
Chemical Communications
Synthesis and shaping of metal–organic frameworks: a review.

* Corresponding authors
a State Key Laboratory of Safety and Control for Chemicals, SINOPEC Research Institute of Safety Engineering Co., Ltd., Qingdao City, Shandong Province, China E-mail: [email protected]
Metal–organic frameworks (MOFs) possess excellent advantages, such as high porosity, large specific surface area, and an adjustable structure, showing good potential for applications in gas adsorption and separation, catalysis, conductivity, sensing, magnetism, etc. However, they still suffer from significant limitations in terms of the scale-up synthesis and shaping, hindering the realization of large-scale commercial applications. Despite some attempts having been devoted to addressing this, challenges remain. In this paper, we outline the advantages and drawbacks of existing synthetic routes such as electrochemistry, microwave, ultrasonic radiation, green solvent reflux, room temperature stirring, steam-assisted transformation, mechanochemistry, and fluid chemistry in terms of scale-up production. Then, the shaping methods of MOFs such as extrusion, mechanical compaction, rolling granulation, spray drying, gel technology, embedded granulation, phase inversion, 3D printing and other shaping methods for the preparation of membranes, coatings and nanoparticles are discussed. Finally, perspectives on the large-scale synthesis and shaping of MOFs are also proposed. This work helps provide in-depth insight into the scale-up production and shaping process of MOFs and boost commercial applications of MOFs.

Article information
Download citation, permissions.
Y. Li, G. Wen, J. Li, Q. Li, H. Zhang, B. Tao and J. Zhang, Chem. Commun. , 2022, 58 , 11488 DOI: 10.1039/D2CC04190A
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author, advertisements.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

The Chemistry and Applications of Metal–Organic Frameworks (MOFs) as Industrial Enzyme Immobilization Systems
Allison r. m. silva.
1 Departamento de Engenharia Química, Campus do Pici, Universidade Federal do Ceará, Bloco 709, Fortaleza 60455760, Brazil; moc.liamg@smnaurnosilla (A.R.M.S.); [email protected] (J.Y.N.H.A.); rb.ude.balinu.onula@kcire (J.E.S.S.); moc.liamtoh@ahledagez (J.G.L.N.)
Jeferson Y. N. H. Alexandre
José e. s. souza, josé g. lima neto, paulo g. de sousa júnior.
2 Centro de Ciências, Departamento de Química Orgânica e Inorgânica, Campus do Pici, Universidade Federal do Ceará, Fortaleza 60455970, Brazil; moc.liamg@jsdgoluap
Maria V. P. Rocha
José c. s. dos santos.
3 Instituto de Engenharias e Desenvolvimento Sustentável, Universidade da Integração Internacional da Lusofonia Afro-Brasileira, Campus das Auroras, Redencao CEP 62790970, Brazil
Enzymatic biocatalysis is a sustainable technology. Enzymes are versatile and highly efficient biocatalysts, and have been widely employed due to their biodegradable nature. However, because the three-dimensional structure of these enzymes is predominantly maintained by weaker non-covalent interactions, external conditions, such as temperature and pH variations, as well as the presence of chemical compounds, can modify or even neutralize their biological activity. The enablement of this category of processes is the result of the several advances in the areas of molecular biology and biotechnology achieved over the past two decades. In this scenario, metal–organic frameworks (MOFs) are highlighted as efficient supports for enzyme immobilization. They can be used to ‘house’ a specific enzyme, providing it with protection from environmental influences. This review discusses MOFs as structures; emphasizes their synthesis strategies, properties, and applications; explores the existing methods of using immobilization processes of various enzymes; and lists their possible chemical modifications and combinations with other compounds to formulate the ideal supports for a given application.
1. Introduction
Enzymes have been widely used as natural biocatalysts in the pharmaceutical, chemical, and food industries, in addition to their well-known applications in medicine and in effluent and solid-waste treatment systems [ 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 ]. This is mainly due to the diversity of reactions enabled by biocatalysts, as well as their high efficiency, specificity, and selectivity [ 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 ]. Furthermore, enzymes are biocompatible and biodegradable structures that can be derived from renewable resources [ 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 ]. Unlike conventional organic syntheses, in enzymatic biocatalysis, the reactions of multifunctional molecules are carried out without the need for previous activation or the use of temporary protection for functional groups, resulting in more economical processes and less waste generation [ 29 , 30 , 31 , 32 , 33 , 34 ].
However, there are clear hurdles to the use of free enzymes, such as degradation (or denaturation) at high temperatures, the need for strict pH control during reactions, their difficult recovery and reuse, high production costs, and their instability under unfavorable environmental conditions, all of which hinder a more widespread implementation across different industries [ 19 , 20 , 22 , 30 , 31 , 35 , 36 , 37 , 38 , 39 ]. A suitable approach used for overcoming these problems is the immobilization of enzymes onto insoluble or solid supports [ 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 ]. Making them insoluble improves their operational characteristics under adverse conditions, which, in turn, enables their employment in media under more extreme temperatures, under comprehensive pH ranges, and in the presence of organic solvents instead of water [ 50 ]. Immobilization also allows for higher product quality and lower processing costs [ 51 ].
Another benefit to immobilization is the more efficient handling of enzymes through solid matrices in comparison to liquid-phase counterparts, which facilitates the separation of final products and reduces their contamination [ 52 ]. Additionally, immobilized enzymes show very little to no allergenicity, high recoveries, and a reuse capacity, rendering processes more economical [ 29 , 53 ]. To increase the stability of the enzymes during storage and make them more resistant to operational conditions, several types of support for enzyme immobilization have been studied, including magnetic nanoparticles, sol-gels, mesoporous silica, and polymers [ 17 , 27 , 43 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 . Immobilization technologies can also prevent subunit dissociation, aggregation, autolysis, and proteolysis, apart from delivering more suitable reaction microenvironments [ 73 , 74 , 75 , 76 ].
However, some challenges have also been observed in these techniques, such as low loading efficiency and enzyme denaturation due to incompatible incorporation processes [ 77 ]. In addition, conventional supports can present irregular non-uniform structures, which can impair the activity of the immobilized enzymes [ 78 , 79 , 80 ]. Among the several materials that can be used as supports for immobilized enzymes, metal–organic frameworks (MOFs) can be highlighted. These are an emerging class of porous materials built from the self-assembly of certain organic ligands and metal ions or specific clusters [ 81 , 82 , 83 , 84 ]. Their use as immobilization supports has been encouraged due to their inherent unique properties, such as structural flexibility, adjustable pore size, large surface area, and the possibility of post-synthetic modifications, among others [ 85 ]. The scientific relevance of using MOFs as support for enzymes can be observed by the significant increase in the number of published articles on these materials ( Figure 1 ).

Growth in the number of published articles retrieved on Scopus over 10 years using the following keywords: (1) “metal-organic frameworks” and “MOF”; and (2) “metal-organic frameworks”, “MOF”, “enzyme”, and “immobilization”. The search was carried out on 7 January 2022, and returned (1) 45,925 and (2) 371 documents.
Furthermore, as observed in Figure 1 , it is possible to discuss possibilities not yet evaluated for MOF applications as enzyme supports. As the figure presents and compares the number of MOF-related publications over the past 10 years, it is clear to see that the research on the topic is being carried out at an increasing pace, and it is possible to identify a vast field of present and future possibilities.
MOFs’ flexible structure size and porous environment, as well as their network of binding and interaction sites, allow for the immobilization of most enzymes and facilitates the mass transfer of substrates and products [ 86 ]. These materials have the highest surface areas ever reported for this specific application and can deliver high immobilization efficiencies due to their vast number of functional sites and pores [ 87 , 88 ]. Furthermore, MOFs can behave similarly to enzymes due to their inherent catalytic groups [ 86 ]. It is important to highlight, however, that for an efficient immobilization to occur, the enzyme confinement method must be carefully chosen, as any structural modification of the enzymes can lead to a significant reduction in their catalytic activity. Moreover, in case the interaction between the enzyme and the MOF is weak, enzyme leaching may occur. Therefore, it is essential to also evaluate the governing support–enzyme interactions [ 83 ].
Several authors have published reviews that discuss biocatalysts composed of enzymes and MOFs, addressing the most common methods of synthesis and immobilization of these composites, as well as their several applications [ 86 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 ]. In this scenario, this review intends to update the discussion of MOFs as highly relevant materials for a wide range of applications, as well as to discuss their roles and mechanisms of action as supports for enzymatic immobilization, and the different combinations for the formation of enzyme–MOF composites to diverse ends, such as catalysis, medicine, and in biosensor manufacturing, among others.
2. Metal–Organic Structures: Synthesis Strategies
MOFs are classes of chemicals that contain metallic ions (or coordinated metals) and organic ligands in their structures [ 100 , 101 , 102 ]. Thus, a MOF can be distinguished by a coordinated network with organic ligands (that can be mono-, di-, or trivalent, or tetravalent) containing empty spaces, or ‘pores’ [ 100 , 103 , 104 ]. The metal–ligand chemical bonds present in the composition of MOFs are predominantly of covalent nature and of the Lewis acid–base type (metal ion and ligand, in that order), given that they can generate a coordination composition [ 103 , 104 ]. Thus, the choice of metal and ligands ultimately determines the structure and pore size in MOFs.
There are no MOFs readily available in nature, except for stepanovite and zhemchuzhnikovite minerals [ 105 ]. Thus, the low functional stability of these materials in natural environments with characteristics of high crystallinity, microporosity (partially), high permanent surface area, and low thermal and chemical stability, in addition to their porosity and density, can substantiate both the interest in this field and the need for further studies on the use of MOFs in different areas [ 103 , 105 , 106 , 107 ].
As mentioned above, the unique characteristics of these materials combined with their wide range of applications reinforce the need for the development and improvement of synthesis techniques [ 105 , 106 , 107 ]. Currently, MOFs can be synthesized by different strategies, such as reticular synthesis [ 108 ], hydrothermal (solvothermal) routes [ 109 , 110 , 111 ], diffusion [ 112 , 113 , 114 ], electrochemistry [ 111 , 115 , 116 ], microwaves [ 117 , 118 ], mechanochemistry [ 119 , 120 ], heating, and ultrasound [ 111 ]. Figure 2 shows a schematic representation of the main strategies for MOF synthesis.

Schematic representation of strategies of ( a ) hydrothermal–solvothermal synthesis, ( b ) diffusion synthesis, ( c ) electrochemical synthesis, ( d ) microwave-assisted synthesis, ( e ) mechanochemical synthesis, and ( f ) sonochemistry synthesis.
2.1. Methods of Synthesis
2.1.1. reticular synthesis.
Professor Omar Yaghi et al. [ 121 ] developed a synthesis strategy based on modular chemistry, known as reticular synthesis [ 121 ]. In this methodology, polytopic organic molecules bind to transition metal ions [ 121 , 122 ]. Subsequently, secondary building units (SBUs) are covalently linked across the entirety of the crystal [ 121 , 122 , 123 ]. SBUs are complexes or clusters in which ligand coordination modes and metal coordination geometries can be employed to modify these fragments into extended porous networks using polytopic structures [ 121 , 122 , 123 ].
In a later work, Yaghi et al. [ 108 ] discuss that reticular chemistry refers to the arrangement of pre-established coordinate structures through rigid molecular building blocks (the SBUs) that replicate and remain united through metal–ligand bonds [ 108 ]. Furthermore, in the same work, the authors redefined the term SBU, which was initially used to characterize fragments of zeolites, but was then defined around the geometry of the units classified as extension points [ 108 ].
In this way, for the construction of a broad network, SBUs must be structured in the correct mining, as this structuring guarantees the three-dimensionality of the material, so the geometry of the binder can directly influence the structure of the material [ 108 , 121 ].
2.1.2. Hydrothermal–Solvothermal Synthesis
Hydrothermal synthesis was initially used for the production of zeolites. Later, it was also incorporated in the synthesis of MOFs [ 111 ]. Jarrah and Farhadi (2019) used hydrothermal synthesis to synthesize a MIL-101(Cr) and P2W18@MIL-101(Cr) nanohybrid. The nanohybrid was used in an adsorption test with the following organic dyes: methylene blue, rhodamine B, and methyl orange. The results indicated that the material obtained showed fast selective adsorption for systems with different dye concentrations [ 124 ].
In this technique, soluble impellers are used in a reactor, where the system operates under high pressures and temperatures ( Figure 2 a) [ 111 , 125 ]. The hydrothermal and solvothermal methods employed are dependent on the solvent used. In general, processes that use water as a solvent are termed hydrothermal processes [ 124 ], while those that use other solvents are classified as solvothermal processes [ 126 ].
The main advantages of this method reside in the good control of the morphology and the composition of the MOF [ 111 ]. It is worth mentioning that the rate of cooling can influence the properties of the synthesized material [ 109 ]. The main disadvantages of these methods include the processing time and the operating costs, making it difficult to reproduce them on industrial scales [ 111 , 124 , 125 , 126 ].
2.1.3. Diffusion Synthesis
The synthesis method via diffusion is based on the gradual transport of several interacting species [ 112 ]. Diffusion-based methods of synthesis can be subdivided into two strategies [ 111 ]. In the first strategy, liquid solvent diffusion is performed [ 125 ]. First, two layers are formed at different density levels. The precipitating solvent resides in one of these layers, and then the final product in the solvent sits in the other [ 111 ]. This way, through the contact between the two interfaces, the gradual diffusion of the precipitant between the separated layer takes place, thus facilitating the crystal development ( Figure 2 b) [ 127 ].
In the second strategy, gradual diffusion occurs through physical barriers [ 128 ]. In addition, gels can also be used as an environment for diffusion and crystallization. This material is used because it mitigates the slow rate of diffusion and hinders the sole precipitation of MOFs [ 111 , 127 , 128 ].
2.1.4. Electrochemical Synthesis
Electrochemical synthesis is widely used on an industrial scale to produce MOFs [ 129 ]. This methodology is based on principles of green chemistry since, when compared to the solvothermal method, for example, it imparts low costs, operating at lower pressures and temperatures, and requires shorter synthesis times, while presenting higher selectivity [ 130 ]. It is worth mentioning that during the crystallization step, issues may occur due to the development of metal ions in situ near the surface of the support, which reduces the agglomeration of crystals [ 131 , 132 ]. Figure 2 c shows a schematic representation of an electrochemical synthesis of MOFs.
As with the hydrothermal method, the cracking process is thermally induced during temperature decay. However, as mentioned above, electrochemical synthesis occurs at milder temperatures, as compared to the former technique. According to Mueller and co-workers [ 111 ], the less abrupt cooling may favor the process of MOF formation [ 111 , 130 , 131 ].
The main disadvantage of this synthesis method compared to the hydrothermal route is the need for controlling a more significant number of variables, since parameters such as voltage and pulse, for example, need to be carefully adjusted [ 130 , 131 , 133 ].
2.1.5. Microwave-Assisted Synthesis
The microwave-assisted technique is widely used for synthesizing small particles of oxides and metals [ 134 ]. Chen et al. [ 135 ], for example, performed the synthesis of MOF-74(Ni) with different methods, such as hydrothermal and microwave-assisted methods. The researchers evaluated the performance of these materials in the adsorption of CO 2 /N 2 and verified that the MOF-74(Ni) synthesized by microwaves presented better adsorption performance. In addition, the authors reported that the protocol studied proved to be easy to conduct, and was also faster when compared to the other methods studied [ 135 ].
Through this process, it is also possible to increase the temperature of the solution, thus facilitating the formation of nanometric metal crystals [ 134 , 136 ]. It is worth mentioning that this strategy apparently cannot be directly used to synthesize MOF crystals [ 136 ]. However, it can speed up the synthesis process and adequately control the size and shape of MOFs [ 137 ]. Figure 2 d presents a schematic representation of the use of microwaves in MOF synthesis.
Another aspect that needs to be considered is the control of parameters for solvent evaporation. Since temperature expansion can increase the solubility of crystals in saturated solutions, the process facilitates the formation of crystals during the cooling phase [ 134 , 136 , 137 ].
2.1.6. Mechanochemical Synthesis
The mechanophysical strategy employs mechanical forces as a precursor of chemical reactions ( Figure 2 e). In this type of synthesis, chemical transformation is preceded by the mechanical rupture of intermolecular bonds [ 138 , 139 ]. Synthetic chemistry has employed mechanical activation in multicomponent reactions (ternary and higher) to form co-crystals with applications in the fields of pharmacy, organic synthesis, inorganic solid-state chemistry, and polymer science, among others [ 140 ].
Thus, several reasons are highlighted for using this strategy in the synthesis of MOFs. The main advantage of this method is the redced possible environmental impacts caused by the process. Syntheses in the absence of organic solvents can be carried out at room temperature, for example. Another positive aspect is reduced synthesis time [ 112 , 138 , 139 , 140 ].
2.1.7. Sonochemical Synthesis
This methodology uses frequencies between 10 MHz and 20 KHz, which are higher than those detectable by the human ear ( Figure 2 f) [ 125 ]. The synthesis media can be close to a solid consistency if the cavitation and the microjets emitted during the reactions have the capacity for deterioration, activation, and interface variation [ 141 ], as well as for dispersion and agglomeration [ 142 ]. Alternatively, a liquid acts under pressure, specific temperature, and homogeneous conditions, or it is the interface that acts under the pressure of the medium, in case of forcing [ 141 ].
The main advantages of using sonochemical synthesis are the speed of synthesis, energy efficiency, process simplicity, and room-temperature reaction environments [ 111 , 138 , 141 , 142 ]. Yu et al. [ 143 ] employed the sonochemical route in the synthesis of Zn-based porphyrins MOF-525 and MOF-545. The authors obtained both porphyrins at high purity, and processing times were of 2.5 h and 0.5 h, respectively. It is worth noting that the materials showed excellent results also in the hydrolysis of dimethyl-4-nitrophenyl phosphate (DMNP) and in the adsorption of bisphenol-A (BPA), when compared to samples obtained conventionally [ 143 ]. Table 1 lists some methods of MOFs synthesis and their characteristics.
Metal–organic frameworks: a summary of different synthesis strategies and their applications.
3. Metal–Organic Frameworks
3.1. properties.
Metal–organic frameworks (MOFs) have plated several roles in many industries and have become promising materials in the areas of catalysis, drug delivery, sensors, biological markers, pesticides, and others [ 153 ]. Their wide application is linked to their key physical properties and versatility, which are evidenced by organic structures linked to a central ion and, more specifically, a metallic cation [ 125 ]. The coordination sphere has a well-defined geometry, leading to the creation of crystals originating from this spatial arrangement, allowing pores to form in a polymerized manner. A scheme of the above definition is shown in Figure 3 [ 84 ].

Representation of the formation of a crystalline structure of metal–organic structures (MOFs) based on organic ligands being coupled to a metallic center.
Metal–organic frameworks show a wide variety of physicochemical and biological properties due to the versatility of their compositions ( Figure 4 ) [ 154 ]. The binding of a metal ion or cluster to a flexible chain of organic polymers creates excellent magnetic properties in these composites that can be widely explored. This also facilitates the removal of these nanomaterials from their respective reaction media [ 99 ]. MOFs are also excellent precursors of chemical synthesis, depending on the chemical groups present in their organic part, where they act as activators or inhibitors of reaction points [ 155 ]. Additionally, they can act as electron donors or acceptors due to the properties of these structures being associated with coordination polymers, which behave as Lewis acids [ 156 ]. Many of these structures can interact with ionic or organic membranes and selectively migrate carrying ligands or macromolecules in biological media from one region to another [ 107 ]. The semiconduction properties of these materials also enable their use in the development of cutting-edge nanotechnology materials and processes. Owing to their excellent thermal capacity, new devices that require high sensitivity, easy detection and mapping, and good thermal stability can also be produced based on these inherent characteristics [ 157 ].

Representation scheme of composite properties based on metal–organic framework (MOF) structures, highlighting their thermal capacity, chirality, high stability, semiconductivity, luminescence, magnetism, catalytic power, and porosity.
The ability of MOFs to act as catalysts or supports for the immobilization of biocatalysts renders these composites widely employable in chemical syntheses [ 158 ]. The chirality of these structures also enables favorable interactions with optically active materials, allowing for enhanced selectivity for these materials when in biological media [ 159 ]. Their thermal capacity, based on the metallic components, enables MOFs to integrate structures that require rapid cooling or heating [ 160 ]. Their semiconduction properties are also associated with the metallic center or the semiconducting organic ligands of these polymers, which allows for their use in nanotechnological applications [ 161 ].
Additionally, high porosity is one of the properties that add the most value to these materials, as pore sizes can be adjusted at the time of synthesis, according to the method and chemical precursors used [ 162 ]. The pores on the contact surface can act as housings to small molecules to be carried in fluids and organisms, and even to other organic molecules responsible for a given specific activity [ 163 ]. Luminescence, another key property, is characterized by the emission of light from the excited compounds. In MOFs, this is not only associated with the type of metal present in their composition but can be potentialized by organic ligands that present ideal chromophores for this property, such as aromatic structures [ 164 , 165 ].
3.2. Applications
3.2.1. adsorption.
Adsorption is a fairly easy and low-cost technique that has been widely used, among other ends, to remove aquatic contaminants ( Figure 5 ) [ 162 ]. MOFs are materials that can be successfully used in this technique due to their good adsorbent properties. More specifically, they have been employed in the removal of excess biological compounds, antibiotics, pesticides, gases, and other toxic pollutants, such as heavy metals [ 163 ]. Pan et al. [ 166 ], and Ghanem et al. [ 167 ] reported the adsorption process of organophosphate compounds used as herbicides, glufosinate (GLUF), glyphosate (GLY), and bialaphos (BIA) via MOFs. When metabolized, these compounds form derivatives that are frequently found in underground water bodies and in the soil, and that cause several environmental problems. They are also difficult to remove due to their high solubility and polarity. The adsorption process described made use of the magnetic properties of these MOFs, their high structural porosity, available surface area, and the possibility of compounds being quickly bound to the metallic center [ 165 ]. Thus, this becomes a viable technique, both from an environmental perspective and from an economic point of view, since MOFs can be reused for many cycles.

Schematic diagram showing pollutant adsorption on the surface of metal–organic frameworks (MOFs), where the contaminant particles can bind to the material, leaving a pollutant-free aqueous medium.
Antibiotics are drugs used to treat human and animal infections and have become an emerging environmental problem due to their excessive and incorrect disposal [ 168 ]. These compounds can be removed from aquatic systems using the MOF adsorption method, as reported in [ 169 ]. In addition to aiding the elimination of the aquatic contamination, these materials could also be used to remove polluting gases from the atmosphere via gas adsorption [ 170 ]. Many other materials are already widely used for this purpose, such as activated carbon and zeolites. However, they have shown a reduced ability to adsorb carbon dioxide [ 171 ]. Thus, materials made from metal–organic frameworks are highly promising, given their properties of adjustable pore size, easy handling and application, reuse, and selectivity [ 172 ]. In recent years, this versatility has led to a great interest in MOF, resulting in the use of these materials for different purposes. When associated with simple techniques, such as adsorption, many new options can be enabled.
3.2.2. Catalysis
There has always been high demand for cheaper and faster processes in several industries. Therefore, the use of catalysts is widely studied for the optimization of industrial processes. MOFs, for example, can be used as catalysts for chemical reactions [ 173 ]. Given the aforementioned properties, they can provide high selectivity of substrates, and can be easily separated from reaction media and vastly reused ( Figure 6 ). In the literature, several types of chemical reactions at small and large scales have been catalyzed by MOFs, including conventional catalysis [ 174 , 175 ], biocatalysis [ 173 , 176 , 177 , 178 ], and electrocatalysis [ 174 , 179 ]. The development and employment of these materials at industrial scales are significant, as they are excellent catalysts. However, it is still necessary to address the stability of MOFs under various reaction conditions, such as pH, temperature, and organic solvents, which has currently been a challenge for researchers.

A simplified representation of the separation of metal–organic structures (MOFs) from their reaction media by their magnetic properties, which enables their simplified removal—an excellent characteristic for catalysts.
3.2.3. Drug Delivery
The number of biomedical applications of structures based on MOFs has been growing throughout the years due to the excellent versatility of these materials, high porosity, and large available surface area [ 132 ]. One of these key applications is in drug loading, which allows MOFs to work as carriers of the active compounds of various drugs through the body, from small organic molecules to macromolecules, such as nucleic acids and proteins ( Figure 7 ) [ 180 ]. One issue related to this application is the toxicity of MOFs and the materials’ lack of full biocompatibility with the organism [ 181 ]. One advantage is that, due to their high loading capacity, they can be monitored in the body, allowing for the mapping of the reaction mechanism of different drugs, especially in the development of new drugs.

Representation of a metal–organic framework (MOF) as a drug-administrating carrier in tumor cells. They can be used as identifiers of the regions of inflammation and, due to their luminescence, can make it easy to detect the exact region of drug action.
3.2.4. Sensors
Biosensors are promising tools which can detect quick, selective, and sensitive molecules [ 182 ]. Due to the insulating characteristics of MOFs, they show great potential in the preparation of electrochemical sensors supported by carbon, which extends their application to the detection of analytes in different industrial fields, including environmental and biomedical fields, among others [ 183 , 184 , 185 ]. MOFs are great detectors of pollutants due to their affinity for specific groups of organic molecules [ 186 ]. Organic solvents, aromatic compounds, and heavy metal ions can also be detected using MOFs made from lanthanides [ 153 , 187 ].
Due to their adjustable pore size and high surface area, MOFs can also provide an ideal environment to accommodate analytes, allowing them to selectively absorb and release specific substrates through size recognition, effectively increasing signal and detection capabilities [ 188 , 189 ]. In addition, features such as the presence of metal coordination sites and lattice structures make them superior materials for the production of electrode coatings and for analyte detection [ 189 ]. Furthermore, there is the possibility of promoting the enhancement of their sensitivity to certain analytes through functionalization by immobilizing functional sites, initiating specific coordination, or promoting hydrogen bonding interactions with the target analyte [ 188 ].
MOF composites, formed by the incorporation of active biomolecules, such as antibodies, enzymes, and nucleic acids, can improve the selectivity, sensitivity, and detection limits of electrochemical sensors [ 190 , 191 ]. Biomolecule–MOF composites have been designed with an innovative focus on the detection of compounds of interest depending on the application sector. Some key compounds include uric acid [ 192 , 193 ], glucose [ 194 ], microRNAs [ 195 ], H 2 O 2 [ 196 ], carcinoembryonic antigens [ 197 ], acetaminophen, and dopamine [ 193 ]. The main biomacromolecules are enzymes, as they provide more ecological, economical, and sustainable processes [ 29 ].
Enzymes can be incorporated into the structure of metal–organic structures and lead to the formation of sensitive electrochemiluminescence biosensors [ 88 , 198 ]. Examples include the manufacture of structures responsible for the detection of oncoproteins related to tumor proliferation ( Figure 8 ), MOF enzymes of environmental interest [ 199 ], and other applications of industrial interest (such as the immobilization of enzymes for biocatalysis and the monitoring of biochemical reactions) [ 200 ].

Illustration of the mapping of overexpressed macromolecules in tumor cell lines through luminescent metal–organic framework (MOF) composites. This is a widely explored property, which was enabled by their metallic centers and mapped by confocal microscopy.
Wang et al. [ 201 ] developed an enzymatic sensor for the photoelectrochemical detection of hypoxanthine using a nanoscale porphyrin MOF (Al-TCPP(Zn)) modified with the xanthine oxidase enzyme. Al-TCPP(Zn) exhibited an O 2 -dependent cathodic photocurrent, and this signal could be used for photoelectrochemical detection. After the addition of hypoxanthine, the produced biosensor delivered better responses due to the photoreduction of the H 2 O 2 product catalyzed by xanthine oxidase. For the photoelectrochemical detection of hypoxanthine, the proposed sensor exhibited low detection limits, which was comparable to, or even better than, previous methods in terms of linear range and limits of determination; the selectivity was tested against several interferences, showing to have only been slightly affected. The authors also pointed out the reusability of the biosensor.
In Wang et al. [ 202 ], a glucose sensor for cascade biocatalysis constructed via the double confinement of enzymes in a nanocage-based zeolite imidazole (NC-ZIF) structure was evaluated. The enzyme@NC-ZIF showed good mass transport rates and excellent enzyme conformational versatility, due to the increased mesoporosity of the structure. The produced GOx/Hemin@NC-ZIF achieved good efficiency in catalytic cascade reactions in colorimetric and electrochemical glucose biosensors, enabling long-term quantitative analysis and continuous real-time monitoring of glucose in transpiration. Although the GOx/Hemin@NC-ZIF is very promising as a sensor, the method is limited to sweat tests, requiring further studies in order for other body fluids to be applied in innovative physiological and clinical investigations.
3.2.5. Hydrogen Storage
MOFs can store hydrogen due to the large available surface of these materials [ 203 ]. Their hybrid metallic and molecular composition allows for several adjustments, such as the functionalization of possible ligands and their storage under variable temperatures [ 204 ]. MOFs have also become very promising in replacing noble metals during hydrogenation syntheses as Pt, the most commonly used metal to this end, is expensive and, even when compared to MOFs, shows lower yields in hydrogen trapping [ 205 ]. Therefore, a straightforward application of these hybrid nanomaterials is indicated, as they possess pores that serve as “gas pockets”, holding hydrogen atoms for synthesis ( Figure 9 ).

Scheme of metal–organic frameworks (MOFs) capturing hydrogen via their adsorbent properties, a function that can be used for hydrogen storage.
3.2.6. Environmental Applications
The environmental applications of metal–organic structures have been widely explored in recent years, as the growing drive to minimize the impacts of chemical residues has become the focus of extensive research around the world [ 206 ]. MOFs are used as efficient removers of heavy metals in fluids and aquatic environments [ 191 ]. They have been used to remove harmful gases and pollutants [ 207 ], such as carbon dioxide [ 208 ], based on their adsorption capacity [ 209 ]. Ma and colleagues [ 210 ] synthesized a MOF compound given its application as a biosensor of organophosphate pesticides, i.e., common pollutants in the agro-industry. These nanomaterials played a substantial role in the detection and removal of organic substances and solvents [ 211 ], organic dyes [ 212 ], antibiotics [ 213 ], volatile organic compounds [ 210 ] and other contaminants of industrial effluents [ 214 ].
Another essential environmental application is the detection of ammonia levels as a result of bioaccumulation, which has drawn the attention of environmentalists. Depending on concentration ranges, this can cause serious problems in aquatic food chains [ 215 ]. Thus, metal–organic structures are an excellent alternative for identifying levels of environmental pollutants [ 216 ] and in the treatment of effluents [ 217 ]. Their easy synthesis and high reuse rates render them particularly more accessible and targeted in the environmental area, which can be noted by the increase in the number of works published in recent years on this application [ 218 ].
All the applications discussed in this work present several possibilities of exploration in the industrial sector ( Table 2 ). The flexible topology of these materials enables new architectures and, consequently, new properties and applications for MOFs, in addition to those that already exist and are extensively studied.
General applications of composite metal–organic frameworks (MOFs) reported in the scientific literature, and their main areas of interest, such as environmental and biomedical industries, among others.
Thus, it is clear that nanomaterials have been widely used in different areas, which reinforces the need to develop, synthesize, and apply MOFs. A disadvantage of their use is still the high associated costs, with processes becoming economically unfeasible depending on their chemical composition, compared to other conventional structures. However, these nanoparticles are still very promising because such costs can potentially be counterbalanced by the number of possible reuses, the ease of synthesis, the wide range of applications, and the highly flexible structure for different processes. This is reinforced by a series of previously discussed properties, and those not yet tested in association with these materials, bringing the growing use of MOFs in complex industrial processes that benefit from the advancement of nanotechnology into perspective.
4. Enzyme Immobilization with Metal–Organic Frameworks (MOFs)
The immobilization of enzymes onto nanomaterials has revolutionized the use of these macromolecules in various industrial fields, which have been more recently enhanced by the advent of metal–organic frameworks [ 247 ]. The efficient immobilization of enzymes, i.e., its support and methods, is the result of perfect matching of factors depending on the enzyme [ 248 ]. Furthermore, the choice and success of the immobilization methods in the reaction depends of the different properties of the substrates and products, as well as the diversified applications of the products obtained. In addition, all methods have advantages and limitations. Consequently, the optimal immobilization conditions for a given enzyme are determined using experimental assays.
In addition to the main factors mentioned that influence the immobilization process, other parameters are important, such as pH, temperature, ionic strength, charge, and porosity of the support. These factors have a lesser or greater effect depending on the immobilization method. As previously mentioned, MOF characteristics of structural versatility, such as the porosity, large surface area, and organic–inorganic hybridity organization, render MOFs excellent candidates for enzyme immobilization using the most diverse methods ( Figure 10 ) [ 93 , 99 , 247 , 249 , 250 , 251 ]. Regarding the porosity of the support, the mesoporous MOFs have been designed and constructed to obtain a high enzyme loading capacity and to reduce the diffusion resistance of reactants and products during the reaction. According to Xia et al. [ 93 ], the size of the pore openings may allow MOFs to gain size selectivity.

Representation of different techniques of enzyme immobilization onto MOFs.
In Subtopics 4.1–4.4, immobilization studies using MOFs with different methods are presented.
4.1. In Situ Synthesis
In this method, the enzymes of interest and MOF materials (metal ions and organic ligands) are mixed under mild operating conditions in a suitable solution [ 93 ]. Using this immobilization technique, Wu, Yang, and Ge [ 252 ] assessed the stability behavior of some enzymes in organic solvents and compared these results with those obtained with the same proteins in their free form. To this end, lipase B from Candida antarctica , horseradish peroxidase, and cytochrome C were immobilized on the composite ZIF-8. The results showed that, even though the enzymes had different properties, the three immobilized macromolecules showed far superior stabilities in dimethyl formaldehyde, dimethyl sulfoxide, ethanol, and methanol compared to their free counterparts. Furthermore, the immobilized enzymes preserved almost 100% of their initial activity after incubation in the organic solvent, showing that the immobilization strategy protected them against potential denaturation due to the solvents used.
Another study considering MOF parameters was performed by Gascón et al. [ 253 ]. The researchers studied the synthesis and in situ strategies used to immobilize beta-glucosidase and laccase in nanocrystalline MOF platforms which aim to increase the activity of the tested enzymes. According to the results obtained, the immobilization stages in MOF nanocrystals favored the efficiency and the specific activity of the enzymes. Derivatives formed from in situ strategies showed an enzymatic charge above 85% and a loss of enzymatic activity of around 5%. Furthermore, the studied immobilization methodology effectively preserved the enzyme activity in a non-aqueous medium (N, N-dimethylformamide). Therefore, the researchers concluded that enzymes can be effectively immobilized in MOF nanocrystals and that in situ immobilization is a viable alternative in the preparation of immobilized biocatalysts.
Even though the in situ approach to immobilizing enzymes in MOF was efficiently conducted and requires mild reaction conditions, not all MOFs are ideal for this process. This is because the mode of enzyme dispersion and their subsequent location on the support can negatively affect the immobilization reactions [ 252 ].
4.2. Covalent Bonding
Unlike the in situ strategy, immobilization by covalent bonding occurs when the already-synthesized MOF is coated with substances capable of binding to the amino groups on the enzyme surface [ 254 ]. Many MOFs are susceptible to modification with functional groups to turn them into immobilization matrices [ 93 ].
Using this strategy, Cao and collaborators [ 255 ] immobilized soy epoxide hydrolase in UiO-66-NH2 MOF with glutaraldehyde as a binding agent, later applying this derivative in the biosynthesis of a (R)-1, 2-octanediol enantiomer. The results showed that the derivative presented a remarkable enzymatic load (87.3 mg/g), and recovered activity of 88%, as well as operational stabilities related to pH, temperature, and contact with organic solvents comparable to the frozen form of the enzyme under study. In addition to the improvements in the enzymatic characteristics associated with immobilization, the protein, when tested for the synthesis of (R)-1, 2-octanediol, delivered an enantiomeric excess of 81.2%. Therefore, the authors concluded that the immobilization of soy epoxide hydrolase on MOFs via covalent bonding showed strong potential for both improving enzyme characteristics and for being applied in enantiomeric reactions.
While seeking to further optimize the preparation and reuse of enzymes immobilized in MOFs, Wang et al. [ 251 ] incorporated iron oxide during MOF synthesis and used the final support to immobilize a Candida rugosa lipase via covalent bonding. The methodology employed by the researchers is justified by the ease of separating the derivative from a given reaction medium with the aid of a simple magnetic field. The derivative obtained was tested for the hydrolysis of olive oil and delivered a conversion rate of more than 65% after 6 h of reaction at 65 °C. Furthermore, the enzyme immobilized in the composite retained about 60% of its initial activity after 10 consecutive reaction cycles. Therefore, according to the above article, the synthesized support had both a large surface area and strong magnetic characteristics, which render this specific composite a good candidate support for enzyme immobilization.
4.3. Surface Immobilization
Surface immobilization (or adsorption) is the most widely used immobilization technique [ 254 ] due to the relatively low associated costs and the easy-to-perform methodology [ 93 ]. Because it is a versatile process, adsorption can be used to immobilize different enzymes on different supports, including MOFs [ 93 , 254 , 256 , 257 ]. In this technique, enzymes bind to the support through weak interactions such as van der Waals forces, hydrogen bonds, or electrostatic forces; therefore, they can be easily removed from the support via variations in pH and temperature, for example [ 93 ]. However, physical adsorption is still widely used and investigated due to its simplicity and the non-requirement for complex reagents [ 93 , 254 ].
In an attempt to compare advantages and disadvantages of this technique, Cao et al. [ 257 ] immobilized a lipase from Bacillus subtilis in a Cu-BTC-based MOF via physical adsorption and used the obtained derivative in an esterification reaction. The researchers obtained excellent results and demonstrated that the derivatives showed high operational stability and good enzymatic activity. Even after 10 consecutive reaction cycles, the lipase retained 90.7% of its initial activity and 99.6% of its initial conversion.
Another study on surface immobilization was performed by Pang and co-workers [ 256 ]. The researchers studied the support synthesis and the subsequent laccase immobilization on mesoporous Zr-MOF. According to the results, the laccase@Zr-MOF complex exhibited an adsorption capacity of 221.83 mg/g, wide temperature and pH distributions, and better stability when compared to that of the free laccase. In addition, the immobilized enzyme was able to maintain about 50% of its activity after 10 reaction cycles of contact between the derivative and ABTS, and retained 55.4% of its initial activity after three weeks of storage. With these numbers, the authors concluded that the immobilization method was successfully employed and that the synthesized support is a potential candidate for laccase immobilization via physical adsorption.
4.4. Entrapment
The immobilization strategy using entrapment or encapsulation is based on the confinement of the enzyme to a microenvironment located inside the support [ 93 ]. Contrary to other techniques, immobilization by entrapment causes isolation of the enzyme from the reaction medium, and also gives the protein better stability against potential denaturation caused by organic solvents, high temperatures, or sudden changes in pH [ 93 , 173 ]. Furthermore, using a MOF as support for this type of immobilization has extra advantages compared to other matrices: (i) MOFs can be synthesized according to their most suitable pore size (supports can have specific sizes for each type of substrate to allow for the efficient insertion and binding of the immobilized enzyme, reducing diffusional limitations); (ii) large enzyme loads can be achieved using MOFs as a consequence of their pore size; and (iii) encapsulated enzymes show a lower tendency to detach from the support [ 173 ].
Making use of such advantages, Li et al. [ 258 ] encapsulated a nerve agent detoxifying enzyme (organophosphorus acid hydrolase) in a mesoporous zirconium–MOF composite. The researchers reported that the synthesized support exhibited high enzyme loading capacity (12 wt%) and considerably improved thermal and storage stabilities.
In another study, Lian and co-workers [ 259 ] immobilized two enzymes in a tandem nanoreactor using a hierarchically structured MOF (PCN-888). The immobilized enzymes were glucose oxidase (GOx) and horseradish peroxidase (HRP). For the immobilization of both proteins to be successful, the researchers had to follow an encapsulation order: GOx followed by HRP. In the described process, the largest pores of the MOF (6.2 nm) were used to accommodate glucose oxidase, the 5.0 nm cavities accommodated horseradish peroxidase, and the smallest cavities (2.0 nm) remained unobstructed and accessible for the input of substrates and the output of products. Therefore, from the results, it was possible to conclude that the MOF was able to protect both enzymes against potential denaturation and considerably increased their operational stabilities ( Table 3 ).
Advantages and disadvantages of different enzyme immobilization strategies in/onto MOF.
5. Future Trends
The application of MOFs combined with biocatalytic agents, including natural enzymes, is relatively recent. This integration has demonstrated an interesting synergistic performance in biocatalysis, due to the increased stability and reusability of encapsulated biocatalysts and the expansion of their applications into other fields [ 86 , 260 ]. Since the porosity properties of MOFs were identified, their investigation has developed exponentially [ 83 ]. However, although significant progress has been made, the investigation on enzyme–MOF composites is still in early stages, with many challenges still being a hurdle to the expansion of their applications [ 260 ]. The performance of this composites is influenced by several factors, including conformation; biomolecule activity and size; morphology; and the structural irregularity of particles in the design, preparation, and analysis of functionalized MOFs [ 82 , 86 ].
The use of MOFs for enzyme encapsulation is a fast developing field, and a significant increase in the number of studies on their properties in a short period of time leads us to believe that new highly effective biocatalysts are on the verge of being developed [ 261 ]. Great efforts have been made to this end; however, addressing the existing obstacles and improving current strategies are necessary so that enzyme–MOF composites can be fully suitable for practical applications [ 86 , 262 ]. There are expectations of future investigations in this area [ 260 ]. Challenges include the low diversity of biocompatible organic ligands and the toxicity of metals, in addition to the potential application of metals and ligands that have not yet been employed to this end [ 261 , 263 ].
To meet enzyme requirements of high activity and stability for practical applications and to elucidate the catalytic behavior of enzyme–MOF systems, it is necessary to investigate and improve the spatial structure of enzymes in MOFs [ 260 , 262 ]. This includes the establishment of spatial distributions that allow the confinement of multiple enzymes in MOFs, since the effective control over the location and orientation of enzymes can contribute to an increase in catalytic efficiency and a reduction in the resistance to the mass transfer of reagents [ 262 ]. In addition, exploring the suitable pore size and distribution profiles of MOFs is certainly an essential step in the encapsulation of several enzymes. Appropriate pore sizes can be optimized to meet specific criteria of enzyme accommodation, improving catalytic properties [ 260 ].
This review aimed to gather and discuss key information on MOFs, such as their synthesis, properties, and roles in enzyme encapsulation. We believe that the discussions, methodologies, and case studies presented can be helpful to readers and researchers interested in this topic. We also believe that this work can be used as a tool in the development of MOF-based materials for diverse applications, especially those related to enzymatic biocatalysis.
6. Conclusions
This review systematically reported on the mechanisms of action, latest advances, challenges, and future perspectives of the use of MOFs as support substrates in enzyme immobilization. MOFs are considered excellent candidates to support immobilization routes. This is because they present a wide variety of physicochemical and biological properties owing to the versatility of their composition. These impart properties include structural flexibility, adjustable pore size, large surface area, and the possibility of post-synthetic modifications, among others.
The chemistry of MOFs has developed exponentially since the porosity properties of these materials were identified. However, progress still needs to be made regarding the stability of MOFs under different reaction conditions (such as pH, temperature, and organic solvents), and in the storage of this material, constituting the most challenging aspects of their research. The elucidation of the different interactions between the MOF ‘housing’ and the enzymes that reside in their microenvironments during the various encapsulation processes is also paramount, since this can guide the construction of enzyme-MOF composites of high stability and bioactivity.
As the design and synthesis of MOFs with specific functionality at predetermined pore locations improve, interactions with biomolecules become more specific, resulting in more selective structures. Additionally, the recent methodologies and technologies based on computational chemistry can contribute to the development of new versatile projects of enzyme–MOF composites of high efficiency. However, to scale up laboratory-scale processes to larger scales, a more comprehensive understanding of the nature of enzyme–MOF composites is still required.
According to the discussion presented in this article, it can be concluded that enzymes immobilized on MOF supports clearly show better catalytic activity and operational stability than when compared to those obtained with their free form. In addition, such composites show an excellent maintenance of their initial activity after incubation in organic solvents by reaching a maximum percentage, which confirms that the immobilization strategies protect these proteins against possible solvent-related denaturation. Finally, it is expected that this review article, having presented synthesis strategies, properties, and applications of both MOFs and enzyme–MOF composites, can be a significant contribution to the advancement of the research on supports for enzymatic catalysis.

Acknowledgments
We gratefully acknowledge the financial support of the following Brazilian Agencies for Scientific and Technological Development: Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Ensino Superior (CAPES) (finance code 001).
Funding Statement
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, project number 311062/2019-9). Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP, project number PS1-00186-00216.01.00/21).
Author Contributions
Writing—original draft preparation, A.R.M.S., J.Y.N.H.A., J.E.S.S., J.G.L.N., P.G.d.S.J., M.V.P.R. and J.C.S.d.S.; writing—review and editing, M.V.P.R. and J.C.S.d.S. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 03 June 2024
ChatMOF: an artificial intelligence system for predicting and generating metal-organic frameworks using large language models
- Yeonghun Kang ORCID: orcid.org/0000-0001-5191-5735 1 &
- Jihan Kim ORCID: orcid.org/0000-0002-3844-8789 1
Nature Communications volume 15 , Article number: 4705 ( 2024 ) Cite this article
1779 Accesses
18 Altmetric
Metrics details
- Computational methods
- Metal–organic frameworks
- Structure prediction
ChatMOF is an artificial intelligence (AI) system that is built to predict and generate metal-organic frameworks (MOFs). By leveraging a large-scale language model (GPT-4, GPT-3.5-turbo, and GPT-3.5-turbo-16k), ChatMOF extracts key details from textual inputs and delivers appropriate responses, thus eliminating the necessity for rigid and formal structured queries. The system is comprised of three core components (i.e., an agent, a toolkit, and an evaluator) and it forms a robust pipeline that manages a variety of tasks, including data retrieval, property prediction, and structure generations. ChatMOF shows high accuracy rates of 96.9% for searching, 95.7% for predicting, and 87.5% for generating tasks with GPT-4. Additionally, it successfully creates materials with user-desired properties from natural language. The study further explores the merits and constraints of utilizing large language models (LLMs) in combination with database and machine learning in material sciences and showcases its transformative potential for future advancements.
Similar content being viewed by others
Augmenting large language models with chemistry tools
Autonomous chemical research with large language models
Dataset of solution-based inorganic materials synthesis procedures extracted from the scientific literature
Introduction.
The realm of generative artificial intelligence (AI) is witnessing an unprecedented surge, predominantly fostered by a new generation of computational tools known as large-scale language models (LLMs) 1 , 2 , 3 , 4 , 5 . These innovative models are deeply rooted in a novel architectural design paradigm, referred to as transformer models 6 . Their capabilities, however, stretch far beyond the domain of basic language tasks. These models are programmed to perform tasks that mimic certain aspects of human cognition, such as processing and applying new information based on few or no examples (few-shot and zero-shot learning) 3 , 5 , 7 . This is achieved through the understanding of vast volumes of text data, underscoring the immense potential held by these models. A significant advancement in this swiftly changing domain is the rise of autonomous LLM agents that employ LLMs for diverse functions 8 ( https://github.com/yoheinakajima/babyagi ; https://github.com/Significant-Gravitas/Auto-GPT ). The application of LLMs for analyzing information, extracting pertinent details, and creating responses is becoming increasingly popular in several research fields, owing to their capacity for independent operation 9 , 10 , 11 , 12 . They harness the capabilities of LLMs using prompt engineering 13 , 14 , 15 , fine-tuned them 16 , 17 , 18 , or meld them with other scientific tools 8 , 19 , 20 , 21 . These systems are highly effective in autonomously processing data and producing outcomes without the need for human involvement.
Despite marked progress in application of LLM across diverse fields such as chemistry 20 , 22 , 23 , 24 , 25 , 26 , medicine 27 , 28 , and biology 29 , 30 , the full potential of its advanced technology within materials science remains largely untapped. This limitation primarily stems from two considerable challenges. Firstly, the inherent complexity of these advanced materials, such as zeolite, porous polymer networks, covalent-organic framework, and metal-organic framework, poses a significant hurdle. These materials often lack suitable text-compatible input representations, which hinders the ability to fully capture their intricate properties 31 , 32 , 33 , 34 . This difficulty in encoding materials for LLMs restricts their understanding and processing capabilities. Secondly, there is a notable scarcity of material-specific training data in the field. In comparison to other disciplines, materials science lags behind due to fewer dedicated databases and their associated data, exacerbating the challenge of representing this scant data in a text format suitable for LLMs. Despite these obstacles, there are ongoing attempts to leverage the capabilities of LLMs in materials science 15 , 18 , 22 . However, so far, these efforts have primarily focused on extracting data from scientific literature and generating responses based on this extracted data, with the actual material itself remaining a largely untouched resource. As such, the exploration and realization of the full potential of LLMs within the sphere of materials science still beckons.
In this work, we highlight the development of a methodology that employs AI systems to automate the generation of new materials and the prediction of their properties, focusing specifically on metal-organic frameworks (MOFs) 35 , 36 , 37 . MOFs are used in many chemical applications 38 , 39 , 40 , 41 due to their large porosity 42 , 43 , 44 , high surface area 43 , and exceptional tunability 45 . To this end, we have developed the artificial intelligence system for MOFs (called ChatMOF), which holds the potential to predict MOF properties from text-based inquiries and to generate MOFs with specified properties (i.e., inverse design). Recently, there has been a rising interest in combining LLMs with traditional machine learning models in the materials field 46 , and this pioneering approach can potentially significantly bridge the gap between the novice users and the computational and machine learning tools, which can potentially facilitate the progress in developing new materials for various applications.
Design of ChatMOF
The effectiveness of autonomous LLM agents is predicated on its capability to accurately extract essential details from textual inputs and offer relevant responses, irrespective of the presence of a rigidly structured query 22 . This concept is clearly illustrated in ChatMOF, as demonstrated in Fig. 1a . A user may pose a query in textual form regarding the properties of a material, to which ChatMOF responds by supplying a detailed description related to the material in question. Moreover, the operational scope of this system extends beyond the simple retrieval of information. When a user expresses the need to generate a MOF with specific properties, ChatMOF is capable of generating the requested material structure accordingly.

a A Conceptual image that explains the ChatMOF. When a user poses a textual question about the properties of a MOF, an appropriate answer is provided by ChatMOF. If a user desires to generate a new MOF, ChatMOF is capable of creating a new MOF that satisfies the condition. b The schematic image of ChatMOF. ChatMOF comprises three core components: an agent, toolkit, and an evaluator. Upon receiving a query from human, the agent formulates a plan and selects a suitable toolkit. Subsequently, the toolkit generates outputs following the proposed plan, and the evaluator makes these results into a final response.
In the context of ChatMOF, LLM functions as central coordinators, managing and evaluating processes, similar to how a central processing unit (CPU) operates in computing. While the LLM excels in general reasoning tasks, its performance in specialized areas is less robust 20 . Supplementary Fig. S1 illustrates this by presenting a direct MOF-related question to the LLM, highlighting the constraints faced when engaging an LLM in specialized tasks. Nonetheless, LLM demonstrates remarkable proficiency in assimilating and leveraging diverse databases and machine learning models. This strength originates from their inherent reasoning abilities and fluid processing capabilities 47 , 48 . ChatMOF uses the LLM to systematically organize and apply various tools for information gathering, similar to a well-executed algorithm in computer programming. This synergy allows the system to precisely predict material properties, retrieve synthesis methods from a text-mined database, and fabricate new materials with preset properties.
As depicted in Fig. 1b , ChatMOF is composed of three main components: an agent, toolkit, and an evaluator. The agent processes human queries through four primary operational stages (i.e., data analysis, action determination, input management, and result observation), following the methodology outlined in the ReAct 49 and MRKL papers 50 . Initially, the user’s query is established as the objective, followed by systematic planning to determine the steps to meet this objective. Subsequently, ChatMOF decides on the appropriate tool to employ from the available options. After the chosen tool is executed, the observed results serve as the basis for evaluating whether a final answer can be generated. If feasible, the final answer is presented, otherwise, the process cycles back to the thought step to formulate a new strategy.
ChatMOF employs an assortment of tools to acquire, predict, or generate material information. These tools can be primarily classified into four categories: table-searcher, internet-searcher, predictor, generator, and utilities. Table-searcher involves obtaining desired information from existing data. The predictor utilizes machine learning models to obtain specified properties. The generator refers to the tool that constructs material structures fulfilling certain properties. Lastly, the utilities encompass a variety of aids like calculators, file saving and reading functions, visualizer, and internet-searcher.
Due to the facile synthesis MOF structures, there are many different database associated with the MOF structures: (1) computational-ready experimental MOFs (CoREMOF) 51 , 52 and (2) quantum MOF (QMOF) database 53 . The CoREMOF database is an archive of synthesized materials present in a CSD MOF subset 54 , encompassing computations of various properties of MOFs including geometric descriptors. The QMOF database is populated with electrical property data, such as band gap, formation energy, HOMO, and LUMO, derived through DFT calculations. When a user demands these electrical properties, ChatMOF seeks and extracts them from the QMOF database.
As such, if a user seeks information about a specific MOF that is included in these databases, ChatMOF can locate and deliver the desired information from the pre-tabulated data. Figure 2 provides an illustrative example of a table-search operation conducted by ChatMOF. In response to a user query, ChatMOF automatically determines the optimal method to extract the necessary data. Subsequently, it creates a Python code tailored to retrieve specific information from the database in accordance with the premeditated strategy. This code typically uses the “pandas 55 ” library to extract or filter relevant details. The drafted code is then executed within the ChatMOF’s designated executor. After processing the results of this operation, ChatMOF automatically determines the subsequent procedures needed to produce the final answer, which is then delivered as the requested response.

The human’s question prompts the system to devise a strategy. From this, Python code is generated and executed to extract the desired information, which is then returned as the final answer. Tested on November 18, 2023 at 17:41 KST with GPT-4.
The versatility of ChatMOF extends to handling diverse table data derived from text mining or rule-based coding processes. For questions related to the building blocks of a MOF, the MOFkey 31 database proves to be instrumental. This particular database leverages rule-based methods to obtain insights about the organic linkers and metal clusters of a MOF, providing details about its topology and the potential presence or absence of interpenetration. In addition, for users seeking guidance on MOF synthesis, the DigiMOF 56 database becomes a valuable resource. DigiMOF provides an array of synthesis conditions, extracted via text mining techniques from MOF-related academic papers, and includes information on organic and metal precursors, and solvent.
The accuracy of the look-up table search is contingent on the pre-calculated values available in the specific files. And for queries regarding the properties of MOFs that are not available, computational simulation can serve as an attractive alternative method, but unfortunately, simulations are a time-intensive process and an abundance of computational resources 57 . The best resolution to such challenges is the application of machine learning models, which enable high-accuracy predictions grounded in extensive data. In the case of pre-trained machine learning models, predictions are quick and can be made for a significant volume of substances simultaneously, making it an excellent toolkit for integration into ChatMOF.
As an appropriate tool for the prediction task, ChatMOF uses the MOFTransformer 58 , 59 model that has been developed in our group for the universal prediction of MOF properties. This model leverages both local features, such as atoms and bonds, and global features like surface area and topology. Having undergone pre-training with one million hypothetical MOFs and subsequent fine-tuning for specific properties, MOFTransformer shows high performance in predicting various properties. Moreover, it affords the convenience of predicting the desired property by retaining the model structure while altering the weights of a model that’s been fine-tuned for the desired property.
The key to generating accurate responses is selecting the appropriate fine-tuned model with the MOFTransformer and the material to which it will be applied, based on the query. Similar to HuggingGPT 8 , ChatMOF does not directly compute the material properties, but it rather selects the appropriate machine learning model. Figure 3 shows the example prompt for ChatMOF using the MOFTransformer predictor. When a user requests information like “Identify the MOF with the highest hydrogen diffusivity at 77 K, 1 bar,” the agent develops a strategy to address the query. It chooses the most suitable fine-tuned MOF transformer model, in this instance, “hydrogen_diffusivity_dilute_77K,” tailored to the task. The agent then identifies the materials for prediction (here, all materials) and utilizes the selected model for making predictions. Subsequently, we conduct a table search to identify the substance with the highest value, as determined by the machine learning model. This process takes into account specific aspects of the fine-tuning model, including units, conditions, and the logarithmic scale. Since the hydrogen diffusivity model outputs logarithmic values, we execute code to identify the substance with the highest value, converting these values back to their exponential form to obtain the final result.

The predictor sets up a plan to solve the question, an appropriate model, and target material. Based on this, it uses machine learning to predict the value, which is then used to derive the final answer. Tested on November 20, 2023 at 10:59 KST with GPT-4.
Finally, a key aspiration among researchers in the field of MOFs is the inverse design of MOFs exhibiting desired properties. In materials science, various generative models, including Generative Adversarial Networks (GAN) 60 , 61 and Diffusion models 62 , have been employed for inverse design. However, due to the inherent complexity of MOFs, which includes a large number of atoms, large void fraction, and complex topologies, an atom-by-atom inverse design approach has been elusive. As a workaround, MOF inverse design has been facilitated top-down approaches leveraging through genetic algorithms 63 , 64 , 65 , Variational Autoencoders 66 (VAE), or reinforcement learning 67 for the selection of building blocks and their placement into suitable topologies.
Genetic algorithms are notably suitable for integration with the LLM. As a bio-inspired optimization methodology, genetic algorithms operate on a selection, mutation, and crossover principle, making them adaptable and efficient 68 . For their application to MOFs, these frameworks must be delineated by genes comprising topology and building blocks. For instance, a representative MOF, HKUST-1, can be depicted as tbo+N17 + N10, with tbo representing topology and N17 and N10 representing the building block notations. As these gene representations are textual, they facilitate the application of genetic algorithms using an LLM.
Figure 4 showcases the utilization of a genetic algorithm by ChatMOF to fabricate a MOF per user specifications. Upon receiving a user query, the system formulates a strategy, optimized through algorithmic processes, based on genetic algorithms. It also identifies the target property and determines the loss function most suited for the objective, such as choosing the maximum, minimum, or closest value. Following the algorithmic strategy, ChatMOF algorithmically selects parent genes from the existing database, aligning with the predefined loss function. In the genetic algorithm employed, parent genes demonstrating high potential based on the desired target characteristics are selected, enhancing the probability that the resultant child gene will exhibit the targeted property more prominently. These children are then transformed into a structure file, and their properties are estimated through machine learning. This procedure is reiterated a fixed number of times, generating multiple generations of children with each generation yielding MOFs progressively nearer to the target. From the created structures, the one that aligns most closely with the question is finally chosen and presented as the response.

The generator establishes a plan, objective, and property for the human question. Based on this, it finds parents that satisfy the objective. It uses a genetic algorithm to create children genes and generate structures. This is repeated for a number of cycles to generate new MOFs, which are used to derive the final answer. The full process of the example is provided in Supplementary Note S1 and Supplementary Data 1 . Tested on October 13, 2023 at 14:26 KST with GPT-4.
Moreover, ChatMOF is engineered to perform a diverse set of utilities, which extend beyond the realms of LLM. This includes capabilities such as file search, internet search, and even simple calculations. These additional functionalities are primarily enabled by leveraging the varied capabilities provided by LangChain ( https://github.com/hwchase17/langchain ), enhancing the overall functionality and utility of ChatMOF. Additionally, the development of unit-conversion and visualization tools has broadened ChatMOF’s range of capabilities. Thus, it is not merely a material analysis tool, but a comprehensive system that can accommodate a wide array of tasks and operations.
Furthermore, ChatMOF incorporates the Atomic Simulation Environment (ASE) 69 library as an integral tool to facilitate diverse operations on material structure data. The ASE library holds considerable importance in the field of materials science due to its capabilities, including atom manipulation, cell information acquisition, and visualization, among others. Similar to the function of a table searcher, when confronted with a query, ChatMOF devises a strategic plan and constructs suitable Python code utilizing the ASE library to fulfil the query’s demands. Subsequently, this code is executed.
With these capabilities, ChatMOF is programmed to efficiently process intricate and multi-step tasks. As depicted in Fig. 5 , ChatMOF efficiently responds to the query “Provide the CO 2 Henry coefficient of XEGKUR at 298 K in mol/cm 3 Pa”. Initially, ChatMOF employs a predictor to ascertain the Henry coefficient of CO 2 . It then employs a unit conversion tool to perform the conversion from mol/KgPa into mol/cm 3 Pa units. In this conversion process, ChatMOF identifies the requirement for additional data about the density of XEGKUR. It then conducts a table search to obtain this necessary Density value. With the density figure in hand, ChatMOF applies the unit conversion tool to transform g/cm 3 to kg/cm 3 , ultimately synthesizing all this information to arrive at the final answer.

ChatMOF uses 4 different tools (predictor, unit-converter, table searcher, python repl) to solve the problem. The full process of the example is provided in Supplementary Data 1 . Tested on November 25, 2023 at 18:23 KST with GPT-4.
Further illustrating ChatMOF’s capabilities, Supplementary Figs. S4 and S5 display its approach in addressing complex, non-intuitive problems that are challenging to translate into structured data. When processing complex inquiries, such as “What is the relationship between the amount of H 2 absorption and accessible volume in a MOF ChatMOF identifies the core issue—the interplay between these two properties—and determines the most effective approach to generate an accurate response. Additionally, leveraging its material domain expertise, the LLM calculates the absorptions of O 2 and N 2 , subsequently deriving the selectivity ratio, to answer questions like “Find the O 2 /N 2 selectivity of XEGKUR at 298 K, 1 bar” without requiring extra information. These scenarios highlight ChatMOF’s proficiency not just in translating natural language into structured data, but in interpreting and strategically processing complex queries to develop a tailored and efficient resolution plan.
To evaluate performance of ChatMOF, analysis was conducted for “search task”, “prediction task”, and “generation task”. For evaluation purposes, questions for ChatMOF were created utilizing GPT-4 to generate various sentences about the given properties of a MOF. This approach of utilizing GPT-4 for question generation aimed to minimize author involvement, increase measurement fairness, and generate a variety of questions. The prompts used to elicit these questions are displayed in Supplementary Note S2 and S3 . The respective questions for each task can be found in Supplementary Tables S1 – 3 . In this investigation, the accuracy measure ensures that the AI’s process of reasoning, from devising a plan to tackle the problem to choosing the toolkit, and then deciphering the results, remains untainted by logical discrepancies. This is because ChatMOF is intended to imitate the expert’s systematic approach to problem-solving.
The accuracy analysis of ChatMOF involved the use of three labels: “True”, “False (token limit exceeded)”, and “False (logic error)”. The “True” label indicates that ChatMOF’s processes correctly matched the logic required to produce an accurate answer. The term “False (Token Limit Exceeded)” was used when the token count in LLM surpassed the maximum allowance of 4000, thus obstructing further progress. Lastly, the “False (Logic Error)” label designated situations where an error in ChatMOF’s logic resulted in an incorrect response or an anomaly. Such situations typically arise due to the formulation of a flawed procedure for obtaining an answer or due to an error in interpreting the output, causing the system to deviate from the intended outcome. When encountering the ‘token limit exceeded’ error, it arises within the LLM, causing the process to halt without further progress. This error mainly influences the recall value because it halts the analysis once the token limit is exceeded. As for the ‘logic error’, it stems from the reasoning abilities of the LLM. This leads to incorrect responses, which in turn affect the precision value. This error type directly challenges the LLM’s capability to provide accurate and logical answers.
Figure 6 presents the accuracy measurements for the three tasks using ChatMOF with GPT-4. Accuracy was measured for 100 sample questions for the search and prediction tasks, and 10 sample questions for the generation task. The number in the bar graph indicates the number of questions in each class. Both the search and prediction tasks rendered accurate answers with high frequency. Excluding ‘Token Limit Exceeded’ instances (4 out of 100, 6 out of 100, and 2 out of 100, for search, prediction, and generation tasks respectively), they exhibit high accuracies of 96.9% and 95.7%, respectively. For the generation task, the accuracy stood at 87.5%. Due to the inherent complexity of this task, compared to the other two, the accuracy rate observed is lower. Regardless, all three tasks report high accuracy rates, and these tasks carry significant weight because these questions cannot be effectively answered by directly querying LLMs. (Refer to Supplementary Fig. 1 ). This is because LLM often fall short in providing precise information due to their lack of detailed material-specific data, particularly in the case of properties that are challenging to ascertain via internet searches.

Accuracies were evaluated based on three labels: True, False (token limit exceeded), and False (logic error). False (token limit exceeded) indicates that the LLM has exceeded the maximum token allowance, while False (Logic Error) indicates that the ChatMOF’s logic has resulted in an incorrect response or anomaly. The number in the bar represents the count of each label.
Also ChatMOF, when integrated with GPT-4, exhibits superior performance compared to its integration with GPT-3.5-turbo. As evidenced in Supplementary Fig. S6 , the accuracy of ChatMOF with GPT-3.5-turbo stands at 95%, 91%, and 77.8% for the search, prediction, and generation tasks respectively, excluding instances of “Token Limit Exceeded”. Across all tasks, GPT-4 consistently outperforms GPT-3.5-turbo in accuracy. The enhanced accuracy of GPT-4 can be attributed to its improved algorithms and processing capabilities, particularly during the planning phase. Supplementary Fig. S7 illustrates the distinct approaches that GPT-4 and GPT-3.5-turbo take when presented with the same query: “How does the pore limiting diameter of YUSGID_clean compare with other materials?”. While GPT-3.5-turbo processes values for all materials mentioned in the query, leading to a token error and subsequent inability to provide an answer, GPT-4 employs a more comprehensive algorithm. The algorithm analyzes the distribution of all materials, using metrics such as mean, variance, and quartile values of the property in question. This approach enables GPT-4 to determine the relative position of the target material in the overall distribution, thus delivering a more informative response to the user.
It is worth noting that the primary cause of encountering token limit errors is influenced by LLM’s coding proficiency rather than the specific maximum token count utilized. Supplementary Fig. S8 illustrates the accuracies of employing GPT-3.5-turbo-16k (with a maximum token limit of 16,835), which boasts a greater maximum token limit compared to GPT-3.5-turbo (with a maximum tokens limit of 4097). Despite the augmented maximum token capacity, the frequency of surpassing this limit has not decreased. This phenomenon arises from suboptimal code execution, as indicated in the comparison with GPT-4 (see Supplementary Fig. S7 ). These results print the entire table and greatly exceed the typical maximum token threshold, thereby leading to token limit errors. Consequently, it becomes imperative to enhance the performance of the LLM itself, rather than merely expanding the token count, in order to improve overall performance.
For the “search task,” the writing of code utilizing the pandas library significantly impacts the accuracy. ‘Token Limit Exceeded’ generally occurs when the output code surpasses the permissible token count. This frequently arises when all relevant materials that satisfy a given condition are provided (for example, when a list of materials with a particular property is listed), or when the question contains a comparative clause such as “compared to other materials.” ‘Logic Error’ typically appears due to an incorrect algorithmic approach or a code error. An instance of this would be when a request to provide 10 specific items is met with a misguided strategy that solely aims to “extract high values,” failing to retrieve the specified number of items.
During the “prediction task,” difficulties often occur in the interpretation process of the observed values using machine learning techniques. Both the ‘Token Limit Exceeded’ and ‘Logic Error’ occurrences can stem from the effort to draw the correct answer from the table based on the estimated values. ‘Logic Errors’ can manifest not only during the table search phase but also during the strategy formulation stage. An incorrect algorithm could either cause the loading of an unsuitable model or generate an input that is incompatible with the intended model.
Inverse design validation
The inverse design process is bifurcated into two stages: planning for a genetic algorithm and executing it. Predominantly, errors in inverse design surface during the planning stage. In this phase, the goal and properties of the genetic algorithm are defined, and code is devised for selecting parental genes for use in the genetic algorithm. As indicated in Fig. 6 and Supplementary Figs. S6 and S8 , ChatMOF demonstrates considerable accuracy (7 out 10) in this aspect of inverse design. The primary errors during planning are encountered in the selection of parental genes. These errors typically arise when a parent gene is not retrievable from the database. When the objective function targets a maximum or minimum value, an appropriate parent gene is usually identifiable. However, issues occur if the objective function is inaccurately set, leading either to an inability to find any suitable parent genes or to an overwhelming abundance of them. A lack of suitable parents aligning with the set objective can easily result in a logic error, while an excess of such parents may trigger a token limit error.
The execution phase of the genetic algorithm using LLM plays a crucial role in the efficiency of material generation. It is worth noting that the performance of the LLM is critical to the efficiency of the genetic algorithm execution. Supplementary Fig. S9 illustrates the degree of overlap between these children and their parents, as well as the number of children generated by GPT-4 and GPT-3.5-turbo. It is observed that GPT-3.5-turbo tends to generate children that are almost identical to the parents, despite instructions not to duplicate an existing parent. This indicates that GPT-3.5-turbo is not suitable for executing genetic algorithms. On the other hand, GPT-4 shows a much lower overlap percentage of around 30% between parents and children. One issue with the generated children is their inconsistent number. Although they are intended to create 100 new children per topology, typically only about 60 are generated. This inconsistency is due to limits in the model’s token count and GPT’s generation capabilities. When compared to traditional code-based algorithms, which can generate the exact number of children without repetition, LLMs currently need to improve to match this efficiency in genetic algorithms.
Nevertheless, it is worth noting that ChatMOF has successfully generated materials that meet user requirements. Figure 7 shows the distribution of MOFs generated by the genetic algorithm under two different scenarios. Figure 7a displays the structures generated in response to the question, ‘Can you generate structures with the largest surface area?’ In this case, ChatMOF is configured to interpret the property as accessible surface area, with the setting adjusted to maximize this parameter. The MOFs initially have a wide distribution, averaging 3748 m 2 /g. However, by the third generation, the average peaks at 5554 m 2 /g. Similarly, Fig. 7b illustrates structures that aim for a hydrogen uptake of approximately 500 cm 3 /cm 3 at 100 bar and 77 K in response to the question, ‘Can you generate structures with a hydrogen uptake of about 500 cm 3 /cm 3 at 100 bar and 77 K?’ The final generation of the LLM genetic algorithm is narrowly focused around 500 cm 3 /cm 3 , indicating its effectiveness. The initial range was between 250 and 650 cm 3 /cm 3 .
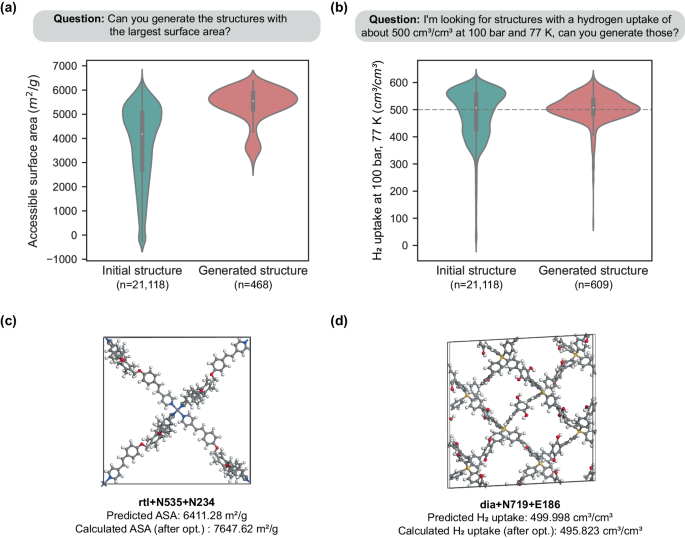
a Violin plot illustrating the distribution of initial and generated structures for a question related to the maximum surface area value. The inner box represents the interquartile range (IQR) and the white horizontal line inside the box indicates the median of the distribution. The number in parentheses represents the number of data. b Violin plot depicting the distributions of initial and generated structures in response to a query about hydrogen uptake values close to 500 cm 3 /cm 3 . The gray horizontal line indicates the location of 500 cm 3 /cm 3 . c Illustration of the MOF with the largest surface area as generated by ChatMOF. ASA stand for accessible surface area. d Representation of the MOF with an H 2 uptake value closest to 500 cm 3 /cm 3 at 298 K, 1 bar, as generated by ChatMOF. The term “opt” stands for the geometric optimization of structures via molecular dynamics simulation. The data used to plot the violin is provided in Source Data 1. Tested on October 13, 2023 at 14:26 KST with GPT-4.
Figures 7c, d depict the final structures for the queries in 7(a) and 7(b). The optimal structure in 7(c), rtl+N535 + N234, boasts the highest surface area amongst the generated MOFs. The predicted value stands at 6411.28 m 2 /g. Upon performing a geometric optimization and calculating accessible surface area using Zeo + + 70 , the surface area is revealed to have 7647.62 m 2 /g. This value is notably higher when compared to the CoREMOF database. Supplementary Fig. S10 illustrates the distribution of accessible surface areas within CoREMOF. This particular structure’s surface area ranks the third-highest position in the CoREMOF ranking. In a similar vein, the optimal configuration of dia+N719 + E186, showcased in Fig. 7d , possesses a predicted H 2 uptake of 499.998 cm 3 /cm 3 at 1 bar and 298 K, mirroring the stipulated target of 500 cm 3 /cm 3 . Following geometric optimization of this structure, its uptake was calculated using RASPA, yielding a value strikingly close to the goal, at 495.823 cm 3 /cm 3 .
Despite its successes, the generation task of ChatMOF does present some limitations. Chief among these is the decrease in gene diversity due to constraints on input and output tokens. The token count restricts the number of parent and child structures to around 100, a fraction compared to inversed design studies that employ conventional genetic algorithm procedures that generate upwards of 100,000 structures for each generation. Other constraints, such as the limited number of topologies and cycles, stem from resource and time restrictions. Yet, despite these limitations, ChatMOF’s algorithms effectively generate MOFs that align well with the objective function, demonstrating its operational efficacy.
The investigation into the role of generative AI in materials science, specifically through the lens of ChatMOF, unveils substantial potential for predicting and generating MOFs. This unique system, which bridges the transformative capabilities of AI and the intricate facets of materials science, demonstrates exceptional performance across various tasks. The accuracy analysis reports high success rates, notably 96.9% and 95.7% for the search and prediction tasks, respectively. Meanwhile, the more complex structure generation task, despite its intricacy, yields a notable accuracy rate of 87.5%. These promising results highlight ChatMOF’s effectiveness, even in managing the most demanding tasks. Despite certain limitations, like the reliance on the number of pre-trained weights, ChatMOF represents significant progress towards achieving higher autonomy in AI for materials science. As the technology advances, and with structured improvements in model capacity and data sharing on online platforms, it is possible to further optimize ChatMOF’s performance, facilitating remarkable progress in MOF research.
Prompt engineering
Prompt engineering emerges as a vital strategy within the realm of artificial intelligence to enhance and calibrate language models for bespoke tasks and results 71 . It consists of crafting high-caliber prompts that steer machine learning models towards producing precise outcomes 72 . This task necessitates selecting the apt prompt category, refining their size and architecture, and sequencing them effectively in relation to the task at hand. To channel this innovation into the field of materials science, we developed a suite of prompts for agent and toolkit, all delineated in Supplementary Note S4 – 11 . These prompts are combined with LLM through the LangChain library in the ChatMOF system.
Every prompt derives its structure from insights within the MRKL 50 and ReAct 49 papers. Each agent and toolkit operates on a sequence encompassing a question/thought/input/observation/final thought/final answer format for problem resolution, with every tool tailored to deliver a format that caters to its specific function. The prediction tool configures the MOFTransformer model and designates the MOF target for forecasting, utilizing the “property” and “material” format, while the generator configures the inverse-design process that setting the aim, searching the parent gene, and facilitating child gene creation through the object/search look-up table/genetic algorithm format. Importantly, the table search provides both the header and the first five lines from the table, and the predictor and generator are provided with a list of predictable properties to guide the LLM to formulate a response within the prescribed bounds. Each prompt is reinforced by two to three exemplars, affording the LLM multiple attempts to refine its proficiency in generating precise responses.
Details of ChatMOF
ChatMOF is a code that links the LLM and tools (Machine learning, python-library, database, etc) together and the process of manipulating them to generate the user-desired output. For the roles of agent, evaluator, and toolkit within ChatMOF, various LLMs (GPT-4, GPT-3.5-turbo, llama2-7B-chat, llama-2-13B-chat) are employed. To minimize the influence of existing examples and evaluate the effectiveness of the LLM, the ChatMOF system employs the LLM without any fine-tuning. During the experiments, the temperature parameter was calibrated to 0.1.
The search functionality within ChatMOF utilizes the CoREMOF structure 51 , augmented with geometric characteristics obtained via ZEO++ 70 calculations performed at high accuracy (indicated by the “-ha” flag), and incorporates a hard sphere model with a diameter of 3.31 Å (nitrogen). In instances of code discrepancies, corrections are made up to a threshold of three attempts. The predictor module within ChatMOF leans on MOFTransformer, trained on insights from four academic articles 53 , 73 , 74 , 75 cited in the MOFTransformer publication.
The example of ChatMOF was tested with 0.2.0 version, and the accuracy measurement was tested with 0.0.0 version.
Genetic algorithm
The genetic algorithm was executed by running the LLM with prompts in Supplementary Note S8 and generating structure files with PORMAKE 63 library. The generative aspect of ChatMOF is structured around three iterative cycles. This generator employs a genetic algorithm across nine unique topologies, namely pcu, dia, acs, rtl, cds, srs, ths, bcu, and fsc. For every topology, a batch of 100 offspring genes arises from a set of 100 parental genes, chosen from a foundational group of 2000 MOFs. Structures are then formulated based on these newly minted genes, followed by value computation via the predictor. This cycle refines the pool of parental genes, and after the designated cycles, an optimized target structure is procured from the cumulative data.
Simulation details for inverse design
MOF structures were optimized using the Universal Force Field (UFF) 76 within the Forcite module of Materials Studio 77 , without assigning partial charges to the MOF atoms. The procedure for measuring hydrogen uptake was aligned with the approach used by MOFTransformer 58 . The RASPA 78 software facilitated the calculations. A united atom model was utilized for H 2 , and the pseudo-Feynman–Hibbs approach 79 , 80 described its low-temperature behavior, adjusting Lenard–Jones potentials at 77 K. Aside from H 2 , the UFF force field with Lorentz–Berthelot mixing rules and a 12.8 Å cutoff was applied, with no partial charges assigned to the MOF atoms. Grand Canonical Monte Carlo (GCMC) simulations were conducted at 100 bar and 77 K, spanning 10,000 production cycles after 5000 cycles of equilibration.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Procedures and answers to questions in LLM-specific ChatMOF (GPT-4, GPT-3.5-turbo, GPT-3.5-turbo-16k, Llama-2-7B-chat-hf, Llama-2-13B-chat-hf) are available in the experimental section of the GitHub repository under https://github.com/Yeonghun1675/ChatMOF.git . The fine-tuned model used in ChatMOF is available at https://figshare.com/articles/dataset/Fine-tuned_MOFTransformer_model/24288919 , and the hMOF used in the genetic algorithm is available at https://figshare.com/articles/dataset/Database_for_ChatMOF/24287731 . Source data are provided with this paper.
Code availability
The ChatMOF library is available at GitHub ( https://github.com/Yeonghun1675/ChatMOF.git .) ref. 81 . An online demo is available at https://chatmof-online.streamlit.app/ .
Kenton, J. D. M.-W. C. & Toutanova, L. K. Bert: Pre-training of deep bidirectional transformers for language understanding. in Proceedings of naacL-HLT (2019).
Bommasani, R. et al. On the opportunities and risks of foundation models. Preprint at https://arxiv.org/abs/2108.07258 (2021).
Brown, T. et al. Language models are few-shot learners. Adv. neural Inf. Process. Syst. 33 , 1877–1901 (2020).
Google Scholar
Touvron, H. et al. Llama: Open and efficient foundation language models. Preprint at https://arxiv.org/abs/2302.13971 (2023).
Bubeck, S. et al. Sparks of artificial general intelligence: early experiments with gpt-4. Preprint at https://arxiv.org/abs/2303.12712 (2023).
Vaswani, A. et al. Attention is all you need. Advances in Neural Information Processing Systems 30 , (2017).
Liu, P. et al. Pre-train, prompt, and predict: a systematic survey of prompting methods in natural language processing. ACM Comput. Surv. 55 , 1–35 (2023).
Shen, Y. et al. Hugginggpt: solving AI tasks with ChatGPT and its friends in huggingface. in NeurIPS 2023 poseter (2023).
Khan, R. A., Jawaid, M., Khan, A. R. & Sajjad, M. ChatGPT-Reshaping medical education and clinical management. Pak. J. Med. Sci. 39 , 605 (2023).
Article PubMed PubMed Central Google Scholar
Taylor, R. et al. Galactica: a large language model for science. in CoRR (2022).
Hendrycks, D. et al. Aligning AI with shared human values. in International Conference on Learning Representations (2021).
Hendrycks, D. et al. Measuring massive multitask language understanding. in International Conference on Learning Representations (2021).
Reynolds, L. & McDonell, K. Prompt programming for large language models: beyond the few-shot paradigm. in Extended Abstracts of the 2021 CHI Conference on Human Factors in Computing Systems (2021).
Polak, M. P. & Morgan, D. Extracting accurate materials data from research papers with conversational language models and prompt engineering. Nat. Commun . 15 , 1569 (2024).
Zheng, Z., Zhang, O., Borgs, C., Chayes, J. T. & Yaghi, O. M. ChatGPT chemistry assistant for text mining and prediction of MOF synthesis. J. Am. Chem. Soc. 145 , 18048–18062 (2023).
Article CAS PubMed PubMed Central Google Scholar
Ziegler, D. M. et al. Fine-tuning language models from human preferences. Preprint at https://arxiv.org/abs/1909.08593 (2019).
Wei, J. et al. Finetuned language models are zero-shot learners. in International Conference on Learning Representations (2022).
Dagdelen, J. et al. Structured information extraction from scientific text with large language models. Nat. Commun . 15 , 1418 (2024).
Wu, Q. et al. Autogen: enabling next-gen llm applications via multi-agent conversation framework. Preprint at https://arxiv.org/abs/2308.08155 (2023).
Bran, A. M. et al. Augmenting large language models with chemistry tools. in NeurIPS 2023 AI for Science Workshop (2023).
Mahjour, B., Hoffstadt, J. & Cernak, T. Designing chemical reaction arrays using phactor and ChatGPT. Organic Process Research & Development 27 , 1510–1516 (2023).
Jablonka, K. M. et al. 14 Examples of how LLMs can transform materials science and chemistry: a reflection on a large language model hackathon. Digit. Discov. 2 , 1233–1250 (2023).
Guo, T. et al. What can large language models do in chemistry? a comprehensive benchmark on eight tasks. Adv. Neural. Inf. Process. Syst. 36 , 59662–59688 (2023).
Ouyang, S. et al. Structured chemistry reasoning with large language models. Preprint at https://arxiv.org/abs/2311.09656 (2023).
White, A. D. et al. Assessment of chemistry knowledge in large language models that generate code. Digit. Discov. 2 , 368–376 (2023).
Bran, A. M. & Schwaller, P. Transformers and large language models for chemistry and drug discovery. Preprint at https://arxiv.org/abs/2310.06083 (2023).
Lee, P., Bubeck, S. & Petro, J. Benefits, limits, and risks of GPT-4 as an AI chatbot for medicine. N. Engl. J. Med. 388 , 1233–1239 (2023).
Article PubMed Google Scholar
Waisberg, E. et al. GPT-4: a new era of artificial intelligence in medicine. Ir. J. Med. Sci. (1971-) 192 , 3197-3200 (2023).
Nori, H., King, N., McKinney, S. M., Carignan, D. & Horvitz, E. Capabilities of gpt-4 on medical challenge problems. Preprint at https://arxiv.org/abs/2303.13375 (2023).
Wang, Y., Zhao, Y. & Petzold, L. Are large language models ready for healthcare? a comparative study on clinical language understanding. In Machine Learning for Healthcare Conference (2023).
Bucior, B. J. et al. Identification schemes for metal–organic frameworks to enable rapid search and cheminformatics analysis. Cryst. Growth Des. 19 , 6682–6697 (2019).
Article CAS Google Scholar
Hu, T., Song, H., Jiang, T. & Li, S. Learning representations of inorganic materials from generative adversarial networks. Symmetry 12 , 1889 (2020).
Article ADS Google Scholar
Ward, L., Agrawal, A., Choudhary, A. & Wolverton, C. A general-purpose machine learning framework for predicting properties of inorganic materials. npj Comput. Mater. 2 , 1–7 (2016).
Article Google Scholar
Calfa, B. A. & Kitchin, J. R. Property prediction of crystalline solids from composition and crystal structure. AIChE J. 62 , 2605–2613 (2016).
Article ADS CAS Google Scholar
James, S. L. Metal-organic frameworks. Chem. Soc. Rev. 32 , 276–288 (2003).
Article CAS PubMed Google Scholar
Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 43 , 5415–5418 (2014).
Zhou, H.-C., Long, J. R. & Yaghi, O. M. Introduction to metal–organic frameworks. Chem. Rev. 112 , 673–674 (2012).
Freund, R. et al. The current status of MOF and COF applications. Angew. Chem. Int. Ed. 60 , 23975–24001 (2021).
Kumar, S. et al. Green synthesis of metal–organic frameworks: a state-of-the-art review of potential environmental and medical applications. Coord. Chem. Rev. 420 , 213407 (2020).
Qian, Q. et al. MOF-based membranes for gas separations. Chem. Rev. 120 , 8161–8266 (2020).
Lee, J. et al. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 38 , 1450–1459 (2009).
Zhang, X. et al. A historical overview of the activation and porosity of metal–organic frameworks. Chem. Soc. Rev. 49 , 7406–7427 (2020).
Deng, H. et al. Large-pore apertures in a series of metal-organic frameworks. Science 336 , 1018–1023 (2012).
Article ADS CAS PubMed Google Scholar
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal-organic frameworks. Science 341 , 1230444 (2013).
Wang, C., Liu, D. & Lin, W. Metal–organic frameworks as a tunable platform for designing functional molecular materials. J. Am. Chem. Soc. 135 , 13222–13234 (2013).
Liu, Y. et al. Data quantity governance for machine learning in materials science. Natl. Sci. Rev . 10 , nwad125 (2023).
Wei, J. et al. Chain-of-thought prompting elicits reasoning in large language models. Adv. Neural Inf. Process. Syst. 35 , 24824–24837 (2022).
Yao, S. et al. Tree of thoughts: Deliberate problem solving with large language models. in Thirty-seventh Conference on Neural Information Processing Systems (2023).
Yao, S. et al. ReAct: Synergizing reasoning and acting in language models. in The Eleventh International Conference on Learning Representations (2022).
Karpas, E. et al. MRKL systems: a modular, neuro-symbolic architecture that combines large language models, external knowledge sources and discrete reasoning. Preprint at https://arxiv.org/abs/2205.00445 (2022).
Chung, Y. G. et al. Computation-ready, experimental metal–organic frameworks: A tool to enable high-throughput screening of nanoporous crystals. Chem. Mater. 26 , 6185–6192 (2014).
Chung, Y. G. et al. Advances, updates, and analytics for the computation-ready, experimental metal–organic framework database: CoRE MOF 2019. J. Chem. Eng. Data 64 , 5985–5998 (2019).
Rosen, A. S. et al. Machine learning the quantum-chemical properties of metal–organic frameworks for accelerated materials discovery. Matter 4 , 1578–1597 (2021).
Moghadam, P. Z. et al. Development of a Cambridge Structural Database subset: a collection of metal–organic frameworks for past, present, and future. Chem. Mater. 29 , 2618–2625 (2017).
McKinney, W. pandas: a foundational Python library for data analysis and statistics. Python high. Perform. Sci. Comput. 14 , 1–9 (2011).
Glasby, L. T. et al. DigiMOF: a database of metal–organic framework synthesis information generated via text mining. Chem. Mater 35 , 4510–4524 (2023).
Altintas, C., Altundal, O. F., Keskin, S. & Yildirim, R. Machine learning meets with metal organic frameworks for gas storage and separation. J. Chem. Inf. Model. 61 , 2131–2146 (2021).
Kang, Y., Park, H., Smit, B. & Kim, J. A multi-modal pre-training transformer for universal transfer learning in metal–organic frameworks. Nat. Mach. Intell. 5 , 309–318 (2023).
Park, H., Kang, Y. & Kim, J. Enhancing structure–property relationships in porous materials through transfer learning and cross-material few-shot learning. ACS Appl. Mater. Interfaces 15 , 56375–56385 (2023).
Kim, B., Lee, S. & Kim, J. Inverse design of porous materials using artificial neural networks. Sci. Adv. 6 , eaax9324 (2020).
Article ADS CAS PubMed PubMed Central Google Scholar
Long, T. et al. Constrained crystals deep convolutional generative adversarial network for the inverse design of crystal structures. npj Comput. Mater. 7 , 66 (2021).
Xie, T., Fu, X., Ganea, O.-E., Barzilay, R. & Jaakkola, T. Crystal diffusion variational autoencoder for periodic material generation. In ICLR 2022 Poster https://openreview.net/forum?id=03RLpj-tc_ (2022).
Lee, S. et al. Computational screening of trillions of metal–organic frameworks for high-performance methane storage. ACS Appl. Mater. Interfaces 13 , 23647–23654 (2021).
Lim, Y., Park, J., Lee, S. & Kim, J. Finely tuned inverse design of metal–organic frameworks with user-desired Xe/Kr selectivity. J. Mater. Chem. A 9 , 21175–21183 (2021).
Park, J., Lim, Y., Lee, S. & Kim, J. Computational design of metal–organic frameworks with unprecedented high hydrogen working capacity and high synthesizability. Chem. Mater. 35 , 9–16 (2022).
Yao, Z. et al. Inverse design of nanoporous crystalline reticular materials with deep generative models. Nat. Mach. Intell. 3 , 76–86 (2021).
Park, H., Majumdar, S., Zhang, X., Kim, J. & Smit, B. Inverse design of metal–organic frameworks for direct air capture of CO 2 via deep reinforcement learning. Digital Discovery 3 , 728–741 (2024).
Katoch, S., Chauhan, S. S. & Kumar, V. A review on genetic algorithm: past, present, and future. Multimed. Tools Appl. 80 , 8091–8126 (2021).
Larsen, A. H. et al. The atomic simulation environment—a Python library for working with atoms. J. Phys.: Condens. Matter 29 , 273002 (2017).
Willems, T. F., Rycroft, C. H., Kazi, M., Meza, J. C. & Haranczyk, M. Algorithms and tools for high-throughput geometry-based analysis of crystalline porous materials. Microporous Mesoporous Mater. 149 , 134–141 (2012).
Gu, J. et al. A systematic survey of prompt engineering on vision-language foundation models. in CoRR (2023).
White, J. et al. A prompt pattern catalog to enhance prompt engineering with chatgpt. in CoRR (2023).
Orhan, I. B., Daglar, H., Keskin, S., Le, T. C. & Babarao, R. Prediction of O 2 /N 2 selectivity in metal–organic frameworks via high-throughput computational screening and machine learning. ACS Appl. Mater. Interfaces 14 , 736–749 (2021).
Moosavi, S. M. et al. Understanding the diversity of the metal-organic framework ecosystem. Nat. Commun. 11 , 1–10 (2020).
Nandy, A. et al. MOFSimplify, machine learning models with extracted stability data of three thousand metal–organic frameworks. Sci. Data 9 , 74 (2022).
Rappé, A. K., Casewit, C. J., Colwell, K., Goddard, W. A. III & Skiff, W. M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 114 , 10024–10035 (1992).
Biovia, D. S. Materials studio. R2 (Dassault Systèmes BIOVIA, 2017).
Dubbeldam, D., Calero, S., Ellis, D. E. & Snurr, R. Q. RASPA: molecular simulation software for adsorption and diffusion in flexible nanoporous materials. Mol. Simul. 42 , 81–101 (2016).
Feynman, R. P., Hibbs, A. R. & Styer, D. F. Quantum Mechanics And Path Integrals (Courier Corporation, 2010).
Fischer, M., Hoffmann, F. & Fröba, M. Preferred hydrogen adsorption sites in various MOFs—a comparative computational study. ChemPhysChem 10 , 2647–2657 (2009).
Kang, Y. ChatMOF. Zenodo https://doi.org/10.5281/zenodo.10806289 (2024).
Download references
Acknowledgements
Y.K. and J.K. acknowledge funding from the National Research Foundation of Korea (NRF) under Project Number 2021M3A7C208974513 and 2021R1A2C2003583. This work was supported by the National Supercomputing Center with supercomputing resources including technical support (KSC-2022-CRE-0515 and KSC-2023-CRE-0065).
Author information
Authors and affiliations.
Department of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology (KAIST), 291, Daehak-ro, Yuseong-gu, Daejeon, Republic of Korea
Yeonghun Kang & Jihan Kim
You can also search for this author in PubMed Google Scholar
Contributions
Y.K. developed ChatMOF and wrote the manuscript with J.K. The manuscript was written through the contributions of all authors. All authors have given approval for the final version of the manuscript.
Corresponding author
Correspondence to Jihan Kim .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Wenjie Zhang, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, description of additional supplementary files, supplementary data 1, reporting summary, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Kang, Y., Kim, J. ChatMOF: an artificial intelligence system for predicting and generating metal-organic frameworks using large language models. Nat Commun 15 , 4705 (2024). https://doi.org/10.1038/s41467-024-48998-4
Download citation
Received : 17 August 2023
Accepted : 15 May 2024
Published : 03 June 2024
DOI : https://doi.org/10.1038/s41467-024-48998-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
Metal ions or clusters that have been bonded with organic linkers to create one- or more-dimensional structures are referred to as metal-organic frameworks (MOFs). Reticular synthesis also forms MOFs with properly designated components that can result in crystals with high porosities and great chemical and thermal stability. Due to the wider surface area, huge pore size, crystalline nature ...
Metal organic frameworks (MOFs) are considered as a group of compounds, either metal ions or clusters, harmonized with organic ligands to form one or some dimensional structures. ... However, literature review showed that some regular structural motifs in MOF preparation existed which is an attempt to predict their architectures [13], [14], ...
Metal-organic frameworks (MOFs) are a class of porous materials with unprecedented chemical and structural tunability. Their synthetic versatility, long-range order, and rich host-guest ...
Metal-organic frameworks (MOFs) possess excellent advantages, such as high porosity, large specific surface area, and an adjustable structure, showing good potential for applications in gas adsorption and separation, catalysis, conductivity, sensing, magnetism, etc. However, they still suffer from significant limitations in terms of the scale-up synthesis and shaping, hindering the ...
Metal-organic frameworks (MOFs), also known as porous coordination polymers (PCPs), are constructed by organic linkers and metal ions or clusters and have emerged as a new type of crystalline materials with large surface area (typically ranging from 1000 to 10,000 m 2 /g), high porosity, tunable structures, and flexible tailorability, compared with traditional porous materials such as ...
1. Introduction. Metal-organic frameworks (MOFs) is a new type of microporous crystalline material formed by coordination bonds between metal ions or clusters and organic linkers (Kreno et al., 2012).According to metal ions or metal clusters, organic ligands and synthesis methods, MOFs can be roughly classified into four types, namely isoreticular metal-organic frameworks (IR-MOFs), Materials ...
Background. Metal-organic frameworks (MOFs) are made by linking inorganic and organic units by strong bonds (reticular synthesis). The flexibility with which the constituents' geometry, size, and functionality can be varied has led to more than 20,000 different MOFs being reported and studied within the past decade.
Metal-organic frameworks (MOFs) are an emerging class of porous materials created by the assembly of inorganic connectors and organic linkers. They have potential applications in fields such as gas storage as well as separation, sensing, catalysis, and drug delivery due to its properties such as flexibility, porosity, high surface area and functionality. Among the various synthetic ...
The canonical metal-organic framework ZIF-8 does not melt and form a glass. Here, authors incorporated two additional organic linkers into ZIF-8 to generate meltable derivatives which can be ...
Fan et al. [30] performed microwave-assisted syn-thesis of zirconium-based metal-organic frameworks. They compared the yield and porosity by changing quantity of modulator, reaction time and temperature. The reaction was completed in 2-2.5 h in microwave which took 24 h for completion in solvothermal method.
Metal-organic frameworks represent a porous class of materials that are build up from metal ions or oligonuclear metallic complexes and organic ligands. They can be considered as sub-class of coordination polymers and can be extended into one-dimension, two-dimensions, and three-dimensions. Depending on the size of the pores, MOFs are divided ...
1. Introduction. Metal-organic frameworks (MOFs) are produced by the formation of chemical bonds between organic ligands as linkers and metal ions as nodes, leading to generation of periodic network crystalline structures with high porosities and large surface areas, which promote their potential applications in various fields of science and technology. 1 In recent years, absorbent materials ...
Metal-organic frameworks (MOFs), formed by the coordination of organic ligands and metal nodes as periodic structural units with structural diversity and multifunctional tunable properties, provide a fertile possibility for optimizing the electronic structure of metal sites by tuning the first and/or second coordination shell, ultimately ...
Abstract. Metal organic frameworks (MOFs) are considered as a group of compounds, either metal ions or clusters, harmonized with organic ligands to form one or some dimensional structures. In addition to resilient bonds between inorganic and organic units, reticular synthesis creates MOFs, accurate selection of constituents of which can produce ...
In this Review we survey the molecular sieving behaviour of metal-organic framework (MOF) and covalent organic framework (COF) membranes, which is different from that of classical zeolite membranes.
Within the extensive array of metal-organic frameworks (MOFs), Zr-based MOFs stand out as particularly promising materials due to their diverse structures, exceptional stability, and intriguing properties. These MOFs are currently in the early stages of development, but noteworthy strides have been achieved in recent years.
Metal-organic frameworks (MOFs) are intrinsically porous extended solids formed by coordination bonding between organic ligands and metal ions or clusters. High electrical conductivity is rare in MOFs, yet it allows for diverse applications in electrocatalysis, charge storage, and chemiresistive sensing, among others. In this Review, we discuss the efforts undertaken so far to achieve ...
Metal-organic framework (MOF) is a pr omising. class of materials composed of metal centres or clust ers. and organic linkers. These are porous crystalline. materials in which metal is locked into ...
As a library, NLM provides access to scientific literature. Inclusion in an NLM database does not imply endorsement of, or agreement with, the contents by NLM or the National Institutes of Health. ... A Comprehensive Review of Metal-Organic Framework: Synthesis, Characterization, and Investigation of Their Application in Electrochemical ...
The term Metal-Organic Framework, or MOF, has come to define compounds where metal ions, or clusters, are bridged by coordinating organic linkers to form extended structures in up to three dimensions. ... This review seeks to highlight the main similarities and differences between MOFs and MOCs in a range of areas. 2 To summarize the entire MOF ...
Search life-sciences literature (44,160,796 articles, preprints and more) Search. Advanced search. Feedback ... Metal-organic frameworks typically have low heat of adsorptions (i.e. 4-7 kJ/mol), whereas for storing significant quantities of hydrogen at near-ambient temperatures, 15-25 kJ/mol is likely required. ... In this review we explore the ...
info_outlined. Nodes are locations in the document that facilitate reading from beginning to end. You can navigate node by node or select one to jump to.
In the literature, several types of chemical reactions at small and large scales have been catalyzed by MOFs, including conventional catalysis [174 ... Jahani S., Omidi A., Khatami M. A Review on Metal-Organic Frameworks: Synthesis and Applications. TrAC Trends Anal. Chem. 2019; 118:401-425. doi: 10.1016/j.trac.2019.06.007. [Google ...
1. Introduction. Metal Organic Frameworks (MOFs) constitute a class of solid porous materials, which consist of metal ions or metallic clusters, which act as nodes, and polydentate organic ligands, which act as linkers between the nodes. The metal nodes (metal ions or metallic clusters) act as connection points and the organic ligands bridge ...
This review focuses on the developments of the adsorptive separation of methane/nitrogen, ethene/ethane, propene/propane mixtures as well as on the separation of C8 aromatics with a wide variety of materials, including carbonaceous materials, zeolites, metal-organic frameworks and porous- organic frameworks. Expand
ChatMOF is an artificial intelligence (AI) system that is built to predict and generate metal-organic frameworks (MOFs). By leveraging a large-scale language model (GPT-4, GPT-3.5-turbo, and GPT-3 ...