- Dermatology
- Gastroenterology
- Geriatric Medicine and Gerontology
- Gynecology and Obstetrics
- Heart and Vascular
- Neurology and Neurosurgery
- Ophthalmology
- Orthopaedics
- Otolaryngology–Head and Neck Surgery
- Physical Medicine and Rehabilitation
- Plastic and Reconstructive Surgery
- Psychiatry and Behavioral Sciences
- Pediatric Specialties
- Pediatric Diabetes and Endocrinology
- Pediatrics Florida
- Pediatric Gastroenterology and GI Surgery
- Pediatric Heart
- Pediatrics Maryland/DC
- Pediatric Neurology & Neurosurgery
- Pediatric Orthopaedics
- Physician Affiliations
- Health Care Technology
- High-Value Health Care
- Clinical Research Advancements
- Precision Medicine Excellence
- Patient Safety

New Research on HPV
The vast majority of people who contract mouth and throat HPV infections, or oral HPV, clear the infection within a few months or years, shows a new study led by Johns Hopkins researchers. However, a fraction of these people retain the infection long-term, which increases their risk for developing HPV-related head and neck cancer. These findings, published in JNCI Cancer Spectrum in June 2020, represent the longest study of oral HPV infection over time, and could help inform policies to screen for this potentially deadly virus.
HPV is also an established cause of cervical cancer. Cervical HPV testing has proven to be an effective method of screening, and is therefore a standard part of gynecologic exams.
While it is known that HPV infection is responsible for oropharyngeal cancer, the most common type of head and neck cancer, how it plays out over time from mere infection of the mouth and throat to cancer has been unknown.

To investigate this question, Gypsyamber D’Souza , professor in the Department of Epidemiology at the Johns Hopkins Bloomberg School of Public Health, and Carole Fakhry , professor in the Department of Otolaryngology–Head and Neck Surgery, and their colleagues evaluated oral HPV infection over time among men and women who have HIV and those at elevated risk of contracting it, in two national longitudinal studies (Multicenter AIDS Cohort Study and the Women’s Interagency HIV Study).
In this study, oral rinse samples were tested for presence of HPV between 2009 and 2016.
They found that for those who had an oral HPV infection detected during the study, the vast majority of infections were no longer present in less than a year and a half, suggesting that the immune system cleared the infection quickly in most cases. However, for 5.5% of individuals, the infection lingered for seven years or more.
For one person in the study, the researchers were able to track from infection to cancer. More than four and a half years after his infection was detected, the viral load rose, eventually culminating in an HPV-related oropharyngeal cancer diagnosis.
D’Souza and Fakhry note that, as testing for HPV has become a standard part of gynecologic care, screening for the virus could eventually be part of methods to identify patients at risk of HPV-related oropharyngeal cancers. But as most people with oral HPV infection clear those infections and do not develop cancer, more work is needed to identify who is at increased risk of cancer and what surveillance or treatment would be appropriate in this group.
“We may eventually be able to pick up microscopic disease far before we could clinically detect it,” Fakhry says, “leading to better outcomes for our patients.”
To refer a patient or learn more, call 443-997-6467 .
- About Johns Hopkins Medicine
- Contact Johns Hopkins Medicine
- Centers & Departments
- Maps & Directions
- Find a Doctor
- Patient Care
- Terms & Conditions of Use
- Privacy Statement
Connect with Johns Hopkins Medicine
Join Our Social Media Communities >
Clinical Connection
- Otolaryngology—Head and Neck Surgery
- Contact Johns Hopkins
© The Johns Hopkins University, The Johns Hopkins Hospital, and Johns Hopkins Health System. All rights reserved.
Privacy Policy and Disclaimer
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 14 September 2021
Human papillomaviruses: diversity, infection and host interactions
- Alison A. McBride ORCID: orcid.org/0000-0001-5607-5157 1
Nature Reviews Microbiology volume 20 , pages 95–108 ( 2022 ) Cite this article
10k Accesses
131 Citations
32 Altmetric
Metrics details
- Viral infection
- Virus–host interactions
Human papillomaviruses (HPVs) are an ancient and highly successful group of viruses that have co-evolved with their host to replicate in specific anatomical niches of the stratified epithelia. They replicate persistently in dividing cells, hijack key host cellular processes to manipulate the cellular environment and escape immune detection, and produce virions in terminally differentiated cells that are shed from the host. Some HPVs cause benign, proliferative lesions on the skin and mucosa, and others are associated with the development of cancer. However, most HPVs cause infections that are asymptomatic and inapparent unless the immune system becomes compromised. To date, the genomes of almost 450 distinct HPV types have been isolated and sequenced. In this Review, I explore the diversity, evolution, infectious cycle, host interactions and disease association of HPVs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others

Cell-type-specific consequences of mosaic structural variants in hematopoietic stem and progenitor cells
Pro-CRISPR PcrIIC1-associated Cas9 system for enhanced bacterial immunity

CRISPR/Cas9 therapeutics: progress and prospects
de Villiers, E. M., Fauquet, C., Broker, T. R., Bernard, H. U. & zur Hausen, H. Classification of papillomaviruses. Virology 324 , 17–27 (2004).
PubMed Google Scholar
Karamanou, M., Agapitos, E., Kousoulis, A. & Androutsos, G. From the humble wart to HPV: a fascinating story throughout centuries. Oncol. Rev. 4 , 133–135 (2010).
Google Scholar
zur Hausen, H. Condylomata acuminata and human genital cancer. Cancer Res. 36 , 794–794 (1976). This paper describes the association of HPV and cancer that led to the Nobel Prize in 2008 .
CAS PubMed Google Scholar
Antonsson, A., Forslund, O., Ekberg, H., Sterner, G. & Hansson, B. G. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J. Virol. 74 , 11636–11641 (2000). This paper is the first indication that HPVs are ubiquitous and commensal .
CAS PubMed PubMed Central Google Scholar
Antonsson, A., Karanfilovska, S., Lindqvist, P. G. & Hansson, B. G. General acquisition of human papillomavirus infections of skin occurs in early infancy. J. Clin. Microbiol. 41 , 2509–2514 (2003).
PubMed PubMed Central Google Scholar
Tirosh, O. et al. Expanded skin virome in DOCK8-deficient patients. Nat. Med. 24 , 1815–1821 (2018).
Pastrana, D. V. et al. Metagenomic discovery of 83 new human papillomavirus types in patients with immunodeficiency. mSphere 3 , e00645-18 (2018). Together with Tirosh et al. (2018), this paper presents the recent discovery of many new HPV types .
de Martel, C., Plummer, M., Vignat, J. & Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 141 , 664–670 (2017).
Lambert, P. F., Munger, K., Rosl, F., Hasche, D. & Tommasino, M. Beta human papillomaviruses and skin cancer. Nature 588 , E20–E21 (2020).
Van Doorslaer, K. Evolution of the Papillomaviridae. Virology 445 , 11–20 (2013).
Shah, S. D., Doorbar, J. & Goldstein, R. A. Analysis of host–parasite incongruence in papillomavirus evolution using importance sampling. Mol. Biol. Evol. 27 , 1301–1314 (2010).
Bravo, I. G. & Felez-Sanchez, M. Papillomaviruses: viral evolution, cancer and evolutionary medicine. Evol. Med. Public Health 2015 , 32–51 (2015).
Chen, Z. et al. Niche adaptation and viral transmission of human papillomaviruses from archaic hominins to modern humans. PLoS Pathog. 14 , e1007352 (2018).
Auslander, N., Wolf, Y. I., Shabalina, S. A. & Koonin, E. V. A unique insert in the genomes of high-risk human papillomaviruses with a predicted dual role in conferring oncogenic risk. F1000Res 8 , 1000 (2019).
Burk, R. D., Harari, A. & Chen, Z. Human papillomavirus genome variants. Virology 445 , 232–243 (2013).
Pimenoff, V. N., de Oliveira, C. M. & Bravo, I. G. Transmission between archaic and modern human ancestors during the evolution of the oncogenic human papillomavirus 16. Mol. Biol. Evol. 34 , 4–19 (2017).
Hirose, Y. et al. Within-host variations of human papillomavirus reveal APOBEC signature mutagenesis in the viral genome. J. Virol. 92 , e00017-18 (2018).
Moody, C. A. & Laimins, L. A. Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation. PLoS Pathog. 5 , e1000605 (2009). This paper shows that HPVs activate the DNA damage response to replicate their genomes in differentiated cells .
Egawa, N. & Doorbar, J. The low-risk papillomaviruses. Virus Res. 231 , 119–127 (2017).
Rowson, K. E. & Mahy, B. W. Human papova (wart) virus. Bacteriol. Rev. 31 , 110–131 (1967). This paper is a fascinating documentation of early human transmission studies .
Dreer, M. et al. Interaction of NCOR/SMRT repressor complexes with papillomavirus E8^E2C proteins inhibits viral replication. PLoS Pathog. 12 , e1005556 (2016). This paper describes how HPV E8^E2 protein restricts replication of many HPV types .
Zhou, J., Liu, W. J., Peng, S. W., Sun, X. Y. & Frazer, I. Papillomavirus capsid protein expression level depends on the match between codon usage and tRNA availability. J.Virol. 73 , 4972–4982 (1999).
Hernandez-Alias, H.-A., Benisty, H., Schaefer, M. H. & Serrano, L. Translational adaptation of human viruses to the tissues they infect. Cell 34 , 108872 (2021).
CAS Google Scholar
Roberts, J. N. et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 13 , 857–861 (2007).
Day, P. M. & Schelhaas, M. Concepts of papillomavirus entry into host cells. Curr. Opin. Virol. 4 , 24–31 (2014).
Herfs, M., Soong, T. R., Delvenne, P. & Crum, C. P. Deciphering the multifactorial susceptibility of mucosal junction cells to HPV infection and related carcinogenesis. Viruses 9 , 85 (2017).
PubMed Central Google Scholar
Quint, K. D. et al. Human Beta-papillomavirus infection and keratinocyte carcinomas. J. Pathol. 235 , 342–354 (2015).
Ryser, M. D., Myers, E. R. & Durrett, R. HPV clearance and the neglected role of stochasticity. PLoS Comput. Biol. 11 , e1004113 (2015).
Xie, J., Zhang, P., Crite, M. & DiMaio, D. Papillomaviruses go retro. Pathogens 9 , 267 (2020).
CAS PubMed Central Google Scholar
DiGiuseppe, S. et al. Incoming human papillomavirus type 16 genome resides in a vesicular compartment throughout mitosis. Proc. Natl Acad. Sci. USA 113 , 6289–6294 (2016).
Uhlorn, B. L. et al. Vesicular trafficking permits evasion of cGAS/STING surveillance during initial human papillomavirus infection. PLoS Pathog. 16 , e1009028 (2020).
Pyeon, D., Pearce, S. M., Lank, S. M., Ahlquist, P. & Lambert, P. F. Establishment of human papillomavirus infection requires cell cycle progression. PLoS Pathog. 5 , e1000318 (2009).
Aydin, I. et al. Large scale RNAi reveals the requirement of nuclear envelope breakdown for nuclear import of human papillomaviruses. PLoS Pathog. 10 , e1004162 (2014).
Guion, L., Bienkowska-Haba, M., DiGiuseppe, S., Florin, L. & Sapp, M. PML nuclear body-residing proteins sequentially associate with HPV genome after infectious nuclear delivery. PLoS Pathog. 15 , e1007590 (2019).
Day, P. M., Baker, C. C., Lowy, D. R. & Schiller, J. T. Establishment of papillomavirus infection is enhanced by promyelocytic leukemia protein (PML) expression. Proc. Natl Acad. Sci. USA 101 , 14252–14257 (2004).
Corpet, A. et al. PML nuclear bodies and chromatin dynamics: catch me if you can! Nucleic Acids Res. 48 , 11890–11912 (2020).
Hofmann, S., Stubbe, M., Mai, J. & Schreiner, S. Double-edged role of PML nuclear bodies during human adenovirus infection. Virus Res. 295 , 198280 (2021).
Ozbun, M. A. Human papillomavirus type 31b infection of human keratinocytes and the onset of early transcription. J. Virol. 76 , 11291–11300 (2002).
Coursey, T. L. & McBride, A. A. Hitchhiking of viral genomes on cellular chromosomes. Annu. Rev. Virol. 6 , 275–296 (2019).
White, E. A. Manipulation of epithelial differentiation by HPV oncoproteins. Viruses 11 , 369 (2019).
Moore, P. S. & Chang, Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat. Rev. Cancer 10 , 878–889 (2010).
Xue, Y. et al. HPV16 E2 is an immediate early marker of viral infection, preceding E7 expression in precursor structures of cervical carcinoma. Cancer Res. 70 , 5316–5325 (2010).
Klumpp, D. J. & Laimins, L. A. Differentiation-induced changes in promoter usage for transcripts encoding the human papillomavirus type 31 replication protein E1. Virology 257 , 239–246 (1999).
Johansson, C. & Schwartz, S. Regulation of human papillomavirus gene expression by splicing and polyadenylation. Nat. Rev. Microbiol. 11 , 239–251 (2013).
Sakakibara, N., Chen, D. & McBride, A. A. Papillomaviruses use recombination-dependent replication to vegetatively amplify their genomes in differentiated cells. PLoS Pathog. 9 , e1003321 (2013).
Pyeon, D., Lambert, P. F. & Ahlquist, P. Production of infectious human papillomavirus independently of viral replication and epithelial cell differentiation. Proc. Natl Acad. Sci. USA 102 , 9311–9316 (2005).
Porter, S. S. et al. Histone modifications in papillomavirus virion minichromosomes. mBio 12 , e03274-20 (2021).
Bryan, J. T. & Brown, D. R. Transmission of human papillomavirus type 11 infection by desquamated cornified cells. Virology 281 , 35–42 (2001).
Egawa, N. et al. Dynamics of papillomavirus in vivo disease formation & susceptibility to high-level disinfection — implications for transmission in clinical settings. EbioMed. 63 , 103177 (2021).
Roden, R. B. S., Lowy, D. R. & Schiller, J. T. Papillomavirus is resistant to desiccation. J. Infect. Dis. 176 , 1076–1079 (1997).
Ozbun, M. A. et al. Infectious titres of human papillomaviruses (HPVs) in patient lesions, methodological considerations in evaluating HPV infectivity and implications for the efficacy of high-level disinfectants. EbioMed. 63 , 103165 (2021).
Spurgeon, M. E. et al. A novel in vivo infection model to study papillomavirus-mediated disease of the female reproductive tract. mBio 10 , e00180-19 (2019).
Bravo, I. G. & Alonso, A. Mucosal human papillomaviruses encode four different E5 proteins whose chemistry and phylogeny correlate with malignant or benign growth. J. Virol. 78 , 13613–13626 (2004).
Bergvall, M., Melendy, T. & Archambault, J. The E1 proteins. Virology 445 , 35–56 (2013).
Wu, S. C. et al. in Human Papillomavirus: Proving and Using a Viral Cause for Cancer (eds Jenkins, D. & Bosch, F. X.) 53–65 (Academic, 2020).
Dreer, M., van de Poel, S. & Stubenrauch, F. Control of viral replication and transcription by the papillomavirus E8^E2 protein. Virus Res. 231 , 96–102 (2017).
Doorbar, J., Campbell, D., Grand, R. J. & Gallimore, P. H. Identification of the human papilloma virus-1a E4 gene products. EMBO J. 5 , 355–362 (1986).
Doorbar, J., Ely, S., Sterling, J., McLean, C. & Crawford, L. Specific interaction between HPV-16 E1–E4 and cytokeratins results in collapse of the epithelial cell intermediate filament network. Nature 352 , 824–827 (1991).
Doorbar, J. The E4 protein; structure, function and patterns of expression. Virology 445 , 80–98 (2013).
DiMaio, D. & Petti, L. M. The E5 proteins. Virology 445 , 99–114 (2013).
Scott, M. L. et al. Human papillomavirus 16 E5 inhibits interferon signaling and supports episomal viral maintenance. J. Virol. 94 , e01582-19 (2020).
Roman, A. & Munger, K. The papillomavirus E7 proteins. Virology 445 , 138–168 (2013).
Brimer, N., Drews, C. M. & Vande Pol, S. B. Association of papillomavirus E6 proteins with either MAML1 or E6AP clusters E6 proteins by structure, function, and evolutionary relatedness. PLoS Pathog. 13 , e1006781 (2017).
Katzenellenbogen, R. Telomerase induction in HPV infection and oncogenesis. Viruses 9 , 180 (2017).
Brimer, N., Lyons, C., Wallberg, A. E. & Vande Pol, S. B. Cutaneous papillomavirus E6 oncoproteins associate with MAML1 to repress transactivation and NOTCH signaling. Oncogene 31 , 4639–4646 (2012).
Jackson, S. & Storey, A. E6 proteins from diverse cutaneous HPV types inhibit apoptosis in response to UV damage. Oncogene 19 , 592–598 (2000).
Campos, S. K. Subcellular trafficking of the papillomavirus genome during initial infection: the remarkable abilities of minor capsid protein L2. Viruses 9 , 370 (2017).
DiGiuseppe, S., Bienkowska-Haba, M., Guion, L. G. & Sapp, M. Cruising the cellular highways: how human papillomavirus travels from the surface to the nucleus. Virus Res. 231 , 1–9 (2017).
Buck, C. B., Day, P. M. & Trus, B. L. The papillomavirus major capsid protein L1. Virology 445 , 169–174 (2013).
Hsu, J. Y., Chen, A. C., Keleher, A., McMillan, N. A. & Antonsson, A. Shared and persistent asymptomatic cutaneous human papillomavirus infections in healthy skin. J. Med. Virol. 81 , 1444–1449 (2009).
Birkeland, S. A. et al. Cancer risk after renal transplantation in the Nordic countries, 1964–1986. Int. J. Cancer 60 , 183–189 (1995).
Leiding, J. W. & Holland, S. M. Warts and all: human papillomavirus in primary immunodeficiencies. J. Allergy Clin. Immun. 130 , 1030–1048 (2012).
Rollison, D. E., Viarisio, D., Amorrortu, R. P., Gheit, T. & Tommasino, M. An emerging issue in oncogenic virology: the role of beta human papillomavirus types in the development of cutaneous squamous cell carcinoma. J. Virol. 93 , e01003-18 (2019).
Strickley, J. D. et al. Immunity to commensal papillomaviruses protects against skin cancer. Nature 575 , 519–522 (2019).
de Koning, M. N. et al. High prevalence of cutaneous warts in elementary school children and the ubiquitous presence of wart-associated human papillomavirus on clinically normal skin. Br. J. Dermatol. 172 , 196–201 (2015).
Egawa, N., Egawa, K., Griffin, H. & Doorbar, J. Human papillomaviruses; epithelial tropisms, and the development of neoplasia. Viruses 7 , 3863–3890 (2015).
Steben, M. & Garland, S. M. Genital warts. Best Pract. Res. Clin. Obstet. Gynaecol. 28 , 1063–1073 (2014).
Gravitt, P. E. The known unknowns of HPV natural history. J. Clin. Invest. 121 , 4593–4599 (2011).
DiGiuseppe, S., Bienkowska-Haba, M., Guion, L. G. M., Keiffer, T. R. & Sapp, M. Human papillomavirus major capsid protein L1 remains associated with the incoming viral genome throughout the entry process. J. Virol. 91 , e00537-17 (2017).
Guion, L. G. & Sapp, M. The role of promyelocytic leukemia nuclear bodies during HPV infection. Front. Cell Infect. Microbiol. 10 , 35 (2020).
Stepp, W. H., Meyers, J. M. & McBride, A. A. Sp100 provides intrinsic immunity against human papillomavirus infection. mBio 4 , e00845-13 (2013).
Florin, L., Schafer, F., Sotlar, K., Streeck, R. E. & Sapp, M. Reorganization of nuclear domain 10 induced by papillomavirus capsid protein l2. Virology 295 , 97–107 (2002).
Ferreira, A. R., Ramalho, A. C., Marques, M. & Ribeiro, D. The interplay between antiviral signalling and carcinogenesis in human papillomavirus infections. Cancers (Basel) 12 , 646 (2020).
Roden, R. B. S. & Stern, P. L. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat. Rev. Cancer 18 , 240–254 (2018).
Gravitt, P. E. Unraveling the epidemiology of oral human papillomavirus infection. Ann. Intern. Med. 167 , 748–749 (2017).
Schiller, J. & Lowy, D. Explanations for the high potency of HPV prophylactic vaccines. Vaccine 36 , 4768–4773 (2018). This paper explains why the HPV vaccine is so immunogenic, and successful .
Beziat, V. Human genetic dissection of papillomavirus-driven diseases: new insight into their pathogenesis. Hum. Genet. 139 , 919–939 (2020).
Venuti, A., Lohse, S., Tommasino, M. & Smola, S. Cross-talk of cutaneous beta human papillomaviruses and the immune system: determinants of disease penetrance. Philos. Trans. R. Soc. London Ser. B, Biol. Sci. 374 , 20180287 (2019).
McDermott, D. H. & Murphy, P. M. WHIM syndrome: immunopathogenesis, treatment and cure strategies. Immunol. Rev. 287 , 91–102 (2019).
Beziat, V. et al. Humans with inherited T cell CD28 deficiency are susceptible to skin papillomaviruses but are otherwise healthy. Cell 184 , 3812–3828.e30 (2021). This paper identifies deficiency in the T cell co-stimulatory molecule CD28 as responsible for the ‘tree man’ syndrome .
Park, S., Park, J. W., Pitot, H. C. & Lambert, P. F. Loss of dependence on continued expression of the human papillomavirus 16 E7 oncogene in cervical cancers and precancerous lesions arising in fanconi anemia pathway-deficient mice. mBio 7 , e00628-16 (2016).
Hammer, A. et al. Whole tissue cervical mapping of HPV infection: molecular evidence for focal latent HPV infection in humans. Papillomavirus Res. 7 , 82–87 (2019). This paper presents an analysis of HPV latency in human cervical infection .
Doorbar, J. Latent papillomavirus infections and their regulation. Curr. Opin. Virol. 3 , 416–421 (2013).
Amella, C. A. et al. Latent infection induced with cottontail rabbit papillomavirus. A model for human papillomavirus latency. Am. J. Pathol. 144 , 1167–1171 (1994).
Maglennon, G. A., McIntosh, P. & Doorbar, J. Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology 414 , 153–163 (2011).
Lanfredini, S. et al. HPV8 field cancerization in a transgenic mouse model is due to Lrig1 + keratinocyte stem cell expansion. J. Investig. Dermatol. 137 , 2208–2216 (2017).
Viens, L. J. et al. Human papillomavirus-associated cancers — United States, 2008–2012. MMWR 65 , 661–666 (2016).
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr. Eval. Carcinog. Risks. Hum. 100 , 1–441 (2012).
Howley, P. M. & Pfister, H. J. Beta genus papillomaviruses and skin cancer. Virology 479–480 , 290–296 (2015).
Herfs, M. et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc. Natl Acad. Sci. USA 109 , 10516–10521 (2012).
Yang, E. J. et al. Microanatomy of the cervical and anorectal squamocolumnar junctions: a proposed model for anatomical differences in HPV-related cancer risk. Mod. Pathol. 28 , 994–1000 (2015).
Doorbar, J. & Griffin, H. Refining our understanding of cervical neoplasia and its cellular origins. Papillomavirus Res. 7 , 176–179 (2019).
Reich, O. & Regauer, S. Thin HSIL of the cervix: detecting a variant of high-grade squamous intraepithelial lesions with a p16INK4a antibody. Int. J. Gynecol. Pathol. 36 , 71–75 (2017).
Roberts, S., Evans, D., Mehanna, H. & Parish, J. L. Modelling human papillomavirus biology in oropharyngeal keratinocytes. Philos. Trans. R. Soc. London B Biol. Sci. 374 , 20180289 (2019).
Doorbar, J., Egawa, N., Griffin, H., Kranjec, C. & Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 25 (Suppl 1), 2–23 (2015).
Klingelhutz, A. J. & Roman, A. Cellular transformation by human papillomaviruses: lessons learned by comparing high- and low-risk viruses. Virology 424 , 77–98 (2012).
Mesri, E. A., Feitelson, M. A. & Munger, K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host Microbe 15 , 266–282 (2014).
Mittal, S. & Banks, L. Molecular mechanisms underlying human papillomavirus E6 and E7 oncoprotein-induced cell transformation. Mutat. Res. 772 , 23–35 (2017).
McBride, A. A. & Warburton, A. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog. 13 , e1006211 (2017).
Tommasino, M. HPV and skin carcinogenesis. Papillomavirus Res. 7 , 129–131 (2019).
de Sanjose, S., Brotons, M., LaMontagne, D. S. & Bruni, L. Human papillomavirus vaccine disease impact beyond expectations. Curr. Opin. Virol. 39 , 16–22 (2019).
Drolet, M. et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet 394 , 497–509 (2019).
Stern, P. L. & Roden, R. B. Opportunities to improve immune-based prevention of HPV-associated cancers. Papillomavirus Res. 7 , 150–153 (2019).
Walling, E. B. et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics https://doi.org/10.1542/peds.2015-3863 (2016).
Article PubMed Google Scholar
Robles, C. et al. Determinants of human papillomavirus vaccine uptake by adult women attending cervical cancer screening in 9 European countries. Am. J. Prev. Med. https://doi.org/10.1016/j.amepre.2020.08.032 (2020).
Van Doorslaer, K. et al. The Papillomavirus Episteme: a major update to the papillomavirus sequence database. Nucleic Acids Res. 45 , D499–D506 (2017). This paper describes the Papillomavirus Episteme, the HPV sequence database .
Hughes, F. J. & Romanos, M. A. E1 protein of human papillomavirus is a DNA helicase/ATPase. Nucleic Acids Res. 21 , 5817–5823 (1993).
Ustav, M., Ustav, E., Szymanski, P. & Stenlund, A. Identification of the origin of replication of bovine papillomavirus and characterization of the viral origin recognition factor E1. EMBO J. 10 , 4321–4329 (1991).
Androphy, E. J., Lowy, D. R. & Schiller, J. T. Bovine papillomavirus E2 trans -activating gene product binds to specific sites in papillomavirus DNA. Nature 325 , 70–73 (1987).
Melendy, T., Sedman, J. & Stenlund, A. Cellular factors required for papillomavirus DNA replication. J.Virol. 69 , 7857–7867 (1995).
Ustav, M. & Stenlund, A. Transient replication of BPV-1 requires two viral polypeptides encoded by the E1 and E2 open reading frames. EMBO J. 10 , 449–457 (1991).
Mohr, I. J. et al. Targeting the E1 replication protein to the papillomavirus origin of replication by complex formation with the E2 transactivator. Science 250 , 1694–1699 (1990).
Skiadopoulos, M. H. & McBride, A. A. Bovine papillomavirus type 1 genomes and the E2 transactivator protein are closely associated with mitotic chromatin. J. Virol. 72 , 2079–2088 (1998).
McBride, A. A. The papillomavirus E2 proteins. Virology 445 , 57–79 (2013).
Piirsoo, M., Ustav, E., Mandel, T., Stenlund, A. & Ustav, M. Cis and trans requirements for stable episomal maintenance of the BPV-1 replicator. EMBO J. 15 , 1–11 (1996).
Davy, C. E. et al. Identification of a G2 arrest domain in the E1^E4 protein of human papillomavirus type 16. J. Virol. 76 , 9806–9818 (2002).
Moody, C. A. Impact of replication stress in human papillomavirus pathogenesis. J. Virol. https://doi.org/10.1128/JVI.01012-17 (2019).
Article PubMed PubMed Central Google Scholar
Huibregtse, J. M., Scheffner, M. & Howley, P. M. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 10 , 4129–4135 (1991).
Adelstein, D. et al. NCCN Guidelines Insights: head and neck cancers, version 2.2017. J. Natl Compr. Cancer Netw. 15 , 761–770 (2017).
Buck, C. B. et al. Arrangement of L2 within the papillomavirus capsid. J. Virol. 82 , 5190–5197 (2008).
Wang, J. W. & Roden, R. B. L2, the minor capsid protein of papillomavirus. Virology 445 , 175–186 (2013).
Cubie, H. A. Diseases associated with human papillomavirus infection. Virology 445 , 21–34 (2013). This paper is a highly cited review of HPV-associated disease .
Moscicki, A. B. et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J. Pediatr. 132 , 277–284 (1998).
Gissmann, L., Pfister, H. & Zur Hausen, H. Human papilloma viruses (HPV): characterization of four different isolates. Virology 76 , 569–580 (1977).
zur Hausen, H., Meinhof, W., Scheiber, W. & Bornkamm, G. W. Attempts to detect virus-specific DNA in human tumors. I. Nucleic acid hybridizations with complementary RNA of human wart virus. Int. J. Cancer. 13 , 650–656 (1974).
Download references
Acknowledgements
Work in the author’s laboratory is funded by the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases (NIAID) (grant number ZIA1000713). The author thanks R. Kissinger (NIAID) for drawing a draft for Fig. 2.
Author information
Authors and affiliations.
Laboratory of Viral Diseases, National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA
Alison A. McBride
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Alison A. McBride .
Ethics declarations
Competing interests.
The author declares no competing interests.
Additional information
Peer review information.
Nature Reviews Microbiology thanks J. Doorbar, S. Graham and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
International Agency for Research on Cancer: https://www.iarc.who.int
International Committee on Taxonomy of Viruses: https://talk.ictvonline.org
International HPV Reference Center: https://ki.se/en/labmed/international-hpv-reference-center
Papillomavirus Episteme: https://pave.niaid.nih.gov
Layers of stratified keratinocytes that form the outer layer of the skin.
The moist mucous epithelium that is present at the entrance to body cavities.
‘High-risk’ human papillomavirus (HPV) types that are associated with the development of several human cancers.
A tumour suppressor protein that restricts cell growth when cells are damaged; p53 is very often mutated in non-human papillomavirus (non-HPV) cancers.
The complementary recognition module of PDZ domains, which are interaction modules found in many proteins.
A long-term viral infection that maintains a reservoir of host cells containing the viral genome, evades immune clearance and often produces virus particles.
An epithelial cell of the epidermis that produces keratin.
A thin, non-cellular layer that lies between the dermis and the epidermis of the skin.
The junction between squamous and columnar epithelial cells in the cervix, anus and similar tissues. Also known as the squamocolumnar junction or transition zone.
A single-layer, glandular epithelium that lines the endocervix.
The viral genome is dormant in cells and does not produce virus.
A protein complex that sorts and traffics proteins between the endosome and trans-Golgi network.
Small, interferon-inducible nuclear bodies involved in many cell processes; they consist of a scaffold of PML protein but contain many other proteins.
Proteins that dysregulate the host cell, and by doing so promote carcinogenesis.
Small circular DNA molecules that are assembled in chromatin.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
McBride, A.A. Human papillomaviruses: diversity, infection and host interactions. Nat Rev Microbiol 20 , 95–108 (2022). https://doi.org/10.1038/s41579-021-00617-5
Download citation
Accepted : 27 July 2021
Published : 14 September 2021
Issue Date : February 2022
DOI : https://doi.org/10.1038/s41579-021-00617-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
A broad wastewater screening and clinical data surveillance for virus-related diseases in the metropolitan detroit area in michigan.
- Brijen Miyani
- Irene Xagoraraki
Human Genomics (2024)
The potential and promise for clinical application of adoptive T cell therapy in cancer
- Yeteng Zheng
Journal of Translational Medicine (2024)
Co-infection of distinct papillomavirus types in a captive North American porcupine
- Bert Vanmechelen
- Jennifer Lahoreau
Virology Journal (2023)
The interactions between PML nuclear bodies and small and medium size DNA viruses
- Boris Ryabchenko
- Vojtěch Šroller
- Sandra Huérfano
Transcriptional activity of the long control region in human papillomavirus type 33 intratype variants
- Eszter Gyöngyösi
- Brigitta László
- György Veress
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
Skip to content
Questions and Answers about HPV and the Vaccine
Vaccine education center.
Many people have questions about human papillomavirus (HPV) and the vaccine that prevents it. Here, you can find a compilation of some common questions. Can't find what you're looking for? Ask your HPV questions here.
HPV infection - general
Infection-related questions, at what age can someone be infected with hpv.
Anyone can be infected with HPV regardless of their age. For example, if a pregnant woman has HPV, her baby can be born with an HPV infection.
If someone is infected with one type of HPV and their immune system clears it, are they immune to other types of HPV too?
Immunity to one type of HPV does not afford protection against the other types. The current vaccine protects against 9 different types of HPV, which protects against the types most likely to cause disease.
If a person is diagnosed with cervical HPV, does that mean they also have HPV anally if they’ve had anal intercourse, and is there a test for the presence of HPV anally?
A person found to have HPV in cells of the cervix may or may not have the infection in cells of the anus. The virus does not travel in the body; however, people often do not know when they were infected. So, it is possible that the cells of the anus could have been infected if the HPV exposure that resulted in cervical infection occurred during relations that also involved anal intercourse. In some cases, anal cells can be isolated to check for changes suggestive of cancer. However, because this type of “pap” test does not only detect changes caused by HPV, but those caused by other pathogens as well, its use is limited to certain high-risk populations, and it may or may not be able to confirm an HPV infection.
If a person is diagnosed with genital HPV, does that mean they also have HPV orally, and is there a test for the presence of HPV orally?
The HPV virus does not travel to other parts of the body, so a genital infection does not automatically mean an oral infection. Unfortunately, no test is available to check for oral HPV at this time.
How long does an HPV infection last?
HPV infections can last up to 24 months before the immune system eliminates the infection. During this time, most people do not know they are infected. This is why it is difficult to stop transmission of the virus.
What does it mean when people say an HPV infection “cleared?” Is the infection gone or is it dormant? Can it still be spread to someone else?
HPV virus can cause persistent infections. This means that when a person is infected, the virus is reproducing in the cells that line the infected area. It does not live silently inside of cells like herpes viruses. This means that when the immune system “clears” the infection, it is no longer present, so it cannot be spread to someone else.
However, what is important to understand is that many people have HPV infections without symptoms, so they do not realize they are infected. Further, since a Pap test checks for cellular changes and not the presence of virus, a “negative” Pap test does not mean that no HPV infection is present. It only means that the cells that line the cervix do not currently show signs of damage caused by a persistent HPV infection. This is why it is important to get regular Pap tests.
How does HPV cause cancer?
HPV infects epithelial cells that line mucosal surfaces of the body. When HPV enters these cells, such as in the throat, genital tract or anus, it causes the cells to produce HPV proteins. In most cases, the immune system recognizes the cells that are infected and eliminates them, clearing the infection. However, in some instances a persistent infection occurs causing the cells to mutate, or change. These mutations can ultimately lead to cancer.
Am I really at risk of getting HPV?
HPV is spread through genital contact, most often, but not always, during sex. It can also spread through oral sex. Most people don’t know they have HPV, so they often don’t realize they are spreading the virus. Since HPV is so common, if you are intimate with anyone, the best way to reduce your chance of getting infected is to be vaccinated with the HPV vaccine.
How common is HPV?
HPV is one of the most common sexually transmitted diseases among both men and women in the United States. Currently, about 42.5 million Americans are infected with HPV, and every year, about 13 million new infections occur.
HPV causes cervical cancer, one of the most common cancers in women. Every year in the United States, approximately 13,000 women get cervical cancer and about 4,000 women die from the disease. Worldwide, the total number of deaths from cervical cancer every year is more than 300,000. HPV is also known to cause genital warts as well as cancers of the penis, vagina, vulva, anus and oropharynx.
How do I know if my partner or I have been exposed to HPV?
Because most people do not develop symptoms of infection, they do not know they are infected. To avoid or decrease the chance of exposure, you can abstain from sexual activity, limit the number of sexual partners you have and use condoms. Unfortunately, other than abstinence, none of these methods offers complete protection.
Is HPV deadly?
Yes, in some people the virus causes changes in cells that lead to the development of potentially fatal cancers.
Once a person has HPV, can he or she get rid of it?
Yes, in fact, most people (9 of every 10) do clear the infection within two years, often never having symptoms. Those who don't clear the infection (the remaining 1 of every 10 people) may suffer from genital warts, cervical cancer or other cancers.
Can someone be infected with more than one type of HPV?
Yes, you can be infected with more than one type of HPV at a time.
Do I need to worry about HPV if my boyfriend and I always use a condom?
If your boyfriend has an HPV infection (with or without symptoms), you can still be infected with HPV even when using a condom for two reasons. First, because condoms aren’t foolproof at containing the virus, you could still be infected and, second, while HPV is most often transmitted during sexual intercourse, it can also be transmitted during oral sex or during genital-to-genital contact.
Symptom-related questions
How long does it take for symptoms of hpv to appear.
People can be infected with HPV for years, or even decades, before they experience any symptoms of infection. This is why women should get regular Pap screenings. Because Pap screenings show early signs of changes in cells of the cervix (precancerous changes), treatment is often more successful than after physical symptoms, such as bleeding and pain, appear.
If someone has genital warts, does that mean HPV virus is still present?
Yes. If a person has outward signs of infection, such as genital warts, he or she can transmit the virus. However, it is important to realize that people can also transmit HPV when they do not have any symptoms. Additionally, even if someone has genital warts removed, they may still be infected and able to transmit the virus.
Even though I got the HPV vaccine, I got genital warts. Will I always have them?
Even if you had the HPV vaccine, you could still develop genital warts if you were infected with a strain of HPV not contained in the vaccine. You may want to consider visiting your healthcare provider to confirm the diagnosis of genital warts. If you do have genital warts, your doctor can go over treatment options with you depending on your particular situation. You can read the information about treating genital warts from the Centers for Disease Control and Prevention.
Regarding whether you will always have genital warts, it is difficult to say. In most people, their immune system will eventually clear the infection and the warts will go away, but for some, they may remain. We have no way of telling whether an individual’s immune system is likely to clear the infection or not.
How soon will genital warts appear if I get infected with HPV? Am I really at risk of getting HPV?
Genital warts typically develop four weeks to eight months after contracting one of the types of HPV that cause genital warts. However, HPV can also replicate without causing symptoms for several years before genital warts appear.
How soon after an HPV infection does cervical cancer develop?
Progression from an initial HPV infection to cancer requires prolonged infection with one of the types of HPV that causes cancer. For this reason, cervical cancer typically develops 20 to 25 years after the initial HPV infection. Regular Pap tests and HPV tests will help your doctor monitor for precancerous changes to the cells of the cervix.
Transmission-related questions
I recently had an leep procedure to remove high-risk cells from my cervix following a positive pap test. can i still pass hpv on to my partner after having this procedure done.
The loop electrosurgical excision procedure (LEEP) does not rid you of HPV. It rids you of some cells that are showing signs of changes resulting from long-term infection. If you are with the same partner that you were with prior to your diagnosis, it is possible the partner was already exposed to the type of HPV you are infected with. If you are with a new partner and that person was not previously exposed to the type of HPV that you have (either naturally or through vaccination), you might expose your partner.
Can a person spread HPV to someone after getting the HPV vaccine?
The HPV vaccine protects against nine types of HPV. Two earlier versions protected against two or four types. The types of HPV in the vaccine protect against the most common causes of cancer and genital warts. If, after being vaccinated, a person is exposed with a type of HPV that was included in the vaccine, he or she is unlikely to be infected and, therefore, wouldn’t spread the virus. However, if a vaccinated person is exposed to an HPV type not in the vaccine, they could potentially be infected and spread the virus to others.
Is it possible for a person who says they are a virgin to spread HPV?
It is possible to spread the virus through intimate contact that does not include intercourse, such as genital-to-genital contact or oral-to-genital contact. So, it is possible that someone who has not had intercourse could be infected with HPV and spread it to others.
If I am infected with HPV orally, can I pass the virus to my children if I kiss them?
While the studies looking at HPV transmission orally are minimal, it is generally agreed upon by the scientific community that HPV is spread orally through more intimate forms of engagement, such as oral sex or "open-mouth" (French) kissing, so kissing your children would not be likely to spread the virus to them if you have an oral HPV infection.
I have heard HPV can be transmitted by skin-to-skin contact. So, if a woman has HPV, can I or my children get it by being around her?
No. HPV is not transmitted by simply being near or touching someone who has it. The reference to skin-to-skin contact refers to intimate interactions, such as genital-to-genital or oral-to-genital contact.
I have never been diagnosed with HPV or genital warts, so how could my child have recurrent respiratory papillomatosis?
Recurrent respiratory papillomatosis, or RRP, is chronic infection of the vocal cords and lungs caused by passage through a birth canal infected with HPV. RRP is primarily caused by two types of HPV that also commonly cause genital warts, types 6 and 11. Because many people are infected with HPV and never have symptoms, they do not know they have an HPV infection. Therefore, unfortunately, it is possible that you could have had an undiagnosed HPV infection during delivery that led to your child’s infection.
I have been in a monogamous relationship for more than 20 years; however, I was recently diagnosed with genital warts. My wife has never had them. How could I have gotten them?
Your question is a common one. Almost everyone who is sexually active will be infected with HPV at some point. For many, they may never know when or how they were infected for a few reasons. First, symptoms can appear years after the initial infection. Second, the disease can be transmitted without having intercourse. Skin-to-skin contact or oral sex can also transmit the virus. Finally, even people who do not know they are infected and those who do not have any symptoms may still transmit the virus.
If a woman is exposed to HPV through oral sex with a man who has had genital warts, can she get HPV and if so, will her infection occur in the oral or genital region?
Yes. A woman can be exposed to HPV if she has oral sex with a man who has an HPV infection (with or without current symptoms). If this happens, the infection will occur in the mucosal areas of her mouth, such as in cells in her throat. In most cases, the woman’s immune system will clear the infection without any symptoms. In very rare cases, the virus will persist and cause a condition known as recurrent respiratory papillomatosis (RRP). People with RRP develop warts in their throat which can become large enough that they cause hoarseness or trouble breathing.
Can you get HPV from someone who does not have any symptoms of HPV?
Yes, in fact, most people do not know when they are infected with HPV. So, even if your partner does not have any symptoms of an HPV infection, he or she can still pass the virus to you.
I have heard that you do not need to have intercourse to get HPV. Is that true?
Yes. Although most infections occur following intercourse, HPV may also be passed on during oral sex and genital-to-genital contact. Even more rarely, a mom can transmit the virus to her baby during birth.
Can someone get HPV during masturbation?
HPV is transmitted through intimate interactions between an infected person and an uninfected person. They do not have to have intercourse. Genital-to-genital contact can spread the virus. Because masturbation involves touching one’s self, it will not cause someone to become infected with HPV.
Can a woman pass HPV to a male partner through intercourse?
Yes, a woman can pass the infection to a partner as well as to her baby during birth, although the latter is fairly uncommon. While the infection is most commonly transmitted through intercourse, the virus can also be passed to one's partner during genital-to-genital contact or oral sex.
HPV and pregnancy
Does having hpv put my unborn baby at risk.
In rare instances, mothers with genital HPV can pass the virus to their baby during vaginal delivery. A small number of these babies go on to develop recurrent respiratory papillomatosis (RRP), a condition in which tumors grow in the throat or lungs, sometimes causing hoarseness, difficulty breathing, talking, and swallowing. While the tumors can be surgically removed, they tend to grow back. Some people with RRP require regular surgical intervention. RRP can also cause a disease of the lungs that resembles cystic fibrosis.
A link between HPV and miscarriage, premature delivery or other complications has not been found.
Consult your doctor if you have any concerns.
- Read more about HPV and Pregnancy»
- Read more about RRP»
Can I get the HPV vaccine if I am pregnant?
Although the HPV vaccine has not been found to cause harm to a woman or her fetus, it is recommended to wait until after delivery to start or continue with the series.
If you got the vaccine while you were pregnant, you do not need to take any special precautions.
I started getting the HPV vaccine and now I am pregnant. Can I still get the other doses of vaccine?
You should wait until after you deliver to get the remaining doses of vaccine. There is no indication that the vaccine causes harm to you or your unborn baby, but it is recommended to wait just to be safe. After you deliver, you can get the remaining doses.
HPV testing and treatment
The Centers for Disease Control and Prevention (CDC) has a helpful resource for understanding Pap and HPV tests .
What tests can a woman have related to HPV?
Two tests for women are available:
- Pap test — A Pap test is done by scraping some cells from the cervix and examining them microscopically. A normal result means your cells looked as expected; an abnormal result means that the cells appeared to have undergone some changes. This does not mean you have cervical cancer. In some cases the cell changes are minor and will return to normal when tested in the future. In other cases the changes are more dramatic and need to be monitored more closely.
- HPV test — The HPV test determines if the human papillomavirus is present in the cervix.
The National Cancer Institute has a useful information page about the different test results and what they mean .
If there are no screening tests for men, how can they tell if they have HPV and if so, what is the treatment?
Although there is no approved test for men to know their "HPV status," most HPV infections resolve without causing any problems. The problems caused by HPV in men can include genital warts, anal and penile cancers, or cancers of the oropharynx. There are ways to check for those:
- Genital warts - If you notice abnormalities in the area of your penis, scrotum or anus, such as warts or blisters, see your healthcare provider.
- Anal cancers - Gay, bisexual, and HIV-positive men may consider annual screening by digital rectal exam . Although it is not a formal recommendation, these men are at higher risk.
- Penile cancers - No screening tests are currently available, but early signs can include color changes or build-up or thickening of the tissue.
- Cancers of the oropharynx - Signs include issues associated with the throat including pain, constant coughing, voice changes or hoarseness, lumps or masses in the necks, and trouble swallowing or breathing.
Although no specific treatments for HPV exist, supportive treatments for the health problems caused by HPV are available.
Is there a treatment for HPV?
No antiviral drugs are available to treat HPV. Most HPV infections, however, clear on their own in a few years without causing any health problems. While there are no treatments for the infection, there are supportive treatments for the health problems caused by HPV, such as genital warts and cancers.
If a person was treated for HPV, are they protected against a future HPV infection?
No. While the symptoms of HPV can be treated, currently, there is not a way to treat the infection. For example, genital warts can be removed, but they may return. If a woman has changes to the cells of her cervix, she may have a procedure to remove or kill the abnormal cells. However, some cells may still contain HPV. There is not a way to know for certain, which is why regular follow-ups are important.
Likewise, a person who has been treated for HPV can still be infected with other types of HPV.
The vaccine may be protective against strains to which the individual was not previously exposed, so some people can still benefit from vaccination after having HPV.
If I got the LEEP procedure done and my tests have come back negative, am I still infected or is the virus dormant, and can I still pass it to future partners? Will I always have HPV in my body?
The LEEP procedure does not rid you of an HPV infection, and therefore, it is important to get follow-up testing as suggested by your healthcare provider. HPV virus does not have a state of dormancy, so if you are still infected, the virus will continue to replicate. But because the virus can only be detected indirectly using the HPV test or the Pap test to look at cervical cells, it can be difficult to tell whether someone without symptoms is infected.
In many cases, the infected person’s immune system overcomes the infection after having LEEP, so they are no longer infected. This can depend on factors, such as the type of HPV that caused the infection and individual differences between people. For example, some people will be HPV-free within six months of the procedure; whereas, others may still be infected up to 18 months later.
However, if you are still infected, you can transmit the virus to future partners who are not immune to that type of HPV.
If I am infected with HPV, will getting vaccinated make the infection go away?
No. The vaccine only protects people against types of HPV to which they were not previously exposed. It does not treat an existing infection or protect against that type of HPV.
Does a negative Pap test mean that I am not infected with HPV?
No. A Pap test is one in which cells isolated from the cervix are examined under a microscope for precancerous changes caused by a persistent, or long-term, HPV infection. So, a negative Pap test is good news in that it means the cervical cells appear normal, but it does not give any information about a person’s HPV status.
A test that specifically detects HPV is also available. Although this test does measure the presence of HPV virus in the cervical cells, it does not provide information about whether that infection will remain long term or eventually cause cancer. Because many younger women get an HPV infection that is cleared by their immune systems, the HPV test can often be positive, causing unnecessary concern; therefore, it is not recommended for most women younger than 30 years of age.
I get regular Pap tests and they have always been normal, so how could my child have developed recurrent respiratory papillomatosis?
Pap tests identify changes to cervical cells that could lead to cervical cancer; however, the types of HPV that cause cervical cancer are rarely associated with recurrent respiratory papillomatosis (RRP). Therefore, you could have had an infection with one of the types that cause RRP and continue to have normal Pap tests. Also, HPV can infect cells without causing the types of precancerous changes that lead to an abnormal Pap smear.
My boyfriend recently had warts on his penis. When I went to the clinic and had a Pap test, the results were normal. Does this mean that I did not get infected, or is there still a chance I could get genital warts?
The types of HPV that cause genital warts typically differ from those that cause cervical cancer. Since a Pap test is meant to identify cellular changes that could potentially lead to cervical cancer, it does not provide information about HPV infections with types that cause genital warts. For this reason, your Pap test results do not mean that you did not get infected with HPV when your boyfriend had it. The good news is that for many people, the infection will clear without any symptoms, so you may never experience genital warts like your boyfriend did.
What happens if my Pap test is abnormal?
If you have an abnormal Pap test, an HPV test may be suggested to determine if human papillomavirus DNA is present in the cells of the cervix. If the results of the HPV test are positive, your doctor will determine how frequently you should be tested. In addition to HPV and Pap tests, a colposcopy or biopsy may be suggested. A colposcopy visualizes the cells of the cervix and a biopsy takes a sample of cervical cells.
For more information about understanding your test results, see the National Cancer Institute’s information, “HPV and Pap Test Results: Next Steps after an Abnormal Cervical Cancer Screening Test”
How frequently should you get a Pap test?
Women are recommended to get their first Pap test at age 21, and then once every three years until they turn 29. Women who are 30 to 65 years old should have both Pap and HPV tests performed every five years, or a Pap test alone every three years. Women who have an irregular Pap test or who are at risk due to other factors, such infection with Human Immunodeficiency Virus (HIV) or previous diagnosis of cervical cancer, may be required to get tested more frequently.
Find out more on the CDC’s page, “What should I know about screening?”
Find out if you qualify for free or reduced cost screening through the National Breast and Cervical Cancer Early Detection Program (NBCCEDP) .
Can HPV tests replace Pap tests?
No, HPV tests should not replace routine Pap tests for two reasons:
- The tests are not measuring the same thing. Pap tests detect changes in the cells of the cervix that could lead to cancer, whereas HPV tests detect human papillomavirus DNA in the cells of the cervix. A positive HPV test could be the result of a recent infection or a chronic infection.
- The tests are recommended for slightly different age groups; routine Pap tests are recommended for all women 21 years and older, whereas HPV tests are recommended for women 30 years and older and only those women between 21 and 29 who have had an irregular Pap test.
Is there a test to determine if I have HPV?
Yes. The HPV test is used to determine if HPV DNA is present in the cells of the cervix. Positive results mean that your cervix has the types of HPV commonly linked to cervical cancer; however, a positive result does not mean you have cervical cancer. Based on the results, your doctor will determine how frequently you should be tested and whether other tests should be performed. Currently, HPV tests are recommended for all women 30 years and older and any woman 21 to 29 years old who has had an irregular Pap test.
When a person is tested for STDs is HPV testing included?
Sexually transmitted disease (STD) testing is not the same for every person as it depends upon individual risk factors. For HPV, there is no test for males. For females, HPV can be detected by either Pap tests or an HPV test. The Mayo Clinic has a good discussion regarding how to determine what STD tests you may need and what is available .
I got all necessary doses of the HPV vaccine. Do I still need to get Pap tests?
Yes. The HPV vaccine does not contain all types of HPV that can cause cervical cancer; therefore, it is important to continue getting Pap tests.
If I have had an abnormal Pap test in the past, can I still get the HPV vaccine?
Yes. You should still get the HPV vaccine even if you have had an abnormal Pap test because even if you have been infected with HPV, it is not likely that you have been infected with all of the types that the vaccine protects against. So, you can still benefit from protection afforded by the HPV vaccine.
HPV vaccine
How long after receiving the hpv vaccine does it take for the vaccine to work.
The immune system takes one to two weeks to generate immunity to vaccines or infections. In the case of HPV vaccine, the first dose (and the second one if the person is on the three-dose series) generates a primary immune response, so people will have some immunity, but protection can vary from one person to another. The last dose (given at least six months after the first dose) is important because it enhances the memory immune response. A person will have the greatest protection beginning about one to two weeks after receiving their last dose of the vaccine.
Questions about who should get HPV vaccine
Who should get the hpv vaccine and how many doses.
The HPV vaccine is recommended for adolescents between 9 and 12 years of age, and all teenagers and adults between 13 and 26 years of age who did not get the vaccine when they were younger. Individuals between 27 and 45 years of age can also discuss vaccination with their healthcare provider and receive the vaccine if they decide it can protect them from HPV infection.
- Younger than 15 years old: Two doses separated by 6 months
- 15 years and older: Three doses of HPV vaccine with the second dose given one to two months after the first, and the third dose given six to 12 months after the first
Learn more about why adolescents are recommended to get this vaccine by watching this short video.
Learn more about the recommendations related to those older than 26 years of age by watching this short video.
If I have received the first dose of HPV vaccine, is it safe to be intimate? Am I protected from HPV?
People who have received one dose of the HPV vaccine may have some protection, but the additional dose or doses (depending upon age) offer additional protection. Further, if you or your partner were already infected with a type of HPV, the vaccine will not prevent transmission of that HPV type.
I think I had the HPV vaccine about six years ago, but I am not certain. Should I get the shot? And if I do, but I was vaccinated before, will anything happen?
You should talk with your healthcare provider to see if they know whether you were vaccinated and if so, what type of HPV vaccine you received and how many doses were given. However, if that is not an option and you are uncertain, you can still get the vaccine. Extra doses are not likely to have negative effects.
I had the HPV vaccine but have since given birth to a child. Do I need the HPV vaccine again?
No, people who have been vaccinated against HPV do not need to be revaccinated after giving birth.
If someone already has HPV, does it help to get the HPV vaccine?
Yes. Typically, people with HPV have not been infected with all of the types contained in the vaccine, so the vaccine could protect them from types to which they have not been exposed previously. However, the vaccine will not help treat or protect against types of HPV to which the person has already been exposed.
I have received two doses of the HPV vaccine but missed my third dose. Do I need to start again?
For those 15 years of age and younger, the HPV vaccine is now given in two doses. So, depending on your age, you may not need a third dose:
- If you are under 15 years old and your first two doses were at least six months apart, you do not need a third dose.
- If you are 15 years or older, you still need the third dose; it should be separated from the first dose by six to 12 months. It does not need to be restarted if a longer period of time has passed.
I have heard there is an HPV vaccine that protects against more types of HPV, but I had one of the original versions. Do I need to get it again?
The HPV vaccine protects against nine types of HPV (Gardasil 9 ® ). The CDC does not recommend giving this vaccine to people who already had the earlier HPV vaccines (Cervarix® or Gardasil ® -4). However, because the vaccine protects against additional types of the virus, individuals may still reasonably get the vaccine. In this case, the person should speak with their healthcare provider regarding the relative benefits associated with this choice.
I had two doses of the HPV vaccine a while ago. Now, I hear there is a different one that protects against more types of HPV. Should I get that one and if so, do I need to get all three doses of the newer one?
The newer version, Gardasil 9 ® , is the only version currently available, so you can be protected against more types of the virus by getting the vaccine. The 9-valent vaccine can be used in place of either of the previous two HPV vaccines (Gardasil ® -4 and Cervarix ® ) to complete a vaccination series, so, you do not need to start over again. You would just get the last dose with the current vaccine option. Cervarix and Gardasil-4 are no longer available in the United States.
If you are younger than 15 years old and your first two doses were separated by at least six months, you do not need any additional doses.
I am in my early 20s and would like to get the HPV vaccine, but I don’t know where to get it. What do you suggest?
You can start by checking with your primary healthcare provider. If you cannot get the vaccine from their office, you can also check with your gynecologist, the local health department or a local pharmacy. The manufacturer, Merck, also has an adult vaccine locator on their website that might be of help.
I am concerned that giving the HPV vaccine to young girls will lead them to become sexually active at an earlier age or sexually promiscuous at a later age. Has this been studied?
Yes. A few studies have looked at this and none have found that receiving the HPV vaccine causes girls to become promiscuous or engage in sexual activity at an earlier age. One such study by Robert Bednarczyk and colleagues, published October 2012 in Pediatrics , compared the medical records of 493 girls who received the HPV vaccine and 905 who didn’t. The study found no differences between the two groups in regard to the incidence of pregnancies, tests for or diagnosis of sexually transmitted diseases (STDs), and contraceptive counseling. Based on these results, the authors of the study reported that the HPV vaccine “was not associated with increased sexual activity-related outcomes.”
I heard that even people who have not received the HPV vaccine have less chance of getting HPV since the vaccine came out. Please explain how this occurred, and why I need to get the HPV vaccine?
The HPV vaccine was introduced in 2006, and according to an article published in the July 2012 issue of Pediatrics , use of the HPV vaccine resulted not only in lower rates of infection among those who were vaccinated, but also, to some degree, in those who have not been vaccinated. This phenomenon is commonly known as herd immunity.
You should still consider getting the vaccine because while herd immunity might lessen your chance of coming into contact with the virus, the vaccine will significantly decrease your chance of infection if you do come into contact with it. Unfortunately, despite the decreases in transmission resulting from HPV vaccination, millions of people are still infected with HPV and many do not know they are infected.
I didn't get the last dose of the HPV vaccine. Do I need to start over again?
No. You can just resume where you left off.
My daughter is not sexually active. Why should I even consider getting her vaccinated against HPV now?
The HPV vaccine is recommended before the start of sexual activity for two reasons:
- Young people tend to get infected more frequently; in fact, about half of all new infections are diagnosed in girls and young women between 15 and 24 years of age.
- It takes at least six months to complete the series, so even though your daughter may not be active now, or even in six months, it is better to have the series completed sooner rather than later.
I am already sexually active; should I still get the HPV vaccine?
Yes. The reason to get the HPV vaccine even if you are already sexually active is that you are not likely to have been exposed to all of the HPV types contained in the vaccine.
Why does my son need an HPV vaccine since I heard it prevents cervical cancer?
Although HPV is a known cause of cervical cancer, the virus can also cause other cancers of the reproductive tract, anal cancer, penile cancer, genital warts, and on occasion, cancers of the head and neck. In fact, about 4 of every 10 cases of HPV-related cancers occur in boys or men. Because vaccinating boys will also decrease the spread of the virus, they will not only protect themselves, but also their sexual partners.
Can my 11-year-old get the HPV vaccine at the same time as other vaccines?
Yes. The HPV vaccine can be given at the same time as other vaccines recommended at this age, including the vaccine for tetanus, diphtheria, and pertussis (Tdap) and the one for meningococcus. If it is influenza vaccine season, this vaccine can be given as well.
Can I have the vaccine if I'm not a virgin anymore? And will it still be effective?
Yes, you can still get the HPV vaccine even if you have had sexual intercourse. While you may have been exposed to one or more types of HPV, it is unlikely that you would have been exposed to all of the types that the vaccine protects against, so it may still be of benefit for you.
I am 33 years old. Can I get the HPV vaccine?
In October 2018, the vaccine was licensed for people up to 45 years of age, so inquire with your provider.
I finished all doses of the HPV vaccine before I became sexually active, but recently, I had an HPV DNA test that was positive. How can that be, and will the infection go away?
Because the HPV vaccine does not protect against all types of HPV, it is possible that a fully vaccinated person could be infected with a type of HPV that is not contained in the vaccine. Most people will clear any type of HPV infection— but it may take months to do so. In a few people, however, HPV infection will persist and possibly become cancerous. We have no way of knowing who will be affected over the long term. That said, the vaccine protects against the most common types that cause cancer or genital warts.
Questions about HPV vaccine safety
I don’t want to get the hpv vaccine for my child because i have heard that all of the safety studies were completed by the vaccine manufacturer. is this true.
Vaccine safety is studied by many, many groups not just those who manufacture vaccines. The FDA reviews all data associated with studies completed by vaccine manufacturers as well as visiting manufacturing sites and continuing to monitor the vaccine as long as it is being made. Additionally, the CDC has systems in place to monitor vaccine safety, including:
- Vaccine Adverse Events Reporting System (VAERS) which allows anyone to report side effects, allowing CDC scientists to watch for trends.
- Vaccine Safety Datalink (VSD) is a collaboration with eight large healthcare organizations from various parts of the United States. Health records are monitored for vaccine receipt and illnesses to study vaccine safety.
- Clinical Immunization Safety Assessment Project (CISA) is a national group of vaccine experts from the CDC, seven medical research centers, and other experts who conduct research around specific vaccine safety concerns, provide consultations for individual healthcare providers on specific patients, and review adverse event data. Vaccine manufacturers do not have a role in these studies.
Additionally, the National Academy of Medicine (NAM), previously called the Institute of Medicine (IOM), periodically conducts comprehensive reviews of the literature to monitor vaccine safety. The NAM completed a review related to adverse effects of vaccines, which included HPV, in 2012. Their findings are available online.
Millions of doses of HPV vaccine have been given safely throughout the world, including in the U.S. What we know from all of these data is that the vaccine is safe and it is working to decrease transmission of HPV, genital warts, cervical changes that cause cancer, and juvenile-onset recurrent respiratory papillomatosis.
Can the HPV vaccine cause cancer?
No. Because the HPV vaccine is made using only a single protein from each type of the virus, it can’t cause HPV infection, and, therefore, it can’t cause cervical cancer or other cancers.
My son received the first dose of HPV vaccine and then two months later he was ill with severe stomach pains, rash and a headache. Could this illness have been caused by the vaccine?
It is not likely that your son’s symptoms were the result of his HPV vaccination for a couple of reasons. First, the length of time between the dose and the appearance of symptoms is not what one would expect if the vaccine was the cause. Second, of the three symptoms you mentioned, the only one that was consistently reported in HPV vaccine recipients was headache, and that was typically reported within 15 days of the first dose.
Does the HPV vaccine cause infertility?
No. HPV infections do not cause infertility, except indirectly in cases when they progress to cervical cancer, so it is not biologically plausible that the HPV vaccine would lead to infertility. To the contrary, since the HPV vaccine decreases the number of cases of cervical cancer, it may indirectly decrease the number of women unable to have a baby.
I heard stories of girls developing different illnesses after getting the HPV vaccine. Are these stories true?
The known side effects of the HPV vaccine include pain, redness or swelling at the injection site. In addition, because teens tend to faint more easily, fainting has been associated with vaccines given to this age group. Because of this, vaccine recipients should remain seated or lying down at the doctor’s office for about 15 minutes after getting the vaccine.
Reports of blood clots, strokes, heart attacks, chronic fatigue syndrome, infertility or premature ovarian failure, and even death have occurred after receipt of this vaccine; however, reviews of individual cases as well as controlled studies looking at groups of people who did and did not get the vaccine have shown that none of these problems were caused by the HPV vaccine.
My daughter is afraid to get the HPV vaccine because one of her friends said it hurts more than other vaccines. What can I tell her?
The HPV vaccine contains higher concentrations of salt than other vaccines, so they may hurt a bit more when they are administered. However, you can suggest one of the following to make your daughter more comfortable while getting the shot:
- Relax the muscle and look away while the shot is given. Take a few short, deep breaths and then a few longer breaths during the vaccine administration.
- Rub an alcohol pad on the opposite wrist right before the vaccine is given and then have her blow on it while the vaccine is administered.
- Use a distraction — friends, music, books, cell phones, or electronic games may work to distract your daughter during the vaccine administration.
- Finally, remind your daughter that the pain of the vaccine is minor compared to the pain associated with the disease.
What are the reactions to an HPV shot?
The HPV vaccine may cause redness, swelling and tenderness at the site of the injection. Some people may faint when they get the vaccine, so people are advised to stay at the doctor's office for 15-20 minutes after getting the vaccine.
Why did my son have to wait 15 minutes after getting the HPV vaccine?
Some teens are more prone to fainting after getting the vaccine; therefore, all teens are recommended to wait at the doctor's office for 15 minutes to be sure they are okay.
Questions about how HPV vaccine is made and works
Q. how long does it take for someone to be protected after getting the hpv vaccine.
A. It takes about two weeks after the first dose of vaccine for the immune system to generate an immune response. The additional doses make that response stronger, particularly the last one which fortifies the memory response.
Q. If I got the HPV vaccine, do I need to use protection?
A. It is important to understand that the HPV vaccine does not protect against other STDs, such as syphilis, chlamydia, gonorrhea, and herpes, nor does it protect against types of HPV to which one was already exposed. For these reasons, using protection is still prudent to consider.
I have had one dose of the HPV vaccine. Will I be protected if I become sexually active?
While you may have some protection after receiving the first dose of HPV vaccine, your best level of protection will occur after you receive all recommended doses.
I did not tell the doctor that I am sexually active before getting the HPV vaccine. Will it still work?
The HPV vaccine will not protect you against types of HPV to which you may have already been exposed; however, it will protect you against types to which you were not previously exposed. Since the vaccine protects against nine types of HPV, it is likely that you can still benefit from receiving the vaccine. For this reason, knowing your sexual activity status is not a requirement for deciding whether or not you should get the HPV vaccine.
How long does immunity last if you receive all doses of the HPV vaccine?
We do not know for sure whether immunity will last a lifetime; however, the data are reassuring. First, the vaccine has been studied for more than 15 years at this point, and immunity doesn’t appear to wane. Second, the immune responses generated by the vaccine are stronger than those invoked after natural infection. Finally, the hepatitis B vaccine, which is made using a technology similar to the HPV vaccine, induces a memory response that lasts at least 30 years.
If I got all necessary doses of the HPV vaccine, can I still develop genital warts?
Yes, it is possible. Although the HPV vaccine protects against the two strains of HPV that most commonly cause genital warts, it will still only prevent about 9 of every 10 cases of genital warts. Therefore, someone could still get genital warts if they are infected with a type of HPV that causes genital warts but was not in the vaccine.
I heard that the cervical cancer vaccine does not prevent all cases of cervical cancer. If this is true, aren’t people getting a false sense of security?
The strains of HPV included in the vaccine will prevent about 9 of 10 cases of cervical cancer. However, because a possibility of getting cervical cancer from one of the types of HPV not contained in the vaccine still exists, women should continue to get regular Pap tests. In addition, the vaccine does not protect against other sexually transmitted diseases, so practicing safe sex is also important.
If my partner and I had the HPV vaccine, do we still need to use condoms?
Yes. The HPV vaccine does not prevent all types of HPV or other types of sexually transmitted diseases. The Centers for Disease Control and Prevention (CDC) has a helpful webpage about the use of condoms .
Will an HPV booster shot ever be required?
HPV booster doses are not expected to be necessary; however, public health officials will continue to monitor rates of disease to watch for waning immunity.
Can the HPV vaccine help me get rid of genital warts?
If you already have genital warts, the HPV vaccine will not treat them. However, the vaccine may still protect you against other types of HPV to which you were not previously exposed. Consult your doctor about medicines and procedures that may be used to treat genital warts.
Can the HPV vaccine cause HPV?
No. The HPV vaccine is made using a protein from the surface of the HPV virus. Although the protein folds itself to look like a viral particle in a microscope, it does not contain any genetic material, so it cannot replicate and cause an infection. Because the proteins look like a viral particle, scientists refer to them as “virus-like particles.”

Does the HPV vaccine protect me against any other sexually transmitted diseases (STDs)?
No. The vaccine does not protect against any other STD. In fact, since there are more than 100 types of HPV, it does not even protect against all types of HPV.
Free movie! Watch Someone You Love: The HPV Epidemic compliments of the Vaccine Education Center. The 80-minute film tells the powerful story of five incredible women whose lives were forever changed by human papillomavirus (HPV) and cervical cancer. Those stories also provide an opportunity to learn more about HPV disease and common issues faced by families.
In addition, the VEC interviewed the filmmakers, during which they opened up about filming the emotional documentary, getting to know the women featured, and the impact of the women’s stories on their own lives. Watch all or parts of that interview here .
VEC resources
- Human Papillomavirus: What you should know Q&A sheet: English [PDF, 303KB] | Spanish [PDF, 317KB] | Japanese [PDF, 577KB]
Professional and advocacy groups
Various professional and advocacy groups provide reliable information about HPV and the HPV vaccine; several are compiled below.
Centers for Disease Control and Prevention
The CDC has several sources of information related to HPV and the HPV vaccine:
- Cervical Cancer Screening
- Genital HPV Infection webpage
- Human papillomavirus website
- National Breast and Cervical Cancer Early Detection Program (NBCCEDP)
National Institutes of Health
The NIH also has several sources of information related to HPV and the HPV vaccine:
- HPV and Cancer
Other resources
- National Cervical Cancer Coalition
- National Foundation for Infectious Diseases (NFID)
- American Sexual Health Association
- The Yellow Umbrella
- Cervivor – a group that advocates for, assists and empowers survivors of cervical cancer
Videos and podcasts
- Talking about Vaccines with Dr. Paul Offit – HPV
- “Should You Get the HPV Vaccine?” Video Illustration — Dr. Mike Evans, St. Michael’s Hospital, Toronto, Canada
Materials in this section are updated as new information and vaccines become available. The Vaccine Education Center staff regularly reviews materials for accuracy.
You should not consider the information in this site to be specific, professional medical advice for your personal health or for your family's personal health. You should not use it to replace any relationship with a physician or other qualified healthcare professional. For medical concerns, including decisions about vaccinations, medications and other treatments, you should always consult your physician or, in serious cases, seek immediate assistance from emergency personnel.
- Research Article
- Open access
- Published: 17 November 2022
HPV knowledge and vaccine acceptability: a survey-based study among parents of adolescents (KAPPAS study)
- Noelia López ORCID: orcid.org/0000-0001-8568-4752 1 ,
- Ignacio Salamanca de la Cueva 2 ,
- Elena Taborga 3 ,
- Auxiliadora Fernández de Alba 2 ,
- Inés Cabeza 4 ,
- Reyes Mazas Raba 5 ,
- Josep Marès 6 ,
- Patricia Company 7 ,
- Bruno Herrera 1 &
- Manuel Cotarelo 1
Infectious Agents and Cancer volume 17 , Article number: 55 ( 2022 ) Cite this article
6678 Accesses
4 Citations
1 Altmetric
Metrics details
Human papillomavirus (HPV) infection is recognized as one of the major causes of infection-related cancer worldwide. In Spain, the HPV vaccination program started in 2007 and until 2022, it targeted 12-year-old girls.
This was a cross-sectional, multicenter survey-based research carried out at 24 pediatric offices to describe HPV knowledge and vaccine acceptability in parents of children aged between 9 and 14 years-old in Spain. Parents were randomly selected from the medical records following specific quotas to ensure representativeness. The survey included five sections that aim to collect information about sociodemographic characteristics, knowledge of HPV, knowledge and acceptability of vaccines in general, HPV vaccination knowledge and HPV vaccine acceptability. Each section was constituted by a number of close questions with different answer options. Specific scores were assigned to each possible answer to these questions. Based on these scores, four composite variables were created to assess HPV knowledge, HPV vaccine knowledge, HPV vaccine acceptability and vaccines knowledge and acceptability in general. A latent class analysis was performed to identify different group of respondents according to their HPV vaccine acceptability.
A total of 1405 valid surveys were included, with 86.19% of the respondents being mothers. The mean score of HPV knowledge was 28.92 out of 40 (maximum value) (95% CI 28.70–29.20) and the mean score of HPV vaccine acceptability was 3.37 out of 5 (maximum value). One third of parents still need more information to take a final decision about HPV vaccination in their children. Parents perceived that females were more likely to become infected than males and tended to associate HPV infection mainly with cervical cancer, showing a. a lack of information about other HPV-related diseases affecting males.
Conclusions
This study results highlight the need for future actions and educational initiatives to raise awareness of HPV consequences in both genders and to contribute to achieving the elimination of HPV-related diseases beyond cervical cancer.
Human papillomavirus (HPV) infection is recognized as one of the major causes of infection-related cancer worldwide, as well as a causal factor of other diseases such as genital warts or recurrent respiratory papillomatosis [ 1 ].
Nowadays, HPV infections are regarded as the most common sexually transmitted infections in the world. Indeed, 80% of sexually active people will become infected at some point in their lifetime. However, most of these infections are typically controlled immunologically within 1–2 years, although if they persist, they can cause different types of cancer, such as cervical cancer or oropharyngeal cancer [ 1 , 2 , 3 ]. Although the HPV-related burden of disease used to be higher in females than in males in most countries, the latest epidemiological studies point to an increasing trend in the incidence of anal and oropharyngeal cancer in men [ 4 ]. Immunization against HPV infection is the most promising strategy for the prevention of one of the most common sexually transmitted infections worldwide [ 1 ].
In 2020, the World Health Organization/The United Nations Children's Emergency Fund (WHO/UNICEF) published the Estimates of National HPV Immunization Coverage from 2010 to 2019. According to this publication, 107 (55%) of the 194 WHO Member States had introduced HPV vaccination [ 5 ]. America and Europe are by far the WHO regions with the most introductions, and 85% and 77% of their countries, respectively, have already introduced the HPV vaccination, with almost one third of the programs (33 out of 107) being gender-neutral [ 5 ]. Many countries have recognized the value of HPV gender-neutral vaccination programs for the purpose of achieving the goal of eliminating not only cervical cancer but all HPV-related diseases [ 6 ]. According to the latest data published by the European Cancer Organization in 2020, 26 countries in the European region of the WHO are or will be including boys in their national HPV vaccination programs [ 6 ]. This represents almost half (48%) of all the countries in this region [ 6 ]. In Spain, HPV vaccination was introduced into the national immunization program in 2007–2008. Until 2022, it targeted 12-year-old girls [ 7 ], with a mean vaccination coverage rate (VCR) of 81.8% in 2020 (latest data available) [ 8 ].
The latest vaccination calendar published by the Spanish Association of Pediatricians (AEP), dated January 2022, recommends HPV vaccination for girls and boys at the age of 12 years [ 9 ]. However, although this Society [ 9 ] recommends including boys in HPV vaccination programs since 2018, the funded Spanish Immunization Program continues to target only female adolescents and specific high-risk groups. Regardless of gender, it is important to stress that the parents of adolescents play a key role in vaccination decision-making, and the success of the vaccination program relies largely on parental decision-making [ 10 ].
Our group published a systematic literature review in 2020 about HPV knowledge and vaccine acceptability among European adolescents and their parents. This review concluded that since HPV knowledge and vaccine acceptability were still modest and varied widely between studies across EU countries, coordinated efforts should be made to provide the relevant population with information to allow informed decision-making on HPV vaccination [ 10 ]. If the information received by the parents is not properly balanced there might be a negative impact on HPV vaccine acceptability and therefore the HPV-related disease elimination goal would not be achievable [ 6 ].
To our knowledge, as yet no studies to assess knowledge of HPV and vaccine acceptability among parents of children (girls and boys) have been performed at national level in Spain. Spanish studies already published are mainly regional and have focused exclusively on describing acceptability in the female or adult populations at regional level [ 11 , 12 , 13 ]. As the VCR differs substantially between regions, a national study to evaluate knowledge of HPV and vaccine acceptability for girls and boys is called for.
The objective of this study, KAPPAS study (Knowledge and Acceptability of Papillomavirus Vaccines in Parents of Adolescents in Spain ), was to describe HPV knowledge and HPV vaccination acceptability among parents of girls and boys aged between 9 and 14 years living in Spain. Moreover, the study assessed the correlation between HPV knowledge and HPV vaccine acceptability and the influence of different sociodemographic variables.
This paper focuses on the results of the level of HPV knowledge and vaccine acceptability in parents of adolescents in Spain. The analysis of the factors involved in HPV knowledge and vaccine acceptability has been addressed in a separate publication [ 14 ].
Study design and setting
This was a cross-sectional, multicenter survey-based research carried out at twenty-four (public and private) pediatric offices in Spain between May 2019 and April 2020. Sample size and sample distribution was estimated to assess primary and secondary endpoints with appropriate precision (≤ 5%) per each stratum of interest.
Due to the COVID-19 pandemic, only surveys completed before 16 April were considered valid for the analysis to ensure external validity of our results; as COVID-19 pandemic could interfere in vaccine perceptions in the overall population. The study recruited the fathers, mothers or legal guardians of children (girls and/or boys) aged between 9 and 14 years who had been living in Spain for at least the last 12 months.
The study obtained the favorable approval of the reference Investigational Ethical Committee (IEC). The rest of IECs requested the evaluation or only the registration of the protocol as necessary per their local guidance.
Survey development
A structured survey, in Spanish language, was developed to collect epidemiological variables as well as knowledge- and acceptance-related measurements. It was designed based on a previous systematic literature review [ 11 ] carried out by our group to identify published studies and items used to evaluate parental and/or adolescent HPV knowledge and/or HPV vaccination acceptability. Based on the inputs found in the systematic review, a draft questionnaire was developed and then validated by an Expert Committee comprised of 4 pediatricians who were experts in adolescents and HPV. The draft questionnaire was afterwards tested through cognitive debriefing methodology on a representative sample of 12 parents of children between 9 and 14 years old following a fine-tuning of the wording and comprehensiveness of the questionnaire according to participants’ perceptions and suggestions. The Experts Committee validated the final version of the questionnaire.
The final survey (Additional file 1 : Material S1) included five sections: (1) sociodemographic characteristics (15 items); (2) knowledge of HPV (9 items); (3) knowledge of vaccines and their acceptability in general (5 items); (4) HPV vaccination knowledge (8 items); (5) HPV vaccine acceptability (7 items). All questions were closed with multiple choice of answers, using the appropriate scale of response according to the specific type of question (yes/no, yes/no/not sure, ordinal scale of level of agreement or specific response options, when needed) (Additional file 1 : Material S2).
Data collection
Representative centers across different Spanish regions were invited to participate. A preliminary selection of sites was performed by the Committee of Experts of the study-a stratification based on HPV VCR was performed to ensure adequate representativeness. To those centers preliminary selected, a feasibility questionnaire was sent, to ensure that their willingness and availability to participate in this study, and to ensure that they had enough patients between 9 and 14 y.o in order to be able to send the questionnaire to the parents of those patients.. Active recall recruitment process was designed to prevent any selection bias such as chronically ill patients who might attend pediatricians’ offices more frequently (Additional file 1 : Figure S1). All children aged between 9 and 14 years were identified from investigator databases or medical records and were divided into 4 stratification quotas based on gender and age (males 9–11 y.o; females 9–11 y.o,; males 12–14 y.o and females 12–14 y.o). Spanish regions were classified into 2 categories according to their HPV VCR: low VCR: < 77.8% / high VCR: ≥ 77.8%). In 2107, when the protocol was drafted, the mean national VCR was 77.8%.
The investigators actively invited parents following the order generated by a randomization tool by telephone and according to the stratification quotas to ensure representativeness. The survey could be completed either online or in a paper format in an autonomous manner. The investigator sent the participant an e-mail/mobile text message with a link to the survey and a code. Each participant could access the link, introduce the code and confirm his/her acceptance to participate and afterwards fill the online survey (Additional file 1 : Figure S1). For the paper-pen option, the participant could either collect the questionnaire in the office or print it directly from the same link. After completion, the questionnaire could be sent through pre-paid envelopes or be delivered at the doctor’s office. Per protocol, the survey should not be completed in the presence of the pediatrician, nor should anyone from his team interfere with the participant’s answers.
Statistical analysis
A descriptive analysis of the qualitative and quantitative variables was performed. The qualitative variables were described by means of frequencies and percentages. Normality data test were performed to choose the appropriate statistical tests accordingly. The quantitative variables were described by n, mean, standard deviation, 95% confidence interval (CI), median, interquartile interval (25 th and 75 th percentiles) and minimum and maximum according to the distribution. The CI was calculated with the Clopper-Pearson method for binomial proportions and the Sison and Glaz (1995) method for multinomial proportions. The Pearson’s correlation coefficient was calculated to study the correlation between quantitative variables. 95% CI were built using a bootstrapping method (N = 1000 iterations).
Four composite variables on knowledge and acceptability were created based on the responses to the items in sections 2–5 of the questionnaire. The points were summated to create a total score and the results were described with the mean and 95% CI. Details on score assignment can be found in Additional file 1 : Material S2.
Degree of HPV knowledge total score ranged from 0 to 40, Degree of HPV vaccine acceptability ranged from 0 to 5, Degree of HPV vaccination knowledge ranged from 0 to 21 and Degree of knowledge of vaccines and their acceptability in general ranged from minus 10 to 10.
In order to identify the different profiles of parents who answered the survey, a latent class analysis (LCA) was conducted. The different response patterns were used to classify parents into groups (classes) according to answer similarities. LCA uses categorical data to create the groups, and the results provide the probability of a respondent belonging to each class. The questions included in the LCA were selected based on the previous assessment on their relevance in HPV vaccine acceptability: 2.4, 2.5, 3.1–3.5, 4.6, 4.7 and 5.1–5.4.
A total of 3110 participants were selected and contacted. 1071 did not answer and 555 refused participation, which translates into a response rate of 47.7%. After exclusion of unanswered and the invalid surveys, (n = 79) 1405 surveys were considered valid for the analysis (1116 online and 289 paper-based) (see reasons of invalid surveys in Additional file 1 : Figure S2).
Sociodemographic characteristics
Most of the recruitment sites were public (68.0%) and were located in a region considered as low VCR (55.9%) (Table 1 ). Distribution according to gender and age was similar in each stratification group.
Most respondents were mothers (86.2%), between 40 and 49 years (69.1%), with a university degree (35.6%), in full-time employment (61.8%), living in a place with more than 50,000 inhabitants (37.0%), of Spanish nationality (97.4%), married (81.7%), with 2 children (63.1%) and not vaccinated against HPV (76.6%). Only 7.6% of the parents stated that they had been vaccinated against HPV, and 15.8% of them were unsure of their vaccination status (Table 1 ). Of the children about whom the survey was completed, 736 (52.4%) were girls with a mean age of 11.5 (SD: 1.6) years. Eight hundred and ninety-five (63.7%) of the children had not been vaccinated against HPV and 391 (27.8%) had. For the rest, the vaccination status was reported as “unknown”.
HPV knowledge
The majority of the respondents (90.7%) had heard of HPV infection. The pediatrician (44.8%) was the most common source of information, followed by family and friends (40.4%) and the Internet (39.3%).
The participants had a medium-to-high degree of HPV knowledge, with a mean score of 28.9 out of 40 (95% CI 28.7–29.2). Additional data are provided in the Additional file 1 : Figure S3 and S4. In general, the parents agreed that HPV was a serious health problem (39.9% strongly agree, 53.1% agree) and that it was one of the most common sexually transmitted diseases (17.9% strongly agree, 52.2% agree).
The respondents correctly answered that HPV is a sexually transmitted disease (89.2%), although 10.8% were not sure how it is transmitted (Additional file 1 : Figure S5). Most of the parents considered than women (89.2%) or girls (69.1%) could get infected by HPV. In contrast, only 50.2% and 67.2% considered that boys and men could be infected, respectively (data not shown). Regarding possible diseases related to HPV infection, the majority of the respondents considered HPV infection to be related to cervical cancer (73.7%). However, in Fig. 1 it is shown that parents were less aware of the role of HPV in other diseases.
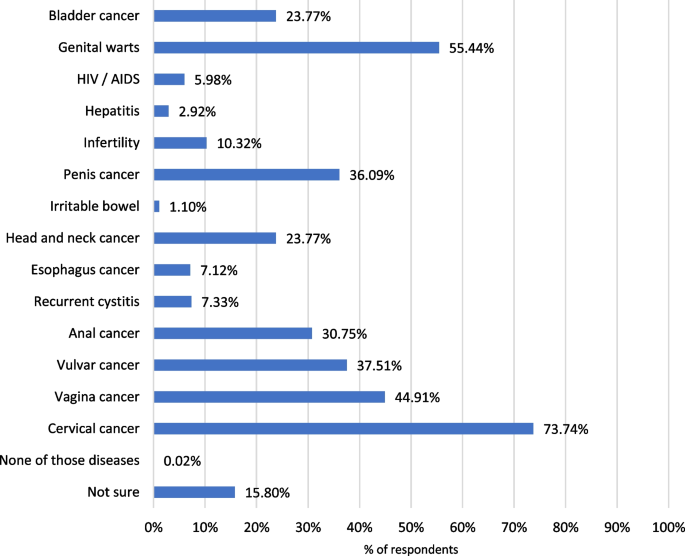
Diseases related to HPV
In terms of HPV prevention; most of the parents answered that it could be prevented by the HPV vaccine (87.2%) and condom use (80.9%). A lower proportion stated that delayed sexual debut (10.2%), personal hygiene (13.9%) and monogamy (14.2%) were also reported mechanisms for preventing HPV infections. Seven percent were not sure how to prevent this infection (data not shown). Additional data are provided in the Additional file 1 : Figure S5.
In order to obtain more information about HPV infection, most parents would rather consult healthcare professionals such as pediatricians (80.1%), family doctors (68.3%) and gynecologists (78.1%). Only 19.8% of the respondents would consult the Internet or the social media (Data not shown).
HPV vaccine acceptability
The respondents had a medium-to-high degree of HPV vaccine acceptability, translating into a mean score of 3.37 out of 5 (95% CI 3.30–3.44) (Additional file 1 : Figure S3).
In general, the participants presented a high level of agreement (strongly agree + agree) in considering that HPV vaccination is necessary in girls and boys. However, the results revealed that a higher proportion of parents considered it necessary in girls compared to boys (Fig. 2 and Additional file 1 : S8).
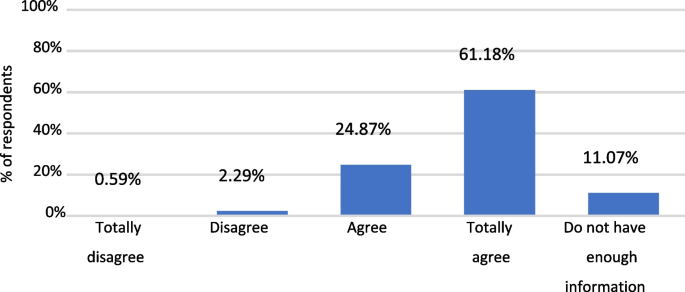
Answers to question: “I would vaccinate my son/daugther against HPV”
The main reasons for having the child vaccinated were to protect them against sexually transmitted diseases (67.4%) or against cancer and/or genital warts (77.4%), whereas the reasons for not having them vaccinated included lack of information (27.9%), fear of possible adverse events (20.9%) and other unspecified reasons (29.3%) (Additional file 1 : Figure S7).
With regard to the type of information needed for the HPV vaccination to be acceptable for parents who initially disagreed to vaccinate their daughter/son: 55.7% would request information about vaccine safety; 54.5% would need a doctor’s recommendation, 51.8% about HPV vaccine efficacy, 49% about the HPV vaccine in general and 46.1% about HPV infection (Data not shown).
The proportion of parents that would consult a pediatrician to obtain further information about the HPV vaccine represented 93.6% (Fig. 3 ).
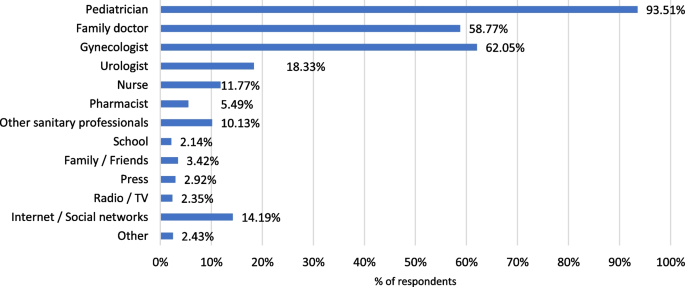
Main sources to be consulted for obtaining more information about HPV vaccine, according to participant’s opinion
Knowledge of HPV vaccination
The parents evinced an intermediate-to-high degree of knowledge of HPV vaccination, with a mean score of 15.5 out of 21 (95% CI 15.3–15.6) (Additional file 1 : Figure S3 and S4).
As it was observed with HPV infection, 92.1% of the respondents had heard of the HPV vaccine, the main source of information being the pediatrician (62.3%). Family and friends (34.5%), the gynecologist (27.8%) and the Internet (25.1%) were also mentioned as sources of information about the HPV vaccination.
Only 57.5% of the participants provided a correct answer indicating that the HPV vaccine was funded only for girls, whereas up to 25.1% of the parents did not know if the HPV vaccine was funded as part of the Spanish vaccination program (data not shown).
When the parents were questioned about the recommended age for vaccination, most of them answered correctly, with a mean (SD) reported age of 12.10 (1.21) years.
As occurred with HPV infection, 90.8% and 55.2% of the parents knew that girls and women, respectively, can be vaccinated against HPV. In contrast, the proportion of participants that considered that male populations could be eligible for the HPV vaccine was lower: only 60.1% and 37.9% of the parents considered that boys and men, respectively, could be vaccinated (Data not shown).
Up to 75.9% of the parents concurred (strongly agree + agree) in considering the HPV vaccine as effective, and 76.8% agreed that its benefits outweigh the risks. Nevertheless, it is important to point out that nearly 20% of the parents opined that they lacked sufficient information to answer (data not shown).
The HPV vaccine results tallied with the answers in the HPV knowledge section, HPV-related diseases. Thus, 80.0% of the participants considered that cervical cancer could be prevented with the vaccine, although the percentage was much lower for other diseases, such as genitals warts or anal cancer, that also affect males (Fig. 4 ). Additional data are shown in Additional file 1 : Figure S6.

Diseases that can be prevented by HPV vaccination, according to participant’s opinion
Knowledge and acceptability of vaccines in general
Knowledge and acceptability of vaccines was high, 6.6 out of 10 (95% CI 6.4–6.8). In fact, 25.8% of the participants obtained the maximum score (10 points).
More details are included in Additional file 1 : Figure S9.
Correlations
There were significant and positive correlations between all variables (vaccine knowledge, HPV vaccine knowledge and HPV vaccine acceptability), and parents who scored high in one variable tended to score high in the other variables (Fig. 5 ).
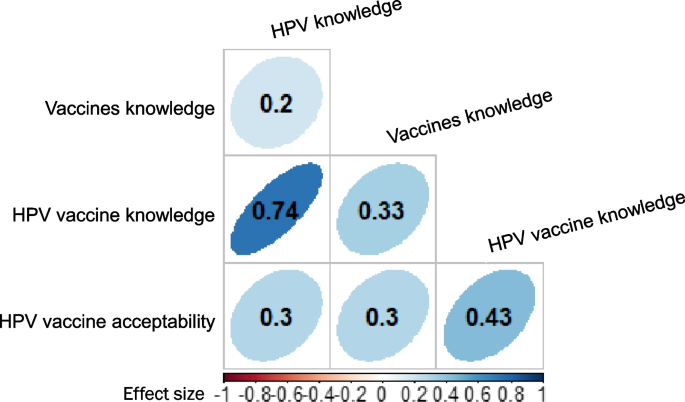
Correlations between knowledge and vaccine acceptability. p < 0.001 for all correlations. The colour intensity and shape indicate the strength of the correlation
The highest correlation was observed between HPV knowledge and HPV vaccine knowledge (0.7), followed by the correlation between HPV vaccination knowledge and HPV vaccine acceptability (0.4). Lower correlations were observed between other pairs of variables, although they were statistically significant (Fig. 5 ).
Typologies of respondents
The results of the LCA analysis allowed us to conclude that there were 4 groups of parents, according to different response patterns:
Class 1 (the probability of belonging to this group was 0.47): they consider that vaccines are useful, effective, beneficial and also that parents who do not have their children vaccinated put other people at risk. On the other hand, they think that both girls and boys should be vaccinated and that their doctor has recommended such vaccination. This group of parents showed a high agreement in considering HPV as a sexually transmitted disease and a serious health problem.
Class 2 (probability: 0.34): they consider that vaccines may be useful, effective, beneficial and also that parents who do not have their children vaccinated put other people at risk but that they may need more information. They think that it might be necessary to have both girls and boys vaccinated and that their doctor has recommended such vaccination. This group of parents agreed in considering HPV as a sexually transmitted disease and a serious health problem. Although these parents may have their sons vaccinated, they may need further information about HPV or its vaccine.
Class 3 (probability: 0.15): this group of parents are very unsure or lack sufficient information about HPV and its vaccine. While they are not afraid of having their child vaccinated, this lack of information could increase their indecision.
Class 4 (probability: 0.04): this group of parents have a higher probability of not accepting the vaccine as they believed that vaccines are not useful, are not safe and are ineffective. In addition, they did not regard HPV as a common sexually transmitted disease and a serious health problem. Furthermore, they tended to think that there is no need to have boys and girls vaccinated. In this group of parents, a high proportion stated that their doctor did not recommend the HPV vaccine.
According to our results, thirteen years after the beginning of the HPV vaccination program in Spain, the degree of knowledge of HPV among parents of adolescents is still modest, although HPV vaccine acceptability is medium–high. There was still a clear tendency to relate HPV to girls and females [ 6 ].
Parents perceived that females were more likely to become infected than males and tended to associate HPV infection mainly with cervical cancer. In addition, there was a lack of information about other HPV-related diseases, and even more about those affecting males. These results contrast with the real burden of HPV-related diseases, which is substantial in both genders, causing not only cervical cancer but also anal, penile, vaginal, vulval and oropharyngeal cancers, in addition to genital warts and recurrent respiratory papillomatosis (RRP) [ 6 ]. In this context, it is important to highlight that the current objective of HPV vaccination programs in high-income countries is the elimination HPV-related diseases, not only cervical cancer [ 15 ]. To this aim establishing gender-neutral vaccination program would therefore seem to be crucial.
Our findings are similar to those of other HPV vaccine awareness studies performed in other European countries, such as the outcomes of our own systematic review published in 2020 [ 11 ]. This review found that HPV knowledge and acceptability of the HPV vaccine continued to be modest and varied widely across EU countries, with insufficient information and safety concerns being the main barriers to vaccination acceptability [ 11 ]. A more recent review [ 16 ] also identified a modest degree of HPV and HPV vaccine knowledge among the male population. In line with the results of previous studies [ 11 , 16 , 17 , 18 ], the KAPPAS study emphasizes the need to implement actions to provide the relevant population with information, including the possible impact of HPV in males, and therefore empower them to make informed decisions. The significant and positive correlations between HPV knowledge and HPV vaccine knowledge as well as between HPV vaccination knowledge and HPV vaccine acceptability found in our study also underscore the importance of awareness-raising campaigns.
In our study, the LCA revealed that approximately more than one third of the parents may still have insufficient information about HPV and its vaccine and evince a certain indecision in vaccinating their children, particularly boys. This result reinforces the need for educational activities targeting parents that are still hesitant to HPV vaccination to ensure the success of HPV immunization programs. On the other hand, in our study, the percentage of parents who present a higher probability of not accepting the HPV vaccine or vaccines in general is very small (3.80%). This correlated with the high VCR in our country of HPV and the rest of the vaccines, one of the highest in Europe. Our LCA analysis may also help healthcare professionals to identify different types of parents and provide them with balanced information targeting their needs in order to enable them to take informed decisions about their children’s HPV vaccination.
Similar results were published in 2015 in an pan-European study that examined A study published in 2015 examining parental views of HPV vaccination of sons in France, Germany, Italy and the UK [ 19 ] found that approximately three quarters of the parents in the UK, Germany and Italy were in favor of the HPV vaccination for their sons. The favorable parents sought to protect their sons from the disease and regarded gender equality as important. Parents in doubt about male HPV vaccination needed more information about HPV diseases and HPV vaccination among males. The rejecting parents were generally skeptical of vaccines and feared the side effects of vaccination. Although all these countries now include boys in their HPV immunization programs, this study was conducted before this inclusion and examined countries with significant differences in vaccination coverage [ 20 , 21 ]. Parents in countries with high HPV vaccination coverage rates (UK and Italy) tended to recognize the importance of national vaccination programs. Parents in countries with limited HPV vaccination coverage rate (Germany and France) felt a greater need for information from healthcare professionals (HCP) and the public health authorities. The authors concluded that by providing brief information about HPV in both genders, parental acceptance of HPV vaccination for sons could be as high as for girls [ 19 ]. This was confirmed by the recent WHO/UNICEF estimations, published in 2020, Bruni et al. [ 5 ] which have shown that the VCR in boys and girls is similar in countries with gender-neutral HPV vaccination programs.
Our study is the first of its kind conducted in Spain at a national level that sheds some light on the knowledge and acceptability of HPV and its vaccine.
Some limitations derived from the nature of this study should be borne in mind. Firstly, the potential sources of bias in this study comprised a selection bias due to the parents’ acceptance to participate in the survey and the inclusion of the population entered in medical records. In addition, parent-reported information is subjective and may be affected by social desirability, imprecision or mistakes in interpreting the questions.
More than 12 years after the implementation of the HPV vaccination program in our country, parental HPV knowledge and vaccine acceptability is medium-to-high. However, HPV is still associated with the female gender, with important lack of knowledge of HPV consequences in males. Moreover, our results also point out that more than one third of parents still need more information to vaccinate their children against HPV. Providing parents with adequate and well-balanced information is crucial to ensure the success of HPV vaccination programs.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due participants privacy protection but are available from the corresponding author on reasonable request.
Abbreviations
Spanish Association of Pediatricians
Confidence interval
Coronavirus disease
European Union
Healthcare professionals
Human papillomavirus
Investigational ethical committee
Latent class analysis
Recurrent respiratory papillomatosis
United Kingdom
United Nations International Children's Emergency Fund
Vaccine coverage rate
World Health Organization
Bosch FX, Broker TR, Forman D, et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31(Suppl 7):H1–31.
Article PubMed PubMed Central Google Scholar
Schiffman M, Doorbar J, Wentzensen N, et al. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers. 2016;2:16086.
Article PubMed Google Scholar
Weaver BA. Epidemiology and natural history of genital human papillomavirus infection. J Am Osteopath Assoc. 2006;106:S2–8.
PubMed Google Scholar
Hartwig S, St Guily JL, Dominiak-Felden G, et al. Estimation of the overall burden of cancers, precancerous lesions, and genital warts attributable to 9-valent HPV vaccine types in women and men in Europe. Infect Agent Cancer. 2017;12:19.
Bruni L, Saura-Lazaro A, Montoliu A, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med. 2021;144:106399.
European Cancer Organization. Viral Protection: Achieving the Possible. A Four Step Plan for Eliminating HPV Cancers in Europe. https://www.europeancancer.org/resources/159:viral-protection-achievingthe-possible-a-four-step-plan-for-eliminating-hpv-cancers-in-europe.html . Accessed 25 March 2021.
Ministerio de Sanidad Servicios Sociales e Igualdad. Spanish Vaccination Calendar 2021. https://www.sanidad.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/calendario-y-coberturas/home.htm . Accessed 22 March 2021.
Ministerio de Sanidad. Servicios Sociales e Igualdad - Profesionales - Vacunas Coberturas de Vacunación. 2022. https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/calendario-y-coberturas/coberturas/docs/Tabla11.pdf . Accessed 03 March 2022.
García FJA, Ortega MJC, Aldeán JÁ, Garcés-Sánchez M, Llanos EG, et al. en representación del Comité Asesor de Vacunas de la Asociación Española de Pediatría (CAV-AEP). Immunisation schedule of the Pediatric Spanish Association: 2022 recommendations. Anales de Pediatría (English Edition). 2022;96(1):59.e1–59.e10.
Lopez N, Garces-Sanchez M, Panizo MB, et al. HPV knowledge and vaccine acceptance among European adolescents and their parents: a systematic literature review. Public Health Rev. 2020;41:10.
Navarro-Illana P, Caballero P, Tuells J, et al. Acceptability of human papillomavirus vaccine in mothers from Valencia (Spain). An Pediatr (Barc). 2015;83:318–27.
Article CAS Google Scholar
Caballero-Perez P, Tuells J, Rementeria J, et al. Acceptability of the HPV vaccine among Spanish university students in the pre-vaccine era: a cross-sectional study. Rev Esp Quimioter. 2015;28:21–8.
Navarro-Illana P, Navarro-Illana E, Vila-Candel R, Diez-Domingo J. Drivers for human papillomavirus vaccination in Valencia (Spain). Gac Sanit. 2018;32:454–8.
López N, Salamanca de la Cueva I, Vergés E, Suárez Vicent E, Sánchez A, López AB, Panizo-Santos MB, Garcés-Sánchez M, Montesdeoca A, Rivera AJ, Cotarelo MS. Factors influencing HPV knowledge and vaccine acceptability in parents of adolescent children: results from a survey-based study (KAPPAS study). Hum Vaccines Immunother. 2022;18(1):2024065.
Article Google Scholar
European. Cancer Organisation. Eliminating HPV-Caused Cancers and Diseases in Europe. 2019. Report available in: https://www.europeancancer.org/resources/51:eliminating-hpv-caused-cancers-and-diseases-in-europe-case-for-action.html . Accessed 03 March 2022.
Radisic G, Chapman J, Flight I, Wilson C. Factors associated with parents’ attitudes to the HPV vaccination of their adolescent sons: a systematic review. Prev Med. 2017;95:26–37.
Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics. 2016;138:e20153863.
Rodriguez AM, Do TQN, Goodman M, et al. Human papillomavirus vaccine interventions in the U.S.: a systematic review and meta-analysis. Am J Prev Med. 2019;56:591–602.
Lee Mortensen GAM, Idtaleb L. Parental attitudes towards male human papillomavirus vaccination: a pan-European cross-sectional survey Infectious Disease epidemiology. BMC Public Health. 2015;15:1–10. https://doi.org/10.1186/s12889-015-1863-6 .
Ministerio della salute. Piano nazionale prevenzione vaccinale. http://www.salute.gov.it/portale/vaccinazioni/dettaglioContenutiVaccinazioni.jsp?lingua=italiano&id=4828&area=vaccinazioni&menu=vuoto . Accessed 1 Sept 2021.
Public Health England. NHS Public Health functions agreement 2019–2020. HPV programme. 2019. https://www.england.nhs.uk/wp-content/uploads/2017/04/Service-Specification-No.11-Human-Papillomavirus-Virus-v2-080819.pdf . Accessed 7 Sept 2021.
Download references
Acknowledgements
Investigators of the KAPPAS study: Magdalena Aga (CS Repélega); Isabel Cañabate (CS Churriana); Cynthia Crespo (CAP Montclar); Mara Garcés (CS Nazaret); Ana Belén López (IHP Córdoba); Gema Belén López (IHP Córdoba); María Martín (CS La Cala de Mijas); Abián Montesdeoca (CS Guanarteme); Ana María Nocea (CS Condes de Barcelona); Mª Belén Panizo (CS Illescas); Lizeth Peña (CS Pla Vinalopó); Victoria Planelles (CS Paiporta); Almudena Sánchez (CAP Les Hortes); Eva Suarez (CS Burriana II); Isabel Úbeda (CS L'Eliana); Edelmiro Verges (CS Binissalem, CS Alaro). Editorial assistance and medical writing support were provided by Esther Tapia, PhD and Adelphi Targis, SL.
This study has been funded and sponsored by MSD Spain.
Author information
Authors and affiliations.
Medical Affairs Department, MSD Spain, C/Josefa Valcarcel, 38, 28027, Madrid, Spain
Noelia López, Bruno Herrera & Manuel Cotarelo
Instituto Hispalense de Pediatría, Seville, Spain
Ignacio Salamanca de la Cueva & Auxiliadora Fernández de Alba
Centro de Salud de Villalegre - La Luz, Avilés, Asturias, Spain
Elena Taborga
Healthcare Centre Galdakao, Vizcaya, Spain
Inés Cabeza
Healthcare Centre Gama, Cantabria, Spain
Reyes Mazas Raba
Institut Pediàtric MARES-RIERA, Girona, Spain
Josep Marès
Healthcare Centre Pla, Elche, Alicante, Spain
Patricia Company
You can also search for this author in PubMed Google Scholar
Contributions
NL was responsible for original idea and design of the study, reviewed the results, and actively prepared and reviewed the manuscript. MC and BH reviewed results and manuscript. ISdlC participated in the design of the study and recruitment, reviewed results and reviewed the manuscript. The other authors participated in the recruitment, reviewed results and manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Noelia López .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Comité de Ética de la investigación con medicamentos de Euskadi (CEIm-E) reference ethics committee on 08th January, 2019.
Acceptance to participate was obtained from all subjects involved in the study.
Consent for publication
Not applicable.
Competing interests
NL, BH, and MC are full-time employee of MSD Spain. ISdlC has received grants and/or honoraria as a consultant/advisor or attending conferences and practical courses from GlaxoSmithKline, Sanofi Pasteur, MSD and Pfizer. ET has received honoraria from MSD as an investigator for this study. AFdA has received honoraria from MSD as an investigator for this study. IC has received honoraria from MSD as an investigator for this study and grants for medical education activities. She has also participated as speaker for other pharmaceutical companies. RMR has received honoraria from MSD as investigator for this study. JM has received grants from MSD as an investigator for this study and payments for lectures including service on speaker bureau and as a board membership from GlaxoSmithKline, Sanofi Pasteur, MSD and Pfizer. PC has received honoraria from MSD as investigator for this study.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: material s1..
Questionnaire used in the KAPPAS study. Material S2 . Scores Keys assigned to each item to compute global scores. Material S3 . Additional information of methodology and results. Figure S1 . Recruitment and data collection process. Figure S2 . Flowchart of participants. Figure S3 . Total scores and distribution of respondents with regard to A) HPV knowledge, B) HPV vaccine knowledge, C) HPV vaccine acceptability, D) knowledge and acceptability of vaccines in general. Figure S4 . Box representation of total scores. Figure S5 . Wrong/Right answers for HPV knowledge. Figure S6 . Wrong/Right answers for HPV vaccine knowledge. Figure S7 . Reasons to vaccinated and to not vaccinate the child. Figure S8 : Responses to questions related to HPV vaccine acceptability. Figure S9 : Responses to questions related to Knowledge and acceptability of vaccines in general.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
López, N., de la Cueva, I.S., Taborga, E. et al. HPV knowledge and vaccine acceptability: a survey-based study among parents of adolescents (KAPPAS study). Infect Agents Cancer 17 , 55 (2022). https://doi.org/10.1186/s13027-022-00467-7
Download citation
Received : 07 October 2021
Accepted : 31 October 2022
Published : 17 November 2022
DOI : https://doi.org/10.1186/s13027-022-00467-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- HPV vaccine
- Vaccination
- Acceptability
- HPV-related diseases
Infectious Agents and Cancer
ISSN: 1750-9378
- General enquiries: [email protected]
What questions do people ask on a human papillomavirus website? A comparative analysis of public and private questions
Affiliation.
- 1 Department of Obstetrics and Gynecology, St. Vincent's Hospital, The Catholic University of Korea, Suwon, Korea.
- PMID: 22253561
- PMCID: PMC3258556
- DOI: 10.7150/ijms.3420
Objective: In 2004, we launched the question and answer (Q&A) section on a human papillomavirus (HPV) website (www.hpvkorea.org) that provides ample and regularly updated information about HPV. The purpose of this study is to collect data pertaining to questions posed on this website about HPV and its related diseases and analyze the type of questions and frequency before and after introduction of HPV vaccine in Korea. Using these results, we intend to determine the clinical and practical implications for doctors treating HPV and for HPV website providers.
Method: Data were collected from March 2004 to July 2011. This study analyzed all the questions that were asked on the website during this period. The questions were categorized into 2 groups, according to whether they were asked publicly or privately. The 10 categories for classification were determined on the basis of the contents of the questions by 4 researchers with medical degrees (Ph.D.) related to HPV research. The frequency of the questions was separately determined for the public and private question formats. Also, we compared the type of questions and frequency before and after introduction of HPV vaccine in Korea and evaluated the changes in the 2 groups over the 2 periods studied.
Results: Of the 3,062 subjects who visited the HPV website, 2,330 subjects asked public questions and 732 asked private questions. The most frequent question was "I have been infected with HPV, and I want to know about the treatment options for HPV infection and cervical dysplasia" (n = 1156, 37.8%), and the second most common question was "What are the transmission routes of HPV?" (n = 684, 22.3%). The third most common question was "How long does it take for HPV infection to spontaneously remit?" (n = 481, 15.7%).Of the 2,330 public questions, the most common question types pertained to the treatment of HPV and cervical dysplasia, HPV transmission, HPV remission, and risk of cervical cancer (in that order). Of the 732 private questions, the most frequent question types pertained to the HPV transmission, treatment of HPV and cervical dysplasia, genital warts, and HPV & pregnancy (in that order). The type and frequency of public and private questions showed statistical differences between the 2 groups (p < 0.001).
Conclusion: Our results show that when people consult an internet site about HPV, they actually want to seek about "treatment of HPV and cervical dysplasia", "HPV transmission", "HPV remission", "genital warts", and "risk of cervical cancer" (in this order). Also, our results showed that "genital warts" and "HPV & pregnancy" may have been considered embarrassing topics. Thus, these findings can be used to make informed recommendations for future clinical or internet-based communications with patients and the general public.
Keywords: cervix; dysplasia; genital warts; human papillomavirus.
Publication types
- Comparative Study
- Evaluation Study
- Alphapapillomavirus / physiology*
- Data Collection / methods
- Papillomavirus Infections / complications
- Papillomavirus Infections / diagnosis
- Papillomavirus Infections / prevention & control
- Papillomavirus Infections / transmission
- Patient Education as Topic / methods*
- Public Sector*
- Surveys and Questionnaires*
- Uterine Cervical Dysplasia / diagnosis
- Uterine Cervical Dysplasia / etiology
- Uterine Cervical Dysplasia / prevention & control
- Uterine Cervical Dysplasia / virology
- Uterine Cervical Neoplasms / diagnosis
- Uterine Cervical Neoplasms / etiology
- Uterine Cervical Neoplasms / virology
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Knowledge of human papillomavirus vaccination: A multi-institution, cross-sectional study of allopathic and osteopathic medical students
Roles Conceptualization, Formal analysis, Funding acquisition, Investigation, Project administration, Supervision, Writing – review & editing
* E-mail: [email protected]
Affiliation Department of Psychiatry and Behavioral Neuroscience, The University of Chicago Medicine, Chicago, Illinois, United States of America
Roles Visualization, Writing – original draft, Writing – review & editing
Affiliation Pritzker School of Medicine, The University of Chicago Medicine, Chicago, Illinois, United States of America
Roles Conceptualization, Investigation, Methodology, Project administration, Writing – review & editing
Affiliation Department of Psychology, College of Health Professions, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois, United States of America
Roles Conceptualization, Methodology, Writing – review & editing
Affiliation Department of Medicine, Section of Infectious Diseases and Global Health, The University of Chicago Medicine, Chicago, Illinois, United States of America
Roles Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – review & editing
Affiliation Department of Pharmaceutical Sciences, College of Pharmacy, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois, United States of America
- Samuel R. Bunting,
- Samantha Morris,
- Julia Chael,
- Brian A. Feinstein,
- Aniruddha Hazra,
- Sarah S. Garber

- Published: January 11, 2023
- https://doi.org/10.1371/journal.pone.0280287
- Reader Comments
Human papillomavirus (HPV) vaccination is a well-established and successful tool for preventing HPV-related cancers. However, vaccine uptake remains low, influenced by patient hesitancy around safety concerns and little opportunity to discuss the vaccine with trusted healthcare providers. We conducted a national, cross-sectional study of allopathic and osteopathic medical students regarding knowledge of HPV vaccination guidelines March-April 2021. Analysis sought to identify gaps in knowledge as well as demographic and academic correlates of knowledge. A total of 718 students participated (response rate = 50.8%). While 92.8% of participants identified the connection between HPV and cervical cancer, lower percentages associated HPV with vaginal/vulvar (67.7%), anal (63.3%), and penile (53.9%) cancers. Low percentages of participants correctly identified age of HPV vaccine eligibility (33.3%) and how many doses are needed for full protection (48.1%). This study identifies specific knowledge gaps in medical students’ training on HPV-related cancers and HPV vaccination guidelines. Through addressing these gaps, we may improve HPV vaccine uptake and decrease the incidence of HPV-related cancers.
Citation: Bunting SR, Morris S, Chael J, Feinstein BA, Hazra A, Garber SS (2023) Knowledge of human papillomavirus vaccination: A multi-institution, cross-sectional study of allopathic and osteopathic medical students. PLoS ONE 18(1): e0280287. https://doi.org/10.1371/journal.pone.0280287
Editor: Malik Sallam, The University of Jordan, JORDAN
Received: August 14, 2022; Accepted: December 23, 2022; Published: January 11, 2023
Copyright: © 2023 Bunting et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the paper and its Supporting Information files.
Funding: This work was supported with unrestricted research funding from Gilead Sciences under award number IN-US-412-9042 (PI: Garber, Co-I: Bunting). Brian Feinstein’s time was supported by a grant from the National Institute on Drug Abuse (K08DA045575). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. The funders had no input on study design, implementation, data analysis, interpretation of results, drafting of the report, or the decision of where to submit the report for publication. There was no additional external funding received for this study.
Competing interests: The authors declare that they have no relevant conflicts of interest to disclose.
Introduction
Each year in the United States (U.S.), an estimated 35,900 people are diagnosed with a Human Papillomavirus (HPV)-related cancer [ 1 ]. An estimated 90% of these cases could be prevented by HPV vaccination [ 1 ]. However, a 2020 U.S. nationally representative survey found that only 75% of adolescents aged 13–17 received at least 1 dose of the HPV vaccine, only 59% were fully vaccinated, and vaccination rates were lower among male compared to female adolescents [ 2 ]. Furthermore, from 2015 to 2018, there was a 79% increase in the percentage of parents who cited safety concerns as a primary reason to refuse initiation of the HPV vaccine for their adolescents [ 3 ]. In the general population, HPV knowledge is relatively limited, particularly surrounding its association with cancer as upwards of 77% of people were unaware of the links between HPV and anal, penile, and oral cancers [ 4 ].
Improving HPV vaccination rates is a U.S. public health priority. Previous studies have found that physicians may hesitate to mention HPV vaccinations out of fear that patients will refuse the vaccine as well as inadequate time during visits to sufficiently explain the benefits of vaccination [ 5 ]. However, evidence suggests that when physicians do take the time to discuss the merits of the HPV vaccine, they may positively encourage their patients to receive the vaccine [ 6 , 7 ]. Among 18–26 year olds, trust in the clinician and clinician recommendation were major factors influencing vaccination decisions [ 6 ]. In a study of college students, clinician recommendation to receive the HPV vaccine resulted in participants being nearly five times more likely to be vaccinated [ 7 ].
Patient eligibility for the HPV vaccine has expanded since U.S. Food and Drug Administration (FDA) approval in 2006. While the vaccine was initially aimed at adolescent and pre-teen girls, recommendation has expanded to now include both male and female patients, starting as young as age 9 and extending through age 45. Current guidelines recommend patients aged 11–12 receive 2 doses and patients aged 15–26 receive 3 vaccine doses [ 1 ]. Patients aged 27–45 are advised to engage in shared decision making with their providers to determine whether vaccination against HPV is appropriate based on individual risk factors and values.
While the link between HPV and cervical cancer is widely-known, HPV is also responsible for oropharyngeal, anal, penile, vulvar, and vaginal cancers, which account for over two-thirds of annual HPV-related cancers [ 1 ]. Previous studies of medical students have found that many, 59.7% of students, were not aware of the connection between HPV and these other cancers [ 8 , 9 ]. Given the power of clinician recommendation and knowledgeable explanation of the risks and benefits of HPV vaccination, medical education about HPV vaccination is imperative to ensure vaccination rates meet the public health need.
Previous studies have found knowledge of HPV and HPV vaccination increased as medical students progressed through training [ 8 , 10 ]. Medical students who had personally received the HPV vaccine also reported significantly greater willingness to vaccinate adolescents before ages 15–16, as well as greater willingness to discuss vaccination during visits [ 11 ]. However, much of this research was conducted prior to expansion of indications for HPV-vaccination beyond adolescent and pre-teen girls. Continued investigation is needed to assess current medical students’ knowledge of HPV vaccination with the ultimate goals of continuing to increase HPV vaccination rates and decrease the incidence of HPV-related cancers.
Furthermore, most of the previous research on medical students’ HPV vaccine knowledge was conducted with allopathic medical students. However, osteopathic and allopathic medical students differ in their medical school curricula and clinical experiences. Both types of students learn scientific, evidence-based practices and treatments at four-year medical training programs that are examined by the same state licensing boards (but using different exams). That said, osteopathic medical practice centers holistic medicine and musculoskeletal manipulative medicine techniques, while allopathic medicine focuses primarily on symptom alleviation and disease treatment through pharmaceutical interventions [ 12 ]. Osteopathic medical education places a specific emphasis on preventive medicine and health promotion, such as vaccinations.
The goals of the current study were to address the following, specific research questions: 1) What are the specific knowledge gaps among medical students regarding U.S. Center for Disease Control (CDC) HPV vaccinations guidelines?; 2) What are the demographic and academic correlates of knowledge of HPV vaccination guidelines?; and 3) Are there specific knowledge deficits that remain among medical students in their final year of training?
Study population and procedure
The study participants were recruited from a cross-sectional sample of students from 16 U.S. allopathic (10) and osteopathic (6) medical schools, which represented a convenience sample of institutions selected for regional diversity. School administrators disseminated study information to potential participants between March–April 2021. Study information sent to students deliberately avoided any mention of HPV to reduce the risk of selection bias. Inclusion criteria for this study were: 1) 18 years of age or older, and 2) currently enrolled in a U.S. allopathic or osteopathic medical school. Interested students who met both inclusion criteria were sent a follow-up email with instructions to access the study. The survey was completed online using QualtricsXM (Provo, UT). Upon completion of the study, participants were sent a debrief message and a $10.00 gift card to an online retailer as compensation.
Instrument development
The study instrument was developed specifically for use in this study. We developed items related to knowledge of HPV informed by the guidelines and recommendations for HPV immunization as developed by the U.S. Preventive Services Task Force (USPSTF) [ 1 , 13 ]. Items were reviewed by an infectious disease physician with content expertise for accuracy. A focus group of 10 medical students from a single, Midwestern allopathic medical education program completed the instrument and provided feedback on item wording and study administration. We incorporated this feedback prior to distribution of the instrument to the larger sample.
The first section of the study presented students with a list of cancer diagnoses (e.g., cervical, colon, vaginal/vulvar) and asked participants to indicate which cancers could be caused by HPV. The next section presented a series of multiple-choice questions and participants were instructed to select the single best answer for each. Multiple choice questions were developed based on review of the CDC and USPSTF guidelines for HPV-vaccination [ 1 , 13 ]. Multiple choice questions are listed in Table 2 and the complete study instrument is attached as a S1 File .
Statistical analysis
We calculated descriptive statistics to describe the sample as well as all items included on the knowledge assessment. Next, we calculated the percentage of respondents who correctly answered each item. The percentage who answered correctly was compared between the group of participants in the pre-clinical phase (years 1/2) and clinical-phase (years 3/4) of medical training utilizing Pearson’s chi-squared tests (χ 2 ). The total number of correct items was summed and divided by the total number of items to create a percentage of correct knowledge for each participant. The knowledge percentage was compared between demographic and training variables utilizing a one-way analysis of variance (ANOVA) for independent variables with three or more levels and independent samples t -tests for type of training, which only had two levels. Multivariable analyses were completed using analysis of covariance (ANCOVA) adjusting for all demographic and training variables. Data management and analysis was conducted using IBM SPSS v27 (IBM Corp., Armonk, NY). The pre-determined level of significance was a p -value < 0.05. This study was reviewed and approved by the Institutional Review Board of Rosalind Franklin University (protocol: COP-20-256) on November 2, 2020 and informed consent was obtained from all participants before beginning the study via the online study instrument.
A total of 1,592 students indicated interest in participating in this study. Of these interested students, 808 completed the study (response rate = 50.8%). We removed 90 due to incomplete responses. This left a final analytic sample of 718.
Demographics
The largest proportion of the participants in this study were in their first year of training ( n = 204, 28.4%) and approximately half were in allopathic medical education programs ( n = 382, 53.2%). Over half of participants were White ( n = 416, 57.9%), most were heterosexual ( n = 604, 84.1%), and approximately two-thirds were cisgender women ( n = 450, 62.7%). Regionally, the greatest number of participants were training in the Midwestern U.S. ( n = 361, 50.3%). Full demographic information is provided in Table 1 . The mean age of participants was 26.3 years ( SD = 3.25). Comparison of demographics between allopathic and osteopathic student groups is presented in S1 Table . We also compared demographics of the sample responding to the survey to the demographics of medical students nationally to ensure representation without identifying major discrepancies ( S2 Table ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0280287.t001
Specific cancer diagnoses
Participants were asked to indicate which cancers HPV could cause from a provided list of 12 different cancers- the correct answers were cervical, vaginal/vulvar, anal, and penile cancers ( Fig 1 ). We found that 92.8% of participants correctly indicated that HPV could be a causative etiology for cervical cancer ( n = 666). However, we found that lower percentages of participants correctly indicated HPV could be causative for vaginal/vulvar cancer (67.7%, n = 486), anal cancer (63.4%, n = 455), and penile cancer (53.9%, n = 387). We found that 10.7% ( n = 77) of participants indicated HPV was a causative etiology for testicular cancer and 10.0% ( n = 72) indicated this for colorectal cancer, both of which were incorrect.
Percentage of respondents indicating an association between HPV and each cancer type. Those indicated with an asterisk have a well-documented relationship with HPV. Labels for each bar indicate the number of participants indicating a relationship between HPV and the listed malignancy.
https://doi.org/10.1371/journal.pone.0280287.g001
Multiple choice items
A greater percentage of students in the clinical-phase of training correctly answered six of the eight multiple choice items ( Table 2 ). Specifically, greater percentages of clinical-phase students correctly identified the age at which patients are no longer indicated to receive the HPV vaccine (>45 years; 65.2% vs. 55.6%, χ 2 [1, N = 718] = 7.05, p = .008), that three HPV vaccine doses were needed to confer protection for a 16 year old patient (88.3% vs. 67.9%, χ 2 [1, N = 718] = 54.6, p < .001), and that a patient should also be considered for the Hepatitis A vaccine (84.9% vs. 78.6%, χ 2 [1, N = 718] = 4.54, p = .03) in addition to the HPV vaccine.
https://doi.org/10.1371/journal.pone.0280287.t002
In addition, a greater percentage of clinical-phase students correctly responded that women who have received the HPV vaccine still require pap-smears (98.8% vs. 96.4%, χ 2 [1, N = 718] = 4.62, p = .03), that the HPV vaccine does not protect against all strains of HPV (98.5% vs. 91.3%, χ 2 [1, N = 718] = 17.5, p < .001), and that HPV serology is not required prior to receiving the HPV vaccine (88.3% vs. 64.4%, χ 2 [1, N = 718] = 41.3, p < .001).
Knowledge comparisons
In the univariate ANOVA analyses ( Table 3 ) we found that knowledge of HPV differed based on a participant’s year in training ( F [3,714] = 32.6, p < .001). Students in the first year of training reported the lowest knowledge percentage compared to all other years (all p < .001; M = 74.3% [72.6–76.0]) as compared to those in the second (79.4% [77.6–81.3]), third (84.2% [82.6–85.7]) and fourth years ( M = 84.6% [82.9–86.2]) of training). We also found that knowledge of the HPV vaccine differed based on the participants’ gender identity ( F [2,717] = 3.61, p = .03). Cisgender women ( M = 81.8% [80.0–82.2]) reported higher knowledge of the HPV vaccine compared to cisgender men ( M = 78.7% [77.1–80.2], p = .03). In the bivariate comparison of knowledge between allopathic ( M = 81.3% [80.1–82.5]) and osteopathic ( M = 79.0% [77.6–80.4]) medical students, we found that allopathic students reported a higher average knowledge score ( t [716] = 2.53, p = .01).
https://doi.org/10.1371/journal.pone.0280287.t003
In the adjusted analyses, the effect of participants’ year in training was maintained ( F [3,713] = 32.4, p < .001). First-year students reported lower knowledge compared to all other years (all p < .001). The effect of participant gender identity was also maintained ( F [2,713] = 5.57, p < .001) with cisgender women reporting higher knowledge compared to cisgender men. No additional effects of demographic or training characteristics were identified on HPV knowledge ( Table 3 ).
HPV-associated cancers continue to cause mortality and morbidity in the U.S. despite wide availability of effective prevention with the HPV vaccine. Clinicians play a key role in ensuring that HPV vaccination reaches all eligible patients [ 14 ]. Medical education about the HPV vaccine is crucial so medical students can identify vaccine candidates, educate their patients about the risks and benefits, and respond to patient questions. To the best of our knowledge, this is the first multi-regional and multi-institutional study of both allopathic and osteopathic medical students’ knowledge of HPV vaccination guidelines. The current findings may be used to improve medical education about HPV-associated cancers and HPV vaccination.
Most participants (92.8%) correctly identified the connection between HPV and cervical cancer. However, smaller percentages correctly identified the link between HPV and vaginal/vulvar, anal, and penile cancers, echoing previous work [ 8 , 9 ]. Importantly, this previous study grouped penile and vaginal/vulvar cancer as genital cancers whereas we separated these two diagnoses. In our study, two-thirds of students linked HPV to vaginal/vulvar cancers, whereas just over half linked HPV to penile cancers. This suggests difficulty identifying HPV-related cancers in the male genital tract that both reflects the predominant societal narrative that so closely links HPV to women’s health and suggests need for concentrated educational efforts that focus specifically on HPV and anal/penile cancers [ 15 , 16 ].
The burden of HPV infection and HPV-related disease among men who have sex with men (MSM) remains disproportionate, and recent work has found that HPV vaccination was more common among MSM who were using HIV PrEP compared to those who were not [ 17 ]. While this is encouraging, it suggests there is a significant need to broadly improve HPV vaccination among all patients who are at risk, not just those who are already engage in preventive sexual healthcare. Research conducted among heterosexual patients found only 11.5% had received one dose of the HPV vaccination, underscoring the importance of education to broadly improve uptake of the HPV vaccine [ 18 ].
Broadly, knowledge of HPV vaccination guidelines improved with increasing years of training as second, third, and fourth-year medical students performed better than first-year students. This trend in increasing knowledge is intuitive given the progressive concentration on clinical medicine as training progresses, and these results are consistent with those from prior studies [ 8 , 10 ]. We found that the only questions without difference between year of training were those answered incorrectly by most students in either group. Specifically, we found that fewer than half (33.0%) of the students in this study, even those in the clinical years of training (36.6%), correctly identified patients could begin receiving the HPV vaccine at age 9. Additionally, only 48.1% of participants, including 48.0% of those in clinical training, were correct in identifying three HPV vaccine doses conferred full protection for a 16-year-old patient. The persistence of incorrect knowledge in this later phase of training suggests the need for additional training about eligibility for the HPV vaccine.
These knowledge gaps thus present an opportunity for educational intervention [ 19 – 21 ]. There is a demonstrable benefit that increased training among first- and second-year medical students can improve comfort and knowledge in counseling on HPV vaccination [ 22 , 23 ]. A follow-up study to the workshop curricula that was implemented by Evans et al. demonstrated that a year and a half later, these students retained their knowledge and continued to better advocate for the vaccine than their peers who did not participate in the workshop, and these students were also able to integrate their knowledge into increasing HPV vaccination rates in student-run free clinics [ 22 , 24 ].
We found several interesting demographic trends. Greater HPV knowledge was demonstrated among cisgender women as compared to cisgender men, without difference based on sexual orientation, region, or type of training. Women remain more likely to have received the HPV vaccine, and this first-hand experience is related to their greater current knowledge and future discussions of the vaccine with patients [ 11 ]. To standardize provider knowledge and to ensure that all students possess a similar foundation of knowledge related to HPV vaccination, school-wide initiatives are necessary. Even a single lecture by an expert can improve attitudes with HPV vaccination, especially in students who themselves did not have it [ 24 – 26 ]. Other curricula have had similar success, including a role-playing workshop conducted with 28 medical students and residents that showed improved participant knowledge, comfort, and confidence discussing the HPV vaccine [ 21 , 27 , 28 ].
Limitations
The results of the present study should be interpreted in the context of several limitations which invite future study. First, there are slight differences in terms of the demographic composition of the study sample and those of allopathic and osteopathic medical students nationally. Relatedly, our sample is also over-representative of students attending medical school in the Midwest. For this reason, we included region as a covariate in the ANCOVA to control for any variance introduced by this variable, and its effect was not statistically significant. Second, our HPV knowledge assessment was developed specifically for this study and has not been validated or linked to clinical practice outcomes. However, the instrument was developed based on current guidelines and reviewed for accuracy by an infectious disease physician. Given that the purpose of the present study was to identify knowledge gaps to guide future educational interventions, this type of knowledge assessment was appropriate. However, we did not include oropharyngeal cancers as one of the options in our assessment of knowledge of the association of cancer types with HPV. Recent work has found knowledge gaps among medical students with respect to the association of HPV and oropharyngeal cancers [ 9 ]. This is an important area for future study to ensure that medical education curricula present comprehensive training on the malignancies associated with HPV.
Third, our survey did not assess students’ personal HPV vaccination status, which may introduce respondent bias but could also represent an avenue of future inquiry into HPV vaccination knowledge and practice patterns. Future study is also needed to directly link knowledge deficits to clinical decision-making regarding HPV vaccination as well as to develop common standards for medical education about HPV vaccination. Finally, we also acknowledge the limitation of studying medical students given their inability to practice independently. However, studying this population is essential for curriculum design, improvement, and preparation for practice. Additional work is needed to determine whether the pattern of findings identified in this study are also present among practicing physicians.
Increased knowledge about HPV-related cancers and HPV vaccination as well as confidence discussing the HPV vaccine can positively impact its uptake and continue to decrease the prevalence of HPV-related cancers. This begins with giving medical students a strong foundation on the effects of HPV infection, the benefits of vaccination, and when/to whom it should be administered. While many studies have focused on the general knowledge base of medical students in specific regions and at specific institutions, we demonstrate multi-regional osteopathic and allopathic knowledge of HPV vaccination rationale and guidelines, highlighting the gaps in knowledge where educational interventions are needed. We found deficits in medical students’ ability to link HPV to vaginal, vulvar, anal, and especially penile cancers, including markedly decreased knowledge in the pre-clinical years. The focus of future work should now shift to the development and implementation of curricular interventions. Students could greatly benefit from lectures and workshops dedicated specifically to non-cervical HPV-related cancers and how to discuss the HPV vaccine with patients. With a new cohort of physicians prepared to address HPV vaccination, we may more successfully decrease the prevalence of deaths from cancers preventable with the HPV vaccine.
Supporting information
S1 table. allopathic and osteopathic sample student demographics..
Comparison of sample demographics of allopathic and osteopathic medical student populations in this study.
https://doi.org/10.1371/journal.pone.0280287.s001
S2 Table. Study and national demographics.
Comparison of sample demographics to national allopathic and osteopathic medical student populations.
https://doi.org/10.1371/journal.pone.0280287.s002
S1 File. Study instrument.
This file contains the study instrument that was used to collect data for this study.
https://doi.org/10.1371/journal.pone.0280287.s003
S2 File. Data file.
This file contains the raw data collected in this study.
https://doi.org/10.1371/journal.pone.0280287.s004
Acknowledgments
The authors would like to thank all of the University administrators who assisted with distribution of study information as well as all of the students who took the time to participate in this study.
- 1. Centers for Disease Control & Prevention. Cancers Caused by HPV Are Preventable 2021. Available from: https://www.cdc.gov/hpv/hcp/protecting-patients.html .
- View Article
- PubMed/NCBI
- Google Scholar
- 12. Wu P, Siu Jonathan. Brief Guide to Osteopathic Medicine, For Students, By Students. Chevy Chase, MD: American Association of Colleges of Osteopathic Medicine, 2015. Available at https://www.aacom.org/docs/default-source/cib/bgom.pdf . Accessed November 25, 2022.
Disclaimer » Advertising
- HealthyChildren.org

- Previous Article
- Next Article
Participants
Experimental procedures, data analysis, interest in hpv vaccine topics, confidence by hpv vaccine topic, correlates of hpv vaccine confidence, correlates of hpv vaccine motivation, conclusions, questions and concerns about hpv vaccine: a communication experiment.
POTENTIAL CONFLICT OF INTEREST: Dr Brewer has served on paid advisory boards of and received research grants from Merck, Pfizer, and GlaxoSmithKline; the other authors have indicated they have no potential conflicts of interest to disclose.
FINANCIAL DISCLOSURE: Dr Brewer has served on paid advisory boards of or received research grants from Merck, Pfizer, and GlaxoSmithKline; the other authors have indicated they have no financial relationships relevant to this article to disclose.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Parth D. Shah , William A. Calo , Melissa B. Gilkey , Marcella H. Boynton , Susan Alton Dailey , Karen G. Todd , Meagan O. Robichaud , Marjorie A. Margolis , Noel T. Brewer; Questions and Concerns About HPV Vaccine: A Communication Experiment. Pediatrics February 2019; 143 (2): e20181872. 10.1542/peds.2018-1872
Download citation file:
- Ris (Zotero)
- Reference Manager
Video Abstract
We sought to identify effective responses to parents’ questions and concerns about human papillomavirus (HPV) vaccine.
In 2017–2018, we surveyed a national sample of 1196 US parents of children aged 9 to 17 years. We recorded brief videos of a pediatrician providing messages that addressed 7 HPV vaccination topics that commonly elicit questions or concerns (eg, recommended age). We randomly assigned parents to 1 of the message topics; parents then viewed 4 videos on that topic in random order and evaluated the messages.
Parents were more confident in HPV vaccine when they were exposed to messages that addressed lack of knowledge about HPV vaccine ( b = 0.13; P = .01), messages that included information about cancer prevention ( b = 0.11; P < .001), messages that required a higher reading level ( b = 0.02; P = .01), and messages that were longer ( b = 0.03; P < .001). Parents were less confident in HPV vaccine when exposed to messages in which urgency was expressed ( b = −0.06; P = .005). Analyses conducted by using HPV vaccine motivation as an outcome revealed the same pattern of findings.
We provide research-tested messages that providers can use to address parents’ HPV vaccination questions and concerns about 7 common topics. Important principles for increasing message effectiveness are to include information on the benefits of vaccination (including cancer prevention) and avoid expressing urgency to vaccinate when addressing parents' questions or concerns. Additionally, providers may need to be prepared to have longer conversations with parents who express concerns about HPV vaccine, especially regarding safety and side effects.
A variety of messages are available for providers to use when they address parents’ questions and concerns about HPV vaccine. However, little is known about which messages may be more effective.
Parents most often want information about safety, yet this concern is more challenging to address. Effective answers to HPV vaccination questions emphasize benefits (including cancer prevention) but do not to express urgency. We present brief, research-tested messages based on these principles.
Since the vaccine’s introduction >10 years ago, human papillomavirus (HPV) vaccine series completion in the United States has increased to 49% of adolescents aged 13 to 17 in 2017 1 ; however, the rate of series completion remains far less than the Healthy People 2020 goal of 80% coverage of adolescents aged 13 to 15, 1 , 2 and follow-through (ie, completion among children who have initiated HPV vaccination) may even be dropping. 3 To date, randomized control trials have identified communication techniques providers can use to bring up HPV vaccination and make effective recommendations to parents. 4 , 5 However, little research has been focused on how providers can effectively address questions and ease concerns parents may have after the initial recommendation. Two recent environmental scans of continuing medical education and educational resources about HPV vaccination identified messages developed by the Centers for Disease Control and Prevention (CDC), American Cancer Society, American Academy of Pediatrics, and vaccination experts to help aid providers to communicate information about HPV vaccine. 6 , 7 These messages vary considerably and are focused on how providers can recommend vaccination, answer parents’ concerns or questions, or persuade hesitant parents. To date, little research has been focused on which of these HPV vaccine messages are effective and why. 8 We conducted an online video-messaging experiment with parents of preteenagers and teenagers to identify messages that providers can use to effectively address common parental questions and concerns about HPV vaccine and to identify characteristics of messages that explain their greater efficacy.
Participants in the study were members of an existing, national online-probability panel of 55 000 US adults who were noninstitutionalized and recruited through address-based sampling. 9 Panel members without a computer and Internet access received these resources; those who already had a computer and internet access received points for completing the survey, which could be redeemed for cash, products, or sweepstakes entries. Eligible respondents were parents of at least 1 child aged 9 to 17 years who either had not initiated the HPV vaccine series or had received only the first dose. From November 2017 to January 2018, representatives of the survey company contacted a random sample of 2857 parents via e-mail. Of these parents, 1834 visited the survey Web site and had a child who was of eligible age (9–17 years) and had ≤1 dose of HPV vaccine. Of these respondents, 1313 (72%) met eligibility criteria, provided informed consent, and completed some portion of the survey. After we excluded 50 panelists who did not complete at least two-thirds of the survey, our sample included 1263 parents. The response rate was 61% and based on the American Association for Public Opinion Research response rate 4. 10 For the current study, we excluded 67 parents who did not provide data on key variables (eg, confidence and motivation: 30 parents) or were unable to properly view the video messages (37 parents) to arrive at a final analytic sample of 1196 parents. Parents who did not respond to the survey and those who were excluded did not differ from this study’s analytic sample on the key demographics included in our analyses (χ 2 and t tests were all P > .05). The University of North Carolina Institutional Review Board approved the study protocol.
More detail about the experimental design appears in the Supplemental Information and is briefly described here. Parents were exposed to 2 different video-messaging experiments during the survey. In the first experiment, we randomly assigned all parents to conditions by employing different vaccine recommendation strategies. In the second experiment, we randomly assigned all parents to messages in which questions or concerns about different HPV vaccine topics were answered (the focus of this study).
In the experiment, we evaluated 28 messages pertaining to 7 topics about HPV vaccination (4 messages per topic). Four topics were related to lack of knowledge (diseases prevented by HPV vaccine, the age to start HPV vaccine series, vaccinations for boys and girls, and national recommendations for HPV vaccine), and 3 topics were related to concerns (safety and side effects, vaccination for children who are not sexually active, and school requirements for vaccination). We developed these messages from a library of 267 unique messages that were identified in an environmental scan of educational materials about HPV vaccination. 7 Each message was coded on 5 characteristics ( Table 1 ; Supplemental Information ).
HPV Vaccination Topics, Wording, and Characteristics
C, message about cancer prevention; L, length of video message in seconds; P, message contained first-person or second-person pronouns; R, reading level; U, message is urgent.
Median reading level of all messages.
Overall percentage of messages that were about cancer prevention.
Overall percentage of messages in which urgency was expressed.
Overall percentage of messages that contained first-person or second-person pronouns.
Randomization
The survey software randomly assigned parents to receive video messages about 1 of 7 topics about HPV vaccine that parents had reported wanting to learn more about ( Supplemental Table 6 ). Once randomly assigned, parents watched 4 prerecorded video messages about that topic in random order, all of which were delivered by a board-certified female pediatrician (K.T.). After each video message, parents answered questions about how it affected them. After being randomized to conditions, the samples did not differ on key demographics in 9 of 9 tests (χ 2 tests and analysis of variance were all P > .05).
Survey Item Development
Survey items were previously validated in studies of parents, adolescents, and health care providers (P.R., A.M., J.K., N.B., unpublished observations). 11 , – 17 When needed, we also adapted items from other sources 18 , – 21 or developed new survey items. We cognitively tested the survey instrument with a convenience sample of 16 parents of adolescents who were aged 9 to 17 years to ensure that participants understood the items as we intended. We pretested the instrument with 31 parents from the national panel to ensure proper survey functionality. The full survey instrument is available on request from Dr Brewer.
After each video message, the survey assessed parents’ confidence in and motivation to get HPV vaccines for their children after hearing that message. Items included, “How much would hearing your doctor or health care provider say this increase your confidence in the HPV vaccine?” and “How much would hearing your doctor or health care provider say this make you want to get the [next dose of the] HPV vaccine for your child?” The items had 4-point response scales that ranged from “not at all” (coded as 1) to “a lot” (coded as 4).
Sociodemographic Characteristics
The survey assessed parents’ attitudes toward vaccines in general (4 items; Cronbach’s α = 0.84) and trait reactance (3 items; Cronbach’s α = 0.61). 20 , 22 All items had 5-point responses that ranged from “strongly disagree” (coded as 1) to “strongly agree” (coded as 5). The survey company provided parent's demographic characteristics, including sex, age, race and ethnicity, and education. For demographic and health characteristics of the parent’s index child (ie, reported by the parent), the survey assessed sex, age, and HPV vaccination status (0 doses or ≥1 dose). Sociodemographic characteristics appear in Table 2 .
Participant Characteristics, N = 1196
Samples that were randomized to topic conditions did not differ on any key demographics.
We used Stata 15.1 (Stata Corp, College Station, TX) for analyses. Statistical tests were 2-tailed with a critical Cronbach’s α of 0.05. We calculated the percentage of parents who wanted to learn about the 7 HPV vaccination topics and which topics parents most wanted to learn about from their children’s health care providers ( Table 3 ). We also calculated the percentage of parents who were more confident in HPV vaccine after message exposure ( Fig 1 ). We defined the proportion of parents who were more confident as those who responded with “moderately” or "a lot” to the confidence items. To identify correlates of parents’ confidence in HPV vaccine or motivation to get HPV vaccine for their children, we constructed multilevel linear models to account for within-subject, repeated-measures of study outcomes ( Table 4 ). For each outcome, we first evaluated intercept-only models. Next, we examined each message (level 1 variables) and parental or child sociodemographic characteristics (level 2 variables) as predictors in separate unadjusted models. Any variable with P ≤.10 in the unadjusted models was included in an adjusted multilevel linear model. We report associations as unstandardized regression coefficients ( b ). We specified unstructured covariance matrices in model estimations and used Huber-White sandwich estimators to account for possible nonnormality in the distribution of the errors in the regression models. Finally, in exploratory analyses, we stratified these models on the child’s HPV vaccination status ( Supplemental Table 7 ).
HPV Vaccine Information Wanted From Child’s Health Care Provider
For last column, parents could choose only 1 topic. Data are for 1189 to 1195 parents (<1% missing).

Proportion of parents who were more confident in HPV vaccine after message exposure.
Correlates of Parents’ Confidence in and Motivation to Get HPV Vaccine for Their Children After Message Exposure
Regression coefficients ( b ) are unstandardized. Confidence and motivation had 4-point response scales of 1 (not at all) to 4 (a lot). Topics for lack of knowledge were: diseases prevented by HPV vaccine, the age to start the HPV vaccine series, vaccination for boys and girls, and national recommendations for HPV vaccination. Topics for concerns were: safety and side effects, vaccination for children not sexually active, and school requirements for HPV vaccination. Parent attitude toward vaccines was assessed by using the Vaccine Confidence Scale that measure attitudes toward adolescent vaccination. 22 Parent trait reactance was assessed by using a brief scale that measures resistance that arises when a person feels their autonomy is threatened. 20 Intraclass correlation for the confidence model was 0.79, and intraclass correlation for the motivation model was 0.82. —, not applicable.
P < .05; ** P < .001.
Missing cases for each variable ranged from 0% to 2%. We generated 20 data sets using multiple imputation by chained equations to estimate plausible values for missing data 23 and used augmented regression procedures to avoid perfect prediction for incomplete categorical variables. 24 We report multilevel linear model results from the pooled multiply imputed analyses. To examine the effect of nonresponse, we compared unweighted model results with weighted model results to adjust the sample to reflect the general US population. Because using survey weights did not meaningfully change our findings, we present unweighted analyses.
Most parents wanted to talk with their children’s health care providers about the 7 HPV vaccine topics, ranging from 68% who wanted to talk about safety and side effects to 84% who wanted to talk about the diseases prevented by HPV vaccine ( Table 3 ). When asked which topic they most wanted information about from their children’s health care providers, parents prioritized safety and side effects (44%), diseases prevented by HPV vaccine (18%), and the age at which to start HPV vaccination (12%). Parents placed the lowest priority on discussing vaccination for boys and girls (8%), vaccination for children who were not sexually active (7%), school requirements for vaccination (6%), and national recommendations for HPV vaccine (5%).
Among parents whose children had not yet received HPV vaccine, the proportion who were moderately or a lot more confident in HPV vaccine after message exposure ranged from 25% to 46% ( Fig 1 ). Confidence was highest after the parents were exposed to messages about the diseases prevented by HPV vaccine (46%) and vaccination for boys and girls (44%). Confidence was lowest after parents were exposed to messages about safety and side effects (30%) or school requirements for HPV vaccination (25%). Among parents of children who had initiated the HPV vaccine series, the proportion of those who were more confident in HPV vaccine after message exposure ranged from 57% to 71% ( Fig 1 ). Confidence was highest after parents were exposed to messages about the age to start HPV vaccination (71%). For the other topics, approximately three-fifths of parents were more confident after message exposure.
With respect to message characteristics, parents who were exposed to messages that adressed lack of knowledge ( b = 0.13; P = .01) were more confident in HPV vaccine compared with parents who were exposed to messages addressing concerns in adjusted analyses ( Table 4 ). Parents who were exposed to messages that required a higher reading grade level ( b = 0.02; P = .01) or messages that were longer ( b = 0.03; P < .001) were more confident in HPV vaccine. Additionally, parents who were exposed to messages about cancer prevention ( b = 0.11; P < .001) were more confident in HPV vaccine. In contrast, parents who were exposed to messages in which urgency was expressed ( b = −0.06; P = .005) had lower confidence in HPV vaccine. Finally, parents who were exposed to messages that contained first-person and second-person pronouns had lower confidence in HPV vaccine, but this association was only significant in bivariate analyses.
With respect to parental characteristics, mothers ( b = 0.16; P = .002) and parents who were black ( b = 0.30; P = .001) were more confident in HPV vaccine after message exposure than fathers and parents who were white, respectively. Parents who had more positive attitudes toward vaccines ( b = 0.39; P < .001) and those with children who had initiated the HPV vaccine series ( b = 0.54; P < .001) were more confident in HPV vaccine after message exposure. In contrast, parents who had higher trait reactance ( b = −0.09; P = .03) or older children ( b = −0.04; P < .001) were less confident in HPV vaccine after message exposure.
Findings for motivation with respect to parents exposed to messages that addressed lack of knowledge ( b = 0.17; P = .001), messages that required a higher reading grade level ( b = 0.01; P = .03), messages that were longer ( b = 0.03; P < .001), messages about cancer prevention ( b = 0.08; P < .001), messages in which urgency was expressed ( b = −0.05; P = .005), and messages that contained first-person and second-person pronouns were similar to findings for confidence in adjusted analysis ( Table 4 ). Additionally, with respect to parent and child characteristics, our findings for motivation with respect to mothers ( b = 0.14; P = .007), parents who were black ( b = 0.25; P = .004), parents’ attitudes toward vaccines ( b = 0.39; P < .001), parents’ trait reactance ( b = −0.11; P = .004), children who had initiated the HPV vaccine series ( b = 0.54; P < .001), and older children ( b = −0.04; P < .001) were similar to our findings for confidence.
Providers’ vaccine recommendations are uniquely powerful in motivating patients to undergo vaccination, 25 but little is known about how providers can effectively clarify parents’ questions and ease their concerns after initial recommendations. In our national study, most parents wanted to learn more information from their children’s health care providers about each of the 7 HPV vaccine topics, with a priority on learning more about safety and side effects, diseases prevented by HPV vaccine, and the age at which to start vaccination. Additionally, brief messages that addressed common questions and concerns boosted the confidence of the majority of parents with children who had initiated HPV vaccination. Even among parents who had not initiated HPV vaccination for their children, many reported greater confidence after considering the messages.
Our findings suggest general communication principles when responding to parents’ questions and concerns about HPV vaccination. First, communication regarding HPV vaccine may be more effective if providers include information about cancer prevention. Messages that referred to cancer prevention were more effective in increasing confidence and motivating parents to have their children vaccinated. In addition, messages that referred to cancer prevention worked well among both parents of children who were unvaccinated and parents of children who were vaccinated. Previous studies have drawn the same conclusion, 8 , 26 , – 29 but they have focused on recommendations or general informational statements (rather than addressing questions and concerns) and focused on the prevention of specific cancers in either boys or girls (rather than HPV-related cancer prevention in children in general), had smaller samples, or were reliant on relatively few messages.
Second, urgency is important when first raising the topic of vaccination and when recommending it, 4 , 30 but expressing urgency for vaccination when addressing questions and concerns may be counterproductive. We found that messages that expressed urgency were less effective among parents of unvaccinated children. One reason may be that parents who are hesitant feel inappropriately rushed or that their concerns are not being treated with appropriate care. 31 Third, the use of personal pronouns may undermine the impact of explanations. We found that using self-referential language (such as “my clinic,” “we can prevent,” and “your child”) in answers to questions and concerns did not help and could have possibly reduced the impact of messages. Other studies have yielded similarly poor performance of messages about providers getting the vaccine for their own children. 8 , 32
Finally, providers may need to prepare to engage in longer discussions about HPV vaccination when parents express concerns. In our study, parents indicated that they most wanted to speak with their children’s health care providers about the safety and side effects of HPV vaccine. However, messages that addressed low knowledge levels were more effective than messages that addressed concerns. One explanation may be that these topics (safety and side effects, vaccination for children who are not sexually active, and school requirements for HPV vaccination) are inherently challenging to address. Another explanation may be that our brief messages may not have been sufficiently detailed to address these concerns. Patients say that they find value in receiving additional information, such as from fact sheets, and in participating in motivational interviewing. 33 , 34 A final explanation may be that our messages addressing low knowledge more often included information about the benefits of vaccination. As such, reiterating vaccination benefits (including cancer prevention) when addressing concerns may also improve the impact of messages.
The study’s strengths include its large national sample of parents of children who were aged 9 to 17 years and its experimental study design. Parents were exposed to video messages presented by a physician rather than reading message text, which more closely reflects the experience of a clinical visit and lends ecological validity to parental responses. Limitations include our examination of vaccine confidence and perceived motivation as a proxy for behavior intention. Although intention is 1 of the strongest predictors of behavior, barriers to action and other impediments can reduce the strength of the association. 35 Future research should be conducted to confirm whether providers easing parents’ concerns in clinical settings yields higher uptake of HPV vaccine. Interventions used to increase parents’ confidence in and motivation to get HPV vaccine alone may not increase vaccine uptake; however, they could be used to increase uptake in combination with clear, strong provider recommendations to get the vaccine, as other studies have shown. 4 , 5 , 25 The validity of our findings must also be interpreted in light of the fact that parents were exposed to a separate experiment of prerecorded videos of vaccine recommendation strategies before they viewed the messages to which they were randomly assigned in our current study. Although randomization in both experiments revealed no observable selection bias in our 7 topic conditions, parents were likely primed to pay closer attention to our messages, which could have influenced the processing of these messages. Finally, because we assigned parents to view messages about topics they had expressed interest in learning about, the effectiveness of our messages in other contexts remains to be established.
Seven brief messages that providers can use to address parents’ questions and ease concerns are shown in Table 5 . For the most part, they are the most effective messages in eliciting confidence and motivation for each HPV vaccine topic. We adjusted the messages in places to align with the previously described communication principles. Providers may take these messages as a starting point and elaborate as needed. In our study, messages elicited higher confidence when they were longer and required a higher reading grade level. We aimed for shorter messages that providers could remember. However, parents seeking information may prefer longer answers (ie, those that translate to a longer discussion). In a study such as ours, the trade-off between a discussion about vaccination and receiving other care may not have been as salient as it would be in a clinical visit. Our study sample was also skewed to parents with higher levels of educational attainment, which could also explain the preference for more complex messages. As a means of conveying complex information about HPV vaccination, providers may consider using validated visual aids in their conversations with parents, 36 particularly those who may have lower levels of health literacy.
Refined Example Messages Used to Address Parents’ Common Questions and Concerns About HPV Vaccine
Reasons for the low uptake of HPV vaccination in the United States and other countries are well known and have been known for the last decade. 37 , 38 Previous research has firmly established the importance of a provider’s clear recommendation for increasing vaccine uptake 25 ; however, more work is needed to establish how to communicate information that can be used to effectively address questions and concerns that may come up afterward. In our study, we provide examples of brief messages providers could employ in their discussions with parents about HPV vaccine. We also identify general communication principles, such as including information about the benefits of vaccination and cancer prevention and avoiding expressions of urgency to vaccinate when addressing parents’ questions or concerns.
Centers for Disease Control and Prevention
human papillomavirus
Dr Shah prepared the data, conducted statistical analyses, drafted the initial manuscript, and critically reviewed and revised the manuscript; Drs Brewer, Gilkey, Calo, and Todd and Ms Alton Dailey conceptualized and designed the study, developed the survey instrument, supervised data collection, and critically reviewed and revised the manuscript; Dr Boynton supervised data analyses and critically reviewed and revised the manuscript for important methodological content; Ms Robichaud and Ms Margolis assisted in the conceptualization and development of the survey instrument and data collection and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FUNDING: Funded by the Centers for Disease Control and Prevention (with Dr Brewer as principal investigator; grant 3U48DP005017-03S6) and supported by Cooperative Agreement U48 DP005017-01S8 from the Centers for Disease Control and Prevention and the National Cancer Institute. Dr Shah’s time was partially supported by a National Research Service Award Post-Doctoral Traineeship from the Agency for Healthcare Research and Quality, which was sponsored by the Cecil G. Sheps Center for Health Services Research at the University of North Carolina at Chapel Hill (grant T32-HS000032). Funders played no role in (1) the study design; (2) the collection, analysis, and interpretation of data; (3) the writing of the report; or (4) the decision to submit the article for publication. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality, the Centers for Disease Control and Prevention, or the National Cancer Institute. Funded by the National Institutes of Health (NIH).
Competing Interests
Supplementary data.
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- Editorial Policies
- Journal Blogs
- Pediatrics On Call
- Online ISSN 1098-4275
- Print ISSN 0031-4005
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Research article
- Open access
- Published: 22 March 2013
Gaps in detailed knowledge of human papillomavirus (HPV) and the HPV vaccine among medical students in Scotland
- Sarah M McCusker 1 ,
- Ishbel Macqueen 1 ,
- Graham Lough 1 ,
- Alasdair I MacDonald 1 ,
- Christine Campbell 2 &
- Sheila V Graham 1
BMC Public Health volume 13 , Article number: 264 ( 2013 ) Cite this article
7822 Accesses
36 Citations
4 Altmetric
Metrics details
A vaccination programme targeted against human papillomavirus (HPV) types16 and 18 was introduced in the UK in 2008, with the aim of decreasing incidence of cervical disease. Vaccine roll out to 12–13 year old girls with a catch-up programme for girls aged up to 17 years and 364 days was accompanied by a very comprehensive public health information (PHI) campaign which described the role of HPV in the development of cervical cancer.
A brief questionnaire, designed to assess acquisition of knowledge of HPV infection and its association to cervical cancer, was administered to two different cohorts of male and female 1 st year medical students (school leavers: 83% in age range 17–20) at a UK university. The study was timed so that the first survey in 2008 immediately followed a summer's intensive PHI campaign and very shortly after vaccine roll-out (150 students). The second survey was exactly one year later over which time there was a sustained PHI campaign (213 students).
We addressed three research questions: knowledge about three specific details of HPV infection that could be acquired from PHI, whether length of the PHI campaign and/or vaccination of females had any bearing on HPV knowledge, and knowledge differences between men and women regarding HPV. No female student in the 2008 cohort had completed the three-dose vaccine schedule compared to 58.4% of female students in 2009. Overall, participants’ knowledge regarding the sexually transmitted nature of HPV and its association with cervical cancer was high in both year groups. However, in both years, less than 50% of students correctly identified that HPV causes over 90% of cases of cervical cancer. Males gave fewer correct answers for these two details in 2009. In 2008 only around 50% of students recognised that the current vaccine protects against a limited subset of cervical cancer-causing HPV sub-types, although there was a significant increase in correct response among female students in the 2009 cohort compared to the 2008 cohort.
Conclusions
This study highlights a lack of understanding regarding the extent of protection against cervical cancer conferred by the HPV vaccine, even among an educated population in the UK who could have a vested interest in acquiring such knowledge. The intensive PHI campaign accompanying the first year of HPV vaccination seemed to have little effect on knowledge over time. This is one of the first studies to assess detailed knowledge of HPV in both males and females. There is scope for continued improvements to PHI regarding the link between HPV infection and cervical cancer.
Peer Review reports
Anogenital human papillomavirus (HPV) infection is the most prevalent viral sexually transmitted infection (STI) in the world today [ 1 ]. Infection is extremely common, most prevalent among sexually active young adults and usually follows a benign course. Persistent infection with “high risk” (HR) HPV causes cervical disease that can lead to cervical cancer [ 2 ]. HR-HPV types, of which there are 15, cause over 99% of cervical cancers [ 3 ].
September 2008 saw the introduction of a UK nationwide HPV immunisation programme [ 4 ]. The vaccine is now offered routinely in secondary school, to girls aged 12 – 13 years. A three-year “catch up” campaign, delivered in GP surgeries and dedicated vaccination centres, has allowed older girls up to the age of 17 and 364 days access to the vaccine. Boys are not vaccinated. In the UK, between 2008 and 2012, the bivalent Cervarix vaccine [ 5 ] was used to protect against the two HR-HPV types, HPV 16 and 18, that cause around 70% of cervical cancers [ 1 ]. The vaccine was provided in three doses over six months to elicit a protective immune response in vaccinees prior to exposure to the virus [ 6 ].
Vaccine uptake in the UK has been excellent, at > 90% for 12–13 year-old girls. However, uptake rates for the catch-up programme are lower at less than 60% overall [ 7 ]. Vaccination is accompanied by targeted public health information (PHI), in the form of leaflets and/ or information sharing sessions directed by trained school nurses. Details of HPV infection and its association with cervical cancer are explained: HPV is sexually transmitted, HPV causes all cervical cancers, HPV vaccine protects against two types of HPV. Therefore, young women who have been vaccinated may be expected to have increased knowledge of HPV and its association with cervical cancer compared to the unvaccinated population. At the launch of the vaccination programme these routine information sources were supplemented by an extremely intensive media campaign as well as a specially developed Scottish website ( http://www.fightcervicalcancer.org.uk/ ) and associated literature. The website has now been withdrawn and replaced with information on immunisation ( http://www.immunisationscotland.org.uk/vaccines-and-diseases/hpv.aspx ). Although these efforts were largely targeted to women, young men would also have been expected to have some exposure to the media campaign because of its intensity and range (cinema, television, radio, billboards, newspaper adverts) in 2008 [ 8 ].
Correct knowledge about HPV infection and cervical cancer could be important to inform decision making regarding uptake of the vaccine. Prior to vaccine roll out many studies revealed a lack of awareness of HPV (i.e. whether respondents had heard of the virus) and a lack of knowledge of the association between HPV and cervical cancer in the UK and a range of other countries [ 9 – 14 ]. Recent studies have demonstrated increased awareness of HPV following the introduction of the vaccine [ 15 , 16 ]. However, fewer studies have investigated acquisition of detailed knowledge of HPV infection. Those that have, indicate low levels of more detailed knowledge of HPV infection and links to cervical cancer [ 17 – 20 ]. Such knowledge appears to be greatest in those with higher education levels [ 19 ].
This study was conducted at the University of Glasgow, Scotland. Prospective medical students at this university are admitted to study on the basis of interview where depth of knowledge of current topics in medicine is assessed. The HPV vaccine was one of the main topics for 2008 and 2009 so new medical students at the university could be expected to acquire some knowledge of HPV. HPV knowledge was assessed in two groups of first year medical students, mean age range 17–20 years of age. The timings of the surveys were specifically chosen. The first survey was carried out in November 2008 immediately following the roll-out of the HPV vaccine and a summer’s intensive media campaign that aimed to inform the public about HPV and the HPV vaccine. The second cohort was surveyed one year later when many more females had received the vaccine and associated PHI. The multimedia PHI campaign was sustained over this one year period. Moreover, media interest in the death from cervical cancer of the celebrity Jade Goody [ 21 , 22 ], and increased discussion of HPV in the media, at the start of 2009 coincided with the timing of interviews for medical school of the 2009 student cohort.
We sought to answer three research questions. The first question we addressed was knowledge about three specific details of HPV infection that could be acquired from reading of PHI leaflets accompanying the HPV vaccination programme and from PHI in an intensive media campaign between 2008 and 2009. The second research question was whether vaccination of females in the group and a year’s PHI campaign had any bearing on HPV knowledge. In contract to policy in the rest of the UK where screening starts at age 25, from age 20, Scottish (and Welsh) women are offered three-yearly cervical screening for the detection of HPV-associated cervical disease. 73.7% of eligible women were screened in the past three and a half years (as of March 31 st , 2010), which is a rise in uptake of around 4.5 percent in comparison to previous years [ 23 ]. Arguments for and against screening before the age of 25 can be found at http://www.cks.nhs.uk/cervical_screening/management/scenario_when_to_offer_cervical_screening/ . The final research question was to find out if men and women acquired similar levels of knowledge about HPV from the same or different sources. This study is among the first to compare knowledge of details of HPV of an educated mixed gender cohort at a time of an extremely intensive media campaign then exactly one year later in a similar cohort where most of the females had received at least one dose of the HPV vaccine and following a sustained PHI campaign.
Study design
A questionnaire survey was administered to first year medical students at a UK university (University of Glasgow). Medical students were chosen as a "best case scenario" of HPV knowledge acquisition among young adults because they are an educated population and may have a greater awareness of current medical issues: entry into medical school at Glasgow is by interview where prospective students are questioned on knowledge of current topics in human health. Moreover, as health professionals of the future, they may have high motivation to pay attention to and acquire current PHI. Student knowledge and opinion was assessed in 2008 in that year’s intake of medical students i.e. immediately following the implementation of HPV vaccine delivery in the UK, and then one year later in the 2009 intake of students. The same questionnaire was administered in both years.
Survey questionnaire
An 8-item closed-ended questionnaire, with, for females, a 3-item data gathering section to determine stage of vaccination, intention to attend smear testing and smear testing status, was developed from a literature review and in consultation with a vaccine specialist at Health Protection Scotland and NHS public health information specialists. No studies examining detailed knowledge of HPV in the UK were reported in the literature at the time of this study so three questions relating to HPV were designed to address specific points regarding HPV knowledge discussed in leaflets delivered to vaccinees and in other PHI. The draft questionnaire was piloted on students in the final year honours virology classes of both years. The questionnaire was then reviewed by members with relevant expertise of the University of Glasgow Ethics Committee and modified according to feedback received. The survey was administered at the same time of year, in November, first in 2008 and then in 2009. This timing was chosen because it preceded taught modules on cancer, including cervical cancer. The questionnaire was completed in a ten minute period at the end of a teaching session in a lecture theatre. One of the researchers introduced the study and explained the ethical permissions and voluntary nature of participation. Students made aware of how anonymity would be ensured. Although signed informed consent was given, the consent forms were collected separately to the questionnaires so that no questionnaire answers could be traced back to an individual. Ethical approval was obtained from the University of Glasgow’s Faculty of Biomedical and Life Sciences Ethics Committee.
In 2008 the timing of the survey was chosen to be immediately following first roll out of the vaccine (September 2008) after a summer's intensive public health campaign. This included television, radio, internet, media, cinema and billboard adverts and age-directed leaflets available in public places, community and health centres and GP surgeries. Few females in the population were vaccinated in 2008. In contrast, in 2009, many of the female students had been offered the vaccine, delivered in association with appropriate PHI. Moreover, there was a sustained high level PHI campaign throughout 2009. In particular, during this period there was extensive media coverage of the illness and death due to cervical cancer of the celebrity Jade Goody [ 21 , 22 ].
The study group
In 2008 all 150 students (100%) and 213 out of 217 students in 2009 (98.2%) who attended the taught sessions at which the questionnaires were given out responded to the questionnaire. In 2008 the survey population consisted of 100 female and 50 male participants while in 2009 the survey population contained 114 female and 99 male students. In both survey populations the majority of students were between seventeen and twenty years of age (84% in 2008; 83% in 2009) (Table 1 ). In 2008, 90% and in 2009, 87% of students were from the UK. In 2008, 91% of the female students had not been vaccinated and no student had received all three vaccine doses. In contrast, in 2009 58.4% of female students had received three vaccine doses (Table 1 ). All students who filled in the questionnaire did so completely except for up to two non-responders to questions regarding uptake of smear testing.
Data collation and analysis
Data were input into Microsoft Excel 11.5. Exact binomial, Randomisation and Chi-Squared tests were used to compare between group data. Differences were considered significant if p< 0.05. Analyses focused on questions pertaining to knowledge of HPV and the HPV vaccine.
Knowledge of details of HPV and its association with cervical cancer
In 2008, 97.3% of the class marked as true the statement "HPV is sexually transmitted between males and females" (Table 2 ). Only four students did not consider that HPV is sexually transmitted between males and females. However, in 2009, 10% of students did not consider that HPV was sexually transmitted indicating a decrease in knowledge acquisition, especially in the male population, in the second group of students. The medical students were asked to estimate what percentage of cervical cancers is due to HPV. Less than half of all males and females in both survey populations answered correctly. There was some difference in the proportion of correct answers given by males in 2009 compared to 2008 (Table 2 ). When asked the question "There are at least 15 different types of HPV that can cause cervical cancer. How many of these types do you think the vaccine protects against?" the majority of female students in 2008 and 2009 answered correctly that the vaccine protects against less that 5 HPV types. There was a statistically significant positive difference in the response of females to this question over time (73% answered correctly in 2009 compared to 50% of females in 2008) (Table 2 ). Male responses in both years were very similar to the 2008 female responses and showed no statistical difference comparing responses in 2008 with 2009.
Assessing potential impact of the vaccine on uptake of cervical smear testing
In 2008, 94% and in 2009, 87.3% of the students assessed the cervical screening programme as “very important”. In Scotland, women are invited for their first smear test at age 20. Some of the female students in the survey populations had already attended for testing: in 2008, 16.0% (n=16) of students (97% of those eligible for screening (i.e. ≥20 years of age)) had been for a smear test while in 2009, 13.3% (n=15) (68% of those eligible for screening) had been tested. In both years the majority of women answered positively when asked if they would go for a smear test when invited. Importantly, in 2009, 94% of females who had been fully vaccinated said they would attend for a smear test when invited.
Exposure to public health information on the HPV vaccine
The impact of the HPV vaccine campaign was also examined (Table 3 ). Most students, male and female, in both years claimed to have been exposed to PHI on the HPV vaccine, reporting television advertising and word of mouth as the main source in the 2008 survey. The 2009 survey included more options due to an increased range of PHI resources available since the start of the vaccination programme and most students reported having seen these. However, in this second year, a much lower percentage of male students reported receiving information from television adverts but a greater percentage reported receiving information by word of mouth. More males in this year were unable to rate PHI on the HPV vaccine.
Many studies in different countries in a range of socio-economic groups have demonstrated an overall lack of awareness of HPV and its link with cervical cancer [ 9 – 11 , 16 , 24 , 25 ]. This study represents one of the first reports of the knowledge of educated young people in the UK of details about HPV infection, the HPV vaccine and cervical cancer, and importantly, how knowledge changed within the first year of introduction of the vaccination programme. The surveys were carefully timed to question the students when they would have been maximally exposed to PHI and media information on HPV and the HPV vaccine. Moreover, the students may have had more motivation to pay attention to details in the PHI than most other young people of this age group because their successful entry to medical school could depend on their displaying clear knowledge and understanding of facts on HPV at interview. There are few studies comparing detailed knowledge of HPV in males and females. The young male population could have a role in encouraging their female peers to be vaccinated so both genders were questioned in each year’s cohort. The study included unvaccinated women and women who were in the age group between being offered the vaccine and being called for cervical screening. The vast majority (99%) of the students, male and female, questioned in each year indicated that they had seen or heard information on the HPV vaccine indicating some efficacy of PHI. The questionnaire assumed that the students would have some knowledge of HPV and cervical cancer and indeed it was clear that the level of knowledge of this educated population was probably greater than that of the general population [ 10 ] as recently suggested [ 26 ].
Knowledge about specific details of HPV infection
The UK PHI leaflets accompanying HPV vaccination note the sexually transmitted nature of HPV. Surprisingly, although most students seemed to appreciate this fact, in agreement with another study [ 27 ], the 2009 survey showed a small decrease in the percentage of students who understood that HPV was sexually transmitted. This was despite a significant step-up in the PHI campaign surrounding HPV and its link with cervical cancer from the summer of 2008 through to autumn 2009 and the media coverage early in 2009 of the death from cervical cancer of the young celebrity Jade Goody [ 21 , 22 ]. However, the level of knowledge of the sexually-transmitted nature of HPV in our cohorts was much higher than that in another study of a similar age group of school students in Germany where less than 50% understood that HPV was a sexually transmitted infection [ 28 ]. It is becoming increasingly clear from similar surveys in a number of countries that even among educated young people there is a lack of understanding of the sexually transmitted nature of HPV infection [ 18 , 19 , 29 – 31 ].
PHI leaflets clearly state that the vaccine protects against 70% of cases of cervical cancer. However, it is not made clear that the remaining 30% of cases are caused by other HR-HPV types. Consequently, anecdotal evidence suggests that the public may believe it is possible to develop cervical cancer without being infected with HPV [ 16 , 32 , 33 ]. Students were asked to guess what percentage of cervical cancers was caused by HPV. Only around half of men and women in 2008 understood that HPV caused between 80 and 100% of cervical cancers. There was a decrease in understanding this fact between the 2008 and 2009 surveys and a greater percentage of men responded incorrectly in 2009.
Relationship between vaccination and HPV knowledge
The vaccine PHI intended to inform the public that the vaccine protected against two types of HPV. However, in 2008 around half the survey population did not appear to appreciate this fact. Male understanding did not increase by 2009 but females gave a larger number of correct responses in that year. This could be due to the increased percentage of vaccinated female students in 2009 that correlated with an increased number who claimed to have seen or heard HPV information in formal education, as expected due to the PHI delivered to vaccinees. However, as noted above, a lower percentage of the 2009 cohort of women realised that HPV caused most cervical cancers. Therefore our evidence is equivocal that PHI delivered with the vaccine improved HPV knowledge.
Acquiring knowledge: gender differences in HPV knowledge
Although there was some evidence of gender differences in HPV knowledge, especially in the 2009 cohort, gender did not have a significant difference on the sources of information on HPV. However, a much higher percentage of male students reported receiving information from word of mouth and more males in 2009 compared to 2008 were unable to rate PHI on the HPV vaccine. Interestingly, although the males in the study displayed knowledge of HPV association with cervical cancer, the majority answered ‘no’ to a question included in the survey “Do you think males should be offered the vaccine?” This could indicate perception of HPV vaccination as a female issue.
Limitations of the study
The study has some limitations. Prior to administering the questionnaire it is possible that some students had begun to prepare for the oncology portion of their course by reading recommended materials and had thus acquired a greater appreciation of the subject prior to questioning. Some homogenisation of answers could have occurred through students comparing answers in class. The restricted time period (ten minutes) for filling in the questionnaire was designed to avoid this. In itself this may have introduced another limitation because the short time period may have led the students to provide rapid, poorly considered responses.
Public knowledge of HPV, cervical cancer and vaccination is central to ensuring good vaccination coverage in the female population and success of future HPV testing strategies that will reduce the burden of disease. This survey indicates that PHI has been somewhat successful in delivering key elements of HPV knowledge for both genders in the young educated population studied, but that there are still important knowledge gaps especially in males. Continued efforts in pressing home the main messages regarding HPV, the HPV vaccine, and cervical screening are required.
Ethical approval
University of Glasgow Faculty of Biomedical Sciences Ethics Committee project approval number FBLS 0825 17/11/2008 and FBLS 0930 01/10/2009.
Abbreviations
- Human papillomavirus
Public health information
Sexually transmitted infection
zur Hausen H: Papillomaviruses in the causation of human cancers - a brief historical account. Virol. 2009, 384: 260-265. 10.1016/j.virol.2008.11.046.
Article CAS Google Scholar
Woodman CBJ, Collins SI, Young LS: The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007, 7: 11-22. 10.1038/nrc2050.
Article CAS PubMed Google Scholar
Munoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsague X, Shah KV: Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003, 348: 518-527. 10.1056/NEJMoa021641.
Article PubMed Google Scholar
published on the Department of Health. 2011, http://www.dh.gov.uk/en/Publichealth/Immunisation/keyvaccineinformation ,
Szarewski A: HPV vaccine: Cervarix. Expert Opin Biol Ther. 2010, 10: 477-487. 10.1517/14712591003601944.
Stanley MA: Human papillomavirus vaccines. Rev Med Virol. 2006, 16: 139-149. 10.1002/rmv.498.
published on the Department of Health. 2011, https://www.wp.dh.gov.uk/immunisation/files/2013/01/2900783_HPVMonthlySurvey_Dec12_acc.pdf ,
published on Health Scotland. 2012, http://www.healthscotland.com/health/topics/immunisation/HPV.aspx ,
Trim K, Nagji N, Elit L, Roy K: Parental knowledge, attitudes and behaviours towards human papillomavirus vaccination for their children: a systematic review from 2001 to 2011. Obs Gynecol International. 2012, 2012: 1-12.
Article Google Scholar
Cuschieri K, Horne A, Szarewski A, Cubie HA: Public awareness of human papillomavirus. J Med Screen. 2006, 13: 201-207.
CAS PubMed Google Scholar
Marlow LAV, Waller J, Wardle J: Public awareness that HPV is a risk factor for cervical cancer. Br J Cancer. 2007, 97: 691-694. 10.1038/sj.bjc.6603927.
Article CAS PubMed PubMed Central Google Scholar
Klug SJ, Hukelmann M, Blettner M: Knowledge about infection with human papillomavirus: A systematic review. Prev Med. 2008, 46: 87-98. 10.1016/j.ypmed.2007.09.003.
Tiro JA, Meissner HI, Kobrin S, Chollette V: What do women in the U.S. know about human papillomavirus and cervical cancer?. Cancer Epiedmiol Biomarkers Prev. 2006, 16: 288-294.
Pitts M, Clarke T: Human papillomavirus infections and risks of cervical cancer: what do women know?. Health Educ Res. 2002, 17: 706-714. 10.1093/her/17.6.706.
Cooper Robbins SC, Bernard D, McCafferty K, Brotherton J, Garland S, Skinner SR: "Is cancer contagious?": Australian adolescent girls and their parents: Making the most of limited information about HPV and HPV vaccination. Vaccine. 2010, 28: 3398-3408. 10.1016/j.vaccine.2010.02.078.
Hilton S, Smith E: "I thought cancer was one of those random things. I didn't know cancer could be caught." Adolescent girls' understanding and experiences of the HPV programme in the UK. Vaccine. 2011, 29: 4409-4415. 10.1016/j.vaccine.2011.03.101.
Article PubMed PubMed Central Google Scholar
Licht AS, Murphy JM, Hyland AJ, Fix BV, Hawk LW, Mahoney MC: Is use of the human papillomavirus vaccine among female college students related to human papillomavirus knowledge and perception?. Sex Transm Infect. 2009, 86: 74-78.
Bowyer HL, Marlow LAV, Hibbets S, Pollock KG, Waller J: Knowledge and awareness of HPV and the HPV vaccine among young women in the first routinely vaccinated cohort in England. Vaccine. 2013, 31: 1051-1056. 10.1016/j.vaccine.2012.12.038.
Marlow LAV, Zimet GD, McCafferty KJ, Ostini R, Waller J: Knowledge of human papillomavirus (HPV) and HPV vaccination: An international comparison. Vaccine. 2013, 31: 763-769. 10.1016/j.vaccine.2012.11.083.
Caskey R, Lindau ST, Alexander GC: Knowledge and early adoption of the HPV vaccine among girls and young women: results of a national survey. J Adolesc Health. 2009, 45: 453-462. 10.1016/j.jadohealth.2009.04.021.
Metcalfe D, Price C, Powell J: Media coverage and public reaction to a celebrity cancer diagnosis. J Pub Health. 2010, 5: 1-6.
Google Scholar
Bell L, Searle C: The reporting of cervical cancer in the mass media: a study of UK newspapers. Eur J Cancer Care. 2010, 20: 389-394.
published on Information Services Division, Scotland. 2011, http://www.isdscotland.org/Health-Topics/Cancer/Cervical-Screening/ ,
Walsh CD, Gera A, Shah M, Sharma A, Powell JE, Wilson S: Public knowledge and attitudes towards Human Papilloma Virus (HPV) vaccination. BMC Publ Health. 2008, 8: 368-377. 10.1186/1471-2458-8-368.
Das A, Madhwapathi V, Davies P, Brown G, Dearnley E, Spencer A, Williams H: Knowledge and acceptability of the HPV vaccine by school children and their parents in Birmingham. Vaccine. 2010, 28: 1440-1446. 10.1016/j.vaccine.2009.11.041.
Schmeink CE, Gosens KC, Melchers WJ, Massuger LF, Bekkers RL: Young adults awareness of HPV and vaccine acceptance after introduction of the HPV vaccine in the Dutch national vaccination program. Eur J Gynaecol Oncol. 2011, 32: 418-426.
Williams K, Forster A, Marlow L, Waller J: Attitudes towards human papillomavirus vaccination: a qualitative study of vaccinated and unvaccinated girls aged 17–18 years. J Fam Plann Reprod Health Care. 2011, 37: 22-25. 10.1136/jfprhc.2010.0017.
Blödt S, Holmberg C, Müller-Nordhorn J, Rieckmann N: Human papillomavirus awareness, knowledge and vaccine acceptance: A survey among 18–25 year old male and female vocational school students in Berlin, Germany. Eur J Public Health. 2012, 22: 808-813. 10.1093/eurpub/ckr188.
Samkange-Zeeb F, Spallek L, Klug SJ, Zeeb H: HPV infection awareness and self-reported HPV vaccination coverage in female adolescent students in two German cities. J Comm Health. 2012, 37: 1151-1156. 10.1007/s10900-012-9589-1.
Juntasopeepun P, Suwan N, Phianmongkhol Y, Srisomboon J: Factors influencing acceptance of human papillomavirus vaccine among young female college students in Thailand. Int J Gynaecol Obstet. 2012, 118: 247-250. 10.1016/j.ijgo.2012.04.015.
Ghotbi N, Anai A: Assessment of the knowledge and attitudes of female students towards cervical cancer prevention at an international university in Japan. Asian Pac J cancer Prev. 2012, 13: 897-900. 10.7314/APJCP.2012.13.3.897.
Mosavel M, El-Shaarawi N: "I haven't heard that one": young girls' knowledge and perception of cervical cancer. J Health Comm. 2007, 12: 707-719. 10.1080/10810730701671985.
Lloyd GP, Marlow LAV, Waller J, Miles A, Wardle J: An experimental investigation of the emotional and motivational impact of HPV information in adolescents. J Adolesc Health. 2009, 45: 532-534. 10.1016/j.jadohealth.2009.06.003.
Pre-publication history
The pre-publication history for this paper can be accessed here: http://www.biomedcentral.com/1471-2458/13/264/prepub
Download references
Acknowledgements
We would like to thank Dr. Susan Jamieson for kindly arranging for us to administer the survey. We thank the Year 1 students of medicine at the University of Glasgow in 2008/9 and 2009/10 who took part in the study and the final year virology students in 2008/9 on whom the study was piloted. We thank Dr Katy Sinka, Health Protection Scotland for advice on the questionnaire design and the manuscript. We are grateful to Ms Claire Scott, Senior, Public Health and Health Improvement (Cancer), NHS, Greater Glasgow and Clyde and West of Scotland Cancer Network and Ms Isabel Gavin, National Services Division, for advice on public health issues relating to HPV vaccination. We thank Prof Heather Cubie, Dr Kate Cuschieri and Dr Becky Devine for critical reading of the manuscript. Graham and Campbell are members of the Scottish HPV Investigators Network ( http://www.shine.mvm.ed.ac.uk/index.shtml ).
Author information
Authors and affiliations.
MRC-University of Glasgow Centre for Virus Research, Institute of Infection Immunity and Inflammation, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, Scotland, G12 8TT, UK
Sarah M McCusker, Ishbel Macqueen, Graham Lough, Alasdair I MacDonald & Sheila V Graham
Centre for Population Health Sciences, The University of Edinburgh, Room 309, Doorway 1, Medical Quad, Teviot Place, Edinburgh, Scotland, EH8 9AG, UK
Christine Campbell
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Sheila V Graham .
Additional information
Competing interests.
We declare no conflict of interest.
Authors’ contributions
SMcC compared the data analytically from both cohorts and wrote the first draft of the paper. GL conducted the survey and analysed data in 2008. IM conducted the survey and analysed data in 2009. AMcD carried out the statistical analyses. CC advised on analysis and co-wrote the paper. SG supervised the study and co-wrote the paper. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
McCusker, S.M., Macqueen, I., Lough, G. et al. Gaps in detailed knowledge of human papillomavirus (HPV) and the HPV vaccine among medical students in Scotland. BMC Public Health 13 , 264 (2013). https://doi.org/10.1186/1471-2458-13-264
Download citation
Received : 11 October 2012
Accepted : 11 March 2013
Published : 22 March 2013
DOI : https://doi.org/10.1186/1471-2458-13-264
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Medical students
- Public health
BMC Public Health
ISSN: 1471-2458
- General enquiries: [email protected]
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Questions about HPV Vaccine Safety
Questions and concerns, safety monitoring for hpv vaccines.
All vaccines used in the United States are required to go through years of extensive safety testing before they are approved and licensed by the U.S. Food and Drug Administration (FDA). After vaccines are licensed, CDC and FDA continue to monitor for any rare or new problems that may happen after vaccination.
CDC uses three systems to monitor the safety of vaccines after they are licensed:
- The Vaccine Adverse Event Reporting System (VAERS)
- The Vaccine Safety Datalink (VSD)
- The Clinical Immunization Safety Assessment (CISA) Project
Learn more about the vaccine safety monitoring systems and how these systems work together to protect America’s health.
The Vaccine Adverse Event Reporting System (VAERS) is a national vaccine safety monitoring system co-managed by CDC and FDA. The system accepts reports of adverse events following vaccination from vaccine manufacturers, healthcare professionals, and the public. It serves as an early warning system to detect possible safety problems that require further evaluation; however, the safety data the system provides has limitations.
VAERS data limitations include reporting biases, inconsistent data quality and completeness, and a lack of unvaccinated comparison groups. Because of these limitations, VAERS generally cannot determine if a vaccine caused a reported adverse event. While some reported adverse events may be caused by vaccination, others may be coincidental and not related to vaccination. Learn more about VAERS and the safety data it provides .
Over 100 million doses of HPV vaccines were distributed in the United States from June 2006 through December 2017. To date, most of CDC’s HPV vaccine safety monitoring and research has focused on Gardasil because it has accounted for the majority of HPV vaccine doses distributed in the United States. These safety efforts continue, now focusing on Gardasil 9.
Among all reports to the Vaccine Adverse Event Reporting System (VAERS) following HPV vaccines, the most frequently reported symptoms overall were:
- Pain, redness, and swelling in the arm where the shot was given
Of the reports to VAERS, 6% were classified as “serious.” Of the other reports to VAERS relating to HPV, about 22% of the reports were not related to health problems, but were reported for reasons such as improper vaccine storage or the vaccine being given to someone for whom it was not recommended.
Gardasil 9 ® Gardasil 9 is currently the only HPV vaccine available in the United States. From its licensure in December 2014 through December 2017, over 28 million doses of Gardasil 9 have been distributed in the U.S. During the same period, VAERS received 7,244 U.S. reports of adverse events following Gardasil 9 vaccination. Overall, 97% of reports were classified as non-serious reports; 3% have been classified as serious.
Gardasil ® From its licensure in June 2006 through December 2017more than 80 million doses of Gardasil were distributed in the United States. During the same period, VAERS received 36,142 U.S. reports of adverse events following Gardasil vaccination. Overall, 93% were non-serious reports; 7% have been classified as serious. Gardasil is no longer available in the United States .
Cervarix ® From its licensure in October 2009 through December 2017, about 720,000 doses of Cervarix were distributed in the United States. During the same period, there have been 245 U.S. VAERS reports of adverse events following Cervarix vaccination. Overall, 96% were non-serious reports; 4% have been classified as serious. Cervarix is no longer available in the United States .
Which adverse events are considered “serious”?
By regulation, an adverse event is defined as serious if it involves any of the following outcomes:
- A life-threatening adverse event
- A persistent or significant disability or incapacity
- A congenital anomaly or birth defect
- Hospitalization, or prolongation of existing hospitalization
Learn more about adverse events .
Some deaths among people who received an HPV vaccine have been reported to the Vaccine Adverse Events Reporting System (VAERS) . This does not mean that the vaccine caused the death, only that the death occurred after the person got the vaccine. CDC and FDA investigate all reports of death following vaccination.
Gardasil 9 From December 1, 2014 through December 31, 2017, when about 28 million doses of Gardasil-9 had been distributed in the United States, VAERS received 7 reports of death. Among these reports, only 2 were verified through medical record review, autopsy reports or death certificates. The other reports were considered hearsay (based on secondhand information), meaning there was not enough information to confirm whether the death occurred.
Gardasil Also, from June 2006 through December 2017, when about 80 million doses of HPV vaccine had been distributed in the United States, VAERS received 183 reports of death after people received Gardasil. Among the 183 reports of death, CDC researchers were able to review medical records, autopsy reports or death certificates among 67 reports and verified that the person had died. The remaining 116 reports could not be further studied because there was not enough information included in the report to verify that a person had died.
After careful review of every reported death that has happened after Gardasil or Gardasil-9 vaccination, CDC concluded there was no pattern of death occurring with respect to time after vaccination, and there was no consistent vaccine dose number or combination of vaccines given among the reports. In summary, the evidence did not suggest a causal link between Gardasil and the reported deaths.
The Vaccine Safety Datalink (VSD) also conducted a study evaluating death 30 days following Gardasil. After a review of all the participating health plans’ data to identify death occurring 0-30 days after Gardasil vaccination, there were 13 deaths identified of which 9 were due to external causes such as accident, homicide, or suicide. Of the 4 remaining deaths, two were determined unrelated to vaccination and the other two did not have sufficient evidence to confirm or rule out whether Gardasil caused the death. In analyses that included comparisons of the rate of death following Gardasil in this study to national rates of death for all causes in the United States, the risk of death was not increased during the 30 days following vaccination and no deaths were found to be causally associated with vaccination after clinical review. Source: Vaccination and 30-day Mortality Risk in Children, Adolescents, and Young Adults. [Pediatrics. 2016]
Yes. When fainting (or syncope) was found to happen after vaccination, FDA changed Gardasil’s guidance for doctors to include information about preventing falls and injuries from fainting after HPV vaccination. CDC and the Advisory Committee on Immunization Practices included this guidance in the recommendations for HPV vaccination. CDC continues to remind doctors and nurses to observe this guidance and to share this information with all their patients.
General HPV Vaccine Safety
Yes. The safety of HPV vaccine has been well studied. All three HPV vaccines went through years of extensive safety testing before it was licensed by the FDA, which only licenses a vaccine if it is safe, effective, and the benefits outweigh any risks.
- Gardasil 9 was studied in more than 15,000 women and men.
- Gardasil was studied in 29,000 women and men.
- Cervarix was studied in more than 30,000 women.
After licensure, HPV vaccine safety monitoring by CDC and FDA continues to look for rare or new problems that may happen after vaccination. Since HPV vaccine became available in 2006, there have been many large safety studies conducted in the United States and other countries with reassuring findings. There have been no confirmed safety signals (i.e. higher than expected number of adverse events) observed, with the exception of syncope (fainting).
Fainting and related symptoms (such as jerking movements) can happen after any medical procedure, including vaccination. Some people, especially teens, faint after being vaccinated. To prevent fainting-related injuries, people receiving HPV vaccines should sit or lie down during vaccination, then patients should be observed for 15 minutes after receiving the shot.
Although rare, a severe allergic reaction called anaphylaxis can also occur following HPV vaccines.
CDC continues to monitor the safety of HPV vaccines and provides updates to the Advisory Committee on Immunization Practices (ACIP), as well as the World Health Organization’s Global Advisory Committee for Vaccine Safety (GACVS). See an overview of CDC’s vaccine safety publications .
Gardasil 9 is the only HPV vaccine currently available for use in the United States.
While only 9vHPV has been available for use in the U.S. since late 2016, safety studies of 4vHPV (Gardasil) have provided important safety information relevant for 9vHPV. Both 9vHPV and 4vHPV are manufactured using a similar process, and contain 4 of the same antigens: HPV types 6, 11, 16, and 18. The 9vHPV vaccine adds 5 additional antigens, providing protection against 9 types of cancer-causing human papillomaviruses. Studies of 2vHPV (Cervarix) have also shown a favorable safety profile. While the vaccines target different HPV types, all three HPV vaccines are similar and made from a single protein of the HPV virus.
Vaccines, like any medicine, can have side effects. Some people who get an HPV vaccine have no side effects at all. Some people report having mild side effects, like a sore arm from the shot for a day or two. The most common side effects are usually mild and go away on their own.
Common side effects of HPV vaccine:
- Pain, redness, or swelling in the arm where the shot was given
- Headache or feeling tired
- Muscle or joint pain
Some people should not get HPV vaccine (Gardasil 9) or should wait.
- Anyone who has ever had a life-threatening allergic reaction to any component of HPV vaccine, or to a previous dose of HPV vaccine, should not get the vaccine. Anyone with severe allergies, including an allergy to yeast, should talk to their doctor before getting the vaccine.
- HPV vaccine is not recommended for pregnant women. However, receiving HPV vaccine when pregnant is not cause for alarm. Women who are breastfeeding may get the vaccine.
- People who are mildly ill (low-grade fever of less than 101 degrees, a cold, runny nose, or cough) when a dose of HPV vaccine is planned can still be vaccinated. People with a moderate or severe illness should wait until they are better.
If you or your child has a severe allergic reaction or other health emergency, call 9-1-1 or go to the nearest hospital.
Look for any signs or symptoms that concern you, such as signs of a severe allergic reaction, very high fever, or behavior changes. Signs of a severe allergic reaction can include hives, swelling of the face and throat, difficulty breathing, a fast heartbeat, dizziness, and weakness. These would start a few minutes to a few hours after the shot is given.
After seeing a doctor, the reaction should be reported to the Vaccine Adverse Event Reporting System (VAERS) . This system is used to report any side effect or adverse event following vaccination. Your doctor can file this report, or you can do it yourself through the VAERS website or by calling 1-800-822-7967.
Reproductive Concerns
CDC is aware of public concern about the safety of HPV vaccine. Since the vaccine’s introduction in 2006, vaccine safety monitoring and studies conducted by CDC, FDA, and other organizations have documented a reassuring safety record. There is no current evidence that HPV vaccines cause reproductive problems in women.
What is primary ovarian insufficiency (POI)?
Also known as “premature menopause,” this is a condition in which a woman’s ovaries stop functioning before age 40. Causes of primary ovarian insufficiency include:
- Chemicals in the environment
- Cancer treatments
- Cigarette smoking
- Autoimmune disorders
- Some viral infections
However, in many cases it’s not possible to determine the cause. CDC and FDA have not found any proof that HPV vaccines cause POI.
How has CDC and FDA addressed the concern of HPV vaccines and POI?
As part of ongoing safety monitoring of HPV vaccines, CDC has reviewed reports of POI to VAERS following both Gardasil 9 and Gardasil vaccination. CDC has also conducted additional safety research on HPV vaccine in the VSD.
Gardasil 9 Between December 1, 2014 and Dec 31, 2017, when 28 million doses of Gardasil 9 had been distributed in the United States, VAERS received 3 reports of POI following Gardasil 9 vaccination. The 3 reports were determined to be hearsay reports (based on secondhand information), meaning there was not enough information to confirm a diagnosis of POI.
Gardasil Between January 2009 and December 2015, more than 60 million doses of Gardasil were distributed for use in the United States. During this time period, VAERS received 17 reports of POI following Gardasil vaccination. Two of these reports had a physician diagnosis of POI; the remaining 15 reports were considered hearsay reports (based on secondhand information), meaning there was not enough information to confirm the diagnosis. Source: Post-licensure safety monitoring of quadrivalent human papillomavirus vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2009-2015. [Vaccine. 2018]
FDA and CDC reviewed the confirmed POI reports, investigating whether or not there was a pattern that might indicate the vaccine was causing the problem. There were no patterns found, making it unlikely the vaccine was the cause.
CDC’s Clinical Immunization Safety Assessment Project (CISA) also reviewed current literature regarding HPV vaccines and POI. The review found that the current evidences is insufficient to suggest or support that HPV vaccinations cause POI. Source: Primary Ovarian Insufficiency and Human Papilloma Virus Vaccines: A Review of the Current Evidence. [Am J Obstet Gynecol. 2020]
To examine this issue further, CDC conducted a VSD study of reports of POI following adolescent vaccination, including HPV vaccination among girls 9-26 years of age. Among 199,078 girls followed, only one confirmed case of POI was identified where the patient received HPV vaccine. This patient received HPV vaccine 23 months before her first clinical evaluation of having a delayed first period. Overall, the study found no increased risk of POI following HPV vaccination or any adolescent vaccination. Source: Primary Ovarian Insufficiency and Adolescent Vaccination. [Pediatrics. 2018]
CDC and FDA continue to closely monitor the safety of HPV vaccines.
HPV vaccination prevents infection with the HPV types that most commonly cause cervical cancer. In some cases, women develop cervical cancer before starting or finish having children. Treatment for cervical cancer (removal of the cervix and uterus, chemotherapy, and/or radiation) can keep a woman from being able to become pregnant. Preventing cervical cancer through HPV vaccination reduces this risk.
CDC works closely with the American Academy of Pediatrics (AAP) and the American Academy of Family Physicians (AAFP) on HPV vaccination. Both organizations have information about HPV vaccine available on their websites. Also, the American College of Obstetrics and Gynecologists (ACOG) offers additional resources.
AAP HPV Vaccine Tools and Resources
AAFP HPV Vaccine Summary
ACOG HPV Guidelines and Recommendations
HPV vaccines are not approved or recommended for pregnant women. However, some pregnant women receive HPV vaccines because they don’t know that they are pregnant at the time of vaccination, or otherwise receive an HPV vaccine when they shouldn’t have.
CDC and vaccine manufacturers have monitored and studied HPV vaccine safety in women who received the vaccine when they were pregnant. The manufacturers for each vaccine have established pregnancy registries to follow outcomes for those women who were mistakenly vaccinated. Close monitoring has not found any health concerns. If a woman receives HPV vaccine and later learns that she is pregnant, there is no reason to be alarmed.
Any woman who learns she was pregnant at the time she received an HPV vaccine is encouraged to contact the vaccine manufacturer. This will help us learn how pregnant women respond to the vaccine.
- If a pregnant woman receives a dose of Gardasil 9 no intervention is needed. If she found out she was pregnant when starting the vaccination series, the remainder of the 3-dose series should be delayed until completion of pregnancy. She may contact Merck at 1-877-888-4231 if she has questions related to getting the vaccine while pregnant.
- Doctors should report Gardasil 9 vaccination during pregnancy as early in the pregnancy as possible using the Merck Pregnancy Registries or calling 1-800-986-8999.
- Pregnant women who received Gardasil 9 and their physician can also report to the Vaccine Adverse Event Reporting System (VAERS).
As part of ongoing safety monitoring of HPV vaccines, CDC has monitored pregnancy outcomes for women who were mistakenly given HPV vaccines during their pregnancies. Both VAERS and the VSD have supported these efforts:
From December 2014 through December 2017, when approximately 28 million doses of Gardasil-9 were distributed in the United States, VAERS received 82 reports of pregnant women vaccinated with Gardasil 9; 60 reports did not describe an adverse event and were submitted only to report the occurrence. Of those reporting an adverse event, the most frequently reported were miscarriage and injection site reactions (three reports each; 3.7%). The observed number of VAERS reports of miscarriages were not considered to be unusual as miscarriage may occur in up to 1/3 of all pregnancies. Injection site reactions after vaccination, such as soreness or swelling where the shot was given, are the most common side effects. They are usually mild and go away on their own. Overall, researchers found no unexpected patterns of adverse event reporting. Source: Safety of 9-valent human papillomavirus vaccine administration among pregnant women: Adverse events reported in the Vaccine Adverse Event Reporting System (VAERS), 2014-2017. [Vaccine. 2019]
In a review of reports to VAERS between June 2006 and December 2013, there were 147 reports of Gardasil administered to pregnant women. There were no unexpected patterns of adverse events in developing babies, nor were there any reported maternal or infant deaths. The most frequent adverse event reported was fever. Source: Safety of quadrivalent human papillomavirus vaccine (Gardasil) in pregnancy: adverse events among non-manufacturer reports in the Vaccine Adverse Event Reporting System, 2006-2013. [Vaccine, 2015]
VSD conducted a study evaluating the risk of miscarriage after inadvertent Gardasil vaccination of pregnant women. Between 2008 and 2014, 2,800 pregnancies with Gardasil exposure were identified. Researchers found that inadvertent Gardasil exposure before and during pregnancy was not significantly associated with an increased risk of miscarriage. Source: Risk of Spontaneous Abortion After Inadvertent Human Papillomavirus Vaccination in Pregnancy. [Obstet Gynecol. 2018]
VSD also conducted a study looking at maternal and infant outcomes following Gardasil vaccination of women who were mistakenly vaccinated while pregnant. Between 2007 and 2013, 720 women received Gardasil at some point two weeks before their last menstrual cycle or two weeks after, and 638 women were mistakenly given Gardasil while pregnant. Administration of Gardasil during pregnancy or right before becoming pregnant was not associated with adverse pregnancy or birth outcomes. Source: Maternal and Infant Outcomes After Human Papillomavirus Vaccination in the Periconceptional Period or During Pregnancy. [Obstet Gynecol 2017]
Other Medical Concerns
Guillain-Barré syndrome (GBS) is a rare disorder where a person’s own immune system damages nerve cells, causing muscle weakness and sometimes paralysis. Most people recover fully from GBS, but some experience long-term nerve damage.
The Vaccine Adverse Event Reporting System (VAERS) continually monitor reports of GBS following Gardasil 9. Between December 1, 2014 and December 31, 2017, when approximately 28 million doses of Gardasil 9 had been given out in the United States, there were 4 confirmed reports of GBS. Source: Safety monitoring of 9-valent human papillomavirus vaccine in the Vaccine Adverse Event Reporting System (VAERS). [Pediatrics. 2019]
The Vaccine Safety Datalink (VSD) also monitored for GBS following Gardasil 9 vaccination. Between October 2015 through October 2017, in VSD nearly 840,000 doses of Gardasil 9 were administered and researchers did not find a safety signal of GBS after HPV vaccination. Source: Near Real-Time Surveillance to Assess the Safety of the 9-valent Human Papillomavirus Vaccine. [Pediatrics. 2019]
Gardasil VSD conducted monitoring for GBS following Gardasil vaccination from August 2006 to December 31, 2015. During this time, 2,773,185 doses of Gardasil were administered to males and females aged 9-26 years. Using medical records to confirm cases of GBS, there was 1 GBS case in a male. The study provides evidence that the risk of getting GBS following HPV vaccination is extremely rare. Source: Risk of Guillain-Barré Syndrome following quadrivalent human papillomavirus vaccine in the Vaccine Safety Datalink. [Vaccine. 2017]
POTS is a condition that causes lightheadedness or fainting and a rapid increase in heartbeat upon standing. The cause is unknown, but doctors think POTS may be associated with a number of risk factors and syndromes, including recent viral illness, head trauma, physical deconditioning, and nervous system problems.
Gardasil 9, Gardasil, and Cervarix From June 2006 through August 2015, more than 80 million HPV vaccine doses were distributed in the United States. During this time period, VAERS received 29 reports of POTS following HPV vaccination. Of these reports, 28 followed Gardasil vaccination, which accounted for the majority of HPV vaccinations in the United States during this time period. CDC’s safety review did not detect any unusual or unexpected patterns among the cases. Source: Reports of Postural Orthostatic Tachycardia Syndrome After Human Papillomavirus Vaccination in the Vaccine Adverse Event Reporting System. [J Adolesc Health. 2017]
In November 2015, the European Medicine’s Agency completed a detailed review of available POTS data from young women who received HPV vaccines. The review found that the evidence does not support a causal link between HPV vaccines and POTS. Source: Review concludes evidence does not support that HPV vaccines cause CRPS or POTS. [European Medicines Agency. 2015] [PDF – 2 pages]
Ongoing safety monitoring through VAERS has not detected any safety concerns related to POTS following HPV vaccination.
VAERS has also conducted a formal review of POTS following Gardasil 9 vaccination. Between December 1, 2014 and December 31, 2017, when over 28 million doses of Gardasil 9 had been given out in the United States, VAERS received 17 reports of POTS. Among those, 6 reports partially met diagnostic criteria of POTS.
The Vaccine Safety Datalink (VSD) is currently conducting a study to describe POTS among 9 to 30 year-olds who received any adolescent vaccination.
Some people who get the HPV vaccine may have some pain in the arm where the shot was given. Usually this pain is mild and goes away quickly. Swelling and redness also sometimes occur after HPV vaccination.
CDC is aware of reports (in Japan and elsewhere) of chronic pain following HPV vaccines. Some of these reports were described as potential cases of Complex Regional Pain Syndrome (CRPS), a rare condition of persistent pain that usually affects arms, legs, hands, or feet after an injury or trauma to that limb.
Gardasil 9, Gardasil, and Cervarix
CDC reviewed reports to VAERS of CRPS following Gardasil 9 vaccination between December 1, 2014 and December 31, 2017. Of the 7,244 reports following HPV vaccination, there was 1 report of CRPS; however, because of incomplete information, the report could only be classified as “possible CRPS.”
A safety review of reports to VAERS from June 2006 through July 2015 identified 22 reports of CRPS following HPV vaccination. At that time, over 67 million doses of HPV vaccine had been distributed in the United States. The findings included 21 reports of CRPS following Gardasil vaccination and 1 report following Cervarix vaccination. The review concluded that CRPS following HPV vaccination is rare. Source: HPV Vaccination and Complex Regional Pain Syndrome: Lack of Evidence. [EBioMedicine. 2015]
In November 2015, the European Medicines Agency completed a detailed review of available data on CRPS in young women who received HPV vaccines. The review found that the evidence does not support a causal link between HPV vaccines and CRPS. Source: Review concludes evidence does not support that HPV vaccines cause CRPS or POTS. [European Medicines Agency. 2015][PDF – 2 pages}
The Vaccine Safety Datalink (VSD) is currently conducting a study to describe CRPS among 9 to 30 year-olds who received any adolescent vaccination.
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a disabling and complex illness. People with ME/CFS have overwhelming fatigue that is not improved by rest. While researchers have not yet identified a cause of ME/CFS, some symptoms can be treated or managed. CDC is aware of reports of chronic fatigue syndrome following HPV vaccines and continues to monitor for any unusual or unexpected patterns among reported cases.
The Vaccine Safety Datalink (VSD) is currently conducting a study to describe CFS among 9-36 year-olds who received any vaccination.
Gardasil In a review of reports to the Vaccine Adverse Event Reporting System (VAERS) between June 2006 and September 2015, there were 20 reports of ME/CFS following Gardasil vaccination. At that time, over 80 million doses of Gardasil had been distributed in the United States. No unusual or unexpected patterns of reporting of CFS following HPV vaccine were detected.
In addition, a 2017 study published by the Norwegian Institute of Public Health observed no increased risk of ME/CFS among girls given HPV vaccine through the Norwegian national immunization program between 2009 and 2014. Source: HPV vaccination and risk of chronic fatigue syndrome/myalgic encephalomyelitis: A nationwide register-based study from Norway. [Vaccine. 2017]
- Human Papillomavirus (HPV) Learn more about this common virus that can lead to certain types of cancer later in life, and how you can protect against it.
- Who should not get this vaccine? Some people should not get certain vaccines or should wait before getting them. Read the CDC guidelines for each vaccine.
- HPV Vaccine - Safety Information Safety information on HPV vaccine, including safety studies, common side effects, vaccine information statements, and more.
- Frequently Asked Questions About Vaccine Safety and Children Find the answers to questions about the safety of vaccines for children.
To receive email updates about this page, enter your email address:
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Supplements
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
Understanding Human Papillomavirus (HPV) Infection Symptoms
- HPV Infection Symptoms
- Common Symptoms
- HPV and Cervical Cancer
- Other Complications
- Next in HPV Guide How HPV Is Diagnosed
Human papillomavirus (HPV) is a sexually transmitted infection (STI). In the United States, HPV affects about 40% of people aged 15-59. It's so common that just about all sexually active people get it at some point.
In as many as nine cases out of 10, HPV doesn't cause health issues. And it usually clears up on its own within two years. But in cases where it doesn't, it can lead to genital warts, cancer, and other health problems.
This article discusses symptoms and complications of HPV, as well as how to prevent getting or spreading it.
Mypurgatoryyears / Getty Images
Understanding HPV Infection Symptoms
It's relatively common to have HPV and not know it. Most of the time, the immune system can clear the virus without symptoms or complications. HPV is a group of more than 200 viruses. Symptoms vary, depending on the type of HPV, but can include warts that may itch or cause other problems.
The virus can only survive in squamous epithelial cells on the surface of the skin and moist surfaces (mucosal surfaces) found in and around the:
- Vulva , vagina, cervix
- Inner foreskin and urethra of the penis
- Inner lining of the nose, mouth, and throat
- Windpipe and bronchi
- Inner eyelids
HPV spreads through sexual contact . There's currently no routine screening for HPV in men since there's no approved test. Women can get routine Pap testing to check for cervical changes, including early cervical cancer . HPV testing can take place along with your Pap test. There's also an effective HPV vaccine generally recommended for people between the ages of 11 and 26.
Common Symptoms of HPV Infection
The most common thing is to have no symptoms at all. Certain low-risk HPV infections can cause a variety of warts . Viral warts are benign , but they can be a nuisance. Warts often resolve on their own within one to five years.
Genital Warts
This photo contains content that some people may find graphic or disturbing.
Reproduced with permission from © DermNet and © Te Whatu Ora dermnetnz.org 2023.
Genital warts can be small or large and raised or flat. They can be dome-shaped or resemble small cauliflowers. Genital warts can appear on the:
Genital warts sometimes cause itching. Depending on their location, they can cause pain when you move your bowels or when you have sex.
Common Warts
Dmitry Epov / Getty Images
Common warts ( verruca vulgaris ) grow on the fingers, around the nails, and on the backs of the hands. The bumps are rough and may have black dots that look like seeds. They can range from the size of a pinhead to the size of a pea.
Plantar Warts
Alena Ivochkina / Getty Images
Plantar warts appear on the soles of the feet. They're hard and can grow rather large. They sometimes have black dots and appear in clusters (mosaic warts). Since feet carry the weight of your whole body, plantar warts push inward instead of growing outward like other types of warts, which can cause tenderness and pain.
Reproduced with permission from © DermNet dermnetnz.org 2023.
Flat warts are smooth, slightly raised, and small, usually just millimeters wide. These warts can grow anywhere but tend to appear on the face, especially the forehead and cheeks. Men may get these in the beard area, and women may get them on their legs. Flat warts typically appear in multiples. You can get 20 to 100 at a time.
HPV Infections and Cervical Cancer
Some low-risk strains of HPV may lead to abnormal changes in the cells on the surface of the cervix ( cervical dysplasia ) that are not precancerous. However, research suggests that HPV causes about 99% of all cases of cervical cancer.
Cervical cancer happens when the immune system can't clear high-risk strains of HPV. Over time, these cells can turn precancerous , then cancerous. Routine Pap screening can allow healthcare providers to detect and remove precancerous cells before they become cancerous. And HPV vaccines can prevent the types of HPV most likely to cause cervical cancer.
Other Possible HPV Complications
HPV types 16 and 18 cause most cancers related to HPV, including cancer of the cervix, vagina, vulva, penis, and anus. Some strains can also lead to cancer of the throat, tongue, and tonsils ( oropharyngeal cancer ). Research suggests that HPV causes about 65% of vaginal cancers , 50% of vulvar cancers , 45%-90% of oropharyngeal cancers, and 90% of anal cancers .
HPV is also associated with some oral benign proliferative (rapid growth) and malignant (cancerous) lesions of the head, neck, middle part of the throat (oropharynx), tonsils, and tongue.
A Word From Verywell
Given the safety and efficacy of the HPV vaccine, it is important to consider vaccinating your children to protect them from developing certain cancers later in life.
How to Prevent an HPV Infection
HPV can be transmitted through skin-to-skin sexual contact as well as vaginal, oral, or anal sex. Since there are so many types of HPV, you can get infected multiple times or get more than one strain at a time.
HPV vaccines are highly effective in protecting against seven types of HPV that are most likely to cause cancer and two low-risk types that cause most genital warts.
Infections with types that cause most HPV genital warts and cancers dropped 88% among teen girls and 81% among adult women after being vaccinated. And among women who have been vaccinated, cervical pre-cancers most often linked to cervical cancer have dropped by 40%.
The best time to get vaccinated is between 9 and 26. If you haven't had one by then, you've likely already been exposed to HPV. However, if you're older than 26 and haven't been vaccinated, you can speak with your healthcare provider about the potential benefits of getting vaccinated now. Vaccines won't help if you're already infected.
It's less effective than the vaccine, but you can lower your risk of getting HPV by using a condom or dental dam during vaginal, oral, and anal sex.
HPV is the most common STI. It spreads so easily that just about every sexually active person gets it at some point. Most of the time, the immune system can clear the infection without symptoms or complications. However, some low-risk strains can cause troublesome warts. High-risk strains that don't clear up can lead to cervical cancer or other types of cancer in females and males.
HPV vaccines effectively prevent the strains most likely to cause warts and cancer. There are no HPV tests for men, but women can get tested along with a Pap test. Speak to a healthcare provider if you think you may have HPV or have screening questions.
Lewis RM, Laprise JF, Gargano JW, et al. Estimated prevalence and incidence of disease-associated HPV types among 15–59-year-olds in the United States . Sex Transm Dis . 2021;48(4):273-277. doi:10.1097/OLQ.0000000000001356
Centers for Disease Control and Prevention. Human papillomavirus (HPV) statistics .
Centers for Disease Control and Prevention. Genital HPV infection - Basic fact sheet .
National Cancer Institute. HPV and cancer .
American Cancer Society. HPV and HPV testing .
Centers for Disease Control and Prevention. HPV and men - fact sheet .
Centers for Disease Control and Prevention. HPV vaccine .
MedlinePlus. HPV .
Okunade KS. Human papillomavirus and cervical cancer . J Obstet Gynaecol . 2020;40(5):602-608. doi:10.1080/01443615.2019.1634030
American Academy of Dermatology Association. Genital warts: Overview .
American Academy of Dermatology Association. Warts: Signs and symptoms .
American Podiatric Medical Association. What are warts?
NYU Langone Health. Types of human papillomavirus .
Petca A, Borislavschi A, Zvanca ME, et al. Non-sexual HPV transmission and role of vaccination for a better future (Review) . Exp Ther Med. 2020;20(6):186. doi:10.3892/etm.2020.9316
Mravak-Stipetić M, Sabol I, Kranjčić J, Knežević M, Grce M. Human papillomavirus in the lesions of the oral mucosa according to topography . PLOS ONE. 2013;8(7):e69736. doi:10.1371/journal.pone.0069736
Children's Hospital of Philadelphia. Human papillomavirus (HPV).
U.S. Department of Health and Human Services. Office on Women's Health. Pap and HPV tests.
U.S. National Library of Medicine. Warts: overview .
By Ann Pietrangelo Pietrangelo is a health writer who has authored two books: one focused on multiple sclerosis and the other on triple-negative breast cancer.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.177(5); 2007 Aug 28

Human papillomavirus, vaccines and women's health: questions and cautions
The federal government's recently announced $300 million investment toward a program for vaccinating girls and women with the currently available human papillomavirus (HPV) vaccine Gardasil, framed by some as a way to prevent cervical cancer in Canada, has generally been welcomed by a wide range of commentators. However, although HPV infection is necessary for the development of cervical cancer and the vaccine may prevent primary infection with HPV types 16 and 18 (currently thought to be the cause of about 70% of cervical cancer cases 1 ), we propose that these facts be assessed within a broad context before immunization policies are implemented. A careful review of the literature, including that submitted by the manufacturer with its application for approval of Gardasil, reveals a sufficient number of unanswered questions to lead us to conclude that a universal immunization program aimed at girls and women in Canada is, at this time, premature and could possibly have unintended negative consequences for individuals and for society as a whole.
In this article we summarize some of the main questions and concerns that need to be addressed before there is a full-scale rollout of an HPV vaccination program (for supplementary material go to www.cwhn.ca/resources/cwhn/hpv-brief.html ). These closely reflect issues raised in the analytical framework created by Erickson and colleagues 2 in the context of the development of the National Immunization Strategy and support efforts to ensure a comprehensive and systematic evaluation of all relevant factors before decisions regarding the implementation of a new immunization program are made. As well, they echo some of the research questions identified as important in the final report from the Canadian Human Papillomavirus Vaccine Research Priorities Workshop, held in Quebec City in 2005. 3 We hope raising these questions now will contribute to the deliberations necessary to ensure a responsible and transparent evidence-based decision-making process.
General questions and cautions
• There is no epidemic of cervical cancer in Canada to warrant the sense of urgency for a vaccination program initiated by the federal finance minister's announcement. According to 2006 Canadian cancer statistics, 4 cervical cancer is the 11th most frequent cancer affecting Canadian women and the 13th most common cause of cancer-related deaths, accounting for approximately 400 deaths per year. Both the incidence and mortality of cervical cancer have been declining in Canada, as in other resource-rich countries, although recently at a somewhat slower rate than has been observed in previous decades. 5 However, the incidence and mortality still vary between different groups of women, being notably higher among Aboriginal women than among non-Aboriginal women.
• Invasive cervical cancer typically follows a slowly progressive course that can be halted at one of various stages. The dramatic decrease in deaths from cervical cancer in Canada, even before the development of any vaccine, represents a public health success ( Figure 1 ). Research attributes this to improved reproductive health practices and the widespread availability of publicly funded programs for Papanicolaou smear testing. 6 In fact, the public funding of such programs has also significantly reduced health inequities among women. 6 Consequently, deaths from cervical cancer — relatively rare in Canada but always unfortunate and not distributed evenly among women — must be considered as a failure in the adequate support of both the primary care and reproductive health services that would guarantee healthy living conditions for all women. Improvements here, as well as steps to ensure that all women receive appropriate Pap testing and follow-up, are needed.

Figure 1: Avoidable mortality (age-standardized expected years of life lost per 100 000 people) due to cervical cancer in Canada from 1971 to 1996, by income quintile. Reproduced, with permission, from James et al. 6 Copyright © 2007, BMJ Publishing Group Ltd.
• Most HPV infections are cleared spontaneously. Recent research using available molecular detection technologies has suggested that clearance occurs within 1 year for about 70% of infected women, and within 2 years for 90%. 7 Thus, HPV infection and cervical cancer must not be conflated: cervical cancer will not develop in most women who are infected with even a high-risk strain of HPV. 8 Unfortunately, there are no data on clearance rates among girls, nor even about the actual HPV prevalence rates among youth and children, yet this is critical information for developing, and subsequently evaluating, policy proposals.
• The nature of a vaccination program is necessarily dependent on the definition of clear and tangible goals. To date, such goals have not been made explicit with regard to a Canadian initiative. Is the aim of the vaccination program the eradication of high-risk HPV types from the population? Or is it to reduce the number of deaths from cervical cancer? These different goals require different strategies. For example, pathogen eradication would imply a herd-immunity goal, thus possibly necessitating the vaccination of boys and young men. In contrast, the reduction of deaths from cervical cancer would suggest the need for a vaccine directed against more than the 2 high-risk HPV types in Gardasil, which may account for only somewhat more than two-thirds of cervical cancer cases.
• Information about the efficacy of Gardasil remains uncertain. Its real-world effectiveness is even less clear. To date, only a handful of randomized controlled trials of sufficient quality to qualify for systematic review have been reported. 9 Interestingly, each of the reported HPV vaccine trials, whether of Gardasil or its potential competitor Cervarix, was funded in whole or in part by the vaccine's manufacturer. Although Rambout and colleagues, 9 in their systematic review (see page 469), find that overall the vaccine is highly efficacious in the short term, particularly when all clinical outcomes are pooled, they also note that some methodologic weaknesses in the trial reports, combined with the limits in currently available data, continue to leave many information gaps. This situation is not unusual at this juncture in the development of new pharmaceutical products; however, it does caution against making overly optimistic descriptions of benefits and downplaying potential risks.
• We would add a number of questions to those raised by Rambout and colleagues. Specifically, what is the length of immunologic protection the vaccine confers against HPV types 16 and 18? Will boosters be needed to maintain this limited coverage, and if so, when? Other questions with regard to effectiveness centre on concerns about the possibility of short-term immunity altering the natural history of viral infection, as seems to be the situation with chicken pox: protection has been of shorter duration than expected, and viral infections in older people have been more severe than those in children. 10
• Furthermore, we lack data on the effectiveness of the HPV vaccine when co-administered with other immunizations, as may occur in real practice. As well, will such factors as a person's nourishment, smoking status and general health (e.g., comorbidities) influence the safety or usefulness of the HPV vaccine? Perhaps more importantly, might misunderstandings about what the vaccine does and does not do lead to reductions in safer sex practices and Pap screening rates? These are among the questions raised at the Research Priorities Workshop in Quebec City in November 2005, and they remain pertinent — and unanswered.
• Relatively few girls (about 1200 aged 9–15 years) were enrolled in the clinical trials of Gardasil, the youngest of whom were followed for only 18 months. 11 Based on the assumption that they will not yet have been exposed to HPV viruses, girls in this age group represent the priority target population for mass vaccination. Clearly, this is a thin information base on which to construct a policy of mass vaccination for all girls aged 9–13, as per the National Advisory Committee on Immunization's recommendations. 1
• Gardasil is the most expensive childhood vaccine proposed for mass use; it currently costs $404 for the 3 required doses. Yet, the cost-effectiveness analyses of proposed vaccination programs needed to evaluate this expense are missing. The lack of effectiveness data makes it difficult to estimate what reduction in repeat testing or colposcopy can be anticipated to counter some of the vaccination costs and precludes determining whether vaccination will have any “added value.” Girls and women, even if vaccinated, will still need to practise safer sex and have access to existing care programs for Pap testing as well as for other reproductive health care. In similar need of analysis are possible lost-opportunity costs and assessments of the impact on other health care priorities of devoting limited resources to HPV vaccination programs.
General recommendations
We propose a number of general recommendations that should be considered before a universal HPV vaccination program is developed and implemented ( Box 1 ).

To be clear, if and when evidence shows that an HPV vaccination program can be successfully implemented in Canada, it must be publicly funded. Lack of financial resources must not preclude any girl or woman from receiving what has been sanctioned by health officials. However, concern about how public funds are used to promote and protect the health of girls and women must consider broader issues, such as the needs of the marginalized and most vulnerable groups in society. Government support for HPV vaccinations must not perpetuate existing health inequities. Instead, such programs ought to reduce health inequities through thoughtful, comprehensive, evidence-based approaches that permit those most at risk to benefit.
To promote and protect women's health most effectively, and to work toward the prevention of deaths from cervical cancer in Canada, we should not focus only on a universal HPV vaccination program at this time when there is an urgent need for prompt and clear answers to the many questions outlined in this article.
Gardasil represents the first of what will likely be many vaccines targeting high-risk HPV strains, and how we proceed now will set a precedent for others. The foundation of a successful vaccination program must be solid, evidence-based research, and we now have the exciting opportunity to complete this work and develop a model for current and future HPV vaccination programs with clearly defined and measurable health outcomes. We must be certain that spending an estimated $2 billion to vaccinate a population of girls and women in Canada who are already mostly well protected by their own immune systems, safer sex practices and existing screening programs will not perpetuate the existing gaps in care and leave the actual rate of deaths from cervical cancer unchanged. Worse would be the emergence of iatrogenic effects, such as an increase in cervical cancer rates, if a false sense of security led girls and women to stop having regular Pap screening and to view vaccination as a simple fix.
In developing a model HPV vaccination program, governments should start by educating the public about the reality of cervical cancer, HPV infection and vaccinations, to quell anxieties about cervical cancer and HPV and to emphasize the importance of healthy personal practices, including use of barrier methods, good nutrition, smoking cessation and regular Pap smears and screening for sexually transmitted infections. As well, federal, provincial and territorial policies for reproductive health care should be reviewed, including an assessment of the place of any vaccination program within existing services for the prevention and management of cervical cancer.
The latter will require a definition of the goals of any potential mass vaccination program. If the aim is cervical cancer reduction, then the possibility of favouring safe and effective vaccines that cover a broad range of high-risk viral strains should be considered. If the objective is to eliminate HPV infections, then data on how to include boys and men as well as girls and women, and how to manage newly identified oncogenic HPV types within an immunization program, are essential. Head-to-head comparisons of different vaccines carried out in unbiased research programs free of conflict of interest will be most useful here to obtain data for evidence-based policy and health care decision-making.
Canada already has thoughtful and useful frameworks for developing vaccination and cancer prevention policies. Their use in amassing and evaluating the scientific (molecular, epidemiologic, immunologic) and social evidence related to HPV vaccines, and for assessing potential benefits and harms expected from widespread immunization with the HPV vaccine, is urgent before governments allocate huge sums of already limited health care dollars to such programs. It is time to take a breath and reflect on what we know and what we don't know, and to develop a plan based on solid, reliable evidence that adds value for everyone. Individual girls and women, as well as policy-makers, can make truly informed decisions about vaccinations only when they have all the evidence, and today, there are more questions than answers.
@ See related articles pages 456, 462, 464, 469 and 480
Acknowledgments
We thank Judy Norsigian, Robin Barnett and Hans Krueger, as well as members of Women and Health Protection, for their thoughtful contributions.
Published at www.cmaj.ca on Aug. 1, 2007. Revised Aug. 3, 2007.
This article has been peer reviewed.
All of the authors are members of the Canadian Women's Health Network Writing Group and have collaborated, and shared texts, with these and other individuals and groups during the development of this material. Women and Health Protection and the Canadian Women's Health Network are both supported by the Women's Health Contribution Program, Health Canada. The opinions expressed in this article are those of the authors and not necessarily those of Health Canada.
Competing interests: None declared.
Correspondence to : Dr. Abby Lippman, Department of Epidemiology, Biostatistics, and Occupational Health, McGill University, 1020 av. des Pins Ouest, Montréal QC H3A 1A2; fax 514 398-4503; [email protected]
- MUSC Health MyChart
- Schedule an Appointment
- Adult Patient Care
- Children's Health
- Education at MUSC
- Research at MUSC
- Latest News
- Pancreatic Cancer
Research study at Hollings points to new combination strategy for pancreatic cancer

Pancreatic ductal adenocarcinoma (PDAC) is the most common form of pancreatic cancer and is projected to become the second-leading cause of cancer-related death by 2030. Although progress has been made in improving outcomes, the five-year survival rate remains stubbornly low at just 13%.
Now, researchers at MUSC Hollings Cancer Center have identified a promising therapeutic strategy. Published in Cell Death and Differentiation , the study describes a new role of HSP70, or heat shock protein 70, and suggests that simultaneously blocking HSP70 and a survival mechanism called autophagy may reduce the growth of pancreatic cancer. This is the inaugural publication for the Barnoud Lab, headed by Tim Barnoud, Ph.D ., who joined Hollings in late 2021. Co-first authors on the paper are Colleen Quaas, Ph.D., and Giulia Ferretti, Ph.D., who have both been awarded fellowships by Hollings. Ferretti is a current HCC Abney Fellow while Quaas has completed her T32 ITOS fellowship and is headed a few blocks north to a faculty position at The Citadel. Barnoud said he’s grateful for Hollings’ commitment to educating the next generation of scientists by funding these fellowships. Not only do they provide opportunities for young researchers, but by funding salary support, they enable his lab to direct more funds toward the research. Quaas pointed out that this investigation required a significant amount of research effort and collaboration from everyone in the lab as well as other labs at Hollings. “A lot of techniques were involved, and one of the benefits of the fellowship is that we were able to have the funds to use a lot of really cutting-edge techniques – such as live cell video-microscopy to study mitochondrial dynamics in real-time – and to perform all of the preclinical efficacy studies of our inhibitors in mouse models. And we’re fortunate that we have an environment at Hollings where we are all collaborative and work really well together,” she said.
Why is HSP70 important in cancer?
Cells utilize heat shock proteins (HSPs) especially when they are under stress. These HSPs act as “chaperones,” ensuring that other proteins are folded correctly in order to do their jobs when facing a stressful environment. Barnoud explained that cancer cells, because they grow and divide at abnormally fast rates, are in a constant state of stress, which includes instances where they have limited oxygen and nutrients necessary to grow. Because of this, they produce significantly more HSP70 than normal cells. Researchers’ attention was drawn to HSP70 as a therapeutic target, although there were still unknowns about everything it was doing in cancer cells. During his postdoctoral fellowship at The Wistar Institute in Philadelphia, Barnoud found that there was an especially large amount of HSP70 in the mitochondria of tumor cells, including pancreatic cancer cells. Mitochondria, the “powerhouse” of the cell, supplies the energy that cells need to live. However, HSP70 wasn’t showing up in the mitochondria of normal cells. “This was quite surprising. But these findings led us to ask a simple question: What is HSP70 doing in the mitochondria of cancer cells?” Barnoud said. At his lab at Hollings, Barnoud set out to find out. Mitochondrial dynamics, a process that regulates the size, shape and position of mitochondria within cells, has been implicated in pancreatic cancer progression and metastasis. The mechanisms that regulate mitochondrial dynamics still aren’t fully understood, however. Barnoud’s lab showed that inhibiting HSP70 with a small-molecule inhibitor impaired the function of a protein called DRP1, which is critical for mitochondrial health and integrity. The buildup of compromised mitochondria in turn can lead to the death of cancer cells. However, another consequence of HSP70 inhibition is the buildup of reactive oxygen species and oxidative stress in the mitochondria, which can activate a critical metabolic sensor in cells known as AMPK. In turn, AMPK activation triggers a survival mechanism known as autophagy, which cancer cells often use to combat a variety of stresses, including chemotherapy. “Autophagy is an interesting way for pancreatic cancer cells to survive, in that cells essentially undergo ‘self-eating’ to obtain critical nutrients needed for important biological processes,” Barnoud said. “What was fascinating is that blocking HSP70 made this self-eating process go into over-drive in order for the pancreatic cancer cells to survive the stress we were throwing at them,” Ferretti said. The team showed that blocking autophagy improved the efficacy of HSP70 inhibition and slowed the growth of pancreatic tumors in mice. As with other cancers, research is showing that a multi-faceted intervention is more effective. “The exciting part of our story is that there is an FDA-approved drug that can block autophagy. Now, more potent autophagy-specific inhibitors are currently in clinical trials for pancreatic cancer,” Barnoud said. “The long-term goal of the lab is to advance the first small molecule HSP70 inhibitor to the clinic, which we hope will be beneficial in the fight against pancreatic cancer but perhaps also for other cancers that are ‘addicted’ to HSP70.”
TB was supported by NIH NCI R00 CA241367. Portions of the study were also performed with support from the MUSC Digestive Disease Research Core Center (P30 DK123704) and the MUSC COBRE in Digestive and Liver Disease Animal Models Core and the Imaging Core. Additional funding was awarded in the form of a pilot project to TB from the MUSC COBRE in Digestive and Liver Disease (P20 GM130457). GDSF was supported by the MUSC Hollings Cancer Center (MUSC) Postdoctoral Fellowship Program and CEQ was supported by NIH NCI T32 CA193201. JR-B was supported by NIH NINDS K01 NS119351, a Rally Foundation Career Development Award (20CDN46), a V Foundation Scholar Award (V2022-008), and a Vince Lombardi Cancer Foundation Grant. AAD was supported by NIH K01 CA245231 and ACS PF-1818301-TBG. GAH was supported by a 2022 Pancreatic Cancer Action Network Career Development Award in memory of Skip Viragh (22-20-HOBB), a 2022 Concern Foundation Career Development Award, and by NIGMS P20 GM130457. JPO is supported in part by a Merit Review Award (1I01BX002095) from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service and by NIH awards (R01 CA212608, P30 CA138313, and P20 GM130457). JMS was supported by NIH awards S10 OD030245 and P30 CA010815. DFK was supported by NIH 1U54 CA274499-01-9941. OS was supported, in part, by NIH R01 CA251374 and NIH R01 CA267101. Support for the MUSC Core Facilities used in this study was provided in part by the MUSC Hollings Cancer Center Support Grant P30 CA138313, including support by the Biorepository & Tissue Analysis Shared Resource and the Cell & Molecular Imaging Shared Resource. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the U.S. Department of Veterans Affairs, not the United States government. Open access funding provided by the Carolinas Consortium.

PurpleStride
The annual PurpleStride walk raises money for pancreatic cancer research at Hollings Cancer Center.

Overcoming Drug Resistance
Breast cancer often develops resistance to previously effective drugs. Hollings researchers showed how the effectiveness of one drug might be restored.
Improving Cancer Immunotherapy
Hollings researchers use patented technology to improve immunotherapy by prolonging survival of cancer-fighting T-cells.
Related Links
More MUSC Hollings Cancer Center news
Subscribe to our newsletter
Hollings Horizons magazine
About the Author
Staff Report
Categories: Cancer , Research

IMAGES
VIDEO
COMMENTS
To investigate this question, Gypsyamber D'Souza, professor in the Department of Epidemiology at the Johns Hopkins Bloomberg School of Public Health, and Carole Fakhry, professor in the Department of Otolaryngology-Head and Neck Surgery, and their colleagues evaluated oral HPV infection over time among men and women who have HIV and those at elevated risk of contracting it, in two national ...
Gynecologist and researcher Rebecca Perkins, MD, is on a mission to make human papillomavirus ( HPV) vaccinations as common as any other childhood vaccine - especially in low-income and minority communities. With funding from an American Cancer Society grant, Perkins serves as lead investigator of a pilot educational program designed to ...
HPV-16, HPV-52, and HPV-53 were the most common high-risk HPV types found among FSWs . Since there are no definite antiviral treatments for HPV, the high prevalence of genital HPV has been a great concern in the world . Another research evaluating 645 sexually active innercity young females reported a cervical HPV prevalence of 54% ...
Human papilloma virus (HPV) is an infectious agent belonging to the virus family Papillomaviridae, members of which have tropism for cutaneous epithelium and mucosal epithelium. There are more ...
Introduction. Human papillomavirus (HPV) is a common sexually transmitted virus and infects a majority of women during their lifetime [].The virus is classified into high-risk and low-risk types based on oncogenic potential [].In the vast majority of cases, the infection is cleared without symptoms, but in some women, infection with high-risk HPV becomes persistent and causes precancerous ...
Finally, immune therapy holds great promise for the treatment of existing HPV positive lesions including oropharyngeal lesions and further efforts in this area need to be encouraged (see outstanding questions). We feel that investigation of these areas is of high importance for HPV research in the next decade.
The potential merit of this study is that HPV-related practical questions submitted by online visitors were collected. Thus, we could determine what people really want to know about HPV and its related diseases. To our knowledge, our study is the first to systematically investigate the type and frequency of such questions.
Knowledge and awareness about HPV and its vaccine were measured using 13 questions with one point for each question. The overall median knowledge score was 2 (IQR = 5) in the whole population.
Human papillomaviruses (HPVs) are a diverse group of viruses that replicate in specific anatomical niches of the stratified epithelia. Most HPVs cause asymptomatic infections, some cause benign ...
HPV type would receive the full benefit of vaccination. Those who already have been infected with one or more HPV types would still get protection from the vaccine types they have not acquired. HPV vaccine can be given to people who have had an abnormal Pap test or genital warts. However, the vaccine is not a treatment
HPV infects epithelial cells that line mucosal surfaces of the body. When HPV enters these cells, such as in the throat, genital tract or anus, it causes the cells to produce HPV proteins. In most cases, the immune system recognizes the cells that are infected and eliminates them, clearing the infection.
Module 5 groupwork. For this small group discussion, you will develop a research question to investigate HPV. You will then determine whether you would use an observational or experimental design to investigate the research question, and you will support your rationale.
Background Human papillomavirus (HPV) infection is recognized as one of the major causes of infection-related cancer worldwide. In Spain, the HPV vaccination program started in 2007 and until 2022, it targeted 12-year-old girls. Methods This was a cross-sectional, multicenter survey-based research carried out at 24 pediatric offices to describe HPV knowledge and vaccine acceptability in ...
Aliya Klein was a 2018 summer Cancer Research Training Awardee at the Center for Global Health. In this blog, she documents how she used World RePORT, an open-access and interactive database of international research projects, grants, and collaborations dedicated to improving health, to answer questions about HPV-related research funding.. Each year, more than half-a-million women around the ...
The proposed HTA will address the following research questions. For the purposes of this review, the diagnostic efficacy of primary HPV testing as a primary screening tool for cervical cancer includes evidence regarding the diagnostic test accuracy and clinical utility (including safety and other clinical outcomes) of that screening strategy. Details on the specific interventions and outcomes ...
Objective: In 2004, we launched the question and answer (Q&A) section on a human papillomavirus (HPV) website (www.hpvkorea.org) that provides ample and regularly updated information about HPV. The purpose of this study is to collect data pertaining to questions posed on this website about HPV and its related diseases and analyze the type of questions and frequency before and after ...
Human papillomavirus (HPV) vaccination is a well-established and successful tool for preventing HPV-related cancers. However, vaccine uptake remains low, influenced by patient hesitancy around safety concerns and little opportunity to discuss the vaccine with trusted healthcare providers. We conducted a national, cross-sectional study of allopathic and osteopathic medical students regarding ...
We provide research-tested messages that providers can use to address parents' HPV vaccination questions and concerns about 7 common topics. Important principles for increasing message effectiveness are to include information on the benefits of vaccination (including cancer prevention) and avoid expressing urgency to vaccinate when addressing ...
Background A vaccination programme targeted against human papillomavirus (HPV) types16 and 18 was introduced in the UK in 2008, with the aim of decreasing incidence of cervical disease. Vaccine roll out to 12-13 year old girls with a catch-up programme for girls aged up to 17 years and 364 days was accompanied by a very comprehensive public health information (PHI) campaign which described ...
Some people should not get certain vaccines or should wait before getting them. Read the CDC guidelines for each vaccine. Safety information on HPV vaccine, including safety studies, common side effects, vaccine information statements, and more. Find the answers to questions about the safety of vaccines for children.
This WHO and HRP guideline is designed to help countries make faster progress, more equitably, on the screening and treatment of cervical cancer. This document includes guidance on an important additional option for cervical screening, the use of mRNA (messenger RNA) HPV testing.
Targeted advertising using social networking sites, like Facebook, is rapidly increasing to recruit research participants [38, 39]. For a HPV vaccine effectiveness study in Australia, it was shown to be a rapid and cost-effective way of recruiting . Inspired by the study we decided to use a similar strategy.
Research suggests that HPV causes about 65% of vaginal cancers, 50% of vulvar cancers, 45%-90% of oropharyngeal cancers, and 90% of anal cancers. HPV is also associated with some oral benign proliferative (rapid growth) and malignant (cancerous) lesions of the head, neck, middle part of the throat (oropharynx), tonsils, and tongue.
This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Question: Create a research question to investigate HPV. Explain whether you would use an observational or experimental design to investigate the research question. Be sure to justify your choice.
General questions and cautions. • There is no epidemic of cervical cancer in Canada to warrant the sense of urgency for a vaccination program initiated by the federal finance minister's announcement. According to 2006 Canadian cancer statistics, 4 cervical cancer is the 11th most frequent cancer affecting Canadian women and the 13th most ...
Not only do they provide opportunities for young researchers, but by funding salary support, they enable his lab to direct more funds toward the research. Quaas pointed out that this investigation required a significant amount of research effort and collaboration from everyone in the lab as well as other labs at Hollings.