/10.1016/j.ijbiomac.2019.05.117
Do BC, Dang TT, Berrin JG et al (2009) Cloning, expression in Pichia pastoris , and characterization of a thermostable GH5 mannan endo-1,4-beta-mannosidase from Aspergillus niger BK01. Microb Cell Fact 8:59. https://doi.org/10.1021/ac60111a017
Hempel N, Petrick I, Behrendt F (2012) Biomass productivity and productivity of fatty acids and amino acids of microalgae strains as key characteristics of suitability for biodiesel production. J Appl Phycol 24:1407–1418. https://doi.org/10.1007/s10811-012-9795-3
García R, Pizarro C, Lavín AG, Bueno JL (2013) Biomass proximate analysis using thermogravimetry. Bioresour Technol. https://doi.org/10.1016/j.biortech.2013.03.197
Article PubMed Google Scholar
Dubois M, Gilles KA, Hamilton JK et al (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. https://doi.org/10.1139/o59-099
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin. J Biol Chem. https://doi.org/10.1016/0304-3894(92)87011-4
Zheng JQ, Wang JZ, Shi CW et al (2014) Characterization and antioxidant activity for exopolysaccharide from submerged culture of Boletus aereus . Process Biochem 49:1047–1053. https://doi.org/10.1016/j.procbio.2014.03.009
Bramhachari PV, Dubey SK (2006) Isolation and characterization of exopolysaccharide produced by Vibrio harveyi strain VB23. Lett Appl Microbiol 43:571–577. https://doi.org/10.1111/j.1472-765X.2006.01967.x
Sert BS, Inan B, Özçimen D (2018) Effect of chemical pre-treatments on bioethanol production from Chlorella minutissima. Acta Chim Slov 65(1):160–165. https://doi.org/10.17344/acsi.2017.3728
Freitas BCB, Morais MG, Costa JAV (2017) Chlorella minutissima cultivation with CO 2 and pentoses: effects on kinetic and nutritional parameters. Bioresour Technol 244:338–344. https://doi.org/10.1016/j.biortech.2017.07.125
Milledge JJ, Heaven S (2011) Disc stack centrifugation separation and cell disruption of microalgae: a technical note. Environ Nat Resour Res. https://doi.org/10.5539/enrr.v1n1p17
Banerjee A, Sharma R, Chisti Y, Banerjee UC (2002) Botryococcus braunii : a renewable source of hydrocarbons and other chemicals. Crit Rev Biotechnol 22:245–279
Choi HJ, Yu SW (2015) Influence of crude glycerol on the biomass and lipid content of microalgae. Biotechnol Biotechnol Equip 29:506–513. https://doi.org/10.1080/13102818.2015.1013988
Yang JS, Rasa E, Tantayotai P et al (2011) Mathematical model of Chlorella minutissima UTEX2341 growth and lipid production under photoheterotrophic fermentation conditions. Bioresour Technol 102:3077–3082. https://doi.org/10.1016/j.biortech.2010.10.049
Kim S, Park J, eun, Cho YB, Hwang SJ, (2013) Growth rate, organic carbon and nutrient removal rates of Chlorella sorokiniana in autotrophic, heterotrophic and mixotrophic conditions. Bioresour Technol 144:8–13. https://doi.org/10.1016/j.biortech.2013.06.068
Kumar K, Dasgupta CN, Das D (2014) Cell growth kinetics of Chlorella sorokiniana and nutritional values of its biomass. Bioresour Technol 167:358–366. https://doi.org/10.1016/j.biortech.2014.05.118
Harun R, Singh M, Forde GM, Danquah MK (2010) Bioprocess engineering of microalgae to produce a variety of consumer products. Renew Sustain Energy Rev 14:1037–1047
Yim JH, Kim SJ, Ahn SH, Lee HK (2003) Optimal conditions for the production of sulfated polysaccharide by marine microalga Gyrodinium impudicum strain KG03. Biomol. Eng 20(4–6):273–280. https://doi.org/10.1016/S1389-0344(03)00070-4
Raposo MFDJ, De Morais AMMB, De Morais RMSC (2014) Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum . Life Sci 101:56–63. https://doi.org/10.1016/j.lfs.2014.02.013
Miqueleto AP, Dolosic CC, Pozzi E et al (2010) Influence of carbon sources and C/N ratio on EPS production in anaerobic sequencing batch biofilm reactors for wastewater treatment. Bioresour Technol 101:1324–1330. https://doi.org/10.1016/j.biortech.2009.09.026
Maalej H, Hmidet N, Boisset C et al (2015) Optimization of exopolysaccharide production from Pseudomonas stutzeri AS22 and examination of its metal-binding abilities. J Appl Microbiol 118:356–367. https://doi.org/10.1111/jam.12688
Yeh KL, Chang JS, Chen WM (2010) Effect of light supply and carbon source on cell growth and cellular composition of a newly isolated microalga Chlorella vulgaris ESP-31. Eng Life Sci 10:201–208. https://doi.org/10.1002/elsc.200900116
Grosu-Tudor SS, Zamfir M (2014) Exopolysaccharide production by selected lactic acid bacteria isolated from fermented vegetables. Sci Bull Ser F Biotechnol 18:107–114
Google Scholar
Dineshkumar R, Kumaravel R, Gopalsamy J et al (2018) Microalgae as bio-fertilizers for rice growth and seed yield productivity. Waste and Biomass Valorization 9:793–800. https://doi.org/10.1007/s12649-017-9873-5
Servel MO, Claire C, Derrien A et al (1994) Fatty acid composition of some marine microalgae. Phytochemistry 36:691–693. https://doi.org/10.1016/S0031-9422(00)89798-8
Koçer AT, Mutlu B, Özçimen D (2019) Investigation of biochar production potential and pyrolysis kinetics characteristics of microalgal biomass. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-019-00411-7
Caporgno MP, Trobajo R, Caiola N et al (2015) Biogas production from sewage sludge and microalgae co-digestion under mesophilic and thermophilic conditions. Renew Energy 75:374–380. https://doi.org/10.1016/j.renene.2014.10.019
Liang K, Zhang Q, Gu M, Cong W (2013) Effect of phosphorus on lipid accumulation in freshwater microalga Chlorella sp. J Appl Phycol 25:311–318. https://doi.org/10.1007/s10811-012-9865-6
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Ruangsomboon S (2012) Effect of light, nutrient, cultivation time and salinity on lipid production of newly isolated strain of the green microalga, Botryococcus braunii KMITL 2. Bioresour Technol 109:261–265. https://doi.org/10.1016/j.biortech.2011.07.025
Molino A, Iovine A, Casella P et al (2018) Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int J Environ Res Public Health 15:1–21. https://doi.org/10.3390/ijerph15112436
Agrawal A, Chakraborty S (2013) A kinetic study of pyrolysis and combustion of microalgae Chlorella vulgaris using thermo-gravimetric analysis. Bioresour Technol 128:72–80. https://doi.org/10.1016/j.biortech.2012.10.043
Miao X, Wu Q, Yang C (2004) Fast pyrolysis of microalgae to produce renewable fuels. J Anal Appl Pyrolysis 71:855–863. https://doi.org/10.1016/j.jaap.2003.11.004
Kent M, Welladsen HM, Mangott A, Li Y (2015) Nutritional evaluation of Australian microalgae as potential human health supplements. PLoSONE 10(2):1–14. https://doi.org/10.1371/journal.pone.0118985
Arbeláez AA, Giraldo ND, Pérez JF, Atehortúa L (2019) Pyrolysis kinetics using TGA and simulation of gasification of the microalga Botryococcus braunii . Bioenergy Res 12:1077–1089. https://doi.org/10.1007/s12155-019-10037-2
Koçer AT, Özçimen D (2018) Investigation of the biogas production potential from algal wastes. Waste Manag Res 36:1100–1105. https://doi.org/10.1177/0734242X18798447
Karakaş C, Özçimen D, İnan B (2017) Potential use of olive stone biochar as a hydroponic growing medium. J Anal Appl Pyrolysis 125:17–23. https://doi.org/10.1016/j.jaap.2017.05.005
Koçer AT, Özçimen D (2021) Determination of combustion characteristics and kinetic parameters of Ulva lactuca and its biochar. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-01245-4
Wang B (2011) Chemical characterization and ameliorating effect of polysaccharide from Chinese jujube on intestine oxidative injury by ischemia and reperfusion. Int J Biol Macromol 48:386–391. https://doi.org/10.1016/j.ijbiomac.2010.12.005
Dertli E, Toker OS, Durak MZ et al (2016) Development of a fermented ice-cream as influenced by in situ exopolysaccharide production: rheological, molecular, microstructural and sensory characterization. Carbohydr Polym 136:427–440. https://doi.org/10.1016/j.carbpol.2015.08.047
Sekkal M, Huvenne JP, Legrand P et al (1993) Direct structural identification of polysaccharides from red algae by FTIR microspectrometry I: localization of agar in Gracilaria verrucosa sections. Mikrochim Acta 112:1–10. https://doi.org/10.1007/BF01243315
Kumar CG, Joo HS, Choi JW et al (2004) Purification and characterization of an extracellular polysaccharide from haloalkalophilic Bacillus sp. I-450. Enzyme Microb Technol 34:673–681. https://doi.org/10.1016/j.enzmictec.2004.03.001
Solmaz KB, Ozcan Y, Dogan NM et al (2018) Characterization and production of extracellular polysaccharides (EPS) by Bacillus pseudomycoides U10. Environ - MDPI 5:1–16. https://doi.org/10.3390/environments5060063
Ismail B, Nampoothiri KM (2010) Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Arch Microbiol 192:1049–1057. https://doi.org/10.1007/s00203-010-0636-y
Ahmed Z, Wang Y, Anjum N et al (2013) Characterization of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir - part II. Food Hydrocoll 30:343–350. https://doi.org/10.1016/j.foodhyd.2012.06.009
Botelho PS, Maciel MIS, Bueno LA et al (2014) Characterisation of a new exopolysaccharide obtained from of fermented kefir grains in soymilk. Carbohydr Polym 107:1–6. https://doi.org/10.1016/j.carbpol.2014.02.036
García-Cubero R, Cabanelas ITD, Sijtsma L et al (2018) Production of exopolysaccharide by Botryococcus braunii CCALA 778 under laboratory simulated Mediterranean climate conditions. Algal Res 29:330–336. https://doi.org/10.1016/j.algal.2017.12.003
Vanhooren PT, Vandamme EJ (1999) L-Fucose: occurrence, physiological role, chemical, enzymatic and microbial synthesis. J Chem Technol Biotechnol 74:479–497. https://doi.org/10.1002/(SICI)1097-4660(199906)74:6%3c479::AID-JCTB76%3e3.0.CO;2-E
Ragavan ML, Das N (2019) Optimization of exopolysaccharide production by probiotic yeast Lipomyces starkeyi VIT-MN03 using response surface methodology and its applications. Ann Microbiol 69:515–530. https://doi.org/10.1007/s13213-019-1440-9
Hu X, Shi Y, Zhang P et al (2016) d-Mannose: properties, production, and applications: an overview. Compr Rev Food Sci Food Saf 15:773–785. https://doi.org/10.1111/1541-4337.12211
Pierre G, Delattre C, Dubessay P et al (2019) What is in store for EPS microalgae in the next decade? Molecules 24:1–25. https://doi.org/10.3390/molecules24234296
Quinn JC, Davis R (2015) The potentials and challenges of algae based biofuels: a review of the techno-economic, life cycle, and resource assessment modeling. Bioresour Technol 184:444–452. https://doi.org/10.1016/j.biortech.2014.10.075
Amer L, Adhikari B, Pellegrino J (2011) Technoeconomic analysis of five microalgae-to-biofuels processes of varying complexity. Bioresour Technol 102:9350–9359. https://doi.org/10.1016/j.biortech.2011.08.010
Download references
This study was supported by General Directorate of Agricultural Research and Policies of Republic of Turkey Ministry of Food, Agriculture and Livestock (Project No: TAGEM-15/AR-GE/35). Anıl Tevfik Koçer is also supported by TUBITAK-BIDEB 2211 National Scholarship Programme for PhD Students and 100/2000 YÖK Doctorate Scholarship.
Author information
Authors and affiliations.
Department of Bioengineering, Yıldız Technical University, Istanbul, Turkey
Anıl Tevfik Koçer, Benan İnan, Didem Özçimen & İbrahim Işıldak
Department of Bioengineering, Gebze Technical University, Kocaeli, Turkey
Sedef Kaptan Usul
Department of Food Engineering, Yıldız Technical University, Istanbul, Turkey
Mustafa Tahsin Yılmaz
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Anıl Tevfik Koçer, Benan İnan, Sedef Kaptan Usul, Didem Özçimen, Mustafa Tahsin Yılmaz and İbrahim Işıldak. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Benan İnan .
Ethics declarations
Ethics approval, consent to participate, consent for publication, conflict of interest.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Ernani Pinto
Rights and permissions
Reprints and permissions
About this article
Koçer, A.T., İnan, B., Kaptan Usul, S. et al. Exopolysaccharides from microalgae: production, characterization, optimization and techno-economic assessment. Braz J Microbiol 52 , 1779–1790 (2021). https://doi.org/10.1007/s42770-021-00575-3
Download citation
Received : 29 December 2020
Accepted : 07 July 2021
Published : 11 September 2021
Issue Date : December 2021
DOI : https://doi.org/10.1007/s42770-021-00575-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Exopolysaccharide
- Chlorella minutissima
- Chlorella sorokiniana
- Botryococcus braunii
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
The second pathway of microbial exopolysaccharide biosynthesis is ATP- binding cassette (ABC) transporter dependent pathway, which is mainly involved in the synthesis of capsular polysaccharide (CPS) [12].Capsular polysaccharide synthesized through the ABC transporter dependent pathway is assembled by the action of glycosyltransferases located at the inner membrane of the cytoplasmic face.
1. Introduction. Bacterial exopolysaccharides (EPSs) have recently received significant attention due to their unique structure and properties and the prospects for use in various fields of science, industry, medicine, and technology [1,2,3,4,5,6].The term exopolysaccharide was first introduced by Sutherland in 1972 [] for high molecular weight carbohydrate biopolymers produced by microorganisms.
Exopolysaccharide from bacillus cereus VK1 has been reported to efficiently adsorb mercury; several other researches have reported exopolysaccharide to be effective in adsorption of heavy metals [163]. A novel exopolysaccharide from lactic acid bacteria was developed by the researchers and investigated the adsorption capacity of methylene blue.
Exopolysaccharides are exploited from both bacterial and archeabacteria sources [8].Dextran, xanthan, and gellan gum are three common exopolysaccharides produced from these prokaryotes (Table 1).Thermophilic bacteria such as Bacillus thermantarcticus and Geobacillu sthermodenitrificans are also reported as competitive producers of EPS. . Exopolysaccharides occurs in thermophilic and halophilic ...
Exopolysaccharide-Degrading Bacteria in Two Soils Wang, Xiaoqing Wang, X. (2013). Identification and Cultivation of Exopolysaccharide-Degrading Bacteria in Two ... Without their support, this thesis would not be possible. In particular, I would like to express my deepest gratitude to my supervisor, Dr. Peter Dunfield, for providing me the ...
Table 4.1 Exopolysaccharide production by 37 LAB cereal isolates on SucMRS agar. Table 4.2 Amounts of EPS (dextran, 5 x106 - 4 x107 Da) produced by the strongest LAB producer strains, all of which were from the Weissella genera. Table 4.3 Formation of acetic and lactic acids, fructose, and ethanol, as well as
Exopolysaccharides (EPSs) from microorganisms are essential harmless natural biopolymers used in applications including medications, nutraceuticals and functional foods, cosmetics, and insecticides. Several microbes can synthesize and excrete EPSs with chemical properties and structures that make them suitable for several important applications. Microbes secrete EPSs outside their cell walls ...
Exopolysaccharide also provides important nutrients to the plant for healthy growth and development through successful colonization of plant roots by EPS-producing microbes, which helps to hold ...
PDF | On Dec 30, 2019, Mariam Zaheer and others published Bacterial Exopolysaccharides: sources, production and applications | Find, read and cite all the research you need on ResearchGate
Carbohydr ates and microbial exopolysaccharides are. used in food industr y to improve the rheological prope r-. ties and create specific characteristics; including. cryoprotec tion, sweetening, h ...
But desulfated exopolysaccharide consumed periodate (0.942 mol) to produce formic acid (0.000801 mol) per one mole of anhydrosugar. HPLC analysis of EPS from Bacillus altitudinis MSH2014 shows erythritol, glycerol, and erytheric acid presence (Table 1). The presence of a small amount of glycerol and free erythritol in large amount partially ...
Exopolysaccharides (EPSs) are naturally occurring high-molecular-weight carbohydrates that have been widely studied for their biological activities, including antioxidant, immunomodulatory, anticancer and gut microbiota regulation activities. Polysaccharides are abundant in nature and can be derived from animals, plants, algae, and microorganisms, but among polysaccharides with potential uses ...
This review offers insight on the current research trend of nine commonly used EPSs, their biosynthesis pathways, their characteristics, and the biomedical applications of these relevant bioproducts. Keywords: alginate, bacteria, biomedical, cellulose, dextran, exopolysaccharides, gellan, hyaluronic acid, levan, xanthan gum.
A Review on Production of Exopolysaccharide and Biofilm in Probiotics Like Lactobacilli and Methods of Analysis Pegah Hooshdar 1, Rouha K. Kermanshahi 1, Parinaz Ghadam 2, Kianoush Khosravi-Darani 3,* 1 Department of Microbiology, Faculty of Biological Sciences, University of Alzahra, Tehran, Iran
condition. The present study was to obtain exopolysaccharide producing bacteria from direct seeded rice. Soil samples were collected from rice rhizospheric region (0-15cm depth). Thirty-seven organisms were -seven Gram -ve in nature. After screening with Polyethylene glycol (PEG-6000), six isolates tolerate maximum level of stress (-0.73MPa).
Carbohydr Res 342:933-942 Vaishnav AM (2017) Bacterial exopolysaccharide production from fruits and potato waste (Ph.D. Thesis). Gujarat University, Gujarat, India Vaishnav AM, Upadhyay KH, Tipre DR, Dave SR (2016) Characterization of potent exopolysaccharide producing bacteria isolated from fruit pulp and potato peels and enhancement in ...
exopolysaccharide synthesis. For many micro-organisms, nutrient imbalance in the presence of large amounts of utilizable carbohydrate leads to increased poly- ... thesis; at optimal incubation temperature for the bacteria (37°C) the cells were normal, synthesizing the three polymers dependent on isoprenoid lipid-linked inter- ...
Many microbial exopolysaccharides (EPS) have been reported in the last decade, and their fermentation processes, functional properties and applications, structural characterization, and biological activities have been extensively studied. Despite the great diversity of biological activities already described for EPS, only a few have been exploited industrially. The main reason for this is that ...
Keywords: exopolysaccharide, Lactiplantibacillus, wound healing, probiotics, chemical characterization. Citation: Zaghloul EH and Ibrahim MIA (2022) Production and Characterization of Exopolysaccharide From Newly Isolated Marine Probiotic Lactiplantibacillus plantarum EI6 With in vitro Wound Healing Activity. Front.
Characterization of a particular exopolysaccharide is consistent with identifying the anomers (if any), monomeric units, the type of linkage between the monomers, and the linkage sequence. Methylation analysis is a tool used in knowing the monomeric linkages present in EPS . Sequencing techniques include mass spectrometry and Nuclear Magnetic ...
3.1. Exopolysaccharide Composites with Natural Materials. EPS itself is a natural biopolymer that is combined with other natural biomolecules, including proteins, enzymes, lipids, carbohydrates, etc., and thus in most cases, the composite has healthcare-associated applications, including medical sealants and scaffolds in tissue recovery and repair.
Microalgae cultivation for exopolysaccharide production has getting more attention as a result of their high hydrocarbon biosynthesis skill. The aim of this study is to examine the exopolysaccharide production potential of different species of microalgae. In this context, exopolysaccharides were produced from Chlorella minutissima, Chlorella sorokiniana and Botryococcus braunii microalgae and ...
Download full-text PDF Read ... thesis by Nostoc strains as ... frequently has uronic acids and non-sugar groups Table 1 List of various microbial and plant sources exploited for exopolysaccharide ...
stacks.stanford.edu