Assessment of Body Composition in Athletes: A Narrative Review of Available Methods with Special Reference to Quantitative and Qualitative Bioimpedance Analysis
Affiliations.
- 1 Department for Life Quality Studies, Università degli Studi di Bologna, 47921 Rimini, Italy.
- 2 Department of Biomedical and Neuromotor Sciences, Università degli Studi di Bologna, 40126 Bologna, Italy.
- 3 School of Sport and Exercise Sciences, Faculty of Psychology, "eCampus" University, 22060 Novedrate, Italy.
- 4 Department of Physical Education, São Paulo State University, Presidente Prudente 19060-900, SP, Brazil.
- 5 Department of Biomedical Sciences for Health, Università degli Studi di Milano, 20133 Milan, Italy.
- PMID: 34065984
- PMCID: PMC8150618
- DOI: 10.3390/nu13051620
Body composition is acknowledged as a determinant of athletic health and performance. Its assessment is crucial in evaluating the efficiency of a diet or aspects related to the nutritional status of the athlete. Despite the methods traditionally used to assess body composition, bioelectric impedance analysis (BIA) and bioelectric impedance vector analysis (BIVA) have recently gained attention in sports, as well as in a research context. Only until recently have specific regression equations and reference tolerance ellipses for athletes become available, while specific recommendations for measurement procedures still remain scarce. Therefore, the present narrative review summarizes the current literature regarding body composition analysis, with a special focus on BIA and BIVA. The use of specific technologies and sampling frequencies is described, and recommendations for the assessment of body composition in athletes are provided. Additionally, the estimation of body composition parameters (i.e., quantitative analysis) and the interpretation of the raw bioelectrical data (i.e., qualitative analysis) are examined, highlighting the innovations now available in athletes. Lastly, it should be noted that, up until 2020, the use of BIA and BIVA in athletes failed to provide accurate results due to unspecific equations and references; however, new perspectives are now unfolding for researchers and practitioners. In light of this, BIA and especially BIVA can be utilized to monitor the nutritional status and the seasonal changes in body composition in athletes, as well as provide accurate within- and between-athlete comparisons.
Keywords: BIVA; bioelectric impedance vector analysis; hydration; localized BIA; nutritional status; phase angle; segmental bioimpedance; tolerance ellipses.

Publication types
- Body Composition*
- Electric Impedance*
- Sports Medicine / methods
- Open access
- Published: 03 May 2024
Discriminating factors of body composition characteristics for academic performance in nursing college students: a cross-sectional study
- Andrew Ke-Ming Lu 1 , 2 na1 ,
- Shi-Yen Tsai 3 na1 ,
- Ching-Yi Lin 4 &
- Jeng-Long Hsieh 1 , 2
BMC Nursing volume 23 , Article number: 305 ( 2024 ) Cite this article
247 Accesses
Metrics details
Poor body composition may affect health status, and better body composition is often associated with better academic performance. Nursing students face heavy academic and practical pressures, and the relationship between body composition and academic performance in this group is not fully understood.
This cross-sectional observational study used de-identified student data from a university of technology in southern Taiwan to analyze the correlation between body composition characteristics and academic performance using regression models.
A total of 275 nursing college students were divided into four groups according to academic performance. The group with the lowest academic performance had a lower percentage of body fat ( P < 0.05) but a higher percentage of muscle mass ( P < 0.05) than the other three groups. Academic performance was positively correlated with percentage of body fat ( R = 0.16, P < 0.01) and body age ( R = 0.41, P < 0.01), but was negatively correlated with percentage of muscle mass ( R = − 0.16, P < 0.01). Percentage of body fat, visceral fat area, and body age were significant discriminators of academic performance ( P < 0.05).
Conclusions
The relationship between academic performance and body composition among nursing college students is not straightforward. Contrary to our initial hypothesis, students with higher academic performance tended to have a higher percentage of body fat and a lower percentage of muscle mass. Percentage of body fat, visceral fat area, and body age were significant discriminators of academic performance, indicating that body composition should be considered an important factor in nursing education and practice.
Peer Review reports
Introduction
Body composition is a term that refers to the relative proportions of different tissues in the body, including fat, muscle, water, and others [ 1 ]. Maintaining a healthy body composition is essential for overall good health [ 2 ]. Excessive body fat has been linked to many health problems, such as cardiovascular disease, high blood pressure, and diabetes [ 3 ]. Visceral fat, also known as intra-abdominal fat or deep abdominal fat, surrounds the organs and is stored within the abdominal cavity [ 4 ]. High levels of visceral fat are associated with an increased risk for several health problems [ 5 ]. Measuring and monitoring visceral fat area can be a useful tool in assessing health risks associated with excess visceral fat [ 6 ]. In contrast, greater muscle mass can increase the body’s metabolic rate and energy expenditure, which increases caloric burn [ 7 ], and may improve balance and stability, preventing accidental injuries such as falls and fractures [ 8 ]. A large-scale epidemiological study of body composition among Taiwanese participants found that Taiwanese people have a relatively lower body mass index (BMI) but a higher percentage of body fat than Caucasians [ 9 ]. There are also studies indicating that high obesity and BMI among young people are associated with increased mortality rates [ 10 ]. In summary, proper management of body fat and muscle mass can help prevent many health issues and promote overall health.
Several factors may affect body composition characteristics among nursing students. First, nursing students often face rigorous coursework, practice, and other study-related pressures that can lead to physical and mental fatigue [ 11 ]. Fatigued individuals may experience a lack of motivation to engage in physical activity [ 12 ], which in turn leads to weight gain and changes in body composition. Second, students may spend extended periods of time sitting in class or in front of computers to study [ 13 ], which leads to poor posture and decreased muscle mass [ 14 ]. Third, the lack of exercise due to time pressure from undertaking studies and internships may contribute to an unhealthy body composition [ 15 ]. Regular exercise is important for maintaining muscle mass, burning calories, and reducing the risk of chronic diseases [ 16 ]. The lack of exercise can lead to muscle wasting, decreased metabolism, and increased body fat [ 17 ]. Fourth, one study demonstrated that almost a third of nursing students had poor sleep habits [ 18 ]. Abnormal sleep patterns have also been linked to a decrease in lean muscle mass and an increase in body fat, which negatively affects body composition [ 19 ].
Higher BMI and body fat levels have been linked to lower academic achievements in children and adolescents [ 20 ], whereas healthy body composition has been associated with higher academic performance [ 21 ]. Similarly, overweight and obese students among adolescents aged 12–17 years have been found to achieve lower grades and experience increased missed days due to illness [ 22 ]. Furthermore, a higher percentage of body fat has been associated with lower cognitive performance and reduced memory recall abilities, which may negatively impact academic performance [ 23 ]. On the other hand, a lower percentage of muscle mass has been linked to poor cognitive function [ 24 ]. Overall, body composition impacts physical fitness, energy levels, and overall health, which in turn influence academic performance [ 25 ]. Therefore, maintaining a healthy body composition through proper nutrition and regular physical activity is likely to positively affect academic performance [ 26 ].
We were interested in exploring whether there is a correlation between students’ health by measuring body composition (including factors such as percentage of body fat, muscle mass, etc.) and their academic performance. The rationale of the study is relevant to the nursing profession, given that this study involved students in a college of nursing, one may be particularly interested in understanding the relationship between physical health as reflected in body composition and their academic success and potentially future performance as health care professionals. Further, provide educational strategies and intervention measures. Identifying discriminating factors in body composition that may influence academic performance can inform the development of targeted interventions or educational strategies. For example, if certain body composition characteristics are found to be associated with better academic performance, educational programs can be designed to promote and support these characteristics. Our hypothesis that maintaining a healthy body composition would lead to better academic performance. By examining this relationship, we aimed to provide nursing educators with a deeper understanding of the health status of their students and to offer health promotion advice to optimize academic performance. The broader goal of our research was to promote the academic and professional success of nursing students by improving their overall health and well-being.
Research design
We performed a cross-sectional study to investigate the association between body composition characteristics, academic performance, conduct grade, and sick leave among nursing college students. This study was correlational in that it evaluated the relationship between variables, and cross-sectional in that it collected and analyzed data from several variables at a specific point in time. Academic performance was regarded as the criterion variable, and body composition characteristics were used as discriminators.
Data collection
The research data for this study were obtained from a medical technology university in southern Taiwan. Body composition data were obtained from the Healthcare Information Technology Education Center at the College of Nursing. Body composition data is a service provided by the College of Nursing for students to monitor their own health status. There will be a fixed time for students to do monitoring every semester. Student academic grades, conduct grades, and sick leaves records were obtained from the Office of Institutional Research database. The student’s academic grades are the average score of all academic subjects in the semester; the conduct grades are based on the basic score of 85 out of 100 set by the school, with bonus and subtraction points based on absences, rewards and punishment records; the sick leave records are the total hours of periods of leave due to illness in the semester. Students signed a data use consent form upon enrollment. All data were de-identified and approved by the Institutional Review Board (IRB). Body composition and academic data were linked by extracting academic grades, conduct grades, and sick leaves records corresponding to the semester when body composition was measured. Our data concatenation is performed through the number generated after de-identified. The research data were collected from July 2020 to July 2022 and included nursing college students aged 18 to 25 who were enrolled including the second year of the four-year system, the first year of the two-year system, and the fourth year of the five-year system. Although the systems are different, the nursing students targeted are all in the classroom learning stage and face the similar request from school work. The four-year bachelor’s degree program is designed for students who have completed senior high school; the five-year associate degree program (junior college) is designed for students who have completed junior high school; the two-year bachelor’s degree program is designed for students who have completed associate degree program. There are different ways to enter the nursing field in Taiwan. All course designs include classroom learning and clinical internships to cultivate talents in nursing and related medical fields. This study included 275 nursing students. To mitigate potential research biases, this study has implemented exclusion criteria targeting specific conditions within the dataset. These criteria include age restrictions, with participants under 18 and over 25 years old being excluded. Additionally, individuals with significant medical issues, such as cancer, severe movement disorders, and mental illnesses, have been excluded from the study cohort. The average age of these 275 students (203 females, 72 males) was 19.26 ± 1.94 years, the average height was 162.33 ± 8.67 cm, the average weight was 59.01 ± 13.81 kg, and the average BMI was 22.31 ± 4.48.
Body composition measurements
The Body Composition Analyzer (ACCUNIQ BC710, Selvas Healthcare, Republic of Korea) uses the bioelectrical resistance method to measure and analyze the body fat and non-fat content of the body trunk and limbs. This instrument can accurately measure body fat, water content, muscle, and bone weight, which is used to understand the health status. In addition to evaluating overall body composition, the instrument also analyzes the condition of individual body parts and provides complete evaluation results for the left/right arm, left/right leg, waist/hip ratio, upper/lower limb balance, and left/right limb balance. Body age is determined by taking into account factors such as weight, percentage of body fat, and skeletal muscle percentage. This calculation produces a reference point to evaluate whether your body age is higher or lower than your chronological age. During measurement, participants wore light clothes, removed metal objects in contact with the body, maintained the correct posture when being measured, and avoided speech and movement. We analyzed BMI, percentage of body fat (%), visceral fat area (cm 2 ), percentage of muscle mass (%), and body age.
Statistical analysis
We present continuous variables as mean ± standard error and categorical variables as proportions. Spearman rank correlation was used to investigate the relationship between variables. We classified all students into four groups based on their academic performance: Group A (academic performance ≥ 80), Group B (academic performance ≥ 70 and < 80), Group C (academic performance ≥ 60 and < 70), and Group D (academic performance < 60). Rationale for dividing the students into four groups was based on GPA grading levels at our schools. We performed ANOVA to compare the mean difference in body composition between the groups and performed multiple comparisons using a post-hoc t -test. Linear regression analysis was used to explore the relationship between body composition characteristics and academic performance. In addition, we used ordinal logistic regression, and computed odds ratios and their corresponding 95% confidence intervals, to explore the potential association between body composition and academic performance. The significance level was set at 95% ( P < 0.05). Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
The demographic characteristics are shown in Table 1 . We included a total of 275 students in the study. All 275 students were systematically categorized into distinct groups, denoted as Group A, Group B, Group C, and Group D, based on their academic performance falling into the respective ranges of > 80, 70–79, 60–69, and < 60 out of 100. Group A includes 112 females and 24 males, with an average age of 20.02 ± 1.7 years; Group B includes 67 females and 23 males, with an average age of 18.69 ± 1.95 years; Group C includes 19 females and 18 males, with an average age of 17.95 ± 1.78 years; and Group D includes 5 females and 7 males, with an average age of 18.92 ± 1.08 years. The majority of the participants of this study were female students, and Group A was slightly older than Groups B, C, and D. Regarding academic performance, the average scores for Groups A through D were 85.37 ± 3.42, 75.25 ± 3.09, 65.72 ± 2.57, and 53.3 ± 6.1 out of 100, respectively. In terms of conduct grade, the average scores for Groups A through D were 86.6 ± 3.3, 84.68 ± 5.64, 78.04 ± 8.38, and 70.5 ± 9.74 out of 100, respectively. The four groups differed in academic performance and conduct grades, ranked in descending order from group A–D. The average numbers of sick leaves during the semester for Groups A through D were 2.87 ± 6.98, 6.86 ± 11.51, 21 ± 27.76, and 53 ± 54.54 h, respectively. Both Group C and Group D had significantly higher rates of sick leave than Group A and Group B.
The body composition characteristics of nursing college students are shown in Table 2 . For Groups A through D, the body mass index (BMI) values were 22.24 ± 4.39, 22.19 ± 4.08, 23.49 ± 5.52, and 20.3 ± 4.39 kg/m², respectively. The percentage of body fat for these groups was 23.93 ± 7.48, 23.5 ± 7.18, 22.32 ± 9.49, and 16.32 ± 9.36%, respectively. Visceral fat area values for Groups A through D were 40.9 ± 27.03, 33.89 ± 31.15, 37.32 ± 42.3, and 39.08 ± 32.53 cm², respectively. Percentage of muscle mass values were 70.3 ± 7.41, 70.74 ± 7.13, 71.92 ± 9.4, and 77.87 ± 9.3%, respectively. Additionally, the body age for Groups A through D was 20 ± 2.01, 18.7 ± 2.06, 17.81 ± 1.52, and 18.92 ± 1.08, respectively. We compared BMI, percentage of body fat, visceral fat area, percentage of muscle mass, and body age between groups, and found that Group D had significantly lower percentage of body fat than Groups A, B, and C, and significantly higher percentage of muscle mass than Groups A, B, and C. However, Group A had a significantly higher body age than Groups B and C.
We then performed correlation analyses between body composition and academic performance, conduct grades, and amount of sick leave (Table 3 ). Academic performance was positively correlated with conduct grades ( R = 0.58, P < 0.01) and negatively correlated with the amount of sick leave ( R = − 0.53, P < 0.01). Conduct grades were also negatively correlated with the amount of sick leave ( R = − 0.60, P < 0.01). Notably, academic performance was positively correlated with percentage of body fat ( R = 0.16, P < 0.01) and with body age ( R = 0.41, P < 0.01), but was negatively correlated with percentage of muscle mass ( R = − 0.16, P < 0.01). Similarly, conduct grades were positively correlated with percentage of body fat ( R = 0.16, P < 0.01) and with body age ( R = 0.16, P < 0.01), but were negatively correlated with percentage of muscle mass ( R = − 0.16, P < 0.01).
The results of the univariate linear regression analysis performed to identify factors influencing academic performance are shown in Table 4 . The adjusted R² is 25.44%. The regression model revealed that percentage of body fat (β = 0.19, P = 0.01) and body age (β = 1.85, P < 0.01) were significantly and positively associated with academic performance, while percentage of muscle mass was significantly negatively associated with academic performance (β = − 0.20, P < 0.01).
We performed a multivariate stepwise linear regression analysis with academic performance as the dependent variable. Based on the results of univariate analyses, we included BMI, percentage of body fat, visceral fat area, and body age as independent variables. The final equation included percentage of body fat ( P < 0.01), visceral fat area ( P = 0.01), and body age ( P < 0.01) as significant discriminators. The detailed results are given in Table 5 .
Furthermore, we used multiple ordinal regression analysis to examine the factors associated with academic performance among nursing college students (Table 6 ). BMI, percentage of body fat, visceral fat area, and body age were included as independent variables, and academic performance as the dependent variable. Our analysis revealed that percentage of body fat ( P < 0.01), visceral fat area ( P = 0.03), and body age ( P < 0.01) were significant discriminators of academic performance.
Our study results contradicted our hypothesis that nursing college students with better academic performance would have better body composition. Rather we, found that academic performance was positively correlated with percentage of body fat and negatively correlated with percentage of muscle mass. Furthermore, we found that percentage of body fat, visceral fat area, and body age had significant discriminatory ability on academic performance. We also found that academic performance was positively correlated with conduct grades and negatively correlated with the amount of sick leave.
One study demonstrates a trade-off between academic performance and physical health outcomes. Students who reported spending more time studying have been reported to have worse sleep habits [ 27 ], which may affect body composition. Additionally, study time is positively correlated with body fat, and weight gain has been linked to increased academic load [ 28 , 29 ]. However, the results of our study contradict those of others that show a positive correlation between academic performance and physical health outcomes [ 30 ]. For example, a study found that higher academic performance was associated with better health-related behaviors and lower obesity rates [ 31 ]. Other studies found a correlation between healthy weight and improved academic performance [ 32 ], and observed that non-obese teens have higher average academic performance than obese teens [ 33 ]. These discrepancies may arise from differences between populations, countries, educational systems, and cultures studied. In particular, our results may be partially explained by our study population, which consisted of nursing college students who face heavy academic and internship pressures. Over 80% of nurses in Taiwan graduate from technical colleges (Universities of Technology and Junior Colleges), and our research may thus represent the health condition of nursing students in Taiwan’s technical education system only.
The present study has uncovered notable associations between body composition metrics, namely percentage of body fat, visceral fat area, and body age, and academic performance among nursing students. Surprisingly, our findings reveal that nursing students exhibiting superior academic performance tend to possess higher percentage of body fat, increased visceral fat area, and an older body age. In seeking to interpret this phenomenon, it is postulated that nursing students experience elevated levels of academic rigor, coupled with substantial physical and psychological stress throughout their educational journey. Notably, high-achieving students often dedicate extensive time to scholastic endeavors, potentially impacting their overall body composition. The pervasive academic demands on nursing students, including rigorous coursework and demanding clinical experiences, may necessitate extended periods of focused study. This heightened commitment to academic pursuits, in turn, could contribute to the observed correlations with body composition metrics. The intricate interplay between academic achievement and physiological parameters suggests a complex relationship, warranting nuanced consideration. It is imperative to approach these findings with a degree of caution and maintain an open-minded perspective. The individuality inherent to each student, encompassing unique learning styles and lifestyle patterns, introduces a multitude of variables that may influence body composition outcomes. Consequently, while our study provides valuable insights into potential associations between body composition and academic performance among nursing students, further research is warranted to unravel the intricate dynamics underlying these correlations. A comprehensive exploration of diverse factors, encompassing lifestyle choices, stress management, and socio-cultural influences, is essential to elucidate the multifaceted nature of this intriguing relationship.
The relationship between physical health or body composition and academic performance may have potential implications for the field of education, several comprehensive approaches can be applied to student well-being as follows. (1) Comprehensive wellness programs and health education courses: These programs can incorporate physical activity plans, nutrition education, stress management, sleep hygiene and mental health support to educate students on the importance of maintaining a healthy lifestyle. (2) Physical Education and Active Learning: Reinforcing the importance of physical education in schools and ensuring students have regular opportunities for physical activity. Consideration should also be given to incorporating active learning strategies into the classroom. (3) Support for Healthy Nutrition: Providing healthy and nutritious food choices in school cafeterias to help students maintain optimal physical health. (4) Physical and Mental Health Services: Offering on-site physical and mental health services within educational institutions. Early identification and intervention for health problems can have a positive impact on academic outcomes. (5) Research and Ongoing Evaluation: Encouraging ongoing research to further understand the subtle links between physical health and academic performance. This holistic perspective supports the development of well-rounded individuals who will be able to meet their cognitive, emotional, and physical needs throughout their educational journey.
Our study found that Taiwanese nursing students with better academic performance intended to have a high body fat rate, low muscle mass rate, high visceral fat area and high body age. We believe those may be due to the following reasons: culture and eating habits, Taiwan’s dietary habits may include foods high in fat and sugar, which may affect body composition; Taiwan’s nursing academic environment is competitive, and nursing students may experience high academic pressure, resulting in ignoring normal diet and essential nutrition; Furthermore, the lifestyle of nursing students may be relatively busy, and they may not be able to maintain regular physical activities and exercise during the semester. All of which may lead to higher physical age, a decrease in muscle mass rate, accumulation of visceral fat as well as high body fat rate.
Understanding the body composition and academic performance of nursing students has significant clinical implications and applications, particularly in nursing education and practice. First, the body composition of nursing students can provide valuable health information, including percentage of body fat and percentage of muscle mass. These data can be used to assess health risks such as obesity and muscle weakness, and provide guidance on nursing education and lifestyle advice. Additionally, such data can be used to design nutrition and exercise programs for nursing students. Second, knowledge of the body composition of nursing students can inform nursing practice, such as assessing students’ physical load and work capacity in practical nursing work. Finally, body composition data can be used for risk assessment in actual work environments, including that of stress, fatigue, and work pressure. We will investigate the relationship between factors such as mental health indicators, exercise, drinking, and smoking habits, and other multivariable factors, on student performance. These findings will provide the guideline for the improvement of nursing education and nursing practice.
Limitations and conclusions
Our study is subject to some limitations. First, causality cannot be determined from a cross-sectional study, and future cohort studies are therefore needed to confirm our findings. Second, many factors influence academic performance, including psychological factors, learning environment, study habits, and personal traits. Accordingly, our study can only partially explain the relationship between body composition and academic performance of nursing students. Third, the nursing college students enrolled in the present study are predominantly female, and gender differences may need to be further discussed.
In conclusion, our study found that the relationship between academic performance and body composition among nursing college students is not straightforward. Contrary to the initial hypothesis, students with higher academic performance had a higher percentage of body fat and a lower percentage of muscle mass. Additionally, the present study found that percentage of body fat, visceral fat area, and body age are significant discriminators of academic performance among nursing college students. These findings suggest that body composition should be considered an important factor in nursing education and nursing practice. Finally, the study also found that academic performance was positively correlated with conduct grades and negatively correlated with the amount of sick leave, which highlights the importance of health and wellness in academic success.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Holmes CJ, Racette SB. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: an overview of current methodology. Nutrients 2021, 13(8).
Hernández-Reyes A, Cámara-Martos F, Molina-Luque R, Romero-Saldaña M, Molina-Recio G, Moreno-Rojas R. Changes in body composition with a hypocaloric diet combined with sedentary, moderate and high-intense physical activity: a randomized controlled trial. BMC Womens Health. 2019;19(1):167.
Article PubMed PubMed Central Google Scholar
Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and Cardiovascular diseases. Circ Res. 2020;126(11):1477–500.
Article PubMed Google Scholar
Frank AP, de Souza Santos R, Palmer BF, Clegg DJ. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J Lipid Res. 2019;60(10):1710–9.
Article CAS PubMed Google Scholar
Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8(7):616–27.
Kihara S, Matsuzawa Y. Fat distribution and Cardiovascular Disease Risk. Curr Cardiovasc Risk Rep. 2015;9(3):8.
Article Google Scholar
Marcus JB. Chap. 10 - Weight Management: Finding the Healthy Balance: Practical Applications for Nutrition, Food Science and Culinary Professionals. In: Culinary Nutrition edn. Edited by Marcus JB. San Diego: Academic Press; 2013: 431–473.
Papalia GF, Papalia R, Diaz Balzani LA, Torre G, Zampogna B, Vasta S, Fossati C, Alifano AM, Denaro V. The effects of Physical Exercise on Balance and Prevention of Falls in Older people: a systematic review and Meta-analysis. J Clin Med 2020, 9(8).
Chang CJ, Wu CH, Chang CS, Yao WJ, Yang YC, Wu JS, Lu FH. Low body mass index but high percent body fat in Taiwanese subjects: implications of obesity cutoffs. Int J Obes Relat Metab Disord. 2003;27(2):253–9.
Allison DB, Zhu SK, Plankey M, Faith MS, Heo M. Differential associations of body mass index and adiposity with all-cause mortality among men in the first and second National Health and Nutrition Examination Surveys (NHANES I and NHANES II) follow-up studies. Int J Obes Relat Metab Disord. 2002;26(3):410–6.
Valero-Chillerón MJ, González-Chordá VM, López-Peña N, Cervera-Gasch Á, Suárez-Alcázar MP, Mena-Tudela D. Burnout syndrome in nursing students: an observational study. Nurse Educ Today. 2019;76:38–43.
Schultchen D, Reichenberger J, Mittl T, Weh TRM, Smyth JM, Blechert J, Pollatos O. Bidirectional relationship of stress and affect with physical activity and healthy eating. Br J Health Psychol. 2019;24(2):315–33.
Caromano F, Amorim C, Rebelo C, Contesin A, Favero F, Costa J, Kawai M, Voos M. Prolonged sitting and physical discomfort in university students. Acta Fisiátrica. 2015;22:176–80.
Park JH, Moon JH, Kim HJ, Kong MH, Oh YH. Sedentary lifestyle: overview of updated evidence of potential health risks. Korean J Fam Med. 2020;41(6):365–73.
Kljajević V, Stanković M, Đorđević D, Trkulja-Petković D, Jovanović R, Plazibat K, Oršolić M, Čurić M, Sporiš G. Physical activity and physical fitness among University Students-A Systematic Review. Int J Environ Res Public Health 2021, 19(1).
Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2(2):1143–211.
Bowden Davies KA, Pickles S, Sprung VS, Kemp GJ, Alam U, Moore DR, Tahrani AA, Cuthbertson DJ. Reduced physical activity in young and older adults: metabolic and musculoskeletal implications. Ther Adv Endocrinol Metab. 2019;10:2042018819888824.
Gallego-Gómez JI, González-Moro MTR, González-Moro JMR, Vera-Catalán T, Balanza S, Simonelli-Muñoz AJ, Rivera-Caravaca JM. Relationship between sleep habits and academic performance in university nursing students. BMC Nurs. 2021;20(1):100.
Stich FM, Huwiler S, D’Hulst G, Lustenberger C. The potential role of Sleep in promoting a healthy body composition: underlying mechanisms determining muscle, Fat, and bone Mass and their Association with Sleep. Neuroendocrinology. 2022;112(7):673–701.
Martin A, Booth JN, McGeown S, Niven A, Sproule J, Saunders DH, Reilly JJ. Longitudinal associations between childhood obesity and academic achievement: systematic review with focus group data. Curr Obes Rep. 2017;6:297–313.
Shi Y, Yu H, Di S, Ma C. Body Mass Index and academic achievement among Chinese secondary school students: the mediating effect of Inhibitory Control and the moderating effect of Social Support. Front Psychol. 2022;13:835171.
Pan L, Sherry B, Park S, Blanck HM. The association of obesity and school absenteeism attributed to illness or injury among adolescents in the United States, 2009. J Adolesc Health. 2013;52(1):64–9.
Anand SS, Friedrich MG, Lee DS, Awadalla P, Després JP, Desai D, de Souza RJ, Dummer T, Parraga G, Larose E, et al. Evaluation of adiposity and cognitive function in adults. JAMA Netw Open. 2022;5(2):e2146324.
Sui SX, Williams LJ, Holloway-Kew KL, Hyde NK, Pasco JA. Skeletal Muscle Health and cognitive function: a narrative review. Int J Mol Sci 2020, 22(1).
Donnelly JE, Hillman CH, Castelli D, Etnier JL, Lee S, Tomporowski P, Lambourne K, Szabo-Reed AN. Physical activity, fitness, cognitive function, and academic achievement in children: a systematic review. Med Sci Sports Exerc. 2016;48(6):1197–222.
Hermassi S, Hayes LD, Sanal-Hayes NEM, Schwesig R. Differences in Health-Related Physical Fitness and Academic School Performance in Male Middle-School students in Qatar: a preliminary study. Front Psychol. 2022;13:791337.
Vik FN, Nilsen T, Øverby NC. Associations between sleep deficit and academic achievement - triangulation across time and subject domains among students and teachers in TIMSS in Norway. BMC Public Health. 2022;22(1):1790.
Calestine J, Bopp M, Bopp CM, Papalia Z. College Student Work habits are related to physical activity and fitness. Int J Exerc Sci. 2017;10(7):1009–17.
PubMed PubMed Central Google Scholar
Economos CD, Hildebrandt ML, Hyatt RR. College freshman stress and weight change: differences by gender. Am J Health Behav. 2008;32(1):16–25.
Hraste M, De Giorgio A, Jelaska PM, Padulo J, Granić I. When mathematics meets physical activity in the school-aged child: the effect of an integrated motor and cognitive approach to learning geometry. PLoS ONE. 2018;13(8):e0196024.
Flueckiger L, Lieb R, Meyer AH, Mata J. How health behaviors relate to academic performance via affect: an intensive longitudinal study. PLoS ONE. 2014;9(10):e111080.
Anderson AS, Good DJ. Increased body weight affects academic performance in university students. Prev Med Rep. 2017;5:220–3.
Hermassi S, Hayes LD, Schwesig R. Differences in Fitness and Academic Attainment between Obese, and Non Obese School-Age Adolescent Handball Players: An Explorative, Cross-Sectional Study. In: Applied Sciences vol. 11; 2021.
Download references
Acknowledgements
Special thanks to all participants in this study. The authors would like to express their appreciation to all members of the nursing college who assisted with the research. We would also like to thank the Healthcare Information Technology Education Center and the Office of Institutional Research in the Chung Hwa University of Medical Technology for their valuable help in processing the data.
This work was financially supported by two funding sources. (1) Chung Hwa University of Medical Technology, within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. (2) Kuo General Hospital in Tainan, Taiwan.
Author information
Andrew Ke-Ming Lu and Shi-Yen Tsai contributed equally to this study and should both be listed as the first authors.
Authors and Affiliations
Department of Nursing, College of Nursing, Chung Hwa University of Medical Technology, Tainan, Taiwan
Andrew Ke-Ming Lu & Jeng-Long Hsieh
Healthcare Information Technology Education Center, College of Nursing, Chung Hwa University of Medical Technology, Tainan, Taiwan
Department of Nursing, Kuo General Hospital, Tainan, Taiwan
Shi-Yen Tsai
Library and Information Office, Chung Hwa University of Medical Technology, Tainan, Taiwan
Ching-Yi Lin
You can also search for this author in PubMed Google Scholar
Contributions
All of the authors have contributed significantly and intellectually to the study. The study idea was generated by JLH, who was the main supervisor of the study. AKML, SYT, and JLH designed the study, while CYL carried out the data collection and provided statistical suggestions. AKML conducted the data analysis, structured the results. AKML and JLH contributed intellectually to the discussion. AKML was also the primary writer of the manuscript, while JLH provided critical revision and supervision. Finally, all authors have read and approved the final version of the manuscript.
Corresponding author
Correspondence to Jeng-Long Hsieh .
Ethics declarations
Ethics approval and consent to participate.
The health data collected by the Healthcare Information Technology Education Center had obtained the consent of the participants, and all data had been de-identified. When students enroll, they have obtained consent for the use of school data analysis by the database from Office of Institutional Research, and all data has been de-identified. All participants provided informed consent. All methods were performed in accordance with relevant guidelines and regulations. This study was approved by the Institutional Review Boards (IRB) of Jianan Psychiatric Center and Kuo General Hospital.
Consent of publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lu, AM., Tsai, SY., Lin, CY. et al. Discriminating factors of body composition characteristics for academic performance in nursing college students: a cross-sectional study. BMC Nurs 23 , 305 (2024). https://doi.org/10.1186/s12912-024-01969-y
Download citation
Received : 03 May 2023
Accepted : 22 April 2024
Published : 03 May 2024
DOI : https://doi.org/10.1186/s12912-024-01969-y

Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Body composition
- Academic performance
- Nursing college students
BMC Nursing
ISSN: 1472-6955
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 27 October 2023
- Epidemiology
Body composition and cancer survival: a narrative review
- Patrick T. Bradshaw ORCID: orcid.org/0000-0001-7761-3129 1
British Journal of Cancer volume 130 , pages 176–183 ( 2024 ) Cite this article
4905 Accesses
1 Altmetric
Metrics details
- Cancer epidemiology
Interest in understanding the relationship between body composition and cancer survival has remained strong for decades, with a number of recent systematic reviews on the topic. However, the current state of evidence is based on heterogeneous exposure definitions based on anthropometry, yielding inconsistent findings with regard to this association. Recently the field has taken an exciting direction with the application of radiological assessments to measure specific aspects of body composition, yet reconciliation of findings from these modern assessment tools with those from the historic use of anthropometric data proves challenging. In this paper, I briefly review the biological basis for a link between body composition and cancer survival and summarize the epidemiological evidence with consideration to specific exposure measures. As enthusiasm is building around novel assessments, I conclude with a discussion of issues that researchers should be aware of when interpreting results from these new modalities.
Similar content being viewed by others

Early-life body mass index and risks of breast, endometrial, and ovarian cancers: a dose–response meta-analysis of prospective studies

Longitudinal body mass index and cancer risk: a cohort study of 2.6 million Catalan adults

Body shape phenotypes of multiple anthropometric traits and cancer risk: a multi-national cohort study
Introduction.
The relationship between body composition and cancer survival has been investigated for decades, as noted by a recent review and meta-analysis that included results published over 30 years ago [ 1 ]. Most previous studies in this area have focused on the characteristic of excess adiposity, typically assessed with metrics derived from anthropometric measurements [ 1 , 2 , 3 ]. The specific interest in the association between survival outcomes and fat mass stems from the understanding that excess adiposity is a risk factor for a number of high-burden cancers [ 4 ], the plausible biological mechanisms that may link it to cancer survival [ 5 , 6 , 7 , 8 ], and its relationships with other high-burden comorbidities experienced by cancer patients such as diabetes and cardiovascular disease [ 9 , 10 ]. These connections are especially concerning given the rapid increase in obesity prevalence among cancer survivors [ 11 ], but also because some cancer survivors experience significant weight gain around and immediately after diagnosis [ 12 , 13 ]. Despite their prolific use, anthropometric measures of body composition have well-known limitations [ 14 ] which may play a role in apparently paradoxical findings noted in the body composition literature [ 15 ]. In response, researchers have recently shifted focus to more direct measures of both fat and muscle tissue from clinical assessments that are able to capture the amount and characteristics of the quality of various tissues simultaneously. While there are plausible links suggesting that elevated adiposity is linked to greater risk of death, there is similarly strong evidence suggesting greater lean mass, in particular muscle, is associated with a reduction in risk [ 16 ]. A better understanding of the multi-dimensional nature of the body composition-survival relationship would help resolve some of the ongoing confusion in the field [ 15 , 17 ].
In this report, I will begin by reviewing the relevant biological mechanisms thought to link adiposity and muscle tissue to cancer survival. I will then summarize the epidemiological evidence of the relationship between survival and several common measures of body composition across cancer sites. Throughout the paper I will highlight important considerations for these different body composition metrics regarding the assessment and interpretation of the associations.
Potential mechanisms
As illustrated in Fig. 1 , adipose tissue, especially visceral fat, is metabolically active and has a number of sequelae that are believed to influence the etiology and prognosis of several cancers in a complex interplay [ 18 ]. Excess adiposity is associated with higher levels of several mitogenic factors, such as insulin and insulin like growth factors [ 19 , 20 ] which can encourage proliferation of cancerous cells. An increase in fat mass is associated with elevated levels of serum free fatty acids through several mechanisms that encourage lipolysis. One such mechanism is that visceral adipose tissue is less sensitive to the antilipolytic effect of insulin and more sensitive to the lipolytic effects of catecholamines [ 21 ]; this may be particularly important for cancer patients as catecholamine levels are increased by psychosocial stress, surgery, and treatment [ 22 ]. Adipocytes are also known to secrete a variety of cytokines including the lipolysis stimulating tumor necrosis factor alpha (TNF-α) [ 23 ]. The increase in free fatty acids driven by adipocytes in both subcutaneous and visceral adipose tissue is thought to inhibit insulin’s effect on glucose uptake and oxidation [ 24 ] thereby resulting in a state of insulin resistance, and a subsequent compensatory increase in insulin secretion by the pancreas in an effort to maintain glucose homeostasis [ 25 ]. This increase in insulin precipitates a decrease in insulin-like growth factor binding proteins (IGF-BPs) and a successive increase in bioavailable insulin-like growth factor I (IGF-I) [ 25 ]. Both insulin and IGF-I, as well as TNF-α, bind to membrane-bound receptors on cells that stimulate cellular proliferation and inhibit apoptosis, thereby providing a mechanism for tumor development [ 18 , 26 ]. This pathway is especially relevant to certain cancers, such as breast [ 27 ], as mammary cell carcinomas typically exhibit an over-expression of insulin receptors [ 28 ] and IGF-I receptors [ 29 ] making them very susceptible to the proliferative effects of these hormones. Although visceral fat has been implicated in obesity-related insulin resistance, there is evidence that subcutaneous fat, specifically deep subcutaneous adipose tissue, may also play a role. Subcutaneous fat around the abdomen is comprised of two layers of superficial and deep tissue separated by fascia [ 30 ]. Deep subcutaneous adipose tissue shares several similarities with visceral adipose tissue including a comparable fatty acid composition [ 31 ] and a strong association with insulin resistance [ 32 ]. This suggests the mechanisms mentioned above may also be relevant for certain patterns of subcutaneous fat distribution.
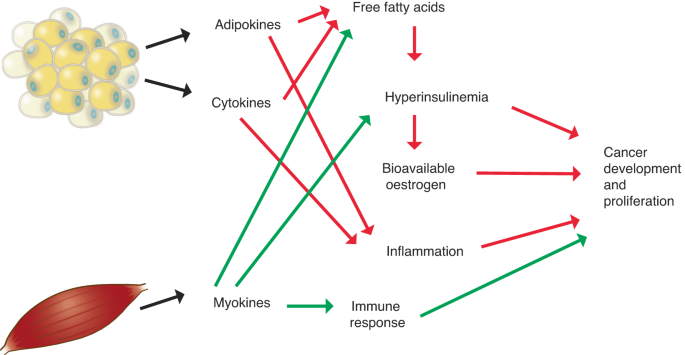
Green arrows denote beneficial direction of associations, red arrows denote deleterious direction of associations.
Obesity-related inflammation may also provide a pathway through which excess adiposity influences carcinogenesis [ 33 , 34 ]. In addition to TNF-α, inflammatory markers produced in response to excess adipose tissue include C-reactive protein (CRP) and interleukin-6 (IL-6), all of which have been implicated in carcinogenesis through various mechanisms [ 18 ]. Obesity is also involved in the regulation of other adipokines with the potential for tumor promotion [ 35 ]. Circulating levels of adiponectin, an adipocyte-specific protein with anti-inflammatory and insulin sensitizing effects [ 36 ], are lower in obese individuals while levels of leptin, which has potential to act as a growth factor, are positively related to adiposity [ 35 ]. In addition to the systemic inflammatory effects of elevated adiposity, mounting evidence suggests that fat cells surrounding the tumor may have important influences on local inflammation in the tumor microenvironment [ 18 , 34 ]. This local inflammation is especially relevant for malignancies that occur in close proximity to adipose tissue depots, such as breast cancer [ 34 ].
Sex hormones are powerful mitogens which stimulate cellular proliferation therefore increasing the likelihood of a DNA mutation during cell division and encouraging replication of aberrant cells [ 37 , 38 , 39 ]. Aromatization of androgens in adipose tissue yields estrone which is subsequently converted to estradiol, the most metabolically active estrogen [ 40 ]. This pathway represents a significant source of estrogen for males and postmenopausal females, in contrast to premenopausal women where ovarian production of estradiol overshadows adipose-mediated formation [ 21 ]. The association between fat mass and sex hormones and related binding proteins, especially estradiol and sex hormone binding globulin (SHBG), are thought to play a significant role in carcinogenesis [ 41 ]. The availability of estradiol to target tissues is primarily determined by the amount of circulating SHBG. Approximately half of the estradiol in the blood is bound to SHBG, the remainder bound to albumin or freely circulating [ 42 ] A common consequence of obesity-related hyperinsulinemia is a reduction in SHBG, resulting in an increase in bioavailable estrogen allowing more free or albumin-bound estradiol to bind with estrogen receptors [ 41 ]. The combined effect of unregulated estradiol exposure and reduction in SHBG has been shown to result in a greater than two-fold increase in free estradiol among obese postmenopausal women compared to women of normal weight [ 42 ]. Excess adiposity, assessed by body mass index (BMI, weight in kilograms divided by squared height in meters), has been shown to be positively associated with estrone, estradiol, free estradiol, free testosterone and prolactin and negatively associated with SHBG [ 21 , 43 ]. Besides its mitogenic potential, there is also evidence that estrogen metabolism generates free radicals which may inflict DNA damage thereby initiating carcinogenesis [ 44 , 45 ]. The influence of obesity-driven hormone dysregulation is particularly relevant for treatment of cancers with a hormonal etiology. In particular, aromatase inhibitor therapy has been shown to be less effective in female breast cancer patients with obesity [ 46 ]. In addition, while adiposity is associated with lower testosterone levels in males [ 47 ], increased exposure to obesity-related growth factors and adipokines is related to activation of androgen receptors which may influence prostate cancer progression [ 48 ].
In contrast to adipose tissue, muscle mass is associated with a generally favorable metabolic and inflammatory profile. Muscle cells produce a number of proteins called myokines that have anti-inflammatory and insulin-sensitizing influences in opposition to the effects of adipokines [ 49 ]. These, and other factors, may be due in part to the relationship between muscle and physical activity [ 50 ]. Physical activity is associated with an increase in insulin sensitivity by increasing expression of the GLUT-4 glucose transporter in the plasma membrane of skeletal muscle [ 51 , 52 , 53 , 54 , 55 ] and by reducing the level of free fatty acids, which have been linked to impaired insulin function [ 56 ]. This increase in insulin sensitivity precipitates a decrease in insulin secretion, which is a possible mechanism for the observed increase in IGF-BPs [ 57 ] and decrease in IGF observed among physically active individuals [ 58 ]. The ability of physical activity to mediate these metabolic hormones and growth factors suggests another potential pathway for the observed protective effect of this exposure [ 59 , 60 ]. This reduction in IGF may yield additional cancer protection as it may reduce sex hormone exposure by encouraging an increase in SHBG production by the liver [ 61 ]. The beneficial effects of physical activity on cancer outcomes may also include improvement of the immune response [ 62 ]. Regular physical activity has been associated with increases in number and cytotoxicity of natural killer cells, as well as favorable shifts in several inflammatory markers including IL-6, CRP, and TNF-α [ 63 ].
Obscuring our understanding of the muscle and cancer survival relationship is the fact that muscle mass is often altered by the presence of malignancy. Tumor-driven inflammation precipitates a catabolic condition known as cachexia [ 64 ], which results in loss of both fat and muscle tissue [ 16 ]. Muscle loss often culminates in a state referred to as sarcopenia, which should be noted can also manifest in the absence of cachexia and is frequently observed in aging populations [ 65 ]. Importantly, cancer patients often present with a high proportion of adipose tissue and low amount of muscle mass together, a condition termed “sarcopenic obesity” [ 16 , 66 ]. This combination of low muscle and high fat is associated with greater systemic inflammation and metabolic dysfunction [ 67 , 68 , 69 ], which complicates the interpretation of the relationship between survival and the individual tissue measures. Importantly, sarcopenic obesity can occur at any BMI, even within the normal weight range [ 16 ], making anthropometric assessments especially unreliable tools to classify body composition phenotypes relevant for cancer survival.
Body mass index
Anthropometric measures such as height and weight are easily gathered and can be collected with reasonable accuracy, making them attractive for large epidemiological studies [ 14 ]. BMI is the current standard for the determination of weight status in populations. Individuals may be classified into underweight (<18.5), normal weight (18.5–<25), overweight (25–<30) and obese (≥30) categories; the latter category potentially broken further into Class 1 (30–<35), Class 2 (35–<40), and Class 3 obesity (≥40) [ 70 ]. Correlations between BMI and directly-measured percent body fat have been noted to range from 0.58 to 0.75 among individuals without cancer [ 71 ], making BMI only moderately associated with adiposity status. Notably, BMI tends to underestimate obesity status when compared to direct measures of adiposity or obesity-related biomarkers [ 72 , 73 ]. Studies have also shown significant variations in body fat within levels of BMI, which may be particularly true among cancer patients. One study showed that BMI only classified 26% of a cohort of cancer patients as obese while 59% had excess fat mass by direct measure [ 74 ]. In this study 31% of those with BMI in the normal range (18.5–<25) had either objectively-measured obesity, low muscle mass, or both, making the normal weight referent category for outcomes analyses a very heterogeneous mix of body composition phenotypes.
A number of systematic reviews have summarized the literature on cancer survival in relation to BMI around the time of diagnosis. A large meta-analysis that involved studies for 15 cancer sites recently considered survival outcomes among those with obesity (BMI ≥ 30) compared to those without obesity. For all cancers combined, authors reported a modestly increased risk of overall mortality (pooled hazard ratio (HR) [95% confidence interval]: 1.14 [1.09, 1.19]) was well as cancer specific death (pooled HR: 1.17 [1.12–1.23]) [ 1 ]. Analyses of individual cancers indicated an increased risk of overall mortality for breast, colorectal, and uterine cancer (all HRs around 1.2), while a decreased risk of death among lung, renal cell carcinoma, and melanoma cancer survivors (HRs ranging from 0.74 to 0.86).
Most recently, the Global Cancer Update Program (CUP global) group considered the relationship between a number of anthropometric measures of adiposity and breast cancer outcomes. Elevated BMI was associated with greater all-cause mortality (across 64 studies, pooled relative risk (RR) per 5 kg/m 2 : 1.07 [1.05–1.10]) as well as breast-cancer specific survival (39 studies, RR: 1.10 [1.06–1.14]), recurrence (63 studies, RR: 1.05 [1.03–1.08]), and incidence of second primary cancers (11 studies, RR: 1.14 [1.04–1.26]) [ 3 ]. The authors found evidence of a nonlinear “J-shaped” relationship between BMI and survival, with the lowest risk occurring around the threshold for overweight status (BMI 25 vs. 20, RR: 0.95 [0.91–0.99]) with an uptick as BMI increased into the Class 2 obese range (BMI 35 vs. 20, RR: 1.21 [1.12–1.30]). A similar but less pronounced dose-response pattern was also reported for breast cancer-specific survival. In total, these findings were graded as providing “strong” and “probable” evidence for a link between BMI and breast cancer outcomes, while trends regarding recurrence and non-breast cancer related death (including cardiovascular disease) were considered to provide suggestive evidence.
Generally concordant findings have been reported in a recent review of studies focused on colorectal cancer survivors. A similar “J-shaped” relationship between BMI and a number of survival outcomes has been reported among colorectal cancer survivors [ 2 ]. Risk of death from any cause was elevated at the extremes of the BMI range; compared to normal weight status those with BMI < 18.5 or ≥35 were at greater risk of death from any cause (summary HR: 1.26 [1.15–1.37] and HR: 1.12 [1.02–1.22], respectively). However, those in the overweight range displayed the lowest risk of death (HR: 0.92 [0.86–0.99]) [ 2 ]. Similar patterns were observed for disease free survival and colorectal cancer-specific deaths.
Over the last decade, several other meta-analyses have reported similarly unexpected associations between BMI and cancer-specific survival across different cancer sites. Among individuals with kidney cancer, obesity and overweight status was associated with lower risk of cancer-specific survival compared to those with normal weight status (HR: 0.85 [0.79, 0.93]), but a large increase in risk was noted among underweight individuals (HR: 2.16 [1.15, 4.04]) [ 75 ]. A qualitative summary of studies of head and neck cancer survivors showed a similar association, with HRs comparing obesity to normal weight around 0.7 for most reports considered [ 76 ]. Notably, these are similar to the magnitude of the pooled HR reported in the sub-analysis of head and neck cancer survivors (HR: 0.59 [0.33–1.05]) in the recent Petrelli meta-analysis [ 1 ].
Circumference measures of central adiposity
Although not as straightforward to measure as height and weight, waist circumference (WC) is a common measure of central adiposity, with higher values tending to indicate a greater deposit of metabolically active visceral fat tissue [ 77 ]. Common thresholds for risk stratification by WC are 102 cm for males and 88 cm for females [ 78 ]. Despite their greater specificity for capturing regional fat distribution, these measures possess important shortcomings. Although these assessments offer a more refined measure of body composition, they are not always practical, and there can be significant variability across different measurement protocols. Waist circumference also tends to be strongly correlated with overall body size measures such as BMI [ 79 ], and so its statistical application as an independent measure of central adiposity requires careful consideration [ 80 ]. Furthermore, although WC is driven strongly by visceral fat mass, it cannot distinguish between subcutaneous and visceral adipose tissue around the abdomen. Waist-hip ratio (WHR), a popular alternative to simple WC, may be more problematic, as larger values can be due to greater abdominal adiposity, reduced gluteal muscle mass, or greater subcutaneous fat deposition around the hips and buttocks [ 14 ]. These different tissue depots may have important characteristics [ 81 , 82 ] that become muddled in a single ratio metric.
Studies relating cancer outcomes to anthropometric assessments of central adiposity are much less common in the extant literature. In a recent meta-analysis, Cheng et al. reported moderately strong summary associations between elevated central adiposity and all-cause mortality (pooled HR: 1.30 [1.15–1.46]) and breast cancer-specific death (HR: 1.26 [1.03–1.55]) across 14 studies of breast cancer survivors [ 83 ]. For colorectal cancer survival, the authors also noted an increased hazard of all-cause mortality (HR: 1.24 [1.04, 1.47]) and colorectal cancer-specific mortality (HR: 1.27 [1.08, 1.49]) [ 83 ]. It is important to note that these pooled estimates included results from studies that used either WC or WHR. As these likely reflect different phenotypes of central adiposity [ 14 ], the heterogeneity of the exposure definition obscures the interpretation. However, the previously mentioned meta-analysis of breast cancer outcomes by the CUP global group did conduct separate analyses for WC and WHR and also reported strong evidence for relationships between all-cause mortality and WC (per 10 cm, summary RR: 1.18 [1.07–1.31]) or WHR (per 0.1 unit, RR: 1.30 [1.20–1.40]), with comparable findings for breast cancer-specific deaths [ 3 ]. Despite the strength of this evidence, the number of studies summarized for each of these measures in the CUP global study was small, ranging from 3 (WC and breast cancer specific mortality) to 8 (WHR and all-cause mortality). Cheng et al. also report that elevated visceral adiposity is associated with greater overall mortality (summary HR: 1.24 [1.04, 1.47]) and cancer-specific death (HR: 1.27 [1.08, 1.49]) among colorectal cancer patients, but modest to null findings for prostate cancer patients [ 83 ].
Weight change
Weight change is an alternative anthropometric measure of adiposity, as weight gain during adulthood reflects a state of sustained positive energy balance and the accumulation of adipose tissue [ 80 ]. Conversely, intentional weight loss among those with excess adiposity has been shown to have beneficial effects on the aforementioned biomarkers of obesity-driven inflammation and insulin resistance [ 84 , 85 ]. Weight change metrics used by researchers have varied somewhat, and an understanding of the various expressions is required when interpreting findings across the broad literature. Weight change can be derived from expressions of anthropometric variables such as weight (in pounds or kilograms) or BMI, representing absolute or percentage changes over time [ 86 ]. Because height scaling equally affects both the numerator and denominator in the calculation of percent change of BMI, it is mathematically equivalent to percent weight change if height is constant over time. However, absolute changes in these variables do not share this property. An important consequence of this difference is that percent weight change over time, as well as absolute BMI change over time, implies larger absolute weight changes for smaller individuals. Taking into account measurement error and normal fluctuations in fluid balance, a threshold of <3% has been proposed for defining weight maintenance [ 86 ]. More commonly, an absolute weight change of <5% is used to define weight maintenance in the literature, with losses more than 5% classified as any weight loss, and gains frequently divided into 5– < 10% (moderate gain) and ≥10% (large gain).
Although the implications of weight gain are fairly clear, interpretation of findings related to weight loss in epidemiological studies is challenging, as intentionality is not typically assessed. Intentional weight loss is typically due to the purposeful adoption of dietary and physical activity practices, while unintentional weight loss is thought to reflect disease progression. Notably, weight stability may also not be a reliable measure of ideal body composition, as incident sarcopenia and myosteatosis after diagnosis has been reported among weight stable cancer patients [ 87 ]. Therefore, considering weight stable individuals as the reference group in cancer survival studies likely shares the problems noted for the normal weight BMI category [ 74 ].
Survival in relation to weight gain after breast cancer diagnosis has been examined extensively in the epidemiological literature. A 2015 meta-analysis of 12 cohort studies of post-diagnosis weight gain and breast cancer survival reported that weight gain of 5% or more from baseline levels was associated with greater risk of mortality from any cause compared to those who maintained weight [ 88 ]. However, the more granular analyses presented by the authors showed that this was driven by extreme weight gain (gain of ≥10%, HR: 1.23 [1.09, 1.39]) with a near-null effect for those gaining 5– < 10% of their baseline weight. Notably, the more recent review by the CUP global group concluded that evidence for a link between postdiagnosis BMI or weight changes was inconclusive, and required further study [ 3 ].
Among colorectal cancer survivors, a meta-analysis included 3 studies that examined the association between survival endpoints and weight change [ 89 ]. The pooled estimates did not suggest an association between any weight gain and overall mortality (pooled HR: 1.03 [0.86, 1.19]) or colorectal cancer-specific survival (HR: 1.02 [0.84, 1.20]) [ 89 ]. However, in individual studies, associations were most pronounced between larger weight gain (absolute change of around 5 kg or more) and colorectal cancer-specific survival [ 90 , 91 ] and overall mortality [ 90 ] for pre- to post-diagnosis change, as well as overall mortality for post-diagnosis changes [ 92 ]. Although not summarized in that meta-analysis, weight loss in the individual studies was associated with a greater risk of mortality outcomes. Subsequently, in a large health system cohort of colorectal cancer patients, Meyerhardt et al. found a greater risk of cancer-specific death (≥10% loss, HR: 3.20 [2.33, 4.39]) and overall mortality (HR: 3.27 [2.56, 4.18]) among those who lost weight after diagnosis [ 93 ]. The association between weight gain and colorectal cancer survival was near-null (≥10% gain HR: 0.93 [0.63, 1.37]), but suggestive for overall mortality (HR: 1.20 [0.91–1.58]). The relationship between weight change and colorectal cancer survival seems to be somewhat more complex than for breast cancer, but indicates a less pronounced convex pattern.
Imaging based measures of body composition
The advent of methods for body composition assessment based on routine clinical imaging has revolutionized this area of research [ 94 , 95 ]. These tools offer a number of advantages over anthropometry including not requiring protocols and logistics involved with measurement of anthropometric data, or relying on inaccurate self-reported values. In addition, assembling cohorts of sufficient size is efficient and feasible for such investigations as they use existing images from the medical record [ 94 ] and so do not require prospective recruitment. While such studies possess a number of attractive features, their utility in understanding the relationship between cancer outcomes and body composition may be limited to malignancies where imaging in relevant anatomical regions is standard in the course of diagnosis and treatment. These techniques allow for accurate and reliable quantification of the amounts of subcutaneous, visceral, and intra-muscular adipose tissue, as well as skeletal muscle tissue. These are typically measured as cross-sectional areas (cm 2 ) from a single image around the L3 vertebra [ 94 ]. These quantities are sometimes expressed as index measures by dividing cross-sectional area by squared height to resemble BMI [ 94 ]. In addition to quantity, adipose tissue and muscle tissue quality may also be calculated by a measure of tissue radiodensity (in Hounsfield units). Greater adipose tissue radiodensity indicates fat cells with less lipid content and potentially more inflammation, while lower muscle radiodensity reflects more fatty infiltration into a given quantity of muscle tissue, and fewer myocytes.
The review by Cheng et al. recently considered 203 studies across 10 cancer sites for evidence linking imaging-based measures of adipose tissue quantity to cancer progression and survival. With the 128 reports included in the meta-analysis, the authors reported modest to near-null summary HRs across all measures of adipose tissue quantity (area or index measures of total, visceral, and subcutaneous tissue) for most cancer sites [ 83 ]. Without regard to statistical significance, a few suggestive associations were also reported: in breast cancer, a greater hazard of overall mortality was observed for subcutaneous fat (pooled HR: 1.36 [0.9, 2.05]), and for progression free survival for visceral fat (HR: 1.20 [0.4, 3.57]) [ 83 ]. Interestingly, HR point estimates below the null were observed for visceral or subcutaneous fat for some ovarian and prostate cancer survival outcomes, although the CI crossed the null for these measures. The authors cited study heterogeneity and some methodological limitations as possible factors for the noted inconsistencies.
Adipose tissue quality also seems to be related to mortality, even independent of adipose tissue quantity. In a recent study of colorectal cancer patients, visceral adipose tissue density (VATD) and subcutaneous adipose tissue density (SATD) were positively associated with overall mortality (per 8 HU in VAT: HR: 1.21 [1.11, 1.32]; per 9 HU in SAT, HR: 1.18 [1.11, 1.26]) with similar associations for colorectal cancer mortality [ 96 ]. Another report found that that SATD was associated with greater mortality among a cohort of breast cancer patients (high vs. mid SATD values, HR: 1.45 [1.15, 1.81]), while the association with VAT was more modest (high vs. mid VATD, HR: 1.16 [0.90, 1.50]) [ 97 ].
A recent review examined the association between muscle and cancer survival, and reported consistent evidence that low muscle quantity was related to poor survival [ 98 ]. Unfortunately, differences in study population, methodology, and other factors prevented a formal meta-analysis of these associations. Muscle quality, assessed by skeletal muscle radiodensity is also emerging as an important prognostic factor, with several studies noting inverse associations between muscle density and mortality, sometimes independent of quantity [ 9 , 99 , 100 , 101 , 102 ].
Biological plausibility and volumes of epidemiological evidence suggest a link between body composition and cancer survival, but several issues remain. Reports focused on BMI have been inconsistent and sometimes suggest a contradictory message regarding weight status to what public health officials endorse for general health. The finding that the optimal BMI lies above the upper limit of the normal-weight range in those with chronic disease has been termed the “obesity paradox” or “overweight paradox” which has fueled a robust controversy [ 17 , 103 ]. However, it should be noted that in this paradox, the nadir of the J-shaped BMI-mortality curve is often shifted to the right rather than suggesting a monotonic decrease in risk with greater BMI (although the latter has been observed). Selection bias has been suggested as the culprit for this paradox [ 17 ], but the biasing relationships may have to be unreasonable for this to be the case [ 104 ]. Emerging evidence has instead implicated measurement error as the underlying issue, as BMI is a poor metric of the putative aspects of body composition. It has specifically been shown that the observed shifts in the mortality curve may be consistent with opposing relationships between constituent expressions of fat and lean mass [ 105 ], and so these observations are not as contrary to general messaging as they might initially appear. In fact, some researchers have proposed this phenomena be re-named the “BMI paradox” to emphasize that the confusion is driven by BMI’s inherent limitations as a relevant measure of adiposity quantity and distribution [ 106 ]. Findings for central measures of adiposity, such as WC, and weight gain after diagnosis, which are more precise measures of adiposity, seem to be somewhat more consistent, but the evidence base for these metrics is relatively small.
Imaging-based body composition assessment offers an exciting opportunity to study multiple characteristics of different fat and muscle tissues in clinical populations. To understand the interplay between this myriad of tissue quantity and quality metrics, the analysis of body composition would ideally consider all of these variables together rather than analyzing individual factors in serial fashion. Paradoxical or null relationships between adiposity and survival are often hypothesized to result from potentially protective factors related to the greater muscle mass that tends to occur with higher fat mass [ 98 ]. In fact, the recent review of imaging based adiposity and cancer survival pointed out that only 11 of the 128 studies included in the study adjusted for muscle in their individual analyses, with the authors concluding that this potentially contributed to the noted heterogeneity across reports [ 83 ]. Simultaneous consideration of all tissues is also suggested by biology, given our understanding of the interrelationships between fat and muscle [ 49 ].
Although imaging-based measures of body composition represent an important advancement in the field, some points regarding their interpretation bear mentioning. CT-based body composition measures provide insight into the distribution of fat and muscle in the regions being imaged. Although these metrics correlate strongly with total body volume of these tissues, they do not quantify the exact amount [ 107 ]. Another ongoing consideration in imaging-based body composition research is the appropriate expression of tissue quantity that is measured in cross-sectional areas. It has become common, although not standard, for researchers to scale area measures by squared height as in the practice of calculating BMI from weight and height. This results in measures of visceral and adipose tissue index (VATI and SATI, respectively), and skeletal muscle index (SMI). The goal of this transformation is to create measures of body composition quantity that are independent of stature. However, the use of squared height in the calculation of BMI was derived to make the resulting weight-based measure independent of height, which is not without controversy [ 108 ]. Interestingly, weight-based measures of body composition, such as total lean mass and total fat mass, do lend themselves to BMI-style normalization by squared height. The resulting metrics, fat mass index (FMI) and lean mass index (LMI) have the appealing property of summing to BMI, thus representing a decomposition into its constituent tissue compartments [ 109 ]. It is not clear that applying the same normalization to individual area-based measures achieves the same goal, and other scaling factors may be more appropriate [ 110 ]. Ultimately, different uses of area-based and height-adjusted index measures may contribute to the variations noted in the literature on adiposity and survival [ 83 ].
Research based on imaging modalities presents challenges to clinical and epidemiological research, some of which have been acknowledged in the radiology literature [ 111 ]. However, there are several specifically relevant to opportunistic assessments of body composition in connection with cancer survival. As these metrics are derived from images that are only obtained in the course of the cancer diagnosis, they are unable to be used to characterize the relationship between these body composition features across the entire cancer continuum. Examination of the associations between body composition and incident disease would clarify their relationship to the etiology of cancer subtypes or other factors related to disease severity at diagnosis. As another consequence, if adjustment for body composition before diagnosis is required, researchers are still relegated to the use of anthropometric variables, frequently BMI, which is discordant with the focus on specific fat and muscle characteristics. Furthermore, relatively few patients receive repeated scans, at least to the degree to accurately characterize longitudinal trajectories of body composition. Thus, studies that have utilized repeated body composition assessments have been much smaller than those focused on baseline values [ 87 , 112 ]. In addition, those with repeated radiological measurements may tend to be less likely to have lower stage disease than those who do not [ 87 ], and so results of such analyses may not be generalizable to all cancer patients.
The field of body composition and cancer survival is rapidly evolving with the availability of detailed tissue assessments in clinical populations. Reconciling evidence on body composition from observational studies across a broad array of assessment methodologies is an ongoing challenge when designing meaningful interventions for cancer survivors [ 95 , 113 ]. While the shift toward more direct measures of body composition for survival research is a clear step forward, researchers and clinicians should to work to identify consistent and meaningful clinical targets that align with general public health guidelines.
Petrelli F, Cortellini A, Indini A, Tomasello G, Ghidini M, Nigro O, et al. Association of Obesity With Survival Outcomes in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open. 2021;4:e213520.
Article PubMed PubMed Central Google Scholar
Li Y, Li C, Wu G, Yang W, Wang X, Duan L, et al. The obesity paradox in patients with colorectal cancer: a systematic review and meta-analysis. Nutr Rev. 2022;80:1755–68.
Article PubMed Google Scholar
Chan DSM, Vieira R, Abar L, Aune D, Balducci K, Cariolou M, et al. Postdiagnosis body fatness, weight change and breast cancer prognosis: Global Cancer Update Program (CUP global) systematic literature review and meta-analysis. Int J Cancer. 2023;152:572–99.
Article CAS PubMed Google Scholar
Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15:484–98.
Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28.
Demark-Wahnefried W, Platz EA, Ligibel JA, Blair CK, Courneya KS, Meyerhardt JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomark Prev. 2012;21:1244–59.
Article Google Scholar
Parekh N, Chandran U, Bandera EV. Obesity in cancer survival. Annu Rev Nutr. 2012;32:311–42.
Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N. Y Acad Sci. 2012;1271:37–43.
Article CAS PubMed PubMed Central Google Scholar
Brown JC, Caan BJ, Prado CM, Weltzien E, Xiao J, Cespedes Feliciano EM, et al. Body Composition and Cardiovascular Events in Patients With Colorectal Cancer: A Population-Based Retrospective Cohort Study. JAMA Oncol. 2019;5:967–72.
Cespedes Feliciano EM, Chen WY, Bradshaw PT, Prado CM, Alexeeff S, Albers KB, et al. Adipose Tissue Distribution and Cardiovascular Disease Risk Among Breast Cancer Survivors. J Clin Oncol. 2019;37:2528–36.
Greenlee H, Shi Z, Sardo Molmenti CL, Rundle A, Tsai WY. Trends in Obesity Prevalence in Adults With a History of Cancer: Results From the US National Health Interview Survey, 1997 to 2014. J Clin Oncol. 2016;34:3133–40.
Winkels RM, Snetselaar T, Adriaans A, van Warmerdam LJC, Vreugdenhil A, Slooter GD, et al. Changes in body weight in patients with colorectal cancer treated with surgery and adjuvant chemotherapy: An observational study. Cancer Treat Res Commun. 2016;9:111–5.
van den Berg MM, Winkels RM, de Kruif JT, van Laarhoven HW, Visser M, de Vries JH, et al. Weight change during chemotherapy in breast cancer patients: a meta-analysis. BMC Cancer. 2017;17:259.
Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35:83–92.
Prado CM, Gonzalez MC, Heymsfield SB. Body composition phenotypes and obesity paradox. Curr Opin Clin Nutr Metab Care. 2015;18:535–51.
Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016;75:188–98.
Lajous M, Banack HR, Kaufman JS, Hernan MA. Should patients with chronic disease be told to gain weight? The obesity paradox and selection bias. Am J Med. 2015;128:334–6.
Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and cancer: local and systemic mechanisms. Annu Rev Med. 2015;66:297–309.
Zimmet PZ. Hyperinsulinemia-how innocent a bystander? Diabetes Care. 1993;16:56–70.
Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–89.
Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738.
Wackerhage H, Christensen JF, Ilmer M, von Luettichau I, Renz BW, Schonfelder M. Cancer catecholamine conundrum. Trends Cancer. 2022;8:110–22.
Green A, Rumberger JM, Stuart CA, Ruhoff MS. Stimulation of lipolysis by tumor necrosis factor-alpha in 3T3-L1 adipocytes is glucose dependent: implications for long-term regulation of lipolysis. Diabetes. 2004;53:74–81.
Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Investig. 1996;97:2859–65.
Kaaks R, Lukanova A. Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc. 2001;60:91–106.
Blackburn GL, Waltman B. Obesity and Insulin Resistance. In: A. McTiernan (ed). Cancer Prevention and Management Through Exercise and Weight Control, Boca Raton, FL; CRC Press: 2006. Ch. 20, p. 301–316.
Bruning PF, Bonfrer JM, van Noord PA, Hart AA, de Jong-Bakker M, Nooijen WJ. Insulin resistance and breast-cancer risk. Int J Cancer. 1992;52:511–6.
Papa V, Belfiore A. Insulin receptors in breast cancer: biological and clinical role. J Endocrinol Investig. 1996;19:324–33.
Article CAS Google Scholar
Papa V, Gliozzo B, Clark GM, McGuire WL, Moore D, Fujita-Yamaguchi Y, et al. Insulin-like growth factor-I receptors are overexpressed and predict a low risk in human breast cancer. Cancer Res. 1993;53:3736–40.
CAS PubMed Google Scholar
Walker GE, Verti B, Marzullo P, Savia G, Mencarelli M, Zurleni F, et al. Deep Subcutaneous Adipose Tissue: A Distinct Abdominal Adipose Depot. Obesity. 2007;15:1933–43.
Lundbom J, Hakkarainen A, Lundbom N, Taskinen MR. Deep subcutaneous adipose tissue is more saturated than superficial subcutaneous adipose tissue. Int J Obes (Lond). 2013;37:620–2.
Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab. 2000;278:E941–8.
Bullo M, Garcia-Lorda P, Megias I, Salas-Salvado J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res. 2003;11:525–31.
Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J Clin Oncol. 2016;34:4270–6.
van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomark Prev. 2009;18:2569–78.
Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer. 2006;94:1221–5.
Preston-Martin S, Pike MC, Ross RK, Jones PA, Henderson BE. Increased cell division as a cause of human cancer. Cancer Res. 1990;50:7415–21.
Dickson RB, Stancel GM. Chapter 8: Estrogen receptor-mediated processes in normal and cancer cells. J Natl Cancer Inst Monogr. 2000;2000:135–45.
Flototto T, Djahansouzi S, Glaser M, Hanstein B, Niederacher D, Brumm C, et al. Hormones and hormone antagonists: mechanisms of action in carcinogenesis of endometrial and breast cancer. Horm Metab Res. 2001;33:451–7.
Judd HL, Shamonki IM, Frumar AM, Lagasse LD. Origin of serum estradiol in postmenopausal women. Obstet Gynecol. 1982;59:680–6.
Kaaks R, McTiernan A. Obesity and Sex Hormones. In: A. McTiernan (ed). Cancer Prevention and Management Through Exercise and Weight Control, Boca Raton, FL; CRC Press: 2006. Ch. 19, p. 289–300.
Key TJ, Allen NE, Verkasalo PK, Banks E. Energy balance and cancer: the role of sex hormones. Proc Nutr Soc. 2001;60:81–9.
McTiernan A, Rajan KB, Tworoger SS, Irwin M, Bernstein L, Baumgartner R, et al. Adiposity and sex hormones in postmenopausal breast cancer survivors. J Clin Oncol. 2003;21:1961–6.
Roy D, Liehr JG. Estrogen, DNA damage and mutations. Mutat Res. 1999;424:107–15.
Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocr Rev. 2000;21:40–54.
Lee K, Kruper L, Dieli-Conwright CM, Mortimer JE. The Impact of Obesity on Breast Cancer Diagnosis and Treatment. Curr Oncol Rep. 2019;21:41.
Kelly DM, Jones TH. Testosterone and obesity. Obes Rev. 2015;16:581–606.
Olivas A, Price RS. Obesity, Inflammation, and Advanced Prostate Cancer. Nutr Cancer. 2021;73:2232–48.
Kirk B, Feehan J, Lombardi G, Duque G. Muscle, Bone, and Fat Crosstalk: the Biological Role of Myokines, Osteokines, and Adipokines. Curr Osteoporos Rep. 2020;18:388–400.
Eckel J. Myokines in metabolic homeostasis and diabetes. Diabetologia. 2019;62:1523–8.
Ivy JL. Role of exercise training in the prevention and treatment of insulin resistance and non-insulin-dependent diabetes mellitus. Sports Med. 1997;24:321–36.
Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–61.
Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol. 2000;88:774–87.
Borghouts LB, Keizer HA. Exercise and insulin sensitivity: a review. Int J Sports Med. 2000;21:1–12.
Perez-Martin A, Raynaud E, Mercier J. Insulin resistance and associated metabolic abnormalities in muscle: effects of exercise. Obes Rev. 2001;2:47–59.
Roden M. How free fatty acids inhibit glucose utilization in human skeletal muscle. N Physiol Sci. 2004;19:92–96.
CAS Google Scholar
McCarty MF. Up-regulation of IGF binding protein-1 as an anticarcinogenic strategy: relevance to caloric restriction, exercise, and insulin sensitivity. Med Hypotheses. 1997;48:297–308.
Allen NE, Appleby PN, Kaaks R, Rinaldi S, Davey GK, Key TJ. Lifestyle determinants of serum insulin-like growth-factor-I (IGF-I), C-peptide and hormone binding protein levels in British women. Cancer Causes Control. 2003;14:65–74.
Yee D, Paik S, Lebovic GS, Marcus RR, Favoni RE, Cullen KJ, et al. Analysis of insulin-like growth factor I gene expression in malignancy: evidence for a paracrine role in human breast cancer. Mol Endocrinol. 1989;3:509–17.
Fair AM, Dai Q, Shu XO, Matthews CE, Yu H, Jin F, et al. Energy balance, insulin resistance biomarkers, and breast cancer risk. Cancer Detect Prev. 2007;31:214–9.
McTiernan A, Ulrich C, Slate S, Potter J. Physical activity and cancer etiology: associations and mechanisms. Cancer Causes Control. 1998;9:487–509.
Wetmore CM, Ulrich CM. Mechanisms Associating Physical Activity with Cancer Incidence: Exercise and Immune Function. In: A. McTiernan (ed). Cancer Prevention and Management Through Exercise and Weight Control, Boca Raton, FL; CRC Press: 2006. Ch. 9, p. 157–176.
Hojman P. Exercise protects from cancer through regulation of immune function and inflammation. Biochem Soc Trans. 2017;45:905–11.
Aversa Z, Costelli P, Muscaritoli M. Cancer-induced muscle wasting: latest findings in prevention and treatment. Ther Adv Med Oncol. 2017;9:369–82.
von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–33.
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. lancet Oncol. 2008;9:629–35.
Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: A Critical appraisal of the current evidence. Clin Nutr. 2012;31:583–601.
Lu CW, Yang KC, Chang HH, Lee LT, Chen CY, Huang KC. Sarcopenic obesity is closely associated with metabolic syndrome. Obes Res Clin Pr. 2013;7:e301–7.
Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14:513–37.
World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253.
Google Scholar
Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–39.
Okorodudu DO, Jumean MF, Montori VM, Romero-Corral A, Somers VK, Erwin PJ, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes (Lond). 2010;34:791–9.
Shah NR, Braverman ER. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PloS ONE. 2012;7:e33308.
Gonzalez MC, Pastore CA, Orlandi SP, Heymsfield SB. Obesity paradox in cancer: new insights provided by body composition. Am J Clin Nutr. 2014;99:999–1005.
Kim LH, Doan P, He Y, Lau HM, Pleass H, Patel MI. A Systematic Review and Meta-Analysis of the Significance of Body Mass Index on Kidney Cancer Outcomes. J Urol. 2021;205:346–55.
Hollander D, Kampman E, van Herpen CM. Pretreatment body mass index and head and neck cancer outcome: A review of the literature. Crit Rev Oncol Hematol. 2015;96:328–38.
Molarius A, Seidell JC. Selection of anthropometric indicators for classification of abdominal fatness-a critical review. Int J Obes Relat Metab Disord. 1998;22:719–27.
Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311:158–61.
Mahmoud I, Al-Wandi AS, Gharaibeh SS, Mohamed SA. Concordances and correlations between anthropometric indices of obesity: a systematic review. Public Health. 2021;198:301–6.
Hu FB. Ch 5: Measurements of Adiposity and Body Composition. In: Frank Hu, editor. Obesity Epidemiology. https://doi.org/10.1093/acprof:oso/9780195312911.003.0005 0 New York: Oxford University Press; 2008.
Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–85.
Dulloo AG, Jacquet J, Solinas G, Montani JP, Schutz Y. Body composition phenotypes in pathways to obesity and the metabolic syndrome. Int J Obes (Lond). 2010;34:S4–17.
Cheng E, Kirley J, Cespedes Feliciano EM, Caan BJ. Adiposity and cancer survival: a systematic review and meta-analysis. Cancer Causes Control. 2022;33:1219–46.
Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, Inflammation, and Cancer. Annu Rev Pathol. 2016;11:421–49.
Tahrani AA, Morton J. Benefits of weight loss of 10% or more in patients with overweight or obesity: A review. Obes (Silver Spring). 2022;30:802–40.
Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. Int J Obes (Lond). 2006;30:391–9.
Brown JC, Caan BJ, Cespedes Feliciano EM, Xiao J, Weltzien E, Prado CM, et al. Weight stability masks changes in body composition in colorectal cancer: a retrospective cohort study. Am J Clin Nutr. 2021;113:1482–9.
Playdon MC, Bracken MB, Sanft TB, Ligibel JA, Harrigan M, Irwin ML. Weight Gain After Breast Cancer Diagnosis and All-Cause Mortality: Systematic Review and Meta-Analysis. J Natl Cancer Inst. 2015;107:djv275.
Otto SJ, Korfage IJ, Polinder S, van der Heide A, de Vries E, Rietjens JA, et al. Association of change in physical activity and body weight with quality of life and mortality in colorectal cancer: a systematic review and meta-analysis. Support Care Cancer. 2015;23:1237–50.
Baade PD, Meng X, Youl PH, Aitken JF, Dunn J, Chambers SK. The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland, Australia. Cancer Epidemiol Biomark Prev. 2011;20:1410–20.
Campbell PT, Newton CC, Dehal AN, Jacobs EJ, Patel AV, Gapstur SM. Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012;30:42–52.
Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Nelson H, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008;26:4109–15.
Meyerhardt JA, Kroenke CH, Prado CM, Kwan ML, Castillo A, Weltzien E, et al. Association of Weight Change after Colorectal Cancer Diagnosis and Outcomes in the Kaiser Permanente Northern California Population. Cancer Epidemiol Biomark Prev. 2017;26:30–7.
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006.
Brown JC, Cespedes Feliciano EM, Caan BJ. The evolution of body composition in oncology-epidemiology, clinical trials, and the future of patient care: facts and numbers. J Cachexia Sarcopenia Muscle. 2018;9:1200–8.
Feliciano EMC, Winkels RM, Meyerhardt JA, Prado CM, Afman LA, Caan BJ. Abdominal adipose tissue radiodensity is associated with survival after colorectal cancer. Am J Clin Nutr. 2021;114:1917–24.
Cheng E, Caan BJ, Chen WY, Irwin ML, Prado CM, Cespedes Feliciano EM. Adipose tissue radiodensity and mortality among patients with nonmetastatic breast cancer. Clin Nutr. 2022;41:2607–13.
Cespedes Feliciano EM, Kroenke CH, Caan BJ. The Obesity Paradox in Cancer: How Important Is Muscle? Annu Rev Nutr. 2018;38:357–79.
Sjoblom B, Gronberg BH, Wentzel-Larsen T, Baracos VE, Hjermstad MJ, Aass N, et al. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non-small cell lung cancer. Clin Nutr. 2016;35:1386–93.
Kroenke CH, Prado CM, Meyerhardt JA, Weltzien EK, Xiao J, Cespedes Feliciano EM, et al. Muscle radiodensity and mortality in patients with colorectal cancer. Cancer. 2018;124:3008–15.
Vrieling A, Kampman E, Knijnenburg NC, Mulders PF, Sedelaar JPM, Baracos VE, et al. Body Composition in Relation to Clinical Outcomes in Renal Cell Cancer: A Systematic Review and Meta-analysis. Eur Urol Focus. 2018;4:420–34.
Maurits JSF, Sedelaar JPM, Mulders PFA, Aben KKH, Kiemeney L, Vrieling A. Skeletal muscle radiodensity and visceral adipose tissue index are associated with survival in renal cell cancer—A multicenter population-based cohort study. Clin Nutr. 2022;41:131–43.
Stevens J, Bradshaw PT, Truesdale KP, Jensen MD. Obesity Paradox should not interfere with public health efforts. Int J Obes. 2015;39:80–1.
Glymour MM, Vittinghoff E. Commentary: Selection bias as an explanation for the obesity paradox: just because it’s possible doesn’t mean it’s plausible. Epidemiology. 2014;25:4–6.
Bradshaw PT, Zevallos JP, Wisniewski K, Olshan AF. A Bayesian Sensitivity Analysis to Partition Body Mass Index Into Components of Body Composition: An Application to Head and Neck Cancer Survival. Am J Epidemiol. 2019;188:2031–9.
Caan BJ, Cespedes Feliciano EM, Kroenke CH. The Importance of Body Composition in Explaining the Overweight Paradox in Cancer-Counterpoint. Cancer Res. 2018;78:1906–12.
MacDonald AJ, Greig CA, Baracos V. The advantages and limitations of cross-sectional body composition analysis. Curr Opin Support Palliat Care. 2011;5:342–9.
Diverse Populations Collaborative, G. Weight-height relationships and body mass index: some observations from the Diverse Populations Collaboration. Am J Phys Anthropol. 2005;128:220–9.
Kyle UG, Schutz Y, Dupertuis YM, Pichard C. Body composition interpretation. Contributions of the fat-free mass index and the body fat mass index. Nutrition. 2003;19:597–604.
Brown JC, Heymsfield SB, Caan BJ. Scaling of computed tomography body composition to height: relevance of height‐normalized indices in patients with colorectal cancer. J Cachexia Sarcopenia Muscle. 2022;13:203–9.
Dewey M, Bosserdt M, Dodd JD, Thun S, Kressel HY. Clinical Imaging Research: Higher Evidence, Global Collaboration, Improved Reporting, and Data Sharing Are the Grand Challenges. Radiology. 2019;291:547–52.
Cooper AB, Slack R, Fogelman D, Holmes HM, Petzel M, Parker N, et al. Characterization of Anthropometric Changes that Occur During Neoadjuvant Therapy for Potentially Resectable Pancreatic Cancer. Ann Surg Oncol. 2015;22:2416–23.
Anderson AS, Martin RM, Renehan AG, Cade J, Copson ER, Cross AJ, et al. Cancer survivorship, excess body fatness and weight-loss intervention-where are we in 2020? Br J Cancer. 2021;124:1057–65.
Download references
Author information
Authors and affiliations.
School of Public Health, Division of Epidemiology, University of California Berkeley, Berkeley, CA, USA
Patrick T. Bradshaw
You can also search for this author in PubMed Google Scholar
Contributions
Dr PTB conducted the literature review, and wrote and edited the paper.
Corresponding author
Correspondence to Patrick T. Bradshaw .
Ethics declarations
Competing interests.
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Bradshaw, P.T. Body composition and cancer survival: a narrative review. Br J Cancer 130 , 176–183 (2024). https://doi.org/10.1038/s41416-023-02470-0
Download citation
Received : 01 April 2023
Revised : 07 October 2023
Accepted : 16 October 2023
Published : 27 October 2023
Issue Date : 10 February 2024
DOI : https://doi.org/10.1038/s41416-023-02470-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
These techniques are now recognized as golden standard for body composition analysis. 25 27. The purpose of this paper is to give a brief introduction to the most commonly used methods for body composition analysis and a review of an MRI-based body composition analysis technique, comparing its performance to other methods.
This simple two-compartment (2C) model of the human body has become the mainstay of body composition assessment in clinical nutrition. A little later, in 1940s and 1950s, the 2C model was ...
TBW is under tight physiological control and in healthy humans the hydration of the non-fat compartment of the body, the fat-free mass (FFM), is relatively constant at 0.732 mL/g FFM. If FFM is ...
The emergence of obesity and related chronic diseases in the 1970s [ 9] prompted a new wave of body composition research aimed at developing methods of phenotyping people for adiposity and ...
Body composition is a key component for maintaining good general health and longevity. It can be influenced by a variety of factors, including genetics, environment, and lifestyle choices. ... Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial ...
In addition, research has shown that the combination of both RT and aerobic exercise (i.e., concurrent training) can be an effective approach to optimize body recomposition ( 5,57 ). Thus, practitioners, coaches, and trainers commonly recommend concurrent training for individuals aiming to gain muscle and lose fat ( 24 ).
Body composition is acknowledged as a determinant of athletic health and performance. Its assessment is crucial in evaluating the efficiency of a diet or aspects related to the nutritional status of the athlete. ... (BIA) and bioelectric impedance vector analysis (BIVA) have recently gained attention in sports, as well as in a research context ...
Body composition is acknowledged as a determinant of athletic health and performance. Its assessment is crucial in evaluating the efficiency of a diet or aspects related to the nutritional status of the athlete. Despite the methods traditionally used to assess body composition, bioelectric impedance analysis (BIA) and bioelectric impedance vector analysis (BIVA) have recently gained attention ...
Employing higher protein and RET concomitantly can, as shown, even increase muscle mass and reduce fat mass; such a pattern of weight loss has been referred to as high-quality weight loss or body ...
Abstract —Bioelectrical impedance (BIA) is a painless, non-invasive, and easily portable technique that may. clarify how th e human body is operational. Body. composition (BC) assessment is ...
1Obesity and Body Composition Research Center, Zhejiang University School of Public Health, ... first century, on average 1481 body composition papers were published annually worldwide.
most widely used way to estimate body fat is the. body mass index (BMI)—body weight normal-. ized by height squared (kg/m ). Being a very. simple and inexpensive method, it is the basis. for WHO ...
Poor body composition may affect health status, and better body composition is often associated with better academic performance. Nursing students face heavy academic and practical pressures, and the relationship between body composition and academic performance in this group is not fully understood. This cross-sectional observational study used de-identified student data from a university of ...
In our study, body fat in men and women were 18.33 and 19.82. The body fat percentage in men and women were 25.74% and 34.01%. Visceral fat area in men and women were 91.98 and 77 cm 2. And, with the increase of age, body fat, body fat percentages and visceral fat area also increased, both in men and in women.
Change in body composition due to dietary, exercise, and lifestyle interventions is of great importance to the decision-making Models of body composition with 2 (2-C), 3 (3-C), or 4 (4-C ...
By Carey Phillips March 10, 2023. NATICK, Mass. — The U.S. Army Research Institute of Environmental Medicine (USARIEM) cross-divisional team recently completed the U.S. Army Center for Initial ...
DOI: 10.1530/endoabs.99.p238 Corpus ID: 269689835; Musculoskeletal health and body composition in patients discontinuing long-term prednisolone treatment - prospective data from the replace Study
The advent of methods for body composition assessment based on routine clinical imaging has revolutionized this area of research [94, 95]. These tools offer a number of advantages over ...