- Lupe_Grau Cancel

Medicinal product or (substance-based) medical device?
The classification of whether a device is a medicinal product or a substance-based medical device has far-reaching regulatory consequences. This classification is so demanding that there are regular disputes with authorities and notified bodies, and in 2023, even the European Court of Justice had to rule on the matter.
This article helps manufacturers, authorities, and notified bodies to "qualify" a device. It addresses the ECJ judgment mentioned above, as well as regulatory requirements and guidance. You will learn what a presentation medicinal product is and in which cases the national courts will still be asked.
1. Summary for readers in a hurry
- A device that achieves its intended medical purpose (almost) exclusively physically counts as a (substance-based) medical device .
- A device that achieves its intended medical purpose pharmacologically, immunologically, or metabolically qualifies as a medicinal product .
If the main physical mode of action is not clearly proven, the device must be treated as a medicinal product.
Further information
Here, you will find a compact overview of the regulatory requirements for substance-based medical devices .
a) What are substance-based medical devices?
Even though the MDR recognizes the existence of "devices consisting of substances or combinations of substances" with classification rules 3 and 21 and provides a legal framework, it still fails to define substance-based medical devices clearly. For this reason, the MDCG provides in document MDCG 2022-5 and provides the following definition:
Definition „Substance-based medical device“
“A substance-based medical device is a medical device which:
· is composed of substances that are permitted in a medical device, and
· does not achieve its main intended effect by pharmacological, metabolic or immunological means.”
Quelle: MDCG 2022-5 / Part: 3.1
Substance-based medical devices, therefore, consist of substances and are similar to medicinal products in their presentation and dosage form. However, they do not achieve their main intended effect via a pharmacological, metabolic, or immunological mechanism but primarily by physical means.
Examples of substance-based medical devices include:
- Saline nasal or throat sprays
- Mucous membrane-moistening syrups, throat sprays, or lozenges
- Artificial tears
- Oral products to neutralize gastric acid
- Devices with a defoaming effect for gastrointestinal complaints caused by gas
b) What are medicinal products?
The definition for medicinal products is provided by Directive 2001/83/EC:
Definition medicinal product
“a) Any substance or combination of substances presented for treating or preventing disease in human beings. b) Any substance or combination of substances which may be administered to human beings with a view to making a medical diagnosis or to restoring, correcting or modifying physiological functions in human beings is likewise considered a medicinal product.”
Source: Directive 2001/83/EC Article 1(2)
This definition consists of two parts: part a) relating more to the presentation or get-up of a device (presentation medicinal product) and part b) relating to the function (functional medicinal product). Thus, a device is a medicinal product if it falls under part (a), part (b), or both parts of the definition.
The terms "presentation medicinal product" and "functional medicinal product" are not part of the definition of the term but are rather common terms for the different preparations according to the BfArM . This distinction is primarily used for delineation in case law.
- Accordingly, presentation medicinal products are those whose designation or presentation (advertising) gives the average informed consumer the impression that they are intended to cure or prevent human diseases, irrespective of their actual mode of action.
- On the other hand, functional medicinal products have a significant influence on the physiological functions of the human organism through their effect.
Consequently, this means that only functional medicinal products are, by definition, characterized by an actual (scientifically verifiable) pharmacological, immunological, or metabolic mode of action.
Examples of medicinal product groups are:
- Anticoagulants
- Cytostatics
- x-ray contrast media
c) Distinction between medicinal products and medical devices
Since substance-based medical devices also consist of substances and are intended for use in the treatment, alleviation, or prevention of human disease, a clear demarcation between medical devices and (presentation) medicinal products is often not readily possible.
Products for which it is not clear from the outset whether they are medical devices or medicinal products are referred to as borderline products in accordance with MDCG 2022-5. The principal mode of action is decisive in the demarcation of these devices.
A device cannot fall under both legal regulations, i.e., it cannot be a medicinal product and a medical device at the same time .
In the case that a device can fall under both the definition of "medical device" and the definition of " medicinal product," the device is a medicinal product according to the so-called doubt rule:
“In cases of doubt, where, taking into account all its characteristics, a product may fall within the definition of a ‘medicinal product’ and within the definition of a product covered by other Community legislation the provisions of this Directive shall apply.”
Source: Directive 2001/83/EC Article 2(2)
3. The momentous ECJ judgment
A) the facts.
At the request of the Federal Administrative Court, the European Court of Justice dealt with various questions on the distinction between substance-based medical devices and medicinal products. The starting point was legal disputes concerning the qualification of preparations declared as medical devices for use in the nasal mucosa, for which, however, according to the German Federal Institute for Drugs and Medical Devices (BfArM), prior authorization as a drug was required.
b) The ECJ's decision
The ECJ clarifies once again in its judgment of January 19, 2023:
- The main intended effect of substance-based medical devices is predominantly physical.
- In contrast, medicinal products act in a pharmacological, metabolic, or immunological manner.
The rule of doubt , according to Article 2 (2) of Directive 2001/83/EC, applies equally to functional and presentation medicinal products.
However, suppose the main intended effect cannot be clearly explained based on the available scientific evidence for a device. In that case, the latter is to be classified neither as a medical device nor as a medicinal product - according to the ECJ.
Borderline products
How are these borderline products to be classified in this case in the future?
According to the ECJ judgment, the possible classification as presentation medicinal products remains, because according to the definition, these are products that are not intended to cure or prevent human diseases based on their proven mode of action but based on their presentation and their advertised properties.
“Where the principal mode of action of a product is not scientifically established, that product cannot meet the definition of the concept of ‘medical device’, within the meaning of Directive 93/42, as amended by Directive 2007/47, or that of ‘medicinal product by function’, as referred to in Directive 2001/83, as amended by Directive 2004/27. It is for the national courts to assess, on a case-by-case basis, whether the conditions relating to the definition of the concept of ‘medicinal product by presentation’, as referred to in Directive 2001/83, as amended by Directive 2004/27, are satisfied.”
ECJ judgment
In this context, the ECJ attributed a higher level of consumer protection to the Directive on medicinal products (Directive 2001/83/EC), since the placing on the market of medicinal products requires the prior granting of an authorization by the authorities.
4. Possible consequences for manufacturers
The ECJ judgment could have significant consequences for the manufacturers of many substance-based medical devices on the market. Mistakes in qualification and classification that are recognized too late can cost time, effort, and money. In addition, manufacturers risk legal disputes.
In the absence of scientific evidence that the main intended effect in or on the human body is achieved by physical means and not by pharmacological, immunological, and metabolic means, there is a risk that these borderline products will regularly have to be classified as presentation medicinal products and consequently be subject to the legal regulation for medicinal products .
Scientific proof is crucial
In order to prevent costly and time-consuming legal disputes on delimitation, it is important to prove the effect of the product scientifically. The following applies here:
- Only a product that fulfills its intended use through exclusively physical mechanisms of action falls under the definition of "medical device" as defined by the MDR.
- Suppose substances are used that can basically have a pharmacological, immunological, or metabolic effect. In that case, special care must be taken: In this case, it must be demonstrated that these substances play no role in fulfilling the main intended effect of the product (e.g., flavoring and preservatives).
- A product that achieves its main intended effect solely by pharmacological, immunological, or metabolic means falls within the definition of "medicinal product" as defined in Directive 2001/83/EC.
- If a product can fall under both the definition of "medical device" and the definition of "medicinal product," the rule of doubt, according to Article 2 (2) of Directive 2001/83/EC, applies, and the device is classified as a medicinal product.
- If the mode of action is unclear or scientific evidence is lacking, the product falls neither under the definition of "medical device" nor under the definition of "medicinal product."
- Suppose a product corresponds to a medical device in its presentation but uses a pharmacological, metabolic, or immunological effect of an ingredient to achieve the intended use. In that case, the device must be approved as a medicinal product.
5. Delimitation by means of MDCG 2022-5
The Medical Devices Coordination Group clarified in its guidance on the demarcation between medical devices and medicinal products even before the ECJ ruling was announced: A product cannot be classified as a medical device unless it is clearly demonstrated that its main intended effect is not based on a primarily pharmacological, immunological, or metabolic mode of action.
a) The main intended effect decides
The decisive criterion for differentiating substance-based medical devices from medicinal products is the main intended effect of a device.
The MDCG document defines the main intended effect (as defined in Article 1(6)(b) of the MDR) as the principle by which the device achieves its main intended effect , i.e., pharmacological, immunological, metabolic, physical, or other.
This effect must be demonstrated objectively and in accordance with the latest scientific knowledge. The manufacturer's claims for its device are irrelevant.
However, the MDCG 2022-5 guidance document does not explain what this evidence might look like in concrete terms. This poses significant challenges for manufacturers of substance-based medical devices!
b) Pharmacological, metabolic, immunological, physical
At least: In this context, the MDCG sets out in section 1.2.2 what it understands by "pharmacological," "immunological," "metabolic," and "physical," and gives examples.
‘Pharmacological means’ is understood as an interaction typically at a molecular level between a substance or its metabolites and a constituent of the human body which results in initiation, enhancement, reduction or blockade of physiological functions or pathological processes ‘Immunological means’ is understood as an action initiated by a substance or its metabolites on the human body and mediated or exerted (i.e., stimulation, modulation, blocking, replacement) by cells or molecules involved in the functioning of the immune system. ‘Metabolic means’ is understood as an action of a substance or its metabolites which involves an alteration, including stopping, starting, or changing the rate, extent, or nature of a biochemical process, whether physiological or pathological,
Source: MDCG Paragraph 1.2.2
Examples of physical means by which a device achieves its principal intended effect include:
- mechanical action
- physical barriers, e.g., building up a film
- heat transfer
- replacement and support of organs and body functions
- hydration or dehydration
- change of the pH-value
The MDCG document provides manufacturers with a decision diagram for correct qualification. We have expanded this decision tree for you to include the definitions of pharmacological, immunological, metabolic, or physical principal mode of action.
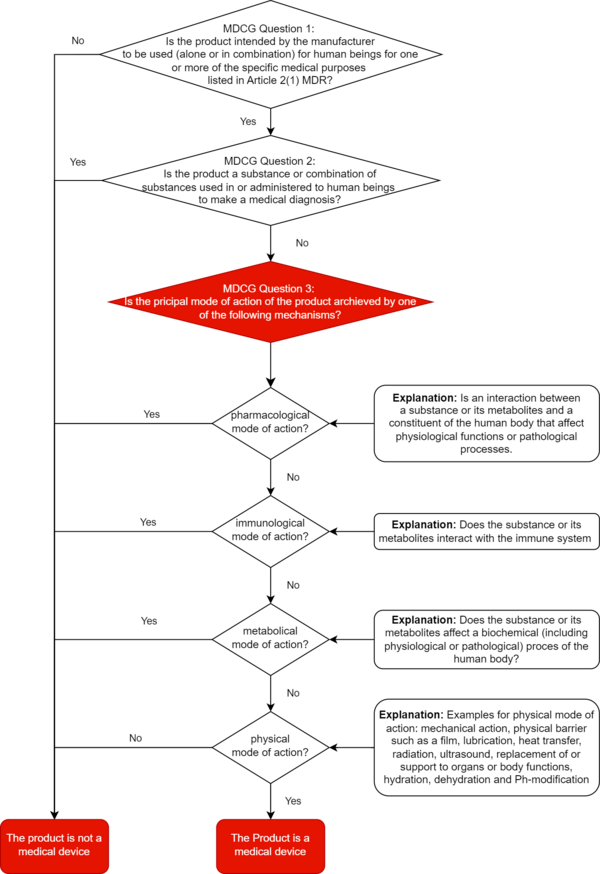
6. Instructions for manufacturers
A) qualification and classification in everyday practice.
With the introduction of Rule 21 of the MDR, Annex VIII , many substance-based medical devices that were regularly classified as Class I under the MDD will be reclassified higher. This makes the involvement of a notified body necessary.
The justification for qualification as a medical device must already be submitted for the application to a notified body.
For the qualification as a substance-based medical device, the mechanism of action is decisive.
Borderline products are difficult to distinguish. Without sufficiently substantiated qualification, manufacturers risk classification of these products by national courts and thus delays in placing them on the market.
Tip 1: Qualify and classify the device at an early stage
Start in time! The first step is to qualify and classify a product as a (substance-based) medical device. Only then should you ensure conformity with the MDR's requirements.
When qualifying substance-based medical devices, consider the specific classification rules 3 and 21, among others.
Mistakes in qualification and classification that are recognized too late can cost you time, effort, and money. In addition, you risk legal disputes.
We support you with an expert opinion in qualifying your device and collecting and documenting the necessary evidence.
Contact us right away.
Tip 2: Check evidence documents
If you know the main intended effect of your device, you should check the modes of action of the individual substances that contribute to it. Only devices with physical principal modes of action are covered by the MDR.
Make sure you have all the evidence to support the principal mode of action. For medical devices, this must be physical.
Use the definitions of terms for the principal mode of action in MDCG 2022-5 as a guide.
7. Conclusion
Although the January 2023 ECJ judgment did not consider MDCG document 2022-5 in its rulings, it confirmed the requirements for the distinction between medical devices and medicinal products.
Accordingly, the decisive criterion is a product's principal mode of action to fulfill its intended use.
The MDCG defines what it means by pharmacological, immunological, and metabolic principal mode of action, thus underpinning the obligation of manufacturers to exclude these modes of action in medical devices.
In order to qualify a substance-based medical device as a medical device, the physical mode of action must also be confirmed. Only this proof refers to the applicability of the MDR. It remains to be seen what form this proof will take for the large number of substance-based medical devices on the market.
This article was written in collaboration with Alexander Müller, who is currently working as a student trainee at the Johner Institute.
We need your feedback
Due to the implementation of Rule 21, many substance-based medical devices will be classified higher in the future, which makes the involvement of a notified body for conformity assessment necessary. When an application is submitted, the notified body will check whether the product is a medical device as defined in the MDR and whether it is correctly classified.
According to the outputs of a survey of notified bodies conducted by the European Commission, incorrect qualification and classification of medical devices is the third most common reason for rejected MDR applications. In addition, the notified bodies explicitly criticized the lack of evidence of a non-pharmacological or non-metabolic mode of action of ingredients.
We would like to ask you for your experiences in order to get a picture of the actual situation on the market. Are you a manufacturer of substance-based medical devices? Then, we look forward to your brief feedback!
Participate in the survey

Manuela Reinhold

A quick overview: Our
Starter-Kit
Always up to date: Our
Back To Top
Privacy settings
We use cookies on our website. Some of them are essential, while others help us improve this website and your experience.
Individual Cookie Settings
Only accept required cookies.
Privacy Notes Imprint
Here is an overview of all cookies use
Required Cookies
These cookies are needed to let the basic page functionallity work correctly.
Show Cookie Informationen
Hide Cookie Information
Provide load balancing functionality.
Provides functions across pages.
Hubspot Forms
Used for the google recaptcha verification for online forms.
Cookies for Statistics
Statistic cookies anonymize your data and use it. These information will help us to learn, how the users are using our website.
Google Analytics
Tracking and analys of traffic on our websites.
Cookies for Marketing
Marketing cookies from thrid parties will be used to show personal advertisment. They use them to track users outside of their own web page.
Keeping track of a visitor's identity. It is passed to HubSpot on form submission and used when deduplicating contacts. It contains an opaque GUID to represent the current visitor. It also introduces cookies from linked in for marketing reasons.
LinkedIn conversion tracking.
Cookies for external Content
Content for Videoplatforms und Social Media Platforms will be disabled automaticly. To see content from external sources, you need to enable it in the cookie settings.
Google Maps
Used to display google maps on our Websites. Google uses cookies to identify and track users.
EudraVigilance Medicinal Product Dictionary (EVMPD)
Extended Medicinal Product Dictionary (XEVMPD)
- Create a list of all medicines authorised and registered in the EU
- Identify medicines accurately, especially medicines included in reports of suspected adverse reactions
- Co-ordinate the regulation and safety monitoring of medicines across the EU
Use of the Extended EudraVigilance Investigational Medicinal Product Dictionary (XEVIMPD) for Sponsors of clinical trials
The EudraVigilance Registration Team will assist if further information is required ( [email protected] )
The population of the EVMPD should take place in accordance with a timetable discussed and agreed by each MAH / Sponsor and the EMA. Please contact the EMA at [email protected] to initiate the process.
- Skip to main content
- assistive.skiplink.to.breadcrumbs
- assistive.skiplink.to.header.menu
- assistive.skiplink.to.action.menu
- assistive.skiplink.to.quick.search
SNOMED CT Document Library
- Remove Read Confirmation
- A t tachments (8)
- Scaffolding History
- Page History
- Resolved comments
- Page Information
- View in Hierarchy
- View Source
- View Scaffolding XML
- Export to PDF
- Export to Word
- Hide Inline Comments
- Copy with Scaffolding XML
- Viewtracker
- Read Confirmation
Unit of Presentation Attributes
- Created by Linda Bird on 2021-Jun-04
The following sections discuss the attribute concepts that are used to represent the unit of presentation of concepts in the medicinal product hierarchy.
Unit of Presentation
A unit of presentation is a qualitative concept that describes a countable entity in which the clinical drug is presented, or by which it is bounded. It is used to support expression of presentation strength, where it provides the denominator for the strength ratio, and to differentiate different clinical drug products when the "intimate container" (see below) is clinically important (e.g. differentiating pre-filled syringes from ampoules for a solution for injection product). As described in the Strength section above and detailed further in Appendix A, there are various patterns for describing how unit of presentation and expression of strength relate together, based on whether the unit of presentation relates to the basic dose form or the intimate container (which is therefore the countable unit) of the medicinal product. As the countable entity for a medicinal product, unit of presentation is also important in describing packages, which although out of scope of the international edition, may be of major importance for national extensions describing medicinal products. There are three types of unit of presentation:
- in this type, the solid dosage form, because of its discrete nature, is the countable unit; it provides the physical boundary in which the active ingredient substance(s) of the medicinal product are presented
- in this type, the countable unit is the "actuation" provided by the metering valve; it is the valve that determines (bounds) the physical amount of the active ingredient substance(s) of the medicinal product are presented
- see below for detail
Intimate container
The "intimate container" of a medicinal product is the receptacle or vessel used to contain (or bound) liquid and some solid or semi-solid medicinal products into countable entities. A medicinal product presented in an intimate container will almost always have at least one layer of additional packaging added to it in order to make it into a packaged medicinal product; this external packaging is not described in the international edition. For example: an ampoule is an intimate container to present a solution for injection dosage form; the ampoule will always be supplied in a box or a moulded carton, possibly additionally with a blister strip as intermediate packaging. Particularly for liquid parenteral products for nebuliser liquids, and for some semi-solid presentations, the intimate container/unit of presentation may have clinical significance: providing a patient heparin in a pre-filled syringe is different from supplying that same concentration of heparin in a (multi-dose) vial. Similarly, hormone replacement gels may be supplied in single dose sachets to provide the correct administration amount.
IDMP Compatibility
In IDMP, the " one countable instance of a whole of medicinal product " is managed through the information model: it is (generally) one instance of the Manufactured Item, with its manufactured dose form and unit of presentation or one instance of the Pharmaceutical Product (with its administrable dose form and unit of presentation). The Manufactured Item is therefore the concept/class that most closely resembles the SNOMED CT Clinical Drug, but both Manufactured Item and Pharmaceutical Product contain the key "unit of presentation" attribute. However, the Manufactured Item is a representation of something that is real, with (at least in theory) all its excipient substances described and therefore is not directly compatible to the Clinical Drug - indeed the Clinical Drug could be seen as a grouper concept for similar Manufactured Items, if excipient substances etc. and packaging are disregarded. The unit of presentation in IDMP is what specifies the "real world" units in which the quantity of the manufactured item is described. The unit of presentation can be specified in accordance with ISO 11239 and ISO/TS 20440 and its resulting terminology [implemented through EDQM. IDMP goes on to state: "For items where their quantity is a measured quantity of weight or volume, the "unit of presentation" shall not be given since it is the same as the units of that quantity (that is ml, mg or %). For solid dose forms and other items that are measured by counting integer quantities, the unit for quantity shall be "unit" and the "unit of presentation" shall be the item that is counted." In EDQM, unit of presentation is defined as the "Qualitative term describing the discrete countable entity in which a pharmaceutical product or manufactured item is presented, in cases where strength or quantity is expressed referring to one instance of this countable entity."
EXAMPLE 1: To describe strength: "Contains 100 mg per tablet" ('tablet' is the unit of presentation). EXAMPLE 2: To describe quantity: "Contains 100 mL per bottle" ('bottle' is the unit of presentation).
Unit of Presentation is therefore sometimes known as "the countable unit".
Powered by a free Atlassian Confluence Community License granted to SNOMED International. Evaluate Confluence today .
- Powered by Atlassian Confluence 7.19.21
- Printed by Atlassian Confluence 7.19.21
- Report a bug
- Atlassian News
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Afr J Tradit Complement Altern Med
- v.10(5); 2013

The Role and Place of Medicinal Plants in the Strategies for Disease Prevention
Abayomi sofowora.
1 C/O Department of Pharmacognosy, Obafemi Awolowo University, Ile-Ife, Nigeria
Eyitope Ogunbodede
2 Department of Preventive and Community Dentistry, Obafemi Awolowo University, Ile-Ife, Nigeria
Adedeji Onayade
3 Department of Community Health, Obafemi Awolowo University, Ile-Ife, Nigeria
Medicinal plants have been used in healthcare since time immemorial. Studies have been carried out globally to verify their efficacy and some of the findings have led to the production of plant-based medicines. The global market value of medicinal plant products exceeds $100 billion per annum. This paper discusses the role, contributions and usefulness of medicinal plants in tackling the diseases of public health importance, with particular emphasis on the current strategic approaches to disease prevention. A comparison is drawn between the ‘whole population’ and ‘high-risk’ strategies. The usefulness of the common-factor approach as a method of engaging other health promoters in propagating the ideals of medicinal plants is highlighted. The place of medicinal plants in preventing common diseases is further examined under the five core principles of the Primary Health Care (PHC) approach. Medicinal plants play vital roles in disease prevention and their promotion and use fit into all existing prevention strategies. However, conscious efforts need to be made to properly identify, recognise and position medicinal plants in the design and implementation of these strategies. These approaches present interesting and emerging perspectives in the field of medicinal plants. Recommendations are proposed for strategising the future role and place for medicinal plants in disease prevention.
Introduction
The emphasis on the use of medicinal plants had hitherto been placed on the treatment rather than prevention of diseases. However, there exists in the literature considerable report in recent times on research work on the use of medicinal plants and their constituents in disease prevention. A World Health Organisation (WHO) Expert Group defined Traditional Medicine as the sum total of all knowledge and practices, whether explicable or not, used in diagnosis, prevention and elimination of physical, mental, or social imbalance and relying exclusively on practical experience and observation handed down from generation to generation, whether verbally or in writing ( WHO, 1976 ). For Africa, this may be extended further by including an expression, such as ‘while bearing in mind the original concept of nature which includes the material world, the sociological environment whether living or dead and the metaphysical forces of the universe’.
Over 90% of traditional medicine recipes/remedies contain medicinal plants but this paper will address, specifically, the medicinal plants that have been implicated with preventive measures in disease control strategies. However, it must be noted that only a very thin divide exists between treatment and prevention in some cases. A quick example is the fact that by treating mild elevation of blood pressure renal disease can be prevented.
What is a medicinal plant?
A medicinal plant is any plant which, in one or more of its organs, contains substances that can be used for therapeutic purposes or which are precursors for the synthesis of useful drugs. This description makes it possible to distinguish between medicinal plants whose therapeutic properties and constituents have been established scientifically, and plants that are regarded as medicinal but which have not yet been subjected to a thorough scientific study.
A number of plants have been used in traditional medicine for many years. Some do seem to work although there may not be sufficient scientific data (double-blind trials, for example) to confirm their efficacy. Such plants should qualify as medicinal plants. The term ‘crude drugs of natural or biological origin’ is used by pharmacists and pharmacologists to describe whole plants or parts of plants which have medicinal properties. A definition of medicinal plants for the purpose of this presentation should include the following ( Sofowora 2008 ; Evans, 2008 ):
- plants or plant parts used medicinally in galenical preparations (e.g. decoctions, infusions, etc.) e.g. Cascara bark;
- plants used for extraction of pure substances either for direct medicinal use or for the hemi-synthesis of medicinal compounds (e.g. hemi-synthesis of sex hormones from diosgenin obtained from Dioscorea yams);
- food, spice, and perfumery plants used medicinally, e.g. ginger;
- microscopic plants, e.g. fungi, actinomycetes, used for isolation of drugs, especially antibiotics. Examples are ergot ( Claviceps purpurea growing on rye) or Streptomyces griseus; and
- fibre plants, e.g. cotton, flax, jute, used for the preparation of surgical dressings.
The growing importance of medicinal plants can be appreciated from the economic stand point when the following facts are considered:
- Global trade in herbs is over USD 100 Billion per annum
- India and China's medicinal plant trade is about two to five billion US dollars annually
- In Germany, it is over one billion US dollars annually
- Rose Periwinkle which is endemic to Madagascar fetches US$100 million per annum
- China trades in 7,000 species and 700,000 tons of medicinal plants per annum
- India trades in 7,000 species of medicinal plants
- Morocco exports 58.7 tons of medicinal plants annually
- In the last 5 years, sales of medicinal plants doubled in China, tripled in India and grew by 25% in Europe.
A Presidential Initiative Committee on the Development, Promotion, and Commercialisation of Nigerian Herbal Medicinal Products was inaugurated on 30th May 2006 and was given a target of US$1billion sales of medicinal plants and its products within 10 years for Nigeria. Based on current research and financial investments, medicinal plants will, seemingly, continue to play an important role as a health aid ( Hoareau and DaSilva, 1999 ). The use of traditional medicine and medicinal plants in most developing countries, as a normative basis for the maintenance of good health, has been widely observed ( UNESCO, 1996 ). Furthermore, an increasing reliance on the use of medicinal plants in the industrialised societies has been traced to the extraction and development of several drugs and chemotherapeutics from these plants as well as from traditionally used rural herbal remedies ( UNESCO, 1998 ). Moreover, in these societies, herbal remedies have become more popular in the treatment of minor ailments, and also on account of the increasing costs of personal health maintenance. Indeed, the market and public demand has been so great that there is a great risk that many medicinal plants today face either extinction or loss of genetic diversity.
Preventive strategies
Health promotion, disease prevention and chronic disease management are proactive approaches to health care that stresses prevention at different points along the health care continuum. Health promotion and disease prevention strategies focus on keeping people well and preventing diseases from occurring. These strategies are referred to as primary prevention activities. Prevention is categorised into three levels (Commission on Chronic Illness, 1957):
- Health promotion/education, and
- Specific protective measures (such as immunisation)
- Secondary Prevention , which seeks to lower the rate of established cases of a disorder or illness in the population (prevalence). This level essentially involves measures that ensure early diagnosis (such as screening) and prompt management
- Disability limitation and
- Rehabilitation
The secondary and tertiary prevention activities focus on maintaining the health of individuals with chronic conditions, delaying progression of their conditions, and preventing complications.
Disease prevention should focus on strategies that reduce the risk of disease, identify risk factors, or detect disease in its early, most treatable stages. Examples of disease prevention activities include well-baby visits, immunisations, calcium and Vitamin D supplements to reduce the risk of osteoporosis, blood pressure and cholesterol assessments during annual health exams, and screening for illnesses such as breast, cervical, colorectal and prostate cancer ( Family Health Teams, 2006 ).
Public health, diet, food production and the environment are deeply interrelated, and understanding these relationships is crucial in pursuing a liveable future. Sometimes therefore, there is only a thin line between treatment and prevention of certain diseases. For example, treatment of mild hypertension will prevent many chronic renal diseases. This is also true for obesity, cancers, coronary heart diseases (CHDs) as well as diabetes and its sequelae, though these are non-communicable diseases.
The burden of healthcare and its human and financial resources requirement
In developing countries all over the world, large numbers of people die daily of preventable or curable diseases because of the lack of even simple health care. Diseases in these countries are often associated with malnutrition. As a result, those that do survive often never recover completely from the effects. The developing world is not a homogenous entity, but is made up of a variety of widely differing countries and areas which are at different stages of development. Nevertheless, these developing countries have certain features in common, including extremely limited resources, poor communications, vast distances, low levels of education, and individual and community poverty. These factors act together to keep these countries in a perpetual state of poverty. Yet, their populations continue to rise, especially in the rural regions which usually account for about 80 per cent of the total population.
Another special characteristic of the developing world is the nomadic lifestyle of some of its people. Some 50 to 100 million nomads have been estimated to be present in the world, and 90 per cent of these live in Africa or Asia. Nomads have their own needs and problems peculiar to their lifestyle. Because of their constant movement and dispersion it is difficult for conventional health services to reach these people. The striking difference between the developed and the developing world in terms of health care is reflected in the differing life expectancy of their populations. For example, according to WHO (2012) , in 2009 the life expectancy at birth was estimated at 51 years in Angola and Burkina Faso, while for Malawi it was 47 years (African countries) compared with 80 years in the United Kingdom, USA and Austria. Although the situation is improving in some African countries, this gap is still wide. Twenty-six African countries are ranked among the ‘Low Income Group’, 17 in the ‘Lower Middle Income Group’ and 9 in the ‘Upper Middle Income Group’, while only Equatorial Guinea is ranked in the ‘High Income Group’ ( WHO, 2012 ). Several of the people in Africa earn less than US$2 per day.
Any strategy for disease prevention in the developing countries, especially in Africa, must take these socioeconomic factors into account. With the abundant biodiversity of plants in the African Region and the relative lower cost of using plant derived medicines instead of processed synthetic drugs, medicinal plants should have a role to play in disease prevention strategies in Africa.
Strategies planned for traditional medicine development (First decade and second decade for Traditional Medicine in Africa)
For WHO, the main principles of the strategy for developing traditional medicine (TM) globally were thus:
- Policy: Integrate TM/CAM with national health care systems, as appropriate, by developing and implementing national TM/CAM policies and programmes
- Safety, efficacy and quality: Promote the safety, efficacy and quality of TM/CAM by expanding the knowledge-base on TM/CAM, and by providing guidance on regulatory and quality assurance standards
- Access: Increase the availability and affordability of TM/CAM, with an emphasis on access for poor populations
- Rational use: Promote therapeutically sound use of appropriate TM/CAM by providers and consumers
Strategies for development of TM in the African Region (WHO-AFRO, Brazzaville)
For WHO-AFRO, the priority interventions for the development of TM during first and second decades (i.e. 2001–2010 and 2011–2020) for African TM are as follows:
- Policy formulation;
- Capacity building;
- Research promotion;
- Support for the local production of Traditional Medicines including cultivation of medicinal plants;
- Protection of intellectual property rights and traditional medical knowledge.
Global Disease Burden
Diseases have been grouped as communicable or non-communicable based on the involvement or otherwise of a transmissible biologic disease causing agent. Until recently, communicable diseases (CDs) were the major causes of ill-health and deaths in the developing (low and middle resource) countries while non-communicable diseases were prevalent in the developed (high resource) countries, where improvement in living conditions and widespread deployment of technology had brought the CDs under control. However, the optimism that communicable diseases would be less of a health problem in the developed countries appears to have been misplaced with the appearance of new infectious diseases and re-emergence of older disease agents. Similarly, non-communicable diseases are already a major cause of morbidity and mortality as a consequence of globalisation and changing lifestyle in developing countries. Globally therefore, NCDs and CDs account for about equal quantities of morbidity and mortality, thus making all countries to currently face the double disease burden. The overall picture is, however, graver for low and middle income countries in terms of the health and socio-economic impacts.
For example, estimates of projected causes of all deaths for the African Region indicate that injuries and chronic communicable diseases accounted for 30% of all deaths for 2005. ( Figure 1 )

Projected deaths by cause, all ages, WHO African Region, 2005
Source : Moeti (2008) . Data estimated by WHO using standard methods to maximize cross-border comparability. They are not necessarily the official statistics of Member States.
Medicinal plants and disease prevention
Strategies for the prevention of communicable diseases.
Three core approaches - surveillance, outbreak investigations and immunisation - are fundamental to the prevention of communicable diseases. While medicinal plants may appear to have limited role in these approaches, several medicinal plants and traditional medicines derived from them have been used to enhance immune response to several disease agents ( Di Pierro et al. , 2012 ; Ramakrishna et al. , 2011 ).
Strategies for Prevention of Non-Communicable Diseases
The WHO 2008 to 2013 Action Plan for the Global Strategy for the Prevention and Control of NCDs articulated an intersectoral, multi-level plan to curb the rising global prevalence of NCDs with particular focus on the low and middle income countries. The overall foci of the plan were to
- map the emerging NCD epidemic and ascertain their social, economic, behavioural and political determinants;
- reduce the level of exposure of individuals and population to the common modifiable risk factors - tobacco use, unhealthy diet, physical inactivity, etc.; and
- strengthen health care for people with non-communicable diseases through the development of evidence-based norms, standards and guidelines for cost effective interventions.
Related to the third focus above, an examination of the causal chain of risk factors for NCDs ( Figure 2 ) is helpful in illuminating the potential role of medicinal plants in the prevention of NCDs. Medicinal plants have specific roles in strengthening health care opportunities for people with NCDs as well as in the management of the biologic risk factors for NCDs, especially in the early stage ( Jung et al. , 2012 ; Tan et al. , 2010 ).

Causal chain of risk factors for NCDs
The ‘whole population’ and ‘high-risk’ strategies
Two main types of approaches have been advocated in tackling major public health problems. The whole-population strategy targets the community as a whole to control the occurrence of new diseases in the population. The high-risk strategy on the other hand aims to identify individuals most at risk for a disease or outcome and then target preventive efforts at that group. These were first defined by Geoffrey Rose ( Rose, 1985 ). In promoting the use of medicinal plants in disease prevention, the whole-population strategy will have the global community as the target, whereas the high-risk strategy may focus on rural communities, especially in the developing countries. It may also on the other hand wish to focus on refining medicinal plants for use in specific disease conditions that presently defy conventional treatment, such as cancer, HIV infection and AIDS.
Utilising the Common Factor approach
The common risk factor approach aims at bringing together several health promoters working on eliminating common-risk factors as a way of preventing diseases. ( Sheiham and Walt, 2000 ) Poor Diet for example can lead to obesity, diabetes, cancers, and dental caries. Hence, nutritionists, diabetologists, oncologists, dental practitioners can work together with diet as common theme. A modified form of this approach ( Figure 3 ) can be a useful tool in engaging other health promoters, in tackling the different forms of disease, and in propagating the ideals of medicinal plants. Working with various groups, for example, appropriate medicinal plants can be incorporated into the diets to alleviate disease and suffering. This approach will enable those working to promote the use of medicinal plants to collaborate with other health promoters in areas such as malaria, diabetes, cancers, cardiovascular diseases, tuberculosis, HIV/AIDS, oral diseases, dermatological problems, etc.

The Common Factor Approach (Adapted from Sheiham and Walt, 2000 )
The Primary Health Care (PHC) Approach
Primary Health Care was defined in Alma Ata ( WHO, 1978 ) as essential care based on practical, scientifically sound and socially acceptable method and technology made universally accessible to individuals and families in the community through their full participation and at a cost they and the country can afford to maintain in the spirit of self-reliance and self-determination. The PHC philosophy recognises that each discipline contributes to health and health services delivery within a PHC model, both in a unique sense and through collaborative interdisciplinary practice.
The five core principles of the PHC approach include the following:
- Equitable distribution
- Community Participation (as issues that local people identify rather than predetermined services introduced by professionals, working within existing community organisations and local government structures, etc.)
- Focus on Prevention
- Appropriate Technology
- Multisectoral Approach - Emphasis should be made that the reason for the failure of many programmes is due to the fact that they operate in isolation, separate from the general health care structure and without the support of other relevant sectors. The need for programme cooperation and collaboration cannot be over-emphasised.
The elements (or components) of PHC include (but not limited to) Immunisation, Maternal and Child Health (MCH) Care, Essential Drugs, Food and Nutrition, Education, Common Illnesses and injury, Water and Sanitation, Endemic Infectious Diseases, Mental health and Oral health.
All African countries have adopted PHC as the over-arching strategy to achieve health for their citizens. Strategies for the promotion of medicinal plants for the prevention of diseases in Africa must therefore take into cognisance the PHC approach. It must essentially follow the 5-key principles outlined above and be integrated into the elements. Medicinal plants will be useful for Maternal and Child health care, as essential drugs, in food and nutrition, for common illnesses and injury, for endemic infectious diseases, mental health and oral health.
Medicinal plants also fit perfectly into the modelling for priorities in Primary Health Care as proposed by McDonald and Ollerenshaw (2011) . ( Figure 5 )

Model for Priority setting in Primary Health Care ( McDonald & Ollerenshaw, 2011 )
In summary, from the above considerations of available strategies, medicinal plants can play vital roles in disease prevention and their promotion and use fit into all existing prevention strategies. However, conscious efforts need to be made to properly identify, recognise and position medicinal plants in the design and implementation of these strategies. These approaches present interesting and emerging perspectives in the field of medicinal plants.
Ethnobotanical Studies on Medicinal Plants Used in Disease Prevention
In order that a comprehensive compilation of medicinal plants that can be used in disease prevention is obtained, collation of original data from the traditional custodians of such knowledge is essential ( Tan et al. , 2010 ). This is especially so in the case of African Traditional Medicine (ATM) where information is passed on from generation to generation orally about the plants used. Unlike in Chinese Traditional Medicine (CTM) and the Indian systems of medicine (Ayurveda, Unani and Sithda) where the information is available in books (and now online), a lot of the information on African traditional medicine is yet to be documented. Efforts are, however, being made by WHO-AFRO to augment the various isolated databases on medicinal plants through the provision of guidelines for documentation of herbal recipes ( WHO/AFRO 2012 ). Specific ethnobotanic surveys at village level using some of the methods described by Sofowora (2008) can be used. Such a survey by Biswas et al. (2011) on medicinal plants used for preventive medicinal purposes in Muktipara village, Chuadanga District of Bangladesh yielded 11 authentic plants including Azadirachta indica and Moringa oleifera which are quite common in Africa.
A similar survey conducted by Rahmatulla et al. (2011) among the Chakma residents of Hatimara (south) village of Rangamati district, also in Bangladesh, indicated that the mode of consumption of the plants differed to some extent. Some plants, like Spilanthes calva or Commelina paludosa , the leaves were boiled, mixed with crushed peppers and taken. The authors found that the addition of peppers did not serve any therapeutic purposes. Rather, peppers, particularly hot peppers, were added to make the dish more palatable and to impart flavour to the dish (Rahmatullah et al. , 2009; Sofowora 2008 ; Abel and Busia, 2005 ). The juice of young leaves of Centella asiatica or juice of leaves of Solena amplexicaulis was taken in the raw state. The fruits of Gymnopetalum cochinchinense were used for prevention of ulcer, and Solanum torvum as a preventive measure against leucorrhoea, typhoid and tonsillitis. The barks and seeds of Saraca were mashed and taken in the raw state as prevention for irregular menstruation and menorrhagia.
International ethnobotanical surveys sponsored by ACCT (Agence de Cooperation Culturelle et Technique) into 17 Francophone African countries and 5 into Anglophone African countries sponsored by the African Union (AU/STRC) and most of which were led by Professor Edouard Adjanohoun (Benin now France) and Professor Laurent Ake Assi have been published. These need to be searched for plants used in disease prevention as they were general ethnobotanic surveys which probably emphasised more enquiries on the use of plants to treat disease. All the data from such surveys especially the new ones need to be stored in a database where it is not yet done and protected for only authorised access.
Medicinal plants used to prevent cancer
Yasukawa (2012) has reviewed the chemopreventive activity of natural sources, foods, supplements, crude drugs and Kampo medicines (traditional Japanese herbal prescriptions). In that review, he observed that cancer chemoprevention is currently one of the most urgent projects in public health. Cancer chemoprevention is defined as the use of specific natural and synthetic chemical agents to reverse or suppress carcinogenesis and prevent the development of invasive cancers. Recently, dietary non-nutrient compounds have demonstrated important effects as chemo-preventive agents, and considerable work on the cancer chemopreventive effects of such compounds in animal models has been undertaken. Epidemiological surveys have shown that the majority of human cancers are related to two factors, namely, diet and smoking ( Banning, 2005 ; Hirayama, 1984 ). However, in the general population, daily consumption of certain foods has also been shown to have anticancer effects. This highlights the importance of environmental factors such as diet in cancer chemoprevention ( Banning, 2005 ). An understanding of the mechanisms of carcinogenesis is essential for cancer chemoprevention. Most cancer prevention research is based on the concept of multistage carcinogenesis: initiation → promotion → progression ( Pitot and Dragan (1991) ]; Morse and Stoner, 1993 ). ( Figure 6 )

Stages in Cancer Prevention Research
In contrast to both the initiation and progression stages, animal studies have indicated that the promotion stage occurs over a long time period and may be reversible, at least early on. For this reason, it is expected that inhibition of tumour promotion should be an efficient approach to cancer control ( Murakami, et al. , 1996 ). Yasukawa and his team have found in the search for potential anti-tumour promoters (cancer chemopreventive agents) from edible plants and fungi, and from crude drugs, that various triterpene alcohols and sterols and their oxygenated derivatives showed inhibitory effects on mouse ear inflammation induced by 12- O tetradecanoylphorbol-13-acetate (TPA). Primary prevention of cancer aims to avoid the development of cancer. Therefore, initiation and/or promotion of carcinogenesis should be inhibited. However, the adult population bears tumour cells that cannot revert to normal cells, and thus effective strategies to prevent cancer include avoiding continuous contact between these cells and promoters and/or aggressively inhibiting the tumour promoter effects. Therefore, to prevent cancer, it is essential to find plants that contain effective compounds (anti-tumour promoters) that delay, inhibit or block tumour promotion, which is a reversible and long-term process ( Yasukawa, 2012 ). A few examples of such plants of interest are shown below:
(Pygeum) Prunus spp (Family Rosaceae) e.g. African Prune or African Plum Tree
Prostate cancer is a very good example for chemoprevention because prostate cancer is typically slow growing and is usually diagnosed in elderly males. The extract of the bark of Pygeum africanum (Prunus Africana ) has been used in Europe as a prevention and treatment of prostate disorders including benign prostatic hypertrophy (BPH). In tissue culture, ethanolic extracts (30%) of the bark inhibited the growth of PC-3 and LNCaP cells; induced apoptosis and altered cell kinetics; down-regulated ERalpha and PKC-alpha protein, and demonstrated good binding ability to both mouse uterine oestrogen receptors and LNCaP human androgen receptors. TRAMP mice fed with P. africanum showed a significant reduction (P = 0.034) in prostate cancer incidence (35%) compared to casein fed mice (62.5%). P. africanum therefore has a significant role in regulation of prostate cancer both in vitro and in vivo and therefore may be a useful supplement for people at high risk for developing prostate cancer ( Shenouda et al, 2007 ). Katz (2002) had observed that the consumption of isoflavones found in legumes and other plants is related to lower rates of BPH and prostate cancer among Asian men ( Katz, 2002 ). The methanol extract of Prunus jamasakura Sieb. ex Koidz. inhibited two-stage carcinogenesis by DMBA/TPA in mouse skin ( Yasukawa, et al. , 1998 ). Octacosyl ferulate, from the active fraction of the plant, inhibited tumour promotion by DMBA/TPA in mouse skin (Yasukawa, et al. , 1998) ( Figure 7 ). The compound also inhibited the phosphorylation of histone by protein kinase C (PKC) in a concentration-dependent manner.

Octacosyl ferulate from Pruni Cortex
Azadirachta indica (Family Meliaceae) Neem
Over 60 different types of biochemicals including terpenoids and steroids have been purified from this plant. The anticancer properties of the plant have been studied largely in terms of its preventive, protective, tumour-suppressive, immunomodulatory and apoptotic effects against various types of cancer and their molecular mechanisms ( Paul et al, 2011 ). Triple-negative breast cancer (TNBC) accounts for 15–20% of all breast tumours and these breast tumours are usually aggressive and highly metastatic. Unfortunately, treatment options for TNBCs are limited. A novel compound, 2′-3′-dehydrosalannol (DHS) isolated from A. indica uncrushed leaves, inhibited growth and induced apoptosis in TNBC cell lines. Molecular analysis suggested that DHS inhibited cathepsin-mediated pro-survival signalling [pAKT: phosphorylated protein kinase B; BCL-2: B-cell lymphoma 2 and cyclin D1] and induced pro-apoptotic markers such as BAX [BCL-2-associated X protein] and cleaved caspase-3 ( Boopalan et al , 2012 , Malathi et al, 2002 ). Also, Neem leaves were found to inhibit tumour promotion by DMBA/TPA in mouse skin ( Arora et al. , 2011 ). Inhibition of carcinogenesis in response to neem treatment was accompanied by an over expression of signal transducer and activator of transcription 1 (STAT1) and activator protein 1 (AP-1) and decrease in nuclear factor-kappa B (NF-κB) expression ( Arora et al. , 2011 ). In a recent study, Bharati et al , (2012) evaluated the anticarcinogenic potential of aqueous A. indica leaf extract against N-nitrosodiethylamine (NDEA)-induced hepatocarcinogenesis. They reported a significant reduction in tumour incidence (33%), tumour multiplicity (42%), and increase in survival (34%) upon administration of the aqueous extract to NDEA-abused mice. Transmission and scanning electron microscopic investigations showed severe alterations in organelle organisation, cellular arrangement, degree of differentiation, cellular metabolism, and morphology of the hepatocytes. They concluded that these changes appeared to be distinctly delayed upon supplementation with the leaf extract of the plant. The results suggest that A. indica may have anticancer potential against NDEA-induced hepatic cancer.
Rosmarinus officinalis L (Family Labiatae) Rosemary
Colorectal cancer is the second leading cause of cancer death in Australia. Ngo et al. (2011) reviewed scientific evidence from all studies published from 1996 to March 2010 and which examined the protective effects of rosemary on colorectal cancer and other types of cancer. They concluded that evidence from animal and cell culture studies demonstrates the anticancer potential of rosemary extract as well as only the following constituents of it: carnosol, carnosic acid, ursolic acid, and rosmarinic acid. The reported anticancer properties were found to arise through the molecular changes in the multiple-stage process of cancer development, which are dose related and not tissue or species specific. López-Jiménez (2013) demonstrated that the anti-angiogenic activity of carnosol and carnosic acid could contribute to the chemopreventive, antitumoral and antimetastatic activities of rosemary extracts and suggested their potential in the treatment of other angiogenesis-related malignancies. Ursolic acid and carnosol were isolated from the methanol extract of Rosemary, and inhibited DMBA/TPA-promoted two-stage carcinogenesis in mouse skin ( Huang et al. , 1994 ) ( Figure 8 ).

Terpenoids from rosemary
Vitis vinifera L. (Family Vitaceae ) Grape
Dietary intake of foods rich in antioxidant properties is suggested to be cancer protective. Foods rich in antioxidant properties include grape ( Vitis vinifera ). Grape skin and seed extracts exert strong free radical scavenging and chelating activities and inhibit lipid oxidation in various food and cell models in vitro. The use of grape antioxidants are promising against a broad range of cancer cells by targeting epidermal growth factor receptor (EGFR) and its downstream pathways, inhibiting over-expression of COX-2 and prostaglandin E2 receptors, or modifying oestrogen receptor pathways, resulting in cell cycle arrest and apoptosis ( Zhou and Raffoul, 2012 ).
Filip et al. (2011) in their studies on Photoprotective effects of two natural products on ultraviolet B-induced oxidative stress and apoptosis in SKH-1 mouse skin reported that their results suggest that Calluna vulgaris and Vitis vinifera extracts might be chemopreventive candidates for reducing UV-induced risk for skin cancer.
Currants and Sultanas ( Vitis vinifera L.) are dried vine products produced in Greece. Kaliora et al. (2008) investigated the gastric cancer preventive activity of methanol extracts obtained from currants from three different origins in Greece (Vostizza, Nemea, and Messinia), as well as methanol extracts obtained from Sultanas cultivated on the island of Crete as to inhibition of cell proliferation, induction of apoptosis, and inhibition of inflammation. All extracts from 500 micrograms of dried raisins studied suppressed cell proliferation, significantly those obtained from Sultanas from Crete and currants from Nemea. The French eat higher levels of animal fat, but their incidence of heart disease remains surprisingly low. This ‘French Paradox’ is thought to be due to the benefits they derive from consuming red wine. The ethanol extract of grapes inhibited tumour promotion by DMBA/TPA in mouse skin ( Alam et al. , 2002 ). Resveratrol, in a dose-dependent manner, reduced the incidence, total number and multiplicity of visible hepatocyte nodules ( Bishayee and Dhir, 2009 ) ( Figure 9 ).

Resveratrol from grape
On the other hand, grape seeds are a rich source of monomeric, dimeric and oligomeric proanthocyanidins. The polyphenolic fraction of grape seeds suppressed tumour promotion by DMBA/TPA in mouse skin ( Bomser, et al. , 1999 ; Zhao, et al. , 1999 ).
Glycine max or G. soya (Family Leguminosae) Soya milk
Genistein, the most abundant phytoestrogen in soybeans, may bind to oestrogen receptors and perform anticancer activities. Choriocarcinoma is a malignant, trophoblastic and aggressive cancer of the placenta. Liu et al (2011) investigated the effect of genistein on the invasive potential of the choriocarcinoma cell line JAR and its underlying mechanism and found that genistein inhibited JAR cell invasion in a dose-dependent manner by a matrigel invasion assay. Their findings have significant implications for the prevention and therapy of choriocarcinoma. However, Khan et al (2012) tested the hypothesis that Soy isoflavone consumption may protect against breast cancer development. They found a lack of efficacy for breast cancer prevention and a possible adverse effect in premenopausal women. Soy milk inhibited 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanoneinduced mammary carcinogenesis in rats ( Ohta et al. , 2000 ). Soy beans contain high amounts of isoflavonoids and saponins; isoflavonoids have been shown to have phytoestrogenic activity (Moliteni et al. , 1995; Katz, 2002 ).
Zingiber officinale Roscoe (Family Zingiberaceae) Ginger
Dehydrozingerone a pungent constituent of ginger is a vanillyl ketone. Structurally, it is representative of half the chemical structure of curcumin which is a promising phytochemical for the inhibition of malignant tumours, including colon cancer. Yogosawa et al. (2012) evaluated the antiproliferative effects of dehydrozingerone against HT-29 human colon cancer cells, and it was found that it dose-dependently inhibited growth at the G2/M phase with up-regulation of p21. Dehydrozingerone additionally led to the accumulation of intracellular ROS, although most radical scavengers could not clearly repress the cell-cycle arrest at the G2/M phase. Their results suggest that analogues of dehydrozingerone may be potential chemotherapeutic agents for colon cancer ( Škrovánková, 2012 ). Kurapati et al. (2012) investigated the combinatorial cytotoxic effects of Curcuma longa and Zingiber officinale on the PC-3M prostate cancer cell line. The two extracts separately showed significant inhibitory effects on colony-forming ability. However, when both the agents were tested together at the same concentrations, the combined effects were much more significant than their individual ones, suggesting the role of multiple components and their synergistic mode of actions to elicit stronger beneficial effects.
Hexahydrocurcumin, extracted from Zingiber officinale, was shown to be cytotoxic to colorectal cancer cells by Chen et al. (2011) . Treatment of SW480 cells with hexahydrocurcumin (100 microM) resulted in a massive accumulation of the cells in the G1/G0 phase of the cell cycle. This compound could prove useful in cancer prevention.
The protective and/or preventive activity of various spices against various cancers and gastric ulcer has been reviewed by Sumbul et al. (2011) and by Sung et al. (2012) .
Topical application of an ethanol extract of ginger inhibited TPA-induced tumour promotion during two-stage carcinogenesis in mouse skin ( Katiyar et al. , 1996 ). Pre-application of an ethanol extract of ginger onto the skin of SENCAR mice resulted in significant inhibition of TPA-induced epidermal ODC, COX and lipoxygenase activities as well as ODC mRNA expression in a dose-dependent manner. Topical application of [6]-gingerol inhibited tumour promotion by DMBA/TPA in mouse skin, and also suppressed TPA-induced epidermal ODC activity and inflammation ( Park et al. , 1998 ) ( Figure 10 ).

Some phenolics from Zingiber officinale
Food Supplements
Allium cepa and a. sativum ( family liliaceae) onion and garlic.
Antony and Singh (2011) have reviewed the mechanisms and targets of cancer chemoprevention by diallyl trisulfide (DATS) extracted from Allium species, while Zhou et al (2011) in a meta-analysis, in which they pooled analysis of all studies, concluded that the consumption of large amounts of Allium vegetables (in a comparison of the highest and lowest consumption groups) reduced the risk for gastric cancer (odds ratio, 0.54; 95% confidence interval, 0.43–0.65). Allium sativum (garlic) and its chemical constituents, especially Allicin, Dially Disulfide, Diallyl Trisulfide, have also been shown in recent studies to be chemopreventive agents for lung cancer and breast cancer ( Chu et al. , 2012 ; Nkrumah-Elie et al. , 2012 ; Li et al. , 2012 ). Allicin was also reported by Wang et al. (2012) to induce apoptosis in EL-4 cells in a time-and concentration-dependent manner, in which the mitochondrial pathway might play a central role.
Onion oil inhibited tumour promotion by DMBA/TPA ( Belman, 1983 ; Perchellt et al. , 1990 ) and by BP/croton oil ( Sadhana et al. , 1988 ) in mouse skin, while garlic oil inhibited tumour promotion by DMBA/TPA in mouse skin ( Belman, 1983 ; Perchellt et al. , 1990 ).
Panax ginseng C.A. Mayer (Family Araliaceae). Ginseng
Li et al. (2012) characterised a homogeneous polysaccharide (PGPW1) from the root of Panax ginseng with molecular weight as 3.5×10(5) Da. PGPW1 contained Glcucose, Galactose, Mannose and Arabinose in the molar ratio of 3.3:1.2:0.5:1.1. It dose-dependently displayed potent anti-proliferation and anti-metastatic activities. They also found that the attenuated expression of M3 muscarinic receptor on the surface of T24 cells by PGPW1 would contribute to its antitumour functions. All the data indicated the potential of its clinical application for the prevention and treatment of bladder cancer metastasis. Dong et al. (2011) have evaluated the cytotoxic potency of ginsenosides and their synthetic derivatives against a variety of cancer cells with a view to determining the structure activity relationship. The results clearly indicated that the compound with less polar chemical structures possesses higher cytotoxic activity towards cancer cells. In their own investigation on ginsenoside Rp1, Kang et al. (2011) found that it inhibited breast cancer cell proliferation and inhibited both anchorage-dependent and -independent breast cancer cell colony formation. In addition, Rp1 induced cycle arrest and apoptosis-mediated cell growth suppression. Rp1 also decreased the stability of the IGF-1R protein in breast cancer cells. They therefore suggested that Rp1 has potential as an anticancer drug and that IGF-1R is an important target for treatment and prevention of breast cancer. In their own studies, Cui et al. (2010) found that when American ginseng was tested using the azoxymethane (AOM)/dextran sulphate sodium (DSS) mouse model of ulcerative colitis, they demonstrated that ginseng can suppress colon cancer associated with colitis.
Oral administration of white and red ginseng ( Panax ginseng C.A. Mayer) suppressed colon carcinogenesis by 1,2-dimethylhydrazine (DMH) in rat (Fukushima, et al., 2001). The ginsenosides Rg3, Rg5 and Rh2 are active components in ginseng, and act either singularly or synergistically in cancer prevention ( Yun, et al., 2001 ). The methanol extract of san-chi ginseng ( P. notoginseng (Burk.) F.H. Chen) suppressed skin carcinogenesis by DMBA/TPA, liver carcinogenesis by DEN/Phenobarbital, lung carcinogenesis by 4NQO/glycerol in mice ( Konoshima et al. , 1996 ). Moreover, the methanol extract of san-chi inhibited skin carcinogenesis by NOR-1/TPA, as well as DMBA/fumonisin B1 in mice ( Konoshima et al. , 1999 ). The ginsenoside Rg1 slightly suppressed tumour promotion by DMBA/TPA in mouse skin ( Konoshima et al. , 1996 ) ( Figure 11 ).

Ginsenosides from Ginseng
Prevention and treatment of male osteoporosis due to androgen deficiency by using the medicinal plant: Eurycoma longifolia
Osteoporosis in elderly men is now becoming an alarming health issue due to its relation with a higher mortality rate compared to osteoporosis in women. Androgen deficiency (hypogonadism) is one of the major factors of male osteoporosis and it can be treated with testosterone replacement therapy (TRT). However, one medicinal plant, Eurycoma longifolia Jack ( EL ), can be used as an alternative treatment to prevent and treat male osteoporosis without causing the side effects associated with TRT. EL exerts proandrogenic effects that enhance testosterone level, as well as stimulate osteoblast proliferation and osteoclast apoptosis. This will maintain bone remodelling activity and reduce bone loss. Phytochemical components of EL may also prevent osteoporosis via its antioxidative property ( Figure 12 ). Hence, EL has the potential as a complementary treatment for male osteoporosis ( Effendy et al. , 2012 ).

Some of the chemical constituents isolated from the root of Eurycoma longifolia Jack (Source: Effendy et al., 2012 )
Plants used for the prevention of Coronary Heart Disease (CHD)
Coronary Heart Disease (CHD) is the primary contributor to morbidity and mortality worldwide. A great deal of research is now focused on identifying new therapeutic alternatives to prevent and treat CHD. The most consistent recommendations from a Public Health perspective involve multiple changes in diet and exercise. Medicinal plants are also a viable option for its prevention and treatment. Clinical and preclinical data on some medicinal plants used as dietary supplements show that they may be useful in the strategies to reduce the prevalence and mortality of CHD either in the general population or in the subsets of individuals at high risk. Such plants include, for example, artichoke, garlic, gingko, guggul, hawthorn and tea.
Artichoke Cynara scolymus L. (Family Asteraceae)
Dried leaves and lower part of the flower of this plant contain 6% phenolic acids, 5% sequiterpene latones. Three sesquiterpenes, cynaropicrin, aguerine B, and grosheimin were isolated as the active components of the artichoke extract which reduced serum triglyceride levels in olive oil-loaded mice. Aqueous and ethanolic extracts of artichoke also reduced intracellular oxidative stress stimulated by inflammatory mediators such as tumour necrosis factor alpha (TNFa) and lipopolysacchaide (LPS), as well as ox-low density lipoprotein (ox-LDL) in endothelial cells and monocytes.
Garlic ( Alium sativum , (Family Liliaceae)
Over 35 randomised clinical trials on garlic were carried out to investigate the effect of garlic on cardiovascular end points. Overall, there is evidence from randomised controlled trials (RCT) in adults that the use of garlic preparations can lead to a small but statistically significant reduction in total cholesterol levels compared with controls.
Guggul Commiphora mukul .(Jacq) Engler, (Family Burseraceae)
The Mukul Myrrh tree has been used as far back as 600 BC. According to the recent WHO Monograph for guggul, the plant is useful for the treatment of hyperlipideamia and hypercholesterolemia. The plant sterols, E- and Z-guggulesterone, are believed to be the bioactive compounds.
Strategies for combating micronutrient deficiencies: Food-based approaches
Multiple micronutrient deficiencies abound in developing countries ( Ramakrishnan, 2002 ). They are caused by inadequate intakes but genetic, parasitic and infectious diseases may also play a role ( Fishman, et al. , 2000 ; Stoltzfus, 2001 ). Micro nutrient deficiencies can have major adverse health consequences, contributing to impairment in growth, immune competence, mental and physical development, and poor reproductive outcomes ( Viteri and Gonzales, 2002 ) that cannot always be reversed by nutrition interventions. Inadequate intakes of certain micronutrients such as iodine, selenium and zinc can also be exacerbated by environmental factors, as their content in plant based foods is dependent on soil trace elements ( Gibson and Hotz, 2001 ).
There is therefore a strong need for strategies to reduce micronutrient deficiencies in developing countries. Strategies commonly used are supplementation and food-based approaches, preferably in conjunction with public health interventions such as promotion and support of breast feeding and control of infectious and parasitic diseases. Fruits and vegetables are a good source of vitamins and minerals. Faber and Laurie (2011) described a home garden strategy that integrates gardening activities with nutrition education, using community based growth monitoring as an entry point in South Africa. A positive effect was observed that Provitamin A rich vegetables and fruits contributed significantly towards achieving the recommended dietary intake of Vitamin A and other micronutrients. They concluded that home gardening is a long term strategy that contributes to combating Vitamin A and other nutritional deficiencies. This is only one of the several individual investigations reported by Thompson and Amoroso (2011) to pinpoint the fact that food based strategies are viable, sustainable and long term solutions to overcoming micronutrient malnutrition.
The strategy of using antioxidant activity of medicinal plants in prevention of diseases
Oxidative stress, caused by reactive oxygen species, plays an important role in many chronic and degenerative diseases, such as atherosclerosis, ischemic heart disease, cancer, diabetes mellitus, neurodegenerative diseases and ageing ( Azizova, 2002 ). The body's non-enzymatic antioxidant defence system includes some antioxidants, such as vitamin C, vitamin E, vitamin K and glutathione. Some synthetic antioxidants, widely used in food industry to protect food from oxidation and spoiling, are harmful because of their potential toxicity and carcinogenicity ( Botterweck et al. , 2000 ). However, natural antioxidants in fruits and vegetables are inversely related with the risk of the chronic diseases mentioned above ( Leifert and Abeywardena, 2008 ). Natural antioxidants, therefore, provide alternative strategy to prevention as well as treatment of these diseases. Phenolic compounds because of their oxidative activity are potential agents for preventing and treating many oxidative stress-related diseases. The antioxidant activity of polyphenols is mainly due to their redox properties, which allow them to act as reducing agents, hydrogen donors, singlet oxygen quenchers, metal chelators and reductants of ferryl hemoglobin ( Kratchanova et al. , 2010 ). Some medicinal plants possess more potent antioxidant activity than common dietary plants ( Cai et al. , 2004 ). Therefore, their extract, if not toxic, can serve as food additive and can be used for disease prevention ( Liu, 2003 ; Liu et al. , 2008 ).
Oral Diseases prevention with medicinal plants
Oral diseases are major health problems with dental caries and periodontal diseases among the most prevalent, preventable global infectious diseases. Oral health influences the general quality of life and poor oral health is linked to chronic conditions and systemic diseases. The association between oral diseases and the oral microbiota is well established. Although there are more than 750 species of bacteria that inhabit the oral cavity, most are normal commensals and only a few are implicated in oral diseases. The development of dental caries involves acidogenic and aciduric Gram-positive bacteria (mutans streptococci, lactobacilli and actinomycetes). Periodontal diseases have been linked to anaerobic Gram-negative bacteria ( Porphyromonas gingivalis, Actinobacillus, Prevotella and Fusobacterium ). Given the incidence of oral disease, increased resistance by bacteria to antibiotics, adverse effects of some antibacterial agents currently used in dentistry and financial considerations in developing countries, there is a need for alternative prevention and treatment options that are safe, effective and economical. While several agents are commercially available, these chemicals can alter oral microbiota and have undesirable side-effects, including tooth staining. Hence, the search for alternative products continues and natural phytochemicals isolated from plants used as traditional medicines are considered as good alternatives. Palombo (2011) concluded that there is considerable evidence that plant extracts, essential oils and purified phytochemicals have the potential to be developed into agents that can be used as preventive or treatment therapies for oral diseases. While it is encouraging to see a number of clinical trials of such products, further studies of the safety and efficacy of these agents will be important to establish whether they offer therapeutic benefits, either alone or in combination with conventional therapies, that can help to reduce the overall burden of oral diseases worldwide. In particular, studies that address issues such as adequate statistical power, blinding, standardisation of extracts or purified compounds, and quality control would be of great value ( Palombo, 2011 )
Some miscellaneous practices for the prevention of diseases with medicinal plants in Africa
According to Koumare (2008) repeated sore throat especially in children is often prevented in Mali and other African countries by using gargles made with the following plants: Spilanthes sp, Guiera senegalensis and Waltheria indica . Traore (1975) had also compiled some cultural practices of the Malian people in the prevention of dental problems with medicinal plants ( Troure, 1975 ). The efficacy of chewing sticks used for preventive dental care had been reported by Sofowora and others ( Sofowora, 2008 ). It is common knowledge among women in Africa, especially in Burkina Faso, to rub the skin of new borne babies with various medicinal plants (e.g. Pterocarpus osun ) soaked in oil to prevent them from bacterial infection as they are carried by various people. Also, because the mothers do not know whether the visitors carrying the newborn have evil intentions, the preparation with which the baby is rubbed often contains plants with occult powers to ward off any spiritual attack on the baby by visitors. Various antenatal practices using medicinal plants exist in Africa. The pregnant woman is made to bathe in some decoctions of herbs and required to drink a little of it periodically throughout the period of pregnancy to sustain her. There are also herbs to be taken just before delivery to hasten or to ease delivery. However, the use of herbs in late pregnancy could be for their oxytocic properties. Some preparations for preventing road accidents exist in Africa just as there are similar preparations utilising the occult power of herbs to eject a person from an accident vehicle before the accident ( Egbe in Yoruba). These latter preventive uses of medicinal plants involving the occult power of herbs may be beyond present capabilities of scientific experimentation to prove their efficacy.
Conclusions
Efforts must be geared towards measures that will enhance the effectiveness, efficacy and rational use of medicinal plants, especially through the integration into national, regional and local health policies and programmes. Most African countries, for example, hinge their health care system on the Primary Health Care (PHC) strategy and it is necessary to incorporate the use of medicinal plants into all the components of PHC in these countries. The following are also recommended: a) Collation of data from books, research articles, conducting of ethnobotanical surveys (because Africans have only recently started documentation of medicinal plants and their uses; oral tradition had been the mainstay) specifically to look for plants used in preventing diseases in our communities as was done in Muktipara village of Sri Lanka. Database searches on medicinal plants will also yield useful results ( Table 1 ); b) collaborative research with Institutes for preventive medicine as well as departments of preventive dentistry in teaching hospitals; and c) coordination of the research to avoid duplication of efforts.
plants with potentials in preventive medicine practice

A Health Promotion Model for Medicinal Plants (Adapted from Nutbeam, 1998 )
Acknowledgements
We would like to thank the organisers of the third CONGRESS INTERNATIONAL DE PHYTOTHERAPIE DE OUAGADOUGOU (CIPO) for inviting Professor Sofowora as a plenary lecturer to present this paper in Ouagadougou on 9th October, 2012. The assistance of the Nigeria Natural Medicine Development Agency (NNMDA) in the generation of the table of plants used in disease prevention through their virtual library as well as the provision of some original research articles by Professor (Mrs.)' Lanre Omobuwajo is hereby acknowledged.
- Open access
- Published: 23 January 2023
Customer-centric product presentations for monoclonal antibodies
- Beate Bittner 1
AAPS Open volume 9 , Article number: 3 ( 2023 ) Cite this article
4421 Accesses
2 Citations
Metrics details
Delivering customer-centric product presentations for biotherapeutics, such as monoclonal antibodies (mAbs), represents a long-standing and paramount area of engagement for pharmaceutical scientists. Activities include improving experience with the dosing procedure, reducing drug administration-related expenditures, and ultimately shifting parenteral treatments outside of a controlled healthcare institutional setting. In times of increasingly cost-constrained markets and reinforced with the coronavirus pandemic, this discipline of “Product Optimization” in healthcare has gained momentum and changed from a nice-to-have into a must.
This review summarizes latest trends in the healthcare ecosystem that inform key strategies for developing customer-centric products, including the availability of a wider array of sustainable drug delivery options and treatment management plans that support dosing in a flexible care setting. Three disease area archetypes with varying degree of implementation of customer-centric concepts are introduced to highlight relevant market differences and similarities. Namely, rheumatoid arthritis and inflammatory bowel disease, multiple sclerosis, and oncology have been chosen due to differences in the availability of subcutaneously dosed and ready-to-use self-administration products for mAb medicines and their follow-on biologics.
Different launch scenarios are described from a manufacturer’s perspective highlighting the necessity of platform approaches. To unfold the full potential of customer-centric care, value-based healthcare provider reimbursement schemes that incentivize the efficiency of care need to be broadly implemented.
Introduction
Over the past quarter century, biotherapeutics, such as monoclonal antibodies (mAbs), have become a prevailing novel treatment modality (Lu et al. 2020 ) and as such significantly contribute to both the costs (Hernandez et al. 2018 ) and the environmental impact (Amasawa et al. 2021 ) of healthcare. Inherent to their physicochemical properties, mAbs must be administered parenterally, a circumstance that can be considered inconvenient (Fernández et al. 2017 ) and frequently mandates more burdensome in-clinic dosing (Bohra et al. 2020 ).
In times of continuously growing cost pressure on healthcare and major implications of the pandemic on established medical services (Arsenault et al. 2022 ), any effort in minimizing the dosing complexity of parenteral administration has the potential to reduce expenditures for the drug administration procedure. For instance, if permitted by the safety profile of a biological medicine, an attempt to lessen in-clinic time during an intravenous dosing day is the provision of fast infusion regimens. This approach can improve cost-effectiveness of the treatment, as it allows more people to be treated in the clinic within a given time frame (Spadaro et al. 2017 ). To further facilitate intravenous dosing and to aid operators with obtaining central vascular access, new device types, such as a handheld assistive artificial intelligence-enabled, ultrasound-guided robotic device for intravenous catheterization, are currently undergoing early development (Brattain et al. 2021 ).
An alternative concept for optimizing hospital resource utilization that has been in the center of interest of many drug delivery researchers in recent years represents reformulating a biological medication for application via a less-invasive administration route. In 2014, Tetteh et al. ( 2014 ) developed a regression-based algorithm that included an estimate on how the administration route of biotherapeutics, including mAbs, may impact on healthcare delivery expenditures. The analysis suggests that assuming no other change (that is, in-clinic dosing, dosing frequency), subcutaneous or intramuscular administration of biologics lowers total healthcare delivery costs as compared to intravenous infusions.
A more significant step in reducing dosing regimen-related inconvenience, healthcare institutional spending and in improving affordability and access to mAb treatments is to shift dosing outside of the clinical setting (Wolfromm et al. 2017 ; Bittner et al. 2018 ; Bittner and Schmidt 2021 ). Here, subcutaneous at-home- and self-administration has become an established dosing regimen for mAbs with a favorable safety and tolerability profile across different disease areas (Gottlieb et al. 2016 , Raffaelli et al. 2019 , Van den Bemt et al. 2019 , Timmermann et al. 2020 , Jappe et al. 2021 , Bagel et al. 2022 ). Especially low-volume subcutaneous injections can be self-administered by means of a variety of prefilled syringes or pen device types, thus accounting for personal priorities and capabilities (Anderson and Redondo 2011 ; Vermeire et al. 2018 ). While these automated dosing aids support convenience with an at-home dosing regimen, adherence and persistence to subcutaneous administration in an unsupervised setting varies between medical products (Tkacz et al. 2014 ; Nieto et al. 2021 ). Besides disease severity or prior experience with subcutaneous administration, also the dosing schedule can contribute to compliance issues with the prespecified application procedures (Tkacz et al. 2014 ).
The situation is complicated specifically for high-dose mAbs requiring comparatively large administration volumes and for mAbs dosed in combination therapy. While user preference assessments demonstrate that even in the hospital setting, high-dose subcutaneous injections with individual dose volumes between 5 and 15 milliliters (mL) are preferred over intravenous infusion regimens (Pivot et al. 2014 ; Rummel et al. 2017 ; O'Shaughnessy et al. 2021 ; Usmani et al. 2021 ), customized dosing schemes for at-home administration still remain to be developed by manufacturers in close cooperation with healthcare providers and regulators. This is particularly the case for mAbs that exhibit severe infusion-related reactions (IRRs) mainly occurring during the first dosing cycles (Rombouts et al. 2020 ).
To improve adherence to parenteral dosing in a non-controlled setting, the individual demands of people treated with mAbs have to be accounted for by manufacturers. In addition to interventions that address educational, behavioral or psychological barriers against complying with the dosing regimen (Remington et al. 2013 ), providing personalized and thus customer-centric product presentations and administration schemes has the potential to enhance adherence to parenteral home administration overall (Ridyard et al. 2016 ).
The term customer centricity is applied across industries highlighting that in order to achieve sustainable product offerings, satisfying the needs of “the customer” has to be the ultimate focus for any development decision (Pardo-Jaramillo et al. 2020 ). For medicinal products, “the customer” is classically defined as “the patient,” but increasingly the entire healthcare ecosystem is referred to using this term. Thus, in addition to people receiving treatment for a diagnosed medical condition, this ecosystem comprises professional healthcare providers and institutions, regulators, payers (Pidun et al. 2021 ), and ultimately society as a whole.
In the pharmaceutical industry, efforts to ameliorate the product profile of an established medicine are driven by the cross-functional discipline of “Product Optimization” (Bittner and Schmidt 2022 ). “Product Optimization” also referred to as “Formulation and Device Lifecycle Management” aims at improving the drug delivery profile and product presentation for a medicine that is either already on the market or in late-stage clinical development and thus at providing a more customer-centric presentation.
This review is written from a manufacturer’s perspective and summarizes key trends in the healthcare ecosystem that define customer-centric drug delivery requirements for mAbs across different therapeutic areas, illustrates approaches to obtain insights into emerging drug delivery necessities, and compares the latest developments in three distinct disease area archetypes.

Drug delivery as treatment enabler versus as customer-centric differentiator
Drug delivery is commonly explained as the “method or process of administering a pharmaceutical compound to achieve a therapeutic effect” (Gupta and Kumar 2012 ; Tiwari et al. 2012 ). This definition predominantly refers to drug delivery technologies that enable the administration of pharmaceutical products per se. Such is particularly the case when developing formulation or device technologies for novel treatment modalities with previously unexplored physicochemical and pharmacokinetic properties. Frequently, in these instances, there is neither experience available on how a galenical formulation can impact bioavailability, safety or efficacy of the medication, nor on what would be the most preferred product profile from a customer perspective. Focus of drug delivery scientists is therefore on progressing a product presentation that achieves the required target exposure via an appropriate administration route. Especially with new and possibly disease-modifying biological molecules, technologies that go beyond treatment enablement may either have not yet been identified or are still in early development (Blanco and Gardinier 2020 ). Aspects of convenient dosing or efficient healthcare resource utilization are of secondary priority at this stage. Consequently, any formulation and device optimization that would delay initial molecule launch and thus availability of the medication for customers is typically introduced as a lifecycle management (Bittner and Schmidt 2022 ).
“Customer-centric” drug delivery, as defined in this article, becomes an important aspect for indications with a variety of more mature medications with similar physicochemical properties available from a given compound class. Here, treatment enabling technologies have been established over time (Shams et al. 2021 ). With emerging customer insights on needs for improving the product presentation, drug delivery efforts predominantly focus on differentiating the medication with a more convenient and cost-efficient profile (Roy et al. 2021 ; Schreiber et al. 2022 ).
In addition to achieving a user-oriented drug delivery profile, manufacturers engage in offering medicines with overall more sustainable presentations. Activities entail the implementation of measures to reduce drug wastage via more economic dosing regimens and supply chain concepts (Hendrikx et al. 2017 ; Tat and Heydari 2021 ).
Over time, and on the basis of progressively established platform technologies, this initially stepwise approach is emerging into a situation where novel products are available with the best feasible drug delivery profile from initial molecule launch onwards.
Healthcare trends that define customer-centric mAb product presentations
Today, people diagnosed with a chronic condition are increasingly well informed about particularities of their disease and willing to actively participate in the design of healthcare processes (Longtin et al. 2010 ). Resulting customer-centric concepts commonly include aspects of disease self-management (Holmes et al. 2019 ) and as such mandate measures that allow unsupervised parenteral dosing. In this case, providers, in order to support dosing in a remote setting, require reassurance that high-quality disease monitoring and adherence to treatment procedures is maintained. Assuming compliance and adequate support services are guaranteed for parenteral administration outside of a healthcare institution, the underlying societal benefits of at-home- and particularly the economic benefits of self-administration (Franken et al. 2020 ) are pivotal elements of value-based healthcare (Dainty et al. 2018 , Teisberg et al. 2020 ).
From a regulator’s perspective, mAbs suitable for home administration must possess an appropriate safety profile and must be available in a product presentation that supports unsupervised administration (Bittner and Schmidt 2022 ). As data derived from registrational clinical trial investigations alone may not exhaustively reveal feasibility of a flexible care setting, additional sources of information are being considered. In assessing the risk benefit of a medicinal product, regulators progressively encourage manufacturers to actively implement real-world data (RWD) and real-world evidence (RWE) during development processes and to share “patient-provided information” for evaluation as part of drug and medical device applications (United Stated Food and Drug Administration 2018 ). The United States Food and Drug Administration (FDA) defines RWD as “data relating to patient health status and/or the delivery of health care routinely collected from a variety of sources” and RWE as “the clinical evidence about the usage and potential benefits or risks of a medical product derived from analysis of RWD” (United States Food and Drug Administration 2022b ). According to the FDA, RWD sources can include registries, collections of electronic health records, or administrative and medical claims databases. Besides prescription information, medical diagnoses, bills submitted to payer organizations, costs, charges and reimbursement amounts, such databases also include insights into procedures and treatments performed (Rocco et al. 2017 ; Park and Lee 2021 ). Globally, clinical trial results are therefore supplemented by RWD and RWE (Hiramatsu et al. 2021 ). This evidence also serves as a source for estimating the impact of drug delivery modalities on healthcare resource utilization (Stearns et al. 2019 ).
Likewise, the application of robust RWE to supplement experimental evidence in coverage decisions is being considered globally (Facey et al. 2020 ). In a literature review on the US healthcare system conducted by Hampson et al. ( 2018 ), comparative clinical effectiveness and network meta-analysis for quantitative indirect comparisons were identified as pivotal sources for initial payer coverage and Heath Technology Assessment decisions. Here, “patient-reported data” may be used as a complimentary data source. For reassessments, that is reconsidering coverage, formulary placement or payment terms, as well as in the context of outcomes-based contracting, RWE can play a role in further defining the clinical or economic value of an intervention, beyond the evidence generated in the clinical trial setting. Notably, in their review, a number of challenges associated with the use of RWE for healthcare payment decisions were identified. These include aspects of reporting bias, incomplete data, lack of universally accepted methodological standards, lack of investigator expertise, or obsolete evidence hierarchies. Similar findings were made in a US payer interview conducted by Timbie et al. ( 2021 ). The evaluation identified the evidence from rigorous clinical trials as a prioritized source for assessing efficacy and short-term safety findings. Some payers, however, “felt that RWE was particularly helpful when the long-term durability of devices or rare adverse events were key considerations in coverage decisions”.
Figure 1 highlights key healthcare trends that inform customer-centric drug delivery needs for monoclonal antibodies. Insights reveal the need for technologies that enable dosing and data collection in a flexible care setting. Here, contingent upon the medication’s safety and tolerability, medication administration may take place in the clinic, a physician’s office, a community or infusion center or in the patient’s home (Bittner and Schmidt 2021 ).
Key healthcare trends that inform customer-centric drug delivery needs for monoclonal antibodies. Need for technologies that enable dosing and data collection in a flexible care setting (FCS)
Customer-centric product presentations that enable dosing in a flexible care setting
Reliable application of biotherapeutics in a flexible care setting depends on adequate user training and education and mandates treatment initiation under professional supervision (Highlights of Prescribing Information (HPI) Humira®, Hizentra®, Kesimpta®: United Stated Food and Drug Administration 2022a ). Establishing convenient drug delivery schemes and electronic data capturing tools permit physicians to make accurate treatment decisions while their patient is dosed outside of a controlled environment (Eun-Young 2017 ; El-Sappagh et al. 2019 ; Sebastian et al. 2019 ). Especially, if home dosing is facilitated with mechanisms to collect “patient-provided information,” such data could have the potential to reduce payer’s uncertainty around adherence and to complement value-based reimbursement models.
Beyond professional support services and electronic data capturing and monitoring tools, drug delivery improvements that reduce supply chain complexity, permit simple and intuitive drug administration, and facilitate medication storage and disposal represent a crucial element of pharmaceutical research and development.
Figure 2 illustrates drug delivery improvements for mAbs that reduce dosing complexity and enable dosing and data collection in a flexible care setting. Aspects include (1) improving the product presentation, (2) reducing the overall burden of parenteral drug administration, and (3) complementing combination therapy. In this order, product optimizations are expected to possess increasing potential to facilitate remote parenteral care.
Drug delivery improvements for mAbs that reduce dosing complexity and enable dosing and data collection in a flexible care setting
Attempts in advancing the product presentation entail optimizing storage conditions and shelf-life of the medication (Kuzman et al. 2021 ), as well as improving its packaging to account for an easy to store and to handle offering (Zadbuke et al. 2013 ). Product optimizations that lessen the burden associated with parenteral administration of biotherapeutics comprise fast intravenous infusion regimens (Al Zahrani et al. 2009 ), fixed dosing regimens instead of body size-adjusted dosing (Egorin 2003 ), subcutaneous dosing alternatives to more invasive intravenous infusions (Bittner et al. 2018 ), or automated injection devices (Vijayaraghavan 2020 ). Connected devices and accompanying health apps are being implemented to support adherence in an out-patient setting and can be utilized to collect RWE on possible adverse events and share it back with the treating physician (Bittner et al. 2019 ). Drug delivery improvements to complement combination therapy involve the development of fixed-dose combinations with two or more mAbs co-formulated in the same dosing vehicle, or dual chamber bags (Allmendinger 2021 ). Details on underlying concepts and scientific nonclinical and clinical development approaches have been summarized previously and are therefore off-scope for this article (Bittner and Schmidt 2022 ).
Gaining insights into customer needs for product optimization of biological medicines
To inform investments into customer-centric product optimizations for existing medicines, manufacturers regularly collect insights on possible challenges with their medications’ drug delivery profile. For marketed mAbs or for biologics in development in an indication with similar treatment modalities already employed, information on how the product might be improved is typically already available from secondary sources and thus can be estimated based on existing analogues. Notably, customers’ desires evolve with the maturity of a market based on emerging sophistication of drug delivery technologies and comparisons with other indications with biotherapeutics with similar drug delivery requirements. The journey of product optimizations is therefore flexible and adaptable to change at all times.
An understanding of user and provider preferences for one over another drug delivery methodology is gained either as part of pivotal registrational clinical trials or in smaller dedicated usability studies (Li and Easton 2018 ). Here, electronic apps with dosing reminders and tools to collect “patient-reported outcomes” in the home setting (El Emam et al. 2009 ) provide early insights into possible challenges and opportunities of a product optimization. Additionally, treatment satisfaction and quality-of-life surveys (Kempton et al. 2021 ), as well as time and motion studies (De Cock et al. 2014 ; Pivot et al. 2014 ) that assess the impact of the drug administration procedure on the efficiency of dosing in a prespecified setting represent an appreciable source of information.
For marketed medications and relevant reference products with a similar drug delivery profile in other indications, insights into the practicality of the drug delivery profile are frequently derived from direct user or provider feedback to the manufacturer of a medicinal product. This encompasses anecdotal reports on challenges with the drug administration instructions and at times even suggestions on how to improve these. Input is additionally tracked via established complaint management processes (Hake et al. 2019 ) in which customer feedback is collected and reviewed systematically over time. Verifiable customer co-creation (Adelman et al. 2019 ; Peng et al. 2022 ) may be realized via satisfaction surveys or customer workshops designing the most appropriate drug delivery system for a drug and as part of well-defined human factor trials (Lageat et al. 2021 ).
Key developments in providing customer-centric product presentations for mAbs across different disease areas
Disease area archetypes.
The availability of product presentations and dosing regimens that support administration of mAbs in a flexible care setting varies considerably across indications. Three different disease area archetypes have been selected to describe the status and key developments on the journey to increasing customer centricity of mAb medications. Table 1 illustrates the relevant attributes of these different markets. Disease areas comprise rheumatoid arthritis (RA) and inflammatory bowel disease (IBD), multiple sclerosis (MS), and oncology (ONC).
Disease area archetype 1 (rheumatoid arthritis and inflammatory bowel disease)—mature markets with a variety of established mAb treatments and corresponding follow-on biologics available for self-administration
Disease area archetype 1 encompasses RA and IBD, representing mature subcutaneous self-administration markets with established originator mAbs and other long-standing biological treatments and corresponding biosimilars either already approved or in development (De Figueiredo et al. 2021 ; Findeisen et al. 2021 ; Radu and Bungau 2021 ). Authorized mAb treatments for both RA and IBD include the tumor necrosis factor-α inhibitors infliximab, adalimumab, golimumab, and certolizumab pegol. The B-cell-depleting therapy rituximab and the interleukin-6 receptor antagonists tocilizumab and sarilumab are indicated for the treatment of RA (Senolt 2019 ). The interleukin-12 and interleukin-23 inhibitor ustekinumab, the α4β7 integrin antagonist vedolizumab, and the α4 integrin antagonist natalizumab are authorized for the treatment of IBD (Mao et al. 2018 ; Wyant et al. 2016 ).
In 1998, the chimeric mAb infliximab was the first tumor necrosis factor-α inhibitor authorized by the FDA for the treatment of Crohn’s disease (CD) (Melsheimer et al. 2019 ). Branded infliximab was and is still solely available with an intravenous dosing regimen as a powder for reconstitution (HPI Remicade®: United Stated Food and Drug Administration 2022a ). The first fully human recombinant immunoglobulin G1 mAb in RA, adalimumab, was already introduced with a subcutaneous dosing regimen at initial product approval in 2002 (Marušić and Klemenčić 2018 ). The majority of branded injectables in the field are available with either both an intravenous and a subcutaneous formulation (golimumab, tocilizumab, ustekinumab, vedolizumab; HPIs Simponi®, Simponi Aria® Actemra®, Stelara®, Entyvio®: United Stated Food and Drug Administration 2022a , SMPC Entyvio®: European Medicines Agency 2021 ) or a subcutaneous formulation only (adalimumab, certolizumab pegol, sarilumab; HPIs Humira®, Cimzia®, Kevzara®: United Stated Food and Drug Administration 2022a ). The fixed-dose regimens omit the need for body size-normalized dose calculation. Subcutaneous injection volumes do not exceed 2 mL and dosing frequencies range from weekly to every 4 weeks. The subcutaneous administration regimen for ustekinumab consists of an intravenous loading dose followed by every 8 weeks subcutaneous maintenance doses. Ready-to-use prefilled syringes or pen devices provide users with different dosing alternatives as per their personal requirements. Branded natalizumab, infliximab, and rituximab can only be given intravenously with maintenance dosing frequencies between every 4 weeks and every 6 months (HPIs Tysabri®, Remicade®, Rituxan®: United Stated Food and Drug Administration 2022a ). These infusion regimens represent an alternative for people who prefer intravenous over subcutaneous dosing (Allen et al. 2010 ) or value a lower treatment frequency independent of the administration route (Huynh et al. 2014 ). In the clinic, less frequent dosing can be a contributor to a more convenient and cost-efficient treatment management scheme (Tetteh and Morris 2014 ), especially if medical examinations can be combined with a dosing day. Most notably, the fact that a variety of injectable medications are approved both for the treatment of RA and IBD increases healthcare provider’s general familiarity with an injection device type and offers the possibility for leveraging learnings on challenges with the injections (Chilton and Collett 2008 ; Domańska et al. 2017 ; Gely et al. 2019 ) across indications. Table 2 summarizes the mAb presentations authorized for the treatment of adults diagnosed with RA or IBD (CD and ulcerative colitis (UC)).
Prominent customer-centric product optimizations in disease area archetype 1 include changing the composition of the subcutaneous formulation for adalimumab. Accounting for "patient-reported pain" immediately following injection, the manufacturer changed the chemical buffer to help stabilize and preserve the mAb. This seemingly small change was shown to result in a reduction of pain at the injection site and ultimately in a significantly improved adherence and time on treatment overall (Bergman et al. 2020 ; Patel and Luu 2020 ). The finding is all the more important as decreased persistence to anti-tumor necrosis factor therapy had been reported to be associated with poorer clinical outcomes (Bluett et al. 2015 ). Additional product optimizations introduced for branded adalimumab comprise a reduced injection volume with a higher concentrated dosing solution, higher needle gauge, or modifications in the material of the injection devices (St Clair-Jones et al. 2020 ).
With the aim to optimize experience with the dosing procedure, to reduce the fear associated with needle use, and to aid people with impaired dexterity, in 2016, certolizumab pegol’s product presentations were complemented with a button-free autoinjector characterized by a wide, non-slip grip (Bailey et al. 2020 ). Supported with adequate training, the device could improve user confidence and satisfaction with subcutaneous self-administration. The introduction of a mini cartridge to be applied by means of a reusable autoinjector for the biologic etanercept in 2017 (Collier et al. 2017 , Sedo 2018 ) may in the future also serve as a platform for mAbs. The product also utilizes an improved dosing solution that had been shown to lessen injection site pain as compared to the previous formulation (Cohen et al. 2019 ). Preference assessments comparing the novel reusable with the existing disposable automated pen device revealed perceived advantages for both injection aids, thus giving users the choice between two devices according to personal priorities (Collier et al. 2017 ).
To further facilitate compliance with at-home dosing, companies are implementing so-called patient support programs and app-based assistance tools including customized dosing reminders or injection and symptom trackers (Graigner et al. 2017 , Lambrecht et al. 2021 ). Branded certolizumab pegol offers the option to apply the first partially reusable electromechanical injection device “of its kind available for use with biologic treatment in rheumatology and dermatology in Europe” (UCB 2021 ). Device design was actually guided by intended user feedback through human factor evaluations (Domańska et al. 2018 ). The injector was found to be preferred due to its ease-of-use over other subcutaneous devices in a study with certolizumab pegol-treated people from the Netherlands, Denmark, and Sweden (Pouls et al. 2020 ).
Manufacturers of follow-on biologics for mAbs in RA and IBD focus efforts on either using established injection device platforms or on customizing technologies to differentiate their products via unique, distinctive drug delivery characteristics. As for the branded counterparts, user and healthcare provider satisfaction and usability studies are part of the autoinjector development and commercialization strategy (Thakur et al. 2016 ; Tischer and Mehl 2018 ; Fleischmann et al. 2022 ). This iterative co-creation with customers is particularly important in disease areas in which people report problems with manual dexterity, pain linked to joint swelling in the hands, and general challenges with the self-injection procedure (Keininger and Coteur 2011 ).
Celltrion’s infliximab biosimilar received European Medicines Agency (EMA) approval for a subcutaneous dosing alternative in 2019 and FDA review is anticipated to be completed as a next milestone (Rose 2021 ; Verma et al. 2021 ), while branded infliximab is only available with an intravenous infusion regimen. This subcutaneous self-administered infliximab product presentation paired with telemedicine support and increasingly available RWD is suggested to lessen the time spent for travel and hospital attendance during dosing days and as such to reduce the pressure on healthcare systems (Ahmed et al. 2021 ; Perry and Jang 2020 ; Schreiber et al. 2022 ).
In aspiring to relieve the burden of parenteral dosing, the feasibility of oral dosing of mAbs is being examined (Philippart et al. 2016 ; New 2020 Abramson et al. 2022 ). Different to injectable dosage forms, the individual dose level that can be administered orally is markedly reduced due to limited fill volumes of ingested oral dosage forms. Consequently, this approach is particularly interesting for mAbs in immunology, as inherent to their comparatively low-dose levels, a practicable oral dosing frequency may be achievable.
Different oral delivery technologies have recently advanced to clinical investigational stage. The first approach aims at precise delivery of biotherapeutics to gastrointestinal tissue thus avoiding high systemic exposure and potentially associated side effects. Here, biosimilar infliximab is being assessed for the feasibility of an oral version in the treatment of IBD. The aim is to target release in the colon and to protect the mAb from digestion in the stomach and upper gastrointestinal tract through local stabilization against proteases (Intract Pharma 2022). A second advanced oral delivery approach utilizes an orally ingestible robotic pill that auto-injects the biotherapeutic into the wall of the small intestine (Dhalla et al. 2022 ). The authors report that in an initial clinical trial with octreotide in healthy participants, administration of the pill was safe, well-tolerated, and yielded in an oral bioavailability of 65%. Assuming the scientific concept is confirmed in larger clinical trials and treatment can be realized at commercializable dose levels and dosing regimens, this approach has the potential to provide new clinical strategies in the future (Zhang et al. 2021 ).
Disease area archetype 2—market with a small number of mAbs established for in-clinic or self-administration and no corresponding biosimilars available
MS, the second disease area archetype, is characterized by established non-mAb biological disease-modifying treatments available for subcutaneous self-administration. The majority of mAbs is offered with an intravenous infusion regimen, but the first subcutaneous mAb for self-administration has recently reached the market. To date, no biosimilar mAb is available in the US (United States Food and Drug Administration 2022c ).
More precisely, subcutaneous self-administration with interferon beta (IFNβ) indicated to treat relapsing forms of MS is an established standard in the field (Kieseier 2011 ; Filipi and Jack 2020 ). Back in 1993, the first IFNβ was approved in the US and since then several others have become available (Bayas and Gold 2003 ). Due to their fixed dosing regimens and low injection volumes, IFNβ products are available in ready-to-use prefilled syringes and autoinjectors including devices with electronic adherence aids (Limmroth et al. 2017 ). IFNβ drug administration regimens range from every second day to every second week for the pegylated version that was authorized by the FDA in 2014 (Dashputre et al. 2017 ). In a German real-world study from 2021, this less frequent dosing alternative showed markedly higher scores for treatment satisfaction and convenience compared with previous therapies that included other IFNβ treatments (Menge et al. 2021 ).
The first mAb, natalizumab, an α4 integrin antagonist, entered the MS market in in 2004 with a fixed dose infused intravenously every 4 weeks over 1 h, and by now is available for the treatment of clinically isolated syndrome (CIS), relapsing–remitting MS (RRMS), and active secondary progressive MS (SPMS) (Rudick et al. 2013 , HPI Tysabri®: United Stated Food and Drug Administration 2022a). While people with prior use of subcutaneous interferon regimens commonly value the option for self-administration, especially when facilitated with automated injection devices (Lugaresi et al. 2012 ), the improved efficacy of natalizumab over IFNβ therapy (Rudick and Panzara 2008 ; Lanzillo et al. 2012 ) is considered to outweigh the convenience disadvantage of more invasive intravenous dosing.
In 2014, the FDA approved alemtuzumab, an anti-cluster of differentiation 52 (CD52) mAb, for the treatment of RRMS (Ruck et al. 2015 ). The medicine is available with a fixed-dose intravenous regimen for two treatment courses. During the first treatment course, alemtuzumab is administered over 4 h on five consecutive days and on three consecutive days during the second treatment course 12 months later. Additional treatment courses may be considered with drug administrations of three consecutive days (HPI Lemtrada®: United Stated Food and Drug Administration 2022a ). This comparatively convenient infrequent dosing regimen is to some extent counterbalanced by the need for regularly monitoring the increased risk of autoimmunity (Garnock-Jones 2014 ). While alemtuzumab when delivered via the subcutaneous route may reduce infusion-related adverse events as compared to intravenous dosing (Perumal 2012 ), a subcutaneous formulation is not available for use in MS.
The anti-CD20 mAb ocrelizumab was first authorized in the US in 2017 (Frampton 2017 ) and by now is applied for the treatment of relapsing forms of MS (RMS), including CIS, RRMS, PPMS, and for the treatment of SPMS (Stahnke et al. 2018 , Weinstock-Guttman et al. 2022 , HPI Ocrevus®: United Stated Food and Drug Administration 2022a ). The mAb was introduced with an intravenous fixed-dose regimen. Here, the initial treatment cycle comprises two separate infusions on days 1 and 15, respectively, followed by twice yearly maintenance doses. The United Kingdom’s (UK) National Institute for Health and Care Excellence (NICE) noted in their final appraisal document on “Ocrelizumab for treating relapsing–remitting multiple sclerosis” that based on insights from “patient experts” “patients would value a treatment with less frequent dosing or monitoring,” acknowledging that the intervention is less interruptive for people’s lives compared to other treatments (National Institute for Health and Care Excellence 2018 ).
To optimize satisfaction and quality of life with intravenous mAb treatments and to improve healthcare institutional resource utilization in MS, efforts are made to support home-based and outpatient infusion management (Vijayan et al. 2017 ; Schultz et al. 2021 ; Barrera et al. 2022 ; Räuber et al. 2022 ). It was found that people are generally open to receiving the intravenous treatment at home and that supporting health services need to ensure safety and be efficient, responsive, and flexible. Thus, health services should also allow for administering the medication at individually preferred times during the day (Rath et al. 2021 ). Supporting measures include designing appropriate home health care services for natalizumab or shortening ocrelizumab’s intravenous infusion time from 3.5 to 2 h (Schultz et al. 2019 ; Hartung et al. 2020 ).
In 2020, a second anti-CD20 mAb, ofatumumab, was authorized by the FDA for the treatment of CIS, RRMS, and active SPMS (HPI Kesimpta®: United Stated Food and Drug Administration 2022a ). Notably, the mAb was directly introduced with a subcutaneous formulation for self-administration, a fixed dose, and a dosing volume of 0.4 mL. Using an existing autoinjector platform that was previously applied to other products of the same manufacturer (HPIs Cosyntex®, Elrezi®, Hyrimoz®: United Stated Food and Drug Administration 2022a ), ofatumumab was launched both in a prefilled syringe and in an automated pen injector. In its final appraisal document on “Ofatumumab for treating relapsing multiple sclerosis,” the UK’s NICE notes that they heard from “patient experts” “that a treatment that could be self-administered monthly is less disruptive to people’s lives than treatments administered by intravenous infusions in hospital, so would be valued by people with multiple sclerosis” (National Institute for Health and Care Excellence 2021 ). Launching a mAb in two different presentations accounts for distinct preferences (Kivitz et al. 2018 ; Vermeire et al. 2018 ) already at first introduction of the novel medicine. Additionally, a manufacturer-initiated study revealed user and nurse preference for the autoinjector over their current injectables mainly due to the “ease to perform self-injection with the pen” and “patient able to use independently” (Ross et al. 2021 ).
In 2021, a subcutaneous version of natalizumab with an overall shorter infusion time as compared to the intravenous regimen received marketing authorization in the EU (Summary of Product Characteristics (SMPC) Tysabri®: European Medicines Agency 2021 , López et al. 2021 ). The product is available in prefilled syringes, two of which need to be administered at each dosing day with a monthly dosing regimen; home treatment is not recommended. In the same year, the manufacturer did receive a complete response letter (CRL) from the FDA to their supplemental Biologic License Application for the subcutaneous dosing alternative (BioSpace 2021 ); the underlying reasons for the CRL are unknown to the author of this review. Also, a subcutaneous dosing alternative in development for ocrelizumab has reached Phase 3 clinical development stage (clinicaltrials.gov 2022 ). Table 3 summarizes the mAb presentations authorized for the treatment of adult people diagnosed with MS.
Disease area archetype 3—market with variety of established mAb treatments for healthcare provider administration and a number of corresponding biosimilars available
The ONC area represents the third selected disease area archetype. Here, mAbs for the treatment of malignancies have been on the market for decades, but due to at times severe IRRs and frequently high individual mAb dose levels, products are not yet available for self-administration. Until recently, due to the lack of technologies that facilitate high-dose subcutaneous administration, mAb products were offered as intravenous infusions only. Today, a number of subcutaneous dosing alternatives have been established. The first biosimilar mAbs have been authorized, currently with intravenous dosing regimens only.
More specifically, since the approval of rituximab for the treatment of B-cell malignancies back in 1997 (Pierpont et al. 2018 ), numerous other mAbs have become available and represent an important modality in the treatment of cancer (Zahavi and Weiner 2020 ). The large majority of these mAbs are authorized for intravenous administration and need to be administered by a healthcare professional (Kafatos et al. 2020 ). Depending on the nature and severity of IRRs, in some instances patients have to be monitored closely to provide medical treatment when required (HPIs Rituxan®, Erbitux®: United Stated Food and Drug Administration 2022a , Graham 2009 ). Understandably, these significant drug administration efforts add to the already high expenditures for mAb treatments overall (Chadda et al. 2013 ).
As described for intravenous treatments in MS, also in cancer care, rapid infusion regimens (Atay et al. 2012 , Gozzetti et al. 2020 ) or less frequent dosing regimens (Lala et al. 2020 ) represent an attempt to reduce the expenditures associated with drug administrations as well as the time people treated with mAbs have to spend in the clinic. Here, the pandemic has intensified the elaboration of clinical strategies for optimizing infusion center care (Hanna et al. 2021 ). The organization of home health services is a pivotal step to reduce time and traveling expenditures associated with in-clinic dosing. Notably, efforts are mandated to ensure that costs underlying provider work supporting at-home dosing and monitoring efforts remain within an affordable range (Franken et al. 2020 ).
Initially, mAbs in ONC were made available with a body weight- or body surface area-adjusted dosing regimen (Hendrikx et al. 2017 ); an approach that was based on the way cytotoxic agents with a narrow therapeutic window are being administered (Egorin 2003 ). With the increasing understanding of the pharmacokinetic-pharmacodynamic and -safety correlation (Paci et al. 2020 ), attempts are made to either develop mAbs with a fixed dosing regimen from the very beginning (Garg et al. 2014 ) or to change from body size-based dosing to fixed dosing as a lifecycle management activity following initial launch (Freshwater et al. 2017 ; Bei et al. 2020 ).
The subsequent step towards more customer-friendly drug delivery of mAbs in ONC represented the development of subcutaneous dosing options for mAbs (Bittner and Schmidt 2012 ). Immanent to the at times high individual dose levels, compared to mAbs in immunology for example (refer to disease area archetype 1), developing subcutaneous injection regimens was initially complicated due to a number of technical challenges. With the introduction of methodologies to achieve high-concentration solutions (Mahler et al. 2009 ; Jiskoot et al. 2022 ) and the co-administration of the dispersion enhancer hyaluronidase (Frost 2007 ), the first moves were made to reduce the overall dosing volume and to facilitate spreading of an injected fluid in the interstitial space.
Approved high-volume subcutaneous treatments that apply these technologies can maintain the infrequent dosing regimen of the initially marketed intravenous presentations. Up until September 2022, the subcutaneous administration alternatives for rituximab in B-cell malignancies (11.7 and 13.4 mL; FDA approval in 2017), trastuzumab in HER2-positive early and metastatic breast cancer (5 mL, FDA approval in 2019), and daratumumab in multiple myeloma (15 mL; FDA approval in 2020) have been authorized in the US (Yelvington 2018 , Center for Drug Evaluation and Research 2020 , Duco 2020 , Kading and Beck 2021 ). These subcutaneous mAb presentations are all available with fixed-dose regimens omitting the need for body size-adjusted dose calculation. Dosing solutions are offered in vial presentations and are injected manually by a healthcare provider using a handheld syringe or an infusion set.
To simplify administration of subcutaneous trastuzumab, a ready-to-use on-body delivery system that is attached to the skin via an adhesive plaster had been developed (Bittner et al. 2012 , Gligorov 2022 ). A small study in 102 participants diagnosed with HER2-positive early breast cancer revealed that subcutaneous at-home injections by a healthcare professional did not introduce new safety signals and respondents agreed that they had benefit from at-home administration to a large (22%) or very large extent (78%) (Denys et al. 2020 ). Time-and-motion and preference assessments demonstrated user preferences of subcutaneous over intravenous dosing in a healthcare institutional setting, regardless of on-body delivery system or handheld syringe delivery (Pivot er al 2014 ). As trastuzumab is not permitted for home- or self-administration, the device was not commercialized at the time of marketing authorization of the subcutaneous trastuzumab formulation in the EU back in 2013.
The aspect that mAbs are increasingly developed for combination therapy (Henricks et al. 2015 ; Peterson et al. 2018 ), a condition that further adds to the complexity of parenteral dosing, makes ONC an intriguing disease area archetype from a drug delivery perspective. Consequently, manufacturers started co-formulating two mAbs within the same dosing vehicle as a fixed-dose combination. The first fixed-dose combination of two mAbs included pertuzumab and trastuzumab and is indicated for the treatment of people diagnosed with with HER2-positive early and metastatic breast cancer. The medication is available with a subcutaneous dosing regimen and has been approved by the FDA in 2020 (Gao et al. 2021 ). It had been shown that patients strongly preferred this fixed-dose combination over sequential intravenous infusion of the individual mAbs in separate formulations (O'Shaughnessy et al. 2021 ). Remarkably, when approving Phesgo, in its press release, the FDA specifically highlights that “…Phesgo offers an out-patient option for patients…” (United States Food and Drug Administration 2020 ), an aspect that is considered very relevant especially in times of the coronavirus pandemic. The first fixed-dose combination of two immunotherapy mAbs, the programmed death receptor-1 inhibitor nivolumab and the lymphocyte activation gene-3 blocking antibody relatlimab, received FDA approval for the treatment of unresectable or metastatic melanoma in 2022 (HPI OpdualagTM: United Stated Food and Drug Administration 2022a ). The formulation is administered as a fixed-dose intravenous infusion regimen.
Table 4 summarizes the high-dose subcutaneous single-active mAb formulations and fixed-dose combinations authorized for the treatment oncological indications in the US.
The initial follow-on biologics for mAbs in ONC indications have been approved by the FDA (Galvão 2020 ). Not only have manufacturers mimicked the originator medications, in some cases they also optimized the product presentation to make it more user-friendly. Improvements include extending the in-use stability to mitigate the impact of cold-chain rupture and exceptional temperature excursions on drug wastage and the quality of the product (Vieillard et al. 2017 ; Park et al. 2020 ). Even without the implementation of product optimizations, biosimilars are considered a customer-centric alternative to their branded counterparts solely based on the potential to increase access to mAb-based cancer medicines globally via reduced product costs compared to the originator mAb (Patel et al. 2018 ; Shelbaya et al. 2021 ). Here, interestingly, actual realization of a switch from branded to follow-on biologic or a switch from one to another biosimilar varies significantly from country to country. Regional differences, such as prescriber and/or patient insecurity concerning efficacy and safety, conservative prescribing patterns, reimbursements and billing policies, supply logistics, and legal considerations have been suggested as limiting factors to broader adoption of biosimilars (Cortes et al. 2020 ; Azuz et al. 2021 ).
The term “customer centricity,” indicating that fulfilling customer demands is as important as creating the product or services themselves (Ceesay 2020 ), is not new and applied across various industries. It has, however, gained increasing attention in healthcare facing high economic pressure, especially in light of the coronavirus pandemic. Here, customer centricity aims at developing convenient medicines that are globally affordable for people, providers, and the healthcare system as whole. For biotherapeutics, such as mAbs, customer-centric product offerings and treatment management concepts ideally facilitate a flexible care setting and thus allow for drug administration and treatment monitoring outside of a controlled healthcare institutional environment. Efforts in the field go beyond the described improvements of the drug delivery profile and product presentation and are in many cases driven by pharmaceutical scientists from different disciplines.
The realization of product optimizations differs across the distinctive disease area archetypes and depends on customer and market needs as well as on the clinical and technical feasibility of the intervention. In an indication in which the identification of disease-modifying medicines represents a major unmet need, initial drug delivery efforts focus on enabling treatment per se. This is for example the case for mAbs in ONC where any new and promising molecule is developed with the aim to offer it to people diagnosed with a given malignancy as soon as feasible. Equally, for mAbs with demonstrated efficacy but unfavorable exposure-related safety findings, product optimizations target a reduction in the incidence and severity of adverse events through lowering post-infusion serum levels. This may be achieved for example by increasing the dosing frequency (Bai et al. 2012 ), a schedule change that reduces convenience and increases healthcare institutional burden associated with shorter dosing intervals.
As per the definition in this review article, the journey to more customer-centric products starts with the availability of an efficacious product with an acceptable risk/benefit ratio. Across the selected disease archetypes, the existence of established drug delivery technology platforms plays a pivotal role in enabling customer-centric product presentations early on, ideally already at initial launch of the mAb. Combining the learnings from these distinct disease area archetypes, Fig. 3 illustrates possible launch scenarios for intravenous and subcutaneous dosing regimens for mAbs. The underlying assumption is that mAbs with dosing volumes of up to approximately 2 mL are conventionally dosed in prefilled syringes and automated pen or autoinjector devices, while higher volume mAbs are provided in vial presentations and possibly in the future in larger automated pen and autoinjector devices or in automated on-body delivery systems.
Possible launch scenarios for intravenous versus subcutaneous dosing regimens for mAbs
In scenario 1, at initial molecule launch, the mAb is available with an intravenous dosing regimen for in-clinic, in-office, or healthcare provider-supervised at-home administration. As a subsequent lifecycle management, a subcutaneous dosing alternative is becoming available, either in a prefilled syringe for self-administration or in a vial presentation for manual or infusion pump-assisted injection by a caregiver. Product presentations vary from case to case depending on the dosing volume and overall feasibility of a stable liquid solution. Most frequently, medications are offered with a fixed dose, unless a body size-, safety-, or response-dependent regimen is justified (Strik et al. 2018 ). In a third step, the manufacturer introduces automated injection devices. This scenario was for example realized for tocilizumab in RA. Initially approved in 2010 with a vial for intravenous infusion and a monthly regimen (HPI Actemra®: United Stated Food and Drug Administration 2022a ), the subcutaneous dosing alternative was made available sequentially with a prefilled syringe in 2013 (Burmester et al. 2014 , Shetty et al. 2014 ), followed by an autoinjector device as a lifecycle management in 2018 (Genentech 2013 ). To keep the injection volume low and account for readily available devices at the time, the subcutaneous dosing frequency was increased to weekly and every 2 weeks with a dosing volume of 0.9 mL (Fettner et al. 2019 ).
Scenario 1 also applies for mAbs in the treatment of malignancies. While intravenously dosed mAbs are an established treatment modality, development of subcutaneous dosing alternatives started only years after the first mAb approval (Salar et al. 2014 ; Jackisch et al. 2019 ). This is due to at times severe and even fatal IRRs and comparatively high individual dose levels that challenged the development of convenient subcutaneous dosing regimens. With increasing knowledge about the general feasibility of high-volume subcutaneous dosing, advances in high-concentration formulations and the co-administration of the dispersion enhancer hyaluronidase, by now, this route of administration has become a key focus area of drug delivery scientists across indications (Bookbinder et al. 2006 ; Mathaes et al. 2016 ). While in ONC, mAb dosing in the clinic is standard practice, today, efforts in disease management support are made to shift treatment and monitoring outside of the clinic (Denys et al. 2020 ). Independent at-home administration for high-dose mAbs has not yet been realized, but represents a thinkable option in the future with the advancement of larger volume on-body delivery systems (Bittner and Schmidt 2021 ).
Notably, next to a lack of technical and clinical feasibility, it is also the healthcare provider reimbursement model applied in a given legislation that challenges subcutaneous administration in a decentralized setting. Roughly speaking, one can discriminate between fee-for-service payment models with separate service-specific payments and models where a medical provider receives a predetermined payment for a sequence of related healthcare services (Einav et al. 2022 ). From a drug delivery perspective, whereas the first model incentivizes the complexity and quantity of care and as such incentivizes more complex intravenous infusions, the latter rewards the quality of care and thus ready-to-use subcutaneous regimens with the potential for at-home administration. Here, any efforts that facilitate shifting treatment outside of the clinic are usually valued.
In launch sequence scenario 2, the mAb enters the market directly with a subcutaneous formulation. Currently, the prerequisite for this approach is that doses are low enough to apply established formulation and device technologies. Products are offered either in a prefilled syringe or vial configuration. Subsequently, upon availability, automated injection devices are introduced as a lifecycle management. This scenario is depicted in how a number of mAb products were introduced into the RA and IBD indications. The sequential market introduction of increasingly optimized product presentations did allow manufacturers to consider user feedback on the selection of a ready-to-use device. The device portfolio was subsequently expanded with additional product offerings for people who prefer one over another injection aid. Examples would be branded golimumab or adalimumab. Golimumab was first approved in the US with a prefilled syringe in 2009 (HPI Simponi®: United Stated Food and Drug Administration 2022a ), followed by the introduction of an autoinjector 4 years later in 2013 (Center for Drug Evaluation and Research 2013 ). This device was favorably evaluated in a prospective study in biologic-naïve people with active RA (Schulze-Koops et al. 2013 ). Likewise, adalimumab was first authorized with a prefilled syringe specifically designed for self-administration for people with stiffness in their hands due to destructive progression of RA as well as with a vial for institutional use in 2002 (HPI Humira®: United Stated Food and Drug Administration 2022a ). Approval of the automated pen device followed sequentially in 2006 (BioSpace 2006 ). A comparison of the pen device with the established prefilled syringe as assessed in a Phase 2 trial in participants diagnosed with RA revealed preference for the automated injector based on its perceived ease of use and resulting convenience (Kivitz et al. 2006 ).
The framework underlying scenario 3, where mAbs are directly and solely launched with a subcutaneous dosing regimen presented in ready-to-use automated injectable presentations, to date comprises mAbs and their follow-on biologics with low dosing volumes that are generally well tolerated. These favorable features enable the use of established drug delivery platforms. Currently, this scenario is being realized for adalimumab follow-on biologics (Ghil et al. 2019 , HPIs Hulio®, Hadlima: United Stated Food and Drug Administration 2022a ). Another example for scenario 3 is the introduction of ofatumumab in MS, where the mAb was directly obtainable with both a prefilled syringe and an automated injection pen. Leveraging an established autoinjector platform, the manufacturer conducted the pivotal Phase 3 study for ofatumumab with a prefilled syringe and bridged to the autoinjector in a Phase 2 trial that demonstrated bioequivalence of ofatumumab administered by the autoinjector versus the prefilled syringe (Bar-Or et al. 2022 ).
A comparison of activities among biosimilar manufacturers qualifying as customer-centric as defined in this article did reveal different focus areas across the designated disease area archetypes. Especially in the event of more than one follow-on biologic accessible for the same originator, the competition for market shares via a customized product profile is expected to increasingly gain momentum. With this, the wider range of injection devices qualified will contribute to fulfilling the needs of a larger user population. Here, the application of established technology platforms for a variety of medications accounts for both, the familiarity of prescribers with the device as well as learnings from challenges associated with their application and how to most appropriately overcome these. For mAbs predominantly available for in-clinic mAb administration, the described seemingly smaller product changes, such as a change from a lyophilized powder for reconstitution to a ready-to-administer liquid formulation or an increased storage time and shelf-life can be an advantage for one over another biosimilar. This is due to for instance improved distribution and handling logistics, reduced drug wastage or more economical resource utilization in the clinic (Smale et al. 2021 ). It is of note in the context of customer-centric biosimilar offerings that depending on the country and associated pricing, reimbursement, and demand-side policies (Rémuzat et al. 2017 ), lower overall treatment costs per se may provide an access advantage over their originator counterpart (Kvien et al. 2022 ) without the need for further optimizing the product profile. In an attempt to facilitate access to treatment, the FDA has designated the first mAb, an adalimumab biosimilar, as interchangeable with the reference product in 2021 (United States Food and Drug Administration 2021 ), meaning that the biological product “may be substituted for the reference product without the involvement of the prescriber” (United States Food and Drug Administration 2017 ).
Summary and outlook
Advancing customer-centric medicinal products is an adaptive process across the lifecycle of a mAb-based medicine that aims to address individual needs of people treated as well as those of the healthcare ecosystem as a whole. The actual realization of product optimizations is in turn influenced by the safety and efficacy profile of a medication, market maturity, and the availability of enabling technologies. Here, the application of platform technologies that have the potential to be utilized for mAbs across different indications offer the possibility to launch a novel mAb already with the most preferred drug delivery profile or even with a variety of different customized options at initial market authorization. This will be especially the case for disease areas in which mAb-based medicines currently are among the investigated targets, such as Alzheimer’s disease or rare diseases (Tambuyzer et al. 2020 ; Lacorte et al. 2022 ).
Subcutaneous at-home administration of low-volume mAb formulations has been feasible for decades and illustrates the long-standing efforts in the field. As a next pivotal step to also warrant high-volume subcutaneous home administration with dosing volumes exceeding 5 mL, on-body delivery systems need to leave the exploratory stage and require implementation into clinical practice. Electronic adherence aids that further engage people treated with mAbs and their care partners into disease management while still guaranteeing a remote contact with the physician should be implemented in parallel. A significant change in dosing paradigm for mAbs would be a shift from parenteral to oral administration, with a variety of technologies in early development. A key prerequisite here is that drug administration schedules can still be managed based on the mAb’s dose level and the cost of goods sold associated with the provision of these at times device-based technologies. Manufacturers need to partner with specialized biotechnology companies and, like with any innovation, need to afford some upfront investment at risk. This way oral delivery platforms may become a reality for mAbs across disease areas.
A field of increasing relevance is the sustainability of novel drug delivery technologies. Manufacturers will have to do their homework to understand whether for example reusable technologies indeed offer a more environmental dosing alternative, or whether possible advantages come with the challenge of reduced user-friendliness.
Pharmaceutical scientists are involved in product optimizations across different disciplines, that is, besides their role as practicing healthcare provider, in formulation and device development, nonclinical and clinical pharmacokinetics and pharmacology, or in regulatory affairs and market access. As such, we have the encouraging opportunity and mandate to leverage existing insights and synergies across different indications and thus avoid repeating assessments and reinventing development and commercialization pathways from the beginning. The work on improving mAb products should involve co-creation not only with customers, but also collaborations between academia, manufacturers, and biotechnology companies.
Availability of data and materials
Not applicable (review article).
Abbreviations
Crohn’s disease
Cluster of differentiation 52
Clinically isolated syndrome
Complete response letter
European Medicines Agency
European Union
- Flexible care setting
Food and Drug Administration
Highlights of Prescribing Information
Inflammatory bowel disease
Interferon beta
Infusion-related reaction
Monoclonal antibody
Multiple sclerosis
National Institute for Health and Care Excellence
Rheumatoid arthritis
Relapsing forms of multiple sclerosis
Relapsing-remitting multiple sclerosis
Real-world evidence
Real-world data
Summary of Product Characteristics
Secondary progressive multiple sclerosis
Ulcerative colitis
United Kingdom
United States
ClinicalTrials.gov (2022) A phase III, non-inferiority, randomized, open-label, parallel group, multicenter study to investigate the pharmacokinetics, pharmacodynamics, safety and radiological and clinical effects of subcutaneous ocrelizumab versus intravenous ocrelizumab in patients with multiple sclerosis (Ocarina II). https://clinicaltrials.gov/ct2/show/NCT05232825 . Accessed 20 Aug 2022.
Abramson A, Frederiksen MR, Vegge A, Jensen B, Poulsen M, Mouridsen B, Jespersen MO, Kirk RK, Windum J, Hubálek F, Water JJ, Fels J, Gunnarsson SB, Bohr A, Straarup EM, Ley MWH, Lu X, Wainer J, Collins J, Tamang S, Ishida K, Hayward A, Herskind P, Buckley ST, Roxhed N, Langer R, Rahbek U, Traverso G. (2022). Oral delivery of systemic monoclonal antibodies, peptides and small molecules using gastric auto-injectors. Nat Biotechnol. 40(1):103-109. https://doi.org/10.1038/s41587-021-01024-0
Adelman DT, Van Genechten D, Megret CM, Truong Thanh XT, Hand P, Martin WA (2019) Co-creation of a lanreotide autogel/depot syringe for the treatment of acromegaly and neuroendocrine tumours through collaborative human factor studies. Adv Ther 36(12):3409–3423. https://doi.org/10.1007/s12325-019-01112-3
Article Google Scholar
Ahmed M, Bankov G, Casey D, Perry ME (2021) CT-P13 subcutaneous infliximab in gastroenterology and rheumatology. Immunotherapy 13(12):1001–1009. https://doi.org/10.2217/imt-2020-0339
Article CAS Google Scholar
Al Zahrani A, Ibrahim N, Al Eid A (2009) Rapid infusion rituximab changing practice for patient care. J Oncol Pharm Pract 15(3):183–186. https://doi.org/10.1177/1078155208100527
Allen PB, Lindsay H, Tham TC (2010) How do patients with inflammatory bowel disease want their biological therapy administered? BMC Gastroenterol 10(10):1. https://doi.org/10.1186/1471-230X-10-1
Allmendinger A (2021) Opportunities in an evolving pharmaceutical development landscape: product differentiation of biopharmaceutical drug products. Pharm Res 38:739–757. https://doi.org/10.1007/s11095-021-03037-5
Amasawa E, Kuroda H, Okamura K, Badr S, Sugiyama H (2021) Cost–benefit analysis of monoclonal antibody cultivation scenarios in terms of life cycle environmental impact and operating cost. ACS Sustain Chem Eng 9(42):14012–14021. https://doi.org/10.1021/acssuschemeng.1c01435
Anderson BJ, Redondo MJ (2011) What can we learn from patient-reported outcomes of insulin pen devices? J Diabetes Sci and Technol 5(6):1563–1571. https://doi.org/10.1177/193229681100500633
Arsenault C, Gage A, Kim MK, Kapoor NR, Akweongo P, Amponsah F, Aryal A, Asai D, Awoonor-Williams JK, Ayele W, Bedregal P, Doubova SV, Dulal M, Gadeka DD, Gordon-Strachan G, Mariam DH, Hensman D, Joseph JP, Kaewkamjornchai P, Eshetu MK, Gelaw SK, Kubota S, Leerapan B, Margozzini P, Mebratie AD, Mehata S, Moshabela M, Mthethwa L, Nega A, Oh J, Park S, Passi-Solar A, Pérez-Cuevas R, Phengsavanh A, Reddy T, Rittiphairoj T, Sapag JC, Thermidor R, Tlou B, Guiñez FV, Bauhoff S, Kruk ME (2022) COVID-19 and resilience of healthcare systems in ten countries. Nat Med 28:1314–1324. https://doi.org/10.1038/s41591-022-01750-1
Atay S, Barista I, Gundogdu F, Akgedik K, Arpaci A (2012) Rapid-infusion rituximab in lymphoma treatment: 2-year experience in a single institution. J Oncol Pract 8(3):141–143. https://doi.org/10.1200/JOP.2011.000319
Azuz S, Newton M, Bartels D, Klindt Poulsen B (2021) Uptake of biosimilar trastuzumab in Denmark compared with other European countries: a comparative study and discussion of factors influencing implementation and uptake of biosimilars. Eur J Clin Pharmacol 77:1495–1501. https://doi.org/10.1007/s00228-021-03155-4
Bagel J, Tatla D, Hellot S, Knapp B, Murphy C, Peterson L, Sebastian M (2022) Bimekizumab self-Injection devices: two multicenter, randomized, open-label studies on self-administration by patients with psoriasis. J Drugs Dermatol 21(2):162–171. https://doi.org/10.36849/jdd.6274
Bai S, Jorga K, Xin Y, Jin D, Zheng Y, Damico-Beyer LA, Gupta M, Tang M, Allison DE, Lu D, Zhang Y, Joshi A, Dresser MJ (2012) A guide to rational dosing of monoclonal antibodies. Clin Pharmacokinet 51(2):119–135. https://doi.org/10.2165/11596370-000000000-00000
Bailey K, Mountian I, Bruggraber R, Sunderland K, Tilt N, Szegvari B (2020) Patient satisfaction with CIMZIA® (certolizumab pegol) AutoClicks® in the UK. Adv Ther 37:1522–1535. https://doi.org/10.1007/s12325-020-01257-6
Bar-Or A, Wiendl H, Montalban X, Alvarez E, Davydovskaya M, Delgado SR, Evdoshenko EP, Giedraitiene N, Gross-Paju K, Haldre S, Herrman CE, Izquierdo G, Karelis G, Leutmezer F, Mares M, Meca-Lallana JE, Mickeviciene D, Nicholas J, Robertson DS, Sazonov DV, Sharlin K, Sundaram B, Totolyan N, Vachova M, Valis M, Bagger M, Häring DA, Ludwig I, Willi R, Zalesak M, Su W, Merschhemke M, Fox EJ (2022) Rapid and sustained B-cell depletion with subcutaneous ofatumumab in relapsing multiple sclerosis: APLIOS, a randomized phase-2 study. Mult Scler 28(6):910–924. https://doi.org/10.1177/13524585211044479
Barrera B, Simpson H, Engebretson E, Sillau S, Valdez B, Gonzalez JP, Winger R, Alvarez E, Gross R, Piquet A, Schreiner T, Corboy J, Pei J, Vollmer T, Nair K (2022) Evaluating the impact of administration of ocrelizumab via home infusion on safety and patient reported outcomes (P16–4.005). Neurology 98(Suppl18):3018
Google Scholar
Bayas A, Gold R (2003) Lessons from 10 years of interferon beta-1b (Betaferon/Betaseron) treatment. J Neurol 250(Suppl4):IV3-8. https://doi.org/10.1007/s00415-003-1402-8
Bei D, Osawa M, Uemura S, Ohno T, Gobburu J, Roy A, Hasegawa M (2020) Benefit-risk assessment of nivolumab 240 mg flat dose relative to 3 mg/kg Q2W regimen in Japanese patients with advanced cancers. Cancer Sci 111(2):528–535. https://doi.org/10.1111/cas.14252
Bergman M, Patel P, Chen N, Jing Y, Saffore CD (2020) Evaluation of adherence and persistence differences between adalimumab citrate-free and citrate formulations for patients with immune-mediated diseases in the United States. Rheumatol Ther 8(1):109–118. https://doi.org/10.1007/s40744-020-00256-x
BioSpace (2006) Abbott Laboratories receives FDA approval for new HUMIRA® delivery device. https://www.biospace.com/article/releases/abbott-laboratories-receives-fda-approval-for-new-humira-r-delivery-device -/. Accessed 20 Aug 2022.
BioSpace (2021) Biogen provides regulatory update on the supplemental biologic license application (sBLA) for subcutaneous administration of TYSABRI® (natalizumab). https://www.biospace.com/article/releases/biogen-provides-regulatory-update-on-the-supplemental-biologic-license-application-sbla-for-subcutaneous-administration-of-tysabri-natalizumab -/. Accessed 20 Aug 2022.
Bittner B, Schmidt S (2012) Subcutaneous administration of monoclonal antibodies in oncology as alternative to established intravenous infusion. Pharm Ind 4:638–643
Bittner B, Schmidt J (2021) Subcutaneous drug delivery devices: enablers of a flexible care setting. In: Chappel E (ed) Drug delivery devices and therapeutic systems. Elsevier Academic Press, London, pp 159–179
Chapter Google Scholar
Bittner B, Schmidt J (2022) Formulation and device lifecycle management: a guidance for researchers and drug developers. Elsevier Academic Press, London
Bittner B, Richter WF, Hourcade-Potelleret F, McIntyre C, Herting F, Zepeda ML, Schmidt J (2012) Development of a subcutaneous formulation for trastuzumab - nonclinical and clinical bridging approach to the approved intravenous dosing regimen. Drug Res 62(9):401–409. https://doi.org/10.1055/s-0032-1321831
Bittner B, Richter W, Schmidt J (2018) Subcutaneous Administration of biotherapeutics: an overview of current challenges and opportunities. BioDrugs 32:425–440. https://doi.org/10.1007/s40259-018-0295-0
Bittner B, Schmit Chiesi C, Kharawala S, Kaur G, Schmidt J (2019) Connected drug delivery devices to complement drug treatments: potential to facilitate disease management in home setting. Med Devices (auckl) 12(12):101–127. https://doi.org/10.2147/MDER.S198943
Blanco MJ, Gardinier KM (2020) New chemical modalities and strategic thinking in early drug discovery. ACS Med Chem Lett 11(3):228–231. https://doi.org/10.1021/acsmedchemlett.9b00582
Bluett J, Morgan C, Thurston L, Plant D, Hyrich KL, Morgan AW, Wilson AG, Isaacs JD, Cordingley L, Barton A (2015) Impact of inadequate adherence on response to subcutaneously administered anti-tumour necrosis factor drugs: results from the Biologics in Rheumatoid Arthritis Genetics and Genomics Study Syndicate cohort. Rheumatology (oxford) 54(3):494–499. https://doi.org/10.1093/rheumatology/keu358
Bohra A, Rizvi QAAK, CYY, Vasudevan A, van Langenberg DR, (2020) Transitioning patients with inflammatory bowel disease from hospital-based to rapid home-based infliximab: a stepwise, safety and patient-orientated process towards sustainability. World J Gastroenterol 26(36):5437–5449. https://doi.org/10.3748/wjg.v26.i36.5437
Bookbinder LH, Hofer A, Haller MF, Zepeda ML, Keller GA, Lim JE, Edgington TS, Shepard HM, Patton JS, Frost GI (2006) A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release 114(2):230–241. https://doi.org/10.1016/j.jconrel.2006.05.027
Brattain LJ, Pierce TT, Gjesteby LA, Johnson MR, DeLosa ND, Werblin JS, Gupta JF, Ozturk A, Wang X, Li Q, Telfer BA, Samir AE (2021) AI-enabled, ultrasound-guided handheld robotic device for femoral vascular access. Biosensors (basel) 11(12):522. https://doi.org/10.3390/bios11120522
Burmester GR, Rubbert-Roth A, Cantagrel A, Hall S, Leszczynski P, Feldman D, Rangaraj MJ, Roane G, Ludivico C, Lu P, Rowell L, Bao M, Mysler EF (2014) A randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study). Ann Rheum Dis 73(1):69–74. https://doi.org/10.1136/annrheumdis-2013-203523
Ceesay LB (2020) Building a high customer experience management organization: towards customer-centricity.Preprints 2020100053. https://doi.org/10.20944/preprints202010.0053.v1
Center for Drug Evaluation and Research (2013) Approval Package for: APPLICATION NUMBER: BLA 125289/S103. SIMPONI® SmartJect® autoinjector. https://www.accessdata.fda.gov/drugsatfda_docs/bla/2013/125289Orig1s103.pdf . Accessed 20 Aug 2022.
Center for Drug Evaluation and Research (2020) FDA approves daratumumab and hyaluronidase-fihj for multiple myeloma. https://www.fda.gov/drugs/drug-approvals-and-data bases/fda-approves-daratumumab-and-hyaluronidase-fih j-multiple-myeloma. Accessed 20 Aug 2022.
Chadda S, Larkin M, Jones C, Sykes D, Barber B, Zhao Z, Gao S, Bengttson NO (2013) The impact of infusion reactions associated with monoclonal antibodies in metastatic colorectal cancer: a European perspective. J Oncol Pharm Pract 19(1):38–47. https://doi.org/10.1177/1078155212451197
Chilton F, Collett RA (2008) Treatment choices, preferences and decision-making by patients with rheumatoid arthritis. Musculoskelet Care 6:1–14. https://doi.org/10.1002/msc.110
Cohen S, Samad A, Karis E, Stolshek BS, Trivedi M, Zhang H, Aras GA, Kricorian G (2019) Chung JB (2019) Decreased injection site pain associated with phosphate-free etanercept formulation in rheumatoid arthritis or psoriatic arthritis patients: a randomized controlled trial. Rheumatol Ther 6:245–254. https://doi.org/10.1007/s40744-019-0152-8
Collier DH, Bitman B, Coles A, Liu L, Kumar S, Judd C (2017) A novel electromechanical autoinjector, AutoTouch™, for self-injection of etanercept: real-world use and benefits. Postgrad Med 129(1):118–125. https://doi.org/10.1080/00325481.2017.1251291
Cortes J, Perez-Garcia JM, Llombart-Cussac A, Curigliano G, El Saghir NS, Cardoso F, Barrios CH, Wagle S, Roman J, Harbeck N, Eniu A, Kaufman PA, Tabernero J, García-Estévez L, Schmid P, Arribas J (2020) Enhancing global access to cancer medicines. CA Cancer J Clin 2:105–124. https://doi.org/10.3322/caac.21597
Dainty KN, Golden BR, Hannam R, Webster F, Browne G, Mittmann N, Stern A, Zwarenstein M (2018) A realist evaluation of value-based care delivery in home care: the influence of actors, autonomy and accountability. Soc Sci Med 206:100–109. https://doi.org/10.1016/j.socscimed.2018.04.006
Dashputre AA, Kamal KM, Pawar G (2017) Cost-effectiveness of peginterferon beta-1a and alemtuzumab in relapsing-remitting multiple sclerosis. J Manag Care Spec Pharm 23(6):666–676. https://doi.org/10.18553/jmcp.2017.23.6.666
De Cock E, Pan YI, Tao S, Baidin P (2014) Time savings with trastuzumab subcutaneous (SC) injection verse trastuzumab intravenous (IV) infusion: a time and motion study in 3 Russian centers. Value Health 17:A653
Denys H, Martinez-Mena CL, Martens MT, D’Hondt RG, Graas ML, Evron E, Fried G, Ben-Baruch NE, Vulsteke C, Van Steenberghe MM (2020) Safety and tolerability of subcutaneous trastuzumab at home administration, results of the phase IIIb open-label BELIS study in HER2-positive early breast cancer. Breast Cancer Res Treat 181(1):97–105. https://doi.org/10.1007/s10549-020-05604-7
Dhalla AK, Al-Shamsie Z, Beraki S, Dasari A, Fung LC, Fusaro L, Garapaty A, Gutierrez B, Gratta D, Hashim M, Horlen K, Karamchedu P, Korupolu R, Liang E, Ong C, Owyang Z, Salgotra V, Sharma S, Syed B, Syed M, Vo AT, Abdul-Wahab R, Wasi A, Yamaguchi A, Yen S, Imran M (2022) A robotic pill for oral delivery of biotherapeutics: safety, tolerability, and performance in healthy subjects. Drug Deliv Transl Res 12(1):294–305. https://doi.org/10.1007/s13346-021-00938-1
Domańska B, Van Lunen B, Peterson L, Mountian I, Schiff M (2017) Comparative usability study for a certolizumab pegol autoinjection device in patients with rheumatoid arthritis. Expert Opin Drug Deliv 14(1):15–22. https://doi.org/10.1080/17425247.2016.1256283
Domańska B, Stumpp O, Poon S, Oray S, Mountian I, Pichon C (2018) Using patient feedback to optimize the design of a certolizumab pegol electromechanical self-injection device: insights from human factors studies. Adv Ther 35(1):100–115. https://doi.org/10.1007/s12325-017-0645-1
Duco MR, Murdock JL, Reeves DJ (2020) Trastuzumab/hyaluronidase-oysk: a new option for patients with HER2-positive breast cancer. Ann Pharmacother 54(3):254–261. https://doi.org/10.1177/1060028019877936
Egorin MJ (2003) Horseshoes, hand grenades, and body-surface area-based dosing: aiming for a target. J Clin Oncol 21(2):182–183
Einav L, Finkelstein A, Ji Y, Mahoney N (2022) Voluntary regulation: evidence from medicare payment reform. Q J Econ 137(1):565–618. https://doi.org/10.1093/qje/qjab035
El Emam K, Jonker E, Sampson M, Krleza-Jerić K, Neisa A (2009) The use of electronic data capture tools in clinical trials: web-survey of 259 Canadian trials. J Med Internet Res 11(1):e8. https://doi.org/10.2196/jmir.1120
El-Sappagh S, Ali F, Hendawi A, Jang JH, Kwak KS (2019) A mobile health monitoring-and-treatment system based on integration of the SSN sensor ontology and the HL7 FHIR standard. BMC Med Inform Decis Mak 19(1):97. https://doi.org/10.1186/s12911-019-0806-z
Eun-Young K (2017) Development and application of direct data capture for monitoring medication compliance in clinical trials. Healthc Inform Res 23(4):249–254. https://doi.org/10.4258/hir.2017.23.4.249
European Medicines Agency (2021) Summary of Product Characteristics. https://www.ema.europa.eu/en/glossary/summary-product-characteristics . Accessed 20 Aug 2022.
Facey K, Rannanheimo P, Batchelor L, Borchardt M, De Cock J (2020) Real-world evidence to support Payer/HTA decisions about highly innovative technologies in the EU—actions for stakeholders. Int J Technol Assess Health Care 36(4):459–468. https://doi.org/10.1017/S026646232000063X
Fernández O, Duran E, Ayuso T, Hernández L, Bonaventura I, Forner M (2017) Treatment satisfaction with injectable disease-modifying therapies in patients with relapsing-remitting multiple sclerosis (the STICK study). PLoS ONE. https://doi.org/10.1371/journal.pone.0185766
Fettner S, Mela C, Wildenhahn F, Tavanti M, Wells C, Douglass W, Mallalieu NL (2019) Evidence of bioequivalence and positive patient user handling of a tocilizumab autoinjector. Expert Opin Drug Deliv 16(5):551–561. https://doi.org/10.1080/17425247.2019.1604678
De Figueiredo SR, Caon AER, Hossne RS, Teixeira FV, Winkler SM, Queiroz NSF (2021) Biosimilars in inflammatory bowel diseases: general concepts and clinical implications. In Azevedo VFF, Moots R (eds), Biosimilars. IntechOpen. https://doi.org/10.5772/intechopen.100452
Filipi M, Jack S (2020) Interferons in the treatment of multiple sclerosis: a clinical efficacy, safety, and tolerability update. Int J MS Care 22(4):165–172. https://doi.org/10.7224/1537-2073.2018-063
Findeisen KE, Sewell J, Ostor AJK (2021) Biological therapies for rheumatoid arthritis: an overview for the clinician. Biologics 15:343–352. https://doi.org/10.2147/BTT.S252575
Fleischmann RM, Bock AE, Zhang W, Godfrey CM, Vranic I, Cronenberger C, Dokoupilová E (2022) Usability study of PF-06410293, an adalimumab biosimilar, by prefilled pen: open-label, single-arm, sub-study of a phase 3 trial in patients with rheumatoid arthritis. Rheumatol Ther 9(3):839–850. https://doi.org/10.1007/s40744-022-00439-8
Frampton JE (2017) Ocrelizumab: first global approval. Drugs 77(9):1035–1041. https://doi.org/10.1007/s40265-017-0757-6
Franken M, Kanters T, Coenen J, de Jong P, Jager A, Uyl-de Groot C (2020) Hospital-based or home-based administration of oncology drugs? A micro-costing study comparing healthcare and societal costs of hospital-based and home-based subcutaneous administration of trastuzumab. The Breast 52:71–77. https://doi.org/10.1016/j.breast.2020.05.001
Freshwater T, Kondic A, Ahamadi M, Li CH, de Greef R, de Alwis D, Stone JA (2017) Evaluation of dosing strategy for pembrolizumab for oncology indications. J Immunother Cancer 16(5):43. https://doi.org/10.1186/s40425-017-0242-5
Frost GI (2007) Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv 4(4):427–440. https://doi.org/10.1517/17425247.4.4.427
Galvão TF, Livinalli A, Lopes LC, Zimmermann IR, Silva MT (2020) Biosimilar monoclonal antibodies for cancer treatment. Cochrane Database Syst Rev CD013539. https://doi.org/10.1002/14651858.CD013539
Gao JJ, Osgood CL, Gong Y, Zhang H, Bloomquist EW, Jiang X, Qiu J, Yu J, Song P, Rahman NA, Chiu HJ, Ricks TK, Rizvi F, Hou S, Wilson W, Abukhdeir AM, Seidman J, Ghosh S, Philip R, Pierce WF, Bhatnagar V, Kluetz PG, Pazdur R, Beaver JA, Amiri-Kordestani L (2021) FDA approval summary: pertuzumab, trastuzumab, and hyaluronidase-zzxf injection for subcutaneous use in patients with HER2-positive breast cancer. Clin Cancer Res 27(8):2126–2129. https://doi.org/10.1158/1078-0432.CCR-20-3474
Garg A, Quartino A, Li J, Jin J, Wada DR, Li H, Cortés J, McNally V, Ross G, Visich J, Lum B (2014) Population pharmacokinetic and covariate analysis of pertuzumab, a HER2-targeted monoclonal antibody, and evaluation of a fixed, non-weight-based dose in patients with a variety of solid tumors. Cancer Chemother Pharmacol 74(4):819–829. https://doi.org/10.1007/s00280-014-2560-3
Garnock-Jones KP (2014) Alemtuzumab: a review of its use in patients with relapsing multiple sclerosis. Drugs 74:489–504. https://doi.org/10.1007/s40265-014-0195-7
Gely C, Marín L, Gordillo J, Mañosa M, Bertoletti F, Cañete F, González-Muñoza C, Calafat M, Domènech E, Garcia-Planella E (2019) Impact of pain associated with the subcutaneous administration of adalimumab. Gastroenterol Hepatol 43(1):9–13. https://doi.org/10.1016/j.gastre.2019.06.008
Genentech (2013) FDA approves the ACTpen for Genentech’s ACTEMRA, a single-dose, prefilled autoinjector for the treatment of rheumatoid arthritis, giant cell arteritis and two forms of juvenile arthritis. https://www.gene.com/media/press-releases/14767/2018-11-26/fda-approves-the-actpen-for-genentechs-a . Accessed 20 Aug 2022.
Ghil J, Zielińska A, Lee Y (2019) Usability and safety of SB5 (an adalimumab biosimilar) prefilled syringe and autoinjector in patients with rheumatoid arthritis. Curr Med Res Opin 35(3):497–502. https://doi.org/10.1080/03007995.2018.1560211
Gligorov J, Pivot X, Ataseven B, De Laurentiis M, Jung KH, Manikhas A, Abdel Azim H, Gupta K, Alexandrou A, Herraez-Baranda L, Tosti N, Restuccia E (2022) Safety and efficacy of adjuvant subcutaneous trastuzumab in human epidermal growth factor receptor 2-positive early breast cancer: Final results of the SafeHER study. Breast 64:151–158. https://doi.org/10.1016/j.breast.2022.03.001
Gottlieb AB, Blauvelt A, Prinz JC, Papanastasiou P, Pathan R, Nyirady J, Fox T, Papavassilis C (2016) Secukinumab self-administration by prefilled syringe maintains reduction of plaque psoriasis severity over 52 weeks: results of the FEATURE trial. J Drugs Dermatol 15(10):1226–1234
Gozzetti A, Bacchiarri F, Sammartano V, Defina M, Sicuranza A, Mecacci B, Zappone E, Cencini E, Fabbri A, Raspadori D, Bocchia M (2020) Long-term safety of rapid daratumumab infusions in multiple myeloma patients. Front Oncol 21(10):570187. https://doi.org/10.3389/fonc.2020.570187
Graham AH (2009) "Administering rituximab: infusion-related reactions and nursing implications. Cancer Nurs Pract 8(2):30–35. https://doi.org/10.7748/cnp2009.03.8.2.30.c6842
Grainger R, Townsley H, White B, Langlotz T, Taylor WJ (2017) Apps for people with rheumatoid arthritis to monitor their disease activity: a review of apps for best practice and quality. JMIR Mhealth Uhealth 5(2):e7. https://doi.org/10.2196/mhealth.6956
Gupta S, Kumar P (2012) Drug delivery using nanocarriers: Indian perspective. Proc Natl Acad Sci, India, Sect B Biol Sci 82:167–206. https://doi.org/10.1007/s40011-012-0080-7
Hake P, Rehse JR, Fettke P (2019) Supporting complaint management in the medical technology industry by means of deep learning. In: Di Francescomarino C, Hanna KS, Segal EM, Barlow A, Barlow B (2021) Clinical strategies for optimizing infusion center care through a pandemic. J Oncol Pharm Pract. 27(1):165–179. https://doi.org/10.1177/1078155220960211
Hampson G, Towse A, Dreitlein WB, Henshall C, Pearson SD (2018) Real-world evidence for coverage decisions: opportunities and challenges. J Comp Eff Res 7(12):1133–1143. https://doi.org/10.2217/cer-2018-0066
Hanna KS, Segal EM, Barlow A, Barlow B (2021) Clinical strategies for optimizing infusion center care through a pandemic. J Oncol Pharm Pract 27(1):165–179. https://doi.org/10.1177/1078155220960211
Hartung HP, Berger T, Bermel RA, Brochet B, Carroll WM, Holmøy T, Karabudak R, Killestein J, Nos C, Patti F, Ross AP, Vanopdenbosch L, Vollmer T, Buffels R, Garas M, Kadner K, Manfrini M, Wang Q, Freedman MS (2020) Shorter infusion time of ocrelizumab: results from the randomized, double-blind ENSEMBLE PLUS substudy in patients with relapsing-remitting multiple sclerosis. Mult Scler Relat Disord 46:102492. https://doi.org/10.1016/j.msard.2020.102492
Hendrikx J, Haanen J, Voest EE, Schellens J, Huitema A, Beijnen JH (2017) Fixed dosing of monoclonal antibodies in oncology. Oncologist 22(10):1212–1221. https://doi.org/10.1634/theoncologist.2017-0167
Henricks LM, Schellens JH, Huitema AD, Beijnen JH (2015) The use of combinations of monoclonal antibodies in clinical oncology. Cancer Treat Rev 41(10):859–867. https://doi.org/10.1016/j.ctrv.2015.10.008
Hernandez I, Bott SW, Patel AS, Wolf CG, Hospodar AR, Sampathkumar S, Shrank WH (2018) Pricing of monoclonal antibody therapies: higher if used for cancer? Am J Manag Care 24(2):109–112
Hiramatsu K, Barrett A, Miyata Y (2021) Current status, challenges, and future perspectives of real-world data and real-world evidence in Japan. Drugs - Real World Outcomes 8:459–480. https://doi.org/10.1007/s40801-021-00266-3
Holmes MM, Stanescu S, Bishop FL (2019) The use of measurement systems to support patient self-management of long-term conditions: an overview of opportunities and challenges. Patient Relat Outcome Meas 10:385–394. https://doi.org/10.2147/PROM.S178488
Huynh TK, Ostergaard A, Egsmose C, Madsen OR (2014) Preferences of patients and health professionals for route and frequency of administration of biologic agents in the treatment of rheumatoid arthritis. Patient Prefer Adher 8:93–99. https://doi.org/10.2147/PPA.S55156
Jackisch C, Stroyakovskiy D, Pivot X, Ahn JS, Melichar B, Chen SC, Meyenberg C, Al-Sakaff N, Heinzmann D, Hegg R (2019) Subcutaneous vs intravenous trastuzumab for patients with ERBB2-positive early breast cancer: final analysis of the HannaH phase 3 randomized clinical trial. JAMA Oncol 5(5):e190339. https://doi.org/10.1001/jamaoncol.2019.0339
Jappe U, Beckert H, Bergmann KC, Gülsen A, Klimek L, Philipp S, Pickert J, Rauber-Ellinghaus MM, Renz H, Taube C, Treudler R, Wagenmann M, Werfel T, Worm M, Zuberbier T (2021) Biologics for atopic diseases: Indication, side effect management, and new developments. Allergologie Select 5:1–25. https://doi.org/10.5414/ALX02197E
Jiskoot W, Hawe A, Menzen T, Volkin DB, Crommelin DJA (2022) Ongoing challenges to develop high concentration monoclonal antibody-based formulations for subcutaneous administration: quo vadis? J Pharm Sci 111(4):861–867. https://doi.org/10.1016/j.xphs.2021.11.008
Kading M, Beck B (2021) Cost analysis of daratumumab therapy: Is there a cost benefit to using the recently approved subcutaneous product versus the IV product? J Oncol Pharm Pract 27(4):978–979. https://doi.org/10.1177/1078155221993218
Kafatos G, Dube S, Burdon P, Lowe K, Leclerc M, Flinois A, Demonty G (2020) The healthcare professionals’ perspective on impact and actions taken following severe infusion reaction events in oncology centers in Europe. Drugs Real World Outcomes 7(2):119–130. https://doi.org/10.1007/s40801-020-00185-9
Keininger D, Coteur G (2011) Assessment of self-injection experience in patients with rheumatoid arthritis: psychometric validation of the self-injection assessment questionnaire (SIAQ). Health Qual Life Outcomes 13(9):2. https://doi.org/10.1186/1477-7525-9-2
Kempton C, Trask P, Parnes A, Niggli M, Campinha-Bacote A, Callaghan MU, O’Connell N, Paz-Priel I, Mahlang JN (2021) Development and testing of the satisfaction questionnaire with intravenous or subcutaneous hemophilia injection and results from the phase 3 HAVEN 3 study of emicizumab prophylaxis in persons with haemophilia A without FVIII inhibitors. Haemophilia 27(2):221–228. https://doi.org/10.1111/hae.14222
Kieseier BC (2011) The mechanism of action of interferon-β in relapsing multiple sclerosis. CNS Drugs 25(6):491–502. https://doi.org/10.2165/11591110-000000000-00000
Kivitz A, Cohen S, Dowd JE, Edwards W, Thakker S, Wellborne FR, Renz CL, Segurado OG (2006) Clinical assessment of pain, tolerability, and preference of an autoinjection pen versus a prefilled syringe for patient self-administration of the fully human, monoclonal antibody adalimumab: the TOUCH trial. Clin Ther 28(10):1619–1629. https://doi.org/10.1016/j.clinthera.2006.10.006
Kivitz A, Baret-Cormel L, van Hoogstraten H, Wang S, Parrino J, Xu C (2018) Stanislav M (2018) Usability and patient preference phase 3 study of the sarilumab pen in patients with active moderate-to-severe rheumatoid arthritis. Rheumatol Ther 5:231–242. https://doi.org/10.1007/s40744-017-0090-2
Kuzman D, Bunc M, Ravnik M, Reiter F, Žagar L, Bončina M (2021) Long-term stability predictions of therapeutic monoclonal antibodies in solution using Arrhenius-based kinetics. Sci Rep 11(1):20534. https://doi.org/10.1038/s41598-021-99875-9
Kvien TK, Patel K, Strand V (2022) The cost savings of biosimilars can help increase patient access and lift the financial burden of health care systems. Semin Arthritis Rheum 52:151939. https://doi.org/10.1016/j.semarthrit.2021.11.009
Lacorte E, Ancidoni A, Zaccaria V, Remoli G, Tariciotti L, Bellomo G, Sciancalepore F, Corbo M, Lombardo FL, Bacigalupo I, Canevelli M, Piscopo P, Vanacore N (2022) Safety and efficacy of monoclonal antibodies for Alzheimer’s disease: a systematic review and meta-analysis of published and unpublished clinical trials. J Alzheimers Dis 87(1):101–129. https://doi.org/10.3233/JAD-220046
Lageat C, Combedazou A, Ramus C, Guerrero K, Frolet C, Glezer S (2021) Formative and validation human factors studies of a new disposable autoinjector for subcutaneous delivery of chronic disease therapies. Expert Opin Drug Deliv 18(11):1761–1775. https://doi.org/10.1080/17425247.2021.1954906
Lala M, Ruosi Li T, de Alwis DP, Sinha V, Mayawala M, Yamamoto N, Siu LL, Chartash E, Aboshady H, Jain L (2020) A six-weekly dosing schedule for pembrolizumab in patients with cancer based on evaluation using modelling and simulation. Eur J Cancer 131:68–75. https://doi.org/10.1016/j.ejca.2020.02.016
Lambrecht A, Vuillerme N, Raab C, Simon D, Messner EM, Hagen M, Bayat S, Kleyer A, Aubourg T, Schett G, Hueber A, Knitza J (2021) Quality of a supporting mobile app for rheumatic patients: patient-based assessment using the user version of the mobile application scale (uMARS). Front Med (lausanne) 22(8):715345. https://doi.org/10.3389/fmed.2021.715345
Lanzillo R, Quarantelli M, Bonavita S, Ventrella G, Lus G, Vacca G, Prinster A, Orefice G, Tedeschi G, Brescia Morra V (2012) Natalizumab vs interferon beta 1a in relapsing-remitting multiple sclerosis: a head-to-head retrospective study. Acta Neurol Scand 126(5):306–314. https://doi.org/10.1111/j.1600-0404.2011.01622.x
Li Z, Easton R (2018) Practical considerations in clinical strategy to support the development of injectable drug-device combination products for biologics. Mabs 10(1):18–33. https://doi.org/10.1080/19420862.2017.1392424
Limmroth V, Reischl J, Mann B, Morosov X, Kokoschka A, Weller I, Schreiner T (2017) Autoinjector preference among patients with multiple sclerosis: results from a national survey. Patient Prefer Adherence 11:1325–1334. https://doi.org/10.2147/PPA.S137741
Longtin Y, Sax H, Leape SSE, Donaldson L, Pittet D (2010) Patient participation: current knowledge and applicability to patient safety. Mayo Clin Proc 85(1):53–62. https://doi.org/10.4065/mcp.2009.0248
López PA, Alonso R, Silva B, Carnero Contentti E (2021) Natalizumab subcutaneous injection for the treatment of relapsing multiple sclerosis patients: a new delivery route. Mult Scler Relat Disord 55:103179. https://doi.org/10.1016/j.msard.2021.103179
Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, Wu HC (2020) Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci 27:1. https://doi.org/10.1186/s12929-019-0592-z
Lugaresi A, Florio C, Brescia-Morra V, Cottone S, Bellantonio P, Clerico M, Centonze D, Uccelli A, di Ioia M, De Luca G, Marcellusi A, Paolillo A (2012) Patient adherence to and tolerability of self-administered interferon β-1a using an electronic autoinjection device: a multicentre, open-label, phase IV study. BMC Neurol 12:7. https://doi.org/10.1186/1471-2377-12-7
Mahler HC, Senner F, Maeder K, Mueller R (2009) Surface activity of a monoclonal antibody. J Pharm Sci 98(12):4525–4533. https://doi.org/10.1002/jps.21776
Mao EJ, Lewin S, Terdiman JP, Beck K (2018) Safety of dual biological therapy in Crohn’s disease: a case series of vedolizumab in combination with other biologics. BMJ Open Gastroenterol 5:e000243. https://doi.org/10.1136/bmjgast-2018-000243
Marušić M, Klemenčić A (2018) Adalimumab – general considerations. J Pharmacol Clin Toxicol 6(2):1104
Mathaes R, Koulov A, Joerg S, Mahler HC (2016) Subcutaneous injection volume of biopharmaceuticals—pushing the boundaries. J Pharm Sci 105(8):2255–2259. https://doi.org/10.1016/j.xphs.2016.05.029
Melsheimer R, Geldhof A, Apaolaza I, Schaible T (2019) Remicade® (infliximab): 20 years of contributions to science and medicine. Biologics 13:139–178. https://doi.org/10.2147/BTT.S207246
Menge T, Rehberg-Weber K, Taipale K, Nastos I, Jauß M (2021) Peginterferon beta-1a was associated with high adherence and satisfaction in patients with multiple sclerosis in a German real-world study. Ther Adv Neurol Disord eCollection. https://doi.org/10.1177/17562864211000461
National Institute for Health and Care Excellence (2018) Ocrelizumab for treating relapsing–remitting multiple sclerosis. Available via technology appraisal guidance [TA533]. https://www.nice.org.uk/guidance/ta533 . Accessed 20 Aug 2022.
National Institute for Health and Care Excellence (2021). Ofatumumab for treating relapsing multiple sclerosis. Available via technology appraisal guidance [TA699]. https://www.nice.org.uk/guidance/ta699 . Accessed 20 Aug 2022.
New R (2020) Oral delivery of biologics via the intestine. Pharmaceutics 13(1):18. https://doi.org/10.3390/pharmaceutics13010018
Nieto JC, Arajol C, Carmona L, Marras C, Cea-Calvo L (2021) Adherence to subcutaneous biological therapies in patients with inflammatory rheumatic diseases and inflammatory bowel disease: a systematic review. Immunotherapy 13(5):433–458. https://doi.org/10.2217/imt-2021-0011
O’Shaughnessy J, Sousa S, Cruz J, Fallowfield L, Auvinen P, Pulido C, Cvetanovic A, Wilks S, Ribeiro L, Burotto M, Klingbiel D, Messeri D, Alexandrou A, Trask P, Fredriksson J, Machackova Z, Stamatovic L (2021) Preference for the fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection in patients with HER2-positive early breast cancer (PHranceSCa): a randomised, open-label phase II study. Eur J Cancer 152:223–232. https://doi.org/10.1016/j.ejca.2021.03.047
Paci A, Desnoyer A, Delahousse J, Blondel L, Maritaz C, Chaput N, Mir O, Broutin S (2020) Pharmacokinetic/pharmacodynamic relationship of therapeutic monoclonal antibodies used in oncology: Part 1, monoclonal antibodies, antibody-drug conjugates and bispecific T-cell engagers. Eur J Cancer 128:107–118. https://doi.org/10.1016/j.ejca.2020.01.005
Pardo-Jaramillo S, Muñoz-Villamizar A, Osuna I, Roncancio R (2020) Mapping research on customer centricity and sustainable organizations. Sustainability 12(19):7908. https://doi.org/10.3390/su12197908
Park J, Lee CH (2021) Clinical study using healthcare claims database. J Rheum Dis 28:119–125. https://doi.org/10.4078/jrd.2021.28.3.119
Park D, Kim J, Yun J, Park SJ (2020) Evaluation of the physico-chemical and biological stability of SB8 (Aybintio), a proposed biosimilar to bevacizumab, under ambient and in-use conditions. Adv Ther 37(10):4308–4324. https://doi.org/10.1007/s12325-020-01465-0
Patel AS, Luu P (2020) Changes in patient reported pain measures with the citrate-free adalimumab formulation in pediatric inflammatory bowel disease patients. JPGN Reports 1(2):e016. https://doi.org/10.1097/PG9.0000000000000016
Patel KB, Arantes LH Jr, Tang WY, Fung S (2018) The role of biosimilars in value-based oncology care. Cancer Manag Res 17(10):4591–4602. https://doi.org/10.2147/CMAR.S164201
Peng Y, Wu T, Chen Z, Deng Z (2022) Value cocreation in health care: systematic review. J Med Internet Res 24(3):e33061. https://doi.org/10.2196/33061
Perry M, Jang M (2020) Budget impact analysis of introducing subcutaneous infliximab CT-P13 SC from the UK payer perspective (AB1185). Ann Rheum Dis 79:1879
Perumal JS, Foo F, Cook P, Khan O (2012) Subcutaneous administration of alemtuzumab in patients with highly active multiple sclerosis. Mult Scler 18(8):1197–1199. https://doi.org/10.1177/1352458511435716
Peterson GM, Thomas J, Yee KC, Kosari S, Naunton M, Olesen IH (2018) Monoclonal antibody therapy in cancer: when two is better (and considerably more expensive) than one. J Clin Pharm Ther 43:925–930. https://doi.org/10.1111/jcpt.12750
Intract Pharma (2020) Intract Pharma Limited and Celltrion Group announce collaboration for development of oral Infliximab. Available via Intract pharma. https://www.intractpharma.com/2020/08/20/intract-pharma-limited-and-celltrion-group-announce-collaboration-for-development-of-oral-infliximab/ . Accessed 20 Aug 2022.
Philippart M, Schmidt J, Bittner B (2016) Oral delivery of therapeutic proteins and peptides: an overview of current technologies and recommendations for bridging from approved intravenous or subcutaneous administration to novel oral regimens. Drug Res 66(3):113–120. https://doi.org/10.1055/s-0035-1559654
Pidun U, Knust N, Kawohl J, Avramakis E, Klar A (2021) The untapped potential of ecosystems in health care. In: BCG Henderson Institute Newsletter: Insights that are shaping business thinking. https://www.bcg.com/publications/2021/five-principles-of-highly-successful-health-care-ecosystems . Accessed 17 Aug 2022.
Pierpont TM, Limper CB, Richards KL (2018) Past, present, and future of rituximab-the world’s first oncology monoclonal antibody therapy. Front Oncol 4(8):163. https://doi.org/10.3389/fonc.2018.00163
Pivot X, Gligorov J, Müller V, Curigliano G, Knoop A, Verma S, Jenkins V, Scotto N, Osborne S, Fallowfield L (2014) Patients’ preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2-positive early breast cancer: final analysis of 488 patients in the international, randomized, two-cohort PrefHer study. Ann Oncol 25(10):1979–1987. https://doi.org/10.1093/annonc/mdu364
Pouls BPH, Kristensen LE, Petersson M, van den Bemt BJF, Ballerini L, Bruggraber R, Karlen H, Mountian I, van Bracht E, Wiegratz S, Jørgensen TS (2020) A pilot study examining patient preference and satisfaction for ava®, a reusable electronic injection device to administer certolizumab pegol. Expert Opin Drug Deliv 17(5):705–711. https://doi.org/10.1080/17425247.2020.1736552
Radu AF, Bungau SG (2021) Management of rheumatoid arthritis: an overview. Cells 10(11):2857. https://doi.org/10.3390/cells10112857
Raffaelli B, Neeb L, Reuter U (2019) Monoclonal antibodies for the prevention of migraine. Expert Opin Biol Ther 19(12):1307–1317. https://doi.org/10.1080/14712598.2019.1671350
Rath L, Campagna MP, Stankovich J, Ellis J, Jokubaitis V, McCarthy D, Nesbitt C, Yeh WZ, Zhong M, Wesselingh R, Monif M, Richards J, Minh VB, Skibina O, Butzkueven H, van der Walt A (2021) Patient preferences for time and location of infusible therapies in multiple sclerosis and neuroimmunologic disorders. Int J MS Care 23(3):114–118. https://doi.org/10.7224/1537-2073.2020-075
Räuber S, Pawlitzki M, Korsen M, Kullmann JS, Thoene D, Pfeuffer S, Rolfes L, Nelke C, Melzer N, Ruck T, Meuth SG (2022) A national, multi-center study in Germany to assess implementation of infusion management, treatment satisfaction and quality of life in MS patients receiving alemtuzumab. Mult Scler Relat Disord 59:103670. https://doi.org/10.1016/j.msard.2022.103670
Remington G, Rodriguez Y, Logan D, Williamson C, Treadaway K (2013) Facilitating medication adherence in patients with multiple sclerosis. Int J MS Care 15(1):36–45. https://doi.org/10.7224/1537-2073.2011-038
Rémuzat C, Kapuśniak A, Caban A, Ionescu D, Radière G, Mendoza C, Toumi M (2017) Supply-side and demand-side policies for biosimilars: an overview in 10 European member states. J Mark Access Health Policy 5(1):1307315. https://doi.org/10.1080/20016689.2017.1307315
Ridyard CH, Dawoud DM, Tuersley LV, Hughes DA (2016) A systematic review of patients’ perspectives on the subcutaneous route of medication administration. Patient 9(4):281–292. https://doi.org/10.1007/s40271-015-0160-x
Rocco P, Kelly AS, Béland D, Kinane M (2017) The new politics of US health care prices: institutional reconfiguration and the emergence of all-payer claims databases. J Health Polit Policy Law 42(1):5–52. https://doi.org/10.1215/03616878-3702746
Rombouts MD, Swart EL, Van Den Eertwegh AJM, Crul M (2020) Systematic review on infusion reactions to and infusion rate of monoclonal antibodies used in cancer treatment. Anticancer Res 40(3):1201–1218. https://doi.org/10.21873/anticanres.14062
Rose J (2021) Subcutaneous infliximab in the U.S.: defining CT-P13 SC's competitive edge. In: Biosimilar Development. https://www.biosimilardevelopment.com/doc/subcutaneous-infliximab-in-the-u-s-defining-ct-p-sc-s-competitive-edge-0001 . Accessed 20 Aug 2022.
Ross A, Besser C, Naval S, Stoneman D, Gaunt H, Barker N (2021) Patient and Nurse preference for Sensoready® autoinjector pen versus other autoinjectors in multiple sclerosis: results from a multicenter survey. ePoster presented at the virtual ACTRIMS Forum, 25–27 February 2021.
Roy A, Geetha RV, Magesh A, Vijayaraghavan R, Ravichandran V (2021) Autoinjector: a smart device for emergency cum personal therapy. Saudi Pharm J 29(10):1205–1215. https://doi.org/10.1016/j.jsps.2021.09.004
Ruck T, Bittner S, Wiendl H, Meuth SG (2015) Alemtuzumab in multiple sclerosis: mechanism of action and beyond. Inti J Mol Sci 16(7):16414–16439. https://doi.org/10.3390/ijms160716414
Rudick RA, Panzara MA (2008) Natalizumab for the treatment of relapsing multiple sclerosis. Biologics 2(2):189–199. https://doi.org/10.2147/btt.s1956
Rudick RA, Polman C, Clifford D, Miller D, Steinman L (2013) Natalizumab: bench to bedside and beyond. JAMA Neurol 70(2):172–182. https://doi.org/10.1001/jamaneurol.2013.598
Rummel M, Kim TM, Aversa F, Brugger W, Capochiani E, Plenteda C, Re F, Trask P, Osborne S, Smith R, Grigg A (2017) Preference for subcutaneous or intravenous administration of rituximab among patients with untreated CD20+ diffuse large B-cell lymphoma or follicular lymphoma: results from a prospective, randomized, open-label, crossover study (PrefMab). Ann Oncol 28(4):836–842. https://doi.org/10.1093/annonc/mdw685
Salar A, Avivi I, Bittner B, Bouabdallah R, Brewster M, Catalani O, Follows G, Haynes A, Hourcade-Potelleret F, Janikova A, Larouche JF, McIntyre C, Pedersen M, Pereira J, Sayyed P, Shpilberg O, Tumyan G (2014) Comparison of subcutaneous versus intravenous administration of rituximab as maintenance treatment for follicular lymphoma: results from a two-stage, phase IB study. J Clin Oncol 32(17):1782–1791. https://doi.org/10.1200/JCO.2013.52.2631
Schreiber S, Ben-Horin S, Alten R, Westhovens R, Peyrin-Biroulet L, Danese S, Hibi T, Takeuchi K, Magro F, An Y, Kim DH, Yoon SW, Reinisch W (2022) Perspectives on subcutaneous infliximab for rheumatic diseases and inflammatory bowel disease: before, during, and after the COVID-19 era. Adv Ther 39:2342–2364. https://doi.org/10.1007/s12325-021-01990-6
Schultz TJ, Thomas A, Georgiou P, Cusack L, Juaton M, Simon L, Naidoo K, Webb K, Karnon J, Ravindran J (2019) Developing a model of care for home infusions of natalizumab for people with multiple sclerosis. J Infus Nurs 42(6):289–296. https://doi.org/10.1097/NAN.0000000000000343
Schultz TJ, Thomas A, Georgiou P, Juaton MS, Cusack L, Simon L, Naidoo K, Webb K, Karnon J, Ravindran J (2021) Home infusions of natalizumab for people with multiple sclerosis: a pilot randomised crossover trial. Ann Clin Transl Neurol 8:1610–1621. https://doi.org/10.1002/acn3.51410
Schulze-Koops H, Giacomelli R, Samborski W, Rednic S, Herold M, Yao R, Govoni M, Vastesaeger N, Weng HH (2013) THU0198 patient evaluations of autoinjectors for delivery of subcutaneous golimumab for treatment of rheumatoid arthritis. Ann Rheum Dis 72:A230–A231
Sebastian S, Roberts J, Waller J, Judge D, Brown C, Davies R, Kachroo S (2019) Remote monitoring of patient-reported outcomes in ulcerative colitis: a prospective real-world pilot study. PharmacoEconomics - Open 3(3):359–365. https://doi.org/10.1007/s41669-019-0121-8
Sedo K (2018) 2017 Global Drug Delivery & Formulation Report: Part 2, Notable Product Approvals of 2017. In: Drug Development and Delivery. https://drug-dev.com/global-report-2017-global-drug-delivery-formulation-report-part-2-notable-product-approvals-of-2017/ . Accessed 20 Aug 2022.
Senolt L (2019) Emerging therapies in rheumatoid arthritis: focus on monoclonal antibodies [version 1; peer review: 2 approved]. F1000Research 8:1549. https://doi.org/10.12688/f1000research.18688.1
Shams S, Martinez JM, Dawson JRD, Flores J, Gabriel M, Garcia G, Guevara A, Murray K, Pacifici N, Vargas MV, Voelker T, Hell JW, Ashouri JF (2021) The therapeutic landscape of rheumatoid arthritis: current state and future directions. Front Pharmacol 12:680043. https://doi.org/10.3389/fphar.2021.680043
Shelbaya A, Kelton JM, Thompson J, Alvir JM, Maculaitis MC, Yang J (2021) Real-world use and acceptance of biosimilar monoclonal antibodies of rituximab in oncology practice in the USA. Future Oncol 17(30):3941–3950. https://doi.org/10.2217/fon-2021-0618
Shetty A, Hanson R, Korsten P, Shawagfeh M, Arami S, Volkov S, Vila O, Swedler W, Shunaigat AN, Smadi S, Sawaqed R, Perkins D, Shahrara S, Sweiss NJ (2014) Tocilizumab in the treatment of rheumatoid arthritis and beyond. Drug Des Devel Ther 28(8):349–364. https://doi.org/10.2147/DDDT.S41437
Smale EM, Egberts TC, Heerdink ER, van den Bemt BJF, Bekker CL (2021) Waste-minimising measures to achieve sustainable supply and use of medication. Sustain Chem Pharm 20:100400. https://doi.org/10.1016/J.SCP.2021.100400
Spadaro G, Vultaggio A, Alberto Bosi A, Reichert D, Janssen J, Lamacchia D, Nappi L, Pecoraro A, Milito C, Ferraro A, Matucci A, Bacchiarri F, Carrai V, Hibbeler A, Speckman E, Guarnieri C, Bongiovanni S, Quinti I (2017) Rapid infusions of human normal immunoglobulin 50g/l are safe and well tolerated in immunodeficiencies and immune thrombocytopenia. Int Immunopharmacol 44:38–42. https://doi.org/10.1016/j.intimp.2016.12.030
St Clair-Jones A, Prignano F, Goncalves J, Paul M, Sewerin P (2020) Understanding and minimising injection-site pain following subcutaneous administration of biologics: a narrative review. Rheumatol Ther 7(4):741–757. https://doi.org/10.1007/s40744-020-00245-0
Stahnke AM, Holt KM (2018) Ocrelizumab: a new B-cell therapy for relapsing remitting and primary progressive multiple sclerosis. Ann Pharmacother 52(5):473–483. https://doi.org/10.1177/1060028017747635
Stearns LJ, Narang S, Albright RE Jr, Hammond K, Xia Y, Richter HB, Paramanandam GK, Haagenson KK, Doth AH (2019) Assessment of health care utilization and cost of targeted drug delivery and conventional medical management vs conventional medical management alone for patients with cancer-related pain. JAMA Netw Open 2(4):e191549. https://doi.org/10.1001/jamanetworkopen.2019.1549
Strik AS, Wang YMC, Ruff LE, Yashar W, Messmer BT, Mould DR (2018) Individualized dosing of therapeutic monoclonal antibodies—a changing treatment paradigm? AAPS J 20:99. https://doi.org/10.1208/s12248-018-0257-y
Tambuyzer E, Vandendriessche B, Austin CP, Brooks PJ, Larsson K, Miller Needleman KI, Valentine J, Davies K, Groft SC, Preti R, Oprea TI, Prunotto M (2020) Therapies for rare diseases: therapeutic modalities, progress and challenges ahead. Nat Rev Drug Discov 19(2):93–111. https://doi.org/10.1038/s41573-019-0049-9
Tat R, Heydari J (2021) Avoiding medicine wastes: introducing a sustainable approach in the pharmaceutical supply chain. J Clean Prod 320:128698. https://doi.org/10.1016/j.jclepro.2021.128698
Teisberg E, Wallace S, O’Hara S (2020) Defining and implementing value-based health care: a strategic framework. Acad Med 95(5):682–685. https://doi.org/10.1097/ACM.0000000000003122
Tetteh EK, Morris S (2014) Evaluating the administration costs of biologic drugs: development of a cost algorithm. Health Econ Rev 4:26. https://doi.org/10.1186/s13561-014-0026-2
Thakur K, Biberger A, Handrich A, Rezk MF (2016) Perceptions and preferences of two etanercept autoinjectors for rheumatoid arthritis: a new European Union-approved etanercept biosimilar (Benepali®) versus etanercept (Enbrel®) - findings from a nurse survey in Europe. Rheumatol Ther 3(1):77–89. https://doi.org/10.1007/s40744-016-0035-1
Timbie JW, Kim AY, Concannon TW (2021) Use of real-world evidence for regulatory approval and coverage of medical devices: a landscape assessment. Value Health 24(12):1792–1798. https://doi.org/10.1016/j.jval.2021.07.003
Timmermann H, Mailänder C (2020) Home self-administration of biologics - a German survey among omalizumab-treated patients with severe asthma and their treating physicians. Pneumologie 74(2):103–111. https://doi.org/10.1055/a-1069-0900
Tischer B, Mehl A (2018) Patients’ and nurses’ preferences for autoinjectors for rheumatoid arthritis: results of a European survey. Patient Prefer Adherence 12:1413–1424. https://doi.org/10.2147/PPA.S169339
Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P, Bannerjee SK (2012) Drug delivery systems: an updated review. Int J Pharm Investig 2(1):2–11. https://doi.org/10.4103/2230-973X.96920
Tkacz J, Ellis L, Bolge SC, Meyer R, Brady BL, Ruetsch C (2014) Utilization and adherence patterns of subcutaneously administered anti-tumor necrosis factor treatment among rheumatoid arthritis patients. Clin Ther 36(5):737–747. https://doi.org/10.1016/j.clinthera.2014.02.019
UCB (2021). UCB Gains CE Mark for ava Connect[®], a first-in-class electromechanical device for use with biologic treatment in rheumatology and dermatology. https://www.ucb.com/stories-media/Press-Releases/article/UCB-Gains-CE-Mark-for-ava-Connect-a-first-in-class-electromechanical-device-for-use-with-biologic-treatment-in-rheumatology-and-dermatology . Accessed 20 Aug 2022.
United States Food and Drug Administration (2017) Biosimilar and interchangeable products. https://www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products#interchange . Accessed 20 Aug 2022.
United States Food and Drug Administration (2018) Statement from FDA Commissioner Scott Gottlieb, M.D., on FDA’s new strategic framework to advance use of real-world evidence to support development of drugs and biologics. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-fdas-new-strategic-framework-advance-use-real-world . Accessed 20 Aug 2022.
United States Food and Drug Administration (2020) FDA approves breast cancer treatment that can be administered at home by health care professional. https://www.fda.gov/news-events/press-announcements/fda-approves-breast-cancer-treatment-can-be-administered-home-health-care-professional . Accessed 20 Aug 2022.
United States Food and Drug Administration (2021) FDA approves Cyltezo, the first interchangeable biosimilar to Humira. https://www.fda.gov/news-events/press-announcements/fda-approves-cyltezo-first-interchangeable-biosimilar-humira . Accessed 20 Aug 2022.
United States Food and Drug Administration (2022a). Drugs@FDA: FDA-Approved Drugs. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm . Accessed 20 Aug 2022a.
United States Food and Drug Administration (2022b). Real-World Evidence. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence . Accessed 20 Aug 2022b.
United States Food and Drug Administration (2022c). Biosimilar Product Information. https://www.fda.gov/drugs/biosimilars/biosimilar-product-information . Accessed 20 Aug 2022c.
Usmani SZ, Mateos MV, Hungria V, Iida S, Bahlis NJ, Nahi H, Magen H, Cavo M, Hulin C, White D, De Stefano V, Fastenau J, Slavcev M, Heuck C, Qin X, Pei H, Masterson T, Lantz K, Gries KS (2021) Greater treatment satisfaction in patients receiving daratumumab subcutaneous vs. intravenous for relapsed or refractory multiple myeloma: COLUMBA clinical trial results. J Cancer Res Clin Oncol 147:619–631. https://doi.org/10.1007/s00432-020-03365-w
Van den Bemt BJF, Gettings L, Domańska B, Bruggraber R, Mountian I, Kristensen LE (2019) A portfolio of biologic self-injection devices in rheumatology: how patient involvement in device design can improve treatment experience. Drug Deliv 26(1):384–392. https://doi.org/10.1080/10717544.2019.1587043
Verma AM, Patel A, Subramanian S, Smith PJ (2021) From intravenous to subcutaneous infliximab in patients with inflammatory bowel disease: a pandemic-driven initiative. Lancet Gastroenterol Hepatol 6(2):88–89. https://doi.org/10.1016/S2468-1253(20)30392-7
Vermeire S, D'heygere F, Nakad A, Franchimont D, Fontaine F, Louis E, Van Hootegem P, Dewit O, Lambrecht G, Strubbe B, Baert F. (2018). Preference for a prefilled syringe or an auto-injection device for delivering golimumab in patients with moderate-to-severe ulcerative colitis: a randomized crossover study. Patient Prefer Adherence. 6(12):1193-1202. https://doi.org/10.2147/PPA.S154181 .
Vieillard V, Paul N, Ibrahim T, Astier A (2017) Extended stability of the rituximab biosimilar CT-P10 in its opened vials and after dilution and storage in polyolefin bags. Ann Pharm Fr 75(6):420–435. https://doi.org/10.1016/j.pharma.2017.06.003
Vijayan S, Adams J, Cook L, Haskins Z, Kermode A (2017) Establishment of the first at-home natalizumab infusion service for the treatment of relapsing remitting multiple sclerosis (RRMS). J Neurol Neurosurg Psychiatry 88:e1
Vijayaraghavan R (2020) Autoinjector device for rapid administration of drugs and antidotes in emergency situations and in mass casualty management. J Int Med Res 8(5):300060520926019. https://doi.org/10.1177/0300060520926019
Weinstock-Guttman B, Bermel R, Cutter G, Freedman MS, Leist TP, Ma X, Kile D, Musch B, Reder AT, Wolinsky JS (2022) Ocrelizumab treatment for relapsing-remitting multiple sclerosis after a suboptimal response to previous disease-modifying therapy: a nonrandomized controlled trial. Mult Scler 28(5):790–800. https://doi.org/10.1177/13524585211035740
Wolfromm A, Mittaine B, Gandrille N, Dallemagne J, Delarue R (2017) Home administration of subcutaneous rituximab is safe and associated with significant cost saving: a single center experience. Blood 130(1):4676. https://doi.org/10.1182/blood.V130.Suppl_1.4676.4676
Wyant T, Fedyk E, Abhyankar B (2016) An Overview of the mechanism of action of the monoclonal antibody vedolizumab. Journal Crohns Colitis 10(12):1437–1444. https://doi.org/10.1093/ecco-jcc/jjw092
Yelvington BJ (2018) Subcutaneous rituximab in follicular lymphoma, chronic lymphocytic leukemia, and diffuse large B-cell lymphoma. J Adv Pract Oncol 9(5):530–534
Zadbuke N, Shahi S, Gulecha B, Padalkar A, Thube M (2013) Recent trends and future of pharmaceutical packaging technology. J Pharm Bioallied Sci 5(2):98–110. https://doi.org/10.4103/0975-7406.111820
Zahavi D, Weiner L (2020) Monoclonal antibodies in cancer therapy. Antibodies 9(3):34. https://doi.org/10.3390/antib9030034
Zhang W, Michalowski CB, Beloqui A (2021) Oral delivery of biologics in inflammatory bowel disease treatment. Front Bioeng Biotechnol 3(9):675194
Download references
Acknowledgements
We thank Johannes Schmidt from F. Hoffmann-La Roche Ltd. for thorough review of the manuscript.
Not applicable.
Author information
Authors and affiliations.
F. Hoffmann-La Roche Ltd., Global Product Strategy - Product Optimization, Grenzacher Strasse 124, CH-4070, Basel, Switzerland
Beate Bittner
You can also search for this author in PubMed Google Scholar
Contributions
Beate Bittner drafted the scope and content of the article, conducted the literature research and wrote the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Beate Bittner .
Ethics declarations
Competing interests.
Beate Bittner is employee of F. Hoffmann-La Roche and owns stock in Roche.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Bittner, B. Customer-centric product presentations for monoclonal antibodies. AAPS Open 9 , 3 (2023). https://doi.org/10.1186/s41120-022-00069-y
Download citation
Received : 21 September 2022
Accepted : 02 December 2022
Published : 23 January 2023
DOI : https://doi.org/10.1186/s41120-022-00069-y
Combined studies
Interface between the regulations on clinical trials of medicinal products, medical devices and in vitro diagnostics.
In the EU, there are legal requirements for the individual authorisation processes of clinical trials of medicinal products, clinical investigations of medical devices and performance studies of in vitro diagnostics (IVDs).
These requirements are laid out in Regulation (EU) 536/2014 on clinical trials of medicinal products for human use (CTR), Regulation (EU) 2017/745 on medical devices (MDR) and Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR) respectively.
In practice, these Regulations may need to be applied together to develop innovative treatments combining medicinal products with medical devices or IVDs.
In this context, combined studies can be understood as studies that involve:
- A clinical trial of a medicinal product in parallel with a performance study of an in vitro diagnostic
- A clinical trial of a medicinal product in parallel with a clinical investigation of a medical device
The ‘COMBINE’ project
Member States' competent authorities for clinical trials and medical devices and the European Commission launched this project in June 2023.
The COMBINE project aims to analyse the root causes of the challenges encountered by sponsors in conducting combined studies and identify possible solutions to these challenges.
The project involves representatives of competent authorities, the European Commission, medical research ethics committees, the European Medicines Agency and relevant medicinal product and medical device sector stakeholders.
The groups affiliated with this project are the Medical Device Coordination Group and relevant clinical trial groups (Clinical Trials Coordination Group, Clinical Trials Coordination and Advisory Group).
The project consists of two phases:
- analysis of the challenges at the interface of MDR/IVDR/CTR.
- possible development of solutions that aim to address some of the challenges.
The analysis phase consists of three strands:
- collecting and analysing the challenges reported by various actors involved;
- mapping relevant national processes and;
- mapping of already ongoing work in this area.
Project Deliverables
The project completed its first phase with the publication of the analysis report which includes proposals for possible solutions to address the most important issues. The next steps of this project will involve developing some of the solutions.
- COMBINE Analysis Phase report
The presentation below includes further information on the projects's background, parties involved, the highlights of the four strands and the next steps.
- Presentation of the COMBINE Project
Before this project, the Member State competent authorities on clinical trials and IVDs jointly endorsed a guidance document about the status of assays used in clinical trials of medicinal products.
MDCG 2022-10 Q&A on the interface between Regulation (EU) 536/2014 on clinical trials for medicinal products for human use (CTR) and Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR)
Latest updates
- News announcement
- 17 May 2024
- 14 May 2024
- 10 April 2024
- 13 March 2024
Share this page
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
- Environment
- Wildlife, animals, biodiversity and ecosystems
- Animal and plant health
Legal requirements for placing a veterinary medicine on the market
Placing a veterinary medicine on the market, including non-medicinal products, medicinal words and phrases, how to obtain advice, report non-compliance.
Legislation
The Veterinary Medicines Regulations 2013 (legislation.gov.uk) (VMR), as amended, set out the UK controls on veterinary medicines, including their manufacture, advertising, marketing, supply and administration.
It is the responsibility of anyone engaged in these activities to comply with the VMR.
The VMR is available on Legislation.gov.uk .
Requirement for a Marketing Authorisation (MA)
The VMR require that any person who places a veterinary medicine on the market does so in accordance with an MA .
It is an offence to place a veterinary medicine on the market unless that product has been granted an MA.
Definition of Veterinary Medicine
A Veterinary Medicine is legally defined as:
- any substance or combination of substances presented as having properties for treating or preventing disease in animals
- any substance or combination of substances that may be used in, or administered to, animals with a view either to restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action, or to making a medical diagnosis
- any substance or combination of substances that may be used for the purpose of euthanising an animal
This means that a product may be a veterinary medicine if it is:
- medicinal by presentation; in that product information, such as product labels or marketing material, gives the averagely well-informed person the impression that the product treats or prevents disease
- medicinal by function; in that it contains a substance that would have a medicinal effect
Medicinal by presentation
A product is medicinal by presentation if its appearance gives the averagely well informed person the impression that the product treats or prevents disease, or they gain that impression.
Principally, if a person placing a product on the market, or the manufacturer, or a connected third party, expressly indicates or recommends the product for treating or preventing disease, this would render the product as medicinal by presentation. This includes product labels, leaflets, websites and social media advertisements or oral recommendations, and any other forms of literature relating to the product issued before, during or after the sale.
UK case law has established that:
- the concept of presentation of a product must be broadly understood
- the presentation will be that of the manufacturer but is not limited to the terms or manner in which the manufacturer chooses to package, describe or classify the product
- when considering whether a product is medicinal by presentation, regard should be given to the warnings, express indications and recommendations on the packaging but they are not conclusive of the position
- the external form of the product may be relevant to establishing the manufacturer’s intention but may also be material to the impression gained by the averagely well informed person
- the method of administration is an aspect of the presentation
- if a product is not only used externally but is used internally this may be relevant to its presentation and function
A product which is medicinal by presentation must have a MA granted by the SoS before it can be placed on the market unless it is covered by Schedule 6 to the VMR Exemptions for small pet animals .
Medicinal words and phrases
If a product claims it will treat, prevent or control a disease it is medicinal by presentation. Certain words are considered medicinal as they’re normally associated with authorised medicines. The whole presentation of the product, including the packaging, will determine whether the words used make the product appear medicinal.
For guidance on marketing non-medicinal products: Digital media checklist ( PDF , 74.8 KB , 1 page )
Diseases and adverse conditions
If a product label refers, explicitly or implicitly, to the treatment or prevention of a disease or adverse condition, or to improving the state of health of the animal treated, it is making a medicinal claim.
References to the nutritional maintenance of a healthy animal, healthy digestive system or healthy respiratory system would not normally be regarded as medicinal claims.
Complying with legal requirements - medicinal by presentation: issues of difficulty
The following is guidance on particular points of difficulty and is not a definitive account of legal requirements.
Marketing and other promotional material
Claims made by a third party, such as magazine reviews or articles published by independent analysts, will be regarded as those of the company marketing the product where evidence confirms that the third party has a connection to the marketing company via solicitation, endorsement, sponsorship or funding.
Disclaimers
Disclaimers, for example on packaging or other marketing material, are not sufficient to prevent a product from being considered medicinal by presentation.
Reference to studies
References in marketing material to studies may cause a product to be considered medicinal if the study indicates that the product, or one of its ingredients, may have a medicinal effect or purpose.
Customer testimonials
If customer testimonials are used in connection with the marketing of a product and report results containing medicinal claims, the claims will be regarded as those of the company marketing the product.
Websites and social media
Websites and social media sites, including any chat room or forum, are considered in the same way as any other form of advertising and should not make medicinal claims for products that do not hold an MA.
UK based websites advertising a non-UK authorised veterinary medicine, intended for sale and administration outside the UK, must clearly indicate that the products will not be sold to UK customers.
For guidance on what internet marketing material to check see the Medicinal words and phrases ( PDF , 103 KB , 5 pages )
False and misleading claims
The VMR do not cover any claim made for an unauthorised veterinary medicine that is thought to be misleading or false but does not imply a medicinal effect.
False or misleading advertising claims about a product that is not a veterinary medicine are dealt with by local Trading Standards Officers.
Product form
The form in which a product is presented and the instructions for administration will be considered when deciding if a product is medicinal by presentation. For example, a vitamin supplement administered by injection may be considered to be medicinal by the nature of its presentation.
Packaging presentation
The appearance and design of packaging and its similarity to that of authorised medicines will be considered when deciding if a product is medicinal by presentation.
Medicinal by function
A product is medicinal by function if it is used or administered to animals with a view to restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action or making a medical diagnosis. Risk to health is a factor that must be taken into account when classifying a product as medicinal by function.
A product which is medicinal by function must have an MA granted by the SoS before it can be placed on the market. This requirement does not apply to products marketed under Schedule 6 to the VMR.
Specific topics
Feeding stuffs intended for particular nutritional purposes.
The Animal Feed (England) Regulations are enforced by local Trading Standards Officers.
Your local office (contact your local council for details) will be able to provide individual advice on these Regulations.
The Animal Feed (England) Regulations apply in England only, separate but parallel legislation is in force in Scotland, Wales and Northern Ireland.
Nutraceuticals
A nutraceutical product is a food or naturally occurring food supplement marketed as having a beneficial effect on health and is treated like any other product. They require an MA if medicinal claims are made or if they contain certain ingredients that exert a pharmacological effect on the target animal.
Biocides, insecticides and repellents
The following require an MA:
- a veterinary product administered to an animal, which contains a substance that kill insects or external parasites, such as pyrethrins, pyrethroids or organophosphate compounds, as they are medicinal by function
- a veterinary product claiming to have, or which has, the function of, control of internal parasites
- a veterinary product administered to an animal, claiming to treat or prevent a disease caused by a viral, bacterial or fungal infection
The following do not require an MA:
- a product containing a repellent, such as diethyltoluamide or ethylhexanediol, provided they claim only to repel external insects
- a product applied only to housing or bedding
- a topical disinfectant applied to intact skin provided they do not claim to treat or prevent disease
The marketing of these products are covered by legislation on biocides. For further information refer to the Chemicals Regulation Directorate , email [email protected] or [email protected] .
A shampoo for animals will be considered medicinal if it contains an insecticide or an ingredient which has a pharmacological effect or is presented as an insecticidal shampoo. Reference to skin conditions such as seborrhoea and dermatitis are medicinal and should not be made in connection with an unauthorised shampoo.
Cosmetic products
Cosmetic products for animals are subject to the general definition of veterinary medicines. Products that do not make specific medicinal claims and are used for cosmetic purposes only, such as colouring shampoos and hoof oils, are not considered to be veterinary medicines as long as they do not contain any pharmacologically active ingredients.
Teat and udder products
A product applied internally to teats and udders for the prevention of mastitis is considered to be a veterinary medicine.
A product applied topically to disinfect teats and udders and for which no medicinal claims are made, does not require an MA. These are regarded as biocidal products and dealt with by the Health and Safety Executive (HSE) under the Biocidal Products Regulations.
Disinfectants
A product labelled as a disinfectant which does not claim to treat or prevent disease does not require an MA. However, disinfectants may be regarded as biocidal products and can be dealt with by the HSE under the Biocidal Products Regulations.
Herbal products
Herbal products are treated like any other products. They require an MA if they are medicinal by presentation or function. For example, a product containing pyrethrum, pyrethrins or alkaloids, such as digoxin from Digitalis sp., would be considered medicinal by function.
Homeopathic remedies
A new homeopathic veterinary remedy placed on the market must either be registered under the homeopathic registration scheme or have a full MA. A homeopathic product on the market prior to 1 January 1994 may remain on the market provided no medicinal claims are made.
Diagnostic tools (testing kits)
Any substance, or combination of substances administered to animals with a view to making a medical diagnosis is a veterinary medicine and therefore requires an MA.
The withdrawal of fluid or tissue for diagnostic purposes and laboratory diagnostic tests are not considered medicinal.
Manufactured colostrum, including that from cows that have been treated to ensure the colostrum will contain particular antibodies, require an MA.
A colostrum or colostrum based product containing pure colostrum, provided that no reference is made to disease, immunoglobulin, antibodies, IgA, IgG or immunity, does not require an MA.
Products excluded from the scope of the Regulations
The VMR do not apply to:
- a veterinary medicinal product based on radio-active isotopes
a product intended for administration in the course of a procedure licensed under the Animals (Scientific Procedures) Act 1986, except that, if the animals are to be put into the human food chain, the only products that may be administered to the animals are:
- authorised veterinary medicinal products administered in accordance with their marketing authorisation; or
- products administered in accordance with an animal test certificate granted under VMR paragraph 9 of Schedule 4.
Obtaining advice
If you are in any doubt as to whether a specific product requires an MA you will be able to obtain confirmation from the VMD through a formal process. There is a fee for this procedure, the details can be found on the Fees applied to veterinary medicine authorisation applications in GB .
It is not mandatory to seek formal confirmation of a product’s status before it is placed on the UK market. However, should a product be placed on the market without an MA and it is deemed to be a veterinary medicine, enforcement action will be taken which could result in the product being seized without compensation.
How to apply
Each Advice application form (ODT, 46.1KB) should be signed by the applicant or in the case of a corporate body by a proper officer and be accompanied by the supporting information referred to in the application form. Applications should be emailed to: [email protected]
All relevant information submitted in support of such applications is treated as commercially confidential.
Each application will be acknowledged and validated to check that all necessary information has been supplied within 14 calendar days of receipt.
The application will be considered by us once it has passed validation. You will be informed within 30 calendar days whether an MA is required, or you will be asked to provide further information. You will receive written confirmation of this.
This decision is only valid based on the documentation submitted as part of the application. Small changes to any of the information provided could invalidate the decision.
On receipt of a valid application, the VMD will send an invoice to the applicant for each product listed in the application.
The fee is £885.
Reporting non-compliance
Use our online reporting forms to:
- report any suspected breach of the VMR
- Report an animal product that is marketed as a medicine
The VMD’s Enforcement Section coordinates reports of suspected breaches of the VMR. The information and intelligence we receive is analysed and may be shared with our enforcement partners with the aim of protecting public health, animal health and the environment, and to promote animal welfare by assuring the safety, quality and efficacy of veterinary medicines.
Anonymous reporting
You can submit an anonymous report to us through:
- the online reporting form and selecting the anonymous option
- our telephone Hotline on 01932 338 338
Please be aware that if you use this option and there is not enough information in your report, we may be limited on the action we can take regarding your concern.
We do not disclose where reports originate.
Other Legislation
If a product does not fall within the definition of a veterinary medicine care should be taken to ensure that it meets the requirements of any legislation which might be relevant, such as:
- The Food and Environment Protection Act as amended
- The Control of Pesticides Regulations as amended
- The Biocidal Products Regulations as amended
- The Animal Feed (England) Regulations as amended
Contact and further guidance
For further information regarding any of the above topics contact the Enforcement Section, [email protected]
For information relating to authorised veterinary products see the Product Information Database or email your enquiry to [email protected] .
For information on authorisation routes and how to apply for a marketing authorisation refer to the guidance; Marketing authorisations for veterinary medicines .
For information on advertising; Advertise veterinary medicines legally .
For information on the retail of veterinary medicine; Retail of veterinary medicines .
Related content
Is this page useful.
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. Please fill in this survey (opens in a new tab) .
Victorian medicinal cannabis patients will be allowed behind the wheel to test road safety
In 2016, Australia legalised access to medicinal cannabis, allowing patients to access dozens of different cannabis products through prescriptions.
Now the Victorian government wants to take another step in opening the door for medicinal cannabis patients in the state to be able to drive.
In a bill passed last year, the government committed to launch a closed-circuit trial to research the impairment that medicinal cannabis causes on driving.
A push to change driving laws around medicinal cannabis has also been seen in other states such as Western Australia , while in Tasmania it is legal to drive as long as a person is not impaired by the drug.
But how does medical cannabis legislation work, and what are the risks to road safety?
Medical cannabis in Victoria explained
Any Victorian patient, with any medical condition, can be prescribed medicinal cannabis by their doctor if they believe it is clinically appropriate.
There are two groups of medicinal cannabis patients, with different driving rules applying to each of them.
Medicinal cannabis patients using cannabidiol or CBD products have always been allowed to drive in Victoria as long as they are not impaired.
However, some medicinal cannabis products contain a compound known as Delta-9-tetrahydrocannabinol or THC, which is responsible for the "high" feeling associated with cannabis and could impair driving ability.
It is currently an offence in Victoria for a person to drive with any amount of THC in their system, whether the THC comes from medicinal cannabis or not.
Victoria Police currently conducts random roadside drug testing throughout Victoria, with saliva tests that are positive for THC resulting in a drug-driving charge.
Drivers with THC in their system faced mandatory licence suspensions and fines if caught.
The new trial by the government will seek to test just how impaired people with medicinal cannabis in their system are whilst driving.
How will the trial work?
Drivers who use medicinal cannabis will be taken around driving courses with an instructor at special closed road facilities such as METEC in Bayswater North and AARC in Wensleydale.
Minister for Roads and Road Safety Melissa Horne said the trial, conducted in partnership with Swinburne University, would be a world-first.
"There is nowhere in the world that actually has got that standard way of measuring impairment through medicinal cannabis," Ms Horne said.
"It is a basic human right — we've got a legally prescribed drug, let us be able to measure what that looks like in a road safety environment."
About 70 participants will take place in the trial, due to begin in September this year.
When first announced last year, the trial was praised by legal groups such as the Australian Lawyers Alliance, who have dubbed driving laws penalising medicinal cannabis users as "outdated and unfair".
"Cases are coming before the courts every week where people are losing their licence and their livelihood because they are taking prescribed medicinal cannabis and driving," Australian Lawyers Alliance spokesperson Greg Barns said.
"Drivers who take opioids or other prescription medication do not find themselves in court or risk losing their license, and neither should drivers who have taken a prescribed and legal dose of cannabis."
Ms Horne would not however say when any potential law changes would be passed if the trial is found to be successful, with results due in 2026.
Is it dangerous to drive with THC in your system?
According to the VicRoads website, THC is a psychoactive substance that has been shown to impair cognitive and motor function, increasing your risk of being involved in a motor vehicle crash.
A meta-analysis of studies conducted in 2021 found cannabis increased the risk of crashes somewhere between 11 and 42 per cent.
Researchers found the added crash risk from cannabis similar to that of the legal blood alcohol content (BAC) limit for driving, with the high end of estimates similar to that of prescription drugs like antidepressants.
Internationally, there is a lack of research on the effects of cannabis on driving impairment.
In the United States, where recreational cannabis is legal in some states, the drug is the second most frequently found substance in the bodies of drivers involved in fatal motor vehicle accidents after alcohol.
A study of emergency department presentations in Canada , which legalised cannabis in 2018, showed no evidence of significant changes in traffic-injury emergency departments.
Driving while drug-impaired remains illegal in both countries, but the measurement of what constitutes impairment differs across state and country lines.
In Canada, any reading above 2 nanograms (ng) of THC per millilitre of blood while driving is considered an offence, while in the US state of Colorado that figure is 5ng or above.
Meanwhile, in states like New York and California, penalties are based on whether law enforcement can prove a driver has been impaired by a drug.
For now, the Victorian government has not made clear what any introduced driving laws may look like, should the trial be successful.
How have medicinal cannabis advocates reacted?
Legalise Cannabis MPs have criticised the government for moving too slowly on the issue, saying millions of dollars are being spent to conduct research that has "already been done".
"In 2023, Dan Andrews promised an answer 'in coming months' followed by a guarantee to have it fixed by 2024. Now, with a new premier, it's mid-2026 at best. She's in the slow lane," MP Rachel Payne said.
“Given the 10-year time blowout, I am calling on the premier to follow Tasmania’s lead and allow Victorians, unimpaired and prescribed medical cannabis, to drive without fear of recrimination."
Ms Payne noted a similar study that had already been conducted by Swinburne University, where 40 people were tested on a virtual driving simulator after consuming medical cannabis , as opposed to a real vehicle and road as planned in the new trial.
The Australian Legal Alliance (ALA) has also called for the prosecution of medicinal cannabis patients who are driving while unimpaired to be paused while the trial is undertaken.
ALA national president Shaun Marcus said drivers were being prosecuted merely for having a substance in their body rather than potentially dangerous impaired driving.
"That is not what road safety legislation was designed to protect," Mr Marcus said.
"The ALA is calling upon the government to not prosecute those persons currently before the courts between now and the end of the trial.
"We see the detrimental effects and over-representation of certain communities before the courts, and we say it really should change quicker."
- X (formerly Twitter)
Related Stories
Imogen is scared she'll lose her licence for driving after taking her prescribed meds.
John breaks the law every day but says medicinal cannabis laws mean he doesn't have a choice
Cannabis driving laws are lagging behind the science, and medicinal users are not in the driving seat
- Road Accidents and Incidents
The browser version you are using is not recommended for this site. Please consider upgrading to the latest version of your browser by clicking one of the following links.
Support for Thunderbolt™ Share
/apps/intel/support/template/supportDynamicHubPage
Find support information for Thunderbolt™ Share, which may include featured content, downloads, specifications, or warranty.
Compatibility
Connectivity
Error Messages
Identify My Product
Install & Setup
Product Codes & Spare Parts
Product Information & Documentation
Troubleshooting
Warranty & RMA
Maintenance & Performance
Product Comparison
Last Reviewed
No results found for
Description
View Details
View download options
Latest Drivers & Software
Get product specification for this product, need more help.
Contact support

COMMENTS
B. Dose Forms, Units of Presentation, Routes of Administration, and Packaging — ISO 11239 . ... Medicinal product name can be either an invented/trade name/proprietary name, a common ...
EMA splitting of the Full Presentation Name of the medicinal product best practice EMA/327516/2014 Page 7/26 2.1. Definition of Product name Considering the definition of product name in the Article 1 (20) of the Directive 2001/83/EC and in line with the SmPC Guideline recommendation, the name of the medicinal product should be expressed as
medicinal product on a case by case basis, taking into account all relevant factors in relation to its ... presentation and function. However, in accordance with Article 2(2) of the Directive, where doubt remains as to its classification as a medicine or another type of product, it will be classified as a medicinal product.
the world for identifying medicinal products. The initial driver for IDMP was pharmacovigilance, but during its development it was realised that the project would be ideal for a much wider range of uses in the regulation of medicinal products. The most noticeable change in the Standard Terms database is the way in which Pharmaceutical dose
The terms "presentation medicinal product" and "functional medicinal product" are not part of the definition of the term but are rather common terms for the different preparations according to the BfArM. This distinction is primarily used for delineation in case law.
non-clinical and clinical data of dossiers for medicinal products for human use, authorised before 1 July 2003. Furthers guidance is given in section "presentation of the application" and the corresponding Annex to the Question & Answer document. If the original Part II contained data on bioequivalence, then this data should be extracted
Use of the Extended EudraVigilance Investigational Medicinal Product Dictionary (XEVIMPD) for Sponsors of clinical trials . On 11 June 2011, the Commission published in the Official Journal (OJ 2011/C 172/01) a Communication on the detailed guidance on the collection, verification and presentation of adverse event/reaction reports arising from ...
2. I. General objective of section 1. This section should provide the (invented) name of the product followed by strength and pharmaceutical form. When referring to properties of the active substance (rather than those of the product) throughout the SmPC text, the international non-proprietary name (INN) or usual common name of the active ...
Medicinal product. A substance or combination of substances that is intended to treat, prevent or diagnose a disease, or to restore, correct or modify physiological functions by exerting a pharmacological, immunological or metabolic action.
Package presentations. Information about presentations can be found in the website of the European Medicines Agency under the section "Product Information". Likewise, presentations on which there has been a Commission decision are referred in the Summary of Product Characteristics (Annex I to the Commission Decision granting the marketing ...
Medicinal products may well fall under both limbs of the definition but the European Court of Justice (ECJ) has confirmed that falling under either limb is sufficient to classify a product as a ... "Directive 65/65 (now Directive 2001/83) provides two definitions of the term "medicinal product": one relating to presentation, the other to ...
A unit of presentation is a qualitative concept that describes a countable entity in which the clinical drug is presented, or by which it is bounded. It is used to support expression of presentation strength, where it provides the denominator for the strength ratio, and to differentiate different clinical drug products when the "intimate ...
In this commentary I briefly discuss the term advanced therapy medicinal products (ATMPs). The last two words, medicinal products, correctly indicate that we are dealing with medicines. However, oftentimes ATMPs and products within the ATMP family are erroneously called therapies, which may raise confusion, as illustrated with some examples, and may lead to ignorance of the importance of ...
The registration of herbal medicinal products needs sufficient evidence for the medicinal use of the product throughout a period of at least 30 years in the European Union (EU), at least 15 years within the EU, and 15 years elsewhere for products from outside the EU. With regard to the manufacturing of these products and their quality, products ...
The revision gave guidance on sentinel dosing (where one person in a first cohort of participants receives a single dose of investigational product in advance of the full study cohort) and the staggering of subjects (that includes a specified follow-up interval between administration of the product to a subject, or small group of subjects, and ...
A definition of medicinal plants for the purpose of this presentation should include the following (Sofowora 2008; Evans, 2008): ... Promotion, and Commercialisation of Nigerian Herbal Medicinal Products was inaugurated on 30th May 2006 and was given a target of US$1billion sales of medicinal plants and its products within 10 years for Nigeria.
Delivering customer-centric product presentations for biotherapeutics, such as monoclonal antibodies (mAbs), represents a long-standing and paramount area of engagement for pharmaceutical scientists. Activities include improving experience with the dosing procedure, reducing drug administration-related expenditures, and ultimately shifting parenteral treatments outside of a controlled ...
In practice, these Regulations may need to be applied together to develop innovative treatments combining medicinal products with medical devices or IVDs. Combined studies. In this context, combined studies can be understood as studies that involve: A clinical trial of a medicinal product in parallel with a performance study of an in vitro ...
The European Medicines Agency (EMA) is a decentralised body of the EU. The mission of the Agency is to foster scientific excellence in the evaluation and supervision of medicines, for the benefit of public and animal health serving over 500 million users of medicinal products. Responsible for centralised procedure and co-ordination of EU ...
The whole presentation of the product, including the packaging, will determine whether the words used make the product appear medicinal. For guidance on marketing non-medicinal products: Digital ...
Step 8: Determine Follow-Up Questions and Provide Answers. At the end of your product presentation, prospects or investors are likely to have a handful of questions about your product. Typically prospective customers ask questions to know if the product is a right fit for their organization.
How big business pushes a product it's not allowed to advertise. It's not your typical act of corporate generosity, giving away $3 million worth of cannabis. That's what's on offer from ...
cell medicinal product, tissue engineering or combined ATMP; • The CAT is not a classification body; hence, it cannot express opinions on whether a product is or is not a medicinal product. • The ATMP classification is a non-mandatory procedure that can be used by developers to clarify the applicable regulatory framework The ATMP ...
However, some medicinal cannabis products contain a compound known as Delta-9-tetrahydrocannabinol or THC, which is responsible for the "high" feeling associated with cannabis and could impair ...
OpenAi Chief Technology Officer Mira Murati introduced the company's product upgrades on stage and in a live-stream presentation on Monday.
Medicinal products used in combination with a medical device (Art 117) • MDR entered into application on 26. th. May 2021 • Almost 1-year experience with the transitioning from MDD to MDR. 10. MDR applies since 26 May 2021. Update of the. Questions and answers on implementation. of the medical devices and in vitro diagnostic medical devices
// No product or component can be absolutely secure. // Your costs and results may vary. // Performance varies by use, configuration and other factors. // See our complete legal Notices and Disclaimers. // Intel is committed to respecting human rights and avoiding causing or contributing to adverse impacts on human rights.
ATMPs. Gene therapy MP, Cell therapy MP and Tissue engineered products. Are medicinal products. ATMPs are authorised in the EU via the centralised procedure. Principles of existing legislation on medicines apply to advanced therapies: marketing authorisation. demonstration of Quality, Safety & Efficacy. GMP, GCP (adapted to ATMPs)
medicinal products for human use' and 'Chapter 3: Process for the electronic submission of information on medicinal products for human use' of the EU ISO IDMP Implementation Guide, the EMA will develop an online training course comprising of series of presentations, videos and step-by-step guides. Access to this training will be free of charge.