Atoms: History, Structure and Application Essay
Introduction, history of atoms, structure of atoms, applications of the atom, works cited.
Any object that exists in this world is composed of atoms. Timber, water, air, metals and anything else that exists in this world is thus made up of atoms. Atoms are the basic or the fundamental building blocks of all items. Scientifically, all objects existing in this world are referred to as matter. Therefore, atoms are defined as the “basic building blocks of matter” (Wong 1). From the definition, it is apparent that atoms cannot be divided into smaller particles since they are the basic particles. This property of atoms was the motivation behind their name because the word atom is a Greek word meaning indivisible. At this point, it is important to note that breaking of an atom will result in a change in the chemical composition of the atom. A number of atomic applications use this idea to make use of atoms. This paper explains what atoms are, investigates the history of atoms, looks into the intricate details about the structure of the atom, and it also gives the areas that atomic knowledge has been applied.
The history of atomic study gives an insight of how philosophers’ come up with useful ideal. The model of the atom does not give an absolute atomic understanding, but it gives an abstract methodology that has been used to make useful scientific predictions and explanations (Walker 1). The father of atomic knowledge is a Greek philosopher named Democritus who developed the atom from the idea that breaking of a piece of matter to form two pieces and repeating the same severally must end at a point when the matter cannot break any further. He explained that at this point, one would have the basic particle of matter, which he named the atom. However, his ideas were dismissed by influential philosophers like Aristotle and thus they were not advanced for many years. This happened around 460 years BC. During the 1800’s John Dalton from England showed that matter was indeed made up of lumpy fundamental particles (Fowler 1). J.J. Thompson was the first scholar to come up with a model of the atom after discovering the electron. He discovered that the electron was negatively charged and thus he suggested that the atom has a positive charge. Thompson’s atomic model was conceived in the year 1897. Three years later, another physicist, a German professor named Planck, showed that atomic vibrations produced measurable energy. In the year 1905, a physicist named Albert Einstein showed that absorption of light by atoms is able to release electrons from atoms, by a process known as photoelectric effect. His photoelectric invention earned him a “Nobel Prize for physics in 1921” (Walker 1). In the year 1911, Earnest Rutherford discovered the proton, the particles that make up the nucleus and most of the mass of an atom. In the year 1912, Bohr, a Danish physicist came up with the atomic theory that explains some of the unclear issues surrounding the Rutherford atomic model. One of those issues is why the electrons do not collapse into the nucleus, which Bohr explained by saying that electrons orbit the nucleus of the atom in energy levels.
As you can see, the history of the atom is long. A number of other scholars made great contributions to the idea of the atom. The names mentioned in the above paragraph were the earliest scholars and philosophers who made contributions towards the idea of the atom. Other people who made commendable contributions to the idea of the atom in the later years are Arnold Sommerfeld, Wolfgang Pauli, Louis de Broglie, Erwin Schrodinger, Max Born, Werner Heisenberg, Paul Dirac, Carl Anderson, Hideki Yukawa, Cecil Powell, Richard Feynman, Julian Shwinger, Sin-Itiro, Murray Gell-Mann, Yuval Ne’man (Fowler 1).
Atoms are composed of three particles with different properties. These are the protons, the electrons, and the protons. As mentioned earlier, electrons are negatively charged particles that are very light and contribute the least to the atomic mass. Protons are heavy and contribute to a large proportion of atomic mass. Unlike the electrons, the protons have a positive charge. The third type of particles; the neutrons are heavy and contribute a large proportion of atomic weight. As their name suggests, the neutrons have no electric charge and thus they are neutral. Each atom is composed of these particles, which are arranged in a given combination (Gagnon 1). For instance, a hydrogen atom, perhaps the lightest atom, is composed of two particles: one electron and one proton. The structure of the aforementioned atom is thus as shown below:
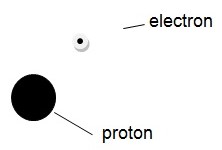
It is important to note that atoms are very small. A hydrogen atom, illustrated above, has an approximate diameter of 5 x 10 -8 millimeters (Carpi 1). Protons and neutrons are similar in most of their properties. They both behave like miniature billiard balls. On the other hand, electrons have the characteristics of waves. The atomic structure can therefore, be depicted as a nucleus, which is positively charged and surrounded by a negatively charged wave. The diagram above would, therefore, be more accurate if the electron is shown as a cloud surrounding the protons. It is also important to note that the atoms of other elements (substances) may have many protons, neutrons and electrons, although the configuration of the particles in the atom is similar for all atoms. With this fact, one may wonder why the nuclei of atoms with multiple protons do not disintegrate. The reason the nucleus does not disintegrate is that every nucleus with more than one proton has an equal number of neutrons that hold the neutrons together (Carpi 1). The latter serve this purpose because the protons are supposed to repel by the laws of electromagnetism. Additionally, electronically neutral atoms have the same number of electrons and protons, which have opposite electromagnetic charges.
Atoms that have a large number of sub-atomic particles (neutrons, electrons, and protons) are larger than their counterparts that have fewer sub-atomic particles. This is the reason why the hydrogen atom was earlier on introduced as the lightest atom.
This discussion has so far highlighted the atomic structure of neutral atoms. It is however, possible to have atoms that have electrical charges. Since the positive charge of an atom cannot be changed, the acquisition of an electrical charge in an atom is only possible through the loss of acquisition of electrons. An atom that has gained an electron will have more electrons than protons and thus it will bear a negative charge. Similarly, an atom that has lost an electron will have more protons than electrons and thus it will have a positive electric charge. An atom that bears a charge is known as an ion (Carpi 1). The following diagrams illustrate two hydrogen ions:

H + : This hydrogen ion bears a positive charge.
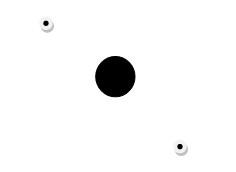
H – : This hydrogen ion bears a negative charge.
Unlike the protons, the number of neutrons in the nucleus of a given atom can be changed. Two atoms that have different numbers of neutrons are known as isotopes. It is thus apparent that isotopes have different atomic masses. A hydrogen isotope known as deuterium is shown below.
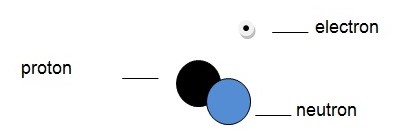
Among the main applications of the invention of atoms is the generation of electricity that was possible due to atomic knowledge. Atomic knowledge has also been used by states to get military power through production of atomic bombs. Atom-related inventions like the photoelectric effect have had a variety of applications like their use in image sensors, photomultipliers, and spectroscopy (Freudenrich 1). Other physical properties like electromagnetism have been used in a number of applications and they have their root in the invention of the atom. The invention of the atom made scientists understand the properties of different elements. This has made scientists use different elements in different applications. For instance, radioactive materials, which were better understood because of the prior inventions about the properties of the atoms, are used to generate electricity. Other elements like gold, silver, and copper were better understood and their properties like being shiny and being non-reactive utilized well. Other inventions like the Cathode Ray Tube, which has been used to make various electronics like television sets, computer monitors, etc., have been possible due to the invention of the atom. From this discussion of the applications of the atom, it is apparent that the applications are as many as the various atom-related inventions that followed the invention of the atom.
The atom is among the scientific ideas that have attracted the most scientific research in the 19 th and 20 th century. Most of the inventions related to the atom were characterized with so much criticism that some of the inventors did not believe in them. For instance, the first inventor of the positron, an electron with a positive charge did not believe that his invention was true. Additionally, after the invention of the atom, a great and influential Greek philosopher – Aristotle – dismissed the invention, making the invention of the atom stay without advancement for centuries. The atom is also among the greatest scientific ideas ever invented in terms of its application. The invention of the atom led to other innumerable inventions that have greatly been applied to make life easier for human beings. The atom is, perhaps, the greatest invention of all times.
From the discussion above, it is apparent that the atom is composed of three sub-atomic particles: the neutrons, the electrons, and the protons. The hydrogen atom is the lightest and the most basic atom consisting of one proton and one electron. This property has made hydrogen to be popular among scholars who use it in their experiments. It is especially used in experiments that investigate atomic properties.
One atomic property that has made the atom to be of great use to scientists and humans as a whole is the ability to change the structure of atoms by creating ions and isotopes. An ion has been defined in this discussion as an atom with a positive charge while an isotope is negatively charged.
Carpi, Anthony. Atomic Structure . 1999. Web.
Fowler, Michael. Evolution of the Atomic Concept and the Beginnings of Modern Chemistry . 2008. Web.
Freudenrich, Craig. How Atoms Work . 2011. Web.
Gagnon, Steve. What are atoms . 2011. Web.
Walker, Jim. Atoms (A short history of the knowledge of the atom) . 2004. Web.
Wong, Ling. Structure of the atom . 2010. Web.
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2022, April 25). Atoms: History, Structure and Application. https://ivypanda.com/essays/atoms-history-structure-and-application/
"Atoms: History, Structure and Application." IvyPanda , 25 Apr. 2022, ivypanda.com/essays/atoms-history-structure-and-application/.
IvyPanda . (2022) 'Atoms: History, Structure and Application'. 25 April.
IvyPanda . 2022. "Atoms: History, Structure and Application." April 25, 2022. https://ivypanda.com/essays/atoms-history-structure-and-application/.
1. IvyPanda . "Atoms: History, Structure and Application." April 25, 2022. https://ivypanda.com/essays/atoms-history-structure-and-application/.
Bibliography
IvyPanda . "Atoms: History, Structure and Application." April 25, 2022. https://ivypanda.com/essays/atoms-history-structure-and-application/.
- Scientific Theories of Atoms and Their Structure
- The Definition of ETC and Chemiosmosis
- The Discovery and Deciphering of the Atom
- Clay Spheres: The Mass and the Diameter Relationship
- Moments Principles for Parallel and Non-Parallel Forces
- Fourier Transformations and Images Analysis
- Collisions in One Dimension: A Physical Experiment
- Three Non-Parallel Forces in Equilibrium
- IIT JEE Study Material
- Atomic Structure

Atomic Structure - Discovery of Subatomic Particles
The atomic structure refers to the structure of an atom comprising a nucleus (centre) in which the protons (positively charged) and neutrons (neutral) are present. The negatively charged particles called electrons revolve around the centre of the nucleus .
Download Complete Chapter Notes of Structure of Atom Download Now
The history of atomic structure and quantum mechanics dates back to the times of Democritus, the person who first proposed that matter is composed of atoms. The study of the structure of an atom gives a great insight into the entire class of chemical reactions, bonds and their physical properties. The first scientific theory of atomic structure was proposed by John Dalton in the 1800s.
Atomic Structure Quick Revision for the JEE

Structure of Atom – Important Topics

Table of Contents
What is atomic structure, atomic models, dalton’s atomic theory, thomson atomic model, rutherford atomic theory, subatomic particles, atomic structure of isotopes, bohr’s atomic theory, dual nature of matter.
The advances in atomic structure and quantum mechanics have led to the discovery of other fundamental particles. The discovery of subatomic particles has been the base for many other discoveries and inventions.
The atomic structure of an element refers to the constitution of its nucleus and the arrangement of the electrons around it. Primarily, the atomic structure of matter is made up of protons , electrons and neutrons.
The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. The atomic number of an element describes the total number of protons in its nucleus.

Neutral atoms have equal numbers of protons and electrons. However, atoms may gain or lose electrons in order to increase their stability, and the resulting charged entity is called an ion.
Atoms of different elements have different atomic structures because they contain different numbers of protons and electrons . This is the reason for the unique characteristics of different elements.
In the 18th and 19th centuries, many scientists attempted to explain the structure of the atom with the help of atomic models. Each of these models had its own merits and demerits and was pivotal to the development of the modern atomic model . The most notable contributions to the field were by the scientists such as John Dalton, J.J. Thomson, Ernest Rutherford and Niels Bohr. Their ideas on the structure of the atom are discussed in this subsection.
The English chemist John Dalton suggested that all matter is made up of atoms, which were indivisible and indestructible. He also stated that all the atoms of an element were exactly the same, but the atoms of different elements differ in size and mass.
Chemical reactions, according to Dalton’s atomic theory, involve a rearrangement of atoms to form products. According to the postulates proposed by Dalton, the atomic structure comprises atoms, the smallest particle responsible for the chemical reactions to occur.
The following are the postulates of his theory:
- Every matter is made up of atoms.
- Atoms are indivisible.
- Specific elements have only one type of atom in them.
- Each atom has its own constant mass that varies from element to element.
- Atoms undergo rearrangement during a chemical reaction.
- Atoms can neither be created nor destroyed but can be transformed from one form to another.
Dalton’s atomic theory successfully explained the Laws of chemical reactions , namely, the Law of conservation of mass, the Law of constant properties, the Law of multiple proportions and the Law of reciprocal proportions.
Demerits of Dalton’s Atomic Theory
- The theory was unable to explain the existence of isotopes.
- Nothing about the structure of the atom was appropriately explained.
- Later, scientists discovered particles inside the atom that proved the atoms are divisible.
The discovery of particles inside atoms led to a better understanding of chemical species; these particles inside the atoms are called subatomic particles. The discovery of various subatomic particles is as follows:
The English chemist Sir Joseph John Thomson put forth his model describing the atomic structure in the early 1900s.
He was later awarded the Nobel Prize for the discovery of “electrons” . His work is based on an experiment called the cathode ray experiment . The construction of working of the experiment is as follows:
Cathode Ray Experiment
It has a tube made of glass which has two openings, one for the vacuum pump and the other for the inlet through which a gas is pumped in.

The role of the vacuum pump is to maintain a “partial vacuum” inside the glass chamber. A high-voltage power supply is connected using electrodes, i.e., cathode and anode , which are fitted inside the glass tube.
Observations:
- When a high voltage power supply is switched on, there are rays emerging from the cathode towards the anode. This was confirmed by the ‘Fluorescent spots’ on the ZnS screen used. These rays were called “Cathode Rays”.
- When an external electric field is applied, the cathode rays get deflected towards the positive electrode, but in the absence of an electric field, they travel in a straight line.

- With all this evidence, Thompson concluded that cathode rays are made of negatively charged particles called “electrons”.
- On applying the electric and magnetic field upon the cathode rays (electrons), Thomson found the charge-to-mass ratio (e/m) of electrons. (e/m) for electron: 17588 × 10 11 e/bg.
From this ratio, the charge of the electron was found by Mullikin through an oil drop experiment . [Charge of e – = 1.6 × 10 -16 C and Mass of e – = 9.1093 × 10 -31 kg].
Conclusions:
Based on conclusions from his cathode ray experiment, Thomson described the atomic structure as a positively charged sphere into which negatively charged electrons were embedded.
It is commonly referred to as the “plum pudding model” because it can be visualised as a plum pudding dish where the pudding describes the positively charged atom and the plum pieces describe the electrons.
Thomson’s atomic structure described atoms as electrically neutral, i.e., the positive and the negative charges were of equal magnitude.
Limitations of Thomson’s Atomic Structure: Thomson’s atomic model does not clearly explain the stability of an atom. Also, further discoveries of other subatomic particles couldn’t be placed inside his atomic model.
Rutherford, a student of J. J. Thomson, modified the atomic structure with the discovery of another subatomic particle called “Nucleus” . His atomic model is based on the Alpha ray scattering experiment.
Alpha Ray Scattering Experiment
Construction:.
- A very thin gold foil of 1000 atoms thick is taken.
- Alpha rays (doubly charged Helium He 2+ ) were made to bombard the gold foil.
- Zn S screen is placed behind the gold foil.
- Most of the rays just went through the gold foil, making scintillations (bright spots) in the ZnS screen.
- A few rays got reflected after hitting the gold foil.
- One in 1000 rays got reflected by an angle of 180° (retraced path) after hitting the gold foil.
- Since most rays passed through, Rutherford concluded that most of the space inside the atom is empty.
- A few rays got reflected because of the repulsion of its positive with some other positive charge inside the atom.
- 1/1000th of the rays got strongly deflected because of a very strong positive charge in the centre of the atom. He called this strong positive charge “nucleus”.
- He said most of the charge and mass of the atom resides in the nucleus.
Rutherford’s Structure of Atom
Based on the above observations and conclusions, Rutherford proposed his own atomic structure , which is as follows.
- The nucleus is at the centre of an atom, where most of the charge and mass is concentrated.
- The atomic structure is spherical.
- Electrons revolve around the nucleus in a circular orbit, similar to the way planets orbit the sun.
Limitations of the Rutherford Atomic Model
- If electrons have to revolve around the nucleus, they will spend energy and that too against the strong force of attraction from the nucleus, a lot of energy will be spent by the electrons, and eventually, they will lose all their energy and will fall into the nucleus so the stability of atom is not explained.
- If electrons continuously revolve around the ‘nucleus, the type of spectrum expected is a continuous spectrum. But in reality, what we see is a line spectrum.
Atomic Structure – Rutherford’s Model, J.J Thomson’s Model

- Protons are positively charged subatomic particles. The charge of a proton is 1e, which corresponds to approximately 1.602 × 10 -19
- The mass of a proton is approximately 1.672 × 10 -24
- Protons are over 1800 times heavier than electrons.
- The total number of protons in the atoms of an element is always equal to the atomic number of the element.
- The mass of a neutron is almost the same as that of a proton, i.e., 1.674×10 -24
- Neutrons are electrically neutral particles and carry no charge.
- Different isotopes of an element have the same number of protons but vary in the number of neutrons present in their respective nuclei.
- The charge of an electron is -1e, which approximates to -1.602 × 10 -19
- The mass of an electron is approximately 9.1 × 10 -31 .
- Due to the relatively negligible mass of electrons, they are ignored when calculating the mass of an atom.
Nucleons are the components of the nucleus of an atom. A nucleon can either be a proton or a neutron. Each element has a unique number of protons in it, which is described by its unique atomic number . However, several atomic structures of an element can exist, which differ in the total number of nucleons.
These variants of elements having a different nucleon number (also known as the mass number) are called isotopes of the element. Therefore, the isotopes of an element have the same number of protons but differ in the number of neutrons.
The atomic structure of an isotope is described with the help of the chemical symbol of the element, the atomic number of the element and the mass number of the isotope. For example, there exist three known naturally occurring isotopes of hydrogen , namely, protium, deuterium and tritium. The atomic structures of these hydrogen isotopes are illustrated below.

The isotopes of an element vary in stability. The half-lives of isotopes also differ. However, they generally have similar chemical behaviour owing to the fact that they hold the same electronic structures .

Atomic Structures of Some Elements
The structure of an atom of an element can be simply represented via the total number of protons, electrons and neutrons present in it. The atomic structures of a few elements are illustrated below.
The most abundant isotope of hydrogen on the planet Earth is protium. The atomic number and the mass number of this isotope are 1 and 1, respectively.
Structure of Hydrogen Atom: This implies that it contains one proton, one electron and no neutrons (Total number of neutrons = Mass number – Atomic number)
Carbon has two stable isotopes – 12C and 13C. Of these isotopes, 12C has an abundance of 98.9%. It contains 6 protons, 6 electrons and 6 neutrons.
Structure of Carbon Atom: The electrons are distributed into two shells, and the outermost shell (valence shell) has four electrons. The tetravalency of carbon enables it to form a variety of chemical bonds with various elements.
There exist three stable isotopes of oxygen – 18O, 17O and 16O. However, oxygen-16 is the most abundant isotope.
Structure of Oxygen Atom: Since the atomic number of this isotope is 8 and the mass number is 16, it consists of 8 protons and 8 neutrons. 6 out of the 8 electrons in an oxygen atom lie in the valence shell.
Neils Bohr put forth his model of the atom in the year 1915. This is the most widely used atomic model to describe the atomic structure of an element which is based on Planck’s theory of quantization .
Postulates:
- The electrons inside atoms are placed in discrete orbits called “stationery orbits”.
- The energy levels of these shells can be represented via quantum numbers.
- Electrons can jump to higher levels by absorbing energy and move to lower energy levels by losing or emitting their energy.
- As long as an electron stays in its own stationery, there will be no absorption or emission of energy.
- Electrons revolve around the nucleus in these stationary orbits only.
- The energy of the stationary orbits is quantised.
Limitations of Bohr’s Atomic Theory:
- Bohr’s atomic structure works only for single electron species such as H, He+, Li2+, Be3+, ….
- When the emission spectrum of hydrogen was observed under a more accurate spectrometer, each line spectrum was seen to be a combination of a number of smaller discrete lines.
- Both Stark and Zeeman’s effects couldn’t be explained using Bohr’s theory.
Heisenberg’s uncertainty principle: Heisenberg stated that no two conjugate physical quantities could be measured simultaneously with 100% accuracy. There will always be some error or uncertainty in the measurement.
Drawback: Position and momentum are two such conjugate quantities that were measured accurately by Bohr (theoretically).
Stark effect: Phenomenon of deflection of electrons in the presence of an electric field.
Zeeman effect: Phenomenon of deflection of electrons in the presence of a magnetic field.
The electrons, which were treated to be particles, and the evidence of the photoelectric effect show they also have a wave nature. This was proved by Thomas Young with the help of his double-slit experiment .
De-Broglie concluded that since nature is symmetrical, so should light or any other matter wave be.
Quantum Numbers
- Principal Quantum Number (n): It denotes the orbital number or shell number of an electron.
- Azimuthal Quantum Numbers ( l ): It denotes the orbital (sub-orbit) of the electron.
- Magnetic Quantum Number: It denotes the number of energy states in each orbit.
- Spin Quantum number(s): It denotes the direction of spin, S = -½ = Anticlockwise and ½ = Clockwise.
Electronic Configuration of an Atom
The electrons have to be filled in the s, p, d and f in accordance with the following rule.
1. Aufbau’s principle: The filling of electrons should take place in accordance with the ascending order of energy of orbitals.
- Lower energy orbital should be filled first, and higher energy levels.
- The energy of orbital α(p + l) value it two orbitals have the same (n + l ) value, E α n
- Ascending order of energy 1s, 2s, 2p, 3s, 3p, 4s, 3d, . . .
2. Pauli’s exclusion principle: No two electrons can have all four quantum numbers to be the same, or if two electrons have to be placed in an energy state, they should be placed with opposite spies.
3. Hund’s rule of maximum multiplicity: In the case of filling degenerate (same energy) orbitals, all the degenerate orbitals have to be singly filled first, and then, only pairing has to happen.
Atomic Structure Solved Problems and Solutions

Atomic Structure – Important Questions

Structure of Atom Class 11 – Full Chapter Revision

Structure of Atom – Top 12 Most Important JEE Main Questions

Frequently Asked Questions on Atomic Structure
What are subatomic particles.
Subatomic particles are the particles that constitute an atom. Generally, this term refers to protons, electrons and neutrons.
How do the atomic structures of isotopes vary?
They vary in terms of the total number of neutrons present in the nucleus of the atom, which is described by their nucleon numbers.
What are the shortcomings of Bohr’s atomic model?
According to this atomic model, the structure of an atom offers poor spectral predictions for larger atoms. It also failed to explain the Zeeman effect. It could only successfully explain the hydrogen spectrum.
How can the total number of neutrons in the nucleus of a given isotope be determined?
The mass number of an isotope is given by the sum of the total number of protons and neutrons in it. The atomic number describes the total number of protons in the nucleus. Therefore, the number of neutrons can be determined by subtracting the atomic number from the mass number.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all JEE related queries and study materials
Your result is as below
Request OTP on Voice Call

Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
- Share Share
Register with Aakash BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
Biology library
Course: biology library > unit 2.
- Elements and atoms
- Matter, elements, and atoms
- Introduction to the atom
Atomic structure
- Atomic number, atomic mass, and isotopes
- (Choice A) Protons, neutrons, and photons A Protons, neutrons, and photons
- (Choice B) Positrons, neutrons, and electrons B Positrons, neutrons, and electrons
- (Choice C) Protons, electrons, and positrons C Protons, electrons, and positrons
- (Choice D) Protons, neutrons, and electrons D Protons, neutrons, and electrons
Home — Essay Samples — Science — Atom — The Structure of an Atom
The Structure of an Atom
- Categories: Atom
About this sample

Words: 454 |
Published: Jan 15, 2019
Words: 454 | Page: 1 | 3 min read
Works Cited
- Chang, R. (2010). Chemistry (10th ed.). McGraw Hill.
- Housecroft, C. E., & Sharpe, A. G. (2018). Inorganic Chemistry (5th ed.). Pearson.
- Huheey, J. E., Keiter, E. A., & Keiter, R. L. (2014). Inorganic chemistry: principles of structure and reactivity (4th ed.). Pearson.
- Kotz, J. C., Treichel Jr, P. M., & Townsend, J. R. (2016). Chemistry and Chemical Reactivity (9th ed.). Cengage Learning.
- Martin, G. J., & Cockett, M. C. R. (2000). Essential Chemistry for Cambridge IGCSE (2nd ed.). Oxford University Press.
- McMurry, J., & Fay, R. C. (2017). Chemistry (7th ed.). Pearson.
- Moore, J. W., & Stanitski, C. L. (2017). Chemistry: The Molecular Science (5th ed.). Cengage Learning.
- Petrucci, R. H., Herring, F. G., Madura, J. D., & Bissonnette, C. (2017). General Chemistry: Principles and Modern Applications (11th ed.). Pearson.
- Silberberg, M. S. (2016). Chemistry: The Molecular Nature of Matter and Change (8th ed.). McGraw Hill Education.
- Zumdahl, S. S., & DeCoste, D. J. (2016). Chemistry (10th ed.). Cengage Learning.

Cite this Essay
Let us write you an essay from scratch
- 450+ experts on 30 subjects ready to help
- Custom essay delivered in as few as 3 hours
Get high-quality help

Dr. Karlyna PhD
Verified writer
- Expert in: Science

+ 120 experts online
By clicking “Check Writers’ Offers”, you agree to our terms of service and privacy policy . We’ll occasionally send you promo and account related email
No need to pay just yet!
Related Essays
3 pages / 1184 words
3 pages / 1456 words
5 pages / 2673 words
6 pages / 2684 words
Remember! This is just a sample.
You can get your custom paper by one of our expert writers.
121 writers online

Still can’t find what you need?
Browse our vast selection of original essay samples, each expertly formatted and styled
Related Essays on Atom
The study of atoms has been central to our understanding of matter and the universe. It is hard to imagine modern technology, medicine, and energy without the knowledge of atomic properties, structures, and reactions. As a [...]
J.J. Thomson was born on December 18, 1856, in Cheetham Hill, England, near Manchester. His father was a bookseller who planned for Thomson to be an engineer. When an apprenticeship at an engineering firm couldn’t be found, [...]
We can clearly see the Periodic Table everywhere. It is one of the most important and successful chemistry discoveries to date. To make the completed and latest periodic table, there are a lot of scientists and chemists who have [...]
“The Speed of Trust” does not only explain how important trust is in different kinds of relationship but also how trust -when fully developed- can make a person successful and prosperous. The author Stephen Covey discusses the [...]
Light arrives on our planet after a speedy trip from the Sun, 149 million km (93 million miles away). Light travels at 186,000 miles (300,000 km) per second, so the light you’re seeing now was still tucked away in the Sun about [...]
Magnetism is a very interesting topic to talk about because of magnets. Some of these magnets lose their magnetism over time. This is an essential fact to consumers because it is better to buy a real-magnet rather than a [...]
Related Topics
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Where do you want us to send this sample?
By clicking “Continue”, you agree to our terms of service and privacy policy.
Be careful. This essay is not unique
This essay was donated by a student and is likely to have been used and submitted before
Download this Sample
Free samples may contain mistakes and not unique parts
Sorry, we could not paraphrase this essay. Our professional writers can rewrite it and get you a unique paper.
Please check your inbox.
We can write you a custom essay that will follow your exact instructions and meet the deadlines. Let's fix your grades together!
Get Your Personalized Essay in 3 Hours or Less!
We use cookies to personalyze your web-site experience. By continuing we’ll assume you board with our cookie policy .
- Instructions Followed To The Letter
- Deadlines Met At Every Stage
- Unique And Plagiarism Free

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

2: Atomic Structure
- Last updated
- Save as PDF
- Page ID 151365

- Kathryn Haas
- Duke University
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
- 2.1.1: The Periodic Table
- 2.1.2: Discovery of Subatomic Particles and the Bohr Atom
- 2.2.1: Particle in a Box
- 2.2.2: Quantum Numbers and Atomic Wave Functions
- 2.2.3: Aufbau Principle
- 2.2.4: Shielding
- 2.3.1: Ionization energy
- 2.3.2: Electron Affinity
- 2.3.3: Covalent and Ionic Radii
- 2.4: Problems

How to Write An Atomic Essay: A Beginner's Guide
Jerine nicole, ultimate guide table of contents.
If you've been hanging out in the creator space on Twitter, you might have seen the ship icon on many users' profiles.
The 🚢 ship icon symbolizes one of the fastest-growing writing communities on the Internet called Ship 30 for 30. The mission is to empower 1,000,000 writers to write online. And so far, 1000+ writers have taken on this writing challenge.
There's only one goal when you join: publish 30 atomic essays in 30 days.

So, what's an atomic essay?
It's a 250-word, single-idea essay published in a visual screenshot. It was Dickie's solution to the need to write “something longer than a Twitter thread, but shorter than a blog post." Dickie, one of the co-creators of Ship 30 for 30, built his Twitter audience of 50k+ in less than a year from writing daily, and is now helping others to do the same.
But why write an atomic essay?
The Atomic Essay format is the sweet spot for writing simple and concise messages, and gathering data without waiting for the lengthy feedback that comes with weekly blog posts.
Writing an atomic essay lets you refine ideas before spending more time and energy on a 1000-word blog post or anything on the Internet, for that matter.
This is what Nicolas Cole, one of the co-creators of Ship 30 for 30, calls data-driven writing.
Cole is a viral online writer with more than 100 million views. His work has been featured in TIME, Forbes, Inc, Harvard Business Review, and more. He and Dickie teach in Ship 30 for 30 that for you to become a successful online writer too, it’s crucial for you to gather feedback and let data drive your writing.
Atomic Essays, then, are how you test your ideas with readers before you decide to invest more time in that direction—whether it’s a long article, a business idea, a book, an online course, or even creating a whole new category .
In short, writing Atomic Essays will help you:
- Think clearly
- Publish with prolific momentum
- Eliminate the friction of sharing ideas online
- And create your niche by gathering data
The 30-day writing challenge has helped so many new creators build an audience, launch digital products, and create new categories for themselves on the Internet.
And they all started from writing atomic essays.
Here are seven steps you can take to write your very first Atomic Essay today.
Step 1: Pick a specific topic you want to talk about.
With so many (or little) things you can write about, you might suffer from analysis paralysis .
The solution to this problem?
Start by answering a question.
For example, " What was a time you thought about giving up but pushed through the end and accomplished something? "
By answering a simple question, your creativity muscle will immediately start to engage.
Now, you have a starting point.
Some people write about their expertise. For example, shipper Julia Saxena is known for her copywriting atomic essays . Or, if you want to explore your inner J.K Rowling, you can experiment with fiction stories. Shipper Sangeeth Kar does a beautiful job at touching our hearts with his atomic fiction essays .
Atomic essays give you publishing constraints and the freedom to explore whatever topic you want.
Here are some writing prompts that will help your creative juices going today, no matter what industry you’re in :
- Write about what you’re consuming. Are you watching a lot of YouTube or TikTok videos? Or maybe you just listened to the new Tim Ferris podcast episode?
- Respond or expand on someone else’s writing. Maybe you read a new post that you didn’t quite agree with? Or maybe it was a piece of content that you deeply resonated with and you want to share your perspective on it. Translate that into writing and tell the world why.
- Curate “the best of” any industry/topic. Maybe you’ve been secretly learning how to code. Along the way, you have probably compiled handfuls of helpful resources for yourself. So, why not write about them and share them publicly? People appreciate creators who have already done the hard work for them by curating the most relevant resources on a given topic.
- Teach a reader “How to” do something. Do you have specific knowledge in your industry? Maybe you know how to invest in cryptocurrencies, or maybe you know how to be productive without using an over-complicated system. Share your secrets and you just might change someone’s life.
- Share a powerful life lesson you learned. Tell your readers what you learned by sharing a personal story and how it changed you (or not). Don’t be scared to be vulnerable if you know people will be able to relate.
There are infinite ways to convert one topic into multiple different angles, a tactic that Cole and Dickie call their Endless Idea Generator (which they share with Shippers inside the program). But for now, focus on brainstorming around ideas you can’t stop thinking about.
Step 2: Decide who you're writing for.
One of the first key lessons in Ship 30 for 30 is to know who you're writing for.
Say your expertise is on cryptocurrencies. You have to decide whether you're writing for your grandma (someone who is brand new to “internet money”), for people with some crypto knowledge already, or for crypto experts. These three different audiences all need different things in order to resonate with your content.
Cole calls these your “audience buckets”:
- General Audience
- Niche Audience
- Industry Audience
When you're writing for a general audience , these are topics that most, if not everyone can relate to. So writing about health, money, and relationships will allow you to reach more people simply because more people are interested in those types of “general” topics.
Whereas, if you want to write about health for a niche audience like fitness influencers, you might assume they have more health knowledge than the average person, which means you need to speak to them in a way that keeps their “working knowledge” in mind.
Or maybe you have a prediction on the trends of the creator economy or the future of work, and you want to write for an industry audience.
As Cole says, “The size of the question dictates the size of the audience.”
How to apply this:
- Ask yourself, "who am I writing this topic for?"
- Are you trying to reach a mass audience, or are you trying to reach a smaller audience?
There is no right or wrong answer here. It all depends on what you're hoping to explore with your topic.
Step 3: Craft an intriguing headline.
If YouTube videos have a 5-second "hook,” effective Atomic Essays have an intriguing headline.
Having a compelling headline is how you catch someone's attention with your writing on the Internet. If your headline is not-so-good, very few will read the rest of your essay. Writing great headlines takes months, if not years of practice. For example, Ship 30 for 30 co-founder, Nicolas Cole, has been writing online for a decade.
Ship 30 for 30 provides an in-depth guide for creating intriguing headlines that Cole learned from his time writing for Inc Magazine . But in a nutshell, these are the five things that need to be in your headline:
- Who are you writing for?
- What are you writing about?
- How are you making the reader feel?
- What is the outcome/the promise you’re giving to the reader?
- How many or how much information can the reader expect from you?
Here are some examples of great headlines that shippers have come up with:

Your headline is the North Star of your Atomic Essay as it tells the reader where your story is going.
Step 4: Outline the key points of the core message of your essay.
Your key points are the meat of your Atomic Essay.
After reading your headline, readers will skim , not read, the key points of your essay. Only then will they decide to engage in your essay. So, you have another few seconds here to earn your readers' trust.
For example, if your headline says, "7 Ways to Simplify Your Daily Morning Routine", there have to be seven key points in your essay. If not, you just created distrust between yourself and the reader. And it’s tough to earn that trust back.
Here's an example of an atomic essay where the Shipper that matches their headline with their key points:

When you deliver your promise to the reader through your writing, you earn the reader’s trust, and they will come back for more.
- Once you have a headline, come up with the points you want to talk about.
- Constantly ask yourself this question: “Are my key points relevant to my headline?”
If your key points don't relate to your headline, it's time to develop new ideas that do.
Step 5: Expand on your main points.
Your readers need to know what you're talking about.
When you expand on your main points, you slowly build your credibility and authority. The core message of each main point can include:
- A personal story that gives the readers context of your essay
- Research to make your essay look more credible
- Background of where you got this idea from
This is where all the juicy context comes in as readers engage with your work. This is where you get to deliver the promise that you told your reader by reading your headline and main key points.
Here are examples of the templates that Dickie and Cole provide Shippers when it comes to expanding their core message:

This is where your creativity shines as you showcase your knowledge and ideas to the readers.
Step 6: Edit your essay to appeal to your readers.
Writing and editing are two different tasks.
When you're writing, you are tapping into your creativity. This is where you put all your ideas into a tangible piece of paper (digital or not) with no judgment. But once you have strong ideas that you have cultivated by following the previous steps, it’s time to edit your essay.
Edit your essay to make it easy to read, and readers will appreciate you.

When you edit intentionally, you're stepping into the reader’s shoes.
Remember, your essay isn't about you. It's about your readers. When you're editing, you have to look at your essay and have the courage to ask yourself, "Am I making this easy to read?"
If the answer is no, it's time to summon your inner designer.
You don't have to be a designer to know what looks "good or bad." When you're browsing on the Internet, you do this unconsciously. You scroll past the things that don't appeal to you. You just have to be more conscious about it when it comes to your writing.
Here are the best practices for atomic essays that (actually) catch attention:
- Capitalized title
- Bolded key points
- Use of colors
- Use of emojis
- Properly formatted essay
- Visually appealing
Here are some examples from some the Shippers from previous cohorts:

Look at your final draft and ask these questions:
- Is this essay visually appealing?
- Am I making it easy for the reader to read this?
Step 7: Publish your essay on one or multiple platforms and gather data
The ship 30 for 30 writing challenge has one goal: to publish 30 atomic essays in 30 days.
The more essays you publish, the more data that you can gather.
Cole and Dickie believe data helps you:
- Understand what readers are enjoying from you
- Decide whether to make more of that same content or try a different topic
- Steer the direction of your online journey
Data is a very important metric you want to learn to pay attention to.
Without data, it will be tough for you to understand the writing that gains attention. Not only do Shippers get a Notion template to track their data, but Cole and Dickie also teach them how to interpret the data.

Here are some tools Shippers use to track their atomic essays data on Twitter:
- Typeshare.co
- Twitter Analytics
- Google Sheets
- As soon as you hit publish, fill out your template according to the bucket.
- Track your audience bucket, category, format, approach and engagement rate over time.
Cole and Dickie are bullish on writing daily because building a daily writing habit is the single fastest way to gaining leverage on the Internet.
Some shippers have validated their ideas and launched their digital products after their first cohort . Some shippers, like me , used data to create a whole new category . Cole’s take on cutting through the online noise is by being different.
But Shippers couldn't have confidently and successfully launched "that thing" they wanted to launch without data.
As the founders like to say, “You can’t steer a stationary ship.”
If you liked this guide, try Ship 30 for 30’s free 10-day email course to get your ship moving.
You might also like...
How to write a press release: 6 tips to start writing breaking news that captivates readers, 3 essential writing routine lessons from legendary copywriter eugene schwartz, how to write a 60,000 word book in 30 days, 6 proven single-sentence openers to hook your reader’s attention, how to write headlines readers can’t help but click, how to start writing on substack: the complete guide.
- 🐦 Follow Dickie Bush on Twitter 🐦 Follow Nicolas Cole Twitter
- 📺 Ship 30 for 30 Youtube
- 🎧 Digital Writing Podcast
🔒 Privacy Policy 💼 Terms & Conditions

IMAGES
VIDEO
COMMENTS
Figure 2.2.1 2.2. 1: The Structure of the Atom. Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different.
Most of the atom is empty space. The rest consists of three basic types of subatomic particles: protons, neutrons, and electrons.The protons and neutrons form the atom's central nucleus. (The ordinary hydrogen atom is an exception; it contains one proton but no neutrons.) As their names suggest, protons have a positive electrical charge, while neutrons are electrically neutral—they carry ...
ATOMIC STRUCTURE ESSAY ASSIGNMENT By Edi Dokou 14. Introduction In this essay I would be explaining the distribution of mass and charges within an atom,the fundamental differences between protons, neutrons and electrons in terms of their mass and charge and also the distribution of mass and charges within an atom.
Unit 12 - Atomic Structure and Bonding Essay By Liam Rowsell The motive for this essay is to demonstrate my understanding of atomic structure by discussing its components and electron configuration, and how they can be determined when there isn't all the information. This essay is based on two scenarios which have been uploaded on ePearl
Three important kinds of radiation are α particles (helium nuclei), β particles (electrons traveling at high speed), and γ rays (similar to x-rays but higher in energy). 2.2: The Discovery of Atomic Structure is shared under a license and was authored, remixed, and/or curated by LibreTexts. Atoms, the smallest particles of an element that ...
A number of atomic applications use this idea to make use of atoms. This paper explains what atoms are, investigates the history of atoms, looks into the intricate details about the structure of the atom, and it also gives the areas that atomic knowledge has been applied. We will write a custom essay on your topic. 809 writers online.
Atomic radii are typically 30-300 pm. Figure 1.2.1 1.2. 1: The structure of the nuclear atom with a central nucleus and surrounding electrons. The nucleus is itself composed of two kinds of particles. Protons are the carriers of positive electric charge in the nucleus; the proton charge is exactly the same as the electron charge, but of ...
Thomson atomic model. (Show more) atomic model, in physics, a model used to describe the structure and makeup of an atom. Atomic models have gone through many changes over time, evolving as necessary to fit experimental data. For a more in-depth discussion of the history of atomic models, see atom: development of atomic theory.
Atom - Development, Theory, Structure: The concept of the atom that Western scientists accepted in broad outline from the 1600s until about 1900 originated with Greek philosophers in the 5th century bce. Their speculation about a hard, indivisible fundamental particle of nature was replaced slowly by a scientific theory supported by experiment and mathematical deduction.
The atomic structure of an element refers to the constitution of its nucleus and the arrangement of the electrons around it. Primarily, the atomic structure of matter is made up of protons, electrons and neutrons. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom.
Over the centuries, this concept has evolved through the contributions of groundbreaking scientists like John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr, leading to our modern understanding of atomic structure and behavior. In this essay, we will delve into the fascinating history of atomic theory, exploring its key milestones and ...
ATOMIC STRUCTURE. By Wasima Uddin In this essay, I will explain the distribution of mass and charges within an atom, highlighting the distinct characteristics of protons, neutrons, and electrons concerning their mass and charge.
Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere.
The purpose of this essay is to discuss Atomic Structure and Bonding. The essay will explain the concepts of "mass number" and "relative atomic mass", nucleon numbers, demonstrating how to deduce the electronic structure, explaining what ions are, and explaining what ionic bonds are. The research was conducted independently via online ...
Published: Jan 15, 2019. An atom has protons and electrons. The protons are placed in the nucleus of the atom while the electrons are situated in the orbital shell of the atom. The way these electrons are positioned is known as the electron configuration. Each shell can hold a certain amount of electrons before moving onto the next shell.
General periodic trends provide chemists with an invaluable tool to quickly predict an element's properties. These trends exist because of the similar atomic structure of the elements within their respective group families or periods. 2.3.1: Ionization energy. 2.3.2: Electron Affinity. 2.3.3: Covalent and Ionic Radii.
However, this essay will explain only the basics, starting with the structure of atoms. It will also explain how atoms can be identified with different information, such as the atomic number of an element. Many terms will be clarified such as mass number, relative atomic mass and neutron number; and the differences between these will be explored.
Essay unit 12 atomic structure - distinction received presentation; Unit 15 - Hydrocarbons Essay Final; Unit 3: Academic Writing Skills; Preview text. The intricate complexity of the atomic realm is governed by the distribution of mass and charges within an atom. The number of protons and neutrons in atomic nuclei, the number
Step 3: Craft an intriguing headline. If YouTube videos have a 5-second "hook," effective Atomic Essays have an intriguing headline. Having a compelling headline is how you catch someone's attention with your writing on the Internet. If your headline is not-so-good, very few will read the rest of your essay.
Atomic Structure and Bonding - Scenarios 1 & 2. For this essay, I will be discussing atomic structure, the associated components as well as electron coniguraion and how they can be determined even without full informaion. Informaion has been provided by scenarios typed for an 'assessor guidance' worksheet via ePearl. Scenario 1
Atomic Structure Essay. Atomic structure is a fundamental concept in chemistry that deals with the composition and organization of atoms, the building blocks of matter. It is a key area of study that provides a foundation for understanding the properties and behavior of matter.